As filed with the Securities and Exchange Commission on October 17, 2022.
Registration Statement No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_____________________________________________
FORM F-4
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
_____________________________________________
Moolec Science SA
(Exact name of Registrant as Specified in its articles of association)*
_____________________________________________
N/A
(Translation of registrant name into English)
_____________________________________________
Grand Duchy of Luxembourg | | 1119 | | Not Applicable |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (IRS Employer
Identification Number) |
17, Boulevard F.W. Raiffeisen
L-2411 Luxembourg,
Grand Duchy of Luxembourg
+352 26 49 65 65
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Cogency Global Inc.
122 East 42nd Street, 18th Floor
New York, NY 10168
+1 212 947 7200
(Address, including zip code, and telephone number, including area code, of agent of service)
_____________________________________________
With copies to:
Leib Orlanski
Matthew Ogurick
K&L Gates LLP
599 Lexington Avenue
New York, NY 10022
(212) 536-3901 | | Matthew Poulter
Pierre-Emmanuel Perais
Linklaters LLP
1290 Avenue of the Americas
New York, NY 10104
(212)-903-9014 |
_____________________________________________
Approximate date of commencement of proposed sale of the securities to the public: As soon as practicable after the effectiveness of this registration statement and upon completion of the business combination described in the enclosed proxy statement/prospectus.
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If applicable, place an X in the box to designate the appropriate rule provision relied upon in conducting this transaction:
| | Exchange Act Rule 13e- 4(i) (Cross- Border Issuer Tender Offer) | | ☐ | | |
Exchange Act Rule 14d- 1(d) (Cross- Border Third Party Tender Offer) | | ☐ | |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
| | Emerging growth company | | ☒ | | |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, pursuant to said Section 8(a), may determine.
Table of Contents
Information contained herein is subject to completion or amendment. A registration statement relating to these securities has been filed with the Securities and Exchange Commission. These securities may not be sold nor may offers to buy be accepted prior to the time the registration statement becomes effective. This proxy statement/prospectus shall not constitute an offer to sell or the solicitation of an offer to buy nor shall there be any sale of these securities in any jurisdiction in which such offer, solicitation or sale would be unlawful.
PRELIMINARY PROXY STATEMENT FOR SPECIAL MEETING OF LIGHTJUMP ACQUISITION CORPORATION AND PROSPECTUS FOR ORDINARY SHARES AND WARRANTS OF MOOLEC SCIENCE LIMITED SUBJECT TO COMPLETION, DATED October 17, 2022
LightJump Acquisition Corporation
2735 Sand Hill Road, Suite 110
Menlo Park, CA 94025
Dear LightJump Acquisition Corporation Stockholder:
You are cordially invited to attend the special meeting of stockholders of LightJump Acquisition Corporation which we refer to as “we,” “us,” “our,” “LightJump” or “SPAC” to be held at [•], Eastern time, on [•], 2022. The special meeting will be conducted via live webcast at [______________].
At the special meeting, our stockholders will be asked to consider and vote upon several proposals relating to LightJump’s proposed business combination with Moolec Science Limited, a private limited company incorporated under the laws of England and Wales (“Moolec” or the “Company”), and Moolec Science SA, a public limited liability company (société anonyme) governed by the laws of the Grand Duchy of Luxembourg, with its registered office at 17, Boulevard F.W. Raiffeisen, L-2411 Luxembourg, Grand Duchy of Luxembourg and registered with the Luxembourg Trade and Companies’ Register (Registre de Commerce et des Sociétés, Luxembourg) under number B268440 (“Holdco”). The proposals include approval of the Business Combination Agreement, dated as of June 14, 2022, among LightJump, Moolec, Holdco and Moolec Acquisition, Inc., a Delaware corporation (“Merger Sub”) (the “Business Combination Agreement”), by which, among other things, LightJump and Moolec would become subsidiaries of Holdco, shares of SPAC Common Stock would be converted into Holdco ordinary shares and SPAC Warrants would be converted into Holdco warrants, which we refer to as the “Business Combination Proposal.” We refer to the transactions contemplated by the Business Combination Agreement as the “Business Combination.” It is expected that, if the Business Combination is consummated, Holdco’s shares and warrants would all be listed on Nasdaq, and Holdco would become a publicly-held company. The accompanying proxy statement/prospectus describes the Business Combination Agreement, the Business Combination and related transactions in detail, and you should read it carefully. Please pay particular attention to the section entitled “Risk Factors”, beginning on page 50.
Our board of directors has unanimously approved and adopted the Business Combination Agreement and unanimously recommends that our stockholders vote FOR all of the proposals to be presented at the special meeting. Our Sponsor, directors and officers have agreed to vote all of their shares, and not seek redemption of such shares, which represent approximately 54.4% of the outstanding shares, in favor of all such proposals. When you consider the board of directors’ recommendation and such persons’ agreement to vote in favor, you should keep in mind that our directors and our officers have interests in the Business Combination that may conflict with your interests as a stockholder. See the section entitled “The Business Combination — Interests of LightJump’s Directors and Officers in the Business Combination.”
Pursuant to LightJump’s Amended and Restated Certificate of Incorporation, we are providing our Public Stockholders with the opportunity to redeem all or a portion of their shares of SPAC Common Stock for cash upon consummation of the Business Combination. The per share redemption price will be equal to the aggregate amount then on deposit in the Trust Account that holds the proceeds of our IPO and related private placement, including interest (net of taxes payable), divided by the number of then outstanding public shares, which excludes the shares owned by LightJump’s Initial Stockholders, who have waived their redemption rights. For illustrative purposes, based on funds in the Trust Account of approximately $27,993,798 on July 12, 2022 and 2,767,210 public shares outstanding, the estimated per share redemption price would have been approximately $10.12. Holders of SPAC Common Stock issued in LightJump’s initial public offering (“Public Shares”) may elect to redeem their Public Shares even if they vote for approval of the Business Combination Agreement and the other proposals presented at the special meeting. There are no redemption rights with respect to our outstanding warrants.
Table of Contents
Your vote is very important. Whether or not you plan to attend the special meeting, please vote by telephone or online, or complete, sign, date and return the enclosed proxy card in the postage-paid envelope provided. If you are a holder of record of SPAC Common Stock, you may also cast your vote at the special meeting. If you hold your shares in “street name” through a bank, broker or other nominee, you will need to follow the instructions provided to you by your bank, broker or other nominee to ensure that your shares are represented and voted at the special meeting.
If you vote by proxy, your shares will be voted in accordance with your instructions. If you sign and return your proxy card without indicating how you wish to vote, your proxy will be voted in favor of each of the proposals presented at the special meeting. If you fail to vote by proxy or to instruct your bank, broker or other nominee how to vote, and do not vote in person at the special meeting, your shares will not be counted for purposes of determining whether a quorum is present at the special meeting of stockholders and, if a quorum is present, will have the same effect as a vote “AGAINST” approval of the Business Combination Agreement.
On behalf of our board of directors, I thank you for your support and look forward to the successful completion of the Business Combination.
| | Sincerely, |
| | | |
, 2022 | | Robert Bennett
Chief Executive Officer |
LightJump shareholders should be aware that Nomura Securities International, Inc. (“Nomura”) has resigned from its role as exclusive financial advisor to Moolec in connection with the Business Combination, effective as of April 27, 2022. Nomura delivered notice of its resignation to the Securities and Exchange Commission pursuant to Section 11(b)(1) of the Securities Act of 1933, as amended, on July 22, 2022 and has disclaimed any responsibility for any portion of this proxy statement/prospectus. Shareholders should not place any reliance on the participation of Nomura prior to such resignation as Moolec’s exclusive financial advisor.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES REGULATORY AGENCY HAS APPROVED OR DISAPPROVED THE TRANSACTIONS DESCRIBED IN THIS PROXY STATEMENT/PROSPECTUS, PASSED UPON THE MERITS OR FAIRNESS OF THE BUSINESS COMBINATION OR RELATED TRANSACTIONS OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE DISCLOSURE IN THIS PROXY STATEMENT/PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY CONSTITUTES A CRIMINAL OFFENSE.
Investing in our securities involves a high degree of risk. Before making an investment decision, please read the information under the section entitled “Risk Factors” elsewhere in this proxy statement/prospectus and under similar headings or in any amendment or supplement to this proxy statement/prospectus.
This proxy statement/prospectus is dated , 2022, and is expected to be first mailed or otherwise delivered to LightJump shareholders on or about , 2022
Table of Contents
LightJump Acquisition Corporation
2735 Sand Hill Road, Suite 110
Menlo Park, CA 94025
Telephone: (650) 515-3930
NOTICE OF SPECIAL MEETING OF STOCKHOLDERS TO BE HELD ON [•], 2022
To the Stockholders of LightJump Acquisition Corporation:
NOTICE IS HEREBY GIVEN that a special meeting of stockholders of LightJump Acquisition Corporation, a Delaware corporation (“LightJump”), will be held on [•], 2022, at [•], Eastern time. The special meeting will be completely virtual. There will be no physical meeting location and the special meeting will only be conducted via live webcast at the following address: [•]. You are cordially invited to attend the special meeting of stockholders for the following purposes:
• Proposal 1 (Business Combination Proposal): to approve and adopt the Business Combination Agreement, dated as of June 14, 2022, by and among LightJump, Moolec, Holdco, and Merger Sub, providing for, among other things, LightJump and Moolec to become subsidiaries of Holdco and all stockholders of LightJump and Moolec to become shareholders of Holdco, which is expected to become a public company listed on Nasdaq (the “Business Combination”), a copy of which is attached to the accompanying proxy statement/prospectus as Annex A.
• Proposal 2 (Stockholder Adjournment Proposal): to approve a proposal to adjourn the special meeting of stockholders to a later date or dates, if necessary, to permit further solicitation and vote of proxies if, based upon the tabulated vote at the time of the special meeting of stockholders, there are not sufficient votes to approve one or more proposals presented at the meeting or if holders of SPAC Common Stock have elected to redeem a number of shares such that the minimum available cash condition to the obligation to closing of the Business Combination would not be satisfied.
Only holders of record of our common stock at the close of business on [•], 2022 are entitled to notice of the special meeting of stockholders and to vote at the special meeting of stockholders and any adjournments or postponements of the special meeting of stockholders. A complete list of our stockholders of record entitled to vote at the special meeting of stockholders will be available for ten days before the special meeting of stockholders (i) on a reasonably accessible electronic network or (ii) at our principal executive offices for inspection by stockholders during ordinary business hours for any purpose germane to the special meeting of stockholders.
Pursuant to LightJump’s Amended and Restated Certificate of Incorporation, holders of SPAC Common Stock who own shares issued in LightJump’s public offering have certain rights to redeem their shares for cash upon the consummation of the Business Combination. For a description of these redemption rights, including certain limitations, and the procedure for electing redemption, see the section entitled “The Special Meeting of LightJump Holders — Redemption Rights”. Holders of warrants to purchase shares of SPAC Common Stock do not have redemption rights with respect to such warrants in connection with the Business Combination.
The Business Combination will be consummated only if a majority of the outstanding shares of SPAC Common Stock are voted in favor of Proposal 1 (Business Combination Proposal) at the special meeting of stockholders. We have no specified maximum redemption threshold under our Amended and Restated Certificate of Incorporation. In no event, however, will LightJump redeem shares of SPAC Common Stock in an amount that would cause LightJump’s net tangible assets to be less than $5,000,001.
Table of Contents
The board of directors of LightJump, has unanimously approved (1) the Business Combination Agreement, dated as of June 14, 2022, by and among LightJump, Moolec, Holdco, and Merger Sub, a copy of which is attached hereto as Annex A and is hereby incorporated by reference into this proxy statement/prospectus and (2) the Business Combination. Capitalized terms used in this proxy statement/prospectus have the meanings set forth in the section entitled “Frequently Used Terms”.
Pursuant to the Business Combination Agreement and related agreements, on the Closing Date:
• all the issued Company Ordinary Shares held by Company Shareholders shall be transferred and for purposes of the 1915 Law, contributed in kind to Holdco, free and clear of all Liens (other than the Company Shareholders’ Agreements Liens that will expire on or prior to the Closing Date), and Company Shareholders shall subscribe for and, as consideration for the contribution, shall be issued, in accordance with the Exchange Ratio (save that the Holdco Ordinary Shares to be issued shall be reduced by the number of Holdco Ordinary Shares already held by Company Shareholders immediately prior to the Exchange), 27,500,000 of Holdco Ordinary Shares; provided, however, that no fractional Holdco Ordinary Shares shall be issued pursuant to the Exchange. For Luxembourg law purposes, a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued a report on the contributions in kind relating to the contribution of the Company Ordinary Shares prepared in accordance with article 420-10 of the 1915 Law;
• each Company SAFE Holder shall have contributed all of its rights and obligations under each Original SAFE to Holdco in consideration for the issuance by Holdco of a simple agreement for future equity on substantively identical terms (mutatis mutandis) with such adjustments (if any) required under Luxembourg law. For Luxembourg law purposes, a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued a report on the contributions in kind relating to the contribution of the Original SAFEs prepared in accordance with article 420-10 of the 1915 Law;
• each Company Shareholder shall cease to be the holder of such Company Ordinary Shares, subject to the submission of all filings required under Law (including any filings required to pay stamp duties), and Holdco will be recorded as the registered holder of all Company Ordinary Shares so exchanged and transferred and will be the legal and beneficial owner thereof; and
• immediately prior to the Merger Effective Time but after the Exchange Effective Time, each Company SAFE Holder shall receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the respective Company SAFE, with such adjustments (if any) required under Luxembourg law. See the section entitled “The Business Combination — The Structure of the Business Combination” included in this proxy statement/prospectus for more information.
Upon consummation of the Business Combination, Moolec and LightJump will each be direct subsidiaries of Holdco.
SPAC Common Stock, SPAC Units and SPAC Warrants are currently listed and traded on Nasdaq under the symbols “LJAQ”, “LJAQU” and “LJAQW”, respectively. Holdco intends to apply for listing, to be effective at the time of the Closing, of the Holdco Ordinary Shares and Holdco Warrants on Nasdaq under the symbols “MLEC” and “MLECW”, respectively. This proxy statement/prospectus provides stockholders of LightJump with detailed information about the proposed Business Combination and other matters to be considered at the special meeting of LightJump. We encourage you to read this entire document, including the Annexes and other documents referred to herein, carefully and in their entirety. You should also carefully consider the risk factors described in the section entitled “Risk Factors” beginning on page 50 of this proxy statement/prospectus.
Table of Contents
Your attention is directed to the proxy statement/prospectus accompanying this notice (including the financial statements and annexes attached thereto) for a more complete description of the proposed Business Combination and related transactions and each of our proposals. We encourage you to read this proxy statement/prospectus carefully. If you have any questions or need assistance voting your shares, please call our proxy solicitor, Advantage Proxy, Inc., at (877) 870-8565. Banks and brokers may reach Advantage Proxy, Inc. at the same, or by email at ksmith@advantageproxy.com.
| | By Order of the Board of Directors, |
| | | |
[•], 2022 | | Robert Bennett
Chairman of the Board of Directors |
Table of Contents
i
Table of Contents
ABOUT THIS PROXY STATEMENT/PROSPECTUS
This document, which forms part of a registration statement on Form F-4 filed with the SEC by Holdco, constitutes a prospectus of Holdco under Section 5 of the Securities Act, with respect to the Holdco Ordinary Shares to be issued to the holders of SPAC Common Stock if the Business Combination described herein is consummated. With respect to LightJump and the holders of SPAC Common Stock, this proxy statement/prospectus serves as and constitutes:
• a notice of meeting and a proxy statement under Section 14(a) of Exchange Act with respect to the special meeting of LightJump Holders being held on , 2022, where LightJump Holders will vote on, among other things, the proposed Business Combination and related transactions and each of the below proposals; and
• a prospectus of Holdco under Section 5 of the Securities Act, with respect to the Holdco Ordinary Shares and Holdco Warrants to be issued to the holders of SPAC Common Stock and SPAC Warrants if the Business Combination described herein is consummated.
This proxy statement/prospectus does not serve as a prospectus for the Holdco Ordinary Shares that the Company Shareholders will receive in the Business Combination, as such shares will be offered to such holders in a private offering. This document does not constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction or to any person to whom it would be unlawful to make such offer.
This proxy statement/prospectus includes trademarks, tradenames and service marks, certain of which belong to us or Moolec and others that are the property of other organizations. Solely for convenience, trademarks, tradenames and service marks referred to in this proxy statement/prospectus appear without the®, TM and SM symbols, but the absence of those symbols is not intended to indicate, in any way, that LightJump or Moolec will not assert their rights or that the applicable owner will not assert its rights to these trademarks, tradenames and service marks to the fullest extent under applicable law. Neither LightJump nor Moolec intend that their use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of LightJump or Moolec by, these other parties.
MARKET AND INDUSTRY DATA
This proxy statement/prospectus contains estimates, projections, and other information concerning Moolec’s industry and business, as well as data regarding market research, estimates, and forecasts prepared by Moolec’s management. Information that is based on estimates, forecasts, projections, market research, or similar methodologies is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. The industry in which Moolec operates is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section titled “Risk Factors.” Unless otherwise expressly stated, Moolec obtained industry, business, market, and other data from reports, research surveys, studies, and similar data prepared by market research firms and other third parties, industry and general publications, government data, and similar sources. In some cases, Moolec does not expressly refer to the sources from which this data is derived. In that regard, when Moolec refers to one or more sources of this type of data in any paragraph, you should assume that other data of this type appearing in the same paragraph is derived from sources that Moolec paid for, sponsored, or conducted, unless otherwise expressly stated or the context otherwise requires. While Moolec has compiled, extracted, and reproduced industry data from these sources, Moolec has not independently verified the data. Forecasts and other forward-looking information with respect to industry, business, market, and other data are subject to the same qualifications and additional uncertainties regarding the other forward-looking statements in this proxy statement/prospectus. See “Cautionary Note Regarding Forward-Looking Statements.”
1
Table of Contents
Frequently used Terms
“1915 Law” means the Luxembourg law of August 10, 1915 on commercial companies, as amended.
“A&R Holdco Organizational Documents” means the amended and restated Holdco Organizational Documents to be amended immediately prior to the consummation of the Merger and the Exchange at the general meeting of the sole shareholder of Holdco in the form set forth on Exhibit C to the Business Combination Agreement.
“Accounting Principles” means GAAP in case of SPAC, IFRS in case of the Company and the Subsidiaries of the Company under the Financial Statements, in each case, as in effect from time to time.
“Action” means any material litigation, proceeding, cause of action, lawsuit, audit, assessment or reassessment, petition, complaint, charge, grievance, prosecution, demand, hearing, written notice, inquiry, investigation, subpoena, summons, inspection, or administrative or other similar proceeding, mediation or arbitration (including any appeal or application for review) of any kind or nature, in law or in equity.
“Ancillary Agreements” means the Exchange Agreements, the Registration Rights and Lock-Up Agreement, the Backstop Agreement, the Transaction Support Agreement and all other agreements, certificates and instruments executed and delivered by SPAC, Holdco, Merger Sub or the Company in connection with the Transactions and specifically contemplated by the Business Combination Agreement.
“Antitrust Laws” means any Laws that are designed to prohibit, restrict or regulate actions having the purpose or effect of monopolization or restraint of trade or lessening of competition or creation or strengthening of a dominant position through merger or acquisition, including Laws of any jurisdiction or Governmental Authority outside of the United States.
“Backstop Agreement” means the agreement dated June 14, 2022 by and between Union Group Ventures Limited, UG Holdings, LLC, THEO I SCSp and LightJump One Founders, LLC, guaranteeing, severally but not jointly, the funding of certain amounts as set forth therein.
“Business Combination” means the transactions contemplated by the Business Combination Agreement, including the Merger and the Exchange.
“Business Combination Agreement” means the Business Combination Agreement, dated as of June 14, 2022, as may be amended, by and among LightJump, Moolec, Holdco and Merger Sub.
“Business Combination Proposal” means the proposal to approve the adoption of the Business Combination Agreement and the Business Combination.
���Business Day” means any day on which the principal offices of the SEC in Washington, D.C. are open to accept filings and on which banks are not required or authorized to close in the City of New York in the United States of America, in London, England or Luxembourg in the Grand Duchy of Luxembourg; provided that banks shall not be deemed to be authorized or obligated to be closed due to a “shelter in place,” “non-essential employee” or similar closure of physical branch locations at the direction of any Governmental Authority if such banks’ electronic funds transfer systems (including for wire transfers) are open for use by customers on such day.
“Certificate of Merger” means the certificate of merger that SPAC shall cause to be executed, acknowledged and filed with the Secretary of State of the State of Delaware in accordance with the applicable provisions of the DGCL to effectuate the Merger.
“Closing” means the consummation of the Business Combination.
“Closing Date” means the date upon which the Closing is to occur.
“Code” means the Internal Revenue Code of 1986, as amended.
“Combined Company” means Holdco and its consolidated subsidiaries after giving effect to the Business Combination.
“Company” or “Moolec” means Moolec Science Limited, a private limited company incorporated under the laws of England and Wales.
2
Table of Contents
“Company Disclosure Schedule” means the disclosure schedule provided by Moolec in connection with the Business Combination Agreement.
“Company Material Adverse Effect” means any Effects that, individually or in the aggregate with all other Effects, (a) is or would reasonably be expected to be materially adverse to the business, condition (financial or otherwise), assets, liabilities or operations of the Company and the Company Subsidiaries taken as a whole or (b) does or would prevent, materially delay or materially impede the performance by the Company of its obligations under this Business Combination Agreement or the consummation of the Exchange, Merger or any of the other Transactions; provided, however, that none of the following shall be deemed to constitute, alone or in combination, or be taken into account in the determination of whether there has been or will be, a Company Material Adverse Effect: (i) any enactment of, change or proposed change in or change in the interpretation of any Law or Accounting Principles; (ii) Effects generally affecting the industries or geographic areas in which the Company or any of the Company Subsidiaries operate; (iii) any downturn in general economic conditions, including changes in the credit, debt, securities, financial or capital markets (including changes in interest or exchange rates, prices of any security or market index or commodity or any disruption of such markets); (iv) acts of war (whether or not declared), sabotage, civil unrest, terrorism, curfews, riots, demonstrations or public disorders, or any escalation or worsening of any such acts of war, sabotage, civil unrest, terrorism, curfews, riots, demonstrations or public disorders, or changes in global, national, regional, state or local political or social conditions; (v) any hurricane, tornado, flood, earthquake, natural disaster, or other acts of God; (vi) Effects arising from or relating to epidemics, pandemics, or disease outbreaks, including COVID-19 or any COVID-19 Measures; (vii) any actions taken or not taken by the Company or any of the Company Subsidiaries as specifically required by this Agreement or any Ancillary Agreement, (viii) the announcement or execution, pendency, negotiation or consummation of the Merger, the Exchange or any of the other Transactions (including the impact thereof on relationships with customers, suppliers, employees or Governmental Authorities); provided that this clause (viii) shall not apply in determining a Company Material Adverse Effect resulting from a breach of the representations and warranties set forth in the Business Combination Agreement; (ix) any failure by the Company or any of the Company Subsidiaries to meet any projections, forecasts, guidance, estimates, milestones, budgets or financial or operating predictions of revenue, earnings, cash flow or cash position, provided that this clause (ix) shall not prevent a determination that any change, event, or occurrence underlying such failure has resulted in a Company Material Adverse Effect; (x) any pending or initiated action against the Company, any of the Company Subsidiaries or any of their respective officers or directors, in each case, arising out of or relating to the execution of this Agreement, any Ancillary Agreements or any of the Transactions (other than any action (A) commenced by any Party to enforce its rights under this Agreement or any Ancillary Agreement to which it is a party or (B) resulting from or arising from a breach of the representations and warranties set forth in the Business Combination Agreement); (xi) any action taken by SPAC; or (xii) any actions taken, or failures to take action, or such other changes or events, in each case, which SPAC has specifically requested or to which it has specifically consented or which actions are specifically contemplated by this Agreement or any Ancillary Agreement, in each case, except in the cases of clauses (i) through (vi), to the extent that the Company and the Company Subsidiaries, taken as a whole, are disproportionately affected thereby as compared with other participants in the industries or geographic areas in which the Company and the Company Subsidiaries operate.
“Company Ordinary Shares” means the Company’s ordinary shares, with a nominal value of £0.01 per share representing the entire issued share capital of the Company.
“Company Organizational Documents” means the memorandum and articles of association of the Company, as amended, modified or supplemented from time to time.
“Company Requisite Approvals” means Company Board Approval and Company Shareholder Approval.
“Company SAFE” means each of the simple agreement for future equity by and between the Company and the Company SAFE Holder named therein (an “Original SAFE”) or any simple agreement for future equity between Holdco and that Company SAFE Holder issued in consideration for the contribution by the Company SAFE Holder of its rights in the Original SAFE to Holdco (in which case the Original SAFE will cease to be a “Company SAFE”) with such adjustments (if any) required under Luxembourg law.
3
Table of Contents
“Company SAFE Holder” means each Person that has entered into a Company SAFE.
“Company Shareholders” means the holders of all of the Company Ordinary Shares and all other shares being Equity Interest as of immediately prior to the Exchange Effective Time.
“Company Shareholders’ Agreements Liens” means the Liens under the Company Shareholders’ Agreements, each of which will expire on or prior to the Closing Date in accordance with the Termination Agreements.
“Company Subsidiaries” means the subsidiaries of the Company.
“Company Transaction Expenses” means the reasonable and documented Transaction Expenses of the Company or any of its affiliates, including, without limitation, (a) Transaction Expenses incurred in the negotiation and preparation of this Agreement, the Ancillary Agreements and the other documents contemplated hereby and thereby and the performance and compliance with all agreements and conditions contained herein and therein, (b) Transaction Expenses incurred in preparing and obtaining the PCAOB Financials, (c) Transaction Expenses incurred in connection with obtaining the consent or approval of any person or Governmental Authority in connection with the Transactions, (d) Transaction Expenses incurred in connection with the Transactions (including the formation of Holdco, Merger Sub and the structuring, negotiation and documentation of the Exchange and Merger) and (e) Transaction Expenses incurred in connection with obtaining the D&O Tail Policy. The Company Transaction Expenses include the fees, expenses and disbursements of legal counsel, auditors and accountants, due diligence expenses, advisory and consulting fees and expenses, and other third-party fees.
“Continental” means Continental Stock Transfer & Trust Company, LightJump’s transfer agent and warrant agent.
“COVID-19” means the novel coronavirus known as SARS-CoV-2 or COVID-19, and any evolutions, mutations thereof or related or associated epidemics, pandemic or disease outbreaks.
“COVID-19 Measures” means any quarantine, “shelter in place,” “stay at home,” workforce reduction, social distancing, delay, shut down (including the shutdown of air cargo routes), closure, sequester, safety or similar Law, directive, guideline or recommendation promulgated by any Governmental Authority, in each case with or in response to COVID-19.
“Deferred Fees” means the amount of deferred fees held in the Trust Account in connection with SPAC’s IPO payable to the underwriters or other advisors upon consummation of a business combination.
“DGCL” means the Delaware Corporation Law, as amended from time to time.
“D&O Tail Policy” means a fully-paid “tail” insurance policy for a term of six years from the Closing Date with terms and scope of coverage at least as favorable as the Company’s directors and officers insurance policy and SPAC’s directors and officers insurance policy covering those persons thereunder.
“EarlyBird Amendment” means that certain amendment dated June 14, 2022 to the engagement letter between the SPAC and EarlyBirdCapital, Inc. (“EarlyBird”) dated January 8, 2021 (as amended from time to time, the “EarlyBird Engagement Letter”).
“EarlyBird Cash Fees” means all cash fees and expenses payable to EarlyBird pursuant to the EarlyBird Engagement Letter.
“EarlyBird Share Fees” means the shares of Holdco to be issued to EarlyBird pursuant to the EarlyBird Engagement Letter.
“Effects” means, collectively, events, circumstances, changes and effects.
“Equity Interest” means all shares of capital stock, common stock, preferred stock, units, ownership interests and any other equity ownership or participation in any Person, including all options, warrants, preemptive rights, calls, convertible securities, simple agreements to acquire future equity, conversion rights or other rights, agreements, arrangements or commitments of any character relating to the issued or unissued capital stock of any Person.
“Exchange” means the transactions contemplated in the Business Combination Agreement to occur at the Exchange Effective Time.
4
Table of Contents
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Exchange Agreements” mean those certain individual Contribution and Exchange Agreements, each dated as of June 14, 2022, and entered into by and among Holdco, the Company and each of the Company Shareholders.
“Exchange Consideration” means $325,000,000.
“Exchange Effective Time” means the time on which the issuance of the new Holdco Ordinary Shares pursuant to the Holdco Delegate Resolutions is effective on the Closing Date, which shall be the effective time of the contribution and exchange of the Company Ordinary Shares held by the Company Shareholders and exchanged for Holdco Ordinary Shares, as applicable and as contemplated under the Exchange Agreements, and which shall occur immediately prior to the Merger Effective Time.
“Exchange Issuance” means the issuance of the Holdco Ordinary Shares to the Company Shareholders in connection with the Exchange.
“Exchange Ratio” means 0.66787343, the ratio used for determining the number of aggregate Holdco Ordinary Shares for which the aggregate Company Ordinary Shares shall be converted in accordance with Section 2.02(a).
“Export Control Laws” means export control laws and regulations of any jurisdiction applicable to SPAC, Holdco, Merger Sub or the Company including the U.S. Export Administration Regulations, 15 C.F.R. §§ 730, et seq., as amended, and any other equivalent or comparable export control laws and regulations of other countries.
“Extension Amendment” means an amendment to the SPAC COI to extend the date by which the SPAC must consummate the Transactions from July 12, 2022 to January 12, 2023.
“Extension Amendment Fees” means all fees and expenses incurred by SPAC solely relating to the Extension Amendment.
“Founder Shares” mean the SPAC Common Stock issued prior to the IPO.
“GAAP” means generally accepted accounting principles as in effect in the United States from time to time.
“Governmental Authority” means any U.S. federal, state, county or local or non-U.S. government, governmental, national, regulatory or administrative authority, agency, instrumentality or commission or any court, tribunal, or judicial or arbitral body.
“HMRC” means HM Revenue and Customs.
“Holdco” means Moolec Science SA, a public limited liability company (société anonyme) governed by the laws of the Grand Duchy of Luxembourg, with its registered office at 17, Boulevard F.W. Raiffeisen, L-2411 Luxembourg, Grand Duchy of Luxembourg and registered with the Luxembourg Trade and Companies’ Register (Registre de Commerce et des Sociétés, Luxembourg) under number B268440.
“Holdco Board” means the board of directors of Holdco.
“Holdco Board Approval” means one or several Holdco Board resolutions with respect to the approval of the Transaction and the Transaction Documents to which Holdco is or will be a party, including, for the avoidance of doubt, (a) the approval by the Holdco Board of the issuance on the Closing Date (and conditional on Closing) by a delegate of (i) new Holdco Ordinary Shares following the Merger as Merger Consideration, and (ii) the Key Staff Participation, both under the authorized share capital of Holdco and pursuant to the Holdco Delegate Merger Resolutions and (b) the issuance of new Holdco Ordinary Shares (x) to the Company Shareholders as part of the Exchange and (y) to the Company SAFE Holders as part of the exercise of the Original SAFE, both by a delegate under the authorized share capital of Holdco and pursuant to the Holdco Delegate Exchange Resolutions.
“Holdco Delegate Exchange Resolutions” means the resolutions taken on the Closing Date by the delegate appointed by the Holdco Board pursuant to the Holdco Board Approval in order to issue on the Closing Date new Holdco Ordinary Shares in the context of the Exchange as well as the exercise of the Original SAFE, under the authorized share capital of Holdco.
5
Table of Contents
“Holdco Delegate Merger Resolutions” means the resolutions taken on the Closing Date by the delegate appointed by the Holdco Board pursuant to the Holdco Board Approval in order to issue on the Closing Date (i) the Merger Consideration in the form of new Holdco Ordinary Shares and (ii) the Key Staff Participation.
“Holdco Delegate Resolutions” means the Holdco Delegate Exchange Resolutions and the Holdco Delegate Merger Resolutions.
“Holdco Ordinary Shares” means the ordinary shares of Holdco, each having a nominal value in U.S. dollars of $0.01.
“Holdco Organizational Documents” means the articles of association of Holdco as amended, modified or supplemented from time to time, including as contemplated by the Holdco Delegate Resolutions and the Holdco Shareholder Approval.
“Holdco Requisite Approvals” means the Holdco Board Approval, the Holdco Delegate Resolutions and the Holdco Shareholder Approval, as applicable.
“Holdco Shareholder Approval” means the approval of the sole shareholder of Holdco, at an extraordinary general meeting of the shareholders of Holdco, to be held in front of a Luxembourg notary prior to the Closing Date to inter alia implement (i) a sufficiently large authorized share capital for the issuance of new Holdco Ordinary Shares in the context of the Merger and the issuance of new Holdco Ordinary Shares in the context of the Exchange, exercise of the Original SAFE and issuance of the Key Staff Participation and (ii) the A&R Holdco Organizational Documents as contemplated by the Business Combination Agreement.
“IFRS” means the International Financial Reporting Standards, as issued by the IFRS Foundation and the International Accounting Standards Board (“IASB”).
“Import Control Laws” means import control laws and regulations of any jurisdiction applicable to SPAC, Holdco, Merger Sub or the Company, including those administered by U.S. Customs and Border Protection and U.S. Immigration and Customs Enforcement (19 U.S.C. §§ 1-4454 and 19 C.F.R. §§ 1-199), and any other equivalent or comparable import control laws and regulations of other countries.
“Intellectual Property” means: (a) patents, patent applications and patent disclosures, together with all reissues, continuations, continuations-in-part, divisionals, revisions, extensions or reexaminations thereof; (b) trademarks and service marks, trade dress, logos, trade names, corporate names, brands, slogans, and other source identifiers, and all applications, registrations, and renewals in connection therewith, together with all of the goodwill associated with the foregoing; (c) copyrights, and other works of authorship (whether or not copyrightable), and moral rights, and registrations and applications for registration, renewals and extensions thereof; (d) trade secrets and know-how (including ideas, formulas, compositions, inventions (whether or not patentable or reduced to practice)), customer and supplier lists, improvements, protocols, processes, methods and techniques, research and development information, industry analyses, algorithms, architectures, layouts, drawings, specifications, designs, plans, methodologies, proposals, industrial models, technical data, financial and accounting data (including pricing and cost information), and all other data, databases and database rights; (e) Internet domain names and social media accounts; (f) rights of privacy and publicity and all other intellectual property or proprietary rights of any kind or description recognized under applicable Laws; (g) copies and tangible embodiments of any of the foregoing, in whatever form or medium; and (h) all legal rights arising from items (a) through (f), including the right to prosecute and perfect such interests and rights to sue, oppose, cancel, interfere, and enjoin based upon such interests, including such rights based on past infringement, if any, in connection with any of the foregoing.
“IPO” means LightJump’s initial public offering of units, consummated on January 12, 2021.
“IPO Shares” means SPAC Common Stock sold in the IPO.
“Key Staff Participation” means 243,774 Holdco Ordinary Shares that will be freely allotted to the Company’s Chief Financial Officer (“CFO”) in order to satisfy the Company’s obligations under the CFO’s consulting agreement (the “CFO Consulting Agreement”).
6
Table of Contents
“Law” means any federal, national, state, county, municipal, provincial, local, foreign or multinational, statute, constitution, common law, ordinance, code, decree, order, judgment, rule, regulation, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Authority.
“Lien” means any lien, security interest, mortgage, deeds of trust, pledge, adverse claim, usufruct, option, right of first refusal, right of first offer, charge, claim, equitable interest, easement, encroachment, lease or sublease, or restriction on the right to vote, sell, transfer or otherwise dispose of any capital stock, shares, or other voting securities, or similar encumbrance (other than those created under applicable securities Laws, and not including any license of Intellectual Property).
“LightJump Holder” means a holder of SPAC Common Stock.
“LightJump Initial Stockholders” means Sponsor (with respect to its 3,450,000 Founder Shares) and EarlyBird (with respect to its 120,000 shares issued as nominal consideration in connection with its role as underwriter).
“Merger” means the merging of Merger Sub with and into LightJump, with LightJump surviving such merger and becoming a direct wholly-owned subsidiary of Holdco.
“Merger Effective Time” means such time as the Certificate of Merger has been duly filed with the Secretary of State of the State of Delaware or at such later time as may be agreed by the Company and SPAC in writing and specified in the Certificate of Merger in accordance with the DGCL.
“Merger Sub” means Moolec Acquisition, Inc., a Delaware corporation.
“Nasdaq” means the Nasdaq Capital Market, the Nasdaq Global Market or the Nasdaq Global Select Market, as may be applicable.
“Net Available Assets” means an amount, determined as of the Closing (or as of another specified time), equal to (i) the total amount of Trust Account Cash remaining as of the Redemption Closing Time plus (ii) the aggregate amount of any proceeds received by Holdco, SPAC or their respective subsidiaries in connection with any financing, funding, contribution or other amounts raised from introductions made by EarlyBird to Holdco, SPAC or their respective subsidiaries prior to the Closing in connection with the transactions contemplated by the Business Combination Agreement (including any funds received as a result of a sale of the securities of Holdco, SPAC or any of their respective subsidiaries); provided that the Net Available Assets shall be determined assuming (a) all payments required to be made to the holders of the SPAC Common Stock exercising Redemption Rights as of the Redemption Closing Time have been paid, (b) no SPAC Transaction Expenses or any EarlyBird Cash Fees have been paid from the Trust Account (and if any such amounts have previously been paid from the Trust Account, such payments shall be added back to Net Available Assets) and (c) no amounts have been funded pursuant to the obligations under the Backstop Agreement.
“Payment Spreadsheet” means a spreadsheet that shall be delivered by the Company to SPAC pursuant to Section 3.01(b) at least five (5) Business Days prior to the Closing (except as may otherwise be agreed in writing by the Company and LightJump), which shall set forth, (a) the initial allocation of the Exchange Consideration among the Company Shareholders, (b) the adjustment to the allocation of the Exchange Consideration described in (a) of this definition to account for the economic rights of certain Company Shareholders under the Company Shareholders’ Agreements, in accordance with the Exchange Agreements, (c) any applicable share premium, and (d) the number of Holdco Ordinary Shares, as applicable, issuable to each Company Shareholder in connection with the Exchange.
“PCAOB” means the Public Company Accounting Oversight Board and any division or subdivision thereof.
“Permitted Liens” means: (a) such imperfections of title, easements, encumbrances, Liens or restrictions that, individually or in the aggregate, do not materially affect, impair or interfere with the use, ownership, value and maintenance of, or the access to any property affected thereby or the conduct of the business of the Company and/or the Company Subsidiaries; (b) materialmen’s, mechanics’, carriers’, workmen’s, warehousemen’s, repairmen’s, landlord’s and other similar Liens arising or incurred in the ordinary course of business to the extent relating to amounts not yet due and payable, or deposits to obtain the release of such Liens; (c) any Liens for Taxes due and not yet payable, or being contested in good faith; (d) zoning, entitlement, conservation restriction and other land use and environmental regulations promulgated by Governmental Authorities; (e) any Liens not created by the
7
Table of Contents
Company that affect the underlying fee interest of any leased real property, including master leases or ground leases and any set of facts that an accurate up-to-date survey would show; (f) non-exclusive licenses, sublicenses or other rights to Intellectual Property owned by or licensed to the Company or the Company Subsidiaries granted to any licensee in the ordinary course of business; (g) any non-monetary Liens, encumbrances and restrictions on real property (including easements, covenants, rights of way and similar restrictions of record) that individually or in the aggregate, do not materially affect, impair or interfere with the use, ownership, value and maintenance of or the access to any real property affected thereby or the conduct of the business of the Company and/or the Company Subsidiaries; (h) any Liens identified in the Financial Statements; or (i) any Liens on leases, subleases, easements, licenses, rights of use, rights to access and rights of way arising from the provisions of such agreements or benefiting or created by any superior estate, right or interest.
“Private Placement Warrants” means the warrants to purchase SPAC Common Stock purchased in a private placement in connection with the IPO.
“Public Share” means a share of SPAC Common Stock issued as part of a SPAC Unit in the IPO.
“Public Stockholders” means the holders of Public Shares that were offered as part of the IPO.
“Redemption Rights” means the redemption rights provided for in Article V, Section 3 of the SPAC COI.
“Registration Rights and Lock-Up Agreement” means that certain Registration Rights and Lock-Up Agreement to be entered into in connection with the Closing by and among SPAC, Holdco, Sponsor, each of the persons and entities listed on Exhibit A attached thereto and the Company Shareholders, Company SAFE Holders and CFO, substantially in the form attached to the Business Combination Agreement as Exhibit A.
“SEC” means the U.S. Securities and Exchange Commission.
“Securities Act” means the Securities Act of 1933, as amended.
“SPAC” or “LightJump” means LightJump Acquisition Corporation, a Delaware corporation.
“SPAC Articles” means the Amended and Restated Memorandum and Articles of Association of SPAC, as amended, modified or supplemented from time to time.
“SPAC COI” means the Amended and Restated Certificate of Incorporation of SPAC, as amended, modified or supplemented from time to time.
“SPAC Material Adverse Effect” means any Effects that, individually or in the aggregate with all other Effects, (a) is or would reasonably be expected to be materially adverse to the business, condition (financial or otherwise), assets, liabilities or operations of SPAC or (b) does or would prevent, materially delay or materially impede the performance by SPAC of its obligations under the Business Combination Agreement or any of the Ancillary Agreements or the consummation of the Merger or any of the other Transactions; provided, however, that none of the following shall be deemed to constitute, alone or in combination, or be taken into account in the determination of whether there has been or will be, a SPAC Material Adverse Effect: (i) any enactment of, change or proposed change in or change in the interpretation of any Law or Accounting Principles; (ii) Effects generally affecting the industries or geographic areas in which SPAC operates, (iii) any downturn in general economic conditions, including changes in the credit, debt, securities, financial or capital markets (including changes in interest or exchange rates, prices of any security or market index or commodity or any disruption of such markets); (iv) acts of war (whether or not declared), sabotage, civil unrest, terrorism, curfews, riots, demonstrations or public disorders, or any escalation or worsening of any such acts of war, sabotage, civil unrest, terrorism, curfews, riots, demonstrations or public disorders, or changes in global, national, regional, state or local political or social conditions; (v) any hurricane, tornado, flood, earthquake, natural disaster, or other acts of God; (vi) Effects arising from or relating to epidemics, pandemics, or disease outbreaks, including COVID-19 or any COVID-19 Measures; (vii) any actions taken or not taken by SPAC as specifically required by the Business Combination Agreement or any Ancillary Agreement; (viii) the announcement or execution, pendency, negotiation or consummation of the Merger or any of the other Transactions (including the impact thereof on relationships with Governmental Authorities); provided that this clause
8
Table of Contents
(viii) shall not apply in determining a SPAC Material Adverse Effect resulting from a breach of the representations and warranties set forth in Section 5.05 of the Business Combination Agreement; (ix) any pending or initiated Action against SPAC or any of its officers or directors, in each case, arising out of or relating to the execution of the Business Combination Agreement t or the Transactions (other than any Action (A) commenced by any Party to the Business Combination Agreement to enforce its rights under the Business Combination Agreement or any Ancillary Agreement to which it is a party or (B) resulting from or arising from a breach of the representations and warranties set forth in Section 5.05 of the Business Combination Agreement); (x) any action taken or not taken by the Company or any of the Company Subsidiaries; or (xi) any actions taken, or failures to take action, or such other changes or events, in each case, which the Company has specifically requested or to which it has specifically consented or which actions are specifically contemplated by the Business Combination Agreement, in each case, except in the cases of clauses (i) through (vi), to the extent that SPAC is disproportionately affected thereby as compared with other participants in the industries or geographic areas in which SPAC operates.
“SPAC Common Stock” means SPAC’s common stock, par value $0.0001 per share.
“SPAC Organizational Documents” means the SPAC COI, the bylaws of SPAC and the Trust Agreement, in each case as amended, modified or supplemented from time to time.
“SPAC Proposals” means proposals made to the LightJump Holders pursuant to the SPAC Organizational Documents and applicable Law to approve and adopt (a) the Business Combination Agreement and the Transactions, including the Merger, (b) the Extension Amendment and (c) any other proposals the Parties deem in good faith are necessary or desirable to effect the Transactions.
“SPAC Stockholder Approvals” means (a) with respect to the Merger, the affirmative vote of a majority of the LightJump Holders who attend and vote at the special meeting of LightJump Holders; and (b) with respect to any other Proposals proposed to the SPAC Shareholders, the requisite approval required under the SPAC Organizational Documents, the DGCL or other applicable Law.
“SPAC Transaction Expenses” means the reasonable and documented Transaction Expenses of SPAC or any of its affiliates, including (a) any and all Transaction Expenses incurred in the negotiation and preparation of this Agreement, the Ancillary Agreements and the other documents contemplated hereby and thereby and the performance and compliance with all agreements and conditions contained herein and therein, (b) the Sponsor Advanced Funds and (c) the preparation, printing and mailing of the Proxy Statement/Prospectus and the Registration Statement. For the avoidance of doubt, the Parties acknowledge and agree that the SPAC Transaction Expenses include the fees, expenses and disbursements of legal counsel, auditors and accountants, due diligence expenses, advisory and consulting fees and expenses, other third-party fees and any Deferred Fees. For the avoidance of doubt, the Parties acknowledge and agree that (i) any expenses incurred by SPAC in its pursuit of potential acquisition or business targets other than the Company or that were not incurred by SPAC in connection with or in furtherance of the Transactions, (ii) the EarlyBird Cash Fees, (iii) the EarlyBird Share Fees and (iv) the Extension Amendment Fees will not constitute SPAC Transaction Expenses (and will be paid pursuant to Section 10.03 of the Business Combination Agreement).
“SPAC Transaction Expenses Cap” means $3,000,000; provided that, for every $10.00 by which the amount equal to the (i) Net Available Assets minus (ii) the EarlyBird Cash Fees exceeds $10,000,000 (up to $25,000,000), the SPAC Transaction Expenses Cap shall be increased by $1.00. If the amount equal to the (i) Net Available Assets minus (ii) the EarlyBird Cash Fees is $25,000,000 or greater, then SPAC Transaction Expenses Cap means $4,500,000.
“SPAC Unit” means a unit comprising one SPAC Common Stock and one SPAC Warrant.
“SPAC Warrant Agreement” means that certain warrant agreement, dated as of January 12, 2021, by and between SPAC and the Trustee.
“SPAC Warrants” means warrants to purchase SPAC Common Stock as contemplated under the SPAC Warrant Agreement, with each warrant exercisable for the number of SPAC Common Stock stated in the applicable SPAC Warrant at an exercise price per SPAC Common Stock of $11.50.
9
Table of Contents
“SPAC Warrant Amendment and Assignment” means the amendment and assignment to the SPAC Warrant Agreement, substantially in the form attached to the Business Combination Agreement as Exhibit D.
“Sponsor” means LightJump One Founders, LLC, a Delaware limited liability company.
“Sponsor Advanced Funds” means the amount equal to all contributions made by Sponsor to SPAC prior to the Closing to pay for expenses and fees of SPAC (not including the Extension Amendment Fees), including contributions deemed to be made by paying for such expenses and fees directly on SPAC’s behalf.
“Stockholder Adjournment Proposal” means the proposal to adjourn the special meeting of stockholders to a later date or dates, if necessary, to permit further solicitation and vote of proxies if, based upon the tabulated vote at the time of the special meeting of stockholders, there are not sufficient votes to approve one or more proposals presented at the meeting or if holders of SPAC Common Stock have elected to redeem a number of shares such that the minimum available cash condition to the obligation to closing of the Business Combination would not be satisfied.
“Tax” or “Taxes” means any and all federal, state, provincial, local and foreign income, profits, franchise, gross receipts, environmental, capital stock, shares, severances, stamp, payroll, sales, employment, unemployment, disability, use, real property, personal property, unclaimed property, withholding, excise, production, occupancy and other Taxes, VAT, duties or assessments of any nature whatsoever, whenever and wherever imposed, administered, collected or assessed directly or indirectly against or attributable directly or primarily to a company or any other person, together with all interest, fines, costs, charges, surcharges, penalties and additions imposed with respect to such amounts and any interest in respect of such penalties and additions.
“Termination Agreements” means those certain termination agreements entered into by the Company and one or more of the Company Shareholders and pursuant to which all the Company Shareholders’ Agreements and the Company Shareholders’ Agreements Liens will automatically expire on or prior to the Closing Date in accordance with the terms thereunder.
“Transaction Documents” means the Business Combination Agreement, including all schedules and exhibits thereto, the Company Disclosure Schedule, the Ancillary Agreements, and all other agreements, certificates and instruments executed and delivered by SPAC, Holdco, Merger Sub or the Company in connection with the Transactions and specifically contemplated by this Agreement.
“Transaction Expenses” means (a) all out-of-pocket fees, costs and expenses (including all fees, costs and expenses of outside counsel, accountants, investment bankers, experts and consultants to a Party and its affiliates and all fees, costs and expenses in connection with newly issued equity and/or debt financing in connection with the Transactions) incurred by a Party or on its behalf in connection with or related to the authorization, preparation, review, negotiation, execution and performance of this Agreement and the other Transaction Documents and consummation of the Transactions, the Proxy Statement/Prospectus, the Registration Statement and the solicitation of the SPAC Shareholders and Company Shareholders and the preparation of any required filings or notices under applicable Antitrust Laws, if any, and (b) the premiums, commissions and other fees paid or payable in connection with obtaining any directors’ and officers’ “tail” insurance policy.
“Transaction Proposals” means the Business Combination Proposal, and the Stockholder Adjournment Proposal.
“Transaction Support Agreement” means the transaction support agreement, dated as of June 14, 2022, by and among Moolec, Holdco, SPAC, Sponsor, SPAC Holders, and the Company SAFE Holders, as amended, modified or supplemented from time to time.
“Transactions” means the transactions contemplated by the Transaction Documents, including the Exchange and the Merger.
“Trust Account” means the trust account that holds a portion of the proceeds of the IPO and the simultaneous sale of the Private Placement Warrants.
10
Table of Contents
“Trust Account Cash” means the total amount of cash held in the Trust Account that was raised as a result of the IPO of the SPAC (not including any interest paid with respect to such cash or amounts contributed into the Trust Account from other transactions).
“Trust Agreement” means that certain Investment Management Trust Agreement, dated as of January 12, 2021, by and between SPAC and Continental.
“VAT” means value added Tax.
11
Table of Contents
QUESTIONS AND ANSWERS ABOUT THE BUSINESS COMBINATION AND THE SPECIAL MEETING
The following questions and answers briefly address some commonly asked questions about the proposals to be presented at the special meeting of stockholders, including with respect to the proposed Business Combination. The following questions and answers may not include all the information that is important to LightJump Holders. Stockholders are urged to read carefully this entire proxy statement/prospectus, including the financial statements and annexes attached hereto and the other documents referred to herein.
Q. Why am I receiving this proxy statement/prospectus?
A. LightJump and Moolec have entered into the Business Combination Agreement with Holdco and Merger Sub, which provides for the Business Combination in which, among other transactions, Moolec and LightJump will become direct subsidiaries of Holdco. A copy of the Business Combination Agreement is attached to this proxy statement/prospectus as Annex A. In addition, the Company Shareholders have entered into the Exchange Agreements with Holdco. If you are a LightJump Holder, you are receiving this proxy statement/prospectus because you hold SPAC Common Stock as of the record date for the special stockholder meeting at which LightJump Holders will be asked to approve the Business Combination Agreement, among other things.
Q. What will happen in the Business Combination?
Pursuant to the Business Combination Agreement and related agreements, on the Closing Date:
• all the issued Company Ordinary Shares held by Company Shareholders shall be transferred and for purposes of the 1915 Law, contributed in kind to Holdco, free and clear of all Liens (other than the Company Shareholders’ Agreements Liens that will expire on or prior to the Closing Date), and Company Shareholders shall subscribe for and, as consideration for the contribution, shall be issued, in accordance with the Exchange Ratio (save that the Holdco Ordinary Shares to be issued shall be reduced by the number of Holdco Ordinary Shares already held by Company Shareholders immediately prior to the Exchange), 27,500,000 of Holdco Ordinary Shares; provided, however, that no fractional Holdco Ordinary Shares shall be issued pursuant to the Exchange. For Luxembourg law purposes, a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued a report on the contributions in kind relating to the contribution of the Company Ordinary Shares prepared in accordance with article 420-10 of the 1915 Law;
• each Company SAFE Holder shall have contributed all of its rights and obligations under each Original SAFE to Holdco in consideration for the issuance by Holdco of a simple agreement for future equity on substantively identical terms (mutatis mutandis) with such adjustments (if any) required under Luxembourg law. For Luxembourg law purposes, a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued a report on the contributions in kind relating to the contribution of the Original SAFEs prepared in accordance with article 420-10 of the 1915 Law;
• each Company Shareholder shall cease to be the holder of such Company Ordinary Shares, subject to the submission of all filings required under Law (including any filings required to pay stamp duties), and Holdco will be recorded as the registered holder of all Company Ordinary Shares so exchanged and transferred and will be the legal and beneficial owner thereof; and
• immediately prior to the Merger Effective Time but after the Exchange Effective Time, each Company SAFE Holder shall receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the respective Original SAFE, with such adjustments (if any) required under Luxembourg law.
Upon consummation of the Business Combination, Moolec and LightJump will each be direct subsidiaries of Holdco.
SPAC Common Stock, SPAC Units and SPAC Warrants are currently listed and traded on Nasdaq under the symbols “LJAQ”, “LJAQU” and “LJAQW”, respectively. Holdco intends to apply for listing, to be effective at the time of the Closing, of the Holdco Ordinary Shares and Holdco Warrants on Nasdaq under the symbols
12
Table of Contents
“MLEC” and “MLECW”, respectively. This proxy statement/prospectus and its Annexes contain important information about the proposed Business Combination and the other proposals to be acted upon at the special meeting. You should read this proxy statement/prospectus and its annexes carefully and in their entirety.
Q. When and where is the LightJump special meeting?
A. The special meeting will be held at [•], Eastern time, on [•], 2022, via live webcast at https://www.cstproxy.com/lightjumpacquisition/2022. The special meeting will be completely virtual. You will be able to attend the special meeting online, vote and submit your questions during the special meeting by visiting https://www.cstproxy.com/lightjumpacquisition/2022, subject to the instructions below. If you plan to attend the virtual online special meeting, you will need the control number found on your proxy card, voting instruction form or notice.
Any stockholder wishing to attend the special meeting must register in advance. To register for and attend the special meeting, please follow these instructions as applicable to the nature of your ownership of SPAC Common Stock:
Record Owners. If you are a record holder and you wish to attend the special meeting, go to https://www.cstproxy.com/lightjumpacquisition/2022, enter the control number you received on your proxy card or notice of the meeting and click on the “Click here to preregister for the online meeting” link at the top of the page. You will need to log back into the meeting site using your control number immediately prior to the start of the special meeting. You must register before the meeting starts.
Beneficial Owners. Beneficial owners who wish to attend the special meeting must obtain a legal proxy from the stockholder of record and e-mail a copy of their legal proxy to proxy@continentalstock.com. Beneficial owners should contact their bank, broker, or other nominee for instructions regarding obtaining a legal proxy. Beneficial owners who e-mail a valid legal proxy will be issued a meeting control number that will allow them to register to attend and participate in the special meeting. You will receive an e-mail prior to the meeting with a link and instructions for entering the special meeting. Beneficial owners should contact Continental Stock Transfer & Trust Company on or before [•] p.m. Eastern Time on [•], 2022.
Q. What matters will LightJump Holders consider at the special meeting of stockholders?
A. At the LightJump special meeting of stockholders, LightJump will ask its stockholders to vote in favor of the following proposals:
• Proposal 1 (The Business Combination Proposal) — a proposal to approve and adopt the Business Combination Agreement and the Business Combination.
• Proposal 2 (Stockholder Adjournment Proposal) — a proposal to adjourn the special meeting to a later date or dates, if necessary, to permit further solicitation and vote of proxies if, based upon the tabulated vote at the time of the special meeting, there are not sufficient votes to approve Proposal 1 (Business Combination Proposal).
Q. What happens if Proposal 1 (Business Combination Proposal) is not approved?
A. If Proposal 1 (Business Combination Proposal) is not approved and LightJump does not consummate a business combination by January 12, 2023, LightJump will be required to dissolve and liquidate, and the holders of Public Shares will be entitled to redeem their Public Shares for a pro rata share of the amount on deposit in the Trust Account.
Q. Are the proposals conditioned on one another?
A. The Closing of the Business Combination is conditioned on the approval of the Business Combination Proposal. The Stockholder Adjournment Proposal is not conditioned on the approval of any other proposal set forth in this proxy statement/prospectus. It is important for you to note that, in the event that the Business Combination Proposal does not receive the requisite vote for approval, LightJump will not consummate the Business Combination.
13
Table of Contents
Q. Why is LightJump proposing the Business Combination Proposal?
A. LightJump was organized for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. Although LightJump was primarily focused on technology or technology enabled businesses, LightJump is not limited to any particular industry or sector. See the section entitled “The Business Combination — LightJump’s Board of Directors’ Reasons for the Approval of the Business Combination.”
Q. Who is Moolec?
A. Moolec is a science-based food ingredient company that focuses on developing real animal proteins in plants using Molecular Farming, a disruptive, scalable, affordable, and sustainable technology. Its purpose is to upgrade taste, nutrition, and affordability of alternative protein products while building a more sustainable and equitable food system. Moolec was founded in 2020 as a spin-off from a privately owned entity, Bioceres Group S.A., which has since provided Moolec with a scientific team and certain intellectual property (Chymosin SPC and GLA patents, as well as trademarks).
Q. Did LightJump’s board of directors obtain a third-party valuation or fairness opinion in determining whether or not to proceed with the Business Combination?
A. In approving the Business Combination, LightJump’s board of directors did not initially obtain a fairness opinion. However, it was decided to include the fairness opinion as a condition of closing. LightJump’s board of directors retained Scura Partners, LLC (“Scura Partners”) to evaluate the fairness, from a financial point of view, of the Merger Consideration to be paid to the LightJump Holders. Scura Partners delivered a written fairness opinion to LightJump dated October 15, 2022, in which it concluded that, as of such date and based on and subject to the matters described therein, the Merger Consideration to be paid to the LightJump Holders was fair, from a financial point of view, to such stockholders. Scura Partners also concluded that the fair market value of Moolec equals or exceeds 80% of the amount held by the LightJump in trust for benefit of its holders of Public Shares (excluding any deferred underwriting commissions and taxes payable on interest earned on the trust account). See “The Business Combination — Fairness Opinion of Scura Partners.”
Q. What equity stake will current LightJump Holders and current Company Shareholders have in Holdco after the Closing?
A. It is anticipated that, upon completion of the Business Combination, (i) the LightJump Holders will own approximately 13.47% of the issued and outstanding Holdco Ordinary Shares, including 7.14% shares subject to certain lock-up arrangements pursuant to the Registration Rights Agreement and Lock-Up Agreement and (ii) the Company Shareholders, CFO and SAFE Holders will own approximately 82.58%, 0.62%, and .69%, of the issued and outstanding Holdco Ordinary Shares, respectively. Certain figures included in this section have been rounded for ease of presentation and, as a result, percentages may not sum to 100%.
The following table presents the share ownership of various holders of Holdco Ordinary Shares upon the closing of the Business Combination and are based on the assumptions that (i) the Closing Date shall be [•], 2022, (ii) no additional equity securities of LightJump are issued at or prior to Closing and (iii) the following redemption scenarios:
No Redemptions. This scenario assumes that none of LightJump’s existing Public Stockholders will exercise their redemption rights in connection with the approval of the Business Combination with respect to their Public Shares.
50% Redemptions. This scenario assumes that LightJump’s existing Public Stockholders exercise their redemption rights with respect to 1,383,605 Public Shares (50% of the currently issued and outstanding unredeemed Public Shares) in connection with the approval of the Business Combination, at a price of $10.12 per share.
Maximum Redemptions. This scenario assumes that 100% of LightJump’s existing Public Stockholders exercise their redemption rights with respect to their public shares in connection with the approval of the Business Combination, at a price of $10.12 per share and assumes that the obligations under the Backstop Agreement will be satisfied by making a cash contribution to Holdco. See “Certain Agreements Related to the Business Combination — Backstop Agreement.”
14
Table of Contents
| | No Redemptions(1) | | 50% Redemptions(1) | | Maximum
Redemptions(1)(2) |
Shareholders of Holdco Post Business Combination(1) | | Number of
Holdco
Ordinary
Shares | | % of
Total | | Number of
Holdco
Ordinary
Shares | | % of
Total | | | | % of
Total |
BG Farming Technologies Limited | | 15,275,000 | | 38.8 | % | | 15,275,000 | | 40.2 | % | | 15,275,000 | | | 40.6 | % |
Union Group Ventures Ltd. | | 15,275,000 | | 38.8 | % | | 15,275,000 | | 40.2 | % | | 15,525,000 | (4)(7) | | 41.3 | % |
Bioceres Crop Solutions Corp. | | 1,950,000 | | 5.0 | % | | 1,950,000 | | 5.1 | % | | 1,950,000 | | | 5.2 | % |
SAFE Holders | | 274,951 | | .7 | % | | 274,951 | | .7 | % | | 524,951 | (5)(7) | | 1.4 | % |
Initial Stockholders(3) | | 2,535,000 | | 6.4 | % | | 2,535,000 | | 6.7 | % | | 3,035,000 | (6)(7) | | 8.1 | % |
LightJump Public Stockholders | | 2,767,210 | | 7.0 | % | | 1,383,605 | | 3.6 | % | | 0 | | | 0 | % |
Key Staff Participation | | 243,774 | | .6 | % | | 243,774 | | .6 | % | | 243,774 | | | .7 | % |
UG Holdings LLC | | 1,035,000 | | 2.6 | % | | 1,035,000 | | 2.7 | % | | 1,035,000 | | | 2.8 | % |
Total | | 39,355,935 | | 99.9 | % | | 37,972,330 | | 99.8 | % | | 37,588,725 | | | 100.1 | % |
Q. Who will be the officers and directors of Holdco if the Business Combination is consummated?
A. It is anticipated that, at the Closing, Holdco’s board of directors will be composed of seven members, including [•]. See the section entitled “Management of Holdco after the Business Combination” for additional information.
Q. What conditions must be satisfied to complete the Business Combination?
A. There are a number of closing conditions in the Business Combination Agreement, including, without limitation, that LightJump Holders have approved and adopted the Business Combination Agreement. For a summary of the conditions that must be satisfied or waived prior to completion of the Business Combination, please see the section entitled “The Business Combination Agreement.”
Q. What happens if I sell my shares of SPAC Common Stock before the special meeting of stockholders?
A. If you transfer your shares of SPAC Common Stock after the record date, but before the special meeting of stockholders, unless the transferee obtains from you a proxy to vote those shares, you will retain your right to vote at the special meeting of stockholders. However, you will not be entitled to redeem your Public Shares for cash or to receive any Holdco Ordinary Shares following the Closing because only LightJump Holders on the date of the Closing will be entitled to either redeem their Public Shares for cash or to receive Holdco Ordinary Shares in connection with the Closing.
15
Table of Contents
Q. What vote is required to approve the proposals presented at the special meeting of stockholders?
A. The approval of Proposal 1 (Business Combination Proposal) requires the affirmative vote of the holders of a majority of all outstanding shares of SPAC Common Stock entitled to vote thereon at the special meeting of stockholders. Accordingly, a LightJump Holder’s failure to vote by proxy or in person at the special meeting of stockholders or to instruct its broker how to vote, or an abstention from voting, will have the same effect as a vote “AGAINST” the Business Combination Proposal.
The approval Proposal 2 (Stockholder Adjournment Proposal) requires the affirmative vote of the holders of a majority of the shares of SPAC Common Stock that are voted thereon at the special meeting of stockholders. Accordingly, a LightJump Holder’s failure to vote by proxy or to vote in person at the special meeting of stockholders or to instruct its broker how to vote, or an abstention from voting, will have no effect on the outcome of any vote on the Stockholder Adjournment Proposal.
Q. Do Company Shareholders need to approve the Business Combination?
A. As required pursuant to Moolec’s organizational documents, each of the Company Shareholders have approved the Exchange and related transactions in the Exchange Agreements, as further described below. Each of the Company Shareholders have entered into the Exchange Agreements and each of the Company Shareholders and Company SAFE Holders have entered into the Transaction Support Agreement. For more information about the terms and conditions of the Exchange Agreements and the Transaction Support Agreement please see the section entitled “Certain Agreements Related to the Business Combination.”
Q. May LightJump, the Sponsor or LightJump’s directors, officers or advisors, or their affiliates, purchase shares in connection with the Business Combination?
A. In connection with the stockholder vote to approve Proposal 1 (Business Combination Proposal) and the other proposals, LightJump and its affiliates may privately negotiate transactions to purchase shares prior to the Closing from stockholders who would have otherwise elected to have their shares redeemed for a pro rata portion of the Trust Account upon consummation of the Business Combination. Such a purchase would include a contractual acknowledgement that such stockholder, although still the record holder of such shares, is no longer the beneficial owner thereof and therefore agrees not to exercise its redemption rights. While they have no current plans to do so, the Sponsor, LightJump’s directors, officers or advisors, or their affiliates reserve the right to purchase shares in privately negotiated transactions from LightJump Holders who have already elected to exercise their redemption rights, in which event such selling stockholders would be required to revoke their prior elections to redeem their shares. Any such transaction would be separately negotiated at the time of the transaction. The consideration for any such transaction would consist of cash and/or SPAC Common Stock owned by the Sponsor and/or LightJump’s directors, officers, advisors, or their affiliates. The purpose of these purchases would be to increase the amount of cash available to LightJump for use in the Business Combination. None of LightJump, the Sponsor or LightJump’s directors, officers or advisors, or their respective affiliates, will make any such purchases when they are in possession of any material non-public information not disclosed to the seller.
Q. Will LightJump or Holdco issue additional equity securities in connection with the consummation of the Business Combination?
A. Holdco or LightJump may, with the consent of Moolec, enter into equity financings in connection with the proposed Business Combination with their respective affiliates or any third parties if LightJump determines that the issuance of additional equity is necessary or desirable in connection with the consummation of the Business Combination. Any equity issuances could result in dilution of the relative ownership interest of the non-redeeming LightJump Holders or Company Shareholders.
Q. How many votes do I have at the special meeting of stockholders?
A. LightJump Holders are entitled to one vote at the special meeting for each share of SPAC Common Stock held of record as of the record date. As of the close of business on the record date, there were 2,767,210 outstanding shares of SPAC Common Stock. SPAC Warrants and SPAC Units do not entitle their holders to vote.
16
Table of Contents
Q. How will the Sponsor, directors and officers vote?
A. In connection with LightJump’s IPO, LightJump entered into agreements with the Sponsor, officers and directors, pursuant to which each agreed to vote their shares of SPAC Common Stock in favor of Proposal 1 (Business Combination Proposal). LightJump’s Sponsor, officers and directors, who currently own approximately 54.4% of the outstanding shares of SPAC Common Stock, have agreed to vote their SPAC Common Stock, as well as any shares of SPAC Common Stock they may purchase prior to the special meeting of stockholders, in favor of Proposal 1 (Business Combination Proposal) and Proposal 2 (Stockholder Adjournment Proposal). As a result, LightJump would not require any additional votes in favor of such proposals in order to have the Business Combination Proposal and Stockholder Adjournment Proposal approved.
Q. What interests do LightJump’s current officers and directors have in the Business Combination?
A. LightJump’s directors and executive officers have interests in the Business Combination that are different from, in addition to, or in conflict with, yours. These interests include:
• The Sponsor beneficially owns 3,450,000 Founder Shares, all of which are beneficially owned by our Chairman and Chief Executive Officer, and such shares would become worthless if LightJump does not complete a business combination within the applicable time period, as such LightJump Initial Stockholders have waived any right to liquidation proceeds with respect to these shares. The Sponsor paid an aggregate of $25,000 (or $0.009 per share) for the 3,450,000 Founder Shares. Such shares have an aggregate market value of approximately $[•] based on the closing price of SPAC Common Stock of $[•] on Nasdaq on [•], the record date for the special meeting of stockholders.
• The Sponsor also beneficially owns 4,210,000 SPAC Warrants, for which it paid $4,210,000 and which will expire and be worthless if LightJump does not complete a business combination within the applicable time period.
• LightJump’s officers and directors have invested an aggregate of $4,235,000, which will be lost in the event that the Business Combination is not approved and concluded.
• LightJump’s directors will not receive reimbursement for the out-of-pocket expenses ($0.00 as of the date hereof) incurred by them on LightJump’s behalf incident to identifying, investigating and consummating a business combination, unless a business combination is consummated.
• The Sponsor and its affiliates can earn a positive rate of return on their investments, even if the Public Stockholders experience a negative rate of return on their investments in LightJump and Holdco, as the Sponsor has purchased 3,450,000 Founder Shares for an aggregate of $25,000 (or $0.009 per share).
• Certain of LightJump’s directors could potentially continue as directors of Holdco if the Business Combination is completed.
• Because the Sponsor and the LightJump directors will benefit from the completion of a business combination, they may be incentivized to recommend and complete a business combination of a less favorable target company or on terms less favorable to LightJump Holders, rather than liquidate LightJump.
• LightJump would be unable to indemnify its current directors and officers or continue to provide directors’ and officers’ liability insurance if the Business Combination is not completed.
None of the Sponsor nor any of LightJump’s directors, officers, or their affiliates will receive any additional securities pursuant to any anti-dilution adjustment provisions based on any potential additional investments.
These interests may have influenced LightJump’s directors in approving the Business Combination and making their recommendation to vote in favor of the approval of the Business Combination Proposal. Please read the section entitled “The Business Combination — Interests of LightJump’s Directors and Officers in the Business Combination.”
17
Table of Contents
Q. What are the potential impacts on the Business Combination and the Transactions resulting from the resignation of Nomura.
A. On June 29, 2022, Nomura Securities International, Inc. (“Nomura”) resigned from its role as exclusive financial advisor to Moolec, effective as of April 27, 2022, and waived its entitlement to the payment of any fees or expense reimbursements. On March 30, 2022, prior to the resignation of Nomura, the SEC issued proposed rules (the “SPAC Rule Proposals”) relating to, among other items, disclosures in SEC filings and the treatment of investment banks in connection with business combination transactions involving special purpose acquisition companies. The uncertainty related to the treatment of investment banks following the SPAC Rule Proposals was the reason Nomura gave for its resignation. Certain customary provisions related to the engagement letter survive the resignation of Nomura, including customary obligations with respect to use of information, indemnification and governing law. The resignation of Nomura will have no impact on the Business Combination and the Transactions. Please see the section entitled “The Business Combination — The Background of the Business Combination” for a discussion regarding the resignation of Nomura.
Q. Do I have redemption rights?
A. If you are a Public Stockholder, you may redeem all or a portion of your Public Shares for cash upon consummation of the Business Combination. The per share redemption price will be equal to the aggregate amount then on deposit in the Trust Account that holds certain of the proceeds of our IPO and simultaneous private placement, including interest (net of taxes payable), divided by the number of then outstanding public shares. All of the LightJump Initial Stockholders have agreed to waive their redemption rights with respect to their SPAC Common Stock in connection with the completion of LightJump’s initial business combination, and such shares will be excluded from the pro rata calculation used to determine the per-share redemption price. For illustrative purposes, based on funds in the Trust Account of approximately $27,993,798 on July 12, 2022 and 2,767,210 Public Shares outstanding, the estimated per share redemption price would have been approximately $10.12. In no event, however, will LightJump redeem shares of SPAC Common Stock in an amount that would cause LightJump’s net tangible assets to be less than $5,000,001.
Q. Will how I vote affect my ability to exercise redemption rights?
A. No. You may exercise your redemption rights whether you vote your SPAC Common Stock for or against Proposal 1 (Business Combination Proposal) or any other proposal described in this proxy statement/prospectus, abstain from voting or do not vote your shares. As a result, Proposal 1 (Business Combination Proposal) can be approved by stockholders who will redeem their SPAC Common Stock and no longer remain stockholders, leaving stockholders who choose not to redeem their SPAC Common Stock holding shares in a company with a less liquid trading market, fewer stockholders, less cash and the potential inability to meet the listing standards of Nasdaq.
Q. How do I exercise my redemption rights?
A. In order to exercise your redemption rights, you must, prior to 5:00 p.m. Eastern time on [•], 2022 (two Business Days before the special meeting), (i) submit a written request to Continental Stock Transfer & Trust Company, LightJump’s transfer agent, that LightJump redeem your SPAC Common Stock for cash, and (ii) deliver your stock to LightJump’s transfer agent physically or electronically through the Depository Trust Company (“DTC”). Your request should be submitted to the transfer agent at the following address:
Continental Stock Transfer & Trust Company
1 State Street Plaza, 30th Floor
New York, New York 10004
Attention: Mark Zimkind
Email: mzimkind@continentalstock.com
Electronic delivery of your stock generally will be faster than delivery of physical stock certificates.
A physical stock certificate will not be needed if your stock is delivered to LightJump’s transfer agent electronically. In order to obtain a physical stock certificate, a stockholder’s broker and/or clearing broker, DTC and LightJump’s transfer agent will need to act to facilitate the request. It is LightJump’s understanding that stockholders should generally allot at least one week to obtain physical certificates from the transfer
18
Table of Contents
agent. However, because LightJump does not have any control over this process or over the brokers or DTC, it may take significantly longer than one week to obtain a physical stock certificate. If it takes longer than anticipated to obtain a physical certificate, stockholders who wish to redeem their shares and do not deliver their stock electronically may be unable to obtain physical certificates by the deadline for exercising their redemption rights and thus will be unable to redeem their shares.
Any demand for redemption, once made, may be withdrawn at any time until the deadline for exercising redemption requests and thereafter, with LightJump’s consent, until the vote is taken with respect to the Business Combination. If you delivered your shares for redemption to LightJump’s transfer agent and decide within the required timeframe not to exercise your redemption rights, you may request that LightJump’s transfer agent return the shares (physically or electronically). Such requests may be made by contacting LightJump’s transfer agent at the street or e-mail address above.
Q. What are the U.S. federal income tax consequences of exercising my redemption rights?
A. In the event that a U.S. Holder (defined below) elects to redeem its SPAC Common Stock for cash as described in the redemption provisions herein, the treatment of the transaction for U.S. federal income tax purposes will depend on whether the redemption qualifies as a sale of the SPAC Common Stock under Section 302 of the Code. If the redemption qualifies as a sale of the SPAC Common Stock, and the SPAC Common Stock is held as a capital asset by the U.S. Holder on the date of the redemption, the U.S. Holder will be treated as recognizing capital gain or loss equal to the difference between the amount realized on the redemption and such U.S. Holder’s adjusted tax basis in the SPAC Common Stock surrendered in such redemption transaction. Any such capital gain or loss generally will be long-term capital gain or loss if the U.S. Holder’s holding period for the SPAC Common Stock redeemed exceeds one year. Long-term capital gains recognized by non-corporate U.S. Holders may be eligible to be taxed at preferential rates. The deductibility of capital losses is subject to certain limitations. See the sections entitled “Material U.S. Federal Income Tax Considerations — U.S. Holders — Exercise of Redemption Rights”, “Material U.S. Federal Income Tax Considerations — U.S. Holders — Gain or Loss on Redemption Treated as a Sale of SPAC Common Stock”, and “Material U.S. Federal Income Tax Considerations — U.S. Holders — Taxation of Redemption Treated as a Distribution.” For tax considerations applicable to Non-U.S. Holders (defined below), see the sections entitled “Material U.S. Federal Income Tax Considerations — Non-U.S. Holders — Exercise of Redemption Rights”, “Material U.S. Federal Income Tax Considerations — Non-U.S. Holders — Gain or Loss on Redemption Treated as a Sale of SPAC Common Stock”, and “Material U.S. Federal Income Tax Considerations — Non-U.S. Holders — Taxation of Redemption Treated as a Distribution.”
Q. What are the U.S. federal income tax consequences of not exercising my redemption rights and participating in the Business Combination?
A. Subject to the limitations and qualifications described in “Material U.S. Federal Income Tax Considerations,” including the discussion on Section 367(a) of the Code, if the Merger qualifies as a “reorganization” within the meaning of Section 368 of the Code, a U.S. Holder should not recognize gain or loss on the exchange of SPAC Common Stock or SPAC Warrants for Holdco Ordinary Shares or Holdco Warrants, as applicable, pursuant to the Merger; provided, however, that if the Merger does not qualify as a “reorganization” within the meaning of Section 368 of the Code, it is intended that the Merger, together with the Exchange, will be treated as an exchange described in Section 351(a) of the Code. If the Merger qualifies as part of an exchange subject to Section 351 of the Code, a U.S. Holder that exchanges its SPAC Common Stock in the Merger for Holdco Ordinary Shares generally should not recognize gain or loss on such exchange, subject to the discussion regarding the treatment of U.S. Holders that exchange both SPAC Common Stock and SPAC Warrants and further subject to the discussion on Section 367(a) of the Code. For a detailed discussion of U.S. federal income tax consequences of the Merger to U.S. Holders, see the section entitled “Material U.S. Federal Income Tax Considerations — U.S. Holders.” For U.S. federal income tax consequences applicable to Non-U.S. Holders, see the section entitled “Material U.S. Federal Income Tax Considerations — Non-U.S. Holders.” All holders of SPAC securities should review the entirety of the section entitled “Material U.S. Federal Income Tax Considerations” and consult with their tax advisors regarding the U.S. federal income tax consequences of the Merger and considerations relating to the ownership and disposition of Holdco securities.
19
Table of Contents
Q: If I hold SPAC Warrants, can I exercise redemption rights with respect to my warrants?
A: No. There are no redemption rights with respect to the SPAC Warrants.
Q: If I hold SPAC Units, can I exercise redemption rights with respect to my SPAC Units?
A: No. Holders of outstanding SPAC Units must separate the underlying SPAC Common Stock and SPAC Warrants prior to exercising redemption rights with respect to the SPAC Common Stock.
Q: Do I have appraisal rights if I object to the proposed Business Combination?
A: No. There are no appraisal rights available to holders of shares of SPAC Common Stock in connection with the Business Combination.
Q: What happens to the funds held in the Trust Account upon consummation of the Business Combination?
A: If the Business Combination is consummated, the funds held in the Trust Account will be released to pay LightJump Holders who properly exercise their redemption rights, and any remaining balance will be released to Holdco to be used for general corporate purposes (including repayment SPAC Transaction Expenses and Sponsor Advanced Loans) following the Business Combination.
Q: What happens if the Business Combination is not consummated?
A: There are certain circumstances under which the Business Combination Agreement may be terminated. See the section entitled “The Business Combination Agreement” for information regarding the parties’ specific termination rights. In addition, the Business Combination will not be consummated if the Business Combination Proposal is not approved or the other conditions to closing are not satisfied or waived.
In order to provide more time to consummate the Business Combination, in July 2022 the LightJump Holders approved an amendment to the SPAC COI to extend the deadline for LightJump to complete its initial business combination to January 12, 2023. If, as a result of the termination of the Business Combination Agreement or otherwise, LightJump is unable to complete a business combination by January 12, 2023, or amend the SPAC COI to further extend the date by which LightJump must consummate an initial business combination, the SPAC COI provides that LightJump will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten Business Days thereafter subject to lawfully available funds therefor, redeem 100% of the IPO Shares in consideration of a per-share price, payable in cash, equal to the quotient obtained by dividing (A) the aggregate amount then on deposit in the Trust Account, including interest (which shall be net of taxes payable), by (B) the total number of then outstanding IPO Shares, which redemption will completely extinguish rights of the holders of IPO Shares (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the remaining stockholders and the Board of Directors in accordance with applicable law, dissolve and liquidate, subject in each case to the Corporation’s obligations under the DGCL to provide for claims of creditors and other requirements of applicable law. See the section entitled “Risk Factors — Risks Related to LightJump and the Business Combination.” The LightJump Initial Stockholders have waived any right to any liquidation distribution with respect to their shares of SPAC Common Stock.
In the event of liquidation, there will be no distribution with respect to outstanding SPAC Warrants. Accordingly, the SPAC Warrants will expire worthless.
Q: When is the Business Combination expected to be completed?
A: It is currently anticipated that the Business Combination will be consummated promptly following the special meeting of stockholders, provided that all other conditions to the consummation of the Business Combination have been satisfied or waived.
For a description of the conditions to the completion of the Business Combination, see the section entitled “LightJump Stockholder Proposal No. 1 — The Business Combination Proposal.”
20
Table of Contents
Q: What do I need to do now?
A: You are urged to carefully read and consider the information contained in this proxy statement/prospectus, including the financial statements and annexes attached hereto, and to consider how the Business Combination will affect you as a stockholder. You should then vote as soon as possible in accordance with the instructions provided in this proxy statement/prospectus on the enclosed proxy card or, if you hold your shares through a brokerage firm, bank or other nominee, on the voting instruction form provided by the broker, bank or nominee.
Q: How do I vote?
A: If you were a holder of record of SPAC Common Stock on [•], 2022, the record date for the special meeting of stockholders, you may vote by telephone, online or by completing, signing, dating and returning the enclosed proxy card in the postage-paid envelope provided. If you hold your shares in “street name,” which means your shares are held of record by a broker, bank or other nominee, you should contact your broker, bank or nominee to ensure that votes related to the shares you beneficially own are properly counted. In this regard, you must provide the record holder of your shares with instructions on how to vote your shares or, if you wish to attend the special meeting of stockholders and vote online, obtain a proxy from your broker, bank or nominee.
Q: What will happen if I abstain from voting or fail to vote at the special meeting?
A: At the special meeting of stockholders, LightJump will count a properly executed proxy marked “ABSTAIN” with respect to a particular proposal as present for purposes of determining whether a quorum is present. For purposes of approval, an abstention or failure to vote will have the same effect as a vote “AGAINST” Proposal 1 (Business Combination Proposal) and will have no effect Proposal 2 (Stockholder Adjournment Proposal).
Q: What will happen if I sign and return my proxy card without indicating how I wish to vote?
A: If you sign and return your proxy card without indicating how you wish to vote, your proxy will be voted in favor of each of the proposals presented at the special meeting.
Q. Do I need to attend the special meeting of stockholders to vote my shares?
A. No. You are invited to attend the special meeting to vote on the proposals described in this proxy statement/prospectus. However, you do not need to attend the special meeting of stockholders to vote your shares. Instead, you may submit your proxy by telephone, online or by signing, dating and returning the enclosed proxy card in the pre-addressed postage-paid envelope. Your vote is important. LightJump encourages you to vote as soon as possible after carefully reading this proxy statement/prospectus.
Q. If I am not going to attend the special meeting of stockholders virtually by telephone or online, should I vote by proxy instead?
A. Yes. After carefully reading and considering the information contained in (and incorporated by reference into) this proxy statement/prospectus, please vote by telephone or online, or submit your proxy, as applicable, by completing, signing, dating and returning the enclosed proxy card in the postage-paid envelope provided.
Q. If my shares are held in “street name,” will my broker, bank or nominee automatically vote my shares for me?
A. No. If your broker holds your shares in its name and you do not give the broker voting instructions, under the applicable stock exchange rules, your broker may not vote your shares on any of the proposals. If you do not give your broker voting instructions and the broker does not vote your shares, your shares will not be counted for purposes of determining the presence of a quorum at the special meeting of stockholders, and it will have the same effect as a vote “AGAINST” Proposal 1 (Business Combination Proposal) and will have no effect on Proposal 2 (Stockholder Adjournment Proposal). However, in no event will your broker’s failure to vote your shares have the effect of exercising your redemption rights, which may only be exercised as described under “The Special Meeting of LightJump Holders — Redemption Rights.”
21
Table of Contents
Q. May I change my vote after I have voted by proxy?
A. Yes. If you vote by telephone or online, only your latest telephone or online proxy that is timely submitted prior to the meeting will be counted. If you vote by signing and returning a proxy card, you may change your vote by completing a new proxy card with a later date. You may also revoke your proxy and change your vote by virtually attending the meeting and voting online. You also may revoke your proxy by sending a notice of revocation to Advantage Proxy, Inc. at P.O. Box 13581, Des Moines, Washington 98198, provided such revocation is received prior to the vote at the special meeting. If your shares are held in street name by a broker or other nominee, you must contact the broker or nominee to change or revoke your vote.
Q. What should I do if I receive more than one set of voting materials?
A. If you hold your shares in more than one brokerage account, you will receive a separate voting instruction card for each brokerage account in which you hold shares. If you are a holder of record and your shares are registered in more than one name, you will receive more than one proxy card. You may also receive multiple copies of this proxy statement/prospectus. Please complete, sign, date and return each proxy card and voting instruction card that you receive in order to cast your vote with respect to all of your shares.
Q. What is the quorum requirement for the special meeting of stockholders?
A. A quorum will be present at the special meeting of stockholders if a majority of the SPAC Common Stock outstanding and entitled to vote at the meeting is represented in person or by proxy. In the absence of a quorum, a majority of LightJump Holders, present in person or represented by proxy, and voting thereon will have the power to adjourn the special meeting to another date.
Your shares will be counted towards the quorum only if you submit a valid proxy (or your broker, bank or other nominee submits one on your behalf) or if you vote in person at the special meeting of stockholders. Abstentions will be counted towards the quorum requirement.
Q. What happens to SPAC Warrants I hold if I vote my shares of SPAC Common Stock against approval of Proposal 1 (Business Combination Proposal) and/or validly exercise my redemption rights with respect to my SPAC Common Stock?
A. Regardless of how, or whether, you vote, if the Business Combination is completed, all of your SPAC Warrants will convert into Holdco Warrants as described in this proxy statement/prospectus, even if you redeem your shares of SPAC Common Stock for cash. If the Business Combination is not completed, you will continue to hold your SPAC Warrants, and if LightJump does not otherwise consummate an initial business combination by January 12, 2023 or amend the SPAC COI to further extend the date by which LightJump must consummate an initial business combination, LightJump will be required to dissolve and liquidate, and your warrants will expire worthless.
Q. What happens to SPAC Units I hold if I vote my shares of SPAC Common Stock against approval of the Business Combination Proposal and/or validly exercise my redemption rights?
A. Regardless of how you vote and in connection with the consummation of the Business Combination and immediately prior to the Merger Effective Time, the SPAC Units will automatically separate into their component parts and holders of SPAC Units will receive one Holdco Ordinary Share for each Ordinary Share and one Holdco Warrant for each SPAC Warrant. If you redeem your shares of SPAC Common Stock for cash, and still hold other SPAC Units, the SPAC Units will be converted into Holdco Ordinary Shares and Holdco Warrants as described in this proxy statement/prospectus. If the Business Combination is not completed, you will continue to hold your SPAC Units, and if LightJump does not otherwise consummate an initial business combination by January 12, 2023 or amend the SPAC COI to further extend the date by which LightJump must consummate an initial business combination, LightJump will be required to dissolve and liquidate, and you will receive the corresponding pro rata amount from the Trust Account that corresponds to the SPAC Common Stock components of the SPAC Units that you hold.
22
Table of Contents
Q. Who will solicit and pay the cost of soliciting proxies?
A. LightJump will pay the cost of soliciting proxies for the special meeting. LightJump has engaged Advantage Proxy, Inc. to assist in the solicitation of proxies for the special meeting. LightJump has agreed to pay Advantage Proxy, Inc. a fee of $[•]. LightJump will reimburse Advantage Proxy, Inc. for reasonable out-of-pocket expenses and will indemnify Advantage Proxy, Inc. and its affiliates against certain claims, liabilities, losses, damages and expenses. LightJump also will reimburse banks, brokers and other custodians, nominees and fiduciaries representing beneficial owners of shares of SPAC Common Stock for their expenses in forwarding soliciting materials to beneficial owners of SPAC Common Stock and in obtaining voting instructions from those owners. LightJump’s directors, officers and employees may also solicit proxies by telephone, by facsimile, by mail, on the internet or in person. They will not be paid any additional amounts for soliciting proxies.
Q. Who can help answer my questions?
A. If you have questions about the stockholder proposals, or if you need additional copies of this proxy statement/prospectus, or the proxy cards you should contact LightJump’s proxy solicitor at:
Advantage Proxy, Inc.
P.O. Box 13581
Des Moines, WA 98198
Attn: Karen Smith
Toll Free Telephone: (877) 870-8565
Main Telephone: (206) 870-8565
E-mail: ksmith@advantageproxy.com
You may also contact LightJump at:
LightJump Acquisition Corporation
2735 Sand Hill Road, Suite 110
Menlo Park, California 94025
Telephone: (650) 515-3930
To obtain timely delivery, LightJump Holders and warrant holders must request the materials no later than five Business Days prior to the special meeting.
You may also obtain additional information about LightJump from documents filed with the SEC by following the instructions in the section entitled “Where You Can Find More Information.”
23
Table of Contents
Summary of the Proxy Statement/Prospectus
This summary highlights selected information from this proxy statement/prospectus and does not contain all of the information that is important to you. To better understand the Business Combination and the proposals to be considered at the special meeting you should read this entire proxy statement/prospectus carefully, including the annexes. See also the section entitled “Where You Can Find More Information.” Certain figures included in this section have been rounded for ease of presentation and, as a result, percentages may not sum to 100%.
Parties to the Business Combination
LightJump Acquisition Corporation
LightJump is a blank check company formed in Delaware on July 28, 2020, for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses, without limitation as to business, industry or sector. The SPAC Common Stock, SPAC Units and SPAC Warrants are currently listed and traded on Nasdaq under the symbols “LJAQ”, “LJAQU” and “LJAQW”, respectively. The closing prices of the publicly traded common stock, warrants and units of LightJump on June 13, 2022, the date preceding LightJump’s public announcement of the entry into the Business Combination Agreement, were $9.97, $10.01 and $0.1167, respectively.
At the Closing, the outstanding shares of SPAC Common Stock will convert into Holdco Ordinary Shares, the outstanding SPAC Warrants will convert into Holdco Warrants and any outstanding SPAC Units will separate and convert into Holdco Ordinary Shares and Holdco Warrants.
The mailing address of LightJump’s principal executive offices is 2735 Sand Hill Road, Suite 110, Menlo Park, California 94025, and its telephone number is (650) 515-3930.
Moolec
Moolec Science Limited is a private limited company incorporated under the laws of England and Wales with its registered office at the Innovation Centre, Gallows Hill, Warwick, CV34 6UW, United Kingdom.
Moolec is a science-based food ingredient company that focuses on developing real animal proteins in plants using Molecular Farming, a disruptive, scalable, affordable, and sustainable technology. Its purpose is to upgrade taste, nutrition, and affordability of alternative protein products while building a more sustainable and equitable food system. Moolec was founded in 2020 as a spin-off from a privately owned entity, Bioceres Group S.A., which has since provided Moolec with a scientific team and certain intellectual property (Chymosin SPC and GLA patents, as well as trademarks). Moolec operates in the United States, Europe and South America. The mailing address of Moolec’s principal executive office is Innovation Centre, Gallows Hill, Warwick, CV34 6UW, United Kingdom and its telephone number is +(44) 33 0001 0162.
For more information about Moolec, see the sections entitled “Information About Moolec” and “Moolec’s Management’s Discussion and Analysis of Financial Condition and Results of Operation.”
Holdco
Moolec Science SA was incorporated under the laws of the Grand Duchy of Luxembourg on May 23, 2022 as a public limited liability company (société anonyme) governed by the laws of the Grand Duchy of Luxembourg, with its registered office at 17, Boulevard F.W. Raiffeisen, L-2411 Luxembourg, Grand Duchy of Luxembourg and registered with the Luxembourg Trade and Companies’ Register (Registre de Commerce et des Sociétés, Luxembourg) under number B268440. Holdco owns no material assets and does not operate any business and was incorporated for purposes of the Business Combination. Prior to the consummation of the Business Combination, the directors of Holdco are Gastón Paladini, Oscar Alejandro Leôn Bentancor and Joost Anton Mees. Prior to the consummation of the Business Combination, the shareholders of Holdco are BG Farming Technologies Limited, Union Group Ventures Limited and Bioceres Crop Solutions Corp., holding 47%, 47% and 6%, respectively, of the currently outstanding Holdco Ordinary Shares. Holdco expects to apply to list its Holdco Ordinary Shares and Holdco Warrants on Nasdaq under the symbol “MLEC” and “MLECW”, respectively.
24
Table of Contents
The address of Holdco’s registered office is 17, Boulevard F.W. Raiffeisen, L-2411 Luxembourg, Grand Duchy of Luxembourg. After the consummation of the Business Combination, its principal executive office will remain at 17, Boulevard F.W. Raiffeisen, L-2411 Luxembourg, Grand Duchy of Luxembourg.
Holdco qualifies as an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), which means that it can take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies.
Upon the effectiveness of the registration statement of which this proxy statement/prospectus forms a part, Holdco will report under the Exchange Act as a non-U.S. company with foreign private issuer status. Even after Holdco no longer qualifies as an emerging growth company, as long as Holdco continues to qualify as a foreign private issuer under the Exchange Act, Holdco will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
• the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act;
• the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and
• the rules under the Exchange Act requiring the filing with the Securities and Exchange Commission, or SEC, of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events.
In addition, Holdco will not be required to file annual reports and financial statements with the SEC as promptly as U.S. domestic companies whose securities are registered under the Exchange Act, and is not required to comply with Regulation FD, which restricts the selective disclosure of material information.
As a foreign private issuer, Holdco will be permitted to follow home country corporate governance practices instead of certain corporate governance practices required by Nasdaq for U.S. domestic issuers.
Merger Sub
Moolec Acquisition, Inc. is a Delaware corporation and a direct wholly owned subsidiary of Holdco. Merger Sub was formed solely in contemplation of the Business Combination, has not commenced any operations, has only nominal assets and no liabilities or continent liabilities, nor any outstanding commitments other than in connection with the Business Combination.
The mailing address of Merger Sub’s principal executive office is c/o The Corporation Trust Company, 1209 Orange Street, Wilmington, Delaware 19801, New Castle County.
The Business Combination
Pursuant to the Business Combination Agreement, LightJump, Holdco, Merger Sub and Moolec will enter into the Business Combination, pursuant to which, among other things, (a) pursuant to the Exchange Agreements, each of the Company Shareholders, effective on the Exchange Effective Time, will contribute its respective Company Ordinary Shares to Holdco in exchange for Holdco Ordinary Shares to be subscribed for by each such Company Shareholder (such contributions and exchanges of Company Ordinary Shares for Holdco Ordinary Shares, collectively, the “Exchange”), (b) as a result of the Exchange, Moolec will become a wholly-owned subsidiary of Holdco, (c) immediately prior to the consummation of the Merger but after the Exchange Effective Time, each of the Company SAFE Holders will receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the respective Company SAFE, with such adjustments (if any) required under Luxembourg law, (d) following the consummation of the Exchange, Merger Sub will merge with and into LightJump, with LightJump surviving such merger and becoming a direct wholly-owned subsidiary of Holdco (the “Merger”) and, in the context of the Merger, all SPAC Common Stock outstanding shall be converted into the right to receive the Merger Consideration in the form of Holdco Ordinary Shares pursuant to a share capital increase of Holdco, as set forth in
25
Table of Contents
the Business Combination Agreement, and (e) Moolec’s CFO will be freely allotted the Key Staff Participation to satisfy the requirements under the CFO Consulting Agreement. Capitalized terms used but not defined herein shall have the respective meanings set forth in the Business Combination Agreement.
Upon the terms and subject to the conditions set forth in the Business Combination Agreement and the Exchange Agreements at the Exchange Effective Time, the Exchange will take place based on an exchange ratio of 0.66787343 used to determine the number of aggregate Holdco Ordinary Shares valued at $10.00 per Holdco Share for which the aggregate Company Ordinary Shares will be exchanged (the “Exchange Consideration”). The valuation of the Company Ordinary Shares contributed to Holdco by the Company Shareholders against new Holdco Ordinary Shares pursuant to the Exchange shall be deemed to be, as of the Exchange Effective Time, the sum of US$325,000,000.
Pursuant to the Exchange Agreements, each Company Shareholder has also agreed to not transfer any of its Company Ordinary Shares before the earlier to occur of the Exchange and the termination of the Business Combination Agreement pursuant to its terms.
In connection with the closing of the transactions contemplated by the Transaction Documents, including the Transactions, each of Union Group Ventures Limited, THEO I SCSp and Sponsor has entered into the Backstop Agreement, agreeing, severally but not jointly, to provide the funding of certain amounts as set forth therein.
The Business Combination Agreement
On June 14, 2022, LightJump, Moolec, Merger Sub and Holdco entered into the Business Combination Agreement, which contains customary representations and warranties, covenants, closing conditions, termination fee provisions and other terms relating to the Merger and Exchange and the other transactions contemplated thereby, as summarized below. Following the effectiveness of the transactions contemplated by the Merger, the parties will consummate the Business Combination and LightJump and Moolec will become direct wholly-owned subsidiaries of Holdco. Pursuant to the Business Combination Agreement, each of the following transactions will occur, in the following order:
• At the Exchange Effective Time, subject to the receipt of the Company Requisite Approvals, the Holdco Requisite Approvals and the delivery of the Exchange Auditor Report, and in accordance with the Holdco Delegate Resolutions:
• all issued Company Ordinary Shares held by the Company Shareholders shall be transferred and for the purposes of the 1915 Law, contributed in kind to Holdco, free and clear of all Liens (other than the Company Shareholders’ Agreements Liens that will expire on or prior to the Closing Date), and the Company Shareholders shall subscribe for and, as consideration for the contribution, shall be issued, in accordance with the Exchange Ratio (save that the Holdco Ordinary Shares to be issued shall be reduced by the number of Holdco Ordinary Shares already held by the Company Shareholders immediately prior to the Exchange), the Exchange Issuance;
• The Exchange Issuance shall be allocated among the Company Shareholders in accordance with the terms of the Payment Spreadsheet and Exchange Agreements;
• each Company SAFE Holder shall have contributed all of its rights and obligations under each Original SAFE to Holdco in consideration for the issuance by Holdco of a simple agreement for future equity on substantively identical terms (mutatis mutandis) with such adjustments (if any) required under Luxembourg law. For Luxembourg law purposes, a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued a report on the contributions in kind relating to the contribution of the Original SAFEs prepared in accordance with article 420-10 of the 1915 Law;
• each Company Shareholder shall cease to be the holder of such Company Ordinary Shares, subject to the submission of all filings required under Law (including any filings required to pay stamp duties), and Holdco will be recorded as the registered holder of all the Company Ordinary Shares so exchanged and transferred and will be the legal and beneficial owner thereof.
26
Table of Contents
• Immediately prior to the Merger Effective Time but after the Exchange Effective Time, each Company SAFE Holder shall receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the respective Original SAFE, with such adjustments (if any) required under Luxembourg law;
• SPAC shall cause the Certificate of Merger to be executed, acknowledged and filed with the Secretary of State of the State of Delaware in accordance with the applicable provisions of the DGCL in order to effectuate the Merger. The Merger shall become effective at such time as the Certificate of Merger has been duly filed with the Secretary of State of the State of Delaware or at such later time as may be agreed by the Company and SPAC in writing and specified in the Certificate of Merger in accordance with the DGCL;
• At the Merger Effective Time, by virtue of the Merger and the Holdco Requisite Approvals, subject to the Merger Auditor Report, and without any further action on the part of SPAC, Merger Sub, Holdco or the Company or the holders thereunder:
• each SPAC Common Stock issued and outstanding immediately prior to the Merger Effective Time, excluding those have been redeemed subject to any redemption rights, shall be exchanged with Holdco (which exchange, for purposes of the 1915 Law, shall include, for the avoidance of doubt, a contribution-in-kind of each such shares of SPAC Common Stock from the holders of SPAC Common Stock to Holdco), against the issue by Holdco of new Holdco Ordinary Shares (such issuance, the “Merger Issuance”), under the authorized share capital of Holdco (pursuant to the Holdco Delegate Merger Resolutions) and subscribed by the contributing holders of SPAC Common Stock by virtue of the Merger and in accordance with the 1915 Law for one validly issued and fully paid Holdco Ordinary Share (the “Merger Consideration”), delivered by Holdco;
• as a result of the Merger, all SPAC Common Stock shall cease to be outstanding, shall be cancelled and shall cease to exist;
• each share of common stock, par value $0.01 of Merger Sub issued and outstanding immediately prior to the Merger Effective Time shall be converted and exchanged for one (1) validly issued, fully paid and nonassessable ordinary share, par value $0.01 per share, of the Surviving Company; and
• Each SPAC Warrant that is outstanding immediately prior to the Merger Effective Time shall, pursuant to the SPAC Warrant Agreement, cease to represent a right to acquire one SPAC Common Stock and shall be converted in accordance with the terms of such SPAC Warrant Agreement, at the Merger Effective Time, into a right to acquire one Holdco Ordinary Share on substantially the same terms as were in effect immediately prior to the Merger Effective Time under the terms of the SPAC Warrant Agreement.
The parties to the Business Combination Agreement will hold the closing on the date of the Merger Effective Time, following the satisfaction or waiver (to the extent such waiver is permitted by applicable law) of the conditions set forth in the Business Combination Agreement (other than those conditions that by their nature are to be satisfied at Closing, but subject to the satisfaction or waiver of those conditions at such time).
The Combined Company is expected to have an implied initial enterprise value of $[•].
For more information, see the section entitled “The Business Combination Agreement — Consideration to be Received in the Business Combination.”
27
Table of Contents
Conditions to the Closing
General Conditions
Under the Business Combination Agreement, the obligations of the parties to consummate the Business Combination are conditioned on the satisfaction or waiver (where permissible) of the following conditions at or prior to the Closing:
(a) The SPAC Proposals shall have been approved and adopted by the requisite affirmative vote of the shareholders of LightJump in accordance with this proxy statement/prospectus, DGCL, the SPAC Organizational Documents and the rules and regulations of the Nasdaq Capital Market;
(b) the Company Requisite Approvals shall have been obtained and delivered to LightJump in a form and substance reasonably acceptable to LightJump;
(c) the Holdco Requisite Approvals shall have been obtained and delivered to LightJump in a form and substance reasonably acceptable to LightJump;
(d) a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued (i) at or before the Merger Effective Time a report on the contributions in kind relating to the Merger Issuance prepared in accordance with article 420-10 of the 1915 Law (the “Merger Auditor Report”), (ii) at or before the Exchange Effective Time, in accordance with the Exchange Agreements, a report on the contributions in kind relating to the Exchange Issuance prepared in accordance with article 420-10 of the 1915 Law (the “Exchange Auditor Report”);
(e) (A) Any waiting period under any Antitrust Laws applicable to the Transactions shall have expired or been earlier terminated, and (B) all other consents of (or filings or registrations with) any Governmental Authority required in connection with the execution, delivery and performance of the Business Combination Agreement set forth on Schedule 9.01 to the Business Combination Agreement shall have been obtained;
(f) no Governmental Authority shall have enacted, issued, promulgated, enforced or entered any Law, rule, regulation, judgment, decree, executive order or award which is then in effect and has the effect of making the Transactions, including the Merger or Exchange, illegal or otherwise prohibiting consummation of the Transactions, including the Merger or Exchange;
(g) this Registration Statement shall have been declared effective by the SEC under the Securities Act. No stop order suspending the effectiveness of the Registration Statement shall be in effect, and no proceedings for purposes of suspending the effectiveness of the Registration Statement shall have been initiated by the SEC and not withdrawn;
(h) the Holdco Ordinary Shares shall have been approved for listing on Nasdaq, subject to official notice of issuance;
(i) LightJump shall have at least $5,000,001 of net tangible assets after giving effect to the amounts funded under the Backstop Agreement and following the exercise of Redemption Rights in accordance with the SPAC Organizational Documents;
(j) the delivery of the Financial Advisor Opinion pursuant to Section 8.19 of the Business Combination Agreement;
(k) the delivery of an agreement, which shall be in a form mutually agreeable to Moolec and LightJump, between the CFO and Moolec by which the CFO agrees that the issuance of the Key Staff Participation satisfies all obligations of Moolec relating to the issuance of shares in Moolec to the CFO under the CFO Consulting Agreement; and
(l) all parties to the Registration Rights and Lock-Up Agreement shall have delivered, or cause to be delivered, copies of the Registration Rights and Lock-Up Agreement duly executed by all such parties.
28
Table of Contents
Conditions to the Obligations of LightJump
The obligations of LightJump to consummate the Transactions, including the Merger, are subject to the satisfaction or waiver (where permissible) at or prior to the Closing of the following additional conditions:
(a) certain representations and warranties of Moolec shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date and all other representations and warranties of Moolec shall be true and correct as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date, except where the failure of such representations and warranties to be true and correct does not result in a Company Material Adverse Effect;
(b) certain representations and warranties of Holdco and Merger Sub shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date and all other representations and warranties of Holdco and Merger Sub shall be true and correct as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date, except where the failure of such representations and warranties to be true and correct would be materially adverse to Holdco or Merger Sub;
(c) Moolec, Holdco and Merger Sub shall have performed or complied in all material respects with all agreements and covenants (other than Section 7.01(c)) of the Business Combination Agreement on or prior to the Exchange Effective Time and the Merger Effective Time, as applicable (including, for the avoidance of doubt and without limitation, Section 8.13 of the Business Combination Agreement); provided, that Holdco shall have performed or complied in all respects with the agreements and covenants set forth in Section 7.01(c) of the Business Combination Agreement;
(d) (i) Moolec, Holdco and Merger Sub shall have delivered to LightJump a certificate, dated as of the date of the Merger Effective Time, signed by an officer of Moolec, certifying as to the satisfaction of the conditions specified in Section 9.02(a), Section 9.02(b) and Section 9.02(d) of the Business Combination Agreement and (ii) Moolec, Holdco and Merger Sub shall have delivered to LightJump a certificate, dated as of the Exchange Effective Time, signed by an officer of Moolec, certifying as to the satisfaction of the conditions specified in Section 9.02(a), Section 9.02(b) and Section 9.02(d) of the Business Combination Agreement;
(e) no Company Material Adverse Effect shall have occurred between the date of the Business Combination Agreement and the Merger Effective Time and no Company Material Adverse Effect shall have occurred between the Merger Effective Time and the Exchange Effective Time;
(f) Moolec shall have delivered the PCAOB Financials as contemplated in Section 8.13 of the Business Combination Agreement;
(g) as of immediately prior to the Closing Date, there shall be no Company Ordinary Shares or other Equity Interest of Moolec outstanding other than Company Ordinary Shares and other Equity Interests that are subject to an Exchange Agreement (which shall provide that such security shall be either cancelled or converted into shares of Company Ordinary Shares to be transferred to Holdco in accordance with Section 3.02 of the Business Combination Agreement);
(h) LightJump shall have received evidence to its satisfaction that the actions contemplated in Section 8.17 of the Business Combination Agreement have been taken;
(i) each of the employees of Moolec listed on 8.04(b) of the Company Disclosure Schedule shall have executed and delivered to Moolec or Holdco employment agreements executed in compliance with Section 8.04(b) of the Business Combination Agreement with such agreements being in full force and effect;
(j) Holdco shall have delivered to LightJump and Trustee, the SPAC Warrant Amendment and Assignment executed by Holdco; and
(k) Each of Moolec, the Company Shareholders, Holdco, and Merger Sub shall have delivered, or caused to be delivered, each of the Ancillary Agreements to which such Person is a party and that, by its terms, is required to be executed and delivered at the Closing Date, in each case, duly executed by such Person.
29
Table of Contents
Conditions to the Obligations of Moolec
The obligations of Moolec to consummate the Transactions, including the Merger, are subject to the satisfaction or waiver (where permissible) at or prior to the Closing of the following additional conditions:
(a) certain representations and warranties of SPAC shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date and all other representations and warranties of SPAC shall be true and correct as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date, except where the failure of such representations and warranties to be true and correct does not result in a SPAC Material Adverse Effect;
(b) certain representations and warranties of Holdco and Merger Sub shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date and all other representations and warranties of Holdco and Merger Sub shall be true and correct as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date, except where the failure of such representations and warranties to be true and correct would be materially adverse to Holdco or Merger Sub;
(c) SPAC shall have performed or complied in all material respects with all agreements and covenants required by the Business Combination Agreement to be performed or complied with by or prior to the Exchange Effective Time and the Merger Effective Time, as applicable.
(d) (i) SPAC shall have delivered to Moolec a certificate, dated as of the date of the Merger Effective Time, signed by the Chief Executive Officer of SPAC, certifying as to the satisfaction of the conditions specified in Section 9.03(a), Section 9.03(b) and Section 9.03(d) of the Business Combination Agreement and (ii) SPAC shall have delivered to Moolec a certificate, dated as of the Exchange Effective Time, signed by the Chief Executive Officer of SPAC, certifying as to the satisfaction of the conditions specified in Section 9.03(a), Section 9.03(b) and Section 9.03(d) of the Business Combination Agreement;
(e) no SPAC Material Adverse Effect shall have occurred between the date of the Business Combination Agreement and the Merger Effective Time and no SPAC Material Adverse Effect shall have occurred between the Merger Effective Time and the Exchange Effective Time;
(f) SPAC shall have delivered to Holdco and Trustee, the SPAC Warrant Amendment and Assignment executed by SPAC;
(g) SPAC shall have delivered or caused to be delivered, each of the Ancillary Agreement to which such Person is a party and that, by its terms is required to be executed and delivered at the Closing, in each case, duly executed by such Person; and
(h) Sponsor shall have transferred to UG Holdings, LLC (or its designated affiliate) 1,035,000 shares of SPAC Common Stock, as contemplated by the Backstop Agreement.
Termination of the Business Combination Agreement
The Business Combination Agreement may be terminated and the Transactions may be abandoned at any time prior to the Merger Effective Time, notwithstanding any requisite approval and adoption of the Business Combination Agreement and the Transactions by the Company Shareholders or the LightJump Holders as follows:
(i) by mutual written consent of SPAC and Moolec;
(ii) by either SPAC or Moolec if the Merger Effective Time shall not have occurred prior to the later of (x) 5:00 p.m. (New York time) on January 12, 2023 and (y) the last day of the extended time period that LightJump is able, under its organization documents, to consummate a business combination if LightJump successfully extends such date;
(iii) by either SPAC or Moolec if any Governmental Authority shall have enacted, issued, promulgated, enforced or entered any injunction, order, decree or ruling (whether temporary, preliminary or permanent) which has become final and non-appealable and has the effect of making consummation of the Transactions illegal or otherwise preventing or prohibiting consummation of the Transaction or the Merger;
30
Table of Contents
(iv) by either SPAC or Moolec if any of the SPAC Proposals shall fail to receive the requisite vote for approval at the special meeting of stockholders of SPAC;
(v) by SPAC upon a breach of any representation, warranty, covenant or agreement set forth in the Business Combination Agreement on the part of Moolec, Holdco or Merger Sub that remains uncured for more than 30 days after written notice of such breach is provided by SPAC to Moolec, or if any representation or warranty of Moolec, Holdco or Merger Sub shall have become untrue, in either case such that the conditions relating to representations and warranties and certain covenants and agreements would not be satisfied; and
(vi) by Moolec upon any breach of any representation, warranty, covenant or agreement set forth in the Business Combination Agreement on the part of SPAC that remains uncured for more than 30 days after written notice of such breach is provided by Moolec to SPAC, or if any representation or warranty of SPAC shall have become untrue, in either case such that the conditions relating to representations and warranties and certain covenants and agreements would not be satisfied.
In the event that the Business Combination Agreement is terminated, all Transaction Expenses incurred in connection with the Business Combination Agreement, the Ancillary Agreements, and the Transactions shall be paid by the party incurring such Transaction Expenses.
If the Transactions are consummated:
(i) holders of Public Shares exercising redemption rights will be paid their pro rata amount of the Trust Account in exchange for their shares;
(ii) as promptly as practicable after the Closing, Holdco shall transfer or cause to be transferred to Sponsor or its designee an amount in cash equal to the Sponsor Advanced Funds so long as the unpaid SPAC Transaction Expenses as of the Closing do not exceed the applicable SPAC Transaction Expenses Cap;
(iii) Holdco shall pay or cause to be paid, (x) the Company Transaction Expenses, (y) the EarlyBird Cash Fees, unless the Net Available Assets are equal to less than $200,000, then, in such event, Sponsor and Holdco shall pay 50% of all EarlyBird Cash Fees, and (z) the SPAC Transaction Expenses that are unpaid as of the Closing up to an amount not to exceed the SPAC Transaction Expenses Cap; and
(iv) Sponsor shall pay or cause to be paid, (x) all unpaid SPAC Transaction Expenses in excess of the applicable SPAC Transaction Expenses Cap; (y) any expenses incurred by LightJump in its pursuit of potential acquisition or business targets other than Moolec or that were not incurred by LightJump in connection with or in furtherance of the Transactions, other than the Sponsor Advanced Funds and (z) all Extension Amendment Fees. Within five (5) days following the six (6) month anniversary of the Closing, Holdco shall issue the EarlyBird Share Fees.
Other Agreements Related to the Business Combination Agreement
Company Shareholder Contribution and Exchange Agreements
Concurrently with the execution of the Business Combination Agreement, each Company Shareholder entered into an Exchange Agreement with Moolec and Holdco pursuant to which, among other things, each Company Shareholder consented to the Exchange and all transaction contemplated under the Business Combination Agreement and the Transaction Documents to which it is a party and, consequently, agreed to (i) contribute its Company Ordinary Shares to Holdco in exchange for Holdco Ordinary Shares, (ii) not transfer any of its Company Ordinary Shares before the earlier of (a) one year from June 14, 2022 and (b) the implementation of the Exchange or termination of the Exchange Agreement. The Company Shareholders who entered into the Exchange Agreements collectively represent 100% of the issued and outstanding Company Ordinary Shares.
The Exchange Agreements are subject to customary conditions, covenants, representation and warranties.
For more information about the Exchange Agreements, see the section entitled “Certain Agreements Related to the Business Combination — Company Shareholder Contribution and Exchange Agreements.”
31
Table of Contents
Backstop Agreement
Concurrently with the execution of the Business Combination Agreement, Union Group Ventures Limited, THEO I SCSp, UG Holdings, LLC and Sponsor entered into the Backstop Agreement, pursuant to which, among other things, the parties agreed to provide, on a several (and not joint) basis, the funding necessary to backstop an aggregate amount equal to $10,000,000, conditioned upon Closing on the terms and subject to the conditions set forth in the Backstop Agreement.
For more information about the Backstop Agreement, see the section entitled “Certain Agreements Related to the Business Combination — Backstop Agreement.”
Transaction Support Agreement
Concurrently with the execution of the Business Combination Agreement, LightJump, Sponsor, Moolec, Holdco, certain Company Shareholders and the SAFE Holders and certain LightJump Holders entered into the Transaction Support Agreement pursuant to which, among other things, the Sponsor and certain LightJump Holders have agreed with LightJump, Holdco, Moolec and the Company Shareholders to (i) waive certain rights, and (ii) take certain actions to support the Transactions. Additionally, during the interim period (from June 14, 2022 until the earlier of (i) the Closing or (ii) termination of the Business Combination Agreement), each LightJump Holder has agreed not to transfer any Sponsor shares that she, he or it Beneficially Owns (as defined under Rule 13d-3 of the Exchange Act) without the prior written consent of Holdco, with the exception of certain permitted transfers described in the Transaction Support Agreement. Each SAFE Holder has agreed to execute all documentation and perform all necessary actions reasonably required by Moolec and/or Holdco as may be necessary or desirable in connection with the issuance by Holdco of Holdco Ordinary Shares to each SAFE Holder, substantially in accordance with the Original SAFE.
For more information about the Transaction Support Agreement, see the section entitled “Certain Agreements Related to the Business Combination — Transaction Support Agreement.”
Assignment, Assumption and Amendment to Warrant Agreement
In connection with the Merger, Holdco will assume the obligations of SPAC under the Warrant Agreement by executing the SPAC Warrant Amendment and Assignment.
For more information about the SPAC Warrant Amendment and Assignment, see the section entitled “Certain Agreements Related to the Business Combination — Assignment, Assumption and Amendment to Warrant Agreement.”
Registration Rights and Lock-Up Agreement
In connection with the closing of the Transactions, the Sponsor, Holdco, the CFO, the Company Shareholders and SAFE Holders will enter into the Registration Rights and Lock-Up Agreement pursuant to which, among other things, the Sponsor, the CFO and the Company Shareholders and SAFE holders shall have customary demand and piggyback registration rights in connection with the Holdco Ordinary Shares issued to them in the Merger or the Exchange. Additionally, the Holdco Ordinary Shares held by each party to the Registration Rights and Lock-Up Agreement will be subject to a lock-up until (i) the date that is 365 days from the Closing Date, and (ii) such date on which Holdco completes a liquidation, merger, share exchange or other similar transaction that results in all of the shareholders of Holdco having the right to exchange their Holdco Ordinary Shares for cash, securities or other property, provided that if the share price of the Holdco Ordinary Shares exceeds $12.00 per Holdco Ordinary Share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-day trading period, the parties to the Registration Rights and Lock-Up Agreement may transfer up to 50% of the Holdco Ordinary Shares subject to the Registration Rights and Lock-Up Agreement.
For more information about the Registration Rights and Lock-Up Agreement, see the section entitled “Certain Agreements Related to the Business Combination — Registration Rights and Lock-Up Agreement.”
32
Table of Contents
Interests of Certain Persons in the Business Combination
In considering the recommendation of LightJump’s board of directors to vote in favor of the Business Combination, LightJump’s stockholders should be aware that, aside from their interests as stockholders, the Sponsor and LightJump’s directors and officers have interests in the Business Combination that are different from, or in addition to, those of other stockholders and warrant holders generally. LightJump’s directors were aware of and considered these interests, among other matters, in evaluating the Business Combination, and in recommending to stockholders that they approve the Business Combination. Stockholders should take these interests into account in deciding whether to approve the Business Combination. These interests include, among other things:
• the Sponsor’s beneficial ownership of an aggregate of 3,450,000 Founder Shares, all of which are beneficially owned by our Chairman and Chief Executive Officer, which shares would become worthless if LightJump does not complete a business combination within the applicable time period, as the LightJump Initial Stockholders, including the Sponsor, have waived any right to liquidation proceeds from the Trust Account with respect to these shares. The Sponsor paid an aggregate of $25,000 (or $0.009 per share) for the 3,450,000 Founder Shares. Such shares have an aggregate market value of approximately $[•] based on the closing price of SPAC Common Stock of $[•] on Nasdaq on [•], 2022, the record date for the special meeting of stockholders;
• the Sponsor’s beneficial ownership of an aggregate of 4,210,000 warrants, acquired for an aggregate purchase price of $4,210,000, which warrants would become worthless if LightJump does not complete a business combination within the applicable time period;
• LightJump’s directors will not receive reimbursement for any out-of-pocket expenses incurred by them on LightJump’s behalf incident to identifying, investigating and consummating a business combination to the extent such expenses exceed the amount not required to be retained in the Trust Account, unless a business combination is consummated. As of [•], no out-of-pocket expenses have been incurred by LightJump’s directors incident to identifying, investigating and consummating a business combination;
• the potential continuation of certain of LightJump’s directors as directors of Holdco; and
• the continued indemnification of current directors and officers of LightJump and continuation of directors’ and officers’ liability insurance after the Business Combination.
These interests may influence LightJump’s directors in making their recommendation to vote in favor of the approval of the Business Combination Proposal and the other proposals described in this proxy statement/prospectus. You should also read the section entitled “The Business Combination — Interests of LightJump’s Directors and Officers in the Business Combination.”
Reasons for the Approval of the Business Combination
After careful consideration, LightJump’s board of directors recommends that LightJump’s stockholders vote “FOR” each proposal being submitted to a vote of the LightJump Holders at the special meeting. For a description of LightJump’s reasons for the approval of the Business Combination and the recommendation of LightJump’s board of directors, see the section entitled “The Business Combination — LightJump’s Board of Directors’ Reasons for the Approval of the Business Combination.”
Redemption Rights
Pursuant to the SPAC COI any holders of Public Shares may demand that such shares be redeemed in exchange for a pro rata share of the aggregate amount on deposit in the Trust Account, less franchise and income taxes payable, calculated as of two business days prior to the consummation of the Business Combination. If demand is properly made and the Business Combination is consummated, these shares, immediately prior to the Business Combination, will cease to be outstanding and will represent only the right to receive a pro rata share of the aggregate amount on deposit in the Trust Account which holds the proceeds of LightJump’s IPO as of two Business Days prior to the consummation of the Business Combination, less franchise and income taxes payable, upon the consummation of the Business Combination. For illustrative purposes, based on funds in the Trust Account of approximately $27,993,798 on July 12, 2022 and 2,767,210 public shares outstanding, the estimated per share redemption price would have been approximately $10.12.
33
Table of Contents
If you exercise your redemption rights, your shares of SPAC Common Stock will cease to be outstanding immediately prior to the Business Combination and will only represent the right to receive a pro rata share of the aggregate amount on deposit in the Trust Account. You will no longer own those shares. You will be entitled to receive cash for these shares only if you properly demand redemption. See the section entitled “The Special Meeting of LightJump Holders — Redemption Rights.”
Impact of the Business Combination on Holdco’s Public Float
It is anticipated that, upon completion of the Business Combination, (i) LightJump’s existing stockholders, including the Sponsor, will own approximately 6.14% of the issued and outstanding Holdco Ordinary Shares, including 2,415,000 Holdco Ordinary Shares held by the Sponsor that will be subject to certain lock-up arrangements pursuant to the Registration Rights and Lock-Up Agreement, (ii) the Company Shareholders will own approximately 82.58% of the issued and outstanding Holdco Ordinary Shares, all of which will be subject to certain lock-up arrangements pursuant to the Registration Rights and Lock-Up Agreement, (iii) the CFO will own approximately .62% of the issued and outstanding Holdco Ordinary Shares which will be subject to certain lock-up arrangements pursuant to the Registration Rights and Lock-Up Agreements and (iv) the SAFE Holders will own approximately .69% of the issued and outstanding Holdco Ordinary Shares. These relative percentages assume that (i) none of the holders of the Public Shares exercise their redemption rights in connection with the approval of the Business Combination, (ii) the provisions relating to the funding pursuant to the Backstop Agreement are not triggered and (iii) no additional equity securities of LightJump are issued at or prior to Closing. If the actual facts are different than these assumptions, the percentage ownership retained by the LightJump Holders will be different. Certain figures included in this section have been rounded for ease of presentation and, as a result, percentages may not sum to 100%.
The following table presents the share ownership of various holders of Holdco Ordinary Shares upon the closing of the Business Combination and are based on the assumptions that (i) the Closing Date shall be [•], 2022, (ii) no additional equity securities of LightJump are issued at or prior to Closing and (iii) the following redemption scenarios:
No Redemptions. This scenario assumes that none of LightJump’s existing Public Stockholders will exercise their redemption rights in connection with the approval of the Business Combination with respect to their Public Shares.
50% Redemptions. This scenario assumes that LightJump’s existing Public Stockholders exercise their redemption rights with respect to 1,383,605 Public Shares (50% of the currently issued and outstanding unredeemed Public Shares) in connection with the approval of the Business Combination, at a price of $10.12 per share.
Maximum Redemptions. This scenario assumes that 100% of LightJump’s existing Public Stockholders exercise their redemption rights with respect to their public shares in connection with the approval of the Business Combination, at a price of $10.12 per share and assumes that the obligations under the Backstop Agreement will be satisfied by making a cash contribution to Holdco. See “Certain Agreements Related to the Business Combination — Backstop Agreement.”
34
Table of Contents
| | No Redemptions(1) | | 50% Redemptions(1) | | Maximum
Redemptions(1)(2) |
Shareholders of Holdco Post Business Combination(1) | | Number of
Holdco
Ordinary
Shares | | % of
Total | | Number of
Holdco
Ordinary
Shares | | % of
Total | | | | % of
Total |
BG Farming Technologies Limited | | 15,275,000 | | 38.8 | % | | 15,275,000 | | 40.2 | % | | 15,275,000 | | | 40.6 | % |
Union Group Ventures Ltd. | | 15,275,000 | | 38.8 | % | | 15,275,000 | | 40.2 | % | | 15,525,000 | (4)(7) | | 41.3 | % |
Bioceres Crop Solutions Corp. | | 1,950,000 | | 5.0 | % | | 1,950,000 | | 5.1 | % | | 1,950,000 | | | 5.2 | % |
SAFE Holders | | 274,951 | | .7 | % | | 274,951 | | .7 | % | | 524,951 | (5)(7) | | 1.4 | % |
Initial Stockholders(3) | | 2,535,000 | | 6.4 | % | | 2,535,000 | | 6.7 | % | | 3,035,000 | (6)(7) | | 8.1 | % |
LightJump Public Stockholders | | 2,767,210 | | 7.0 | % | | 1,383,605 | | 3.6 | % | | 0 | | | 0 | % |
Key Staff Participation | | 243,774 | | .6 | % | | 243,774 | | .6 | % | | 243,774 | | | .7 | % |
UG Holdings LLC | | 1,035,000 | | 2.6 | % | | 1,035,000 | | 2.7 | % | | 1,035,000 | | | 2.8 | % |
Total | | 39,355,935 | | 99.9 | % | | 37,972,330 | | 99.8 | % | | 37,588,725 | | | 100.1 | % |
For more information, see the section entitled “Unaudited Pro Forma Condensed Combined Financial Information.”
35
Table of Contents
Organizational Structure
Prior to the Business Combination
The following diagram shows the current ownership structure of LightJump (excluding the impact of the shares underlying the SPAC Warrants).

The following diagram shows the current structure of Moolec and Holdco:
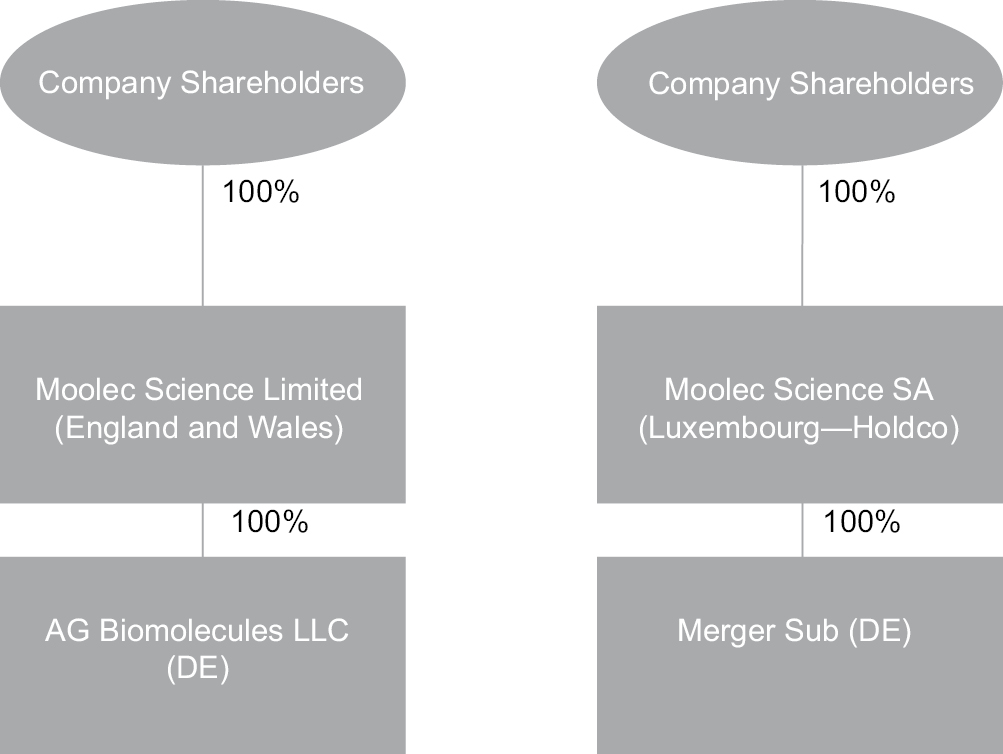
36
Table of Contents
The following diagram shows the pro forma ownership percentages (excluding the impact of the shares underlying the Holdco Warrants) and structure of Holdco immediately following the consummation of the Business Combination. The relative percentages assume that (i) none of LightJump’s existing Public Stockholders exercise their redemption rights in connection with the approval of the Business Combination with respect to their Public Shares, (ii) no additional equity securities of LightJump are issued prior to the Closing and (iii) a Closing Date of [•].
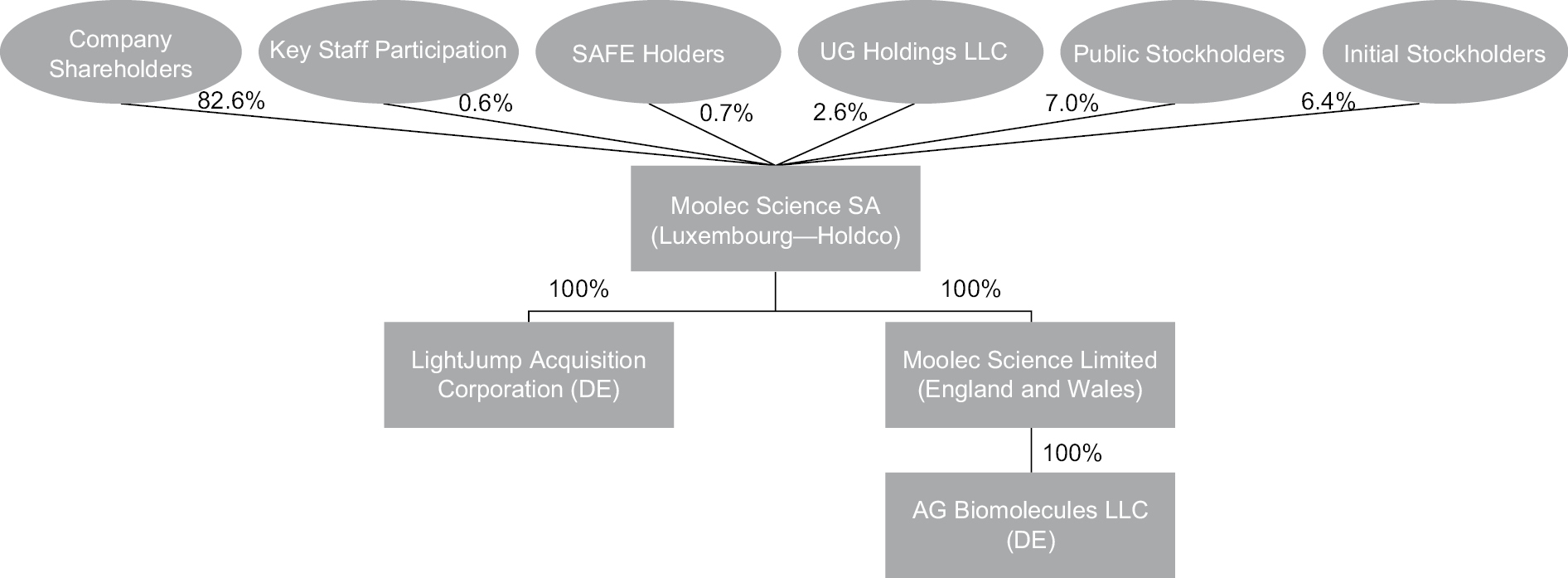
Board of Directors of Holdco Following the Business Combination
At the Merger Effective Time, the Holdco Board is expected to be comprised of seven members, including [•].
Material Tax Consequences
For a detailed discussion of certain U.S. federal income tax consequences and Luxembourg tax consequences and of the Business Combination, see the sections titled “Material U.S. Federal Income Tax Considerations” and “Material Luxembourg Income Tax Considerations” in this proxy statement/prospectus.
Accounting Treatment
The Business Combination will be accounted for as a capital reorganization in accordance with IFRS as issued by the IASB. Under this method of accounting, LightJump will be treated as the “acquired” company for financial reporting purposes and Moolec will be the accounting “acquirer.” This determination was primarily based on the assumptions that Moolec’s shareholders will hold a majority of the voting power of the Combined Company, Moolec’s operations will substantially comprise the ongoing operations of the Combined Company, Moolec’s designees are expected to comprise a majority of the governing body of the Combined Company, and Moolec’s senior management will comprise the senior management of the Combined Company. However, LightJump does not meet the definition of a “business” pursuant to IFRS 3, Business Combinations, and thus, for accounting purposes, the Business Combination will be accounted for as a capital reorganization. The net assets of LightJump will be stated at historical cost, with no goodwill or other intangible assets recorded.
Other Shareholder Proposals
In addition to the Business Combination Proposal, LightJump Holders will be asked to vote on the Stockholder Adjournment Proposal. For more information about the Stockholder Adjournment Proposal, see the section entitled “Stockholder Proposal No. 2 — The Stockholder Adjournment Proposal.”
Appraisal or Dissenters’ Rights
No appraisal or dissenters’ rights are available to holders of shares of SPAC Common Stock or SPAC Warrants in connection with the Business Combination.
37
Table of Contents
Date, Time and Place of the Special Meeting
The special meeting of stockholders of LightJump will be held at [•] a.m., Eastern time, on [•], 2022, at [•], or such other date, time and place to which such meetings may be adjourned or postponed, for the purpose of considering and voting upon the proposals. [The special meeting will be completely virtual].
Record Date and Voting
You will be entitled to vote or direct votes to be cast at the special meeting of stockholders if you owned shares of SPAC Common Stock at the close of business on [•], 2022, which is the record date for the special meeting of stockholders. You are entitled to one vote for each share of SPAC Common Stock that you owned as of the close of business on the record date. If your shares are held in “street name” or are in a margin or similar account, you should contact your broker, bank or other nominee to ensure that votes related to the shares you beneficially own are properly counted. On the record date, there were 2,767,210 shares of SPAC Common Stock outstanding and 6,900,000 outstanding Public Warrants.
LightJump’s Sponsor, officers and directors have agreed to vote all of their Common Stock and any Public Shares acquired by them in favor of the Business Combination Proposal and the other proposals described in this proxy statement/prospectus. LightJump’s issued and outstanding warrants do not have voting rights at the special meeting of stockholders.
Proxy Solicitation
Proxies may be solicited by mail. LightJump has engaged Advantage Proxy, Inc. to assist in the solicitation of proxies. If a stockholder grants a proxy, it may still vote its shares in person if it revokes its proxy before the special meeting. A stockholder may also change its vote by submitting a later-dated proxy as described in the section entitled “The Special Meeting of LightJump Holders — Revocability of Proxies.”
Quorum and Required Vote for Proposals for the Special Meeting
A quorum of LightJump’s stockholders is necessary to hold a valid meeting. A quorum will be present at the special meeting of stockholders if a majority of the SPAC Common Stock outstanding and entitled to vote at the meeting is represented in person or by proxy.
The approval of the Business Combination Proposal requires the affirmative vote of the holders of at least a majority of all then outstanding shares of SPAC Common Stock entitled to vote thereon at the special meeting of stockholders. The Stockholder Adjournment Proposal, if presented, requires the affirmative vote of the holders of a majority of the shares of SPAC Common Stock that are voted thereon at the special meeting of stockholders. Accordingly, a LightJump stockholder’s failure to vote by proxy or to vote in person at the special meeting of stockholders, or an abstention from voting, will have the same effect as a vote “AGAINST” the Business Combination Proposal and will have no effect on the outcome of any vote on the Stockholder Adjournment Proposal.
Recommendation to LightJump Shareholders
LightJump’s board of directors believes that each of the Business Combination Proposal and the Stockholder Adjournment Proposal, is in the best interests of LightJump and its stockholders and recommends that its stockholders vote “FOR” each of the proposals to be presented at the special meeting.
Summary Risk Factors
In evaluating the proposals set forth in this proxy statement/prospectus, you should carefully read this proxy statement/prospectus, including the annexes, and especially consider the factors discussed in the section entitled “Risk Factors.” Some of the risks related to LightJump and Moolec are summarized below:
LightJump
• LightJump identified a material weakness in its internal control over financial reporting. This material weakness could continue to adversely affect its ability to report its results of operations and financial condition accurately and in a timely manner.
38
Table of Contents
• LightJump has no operating or financial history and its results of operations and those of Holdco may differ significantly from the unaudited pro forma financial data included in this proxy statement.
• LightJump may not be able to complete its initial business combination or amend its charter prior to January 12, 2023, in which case LightJump would cease all operations except for the purpose of winding up and LightJump would redeem its public shares and liquidate. If this occurs, LightJump’s Public Stockholders may only receive $10.12 per share of SPAC Common Stock, or less than such amount in certain circumstances, and the SPAC Warrants will expire worthless.
• If a LightJump Holder wishing to redeem its SPAC Common Stock in connection with the Business Combination fails to comply with the procedures for tendering its shares, such shares may not be redeemed.
• The Sponsor and LightJump’s directors, officers, advisors or their affiliates may elect to purchase shares from LightJump Holders, which may influence a vote on the Business Combination and reduce the public “float” of SPAC Common Stock.
• LightJump Holders cannot be sure of the market value of the Holdco Ordinary Shares to be issued upon completion of the Business Combination.
• The Holdco Ordinary Shares to be received by LightJump’s Holders as a result of the Business Combination will have different rights from shares of SPAC Common Stock.
• LightJump’s Sponsor, officers and directors have agreed to vote in favor of the Business Combination, regardless of how the other LightJump Holders vote. As a result, LightJump would not need any additional votes in favor of such proposals in order to approve the Business Combination.
• The Sponsor and LightJump’s executive officers and directors have potential conflicts of interest in recommending that stockholders vote in favor of approval of the Business Combination Proposal and approval of the other proposals described in this proxy statement/prospectus.
• The exercise of discretion by LightJump’s directors and officers in agreeing to changes to the terms of or waivers of closing conditions in the Business Combination Agreement may result in a conflict of interest when determining whether such changes to the terms of the Business Combination Agreement or waivers of conditions are appropriate and in the best interests of LightJump securityholders.
• Subsequent to the completion of the Business Combination, Holdco may be required to take write-downs or write-offs, restructuring and impairment or other charges that could have a significant negative effect on Holdco’s financial condition, results of operations and stock price, which could cause you to lose some or all of your investment.
• LightJump Holders’ ownership and voting interest in Holdco will be significantly reduced from their interest in LightJump, and the LightJump Holders will exercise little influence over management.
Moolec
• Moolec is an early-stage company with a history of losses and Moolec may not achieve or maintain profitability.
• Moolec has a limited operating history, which makes it difficult to evaluate its current business and prospects and may increase the risk of investment.
• Moolec is wholly dependent on the success of its technologies, including its molecular farming technologies, and Moolec has limited data on the performance of its technologies to date.
• Moolec will likely require additional financing to achieve its goals, and failure to obtain necessary capital when needed on acceptable terms, or at all, may force Moolec to delay, limit, reduce or terminate its product manufacturing, development and other operations.
• To compete effectively, Moolec must introduce new products that achieve market acceptance and improve the output of its technology.
39
Table of Contents
• The successful commercialization of Moolec’s products depends on its ability to produce high-quality products cost-effectively on a large scale and to accurately forecast demand for its products, and Moolec may be unable to do so.
• The successful commercialization of Moolec’s products may face challenges from public perceptions of plant engineered products and ethical, legal, environmental, health and social concerns.
• Moolec’s field trials and research could be negatively affected by weather and climatic variations, disease or pests, or acts of protest or vandalism which could lower the expected yield, and delay the production and product candidates.
• If Moolec’s genetically engineered plants do not express a sufficient yield of an animal protein, Moolec may not be able to take its products to market in a timely manner or successfully operate its business.
• If Moolec fails to develop and maintain the Moolec brand, its business could suffer.
• The overall agricultural industry is susceptible to commodity price changes and Moolec is exposed to market risks from changes in commodity prices.
• Adverse weather conditions, natural disasters, crop disease, pests and other natural conditions can impose significant costs and losses on Moolec’s business.
• Climate Change may negatively affect Moolec’s business and operations.
• Moolec and its customers depend on patents, copyrights, trademarks, know-how, trade secrets, and other forms of intellectual property protections, but these protections may not be adequate.
• Patents and patent applications of biotechnology involve highly complex legal and factual questions, which, if determined adversely to Moolec, could negatively impact its competitive position.
• Moolec will not seek to protect its intellectual property right in all jurisdictions throughout the world and Moolec may not be able to adequately enforce its intellectual property rights even in the jurisdictions where Moolec seeks protection.
• Moolec may be unsuccessful in developing, licensing or acquiring intellectual property that may be required to develop and commercialize its future products.
• The regulatory environment in the United States for Moolec’s current and potential future products is evolving and may change in the future, negatively impacting its speed to market and cost to launch its potential future products.
• The regulatory environment outside the United States varies greatly from jurisdiction to jurisdiction and there is less certainty how Moolec’s products will be regulated.
40
Table of Contents
Selected Historical Financial Data of LightJump
The following tables summarize certain financial data for LightJump’s business and should be read in conjunction with the section entitled “LightJump Management’s Discussion and Analysis of Financial Condition and Results of Operations” and LightJump’s audited and unaudited financial statements, and the notes related thereto, which are included elsewhere in this proxy statement/prospectus.
LightJump’s balance sheet data as of June 30, 2022 and the statement of operations data for the six months ended June 30, 2022 and 2021 are derived from LightJump’s unaudited financial statements included elsewhere in this proxy statement/prospectus.
LightJump’s balance sheet data as of December 31, 2021 and 2020 and statement of operations data for the year ended December 31, 2021 and the period from July 28, 2020 (inception) through December 31, 2020 are derived from LightJump’s audited financial statements included elsewhere in this proxy statement/prospectus.
