THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (“SEC”) DOES NOT PASS UPON THE MERITS OF OR GIVE ITS APPROVAL TO ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SELLING LITERATURE. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION; HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THIS INVESTMENT INVOLVES A DEGREE OF RISK THAT MAY NOT BE SUITABLE FOR ALL PERSONS. ONLY THOSE INVESTORS WHO CAN BEAR THE LOSS OF A SIGNIFICANT PORTION OF THEIR INVESTMENT SHOULD PARTICIPATE IN THE INVESTMENT. (SEE “RISK FACTORS” BELOW.)
Offering Circular
For
Starpax Biopharma Inc.
A Canadian Corporation
February 8, 2023
SECURITIES OFFERED : Equity in the form of 4,000,000 Common Shares
PRICE PER SHARE : $6.25 per Common Share
MAXIMUM OFFERING AMOUNT : $25,000,000.00
MINIMUM OFFERING AMOUNT : Not Applicable (No Minimum Offering Amount)
MINIMUM INVESTMENT : $500.00
CONTACT INFORMATION :
2500-1000 boul. René-Lévesque West
Montréal, Québec, Canada H3B 5C9
PHONE (514) 427-3004
Starpaxbiopharma.com
(Please request President and CEO, Michael Gareau)
Generally, no sale may be made to you in this Offering if the aggregate purchase price you pay is more than ten (10%) percent of the greater of your annual income or net worth. Different rules apply to accredited investors and non-natural persons. Before making any representation that your investment does not exceed applicable thresholds, Investors are encouraged to review rule 251(d)(2)(i)(C) of Regulation A. For general information on investing, Investors are encouraged to refer to www.investor.gov.
Starpax Biopharma Inc. (the “Company” or the “Issuer”), a biopharmaceutical research and development company, has conceived an unprecedented platform technology intended to treat cancer using its living self-propelled Starpax Magnetodrones that are sensitive to magnetic fields and transport anticancer drugs attached to their surface. The Magnetodrones are injected directly into the tumor. The trajectory is controlled by the Starpax PolarTrak that generates precision 3D guidance monopole magnetic field vectors in order to keep them captive in a tumor and force them to spread and release the anticancer drug throughout the volume of the tumor without circulating in the blood system, avoiding side effects usually resulting from systemic cancer treatments.
The Company was founded as Starpax Medical Inc. on December 19, 2017, under the laws of Québec, Canada. The Company changed its name to Starpax Biopharma Inc. on June 9, 2022 (See Exhibit 2A “Articles of Consolidation and Other Corporate Documents”).
The Company is run by a board of directors, comprised of seven (7) directors (the “Board” collectively, “Director” when referring to a director). The day-to-day management of the Company is vested in the officers of the Company (the “Officers”). The Board of Directors oversees the corporate conduct and represents the interests of the Company’s shareholders. It provides guidance and advice to the CEO and the executive team.
The share capital of the Company is made up of an unlimited number of common shares (the “Common Shares” or the “Shares”). The minimum investment amount per Investor is Five Hundred Dollars ($500.00), representing 80 Common Shares at Six Dollars and Twenty-Five Cents ($6.25) per Share.
Sales of the Shares pursuant to the Offering will commence immediately upon qualification of the Offering by the Securities and Exchange Commission (the “Effective Date”) and will terminate at the discretion of the Board or twelve (12) months following the Effective Date, whichever is earlier. The maximum amount of the Offering shall not exceed Twenty-Five Million Dollars ($25,000,000) in any twelve (12) month period (“Maximum Offering Amount”) in accordance with Tier II of Regulation A as set forth under the Securities Act of 1933, as amended, (“Reg A Tier II” or “Tier II”). The Company intends to offer the Shares described herein on a continuous and ongoing basis pursuant to Rule 251(d)(3)(i)(F). Further, the acceptance of Investor subscriptions, may be briefly paused at times to allow the Company to effectively and accurately process and settle subscriptions that have been received. (See “Summary of the Offering” below.) The Company may increase the Maximum Offering Amount at its sole and absolute discretion, subject to qualification by the SEC of a post-qualification amendment.
Prior to this Offering, there has been no public market for the Shares, and none is expected to develop for the foreseeable future, however, the Company reserves the right to list the Shares on an exchange in the future. The Offering price does not bear any relationship to the value of the assets of the Company. Investing in the Company through the purchase of Shares involves risks, some of which are set forth below. See the section titled “Risk Factors” to read about the factors an Investor should consider prior to purchasing Shares.
Investors who purchase Shares will become shareholders of the Company (“Investors” or “Shareholders” subject to the terms of the Articles of Consolidation and the Bylaws of the Company (see Exhibit 2A Articles of Consolidation and Other Corporate Documents” and Exhibit 2B “Bylaws”) once the Company deposits the Investor’s investment into the Company’s main
operating account and the Investor is properly registered in the share register held on its behalf by its registrar and transfer agent.
The Directors and Officers will receive compensation from the Company (see “Risk Factors” below starting on Page 4, and “Compensation of Directors and Officers” below). Investing in the Shares is speculative and involves substantial risks, including the risk of complete loss. Prospective Investors should purchase these securities only if they can afford a complete loss of their investment (see “Risk Factors” below).
As of the date of this Offering Circular, the Company has engaged KoreConX as registrar and transfer agent in relation to this Offering. The Company has engaged North Capital as escrow agent for this Offering.
NO PERSON HAS BEEN AUTHORIZED IN CONNECTION WITH THIS OFFERING TO GIVE ANY INFORMATION OR TO MAKE ANY REPRESENTATIONS OTHER THAN THAT INFORMATION AND THOSE REPRESENTATIONS SPECIFICALLY CONTAINED IN THIS OFFERING CIRCULAR; ANY OTHER INFORMATION OR REPRESENTATIONS SHOULD NOT BE RELIED UPON. ANY PROSPECTIVE PURCHASER OF THE SECURITIES WHO RECEIVES ANY OTHER INFORMATION OR REPRESENTATIONS SHOULD CONTACT THE COMPANY IMMEDIATELY TO DETERMINE THE ACCURACY OF SUCH INFORMATION AND REPRESENTATIONS. NEITHER THE DELIVERY OF THIS OFFERING CIRCULAR NOR ANY SALES HEREUNDER SHALL, UNDER ANY CIRCUMSTANCES, CREATE AN IMPLICATION THAT THERE HAS BEEN NO CHANGE IN THE AFFAIRS OF THE COMPANY OR IN THE INFORMATION SET FORTH HEREIN SINCE THE DATE OF THIS OFFERING CIRCULAR SET FORTH ABOVE.
THE INFORMATION CONTAINED IN THIS OFFERING CIRCULAR HAS BEEN SUPPLIED BY THE COMPANY. THIS OFFERING CIRCULAR CONTAINS SUMMARIES OF DOCUMENTS NOT CONTAINED IN THIS OFFERING CIRCULAR, BUT ALL SUCH SUMMARIES ARE QUALIFIED IN THEIR ENTIRETY BY REFERENCES TO THE ACTUAL DOCUMENTS. COPIES OF DOCUMENTS REFERRED TO IN THIS OFFERING CIRCULAR, BUT NOT INCLUDED AS AN EXHIBIT, WILL BE MADE AVAILABLE TO QUALIFIED PROSPECTIVE INVESTORS UPON REQUEST.
RULE 251(D)(3)(I)(F) DISCLOSURE. RULE 251(D)(3)(I)(F) PERMITS REGULATION A OFFERINGS TO CONDUCT ONGOING CONTINUOUS OFFERINGS OF SECURITIES FOR MORE THAN THIRTY (30) DAYS AFTER THE QUALIFICATION DATE IF: (1) THE OFFERING WILL COMMENCE WITHIN TWO (2) DAYS AFTER THE QUALIFICATION DATE; (2) THE OFFERING WILL BE MADE ON A CONTINUOUS AND ONGOING BASIS FOR A PERIOD THAT MAY BE IN EXCESS OF THIRTY (30) DAYS OF THE INITIAL QUALIFICATION DATE; (3) THE OFFERING WILL BE IN AN AMOUNT THAT, AT THE TIME THE OFFERING CIRCULAR IS QUALIFIED, IS REASONABLY EXPECTED TO BE OFFERED AND SOLD WITHIN ONE (1) YEAR FROM THE INITIAL QUALIFICATION DATE; AND (4) THE SECURITIES MAY BE OFFERED AND SOLD ONLY IF NOT MORE THAN THREE (3) YEARS HAVE ELAPSED SINCE THE INITIAL QUALIFICATION DATE OF THE OFFERING, UNLESS A NEW OFFERING CIRCULAR IS SUBMITTED AND FILED BY THE COMPANY PURSUANT TO RULE 251(D)(3)(I)(F) WITH THE SEC COVERING THE REMAINING SECURITIES OFFERED UNDER THE PREVIOUS OFFERING; THEN THE SECURITIES MAY CONTINUE TO BE OFFERED AND SOLD UNTIL THE EARLIER OF THE QUALIFICATION DATE OF THE NEW OFFERING CIRCULAR OR THE ONE HUNDRED EIGHTY (180) CALENDAR DAYS AFTER THE THIRD ANNIVERSARY OF THE INITIAL QUALIFICATION DATE OF THE PRIOR OFFERING CIRCULAR. THE COMPANY INTENDS TO OFFER THE SHARES DESCRIBED HEREIN ON A CONTINUOUS AND ONGOING BASIS PURSUANT TO RULE 251(D)(3)(I)(F). THE COMPANY INTENDS TO COMMENCE THE OFFERING IMMEDIATELY AND NO LATER THAN TWO (2) DAYS FROM THE INITIAL QUALIFICATION DATE. THE COMPANY REASONABLY EXPECTS TO OFFER AND SELL THE SECURITIES STATED IN THIS OFFERING CIRCULAR WITHIN ONE (1) YEAR FROM THE INITIAL QUALIFICATION DATE.
There are no selling shareholders within this Offering.
The Company will commence sales of the Shares immediately upon qualification of the Offering by the SEC. The Company approximates that sales will commence during Q1 – 2023.
| | | Price to Public* | | Commissions** | | Proceeds to Other Persons | | Proceeds to the Company |
| Amount to be Raised per Share | | $ | 6.25 | | | $ | 0.1875 | | | $ | 0.00 | | | $ | 6.0625 | |
Minimum Investment
Amount | | $ | 500.00 | | | $ | 15.00 | | | $ | 0.00 | | | $ | 485.00 | |
| Minimum Offering Amount | | | Not Applicable | | | | Not Applicable | | | | Not Applicable | | | | Not Applicable | |
| Maximum Offering Amount | | $ | 25,000,000 | | | $ | 750,000 | | | $ | 0.00 | | | $ | 24,250,000 | |
*The Board of Directors and management set the Share price based on public market data, Company forecasts, valuation of comparable pre-revenue pharmaceutical companies in preclinical stage, consultations with experts, as well as the price per Share of recent private offerings from the Company.
** The Company is not using an underwriter for the sale of Shares. These commissions listed are those for Justly Markets, a FINRA broker-dealer. Justly Markets is entitled to 1% on all passive sales of securities as placement agent. If securities are sold through the efforts of Justly Markets, 5% will be due to Justly Markets (instead of 1%) up to a maximum of $500,000– for potential maximum commissions of $750,000. The commissions due to Justly Markets are conditional on the services provided by Justly Markets with respect to any one sale. See “Plan of Distribution” below.
FORWARD LOOKING STATEMENTS
Investors should not rely on forward-looking statements in this Offering Circular because they are inherently uncertain. This Offering Circular contains forward-looking statements that involve risks and uncertainties. The use of words such as “anticipated”, “projected”, “forecasted”, “estimated”, “prospective”, “believes”, “expects,” “plans”, “future”, “intends”, “by design”, “designed”, “should”, “can”, “could”, “might”, “potential”, “continue”, “may”, “will”, and similar expressions identify these forward-looking statements. Investors should not place undue reliance on these forward-looking statements, which may apply only as of the date of this Offering Circular, and the Company undertakes no obligation to publicly update or revise any forward-looking information, other than as required by applicable law.
TABLE OF CONTENTS
SUMMARY OF THE OFFERING
The following information is only a brief summary of, and is qualified in its entirety by, the detailed information appearing elsewhere in this Offering Circular. This Offering Circular, together with the exhibits attached including, but not limited to, the Amended Articles of Incorporation and Company Bylaws, copies of which are attached hereto as Exhibit 2A and Exhibit 2B, respectively, and should be carefully read in their entirety before any investment decision is made. If there is a conflict between the terms contained in this Offering Circular and these documents, Amended Articles of Incorporation and Bylaws shall prevail and control, and no Investor should rely on any reference herein to the Amended Articles of Incorporation or Bylaws without consulting the actual underlying documents.
| COMPANY INFORMATION | | Starpax Biopharma Inc. has a principal place of business located at 6615 Abrams Street,
Montréal, Québec, Canada H4S 1V9. |
| MANAGEMENT | | The Company is managed by a Board of Directors. The Board is comprised of seven (7) Directors. The Company has five (5) Officers who manage the day-to-day operations. See “Risk Factors” and “Directors, Officers, and Significant Employees” below. |
| THE OFFERING | | This Offering is the first capital raise by the Issuer under Regulation A. The Company is selling Company equity in the form of Common Shares through this Offering. The Company will use the Proceeds of this Offering to execute Phase I and Phase II clinical trials regarding the Starpax Technology. See “Use of Proceeds” below. |
| SECURITIES BEING OFFERED | | The Shares are being offered at a purchase price of $6.25 per Share. The Minimum Investment Amount for any Investor is $500.00. Upon purchase of the Shares and becoming a Shareholder of the Company, a Shareholder is granted the right to vote, receive all dividends declared, and share the remaining property of the Company upon its liquidation. See “Description of the Securities” below. |
| COMPENSATION TO DIRECTORS | | The Company compensates the Directors and Officers with salaries and/or stock options for their roles as Directors and Officers. For more information on this compensation see “Compensation of the Director and Officers” section below. The Directors, Officers, and employees of the Company will not be compensated through commissions for the sale of the Shares through this Offering. |
| PRIOR EXPERIENCE OF COMPANY MANAGEMENT | | The Company is overseen by a seven-person, highly experienced Board of Directors, which includes three retired presidents of divisions of large pharmaceutical companies (Johnson & Johnson France, Pfizer Canada, Eli Lilly Canada), a retired managing director and chief operating officer of J.P. Morgan, EMEA Investment Bank (London, UK), and the former president and managing director of a high-performing private equity fund. |
| INVESTOR SUITABILITY STANDARDS | | The Shares will not be sold to any person unless they are a “Qualified Purchaser”. A Qualified Purchaser includes: (1) an “Accredited Investor” as that term is defined in Rule 501(a) of Regulation D promulgated under the Securities Act of 1933 (the “Securities Act”); or (2) all other Investors who meet the investment limitations set forth in Rule 251(d)(2)(i)(C) of Regulation A. Such persons as stated in (2) above must conform with the “Limitations on Investment Amount” as described in this Summary below. Each person acquiring Shares may be required to represent that he, she, or it is purchasing the Shares for his, her, or its own account for investment purposes and not with a view to resell or distribute the securities.