The historical results presented below are not necessarily indicative of the results to be expected for any future period. You should read the following selected financial information in conjunction with LightJump’s financial statements and related notes and the section entitled “LightJump Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained elsewhere in this proxy statement/prospectus.
| | For the
Six Months
Ended
June 30,
2022 | | For the
Six Months
Ended
June 30,
2021 | | For the
Year Ended
December 31,
2021 | | For the period
from July 28,
2020
(inception)
through
December 31,
2020 |
Income Statement Data: | | | | | | | | | | | | |
Formation and operating costs | | 1,973,436 | | | 1,037,402 | | | 1,716,659 | | | 13,802 | |
Loss from operations | | (1,973,436 | ) | | (1,037,402 | ) | | (1,716,659 | ) | | (13,802 | ) |
| | | | | | | | | | | | | |
Other income | | | | | | | | | | | | |
Change in fair value of warrant liability | | 1,936,782 | | | 216,765 | | | 1,663,532 | | | — | |
Change in fair value of overallotment liability | | — | | | — | | | (54,000 | ) | | — | |
Trust interest income | | 187,600 | | | 6,361 | | | 13,319 | | | — | |
Total other income | | 2,124,382 | | | 223,126 | | | 1,622,851 | | | — | |
Income (loss) before income tax provision | | 150,946 | | | (814,276 | ) | | (93,808 | ) | | (13,802 | ) |
Income tax provisions | | (3,679 | ) | | — | | | — | | | — | |
Net income (loss) | | 147,267 | | | (814,276 | ) | | (93,808 | ) | | (13,802 | ) |
| | | | | | | | | | | | | |
Basic and diluted weighted average shares outstanding, common stock subject to redemption | | 13,800,000 | | | 12,926,667 | | | 13,369,315 | | | — | |
Basic and diluted income (loss) per share | | 0.01 | | | (0.05 | ) | | (0.01 | ) | | — | |
Basic and diluted weighted average shares outstanding, non-redeemable common stock | | 3,570,000 | | | 3,525,667 | | | 3,552,740 | | | 3,098,571 | |
Basic and diluted net income (loss) per share | | 0.01 | | | (0.05 | ) | | (0.01 | ) | | (0.00 | ) |
| | June 30,
2022 | | December 31,
2021 | | December 31,
2020 |
Balance Sheet Data: | | | | | | | | |
Cash and marketable securities held in Trust Account | | 138,200,919 | | | 138,013,319 | | | — |
Total assets | | 138,428,129 | | | 138,169,512 | | | 312,398 |
Total liabilities | | 3,425,162 | | | 3,313,812 | | | 300,000 |
Preferred stock, $0.0001 par value; 1,000,000 shares authorized; none issued and outstanding | | — | | | — | | | — |
Common stock, $0.0001 par value; 99,000,000 shares authorized; 3,570,000 shares issued and outstanding (excluding 13,800,000 shares and 0 shares subject to possible redemption as of December 31, 2021 and 2020, respectively) | | 357 | | | 357 | | | 357 |
Total stockholders’ deficit | | (2,997,033 | ) | | (3,144,300 | ) | | 12,398 |
41
Table of Contents
Selected historical financial data of Moolec
The following table contains selected historical financial data of Moolec as of June 30, 2022 and for the year ended June 30, 2022, and as of June 30, 2021, and for the period from January 1, 2021 through June 30, 2021 and as of December 31, 2020 and for the period from inception on August 21, 2020 through December 31, 2020. Such data as of June 30, 2022 and for the year ended June 30, 2022 and as of June 30, 2021 and for the period from January 1, 2021 through June 30, 2021 and as of December 31, 2020 and for the period from inception on August 21, 2020 through December 31, 2020 have been derived from the audited financial statements of Moolec included elsewhere in this proxy statement/prospectus. The information below is only a summary and should be read in conjunction with the section entitled “Moolec Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Moolec’s financial statements, and the notes and schedules related thereto, which are included elsewhere in this proxy statement/prospectus.
Consolidated Statement of Operations:
| | For the year ended
June 30,
2022 | | From the
period of
January 1,
2021
through
June 30,
2021 | | From inception
on August 21,
2020
through
December, 31
2020 |
Continuing operations | | | | | | | | | |
Research and development expense | | (985,158 | ) | | (601,942 | ) | | (179,061 | ) |
Sales and marketing expense | | (105,060 | ) | | (80,221 | ) | | (10,938 | ) |
Administrative expense | | (2,523,230 | ) | | (820,946 | ) | | (127,886 | ) |
Other operating expense | | (38,985 | ) | | (93,252 | ) | | (2,639 | ) |
Loss from operations | | (3,652,433 | ) | | (1,596,361 | ) | | (320,524 | ) |
| | | | | | | | | | |
Share of loss from associate | | — | | | (390,453 | ) | | — | |
| | | | | | | | | | |
Finance costs | | (874,472 | ) | | — | | | — | |
| | | | | | | | | | |
Loss before Income tax | | (4,526,905 | ) | | (1,986,814 | ) | | (320,524 | ) |
| | | | | | | | | | |
Income tax | | — | | | — | | | — | |
Loss for the period | | (4,526,905 | ) | | (1,986,814 | ) | | (320,524 | ) |
| | | | | | | | | | |
Total comprehensive loss for the year/period | | (4,526,905 | ) | | (1,986,814 | ) | | (320,524 | ) |
Basic and diluted profit (loss) per share | | (0.09 | ) | | (0.04 | ) | | (0.01 | ) |
42
Table of Contents
Consolidated Statements of Financial Position:
| | As of
June 30,
2022 | | As of
June 30,
2021 |
ASSETS | | | | | | |
Non-current assets | | | | | | |
Intangible Assets | | 4,598,930 | | | 4,598,930 | |
Fixed Assets | | 8,918 | | | 10,617 | |
Investment in associate | | — | | | — | |
Total non-current assets | | 4,607,848 | | | 4,609,547 | |
Current assets | | | | | | |
Cash and cash equivalents | | 1,081,808 | | | 980,527 | |
Receivables from related parties | | — | | | 289,888 | |
Prepayments | | 2,061 | | | 358 | |
Total current assets | | 1,083,869 | | | 1,270,773 | |
TOTAL ASSETS | | 5,691,717 | | | 5,880,320 | |
LIABILITIES AND EQUITY | | | | | | |
Equity | | | | | | |
Share capital | | 638,297 | | | 638,297 | |
Share premium | | 6,961,703 | | | 6,961,703 | |
Equity settled share based payment | | 838,576 | | | — | |
Accumulated deficit | | (6,834,243 | ) | | (2,307,338 | ) |
Total equity | | 1,604,333 | | | 5,292,662 | |
Liabilities | | | | | | |
Current liabilities | | | | | | |
Accounts payable | | 1,226,213 | | | 587,658 | |
Other current liabilities | | 1,171 | | | — | |
Simple Agreement for Future Equity (“SAFE”) | | 2,860,000 | | | — | |
Total current liabilities | | 4,087,384 | | | 587,658 | |
TOTAL LIABILITIES | | 4,087,384 | | | 587,658 | |
TOTAL LIABILITIES AND EQUITY | | 5,691,717 | | | 5,880,320 | |
43
Table of Contents
SELECTED UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
LightJump is providing the following selected unaudited pro forma condensed combined financial information to aid you in your analysis of the financial aspects of the Transactions.
The following selected unaudited pro forma condensed combined statement of financial position as of June 30, 2022, combines the historical balance sheet of LightJump as of June 30, 2022, with the historical consolidated balance sheet of Moolec as of June 30, 2022, giving pro forma effect to the Business Combination, as if it had occurred as of June 30, 2022.
The following selected unaudited pro forma condensed combined statement of operations for the year ended June 30, 2022, combine the historical statement of operations of LightJump for the twelve-month period ended June 30, 2022, and the historical consolidated statements of operations of Moolec for year ended June 30, 2022, giving pro forma effect to the Business Combination as if it had occurred on July 1, 2021, the beginning of the earliest period presented. For more information about the Business Combination see the section titled “The Business Combination.”
The selected unaudited pro forma condensed combined financial information has been prepared assuming:
i) the impact of the Extension Amendment, whereby the remaining balance of the Trust Account was reduced from $138,200,919 as of June 30, 2022 to $27,993,797 (which includes an additional $276,721 contributed by the Sponsor in connection with the Extension Amendment), as of July 12, 2022;
ii) no additional shares issued following the Business Combination pursuant to any employee share plan of Moolec. See “Management of Moolec — Moolec Share Option Plan”; and
iii) three alternative levels of redemption of Public Shares into cash:
Scenario 1 — Assuming no redemptions for cash: This presentation assumes that no LightJump shareholders exercise redemption rights with respect to their Public Shares upon consummation of the Business Combination;
Scenario 2 — Assuming redemptions of 50% of the outstanding Public Shares for cash: This presentation assumes that LightJump shareholders exercise their redemption rights with respect to 1,383,605 Public Shares upon consummation of the Business Combination at a redemption price of approximately $10.12 per share. Scenario 2 includes all adjustments contained in Scenario 1 and presents additional adjustments to reflect the effect of the redemptions of 50% of the Public Shares;
Scenario 3 — Assuming redemptions of 100% of outstanding Public Shares for cash: This presentation assumes that LightJump shareholders exercise their redemption rights with respect to a maximum of 2,767,210 Public Shares upon consummation of the Business Combination at a redemption price of approximately $10.12 per share. Scenario 3 includes all adjustments contained in Scenario 1, presents additional adjustments to reflect the effect of the maximum redemptions and assumes that the obligations under the Backstop Agreement will be satisfied by making a cash contribution to Holdco. See “Certain Agreements Related to the Business Combination — Backstop Agreement.”
The adjustments presented in the selected unaudited pro forma condensed combined financial statements have been identified and presented to provide relevant information necessary for an accurate understanding of the Combined Company upon consummation of the Transactions.
The historical financial statements of Moolec have been prepared in accordance with IFRS as issued by the IASB and in its presentation currency of the U.S. dollar. The historical financial statements of LightJump have been prepared in accordance with GAAP in its presentation currency of the U.S. dollar. The condensed combined pro forma financial information reflects IFRS, the basis of accounting used by the registrant, Holdco, and all material accounting policy differences have been identified in converting LightJump’s historical financial statements to IFRS in the pro forma section. The adjustments presented in the selected unaudited pro forma condensed combined financial information have been identified and presented to provide relevant information necessary for an accurate
44
Table of Contents
understanding of the Combined Company after giving effect to the Business Combination. LightJump and Moolec did not have any historical relationship prior to the Business Combination. Accordingly, no pro forma adjustments were required to eliminate activities between the companies.
This information should be read together with LightJump’s and Moolec’s financial statements and related notes, “LightJump Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Moolec’s Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other financial information included elsewhere in this proxy statement/prospectus.
The selected unaudited pro forma condensed combined financial information is presented for illustrative purposes only. Such information is only a summary and should be read in conjunction with the section titled “Unaudited Pro Forma Combined Financial Information.” The financial results may have been different had the companies always been combined. You should not rely on the selected unaudited pro forma condensed combined financial information as being indicative of the historical results that would have been achieved had the companies always been combined or the future results that the Combined Company will experience.
Consolidated Statements of Operations (In thousands of United States Dollars):
| | For the period ended on
June 30, 2022 | | Scenario 1 –
Assuming no
redemptions | | Scenario 2 –
Assuming
50% of
redemptions | | Scenario 3 –
Assuming
100% of
redemptions |
| | | Moolec
(Historical) | | Light Jump
(Historical)(1)(2) | | Pro Forma
Combined | | Pro Forma
Combined | | Pro Forma
Combined |
Continuing operations | | | | | | | | | | | | | | | |
Research and development expense | | (985 | ) | | — | | | (985 | ) | | (985 | ) | | (985 | ) |
Sales and marketing expense | | (105 | ) | | — | | | (105 | ) | | (105 | ) | | (105 | ) |
Administrative expense | | (2,523 | ) | | — | | | (4,054 | ) | | (4,054 | ) | | (4,054 | ) |
Other operating expense | | (39 | ) | | (2,653 | ) | | (47,528 | ) | | (45,772 | ) | | (43,436 | ) |
Loss from operations | | (3,652 | ) | | (2,653 | ) | | (52,672 | ) | | (50,916 | ) | | (48,580 | ) |
| | | | | | | | | | | | | | | | |
Finance results | | (875 | ) | | 7,784 | | | 7,575 | | | 7,575 | | | 7,575 | |
| | | | | | | | | | | | | | | | |
Profit / (Loss) before
Income tax | | (4,527 | ) | | 5,131 | | | (45,097 | ) | | (43,341 | ) | | (41,005 | ) |
Income tax | | — | | | (4 | ) | | (4 | ) | | (4 | ) | | (4 | ) |
Total profit / (loss) for the period | | (4,527 | ) | | 5,127 | | | (45,101 | ) | | (43,345 | ) | | (41,009 | ) |
Basic and diluted profit/(loss)
per share | | (0.09 | ) | | 1.44 | | | (1.15 | ) | | (1.14 | ) | | (1.09 | ) |
45
Table of Contents
Consolidated Statements of Financial Position (In thousands of United States Dollars):
| | | | | | Pro Forma Combined |
| | | Moolec
As of
June 30,
2022 | | Light Jump
As of
June 30,
2022(1) | | Scenario 1 –
Assuming no redemptions | | Scenario 2 –
Assuming 50% of redemptions | | Scenario 3 –
Assuming 100% of redemptions |
Intangible Assets | | 4,599 | | | — | | | 4,599 | | | 4,599 | | | 4,599 | |
Investment held in Trust
Account | | — | | | 138,201 | | | — | | | — | | | — | |
Fixed Assets | | 9 | | | — | | | 9 | | | 9 | | | 9 | |
Total non-current assets | | 4,608 | | | 138,201 | | | 4,608 | | | 4,608 | | | 4,608 | |
Cash and cash equivalents | | 1,082 | | | 5 | | | 17,074 | | | 5,590 | | | 4,510 | |
Prepayments | | 2 | | | 222 | | | 724 | | | 724 | | | 724 | |
Total current assets | | 1,084 | | | 227 | | | 17,798 | | | 6,314 | | | 5,234 | |
Total assets | | 5,692 | | | 138,428 | | | 22,406 | | | 10,922 | | | 9,842 | |
| | | | | | | | | | | | | | | | |
Share capital | | 638 | | | — | | | 4 | | | 4 | | | 4 | |
Share premium | | 6,962 | | | — | | | 74,007 | | | 60,639 | | | 57,097 | |
Equity settled share based payment | | 839 | | | — | | | 475 | | | 475 | | | 475 | |
Accumulated deficit | | (6,834 | ) | | (3,357 | ) | | (53,546 | ) | | (51,662 | ) | | (49,200 | ) |
Total equity | | 1,605 | | | (3,357 | ) | | 20,940 | | | 9,456 | | | 8,376 | |
Common stock subject to
possible redemption | | — | | | 138,000 | | | — | | | — | | | — | |
Warrant liabilities | | — | | | 621 | | | 621 | | | 621 | | | 621 | |
Total non-current liabilities | | — | | | 138,621 | | | 621 | | | 621 | | | 621 | |
Accounts payable | | 1,226 | | | 1,969 | | | 840 | | | 840 | | | 840 | |
Other current liabilities | | 1 | | | 300 | | | 1 | | | 1 | | | 1 | |
Income tax payable | | — | | | 4 | | | 4 | | | 4 | | | 4 | |
Promissory note – related party | | — | | | 891 | | | — | | | — | | | — | |
Simply Agreement for Future Equity (“SAFE”). | | 2,860 | | | — | | | — | | | — | | | — | |
Total current liabilities | | 4,087 | | | 3,164 | | | 845 | | | 845 | | | 845 | |
Total liabilities and equity | | 5,692 | | | 138,428 | | | 22,406 | | | 10,922 | | | 9,842 | |
46
Table of Contents
Cautionary Note Regarding Forward-Looking Statements
Certain statements in this proxy statement/prospectus may constitute “forward-looking statements” for purposes of the federal securities laws. Our forward-looking statements include, but are not limited to, statements regarding our or our management team’s expectations, hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. Forward-looking statements in this proxy statement/prospectus may include, for example, statements about:
• our ability to consummate the Business Combination;
• the benefits of the Business Combination;
• the Combined Company’s financial performance following the Business Combination;
• the ability to obtain or maintain the listing of the Holdco Ordinary Shares or Holdco Warrants on Nasdaq, following the Business Combination;
• changes in Moolec’s strategy, future operations, financial position, estimated revenues and losses, projected costs, prospects and plans;
• Moolec’s ability to develop and launch new products and services
• Moolec’s ability to successfully and efficiently integrate future expansion plans and opportunities;
• The availability of raw materials used in Moolec’s products and it ability to source such raw materials;
• Moolec’s ability to grow its business in a cost-effective manner;
• Moolec’s product development timeline and expected research and development (“R&D”);
• the implementation, market acceptance and success of Moolec’s business model;
• developments and projections relating to Moolec’s competitors and industry;
• Moolec’s approach and goals with respect to technology;
• Moolec’s expectations regarding its ability to obtain and maintain intellectual property protection and not infringe on the rights of others;
• the impact of the COVID-19 pandemic on Moolec’s business;
• the risks that uncertainty and instability resulting from the conflict between Russia and Ukraine could adversely affect Moolec’s business, financial condition and operations, in addition to global macroeconomic indications;
• changes in applicable laws or regulations; and
• the outcome of any known and unknown litigation and regulatory proceedings.
These forward-looking statements are based on information available as of the date of this proxy statement/prospectus, and current expectations, forecasts and assumptions, and involve a number of judgments, risks and uncertainties. Accordingly, forward-looking statements should not be relied upon as representing our views as of any subsequent date, and we do not undertake any obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
47
Table of Contents
You should not place undue reliance on these forward-looking statements in deciding how to vote your shares or instruct how your vote should be cast on the proposals set forth in this proxy statement/prospectus. As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward-looking statements. Some factors that could cause actual results to differ include:
• the occurrence of any event, change or other circumstances that could delay the Business Combination or give rise to the termination of the Business Combination Agreement;
• the outcome of any legal proceedings that may be instituted against LightJump, Holdco or Moolec following announcement of the proposed Business Combination and transactions contemplated thereby;
• the inability to complete the Business Combination due to the failure to obtain approval of the stockholders of LightJump or to satisfy other conditions to the Closing in the Business Combination Agreement;
• the ability to obtain or maintain the listing of the Holdco Ordinary Shares on Nasdaq following the Business Combination;
• the risk that the proposed Business Combination disrupts current plans and operations of Moolec as a result of the announcement and consummation of the transactions described herein;
• our ability to recognize the anticipated benefits of the Business Combination, which may be affected by, among other things, competition and the ability of Moolec to grow and manage growth profitably following the Business Combination;
• costs related to the Business Combination;
• changes in applicable laws or regulations;
• the effects of the COVID-19 pandemic on Moolec’s business;
• the risks that uncertainty and instability resulting from the conflict between Russia and Ukraine could adversely affect Moolec’s business, financial condition and operations, in addition to global macroeconomic indications;
• the ability to implement business plans, forecasts, and other expectations after the completion of the proposed transaction, and identify and realize additional opportunities;
• the risk of downturns and the possibility of rapid change in the highly competitive industry in which Moolec operates;
• the risk that Moolec and its current and future collaborators are unable to successfully develop and commercialize Moolec’s products or services, or experience significant delays in doing so;
• the risk that the post-combination company may never achieve or sustain profitability;
• the risk that the post-combination company will need to raise additional capital to execute its business plan, which may not be available on acceptable terms or at all;
• the risk that the post-combination company experiences difficulties in managing its growth and expanding operations;
• the risk that third-party suppliers and manufacturers are not able to fully and timely meet their obligations;
• the risk of disruption at any of Moolec’s manufacturing facilities;
• the risk that Moolec is unable to secure or protect its intellectual property;
• the possibility that LightJump or Moolec may be adversely affected by other economic, business, and/or competitive factors; and
• other risks and uncertainties described in this proxy statement/prospectus, including those under the section entitled “Risk Factors.”
48
Table of Contents
Trademarks, tradenames and service Marks
This proxy statement/prospectus contains trademarks, trade names and service marks that are the property of Moolec, as well as, for informational purposes, trademarks, trade names, and service marks that are the property of other organizations. Solely for convenience, certain trademarks, trade names, and service marks referred to in this report appear without the ®, ™ and SM symbols, but those references are not intended to indicate that we or the applicable owner, as the case may be, will not assert, to the fullest extent under applicable law, our or their rights to such trademarks, trade names, and service marks.
49
Table of Contents
Risk Factors
In addition to the other information contained in (or incorporated by reference into) this proxy statement/prospectus, including the matters addressed under the heading “Forward-Looking Statements,” you should carefully consider the following risk factors in deciding how to vote on the proposals presented in this proxy statement/prospectus. The occurrence of one or more of the events or circumstances described in these risk factors, alone or in combination with other events or circumstances, may have a material adverse effect on Holdco’s business, reputation, revenue, financial condition, results of operations and future prospects, in which event the market price of the Holdco Ordinary Shares could decline, and you could lose part or all of your investment. Unless otherwise indicated, reference in this section and elsewhere in this proxy statement/prospectus to the Moolec business being adversely affected, negatively impacted or harmed will include an adverse effect on, or a negative impact or harm to, the business, reputation, financial condition, results of operations, revenue and future prospects of Holdco.
Risks Related to Moolec
References in this section to “we”, “our”, “us” or “Moolec” generally refer to Moolec Science Limited.
Risks Relating to Our Business and Operations
We are an early-stage company with a history of losses and we may not achieve or maintain profitability.
Our net losses for the year ended June 30, 2022 were U.S.$4,526,905. As of June 30, 2022, we had an accumulated deficit of U.S.$6,834,243. We will need to generate significant revenues to achieve profitability, and we may not be able to achieve and maintain profitability in the near future or at all. Since we are a pre revenue company, we expect to continue without generating revenues in the near future. Our future success will depend, in part, on our ability to grow revenue associated with further research and development (“R&D”), production partnerships and direct sale to distributors and food producers.
The net losses we incur may fluctuate significantly from year-to-year such that a period-to-period comparison of our results of operations may not be a good indication of our future performance.
We cannot ensure that we will generate increased revenues, successfully commercialize products or generate revenue from licensing needed to attain a level of profitable operations. Based on our history of losses we do not expect that we will be able to fund our longer-term capital and liquidity needs through our cash balances and operating cash flow alone. Our current source of investment comes mainly from our shareholders Bioceres Group and Union Group. To fund our longer-term capital and liquidity needs, we expect we will need to secure additional capital. Our business plan and financing needs are subject to change depending on, among other things, the success of our efforts to grow revenue and our efforts to continue to effectively manage expenses.
We have a limited operating history, which makes it difficult to evaluate our current business and prospects and may increase the risk of investment.
We are an early-stage food-technology company with a limited operating history that to date has been focused primarily on R&D, conducting field trials, pursuing initial commercialization efforts and building our management team. Investment in food technology development is a highly speculative endeavor. It entails substantial upfront R&D investment and there is significant risk that we will not be able to edit the genes in a particular plant to express a desired trait, or, once edited, we will not be able to replicate that trait across entire crops in order to commercialize the product candidate. Moreover, the regulatory pathway for our product candidates can be uncertain and could add significant additional cost and time to development.
Our limited operating history may make it difficult to evaluate our current business and our prospects. We have encountered, and will continue to encounter, risks and difficulties frequently experienced by growing companies in rapidly developing and changing industries, including challenges in forecasting accuracy, determining appropriate investments of our limited resources, gaining market acceptance of the products made using our plant Molecular Farming or plant grown proteins, managing a complex regulatory landscape and developing new product candidates. We may also face challenges in scaling our supply chain in a cost-effective manner, as we will likely rely on contracting with third parties to bring our products to market. Currently, we have only one ongoing production contract with farmers, Koompin Farms, in American Falls, Idaho, United States, for the production of one to two
50
Table of Contents
acre of Safflower GLA crop. In addition, there is limited crushing and processing capacity for our soybean-based and pea-based products which could restrict our ability to scale production of these products. We may be required to adjust our current operating model in order to efficiently scale our operations. We may not be able to fully implement or execute our business strategy or realize, in whole or in part within our expected time frames, the anticipated benefits of our growth strategies.
We are wholly dependent on the success of our technologies, including our molecular farming technologies, and we have limited data on the performance of our technologies to date.
We do not currently have any products or technologies approved for sale and we are still in the early stages of development. To date, we have limited data on the ability of our technologies, which consist of developing a nuclear stable transformation of a crop to express an animal protein within the protein bodies of the seed in order to produce a product consisting not only of the alternative protein but the plant protein as well, towards which we have devoted substantial resources to date.
We may not be successful in developing our technologies in a manner sufficient to support our expected scale-ups and future growth, or at all. We expect that a substantial portion of our efforts and expenditures over the next few years will be devoted to the development of technologies designed to enable us to market industrial-scale manufacturing processes. We cannot guarantee that we will be successful in developing these technologies on the timeline we expect, or at all, and we may not be able to achieve our anticipated growth, revenues or profitability needed to continue our operations. If we are able to successfully develop our technologies, we cannot ensure that we will obtain regulatory approval or that, following approval, upon commercialization our technologies will achieve market acceptance. Any such delay or failure would materially and adversely affect our financial condition, results of operations and prospects. See “Business of Moolec and Certain Information About Moolec — Products — Development Timelines.”
We will likely require additional financing to achieve our goals, and failure to obtain necessary capital when needed on acceptable terms, or at all, may force us to delay, limit, reduce or terminate our product manufacturing, development and other operations.
We believe that we will continue to expend substantial resources for the foreseeable future as we expand into additional markets we may choose to pursue. These expenditures are expected to include costs associated with R&D, the acquisition or expansion of manufacturing and supply capabilities, as well as the marketing and selling of new products. In addition, other unanticipated costs may arise.
Our operations may change because of factors that are currently unknown to us, and we may need to seek additional funds sooner than planned, including through public equity or debt financings or other sources, such as strategic collaborations. Such financing may result in dilution to shareholders, imposition of debt covenants and repayment obligations, or other restrictions that may adversely affect our business. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Our future capital requirements depend on many factors, including:
• the number and characteristics of any additional products or manufacturing processes we develop or acquire to serve new or existing markets;
• the expenses associated with our marketing initiatives;
• investment in manufacturing to expand manufacturing and production capacity;
• the costs required to fund domestic and international growth;
• the scope, progress, results and costs of researching and developing future products or improvements to existing products or manufacturing processes;
• any lawsuits related to our products that may be commenced against us;
• the expenses needed to attract and retain skilled personnel;
• the costs associated with being a public company; and
• the timing, receipt and amount of sales of future products.
51
Table of Contents
Additional funds may not be available when we need them, on terms that are acceptable to us, or at all. If adequate funds are not available on a timely basis, we may be required to:
• delay, limit, reduce or terminate our manufacturing, research and development activities or growth and expansion plans; and
• delay, limit, reduce or terminate the expansion of sales and marketing capabilities or other activities that may be necessary to generate revenue and increase profitability.
If we fail to effectively start or expand our manufacturing and production capacity, our business and operating results and our brand reputation could be harmed.
If we do not have sufficient capacity to meet our customers’ demands and to satisfy increased demand, we will need to expand our operations, supply and manufacturing capabilities. However, there is risk in our ability to effectively scale production processes and effectively manage our supply chain requirements. As we are currently a pre-revenue company, our business, which primarily consists of R&D, is not materially impacted by any supply chain disruptions. However, as we begin to scale our production, we must accurately forecast demand for our products in order to ensure we have adequate available manufacturing capacity. We currently do not know what impact any current supply chain disruptions, including but not limited to labor shortages, consumer demand, challenges related to Russia’s invasion of Ukraine, or export restrictions will have on our business. Our forecasts are based on multiple assumptions which may cause our estimates to be inaccurate and affect our ability to obtain adequate manufacturing capacity (whether our own manufacturing capacity or co-manufacturing capacity) in order to meet the demand for our products, which could prevent us from meeting increased customer demand and harm our brand and our business and in some cases may result in penalties we must pay customers or distributors if we are unable to fulfill orders placed by them in a timely manner or at all.
However, if we overestimate our demand and overbuild our capacity, we may have significantly underutilized assets and may experience reduced margins. If we do not accurately align our manufacturing capabilities with demand, if we experience disruptions or delays in our supply chain, or if we cannot obtain raw materials of sufficient quantity and quality at reasonable prices and in a timely manner, our business, financial condition and results of operations may be materially adversely affected.
We do not operate out of privately owned research facilities and our research capabilities are subject to service agreements with private companies and research universities.
We conduct our research by means of service agreements and contracts with universities and private companies. We cannot guarantee that these arrangements will be successful and yield the expected results, and that the engaged third parties will not make errors in their research activities or breach their obligations to us.
Our current operations depend on third-party agreements for necessary supplies in order to scale our production and increase our stock of seed related to our product development.
Currently, our operations require an increase in the stock of our seed and product development for the safflower platform, as well as R&D of the soy and pea platform since we have not yet reached the production stage. The seeds that we are developing in R&D stages are the primary raw materials necessary for the development of our portfolio.
In addition, our current operations require the use of chemical solvents, equipment, filters and other research materials in order to increase our stock of viable seeds. The suppliers of these products manufacture their products at a limited number of facilities. A natural disaster, fire, power interruption, work stoppage or other calamity affecting any of these facilities, or any interruption in their operations, could negatively impact our ability to obtain required quantities of plant proteins in a timely manner, or at all, which could materially reduce our net product sales and have a material adverse effect on our business and financial condition.
We do not have any contracts with customers.
We do not currently have customers that have committed to long-term or short-term volume purchase commitments. As a result, we could have periods in the future whereby we have no or limited orders for our products, but will continue to have fixed costs. We may not be able to find new customers in a timely manner if we experience periods of limited purchase orders. Periods of no or limited purchase orders for our products in the future could adversely affect our business, financial condition and results of operations.
52
Table of Contents
We do not currently have any customer base and the inability to attract a customer base could negatively impact our sales and profitability.
If customers do not perceive our product offerings to be of sufficient value, quality or innovation, or if we fail to offer innovative and relevant product offerings, and if we fail to develop a suitable pricing strategy, we may not be able to attract customers to purchase our products. We are focused on using animal DNA within plants to create animal proteins within the plants themselves. This is a technology that has not been widely introduced to the market and will require sufficient education to the market to show the difference between our technology and other alternative proteins. In addition, our products could face difficulties in some jurisdictions due to regulatory requirements. If producers face challenges in adopting our products, if we are unable to adequately educate our customers and develop commercial relationships while emphasizing the difference in our technology, it may negatively impact our sales and profitability.
We face significant competition and many of our competitors have substantially greater financial, technical and other resources than we do.
The alternative protein market is highly competitive, and we face significant direct and indirect competition in several aspects of our business. Our main competitors are Motif FoodWorks, Impossible Foods Inc., Givaudan Group, International Flavors & Fragrances, Nobell Foods, Kyomei, DSM and Hansen. Mergers and acquisitions in the plant science, specialty food ingredient and agricultural biotechnology, and seed industries may result in even more resources being concentrated among a smaller number of our competitors.
Most of these competitors have substantially greater financial, technical, marketing, sales, distribution, supply chain infrastructure, and other resources than we do, such as larger R&D staff, more experienced marketing, manufacturing, and supply chain organizations and more well-established sales forces. As a result, we may be unable to compete successfully against our current or future competitors, which may result in price reductions, reduced margins and the inability to achieve market acceptance for our products. We expect to continue to face significant competition in the markets in which we intend to commercialize our products.
Many of our competitors engage in ongoing R&D, and technological developments by our competitors could render our products less competitive or obsolete, resulting in reduced sales compared to our expectations. Our ability to compete effectively and to achieve commercial success depends, in part, on our ability to: control manufacturing and marketing costs; effectively price and market our products; successfully develop an effective marketing program and an efficient supply chain; develop new products with properties attractive to customers; and commercialize our products quickly without incurring major regulatory costs. We may not be successful in achieving these factors and any such failure may adversely affect our business, results of operations and financial condition.
We also anticipate increased competition in the future as new companies enter the market and new technologies become available, particularly in the area of molecular farming, gene editing, fermentation (synthetic biology) and vertical farming. Our technology may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors that are more effective or that enable them to develop and commercialize products more quickly or with lower expense than we are able to. If for any reason our technology becomes obsolete or uneconomical relative to our competitors’ technologies, this would threaten or limit our ability to generate revenues from the commercialization of our products.
To compete effectively, we must introduce new products that achieve market acceptance and improve the output of our technology.
In order to become competitive and create revenue, we must introduce new products from our pipeline of product candidates and prove that our technology is economically feasible and accepted. If we fail to anticipate or respond to technological developments, market requirements, or consumer preferences, or if we are significantly delayed in developing and introducing products, we may not be able to increase our revenue.
Development of successful agricultural products using plant engineering and precision fermentation technologies requires significant levels of investment in R&D, including laboratory, greenhouse and field testing, to demonstrate product effectiveness and can take several years or more. We must commit significant resources and may incur obligations (such as royalty obligations or milestone fees) to develop new products before knowing whether our investments will result in products the market will accept and without knowing the levels of revenue, if any, that may be derived from these products.
53
Table of Contents
Development of new or improved agricultural products involve risks of failure inherent in the development of products based on innovative and complex technologies. These risks include the possibility that:
• we may face challenges to scale up seed inventories, such as climate events impacting seed multiplication ramp up curves and industrial costs higher than estimated;
• farmers may not choose to adopt our seeds;
• our products may not perform as expected in the field;
• our products may not receive necessary regulatory permits and governmental clearances in the markets in which we intend to sell them;
• our seed processing and production may not have the expected results;
• consumer preferences, which are unpredictable and can vary greatly, may change quickly, making our products no longer desirable;
• our competitors may develop new products that taste better or have other more appealing characteristics than our products;
• our products may be viewed as too expensive by our customers as compared to competitive products;
• our products may be difficult to produce on a large scale or may not be economical to grow;
• intellectual property and other proprietary rights of third parties may prevent us or our collaborators from marketing and selling our products;
• we may be unable to patent or otherwise obtain intellectual property protection for our discoveries in the necessary jurisdictions;
• we or our collaborators may be unable to fully develop or commercialize products in a timely manner or at all;
• third parties may develop superior or equivalent products; and
• natural and climate risks may impact our business.
Accordingly, if we experience any significant delays in the development or introduction of new products or if our new products do not achieve market acceptance, our business, operating results and financial condition would be adversely affected. See “Business of Moolec and Certain Information About Moolec — Products — Development Timelines.”
Collaboration agreements we may seek to enter into with third parties may not be accomplished or successful.
We may seek collaboration arrangements with third parties for the development or commercialization of our products. We will face, to the extent that we decide to enter collaboration arrangements, significant competition in seeking appropriate partners. Moreover, collaboration arrangements are complex and time-consuming to negotiate, document, implement and maintain. We may not be successful in our efforts to establish and implement collaboration or other alternative arrangements should we so chose to enter such arrangements. The terms of any collaborations or other arrangements that we may establish may not be favorable to us. Any collaboration/marketing/wholesale/research agreements that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize our product candidates.
The successful commercialization of our products depends on our ability to produce high-quality products cost-effectively on a large scale and to accurately forecast demand for our products, and we may be unable to do so.
The production of commercial-scale quantities of seeds and products requires the multiplication of the plants or seeds through a succession of plantings and seed harvests. The cost-effective production of high-quality, high-volume quantities of any product candidates we successfully develop depends on our ability to scale our production processes to produce plants and seeds in enough quantity to meet demand. We cannot assure that our
54
Table of Contents
existing or future seed production techniques will enable us to meet our large-scale production goals cost-effectively. Even if we are successful in developing ways to minimize yield drag and enhance quality, we may not be able to do so cost-effectively or on a timely basis, which could adversely affect our ability to achieve profitability. If we are unable to maintain or enhance the quality of our plants and seeds as we increase our production capacity, including through the expected use of third parties to have access to industrial availability for processing and purification, we may experience reductions in customer or farmer demand, higher costs and increased inventory write-offs.
In addition, because of the length of time it takes to produce commercial quantities of marketable seeds, we will need to make seed production decisions well in advance of product sales. The process that we may use to concentrate and isolate proteins is based on technologies currently being used in the industry. We have access to a pilot plant where the wet-concentration process for proteins recovery is being validated. Our ability to accurately forecast supply can be adversely affected by several factors outside of our control, including unexpected challenges in the process, changes in market conditions, environmental factors, such as pests and diseases, and adverse weather conditions. A shortfall in the supply of our products may reduce product revenue, damage our reputation in the market and adversely affect relationships. Any product surplus we have on hand may negatively impact cash flows, reduce the quality of our inventory and ultimately result in write-offs of inventory. While we estimate that the potential size of our target markets for our products is significant, that estimate has not been independently verified and is based on certain assumptions that may not prove to be accurate. Our ability to accurately forecast demand is dependent on the timing of customer decisions, qualification cycles, and other factors outside of our control. As a result, these estimates could differ materially from actual market sizes, which could result in decreased demand for our products and therefore adversely impact our future business prospects, results of operation and financial condition.
Our Products are subject to specific measures with regard to their maintenance in storage.
Our products are subject to specific measures with regard to their maintenance in storage. The final product will be stored in sealed plastic bags to prevent changes in moisture that can lead to the acceleration of oxidation, formation of lumps, alterations of the characteristics of the product. Sealed bags also prevent the powder from any contact with insects or odors, and delay oxidation. The temperature should be kept below a specific temperature to prevent microbial growth and oxidation. Direct sunlight in the storage environment should be avoided. Brown paper bags with a plastic layer inside are advised since they prevent the isolates from light. A change in any of these conditions may affect the shelf life and the properties of the product significantly, which may consequently adversely affect our business. See the section titled “Business of Moolec and Certain Information about Moolec — Products.”
The successful commercialization of our products may face challenges from public perceptions of plant engineered products and ethical, legal, environmental, health and social concerns.
The successful commercialization of our products depends, in part, on public acceptance of molecular farming/plant engineered agricultural products. Our products will be genetically modified organisms (“GMOs”) and any negative social perception about GMOs or our technology may negatively impact our business.
Consumers may not understand the nature of our technologies, which consist of the nuclear stable transformation of a crop to express an animal protein within the protein bodies of the seed for the product to consist in an ingredient containing not only the alternative protein but the plant protein as well. Consumers may also not understand the scientific distinction between our molecular farming products and other plant-based protein or alternative protein products. As a result, they may transfer negative perceptions and attitudes regarding our products. A lack of understanding of our technologies may also make consumers more susceptible to the influence of negative information provided by opponents of biotechnology. Some opponents of biotechnology actively seek to raise public concern about gene editing or GMOs by claiming that plant products developed using biotechnology are unsafe for consumption or their use, pose a risk of damage to the environment, or creates legal, social and ethical dilemmas. The commercial success of our products may be adversely affected by such claims, even if unsubstantiated. In addition, opponents of biotechnology have vandalized the fields of farmers planting biotech seeds and facilities used by biotechnology companies. Any such acts of vandalism targeting the fields of our farmers, our field-testing sites or our research, production or other facilities, could adversely affect our sales and our costs.
Negative public perceptions about molecular farming and GMO products in general can also affect the regulatory environment in the jurisdictions in which we are targeting the sale of our products and the commercialization of our product candidates. Any increase in such negative perceptions or any restrictive government regulations in
55
Table of Contents
response thereto, could have a negative effect on our business and may delay or impair the sale of our products or the development or commercialization of our product candidates. Even in light of compliance with regulatory protocols or following receipt of confirmation of non-regulated status in a jurisdiction, public pressure may lead to increased regulation of products produced using biotechnology, further legislation regarding novel trait development technologies, or administrative litigation concerning prior regulatory determinations, each of which could adversely affect our ability to sell our product or commercialize our product candidates. In addition, labeling requirements could heighten public concerns and make consumers less likely to purchase food products containing plant bioengineered/molecular farming and fermented ingredients.
Market perception and consumer preferences for our products are difficult to predict and may adversely affect our business
There are currently many competitors in the plant-based protein market, however our product will be one of the first to integrate animal-proteins into plants. We expect to be the first to produce and commercialize an ingredient containing functional bovine and plant proteins coming from a single source for the food market. There is a risk that our product will not be as in demand as more traditional plant-based proteins or lab grown meat. A change in preferences for our products could reduce our sales or our market share and could harm our business and financial condition.
Consumer preferences for our products are difficult to predict and may change, and if we are unable to respond quickly to new trends, our business may be adversely affected.
Our business is focused on the development, manufacturing, marketing, and distribution of plant-based products which include animal proteins using animal-free ingredients. Consumer demand could change based on a number of possible factors, including dietary habits and nutritional values, concerns regarding the health effects of ingredients, and shifts in preference for various product attributes. If consumer demand for our products decreased, our business and financial condition would suffer. In addition, sales of plant-based animal proteins will be subject to evolving consumer preferences to which we may not be able to accurately predict or respond. Consumer trends that we believe favor sales of our products could change based on a number of possible factors, including economic factors and social trends a more sustainable and healthy approach. Views towards healthy eating and plant-based products are trendy in nature, with constantly changing consumer perceptions, however utilizing animal-proteins within plants has not been introduced into the market.
Our success depends, in part, on our ability to anticipate the tastes and dietary habits of consumers and other consumer trends and to offer products that appeal to their needs and preferences on a timely and affordable basis. A change in consumer discretionary spending, due to economic downturn or other reasons, may also adversely affect our sales and its business, financial condition and results of operations. A significant shift in consumer demand away from our products could reduce sales or market share and the perception of our brand, which would harm our business and financial condition.
Our business activities are currently conducted in a limited number of locations, which make us susceptible to damage or business disruptions caused by natural disasters or acts of vandalism.
Our business activities are currently conducted through third-party contracts with research centers, laboratories and universities in a limited number of locations. These include, among others, (i) Washington State University, Genomics Laboratory, Department of Horticulture located in Pullman Washington, USA; (ii) Wisconsin Crop Innovation Center — University of Wisconsin-Madison — located in Middleton, Wisconsin, USA; (iii) INDEAR research and development center located in Rosario, Santa Fe, Argentina; (iv) INMET research and development center located in Rosario, Santa Fe, Argentina; (v) Artes laboratory located in Langenfeld, Rheinland, Germans; (vi) HAN Biocentre — University of Applied Sciences in Nijmegen — located in Nijmegen, the Netherlands; (vii) Future Foods laboratory, located in Malden, the Netherlands and (viii) Premas Biotech — research center located in Manesar, India. Our seed production, field-testing and production and research take place primarily in Argentina, the United States and the Netherlands, with concentration in certain geographic regions. These geographic regions are subject to climate disasters or other natural disasters that could negatively affect our business and the development and increase of our seed production. For more information regarding our business activities and contracts with certain research facilities please see “Business of Moolec and Certain Information about Moolec — Research and Development.”
56
Table of Contents
A natural disaster, such as a hurricane, drought, fire, flood, tornado, earthquake, or other intentional or negligent acts, including acts of vandalism, could damage or destroy our equipment, inventory, development projects, field trials or data, and cause us to incur significant additional expenses to repair or replace the damaged physical facilities, which in the case of seed production may be the result of years of development work that is not easily or quickly reproduced, and increase the development schedule for our pipeline of product candidates. If our early testing of pipeline products is unsuccessful, we may be unable to complete the development of product candidates on a timely basis or at all.
Our field trials and research could be negatively affected by weather and climatic variations, disease or pests, or acts of protest or vandalism which could lower the expected yield, and delay the production and product candidates.
We rely on early testing and research, including laboratory research, greenhouse activities and field trials, to demonstrate the efficacy of product candidates that we develop and evaluate. Field trials allow us to test product candidates in the field as well as to increase seed production, and to measure performance across multiple geographies and conditions. Successful completion of early testing is critical to the success of our product development efforts. Field trials for event selection and field trials for gathering regulatory information are expected to be carried out in 2023 and 2024 in the United States and Argentina. If our ongoing or future testing is unsuccessful or produces inconsistent results or unanticipated adverse effects on the agronomic performance of our crops, or if the testing does not produce reliable data, our product development efforts could be delayed, subject to additional regulatory review or abandoned entirely. In addition, in order to support our commercialization efforts, it is necessary to collect data across multiple growing seasons and from different geographies. Even in cases where initial field trials are successful, we cannot be certain that additional field trials conducted on a greater number of acres or in different geographies will also be successful. Many factors that are beyond our control may adversely affect the success of these field trials, including unique geographic conditions, weather and climatic variations, disease or pests, or acts of protest or vandalism. Field trials, which may take years to be concluded, are costly, and any field trial failures that we may experience may not be covered by insurance and, therefore, could result in increased costs, which may negatively impact our business and results of operations.
If our genetically engineered plants do not express a sufficient yield of an animal protein, we may not be able to take our products to market in a timely manner or successfully operate our business.
We rely heavily on research and development of our products. Our process of molecular farming involves introducing DNA within a plant genome in order to express animal proteins within the plant without using any animal products. This process requires significant research and testing in order to have a product that produces a sufficient amount of the animal protein in order to be commercially viable. If the tests of our products do not yield a sufficient amount of the animal protein within the plant, we may be required to begin the research and development process anew for certain products or spend additional time in order to have the necessary outcome.
Our research and testing to produce the expected result of animal protein yields within our molecular farmed plants can also be affected by laboratory conditions, adverse climate conditions during planting and harvesting, pests, vandalism or other conditions whereby the expected yield of animal proteins within the plant are not what we would expect. Such conditions may delay our ability to take our products to market or require additional time within our research and development stage in order to prepare a viable product. Such delays may have an adverse effect on our business and operations.
If we are unable to attract, train and retain employees, we may not be able to grow or successfully operate our business.
Our success depends in part on our ability to attract, train and retain a sufficient number of employees who understand and appreciate our culture and business model, and can effectively represent our brand and establish credibility with potential business partners and customers. The ability, expertise, motivation, judgment and discretion of our employees, including our technical team, are key for the development of our business. In addition, our ability to manage our anticipated growth depends on our ability to recruit and retain qualified personnel prepared to face major challenges with regard to technology and innovation. Our ability to retain our employees is influenced by the economic environment and the fact that our operations are spread in different locations.
57
Table of Contents
Food safety and food-borne illness incidents or advertising or product mislabeling may materially and adversely affect our business by exposing us to lawsuits, product recalls or regulatory enforcement actions, increasing our operating costs and reducing demand for our product offerings.
Selling food for human consumption involves inherent legal and other risks, and there is increasing governmental scrutiny of and public awareness regarding food safety. Unexpected side effects, illness, injury or death related to allergens, food-borne illnesses or other food safety incidents caused by products we sell, or involving our suppliers, could result in the discontinuance of sales of these products or our relationships with such suppliers, or otherwise result in increased operating costs, regulatory enforcement actions or harm to our reputation. Shipment of adulterated or misbranded products, even if inadvertent, can result in criminal or civil liability. Such incidents could also expose us to product liability, negligence or other lawsuits, including consumer class action lawsuits. Any claims brought against us may exceed or be outside the scope of our existing or future insurance policy coverage or limits. Any judgment against us that is more than our policy limits or not covered by our policies or not subject to insurance would have to be paid from our cash reserves, which would reduce our capital resources.
The occurrence of food-borne illnesses or other food safety incidents could also adversely affect the price and availability of affected ingredients, resulting in higher costs, disruptions in supply and a reduction in our sales. Furthermore, any instances of food contamination or regulatory noncompliance, whether or not caused by our actions, could compel us, our suppliers, our distributors or our customers, depending on the circumstances, to conduct a recall in accordance with FDA regulations, and comparable state laws. Food recalls could result in significant losses due to their costs, the destruction of product inventory, lost sales due to the unavailability of the product for a period of time and potential loss of existing distributors or customers and a potential negative impact on our ability to attract new customers due to negative consumer experiences or because of an adverse impact on our brand and reputation. The costs of a recall could exceed or be outside the scope of our existing or future insurance policy coverage or limits.
In addition, food companies have been subject to targeted, large-scale tampering as well as to opportunistic, individual product tampering, and we, like any food company, could be a target for product tampering. Forms of tampering could include the introduction of foreign material, chemical contaminants and pathological organisms into consumer products as well as product substitution. Recently issued FDA regulations will require companies like us to analyze, prepare and implement mitigation strategies specifically to address tampering designed to inflict widespread public health harm. If we do not adequately address the possibility, or any actual instance, of product tampering, we could face possible seizure or recall of our products and the imposition of civil or criminal sanctions, which could materially adversely affect our business, financial condition and operating results.
If we are sued for defective products and if such lawsuits were determined adversely, we could be subject to substantial damages, for which insurance coverage may not be available.
We may be held liable if any product we develop, or any product that uses or incorporates any of our technologies, is found unsuitable during marketing, sale or consumption. For example, the detection of an unintended trait in a commercial seed variety or the crops and products produced may result in governmental actions such as mandated crop destruction, product recalls or environmental cleanup or monitoring. The costs of these things could exceed or be outside the scope of our existing or future insurance policies. Concerns about seed quality could also lead to additional regulations being imposed on our business, such as regulations related to testing procedures, mandatory governmental reviews of biotechnology advances, or additional regulations relating to the integrity of the food supply chain from the farm to the finished product. See “Business of Moolec and Certain Information about Moolec — Legal Proceedings.”
Our brand has limited awareness among the general public.
We have not conducted a dedicated and significant marketing campaign to educate consumers on the Moolec brand and it still has limited awareness among the general public.
We will need to dedicate significant resources in order to effectively plan, coordinate, and execute a marketing campaign and to add additional sales and marketing staff. Substantial advertising and promotional expenditures may be required to improve our brand’s market position or to introduce new products to the market. However, an increase in our marketing and advertising efforts may not enhance our reputation, or lead to an increase in brand awareness.
58
Table of Contents
Further, we compete against other large, well-capitalized food companies who have significantly more resources than we do. Therefore, we may have limited success, or none at all, in increasing awareness and reputation of the Moolec brand.
If we fail to develop and maintain our Moolec brand, our business could suffer.
We believe our future growth could be affected by the perception of our brand and its value. Maintaining, promoting and positioning our brand and its reputation will depend on, among other factors, the success of our plant-based animal proteins, food safety, quality assurance, marketing efforts, and our ability to provide a consistent, high-quality customer experience. Any negative publicity, regardless of its accuracy, could adversely affect our business. Brand value is based on perceptions of subjective qualities, and any incident that erodes the loyalty of customers or suppliers, including adverse publicity, product recall or a governmental investigation or litigation, could significantly reduce the value of our Moolec brand and significantly damage our business, financial condition and results of operation.
We rely on information technology systems and any inadequacy, failure, interruption or security breaches of those systems may impair our ability to operate our business effectively.
We are increasingly dependent upon third-party information technology systems to operate our business. These systems may contain confidential information (including personal data, trade secrets or other intellectual property, or proprietary business information). The nature of digital systems, both internally and externally, makes them potentially vulnerable to disruption or damage from human error and/or security breaches, which include, but are not limited to, ransomware, data theft, denial of service attacks, sabotage, industrial espionage, and computer viruses. Such events may be difficult to detect, and once detected, their impact may be difficult to assess and address.
In addition, the information technology systems we use may be vulnerable to damage or interruption from circumstances beyond our control, including fire, natural disasters, power outages, systems failures and viruses. If we are unable to execute our disaster recovery plans or if our plans prove insufficient for a particular situation or take longer than expected to implement in a crisis situation, it could have a material adverse effect on our business, financial condition and results of operations, and our business interruption insurance may not adequately compensate us for losses that may occur.
We are also subject to numerous laws and regulations designed to protect personal data, such as the European national laws implementing the General Data Protection Regulation as well as the UK General Data Protection Regulation. These data protection laws introduced more stringent data protection requirements and significant potential fines, as well as increased our responsibility and potential liability in relation to personal data that we process. We have put mechanisms in place to ensure compliance with applicable data protection laws but there can be no guarantee of their effectiveness.
Disruptions in the worldwide economy, including Russia’s recent invasion of Ukraine and the impact of pandemics, epidemics or disease outbreaks may adversely affect our business, results of operations and financial condition.
The global economy can be negatively impacted by a variety of factors such as the spread or fear of spread of contagious diseases (such as the recent COVID-19 pandemic) in locations where our products are sold, man-made or natural disasters, actual or threatened war, terrorist activity, political unrest, civil strife and other geopolitical uncertainty. These economic conditions can arise suddenly and the full impact of such conditions can be difficult to predict. In addition, geopolitical and domestic political developments, such as existing and potential trade wars and other events beyond our control, such as Russia’s recent invasion of Ukraine, can increase levels of political and economic unpredictability globally and increase the volatility of global financial markets.
In addition, our Argentine operations could be affected by any general business restrictions imposed by the government due to its political and economic instability. These adverse and uncertain economic conditions may impact distributor, retailer, foodservice and consumer demand for our products. In addition, our ability to manage normal commercial relationships with our suppliers, distributors, customers and consumers and creditors may suffer. Consumers may shift purchases to lower-priced or other perceived value offerings during economic downturns as a result of various factors, including job losses, inflation, higher taxes, reduced access to credit, change in federal economic policy and international trade disputes. A decrease in consumer discretionary spending may also result in consumers reducing the frequency and amount spent on food prepared away from home. Distributors and customers
59
Table of Contents
may become more conservative in response to these conditions and seek to reduce their inventories. Our results of operations depend upon, among other things, our ability to maintain and increase sales volume with our existing and future customers and our ability to attract new consumers, the financial condition of consumers and our ability to provide products that appeal to consumers at the right price. Decreases in demand for products without a corresponding decrease in costs would put downward pressure on margins and would negatively impact financial results. Prolonged unfavorable economic conditions or uncertainty may adversely affect our sales and profitability and may result in consumers making changes to their discretionary spending behavior on a more long-lasting or even permanent basis.
Risks Relating to Our Industry
The overall agricultural industry is susceptible to commodity price changes and we are exposed to market risks from changes in commodity prices.
Conditions in the United States agricultural industry and the Argentine agricultural industry significantly impact our operating results. Changes in the prices of commodities could result in higher overall cost along the agricultural and commercial supply chains, which may negatively affect our ability to commercialize our products. We will be susceptible to changes in costs in the agricultural industry as a result of factors beyond our control, such as general economic conditions, seasonal fluctuations, weather conditions, demand, food safety concerns, product recalls and government regulations. As a result, we may not be able to anticipate or react to changing costs by adjusting our practices, which could cause our operating results to deteriorate.
We are subject to industry-specific risks, which could adversely affect our operating results.
We are subject to industry-specific risks which include, but are not limited to, product safety and quality, launch of new products by other industries that can replace the functionalities of our production; shifting consumer preferences, federal, state, and local regulations on manufacturing or labeling; socially acceptable and sustainable farming practices; environmental, health, and safety regulations; and customer product liability claims. The liability which could result from certain of these risks may not always be covered by, or could exceed liability insurance related to product liability and food safety matters maintained by us. Risks to our reputation may exist due to potential negative publicity caused by product liability, food safety, occupational health and safety, workforce diversity, and environmental matters.
Our products are used as ingredients in food production. We are subject to risks associated with economic, product quality, food safety or other factors inherent to the food industry, which may adversely affect us. In addition, as the Company increases its investment in flavors and ingredients businesses, it is exposed to increased risks related to rapidly changing consumer preferences and the impacts these changes could have on the success of certain of our customers.
Adverse weather conditions, natural disasters, crop disease, pests and other natural conditions can impose significant costs and losses on our business.
The ability to grow our products is vulnerable to adverse weather conditions, including windstorms, floods, drought and temperature extremes, which are quite common but difficult to predict, the effects of which may be influenced and intensified by ongoing global climate change. Unfavorable growing conditions can reduce both crop size and crop quality. In extreme cases, entire harvests may be lost in some geographic areas. Such adverse conditions can result in harvesting delays or loss of crops for farmers and cause us to be delayed, or to fail entirely in delivering product to customers, resulting in loss of revenue. Furthermore, significant fluctuations in market prices for agricultural inputs and crops could also have an adverse effect on the prices of our products.
The ability to grow our products is also vulnerable to crop disease and to pests, which may vary in severity and effect, depending on the stage of production at the time of infection or infestation, the type of treatment applied, climatic conditions and the risks associated with ongoing global climate change. The costs to control disease and other infestations vary depending on the severity of the damage and the extent of the plantings affected. Moreover, there can be no assurance that available technologies to control such infestations will continue to be effective. These infestations can also increase costs, decrease revenues and lead to additional charges to earnings, which may have a material adverse effect on our business, financial position and results of operations.
60
Table of Contents
Climate Change may negatively affect our business and operations.
There is concern that carbon dioxide and other greenhouse gases in the atmosphere may adversely impact global temperatures, weather patterns and the frequency and severity of extreme weather and natural disasters. If climate change negatively affects agricultural productivity, we may be subject to decreased availability or less favorable pricing for certain commodities that are necessary for its products, such as our soy and pea plants, among others. Due to climate change, we may also be subjected to decreased availability of water, deteriorated quality of water or less favorable pricing for water, which could adversely impact manufacturing operations.
Risks Relating to Intellectual Property
Agreements with our collaborators and third parties may not adequately prevent disclosure of trade secrets, know-how and other proprietary information, which could materially adversely affect our technology and harm our business.
We rely on a combination of intellectual property laws and agreements with our collaborators and third parties to protect and otherwise seek to control access to, and distribution of, our proprietary information. These measures may not prevent disclosure, infringement, or misappropriation of our confidential information. Our confidentiality and nondisclosure agreements or covenants may not be enforceable under applicable law and, even if they are enforceable, may be breached, and we may not have adequate remedies for such a breach that would effectively prevent the further dissemination of our confidential information. We also have limited control over the protection of trade secrets used by our collaborators and could lose future trade secret protection if any unauthorized disclosure of such information occurs. Enforcement of any claim that a party illegally disclosed confidential information or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, others may independently discover our trade secrets and proprietary information, and in such cases, we may not be able to assert any trade secret rights against such parties. Laws regarding trade secret rights in certain markets where we operate may afford little or no protection of our trade secrets. If any of our trade secrets were to be disclosed to or independently developed by a competitor, or if we otherwise were to lose protection for our trade secrets or proprietary know-how, the value of this information may be greatly reduced, and our business could be adversely impacted.
We and our customers depend on patents, copyrights, trademarks, know-how, trade secrets, and other forms of intellectual property protections, but these protections may not be adequate.
We rely on a combination of know-how, trade secrets, patents, copyrights, trademarks, and other intellectual property laws, nondisclosure and other contractual provisions, and technical measures to protect many of our products, services and intangible assets. These proprietary rights are important to our ongoing operations. There can be no assurance that these protections will provide uniqueness or meaningful competitive differentiation in our offerings or otherwise be commercially valuable or that we will be successful in obtaining additional intellectual property or enforcing our intellectual property rights against unauthorized users. Our exclusive rights under certain of our products and services are protected by patents, and when patents covering a product or service expire, loss of exclusivity may occur, which may force us to compete with third parties, thereby negatively affecting our revenue and profitability.
Our proprietary rights may be invalidated, circumvented, or challenged. We may in the future be subject to proceedings seeking to oppose or limit the scope of our patent applications or issued patents. In addition, in the future, we may need to take legal actions to enforce our intellectual property rights, to protect our trade secrets, or to determine the validity or scope of the proprietary rights of others. Legal proceedings are inherently uncertain, and the outcome of such proceedings may be unfavorable to us. Any legal action regardless of outcome might result in substantial costs and diversion of resources and management attention. Although we use reasonable efforts to protect our proprietary and confidential information, there can be no assurance that our confidentiality and non-disclosure agreements will not be breached, our trade secrets will not otherwise become known by competitors, or that we will have adequate remedies in the event of unauthorized use or disclosure of proprietary information. Even if the validity and enforceability of our intellectual property is upheld, an adjudicator might construe our intellectual property not to cover the alleged infringement. In addition, intellectual property enforcement may be unavailable or practically ineffective in some countries. There can be no assurance that our competitors will not independently develop technologies that are substantially equivalent or superior to our technology or that third parties will not
61
Table of Contents
design around our intellectual property claims to produce competitive offerings. The use of our technology or similar technology by others could reduce or eliminate any competitive advantage we have developed, cause us to lose sales, or otherwise harm our business.
For example, Impossible Foods filed a lawsuit against Motif FoodWorks, claiming that it infringed on at least one of Impossible Foods’ patents and, subsequently, Motif FoodWorks filed an administrative claim challenging certain of Impossible Foods’ products. Although this proceeding is still ongoing, similar cases can be brought against other parties that rely heavily on intellectual property rights within the alternative protein landscape, including us. While we are not party to such suits at this time, we may become subject to such litigation in the future. Such cases could also adversely impact public perception and the demand for alternative protein products, which could subsequently result in decreased demand for our products and adversely affect our business and operations.
We have patents granted and patent applications, as owner or as licensee, in the United States, China, India, Canada, Germany, Spain, France, United Kingdom, Brazil, Mexico, Australia, New Zealand, Japan, Hong Kong, Malaysia, and Argentina and trademarks and service marks in the United States, Europe, Canada, the United Kingdom and Argentina, some of which have been registered or issued, and also claim common law rights in various trademarks and service marks. There can be no assurance that third parties might not oppose our future applications to register intellectual property. It is possible that in some cases we may be unable to obtain the registrations for trademarks, service marks, and patents for which we have applied, and a failure to obtain trademark and patent registrations in the United States, the United Kingdom, the European Union, and Argentina or other countries could limit our ability to protect our trademarks and proprietary technologies and impede our marketing efforts in those jurisdictions.
License agreements with third parties control our rights to use certain patents, software, and information technology systems and proprietary technologies owned by third parties, some of which are important to our business. Termination of these license agreements for any reason could result in the loss of our rights to this intellectual property, causing an adverse change in our operations or the inability to commercialize certain offerings.
Patents and patent applications of biotechnology involve highly complex legal and factual questions, which, if determined adversely to us, could negatively impact our competitive position.
The patent positions of biotechnology companies and other actors in our fields of business can be highly uncertain and involve complex scientific, legal and factual analyses. The interpretation and breadth of claims allowed in some patents covering biological compositions may be uncertain and difficult to determine and are often affected materially by the facts and circumstances that pertain to the patented compositions and the related patent claims. The issuance and scope of patents cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated, narrowed or circumvented. Challenges to our or our licensors’ patents and patent applications, if successful, may result in the denial of our or our licensors’ patent applications or the loss or reduction in their scope. In addition, defending against such challenges may be costly and involve the diversion of significant management time. Accordingly, rights under any of our patents may not provide us with enough protection against competitive products or processes and any loss, denial or reduction in scope of any of such patents and patent applications may have a material adverse effect on our business.
We will not seek to protect our intellectual property right in all jurisdictions throughout the world and we may not be able to adequately enforce our intellectual property rights even in the jurisdictions where we seek protection.
Filing, prosecuting and defending patents in all countries and jurisdictions throughout the world would be prohibitively expensive. Patent prosecution must be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Our intellectual property rights in some countries outside the United States could be less extensive than those in the United States, assuming that rights are obtained in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from using our inventions in countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions.
62
Table of Contents
Competitors may use our technologies in jurisdictions where we or our licensors do not pursue and obtain patent protection. Further, competition may export otherwise infringing products to territories where we or our licensors have patent protection, but where the ability to enforce those patent rights is not as strong as in the United States. These products may compete with our products and our intellectual property rights and such rights may not prevent such competition.
In addition, changes in, or different interpretations of, patent laws in the United States and other countries may permit others to use our discoveries or to develop and commercialize our technology and products without providing any notice or compensation to us or may limit the scope of patent protection that we or our licensors are able to obtain. The laws of some countries do not protect intellectual property rights to the same extent as United States laws and those countries may lack adequate rules and procedures for defending our intellectual property rights.
Furthermore, proceedings to enforce our patent rights and other intellectual property rights around the world could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our or our licensors’ patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded to us, if any, may not be commercially meaningful, while the damages and other remedies we may be ordered to pay such third parties may be significant. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
In addition, there is a possibility that our technologies may be viewed by competitors as an infringement on their intellectual property rights, even in a case where they do not. This may lead to litigation that could create a significant cost for us and negatively impact our business and operations as well as our public perception and goodwill.
We may be unsuccessful in developing, licensing or acquiring intellectual property that may be required to develop and commercialize our future products.
Our programs may involve additional product candidates that may require the use of intellectual property or proprietary rights held by third parties and the growth of our business may depend in part on our ability to acquire, license or use these intellectual property and proprietary rights.
However, we may be unable to acquire or license any third-party intellectual property or proprietary rights that may be key to development. Even if we can acquire or license such rights, we may be unable to do so on commercially reasonable terms. The licensing and acquisition of third-party intellectual property and proprietary rights is a competitive area, and several more established companies are also pursuing strategies to license or acquire third-party intellectual property and proprietary rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, capital resources and agricultural development and commercialization capabilities.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license intellectual property and proprietary rights to us. We also may be unable to license or acquire third-party intellectual property and proprietary rights on terms that would allow us to make an appropriate return on our investment or at all. If we are unable to successfully acquire or license rights to required third-party intellectual property and proprietary rights or maintain the existing intellectual property and proprietary rights we have, we may have to cease development of the relevant program, product or product candidate, which could have a material adverse effect on our business.
Risks Relating to Laws and Regulation
The regulatory environment in the United States for our current and potential future products is evolving and may change in the future, negatively impacting our speed to market and cost to launch our potential future products.
Changes in applicable regulatory requirements could result in a substantial increase in the time and costs associated with developing our products and negatively impact our operating results. While the USDA and U.S. Food and Drug Administration (“FDA”) currently have petition processes that we have initiated, these processes and the manner in which the USDA and FDA interpret their regulations may change in the future, negatively impacting our speed to market and cost to launch our potential future products. We cannot predict whether advocacy groups
63
Table of Contents
will challenge existing regulations and USDA or FDA determinations or whether the USDA or FDA will alter the manner in which it interprets its own regulations or institutes new regulations, or otherwise modify regulations in a way that will subject our products to more burdensome standards, thereby substantially increasing the time and costs associated with developing our product candidates.
The regulatory environment outside the United States varies greatly from jurisdiction to jurisdiction and there is less certainty how our products will be regulated.
The regulatory environment around gene editing in plants, particularly in our case which includes the addition of animal-proteins without the use of animals, to create food ingredients is greatly uncertain outside of the United States and varies greatly from jurisdiction to jurisdiction. Each jurisdiction may have its own regulatory framework regarding genetically modified foods, which may include restrictions and regulations on planting and growing genetically modified plants and in the consumption and labeling of genetically modified foods, and which may encapsulate our products. To the extent regulatory frameworks outside of the United States are not receptive to our gene-editing technologies, this may limit our ability to expand into other global markets.
Complying with the regulatory requirements outside the United States will be costly and time-consuming, and there is no guarantee we will be able to commercialize our products in other jurisdictions apart from the United States and Argentina.
We cannot predict whether or when any jurisdiction will change its regulations with respect to our products. Advocacy groups have engaged in publicity campaigns and filed lawsuits in various countries against companies and regulatory authorities, seeking to halt regulatory approval or clearance activities or influence public opinion against genetically engineered (GMOs) products. In addition, governmental reaction to negative publicity concerning our products could result in greater regulation of genetic research and derivative products or regulatory costs that render our products cost prohibitive.
The scale of the commodity food and agricultural industry may make it difficult to monitor and control the distribution of our products. As a result, our products may be sold inadvertently within jurisdictions where they are not approved for distribution. Such sales may lead to regulatory challenges or lawsuits against us, which could result in significant expenses and management attention.
Government policies and regulations, particularly those affecting the agricultural sector and related industries, could adversely affect our operations and profitability.
Agricultural production and trade flows are subject to government policies and regulations. Governmental policies and approvals of technologies affecting the agricultural industry, such as taxes, tariffs, duties, subsidies, incentives and import and export restrictions on agricultural commodities and commodity products can influence the planting of certain crops, the location and size of crop production, and the volume and types of imports and exports. In addition, as we grow our business, we may be required to secure additional permits and licenses. Finally, future government policies in the United States or in other countries may discourage our customers from using our products or encourage the use of products more advantageous to our competitors, which would put us at a commercial disadvantage and could negatively impact our future revenues and results of operations.
We may use biological materials in our business and are subject to numerous environmental, health and safety laws and regulations. Compliance with such laws and regulations and any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
We are subject to numerous federal, state, local and foreign environmental, health and safety laws and regulations, including those governing laboratory procedures, the handling, use, storage, treatment, manufacture and disposal of hazardous materials and wastes, discharge of pollutants into the environment and human health and safety matters. Our R&D processes involve the controlled use of hazardous materials, including biological materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, or may otherwise be required to remediate such contamination, and our liability may exceed any insurance coverage and our total assets. Compliance with environmental, health and safety laws and regulations may be expensive and may impair our R&D efforts. If we fail to comply with these requirements, we could incur substantial costs and liabilities, including civil or criminal fines and penalties, clean-up costs or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance. In addition, we cannot predict
64
Table of Contents
the impact on our business of new or amended environmental, health and safety laws or regulations or any changes in the way existing and future laws and regulations are interpreted and enforced. These current or future laws and regulations may impair our research, development or production efforts or result in increased expense of compliance.
Tax legislative or regulatory initiatives, new interpretations or developments concerning existing tax laws, or challenges to our tax positions could adversely affect our results of operations and financial condition.
We currently have limited operations, however we have relationships with multiple jurisdictions throughout the world including the United Kingdom, Luxembourg, the United States, Argentina and the Netherlands. As such, we are subject to the tax laws and regulations of various jurisdictions, including U.S. federal, state, and local governments and could be potentially subject to additional tax laws and regulations in additional jurisdictions. From time to time, various legislative initiatives may be proposed that could adversely affect our tax positions, and existing legislation, such as the 2017 U.S. Tax Cuts and Jobs Act, may be subject to additional regulatory changes or new interpretations. There can be no assurance that our effective tax rate or tax payments will not be adversely affected by these initiatives.
In addition, the tax laws of several of the countries we operate in, including Argentinian and U.S. federal, state and local tax laws and regulations are extremely complex and subject to varying interpretations. It is possible that the outcomes from examinations or interpretations by taxing authorities or changes in laws, rules, regulations, or interpretations by taxing authorities could have a material adverse effect on our financial condition or results of operations.
We are subject to governmental export and import controls that could impair our ability to compete in international markets and subject us to liability if we are not in compliance with applicable laws.
Our products are subject to Export Control Laws and Import Control Laws and regulations of the jurisdictions in which we operate. Exports of our products must be made in compliance with these laws and regulations. If we fail to comply with these laws and regulations, we and certain of our employees could be subject to substantial civil or criminal penalties, including the possible loss of export or import privileges; fines, which may be imposed on us and responsible employees or managers; and, in extreme cases, the incarceration of responsible employees or managers.
In addition, changes in our products or changes in applicable export or import laws and regulations may create delays in the introduction, provision, or sale of our products in international markets, prevent customers from using our products or, in some cases, prevent the export or import of our products to certain countries, governments or persons altogether. Any limitation on our ability to export, provide, or sell our products could adversely affect our business, financial condition and results of operations.
Failure to comply with the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act 2010 and similar laws associated with our activities in other jurisdictions could subject us to penalties and other adverse consequences.
As we expect a substantial portion of our future revenues to come from jurisdictions outside of the United States, we face significant risks if we fail to comply with the U.S. Foreign Corrupt Practices Act (“FCPA”), the U.K. Bribery Act 2010 (the “Bribery Act”) and other laws that prohibit improper payments or offers of payment to governments and their officials and political parties by us and other business entities for the purpose of obtaining or retaining business. In many countries, particularly in countries with developing economies, it may be a local custom that businesses operating in such countries engage in business practices that are prohibited by the FCPA, the Bribery Act or other laws and regulations. Although we have implemented company policy requiring employees and consultants to comply with the FCPA, the Bribery Act and similar laws, such policy may not be effective at preventing all potential FCPA, Bribery Act or other violations. In addition, we cannot guarantee the compliance by our partners, resellers, suppliers and agents with applicable laws, including the FCPA and the Bribery Act. Therefore, there can be no assurance that none of our employees or agents will take actions in violation of our policies or of applicable laws, for which we may be ultimately held responsible. Any violation of the FCPA or the Bribery Act and related policies could result in severe criminal or civil sanctions, which could have a material and adverse effect on our reputation, business, financial condition and results of operations.
65
Table of Contents
Risks Related to Holdco
Holdco will incur increased costs as a result of operating as a public company whose shares will be listed on the Nasdaq stock exchange, and its management will devote substantial time to new compliance initiatives.
If Holdco completes the Business Combination and becomes a public company, it will incur significant legal, accounting and other expenses that it did not incur as a private company, and these expenses may increase even more after Holdco is no longer an emerging growth company, as defined in Section 2(a) of the Securities Act. As a public company, Holdco will be subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, as well as rules adopted, and to be adopted, by the SEC and Nasdaq. Holdco’s management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, Holdco expects these rules and regulations to substantially increase its legal and financial compliance costs and to make some activities more time consuming and costly. The increased costs will increase Holdco’s net loss. For example, these rules and regulations could make it more difficult and more expensive for Holdco to obtain director and officer liability insurance and as a result, Holdco may be forced to accept reduced policy limits or incur substantially higher costs to maintain the same or similar coverage. Holdco cannot predict or estimate the amount or timing of additional costs it may incur to respond to these requirements. The impact of these requirements could also make it more difficult for Holdco to attract and retain qualified persons to serve on its board of directors or as executive officers. Furthermore, due to the increase in the shareholders base of Holdco, costs related to shareholders’ communication will increase as a result of the listing of Holdco shares on the Nasdaq stock exchange.
Holdco’s management has limited experience in operating a public company.
Holdco’s executive officers have limited experience in the management of a publicly traded company. Holdco’s management team may not successfully or effectively manage its transition to a public company that will be subject to significant regulatory oversight and reporting obligations under federal securities laws. Their limited experience in dealing with the increasingly complex laws pertaining to public companies could be a significant disadvantage in that it is likely that an increasing amount of their time may be devoted to these activities which will result in less time being devoted to the management and growth of the Combined Company. Holdco may not have adequate personnel with the appropriate level of knowledge, experience, and training in the accounting policies, practices or internal controls over financial reporting required of public companies in the United States. The development and implementation of the standards and controls necessary for the Combined Company to achieve the level of accounting standards required of a public company in the United States may require costs greater than expected. It is likely that the Combined Company will be required to expand its employee base and hire additional employees to support its operations as a public company, which will increase its operating costs in future periods.
There can be no assurance that the Holdco Ordinary Shares that will be issued in connection with the Business Combination or the Holdco Warrants will be approved for listing on Nasdaq or, if approved, will continue to be so listed following the closing of the Business Combination, or that Holdco will be able to comply with the continued listing standards of Nasdaq.
Holdco’s eligibility for listing may depend on, among other things, the number of shares of SPAC Common Stock that are redeemed. Holdco intends to apply for the listing of the Holdco Ordinary Shares and Holdco Warrants on Nasdaq. If Nasdaq denies its application for failure to meet the listing standards, or if Holdco elects to waive the condition for listing, Holdco and its shareholders could face significant material adverse consequences including:
• a limited availability of market quotations for its securities;
• reduced liquidity for its securities;
• a determination that Holdco Ordinary Shares are a “penny stock” which will require brokers trading in the Holdco Ordinary Shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for its securities;
• a limited amount of news and analyst coverage; and
• a decreased ability to issue additional securities or obtain additional financing in the future.
66
Table of Contents
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” If the Holdco Ordinary Shares and the public warrants of the Combined Company are listed on Nasdaq, they will be covered securities. Although the states are preempted from regulating the sale of the Combined Company’s securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. Certain state securities regulators view blank check companies unfavorably and might use these powers, or threaten to use these powers, to hinder the sale of securities of blank check companies in their states. Further, if the Combined Company was not listed on Nasdaq, its securities would not be covered securities and it would be subject to regulation in each state in which it offers its securities.
Because the Combined Company will become a publicly traded company by virtue of a merger as opposed to an underwritten initial public offering, the process doesn’t use the services of one or more underwriters which could result in less diligence being conducted.
In an underwritten initial public offering, underwriters typically conduct diligence on the company being taken public in order to establish a due diligence defense against liability claims under federal securities laws. Because LightJump is already a publicly-traded company, an underwriter has not been engaged. The Sponsor has an inherent conflict as their equity interests in LightJump are worthless absent a business combination. While private investors and management in a business combination undertake a certain level of due diligence, it is not necessarily the same level of due diligence undertaken by an underwriter in a traditional initial public offering and, therefore, there could be a heightened risk of an incorrect valuation of the business or material misstatements or omissions in this proxy statement/prospectus.
Additional Risk Factors Related to the Business Combination
LightJump has no operating or financial history and its results of operations and those of the Combined Company may differ significantly from the unaudited pro forma financial data included in this proxy statement.
LightJump has no operating history and no revenues. This proxy statement/prospectus includes unaudited pro forma condensed combined financial statements for the Combined Company. The unaudited pro forma condensed combined statement of operations of the Combined Company combines the historical audited results of operations of LightJump for the twelve-month period ended June 30, 2022, using its Consolidated Statements of Operations for the six-month period ended June 30, 2022, added with its Consolidated Statements of Operations for the fiscal year ended December 31, 2021, net of its Consolidated Statements of Operations for the six-month period ended June 30, 2021, with the historical audited results of operations of Moolec for the year ended June 30, 2022, and gives pro forma effect to the Business Combination as if it had been consummated on July 1, 2021. The unaudited pro forma condensed combined balance sheet of the Combined Company combines the historical balance sheets of LightJump as of June 30, 2022 and of Moolec as of June 30, 2022, and gives pro forma effect to the Business Combination as if it had been consummated on June 30, 2022.
The unaudited pro forma condensed combined financial statements are presented for illustrative purposes only, are based on certain assumptions, address a hypothetical situation and reflect limited historical financial data. Therefore, the unaudited pro forma condensed combined financial statements are not necessarily indicative of the results of operations and financial position that would have been achieved had the Business Combination been consummated on the dates indicated above, or the future consolidated results of operations or financial position of the Combined Company. Accordingly, the Combined Company’s business, assets, cash flows, results of operations and financial condition may differ significantly from those indicated by the unaudited pro forma condensed combined financial statements included in this document. For more information, please see the section entitled “Unaudited Pro Forma Condensed Combined Financial Information.”
A market for Holdco’s securities may not continue, which would adversely affect the liquidity and price of its securities.
Following the Business Combination, the price of Holdco’s securities may fluctuate significantly due to the market’s reaction to the Business Combination and general market and economic conditions. An active trading market for Holdco’s securities following the Business Combination may never develop or, if developed, it may not be sustained. In addition, the price of Holdco’s securities after the Business Combination can vary due to
67
Table of Contents
general economic conditions and forecasts, its general business condition and the release of its financial reports. Additionally, if its securities are not listed on, or become delisted from, Nasdaq for any reason, and are quoted on the OTC Bulletin Board, an inter-dealer automated quotation system for equity securities that is not a national securities exchange, the liquidity and price of its securities may be more limited than if it were quoted or listed on Nasdaq or another national securities exchange. You may be unable to sell your securities unless a market can be established or sustained.
If, following the Business Combination, securities or industry analysts do not publish or cease publishing research or reports about the Combined Company, its business, or its market, or if they change their recommendations regarding Holdco Ordinary Shares adversely, then the price and trading volume of Holdco Ordinary Shares could decline.
The trading market for Holdco Ordinary Shares will be influenced by the research and reports that industry or securities analysts may publish about the Combined Company, its business, its market, or its competitors. Securities and industry analysts do not currently, and may never, publish research on LightJump or the Combined Company. If no securities or industry analysts commence coverage of the Combined Company, Holdco Ordinary Share price and trading volume would likely be negatively impacted. If any of the analysts who may cover the Combined Company change their recommendation regarding Holdco Ordinary Shares adversely, or provide more favorable relative recommendations about Holdco’s competitors, the price of Holdco Ordinary Shares would likely decline. If any analyst who may cover LightJump were to cease coverage of the Combined Company or fail to regularly publish reports on it, Holdco could lose visibility in the financial markets, which could cause Holdco Ordinary Share price or trading volume to decline.
The JOBS Act permits “emerging growth companies” like Holdco to take advantage of certain exemptions from various reporting requirements applicable to other public companies that are not emerging growth companies.
Holdco currently qualifies as an “emerging growth company” as defined in Section 2(a)(19) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012, which we refer to as the “JOBS Act.” As such, Holdco takes advantage of certain exemptions from various reporting requirements applicable to other public companies that are not emerging growth companies for as long as it continues to be an emerging growth company, including the exemption from the auditor attestation requirements with respect to internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act. As a result, Holdco shareholders may not have access to certain information they deem important. Holdco expects to remain an emerging growth company for the foreseeable future.
Holdco cannot predict if investors will find Holdco Ordinary Shares less attractive because it relies on these exemptions. If some investors find Holdco Ordinary Shares less attractive as a result, there may be a less active trading market and share price for Holdco Ordinary Shares may be more volatile. When Holdco no longer qualifies as an emerging growth company Holdco may incur increased legal, accounting and compliance costs associated with Section 404 of the Sarbanes-Oxley Act.
The resignation of Nomura as Moolec’s exclusive financial advisor may indicate that it is unwilling to be associated with the disclosure in this proxy statement/prospectus and the underlying business analysis related to the transaction.
On June 29, 2022, Nomura Securities International, Inc. (“Nomura”) resigned from its role as exclusive financial advisor to Moolec, effective as of April 27, 2022, and waived its entitlement to the payment of any fees or expense reimbursements. On March 30, 2022, prior to the resignation of Nomura, the SEC issued proposed rules (the “SPAC Rule Proposals”) relating to, among other items, disclosures in SEC filings and the treatment of investment banks in connection with business combination transactions involving special purpose acquisition companies. The uncertainty related to the treatment of investment banks following the SPAC Rule Proposals was the reason Nomura gave for its resignation. Nomura did not have a role in the negotiation of any materials related to or arising out of the Transactions and had no role in any subsequent preparation of the disclosure in this proxy statement/prospectus. Neither Moolec or LightJump relied on Nomura in the preparation and analysis of materials related to the Transactions and the Business Combination.
We note that Nomura was provided with the disclosures in this proxy statement/prospectus pertaining to its roles and resignation, however we did not receive a response from Nomura whether they agree with either the risks or the conclusions stated herein that are associated with its role and resignation. While Nomura did not provide
68
Table of Contents
any additional detail in its resignation letters either to Moolec or to the SEC, such resignation may be an indication by Nomura that it does not want to be associated with the disclosure in this proxy statement/prospectus, including any discussion related to reasons for its withdrawal, or the underlying business analysis related to the transaction. Shareholders and investors should not place any reliance on the fact that Nomura has been previously involved with this transaction solely as it was engaged as the exclusive financial advisor to Moolec. Please see the section entitled “The Business Combination — The Background of the Business Combination” for a discussion regarding the resignation of Nomura.
Risks Related to Investment in a Luxembourg Company and Holdco’s Status as a Foreign Private Issuer
As a foreign private issuer, Holdco will be exempt from a number of U.S. securities laws and rules promulgated thereunder and will be permitted to publicly disclose less information than U.S. public companies must. This may limit the information available to holders of the Holdco Ordinary Shares.
Holdco will qualify as a “foreign private issuer,” as defined in the SEC’s rules and regulations, and, consequently, Holdco will not be subject to all of the disclosure requirements applicable to public companies organized within the United States. For example, Holdco will be exempt from certain rules under the Exchange Act that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act. In addition, Holdco’s officers and directors are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of Holdco’s securities. For example, some of Holdco’s key executives may sell a significant amount of Holdco Ordinary Shares and such sales will not be required to be disclosed as promptly as public companies organized within the United States would have to disclose. Accordingly, once such sales are eventually disclosed, the price of Holdco Ordinary Shares may decline significantly. Moreover, Holdco will not be required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. public companies. Holdco will also not be subject to Regulation FD under the Exchange Act, which would prohibit Holdco from selectively disclosing material nonpublic information to certain persons without concurrently making a widespread public disclosure of such information. Accordingly, there may be less publicly available information concerning Holdco than there is for U.S. public companies.
As a foreign private issuer, Holdco will file an annual report on Form 20-F within four months of the close of each fiscal year ended June 30 and furnish reports on Form 6-K relating to certain material events promptly after Holdco publicly announces these events. However, because of the above exemptions for foreign private issuers, which Holdco intends to rely on, Holdco shareholders will not be afforded the same information generally available to investors holding shares in public companies that are not foreign private issuers.
Holdco may lose its foreign private issuer status in the future, which could result in significant additional costs and expenses. This would subject Holdco to GAAP reporting requirements which may be difficult for it to comply with.
As a “foreign private issuer,” Holdco would not be required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act and related rules and regulations. Under those rules, the determination of foreign private issuer status is made annually on the last Business Day of an issuer’s most recently completed second fiscal quarter, and, accordingly, the next determination will be made with respect to Holdco on December 31, 2022.
In the future, Holdco could lose its foreign private issuer status if a majority of its ordinary shares are held by residents in the United States and it fails to meet any one of the additional “business contacts” requirements. Although Holdco intends to follow certain practices that are consistent with U.S. regulatory provisions applicable to U.S. companies, Holdco’s loss of foreign private issuer status would make such provisions mandatory. The regulatory and compliance costs to Holdco under U.S. securities laws if it is deemed a U.S. domestic issuer may be significantly higher. If Holdco is not a foreign private issuer, Holdco will be required to file periodic reports and prospectuses on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. For example, Holdco would become subject to the Regulation FD, aimed at preventing issuers from making selective disclosures of material information. Holdco also may be required to modify certain of its policies to comply with good governance practices associated with U.S. domestic issuers. Such conversion and modifications will involve additional costs.
69
Table of Contents
In addition, Holdco may lose its ability to rely upon exemptions from certain corporate governance requirements of Nasdaq that are available to foreign private issuers. For example, Nasdaq’s corporate governance rules require listed companies to have, among other things, a majority of independent board members and independent director oversight of executive compensation, nomination of directors, and corporate governance matters. As a foreign private issuer, Holdco would be permitted to follow home country practice in lieu of the above requirements. As long as Holdco relies on the foreign private issuer exemption to certain of Nasdaq’s corporate governance standards, a majority of the directors on its board of directors are not required to be independent directors, its compensation committee is not required to be comprised entirely of independent directors, and it will not be required to have a nominating committee. Also, Holdco would be required to change its basis of accounting from IFRS as issued by the IASB to GAAP, which may be difficult and costly for it to comply with. If Holdco loses its foreign private issuer status and fails to comply with U.S. securities laws applicable to U.S. domestic issuers, Holdco may have to de-list from Nasdaq and could be subject to investigation by the SEC, Nasdaq and other regulators, among other materially adverse consequences.
Holdco is organized under the laws of the Grand Duchy of Luxembourg and a substantial amount of its assets are not located in the United States. It may be difficult for you to obtain or enforce judgments or bring original actions against Holdco or the members of its board of directors in the United States.
Holdco is incorporated under the laws of the Grand Duchy of Luxembourg. In addition, a substantial amount of its assets are located outside the United States. Furthermore, some of the members of Holdco’s board of directors and officers reside outside the United States and a substantial portion of Holdco’s assets are located outside the United States. Investors may not be able to effect service of process within the United States upon Holdco or these persons or enforce judgments obtained against Holdco or these persons in U.S. courts, including judgments in actions predicated upon the civil liability provisions of the U.S. federal securities laws. Likewise, it also may be difficult for an investor to enforce in U.S. courts judgments obtained against Holdco or these persons in courts located in jurisdictions outside the United States, including judgments predicated upon the civil liability provisions of the U.S. federal securities laws. Awards of punitive damages in actions brought in the United States or elsewhere are generally not enforceable in the Grand Duchy of Luxembourg.
As there is no treaty in force on the reciprocal recognition and enforcement of judgments in civil and commercial matters between the United States and the Grand Duchy of Luxembourg, courts in the Grand Duchy of Luxembourg will not automatically recognize and enforce a final judgment rendered by a U.S. court. However, a party who received such favorable judgment in a U.S. Court may initiate enforcement proceedings in the Grand Duchy of Luxembourg (exequatur) by requesting enforcement of the U.S. judgment by the District Court (Tribunal d’Arrondissement) pursuant to Section 678 of the New Luxembourg Code of Civil Procedure. The District Court will authorize the enforcement in Luxembourg of the U.S. judgment if it is satisfied that all of the following conditions are met:
• the U.S. judgment is enforceable (exécutoire) in the United States;
• the U.S. court awarding the judgment had jurisdiction to adjudicate the applicable matter under applicable U.S. federal or state jurisdictions rules, and the jurisdiction of the U.S. court is recognized by Luxembourg private international and local law;
• the U.S. court has applied the substantive law as designated by the Grand Duchy of Luxembourg conflict of laws rules according to certain Luxembourg case law, it is admitted that the Grand Duchy of Luxembourg courts which are asked to grant an exequatur do not have to verify whether the substantive law actually applied by the U.S. court awarding the judgment was the law which would have been applied;
• the U.S. judgment does not contravene international public policy or order as understood under the laws of the Grand Duchy of Luxembourg;
• the U.S. court has acted in accordance with its own procedural rules and laws;
• the U.S. judgment was granted following proceedings where the counterparty had the opportunity to appear, and if it appeared, to present a defense; and
• the U.S. judgment was not granted pursuant to an evasion of Grand Duchy of Luxembourg law (fraude à la loi luxembourgeoise).
70
Table of Contents
Please note that the Grand Duchy of Luxembourg case law is constantly evolving. Some of the conditions of admissibility described above may change, and additional conditions could be required to be fulfilled by the Grand Duchy of Luxembourg courts while other conditions may not be required by Luxembourg courts in the future.
Subject to the conditions described above, courts of the Grandy Duchy of Luxembourg tend not to review the merits of a foreign judgment, although such a review is not statutorily prohibited.
If an original action is brought in the Grand Duchy of Luxembourg, the Grand Duchy of Luxembourg courts may refuse to apply the law designated and applied in the original action if (i) the choice of such law was not bona fide or if the foreign law was not pleaded or proved or if pleaded and proved, the foreign law was contrary to the Grand Duchy of Luxembourg mandatory provisions (lois impératives) or incompatible with the Grand Duchy of Luxembourg public policy rules, and (ii) its application is manifestly incompatible with the Grand Duchy of Luxembourg international policy rules. In an action brought in the Grand Duchy of Luxembourg on the basis of U.S. federal or state securities laws, the Grand Duchy of Luxembourg courts may not have the requisite power to grant the remedies sought. Also, an exequatur may be refused if it involves punitive damages.
Litigation in the Grand Duchy of Luxembourg also is subject to rules of procedure that differ from the U.S. rules, including, with respect to the taking and admissibility of evidence, the conduct of the proceedings and the allocation of costs. Proceedings in the Grand Duchy of Luxembourg would in principle have to be conducted in the French or German language, and all documents submitted to the court would, in principle, have to be translated into French or German. For these reasons, it may be difficult for a U.S. investor to bring an original action in a Grand Duchy of Luxembourg court predicated upon the civil liability provisions of the U.S. federal securities laws against Holdco, the members of its board of directors, its officers, or the experts named herein. In addition, even if a judgment against Holdco, the non-U.S. members of its board of directors, its officers, or the experts named in this proxy statement/prospectus based on the civil liability provisions of the U.S. federal securities laws is obtained, a U.S. investor may not be able to enforce it in U.S. or the Grand Duchy of Luxembourg courts.
Further, in the event of any proceedings being brought in the Grand Duchy of Luxembourg court in respect of a monetary obligation expressed to be payable in a currency other than the Euro, a Grand Duchy of Luxembourg court would have power to give judgment expressed as an order to pay a currency other than the Euro. However, enforcement of the judgment against any party in the Grand Duchy of Luxembourg would be available only in Euros and for such purposes all claims or debts would be converted into Euros.
The amended and restated articles of association of Holdco to be adopted in connection with the Business Combination contain specific indemnification provisions stating that every person who is, or has been, a member of the board of directors or officer (mandataire) of Holdco shall be indemnified by Holdco to the fullest extent permitted by Luxembourg law against liability and against all expenses reasonably incurred or paid by such director or officer in connection with any claim, action, suit or proceeding in which such director or officer becomes involved as a party or otherwise by virtue of his or her being or having been a director or officer and against amounts paid or incurred by him or her in the settlement thereof.
Luxembourg and European insolvency and bankruptcy laws are substantially different from U.S. insolvency and bankruptcy laws and may offer Holdco’s shareholders less protection than they would have under U.S. insolvency and bankruptcy laws.
As a company organized under the laws of the Grand Duchy of Luxembourg and with its registered office in the Grand Duchy of Luxembourg, Holdco is subject to the Grand Duchy of Luxembourg insolvency and bankruptcy laws in the event any insolvency proceedings are initiated against it including, among other things, Council and European Parliament Regulation (EU) 2015/848 of 20 May 2015 on insolvency proceedings (recast). Should courts in another European country determine that the insolvency and bankruptcy laws of that country apply to Holdco in accordance with and subject to such European Union (“EU”) regulations, the courts in that country could have jurisdiction over the insolvency proceedings initiated against Holdco. Insolvency and bankruptcy laws in the Grand Duchy of Luxembourg or the relevant other European country, if any, may offer Holdco’s shareholders less protection than they would have under U.S. insolvency and bankruptcy laws and make it more difficult for them to recover the amount they could expect to recover in a liquidation under U.S. insolvency and bankruptcy laws.
71
Table of Contents
The rights of Holdco’s shareholders may differ from the rights they would have as shareholders of a United States corporation, which could adversely impact trading in Holdco’s Ordinary Shares and its ability to conduct equity financings.
Holdco’s corporate affairs are governed by Holdco’s articles of association and the laws of the Grand Duchy of Luxembourg, including the Luxembourg Company Law (loi du 10 aoûit 1915 sur les societes commerciales, telle que modifiée). The rights of Holdco’s shareholders and the responsibilities of its directors and officers under Luxembourg law are different from those applicable to a corporation incorporated in the United States. For example, under Delaware law, the board of directors of a Delaware corporation bears the ultimate responsibility for managing the business and affairs of a corporation. In discharging this function, directors of a Delaware corporation owe fiduciary duties of care and loyalty to the corporation and its shareholders. Luxembourg law imposes a duty on directors of a Luxembourg company to: (i) act in good faith with a view to the best interests of a company; and (ii) exercise the care, diligence, and skill that a reasonably prudent person would exercise in a similar position and under comparable circumstances. Additionally, under Delaware law, a shareholder may bring a derivative action on behalf of a company to enforce a company’s rights. Under Luxembourg law, the board of directors has sole authority to decide whether to initiate legal action to enforce a company’s rights (other than, in certain circumstances, an action against members of Holdco’s board of directors, which may be initiated by the general meeting of the shareholders, or, subject to certain conditions, by minority shareholders holding together at least 10% of the voting rights in the company). See “Comparison of Stockholder Rights” for an additional explanation of the differences. Further, under Luxembourg law, there may be less publicly available information about Holdco than is regularly published by or about U.S. issuers. In addition, Luxembourg laws governing the securities of Luxembourg companies may not be as extensive as those in effect in the United States, and Luxembourg laws and regulations in respect of corporate governance matters might not be as protective of minority shareholders as are state corporation laws in the United States. Therefore, Holdco’s shareholders may have more difficulty in protecting their interests in connection with actions taken by Holdco’s directors, officers or principal shareholders than they would as shareholders of a corporation incorporated in the United States. As a result of these differences, Holdco’s shareholders may have more difficulty protecting their interests than they would as shareholders of a U.S. issuer.
Non-Luxembourg resident holders of Holdco Ordinary Shares could be subject to adverse Grand Duchy of Luxembourg income tax consequences.
The tax position of the holders of Holdco Ordinary Shares may vary according to their particular financial and tax situation. The tax structuring of Holdco and/or its investments may not be tax-efficient for a particular prospective holder of Holdco Ordinary Shares. No assurances can be given that amounts distributed or allocated to the holders of Holdco Ordinary Shares will have any particular characteristics or that any specific tax treatment will apply. Furthermore, no assurances can be given that any particular investment structure in which Holdco has a direct or indirect interest will be suitable for all holders of Holdco Ordinary Shares and, in certain circumstances, such structures may lead to additional costs or reporting obligations for some or all of the holders of Holdco Ordinary Shares.
Non-Luxembourg resident holders of Holdco Ordinary Shares that have neither a permanent establishment nor a permanent representative in the Grand Duchy of Luxembourg to which or whom the Holdco Ordinary Shares are attributable, are generally not subject to any income tax in the Grand Duchy of Luxembourg on gains realized upon the sale, repurchase or redemption of the Holdco Ordinary Shares.
Non-Luxembourg resident holders of Holdco Ordinary Shares will only be subject to the Grand Duchy of Luxembourg income tax on capital gains in the event they hold a substantial participation in Holdco (i.e. more than 10% of the issued shares of Holdco, either alone or together with certain close relatives, at any time during the five-year period preceding the disposition of Holdco Ordinary Shares) and (a) the disposition of Holdco Ordinary Shares (including liquidation) takes place within six months after acquisition or (b) in case of a disposition of Holdco Ordinary Shares after six months or more, such holder had been a Grand Duchy of Luxembourg resident taxpayer for more than fifteen years and has become a non-Luxembourg taxpayer less than five years before the disposition of Holdco Ordinary Shares occurs. Nevertheless, holders should consult their own tax advisors to determine which double tax treaties concluded by the Grand Duchy of Luxembourg, if any, apply in order to determine which state (residency state or the Grand Duchy of Luxembourg) has the right to tax any such capital gains.
72
Table of Contents
Risks Related to LightJump and the Business Combination
LightJump has identified a material weakness in its internal control over financial reporting. If LightJump is unable to develop and maintain an effective system of internal control over financial reporting, LightJump may not be able to accurately report its financial results in a timely manner, which may adversely affect investor confidence and materially and adversely affect its business.
On April 12, 2021, the Acting Director of the Division of Corporation Finance and Acting Chief Accountant of the SEC together issued a statement regarding the accounting and reporting considerations for warrants issued by special purpose acquisition companies entitled “Staff Statement on Accounting and Reporting Considerations for Warrants Issued by Special Purpose Acquisition Companies (“SPACs”) (the “SEC Statement”). Following the issuance of the SEC Statement and after LightJump consulted with management and its audit committee, LightJump concluded that, in light of the SEC Statement, it was appropriate to restate its previously issued audited balance sheet as of January 12, 2021 and its unaudited financial statements for the three months ended March 31, 2021 and for the six months ended June 30, 2021 to account for the Private Warrants as liabilities measured at fair value, rather than equity securities (the “Restatement”). See “— Certain of LightJump’s warrants are accounted for as liabilities and the changes in value of such warrants could have a material effect on its financial results.” As a result of these events, which led to the Restatement, LightJump identified a material weakness in its internal control over financial reporting.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of LightJump’s annual or interim financial statements will not be prevented, or detected and corrected on a timely basis. Effective internal controls are necessary for LightJump to provide reliable financial reports and prevent fraud. LightJump continues to evaluate steps to remediate the material weakness. If LightJump identifies any new material weakness in the future, any such newly identified material weakness could limit its ability to prevent or detect a misstatement of our accounts or disclosures that could result in a material misstatement of its annual or interim financial statements. In such case, LightJump may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports in addition to applicable stock exchange listing requirements, investors may lose confidence in its financial reporting and the price of LightJump’s securities may decline as a result. LightJump cannot assure you that the measures taken to date, or any measures that may be taken in the future, will be sufficient to avoid potential future material weaknesses.
Certain of LightJump’s warrants are accounted for as liabilities and the changes in value of such warrants could have a material effect on its financial results.
The SEC Statement focused on certain settlement terms and provisions related to certain tender offers following a business combination, which terms are similar to those contained in the warrant agreement governing LightJump’s Private Warrants. LightJump evaluated the accounting treatment of its 4,210,000 Private Warrants, and determined to classify such warrants as derivative liabilities measured at fair value, with changes in fair value each period reported in earnings. Due to the recurring fair value measurement, LightJump expects that it will recognize non-cash gains or losses on its Private Warrants each reporting period and that the amount of such gains or losses could be material.
LightJump’s accounting treatment of the 4,210,000 Private Warrants is based on its current interpretation of the SEC Statement and other guidance and may change in light of any further interpretive guidance, as may be applicable.
LightJump may not be able to complete its initial business combination or amend its charter prior to January 12, 2023, in which case LightJump would cease all operations except for the purpose of winding up and LightJump would redeem its public shares and liquidate. If this occurs, LightJump’s Public Stockholders may only receive $10.12 per share of SPAC Common Stock, or less than such amount in certain circumstances, and the SPAC Warrants will expire worthless.
The SPAC COI currently provides that LightJump must complete its initial business combination by January 12, 2023. LightJump may not be able to complete its initial business combination, or amend the SPAC COI to extend the time to consummate its initial business combination, within such time period. LightJump’s ability to
73
Table of Contents
complete its initial business combination may be negatively impacted by general market conditions, volatility in the capital and debt markets and the other risks described herein. If LightJump has not completed its initial business combination within such time period, it will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten Business Days thereafter, redeem the Public Shares at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account and not previously released to LightJump to pay taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding Public Shares which redemption will completely extinguish LightJump Holders’ rights as stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of LightJump’s remaining stockholders and board of directors, dissolve and liquidate, subject in each case to LightJump’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. In such case, the LightJump Holders may only receive $10.12 per public share of SPAC Common Stock, and the SPAC Warrants will expire worthless. In certain circumstances, the LightJump Holders may receive less than $10.12 per share on the redemption of their shares.
If a LightJump Holder wishing to redeem its SPAC Common Stock in connection with the Business Combination fails to comply with the procedures for tendering its shares, such shares may not be redeemed.
This proxy statement/prospectus describes the procedures that must be complied with in order for a LightJump Holder to validly redeem its SPAC Common Stock. In the event that a stockholder fails to comply with these procedures, its shares may not be redeemed.
The Sponsor and LightJump’s directors, officers, advisors or their affiliates may elect to purchase shares from LightJump Holders, which may influence a vote on the Business Combination and reduce the public “float” of SPAC Common Stock.
The Sponsor and LightJump’s directors, officers, advisors or their affiliates may purchase shares of SPAC Common Stock in privately negotiated transactions or in the open market prior to the completion of the Business Combination, although they are under no obligation to do so and they have no current plans to do so. Such a purchase may include a contractual acknowledgement that such stockholder, although still the record holder of such shares, is no longer the beneficial owner thereof and therefore agrees not to exercise its redemption rights. In the event that the Sponsor and LightJump’s directors, officers, advisors or their affiliates purchase shares in privately negotiated transactions from LightJump Holders who have already elected to exercise their redemption rights, such selling stockholders would be required to revoke their prior elections to redeem their shares. Any such transaction would be separately negotiated at the time of the transaction. The consideration for any such transaction would consist of cash and/or SPAC Common Stock owned by the Sponsor and/or LightJump’s directors, officers, advisors, or their affiliates. The purpose of such purchases could be to decrease the number of shareholders that have elected to exercise their redemption rights. This may result in the completion of the Business Combination that may not otherwise have been possible.
In addition, if such purchases are made, the public “float” of SPAC Common Stock and the number of beneficial holders of LightJump’s securities may be reduced, possibly making it difficult for Holdco to obtain the quotation, listing or trading of its securities on a national securities exchange.
LightJump Holders cannot be sure of the market value of the Holdco Ordinary Shares to be issued upon completion of the Business Combination.
The holders of shares of SPAC Common Stock issued and outstanding immediately prior to the Closing of the Business Combination (other than any redeemed shares) will receive one Holdco Share in exchange for each share of SPAC Common Stock held by them, rather than a number of shares with a particular fixed market value. The market value of SPAC Common Stock at the time of the Business Combination may vary significantly from its price on the date the Business Combination Agreement was executed, the date of the Registration Statement of which this proxy statement/prospectus is a part or the date on which LightJump Holders vote on the Business Combination. Because the exchange ratio of the shares will not be adjusted to reflect any changes in the market prices of SPAC Common Stock, the market value of the Holdco Ordinary Shares issued to LightJump Holders in the Business Combination and the SPAC Common Stock surrendered in the Business Combination may be higher or lower than the value of these shares on earlier dates. The entirety of the consideration to be received by LightJump Holders with respect
74
Table of Contents
to their shares of SPAC Common Stock will be Holdco Ordinary Shares. Following consummation of the Business Combination, the market price of Holdco’s securities may be influenced by many factors, some of which are beyond its control, including those described above and the following:
• changes in financial estimates by analysts;
• announcements by it or its competitors of significant contracts, productions, acquisitions or capital commitments;
• fluctuations in its quarterly financial results or the quarterly financial results of companies perceived to be similar to it;
• general economic conditions;
• changes in market valuations of similar companies;
• terrorist acts;
• changes in its capital structure, such as future issuances of securities or the incurrence of additional debt;
• future sales of Holdco Ordinary Shares;
• regulatory developments in the United States, foreign countries or both;
• litigation involving Holdco, its subsidiaries or its general industry; and
• additions or departures of key personnel.
In addition, it is possible that the Business Combination may not be completed until a period of time has passed after the LightJump special meeting of stockholders. As a result, the market value of SPAC Common Stock may vary significantly from the date of the special meeting to the date of the Closing of the Business Combination. You are urged to obtain up-to-date prices for SPAC Common Stock. There is no assurance that the Business Combination will be completed, that there will not be a delay in the completion of the Business Combination or that all or any of the anticipated benefits of the Business Combination will be obtained.
The Holdco Ordinary Shares to be received by LightJump’s Holders as a result of the Business Combination will have different rights from shares of SPAC Common Stock.
Following completion of the Business Combination, the LightJump Holders will no longer be stockholders of LightJump but will instead be shareholders of Holdco. There will be important differences between your current rights as a LightJump Holder and your rights as a Holdco shareholder. See “Comparison of Shareholder Rights” for a discussion of the different rights associated with the securities.
LightJump’s Sponsor, officers and directors have agreed to vote in favor of the Business Combination, regardless of how the other LightJump Holders vote.
LightJump’s Sponsor, officers and directors, who currently own approximately 54.4% of the outstanding shares of SPAC Common Stock, have agreed to vote their SPAC Common Stock in favor of the Business Combination. Accordingly, it is more likely that the necessary stockholder approval to complete the Business Combination will be received than would be the case if LightJump’s Sponsor, officers and directors had agreed to vote their shares in accordance with the majority of the votes cast by the public LightJump Holders.
The Sponsor and LightJump’s executive officers and directors have potential conflicts of interest in recommending that stockholders vote in favor of approval of the Business Combination Proposal and approval of the other proposals described in this proxy statement/prospectus.
• When you consider the recommendation of LightJump’s board of directors in favor of approval of Proposal 1 (Business Combination Proposal) you should keep in mind that certain of LightJump’s
75
Table of Contents
directors and officers have interests in the Business Combination that are different from, or in addition to, your interests as a stockholder. LightJump’s directors and executive officers have interests in the Business Combination that are different from, in addition to, or in conflict with, yours. These interests include:
• The Sponsor beneficially owns 3,450,000 Founder Shares, all of which are beneficially owned by our Chairman and Chief Executive Officer, and such shares would become worthless if LightJump does not complete a business combination within the applicable time period, as such LightJump Initial Stockholders have waived any right to liquidation proceeds with respect to these shares. The Sponsor paid an aggregate of $25,000 (or $0.009 per share) for the 3,450,000 Founder Shares. Such shares have an aggregate market value of approximately $[•] based on the closing price of SPAC Common Stock of $[•] on Nasdaq on [•], the record date for the special meeting of stockholders.
• The Sponsor also beneficially owns 4,210,000 SPAC Warrants, for which it paid $4,210,000 and which will expire and be worthless if LightJump does not complete a business combination within the applicable time period.
• LightJump’s officers and directors have invested an aggregate of $4,235,000, which will be lost in the event that the Business Combination is not approved and concluded.
• LightJump’s directors will not receive reimbursement for the out-of-pocket expenses ($0.00 as of the date hereof) incurred by them on LightJump’s behalf incident to identifying, investigating and consummating a business combination, unless a business combination is consummated.
• The Sponsor and its affiliates can earn a positive rate of return on their investments, even if the Public Stockholders experience a negative rate of return on their investments in LightJump and Holdco, as the Sponsor has purchased 3,450,000 Founder Shares for an aggregate of $25,000 (or $0.009 per share).
• Certain of LightJump’s directors could potentially continue as directors of Holdco if the Business Combination is completed and could potentially receive the same compensation that Holdco pays to its other directors, if any.
• Because the Sponsor and the LightJump directors will benefit from the completion of a business combination, they may be incentivized to recommend and complete a business combination of a less favorable target company or on terms less favorable to LightJump Holders, rather than liquidate LightJump.
• LightJump would be unable to indemnify its current directors and officers or continue to provide directors’ and officers’ liability insurance if the Business Combination is not completed.
These interests may have influenced LightJump’s directors in approving the Business Combination and making their recommendation to vote in favor of the approval of the Business Combination Proposal. Please read the section entitled “The Business Combination — Interests of LightJump’s Directors and Officers in the Business Combination.”
The LightJump board of directors considered these interests when reviewing the proposed terms of the Business Combination and how they might impact the negotiations with Moolec but determined that the transaction was still in the best interests of LightJump and its stockholders. Nonetheless, these interests may influence LightJump’s directors in making their recommendation to vote in favor of Proposal 1 (Business Combination Proposal) and the other proposals described in the Registration Statement of which this proxy statement/prospectus is a part. You should also read the section entitled “The Business Combination.”
These interests may influence LightJump’s directors in making their recommendation to vote in favor of Proposal 1 (Business Combination Proposal) and the other proposals described in the Registration Statement of which this proxy statement/prospectus is a part. You should also read the section entitled “The Business Combination.”
76
Table of Contents
The exercise of discretion by LightJump’s directors and officers in agreeing to changes to the terms of or waivers of closing conditions in the Business Combination Agreement may result in a conflict of interest when determining whether such changes to the terms of the Business Combination Agreement or waivers of conditions are appropriate and in the best interests of LightJump securityholders.
In the period leading up to the Closing, events may occur that would require LightJump to agree to amend the Business Combination Agreement, to consent to certain actions or to waive one or more closing conditions. Such events could arise because of changes in the course of Moolec’s business, a request by Moolec to undertake actions that would otherwise be prohibited by the terms of the Business Combination Agreement or the occurrence of other events that would have a material adverse effect on Moolec’s business and would entitle LightJump to terminate the Business Combination Agreement. In any of such circumstances, it would be in LightJump’s discretion, acting through its board of directors, to grant LightJump’s consent or waive its rights. The existence of the financial and personal interests of the directors described elsewhere in this proxy statement/prospectus may result in a conflict of interest on the part of one or more of the directors between what he may believe is best for LightJump and its securityholders and what he may believe is best for himself or his affiliates in determining whether or not to take the requested action. As of the date of this proxy statement/prospectus, LightJump does not believe there will be any changes to the Business Combination Agreement after stockholder approval has been obtained. While certain changes could be made without further stockholder approval, if there is a change to the terms of the transaction that would have a material impact on the stockholders, LightJump will be required to circulate an amended proxy statement/prospectus or supplement thereto and resolicit the vote of its stockholders with respect to the Business Combination Proposal.
Subsequent to the completion of the Business Combination, Holdco may be required to take write-downs or write-offs, restructuring and impairment or other charges that could have a significant negative effect on Holdco’s financial condition, results of operations and stock price, which could cause you to lose some or all of your investment.
Although LightJump has conducted due diligence procedures on Moolec, we cannot assure you that our diligence surfaced all material issues that may be present inside Moolec or that it would be possible to uncover all material issues through a customary amount of due diligence. Moreover, even if LightJump’s due diligence accurately identified certain risks, unexpected risks may arise and previously known risks may materialize in a manner not consistent with LightJump’s preliminary risk analysis. As a result of these events, Holdco may be forced to write-down or write off assets, restructure its operations, or incur impairment or other charges that could result in Holdco reporting losses. Even though these charges may be non-cash items and would not have an immediate impact on Holdco’s liquidity, the fact that Holdco reports charges of this nature could contribute to negative market perceptions about Holdco or its securities. Accordingly, any stockholders who choose to remain stockholders following the Business Combination could suffer a reduction in the value of their shares. Such stockholders are unlikely to have a remedy for such reduction in value unless they are able to successfully claim that the reduction was due to the breach by LightJump’s officers or directors of a duty of care or other fiduciary duty owed to them, or if they are able to successfully bring a private claim under securities laws that the proxy statement/prospectus relating to the Business Combination contained an actionable material misstatement or material omission.
LightJump Holders’ ownership and voting interest in Holdco will be significantly reduced from their interest in LightJump, and the LightJump Holders will exercise little influence over management.
After the completion of the Business Combination, LightJump Holders will own a significantly smaller percentage of Holdco than they currently own of LightJump. Upon completion of the Business Combination, it is anticipated that the LightJump Holders will own approximately 13.5% of the issued and outstanding Holdco Ordinary Shares immediately after the consummation of the Business Combination, assuming that none of the existing LightJump Holders exercise their redemption rights. Consequently, LightJump Holders, as a group, will have reduced ownership and voting power in Holdco compared to their ownership and voting power in LightJump.
77
Table of Contents
LightJump may be subject to U.S. foreign investment regulations which may impose conditions on or limit certain investors’ ability to purchase LightJump securities, potentially making the securities less attractive to investors. LightJump’s future investments in U.S. companies may also be subject to U.S. foreign investment regulations.
The Committee on Foreign Investment in the U.S. (“CFIUS”) is an interagency committee authorized to review certain transactions involving acquisitions and investments in the U.S. by foreign persons in order to determine the effect of such transactions on the national security of the U.S. CFIUS has jurisdiction to review transactions that could result in control of a U.S. business directly or indirectly by a foreign person, certain non-controlling investments that afford the foreign investor non-passive rights in a “TID U.S. business” (defined as a U.S. business that (1) produces, designs, tests, manufactures, fabricates, or develops one or more critical technologies; (2) owns or operates certain critical infrastructure; or (3) collects or maintains directly or indirectly sensitive personal data of U.S. citizens), and certain acquisitions, leases, and concessions involving real estate even with no underlying U.S. business. Certain categories of acquisitions of and investments in a U.S. business also may be subject to a mandatory notification requirement.
If under such rules and regulations CFIUS finds that the Business Combination may affect national security, LightJump could be subject to such foreign ownership restrictions and/or CFIUS review. If the Business Combination with Moolec falls within the scope of foreign ownership restrictions, LightJump may be unable to consummate the Business Combination. In addition, if the Business Combination falls within CFIUS’s jurisdiction, LightJump may be required to make a mandatory filing or determine to submit a voluntary notice to CFIUS, or to proceed with the Business Combination without notifying CFIUS and risk CFIUS intervention, before or after closing of the Business Combination. Although LightJump does not believe it is a “foreign person” or a U.S. business that may affect national security, CFIUS may take a different view and decide to block or delay the Business Combination, impose conditions to mitigate national security concerns with respect to the Business Combination, order us to divest all or a portion of a U.S. business of the combined company if LightJump had proceeded without first obtaining CFIUS clearance, or impose penalties if CFIUS believes that the mandatory notification requirement applied. The foreign ownership limitations, and the potential impact of CFIUS, may prevent LightJump from consummating the Business Combination with Moolec.
Moreover, the process of government review, whether by CFIUS or otherwise, could be lengthy. Because LightJump has only a limited time to complete the Business Combination, LightJump’s failure to obtain any required approvals within the requisite time period may require LightJump to liquidate. If LightJump liquidates, LightJump’s Public Stockholders may only receive $10.12 per share of SPAC Common Stock, or less than such amount in certain circumstances, and the SPAC Warrants will expire worthless. This will also cause you to lose any potential investment opportunity in Moolec and the chance of realizing future gains on your investment through any price appreciation in the combined company.
In addition, CFIUS could choose to review past or proposed transactions involving new or existing foreign investors in LightJump or in the Sponsor, even if a filing with CFIUS is or was not required at the time of the Business Combination. Any review and approval of an investment or transaction by CFIUS may impact transaction certainty, timing, feasibility, and cost, among other things. CFIUS policies and practices are rapidly evolving, and in the event that CFIUS reviews one or more proposed or existing investment by investors, there can be no assurances that such investors will be able to maintain, or proceed with, such investments on terms acceptable to such investors. CFIUS could seek to impose limitations or restrictions on, or prohibit, investments by such investors (including, but not limited to, limits on purchasing LightJump’s stock, limits on information sharing with such investors, requiring a voting trust, governance modifications, or forced divestiture, among other things).
These restrictions on the ability of foreign persons to invest in LightJump could limit LightJump’s ability to engage in strategic transactions that could benefit LightJump’s stockholders, including a change of control, and could also affect the price that an investor may be willing to pay for LightJump’s shares or the securities after the Business Combination.
78
Table of Contents
U.S. Tax Risk Factors
There may be tax consequences of the Business Combination that may adversely affect holders of SPAC Common Stock or SPAC Warrants.
Subject to the limitations and qualifications described in “Material U.S. Federal Income Tax Considerations,” including the discussion on Section 367(a) of the Code, the exchange of SPAC Common Stock for Holdco Ordinary Shares pursuant to the Merger generally is intended to qualify as a tax-free exchange for U.S. federal income tax purposes. However, to the extent the Merger does not so qualify, it could result in the imposition of substantial taxes on SPAC Holders. In addition, as more fully described in the section titled “Material U.S. Federal Income Tax Considerations — U.S. Holders — The Business Combination,” the appropriate U.S. federal income tax treatment of SPAC Warrants in connection with the Merger is uncertain; as a result, the Merger may be a taxable transaction for U.S. federal income tax purposes to holders of SPAC Warrants.
The IRS may not agree that Holdco should be treated as a non-U.S. corporation for U.S. federal income tax purposes.
Under current U.S. federal income tax law, a corporation is generally considered for U.S. federal income tax purposes to be a tax resident in the jurisdiction of its organization or incorporation. Accordingly, under generally applicable U.S. federal income tax rules, Holdco, which is incorporated under and governed by the laws of Luxembourg, would be classified as a non-U.S. corporation (and, therefore, not a U.S. tax resident) for U.S. federal income tax purposes. Section 7874 of the Code, however, contains rules that may cause a non-U.S. corporation to, in certain circumstances, be treated as a U.S. corporation for U.S. federal income tax purposes. If Holdco were to be treated as a U.S. corporation for U.S. federal income tax purposes, it could be subject to substantial U.S. tax liability, in addition to tax liability in its country of residence, and the gross amount of any dividend payments to its Non-U.S. Holders could be subject to U.S. withholding tax.
As more fully described in the section titled “Material U.S. Federal Income Tax Considerations — Tax Residence of Holdco for U.S. Federal Income Tax Purposes,” Holdco is not currently expected to be treated as a U.S. corporation for U.S. federal income tax purposes. However, whether the requirements for such treatment have been satisfied must be finally determined at the completion of the Business Combination, by which time there could be adverse changes to the relevant facts and circumstances. Further, the rules for determining ownership under Section 7874 are complex, unclear and the subject of ongoing regulatory change. Accordingly, there can be no assurance that the IRS would not assert a contrary position to those described above or that such an assertion would not be sustained by a court in the event of litigation.
The IRS may take the position that Section 367(a) of the Code requires a U.S. Holder to recognize gain (but not loss) with respect to the exchange of shares of SPAC Common Stock for Holdco Ordinary Shares pursuant to the Merger.
Section 367(a) of the Code generally requires a U.S. holder of stock in a U.S. corporation to recognize gain (but not loss) when such stock is exchanged for stock of a non-U.S. corporation in an exchange that would otherwise qualify for nonrecognition treatment unless certain conditions are met. Although it is currently expected that these conditions will be met, U.S. Holders are cautioned that the potential application of Section 367(a) of the Code to the Merger is complex and depends on factors that cannot be determined until the closing of the Merger and the interpretation of legal authorities and facts relating to the Business Combination. Accordingly, there can be no assurance that the IRS will not take the position that Section 367(a) of the Code applies to cause U.S. Holders to recognize gain as a result of the Merger or that a court will not agree with such a position of the IRS in the event of litigation. U.S. Holders should consult with their own tax advisors regarding the potential application of Section 367(a) of the Code in their particular situation.
79
Table of Contents
If Holdco were a passive foreign investment company for U.S. federal income tax purposes for any taxable year, U.S. Holders of Holdco Ordinary Shares could be subject to adverse U.S. federal income tax consequences.
If Holdco is or becomes a “passive foreign investment company,” or a PFIC, within the meaning of Section 1297 of the Code for any taxable year during which a U.S. Holder holds Holdco Ordinary Shares, certain adverse U.S. federal income tax consequences may apply to such U.S. Holder. PFIC status depends on the composition of a company’s income and assets and the fair market value of its assets from time to time, as well as on the application of complex statutory and regulatory rules that are subject to potentially varying or changing interpretations. Holdco has not made a determination as to whether it currently is, or in the future may become, a PFIC, but there is a possibility that it may be classified as a PFIC for its taxable year that includes the date of the Merger or in the foreseeable future. There can be no assurance that Holdco will not be treated as a PFIC for any taxable year.
If Holdco were treated as a PFIC, a U.S. Holder of Holdco Ordinary Shares may be subject to adverse U.S. federal income tax consequences, such as taxation at the highest marginal ordinary income tax rates on capital gains and on certain actual or deemed distributions, interest charges on certain taxes treated as deferred, and additional reporting requirements. Certain elections (including a qualified electing fund (“QEF”) or a mark-to-market election) may be available to U.S. Holders of Holdco Ordinary Shares to mitigate some of the adverse tax consequences resulting from PFIC treatment, but U.S. Holders will not be able to make similar elections with respect to the Holdco Warrants. There is no assurance that Holdco will provide the information necessary for a U.S. Holder to make a QEF election with respect to the U.S. Holder’s Holdco Ordinary Shares.
80
Table of Contents
UNAUDITED PRO FORMA COMBINED FINANCIAL INFORMATION
Introduction
LightJump is providing the following unaudited pro forma condensed combined financial information to aid you in your analysis of the financial aspects of the Transactions.
The following unaudited pro forma condensed combined statements of financial position as of June 30, 2022, combines the historical unaudited consolidated balance sheet of LightJump as of June 30, 2022, with the historical audited consolidated balance sheet of Moolec as of June 30, 2022, giving pro forma effect to the Business Combination, as if it had occurred as of June 30, 2022.
The following unaudited pro forma condensed combined statements of operations for the year ended June 30, 2022, combine the historical statement of operations of LightJump for the twelve-month period ended June 30, 2022 using its unaudited consolidated statements of operations for the six-month period ended June 30, 2022, added with its audited consolidated statements of operations for the fiscal year ended December 31, 2021, net of its unaudited consolidated statements of operations for the six-month period ended June 30, 2021, and the historical audited consolidated statements of operations of Moolec for year ended June 30, 2022, giving pro forma effect to the Business Combination as if it had occurred on July 1, 2021, the beginning of the earliest period presented. For more information about the Business Combination see the section titled “The Business Combination.”
The unaudited pro forma condensed combined statement of financial position as of June 30, 2022 has been derived from:
• the historical unaudited financial statements of LightJump as of June 30, 2022 and the related notes thereto included elsewhere in this proxy statement/prospectus; and
• the historical audited consolidated financial statements of Moolec as of June 30, 2022 and the related notes thereto included elsewhere in this proxy statement/prospectus.
The unaudited pro forma condensed combined statements of operations have been derived from:
• the historical audited financial statements of LightJump for the year ended December 31, 2021 and the related notes thereto included elsewhere in this proxy statement/prospectus;
• the historical unaudited financial statements of LightJump as of and for the six months ended June 30, 2022 and 2021 and the related notes thereto included elsewhere in this proxy statement/prospectus; and
• the historical audited consolidated financial statements of Moolec as of and for the year ended June 30, 2022 and the related notes thereto included elsewhere in this proxy statement/prospectus.
The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X as in effect on the date of this proxy statement/prospectus which incorporates requirements to depict the accounting for the transaction (“Transaction Accounting Adjustments”). Moolec and LightJump have elected not to present any estimates related to potential synergies and other transaction effects that are reasonably expected to occur or have already occurred and will only be presenting Transaction Accounting Adjustments in unaudited pro forma condensed combined financial information.
This information should be read together with the consolidated financial statements of Moolec and its related notes and LightJump’s financial statements and related notes, “Moolec Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “LightJump Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other financial information included elsewhere in this proxy statement/prospectus.
81
Table of Contents
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENTS OF OPERATIONS
AS OF JUNE 30, 2022
(In thousands of United States Dollars)
| | For the period ended on
June 30, 2022 | | Scenario 1 –
Assuming no
redemptions | | Scenario 2 –
Assuming 50%
of redemptions | | Scenario 3 –
Assuming 100%
of redemptions |
| | | Moolec
(Historical) | | LightJump
(Historical)(1)(2) | | Transaction Accounting Adjustments | | | | Pro Forma Combined | | Transaction Accounting Adjustments | | | | Pro Forma Combined | | Transaction Accounting Adjustments | | | | Pro Forma Combined |
Continuing operations | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development expense | | (985 | ) | | — | | | — | | | | | | (985 | ) | | — | | | | | | (985 | ) | | — | | | | | | (985 | ) |
Sales and marketing expense | | (105 | ) | | — | | | — | | | | | | (105 | ) | | — | | | | | | (105 | ) | | — | | | | | | (105 | ) |
Administrative expense | | (2,523 | ) | | — | | | (1,531 | ) | | (2 | ) | | (4,054 | ) | | (1,531 | ) | | (2 | ) | | (4,054 | ) | | (1,531 | ) | | (2 | ) | | (4,054 | ) |
Other operating expense | | (39 | ) | | (2,653 | ) | | (44,836 | ) | | (1 | ) | | (47,528 | ) | | (43,080 | ) | | (1 | ) | | (45,772 | ) | | (40,744 | ) | | (1 | ) | | (43,436 | ) |
Loss from operations | | (3,652 | ) | | (2,653 | ) | | (46,367 | ) | | | | | (52,672 | ) | | (44,611 | ) | | | | | (50,916 | ) | | (42,275 | ) | | | | | (48,580 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Finance results | | (875 | ) | | 7,784 | | | 860 | | | (3 | ) | | 7,575 | | | 860 | | | (3 | ) | | 7,575 | | | 860 | | | (3 | ) | | 7,575 | |
| | | | | | | | | (194 | ) | | (4 | ) | | | | | (194 | ) | | (4 | ) | | | | | (194 | ) | | (4 | ) | | | |
Profit/(Loss) before
Income tax | | (4,527 | ) | | 5,131 | | | (45,701 | ) | | | | | (45,097 | ) | | (43,945 | ) | | | | | (43,341 | ) | | (41,609 | ) | | | | | (41,005 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income tax | | — | | | (4 | ) | | — | | | | | | (4 | ) | | — | | | | | | (4 | ) | | — | | | | | | (4 | ) |
Total profit/(loss) for the period | | (4,527 | ) | | 5,127 | | | (45,701 | ) | | | | | (45,101 | ) | | (43,945 | ) | | | | | (43,345 | ) | | (41,609 | ) | | | | | (41,009 | ) |
Basic and diluted profit/(loss) per share | | (0.09 | ) | | 1.44 | | | | | | | | | (1.15 | ) | | | | | | | | (1.14 | ) | | | | | | | | (1.09 | ) |
82
Table of Contents
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENTS OF FINANCIAL POSITION
AS OF JUNE 30, 2022
(In thousands of United States Dollars)
| | As of June 30, 2022 | | Scenario 1 –
Assuming no
redemptions | | Scenario 2 –
Assuming 50% of
redemptions | | Scenario 3 –
Assuming 100% of redemptions |
| | | Moolec (Historical) | | Light Jump
(Historical)(1) | | Transaction Accounting Adjustments | | | | Pro Forma Combined | | Transaction Accounting Adjustments | | | | Pro Forma Combined | | Transaction Accounting Adjustments | | | | Pro Forma Combined |
ASSETS | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Non-current assets | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Intangible Assets | | 4,599 | | | — | | | — | | | | | | 4,599 | | | — | | | | | | 4,599 | | | — | | | | | | 4,599 | |
Investment held in Trust Account | | — | | | 138,201 | | | (110,507 | ) | | (1 | ) | | — | | | (124,354 | ) | | (1 | ) | | — | | | (138,201 | ) | | (1 | ) | | — | |
| | | | | | | | | (27,694 | ) | | (1 | ) | | | | | (13,847 | ) | | (1 | ) | | | | | — | | | (1 | ) | | | |
Fixed Assets | | 9 | | | — | | | — | | | | | | 9 | | | — | | | | | | 9 | | | — | | | | | | 9 | |
Total non-current assets | | 4,608 | | | 138,201 | | | (138,201 | ) | | | | | 4,608 | | | (138,201 | ) | | | | | 4,608 | | | (138,201 | ) | | | | | 4,608 | |
Current assets | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | 1,082 | | | 5 | | | 27,694 | | | (1 | ) | | 17,074 | | | 13,847 | | | (1 | ) | | 5,590 | | | — | | | (1 | ) | | 4,510 | |
| | | | | | | | | — | | | (2 | ) | | | | | — | | | (2 | ) | | | | | 10,000 | | | (2 | ) | | | |
| | | | | | | | | (11,707 | ) | | (3 | ) | | | | | (9,344 | ) | | (3 | ) | | | | | (6,577 | ) | | (3 | ) | | | |
Prepayments | | 2 | | | 222 | | | 500 | | | (3 | ) | | 724 | | | 500 | | | (3 | ) | | 724 | | | 500 | | | (3 | ) | | 724 | |
Total current assets | | 1,084 | | | 227 | | | 16,487 | | | | | | 17,798 | | | 5,003 | | | | | | 6,314 | | | 3,923 | | | | | | 5,234 | |
TOTAL ASSETS | | 5,692 | | | 138,428 | | | (121,714 | ) | | | | | 22,406 | | | (133,198 | ) | | | | | 10,922 | | | (134,278 | ) | | | | | 9,842 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
LIABILITIES AND EQUITY | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Equity | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Share capital | | 638 | | | — | | | (634 | ) | | (5 | ) | | 4 | | | (634 | ) | | (5 | ) | | 4 | | | (634 | ) | | (5 | ) | | 4 | |
Share premium | | 6,962 | | | — | | | 27,947 | | | (2 | ) | | 74,007 | | | 13,973 | | | (2 | ) | | 60,639 | | | 10,000 | | | (2 | ) | | 57,097 | |
| | | | | | | | | 34,158 | | | (4 | ) | | | | | 34,764 | | | (4 | ) | | | | | 35,195 | | | (4 | ) | | | |
| | | | | | | | | 3,749 | | | (5 | ) | | | | | 3,749 | | | (5 | ) | | | | | 3,749 | | | (5 | ) | | | |
| | | | | | | | | 1,191 | | | (6 | ) | | | | | 1,191 | | | (6 | ) | | | | | 1,191 | | | (6 | ) | | | |
Equity settled share based payment | | 839 | | | — | | | (364 | ) | | (5 | ) | | 475 | | | (364 | ) | | (5 | ) | | 475 | | | (364 | ) | | (5 | ) | | 475 | |
Accumulated deficit | | (6,834 | ) | | (3,357 | ) | | (178 | ) | | (1 | ) | | (53,546 | ) | | (189 | ) | | (1 | ) | | (51,662 | ) | | (201 | ) | | (1 | ) | | (49,200 | ) |
| | | | | | | | | (277 | ) | | (2 | ) | | | | | (138 | ) | | (2 | ) | | | | | — | | | (2 | ) | | | |
| | | | | | | | | (1,531 | ) | | (3 | ) | | | | | (1,531 | ) | | (3 | ) | | | | | (1,531 | ) | | (3 | ) | | | |
| | | | | | | | | (44,836 | ) | | (4 | ) | | | | | (43,080 | ) | | (4 | ) | | | | | (40,744 | ) | | (4 | ) | | | |
| | | | | | | | | 3,357 | | | (4 | ) | | | | | 3,357 | | | (4 | ) | | | | | 3,357 | | | (4 | ) | | | |
| | | | | | | | | 110 | | | (5 | ) | | | | | 110 | | | (5 | ) | | | | | 110 | | | (5 | ) | | | |
Total equity | | 1,605 | | | (3,357 | ) | | 22,692 | | | | | | 20,940 | | | 11,208 | | | | | | 9,456 | | | 10,128 | | | | | | 8,376 | |
Liabilities | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Current liabilities | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Accounts payable | | 1,226 | | | 1,969 | | | (2,355 | ) | | (3 | ) | | 840 | | | (2,355 | ) | | (3 | ) | | 840 | | | (2,355 | ) | | (3 | ) | | 840 | |
Other current liabilities | | 1 | | | 300 | | | (300 | ) | | (6 | ) | | 1 | | | (300 | ) | | (6 | ) | | 1 | | | (300 | ) | | (6 | ) | | 1 | |
Income tax payable | | — | | | 4 | | | — | | | | | | 4 | | | — | | | | | | 4 | | | — | | | | | | 4 | |
Promissory note – related
party | | — | | | 891 | | | (891 | ) | | (6 | ) | | — | | | (891 | ) | | (6 | ) | | — | | | (891 | ) | | (6 | ) | | — | |
Simply Agreement for Future Equity (“SAFE”). | | 2,860 | | | — | | | (2,860 | ) | | (5 | ) | | — | | | (2,860 | ) | | (5 | ) | | — | | | (2,860 | ) | | (5 | ) | | — | |
Total current liabilities | | 4,087 | | | 3,164 | | | (6,406 | ) | | | | | 845 | | | (6,406 | ) | | | | | 845 | | | (6,406 | ) | | | | | 845 | |
Non-Current liabilities | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Common stock subject to possible redemption | | — | | | 138,000 | | | (110,329 | ) | | (1 | ) | | — | | | (124,165 | ) | | (1 | ) | | — | | | (138,000 | ) | | (1 | ) | | — | |
| | | | | | | | | (27,671 | ) | | (2 | ) | | | | | (13,835 | ) | | (2 | ) | | | | | — | | | (2 | ) | | | |
Warrant liabilities | | — | | | 621 | | | — | | | | | | 621 | | | — | | | | | | 621 | | | — | | | | | | 621 | |
Total Non-Current
liabilities | | — | | | 138,621 | | | (138,000 | ) | | | | | 621 | | | (138,000 | ) | | | | | 621 | | | (138,000 | ) | | | | | 621 | |
TOTAL LIABILITIES | | 4,087 | | | 141,785 | | | (144,406 | ) | | | | | 1,466 | | | (144,406 | ) | | | | | 1,466 | | | (144,406 | ) | | | | | 1,466 | |
TOTAL LIABILITIES AND EQUITY | | 5,692 | | | 138,428 | | | (121,714 | ) | | | | | 22,406 | | | (133,198 | ) | | | | | 10,922 | | | (134,278 | ) | | | | | 9,842 | |
See accompanying notes to the unaudited pro forma condensed combined financial information.
83
Table of Contents
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Description of Business Combination
On June 14, 2022, LightJump, Moolec, Merger Sub and Holdco entered into the Business Combination Agreement. Pursuant to the Business Combination Agreement, each of the following transactions occurred, or will occur, in the following order:
• At the Exchange Effective Time, subject to the receipt of the Company Requisite Approvals, the Holdco Requisite Approvals and the delivery of the Exchange Auditor Report, and in accordance with the Holdco Delegate Resolutions:
• all issued Company Ordinary Shares held by the Company Shareholders shall be transferred and for the purposes of the 1915 Law, contributed in kind to Holdco, free and clear of all Liens (other than the Company Shareholders’ Agreements Liens that will expire on or prior to the Closing Date), and the Company Shareholders shall subscribe for and, as consideration for the contribution, shall be issued, in accordance with the Exchange Ratio (save that the Holdco Ordinary Shares to be issued shall be reduced by the number of Holdco Ordinary Shares already held by the Company Shareholders immediately prior to the Exchange), the Exchange Issuance;
• the Exchange Issuance shall be allocated among the Company Shareholders in accordance with the terms of the Payment Spreadsheet and Exchange Agreements;
• each Company SAFE Holder shall have contributed all of its rights and obligations under each Original SAFE to Holdco in consideration for the issuance by Holdco of a simple agreement for future equity on substantively identical terms (mutatis mutandis) with such adjustments (if any) required under Luxembourg law. For Luxembourg law purposes, a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued a report on the contributions in kind relating to the contribution of the Original SAFEs prepared in accordance with article 420-10 of the 1915 Law;
• each Company Shareholder shall cease to be the holder of such Company Ordinary Shares, subject to the submission of all filings required under Law (including any filings required to pay stamp duties), and Holdco will be recorded as the registered holder of all the Company Ordinary Shares so exchanged and transferred and will be the legal and beneficial owner thereof.
• Immediately prior to the Merger Effective Time but after the Exchange Effective Time, each Company SAFE Holder shall receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the respective Original SAFE, with such adjustments (if any) required under Luxembourg law;
• SPAC shall cause the Certificate of Merger to be executed, acknowledged and filed with the Secretary of State of the State of Delaware in accordance with the applicable provisions of the DGCL in order to effectuate the Merger. The Merger shall become effective at such time as the Certificate of Merger has been duly filed with the Secretary of State of the State of Delaware or at such later time as may be agreed by the Company and SPAC in writing and specified in the Certificate of Merger in accordance with the DGCL;
• At the Merger Effective Time, by virtue of the Merger and the Holdco Requisite Approvals, subject to the Merger Auditor Report, and without any further action on the part of SPAC, Merger Sub, Holdco or the Company or the holders thereunder:
• each SPAC Common Stock issued and outstanding immediately prior to the Merger Effective Time, excluding those have been redeemed subject to any redemption rights, shall be exchanged with Holdco (which exchange, for purposes of the 1915 Law, shall include, for the avoidance of doubt, a contribution-in-kind of each such shares of SPAC Common Stock from the holders of SPAC Common Stock to Holdco), against the issue by Holdco of new Holdco Ordinary Shares (such issuance, the “Merger Issuance”), under the authorized share capital of Holdco (pursuant to the Holdco Delegate Merger Resolutions) and subscribed by the contributing holders of SPAC Common Stock by virtue of the Merger and in accordance with the 1915 Law for one validly issued and fully paid Holdco Ordinary Share (the “Merger Consideration”), delivered by Holdco;
84
Table of Contents
• as a result of the Merger, all SPAC Common Stock shall cease to be outstanding, shall be cancelled and shall cease to exist;
• each share of common stock, par value $0.01, of Merger Sub issued and outstanding immediately prior to the Merger Effective Time shall be converted and exchanged for one (1) validly issued, fully paid and nonassessable ordinary share, par value $0.01 per share, of the Surviving Company; and
• each SPAC Warrant that is outstanding immediately prior to the Merger Effective Time shall, pursuant to the SPAC Warrant Agreement, cease to represent a right to acquire one SPAC Common Stock and shall be converted in accordance with the terms of such SPAC Warrant Agreement, at the Merger Effective Time, into a right to acquire one Holdco Ordinary Share on substantially the same terms as were in effect immediately prior to the Merger Effective Time under the terms of the SPAC Warrant Agreement.
For more information on the Business Combination, please see the section entitled “The Business Combination Agreement.”
Basis of Presentation
The adjustments presented on the pro forma combined financial statements have been identified and presented to provide an understanding of the Combined Company upon consummation of the Business Combination for illustrative purposes.
The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X, the existing pro forma adjustment criteria with simplified requirements to depict the accounting for the transaction (“Transaction Accounting Adjustments”) and present the reasonably estimable synergies and other transaction effects that have occurred or are reasonably expected to occur (“Management’s Adjustments”). LightJump and Moolec have elected not to present Management’s Adjustments and will only be presenting Transaction Accounting Adjustments in the following unaudited pro forma condensed combined financial information.
LightJump does not meet the definition of a “business” pursuant to IFRS 3, Business Combinations, as it is an empty listed shell holding only cash raised as part of its original equity issuance. As a result, the Business Combination does not qualify as a “business combination” within the meaning of IFRS 3, Business Combinations; rather, the Business Combination will be accounted for as a capital reorganization in accordance with IFRS 2, Share-Based Payments. See Note 3-Accounting for the Business Combination for more details.
The pro forma condensed combined financial information is for illustrative purposes only. The financial results may have been different had the companies always been combined. You should not rely on the unaudited pro forma condensed combined financial information as being indicative of the historical results that would have been achieved had the companies always been combined or the future results that the Combined Company will experience. LightJump and Moolec have not had any historical relationship prior to the Business Combination. Accordingly, no pro forma adjustments were required to eliminate activities between the companies.
The historical financial statements of Moolec have been prepared in accordance with IFRS as issued by the IASB and in its presentation currency of the U.S. dollar. The historical financial statements of LightJump have been prepared in accordance with GAAP in its presentation currency of the U.S. dollar. The condensed combined pro forma financial information reflects IFRS, the basis of accounting used by the registrant, Holdco, and all material accounting policy differences have been identified in converting LightJump’s historical financial statements to IFRS in the pro forma section, see “— GAAP to IFRS conversion of LightJump’s financial information as of June 30, 2022 and for the year ended June 30,2022.” The adjustments presented in the selected unaudited pro forma condensed combined financial information have been identified and presented to provide relevant information necessary for an accurate understanding of the Combined Company after giving effect to the Business Combination. LightJump and Moolec did not have any historical relationship prior to the Business Combination. Accordingly, no pro forma adjustments were required to eliminate activities between the companies.
85
Table of Contents
The selected unaudited pro forma condensed combined financial information has been prepared assuming three alternative levels of redemption of LightJump Public Shares into cash:
Scenario 1 — Assuming no redemptions for cash: This presentation assumes that no holders of Public Shares exercise redemption rights with respect to their upon consummation of the Business Combination;
Scenario 2 — Assuming redemptions of 50% of the outstanding Public Shares for cash: This presentation assumes that holders of Public Shares exercise their redemption rights with respect to 1,383,605 Public Shares upon consummation of the Business Combination at a redemption price of approximately $10.12 per share. Scenario 2 includes all adjustments contained in Scenario 1 and presents additional adjustments to reflect the effect of the redemptions of 50% of the Public Shares; and
Scenario 3 — Assuming redemptions of 100% of outstanding Public Shares for cash: This presentation assumes that holders of Public Shares exercise their redemption rights with respect to a maximum of 2,767,210 Public Shares upon consummation of the Business Combination at a redemption price of approximately $10.12 per share. Scenario 3 includes all adjustments contained in Scenario 1, presents additional adjustments to reflect the effect of the maximum redemptions and assumes that the obligations under the Backstop Agreement will be satisfied by making a cash contribution to Holdco. See “Certain Agreements Related to the Business Combination — Backstop Agreement.”
Included in the shares outstanding and weighted average shares outstanding as presented in the pro forma combined financial statements are an aggregate of 32,500,000 Holdco Ordinary Shares to be issued to Moolec shareholders under Scenario 1, Scenario 2 and Scenario 3.
After the Business Combination, assuming no redemptions of Public Shares for cash, LightJump’s current shareholders will own approximately 13.5% of the outstanding Holdco Ordinary Shares and the former shareholders of Moolec will own approximately 82.6% of the outstanding Holdco Ordinary Shares. Assuming 50% redemption by Public Stockholders of 1,383,605 shares of SPAC Common Stock, LightJump shareholders will own approximately 10.3% of the outstanding Holdco Ordinary Shares and the former shareholders of Moolec will own approximately 85.6% of the outstanding Holdco Ordinary Shares. Assuming 100% redemption by Public Stockholders of 2,767,210 Public Shares, LightJump shareholders will own approximately 8.1% of the outstanding Holdco Ordinary Shares and the former shareholders of Moolec will own approximately 87.1% of the outstanding Holdco Ordinary Shares (in each case, not giving effect to any shares issuable upon the exercise or conversion of warrants).
The pro forma adjustments do not have an income tax effect as they are either (i) incurred by legal entities that are not subject to a corporate income tax, or (ii) permanently nondeductible or nontaxable based on the laws of the relevant jurisdiction.
Accounting for the Business Combination
The Business Combination will be accounted for as a capital reorganization in accordance with IFRS. Under this method of accounting, LightJump will be treated as the “acquired” company for financial reporting purposes, and Moolec will be the accounting “acquirer.” This determination was primarily based on the assumptions that:
• Moolec’s shareholders will hold a majority of the voting power of the Combined Company;
• Moolec’s operations will substantially comprise the ongoing operations of the Combined Company;
• Moolec’s designees are expected to comprise a majority of the governing body of the Combined Company; and
• Moolec’s senior management will comprise the senior management of the Combined Company.