Each prospective purchaser of Shares may be required to furnish such information or certification as the Company may require in order to determine whether any person or entity purchasing Shares is an Accredited Investor if such is claimed by the Investor. |
| LIMITATIONS ON INVESTMENT AMOUNT | | For Qualified Purchasers who are Accredited Investors, there is no limitation as to the amount invested through the purchase of Shares. For non-Accredited Investors, the aggregate purchase price paid to the Company for the purchase of the Shares cannot be more than 10% of the greater of the purchaser’s (1) annual income or net worth as determined under Rule 501(a) of Regulation D, if purchaser is a natural person; or (2) revenue or net assets for the purchaser’s most recently completed fiscal year if purchaser is a non- natural person. Different rules apply to Accredited Investors and non-natural persons. Each Investor should review Rule 251(d)(2)(i)(C) of Regulation A as determined under Rule 501(a) of Regulation D before purchasing the Shares. |
| NON-FOREIGN SALES OF SECURITIES | | The Company will only sell the Shares offered through this Offering to United States investors. |
| COMMISSIONS FOR SELLING SHARES | | The Shares will be offered and sold directly by the Company, the Board, the Officers, and Company’s employees. No commissions for selling the Shares will be paid to the Company, the Board, the Officers, or the Company’s employees. The Company is not using an underwriter for the sale of Shares. These commissions listed are those for Justly Markets, a FINRA broker- dealer. Justly Markets is entitled to 1% on all passive sales of securities as placement agent. If securities are sold through the efforts of Justly Markets, 5% will be due to Justly Markets (instead of 1%) up to a maximum of$500,000 – for potential maximum commissions of $750,000. The commissions due to Justly Markets are conditional on the services provided by Justly Markets with respect to any one sale. See “Plan of Distribution” below. |
| NO LIQUIDITY | | The Company does not currently have plans to list any Shares on any securities market or exchange, however, the Company reserves the right to list the Shares on an exchange in the future. Additionally, the Shares will be transferable, in accordance with Federal and state securities laws, and Canadian law. However, the Shares will not be listed for trading on any exchange or automated quotation system. (See “Description of the Securities” below.) Prospective Investors are urged to consult their own legal advisors with respect to secondary trading of the Shares. See “Risk Factors” below. |
| COMPANY EXPENSES | | Except as otherwise provided herein, the Company shall bear all costs and expenses associated with the Offering, the operation of the Company, including, but not limited to, the annual preparation of the Company's tax returns, any state and federal income tax due, accounting fees, filing fees, independent audit reports, other costs and expenses, and other advisory fees. |
The Company currently develops several technologies, the purposes of which are to treat cancers in humans.
Defined terms used in this Offering Circular:
The Starpax cancer treatment technology consists of two major elements that cannot be dissociated:
1) The first element consists of living self-moving Magnetodrones sensitive to magnetic fields swimming in the tumor without circulating in the blood system and transporting the therapeutic anticancer agent attached to their surface. The Magnetodrones are injected directly into the tumor. This element will be referred to throughout this Offering Circular as “Magnetodrones” without notification to the reader that Magnetodrones is a trademark of the Company by use of the “TM” symbol.
2) The second element is a medical device in which the patient is installed (the Starpax PolarTrak) that generates precision 3D guidance monopole magnetic field vectors in order to keep the Magnetodrones captive inside the tumor in order for the Magnetodrones not to escape to the rest of the body and forces them to distribute in three dimensions throughout the tumor volume including hypoxic zones where the stem cells are located. This element will be referred to throughout this Offering Circular as “PolarTrak” without notification to the reader that PolarTrak is a trademark of the Company by use of the “TM” symbol.
This Offering Circular will refer to “anti-cancer drug”, therapeutic agent”, “drug”, “therapeutic anti-cancer drug”, or some derivation or combination of these terms to mean the pharmaceutical attached to the Magnetodrones whose sole purpose is to kill the cancerous cells in a tumor.
“Starpax Dose” refers to the dose given to patients containing the Magnetodrones already combined with a therapeutic agent.
“GMP” refers to Good Manufacturing Practices. GMP are a set of regulations set by the relevant authorities, in this case Health Canada and/or the FDA, that regulate manufacturers, packagers, labelers, testers, distributors, importers, wholesalers of drugs. These regulations dictate the standards and processes required for facilities that handle drugs in this way. The Company and its facilities are currently regulated by GMP.
“NDA refers to a New Drug Application with the Food and Drug Administration.
“IND” refers to an Investigational New Device with the Food and Drug Administration.
“BLA” refers to Biologics License Applications and the associated process with the Food and Drug Administration.
“CDER” refers to Center for Drug Evaluation and Research at the Food and Drug Administration.
“PMA” refers to the Premarket Approval which is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices
“De Novo” refers to De Novo request to the FDA providing a marketing pathway to classify novel medical devices for which general controls alone, or general and special controls, provide reasonable assurance of safety and effectiveness for the intended use, but for which there is no legally marketed predicate device.
“510k” refers to the 510(k) process with the FDA which is a premarket submission made to FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, to a legally marketed device (section 513(i)(1)(A) FD&C Act) that is not subject to premarket approval.
“product candidate(s)” refers to the Company products that have applications with the Food and Drug Administration, which require clinical trials for ultimate approval or clearance by the Food and Drug Administration. Current product candidates of the Company include the Magnetodrones combined with SN38. See below.
“Starpax Care Center(s)” or “Starpax Cancer Center(s)” refers to the Company-owned centers where the Starpax Treatments will be administered.
“3D” refers to three dimensions in space.
Collectively, the Magnetodrones and PolarTrak, and other associated technologies will be referred to as the “Starpax Technology” or “Technology.”
“Starpax Treatment” and “Starpax Cancer Treatment” – refers to the utilization of the Starpax Technology, including the Magnetodrones and PolarTrak to treat cancers in humans. These terms may be used to refer to a single treatment or the collective treatments over a given time period.
On January 19, 2023, the Company amended its Articles of Incorporation to consolidate Class A and B Common Shares into a single class of common stock. Throughout this document, any reference to “Class A Common Shares” or “Class B Common Shares” is referring to shares of the Company’s common stock prior to January 19, 2023. “Common Shares” or the “Shares” will refer to the Shares of common stock after January 19, 2023, as being offered through this Offering.
All results, assertions, deductions, technical, and/or scientific or medical forward-looking claims by Starpax in this document are based either on independent 3rd party preclinical work conducted at reputable Canadian 1st-tier research institutions, good laboratory practice (“GLP”) certified labs, clinical research organization, papers from the scientific literature or by design intended use of the technology on humans.
The Starpax Technology is currently an investigational product and is not yet approved for commercial use.
RISK FACTORS
IN INVESTING IN STARPAX, INVESTORS FACE RISKS THAT ARE SIMILAR TO ANY OTHER INVESTMENT IN A BIOPHARMACEUTICAL, BIOTHECHNOLOGY, OR ANY OTHER KIND OF MANUFACTURING COMPANIES. THE RISK FACTORS LISTED IN THIS SECTION MAY NOT BE EXHAUSTIVE AS MANY RISKS MAY STILL BE UNKNOWN AS OF THE DATE OF THIS OFFERING CIRCULAR. THE COMPANY MAKES NO REPRESENTATION AS TO THE RELEVANCY OR COMPLETENESS OF THESE RISK FACTORS AND INVESTORS SHOULD CONSULT WITH INVESTOR’S LEGAL, ACCOUNTING, AND OTHER ADVISORS WHEN MAKING AN INVESTMENT DECISION.
The Company may continue to be impacted by the COVID-19 pandemic.
As a result of the pandemic, the Company has at times experienced delays in reception of manufacturing equipment, shortage of qualified workers, reduced access to essential workers at expected clinical sites and costs increases for raw material, wages or other purchases. The Company might be affected by shortages of raw materials, parts and other supplies and there can be no assurance that the Company will be able to avoid supply chain shortages in the future. The risk exists that further COVID-19 developments may negatively impact operations if the Company should suffer supply chain shortages, absenteeism of workers or facility shutdowns due to the pandemic or clinical site and external laboratories access restrictions. Governmental restrictions, including travel restrictions, quarantines, shelter-in-place orders, business closures, new safety requirements or regulations, or restrictions on the import or export of certain materials, or other operational issues related to the COVID-19 pandemic may have an adverse effect on business and results of operations. The evolving nature of the circumstances is such that it is impossible, at this stage, to determine the full and overall impact the COVID-19 pandemic may have, but it could further disrupt production and cause delays in the supply and delivery of investigational products used in clinical trials, adversely affect employees and disrupt operations and manufacturing activities, interrupt clinical trials, all of which may have a material adverse effect on business. The Company has ascertained that certain risks associated with further COVID-19 developments may adversely impact operations and liquidity, and business and share price may also be affected by the COVID-19 pandemic. Due to the general unknown nature surrounding the pandemic, the Company cannot reasonably estimate the potential for any future impacts on operations or liquidity.
Risks related to Operations
The Company’s past experience may not be indicative of future performance.
The Company faces many risks. Past experience may not be indicative of future performance, and the Company has included forward-looking statements about business, plans and prospects that are subject to change. Forward-looking statements are particularly located in, but not limited to, the sections “Description of the Business” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” In addition to the other risks or uncertainties, the risks described below may affect operating results, financial condition, and cash flows. If any of these risks occur, either alone or in combination with other factors, the business, financial condition, or operating results could be adversely affected, and the fair market value of the Shares may decline. The Company has limited operating history and future results are uncertain. Moreover, readers should note that this is not an exhaustive list of the risks the Company faces; some risks are unknown or not quantifiable, and other risks that the Company currently perceives as immaterial may ultimately prove more significant than expected. Statements about plans, predictions or expectations should not be construed to be assurances of performance or promises to take a given course of action.
The Company is in its research and development phase and has never generated profits.
Since the Company’s formation in 2017, the Company has incurred operating losses and negative cash flows from operations. The Company’s net loss was approximately $4.5 million and $3 million for 2021 and 2020, respectively. As of June 30, 2022, the Company had an accumulated deficit of approximately $14 million. As of the Date of this Offering Circular, the Company has never been profitable.
To date, the Company has financed operations primarily through the sale of Common Shares. The Company has devoted substantially all efforts to research and development, including the development and validation of the Starpax Technology, development of the “Good Manufacturing Practices” (“GMP”) compliant manufacturing plant, research and development (“R&D”) and quality control (“QC”) laboratories. The Company has not completed development of or commercialized the Starpax Technology nor any product candidate or medical device. The Company expects to continue to incur significant expenses and incur operating losses for at least the next 3 years. The Company anticipates that expenses and losses will increase substantially when the Company:
| • | initiates Phase I and Phase II clinical trials of its product candidates; |
| • | continues the R&D of product candidates; |
| • | seeks to discover additional product candidates; and |
| • | adds operational, financial and management information systems, and personnel, including personnel to support product development and manufacturing efforts. |
Profitability in large part depends on (1) the success of the planned clinical trials; (2) receiving NDA or BLA from the FDA for its product candidates or market approval for its medical device from the FDA; (3) and the execution of the Company’s business plan by opening Starpax Care Centers. This will require the Company, alone or with collaborators, to be successful in a range of challenging activities, including completing preclinical testing, Phase I and Phase II clinical trials of the Company’s product candidates, obtaining regulatory approval for the Starpax Technology (product candidates and medical devices) and manufacturing, marketing, and offering the Starpax Treatment for which regulatory approval is to be obtained. The Company may never succeed in these activities and the Company may never generate revenues that are significant or large enough to achieve profitability.
Even if the Company does achieve profitability, the Company may not be able to sustain or increase profitability on a periodic or annual basis. Failure to become and remain profitable would diminish the value of the Company and could impair the ability to raise capital, expand business, diversify product offerings, or continue operations. A decline in the value of the Company may also cause Investors to lose all or part of their investment.
The Company may require additional funding to develop and commercialize the products past the Clinical Trial phase.
The Company expects expenses to increase significantly as the product candidates advance in clinical development, and as the Company expands operations. As part of the regulatory process, the Company must conduct clinical trials for each product candidate to demonstrate safety and efficacy to the satisfaction of the FDA and other regulatory authorities. The number and design of the required clinical trials varies depending upon the product candidate, the condition being evaluated, and the trial results themselves. Therefore, it is difficult to accurately estimate the cost of the clinical trials. Clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. The clinical trial process is also time consuming. The Company estimates that clinical trials of product candidates will take at least several years to complete. Because of numerous risks and uncertainties involved in business, the timing or amount of increased development expenses cannot be accurately predicted, and expenses could increase beyond expectations if the Company is required by the FDA, or comparable non- U.S. regulatory authorities, to perform studies or clinical trials in addition to those the Company currently anticipates. Furthermore, failure can occur at any stage of the trials, and the Company could encounter problems that cause the Company to abandon or repeat clinical trials.