Another determining factor was that LightJump does not meet the definition of a “business” pursuant to IFRS 3, Business Combinations, and thus, for accounting purposes, the Business Combination will be accounted for as a capital reorganization. The net assets of LightJump will be stated at historical cost, with no goodwill or other intangible assets recorded. The deemed costs of the shares issued by Moolec, which represents the fair value of the shares that Moolec would have had to issue for the ratio of ownership interest in the Combined Company to be the same as if the Business Combination had taken the legal form of Moolec acquiring shares of LightJump, in excess of the net assets of LightJump will be accounted for as stock-based compensation under IFRS 2 Share-based payment.
86
Table of Contents
GAAP to IFRS conversion of LightJump’s financial information as of June 30, 2022 and for the year ended June 30, 2022
The historical financial information of LightJump has been adjusted to give effect to the differences between GAAP and IFRS as issued by the IASB for the purposes of the unaudited pro forma condensed combined financial information. The identified adjustments to convert LightJump’s financial statements from GAAP to IFRS for purposes of the unaudited pro forma condensed combined financial information were to: (i) reclassify LightJump’s Common Stock subject to redemption to non-current financial liabilities for $138 million, and (ii) to recognize LightJump’s public warrants as non-current financial liabilities under IAS 32 (for $360 thousand as of June 30, 2022 against accumulated deficit), given that they do not meet the definition of equity under IFRS, resulting in a recognition of a $4.3 million gain in finance results within the statements of operations for the year ended June 30, 2022.
Adjustments to Unaudited Pro Forma Condensed Combined Statement of Operations for the Year Ended June 30, 2022
The pro forma adjustments, included in the unaudited pro forma condensed combined statement of operations for the twelve months ended June 30, 2022 are as follows:
(1) Represents the preliminary estimated expense recognized, in accordance with IFRS 2, for the excess of the deemed costs of shares issued by Moolec over the fair value of LightJump’s identifiable net assets at the date of the Business Combination. For reference on the calculation see “— Adjustments to Unaudited Pro Forma Condensed Combined Statements of Financial Position as of June 30, 2022” below.
(2) Represents the preliminary estimated Company Transaction Expenses.
(3) To reflect the elimination of finance expenses recorded in the consolidated statement of operations of Moolec for financial liabilities associated with the Company SAFEs of $0.9 million.
(4) To reflect the elimination of interest income on marketable securities held in the Trust Account.
The calculation of weighted average shares outstanding for basic and diluted net loss per share assumes that the Business Combination was closed as of July 1, 2021. The pro forma loss per share is calculated based on pro forma net loss divided by the weighted average pro forma basic and diluted number of shares. The pro forma diluted loss per share does not consider the impact of securities other than the ordinary shares as such other securities would be anti-dilutive due to the pro forma net loss position, and thus pro forma basic and diluted loss per share are the same value.
The unaudited pro forma condensed combined financial information has been prepared assuming three alternative levels of redemption of Public Shares:
| | | | Scenario
1 – Assuming
no redemptions | | Scenario
2 – Assuming
50% of
redemptions | | Scenario
3 – Assuming
100% of
redemptions |
Weighted average shares outstanding –
basic and diluted | | | | | | |
Company Shareholders | | 32,500,000 | | 32,500,000 | | 32,500,000 |
LightJump Public Stockholders | | 2,767,210 | | 1,383,605 | | 0 |
LightJump Initial Stockholders | | 3,570,000 | | 3,570,000 | | 3,570,000 |
Other Shareholders(1) | | 518,725 | | 518,725 | | 518,725 |
LightJump Backstop Shareholders(2) | | 0 | | 0 | | 500,000 |
Moolec Backstop Shareholders(3) | | 0 | | 0 | | 500,000 |
Total | | 39,355,935 | | 37,972,330 | | 37,588,725 |
87
Table of Contents
Adjustments to Unaudited Pro Forma Condensed Combined Statement of Financial Position as of June 30, 2022
The pro forma notes and adjustments, based on preliminary estimates that could change materially as additional information is obtained, are as follows:
(1) To reflect the release of cash from marketable securities held in the Trust Account according to the redemption on every scenario. For Scenario 1, the 0% redemption of Public Shares and corresponding release of $0 of the $27.7 million held in the Trust Account. For Scenario 2 the 50% redemption of Public Shares and corresponding release cash held within the Trust Account. For Scenario 3 the 100% redemption of Public Shares and corresponding release of the entire amount within the Trust Account. Further, the redemption of 11,032,790 shares of SPAC Common Stock in connection with the Extension Amendment has been reflected as a reduction of other non-current liabilities. As a result of the redemption, $110.5 million (or approximately $10.02 per share) was released from the Trust Account to pay redeeming stockholders. For more information regarding the Extension Amendment, See “Business of LightJump and Certain Information about LightJump — Overview.”
(2) To reclassify other liabilities related to shares of SPAC Common Stock subject to redemption to permanent equity at the closing of the Business Combination also considering the impact of the Extension Amendment. For Scenario 1, it is assumed that no holders of Public Shares exercise their redemption rights. For Scenario 2, it is assumed that 50% of Public Shares, or 1,383,605 Public Shares are redeemed for a cash amount of $13.8 million by holders of Public Shares. For Scenario 3, it is assumed that the maximum number of shares of SPAC Common Stock, or 2,767,210 Public Shares are redeemed for cash by the holders of Public Shares and a cash amount of $27.7 million would be paid to the redeeming holders of Public Shares. The $27.7 million, or 2,767,210 Public Shares, represents the maximum redemption amount. In addition, for Scenario 3 it is assumed that $10.0 million would be incorporated in cash pursuant to the Backstop Agreement.
(3) Reflects the preliminary estimated transaction costs to be incurred by LightJump and Moolec of approximately (i) $9.2 million and $2.5 million, respectively, in Scenario 1; (ii) $6.8 million and $2.5 million, respectively, in Scenario 2; and (iii) $4.1 million and $2.5 million, respectively, in Scenario 3.
$1.9 million of the estimated LightJump transaction costs have been accrued as of the date of the pro forma balance sheet. The remaining amount of $7.3 million, $4.9 million or $2.2 million for Scenarios 1, 2 and 3 respectively, is reflected as an adjustment between;
(i) share premium for the cost specifically attributable to the issue of new shares (using a criteria of percent of shares issued); and
(ii) the remaining amount to accumulated deficit included in the Listing Cost (as noted below in (4).
$0.5 million of the estimated Moolec transaction costs have accrued as of the date of the pro forma balance sheet, and $0.5 million is reflected as prepaid insurance for officers and directors’ insurance. The remaining amount of $1.5 million is reflected as an adjustment to accumulated deficit.
(4) The listing fees adjustment connected to the LightJump merger adjustment (described above) is accounted for under IFRS 2. As the fair value of relevant service cost cannot be reliably measured
88
Table of Contents
directly, the fair value is measured by reference to the fair value of the equity instrument issued by Holdco (i.e., shares), which is derived from the fair value of LightJump’s shares of SPAC Common Stock. The differences in the estimated fair value of those equity instruments over the fair value of identifiable net assets of LightJump represents a service for listing of 3,570,000 new shares (at a price of $10.00 per share) and is accounted for as a share-based payment expense in accordance with IFRS 2. The cost of the service, which is a non-cash and non-recurring expense, is preliminarily estimated to be the roundup amounts of: $44.8 million in the no redemptions scenario, $42.9 million in the 50% redemption scenario and $40.8 million in the maximum redemptions scenario and have been calculated as follows:
| | | | | | Scenario
1 – Assuming
no redemptions | | Scenario
2 – Assuming
50% of
redemptions | | Scenario
3 – Assuming
100% of
redemptions |
Fair value of Moolec | | A | | 325.0 | | | 325.0 | | | 325.0 | |
Equity Interest in Moolec that will be issued to shareholders of
LightJump | | B | | 16.1 | % | | 13.0 | % | | 12.2 | % |
Equity Interest in Moolec of the
Moolec Shareholders after the Business Combination | | C | | 82.6 | % | | 85.6 | % | | 86.5 | % |
Deemed costs of shares issued by Moolec | | AxB/C | | 63.3 | | | 49.4 | | | 45.8 | |
Less: SPAC net assets | | | | 18.5 | | | 6.5 | | | 5.0 | |
IFRS 2 charge for listing services | | | | 44.8 | | | 42.9 | | | 40.8 | |
The adjustment of the listing fees on accumulated deficit considers the elimination of LightJump’s net assets of $3.4 million.
(5) Represents the conversion of the Company SAFE in the aggregate amount of $2.9 million into 274,951 Holdco Ordinary Shares (including the positive effect on accumulated deficit of $110 thousand due to remeasurement at fair value at transaction date) and the issuance of 243,774 Holdco Ordinary Shares pursuant to the Key Staff Participation for $0.4 million as well as the adjustment to remove the historical share capital of Moolec and recognize the share capital of 32,500,000 shares with a par value of $0.001.
(6) Represents the write-off of LightJump related party liabilities as of June 30, 2022 for $1.2 million according to the Business Combination Agreement.
89
Table of Contents
COMPARATIVE PER SHARE DATA
The following table sets forth the historical comparative share information for Moolec and LightJump on a stand-alone basis and pro forma combined per share information after giving effect to the Business Combination, (1) assuming no LightJump shareholders exercise redemption rights with respect to their SPAC Common Stock upon the consummation of the Business Combination, the SPAC Transaction Expenses do not exceed the SPAC Transaction Expenses Cap, and no additional equity securities of LightJump are issued at or prior to Closing; (2) assuming that LightJump shareholders exercise their redemption rights with respect to 1,383,605 Public Shares upon consummation of the Business Combination at a redemption price of approximately $10.12 per share; and (3) assuming that LightJump shareholders exercise their redemption rights with respect to a maximum of 2,767,210 Public Shares upon consummation of the Business Combination, the SPAC Transaction Expenses do not exceed the SPAC Transaction Expenses Cap, no additional equity securities of LightJump are issued at or prior to Closing.
The financial statements of Moolec have been prepared in accordance with IFRS as issued by the IASB and in its functional and presentation currency of U.S. dollars. The historical financial statements of LightJump have been prepared in accordance with GAAP in its functional and presentation currency of U.S. dollars.
The historical information should be read in conjunction with the information in the sections entitled “Selected Historical Financial and Other Data of LightJump” and “Selected Historical Combined Financial Data of Moolec” and the historical financial statements of LightJump and Moolec included elsewhere in this proxy statement/prospectus. The pro forma combined per share information is derived from, and should be read in conjunction with, the information contained in the section of this proxy statement/prospectus entitled “Unaudited Pro Forma Condensed and Combined Financial Information.”
The pro forma combined share information below does not purport to represent what the actual results of operations or the earnings per share would been had the companies been combined during the periods presented, nor to project the Combined Company’s results of operations or earnings per share for any future date or period. The pro forma combined shareholders’ equity per share information below does not purport to represent what the value of LightJump and Moolec would have been had the companies been combined during the periods presented.
| | (in U.S. dollars, in thousands, except share and per share data) |
| | | | | | | Combined Pro Forma |
| | | Moolec
(Historical) | | LightJump
(Historical)(2) | | Scenario 1 –
Assuming no redemptions | | Scenario 2 –
Assuming 50% of redemptions | | Scenario 3 –
Assuming 100% of redemptions |
As of and for the year ended June 30, 2022 | | | | | | | | | | | | | | | |
Book value per share(1) | | 0.03 | | | (0.94 | ) | | 0.53 | | | 0.25 | | | 0.22 | |
Cash dividends per share | | 0.00 | | | 0.00 | | | 0.00 | | | 0.00 | | | 0.00 | |
| | | | | | | | | | | | | | | | |
Weighted average
shares: | | | | | | | | | | | | | | | |
Weighted average of outstanding shares – basic and diluted | | 48,661,908 | | | 3,570,000 | | | 39,355,935 | | | 37,972,330 | | | 37,588,725 | |
Earnings (loss) per
share: | | | | | | | | | | | | | | | |
Earnings (loss) per outstanding shares,
basic and diluted | | (0.09 | ) | | 1.44 | | | (1.15 | ) | | (1.14 | ) | | (1.09 | ) |
90
Table of Contents
The Special Meeting of LightJump Holders
The LightJump Special Meeting
LightJump is furnishing this proxy statement/prospectus to LightJump Holders as part of the solicitation of proxies by its board of directors for use at LightJump’s special meeting of stockholders to be held on [•], 2022, and at any adjournment or postponement thereof. This proxy statement/prospectus is first being furnished to the LightJump Holders on or about [•], 2022. This proxy statement/prospectus provides you with information you need to know to be able to vote or instruct your vote to be cast at the special meeting of stockholders.
Date, Time and Place of the Special Meeting
The special meeting of stockholders of LightJump will be held at [•] a.m., Eastern time, on [•], 2022, at [•], or such other date, time and place to which such meetings may be adjourned or postponed, for the purpose of considering and voting upon the proposals. [The special meeting will be completely virtual].
Purpose of the Special Meeting
At the LightJump special meeting of stockholders, LightJump will ask the LightJump Holders to vote in favor of the following proposals:
• Proposal 1 (The Business Combination Proposal) — a proposal to approve and adopt the Business Combination Agreement and the Business Combination.
• Proposal 2 (Stockholder Adjournment Proposal) — a proposal to adjourn the special meeting to a later date or dates, if necessary, as described herein.
Recommendation of the LightJump Board of Directors
LightJump’s board of directors believes that each of the Business Combination Proposal and the Stockholder Adjournment Proposal is in the best interests of LightJump and its stockholders and recommends that its stockholders vote “FOR” each of the proposals to be presented at the special meeting.
When you consider the recommendation of LightJump’s board of directors in favor of approval of Proposal 1 (The Business Combination Proposal), and Proposal 2 (Stockholder Adjournment Proposal), you should keep in mind that certain of LightJump’s directors and officers have interests in the Business Combination that are different from, or in addition to, your interests as a stockholder. These interests include, among other things:
• the beneficial ownership of the Sponsor of an aggregate of 3,450,000 SPAC Common Stock, acquired for an aggregate purchase price of $25,000, which shares would become worthless if LightJump does not complete a business combination within the applicable time period, as the LightJump Initial Stockholders have waived any right to liquidation proceeds with respect to these shares. Such shares have an aggregate market value of approximately $[•] based on the closing price of SPAC Common Stock of $[•] on Nasdaq on [•], 2022, the record date for the special meeting of stockholders;
• Sponsor’s beneficial ownership of an aggregate of 4,210,000 warrants, acquired for an aggregate purchase price of $4,210,000, which warrants would become worthless if LightJump does not complete a business combination with the applicable time period. Such warrants have an aggregate market value of approximately $[•] based on the closing price of the SPAC Warrants of $[•] on Nasdaq on [•], 2022, the record date for the special meeting of stockholders.
• LightJump’s directors will not receive reimbursement for any out-of-pocket expenses incurred by them on LightJump’s behalf incident to identifying, investigating and consummating a business combination to the extent such expenses exceed the amount not required to be retained in the Trust Account, unless a business combination is consummated. As of [•], no out-of-pocket expenses have been incurred by LightJump’s directors incident to identifying, investigating and consummating a business combination;
91
Table of Contents
• the potential continuation of certain of LightJump’s directors as directors of Holdco and potential receipt of the same compensation that Holdco pays to its other directors, if any; and
• the continued indemnification of current directors and officers of LightJump and the continuation of directors’ and officers’ liability insurance after the Business Combination.
These interests may influence LightJump’s directors in making their recommendation to vote in favor of the approval of the Business Combination Proposal and the other proposals described in this proxy statement/prospectus. You should also read the section entitled “The Business Combination — Interests of LightJump’s Directors and Officers in the Business Combination.”
Record Date and Voting
You will be entitled to vote or direct votes to be cast at the special meeting of stockholders if you owned shares of SPAC Common Stock at the close of business on [•], 2022, which is the record date for the special meeting of stockholders. You are entitled to one vote for each share of SPAC Common Stock that you owned as of the close of business on the record date. If your shares are held in “street name” or are in a margin or similar account, you should contact your broker, bank or other nominee to ensure that votes related to the shares you beneficially own are properly counted. On the record date, there were 6,337,210 shares of SPAC Common Stock outstanding.
LightJump’s Sponsor, officers and directors have agreed to vote all of their SPAC Common Stock and any Public Shares acquired by them in favor of the Business Combination Proposal and the other proposals described in this proxy statement/prospectus. As a result, LightJump would not need any additional votes in favor of such proposals in order to have the Business Combination approved. LightJump’s issued and outstanding warrants do not have voting rights at the special meeting of stockholders.
Voting Your Shares
Each share of SPAC Common Stock that you own in your name entitles you to one vote on each of the proposals for the special meeting of stockholders. Your one or more proxy cards show the number of shares of SPAC Common Stock that you own.
If you are a holder of record, there are two ways to vote your shares of SPAC Common Stock at the special meeting of stockholders:
• You can vote by proxy, by telephone, online, or by completing, signing and returning the enclosed proxy card(s) in the postage-paid envelope provided. If you hold your shares in “street name” through a bank, broker or other nominee, you will need to follow the instructions provided to you by your bank, broker or other nominee to ensure that your shares are represented and voted at the applicable special meeting(s). If you vote by proxy, your shares will be voted in accordance with your instructions. If you sign and return the proxy card but do not give instructions on how to vote your shares, your shares of SPAC Common Stock will be voted as recommended by LightJump’s board of directors, which is to say “FOR” each of the proposals presented at the special meeting of stockholders.
• You can attend the special meeting and vote virtually. However, if your shares of SPAC Common Stock are held in the name of your broker, bank or other nominee, you must get a proxy from the broker, bank or other nominee.
Who Can Answer Your Questions About Voting Your Shares
If you have any questions or need assistance voting your shares, please call our proxy solicitor, Advantage Proxy, Inc., at (877) 870-8565. Banks and brokers may reach Advantage Proxy, Inc. at the same, or by email at ksmith@advantageproxy.com.
Quorum and Vote Required for the Proposals
A quorum of LightJump’s stockholders is necessary to hold a valid meeting. A quorum will be present at the special meeting of stockholders if a majority of the SPAC Common Stock outstanding and entitled to vote at the meeting is represented in person or by proxy.
92
Table of Contents
The approval of the Business Combination Proposal requires the affirmative vote of the holders of at least a majority of all then outstanding shares of SPAC Common Stock entitled to vote thereon at the special meeting of stockholders. The Stockholder Adjournment Proposal, if presented, requires the affirmative vote of the holders of a majority of the shares of SPAC Common Stock that are voted thereon at the special meeting of stockholders. Accordingly, a LightJump stockholder’s failure to vote by proxy or to vote in person at the special meeting of stockholders, or an abstention from voting, will have the same effect as a vote “AGAINST” the Business Combination Proposal and will have no effect on the outcome of any vote on the Stockholder Adjournment Proposal.
Shares that are Not Voted and Abstentions
Under the rules of various national and regional securities exchanges, your broker, bank or nominee cannot vote your shares with respect to non-discretionary matters unless you provide instructions on how to vote in accordance with the information and procedures provided to you by your broker, bank or nominee. LightJump believes the proposals presented to its stockholders will be considered non-discretionary and therefore your broker, bank or nominee cannot vote your shares without your instruction. If you do not provide instructions with your proxy, your bank, broker or other nominee will not vote your shares, and they will not be represented at the special meeting or counted for purposes of determining the presence of a quorum. Shares that are not voted will have the same effect as a vote “AGAINST” Proposal 1 (The Business Combination Proposal) and no effect on Proposal 2 (Stockholder Adjournment Proposal).
Abstentions will be counted for purposes of determining the presence of a quorum at LightJump’s special meeting of stockholders. Abstentions will have the same effect as a vote “AGAINST” Proposal 1 (The Business Combination Proposal) and no effect on Proposal 2 (Stockholder Adjournment Proposal).
Revocability of Proxies
If you vote by telephone or online, only your latest telephone or online proxy that is timely submitted prior to the meeting will be counted. If you vote by signing and returning a proxy card, you may change your vote by completing a new proxy card with a later date. You may also revoke your proxy and change your vote by virtually attending the meeting and voting online. You also may revoke your proxy by sending a notice of revocation to Advantage Proxy, Inc. at P.O. Box 13581, Des Moines, Washington 98198, provided such revocation is received prior to the vote at the special meeting. If your shares are held in street name by a broker or other nominee, you must contact the broker or nominee to change or revoke your vote.
Redemption Rights
Pursuant to the SPAC COI, any holders of Public Shares may demand that such shares be redeemed in exchange for a pro rata share of the aggregate amount on deposit in the Trust Account, less taxes payable, calculated as of two Business Days prior to the consummation of the Business Combination. If demand is properly made and the Business Combination is consummated, these shares, immediately prior to the Business Combination, will cease to be outstanding and will represent only the right to receive a pro rata share of the aggregate amount on deposit in the Trust Account which holds the proceeds of LightJump’s IPO and concurrent private placements as of two Business Days prior to the consummation of the Business Combination, less taxes payable, upon the consummation of the Business Combination. For illustrative purposes, based on funds in the Trust Account of approximately $27,993,798 on July 12, 2022 and 2,767,210 public shares outstanding, the estimated per share redemption price would have been approximately $10.12.
If you exercise your redemption rights, your shares of SPAC Common Stock will cease to be outstanding immediately prior to the Business Combination and will only represent the right to receive a pro rata share of the aggregate amount on deposit in the Trust Account. You will no longer own those shares. You will be entitled to receive cash for these shares only if you properly demand redemption. See the section entitled “The Special Meeting of LightJump Holders — Redemption Rights.”
93
Table of Contents
In order to exercise your redemption rights, you must, prior to 5:00 p.m. Eastern time on [•], 2022 (two Business Days before the special meeting), (i) submit a written request to Continental Stock Transfer & Trust Company, LightJump’s transfer agent, that LightJump redeem your SPAC Common Stock for cash, and (ii) deliver your stock to LightJump’s transfer agent physically or electronically through the DTC. Your request should be submitted to the transfer agent at the following address:
Continental Stock Transfer & Trust Company
1 State Street Plaza, 30th Floor
New York, New York 10004
Attention: Mark Zimkind
Email: mzimkind@continentalstock.com
Electronic delivery of your stock generally will be faster than delivery of physical stock certificates.
A physical stock certificate will not be needed if your stock is delivered to LightJump’s transfer agent electronically. In order to obtain a physical stock certificate, a stockholder’s broker and/or clearing broker, DTC and LightJump’s transfer agent will need to act to facilitate the request. It is LightJump’s understanding that stockholders should generally allot at least one week to obtain physical certificates from the transfer agent. However, because LightJump does not have any control over this process or over the brokers or DTC, it may take significantly longer than one week to obtain a physical stock certificate. If it takes longer than anticipated to obtain a physical certificate, stockholders who wish to redeem their shares and do not deliver their stock electronically may be unable to obtain physical certificates by the deadline for exercising their redemption rights and thus will be unable to redeem their shares.
Any demand for redemption, once made, may be withdrawn at any time until the deadline for exercising redemption requests and thereafter, with LightJump’s consent. If you delivered your shares for redemption to LightJump’s transfer agent and decide within the required timeframe not to exercise your redemption rights, you may request that LightJump’s transfer agent return the shares (physically or electronically). Such requests may be made by contacting LightJump’s transfer agent at the street or e-mail address above.
Appraisal or Dissenters’ Rights
No appraisal or dissenters’ rights are available to holders of shares of SPAC Common Stock in connection with the Business Combination.
Solicitation of Proxies
LightJump will pay the cost of soliciting proxies for the special meeting. LightJump has engaged Advantage Proxy, Inc. to assist in the solicitation of proxies for the special meeting. LightJump has agreed to pay Advantage Proxy, Inc. a fee of [$•]. LightJump will reimburse Advantage Proxy, Inc. for reasonable out-of-pocket expenses and will indemnify Advantage Proxy, Inc. and its affiliates against certain claims, liabilities, losses, damages and expenses. LightJump also will reimburse banks, brokers and other custodians, nominees and fiduciaries representing beneficial owners of shares of SPAC Common Stock for their expenses in forwarding soliciting materials to beneficial owners of SPAC Common Stock and in obtaining voting instructions from those owners. LightJump’s directors, officers and employees may also solicit proxies by telephone, by facsimile, by mail, on the internet or in person. They will not be paid any additional amounts for soliciting proxies.
Stock Ownership
As of the record date, the Sponsor beneficially own an aggregate of 54.4% of the outstanding shares of SPAC Common Stock. The Sponsor and LightJump’s officers and directors have agreed to vote all of their SPAC Common Stock in favor of Proposal 1 (The Business Combination Proposal) and the other proposals described in this proxy statement/prospectus. Accordingly, all proposals are expected to be approved even if every other holder of SPAC Common Stock were to vote against the proposals.
Certain EarlyBird designees own 120,000 shares of SPAC Common Stock, which shares would become worthless if LightJump does not complete a business combination within the applicable time period, as the designees of EarlyBird have waived any right to liquidation proceeds with respect to these shares.
94
Table of Contents
The Business Combination
The Background of the Business Combination
The terms of the Business Combination are the result of negotiations between representatives of LightJump and Moolec. The following is a brief description of the background of these negotiations and the resulting Business Combination.
LightJump is a blank check company formed under the laws of the State of Delaware on July 28, 2020, for the purpose of effecting a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization, or other similar business combination with one or more businesses or entities in any industry or geographic location. The proposed Business Combination with Moolec is the result of an extensive search for a potential business combination using the investing and operating experience of LightJump’s board of directors and management team. The terms of the Business Combination are the result of arm’s-length negotiations between representatives of LightJump and Moolec. The following is a brief discussion of the background of these negotiations, the Business Combination, and related transactions.
On January 12, 2021, LightJump closed its IPO of 12,000,000 SPAC Units at $10.00 per unit, generating total gross proceeds of $120,000,000. Each SPAC Unit consists of one share of SPAC Common Stock and one-half of one SPAC Warrant. Each whole SPAC Warrant entitles the holder thereof to purchase one share of SPAC Common Stock at a price of $11.50 per share.
Simultaneously with the closing of our IPO, Sponsor purchased an aggregate of 3,850,000 warrants at a price of $1.00 per warrant, for an aggregate purchase price of $3,850,000 in a private placement. The proceeds from the private placement of the Private Warrants were added to the proceeds of the IPO and placed in a U.S.-based trust account maintained by Continental, as trustee.
On January 15, 2021, LightJump announced that it completed the sale of an additional 1,800,000 SPAC Units pursuant to the underwriters’ over-allotment option granted in connection with LightJump’s IPO. The additional SPAC Units were sold at $10.00 per unit resulting in additional gross proceeds to LightJump of $18 million.
Simultaneously with the closing of the exercise of the overallotment option, LightJump completed the sale of an aggregate of 360,000 Private Warrants to the Sponsor at a purchase price of $1.00 per Private Placement Warrant, generating gross proceeds of $360,000.
Of the proceeds received from the consummation of the IPO and a simultaneous private placement of warrants, $138,000,000 (or $10.00 per unit sold in the public offering) was placed in the Trust Account.
Prior to the closing of the IPO, neither LightJump, nor anyone on its behalf, had selected any business combination target or initiated any substantive discussions, directly or indirectly, with respect to identifying any business combination target.
After the IPO, LightJump’s officers and directors commenced an active search for prospective businesses or assets to acquire in the initial business combination. In the prospectus for the IPO, LightJump identified the following general criteria and guidelines that it believed would be important in evaluating acquisition opportunities, although it indicated that it may decide to enter an initial business combination with a target business that does not meet these criteria and guidelines. LightJump intended to acquire companies or assets that it believed had the following attributes:
Category creating companies with defensible moats, large total addressable markets, with the ability to create disruptive business plans that could reshape industries, SaaS business software companies, Blockchain companies, and Cyber-Security companies.
95
Table of Contents
As disclosed in LightJump’s prospectus for the IPO, these criteria were not intended to be exhaustive. Any evaluation relating to the merits of a particular initial business combination was to be based, to the extent relevant, on these general guidelines as well as other considerations, factors, and criteria that our management deemed relevant.
During the search process from the consummation of LightJump’s IPO through the signing of the Business Combination Agreement on June 14, 2022, LightJump reviewed hundreds of companies, originated term sheets to companies, and explored ideas with many investment banks. As part of that process, LightJump engaged in discussions with representatives of over 50 potential business combination targets and conducted analysis and due diligence on a significant subset thereof. From the date of the IPO through June 14, 2022, representatives of LightJump entered into over 30 non-disclosure or confidentiality agreements with potential business combination targets, exchanged drafts of letters of intent with three alternative business combination targets and/or their advisors and entered into two letters of intents with alternative business combination targets. LightJump or the target ultimately determined to abandon each of the alternative acquisition opportunities either because the target pursued an alternative business combination or strategy, or LightJump concluded that the target business could not achieve its requirements at closing.
LightJump reviewed targets in industries that included space, automotive EV, and other technology-oriented businesses, and engaged with experts in these areas.
During that time Mr. Rich, an independent board member, helped source deals through his deal network, including an internet technology services company. Messrs. Bennett, Ver Ploeg and Bunker also sourced several potential deals. LightJump reviewed many businesses including businesses with large market opportunities and companies that had the potential for growth that LightJump was looking for in a SPAC candidate.
From March of 2021 to August of 2021, LightJump focused its efforts on an automotive enterprise SaaS company and spent these months researching this marketplace. During this time, the PIPE market became overly competitive and the company could not achieve its PIPE objectives in the PIPE market. Following that in the fall of 2021, LightJump focused on battery and energy related deals, including a European company it focused its efforts on.
On December 22, 2021, EarlyBird introduced LightJump to Moolec as an opportunity to review. The initial call originated by EarlyBird initiated a follow up call for after the holidays. During the holiday period of 2021, Messrs Bennett, Ver Ploeg and Bunker discussed the future of the alternative protein market compared to other markets that LightJump was investigating including more industrial opportunities, as well as continuing to look at the European battery market.
The discussions about the opportunity were initially conducted by LightJump with Mr. Bransfield at Union Acquisition Group, an affiliate of a shareholder of Moolec. Mr. Bransfield discussed with Mr. Bennett the possibility of offering some form of backstop agreement that Union Acquisition Group and other affiliates of Moolec, including Bioceres affiliates, would offer up to ensure a closing of the business combination. LightJump felt that the backstop was beneficial in assuring a closing condition and a very important element to pursuing the transaction given its experience with other target companies that had uncertainty in closing.
On January 11, 2022 Messrs Bennett, Ver Ploeg and Bunker reached out to a person with significant industry experience to discuss the business, and the general industry that Moolec operated under. On this call, the parties discussed the global potential of a transgenic provider of plants to the meat industry. The parties agreed that this market, which continues to evolve, could continue to be a large and dominant part of the global food change. Because of this call, Messrs. Bennett, Ver Ploeg, and Bunker determined that more research was warranted and an additional call had already been set up for the next day with another person with significant industry experience.
On January 12, 2022, Messrs Bennett, Ver Ploeg and Bunker reached out to another person with significant industry experience and discussed in detail the opportunity for Moolec Science Limited. This person, with experience in the food industry, discussed with the LightJump team the opportunity that could be provided from a provider of a transgenic produce that introduced animal proteins into a commodity style agricultural product such as soy or wheat. This person agreed that the market was evolving into a large opportunity with a large global market that could be tapped.
96
Table of Contents
On January 18, 2022, LightJump engaged in a video conference with Moolec, its management team and board members to discuss the opportunity of using the LightJump as the SPAC vehicle for going public. One point interest to LightJump was that Moolec was not a startup per-se but a spinout from a parent company, Bioceres, S.A., a well-respected agricultural solutions company.
On February 11, 2022, Moolec’s board of directors held a meeting in which it discussed the possibility of a potential business combination with a SPAC.
On March 21, 2022, Moolec had a meeting with Linklaters to discuss a potential business combination with the SPAC and formally engaged Linklaters as its outside legal counsel to assist it in a potential business combination pursuant to the engagement letter entered into on March 22, 2021.
After that date, LightJump continued to pursue other opportunities in the marketplace during February and March, including an industrial opportunity.
By the end of March, LightJump decided to follow up on the progress of Moolec and on March 25, 2022 Eric Ver Ploeg from LightJump visited Moolec at the San Francisco ag-tech food show, discussed with industry experts Moolec’s position in the industry and further researched the company. At that show, Mr. Ver Ploeg met with Mr. Bransfield from Union Acquisition Group and other exhibitors at the show. Mr. Ver Ploeg gained more industry knowledge associated with the ag-tech food business and the opportunity Moolec has in that sector. Besides meeting Moolec’s management team and Mr. Bransfield, Mr. Ver Ploeg discussed the transgenic seed opportunity, as well as fermenting opportunities, and other alternative protein opportunities with other exhibitors at the show, attended panels, and attended the panel led by Gastón Paladini, CEO of Moolec, who was speaking at the show. That panel included CEOs from other leading alternative protein companies.
Upon return from the Ag-Tech food show in San Francisco, Messrs. Bennett, Ver Ploeg and Bunker re-convened on a conference call to discuss the opportunity provided by Moolec, versus other opportunities LightJump was looking at. Mr. Ver Ploeg discussed the private enterprise values of some of the peer group companies in the ag-tech space, including companies providing cheese alternatives using plant proteins that were garnering a lot of attention. Mr. Ver Ploeg discovered that while most companies were providing meat alternatives through fermentation, Moolec had a unique market position by focusing on using transgenic seeds combining animal proteins into crops. This strategy Mr. Ver Ploeg discovered could create a breakthrough in cost and volume considering fermenting animal proteins was a slow and somewhat expensive process.
On April 1, 2022, Mr. Bennett conversed with representatives of EarlyBird about the transaction and discussed the potential terms of the transaction. A relevant structure feature was the backstop and that the Moolec business was a novel approach to scaling the alternative proteins business. Furthermore, Mr. Bennett discussed the fact that Moolec was jointly owned by Bioceres, S.A., a well-respected provider of ag-tech, and the parent company of Bioceres Crop Solutions Corp. (BIOX), a publicly traded company on Nasdaq. Also, Mr. Bennett discussed that Union Acquisition Group, who had used a SPAC to take Bioceres Crop Solutions Corp. public, was knowledgeable in the SPAC market.
At that time, LightJump’s team started to review the comparable valuations in the private markets, including many notable alternative protein companies’ valuations and market potential in the marketplace, and reviewed an analysis on the current market comps.
On April 1, 2022, LightJump executed a letter of intent (“LOI”) with Moolec at a $325 Million Enterprise Value which included the $10 Million backstop agreement that had been discussed. On April 5, 2022, LightJump convened a board meeting to review the LOI with the board of directors. Attending the meeting was Messrs Bennett, Ver Ploeg, Bunker, Rich, Keyes, and Hamer. At that meeting, Mr. Ver Ploeg presented the valuation comps and Mr. Bennett reviewed the current presentation that had been received by LightJump from Moolec. At the meeting, the company had in depth discussions on the market opportunities and the novel solution provided by Moolec, and the benefits offered through Moolec’s ownership structure with significant experience in the Agricultural market (Bioceres), the SPACs and financial market (Union). The directors discussed that Moolec had two products with existing approvals from Bioceres: (i) Chymosin, an alternative vegetarian rennet replacement and (ii) GLA, a nutritional oil, and that both products were far along in their approval cycle and closer to market traction, giving the company an opportunity to reach market acceptance at a much faster pace than a traditional startup. The directors believed that those two products, combined with the companies’ meat replacing transgenic seed products gave the company a unique market position.
97
Table of Contents
On April 21, 2022, LightJump initiated another call with EarlyBird to discuss the transaction metrics and the market opportunity that the company had in the marketplace. On this call, the parties discussed Union Acquisition Group’s experience in the SPAC market, the backstop guarantee and the market opportunity in the alternative protein marketplace.
On April 22, 2022, LightJump’s Messrs Bennett and Ver Ploeg reached out to a fairness opinion provider to discuss the feasibility of a fairness opinion and engaging the firm to perform it. During this call the parties discussed the alternative proteins marketplace and the valuations placed on companies in the space. Also on April 22, 2022, Mr. Bennett and Mr. Bransfield, discussed the capital raising opportunities in the marketplace and the unique position the company had in the alternative proteins industry.
On April 23, 2022, Mr. Bennett visited Amit Dhingra, the Chief Science Officer of Moolec, at Texas A&M University, to discuss the transgenic seed work being performed by Moolec. Mr. Bennett and Mr. Dhingra met for breakfast and spent the day reviewing the work being performed by Moolec and the vision for the Moolec products being developed from the transgenic seed programs. Mr. Dhingra’s expertise in transgenic seed work gives Moolec a key resource to develop the R&D products required to launch the transgenic seed products. Mr. Dhingra manages R&D programs for the company at multiple university and R&D locations across the USA.
Messrs Bennett, Bunker and Ver Ploeg continued their due diligence work and on April 25, 2022, initiated a call with another person with significant experience in the industry to discuss transgenic seeds, and market potential. On this call, the parties discussed the market opportunities presented by the company.
On April 27, 2022, the LightJump team, Messrs Bennett, Ver Ploeg, and Bunker discussed the transaction with Federico Trucco, a Moolec board member and the CEO of Bioceres, S.A., as well as the management team from Moolec and members of Union Acquisition Group. Mr. Trucco relayed to the team his vision for Moolec and his experience running Bioceres, S.A.
During this period, Linklaters, Moolec’s counsel, and K&L Gates, LightJump’s counsel, worked on a proposed Business Combination Agreement. During this time, LightJump had multiple conversations with the management team at Moolec to finalize the business points to be covered in such an agreement.
On June 13, 2022, LightJump convened a board meeting to approve the Business Combination Agreement, discussed in general the market and reviewed documents presented to them by LightJump’s management team discussing the market opportunity, comparable companies and their valuations and had detailed conversations thereof. Furthermore, LightJump’s counsel, K&L Gates, attended the meeting and reviewed the details of the business combination agreement, the details of the backstop agreement and the other details of the transaction. Also at the meeting, it was decided to seek and hire a consultant or advisory firm to prepare a fairness opinion for the transaction. However, in lieu of the extensive due diligence performed by the members of the Board, it was decided to engage the fairness opinion provider after the signing of the Business Combination Agreement with and include the fairness opinion as a condition of closing. At the end of the meeting, the Board of Directors unanimously approved the transaction.
On June 14, 2022, considering both boards of both companies had reviewed and approved the transaction, LightJump and Moolec Sciences executed the Business Combination Agreement.
On April 27, 2021, Moolec entered into an engagement letter (the “Nomura Engagement Letter”) with Nomura Securities International, Inc. (“Nomura”). Pursuant to the terms of the Nomura Engagement Letter, Moolec agreed to engage Nomura as (i) exclusive financial advisor to Moolec in connection with any potential business combination or sale transaction involving Moolec and any other third party that is a special purpose acquisition company and (ii) exclusive placement agent in connection with the offer and proposed sale, through a private placement to third parties, whether in one or a series of transactions, of Moolec’s common equity, preferred equity (including perpetual, redeemable, convertible or any combination thereof), convertible debentures, any other equity-linked securities, mezzanine financing and/or debt securities. The terms of the Nomura Engagement Letter included fees to be paid by Moolec to Nomura of (i) 1.00% of the transaction value of any business combination or sale transaction involving Moolec and any other third party special purpose acquisition company, (ii) a placement fee, payable in cash, equal to 6% of the gross proceeds received or to be received by Moolec on the closing date of any placement of securities and (iii) reimbursement to Nomura by Moolec for all expenses incurred by entering into and performing services pursuant to the Nomura Engagement Letter.
98
Table of Contents
On June 29, 2022, Nomura delivered to Moolec a letter agreement terminating its engagement with Moolec (the “Nomura Termination Letter”). On March 30, 2022, prior to the resignation of Nomura, the SEC issued proposed rules (the “SPAC Rule Proposals”) relating to, among other items, disclosures in SEC filings and the treatment of investment banks in connection with business combination transactions involving special purpose acquisition companies. The uncertainty related to the treatment of investment banks following the SPAC Rule Proposals was the reason Nomura gave for its resignation. Pursuant to the Nomura Termination Letter, Nomura resigned from, and ceased or refused to act in, every capacity and relationship contemplated under the Nomura Engagement Letter, effective as of April 27, 2022, and waived its entitlement to the payment of any fees or expense reimbursements included in the Nomura Engagement Letter. Certain provisions of the Nomura Engagement Letter survive the termination of the Nomura Engagement Letter. These provisions include customary obligations with respect to use of information, indemnification and governing law. For example, Moolec is obligated to indemnify, defend and hold Nomura, or any of its respective affiliates, current and former directors, officers, employees, agents and representatives, and the successors and assigns of all the foregoing persons, harmless from and against any losses, claims, damages, liabilities and expenses in connection with (i) any untrue or alleged untrue statement of a material fact contained in any offering materials prepared or reviewed and approved by Moolec in connection with any placement of securities or (ii) any matter in any way directly or indirectly relating to or referred to in the Nomura Engagement Letter or arising out of the matters contemplated by the Nomura Engagement Letter. Due to Nomura’s resignation prior to the consummation or the Business Combination or the Transactions, Moolec does not expect there to be any material obligations of Moolec or LightJump that arise out of the Nomura Engagement Letter. Moreover, as of the date of this proxy statement/prospectus, neither Moolec or LightJump is a party to any agreement that would require the payment of any fees to, or require the reimbursement of any expenses of Nomura with respect to the Business Combination or the Transactions. In addition, Nomura delivered notice of resignation to the SEC pursuant to Section 11(b)(1) under the Securities Act on July 22, 2022.
As a result of this resignation and the associated waiver of fees, there are no transaction fees payable by Moolec or LightJump at the consummation of the Business Combination to Nomura. We note that the resignation of Nomura will have no impact on the Business Combination or the Transactions. While Nomura reviewed drafts of certain marketing materials and presentations of Moolec, such review was in its capacity as financial advisor to Moolec related to Moolec’s general marketing strategy. Nomura was not involved in the preparation of any disclosure that is included in this registration statement, including but not limited to the disclosure regarding any material that was prepared by Moolec’s management and reviewed by the board of directors of LightJump in connection with its decision to move forward with the Business Combination. Nomura did not have a role in the negotiation of any materials related to or arising out of the Transactions and had no role in any subsequent preparation of disclosure included in this proxy statement/prospectus. We note that Nomura was provided with the disclosures in this proxy statement/prospectus pertaining to its roles and resignation, however we did not receive a response from Nomura whether they agree with either the risks or the conclusions stated herein that are associated with its role and resignation.
Shareholders and investors should not place any reliance on the fact that Nomura had previously been engaged by Moolec. Moolec and LightJump did not rely on Nomura, in its role as exclusive financial advisor to Moolec, in any capacity in connection with the Business Combination. LightJump Holders or investors may believe that when financial institutions, such as Nomura, are named in a proxy statement/prospectus, the involvement of such institutions typically presumes a level of due diligence and independent analysis on the part of such financial institution and that the naming of such financial institutions generally means that a financial institution has done a level of due diligence ordinarily associated with a professional engagement. Both the Nomura Termination Letter with respect to its engagement with Moolec and the notice of resignation to the SEC pursuant to Section 11(b)(1) under the Securities Act, stated that Nomura is not responsible for any part of this registration statement. While Nomura did not provide any additional detail in its resignation letters either to Moolec or to the SEC, such resignation may be an indication by Nomura that it does not want to be associated with the disclosure in this proxy statement/prospectus, including any discussion related to reasons for its withdrawal, or the underlying business analysis related to the transaction. Accordingly, LightJump holders and investors should not place any reliance on the fact that Nomura has been previously involved with this transaction solely as it was engaged as the exclusive financial advisor to Moolec.
99
Table of Contents
LightJump’s Board of Directors’ Reasons for the Approval of the Business Combination
In reaching its unanimous decision to approve the Business Combination Agreement and the transactions contemplated by the Business Combination Agreement, LightJump’s board of directors considered a range of factors, including, but not limited to, the factors discussed below. In light of the number and wide variety of factors considered in connection with its evaluation of the combination, LightJump’s board of directors did not consider it practicable to, and did not attempt to, quantify or otherwise assign relative weights to the specific factors that it considered in reaching its determination and supporting its decision. LightJump’s board of directors viewed its decision as being based on all of the information available and the factors presented to and considered by it. In addition, individual directors may have given different weight to different factors.
This explanation of LightJump’s reasons for the approval by LightJump’s board of directors of the Business Combination and all other information presented in this section is forward-looking in nature and, therefore, should be read in light of the factors discussed under the section titled “Cautionary Note Regarding Forward-Looking Statements.”
Before reaching its decision, the LightJump board of directors reviewed the results of the due diligence conducted by our management, which included:
• extensive meetings and calls with Moolec’s management and advisors to understand and analyze Moolec’s business and to understand Moolec’s product pipeline and related assumptions;
• consultation with industry experts regarding competitive landscape, industry outlook, company reputation, business model and scientific validity;
• consultation with Moolec’s management and legal and financial advisors;
• consultation with LightJump’s legal and accounting advisors;
• review of Moolec’s material contracts and financial, tax, legal, accounting, environmental, and intellectual property due diligence;
• review of Moolec’s financial statements;
• financial review and analysis of Moolec and the Business Combination;
• study of analyst reports and market trends in the industries;
• research on comparable private companies;
• research on comparable private companies;
• research on comparable transactions.
In approving the combination, LightJump’s board of directors did not initially obtain a fairness opinion. However, it was decided to include the fairness opinion as a condition of closing, which was received on October 15, 2022. See “— Fairness Opinion of Scura Partners.” The officers and directors of LightJump have substantial experience in evaluating the operating and financial merits of companies from a wide range of industries and concluded that their experience and background enabled them to make the necessary analyses and determinations regarding the Business Combination. In performing such analyses and making determinations in connection with the Business Combination, LightJump’s management did not review prospective or projected financial information of Moolec.
LightJump’s management also considered a comparable company analysis to assess the potential value that the public markets would likely ascribe to Moolec, and this analysis was discussed with LightJump’s board. These companies were selected by LightJump as publicly traded companies having businesses that were considered, in certain respects, to be similar to the combined company’s business.
LightJump looked at several categories of potentially comparable public companies, including disruptive food companies that are creating new categories of consumption in the broader food industry through technological or business model changes. Specifically, the companies we evaluated included Freshpet, Inc., The Simply Good Foods Company, Celsius Holdings, Inc., BellRing Brands, Inc., Beyond Meat, Inc., Oatly Group AB, Tattooed Chef, Inc., AppHarvest, Inc., Village Farms International, Inc., Else Nutrition Holdings Inc. and Burcon NutraScience
100
Table of Contents
Corporation. Due to Moolec’s innovative technology, strong management team, commercial support from Bioceres SA, financial support from Union Acquisition Group, and growth potential, we believe that Moolec should trade comparably, relative to its startup nature, to similar disruptive food companies, as it executes on its business plan. As detailed in the table below, pre-money enterprise value is $325 million. We also looked at the valuation on a post-money basis under two separate redemption scenarios, which we viewed as the best-and-worst case scenarios. This is also outlined in the table below:
| | Comparable Companies |
($ in Millions) | | Total Market Capitalization(1) | | Total Enterprise Value(2) |
Disruptive Food Companies | | | | | | | | |
Freshpet, Inc. | | $ | 5,032 | | | $ | 4,966 | |
The Simply Good Foods Company | | | 4,776 | | | | 5,204 | |
Celsius Holdings, Inc. | | | 4,491 | | | | 4,477 | |
BellRing Brands, Inc. | | | 3,374 | | | | 6,636 | |
Beyond Meat, Inc. | | | 2,744 | | | | 3,168 | |
Oatly Group AB | | | 2,521 | | | | 2,125 | |
Tattooed Chef, Inc. | | | 839 | | | | 762 | |
AppHarvest, Inc. | | | 471 | | | | 458 | |
Village Farms International, Inc. | | | 400 | | | | 441 | |
Else Nutrition Holdings Inc. | | | 166 | | | | 142 | |
Burcon NutraScience Corporation | | | 103 | | | | 93 | |
Total Peer Group Average | | $ | 2,265 | | | $ | 2,588 | |
| | | | | | | | | |
Moolec Science(3)(4) | | | | | | | | |
Pre-Transaction | | $ | 325 | | | $ | 325 | |
(% Discount to Peer Group)(5) | | | 85.7 | % | | | 87.4 | % |
Post-Transaction (100% Redemption Rate)(6)(7) | | $ | 376 | | | $ | 373 | |
Post-Transaction (% Discount to Peer Group) | | | 83.4 | % | | | 85.6 | % |
Post-Transaction (0% Redemption Rate) | | $ | 504 | | | $ | 386 | |
Post-Transaction (% Discount to Peer Group) | | | 77.7 | % | | | 85.1 | % |
Although none of the selected companies reviewed in this analysis were directly comparable to Moolec, the companies operate in the broadly defined food industry and are each innovating on at least one important technological or business model attribute and therefore LightJump believes that the selected companies provide the most relevant basis to evaluate the proposed valuation of Moolec. The selected companies generally had products that were farther along in their development or had revenue producing products whereas Moolec is prerevenue. While LightJump looked for prerevenue disruptive food public companies to include in its analysis, LightJump did not identify any prerevenue public companies that (i) had similar products, technology platforms, or partnerships and (ii) were publicly traded with readily available financial or other information to include. Given Moolec’s unique technology platform and business strategy, and the limited number of directly comparable public companies, LightJump believed that it was important to consider both quantitative and qualitative information in its analysis of
101
Table of Contents
Moolec versus comparable companies. With respect to quantitative information, LightJump reviewed the selected companies’ total market capitalization and total enterprise value in its analysis. Additionally, with respect to qualitative information, LightJump reviewed the operational, business and/or financial characteristics of Moolec and the selected companies to provide a context in which to consider the results of the quantitative analysis. In its review of qualitative information, Moolec’s technology platform, disruptive nature, strong barriers to competitive entry, management team, commercial support from Bioceres SA, financial support from Union Acquisition Group, product pipeline, and total addressable market were key qualitative factors. LightJump’s board considered this analysis and viewed Moolec to be favorable compared to such other companies.
LightJump’s board of directors considered a number of factors pertaining to the Business Combination as generally supporting its decision to enter into the Business Combination Agreement and the transactions contemplated thereby, including, but not limited to, the following:
• Strong Product Concept. LightJump’s board of directors considered Moolec’s positioning as one of the first companies to offer scalable, molecular farming solutions as an alternative to plant-based, fermentation-based, and cultured meat foods;
• Significant Market Opportunity. LightJump’s board of directors considered Moolec’s focus on the molecular farming market with the potential for Moolec to achieve a meaningful share of the alternative protein market. In addition, LightJump’s board of directors believes Moolec’s molecular farming techniques may make Moolec more competitive than the competitors in the plant-based, fermentation, and cultured meat categories;
• Ownership of Intellectual Property (“IP”). LightJump’s board of directors considered the competitive advantages offered by the significant amount of IP owned versus IP outsourced by Moolec. LightJump’s board of directors also considered the potential difficulty competitors may have in replicating Moolec’s IP and the extensive portfolio of patent applications Moolec has filed to protect its innovations;
• Complementary and Experienced Management Teams. LightJump’s management and board of directors believe that the complementary business, industry and investing experience of LightJump’s board members and Moolec’s management team will help to accelerate the growth for the Combined Company. In addition, Moolec has a strong management team, which is expected to remain with the Combined Company to seek to execute Moolec’s strategic and growth goals;
• Moolec’s Business Model. LightJump’s board of directors and management considered the Company’s technology platform, large addressable market, multiple partnerships, product pipeline, and intellectual property portfolio which the board of directors believes can provide strong growth prospects for the Combined Company;
• Financial Condition. LightJump’s board of directors also considered factors such as Moolec’s outlook, financial plan and capital structure, relative capital efficiency, as well as valuations and trading of comparable publicly traded companies;
• Due Diligence. LightJump’s management conducted due diligence examinations of Moolec and discussions with Moolec’s management and LightJump’s financial and legal advisors concerning LightJump’s due diligence examination of Moolec;
• Other Alternatives. LightJump’s board of directors believes, after a thorough review of other business combination opportunities reasonably available to LightJump, that the proposed Business Combination represents the best available business combination opportunity for LightJump based upon the process utilized to evaluate and assess other potential combination targets, and LightJump’s board of directors’ belief that such process has not presented a better available alternative; and
• Negotiated Transaction. The financial and other terms of the Business Combination Agreement and the fact that such terms and conditions are reasonable and were the product of arm’s length negotiations between LightJump and Moolec.
102
Table of Contents
In the course of its deliberations, LightJump’s board of directors considered a variety of uncertainties, risks and other potentially negative reasons relevant to the Business Combination, including the below:
• Benefits May Not Be Achieved. The risk that the potential benefits of the Business Combination may not be fully achieved or may not be achieved within the expected timeframe;
• Development Stage Company. Moolec’s status as early stage company and the risks associated with executing on its business plan, including, without limitation, the risks associated with Moolec’s scale-up plans, and Moolec’s ability to meet its development timelines, execute on its market strategy and integrate a complex supply chain;
• Redemption Risk. The risk that a significant number of LightJump Holders may elect to redeem their shares prior to the consummation of the Business Combination pursuant to LightJump’s existing charter, which may potentially make the Business Combination more difficult to complete and would reduce the amount of cash available to the Combined Company to fund its business plan following the consummation of the Business Combination;
• LightJump Holders Receiving a Minority Position. The fact that LightJump Holders will hold a minority position in the Combined Company;
• Fees and Expenses. The significant fees and expenses associated with completing the Business Combination and the substantial time and effort of LightJump’s management required to complete the Business Combination;
• Interests of LightJump’s Directors and Officers. The interests of LightJump’s directors and officers in the Business Combination, and the potential conflicts of interest they present (see “— Interests of Certain Persons in the Business Combination”); and
• Other Risk Factors. Various other risk factors associated with Moolec’s business, as described in the section entitled “Risk Factors” appearing elsewhere in this document.
After considering the foregoing potentially negative and potentially positive reasons, LightJump’s board of directors concluded, in its business judgment, that the potentially positive reasons relating to the Business Combination and the other related transactions outweighed the potentially negative reasons. The LightJump board of directors recognized that there can be no assurance about future results, including results considered or expected as disclosed in the foregoing discussion.
The above discussion of the material factors considered by LightJump’s board of directors sets forth the principal factors it considered but is not intended to be exhaustive.
Fairness Opinion of Scura Partners
LightJump retained Scura Partners to evaluate the fairness, from a financial point of view, to LightJump shareholders of the merger consideration to be paid to such holders in the Transaction.
On October 6, 2022, at a meeting of the LightJump board of directors, Scura Partners rendered to the LightJump board of directors an oral opinion, which was confirmed by delivery of a written opinion dated October 15, 2022, to the effect that, as of that date and based on and subject to the assumptions made, procedures followed, matters considered and qualifications and limitations on the review undertaken described in such opinion, (i) the consideration in the BCA is fair from a financial point of view to the Lightjump shareholders and (ii) the fair market value of Moolec equals or exceeds 80% of the amount held by the LightJump in trust for benefit of its holders of Public Shares (excluding any deferred underwriting commissions and taxes payable on interest earned on the trust account).
The full text of Scura Partners’ written opinion, dated October 15, 2022, which describes the assumptions made, procedures followed, matters considered, and qualifications and limitations on the review undertaken, is attached as Annex B and is incorporated by reference in this document. The summary of the written opinion of Scura Partners, dated October 15, 2022, set forth in this proxy statement/prospectus is qualified in its entirety by reference to the full text of Scura Partners’ opinion attached as Annex B. Scura Partners’ opinion was rendered to the LightJump board of directors in connection with its evaluation of the BCA and did not address any terms or other aspects (other than the consideration to the extent expressly specified in Scura
103
Table of Contents
Partners’ opinion) of the transaction contemplated by the BCA. Scura Partners’ opinion did not address LightJump’s underlying business decision to effect such transaction or the relative merits of such transaction as compared to any alternative business strategies or transactions that might be available to LightJump and did not address any legal, regulatory, tax, or accounting matters. Scura Partners’ opinion is not intended to and does not constitute a recommendation to any LightJump stockholder as to how such stockholder should vote or act with respect to the transaction or any matter relating thereto.
With respect to the bullets listed below and in connection with rendering its opinion, although Scura Partners considered internal information provided to it with respect to the business, earnings, cash flow, assets and liabilities of Moolec and discussions conducted with the management and representatives of Moolec, Scura Partners created its own financial models that served as the basis for its analysis set forth below and the opinion it rendered to LightJump’s board of directors.
In connection with its opinion, Scura Partners:
• reviewed certain internal information relating to the business, earnings, cash flow, assets and liabilities of Moolec furnished to Scura Partners by LightJump;
• prepared financial models and discussed with the management of LightJump and the management of Moolec;
• conducted discussions with members of the management and representatives of LightJump and of Moolec concerning the information described in the two foregoing bullet points;
• reviewed LightJump’s and Moolec’ capital structure furnished to Scura Partners by the management of LightJump both on a standalone basis pre-transaction and on a pro forma basis giving effect to the transaction contemplated by the BCA;
• reviewed publicly available financial and stock market data of certain other companies in lines of business that Scura Partners deemed relevant;
• reviewed the BCA, dated as of June 14, 2022; and
• conducted such other financial studies and analyses and took into account such other information as Scura Partners deemed appropriate.
Scura Partners, with LightJump’s consent, relied upon the information supplied to, discussed with or reviewed by Scura Partners for purposes of its opinion being complete and accurate in all material respects. Scura Partners did not assume any responsibility for independent verification of, and did not independently verify, any of such information.
With LightJump’s consent, Scura Partners relied upon, without independent verification, the assessment by LightJump and its legal, tax, regulatory and accounting advisors with respect to legal, tax, regulatory and accounting matters. In addition, Scura Partners relied upon, with LightJump’s consent, the assessments of the management of LightJump as to the existing technology, products and services of Moolec and the validity of, and risks associated with, the future technology, products and services of Moolec. Scura Partners assumed, with LightJump’s consent, that there will be no developments with respect to any of the foregoing that would affect Scura Partners’ analyses or opinion. With LightJump’s consent, Scura Partners assumed that any adjustments to the consideration in accordance with the BCA or otherwise would not be material to Scura Partners’ analysis or its opinion. In addition, Scura Partners relied upon, with LightJump’s consent, the assessments of the management of LightJump as to LightJump’s ability to retain key employees of Moolec. In addition, with LightJump’s consent, Scura Partners did not make any independent evaluation or appraisal of any of the assets or liabilities (contingent, derivative, off-balance-sheet, or otherwise) of Moolec or LightJump, nor had Scura Partners been furnished with any such evaluation or appraisal.
Further, Scura Partners’ opinion was necessarily based on economic, monetary, market and other conditions as in effect on, and the information made available to Scura Partners as of the date of its opinion. Scura Partners assumed no responsibility for updating its opinion based on developments after the date of its opinion. As stated above, Scura Partners’ opinion did not address the underlying business decision to effect the transactions contemplated by the BCA or the relative merits of the same as compared to any alternative business strategies or transactions that might be available to LightJump and does not address any legal, regulatory, tax, or accounting matters.
104
Table of Contents
With LightJump’s consent, Scura Partners did not opine on what the value of the shares of Holdco would be when issued pursuant to the transactions contemplated by the BCA. Scura Partners did not express any opinion as to fair value or the solvency of Moolec, Holdco or LightJump following the closing of the transactions contemplated by the BCA. In rendering its opinion, Scura Partners assumed, with LightJump’s consent, that the transaction contemplated by the BCA, will be consummated in accordance with its terms without any waiver or modification that could be material to Scura Partners’ analysis, and that the parties to the BCA will comply with all the material terms of the BCA. Scura Partners assumed, with LightJump’s consent, that all governmental, regulatory, or other consents and approvals necessary for the completion of the transaction contemplated by the BCA will be obtained except to the extent that could not be material to Scura Partners’ analysis.
Summary of the Financial Analyses of Scura Partners
In preparing its opinion to the LightJump board of directors, Scura Partners performed a variety of financial and comparative analyses, and, for purposes of its opinion, used only those financial models that Scura Partners created. The summary set forth below does not purport to be a complete description of the financial analyses performed or factors considered by, and underlying Scura Partners’ opinion, nor does the order of the financial analyses described represent the relative importance or weight given to those financial analyses. The preparation of a financial opinion or analysis is a complex process involving various determinations as to the most appropriate and relevant methods of financial analysis and the application of those methods to the particular circumstances and, therefore, a financial opinion or analysis is not readily susceptible to summary description. In arriving at its opinion, Scura Partners considered the results of all of the analyses undertaken by it and assessed as a whole and did not draw, in isolation, conclusions from or with regard to any particular factor or method of analysis considered by it. Rather, Scura Partners made its determination as to fairness on the basis of its experience and professional judgment after considering the results of all of the analyses. Accordingly, Scura Partners believes that its analyses and factors summarized below must be considered as a whole and in context. Scura Partners further believes that selecting portions of its analyses and factors or focusing on information presented in tabular format, without considering all analyses and factors or the narrative description of the analyses and factors, could create a misleading or incomplete view of the processes underlying its analyses and opinion.
In performing its analyses, Scura Partners considered industry performance, general business, economic, market and financial conditions and other matters, existing as of the date of its opinion, many of which are beyond the control of LightJump and Moolec. No company, business or transaction reviewed is identical or directly comparable to LightJump, Moolec or their respective businesses or the transaction. Accordingly, an evaluation of these analyses is not entirely mathematical. Rather, the analyses involve complex considerations and judgments concerning business, financial and operating characteristics and other factors that could affect the public trading, acquisition or other values of the companies, businesses or transactions reviewed or views regarding the comparability of such companies, businesses or transactions. Accordingly, such analyses may not necessarily include all companies, businesses or transactions that could be deemed relevant. The estimates of the future performance of LightJump and Moolec in or underlying Scura Partners’ analyses and the ranges of valuations resulting from any particular analysis are not necessarily indicative of actual values or predictive of future results or values, which may be significantly more or less favorable than those estimates or those suggested by the analyses. In addition, analyses relating to the value of businesses or securities do not purport to be appraisals or to reflect the prices at which a company may actually be sold or the prices at which any securities have traded or may trade at any time in the future. Accordingly, the assumptions and estimates used in, and the ranges of valuations resulting from, any particular analysis described below are inherently subject to substantial uncertainty and should not be taken as the views of Scura Partners regarding the actual values of Moolec or LightJump. Except as otherwise noted, the following quantitative information, to the extent that it is based on market data, is based on market data as it existed on or before October 6, 2022 and is not necessarily indicative of current market conditions.
The type and amount of consideration payable in the transaction contemplated by the BCA was determined through negotiations between LightJump and Moolec, rather than by any financial advisor, and was approved by the LightJump board of directors. The decision to enter into the BCA was solely that of the LightJump board of directors and the Moolec board of directors. Receipt of Scura Partners’ opinion and analyses should not be viewed as determinative of the views of the LightJump board of directors or management with respect to the transaction or the consideration payable in the transaction.
105
Table of Contents
Financial Analyses
The summary of the financial analyses described in this section entitled “— Financial Analyses” is a summary of the material financial analyses provided by Scura Partners in connection with its opinion, dated October 15, 2022, to the LightJump board of directors. The summary set forth below is not a comprehensive description of all analyses undertaken by Scura Partners in connection with its opinion, nor does the order of the analyses in the summary below indicate that any analysis was given greater weight than any other analysis. The financial analyses summarized below include information presented in tabular format. In order to fully understand Scura Partners’ financial analyses, the tables must be read together with the text of each summary. The tables alone do not constitute a complete description of the financial analyses performed by Scura Partners. Considering the data set forth in the tables below without considering the full narrative description of the financial analyses, including the methodologies and assumptions underlying the analyses, could create a misleading or incomplete view of the financial analyses performed by Scura Partners. Future results may differ from those described and such differences may be material.
The financial data utilized for LightJump in the financial analyses described below were based on, among other things: (i) certain internal information relating to the business, earnings, cash flow, assets and liabilities of Moolec furnished to Scura Partners by LightJump; (ii) certain internal information relating to expenses expected to result from the Transaction furnished to Scura Partners by LightJump; (iii) LightJump’s and Moolec’s capital structure furnished to us by the management of LightJump both on a standalone basis pre-Transaction and on a pro forma basis giving effect to the Transaction; (iv) publicly available financial and stock market data of certain other companies in lines of business that Scura Partners deemed relevant; and (v) such other financial studies and analyses and such other information as Scura Partners deemed appropriate. Scura Partners assumed that Moolec would have net debt less cash of $0 at closing. As a result, the terms “Enterprise Value’ and “Equity Value” are identical in this analysis.
Moolec Financial Analyses
Comparable Public Companies Analysis
Using public filings and other publicly available information, Scura Partners compared certain financial information of Moolec to corresponding financial information for selected publicly traded companies that, based on Scura Partners’ professional judgment and experience, Scura Partners considered generally relevant for purposes of analysis. Scura Partners included companies in the plant-based products, ingredients and bioscience industries. Scura Partners did not include companies where there was a lack of forecasts over the periods of 2027, 2028 and 2029. The selected companies used in this analysis were as follows: Beyond Meat, Inc., Bioceres Crop Solutions Corp., Burdon NutraScience Corporation, Calyxt, Inc., Corteva, Inc., Koninklijke DSM N.V., Oatly Group AB and Yield 10 Bioscience.
Scura Partners selected the companies above because, among other things, the selected companies operate businesses similar in certain respects to the business of Moolec.
As set forth in the table below, for each selected company, Scura Partners calculated its enterprise value (defined as equity market value, plus debt, lease liabilities, preferred stock and minority interests, less cash and cash equivalents) as a multiple of its Revenue for fiscal year ends 2027, 2028 and 2029 (“Enterprise Value/2027 Revenue”, “Enterprise Value/2028 Revenue”, and “Enterprise Value/2029 Revenue”).
The mean Enterprise Value/2027 Revenue, Enterprise Value/2028 Revenue, and Enterprise Value/2029 Revenue multiples observed for the supply chain solutions peers were 1.6x, 1.5x and 2.2x, respectively. The median Enterprise Value/2027 Revenue, Enterprise Value/2028 Revenue, and Enterprise Value/2029 Revenue multiples observed for the supply chain solutions peers were 0.9x, 0.8x and 0.7x, respectively.
106
Table of Contents
(U.S. dollars in millions)
| | Enterprise Value | | Revenue 2027 | | Revenue 2028 | | Revenue 2029 | | Enterprise
Value/
Revenue
2027 | | Enterprise
Value/
Revenue
2028 | | Enterprise
Value/
Revenue
2029 |
Beyond Meat, Inc. (NasdaqGS:BYND) | | $ | 1,727 | | $ | 1,971 | | $ | 2,223 | | $ | 2,467 | | 0.9x | | 0.8x | | 0.7x |
Bioceres Crop Solutions Corp. (NasdaqGS:BIOX) | | $ | 988 | | $ | 972 | | $ | 1,107 | | $ | 1,321 | | 1.0x | | 0.9x | | 0.8x |
Burcon NutraScience Corporation (TSX:BU) | | $ | 37 | | $ | 5 | | $ | 6 | | $ | 3 | | 7.4x | | 6.4x | | 11.9x |
Calyxt, Inc. (NasdaqCM:CLXT) | | $ | 11 | | $ | 138 | | $ | 228 | | $ | 329 | | 0.1x | | 0.0x | | 0.0x |
Corteva, Inc. (NYSE:CTVA) | | $ | 43,788 | | $ | 20,305 | | $ | 20,712 | | $ | 21,775 | | 2.2x | | 2.1x | | 2.0x |
Koninklijke DSM N.V. (ENXTAM:DSM) | | $ | 22,293 | | $ | 10,598 | | $ | 11,106 | | $ | 11,662 | | 2.1x | | 2.0x | | 1.9x |
Oatly Group AB (NasdaqGS:OTLY) | | $ | 1,515 | | $ | 1,815 | | $ | 1,992 | | | — | | 0.8x | | 0.8x | | — |
Yield10 Bioscience, Inc. (NasdaqCM:YTEN) | | $ | 7 | | $ | 64 | | $ | 73 | | $ | 82 | | 0.1x | | 0.1x | | 0.1x |
Expected Revenue was not available for Oatly Group AB 2029.
The mean Enterprise Value/2027 Revenue, Enterprise Value/2028 Revenue, and Enterprise Value/2029 Revenue multiples observed for the supply chain solutions peers were 4.7x, 9.6x and 3.4x, respectively. The median Enterprise Value/2027 Revenue, Enterprise Value/2028 Revenue, and Enterprise Value/2029 Revenue multiples observed for the supply chain solutions peers were 4.7x, 4.7x and 1.9x, respectively.
As set forth in the table below, for each selected company Scura Partners also calculated its enterprise value (defined as equity market value, plus debt, lease liabilities, preferred stock and minority interests, less cash and cash equivalents) as a multiple of its EBITDA (defined as estimated earnings before interest, taxes, depreciation and amortization, and adjusted for lease expenses (excluding short-term and variable expenses) for companies reporting in GAAP).
(U.S. dollars in millions)
| | Enterprise Value | | EBITDA 2027 | | EBITDA 2028 | | EBITDA 2029 | | Enterprise
Value/
EBITDA
2027 | | Enterprise
Value/
EBITDA
2028 | | Enterprise
Value/
EBITDA
2029 |
Beyond Meat, Inc. (NasdaqGS:BYND) | | $ | 1,727 | | $ | 366 | | $ | 446 | | $ | 525 | | 4.7x | | 3.9x | | 3.3x |
Bioceres Crop Solutions Corp. (NasdaqGS:BIOX) | | $ | 988 | | | — | | | — | | | — | | — | | — | | — |
Burcon NutraScience Corporation (TSX:BU) | | $ | 37 | | | — | | $ | 1 | | | — | | — | | 36.8x | | — |
Calyxt, Inc. (NasdaqCM:CLXT) | | $ | 11 | | $ | 31 | | $ | 73 | | $ | 120 | | 0.3x | | 0.1x | | 0.1x |
Koninklijke DSM N.V. (ENXTAM:DSM) | | $ | 22,293 | | $ | 2,059 | | $ | 2,118 | | $ | 2,310 | | 10.8x | | 10.5x | | 9.7x |
Oatly Group AB (NasdaqGS:OTLY) | | $ | 1,515 | | $ | 239 | | $ | 285 | | | — | | 6.3x | | 5.3x | | — |
Yield10 Bioscience, Inc. (NasdaqCM:YTEN) | | $ | 7 | | $ | 7 | | $ | 11 | | $ | 15 | | 1.1x | | 0.7x | | 0.5x |
Expected EBITDA was not available for Bioceres Crop Solutions Corp., and for partial years for Burcon NutraScience Corporation (TSX:BU) and Oakley.
Using its professional judgment and experience, Scura Partners applied a range of mean multiples of estimated EV/Revenue for 2027, 2028 and 2029 to Moolec’s expected Revenue for those years to produce a range of enterprise value for Moolec between $210 and $703 million.
Using its professional judgment and experience, Scura Partners applied a range of median multiples of estimated EV/Revenue for 2027, 2028 and 2029 to Moolec’s expected Revenue for those years to produce a range of enterprise value for Moolec between $110 and $239 million.
Using its professional judgment and experience, Scura Partners applied a range of mean multiples of estimated EV/EBITDA for 2027, 2028 and 2029 to Moolec’s expected EBITDA for those years to produce a range of enterprise value for Moolec between $258 and $511 million.
107
Table of Contents
Using its professional judgment and experience, Scura Partners applied a range of median multiples of estimated EV/EBITDA for 2027, 2028 and 2029 to Moolec’s expected EBITDA for those years to produce a range of enterprise value for Moolec between $257 and $1,042 million.
Precedent Transactions Analysis
Scura Partners did not identify transactions involving companies with publicly available financial information that Scura Partners believed, based on its experience and professional judgment, to be generally relevant for purposes of this analysis.
Discounted Cash Flow Analysis
Scura Partners performed a discounted cash flow (“DCF”) analysis of Moolec using a financial forecast that Scura Partners developed with the guidance of LightJump’s management and other information and data provided by the LightJump’s management to calculate the estimated present value of the future unlevered after-tax free cash flows projected to be generated by Moolec through the end of 2032. Based on the information provided by LightJump’s management, with the consent of the LightJump board of directors, Scura Partners assumed that the Business Combination would close on or about December 31, 2022. Scura Partners performed DCF analyses for two sets of periods (i) the term of January 1, 2023 through 2032 and (ii) the stub period as of 2032.
In performing the DCF analysis of Moolec, Scura Partners utilized a range of discount rates between 11.2% and 15.54%. A mid-range discount rate of 13.2% was calculated based on an estimate of Moolec’ weighted average cost of capital (“WACC”), arrived at using the capital asset pricing model, incorporating:
(i) an unlevered beta of 1.06x based on the unlevered beta of comparable companies;
(ii) a risk-free rate of return of 4.00% based on the yield on the 20-year U.S. treasury bond as of October 5, 2022;
(iii) a market return (Rm) of 8.92% based on the 20 year average return on the S&P 500 index.;
(iv) a size premium of between 2% and 4% estimated based on the market return premium assigned to businesses (public companies) with an enterprise value between $200 million and $1.0 billion;
(v) a country risk premium of between 0% and 11% estimated based on the current country risk premium ascribed to Argentina of approximately 11% and the apportion of that risk to be allocated to Moolec’s anticipated business; and
(vi) the assumption that Moolec’ capital structure would be funded with equity through the end of each set of periods.
The assumption that a portion of the capital structure would be funded by debt would have led to a lower WACC and a higher DCF net present value for Moolec. Such a change would not have affected Scura Partners’ opinion. Scura Partners estimated the range of discount rates using the mid-range discount rate and based on its experience and professional judgement.
In performing the DCF analysis of Moolec to arrive at a range of terminal values, Scura Partners applied terminal value multiples of between 6.1x and 14.1x to EBITDA in the final year. Scura Partners derived a mid-range terminal value multiple of 10.1x based on current EBITDA trading multiples for selected publicly traded companies with anticipated growth rates similar to the growth rate for Moolec anticipated for the terminal year. Based on its experience and professional judgement, Scura Partners estimated a range of terminal value multiples using the mid-range terminal value multiple.
In performing the DCF analysis of Moolec, Scura Partners also conducted the analysis assuming terminal years of 2028, 2929, 2030, 2031 and 2032. Scura Partners assumed a cash tax rate for Moolec of 25.5%.
After-tax free cash flows from each period in each set were discounted to December 31, 2022 using the mid-period convention. Terminal value for each set were discounted from the end of the relevant fiscal year to December 31, 2022.
108
Table of Contents
Using an assumption of cash and cash equivalents of $0 million, the Discounted Cash Flow Analysis indicated the following implied total equity value for Moolec:
(U.S. dollars in millions)
| | EBITDA Exit Multiple |
| | | 6.1x | | 8.1x | | 10.1x | | 12.1x | | 14.1x |
Equity Value | | $ | 705 | | $ | 857 | | $ | 1,008 | | $ | 1,159 | | $ | 1,310 |
(U.S. dollars in millions)
| | Weighted Average Cost of Capital |
| | | 11.2% | | 12.2% | | 12.2% | | 12.2% | | 12.2% |
Equity Value | | $ | 1,196 | | $ | 1,097 | | $ | 1,008 | | $ | 926 | | $ | 852 |
(U.S. dollars in millions)
| | Terminal Value Year |
| | | 2028 | | 2029 | | 2030 | | 2031 | | 2032 |
Equity Value | | $ | 544 | | $ | 703 | | $ | 841 | | $ | 921 | | $ | 1,008 |
(U.S. dollars in millions)
| | Size Premium |
| | | 1.0% | | 1.5% | | 2.0% | | 2.5% | | 3.0% |
Equity Value | | $ | 1,097 | | $ | 1,051 | | $ | 1,008 | | $ | 966 | | $ | 926 |
(U.S. dollars in millions)
| | Country Risk Premium |
| | | 0.0% | | 2.0% | | 5.0% | | 8.0% | | 11.0% |
Equity Value | | $ | 1,196 | | $ | 1,008 | | $ | 784 | | $ | 614 | | $ | 483 |
The terminal value represents approximately 76.0% of Moolec’s equity value for the set of periods from the stub period through FY2032, assuming the mid-range size premium, country premium, WACC and terminal value multiple.
General
In connection with Scura Partners’ services as a financial advisor to the LightJump board of directors, LightJump agreed to pay, and has paid in full, Scura Partners an aggregate fee of $150,000. In addition, LightJump has agreed to reimburse certain of Scura Partners’ expenses arising, and to indemnify Scura Partners against certain liabilities that may arise, out of Scura Partners’ engagement.
Scura Partners, as part of its investment banking business, is continually engaged in the valuation of businesses and their securities in connection with mergers and acquisitions, private placements, leveraged buyouts, and valuations for estate, corporate and other purposes. In the two-year period prior to the date of Scura Partners’ opinion, Scura Partners has not provided any investment banking services to LightJump. In the two-year period prior to the date of Scura Partners’ opinion, Scura Partners has not been engaged to provide financial advisory or other services to Moolec and Scura Partners has not received any compensation from Moolec during this period. In addition, in the ordinary course, Scura Partners’employees may trade securities of LightJump, Moolec and certain of their respective affiliates for their own accounts and, accordingly, may at any time hold a long or short position in such securities. The issuance of Scura Partners’ opinion was approved by the opinion committee of Scura Partners.
Scura Partners is an internationally recognized investment banking firm providing a full range of financial advisory and other services. Scura Partners was selected to act as investment banker to LightJump because of its qualifications, expertise and reputation in investment banking and mergers and acquisitions generally and in particular, as an advisor to companies in LightJump’s sector, as well as its familiarity with the business of LightJump.
109
Table of Contents
Scura Partners’ opinion is not intended to and does not constitute a recommendation to any LightJump stockholder as to how such stockholder should vote or act with respect to the transaction or any matter relating thereto.
The financial and comparative analyses and financial models that Scura Partners used or relied on (the “Fairness Opinion Financial Information”) was prepared by Scura Partners. Price Waterhouse & Co. S.R.L. has not audited, reviewed, examined compiled nor applied agreed-upon procedures with respect to the Fairness Opinion Financial Information for the purposes of its inclusion herein, and , accordingly, Price Waterhouse & Co. S.R.L. does not provide any form of assurance with respect thereto for the purpose of this proxy statement/prospectus. The Price Waterhouse & Co. S.R.L. reports included in this proxy statement/prospectus relates solely to Moolec’s previously issued financial statements. It does not extend to the Fairness Opinion Financial Information and should not be read to do so.
Interests of LightJump’s Directors and Officers in the Business Combination
When you consider the recommendation of LightJump’s board of directors in favor of approval of the Business Combination Proposal, you should keep in mind that certain of LightJump’s directors and officers have interests in the Business Combination that are different from, or in addition to, your interests as a stockholder. These interests include, among other things:
• the beneficial ownership of the Sponsor of an aggregate of 3,450,000 SPAC Common Stock, acquired for an aggregate purchase price of $25,000, which shares would become worthless if LightJump does not complete a business combination within the applicable time period, as the LightJump Initial Stockholders have waived any right to liquidation proceeds with respect to these shares. Such shares have an aggregate market value of approximately $[•] based on the closing price of SPAC Common Stock of $[•] on Nasdaq on [•], 2022, the record date for the special meeting of stockholders;
• Sponsor’s beneficial ownership of an aggregate of 4,210,000 warrants, acquired for an aggregate purchase price of $4,210,000, which warrants would become worthless if LightJump does not complete a business combination with the applicable time period. Such warrants have an aggregate market value of approximately $[•] based on the closing price of the SPAC Warrants of $[•] on Nasdaq on [•], 2022, the record date for the special meeting of stockholders.
• LightJump’s directors will not receive reimbursement for any out-of-pocket expenses incurred by them on LightJump’s behalf incident to identifying, investigating and consummating a business combination to the extent such expenses exceed the amount not required to be retained in the Trust Account, unless a business combination is consummated. As of [•], no out-of-pocket expenses have been incurred by LightJump’s directors incident to identifying, investigating and consummating a business combination;
• the potential continuation of certain of LightJump’s directors as directors of Holdco; and
• the continued indemnification of current directors and officers of LightJump and the continuation of directors’ and officers’ liability insurance after the Business Combination.
The Sponsor’s and/or its affiliates’ aggregate at-risk investment that depends on completion of a business combination totals $6,130,300, which consists of the following:
• $25,000 investment by Sponsor in the Founder Shares. See “Certain LightJump Relationships and Related Persons Transactions”;
• $4,210,000 investment by Sponsor in SPAC Warrants. See “Certain LightJump Relationships and Related Persons Transactions”;
• $350,000 loan from Moolec to Sponsor, of which $76,721 is drawn and outstanding. See “Certain LightJump Relationships and Related Persons Transactions — Promissory Note — Moolec”; and
110
Table of Contents
• $1,545,300 in promissory notes or advances by Sponsor, or its affiliates, to LightJump, including a $125,000 advance by First Lexington, LLC. See “Certain LightJump Relationships and Related Person Transactions — Promissory Note — Related Party” and “Certain LightJump Relationships and Related Party Transactions — Advances from Related Party.”
• The remaining amount consists of $1,420,300 in other promissory notes or advances to LightJump. See “Certain LightJump Relationships and Related Person Transactions — Promissory Note — Related Party.” Such amount includes $200,000 that was used to fund the Extension Amendment. For more information regarding the Extension Amendment, see “Business of LightJump and Certain Information about LightJump — Overview.”
Pursuant to the terms of the Business Combination, the Sponsor Advanced Funds will only be reimbursed by the Combined Company if the unpaid SPAC Transaction Expenses as of the Closing do not exceed the applicable SPAC Transaction Expenses Cap. See “The Business Combination Agreement — Expenses.”
The following table presents the share ownership of Sponsor upon the closing of the Business Combination and is based on the assumptions that (i) the Closing Date shall be [•], 2022, (ii) no additional equity securities of LightJump are issued at or prior to Closing, (iii) exercise of all outstanding SPAC Warrants and (iv) the following redemption scenarios:
No Redemptions. This scenario assumes that none of LightJump’s existing Public Stockholders will exercise their redemption rights in connection with the approval of the Business Combination with respect to their Public Shares.
50% Redemptions. This scenario assumes that LightJump’s existing Public Stockholders exercise their redemption rights with respect to 1,383,605 Public Shares (50% of the currently issued and outstanding unredeemed Public Shares) in connection with the approval of the Business Combination, at a price of $10.12 per share.
Maximum Redemptions. This scenario assumes that 100% of LightJump’s existing Public Stockholders exercise their redemption rights with respect to their public shares in connection with the approval of the Business Combination, at a price of $10.12 per share and assumes that the obligations under the Backstop Agreement will be satisfied by making a cash contribution to Holdco. See “Certain Agreements Related to the Business Combination — Backstop Agreement.”
| | No Redemptions(1) | | 50% Redemptions(1) | | Maximum Redemptions(1)(2) |
Shareholders of Holdco Post Business Combination(1) | | Number of
Holdco
Ordinary Shares | | % of
Total | | Number of
Holdco
Ordinary Shares | | % of
Total | | Number of
Holdco
Ordinary
Shares | | % of
Total |
Sponsor | | 6,625,000 | | 13.1 | % | | 6,625,000 | | 13.5 | % | | 7,125,0003 | | 14.6 | % |
Total | | 50,465,935 | | 100 | % | | 49,082,330 | | 100 | % | | 48,698,725 | | 100 | % |
For more information, see the section entitled “Unaudited Pro Forma Condensed Combined Financial Information.”
Regulatory Approvals Required for the Business Combination
Non-U.S. regulatory bodies and U.S. state attorneys general could take action under other applicable regulatory laws as they deem necessary or desirable in the public interest, including, without limitation, seeking to enjoin or otherwise prevent the completion of the Business Combination or permitting completion subject to regulatory conditions. Private parties may also seek to take legal action under regulatory laws under some circumstances. There
111
Table of Contents
can be no assurance that a challenge to the Business Combination on antitrust grounds will not be made or, if such a challenge is made, that it would not be successful. LightJump and Moolec are not aware of any other regulatory approvals in the United States, the United Kingdom, Luxembourg or elsewhere required for the consummation of the Business Combination.
Litigation Relating to the Business Combination
There have been no proceedings brought against LightJump in relation to the Business Combination or the Business Combination Agreement.
Listing of Holdco Ordinary Shares
Approval of the listing on Nasdaq of the Holdco Ordinary Shares to be issued in the Business Combination, subject to official notice of issuance, is a condition to each party’s obligation to complete the Business Combination.
Accounting Treatment of the Business Combination
The Business Combination will be accounted for as a capital reorganization in accordance with IFRS as issued by the IASB. Under this method of accounting, LightJump will be treated as the “acquired” company for financial reporting purposes and Moolec will be the accounting “acquirer.” This determination was primarily based on the assumptions that Moolec’s shareholders will hold a majority of the voting power of the Combined Company, Moolec’s operations will substantially comprise the ongoing operations of the Combined Company, Moolec’s designees are expected to comprise a majority of the governing body of the Combined Company, and Moolec’s senior management will comprise the senior management of the Combined Company. However, LightJump does not meet the definition of a “business” pursuant to IFRS 3, Business Combinations, and thus, for accounting purposes, the Business Combination will be accounted for as a capital reorganization. The net assets of LightJump will be stated at historical cost, with no goodwill or other intangible assets recorded.
112
Table of Contents
The Business Combination Agreement
This section of the proxy statement/prospectus describes the material provisions of the Business Combination Agreement, but does not purport to describe all of the terms of the Business Combination Agreement. This summary is qualified in its entirety by reference to the Business Combination Agreement, a copy of which is attached as Annex A hereto. Certain figures included in this section have been rounded for ease of presentation and, as a result, percentages may not sum to 100%.
The Business Combination
On June 14, 2022, LightJump, Moolec, Merger Sub and Holdco entered into the Business Combination Agreement, which contains customary representations and warranties, covenants, closing conditions and other terms relating to the Exchange and Merger and the other transactions contemplated thereby, as summarized below. Capitalized terms used in this section but not otherwise defined herein have the meanings given to them in the Business Combination Agreement.
The Structure of the Business Combination
Following the effectiveness of the transactions contemplated by the Merger, the parties will consummate the Business Combination and LightJump and Moolec will become direct wholly-owned subsidiaries of Holdco. Pursuant to the Business Combination Agreement, each of the following transactions will occur, in the following order:
• At the Exchange Effective Time, subject to the receipt of the Company Requisite Approvals, the Holdco Requisite Approvals and the delivery of the Exchange Auditor Report, and in accordance with the Holdco Delegate Resolutions:
• all issued Company Ordinary Shares held by the Company Shareholders shall be transferred and for the purposes of the 1915 Law, contributed in kind to Holdco, free and clear of all Liens (other than the Company Shareholders’ Agreements Liens that will expire on or prior to the Closing Date), and the Company Shareholders shall subscribe for and, as consideration for the contribution, shall be issued, in accordance with the Exchange Ratio (save that the Holdco Ordinary Shares to be issued shall be reduced by the number of Holdco Ordinary Shares already held by the Company Shareholders immediately prior to the Exchange), the Exchange Issuance;
• The Exchange Issuance shall be allocated among the Company Shareholders in accordance with the terms of the Payment Spreadsheet and Exchange Agreements;
• each Company SAFE Holder shall have contributed all of its rights and obligations under each Original SAFE to Holdco in consideration for the issuance by Holdco of a simple agreement for future equity on substantively identical terms (mutatis mutandis) with such adjustments (if any) required under Luxembourg law. For Luxembourg law purposes, a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued a report on the contributions in kind relating to the contribution of the Original SAFEs prepared in accordance with article 420-10 of the 1915 Law;
• each Company Shareholder shall cease to be the holder of such Company Ordinary Shares, subject to the submission of all filings required under Law (including any filings required to pay stamp duties), and Holdco will be recorded as the registered holder of all the Company Ordinary Shares so exchanged and transferred and will be the legal and beneficial owner thereof.
• Immediately prior to the Merger Effective Time but after the Exchange Effective Time, each Company SAFE Holder shall receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the respective Company SAFE, with such adjustments (if any) required under Luxembourg law;
• SPAC shall cause the Certificate of Merger to be executed, acknowledged and filed with the Secretary of State of the State of Delaware in accordance with the applicable provisions of the DGCL in order to effectuate the Merger. The Merger shall become effective at such time as the Certificate of Merger
113
Table of Contents
has been duly filed with the Secretary of State of the State of Delaware or at such later time as may be agreed by the Company and SPAC in writing and specified in the Certificate of Merger in accordance with the DGCL;
• At the Merger Effective Time, by virtue of the Merger and the Holdco Requisite Approvals, subject to the Merger Auditor Report, and without any further action on the part of SPAC, Merger Sub, Holdco or the Company or the holders thereunder:
• each SPAC Common Stock issued and outstanding immediately prior to the Merger Effective Time, excluding those have been redeemed subject to any redemption rights, shall be exchanged with Holdco (which exchange, for purposes of the 1915 Law, shall include, for the avoidance of doubt, a contribution-in-kind of each such shares of SPAC Common Stock from the holders of SPAC Common Stock to Holdco), against the issue by Holdco of new Holdco Ordinary Shares (such issuance, the “Merger Issuance”), under the authorized share capital of Holdco (pursuant to the Holdco Delegate Merger Resolutions) and subscribed by the contributing holders of SPAC Common Stock by virtue of the Merger and in accordance with the 1915 Law for one validly issued and fully paid Holdco Ordinary Share (the “Merger Consideration”), delivered by Holdco;
• as a result of the Merger, all SPAC Common Stock shall cease to be outstanding, shall be cancelled and shall cease to exist;
• each share of common stock, par value $0.01 of Merger Sub issued and outstanding immediately prior to the Merger Effective Time shall be converted and exchanged for one (1) validly issued, fully paid and nonassessable ordinary share, par value $0.01 per share, of the Surviving Company.
• Each SPAC Warrant that is outstanding immediately prior to the Merger Effective Time shall, pursuant to the SPAC Warrant Agreement, cease to represent a right to acquire one SPAC Common Stock and shall be converted in accordance with the terms of such SPAC Warrant Agreement, at the Merger Effective Time, into a right to acquire one Holdco Ordinary Share on substantially the same terms as were in effect immediately prior to the Merger Effective Time under the terms of the SPAC Warrant Agreement.
The parties to the Business Combination Agreement will hold the closing on the date of the Merger Effective Time, following the satisfaction or waiver (to the extent such waiver is permitted by applicable law) of the conditions set forth in the Business Combination Agreement (other than those conditions that by their nature are to be satisfied at Closing, but subject to the satisfaction or waiver of those conditions at such time).
The following diagram shows the current ownership structure of LightJump (excluding the impact of the shares underlying the SPAC Warrants).

114
Table of Contents
The following diagram shows the current structure of Moolec and Holdco:

The following diagram shows the pro forma ownership percentages (excluding the impact of the shares underlying the Holdco Warrants) and structure of Holdco immediately following the consummation of the Business Combination. The relative percentages assume that (i) none of LightJump’s existing Public Stockholders exercise their redemption rights in connection with the approval of the Business Combination with respect to their Public Shares, (ii) no additional equity securities of LightJump are issued prior to the Closing and (iii) a Closing Date of [•].

Consideration to be received in the Business Combination
At the Exchange Effective Time, the Company Shareholders shall make a contribution in kind to Holdco of all the issued and outstanding Company Ordinary Shares and subscribe and be issued an aggregate number of Holdco Ordinary Shares equal to the total number of issued and outstanding Company Ordinary Shares, multiplied by the Exchange Ratio (save that the Holdco Ordinary Shares to be issued shall be reduced by the number of Holdco Ordinary Shares already held by the Company Shareholders immediately prior to the Exchange), which is equal to an aggregate of 32,500,000 Holdco Ordinary Shares. The aggregate amount of Holdco Ordinary Shares to be issued by Holdco as part of the Exchange shall be allocated among the Company Shareholders in accordance with their respective Exchange Agreements.
115
Table of Contents
At the Merger Effective Time, each share of SPAC Common Stock issued and outstanding immediately prior to the Merger Effective Time will automatically be converted into one (1) validly issued and fully paid Holdco Ordinary Share, which will be valued at $10.00 per share and each SPAC Warrant will automatically be converted into one (1) validly issued Holdco Warrant.
Ownership of the Combined Company Upon Completion of the Business Combination
Following the Business Combination, each of LightJump and Moolec shall be direct wholly-owned subsidiaries in Holdco.
Representation and Warranties
The Business Combination Agreement contains customary representations, warranties and covenants of D-Moolec and Holdco, LightJump, and Merger Sub relating to, among other things, their ability to enter into the Business Combination Agreement and their outstanding capitalization.
Conduct of Business Pending Consummation of the Business Combination; Covenants
Conduct of Business by Moolec, Holdco and Merger Sub pending the Merger
From the date of the Business Combination Agreement and the Closing or the earlier termination of the Business Combination Agreement, except as (i) expressly contemplated or permitted by any other provision of the Business Combination Agreement or any Ancillary Agreement (ii) as set forth in the Company Disclosure Schedule, and (iii) as required by applicable Law (including COVID-19 Measures or as may be requested or compelled by any Governmental Authority), unless SPAC shall otherwise consent in writing, which consent shall not be unreasonably withheld, delayed or conditioned: (A) the Company shall, and shall cause the Company Subsidiaries to, conduct their business in all material respects in the ordinary course of business and in a manner consistent with past practice; provided that, in the case of actions that are taken (or omitted to be taken) reasonably in response to an emergency or urgent condition or conditions arising from COVID-19, the Company and the Company Subsidiaries shall not be deemed to be acting outside the ordinary course of business, so long as such actions or omissions are reasonably designed to (1) protect the health or welfare of the Company’s employees, directors, officers or agents or (2) comply with clause (B) herein, and in each case, the Company promptly notifies SPAC prior to taking such actions and reasonably takes into account the reasonable requests of SPAC in further acts or omissions of the Company with respect to such condition or conditions arising from COVID-19; and (B) the Company shall use its reasonable best efforts to preserve substantially intact the business organization of the Company and the Company Subsidiaries and shall use its commercially reasonable efforts to keep available the services of the current officers, key employees and consultants of the Company and the Company Subsidiaries and to preserve the current relationships of the Company and the Company Subsidiaries with customers, suppliers and other persons with which the Company or any Company Subsidiary has significant business relations.
By way of amplification and not limitation, except as (x) expressly contemplated or permitted by any other provision of this Agreement or any Ancillary Agreement, (y) as set forth in the Company Disclosure Schedule, and (z) as required by applicable Law (including COVID-19 Measures or as may be requested or compelled by any Governmental Authority), the Company shall not, and shall cause each Company Subsidiary, Holdco and Merger Sub not to, between the date of this Agreement and the Closing or the earlier termination of this Agreement, directly or indirectly, do any of the following without the prior written consent of SPAC which consent shall not be unreasonably withheld, delayed or conditioned: (i) amend or otherwise change its memorandum of association, articles of association, certificate of incorporation, by-laws or equivalent organizational documents; (ii) issue, sell, pledge, dispose of, grant or encumber, solicit, propose, negotiate with respect to, or authorize the issuance, sale, pledge, disposition, grant or encumbrance of, (A) any shares of any class of capital stock of the Company or any Company Subsidiary, if any, (B) any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital stock, or any other ownership interest (including any phantom interest) or (C) except in the ordinary course of business, any assets of the Company or any Company Subsidiary; (iii) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital stock; (iv) reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly,
116
Table of Contents
any of its capital stock, other than redemptions of equity securities from former employees upon the terms set forth in the underlying agreements governing such equity securities; (v) merge or consolidate with any other person or restructure, reorganize, dissolve or completely or partially liquidate or otherwise enter into any agreements or arrangements imposing material changes or restrictions on its assets, operations or businesses; (vi) acquire or invest (including, without limitation, by merger, consolidation, or acquisition of stock or assets or any other business combination) any corporate partnership, other business organization, or any division thereof, in each case, for an aggregate purchase price that exceeds $500,000; (vii) incur any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise become responsible for, the obligations of any person, or make any loans or advances, or intentionally grant any security interest in any of its assets, in each case, except in the ordinary course of business and consistent with past practice; (viii) transfer, sell, lease, license, mortgage, pledge, surrender, encumber, divest, cancel abandon or allow to lapse or expire or otherwise dispose of any of its material assets, properties, licenses, operations, rights, production lines, businesses or interests therein, except for sales or other dispositions in the ordinary course of business consistent with past practice; (ix) (A) increase the compensation or benefit payable to any current or former director, officer, employee or consultant of the Company or any Company Subsidiary, other than (1) health and welfare plan renewals in the ordinary course of business consistent with past practices or (2) increases in base salary or wage of employees in the ordinary course of business whose cash portion of the annual base salary or wage is not in excess of $200,000; (B) take any action to accelerate or commit to accelerate the funding, payment, or vesting of any compensation or benefits to any current or former director, officer, employee or consultant; (C) hire or otherwise enter into any employment or consulting agreement or arrangement with any person or terminate (other than for cause) any current or former director, officer, employee or consultant provider whose cash portion of the annual base salary or wage would exceed $200,000; or (D) enter into any new, or materially amend any existing employment or severance or termination agreement with any current or former director, officer, employee or consultant provider whose cash portion of the annual base salary or wage would exceed $200,000; (x) adopt, enter into, materially amend and/or terminate any Plan or any plan, program, agreement, arrangement or policy for the current or future benefit of any current or former director, officer, employee, consultant or individual independent contractor that would be a Plan if it were in existence on the date hereof (including any existing employment or severance or termination agreement with any current or former director, officer, employee or consultant), except as may be required by applicable Law or health and welfare Plan renewals in the ordinary course of business and consistent with past practice; (xi) materially amend other than reasonable and usual amendments in the ordinary course of business, with respect to accounting policies or procedures, other than as required by the Accounting Principles; (xii) make change or revoke any material Tax election, change any annual Tax accounting period, adopt or change any method of Tax accounting, materially amend any Tax Returns or file claims for Tax refunds except in the ordinary course of business, enter into any closing agreement, waive or extend any statute of limitations period in respect of an amount of Taxes, settle any Tax claim, audit or assessment, or surrender any right to claim a Tax refund, offset or other reduction in Tax liability; (xiii) materially amend or modify, or consent to the termination (excluding any expiration in accordance with its terms) of any Material Contract or materially amend or modify, waive, or consent to the termination (excluding any expiration in accordance with its terms) of the Company’s or any Company Subsidiary’s material rights thereunder, in each case in a manner that is adverse to the Company or any Company Subsidiary, taken as a whole, except in the ordinary course of business; (xiv) intentionally permit any material item of Company IP to lapse or to be abandoned, invalidated, dedicated to the public, or disclaimed, or otherwise become unenforceable or fail to perform or make any applicable filings, recordings or other similar actions or filings, or fail to pay all required fees and Taxes required to maintain and protect its interest in each and every material item of Company IP; (xv) create or incur any Lien material to the Company, any Company Subsidiary, Holdco or Merger Sub other than Permitted Liens incurred in the ordinary course of business consistent with past practice; (xvi) make any loans, advances, or guarantees in any person (other than the Company or any Company Subsidiaries); (xvii) fail to make or authorize any budgeted capital expenditures or make or authorize any unbudgeted capital expenditures, in each case in excess of 10% of the aggregate amount of budgeted capital expenses; (xviii) fail to pay or satisfy when due any material account payable or other material liability, other than in the ordinary course of business consistent with past practice or any such liability that is being contested in good faith by the Company or any Company Subsidiary or otherwise materially change the manner by which the Company or any Company Subsidiary manages its working capital; (xix) fail to keep current and in full force and effect, or comply in all material respects with the requirements of any Company Permit issued to the Company or any Company Subsidiary by any Governmental Authority; (xx) take any steps for liquidation, winding-up, freeze of proceedings, arrangements with creditors or similar action or proceeding by or in
117
Table of Contents
respect of the Company or any Company Subsidiary; (xxi) take any actions or omit to take any actions that would, individually or in the aggregate, reasonably be expected to result in any of the conditions set forth in Article IX not being satisfied; (xxii) engage in any dealings or transactions (i) with any Restricted Person in violation of applicable laws; (ii) involving any Sanctionable Activity; or (iii) otherwise in violation of Sanction Laws, Export Control Laws or Import Control Laws; or (xxiii) enter into any formal or informal agreement or otherwise make a binding commitment to do any of the foregoing.
By way of amplification and not limitation, Holdco shall not, and the Company Shareholders or the Company, as the case may be, shall not permit Holdco to, between the date of the Business Combination and the Closing or the earlier termination of the Business Combination Agreement, directly or indirectly, except as expressly contemplated or permitted by any other provision of the Business Combination Agreement, any Ancillary Agreements or as may be required by applicable Law (including as may be requested or compelled by any Governmental Authority), do any of the following without the prior written consent of SPAC which consent shall not be unreasonably withheld, delayed or conditioned: (i) engage in any business or activity other than the consummation of the Exchange; (ii) amend or otherwise change the Holdco Organizational Documents except for the A&R Holdco Organizational Documents or as otherwise required to implement the Exchange and issuance of Merger Consideration; (iii) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital stock; (iv) reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of the Holdco Ordinary Shares; (v) issue, sell, pledge, dispose of, grant or encumber, or authorize, solicit, propose, or negotiate with respect to the issuance, sale, pledge, disposition, grant or encumbrance of, any shares of any class of capital or other securities of Holdco or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital, or any other ownership interest (including, without limitation, any phantom interest), of Holdco; (vi) liquidate, dissolve, reorganize or otherwise wind up the business and operations of Holdco; (vii) amend any Exchange Agreement or any other agreement related to the Exchange; (viii) permit any Company Shareholder who owns Holdco Ordinary Shares both prior to the Exchange and pursuant to the Exchange to transfer, sell, lease, license, mortgage, pledge, surrender, encumber, divest, cancel, abandon or otherwise dispose of any Holdco Ordinary Shares, or recognize any such transfer, sale, lease, license, mortgage, pledge, surrender, encumbrances, divestment, cancellation, abandonment or other disposition of Holdco Ordinary Shares; (ix) transfer, sell, lease, license, mortgage, pledge, surrender, encumber, divest, cancel, abandon or allow to lapse or expire or otherwise dispose of any Company Ordinary Shares acquired pursuant to the Exchange and any such attempted action shall be null and void and Company will not inscribe any such transfer (of any kind as contemplated in this provision) in the shareholder register of Company; (x) acquire or hold any equity securities or rights thereto in any person other than the Company, pursuant to the Exchange, and Merger Sub; or (xi) enter into any formal or informal agreement or otherwise make a binding commitment to do any of the foregoing.
Conduct of Business by LightJump pending the Merger
From the date of the Business Combination Agreement and the Closing or earlier termination of the Business Combination Agreement, except as expressly contemplated by any other provision of the Business Combination Agreement or any Ancillary Agreement and as required by applicable Law (including as may be requested or compelled by any Governmental Authority), unless the Company shall otherwise consent in writing (which consent shall not be unreasonably withheld, delayed or conditioned), the business of SPAC shall be conducted in the ordinary course of business and in a manner consistent with past practice and SPAC shall not, directly or indirectly, take any action that would reasonably be likely to materially delay or prevent the Transactions. By way of amplification and not limitation, except as expressly contemplated by any other provision of the Business Combination Agreement or any Ancillary Agreement or as required by applicable Law (including as may be requested or compelled by any Governmental Authority), SPAC shall not, between the date of the Business Combination Agreement and the Closing or the earlier termination of the Business Combination Agreement, directly or indirectly, do any of the following without the prior written consent of the Company, which consent shall not be unreasonably withheld, delayed or conditioned: (i) amend or otherwise change the SPAC Organizational Documents (other than in connection with a SPAC Extension Proposal, if any); (ii) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, shares, property or otherwise, with respect to any of its shares, other than pursuant to the Redemption Rights from the Trust Fund that are required pursuant to the SPAC Organizational Documents; (iii) reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of the SPAC
118
Table of Contents
Common Stock or SPAC Warrants except for the Redemption Rights from the Trust Fund that are required pursuant to the SPAC Organizational Documents; (iv) issue, sell, pledge, dispose of, grant or encumber, or authorize, solicit, propose, or negotiate with respect to the issuance, sale, pledge, disposition, grant or encumbrance of, any shares of any class of shares or other securities of SPAC or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such shares, or any other ownership interest (including, without limitation, any phantom interest), of SPAC; (v) reclassify, combine, split or subdivide, directly or indirectly, any of its shares (except pursuant to the Redemption Rights); (vi) acquire (including, without limitation, by merger, consolidation, or acquisition of stock, shares or assets or any other business combination) any corporation, partnership, other business organization or any division thereof or any material amount of assets or enter into any strategic joint ventures, partnerships or alliances with any other person; (vii) incur any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise become responsible for, the obligations of any person, or make any loans or advances, or intentionally grant any security interest in any of its assets; (viii) make any change in any method of financial accounting or financial accounting principles, policies, procedures or practices, except as required by a concurrent amendment in GAAP or applicable Law made subsequent to the date hereof, as agreed to by its independent accountants; (ix) make, change or revoke any material Tax election, change any annual Tax accounting period, adopt or change any method of Tax accounting, amend any Tax Returns or file claims for Tax refunds, enter into any closing agreement, waive or extend any statute of limitations period in respect of an amount of Taxes, settle any Tax claim, audit or assessment, or surrender any right to claim a Tax refund, offset or other reduction in Tax liability; (x) liquidate, dissolve, reorganize or otherwise wind up the business and operations of SPAC; (xi) amend the Trust Agreement or any other agreement related to the Trust Account; or (xii) enter into any formal or informal agreement or otherwise make a binding commitment to do any of the foregoing.
From the date of the Business Combination Agreement and the Closing or the earlier termination of the Business Combination Agreement, SPAC shall use reasonable best efforts to maintain the EarlyBird Amendment in full force and effect (and SPAC shall not take any action to cause the EarlyBird Amendment not to be in full force and effect and shall not refrain from taking any commercially reasonable action to maintain the EarlyBird Amendment in full force and effect) and SPAC shall not amend or otherwise change the EarlyBird Engagement Letter without the prior written consent of the Company and any such amendment or change shall be in a form that is mutually agreeable to the SPAC and the Company. For the avoidance of doubt, nothing in the Business Combination Agreement shall prevent, limit or restrict SPAC or its affiliates from taking all actions required to raise funds for the benefit of Parties (after the Closing), including by raising funds as contemplated by the EarlyBird Engagement Letter.
Board of Directors
At the Merger Effective Time, the Holdco Board is expected to be composed of seven directors, including [•].
Conditions to Closing the Business Combination
General Conditions
Under the Business Combination Agreement, the obligations of the parties to consummate the Business Combination are conditioned on the satisfaction or waiver (where permissible) of the following conditions at or prior to the Closing:
(a) The SPAC Proposals shall have been approved and adopted by the requisite affirmative vote of the shareholders of LightJump in accordance with this proxy statement/prospectus, the proxy statement/prospectus filed with the SEC in connection with the Extension Amendment, DGCL, the SPAC Organizational Documents and the rules and regulations of the Nasdaq Capital Market;
(b) the Company Requisite Approvals shall have been obtained and delivered to LightJump in a form and substance reasonably acceptable to LightJump;
(c) the Holdco Requisite Approvals shall have been obtained and delivered to LightJump in a form and substance reasonably acceptable to LightJump;
119
Table of Contents
(d) a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued (i) at or before the Merger Effective Time a report on the contributions in kind relating to the Merger Issuance prepared in accordance with article 420-10 of the 1915 Law (the “Merger Auditor Report”), and (ii) at or before the Exchange Effective Time, in accordance with the Exchange Agreements, a report on the contributions in kind relating to the Exchange Issuance prepared in accordance with article 420-10 of the 1915 Law (the “Exchange Auditor Report”);
(e) (A) Any waiting period under any Antitrust Laws applicable to the Transactions shall have expired or been earlier terminated, and (B) all other consents of (or filings or registrations with) any Governmental Authority required in connection with the execution, delivery and performance of the Business Combination Agreement set forth on Schedule 9.01 to the Business Combination Agreement shall have been obtained;
(f) no Governmental Authority shall have enacted, issued, promulgated, enforced or entered any Law, rule, regulation, judgment, decree, executive order or award which is then in effect and has the effect of making the Transactions, including the Merger or Exchange, illegal or otherwise prohibiting consummation of the Transactions, including the Merger or Exchange;
(g) this Registration Statement shall have been declared effective by the SEC under the Securities Act. No stop order suspending the effectiveness of the Registration Statement shall be in effect, and no proceedings for purposes of suspending the effectiveness of the Registration Statement shall have been initiated by the SEC and not withdrawn;
(h) the Holdco Ordinary Shares shall have been approved for listing on Nasdaq, subject to official notice of issuance;
(i) LightJump shall have at least $5,000,001 of net tangible assets after giving effect to the amounts funded under the Backstop Agreement and following the exercise of Redemption Rights in accordance with the SPAC Organizational Documents;
(j) the delivery of the Financial Advisor Opinion pursuant to Section 8.19 of the Business Combination Agreement;
(k) the delivery of an agreement, which shall be in a form mutually agreeable to Moolec and LightJump, between the CFO and Moolec by which the CFO agrees that the issuance of the Key Staff Participation satisfies all obligations of Moolec relating to the issuance of shares in Moolec to the CFO under the CFO Consulting Agreement; and
(l) all parties to the Registration Rights and Lock-Up Agreement shall have delivered, or cause to be delivered, copies of the Registration Rights and Lock-Up Agreement duly executed by all such parties.
Conditions to the Obligations of LightJump
The obligations of LightJump to consummate the Transactions, including the Merger, are subject to the satisfaction or waiver (where permissible) at or prior to the Closing of the following additional conditions:
(a) certain representations and warranties of Moolec shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date and all other representations and warranties of Moolec shall be true and correct as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date, except where the failure of such representations and warranties to be true and correct does not result in a Company Material Adverse Effect;
(b) certain representations and warranties of Holdco and Merger Sub shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date and all other representations and warranties of Holdco and Merger Sub shall be true and correct as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date, except where the failure of such representations and warranties to be true and correct would be materially adverse to Holdco or Merger Sub;
120
Table of Contents
(c) Moolec, Holdco and Merger Sub shall have performed or complied in all material respects with all agreements and covenants (other than Section 7.01(c)) of the Business Combination Agreement on or prior to the Exchange Effective Time and the Merger Effective Time, as applicable (including, for the avoidance of doubt and without limitation, Section 8.13 of the Business Combination Agreement; provided, that Holdco shall have performed or complied in all respects with the agreements and covenants set forth in Section 7.01(c) of the Business Combination Agreement;
(d) (i) Moolec, Holdco and Merger Sub shall have delivered to LightJump a certificate, dated as of the date of the Merger Effective Time, signed by an officer of Moolec, certifying as to the satisfaction of the conditions specified in Section 9.02(a), Section 9.02(b) and Section 9.02(d) of the Business Combination Agreement and (ii) Moolec, Holdco and Merger Sub shall have delivered to LightJump a certificate, dated as of the Exchange Effective Time, signed by an officer of Moolec, certifying as to the satisfaction of the conditions specified in Section 9.02(a), Section 9.02(b) and Section 9.02(d) of the Business Combination Agreement;
(e) no Company Material Adverse Effect shall have occurred between the date of the Business Combination Agreement and the Merger Effective Time and no Company Material Adverse Effect shall have occurred between the Merger Effective Time and the Exchange Effective Time;
(f) Moolec shall have delivered the PCAOB Financials as contemplated in Section 8.13 of the Business Combination Agreement;
(g) as of immediately prior to the Closing Date, there shall be no Company Ordinary Shares or other Equity Interest of Moolec outstanding other than Company Ordinary Shares and other Equity Interests that are subject to an Exchange Agreement (which shall provide that security shall be either cancelled or converted into shares of Company Ordinary Shares to be transferred to Holdco in accordance with Section 3.02 of the Business Combination Agreement;
(h) LightJump shall have received evidence to its satisfaction that the actions contemplated in Section 8.17 of the Business Combination Agreement have been taken;
(i) each of the employees of Moolec listed on 8.04(b) of the Company Disclosure Schedule shall have executed and delivered to Moolec or Holdco employment agreements executed in compliance with Section 8.04(b) of the Business Combination Agreement with such agreements being in full force and effect;
(j) Holdco shall have delivered to LightJump and Trustee, the SPAC Warrant Amendment and Assignment executed by Holdco; and
(k) Each of Moolec, the Company Shareholders, Holdco, and Merger Sub shall have delivered, or caused to be delivered, each of the Ancillary Agreements to which such Person is a party and that, by its terms, is required to be executed and delivered at the Closing Date, in each case, duly executed by such Person.
Conditions to the Obligations of Moolec
The obligations of Moolec to consummate the Transactions, including the Merger, are subject to the satisfaction or waiver (where permissible) at or prior to the Closing of the following additional conditions:
(a) certain representations and warranties of SPAC shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date and all other representations and warranties of SPAC shall be true and correct as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date, except where the failure of such representations and warranties to be true and correct does not result in a SPAC Material Adverse Effect;
(b) certain representations and warranties of Holdco and Merger Sub shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and as of the Closing Date and all other representations and warranties of Holdco and Merger Sub shall be true and correct as of the
121
Table of Contents
Exchange Effective Time, the Merger Effective Time and as of the Closing Date, except where the failure of such representations and warranties to be true and correct would be materially adverse to Holdco or Merger Sub;
(c) SPAC shall have performed or complied in all material respects with all agreements and covenants required by the Business Combination Agreement to be performed or complied with by or prior to the Exchange Effective Time and the Merger Effective Time, as applicable;
(d) (i) SPAC shall have delivered to Moolec a certificate, dated as of the date of the Merger Effective Time, signed by the Chief Executive Officer of SPAC, certifying as to the satisfaction of the conditions specified in Section 9.03(a), Section 9.03(b) and Section 9.03(d) of the Business Combination Agreement and (ii) SPAC shall have delivered to Moolec a certificate, dated as of the Exchange Effective Time, signed by the Chief Executive Officer of SPAC, certifying as to the satisfaction of the conditions specified in Section 9.03(a), Section 9.03(b) and Section 9.03(d) of the Business Combination Agreement;
(e) no SPAC Material Adverse Effect shall have occurred between the date of the Business Combination Agreement and the Merger Effective Time and no SPAC Material Adverse Effect shall have occurred between the Merger Effective Time and the Exchange Effective Time;
(i) SPAC shall have delivered to Holdco and Trustee, the SPAC Warrant Amendment and Assignment executed by SPAC;
(ii) SPAC shall have delivered or caused to be delivered, each of the Ancillary Agreement to which such Person is a party and that, by its terms is required to be executed and delivered at the Closing, in each case, duly executed by such Person; and
(iii) Sponsor shall have transferred to UG Holdings, LLC (or its designated affiliate) 1,035,000 shares of SPAC Common Stock, as contemplated by the Backstop Agreement.
Termination of the Business Combination Agreement
The Business Combination Agreement may be terminated and the Transactions may be abandoned at any time prior to the Merger Effective Time, notwithstanding any requisite approval and adoption of the Business Combination Agreement and the Transactions by the Company Shareholders or the LightJump Holders as follows:
(vii) by mutual written consent of SPAC and Moolec;
(viii) by either SPAC or Moolec if the Merger Effective Time shall not have occurred prior to the later of (x) 5:00 p.m. (New York time) on January 12, 2023 and (y) the last day of the extended time period that LightJump is able, under its organization documents, to consummate a business combination if LightJump successfully extends such date;
(ix) by either SPAC or Moolec if any Governmental Authority shall have enacted, issued, promulgated, enforced or entered any injunction, order, decree or ruling (whether temporary, preliminary or permanent) which has become final and non-appealable and has the effect of making consummation of the Transactions illegal or otherwise preventing or prohibiting consummation of the Transaction or the Merger;
(x) by either SPAC or Moolec if any of the SPAC Proposals shall fail to receive the requisite vote for approval at the special meeting of stockholders of SPAC;
(xi) by SPAC upon a breach of any representation, warranty, covenant or agreement set forth in the Business Combination Agreement on the part of Moolec, Holdco or Merger Sub that remains uncured for more than 30 days after written notice of such breach is provided by SPAC to Moolec, or if any representation or warranty of Moolec, Holdco or Merger Sub shall have become untrue, in either case such that the conditions relating to representations and warranties and certain covenants and agreements would not be satisfied; and
122
Table of Contents
(xii) by Moolec upon any breach of any representation, warranty, covenant or agreement set forth in the Business Combination Agreement on the part of SPAC that remains uncured for more than 30 days after written notice of such breach is provided by Moolec to SPAC, or if any representation or warranty of SPAC shall have become untrue, in either case such that the conditions relating to representations and warranties and certain covenants and agreements would not be satisfied.
Amendment; Waiver and Extension of the Business Combination Agreement
The Business Combination Agreement may be amended in writing by all parties thereto at any time prior to the Merger Effective Time by action or on behalf of the parties’ respective board of directors; provided, however that after approval of the Business Combination Agreement by the stockholders of SPAC and Merger Sub, no amendment shall be made that requires a further vote of such stockholders without such further approval. The Business Combination Agreement may not be amended except by an instrument in writing signed by each of the parties.
At any time prior to the Merger Effective Time, (a) SPAC may in its sole discretion (i) extend the time for the performance of any obligation or other act of the Company, Holdco or Merger Sub, (ii) waive any inaccuracy in the representations and warranties of the Company, Holdco or Merger Sub contained herein or in any document delivered by the Company, Holdco or Merger Sub pursuant hereto and (iii) waive compliance with any agreement of the Company, Holdco or Merger Sub or any condition to its own obligations contained herein and (b) the Company may in its sole discretion (i) extend the time for the performance of any obligation or other act of SPAC, (ii) waive any inaccuracy in the representations and warranties of SPAC contained herein or in any document delivered by SPAC pursuant hereto and (iii) waive compliance with any agreement of SPAC or any condition to its own obligations contained herein. Any such extension or waiver shall be valid if set forth in an instrument in writing signed by the Party or Parties to be bound thereby.
Governing Law; Consent to Jurisdiction
The Business Combination Agreement is governed by, and construed with, the Laws of the State of Delaware applicable to contracts executed in and to be performed in that State, except to the extent mandatorily governed by the laws of the Grand Duchy of Luxembourg or the United Kingdom, including the provisions relating to the Exchange. All legal actions and proceedings arising out of or relating to the Business Combination Agreement shall be heard and determined exclusively in the Court of Chancery of the State of Delaware or, if (and only if) the court of Chancery of the State of Delaware declines to accept jurisdiction over a particular matter, the Superior Court of the State of Delaware (Complex Commercial Division) or, if (and only if) the Superior Court of the State of Delaware (Complex Commercial Division) declines to accept jurisdiction over a particular matter, any federal court sitting in the State of Delaware, and any appellate courts therefrom (collectively, the “Chosen Courts”), with regard to any such Action arising out of or relating to this Agreement and the transactions contemplated hereby.
The Parties to the Business Combination Agreement. (a) irrevocably submit to the exclusive jurisdiction of the aforesaid courts for themselves and with respect to their respective properties for the purpose of any Action arising out of or relating to the Business Combination Agreement brought by any Party, and (b) agree not to commence any Action relating thereto except in the Chosen Courts, other than Actions in any court of competent jurisdiction to enforce any judgment, decree or award rendered by any such court in the State of Delaware as described in the Business Combination Agreement.
Expenses
In the event that the Business Combination Agreement is validly terminated, all Transaction Expenses incurred in connection with the Business Combination Agreement, the Ancillary Agreements, and the Transactions shall be paid by the party incurring such Transaction Expenses.
If the Transactions are consummated:
(i) as promptly as practicable after the Closing, Holdco shall transfer or cause to be transferred to Sponsor or its designee an amount in cash equal to the Sponsor Advanced Funds so long as the unpaid SPAC Transaction Expenses as of the Closing do not exceed the applicable SPAC Transaction Expenses Cap;
123
Table of Contents
(ii) Holdco shall pay or cause to be paid: (x) the Company Transaction Expenses, (y) the EarlyBird Cash Fees, unless the Net Available Assets are equal to or less than $200,000, then, in which case, Sponsor and Holdco shall pay 50% of all EarlyBird Cash Fees, and (z) the SPAC Transaction Expenses that are unpaid as of the Closing up to an amount not to exceed the SPAC Transaction Expenses Cap; and
(iii) Sponsor shall pay or cause to be paid, (x) all unpaid SPAC Transaction Expenses in excess of the applicable SPAC Transaction Expenses Cap; (y) any expenses incurred by LightJump in its pursuit of potential acquisition or business targets other than Moolec or that were not incurred by LightJump in connection with or in furtherance of the Transactions, other than the Sponsor Advanced Funds and (z) all Extension Amendment Fees.
(iv) Within five (5) days following the six (6) month anniversary of the Closing, Holdco shall issue the EarlyBird Share Fees.
Vote Required for Approval
The Business Combination Proposal will be approved and adopted only if the holders of a majority of the outstanding shares of SPAC Common Stock entitled to vote thereon at the special meeting vote “FOR” the Business Combination Proposal. Adoption of the Business Combination Proposal is not conditioned upon the adoption of any of the other proposals.
Recommendation of the Board
LIGHTJUMP’s BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS VOTE “FOR” THE APPROVAL OF PROPOSAL 1 (THE BUSINESS COMBINATION PROPOSAL).
124
Table of Contents
Certain Agreements Related to the Business Combination
Company Shareholder Contribution and Exchange Agreements
Concurrently with the execution of the Business Combination Agreement, each Company Shareholder entered into an Exchange Agreement with Moolec and Holdco pursuant to which, among other things, each Company Shareholder consented to the Exchange and all transaction contemplated under the Business Combination Agreement and the Transaction Documents to which it is a party and, consequently, agreed to (i) contribute its Company Ordinary Shares to Holdco in exchange for Holdco Ordinary Shares, (ii) not transfer any of its Company Ordinary Shares before the earlier of (a) one year from June 14, 2022 and (b) the implementation of the Exchange or termination of the Exchange Agreement. The Company Shareholders who entered into the Exchange Agreements collectively represent 100% of the issued and outstanding Company Ordinary Shares.
Backstop Agreement
Concurrently with the execution of the Business Combination Agreement, Union Group Ventures Limited, THEO I SCSp, UG Holdings, LLC and Sponsor entered into the Backstop Agreement, pursuant to which, among other things, Sponsor, Union Group Ventures limited and THEO I SCSp agreed, on a several (and not joint) basis, to backstop an aggregate amount equal to $10,000,000, conditioned upon Closing on the terms and subject to the conditions set forth in the Backstop Agreement.
In the case that the Net Available Assets minus the EarlyBird Fee is less than $10,000,000, each of the Sponsor, Union Group Ventures Limited and Theo I SCSp shall comply with their respective obligations under the Backstop Agreement by (i) contributing cash to Holdco immediately prior to Closing or, (ii) arranging for and obtaining written commitments from Public Stockholders seeking to exercise their election to redeem their Public Shares to reverse the redemption election. This could be in exchange for guarantee of payment or other agreed form of consideration.
If the Sponsor, Union Group Ventures Limited or THEO I SCSp elect to fulfil their obligations through a cash contribution to Holdco, then Holdco will issue a number of Holdco Ordinary Shares with value equal to the cash amount contributed, with each Holdco Ordinary Share being valued at $10.00 per share. Such issuances will have a dilutive effect on current LightJump Holders. See section entitled “Parties to the Business Combination — Impact of the Business Combination on Holdco’s Public Float”, which includes a table reflecting the post-Business Combination ownership of Holdco, inclusive of the potential issuance pursuant to the Backstop Agreement.
Sponsor will also be able to elect to have Union Group Ventures Limited or THEO I SCSp fund all or any portion of the Sponsor’s obligations under the Backstop Agreement. If the Sponsor makes such election, then Sponsor will be required to transfer a number of shares of SPAC Common Stock corresponding to the amount of such election to Union Group Ventures Limited and/or THEO I SCSp, with each share valued at $10.00 per share.
The Backstop Agreement benefits LightJump Holders by ensuring LightJump satisfies the SPAC Net Tangible Assets (as defined in the Business Combination Agreement) condition to close in the Business Combination Agreement in the event that redemption cause the SPAC Net Tangible Assets amount to fall below $5,000,001. The Backstop Agreement also benefits LightJump Holders post-Business Combination, because the Combined Company will have additional cash to fund working capital and other business needs.
The parties to the Backstop Agreement, including Sponsor, will benefit from the backstop transactions and the consummation of the Business Combination, which benefits are different from, or in addition to, those available to LightJump Holders and warrant holders generally. See the section entitled “The Business Combination — Interests of LightJump’s Directors and Officers in the Business Combination.”
Transaction Support Agreement
Concurrently with the execution of the Business Combination Agreement, LightJump, Sponsor, Moolec, Holdco, certain Company Shareholders and the SAFE Holders and the LightJump Holders entered into the Transaction Support Agreement pursuant to which, among other things, the Sponsor and certain LightJump Holders have agreed with LightJump, Holdco, Moolec and the Eligible Company Shareholders to (i) waive certain rights, and (ii) take certain actions to support the Transactions. Additionally, during the interim period (from June 14, 2022 until the earlier of (i) the Closing or (ii) termination of the Business Combination Agreement), each LightJump Investor has agreed not to transfer any Sponsor shares that she, he or it Beneficially Owns (as defined under Rule 13d-3 of
125
Table of Contents
the Exchange Act) without the prior written consent of Holdco, with the exception of certain permitted transfers described in the Transaction Support Agreement. Each SAFE Holder has agreed to execute all documentation and perform all necessary actions reasonably required by Moolec and/or Holdco as may be necessary or desirable in connection with the issuance by Holdco of Holdco Ordinary Shares to each SAFE Holder, substantially in accordance with the respective Original SAFE, with such adjustments (if any) required under Luxembourg law.
Assignment, Assumption and Amendment to Warrant Agreement
In connection with the Merger, Holdco, LightJump and Continental, as warrant agent, will enter into the SPAC Warrant Amendment and Assignment pursuant to which, as of the Merger Effective Time, (i) each SPAC Warrant that is outstanding immediately prior to the Merger Effective Time will no longer represent a right to acquire one share of SPAC Common Stock and will instead represent the right to acquire the same number of Holdco Ordinary Shares under substantially the same terms as set forth in the SPAC Warrant Agreement entered into in connection with LIghtJump’s IPO and (ii) LightJump will assign to Holdco all of LightJump’s right, title and interest in and to the existing SPAC Warrant Agreement and Holdco will assume, and agree to pay, perform, satisfy and discharge in full, all of LightJump’s liabilities and obligations under the existing Warrant Agreement arising from and after the Merger Effective Time.
Registration Rights and Lock-Up Agreement
In connection with the closing of the Transactions, the Sponsor, Holdco, the CFO and the Company Shareholders and SAFE Holders will enter into the Registration Rights and Lock-Up Agreement pursuant to which, among other things, the Sponsor, the CFO and the Company Shareholders and SAFE holders shall have customary demand and piggyback registration rights in connection with the Holdco Ordinary Shares issued to them in the Merger or the Exchange. Additionally, the Holdco Ordinary Shares held by each party to the Registration Rights and Lock-Up Agreement will be subject to a lock-up until (i) the date that is 365 days from the Closing Date, and (ii) such date on which Holdco completes a liquidation, merger, share exchange or other similar transaction that results in all of the shareholders of Holdco having the right to exchange their Holdco Ordinary Shares for cash, securities or other property, provided that if the share price of the Holdco Ordinary Shares exceeds $12.00 per Holdco Ordinary Share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-day trading period, the parties to the Registration Rights and Lock-Up Agreement may transfer up to 50% of the Holdco Ordinary Shares subject to the Registration Rights and Lock-Up Agreement.
Amendment to Business Combination Marketing Agreement
On January 8, 2021, the SPAC entered into the BCMA with EarlyBird. It is a condition to the consummation of the transactions contemplated by the Business Combination Agreement that SPAC and EarlyBird enter into an amendment to the BCMA to provide for, among other things, a change in the compensation to be payable to EarlyBird upon the Closing of the Business Combination. On June 14, 2022, the SPAC and EarlyBird executed an amendment to the BCMA (the “Amendment”) whereby the SPAC shall pay to EarlyBird (A) a Cash Fee at Closing equal to (i) 20% of the aggregate gross proceeds (up to a maximum of $3,830,000) held in the Trust Account (after redemptions and reduction of all additional payments included in the Trust Account to accommodate all extensions) and received by the SPAC in any financing in connection with the Business Combination regardless of the source of such funds, plus (ii) $1,000,000 and (B) in consideration of EarlyBird introducing the Company to Registrant, Holdco shall issue to EarlyBird a number of ordinary shares of Holdco equal to $2,000,000 divided by the lesser of (y) the volume weighted average price of Holdco’s ordinary shares for the ten trading days preceding the six month anniversary of the Closing and (z) $10.00, up to a maximum of 600,000 shares (the “Share Fee”). The Share Fee shall be issued to EarlyBird within five Business Days of the six-month anniversary of the Closing, and Holdco shall register the resale of the ordinary shares issued to EarlyBird as promptly as practicable after their issuance. The Sponsor shall forfeit to Holdco for cancellation the same number of shares of common stock payable to EarlyBird under such Share Fee.
126
Table of Contents
Material Luxembourg Income Tax Considerations
The following is a general description of certain Luxembourg tax considerations relating to Holdco and the holders of Holdco Ordinary Shares and Holdco Warrants. It does not purport to be a complete analysis of all tax considerations in relation to the Holdco Ordinary Shares and Holdco Warrants. Prospective purchasers should consult their own tax advisers as to which countries’ tax laws could be relevant to acquiring, holding and disposing of the securities and the consequences of such actions under the tax laws of those countries. This overview is based upon the law as in effect on the date of this document and is subject to any change in law that may take effect after such date, even with retroactive effect.
The comments below are intended as a basic overview of certain tax consequences in relation to Holdco and the purchase, ownership and disposition of Holdco Ordinary Shares and Holdco Warrants under Luxembourg law. Persons who are in any doubt as to their tax position should consult a professional tax adviser.
Please be aware that the residence concept used under the respective headings below applies for Luxembourg income tax assessment purposes only. Any reference in the present section to a tax, duty, levy impost or other charge or withholding of a similar nature refers to Luxembourg tax law and/or concepts only. Also, please note that a reference to Luxembourg income tax generally encompasses corporate income tax (impôt sur le revenu des collectivités), municipal business tax (impôt commercial communal), a solidarity surcharge (contribution au fonds pour l’emploi) as well as personal income tax (impôt sur le revenu des personnes physiques). Corporate taxpayers may further be subject to net worth tax (impôt sur la fortune), as well as other duties, levies and taxes. Corporate income tax, municipal business tax, net wealth tax and the solidarity surcharge invariably apply to most corporate taxpayers resident in Luxembourg for tax purposes. Individual taxpayers are generally subject to personal income tax and solidarity surcharge. Under certain circumstances, where individual taxpayers act in the course of the management of a professional or business undertaking, municipal business tax may apply as well.
Taxation of Holdco
Holdco is subject to Luxembourg tax on its worldwide profits at the current combined ordinary rate of 24.94% for Luxembourg City, including the 17% corporate income tax, a 6.75% municipal business tax (rate in the municipality of Luxembourg City in 2022) and a solidarity surcharge (together the “Income Tax”).
In principle, dividends and capital gains realized by Holdco are fully subject to Income Tax in Luxembourg.
However, provided the conditions of the Luxembourg participation exemption regime are met, dividends or capital gains realized by Holdco upon the disposal of shares are not taxable in Luxembourg.
Luxembourg net wealth tax (“NWT”) will be due annually by Holdco at the rate of 0.5% on its total net asset value below or equal to € 500 million. The tranche above € 500 million will be taxed at a rate of 0.05%. Net worth is assessed based on the unitary value (valeur unitaire), as determined on January 1st of each year. The unitary value is in principle calculated as the difference between (i) assets estimated at their fair market value (valeur estimée de réalisation), and (ii) liabilities vis-à-vis third parties.
Shareholdings qualifying for the Luxembourg participation exemption regime are excluded from the NWT basis provided that, Holdco holds a direct shareholding in a qualifying subsidiary representing at least 10% of the qualifying subsidiary’s share capital or having an acquisition cost (including both share capital and share premium) of at least € 1.2 million; there is no minimum holding period requirement.
Companies for which the sum of fixed financial assets (i.e., financial assets notably including shares and loans, transferable securities and cash) exceeds 90% of their total balance sheet and € 350,000 are liable to a minimum annual NWT of € 4,815. Other companies are liable to a minimum progressive tax (in an amount up to € 32,100), depending on the total assets on their balance sheet.
Withholding taxation
Any dividend distributed by Holdco to its shareholders will in principle be subject to a 15% withholding tax unless an exemption or a reduced treaty rate applies.
127
Table of Contents
Luxembourg taxation of the holders
Luxembourg tax residence of the holders
Holders will not be deemed to be resident, domiciled or carrying on business in Luxembourg solely by reason of holding, execution, performance, delivery, exchange and/or enforcement of the Holdco Ordinary Shares or Holdco Warrants.
Taxation of Luxembourg non-residents
Holders who are non-residents of Luxembourg and who do not have a permanent establishment, a permanent representative, or a fixed place of business in Luxembourg with which the holding of the Holdco Ordinary Shares or Holdco Warrants is connected, are not liable to any Luxembourg income tax, whether they receive payments upon redemption or repurchase of the Holdco Ordinary Shares or Holdco Warrants, or realize capital gains on the sale of any Holdco Ordinary Shares or Holdco Warrants, unless they sell a participation of more than 10% in Holdco within 6 months of its acquisition.
Taxation of Luxembourg residents
Holders who are Luxembourg resident individuals will generally be subject to income tax on income derived from the Holdco Ordinary Shares and Holdco Warrants. Capital gains realized upon the disposal, sale or redemption of the Holdco Ordinary Shares and Holdco Warrants by individual resident holders acting in the course of the management of their private wealth are in principle not subject to income tax (except if the gain has been realized within 6 months of the acquisition of the Holdco Ordinary Shares or Holdco Warrants), to the extent they do not hold a participation of more than 10% in Holdco.
Holders who are Luxembourg resident companies (société de capitaux) or foreign entities which have a permanent establishment or a permanent representative in Luxembourg with which the holding of the Holdco Ordinary Shares or Holdco Warrants is connected, must include in their taxable income any income (including dividend) and the difference between the sale or redemption price and the lower of the cost or book value of the Holdco Ordinary Shares and Holdco Warrants sold or redeemed.
A holder who is a Luxembourg resident company benefiting from a special tax regime, such as (i) a specialized investment fund governed by the amended law of February 13, 2007, (ii) a family wealth management company governed by the amended law of May 11, 2007, (iii) an undertaking for collective investment governed by the amended law of December 17, 2010 or (iv) a reserved alternative investment fund treated as a specialized investment fund for Luxembourg tax purposes governed by the amended law of July 23, 2016 is exempt from income tax in Luxembourg and profits derived from the Holdco Ordinary Shares and Holdco Warrants are thus not subject to Luxembourg income tax.
Net Wealth Tax
A Luxembourg resident as well as a non-resident who has a permanent establishment or a permanent representative in Luxembourg to which the Holdco Ordinary Shares or Holdco Warrants are attributable, are subject to Luxembourg NWT on such Holdco Ordinary Shares or Holdco Warrants, except if the holder is (i) a resident or non-resident individual taxpayer, (ii) a securitization company governed by the amended law of March 22, 2004 on securitization, (iii) a company governed by the amended law of June 15, 2004 on venture capital vehicles, (iv) a professional pension institution governed by the amended law dated July 13, 2005, (v) a specialized investment fund governed by the amended law of February 13, 2007, (vi) a family wealth management company governed by the law of May 11, 2007, (vii) an undertaking for collective investment governed by the amended law of December 17, 2010 or (viii) a reserved alternative investment fund governed by the amended law of July 23, 2016.
However, (i) a securitization company governed by the amended law of March 22, 2004 on securitization, (ii) a company governed by the amended law of June 15, 2004 on venture capital vehicles, (iii) a professional pension institution governed by the amended law dated July 13, 2005 and (iv) a reserved alternative investment fund treated as a venture capital vehicle governed by the amended law of July 23, 2016 remain subject to minimum NWT.
128
Table of Contents
The minimum NWT tax is levied on companies having their statutory seat or central administration in Luxembourg. For entities for which the sum of fixed financial assets, receivables against related companies, transferable securities and cash at bank exceeds 90% of their total gross assets and € 350,000, the minimum NWT is currently set at € 4,815. For all other companies having their statutory seat or central administration in Luxembourg which do not fall within the scope of the € 4,815 minimum NWT, the minimum NWT ranges from € 535 to € 32,100, depending on the company’s total gross assets.
Other Taxes
No stamp, value, issue, registration, transfer or similar taxes or duties will be payable in Luxembourg by shareholders in connection with the issue of the Holdco Ordinary Shares and Holdco Warrants, nor will any of these taxes be payable as a consequence of a subsequent transfer, exchange or redemption of the Holdco Ordinary Shares or Holdco Warrants, unless the documents relating to the Holdco Ordinary Shares or Holdco Warrants are (i) voluntarily registered in Luxembourg or (ii) appended to a document that requires obligatory registration in Luxembourg.
There is no Luxembourg value added tax payable in respect of payments in consideration for the issuance of the Holdco Ordinary Shares or Holdco Warrants or in respect of the payment under the Holdco Ordinary Shares or Holdco Warrants or the transfer of the Holdco Ordinary Shares or Holdco Warrants. Luxembourg value added tax may, however, be payable in respect of fees charged for certain services rendered to the Holdco if, for Luxembourg value added tax purposes, such services are rendered or are deemed to be rendered in Luxembourg and an exemption from Luxembourg value added tax does not apply with respect to such services.
No Luxembourg inheritance tax is levied on the transfer of the Holdco Ordinary Shares or Holdco Warrants upon the death of a holder in cases where the deceased was not a resident of Luxembourg for inheritance tax purposes. Where a holder is a resident of Luxembourg for tax purposes at the time of his death, the Holdco Ordinary Shares and Holdco Warrants are included in such holder’s taxable estate for inheritance tax assessment purposes. No Luxembourg gift tax will be levied on the transfer of Holdco Ordinary Shares or Holdco Warrants by way of gift unless the gift is registered in Luxembourg.
129
Table of Contents
Material U.S. Federal Income Tax Considerations
Subject to the limitations and qualifications set forth herein (including the limitations and qualifications set forth in the opinion attached as Exhibit 8.1), the following is a discussion of the material U.S. federal income tax considerations of the Merger to holders of SPAC Common Stock and SPAC Warrants (collectively, the “SPAC securities”) with respect to (i) an election by the holders to redeem for cash their shares of SPAC Common Stock, (ii) the Merger pursuant to the Business Combination, and (iii) the ownership and disposition of Holdco Ordinary Shares and Holdco Warrants received in the Merger (collectively, the “Holdco securities”).
This discussion is limited to considerations relevant to holders that hold SPAC securities (and, after the Merger, Holdco securities) as “capital assets” for U.S. federal income tax purposes (generally, property held for investment). This discussion does not address all aspects of U.S. federal income taxation that may be relevant to holders in light of their individual circumstances (including consequences under the alternative minimum tax or net investment income tax) and does not address state, local, non-U.S. or other tax laws (such as estate or gift tax laws). This discussion also does not address:
• banks or other financial institutions, underwriters, or insurance companies;
• traders in securities who elect to apply a mark-to-market method of accounting;
• real estate investment trusts and regulated investment companies;
• controlled foreign corporations or passive foreign investment companies;
• tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts;
• expatriates or former long-term residents of the United States;
• subchapter S corporations, partnerships or other pass-through entities or investors in any such entities;
• dealers or traders in securities, commodities or currencies;
• grantor trusts;
• U.S. persons whose “functional currency” is not the U.S. dollar;
• persons who received shares of SPAC Common Stock through the issuance of restricted stock under an equity incentive plan or through a tax-qualified retirement plan or otherwise as compensation;
• persons who own (directly or through attribution) 5% or more (by vote or value) of the outstanding shares of SPAC Common Stock, or, after the Merger, the outstanding Holdco Ordinary Shares; or
• persons holding SPAC securities, or, after the Merger, Holdco securities, as a position in a “straddle,” as part of a “synthetic security” or “hedge,” as part of a “conversion transaction,” or other integrated investment or risk reduction transaction.
If a partnership, including for this purpose any entity or arrangement that is treated as a partnership for U.S. federal income tax purposes, holds SPAC securities (or, after the Merger, Holdco securities), the U.S. federal income tax treatment of a partner in such partnership will generally depend on the status of the partner and the activities of the partnership. A holder that is a partnership and the partners in such partnership should consult their own tax advisors with regard to the U.S. federal income tax consequences of the Merger and the subsequent ownership and disposition of Holdco securities.
This discussion is based on the tax laws of the United States, including the Internal Revenue Code of 1986, as amended (the “Code”), its legislative history, existing and proposed regulations thereunder, published rulings and court decisions, all as of the date hereof and all subject to change at any time, possibly with retroactive effect.
130
Table of Contents
ALL HOLDERS OF SPAC SECURITIES SHOULD CONSULT WITH THEIR TAX ADVISORS REGARDING THE TAX CONSEQUENCES OF THE BUSINESS COMBINATION AND CONSIDERATIONS RELATING TO THE OWNERSHIP AND DISPOSITION OF HOLDCO SHARES AND HOLDCO WARRANTS, INCLUDING THE EFFECTS OF U.S. FEDERAL, STATE, AND LOCAL AND NON-U.S. TAX LAWS.
Tax Residence of Holdco for U.S. Federal Income Tax Purposes
Under current U.S. federal income tax law, a corporation is generally considered for U.S. federal income tax purposes to be a tax resident in the jurisdiction of its organization or incorporation. Accordingly, under generally applicable U.S. federal income tax rules, Holdco, which is organized under the laws of Luxembourg, is classified as a non-U.S. corporation (and, therefore, not a U.S. tax resident) for U.S. federal income tax purposes. Section 7874 of the Code, however, contains rules that may cause a non-U.S. corporation to be treated as a U.S. corporation for U.S. federal income tax purposes. These rules are complex and require an analysis of all relevant facts and circumstances, and there is limited guidance on their application.
Under Section 7874 of the Code, a corporation created or organized outside the United States (i.e., a non-U.S. corporation) will nevertheless be treated as a U.S. corporation for U.S. federal income tax purposes (and, therefore, as a U.S. tax resident subject to U.S. federal income tax on its worldwide income) if, pursuant to a plan or series of related transactions, each of the following three conditions are met: (i) the non-U.S. corporation acquires, directly or indirectly, substantially all of the properties held directly or indirectly by a U.S. corporation; (ii) after the acquisition, the non-U.S. corporation’s “expanded affiliated group” does not have substantial business activities in the non-U.S. corporation’s country of organization or incorporation relative to the expanded affiliated group’s worldwide activities (as determined under the Treasury Regulations); and (iii) subject to the Third Country Rule discussed below, after the acquisition, the percentage (by vote or value) of the shares of the acquiring non-U.S. corporation held by former shareholders and security holders of the U.S. corporation by reason of holding shares and securities (including rights to acquire shares and securities) of the U.S. corporation (which includes the receipt of the non-U.S. corporation’s shares in the acquisition) (the “Section 7874 Percentage”) is at least 80%. The third requirement is referred to herein as the “Ownership Test.”