After the planned clinical trials and commercialization of the Starpax Technology, as the Company expands its business, the Company will need to retain additional employees with the necessary skills including employees for planned establishment of Starpax Care Centers across the United States and later Europe and Canada.
Even if the Company’s product candidates and medical devices are approved for commercial sale, the Company anticipates incurring significant costs associated with the commercial launch of, and the related commercial-scale manufacturing requirements for, the product candidate. As a result, the Company expects to continue to incur significant and increasing operating losses and negative cash flows for the foreseeable future. Because of the numerous risks and uncertainties associated with biopharmaceutical product development and medical device development and commercialization, the Company is unable to accurately predict the timing or amount of future expenses or when, or if, the Company will be able to achieve or maintain profitability. These losses have had and will continue to have an adverse effect on the financial position and working capital.
The Company currently has no committed sources of funding. If the Company is unable to raise capital in sufficient amounts when needed or on attractive terms, it would be forced to delay development programs.
The Company may experience delays before initiating clinical trials.
Before initiating clinical trials, the Company could be affected by various circumstances that could result in additional delays and costs. This includes but is not limited to, obtaining authorization from governmental health agencies and ethical review boards of the hospital to start the clinical trials, obtaining compliance certifications for operating the medical device and GMP manufacturing equipment, securing adequate insurance coverage for the clinical trials, contracting a competent Contract Research Organization (“CRO”), securing access to essential and qualified workers at expected clinical sites. With respect to the GMP plant and the commissioning of its production equipment, the Company could experience delays in receiving (or shortages of) equipment, parts, tools, raw materials and consumables. It could face technical issues related to the installation, validation, and commissioning of equipment, and in the development and validation of methods, scale-up process, operations, as well as experiencing broken parts, engineering design errors or other type of delays, including from its general contractor. The auditing of the manufacturing plant by Health Canada or FDA may require some changes to be a GMP compliant facility for biopharmaceutical manufacturing. Although the occurrence of one or more of these circumstances could cause business, financial performance, and cash position to be negatively impacted, the Company cannot reasonably estimate the potential for any future impacts on operations or liquidity due to the general unknown nature surrounding these risks.
The Company’s future products may not be commercially successful.
Even if the Company is successful in further validating the Starpax Technology and continuing to build a Company pipeline, the potential product candidates that the Company identifies may not be suitable for clinical development for many possible reasons, including harmful side effects, limited efficacy or other characteristics that indicate that such product candidates or medical devices are unlikely to be products that will receive marketing approval and achieve market acceptance, even if the Company uses already-approved drugs in its Technology. If the Company does not successfully develop and commercialize product candidates and medical devices based upon the Starpax Technology, the Company will not obtain product revenues in future periods, which likely would result in significant harm to financial position and adversely affect the fair market value of the Shares.
A failure by Starpax to hire and retain an appropriately skilled and adequate workforce could adversely impact the ability of the facility to operate and function efficiently.
The Company’s operations will depend, in part, on its ability to attract and retain an appropriately skilled and sufficient workforce to operate its development and manufacturing facility as well as its R&D facility. These employees may voluntarily terminate their employment with the Company at any time. Laboratories and manufacturing plants are located in a growing biotechnology and medical device hub and competition for skilled workers will continue to increase as the industry undergoes further growth in the area. There can be no assurance that the Company will be able to retain key personnel, or to attract and retain additional qualified employees. The Company’s inability to attract and retain key personnel as the Company grows may have a material adverse effect on the business.
The Company may be unable to manage future growth effectively, which could make it difficult to execute business strategy.
The Company intends to grow business operations as regulatory approvals of the product candidates and medical devices are granted by regulatory authorities This will increase demand and increase the number of employees to accommodate such potential growth, which may cause the Company to experience periods of rapid growth and expansion. This potential future growth could create a strain on organizational, administrative and operational infrastructure, including manufacturing operations, quality control, technical support and other administrative functions. The Company’s ability to manage growth properly will require the Company to continue to improve operational, financial and management controls.
As the commercial operations and opening of Starpax Care Centers grows, the Company will need to continue to increase capacity for manufacturing, billing, reimbursement and general process improvements, and expand internal quality assurance program, among other things. The Company may also need to purchase additional equipment, some of which can take several months or more to procure, set up and validate, and increase the Company’s manufacturing, maintenance, software and computing capacity to meet increased demand. These increases in scale, expansion of personnel, purchase of equipment or process enhancements may not be successfully implemented.
The Company may be subject to various litigation claims and legal proceedings.
The Company, as well as certain of its Directors and Officers, may be subject to claims or lawsuits during the ordinary course of business. Regardless of the outcome, these lawsuits may result in significant legal fees and expenses and could divert management’s time and other resources. If the claims contained in these lawsuits are successfully asserted against the Company, the Company could be liable for damages and be required to alter or cease certain of business practices. Any of these outcomes could cause business, financial performance and cash position to be negatively impacted.
The Company relies extensively on information technology systems that are vulnerable to damage and interruption.
The Company relies on information technology systems and infrastructure to process transactions, summarize results and manage business, including maintaining client and supplier information. Additionally, the Company utilizes third parties, including cloud providers, to store, transfer and process data. Information technology systems, as well as the systems of suppliers and other partners, whose systems the Company does not control, are vulnerable to outages and an increasing risk of continually evolving deliberate intrusions to gain access to Company sensitive information. Likewise, data security incidents and breaches by employees and others with or without permitted access to systems pose a risk that sensitive data may be exposed to unauthorized persons or to the public. A cyber-attack or other significant disruption involving Company information technology systems, or those of vendors, suppliers and other partners, could also result in disruptions in critical systems, corruption or loss of data and theft of data, funds or intellectual property. A security breach of any kind, including physical or electronic break-ins, computer viruses and attacks by hackers, employees or others, could expose the Company to risks of data loss, litigation, government enforcement actions, regulatory penalties and costly response measures, and could seriously disrupt Company operations. The Company may be unable to prevent outages or security breaches in Company systems. The Company remains potentially vulnerable to additional known or yet unknown threats as, in some instances, the Company, suppliers and other partners may be unaware of an incident or its magnitude and effects. The Company also faces the risk that the Company exposes the vendors or partners to cybersecurity attacks. Any or all of the foregoing could harm the Company’s reputation and adversely affect the results of operations and business reputation.
Future financing may result in ownership dilution to the Company’s existing Shareholders and may not be on terms similar to past financings, including this Offering.
The sale of a substantial number of Common Shares to future investors, or anticipation of such sales, could make it more difficult for the Company to sell equity or equity-related securities in the future at a time and at a price that might otherwise wish to effect sales. Until such time as the Company can generate substantial development, the Company expects to finance cash needs through a combination of equity offerings and other arrangements. Sources of funds may not be available or, if available, may not be available on terms satisfactory to the Company.
If the Company raises additional funds by issuing equity securities, existing Shareholders may, subject to the Anti-Dilution Agreement, experience dilution. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting the Company’s ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any debt financing or additional equity that the Company raises may contain terms, such as liquidation and other preferences, which are not favorable to Shareholders. If the Company raises additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish valuable rights to technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable. Should the financing the Company requires to sustain working capital needs be unavailable or prohibitively expensive when the Company requires it, the business, operating results, financial condition, and prospects could be materially and adversely affected, and the Company may be unable to continue operations.
To the extent the Company raises additional capital through a public or private offering and sale of equity securities, but subject to the provisions of the anti-dilution agreement (see “Dilution” below), Investors’ ownership interest may be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect an Investor’s rights as a Shareholder. Sales of Common Shares offered through current or future equity offerings may result in substantial dilution to the Shareholders.
Any government funding programs may impose requirements that could affect the Company’s business, financial condition, and results of operations.
The Company has applied for government grants to support some of the clinical development activities for the product candidates. Often government grants include provisions that reflect the government’s substantial rights and remedies, many of which are not typically found in commercial contracts, including powers of the government to potentially require repayment of all or a portion of the grant award proceeds, in certain cases with interest, in the event the Company violates certain covenants pertaining to various matters.
The Company is currently eligible for certain tax credits that may no longer be available in the future.
The Company currently has access to certain tax credits to fund some of its R&D expenses and investments. In the future, the governments may eliminate those tax credits or change the eligibility criteria, therefore negatively affecting the Company’s access to this type of funding. Should the Company lose access to current or future tax credits or is eligible to less, the business, operating results, financial condition, and prospects could be materially and adversely affected.
Risks Related to Intellectual Property
If the Company is unable to obtain and maintain intellectual property protection for the Starpax Technology and products, the ability to successfully commercialize the Starpax Technology and products may be impaired.
The Company currently owns 31 patents or pending patents, therefore success depends in part on the Company’s ability to maintain and obtain patent and other intellectual property protections in the United States and other countries with respect to the Starpax Technology and products. The Company seeks to protect its proprietary position by filing patent applications in the United States and abroad related to the Starpax Technology and product candidates, and by maintenance of trade secrets and copyrights through proper procedures.
The patent prosecution process is expensive and time-consuming, and the Company may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost, in a timely manner, or in all jurisdictions. It is also possible that the Company will fail to identify patentable aspects of R&D output before it is too late to obtain patent protection.
The patent position of biotechnology and pharmaceutical and medical device companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, the laws of foreign countries may not protect Company rights to the same extent as the laws of the United States and the Company may fail to seek or obtain patent protection in all major markets. Even if, to the Company’s knowledge, the Company were the first to file patent on the underlying inventions of the Starpax Technology, the Company cannot know with certainty whether the Company was the first to make the inventions claimed in the Company’s owned patents or pending patent applications, or that the Company was the first to file for patent protection of such inventions. Nor can the Company know whether those from whom the Company licenses patents were the first to make the inventions claimed or were the first to file. As a result, the issuance, scope, validity, enforceability and commercial value of patent rights are uncertain. Pending and future patent applications may not result in patents being issued which protect the Company’s Technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of patents or narrow the scope of the Company’s patent protection. Even if the Company’s pending or future patent applications issue as patents, they may not issue in a form that will provide the Company with any meaningful protection, prevent competitors from competing with the Company or otherwise provide the Company with any competitive advantage. Competitors may be able to circumvent patents by developing similar or alternative technologies or products in a non-infringing manner.
The Company may become involved in lawsuits to protect or enforce patents or other intellectual property.
Commercial success depends upon the Company’s ability, and the ability of the collaborators, to develop, manufacture, market and sell product candidates and use proprietary technologies without infringing the proprietary rights of third parties. There is considerable intellectual property litigation in the biotechnology and pharmaceutical and medical device industry. Competitors may infringe the Company’s issued patents or other intellectual property. To counter infringement or unauthorized use, the Company may be required to file infringement claims, which can be expensive and time-consuming. Any claims the Company asserts against perceived infringers could provoke these parties to assert counterclaims against the Company alleging that the Company infringes their intellectual property. In addition, in a patent infringement proceeding, a court may decide that a patent of the Company is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that the Company’s patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of the Company’s patents at risk of being invalidated or interpreted narrowly, which could adversely affect the Company and its collaborators.
The Company may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to the Company’s products and technology, including interference or derivation proceedings before the United States Patent and Trademark Office (“USPTO”) and similar bodies in other countries. Third parties may assert infringement claims against the Company based on existing intellectual property rights and intellectual property rights that may be granted in the future.
If the Company is found to infringe on a third party’s intellectual property rights, the Company could be required to obtain a license from such third party to continue developing and marketing the products and Starpax Technology. However, the Company may not be able to obtain any required license on commercially reasonable terms or at all. Even if the Company were able to obtain a license, it could be non-exclusive, thereby giving potential competitors access to the same technologies licensed to the Company. The Company could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, the Company could be found liable for monetary damages. A finding of infringement could prevent the Company from commercializing product candidates or force the Company to cease some of the business operations, which could materially harm the business. Claims that the Company has misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on the business.
In addition, the uncertainties associated with litigation could have a material adverse effect on the Company’s ability to raise the funds necessary to continue clinical trials, continue research programs, license necessary technology from third parties, or enter into development partnerships that would help the Company bring product candidates to market. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of the Company’s confidential information could be compromised by disclosure during this type of litigation.
If the Company is unable to protect trade secrets, business and competitive position would be harmed.
The Company also seeks to enter into confidentiality and invention or patent assignment agreements with the employees, consultants, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. The Company has implemented procedural and physical mechanisms to deter external intrusions and violation from inside the Company’s organization. Despite these efforts, any of these parties may breach the agreements and disclose proprietary information, including trade secrets, and the Company may not be able to obtain adequate remedies for such breaches.
Trade secrets may also be obtained by third parties by other means, such as breaches of the Company’s physical or computer security systems. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of the Company’s trade secrets were to be lawfully obtained or independently developed by a competitor, the Company would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with the Company. If any of the Company’s trade secrets were to be disclosed to or independently developed by a competitor, competitive position would be harmed.
Risks related to Regulations
The Company currently is dependent on the success of product candidates and medical devices in the clinical trial process. If product candidates or medical devices do not receive regulatory approval or are not successfully commercialized, the business may be harmed.
The Company expects that a substantial portion of efforts and expenditures over the next few years will be devoted to its product candidates and medical devices. Accordingly, business currently depends heavily on the successful development, regulatory approval, and commercialization of these product candidates and medical devices, which may not receive regulatory approval or be successfully commercialized even if regulatory approval is received. After approval, the research, testing, manufacturing, labeling, approval, sale, marketing and commercial use of product candidates and medical devices are and will remain subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries that each have differing regulations. The Company is not permitted to market any product in the United States unless and until the Company receives approval from the FDA, or in any foreign countries unless and until the Company receives the requisite approval from regulatory authorities in such countries. The Company has never submitted a NDA, BLA, PMA, de Novo, or 510k applications to the FDA or comparable applications to other regulatory authorities and does not expect to be in a position to do so for the foreseeable future. Obtaining approval of an NDA, BLA PMA, de Novo, or 510k applications is an extensive, lengthy, expensive, and inherently uncertain process, and the FDA may delay, limit or deny approval of Company products for many reasons.
Because the Company has limited financial and managerial resources, focus is limited to the development of current product candidates. As a result, the Company may forego or delay pursuit of opportunities with other technologies or product candidates that later prove to have greater commercial potential. Resource allocation decisions may cause the Company to fail to capitalize on viable commercial products or profitable market opportunities. Spending may not yield any commercially viable products.
The Company has based the R&D efforts largely on technologies and product candidates and medical devices derived from such technologies. Notwithstanding large investment to date and anticipated future expenditures in these technologies, the Company has not yet developed, and may never successfully develop, any marketed products using these technologies. As a result, the Company may fail to address or develop product candidates and medical devices based on other scientific approaches that may offer greater commercial potential or for which there is a greater likelihood of success.
The Company also may not be successful in efforts to identify or discover additional product candidates and medical devices using the Starpax Technology. Research programs to identify new product candidates and medical devices require substantial technical, financial, and human resources. These research programs may initially show promise in identifying potential product candidates and medical devices yet fail to yield product candidates for clinical development.
There may be risks of failure proceeding through clinical trials.
Starpax product candidates and medical devices are not yet in clinical development. The Company’s ability to generate product sales revenues for the Company’s own products, which the Company does not expect will occur for several years, will depend heavily on the successful development and eventual commercialization of product candidates and medical devices.
Clinical trials are expensive, time consuming, uncertain and susceptible to change, delay or termination.
Preclinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and has an uncertain outcome. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. The Company may experience numerous unforeseen events during, or as a result of, preclinical testing and the clinical trial process that could delay or prevent the commercialization of product candidates.
Significant clinical trial delays could allow competitors to bring products to market before the Company and impair the ability to commercialize the Starpax Technology and product candidates and medical devices based on the Starpax Technology. Poor clinical trial results or delays may make it impossible to license a product candidate, or reduce its attractiveness to prospective licensees, so that the Company will be unable to successfully develop and commercialize such a product candidate
After the completion of clinical trials, and the Company’s products are commercialized, failure to comply with regulatory requirements could adversely affect business and results of operations.
Manufacturing operations will be regulated, and the Company must comply with the regulatory requirements of various local, state, provincial, national and international regulatory bodies having jurisdiction in the locality in which the Company manufactures products. In particular, the Company is subject to laws and regulations concerning development, testing, manufacturing processes, equipment and facilities, including compliance with GMPs or ISO13485 import and export, and product registration and listing, among other things. As a result, the facility is subject to regulation by the FDA, Health Canada, as well as regulatory bodies of other jurisdictions. As the Company progresses in pharmaceutical development, the Company may be exposed to more complex and newer regulatory and administrative requirements and legal risks, any of which may require expertise in which the Company has little or no experience. It is possible that compliance with new regulatory requirements could impose significant compliance costs. Such costs could have a material adverse effect on the business, financial condition and results of operations.
The Company’s operations are also subject to a variety of environmental, health and safety laws and regulations, including those of the local, provincial, state, and federal agencies. These laws and regulations govern, among other things, air emissions, wastewater discharges, the use, handling and disposal of hazardous substances and wastes, soil and groundwater contamination, and employee health and safety. Any failure to comply with environmental, health and safety requirements could result in the limitation or suspension of production or monetary fines or civil or criminal sanctions, or other future liabilities. The Company is also subject to laws and regulations governing the destruction and disposal of raw materials and the handling and disposal of regulated material.
Product candidates will be subject to the regulatory approvals discussed above, but the facility is subject to governmental approval for the testing or manufacturing of products. If the manufacturing facility or medical devices are not able to demonstrate compliance with GMPs or ISO13485 and other related industry requirements, pass other aspects of pre-approval inspections or properly scale up to produce commercial supplies, the FDA or other regulatory agencies can delay approval of product candidate(s).
In addition, if new legislation or regulations are enacted or existing legislation or regulations are amended or are interpreted or enforced differently, the Company may be required to obtain additional approvals or operate according to different manufacturing or operating standards. This may require a change in development and manufacturing techniques or additional capital investments in the facility. Any related costs may be significant. If the Company fails to comply with applicable regulatory requirements in the future, then the Company may be subject to warning letters and/or civil or criminal penalties and fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, restrictions on the import and export of the products, debarment, exclusion, disgorgement of profits, operating restrictions and criminal prosecution and the loss of contracts and resulting revenue losses. Inspections by regulatory authorities that identify any deficiencies could result in remedial actions, production stoppages or facility closure, which would disrupt the manufacturing process and supply of product to the customers. In addition, such failure to comply could expose the Company to contractual and product liability claims, including claims for reimbursement for lost or damaged active pharmaceutical ingredients or recall or other corrective actions, the cost of which could be significant.
The FDA and comparable government authorities having jurisdiction in the countries in which the Company intends to market its products have the authority to withdraw product approval or suspend manufacture if there are significant problems with raw materials, parts or supplies, quality control and assurance or the product the Company manufactures is adulterated or misbranded. If manufacturing facilities, medical device and services are not in compliance with the FDA and comparable government authorities, the Company may be unable to obtain or maintain the necessary approvals to continue manufacturing products, which would materially adversely affect the financial condition and results of operations.
Environmental Regulations.
The Company’s GMP manufacturing plant, Starpax Cancer Care Centers, and other facilities are or will be subject to various federal, state/province, and local and laws and regulations to protect the environment. Various states and governmental agencies are considering, and some have adopted, laws and regulations regarding environmental control which could adversely affect our business. Compliance with such legislation and regulations, together with any penalties resulting from noncompliance therewith, will increase the Company’s operating costs.
Government Regulations.
In addition to the regulation of the Company’s manufacturing plant and R&D facilities, the Company’s anticipated business of operating the Starpax Cancer Centers will be subject to extensive governmental regulation under which, among other things, will regulate the practice of medicine within the Starpax Cancer Centers. Governmental regulation also may limit or otherwise affect the market for the Starpax Cancer Centers and the manner in which the Company develops and deploys the commercialization strategy. Governmental regulations relating to environmental matters could also affect our operations. The nature and extent of various regulations, the nature of other political developments and their overall effect upon the Company are not predictable.
Included in these government regulations will be import/export laws and controls. The Company’s main manufacturing plant is located in Canada, for use in Starpax Cancer Centers to be located throughout the United States. This will require the Company to comply with import and export laws of at least these two jurisdictions. Any changes in these import and export laws could result in difficulties or inability of the Company to get the Starpax Technology to the Starpax Cancer Centers once commercialization of the Starpax Technology has begun.
Risks related to commercialization of the Starpax Technology
If the market chooses to buy competing products, the Company may fail.
The manufacture of biologics, drugs, medical devices and the methods of such manufacture are intensely competitive fields. Each of these fields is characterized by extensive research efforts, which result in rapid technological progress that can render existing technologies obsolete or economically non-competitive. If competitors succeed in developing more effective technologies or render technologies obsolete or non-competitive, business will suffer. Many universities, public agencies and established pharmaceutical, biotechnology, and other life sciences companies with substantially greater resources than the Company has are developing and using technologies and are actively engaging in the development of products competitive with Company technologies and products. To remain competitive, the Company must continue to invest in new technologies and improve existing technologies. To make such renewing investment the Company will need to obtain additional financing. If the Company is unable to secure such financing, the Company will not have sufficient resources to continue such investment. In addition, competitors may also have significantly greater experience in the discovery and development of products, as well as in obtaining regulatory approvals of those products in the United States and in foreign countries. Current and potential future competitors also have significantly more experience in commercially deploy technologies or therapeutics that have been approved for marketing. Mergers and acquisitions in the pharmaceutical and biotechnology industries could result in even more resources being concentrated among a small number of competitors.
For cancer product candidates, not only does the Company compete with companies engaged in various cancer treatments including surgery, thermal, radiotherapy, hormonotherapy and chemotherapy, but the Company also competes with various companies that have developed or are trying to develop different technologies such as but not limited to nanocarriers, precision medicine, immune check points inhibitors, oncolytic virus, immunology treatment in oncology. Certain of the Company’s competitors have substantially greater capital resources, large customer bases, broader product lines, sales forces, greater marketing and management resources, larger research and development staffs with extensive facilities and equipment than the Company does and have more established reputations as well as global distribution channels.
The availability of competitors’ products could limit the demand, and the price the Company is able to charge, for any product candidate the Company develops. The inability to compete with existing or subsequently introduced therapies would have an adverse impact on the business, financial condition and prospects.
Established pharmaceutical companies may invest heavily to accelerate discovery and development of novel compounds or to in-license novel compounds that could make product candidates less competitive. In addition, any new products that compete with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome price competition and to be commercially successful.
Product liability lawsuits against the Company could cause the Company to incur substantial liabilities and to limit commercialization of any products that the Company may develop.
The Company faces the risk of product liability exposure in connection with the testing of the product candidates and medical devices in human clinical trials and will face an even greater risk if the Company commercially sells any products that the Company may develop. If the Company cannot successfully defend itself against claims that the Company’s product candidates, medical device or products caused injuries, the Company will incur substantial liabilities.
Prior to commencing human clinical trials and after regulatory approval, the Company will seek to obtain insurance coverage. Such insurance coverage is expensive and may not be available in coverage amounts the Company seeks or at all. If the Company obtains such coverage, the Company may in the future be unable to maintain such coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
The Company intends to use its own manufacturing facility. Any manufacturing problems experienced by the Company could result in a delay or interruption in the supply of the Company’s products.
The Company is preparing to manufacture clinical product candidates in its own facilities. It currently does not have any alternative manufacturer. If the Company experiences disruption in distribution, considering the short shelf-life of the product candidate(s), the Company could be forced to interrupt supply for the patient treatments, negatively impacting timelines, and clinical trials or commercialization costs. If the Company changes manufacturers at any point during the development process or after approval of a product candidate or medical device, the Company will be required to demonstrate comparability between the product manufactured by the old manufacturer and the product manufactured by the new manufacturer. If the Company is unable to do so the Company may need to conduct additional clinical trials with product manufactured by the new manufacturer.
The Company may not be able to manufacture products at the scale required for commercial success.
If the Company is not able to manufacture sufficient quantities of clinical product candidate(s), or fails to develop product candidate(s) with specifications or quality attributes adequate for the clinical trials or the commercialization of the Company’s clinical product candidate(s), development or commercial activities would be impaired. In addition, the manufacturing facility where clinical product candidates are manufactured is subject to ongoing, periodic inspection by the FDA or other comparable regulatory agencies (including Health Canada) to ensure compliance with current Good Manufacturing Practice regulations. Any failure to follow and document the manufacturer’s adherence to such GMP regulations or other regulatory requirements may lead to significant delays in the availability of investigational product substance for clinical trials, which may result in the termination of or a hold on a clinical trial or may delay or prevent filing or approval of marketing applications for the Company’s clinical product candidates.
Reliance on third-party manufacturers and suppliers entails risks to which the Company would not be subject if the Company manufactures the clinical product candidates itself, including:
| • | reliance on the third parties for regulatory compliance and quality assurance; |
| • | the possible breach of the manufacturing agreements by the third parties because of factors beyond Company control or the insolvency of any of these third parties or other financial difficulties, labor unrest, natural disasters or other factors adversely affecting their ability to conduct their business; and |
| • | possibility of termination or non-renewal of the agreements by the third parties, at a time that is costly or inconvenient for the Company, because of breach of the manufacturing agreement or based on their own business priorities. |
The Company currently leases the space where the R&D laboratories and GMP manufacturing plant are located.
The Company currently leases the space where the R&D laboratories and GMP plant are located. The lease is for a term of 5 years. The Company currently has an option to extend the lease for an additional 5 years after the expiration of the primary term. In the event the lease expires, or if the lessor of the space does not wish to extend the lease past the option period, the Company could incur delays in manufacturing and R&D which may render the products unavailable for the clinical trials or to patients if commercialization of the Starpax Technology has already begun. This could result in significant losses which may cause the Company to (1) seek future financings to remedy; (2) lose market share and customer confidence; (3) utilize a third-party manufacturer for the manufacturing of the Company’s products; (4) incur significant costs in developing a second manufacturing plant, subject to GMP.
Risks Relating to Common Shares
Provisions of the Articles of Consolidation, bylaws and provisions of applicable Québec law may discourage, delay or prevent a merger, or other change in control.
The Board of Directors is authorized to issue additional Common Shares and, if the Company were to amend its Articles, the Board of directors could be authorized to issue preferred Shares. Any additional issuance of Common Shares could have the effect of impeding or discouraging the acquisition of control of the Company by means of a merger, tender offer, proxy contest or otherwise, including a transaction in which Shareholders would receive a premium over the market price for their Shares, and thereby protect the continuity of management. Specifically, if in the due exercise of its fiduciary obligations, the Board of Directors were to determine that a takeover proposal was not in the Company’s best interest, Common Shares could be issued by the Board of Directors without Shareholder approval in one or more transactions that might prevent or render more difficult or costly the completion of the takeover that a Shareholder may determine to be in his, her or its best interest, including attempts that might result in a premium over the market price for the Shares held by the Shareholders, by:
| • | diluting the voting or other rights of the proposed acquirer or insurgent Shareholder group, |
| • | putting a substantial voting bloc in institutional or other hands that might undertake to support the incumbent Board of Directors, or |
| • | effecting an acquisition that might complicate or preclude the takeover. |
The Company does not anticipate paying cash dividends for the foreseeable future.
The Company has never declared or paid any cash dividends or distributions on the Company’s Share capital. The Company currently intends to retain future earnings to support operations and to finance expansion and therefore the Company does not anticipate paying any cash dividends on Common Shares in the foreseeable future.
There is no current public market for the Shares.