The Treasury Regulations promulgated under Section 7874 include a rule that generally provides that, if (i) there is an acquisition of a domestic company by a non-U.S. corporation in which the Section 7874 Percentage is at least 60% (without the application of the Third Party Rule, as defined below), and (ii) in a related acquisition, such non-U.S. corporation acquires another non-U.S. corporation and the acquiring non-U.S. corporation is not subject to tax as a resident in the foreign country in which the acquired non-U.S. corporation was subject to tax as a resident prior to the acquisitions, then stock of the acquiring non-U.S. corporation held by former shareholders of the acquired non-U.S. corporation by reason of having held stock in the acquired non-U.S. corporation is excluded in applying the Ownership Test. This rule is referred to herein as the “Third Country Rule.” If applicable, the Third Country Rule increases the Section 7874 Percentage and generally results in the acquiring non-U.S. corporation meeting the Ownership Test.
For purposes of Section 7874 of the Code, immediately after the completion of the Business Combination, the first two general conditions described above may be met because (i) Holdco could be viewed as directly acquiring substantially all of the assets of SPAC through the Merger, and (ii) Holdco, including its expanded affiliated group, will not have substantial business activities in Luxembourg for purposes of Section 7874. Furthermore, because Holdco is expected to be a tax resident in Luxembourg and not England (the jurisdiction in which the Company is tax resident), the Third Country Rule could apply to the Business Combination if the Section 7874 Percentage were at least 60%. As a result, the application of Section 7874 to the Business Combination depends on the Section 7874 Percentage.
Based on the terms of the Business Combination, the rules for determining share ownership and calculating the Section 7874 Percentage under Section 7874 and the Treasury Regulations promulgated thereunder, and certain factual assumptions, immediately after the Business Combination, former SPAC Holders and security holders are expected to be treated as holding less than 60% (by both vote and value) of the Holdco Ordinary Shares by reason of their ownership of SPAC Common Stock and other securities. However, because the determination of whether the Ownership Test will be met must be finally made at the completion of the Business Combination, by which time there could be adverse changes to the relevant facts and circumstances, no representation is being made to you that Holdco will be treated as a non-U.S. corporation for U.S. federal income tax purposes. Neither SPAC nor Holdco
131
Table of Contents
has sought nor will seek any ruling from the IRS or any opinion from any tax advisor as to such tax treatment, and the closing of the Business Combination is not conditioned upon achieving, or receiving a ruling from any tax authority or opinion from any tax advisor in regards to, any particular tax treatment. Further, there can be no assurance that your, SPAC’s or Holdco’s tax advisors, the IRS, or a court will conclude that Holdco is not treated as a U.S. corporation pursuant to Section 7874. As noted above, the rules under Section 7874 are complex and require an analysis of all relevant facts and circumstances, and there is limited guidance as to their application. Further, whether the Ownership Test has been satisfied must be finally determined after the completion of the Business Combination, by which time there could be adverse changes to the relevant facts and circumstances.
The computation of the Section 7874 Percentage is subject to various complex adjustments for which there is limited guidance. For example, for purposes of determining the Section 7874 Percentage, (i) any “non-ordinary course distributions” (within the meaning of the Treasury Regulations) made by the acquired U.S. corporation during the 36 months preceding the acquisition, including certain dividends and share repurchases, (ii) shares of the acquiring non-U.S. corporation’s stock attributable to the foreign acquirer’s passive assets (as determined under applicable rules), and (iii) any shares held by shareholders of the acquiring non-U.S. corporation that were issued for cash in a public offering related to the acquisition or other passive assets, in each case, are disregarded. In addition, changes to the rules in Section 7874 or the Treasury Regulations promulgated thereunder, or other changes in law, could adversely affect Holdco’s status as a non-U.S. corporation for U.S. federal income tax purposes. Accordingly, there can be no assurance that the IRS will not take a contrary position to one of those described above or that a court will not agree with a contrary position of the IRS in the event of litigation.
If Holdco were to be treated as a U.S. corporation for U.S. federal income tax purposes, it could be subject to substantial U.S. tax liability, in addition to tax liability in its country of residence, and the gross amount of any dividend payments to its Non-U.S. Holders could be subject to 30% U.S. withholding tax, depending on the application of any income tax treaty that might apply to reduce the withholding tax.
The remainder of this discussion assumes that Holdco will not be treated as a U.S. corporation for U.S. federal income tax purposes. However, neither SPAC nor Holdco is representing to you that Holdco will not be treated as a U.S. corporation for U.S. federal income tax purposes under Section 7874.
U.S. Holders
This section applies to you if you are a U.S. Holder. For purposes of this discussion, the term “U.S. Holder” means a beneficial owner of SPAC securities and, after the Merger, Holdco securities received in the Merger, that is, for U.S. federal income tax purposes:
• an individual who is a United States citizen or resident of the United States;
• a corporation (including an entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia;
• an estate the income of which is includible in gross income for U.S. federal income tax purposes regardless of its source; or
• a trust (A) the administration of which is subject to the primary supervision of a United States court and which has one or more United States persons (within the meaning of the Code) who have the authority to control all substantial decisions of the trust or (B) that has in effect a valid election under applicable Treasury regulations to be treated as a United States person.
The Business Combination
Subject to the discussion below of SPAC Warrants and Section 367(a) of the Code, for U.S. federal income tax purposes (a) the Merger is intended to be treated as a “reorganization” within the meaning of Section 368(a)(1)(A) by reason of Section 368(a)(2)(E) of the Code (collectively, Sections 368(a)(1)(A) and 368(a)(2)(E) of the Code, “Section 368 of the Code”), or (b) if the Merger does not meet the requirements to be treated as a “reorganization” within the meaning of Section 368 of the Code, it is intended that the Merger will, together with the Exchange, be treated as an exchange described in Section 351(a) of the Code. The parties to the Business Combination Agreement have adopted the Business Combination Agreement as a “plan of reorganization” within the meaning of Treasury Regulations Sections 1.368-2(g) and 1.368-3(a).
132
Table of Contents
The provisions of Section 351(a) of the Code are complex and qualification as a non-recognition transaction thereunder could be adversely affected by events or actions that occur following the consummation of the Business Combination that are beyond SPAC’s control. For example, if more than 20% of the Holdco Ordinary Shares were in the aggregate subject to one or more arrangements or agreements to be sold or disposed of at the time of their issuance in the Business Combination, one of the requirements for Section 351(a) treatment would not be met. If the Merger qualifies as part of an exchange subject to Section 351 of the Code, a U.S. Holder that exchanges its SPAC Common Stock in the Merger for Holdco Ordinary Shares generally should not recognize any gain or loss on such exchange, subject to Section 367(a) of the Code discussed below and further subject to the discussion below regarding the treatment of U.S. Holders that exchange both SPAC Common Stock and SPAC Warrants. In such case, assuming gain recognition is not required under Section 367(a) of the Code as described below, the aggregate adjusted tax basis of the Holdco Ordinary Shares received in the Merger by a U.S. Holder would be equal to the adjusted tax basis of the SPAC Common Stock surrendered in the Merger in exchange therefor. The holding period of the Holdco Ordinary Shares would include the holding period of the SPAC Common Stock surrendered in the Merger in exchange therefor.
There are many requirements that must be satisfied in order for the Merger to qualify as a “reorganization” under Section 368 of the Code, some of which are based upon factual determinations and others that are fundamental to corporate reorganizations. For example, it is unclear as a matter of law whether a blank check company such as SPAC can satisfy the “continuity of business enterprise” requirement under Section 368 of the Code. In addition, reorganization treatment could be adversely affected by events or actions, some of which are outside the control of SPAC. For example, the requirements for reorganization treatment could be affected by the magnitude of SPAC Common Stock redemptions that occur in connection with the Business Combination. Accordingly, no representation is being made to you with respect to the Merger’s qualification as a reorganization under Section 368 of the Code. If the Merger qualifies as a reorganization under Section 368 of the Code, the consequences to U.S. Holders of SPAC Common Stock would generally be the same as described in the preceding paragraph.
The completion of the Merger is not conditioned on the receipt of an opinion of counsel regarding the U.S. federal income tax consequences of the Merger or the Business Combination, and neither SPAC nor Holdco intends to request a ruling from the IRS regarding the U.S. federal income tax consequences of the Merger or the Business Combination. Accordingly, no assurance can be given that the IRS will not challenge the views expressed herein or that a court would not sustain such a challenge.
The appropriate U.S. federal income tax treatment of SPAC Warrants in connection with the Merger is uncertain. If the exchange of SPAC Warrants for Holdco Warrants qualifies as part of a “reorganization” within the meaning of Section 368 of the Code, subject to Section 367(a) of the Code discussed below, a U.S. Holder of SPAC Warrants generally should not recognize any gain or loss on any such deemed transfer of SPAC Warrants, and such U.S. Holder’s basis in the Holdco Warrants deemed received should be equal to the U.S. Holder’s basis in its SPAC Warrants deemed transferred therefor. However, the requirements for qualification of the Merger as a “reorganization” under Section 368 of the Code are more stringent in certain respects than the requirements for qualification as an exchange under Section 351 of the Code, and there can be no assurance that the Merger will also qualify under Section 368 of the Code. If a U.S. Holder of SPAC Warrants is treated as transferring its SPAC Warrants and shares of SPAC Common Stock to Holdco in exchange for Holdco Warrants and Holdco Ordinary Shares in an exchange governed only by Section 351(a) of the Code (and not by Section 368 of the Code), the U.S. Holder should be required to recognize gain (but not loss) in an amount equal to the lesser of (i) the amount of gain realized by such holder (generally, the excess of (x) the sum of the fair market values of the Holdco Warrants and the Holdco Ordinary Shares received by such holder over (y) such holder’s aggregate adjusted tax basis in the SPAC Warrants and SPAC Common Stock exchanged therefor) and (ii) the fair market value of the Holdco Warrants received by such holder in such exchange. Any such gain would be capital gain, and generally will be long-term capital gain if the U.S. Holder’s holding period for the SPAC Common Stock and SPAC Warrants, if any exceeds one year at the time of the Merger.
U.S. Holders of SPAC Warrants are urged to consult with their tax advisors regarding the treatment of their SPAC Warrants in connection with the Merger.
133
Table of Contents
Section 367(a)
Section 367(a) of the Code and the Treasury Regulations promulgated thereunder impose certain additional requirements for qualifying for tax-deferred treatment under Sections 351 or 368 of the Code with respect to transactions where a U.S. person transfers stock or securities in a U.S. corporation to a non-U.S. corporation in exchange for stock or securities in a non-U.S. corporation. U.S. Holders of SPAC Common Stock will be deemed to transfer shares of such stock to Holdco in exchange for Holdco Ordinary Shares, so that these requirements will apply.
In general, Section 367(a) requires a U.S. Holder to recognize gain (but not loss) on the exchange of SPAC Common Stock for Holdco Ordinary Shares by a U.S. Holder in the Merger unless each of the following conditions is met: (i) the U.S. corporation complies with certain reporting requirements; (ii) no more than 50% of both the total voting power and the total value of the stock of Holdco is received in the exchange, in the aggregate, by “U.S. transferors” (as defined in the Treasury Regulations and computed taking into account direct, indirect and constructive ownership); (iii) no more than 50% of each of the total voting power and the total value of the stock of Holdco is owned, in the aggregate, immediately after the exchange by “U.S. persons” (as defined in the Treasury Regulations) that are either officers or directors or “five-percent target shareholders” (as defined in the Treasury Regulations and computed taking into account direct, indirect and constructive ownership) of SPAC; (iv) either (A) the U.S. Holder is not a “five-percent transferee shareholder” (as defined in the Treasury Regulations and computed taking into account direct, indirect and constructive ownership) of Holdco or (B) the U.S. Holder is a “five-percent transferee shareholder” of Holdco and enters into an agreement with the IRS to recognize gain on the transferred shares under certain circumstances; and (v) the “active trade or business test” as defined in Treasury Regulation Section 1.367(a)-3(c)(3) is satisfied. The active trade or business test generally requires (A) Holdco or any qualified subsidiary of Holdco to be engaged in an “active trade or business” outside of the United States for the 36-month period immediately before the transfer and neither the transferors nor Holdco to have an intention to substantially dispose of or discontinue such trade or business and (B) the fair market value of Holdco to be at least equal to the fair market value of SPAC, as specifically determined for purposes of Section 367 of the Code, at the time of the transfer. The satisfaction of the foregoing conditions depends on an interpretation of legal authorities and facts relating to the Business Combination, and there is limited guidance regarding the application of these requirements to facts similar to the Business Combination. In addition, the determination of whether Section 367(a) of the Code will apply to U.S. Holders of SPAC Common Stock cannot be made until the Merger is completed, and no rulings will be sought regarding the tax consequences of the Business Combination. Accordingly, there can be no assurance that Section 367(a) of the Code will not apply to U.S. Holders of SPAC Common Stock that participate in the Merger to cause them to recognize taxable gain as a result of the Merger. As a result, no representation is being made to you, with respect to whether gain will be recognized by a U.S. Holder of SPAC Common Stock under Section 367(a) of the Code.
To the extent that a U.S. Holder of SPAC Common Stock is required to recognize gain under Section 367(a) for any of the foregoing reasons, such U.S. Holder would recognize gain, if any, in the Merger in an amount equal to the excess of (i) the sum of the fair market value of the Holdco Ordinary Shares (and, if such holder’s SPAC Warrants convert to Holdco Warrants, the fair market value of the Holdco Warrants) received by such holder, over (ii) such holder’s adjusted tax basis in the SPAC Common Stock (and SPAC Warrants, if any) exchanged therefor. Any such gain would be capital gain, and generally will be long-term capital gain if the U.S. Holder’s holding period for the SPAC Common Stock (and SPAC Warrants, if any) exceeds one year at the time of the Merger.
Exercise of Redemption Rights
In the event that a U.S. Holder elects to redeem its SPAC Common Stock for cash as described in the redemption provisions herein, the treatment of the transaction for U.S. federal income tax purposes will depend on whether the redemption qualifies as sale of the SPAC Common Stock under Section 302 of the Code. If the redemption qualifies as a sale of the SPAC Common Stock, the U.S. Holder will be treated as described under “— U.S. Holders — Gain or Loss on Redemption Treated as a Sale of SPAC Common Stock” below. If the redemption does not qualify as a sale of the SPAC Common Stock, the U.S. Holder will be treated as described under “— U.S. Holders — Taxation of Redemption Treated as a Distribution” below.
134
Table of Contents
Whether a redemption qualifies for sale treatment will depend largely on the total number of shares of SPAC Common Stock treated as held by the U.S. Holder (including any SPAC Common Stock constructively owned by the U.S. Holder as a result of owning SPAC Warrants) relative to all of the shares of SPAC Common Stock outstanding both before and after the redemption. The redemption of SPAC Common Stock generally will be treated as a sale of the SPAC Common Stock (rather than as a corporate distribution) if the redemption (i) is “substantially disproportionate” with respect to the U.S. Holder, (ii) results in a “complete termination” of the U.S. Holder’s interest in SPAC or (iii) is “not essentially equivalent to a dividend” with respect to the U.S. Holder. These tests are explained more fully below.
In determining whether any of the foregoing tests are satisfied, a U.S. Holder takes into account not only SPAC Common Stock actually owned by the U.S. Holder, but also shares of SPAC Common Stock that are constructively owned by it. A U.S. Holder may constructively own, in addition to stock owned directly, stock owned by certain related individuals and entities in which the U.S. Holder has an interest or that have an interest in such U.S. Holder, as well as any stock the U.S. Holder has a right to acquire by exercise of an option, which would generally include SPAC Common Stock which could be acquired pursuant to the exercise of the SPAC Warrants. In order to meet the substantially disproportionate test, the percentage of SPAC’s outstanding voting stock actually and constructively owned by the U.S. Holder immediately following the redemption of the SPAC Common Stock must, among other requirements, be less than 80% of the percentage of SPAC’s outstanding voting stock actually and constructively owned by the U.S. Holder immediately before the redemption. There will be a complete termination of a U.S. Holder’s interest if either (i) all of the shares of the SPAC Common Stock actually and constructively owned by the U.S. Holder are redeemed or (ii) all of the shares of the SPAC Common Stock actually owned by the U.S. Holder are redeemed and the U.S. Holder is eligible to waive, and effectively waives in accordance with specific rules, the attribution of stock owned by certain family members and the U.S. Holder does not constructively own any other SPAC Common Stock. The redemption of the SPAC Common Stock will not be essentially equivalent to a dividend if a U.S. Holder’s redemption results in a “meaningful reduction” of the U.S. Holder’s proportionate interest in SPAC. Whether the redemption will result in a meaningful reduction in a U.S. Holder’s proportionate interest in SPAC will depend on the particular facts and circumstances. However, the IRS has indicated in a published ruling that even a small reduction in the proportionate interest of a small minority stockholder in a publicly held corporation who exercises no control over corporate affairs may constitute such a “meaningful reduction.” A U.S. Holder should consult with its own tax advisors as to the tax consequences of a redemption.
If none of the foregoing tests is satisfied, then the redemption will be treated as a corporate distribution and the tax effects will be as described under “— U.S. Holders — Taxation of Redemption Treated as a Distribution” below. After the application of those rules, any remaining tax basis of the U.S. Holder in the redeemed SPAC Common Stock will be added to the U.S. Holder’s adjusted tax basis in its remaining SPAC Common Stock, or, if it has none, to the U.S. Holder’s adjusted tax basis in its SPAC Warrants or possibly in other SPAC Common Stock constructively owned by it.
Gain or Loss on Redemption Treated as a Sale of SPAC Common Stock
If the redemption qualifies as a sale of SPAC Common Stock, a U.S. Holder must treat any gain or loss recognized as capital gain or loss. Any such capital gain or loss will be long-term capital gain or loss if the U.S. Holder’s holding period for the SPAC Common Stock so disposed of exceeds one year. Generally, a U.S. Holder will recognize gain or loss in an amount equal to the difference between (i) the amount of cash received in such redemption and (ii) the U.S. Holder’s adjusted tax basis in its SPAC Common Stock so redeemed. A U.S. Holder’s adjusted tax basis in its SPAC Common Stock generally will equal the U.S. Holder’s acquisition cost (that is, the portion of the purchase price of a SPAC Unit allocated to a share of SPAC Common Stock or the purchase price of a share of SPAC Common Stock purchased in the open market) less any prior distributions treated as a return of capital. Long-term capital gain realized by a non-corporate U.S. Holder generally will be taxable at a reduced rate. The deduction of capital losses is subject to limitations.
Taxation of Redemption Treated as a Distribution
If the redemption does not qualify as a sale of SPAC Common Stock, the U.S. Holder will be treated as receiving a distribution. In general, any distributions to U.S. Holders generally will constitute dividends for U.S. federal income tax purposes to the extent paid from SPAC’s current or accumulated earnings and profits,
135
Table of Contents
as determined under U.S. federal income tax principles. Distributions in excess of current and accumulated earnings and profits will constitute a return of capital that will be applied against and reduce (but not below zero) the U.S. Holder’s adjusted tax basis in SPAC Common Stock. Any remaining excess will be treated as gain realized on the sale or other disposition of the SPAC Common Stock and will be treated as described under “— U.S. Holders — Gain or Loss on Redemption Treated as a Sale of SPAC Common Stock.” Dividends paid to a U.S. Holder that is a taxable corporation generally will qualify for the dividends received deduction if the requisite holding period is satisfied, subject to the “extraordinary dividend” provisions of the Code (which could cause a reduction in the tax basis of such corporate U.S. Holder’s SPAC Common Stock and increase the amount of gain or decrease the amount of loss recognized by such U.S. Holder in connection with a disposition of its shares). With certain exceptions, and provided certain holding period requirements are met, dividends paid to a non-corporate U.S. Holder generally will constitute “qualified dividends” that will be subject to tax at the maximum tax rate accorded to long-term capital gains.
Distributions on Holdco Ordinary Shares
Subject to the discussion below under “— Passive Foreign Investment Company Rules,” the gross amount of any distribution on Holdco Ordinary Shares that is made out of Holdco’s current or accumulated earnings and profits (as determined for U.S. federal income tax purposes) will generally be taxable to a U.S. Holder as ordinary dividend income on the date such distribution is actually or constructively received by such U.S. Holder. Any such dividends paid to corporate U.S. Holders generally will not qualify for the dividends-received deduction that may otherwise be allowed under the Code. To the extent that the amount of the distribution exceeds Holdco’s current and accumulated earnings and profits (as determined under U.S. federal income tax principles), such excess amount will be treated first as a non-taxable return of capital to the extent of the U.S. Holder’s tax basis in its Holdco Ordinary Shares, and thereafter as capital gain recognized on a sale. However, Holdco does not maintain calculations of its earnings and profits in accordance with U.S. federal income tax principles. U.S. Holders should therefore assume that any distribution by Holdco with respect to Holdco Ordinary Shares will be reported as ordinary dividend income. U.S. Holders should consult their tax advisors with respect to the appropriate U.S. federal income tax treatment of any distribution received from Holdco.
Subject to the discussion below under “— Passive Foreign Investment Company Rules,” dividends received by non-corporate U.S. Holders (including individuals), from a “qualified foreign corporation” may be eligible for preferential rates of taxation, provided that certain holding period requirements and other conditions are satisfied. For these purposes, a non-U.S. corporation will be treated as a qualified foreign corporation if it is eligible for the benefits of a comprehensive income tax treaty with the United States which is determined by the U.S. Treasury Department to be satisfactory for purposes of these rules and which includes an exchange of information provision. The IRS has issued guidance pursuant to which foreign corporations eligible for benefits of the U.S. income tax treaty with Luxembourg, among other countries, are qualified foreign corporations. However, no examination has been made, and there can be no assurances that, Holdco will be eligible for benefits of the U.S. income tax treaty with Luxembourg or for benefits of an applicable comprehensive income tax treaty of any other country with the United States. A non-U.S. corporation is also treated as a qualified foreign corporation with respect to dividends paid by that corporation on shares that are readily tradable on an established securities market in the United States. U.S. Treasury Department guidance indicates that shares listed on the Nasdaq (on which Holdco intends to apply to list the Holdco Ordinary Shares) will be considered readily tradable on an established securities market in the United States. Even if the Holdco Ordinary Shares are listed on Nasdaq, there can be no assurance that the Holdco Ordinary Shares will be considered readily tradable on an established securities market in future years. Non-corporate U.S. Holders that (i) do not meet a minimum holding period requirement during which they are not protected from the risk of loss or (ii) elect to treat the dividend income as “investment income” pursuant to Section 163(d)(4) of the Code (dealing with the deduction for investment interest expense), will not be eligible for the preferential rates of taxation regardless of Holdco’s status as a qualified foreign corporation. In addition, the rate reduction will not apply to dividends if the recipient of a dividend is obligated to make related payments with respect to positions in substantially similar or related property. This disallowance applies even if the minimum holding period has been met. Finally, Holdco will not constitute a qualified foreign corporation for purposes of these rules if it is a PFIC for the taxable year in which it pays a dividend or for the preceding taxable year. See the discussion below under “— Passive Foreign Investment Company Rules.”
136
Table of Contents
Subject to certain conditions and limitations, withholding taxes, if any, on dividends paid by Holdco may be treated as foreign taxes eligible for credit against a U.S. Holder’s U.S. federal income tax liability under the U.S. foreign tax credit rules. For purposes of calculating the U.S. foreign tax credit, dividends paid on Holdco Ordinary Shares will generally be treated as income from sources outside the United States and will generally constitute passive category income. The rules governing the U.S. foreign tax credit are complex. U.S. Holders should consult their tax advisors regarding the availability of the U.S. foreign tax credit under particular circumstances.
Sale, Exchange, Redemption or Other Taxable Disposition of Holdco Securities
Subject to the discussion below under “— Passive Foreign Investment Company Rules,” a U.S. Holder will generally recognize gain or loss on any sale, exchange, redemption, or other taxable disposition of Holdco Ordinary Shares or Holdco Warrants in an amount equal to the difference between the amount realized on the disposition and such U.S. Holder’s adjusted tax basis in the Holdco Ordinary Shares or Holdco Warrants. Any gain or loss recognized by a U.S. Holder on a taxable disposition of Holdco Ordinary Shares or Holdco Warrants will generally be capital gain or loss and will be long-term capital gain or loss if the holder’s holding period in the Holdco Ordinary Shares or Holdco Warrants exceeds one year at the time of the disposition. Preferential tax rates may apply to long-term capital gains of non-corporate U.S. Holders (including individuals). The deductibility of capital losses is subject to certain limitations. Any gain or loss recognized by a U.S. Holder on the sale or exchange of Holdco Ordinary Shares or Holdco Warrants will generally be treated as U.S. source gain or loss.
Exercise or Lapse of a Holdco Warrant
Except as discussed below with respect to the cashless exercise of a Holdco Warrant, a U.S. Holder generally will not recognize gain or loss upon the acquisition of a Holdco Ordinary Share on the exercise of a Holdco Warrant for cash. A U.S. Holder’s tax basis in a Holdco Ordinary Share received upon exercise of the Holdco Warrant generally will be an amount equal to the sum of the U.S. Holder’s tax basis in the Holdco Warrant exchanged therefor and the exercise price. The U.S. Holder’s holding period for a Holdco Ordinary Share received upon exercise of the Holdco Warrant will begin on the date following the date of exercise (or possibly the date of exercise) of the Holdco Warrants and will not include the period during which the U.S. Holder held the Holdco Warrants. If a Holdco Warrant is allowed to lapse unexercised, a U.S. Holder generally will recognize a capital loss equal to such holder’s tax basis in the Holdco Warrant.
The tax consequences of a cashless exercise of a warrant are not clear under current tax law. A cashless exercise may be tax-free, either because the exercise is not a gain realization event or because the exercise is treated as a recapitalization for U.S. federal income tax purposes. In either tax-free situation, a U.S. Holder’s basis in the Holdco Ordinary Shares received would equal the holder’s basis in the Holdco Warrant. If the cashless exercise were treated as not being a gain recognition event, a U.S. Holder’s holding period in the Holdco Ordinary Shares would be treated as commencing on the date following the date of exercise (or possibly the date of exercise) of the Holdco Warrant. If the cashless exercise were treated as a recapitalization, the holding period of the Holdco Ordinary Share would include the holding period of the Holdco Warrant.
It is also possible that a cashless exercise could be treated in part as a taxable exchange in which gain or loss would be recognized. In such event, a U.S. Holder would recognize gain or loss with respect to the portion of the exercised Holdco Warrants treated as surrendered to pay the exercise price of the Holdco Warrants (the “surrendered warrants”). The U.S. Holder would recognize capital gain or loss with respect to the surrendered warrants in an amount generally equal to the difference between (i) the fair market value of the Holdco Ordinary Shares that would have been received with respect to the surrendered warrants in a regular exercise of the Holdco Warrants and (ii) the sum of the U.S. Holder’s tax basis in the surrendered warrants and the aggregate cash exercise price of such warrants (if they had been exercised in a regular exercise). In this case, a U.S. Holder’s tax basis in the Holdco Ordinary Shares received would equal the U.S. Holder’s tax basis in the Holdco Warrants exercised plus (or minus) the gain (or loss) recognized with respect to the surrendered warrants. A U.S. Holder’s holding period for the Holdco Ordinary Shares would commence on the date following the date of exercise (or possibly the date of exercise) of the Holdco Warrant.
137
Table of Contents
Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise, there can be no assurance which, if any, of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court of law. Accordingly, U.S. Holders should consult their tax advisors regarding the tax consequences of a cashless exercise.
Possible Constructive Distributions
The terms of Holdco Warrants provide for an adjustment to the number of Holdco Ordinary Shares for which the warrant may be exercised or to the exercise price of the warrant in certain events. An adjustment which has the effect of preventing dilution generally is not taxable. The U.S. Holders of the Holdco Warrants would, however, be treated as receiving a constructive distribution from Holdco if, for example, the adjustment increases the warrant holders’ proportionate interest in Holdco’s assets or earnings and profits (e.g., through an increase in the number of Holdco Ordinary Shares that would be obtained upon exercise) as a result of a distribution of cash to the holders of Holdco Ordinary Shares which is taxable to the U.S. Holders of such shares as described under “— Distributions on Holdco Ordinary Shares” above. Such constructive distribution would be subject to tax as described under that section in the same manner as if the U.S. Holders of the Holdco Warrants received a cash distribution from Holdco equal to the fair market value of such increased interest. The rules regarding constructive distributions are complex. U.S. Holders should consult their own tax advisors regarding the application of the rules to them in light of their own circumstances.
Passive Foreign Investment Company Rules
Generally. The treatment of U.S. Holders of the Holdco Ordinary Shares could be materially different from that described above if Holdco is treated as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. A PFIC is any foreign corporation with respect to which either: (i) 75% or more of the gross income for a taxable year constitutes passive income for purposes of the PFIC rules, or (ii) 50% or more of such foreign corporation’s assets in any taxable year (generally based on the quarterly average of the value of its assets during such year) is attributable to assets, including cash, that produce passive income or are held for the production of passive income. Passive income generally includes dividends, interest, certain royalties and rents, annuities, net gains from the sale or exchange of property producing such income and net foreign currency gains. The determination of whether a foreign corporation is a PFIC is based upon the composition of such foreign corporation’s income and assets (including, among others, its proportionate share of the income and assets of any other corporation in which it owns, directly or indirectly, 25% (by value) of the stock), and the nature of such foreign corporation’s activities. A separate determination must be made after the close of each taxable year as to whether a foreign corporation was a PFIC for that year. Once a foreign corporation qualifies as a PFIC it is, with respect to a shareholder during the time it qualifies as a PFIC, and subject to certain exceptions, always treated as a PFIC with respect to such shareholder, regardless of whether it satisfied either of the qualification tests in subsequent years.
Holdco has not made a determination as to whether it currently is, or in the future may become, a PFIC, but there is a possibility that it may be classified as a PFIC for its taxable year that includes the date of the Merger or in the foreseeable future. The tests for determining PFIC status are applied annually after the close of the taxable year, and it is difficult to predict accurately future income and assets relevant to this determination. The fair market value of the assets of Holdco is expected to depend, in part, upon (a) the market value of the Holdco Ordinary Shares, and (b) the composition of the assets and income of Holdco. Further, because Holdco may value its goodwill based on the market value of the Holdco Ordinary Shares, a decrease in the market value of the Holdco Ordinary Shares and/or an increase in cash or other passive assets (including as a result of the Merger) would increase the relative percentage of its passive assets. Moreover, Holdco may be classified as a PFIC for its taxable year that includes the date of the closing of the Merger as a result of interest income that Holdco earns on its deposits, which generally will be treated as passive income. The application of the PFIC rules is subject to uncertainty in several respects and, therefore, no assurances can be provided that the IRS will not assert that Holdco is a PFIC for the taxable year that includes the date of the Merger or in a future year.
If Holdco is or becomes a PFIC during any year in which a U.S. Holder holds Holdco Ordinary Shares, there are three separate taxation regimes that could apply to such U.S. Holder under the PFIC rules, which are the (i) excess distribution regime (which is the default regime), (ii) QEF regime, and (iii) mark-to-market regime. A U.S. Holder who holds (actually or constructively) stock in a foreign corporation during any year in which such corporation qualifies as a PFIC is subject to U.S. federal income taxation under one of these three regimes. The
138
Table of Contents
effect of the PFIC rules on a U.S. Holder will depend upon which of these regimes applies to such U.S. Holder. However, dividends paid by a PFIC are generally not eligible for the lower rates of taxation applicable to qualified dividend income (“QDI”) under any of the foregoing regimes.
Excess Distribution Regime. If a U.S. Holder does not make a QEF election or a mark-to-market election, as described below, such U.S. Holder will be subject to the default “excess distribution regime” under the PFIC rules with respect to (i) any gain realized on a sale or other disposition (including a pledge) of Holdco Ordinary Shares, and (ii) any “excess distribution” received on Holdco Ordinary Shares (generally, any distributions in excess of 125% of the average of the annual distributions on Holdco Ordinary Shares during the preceding three years or a holder’s holding period, whichever is shorter). Generally, under this excess distribution regime:
• the gain or excess distribution will be allocated ratably over the period during which a U.S. Holder held the Holdco Ordinary Shares;
• the amount allocated to the current taxable year, will be treated as ordinary income; and
• the amount allocated to prior taxable years will be subject to the highest tax rate in effect for that taxable year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year.
The tax liability for amounts allocated to years prior to the year of disposition or excess distribution will be payable generally without regard to offsets from deductions, losses and expenses. In addition, gains (but not losses) realized on the sale of Holdco Ordinary Shares cannot be treated as capital gains, even if a U.S. Holder holds the shares as capital assets. Further, no portion of any distribution will be treated as QDI.
QEF Regime. A QEF election is effective for the taxable year for which the election is made and all subsequent taxable years and may not be revoked without the consent of the IRS. If a U.S. Holder makes a timely QEF election with respect to its direct or indirect interest in a PFIC, the U.S. Holder will be required to include in income each year a portion of the ordinary earnings and net capital gains of the PFIC as QEF income inclusions, even if such amount is not distributed to the U.S. Holder. Thus, the U.S. Holder may be required to report taxable income as a result of QEF income inclusions without corresponding receipts of cash. Holdco shareholders that are U.S. Holders subject to U.S. federal income tax should not expect that they will receive cash distributions from Holdco sufficient to cover their respective U.S. tax liability with respect to such QEF income inclusions. In addition, U.S. Holders of Holdco Warrants will not be able to make a QEF election with respect to their warrants.
The timely QEF election also allows the electing U.S. Holder to: (i) generally treat any gain recognized on the disposition of its shares of the PFIC as capital gain; (ii) treat its share of the PFIC’s net capital gain, if any, as long-term capital gain instead of ordinary income; and (iii) either avoid interest charges resulting from PFIC status altogether, or make an annual election, subject to certain limitations, to defer payment of current taxes on its share of PFIC’s annual realized net capital gain and ordinary earnings subject, however, to an interest charge on the deferred tax computed by using the statutory rate of interest applicable to an extension of time for payment of tax. In addition, net losses (if any) of a PFIC will not pass through to Holdco shareholders and may not be carried back or forward in computing such PFIC’s ordinary earnings and net capital gain in other taxable years. Consequently, a U.S. Holder may over time be taxed on amounts that as an economic matter exceed Holdco’s net profits.
A U.S. Holder’s tax basis in Holdco Ordinary Shares will be increased to reflect QEF income inclusions and will be decreased to reflect distributions of amounts previously included in income as QEF income inclusions. No portion of the QEF income inclusions attributable to ordinary income will be treated as QDI. Amounts included as QEF income inclusions with respect to direct and indirect investments generally will not be taxed again when distributed. U.S. Holders should consult their tax advisors as to the manner in which QEF income inclusions affect a U.S. Holder’s allocable share of Holdco’s income and basis in Holdco Ordinary Shares.
In order to comply with the requirements of a QEF election, a U.S. Holder must receive certain information from Holdco. There is no assurance that Holdco will provide such information. There is also no assurance that Holdco will have timely knowledge of its status as a PFIC in the future or of the required information to be provided. In addition, if Holdco holds an interest in a lower-tier PFIC (including, without limitation, in any PFIC subsidiaries), U.S. Holders will generally be subject to the PFIC rules described above with respect to any such lower-tier PFICs.
139
Table of Contents
There can be no assurance that a portfolio company or subsidiary in which Holdco holds an interest will not qualify as a PFIC, or that a PFIC in which Holdco holds an interest will provide the information necessary for a QEF election to be made by a U.S. Holder (in particular if Holdco does not control that PFIC).
Mark-to-Market Regime. Alternatively, a U.S. Holder may make an election to mark marketable shares in a PFIC to market on an annual basis. PFIC shares generally are marketable if: (i) they are “regularly traded” on a national securities exchange that is registered with the SEC or on the national market system established under Section 11A of the Exchange Act; or (ii) they are “regularly traded” on any exchange or market that the Treasury Department determines to have rules sufficient to ensure that the market price accurately represents the fair market value of the stock. It is expected that Holdco Ordinary Shares, which are expected to be listed on Nasdaq, will qualify as marketable shares for the PFIC rules purposes, but there can be no assurance that Holdco Ordinary Shares will be “regularly traded” for purposes of these rules. Pursuant to such an election, a U.S. Holder would include in each year as ordinary income the excess, if any, of the fair market value of such stock over its adjusted basis at the end of the taxable year. A U.S. Holder may treat as ordinary loss any excess of the adjusted basis of the stock over its fair market value at the end of the year, but only to the extent of the net amount previously included in income as a result of the election in prior years. A U.S. Holder’s adjusted tax basis in the PFIC shares will be increased to reflect any amounts included in income, and decreased to reflect any amounts deducted, as a result of a mark-to-market election. Any gain recognized on a disposition of Holdco Ordinary Shares will be treated as ordinary income and any loss will be treated as ordinary loss (but only to the extent of the net amount of income previously included as a result of a mark-to-market election). A mark-to-market election only applies for the taxable year in which the election was made, and for each subsequent taxable year, unless the PFIC shares ceased to be marketable or the IRS consents to the revocation of the election. U.S. Holders should also be aware that the Code and the Treasury Regulations do not allow a mark-to-market election with respect to stock of lower-tier PFICs that is non-marketable. There is also no provision in the Code, Treasury Regulations or other published authority that specifically provides that a mark-to-market election with respect to the stock of a publicly-traded holding company (such as Holdco) effectively exempts stock of any lower-tier PFICs from the negative tax consequences arising from the general PFIC rules and, as a result, a mark-to-market election may not mitigate the adverse implications of the excess distribution regime with respect to an investment in the Holdco Ordinary Shares if Holdco were classified as a PFIC as a result of owning interests in PFIC subsidiaries. We advise U.S. Holders to consult their own tax advisors to determine whether the mark-to-market tax election is available to them and the consequences resulting from such election. In addition, U.S. Holders of Holdco Warrants will not be able to make a mark-to-market election with respect to their warrants.
PFIC Reporting Requirements. A U.S. Holder of Holdco Ordinary Shares will be required to file an annual report on IRS Form 8621 containing such information with respect to its interest in a PFIC as the IRS may require. Failure to file IRS Form 8621 for each applicable taxable year may result in substantial penalties and result in the U.S. Holder’s taxable years being open to audit by the IRS until such Forms are properly filed.
Non-U.S. Holders
This section applies to you if you are a Non-U.S. Holder. For purposes of this discussion, the term “Non-U.S. Holder” means a beneficial owner of SPAC securities, and, after the Merger, Holdco securities received in the Merger, that is neither a U.S. Holder nor a partnership (or an entity or arrangement treated as a partnership) for U.S. federal income tax purposes, including:
• a nonresident alien individual, other than certain former citizens and residents of the United States;
• a foreign corporation; or
• a foreign estate or trust;
but generally does not include an individual who is present in the United States for 183 days or more in the taxable year of disposition. A holder who is such an individual should consult his or her tax advisor regarding the U.S. federal income tax consequences of the sale or other disposition of SPAC securities or Holdco Ordinary Shares or Holdco Warrants.
140
Table of Contents
Ownership and Disposition of Holdco Securities
Assuming that Holdco is not treated as a U.S. corporation under the rules discussed above under “— Tax Residence of Holdco for U.S. Federal Income Tax Purposes”, a Non-U.S. Holder of Holdco Ordinary Shares will not be subject to U.S. federal income tax or, subject to the discussion below under “— Information Reporting and Backup Withholding,” U.S. federal withholding tax on any dividends received on Holdco Ordinary Shares or any gain recognized on a sale or other disposition of Holdco Ordinary Shares (including, any distribution to the extent it exceeds the adjusted basis in the Non-U.S. Holder’s Holdco Ordinary Shares) unless the dividend or gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States (or, under certain income tax treaties, is attributable to a United States permanent establishment or fixed base maintained by the Non-U.S. Holder). In addition, special rules may apply to a Non-U.S. Holder who is an individual present in the United States for 183 days or more during the taxable year of the sale or disposition, and certain other requirements are met. Such holders should consult their own tax advisors regarding the U.S. federal income tax consequences of the sale or disposition of Holdco Ordinary Shares.
Dividends and gains that are effectively connected with a Non-U.S. Holder’s conduct of a trade or business in the United States (or, under certain income tax treaties, that are attributable to a United States permanent establishment or fixed base maintained by the Non-U.S. Holder) generally will be subject to U.S. federal income tax at the same regular U.S. federal income tax rates applicable to a comparable U.S. Holder. A Non-U.S. Holder that is a corporation may also be subject to a branch profits tax at a rate of 30% (or such lower rate provided by an applicable tax treaty) on its effectively connected earnings and profits for the taxable year, as adjusted for certain items.
The U.S. federal income tax treatment of a Non-U.S. Holder’s exercise of a Holdco Warrant, or the lapse of a Holdco Warrant held by a Non-U.S. Holder, generally will correspond to the U.S. federal income tax treatment of the exercise or lapse of a warrant by a U.S. Holder, as described under “— U.S. Holders — Exercise or Lapse of a Holdco Warrant,” above, although to the extent a cashless exercise results in a taxable exchange, the consequences would be similar to those described in the preceding paragraphs above for a Non-U.S. Holder’s gain on the sale or other disposition of the Holdco Ordinary Shares and Holdco Warrants.
Exercise of Redemption Rights
The characterization for U.S. federal income tax purposes of the redemption of a Non-U.S. Holder’s SPAC Common Stock as described in the redemption provisions herein generally will correspond to the U.S. federal income tax characterization of such a redemption of a U.S. Holder’s SPAC Common Stock, as described under “— U.S. Holders — Exercise of Redemption Rights.”
Gain or Loss on Redemption Treated as a Sale of SPAC Common Stock
If the redemption qualifies as a sale of SPAC Common Stock, a Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax in respect of gain recognized on a sale of its SPAC Common Stock, unless:
• the gain is effectively connected with the conduct of a trade or business by the Non-U.S. Holder within the United States (and, if provided by an applicable income tax treaty, is attributable to a United States permanent establishment or fixed base maintained by the Non-U.S. Holder), in which case the Non-U.S. Holder will generally be subject to the same treatment as a U.S. Holder with respect to the redemption, and a corporate Non-U.S. Holder may be subject to the branch profits tax at a 30% rate (or lower rate as may be specified by an applicable income tax treaty);
• the Non-U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year in which the redemption takes place and certain other conditions are met, in which case the Non-U.S. Holder will be subject to a 30% tax on the individual’s net capital gain for the year; or
• SPAC is or has been a “U.S. real property holding corporation” for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of disposition or the period that the Non-U.S. Holder held SPAC Common Stock, and, in the case where shares of SPAC Common Stock are regularly traded on an established securities market, the Non-U.S. Holder has owned, directly or constructively, more than 5% of SPAC Common Stock at any time within the shorter of the five-year period preceding the disposition or such Non-U.S. Holder’s holding period for the shares of SPAC Common Stock. We do not believe we are or have been a U.S. real property holding corporation.
141
Table of Contents
Taxation of Redemption Treated as a Distribution
If the redemption does not qualify as a sale of SPAC Common Stock, the Non-U.S. Holder will be treated as receiving a distribution. In general, any distributions to a Non-U.S. Holder of SPAC Common Stock, to the extent paid out of SPAC’s current or accumulated earnings and profits (as determined under U.S. federal income tax principles), will constitute dividends for U.S. federal income tax purposes and, provided such dividends are not effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States, (or, if provided by an applicable income tax treaty, are not attributable to a United States permanent establishment or fixed base maintained by the Non-U.S. Holder), SPAC will be required to withhold tax from the gross amount of the dividend at a rate of 30%, unless such Non-U.S. Holder is eligible for a reduced rate of withholding tax under an applicable income tax treaty and provides proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN or W-8BEN-E). Any distribution not constituting a dividend will be treated first as reducing (but not below zero) the Non-U.S. Holder’s adjusted tax basis in its shares of SPAC Common Stock and, to the extent such distribution exceeds the Non-U.S. Holder’s adjusted tax basis, as gain realized from the sale or other disposition of the SPAC Common Stock, which will be treated as described under “— Non-U.S. Holders — Gain or Loss on Redemption Treated as a Sale of SPAC Common Stock.”
Dividends paid to a Non-U.S. Holder that are effectively connected with such Non-U.S. Holder’s conduct of a trade or business within the United States (and, if provided by an applicable income tax treaty, are attributable to a United States permanent establishment or fixed base maintained by the Non-U.S. Holder) generally will not be subject to United States withholding tax, provided such Non-U.S. Holder complies with certain certification and disclosure requirements. Instead, such dividends generally will be subject to U.S. federal income tax, net of certain deductions, at the same individual or corporate rates applicable to U.S. Holders (subject to an exemption or reduction in such tax as may be provided by an applicable income tax treaty). If the Non-U.S. Holder is a corporation, dividends that are effectively connected income may also be subject to the branch profits tax at a 30% rate (or lower rate as may be specified by an applicable income tax treaty).
Information Reporting and Backup Withholding
In general, information reporting requirements will apply to dividends received by U.S. Holders with respect to their Holdco Ordinary Shares (including constructive dividends), and the proceeds received on the disposition of Holdco Ordinary Shares and Holdco Warrants effected within the United States (and, in certain cases, outside the United States), in each case, other than U.S. Holders that are exempt recipients (such as corporations). Information reporting requirements will also apply to redemptions from U.S. Holders of SPAC Common Stock. Backup withholding (currently at a rate of 24%) may apply to such amounts if the U.S. Holder fails to provide an accurate taxpayer identification number (generally on an IRS Form W-9 provided to the paying agent or the U.S. Holder’s broker) or is otherwise subject to backup withholding.
Certain U.S. Holders holding specified foreign financial assets with an aggregate value in excess of the applicable dollar threshold are required to report information to the IRS relating to Holdco securities, subject to certain exceptions (including an exception for Holdco securities held in accounts maintained by U.S. financial institutions), by attaching a complete IRS Form 8938, Statement of Specified Foreign Financial Assets, to their tax return, for each year in which they hold Holdco securities. In addition to these requirements, U.S. Holders may be required to annually file FinCEN Report 114 (Report of Foreign Bank and Financial Accounts) with the U.S. Department of Treasury. U.S. Holders should consult with their own tax advisors regarding information reporting requirements relating to their ownership of Holdco securities.
Dividends paid with respect to Holdco Ordinary Shares (including constructive dividends) and proceeds from the sale or other disposition of Holdco Ordinary Shares and Holdco Warrants received in the United States by a Non-U.S. Holder through certain U.S.-related financial intermediaries may be subject to information reporting and backup withholding unless such Non-U.S. Holder provides to the applicable withholding agent the required certification as to its non-U.S. status, such as by providing a valid IRS Form W-8BEN, IRS Form W-8BEN-E or IRS Form W-ECI, or otherwise establishes an exemption, and otherwise complies with the applicable requirements of the backup withholding rules.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or credit against a holder’s U.S. federal income tax liability, if any, provided the required information is timely furnished to the IRS.
142
Table of Contents
FATCA
Sections 1471 through 1474 of the Code and the Treasury regulations and administrative guidance promulgated thereunder (commonly referred to as the “Foreign Account Tax Compliance Act” or “FATCA”) generally impose U.S. federal withholding tax of 30% on certain withholdable payments to a “foreign financial institution” (as defined in the Code) (an “FFI”) unless the FFI (1) enters into an agreement with the IRS (or is subject to an applicable intergovernmental agreement) to withhold on certain payments and to collect and provide to the IRS (or local revenue authorities, as required under an applicable intergovernmental agreement) information regarding United States persons who hold accounts with the FFI and its affiliates (including certain foreign entities owned by United States persons), and (2) provides the payor with a properly completed IRS Form W-8BEN-E to document its status or the FFI otherwise qualifies for an exemption. This IRS Form W-8BEN-E must include the FFI’s Global Intermediary Identification Number, which is obtained by registering with the IRS. FATCA may also impose a 30% withholding tax on withholdable payments to a “non-financial foreign entity” (as defined in the Code) unless the entity provides the withholding agent with a properly completed IRS Form W-8BEN-E certifying that it does not have any “substantial United States owners” (as defined in the Code) or identifying its direct and indirect substantial United States owners or the entity otherwise qualifies for an exemption.
For purposes of FATCA, “withholdable payments” generally include U.S.-source payments otherwise subject to non-resident withholding tax (e.g., U.S. source interest or U.S. source dividends, such as dividends on SPAC Common Stock), and, subject to the proposed Treasury regulations discussed below, payments of gross proceeds from the sale or other disposition of any property of a type that can produce U.S. source interest or dividends (e.g., retirements and redemptions of indebtedness, sales of securities, or redemptions of stock, such as redemptions of SPAC Common Stock), even if the payment would otherwise not be subject to U.S. non-resident withholding tax (e.g., because it is capital gain).
While withholding under FATCA would have applied also to payments of gross proceeds from the sale or other disposition of property, proposed Treasury regulations eliminate FATCA withholding on payments of gross proceeds entirely. Taxpayers generally may (but are not required to) rely on these proposed Treasury regulations until final Treasury regulations are issued. Redeeming Non-U.S. Holders are encouraged to consult with their own tax advisors regarding the possible application of FATCA to redemptions of SPAC Common Stock.
143
Table of Contents
Proposal no. 1 — The Business Combination Proposal
As discussed in this proxy statement/prospectus, LightJump Holders are being asked to consider and vote on the Business Combination Proposal. You should read carefully this proxy statement/prospectus in its entirety for more detailed information concerning the Business Combination. In particular, you are directed to the Business Combination Agreement, which is attached as Annex A to this proxy statement/prospectus.
Vote Required for Approval
The Business Combination Proposal will be approved and adopted only if the holders of a majority of the outstanding shares of SPAC Common Stock entitled to vote thereon at the special meeting vote “FOR” the Business Combination Proposal. Adoption of the Business Combination Proposal is not conditioned upon the adoption of any of the other proposals.
Recommendation of the Board
LIGHTJUMP’s BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS VOTE “FOR” THE APPROVAL OF THE BUSINESS COMBINATION PROPOSAL.
144
Table of Contents
Proposal no. 2 — The Stockholder Adjournment Proposal
Overview
The Stockholder Adjournment Proposal
The Stockholder Adjournment Proposal, if adopted, will allow LightJump’s board of directors to adjourn the special meeting of stockholders to a later date or dates to permit further solicitation of proxies or if more time is necessary to consummate the Business Combination. The Stockholder Adjournment Proposal will only be presented to LightJump’s Holders in the event that, based on the tabulated votes, there are not sufficient votes at the time of the special meeting of stockholders to approve Proposal 1 (Business Combination Proposal). In no event will LightJump’s board of directors adjourn the special meeting of stockholders or consummate the Business Combination beyond the date by which it may properly do so under LightJump’s Amended and Restated Certificate of Incorporation and Delaware law.
Consequences if the Stockholder Adjournment Proposal is Not Approved
If the Stockholder Adjournment Proposal is not approved by LightJump’s Holders, LightJump’s board of directors may not be able to adjourn the special meeting of stockholders to a later date in the event that, based on the tabulated votes, there are not sufficient votes at the time of the special meeting of stockholders to approve Proposal 1 (Business Combination Proposal). If the board of directors cannot adjourn the special meeting and the Business Combination Approval is not approved, LightJump will be unable to consummate the Business Combination and, if LightJump does not consummate another business combination before January 12, 2023, LightJump will be required to dissolve and liquidate, and the holders of Public Shares will be entitled to redeem their Public Shares for a pro rata share of the amount on deposit in the Trust Account.
Vote Required for Approval
The Stockholder Adjournment Proposal will be approved and adopted if the holders of a majority of the shares of SPAC Common Stock represented in person or by proxy and voted thereon at the special meeting vote “FOR” the Stockholder Adjournment Proposal. Adoption of the Stockholder Adjournment Proposal is not conditioned upon the adoption of any of the other proposals.
Recommendation of the Board
LIGHTJUMP’s BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS VOTE “FOR” PROPOSAL NO. 2 (APPROVAL OF THE STOCKHOLDER ADJOURNMENT PROPOSAL).
145
Table of Contents
Business of Moolec and Certain Information about Moolec
References in this section to “we”, “our”, “us” or “Moolec” generally refer to Moolec Science Limited.
Overview
Moolec is a science-based food ingredient company that focuses on developing real animal proteins in plants using Molecular Farming, a disruptive, scalable, affordable, and sustainable technology. Its purpose is to upgrade taste, nutrition, and affordability of alternative protein products while building a more sustainable and equitable food system. We were founded in 2020 as a spin-off from a privately owned entity, Bioceres Group S.A., which provided Moolec with a scientific team and certain intellectual property (Chymosin SPC and GLA patents, as well as trademarks). We operate in the United States, Europe and South America.
Molecular Farming, which can be defined as the production of recombinant proteins in plants with the express intention to utilize (and ultimately commercialize) the protein itself rather than any trait or capability it confers on the plant. Molecular farming therefore differs from other genetic engineering applications such as metabolic engineering (where the expressed protein has a catalytic activity in plants and the value-added product is a particular metabolite) and agronomic engineering (where the expressed protein confers beneficial agronomic properties such as pest or disease resistance, stress tolerance, or increased yields). Molecular Farming enables the synthesis of real animal proteins’ DNA in any seed crop, selecting each protein for its ability to add value in terms of a targeted functionality trait such as clotting, taste, texture, or nutritional value. The resulting proteins can be used as ingredients in consumer food products providing better-tasting, more functional, and affordable animal-free protein alternatives. We believe that this technological platform has the ability to capitalize on the scale that extensive agriculture entails to achieve affordability and that it is also cost-efficient due to the fact that it leverages biology, using plants and their inputs — sun, water, and soil — as small factories to produce animal proteins. Plants are grown through traditional farming practices that result in economies of scale through high productivity volume production.
Moolec targets the fast-growing alternative proteins market trend. Our business model focuses on R&D; manufacturing and farming; developing joint ventures and/or partnerships; technology licensing; co-branding/co-development of CPG products for food service; B2B strategy for selling of the ingredients; recipes and other technological solutions to food producers and/or retailers. Molecular Farming has the potential to modify and enhance all sorts of crops with animal proteins, which could allow us to possibly consider other market opportunities. Such possible market opportunities include milk, egg, chicken, and fish replacements, or other alternative biomaterials and cosmetics.
Our first two products are Chymosin SPC, a bovine protein expressed in safflower that has curdling applications in the cheese industry, and gamma-linoleic acid (“GLA”), a nutritional oil technology. They both have been cleared by regulatory authorities in Argentina and the United States and we are currently ramping up seed inventories. Upon completion of cornerstone milestones in these two products, we expect to accelerate product development efforts to widen our technology reach, by using the two crops that are most broadly used as protein alternatives to develop meat proteins, which are soy and peas. We are currently focused on producing the following four products (i) SPC Chymosin, (ii) GLA Oil, (iii) BEEF+ and (iv) POORK+.
We hold a growing international patent portfolio for our Molecular Farming technology, which is run by a diverse team of experts, such as PhDs and food insiders who have a robust background in the traditional food and meat industries. Research, development and innovation are core elements of our business strategy, and we believe they represent a critical competitive advantage for us.
We are supported by Nasdaq-listed Bioceres Crop Solutions Corp. (NASDAQ: BIOX), a fully integrated provider of crop productivity solutions enabling the transition to a carbon neutral agriculture, Theo I, a life sciences venture capital enterprise, and Union Group, a private equity management firm.
Moolec’s Strength’s and Competitive Positioning
Environmental Advantages. Our goal is to promote a sustainable business model. Plants are highly efficient metabolic factories which operate mainly on sunlight and carbon dioxide. If plants are used to make the protein of choice, instead of a bioreactor, there will be no energy added to the process. The technology will become carbon-fixating instead of carbon emitting. Moreover, Molecular Farming allows for sustainable water handling and can benefit from Ag-space sustainable technologies due to the compatibility of the spaces. It also allows raw
146
Table of Contents
material and product traceability from farm-to-market and clean label approach. Further, Molecular Farming will allow us to produce the traditional by-products that the market is used to (such as sugars, fiber, and oil) promoting a circular production and business model which has the potential to reduce our waste generation. We believe that this technology provides us with a competitive advantage when compared to the traditional protein industry, by producing products in a way that will appeal to the environmentally conscious consumer and providing food with real animal proteins without the negative impact of greenhouse gas emissions and reducing waste.
Consumer’s Shift. The consumer’s concern for health, sustainability, ethical sourcing, animal welfare and convenience are driving food producers and retailers to reconsider their product offerings, which traditionally contain animal-derived products. In this scenario, we intend to continue to develop our brand and products. Initially, animal proteins, which are also considered to be allergens, were replaced by plant-based alternatives to cater towards the free-from market however this soon shifted to replacing the “center of plate protein” for several meals per week making it a more mainstream product which moved it out of the niche stores and into the large retailers. This increased feasibility started helping plant-based products become an accepted part of the regular diet. Usually, consumers start buying these alternatives for one main reason (e.g. health/diet) but later, after an initially positive experience, find other advantages which solidify the product’s presence in the home (e.g., convenience). We believe that we will be able to capitalize on this shift in the consumer’s concern for health and animal welfare, which puts us in an advantageous position when compared to the traditional protein industry.
Industrial Shift. The food industry is going through a significant global protein shift and we are following along this change. This shift is driven mainly by five main pillars, which are supply chain, cost, compliance, operations and innovation, as follows:
• The weaknesses and vulnerabilities in the supply chain have been highlighted by the droughts, trade-sanctions, trade-wars, livestock diseases, Covid-19 pandemic, Suez Canal blockage and political instability and war in a major food exporting country such as Ukraine. When coupled with the increasing desire for local foods, this instability has led companies to reconsider their importing strategy. Moreover, certain ingredients have been difficult to source. Specifically, several animal-derived ingredients including milk-protein, pork and chicken have been difficult to procure due to a lack of exportable volumes (e.g., export ban of pork or chicken due to swine fever or bird flu respectively).
• Many essential ingredients like milk-derivatives, pork and chicken derivatives have gone up significantly in price which is putting pressure on low-cost high-volume markets in the developing nations. Due to the functionality and ease of use of these ingredients, they have been difficult to replace when the ingredient costs have been low. Considering that, for example, milk-derived proteins prices have reached an all-time high, many food formulators/producers have serious incentives to try and replace part, if not all, of the ingredients in the final product. Assembled food products or food service offerings consisting of multiple protein sources like cheese sausages, cheeseburgers, pastas, bakery products provide ample space for partial replacement of certain high-cost ingredients.
• Sourcing compliance has increased the complexity the food production and its supply chain. The pressure to responsibly source certain ingredients is leading to the reduction of use of that specific ingredient whenever possible to limit the exposure to a market prone to shortages in volumes.
• The safety of certain food products is increasingly making daily operations complex due to the stricter control of cross-contamination of specific product claims (e.g., organic, allergen-free, animal-free, GMO-free, etc.). This means that certain production lines are moving to a system in which either it can process all of the above, or to a line in which it is not allowed to process specific ingredients in order to maintain the free-from status.
• Pressure for innovation to solve certain problems related to the previous pillars or demand-related topics is also generating opportunities for plant-based proteins. Due to increased routes into higher volume applications of the traditional plant-proteins, other ingredient suppliers have been developing a wider plant-based offering to find applications that other plant-based proteins have failed due to technical or marketing concerns.
We believe that by addressing the main concerns with supply chain issues, price and food safety concerns, our innovative Molecular Farming technology puts us at an advantage when compared to other alternative protein companies as well as the traditional protein industry. We will not be subject to certain of the risks caused by supply
147
Table of Contents
chain issues, sourcing compliance, or rising cost of ingredients as our products would use the proteins grown within the plant itself. Our technology also addresses the current challenges faced by the traditional alternative protein and meat industries by being animal free, green and reducing costs.
Taste and Texture. Alternatives to animal-derived foods are edging closer to their targets whilst being acceptable to the consumers who buy plant-based alternatives with conviction. We are committed to prioritizing the growth of this acceptance. To make a global impact on sustainability and health, wider adoption is needed which means that the mainstream consumer, which is not inherently attracted to plant-based alternatives, needs to be drawn in. The prerequisites for a successful product which can be marketed towards committed vegans are significantly different than flexitarian/reducetarian consumers. As a consequence, the plant-based products need to be a convincing alternative to the traditional animal-based product in order to help a group of people to reduce their reliance on animal-derived protein. We believe that if the animal-alternatives can survive for one or two generations, then a gradual shift can start taking place where the food systems are nudged towards products which are perhaps less like meat but offer significant nutritional, cost and sustainability advantages over the initial generations of meat alternatives. If successful, the introduction of animal proteins into plants could help increase the functionality of the bulk of the animal-alternative recipes which are based on plants and seeds. These animal proteins have very specific functions in food where they determine texture and overall behavior in application. In terms of taste, the industry has come a long way in boosting or replacing animal-based flavors with plant or microbial derived ingredients. Using animal-inspired proteins and fats, we can also come closer to developing the tastes more associated with the traditional animal-based products.
We believe that our product will appeal to the consumer that prefers the traditional taste and texture of traditional meat products, but shares our values. A challenge for the alternative protein industry has been the ability to create alternatives that mimic the taste and texture of meat. With our technology the proteins that have a significant role and affect the taste and texture of meat products will be produced within the plants themselves. This places us in a positive position when compared to competitors that use other methods to mimic the taste and texture of meat products.
Nutritional Values. We are a mission-driven business and nutritional value is among our priorities. More data is becoming available regarding general nutrition of specific diets and the relationship with general health and wellbeing. Many foods which are attractive in terms of taste, cost and convenience are not part of a healthy diet. Replacing these foods and attracting the mainstream consumer can be difficult. When making alternatives, it is often easy to replace specific ingredients, but it will not always result in a healthier product. This is true for plant-proteins which need to replace animal-proteins. Due to the decreased ability of plant-proteins to achieve animal-based product experiences, other components such as salt, sugar and fat may need to be increased to balance out the organoleptic perception. However, reliance for these generally unhealthy ingredients can be reduced when animal proteins are produced within the plant itself. Additionally, the animal proteins which are made in plants will have the same protein sequence to the proteins which we have been eating during the evolution of our species. By introducing these animal proteins in plants, we expect to increase the overall nutritional score.
Our products are expected to have less salt, sugar and fat when compared to competitors within the alternative protein industry. For the health-conscious consumer, we expect to have a competitive advantage. We believe that our technology will produce a product that retains the benefits of traditional meat, including certain animal-specific proteins, without the need to include less healthy ingredients needed to create a suitable meat alternative. As discussed above, currently consumers are more health conscious when making decisions related to their food, and we believe we are in a position to capitalize on this trend.
Scalability and Low Costs. We focus on Molecular Farming, as we believe through it we can compete with animal-based products in terms of costs. Considering that our genetically modified seeds generally will not require significant downstream modifications, including farming and industrial modifications that generally occur in the alternative protein industry, we expect operational costs to be in line with the current farming methods and industrialization standards for existing soy, pea and safflower beans. With lower costs associated to our ability to scale and produce our products, we believe we are at an advantage when compared to competitors in the alternative protein space.
Management team and Industry Expertise. We are led by a proven and experienced executive management team with many years of industry experience. We believe this blend of talent gives us tremendous insights and capabilities to create demand and fulfill it in a scalable, profitable and sustainable way and is one of our main strengths in the competitive alternative protein industry. See the section titled “Management of Moolec” in this proxy statement/prospectus.
148
Table of Contents
Market Opportunity/Evolving Food Industry
We believe that consumer awareness of the perceived negative health, environmental and animal-welfare impacts of animal-based meat consumption has resulted in a surge in demand for viable plant-based protein alternatives. A key analogy for both the approach to and the scale of our opportunity is the strategy by which the plant-based industry captured significant market share.
We expect the plant-based industry to grow but we also recognize that a significant impact can be made on the world-wide food system by helping traditional animal-based food producers minimize the amount of animal-derived ingredients they require for the production of nutritious, low cost and sustainable foods. There is more interest from the traditional meat and dairy industry to accept next generation and alternative ingredients which can reduce their dependence on animal proteins whilst maintaining a certain level of quality and preferably reduce costs and increase supply chain stability. We believe working alongside the traditional food industry as a key opportunity to impact global sustainability.
Recent Developments
We are currently operating in a period of economic uncertainty and capital markets disruption, which has been significantly impacted by geopolitical instability, including the ongoing military conflict between Russia and Ukraine. On February 24, 2022, a full-scale military invasion of Ukraine by Russian troops began. Although the length and impact of the ongoing military conflict is highly unpredictable, the conflict in Ukraine has led to market disruptions, including significant volatility in commodity prices, credit and capital markets, as well as supply chain disruptions. Our business, financial condition, and results of operations may be adversely affected by the negative impact on the global economy resulting from the conflict in Ukraine or any other geopolitical tensions.
In recent years and months, the traditional and alternative food industry has experienced several major global events which have significantly disrupted the global supply chain. Beyond the conflict in Ukraine, this includes several years of swine and avian flu in Europe, the current trade conflicts between the United States and China, the COVID-19 pandemic, the Suez Canal blockage, severe climate events and drought in significant agricultural locations and the current labor shortage. The relatively low cost of food, of which consumers have grown accustomed, requires efficient production and supply. These developments and continuing pressure on the price of food and food products has reinforced the dependence on the global food supply chain. For example, food recipes have frequently become complex so that if one ingredient cannot be sourced, the whole production line is unable to produce. Due to strict labeling laws, including in the countries in which we operate, replacement ingredients cannot always be used which can cause major problems which have been exacerbated by the aforementioned global events.
The disruptions and demonstration of the fragility of the global food supply chain has expedited the need for more localized sourcing when possible, but more importantly has increased the attractiveness of technological solutions to produce foods which cannot be produced locally. We believe that these technological solutions, such as molecular farming, can play a major role to resolve the food security issue while also providing additional advantages related to sustainability and cost reduction.
In recent years, the general acceptance of GMOs and the alternative protein segment has increased. The most common GMO crops include soybean, maize, cotton, canola, and alfalfa. The following GMO crops were also planted in different countries in 2018: papaya, eggplant, potato, apple, safflower, pineapple, and sugarcane. In addition, several genetically modified organisms (GMOs) have been approved by the EU commission recently. Since GMOs were first approved for commercial use and planted in United States soil in 1996, their production has rapidly increased; helping to make farming methods far more efficient and productive.
Key Agreements and Partnerships
Grupo Insud
On May 24, 2022, Grupo Insud (“Insud”), a global conglomerate with a strong presence in manufacturing of biosimilars, has formed a joint venture with us through its affiliate INVIM Corporativo S.L. The joint venture plans to use yeast, fungi and other micro-organisms to produce animal-free ingredients that are expected to complement
149
Table of Contents
our molecular farming and plant-based pipeline and enable us to have a unique set of food ingredients formulations. The new products that may result from this joint venture are expected to represent a significant upgrade in nutritional values, while preserving affordability. We expect that combining our respective technological platforms may improve our R&D, since this partnership is expected to permit us to speed up our scientific research and developments and to scale-up our production expertise.
Bioceres
We were founded in 2020 as a spin-off from a privately owned entity, Bioceres Group S.A., which provided Moolec with a scientific team and certain intellectual property (Chymosin SPC and GLA patents, as well as trademarks).
On June 17, 2021, we entered into an agreement with Bioceres to assist us with local operations in Argentina, as well as provide us with additional expertise. Bioceres provided assistance during our inception and early operations in Argentina before incorporating our subsidiary.
Products
The table below sets forth our product portfolio. Our products are in various stages of R&D and not yet available to consumers or in the production stage. We currently have multiple projects in development which will require us to confirm discovery, proof of concept and develop further products, particularly in connection with our plans to develop meat replacements, POORK+ and BEEF+:
Product | | Category | | Description | | Market |
Chymosin SPC | | Dairy Ingredient | | Plant-based Chymosin. Chymosin is a key ingredient for cheese production compulsory for the clotting step. Moolec’s technology allows Chymosin to be produced in Safflower seeds turning this semi-arid crop into a bio-reactor for enzymes. | | Food, dairy (cheese industry) |
GLA SONOVA® | | Nutritional Oil | | Plant-based GLA (Gamma Linolenic Acid). A safflower oil with high levels of GLA (at least 40%). Moolec’s technology allows a high concentration of GLA that is at least twice as concentrated when compared with other standard sources of GLA such as borage oil and evening primrose oil, which concentrations are 20% and 10%, respectively. | | Food, pet food, health & nutrition(1) |
POORK+ | | Meat Replacement | | POORK+ is a plant-based and animal meat free ingredient containing porcine proteins in soybeans. Moolec’s technology allows the enhancement of organoleptic properties and nutrition in alternative meat products by using a more sustainable and affordable approach when compared to traditional animal protein production. | | Food, alternative meat |
150
Table of Contents
Product | | Category | | Description | | Market |
BEEF+ | | Meat Replacement | | BEEF+ is a plant based and animal meat free ingredient containing bovine proteins in peas and yeast (prototype). Moolec’s technology allows the enhancement of organoleptic properties and nutrition in alternative meat products by using a more sustainable and affordable approach when compared to traditional animal protein production. | | Food, alternative meat |
Development Timelines
Our four products included in our product portfolio described above, Chymosin SPC, GLA SONOVA®, POORK+ and BEEF+ are each in different stages of product development. In order to commercialize our products and for them to enter into markets, it is necessary for our products to go through multiple stages of development, including research and development where we aim to locate proteins of interest found in traditional animal foods that can be effectively and efficiently expressed within a plant or a microorganism, the development of a successful prototype, further research and testing to ensure that the prototype can be efficiently scaled-up, production of the product and finally commercialization of the product. The stages of development for our products consist of the following:
i) Discovery: The first phase in the technology development process is the discovery or identification of candidate genes or genetic systems, metabolites, or microorganisms potentially capable of enhancing specified plant characteristics or enabling an agroindustrial biotech solution.
ii) Proof of concept: Upon successful validation of the technologies in model systems (in vitro or in vivo), promising technologies graduate from discovery and are advanced to the proof-of-concept phase. The goal of this phase is to validate a technology within the targeted organism before moving forward with technology escalation activities or extensive field validation.
iii) Early development: In this phase, efficacy field trials are expanded to evaluate the expression level and phenological characteristics of the traits in multiple geographies and growing cycles, as well as other characteristics in order to optimize the technology’s performance in the targeted organisms. The goal of the early development phase is to evaluate the technical feasibility by identifying the best candidate to scale up the seed stock and to start the regulatory field trials.
iv) Advanced development and deregulation: In this phase, extensive field tests are used to fully demonstrate the effectiveness of the technology for its intended purpose. In the case of GM traits, the process of obtaining regulatory approvals from government agencies is started, and includes field trials for environmental, core and food safety data generation. For solutions involving microbial fermentation, industrial-scale runs are conducted.
v) Pre-launch: This phase involves finalizing the regulatory approval process and preparing for the launch and commercialization of the technology. The range of activities in this phase includes seed increases, pre-commercial production, and product and solution testing with selected customers. Usually, a more detailed marketing strategy and preparation of marketing materials occur during this phase.
vi) Product launch: In general, this phase, which is the last milestone of the research and development process, is carried out by the group.
Our Chymosin SPC and GLA SONOVA® products are currently at the “scale-up” stage, described above as Pre-launch, whereby we have already been able to successfully develop the prototype and our conducting further research in greenhouses related to seed multiplication and protein expression. Our meat replacements products, POORK+ and BEEF+, remain in the first developmental stages of research, including Discovery, Proof of concept and Early development.
151
Table of Contents
We do not currently have an exact developmental timeline related to our products and when they will reach the commercialization stage. Our timeline is subject to changes as we proceed with field trials, seed multiplication and additional R&D testing.
The table below describes the current projects that are within our pipeline. We believe that the projects below can continue to advance, becoming products, and reach commercialization, subject to final regulatory approvals and success within each of the stages described above:
Product | | Project | | Application | | Plant/
Microorganism Host | | Function(1) | | Development Stage | | Planned Commercial Launch |
Chymosin SPC | | SPC2 | | Dairy Ingredient | | Safflower | | Texture | | Scale-up | | 2025 |
GLA SONOVA® | | GLASO | | Nutritional Oil | | Safflower | | Nutrition | | Scale-up | | 2025 |
BEEF+ | | YEEA1 | | Meat replacement | | Yeast | | Sensory and Nutrition | | Early development | | 2025 |
BEEF+ | | YEEA2 | | Meat replacement | | Yeast | | Sensory and Nutrition | | Discovery | | 2025 |
BEEF+ | | YEEA3 | | Meat replacement | | Yeast | | Sensory and Nutrition | | Discovery | | 2026 |
POORK+ | | SOOY1 | | Meat replacement | | Soybean | | Sensory and Nutrition | | Proof of concept | | 2027 |
POORK+ | | SOOY2 | | Meat replacement | | Soybean | | Sensory and Nutrition | | Discovery | | 2029 |
POORK+ | | SOOY3 | | Meat replacement | | Soybean | | Sensory and Nutrition | | Discovery | | 2029 |
POORK+ | | SOOY4 | | Meat replacement | | Soybean | | Sensory | | Incubation | | To be determined |
BEEF+ | | PEEA1 | | Meat replacement | | Pea | | Sensory and Nutrition | | Discovery | | 2028 |
We believe, based on available market research, that the Serviceable Available Market (SAM) with respect to products similar to Chymosin SPC, GLA SONOVA®, and our potential meat replacements (POORK+ & BEEF+) could reach $350 million, $1,500 million, and $1,500 million, respectively, by the end of 2025. We must complete the necessary development activities in order to meet the development timelines indicated above. However, delays may occur as a result of the development process. See “Risk Factors — Risks Related to Moolec — Risks Related to our Business and Operations — To compete effectively, we must introduce new products that achieve market acceptance and improve the output of our technology.”
Marketing and Sales
We are currently in the R&D phase of our production and are working to scale up our seed inventory and production. As our product and focus on molecular farming may not be familiar to many potential consumers, we will be required to educate consumers about our brand, respond quickly to concerns and consult on food trends. As we grow our brand, we expect to expand our sales and marketing team in the future by adding dedicated personnel to service new retail customers. In the future we may also add outside sales representative to extend our sales efforts to the extent necessary.
152
Table of Contents
Competition
The alternative protein market is highly competitive. Our primary competitors in this area include Motif FoodWorks, Impossible Foods Inc., Givaudan Group, International Flavors & Fragrances, Nobell Foods, Kyomei, DSM and Hansen.
Intellectual Property
Our business model is focused on R&D and the development of future IP and patents. Our IP strategy consists of conducting research and testing to rapidly acquire IP protection for our molecular farming platform.
Our patents or patent applications relate to plant-based technologies. The main countries in which we seek patent protection, among others, are the United States, Canada, Brazil, Argentina, Australia, India, China, Hong Kong, Japan, Malaysia, New Zealand, Mexico and other countries in Europe.
To date, we have identified and sought patent protection in our capacity as either title holder or exclusive licensee for over 22 plant-based technologies linked to (i) SPC, a plant-based chymosin and key ingredient for cheese production, (ii) GLA, a plant based oil destined for enriching food, pet food, and nutraceutical products (iii) plant-based real porcine and bovine proteins embedded within the matrix of native soy proteins to enhance alternative meat products in terms of taste, color and nutritional values and (iv) methods for producing recombinant animal proteins within yeast. These technologies are currently protected through our 17 patents and 5 current patent applications as owner and or as exclusive licensee.
The table below sets forth the product type/technology for which: (i) our patents granted relate, the jurisdiction of registration, the expiration and the type of patent; (ii) our patents application relate, the jurisdiction in which the registration was applied for, the application date and the type of patent, as well as our licensed patents and trademarks. 10.5% of our patents are proprietary while 89.5% of the patents listed below have been licensed from third parties.
Patents
The table below shows the patents that have been granted as of the date hereof.
Current Owner | | Jurisdiction | | Application/Patent Number | | Filing
Date | | Issue
Date | | Expiration
Date | | Prosecution
Status | | Company
Technology |
Arcadia Biosciences, Inc. | | Argentina | | P060102090 | | 05/22/2006 | | 11/15/2017 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Blue Horse Labs, Inc. | | Germany | | EP06771043A | | 05/22/2006 | | 07/29/2015 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Arcadia Biosciences, Inc. | | Australia | | 2006249983 | | 05/22/2006 | | 06/30/2011 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Arcadia Biosciences, Inc. | | Brazil | | PI06114806 B1 | | 05/22/2006 | | 07/06/2016 | | 07/06/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Arcadia Biosciences, Inc. | | Canada | | 2609367 | | 05/22/2006 | | 08/19/2014 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Arcadia Biosciences, Inc. | | China | | ZI.200680024359.6 | | 05/22/2006 | | 09/05/2012 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Blue Horse Labs, Inc. | | Spain | | EP06771043A | | 05/22/2006 | | 07/29/2015 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Blue Horse Labs, Inc. | | France | | EP06771043A | | 05/22/2006 | | 07/29/2015 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Blue Horse Labs, Inc. | | United Kingdom | | EP06771043A | | 05/22/2006 | | 07/29/2015 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Blue Horse Labs, Inc. | | Hong Kong | | 8108949.7 | | 08/13/2008 | | 12/24/2015 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Arcadia Biosciences, Inc. | | India | | 9948DELNP2007 | | 12/20/2007 | | 07/28/2016 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Arcadia Biosciences, Inc. | | Japan | | 2008513656 | | 11/23/2007 | | 07/06/2012 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Arcadia Biosciences, Inc. | | Mexico | | MXA2007014753 | | 05/22/2006 | | 06/04/2013 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Arcadia Biosciences, Inc. | | Malaysia | | PI20072148 | | 11/30/2007 | | 11/29/2013 | | 11/30/2027 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
153
Table of Contents
Current Owner | | Jurisdiction | | Application/Patent Number | | Filing
Date | | Issue
Date | | Expiration
Date | | Prosecution
Status | | Company
Technology |
Arcadia Biosciences, Inc. | | New Zealand | | 563676 | | 11/23/2007 | | 05/09/2011 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Blue Horse Labs, Inc. | | United States | | 11/438,951 | | 02/11/2011 | | 02/22/2011 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
Blue Horse Labs, Inc. | | United States | | 13/025,345 | | 02/11/2011 | | 06/05/2012 | | 05/22/2026 | | Granted | | Safflower with elevated gamma-linolenic acid (GLA) |
The table below shows current patent applications pending as of the date hereof.
Patent Technology | | Jurisdiction | | Filing Date | | Status |
High expression of animal protein in plants | | United States | | 6/29/2022 | | Pending |
Safflower Transgenic Event SPC (SCP 2.0) | | United States | | 8/20/2020 | | Published |
Safflower Transgenic Event SPC (SCP 2.0) Div. | | United States | | 9/20/2022 | | Pending |
Safflower Transgenic Event SPC (SPC 2.0) | | Argentina | | 05/07/2021 | | Pending |
Methods for the production of recombinant heme proteins in hansenula polymorpha | | United States | | 10/06/2022 | | Pending |
Trademarks
The table below shows our current trademarks as of the date hereof.
Current Owner | | Jurisdiction | | Application Number | | Filing
Date | | Registration Date | | Prosecution Status | | Trademark |
Moolec Science Limited | | US — International Class 5 | | 85/532,292 | | 02/02/2012 | | 04/09/2013 | | Registered | | SONOVA (and DESIGN) (BLACK and WHITE) |
Moolec Science Limited | | US — International Class 5 | | 85/532,568 | | 02/02/2012 | | 04/09/2013 | | Registered | | SONOVA (and DESIGN) (COLOR) |
Moolec Science Limited | | US — International Class 5 | | 85/532,041 | | 02/02/2012 | | 06/10/2014 | | Registered | | SONOVA GLA SAFFLOWER OIL (STYLIZED DESIGN) (COLOR) |
Moolec Science Limited | | US — International Class 5 | | 85/530,341 | | 01/31/2012 | | 06/10/2014 | | Registered | | SONOVA GLA SAFFLOWER OIL (and DESIGN) (BLACK and WHITE) |
Moolec Science Limited | | US — International Class 3 | | 85/508,765 | | 01/04/2012 | | 11/27/2012 | | Registered | | SONOVA |
Moolec Science Limited | | US — International Class 5 | | 85/505,979 | | 12/29/2011 | | 08/14/2012 | | Registered | | SONOVA |
Moolec Science Limited | | Canada | | 1414621 | | 10/15/2008 | | 10/26/2011 | | Registered | | SONÕVA |
Moolec Science Limited | | European Union — Class 29, 42 | | 18535249 | | 08/19/2021 | | 12/22/2021 | | Registered | | Moolec |
Moolec Science Limited | | United Kingdom — Class 29, 42 | | UK00003685139 | | 08/23/2021 | | 08/23/2021 | | Registered | | Moolec |
Moolec Science Limited | | United Kingdom — Class 29, 42 | | UK00003685109 | | 08/23/2021 | | 08/23/2021 | | Registered | | GM4GOOD |
Moolec Science Limited | | European Union — Class 29, 42 | | 18535246 | | 08/19/2021 | | 12/22/2021 | | Registered | | GM4GOOD |
Moolec Science Limited | | European Union — Class 29, 42 | | 18536578 | | 08/19/2022 | | 12/22/2021 | | Registered | | Moolec |
AG Biomolecules LLC | | Argentina — Class 1 | | 3167938 | | 05/30/2012 | | 9/30/2013 | | Registered | | SPC |
AG Biomolecules LLC | | Argentina — Class 31 | | 3167939 | | 05/30/2012 | | 9/30/2013 | | Registered | | SPC |
154
Table of Contents
Research and Development
Our R&D activities are directed toward the ability to find proteins of interest that are found in traditional animal foods and express the desired proteins in a host plant. Our R&D platform is undertaken through service agreements with companies that specialize in the research and development of increasing crop yields, gene expression and biotechnology.
Agrality S.A. (Agrality)
On October 15, 2020, we entered into a service agreement with Agrality for the provision of seed multiplication services.
Ingeniería Metabólica S.A. (INMET)
On October 22, 2020, we entered into a service agreement with INMET for a fermentation project consisting of strain construction and downstream processing of a bovine protein.
HAN Biocentre — University of Applied Sciences in Nijmegen (HAN Biocentre)
On December 16, 2020, we entered into a service agreement with HAN Biocentre for a fermentation project consisting of strain construction and downstream processing of a porcine protein.
Instituto de Agrobiotecnología Rosario S.A.U. (INDEAR)
On January 18, 2021, we entered into a service agreement with INDEAR for transformation services of transgenic events.
On September 9, 2021, we entered into a service agreement with INDEAR for the development of the following: (a) research related to bovine protein expression in soybean plants, (b) seed multiplication of GM safflower containing nutritional oil, and (c) breeding activities related to our safflower’s technologies.
On June 23, 2022, we entered into a service agreement with INDEAR for gene expression analysis and generation advancement services in order to select desirable soybean transgenic lines.
Washington State University (WSU)
On January 29, 2021, we entered into a sponsored research project agreement with WSU for the development of research related to bovine protein expression in pea plants.
Wisconsin Crop Innovation Center (WCIC)
On February 11, 2021, we entered into a service agreement with University of Wisconsin-Madison for soybean transformation services.
Future Foods B.V. (Future Foods)
On May 1, 2021, we entered into a service agreement with Future Foods for food-product development services and application research.
Artes Biotechnology GmbH (Artes)
On September 15, 2021, we entered into a service agreement with Artes for a fermentation project consisting of the development of a yeast-based production cell line.
Flavour Products BV (Flavour Products)
On December 28, 2021, we entered into a service agreement with Flavour Products for the development of flavor profiles for our products.
155
Table of Contents
Premas Biotech PVT Ltd. (Premas)
On May 16, 2022, we entered into a service agreement with Premas for a fermentation project consisting of the development of yeast-based production cell lines for several bovine proteins.
Service agreements with third parties
The table below shows the service agreements of which we are a party which are a primary source of research and development activities.
Counterparty name | | Type of agreement | | Date |
Ingeniería Metabólica S.A. (INMET) | | Service Agreement | | October 5, 2020 |
HAN BIOCENTRE UNIVERSITY OF APPLIED SCIENCE (HAN) | | Service Agreement | | December 16, 2020 |
Instituto de Agrobiotecnología Rosario S.A.U. (INDEAR) | | Service Agreement | | January 18, 2021 |
Future Foods B.V. | | Service Agreement | | May 1, 2021 |
Instituto de Agrobiotecnología Rosario S.A.U. (INDEAR) | | Service Agreement | | September 9, 2021 |
Artes Biotechnology GmbH | | Service Agreement | | September 15, 2021 |
Raw Materials and Material Sourcing
The process of scaling up our seed inventory and production is performed in the United States and Argentina. We have access to different growers in both countries to outsource the production depending on agronomical conditions. Currently, we have safflower productions in American Falls (Idaho, USA) and Monte del Rosario (Córdoba, Argentina).
For seed transformation and seed scaling up at R&D stage (soybean and pea), including greenhouses use, we currently work with three outsourced service centers: WCIC, WSU and INDEAR. We are constantly evaluating the most suitable partners considering the scope of the new R&D programs.
Regulatory Landscape
We are, and will be, subject to laws and regulations in which we operate. This includes laws and regulations governing biotechnology and food companies related to the development, approval, manufacturing, import, export, marketing and sale of our products.
Regulation of Plant Biotechnology Products
United States
Our main focus is the United States market. In the United States, the main agencies with responsibility for regulation of plant biotechnology products are the U.S. Department of Agriculture’s (“USDA”), the Animal and Plant Health Inspection Services (“APHIS”), and the FDA.
As of the date hereof, Moolec has permits from USDA-APHIS for importation, interstate movement, and environmental release of GE plants while we are seeking confirmation from USDA-APHIS-BRS. Moolec GLA safflower oil (GLASO) already has approvals from the FDA for use of GLASO as an ingredient in dietary supplements, an ingredient in nutritional beverages and medical foods, and as an ingredient in pet food. We have also the FDA approval for use of the seed meal from GLA safflower plants in food for cattle and poultry.
Argentina
Our plants require approval under the Ministry of Agriculture, Livestock and Fisheries (“MAGP”), since we plan to produce our crops in both Argentina and the United States in order to have flexibility given the counter seasons of the two locations.
156
Table of Contents
Currently we have permits from the MAGP for importation, movement and environmental release from some of our genetically modified plants. Our chymosin safflower has been fully approved by MAGP for release and production in Argentina and no further permits are required for its production.
Regulation of Food and Ingredient Products
United States
We are subject to laws and regulations administered by various federal, state and local government agencies in the United States, such as the FDA, the Federal Trade Commission, the Environmental Protection Agency, the Occupational Safety and Health Administration, and the USDA, related to any future processing, packaging, distribution, sale, marketing, labeling, quality, safety, and transportation of our products, as well as our occupational safety and health practices.
Among other things, the facilities where are products are grown in the United States may be required to register with the FDA, and comply with regulatory schemes including the Food Safety Modernization Act. We would also be subject to state and local food and safety regulations in connection with the sale of our future products. We are also subject to labor and employment laws, laws governing advertising, privacy laws, safety regulations and other laws.
Other Regulatory Requirements
We are also subject to various federal, state, local and national and transnational laws, regulations and requirements in the jurisdictions in which we operate, relating to not only biotechnology and food and ingredient products. In the future as we continue to grow, we may be subject to other regulations and requirements in the jurisdictions in which we operate relating to, among others, safe working conditions, laboratory and distribution practices, transportation and disposal of hazardous or potentially hazardous substances. In addition, applicable import and export laws will require us to abide by certain standards relating to the cross-border transit of finished goods and raw materials.
The costs associated with our continued compliance with the various applicable federal, state, local, national and transnational regulations to which we are subject, or could become subject could be significant, and the failure to comply with such legal requirements could have an adverse effect on our results of operations and financial condition. See “Risk Factors — Risks Related to Moolec — Risks Related to Laws and Regulations.”
Employees
As of the date hereof, we had [12] full-time and temporary employees located in the United States, Argentina, the Netherlands and the United Kingdom. We employ six scientists and skilled personnel. We have never experienced a labor-related work stoppage. Our work environment is focused on technology, as is our corporate purpose.
Legal Proceedings
From time to time, we may become involved in legal proceedings arising in the ordinary course of our business. As of the date hereof, we are not party to any legal proceedings.
157
Table of Contents
Management of Moolec
References in this section to “we”, “our”, “us” or “Moolec” generally refer to Moolec Science Limited.
Executive Officers
The names, ages and current positions of Moolec’s current executive officers are listed in the table below. Moolec expects that these executive officers will continue as executive officers of Holdco following the Business Combination.
Name | | Age | | Title |
Gastón Paladini | | 41 | | Chief Executive Officer |
José López Lecube | | 39 | | Chief Financial Officer |
Amit Dhingra | | 52 | | Chief Science Officer |
Henk Hoogenkamp | | 35 | | Chief Product Officer |
Gastón Paladini. Upon the closing of the Business Combination, Mr. Paladini will serve as the Chief Executive Officer of Holdco. Mr. Paladini is a Co-Founder, and has been the Chief Executive Officer, of Moolec since its inception in 2020. For the last 10 years, Mr. Paladini has been a board member for Paladini Group, one of the largest meat producers in Latin America where he committed to promoting innovation as a fundamental company value. Fully aware of the current challenges the planet is facing and based on his knowledge and experience in the traditional meat industry, he began to explore the ecosystem of alternative proteins. Before his role as a board member Mr. Paladini has worked for more than a decade at renowned advertising agencies, including Craverolanis and Agulla & Baccetti. He has an MBA from IAE Business School and an Advertising degree from the University of Palermo.
José López Lecube. Upon the closing of the Business Combination, Mr. López will serve as the Chief Financial Officer of Holdco. Mr. López has served as Moolec’s Chief Financial Officer since July 2021. He has over 15 years of corporate development experience in strategic roles for multinational companies with expertise in corporate strategy, finance, and high-impact partnerships. Most recently he worked for Uber Tech Inc. leading key growth initiatives for the LatAm region during 2019 and 2020. Prior to that Mr. López served as a corporate development executive for Lartirigoyen and Cia (a Glencore Agri joint venture) leading M&A, FP&A and corporate strategy during 2017 and 2018. During 2015 and 2016 he was part of the corporate finance team of Archer Daniel Midland Company in Chicago and São Paulo. At the beginning of his career, Mr. López Lecube served as an executive for Schroders Asset Management for four years focusing on institutional markets. He obtained his bachelor’s degree in business administration from the Universidad de San Andrés in Argentina in 2007 and his MBA from the Kellogg School of Business at Northwestern University in the United States in 2015.
Amit Dhingra. Upon the closing of the Business Combination, Mr. Dhingra will serve as the Chief Science Officer. Mr. Dhingra has served as Moolec’s Chief Science Officer since August 2020. In 2016, Mr. Dhingra joined Washington State University (“WSU”) as an assistant professor. He gained tenure and became a full professor and also served as the Interim Chair and Professor of Genomics and Biotechnology in the Department of Horticulture at WSU. He also served as the Chair of the Entrepreneurial Faculty Ambassadors Program, a presidential level task force. Recently he moved his program to Texas A&M University where he is the Head of the Department of Horticultural Sciences and Professor of Genomics and Biotechnology. Mr. Dhingra is a recipient of a national Biology Mentor award conferred by the Council on Undergraduate Research. He serves on the editorial board of five internationally reputed plant science journals. He has been awarded three US and three international patents on regulating ripening in fruits to reduce post-harvest wastage. His research has been featured in the New York Times, The Atlantic, BBC, The Times of London, and several other news outlets. Before serving as the Chief Science Officer for Moolec, Mr. Dhingra founded Phytelligence Inc., an agriculture biotechnology spin-off out of his lab in 2011. Mr. Dhingra completed his B.Sc. in Botany (Hons) from Hindu College in 1991, New Delhi, India and his M.Sc. in Botany (Hons) with specialization in Cytogenetics and Plant Breeding from Raja Balwant Singh College, Agra, India in 1993. He then completed his Ph.D. at the University of Delhi, India and Rutgers University, New Jersey supported by fellowships from the University Grants Commission and The Rockefeller Foundation, USA, respectively in 2000.
Henk Hoogenkamp. Upon the closing of the Business Combination, Mr. Hoogenkamp will serve as the Chief Product Officer. Mr. Hoogenkamp has acted as Moolec’s Chief Product Officer since August 2020. In 2019, Mr. Hoogenkamp started a contract research company active in the development of extruded plant-based ingredients
158
Table of Contents
whilst working for large stock-listed multinationals and many start-ups as a technical and strategic advisor. In 2016, Mr. Hoogenkamp resided in the UK where he worked as a sales executive for the Brecks Food Company, a plant-based ingredient manufacturer and co-manufacturer of vegetarian sausages. In 2014, Mr. Hoogenkamp joined Food Flow Inc., an ingredient company in the Philippines as a technical sales and innovation manager regarding the application of animal and plant-based proteins in local food production until August 2016. Mr. Hoogenkamp has a Bachelor’s degree in Biochemistry and a Master’s degree in Molecular Life Sciences from the HAN University of Applied Sciences in the Netherlands. For his Ph.D. research, he raised funds from both public and private sectors to further research the use of animal by-products for the design of biomedical materials in tissue engineering and regenerative medicine. He split his time between fundamental research for the Radboud University Nijmegen Medical Centre and applied research for Marel Townsend Further Processing.
Moolec Executive Compensation
For the year ended June 30, 2022, Moolec’s executive officers received an aggregate compensation of approximately $363,978. Prior to June 30, 2022, Moolec’s executive officers were subject to consulting agreements rather than employment contracts. During the year beginning July 1, 2022, Moolec’s executive officers entered into/or will enter into new employment contracts with updated terms. For the fiscal year beginning July 1, 2022, the total compensation paid to Moolec’s executive officers consists of a base salary, restricted stock units (“RSUs”), and stock options. In addition, executives are entitled to an annual bonus paid in RSUs subject to performance and board approval. Details of Moolec’s executive officer compensation commencing July 1, 2022 are as follows:
Name | | Basic Salary | | Stock Options(1) | | Annual Bonus(2) |
Gastón Paladini | | $120,000 and $68,000 worth of RSUs | | — | | $141,000 worth of RSUs |
José López Lecube | | $150,000 and $12,800 worth of RSUs | | 365,000 | | $105,820 worth of RSUs |
Amit Dhingra | | $108,000 | | 250,000 | | $54,000 worth of RSUs |
Henk Hoogenkamp | | $148,000 and 16,500 worth of RSUs | | 292,000 | | $82,250 worth of RSUs |
Moolec Share Option Plan
The Moolec Science Limited Employee Share Plan (the “Plan”) was approved and adopted by the Board of Directors of Moolec on August 23, 2021. The Plan sets a limit of 5% of the ordinary share capital to be issued under the Plan. In terms of eligibility, any current or former employee, director or office holder of Moolec or any subsidiary of Moolec may be designated by the Board of Directors as eligible to receive an award under the Plan. Prior to the issuance or transfer of shares to the award holder, the award holder does not have voting rights, the right to receive dividends, nor any other right of a shareholder. Any award granted under the Plan is expected to be exchanged by an equivalent award in the Holdco.
Employment Agreements
We have entered into an employment agreement with certain members of management, including our Chief Science Officer. Our employees consist of skilled personnel in R&D and in the agricultural field as well as experienced scientists and other skilled personnel. During the term of the members of our management team’s employment, and for one year after termination, the employees will typically be bound by non-competition and non-solicitation obligations.
Director Compensation
Moolec does not pay any compensation to directors who are executives or employees of Moolec or to non-executive/employee directors.
159
Table of Contents
Moolec Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis provide information which Moolec’s management believes is relevant to an assessment and understanding of Moolec’s results of operations and financial condition. This discussion and analysis should be read together with the section of this proxy statement/prospectus entitled “Selected Historical Consolidated Financial Information of Moolec” and the audited consolidated financial statements and related notes of Moolec that are included elsewhere in this proxy statement/prospectus. This discussion and analysis should also be read together with the section of this proxy statement/prospectus entitled “Business of Moolec and Information About Moolec” and the unaudited condensed combined pro forma financial information as of and for the year ended June 30, 2022 in the section of this proxy statement/prospectus entitled “Unaudited Pro Forma Condensed Combined Financial Information.” In addition to historical financial information, this discussion and analysis contains forward-looking statements based upon current expectations that involve risks, uncertainties and assumptions. See the section entitled “Cautionary Note Regarding Forward-Looking Statements.” Actual results and timing of selected events may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors” or elsewhere in this proxy statement/prospectus.
Overview
Moolec is a science-based food ingredient company that focuses on developing real animal proteins in plants using Molecular Farming, a disruptive, scalable, affordable, and sustainable technology. Its purpose is to upgrade taste, nutrition, and affordability of alternative protein products while building a more sustainable and equitable food system. We were founded in 2020 as a spin-off from a privately owned entity, Bioceres Group S.A., which has since provided Moolec with a scientific team and certain intellectual property (Chymosin SPC and GLA patents, as well as trademarks). We operate in the United States, Europe and South America.
Molecular Farming, which can be defined as the production of recombinant proteins in plants with the express intention to utilize (and ultimately commercialize) the protein itself rather than any trait or capability it confers on the plant. Molecular farming therefore differs from other genetic engineering applications such as metabolic engineering (where the expressed protein has a catalytic activity in plants and the value-added product is a particular metabolite) and agronomic engineering (where the expressed protein confers beneficial agronomic properties such as pest or disease resistance, stress tolerance, or increased yields). Molecular Farming enables the synthesis of real animal proteins’ DNA in any seed crop, selecting each protein for its ability to add value in terms of a targeted functionality trait such as clotting, taste, texture, or nutritional value. The resulting proteins can be used as ingredients in consumer food products providing better-tasting, more functional, and affordable animal-free protein alternatives. This technological platform has the ability to capitalize on the scale that extensive agriculture entails to achieve affordability. It is also cost-efficient due to the fact that it leverages biology, using plants and their inputs — sun, water, and soil — as small factories to produce animal proteins. Plants are grown through traditional farming practices that result in economies of scale through high productivity volume production.
Moolec targets the fast-growing alternative proteins market trend. Our business model focuses on R&D; manufacturing and farming; developing joint ventures and/or partnerships; technology licensing; co-branding/co-development of CPG products for food service; B2B strategy for selling of the ingredients; recipes and other technological solutions to food producers and/or retailers. Molecular Farming has the potential to modify and enhance all sorts of crops with animal proteins, which could allow us to possibly consider other market opportunities. Such possible market opportunities include milk, egg, chicken, and fish replacements, or other alternative biomaterials and cosmetics.
Our first two products are Chymosin SPC, a bovine protein expressed in safflower that has curdling applications in the cheese industry, and gamma-linoleic acid (“GLA”), a nutritional oil technology. They both have been cleared by regulatory authorities in Argentina and the United States and we are currently ramping up seed inventories. Upon completion of cornerstone milestones in these two products, we expect to accelerate product development efforts to widen our technology reach, by using the two crops that are most broadly used as protein alternatives to develop meat proteins, which are soy and peas. We are currently focused on producing the following four products (i) SPC Chymosin, (ii) GLA Oil, (iii) BEEF+ and (iv) POORK+.
160
Table of Contents
We hold a growing international patent portfolio for our Molecular Farming technology, which is run by a diverse team of experts, such as PhDs and food insiders who have a robust background in the traditional food and meat industries. Research, development and innovation are core elements of our business strategy, and we believe they represent a critical competitive advantage for us.
We are supported by Nasdaq-listed Bioceres Crop Solutions Corp. (NASDAQ: BIOX), a fully integrated provider of crop productivity solutions enabling the transition to a carbon neutral agriculture, Theo I, a life sciences venture capital enterprise, and Union Group, a private equity management firm.
The Business Combination
On June 14, 2022, LightJump, Moolec, Merger Sub and Holdco entered into the Business Combination Agreement, which contains customary representations and warranties, covenants, closing conditions, termination fee provisions and other terms relating to the Merger and Exchange and the other transactions contemplated thereby, as summarized below. Following the effectiveness of the transactions contemplated by the Merger, the parties will consummate the Business Combination and LightJump and Moolec will become direct wholly-owned subsidiaries of Holdco. Pursuant to the Business Combination Agreement, each of the following transactions occurred, or will occur, in the following order:
• At the Exchange Effective Time, subject to the receipt of the Company Requisite Approvals, the Holdco Requisite Approvals and the delivery of the Exchange Auditor Report, and in accordance with the Holdco Delegate Resolutions:
• all issued Company Ordinary Shares held by the Company Shareholders shall be transferred and for the purposes of the 1915 Law, contributed in kind to Holdco, free and clear of all Liens (other than the Company Shareholders’ Agreements Liens that will expire on or prior to the Closing Date), and the Company Shareholders shall subscribe for and, as consideration for the contribution, shall be issued, in accordance with the Exchange Ratio (save that the Holdco Ordinary Shares to be issued shall be reduced by the number of Holdco Ordinary Shares already held by the Company Shareholders immediately prior to the Exchange), the Exchange Issuance;
• the Exchange Issuance shall be allocated among the Company Shareholders in accordance with the terms of the Payment Spreadsheet and Exchange Agreements;
• each Company SAFE Holder shall have contributed all of its rights and obligations under each Original SAFE to Holdco in consideration for the issuance by Holdco of a simple agreement for future equity on substantively identical terms (mutatis mutandis) with such adjustments (if any) required under Luxembourg law. For Luxembourg law purposes, a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued a report on the contributions in kind relating to the contribution of the Original SAFEs prepared in accordance with article 420-10 of the 1915 Law;
• each Company Shareholder shall cease to be the holder of such Company Ordinary Shares, subject to the submission of all filings required under Law (including any filings required to pay stamp duties), and Holdco will be recorded as the registered holder of all the Company Ordinary Shares so exchanged and transferred and will be the legal and beneficial owner thereof.
• Immediately prior to the Merger Effective Time but after the Exchange Effective Time, each Company SAFE Holder shall receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the respective Original SAFE, with such adjustments (if any) required under Luxembourg law;
• SPAC shall cause the Certificate of Merger to be executed, acknowledged and filed with the Secretary of State of the State of Delaware in accordance with the applicable provisions of the DGCL in order to effectuate the Merger. The Merger shall become effective at such time as the Certificate of Merger has been duly filed with the Secretary of State of the State of Delaware or at such later time as may be agreed by the Company and SPAC in writing and specified in the Certificate of Merger in accordance with the DGCL;
161
Table of Contents
• At the Merger Effective Time, by virtue of the Merger and the Holdco Requisite Approvals, subject to the Merger Auditor Report, and without any further action on the part of SPAC, Merger Sub, Holdco or the Company or the holders thereunder:
• each SPAC Common Stock issued and outstanding immediately prior to the Merger Effective Time, excluding those have been redeemed subject to any redemption rights, shall be exchanged with Holdco (which exchange, for purposes of the 1915 Law, shall include, for the avoidance of doubt, a contribution-in-kind of each such SPAC Ordinary Shares from the holders of SPAC Ordinary Shares to Holdco), against the issue by Holdco of new Holdco Ordinary Shares (such issuance, the “Merger Issuance”), under the authorized share capital of Holdco (pursuant to the Holdco Delegate Merger Resolutions) and subscribed by the contributing holders of SPAC Ordinary Shares by virtue of the Merger and in accordance with the 1915 Law for one validly issued and fully paid Holdco Ordinary Share (the “Merger Consideration”), delivered by Holdco;
• as a result of the Merger, all SPAC Common Stock shall cease to be outstanding, shall be cancelled and shall cease to exist;
• each share of common stock, par value $0.01 of Merger Sub issued and outstanding immediately prior to the Merger Effective Time shall be converted and exchanged for one (1) validly issued, fully paid and nonassessable ordinary share, par value $0.01 per share, of the Surviving Company.
• Each SPAC Warrant that is outstanding immediately prior to the Merger Effective Time shall, pursuant to the SPAC Warrant Agreement, cease to represent a right to acquire one SPAC Common Stock and shall be converted in accordance with the terms of such SPAC Warrant Agreement, at the Merger Effective Time, into a right to acquire one Holdco Ordinary Share on substantially the same terms as were in effect immediately prior to the Merger Effective Time under the terms of the SPAC Warrant Agreement.
The parties to the Business Combination Agreement will hold the closing on the date of the Merger Effective Time, following the satisfaction or waiver (to the extent such waiver is permitted by applicable law) of the conditions set forth in the Business Combination Agreement (other than those conditions that by their nature are to be satisfied at Closing, but subject to the satisfaction or waiver of those conditions at such time).
Key Factors Affecting Operating Results
Moolec was formed and incorporated as a spin-off from Bioceres S.A. on August 21, 2020, and therefore we have a limited operating history and no generated revenues. There is no assurance that we will be able to generate revenues in the future, and even if revenues are generated, there is no assurance that we will be able to earn a profit. However, given the current heightened consumer demand for plant-based foods worldwide, we are optimistic in the trajectory of our business given our unique approach to alternative proteins. Moolec is a pre-revenue company and believe that its performance and future success depends on several factors that present significant opportunities for growth but also pose risks and challenges, including those discussed below and in the section of this proxy statement/prospectus entitled “Risk Factors — Risks Related to Moolec’s Business and Industry.”
Basis of Preparation and Significant Accounting Policies
The following is a description of our accounting policies that we believe are most critical to the portrayal of our financial conditions and results of operations and that require significant, difficult, subjective or complex judgments. Our basis of preparation and significant accounting policies are described in our consolidated financial statements (Notes 2 and 3 respectively) included elsewhere in this proxy statement/prospectus, which have been applied consistently to all the periods presented in the consolidated financial statements.
Accounting Standards and Basis of Preparation
The consolidated financial statements that are included present the consolidated financial position of Moolec, which includes its subsidiary, AG Biomolecules LLC (“AG Biomolecules” and together with Moolec, the “Group”). AG Biomolecules is a Delaware limited liability company organized for the purpose of contributing all of Moolec’s business net assets upon commencement of business operations. Subsidiaries are entities that are controlled by
162
Table of Contents
Moolec. The financial statements of AG Biomolecules are included in the consolidated financial statements from the date that control commences. The accounting policies of AG Biomolecules have been changed when necessary to align them with the policies adopted by the company. For more information about AG Biomolecules please refer to Note 5 in the accompanying consolidated financial statements of Moolec included elsewhere in this proxy statement/prospectus. Moolec’s financial statements are prepared in accordance with IFRS as issued by the IASB. For more information about Moolec’s basis of presentation, refer to Note 3 in the accompanying financial statements of Moolec included elsewhere in this proxy statement/prospectus.
Foreign Currency
Transactions entered into by the Group in a currency other than their functional currency, which is the U.S. dollar, are recorded at the relevant exchange rates at the date upon which such transactions occur. Foreign currency monetary assets and liabilities are translated at the prevailing exchange rates as of the final day of each reporting period. Non-monetary assets and liabilities that are measured at fair value in a foreign currency are translated into the functional currency at the exchange rate when the fair value was determined. Non-monetary items that are measured based on historical cost in a foreign currency are translated at the exchange rate at the date of the transaction. Exchange differences arising on the retranslation of unsettled monetary asset and liabilities are recognized immediately in the consolidated statements of operations.
Financial Assets
Non-derivative financial assets, as presented in the consolidated statements of financial position as “Receivable from related parties” and classified as a current asset, intended as the assets that should be realized during the normal operating cycle or over the 12-month period subsequent to the reporting date.
The Group initially recognizes these financial assets at fair value plus any directly attributable transaction costs on the date it originated at which the Group becomes a party to the contractual terms and conditions. Subsequent to initial recognition, these financial assets are measured at amortized cost using the effective interest method less any impairment losses.
Financial Liabilities
Non-derivative financial liabilities, as presented in the consolidated statements of financial position as “Accounts Payable” and classified as a current liability, intended as the liabilities that should be paid during the normal operating cycle or over the 12-month period subsequent to the reporting date.
The Group initially recognizes these financial liabilities at fair value plus any directly attributable transaction costs on the date it originated at which the Group becomes a party to the contractual terms and conditions. Subsequent to initial recognition, these financial liabilities are measured at amortized cost using the effective interest method.
Intangible Assets
Expenditure on internally developed products is capitalized if it can be demonstrated that:
• It is technically feasible to develop the product for it to be sold;
• Adequate resources are available to complete the development;
• There is an intention to complete and sell the product;
• The Group is able to sell the product;
• Sale of the product will generate future economic benefits; and
• Expenditure on the project can be measured reliably.
Development expenditure not satisfying the above criteria and expenditure on the research phase of internal projects are recognized in the consolidated statements of operations.
Capitalized development costs are amortized using the straight-line method over the periods the Group expects to benefit from selling the products developed.
Useful lives and amortization methods are reviewed every year as required by IAS 38.
163
Table of Contents
The research and development process can be divided into several discrete steps or phases, which generally begin with discovery, validation and development and end with regulatory approval and commercial launch. The process for developing seed traits is relatively similar for both genetically modified (“GM”) and non-GM traits. However, the two differ significantly in later phases of development. For example, obtaining regulatory approval for GM seeds is a far more comprehensive and lengthy process than for non-GM seeds. Although breeding programs and industrial biotechnology solutions may have shorter or simpler phases than those described below, the Group has used the industry consensus for seed trait development phases to characterize its technology portfolios, which is generally divided into the following six phases:
i) Discovery: The first phase in the technology development process is the discovery or identification of candidate genes or genetic systems, metabolites, or microorganisms potentially capable of enhancing specified plant characteristics or enabling an agroindustrial biotech solution.
ii) Proof of concept: Upon successful validation of the technologies in model systems (in vitro or in vivo), promising technologies graduate from discovery and are advanced to the proof-of-concept phase. The goal of this phase is to validate a technology within the targeted organism before moving forward with technology escalation activities or extensive field validation.
iii) Early development: In this phase, efficacy field trials are expanded to evaluate the expression level and phenological characteristics of the traits in multiple geographies and growing cycles, as well as other characteristics in order to optimize the technology’s performance in the targeted organisms. The goal of the early development phase is to evaluate the technical feasibility by identifying the best candidate to scale up the seed stock and to start the regulatory field trials.
iv) Advanced development and deregulation: In this phase, extensive field tests are used to fully demonstrate the effectiveness of the technology for its intended purpose. In the case of GM traits, the process of obtaining regulatory approvals from government agencies is started, and includes field trials for environmental, core and food safety data generation. For solutions involving microbial fermentation, industrial-scale runs are conducted.
v) Pre-launch: This phase involves finalizing the regulatory approval process and preparing for the launch and commercialization of the technology. The range of activities in this phase includes seed increases, pre-commercial production, and product and solution testing with selected customers. Usually, a more detailed marketing strategy and preparation of marketing materials occur during this phase.
vi) Product launch: In general, this phase, which is the last milestone of the research and development process, is carried out by the Group.
We determined that the R&D is more likely than not (“probable”) to become a commercialized product and meet the criteria for capitalization under IAS 38 “Intangible Assets” at the end of the phase (iii) “Early development.” During this phase efficacy field trials are performed to determine the technical feasibility of the project by measuring parameters such as expression level and phenological characteristics. Obtaining desired values under these parameters provide the strongest and clearest indication that the technical feasibility is proven.
During phase (iv) “Advanced development and deregulation”, more field trials are performed to gather regulatory information for the “de-regulation” dossier of GM-crops and food safety approval of the end-product. Obtaining regulatory approval to cultivate GM crops (de-regulation) is a lengthy process, but it is not required for the commercialization of the end-product because commercialization is still possible during the period the crop is under regulated status.
In our timeline, during the developmental process, the main purpose for deregulating the GM-crop production is to reduce cost and optimize production and logistics. The commercialization approval of the end-product (ingredient to be used in the food industry), is a separate process from the GM-crops de-regulation and it is subject to standard reviews in order to determine safety for human consumption. We determines if the food safety approval is probable during phase (i) “Discovery” and phase (ii) “Proof of concept”, when Moolec identifies proteins and hosts with a long history of safe use as food for human consumption.
164
Table of Contents
Taxes
Current Income Tax
Current income tax assets and liabilities are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted at the reporting date in the country where the Group operates and generates taxable income.
Current income tax relating to items recognized directly in equity is recognized in equity and not in the consolidated statements of operations. Management periodically evaluates positions taken in the tax returns with respect to situations in which applicable tax regulations are subject to interpretation and establishes provisions where appropriate.
Deferred Tax
Deferred tax is provided using the liability method on temporary differences between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes at the reporting date.
Deferred tax liabilities are recognized for all taxable temporary differences, except:
• When the deferred tax liability arises from the initial recognition of goodwill or an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss, and
• In respect of taxable temporary differences affiliated with investments in subsidiaries, associates, and interests in joint arrangements, when the timing of the reversal of the temporary differences can be controlled and it is probable that the temporary differences will not reverse in the foreseeable future.
Deferred tax assets are recognized for all deductible temporary differences, the carry forward of unused tax credits and any unused tax losses. Deferred tax assets are recognized to the extent that it is probable that taxable profit will be available against which the deductible temporary differences, and the carry forward of unused tax credits and unused tax losses can be utilized, except:
• When the deferred tax asset relating to the deductible temporary difference arises from the initial recognition of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss, and
• In respect of deductible temporary differences affiliated with investments in subsidiaries, associates and interests in joint arrangements, deferred tax assets are recognized only to the extent that it is probable that the temporary differences will reverse in the foreseeable future and taxable profit will be available against which the temporary differences can be utilized.
The carrying amount of deferred tax assets is reviewed at each reporting date and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred tax asset to be utilized. Unrecognized deferred tax assets are re-assessed at each reporting date and are recognized to the extent that it has become probable that future taxable profits will allow the deferred tax asset to be recovered.
Deferred tax assets and liabilities are measured at the tax rates that are expected to apply in the year when the asset is realized or the liability is settled, based on tax rates that have been enacted or substantively enacted at the reporting date.
Deferred tax relating to items recognized outside profit or loss is recognized outside profit or loss. Deferred tax items are recognized in correlation to the underlying transaction either in OCI or directly in equity.
Expenses
Research and Development Costs
Research costs are expensed in the period in which these costs are incurred. Development costs are expensed in the period in which these costs are incurred if they do not meet the criteria for capitalization.
165
Table of Contents
Sales and marketing, administrative and other operating expenses
The Group recognizes expenses in the period in which these costs are incurred and are presented by function on the consolidated statements of profit or loss. Sales and marketing expenses primarily relate to marketing materials and research of the Group to increase brand awareness in the marketplace. Administrative expenses primarily comprise professional fees as it relates to consultancy, accountancy and legal expenses. Other operating expenses relate to those that do not depend on general business operations or relate to the other expense categories.
Results of Operations
Comparison of the Year Ended June 30, 2022 and the irregular period from inception on August 20, 2020 through June 30, 2021
The unaudited results for the period from inception on August 20,2020 to June 30, 2021 have been calculated using the audited financial information for the irregular period from inception on August 21,2020 to December 31, 2020 and the audited financial information for the six months period from January 1, 2021 through June 30, 2021. These unaudited results should be read in conjunction with our audited consolidated financial statements for the six-months period from January 1, 2021 through June 30, 2021, included elsewhere in this proxy statement/prospectus.
The following table sets forth Moolec’s historical operating results for the periods indicated:
Consolidated Statements of
Comprehensive Loss: | | | | | | | | |
(dollars) | | As of and for the year ended June 30, 2022 | | From inception on August 20, 2020 through June 30, 2021(1) | | U.S.$
Changes | | % Changes |
Continuing operations | | | | | | | | | | | | |
Research and development expense | | (985,158 | ) | | (781,003 | ) | | (204,155 | ) | | 26.1 | % |
Sales and marketing expense | | (105,060 | ) | | (91,159 | ) | | (13,901 | ) | | 15.2 | % |
Administrative expense | | (2,523,230 | ) | | (948,832 | ) | | (1,574,398 | ) | | 165.9 | % |
Other operating expense | | (38,985 | ) | | (95,891 | ) | | 56,906 | | | (59.3 | )% |
Loss from operations | | (3,652,433 | ) | | (1,916,885 | ) | | (1,735,548) | | | 90.5 | % |
| | | | | | | | | | | | | |
Share of loss from associate | | — | | | (390,453 | ) | | 390,453 | | | (100.0 | )% |
Finance costs | | (874,472 | ) | | — | | | (874,472 | ) | | 100 | % |
Loss before income tax | | (4,526,905 | ) | | (2,307,338 | ) | | (2,219,567) | | | 96.2 | % |
| | | | | | | | | | | | | |
Income tax | | — | | | — | | | — | | | — | |
Loss for the period | | (4,526,905 | ) | | (2,307,338 | ) | | (2,219,567) | | | 96.2 | % |
| | | | | | | | | | | | | |
Total comprehensive loss for the period | | (4,526,905 | ) | | (2,307,338 | ) | | (2,219,567) | | | 96.2 | % |
Basic and diluted loss per share | | (0.09 | ) | | (0.05 | ) | | (0.04 | ) | | 80.0 | % |
Research and Development Expense
R&D expenses increased by U.S.$204,155 or 26.1% from U.S.$781,003 from inception on August 21, 2020 through June 30, 2021 to U.S.$985,158 during the year ended June 30, 2022, primarily due to advances in our R&D pipeline.
Administrative Expense
Administrative expense increased by U.S.$1,574,398 or 165.9% from U.S.$948,832 from inception on August 21, 2020 through June 30, 2021 to U.S.$2,523,230 during the year ended June 30, 2022, primarily due to an increase in payroll and additional expenses including share based payment and legal fees.
166
Table of Contents
Other Operating Expense
Other operating expense decreased by U.S.$56,906 or 59.3% from U.S.$95,891 from inception on August 21, 2020 through June 30, 2021 to U.S.$38,985 during the year ended June 30, 2022, primarily due to a reduction in non-recurrent related party expenses.
Consolidated statements of
financial position
| | As of June 30, |
(dollars, except for share and per share data) | | 2022 | | 2021 |
Assets | | | | | | |
Non-current assets | | | | | | |
Intangible assets | | 4,598,930 | | | 4,598,930 | |
Fixed assets | | 8,918 | | | 10,617 | |
Investment in associate | | — | | | — | |
Total non-current assets | | 4,607,848 | | | 4,609,547 | |
| | | | | | | |
Current assets | | | | | | |
Cash and cash equivalents | | 1,081,808 | | | 980,527 | |
Receivables from related parties | | — | | | 289,888 | |
Prepayments | | 2,061 | | | 358 | |
Total current assets | | 1,083,869 | | | 1,270,773 | |
Total assets | | 5,691,717 | | | 5,880,320 | |
| | | | | | | |
Liabilities and equity | | | | | | |
Equity | | | | | | |
Share capital | | 638,297 | | | 638,297 | |
Share premium | | 6,961,703 | | | 6,961,703 | |
Equity settled share based payment | | 838,576 | | | — | |
Accumulated deficit | | (6,834,243 | ) | | (2,307,338 | ) |
Total equity | | 1,604,333 | | | 5,292,662 | |
| | | | | | | |
Liabilities | | | | | | |
Current liabilities | | | | | | |
Accounts payable | | 1,226,213 | | | 587,658 | |
Other current liabilities | | 1,171 | | | — | |
Simple Agreement for Future Equity (“SAFE”) | | 2,860,000 | | | — | |
Total current liabilities | | 4,087,384 | | | 587,658 | |
Total liabilities | | 4,087,384 | | | 587,658 | |
Total liabilities and equity | | 5,691,717 | | | 5,880,320 | |
Intangible Assets
Our intangible assets remained at U.S.$4,598,930 from inception on August 21, 2020 through June 30, 2021 and during the year ended June 30, 2022.
Fixed Assets
Our fixed assets decreased by U.S.$1,699 or 16.0% from U.S.$10,617 thousand from inception on August 21, 2020 through June 30, 2021 to U.S.$8,918 during the year ended June 30, 2022, primarily due to depreciation and amortization.
167
Table of Contents
Consolidated Statements of Cash Flows
Presented below is a summary of Moolec’s operating and financing cash flow:
(in United States dollars) | | For the
year ended
June 30,
2022 | | From the period
of August 21,
2020 through
June 30,
2021(1) |
Net cash (used) generated in operating activities | | (1,885,979 | ) | | (1,619,473 | ) |
Net cash generated from financing activities | | 2,000,000 | | | 2,600,000 | |
Net increase in cash and cash equivalents | | 114,021 | | | 980,527 | |
Cash and cash equivalents at the beginning of the year/period | | 980,527 | | | — | |
Effect of exchange rate changes on cash and equivalents | | (12,740 | ) | | — | |
Cash and cash equivalents at end of the year/period | | 1,081,808 | | | 980,527 | |
Net cash used in operating activities
Net cash used in operating activities increased by U.S.$266,506 or 16.5% from U.S.$1,619,473 from the period of August 21, 2020 through June 30, 2021 to U.S.$1,885,979 during the year ended June 30, 2022, primarily due to increases in R&D expenditures, payroll and administrative expenses.
Net cash generated from financing activities
Cash flows from financing activities decreased by U.S.$600,000 or 23% from U.S.$2,600,000 from the period of August 21, 2020 through June 30, 2021 to U.S.$2,000,000 during the year ended June 30, 2022, primarily due to a decrease in cash subscription.
Changes in Equity
(in United States dollars) | | Payments Due by Period |
Share
capital | | Equity settled
share based
payment | | Retained
(deficit) | | Share
Premium | | Total
Equity |
Issue of share capital | | 638,297 | | — | | — | | | 6,961,703 | | 7,600,000 | |
Total comprehensive (loss) | | — | | — | | (2,307,338 | ) | | — | | (2,307,338 | ) |
Balance as of June 30, 2021 | | 638,297 | | — | | (2,307,338 | ) | | 6,961,703 | | 5,292,662 | |
Total comprehensive (loss) | | — | | — | | (4,526,905 | ) | | — | | (4,526,905 | ) |
Equity settled share based payment | | — | | 838,576 | | — | | | — | | 838,576 | |
Balance as of June 30, 2022 | | 638,297 | | 838,576 | | (6,834,243 | ) | | 6,961,703 | | 1,604,333 | |
168
Table of Contents
Certain Moolec Relationships and Related Person Transactions
The table below sets for the entities Moolec has engaged in related party transactions with and their relationships to Moolec.
Related Party | | Relationship to Moolec |
Bioceres S.A. | | A company organized under the laws of Argentina and parent company to Bioceres Crop Solutions Corp and BG Farming Technologies Limited. |
Union Group Ventures Limited | | A company limited by shares and governed by the laws of the British Virgin Islands and shareholder of Moolec. |
Theo I SCSp | | A special limited partnership governed by the laws of the Grand Duchy of Luxembourg and subsidiary of Bioceres S.A. |
Ingenieria Metabólica S.A. | | A company organized under the laws of Argentina and 30% subsidiary of Bioceres S.A. |
Instituto de Agrobiotecnología Rosario S.A.U. | | A Company organized under the laws of Argentina and 98.6% subsidiary of Bioceres S.A. |
Future Foods B.V. | | A Company organized under the laws of the Netherlands which was founded and operated by Moolec’s CPO, Henk Hoogenkamp. |
Commercial Operations
Moolec has conducted commercial operations in the ordinary course of business in arm’s length transactions under market terms with Bioceres Crop Solutions Corp. On June 17, 2021 Moolec entered into a professional services and operations agreement with Bioceres S.A, pursuant to which certain expenses and office space would be made available to Moolec from Bioceres S.A.
Related Party Loans
On July 26, 2022, pursuant to two interest bearing promissory notes, Union Group Ventures Limited agreed to loan to Moolec up to $175,000 and up to $500,000. The loans pursuant to the promissory notes will mature upon consummation of the Business Combination.
On July 26, 2022, pursuant to an interest-bearing promissory note, Theo I SCSp agreed to loan to Moolec up to $175,000. The loan pursuant to the promissory note will mature upon consummation of the Business Combination.
169
Table of Contents
Business of LightJump and Certain Information about LightJump
References in this section to “we”, “our”, “us”, the “Company”, or “LightJump” generally refer to LightJump.
Overview
LightJump is a blank check company formed in Delaware on July 28, 2020, for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses, without limitation as to business, industry or sector.
LightJump’s registration statement on Form S-1 (File No. 333-251435), or LightJump’s registration statement, for LightJump’s IPO was declared effective by the SEC on January 8, 2021. On January 12, 2021, LightJump consummated its IPO of 12,000,000 SPAC Units. Each SPAC Unit consists of one share of SPAC Common Stock, $0.0001 par value per share, and one-half of one SPAC Warrant, with each whole SPAC Warrant entitling the holder to purchase one share of SPAC Common Stock at $11.50 per share. The SPAC Units were sold at an offering price of $10.00 per SPAC Unit, generating gross proceeds of $120,000,000. Simultaneously with the consummation of the IPO and the sale of the SPAC Units, LightJump consummated the private placement of an aggregate of 3,850,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant, generating total proceeds of $3,850,000.
On January 15, 2021, LightJump consummated the sale of an additional 360,000 Private Placement Warrants pursuant to the underwriter’s exercise of its over-allotment option to purchase 1,800,000 additional SPAC Units for gross proceeds of $18,000,000. The Private Placement Warrants were sold at $1.00 per Private Placement Warrant, generating additional gross proceeds of $360,000. Following the closing of the over-allotment option, an aggregate amount of $138,000,000 was placed in LightJump’s Trust Account established in connection with the IPO.
LightJump’s SPAC Units began trading on January 7, 2021 on Nasdaq under the symbol LJAQU. Commencing on March 1, 2021, the securities comprising the units began separate trading. The units, SPAC Common Stock, and SPAC Warrants are trading on Nasdaq under the symbols “LJAQ,” and “LJAQW,” respectively.
On July 11, 2022, LightJump filed an amendment to its Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware. The extension amendment extends the date by which LightJump must consummate its initial business combination from July 12, 2022 to January 12, 2023. LightJump Holders holding 11,032,790 Public Shares exercised their right to redeem such shares for a pro rata portion of the funds in the Trust Account. As a result, $110,507,220.68 (approximately $10.02 per share) was removed from the Trust Account to pay such holders. Following the redemptions, LightJump has 2,767,210 Public Shares outstanding and the aggregate amount remaining in the Trust Account as of July 12, 2022 was $27,993,797.65 (which includes an additional $276,721 contributed by the Sponsor in connection with the Extension).
Permitted Purchases of LightJump’s Securities
Our Sponsor, directors, officers, advisors or their affiliates may purchase shares or public warrants in privately negotiated transactions or in the open market either prior to or following the completion of our initial business combination. There is no limit on the number of shares LightJump Initial Stockholders, directors, officers, advisors or their affiliates may purchase in such transactions, subject to compliance with applicable law and Nasdaq rules. However, they have no current commitments, plans or intentions to engage in such transactions and have not formulated any terms or conditions for any such transactions. If they engage in such transactions, they will not make any such purchases when they are in possession of any material nonpublic information not disclosed to the seller or if such purchases are prohibited by Regulation M under the Exchange Act. We do not currently anticipate that such purchases, if any, would constitute a tender offer subject to the tender offer rules under the Exchange Act or a going-private transaction subject to the going-private rules under the Exchange Act; however, if the purchasers determine at the time of any such purchases that the purchases are subject to such rules, the purchasers will comply with such rules. Any such purchases will be reported pursuant to Section 13 and Section 16 of the Exchange Act to the extent such purchasers are subject to such reporting requirements. None of the funds held in the Trust Account will be used to purchase shares or public warrants in such transactions prior to completion of our initial business combination.
170
Table of Contents
Our Sponsor, officers, directors and/or their affiliates anticipate that they may identify the stockholders with whom our Sponsor, officers, directors or their affiliates may pursue privately negotiated purchases by either the stockholders contacting us directly or by our receipt of redemption requests submitted by stockholders following our mailing of proxy materials in connection with our initial business combination. To the extent that our Sponsor, officers, directors, advisors or their affiliates enter into a private purchase, they would identify and contact only potential selling stockholders who have expressed their election to redeem their shares for a pro rata share of the Trust Account or vote against our initial business combination, whether or not such stockholder has already submitted a proxy with respect to our initial business combination. Our Sponsor, officers, directors, advisors or their affiliates will only purchase shares if such purchases comply with Regulation M under the Exchange Act and the other federal securities laws.
Any purchases by our Sponsor, officers, directors and/or their affiliates who are affiliated purchasers under Rule 10b-18 under the Exchange Act will only be made to the extent such purchases are able to be made in compliance with Rule 10b-18, which is a safe harbor from liability for manipulation under Section 9(a)(2) and Rule 10b-5 of the Exchange Act. Rule 10b-18 has certain technical requirements that must be complied with in order for the safe harbor to be available to the purchaser. Our Sponsor, officers, directors and/or their affiliates will not make purchases of common stock if the purchases would violate Section 9(a)(2) or Rule 10b-5 of the Exchange Act. Any such purchases will be reported pursuant to Section 13 and Section 16 of the Exchange Act to the extent such purchases are subject to such reporting requirements.
Redemption Rights for Holders of Public Shares
Public Stockholders have the opportunity to redeem all or a portion of their shares of common stock upon the completion of our initial business combination at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account as of two Business Days prior to the special meeting including interest earned on the funds held in the Trust Account and not previously released to us to pay all of our taxes, divided by the number of then outstanding public shares, subject to the limitations described herein. The amount in the Trust Account was initially $10.00 per public share at the time of our IPO, and as of the Record Date, is $10.12 per Public Share. The per-share amount we will distribute to investors who properly redeem their shares will not be reduced by the deferred underwriting commissions we will pay to the underwriters. Our Sponsor, officers and directors have entered into a letter agreement with us, pursuant to which they have agreed to waive their redemption rights with respect to any Founder Shares and any public shares held by them in connection with the completion of our initial business combination.
We will complete our initial business combination only if a majority of the outstanding shares of common stock voted are voted in favor of the initial business combination. A quorum for such meeting will consist of the holders present in person or by proxy of shares of outstanding capital stock of the company representing a majority of the voting power of all outstanding shares of capital stock of the company entitled to vote at such meeting. LightJump Initial Stockholders will count toward this quorum and pursuant to the letter agreement, our Sponsor, officers and directors have agreed to vote their Founder Shares and any public shares purchased in favor of our initial business combination. For purposes of seeking approval of the majority of our outstanding shares of common stock voted, non-votes will have no effect on the approval of our initial business combination once a quorum is obtained. As a result, in addition to LightJump Initial Stockholders’ Founder Shares, we would need no additional votes in favor of such proposals in order to have its initial business combination approved. We intend to give approximately 30 days (but not less than 10 days nor more than 60 days) prior written notice of any such meeting, if required, at which a vote shall be taken to approve our initial business combination. These quorum and voting thresholds, and the voting agreements of our Sponsor and officers and directors, may make it more likely that we will consummate our initial business combination. Each Public Stockholder may elect to redeem its public shares irrespective of whether they vote for or against the proposed transaction.
Our amended and restated certificate of incorporation provides that we will only redeem our public shares so long as (after such redemption) our net tangible assets will be at least $5,000,001 either immediately prior to or upon consummation of our initial business combination and after payment of underwriters’ fees and commissions (so that we are not subject to the SEC’s “penny stock” rules) or any greater net tangible asset or cash requirement which may be contained in the agreement relating to our initial business combination. Concurrently with the execution of the Business Combination Agreement, Union Group Ventures Limited, THEO I SCSp, UG Holdings, LLC and Sponsor
171
Table of Contents
entered into the Backstop Agreement, pursuant to which, among other things, the parties guaranteed, on a several (and not joint) basis, to backstop an aggregate amount equal to $10,000,000, conditioned upon Closing on the terms and subject to the conditions set forth in the Backstop Agreement.
On July 8, 2022, Moolec issued a promissory note (the “Note”) to the Sponsor. Pursuant to the Note, Moolec agreed to loan to the Sponsor up to an aggregate principal amount of $350,000 (the “Extension Funds”) to deposit into the SPAC’s Trust Account in connection with the extension of the SPAC’s termination date from July 12, 2022 to January 12, 2023 (or such earlier date as determined by the Board).
The SPAC may draw down from the Extension Funds, at its discretion, to distribute to the Public Stockholders who elect to have their shares redeemed in connection with the consummation of the initial business combination.
The Note bears interest at 20.0% per annum and is repayable in full upon the earlier of (i) the consummation of the initial business combination of the SPAC, (ii) the date of the termination of the Business Combination Agreement and (ii) January 12, 2023.
On July 8, 2022, LightJump held a special meeting of stockholders. At the meeting, LightJump’s stockholders approved the Extension Amendment extending the date by which LightJump must consummate its initial business combination from July 12, 2022 to January 12, 2023. Public Stockholders holding 11,032,790 Public Shares exercised their right to redeem such shares for a pro rata portion of the funds in the Trust Account. As a result, $110,507,220.68 (approximately $10.02 per share) was removed from the Trust Account to pay such holders. Following redemptions, LightJump had 2,767,210 Public Shares outstanding and the aggregate amount remaining in the Trust Account as of July 12, 2022 was $27,993,797.65 (which includes an additional $276,721 contributed by the Sponsor in connection with the Extension).
In the event the aggregate cash consideration we would be required to pay for all shares of common stock that are validly submitted for redemption exceed the aggregate amount of cash available to us, we will not complete the initial business combination or redeem any shares, and all shares of common stock submitted for redemption will be returned to the holders thereof.
Tendering Stock Certificates in Connection with Redemption Rights
We require our Public Stockholders seeking to exercise their redemption rights, whether they are record holders or hold their shares in “street name,” to tender their certificates to our transfer agent prior to the meeting held to approve a proposed initial business combination by a date set forth in the proxy materials mailed to such holders or to deliver their shares to the transfer agent electronically using The Depository Trust Company’s DWAC (Deposit/Withdrawal At Custodian) System, at the holder’s option. The proxy materials that we will furnish to holders of our public shares in connection with our initial business combination will indicate whether we are requiring Public Stockholders to satisfy such delivery requirements. Accordingly, a Public Stockholder would have from the time we send out our proxy materials until the date set forth in such proxy materials to tender its shares if it wishes to seek to exercise its redemption rights. Given the relatively short exercise period, it is advisable for stockholders to use electronic delivery of their public shares.
There is a nominal cost associated with the above-referenced tendering process and the act of certificating the shares or delivering them through the DWAC System. The transfer agent will typically charge the tendering broker $80.00 and it would be up to the broker whether or not to pass this cost on to the redeeming holder. However, this fee would be incurred regardless of whether or not we require holders seeking to exercise redemption rights to tender their shares. The need to deliver shares is a requirement of exercising redemption rights regardless of the timing of when such delivery must be effectuated.
The foregoing is different from the procedures used by many blank check companies. In order to perfect redemption rights in connection with their business combinations, many blank check companies would distribute proxy materials for the stockholders’ vote on an initial business combination, and a holder could simply vote against a proposed initial business combination and check a box on the proxy card indicating such holder was seeking to exercise his or her redemption rights. After the initial business combination was approved, the company would contact such stockholder to arrange for him or her to deliver his or her certificate to verify ownership. As a result, the stockholder then had an “option window” after the completion of the initial business combination during which he or she could monitor the price of the company’s stock in the market. If the price rose above the redemption price,
172
Table of Contents
he or she could sell his or her shares in the open market before actually delivering his or her shares to the company for cancellation. As a result, the redemption rights, to which stockholders were aware they needed to commit before the stockholder meeting, would become “option” rights surviving past the completion of the initial business combination until the redeeming holder delivered its certificate. The requirement for physical or electronic delivery prior to the meeting ensures that a redeeming holder’s election to redeem is irrevocable once the initial business combination is approved.
Any request to redeem such shares, once made, may be withdrawn at any time up to the date set forth in the proxy materials. Furthermore, if a holder of a public share delivered its certificate in connection with an election of redemption rights and subsequently decides prior to the applicable date not to elect to exercise such rights, such holder may simply request that the transfer agent return the certificate (physically or electronically). It is anticipated that the funds to be distributed to holders of our public shares electing to redeem their shares will be distributed promptly after the completion of our initial business combination.
If our initial business combination is not approved or completed for any reason, then our Public Stockholders who elected to exercise their redemption rights would not be entitled to redeem their shares for the applicable pro rata share of the Trust Account. In such case, we will promptly return any certificates delivered by Public Stockholders who elected to redeem their shares.
If our initial proposed initial business combination is not completed, we may continue to try to complete an initial business combination with a different target until January 12, 2023.
Redemption of Public Shares and Liquidation if no Initial Business Combination
If we are unable to complete our initial business combination by January 12, 2023, we will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten Business Days thereafter, redeem 100% of the outstanding public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including any interest not previously released to us but net of franchise and income taxes payable, divided by the number of then outstanding public shares, which redemption will completely extinguish Public Stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of our remaining stockholders and our board of directors, dissolve and liquidate, subject (in the case of (ii) and (iii) above) to our obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. There will be no redemption rights or liquidating distributions with respect to our warrants, which will expire worthless if we fail to complete our initial business combination by January 12, 2023.
Our Sponsor, officers and directors have entered into a letter agreement with us, pursuant to which they have waived their rights to liquidating distributions from the Trust Account with respect to any Founder Shares held by them if we fail to complete our initial business combination by January 12, 2023. However, if our Sponsor, officers or directors acquire public shares, they will be entitled to liquidating distributions from the Trust Account with respect to such public shares if we fail to complete our initial business combination within the allotted time period.
Our Sponsor, officers and directors have agreed, pursuant to a written agreement with us, that they will not propose any amendment to our amended and restated certificate of incorporation (i) to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete our initial business combination by January 12, 2023 or (ii) with respect to any other provision relating to stockholders’ rights or pre-initial business combination activity, unless we provide our Public Stockholders with the opportunity to redeem their shares of common stock upon approval of any such amendment at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account including interest earned on the funds held in the Trust Account and not previously released to us to pay our taxes divided by the number of then outstanding public shares. However, we may not redeem our public shares unless our net tangible assets are at least $5,000,001 either immediately prior to or upon consummation of our initial business combination and after payment of underwriters’ fees and commissions (so that we are not subject to the SEC’s “penny stock” rules). If this optional redemption right is exercised with respect to an excessive number of public shares such that we cannot satisfy the net tangible asset requirement (described above), we would not proceed with the amendment or the related redemption of our public shares at such time.
173
Table of Contents
We expect that all costs and expenses associated with implementing our plan of dissolution, as well as payments to any creditors, will be funded from amounts remaining out of the $5,422 of cash held outside the Trust Account as of June 30, 2022, although we cannot assure you that there will be sufficient funds for such purpose. We will depend on sufficient interest being earned on the proceeds held in the Trust Account to pay any tax obligations we may owe.
If we were to expend all of the net proceeds of our IPO and the sale of the Private Placement Warrants, other than the proceeds deposited in the Trust Account, and without taking into account interest, if any, earned on the Trust Account, the per-share redemption amount received by stockholders upon our dissolution would be approximately $10.12. The proceeds deposited in the Trust Account could, however, become subject to the claims of our creditors which would have higher priority than the claims of our Public Stockholders. We cannot assure you that the actual per-share redemption amount received by stockholders will not be substantially less than $10.00. Under Section 281(b) of the DGCL, our plan of dissolution must provide for all claims against us to be paid in full or make provision for payments to be made in full, as applicable, if there are sufficient assets. These claims must be paid or provided for before we make any distribution of our remaining assets to our stockholders. While we intend to pay such amounts, if any, we cannot assure you that we will have funds sufficient to pay or provide for all creditors’ claims.
Although we seek to have all vendors, service providers, prospective target businesses or other entities with which we do business execute agreements with us waiving any right, title, interest or claim of any kind in or to any monies held in the Trust Account for the benefit of our Public Stockholders, there is no guarantee that they will execute such agreements or even if they execute such agreements that they would be prevented from bringing claims against the Trust Account including but not limited to fraudulent inducement, breach of fiduciary responsibility or other similar claims, as well as claims challenging the enforceability of the waiver, in each case in order to gain an advantage with respect to a claim against our assets, including the funds held in the Trust Account. If any third party refuses to execute an agreement waiving such claims to the monies held in the Trust Account, our management will perform an analysis of the alternatives available to it and will only enter into an agreement with a third party that has not executed a waiver if management believes that such third party’s engagement would be significantly more beneficial to us than any alternative. Examples of possible instances where we may engage a third party that refuses to execute a waiver include the engagement of a third-party consultant whose particular expertise or skills are believed by management to be significantly superior to those of other consultants that would agree to execute a waiver or in cases where management is unable to find a service provider willing to execute a waiver. Marcum LLP, our independent registered public accounting firm, and the underwriters of our IPO will not execute agreements with us waiving such claims to the monies held in the Trust Account.
In addition, there is no guarantee that such entities will agree to waive any claims they may have in the future as a result of, or arising out of, any negotiations, contracts or agreements with us and will not seek recourse against the Trust Account for any reason. Our Sponsor has agreed that it will be liable to us if and to the extent any claims by a third party for services rendered or products sold to us, or a prospective target business with which we have entered into a written letter of intent, confidentiality or similar agreement or business combination agreement, reduce the amount of funds in the Trust Account trust account to below the lesser of (i) $10.00 per public share and (ii) the actual amount per public share held in the Trust Account as of the date of the liquidation of the Trust Account, if less than $10.00 per share due to reductions in the value of the trust assets, less taxes payable, provided that such liability will not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies held in the Trust Account (whether or not such waiver is enforceable) nor will it apply to any claims under our indemnity of the underwriters of our IPO against certain liabilities, including liabilities under the Securities Act. However, we have not asked our Sponsor to reserve for such indemnification obligations, nor have we independently verified whether our Sponsor has sufficient funds to satisfy its indemnity obligations and believe that our Sponsor’s only assets are securities of our company. Therefore, we cannot assure you that our Sponsor would be able to satisfy those obligations. None of our officers or directors will indemnify us for claims by third parties including, without limitation, claims by vendors and prospective target businesses.
In the event that the proceeds in the Trust Account are reduced below $10.00 per public share or (ii) such lesser amount per public share held in the Trust Account as of the date of the liquidation of the Trust Account, due to reductions in value of the trust assets, in each case net of the amount of interest which may be withdrawn to pay taxes, and our Sponsor asserts that it is unable to satisfy its indemnification obligations or that it has no indemnification obligations related to a particular claim, our independent directors would determine whether to
174
Table of Contents
take legal action against our Sponsor to enforce its indemnification obligations. While we currently expect that our independent directors would take legal action on our behalf against our Sponsor to enforce its indemnification obligations to us, it is possible that our independent directors in exercising their business judgment may choose not to do so if, for example, the cost of such legal action is deemed by the independent directors to be too high relative to the amount recoverable or if the independent directors determine that a favorable outcome is not likely. We have not asked our Sponsor to reserve for such indemnification obligations and we cannot assure you that our Sponsor would be able to satisfy those obligations. Accordingly, we cannot assure you that due to claims of creditors the actual value of the per-share redemption price will not be less than $10.00 per public share.
We seek to reduce the possibility that our Sponsor will have to indemnify the Trust Account due to claims of creditors by endeavoring to have all vendors, service providers, prospective target businesses or other entities with which we do business execute agreements with us waiving any right, title, interest or claim of any kind in or to monies held in the Trust Account. Our Sponsor will also not be liable as to any claims under our indemnity of the underwriters of our IPO against certain liabilities, including liabilities under the Securities Act. We have access to up to approximately $5,422 of cash held outside the Trust Account as of June 30, 2022 with which to pay any such potential claims. In the event that we liquidate and it is subsequently determined that the reserve for claims and liabilities is insufficient, stockholders who received funds from our Trust Account could be liable for claims made by creditors.