Despite the possibility of individual transfer transactions between an Investor and a buyer allowing resale of the Shares, there is no public market for the resale of Company Shares and none is expected to arise for the foreseeable future, however, the Company reserves the right list the Shares on an exchange in the future.
The value of the Shares through this Offering may fluctuate after investment and may vary in the future.
The Board of Directors and management set the Share price based on public market data, Company forecasts, valuation of comparable pre-revenue pharmaceutical companies in preclinical stage, consultations with experts, as well as the price per Share of recent private offerings from the Company. However, the price per Share in this Offering bears no relationship to the book or asset values or to any other criteria for valuing Shares and may not be indicative of the proceeds that an Investor would receive upon liquidation. Further, the price at which the Shares would trade if they were to be listed on an exchange or actively traded by broker-dealers may be different than the Offering price per Share.
There is limited liquidity for the Shares
The Shares have no redemption rights and limited transferability rights, therefore impacting liquidity for the Shares. As of the date of this Offering Circular, the Company does not currently have plans to list any Shares on any securities market or exchange, however, the Company reserves the right to list the Shares on an exchange in the future.
DILUTION
Except for one Officer of the Company (Jean-Francois Pruneau, Executive Vice President and Chief Financial Officer), none of the Directors, Officers, or affiliated persons have received Shares within the past calendar year from the date of this Offering Circular.
These Shares were purchased by Jean-Francois Pruneau through the exercise of stock options. Jean-Francois Pruneau was granted 15,000 stock options on February 15, 2022, as a result of his position as an Officer. Jean-Francois Pruneau exercised one third of his options on December 20, 2022 (5,000 options). After the stock split on January 19, 2023, Jean-Francois Pruneau had 50,000 remaining options. After the stock split executed on January 19, 2023, the effective cash cost per Share was $0.0005 per Share. This represents a material disparity of approximately $6.2495 per Share as is being offered to Investors through this Offering.
Anti-Dilution Agreement
On November 23, 2022, the Company entered into an anti-dilution agreement between the Company and Ipax 2 llp. Subject to certain terms, this anti-dilution agreement avoids the dilution of the percentage of Shares that investors, excluding Ipax 2 llp, own in the Company when new Shares are issued from treasury. The anti-dilution agreement is provided hereto as Exhibit 3.
This agreement includes a forced redemption option granted to the Company. Ipax 2 granted the Company an option (the “Forced Redemption Option”) allowing the Company to redeem one share held by Ipax 2 in its share capital, for a price of $0.02 CAD per Share (approximately $0.014), upon each and every issue of a treasury share by the Corporation. Conversely, the Corporation undertakes to exercise the Forced Redemption Option upon each new issue of treasury shares, thus to buying back a number of shares held by Ipax 2 equal to the number of shares so issued.
This agreement will affect sales of the Shares through the Offering. This means that for every sale of a Share through this Offering, the Company will pay $0.02 CAD (approximately $0.014) to Ipax 2 llp.
Under no circumstances will the number of issued and outstanding Shares exceed 75,500,000 while the Forced Redemption Option is in effect.
The Forced Redemption Option will end:
(a) upon the cumulative amount of subscription proceeds for Shares of the Corporation (excluding the shares held by Ipax 2 and Polyvalor, S.E.C.), including the amounts of non-tax subsidies (thus excluding tax credits and tax incentives) and donations from private sources, and the profits realized and dividends paid, all combined, having reached $88 million CAD (approx. $64.24 million USD), such amounts being calculated from the date of incorporation of the Corporation. It is understood that the calculation of the profits made shall exclude donations and subsidies so that these sums are not counted more than once in the aforementioned cumulative amount;*
or,
(b) upon the Corporation having reached the marketing and sale of the first PolarTrak, excluding facilities for testing, clinical trials, pilots, demonstration and showcases.
*As of the date of this Offering Circular, The Company has raised aggregate proceeds of $48,400,349 CAD (approximately $35,332,254.77 USD, based off of an exchange rate of $0.73 USD to $1.00 CAD).
The Company may engage in other financings including future equity raises. In the event the Company sells equity securities subsequent to an Investor’s purchase of Shares through this Offering or future offerings, the Investor’s proportionate ownership of the Company will be diluted only if the Forced Redemption Option is no longer in effect.
PLAN OF DISTRIBUTION
The Offering will be made through general solicitation, direct solicitation, and marketing efforts whereby Investors will be directed to the investment website (investinstarpax.com) to invest. The Company has engaged Justly Markets, an independent FINRA broker-dealer to assist with the Share sales in exchange for a 1% commission fee on the aggregate sales not made through the efforts of Justly Markets. The Offering is conducted on a best-efforts basis. No Commissions or any other renumeration for the Share sales will be provided to the Company, the Directors, any Officer, or any employee of the Company, relying on the safe harbor from broker-dealer registration set forth in Rule 3a4-1 under the Securities Exchange Act of 1934, as amended.
The Company will not limit or restrict the sale of the Shares during this 12-month Offering. No market exists for the Shares and no market is anticipated or intended to exist in the future, therefore there is no plan to stabilize the market for any securities to be offered.
Directors, Officers, and employees of the Company are primarily engaged in the Company’s business of researching and developing the Starpax Technology, and none of them are, or have ever been, brokers nor dealers of securities. The Directors, Officers, and employees will not be compensated in connection with the sale of securities through this Offering. The Company believes that the Directors, Officers, and employees are associated persons of the Company not deemed to be brokers under Exchange Act Rule 3a4-1 because: (1) no Director, Officer, or employee is subject to a statutory disqualification, as that term is defined in section 3(a)(39) of Exchange Act at the time of their participation; (2) no Director, Officer, or employee will be compensated in connection with his participation by the payment of commissions or by other renumeration based either directly or indirectly on transactions in connection with the sale of securities through this Offering; (3) no Director, Officer, or employee is an associated person of a broker or dealer; (4) the Directors, Officers, and employees primarily perform substantial duties for the Company other than the sale or promotion of securities; (5) no Director, Officer, or employee has acted as a broker or dealer within the preceding twelve months of the date of this Offering Circular; (6) no Director, Officer, or employee will participate in selling this Offering after more than twelve months from the Effective Date of the Offering.
Justly Markets (“Justly Markets”) has agreed to act as placement agent to assist in connection with this Offering. Justly Markets is not purchasing or selling any securities offered by this Offering Circular, nor is it required to arrange the purchase or sale of any specific number or dollar amount of securities. However, Justly Markets has agreed to use their best efforts to arrange for the sale of the Shares offered through this Offering Circular. In addition, Justly Markets may engage other brokers to sell the securities on their behalf. Justly Markets will receive compensation for all passive sales of the Shares offered and sold pursuant to this Offering at a rate of 1% of the gross Proceeds for a maximum of $250,000. The Company may pay Justly Markets 5% of the gross Proceeds from the sale of up to $10,000,000 in Shares resulting from the direct selling efforts of Justly Markets not to exceed $500,000.
In the event that Justly Markets’s targeted selling efforts lead to sales of up to $10,000,000 in Shares, Justly Markets will be entitled to 5% of the gross Proceeds from the sale of such Shares not to exceed $500,000. If Justly Markets’s efforts lead to all $10,000,000, the maximum commissions to be charged would be $750,000. There will not be any commissions charged at a combined 6%.
The Company will also publicly market the Offering using general solicitation through methods that include emails to potential Investors, the internet, social media, and any other means of widespread communication.
The Offering Circular will be furnished to prospective investors via download 24 hours per day, 7 days per week on the Company’s investment website at investinstarpax.com and via the EDGAR filing system. The following table shows the total discounts and commissions payable to Justly Markets in connection with this Offering by the Company:
In the event that Justly Markets’s targeted selling efforts lead to sales up to $10,000,000 in, Justly Markets will be entitled to 5% of the Gross Proceeds from the sale of such Shares not to exceed $500,000.
| | | Price Per Share | | Total Offering |
| Public Offering Price | | $ | 6.25 | | | $ | 25,000,000 | |
| Placement Agent Commissions * | | $ | 0.1875 | | | $ | 750,000 | |
Proceeds, Before
Expenses | | $ | 6.0625 | | | $ | 24,250,000 | |
*This represents the maximum potential commissions due to Justly Markets, the commissions actually due may be less than this number conditional on the success of Justly Markets’s targeted sales efforts.
Other Terms
Justly Markets has also agreed to perform the following services in exchange for the compensation discussed above:
- Act as lead broker for the Offering, coordinating efforts of parties involved and providing regulatory guidance;
- Manage the back-end process of the Offering Platform technology that Investors use to invest in the Offering;
| - | Reviewing marketing materials if requested; |
| - | Performing AML/KYC checks on all Investors, and; |
- Providing other financial advisory services normal and customary for Regulation A offerings and coordinate with the Company’s registered transfer agent and legal representatives.
In addition to the commissions described above, the Company will also pay a maximum of $26,750 to Justly Markets for out-of-pocket accountable expenses paid prior to commencing the Offering. This fee will be used for the purpose of coordinating filings with FINRA (Form 5110). Justly Markets has waived any consulting fees related to the filing of the FINRA 5110 letter.
Blue sky filing fees of $15,000, paid to Justly Markets, are subject to remittance of any unused funds in filing Blue Sky state filings.
The Company will forward the fees required for state notice filing fees, estimated to be approximately $15,000. Assuming the full amount of the Offering is raised and that Justly Markets’ targeted selling efforts lead to sales of $10,000,000, the Company estimates that the total commissions of the Offering payable by the Company to Justly Markets will be approximately $750,000. Maximum expected out of pocket expenses total $776,750 (commissions and fees).
The Company has engaged KoreKonX as registrar and transfer agent for this Offering. The Company has engaged North Capital as escrow agent for this Offering.
USE OF PROCEEDS
| | | | 25% | | | | 50% | | | | 75% | | | | 100% | |
| Clinical Trials – Phase 1 | | $ | 1,974,328 | | | $ | 6,581,092 | | | $ | 7,312,324 | | | $ | 7,312,324 | |
| Clinical Trials – Phase 2 | | $ | 0 | | | $ | 0 | | | $ | 4,228,158 | | | $ | 6,604,938 | |
| Preparation for Clinical Trials | | $ | 1,236,368 | | | $ | 1,442,429 | | | $ | 1,545,459 | | | $ | 2,060,613 | |
| PolarTrak | | $ | 700,290 | | | $ | 1,167,150 | | | $ | 1,167,150 | | | $ | 1,520,024 | |
| Capital Expenditures | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 1,489,091 | |
| Administration and Working Capital | | $ | 684,150 | | | $ | 1,125,505 | | | $ | 1,679,960 | | | $ | 2,687,936 | |
Fees and Expenses related
to the Offering | | $ | 1,654,864 | | | $ | 2,183,824 | | | $ | 2,816,949 | | | $ | 3,325,074 | |
| Total | | $ | 6,250,000 | | | $ | 12,500,000 | | | $ | 18,750,000 | | | $ | 25,000,000 | |
| 1. | Clinical Trials – Phase 1 - $7,312,324 |
The Company intends to spend $7,312,324 of the Proceeds on Phase I Clinical Trials (six cancer indications). These funds will be primarily used for chemical products and consumables required in the production of Magnetodrones, hospital fees, wages and salaries of employees in charge of the production of the Magnetodrones, and consultants associated with Research and Development activities.
| 2. | Clinical Trials – Phase 2 - $6,604,938 |
The Company anticipates spending $6,604,938 of the Proceeds on Clinical Trials – Phase 2 for one cancer indication. These funds will be primarily used for chemical products and consumables required in the production of Magnetodrones, wages and salaries of employees in charge of the production of the Magnetodrones, hospital fees and consultants associated with Research and Development activities.
| 3. | Preparation for Clinical Trials – $2,060,613 |
The Company anticipates using approximately $2,060,613 of the Proceeds for the preparation of its Clinical Trials, consisting primarily in wages and salaries of employees in charge of the production of Magnetodrones and chemical products and consumables required for the production of Magnetodrones.
| 4. | PolarTrak Development - $1,520,024 |
The Company anticipates spending $1,520,024 of the Proceeds on the continuing development of the PolarTrak. This includes wages and salaries for engineers, computer scientists, and other professionals critical to the development of the PolarTrak.
| 5. | Capital Expenditures - $1,489,091 |
The Company anticipates using $1,489,091 of the Proceeds for Capital Expenditures, consisting mainly of investments in equipment required in the production of Magnetodrones, as well as leasehold improvements required in its Magnetodrones GMP manufacturing plant.
| 6. | Administration and Working Capital – $2,687,936 |
The Company intends to spend $2,687,936 of the Proceeds on administration and other working capital needs. This consists primarily in wages and salaries of administrative employees, utilities, rent related to its sole facility, professional fees and accounts payable.
| 7. | Fees and Expenses related to the Offering - $3,325,074 |
The Company anticipates using $3,325,074 on fees and expenses related to the Offering, mainly consisting of marketing expenses related to the Offering, credit card and ACH fees, commissions to its financial advisor and other fees related to the Offering (auditor, broker dealer, etc.)
Expected Outside Funding
The maximum offering amount is $25,000,000. In addition to the Proceeds from the Offering, the Company expects approximately $4,400,000 of funds from outside sources. These outside funds are expected to come from the following three sources. The Company intends to use this additional funding for the same uses of the Proceeds as stated above.
| 1. | Drawings from loan agreements – approximately $600,000 |
The Company has access to borrowing capacity on two outstanding loan agreements (Investissement Québec and Economic Development of Canada Agency). The drawing of these funds is non-contingent.
| 2. | Scientific Research and Experimental Tax Incentives – approximately $1,500,000 |
The Company has access to tax credits from the Government of Canada and the Government of Québec applicable on its expenses in research and development activities. The Company expects to collect a total of $1.5 million from both tax authorities. These funds come in the form of a tax credit. As such the receipt of this tax credit is contingent on a review by the tax authority. This review is generally based on (i) Company expenses that are eligible to be claimed under the tax program, and (ii) if deemed necessary by the tax authority, the Company’s successful passing of an audit regarding such eligible expenses. In these two ways, this tax credit is contingent.
| 3. | Contribution from Clinical Trials Hospital - approximately $2,300,000 |
The Company and the hospital where the clinical trials will take place have entered into, in December 2022, an agreement whereby the Company will receive a payment of $2.3 million upon the commencement of its clinical trials. The receipt of these funds is contingent on the Phase I trials commencing.