Under the DGCL, stockholders may be held liable for claims by third parties against a corporation to the extent of distributions received by them in a dissolution. The pro rata portion of our Trust Account distributed to our Public Stockholders upon the redemption of our public shares in the event we do not complete our initial business combination by January 12, 2023 may be considered a liquidating distribution under Delaware law. If the corporation complies with certain procedures set forth in Section 280 of the DGCL intended to ensure that it makes reasonable provision for all claims against it, including a 60-day notice period during which any third-party claims can be brought against the corporation, a 90-day period during which the corporation may reject any claims brought, and an additional 150-day waiting period before any liquidating distributions are made to stockholders, any liability of stockholders with respect to a liquidating distribution is limited to the lesser of such stockholder’s pro rata share of the claim or the amount distributed to the stockholder, and any liability of the stockholder would be barred after the third anniversary of the dissolution.
Furthermore, if the pro rata portion of our Trust Account distributed to our Public Stockholders upon the redemption of our public shares in the event we do not complete our initial business combination by January 12, 2023, is not considered a liquidating distribution under Delaware law and such redemption distribution is deemed to be unlawful (potentially due to the imposition of legal proceedings that a party may bring or due to other circumstances that are currently unknown), then pursuant to Section 174 of the DGCL, the statute of limitations for claims of creditors could then be six years after the unlawful redemption distribution, instead of three years, as in the case of a liquidating distribution. If we are unable to complete our initial business combination by January 12, 2023, we will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten Business Days thereafter, redeem 100% of the outstanding public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including any interest not previously released to us but net of franchise and income taxes payable, divided by the number of then outstanding public shares, which redemption will completely extinguish Public Stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of our remaining stockholders and our board of directors, dissolve and liquidate, subject (in the case of (ii) and (iii) above) to our obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. Accordingly, it is our intention to redeem our public shares as soon as reasonably possible following January 12, 2023 and, therefore, we do not intend to comply with those procedures. As such, our stockholders could potentially be liable for any claims to the extent of distributions received by them (but no more) and any liability of our stockholders may extend well beyond the third anniversary of such date.
Because we will not be complying with Section 280, Section 281(b) of the DGCL requires us to adopt a plan, based on facts known to us at such time that will provide for our payment of all existing and pending claims or claims that may be potentially brought against us within the subsequent 10 years. However, because we are a blank check company, rather than an operating company, and our operations will be limited to searching for prospective target businesses to acquire, the only likely claims to arise would be from our vendors (such as lawyers, investment
175
Table of Contents
bankers, etc.) or prospective target businesses. As described above, pursuant to the obligation contained in our underwriting agreement, we have sought and will continue to seek to have all vendors, service providers (other than our independent auditors), prospective target businesses or other entities with which we do business execute agreements with us waiving any right, title, interest or claim of any kind in or to any monies held in the Trust Account. As a result of this obligation, the claims that could be made against us are significantly limited and the likelihood that any claim that would result in any liability extending to the Trust Account is remote. Further, our Sponsor may be liable only to the extent necessary to ensure that the amounts in the Trust Account are not reduced below (i) $10.00 per public share or (ii) such lesser amount per public share held in the trust account as of the date of the liquidation of the Trust Account, due to reductions in value of the trust assets, in each case net of the amount of interest withdrawn to pay taxes and will not be liable as to any claims under our indemnity of the underwriters of our IPO against certain liabilities, including liabilities under the Securities Act. In the event that an executed waiver is deemed to be unenforceable against a third party, our Sponsor will not be responsible to the extent of any liability for such third-party claims.
If we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, the proceeds held in the Trust Account could be subject to applicable bankruptcy law, and may be included in our bankruptcy estate and subject to the claims of third parties with priority over the claims of our stockholders. To the extent any bankruptcy claims deplete the Trust Account, we cannot assure you we will be able to return $10.00 per share to our Public Stockholders. Additionally, if we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, any distributions received by stockholders could be viewed under applicable debtor/creditor and/or bankruptcy laws as either a “preferential transfer” or a “fraudulent conveyance.” As a result, a bankruptcy court could seek to recover some or all amounts received by our stockholders. Furthermore, our board of directors may be viewed as having breached its fiduciary duty to our creditors and/or may have acted in bad faith, thereby exposing itself and our company to claims of punitive damages, by paying Public Stockholders from the Trust Account prior to addressing the claims of creditors. We cannot assure you that claims will not be brought against us for these reasons.
Our Public Stockholders will be entitled to receive funds from the Trust Account only upon the earlier to occur of: (i) the completion of our initial business combination, (ii) the redemption of any public shares properly tendered in connection with a stockholder vote to amend any provisions of our amended and restated certificate of incorporation (A) to modify the substance or timing of our obligation to offer redemption rights in connection with any proposed initial business combination or to redeem 100% of our public shares if we do not complete our initial business combination by January 12, 2023 or (B) with respect to any other provision relating to stockholders’ rights or pre-initial business combination activity, and (iii) the redemption of all of our public shares if we are unable to complete our business combination by January 12, 2023, subject to applicable law. In no other circumstances will a stockholder have any right or interest of any kind to or in the Trust Account. In the event we seek stockholder approval in connection with our initial business combination, a stockholder’s voting in connection with the initial business combination alone will not result in a stockholder’s redeeming its shares to us for an applicable pro rata share of the Trust Account. Such stockholder must have also exercised its redemption rights as described above. These provisions of our amended and restated certificate of incorporation, like all provisions of our amended and restated certificate of incorporation, may be amended with a stockholder vote.
Legal Proceedings
From time to time, LightJump may become involved in actions, claims, suits, and other legal proceedings arising in the ordinary course of its business. LightJump is not currently involved in any such actions, claims, suits or other legal proceedings.
Employees
We currently have four officers. These individuals are not obligated to devote any specific number of hours to our matters but they intend to devote as much of their time as they deem necessary to our affairs until we have completed our initial business combination. The amount of time they will devote in any time period will vary based on whether a target business has been selected for our initial business combination and the stage of the initial business combination process we are in. We do not intend to have any full-time employees prior to the completion of our initial business combination.
176
Table of Contents
Directors and Executive Officers
LightJump’s directors and executive officers are as follows:
Name | | Age | | Position |
Robert M. Bennett | | 58 | | Chairman and Chief Executive Officer |
William W. Bunker | | 52 | | Vice-Chairman and Chief Financial Officer |
Eric Ver Ploeg | | 56 | | President |
Patrick Brandt | | 49 | | Vice President of Corporate Development |
James W. Keyes | | 66 | | Director |
Jeffrey A. Rich | | 61 | | Director |
Andrew D. Hamer | | 58 | | Director |
Robert M. Bennett, our Chairman and Chief Executive Officer since July 2020 has over 30 years of private equity experience in technology, media and manufacturing businesses. Mr. Bennett has broad experience in building proprietary deal sourcing, raising financing and closing acquisition transactions and then growing those businesses and selling them to strategic acquirers. Since 1997, Mr. Bennett has served as Chief Executive Officer of the First Lexington organization, a private equity sponsor group that has led many transactions. From 2014 to 2017, Mr. Bennett was Chief Executive Officer of ViewMarket, Inc., a company he co-founded that acquired CultureMap, a digital media company. ViewMarket was subsequently sold to Gow Media, LLC in 2017. Since 2017, Mr. Bennett has also served as Chairman and Chief Executive Officer of Jon D. Williams Cotillions, Inc., a national social education provider. From 1997 to 2019, Mr. Bennett was Chief Executive Officer of Long-Lok Fasteners Corporation, a next generation proprietary aerospace fasteners company in which he purchased two additional “bulk up” businesses, Bernic Screw Corp and A&W Screw Corp. The company was sold to Novaria Group, LLC in December 2019. Since 2003, Mr. Bennett has also served as Vice Chairman of Modulant Solutions, an IT services and software company that he co-founded and later acquired Product Data Integration Technologies, Inc. From 1999 to 2003, Mr. Bennett was Chairman of Springbow Solutions, Inc., a company he co-founded that acquired three IT service companies and provided next generation IT, portal and web services. The company was sold to Soflink, Inc. in 2005. In the 1990s, Mr. Bennett acquired and sold various media and manufacturing businesses.
William W. Bunker, our Vice-Chairman and Chief Financial Officer since July 2020, co-founded the largest dating site of the 1990s, which became Match.com and was ultimately sold for $47.5 million to Ticketmaster in 1999. He served as President of the rebranded site Match.com during the transition. After Match.com, he became a co-founder of Critical Watch, an enterprise security company that was sold to Alert Logic in 2015. Mr. Bunker co-founded two seed stage VC funds, Silicon Valley Growth Syndicate in 2013 which he managed actively until 2016 and GrowthX which Mr. Bunker has operated since 2016. He has invested in over 150 startups.
Eric Ver Ploeg, our President since July 2020, has over 20 years of Silicon Valley high-tech startup and investment experience. From 2017 to 2020, Dr. Ver Ploeg was a Managing Director of Deutsche Telekom Capital Partners venture group, a growth stage focused venture capital investor, where he was involved with the firm’s investments in cloud infrastructure company Fastly (NYSE: FSLY), enterprise SaaS company Dynamic Signal, and cyber security company Anomali. From 2015 to early 2017, Dr. Ver Ploeg was acting as an independent venture investor. From 2001 to 2008, Dr. Ver Ploeg was a Managing Director of VantagePoint Venture Partners, where he was involved in the funding of many private companies, including Spatial Wireless and OZ Communications. Dr. Ver Ploeg co-managed the quantitative hedge fund, Recursive Capital, and has made personal investments into private companies in the crypto, fintech, marketplace, and enterprise SaaS sectors. Before beginning his investment career, Dr. Ver Ploeg was the co-founder of two venture-backed startup companies, which raised multiple rounds of venture capital.
Patrick Brandt, our Vice President of Corporate Development since July 2020, has over 20 years of experience in enterprise software with a proven track record of leading companies through rapid growth, expansion, acquisition, and exits. Since 2016 Brandt has been President and a member of the Board of Directors of Shiftsmart, Inc. a labor management platform he co-founded. Additionally, he serves as Chairman of the Board of OneDay, a venture backed video technology start-up. From 2009 to 2015, Brandt served as Chairman and CEO of Telligent Systems, Inc., which became Zimbra, Inc. following the acquisition of the Zimbra assets from VMWare (NYSE: VMW) in 2013; and then in 2015 sold Zimbra to Synacor (NASDAQ: SYNC) and Telligent to Verint Systems, Inc. (NASDAQ: VRNT). Brandt was also the founder and CEO of Skywire Software in 2000.
177
Table of Contents
During his eight-year tenure at Skywire Software, Brandt led the company through organic growth and 11 acquisitions, including the leveraged buyout of Docucorp (NASDAQ: DOCC) in 2007. In July of 2008, Brandt led and negotiated the sale of Skywire Software’s enterprise software business to Oracle (NASDAQ: ORCL) and Skywire Software’s BPO business to Kubra, a private-equity backed outsourcer. Brandt remained on the Board of Directors, and served as Chairman of iWave Software, a spin-off of Skywire Software, until its sale to EMC (NYSE: EMC) in December 2012.
James W. Keyes, a member of the board of directors since December 2020, served as Chairman and Chief Executive Officer of Blockbuster, Inc. (a provider of movie and video game rental services) from 2007 to 2010, leading a successful restructuring of the business and subsequent sale to Dish Networks where he stayed on as a senior advisor to oversee Blockbuster’s integration. Prior to Blockbuster, from 2000 – 2005, Keyes served as President and CEO of 7-Eleven, Inc. The role of global CEO culminated a 21-year career in which he served multiple roles from Chief Financial Officer to Chief Operating Officer and finally CEO of the Fortune 500 company. After leading a successful transformation of 7-Eleven, he oversaw the company’s sale to Ito Yokado in 2005 with a tenfold improvement in market capitalization during his tenure. Mr. Keyes graduated Cum Laude and Phi Beta Kappa from the College of the Holy Cross with a degree in Political Science. He then earned an Masters in Business Administration from the Columbia Graduate School of Business. Mr. Keyes remains active at his alma maters, serving on the Board of Trustees for College of the Holy Cross since 2017 and on the Board of Overseers for the Columbia Business School since 2010. Mr. Keyes has been a member of Dallas Symphony Association’s Board of Governors since 2001, serving as Chairman of the Board from 2004 to 2006. Since 2005 he has served on the Board of Directors and Chairman of the Investment Committee for the Cooper Institute, a nonprofit organization dedicated to promoting health and wellness through research, education and advocacy. Since 2014 he has been on the Board of Directors of Murphy USA, which operates more than 1,470 retail gas stations in 26 states. He has also served on the American Red Cross National Board of Governors and chaired the American Red Cross National Philanthropic Board.
Jeffrey A. Rich, a member of the board of directors since December 2020, has served as an Operating Partner at Sunstone Partners, a San Mateo based growth equity firm, since 2015. Sunstone invests in high growth software and technology enabled services companies, focused in one of four sectors: Cloud and Enterprise IT, Healthcare IT, Internet and Marketing Services, and Cybersecurity. Mr. Rich also serves as the Managing Director of Plumtree Partners LLC, since 2006, which is his private family investment firm. Mr. Rich previously served as the Chief Executive Officer at Affiliated Computer Services, Inc. from 1998 to 2005, where he oversaw the company from its inception through its IPO to become a Fortune 500 company with over $5 billion of annual revenues and 50,000 employees worldwide. Mr. Rich currently serves on the board of directors of Cloudbakers Holdings, LLC, Avertium Holdings, LLC, Nexa Receptionists Holdings, LLC, OSF Global Services, Inc., and BP3 Global, Inc. He also serves on the boards of The Horatio Alger Association, Best Buddies International and the TD Jakes Foundation, each a non-profit organization. He has previously served as a Director of OutMatch Holdings, LLC, Onica Holdings, LLC, Fruition Partners, Inc., Zimbra, Inc., Digital Generation, Inc., RoyaltyShare, Inc., Pegasus Systems, Inc., Affiliated Computer Services, Inc., The Dallas Symphony Association and The University of Michigan Business School. Mr. Rich holds a BBA degree from the University of Michigan Business School and is a member of The Horatio Alger Association and Young President’s Organization (YPO).
Andrew D. Hamer, a member of the board of directors since December 2020, has served as Chief Financial Officer for Velodyne Lidar (NASDAQ: VLDR), the leading lidar provider, since April of 2019. Velodyne Lidar completed its merger with Graf Industrial Corp., a SPAC, in September 2020. He is a seasoned finance executive with over 25 years of financial leadership experience at public and pre-public technology companies. From 2010 to 2016, Mr. Hamer served as the Chief Financial Officer of ON24 Inc. where he was involved with the companies pivot to a SaaS based business model and capital raising efforts. From 2005 to 2010, Mr. Hamer was the Chief Financial Officer of Keynotes Systems (NASDAQ: KEYN) where he was involved in the company’s international expansion that included the 2006 acquisition of Sigos Systems GMBH. From 1997 to 2000, Mr. Hamer was the Director of Finance at Excite@Home where was a core member in the team completing @Home Corporation’s IPO in 1997 and subsequent acquisition and integration of Excite Inc. in 1999. Mr. Hamer graduated with a Master of Accounting at Florida International University, Miami, Florida in 1991; a Bachelor of Science in Accounting from Binghamton University, Binghamton, New York in 1986; and an Associate of Arts in Business from County College of Morris in Randolph, New Jersey in 1984.
178
Table of Contents
Number and Terms of Office of Officers and Directors
Our board of directors is currently divided into three classes with only one class of directors being elected in each year and each class serving a three-year term. The term of office of the first class of directors, currently consisting of Jeffrey A. Rich, will expire at our first annual meeting of stockholders. The term of office of the second class of directors, currently consisting of Andrew D. Hamer and James W. Keyes, will expire at the second annual meeting. The term of office of the third class of directors, currently consisting of Robert M. Bennett and William W. Bunker, will expire at the third annual meeting.
Director Independence
Currently James W. Keyes, Jeffrey A. Rich and Andrew D. Hamer would each be considered an “independent director” under the Nasdaq listing rules, which is defined generally as a person other than an officer or employee of the company or its subsidiaries or any other individual having a relationship, which, in the opinion of the company’s board of directors would interfere with the director’s exercise of independent judgment in carrying out the responsibilities of a director.
Our independent directors will have regularly scheduled meetings at which only independent directors are present.
Any affiliated transactions will be on terms no less favorable to us than could be obtained from independent parties. Our board of directors will review and approve all affiliated transactions with any interested director abstaining from such review and approval.
Officer and Director Compensation
No executive officer has received any cash compensation for services rendered to us. Commencing on the date of this proxy statement/prospectus through the acquisition of a target business or our liquidation of the Trust Account, we will pay our Sponsor $10,000 per month for providing us with office space and certain office and secretarial services. However, this arrangement is solely for our benefit and is not intended to provide our officers or directors compensation in lieu of a salary.
Other than the $10,000 per month administrative fee, the payment of consulting, success or finder fees to our Sponsor, officers, directors, or their affiliates in connection with the consummation of our initial business combination and the repayment of the $150,000 loan made by an affiliate of our chief executive officer to us, no compensation or fees of any kind will be paid to our Sponsor, members of our management team or their respective affiliates, for services rendered prior to or in connection with the consummation of our initial business combination (regardless of the type of transaction that it is). However, they will receive reimbursement for any out-of-pocket expenses incurred by them in connection with activities on our behalf, such as identifying potential target businesses, performing business due diligence on suitable target businesses and business combinations as well as traveling to and from the offices, plants or similar locations of prospective target businesses to examine their operations. There is no limit on the amount of consulting, success or finder fees payable by us upon consummation of an initial business combination. Additionally, there is no limit on the amount of out-of-pocket expenses reimbursable by us; provided, however, that to the extent such expenses exceed the available proceeds not deposited in the Trust Account, such expenses would not be reimbursed by us unless we consummate an initial business combination.
Committees of the Board of Directors
Our board of directors has three standing committees: an audit committee, a nominating committee, and a compensation committee. Subject to phase-in rules and a limited exception, the rules of Nasdaq and Rule 10A-3 of the Exchange Act require that the audit committee of a listed company be comprised solely of independent directors, and the rules of Nasdaq require that the compensation committee of a listed company be comprised solely of independent directors.
179
Table of Contents
Audit Committee
We have established an audit committee of the board of directors, consisting of James W. Keyes, Jeffrey A. Rich and Andrew D. Hamer, each of whom is an independent director under Nasdaq’s listing standards. The audit committee’s duties, which are specified in our Audit Committee Charter, include, but are not limited to:
• reviewing and discussing with management and the independent auditor the annual audited financial statements, and recommending to the board whether the audited financial statements should be included in our Form 10-K;
• discussing with management and the independent auditor significant financial reporting issues and judgments made in connection with the preparation of our financial statements;
• discussing with management major risk assessment and risk management policies;
• monitoring the independence of the independent auditor;
• verifying the rotation of the lead (or coordinating) audit partner having primary responsibility for the audit and the audit partner responsible for reviewing the audit as required by law;
• reviewing and approving all related-party transactions;
• inquiring and discussing with management our compliance with applicable laws and regulations;
• pre-approving all audit services and permitted non-audit services to be performed by our independent auditor, including the fees and terms of the services to be performed;
• appointing or replacing the independent auditor;
• appointing or replacing the independent auditor;
• determining the compensation and oversight of the work of the independent auditor (including resolution of disagreements between management and the independent auditor regarding financial reporting) for the purpose of preparing or issuing an audit report or related work;
• establishing procedures for the receipt, retention and treatment of complaints received by us regarding accounting, internal accounting controls or reports which raise material issues regarding our financial statements or accounting policies; and
• approving reimbursement of expenses incurred by our management team in identifying potential
Financial Experts on Audit Committee
The audit committee will at all times be composed exclusively of “independent directors” who are “financially literate” as defined under Nasdaq’s listing standards. Nasdaq’s standards define “financially literate” as being able to read and understand fundamental financial statements, including a company’s balance sheet, income statement and cash flow statement.
In addition, we must certify to Nasdaq that the committee has, and will continue to have, at least one member who has past employment experience in finance or accounting, requisite professional certification in accounting, or other comparable experience or background that results in the individual’s financial sophistication. The board of directors has determined that Andrew D. Hamer qualifies as an “audit committee financial expert,” as defined under rules and regulations of the SEC.
Compensation Committee
We have established a compensation committee of the board of directors, consisting of James W. Keyes, Jeffrey A. Rich and Andrew D. Hamer, each of whom is an independent director under Nasdaq’s listing standards. The compensation committee’s duties, which are specified in our Compensation Committee Charter, include, but are not limited to:
• reviewing and approving on an annual basis the corporate goals and objectives relevant to our Chief Executive Officer’s compensation, evaluating our Chief Executive Officer’s performance in light of such goals and objectives and determining and approving the remuneration (if any) of our Chief Executive Officer based on such evaluation;
180
Table of Contents
• reviewing and approving the compensation of all of our other executive officers;
• reviewing our executive compensation policies and plans;
• implementing and administering our incentive compensation equity-based remuneration plans;
• implementing and administering our incentive compensation equity-based remuneration plans;
• assisting management in complying with our proxy statement and annual report disclosure requirements;
• approving all special perquisites, special cash payments and other special compensation and benefit arrangements for our executive officers and employees;
• if required, producing a report on executive compensation to be included in our annual proxy statement; and
• reviewing, evaluating and recommending changes, if appropriate, to the remuneration for directors.
Nominating Committee
We have established a nominating committee of the board of directors, consisting of James W. Keyes, Jeffrey A. Rich and Andrew D. Hamer, each of whom is an independent director under Nasdaq’s listing standards. The nominating committee is responsible for overseeing the selection of persons to be nominated to serve on our board of directors. The nominating committee considers persons identified by its members, management, stockholders, investment bankers and others.
Guidelines for Selecting Director Nominees
The guidelines for selecting nominees, which are specified in the Nominating Committee Charter, generally provide that persons to be nominated:
• should have demonstrated notable or significant achievements in business, education or public service;
• should possess the requisite intelligence, education and experience to make a significant contribution to the board of directors and bring a range of skills, diverse perspectives and backgrounds to its deliberations; and
• should have the highest ethical standards, a strong sense of professionalism and intense dedication to serving the interests of the stockholders.
The Nominating Committee will consider a number of qualifications relating to management and leadership experience, background and integrity and professionalism in evaluating a person’s candidacy for membership on the board of directors. The nominating committee may require certain skills or attributes, such as financial or accounting experience, to meet specific board needs that arise from time to time and will also consider the overall experience and makeup of its members to obtain a broad and diverse mix of board members. The nominating committee does not distinguish among nominees recommended by stockholders and other persons.
Code of Ethics
We have adopted a Code of Ethics applicable to our directors, officers and employees. We have filed a copy of our Code of Ethics and our audit and compensation committee charters as exhibits to the registration statement in connection with our IPO. You can review these documents by accessing our public filings at the SEC’s web site at www.sec.gov. In addition, a copy of the Code of Ethics will be provided without charge upon request from us. We intend to disclose any amendments to or waivers of certain provisions of our Code of Ethics in a Current Report on Form 8-K.
181
Table of Contents
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our officers, directors and persons who own more than ten percent of a registered class of our equity securities to file reports of ownership and changes in ownership with the SEC. Officers, directors and ten percent stockholders are required by regulation to furnish us with copies of all Section 16(a) forms they file. Based solely on copies of such forms received, we believe that, during the period from July 28, 2020 (inception) through December 31, 2021, all filing requirements applicable to our officers, directors and greater than ten percent beneficial owners were complied with.
Principal Accountant Fees and Services
The following is a summary of fees paid to Marcum LLP (“Marcum”), for services rendered.
Audit Fees. Audit fees consist of fees billed for professional services rendered for the audit of our year-end financial statements and services that are normally provided by Marcum in connection with regulatory filings. The aggregate fees billed by Marcum for professional services rendered for the audit of our annual financial statements, review of the financial information included in our annual report on Form 10-K for the respective periods and other required filings with the SEC for the year ended December 31, 2021, including services in connection with our IPO totalled $125,560. The above amounts include procedures and audit fees, as well as attendance at audit committee meetings.
Audit-Related Fees. Audit-related services consist of fees billed for assurance and related services that are reasonably related to performance of the audit or review of our financial statements and are not reported under “Audit Fees.” These services include attest services that are not required by statute or regulation and consultations concerning financial accounting and reporting standards. During the year ended December 31, 2021, we did not pay Marcum for consultations concerning financial accounting and reporting standards.
Tax Fees. We paid Marcum $4,893 for tax planning and tax advice during the year ended December 31, 2021.
All Other Fees. We did not pay Marcum for other services during the year ended December 31, 2021.
Pre-Approval Policy
Our audit committee was formed upon the consummation of our IPO. As a result, the audit committee did not pre-approve all of the foregoing services, although any services rendered prior to the formation of our audit committee were approved by our board of directors. Since the formation of our audit committee, and on a going-forward basis, the audit committee has and will pre-approve all auditing services and permitted non-audit services to be performed for us by our auditors, including the fees and terms thereof (subject to the de minimis exceptions for non-audit services described in the Exchange Act which are approved by the audit committee prior to the completion of the audit).
182
Table of Contents
LightJump Management’s Discussion and Analysis of
Financial Condition and Results of Operations
References in this section to “we”, “our”, “us”, “SPAC” or “LightJump” generally refer to LightJump Acquisition Corporation.
Overview
We are a blank check company incorporated as a Delaware corporation on July 28, 2020 and formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. LightJump intends to complete its initial business combination using cash from the proceeds of its IPO and the private placements of the Private Placement Warrants, LightJump’s capital stock, debt or a combination of cash, stock and debt. LightJump’s sponsor is LightJump One Founders, LLC, a Delaware limited liability company.
LightJump consummated its IPO on January 12, 2021. On June 14, 2022, LightJump, Moolec, Holdco and Merger Sub entered into the Business Combination Agreement. The terms of the Business Combination Agreement, which contains customary representations and warranties, covenants, closing conditions, termination fee provisions and other terms relating to the mergers and the other transactions contemplated thereby, are summarized below. Capitalized terms used in this section but not otherwise defined herein have the meanings given to them in the Business Combination Agreement.
Pursuant to the Business Combination Agreement, following the effectiveness of the transactions contemplated by the Exchanges, the parties will consummate the Business Combination and Moolec and LightJump will become direct wholly-owned subsidiaries of Holdco. Pursuant to the Business Combination Agreement, each of the following transactions occurred, or will occur, in the following order:
• At the Exchange Effective Time, subject to the receipt of the Company Requisite Approvals, the Holdco Requisite Approvals and the delivery of the Exchange Auditor Report, and in accordance with the Holdco Delegate Resolutions:
• all issued Company Ordinary Shares held by the Company Shareholders shall be transferred and for the purposes of the 1915 Law, contributed in kind to Holdco, free and clear of all Liens (other than the Company Shareholders’ Agreements Liens that will expire on or prior to the Closing Date), and the Company Shareholders shall subscribe for and, as consideration for the contribution, shall be issued, in accordance with the Exchange Ratio (save that the Holdco Ordinary Shares to be issued shall be reduced by the number of Holdco Ordinary Shares already held by the Company Shareholders immediately prior to the Exchange), the Exchange Issuance;
• The Exchange Issuance shall be allocated among the Company Shareholders in accordance with the terms of the Payment Spreadsheet and Exchange Agreements;
• each Company SAFE Holder shall have contributed all of its rights and obligations under each Original SAFE to Holdco in consideration for the issuance by Holdco of a simple agreement for future equity on substantively identical terms (mutatis mutandis) with such adjustments (if any) required under Luxembourg law. For Luxembourg law purposes, a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued a report on the contributions in kind relating to the contribution of the Original SAFEs prepared in accordance with article 420-10 of the 1915 Law;
• each Company Shareholder shall cease to be the holder of such Company Ordinary Shares, subject to the submission of all filings required under Law (including any filings required to pay stamp duties), and Holdco will be recorded as the registered holder of all the Company Ordinary Shares so exchanged and transferred and will be the legal and beneficial owner thereof.
183
Table of Contents
• Immediately prior to the Merger Effective Time but after the Exchange Effective Time, each Company SAFE Holder shall receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance the respective Company SAFE, with such adjustments (if any) required under Luxembourg law;
• SPAC shall cause the Certificate of Merger to be executed, acknowledged and filed with the Secretary of State of the State of Delaware in accordance with the applicable provisions of the DGCL in order to effectuate the Merger. The Merger shall become effective at such time as the Certificate of Merger has been duly filed with the Secretary of State of the State of Delaware or at such later time as may be agreed by the Company and SPAC in writing and specified in the Certificate of Merger in accordance with the DGCL;
• At the Merger Effective Time, by virtue of the Merger and the Holdco Requisite Approvals, subject to the Merger Auditor Report, and without any further action on the part of SPAC, Merger Sub, Holdco or the Company or the holders thereunder:
• each SPAC Common Stock issued and outstanding immediately prior to the Merger Effective Time, excluding those have been redeemed subject to any redemption rights, shall be exchanged with Holdco (which exchange, for purposes of the 1915 Law, shall include, for the avoidance of doubt, a contribution-in-kind of each such shares of SPAC Common Stock from the holders of SPAC Common Stock to Holdco), against the issue by Holdco of new Holdco Ordinary Shares (such issuance, the “Merger Issuance”), under the authorized share capital of Holdco (pursuant to the Holdco Delegate Merger Resolutions) and subscribed by the contributing holders of SPAC Common Stock by virtue of the Merger and in accordance with the 1915 Law for one validly issued and fully paid Holdco Ordinary Share (the “Merger Consideration”), delivered by Holdco;
• as a result of the Merger, all SPAC Common Stock shall cease to be outstanding, shall be cancelled and shall cease to exist;
• each share of common stock, par value $0.01 of Merger Sub issued and outstanding immediately prior to the Merger Effective Time shall be converted and exchanged for one (1) validly issued, fully paid and nonassessable ordinary share, par value $0.01 per share, of the Surviving Company; and
• Each SPAC Warrant that is outstanding immediately prior to the Merger Effective Time shall, pursuant to the SPAC Warrant Agreement, cease to represent a right to acquire one SPAC Common Stock and shall be converted in accordance with the terms of such SPAC Warrant Agreement, at the Merger Effective Time, into a right to acquire one Holdco Ordinary Share on substantially the same terms as were in effect immediately prior to the Merger Effective Time under the terms of the SPAC Warrant Agreement.
The parties to the Business Combination Agreement will hold the closing on the date of the Merger Effective Time, following the satisfaction or waiver (to the extent such waiver is permitted by applicable law) of the conditions set forth in the Business Combination Agreement (other than those conditions that by their nature are to be satisfied at Closing, but subject to the satisfaction or waiver of those conditions at such time).
Results of Operations
Our entire activity since inception up to June 30, 2022 relates to our formation, the IPO and, since the closing of the IPO, a search for a Business Combination candidate. We will not be generating any operating revenues before the closing and completion of our initial Business Combination, and pursuant to the Business Combination will be a wholly-owned subsidiary of Holdco.
For the three months ended June 30, 2022, we had net loss of $78,739, which consisted of $1,171,347 in formation and operating costs and $3,679 in income tax provision, offset by $175,297 in interest earned from cash in the Trust Account, and $920,990 in an unrealized gain on change in fair value of warrants.
184
Table of Contents
For the three months ended June 30, 2021, we had a net loss of $1,868,255, which consisted of $786,609 in formation and operating costs and $1,085,087 in an unrealized loss on change in fair value of warrants, offset by $3,441 in interest earned from cash in the Trust Account.
For the six months ended June 30, 2022, we had net income of $147,267, which consisted of $187,600 in interest earned from cash in the Trust Account, and $1,936,782 in an unrealized gain on change in fair value of warrants, offset by $1,973,436 in formation and operating costs and $3,679 in income tax provision.
For the six months ended June 30, 2021, we had a net loss of $814,276, which consisted of $1,037,402 in formation and operating costs, offset by $6,361 in interest earned from cash in the Trust Account, and $216,765 in an unrealized gain on change in fair value of warrants.
Liquidity, Capital Resources and Going Concern
As of June 30, 2022, LightJump had $5,422 in its operating bank account and a working capital deficit of approximately $2.6 million.
Prior to the completion of the IPO, LightJump’s liquidity needs had been satisfied through a payment from the Sponsor of $25,000 (see Note 5 in the accompanying financial statements of LightJump included elsewhere in this proxy statement/prospectus) in exchange for the Founder Shares to cover certain offering costs, and a loan under an unsecured promissory note from the Sponsor of $125,000 (see Note 5 in the accompanying financial statements of LightJump included elsewhere in this proxy statement/prospectus). Subsequent to the consummation of the IPO and Private Placement, LightJump’s liquidity needs have been satisfied through the proceeds from the consummation of the Private Placement not held in the Trust Account.
In addition, in order to finance transaction costs in connection with a Business Combination, LightJump’s Sponsor or an affiliate of the Sponsor or certain of LightJump’s officers and directors may, but are not obligated to, provide LightJump Working Capital Loans (see Note 5 in the accompanying financial statements of LightJump included elsewhere in this proxy statement/prospectus). To date, there were no amounts outstanding under any Working Capital Loans.
LightJump can raise additional capital through Working Capital Loans from the LightJump Initial Stockholders, LightJump’s officers, directors, or their respective affiliates (which is described in Note 5 in the accompanying financial statements of LightJump included elsewhere in this proxy statement/prospectus), or through loans from third parties. None of the Sponsor, officers or directors are under any obligation to advance funds to, or to invest in, LightJump. If LightJump is unable to raise additional capital, it may be required to take additional measures to conserve liquidity, which could include, but not necessarily be limited to, curtailing operations, suspending the pursuit of its business plan, and reducing overhead expenses. LightJump cannot provide any assurance that new financing will be available to it on commercially acceptable terms, if at all.
LightJump anticipates that the $5,422 held outside of the Trust Account as of June 30, 2022, might not be sufficient to allow LightJump to operate until January 12, 2023, the period it has to consummate an initial business combination, assuming that a business combination is not consummated during that time. Until consummation of our business combination, LightJump will be using the funds not held in the Trust Account, and any additional Working Capital Loans from the LightJump Initial Stockholders, LightJump’s officers and directors, or their respective affiliates, for identifying and evaluating prospective acquisition candidates, performing business due diligence on prospective target businesses, traveling to and from the offices, plants or similar locations of prospective target businesses, reviewing corporate documents and material agreements of prospective target businesses, selecting the target business to acquire and structuring, negotiating and consummating the business combination.
LightJump was required to complete its Initial Business Combination within 18 months from the date of IPO (January 12, 2021). However, on July 8, 2022, LightJump held a special meeting of stockholders. At the meeting, LightJump’s stockholders approved an amendment to extend the date by which LightJump must consummate its Initial Business Combination from July 12, 2022 to January 12, 2023.
LightJump Holders holding 11,032,790 Public Shares exercised their right to redeem such shares for a pro rata portion of the funds in the Trust Account. As a result, $110,507,220.68 (approximately $10.02 per share) was removed from the Trust Account to pay such holders. Following redemptions, LightJump has 2,767,210
185
Table of Contents
Public Shares outstanding and the aggregate amount remaining in the Trust Account as of July 12, 2022 was $27,993,797.65 (which includes an additional $276,721 contributed by the Sponsor in connection with the Extension).
If LightJump is unable to complete its initial Business Combination within the Combination Period, LightJump will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten Business Days thereafter, redeem 100% of the outstanding public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including any interest not previously released to LightJump (net of taxes payable), divided by the number of then outstanding public shares, which redemption will completely extinguish Public Stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of LightJump’s remaining stockholder and its board of directors, dissolve and liquidate, subject (in the case of (ii) and (iii) above) to LightJump’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. It is not certain that LightJump would be able to complete a business combination with the period of Initial Business Combination and LightJump cannot assure that it will have funds sufficient to pay or provide for creditors’ claims.
These conditions raise substantial doubt about LightJump’s ability to continue as a going concern through the next 12 months, if a business combination is not consummated. These unaudited condensed financial statements do not include any adjustments relating to the recovery of the recorded assets of the classification of the liabilities that might be necessary should LightJump be unable to continue as a going concern.
Critical Accounting Policies and Estimates
The preparation of the unaudited condensed financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the unaudited condensed financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates. We have identified the following as our critical accounting policies:
Warrant Liabilities
We do not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. We evaluate all of our financial instruments, including issued stock purchase warrants, to determine if such instruments are derivatives or contain features that qualify as embedded derivatives, pursuant to ASC 480 and ASC 815-15.
We account for the Public Warrants and Private Warrants collectively (“Warrants”), as either equity or liability-classified instruments based on an assessment of the specific terms of the Warrants and the applicable authoritative guidance in Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the Warrants meet all of the requirements for equity classification under ASC 815, including whether the Warrants are indexed to our own common stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of our control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of issuance of the Warrants and as of each subsequent quarterly period end date while the Warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, such warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, such warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. Changes in the estimated fair value of liability-classified warrants are recognized as a non-cash gain or loss on the statements of operations.
We account for the Private Warrants in accordance with ASC 815-40 under which the Warrants do not meet the criteria for equity classification and must be recorded as liabilities. The fair value of the Private Warrants has been estimated using the Monte Carlo simulation model.
186
Table of Contents
We evaluated the Public Warrants and Rights in accordance with ASC 815-40, “Derivatives and Hedging — Contracts in Entity’s Own Equity,” and concluded that they met the criteria for equity classification and are required to be recorded as part a component of additional paid-in capital at the time of issuance.
Net Income Per Common Share
We comply with accounting and disclosure requirements of FASB ASC Topic 260, Earnings Per Share. Net income (loss) per share is computed by dividing net income (loss) by the weighted average number of shares of our common stock outstanding during the period. We have two classes of shares, redeemable common stock and non-redeemable common stock. Earnings and losses are shared pro rata between the two classes. Our condensed statement of operations applies the two-class method in calculating net income (loss) per share. Basic and diluted net income (loss) per common share for redeemable common stock and non-redeemable common stock is calculated by dividing net income (loss), allocated proportionally to each class of common stock, by the weighted average number of shares of redeemable common stock and non-redeemable common stock outstanding. We do not include our warrants as they are anti-dilutive. As a result, diluted income per share is the same as basic income per share for the period presented.
Common Stock Subject to Possible Redemption
We account for our common stock subject to possible redemption in accordance with the guidance in ASC Topic 480 “Distinguishing Liabilities from Equity.” Common stock subject to mandatory redemption (if any) is classified as a liability instrument and is measured at fair value. Conditionally redeemable common stock (including common stock that feature redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within LightJump’s control) is classified as temporary equity. At all other times, common stock is classified as stockholders’ deficit. Our common stock feature certain redemption rights that is considered to be outside of our control and subject to the occurrence of uncertain future events. Accordingly, common stock subject to possible redemption is presented at redemption value as temporary equity, outside of the stockholders’ deficit section of our condensed balance sheet.
Off-Balance Sheet Financing Arrangements
We have no obligations, assets or liabilities, which would be considered off-balance sheet arrangements as of June 30, 2022. We do not participate in transactions that create relationships with unconsolidated entities or financial partnerships, often referred to as variable interest entities, which would have been established for the purpose of facilitating off-balance sheet arrangements. We have not entered into any off-balance sheet financing arrangements, established any special purpose entities, guaranteed any debt or commitments of other entities, or purchased any non-financial assets.
Contractual Obligations
We do not have any long-term debt, capital lease obligations, operating lease obligations or long-term liabilities, other than an agreement to pay an affiliate of our Sponsor a monthly fee of $10,000 for office space, utilities and administrative support provided to LightJump. Upon completion of the initial Business Combination or LightJump’s liquidation, LightJump will cease paying these monthly fees.
Quantitative and Qualitative Disclosures About Market Risk
As of June 30, 2022, we were not subject to any market or interest rate risk. Following the consummation of our IPO, the net proceeds of our IPO, including amounts in the Trust Account, have been invested in U.S. government treasury bills, notes or bonds with a maturity of 185 days or less or in certain money market funds that invest solely in U.S. treasuries. Due to the short-term nature of these investments, we believe there will be no associated material exposure to interest rate risk.
187
Table of Contents
JOBS Act
We are an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the JOBS Act. As such, we are eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. If some investors find our securities less attractive as a result, there may be a less active trading market for our securities and the prices of our securities may be more volatile.
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We intend to take advantage of the benefits of this extended transition period.
We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of our IPO, (b) in which we have total annual gross revenue of at least $1.07 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
Recent Accounting Standards
In August 2020, the FASB issued ASU 2020-06, Debt-Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU also removes certain settlement conditions that are required for equity-linked contracts to qualify for scope exception, and it simplifies the diluted earnings per share calculation in certain areas. LightJump is reviewing what impact, if any, adoption may have on the financial statements.
188
Table of Contents
Certain LightJump Relationships and Related Person Transactions
References in this section to “we”, “our”, “us”, “SPAC” or “LightJump” generally refer to LightJump Acquisition Corporation.
In September 2020, the Sponsor paid $25,000 in cash, or approximately $0.009 per share, to LightJump in consideration for 2,875,000 shares of common stock, par value $0.0001. On January 11, 2021, LightJump effected a stock dividend of 0.2 shares for each share outstanding, resulting in there being an aggregate of 3,450,000 Founder Shares outstanding. All shares and associated amounts have been retroactively restated to reflect the stock dividend. Up to 450,000 Founder Shares were subject to forfeiture to the extent that the over-allotment option was not exercised in full by the underwriters. In connection with the underwriters’ full exercise of the over-allotment option on January 15, 2021, the 450,000 Founder Shares were no longer subject to forfeiture. In connection with the closing of the IPO and the underwriters’ full exercise of the over-allotment option on January 15, 2021, Sponsor purchased an aggregate of 4,210,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant in a private placement, for a total purchase price of $4,210,000.
The Founder Shares were placed into an escrow account maintained in New York, New York by Continental Stock Transfer & Trust Company, acting as escrow agent. Subject to certain limited exceptions, these shares will not be transferred, assigned, sold or released from escrow (subject to certain limited exceptions set forth below) (i) with respect to 50% of such shares, for a period ending on the earlier of the one-year anniversary of the date of the consummation of an initial Business Combination and the date on which the closing price of the common stock equals or exceeds $12.50 per share (as adjusted for share splits, share dividends, reorganizations and recapitalizations) for any 20 trading days within a 30-trading day period following the consummation of the initial Business Combination and (ii) with respect to the remaining 50% of such shares, for a period ending on the one-year anniversary of the date of the consummation of the initial Business Combination, or earlier, in either case, if, subsequent to our initial Business Combination, the combined company consummates a liquidation, merger, stock exchange or other similar transaction which results in all of the stockholders having the right to exchange their shares of common stock for cash, securities or other property.
Advances from Related Party
As of June 30, 2022, we have received $300,000 in advances from our Sponsor of which $260,000 remains unpaid, including $40,000 accrued for the administrative support fee. These advances are due on demand.
Promissory Note — Moolec
On July 8, 2022, the Sponsor issued a promissory note to Moolec, pursuant to which, Moolec agreed to loan to the Sponsor up to an aggregate principal amount of $350,000 to deposit into the Trust Account in connection with the extension of LightJump’s termination date from July 12, 2022 to January 12, 2023 (or such earlier date as determined by the Board).
Promissory Note — Related Party
On September 30, 2020, LightJump issued an unsecured promissory note to an affiliate of LightJump’s chief executive officer, pursuant to which LightJump may borrow up to an aggregate principal amount of $150,000 to be used for a portion of the expenses of our IPO. This loan is non-interest bearing, unsecured and due at the earlier of July 31, 2021 or the closing of our IPO. The loan will be repaid upon the closing of our IPO out of offering proceeds not held in the Trust Account. As of June 30, 2022, we had borrowed $125,000 under the promissory note with an affiliate of the Sponsor which is still outstanding as of June 30, 2022.
The Company issued several additional promissory notes to the Sponsor in principal of an aggregate of $1,120,300, of which $354,300 were issued after June 30, 2022. All notes are not interest bearing and are repayable upon conclusion of business combination. The principal balance may be prepaid at any time.
189
Table of Contents
Working Capital Loans
In order to finance transaction costs in connection with a business combination, the Sponsor or an affiliate of the Sponsor, or certain of LightJump’s officers and directors may, but are not obligated to, loan LightJump funds as may be required (“Working Capital Loans”). If the LightJump completes a business combination, LightJump would repay the Working Capital Loans. In the event that a business combination does not close, LightJump may use a portion of proceeds held outside the Trust Account to repay the Working Capital Loans but no proceeds held in the Trust Account would be used to repay the Working Capital Loans.
Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. The Working Capital Loans would either be repaid upon consummation of a business combination, without interest, or, at the lender’s discretion, up to $1.5 million of such Working Capital Loans may be convertible into Private Warrants at a price of $1.00 per warrant. As of June 30, 2022, LightJump had no borrowings under the Working Capital Loans.
Administrative Service Fee
Commencing on the date of our IPO, LightJump has agreed to pay the Sponsor a total of $10,000 per month for office space, secretarial and administrative services. Upon completion of the initial business combination or the SPAC’s liquidation, LightJump will cease paying these monthly fees. For the three and six months ended June 30, 2022, the SPAC incurred an aggregate of $30,000 and $60,000 for administrative services.
Other than the foregoing, no compensation of any kind, including any finder’s fee, reimbursement, consulting fee or monies in respect of any payment of a loan, will be paid by us to our Sponsor, officers and directors, or any affiliate of our Sponsor or officers, prior to, or in connection with any services rendered in order to effectuate, the consummation of an initial business combination (regardless of the type of transaction that it is). However, these individuals will be reimbursed for any out-of-pocket expenses incurred in connection with activities on our behalf.
Related Party Policy
Our Code of Ethics requires us to avoid, wherever possible, all related party transactions that could result in actual or potential conflicts of interests, except under guidelines approved by the board of directors (or the audit committee). Related-party transactions are defined as transactions in which (1) the aggregate amount involved will or may be expected to exceed $120,000 in any calendar year, (2) we or any of our subsidiaries is a participant, and (3) any (a) executive officer, director or nominee for election as a director, (b) greater than 5% beneficial owner of our shares of common stock, or (c) immediate family member, of the persons referred to in clauses (a) and (b), has or will have a direct or indirect material interest (other than solely as a result of being a director or a less than 10% beneficial owner of another entity). A conflict of interest situation can arise when a person takes actions or has interests that may make it difficult to perform his or her work objectively and effectively. Conflicts of interest may also arise if a person, or a member of his or her family, receives improper personal benefits as a result of his or her position.
Our audit committee, pursuant to its written charter, is responsible for reviewing and approving related-party transactions to the extent we enter into such transactions. The audit committee will consider all relevant factors when determining whether to approve a related party transaction, including whether the related party transaction is on terms no less favorable to us than terms generally available from an unaffiliated third-party under the same or similar circumstances and the extent of the related party’s interest in the transaction. No director may participate in the approval of any transaction in which he is a related party, but that director is required to provide the audit committee with all material information concerning the transaction. We also require each of our directors and executive officers to complete a directors’ and officers’ questionnaire that elicits information about related party transactions.
These procedures are intended to determine whether any such related party transaction impairs the independence of a director or presents a conflict of interest on the part of a director, employee or officer.
190
Table of Contents
To further minimize conflicts of interest, we have agreed not to consummate an initial business combination with an entity that is affiliated with any of our Sponsor, officers or directors unless we have obtained an opinion from an independent investment banking firm, or another independent entity that commonly renders valuation opinions, that the business combination is fair to our unaffiliated stockholders from a financial point of view. We will also need to obtain approval of a majority of our disinterested independent directors.
Furthermore, other than as described herein, no finder’s fees, reimbursements, consulting fee, monies in respect of any payment of a loan or other compensation will be paid by us to our Sponsor, officers or directors, or any affiliate of our Sponsor or officers, for services rendered to us prior to, or in connection with any services rendered in order to effectuate, the consummation of our initial business combination (regardless of the type of transaction that it is). However, the following payments will be made to our Sponsor, officers or directors, or our or their affiliates, none of which will be made from the proceeds of our IPO held in the Trust Account prior to the completion of our initial business combination:
• Repayment of up to an aggregate of $170,000 in loans made to us by our Sponsor to cover offering-related and organizational expenses;
• Payment to our Sponsor of $10,000 per month until the earlier of (i) the consummation of our initial business combination or (ii) our liquidation, for office space, utilities and secretarial and administrative support;
• Reimbursement for any out-of-pocket expenses related to identifying, investigating and completing an initial business combination; and
• Repayment of non-interest bearing loans which may be made by our Sponsor or an affiliate of our Sponsor or certain of our officers and directors to finance transaction costs in connection with an intended initial business combination. Up to $1,500,000 of such loans may be convertible into warrants, at a price of $1.00 per warrant at the option of the lender.
Our audit committee will review on a quarterly basis all payments that were made to our Sponsor, officers or directors, or our or their affiliates.
In connection with the Business Combination, certain agreements were entered into or will be entered into pursuant to the Business Combination Agreement. The agreements described in this section, or forms of such agreements as they will be in effect substantially concurrently with the completion of the Business Combination, are filed as exhibits to the registration statement of which this proxy statement/prospectus forms a part, and the following descriptions are qualified by reference thereto. These agreements include:
• Form of Exchange Agreement (see the section entitled “Certain Agreements Related to the Business Combination — Company Shareholder Contribution and Exchange Agreements”);
• Backstop Agreement (see the section entitled “Certain Agreements Related to the Business Combination — Backstop Agreement”);
• Form of SPAC Warrant and Assignment Agreement (see the section entitled “Certain Agreements Related to the Business Combination — Assignment, Assumption and Amendment to Warrant Agreement”);
• Transaction Support Agreement (see the section entitled “Certain Agreements Related to the Business Combination — Transaction Support Agreement”);
• Registration Rights and Lock-Up Agreement (see the section entitled “Certain Agreements Related to the Business Combination — Registration Rights and Lock-Up Agreement”); and
• Amendment to Business Combination Marketing Agreement (see the section entitled “Certain Agreements Related to the Business Combination — Amendment to Business Combination Marketing Agreement”).
191
Table of Contents
Management of Holdco after the Business Combination
References in this section to “we”, “our”, “us” and the “Company” generally refer to Moolec and its consolidated subsidiaries, prior to the Business Combination and Holdco and its consolidated subsidiaries after giving effect to the Business Combination.
Management and Board of Directors
The following table sets forth the persons LightJump and Moolec anticipate will become the executive officers and directors of Holdco.
The Holdco Board is expected to be comprised of seven directors. Pursuant to the Business Combination Agreement, the following individuals are expected to comprise the Holdco Board: [•].
For biographical information concerning the executive officers and senior management team, see “Management of Moolec.” For biographical information concerning [•], see “Business of LightJump and Certain Information about LightJump — Directors and Executive Officers.” For biographical information concerning the remaining directors, see below.
Name | | Age | | Position |
[•] | | [•] | | [•] |
[•] | | [•] | | [•] |
[•] | | [•] | | [•] |
[•] | | [•] | | [•] |
[•] | | [•] | | [•] |
[•] | | [•] | | [•] |
[•] | | [•] | | [•] |
Foreign Private Issuer Exemption
As a “foreign private issuer,” as defined by the SEC, Holdco is permitted to follow home country corporate governance practices, instead of certain corporate governance practices required by Nasdaq for U.S. domestic issuers other than with respect to certain voting and committee requirements.
Holdco intends to take all actions necessary for it to maintain compliance as a foreign private issuer under the applicable corporate governance requirements of the Sarbanes-Oxley Act of 2002, the rules adopted by the SEC and the Nasdaq corporate governance rules and listing standards.
Because Holdco is a foreign private issuer, its directors and senior management are not subject to short-swing profit and insider trading reporting obligations under Section 16 of the Exchange Act. They will, however, be subject to the obligations to report changes in share ownership under Section 13 of the Exchange Act and related SEC rules.
Corporate Governance
We will structure our corporate governance in a manner LightJump and Moolec believe will closely align our interests with those of our shareholders following the Business Combination. Notable features of this corporate governance include:
• we will have a majority of independent directors and independent director representation on our audit, compensation and nominating committees immediately following the consummation of the Business Combination, and our independent directors will meet regularly in executive sessions without the presence of our corporate officers or non-independent directors;
• at least one of our directors will qualify as an “audit committee financial expert” as defined by the SEC; and
• we will implement a range of other corporate governance practices, including implementing a robust director education program.
192
Table of Contents
Independence of our Board of Directors1
Audit Committee
[Our audit committee will be responsible for, among other things:
• appointing, compensating, retaining, evaluating, terminating and overseeing our independent registered public accounting firm;
• discussing with our independent registered public accounting firm their independence from management;
• reviewing, with our independent registered public accounting firm, the scope and results of their audit;
• approving all audit and permissible non-audit services to be performed by our independent registered public accounting firm;
• overseeing the financial reporting process and discussing with management and our independent registered public accounting firm the annual financial statements that we file with the SEC;
• overseeing our financial and accounting controls and compliance with legal and regulatory requirements;
• reviewing our policies on risk assessment and risk management;
• reviewing related person transactions; and
• establishing procedures for the confidential anonymous submission of concerns regarding questionable accounting, internal controls or auditing matters.]
The members of Holdco’s audit committee will be designated by Holdco’s Board at the closing of the Business Combination, and each will qualify as independent directors according to the rules and regulations of the SEC and Nasdaq with respect to audit committee membership. In addition, all of the audit committee members will meet the requirements for financial literacy under applicable SEC and Nasdaq rules and at least one of the members will qualify as an “audit committee financial expert,” as such term is defined in Item 407(d) of Regulation S-K. Holdco’s Board will adopt a new written charter for the audit committee, which will be available on Holdco’s website after adoption. The reference to Holdco’s websites in this proxy statement/prospectus does not include or incorporate by reference the information on Holdco’s website into this proxy statement/prospectus.
Compensation Committee
[Our compensation committee will be responsible for, among other things:
• reviewing and approving the corporate goals and objectives, evaluating the performance of and reviewing and approving, (either alone or, if directed by the board of directors, in conjunction with a majority of the independent members of the board of directors) the compensation of our Chief Executive Officer;
• overseeing an evaluation of the performance of and reviewing and setting or making recommendations to our board of directors regarding the compensation of our other executive officers;
• reviewing and approving or making recommendations to our board of directors regarding our incentive compensation and equity-based plans, policies and programs;
• reviewing and approving all employment agreement and severance arrangements for our executive officers;
• making recommendations to our board of directors regarding the compensation of our directors; and
• retaining and overseeing any compensation consultants.]
193
Table of Contents
The members of Holdco’s compensation committee will be designated by Holdco’s Board at the closing of the Business Combination, and each will qualify as independent directors according to the rules and regulations of the SEC and Nasdaq with respect to compensation committee membership, including the heightened independence standards for members of a compensation committee. Holdco’s Board will adopt a new written charter for the compensation committee, which will be available on Holdco’s website after adoption. The reference to Holdco’s website address in this proxy statement/prospectus does not include or incorporate by reference the information on Holdco’s website into this proxy statement/prospectus.
Nominating Committee
[Our nominating committee will be responsible for, among other things:
• identifying individuals qualified to become members of our board of directors, consistent with criteria approved by our board of directors;
• overseeing succession planning for our Chief Executive Officer and other executive officers;
• periodically reviewing our board of directors’ leadership structure and recommending any proposed changes to our board of directors;
• reviews developments in corporate governance practices;
• overseeing an annual evaluation of the effectiveness of our board of directors and its committees; and
• developing and recommending to our board of directors a set of corporate governance guidelines.]
The members of Holdco’s nominating committee will be designated by Holdco’s Board at the closing of the Business Combination, and each will qualify as independent directors according to the rules and regulations of the SEC and Nasdaq with respect to nominating committee membership. Holdco’s Board will adopt a new written charter for the nominating committee, which will be available on Holdco’s website after adoption. The reference to Holdco’s website address in this proxy statement/prospectus does not include or incorporate by reference the information on Holdco’s website into this proxy statement/prospectus.
Risk Oversight
Our board of directors is responsible for overseeing our risk management process. Our board of directors focuses on our general risk management strategy, the most significant risks facing us, and oversees the implementation of risk mitigation strategies by management. Our audit committee is also responsible for discussing our policies with respect to risk assessment and risk management. Our board of directors believes its administration of its risk oversight function has not negatively affected our board of directors’ leadership structure.
Code of Ethics
Holdco’s Board will adopt a Code of Ethics applicable to our directors, executive officers and team members that complies with the rules and regulations of Nasdaq and the SEC. The Code of Ethics will be available on Holdco’s website. In addition, Holdco intends to post on the [Corporate Governance section of its website] all disclosures that are required by law or Nasdaq listing standards concerning any amendments to, or waivers from, any provision of the Code of Ethics. The reference to Holdco’s website address in this proxy statement/prospectus does not include or incorporate by reference the information on Holdco’s website into this proxy statement/prospectus.
Compensation of Directors and Officers
[•]
194
Table of Contents
Description of Holdco’s Securities
As a result of the Business Combination, LightJump shareholders and Moolec shareholders who receive Holdco Ordinary Shares in the Business Combination will become Holdco shareholders. Your rights as Holdco shareholders will be governed by the laws of the Grand Duchy of Luxembourg and Holdco’s articles of association. The following description of the material terms of Holdco’s share capital, including the Holdco Ordinary Shares to be issued in the Business Combination, reflects the anticipated state of affairs immediately upon completion of the Business Combination. We urge you to read the applicable provisions of Luxembourg law and Holdco’s forms of articles of association carefully and in their entirety because they describe your rights as a holder of Holdco Ordinary Shares upon consummation of the Business Combination.
Ordinary Shares
Share Capital
Holdco was incorporated on May 23, 2022 by Exequtive Partners S.A., with an initial share capital of $50,000, represented by five million (5,000,000) Holdco Ordinary Shares with a nominal value of $0.01per share. Effective as of May 27, 2022, Exequtive Partners S.A. sold and transferred the entire share capital of Holdco to BG Farming Technologies Limited, Union Group Ventures Ltd. and Bioceres Crop Solutions Corp.
Based on the assumptions set out elsewhere in this proxy statement/prospectus including the assumptions that (i) none of LightJump’s existing Public Stockholders exercise their redemption rights in connection with the approval of the Business Combination with respect to their Public Shares, (ii) no additional equity securities of LightJump are issued prior to the Closing and (iii) a Closing Date of [•], upon completion of the Exchange but prior to the Merger, Holdco’s share capital will equal $325,000,000.00 represented by (i) 32,500,000 Holdco Ordinary Shares.
Based on the assumptions set out elsewhere in this proxy statement/prospectus including the assumptions that (i) none of LightJump’s existing Public Stockholders exercise their redemption rights in connection with the approval of the Business Combination with respect to their Public Shares, (ii) no additional equity securities of LightJump are issued prior to the Closing and (iii) a Closing Date of [•], immediately following the Merger and the Exchange, Holdco’s issued share capital will equal $393,593,350, represented by (i) 39,355,935 Holdco Ordinary Shares. All issued shares will be fully paid and subscribed for.
A shareholder in a Luxembourg société anonyme holding fully paid up shares is, in principle, not liable, solely because of his, her or its shareholder status, for additional payments to Holdco or its creditors.
Share Issuances
Pursuant to Luxembourg law, the issuance of Holdco Ordinary Shares requires in principle approval by the extraordinary general meeting of shareholders subject to necessary quorum and majority requirements. It is intended for the extraordinary general meeting of shareholders of Holdco, prior to the Business Combination, to approve an authorized capital and authorize the board of directors to (i) realize for any reason whatsoever. including any issue in one or several successive tranches of (a) any subscription and/or conversion rights, including warrants (which may be issued separately or linked to Holdco Ordinary Shares, bonds, options, notes or similar instruments issued by Holdco), convertible bonds, notes or similar instruments as well as (b) new Holdco Ordinary Shares, with or without share premium, against payment in cash or in kind, by conversion of claims on Holdco, by way of conversion of available reserves or in any other manner; (ii) determine the place and date of the issue or the successive issues, the issue price, the terms and conditions of the subscription of and paying up on the new Holdco Ordinary Shares, and (iii) remove or limit the preferential subscription right of the shareholders in case of issue against payment in cash of Holdco Ordinary Shares, warrants (which may be separate or attached to Holdco Ordinary Shares, bonds, notes or similar instruments), convertible bonds, notes or similar instruments, up to the maximum amount of such authorized capital for a maximum period of five years from the date of incorporation or any subsequent resolutions to create, amend, renew, increase or extend the authorized capital. The extraordinary general meeting of shareholders of Holdco may renew or increase such authorized capital and such authorization to the board of directors to issue Holdco Ordinary Shares, each time for a period not exceeding five (5) years.
195
Table of Contents
In addition, upon adopting the amended and restated articles of association of Holdco pursuant to the terms of the Business Combination, Holdco’s shareholders shall have authorized the board of directors of Holdco to allocate existing shares of Holdco without consideration or to issue new shares (“Bonus Shares”) paid-up out of distributable reserves (i) to employees of Holdco or to certain classes of such employees; (ii) to employees of companies or economic interest groupings in which Holdco holds directly or indirectly at least fifty percent (50%) of the share capital or of the voting rights; (iii) to employees of companies or economic interest groupings which hold directly or indirectly at least fifty percent (50%) of the share capital or of the voting rights of Holdco; (iv) to employees of companies or economic interest groupings in which at least fifty percent (50%) of the share capital or of the voting rights are held, directly or indirectly, by a company holding itself, directly or indirectly, at least fifty percent (50%) of the share capital of Holdco; or (v) to members of the corporate bodies of Holdco or any of the other companies or economic interest groupings referred to under items (ii) to (iv) above, for a maximum period of five years from the date of incorporation or any subsequent resolutions to create, renew or increase the authorisation (such period restriction is only applicable in case of an allotment of newly issued shares). The preferential subscription right of existing shareholders is, through their authorization to the board of directors of Holdco, automatically waived in case of issuance of Bonus Shares.
Holdco recognizes only one (1) holder per Holdco Share. In case a Holdco Share is owned by several persons, they shall appoint a single representative who shall represent them in respect of Holdco. Holdco has the right to suspend the exercise of all rights attached to that Holdco Share, except for relevant information rights, until such representative has been appointed.
Upon the consummation of the Business Combination, a delegate of the board of directors of Holdco, who will have been granted powers pursuant to resolutions of the board of directors of Holdco, will resolve on the issuance of Holdco Ordinary Shares out of the authorized capital to LightJump shareholders. When delegating such powers to the delegate, the board of directors will also resolve on the applicable procedures and timelines to which such issuance will be subjected. In the event a proposal of the board of directors to issue new Holdco Ordinary Shares exceeds the limits of Holdco’s authorized share capital, the board of directors must then convene the shareholders to an extraordinary general meeting to be held in front of a Luxembourg notary for the purpose of increasing the issued share capital. Such meeting will be subject to the quorum and majority requirements required for amending the articles of association, it being understood that the articles of association may be amended by a majority of at least two thirds (2/3) of the votes validly cast at such general meeting at which a quorum of more than half (1/2) of Holdco’s share capital is present or represented. If no quorum is reached in a meeting, a second meeting may be convened in accordance with the provisions of Luxembourg law and the articles of association of Holdco, which may deliberate regardless of the quorum and at which resolutions are adopted at a majority of at least two thirds of the votes validly cast. Abstentions and nil votes shall not be taken into account. If the capital call proposed by the board of directors consists of an increase in the shareholders’ commitments, the board of directors must convene the shareholders to an extraordinary general meeting to be held in front of a Luxembourg notary for such purpose. Such meeting will be subject to the unanimous consent of the shareholders.
Preferential Subscription Rights
Under Luxembourg law and in accordance with the articles of association of Holdco, existing shareholders benefit from a preferential subscription right on the issuance of new Holdco Ordinary Shares for cash consideration. However, upon adopting the amended and restated articles of association of Holdco pursuant to the terms of the Business Combination, Holdco’s shareholders shall have, in accordance with Luxembourg law, authorized the board of directors, within the limits of Holdco’s authorized share capital and within a period of five years, to remove or limit any preemptive subscription rights of shareholders in case of issue against payment in cash of Holdco Ordinary Shares, warrants (which may be separate or linked to Holdco Ordinary Shares, bonds, notes or similar instruments issued by Holdco), convertible bonds, notes or similar instruments and Holdco can limit or suppress, subject to the quorum and majority for the amendment of the articles of association. Such Holdco Ordinary Shares may be issued above, at, or below market value, and, following a certain procedure, even below the accounting par value, if applicable, per Holdco Share. New Holdco Ordinary Shares also may be issued by way of incorporation of available reserves, including share premium.
196
Table of Contents
Share Repurchases
Holdco cannot subscribe for its own Holdco Ordinary Shares.
Holdco may, however, repurchase issued Holdco Ordinary Shares or have another person acting in his, her or its own name, but on behalf of Holdco, repurchase issued Holdco Ordinary Shares, subject to the following conditions:
1. prior authorization by a simple majority vote at an ordinary general meeting of shareholders, which authorization sets forth:
a. the terms and conditions of the proposed repurchase and in particular the maximum number of Holdco Ordinary Shares to be repurchased;
b. the duration of the period for which the authorization is given, which may not exceed five years; and
c. in the case of repurchase for consideration, the minimum and maximum consideration per share;
2. redemptions, including shares previously acquired by Holdco and held by it in its portfolio and shares acquired by a person acting in his, her or its own name, but on behalf of Holdco, may not result in the net assets as shown in the annual accounts falling below the amount of the subscribed capital, increased by the reserves which Luxembourg law or the articles of association do not permit to distribute;
3. only fully paid-up Holdco Ordinary Shares may be repurchased; and
4. the offer to repurchase must be made on the same terms to all shareholders in the same situation except for repurchases which have been unanimously decided by a general meeting at which all shareholders were present or represented; similarly, listed companies may purchase their own Holdco Ordinary Shares on the stock exchange without an offer to acquire having to be made to its shareholders.
When the acquisition of Holdco’s own Holdco Ordinary Shares is necessary to avoid serious and imminent harm to Holdco, the prior authorization by a simple majority vote at an ordinary general meeting of shareholders described in paragraph (1) above shall not apply. In such a case, the board of directors must inform the shareholders at the following general meeting of the reasons for, and purpose of, the redemption, the number and nominal value, or failing that, such acquired Holdco Ordinary Share’s accounting par value, the fraction of the subscribed capital such acquired Holdco Ordinary Shares represent, as well as the countervalue of such Holdco Ordinary Shares.
The prior authorization by a simple majority vote at an ordinary general meeting of shareholders described in paragraph (1) above shall also not apply in the case of Holdco Ordinary Shares acquired either by Holdco itself or by a person acting in his, her or its own name, but on behalf of Holdco, for distribution to the employees of Holdco or to the employees of an affiliate of Holdco due to a control relationship (i.e., its subsidiaries or controlling shareholder) or in any of the circumstances listed in article 430-16 of the Luxembourg law of August 10, 1915 on commercial companies, as amended. The distribution of such Holdco Ordinary Shares must be made within 12 months of the acquisition of those shares.
The authorization will be valid for a period ending on the earlier of five years from the date of such shareholder authorization and the date of its renewal by a subsequent general meeting of shareholders. Pursuant to such authorization, the board of directors is authorized to redeem all Holdco Ordinary Shares under the conditions set forth in article 430-15 of the 1915 Law. Such purchases and sales may be carried out for any authorized purpose or any purpose that is authorized by the laws and regulations in force. The purchase price per Holdco Ordinary Share to be determined by the board of directors or its delegate shall represent not more than the fair market value of such Holdco Ordinary Shares.
The voting and dividend rights attached to the repurchased Holdco Ordinary Shares will be suspended as long as such repurchased Holdco Ordinary Shares are held by Holdco.
197
Table of Contents
Voting rights
Each Holdco Ordinary Share entitles the holder thereof to one vote. Neither Luxembourg law nor Holdco’s articles of association contain any restrictions as to the voting of Holdco Ordinary Shares by non-Luxembourg residents. The 1915 Law distinguishes general meetings of shareholders and extraordinary general meetings of shareholders with respect to voting rights.
Meetings
Ordinary General Meeting
In accordance with the 1915 Law and Holdco’s articles of association, there is no quorum requirement at an ordinary general meeting and resolutions are adopted by a simple majority of validly cast votes of the shareholders present or represented for a given duly convened ordinary general meeting. Abstentions and nil votes are not taken into account.
Extraordinary General Meeting
Extraordinary resolutions are required for any of the following matters, among others: (i) an increase or decrease of the authorized or issued capital (except if made by the board of directors under the authorized capital), (ii) a limitation or exclusion of preemptive rights (except if made by the board of directors under the authorized capital), (iii) approval of a statutory merger or de-merger (scission), (iv) Holdco’s dissolution and liquidation, (v) any and all amendments to Holdco’s articles of association and (vi) change of nationality. Pursuant to the 1915 Law and Holdco’s articles of association, for any resolutions to be considered at an extraordinary general meeting of shareholders, the quorum shall be at least half (1/2) of Holdco’s issued share capital at a first duly convened meeting, unless otherwise mandatorily required by law. If the said quorum is not reached, a second meeting may be convened, for which the 1915 Law and the Holdco’s articles of association do not prescribe a quorum. Any extraordinary resolution shall be adopted at a quorate general meeting, except otherwise provided by law, by at least a two-thirds (2/3) majority of the votes validly cast at such meeting by shareholders. Abstentions and nil votes are not taken into account.
Annual Shareholders Meetings
An annual general meeting of shareholders shall be held in the Grand Duchy of Luxembourg within 6 months of the end of the preceding financial year.
Warrants
Pursuant to the SPAC Warrant Amendment and Assignment LightJump will assign to Holdco all of LightJump’s right, title and interest in and to the existing Warrant Agreement and Holdco will assume, and agree to pay, perform, satisfy and discharge in full, all of LightJump’s liabilities and obligations under the existing Warrant Agreement arising from and after the Merger Effective Time.
Each Holdco Warrant is exercisable to purchase one half Holdco Ordinary Share and only whole warrants are exercisable. The exercise price of the Holdco Warrants is $11.50 per share, subject to adjustment as described in the SPAC Warrant Amendment and Assignment. A Holdco Warrant may be exercised only during the period commencing only during the period commencing on the date of the consummation of the transactions contemplated by the Business Combination Agreement, and terminating at 5:00 p.m., New York City time on the earlier to occur of: (x) the date that is five (5) years after the date on which the Business Combination is completed, (y) the redemption date as provided in Section 6.2 of the SPAC Warrant Amendment and Assignment, or (z) the liquidation of Holdco. Redemptions of warrants for cash pursuant to the SPAC Warrant Amendment and Assignment, once the public warrants become exercisable, may be redeemed (i) in whole and not in part, (ii) at a price of $0.01 per warrant, (iii) upon not less than 30 days’ prior written notice of redemption to each warrant holder, and (iv) if, and only if, the reported last sale price of the Holdco Ordinary Shares equals or exceeds $18.00 per share for any 20 trading days within a 30-trading day period ending three Business Days before sending the notice of redemption
198
Table of Contents
to each warrant holder. If the public warrants are called for redemption for cash, management will have the option to require all holders that wish to exercise the public warrants to do so on a “cashless basis,” as described in the SPAC Warrant Amendment and Assignment.
The private warrants will be treated identical to the public warrants.
Dividends
From the annual net profits of Holdco, at least 5% shall each year be allocated to the reserve required by applicable laws (the “Legal Reserve”). That allocation to the Legal Reserve will cease to be required as soon and as long as the Legal Reserve amounts to 10% of the amount of the share capital of Holdco. The general meeting of shareholders shall resolve how the remainder of the annual net profits, after allocation to the Legal Reserve, will be disposed of by allocating the whole or part of the remainder to a reserve or to a provision, by carrying it forward to the next following financial year or by distributing it, together with carried forward profits, distributable reserves or share premium to the shareholders, each Holdco Ordinary Share entitling to the same proportion in such distributions.
The board of directors may resolve that Holdco pays out an interim dividend to the shareholders, subject to the conditions of article 461-3 of the 1915 Law and Holdco’s articles of association. The board of directors shall set the amount and the date of payment of the interim dividend.
Any share premium, assimilated premium or other distributable reserve may be freely distributed to the shareholders subject to the provisions of the 1915 Law and Holdco’s articles of association. In case of a dividend payment, each shareholder is entitled to receive a dividend right pro rata according to his or her respective shareholding. The dividend entitlement lapses upon the expiration of a five-year prescription period from the date of the dividend distribution. The unclaimed dividends return to Holdco’s accounts.
199
Table of Contents
Comparison of Shareholder Rights
| | Delaware | | Luxembourg |
SHAREHOLDER APPROVAL OF BUSINESS COMBINATIONS | | Generally, under the DGCL, completion of a merger, consolidation, dissolution, or the sale, lease, or exchange of substantially all of a corporation’s assets requires approval by the board of directors and by a majority (unless the certificate of incorporation requires a higher percentage) of outstanding stock of the corporation entitled to vote. Mergers in which less than 20% of the acquirer’s stock is issued generally do not require acquirer stockholder approval. Mergers in which one corporation owns 90% or more of a second corporation may be completed without the vote of the second corporation’s board of directors or stockholders. The DGCL also requires a special vote of stockholders in connection with a business combination with an “interested stockholder” as defined in section 203 of the DGCL. | | Under Luxembourg law and the amended and restated articles of association of Holdco to be adopted in connection with the Business Combination (the “Holdco Articles”), the board of directors has the broadest powers to take any action necessary or useful to achieve Holdco’s object. The board of directors’ powers are limited only by law and Holdco’s articles of association. Any type of business combination that would require an amendment to the articles of association, such as a merger, de-merger, dissolution, or voluntary liquidation, requires an extraordinary resolution of a general meeting of shareholders. Transactions such as a sale, lease, or exchange of substantial company assets require only the approval of the board of directors. Neither Luxembourg law nor Holdco Articles contain any provision requiring the board of directors to obtain shareholder approval of a sale, lease, or exchange of substantial assets of Holdco. |
SPECIAL VOTE REQUIRED FOR COMBINATIONS WITH INTERESTED SHAREHOLDERS | | Section 203 of the DGCL generally prohibits a Delaware corporation from engaging in specified corporate transactions (such as mergers, stock and asset sales, and loans) with an “interested stockholder” for three years following the time that the stockholder becomes an interested stockholder. Subject to specified exceptions, an “interested stockholder” is a person or group that owns 15% or more of the corporation’s outstanding voting stock (including any rights to acquire stock pursuant to an option, warrant, agreement, arrangement, or understanding, or upon the exercise of conversion or exchange rights, and stock with respect to which the person has voting rights only), or is an affiliate or associate of the corporation and was the owner of 15% or more of the voting stock at any time within the previous three years. | | Under Luxembourg law, no restriction exists as to the transactions that a shareholder may engage in with Holdco. The transaction must, however, be in Holdco’s corporate interest, which for instance requires that the transactions are made on arm’s length terms. |
200
Table of Contents
| | Delaware | | Luxembourg |
SHAREHOLDER RIGHTS PLAN | | Under the DGCL, the certificate of incorporation of a corporation may give the board of directors the right to issue new classes of preferred shares with voting, conversion, dividend distribution, and other rights to be determined by the board of directors at the time of issuance, which could prevent a takeover attempt and thereby preclude stockholders from realizing a potential premium over the market value of their shares. In addition, Delaware law does not prohibit a corporation from adopting a stockholder rights plan, or “poison pill,” which could prevent a takeover attempt and also preclude stockholders from realizing a potential premium over the market value of their shares. | | Pursuant to Luxembourg law, the shareholders may create an authorized share capital which allows the board of directors to increase the issued share capital in one or several tranches with or without share premium, against payment in cash or in kind, by conversion of claims on Holdco or in any other manner for any reason whatsoever including (i) issue subscription and/or conversion rights in relation to new shares or instruments within the limits of the authorised capital under the terms and conditions of warrants (which may be separate or linked to shares, bonds, notes or similar instruments issued by Holdco), convertible bonds, notes or similar instruments; (ii) determine the place and date of the issue or successive issues, the issue price, the terms and conditions of the subscription of and paying up on the new shares and instruments and (iii) remove or limit the statutory preferential subscription right of the shareholders in case of issue against payment in cash or shares, warrants (which may be separate or attached to shares, bonds, notes or similar instruments), convertible bonds, notes or similar instruments within the limits of such authorized share capital. The board of directors may be further authorized to, under certain conditions, limit, restrict, or waive preferential subscription rights of existing shareholders when issuing new shares within the authorized share capital. The rights attached to the new shares issued within the authorized share capital will be equal to those attached to existing shares and set forth in the articles of association. |
201
Table of Contents
| | Delaware | | Luxembourg |
| | | | | In addition, the board of directors may be further authorized to make an allotment of existing or newly issued shares without consideration to (a) employees of Holdco or certain categories amongst those; (b) employees of companies or economic interest grouping in which Holdco holds directly or indirectly at least fifty per cent (50%) of the share capital or voting rights; (c) employees of companies or economic interest grouping in which at least fifty per cent (50%) of the share capital or voting rights is held directly or indirectly by a company which holds directly or indirectly at least fifty per cent (50%) of the share capital of Holdco; (d) members of the corporate bodies of Holdco or of the companies or economic interest grouping listed in point (b) to (c) above or certain categories amongst those. The authorization to the board of directors to issue additional shares or other instruments as described above within the authorized share capital (and to limit, restrict, or waive, as the case may be, preferential subscription rights) as well as the authorization to allot shares without consideration may be valid for a period of up to five years, starting from either the date of the deed of incorporation of the Company or of the minutes of the extraordinary general meeting resolving upon such authorization. The authorization may be renewed, increased or reduced by a resolution of the extraordinary general meeting of shareholders, with the quorum and majority rules set for the amendment of the articles of association. |
202
Table of Contents
| | Delaware | | Luxembourg |
| | | | | Holdco’s Articles authorize its board of directors to issue Holdco Ordinary Shares within the limits of the authorized share capital at such times and on such terms as the board of directors or its delegates may decide for a period ending five years after the date of the creation of the authorized share capital unless such period is extended, amended or renewed. Accordingly, the board of directors is authorized to issue Holdco Ordinary Shares up to the limits of authorized share capital until such date. Holdco currently intends to seek renewals and/or extensions as required from time to time. |
APPRAISAL RIGHTS | | Under the DGCL, a stockholder of a corporation participating in some types of major corporate transactions may, under varying circumstances, be entitled to appraisal rights pursuant to which the stockholder may receive cash in the amount of the fair market value of his or her shares in lieu of the consideration he or she would otherwise receive in the transaction. | | Neither Luxembourg law nor Holdco Articles provide for appraisal rights. |
SHAREHOLDER CONSENT TO ACTION WITHOUT MEETING | | Under the DGCL, unless otherwise provided in a corporation’s certificate of incorporation, any action that may be taken at a meeting of stockholders may be taken without a meeting, without prior notice, and without a vote if the holders of outstanding stock, having not less than the minimum number of votes that would be necessary to authorize such action, consent in writing. | | A shareholder meeting must always be called if the matter to be considered requires a shareholder resolution under Luxembourg law or Holdco Articles. Pursuant to Luxembourg law, shareholders of a public limited liability company may not take actions by written consent. All shareholder actions must be approved at an actual meeting of shareholders held before a notary public or under private seal, depending on the nature of the matter. Shareholders may vote in person, by proxy or, if the articles of association provide for that possibility, by correspondence. Holdco Articles provide for the possibility of vote by correspondence. |
203
Table of Contents
| | Delaware | | Luxembourg |
MEETINGS OF SHAREHOLDERS | | LightJump’s bylaws provide that annual meetings of stockholders are to be held on a date and at a time fixed by the board of directors. Under the DGCL, a special meeting of stockholders may be called by the board of directors or by any other person authorized to do so in the certificate of incorporation or the bylaws. LightJump’s bylaws provide that a special meeting of the stockholders of LightJump may only be called only by the majority of the entire Board of Directors, the Chief Executive Officer or the President or the Chairman of the Board of Directors of LightJump, and may not be called by any other person. Under the DGCL, a corporation’s certificate of incorporation or bylaws can specify the number of shares that constitute the quorum required to conduct business at a meeting, provided that in no event shall a quorum consist of less than one-third of the shares entitled to vote at a meeting. LightJump’s Bylaws provide that at a stockholders meeting the holders of shares of outstanding capital stock of LightJump representing a majority of the voting power of all outstanding shares of capital stock of LightJump entitled to vote at such meeting shall constitute a quorum for the transaction of business at such meeting, however, if such a quorum is not present LightJump’s bylaws provide that the holders of a majority of the votes entitled to be cast by the stockholders entitled to vote shall have power to adjourn the meeting from time to time, without notice other than announcement at the meeting, until a quorum shall be present or represented. | | Pursuant to Luxembourg law, at least one general meeting of shareholders must be held each year, within six months as from the close of the financial year. The purpose of such annual general meeting is to approve the annual accounts, allocate the results, proceed to statutory appointments and resolve on the discharge of the directors. Other general meetings of shareholders may be convened. Luxembourg law distinguishes between ordinary resolutions to be adopted and extraordinary resolutions to be adopted by the general meeting of shareholders. Extraordinary resolutions relate to proposed amendments to the articles of association and other limited matters. All other resolutions are ordinary resolutions. Pursuant to Luxembourg law, there is no requirement of a quorum for any ordinary resolutions to be considered at a general meeting and such ordinary resolutions shall be adopted by a simple majority of votes validly cast on such resolution. Abstentions are not considered “votes.” Extraordinary resolutions are required for any of the following matters, among others: (i) an increase or decrease of the authorized or issued share capital, (ii) a limitation or exclusion of preemptive rights, (iii) approval of a statutory merger or de-merger (scission), (iv) dissolution, (v) an amendment of the articles of association and (vi) change of nationality. |
204
Table of Contents
| | Delaware | | Luxembourg |
| | | | | Pursuant to Luxembourg law for any extraordinary resolutions to be considered at a general meeting, the quorum shall be at least one half (50%) of the issued share capital. If the said quorum is not present, a second meeting may be convened at which Luxembourg law does not prescribe a quorum. Any extraordinary resolution shall be adopted at a quorate general meeting (except as otherwise provided by mandatory law) by a two-thirds majority of the votes validly cast on such resolution by shareholders. Abstentions are not considered “votes.” 1915 Law provides that if, as a result of losses, net assets fall below half of the share capital of the company, the board of directors shall convene an extraordinary general meeting of shareholders so that it is held within a period not exceeding two months from the time at which the loss was or should have been ascertained by them and such meeting shall resolve on the possible dissolution of the company and possibly on other measures announced in the agenda. The board of directors shall, in such situation, draw up a special report which sets out the causes of that situation and justify its proposals eight days before the extraordinary general meeting. If it proposes to continue to conduct business, it shall set out in the report the measures it intends to take in order to remedy the financial situation of the company. The same rules apply if, as a result of losses, net assets fall below one-quarter of the share capital provided that in such case dissolution shall take place if approved by one-fourth of the votes casts at the extraordinary general meeting. |
205
Table of Contents
| | Delaware | | Luxembourg |
DISTRIBUTIONS AND DIVIDENDS; REPURCHASES AND REDEMPTIONS | | Under the DGCL, the board of directors, subject to any restrictions in the corporation’s certificate of incorporation, may declare and pay dividends out of: • surplus of the corporation, which is defined as net assets less statutory capital; or • if no surplus exists, out of the net profits of the corporation for the year in which the dividend is declared and/or the preceding year. If, however, the capital of the corporation has been diminished by depreciation in the value of its property, or by losses, or otherwise, to an amount less than the aggregate amount of capital represented by the issued and outstanding stock of all classes having a preference upon the distribution of assets, the board of directors shall not declare and pay dividends out of the corporation’s net profits until the deficiency in the capital has been repaired. Under the DGCL, any corporation may purchase or redeem its own shares, except that generally it may not purchase or redeem these shares if such repurchase or redemption would impair the capital of the corporation. A corporation may, however, purchase or redeem out of capital any of its own shares which are entitled upon any distribution of its assets to a preference over another class or series of its shares if such shares will be retired and the capital reduced. Pursuant to the LightJump Amended and Restated Certificate of Incorporation, LightJump will provide all holders of shares of Common Stock sold in the IPO the opportunity to demand that LightJump convert the IPO Shares into cash. If so demanded, LightJump shall, promptly after consummation of the Business Combination, convert such shares into cash at a per share price equal to the quotient determined by dividing | | Under Luxembourg law, the amount and payment of dividends or other distributions is determined by a simple majority vote at a general shareholders’ meeting based on the recommendation of the board of directors, except in certain limited circumstances. Pursuant to Holdco Articles, the board of directors has the power to pay interim dividends or make other distributions in accordance with applicable Luxembourg law. Distributions may be lawfully declared and paid if Holdco’s net profits and/or distributable reserves are sufficient under Luxembourg law. All Holdco Ordinary Shares rank pan passu with respect to the payment of dividends or other distributions unless the right to dividends or other distributions has been suspended in accordance with Holdco Articles or applicable law. Under Luxembourg law, at least 5% of Holdco’s net profits per year must be allocated to the creation of a legal reserve until such reserve has reached an amount equal to 10% of Holdco’s issued share capital. The allocation to the legal reserve becomes compulsory again when the legal reserve no longer represents 10% of Holdco’s issued share capital. The legal reserve is not available for distribution. Pursuant to Luxembourg law, Holdco (or any party acting on its behalf) may repurchase its own shares and hold them in treasury, provided that: • the shareholders at a general meeting have previously authorized the board of directors to acquire its ordinary shares. The general meeting shall determine the terms and conditions of the proposed acquisition and in particular the maximum number of shares to be acquired, the period for which the authorization is given (which may not exceed five years), and, in the case of acquisition for value, the maximum and minimum consideration; |
206
Table of Contents
| | Delaware | | Luxembourg |
| | | (i) the amount then held in the Trust Account including any interest earned on the funds held in the Trust Account net of interest that may be used by the Corporation to pay its franchise and income taxes payable, calculated as of two Business Days prior to the Business Combination, by (ii) the total number of IPO Shares then outstanding; provided, however, that the LightJump will only consummate the Business Combination if it has net tangible assets of at least $5,000,001 upon consummation of the Business Combination. | | • the acquisitions, including shares previously acquired by Holdco and held by it and shares acquired by a person acting in his or her own name but on Holdco’s behalf, may not have the effect of reducing the net assets below the amount of the issued share capital plus the reserves (which may not be distributed by law or under the articles of association); • the shares repurchased are fully paid-up; and • the acquisition offer must be made on the same terms and conditions to all the shareholders who are in the same position, except for acquisitions which were unanimously decided by a general meeting at which all the shareholders were present or represented. In addition, listed companies may repurchase their own shares on the stock exchange without an acquisition offer having to be made to Holdco’s shareholders. No prior authorization by shareholders is required (i) if the acquisition is made to prevent serious and imminent harm to Holdco, provided that the board of directors informs the next general meeting of the reasons for and the purpose of the acquisitions made, the number and nominal values or the accounting value of the shares acquired, the proportion of the subscribed capital which they represent, and the consideration paid for them, and (ii) in the case of shares acquired by either Holdco or by a person acting on its behalf with a view to redistributing the shares to its staff or staff of its controlled subsidiaries, provided that the distribution of such shares is made within twelve months from their acquisition. |
207
Table of Contents
| | Delaware | | Luxembourg |
| | | | | Luxembourg law provides for further situations in which the above conditions do not apply, including the acquisition of shares pursuant to a decision to reduce Holdco’s share capital or the acquisition of shares issued as redeemable shares. Such acquisitions may not have the effect of reducing net assets below the aggregate of subscribed capital and reserves (which may not be distributed by law) and are subject to specific provisions on reductions in share capital and redeemable shares under Luxembourg law. Any shares acquired in contravention of the above provisions must be resold within a period of one year after the acquisition or be cancelled at the expiration of the one-year period. As long as shares are held in treasury, the voting rights attached thereto are suspended. Further, to the extent the treasury shares are reflected as assets on Holdco’s balance sheet a non-distributable reserve of the same amount must be reflected as a liability. Holdco’s articles of association provide that Holdco Ordinary Shares may be acquired in accordance with the law. |
NUMBER OF DIRECTORS | | A typical certificate of incorporation and bylaws would provide that the number of directors on the board of directors will be fixed from time to time by a vote of the majority of the authorized directors. The Board consists of seven directors, divided into three classes, with only one class of directors being elected in each year, and each class serving a three-year term. | | Pursuant to Luxembourg law, the Holdco Board must be composed of at least three directors. They are appointed by the general meeting of shareholders (by proposal of the board of directors, the shareholders, or a spontaneous candidacy) by a simple majority of the votes cast. Directors may be reelected, but the term of their office may not exceed six years. Holdco Articles will provide that the board of directors shall be composed of at least six directors, to be elected by a simple majority vote at a general meeting. Abstentions are not considered “votes.” |
208
Table of Contents
| | Delaware | | Luxembourg |
VACANCIES ON BOARD OF DIRECTORS | | The LightJump Amended and Restated Certificate of Incorporation provides that any newly created directorships resulting from an increase in the number of directors and any vacancies on the Board may be filled solely and exclusively by a majority vote of the remaining directors then in office, even if less than a quorum, or by a sole remaining director (and not by stockholders). Any director so chosen shall hold office for the remainder of the full term of the class of directors in which the new directorship was added or in which the vacancy occurred and until such director’s successor has been duly elected and qualified. | | Holdco Articles provide that in case of a vacancy the remaining members of the board of directors may elect a director to fill the vacancy, on a temporary basis and for a period of time not exceeding the initial mandate of the replaced member of the board of directors, until the next general meeting of shareholders, which shall resolve on the permanent appointment in compliance with the applicable legal provisions and the articles of association. |
REMOVAL OF DIRECTORS; STAGGERED TERM OF DIRECTORS | | Under Delaware law, a board of directors can be divided into classes. The Board is divided into three classes, with only one class of directors being elected in each year and each class serving a three-year term. The LightJump Bylaws provide that directors may be removed with or without cause by a majority vote of the holders of the outstanding shares then entitled to vote at an election of directors. | | Under Luxembourg law, a director may be removed by the general meeting of shareholders (by proposal of the board of directors, the shareholders, or a spontaneous request) by a simple majority of the votes cast, with or without cause. Holdco Articles will provide for a minimum number of five directors. The duration of their mandate will not exceed the date of the annual general meeting of shareholders of Holdco of the next financial year following their appointment. |
COMMITTEES | | LightJump’s bylaws authorizes the Board to designate one or more committees by resolution of the Board. Each committee is to consist of one or more directors. | | Holdco Articles will provide that the board of directors may set up committees and determine their composition, powers, and rules. |
CUMULATIVE VOTING | | Under the DGCL, a corporation may adopt in its certificate of incorporation that its directors shall be elected by cumulative voting. When directors are elected by cumulative voting, a stockholder has a number of votes equal to the number of shares held by such stockholder multiplied by the number of directors nominated for election. The stockholder may cast all of such votes for one director or among the directors in any proportion. LightJump has not adopted cumulative voting rights. | | Not applicable. |
209
Table of Contents
| | Delaware | | Luxembourg |
AMENDMENT OF GOVERNING DOCUMENTS | | Under the DGCL, a certificate of incorporation may be amended if: • the board of directors sets forth the proposed amendment in a resolution, declares the advisability of the amendment and directs that it be submitted to a vote at a meeting of stockholders; and • the holders of at least a majority of shares of stock entitled to vote on the matter approve the amendment, unless the certificate of incorporation requires the vote of a greater number of shares. In addition, under the DGCL, class voting rights exist with respect to amendments to the charter that adversely affect the terms of the shares of a class. Class voting rights do not exist as to other extraordinary matters, unless the charter provides otherwise. Under the DGCL, the board of directors may amend a corporation’s bylaws if so authorized in the charter. The stockholders of a Delaware corporation also have the power to amend bylaws. | | Under Luxembourg law, amendments to Holdco’s articles of association require an extraordinary general meeting of shareholders held in front of a Luxembourg notary at which at least one half (50%) of the share capital is present or represented. The notice of the extraordinary general meeting shall set out the proposed amendments to the articles of association. If the aforementioned quorum is not reached, a second meeting may be convened by means of a notice published in the Luxembourg official electronic gazette (RESA) and in a Luxembourg newspaper 15 days before the meeting. The second meeting shall be validly constituted regardless of the proportion of the share capital present or represented. At both meetings, resolutions will be adopted if approved by at least two-thirds of the votes cast by shareholders (unless otherwise required by Luxembourg law or the articles of association). Where classes of shares exist and the resolution to be adopted by the general meeting of shareholders changes the respective rights attaching to such shares, the resolution will be adopted only if the conditions as to quorum and majority set out above are fulfilled with respect to each class of shares. An increase of the commitments of its shareholders requires the unanimous consent of the shareholders. Holdco Articles provide that for any extraordinary resolutions to be considered at a general meeting, the quorum shall be at least one-half of Holdco’s issued share capital. If said quorum is not present, a second meeting may be convened at which Luxembourg law does not prescribe a quorum. Any extraordinary resolution shall be adopted at a quorate general meeting (save as otherwise provided by mandatory law) by a two-thirds majority of the votes validly cast on such resolution by shareholders. Abstentions are not considered “votes.” |
210
Table of Contents
| | Delaware | | Luxembourg |
| | | | | In very limited circumstances, the board of directors may be authorized by the shareholders to amend the articles of association, albeit always within the limits set forth by the shareholders at a duly convened shareholders’ meeting. This is the case in the context of Holdco’s authorized share capital within which the board of directors is authorized to issue further Holdco Ordinary Shares. The board of directors is then authorized to appear in front of a Luxembourg notary to record the capital increase and to amend the share capital set forth in the articles of association. The above also applies in case of the transfer of Holdco’s registered office outside the current municipality. |
INDEMNIFICATION OF DIRECTORS AND OFFICERS | | The DGCL generally permits a corporation to indemnify its directors and officers against expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with a third-party action, other than a derivative action, and against expenses actually and reasonably incurred in the defense or settlement of a derivative action, provided that there is a determination made by the corporation that the individual acted in good faith and in a manner reasonably believed to be in or not opposed to the best interests of the corporation. Such determination shall be made, in the case of an individual who is a director or officer at the time of the determination: • by a majority of the disinterested directors, even though less than a quorum; • by a committee of disinterested directors designated by a majority vote of disinterested, directors, even though less than a quorum; • by independent legal counsel, regardless of whether a quorum of disinterested directors exists; or • by the stockholders. | | Luxembourg law permits Holdco to keep directors indemnified against any expenses, judgments, fines and amounts paid in connection with liability of a director towards Holdco or a third party for management errors i.e., for wrongful acts committed during the execution of the mandate (mandat) granted to the director by Holdco, except in connection with criminal offences, gross negligence or fraud. |
211
Table of Contents
| | Delaware | | Luxembourg |
| | | Without court approval, however, no indemnification may be made in respect of any derivative action in which an individual is adjudged liable to the corporation. The DGCL requires indemnification of directors and officers for expenses relating to a successful defense on the merits or otherwise of a derivative or third-party action. The DGCL permits a corporation to advance expenses relating to the defense of any proceeding to directors and officers contingent upon those individuals’ commitment to repay any advances, unless it is determined ultimately that those individuals are entitled to be indemnified. | | |
LIMITED LIABILITY OF DIRECTORS | | Delaware law permits limiting or eliminating the monetary liability of a director to a corporation or its stockholders, except with regard to breaches of duty of loyalty, intentional misconduct, unlawful repurchases or dividends, or improper personal benefit. | | Luxembourg law does not provide for an ex ante limitation of liability but it permits Holdco to keep directors indemnified as set out above. |
ADVANCE NOTIFICATION REQUIREMENTS FOR PROPOSALS OF SHAREHOLDERS | | Under the LightJump bylaws, any stockholder may bring proper business before an annual meeting, including nominations to the board of directors, but only if the stockholder gives timely notice, in writing and proper form, of the stockholder’s intention to bring the business before the meeting. | | One or several shareholders holding at least 10% of the share capital may request the addition of one or several items on the agenda of a general meeting. Such request must be addressed to the registered office of Holdco by registered mail at least five days before the general meeting. If one or more shareholders representing at least 10% of the share capital request so in writing, with an indication of the agenda, the convening of a general meeting, the board of directors or the statutory auditor must convene a general meeting. The general meeting must be held within a period of one month from receipt of such request. |
212
Table of Contents
| | Delaware | | Luxembourg |
SHAREHOLDERS’ SUITS | | Under Delaware law, a stockholder may bring a derivative action on a company’s behalf to enforce the rights of a company. An individual also may commence a class action lawsuit on behalf of himself or herself and other similarly situated stockholders if the requirements for maintaining a class action lawsuit under Delaware law are met. An individual may institute and maintain a class action lawsuit only if such person was a stockholder at the time of the transaction that is the subject of the lawsuit or his or her shares thereafter devolved upon him or her by operation of law. In addition, the plaintiff must generally be a stockholder through the duration of the lawsuit. Delaware law requires that a derivative plaintiff make a demand on the directors of the corporation to assert the corporate claim before the lawsuit may be prosecuted, unless such demand would be futile. This provision related to shareholders’ suits is a discussion only with respect to Delaware law and does not apply to claims arising under United States federal securities laws. | | Under Luxembourg law, the board of directors has sole authority to decide whether to initiate legal action to enforce a company’s rights (other than, in certain circumstances, an action against board members). Shareholders generally do not have the authority to initiate legal action on a company’s behalf unless the company fails abusively to exercise its legal rights. However, a company’s shareholders may vote at a general meeting to initiate legal action against directors on grounds that the directors have failed to perform their duties. Luxembourg law does not provide for class action lawsuits. However, it is possible for plaintiffs who have similar but separate claims against the same defendant(s) to bring an action on a “group” basis by way of a joint action. It is also possible to ask the court, under article 206 of the Luxembourg New Civil Procedure Code, to join claims which are closely related and to rule on them together. In addition, minority shareholders holding an aggregate of 10% of the voting rights and who voted against the discharge to a director at the annual general meeting of the company can initiate legal action against the director on behalf of the company. |
213
Table of Contents
Shares Eligible for Future Sale
Immediately following the Closing, Holdco will have [•] Holdco Ordinary Shares authorized and, based on the assumptions set out elsewhere in this proxy statement/prospectus, up to [•] Holdco Ordinary Shares issued and outstanding, assuming that (i) none of LightJump’s existing Public Stockholders exercise their redemption rights in connection with the approval of the Business Combination with respect to their Public Shares, (ii) no additional equity securities of LightJump are issued prior to the Closing and (iii) a Closing Date of [•]. In addition, Holdco is expected to have [•] warrants issued and outstanding, each warrant exercisable for one half Holdco Ordinary Share at $11.50 per share. All of the Holdco Ordinary Shares issued to the LightJump Holders in connection with the Business Combination will be freely transferable by persons other than by Holdco “affiliates” or LightJump’s “affiliates” without restriction or further registration under the Securities Act. Sales of substantial amounts of the Holdco Ordinary Shares in the public market could adversely affect prevailing market prices of the Holdco Ordinary Shares. Prior to the Business Combination, there has been no public market for Holdco Ordinary Shares. Holdco intends to apply for listing of the Holdco Ordinary Shares and Holdco Warrants on Nasdaq, but Holdco cannot assure you that a regular trading market will develop in the Holdco Ordinary Shares and Holdco Warrants.
Lock-up Agreements and Registration Rights
In connection with, and as a condition to the consummation of, the Business Combination, the Sponsor, Holdco, the CFO, the Company Shareholders and SAFE Holders will enter into the Registration Rights and Lock-Up Agreement pursuant to which, among other things, the Sponsor, the CFO and the Company Shareholders and SAFE holders shall have customary demand and piggyback registration rights in connection with the Holdco Ordinary Shares issued to them in the Merger or the Exchange. Additionally, the Holdco Ordinary Shares held by each party to the Registration Rights and Lock-Up Agreement will be subject to a lock-up until (i) the date that is 365 days from the Closing Date, and (ii) such date on which Holdco completes a liquidation, merger, share exchange or other similar transaction that results in all of the shareholders of Holdco having the right to exchange their Holdco Ordinary Shares for cash, securities or other property, provided that if the share price of the Holdco Ordinary Shares exceeds $12.00 per Holdco Ordinary Share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-day trading period, the parties to the Registration Rights and Lock-Up Agreement may transfer up to 50% of the Holdco Ordinary Shares subject to the Registration Rights and Lock-Up Agreement.
For more information about the Registration Rights and Lock-Up Agreement, see the section entitled “Certain Agreements Related to the Business Combination — Registration Rights and Lock-Up Agreement.”
Rule 144
All of Holdco’s equity shares that will be outstanding upon the completion of the Business Combination, other than those equity shares issued to the LightJump Holders in connection with the Business Combination, are “restricted securities” as that term is defined in Rule 144 under the Securities Act, including the shares issued to [Company Shareholders in the Exchanges], and may be sold publicly in the United States only if they are subject to an effective registration statement under the Securities Act or pursuant to an exemption from the registration requirement such as those provided by Rule 144 and Rule 701 promulgated under the Securities Act. In general, beginning 90 days after the date of this proxy statement/prospectus, a person (or persons whose shares are aggregated) who, at the time of a sale, is not, and has not been during the three months preceding the sale, an affiliate of Holdco and has beneficially owned Holdco’s restricted securities for at least six months will be entitled to sell the restricted securities without registration under the Securities Act, subject only to the availability of current public information about Holdco. Persons who are affiliates of Holdco and have beneficially owned Holdco’s restricted securities for at least six months may sell a number of restricted securities within any three-month period that does not exceed the greater of the following:
• 1% of the then outstanding equity shares of the same class; or
• the average weekly trading volume of Holdco Ordinary Shares of the same class during the four calendar weeks preceding the date on which notice of the sale is filed with the SEC.
Sales by affiliates of Holdco under Rule 144 are also subject to certain requirements relating to manner of sale, notice and the availability of current public information about Holdco.
214
Table of Contents
Restrictions on the Use of Rule 144 by Shell Companies or Former Shell Companies
Rule 144 is not available for the resale of securities initially issued by shell companies (other than business combination related shell companies) or issuers that have been at any time previously a shell company. However, Rule 144 also includes an important exception to this prohibition if the following conditions are met:
• the issuer of the securities that was formerly a shell company has ceased to be a shell company;
• the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act;
• the issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials); and
• at least one year has elapsed from the time that the issuer filed Form 20-F type information with the SEC, which is expected to be filed promptly after completion of the Business Combination, reflecting its status as an entity that is not a shell company.
Rule 701
[In general, under Rule 701 of the Securities Act as currently in effect, each of Moolec’s employees, consultants or advisors who purchases equity shares from Holdco in connection with a compensatory stock plan or other written agreement executed prior to the completion of the Business Combination is eligible to resell those equity shares in reliance on Rule 144, but without compliance with some of the restrictions, including the holding period, contained in Rule 144. However, the Rule 701 shares would remain subject to lock-up arrangements and would only become eligible for sale when the lock-up period expires.]
215
Table of Contents
Security Ownership of Certain Beneficial Owners and Management
LightJump
The following table sets forth information regarding the beneficial ownership of LightJump’s common stock as of the record date based on information obtained from the persons named below, with respect to the beneficial ownership of shares of LightJump’s common stock, by:
• each person known by LightJump to be the beneficial owner of more than 5% of the outstanding shares of SPAC Common Stock;
• each of LightJump’s executive officers and directors that beneficially owns shares of common stock; and
• all of LightJump’s officers and directors as a group.
The Sponsor and LightJump’s directors, officers, advisors or their affiliates may purchase shares of SPAC Common Stock in privately negotiated transactions or in the open market prior to the completion of the Business Combination, although they are under no obligation to do so and they have no current plans to do so. Such a purchase may include a contractual acknowledgement that such stockholder, although still the record holder of such shares, is no longer the beneficial owner thereof and therefore agrees not to exercise its redemption rights. In the event that the Sponsor and LightJump’s directors, officers, advisors or their affiliates purchase shares in privately negotiated transactions from LightJump Holders who have already elected to exercise their redemption rights, such selling stockholders would be required to revoke their prior elections to redeem their shares. Any such transaction would be separately negotiated at the time of the transaction. The consideration for any such transaction would consist of cash and/or SPAC Common Stock owned by the Sponsor and/or LightJump’s directors, officers, advisors, or their affiliates. The purpose of such purchases could be to vote such shares in favor of the Business Combination and thereby increase the likelihood of obtaining stockholder approval of the Business Combination. This may result in the completion of the Business Combination that may not otherwise have been possible.
In addition, if such purchases are made, the public “float” of the SPAC Common Stock and the number of beneficial holders of LightJump’s securities may be reduced, possibly making it difficult for Holdco to obtain the quotation, listing or trading of its securities on a national securities exchange.
As of the record date, there were 6,337,210 shares of common stock issued and outstanding. Unless otherwise indicated, all persons named in the table have sole voting and investment power with respect to all shares of common stock beneficially owned by them. The following table does not reflect record or beneficial ownership of the SPAC Warrants.
Name and Address of Beneficial Owner(1) | | Number
of Shares
Beneficially
Owned | | Percentage(2) |
Robert M. Bennett | | 3,450,000 | (3) | | 54.4 | % |
William W. Bunker | | — | (4) | | — | (4) |
Eric Ver Ploeg | | — | (4) | | — | (4) |
Patrick Brandt | | — | (4) | | — | (4) |
James W. Keyes | | — | (4) | | — | (4) |
Jeffrey A. Rich | | — | (4) | | — | (4) |
Andrew D. Hamer | | — | (4) | | — | (4) |
All directors and executive officers as a group (seven individuals) | | 3,450,000 | | | 54.4 | % |
LightJump One Founders, LLC 14755 Preston Road, Suite 520 Dallas, TX 75254 | | 3,450,000 | | | 54.4 | % |
All executive officers and directors as a group (7 individuals) | | | | | | |
216
Table of Contents
The table above does not include the shares of common stock issuable upon exercise of outstanding warrants because the warrants are not exercisable within 60 days of the record date for the Special Meeting.
The following table shows the beneficial ownership of Company Ordinary Shares as of [•], 2022 by:
• each person known by Moolec to beneficially own more than 5% of the outstanding Company Ordinary Shares;
• each of Moolec’s named executive officers and directors; and
• all of Moolec’s executive officers and directors as a group.
Unless otherwise indicated, Moolec believes that all persons named in the table have sole voting and investment power with respect to all shares beneficially owned by them. Except as otherwise noted herein, the number and percentage of Moolec Ordinary Shares beneficially owned is determined in accordance with Rule 13d-3 of the Exchange Act, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rule, beneficial ownership includes any Moolec Ordinary Shares as to which the holder has sole or shared voting power or investment power and also any Moolec Ordinary Shares which the holder has the right to acquire within 60 days of [•], 2022 through the exercise of any option, conversion or any other right. The table does not include stock options and restricted shares held by the executive officers that do not vest or become exercisable, and do not provide voting rights, within 60 days of the date of this proxy statement/prospectus. See the section entitled “Management of Moolec.” As of [•], 2022, there were 48,661,915 Company Ordinary Shares outstanding.
Name and Address of Beneficial Owner(1) | | Amount and
Nature of
Beneficial
Ownership | | Approximate
Percentage of
Outstanding
Shares |
Executive Officers and Directors | | | | | | |
Gastón Paladini(1) | | — | | | — | % |
José López Lecube(2) | | — | | | — | % |
Amit Dhingra(3) | | — | | | — | % |
Henk Hoogenkamp(4) | | — | (12) | | — | % |
Oscar Alejandro León Bentancor(5) | | — | | | — | % |
Juan Sartori(6) | | 22,871,100 | (8) | | 47.0 | % |
Federico Trucco(7) | | — | | | — | % |
| | | — | | | — | % |
All directors and executive officers as a group (eight individuals) | | 22,871,100 | | | 47.0 | % |
Five Percent or More Holders: | | | | | | |
BG Farming Technologies Ltd.(9)(10) | | 22,871,100 | | | 47.0 | % |
Union Group Ventures Limited(11) | | 22,871,100 | | | 47.0 | % |
Bioceres Crop Solutions Corp.(12) | | 2,919,715 | | | 6.0 | % |
217
Table of Contents
The following table shows the beneficial ownership of Holdco Ordinary Shares following the consummation of the Business Combination by:
• each person known to Holdco who will beneficially own more than 5% of the Holdco Ordinary Shares issued and outstanding immediately after the consummation of the Business Combination;
• each person who will become an executive officer or a director of Holdco upon consummation of the Business Combination; and
• all of the executive officers and directors of Holdco as a group upon consummation of the Business Combination.
Except as otherwise noted herein, the number and percentage of Holdco Ordinary Shares beneficially owned is determined in accordance with Rule 13d-3 of the Exchange Act, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rule, beneficial ownership includes any Holdco Ordinary Shares as to which the holder has sole or shared voting power or investment power and also any Holdco Ordinary Shares which the holder has the right to acquire within 60 days of [•], 2022 through the exercise of any option, warrant or any other right.
The expected beneficial ownership of Holdco Ordinary Shares post-Business Combination has been determined based upon the following: (i) none of LightJump’s existing Public Stockholders exercise their redemption rights in connection with the approval of the Business Combination with respect to their Public Shares, (ii) no additional equity securities of LightJump are issued prior to the Closing and (iii) a Closing Date of [•].
Name and Address of Beneficial Owner | | Amount and
Nature of
Beneficial
Ownership | | Approximate
Percentage of
Outstanding
Shares |
Gastón Paladini(1) | | — | | —% |
Jose López Lecube(2) | | 243,774 | | 0.6% |
Amit Dhingra(3) | | — | | —% |
Henk Hoogenkamp(4) | | — | | —% |
Oscar Alejandro León Bentancor(5) | | — | | —% |
[Directors] | | — | | —% |
| | | — | | —% |
| | | — | | —% |
All directors and executive officers as a group ([•] individuals) | | — | | —% |
Five Percent or More Holders: | | | | |
BG Farming Technologies Ltd.(6) | | | | % |
Union Group Ventures Limited | | | | % |
| | | | | % |
218
Table of Contents
Price Range of Securities and Dividends
LIGHTJUMP
Price Range of LightJump Securities
The following table shows, for the periods indicated, the high and low sales prices per share of the SPAC Units, SPAC Common Stock and SPAC Warrants as reported by Nasdaq. Prior to January 8, 2021, there was no established public trading market for LightJump’s securities.
| | SPAC Units | | SPAC Common Stock | | SPAC Warrants |
(in United States dollars) | | High | | Low | | High | | Low | | High | | Low |
Quarter Ended | | | | | | | | | | | | | | | | | | |
2021 | | | | | | | | | | | | | | | | | | |
First Quarter(1)(2) | | | 10.80 | | | 9.87 | | | 9.93 | | | 9.55 | | | 1.32 | | | 0.45 |
Second Quarter | | | 10.25 | | | 9.92 | | | 9.90 | | | 9.68 | | | 1.00 | | | 0.52 |
Third Quarter | | | 10.15 | | | 9.98 | | | 9.86 | | | 9.65 | | | 0.93 | | | 0.50 |
Fourth Quarter | | | 10.20 | | | 10.02 | | | 9.89 | | | 9.78 | | | 0.74 | | | 0.49 |
2022 | | | | | | | | | | | | | | | | | | |
First Quarter | | | 10.14 | | | 9.90 | | | 9.94 | | | 9.84 | | | 0.62 | | | 0.16 |
Second Quarter | | | 10.06 | | | 9.92 | | | 10.00 | | | 9.92 | | | 0.33 | | | 0.06 |
Third Quarter | | | 10.10 | | | 9.53 | | | 10.10 | | | 9.98 | | | 0.12 | | | 0.05 |
Fourth Quarter (through October 14, 2022)(3) | | $ | 10.11 | | $ | 10.08 | | $ | 10.10 | | $ | 10.06 | | $ | 0.07 | | $ | 0.06 |
Dividends
LightJump has not paid any cash dividends on the SPAC Common Stock to date and does not intend to pay cash dividends prior to the completion of the Business Combination.
Moolec
Price Range of Moolec Securities
Historical market price information regarding Moolec is not provided because Moolec is a privately held company and there is no public market for Moolec’s securities.
Dividends
Moolec has not paid any cash dividends on its shares to date and does not intend to pay cash dividends prior to the completion of the Business Combination.
Holdco
Price Range of Holdco Securities
Historical market price information regarding Holdco is not provided because there is no public market for its securities. We are applying to list the Holdco Ordinary Shares and Holdco Warrants on Nasdaq upon the Closing.
Dividends
Holdco has not paid any cash dividends to date and does not intend to pay cash dividends prior to the completion the Business Combination.
219
Table of Contents
Additional Information
Submission of Future Stockholder Proposals
LightJump’s board of directors is aware of no other matter that may be brought before the special meeting. Under Delaware law, only business that is specified in the notice of special meeting to stockholders may be transacted at the special meeting.
LightJump does not expect to hold a 2022 annual meeting of stockholders because it will not be a separate public company if the Business Combination is completed. Alternatively, if by January 12, 2023, LightJump does not consummate a business combination, or further amend the SPAC COI to extend the date by which it must consummate an initial business combination in accordance with the terms thereof, LightJump is required to begin the dissolution process provided for in the SPAC COI. LightJump will liquidate as soon as practicable following such dissolution and will conduct no annual meetings thereafter.
Delivery of Documents to Stockholders
Pursuant to the rules of the SEC, LightJump and servicers that it employs to deliver communications to its stockholders are permitted to deliver to two or more stockholders sharing the same address a single copy of the proxy statement. Upon written or oral request, LightJump will deliver a separate copy of the proxy statement to any stockholder at a shared address to which a single copy of the proxy statement was delivered and who wishes to receive separate copies in the future. Stockholders receiving multiple copies of the proxy statement may likewise request delivery of single copies of the proxy statement in the future. Stockholders may notify LightJump of their requests by calling or writing LightJump at its principal executive offices at (650) 515-3930, or 2735 Sand Hill Road, Suite 110, Menlo Park, California 94025.
Transfer Agent; Warrant Agent; Rights Agent and Registrar
The registrar and transfer agent for the shares of common stock of LightJump and Holdco and the warrant agent for LightJump’s warrants is Continental. LightJump has agreed to indemnify Continental in its roles as transfer agent, warrant agent, and units agent against all liabilities, including judgments, costs and reasonable counsel fees that may arise out of acts performed or omitted for its activities in that capacity, except for any liability due to any gross negligence, willful misconduct or bad faith of the indemnified person or entity.
Legal Matters
The validity of the Holdco Ordinary Shares to be issued in connection with the Business Combination will be passed upon by Linklaters LLP and the material U.S. federal income tax consequences of the Business Combination will be passed upon by K&L Gates.
Experts
The financial statements of Moolec Science Limited as of June 30, 2022 and 2021 and for the year ended June 30, 2022 and for each of the periods from January 1, 2021 through June 30, 2021 and from August 21, 2020 through December 31, 2020 included in this Proxy Statement/Prospectus have been so included in reliance on the report of Price Waterhouse & Co. S.R.L., an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The financial statements of LightJump Acquisition Corporation as of December 31, 2021 and 2020 and for the year ended December 31, 2021 and for the period from July 28, 2020 (inception) through December 31, 2020 included in this proxy statement/prospectus and elsewhere in the registration statement have been so included in reliance upon the report of Marcum LLP, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
220
Table of Contents
Where You Can Find More Information
As a foreign private issuer, after the consummation of the Business Combination, Holdco will be required to file its annual report on Form 20-F with the SEC no later than four months following its fiscal year end. LightJump files annual, quarterly and current reports, proxy statements and other information with the SEC as required by the Exchange Act. You can read LightJump’s SEC filings, including this proxy statement/prospectus, over the Internet at the SEC’s website at http://www.sec.gov.
All documents subsequently filed by LightJump pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, prior to the date on which the Special Meeting is held, shall be deemed to be incorporated by reference into this proxy statement/prospectus.
If you would like additional copies of this proxy statement/prospectus or if you have questions about the Business Combination or the proposals to be presented at the Extraordinary General Meeting, you should contact us by telephone or in writing:
LightJump Acquisition Corporation
2735 Sand Hill Road, Suite 110
Menlo Park, California 94025
Telephone: (650) 515-3930
You may also obtain these documents by requesting them in writing or by telephone from LightJump’s proxy solicitation agent at the following address, telephone number and email:
Advantage Proxy, Inc.
P.O. Box 13581
Des Moines, WA 98198
Attn: Karen Smith
Toll Free Telephone: (877) 870-8565
Main Telephone: (206) 870-8565
E-mail: ksmith@advantageproxy.com
If you are a shareholder of LightJump and would like to request documents, please do so by , 2022 to receive them before the Special Meeting. If you request any documents from us, we will mail them to you by first class mail, or another equally prompt means.
All information in this proxy statement/prospectus relating to LightJump has been supplied by LightJump, and all such information relating to Moolec has been supplied by Moolec. Information provided by either LightJump or Moolec does not constitute any representation, estimate or projection of any other party.
Moolec does not file any annual, quarterly and current reports, proxy statements and other information with the SEC.
None of Moolec or LightJump has authorized anyone to give any information or make any representation about the Business Combination or their companies that is different from, or in addition to, that contained in this proxy statement/prospectus or in any of the materials that have been incorporated in this proxy statement/prospectus. Therefore, if anyone does give you information of this sort, you should not rely on it. If you are in a jurisdiction where offers to exchange or sell, or solicitations of offers to exchange or purchase, the securities offered by this proxy statement/prospectus or the solicitation of proxies is unlawful, or if you are a person to whom it is unlawful to direct these types of activities, then the offer presented in this proxy statement/prospectus does not extend to you.
The information contained in this proxy statement/prospectus speaks only as of the date of this proxy statement/prospectus unless the information specifically indicates that another date applies.
221
Table of Contents
INDEX TO FINANCIAL STATEMENTS
| | Page |
LIGHTJUMP ACQUISITION CORPORATION | | |
Unaudited Financial Statements | | |
For the six month period ended June 30, 2022 | | |
Condensed Balance Sheets as of June 30, 2022 (Unaudited) and December 31, 2021 (Audited) | | F-2 |
Unaudited Condensed Statements of Operations for the Three and Six Months Ended June 30, 2022 and June 30, 2021 | | F-3 |
Unaudited Condensed Statements of Changes in Stockholders’ Deficit for the Three and Six Months
Ended June 30, 2022 and June 30, 2021 | | F-4 |
Unaudited Condensed Statements of Cash Flows for the Six Months Ended June 30, 2022 and June 30, 2021 | | F-5 |
Notes to Unaudited Condensed Financial Statements | | F-6 |
| | | |
Audited Financial Statements | | |
For the year ended December 30, 2021 and the period from July 28, 2020 (inception) through December 31, 2020 | | |
Report of Independent Registered Public Accounting Firm | | F-24 |
Balance Sheets | | F-25 |
Statements of Operations | | F-26 |
Statements of Changes in Stockholders’ (Deficit) Equity | | F-27 |
Statements of Cash Flows | | F-28 |
Notes to Financial Statements | | F-29 |
F-1
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
CONDENSED BALANCE SHEETS
AS OF JUNE 30, 2022 AND DECEMBER 31, 2021
| | June 30,
2022
(Unaudited) | | December 31,
2021
(Audited) |
Assets | | | | | | | | |
Cash | | $ | 5,422 | | | $ | 137,163 | |
Prepaid expenses | | | 221,788 | | | | 19,030 | |
Total current assets | | | 227,210 | | | | 156,193 | |
Investment held in Trust Account | | | 138,200,919 | | | | 138,013,319 | |
Total Assets | | $ | 138,428,129 | | | $ | 138,169,512 | |
| | | | | | | | | |
Liabilities, Redeemable Common Stock, and Stockholders’ Deficit | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable and accrued operating expenses | | | 1,969,060 | | | | 730,607 | |
Due to related party | | | 300,000 | | | | 260,000 | |
Income tax payable | | | 3,679 | | | | — | |
Promissory note – related party | | | 891,000 | | | | 125,000 | |
Total current liabilities | | | 3,163,739 | | | | 1,115,607 | |
Warrant liability | | | 261,423 | | | | 2,198,205 | |
Total liabilities | | $ | 3,425,162 | | | $ | 3,313,812 | |
| | | | | | | | | |
Commitments and Contingencies (Note 7) | | | | | | | | |
Common stock subject to possible redemption, 13,800,000 shares at redemption value of $10.00 as of June 30, 2022 and December 31, 2021 | | | 138,000,000 | | | | 138,000,000 | |
| | | | | | | | | |
Stockholders’ Deficit | | | | | | | | |
Preferred stock, $0.0001 par value; 1,000,000 shares authorized; none issued and outstanding | | | — | | | | — | |
Common stock, $0.0001 par value; 99,000,000 shares authorized; 3,570,000 shares issued and outstanding (excluding 13,800,000 shares subject to possible redemption), as of June 30, 2022 and December 31, 2021 | | | 357 | | | | 357 | |
Additional paid-in capital | | | — | | | | — | |
Accumulated deficit | | | (2,997,390 | ) | | | (3,144,657 | ) |
Total Stockholders’ Deficit | | | (2,997,033 | ) | | | (3,144,300 | ) |
Total Liabilities, Redeemable Common Stock and Stockholders’ Deficit | | $ | 138,428,129 | | | $ | 138,169,512 | |
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-2
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
UNAUDITED CONDENSED STATEMENTS OF OPERATIONS
| | Three Months
Ended June 30, | | Six Months
Ended June 30, |
| | | 2022 | | 2021 | | 2022 | | 2021 |
Formation and operating costs | | $ | 1,171,347 | | | $ | 786,609 | | | $ | 1,973,436 | | | $ | 1,037,402 | |
Loss from operations | | | (1,171,347 | ) | | | (786,609 | ) | | | (1,973,436 | ) | | | (1,037,402 | ) |
| | | | | | | | | | | | | | | | | |
Other income (expense) | | | | | | | | | | | | | | | | |
Change in fair value of warrant liability | | | 920,990 | | | | (1,085,087 | ) | | | 1,936,782 | | | | 216,765 | |
Trust interest income | | | 175,297 | | | | 3,441 | | | | 187,600 | | | | 6,361 | |
Total other income (expense) | | | 1,096,287 | | | | (1,081,646 | ) | | | 2,124,382 | | | | 223,126 | |
| | | | | | | | | | | | | | | | | |
Income (loss) before income tax provision | | | (75,060 | ) | | | (1,868,255 | ) | | | 150,946 | | | | (814,276 | ) |
Income tax provision | | | (3,679 | ) | | | — | | | | (3,679 | ) | | | — | |
Net income (loss) | | $ | (78,739 | ) | | $ | (1,868,255 | ) | | $ | 147,267 | | | $ | (814,276 | ) |
| | | | | | | | | | | | | | | | | |
Basic and diluted weighted average shares outstanding, common stock subject to redemption | | | 13,800,000 | | | | 13,800,000 | | | | 13,800,000 | | | | 12,926,667 | |
Basic and diluted net income (loss) per share | | $ | (0.00 | ) | | $ | (0.11 | ) | | $ | 0.01 | | | $ | (0.05 | ) |
Basic and diluted weighted average shares outstanding, non-redeemable common stock | | | 3,570,000 | | | | 3,570,000 | | | | 3,570,000 | | | | 3,525,667 | |
Basic and diluted net income (loss) per share | | $ | (0.00 | ) | | $ | (0.11 | ) | | $ | 0.01 | | | $ | (0.05 | ) |
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-3
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
UNAUDITED CONDENSED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
THREE AND SIX MONTHS ENDED JUNE 30, 2022 AND 2021
| |
Common Stock
| | Additional
Paid-in
Capital | | Accumulated
Deficit | | Total
Stockholders’
Deficit |
Shares | | Amount | |
Balance as of December 31, 2021 | | 3,570,000 | | $ | 357 | | $ | — | | $ | (3,144,657 | ) | | $ | (3,144,300 | ) |
Net income | | — | | | — | | | — | | | 226,006 | | | | 226,006 | |
Balance as of March 31, 2022 | | 3,570,000 | | $ | 357 | | $ | — | | $ | (2,918,651 | ) | | $ | (2,918,294 | ) |
Net loss | | — | | | — | | | — | | | (78,739 | ) | | | (78,739 | ) |
Balance as of June 30, 2022 | | 3,570,000 | | $ | 357 | | $ | — | | $ | (2,997,390 | ) | | $ | (2,997,033 | ) |
| |
Common Stock
| | Additional
Paid-in
Capital | | Accumulated
Deficit | | Total
Stockholders’
Deficit |
| | | Shares | | Amount | |
Balance as of December 31, 2020 | | 3,570,000 | | $ | 357 | | $ | 25,843 | | | $ | (13,802 | ) | | $ | 12,398 | |
Sale of 4,210,000 Private Placement Warrants, net of fair value of warrant liability | | — | | | — | | | 348,263 | | | | — | | | | 348,263 | |
Remeasurement of common stock subject to redemption value | | — | | | — | | | (374,106 | ) | | | (3,091,047 | ) | | | (3,465,153 | ) |
Net income | | — | | | — | | | — | | | | 1,053,979 | | | | 1,053,979 | |
Balance as of March 31, 2021 | | 3,570,000 | | $ | 357 | | $ | — | | | $ | (2,050,870 | ) | | $ | (2,050,513 | ) |
Net loss | | — | | | — | | | — | | | | (1,868,255 | ) | | | (1,868,255 | ) |
Balance as of June 30, 2021 | | 3,570,000 | | $ | 357 | | $ | — | | | $ | (3,919,125 | ) | | $ | (3,918,768 | ) |
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-4
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
UNAUDITED CONDENSED STATEMENTS OF CASH FLOWS
| | Six Months Ended
June 30, |
| | | 2022 | | 2021 |
Cash flows from Operating Activities: | | | | | | | | |
Net income (loss) | | $ | 147,267 | | | $ | (814,276 | ) |
Adjustments to reconcile net income (loss) to net cash used in operating activities: | | | | | | | | |
Change in fair value of warrant liability | | | (1,936,782 | ) | | | (216,765 | ) |
Interest earned on cash and marketable securities held in Trust Account | | | (187,600 | ) | | | (6,361 | ) |
Changes in current assets and current liabilities: | | | | | | | | |
Prepaid expenses | | | (202,758 | ) | | | (244,094 | ) |
Accounts payable and accrued operating expenses | | | 1,238,453 | | | | 726,854 | |
Income tax payable | | | 3,679 | | | | — | |
Due to related party | | | 40,000 | | | | (160,000 | ) |
Net cash used in operating activities | | | (897,741 | ) | | | (714,642 | ) |
| | | | | | | | | |
Cash Flows from Investing Activities: | | | | | | | | |
Purchase of investment held in Trust | | | — | | | | (138,000,000 | ) |
Net cash used in investing activities | | | — | | | | (138,000,000 | ) |
| | | | | | | | | |
Cash flows from financing activities: | | | | | | | | |
Proceeds from initial public offering, net of underwriters’ fees | | | — | | | | 135,240,000 | |
Proceeds from promissory note from sponsor | | | 766,000 | | | | — | |
Proceeds from issuance of Private Placement Warrants | | | — | | | | 4,210,000 | |
Payment of deferred offering costs | | | — | | | | (403,894 | ) |
Net cash provided by financing activities | | | 766,000 | | | | 139,046,106 | |
| | | | | | | | | |
Net change in cash | | | (131,741 | ) | | | 331,464 | |
Cash, beginning of the period | | | 137,163 | | | | 6,139 | |
Cash, end of the period | | $ | 5,422 | | | $ | 337,603 | |
| | | | | | | | | |
Supplemental disclosure of noncash investing and financing activities: | | | | | | | | |
Initial value of common stock subject to possible redemption | | $ | — | | | $ | 138,000,000 | |
Initial classification of warrant liability | | $ | — | | | $ | 3,531,517 | |
Offering costs included in accrued offering costs | | $ | — | | | $ | — | |
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-5
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1 — Organization, Business Operations and Liquidity
Organization and General
LightJump Acquisition Corporation (the “Company”) is a newly organized blank check company incorporated as a Delaware Company on July 28, 2020. The Company was formed for the purpose of acquiring, merging with, engaging in capital stock exchange with, purchasing all or substantially all of the assets of, engaging in contractual arrangements, or engaging in any other similar business combination with a single operating entity, or one or more related or unrelated operating entities operating in any sector (“Business Combination”).
The Company has selected December 31 as its fiscal year end.
As of June 30, 2022, the Company had not commenced any operations. All activity from July 28, 2020 (inception) through June 30, 2022, relates to the Company’s formation and the initial public offering described below. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company generates non-operating income in the form of interest income on cash and cash equivalents from the proceeds derived from the Initial Public Offering (“IPO”) and will recognize changes in the fair value of its warrant liability as other income (expense).
The Company’s sponsor is LightJump One Founders, LLC, a Delaware limited liability company (the “Sponsor”).
Financing
The registration statement for the Company’s IPO was declared effective on January 8, 2021 (the “Effective Date”). On January 12, 2021, the Company consummated the IPO of 12,000,000 units (each, a “Unit” and collectively, the “Units”) at $10.00 per Unit, generating gross proceeds of $120,000,000, which is discussed in Note 3.
Simultaneously with the closing of the IPO, the Company consummated the sale of 3,850,000 warrants (the “Private Warrants”), at a price of $1.00 per Private Warrant (the “Private Placement”), which is discussed in Note 4.
On January 15, 2021, the underwriters exercised the over-allotment option in full to purchase 1,800,000 Units (the “Over-allotment Units”), generating aggregate gross proceeds of $18,000,000. Simultaneously with the closing of the sale of the Over-allotment Units, the Company consummated the sale of an additional 360,000 Private Warrants at a price of $1.00 per Private Placement Warrant in a private placement to the Sponsor, generating gross proceeds of $360,000.
Transaction costs of the IPO including the exercise of over-allotment amounted to $3,465,153 consisting of $2,760,000 of underwriting fees and $705,153 of other offering costs.
Trust Account
Following the closing of the IPO on January 12, 2021 and the exercise of over-allotment on January 15, 2021, $138,000,000 from the net proceeds of the sale of the Units in the IPO and the sale of the Private Warrants was placed in a trust account (the “Trust Account”) and invested in U.S. government securities, with a maturity of 180 days or less or in money market funds meeting certain conditions under Rule 2a-7 under the Investment Company Act, which invest only in direct U.S. government treasury obligations. Except with respect to interest earned on the funds held in the trust account that may be released to the Company to pay its tax obligations, the proceeds from this Initial Public Offering will not be released from the trust account until the earlier of: (a) the completion of the Company’s initial business combination, (b) the redemption of the Company’s public shares if the Company are unable to complete its initial business combination in the required time period.
F-6
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1 — Organization, Business Operations and Liquidity (cont.)
Initial Business Combination
The Company’s management has broad discretion with respect to the specific application of the net proceeds of the IPO, although substantially all of the net proceeds are intended to be applied generally toward consummating a Business Combination. There is no assurance that the Company will be able to complete a Business Combination successfully. The Company must complete one or more Initial Business Combinations having an aggregate fair market value of at least 80% of the assets held in the Trust Account (as defined below) (excluding the taxes payable on interest earned and less any interest earned thereon that is released for taxes) at the time of the agreement to enter into the Initial Business Combination. However, the Company will only complete a Business Combination if the post-transaction company owns or acquires 50% or more of the outstanding voting securities of the target or otherwise acquires an interest in the target sufficient for it not to be required to register as an investment company under the Investment Company Act 1940, as amended (the “Investment Company Act”).
In connection with any proposed initial Business Combination, the Company will either (1) seek stockholder approval of such initial Business Combination at a meeting called for such purpose at which public stockholder may seek to convert their public shares, regardless of whether they vote for or against the proposed business combination or don’t vote at all, into their pro rata share of the aggregate amount then on deposit in the trust account (net of taxes payable), or (2) provide its public stockholder with the opportunity to sell their public shares to the Company by means of a tender offer (and thereby avoid the need for a stockholder vote) for an amount equal to their pro rata share of the aggregate amount then on deposit in the trust account (net of taxes payable), in each case subject to the limitations described herein.
If the Company determines to engage in a tender offer, such tender offer will be structured so that each stockholder may tender all of his, her or its shares rather than some pro rata portion of his, her or its shares. The decision as to whether the Company will seek stockholder approval of a proposed business combination or will allow stockholder to sell their shares to the Company in a tender offer will be made by the Company, solely in the Company’s discretion, and will be based on a variety of factors such as the timing of the transaction and whether the terms of the transaction would otherwise require us to seek stockholder approval. If the Company determines to allow stockholder to sell their shares to the Company in a tender offer, it will file tender offer documents with the SEC which will contain substantially the same financial and other information about the initial business combination as is required under the SEC’s proxy rules.
The common stock subject to redemption is recorded at a redemption value and classified as temporary equity upon the completion of the IPO, in accordance with Accounting Standards Codification (“ASC”) Topic 480 “Distinguishing Liabilities from Equity.” In such case, the Company will proceed with a Business Combination if the Company has net tangible assets of at least $5,000,001 upon such consummation of a Business Combination and, if the Company seeks stockholder approval, a majority of the issued and outstanding shares voted are voted in favor of the Business Combination.
If a stockholder vote is not required by law and the Company does not decide to hold a stockholder vote for business or other legal reasons, the Company will, pursuant to its Amended and Restated Certificate of Incorporation (the “Amended and Restated Certificate of Incorporation”), conduct the redemptions pursuant to the tender offer rules of the SEC and file tender offer documents with the SEC prior to completing a Business Combination.
If, however, stockholder approval of the transactions is required by law, or the Company decides to obtain stockholder approval for business or legal reasons, the Company will offer to redeem shares in conjunction with a proxy solicitation pursuant to the proxy rules and not pursuant to the tender offer rules. Additionally, each public stockholder may elect to redeem their Public Shares irrespective of whether they vote for or against the proposed transaction.
Notwithstanding the foregoing redemption rights, if the Company seeks stockholder approval of its initial business combination and the Company does not conduct redemptions in connection with its initial business combination pursuant to the tender offer rules, the Amended and Restated Certificate of Incorporation provides that
F-7
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1 — Organization, Business Operations and Liquidity (cont.)
a public stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert or as a “group” (as defined under Section 13 of the Exchange Act), will be restricted from redeeming its shares with respect to more than an aggregate of 15% of the shares sold in our initial public offering, without the Company’s prior consent.
The Company’s sponsor, officers and directors (the “initial stockholder”) have agreed not to propose any amendment to Amended and Restated Certificate of Incorporation that would affect the Company’s public stockholder’s ability to convert or sell their shares to the Company in connection with a business combination as described herein or affect the substance or timing of our obligation to redeem 100% of its public shares if the Company does not complete a business combination within 18 months from the closing of the IPO (the “Combination Period”) unless the Company provides its public stockholder with the opportunity to convert their shares of common stock upon the approval of any such amendment at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account, including interest not previously released to the Company but net of franchise and income taxes payable, divided by the number of then outstanding public shares.
If the Company is unable to complete its initial Business Combination within the Combination Period, the Company will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem 100% of the outstanding public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account, including any interest not previously released to the Company (net of taxes payable), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the Company’s remaining stockholder and its board of directors, dissolve and liquidate, subject (in the case of (ii) and (iii) above) to the Company’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. The Company cannot assure you that it will have funds sufficient to pay or provide for all creditors’ claims.
The Company’s initial stockholder agreed to waive their rights to liquidating distributions from the Trust Account with respect to any founder shares held by them if the Company fails to complete its initial business combination within the Combination Period. However, if the initial stockholder acquires public shares in or after the IPO, they will be entitled to liquidating distributions from the Trust Account with respect to such public shares if the Company fails to complete a Business Combination during the Combination Period.
Business Combination Agreement
On June 14, 2022, the Company, Moolec Science Limited, a private limited company incorporated under the laws of England and Wales (the “Moolec”), Moolec Science SA, a public limited liability company (société anonyme) governed by the laws of the Grand Duchy of Luxembourg with its registered office at 17, Boulevard F.W. Raiffeisen, L-2411 Luxembourg, Grand Duchy of Luxembourg and registered with the Luxembourg Trade and Companies’ Register (Registre de Commerce et des Sociétés, Luxembourg) under number B268440 (“Holdco”), and Moolec Acquisition, Inc., a Delaware corporation (“Merger Sub”) entered into a Business Combination Agreement (the “Business Combination Agreement”).
Pursuant to the Business Combination Agreement, Moolec, Holdco, Merger Sub and the Company will enter into a business combination transaction pursuant to which, among other things, (a) pursuant to the Exchange Agreements, each of the Moolec Shareholders, effective on the Exchange Effective Time, will contribute its respective Moolec Ordinary Shares to Holdco in exchange for Holdco Ordinary Shares to be subscribed for by each such Moolec Shareholder (such contributions and exchanges of the Moolec Ordinary Shares for Holdco Ordinary Shares, collectively, the “Exchange”), (b) as a result of the Exchange, the Moolec will become a wholly-owned subsidiary of Holdco, (c) immediately prior to the consummation of the Merger but after the Exchange Effective Time, each of the Moolec SAFE Holders will receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the applicable Moolec SAFE, (d) following the consummation of the Exchange,
F-8
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1 — Organization, Business Operations and Liquidity (cont.)
Merger Sub will merge with and into Company, with Company surviving such merger and becoming a direct wholly-owned subsidiary of Holdco (the “Merger”) and, in the context of the Merger, all Company Common Stock outstanding shall be converted into the right to receive the Merger Consideration in the form of Holdco Ordinary Shares pursuant to a share capital increase of Holdco, as set forth in this Agreement, and (e) in order to satisfy the Moolec’s obligations under that certain Consulting Agreement, dated June 18, 2021, by and between the Moolec and the Moolec’s Chief Financial Officer (“CFO”), CFO will be freely allotted an aggregate of 243,774 Holdco Ordinary Shares (the “CFO Free Shares”). Capitalized terms used but not defined herein shall have the respective meanings set forth in the Business Combination Agreement.
Upon the terms and subject to the conditions set forth in the Business Combination Agreement and the Exchange Agreements at the Exchange Effective Time, the Exchange will take place based on an exchange ratio of .66787343 used to determine the number of aggregate Holdco Shares valued at $10.00 per Holdco Share for which the aggregate Moolec Ordinary Shares will be exchanged (the “Exchange Consideration”). The valuation of the Moolec Ordinary Shares contributed to Holdco by the Moolec Shareholders against new Holdco Shares pursuant to the Exchange shall be deemed to be, as of the Exchange Effective Time, the sum of US$325,000,000.
Pursuant to the Exchange Agreements, each Moolec Shareholder has also agreed to not transfer any of its Moolec Ordinary Shares before the earlier to occur of the Exchange and the termination of the Business Combination Agreement pursuant to its terms.
Backstop Agreement
Concurrently with the execution of the Business Combination Agreement, Union Group Ventures Limited, THEO I SCSp, UG Holdings, LLC and Sponsor entered into the Backstop Agreement (the “Backstop Agreement”), pursuant to which, among other things, the parties guaranteed, on a several (and not joint) basis, to backstop an aggregate amount equal to $10,000,000 after taking into account the EarlyBird Fee, conditioned upon Closing on the terms and subject to the conditions set forth in the Backstop Agreement.
Amendment to Business Combination Marketing Agreement
On January 8, 2020, the Company entered into that certain Business Combination Marketing Agreement (“BCMA”) with EarlyBirdCapital, Inc. (“EBC”). It is a condition to the consummation of the transactions contemplated by the Business Combination Agreement that the Company and EBC enter into an amendment to the BCMA to provide for, among other things, a change in the compensation to be payable to EBC upon the Closing of the business combination. On June 14, 2022, the Company and EBC executed an amendment to the BCMA (the “Amendment”) whereby the Company shall pay to EBC (A) a cash fee at Closing equal to (i) 20% of the aggregate gross proceeds (up to a maximum of $3,830,000) held in the Trust Account (after redemptions and reduction of all additional payments included in the Trust Account to accommodate all extensions) and received by the Company in any financing in connection with the Business Combination regardless of the source of such funds, plus (ii) $1,000,000 and (B) in consideration of EBC introducing Moolec to the Company, Holdco shall issue to EBC a number of ordinary shares of Holdco equal to $2,000,000 divided by the lesser of (y) the volume weighted average price of Holdco’s ordinary shares for the ten trading days preceding the six month anniversary of the Closing and (z) $10.00, up to a maximum of 600,000 shares (the “Share Fee”). The Share Fee shall be issued to EBC within five business days of the six month anniversary of the Closing, and Holdco shall register the resale of the ordinary shares issued to EBC as promptly as practicable after their issuance. The Sponsor shall forfeit to Holdco for cancellation the same number of shares of common stock payable to EBC under such Share Fee.
Liquidity, Capital Resources and Going Concern
As of June 30, 2022, the Company had $5,422 in its operating bank account and a working capital deficit of approximately $2.6 million.
F-9
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1 — Organization, Business Operations and Liquidity (cont.)
Prior to the completion of the IPO, the Company’s liquidity needs had been satisfied through a payment from the Sponsor of $25,000 (see Note 5) in exchange for the Founder Shares to cover certain offering costs, and a loan under an unsecured promissory note from the Sponsor of $125,000 (see Note 5). Subsequent to the consummation of the IPO and Private Placement, the Company’s liquidity needs have been satisfied through the proceeds from the consummation of the Private Placement not held in the Trust Account.
An affiliate of the Company’s chief executive officer has agreed to loan the Company an aggregate of up to $150,000 to cover expenses related to the Initial Public Offering pursuant to a promissory note (see Note 5). This loan is non-interest bearing and payable on demand. The Company had drawn down $125,000 in 2020, which was still outstanding as of June 30, 2022. The Company issued several additional promissory notes to related parties in principal of an aggregate of $766,000. All notes are not interest bearing and are repayable upon conclusion of business combination. The principal balance may be prepaid at any time.
In addition, in order to finance transaction costs in connection with a Business Combination, the Company’s Sponsor or an affiliate of the Sponsor or certain of the Company’s officers and directors may, but are not obligated to, provide the Company Working Capital Loans (see Note 5). To date, there were no amounts outstanding under any Working Capital Loans.
The Company can raise additional capital through Working Capital Loans (as defined in Note 5) from the initial stockholders, the Company’s officers, directors, or their respective affiliates (which is described in Note 5), or through loans from third parties. None of the Sponsor, officers or directors are under any obligation to advance funds to, or to invest in, the Company. If the Company is unable to raise additional capital, it may be required to take additional measures to conserve liquidity, which could include, but not necessarily be limited to, curtailing operations, suspending the pursuit of its business plan, and reducing overhead expenses. The Company cannot provide any assurance that new financing will be available to it on commercially acceptable terms, if at all.
On July 8, 2022, the Sponsor issued a promissory note to Moolec, pursuant to which, Moolec agreed to loan to the Sponsor up to an aggregate principal amount of $350,000 (the “Extension Funds”) to deposit into the Company’s Trust Account in connection with the extension of the Company’s termination date from July 12, 2022 to January 12, 2023 (or such earlier date as determined by the Board) (the “Extension Combination Period”).
The Company anticipates that the $5,422 held outside of the trust account as of June 30, 2022, might not be sufficient to allow the Company to operate until January 12, 2023, the period it has to consummate an initial business combination, assuming that a business combination is not consummated during that time. Until consummation of our business combination, the Company will be using the funds not held in the trust account, and any additional Working Capital Loans from the initial stockholders, the Company’s officers and directors, or their respective affiliates, for identifying and evaluating prospective acquisition candidates, performing business due diligence on prospective target businesses, traveling to and from the offices, plants or similar locations of prospective target businesses, reviewing corporate documents and material agreements of prospective target businesses, selecting the target business to acquire and structuring, negotiating and consummating the business combination.
If the Company is unable to complete its initial Business Combination within the Extension Combination Period, the Company will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem 100% of the outstanding public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account, including any interest not previously released to the Company (net of taxes payable), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the Company’s remaining stockholder and its board of directors, dissolve and liquidate, subject (in the case of (ii) and (iii) above) to the Company’s
F-10
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1 — Organization, Business Operations and Liquidity (cont.)
obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. It is not certain that The Company would be able to complete a business combination with the period of Initial Business Combination and Company cannot assure that it will have funds sufficient to pay or provide for creditors’ claims.
These conditions raise substantial doubt about the Company’s ability to continue as a going concern through the next 12 months, if a business combination is not consummated. These unaudited condensed financial statements do not include any adjustments relating to the recovery of the recorded assets of the classification of the liabilities that might be necessary should the Company be unable to continue as a going concern.
Risks and Uncertainties
Management is currently evaluating the impact of the COVID-19 pandemic and the Russian-Ukraine war and has concluded that while it is reasonably possible that the virus and war could have a negative effect on the Company’s financial position, results of its operations, and/or search for a target company, the specific impact is not readily determinable as of the date of these unaudited condensed financial statements. The unaudited condensed financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 2 — Basis of Presentation and Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed financial statements are presented in U.S. dollars in conformity with accounting principles generally accepted in the United States of America (“GAAP”) for financial information and pursuant to the rules and regulations of the SEC. Accordingly, they do not include all of the information and footnotes required by GAAP. In the opinion of management, the unaudited condensed financial statements reflect all adjustments, which include only normal recurring adjustments necessary for the fair statement of the balances and results for the periods presented. Operating results for the three and six months ended June 30, 2022 is not necessarily indicative of the results that may be expected through December 31, 2022.
The accompanying unaudited condensed financial statements should be read in conjunction with the audited financial statements and notes thereto included in the Form 10-K filed by the Company with the SEC on April 12, 2022.
Emerging Growth Company Status
The Company is an “emerging growth company”, as defined in Section 2(a) of the Securities Act of 1933, as amended, (the “Securities Act”), as modified by the Jumpstart Business Startups Act of 2012, (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. The Company intends to take advantage of the benefits of this extended transition period.
F-11
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 2 — Basis of Presentation and Significant Accounting Policies (cont.)
Use of Estimates
The preparation of these unaudited condensed financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the unaudited condensed financial statements and the reported amounts of expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the unaudited condensed financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Actual results could differ from those estimates.
Cash
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash and cash equivalents. The Company did not have any cash equivalents at June 30, 2022 and December 31, 2021.
Investment in Trust Account
The proceeds held in the Trust Account will be invested in U.S. government securities, with a maturity of 180 days or less or in money market funds meeting certain conditions under Rule 2a-7 under the Investment Company Act, which invest only in direct U.S. government treasury obligations. Except with respect to interest earned on the funds held in the Trust Account that may be released to the Company to pay its tax obligations, the proceeds from our initial public offering will not be released from the Trust Account until the earlier of: (a) the completion of the Company’s initial business combination, (b) the redemption of the Company’s public shares if the Company is unable to complete its initial business combination within the Extension Combination Period.
At June 30, 2022 and December 31, 2021, the assets held in the Trust Account consisted of mutual funds in the amount of $138,200,919 and $138,013,319, respectively, and the Company had not withdrawn any of the interest income from the Trust Account to pay its tax obligations for the period from July 28, 2020 to June 30, 2022. The mutual funds held in the Trust Account were available for sale and reported at fair value.
The carrying value and fair value of mutual funds held in Trust Account on June 30, 2022 are as follows:
| | Carrying Value | | Fair Value |
U.S. Money Market | | $ | 138,200,919 | | $ | 138,200,919 |
The carrying value and fair value of mutual funds held in Trust Account on December 31, 2021 are as follows:
| | Carrying Value | | Fair Value |
U.S. Money Market | | $ | 138,013,319 | | $ | 138,013,319 |
Fair-Value Measurements
The fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC Topic 820, “Fair Value Measurement,” approximates the carrying amounts represented in the accompanying balance sheet, primarily due to their short-term nature. Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement
F-12
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 2 — Basis of Presentation and Significant Accounting Policies (cont.)
date. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
• Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets;
• Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and
• Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable.
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash accounts in a financial institution, which, at times, may exceed the Federal Depository Insurance Coverage of $250,000. At June 30, 2022 and December 31, 2021, the Company has not experienced losses on these accounts and management believes the Company is not exposed to significant risks on such accounts.
Common Stock Subject to Possible Redemption
The Company accounts for its common stock subject to possible redemption in accordance with the guidance in ASC Topic 480 “Distinguishing Liabilities from Equity.” Common stock subject to mandatory redemption (if any) is classified as a liability instrument and is measured at fair value. Conditionally redeemable common stock (including common stock that features redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) is classified as temporary equity. At all other times, common stock is classified as stockholders’ deficit. The Company’s common stock features certain redemption rights that are considered to be outside of the Company’s control and subject to the occurrence of uncertain future events. Accordingly, common stock subject to possible redemption is presented at redemption value as temporary equity, outside of the stockholders’ deficit section of the Company’s condensed balance sheet.
At June 30, 2022, the common stock reflected in the balance sheets are reconciled in the following table:
Gross Proceeds | | $ | 138,000,000 | |
Less: Proceeds allocated to Public Warrants | | | (5,795,688 | ) |
Less: Issuance costs related to common stock | | | (3,465,153 | ) |
Plus: Remeasurement of carrying value to redemption value | | | 9,260,841 | |
Common stock subject to possible redemption | | $ | 138,000,000 | |
Net Income (Loss) Per Common Share
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, Earnings Per Share. Net income (loss) per share is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the period. The Company has two classes of shares, redeemable common stock and non-redeemable common stock. Earnings and losses are shared pro rata between the two classes
F-13
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 2 — Basis of Presentation and Significant Accounting Policies (cont.)
of shares. The Company’s statement of operations applies the two-class method in calculating net income (loss) per share. Basic and diluted net income (loss) per common share for redeemable common stock and non-redeemable common stock is calculated by dividing net income (loss), allocated proportionally to each class of common stock, attributable to the Company by the weighted average number of shares of redeemable common stock and non-redeemable common stock outstanding.
The Company did not include the warrants as they are anti-dilutive. As a result, diluted income (loss) per share is the same as basic income (loss) per share for the period presented.
Accordingly, basic and diluted income (loss) per common share is calculated as follows:
| | Three Months ended
June 30, | | Six Months ended
June 30, |
| | | 2022 | | 2021 | | 2022 | | 2021 |
Common stock subject to possible redemption Numerator: | | | | | | | | | | | | | | | |
Net income (loss) allocable to common stock subject to possible redemption | | $ | (62,556 | ) | | $ | (1,484,279 | ) | | $ | 117,000 | | $ | (639,780 | ) |
Denominator: | | | | | | | | | | | | | | | |
Weighted average redeemable common stock, basic and diluted | | | 13,800,000 | | | | 13,800,000 | | | | 13,800,000 | | | 12,926,667 | |
Basic and diluted net income (loss) per stock, redeemable common stock | | $ | (0.00 | ) | | $ | (0.11 | ) | | $ | 0.01 | | $ | (0.05 | ) |
| | | | | | | | | | | | | | | | |
Non-redeemable common stock | | | | | | | | | | | | | | | |
Numerator: | | | | | | | | | | | | | | | |
Net income (loss) allocable to common stock not subject to redemption | | $ | (16,183 | ) | | $ | (383,976 | ) | | $ | 30,267 | | $ | (174,496 | ) |
Denominator: | | | | | | | | | | | | | | | |
Weighted average non-redeemable common stock, basic and diluted | | | 3,570,000 | | | | 3,570,000 | | | | 3,570,000 | | | 3,525,667 | |
Basic and diluted net income (loss) per stock, common stock | | $ | (0.00 | ) | | $ | (0.11 | ) | | $ | 0.01 | | $ | (0.05 | ) |
On July 8, 2022, LightJump held a special meeting of stockholders. At the meeting, LightJump’s stockholders approved the Extension Amendment extending the date by which LightJump must consummate its initial business combination from July 12, 2022 to January 12, 2023. Stockholders holding 11,032,790 Public Shares exercised their right to redeem such shares for a pro rata portion of the funds in the Trust Account. As a result, $110,507,220.68 (approximately $10.02 per share) was removed from the Trust Account to pay such holders. Following redemptions, LightJump had 2,767,210 Public Shares outstanding and the aggregate amount remaining in the Trust Account is $27,993,797.65 (which includes an additional $276,721 contributed by the Sponsor in connection with the Extension).
Offering Costs associated with the Initial Public Offering
The Company complies with the requirements of ASC 340-10-S99-1 and SEC Staff Accounting Bulletin (“SAB”) Topic 5A–“Expenses of Offering”. Offering costs consist principally of professional and registration fees incurred through the balance sheet date that are related to the IPO and were charged to stockholders’ deficit upon the completion of the IPO. The Company determined the Public Warrants qualified for equity treatment, and accordingly, offering costs in the aggregate of $3,465,153 have been charged to stockholders’ deficit (consisting of $2,760,000 of underwriting fees and $705,153 of other offering costs).
F-14
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 2 — Basis of Presentation and Significant Accounting Policies (cont.)
Warrant Liability
The Company accounts for the Public Warrants and Private Warrants (as defined in Note 1, 2 and 3) collectively (“Warrants”), as either equity or liability-classified instruments based on an assessment of the specific terms of the Warrants and the applicable authoritative guidance in Financial Accounting Standards Board (“FASB”) ASC 815, “Derivatives and Hedging”. The assessment considers whether the Warrants meet all of the requirements for equity classification under ASC 815, including whether the Warrants are indexed to the Company’s own common stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of issuance of the Warrants and as of each subsequent annual period end date while the Warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, such warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, such warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. Changes in the estimated fair value of liability-classified warrants are recognized as a non-cash gain or loss on the statements of operations.
The Company accounts for the Private Warrants in accordance with ASC 815-40 under which the Private Warrants do not meet the criteria for equity classification and must be recorded as liabilities. The fair value of the Private Warrants has been estimated using the Monte Carlo simulation model. See Note 6 for further discussion of the pertinent terms of the Warrants used to determine the value of the Private Warrants.
The Company evaluated the Public Warrants in accordance with ASC 815-40, “Derivatives and Hedging — Contracts in Entity’s Own Equity” and concluded that they met the criteria for equity classification and are required to be recorded as part a component of additional paid-in capital at the time of issuance.
Income Taxes
The Company accounts for income taxes under ASC 740, “Income Taxes.” ASC 740, Income Taxes, requires the recognition of deferred tax assets and liabilities for both the expected impact of differences between the unaudited condensed financial statements and tax basis of assets and liabilities and for the expected future tax benefit to be derived from tax loss and tax credit carry forwards. ASC 740 additionally requires a valuation allowance to be established when it is more likely than not that all or a portion of deferred tax assets will not be realized. As of June 30, 2022 and December 31, 2021, the Company’s deferred tax asset had a full valuation allowance recorded against it. Our effective tax rate was (4.90)% and 2.44% for the three and six months ended June 30, 2022, and 0.00% and 0.00% for the three and six months ended June 30, 2021. The effective tax rate differs from the statutory tax rate of 21% for the three and six months ended June 30, 2022, due to changes in fair of warrant liabilities, and the valuation allowance on the deferred tax assets.
ASC 740 also clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities. ASC 740 also provides guidance on derecognition, classification, interest and penalties, accounting in interim period, disclosure and transition.
The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of June 30, 2022 and December 31, 2021. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position.
F-15
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 2 — Basis of Presentation and Significant Accounting Policies (cont.)
The Company has identified the United States as its only “major” tax jurisdiction. The Company is subject to income taxation by major taxing authorities since inception. These examinations may include questioning the timing and amount of deductions, the nexus of income among various tax jurisdictions and compliance with federal and state tax laws. The Company’s management does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months.
Recent Accounting Standards
In August 2020, the FASB issued ASU 2020-06, Debt-Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU also removes certain settlement conditions that are required for equity-linked contracts to qualify for scope exception, and it simplifies the diluted earnings per share calculation in certain areas. The Company adopted ASU 2020-06 on January 1, 2022. The adoption did not impact the Company’s financial position, results of operations or cash flows.
Management does not believe that any other recently issued, but not effective, accounting standards, if currently adopted, would have a material effect on the Company’s unaudited condensed financial statements.
Note 3 — Initial Public Offering
Pursuant to the Initial Public Offering, the Company sold 12,000,000 Units at a price of $10.00 per Unit. Each Unit consists of one share of Common Stock, par value $0.0001 per share and one-half of one redeemable warrant (each, a “Public Warrant”). Each whole Public Warrant entitles the holder to purchase one share of Common Stock at a price of $11.50 per share. Each whole warrant will become exercisable 30 days after the completion of the initial Business Combination and will expire five years after the completion of the initial Business Combination, or earlier upon redemption or liquidation.
On January 15, 2021, the Underwriters exercised the over-allotment option in full to purchase 1,800,000 Units. The proceeds of $18,000,000 from the over-allotment was deposited in the Trust Account after deducting the underwriting fees.
The underwriters were paid a cash underwriting fee of 2.0% of the gross proceeds of the IPO and over-allotment, or $2,760,000 in the aggregate.
Note 4 — Private Placement
Simultaneously with the closing of the IPO, the Company’s Sponsor purchased an aggregate of 3,850,000 warrants at a price of $1.00 per warrant, for an aggregate purchase price of $3,850,000, in a private placement. The proceeds from the private placement of the Private Warrants were added to the proceeds of this Initial Public Offering and placed in a U.S.-based trust account maintained by Continental Stock Transfer & Trust Company, as trustee. If we do not complete an initial business combination within the Extension Combination Period, the proceeds from the sale of the Private Warrants will be included in the liquidating distribution to our public stockholders and the Private Warrants will be worthless.
On January 15, 2021, the Underwriters exercised the over-allotment option in full to purchase 1,800,000 Units. Simultaneously with the closing of the exercise of the overallotment option, the Company completed the private sale of an aggregate of 360,000 Private Warrants to the Sponsor at a purchase price of $1.00 per Private Placement Warrant, generating gross proceeds of $360,000.
F-16
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 5 — Related Party Transactions
Founder Shares
On September 11, 2020, the Sponsor paid $25,000, or approximately $0.009 per share, to cover certain offering costs in consideration for 2,875,000 shares of common stock, par value $0.0001 (the “Founder Shares”). On January 11, 2021, the Company effected a stock dividend of 0.2 shares for each share outstanding (the “Dividend”), resulting in there being an aggregate of 3,450,000 Founder Shares outstanding. All shares and associated amounts have been retroactively restated to reflect the stock dividend. Up to 450,000 Founder Shares were subject to forfeiture to the extent that the over-allotment option was not exercised in full by the underwriters. On January 15, 2021, the overallotment was exercised in full and all of the 450,000 shares were no longer subject to forfeiture.
Promissory Note — Related Party
An affiliate of the Company’s chief executive officer has agreed to loan the Company an aggregate of up to $150,000 to cover expenses related to the Initial Public Offering pursuant to a promissory note (the “Note”). This loan is non-interest bearing and payable on demand. The Company had drawn down $125,000 in 2020, which was still outstanding as of both June 30, 2022 and December 31, 2021.
During the first quarter of 2022, the Company entered into three additional promissory notes to related parties: on February 9, 2022, for the amount of $200,000, on February 25, 2022, for the amount of $203,000, and on March 22, 2022 for the amount of $230,000.
During the second quarter of 2022, the Company entered into three additional promissory notes to related parties: on April 7, 2022, for the amount of $3,000, on April 11, 2022, for the amount of $50,000, and on June 29, 2022 for the amount of $80,000.
All notes are not interest bearing and are repayable upon conclusion of business combination. The principal balance may be prepaid at any time.
Due to Related Party
As of June 30, 2022, the Due to Related Party balance of $300,000 represents the Administrative Support Expense (defined below) and additional funds from founders in December 2021.
Working Capital Loans
In order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (“Working Capital Loans”). If the Company completes a Business Combination, the Company will repay the Working Capital Loans. In the event that a Business Combination does not close, the Company may use a portion of proceeds held outside the Trust Account to repay the Working Capital Loans, but no proceeds held in the Trust Account would be used to repay the Working Capital Loans.
Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. The Working Capital Loans would either be repaid upon consummation of a Business Combination, without interest, or, at the lender’s discretion, up to $1.5 million of such Working Capital Loans may be convertible into Private Warrants at a price of $1.00 per warrant. As of June 30, 2022 and December 31, 2021, the Company had no borrowings under the Working Capital Loans.
F-17
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 5 — Related Party Transactions (cont.)
Administrative Support Agreement
Commencing on the date of the final prospectus, the Company has agreed to pay the Sponsor a total of $10,000 per month for office space, secretarial and administrative services (“Administrative Support Expense”). Upon completion of the initial Business Combination or the Company’s liquidation, the Company will cease paying these monthly fees. For the three and six months ended June 30, 2022 the company incurred an aggregate of $30,000 and $60,000 for administrative services. For the three and six and months ended June 30, 2021 the Company has incurred an aggregate of $30,000 and $60,000 for administrative services.
Note 6 — Fair Value Measurements
The following table presents information about the Company’s assets and liabilities that were measured at fair value on a recurring basis as of June 30, 2022, and indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value.
| | June 30,
2022 | | Quoted
Prices In
Active
Markets
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Other
Unobservable
Inputs
(Level 3) |
Assets: | | | | | | | | | | | | |
U.S. Money Market held in Trust Account | | $ | 138,200,919 | | $ | 138,200,919 | | $ | — | | $ | — |
Liabilities: | | | | | | | | | | | | |
Private Warrant Liability | | $ | 261,423 | | $ | — | | $ | — | | | 261,423 |
The following table presents information about the Company’s assets and liabilities that were measured at fair value on a recurring basis as of December 31, 2021, and indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value.
| | December 31,
2021 | | Quoted
Prices In
Active
Markets
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Other
Unobservable
Inputs
(Level 3) |
Assets: | | | | | | | | | | | | |
U.S. Money Market held in Trust Account | | $ | 138,013,319 | | $ | 138,013,319 | | $ | — | | $ | — |
Liabilities: | | | | | | | | | | | | |
Private Warrant Liability | | $ | 2,198,205 | | $ | — | | $ | — | | | 2,198,205 |
The Private Warrants were valued using a Monte Carlo simulation model, which is considered to be a Level 3 fair value measurement. Inherent in an options pricing model are assumptions related to expected stock-price volatility, expected life, risk-free interest rate and dividend yield. The Company estimates the volatility of its common stock based on historical volatility that matches the expected remaining life of the warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected remaining life of the warrants. The expected life of the warrants is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which the Company anticipates to remain at zero.
There were no transfers between Levels 1, 2 or 3 during the six months ended June 30, 2022.
F-18
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 6 — Fair Value Measurements (cont.)
The following table provides quantitative information regarding Level 3 fair value measurements for Private Warrants as of June 30, 2022 and December 31, 2021.
| | June 30,
2022 | | December 31,
2021 |
Exercise price | | 11.50 | | | $ | 11.50 | |
Share price | | 9.99 | | | $ | 9.86 | |
Volatility | | 5.6 | % | | | 9.7 | % |
Expected life | | 5.30 | | | | 5.37 | |
Risk-free rate | | 3.01 | % | | | 1.29 | % |
Dividend yield | | — | % | | | — | % |
The following table presents a summary of the changes in the fair value of the Private Warrants and Representative’s Warrants, a Level 3 liability, measured on a recurring basis for the three and six months ended June 30, 2022 and 2021.
| | Warrant
Liability |
Fair value, January 1, 2022 | | $ | 2,198,205 | |
Change in fair value | | | (1,015,792 | ) |
Fair value, March 31, 2022 | | $ | 1,182,413 | |
Change in fair value | | | (920,990 | ) |
Fair value, June 30, 2022 | | $ | 261,423 | |
| | Warrant
Liability |
Fair value, January 1, 2021 | | $ | — | |
Initial measurement on January 12, 2021, including over-allotment | | | 3,861,737 | |
Change in fair value | | | (1,301,852 | ) |
Fair value, March 31, 2021 | | $ | 2,559,885 | |
Change in fair value | | | 1,085,087 | |
Fair value, June 30, 2021 | | $ | 3,644,972 | |
Note 7 — Commitments and Contingencies
Registration Rights
The holders of the founders’ shares and representative shares issued and outstanding on the date of the Initial Public Offering, as well as the holders of the Private Warrants (and the underlying shares) and any warrants the Company’s sponsor, officers, directors or their affiliates may be issued in payment of working capital loans made to the Company (and all underlying securities), are entitled to registration rights pursuant to an agreement signed prior to or on the effective date of this Initial Public Offering. The holders of a majority of these securities are entitled to make up to two demands that the Company registers such securities. The holders of the majority of the founders’ shares, as well as the holders of the Private Warrants (and the underlying shares) can elect to exercise these registration rights at any time commencing three months prior to the date on which these shares of common stock are to be released from escrow. The holders of a majority of the representative shares, and warrants issued to our sponsor, officers, directors or their affiliates in payment of working capital loans made to the Company (or underlying securities) can elect to exercise these registration rights at any time after the Company consummates a business combination. Notwithstanding anything to the contrary, EarlyBirdCapital Inc. (“EarlyBirdCapital”) may only make a demand on one occasion and only during the five-year period beginning on the effective date of the registration statement of which this prospectus forms a part. In addition, the holders have certain “piggy-back”
F-19
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 7 — Commitments and Contingencies (cont.)
registration rights with respect to registration statements filed subsequent to the consummation of a business combination; provided, however, that EarlyBirdCapital may participate in a “piggy-back” registration only during the seven-year period beginning on the effective date of the registration statement of which this prospectus forms a part. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Business Combination Marketing Agreement
The Company has engaged EBC as an advisor in connection with our business combination to assist in holding meetings with stockholders to discuss the potential business combination and the target business’ attributes, introduce the Company to potential investors that are interested in purchasing its securities in connection with the business combination, assist in obtaining stockholder approval for the business combination and assist with press releases and public filings in connection with the business combination. The Company would pay EBC a cash fee for such services upon the consummation of the business combination in an amount equal to 3.5% of the gross proceeds of the Initial Public Offering. Additionally, the Company would pay EBC a cash fee equal to 1.0% of the total consideration payable in the proposed business combination if EBC introduces the Company to the target business with which it completes the business combination; provided that the foregoing fee would not be paid prior to the date that is 90 days from the effective date of the registration statement.
On June 14, 2022, the Company and EBC executed an amendment to the BCMA (the “Amendment”) whereby the Company shall pay to EBC (A) a cash fee at Closing equal to (i) 20% of the aggregate gross proceeds (up to a maximum of $3,830,000) held in the Trust Account (after redemptions and reduction of all additional payments included in the Trust Account to accommodate all extensions) and received by the Company in any financing in connection with the Business Combination regardless of the source of such funds, plus (ii) $1,000,000 and (B) in consideration of EBC introducing Moolec to the Company, Holdco shall issue to EBC a number of ordinary shares of Holdco equal to $2,000,000 divided by the lesser of (y) the volume weighted average price of Holdco’s ordinary shares for the ten trading days preceding the six month anniversary of the Closing and (z) $10.00, up to a maximum of 600,000 shares (the “Share Fee”). The Share Fee shall be issued to EBC within five business days of the six month anniversary of the Closing, and Holdco shall register the resale of the ordinary shares issued to EBC as promptly as practicable after their issuance. The Sponsor shall forfeit to Holdco for cancellation the same number of shares of common stock payable to EBC under such Share Fee.
As of June 30, 2022 and December 31, 2021, these fees are not accrued.
Representative Shares
On October 1, 2020, the Company issued to designees of EarlyBirdCapital Inc. the 120,000 representative shares for nominal consideration. The Company estimated the fair value of the stock to be $1,200 based upon the price of the founder shares issued to the Sponsor and were treated as underwriters’ compensation and charged directly to stockholders’ deficit. The holders of the representative shares have agreed not to transfer, assign or sell any such shares without the Company’s prior consent until the completion of its initial business combination. In addition, the holders of the representative shares have agreed (i) to waive their conversion rights (or right to participate in any tender offer) with respect to such shares in connection with the completion of our initial business combination and (ii) to waive their rights to liquidating distributions from the trust account with respect to such shares if the Company fails to complete an initial business combination within the Extension Combination Period.
The representative shares have been deemed compensation by FINRA and are therefore subject to a lock-up for a period of 180 days immediately following the date of the effectiveness of the registration statement of which this prospectus forms a part pursuant to Rule 5110(g)(1) of the FINRA Manual. Pursuant to FINRA Rule 5110(g)(1), these securities were not sold during the Initial Public Offering, or sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the economic disposition of the securities by any person for a period of 180 days immediately following the effective date of the registration statement of which this prospectus forms a part or commencement of sales of
F-20
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 7 — Commitments and Contingencies (cont.)
the public offering, except to any underwriter and selected dealer participating in the Initial Public Offering and their bona fide officers or partners, provided that all securities so transferred remain subject to the lockup restriction above for the remainder of the time period.
Promissory Note — Moolec
On July 8, 2022, the Sponsor issued a promissory note to Moolec, pursuant to which, Moolec agreed to loan to the Sponsor up to an aggregate principal amount of $350,000 to deposit into the Company’s Trust Account in connection with the extension of the Company’s termination date from July 12, 2022 to January 12, 2023 (or such earlier date as determined by the Board). The promissory note bears interest at 20.0% per annum and is repayable in full upon the earlier of (i) the consummation of the Initial Business Combination of the Company, (ii) the date of the termination of the Business Combination Agreement and (ii) January 12, 2023.
Note 8 — Warrants
Public Warrants
The Public Warrants will become exercisable 30 days after the completion of a Business Combination. However, no warrants will be exercisable for cash unless the Company has an effective and current registration statement covering the shares of common stock issuable upon exercise of the warrants and a current prospectus relating to such shares of common stock. Notwithstanding the foregoing, if a registration statement covering the shares of common stock issuable upon exercise of the public warrants is not effective within a specified period following the consummation of our initial business combination, warrant holders may, until such time as there is an effective registration statement and during any period when we shall have failed to maintain an effective registration statement, exercise warrants on a cashless basis pursuant to the exemption provided by Section 3(a)(9) of the Securities Act, provided that such exemption is available. If that exemption, or another exemption, is not available, holders will not be able to exercise their warrants on a cashless basis. In the event of such cashless exercise, each holder would pay the exercise price by surrendering the warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the difference between the exercise price of the warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose will mean the average reported last sale price of the shares of common stock for the 5 trading days ending on the trading day prior to the date of exercise. The warrants will expire on the fifth anniversary of our completion of an initial business combination, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation. The warrants will expire five years after the completion of a Business Combination or earlier upon redemption or liquidation.
The Company may call the Public Warrants for redemption:
• in whole and not in part;
• at a price of $0.01 per warrant;
• upon not less than 30 days’ prior written notice of redemption (the “30-day redemption period”) to each warrant holder; and
• if, and only if, the reported last sale price of the shares of common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations and recapitalizations), for any 20 trading days within a 30 trading day period commencing at any time after the warrants become exercisable and ending on the third business day prior to the notice of redemption to warrant holders; and
• if, and only if, there is a current registration statement in effect with respect to the shares of common stock underlying such warrants.
F-21
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 8 — Warrants (cont.)
If the Company calls the Public Warrants for redemption, management will have the option to require all holders that wish to exercise the Public Warrants to do so on a “cashless basis”, as described in the warrant agreement. In such event, each holder would pay the exercise price by surrendering the warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the difference between the exercise price of the warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose shall mean the average reported last sale price of the shares of common stock for the 5 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants.
In addition, if (x) the Company issues additional shares of common stock or equity-linked securities for capital raising purposes in connection with the closing of the initial business combination at an issue price or effective issue price of less than $9.20 per share of common stock (with such issue price or effective issue price to be determined in good faith by our board of directors, and in the case of any such issuance to our sponsor, initial stockholders or their affiliates, without taking into account any founders’ shares held by them prior to such issuance), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of the initial business combination on the date of the consummation of the initial business combination (net of redemptions), and (z) the Market Value is below $9.20 per share, the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the greater of (i) the Market Value or (ii) the price at which the Company issues the additional shares of common stock or equity-linked securities.
Private Warrants
The Private Warrants are identical to the Public Warrants underlying the Units being sold in the Initial Public Offering, except that the Private Warrants, so long as they are held by the Sponsor or their permitted transferees, (i) will not be redeemable by the Company, (ii) may be exercised for cash or on a cashless basis, as described in the Initial Public Offering, in each case so long as they are held by the initial purchasers or any of their permitted transferees. If the Private Warrants are held by holders other than the initial purchasers or any of their permitted transferees, the Private Warrants will be redeemable by the Company and exercisable by the holders on the same basis as the warrants included in the units being sold in this offering.
Note 9 — Stockholders’ Deficit
Preferred Stock — The Company is authorized to issue 1,000,000 shares of preferred stock with a par value of $0.0001 and with such designations, voting and other rights and preferences as may be determined from time to time by the Company’s board of directors. As of June 30, 2022 and December 31, 2021, there were no preferred stock issued or outstanding.
Common Stock — The Company is authorized to issue 99,000,000 shares of common stock with a par value of $0.0001 per share. Holders are entitled to one vote for each share of common stock. On January 11, 2021, the Company effected a stock dividend of 0.2 shares for each share outstanding, resulting in there being an aggregate of 3,450,000 Founder Shares outstanding. All shares and associated amounts have been retroactively restated to reflect the stock dividend. At June 30, 2022 and December 31, 2021, there were 3,570,000 shares of common stock issued and outstanding, excluding 13,800,000 shares subject to possible redemption at June 30, 2022 and December 31, 2021.
Note 10 — Subsequent Events
The Company evaluated subsequent events and transactions that occurred after the balance sheet date up to the date that the unaudited condensed financial statements were issued. Other than disclosed below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the unaudited condensed financial statements.
F-22
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 10 — Subsequent Events (cont.)
On July 8, 2022, LightJump held a special meeting of stockholders. At the meeting, LightJump’s stockholders approved the Extension Amendment extending the date by which LightJump must consummate its initial business combination from July 12, 2022 to January 12, 2023. Stockholders holding 11,032,790 Public Shares exercised their right to redeem such shares for a pro rata portion of the funds in the Trust Account. As a result, $110,507,220.68 (approximately $10.02 per share) was removed from the Trust Account to pay such holders. Following redemptions, LightJump had 2,767,210 Public Shares outstanding and the aggregate amount remaining in the Trust Account is $27,993,797.65 (which includes an additional $276,721 contributed by the Sponsor in connection with the Extension).
On July 8, 2022, the Sponsor issued a promissory note to Moolec, pursuant to which, Moolec agreed to loan to the Sponsor up to an aggregate principal amount of $350,000 to deposit into the Company’s Trust Account in connection with the extension of the Company’s termination date from July 12, 2022 to January 12, 2023 (or such earlier date as determined by the Board). The promissory note bears interest at 20.0% per annum and is repayable in full upon the earlier of (i) the consummation of the Initial Business Combination of the Company, (ii) the date of the termination of the Business Combination Agreement and (ii) January 12, 2023.
F-23
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors of
LightJump Acquisition Corporation
Opinion on the Financial Statements
We have audited the accompanying balance sheets of LightJump Acquisition Corporation (the “Company”) as of December 31, 2021 and 2020, the related statements of operations, changes in stockholders’ equity (deficit) and cash flows for the year ended December 31, 2021 and for the period from July 28, 2020 (inception) through December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for the year ended December 31, 2021 and for the period from July 28, 2020 (inception) through December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph — Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 1 to the financial statements, the Company’s business plan is dependent on the completion of a business combination and the Company’s cash and working capital as of December 31, 2021 are not sufficient to complete its planned activities. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provides a reasonable basis for our opinion.
/s/ Marcum llp
Marcum llp
We have served as the Company’s auditor since 2020.
New York, NY
April 12, 2022
F-24
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
BALANCE SHEETS
FOR THE YEARS ENDED DECEMBER 31, 2020 AND DECEMBER 31, 2021
| | December 31,
2021 | | December 31,
2020 |
Assets | | | | | | | | |
Cash | | $ | 137,163 | | | $ | 6,139 | |
Deferred offering costs | | | — | | | | 306,259 | |
Prepaid expenses | | | 19,030 | | | | — | |
Total current assets | | | 156,193 | | | | 312,398 | |
Investment held in Trust Account | | | 138,013,319 | | | | — | |
Total Assets | | $ | 138,169,512 | | | $ | 312,398 | |
| | | | | | | | | |
Liabilities, Redeemable Common Stock, and Stockholders’ (Deficit) Equity | | | | | | | | |
Current liabilities: | | | | | | | | |
Accrued operating expenses | | | 730,607 | | | | — | |
Due to related party | | | 260,000 | | | | 170,000 | |
Promissory note – related party | | | 125,000 | | | | 125,000 | |
Accrued offering costs | | | — | | | | 5,000 | |
Total current liabilities | | | 1,115,607 | | | | 300,000 | |
Warrant liability | | | 2,198,205 | | | | — | |
Total liabilities | | $ | 3,313,812 | | | $ | 300,000 | |
| | | | | | | | | |
Commitments and Contingencies (Note 7) | | | | | | | | |
Common stock subject to possible redemption, 13,800,000 shares and 0 shares at redemption value of $10.00 as of December 31, 2021 and 2020, respectively | | | 138,000,000 | | | | — | |
| | | | | | | | | |
Stockholders’ (Deficit) Equity: | | | | | | | | |
Preferred stock, $0.0001 par value; 1,000,000 shares authorized; none issued and outstanding | | | — | | | | — | |
Common stock, $0.0001 par value; 99,000,000 shares authorized; 3,570,000 shares issued and outstanding (excluding 13,800,000 shares and 0 shares subject to possible redemption as of December 31, 2021 and 2020, respectively) | | | 357 | | | | 357 | |
Additional paid-in capital | | | — | | | | 25,843 | |
Accumulated deficit | | | (3,144,657 | ) | | | (13,802 | ) |
Total Stockholders’ (Deficit) Equity | | | (3,144,300 | ) | | | 12,398 | |
Total Liabilities, Redeemable Common Stock and Stockholders’ (Deficit) Equity | | $ | 138,169,512 | | | $ | 312,398 | |
The accompanying notes are an integral part of these financial statements.
F-25
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
STATEMENTS OF OPERATIONS
FOR THE PERIOD FROM JULY 28, 2020 THROUGH DECEMBER 31, 2020
AND DECEMBER 31, 2021
| | For the
Year
Ended
December 31,
2021 | | For the
period from July 28,
2020
(inception) through
December 31,
2020 |
Formation and operating costs | | $ | 1,716,659 | | | $ | 13,802 | |
Loss from operations | | | (1,716,659 | ) | | | (13,802 | ) |
| | | | | | | | | |
Other income | | | | | | | | |
Change in fair value of warrant liability | | | 1,663,532 | | | | — | |
Change in fair value of overallotment liability | | | (54,000 | ) | | | — | |
Trust interest income | | | 13,319 | | | | — | |
Total other income | | | 1,622,851 | | | | — | |
| | | | | | | | | |
Net loss | | $ | (93,808 | ) | | $ | (13,802 | ) |
| | | | | | | | | |
Basic and diluted weighted average shares outstanding, common stock subject to redemption | | | 13,369,315 | | | | — | |
Basic and diluted loss per share | | $ | (0.01 | ) | | $ | — | |
Basic and diluted weighted average shares outstanding, non-redeemable common stock | | | 3,552,740 | | | | 3,098,571 | |
Basic and diluted net loss per share | | $ | (0.01 | ) | | $ | (0.00 | ) |
The accompanying notes are an integral part of these financial statements.
F-26
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE PERIOD FROM JULY 28, 2020 (INCEPTION) THROUGH DECEMBER 31, 2020
AND FOR THE YEAR ENDED DECEMBER 31, 2021
| | Common Stock | | Additional Paid-in
Capital | | Accumulated
Deficit | | Total
Stockholders’
Equity
(Deficit) |
Shares | | Amount | |
Balance as of July 28, 2020 (inception) | | — | | $ | — | | $ | — | | | $ | — | | | $ | — | |
Common stock issued to Sponsor | | 3,450,000 | | | 345 | | | 24,655 | | | | — | | | | 25,000 | |
Issuance of 120,000 Representative shares to underwriters | | 120,000 | | | 12 | | | 1,188 | | | | — | | | | 1,200 | |
Net loss | | — | | | — | | | — | | | | (13,802 | ) | | | (13,802 | ) |
Balance as of December 31, 2020 | | 3,570,000 | | $ | 357 | | $ | 25,843 | | | $ | (13,802 | ) | | $ | 12,398 | |
Sale of 4,210,000 Private Placement Warrants, net of fair value of warrant liability | | — | | | — | | | 348,263 | | | | — | | | | 348,263 | |
Remeasurement of common stock subject to possible redemption | | — | | | — | | | (374,106 | ) | | | (3,037,047 | ) | | | (3,411,153 | ) |
Net loss | | — | | | — | | | — | | | | (93,808 | ) | | | (93,808 | ) |
Balance as of December 31, 2021 | | 3,570,000 | | $ | 357 | | $ | — | | | $ | (3,144,657 | ) | | $ | (3,144,300 | ) |
The accompanying notes are an integral part of these financial statements.
F-27
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
STATEMENTS OF CASH FLOWS
| | For the
Year Ended
December 31,
2021 | | For the
Period from
July 28,
2020
(inception)
through December 31,
2020 |
Cash flows from Operating Activities: | | | | | | | | |
Net loss | | $ | (93,808 | ) | | $ | (13,802 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
Change in fair value of warrant liability | | | (1,663,532 | ) | | | — | |
Change in fair value of overallotment of liability | | | 54,000 | | | | — | |
Interest earned on cash and marketable securities held in Trust Account | | | (13,319 | ) | | | — | |
Changes in current assets and current liabilities: | | | | | | | | |
Prepaid expenses | | | (19,030 | ) | | | — | |
Accrued operating expenses | | | 730,607 | | | | — | |
Accrued offering costs | | | — | | | | 5,000 | |
Due to related party | | | 90,000 | | | | — | |
Net cash used in operating activities | | | (915,082 | ) | | | (8,802 | ) |
| | | | | | | | | |
Cash Flows from Investing Activities: | | | | | | | | |
Purchase of investment held in Trust | | | (138,000,000 | ) | | | — | |
Net cash used in investing activities | | | (138,000,000 | ) | | | — | |
| | | | | | | | | |
Cash flows used by financing activities: | | | | | | | | |
Proceeds from initial public offering, net of underwriters’ fees | | | 135,240,000 | | | | — | |
Proceeds from issuance of Private Placement Warrants | | | 4,210,000 | | | | — | |
Payment of deferred offering costs | | | (403,894 | ) | | | — | |
Proceeds from issuance of founder shares | | | — | | | | 25,000 | |
Proceeds from issuance of representative shares | | | — | | | | 1,200 | |
Proceeds from advances from related party | | | — | | | | 170,000 | |
Proceeds from issuance of promissory note to related party | | | — | | | | 125,000 | |
Payment of deferred offering costs | | | — | | | | (306,259 | ) |
Net cash provided by financing activities | | | 139,046,106 | | | | 14,941 | |
| | | | | | | | | |
Net change in cash | | | 131,024 | | | | 6,139 | |
Cash, beginning of the period | | | 6,139 | | | | — | |
Cash, end of the period | | $ | 137,163 | | | $ | 6,139 | |
| | | | | | | | | |
Supplemental disclosure of noncash investing and financing activities: | | | | | | | | |
Initial value of common stock subject to possible redemption | | $ | 138,000,000 | | | $ | — | |
Initial classification of warrant liability | | $ | 3,861,737 | | | $ | — | |
Exercise of overallotment option charged to APIC | | $ | 396,000 | | | $ | — | |
Fair value of representative shares | | $ | — | | | $ | 1,200 | |
The accompanying notes are an integral part of these financial statements.
F-28
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 1 — Organization, Business Operations and Liquidity
Organization and General
LightJump Acquisition Corporation (the “Company”) is a newly organized blank check company incorporated as a Delaware Company on July 28, 2020. The Company was formed for the purpose of acquiring, merging with, engaging in capital stock exchange with, purchasing all or substantially all of the assets of, engaging in contractual arrangements, or engaging in any other similar business combination with a single operating entity, or one or more related or unrelated operating entities operating in any sector (“Business Combination”).
The Company has selected December 31 as its fiscal year end.
As of December 31, 2021, the Company had not commenced any operations. All activity from July 28, 2020 (inception) through December 31, 2021, relates to the Company’s formation and the initial public offering described below. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company generates non-operating income in the form of interest income on cash and cash equivalents from the proceeds derived from the Initial Public Offering (“IPO”) and will recognize changes in the fair value of its warrant liability as other income (expense).
The Company’s sponsor is LightJump One Founders, LLC, a Delaware limited liability company (the “Sponsor”).
Financing
The registration statement for the Company’s IPO was declared effective on January 8, 2021 (the “Effective Date”). On January 12, 2021, the Company consummated the IPO of 12,000,000 units (each, a “Unit” and collectively, the “Units”) at $10.00 per Unit, generating gross proceeds of $120,000,000, which is discussed in Note 3.
Simultaneously with the closing of the IPO, the Company consummated the sale of 3,850,000 warrants (the “Private Warrants”), at a price of $1.00 per Private Warrant (the “Private Placement”), which is discussed in Note 4.
On January 15, 2021, the underwriters exercised the over-allotment option in full to purchase 1,800,000 Units (the “Over-allotment Units”), generating aggregate gross proceeds of $18,000,000. Simultaneously with the closing of the sale of the Over-allotment Units, the Company consummated the sale of an additional 360,000 Private Warrants at a price of $1.00 per Private Placement Warrant in a private placement to the Sponsor, generating gross proceeds of $360,000.
Transaction costs of the IPO including the exercise of over-allotment amounted to $3,465,153 consisting of $2,760,000 of underwriting fees and $705,153 of other offering costs.
Trust Account
Following the closing of the IPO on January 12, 2021 and the exercise of over-allotment on January 15, 2021, $138,000,000 from the net proceeds of the sale of the Units in the IPO and the sale of the Private Warrants was placed in a trust account (the “Trust Account”) and invested in U.S. government securities, with a maturity of 180 days or less or in money market funds meeting certain conditions under Rule 2a-7 under the Investment Company Act, which invest only in direct U.S. government treasury obligations. Except with respect to interest earned on the funds held in the trust account that may be released to the Company to pay its tax obligations, the proceeds from this Initial Public Offering will not be released from the trust account until the earlier of: (a) the completion of the Company’s initial business combination, (b) the redemption of the Company’s public shares if the Company are unable to complete its initial business combination in the required time period.
F-29
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 1 — Organization, Business Operations and Liquidity (cont.)
Initial Business Combination
The Company’s management has broad discretion with respect to the specific application of the net proceeds of the IPO, although substantially all of the net proceeds are intended to be applied generally toward consummating a Business Combination. There is no assurance that the Company will be able to complete a Business Combination successfully. The Company must complete one or more Initial Business Combinations having an aggregate fair market value of at least 80% of the assets held in the Trust Account (as defined below) (excluding the taxes payable on interest earned and less any interest earned thereon that is released for taxes) at the time of the agreement to enter into the Initial Business Combination. However, the Company will only complete a Business Combination if the post-transaction company owns or acquires 50% or more of the outstanding voting securities of the target or otherwise acquires an interest in the target sufficient for it not to be required to register as an investment company under the Investment Company Act 1940, as amended (the “Investment Company Act”).
In connection with any proposed initial Business Combination, the Company will either (1) seek stockholder approval of such initial Business Combination at a meeting called for such purpose at which public stockholder may seek to convert their public shares, regardless of whether they vote for or against the proposed business combination or don’t vote at all, into their pro rata share of the aggregate amount then on deposit in the trust account (net of taxes payable), or (2) provide its public stockholder with the opportunity to sell their public shares to the Company by means of a tender offer (and thereby avoid the need for a stockholder vote) for an amount equal to their pro rata share of the aggregate amount then on deposit in the trust account (net of taxes payable), in each case subject to the limitations described herein.
If the Company determines to engage in a tender offer, such tender offer will be structured so that each stockholder may tender all of his, her or its shares rather than some pro rata portion of his, her or its shares. The decision as to whether the Company will seek stockholder approval of a proposed business combination or will allow stockholder to sell their shares to the Company in a tender offer will be made by the Company, solely in the Company’s discretion, and will be based on a variety of factors such as the timing of the transaction and whether the terms of the transaction would otherwise require us to seek stockholder approval. If the Company determines to allow stockholder to sell their shares to the Company in a tender offer, it will file tender offer documents with the SEC which will contain substantially the same financial and other information about the initial business combination as is required under the SEC’s proxy rules.
The common stock subject to redemption is recorded at a redemption value and classified as temporary equity upon the completion of the IPO, in accordance with Accounting Standards Codification (“ASC”) Topic 480 “Distinguishing Liabilities from Equity.” In such case, the Company will proceed with a Business Combination if the Company has net tangible assets of at least $5,000,001 upon such consummation of a Business Combination and, if the Company seeks stockholder approval, a majority of the issued and outstanding shares voted are voted in favor of the Business Combination.
If a stockholder vote is not required by law and the Company does not decide to hold a stockholder vote for business or other legal reasons, the Company will, pursuant to its Amended and Restated Certificate of Incorporation (the “Amended and Restated Certificate of Incorporation”), conduct the redemptions pursuant to the tender offer rules of the SEC and file tender offer documents with the SEC prior to completing a Business Combination.
If, however, stockholder approval of the transactions is required by law, or the Company decides to obtain stockholder approval for business or legal reasons, the Company will offer to redeem shares in conjunction with a proxy solicitation pursuant to the proxy rules and not pursuant to the tender offer rules. Additionally, each Public stockholder may elect to redeem their Public Shares irrespective of whether they vote for or against the proposed transaction.
Notwithstanding the foregoing redemption rights, if the Company seeks stockholder approval of its initial business combination and the Company does not conduct redemptions in connection with its initial business combination pursuant to the tender offer rules, the Amended and Restated Certificate of Incorporation provides that
F-30
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 1 — Organization, Business Operations and Liquidity (cont.)
a public stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert or as a “group” (as defined under Section 13 of the Exchange Act), will be restricted from redeeming its shares with respect to more than an aggregate of 15% of the shares sold in our initial public offering, without the Company’s prior consent.
The Company’s sponsor, officers and directors (the “initial stockholder”) have agreed not to propose any amendment to Amended and Restated Certificate of Incorporation that would affect the Company’s public stockholder’s ability to convert or sell their shares to the Company in connection with a business combination as described herein or affect the substance or timing of our obligation to redeem 100% of its public shares if the Company does not complete a business combination within 18 months from the closing of the IPO (the “Combination Period”) unless the Company provides its public stockholder with the opportunity to convert their shares of common stock upon the approval of any such amendment at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account, including interest not previously released to the Company but net of franchise and income taxes payable, divided by the number of then outstanding public shares.
If the Company is unable to complete its initial Business Combination within the Combination Period, the Company will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem 100% of the outstanding public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account, including any interest not previously released to the Company (net of taxes payable), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the Company’s remaining stockholder and its board of directors, dissolve and liquidate, subject (in the case of (ii) and (iii) above) to the Company’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. The Company cannot assure you that it will have funds sufficient to pay or provide for all creditors’ claims.
The Company’s initial stockholder agreed to waive their rights to liquidating distributions from the Trust Account with respect to any founder shares held by them if the Company fails to complete its initial business combination within the Combination Period. However, if the initial stockholder acquires public shares in or after the IPO, they will be entitled to liquidating distributions from the Trust Account with respect to such public shares if the Company fails to complete a Business Combination during the Combination Period.
Liquidity, Capital Resources and Going Concern
As of December 31, 2021, the Company had $137,163 in its operating bank account and a working capital deficit of approximately $0.9 million.
Prior to the completion of the IPO, the Company’s liquidity needs had been satisfied through a payment from the Sponsor of $25,000 (see Note 5) in exchange for the Founder Shares to cover certain offering costs, and a loan under an unsecured promissory note from the Sponsor of $125,000 (see Note 5). Subsequent to the consummation of the IPO and Private Placement, the Company’s liquidity needs have been satisfied through the proceeds from the consummation of the Private Placement not held in the Trust Account.
In addition, in order to finance transaction costs in connection with a Business Combination, the Company’s Sponsor or an affiliate of the Sponsor or certain of the Company’s officers and directors may, but are not obligated to, provide the Company Working Capital Loans (see Note 5). To date, there were no amounts outstanding under any Working Capital Loans.
The Company can raise additional capital through Working Capital Loans (as defined in Note 5) from the initial stockholders, the Company’s officers, directors, or their respective affiliates (which is described in Note 5), or through loans from third parties. None of the Sponsor, officers or directors are under any obligation to advance funds to, or to invest in, the Company. If the Company is unable to raise additional capital, it may be required to
F-31
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 1 — Organization, Business Operations and Liquidity (cont.)
take additional measures to conserve liquidity, which could include, but not necessarily be limited to, curtailing operations, suspending the pursuit of its business plan, and reducing overhead expenses. The Company cannot provide any assurance that new financing will be available to it on commercially acceptable terms, if at all.
The Company anticipates that the $137,163 held outside of the trust account as of December 31, 2021, might not be sufficient to allow the Company to operate until July 12, 2022, the period it has to consummate an initial business combination, assuming that a business combination is not consummated during that time. Until consummation of our business combination, the Company will be using the funds not held in the trust account, and any additional Working Capital Loans from the initial stockholders, the Company’s officers and directors, or their respective affiliates, for identifying and evaluating prospective acquisition candidates, performing business due diligence on prospective target businesses, traveling to and from the offices, plants or similar locations of prospective target businesses, reviewing corporate documents and material agreements of prospective target businesses, selecting the target business to acquire and structuring, negotiating and consummating the business combination.
The Company is required to complete its Initial Business Combination within 18 months from the date of IPO (January 12, 2021).
If the Company is unable to complete its initial Business Combination within the Combination Period, the Company will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem 100% of the outstanding public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account, including any interest not previously released to the Company (net of taxes payable), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the Company’s remaining stockholder and its board of directors, dissolve and liquidate, subject (in the case of (ii) and (iii) above) to the Company’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. It is not certain that The Company would be able to complete a business combination with the period of Initial Business Combination and Company cannot assure that it will have funds sufficient to pay or provide for creditors’ claims.
These conditions raise substantial doubt about the Company’s ability to continue as a going concern through the next 12 months, if a business combination is not consummated. These financial statements do not include any adjustments relating to the recovery of the recorded assets of the classification of the liabilities that might be necessary should the Company be unable to continue as a going concern.
Risks and Uncertainties
Management is currently evaluating the impact of the COVID-19 pandemic and has concluded that while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations, and/or search for a target company, the specific impact is not readily determinable as of the date of these financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 2 — Basis of Presentation and Significant Accounting Policies
Basis of Presentation
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”) for annual financial information and in accordance with the instructions to Form 10-K and Article 8 of Regulation S-X of the U.S. Securities and Exchange Commission (“SEC”). In the opinion of management, the accompanying financial statements include all adjustments, consisting of a normal recurring nature, which are necessary for a fair presentation of the financial position, operating results and cash flows for the periods presented.
F-32
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 2 — Basis of Presentation and Significant Accounting Policies (cont.)
Emerging Growth Company Status
The Company is an “emerging growth company”, as defined in Section 2(a) of the Securities Act of 1933, as amended, (the “Securities Act”), as modified by the Jumpstart Business Startups Act of 2012, (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. The Company intends to take advantage of the benefits of this extended transition period.
Use of Estimates
The preparation of financial statement in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statement and the reported amounts of expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Actual results could differ from those estimates.
Cash
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash and cash equivalents. The Company did not have any cash equivalents at December 31, 2021 or December 31, 2020.
Investment in Trust Account
The proceeds held in the Trust Account will be invested in U.S. government securities, with a maturity of 180 days or less or in money market funds meeting certain conditions under Rule 2a-7 under the Investment Company Act, which invest only in direct U.S. government treasury obligations. Except with respect to interest earned on the funds held in the Trust Account that may be released to the Company to pay its tax obligations, the proceeds from our initial public offering will not be released from the Trust Account until the earlier of: (a) the completion of the Company’s initial business combination, (b) the redemption of the Company’s public shares if the Company is unable to complete its initial business combination within 18 months from the closing of our initial public offering.
At December 31, 2021, the assets held in the Trust Account consisted of mutual funds in the amount of $138,013,319 and the Company had not withdrawn any of the interest income from the Trust Account to pay its tax obligations for the period from July 28, 2020 to December 31, 2021. The mutual funds held in the Trust Account were available for sale and reported at fair value.
F-33
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 2 — Basis of Presentation and Significant Accounting Policies (cont.)
The carrying value and fair value of mutual funds held in Trust Account on December 31, 2021 are as follows:
| | Carrying
Value | | Fair
Value |
U.S. Money Market | | $ | 138,013,319 | | $ | 138,013,319 |
Fair Value Measurements
The fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC Topic 820, “Fair Value Measurement,” approximates the carrying amounts represented in the accompanying balance sheet, primarily due to their short-term nature. Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
• Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets;
• Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and
• Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable.
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash accounts in a financial institution, which, at times, may exceed the Federal Depository Insurance Coverage of $250,000. At December 31, 2021, the Company has not experienced losses on these accounts and management believes the Company is not exposed to significant risks on such accounts.
Common Stock Subject to Possible Redemption
The Company accounts for its common stock subject to possible redemption in accordance with the guidance in ASC Topic 480 “Distinguishing Liabilities from Equity.” Common stock subject to mandatory redemption (if any) is classified as a liability instrument and is measured at fair value. Conditionally redeemable common stock (including common stock that features redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) is classified as temporary equity. At all other times, common stock is classified as stockholders’ equity. The Company’s common stock features certain redemption rights that are considered to be outside of the Company’s control and subject to the occurrence of uncertain future events. Accordingly, common stock subject to possible redemption is presented at redemption value as temporary equity, outside of the stockholders’ equity section of the Company’s balance sheet.
F-34
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 2 — Basis of Presentation and Significant Accounting Policies (cont.)
At December 31, 2021, the common stock reflected in the balance sheets are reconciled in the following table:
Gross Proceeds | | $ | 138,000,000 | |
Less: Proceeds allocated to Public Warrants | | | (5,795,688 | ) |
Less: Issuance costs related to common stock | | | (3,465,153 | ) |
Plus: Remeasurement of carrying value to redemption value | | | 9,260,841 | |
Common stock subject to possible redemption | | $ | 138,000,000 | |
Net Loss Per Common Share
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, Earnings Per Share. Net loss per share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the period. The Company has two classes of shares, redeemable common stock and non-redeemable common stock. Earnings and losses are shared pro rata between the two classes of shares. The Company’s statement of operations applies the two-class method in calculating net loss per share. Basic and diluted net loss per common share for redeemable common stock and non-redeemable common stock is calculated by dividing net loss, allocated proportionally to each class of common stock, attributable to the Company by the weighted average number of shares of redeemable common stock and non-redeemable common stock outstanding.
The Company did not include the warrants as they are anti-dilutive. As a result, diluted income per share is the same as basic income per share for the period presented.
Accordingly, basic and diluted income (loss) per common share is calculated as follows:
| | For the
Year ended
December 31,
2021 | | For the
period from
July 28, 2020 (inception)
through
December 31,
2020 |
Common stock subject to possible redemption | | | | | | | | |
Numerator: | | | | | | | | |
Net loss allocable to common stock subject to possible redemption | | $ | (74,113 | ) | | $ | — | |
Denominator: | | | | | | | | |
Weighted average redeemable common stock, basic and diluted | | | 13,369,315 | | | | — | |
Basic and diluted net loss per stock, redeemable common stock | | $ | (0.01 | ) | | $ | — | |
| | | | | | | | | |
Non-redeemable common stock | | | | | | | | |
Numerator: | | | | | | | | |
Net loss allocable to common stock not subject to redemption | | $ | (19,695 | ) | | $ | (13,802 | ) |
Denominator: | | | | | | | | |
Weighted average non-redeemable common stock, basic and diluted | | | 3,552,740 | | | | 3,098,571 | |
Basic and diluted net loss per stock, common stock | | $ | (0.01 | ) | | $ | (0.00 | ) |
Offering Costs associated with the Initial Public Offering
The Company complies with the requirements of ASC 340-10-S99-1 and SEC Staff Accounting Bulletin (“SAB”) Topic 5A — “Expenses of Offering”. Offering costs consist principally of professional and registration fees incurred through the balance sheet date that are related to the IPO and were charged to stockholders’ equity upon the completion of the IPO. The Company determined the Public Warrants qualified for equity treatment, and accordingly, as of December 31, 2021, offering costs in the aggregate of $3,465,153 have been charged to stockholders’ equity (consisting of $2,760,000 of underwriting fees and $705,153 of other offering costs).
F-35
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 2 — Basis of Presentation and Significant Accounting Policies (cont.)
Warrant Liability
The Company accounts for the Public Warrants and Private Warrants (as defined in Note 1, 2 and 3) collectively (“Warrants”), as either equity or liability-classified instruments based on an assessment of the specific terms of the Warrants and the applicable authoritative guidance in Financial Accounting Standards Board (“FASB”) ASC 815, “Derivatives and Hedging”. The assessment considers whether the Warrants meet all of the requirements for equity classification under ASC 815, including whether the Warrants are indexed to the Company’s own common stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of issuance of the Warrants and as of each subsequent annual period end date while the Warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, such warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, such warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. Changes in the estimated fair value of liability-classified warrants are recognized as a non-cash gain or loss on the statements of operations.
The Company accounts for the Private Warrants in accordance with ASC 815-40 under which the Private Warrants do not meet the criteria for equity classification and must be recorded as liabilities. The fair value of the Private Warrants has been estimated using the Monte Carlo simulation model. See Note 6 for further discussion of the pertinent terms of the Warrants used to determine the value of the Private Warrants.
The Company evaluated the Public Warrants in accordance with ASC 815-40, “Derivatives and Hedging — Contracts in Entity’s Own Equity” and concluded that they met the criteria for equity classification and are required to be recorded as part a component of additional paid-in capital at the time of issuance.
Income Taxes
The Company follows the asset and liability method of accounting for income taxes under FASB ASC 740, “Income Taxes”. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. There were no unrecognized tax benefits as of December 31, 2021 and 2020. The Company’s management determined that the United States is the Company’s only major tax jurisdiction. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2021 and 2020. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position.
The Company is subject to income tax examinations by major taxing authorities since inception. These examinations may include questioning the timing and amount of deductions, the nexus of income among various tax jurisdictions and compliance with federal and state tax laws. The Company’s management does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months.
F-36
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 2 — Basis of Presentation and Significant Accounting Policies (cont.)
Recent Accounting Standards
In August 2020, the FASB issued ASU 2020-06, Debt-Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU also removes certain settlement conditions that are required for equity-linked contracts to qualify for scope exception, and it simplifies the diluted earnings per share calculation in certain areas. The Company is reviewing what impact, if any, adoption may have on the financial statements.
Management does not believe that any other recently issued, but not effective, accounting standards, if currently adopted, would have a material effect on the Company’s financial statements.
Note 3 — Initial Public Offering
Pursuant to the Initial Public Offering, the Company sold 12,000,000 Units at a price of $10.00 per Unit. Each Unit consists of one share of Common Stock, par value $0.0001 per share and one-half of one redeemable warrant (each, a “Public Warrant”). Each whole Public Warrant entitles the holder to purchase one share of Common Stock at a price of $11.50 per share. Each whole warrant will become exercisable 30 days after the completion of the initial Business Combination and will expire five years after the completion of the initial Business Combination, or earlier upon redemption or liquidation.
On January 15, 2021, the Underwriters exercised the over-allotment option in full to purchase 1,800,000 Units. The proceeds of $18,000,000 from the over-allotment was deposited in the Trust Account after deducting the underwriting fees.
The underwriters were paid a cash underwriting fee of 2.0% of the gross proceeds of the IPO and over-allotment, or $2,760,000 in the aggregate.
Note 4 — Private Placement
Simultaneously with the closing of the IPO, the Company’s Sponsor purchased an aggregate of 3,850,000 warrants at a price of $1.00 per warrant, for an aggregate purchase price of $3,850,000, in a private placement. The proceeds from the private placement of the Private Warrants were added to the proceeds of this Initial Public Offering and placed in a U.S.-based trust account maintained by Continental Stock Transfer & Trust Company, as trustee. If we do not complete an initial business combination within 18 months from the closing of this Initial Public Offering, the proceeds from the sale of the Private Warrants will be included in the liquidating distribution to our public stockholders and the Private Warrants will be worthless.
On January 15, 2021, the Underwriters exercised the over-allotment option in full to purchase 1,800,000 Units. Simultaneously with the closing of the exercise of the overallotment option, the Company completed the private sale of an aggregate of 360,000 Private Warrants to the Sponsor at a purchase price of $1.00 per Private Placement Warrant, generating gross proceeds of $360,000.
Note 5 — Related Party Transactions
Founder Shares
On September 11, 2020, the Sponsor paid $25,000, or approximately $0.009 per share, to cover certain offering costs in consideration for 2,875,000 shares of common stock, par value $0.0001 (the “Founder Shares”). On January 11, 2021, the Company effected a stock dividend of 0.2 shares for each share outstanding (the “Dividend”), resulting in there being an aggregate of 3,450,000 Founder Shares outstanding. All shares and associated amounts have been retroactively restated to reflect the stock dividend. Up to 450,000 Founder Shares were subject to forfeiture to the extent that the over-allotment option was not exercised in full by the underwriters. On January 15, 2021, the overallotment was exercised in full and all of the 450,000 shares were no longer subject to forfeiture.
F-37
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 5 — Related Party Transactions (cont.)
Promissory Note — Related Party
An affiliate of the Company’s chief executive officer has agreed to loan the Company an aggregate of up to $150,000 to cover expenses related to the Initial Public Offering pursuant to a promissory note (the “Note”). This loan is non-interest bearing and payable on demand. As of both December 31, 2021 and 2020, the Company had drawn down $125,000 during 2020 which is still outstanding in 2021 and 2020.
Due to Related Party
The Due to Related Party balance of $170,000 at December 31, 2020 was repaid in January 2021. As of December 31, 2021, the Due to Related Party balance of $260,000 represents the Administrative Support Expense (defined below) and additional funds from founders for December 2021. For the December 31, 2021 the Company has incurred an aggregate of $120,000 for administrative services.
Working Capital Loans
In order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (“Working Capital Loans”). If the Company completes a Business Combination, the Company would repay the Working Capital Loans. In the event that a Business Combination does not close, the Company may use a portion of proceeds held outside the Trust Account to repay the Working Capital Loans but no proceeds held in the Trust Account would be used to repay the Working Capital Loans.
Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. The Working Capital Loans would either be repaid upon consummation of a Business Combination, without interest, or, at the lender’s discretion, up to $1.5 million of such Working Capital Loans may be convertible into Private Warrants at a price of $1.00 per warrant. As of December 31, 2021 and December 31, 2020, the Company had no borrowings under the Working Capital Loans.
Administrative Support Agreement
Commencing on the date of the final prospectus, the Company has agreed to pay the Sponsor a total of $10,000 per month for office space, secretarial and administrative services (“Administrative Support Expense”). Upon completion of the initial Business Combination or the Company’s liquidation, the Company will cease paying these monthly fees. For the year ended December 31, 2021 the Company has incurred an aggregate of $120,000 for administrative services as of December 31, 2021.
Note 6 — Fair Value Measurements
The following table presents information about the Company’s assets and liabilities that were measured at fair value on a recurring basis as of December 31, 2021, and indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value.
| | December 31,
2021 | | Quoted
Prices
In Active Markets
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Other
Unobservable
Inputs
(Level 3) |
Assets: | | | | | | | | | | | | |
U.S. Money Market held in Trust Account | | $ | 138,013,319 | | $ | 138,013,319 | | $ | — | | $ | — |
Liabilities: | | | | | | | | | | | | |
Private Warrant Liability | | $ | 2,198,205 | | $ | — | | $ | — | | | 2,198,205 |
F-38
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 6 — Fair Value Measurements (cont.)
The Private Warrants were valued using a Monte Carlo simulation model, which is considered to be a Level 3 fair value measurement. Inherent in an options pricing model are assumptions related to expected stock-price volatility, expected life, risk-free interest rate and dividend yield. The Company estimates the volatility of its common stock based on historical volatility that matches the expected remaining life of the warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected remaining life of the warrants. The expected life of the warrants is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which the Company anticipates to remain at zero.
There were no transfers between Levels 1, 2 or 3 during the year ended December 31, 2021.
The following table provides quantitative information regarding Level 3 fair value measurements for Private Warrants as of January 12, 2021 (initial measurement), and December 31, 2021.
| | December 31,
2021 | | January 12,
2021 |
Exercise price | | $ | 11.50 | | | $ | 11.50 | |
Share price | | $ | 9.86 | | | $ | 9.58 | |
Volatility | | | 9.7 | % | | | 16.0 | % |
Expected life | | | 5.37 | | | | 5.71 | |
Risk-free rate | | | 1.29 | % | | | 0.62 | % |
Dividend yield | | | — | % | | | — | % |
The following table presents a summary of the changes in the fair value of the Private Warrants and Representative’s Warrants, a Level 3 liability, measured on a recurring basis.
| | Warrant
Liability |
Fair value, January 1, 2021 | | $ | — | |
Initial measurement on January 12, 2021, including over-allotment | | | 3,861,737 | |
Change in fair value | | | (1,663,532 | ) |
Fair value, December 31, 2021 | | $ | 2,198,205 | |
Note 7 — Commitments and Contingencies
Registration Rights
The holders of the founders’ shares and representative shares issued and outstanding on the date of the Initial Public Offering, as well as the holders of the Private Warrants (and the underlying shares) and any warrants the Company’s sponsor, officers, directors or their affiliates may be issued in payment of working capital loans made to the Company (and all underlying securities), are entitled to registration rights pursuant to an agreement signed prior to or on the effective date of this Initial Public Offering. The holders of a majority of these securities are entitled to make up to two demands that the Company registers such securities. The holders of the majority of the founders’ shares, as well as the holders of the Private Warrants (and the underlying shares) can elect to exercise these registration rights at any time commencing three months prior to the date on which these shares of common stock are to be released from escrow. The holders of a majority of the representative shares, and warrants issued to our sponsor, officers, directors or their affiliates in payment of working capital loans made to the Company (or underlying securities) can elect to exercise these registration rights at any time after the Company consummates a business combination. Notwithstanding anything to the contrary, EarlyBirdCapital Inc. (“EarlyBirdCapital”) may only make a demand on one occasion and only during the five-year period beginning on the effective date of the registration statement of which this prospectus forms a part. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to the consummation of a business
F-39
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 7 — Commitments and Contingencies (cont.)
combination; provided, however, that EarlyBirdCapital may participate in a “piggy-back” registration only during the seven-year period beginning on the effective date of the registration statement of which this prospectus forms a part. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Business Combination Marketing Agreement
The Company has engaged EarlyBirdCapital as an advisor in connection with our business combination to assist in holding meetings with stockholders to discuss the potential business combination and the target business’ attributes, introduce the Company to potential investors that are interested in purchasing its securities in connection with the business combination, assist in obtaining stockholder approval for the business combination and assist with press releases and public filings in connection with the business combination. The Company will pay EarlyBirdCapital a cash fee for such services upon the consummation of the business combination in an amount equal to 3.5% of the gross proceeds of the Initial Public Offering. Additionally, the Company will pay EarlyBirdCapital a cash fee equal to 1.0% of the total consideration payable in the proposed business combination if EarlyBirdCapital introduces the Company to the target business with which it completes the business combination; provided that the foregoing fee will not be paid prior to the date that is 90 days from the effective date of the registration statement. As of December 31, 2021 and 2020, these fees are not accrued.
Representative Shares
On October 1, 2020, the Company issued to designees of EarlyBirdCapital Inc. the 120,000 representative shares for nominal consideration. The Company estimated the fair value of the stock to be $1,200 based upon the price of the founder shares issued to the Sponsor and were treated as underwriters’ compensation and charged directly to stockholders’ equity. The holders of the representative shares have agreed not to transfer, assign or sell any such shares without the Company’s prior consent until the completion of its initial business combination. In addition, the holders of the representative shares have agreed (i) to waive their conversion rights (or right to participate in any tender offer) with respect to such shares in connection with the completion of our initial business combination and (ii) to waive their rights to liquidating distributions from the trust account with respect to such shares if the Company fails to complete an initial business combination within 18 months from the closing of this Initial Public Offering.
The representative shares have been deemed compensation by FINRA and are therefore subject to a lock-up for a period of 180 days immediately following the date of the effectiveness of the registration statement of which this prospectus forms a part pursuant to Rule 5110(g)(1) of the FINRA Manual. Pursuant to FINRA Rule 5110(g)(1), these securities were not sold during the Initial Public Offering, or sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the economic disposition of the securities by any person for a period of 180 days immediately following the effective date of the registration statement of which this prospectus forms a part or commencement of sales of the public offering, except to any underwriter and selected dealer participating in the Initial Public Offering and their bona fide officers or partners, provided that all securities so transferred remain subject to the lockup restriction above for the remainder of the time period.
Note 8 — Warrants
Public Warrants
The Public Warrants will become exercisable 30 days after the completion of a Business Combination. However, no warrants will be exercisable for cash unless the Company has an effective and current registration statement covering the shares of common stock issuable upon exercise of the warrants and a current prospectus relating to such shares of common stock. Notwithstanding the foregoing, if a registration statement covering the shares of common stock issuable upon exercise of the public warrants is not effective within a specified period following the consummation of our initial business combination, warrant holders may, until such time as there is an effective registration statement and during any period when we shall have failed to maintain an effective registration
F-40
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 8 — Warrants (cont.)
statement, exercise warrants on a cashless basis pursuant to the exemption provided by Section 3(a)(9) of the Securities Act, provided that such exemption is available. If that exemption, or another exemption, is not available, holders will not be able to exercise their warrants on a cashless basis. In the event of such cashless exercise, each holder would pay the exercise price by surrendering the warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the difference between the exercise price of the warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose will mean the average reported last sale price of the shares of common stock for the 5 trading days ending on the trading day prior to the date of exercise. The warrants will expire on the fifth anniversary of our completion of an initial business combination, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation. The warrants will expire five years after the completion of a Business Combination or earlier upon redemption or liquidation.
The Company may call the Public Warrants for redemption:
• in whole and not in part;
• at a price of $0.01 per warrant;
• upon not less than 30 days’ prior written notice of redemption (the “30-day redemption period”) to each warrant holder; and
• if, and only if, the reported last sale price of the shares of common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations and recapitalizations), for any 20 trading days within a 30 trading day period commencing at any time after the warrants become exercisable and ending on the third business day prior to the notice of redemption to warrant holders; and
• if, and only if, there is a current registration statement in effect with respect to the shares of common stock underlying such warrants.
If the Company calls the Public Warrants for redemption, management will have the option to require all holders that wish to exercise the Public Warrants to do so on a “cashless basis”, as described in the warrant agreement. In such event, each holder would pay the exercise price by surrendering the warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the difference between the exercise price of the warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose shall mean the average reported last sale price of the shares of common stock for the 5 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants.
In addition, if (x) the Company issues additional shares of common stock or equity-linked securities for capital raising purposes in connection with the closing of the initial business combination at an issue price or effective issue price of less than $9.20 per share of common stock (with such issue price or effective issue price to be determined in good faith by our board of directors, and in the case of any such issuance to our sponsor, initial stockholders or their affiliates, without taking into account any founders’ shares held by them prior to such issuance), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of the initial business combination on the date of the consummation of the initial business combination (net of redemptions), and (z) the Market Value is below $9.20 per share, the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the greater of (i) the Market Value or (ii) the price at which the Company issues the additional shares of common stock or equity-linked securities.
F-41
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 8 — Warrants (cont.)
Private Warrants
The Private Warrants are identical to the Public Warrants underlying the Units being sold in the Initial Public Offering, except that the Private Warrants, so long as they are held by the Sponsor or their permitted transferees, (i) will not be redeemable by the Company, (ii) may be exercised for cash or on a cashless basis, as described in the Initial Public Offering, in each case so long as they are held by the initial purchasers or any of their permitted transferees. If the Private Warrants are held by holders other than the initial purchasers or any of their permitted transferees, the Private Warrants will be redeemable by the Company and exercisable by the holders on the same basis as the warrants included in the units being sold in this offering.
Note 9 — Stockholders’ Equity
Preferred Stock — The Company is authorized to issue 1,000,000 shares of preferred stock with a par value of $0.0001 and with such designations, voting and other rights and preferences as may be determined from time to time by the Company’s board of directors. As of December 31, 2021 and 2020, there were no preferred stock issued or outstanding.
Common Stock — The Company is authorized to issue 99,000,000 shares of common stock with a par value of $0.0001 per share. Holders are entitled to one vote for each share of common stock. On January 11, 2021, the Company effected a stock dividend of 0.2 shares for each share outstanding, resulting in there being an aggregate of 3,450,000 Founder Shares outstanding. All shares and associated amounts have been retroactively restated to reflect the stock dividend. At December 31, 2021 and 2020, there were 3,570,000 shares of common stock issued and outstanding, excluding 13,800,000 and 0 shares subject to possible redemption at December 31, 2021 and 2020, respectively.
Note 10 — Income Tax
The Company’s net deferred tax assets are as follows:
| | December 31, 2021 | | December 31, 2020 |
Deferred tax asset | | | | | | | | |
Organizational costs/Start-up costs | | $ | 321,397 | | | $ | 2,898 | |
Federal net operating loss | | | 39,203 | | | | — | |
Total deferred tax asset | | | 360,600 | | | | 2,898 | |
Valuation allowance | | | (360,600 | ) | | | (2,898 | ) |
Deferred tax asset, net of allowance | | $ | — | | | $ | — | |
The income tax provision consists of the following:
| | December 31, 2021 | | December 31, 2020 |
Federal | | | | | | | | |
Current | | $ | — | | | $ | — | |
Deferred | | | (357,701 | ) | | | (2,898 | ) |
State | | | | | | | | |
Current | | | — | | | | — | |
Deferred | | | — | | | | — | |
Change in valuation allowance | | | 357,701 | | | | 2,898 | |
Income tax provision | | $ | — | | | $ | — | |
F-42
Table of Contents
LIGHTJUMP ACQUISITION CORPORATION
NOTES TO FINANCIAL STATEMENTS
Note 10 — Income Tax (cont.)
The Company’s Federal net operating loss carryforward as of December 31, 2021 amounted to $186,680 and will be carried forward indefinitely.
In assessing the realization of the deferred tax assets, management considers whether it is more likely than not that some portion of all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which temporary differences representing net future deductible amounts become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. After consideration of all of the information available, management believes that significant uncertainty exists with respect to future realization of the deferred tax assets and has therefore established a full valuation allowance. For the year ended December 31, 2021, the change in the valuation allowance was $357,701.
A reconciliation of the federal income tax rate to the Company’s effective tax rate is as follows:
| | December 31,
2021 | | December 31,
2020 |
Statutory federal income tax rate | | 21.00 | % | | 21.00 | % |
State taxes, net of federal tax benefit | | — | | | — | |
Permanent book/tax differences: | | | | | | |
Change in FV of Warrants | | 360.31 | % | | — | |
Change in valuation allowance | | (381.31 | )% | | (21.00 | )% |
Income tax provision | | — | | | — | |
The Company’s effective tax rates for the periods presented differ from the expected (statutory) rates due to transaction costs related to warrants, change in FV of warrants and the recording of full valuation allowances on deferred tax assets and permanent differences.
The Company files income tax returns in the U.S. federal, which remain open and subject to examination since inception.
Note 11 — Subsequent Events
The Company evaluated subsequent events and transactions that occurred after the balance sheet date up to the date that the financial statements were issued. The Company did not identify any subsequent events that would have required adjustment or disclosure in the financial statements.
F-43
Table of Contents

Report of Independent Registered Public Accounting Firm
To the board of directors and shareholders of Moolec Science Ltd.
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of financial position of Moolec Science Ltd. and its subsidiary (the “Company”) as of June 30, 2022 and June 30, 2021, and the related consolidated statements of operations, of changes in equity and of cash flows for the year ended June 30, 2022 and for each of the periods from January 1, 2021 through June 30, 2021 and from August 21, 2020 through December 31, 2020, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2022, June 30, 2021 and December 31, 2020 and the results of its operations and its cash flows for the year ended June 30, 2022 and each of the periods from January 1, 2021 through June 30, 2021 and from August 21, 2020 through December 31, 2020 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the Consolidated Financial Statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Price Waterhouse & Co. S.R.L. | | |
/s/ Sebastian Azagra | | |
Partner | | |
Rosario, Argentina | | |
September 29, 2022 | | |
We have served as the Company’s auditor since 2020.
F-44
Table of Contents
Moolec Science Limited
Consolidated statements of operations
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021
and from inception on August 21, 2020 through December 31, 2020
In USD [$]
| | | | For the
year ended
on June 30,
2022 | | From the
period of
January 1,
2021
through
June 30,
2021 | | From
inception on
August 21,
2020
through
December, 31
2020 |
Continuing operations | | | | | | | | | | | | | | |
Research and development expense | | | | | (985,158 | ) | | | (601,942 | ) | | | (179,061 | ) |
Sales and marketing expense | | | | | (105,060 | ) | | | (80,221 | ) | | | (10,938 | ) |
Administrative expense | | | | | (2,523,230 | ) | | | (820,946 | ) | | | (127,886 | ) |
Other operating expense | | | | | (38,985 | ) | | | (93,252 | ) | | | (2,639 | ) |
Loss from operations | | | | $ | (3,652,433 | ) | | $ | (1,596,361 | ) | | $ | (320,524 | ) |
| | | | | | | | | | | | | | | |
Share of loss from associate | | 6 | | $ | — | | | $ | (390,453 | ) | | $ | — | |
| | | | | | | | | | | | | | | |
Finance costs | | 16 | | $ | (874,472 | ) | | | — | | | | — | |
Loss before Income tax | | | | $ | (4,526,905 | ) | | $ | (1,986,814 | ) | | $ | (320,524 | ) |
| | | | | | | | | | | | | | | |
Income tax | | 17 | | | — | | | | — | | | | — | |
Loss of the year/period | | | | $ | (4,526,905 | ) | | $ | (1,986,814 | ) | | $ | (320,524 | ) |
| | | | | | | | | | | | | | | |
Total comprehensive loss for the year/period | | | | $ | (4,526,905 | ) | | $ | (1,986,814 | ) | | $ | (320,524 | ) |
Basic and diluted loss per share | | 9 | | | (0.09 | ) | | | (0.04 | ) | | | (0.01 | ) |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-45
Table of Contents
Moolec Science Limited
Consolidated statements of financial position
As of June 30, 2022
presented in comparative figures
In USD [$]
| | Notes | | As of
June 30,
2022 | | As of
June 30,
2021 |
ASSETS | | | | | | | | | | |
Non-current assets | | | | | | | | | | |
Intangible Assets | | 7 | | | 4,598,930 | | | | 4,598,930 | |
Fixed Assets | | 8 | | | 8,918 | | | | 10,617 | |
Total non-current assets | | | | $ | 4,607,848 | | | $ | 4,609,547 | |
| | | | | | | | | | | |
Current assets | | | | | | | | | | |
Cash and cash equivalents | | | | | 1,081,808 | | | | 980,527 | |
Receivables from related parties | | 10 | | | — | | | | 289,888 | |
Prepayments | | | | | 2,061 | | | | 358 | |
Total current assets | | | | $ | 1,083,869 | | | $ | 1,270,773 | |
TOTAL ASSETS | | | | $ | 5,691,717 | | | $ | 5,880,320 | |
| | | | | | | | | | | |
LIABILITIES AND EQUITY | | | | | | | | | | |
Equity | | | | | | | | | | |
Share capital | | 9 | | | 638,297 | | | | 638,297 | |
Share premium | | 9 | | | 6,961,703 | | | | 6,961,703 | |
Equity settled share based payment | | 11 | | | 838,576 | | | | — | |
Accumulated deficit | | | | | (6,834,243 | ) | | | (2,307,338 | ) |
Total equity | | | | $ | 1,604,333 | | | $ | 5,292,662 | |
| | | | | | | | | | | |
Liabilities | | | | | | | | | | |
Current liabilities | | | | | | | | | | |
Accounts payable | | 13 | | | 1,226,213 | | | | 587,658 | |
Other current liabilities | | 14 | | | 1,171 | | | | — | |
Simply Agreement for Future Equity (“SAFE”) | | 15 | | | 2,860,000 | | | | — | |
Total current liabilities | | | | $ | 4,087,384 | | | $ | 587,658 | |
TOTAL LIABILITIES | | | | $ | 4,087,384 | | | $ | 587,658 | |
TOTAL LIABILITIES AND EQUITY | | | | $ | 5,691,717 | | | $ | 5,880,320 | |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-46
Table of Contents
Moolec Science Limited
Consolidated statements of cash flows
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021
and from inception on August 21, 2020 through December 31, 2020
In USD [$]
| | From the
period of
July 1,
2021
through
June 30,
2022 | | From the
period of
January 1,
2021
through
June 30,
2021 | | From
inception on
August 21,
2020
through
December, 31
2020 |
Cash flows from operating activities | | | | | | | | | | | | |
Loss for the year/period | | $ | (4,526,905 | ) | | $ | (1,986,814 | ) | | $ | (320,524 | ) |
Adjustment from operating activities | | | | | | | | | | | | |
Share of loss from associate | | | — | | | | 390,453 | | | | — | |
Depreciation and amortization | | | 1,699 | | | | — | | | | — | |
Employee share based payment | | | 838,576 | | | | — | | | | — | |
Change in fair value of Simply Agreement for Future Equity (“SAFE”) | | | 860,000 | | | | — | | | | — | |
Other finance cost | | | 14,472 | | | | — | | | | — | |
| | | | | | | | | | | | | |
Changes in working capital | | | | | | | | | | | | |
Receivables from related parties | | | — | | | | 2,009,220 | | | | (2,299,108 | ) |
Prepayments | | | (1,703 | ) | | | (358 | ) | | | — | |
Accounts Payable | | | 926,711 | | | | 568,026 | | | | 19,632 | |
Other liabilities | | | 1,171 | | | | — | | | | — | |
Net cash (used) generated in operating activities | | $ | (1,885,979 | ) | | $ | 980,527 | | | $ | (2,600,000 | ) |
| | | | | | | | | | | | | |
Cash flows from financing activities | | | | | | | | | | | | |
Proceeds from issue of share capital | | | — | | | | — | | | | 600,000 | |
Proceeds from additional issue of ordinary shares | | | — | | | | — | | | | 2,000,000 | |
Proceeds from SAFE | | | 2,000,000 | | | | — | | | | — | |
Net cash generated from financing activities | | $ | 2,000,000 | | | $ | — | | | $ | 2,600,000 | |
| | | | | | | | | | | | | |
Net increase in cash and cash equivalents | | $ | 114,021 | | | $ | 980,527 | | | $ | | |
Cash and cash equivalents at beginning of the year/period | | | 980,527 | | | | — | | | | — | |
Effect of exchange rate changes on cash and equivalents | | | (12,740 | ) | | | — | | | | — | |
Cash and cash equivalents at end of the year/period | | $ | 1,081,808 | | | $ | 980,527 | | | $ | — | |
| | | | | | | | | | | | | |
Non-cash financing activities | | | | | | | | | | | | |
Acquisition of non-current assets by the issuance of ordinary shares | | $ | — | | | $ | 3,000,000 | | | $ | 2,000,000 | |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-47
Table of Contents
Moolec Science Limited
Consolidated statements of changes in equity
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021
and from inception on August 21, 2020 through December 31, 2020
In USD [$]
| | Share
capital | | Equity
settled share
based
payment | | Retained
(deficit) | | Share
premium | | Total
equity |
CHANGES IN EQUITY | | | | | | | | | | | | | | | | | |
Issue of share capital | | | 600,000 | | | — | | | — | | | | 4,000,000 | | | 4,600,000 | |
Total comprehensive (loss) | | | — | | | — | | | (320,524 | ) | | | — | | | (320,524 | ) |
Balance as of December 31, 2020 | | $ | 600,000 | | $ | — | | $ | (320,524 | ) | | $ | 4,000,000 | | $ | 4,279,476 | |
| | | | | | | | | | | | | | | | | | |
Issue of share capital | | | 38,297 | | | — | | | — | | | | 2,961,703 | | | 3,000,000 | |
Total comprehensive (loss) | | | — | | | — | | | (1,986,814 | ) | | | — | | | (1,986,814 | ) |
Balance as of June 30, 2021 | | $ | 638,297 | | $ | — | | $ | (2,307,338 | ) | | $ | 6,961,703 | | $ | 5,292,662 | |
| | | | | | | | | | | | | | | | | | |
Total comprehensive (loss) | | | — | | | — | | | (4,526,905 | ) | | | — | | | (4,526,905 | ) |
Equity settled share based payment | | | — | | | 838,576 | | | — | | | | — | | | 838,576 | |
Balance as of June 30, 2022 | | $ | 638,297 | | $ | 838,576 | | $ | (6,834,243 | ) | | $ | 6,961,703 | | $ | 1,604,333 | |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-48
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 1. General information
Moolec Science Limited (“the Company’’ or “Moolec Science”) is a private limited liability company incorporated on August 21, 2020 (“date of incorporation”), created to develop affordable alternative proteins using molecular farming technology. Moolec Science is equally owned by BG Farming Technologies Ltd and Union Group Ventures Limited (together referred to as “the Parent” and individually as “BG Farming Technologies” and “Union Group Ventures”, respectively). BG Farming Technologies is 80% owned by Theo 1 SCSp. The Company is registered in Wales and England under company registration number 12828514. Its registered address is Innovation Centre, Gallows Hill, Warwick, Warwickshire, England CV34 6UW.
On December 23, 2020, the Company entered into a share purchase agreement (“SPA”) with BG Farming Technologies where the Company issued an additional 100 ordinary shares in exchange for 100% of equity ownership of AG Biomolecules LLC (“AG Biomolecules” or referred together with Moolec Science as “the Group”). AG Biomolecules is a Delaware limited liability company organized for the purpose of contributing all of the Company’s business net assets upon commencement of business operations. Refer to Note 5 — Subsidiaries and Note 11 — Related Parties for further information regarding the Company’s subsidiary and related party relationships of the Group.
On March 15, 2021, the Company issued an additional 2,919,715 ordinary shares at a par value of $0.013117 (per share amount), to Bioceres Crop Solutions Corp (“Bioceres Crop Solutions”) in exchange for intellectual property related to Gamma Linolenic Acid (“GLA’) assets and licensing rights to Arachidonic Acid (“ARA”) patents. The ownership percentages of Moolec Science after the transaction are as follows: Bioceres Crop Solutions — 6%, BG Farming Technologies — 47% and Union Group Ventures — 47%.
On June 22, 2021 the Company changed its fiscal year end date from December 31 to June 30, to adapt it to the year end date of its shareholders. Therefore, comparative information for the prior fiscal year is presented by the six-month period from January 1 to June 30, 2021 and for the irregular period from the inception date (August 21, 2020) through December 31, 2020.
Note 2. Accounting standards and Basis of preparation
Note 2.1. Compliance with IFRS
These Consolidated Financial Statements of the Group have been prepared in accordance with International Financial Reporting Standards (IFRS) and interpretations issued by the IFRS Interpretations Committee (IFRS IC) applicable to companies reporting under IFRS. The financial statements comply with IFRS as issued by the International Accounting Standards Board (IASB).
Note 2.2.
The Consolidated Financial Statements of the Group as of June 30, 2022, 2021 and December 30, 2020 for the year ended June 30, 2022 and for the period from July 1, 2020 through June 30, 2021 and for the period from the inception on August 21, 2020 through December 31, 2020, have been authorized by the Board of Directors of Moolec Science Limited on September 29, 2022.
Note 2.3. Basis of measurement
The Consolidated Financial Statements have been prepared on the historical cost basis.
The accounting policies set out in Note 3 — Significant accounting policies have been applied in preparing the Consolidated Financial Statements as of June 30, 2022 and 2021.
F-49
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 2. Accounting standards and Basis of preparation (cont.)
Due to the activities of the Group, costs and expenses presented in the consolidated statements of Operations are classified according to their function. The consolidated statements of Financial Position has been prepared on the nature of the transactions, distinguishing: (a) current assets from non-current assets, where current assets are intended as the assets that should be realized, sold or used during the normal operating cycle, or the assets owned with the aim of being sold in the short-term (within 12 months); (b) current liabilities from non-current liabilities, where current liabilities are intended as the liabilities that should be paid during the normal operating cycle or over the 12-month period subsequent to the reporting date.
Note 2.4. Functional and presentation currency
Items included in the Consolidated Financial Statements are measured using the currency of the primary economic market in which the Company operates (“the functional currency”). These Consolidated Financial Statements are presented in US Dollars, which is the Company’s functional currency.
Note 2.5. Use of estimates and judgements
The preparation of the Consolidated Financial Statements in conformity with IFRS requires Management to make judgements, estimates and assumptions that affect the application of accounting policies and the reporting amounts as presented in the Consolidated Financial Statements.
Estimates and underlying assumptions are reviewed on an ongoing basis. Refer to Note 3 — Significant accounting policies for further discussion on accounting treatments applied in preparation of the financial results of the Group as of the reporting period in compliance with IFRS.
Note 2.6. Going concern
Management has evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about the Group’s ability to continue as a going concern after the accompanying Consolidated Financial Statements are issued. The accompanying Consolidated Financial Statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities that might be necessary if the Group is unable to continue as a going concern. The Group presumes it will, for the foreseeable future, be able to realize its assets and discharge its liabilities in the normal course of operations.
The following matters have been considered by Management in determining the appropriateness of the going concern basis of preparation of the accompanying Consolidated Financial Statements.
Liquidity risk
Liquidity risk is the risk that the Group will encounter difficulty in meeting the obligations affiliated with its financial liabilities that are settled by delivering cash or another financial asset. The Group’s approach to managing liquidity is to ensure, as far as possible, that it will always have sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions, without incurring unacceptable losses or risking damage to the Group’ s reputation. Given the Group’s financial position as of June 30, 2022, total current financial assets $1,081,808 as compared to total financial liabilities of $4,087,889 (of which $2,860,000 are part of the SAFE agreement convertible in future Equity), Management expects that the Group will be able to provide the capital needed to keep the Group liquid and able to fulfill its short-term obligations. Group’s financial position as of June 30, 2021, total current financial assets $1,270,415 as compared to total financial liabilities of $587,658.
F-50
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 2. Accounting standards and Basis of preparation (cont.)
Credit risk
Credit risk is the risk of financial loss to the Group if the counterparty to a financial instrument fails to meet its contractual obligations.
As of June 30, 2021, the maximum exposure to credit risk in financial assets of $289,888, was substantially mitigated as the counterparties to the arrangement is Bioceres LLC, who had a special interest in the optimistic financial outlook of the Group. Management did not expect Bioceres LLC, to fail to meet their obligations in the twelve months subsequent to the reporting date. Refer to Note 12 — Related parties for further discussion on the arrangements with Bioceres LLC. As of June 30, 2022, there is no exposure to credit risk in financial assets.
Operational risk
As the Group was recently formed and incorporated on August 21, 2020, there is no operating history and no generated revenues for the periods ended on June 30, 2022 and 2021. There is no assurance that the Group will generate revenues in the marketplace, and even if revenues are generated subsequent to the reporting period, there is no assurance that the Group can earn a profit. However, given the recent heightened consumer demand for plant-based foods worldwide, Management is optimistic in the trajectory of its business given its unique approach to affordable alternative proteins. Therefore, there are no significant doubts on the entity’s ability to continue as a going concern.
Note 3. Summary of significant accounting policies
The accounting policies set out below have been applied to the period presented in the Group’s first IFRS Consolidated Financial Statements since incorporation on August 21, 2020 and have been applied consistently by the Group for the year ended on June 30, 2022 and the six-month period ended June 30, 2021 and the irregular period from inception though December 31, 2020.
Note 3.1. Basis of consolidation of subsidiary
Subsidiaries are entities controlled by the Company. The financial statements of the Company’s subsidiary, AG Biomolecules, are included in the Consolidated Financial Statements from the date that control commences until the date in which control ceases. The accounting policies of subsidiaries have been changed when necessary to align them with the policies adopted by the Company.
Note 3.2. Foreign currency
Transactions entered into by the Group in a currency other than their functional currency are recorded at the relevant exchange rates as of the date upon which such transactions occur. Foreign currency monetary assets and liabilities are translated at the prevailing exchange rates as of the final day of each reporting period. Non-monetary assets and liabilities that are measured at fair value in a foreign currency are translated into the functional currency at the exchange rate when the fair value was determined. Non-monetary items that are measured based on historical cost in a foreign currency are translated at the exchange rate at the date of the transaction. Exchange differences arising on the retranslation of unsettled monetary assets and liabilities are recognized immediately in the consolidated statements of operations.
Note 3.3. Financial liabilities
Non-derivative financial liabilities, as presented in the consolidated statements of financial position as “Accounts Payable” and classified as a current liability, intended as the liabilities that should be paid during the normal operating cycle or over the 12-month period subsequent to the reporting date.
F-51
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 3. Summary of significant accounting policies (cont.)
The Group initially recognizes these financial liabilities at fair value plus any directly attributable transaction costs on the date it originated at which the Group becomes a party to the contractual terms and conditions. Subsequent to initial recognition, these financial liabilities are measured at amortized cost using the effective interest method.
SAFE
According to the clauses set forth in Simple Agreement for Future Equity (“SAFE”) (by which a variable number of shares will be delivered upon the occurrence of certain events), the agreement is accounted for as a financial liability measured to fair value through profit or loss.
Simple Agreement for Future Equity has been valued using an expected present value technique. Under this approach, probabilities and timings are assigned to the various conversion scenarios embedded within the agreements. The probabilities are based on management judgement, which in turn are based on discussions held with senior management of the investees and any other known events (such as fundraising rounds currently in flight). The second stage is to assign an estimate of the valuation of the investment at the forecast conversion date. This is based on management judgement (using the basis of recent negotiations where funding rounds are in flight).
In valuing the Simple Agreement for Future Equity, unobservable inputs arise where judgement is required to estimate the valuation of the underlying investment at a future date. The key quantitative unobservable inputs were the assumptions used in respect of a potential funding round, namely the pre-money valuation. An increase of 10% in the pre-money valuation and the probability of occurrence for the funding round would result in a higher fair value measurement of $143,000, whereas a decrease of 10% in the pre-money valuation and the probability of occurrence for the funding round would result in a lower fair value measurement of $ 169,000. The following table sets forth by level within the fair value hierarchy the Company’s financial liabilities that were accounted for at fair value on a recurring basis as of June 30, 2022.
Liabilities | | Level 1 | | Level 2 | | Level 3 |
Simply Agreement for Future equity | | — | | — | | 2,860,000 |
Total Liabilities | | — | | — | | 2,860,000 |
Note 3.4. Share-based payment arrangements
The grant-date fair value of equity-settled share-based payment arrangements granted to employees is generally recognized as an expense, with a corresponding increase in equity, over the vesting period of the awards. The amount recognized as an expense is adjusted to reflect the number of awards for which the related service is expected to be met, such that the amount ultimately recognized is based on the number of awards that meet related service and non-market performance conditions at the vesting date.
Note 3.5. Financial assets
Non-derivative financial assets, as presented in the consolidated statements of financial position as “Receivable from related parties” and classified as a current asset, intended as the assets that should be realized during the normal operating cycle or over the 12-month period subsequent to the reporting date.
The Group initially recognizes these financial assets at fair value plus any directly attributable transaction costs on the date it originated at which the Group becomes a party to the contractual terms and conditions. Subsequent to initial recognition, these financial assets are measured at amortized cost using the effective interest method less any impairment losses.
F-52
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 3. Summary of significant accounting policies (cont.)
Note 3.6. Intangible assets
Expenditure on internally developed products is capitalized if it can be demonstrated that:
• It is technically feasible to develop the product for it to be sold;
• Adequate resources are available to complete the development;
• There is an intention to complete and sell the product;
• The Group is able to sell the product;
• Sale of the product will generate future economic benefits; and
• Expenditure on the project can be measured reliably.
Development expenditure not satisfying the above criteria and expenditure on the research phase of internal projects are recognized in the consolidated statements of operations as incurred.
Capitalized development costs are amortized using the straight-line method over the periods the Group expects to benefit from selling the products developed.
Useful lives and amortization methods are reviewed every year as required by IAS 38.
The research and development process can be divided into several discrete steps or phases, which generally begin with discovery, validation and development and end with regulatory approval and commercial launch. The process for developing seed traits is relatively similar for both GM and non-GM traits. However, the two differ significantly in later phases of development. For example, obtaining regulatory approval for GM seeds is a far more comprehensive and lengthy process than for non-GM seeds. Although breeding programs and industrial biotechnology solutions may have shorter or simpler phases than those described below, the Group has used the industry consensus for seed-trait development phases to characterize its technology portfolios, which is generally divided into the following six phases:
i) Discovery: The first phase in the technology development process is the discovery or identification of candidate genes or genetic systems, metabolites, or microorganisms potentially capable of enhancing specified plant characteristics or enabling an agro-industrial biotech solution.
ii) Proof of concept: Upon successful validation of the technologies in model systems (in vitro or in vivo), promising technologies graduate from discovery and are advanced to the proof of concept phase. The goal of this phase is to validate a technology within the targeted organism before moving forward with technology escalation activities or extensive field validation.
iii) Early development: In this phase, efficacy field trials are expanded to evaluate the expression level and pheonological characteristics of the traits in multiple geographies and growing cycles, as well as other characteristics in order to optimize the technology’s performance in the targeted organisms. The goal of the early development phase is to evaluate the technical feasibility by identifying the best candidate to scale up the seed stock and to start the regulatory field trials.
iv) Advanced development and deregulation: In this phase, extensive field tests are used to fully demonstrate the effectiveness of the technology for its intended purpose. In the case of GM traits, the process of obtaining regulatory approvals from the government agencies is started, and includes field trials for environmental, core and food safety data generation. For solutions involving microbial fermentation, industrial-scale runs are conducted.
F-53
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 3. Summary of significant accounting policies (cont.)
v) Pre-launch: This phase involves finalizing the regulatory approval process and preparing for the launch and commercialization of the technology. The range of activities in this phase includes seed increases, pre-commercial production, and product and solution testing with selected customers. Usually, a more detailed marketing strategy and preparation of marketing materials occur during this phase.
vi) Product launch: In general, this phase, which is the last milestone of the research and development process, is carried out by the Group.
We determined that the R&D is more likely than not (“probable”) to become a commercialized product and reach compliance with IAS 38 criteria items at the end of the phase iii “Early Development” , when efficacy field trials are performed to determine the technical feasibility of the project by measuring parameters like expression level and phenological characteristics. The obtention of desired values for this set of data represents the strongest and clearest indication that the technical feasibility has been proved.
Note 3.7. Taxes
Current income tax
Current income tax assets and liabilities are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted at the reporting date in the country where the Group operates and generates taxable income.
Current income tax relating to items recognized directly in equity is recognized in equity and not in the consolidated statements of operations. Management periodically evaluates positions taken in the tax returns with respect to situations in which applicable tax regulations are subject to interpretation and establishes provisions where appropriate.
Deferred tax
Deferred tax is provided using the liability method on temporary differences between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes at the reporting date.
Deferred tax liabilities are recognized for all taxable temporary differences, except:
• When the deferred tax liability arises from the initial recognition of goodwill or an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss, and
• In respect of taxable temporary differences affiliated with investments in subsidiaries, associates, and interests in joint arrangements, when the timing of the reversal of the temporary differences can be controlled and it is probable that the temporary differences will not reverse in the foreseeable future.
Deferred tax assets are recognized for all deductible temporary differences, the carry forward of unused tax credits and any unused tax losses. Deferred tax assets are recognized to the extent that it is probable that taxable profit will be available against which the deductible temporary differences, and the carry forward of unused tax credits and unused tax losses can be utilized, except:
• When the deferred tax asset relating to the deductible temporary difference arises from the initial recognition of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss, and
F-54
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 3. Summary of significant accounting policies (cont.)
• In respect of deductible temporary differences affiliated with investments in subsidiaries, associates and interests in joint arrangements, deferred tax assets are recognized only to the extent that it is probable that the temporary differences will reverse in the foreseeable future and taxable profit will be available against which the temporary differences can be utilized.
The carrying amount of deferred tax assets is reviewed at each reporting date and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred tax asset to be utilized. Unrecognized deferred tax assets are re-assessed at each reporting date and are recognized to the extent that it has become probable that future taxable profits will allow the deferred tax asset to be recovered.
Deferred tax assets and liabilities are measured at the tax rates that are expected to apply in the year when the asset is realized or the liability is settled, based on tax rates that have been enacted or substantively enacted at the reporting date.
Deferred tax relating to items recognized outside profit or loss is recognized outside profit or loss. Deferred tax items are recognized in correlation to the underlying transaction either in OCI or directly in equity.
Note 3.8. Share capital
Ordinary shares
Ordinary shares are classified as equity. Incremental costs directly attributable to the issue of ordinary shares and share options are recognized as a deduction from equity, net of any tax effects.
Note 3.9. Subsidiaries
Where the Company holds a controlling interest in an entity, such entity is classified as a subsidiary. The Company exercises control over such an entity if all three of the following elements are present: (i) the Company has the power to direct or cause the direction of the management and policies of the entity, (ii) the Company is exposed to the variable returns of such entity; and (iii) the Company has power to affect the variability of such returns. Control is reassessed whenever facts and circumstances indicate that there may be a change in any of these elements of control. De-facto control exists in situations where the Company has the practical ability to direct the relevant activities of an entity without holding the majority of the voting rights. In determining whether de facto control exists, the Company considers all relevant facts and circumstances, including: (i) the relative share of the Company’s voting rights with respect to both the size and dispersion of other parties who hold voting rights; (ii) substantive potential voting rights held by the Company and by other parties, (iii) other contractual arrangements; and (iv) historic patterns in voting attendance.
The subsidiary of the Company, of which its financial results have been included in the Consolidated Financial Statements, and holds a majority share of the voting rights is as follows:
Name | | Principal
activities | | Country of
incorporation
and principal place of business | | Ref | |
% Equity interest as of
|
June 30,
2022 | | June 30,
2021 |
AG Biomolecules LLC | | Investment in subsidiaries | | United States | | (a) | | 100.00 | % | | 100.00 | % |
F-55
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 3. Summary of significant accounting policies (cont.)
Note 3.10. Investment in associates
Associates are those entities in which the Group has significant influence, but not control, over the financial and operating policies of that associate. Significant influence is presumed to exist when the Group holds between 20 and 50 percent of the voting power of another entity.
Investments in associates are accounted for using the equity method (equity accounted investees) and are recognized initially at cost. The Group’s investment includes goodwill identified on acquisition, net of any accumulated impairment losses. The Consolidated Financial Statements include the Group’s share of the income and expenses and equity movements of equity accounted investees, after adjustments to align the accounting policies with those of the Group, from the date that significant influence commences until the date that significant influence ceases. When the Group’s share of losses exceeds its interest in an equity accounted investee, the carrying amount of that interest, including any long-term investments, is reduced to zero, and the recognition of further losses is discontinued except to the extent that the Group has an obligation or has made payments on behalf of the investee.
Note 3.11. New standards, amendments and interpretations of IFRS
Standards and IFRICs newly applicable for companies with 30 June 2022 year ends are set out below.
Amendments to IFRS 7, IFRS 4, and IFRS 16 — Interest rate benchmark reform — Phase 2 (effective 1 January 2021)
The Phase 2 amendments address issues that arise from the implementation of the reforms, including the replacement of one benchmark with an alternative one. The Phase 2 amendments provide additional temporary reliefs from applying specific IAS 39 and IFRS 9 hedge accounting requirements to hedging relationships directly affected by IBOR reform.
Amendment to IFRS 16, ‘Leases’ — Covid-19 related rent concessions Extension of the practical expedient (effective 1 April 2021)
As a result of the coronavirus (COVID-19) pandemic, rent concessions have been granted to lessees. In May 2020, the IASB published an amendment to IFRS 16 that provided an optional practical expedient for lessees from assessing whether a rent concession related to COVID-19 is a lease modification. On March 31, 2021, the IASB published an additional amendment to extend the date of the practical expedient from June 30, 2021 to June 30, 2022. Lessees can elect to account for such rent concessions in the same way as they would if they were not lease modifications. In many cases, this will result in accounting for the concession as variable lease payments in the period(s) in which the event or condition that triggers the reduced payment occurs.
The amendments listed above did not have any impact on the amounts recognised in the Consolidated Financial Statements.
F-56
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 3. Summary of significant accounting policies (cont.)
New standards and interpretations not yet adopted
Certain new accounting standards and interpretations have been published that are not mandatory for June 30, 2022 reporting period and have not been early adopted by the Group. These standards are not expected to have a material impact on the Group in the current or future reporting periods and on foreseeable future transactions:
A number of narrow-scope amendments to IFRS 3, IAS 16, IAS 37 and some annual improvements on IFRS 1, IFRS 9, IAS 41 and IFRS 16
Amendments to IFRS 3, ‘Business combinations’ update a reference in IFRS 3 to the Conceptual Framework for Financial Reporting without changing the accounting requirements for business combinations.
Amendments to IAS 16, ‘Property, plant and equipment’ prohibit a company from deducting from the cost of property, plant and equipment amounts received from selling items produced while the company is preparing the asset for its intended use. Instead, a company will recognise such sales proceeds and related costs in profit or loss.
Amendments to IAS 37, ‘Provisions, contingent liabilities and contingent assets’ specify which costs a company includes when assessing whether a contract will be loss-making.
Annual improvements make minor amendments to IFRS 1, ‘First-time Adoption of IFRS’, IFRS 9, ‘Financial instruments’, IAS 41, ‘Agriculture’ and the Illustrative Examples accompanying IFRS 16, ‘Leases.
Effective date Annual periods beginning on or after January 1, 2022.
Amendments to IAS 1, ‘Presentation of financial statements’, on classification of liabilities
These narrow-scope amendments to IAS 1, ‘Presentation of financial statements’, clarify that liabilities are classified as either current or noncurrent, depending on the rights that exist at the end of the reporting period.
Classification is unaffected by the expectations of the entity or events after the reporting date (for example, the receipt of a waiver or a breach of covenant).
The amendment also clarifies what IAS 1 means when it refers to the ‘settlement’ of a liability.
Effective date Annual periods beginning on or after January 1, 2024.
Narrow scope amendments to IAS 1, Practice statement 2 and IAS 8
The amendments aim to improve accounting policy disclosures and to help users of the financial statements to distinguish between changes in accounting estimates and changes in accounting policies.
Effective date Annual periods beginning on or after January 1, 2023. However, the IASB plans to publish an exposure draft in the fourth quarter of 2021 proposing the deferral of the effective date to no earlier than January 1, 2024.
Amendment to IAS 12- deferred tax related to assets and liabilities arising from a single transaction
These amendments require companies to recognise deferred tax on transactions that, on initial recognition, give rise to equal amounts of taxable and deductible temporary differences.
Effective date Annual periods beginning on or after January 1, 2023.
F-57
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 3. Summary of significant accounting policies (cont.)
Note 3.12. Loss per share
The Group presents basic and diluted earnings per share (EPS) data for its ordinary shares. Basic EPS is calculated by dividing the profit and loss attributable to ordinary shareholders of the Group by the weighted average number of ordinary shares outstanding during the period. Diluted EPS is determined by adjusting the profit or loss attributed to ordinary shareholders and the weighted average number of ordinary shares outstanding for the effects of all dilutive potential ordinary shares.
As of June 30, 2022 and 2021, Basic EPS and Diluted EPS are the same, with the difference that as of June 30, 2022 SAFE and Employee share based payment are considered dilutive instruments properly included in the profit and loss account, however they do not impact in EPS diluted calculation because the Group has generated net loss to ordinary shareholders (See Note 10). As of December 31, 2020 there are no dilutive instruments.
Note 3.13. Cash and cash equivalents
Cash and cash equivalents include all cash balances.
Note 3.14. Expenses
Research and development costs
Research costs are expensed in the period in which these costs are incurred. Development costs are expensed in the period in which these costs are incurred if they do not meet the criteria for capitalization.
Sales and marketing, administrative and other operating expenses
The Group recognizes expenses in the period in which these costs are incurred and are presented by function on the consolidated statements of operations. Sales and marketing expenses primarily relate to marketing materials and research of the Group to increase brand awareness in the marketplace. Administrative expenses primarily comprise professional fees as it relates to consultancy, accountancy and legal expenses. Other operating expenses relate to those that do not depend on general business operations or relate to the other expense categories.
Note 4. Comparative Information
As per indicated in Note 1 on June 22, 2021 the Company changed its fiscal year end date from December 31 to June 30, to adapt it to the year end date of its shareholders. Therefore, comparative information for the prior fiscal year is presented by the six-month period from January 1 to June 30, 2021 and for the irregular period from the inception date (August 21, 2020) through December 31, 2020.
Note 5. Subsidiaries
On December 23, 2020, the Company obtained control of AG Biomolecules, which is a holding company based in the United States with a unique investment in an equity investee (AGBM S.A.), by acquiring 100% of the shares and voting interests in exchange for 100 of its ordinary shares, valued at $2,000,000. The reporting date of AG Biomolecules is December 31, 2020.
As of June 30, 2022 and 2021, AG Biomolecules (through its investment in AGBM S.A.) contributed revenue of $0 and net loss of $0 and revenue of $0 and net loss of $280,700, respectively (see note 6).
Consequently, the Company consolidates all of AG Biomolecule’s assets, liabilities, revenues and expenses into its Consolidated Financial Statements and all balances are presented appropriately in accordance with IFRS in the Consolidated Financial Statements as of June 30, 2022 and 2021.
F-58
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 6. Investment in associate
On December 10, 2020, AG Biomolecules entered into an agreement with Bioceres S.A. and contributed 137,954,282 Argentine pesos in exchange for Bioceres S.A.’s 49.9998% equity interest in AGBM S.A.. As of December 31, 2020 the investment was valued at acquisition cost of $2,000,000, because there was no subsequent recognition.
AGBM S.A.’s principal place of business and incorporation is in Argentina and is an industrial enzymes company working to produce and commercialize chymosin and safflower industrial by-products from modified safflower seeds using a leading molecular farming technology called SPC®. The industrial plant is capable of processing 6,000 metric tons per year and can produce 2 million liters of commercial-level chymosin (up to 20% of the global market share) annually. The facility also includes 7,500 square feet of offices and laboratories, as well as two warehouses of 9,800 square feet.
On June 28, 2021, AG Biomolecules sold back its equity interest in AGBM S.A. in exchange for the SPC technology rights, patents and a reverse osmosis equipment and for a first offer right to produce chymosin in AGBM S.A. facilities. As of June 30, 2021 AG Biomolecules holds all rights on SPC and technology with a recognized value of $1,598,930 and a fixed asset recognized of $10,617 to produce but no equity interest in AGBM S.A. According to the abovementioned, there is no investment in associates registered subsequent to this transaction and as of June 30, 2022 and 2021.
This transaction enables the Group to scale-up the molecular farming business, by producing and commercializing chymosin, an enzyme used in cheese manufacturing, which is an integral part to ensure the Group’s direction in a strategic growth and business operational model.
A reconciliation of the summarized financial information to the carrying amount of the Group’s interest in AGBM S.A. is as follows as of date of transaction:
In USD ($) | | |
Investment in AGBM S.A. | | |
Balance as of August 21, 2020 | | | — | |
Acquisition – December 10, 2020 | | | 2,000,000 | |
Balance as of December 31, 2020 | | $ | 2,000,000 | |
Share of: | | | | |
Net loss (i) | | | (390,453 | ) |
Sale of investment | | | (1,609,547 | ) |
Balance as of June, 30 2021 | | $ | — | |
Balance as of June, 30 2022 | | $ | — | |
F-59
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 7. Intangible Assets
Intangible assets are initially measured at cost. After initial recognition, intangible assets are measured at cost less any accumulated amortization and any accumulated impairment losses. Development costs are being amortized evenly over their estimated useful life once they are considered completed as we continue developing our intangible assets in order to use it for our future commercial products in development. Carrying amount of intangible assets as of June 30, 2022 and June 30, 2021 was as follows:
| | Original Values | | Net book Values |
| | | As of
June 30,
2022 | | As of
June 30,
2021 | | As of
June 30,
2022 | | As of
June 30,
2021 |
Gamma Linolenic Acid (“GLA’) assets and licensing rights to Arachidonic Acid (“ARA”) | | 3,000,000 | | 3,000,000 | | 3,000,000 | | 3,000,000 |
SPC® technology | | 1,598,930 | | 1,598,930 | | 1,598,930 | | 1,598,930 |
Total Intangible Assets | | 4,598,930 | | 4,598,930 | | 4,598,930 | | 4,598,930 |
Note 8. Fixed Assets
| | Original Values | | Depreciation | | Net book Values |
Chymosin osmosis equipment | | | 10,617 | | | (1,699 | ) | | | 8,918 |
Total Fixed Assets as of June 30, 2022 | | $ | 10,617 | | $ | (1,699 | ) | | $ | 8,918 |
Total Fixed Assets as of June 30, 2021 | | $ | 10,617 | | $ | — | | | $ | 10,617 |
Note 9. Share capital and share premium
As of June 30, 2022 and 2021, the share capital stock and share premium amounts to $7,600,000 each. As of December 31, 2020 the share capital stock and share premium amounts to $4,600,000.
| | Ordinary
Shares |
Issued on August 2020 (i) | | 45,742,000 |
Issued for cash (ii) | | 100 |
Issued for acquisition of AG Biomolecules 2020 (iii) | | 100 |
In issue on December 31, 2020 | | 45,742,200 |
Issued for GLA contributions 2021(iv) | | 2,919,715 |
In issue on June 30, 2021 | | 48,661,915 |
In issue on June 30, 2022 | | 48,661,915 |
Ordinary Shares
(i) On August 21, 2020, the Company was incorporated, and the share capital of the company was made up of 45,742,000 ordinary shares at $0.013 per share, resulting in a total of $600,000.
(ii) On December 23, 2020, the directors of the Company approved the issue of 100 ordinary shares at $0.013 per share to Union Group Ventures for $2,000,000 paid in cash.
(iii) On December 23, 2020, the directors of the Company approved the issue of 100 ordinary shares at $0.013 per share to BG Farming Technologies in exchange for 100% of the stock in AG Biomolecules, at a purchase price of $2,000,000.
F-60
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 9. Share capital and share premium (cont.)
(iv) On March 15, 2021, the directors of the Company approved the issue of 2,919,715 ordinary shares at a par value of $0.013 per share and a premium of $1.01470 per share to Bioceres Crop Solutions in exchange for intellectual property related to Gamma Linolenic Acid (“GLA’) assets and licensing rights to Arachidonic Acid (“ARA”) patents.
Note 10. Net loss per share
Basic loss per share is computed using the weighted-average number of ordinary shares outstanding during the period. Diluted net loss per share is computed using the weighted-average number of ordinary shares and, potentially dilutive, ordinary share equivalents outstanding during the period. The Group’s basic and diluted loss per ordinary share are the same because the Group has generated net loss to ordinary shareholders.
The following table presents the calculation of basic and diluted loss per ordinary share for the periods ended on June 30, 2022 and June 30, 2021 and December 31, 2020 as follows:
Loss attributable to ordinary shareholders (basic and diluted)
Numerator | | June 30,
2022 | | June 30,
2021 | | December 31,
2020 |
Loss for the period, attributable to the owners of
the Group | | (4,526,905 | ) | | (1,986,814 | ) | | (320,524 | ) |
Loss attributable to the ordinary shareholders | | (4,526,905 | ) | | (1,986,814 | ) | | (320,524 | ) |
Weighted-average number of ordinary shares (basic and diluted)
Denominator | | June 30,
2022 | | June 30,
2021 | | December 31,
2020 |
Weighted-average number of ordinary shares | | 48,661,915 | | 47,468,219 | | 45,742,012 |
Net loss attributable to ordinary shareholders per share | | June30,
2022 | | June30,
2021 | | December 31,
2020 |
Basic and Diluted | | (0.09 | ) | | (0.04 | ) | | (0.01 | ) |
Note 11. Share based payment
As of June 30, 2022, the Group had the following shared-based payment arrangements:
Share option plan for executives and senior management: Group 1 granted up to 909,000 underlying ordinary shares. The options have an exercise price of £0.74 and expiration in December 2030 (except one case in June 2031). Group 2 granted up to 544,000 underlying ordinary shares. The options have an exercise price of the higher of £0.74 or the valuation of a Share achieved at the next equity funding round following the date of this Agreement, subject to a 20% Discount and expires in December 2030.
The fair value is defined as the actuarial expected value of the future benefits under the Plan calculated at the date in which benefits are granted and it is estimated using the option valuation method known as ‘binomial trees’. The estimate considers the effects of rotation, the vesting schedule and the possible dilutive effect of the future exercise of options.
F-61
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 11. Share based payment (cont.)
Factor | | Group 1 | | Group 2 |
Fair value of shares | | $ 1.00 | | $ 1.00 |
Exercise price | | $ 1.00 | | higher of
$1.00; and 80% of valuation
of a share
achieved at
the next equity
funding. |
Expected volatility | | 70% | | 70% |
Dividend rate | | — | | — |
Reference risk-free interest rate | | 3.00% | | 3.00% |
Plan duration | | 10 | | 10 |
Fair value of stock options at measurement date | | 0.67 | | 0.18 |
There are no market-related performance conditions or non-vesting conditions that should be considered for determining the fair value of options.
The Group estimates an expected rotation of 2.00% annually at constant value, taking into account historical patterns of executives maintaining their jobs and the probability of exercising the options. This estimate is reviewed at the end of each annual or interim period.
The following table shows the amount and exercise price and the movements of the stock options of executives and managers of the Group for the period ended June 30, 2022.
| | June 30, 2022 |
| | | Group 1 | | Group 2 |
| | | Number of options | | Exercise price | | Number of options | | Exercise price |
As of June 30, 2021 | | — | | — | | — | | — |
Granted during the period | | 909,000 | | £0.74 | | 544,000 | | Higher of
$1.00; and 80%
of valuation
of a share
achieved at
the next equity
funding |
Annulled during the period | | — | | — | | — | | — |
Exercised during the period | | — | | — | | — | | — |
Expired during the period | | — | | — | | — | | — |
As of June 30, 2022 | | 909,000 | | £0.74 | | 544,000 | | Higher of
$1.00; and 80%
of valuation
of a share
achieved at
the next equity
funding |
The charge of the plans based on options recognized is $ 474,562.
F-62
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 11. Share based payment (cont.)
In July 2021, senior management joined the company through a consulting agreement. This agreement established a signing bonus for 365,000 shares at £0.74 for a total of $364,014 disclosed as share-based payment. Subsequently, on July 15, 2022 this agreement was replaced by another one (Note 20 — Subsequent events) leaving without effect the original consulting agreement.
Note 12. Related parties
Balances and transactions between the Group entities, which are related parties, have been eliminated on consolidation and are not disclosed in this note. Transactions between the Group and its directors and/or executive board members and the Company and the Parent are disclosed below.
Transactions with key management personnel
Key management personnel compensation comprised:
In USD ($) | | June 30,
2022 | | June 30,
2021 | | December 31, 2020 |
Short-term employee benefits | | 96,437 | | 109,205 | | 38,355 |
Share based payment | | 838,576 | | — | | — |
Other Related Party Transactions
In USD ($) | | Note | | Transaction
values from
the period
of July 1,
2021 through
June 31,
2022 | | Transaction
values from
the period
of January 1,
2021 through
June 31,
2021 | | Transaction
values from
the period
of August 21,
2020 through
December 31,
2020 |
Share based payment | | | | | | | | |
Key management | | | | 838,576 | | — | | — |
Issue of Additional Shares | | | | | | | | |
Parent of the Company – Bioceres Crop Solutions Corp. | | (i) | | — | | 3,000,000 | | |
Parent of the Company – Union Group Ventures | | (ii) | | — | | — | | 2,000,000 |
Parent of the Company – BG Farming Technologies | | (iii) | | — | | — | | 2,000,000 |
Company’s Cash Balance in Others’ Accounts | | | | | | | | |
Parent of BG Farming Technologies – Bioceres S.A. | | | | — | | — | | 300,000 |
100% Subsidiary of Bioceres S.A. – Bioceres LLC | | (iv) | | — | | 500,000 | | 300,000 |
Expenses Paid on Behalf of the Company | | | | | | | | |
Parent of BG Farming Technologies – Bioceres S.A. | | (v) | | 265,810 | | 509,628 | | 244,029 |
100% Subsidiary of Bioceres S.A. – Bioceres LLC | | (v) | | 156,760 | | 453,282 | | 56,863 |
F-63
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 12. Related parties (cont.)
In USD ($) | | Note | | Transaction
values from
the period
of July 1,
2021 through
June 31,
2022 | | Transaction
values from
the period
of January 1,
2021 through
June 31,
2021 | | Transaction
values from
the period
of August 21,
2020 through
December 31,
2020 |
Services Provided by Other Companies | | | | | | | | |
30% owned by Bioceres S.A. – INMET S.A. – Ingenieria Metabolica S.A | | (vi) | | 252 | | 144,530 | | 160,390 |
98.6% owned by Bioceres S.A. – INDEAR S.A. – Instituto de Agrobiotecnología Rosario | | (vii) | | 57,047 | | 129,695 | | 2,540 |
Owned by Bioceres S.A. – Agrality Inc. | | (viii) | | 85,435 | | — | | — |
Founded and operated by the Company’s CPO – Future Foods B.V. | | (ix) | | 94,857 | | 35,958 | | 17,801 |
Simple Agreement for Future Equity (“SAFE”) | | | | | | | | |
THEO I SCSp | | (Note 15) | | 1,500,000 | | — | | — |
F-64
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 12. Related parties (cont.)
Other Related Party Balances
In USD ($) | | Balance
outstanding
as of
June 30,
2022 | | Balance
outstanding
as of
June 30,
2021 |
100% Subsidiary of Bioceres S.A. – Bioceres LLC | | (385,508 | ) | | 289,888 | |
Parent of BG Farming Technologies – Bioceres S.A. | | — | | | (452,563 | ) |
Note 13. Accounts Payables
| | As of
June 30,
2022 | | As of
June 30,
2021 |
Related Parties (Note 12) | | | (385,508 | ) | | | (452,563 | ) |
Suppliers | | | (840,705 | ) | | | (135,095 | ) |
Total Accounts Payable | | $ | (1,226,213 | ) | | $ | (587,658 | ) |
Note 14. Other liabilities
| | As of
June 30,
2022 | | As of
June 30,
2021 |
Pay as you Earn (“P.A.Y.E.”) | | | (1,171 | ) | | — |
Total Other liabilities | | $ | (1,171 | ) | | — |
Note 15. Simple Agreement for Future Equity (“SAFE”)
The company signed two simple agreements for future equity (referred to as “SAFE” or “SAFEs” in plural), in exchange for the payment by certain investors of the amounts detailed below on December 28, 2021. Both SAFEs were signed with two different investors and for the following amounts:
One SAFE was signed with THEO 1 SCSp for the amount of $1,500,000, from which $1,000,000 was received on January 5, 2022 and $500,000 were received on June 3,2022.
The other SAFE was signed with SERENITY TRADERS LTD. for the amount of $500,000, fully received on January 6, 2022.
Both SAFEs give the investors, in exchange for the payment of the mentioned amounts, the right to a variable number of shares on the Company’s Share Capital subject to the occurrence of a qualified event or a twelve months maturity, whatever happens before, and in the case of a qualified event, specifically the shares of the series of equity securities issued to the investors investing new money in the Company in connection with the closing. These qualified events are defined as Equity Financing of not less than $20,000,000, Change of Control, a Direct Listing, an Initial Public Offering or a De-SPAC Transaction.
As of June 30, 2022, the fair value of Simply Agreement for Future Equity (“SAFE”) amounts to $2,860,000.
F-65
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 15. Simple Agreement for Future Equity (“SAFE”) (cont.)
The following table presents the changes in Level 3 financial instruments as of June 30, 2022:
Balance as of June 30, 2021 | | $ | — |
Additions | | | 2,000,000 |
Losses recognized in the year(i) | | | 860,000 |
Balance as of June 30, 2022 | | $ | 2,860,000 |
Note 16. Finance cost
| | As of
June 30,
2022 | | As of
June 30,
2021 | | As of
December, 31
2020 |
Change in fair value of Simply Agreement for Future Equity (“SAFE”) | | | 860,000 | | | — | | | — |
Exchange rate difference | | | 12,342 | | | — | | | — |
Interest | | | 2,130 | | | — | | | — |
Total finance cost | | $ | 874,472 | | $ | — | | $ | — |
Note 17. Taxes
As of June 30, 2022 and 2021, and December 31, 2020, deferred tax assets aren’t recognised because it is not probable that future taxable amounts will be available to utilize those temporary differences and losses. Therefore, in the present Consolidated Financial Statements, the Company decided not to recognize deferred income tax assets generated by the tax loss carry forward for the periods ended on June 30, 2022 and 2021 and December 31, 2020 for the amounts of $850,855 $377,495 and $69,017 respectively.
Note 18. Capital Management
The Group defines its capital as its shareholder’s equity and debt. The Group’s objectives when managing capital are to safeguard the Group’s ability to continue as a going concern, in order to provide returns for shareholders and benefits for other stakeholders, and to maintain an optimal capital structure to reduce the cost of capital. The Group manages its capital structure and makes adjustments to it in the light of changes in economic conditions. In order to maintain or adjust the capital structure, the Group may issue new shares. The Group is not subject to externally imposed capital requirements. Refer to Note 9 — Share capital and share premium for the Group’s actions related to the management of capital.
Note 19. The Business Combination Agreement
As of June 14, 2022 Moolec Science entered into a Business Combination Agreement by and among Moolec Science, LightJump Acquisition Corp., (SPAC or LightJump) a special purpose acquisition company incorporated under the laws of Delaware, Moolec Science S.A. and Moolec Acquisition, Inc. (Moolec Science S.A. and Moolec Acquisition, Inc are entities under common control together with Moolec Science). As a result of the transactions contemplated by the Business Combination Agreement, each of LightJump Acquisition Corp. and Moolec Science will become direct wholly-owned subsidiaries of Moolec Science S.A. and each of the shareholders of Moolec Science and the shareholders of LightJump Acquisition Corp. are expected to own the majority of the issued and outstanding shares of Moolec Science SA (the “Transaction”).
F-66
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 19. The Business Combination Agreement (cont.)
Transaction closing is subject to customary closing conditions, including approval by LightJump Acquisition Corp.’s shareholders.
The Business Combination will be accounted for as a capital reorganization in accordance with IFRS as issued by the IASB. Under this method of accounting, LightJump will be treated as the “acquired” company for financial reporting purposes and Moolec Science will be the accounting “acquirer”. This determination was primarily based on the assumptions that Moolec Science’s shareholders will hold a majority of the voting power of the Combined Company (Holdco and its consolidated subsidiaries after giving effect to the Business Combination.), Moolec Science’s operations will substantially comprise the ongoing operations of the Combined Company, Moolec Science’s designees are expected to comprise a majority of the governing body of the Combined Company, and Moolec Science’s senior management will comprise the senior management of the Combined Company. However, LightJump does not meet the definition of a “business” pursuant to IFRS 3 Business Combinations, and thus, for accounting purposes, the Business Combination will be accounted for as a capital reorganization. The net assets of LightJump will be stated at historical cost, with no goodwill or other intangible assets recorded.
Note 20. Events after the reporting period
Management has considered subsequent events through September 29, 2022, which is the date in which these Consolidated Financial Statements were issued.
Between July 15, 2022 and August 1, 2022 the company signed four contracts formalizing four corporate officers positions. Three of the new officers were already working with Moolec Science through consulting agreements which are being replaced by their new contracts as officers.
The new positions are: Chief Executive Officer (CEO), Chief Financial Officer (CFO), Chief Product Officer (CPO) and Chief Scientific Officer (CSO).
The new contracts establish similar conditions to the original consulting agreements while adding additional terms establishing updated roles and responsibilities, annual management fees, participation in the Stock Option Plan and Equity Awards, non-disclosure agreements, indemnities and non-competes.
As of July 12, 2022 LightJump One Founders LLC, a SPAC sponsor, signed a promissory note with Moolec Science for $350,000 to be paid in parts and returned at the earlier of: the consummation of the Initial Business Combination of LightJump Acquisition Corp., the termination of the business combination agreement, or January 12, 2023.
Moolec Science has advanced $78,000 to fund payments to the trust account for the benefit of the public stockholders of SPAC and has agreed to fund another $272,000 to fund payments to the trust account upon receipt of request in order to extend the period during which SPAC may consummate its initial business combination from July 12, 2022 to January 12, 2023.
As of July 2022, Moolec Science signed three promissory notes, one with a company’s parent shareholder, THEO I SCSp and two with a company’s shareholder, Union Group Ventures Ltd. One of the promissory notes with Union Group Ventures Ltd. and the one with Theo I SCSp are for the same amount ($175,000) for Moolec Science to fund payments to the trust account for the benefit of the public stockholders of the SPAC upon receipt of requests. These promissory notes need to be paid and returned at the earlier of: the consummation of the Initial Business Combination of LightJump Acquisition Corp., the termination of the business combination agreement, or January 12, 2023.
F-67
Table of Contents
Moolec Science Limited
Notes to the Consolidated Financial Statements
for the year ended June 30, 2022 and for the period of January 1, 2021 through June 30, 2021 and
from inception on August 21, 2020 through December 31, 2020
In USD [$]
Note 20. Events after the reporting period (cont.)
The second promissory note with Union Group Ventures Ltd. is for the sum of $500,000, due and payable by Moolec Science upon the consummation of the business combination agreement. Union Group Ventures Ltd. has agreed to fund Moolec Science for its operations and business objectives until December 2022, upon receipt of requests.
As of 17 August, 2022 funding of $39,000 has been requested and received by Moolec Science from Union Group Ventures Ltd. according to the first promissory note as part of the reimbursement of the first $78,000 advanced to LightJump Acquisition Corp.
As of 22 September, 2022 funding of $500,000 has been requested and received by Moolec Science from Union Group Ventures Ltd. according to the second promissory note.
F-68
Table of Contents
Annex A
Execution Version
BUSINESS COMBINATION AGREEMENT
by and among
LIGHTJUMP ACQUISITION CORPORATION,
MOOLEC SCIENCE SA,
MOOLEC ACQUISITION, INC.,
AND
MOOLEC SCIENCE LIMITED,
Dated as of June 14, 2022
Table of Contents
TABLE OF CONTENTS
| | | | Annex A Page No |
ARTICLE I DEFINITIONS | | A-2 |
Section 1.01 | | Certain Definitions | | A-2 |
Section 1.02 | | Further Definitions | | A-13 |
Section 1.03 | | Construction | | A-15 |
| | | |
ARTICLE II MERGER; POST-MERGER | | A-15 |
Section 2.01 | | The Merger | | A-15 |
Section 2.02 | | Pre-Merger Actions | | A-16 |
Section 2.03 | | Closing; Merger Effective Time | | A-16 |
Section 2.04 | | Effect of the Merger | | A-16 |
Section 2.05 | | Articles; Organizational Documents | | A-17 |
Section 2.06 | | Directors and Officers | | A-17 |
Section 2.07 | | Tax Treatment of the Exchange and the Merger | | A-18 |
Section 2.08 | | Withholding | | A-18 |
| | | |
ARTICLE III EXCHANGE | | A-18 |
Section 3.01 | | Exchange of Securities | | A-18 |
Section 3.02 | | Exchange Consideration | | A-19 |
Section 3.03 | | Exchange of Certificates | | A-19 |
Section 3.04 | | Stock Transfer Books | | A-21 |
Section 3.05 | | SPAC Warrants | | A-21 |
Section 3.06 | | Appraisal Rights | | A-21 |
| | | |
ARTICLE IV REPRESENTATIONS AND WARRANTIES OF THE COMPANY | | A-22 |
Section 4.01 | | Organization and Qualification; Subsidiaries | | A-22 |
Section 4.02 | | Organizational Documents | | A-22 |
Section 4.03 | | Capitalization | | A-22 |
Section 4.04 | | Authority Relative to this Agreement | | A-23 |
Section 4.05 | | No Conflict; Required Filings and Consents | | A-23 |
Section 4.06 | | Permits; Compliance | | A-24 |
Section 4.07 | | Financial Statements | | A-24 |
Section 4.08 | | Absence of Certain Changes or Events | | A-25 |
Section 4.09 | | Absence of Litigation | | A-26 |
Section 4.10 | | Employee Benefit Plans | | A-26 |
Section 4.11 | | Labor and Employment Matters | | A-28 |
Section 4.12 | | Real Property; Title to Assets | | A-29 |
Section 4.13 | | Intellectual Property | | A-30 |
Section 4.14 | | Taxes | | A-31 |
Section 4.15 | | Environmental Matters | | A-33 |
Section 4.16 | | Material Contracts | | A-34 |
Section 4.17 | | Insurance | | A-36 |
Section 4.18 | | Board Approval; Vote Required | | A-36 |
Section 4.19 | | Certain Business Practices | | A-36 |
Section 4.20 | | Product Warranty; Product Liability | | A-37 |
Section 4.21 | | Compliance with Regulatory Laws | | A-37 |
Section 4.22 | | Interested Party Transactions | | A-38 |
Section 4.23 | | Exchange Act | | A-38 |
Annex A-i
Table of Contents
| | | | Annex A Page No |
Section 4.24 | | Brokers | | A-38 |
Section 4.25 | | Sanctions, Import Control, and Export Control Laws | | A-38 |
Section 4.26 | | Exchange Agreements | | A-39 |
Section 4.27 | | Exclusivity of Representations and Warranties | | A-39 |
| | | |
ARTICLE V REPRESENTATIONS AND WARRANTIES OF SPAC | | A-40 |
Section 5.01 | | Corporate Organization | | A-40 |
Section 5.02 | | SPAC Organizational Documents | | A-40 |
Section 5.03 | | Capitalization | | A-40 |
Section 5.04 | | Authority Relative to this Agreement | | A-41 |
Section 5.05 | | No Conflict; Required Filings and Consents | | A-41 |
Section 5.06 | | Compliance | | A-41 |
Section 5.07 | | SEC Filings; Financial Statements; Sarbanes-Oxley | | A-41 |
Section 5.08 | | Absence of Certain Changes or Events | | A-43 |
Section 5.09 | | Absence of Litigation | | A-43 |
Section 5.10 | | Board Approval; Vote Required | | A-43 |
Section 5.11 | | Brokers | | A-43 |
Section 5.12 | | SPAC Trust Fund | | A-43 |
Section 5.13 | | Employees | | A-44 |
Section 5.14 | | Taxes | | A-44 |
Section 5.15 | | Listing | | A-46 |
Section 5.16 | | Prior Business Operation | | A-46 |
Section 5.17 | | SPAC Material Contracts | | A-46 |
Section 5.18 | | Investment Company Act | | A-46 |
Section 5.19 | | Proxy Statement/Prospectus and Registration Statement | | A-46 |
Section 5.20 | | SPAC’s Investigation and Reliance | | A-46 |
| | | |
ARTICLE VI REPRESENTATIONS AND WARRANTIES OF HOLDCO AND MERGER SUB | | A-47 |
Section 6.01 | | Organization | | A-47 |
Section 6.02 | | Organization Documents | | A-47 |
Section 6.03 | | Capitalization | | A-47 |
Section 6.04 | | Authority Relative to this Agreement | | A-48 |
Section 6.05 | | No Conflict; Required Filings and Consents | | A-48 |
Section 6.06 | | Compliance | | A-48 |
Section 6.07 | | Board Approval; Vote Required | | A-49 |
Section 6.08 | | No Prior Operations of Holdco or Merger Sub; Post-Closing Operations | | A-49 |
Section 6.09 | | Brokers | | A-49 |
Section 6.10 | | Proxy Statement/Prospectus and Registration Statement | | A-49 |
Section 6.11 | | Tax Matters | | A-49 |
| | | |
ARTICLE VII CONDUCT OF BUSINESS PENDING THE MERGER | | A-49 |
Section 7.01 | | Conduct of Business by the Company, Holdco and Merger Sub Pending the Merger | | A-49 |
Section 7.02 | | Conduct of Business by SPAC Pending the Merger | | A-53 |
Section 7.03 | | Claims Against Trust Account | | A-54 |
Section 7.04 | | SPAC Public Filings | | A-54 |
| | | |
ARTICLE VIII ADDITIONAL AGREEMENTS | | A-54 |
Section 8.01 | | Proxy Statement; Registration Statement | | A-54 |
Section 8.02 | | SPAC Shareholders’ Meetings | | A-56 |
Section 8.03 | | Access to Information; Confidentiality | | A-58 |
Annex A-ii
Table of Contents
| | | | Annex A Page No |
Section 8.04 | | Employee Matters | | A-58 |
Section 8.05 | | Directors’ and Officers’ Indemnification | | A-59 |
Section 8.06 | | Notification of Certain Matters | | A-60 |
Section 8.07 | | Further Action; Reasonable Best Efforts | | A-60 |
Section 8.08 | | Public Announcements | | A-61 |
Section 8.09 | | Tax Matters | | A-61 |
Section 8.10 | | Stock Exchange Listing | | A-62 |
Section 8.11 | | Delisting and Deregistration | | A-62 |
Section 8.12 | | Antitrust | | A-62 |
Section 8.13 | | PCAOB Financials | | A-63 |
Section 8.14 | | Backstop Agreement | | A-63 |
Section 8.15 | | Exclusivity | | A-63 |
Section 8.16 | | Trust Account | | A-64 |
Section 8.17 | | Treatment of Interested Party Transactions | | A-64 |
Section 8.18 | | EU Securities Regulation | | A-64 |
Section 8.19 | | Opinion of the Financial Advisor | | A-65 |
Section 8.20 | | SPAC Extension Proposal | | A-65 |
| | | |
ARTICLE IX CONDITIONS TO THE MERGER | | A-65 |
Section 9.01 | | Conditions to the Obligations of Each Party | | A-65 |
Section 9.02 | | Conditions to the Obligations of SPAC | | A-66 |
Section 9.03 | | Conditions to the Obligations of the Company | | A-67 |
| | | |
ARTICLE X TERMINATION, AMENDMENT AND WAIVER | | A-68 |
Section 10.01 | | Termination | | A-68 |
Section 10.02 | | Notice of Termination; Effect of Termination | | A-69 |
Section 10.03 | | Expenses | | A-69 |
Section 10.04 | | Amendment | | A-69 |
Section 10.05 | | Waiver | | A-70 |
| | | |
ARTICLE XI GENERAL PROVISIONS | | A-70 |
Section 11.01 | | Notices | | A-70 |
Section 11.02 | | Non-Survival of Representations, Warranties and Covenants | | A-71 |
Section 11.03 | | Severability | | A-71 |
Section 11.04 | | Entire Agreement; Assignment | | A-71 |
Section 11.05 | | Parties in Interest; Non-Recourse | | A-71 |
Section 11.06 | | Governing Law | | A-71 |
Section 11.07 | | Waiver of Jury Trial | | A-72 |
Section 11.08 | | Headings | | A-72 |
Section 11.09 | | Counterparts | | A-72 |
Section 11.10 | | Specific Performance | | A-72 |
Section 11.11 | | Drafting of the Agreement | | A-72 |
Section 11.12 | | Provision Respecting Legal Representation | | A-73 |
Annex A-iii
Table of Contents
EXHIBITS
EXHIBIT A Registration Rights and Lock-Up Agreement
EXHIBIT B-1 Amended and Restated SPAC COI
EXHIBIT B-2 Amended and Restated SPAC Bylaws
EXHIBIT C A&R Holdco Organizational Documents
EXHIBIT D SPAC Warrant Amendment and Assignment
SCHEDULES
SCHEDULE A Company Knowledge Parties
APPENDIXES
APPENDIX A Illustrative Capitalization Table
APPENDIX B Illustrative SPAC Transaction Expenses
Annex A-iv
Table of Contents
This BUSINESS COMBINATION AGREEMENT is made and entered into as of June 14, 2022 (this “Agreement”), by and among LightJump Acquisition Corporation, a Delaware corporation (“SPAC”), Moolec Science Limited, a private limited company incorporated under the laws of England and Wales (the “Company”), Moolec Science SA, a public limited liability company (société anonyme) governed by the laws of the Grand Duchy of Luxembourg, with its registered office at 17, Boulevard F.W. Raiffeisen, L-2411 Luxembourg, Grand Duchy of Luxembourg and registered with the Luxembourg Trade and Companies’ Register (Registre de Commerce et des Sociétés, Luxembourg) under number B268440 (“Holdco”), and Moolec Acquisition, Inc., a Delaware corporation (“Merger Sub”). Each of SPAC, Holdco, the Company, and Merger Sub shall individually be referred to herein as a “Party” and, collectively, the “Parties”.