THE COMPANY RESERVES THE RIGHT TO CHANGE ITS USE OF THE PROCEEDS AT ANY TIME, AT THE SOLE DISCRETION OF THE COMPANY, ITS DIRECTOR(S), OR OFFICERS WITHOUT PRIOR NOTICE TO SHAREHOLDERS OR POTENTIAL INVESTORS.
DESCRIPTION OF THE BUSINESS
Company History
Starpax Biopharma Inc. (“Starpax” or the “Company”) was incorporated under the laws of Québec, Canada on December 19, 2017, under the name Starpax Medical Inc, with one Class A Share issued and outstanding. On June 9, 2022, the Company changed its name to Starpax Biopharma Inc.
In 2016, Michael Gareau was introduced to the concept of a revolutionary technology, virtual monopole magnetic fields vectors. This technology had been in development for 17 years, involving approximately 180 professionals, multiple medical research university institutions, and the Department of Computer and Software Engineering within the Polytechnique Montréal University. In 2017, after in-depth analysis and due diligence, Mr. Gareau decided to acquire, through a Canada-based private equity limited partnership owned almost exclusively by the MG Trust, Medpax Consortium S.E.C. (which changed its name to Ipax 2 llp on September 27, 2021), the exclusive and perpetual licenses of the Technology encompassing two family patents covering virtual monopole magnetic fields vectors. The licenses were assigned by Polyvalor llp (“Polyvalor”) (the investment arm of Polytechnique Montréal University) to Ipax 2 llp. Assignment of these licenses was made in exchange for partnership units in Ipax 2 llp. After this assignment transaction, Polyvalor llp became a unitholder in Ipax 2 llp with 1,500,000 partnership units out of 15,100,000 partnership units issued by Ipax 2 llp
In March 2018, Ipax 2 llp transferred, free of any royalties or debts, all its intellectual property covering the Technology and the patent license rights covering virtual monopole magnetic fields vectors to Starpax in exchange of 15,099,999 Class B Common Shares of Starpax. This intellectual property included ownership of the two patents, know-how, data, analyses, and other relevant intangible assets. Concurrent with this transfer, Polyvalor llp, through a series of transactions, converted its partnership units in Ipax 2 llp to 1,500,000 Class A Common Shares in Starpax, which issuance of Class A Common shares triggered as part of the Anti-dilution provision of the Unanimous Shareholders’ Agreement, the repurchase and cancellation of 1,500,000 Class B Common Shares held by Ipax 2 llp at a price of $0.10 CAD per share (approximately $0.08 USD). The final result was 13,599,999 Class B Common Shares in Starpax owned by Ipax 2 llp and 1,500,000 Class A Common Shares owned by Polyvalor llp, resulting in 15,100,000 total Class A and Class B Common Shares in Starpax issued as of March 2018, (including the one Class A share issued on the date of incorporation). This assignment by Ipax 2 llp to Starpax was free of any royalty or debt.
On January 19, 2023, the Company amended its Articles (i) converting all outstanding Class A Common Shares and Class B Common Shares into shares of a single class called common shares (“Common Shares”) and (ii) executing a 5:1 stock split, subdividing all its issued and outstanding Common Shares into five (5) such Common Shares. (See Exhibit 2)
Over the past four years, Starpax has collaborated with more than 300 professionals to transform the concept of virtual monopole magnetic vectors to a revolutionary multidisciplinary technology for the treatment of solid cancers for humans. Using the virtual monopole magnetic vectors concept as a base, the Company has developed several technologies including, but not limited to, the Starpax Magnetodrones and the PolarTrak. During this time, the Company has expanded its patent portfolio from 2 to 31 issued and pending patents applications (six issued patents and 25 pending applications) in various jurisdictions including the United States Patent and Trademark Office (“USPTO”) the European Patent Office (“EPO”) and “Canadian Intellectual Property Office (“CIPO”) and other filings. These patents include utility patents (including “method” or “process” patents and design patents).
As majority shareholder of Starpax, Ipax 2 llp does not have any shares other than Company Shares in its portfolio. Ipax 2 llp is solely dedicated to its single investment and does not intend to expand its own portfolio.
Summary of the Starpax Technology
ALL RESULTS, ASSERTIONS, DEDUCTIONS, TECHNICAL, AND/OR SCIENTIFIC OR MEDICAL FORWARD-LOOKING CLAIMS BY STARPAX IN THIS DOCUMENT ARE BASED EITHER ON INDEPENDENT 3RD PARTY PRECLINICAL WORK CONDUCTED AT REPUTABLE CANADIAN 1ST-TIER RESEARCH INSTITUTIONS, GLP CERTIFIED LABS, CLINICAL RESEARCH ORGANIZATION, PAPERS FROM THE SCIENTIFIC LITERATURE OR BY DESIGN INTENDED USE OF THE TECHNOLOGY ON HUMANS.
THE STARPAX TECHNOLOGY IS CURRENTLY AN INVESTIGATIONAL PRODUCT AND HAS NOT YET BEEN APPROVED FOR COMMERCIAL USE.
Starpax is a biopharmaceutical company that has conceived an unprecedented platform technology intended to treat cancer using its living self-propelled Starpax Magnetodrones that are sensitive to magnetic fields and transport anticancer drugs attached to their surface. The Magnetodrones are injected directly into the tumor. The trajectory of the Magnetodrones is controlled by the Starpax PolarTrak that generates precision 3D guidance monopole magnetic field vectors in order to keep them captive in a tumor and force them to spread and release the anticancer drug throughout the volume of the tumor without circulating in the blood system, avoiding side effects usually resulting from systemic cancer treatments.
The Company is comprised of 44 professionals as supported by an important independent team of medical and regulatory advisors and co-investigators specializing in oncology. Since its formation and acquisition of the foundational patents, the Company has utilized the services of more than 300 experts to research, develop, and produce the Starpax Technology.
The Starpax Technology consists of two major elements that cannot be dissociated. The first element consists of self-moving living Magnetodrones sensitive to very specific magnetic fields. The Magnetodrones are injected into the tumor. They swim by themselves and transport the therapeutic anti-cancer agent on their surface without circulating into the blood system. The second element is a medical device, the Starpax PolarTrak in which the patient is placed, that generates specific monopole magnetic fields, intended to keep the Magnetodrones captive inside the tumor and force them to distribute in 3D throughout the volume of the tumor. By forcing the Magnetodrones to stay within the tumor, the therapeutic anti-cancer agent does not escape to the body and will be distributed to cancers cells in the tumor, including the cancer stem cells located in the hypoxic zones of the tumor. These Magnetodrones path(s) are influenced by two factors, (1) the monopole magnetic fields generated by the PolarTrak, and (2) their aerotactic ability to take refuge into the hypoxic zones due to the low level of oxygen which is the same as their culture medium.
The Starpax Magnetodrones consists of non-pathogenic magnetotactic, aerotactic proprietary, self- propelled Starpax Bn1-S living bacteria and proprietary Starpax liposomes attached by the Company to the bacterium’s surface and loaded with therapeutic anti-cancer agent. The Magnetodrones are injected into the tumor. The therapeutic anti-cancer agent that the Magnetodrones will transport is SN-38, the active metabolite of Irinotecan, a widely-used anti- cancer drug already approved by the FDA (or other regulatory authorities). The Company does not develop any therapeutic agent but intends to use multiple kinds of FDA-approved therapeutic agents.
For a more detailed description of SN-38, please see “SN-38, The Starpax Therapeutic Agent” below.
Starpax PolarTrak is a therapeutic medical device in which the patient is placed and aligned, and is a complex medical device conceived to generate unique monopole magnetic fields vectors into and around the tumor. The purpose of this alignment within the PolarTrak is to force the Magnetodrones injected into the tumor to spread in 3D throughout the volume of the tumor and keep them captive inside the tumor in order that they do not escape to the rest of the patient’s body. The Company expects to use the Proceeds of this Offering to conduct clinical trials for the Technology in human subjects for six Unmet Medical Needs (defined below) in the following types of cancer indication: (1) rectum non operable; (2) pancreas, (3) head & neck; (4) skin metastasis of breast recurrence; (5) vulva; (6) prostate. Other indications, such as cervical, bladder, liver, and kidney will be considered depending on the timing and availability of medical resources.
The Starpax Technology
ALL RESULTS, ASSERTIONS, DEDUCTIONS, TECHNICAL, AND/OR SCIENTIFIC OR MEDICAL FORWARD-LOOKING CLAIMS BY STARPAX IN THIS OFFERING CIRCULAR ARE BASED EITHER ON INDEPENDENT 3RD PARTY PRECLINICAL WORK CONDUCTED AT REPUTABLE CANADIAN 1ST-TIER RESEARCH INSTITUTIONS, GLP CERTIFIED LABS, CLINICAL RESEARCH ORGANIZATIONS, PAPERS FROM THE SCIENTIFIC LITERATURE, OR BY DESIGN INTENDED USE OF THE TECHNOLOGY ON HUMANS.
THE STARPAX TECHNOLOGY IS CURRENTLY AN INVESTIGATIONAL PRODUCT AND HAS NOT YET BEEN APPROVED FOR COMMERCIAL USE.
The Starpax Technology consists of (1) the Starpax Magnetodrones; and (2) the Starpax PolarTrak device.
The Starpax Magnetodrones
The Magnetodrones consist of unique, proprietary self-propelled Starpax Bn1-S living bacteria, sensitive to magnetic fields, that transport Starpax liposomes attached to their surface and loaded with SN-38. The therapeutic anti-cancer agent that the Magnetodrones will transport is SN-38, the active metabolite of Irinotecan, a widely used anti-cancer drug already approved by the FDA (or other regulatory authorities).
The Starpax Bn1-S bacterium is the result of 22 years of development and more than 800 mutations. The Bn1-S bacterium is at the heart of the Starpax Technology, it is sensitive to very specific magnetic fields created by the Starpax PolarTrak that controls the Magnetodrones trajectory in three dimensions within the tumor with millimeter precision.
This bacterium one of the smallest bacteria in existence, is capable of swimming within interstitial spaces between tumor tissues. It is non-pathogenic, and the bacterium dies approximately 60 minutes after injection because the human body temperature is too high for it to survive. This bacterium is the property of the Company (see “Starpax Ownership of the Magnetodrones” below). The Bn1-S Bacteria and the Starpax Liposomes are produced by the Company in its facilities.
Starpax Ownership of the Magnetodrones
Starpax is the owner of the Magnetodrone Bn1-S bacteria by way of owning the materials used to yield the Magnetodrone Bn1-S bacteria through lab-based manipulation. Because the Magnetodrone Bn1-S bacteria result from the materials used to produce the same, Starpax is the owner of these Magnetodrones under Québec property law (sections 948 and 949 of the Civil Code of Québec).
The Civil Code of Québec, Section 948 states “Ownership of property gives a right to what it produces and to what is united to it, naturally or artificially, from the time of union. This right is called a right of accession. Section 949 states “The fruits and revenues of property belong to the owner, who bears the costs he incurred to produce them.”
The materials, methodologies, and equipment which produce the Magnetodrones are the property of the Company. Through the use of this property to produce the Magnetodrones, the Magnetodrones are deemed the “fruits” of such property. Therefore, the Company owns the Magnetodrones as the “fruits” (fructus) of Company property.
From time to time, by way of contract, the Company puts into place agreements whereby the Company grants a license to users of the Magnetodrones. The “user” in this context refers to anyone who uses the Magnetodrones in any way, not including patients. This does not constitute a sale of the bacteria resulting in a change of ownership. As such, this license does not result in a transfer of ownership of the property from the Company to the user, nor does this transfer a right to benefit from the fruit resulting from the reproduction of the Magnetodrone bacteria, should the client reproduce the Magnetodrones. By way of license, Starpax retains ownership of the Magnetodrone bacteria and the bacteria resulting from the reproduction of the Magnetodrone bacteria.

Pictured Above: an illustration of Starpax Magnetodrones with attached Starpax Liposomes.
Starpax Liposomes
The liposomes are nanoscopic spheres into which the anti-cancer drug is inserted. They are small unilamellar lipid bilayer vesicles, approximately 170nm in diameter. Each liposome encloses an aqueous compartment that contains the active molecule of the anti-cancer therapeutic agent. Liposomes containing the anticancer agent are attached to the surface of the Bn1-S Starpax bacteria. The combination of the Bn1-S Bacterium with the ‘loaded’ liposomes results in the Magnetodrones.

Pictured Above: an illustration of a Starpax Liposome.
The Company manufactures and controls all processes regarding the manufacturing of: (1) the Starpax Liposomes; (2) the Starpax Bn1-S bacteria; (3) attachment of the Liposomes to the Bn1- S bacteria (resulting in the formation of the Magnetodrones); (4) the packaging of the Magnetodrones into vials for treatment (injections). All manufacturing methods are proprietary and owned by the Company in full. The Company does not manufacture the anti-cancer drug, SN- 38. Instead, SN-38 is purchased from a manufacturer with an establishment license from the FDA. “Establishment license” refers to currently registered establishments which manufacture, prepare, propagate, compound or process drugs that are distributed in the U.S. or offered for import to the U.S.
The Magnetodrones are conceived to carry a wide variety of therapeutic agents - however, the Company has conducted research and development using the SN-38 anti-cancer therapeutic agent. For a more detailed description of SN-38, please see “SN-38, The Starpax Therapeutic Agent” below.