WHEREAS, SPAC is a special purpose acquisition company incorporated under the laws of Delaware for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, recapitalization, reorganization or other similar business combination with one or more businesses or entities;
WHEREAS, each of Holdco and Merger Sub is an entity newly formed for the purposes of the transactions proposed herein, Holdco is wholly-owned by the Company Shareholders and Merger Sub is a direct wholly-owned subsidiary of Holdco;
WHEREAS, upon the terms and subject to the conditions of this Agreement and those certain individual Contribution and Exchange Agreements, each dated as of the date hereof (collectively, the “Exchange Agreements”), by and among Holdco, the Company and each of the Company Shareholders, and in accordance with the Companies Act 2006 (as amended from time to time, the “Companies Act”), the Luxembourg Law of 10 August 1915 on commercial companies (as amended from time to time, the “1915 Law”), and the Delaware General Corporation Law (as amended from time to time, the “DGCL”), SPAC, Holdco, Merger Sub and the Company will enter into a business combination transaction pursuant to which, among other things, (a) pursuant to the Exchange Agreements, each of the Company Shareholders, effective on the Exchange Effective Time, will contribute its respective Company Ordinary Shares to Holdco in exchange for Holdco Ordinary Shares to be subscribed for by each such Company Shareholder (such contributions and exchanges of Company Ordinary Shares for Holdco Ordinary Shares, collectively, the “Exchange”), (b) as a result of the Exchange, the Company will become a wholly-owned subsidiary of Holdco and the Company Shareholders will increase the number of issued and outstanding Holdco Ordinary Shares held by them, in accordance with the Exchange Agreements, (c) immediately prior to the consummation of the Merger, each of the Company SAFE Holders will receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the applicable Company SAFE, (d) following the consummation of the Exchange, Merger Sub will merge with and into SPAC, with SPAC surviving such merger and becoming a direct wholly-owned subsidiary of Holdco (the “Merger”) and, in the context of the Merger, all SPAC Common Stock outstanding shall be exchanged with Holdco for the right to receive the Merger Consideration in the form of Holdco Ordinary Shares pursuant to a share capital increase of Holdco, as set forth in this Agreement, and (e) in order to satisfy the Company’s obligations under that certain Consulting Agreement, dated June 18, 2021 (the “CFO Consulting Agreement”), by and between the Company and the Company’s Chief Financial Officer (“CFO”), CFO will be freely allotted an aggregate of 243,774 Holdco Ordinary Shares (the “CFO Free Shares”);
WHEREAS, in connection with the Exchange and Merger, the Parties desire for Holdco to register with the SEC to become a publicly traded company;
WHEREAS, each of the Company Shareholders and Board of Directors of the Company (the “Company Board”) have unanimously (a) determined that the Transactions are in the best interests of the Company and the Company Shareholders and (b) approved this Agreement, the Transaction Documents to which the Company is or will be a party, and the Transactions;
WHEREAS, the Board of Directors of SPAC (the “SPAC Board”) has unanimously (a) determined that the Merger and the other Transactions are in the best interests of SPAC, (b) adopted a resolution approving this Agreement and declaring its advisability and approving the Merger and the other Transactions, and (c) recommended the approval and adoption of this Agreement, the Merger and the other Transactions by the shareholders of SPAC (the “SPAC Shareholders”);
Annex A-1
Table of Contents
WHEREAS, the Board of Directors of Holdco (the “Holdco Board”) has unanimously determined that the Transactions are in the best interests of Holdco and has approved this Agreement, the Transaction Agreements to which Holdco is or will be a party, and the Transactions;
WHEREAS, the Board of Directors of Merger Sub (the “Merger Sub Board”) has unanimously (a) determined that this Agreement, the Merger and the other Transactions are in the best interests of Merger Sub, (b) adopted a resolution approving this Agreement and declaring its advisability and approving the Merger and the other Transactions, and (c) recommended the approval and adoption of this Agreement and the Merger by Holdco (as the sole shareholder of Merger Sub);
WHEREAS, in connection with the Closing, SPAC, Holdco, Sponsor, each of the persons and entities listed on Exhibit A attached thereto and the Company Shareholders, Company SAFE Holders and CFO shall enter into a Registration Rights and Lock-Up Agreement (“Registration Rights and Lock-Up Agreement”) substantially in the form attached hereto as Exhibit A;
WHEREAS, contemporaneously with the execution of this Agreement, each of Union Group Ventures Limited, UG Holdings, LLC, THEO I SCSp and LightJump One Founders, LLC (“Sponsor”) has entered into a Backstop Agreement (the “Backstop Agreement”), guaranteeing, severally but not jointly, the funding of certain amounts as set forth therein;
WHEREAS, pursuant to the Backstop Agreement, immediately prior to the Closing, Sponsor agrees to transfer to UG Holdings, LLC (or its designated affiliate) 1,035,000 shares of SPAC Common Stock.
WHEREAS, for U.S. federal income Tax purposes, the Merger is intended to qualify as a “reorganization” within the meaning of Section 368(a)(1)(A) of the Code by reason of Section 368(a)(2)(E) of the Code, and the Merger and the Exchange are being undertaken as part of a prearranged, integrated plan and are intended to qualify as exchanges described in Section 351 of the Code and the Treasury Regulations promulgated thereunder; and
NOW, THEREFORE, in consideration of the foregoing and the mutual covenants and agreements herein contained, and intending to be legally bound hereby, the Parties hereby agree as follows:
ARTICLE I
DEFINITIONS
SECTION 1.01 Certain Definitions. For purposes of this Agreement:
“Accounting Principles” means GAAP in case of SPAC and IFRS in case of the Company and Company Subsidiaries under the Financial Statements, in each case, as in effect from time to time.
“affiliate” of a specified person means a person who, directly or indirectly through one or more intermediaries, controls, is controlled by, or is under common control with, such specified person.
“Ancillary Agreements” means the Exchange Agreements, the Registration Rights and Lock-Up Agreement, the Backstop Agreement, the Transaction Support Agreement and all other agreements, certificates and instruments executed and delivered by SPAC, Holdco, Merger Sub or the Company in connection with the Transactions and specifically contemplated by this Agreement.
“Anti-Corruption Laws” means (a) the UK Bribery Act 2010, (b) the U.S. Foreign Corrupt Practices Act 1977, as amended, (b) anti-bribery legislation promulgated by the European Union and implemented by its member states, (c) legislation adopted in furtherance of the OECD Convention on Combating Bribery of Foreign Public Officials in International Business Transactions, and (d) other similar laws, regulations, rules and guidance (having the force of law) of any jurisdiction applicable to SPAC, Holdco, Merger Sub or the Company concerning or relating to bribery or corruption.
“Anti-Money Laundering Laws” means all laws, rules, regulations, and guidance (having the force of law) of any jurisdiction applicable to SPAC, Holdco, Merger Sub or the Company concerning terrorist financing or Money Laundering, including, without limitation, (a) the Money Laundering Control Act of 1986, (b) the USA PATRIOT Act, and (b) the Bank Secrecy Act.
Annex A-2
Table of Contents
“Average SPAC Share Price” means the average of the volume weighted averages of the trading prices of the SPAC Common Stock on Nasdaq (as reported by Bloomberg L.P. or, if not reported therein, in another authoritative source mutually selected by the Parties) on each of the ten (10) consecutive trading days ending on (and including) the trading day that is three (3) trading days prior to the date of the Merger Effective Time.
“Business Data” means all business information and data, including Personal Information (whether of employees, contractors, consultants, customers, consumers, or other persons and whether in electronic or any other form or medium) that is accessed, collected, used, processed, stored, shared, distributed, transferred, disclosed, destroyed, or disposed of by any of the Business Systems or otherwise in the course of the conduct of the business of the Company or any Company Subsidiaries.
“Business Day” means any day on which the principal offices of the SEC in Washington, D.C. are open to accept filings and on which banks are not required or authorized to close in the City of New York in the United States of America, in London, England or Luxembourg in the Grand Duchy of Luxembourg; provided that banks shall not be deemed to be authorized or obligated to be closed due to a “shelter in place,” “non-essential employee” or similar closure of physical branch locations at the direction of any Governmental Authority if such banks’ electronic funds transfer systems (including for wire transfers) are open for use by customers on such day.
“Business Systems” means all Software, computer hardware (whether general or special purpose), electronic data processing, information, record keeping, communications, telecommunications, networks, interfaces, platforms, servers, peripherals, and computer systems, including any outsourced systems and processes, that are owned or used in the conduct of the business of the Company or any Company Subsidiaries.
“Code” means the United States Internal Revenue Code of 1986, as amended.
“Company Board Approval” means the Company Board resolutions approving and authorizing (a) this Agreement, the Transaction Documents to which the Company is or will be a party, and the Transactions, and (b) the Exchange Issuance.
“Company IP” means, collectively, all Company-Owned IP and Company-Licensed IP.
“Company-Licensed IP” means all Intellectual Property rights owned or purported to be owned by a third party and licensed to the Company or any Company Subsidiary or to which the Company or any Company Subsidiary otherwise has a right to use.
“Company Material Adverse Effect” means any Effects that, individually or in the aggregate with all other Effects, (a) is or would reasonably be expected to be materially adverse to the business, condition (financial or otherwise), assets, liabilities or operations of the Company and the Company Subsidiaries taken as a whole or (b) does or would prevent, materially delay or materially impede the performance by the Company of its obligations under this Agreement or the consummation of the Exchange, Merger or any of the other Transactions; provided, however, that none of the following shall be deemed to constitute, alone or in combination, or be taken into account in the determination of whether there has been or will be, a Company Material Adverse Effect: (i) any enactment of, change or proposed change in or change in the interpretation of any Law or Accounting Principles; (ii) Effects generally affecting the industries or geographic areas in which the Company or any of the Company Subsidiaries operate; (iii) any downturn in general economic conditions, including changes in the credit, debt, securities, financial or capital markets (including changes in interest or exchange rates, prices of any security or market index or commodity or any disruption of such markets); (iv) acts of war (whether or not declared), sabotage, civil unrest, terrorism, curfews, riots, demonstrations or public disorders, or any escalation or worsening of any such acts of war, sabotage, civil unrest, terrorism, curfews, riots, demonstrations or public disorders, or changes in global, national, regional, state or local political or social conditions; (v) any hurricane, tornado, flood, earthquake, natural disaster, or other acts of God; (vi) Effects arising from or relating to epidemics, pandemics, or disease outbreaks, including COVID-19 or any COVID-19 Measures; (vii) any actions taken or not taken by the Company or any of the Company Subsidiaries as specifically required by this Agreement or any Ancillary Agreement, (viii) the announcement or execution, pendency, negotiation or consummation of the Merger, the Exchange or any of the other Transactions (including the impact thereof on relationships with customers, suppliers, employees or Governmental Authorities); provided that this clause (viii) shall not apply in determining a Company Material Adverse Effect resulting from a breach of the representations and warranties set forth in Section 4.05;
Annex A-3
Table of Contents
(ix) any failure by the Company or any of the Company Subsidiaries to meet any projections, forecasts, guidance, estimates, milestones, budgets or financial or operating predictions of revenue, earnings, cash flow or cash position, provided that this clause (ix) shall not prevent a determination that any change, event, or occurrence underlying such failure has resulted in a Company Material Adverse Effect; (x) any pending or initiated Action against the Company, any of the Company Subsidiaries or any of their respective officers or directors, in each case, arising out of or relating to the execution of this Agreement, any Ancillary Agreements or any of the Transactions (other than any Action (A) commenced by any Party to enforce its rights under this Agreement or any Ancillary Agreement to which it is a party or (B) resulting from or arising from a breach of the representations and warranties set forth in Section 4.05); (xi) any action taken by SPAC; or (xii) any actions taken, or failures to take action, or such other changes or events, in each case, which SPAC has specifically requested or to which it has specifically consented or which actions are specifically contemplated by this Agreement or any Ancillary Agreement, in each case, except in the cases of clauses (i) through (vi), to the extent that the Company and the Company Subsidiaries, taken as a whole, are disproportionately affected thereby as compared with other participants in the industries or geographic areas in which the Company and the Company Subsidiaries operate.
“Company Ordinary Shares” means the Company’s ordinary shares, with a nominal value of £0.01 per share representing the entire issued share capital of the Company.
“Company Organizational Documents” means the memorandum and articles of association of the Company, as amended, modified or supplemented from time to time, including as contemplated in Section 2.05(c).
“Company-Owned IP” means all Intellectual Property rights owned or purported to be owned by the Company or any of the Company Subsidiaries.
“Company Requisite Approvals” means the Company Board Approval.
“Company SAFE” means each of the simple agreement for future equity by and between the Company and the Company SAFE Holder named therein (an “Original SAFE”) or any simple agreement for future equity between Holdco and that Company SAFE Holder issued in consideration for the contribution by the Company SAFE Holder of its rights in the Original SAFE to Holdco (in which case the Original SAFE will cease to be a “Company SAFE”) with such adjustments (if any) required under Luxembourg law.
“Company SAFE Holder” means each Person that has entered into a Company SAFE.
“Company Shareholders” means the holders of all of the Company Ordinary Shares and all other shares being Equity Interest as of immediately prior to the Exchange Effective Time.
“Company Shareholders’ Agreements” means the shareholders’ agreements, put option agreements, pledge agreements and similar agreements entered into by and among the Company and one or more of the Company Shareholders, other than agreements entered into on or about the date hereof by the Company and one or more of the Company Shareholders in connection with any of the Transactions, each of which will expire on or prior to the Closing Date in accordance with the Termination Agreements.
“Company Shareholders’ Agreements Liens” means the Liens under the Company Shareholders’ Agreements, each of which will expire on or prior to the Closing Date in accordance with the Termination Agreements.
“Company Transaction Expenses” means the reasonable and documented Transaction Expenses of the Company or any of its affiliates, including, without limitation, (a) Transaction Expenses incurred in the negotiation and preparation of this Agreement, the Ancillary Agreements and the other documents contemplated hereby and thereby and the performance and compliance with all agreements and conditions contained herein and therein, (b) Transaction Expenses incurred in preparing and obtaining the PCAOB Financials, (c) Transaction Expenses incurred in connection with obtaining the consent or approval of any person or Governmental Authority in connection with the Transactions, (d) Transaction Expenses incurred in connection with the Transactions (including the formation of Holdco, Merger Sub and the structuring, negotiation and documentation of the Exchange and Merger) and (e) Transaction Expenses incurred in connection with obtaining the D&O Tail Policy. For the avoidance of doubt, the Parties acknowledge and agree that the Company Transaction Expenses include the fees, expenses and disbursements of legal counsel, auditors and accountants, due diligence expenses, advisory and consulting fees and expenses, and other third-party fees.
Annex A-4
Table of Contents
“Competing Seller” means a person (including any financial investor or group of financial investors) actively engaged, directly or indirectly, in any one or more of the development, production, marketing, distribution and/or exploitation of any products and/or services, in each case other than the Company, the Company Shareholders and indirect equityholders or any Company Subsidiary.
“Competing SPAC Transaction” means any merger or business combination between SPAC, on the one hand, and a Competing Seller, on the other hand.
“Competing Transaction” means any (a) sale or transfer (except in the ordinary course of business consistent with past practices) of all or substantially all of the assets of the Company or any Company Subsidiary to any person or (b) merger or business combination between the Company or any Company Subsidiary, on the one hand, and any other person, on the other hand.
“Confidential Information” means any information, knowledge or data concerning the businesses and affairs of the Company, the Company Subsidiaries, or any Suppliers or customers of the Company or any Company Subsidiaries or SPAC or its subsidiaries (as applicable) that is not already generally available to the public, including any Intellectual Property rights.
“control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly, or as trustee or executor, of the power to direct or cause the direction of the management and policies of a person, whether through the ownership of voting securities, as trustee or executor, by contract or otherwise.
“COVID-19” means the novel coronavirus known as SARS-CoV-2 or COVID-19, and any evolutions, mutations thereof or related or associated epidemics, pandemic or disease outbreaks.
“COVID-19 Measures” means any quarantine, “shelter in place,” “stay at home,” workforce reduction, social distancing, delay, shut down (including the shutdown of air cargo routes), closure, sequester, safety or similar Law, directive, guideline or recommendation promulgated by any Governmental Authority, in each case with or in response to COVID-19.
“Dataroom” means that certain virtual dataroom named “Moolec Science Limited” and located on Intralinks.
“Deferred Fees” shall mean the amount of deferred fees held in the Trust Account in connection with SPAC’s initial public offering payable to the underwriters or other advisors upon consummation of a business combination.
“Disabling Devices” means undisclosed Software viruses, time bombs, logic bombs, trojan horses, trap doors, back doors, or other computer instructions, intentional devices or techniques that are designed to threaten, infect, assault, vandalize, defraud, disrupt, damage, disable, maliciously encumber, hack into, incapacitate, infiltrate or slow or shut down a computer system or any component of such computer system, including any such device affecting system security or compromising or disclosing user data in an unauthorized manner.
“Dissenting Shares” means the SPAC Common Stock outstanding immediately prior to the Merger Effective Time and held by a SPAC Shareholder who has not voted in favor of the Merger or consented thereto in writing and who has properly demanded appraisal for such SPAC Common Stock in accordance with Section 262 of the DGCL.
“EarlyBird Amendment” means that certain amendment dated June 14, 2022 to the engagement letter between the SPAC and EarlyBirdCapital, Inc. (“EarlyBird”) dated January 8, 2020 (as amended from time to time, the “EarlyBird Engagement Letter”).
“EarlyBird Cash Fees” means all cash fees and expenses payable to EarlyBird pursuant to the EarlyBird Engagement Letter.
“EarlyBird Share Fees” means the shares of Holdco to be issued to EarlyBird pursuant to the EarlyBird Engagement Letter.
“Effects” means, collectively, events, circumstances, changes and effects.
“Employee” means any person employed by the Company or any Company Subsidiary.
Annex A-5
Table of Contents
“Environmental Laws” means any Law relating to: (a) Releases or threatened Releases of Hazardous Substances or materials containing Hazardous Substances; (b) the presence, manufacture, refining, production, generation, handling, transport, use, treatment, recycling, storage, importing, labeling, testing, disposal, cleanup or control of Hazardous Substances or materials containing Hazardous Substances; (c) pollution or protection of the environment or natural resources; or (d) public health and safety or, as it relates to the handling of or exposure to Hazardous Substances, worker/occupational health and safety.
“Equity Interest” means all shares of capital stock, common stock, preferred stock, units, ownership interests and any other equity ownership or participation in any Person, including all options, warrants, preemptive rights, calls, convertible securities, simple agreements to acquire future equity, conversion rights or other rights, agreements, arrangements or commitments of any character relating to the issued or unissued capital stock of any Person.
“Exchange Effective Time” means the time on which the issuance of the new Holdco Ordinary Shares pursuant to the Holdco Delegate Resolutions is effective on the Closing Date, which shall be the effective time of the contribution and exchange of the Company Ordinary Shares held by the Company Shareholders and exchanged for Holdco Ordinary Shares, as applicable and as contemplated under the Exchange Agreements, and which shall occur immediately prior to the Merger Effective Time.
“Exchange Ratio” means 0.66787343, the ratio used for determining the number of aggregate Holdco Ordinary Shares for which the aggregate Company Ordinary Shares shall be converted in accordance with Section 2.02(a).
“Export Control Laws” means export control laws and regulations of any jurisdiction applicable to SPAC, Holdco, Merger Sub or the Company including the U.S. Export Administration Regulations, 15 C.F.R. §§ 730, et seq., as amended, and any other equivalent or comparable export control laws and regulations of other countries.
“Extension Amendment to the SPAC COI” means an amendment to the SPAC COI to extend the date by which the SPAC must consummate the Transactions from July 12, 2022 to a date no later than 6 months after the date hereof.
“Extension Amendment Fees” means all fees and expenses incurred by SPAC solely relating to the Extension Amendment to the SPAC COI.
“GAAP” means generally accepted accounting principles as in effect in the United States from time to time.
“Governmental Authority” means any U.S. federal, state, county or local or non-U.S. government, governmental, national, regulatory or administrative authority, agency, instrumentality or commission or any court, tribunal, or judicial or arbitral body.
“Hazardous Substance(s)” means: (a) any substance, material or waste which is regulated by, or for which liability or standards of conduct may be imposed under, any Environmental Law, including any substance, material or waste which is defined as a “hazardous substance,” “hazardous material,” “hazardous waste,” “solid waste,” “pollutant,” “contaminant,” “toxic substance,” “toxic waste” or other similar term or phrase under any Environmental Law; (b) petroleum and petroleum products, including crude oil and any fractions thereof; (c) natural gas, natural gas liquids, synthetic gas, and any mixtures thereof; (d) polychlorinated biphenyls, asbestos and asbestos-containing materials, urea formaldehyde, toxic mold, and radon; and (e) per- or polyfluoroalkyl substances.
“HMRC” means HM Revenue and Customs.
“Holdco Board Approval” means one or several Holdco Board resolutions with respect to the approval of the Transaction and the Transaction Documents to which Holdco is or will be a party, including, for the avoidance of doubt, (a) the approval by the Holdco Board of the issuance on the Closing Date (and conditional on Closing) by a delegate of (i) new Holdco Ordinary Shares following the Merger as Merger Consideration, and (ii) the CFO Free Shares, both under the authorized share capital of Holdco and pursuant to the Holdco Delegate Merger Resolutions and (b) the issuance of new Holdco Ordinary Shares to the Company Shareholders as part of the Exchange, by a delegate under the authorized share capital of Holdco and pursuant to the Holdco Delegate Exchange Resolutions.
Annex A-6
Table of Contents
“Holdco Delegate Exchange Resolutions” means the resolutions taken on the Closing Date by the delegate appointed by the Holdco Board pursuant to the Holdco Board Approval in order to issue on the Closing Date new Holdco Ordinary Shares in the context of the Exchange, under the authorized share capital of Holdco.
“Holdco Delegate Merger Resolutions” means the resolutions taken on the Closing Date by the delegate appointed by the Holdco Board pursuant to the Holdco Board Approval in order to issue on the Closing Date (i) the Merger Consideration in the form of new Holdco Ordinary Shares and (ii) the CFO Free Shares.
“Holdco Delegate Resolutions” means the Holdco Delegate Exchange Resolutions and the Holdco Delegate Merger Resolutions.
“Holdco Ordinary Shares” means the ordinary shares of Holdco, each having a nominal value in US dollars of $0.01.
“Holdco Organizational Documents” means the Articles of Association of Holdco as amended, modified or supplemented from time to time, including as contemplated by the Holdco Delegate Resolutions and the Holdco Shareholder Approval.
“Holdco Requisite Approvals” means the Holdco Board Approval, the Holdco Delegate Resolutions and the Holdco Shareholder Approval, as applicable.
“Holdco Shareholder Approval” means the approval of the extraordinary general meeting of the shareholders of Holdco, being the Company Shareholders, to be held in front of a Luxembourg notary prior to the Closing Date to inter alia implement the A&R Holdco Organizational Documents as contemplated by 2.05(b).
“IFRS” means the International Financial Reporting Standards, as issued by the IFRS Foundation and the International Accounting Standards Board.
“Import Control Laws” means import control laws and regulations of any jurisdiction applicable to SPAC, Holdco, Merger Sub or the Company, including those administered by U.S. Customs and Border Protection and U.S. Immigration and Customs Enforcement (19 U.S.C. §§ 1-4454 and 19 C.F.R. §§ 1-199), and any other equivalent or comparable import control laws and regulations of other countries.
“Intellectual Property” means: (a) patents, patent applications and patent disclosures, together with all reissues, continuations, continuations-in-part, divisionals, revisions, extensions or reexaminations thereof; (b) trademarks and service marks, trade dress, logos, trade names, corporate names, brands, slogans, and other source identifiers, and all applications, registrations, and renewals in connection therewith, together with all of the goodwill associated with the foregoing; (c) copyrights, and other works of authorship (whether or not copyrightable), and moral rights, and registrations and applications for registration, renewals and extensions thereof; (d) trade secrets and know-how (including ideas, formulas, compositions, inventions (whether or not patentable or reduced to practice)), customer and supplier lists, improvements, protocols, processes, methods and techniques, research and development information, industry analyses, algorithms, architectures, layouts, drawings, specifications, designs, plans, methodologies, proposals, industrial models, technical data, financial and accounting data (including pricing and cost information), and all other data, databases and database rights; (e) Internet domain names and social media accounts; (f) rights of privacy and publicity and all other intellectual property or proprietary rights of any kind or description recognized under applicable Laws; (g) copies and tangible embodiments of any of the foregoing, in whatever form or medium; and (h) all legal rights arising from items (a) through (f), including the right to prosecute and perfect such interests and rights to sue, oppose, cancel, interfere, and enjoin based upon such interests, including such rights based on past infringement, if any, in connection with any of the foregoing.
“Inventory” means all inventories of the Company and the Company Subsidiaries wherever located, including (if any) raw materials, goods consigned to vendors or subcontractors, works in process, finished goods, spare parts, goods in transit, and inventory on consignment.
“knowledge” or “to the knowledge” of a person means in the case of the Company, the actual knowledge of the persons listed on Schedule A (each, a “Company Knowledge Party”) after reasonable inquiry, and in the case of SPAC, the actual knowledge of Robert Bennett, Eric Ver Ploeg and William Bunker, after reasonable inquiry.
Annex A-7
Table of Contents
“Law” means any federal, national, state, county, municipal, provincial, local, foreign or multinational, statute, constitution, common law, ordinance, code, decree, order, judgment, rule, regulation, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Authority.
“Leased Real Property” means all real property leased, subleased, licensed or sublicensed by the Company or Company Subsidiaries as tenant, subtenant, licensee or sublicensee together with, to the extent leased, subleased, licensed, or sublicensed by the Company or Company Subsidiaries, all buildings and other structures, facilities or improvements located thereon and all easements, licenses, rights and appurtenances of the Company or Company Subsidiaries relating to the foregoing.
“Lien” means any lien, security interest, mortgage, deeds of trust, pledge, adverse claim, usufruct, option, right of first refusal, right of first offer, charge, claim, equitable interest, easement, encroachment, lease or sublease, or restriction on the right to vote, sell, transfer or otherwise dispose of any capital stock, shares, or other voting securities, or similar encumbrance (other than those created under applicable securities Laws, and not including any license of Intellectual Property).
“Merger Sub Organizational Documents” means the certificate of incorporation and bylaws of Merger Sub, in each case, as amended, modified or supplemented from time to time.
“Misrepresentation” means an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made.
“Money Laundering” means the acquisition, possession, use, conversion, transfer or concealment of the true nature of property of any description, and legal documents or instruments evidencing title to, or interest in, such property, knowing that such property is an economic advantage from criminal offenses, for the purpose of (a) concealing or disguising the illicit origin of the property or (b) assisting any person who is involved in the commission of the criminal offense as a result of which such property is generated, to evade the legal consequences of such actions.
“Net Available Assets” means an amount, determined as of the Closing (or as of another specified time), equal to (i) the total amount of Trust Account Cash remaining as of the Redemption Closing Time plus (ii) the aggregate amount of any proceeds received by Holdco, SPAC or their respective subsidiaries in connection with any financing, funding, contribution or other amounts raised by the Parties prior to the Closing in connection with the transactions contemplated by this Agreement as a direct result of SPAC’s relationship with EarlyBird (including any funds received as a result of a sale of the securities of Holdco, SPAC or any of their respective subsidiaries); provided that the Net Available Assets shall be determined assuming (a) all payments required to be made to the holders of the SPAC Common Stock exercising Redemption Rights as of the Redemption Closing Time have been paid, (b) no SPAC Transaction Expenses or any EarlyBird Cash Fees have been paid from the Trust Account (and if any such amounts have previously been paid from the Trust Account, such payments shall be added back to Net Available Assets) and (c) no amounts have been funded pursuant to the obligations under the Backstop Agreement.
“Nasdaq” means the Nasdaq Capital Market, the Nasdaq Global Market or the Nasdaq Global Select Market, as may be applicable.
“Open Source Software” means any Software that is licensed pursuant to: (a) any license that is a license now or in the future approved by the open source initiative and listed at http://www.opensource.org/licenses, which licenses include all versions of the GNU General Public License (GPL), the GNU Lesser General Public License (LGPL), the GNU Affero GPL, the MIT license, the Eclipse Public License, the Common Public License, the CDDL, the Mozilla Public License (MPL), the Artistic License, the Netscape Public License, the Sun Community Source License (SCSL), and the Sun Industry Standards License (SISL); or (b) any license to Software that is considered “free” or “open source software” by the open source foundation or the free software foundation.
“Payment Spreadsheet” means a spreadsheet that shall be delivered by the Company to SPAC pursuant to Section 3.01(b) at least five (5) Business Days prior to the Closing (except as may otherwise be agreed in writing by the Company and SPAC), which shall set forth, (a) the initial allocation of the Exchange Consideration among the Company Shareholders, (b) the adjustment to the allocation of the Exchange Consideration described in (a) of this
Annex A-8
Table of Contents
definition to account for the economic rights of certain Company Shareholders under the Company Shareholders’ Agreements, in accordance with the Exchange Agreements and Section 2.01 of the Company Disclosure Schedule, (c) any applicable share premium, and (d) the number of Holdco Ordinary Shares, as applicable, issuable to each Company Shareholder in connection with the Exchange.
“PCAOB” means the Public Company Accounting Oversight Board and any division or subdivision thereof.
“Permitted Liens” means: (a) such imperfections of title, easements, encumbrances, Liens or restrictions that, individually or in the aggregate, do not materially affect, impair or interfere with the use, ownership, value and maintenance of, or the access to any property affected thereby or the conduct of the business of the Company and/or the Company Subsidiaries; (b) materialmen’s, mechanics’, carriers’, workmen’s, warehousemen’s, repairmen’s, landlord’s and other similar Liens arising or incurred in the ordinary course of business to the extent relating to amounts not yet due and payable, or deposits to obtain the release of such Liens; (c) any Liens for Taxes due and not yet payable, or being contested in good faith; (d) zoning, entitlement, conservation restriction and other land use and environmental regulations promulgated by Governmental Authorities; (e) any Liens not created by the Company that affect the underlying fee interest of any leased real property, including master leases or ground leases and any set of facts that an accurate up-to-date survey would show; (f) non-exclusive licenses, sublicenses or other rights to Intellectual Property owned by or licensed to the Company or the Company Subsidiaries granted to any licensee in the ordinary course of business; (g) any non-monetary Liens, encumbrances and restrictions on real property (including easements, covenants, rights of way and similar restrictions of record) that individually or in the aggregate, do not materially affect, impair or interfere with the use, ownership, value and maintenance of or the access to any real property affected thereby or the conduct of the business of the Company and/or the Company Subsidiaries; (h) any Liens identified in the Financial Statements; or (i) any Liens on leases, subleases, easements, licenses, rights of use, rights to access and rights of way arising from the provisions of such agreements or benefiting or created by any superior estate, right or interest.
“person” means an individual, corporation, partnership, limited partnership, limited liability company, company, syndicate, person (including, without limitation, a “person” as defined in Section 13(d)(3) of the Exchange Act), trust, association or entity or government, political subdivision, agency or instrumentality of a government.
“Personal Information” means (a) information related to an identified or identifiable individual (e.g., name, address telephone number, email address, financial account number, government-issued identifier), (b) any other data used or intended to be used or which reasonably allows one to identify, contact, or precisely locate an individual, including any internet protocol address or other persistent identifier, and (c) any other, similar information or data regulated by Privacy/Data Security Laws.
“Privacy/Data Security Laws” means all laws governing the receipt, collection, use, storage, processing, sharing, security, disclosure, or transfer of Personal Information or the security of Company’s Business Systems or Business Data.
“Products” mean any products, developed, manufactured, performed, licensed, sold, distributed, delivered or otherwise made available by or on behalf of the Company or any Company Subsidiary, from which the Company or any Company Subsidiary has derived previously, is currently deriving or is scheduled to derive revenue from the sale or provision thereof.
“Prospectus Regulation” means the Regulation (EU) 2017/1129 of the European Parliament and of the Council of 14 June 2017 on the prospectus to be published when securities are offered to the public or admitted to trading on a regulated market, and repealing Directive 2003/71/EC.
“Redemption Closing Time” means the end of the day of the last day that the Redemption Rights can be exercised as set forth in the Proxy Statement/Prospectus.
“Redemption Rights” means the redemption rights provided for in Article V, Section 3 of the SPAC COI.
“Release” means any spilling, leaking, pumping, pouring, emitting, emptying, discharging, injecting, escaping, leaching, migrating, dumping, disposing, or other release into or through the environment, and any abandonment or discarding of barrels, containers, or other closed receptacles containing any Hazardous Substance.
Annex A-9
Table of Contents
“Representatives” means, with respect to any Person, such Person’s officers, directors, employees, accountants, consultants, legal counsel, agents, financial advisors, lenders, financing sources and other representatives.
“Restricted Person” means: (a) any individual or entity that is a citizen or resident of, located in, or organized under the laws of, or acting for or on behalf of, a Sanctioned Country; (b) the government of any Sanctioned Country; (c) any government that is the subject or target of restrictions under Sanctions Law; or (d) any individual or entity that is, and/or any entity that is owned or controlled directly or indirectly by, or acts for or on behalf of individuals or entities that are designated, on any of the following lists, as updated, substituted, or replaced from time to time:
(i) the United Nations Security Council’s “Consolidated United Nations Security Council Sanctions List”;
(ii) the lists of persons subject to Sanctions Laws, as administered by the U.S. Department of the Treasury, Office of Foreign Assets Control (“OFAC”) including, but not limited to, OFAC’s “Specially Designated Nationals and Blocked Persons List,” the “Foreign Sanctions Evaders,” and the “Sectoral Sanctions Identifications List”;
(iii) the U.S. Department of Commerce, Bureau of Industry and Security’s “Entity List,” “Denied Persons List,” or “Unverified List”;
(iv) the U.S. Department of State’s list of debarred parties and lists of individuals and entities that have been designated pursuant to sanctions and/or non-proliferation statutes that it administers and related executive orders;
(v) the European Union Commission’s “Consolidated list of persons, groups and entities subject to EU financial sanctions” or individuals or entities that are listed in any Annex to EU Council Regulation 833/2014 (as amended);
(vi) Her Majesty’s Treasury of United Kingdom’s “Consolidated List of Financial Sanctions Targets in the UK”; and
(vii) any additional list promulgated, designated, or enforced by a Sanctions Authority.
“Sanctionable Activity” means any condition or activity specifically identified under any Sanctions Laws that serves as a basis to designate any person described by such condition or engaged in such activity as a Restricted Person.
“Sanctioned Country” means at any time, a country or territory that is the target of comprehensive economic or trade sanctions under Sanctions Laws. As of the date of this Agreement, Sanctioned Countries include Cuba, Iran, North Korea, Syria, Government of Venezuela, and the Crimea, Donetsk, and Luhansk regions of Ukraine.
“Sanctions Authority” means (a) the United Nations Security Council; (b) the U.S. Department of the Treasury; (c) the U.S. Department of Commerce; (d) the U.S. Department of State; (e) the European Union Council and/or Commission (including any present or future member state of the European Union with jurisdiction over any Party); (f) Her Majesty’s Treasury of the United Kingdom; and (g) any other Governmental Authority with authority to enact Sanctions Laws in any country and/or territory with jurisdiction over any Party.
“Sanctions Laws” means all economic, trade or financial sanctions Laws enacted, adopted, administered, imposed, or enforced from time to time by any Sanctions Authority.
“Software” means all computer software (in object code or source code format), data and databases, and related documentation and materials.
“SPAC COI” means the Amended and Restated Certificate of Incorporation of SPAC, as amended, modified or supplemented from time to time, including as contemplated by Section 8.20.
Annex A-10
Table of Contents
“SPAC Material Adverse Effect” means any Effects that, individually or in the aggregate with all other Effects, (a) is or would reasonably be expected to be materially adverse to the business, condition (financial or otherwise), assets, liabilities or operations of SPAC or (b) does or would prevent, materially delay or materially impede the performance by SPAC of its obligations under this Agreement or any of the Ancillary Agreements or the consummation of the Merger or any of the other Transactions; provided, however, that none of the following shall be deemed to constitute, alone or in combination, or be taken into account in the determination of whether there has been or will be, a SPAC Material Adverse Effect: (i) any enactment of, change or proposed change in or change in the interpretation of any Law or Accounting Principles; (ii) Effects generally affecting the industries or geographic areas in which SPAC operates, (iii) any downturn in general economic conditions, including changes in the credit, debt, securities, financial or capital markets (including changes in interest or exchange rates, prices of any security or market index or commodity or any disruption of such markets); (iv) acts of war (whether or not declared), sabotage, civil unrest, terrorism, curfews, riots, demonstrations or public disorders, or any escalation or worsening of any such acts of war, sabotage, civil unrest, terrorism, curfews, riots, demonstrations or public disorders, or changes in global, national, regional, state or local political or social conditions; (v) any hurricane, tornado, flood, earthquake, natural disaster, or other acts of God; (vi) Effects arising from or relating to epidemics, pandemics, or disease outbreaks, including COVID-19 or any COVID-19 Measures; (vii) any actions taken or not taken by SPAC as specifically required by this Agreement or any Ancillary Agreement; (viii) the announcement or execution, pendency, negotiation or consummation of the Merger or any of the other Transactions (including the impact thereof on relationships with Governmental Authorities); provided that this clause (viii) shall not apply in determining a SPAC Material Adverse Effect resulting from a breach of the representations and warranties set forth in Section 5.05; (ix) any pending or initiated Action against SPAC or any of its officers or directors, in each case, arising out of or relating to the execution of this Agreement or the Transactions (other than any Action (A) commenced by any Party hereto to enforce its rights under this Agreement or any Ancillary Agreement to which it is a party or (B) resulting from or arising from a breach of the representations and warranties set forth in Section 5.05); (x) any action taken or not taken by the Company or any of the Company Subsidiaries; or (xi) any actions taken, or failures to take action, or such other changes or events, in each case, which the Company has specifically requested or to which it has specifically consented or which actions are specifically contemplated by this Agreement, in each case, except in the cases of clauses (i) through (vi), to the extent that SPAC is disproportionately affected thereby as compared with other participants in the industries or geographic areas in which SPAC operates.
“SPAC Common Stock” means SPAC’s common stock, par value $0.0001 per share.
“SPAC Extension Proposal” means the proposal to be submitted to the SPAC Shareholders pursuant to a definitive proxy statement filed by SPAC with the SEC and provided to the SPAC Shareholders for the purpose of amending the SPAC Organizational Documents to extend the time period for SPAC, to a date that is at least six months after the date hereof, to consummate a business combination.
“SPAC Organizational Documents” means the SPAC COI, the bylaws of SPAC and the Trust Agreement, in each case as amended, modified or supplemented from time to time.
“SPAC Proposals” means proposals made to the SPAC Shareholders pursuant to the SPAC Organizational Documents and applicable Law to approve and adopt (a) this Agreement and the Transactions, including the Merger, (b) the Extension Amendment to the SPAC COI and (c) any other proposals the Parties deem in good faith are necessary or desirable to effect the Transactions.
“SPAC Shareholder Approvals” means (a) with respect to the Merger, the affirmative vote of a majority of the SPAC Shareholders who attend and vote at the SPAC Shareholders’ Meeting; and (b) with respect to any other Proposals proposed to the SPAC Shareholders, the requisite approval required under the SPAC Organizational Documents, the DGCL or other applicable Law.
“SPAC Transaction Expenses” means the reasonable and documented Transaction Expenses of SPAC or any of its affiliates, including (a) any and all Transaction Expenses incurred in the negotiation and preparation of this Agreement, the Ancillary Agreements and the other documents contemplated hereby and thereby and the performance and compliance with all agreements and conditions contained herein and therein, (b) the Sponsor Advanced Funds and (c) the preparation, printing and mailing of the Proxy Statement/Prospectus and the Registration Statement. For the avoidance of doubt, the Parties acknowledge and agree that the SPAC Transaction Expenses include the fees, expenses and disbursements of legal counsel, auditors and accountants, due diligence
Annex A-11
Table of Contents
expenses, advisory and consulting fees and expenses, other third-party fees and any Deferred Fees. For the avoidance of doubt, the Parties acknowledge and agree that (i) any expenses incurred by SPAC in its pursuit of potential acquisition or business targets other than the Company or that were not incurred by SPAC in connection with or in furtherance of the Transactions, (ii) the EarlyBird Cash Fees, (iii) the EarlyBird Share Fees and (iv) the Extension Amendment Fees will not constitute SPAC Transaction Expenses (and will be paid pursuant to Section 10.03). For the avoidance of doubt, Appendix B sets forth an illustrative list of estimated SPAC Transaction Expenses.
“SPAC Transaction Expenses Cap” means $3,000,000; provided that, for every $10.00 by which the amount equal to the (i) Net Available Assets minus (ii) the EarlyBird Cash Fees exceeds $10,000,000 (up to $25,000,000), the SPAC Transaction Expenses Cap shall be increased by $1.00. If the amount equal to the (i) Net Available Assets minus (ii) the EarlyBird Cash Fees is $25,000,000 or greater, then SPAC Transaction Expenses Cap means $4,500,000.
“SPAC Unit” means a unit comprising one SPAC Common Stock and one SPAC Warrant.
“SPAC Warrant Agreement” means that certain warrant agreement, dated as of January 12, 2021 by and between SPAC and the Trustee.
“SPAC Warrants” means warrants to purchase SPAC Common Stock as contemplated under the SPAC Warrant Agreement, with each warrant exercisable for the number of SPAC Common Stock stated in the applicable SPAC Warrant at an exercise price per SPAC Common Stock of $11.50.
“Sponsor Advanced Funds” means the amount equal to all contributions made by Sponsor to SPAC prior to the Closing to pay for expenses and fees of SPAC (not including the Extension Amendment Fees), including contributions deemed to be made by paying for such expenses and fees directly on SPAC’s behalf.
“subsidiary” or “subsidiaries” of the Company, the Surviving Company, SPAC or any other person means an affiliate controlled by such person, directly or indirectly, through one or more intermediaries.
“Supplier” means any person that supplies inventory or other materials or personal property, components, or other goods or services that are utilized in or comprise the Products of the Company or any of the Company Subsidiaries.
“Tax” or “Taxes” means any and all federal, state, provincial, local and foreign income, profits, franchise, gross receipts, environmental, capital stock, shares, severances, stamp, payroll, sales, employment, unemployment, disability, use, real property, personal property, unclaimed property, withholding, excise, production, occupancy and other Taxes, VAT, duties or assessments of any nature whatsoever, whenever and wherever imposed, administered, collected or assessed directly or indirectly against or attributable directly or primarily to a company or any other person, together with all interest, fines, costs, charges, surcharges, penalties and additions imposed with respect to such amounts and any interest in respect of such penalties and additions.
“Tax Return” means any returns and reports (including elections, declarations, disclosures, schedules, estimates and information returns, as well as attachments thereto and amendments thereof) required to be supplied to a Tax authority relating to Taxes.
“Termination Agreements” means those certain termination agreements entered into by the Company and one or more of the Company Shareholders and pursuant to which all the Company Shareholders’ Agreements and the Company Shareholders’ Agreements Liens will automatically expire on or prior to the Closing Date in accordance with the terms thereunder.
“Transaction Documents” means this Agreement, including all Schedules and Exhibits hereto, the Company Disclosure Schedule, the Ancillary Agreements, and all other agreements, certificates and instruments executed and delivered by SPAC, Holdco, Merger Sub or the Company in connection with the Transactions and specifically contemplated by this Agreement.
“Transaction Expenses” means (a) all out-of-pocket fees, costs and expenses (including all fees, costs and expenses of outside counsel, accountants, investment bankers, experts and consultants to a Party and its affiliates and all fees, costs and expenses in connection with newly issued equity and/or debt financing in connection with
Annex A-12
Table of Contents
the Transactions) incurred by a Party or on its behalf in connection with or related to the authorization, preparation, review, negotiation, execution and performance of this Agreement and the other Transaction Documents and consummation of the Transactions, the Proxy Statement/Prospectus, the Registration Statement and the solicitation of the SPAC Shareholders and Company Shareholders and the preparation of any required filings or notices under applicable Antitrust Laws, if any, and (b) the premiums, commissions and other fees paid or payable in connection with obtaining any directors’ and officers’ “tail” insurance policy.
“Transactions” means the transactions contemplated by the Transaction Documents, including the Exchange, the Merger and extension under the SPAC Extension Proposal.
“Transfer Tax” means any sales, use, value-added, business, goods and services, transfer (including any stamp duty or other similar Tax chargeable in respect of any instrument transferring property), documentary, conveyancing or similar Tax or expense or any recording fee, in each case that is imposed as a result of the Transactions, together with any penalty, interest and addition to any such item with respect to such item; provided, however, for the avoidance of doubt, the term Transfer Tax shall not include any income Tax or similar Tax imposed on any direct or indirect equity holder of SPAC, the Company, any Company Subsidiary or Holdco.
“Treasury Regulations” means the United States Treasury regulations issued pursuant to the Code.
“Trust Account Cash” means the total amount of cash held in the Trust Account that was raised as a result of the initial public offering of the SPAC (not including any interest paid with respect to such cash or amounts contributed into the Trust Account from other transactions).
“VAT” means value added Tax.
“Worker” means any person who personally performs work for the Company but who is not in business on their own account or in a client/customer relationship.
SECTION 1.02 Further Definitions. The following terms have the meaning set forth in the Sections set forth below:
Defined Term | | Location of Definition |
1915 Law | | Recitals |
A&R Holdco Organizational Documents | | § 2.05(b) |
Action | | § 4.09 |
Additional SEC Reports | | § 7.04(a) |
Agreement | | Preamble |
Antitrust Laws | | § 8.12(a) |
Blue Sky Laws | | § 4.05(b) |
DGCL | | Recitals |
Certificates | | § 3.03(b) |
CFO | | Recitals |
Claims | | § 7.03 |
Closing | | § 2.03(b) |
Closing Date | | § 2.03(b) |
Companies Act | | Recitals |
Company | | Preamble |
Company Board | | Recitals |
Company Disclosure Schedule | | Article IV |
Company Permits | | § 4.06 |
Company Plan | | § 4.10(b) |
Company Subsidiary | | § 4.01(a) |
Confidentiality Agreement | | § 8.03(b) |
Continuing Employees | | § 8.04(a) |
D&O Indemnified Party | | § 8.05(a) |
Annex A-13
Table of Contents
Defined Term | | Location of Definition |
D&O Tail Policy | | § 8.05(b) |
Data Security Requirements | | § 4.13(h) |
Environmental Permits | | § 4.15 |
ERISA | | § 4.10(a) |
ERISA Affiliate | | § 4.10(c) |
Exchange | | Recitals |
Exchange Act | | § 4.23 |
Exchange Agent | | § 3.03(a) |
Exchange Agreements | | Recitals |
Exchange Auditor Report | | § 9.01(d) |
Exchange Consideration | | § 3.02(a)(i) |
Exchange Fund | | § 3.03(a) |
Exchange Issuance | | § 2.02(a) |
Holdco | | Preamble |
Holdco Board | | Recitals |
Holdco Warrant | | § 3.05 |
Insurance Policies | | § 4.17(a) |
Intended Tax Treatment | | § 2.07 |
IRS | | § 4.10(b) |
Issued Share Capital | | § 4.03(a) |
Lease | | § 4.12(a) |
Lease Documents | | § 4.12(a) |
Letter of Transmittal | | § 3.03(b) |
Material Contracts | | § 4.16(a) |
Merger | | Recitals |
Merger Auditor Report | | § 9.01(d) |
Merger Consideration | | § 3.01(a)(i) |
Merger Effective Time | | § 2.03(a) |
Merger Issuance | | § 3.01(a)(i) |
Merger Sub | | Preamble |
Merger Sub Board | | Recitals |
Merger Sub Common Stock | | § 3.01(a)(iii) |
Outside Date | | § 10.01(b) |
Owned Real Property | | § 4.12(a) |
Party | | Preamble |
PCAOB Financials | | § 8.13 |
Plans | | § 4.10(a) |
Post-Signing Returns | | § 8.09(c)(ii)(A) |
Proxy Statement/Prospectus | | § 8.01(a) |
Redemption | | § 8.01(a) |
Registration Statement | | § 8.01(a) |
Remedies Exceptions | | § 4.04 |
Representatives | | § 8.03(a) |
SEC | | § 5.07(a) |
Securities Act | | § 5.07(a) |
SPAC | | Preamble |
SPAC Board | | Preamble |
SPAC Material Contracts | | § 5.17(a) |
SPAC SEC Reports | | § 5.07(a) |
SPAC Shareholders | | Recitals |
Annex A-14
Table of Contents
Defined Term | | Location of Definition |
SPAC Shareholders’ Meeting | | § 8.01(a) |
Surviving Company | | § 2.01 |
Tail Period | | § 8.05(b) |
Terminating Company Breach | | § 10.01(e) |
Terminating SPAC Breach | | § 10.01(e) |
Trust Account | | § 5.12 |
Trust Agreement | | § 5.12 |
Trust Fund | | § 5.12 |
Trustee | | § 5.12 |
SECTION 1.03 Construction. Unless the context of this Agreement otherwise requires, (i) words of any gender include each other gender, (ii) words using the singular or plural number also include the plural or singular number, respectively, (iii) the terms “hereof,” “herein,” “hereby,” “hereto” and derivative or words of similar import refer to this Agreement as a whole, including the schedules and exhibits, and not to any particular section, subsection, paragraph, subparagraph or clause contained in this Agreement, (iv) the terms “Article,” “Section,” “Schedule” and “Exhibit” refer to the specified Article, Section, Schedule or Exhibit of or to this Agreement, (v) the words “include,” “includes” or “including” shall be deemed to be followed by the words “without limitation”, (vi) the word “or” shall be disjunctive but not exclusive, (vii) references to agreements and other documents shall be deemed to include all subsequent amendments and other modifications thereto, (viii) references to statutes shall include all regulations promulgated thereunder and references to statutes or regulations shall be construed as including all statutory and regulatory provisions consolidating, amending or replacing the statute or regulation, (ix) the word “extent” in the phrase “to the extent” shall mean the degree to which a subject or other thing extends, and such phrase shall not mean simply “if”, (x) references to “dollar”, “dollars” or “$” shall be to the lawful currency of the United States, and (xi) the word “shall” and the word “will” indicate a mandatory obligation.
(a) The language used in this Agreement shall be deemed to be the language chosen by the Parties to express their mutual intent and no rule of strict construction shall be applied against any Party.
(b) Whenever this Agreement refers to a number of days, such number shall refer to calendar days unless Business Days are specified. If any action is to be taken or given on or by a particular calendar day, and such calendar day is not a Business Day, then such action may be deferred until the next Business Day.
(c) All accounting terms used herein and not expressly defined herein shall have the meanings given to them under the applicable Accounting Principles.
(d) Whenever this Agreement states that documents or other information have been “made available to” or “provided to” SPAC (including words of similar import), such words shall mean that such documents or information referenced shall have been posted in the Dataroom, or otherwise provided in writing to SPAC and its Representatives, at least two (2) days prior to the date hereof.
ARTICLE II
MERGER; POST-MERGER
SECTION 2.01 The Merger.
Subject to the receipt of the Holdco Requisite Approvals on or before the Merger Effective Time, and upon the terms and conditions set forth in this Agreement, and in accordance with the DGCL, at the Merger Effective Time, Merger Sub shall be merged with and into SPAC. As a result of the Merger, the separate existence of Merger Sub shall cease and SPAC shall continue as the surviving company of the Merger (the “Surviving Company”). The consummation of the Exchange shall be a condition precedent to the consummation of the Merger.
Annex A-15
Table of Contents
SECTION 2.02 Pre-Merger Actions.
At the Exchange Effective Time, subject to the receipt of the Company Requisite Approvals, the Holdco Requisite Approvals and the delivery of the Exchange Auditor Report, and in accordance with the Holdco Delegate Resolutions;
(a) all the issued Company Ordinary Shares held by the Company Shareholders shall be transferred and for purposes of the 1915 Law, contributed in kind to Holdco, free and clear of all Liens (other than the Company Shareholders’ Agreements Liens that will expire on or prior to the Closing Date), and the Company Shareholders shall subscribe for and, as consideration for the contribution, shall be issued, in accordance with the Exchange Ratio (save that the Holdco Ordinary Shares to be issued shall be reduced by the number of Holdco Ordinary Shares already held by the Company Shareholders immediately prior to the Exchange), the aggregate number of Holdco Ordinary Shares set forth in Section 2.02(a) of the Company Disclosure Schedule (the issuance of the Holdco Ordinary Shares pursuant to this Section 2.02 (the “Exchange Issuance”); provided, however, that no fractional Holdco Ordinary Shares shall be issued pursuant to the Exchange;
(b) the Exchange Issuance shall be allocated among the Company Shareholders in accordance with the terms of the Payment Spreadsheet and Exchange Agreements;
(c) each Company SAFE Holder shall have contributed all of its rights and obligations under each Original SAFE to Holdco in consideration for the issuance by Holdco of a simple agreement for future equity on substantively identical terms (mutatis mutandis) with such adjustments (if any) required under Luxembourg law. For Luxembourg law purposes, a Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued a report on the contributions in kind relating to the contribution of the Original SAFEs prepared in accordance with article 420-10 of the 1915 Law; and
(d) each Company Shareholder shall cease to be the holder of such Company Ordinary Shares, subject to the submission of all filings required under Law (including any filings required to pay stamp duties), and Holdco will be recorded as the registered holder of all the Company Ordinary Shares so exchanged and transferred and will be the legal and beneficial owner thereof.
SECTION 2.03 Closing; Merger Effective Time.
(a) Immediately prior to the Merger Effective Time but after the Exchange Effective Time, each Company SAFE Holder shall receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the Company SAFE.
(b) Subject to the terms and conditions of this Agreement, at the Closing, SPAC shall cause a certificate of merger (the “Certificate of Merger”) to be executed, acknowledged and filed with the Secretary of State of the State of Delaware in accordance with the applicable provisions of the DGCL in order to effectuate the Merger. The Merger shall become effective at such time as the Certificate of Merger has been duly filed with the Secretary of State of the State of Delaware or at such later time as may be agreed by the Company and SPAC in writing and specified in the Certificate of Merger in accordance with the DGCL (the effective time of the Merger being hereinafter referred to as the “Merger Effective Time”).
(c) On the date of the Merger Effective Time, a closing of the Transactions shall be effected remotely by the exchange of documents and signatures in PDF format by electronic mail. The Business Day on which the Merger Effective Time occurs shall be the “Closing Date” and the closing of the Transactions that occur on the Closing Date shall be referred to as the “Closing”.
(d) On the date of the Merger Effective Time, immediately following the Merger, the Company’s CFO shall be allotted the CFO Free Shares.
SECTION 2.04 Effect of the Merger. At the Merger Effective Time, the effect of the Merger shall be as provided in the applicable provisions of the DGCL, the Certificate of Merger and as set forth in this Agreement, including Article III. Without limiting the generality of the foregoing, and subject thereto, at the Merger Effective Time, pursuant to the Merger, (a) all the property, rights, privileges, powers, franchises, licenses and authority of Merger Sub shall vest in SPAC as the Surviving Company, (b) all debts, liabilities, obligations, restrictions,
Annex A-16
Table of Contents
disabilities and duties of Merger Sub shall vest in SPAC as the Surviving Company, (c) for purposes of the 1915 Law a contribution-in-kind of the SPAC Common Stock shall be made to Holdco by the SPAC Shareholders through the Merger against issue of the Merger Consideration by means of a share capital increase realized by Holdco under the authorized share capital (pursuant to the Holdco Delegate Merger Resolutions) by virtue of the foregoing and (d) for purposes of the 1915 Law a capitalization of freely distributable reserves shall be made by Holdco against the issue of the CFO Free Shares by means of a share capital increase realized by Holdco under the authorized share capital (pursuant to the Holdco Delegate Merger Resolutions) by virtue of the foregoing.
SECTION 2.05 Articles; Organizational Documents.
(a) At the Merger Effective Time, (i) the SPAC COI, as in effect immediately prior to the Merger Effective Time, shall be amended and restated in the form of Exhibit B-1, attached hereto and, as so amended and restated, shall be the certificate of incorporation of the Surviving Company, until thereafter amended as provided by applicable Law, and (ii) the SPAC bylaws shall be amended and restated in the form of Exhibit B-2 attached hereto, and, as so amended and restated, shall be the bylaws of the Surviving Company until thereafter amended as provided by applicable Law.
(b) Immediately prior to the consummation of the Merger and the Exchange, the general meeting of shareholders of Holdco shall amend and restate the Holdco Organizational Documents such that the Holdco Organizational Documents are in the form set forth on Exhibit C (“A&R Holdco Organizational Documents”), which A&R Holdco Organizational Documents shall remain in effect and shall otherwise not be amended, restated, modified or waived, in whole or in part, through the Closing, except to reflect the share capital increases following the adoption of the Holdco Delegate Merger Resolutions, as well as, as the case may be, the capital increase following the cash contribution as provided for under the Backstop Agreement, and the Holdco Delegate Exchange Resolutions and thereafter shall be the organizational documents of Holdco until amended as provided by applicable Law.
(c) Immediately following the consummation of the Exchange, Holdco shall cause the Company to take such actions and make such filings as are necessary under the laws of its jurisdiction to amend and restate the Company Organizational Documents such that the Company Organizational Documents are in the form agreed to by the Parties. The Company Organizational Documents shall remain in effect and shall otherwise not be amended, restated, modified or waived, in whole or in part, through the Closing and thereafter shall be the organizational documents of the Company until amended as provided by applicable Law.
SECTION 2.06 Directors and Officers.
(a) The Parties shall cause the directors of the Company Board and the initial officers of the Company immediately following the Exchange Effective Time to be comprised of individuals to be mutually agreed upon by the Parties, each to hold office in accordance with the Company Organizational Documents.
(b) The Parties shall cause and take action as necessary (including by obtaining the Holdco Requisite Approvals) to ensure that, immediately after the Closing,
(i) the Holdco Board shall be comprised of at least seven (7) directors, which shall comprise of (A) five (5) individuals proposed for appointment by the Company at least five (5) Business Days prior to the Closing Date, (B) one (1) individual proposed for appointment by SPAC at least five (5) Business Days prior to the Closing Date (“SPAC Designee”), and (C) one (1) individual that is proposed for appointment by Union Acquisition Group (or a designated affiliate), but who shall be independent under NASDAQ requirements, each to hold office in accordance with the A&R Holdco Organizational Documents; and
(ii) the Holdco Board shall be a declassified board with each director initially serving a term effective from the Closing until the first annual general meeting of the shareholders of Holdco held after the Closing.
(c) The Parties shall cause the initial directors of the Surviving Company and the officers of Surviving Company as of immediately following the Merger Effective Time to be comprised of individuals to be mutually agreed upon by the Parties, each to hold office in accordance with the amended and restated memorandum and articles of association of the Surviving Company.
Annex A-17
Table of Contents
SECTION 2.07 Tax Treatment of the Exchange and the Merger. The Parties agree that for U.S. federal income Tax purposes (and, to the extent applicable, for state and local Tax purposes), (a) the Merger shall be treated as a “reorganization” within the meaning of Section 368(a)(1)(A) of the Code by reason of Section 368(a)(2)(E) of the Code and (b) the Merger and the Exchange are being undertaken as part of a prearranged, integrated plan and shall qualify as exchanges described in Section 351 of the Code and the Treasury Regulations promulgated thereunder (the “Intended Tax Treatment”). The Exchange and the Merger shall be completed in consecutive order such that the Exchange is completed before the Merger Effective Time.
SECTION 2.08 Withholding. Notwithstanding anything in this Agreement to the contrary, (a) Holdco shall be entitled to deduct and withhold from the Holdco Ordinary Shares issued as consideration in the Exchange, from the Merger Consideration issued in the Merger, and from any other consideration it issues in connection with this Agreement, such amounts as it is required to deduct and withhold with respect to the issuance of such consideration under the Code or any applicable provision of state, local or foreign Tax law, and (b) any other Party making payments pursuant to this Agreement and the Transactions shall be entitled to deduct and withhold from such payments such amounts as it is required to deduct and withhold pursuant to any applicable provision of U.S. federal, state, local or foreign Tax law; provided that in each case of clause (a) and (b), the Parties shall cooperate and use reasonable best efforts to reduce, minimize or eliminate any applicable withholding to the extent reasonably permitted under applicable Tax law. Without limiting the foregoing, Holdco may give effect to withholding hereunder by withholding any consideration issued in the form of Holdco Ordinary Shares or other consideration issued in kind, and then selling such portion of Holdco Ordinary Shares or other consideration issued in kind as it may determine and using the proceeds thereof to satisfy applicable withholding obligations and remitting such proceeds to applicable taxing authorities. To the extent that amounts are deducted or withheld under this Section 2.08, such deducted or withheld amounts shall be treated for all purposes of this Agreement as having been issued or paid to the person in respect of which such deduction and withholding was made, and Holdco or any other person deducting or withholding amounts hereunder shall disburse such deducted or withheld amounts to the applicable taxing authorities in accordance with applicable Laws.
ARTICLE III
EXCHANGE
SECTION 3.01 Exchange of Securities.
(a) At the Merger Effective Time, subject to Sections 3.03(h), 3.03(e) and 3.06, by virtue of the Merger and the Holdco Requisite Approvals, subject to the Merger Auditor Report, and without any further action on the part of SPAC, Merger Sub, Holdco or the Company or the holders thereunder:
(i) each SPAC Common Stock issued and outstanding immediately prior to the Merger Effective Time (excluding the SPAC Common Stock to be cancelled in accordance with Section 3.01(b) and the Dissenting Shares) shall be exchanged with Holdco (which exchange, for purposes of the 1915 Law, shall include, for the avoidance of doubt, a contribution-in-kind of each such SPAC Ordinary Shares from the holders of SPAC Ordinary Shares to Holdco), against the issue by Holdco of new Holdco Ordinary Shares (such issuance, the “Merger Issuance”), under the authorized share capital of Holdco (pursuant to the Holdco Delegate Merger Resolutions) and subscribed by the contributing holders of SPAC Ordinary Shares by virtue of the Merger and in accordance with the 1915 Law for one validly issued and fully paid Holdco Ordinary Share (the “Merger Consideration”), delivered by Holdco in accordance with its obligations set forth in Section 3.03;
(ii) As a result of the Merger, all SPAC Common Stock shall cease to be outstanding, shall be cancelled and shall cease to exist and (A) each certificate formerly representing each of the SPAC Common Stock and (B) each book-entry account formerly representing each of the uncertificated SPAC Common Stock shall thereafter, in case of both (A) and (B), only represent the Merger Consideration and the right, if any, to receive pursuant to Section 3.03(h) cash in lieu of fractional shares into which such SPAC Common Stock have been exchanged (and contributed-in-kind) pursuant to this Section 3.01 and any distribution or dividend pursuant to Section 3.03(c); and
Annex A-18
Table of Contents
(iii) each share of common stock, par value $0.01 per share, of Merger Sub (the “Merger Sub Common Stock”) issued and outstanding immediately prior to the Merger Effective Time shall be converted into and exchanged for one (1) validly issued, fully paid and nonassessable ordinary share, par value $0.01 per share, of the Surviving Company.
(b) Each SPAC Common Stock held in the SPAC’s treasury immediately prior to the Merger Effective Time, or held by Holdco or Merger Sub, will be cancelled without payment of any consideration therefor.
SECTION 3.02 Exchange Consideration.
(a) As set forth in the Exchange Agreements:
(i) The valuation of the Company Ordinary Shares transferred to Holdco and for purposes of the 1915 Law, contributed in kind by the Company Shareholders against new Holdco Ordinary Shares, as applicable, pursuant to the Exchange shall be deemed to be, as of the Exchange Effective Time, $325,000,000 (the “Exchange Consideration”). Appendix A sets forth an illustrative calculation of the capitalization of Holdco immediately following the consummation of the Transactions (assuming no Redemptions by the holders of SPAC Common Stock and excluding any Holdco Warrants).
(ii) The Exchange Consideration subscribed for by the Company Shareholders shall be paid in Holdco Ordinary Shares that shall be valued at $10.00 per Holdco Ordinary Share. The Holdco Ordinary Shares making up the Exchange Consideration shall be allocated among the Company Shareholders pursuant to Section 2.02 of the Company Disclosure Schedule and the Payment Spreadsheet.
(b) At least five (5) Business Days prior to the Closing (except as may otherwise be agreed in writing by the Company and SPAC), (i) SPAC shall cause an authorized person of SPAC, solely in his or her capacity as such, to deliver to the Company a certificate certifying SPAC’s good faith estimate of the SPAC Transaction Expenses, including reasonable supporting materials for the amount of each item included in SPAC Transaction Expenses, and (ii) the Company shall cause an authorized officer of the Company, solely in his or her capacity as such, to deliver to SPAC a certificate setting forth: (i) the Company’s good faith estimate of the Company Transaction Expenses, including reasonable supporting materials for the amount of each item included in Company Transaction Expenses and (ii) the Payment Spreadsheet.
SECTION 3.03 Exchange of Certificates.
(a) Exchange Agent. On the Closing Date (and after the Merger Effective Time and the consummation of the transactions contemplated by Section 3.01(a)(i), Section 3.01(a)(ii) and Section 3.01(a)(iii)), Holdco shall deposit with a bank or trust company that shall be designated by SPAC and is reasonably satisfactory to the Company (the “Exchange Agent”), for the benefit of the holders of SPAC Common Stock, for exchange in accordance with this Article III, the number of Holdco Ordinary Shares (in uncertificated form or book-entry form) sufficient to deliver the Merger Consideration consisting of the Holdco Ordinary Shares to be issued to the holders of SPAC Common Stock in the Merger pursuant to this Agreement. In addition, Holdco shall deposit, or cause to be deposited, with the Exchange Agent, as necessary from time to time after the Merger Effective Time, (i) any dividends or other distributions payable pursuant to Section 3.03(c) with respect to the Holdco Ordinary Shares issued pursuant to the Merger for any SPAC Common Stock with a record and payment date after the Merger Effective Time and prior to the surrender of such shares and (ii) cash in lieu of any fractional shares payable pursuant to Section 3.03(h) (all such Holdco Ordinary Shares and cash, together with the amount of any dividends or distributions contemplated pursuant to Section 3.03(c), being hereinafter referred to, collectively, as the “Exchange Fund”). Holdco shall cause the Exchange Agent pursuant to irrevocable instructions, to deliver the Merger Consideration out of the Exchange Fund in accordance with this Agreement. Except as contemplated by this Section 3.03 hereof, the Exchange Fund shall not be used for any other purpose. The Exchange Agent shall invest the cash portion of the Exchange Fund as directed by Holdco; provided that such investments shall be in obligations, funds or accounts typical for (including having liquidity typical for) transactions of this nature. To the extent that there are losses or any diminution of value with respect to such investments, or the Exchange Fund diminishes for any other reason below the level required to make prompt cash payment of any dividends or other distributions payable pursuant to Section 3.03(c) and any cash in lieu of any fractional shares payable pursuant to Section 3.03(h), Holdco shall promptly replace or restore the cash in the Exchange Fund lost through such investments or other events so as to ensure that the Exchange Fund is at all
Annex A-19
Table of Contents
times maintained at a level sufficient to make such cash payments. Any interest and other income resulting from such investment shall become a part of the Exchange Fund, and any amounts in excess of the amounts payable under this Section 3.03(a) shall be promptly returned to Holdco.
(b) Exchange Procedures. As promptly as practicable after the Merger Effective Time, Holdco shall use its reasonable best efforts to cause the Exchange Agent to mail to each holder of record of SPAC Common Stock entitled to receive the Merger Consideration pursuant to Section 3.01 a letter of transmittal, which shall be in a form reasonably acceptable to SPAC and the Company (the “Letter of Transmittal”) and shall specify (i) that delivery shall be effected, and risk of loss and title to the certificates evidencing such SPAC Common Stock (collectively, the “Certificates”) shall pass, only upon proper delivery of the Certificates to the Exchange Agent; and instructions for use in effecting the surrender of the Certificates pursuant to the Letter of Transmittal. Within five (5) Business Days after the surrender to the Exchange Agent of all Certificates held by such holder for cancellation, together with a Letter of Transmittal, duly completed and validly executed in accordance with the instructions thereto and such other documents as may be required pursuant to such instructions, the holder of such Certificates shall be entitled to receive in exchange therefor, and Holdco shall cause the Exchange Agent to deliver (i) the Merger Consideration and (ii) an amount in immediately available funds (or, if no wire transfer instructions are provided, a check) equal to (A) any cash in lieu of fractional shares pursuant to Section 3.03(h) plus (B) any unpaid non-stock dividends and any other dividends or other distributions that such holder has the right to receive pursuant to Section 3.03(c) in accordance with the provisions of this Section 3.03, and the Certificates so surrendered shall forthwith be cancelled. No interest will be paid or accrued on any amount payable upon due surrender of the Certificates. Until surrendered as contemplated by this Section 3.03, each Certificate entitled to receive a portion of the Merger Consideration in accordance with Section 3.01 shall be deemed at all times after the Merger Effective Time, as the case may be, to represent only the right to receive upon such surrender the Merger Consideration that such holder is entitled to receive in accordance with the provisions of Section 3.01.
(c) Distributions with Respect to Unexchanged SPAC Common Stock. No dividends or other distributions declared or made after the Merger Effective Time with respect to the SPAC Common Stock with a record date after the Merger Effective Time shall be paid to the holder of any unsurrendered Certificate (if any) with respect to the SPAC Common Stock represented thereby until the holder of such Certificate (if any) shall surrender such Certificate (if any) in accordance with this Section 3.03. Subject to the effect of escheat, Tax or other applicable Laws, following surrender of any such Certificate (if any), SPAC shall pay or cause to be paid to the holder of the certificates (if any) representing SPAC Common Stock issued in exchange therefor, without interest, (i) promptly, but in any event within five (5) Business Days of such surrender, the amount of dividends or other distributions with a record date after the Merger Effective Time and theretofore paid with respect to such SPAC Common Stock, and (ii) at the appropriate payment date, the amount of dividends or other distributions, with a record date after the Merger Effective Time but prior to surrender and a payment date occurring after surrender, payable with respect to such SPAC Common Stock.
(d) Merger Consideration as Payment in Full. The Merger Consideration payable upon conversion of the SPAC Common Stock in accordance with the terms of this Section 3.03 shall be deemed to have been paid and issued in full satisfaction of all rights pertaining to such SPAC Common Stock.
(e) Adjustments to Merger Consideration. The Merger Consideration shall be adjusted to reflect appropriately the effect of any stock split or share sub-division, reverse stock split or share consolidation, stock dividend or share capitalization, reorganization, recapitalization, reclassification, combination, exchange of shares or other like change with respect to SPAC Common Stock occurring on or after the date hereof and prior to the Merger Effective Time.
(f) Termination of Exchange Fund. Any portion of the Exchange Fund that remains undistributed to the holders of SPAC Common Stock with respect to the Merger Consideration for one year after the Merger Effective Time shall be delivered to Holdco, and any holders of SPAC Common Stock who have not theretofore complied with this Section 3.03 shall thereafter look only to Holdco for the Merger Consideration. Any portion of the Exchange Fund with respect to the Merger Consideration remaining unclaimed by holders of SPAC Common Stock, as may be applicable, as of a date which is immediately prior to such time as such amounts
Annex A-20
Table of Contents
would otherwise escheat to or become property of any government entity shall, to the extent permitted by applicable Law, become the property of Holdco free and clear of any claims or interest of any person previously entitled thereto.
(g) No Liability. None of the Exchange Agent, SPAC, Holdco, Company, the Surviving Company or any of their respective affiliates shall be liable to any holder of SPAC Common Stock for any such SPAC Common Stock (or dividends or distributions with respect thereto) or cash delivered to a public official pursuant to any abandoned property, escheat or similar Law in accordance with this Section 3.03.
(h) Fractional Shares. Notwithstanding any other provision of this Agreement, no fractional shares of Holdco Ordinary Shares will be issued, and any holder of SPAC Common Stock entitled to receive a fractional share of Holdco Ordinary Shares but for this Section 3.03 shall be entitled to receive a cash payment in lieu thereof, which payment shall be calculated by the Exchange Agent and shall represent such holder’s proportionate interest in a share of Holdco Ordinary Shares based on the Average SPAC Share Price.
(i) Lost Certificates. If any Certificate shall have been lost, stolen or destroyed, upon the making of an affidavit of that fact or an appropriate indemnity for lost share certificate by the person claiming such Certificate to be lost, stolen or destroyed, the Exchange Agent will deliver in exchange for such lost, stolen or destroyed Certificate, the Merger Consideration, as the case may be, that such holder is otherwise entitled to receive pursuant to, and in accordance with, the provisions of Section 3.01.
SECTION 3.04 Stock Transfer Books. At the Merger Effective Time, following the recordation of the Transactions in the share records of Holdco, the register of members of SPAC shall be closed and there shall be no further registration of transfers of SPAC Common Stock thereafter on the records and registers of SPAC. From and after the Merger Effective Time, the holders of Certificates (if any) representing SPAC Common Stock outstanding immediately prior to the Merger Effective Time shall cease to have any rights with respect to such SPAC Common Stock, except as otherwise provided in this Agreement or by applicable Law. On or after the Merger Effective Time, any Certificates (if any) presented to the Exchange Agent or Holdco for any reason shall be converted into the Merger Consideration in accordance with the provisions of Section 3.01.
SECTION 3.05 SPAC Warrants. At the Merger Effective Time, each SPAC Warrant that is outstanding immediately prior to the Merger Effective Time shall, pursuant to the SPAC Warrant Agreement, cease to represent a right to acquire one (1) SPAC Common Stock and shall be converted in accordance with the terms of such SPAC Warrant Agreement, at the Merger Effective Time, into a right to acquire one Holdco Ordinary Share (a “Holdco Warrant” and collectively, the “Holdco Warrants”) on substantially the same terms as were in effect immediately prior to the Merger Effective Time under the terms of the SPAC Warrant Agreement. Each of the Parties shall take all lawful action to effect the aforesaid provisions of this Section 3.05, including by executing and delivering the amendment and assignment agreement to the SPAC Warrant Agreement substantially the form attached hereto as Exhibit D (the “SPAC Warrant Amendment and Assignment”).
SECTION 3.06 Appraisal Rights. Notwithstanding anything in this Agreement to the contrary, no Dissenting Shares will be converted into or represent a right to receive the Merger Consideration but instead the applicable SPAC Shareholder will only be entitled to such rights as are provided by the DGCL and, at the Merger Effective Time, such Dissenting Shares will no longer be outstanding, and will be cancelled and cease to exist, and the holders of such Dissenting Shares will cease to have any rights with respect thereto, except the rights set forth in Section 262 of the DGCL; provided, however, that all SPAC Common Stock held by SPAC Shareholders who will have failed to perfect or who effectively will have withdrawn or lost their rights to appraisal of such SPAC Common Stock will thereupon be deemed to have been converted, as of the Merger Effective Time, into the right to receive the Merger Consideration. SPAC will give Holdco, Merger Sub and the Company prompt notice of any demands received by SPAC for the exercise of rights to appraisal, attempted withdrawals of such demands, and any other instruments delivered pursuant to the DGCL and received by the Surviving Company relating to the SPAC Shareholders’ rights to appraisal with respect to the Merger.
Annex A-21
Table of Contents
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Except as set forth in the Company’s disclosure schedule (it being understood and agreed that information disclosed in any section of the Company Disclosure Schedule shall be deemed to be disclosed with respect to any other section of the Company Disclosure Schedule to which such disclosure would reasonably pertain or if its relevance to such other section is reasonably apparent on the face of such disclosure) delivered by Company in connection with this Agreement (the “Company Disclosure Schedule”), the Company hereby represents and warrants to SPAC, Holdco and Merger Sub as follows:
SECTION 4.01 Organization and Qualification; Subsidiaries.
(a) The Company and each subsidiary of the Company (each, a “Company Subsidiary”) is a corporation or other organization duly organized, validly existing and in good standing under the Laws of the jurisdiction of its incorporation or organization (insofar as such concept exists in such jurisdiction) and has the requisite corporate or other organizational power and authority to own, lease and operate its properties and assets and to carry on its business as it is now being conducted. Except as set forth on Section 4.01(a) of the Company Disclosure Schedule, the Company and each Company Subsidiary (i) has all necessary governmental approvals to own, lease and operate its properties and assets and to carry on its business as it is now being conducted, (ii) is duly qualified or licensed as a foreign corporation or other organization to do business where the character of the properties or assets owned, leased or operated by it or the nature of its business makes such qualification or licensing necessary pursuant to applicable Law, and (iii) is in good standing, in each jurisdiction (insofar as such concept exists in such jurisdiction) where the character of the properties or assets owned, leased or operated by it or the nature of its business makes such qualification or licensing necessary, except where the failure to have such governmental approval or be so qualified or licensed or in good standing would not have a Company Material Adverse Effect.
(b) A true and complete list of all the Company Subsidiaries, together with the jurisdiction of organization or incorporation of each Company Subsidiary and the percentage of the outstanding capital stock of each Company Subsidiary owned by the Company and each other Company Subsidiary, in each case, as of the date hereof, is set forth in Section 4.01(b) of the Company Disclosure Schedule. Except with respect to the Company Subsidiaries, the Company does not directly or indirectly own any equity or similar interest in, or any interest convertible into or exchangeable or exercisable for any equity or similar interest in, any other corporation, partnership, joint venture or business association or other entity.
SECTION 4.02 Organizational Documents. The Company has prior to the date of this Agreement made available to SPAC a complete and correct copy of the memorandums of association, articles of association, certificates of incorporation, certificates of formation, by-laws, operating agreements and equivalent organizational documents (including all Company Shareholders’ Agreements), as applicable, each as amended to date, of the Company and each Company Subsidiary. Such memorandums of association, articles of association, certificates of incorporation, certificates of formation, by-laws, operating agreements, registration statements and equivalent organizational documents (including all Company Shareholders’ Agreements) are in full force and effect. The Company and each Company Subsidiary is in all material respects in compliance with all the provisions of their respective memorandum of association, articles of association, certificates of incorporation, certificates of formation, by-laws, operating agreements, registration statements or equivalent organizational documents (including all Company Shareholders’ Agreements).
SECTION 4.03 Capitalization.
(a) As of the date hereof, the Company has an issued share capital of £486,619.15, divided into 48,661,915 shares of £0.01 per share, each fully paid-up (the “Issued Share Capital”). Set forth on Section 4.03(a) of the Company Disclosure Schedule is, with respect to each of the Company and each Company Subsidiary, (i) the identity of each Person who holds any shares of Equity Interest of the Company or of such Company Subsidiary and (ii) the amount, type and class of Equity Interest held by each such Person. Other than as set forth on Section 4.03(a) of the Company Disclosure Schedule, there are no other Equity Interests relating to the issued or unissued capital stock of the Company or any Company Subsidiary or obligating the Company or any Company Subsidiary to issue or sell any shares of capital stock of, or other Equity Interests in, the Company or any Company Subsidiary. Neither the Company nor any Company
Annex A-22
Table of Contents
Subsidiary is a party to, or otherwise bound by, and neither the Company nor any Company Subsidiary has granted, any equity appreciation rights, participations, phantom equity or similar rights, other than the Company Shareholders’ Agreements Liens that will be terminated on or prior to the Closing Date. Other than pursuant to the Transaction Documents and the Company Shareholders’ Agreements that will be terminated on or prior to the Closing Date, there are no voting trusts, voting agreements, proxies, shareholder agreements or other similar agreements with respect to the voting or transfer of the Company Ordinary Shares or any of the equity interests or other securities of the Company or any of the Company Subsidiaries. The Company does not own any equity interests in any person, other than the Company Subsidiaries.
(b) Other than pursuant to the Company Organizational Documents, the respective organizational documents of the Company Subsidiaries, the Company Shareholders’ Agreements, the Company Employee Share Plan or the Transaction Documents, there are no outstanding contractual obligations of the Company or any Company Subsidiary to repurchase, redeem or otherwise acquire any Company Ordinary Shares or any capital stock of any Company Subsidiary or to provide funds to or make any investment (in the form of a loan, capital contribution or otherwise) in any person other than a Company Subsidiary.
(c) Each outstanding share of capital stock of each Company Subsidiary is duly authorized, validly issued, fully paid and, except as set forth in Section 4.03(c) of the Company Disclosure Schedule, each such share is owned by the Company or another Company Subsidiary free and clear of all Liens, other than transfer restrictions under applicable Laws and their respective organizational documents.
(d) The Company Shareholders collectively own directly and beneficially and of record, all the equity of the Company (which are represented by the issued Company Ordinary Shares). Except as set forth in Section 4.03(d) of the Company Disclosure Schedule and Company Shareholders’ Agreements Liens that will be terminated on or prior to the Closing Date, and except for the shares of the Company held by the Company Shareholders, no shares or other equity or voting interest of the Company, or options, warrants or other rights to acquire any such shares or other equity or voting interest, of the Company is authorized or issued.
(e) All issued Company Ordinary Shares and all issued shares of capital stock or other equity securities (as applicable) of each Company Subsidiary have been issued and granted in compliance with (i) applicable securities Laws and other applicable Laws and (ii) any preemptive rights and other similar requirements set forth in applicable contracts to which the Company or any Company Subsidiary is a party.
SECTION 4.04 Authority Relative to this Agreement. The Company has all necessary power and authority to execute and deliver this Agreement and each Ancillary Agreement to which it is a party, to perform its obligations hereunder and thereunder and to consummate the Transactions. The execution and delivery by the Company of this Agreement and the Ancillary Agreements to which it is a party and the consummation by the Company of the Transactions have been duly and validly authorized by all necessary corporate action and no other corporate proceedings on the part of the Company are necessary to authorize this Agreement, each such Ancillary Agreement or to consummate the Transactions (other than the approval of the Exchange, the filing and recordation of appropriate documents as required by the Companies Act or other applicable Law, as the case may be, and approval of the amended and restated Company Organizational Documents by Holdco as the sole shareholder of the Company following the Exchange as contemplated by Section 2.05(c)). This Agreement and each such Ancillary Agreement have been duly and validly executed and delivered by the Company and, assuming the due authorization, execution and delivery by SPAC, Holdco and Merger Sub, constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, except as limited by applicable bankruptcy, insolvency, reorganization, moratorium and other Laws of general application affecting enforcement of creditors’ rights generally, by general equitable principles (the “Remedies Exceptions”). To the knowledge of the Company, no state, provincial, federal, domestic or foreign takeover statute is applicable to the Transactions, except as otherwise contemplated herein.
SECTION 4.05 No Conflict; Required Filings and Consents.
(a) The execution and delivery by the Company of this Agreement and each Ancillary Agreement to which it is a party does not, and subject to receipt of the filing and recordation of documents in connection with the Merger and the Exchange, as required by the Companies Act or other applicable Law, and of the consents, approvals, authorizations or permits, filings and notifications contemplated by Section 4.05 of the Company Disclosure Schedule, the performance of this Agreement and each such Ancillary Agreement by
Annex A-23
Table of Contents
the Company will not (i) conflict with or violate the memorandum of association, articles of association, certificate of incorporation or by-laws or any equivalent organizational documents of the Company or any Company Subsidiary, (ii) conflict with or violate any Law applicable to the Company or any Company Subsidiary or by which any property or asset of the Company or any Company Subsidiary is bound or affected, or (iii) result in any breach of or constitute a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any right of termination, amendment, acceleration or cancellation of, or result in the creation of a Lien (other than any Permitted Lien) on any property or asset of the Company or any Company Subsidiary pursuant to, any Material Contract, except with respect to clauses (a)(ii) and (a)(iii) for any such conflicts, violations, breaches, defaults or other occurrences which would not have a Company Material Adverse Effect.
(b) The execution and delivery by the Company of this Agreement and each Ancillary Agreement to which it is a party does not, and the performance of this Agreement by the Company will not, require any consent, approval, authorization or permit of, or filing with or notification to, a Governmental Authority, other than as contemplated by Section 4.05(b) of the Company Disclosure Schedule, and except (i) for applicable requirements, if any, of the Securities Act, the Exchange Act, state securities or “blue sky” laws (“Blue Sky Laws”) and state takeover Laws, rules and regulations of Nasdaq, the notification requirements of applicable Antitrust Laws, if any, and filing and recordation of appropriate documents in connection with the Merger and the Exchange or other documents as required by the DGCL, the 1915 Law or the Companies Act, and (ii) as and where the failure to obtain such consents, approvals, authorizations or permits, or to make such filings or notifications, would not, individually or in the aggregate, prevent or materially delay consummation of any of the Transactions or otherwise prevent the Company from performing its material obligations under this Agreement and each such Ancillary Agreement.
SECTION 4.06 Permits; Compliance. Except as set forth in Section 4.06 of the Company Disclosure Schedule, each of the Company and the Company Subsidiaries is in possession of all franchises, grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental Authority necessary for each of the Company or the Company Subsidiaries to own, lease and operate its properties or to carry on its business as it is now being conducted (including, without limitation, as applicable, all such permits, licenses, approvals, consents and other authorizations required by the Food and Drug Administration or any other federal, state, local or foreign agencies or bodies engaged in the regulation of food growing, production, development, manufacture, distribution, processing, or any other activities related to the business now operated by the Company and its Subsidiaries) (the “Company Permits”), except where the failure to have such Company Permits would not reasonably be expected to have a Company Material Adverse Effect. No suspension or cancellation of any of the Company Permits is pending or, to the knowledge of the Company, threatened in writing. Except as set forth in Section 4.06 of the Company Disclosure Schedule, neither the Company nor any Company Subsidiary is in conflict with, or in default, breach or violation of, (a) any Law applicable to the Company or any Company Subsidiary or by which any property or asset of the Company or any Company Subsidiary is bound or affected, or (b) any Company Permit, except for any such conflicts, violations, breaches, defaults or other occurrences which would not have a Company Material Adverse Effect. Neither the Company nor any Company Subsidiary has received since its date of formation any written notices from any Governmental Authority alleging violation of any applicable Laws, except for any violations which would not, individually or in the aggregate, result in a Company Material Adverse Effect.
SECTION 4.07 Financial Statements.
(a) The Company has made available to SPAC true and complete copies of (i) the audited consolidated financial statements as of June 30, 2021 and December 31, 2020 and from the period of January 1, 2021 through June 30, 2021 and from inception on August 21, 2020 through December 31, of the Company and the Company and (ii) the unaudited interim condensed consolidated financial statements as of December 31, 2021 and June 30, 2021 and from the period of July 1, 2021 through December 31, 2021 and from August 21, 2020 through December 30, 2020 of the Company and the Company Subsidiaries (collectively, the “Financial Statements”), which are attached as Section 4.07(a) of the Company Disclosure Schedule. Each of the Financial Statements (including the notes thereto) (i) was prepared in accordance with IFRS applied on a consistent basis throughout the periods indicated (except as may be indicated in the notes thereto) and (ii) fairly presents, in all material respects, the financial position, results of operations and cash flows of the Company and the Company Subsidiaries as of the date thereof and for the period indicated therein, except as otherwise noted therein.
Annex A-24
Table of Contents
(b) Except as and to the extent set forth on the Financial Statements, neither the Company nor any Company Subsidiary has any liability or obligation of a nature (whether accrued, absolute, contingent or otherwise) required to be reflected on a balance sheet prepared in accordance with the Accounting Principles except for (i) liabilities that were incurred in the ordinary course of business or in connection with the Transactions since December 31, 2021, (ii) obligations for future performance under any contract to which the Company or any Company Subsidiary is a party or (iii) any other liabilities and obligations which would not, individually or in the aggregate, result in a Company Material Adverse Effect.
(c) Since its date of formation, (i) neither the Company nor any Company Subsidiary nor, to the Company’s knowledge, any director, officer, employee, auditor, accountant or Representative of the Company or any Company Subsidiary, has received or otherwise had or obtained knowledge of any written complaint, allegation, assertion or claim regarding the accounting or auditing practices, procedures, methodologies or methods of the Company or any Company Subsidiary or their respective internal accounting controls, including any such written complaint, allegation, assertion or claim that the Company or any Company Subsidiary has engaged in questionable accounting or auditing practices, and (ii) there have been no internal investigations regarding accounting or revenue recognition discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer, general counsel, the Company Board or any committee thereof.
(d) To the knowledge of the Company, no employee of the Company or any Company Subsidiary has provided since its date of formation or is providing information to any law enforcement agency regarding the commission or possible commission of any crime under applicable Law. None of the Company, any Company Subsidiary or, to the knowledge of the Company, any officer, employee, contractor, subcontractor or agent of the Company or any such Company Subsidiary has discharged, demoted, suspended, threatened, harassed or in any other manner discriminated against an employee of the Company or any Company Subsidiary in the terms and conditions of employment because of any act of such employee described in 18 U.S.C. sec. 1514A(a) or the Equality Act 2010 (as applicable).
(e) The Company and each Company Subsidiary, individually or together with other Company Subsidiaries, maintains systems of internal control over financial reporting that are sufficient to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the Accounting Principles, including policies and procedures sufficient to provide reasonable assurance: (i) that the Company and each Company Subsidiary maintains records that in reasonable detail accurately and fairly reflect, in all material respects, its transactions and dispositions of assets; (ii) that transactions of the Company and each Company Subsidiary are recorded as necessary to permit the preparation of financial statements in conformity with the Accounting Principles; (iii) that receipts and expenditures of the Company and each Company Subsidiary are being made only in accordance with authorizations of management and its board of directors; and (iv) regarding prevention or timely detection of unauthorized acquisition, use or disposition of the assets of the Company and each Company Subsidiary that could have a material effect on its financial statements. The Company has delivered to SPAC a true and complete copy of any disclosure (or, if unwritten, a summary thereof) by any representative of the Company to the Company’s independent auditors since its date of formation and relating to any material weaknesses in internal controls and any significant deficiencies in the design or operation of internal controls that would adversely affect the ability of the Company or any Company Subsidiary to record, process, summarize and report financial data. The Company has no knowledge of any fraud or whistle-blower allegations, whether or not material, that involve management or other employees or consultants who have or had a significant role in the internal control over financial reporting of the Company or any Company Subsidiaries. Since December 31, 2018, there have been no material changes in the Company’s or any of Company Subsidiary’s internal control over financial reporting.
SECTION 4.08 Absence of Certain Changes or Events. To the knowledge of the Company, since December 31, 2021 and prior to the date of this Agreement, except as otherwise reflected in the Financial Statements, actions or omissions taken as a result of COVID-19 and COVID-19 Measures, or in connection with the Transactions or as expressly contemplated or permitted by this Agreement or any Ancillary Agreement, (a) the Company and the Company Subsidiaries have conducted their respective businesses in the ordinary course and in a manner consistent with past practice in all material respects, (b) the Company and the Company Subsidiaries have not sold, assigned or otherwise transferred any right, title, or interest in or to any of their material assets (including Intellectual Property and Business Systems) other than non-exclusive licenses or assignments or transfers in the ordinary course
Annex A-25
Table of Contents
of business, (c) there has not been any Company Material Adverse Effect, and (d) none of the Company or any Company Subsidiary has taken any action that, if taken after the date of this Agreement, would constitute a material breach of any of the covenants set forth in Section 7.01.
SECTION 4.09 Absence of Litigation. Except as otherwise set forth in Section 4.09 of the Company Disclosure Schedule, as of the date hereof, there is no material litigation, proceeding, cause of action, lawsuit, audit, assessment or reassessment, petition, complaint, charge, grievance, prosecution, demand, hearing, written notice, inquiry, investigation, subpoena, summons, inspection, or administrative or other similar proceeding, mediation or arbitration (including any appeal or application for review) of any kind or nature, in law or in equity (an “Action”), pending or, to the knowledge of the Company, threatened in writing against the Company or any Company Subsidiary, or any property or asset of the Company or any Company Subsidiary, before any Governmental Authority where the losses or damages claimed against the Company or any Company Subsidiary exceed $250,000. As of the date hereof, neither the Company nor any Company Subsidiary nor any material property or asset of the Company or any Company Subsidiary is subject to any continuing material order of, consent decree, settlement agreement or other similar written agreement with, or, to the knowledge of the Company, continuing investigation by, any Governmental Authority, or any order, writ, judgment, injunction, decree, determination or award of any Governmental Authority.
SECTION 4.10 Employee Benefit Plans.
(a) Other than mandatory benefits or plans regarding Employers or Workers under applicable Law, Section 4.10(a) of the Company Disclosure Schedule sets forth a true and complete list of all material employee benefit plans (as defined in Section 3(3) of the Employee Retirement Income Security Act of 1974, as amended, and the rules and regulations promulgated thereunder (“ERISA”)) and all other material bonus, stock option, stock purchase, restricted stock, equity or equity-based, incentive, deferred compensation, retiree medical or life insurance, retirement, pension, supplemental retirement, severance, retention, separation, change in control, health, welfare, fringe benefit, sick pay and vacation plans or arrangements or other material employee benefit plans, programs, ex gratia promises, policies, agreements or arrangements, whether or not subject to ERISA, whether formal or informal, whether written or oral, in each case which are maintained, sponsored by, or contributed to by (or for which there is an obligation to contribution to by) the Company or any Company Subsidiary for the benefit of any current or former employee, officer, director, individual independent contractor and/or consultant, or with respect to which the Company or any Company Subsidiary has or could incur any present or future liability (contingent or otherwise) (collectively, the “Plans”).
(b) With respect to each Plan sponsored by the Company or any Company Subsidiary (each, a “Company Plan”), the Company has made available to SPAC, if applicable (i) a true and complete copy of the current plan document and all amendments thereto and each trust or other funding arrangement, (ii) copies of the most recent summary plan description and any summaries of material modifications, (iii) if applicable, a copy of the most recent filed Internal Revenue Service (“IRS”) Form 5500 annual report and accompanying schedules, (iv) copies of the most recently received IRS determination or opinion letter for each such Company Plan, and (v) any material non-routine correspondence from any Governmental Authority and any filing, reporting or any other documentation or correspondence to or with a Governmental Authority with respect to any Company Plan since its date of formation.
(c) None of the Plans is or was within the past six (6) years, nor does the Company, any Company Subsidiary nor any of their respective ERISA Affiliates have or reasonably expect to have any material liability or obligation under (i) a multiemployer plan (within the meaning of Sections 3(37) or 4001(a)(3) of ERISA), or (ii) an “employee pension benefit plan” (as defined in Section 3(2) of ERISA) subject to Title IV of ERISA, Section 412 of the Code or Section 302 of ERISA or any other plan that is subject to Title IV of ERISA. For purposes of this Agreement, “ERISA Affiliate” shall mean any trade, business or any person that, together with the Company or any Company Subsidiary, is treated as a “single employer” for purposes of Section 4001(b)(1) of ERISA and/or Section 414(b),(c), (m) or (o) of the Code.
(d) Neither the execution and delivery of this Agreement nor the other Ancillary Agreements nor the consummation of the Transactions will or could reasonably be expected to (alone or in combination with any other event) (i) result in (A) an increase in the amount of compensation or benefits to or in respect of any current or former employee, officer, director, individual independent contractor or consultant; (B) any payment or benefit becoming due to or in respect of any current or former employee, officer, director, individual independent contractor and/or consultant; (C) the acceleration of the vesting, funding or timing of payment
Annex A-26
Table of Contents
of any compensation or benefits payable to or in respect of any current or former employee, officer, director, individual independent contractor or consultant; or (D) any increased or accelerated funding obligation with respect to any Plan; (ii) limit the right to merge, amend or terminate any Plan; or (iii) give rise to any “excess parachute payment” within the meaning of Section 280G of the Code or any corresponding or similar provision of any other applicable Law. Neither the Company nor any Company Subsidiary has any indemnity or gross-up obligation for any Taxes imposed under Section 4999 or Section 409A of the Code or any corresponding or similar provision of applicable Law or otherwise.
(e) None of the Plans provide for, nor does the Company nor any Company Subsidiary have or reasonably expect to have any material liability or obligation to provide any post-employment or post-service health or welfare benefits or retiree medical or life insurance to any current or former employee, officer, director, individual independent contractor or consultant of the Company or any Company Subsidiary after termination of employment or service except (i) as set forth in any existing employment or severance agreement or (ii) as may be required under applicable Law, including under Section 4980B of the Code and Parts 6 and 7 of Title I of ERISA or similar applicable Law for which the covered individual pays the full cost of coverage.
(f) In all material respects, (i) each Company Plan is and has been established, maintained and administered in accordance with its terms and in compliance with the requirements of all applicable Laws including, without limitation, as applicable, ERISA and the Code, and (ii) other than routine claims for benefits in the ordinary course of business, no actions, litigation, claims, lawsuits, audits, inquiries, arbitrations, investigations, or proceedings are pending or, to the knowledge of Company, threatened, from any Governmental Authority in connection with any Company Plan or by or on behalf of any participant in any Company Plan, or otherwise involving or relating to any Company Plan or the assets of any Company Plan or any trust thereunder or the plan sponsor or plan administrator of any Company Plan (acting in such individual’s capacity as plan sponsor or plan administrator).
(g) Each Company Plan that is intended to be qualified under Section 401(a) of the Code or under similar applicable Law, if any, and any trust forming any part thereof, (i) has timely received a favorable determination letter from the IRS or other applicable Governmental Authority or (ii) is entitled to rely on a favorable opinion letter from the IRS or other applicable Governmental Authority, and to the knowledge of Company, nothing has occurred that would reasonably be expected to result in any revocation of, or an adverse material change to, such determination or opinion letter or otherwise adversely affect the qualified status of such plan or exempt status of such trust.
(h) Except as would not result in material liability to the Company and the Company Subsidiaries, taken as a whole, either individually or in the aggregate, (i) there has not been any non-exempt prohibited transaction (within the meaning of Section 406 of ERISA or Section 4975 of the Code or under similar applicable Law) nor any reportable events (within the meaning of Section 4043 of ERISA or under similar applicable Law) with respect to any Plan to which such sections of ERISA or the Code or such similar applicable Law would apply, and (ii) there have been no acts or omissions by the Company or any Company Subsidiary with respect to any Plan that have given or could reasonably be expected to give rise to any fines, penalties, Taxes or related charges under ERISA, the Code or other applicable Law.
(i) All material liabilities or expenses of the Company or any Company Subsidiary in respect of any Company Plan which have not been paid have been properly accrued on the Company’s or any Company Subsidiary’s most recent financial statements in compliance with the Accounting Principles. With respect to each Company Plan, all material contributions or payments (including all material employer contributions, employee salary reduction contributions, defined benefit plan contributions deferred under the Coronavirus Aid, Relief, and Economic Security Act of 2020, Pub. L. 116-136 (the “CARES Act”) and premium or benefit payments) that are due or are required to be made under the terms of any Company Plan or in accordance with applicable Laws have been made within the time periods prescribed by the terms of each such Company Plan, ERISA, the Code and applicable Laws, as the case may be, except as would not result in material liability to the Company, and all such contributions or payments that are not yet due or required to be made under the terms of any Company Plan or in accordance with applicable Laws have been properly accrued in accordance with the Accounting Principles, applied on a consistent basis, and reflected on the Company’s or any Company Subsidiary’s audited financial statements.
Annex A-27
Table of Contents
SECTION 4.11 Labor and Employment Matters.
(a) As of the date hereof, except as would not be material to the Company or any Company Subsidiary, individually or in the aggregate, all compensation, including wages, commissions and bonuses, due and payable to all Employees of the Company and any Company Subsidiary for services performed on or prior to the date hereof have been paid in full (or accrued in full in the Company’s financial statements).
(b) (i) Except as otherwise set forth in Section 4.11(b) of the Company Disclosure Schedule, there are no Actions pending or, to the knowledge of the Company, threatened against the Company or any Company Subsidiary by any of their respective current or former Employees, independent contractors, applicants for employment, or any class of the foregoing, which Actions would be material to the Company and the Company Subsidiaries, taken as a whole; (ii) neither the Company nor any Company Subsidiary is, nor have been since its date of formation, a party to, bound by, or negotiating any collective bargaining agreement or other contract with a union, works council or labor organization applicable to persons employed by the Company or any Company Subsidiary, nor, to the knowledge of the Company, are there any activities or proceedings of any labor union to organize any such employees; (iii) there are no material unfair labor practice complaints pending against the Company or any Company Subsidiary before the National Labor Relations Board; and (iv) since its date of formation, there has never been, nor, to the knowledge of the Company, has there been any threat of, any strike, slowdown, work stoppage, lockout, concerted refusal to work overtime or other similar labor disruption or dispute affecting, or, to the knowledge of the Company, threatened, by or with respect to any employees of the Company or any Company Subsidiary.
(c) The Company and the Company Subsidiaries are and have been since its date of formation in compliance in all material respects with all applicable Laws relating to the employment, employment practices, employment discrimination, terms and conditions of employment, mass layoffs and plant closings, immigration, pay equity, workers’ compensation, family and medical leave, and occupational safety and health requirements, including those related to wages, hours, collective bargaining and the payment and withholding of Taxes and other sums as required by the appropriate Governmental Authority.
(d) To the Company’s knowledge, the Company and the Company Subsidiaries are in all material respects in compliance with any Laws, recommendations or guidance issued by any applicable Governmental Authority relating in any way to the work of Employees and/or procedures for returning to work for Employees with respect to COVID-19.
(e) Except as set forth in Section 4.11(e) of the Company Disclosure Schedule, to the Company’s knowledge, (i) no Employee, Worker or independent contractor of the Company or any Company Subsidiary is in violation of any term of any employment contract, consulting contract, non-disclosure agreement, common law non-disclosure obligation, noncompetition agreement, non-solicitation agreement, proprietary information agreement or any other agreement with a third party relating to confidential or proprietary information, intellectual property, competition, or related matters; and (ii) the continued employment by the Company and the Company Subsidiaries of their respective Employees, and the performance of the contracts with the Company and the Company Subsidiaries by their respective Workers and independent contractors, will not result in any such violation. Neither the Company nor any of the Company Subsidiaries has received any notice from a Governmental Authority alleging that any such violation has occurred since its date of formation.
(f) All material contracts of employment between the Company and any Company Subsidiary and any person employed by the Company or any Company Subsidiary are terminable by giving the applicable minimum period of notice specified in applicable Law and neither the Company nor any Company Subsidiary is contractually obliged to make any material payment as a consequence of the termination of any such contract.
(g) All material contracts of engagement between the Company and any Company Subsidiary and any person engaged by the Company or any Company Subsidiary are terminable by giving no more than one month’s notice and neither the Company nor any Company Subsidiary is contractually obliged to make any material payment as a consequence of the termination of any such contract.
Annex A-28
Table of Contents
(h) To the Company’s knowledge, no person employed or engaged by the Company or any Company Subsidiary nor any person who was previously a person employed or engaged by the Company or any Company Subsidiary is entitled to:
(i) any material accrued but unpaid holiday pay in respect of the relevant Group Company’s current or any previous holiday years; or
(ii) any material accrued but untaken holiday leave in respect of the relevant Group Company’s previous holiday years.
(i) To the Company’s knowledge, every person employed or engaged by the Company or any Company Subsidiary who requires permission to work in the relevant jurisdiction has current and appropriate permission to work in such jurisdiction.
SECTION 4.12 Real Property; Title to Assets.
(a) Section 4.12(a) of the Company Disclosure Schedule lists the street address of each parcel of Leased Real Property, and also identifies with respect to each Leased Real Property, each lease, sublease, license or other contractual arrangement under which such Leased Real Property is occupied or used (each, a “Lease”), including the date of and legal name of each of the parties to such Lease, and each guaranty, amendment, restatement, modification or supplement thereto (collectively, the “Lease Documents”) and lists all of the material real property owned by the Company or any Company Subsidiary (the “Owned Real Property”). True, correct and materially complete copies of all Lease Documents have been made available to SPAC.
(b) The Leased Real Property and the Owned Real Property constitutes all material interests in real property currently used, occupied or held for use in connection with the business of the Company and/or Company Subsidiaries and necessary for the continued operation of the business of the Company and/or the Company Subsidiaries, as applicable. The Leased Real Property and the Owned Real Property, including all buildings, fixtures and other improvements constituting a part thereof, is in good operating condition, except for ordinary wear and tear, without material structural defects and is in all material respects suitable, sufficient and appropriate for its current and contemplated uses. No Leased Real Property is subject to any sublease, license or right of occupancy in favor of any third party.
(c) The Company and/or the applicable Company Subsidiary has a valid, binding and enforceable, subject to the Remedies Exceptions, leasehold interest under each of the Leases, free and clear of all Liens other than Permitted Liens. Except as set forth in Section 4.12(c) of the Company Disclosure Schedule, the Company and the Company Subsidiaries have good and marketable title to all Owned Real Property, free and clear of all Liens, other than Permitted Liens. Each Lease is in full force and effect and is the valid, binding and enforceable, subject to the Remedies Exceptions, obligation of each party thereto in accordance with its terms. The Company and/or the applicable Company Subsidiary has accepted possession of each individual Leased Real Property and is currently occupying and using same in all material respects pursuant to the terms of the applicable Lease. None of the Company nor any of the Company Subsidiaries, nor to the Company’s knowledge, any other party to any Lease is in material breach or material violation of, or default under, any such Lease.
(d) Except as set forth in Section 4.12(d) of the Company Disclosure Schedule, there are no contractual or legal restrictions that preclude or restrict the ability of the Company or any Company Subsidiary to use any Leased Real Property or Owned Party by such party for the purposes for which it is currently being used, except as would not, individually or in the aggregate, be material to the Company and the Company Subsidiaries, taken as a whole. There are no latent defects or adverse physical conditions affecting the Leased Real Property, and improvements thereon, other than those that would not, individually or in the aggregate, reasonably be expected to be material to the Company and the Company Subsidiaries, taken as a whole.
(e) There do not exist any actual or, to the Company’s knowledge, threatened condemnation or eminent domain proceedings that affect any Leased Real Property or Owned Real Property or any part thereof, and none of the Company nor any of the Company Subsidiaries has received since its date of formation any written notice of the intention of any Governmental Authority in connection with any such condemnation or eminent domain proceedings of any Leased Real Property or Owned Real Property or any part thereof or interest therein.
Annex A-29
Table of Contents
SECTION 4.13 Intellectual Property.
(a) Section 4.13(a) of the Company Disclosure Schedule contains a true, correct and complete list of all of the following: (i) registered Intellectual Property rights and applications for registrations of Intellectual Property rights that are owned or purported to be owned by the Company and/or the Company Subsidiaries (showing in each case, as applicable, the filing date, date of issuance, expiration date and registration or application number, and jurisdiction); (ii) registered Intellectual Property rights and applications for registrations of Intellectual Property rights that are licensed to the Company and/or the Company Subsidiaries (showing in each case, as applicable, the filing date, date of issuance, expiration date and registration or application number, and jurisdiction); and (iii) all contracts or agreements to use any Company-Licensed IP, including for the Software or Business Systems of any other person, that are material to the business of the Company and/or the Company Subsidiaries as currently conducted (other than (x) unmodified, commercially available, “off-the-shelf” Software or (y) Software, Technology or Business Systems with a replacement cost or aggregate annual license and maintenance fees of less than $250,000). To the knowledge of the Company, the Company IP specified on Section 4.13(a) of the Company Disclosure Schedule constitutes all material Intellectual Property rights used in the operation of the business of the Company and the Company Subsidiaries and is sufficient for the conduct of such business as currently conducted and contemplated to be conducted as of the date hereof. To the knowledge of the Company, all actions required for the prosecution, maintenance and protection of the Company IP (including the payment of registry fees and taxes) have been taken by the Company on time.
(b) Except as would not reasonably be expected to have, individually or in the aggregate, a Company Material Adverse Effect, the Company or any one of the Company Subsidiaries solely and exclusively owns and possesses, free and clear of all Liens (other than Permitted Liens), all right, title and interest in the Company-Owned IP and has the right to use pursuant to a valid and binding written license of all Company-Licensed IP and Business Systems, including Software, in each case that are material to the business of the Company and the Company Subsidiaries. All material Company-Owned IP is subsisting and, to the knowledge of the Company, valid and enforceable and nothing has been done or omitted to be done which would or might result in any of it ceasing to be valid. No loss or expiration of any of the Company-Owned IP, or, to the Company’s knowledge, any of the Company-Licensed IP, is threatened in writing.
(c) Except as would not reasonably be expected to have, individually or in the aggregate, a Company Material Adverse Effect, the Company and each of its applicable Company Subsidiaries have taken and take commercially reasonable actions to maintain, protect, and enforce Intellectual Property rights, including the secrecy, confidentiality and value of its trade secrets and other Confidential Information in all material respects. To the knowledge of the Company or any of the Company Subsidiaries, neither the Company nor any Company Subsidiaries have disclosed any trade secrets or other Confidential Information that is material to the business of the Company and any applicable Company Subsidiaries to any other person other than pursuant to a written confidentiality agreement under which such other person agrees to maintain the confidentiality and protect such Confidential Information.
(d) Except as otherwise set forth in Section 4.13(d) of the Company Disclosure Schedule (i) since its date of formation, there have been no claims properly filed and served, or threatened in writing (including email) to be filed, against the Company or any Company Subsidiary in any forum, by any person (A) contesting the validity, use, ownership, enforceability, patentability or registrability of any material Company IP, or (B) alleging any material infringement or misappropriation of, or other conflict with, any Intellectual Property rights of other persons (including any material demands or offers to license any Intellectual Property rights from any other person); (ii) to the Company’s knowledge, the operation of the business of the Company and the Company Subsidiaries (including the Products) has not and does not materially infringe, misappropriate or violate, any Intellectual Property rights of other persons; and (iii) to the Company’s knowledge, no other person has infringed, misappropriated or violated any material Company-Owned IP.
(e) To the extent required under applicable Law to assign or transfer to the Company or any Company Subsidiary all right, title and interest in and to any Intellectual Property, all persons who have contributed, created, conceived, or otherwise developed any Company-Owned IP have executed valid, written agreements with the Company or one of the Company Subsidiaries, pursuant to which such persons agreed to assign to the Company or the applicable Company Subsidiary all of their entire right, title, and interest in and to any
Annex A-30
Table of Contents
Intellectual Property contributed, created, conceived or otherwise developed by such person in the course of and related to his, her or its relationship with the Company or the applicable Company Subsidiary. There are no outstanding Actions, and, to the Company’s knowledge, threatened Actions, for any compensation or other payments to such person in relation to any Company IP that such person has contributed, created, conceived or otherwise developed. To the Company’s knowledge, no employee, independent contractor, or agent of the Company or the Company Subsidiaries has misappropriated any material trade secrets of the Company or the Company Subsidiaries in the course of his or her performance as an employee, independent contractor, or agent, and no employee, independent contractor, or agent of the Company or the Company Subsidiaries is in material default or material breach of any material term of any employment agreement, nondisclosure agreement, assignment of invention agreement, or similar agreement or contract to the extent relating to the protection, ownership, development, use or transfer of Company IP.
(f) To the Company’s knowledge, the Company and Company Subsidiaries have not used any Open Source Software or any modification or derivative thereof in a manner that would (i) grant or purport to grant to any other person any rights to or immunities under any of the Company IP, or (ii) require the Company or any Company Subsidiary to disclose or distribute the source code to any Business Systems or Product components, to license or provide the source code to any of the Business Systems or Product components for the purpose of making derivative works, or to make available for redistribution to any person the source code to any of the Business Systems or Product components at no or minimal charge.
(g) The Company and/or one of the Company Subsidiaries owns, leases, licenses, or otherwise has the legal right to use all Business Systems, and such Business Systems are sufficient for the immediate and anticipated future needs of the business of the Company or any of the Company Subsidiaries as currently conducted by the Company and/or the Company Subsidiaries. The Company, on its behalf and on behalf of each of the Company Subsidiaries, maintains commercially reasonable disaster recovery and business continuity plans, procedures and facilities, and since January 1, 2019, there has not been any material failure with respect to any of the Business Systems that has not been remedied or replaced in all material respects.
(h) The Company and each of the Company Subsidiaries comply in all material respects with (i) all applicable Privacy/Data Security Laws (including any data collected in connection with COVID-19 screening), (ii) any applicable privacy or other policies of the Company and/or the Company Subsidiary, respectively, concerning the collection, dissemination, storage or use of Personal Information, and (iii) except as would not have a Company Material Adverse Effect, all contractual commitments that the Company or any Company Subsidiary has entered into or is otherwise bound with respect to privacy and/or data security (collectively, the “Data Security Requirements”). The Company and the Company Subsidiaries have each implemented reasonable data security safeguards designed to protect the security and integrity of its Business Systems and any Business Data, including utilizing industry standard tools designed to prevent unauthorized access and the introduction of Disabling Devices. To the Company’s knowledge, since January 1, 2019, neither the Company nor any of the Company Subsidiaries has (x) experienced any data security breaches that were required to be reported under applicable Privacy/Data Security Laws or customer contracts; or (y) been subject to or received written notice of any audits, proceedings or investigations by any Governmental Authority or any customer, or received any material claims or complaints regarding the collection, dissemination, storage or use of Personal Information, or the violation of any applicable Data Security Requirements.
SECTION 4.14 Taxes.
(a) The Company and each of the Company Subsidiaries: (i) have duly and timely filed (taking into account any extension of time within which to file) all income Tax Returns and other material Tax Returns required to be filed by any of them as of the date hereof and all such filed Tax Returns are complete and accurate in all material respects; (ii) have timely paid all Taxes that are shown as due on such filed Tax Returns and any other material Taxes that the Company or any of the Company Subsidiaries are otherwise obligated to pay, except with respect to Taxes that are (whether or not such Taxes have been reported on any Tax Returns) not yet due and payable or are otherwise being contested in good faith, and no material penalties or charges are due with respect to the late filing of any Tax Return required to be filed by or with respect to any of them on or before the Closing; (iii) with respect to all material Tax Returns filed by or with respect to any of them, have not waived any statute of limitations with respect to Taxes or agreed to any extension of time with respect to
Annex A-31
Table of Contents
a Tax assessment or deficiency; and (iv) do not have any deficiency, audit, examination, investigation or other proceeding in respect of a material amount of Taxes or material Tax matters pending or proposed or threatened in writing, for a Tax period which the statute of limitations for assessments remains open.
(b) Neither the Company nor any Company Subsidiary is a party to, is bound by or has an obligation under any Tax sharing agreement, Tax indemnification agreement, Tax allocation agreement or similar contract or arrangement (including any agreement, contract or arrangement providing for the sharing or ceding of credits or losses) or has a potential liability or obligation to any person as a result of or pursuant to any such agreement, contract, arrangement or commitment other than an agreement, contract, arrangement or commitment the primary purpose of which does not relate to Taxes.
(c) None of the Company and the Company Subsidiaries will be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting for a taxable period or portion thereof ending on or prior to the Closing Date under Section 481(c) of the Code (or any corresponding or similar provision of state, local or foreign income Tax law); (ii) “closing agreement” as described in Section 7121 of the Code (or any corresponding or similar provision of state, local or foreign income Tax law) executed on or prior to the Closing Date; (iii) installment sale or open transaction disposition made on or prior to the Closing Date; (iv) prepaid amount received or deferred revenue accrued on or prior to the Closing Date; (v) intercompany transaction or excess loss account described in Treasury Regulations under Section 1502 of the Code (or any corresponding or similar provision of state, local or non-United States income Tax law) in existence on or prior to the Closing Date; (vi) any use of an improper method of accounting use for any taxable period or portion thereof ending or ended on or prior to the Closing Date; or (vii) income arising or accruing prior to the Closing and includable after the Closing under Subchapter K, Section 951, 951A or 956 of the Code.
(d) Each of the Company and the Company Subsidiaries has withheld and paid to the appropriate Tax authority all material Taxes required to have been withheld and paid in connection with amounts paid or owing to any current or former employee, independent contractor, creditor, shareholder or other third party and has complied in all material respects with all applicable laws, rules and regulations relating to the payment and withholding of Taxes, including all reporting and record keeping requirements related thereto.
(e) Neither the Company nor any of the Company Subsidiaries has been a member of an affiliated group filing a consolidated, combined or unitary U.S. federal, state, local or foreign income Tax Return (other than a group comprised only of the Company and/or Company Subsidiaries).
(f) Neither the Company nor any of the Company Subsidiaries has any material liability for the Taxes of any person (other than the Company and the Company Subsidiaries) under Treasury Regulation Section 1.1502-6 (or any similar provision of state, local or foreign law), as a transferee or successor, by contract, or otherwise.
(g) Neither the Company nor any of the Company Subsidiaries has any request for a material ruling in respect of Taxes pending between the Company or any Company Subsidiary, on the one hand, and any Tax authority, on the other hand.
(h) The Company has made available to SPAC in the Dataroom true, correct and complete copies of the U.S. federal income Tax Returns filed by the Company and the Company Subsidiaries for Tax years 2018, 2019 and 2020.
(i) Neither the Company nor any of the Company Subsidiaries has within the last two (2) years distributed shares or stock of another person, or had its shares or stock distributed by another person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 or Section 361 of the Code.
(j) Neither the Company nor any of the Company Subsidiaries has engaged in or entered into a “listed transaction” within the meaning of Treasury Regulation Section 1.6011-4(b)(2), or any corresponding or similar provision of state, local or non-United States Law.
Annex A-32
Table of Contents
(k) Neither the IRS nor any other United States or non-United States taxing authority or agency has asserted in writing or, to the knowledge of the Company or any of the Company Subsidiaries, has threatened to assert against the Company or any Company Subsidiary any deficiency or claim for any Taxes or interest thereon or penalties in connection therewith, which is still pending or unresolved.
(l) There are no Tax liens upon any assets of the Company or any of the Company Subsidiaries except for Permitted Liens.
(m) None of the Company and the Company Subsidiaries has received written notice from a non-United States taxing authority that it has a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise has an office or fixed place of business in a country other than the country in which it is organized. None of the Company and the Company Subsidiaries has received written notice from a jurisdiction where it does not file Tax Returns that it is subject to Tax in that jurisdiction. None of the Company and the Company Subsidiaries has made an election under Section 965(h) of the Code.
(n) Equity interests in the Company are not United States real property interests within the meaning of Section 897(c)(1) of the Code.
(o) The Company is, and has been since its formation, treated as a foreign corporation for United States federal income Tax purposes.
(p) Neither the Company nor any of the Company Subsidiaries has taken or agreed to take any action, and does not intend to or plan to take any action, or has any knowledge of any fact or circumstance that could reasonably be expected to prevent the Merger and the Exchange from qualifying for the Intended Tax Treatment.
(q) The Company and each Company Subsidiary has complied in all material respects with all statutory provisions, rules, regulations, orders and directions in respect of VAT under applicable Law, promptly submitted accurate returns, and maintained full and accurate VAT records, invoices, and other requisite documents as required by applicable Law.
(r) Neither the Company nor any Company Subsidiary has been a party to, nor has otherwise been involved in, any transaction, scheme or arrangement (i) the main purpose of which was avoiding, deferring or reducing a liability to Tax or producing a loss for Tax purposes with no corresponding commercial or economic loss, or (ii) that is required to be disclosed to HMRC under any applicable Law for Taxes.
SECTION 4.15 Environmental Matters. Except as set forth in Section 4.15 of the Company Disclosure Schedule, or as would not have a Company Material Adverse Effect, (a) each of the Company and the Company Subsidiaries is and has been since January 1, 2019 in compliance with all applicable Environmental Laws; (b) each of the Company and the Company Subsidiaries has obtained and is in compliance with all permits, licenses, franchises, grants, exemptions, registrations, accreditations and other authorizations required under Environmental Laws (“Environmental Permits”) to own, lease and operate its properties and to carry on its business, and each such Environmental Permit is in full force and effect, free from breach by the Company and the Company Subsidiaries, and will not be adversely affected by the Transactions; (c) neither the Company nor any Company Subsidiary has received written notice from any Governmental Authority regarding any actual or alleged violation of, or liability under, any Environmental Law, the subject of which has not been fully resolved; (d) except for regulatory orders of general applicability, neither the Company nor any Company Subsidiary is subject to any order, writ, judgment, injunction, decree, determination or award applicable to it or with respect to its assets arising under Environmental Law under which any material obligation remains unsatisfied; (e) to the knowledge of the Company, none of the properties currently or formerly owned, leased or operated by the Company or any Company Subsidiary are contaminated with any Hazardous Substance in violation of applicable Environmental Laws as a result of any act or omission by the Company or any Company Subsidiary or in a manner that requires or would reasonably be expected to require reporting, investigation, remediation, monitoring or other response action by the Company or any Company Subsidiary pursuant to applicable Environmental Laws; (f) neither the Company nor any Company Subsidiary has handled, stored, transported, disposed of, arranged for or permitted the disposal of, or Released any Hazardous Substances, or owned or operated any property or facility, in a manner that has given or would reasonably be expected to give rise to material liability under any Environmental Law; (g) the Transactions will not result in any liabilities for site investigation or cleanup, or require the consent of any Person, pursuant to
Annex A-33
Table of Contents
any so-called “transaction-triggered” or “responsible property transfer” requirements in any Environmental Laws; (h) neither the Company nor any Company Subsidiary has, either expressly or by operation of Law, assumed or undertaken any material liability, including any obligation for corrective or remedial action, of any other person relating to Environmental Laws.
SECTION 4.16 Material Contracts.
(a) �� Section 4.16(a) of the Company Disclosure Schedule lists, as of the date of this Agreement, the following types of contracts and agreements to which the Company or any Company Subsidiary is a party, excluding for this purpose, any purchase orders submitted by customers (such contracts and agreements as are required to be set forth Section 4.16(a) of the Company Disclosure Schedule along with any Plan listed on Section 4.10(a) of the Company Disclosure Schedule being the “Material Contracts”):
(i) except for any contracts or agreements by and between or among any of the Company and the Company Subsidiaries, each contract and agreement with consideration paid or payable to the Company or any of the Company Subsidiaries of more than $100,000 in the aggregate, in the prior or current fiscal year;
(ii) each contract and agreement with (A) the Company’s top 10 customers and Suppliers based on the aggregate amounts paid by or to the Company and the Company Subsidiaries in the 12-month period ending on December 31, 2021 or the 12-month period ending on March 31, 2022, and (B) the Company’s Suppliers of raw materials that are material to the production of the Company’s products and services (any of the customers referenced in the foregoing, the “Material Customers” and any of the Suppliers referenced in the foregoing, the “Material Suppliers”);
(iii) except for any contracts or agreements by and between or among any of the Company and the Company Subsidiaries, all broker, distributor, dealer, manufacturer’s representative, franchise, agency, sales promotion, market research, marketing consulting and advertising contracts and agreements to which the Company or any Company Subsidiary is a party and that are material to the business of the Company;
(iv) all contracts that provides for the formation, creation, operation, management or control of any joint venture or partnership (including any commercial partnership) with a third party (and any such third party, a “Material Partner”);
(v) all management contracts (excluding contracts for employment), and contracts with other consultants, that are material to the business of the Company, including any contracts involving the payment of royalties or other amounts calculated based upon the revenues or income of the Company or any Company Subsidiary or income or revenues related to any Product of the Company or any Company Subsidiary to which the Company or any Company Subsidiary is a party, but excluding any such contracts entered into by the Company or any Company Subsidiary in the ordinary course of business;
(vi) all (A) employment agreements pursuant to which an employee is entitled to receive base annual compensation in excess of $150,000; and (B) consulting agreements pursuant to which an independent contractor is entitled to receive annual payments in excess of $150,000; and (C) severance agreements that provide for mandatory or potential severance payments in excess of $150,000.
(vii) except for contracts or agreements relating to trade receivables or by and between or among any of the Company and the Company Subsidiaries, all contracts and agreements evidencing indebtedness for borrowed money in an amount greater than $250,000;
(viii) except for any contracts or agreements by and between or among any of the Company and the Company Subsidiaries, all material definitive partnership, joint venture or similar agreements;
(ix) all contracts and agreements with any Governmental Authority to which the Company or any Company Subsidiary is a party, other than any Company Permits;
(x) all collective bargaining agreements or other contracts with any union, works council or labor organization;
Annex A-34
Table of Contents
(xi) all contracts and agreements that limit, or purport to limit, the ability of the Company or any Company Subsidiary to compete in any material respect in any line of business or with any person or entity or in any geographic area or during any period of time (including all contracts and agreements that restrict or limit the ability of the Company or any Company Subsidiary to sell, manufacture, develop, commercialize, test or research products (directly or indirectly through third parties), or to solicit any potential employee or customer in any manner that is material to the business of the Company and the Company Subsidiaries), in each case of the foregoing, excluding any non-solicitation provisions in contracts that are entered into by the Company or any Company subsidiary in the ordinary course of business consistent with past practices where such non-solicitation provision is not the primary reason for the contract and customary confidentiality agreements and agreements that contain customary confidentiality clauses;
(xii) all leases or master leases of personal property reasonably likely to result in annual payments of $150,000 or more in a 12-month period;
(xiii) all contracts involving the use of any Company-Licensed IP and required to be listed in the Company Disclosure Schedule pursuant to Section 4.13(a)(ii);
(xiv) all contracts or agreements under which the Company has agreed to purchase goods or services from a vendor, supplier or other person on a preferred supplier or “most favored supplier” basis, excluding customary exclusivity agreements or arrangements;
(xv) except for any contracts or agreements by and between or among any of the Company and the Company Subsidiaries, contracts which involve the license or grant of rights to Company-Owned IP by the Company and/or the Company Subsidiaries, but excluding any nonexclusive licenses (or sublicenses) of Company-Owned IP granted to customers in the ordinary course of business;
(xvi) any contract or agreement granting to any Person (other than the Company or any Company Subsidiaries) (A) a right of first refusal, first offer or similar preferential right to purchase or acquire equity interests in the Company or any of the Company’s Subsidiaries or (B) the right to receive or earn milestones payments, royalties or other contingent payments based on any investigation, manufacture, research, testing, development, regulatory filings or approval, sale, distribution, commercial manufacture or other similar occurrences, developments, activities or events;
(xvii) any contract or agreement involving any resolution, conciliation or settlement of any actual or threatened litigation, arbitration, claim or other dispute related to the business of the Company and the Company Subsidiaries (A) with any Governmental Authority, or (B) under which the Company or any Company Subsidiary has any material ongoing obligations; or
(xviii) any contract or agreement requiring capital expenditures on behalf of the Company or any Company Subsidiary after the date of this Agreement in an amount in excess of $500,000 in any 12 month period.
(b) Except as set forth in Section 4.16(b) of the Company Disclosure Schedule, or except as would not be material to the Company and the Company Subsidiaries, taken as a whole, (i) each Material Contract is a legal, valid and binding obligation of the Company or the Company Subsidiaries (subject to the Remedies Exception) and, to the knowledge of the Company, the other parties thereto, and neither the Company nor any Company Subsidiary is in breach or violation of, or default under, any Material Contract nor has any Material Contract been canceled by the other party; (ii) to the Company’s knowledge, no other party to any Material Contract is in breach or violation of, or default under, such Material Contract; and (iii) the Company and the Company Subsidiaries have not received any written or to the knowledge of the Company, oral claim of default under any such Material Contract. The Company has furnished or made available to SPAC true and materially complete copies of all Material Contracts, including amendments thereto that are material in nature, but excluding any Material Contracts in the form of individuals purchase or service orders.
Annex A-35
Table of Contents
(c) Since December 31, 2021, no Material Customer, Material Supplier or Material Partner has notified the Company or any Company Subsidiary in writing (i) indicating that it intends to cease doing business with the Company or any Company Subsidiary, (ii) requesting or otherwise engaging in a material decrease in the volume of business from or to the Company or any Company Subsidiary, or (iii) requesting or otherwise engaging in a material change in pricing charged to or by the Company or any Company Subsidiary.
SECTION 4.17 Insurance.
(a) Section 4.17(a) of the Company Disclosure Schedule sets forth, with respect to each material insurance policy under which the Company or any Company Subsidiary is an insured (the “Insurance Policies”), a named insured or otherwise the principal beneficiary of coverage as of the date of this Agreement (i) the names of the insurer, the principal insured and each named insured, (ii) the policy number, (iii) the period, scope and amount of coverage and (iv) the premium most recently charged.
(b) With respect to each Insurance Policy, except as would not have a Company Material Adverse Effect: (i) each policy is legal, valid, binding and enforceable in accordance with its terms (subject to the Remedies Exceptions) and, except for policies that have expired under their terms in the ordinary course, is in full force and effect; (ii) neither the Company nor any Company Subsidiary is in breach or default, and no event has occurred which, with notice or the lapse of time, would constitute such a breach or default, or permit termination or modification, under the policy and (iii) to the knowledge of the Company, no insurer on the policy has been declared insolvent or placed in receivership, conservatorship or liquidation. The Company and the Company Subsidiaries hold policies of insurance required to be maintained by Material Contracts.
SECTION 4.18 Board Approval; Vote Required.
(a) The Company Board Approval has been duly obtained by resolutions duly adopted by written consent and not subsequently rescinded or modified in any way.
(b) The only vote of the holders of any class of shares in the Issued Share Capital or any governing body of the Company that is necessary to approve this Agreement and the Transactions is (i) the approval of Holdco, as sole shareholder of the Company following the Exchange, to amend and restate the Company Organizational Documents pursuant to Section 2.05(c); and (ii) the Company Board Approval. The Company Board Approval has been obtained.
SECTION 4.19 Certain Business Practices.
(a) None of the Company, any Company Subsidiary or, to the Company’s knowledge, any directors, officers, agents or employees of the Company or any Company Subsidiary, has:
(i) used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to political activity;
(ii) made any unlawful payment to foreign or domestic government officials or employees or to foreign or domestic political parties or campaigns or violated any applicable Anti-Corruption Laws; or
(iii) made any payment in the nature of bribery.
(b) The Company, any Company Subsidiary and, to the Company’s knowledge, their respective directors, officers, agents and employees, are and have been in compliance with Anti-Corruption Laws and Anti-Money Laundering Laws, including with regard to financial recordkeeping and reporting requirements in all jurisdictions in which the Company and any Company Subsidiary conducts business.
(c) None of the Company, any Company Subsidiary, nor, to the Company’s knowledge, any of their respective directors, officers, agents or employees: (i) is or has been subject to any action, suit, claim, proceeding, prosecution, settlement, formal or informal notice, or investigation with respect to Anti-Corruption Laws or Anti-Money Laundering Laws; or (ii) made a voluntary, directed, or involuntary disclosure to any governmental authority or similar agency with respect to any alleged act or omission arising under or relating to any alleged noncompliance with Anti-Corruption Laws or Anti-Money Laundering Laws.
Annex A-36
Table of Contents
(d) The Company as well as its respective affiliates have instituted and maintain in effect policies and procedures reasonably designed to achieve compliance with Anti-Corruption Laws and Anti-Money Laundering Laws.
SECTION 4.20 Product Warranty; Product Liability.
(a) Since its date of formation, there have been no claims exceeding the equivalent of $150,000 made, or to the knowledge of the Company, threatened in writing against the Company or any Company Subsidiary by a customer or any other person alleging that (i) such Product (A) did not comply with any express or implied warranty regarding such Product, (B) contained an unintended Hazardous Substance, or (C) was otherwise contaminated, adulterated, mislabeled, defective or improperly packaged or transported, or (ii) the Company, any Company Subsidiary or any of their licensees, distributors or agents breached any duty to warn, test, inspect or instruct of the risks, limitations, precautions or dangers related to the use, application, or transportation of any such Product.
(b) Except as would not have a Company Material Adverse Effect, neither the Company nor any Company Subsidiary, nor, to the knowledge of the Company, any of its licensees, distributors, suppliers or agents has developed, manufactured, commercialized, produced, formulated, propagated, modified, customized, processed, distributed or sold any Product that did not comply with any express or implied warranty regarding such Product or that contained any unintended Hazardous Substance or that was otherwise adulterated, contaminated, mislabeled, defective, off-specification or improperly packaged or transported or that otherwise did not comply with applicable Law.
(c) Since its date of formation, the Company and the Company Subsidiaries have in all material respects made all declarations and filings required by any Law or Company Permit for the handling and delivery to third parties of Products.
(d) Since its date of formation, there have been no recalls, market withdrawals or replacements (voluntary or involuntary) with respect to any Product or any similar actions, investigations, written notices or written threats of recalls by any Governmental Entity with respect to any Product.
SECTION 4.21 Compliance with Regulatory Laws.
(a) Except as would not result in a Company Material Adverse Effect, the Products are not and have not been adulterated or misbranded within the meaning of the applicable UK laws, Federal Fair Packaging and Label Act, Food Drug and Cosmetic Act, the U.S. Food and Drug Administration (the “FDA”), and their attendant regulations, and similar state and local food and drug laws, as the same may be amended. The Company Products are in compliance, and since its date of formation have been in compliance, in all material respects with (i) the applicable provisions of the applicable UK laws, the Federal Food, Drug, and Cosmetic Act, as amended, and the applicable regulations and requirements adopted by applicable UK regulatory agencies, the FDA, the thereunder, the applicable regulations and requirements adopted by the U.S. Department of Agriculture, all applicable statutes enforced by the U.S. Federal Trade Commission and the applicable regulations and requirements and any applicable requirements established by any other federal, state, county, city, local or foreign Governmental Bodies responsible for regulating food products (collectively, the foregoing Governmental Authorities, the “Food Authorities” and the foregoing Laws, rules, regulations and requirements, the “Food Laws”)), and (ii) all terms and conditions imposed in any Permits granted to the Company or any Company Subsidiary by any Food Authority. The foregoing includes any applicable good manufacturing practices and sanitation requirements, labeling and advertising requirements, requirements relating to food or color additives, food standards, product composition requirements, testing requirements or protocols, recordkeeping or reporting requirements, monitoring requirements, packaging (including co-packing and re-packing) requirements, laboratory controls, storage and warehousing procedures, shipping requirements, import and export requirements, supply chain security, food handling, and shelf-life requirements.
(b) The Company has obtained all material Permits required by Food Authorities with respect to the conduct of the business operated by the Company and such Permits are in good standing as of the date hereof. Except as would not result in a Company Material Adverse Effect, the Company has maintained and retained accurate records with respect to the foregoing, as well as other records required to be kept by Food Authorities or as may be required under any agreement with third parties regarding the Company Products.
Annex A-37
Table of Contents
(c) None of the Company, the Company Products nor the facilities in which the Company Products are made or handled is subject to any warning letter, untitled letter or other adverse written correspondence or written notice from the FDA, Notice of Intended Enforcement by the USDA or other adverse written correspondence or written notice from the USDA (“Adverse Notice”), recall, investigation, penalty assessment or other compliance or enforcement action by any Food Authority or any other Governmental Authority having responsibility for the regulation of food products, beyond any Adverse Notices to which the Company responded and which are not reasonably expected to be material to the Company and its Subsidiaries. To the knowledge of the Company, none of the entities which manufacture, process, package, supply ingredients for or distribute the Company Products is subject to any such adverse action with regard to a Company Product or a facility that manufactures a Company Product, in each case, except as would not result in a Company Material Adverse Effect. In all material respects, the Company audits its suppliers for compliance with all regulations promulgated by Food Authorities to which such suppliers are subject in accordance with the rights granted under any third party agreements with such suppliers.
SECTION 4.22 Interested Party Transactions. Except as set forth in Section 4.22 of the Company Disclosure Schedule and except for employment relationships and the payment of compensation, benefits and expense reimbursements and advances in the ordinary course of business and pursuant to any Plan, no director, officer or other affiliate of the Company or any Company Subsidiary (other than the Company or any Company Subsidiary), to the Company’s knowledge, has, directly or indirectly: (a) an economic interest in any person that furnishes or sells, services or Products that the Company or any Company Subsidiary furnishes or sells, or proposes to furnish or sell; (b) an economic interest in any person that purchases from or sells or furnishes to, the Company or any Company Subsidiary, any goods or services; (c) an economic interest in any person (other than the Company and the Company Subsidiaries) party to any contract or agreement disclosed in Section 4.16(a) of the Company Disclosure Schedule; or (d) any contractual or other arrangement with the Company or any Company Subsidiary, other than contracts or arrangements constituting Transaction Documents or customary indemnity arrangements (collectively the foregoing, the “Interested Party Transactions”); provided, however, that ownership of no more than five percent (5%) of the outstanding voting stock of a publicly traded corporation shall not be deemed an “economic interest in any person” for purposes of this Section 4.22. As of the date of this Agreement, the Company and the Company Subsidiaries have not (i) extended or maintained credit, arranged for the extension of credit or renewed an extension of credit in the form of a personal loan to or for any director or executive officer (or equivalent thereof) of the Company.
SECTION 4.23 Exchange Act; Proxy Statement/Prospectus and Registration Statement.
(a) Neither the Company nor any Company Subsidiary is currently (or has previously been) subject to the requirements of Section 12 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
(b) None of the information relating to the Company supplied by the Company in writing for inclusion in the Proxy Statement/Prospectus or Registration Statement will, as of the date the Registration Statement is declared effective, as of the date the Proxy Statement/Prospectus (or any amendment or supplement thereto) is first mailed to the SPAC Shareholders, at the time of the SPAC Shareholders’ Meeting, or at the Merger Effective Time, contain any misstatement of a material fact or omission of any material fact necessary to make the statements therein, in light of the circumstances under which they are made, not misleading; provided, however, that the Company makes no representation with respect to any forward-looking statements supplied by or on behalf of the Company for inclusion in, or relating to information to be included in the Proxy Statement/Prospectus or Registration Statement.
SECTION 4.24 Brokers. Except as set forth in Section 4.24 of the Company Disclosure Schedules, no broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the Transactions based upon arrangements made by or on behalf of the Company or any Company Subsidiary.
SECTION 4.25 Sanctions, Import Control, and Export Control Laws.
(a) None of the Company, any Company Subsidiary, nor, to the Company’s knowledge, any of their respective directors, officers, employees or agents is a Restricted Person.
(b) None of the Company, any Company Subsidiary, nor, to the Company’s knowledge, any of their respective directors, officers, employees or agents, is in violation of, or has violated since its date of formation, Sanctions Laws, Import Controls Laws, or Export Control Laws.
Annex A-38
Table of Contents
(c) None of the Company, any Company Subsidiary, nor to the Company’s knowledge, any of their respective directors, officers, employees or agents:
(i) is or has been since its date of formation subject to any action, suit, claim, proceeding, prosecution, settlement, formal or informal notice, or investigation with respect to Sanctions Laws, Import Control Laws, or Export Control Laws; or
(ii) made since its date of formation a voluntary, directed, or involuntary disclosure to any governmental authority or similar agency with respect to any alleged act or omission arising under or relating to any alleged noncompliance with Sanctions Laws, Import Control Laws, or Export Control Laws.
(d) Any provision of this Section 4.25 shall not apply to any person if and to the extent that it is or would be unenforceable by or in respect of that person by reason of breach of any provision of Council Regulation (EC) No 2271/1996 of 22 November 1996 (or any law or regulation implementing such Regulation in any member state of the European Union or the United Kingdom).
SECTION 4.26 Exchange Agreements.
Notwithstanding anything in this Article IV to the contrary, (a) each Exchange Agreement (subject to the Remedies Exception) is a legal, valid and binding obligation of the Company, Holdco and, to the knowledge of the Company, each of the Company Shareholders party thereto enforceable against such Persons in accordance with its terms, (b) neither the Company nor Holdco is in breach or violation of, or default under, any Exchange Agreement nor has any Exchange Agreement been terminated or canceled by any Company Shareholder, (c) no Company Shareholder is in breach or violation of, or default under, any Exchange Agreement and (d) the Company and Holdco have not received any written or, to the knowledge of the Company, oral claim of default under any such Exchange Agreement. The Company has furnished or made available to SPAC true and complete copies of all Exchange Agreements, including amendments thereto that are material in nature. The Company has not altered or amended any Exchange Agreement and no Company Shareholder has sought to or threatened in writing or, to the knowledge of the Company, otherwise threatened to renegotiate any Exchange Agreement or threatened non-performance under any Exchange Agreement, in each case, as to which such Company Shareholder is a party.
SECTION 4.27 Exclusivity of Representations and Warranties. Except as otherwise expressly provided in this Article IV (as modified by the Company Disclosure Schedule), the Company hereby expressly disclaims and negates, any other express or implied representation or warranty whatsoever (whether at Law or in equity) with respect to the Company, its affiliates, and any matter relating to any of them, including their affairs, the condition, value or quality of the assets, liabilities, financial condition or results of operations, or with respect to the accuracy or completeness of any other information made available to SPAC, its affiliates or any of their respective Representatives by, or on behalf of, the Company, and any such representations or warranties are expressly disclaimed. Without limiting the generality of the foregoing, except as expressly set forth in this Agreement, neither the Company nor any other person on behalf of the Company has made or makes, any representation or warranty, whether express or implied, with respect to any projections, forecasts, estimates or budgets made available to SPAC, its affiliates or any of their respective Representatives of future revenues, future results of operations (or any component thereof), future cash flows or future financial condition (or any component thereof) of the Company (including the reasonableness of the assumptions underlying any of the foregoing), whether or not included in any management presentation or in any other information made available to SPAC, its affiliates or any of their respective Representatives or any other person, and that any such representations or warranties are expressly disclaimed.
Annex A-39
Table of Contents
ARTICLE V
REPRESENTATIONS AND WARRANTIES OF SPAC
Except as set forth in the SPAC SEC Reports (to the extent the qualifying nature of such disclosure is readily apparent from the content of such SPAC SEC Reports, but excluding any disclosures contained or referenced therein under the captions “Risk Factors,” “Forward-Looking Statements,” “Quantitative and Qualitative Disclosures About Market Risk,” and any other disclosures contained or referenced therein of information, factors, or risks that are predictive, cautionary, or forward-looking in nature) (it being acknowledged that nothing disclosed in such an SEC Report will be deemed to modify or qualify the representations and warranties set forth in Section 5.01, Section 5.03 and Section 5.04), SPAC hereby represents and warrants to the Company as follows:
SECTION 5.01 Corporate Organization.
(a) SPAC is a corporation duly incorporated, validly existing and in good standing under the laws of the state of Delaware and has the requisite corporate power and authority to own, lease and operate its properties and to carry on its business as it is now being conducted. SPAC has all necessary governmental approvals to own, lease and operate its properties and to carry on its business as it is now being conducted, except where the failure to have such governmental approval or be so qualified or licensed and in good standing would not have a SPAC Material Adverse Effect.
(b) SPAC does not directly or indirectly own any equity or similar interest in, or any interest convertible into or exchangeable or exercisable for any equity or similar interest in, any corporation, partnership, joint venture, business association or other person.
SECTION 5.02 SPAC Organizational Documents. SPAC has heretofore furnished to the Company complete and correct copies of the SPAC Organizational Documents. The SPAC Organizational Documents are in full force and effect. SPAC is not in violation of any of the provisions of the SPAC Organizational Documents.
SECTION 5.03 Capitalization.
(a) As of the date hereof, the authorized share capital of SPAC consists of (i) 99,000,000 shares of common stock, par value $0.0001 per share and (ii) 1,000,000 preference shares, par value $0.0001 per share. As of the date of this Agreement, (A) 17,370,000 SPAC Common Stock are issued and outstanding (and 13,800,000 SPAC Common Stock are subject to Redemption Rights), (B) no preference shares are issued and outstanding, and (C) 6,900,000 redeemable SPAC Warrants to purchase 6,900,000 SPAC Common Stock and 4,210,000 private placement SPAC Warrants to purchase 4,210,000 SPAC Common Stock are issued and outstanding. Each SPAC Warrant entitles the holder to purchase one SPAC Common Stock at an exercise price of $11.50.
(b) All outstanding SPAC Common Stock and SPAC Warrants (i) are duly authorized, validly issued, fully paid and nonassessable, (ii) are not subject to any preemptive rights, (iii) have been issued and granted in compliance with all applicable securities Laws and other applicable Laws and (iv) were issued free and clear of all Liens other than transfer restrictions under applicable securities Laws and the SPAC Organizational Documents.
(c) Other than the SPAC Warrants, there are no options, warrants, preemptive rights, calls, convertible securities, conversion rights or other rights, agreements, arrangements or commitments of any character relating to the issued or unissued shares of SPAC or obligating SPAC to issue or sell any shares of, or other equity interests in, SPAC. SPAC is not a party to, or otherwise bound by, and has not granted, any equity appreciation rights, participations, phantom equity or similar rights. There are no voting trusts, voting agreements, proxies, shareholder agreements or other agreements with respect to the voting or transfer of SPAC Common Stock or any of the equity interests or other securities of SPAC. SPAC does not own any equity interests in any person.
(d) Other than Redemption Rights, there are no outstanding contractual obligations of SPAC to repurchase, redeem or otherwise acquire any SPAC Common Stock or to provide funds to or make any investment (in the form of a loan, capital contribution or otherwise) in any persons.
Annex A-40
Table of Contents
SECTION 5.04 Authority Relative to this Agreement. SPAC has all necessary corporate power and corporate authority to execute and deliver this Agreement and each Ancillary Agreement to which it is a party and, subject to obtaining the requisite consent of the SPAC Shareholders to approve the SPAC Proposals, to perform its obligations hereunder and thereunder and to consummate the Transactions. The execution and delivery by SPAC of this Agreement and the Ancillary Agreements to which it is a party and the consummation by SPAC of the Transactions have been duly and validly authorized by all necessary corporate action, including approval by the SPAC Board, and no other corporate proceedings on the part of SPAC is necessary to authorize this Agreement, each such Ancillary Agreement or to consummate the Transactions (other than the requisite consent of the SPAC Shareholders to approve the SPAC Proposals). This Agreement and each such Ancillary Agreement have been duly and validly executed and delivered by SPAC and, assuming due authorization, execution and delivery by the Company, Holdco and Merger Sub, constitutes a legal, valid and binding obligation of SPAC, enforceable against SPAC, in accordance with its terms subject to the Remedies Exceptions.