The Starpax PolarTrak
The PolarTrak is a patented Starpax device in which the patient is placed and aligned. It generates a three-dimensional magnetic sphere around the tumor with virtual monopole magnetic fields vectors directed from the perimeter of the tumor toward a focal point of convergence in the tumor (the “Focal Point”). Monopole magnetic fields make the Magnetodrones movements unidirectional and guide them in three dimensions. For example, the Earth has bipolar magnetic field with a “north” and “south” magnetic poles. Magnetic monopoles only have a single direction magnetic pole, whereby the field only exhibits a “north” or “south” pole. The PolarTrak has six magnetic field generators. By aligning these six magnetic field generators, the PolarTrak can create a magnetic-field sphere around the tumor.

Pictured Above: an illustration of a patient positioned in Starpax PolarTrak
The magnetic fields of the PolarTrak are relatively very weak, safe for human use, and are approximately 1,000 times weaker than an MRI for the patient. After the placement of the patient and alignment in the PolarTrak, the Magnetodrones are injected into the tumor, then the PolarTrak’s magnetic fields are turned on. While on, the PolarTrak moves the Focal Point throughout the volume of tumor, forcing the magnetic-sensitive Magnetodrones to distribute throughout the tumor volume. The magnetic sphere is also designed to keep the Magnetodrones captive inside the tumor, preventing the bacteria from circulating outside the tumor and into the blood system. By trapping the Magnetodrones (and thus the anti-cancer drug attached to them), adverse side effects of these drugs are substantially mitigated or avoided. Throughout the treatment, when the Magnetodrones pass by hypoxic zones, they stop swimming, penetrate and accumulate in the hypoxic zones because their oxygen level is the same as their culture medium. Magnetodrones that have not reached hypoxic zones continue to swim throughout the volume of the tumor guided by the PolarTrak and release the drug they transport along their path. This results in cancer cells in the tumor being attacked, including stem cells in hypoxic zones.

Pictured Above: The Starpax PolarTrak room has been inaugurated at the Jewish General Hospital in Montréal, Québec, Canada, for clinical trials.
The Starpax Treatment Process
The Starpax Technology treatment process to be used in the clinical trials consists of and is simplified into four steps as follows:
| (1) | Delivery of the Magnetodrones to the intervention room (the treatment facility); |
| (2) | Placement and alignment of the target tumor within the PolarTrak |
| (3) | Injection of the Starpax Dose directly into the target tumor |
| (4) | Engagement of the PolarTrak monopole magnetic fields |
| (1) | Delivery of the dose of Magnetodrones to the intervention room |
The Starpax Magnetodrones are manufactured at the Starpax GMP manufacturing plant. Anticancer drug is inserted into the Starpax Liposomes and these Liposomes are attached to the bacteria according to a proprietary Starpax process. Then syringes are filled with millions of Magnetodrones per dose and delivered to the intervention room. The number of Magnetodrones administered in any one treatment will depend on the prescribed dose.

Pictured Above:an illustration of Starpax Magnetodrones in vials ready to deliver to the intervention room.
| (2) | Placement and alignment of the PolarTrak with the target tumor |
Before injection of the Starpax Dose, the patient is placed within the PolarTrak. MRI images of the tumor are uploaded to the PolarTrak. Using this imaging, the magnetic generators are aligned on the patient to where the Focal Point is positioned in the midle of the tumor, waiting to be activated.

Pictured Above: an illustration of the Focal Point of magnetic fields as positioned in the tumor.
| (3) | Injection of the Starpax Dose directly into the target tumor |
The patient is still installed in the PolarTrak and the Magnetodrones are injected directly into the tumor using endoscope; by the mouth, the rectum or in some cases reaching the tumor by percutaneous injection.

Pictured Above: illustration of an injection of the Magnetodrones that is done through endoscopy (oral or rectal) or percutaneously (needle injection).
| (4) | Engagement of the PolarTrak monopole magnetic fields |
After the injection of the Magnetodrones into the tumor via endoscopy or percutaneous injection. The medical doctor engages the PolarTrak, activating the monopole magnetic fields. The PolarTrak has six magnetic field generators that allow for X,Y,Z-axis coordinates for the magnetic field(s), creating a magnetic sphere around the tumor to capture and trap the Magnetodrones within the three dimensions of the tumor. The PolarTrak is designed to create a magnetic Focal Point in the middle of the tumor that Magnetodrones follow during the treatment. This Focal Point will move throughout the volume of the tumor, forcing the Magnetodrones to distibute throughout the volume of the tumor. This is made possible by artificial intelligence creating algorithms that are based on the data transferred from magnetic resonance imaging system (MRI) of the patient’s tumor taken days before.

Pictured Above: an illustration of the magnetic sphere created by the PolarTrak to trap the Magnetodrones within the tumor.
Throughout the treatment, the Magnetodrones take refuge and accumulate in hypoxic zones because the bacteria’s culture oxygen level is similar to the hypoxic zones.

Pictured Above: an illustration of the Magnetodrones following the Focal Point of the magnetic fields that the PolarTrak moves with millimetric precision in 3D throughout the tumor.

Pictured Above: an illustration of the Magnetodrones penetrating and accumulating in the hypoxic zones of the tumor, attacking stem cells within the hypoxic zones.
The patient remains in the PolarTrak for approximately 30 minutes to ensure distribution in the whole tumor volume including hypoxic zones. The Magnetodrones die approximately 60 minutes after injection into the body because the human body temperature is too high for them to survive.
Starpax Solutions to Major Challenges in Systemic Cancer Treatment
The Company has identified five major challenges surrounding cancer treatments. The Starpax Technology addresses each of these issues in very specific ways as discussed below.
Challenge 1: Reaching the tumor
The same difficulties in delivering anti-cancer therapeutic agents to the tumor have persisted for over 100 years. Normally, in traditional chemotherapy, only a small fraction of the injected dose, 0.001% of the anti-cancer drug actually reaches the tumor in patients. Some improvements were achieved in the past few decades using nanoparticles, nano-carriers, antibody drug-conjugates or precision target drugs. However, many recently independent published studies found that a median of only 0.7% of the injected dose using these new methods (or drugs) reached the tumor.
The Starpax solution 1
Since the injection of the Starpax Dose is directly into the tumor, 100% of the Starpax Magnetodrones transporting the therapeutic anticancer agent reach the tumor. When utilizing the Starpax Technology, injection of the therapeutic anti-cancer agent is local and not systemic – meaning the anti-cancer drug is not sent throughout the bloodstream. Magnetodrones transporting the anticancer agent are injected directly into the tumor. By design, 100% of the dose stays in the volume of the tumor.
Challenge 2: Distribution of the therapeutic anti-cancer agent throughout the volume of the tumor
A meta-analysis of 117 scientific studies has shown that 90% of the tumor volume receives little or no drug at all during systemic administration of anti-cancer therapeutic agents. With systemic administration of the anti-cancer agent (through the bloodstream) diffusion of the drug throughout the volume of the tumor is restricted by the formation of hypoxic zones generating the deterioration of the blood vessels, which become dysfunctional.
During tumor formation, cancer cells reproduce rapidly and consume most of the oxygen around them, forming barriers called “hypoxic zones”. Hypoxia causes a slow-proliferating stem-cell-like phenotype of cells, decreases senescence, and results in the creation of chaotic and malfunctioning blood and lymphatic vessels. During tumor growth there is little lymphatic drainage in the tumor interior. The interstitial fluid pressure increases significantly, reflecting the inhibited drainage of the interstitial fluid by both degradation of the lymphatic vessels and pressure-induced lymphatic collapse.
Because traditional systemic chemotherapy relies on the bloodstream to distribute anti-cancer drugs to the tumor, when blood vessels and/or lymphatics are chaotic, malfunctioning, or collapsed, the means of distribution of the therapeutic agents throughout the volume of the tumor is inefficient.
The Starpax solution 2:
The Starpax Magnetodrones are designed to swim in the interstitial spaces of the tumoral tissues. In the context of the Starpax Technology, “Interstitial Spaces” refers to the fluids between the tumor cells. The Magnetodrones are small enough to move through the interstitial spaces of the volume of the tumor, into and through the interstitial spaces, but too big to enter into capillaries. This results in two effects. First, the Magnetodrones carrying the anti-cancer drug do not require blood vessels for distribution. Second, the Magnetodrones can travel between tumoral spaces to deliver the anti-cancer drug throughout the volume of the tumor.
The Magnetodrones are magnetotactic, which means they are sensitive to the very specific magnetic fields created by the Starpax PolarTrak. The PolarTrak generates monopole magnetic fields vectors and uses artificial intelligence to create a magnetic Focal Point that moves in three dimensions (turn right, turn left, go up, go down) in the volume of the tumor with millimeter precision. The Magnetodrones swim in the direction of the moving Focal Point throughout the tumor so that they distribute themselves through the tumoral tissue and interstitial spaces throughout the volume of the tumor – all while spreading the anti-cancer drugs along their path. This is made possible by the proprietary Starpax artificial intelligence creating algorithms that are based on the data transferred from magnetic resonance imaging system (MRI) of the patient’s tumor taken days before.
Challenge 3: Penetration into hypoxic zones where cancer stem cells are located
Oxygen in hypoxic zones is virtually absent. Recent research has shown that hypoxia within tumors is one of the major drivers of metastatic spread of cancer (the major cause of death by the disease).
Hypoxic zones in tumor are not vascularized, meaning it is extremely difficult for the drug to reach and penetrate the hypoxic zones when the drug is distributed through blood vessels (such as through systemic injection of the anti-cancer drugs). This low vascularization hypoxic zones creates a strong resistance to treatments. Several studies have demonstrated that chemotherapy, radiotherapy or immunotherapy are less effective in hypoxic zones.
The Starpax solution 3
The Starpax Bn1-S bacteria fundamental to the Starpax Magnetodrones are aerotactic. This means that they have been developed to live in extremely low oxygen environments. After injection of Starpax Dose, the Magnetodrones follow the Focal Point created by the PolarTrak. When Magnetodrones pass by a hypoxic region, they stop ‘swimming’ and take refuge within the hypoxic zones because the level of oxygen within the hypoxic zone is the same as their culture medium. While in the hypoxic zones, the Magnetodrones deliver the cancer-fighting drug to the stem cells contained in these areas.
One injection of the Starpax Dose contains millions of Magnetodrones. The Magnetodrones are aerotactic (sensitive to low level of oxygen), naturally detect, penetrate and accumulate in hypoxic areas. The Magnetodrones that are not in the hypoxic zones continue to spread throughout the volume of the tumor, gradually releasing the therapeutic anti-cancer agent along their path. Magnetodrones will die within approximately 60 minutes after injection, because the patient’s body temperature is too high for them to survive any longer.
Challenge 4: Side effects
Systemic treatments, such as chemotherapy, immunotherapy, and radiotherapy, have a severe detrimental effect on the patient’s body. Chemotherapy circulates toxic cancer-fighting drugs throughout the bloodstreams distributing these chemicals throughout the patient’s body, resulting in a destruction of all cells affected by the chemotherapy agent (good or bad). Radiotherapy (radiation) destroys all cells within the path of the radiation. Immunotherapy, may result in various and unexpected reactions within a patient’s body, including the infection of healthy tissue resulting in the generation of more cancerous cells.
The Starpax solution 4
The Starpax Magnetodrones are injected directly into the tumor. At that moment, the PolarTrak functions by creating a magnetic sphere around the tumor in order to keep the Magnetodrones captive inside the tumor.
The captive Magnetodrones are forced to distribute the anti-cancer drug only within the interstitial tissues of the tumor without entering the blood system. Magnetodrones are too big to enter in the capillaries blood vessels. By ‘trapping’ the Magnetodrones to the tumor area, the probability of generating side effects is drastically reduced.
Calculation of the Starpax Dose shows a distribution of 800 times fewer toxic molecules in the body than a reference systemic chemotherapy using the same anti-cancer therapeutic agent. The Bn1-S bacterium is non-pathogenic, does not proliferate in human body and dies within approximately 60 minutes after injection. Analysis of the preclinical studies has found no observed side effects on 100% animals, no inflammation and pathological changes in their liver, kidney, heart tissues or any organs.
Challenge 5: The Regulatory approval of new drug has a high failure rate
As of the date of this Offering Circular, the failure rate with the FDA-approval after human clinical trials is 96.6% (according to the FDA). Developing a new drug is a risky challenge. There is a low chance of passing toxicity tests and reaching treatment efficacy, as well as the regulatory risk of non-approval by the relevant regulatory agency.
The Starpax solution 5
The Company does not develop or manufacture anti-cancer drugs to attach to the Magnetdrones. Starpax Technology will use a drug (SN-38) that has been used for over 20 years to treat patients with a common traditional chemotherapy treatment that uses the SN-38 molecule, and is still used currently by most hospitals. Starpax Technology will administer a substantially smaller dose of SN-38 than this common FDA approved systemic chemotherapy treatment that uses the SN-38 molecule, meaning less toxicity. With 100% of the Starpax Dose being injected directly into the tumor and not circulating in the patient’s blood system the Company expects that there will be 50 times more drug inside the tumor and, 800 times fewer toxic molecules in the patient’s body, thus reducing the risk of toxicity and efficacy failure in clinical trials. The Polartrak generates magnetic fields that are 1,000 times weaker than those of an MRI used daily in hospitals, also reducing the risk of failure in clinical trials.
SN-38, The Starpax Therapeutic Agent
The Company’s preclinical studies used a therapeutic agent called SN-38. The SN-38 was encapsulated in the liposomes attached to the surface of the Starpax Bn1-S bacteria.