SECTION 5.05 No Conflict; Required Filings and Consents.
(a) Subject to obtaining the requisite consent of the SPAC Shareholders to approve the SPAC Proposals, the execution and delivery by SPAC of this Agreement and each Ancillary Agreement to which it is a party does not, and the performance of this Agreement and each such Ancillary Agreement by SPAC will not, (i) conflict with or violate the SPAC Organizational Documents, (ii) assuming that all consents, approvals, authorizations and other actions described in Section 5.05(b) have been obtained and all filings and obligations described in Section 5.05(b) have been made, conflict with or violate any Law, rule, regulation, order, judgment or decree applicable to SPAC or by which any of its property or assets is bound or affected, or (iii) result in any breach of, or constitute a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of a Lien on any property or asset of SPAC pursuant to, any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which SPAC is a party or by which SPAC or any of its property or assets is bound or affected, except, with respect to clauses (a)(ii) and (a)(iii) for any such conflicts, violations, breaches, defaults or other occurrences which would not have a SPAC Material Adverse Effect.
(b) The execution and delivery by SPAC of this Agreement and each Ancillary Agreement to which it is a party does not, and the performance of this Agreement and each such Ancillary Agreement by SPAC will not, require any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Authority, except (i) for applicable requirements, if any, of the Securities Act, Exchange Act, Blue Sky Laws, stock exchange, HM Revenue and Customs and state takeover laws, and the notification requirements of applicable Antitrust Laws, if any, and the filing and recordation of appropriate Merger and Exchange documents, and (ii) where the failure to obtain such consents, approvals, authorizations or permits, or to make such filings or notifications, would not, individually or in the aggregate, prevent or materially delay consummation of any of the Transactions or otherwise prevent SPAC from performing its material obligations under this Agreement and each such Ancillary Agreement.
SECTION 5.06 Compliance. SPAC is not or has not been in conflict with, or in default, breach or violation of, (a) any Law applicable to SPAC or by which any property or asset of SPAC is bound or affected, or (b) any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which SPAC is a party or by which SPAC or any property or asset of SPAC is bound. SPAC is in possession of all material franchises, grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental Authority necessary for SPAC to own, lease and operate its properties or to carry on its business as it is now being conducted.
SECTION 5.07 SEC Filings; Financial Statements; Sarbanes-Oxley.
(a) SPAC has filed all forms, reports, schedules, statements and other documents, including any exhibits thereto, required to be filed by it with the Securities and Exchange Commission (the “SEC”) since January 12, 2021, together with any amendments, restatements or supplements thereto (collectively, the “SPAC SEC Reports”). SPAC has heretofore furnished to the Company true and correct copies of all amendments and modifications that have not been filed by SPAC with the SEC to all agreements, documents and other instruments that previously had been filed by SPAC with the SEC and are currently in effect. As of their respective dates, the SPAC SEC Reports (i) complied in all material respects with the applicable requirements
Annex A-41
Table of Contents
of the Securities Act of 1933, as amended (the “Securities Act”), the Exchange Act and the Sarbanes-Oxley Act, and the rules and regulations promulgated thereunder, and (ii) did not, at the time they were filed, or, if amended, as of the date of such amendment, contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. Each director and executive officer of SPAC has filed with the SEC on a timely basis all documents required with respect to SPAC by Section 16(a) of the Exchange Act and the rules and regulations thereunder.
(b) Each of the financial statements (including, in each case, any notes thereto) contained in the SPAC SEC Reports was prepared in accordance with GAAP (applied on a consistent basis) and Regulation S-X and Regulation S-K, as applicable, throughout the periods indicated (except as may be indicated in the notes thereto or, in the case of unaudited financial statements, as permitted by Form 10-Q of the SEC) and each fairly presents, in all material respects, the financial position, results of operations, changes in shareholders equity and cash flows of SPAC as at the respective dates thereof and for the respective periods indicated therein, (subject, in the case of unaudited statements, to normal and recurring year-end adjustments). SPAC has no off-balance sheet arrangements that are not disclosed in the SPAC SEC Reports. No financial statements other than those of SPAC are required by GAAP to be included in the consolidated financial statements of SPAC.
(c) Except as and to the extent set forth in the SPAC SEC Reports, SPAC has no liability or obligation of a nature (whether accrued, absolute, contingent or otherwise) required to be reflected on a balance sheet prepared in accordance with GAAP, except for liabilities and obligations arising in the ordinary course of SPAC’s business.
(d) SPAC is in compliance with the applicable listing and corporate governance rules and regulations of Nasdaq Capital Market.
(e) SPAC has established and maintains disclosure controls and procedures (as defined in Rule 13a-15 under the Exchange Act). Such disclosure controls and procedures are designed to ensure that material information relating to SPAC and other material information required to be disclosed by SPAC in the reports and other documents that it files or furnishes under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC, and that all such material information is accumulated and communicated to SPAC’s principal executive officer and its principal financial officer as appropriate to allow timely decisions regarding required disclosure and to make the certifications required pursuant to Sections 302 and 906 of the Sarbanes-Oxley Act. Such disclosure controls and procedures are effective in timely alerting SPAC’s principal executive officer and principal financial officer to material information required to be included in SPAC’s periodic reports required under the Exchange Act.
(f) SPAC maintains systems of internal control over financial reporting that are sufficient to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP, including policies and procedures sufficient to provide reasonable assurance: (i) that SPAC maintains records that in reasonable detail accurately and fairly reflect, in all material respects, its transactions and dispositions of assets; (ii) that transactions are recorded as necessary to permit the preparation of financial statements in conformity with GAAP; (iii) that receipts and expenditures are being made only in accordance with authorizations of management and its board of directors; and (iv) regarding prevention or timely detection of unauthorized acquisition, use or disposition of its assets that could have a material effect on its financial statements. SPAC has delivered to the Company a true and complete copy of any disclosure (or, if unwritten, a summary thereof) by any representative of SPAC to SPAC’s independent auditors relating to any material weaknesses in internal controls and any significant deficiencies in the design or operation of internal controls that would adversely affect the ability of SPAC to record, process, summarize and report financial data. SPAC has no knowledge of any fraud or whistle-blower allegations, whether or not material, that involve management or other employees or consultants who have or had a significant role in the internal control over financial reporting of SPAC. Since January 12, 2021, there have been no material changes in SPAC internal control over financial reporting.
(g) There are no outstanding loans or other extensions of credit made by SPAC to any executive officer (as defined in Rule 3b-7 under the Exchange Act) or director of SPAC. SPAC has not taken any action prohibited by Section 402 of the Sarbanes-Oxley Act.
Annex A-42
Table of Contents
(h) Neither SPAC (including any employee thereof) nor SPAC’s independent auditors has identified or been made aware of (i) any significant deficiency or material weakness in the system of internal accounting controls utilized by SPAC, (ii) any fraud, whether or not material, that involves SPAC’s management or other employees who have a role in the preparation of financial statements or the internal accounting controls utilized by SPAC or (iii) any claim or allegation regarding any of the foregoing.
(i) As of the date hereof, there are no outstanding SEC comments from the SEC with respect to the SPAC SEC Reports. To the knowledge of SPAC, none of the SPAC SEC Reports filed on or prior to the date hereof is subject to ongoing SEC review or investigation as of the date hereof.
SECTION 5.08 Absence of Certain Changes or Events. Since March 31, 2022, except as expressly contemplated by this Agreement, (a) SPAC has conducted its business in the ordinary course and in a manner consistent with past practice in all material respects and (b) there has not been any SPAC Material Adverse Effect.
SECTION 5.09 Absence of Litigation. There is no Action pending or, to the knowledge of SPAC, threatened against SPAC, or any property or asset of SPAC, before any Governmental Authority. Neither SPAC nor any material property or asset of SPAC is subject to any continuing order of, consent decree, settlement agreement or other similar written agreement with, or, to the knowledge of SPAC, continuing investigation by, any Governmental Authority, or any order, writ, judgment, injunction, decree, determination or award of any Governmental Authority.
SECTION 5.10 Board Approval; Vote Required.
(a) The SPAC Board has unanimously adopted by written consent and not subsequently rescinded or modified in any way, has duly (i) determined that this Agreement, the Ancillary Agreements to which SPAC is a party and the Transactions are fair to and in the best interests of SPAC and the SPAC Shareholders, (ii) approved this Agreement, the Ancillary Agreements to which SPAC is a party and the Transactions and declared their advisability and (iii) recommended that the SPAC Shareholders approve and adopt this Agreement, the Ancillary Agreements to which SPAC is a party and the Transactions, and directed that this Agreement, the Ancillary Agreements to which SPAC is a party and the Transactions, be submitted for consideration by the SPAC Shareholders at the SPAC Shareholders’ Meeting.
(b) The only votes of the holders of any class or series of shares of SPAC necessary to approve the Merger and the Transactions as contemplated by this Agreement are the SPAC Shareholder Approvals.
SECTION 5.11 Brokers. No broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the Transactions based upon arrangements made by or on behalf of SPAC.
SECTION 5.12 SPAC Trust Fund. As of the date of this Agreement, SPAC has no less than $138,025,662.14 in the trust fund established by SPAC for the benefit of its SPAC Shareholders (the “Trust Fund”) maintained in a trust account at Continental Stock Transfer & Trust Company (the “Trust Account”). The monies of such Trust Account are invested in United States Government securities or money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act of 1940, as amended, and held in trust by Continental Stock Transfer & Trust Company (the “Trustee”) pursuant to that certain Investment Management Trust Agreement, dated as of January 12, 2021, by and between SPAC and the Trustee (the “Trust Agreement”). The Trust Agreement has not been amended or modified and is valid and in full force and effect and is enforceable in accordance with its terms, subject to the Remedies Exceptions. SPAC has complied in all material respects with the terms of the Trust Agreement and is not in breach thereof or default thereunder and there does not exist under the Trust Agreement any event which, with the giving of notice or the lapse of time, would constitute such a breach or default by SPAC or the Trustee. There are no separate contracts, agreements, side letters or other understandings (whether written or unwritten, express or implied) (a) between SPAC and the Trustee that would cause the description of the Trust Agreement in the SPAC SEC Reports to be inaccurate in any material respect or (b) to the knowledge of SPAC, that would entitle any person (other than shareholders of SPAC who shall have elected to redeem their SPAC Common Stock pursuant to the SPAC Organizational Documents) to any portion of the proceeds in the Trust Account. Prior to the Closing, none of the funds held in the Trust Account may be released except (i) to pay income and franchise Taxes from any interest income earned in the Trust Account and (ii) upon the exercise of Redemption Rights in accordance with the provisions of the SPAC Organizational Documents. As of the date hereof, there are no Actions pending or, to the knowledge of SPAC, threatened in writing with respect to the Trust Account.
Annex A-43
Table of Contents
There are no claims, proceedings or other Actions pending with respect to, or against, the Trust Fund and, to the knowledge of SPAC, there are no events, circumstances or conditions that would reasonably result in any such claim, proceeding or other Action. Upon consummation of the Merger and notice thereof to the Trustee pursuant to the Trust Agreement, SPAC shall cause the Trustee to, and the Trustee shall thereupon be obligated to, release to SPAC as promptly as practicable, the Trust Funds in accordance with the Trust Agreement at which point the Trust Account shall terminate; provided, however that the liabilities and obligations of SPAC due and owing or incurred at or prior to the Closing shall be paid as and when due, including all amounts payable (A) to SPAC Shareholders who shall have exercised their Redemption Rights, (B) with respect to filings, applications and/or other actions taken pursuant to this Agreement required under Law, (C) to the Trustee for fees and costs incurred in accordance with the Trust Agreement; (D) to third parties (e.g., professionals, printers, etc.) who have rendered services to SPAC in connection with its efforts to effect the Merger and (E) to underwriters to pay deferred underwriting fees incurred in connection with SPAC’s initial public offering. As of the date hereof, assuming the accuracy of the representations and warranties of the Company herein and the compliance by the Company with its respective obligations hereunder, SPAC has no reason to believe that any of the conditions to the use of funds in the Trust Account will not be satisfied or funds available in the Trust Account will not be available to SPAC at the Closing.
SECTION 5.13 Employees. Other than any officers of SPAC as described in the SPAC SEC Reports, SPAC has never employed any employees. Other than consultants and advisors retained in the ordinary course of business (including in connection with the Transactions) or as described in the SPAC SEC Reports, SPAC has never retained any contractors. Other than reimbursement of any out-of-pocket expenses incurred by SPAC’s officers and directors in connection with activities on SPAC’s behalf in an aggregate amount not in excess of the amount of cash held by SPAC outside of the Trust Account, SPAC has no unsatisfied material liability with respect to any employee, officer or director. SPAC has never and does not currently maintain, sponsor, contribute to or have any direct liability under any employee benefit plan (as defined in Section 3(3) of ERISA), nonqualified deferred compensation plan subject to Section 409A of the Code, bonus, stock option, stock purchase, restricted stock, incentive, deferred compensation, retiree medical or life insurance, supplemental retirement, severance, change in control, fringe benefit, sick pay and vacation plans or arrangements or other employee benefit plans, programs or arrangements. Neither the execution and delivery of this Agreement nor the other Ancillary Agreements nor the consummation of the Transactions will (i) result in any payment becoming due to any director, officer or employee of SPAC (other than payments received or deemed to be received by any of the foregoing persons in their capacity as SPAC equityholders), (ii) result in the acceleration of the time of payment or vesting of any such benefits, or (iii) give rise to any “excess parachute payment” within the meaning of Section 280G of the Code. There is no contract, agreement, plan or arrangement to which SPAC is a party which requires payment by any party of a Tax gross-up or Tax reimbursement payment to any person.
SECTION 5.14 Taxes.
(a) SPAC (i) has duly and timely filed (taking into account any extension of time within which to file) all income Tax Returns and other material Tax Returns required to be filed by it as of the date hereof and all such filed Tax Returns are complete and accurate in all material respects; (ii) has timely paid all Taxes that are shown as due on such filed Tax Returns and any other material Taxes that the SPAC is otherwise obligated to pay, except with respect to Taxes that are (whether or not such Taxes have been reported on any Tax Returns) not yet due and payable or are otherwise being contested in good faith, and no material penalties or charges are due with respect to the late filing of any Tax Return required to be filed by or with respect to it on or before the Closing; (iii) with respect to all material Tax Returns filed by or with respect to it, has not waived any statute of limitations with respect to Taxes or agreed to any extension of time with respect to a Tax assessment or deficiency; and (iv) does not have any deficiency, audit, examination, investigation or other proceeding in respect of a material amount of Taxes or material Tax matters pending or proposed or threatened in writing, for a Tax period which the statute of limitations for assessments remains open.
(b) SPAC is not a party to, bound by or has an obligation under any Tax sharing agreement, Tax indemnification agreement, Tax allocation agreement or similar contract or arrangement (including any agreement, contract or arrangement providing for the sharing or ceding of credits or losses) or has a potential liability or obligation to any person as a result of or pursuant to any such agreement, contract, arrangement or commitment other than an agreement, contract, arrangement or commitment the primary purpose of which does not relate to Taxes.
Annex A-44
Table of Contents
(c) SPAC will not be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting for a taxable period or portion thereof ending on or prior to the Closing Date under Section 481(c) of the Code (or any corresponding or similar provision of state, local or foreign income Tax law); (ii) “closing agreement” as described in Section 7121 of the Code (or any corresponding or similar provision of state, local or foreign income Tax law) executed on or prior to the Closing Date; (iii) installment sale or open transaction disposition made on or prior to the Closing Date; (iv) prepaid amount received or deferred revenue accrued on or prior to the Closing Date; (v) intercompany transaction or excess loss account described in Treasury Regulations under Section 1502 of the Code (or any corresponding or similar provision of state, local or non-United States income Tax law) in existence on or prior to the Closing Date; (vi) any use of an improper method of accounting use for any taxable period or portion thereof ending or ended on or prior to the Closing Date; or (vii) income arising or accruing prior to the Closing and includable after the Closing under Subchapter K, Section 951, 951A or 956 of the Code.
(d) SPAC has withheld and paid to the appropriate Tax authority all material Taxes required to have been withheld and paid in connection with amounts paid or owing to any current or former employee, independent contractor, creditor, shareholder or other third party and has complied in all material respects with all applicable laws, rules and regulations relating to the payment and withholding of Taxes, including all reporting and record keeping requirements related thereto.
(e) SPAC has not been a member of an affiliated group filing a consolidated, combined or unitary U.S. federal, state, local or foreign income Tax Return.
(f) SPAC does not have any material liability for the Taxes of any person under Treasury Regulation section 1.1502-6 (or any similar provision of state, local or foreign law), as a transferee or successor, by contract, or otherwise.
(g) SPAC does not have any request for a material ruling in respect of Taxes pending between SPAC, on the one hand, and any Tax authority, on the other hand.
(h) SPAC has made available to the Company true, correct and complete copies of the U.S. federal income Tax Returns filed by SPAC for the 2020 Tax year.
(i) SPAC has not within the last two (2) years distributed shares or stock of another person, or had its shares distributed by another person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 or Section 361 of the Code.
(j) SPAC has not engaged in or entered into a “listed transaction” within the meaning of Treasury Regulation Section 1.6011-4(b)(2), or any corresponding or similar provision of state, local or non-United States Law.
(k) Neither the IRS nor any other United States or non-United States taxing authority or agency has asserted in writing or to the knowledge of SPAC, has threatened to asset against SPAC any deficiency or claim for any Taxes or interest thereon or penalties in connection therewith, which is still pending or unresolved.
(l) There are no Tax liens upon any assets of SPAC except for Permitted Liens.
(m) SPAC has not received written notice from a non-United States taxing authority that it has a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise has an office or fixed place of business in a country other than the country in which it is organized. SPAC has not received written notice from a jurisdiction where it does not file Tax Returns that it is subject to Tax in that jurisdiction. SPAC has not made an election under Section 965(h) of the Code.
(n) Equity interests in the SPAC are not United States real property interests within the meaning of Section 897(c)(1) of the Code.
(o) SPAC is, and has been since its formation, treated as a domestic corporation for United States federal income Tax purposes.
Annex A-45
Table of Contents
(p) SPAC has not taken or agreed to take any action, and does not intend to or plan to take any action, or has any knowledge of any fact or circumstance that could reasonably be expected to prevent the Merger and the Exchange from qualifying for the Intended Tax Treatment.
(q) SPAC has complied in all material respects with all statutory provisions, rules, regulations, orders and directions in respect of VAT under applicable Law, promptly submitted accurate returns, and maintained full and accurate VAT records, invoices, and other requisite documents as required by applicable Law.
(r) SPAC has not been a party to, nor has otherwise been involved in, any transaction, scheme or arrangement (i) the main purpose, or one of the main purposes of which was avoiding, deferring or reducing a liability to Tax or producing a loss for Tax purposes with no corresponding commercial or economic loss, or (ii) that is required to be disclosed to HMRC under any applicable Law for Taxes.
SECTION 5.15 Listing. The issued and outstanding SPAC Units are registered pursuant to Section 12(b) of the Exchange Act and are listed for trading on the Nasdaq Capital Market under the symbol “LJAQ”. The issued and outstanding SPAC Common Stock are registered pursuant to Section 12(b) of the Exchange Act and are listed for trading on the Nasdaq Capital Market under the symbol “LJAQU”. The issued and outstanding SPAC Warrants are registered pursuant to Section 12(b) of the Exchange Act and are listed for trading on the Nasdaq Capital Market under the symbol “LJAQW”. As of the date of this Agreement, there is no Action pending or, to the knowledge of SPAC, threatened against SPAC by the Nasdaq Capital Market or the SEC with respect to any intention by such entity to deregister the SPAC Units, the SPAC Common Stock, or SPAC Warrants or terminate the listing of SPAC on the Nasdaq Capital Market. None of SPAC or any of its affiliates has taken any action in an attempt to terminate the registration of the SPAC Units, the SPAC Common Stock or the SPAC Warrants, under the Exchange Act.
SECTION 5.16 Prior Business Operation. SPAC has limited its activities in all material respects to those activities (a) contemplated in the prospectus of SPAC, dated as of January 12, 2021 and in the Form 10-K of SPAC, for the fiscal year ended December 31, 2021, or (b) otherwise necessary to consummate the Transactions.
SECTION 5.17 SPAC Material Contracts.
(a) The SPAC SEC Reports include true and complete copies of each “material contract” (as such term is defined in Regulation S-K of the SEC) to which SPAC is party (the “SPAC Material Contracts”).
(b) Each SPAC Material Contract is in full force and effect and, to the knowledge of SPAC, is valid and binding upon and enforceable against each of the parties thereto (subject to the Remedies Exception), except insofar as enforceability may be limited by the Remedies Exceptions. True and complete copies of all SPAC Material Contracts have been made available to the Company.
(c) The EarlyBird Amendment is in full force and effect and is valid and binding upon and enforceable against each of the parties thereto. A true and complete copy of the EarlyBird Amendment has been made available to the Company.
SECTION 5.18 Investment Company Act. SPAC is not an “investment company” or a person directly or indirectly “controlled” by or acting on behalf of an “investment company”, or required to register as an “investment company”, in each case within the meaning of the Investment Company Act of 1940.
SECTION 5.19 Proxy Statement/Prospectus and Registration Statement. None of the information relating to SPAC supplied by SPAC in writing for inclusion in the Proxy Statement/Prospectus or Registration Statement will, as of the date the Registration Statement is made effective, as of the date the Proxy Statement/Prospectus (or any amendment or supplement thereto) is first mailed to the SPAC Shareholders, at the time of the SPAC Shareholders’ Meeting, or at the Merger Effective Time, contain any misstatement of a material fact or omission of any material fact necessary to make the statements therein, in light of the circumstances under which they are made, not misleading; provided, however, that SPAC makes no representation with respect to any forward-looking statements supplied by or on behalf of SPAC for inclusion in, or relating to information to be included in the Proxy Statement/Prospectus or Registration Statement.
SECTION 5.20 SPAC’s Investigation and Reliance. SPAC is a sophisticated purchaser and has made its own independent investigation, review and analysis regarding the Company and the Company Subsidiaries and the Transactions, which investigation, review and analysis were conducted by SPAC together with expert advisors, including legal counsel, that it has engaged for such purpose. SPAC and its Representatives have been provided with
Annex A-46
Table of Contents
full and complete access to the Representatives, books and records of the Company and the Company Subsidiaries and other information that they have requested in connection with their investigation of the Company, the Company Subsidiaries and the Transactions. SPAC is not relying on any statement, representation or warranty, oral or written, express or implied, made by the Company, any of the Company Subsidiaries or their Representatives (including the Company Shareholders), except as expressly set forth in Article IV (as modified by the Company Disclosure Schedule). Neither the Company nor any of its shareholders, affiliates or Representatives shall have any liability to SPAC or any of its shareholders, affiliates or Representatives resulting from the use of any information, documents or materials made available to SPAC, whether orally or in writing, in any confidential information memoranda, the Dataroom or other “datarooms,” management presentations, due diligence discussions or in any other form in expectation of the Transactions. Neither the Company nor any of its shareholders, affiliates or Representatives is making, directly or indirectly, any representation or warranty with respect to any estimates, projections or forecasts involving the Company and the Company Subsidiaries.
ARTICLE VI
REPRESENTATIONS AND WARRANTIES OF HOLDCO AND MERGER SUB
Each of Holdco and Merger Sub hereby represents and warrants to SPAC as follows:
SECTION 6.01 Organization. Each of Holdco and Merger Sub is a company duly organized, validly existing and in good standing (insofar as such concept exists in the relevant jurisdiction) under the laws of the jurisdiction of its incorporation and has the requisite corporate power and authority and all necessary governmental approvals to own, lease and operate its properties and to carry on its business as it is now being conducted.
SECTION 6.02 Organization Documents. Each of Holdco and Merger Sub has heretofore furnished to SPAC complete and correct copies of the Holdco Organizational Documents and Merger Sub Organizational Documents, respectively, as of the date of this Agreement. Each of the Holdco Organizational Documents and Merger Sub Organizational Documents are in full force and effect and neither Holdco nor Merger Sub is in violation of any of the provisions of such organizational documents.
SECTION 6.03 Capitalization.
(a) As of the date hereof, the share capital of Holdco consists of 5,000,000 Holdco Ordinary Shares, having a nominal value of $0.01 each. As of the date hereof, the Company Shareholders are the shareholders of Holdco.
(b) As of the date hereof, the authorized share capital of Merger Sub consists of 1,000 shares of Merger Sub Common Stock.
(c) The shares constituting the Merger Consideration being delivered by Holdco hereunder shall be duly and validly issued and fully paid, and each such share or other security shall be issued free and clear of preemptive rights and all Liens, other than transfer restrictions under applicable securities Laws and the A&R Holdco Organizational Documents. The Holdco Ordinary Shares constituting the Merger Consideration being delivered by Holdco hereunder will be issued in compliance with the 1915 Law and will not be subject to or give rise to any preemptive rights or rights of first refusal, other than those which are mandatorily applicable under the 1915 Law.
(d) Except as contemplated by this Agreement, the Holdco Organizational Documents and the Exchange Agreements and except as for the mandatory subscription rights provided for in the 1915 Law, (i) there are no other options, warrants, preemptive rights, calls, convertible securities, conversion rights or other rights, agreements, arrangements or commitments of any character relating to the issued or unissued capital of Holdco or obligating Holdco to issue or sell any shares of, or other equity interests in, Holdco, (ii) Holdco is not a party to, or otherwise bound by, and Holdco has not granted, any equity appreciation rights, participations, phantom equity or similar rights and (iii) there are no voting trusts, voting agreements, proxies, shareholder agreements or other similar agreements with respect to the voting or transfer of the Holdco Ordinary Shares or any of the equity interests or other securities of Holdco. As of the date hereof, except for Merger Sub, Holdco does not own any equity interests in any person.
Annex A-47
Table of Contents
SECTION 6.04 Authority Relative to this Agreement. Each of Holdco and Merger Sub have all necessary power and authority to execute and deliver this Agreement and each Ancillary Agreement to which it is a party, to perform its obligations hereunder and thereunder and to consummate the Transactions. The execution and delivery of this Agreement and such Ancillary Agreements to which they are a party by each of Holdco and Merger Sub and the consummation by each of Holdco and Merger Sub of the Transactions have been duly and validly authorized by all necessary corporate action, and no other corporate proceedings on the part of Holdco or Merger Sub are necessary to authorize this Agreement, each such Ancillary Agreement to which they are a party or to consummate the Transactions (other than (a) the filing and recordation of appropriate Merger and Exchange documents as required by DGCL and the 1915 Law, as the case may be, (b) the Holdco Shareholder Approval, (c) the Holdco Board resolutions approving the issuance on the Closing Date (and conditional on Closing) by a delegate of (A) the Merger Consideration and (B) new Holdco Ordinary Shares in the context of the Exchange; both pursuant to the Holdco Delegate Resolutions,, and (d) the Holdco Delegate Resolutions). This Agreement and each such Ancillary Agreement have been duly and validly executed and delivered by Holdco and Merger Sub and, assuming due authorization, execution and delivery by the Company and SPAC, constitutes a legal, valid and binding obligation of Holdco or Merger Sub, enforceable against Holdco or Merger Sub in accordance with its terms subject to the Remedies Exceptions.
SECTION 6.05 No Conflict; Required Filings and Consents.
(a) The execution and delivery by Holdco and Merger Sub of this Agreement and each Ancillary Agreement to which it is a party does not, and the performance of this Agreement and each such Ancillary Agreement by Holdco and Merger Sub will not, (i) conflict with or violate the Holdco Organizational Documents or Merger Sub Organizational Documents (as the case may be), (ii) assuming that all consents, approvals, authorizations and other actions described in Section 6.05 have been obtained and all filings and obligations described in Section 6.05 have been made, conflict with or violate any Law, rule, regulation, order, judgment or decree applicable to Holdco or Merger Sub or by which any of their respective property or assets is bound or affected or (iii) result in any breach of, or constitute a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of a Lien on any property or asset of Holdco or Merger Sub pursuant to, any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which each of Holdco or Merger Sub is a party or by which Holdco or Merger Sub or any of their respective property or assets is bound or affected, except, with respect to clauses (ii) and (iii), for any such conflicts, violations, breaches, defaults or other occurrences which would not have or reasonably be expected to have a material adverse effect.
(b) The execution and delivery by Holdco and Merger Sub of this Agreement and each Ancillary Agreement to which it is a party does not, and the performance of this Agreement and each such Ancillary Agreement by Holdco or Merger Sub, as applicable, will not, require any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Authority, except (i) for applicable requirements, if any, of the Exchange Act, Blue Sky Laws and state takeover laws, the Antitrust Laws, if any, and filing and recordation of appropriate Merger and Exchange documents as required by DGCL and the 1915 Law, as the case may be and (ii) where the failure to obtain such consents, approvals, authorizations or permits, or to make such filings or notifications, would not, individually or in the aggregate, prevent or materially delay consummation of any of the Transactions or otherwise prevent Holdco and Merger Sub from performing their respective material obligations under this Agreement and each such Ancillary Agreement.
SECTION 6.06 Compliance. Neither Holdco nor Merger Sub is or has been in conflict with, or in default, breach or violation of, (a) any Law applicable to Holdco or Merger Sub or by which any property or asset of Holdco or Merger Sub is bound or affected, or (b) any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which Holdco or Merger Sub is a party or by which Holdco or Merger Sub or any property or asset of Holdco or Merger Sub is bound. Holdco and Merger Sub are in possession of all material franchises, grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental Authority necessary for Holdco and Merger Sub to own, lease and operate their respective properties or to carry on their respective businesses as they are now being conducted.
Annex A-48
Table of Contents
SECTION 6.07 Board Approval; Vote Required.
(a) The Holdco Board has by resolutions duly adopted by written consent and not subsequently rescinded or modified in any way, duly (i) determined that this Agreement and the Transactions are in the best interests of Holdco and (ii) approved this Agreement and the Transactions and shall, on the Closing Date, cause the Holdco Delegate Resolutions to be issued.
(b) The only vote of the holders of any class or series of capital of Holdco that is necessary to approve this Agreement, the Exchange and the Transactions is the Holdco Shareholder Approval.
(c) Merger Sub Board has, by resolutions duly adopted by written consent and not subsequently rescinded or modified in any way, duly (i) determined that this Agreement and the Transactions are fair to and in the best interests of Merger Sub and Holdco (as the sole shareholder of Merger Sub), (ii) approved this Agreement and the Transactions and declared their advisability and (iii) recommended that Holdco (as the sole shareholder of Merger Sub) approve and adopt this Agreement and approve the Transactions and directed that this Agreement and the Transactions be submitted for consideration by Holdco (as the sole shareholder of Merger Sub).
(d) The only shareholder vote of Merger Sub that is necessary to approve this Agreement and the Transactions is the affirmative vote of the Holdco as sole shareholder of Merger Sub.
SECTION 6.08 No Prior Operations of Holdco or Merger Sub; Post-Closing Operations. Holdco and Merger Sub were formed for the sole purposes of entering into this Agreement and the Ancillary Agreements to which they are party and engaging in the Transactions. Since the date of the Holdco Organizational Documents, Holdco has not engaged in any business or activities whatsoever, nor incurred any liabilities, except in connection with this Agreement, the Ancillary Agreements or in furtherance of the Transactions and in connection with its incorporation and sale to the Company Shareholders. Since the date of the Merger Sub Organizational Documents, Merger Sub has not engaged in any business or activities whatsoever, nor incurred any liabilities, except in connection with this Agreement, the Ancillary Agreements or in furtherance of the Transactions. Neither Holdco nor Merger Sub has any employees or liabilities under any Plan.
SECTION 6.09 Brokers. No broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the Transactions based upon arrangements made by or on behalf of Holdco or Merger Sub.
SECTION 6.10 Proxy Statement/Prospectus and Registration Statement. None of the information relating to Holdco or Merger Sub supplied by Holdco or Merger Sub in writing for inclusion in the Proxy Statement/Prospectus or Registration Statement will, as of the date the Registration Statement is declared effective, as of the date the Proxy Statement/Prospectus (or any amendment or supplement thereto) is first mailed to the SPAC Shareholders, at the time of the SPAC Shareholders’ Meeting, or at the Merger Effective Time, contain any misstatement of a material fact or omission of any material fact necessary to make the statements therein, in light of the circumstances under which they are made, not misleading; provided, however, that Holdco and Merger Sub make no representation with respect to any forward-looking statements supplied by or on behalf of Holdco or Merger Sub for inclusion in, or relating to information to be included in the Proxy Statement/Prospectus or Registration Statement.
SECTION 6.11 Tax Matters. To the knowledge of Company, Holdco and the Merger Sub, there is no plan or intention to liquidate SPAC (including a liquidation for Tax purposes) following the Transactions.
ARTICLE VII
CONDUCT OF BUSINESS PENDING THE MERGER
SECTION 7.01 Conduct of Business by the Company, Holdco and Merger Sub Pending the Merger.
(a) The Company agrees that, between the date of this Agreement and the Closing or the earlier termination of this Agreement, except as (i) expressly contemplated or permitted by any other provision of this Agreement or any Ancillary Agreement, (ii) as set forth in Section 7.01(a) of the Company Disclosure Schedule, and (iii) as required by applicable Law (including COVID-19 Measures or as may be requested or compelled by any Governmental Authority), unless SPAC shall otherwise consent in writing, which consent shall not be unreasonably withheld, delayed or conditioned: (A) the Company shall, and shall cause the Company
Annex A-49
Table of Contents
Subsidiaries to, conduct their business in all material respects in the ordinary course of business and in a manner consistent with past practice; provided that, in the case of actions that are taken (or omitted to be taken) reasonably in response to an emergency or urgent condition or conditions arising from COVID-19, the Company and the Company Subsidiaries shall not be deemed to be acting outside the ordinary course of business, so long as such actions or omissions are reasonably designed to (1) protect the health or welfare of the Company’s employees, directors, officers or agents or (2) comply with clause (B) of this Section 7.01, and in each case, the Company promptly notifies SPAC prior to taking such actions and reasonably takes into account the reasonable requests of SPAC in further acts or omissions of the Company with respect to such condition or conditions arising from COVID-19; and (B) the Company shall use its reasonable best efforts to preserve substantially intact the business organization of the Company and the Company Subsidiaries and shall use its commercially reasonable efforts to keep available the services of the current officers, key employees and consultants of the Company and the Company Subsidiaries and to preserve the current relationships of the Company and the Company Subsidiaries with customers, suppliers and other persons with which the Company or any Company Subsidiary has significant business relations.
(b) By way of amplification and not limitation, except as (x) expressly contemplated or permitted by any other provision of this Agreement or any Ancillary Agreement, (y) as set forth in Section 7.01(b) of the Company Disclosure Schedule, and (z) as required by applicable Law (including COVID-19 Measures or as may be requested or compelled by any Governmental Authority), the Company shall not, and shall cause each Company Subsidiary, Holdco and Merger Sub not to, between the date of this Agreement and the Closing or the earlier termination of this Agreement, directly or indirectly, do any of the following without the prior written consent of SPAC which consent shall not be unreasonably withheld, delayed or conditioned:
(i) amend or otherwise change its memorandum of association, articles of association, certificate of incorporation, by-laws or equivalent organizational documents;
(ii) issue, sell, pledge, dispose of, grant or encumber, solicit, propose, negotiate with respect to, or authorize the issuance, sale, pledge, disposition, grant or encumbrance of, (A) any shares of any class of capital stock of the Company or any Company Subsidiary, if any, (B) any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital stock, or any other ownership interest (including any phantom interest) or (C) except in the ordinary course of business, any assets of the Company or any Company Subsidiary;
(iii) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital stock;
(iv) reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of its capital stock, other than redemptions of equity securities from former employees upon the terms set forth in the underlying agreements governing such equity securities;
(v) merge or consolidate with any other person or restructure, reorganize, dissolve or completely or partially liquidate or otherwise enter into any agreements or arrangements imposing material changes or restrictions on its assets, operations or businesses;
(vi) acquire or invest (including, without limitation, by merger, consolidation, or acquisition of stock or assets or any other business combination) any corporate partnership, other business organization, or any division thereof, in each case, for an aggregate purchase price that exceeds $500,000;
(vii) incur any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise become responsible for, the obligations of any person, or make any loans or advances, or intentionally grant any security interest in any of its assets, in each case, except in the ordinary course of business and consistent with past practice;
(viii) transfer, sell, lease, license, mortgage, pledge, surrender, encumber, divest, cancel abandon or allow to lapse or expire or otherwise dispose of any of its material assets, properties, licenses, operations, rights, production lines, businesses or interests therein, except for sales or other dispositions in the ordinary course of business consistent with past practice;
Annex A-50
Table of Contents
(ix) (A) increase the compensation or benefit payable to any current or former director, officer, employee or consultant of the Company or any Company Subsidiary, other than (1) health and welfare plan renewals in the ordinary course of business consistent with past practices or (2) increases in base salary or wage of employees in the ordinary course of business whose cash portion of the annual base salary or wage is not in excess of $200,000; (B) take any action to accelerate or commit to accelerate the funding, payment, or vesting of any compensation or benefits to any current or former director, officer, employee or consultant; (C) hire or otherwise enter into any employment or consulting agreement or arrangement with any person or terminate (other than for cause) any current or former director, officer, employee or consultant provider whose cash portion of the annual base salary or wage would exceed $200,000; or (D) enter into any new, or materially amend any existing employment or severance or termination agreement with any current or former director, officer, employee or consultant provider whose cash portion of the annual base salary or wage would exceed $200,000;
(x) adopt, enter into, materially amend and/or terminate any Plan or any plan, program, agreement, arrangement or policy for the current or future benefit of any current or former director, officer, employee, consultant or individual independent contractor that would be a Plan if it were in existence on the date hereof (including any existing employment or severance or termination agreement with any current or former director, officer, employee or consultant), except as may be required by applicable Law or health and welfare Plan renewals in the ordinary course of business and consistent with past practice;
(xi) materially amend other than reasonable and usual amendments in the ordinary course of business, with respect to accounting policies or procedures, other than as required by the Accounting Principles;
(xii) make change or revoke any material Tax election, change any annual Tax accounting period, adopt or change any method of Tax accounting, materially amend any Tax Returns or file claims for Tax refunds except in the ordinary course of business, enter into any closing agreement, waive or extend any statute of limitations period in respect of an amount of Taxes, settle any Tax claim, audit or assessment, or surrender any right to claim a Tax refund, offset or other reduction in Tax liability;
(xiii) materially amend or modify, or consent to the termination (excluding any expiration in accordance with its terms) of any Material Contract or materially amend or modify, waive, or consent to the termination (excluding any expiration in accordance with its terms) of the Company’s or any Company Subsidiary’s material rights thereunder, in each case in a manner that is adverse to the Company or any Company Subsidiary, taken as a whole, except in the ordinary course of business;
(xiv) intentionally permit any material item of Company IP to lapse or to be abandoned, invalidated, dedicated to the public, or disclaimed, or otherwise become unenforceable or fail to perform or make any applicable filings, recordings or other similar actions or filings, or fail to pay all required fees and Taxes required to maintain and protect its interest in each and every material item of Company IP;
(xv) create or incur any Lien material to the Company, any Company Subsidiary, Holdco or Merger Sub other than Permitted Liens incurred in the ordinary course of business consistent with past practice;
(xvi) make any loans, advances, or guarantees in any person (other than the Company or any Company Subsidiaries);
(xvii) fail to make or authorize any budgeted capital expenditures or make or authorize any unbudgeted capital expenditures, in each case in excess of 10% of the aggregate amount of budgeted capital expenses;
(xviii) fail to pay or satisfy when due any material account payable or other material liability, other than in the ordinary course of business consistent with past practice or any such liability that is being contested in good faith by the Company or any Company Subsidiary or otherwise materially change the manner by which the Company or any Company Subsidiary manages its working capital;
Annex A-51
Table of Contents
(xix) fail to keep current and in full force and effect, or comply in all material respects with the requirements of any Company Permit issued to the Company or any Company Subsidiary by any Governmental Authority;
(xx) take any steps for liquidation, winding-up, freeze of proceedings, arrangements with creditors or similar action or proceeding by or in respect of the Company or any Company Subsidiary;
(xxi) take any actions or omit to take any actions that would, individually or in the aggregate, reasonably be expected to result in any of the conditions set forth in Article IX not being satisfied;
(xxii) engage in any dealings or transactions (i) with any Restricted Person in violation of applicable laws; (ii) involving any Sanctionable Activity; or (iii) otherwise in violation of Sanction Laws, Export Control Laws or Import Control Laws; or
(xxiii) enter into any formal or informal agreement or otherwise make a binding commitment to do any of the foregoing.
(c) By way of amplification and not limitation, Holdco shall not, and the Company Shareholders or the Company, as the case may be, shall not permit Holdco to, between the date of this Agreement and the Closing or the earlier termination of this Agreement, directly or indirectly, except as expressly contemplated or permitted by any other provision of this Agreement, any Ancillary Agreements or as may be required by applicable Law (including as may be requested or compelled by any Governmental Authority), do any of the following without the prior written consent of SPAC which consent shall not be unreasonably withheld, delayed or conditioned:
(i) engage in any business or activity other than the consummation of the Exchange;
(ii) amend or otherwise change the Holdco Organizational Documents except for the A&R Holdco Organizational Documents or as otherwise required to implement the Exchange and issuance of Merger Consideration;
(iii) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital stock;
(iv) reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of the Holdco Ordinary Shares;
(v) issue, sell, pledge, dispose of, grant or encumber, or authorize, solicit, propose, or negotiate with respect to the issuance, sale, pledge, disposition, grant or encumbrance of, any shares of any class of capital or other securities of Holdco or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital, or any other ownership interest (including, without limitation, any phantom interest), of Holdco;
(vi) liquidate, dissolve, reorganize or otherwise wind up the business and operations of Holdco;
(vii) amend any Exchange Agreement or any other agreement related to the Exchange;
(viii) permit any Company Shareholder who owns Holdco Ordinary Shares both prior to the Exchange and pursuant to the Exchange to transfer, sell, lease, license, mortgage, pledge, surrender, encumber, divest, cancel, abandon or otherwise dispose of any Holdco Ordinary Shares, or recognize any such transfer, sale, lease, license, mortgage, pledge, surrender, encumbrances, divestment, cancellation, abandonment or other disposition of Holdco Ordinary Shares;
(ix) transfer, sell, lease, license, mortgage, pledge, surrender, encumber, divest, cancel, abandon or allow to lapse or expire or otherwise dispose of any Company Ordinary Shares acquired pursuant to the Exchange and any such attempted action shall be null and void and Company will not inscribe any such transfer (of any kind as contemplated in this provision) in the shareholder register of Company;
(x) acquire or hold any equity securities or rights thereto in any person other than the Company, pursuant to the Exchange, and Merger Sub; or
(xi) enter into any formal or informal agreement or otherwise make a binding commitment to do any of the foregoing.
Annex A-52
Table of Contents
SECTION 7.02 Conduct of Business by SPAC Pending the Merger.
(a) SPAC agrees that, between the date of this Agreement and the Closing or the earlier termination of this Agreement, except as expressly contemplated by any other provision of this Agreement or any Ancillary Agreement and as required by applicable Law (including as may be requested or compelled by any Governmental Authority), unless the Company shall otherwise consent in writing (which consent shall not be unreasonably withheld, delayed or conditioned), the business of SPAC shall be conducted in the ordinary course of business and in a manner consistent with past practice and SPAC shall not, directly or indirectly, take any action that would reasonably be likely to materially delay or prevent the Transactions. By way of amplification and not limitation, except as expressly contemplated by any other provision of this Agreement or any Ancillary Agreement or as required by applicable Law (including as may be requested or compelled by any Governmental Authority), SPAC shall not, between the date of this Agreement and the Closing or the earlier termination of this Agreement, directly or indirectly, do any of the following without the prior written consent of the Company, which consent shall not be unreasonably withheld, delayed or conditioned:
(i) amend or otherwise change the SPAC Organizational Documents (other than in connection with a SPAC Extension Proposal, if any);
(ii) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, shares, property or otherwise, with respect to any of its shares, other than pursuant to the Redemption Rights from the Trust Fund that are required pursuant to the SPAC Organizational Documents;
(iii) reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of the SPAC Common Stock or SPAC Warrants except for the Redemption Rights from the Trust Fund that are required pursuant to the SPAC Organizational Documents;
(iv) issue, sell, pledge, dispose of, grant or encumber, or authorize, solicit, propose, or negotiate with respect to the issuance, sale, pledge, disposition, grant or encumbrance of, any shares of any class of shares or other securities of SPAC or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such shares, or any other ownership interest (including, without limitation, any phantom interest), of SPAC;
(v) reclassify, combine, split or subdivide, directly or indirectly, any of its shares (except pursuant to the Redemption Rights);
(vi) acquire (including, without limitation, by merger, consolidation, or acquisition of stock, shares or assets or any other business combination) any corporation, partnership, other business organization or any division thereof or any material amount of assets or enter into any strategic joint ventures, partnerships or alliances with any other person;
(vii) incur any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise become responsible for, the obligations of any person, or make any loans or advances, or intentionally grant any security interest in any of its assets;
(viii) make any change in any method of financial accounting or financial accounting principles, policies, procedures or practices, except as required by a concurrent amendment in GAAP or applicable Law made subsequent to the date hereof, as agreed to by its independent accountants;
(ix) make, change or revoke any material Tax election, change any annual Tax accounting period, adopt or change any method of Tax accounting, amend any Tax Returns or file claims for Tax refunds, enter into any closing agreement, waive or extend any statute of limitations period in respect of an amount of Taxes, settle any Tax claim, audit or assessment, or surrender any right to claim a Tax refund, offset or other reduction in Tax liability;
(x) liquidate, dissolve, reorganize or otherwise wind up the business and operations of SPAC;
(xi) amend the Trust Agreement or any other agreement related to the Trust Account; or
(xii) enter into any formal or informal agreement or otherwise make a binding commitment to do any of the foregoing.
Annex A-53
Table of Contents
(b) From the date of this Agreement and the Closing or the earlier termination of this Agreement, SPAC shall use reasonable best efforts to maintain the EarlyBird Amendment in full force and effect (and SPAC shall not take any action to cause the EarlyBird Amendment not to be in full force and effect and shall not refrain from taking any commercially reasonable action to maintain the EarlyBird Amendment in full force and effect) and SPAC shall not amend or otherwise change the EarlyBird Engagement Letter without the prior written consent of the Company and any such amendment or change shall be in a form that is mutually agreeable to the SPAC and the Company. For the avoidance of doubt, nothing in this Agreement shall prevent, limit or restrict SPAC or its affiliates from taking all actions required to raise funds for the benefit of Parties (after the Closing), including by raising funds as contemplated by the EarlyBird Engagement Letter.
SECTION 7.03 Claims Against Trust Account. Each of Holdco, Merger Sub and the Company agrees that, notwithstanding any other provision contained in this Agreement, none of Holdco, Merger Sub or the Company does now have, and shall not at any time prior to the Merger Effective Time have, any claim to, or make any claim against, the Trust Fund, regardless of whether such claim arises as a result of, in connection with or relating in any way to, the business relationship between or among Holdco, Merger Sub, the Company and SPAC, this Agreement, or any other agreement or any other matter, and regardless of whether such claim arises based on contract, tort, equity or any other theory of legal liability (any and all such claims against the Trust Fund are collectively referred to in this Section 7.03 as the “Claims”). Notwithstanding any other provision contained in this Agreement, each of Holdco, Merger Sub and the Company hereby irrevocably waives any Claim it may have, now or in the future and will not seek recourse against the Trust Fund for any reason whatsoever in respect thereof provided, however, that the foregoing waiver will not limit or prohibit any of Holdco, Merger Sub or the Company from pursuing a claim against SPAC or any other person (a) for legal relief against monies or other assets of SPAC held outside of the Trust Account or for specific performance or other equitable relief in connection with the Transactions or (b) for damages for breach of this Agreement against SPAC (or any successor entity) in the event this Agreement is terminated for any reason and SPAC consummates a business combination transaction with another party. In the event that any of Holdco, Merger Sub or the Company commences any Claims against or involving the Trust Fund in violation of the foregoing, SPAC shall be entitled to recover from Holdco, Merger Sub or the Company, as applicable the associated reasonable legal fees and costs in connection with any such action, in the event SPAC prevails in such action or proceeding.
SECTION 7.04 SPAC Public Filings.
(a) Between the date of this Agreement and the Closing or the earlier termination of this Agreement, SPAC will keep current and timely file all of the forms, reports, schedules, statements and other documents required to be filed by SPAC with the SEC, including all necessary amendments and supplements thereto, and otherwise comply in all material respects with applicable securities Laws (the “Additional SEC Reports”). All such Additional SEC Reports (including any financial statements or schedules included therein) (i) shall be prepared in all material respects in accordance with either the requirements of the Securities Act, the Exchange Act and the Sarbanes-Oxley Act, as the case may be, and the rules and regulations promulgated thereunder and (ii) will not, at the time they are filed, or, if amended, as of the date of such amendment, contain any Misrepresentation. As used in this Section 7.04, the term “file” shall be broadly construed to include any manner in which a document or information is furnished, supplied or otherwise made available to the SEC or Nasdaq. Any Additional SEC Reports which discuss or refer to this Agreement or the Transactions shall be subject to the prior review and approval of the Company (not to be unreasonably withheld, delayed or conditioned).
(b) Between the date of this Agreement and the Closing or the earlier termination of this Agreement, SPAC shall use its reasonable best efforts prior to the Merger to maintain the listing of the SPAC Units, the SPAC Common Stock and the SPAC Warrants on the Nasdaq Capital Market.
ARTICLE VIII
ADDITIONAL AGREEMENTS
SECTION 8.01 Proxy Statement; Registration Statement.
(a) As promptly as practicable after the execution and delivery of this Agreement and delivery of the PCAOB Financials, (i) Holdco, the Company and SPAC shall prepare and Holdco shall file with the SEC the proxy statement/prospectus (as amended or supplemented from time to time, the “Proxy Statement/Prospectus”) to be sent to the SPAC Shareholders relating to the general meeting of SPAC (the “SPAC Shareholders’ Meeting”) for the purpose of soliciting proxies from SPAC Shareholders for the matters to be acted upon at
Annex A-54
Table of Contents
the SPAC Shareholders’ Meeting and providing the SPAC Shareholders an opportunity in accordance with the SPAC Organizational Documents to have their SPAC Common Stock redeemed (the “Redemption”) in conjunction with the shareholder vote on the SPAC Proposals and (ii) Holdco, the Company and SPAC shall prepare and Holdco shall file (and the Company and SPAC shall cause Holdco to file) with the SEC a registration statement on Form F-4 or such other applicable form as the Company and SPAC may agree (as amended or supplemented from time to time, the “Registration Statement”), in which the Proxy Statement/Prospectus will be included, in connection with the registration under the Securities Act of the Holdco Ordinary Shares and Holdco Warrants, in each case, to be issued in the Merger. Each Party shall use its reasonable best efforts to cause the Registration Statement and the Proxy Statement/Prospectus to comply with the applicable rules and regulations promulgated by the SEC, including providing any necessary opinions of counsel, to have the Registration Statement declared effective under the Securities Act as promptly as practicable after such filing, and to keep the Registration Statement effective as long as is necessary to consummate the Transactions. Each of Holdco, the Company and SPAC shall furnish all information as may be reasonably requested by the other Parties in connection with any such action and the preparation, filing and distribution of the Registration Statement and the Proxy Statement/Prospectus; provided, however, that no Party shall use any such information for any purposes other than those contemplated by this Agreement unless such Party obtains the prior written consent of the other. SPAC also agrees to use its reasonable best efforts to obtain all necessary state securities law or “Blue Sky” permits and approvals required to carry out the Transactions, and the Company shall furnish all information concerning the Company and the Company Subsidiaries as may be reasonably requested in connection with any such action; provided that, without the prior written consent of the Company, SPAC shall not use any such information for any purposes other than to obtain necessary state securities law or “Blue Sky” permits and approvals.
(b) As promptly as practicable after the Registration Statement shall have become effective, SPAC shall use its reasonable best efforts to cause the Proxy Statement/Prospectus to be mailed to the SPAC Shareholders as of the record date for the SPAC Shareholders’ Meeting. No filing of, or amendment or supplement to, the Registration Statement or the Proxy Statement/Prospectus will be made (in each case including documents incorporated by reference therein) by SPAC, the Company or Holdco without providing the other with a reasonable opportunity to review and comment thereon and each Party shall give reasonable and good faith consideration to any comments made by any other Party and their counsel. Each of SPAC, the Company and Holdco will be given a reasonable opportunity to participate in the response to any SEC comments and to provide comments on that response (to which reasonable and good faith consideration shall be given), including by participating with SPAC, the Company or Holdco or their counsel in any discussions or meetings with the SEC. SPAC shall comply with all applicable rules and regulations promulgated by the SEC, any applicable rules and regulations of Nasdaq, SPAC Organizational Documents, and this Agreement in the preparation, filing and distribution of the Proxy Statement/Prospectus, any solicitation of proxies thereunder, the calling and holding of the SPAC Shareholders’ Meeting and the Redemption.
(c) If at any time prior to the Merger Effective Time, any information relating to SPAC, the Company or Holdco or any of their respective affiliates, directors or officers, should be discovered by SPAC, the Company or Holdco which should be set forth in an amendment or supplement to either the Registration Statement or the Proxy Statement/Prospectus, so that either such document would not include any misstatement of a material fact or omit to state any material fact necessary to make the statements therein, in light of the circumstances under which they are made, not misleading, the Party that discovers such information shall promptly notify the other Parties and an appropriate amendment or supplement describing such information shall be promptly filed with the SEC and, to the extent required by Law, disseminated to the SPAC Shareholders.
(d) Each of SPAC, the Company and Holdco will advise the other Parties promptly after it receives any oral or written request by the SEC for amendment of the Proxy Statement/Prospectus or the Registration Statement, as applicable, or comments thereon and responses thereto, any oral or written comments or requests in relation to the SPAC Shareholders’ Meeting or the Redemption, or requests by the SEC for additional information and each Party will promptly provide the other with copies of any written communication between it or any of its Representatives, on the one hand, and the SEC, any state securities commission or their respective staffs, on the other hand, with respect to the Proxy Statement/Prospectus, the Registration Statement, the Exchange, the Merger, the SPAC Shareholders’ Meeting or the Redemption. SPAC, the Company and
Annex A-55
Table of Contents
Holdco shall use their respective reasonable best efforts, after consultation with each other, to resolve all such requests or comments with respect to the Proxy Statement/Prospectus, the Registration Statement, the SPAC Shareholders’ Meeting or the Redemption, as applicable, as promptly as reasonably practicable after receipt thereof.
(e) Without limiting the generality of the foregoing, each of SPAC, the Company and Holdco shall cooperate with each other in the preparation of each of the Proxy Statement/Prospectus and the Registration Statement, and each of the Company and SPAC shall furnish Holdco with all information concerning it and its affiliates as the providing Party (after consulting with counsel) may deem reasonably necessary or advisable in connection with the preparation of the Proxy Statement/Prospectus or the Registration Statement, as applicable.
(f) With respect to any SPAC Shareholder outreach in connection with the SPAC Shareholders’ Meeting, the Company and the Company Subsidiaries shall use their commercially reasonable efforts to provide to SPAC, and the Company and the Company Subsidiaries shall use their respective commercially reasonable efforts to cause their Affiliates and Representatives, including legal and accounting representatives, to provide to SPAC, all cooperation reasonably requested by SPAC that is customary in connection with SPAC Shareholder outreach for the SPAC Shareholders’ Meeting, which commercially reasonable efforts shall include, among other things, (i) furnishing SPAC promptly following SPAC’s request, with information reasonably available to it regarding the Company and the Company Subsidiaries (including information to be used in the preparation of one or more information packages regarding the business, operations, financial projections and prospects of the Company and the Company Subsidiaries) customary for such outreach activities, (ii) causing each of their Representatives with appropriate seniority and expertise to participate in a reasonable number of meetings (including customary one-on-one meetings), presentations and due diligence sessions and drafting sessions in connection with such outreach activities, (iii) assisting with the preparation of marketing materials and similar documents required in connection with any such outreach activities, (iv) providing reasonable assistance to SPAC in connection with the preparation of pro forma financial information to be included in any marketing materials to be used in any outreach activities, and (v) cooperating with requests for due diligence to the extent customary and reasonable.
(g) SPAC, the Company and Holdco shall notify each other promptly of the time when the Registration Statement has become effective, of the issuance of any stop order or suspension of the qualification of the Holdco Ordinary Shares or Holdco Warrants issuable in connection with the Merger for offering or sale in any jurisdiction, or of the receipt of any comments from the SEC or the staff of the SEC and of any request by the SEC or the staff of the SEC for amendments or supplements to the Proxy Statement/Prospectus or the Registration Statement or for additional information.
SECTION 8.02 SPAC Shareholders’ Meetings.
(a) SPAC shall call the SPAC Shareholders’ Meeting in accordance with the SPAC Organizational Documents and applicable Law for the purposes of voting upon the SPAC Proposals as promptly as practicable after the date on which the SEC has declared the Registration Statement effective for the purpose of voting solely upon the SPAC Proposals. SPAC shall consult with the Company in fixing the record date for the SPAC Shareholders’ Meeting and the date of the SPAC Shareholders’ Meeting, give notice to the Company of the SPAC Shareholders’ Meeting and allow the Company’s representatives and legal counsel to attend the SPAC Shareholders’ Meeting. Without the prior written consent of the Company (not to be unreasonably withheld, delayed or conditioned), the SPAC Proposals shall be the only matters (other than procedural matters) which SPAC shall propose to be acted on by the SPAC Shareholders at the SPAC Shareholders’ Meeting.
(b) Subject to this Section 8.02, SPAC shall include in the Proxy Statement/Prospectus the unanimous recommendation of the SPAC Board that the SPAC Shareholders vote in favor of the SPAC Proposals, shall otherwise use its reasonable best efforts to obtain the approval of the SPAC Proposals at the SPAC Shareholders’ Meeting, including by soliciting from its shareholders proxies as promptly as possible in favor of the SPAC Proposals, and shall take all other action necessary or advisable to secure the required vote or consent of its shareholders therefor and neither the SPAC Board nor any committee thereof shall withdraw or modify, or publicly propose or resolve to withdraw or modify, the recommendation of the SPAC Board that the SPAC Shareholders vote in favor of the SPAC Proposals (a “Change in Recommendation”). SPAC shall provide the Company with (a) updates with respect to the tabulated vote counts received by SPAC, (b) the right to demand postponement or adjournment of the SPAC Shareholders’ Meeting if, based on the tabulated vote count, SPAC will not receive the required approval of its shareholders of the SPAC Proposals; provided, however, that SPAC
Annex A-56
Table of Contents
shall not be permitted to postpone the SPAC Shareholders’ Meeting more than the earlier of (i) five (5) Business Days prior to the Outside Date and (ii) ten (10) days from the date of the first SPAC Shareholders’ Meeting without the prior written consent of the Company (not to be unreasonably withheld, delayed or conditioned), and (c) the right to review and comment on all communication sent to SPAC Shareholders, holders of SPAC Warrants and/or proxy solicitation firms.
(c) Notwithstanding anything to the contrary contained in this Agreement, the SPAC Board may, at any time prior to, but not after, obtaining the SPAC Shareholder Approval, determined in good faith, after consultation with its outside legal counsel, make a Change in Recommendation in response to an Intervening Event (an “Intervening Event Change in Recommendation”) if the failure to take such action would be inconsistent with the fiduciary duties of the SPAC Board to the SPAC Shareholders under applicable Law, provided, that: (i) the Company shall have received written notice from SPAC of SPAC’s intention to make an Intervening Event Change in Recommendation at least three (3) Business Days prior to the taking of such action by SPAC (the “Intervening Event Notice Period”), which notice shall specify the applicable Intervening Event in reasonable detail, (ii) during the Intervening Event Notice Period and prior to making an Intervening Event Change in Recommendation, if requested by the Company, SPAC and its Representatives shall have negotiated in good faith with the Company and its Representatives regarding any revisions or adjustments proposed by the Company to the terms and conditions of this Agreement as would enable SPAC to proceed with its recommendation of this Agreement and the Transactions and not make such Intervening Event Change in Recommendation and (iii) if the Company requested negotiations in accordance with clause (ii), SPAC may make an Intervening Event Change in Recommendation only if the SPAC Board, after considering in good faith any revisions or adjustments to the terms and conditions of this Agreement that the Company shall have, prior to the expiration of the three-Business Day period, offered in writing in a manner that would form a binding contract if accepted by SPAC (and the other applicable parties hereto), continues to determine in good faith that failure to make an Intervening Event Change in Recommendation would be inconsistent with its fiduciary duties to the SPAC Shareholders under applicable Law.
(d) Notwithstanding anything to the contrary contained in this Agreement, the SPAC Board may, at any time prior to, but not after, obtaining the SPAC Shareholder Approval, determined in good faith, based on the advice of its outside legal counsel, make a Change in Recommendation in response to a Superior Proposal (a “Superior Proposal Change in Recommendation”) if the SPAC Board determines after consultation with outside counsel that failure to take such action would be inconsistent with the fiduciary duties of the SPAC Board to the SPAC Shareholders under applicable Law, provided, that: (i) the Company shall have received written notice from SPAC of SPAC’s intention to make a Superior Proposal Change in Recommendation at least three (3) Business Days prior to the taking of such action by SPAC (the “Superior Proposal Notice Period”), which notice shall specify the Superior Proposal in reasonable detail, and (ii) if, prior to the expiration of the Superior Proposal Notice Period, the Company provides SPAC with a written proposal to adjust the terms and conditions of this Agreement, SPAC shall not make a Superior Proposal Change in Recommendation unless the SPAC Board, after considering in good faith such written proposal from the Company, continues to reasonably determine in good faith that such Superior Proposal continues to be a Superior Proposal and after consultation with outside counsel determines that failure to make a Superior Proposal Change in Recommendation would be reasonably likely to be inconsistent with its fiduciary duties to the SPAC Shareholders under applicable Law.
(e) For purposes hereof
(i) An “Intervening Event” shall mean any material event, state of facts, development, change, circumstance, occurrence or effect thereof occurring or arising after the date of this Agreement with respect to the business, operations, financial condition or results of operations of the Company or Company Subsidiaries that is material to the Company and the Company Subsidiaries, taken as a whole, that (i) was not known to or reasonably foreseeable by the SPAC Board as of the date of this Agreement, and (ii) does not relate to any SPAC Acquisition Proposal.
(ii) A “Superior Proposal” shall mean an unsolicited, bona fide written Competing SPAC Transaction that the SPAC Board reasonably determines in good faith (after consultation with its financial advisor and its outside legal counsel, and after taking into account all legal, financial, regulatory and other aspects of the proposal and the Person making the proposal, (i) is reasonably capable of being consummated in accordance with its terms and (ii) would, if consummated, be more favorable to the SPAC Shareholders
Annex A-57
Table of Contents
from a financial point of view than the Transactions (including any adjustments proposed by the Company during the Superior Proposal Notice Period in accordance with Section 8.02); provided, that, for purposes of hereof, “Competing SPAC Transaction” shall be deemed to exclude any issuance, sale or acquisition of less than fifty percent (50%) of the shares of capital stock or other equity securities of the SPAC.
SECTION 8.03 Access to Information; Confidentiality.
(a) From the date of this Agreement until the earlier of the Closing or the termination of this Agreement, the Company and SPAC shall (and shall cause their respective subsidiaries to): (i) provide to the other Party (and the other Party’s Representatives) reasonable access at reasonable times upon prior notice to the officers, employees, agents, properties, offices and other facilities of such Party and its subsidiaries and to the books and records thereof; provided, that such access shall not unreasonably interfere with the business and operations of SPAC and the Company; and (ii) furnish promptly to the other Party such information concerning the business, properties, contracts, assets, liabilities, personnel and other aspects of such Party and its subsidiaries as the other Party or its Representatives may reasonably request. Notwithstanding the foregoing, neither the Company nor SPAC shall be required to provide access to or disclose information where the access or disclosure would jeopardize the protection of attorney-client privilege or contravene applicable Law (it being agreed that the Parties shall use their reasonable best efforts to cause such information to be provided in a manner that would not result in such jeopardy or contravention).
(b) All information obtained by the Parties pursuant to this Section 8.03 shall be kept confidential in accordance with the confidentiality agreement, dated January 12, 2022 (the “Confidentiality Agreement”), between SPAC and the Company.
(c) Notwithstanding anything in this Agreement to the contrary, each Party (and its Representatives) may consult any Tax advisor regarding the Tax treatment and Tax structure of the Transactions and may disclose to any other person, without limitation of any kind, the Tax treatment and Tax structure of the Transactions and all materials (including opinions or other Tax analyses) that are provided relating to such treatment or structure, in each case in accordance with the Confidentiality Agreement.
SECTION 8.04 Employee Matters.
(a) Holdco shall, or shall cause the Company, the Surviving Company and each of their respective subsidiaries, as applicable, to provide the employees of the Company and the Company Subsidiaries who remain employed immediately after the Closing (the “Continuing Employees”) to receive credit for purposes of eligibility to participate and vesting under any employee benefit plan, program or arrangement established or maintained by Holdco, the Company or the Surviving Company or any of their respective subsidiaries, other than any qualified or nonqualified defined benefit plan, for service accrued or deemed accrued prior to the Closing with the Company or any Company Subsidiary; provided, however, that such crediting of service shall not operate to duplicate any benefit or the funding of any such benefit. In addition, Holdco shall use reasonable best efforts to (i) cause to be waived any eligibility waiting periods, any evidence of insurability requirements and the application of any pre-existing condition limitations under each of the employee benefit plans established or maintained by Holdco, the Company, the Surviving Company or any of their respective subsidiaries that cover the Continuing Employees or their dependents following the Closing and (ii) cause any eligible expenses incurred by any Continuing Employee and his or her covered dependents, during the portion of the plan year in which the Closing occurs, under those health and welfare Plans in which such Continuing Employee participates immediately prior to the Closing to be taken into account under those health and welfare benefit plans of Holdco, the Company, the Surviving Company or any of their respective subsidiaries in which such Continuing Employee participates following the Closing for purposes of satisfying all deductible, coinsurance, and maximum out-of-pocket requirements applicable to such Continuing Employee and his or her covered dependents for the applicable plan year. Following the Closing, Holdco shall, or shall cause the Company, the Surviving Company and each of their respective subsidiaries, as applicable, to honor all accrued but unused vacation and other paid time off of the Continuing Employees that existed immediately prior to the Closing.
Annex A-58
Table of Contents
(b) Between the date of this Agreement and the Closing Date,
(i) the Company shall enter into employment agreements with the employees listed on Section 8.04(b) of the Company Disclosure Schedule and if such employment agreements provide for the cash portion of the annual base salary or wage in excess of $200,000, for such employment agreements shall be in a form mutually agreeable to the Company and SPAC (provided that SPAC shall not unreasonably withhold, delay or condition its approval with respect to any employment agreement if such employee’s compensation and benefits are not materially changed from the compensation and benefits provided to such employees prior to the Closing); and
(ii) the Company and SPAC shall cooperate to (i) cause Holdco to adopt and authorize a mutually agreeable equity incentive plan for the benefit of the employees of Holdco and its Subsidiaries after the Closing (the “Equity Incentive Plan”) and (ii) identify the initial recipient of awards and the terms of such awards to be awarded under the Equity Incentive Plan. Holdco shall use reasonable best efforts to obtain the approval of the Holdco Board of the Equity Incentive Plan as of the Closing and to, promptly after the Closing, grant the awards to the individuals identified pursuant to this Section 8.20 (including by taking any actions required to file registration statements on Form S-8 covering any such awards).
(c) Notwithstanding anything in this Section 8.04 to the contrary, nothing contained herein, whether express or implied, is or will be deemed to be an establishment, amendment or other modification of any Plan or any employee benefit plan, program, policy, agreement or arrangement of Holdco, the Company, the Surviving Company and each of their respective subsidiaries or affiliates, or shall prohibit or limit the right of Holdco, the Company, the Surviving Company and each of their respective subsidiaries or affiliates to amend, terminate or otherwise modify any Plan or other employee benefit plan, program, policy, agreement or arrangement. The Parties acknowledge and agree that all provisions contained in this Section 8.04 are included for their sole benefit, and that nothing in this Section 8.04, whether express or implied, shall create (i) any third party beneficiary or other rights in any other person, including any Continuing Employee, any participant in any Plan or employee benefit plan, program, policy, agreement or arrangement of Holdco, the Company, the Surviving Company and each of their respective subsidiaries or affiliates, or any dependent or beneficiary thereof, or (ii) any rights in such person to continued employment with Holdco, the Company, the Surviving Company or any of their respective subsidiaries or affiliates or to any particular term or condition of employment.
SECTION 8.05 Directors’ and Officers’ Indemnification.
(a) To the fullest extent permitted under applicable Law, the A&R Holdco Organizational Documents shall contain provisions no less favorable with respect to indemnification, advancement or expense reimbursement than are set forth in the Company Organizational Documents and the SPAC Organizational Documents, which provisions shall not be amended, repealed or otherwise modified for a period of six (6) years from the Closing Date in any manner that would affect adversely the rights thereunder of individuals who, at or prior to the Merger Effective Time, were directors, officers, employees, fiduciaries or agents of the Company or SPAC (each such individual, a “D&O Indemnified Party” and collectively, the “D&O Indemnified Parties”), unless such modification shall be required by applicable Law. Holdco and the Company agree that with respect to the provisions of the articles, bylaws, limited liability company agreements or other equivalent organizational documents of the Company Subsidiaries relating to indemnification, advancement or expense reimbursement, such provisions shall not be amended, repealed or otherwise modified for a period of six (6) years from the Merger Effective Time in any manner that would affect adversely the rights thereunder of individuals who, at or prior to the Closing Date, were directors, managers, officers, employees, fiduciaries or agents of such Company Subsidiary, unless such modification shall be required by applicable Law.
(b) Holdco shall obtain, fully pay the premium for, and maintain prior to the Closing a fully-paid “tail” insurance policy for a term of six (6) years from the Closing Date (the “D&O Tail Policy”, and the period for the D&O Tail Policy, the “Tail Period”) with terms and scope of coverage at least as favorable as the Company’s directors and officers insurance policy and SPAC’s directors and officers insurance policy covering those persons thereunder; provided, however, that nothing in this Section 8.05 shall relieve Holdco or the Company of its other obligations under this Section 8.05, or allow Holdco or the Company to delay its performance of its obligations under this Section 8.05 and otherwise to provide indemnification for or make any expense advances with respect to the expenses of any claim for indemnification by a D&O Indemnified Party. Holdco shall maintain the D&O
Annex A-59
Table of Contents
Tail Policy in full force and effect for the full term and comply with all obligations thereunder. Such D&O Tail Policy shall be non-cancellable and placed with the incumbent insurers using the policies that were in place as of the date of this Agreement (unless the incumbent insurers will not offer such policies in which case coverage for the Tail Period shall be placed with a substantially comparable insurer with the same or better terms, conditions, exclusions, retentions and limits of the expiring policies). Holdco will instruct the insurers and their brokers that they may communicate directly with the D&O Indemnified Party(ies) regarding such claim, and Holdco and the Company will provide the D&O Indemnified Party(ies) a copy of all insurance policies and coverage correspondence relating to any proceeding involving any D&O Indemnified Party upon request. The D&O Indemnified Parties are express and intended third-party beneficiaries of the provisions of this Section 8.05 and shall be entitled to independently enforce the terms hereof as if they were each a party to this Agreement.
(c) In the event Holdco, the Company, the Surviving Company or any of their respective successors or assigns (i) consolidates with or merges into any other person and shall not be the continuing or surviving company or entity in such consolidation or merger, or (ii) transfers all or substantially all of its properties and assets to any person, then and in any such case proper provision shall be made so that the successors and assigns of Holdco, the Company, the Surviving Company, as the case may be, shall assume the obligations set forth in this Section 8.05.
(d) Holdco shall cause (i) each of its directors to be compensated in substantially the same manner and in the same amount than its other directors and (ii) such compensation to be consistent with compensation of directors of substantially comparable companies, including with respect to size, listed on Nasdaq.
SECTION 8.06 Notification of Certain Matters. The Company shall give prompt notice to SPAC, and SPAC shall give prompt notice to the Company, of any event which a Party becomes aware of between the date of this Agreement and the Closing (or the earlier termination of this Agreement in accordance with Article IX), the occurrence, or non-occurrence of which causes or would reasonably be expected to cause any of the conditions set forth in Article IX to fail. The failure by the Company or SPAC to give notice under this Section 8.06 shall not be deemed to be a breach under this Section 8.06, unless such breach is knowing and in any event shall not give rise to any additional damages above and beyond the breach of the underlying representation, warranty, covenant, condition or agreement, as the case may be.
SECTION 8.07 Further Action; Reasonable Best Efforts
(a) Upon the terms and subject to the conditions of this Agreement, each of the Parties shall use its reasonable best efforts to take, or cause to be taken, appropriate action, and to do, or cause to be done, such things as are necessary, proper or advisable under applicable Laws or otherwise to consummate and make effective the Transactions as soon as practicable, including, without limitation, using its reasonable best efforts to obtain all permits, consents, approvals, authorizations, qualifications and orders of Governmental Authorities and parties to contracts with the Company and the Company Subsidiaries as set forth in Section 4.05 necessary for the consummation of the Transactions and to fulfill the conditions to the Merger. In case, at any time after the Closing, any further action is necessary or desirable to carry out the purposes of this Agreement, the proper officers and directors of each Party shall use their reasonable best efforts to take all such action.
(b) Each of the Parties shall keep each other apprised of the status of matters relating to the Transactions, including promptly notifying the other Parties of any communication it or any of its affiliates receives from any Governmental Authority relating to the matters that are the subject of this Agreement and permitting the other Parties to review in advance, and to the extent practicable consult about, any proposed communication by such Party to any Governmental Authority in connection with the Transactions. No Party shall agree to participate in any meeting with any Governmental Authority in respect of any filings, investigation or other inquiry unless it consults with the other Parties in advance and, to the extent permitted by such Governmental Authority, gives the other Parties the opportunity to attend and participate at such meeting. Subject to the terms of the Confidentiality Agreement, the Parties will use reasonable best efforts to coordinate and cooperate fully with each other in exchanging such information and providing such assistance as the other Parties may reasonably request in connection with the foregoing. Subject to the terms of the Confidentiality Agreement, the Parties will provide each other with copies of all material correspondence, filings or communications, including any documents, information and data contained therewith, between them or any of their Representatives, on the one
Annex A-60
Table of Contents
hand, and any Governmental Authority or members of its staff, on the other hand, with respect to this Agreement and the Transactions. No Party shall take or cause to be taken any action before any Governmental Authority that is inconsistent with or intended to delay its action on requests for a consent or the consummation of the Transactions.
SECTION 8.08 Public Announcements. The initial press release relating to this Agreement shall be a joint press release the text of which has been agreed to by each of SPAC and the Company. Thereafter, between the date of this Agreement and the Closing Date (or the earlier termination of this Agreement in accordance with Article 10.01) unless otherwise prohibited by applicable Law or the requirements of the Nasdaq Capital Market, each of SPAC and the Company shall each use its reasonable best efforts to consult with each other before issuing any press release or otherwise making any public statements (including through social media platforms) with respect to this Agreement, the Merger or any of the other Transactions, and shall not issue any such press release or make any such public statement without the prior written consent of the other Party (such consent not to be unreasonably withheld, conditioned or delayed).
SECTION 8.09 Tax Matters.
(a) No Party has taken (or failed to take) any action or caused any action to be taken (or to fail to be taken) and will not take (or fail to take) any action or will cause any action to be taken (or to fail to be taken) (in each case other than any action provided for or prohibited by this Agreement), or has any knowledge of any fact or circumstance that could reasonably be expected to prevent the Merger and the Exchange from qualifying for the Intended Tax Treatment. Following the Merger, the Surviving Company shall, for at least six (6) months following the Closing Date, either (i) continue SPAC’s “historic business” (with the meaning of Treasury Regulations Section 1.368-1(d)(2)), or (ii) use a significant portion of SPAC’s “historic business assets” (within the meaning of Treasury Regulations Section 1.368-1(d)(3)) in a business. Within two (2) years following the Closing Date, the HoldCo shall not cause the Surviving Company to (i) dispose of more than 50% of the assets held by it at Closing pursuant to one or more distributions or other transfers where the Surviving Company does not receive an exchange of net value in such transfer, (ii) make any distribution or other transfer that fails to satisfy the requirements of Treasury Regulations Section 1.368-2(k)(1)(i) (in the case of a distribution), Treasury Regulations Section 1.368-2(k)(1)(ii) (in the case of a transfer other than a distribution), or (iii) otherwise take any action that would result in an actual or deemed liquidation of the Surviving Company for U.S. federal income tax purposes.
(b) Each Party agrees to act in good faith, consistent with the Intended Tax Treatment and will not take any position on any U.S. Tax Return or otherwise take any U.S. Tax reporting position inconsistent with the Intended Tax Treatment, unless otherwise required by a “determination” within the meaning of Section 1313 of the Code that the Intended Tax Treatment is not correct. For the avoidance of doubt, the preceding sentence shall not be interpreted to prevent a person from reporting transactions hereunder pursuant to other applicable non-recognition provisions of the Code, including under Section 368 of the Code (Section 368(a)(1)(F) of the Code), to the extent any such position is not, in and of itself, conflicting with the qualification of the transactions contemplated hereby for the Intended Tax Treatment.
(c) Tax Covenants.
(i) The Parties agree that this Agreement is a “plan of reorganization” within the meaning of Treasury Regulation Section 1.368-2(g) and 1.368-3(a).
(ii) From the date of this Agreement to the Closing, (x) the Company shall and shall cause each of the Company Subsidiaries to, and (y) SPAC shall:
(A) prepare, in the ordinary course of business consistent with past practice (except as otherwise required by a change in applicable Law), and timely file all Tax Returns required to be filed by it on or before the Closing Date (“Post-Signing Returns”);
(B) deliver drafts of such material Post-Signing Returns to the other Party no later than ten (10) Business Days prior to the date (including extensions) on which such Post-Signing Returns are required to be filed;
Annex A-61
Table of Contents
(C) fully and timely pay all Taxes due and payable in respect of such Post-Signing Returns that are so filed;
(D) �� properly reserve (and reflect such reserve in its books and records and relevant financial statements), in the ordinary course of business consistent with past practice, for all Taxes payable by it for which no Post-Signing Return is due prior to the Closing Date; and
(E) promptly notify the other Party of any material federal, state, local or foreign income or franchise, Action or audit pending or threatened in writing against or with respect to such Party or its subsidiaries in respect of any Tax matter.
(iii) Holdco acknowledges that any SPAC Shareholder who owns five percent (5%) or more of the ordinary shares of Holdco immediately after the Closing, as determined under Section 367 of the Code and the Treasury Regulations promulgated thereunder, may enter into (and cause to be filed with the IRS) a gain recognition agreement in accordance with Treasury Regulations Section 1.367(a)-8. Upon the written request of any such SPAC Shareholder made following the Closing Date, Holdco shall (i) use reasonable best efforts to furnish to such SPAC Shareholder such information as such SPAC Shareholder reasonably requests in connection with such SPAC Shareholder’s preparation of a gain recognition agreement, and (ii) use reasonable best efforts to provide such SPAC Shareholder with the information reasonably requested by such SPAC Shareholder for purposes of determining whether there has been a gain “triggering event” under the terms of such SPAC Shareholder’s gain recognition agreement, in each case, at the sole cost and expense of such requesting SPAC Shareholders.
(d) Transfer Taxes. Any Transfer Taxes incurred in connection with the Transactions shall be paid by HoldCo. The Parties shall reasonably cooperate to establish any available exemption from (or reduction in) any Transfer Tax.
SECTION 8.10 Stock Exchange Listing. The Company, Holdco and SPAC shall use their respective reasonable best efforts to cause the Holdco Ordinary Shares to be issued in exchange of the Company Ordinary Shares, the Merger Consideration, to be issued in the form of Holdco Ordinary Shares and Holdco Warrants and Holdco Ordinary Shares that will become issuable upon the exercise of the Holdco Warrants, to be approved for listing on Nasdaq, subject to official notice of issuance, as promptly as practicable after the date of this Agreement, and in any event prior to the Closing Date.
SECTION 8.11 Delisting and Deregistration. The Company, Holdco and SPAC shall use their respective reasonable best efforts to cause the SPAC Units, SPAC Common Stock and SPAC Warrants to be delisted from Nasdaq (or be succeeded by the respective Holdco securities) and to terminate its registration with the SEC pursuant to Sections 12(b), 12(g) and 15(d) of the Exchange Act (or be succeeded by Holdco) as of the Closing Date or as soon as practicable thereafter.
SECTION 8.12 Antitrust.
(a) To the extent required under any Laws that are designed to prohibit, restrict or regulate actions having the purpose or effect of monopolization or restraint of trade or lessening of competition or creation or strengthening of a dominant position through merger or acquisition, including the Laws of any jurisdiction or Governmental Authority outside of the United States (“Antitrust Laws”), each Party agrees to promptly (but in no event later than fifteen (15) days after the date hereof) make any required filing or application under Antitrust Laws, as applicable. The Parties agree to supply as promptly as reasonably practicable any additional information and documentary material that may be requested pursuant to Antitrust Laws and to take all other actions necessary, proper or advisable to cause the expiration or termination of the applicable waiting periods or obtain required approvals, as applicable under Antitrust Laws as soon as practicable, including by requesting early termination of any waiting periods thereunder.
(b) Each Party shall, in connection with its efforts to obtain all requisite approvals and authorizations for the Transactions under any Antitrust Law, use its reasonable best efforts to: (i) cooperate in all respects with each other Party or its affiliates in connection with any filing or submission and in connection with any investigation or other inquiry, including any proceeding initiated by a private person; (ii) keep the other Parties reasonably informed of any communication received by such Party or its Representatives from, or given by such Party or its Representatives to, any Governmental Authority and of any communication received or given in
Annex A-62
Table of Contents
connection with any proceeding by a private person, in each case regarding any of the Transactions; (iii) permit a Representative of the other Parties and their respective outside counsel to review any communication given by it to, and consult with each other in advance of any meeting or conference with, any Governmental Authority or, in connection with any proceeding by a private person, with any other person, and to the extent permitted by such Governmental Authority or other person, give a Representative or Representatives of the other Parties the opportunity to attend and participate in such meetings and conferences; (iv) in the event a Party’s Representative is prohibited from participating in or attending any meetings or conferences, the other Parties shall keep such Party promptly and reasonably apprised with respect thereto; and (v) use reasonable best efforts to cooperate in the filing of any memoranda, white papers, filings, correspondence or other written communications explaining or defending the Transactions, articulating any regulatory or competitive argument, and/or responding to requests or objections made by any Governmental Authority.
(c) No Party shall take any action that could reasonably be expected to adversely affect or materially delay the approval of any Governmental Authority of any required filings or applications under Antitrust Laws. The Parties further covenant and agree, with respect to a threatened or pending preliminary or permanent injunction or other order, decree or ruling or statute, rule, regulation or executive order that would adversely affect the ability of the Parties to consummate the Transactions, to use reasonable best efforts to prevent or lift the entry, enactment or promulgation thereof, as the case may be. No Party shall permit any of its officers or any other Representatives or agents to participate in any pre-scheduled meeting with any Governmental Authority in respect of any filing, investigation or other inquiry relating to the Transactions unless it consults with the other part in advance and, to the extent permitted by such Governmental Authority, gives the other Party to attend and participate thereat.
SECTION 8.13 PCAOB Financials. The Company shall deliver true and complete copies of (i) the Financial Statements and (iv) the unaudited consolidated balance sheet of the Company and the consolidated Company Subsidiaries as of March 31, 2022, and the reviewed unaudited consolidated statements of income and cash flows of the Company and the consolidated Company Subsidiaries for the 3 month period then ended, in each case (a) prepared in accordance with the International Financial Reporting Standards issued by the International Accounting Standards Board and audited in accordance with the auditing standards of the PCAOB (collectively, the “PCAOB Financials”) not later than 60 days from the date hereof, unless otherwise extended with SPAC’s consent (which consent shall not be unreasonably withheld, conditioned or delayed).
SECTION 8.14 Backstop Agreement. Unless otherwise approved in writing by the Company, none of the Parties shall permit any material amendment or modification to be made to, or any waiver of any provision or remedy under, or any replacement or termination of, the Backstop Agreement in any manner that would have an adverse impact to the consummation of the Transactions hereto or in a manner that would be adverse to any of the Parties. Each of the Parties shall use its respective reasonable best efforts to take, or cause to be taken, all actions and do, or cause to be done, all things necessary, proper or advisable to consummate the transactions contemplated by the Backstop Agreement on the terms and conditions described therein, including using its reasonable best efforts to enforce its rights under the Backstop Agreement to cause all parties thereto to pay to (or as directed by) the applicable amounts under the Backstop Agreement to the extent payable in accordance with its term.
SECTION 8.15 Exclusivity.
(a) From and after the date hereof until the Closing or, if earlier, the valid termination of this Agreement in accordance with Section 10.01, but only to the extent not inconsistent with the fiduciary duties of the SPAC Board, (i) SPAC will not, and will direct its Representatives acting on its behalf not to, directly or indirectly, (A) initiate, seek, solicit, knowingly facilitate or encourage, submit an indication of interest for, any inquiries, proposals or offer to a Competing Seller relating to a Competing SPAC Transaction or (B) participate in any negotiations with a Competing Seller relating to a Competing SPAC Transaction; (ii) SPAC will, and will cause its Representatives to, (A) terminate immediately any negotiations with any Competing Seller relating to a Competing SPAC Transaction and (B) promptly advise the Company in writing of any proposal regarding a Competing SPAC Transaction involving a Competing Seller that it may receive (it being understood that SPAC will not be required to inform the Company of the identity of the person making such proposal or the material terms thereof);
Annex A-63
Table of Contents
(b) From and after the date hereof until the Closing or, if earlier, the valid termination of this Agreement in accordance with Section 10.01, the Company and each Company Subsidiary will not, and will direct their respective Representatives acting on their behalf not to, directly or indirectly, (i) initiate, seek, solicit, knowingly facilitate or encourage, submit an indication of interest for, any inquiries, proposals or offer from any person relating to a Competing Transaction, (ii) participate in any discussions or negotiations with any person regarding, or furnish or make available to any person any information relating to the Company or any Company Subsidiary with respect to, a Competing Transaction, other than to make such person aware of the provisions of this Section 8.15 or (iii) enter into any understanding, arrangement, agreement, agreement in principle or other commitment (whether or not legally binding) with any person relating to a Competing Transaction.
SECTION 8.16 Trust Account. As of the Merger Effective Time, the obligations of SPAC to dissolve or liquidate within a specified time period as contained in the SPAC Certificate of Incorporation will be terminated and SPAC shall have no obligation whatsoever to dissolve and liquidate the assets of SPAC by reason of the consummation of the Merger or otherwise, and no shareholder of SPAC shall be entitled to receive any amount from the Trust Account. At least 48 hours prior to the Merger Effective Time, SPAC shall provide notice to the Trustee in accordance with the Trust Agreement and shall deliver any other documents, opinions or notices required to be delivered to the Trustee pursuant to the Trust Agreement and cause the Trustee prior to the Merger Effective Time to, and the Trustee shall thereupon be obligated to, transfer all funds held in the Trust Account to SPAC (to be held as available cash on the balance sheet of SPAC, and to be used for working capital and other general corporate purposes of the business following the Closing) and thereafter shall cause the Trust Account and the Trust Agreement to terminate.
SECTION 8.17 Treatment of Interested Party Transactions. Prior to Closing,
(a) the Company shall deliver to SPAC fully executed Termination Agreements by and among the Company and the Company Shareholders, which Termination Agreement shall provide that, upon the Closing, no parties to the Termination Agreements shall have any further rights or obligations thereunder;
(b) the Company shall cause all Intellectual Property used in the business of the Company or any Company Subsidiary that is owned by, held in the name of or licensed to any equityholder, Affiliate or Representative of the Company (other than any Company Subsidiary) to be transferred to the Company prior to the Closing and shall otherwise take action as necessary to ensure that, immediately after the Closing, the Company or a Company Subsidiary will have good and valid title to, or valid license to or leasehold interests in, all Intellectual Property used in the business of the Company and the Company Subsidiaries, free and clear of any Liens other than Permitted Liens; and
(c) the Company shall, and shall cause to be taken all actions necessary to, terminate as of the Closing all Interested Party Transactions, in each case without any further liability to the Company or any of the Company Subsidiaries to any Person; provided that the Company shall not be required to terminate any Interested Party Transactions that are on an arm’s length basis and are in the best interest of the Company to maintain outstanding.
SECTION 8.18 EU Securities Regulation. From and after the date of this Agreement and until the earlier of the Closing and the termination of this Agreement pursuant to Section 10.01, the Parties shall not make any offer of securities in the European Union in connection with the Transactions other than in accordance with the provisions of the Prospectus Regulation. In the event that the Parties, following consultation with their respective counsel, determine that a prospectus or a prospectus exemption document (as applicable) may be required to be published in accordance with the provisions of the Prospectus Regulation, each Party shall use its reasonable best efforts take such actions and do such things that such Party (after consultation with counsel) deems reasonably necessary or desirable, including the delivery or execution of any documents or instruments reasonably required or desirable in order for the Company to publish a prospectus or be exempted from the obligation to publish a prospectus or a prospectus exemption document (as applicable) under the Prospectus Regulation. Without limiting the generality of the foregoing, each of the Parties shall use reasonable best efforts to cooperate with each other in good faith in taking any actions or preparing or delivering any documents or instruments pursuant to the preceding sentence and to furnish the others with such information concerning it and its affiliates as the providing Party (after consulting with counsel) may deem reasonably necessary or advisable in connection the foregoing.
Annex A-64
Table of Contents
SECTION 8.19 Opinion of the Financial Advisor. If SPAC determines (in its sole discretion) that an opinion of an independent financial advisor is required for the consummation of the Transactions, SPAC shall use reasonable best efforts to engage a financial advisor to give an opinion substantially to the effect that, as of the date of such opinion and subject to the various assumptions made, procedures followed, matters considered, and limitations, qualifications and other matters considered in connection with the preparation of such opinion, the Merger Consideration to be received by the SPAC Shareholders in the Merger pursuant to this Agreement is fair, from a financial point of view, to the SPAC Shareholders.
SECTION 8.20 SPAC Extension Proposal. SPAC shall use its reasonable best efforts to take all actions necessary (including at the request of the Company) to obtain the approval of the SPAC Extension Proposal. In connection with obtaining the approval, SPAC will prepare, file and mail all required proxy materials to be sent to the SPAC Shareholders seeking approval of the SPAC Extension Proposal.
ARTICLE IX
CONDITIONS TO THE MERGER
SECTION 9.01 Conditions to the Obligations of Each Party. The obligations of the Company, SPAC, Holdco and Merger Sub to consummate the Transactions, including the Merger and Exchange, are subject to the satisfaction or waiver (where permissible) at or prior to the Closing of the following conditions:
(a) SPAC Shareholders’ Approval. The SPAC Proposals shall have been approved and adopted by the requisite affirmative vote of the shareholders of SPAC in accordance with the Proxy Statement/Prospectus, the proxy statement/prospectus filed with the SEC in connection with the Extension Amendment to the SPAC COI, DGCL, the SPAC Organizational Documents and the rules and regulations of the Nasdaq Capital Market.
(b) Company Requisite Approval. The Company Requisite Approvals shall have been obtained and delivered to SPAC in a form and substance reasonably acceptable to SPAC.
(c) Holdco Requisite Approvals. The Holdco Requisite Approvals shall have been obtained and delivered to SPAC in a form and substance reasonably acceptable to SPAC.
(d) Holdco Auditor Reports. A Luxembourg independent auditor (réviseur d’entreprises) of Holdco shall have issued (i) at or before the Merger Effective Time a report on the contributions in kind relating to the Merger Issuance prepared in accordance with article 420-10 of the 1915 Law (the “Merger Auditor Report”), and (ii) at or before the Exchange Effective Time, in accordance with the Exchange Agreements, a report on the contributions in kind relating to the Exchange Issuance prepared in accordance with article 420-10 of the 1915 Law (the “Exchange Auditor Report”).
(e) Regulatory Approvals. (A) Any waiting period under any Antitrust Laws applicable to the Transactions shall have expired or been earlier terminated, and (B) all other consents of (or filings or registrations with) any Governmental Authority required in connection with the execution, delivery and performance of this Agreement set forth on Schedule 9.01 shall have been obtained.
(f) No Order. No Governmental Authority shall have enacted, issued, promulgated, enforced or entered any Law, rule, regulation, judgment, decree, executive order or award which is then in effect and has the effect of making the Transactions, including the Merger or Exchange, illegal or otherwise prohibiting consummation of the Transactions, including the Merger or Exchange.
(g) Registration Statement. The Registration Statement shall have been declared effective by the SEC under the Securities Act. No stop order suspending the effectiveness of the Registration Statement shall be in effect, and no proceedings for purposes of suspending the effectiveness of the Registration Statement shall have been initiated by the SEC and not withdrawn.
(h) Stock Exchange Listing. The Holdco Ordinary Shares shall have been approved for listing on Nasdaq, subject to official notice of issuance.
(i) SPAC Net Tangible Assets. SPAC shall have at least $5,000,001 of net tangible assets after giving effect to the amounts funded under the Backstop Agreement and following the exercise of Redemption Rights in accordance with the SPAC Organizational Documents.
Annex A-65
Table of Contents
(j) Fairness Opinion. The delivery of the Financial Advisor Opinion pursuant to Section 8.19.
(k) CFO Free Shares. The delivery of an agreement, which shall be in a form mutually agreeable to the Company and SPAC, between the CFO and the Company by which the CFO agrees that the issuance of the CFO Free Shares satisfies all obligations of the Company relating to the issuance of shares in the Company to the CFO under the CFO Consulting Agreement.
(l) Registration Rights and Lock-Up Agreement. All parties to the Registration Rights and Lock-Up Agreement shall have delivered, or cause to be delivered, copies of the Registration Rights and Lock-Up Agreement duly executed by all such parties.
SECTION 9.02 Conditions to the Obligations of SPAC. The obligations of SPAC to consummate the Transactions, including the Merger, are subject to the satisfaction or waiver (where permissible) at or prior to the Closing of the following additional conditions:
(a) Representations and Warranties.
(i) The representations and warranties of the Company contained in Section 4.01, Section 4.03, Section 4.04, Section 4.05, Section 4.24 and Section 4.26 shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and the Closing Date as though made on the Exchange Effective Time, the Merger Effective Time and the Closing Date (as applicable), except to the extent that any such representation and warranty expressly speaks as of an earlier date, in which case such representation and warranty shall be true and correct as of such earlier date. All other representations and warranties of the Company contained in this Agreement shall be true and correct (without giving any effect to any limitation as to “materiality” or “Company Material Adverse Effect” or any similar limitation set forth therein) in all respects as of the Exchange Effective Time, the Merger Effective Time and the Closing Date, as though made on and as of the Exchange Effective Time, the Merger Effective Time and the Closing Date (as applicable), except (A) to the extent that any such representation and warranty expressly speaks as of an earlier date, in which case such representation and warranty shall be true and correct as of such earlier date and (B) where the failure of such representations and warranties to be true and correct (whether as of the Exchange Effective Time, the Merger Effective Time and the Closing Date (as applicable) or such earlier date), taken as a whole, does not result in a Company Material Adverse Effect.
(ii) The representations and warranties of each of Holdco and Merger Sub in Section 6.01, Section 6.03, Section 6.04, Section 6.07(b) and Section 6.09 shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and the Closing Date as though made on the Exchange Effective Time, the Merger Effective Time and the Closing Date (as applicable), except to the extent that any such representation and warranty expressly speaks as of an earlier date, in which case such representation and warranty shall be true and correct as of such earlier date. All other representations and warranties of Merger Sub and Holdco contained in this Agreement shall be true and correct (without giving any effect to any limitation as to “materiality” or any similar limitation set forth therein) in all respects as of the Exchange Effective Time, the Merger Effective Time and the Closing Date, as though made on and as of the Exchange Effective Time, the Merger Effective Time and the Closing Date (as applicable), except (A) to the extent that any such representation and warranty expressly speaks as of an earlier date, in which case such representation and warranty shall be true and correct as of such earlier date and (B) where the failure of such representations and warranties to be true and correct (whether as of the Exchange Effective Time, the Merger Effective Time and the Closing Date (as applicable) or such earlier date), taken as a whole, would be materially adverse to Holdco or Merger Sub.
(b) Agreements and Covenants. The Company, Holdco and Merger Sub shall have performed or complied in all material respects with all agreements and covenants (other than Section 7.01(c)) required by this Agreement to be performed or complied with by it on or prior to the Exchange Effective Time and the Merger Effective Time, as applicable (including, for the avoidance of doubt and without limitation, Section 8.13); provided, that Holdco shall have performed or complied in all respects with the agreements and covenants set forth in Section 7.01(c).
Annex A-66
Table of Contents
(c) Officer Certificate. (i) The Company, Holdco and Merger Sub shall have delivered to SPAC a certificate, dated as of the date of the Merger Effective Time, signed by an officer of the Company, certifying as to the satisfaction of the conditions specified in Section 9.02(a), Section 9.02(b) and Section 9.02(d) and (ii) the Company, Holdco and Merger Sub shall have delivered to SPAC a certificate, dated as of the Exchange Effective Time, signed by an officer of the Company, certifying as to the satisfaction of the conditions specified in Section 9.02(a), Section 9.02(b) and Section 9.02(d).
(d) No Company Material Adverse Effect. No Company Material Adverse Effect shall have occurred between the date of this Agreement and the Merger Effective Time and no Company Material Adverse Effect shall have occurred between the Merger Effective Time and the Exchange Effective Time.
(e) PCAOB Financials. The Company shall have delivered the PCAOB Financials as contemplated in Section 8.13.
(f) Capitalization. As of immediately prior to the Closing, there shall be no Company Ordinary Shares or other Equity Interest of the Company outstanding other than Company Ordinary Shares and other Equity Interests that are subject to an Exchange Agreement (which shall provide that security shall be either cancelled or converted into shares of Company Ordinary Shares to be transferred to Holdco in accordance with Section 3.02).
(g) Evidence of Related Party Transactions. SPAC shall have received evidence to its satisfaction that the actions contemplated in Section 8.17 have been taken.
(h) Delivery of Ancillary Agreements
(i) Employment Agreements. Each of the employees of the Company listed on 8.04(b) of the Company Disclosure Schedule shall have executed and delivered to the Company or Holdco employment agreements executed in compliance with Section 8.04(b) with such agreements being in full force and effect.
(ii) Warrant Amendment. Holdco shall have delivered to SPAC and Trustee, the SPAC Warrant Amendment and Assignment executed by Holdco.
(iii) Other Documents. Each of the Company, the Company Shareholders, Holdco, and Merger Sub shall have delivered, or caused to be delivered, each of the Ancillary Agreements to which such Person is a party and that, by its terms, is required to be executed and delivered at the Closing, in each case, duly executed by such Person.
SECTION 9.03 Conditions to the Obligations of the Company. The obligations of the Company to consummate the Transactions, including the Merger, are subject to the satisfaction or waiver (where permissible) at or prior to the Closing of the following additional conditions:
(a) Representations and Warranties. The representations and warranties of SPAC contained in Section 5.01, Section 5.03, Section 5.04 and Section 5.11 shall each be true and correct in all respects as of the Exchange Effective Time, the Merger Effective Time and the Closing Date as though made on the Exchange Effective Time, the Merger Effective Time and the Closing Date (as applicable), except to the extent that any such representation and warranty expressly speaks as of an earlier date, in which case such representation and warranty shall be true and correct as of such earlier date. All other representations and warranties of SPAC contained in this Agreement shall be true and correct (without giving any effect to any limitation as to “materiality” or “SPAC Material Adverse Effect” or any similar limitation set forth therein) in all respects as of the Exchange Effective Time, the Merger Effective Time and the Closing Date, as though made on and as of the Exchange Effective Time, the Merger Effective Time and the Closing Date (as applicable), except (i) to the extent that any such representation and warranty expressly speaks as of an earlier date, in which case such representation and warranty shall be true and correct as of such earlier date and (ii) where the failure of such representations and warranties to be true and correct (whether as of the Exchange Effective Time, the Merger Effective Time and the Closing Date (as applicable) or such earlier date), taken as a whole, does not result in a SPAC Material Adverse Effect.
(b) Agreements and Covenants. SPAC shall have performed or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied with by it on or prior to the Exchange Effective Time and the Merger Effective Time, as applicable.
Annex A-67
Table of Contents
(c) Officer Certificate. (i) SPAC shall have delivered to the Company a certificate, dated as of the Merger Effective Time, signed by the Chief Executive Officer of SPAC, certifying as to the satisfaction of the conditions specified in Section 9.03(a), Section 9.03(b) and Section 9.03(d) and (ii) SPAC shall have delivered to the Company a certificate, dated as of the Exchange Effective Time, signed by the Chief Executive Officer of SPAC, certifying as to the satisfaction of the conditions specified in Section 9.03(a), Section 9.03(b) and Section 9.03(d).
(d) No SPAC Material Adverse Effect. No SPAC Material Adverse Effect shall have occurred between the date of this Agreement and the Merger Effective Time and no SPAC Material Adverse Effect shall have occurred between the Merger Effective Time and the Exchange Effective Time.
(e) Delivery of Ancillary Agreements.
(i) Warrant Amendment. SPAC shall have delivered to Holdco and Trustee, the SPAC Warrant Amendment and Assignment executed by SPAC.
(ii) Other Documents. SPAC shall have delivered, or caused to be delivered, each of the Ancillary Agreements to which such Person is a party and that, by its terms, is required to be executed and delivered at the Closing, in each case, duly executed by such Person.
(f) Transfer of SPAC Common Stock. Sponsor shall have transferred to UG Holdings, LLC (or its designated affiliate) 1,035,000 shares of SPAC Common Stock, as contemplated in the Backstop Agreement.
ARTICLE X
TERMINATION, AMENDMENT AND WAIVER
SECTION 10.01 Termination. This Agreement may be terminated and the Merger and the other Transactions may be abandoned at any time prior to the Merger Effective Time, notwithstanding any requisite approval and adoption of this Agreement and the Transactions by the shareholders of the Company or the shareholders of SPAC, as follows:
(a) by mutual written consent of SPAC and the Company;
(b) by either SPAC or the Company if the Merger Effective Time shall not have occurred prior to 5:00 p.m. (New York time) on the later of (i) the last day of the extended time period for SPAC to consummate a business combination if a SPAC Extension Proposal shall be approved at a relevant SPAC Shareholders’ Meeting and (ii) July 12, 2022 (the “Outside Date”); provided, however, that this Agreement may not be terminated under this Section 10.01(b) by or on behalf of any Party that either directly or indirectly through its affiliates is in breach or violation of any representation, warranty, covenant, agreement or obligation contained herein and such breach or violation is the principal cause of the failure to satisfy a condition set forth in Article IX on or prior to the Outside Date;
(c) by either SPAC or the Company if any Governmental Authority shall have enacted, issued, promulgated, enforced or entered any injunction, order, decree or ruling (whether temporary, preliminary or permanent) which has become final and non-appealable and has the effect of making consummation of the Transactions, including the Merger, illegal or otherwise preventing or prohibiting consummation of the Transactions or the Merger;
(d) by either SPAC or the Company if any of the SPAC Proposals shall fail to receive the requisite vote for approval at the SPAC Shareholders’ Meeting;
(e) by SPAC upon a breach of any representation, warranty, covenant or agreement on the part of the Company, Holdco or Merger Sub set forth in this Agreement, or if any representation or warranty of the Company, Holdco or Merger Sub shall have become untrue, in either case only if the conditions set forth in Section 9.02(a) and Section 9.02(b) would not be satisfied (“Terminating Company Breach”); provided that SPAC has not waived such Terminating Company Breach and SPAC is not then in material breach of any of its representations, warranties, covenants or agreements in this Agreement; provided further that, if such Terminating Company Breach is curable by the Company, Holdco or Merger Sub, SPAC may not terminate this Agreement under this Section 10.01(e) for so long as the Company, Holdco or Merger Sub continues to exercise its reasonable best efforts to cure such breach, unless such breach is not cured within thirty (30) days after written notice of such breach is provided by SPAC to the Company;
Annex A-68
Table of Contents
(f) by the Company upon a breach of any representation, warranty, covenant or agreement on the part of SPAC set forth in this Agreement, or if any representation or warranty of SPAC shall have become untrue, in either case only if the conditions set forth in Section 9.03(a) and Section 9.03(b) would not be satisfied (“Terminating SPAC Breach”); provided that the Company has not waived such Terminating SPAC Breach and the Company, Holdco or Merger Sub is not then in material breach of their representations, warranties, covenants or agreements in this Agreement; provided, further, that, if such Terminating SPAC Breach is curable by SPAC, the Company may not terminate this Agreement under this Section 10.01(f) for so long as SPAC continues to exercise their reasonable best efforts to cure such breach, unless such breach is not cured within thirty (30) days after written notice of such breach is provided by the Company to SPAC; or
SECTION 10.02 Notice of Termination; Effect of Termination.
(a) The Party seeking to terminate this Agreement pursuant to Section 10.01 shall provide written notice of termination to the other Parties in accordance with Section 11.01 specifying the reason for such valid termination, and any such termination in accordance with Section 10.01 shall be effective immediately upon delivery of such written notice to the other Parties.
(b) In the event of the valid termination of this Agreement pursuant to Section 10.01, this Agreement shall forthwith become void, and there shall be no liability under this Agreement on the part of any Party, except that the provisions set forth in this Section 10.02, Article XI, and any corresponding definitions set forth in Article I shall survive such termination; provided, however, that nothing herein shall relieve any Party from any liability for any willful and material breach of this Agreement.
SECTION 10.03 Expenses.
(a) In the event that this Agreement is terminated in accordance with Section 10.01 above, all Transaction Expenses incurred in connection with this Agreement, the Ancillary Agreements and the Transactions shall be paid by the Party incurring such Transaction Expenses.
(b) If the Transactions are consummated:
(i) As promptly as practicable after the Closing, Holdco shall transfer or cause to be transferred to Sponsor or its designee an amount in cash equal to the Sponsor Advanced Funds; provided, that if the unpaid SPAC Transaction Expenses as of the Closing (including the Sponsor Advanced Funds) would exceed the applicable SPAC Transaction Expenses Cap, Holdco shall not be required to transfer the amount of such excess to Sponsor and such excess shall remain with Holdco and its subsidiaries.
(ii) Holdco shall pay or cause to be paid, (x) the Company Transaction Expenses, (y) the EarlyBird Cash Fees (regardless of whether the SPAC Transaction Expenses Cap has been exceeded), provided that if Net Available Assets is equal to or less than $200,000, then each of Sponsor and Holdco shall pay 50% of all EarlyBird Cash Fees, and (z) all SPAC Transaction Expenses that are unpaid as of the Closing up to the SPAC Transaction Expenses Cap. Within five (5) days following the six (6) month anniversary of the Closing, Holdco shall issue the EarlyBird Share Fees.
(iii) Sponsor shall pay or cause to be paid, (x) all unpaid SPAC Transaction Expenses in excess of the applicable SPAC Transaction Expenses Cap (with the Sponsor Advance Funds being subject to Section 10.03(b)(i)), (y) any expenses incurred by SPAC in its pursuit of potential acquisition or business targets other than the Company or that were not incurred by SPAC in connection with or in furtherance of the Transactions, other than the Sponsor Advanced Funds (with Sponsor Advanced Funds being subject to Section 10.03(b)(i)) and (z) all Extension Amendment Fees.
SECTION 10.04 Amendment. This Agreement may be amended in writing by all Parties hereto at any time prior to the Merger Effective Time by action by or on behalf of the Parties’ respective board of directors; provided, however that after approval of this Agreement by the stockholders of the SPAC and Merger Sub, no amendment shall be made that requires a further vote of such stockholders without such further approval. This Agreement may not be amended except by an instrument in writing signed by each of the Parties.
Annex A-69
Table of Contents
SECTION 10.05 Waiver. At any time prior to the Merger Effective Time, (a) SPAC may in its sole discretion (i) extend the time for the performance of any obligation or other act of the Company, Holdco or Merger Sub, (ii) waive any inaccuracy in the representations and warranties of the Company, Holdco or Merger Sub contained herein or in any document delivered by the Company, Holdco or Merger Sub pursuant hereto and (iii) waive compliance with any agreement of the Company, Holdco or Merger Sub or any condition to its own obligations contained herein and (b) the Company may in its sole discretion (i) extend the time for the performance of any obligation or other act of SPAC, (ii) waive any inaccuracy in the representations and warranties of SPAC contained herein or in any document delivered by SPAC pursuant hereto and (iii) waive compliance with any agreement of SPAC or any condition to its own obligations contained herein. Any such extension or waiver shall be valid if set forth in an instrument in writing signed by the Party or Parties to be bound thereby.
ARTICLE XI
GENERAL PROVISIONS
SECTION 11.01 Notices. All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be deemed to have been duly given upon receipt) by delivery in person, by email or by registered or certified mail (postage prepaid, return receipt requested) to the respective Parties at the following addresses (or at such other address for a Party as shall be specified in a notice given in accordance with this Section 11.01):
if to SPAC:
LightJump Acquisition Corporation
2735 Sand Hill Road, Suite 110(2)
Menlo Park, CA 94025
Attn: Robert Bennett
Email: rbennett@lightjumpcapital.com
with a copy (which shall not constitute notice) to:
K&L Gates LLP
10100 Santa Monica Blvd.
Los Angeles, CA 90067
Attention: Leib Orlanski
Email: leib.orlanski@klgates.com
with a copy (which shall not constitute notice) to:
K&L Gates LLP
599 Lexington Avenue
New York NY 10022
Attention: Robert S. Matlin
Email: robert.matlin@klgates.com
if to the Company, Holdco or Merger Sub:
Moolec Science Limited
Innovation Centre, Gallows Hill
Warwick
CV34 6UW
United Kingdom
Attention: Gastón Paladini and Oscar Leon
Email: gaston@moolecscience.com
Annex A-70
Table of Contents
with a copy (which shall not constitute notice) to:
Linklaters LLP
1290 Avenue of the Americas
New York, NY 10104
Attention: Matthew Poulter and Pierre-Emmanuel Perais
Email: matthew.poulter@linklaters.com and pierre-emmanuel.perais@linklaters.com
SECTION 11.02 Non-Survival of Representations, Warranties and Covenants. The representations, warranties, obligations, agreements and covenants in this Agreement or in any certificate, statement or instrument delivered pursuant to this Agreement shall terminate at the Closing (and there shall be no liability after the Closing in respect thereof), except in the case of fraud and that (a) this Article XI and any corresponding definitions included herein shall survive the Closing and (b) this Section 11.02 shall not limit any covenant, obligation or agreement contained herein that by its terms expressly applies or requires performance in whole or in part after the Closing and then only with respect to any breaches occurring after the Closing. Effective as of the Closing, in the case of fraud, there are no remedies available to the Parties with respect to any breach of the representations, warranties, obligations, covenants or agreements of the Parties, except, with respect to those obligations, covenants and agreements contained herein that by their terms apply or require performance in whole or in part after the Closing and the remedies that may be available under Section 11.10.
SECTION 11.03 Severability. If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any rule of law, or public policy, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the Transactions is not affected in any manner materially adverse to any Party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the Parties shall negotiate in good faith to modify this Agreement so as to effect the original intent of the Parties as closely as possible in a mutually acceptable manner in order that the Transactions be consummated as originally contemplated to the fullest extent possible.
SECTION 11.04 Entire Agreement; Assignment. This Agreement and the Ancillary Agreements constitute the entire agreement among the Parties with respect to the subject matter hereof and supersede, except for the Confidentiality Agreement, all prior agreements and undertakings, both written and oral, among the Parties, or any of them, with respect to the subject matter hereof. Neither Party shall assign, grant or otherwise transfer the benefit of the whole or any part of this Agreement or any of the rights hereunder (whether pursuant to a merger, by operation of Law or otherwise) by any Party without the prior express written consent of the other Parties.
SECTION 11.05 Parties in Interest; Non-Recourse. This Agreement shall be binding upon and inure solely to the benefit of each Party, and nothing in this Agreement, express or implied, is intended to or shall confer upon any other person any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement, other than Section 8.05 (which is intended to be for the benefit of the persons covered thereby and may be enforced by such persons). For the avoidance of doubt, this Agreement may only be enforced against, and any claim or cause of action based upon, arising out of, or related to this Agreement or the transactions contemplated hereby may only be brought against, the entities that are expressly named as parties hereto and then only with respect to the specific obligations set forth herein with respect to such party. Except to the extent a named party to this Agreement (and then only to the extent of the specific obligations undertaken by such named party in this Agreement), (i) no past, present or future director, officer, employee, incorporator, member, partner, stockholder, Affiliate, agent, attorney, advisor, sponsor or representative or Affiliate of any named party to this Agreement and (ii) no past, present or future director, officer, employee, incorporator, member, partner, stockholder, Affiliate, agent, attorney, advisor, sponsor or representative or Affiliate of any of the foregoing shall have any liability (whether in contract, tort, equity or otherwise) for any one or more of the representations, warranties, covenants, agreements or other obligations or liabilities of any one or more of the Company, Holdco, Merger Sub, or SPAC under this Agreement or for any claim based on, arising out of, or related to this Agreement or the Transactions.
SECTION 11.06 Governing Law. This Agreement shall be governed by, and construed in accordance with, the Laws of the State of Delaware applicable to contracts executed in and to be performed in that State, except to the extent mandatorily governed by the laws of the Grandy Duchy of Luxembourg or the United Kingdom, including the provisions relating to the Exchange. All legal actions and proceedings arising out of or relating to this Agreement shall be heard and determined exclusively in the Court of Chancery of the State of Delaware or, if (and only if) the Court of Chancery of the State of Delaware declines to accept jurisdiction over a particular matter, the Superior
Annex A-71
Table of Contents
Court of the State of Delaware (Complex Commercial Division) or, if (and only if) the Superior Court of the State of Delaware (Complex Commercial Division) declines to accept jurisdiction over a particular matter, any federal court sitting in the State of Delaware, and any appellate courts therefrom (collectively, the “Chosen Courts”), with regard to any such Action arising out of or relating to this Agreement and the transactions contemplated hereby. The Parties hereby (a) irrevocably submit to the exclusive jurisdiction of the aforesaid courts for themselves and with respect to their respective properties for the purpose of any Action arising out of or relating to this Agreement brought by any Party, and (b) agree not to commence any Action relating thereto except in the Chosen Courts, other than Actions in any court of competent jurisdiction to enforce any judgment, decree or award rendered by any such court in the State of Delaware as described herein. To the fullest extent permitted by applicable Law, each of the Parties further agrees that notice as provided herein shall constitute sufficient service of process and the Parties further waive any argument that such service is insufficient. To the fullest extent permitted by applicable Law, each of the Parties hereby irrevocably and unconditionally waives, and agrees not to assert, by way of motion or as a defense, counterclaim or otherwise, in any Action arising out of or relating to this Agreement or the Transactions, (x) any claim that it is not personally subject to the jurisdiction of the courts in the State of Delaware as described herein for any reason, (y) that it or its property is exempt or immune from jurisdiction of any such court or from any legal process commenced in such courts (whether through service of notice, attachment prior to judgment, attachment in aid of execution of judgment, execution of judgment or otherwise) and (z) that (i) the Action in any such court is brought in an inconvenient forum, (ii) the venue of such Action is improper or (iii) this Agreement, or the subject matter hereof, may not be enforced in or by such courts.
SECTION 11.07 Waiver of Jury Trial. EACH OF THE PARTIES HEREBY WAIVES TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW ANY RIGHT IT MAY HAVE TO A TRIAL BY JURY WITH RESPECT TO ANY LITIGATION DIRECTLY OR INDIRECTLY ARISING OUT OF, UNDER OR IN CONNECTION WITH THIS AGREEMENT OR THE TRANSACTIONS. EACH OF THE PARTIES (A) CERTIFIES THAT NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THAT FOREGOING WAIVER AND (B) ACKNOWLEDGES THAT IT AND THE OTHER HERETO HAVE BEEN INDUCED TO ENTER INTO THIS AGREEMENT AND THE TRANSACTIONS, AS APPLICABLE, BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 11.07.
SECTION 11.08 Headings. The descriptive headings contained in this Agreement are included for convenience of reference only and shall not affect in any way the meaning or interpretation of this Agreement.
SECTION 11.09 Counterparts. This Agreement may be executed and delivered (including executed manually or electronically via DocuSign or other similar services, and delivered by facsimile or portable document format (pdf) transmission) in one or more counterparts, and by the different Parties in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
SECTION 11.10 Specific Performance. The Parties agree that irreparable damage would occur if any provision of this Agreement were not performed in accordance with the terms hereof, and, accordingly, that the Parties shall be entitled to an injunction or injunctions to prevent breaches and threatened breaches of this Agreement or to enforce specifically the performance of the terms and provisions hereof (including the Parties’ obligation to consummate the Transactions) in any Chosen Court without proof of actual damages or otherwise, in addition to any other remedy to which they are entitled at law or in equity as expressly permitted in this Agreement. Each of the Parties hereby further waives (i) any defense in any action for specific performance that a remedy at law would be adequate and (ii) any requirement under any Law to post security or a bond as a prerequisite to obtaining equitable relief.
SECTION 11.11 Drafting of the Agreement. Each Party acknowledges that such Party has had the opportunity to participate in the drafting of this Agreement and to review this Agreement with legal counsel of its choice, and there shall be no presumption that ambiguities shall be construed or interpreted against the drafting Party, and no presumptions shall be made or inferences drawn because of the inclusion of a term not contained in a prior draft of this Agreement or the deletion of a term contained in a prior draft of the Agreement.
Annex A-72
Table of Contents
SECTION 11.12 Provision Respecting Legal Representation.
(a) It is acknowledged by each of the Parties, on its own behalf and on behalf of its respective Representatives and Affiliates, that the Company, the Company Subsidiaries and the Company Shareholders have retained Linklaters LLP (the “Retained Counsel”) to act as their counsel in connection with the Transactions and that the Retained Counsel has not acted as counsel for any other Party in connection with the Transactions and that none of the other Parties has the status of a client of the Retained Counsel for conflict of interest or any other purposes as a result thereof. SPAC hereby agrees, on their own behalf and on behalf of its Representatives and Affiliates, that, in the event that a dispute arises after the Closing between SPAC, the Company and/or their Subsidiaries, on the one hand, and any Company Shareholder and/or any of their respective Affiliates, on the other hand, the Retained Counsel may represent such Company Shareholder and/or their respective Affiliates in such dispute even though the interests of such Company Shareholder or their respective Affiliates may be directly adverse to SPAC, Holdco, the Company or their respective Subsidiaries.
(b) It is acknowledged by each of the Parties, on its own behalf and on behalf of its respective Representatives and Affiliates, that SPAC, Sponsor and their respective Affiliates have retained K&L Gates LLP (the “Other Retained Counsel”) to act as their counsel in connection with the Transactions and that the Other Retained Counsel has not acted as counsel for any other Party in connection with the Transactions and that none of the other Parties has the status of a client of the Other Retained Counsel for conflict of interest or any other purposes as a result thereof. Each Party hereby agrees, on their own behalf and on behalf of their respective Representatives and Affiliates, that, in the event that a dispute arises after the Closing between any Party and/or their Subsidiaries or their respective Affiliates, on the one hand, and Sponsor and/or any of its Affiliates, on the other hand, the Other Retained Counsel may represent Sponsor and/or its Affiliates in such dispute even though the interests of Sponsor and/or any of its Affiliates may be directly adverse to SPAC, Holdco, the Company or their respective Subsidiaries.
[Signature Page Follows.]
Annex A-73
Table of Contents
IN WITNESS WHEREOF, SPAC, the Company, Holdco and Merger Sub have caused this Agreement to be executed as of the date first written above by their respective officers thereunto duly authorized.
| | LIGHTJUMP ACQUISITION CORPORATION |
| | | | | |
| | | By | | /s/ Robert M. Bennett |
| | | Name: | | Robert M. Bennett |
| | | Title: | | Chief Executive Officer |
| | | | | |
| | | Moolec Science Limited |
| | | | | |
| | | By | | /s/ Gastón Paladini |
| | | Name: | | Gastón Paladini |
| | | Title: | | Co-Chairman and Chief Executive Officer |
| | | | | |
| | | MOOLEC ACQUISITION, INC. |
| | | | | |
| | | By | | /s/ Gastón Paladini |
| | | Name: | | Gastón Paladini |
| | | Title: | | President |
| | | | | |
| | | MOOLEC SCIENCE SA |
| | | | | |
| | | By | | /s/ Gastón Paladini |
| | | Name: | | Gastón Paladini |
| | | Title: | | Class A Director |
[Signature Page to Business Combination Agreement]
Annex A-74
Table of Contents
Exhibit A
Form of Registration Rights and Lock-Up Agreement
[Intentionally Omitted]
Annex A-75
Table of Contents
Exhibit B-1
Amended and Restated SPAC COI
[Intentionally Omitted]
Annex A-76
Table of Contents
Exhibit B-2
Amended and Restated SPAC Bylaws
[Intentionally Omitted]
Annex A-77
Table of Contents
Exhibit C
A&R Holdco Organizational Documents
[Intentionally Omitted]
Annex A-78
Table of Contents
Exhibit D
Form of SPAC Warrant Amendment and Assignment
[Intentionally Omitted]
Annex A-79
Table of Contents
Schedule A
Company Knowledge Parties
[Intentionally Omitted]
Annex A-80
Table of Contents
Appendix A
Illustrative Capitalization Table
[Intentionally Omitted]
Annex A-81
Table of Contents
Appendix B
Illustrative SPAC Transaction Expenses
[Intentionally Omitted]
Annex A-82
Table of Contents
Annex B
Fairness Opinion of Scura Partners

2925 Sugan Road
New Hope, PA 18938
October 15, 2022
CONFIDENTIAL
The Board of Directors
LightJump Acquisition Corporation
2735 Sand Hill Road
Menlo Park, CA 94025
Ladies and Gentlemen:
You have requested our opinion as to the fairness, from a financial point of view, to LightJump Acquisition Corp, a Delaware corporation, (the “SPAC”) of the “Consideration” (as defined below) to be paid pursuant to a certain Business Combination Agreement (the “Agreement”) dated June 14, 2022, to which the following are parties: the SPAC, Moolec Science Limited, a private limited company incorporated under the laws of England and Wales (the ”Company”), Moolec Science SA, a public limited liability company governed by the laws of the Grand Duchy of Luxembourg (“Holdco”), and Moolec Acquisition, Inc., a Delaware corporation (“Merger Sub”).
Upon the terms and subject to the conditions of the Agreement and those certain individual Contribution and Exchange Agreements, each dated as of June 14, 2022 (collectively, the “Exchange Agreements”), by and among Holdco, the Company and each of the Company Shareholders, and in accordance with the Companies Act 2006 (as amended from time to time, the “Companies Act”), the Luxembourg Law of 10 August 1915 on commercial companies (as amended from time to time, the “1915 Law”), and the Delaware General Corporation Law (as amended from time to time, the “DGCL”), SPAC, Holdco, Merger Sub and the Company agreed to enter into a business combination transaction pursuant to which, among other things, (a) pursuant to the Exchange Agreements, each of the Company Shareholders, effective on the Exchange Effective Time (as defined in the Agreement), agreed to contribute its respective Company Ordinary Shares to Holdco in exchange for Holdco Ordinary Shares to be subscribed for by each such Company Shareholder (such contributions and exchanges of Company Ordinary Shares for Holdco Ordinary Shares, collectively, the “Exchange”), (b) as a result of the Exchange, the Company will become a wholly-owned subsidiary of Holdco and the Company Shareholders will increase the number of issued and outstanding Holdco Ordinary Shares held by them, in accordance with the Exchange Agreements, (c) immediately prior to the consummation of the “Merger” (as hereinafter defined) , each of the Company SAFE Holders will receive and become holders of issued and outstanding Holdco Ordinary Shares, in accordance with the applicable Company SAFE, (d) following the consummation of the Exchange, Merger Sub will merge with and into the SPAC, with the SPAC surviving such merger and becoming a direct wholly-owned subsidiary of Holdco (the “Merger”) and, in the context of the Merger, all SPAC Common Stock outstanding will be exchanged with Holdco for the right to receive the Merger consideration in the form of Holdco Ordinary Shares pursuant to a share capital increase of Holdco, as set forth in the Agreement, and (e) in order to satisfy the Company’s obligations under that certain Consulting Agreement, dated June 18, 2021 (the “CFO Consulting Agreement”), by and between the Company and the Company’s Chief Financial Officer (“CFO”), CFO will be freely allotted an aggregate of 243,774 Holdco Ordinary Shares (the “CFO Free Shares”);
Upon the closing of the transactions (collectively, the “Transaction”) contemplated by the Agreement, the shares of the Company will be deemed to have a value of US$325 million (the “Consideration”).
You have also requested our opinion as to whether the fair market value of the Company equals or exceeds 80% of the amount held by the SPAC in trust for benefit of its public stockholders (excluding any deferred underwriting commissions and taxes payable on the income earned on the trust account).
Annex B-1
Table of Contents
In arriving at our opinion, we have, among other things: (i) reviewed certain internal information relating to the business, earnings, cash flow, assets and liabilities of the Company furnished to us by the SPAC; (ii) prepared financial models and discussed with the management of the SPAC and the management of the Company; (iii) reviewed certain internal information relating to expenses expected to result from the Transaction furnished to us by the SPAC; (iv) conducted discussions with members of the management and representatives of the SPAC and of the Company concerning the information described in clauses (i), (ii) and (iii); (v) reviewed the SPAC’s and the Company’s capital structure furnished to us by the management of the SPAC both on a standalone basis pre-Transaction and on a pro forma basis giving effect to the Transaction; (vi) reviewed publicly available financial and stock market data of certain other companies in lines of business that we deemed relevant; (vii) reviewed the Agreement; and (viii) conducted such other financial studies and analyses and took into account such other information as we deemed appropriate.
In connection with our review, we have, with your consent, relied on the information supplied to, discussed with or reviewed by us for purposes of this opinion being complete and accurate in all material respects. We have not assumed any responsibility for independent verification of, and have not independently verified, any of such information. With your consent, we have relied upon, without independent verification, the assessment of the SPAC and its legal, tax, regulatory and accounting advisors with respect to legal, tax, regulatory and accounting matters. In addition, we have relied, with your consent, on the assessments of the management of the SPAC as to the existing technology, products and services of the Company and the validity of, and risks associated with, the future technology, products and services of the Company. We have assumed, with your consent, that there will be no developments with respect to any of the foregoing that would affect our analyses or opinion. With your consent, we have assumed that (i) the Transaction does not include a minimum cash consideration, and (ii) any adjustments to the Consideration in accordance with the Agreement or otherwise would not be material to our analysis or this opinion. In addition, we have relied, with your consent, on the assessments of the management of the SPAC as to the SPAC’s ability to retain key employees of the Company. We express no views as to the reasonableness of any financial or other forecasts or the assumptions on which they are based. In addition, with your consent, we have not made any independent evaluation or appraisal of any of the assets or liabilities (contingent, derivative, off-balance-sheet, or otherwise) of the Company or the SPAC, nor have we been furnished with any such evaluation or appraisal.
Our opinion does not address the SPAC’s underlying business decision to effect the Transaction or the relative merits of the Transaction as compared to any alternative business strategies or transactions that might be available to the SPAC and does not address any legal, regulatory, tax, or accounting matters. At your direction, we have not been asked to, nor do we offer any opinion as to any terms of the Agreement or any aspect or implication of the Transaction, except for the fairness of the Consideration in the Transaction from a financial point of view to the SPAC and the fair market value of the Company.
We are not expressing any opinion as to fair value or the solvency of the Company or the SPAC following the closing of the Transaction. In rendering this opinion, we have assumed, with your consent, that the Agreement will not be modified or amended in any material respect, that the Transaction, including any proposed PIPE transaction, will be consummated in accordance with its terms without any waiver or modification that could be material to our analysis, and that the parties to the Agreement will comply with all the material terms of the Agreement. We have assumed, with your consent, that all governmental, regulatory, or other consents and approvals necessary for the completion of the Transaction will be obtained except to the extent that could not be material to our analysis. We also have not been requested to, and have not, participated in the structuring or negotiation of the Transaction.
Our opinion is necessarily based on economic, monetary, market and other conditions as in effect on, and the information made available to us as of, the date hereof, and we assume no responsibility to update this opinion for developments after the date hereof.
We have been engaged by the SPAC to render this opinion and will earn a fee upon delivery of this opinion, which fee is not contingent upon either the conclusion expressed in this opinion or the consummation of the Transaction. Our affiliates, employees, officers, and partners may at any time own securities (long or short) of the SPAC and the Company. In the future we may provide investment banking or other services to the SPAC, the Company or their respective affiliates and may receive compensation for such services.
Annex B-2
Table of Contents
This opinion does not constitute a recommendation as to how any holder of securities should vote or act with respect to the Transaction or any other matter. This opinion does not address the fairness of the Transaction or any aspect or implication thereof to, or any other consideration of or relating to, the holders of any class of securities, creditors, or other constituencies of the SPAC or the Company. In addition, we do not express any opinion as to the fairness of the amount or nature of any compensation to be received by any officers, directors, or employees of any parties to the Transaction, or any class of such persons, whether relative to the Consideration or otherwise. This opinion was approved by the Scura Partners LLC fairness opinion committee.
Based upon and subject to the foregoing, it is our opinion that, as the date hereof, (i) the Consideration in the Transaction is fair from a financial point of view to the SPAC and (ii) the fair market value of the Company equals or exceeds 80% of the amount held by the SPAC in trust for benefit of its public stockholders (excluding any deferred underwriting commissions and taxes payable on interest earned on the trust account).
Very truly yours,
/s/ Scura Partners LLC
Scura Partners LLC
Annex B-3
Table of Contents
Annex C
Proxy Card for Special Meeting
Preliminary Proxy Card
LightJump Acquisition Corporation
THIS PROXY IS SOLICITED BY THE BOARD OF DIRECTORS FOR THE SPECIAL MEETING OF STOCKHOLDERS TO BE HELD ON
[•], 2022
The undersigned, revoking any previous proxies relating to these shares, hereby acknowledges receipt of the Notice and Proxy Statement/Prospectus, dated [•], 2022, in connection with the Special Meeting of Stockholders to be held on [•], 2022 at a.m., local time, at, and hereby appoints, and each of them (with full power to act alone), the attorneys and proxies of the undersigned, with power of substitution to each, to vote all shares of the common stock of LightJump Acquisition Corporation (the “Corporation”) registered in the name provided, which the undersigned is entitled to vote at the Special Meeting of Stockholders, and at any adjournments thereof, with all the powers the undersigned would have if personally present. Without limiting the general authorization hereby given, said proxies are, and each of them is, instructed to vote or act as follows on the proposals set forth in this Proxy Statement/Prospectus.
PLEASE SIGN, DATE AND RETURN THE PROXY IN THE ENCLOSED ENVELOPE. THIS PROXY WILL BE VOTED IN THE MANNER DIRECTED HEREIN BY THE UNDERSIGNED STOCKHOLDER. IF NO DIRECTION IS MADE, THIS PROXY WILL BE VOTED “FOR” EACH OF THE PROPOSALS AND WILL GRANT DISCRETIONARY AUTHORITY TO VOTE UPON SUCH OTHER MATTERS AS MAY PROPERLY COME BEFORE THE MEETING OR ANY ADJOURNMENTS THEREOF. THIS PROXY WILL REVOKE ALL PRIOR PROXIES SIGNED BY YOU. THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” PROPOSALS [1 and 2].
Important Notice Regarding the Availability of Proxy Materials for the Special Meeting of Stockholders to be held on [•], 2022: This notice of meeting and the accompanying proxy statement/prospectus are available at [•].
(Continued and to be marked, dated and signed below)
| | Please mark vote as
indicated in this example ☒ |
LightJump Acquisition Corporation — THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR”
PROPOSALS 1 AND 2.
| | FOR | | AGAINST | | ABSTAIN |
Proposal 1 — The Business Combination Proposal | | ☐ | | ☐ | | ☐ |
To consider and vote upon a proposal to approve and adopt the Business Combination Agreement, dated as of June 14, 2022, as may be amended, by and among LightJump, Moolec, Holdco and Merger Sub and the transactions contemplated thereby, and the Business Combination. | | | | | | |
| | | FOR | | AGAINST | | ABSTAIN |
Proposal 2 — Stockholder Adjournment Proposal | | ☐ | | ☐ | | ☐ |
To consider and vote upon a proposal to adjourn the special meeting of stockholders to a later date or dates, if necessary, to permit further solicitation and vote of proxies if, based upon the tabulated vote at the time of the special meeting of stockholders, there are not sufficient votes to approve one or more proposals presented to stockholders for vote or the LightJump Holders, as defined in the proxy statement/prospectus, [have elected to redeem an amount of the SPAC Common Stock such that the minimum available cash condition to the obligation to closing of the Business Combination would not be satisfied.] | | | | | | |
| | Dated [•], 2022 |
| | |
Stockholder’s Signature | | Stockholder’s Signature |
Signature should agree with name printed hereon. If stock is held in the name of more than one person, EACH joint owner should sign. Executors, administrators, trustees, guardians, and attorneys should indicate the capacity in which they sign. Attorneys should submit powers of attorney.
Annex C-1
Table of Contents
Part II
Information not required in Prospectus
Item 20. Indemnification of Directors and Officers
Article 441-8 of the 1915 Law provides that the directors shall not incur any personal obligation by reason of the commitments of the company.
Article 441-9 of the 1915 Law provides that the directors, the members of the management committee and the managing executive officer shall be liable to the company in accordance with general law for the execution of the mandate given to them and for any misconduct in the management of the company’s affairs. The directors and members of the management committee shall be jointly and severally liable towards either the company or any third parties for damages resulting from this violation of the 1915 Law or the company’s articles of association. The directors and members of the management committee shall be discharged from such liability in the case of a violation to which they were not a party provided no misconduct is attributable to them and they have reported such violation, as regards members of the board of directors, to the first general meeting and, as regards members of the management committee, during the first meeting of the board of directors after they had acquired knowledge thereof.
Holdco’s articles of association, which will become effective immediately prior completion of the Business Combination, will provide that directors of Holdco are not held personally liable for the indebtedness or other obligations of Holdco. As agents of Holdco, they are responsible for the performance of their duties. Subject to the exceptions and limitations listed in Holdco’s articles of association and mandatory provisions of law, every person who is, or has been, a member of the board of directors or officer (mandataire) of Holdco shall be indemnified by Holdco to the fullest extent permitted by law against liability and against all expenses reasonably incurred or paid by him in connection with any claim, action, suit or proceeding which he becomes involved as a party or otherwise by virtue of his or her being or having been a director or officer of Holdco, and against amounts paid or incurred by him or her in the settlement thereof. The words “claim”, “action”, “suit” or “proceeding” shall apply to all claims, actions, suits or proceedings (civil, criminal or otherwise including appeals) actual or threatened and the words “liability” and “expenses” shall include without limitation attorneys’ fees, costs, judgments, amounts paid in settlement and other liabilities. However, no indemnification shall be provided to any director or officer of Holdco (i) against any liability by reason of wilful misfeasance, bad faith, gross negligence or reckless disregard of the duties involved in the conduct of his or her office (ii) with respect to any matter as to which he or she shall have been finally adjudicated to have acted in bad faith and not in the interest of Holdco or (iii) in the event of a settlement, unless the settlement has been approved by a court of competent jurisdiction or by the board of directors of Holdco.
Holdco’s articles of association will provide that the right of indemnification provided by such articles of association shall be severable, shall not affect any other rights to which any director or officer may now or hereafter be entitled, shall continue as to a person who has ceased to be such director or officer and shall inure to the benefit of the heirs, executors and administrators of such a person. Nothing contained in such articles of association shall affect or limit any rights to indemnification to which corporate personnel, including directors and officers, may be entitled by contract or otherwise under law. Holdco shall specifically be entitled to provide contractual indemnification to and may purchase and maintain insurance for any corporate personnel, including directors and officers of Holdco, as Holdco may decide upon from time to time.
II-1
Table of Contents
Item 21. Exhibits and Financial Statement Schedules
Exhibit
Number | | Description |
2.1# | | Business Combination Agreement, dated as of June 14, 2022, by and among LightJump Acquisition Corporation, Moolec Science Limited, Moolec Science SA and Moolec Acquisition, Inc. (included as Annex A to the proxy statement/prospectus) |
3.1* | | Articles of Association of Moolec Science SA |
3.2* | | Form of Amended and Restated Articles of Moolec Science SA |
3.3 | | Amended and Restated Certificate of Incorporation of LightJump Acquisition Corporation dated January 12, 2021 (incorporated by reference to Exhibit 3.1 to LightJump Acquisition Corporation’s Form 8-K, File No. 001-39869, filed with the SEC on January 20, 2021). |
3.4 | | Amendments to the Amended and Restated Certificate of Incorporation of LightJump Acquisition Corporation dated July 11, 2022 (incorporated by reference to Exhibit 3.1 to LightJump Acquisition Corporation’s Form 8-K, File No. 001-39869, filed with the SEC on July 13, 2022). |
4.1* | | Specimen Ordinary Share Certificate of Moolec Science SA |
4.2 | | Warrant Agreement, dated January 12, 2021, by and between LightJump Acquisition Corporation and Continental Stock Transfer & Trust Company, as warrant agent (incorporated by reference to Exhibit 4.1 to LightJump Acquisition Corporation’s Form 8-K, File No. 001-39869, filed with the SEC on January 20, 2021). |
4.3 | | Subscription Agreement for Private Warrants by LightJump One Founders, LLC (incorporated by reference to Exhibit 10.3 to LightJump Acquisition Corporation’s Form 8-K, File No. 001-39869, filed with the SEC on January 20, 2021). |
4.4 | | Form of Assignment, Assumption and Amendment Agreement with respect to the Warrant Agreement between LightJump Acquisition Corporation, Moolec Science SA and Continental Stock Transfer & Trust Company (incorporated by reference to Exhibit 10.4 to LightJump Acquisition Corporation’s Form 8-K, File No. 001-39869, filed with the SEC on June 15, 2022). |
4.5* | | Specimen Warrant Certificate of Moolec Science S.A. |
5.1* | | Legal Opinion of Linklaters LLP |
8.1 | | Form of Tax Opinion of K&L Gates LLP |
10.1 | | Form of Exchange Agreement (incorporated by reference to Exhibit 10.1 to LightJump Acquisition Corporation’s Form 8-K, File No. 001-39869, filed with the SEC on June 15, 2022). |
10.2 | | Transaction Support Agreement, dated as of June 14, 2022 by and among LightJump Acquisition Corporation, LightJump One Founders, LLC, Moolec Science Limited, Moolec Science SA and others (incorporated by reference to Exhibit 10.3 to LightJump Acquisition Corporation’s Form 8-K, File No. 001-39869, filed with the SEC on June 15, 2022). |
10.3 | | Registration Rights Agreement, dated as of January 12, 2021, by and between LightJump Acquisition Corporation and other investors ((incorporated by reference to Exhibit 10.2 to LightJump Acquisition Corporation’s Form 8-K, File No. 001-39869, filed with the SEC on January 20, 2021) |
10.4 | | Form of Registration Rights and Lock-Up Agreement (incorporated by reference to Exhibit 10.5 to LightJump Acquisition Corporation’s Form 8-K, File No. 001-39869, filed with the SEC on June 15, 2022). |
10.6 | | Backstop Agreement (incorporated by reference to Exhibit 10.2 to LightJump Acquisition Corporation’s Form 8-K, File No. 001-39869, filed with the SEC on June 15, 2022) |
10.7 | | Amended to Business Combination Marketing Agreement, dated June 14, 2022 between LightJump Acquisition Corporation and EarlyBirdCapital, Inc.(incorporated by reference to Exhibit 10.6 to LightJump Acquisition Corporations’s Form 8-K, File No. 001-39869, filed with the SEC on June 15, 2022) |
10.8 | | Consulting Agreement, dated as of June 18, 2021 by and between Moolec Science Limited and Jose López Lecube. |
10.9 | | Employment Letter Agreement, dated as of July 15, 2022 by and between Moolec Science Limited and Jose López Lecube |
21.1* | | List of Subsidiaries of Moolec Science SA |
23.1 | | Consent of Price Waterhouse & Co. S.R.L. |
23.2 | | Consent of Marcum LLP |
II-2
Table of Contents
Item 22. Undertakings
(A) Moolec Science SA hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, as amended, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
II-3
Table of Contents
(4) To file a post-effective amendment to the registration statement to include any financial statements required by Item 8.A of Form 20-F at the start of any delayed offering or throughout a continuous offering.
(5) For purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(B) Moolec Science SA hereby undertakes:
(1) that prior to any public reoffering of the securities registered hereunder through use of a prospectus which is a part of this registration statement, by any person or party who is deemed to be an underwriter within the meaning of Rule 145(c), that such reoffering prospectus will contain the information called for by the applicable registration form with respect to reofferings by persons who may be deemed underwriters, in addition to the information called for by the other items of the applicable form.
(2) That every prospectus: (i) that is filed pursuant to paragraph (1) immediately preceding, or (ii) that purports to meet the requirements of Section 10(a)(3) of the Act and is used in connection with an offering of securities subject to Rule 415, will be filed as a part of an amendment to the registration statement and will not be used until such amendment is effective, and that, for purposes of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(C) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(D) The undersigned registrant hereby undertakes (i) to respond to requests for information that is incorporated by reference into the prospectus pursuant to Items 4, 10(b), 11, or 13 of this Form, within one Business Day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This includes information contained in documents filed subsequent to the effective date of the registration statement through the date of responding to the request.
(E) The undersigned registrant hereby undertakes to supply by means of a post-effective amendment all information concerning a transaction and the company being acquired involved therein, that was not the subject of and included in the registration statement when it became effective.
II-4
Table of Contents
Signatures
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Rosario, Province of Santa Fe, Argentina on October 17, 2022.
| | Moolec Science SA |
| | | By: | | Gastón Paladini |
| | | Title: | | Class A Director |
Power of Attorney
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
Signature | | Title | | Date |
/s/ Gastón Paladini | | Class A Director | | October 17, 2022 |
| | | | | |
| | | | | |
/s/ Joost Anton Mees | | Class B Director | | October 17, 2022 |
| | | | | |
| | | | | |
II-5
Table of Contents
Authorized Representative
Pursuant to the requirements of Section 6(a) of the Securities Act of 1933, as amended, this registration statement on Form F-4 has been signed on behalf of the registrant by the undersigned, solely in his capacity as the duly authorized representative of the registrant in the United States, on October 17, 2022.
| | COGENCY GLOBAL INC. |
| | | By: | | /s/ Colleen A. De Vries |
| | | Name: | | Colleen A. De Vries |
| | | Title: | | Sr. Vice President on behalf of
Cogency Global Inc. |
II-6