SN-38 is an antineoplastic drug. It is the active metabolite of the FDA-approved Irinotecan, a drug whose patent has expired and is one of the most used anti-cancer drugs in the world for first line and second line chemotherapy for colorectal cancers and pancreatic cancers. SN-38 prevents DNA unwinding by inhibition of DNA topoisomerase, which results in irreversible double strand breaks and ultimately cell death. It is widely known that SN-38 is one of the most potent CPT analogs (anticancer drugs family called Camptothecin) and shows enhanced anticancer potency against a broad range of solid tumors. Studies have demonstrated that SN-38 has 1,000 times more activity than Irinotecan itself. Instead of intravenous administration in the alkaline environment of the blood, the Starpax Magnetodrones are loaded with SN-38 and injected directly into the tumor, where there is higher level of acidity facilitating better solubility and potency of the SN-38. SN- 38 is the anti-cancer drug that will be used in the Company’s clinical trials. However, the Magnetodrones can be loaded with other anti-cancer drugs as well.
A Starpax Dose with Magnetodrones loaded with SN-38 will be approximately 16% of the dose the body of a patient is currently exposed to when receiving systemic irinotecan. However, with a Starpax Dose, the tumor’s exposure to SN-38 is calculated to be 50 times more than systemic injection of irinotecan.
Irinotecan was first discovered and synthesized in Japan by Yakult Honsha Co, Ltd, in 1983. It initially demonstrated strong activity against a broad variety of experimental tumors. Subsequently, clinical Phase I studies were initiated in Japan in 1986, in Europe in 1990, and in the United States in 1991. Many clinical studies have been published about the particularities, toxicity, and efficacy of the SN-38 as metabolite of Irinotecan.
In the United States, Irinotecan or liposomal Irinotecan is approved for use and is part of standard management guidelines for rectal, pancreatic, gastric, and esophageal cancers. A Trop-2-specific antibody/SN-38 conjugate* (sacituzumab govitecan-hziy) has shown clinical activity in epithelial cancers and been approved for use in the treatment of advanced/metastatic triple negative breast and urothelial cancers. Irinotecan or SN-38 have been shown to have clinical activity against other solid tumors including lung and head and neck cancers, and in vitro and in vivo activity against human prostate and breast cancer cell lines.
*Trop-2 is a transmembrane glycoprotein encoded by the Tacstd-2 gene. It is an intracellular calcium signal transducer that is differentially expressed in many cancers.
Three Therapeutic Benefits in One Treatment
Through its research and development process, the Company has identified three distinct therapeutic effects expected to occur during a single treatment using the Starpax Technology.
Local Chemotherapy:
The first therapeutic effect is local chemotherapy to the tumor. As stated above, by design, the Magnetodrones containing the anti-cancer drug are kept captive inside the tumor, avoiding the circulation of the anti-cancer drug to the rest of the body. The effect of this is a drastic reduction of the usual chemotherapy side effects. The Company’s preclinical studies resulted in 100% of the animal subjects showing little or no observed side effects from the Starpax Treatment.
Hypoxic Area(s) Treatment:
“Hypoxic Area(s) Treatment” means, in the present case, the ability to reach, penetrate and accumulate in hypoxic zones with anti-cancer drugs to kill the cancer stem cells located within the hypoxic zone. Hypoxic zones in tumor are not vascularized, meaning it is extremely difficult for the drug to reach and penetrate the hypoxic zones when the drug is distributed through blood vessels. This low vascularization hypoxic zones creates a strong resistance to treatments.
The Magnetodrones are able to distribute through tumor tissue into interstitial spaces, including within hypoxic zones within the tumor without using blood vessels. The Magnetodrones will then accumulate in the hypoxic zones due to the low level of oxygen within these zones that is the same as their culture medium. As they accumulate, they release the anti-cancer drugs in the hypoxic zones. This is a departure from the status quo of cancer treatment which is extremely ineffective in reaching hypoxic zones.
A preclinical study of human tumors grown in mice shows that the Starpax Bn1-S bacteria fill the tumor hypoxic regions and attack cancer cells in hypoxic “low oxygen” areas.
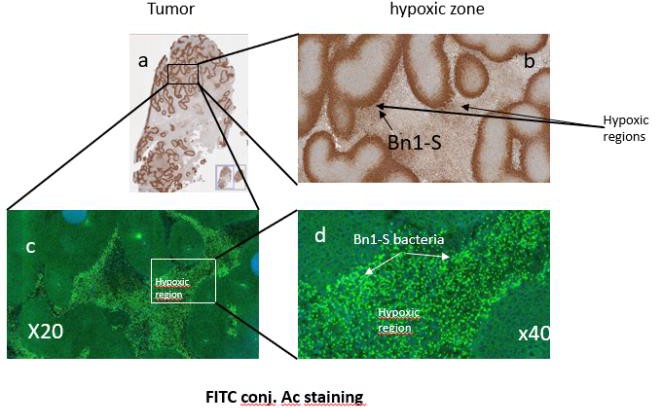
Pictured Above: Actual picture of Starpax Bn1-S bacteria/Magnetodrones accumulating within the hypoxia zones surrounding necrotic dead cells.
Studies demonstrate that radiation therapy, systemic chemotherapy and immunotherapy are ineffective in hypoxic zones where there is a lack of oxygen in the tissues.
Immunotherapy:
Third-party preclinical studies of cancer tumors grown in mice have shown that the Magnetodrones accumulate within tumor hypoxic zones. Additionally, the Bn1-S bacteria, part of the Magnetodrones, introduced in a tumor triggers the immune system, inducing deep infiltration of immune cells (T-cells, NK-cells, neutrophils and macrophages) in the tumor. Also, the preclinical trials have shown that injection of the Bn1-S bacteria into the tumor (alone without any drug) generates significant tumor regression. More independent studies in non-human primates brought data supporting the conclusion that the Starpax Bn1-S bacteria induce activation of immune cells. Based on literature and preclinical results, a treatment using the Starpax Technology may cause a systemic immune response, that could then attack floating cancer cells in the rest of the body and remote metastases, without any anti-cancer drug circulating within the body.
The Company does not count on immunotherapy to eradicate an advanced tumor. However, a patient’s immune response from the injection of the Starpax Bn1-S bacterium may result in the T- cells memorizing the cancer cells antigens. This might result in the reproduction and circulation of these T-cells in the blood system, recognize these antigens in the patient’s body and attack floating cancer cells and metastasis throughout the patient’s body without having the drug circulating in the bloodstream. Immune efficacy will be studied in the Phase II clinical trials.
Starpax believes these three therapeutic effects generate a strong synergy granting its Technology a superior advantage over single therapies.
Summary of the Preclinical Studies Results
The Company has conducted preclinical studies that are very promising and have prompted the Company to undergo this Offering to finance Phase I and Phase II human clinical trials using the Proceeds.
The preclinical safety of the Magnetodrones was assessed in rats, mice and non-human primates. The therapeutic agent encapsulated in the liposomes of the Magnetodrones used in the preclinical studies was SN-38. Preclinical studies were also conducted in two mice models to demonstrate the effect of SN-38 when administrated using the Starpax Technology by the measure of the tumors volume when compared to controls. Future clinical trials, including those financed using the Proceeds of this Offering will utilize SN-38.
The results of these preclinical studies demonstrated (1) a 100% remission rate in treated animals bearing HCT116 human tumors (one of the most aggressive cancer tumor types); (2) no observed side effects in animal test subjects; (3) no infection or inflammation in any organs (4) no organ damage.
For Phase I and Phase II clinical trials, calculation shows that the Starpax Dose is expected to distribute 800 times fewer toxic molecules in the body and 50 times more drug in the tumor than a reference systemic chemotherapy.
Clinical Development
The protocol of a clinical trial of an investigational drug to be conducted in the United States (US) must be submitted in an Investigational New Drug (“IND”) exemption that is reviewed by the US Food and Drug Administration and the study may be allowed to proceed if the proposed study and documentation provided is acceptable and does not subject the study subjects to an unreasonable risk of harm. The protocol of any clinical trial to be conducted in Canada must be approved by Health Canada after the agency has completed an assessment of the available data supporting the clinical trial.
The clinical development of an anticancer treatment encompasses various phases, the first of which consists in evaluating the adverse events and tolerance to a drug when increasing doses of the product are administered to human patients. This type of study is referred to in the industry as a “Phase 1” study.
The protocol of any clinical trial to be conducted in Canada must be approved by Health Canada after the agency has completed an assessment of the available data supporting the clinical trial. The objectives of the phase 2 oncology clinical trials include confirmation that the treatment shows some benefit such as slowing the growth of tumors, the evaluation of the best dose for the new treatment and the type of cancers that can be treated with the drug. Traditionally, the Phase 3 oncology studies have been providing the clinical efficacy and safety evidence for the approval of drug products when compared to standard treatments. However, over the last decade, worldwide regulatory agencies have increasingly considered that clinical trials combining two phases of the development could provide sufficient initial efficacy and safety evidence to grant the approval of anticancer treatments.
One type of study protocol that includes two clinical phases is referred to as an adaptive seamless clinical trial where the first step evaluates the safety of the treatment and a maximum tolerated dose, while the second step of the study assesses the treatment both from an efficacy and safety perspectives. The decisions on how to ‘adapt’ the study phases are made based on planned interim analysis of the data. The guiding principle for Phase I/II dose escalation studies in oncology is to avoid unnecessary exposure of patients to subtherapeutic doses of an agent while preserving safety.
Study Design of the proposed Phase I/II clinical trials
The proposed first-in-human study to be conducted using the Starpax Technology is a seamless Phase I/II study that will evaluate the safety, tolerability, and efficacy of escalating and multiple doses of the Magnetodrones in the treatment of solid cancer tumors following intra-tumoral injection and targeting via the Magnetodrones.
The study will be conducted in adult patients with the following solid tumor malignancies:
| o | Pancreas: local unresectable pancreatic ductal adenocarcinoma |
| o | Prostate: adenocarcinoma of the prostate (localized post Radiation Therapy failure) |
| o | Head & Neck: recurrent head and neck squamous cell carcinoma |
| o | Breast: cutaneous metastasis (breast cancer) |
| o | Colorectal: recurrent, unresectable rectal cancer and non-operable rectal cancer |
| o | Gynecologic: recurrent and non-operable vulva cancer |
Target tumor evaluations will be conducted by imaging scans (MRI or CT), and visual examination depending on the cohort.
The primary objectives of the study are:
(a) To evaluate the safety and tolerability of repeated administrations of escalating doses of Magnetodrones targeted to tumors
(b) To determine the maximum tolerated dose and recommended dosage of the Starpax Magnetodrones for the Phase 2 studies.
(c) To evaluate the effect of treatment on tumor size with repeated administrations of the Magnetodrones on targeted to tumors in each tumor-specific indication
| (d) | To evaluate the usability of the PolarTrak. |
Secondary objectives of the study include the evaluation of the immunogenicity of the Magnetodrones. The study will be conducted in a clinical research unit experienced in the conduct of Phase I/Phase II clinical trials. Each patient will receive one dose per week of Starpax Dose for a total 6 doses over the course of 6 weeks. In patients showing therapeutic benefits from the Starpax Treatments, treatment may be continued beyond 6 weeks, according to the same dose schedule in accordance with the respective hospital ethics committees overseeing administration of the clinical trials.
The initial Phase I/II clinical trial described above with the Starpax Technology will be performed using state of the art adaptive (Bayesian) statistical methodology. This approach monitors response rates in real time and will enable the Company to rapidly focus patient recruitment in the indications showing the best initial therapeutic benefits. This clinical trial methodology is thus designed to seamlessly transform our initial study into a Phase II trial with the potential for an initial product registration.
Considering the characteristic of the Starpax Technology and indications targeted, the Company intends to file for one or more of the FDA and Health Canada accelerated programs such as Fast Track, Breakthrough Technology, Priority Review, etc.
Starpax initial post-approval strategy for additional solid tumor indications
After obtaining FDA approval of a New Drug Application (“NDA”) for a first set of cancer indications corresponding to Unmet Medical Needs, the Company intends to pursue its clinical development to: 1) enlarge the number of solid tumor indications addressable by its technology, 2) justify using the Starpax Treatment as a first line treatment for solid tumors, 3) add other oncology therapeutics to its Magnetodrones and 4) expand the application of its platform technology to non-cancer diseases with substantial unmet medical needs such as cardiovascular indications (ex: ischemic heart disease), pulmonary disease (ex: pulmonary hypertension), neurological disorders (Ex: acute ischemic stroke in the brain) or ocular diseases (diabetic retinopathy).
Special Characteristics of the Company’s Operation that will have a material impact on the Company’s Financial Performance
Regulatory Considerations for the Company’s Products
The Company is subject to regulation of its Products before, during, and after approval by the FDA.
Also included in section 503(g) of the Federal Food, Drug and Cosmetic Act (FD&C Act) and the Code of Federal Regulations (21 CFR part 3), a combination product is a product comprised of two or more different types of medical products (i.e., a combination of a drug, device, and/or biological product with one another). The drugs, devices, and biological products included in combination products are referred to as constituent parts of the combination product.
A combination product is assigned to an Agency center that will have primary jurisdiction for that product’s regulation. The assignment of a combination product to a lead center is based on a determination of which constituent part provides the primary mode of action (PMOA) of the combination product. If the PMOA of a drug/device product combination product is attributable to the drug product, the Center for Drug Evaluation and Research (CDER)would be the lead center responsible for the review of such a drug product and would have primary jurisdiction for the regulation of the combination product.
Since the PMOA of the Starpax Magnetodrones is the drug mode of action, CDER will likely be the lead center for review, with consultations from both the Center for Biologics Evaluation and Research (CBER) with respect to proprietary bacteria carrier well as the Center for Devices and Radiological Health (CDRH) with respect to the PolarTrak device component.
The FDA review process – drug/biologic component.
The clinical phase of drug development focuses on testing new drugs on human volunteers. Phase 1 tests may involve dozens of volunteer subjects and are performed to ascertain if the new drug has side effects and how the human body metabolizes and excretes the drug. Phase 2 tests may involve hundreds of volunteer subjects and are used to gather preliminary data on the efficacy of the new drug on a specific condition or disease. These studies may compare the efficacy of the new drug against established drugs used to treat a condition or against placebos. After Phase 2 trials are completed, a sponsor will typically coordinate with the FDA to plan its Phase 3 trials. Phase 3 trials may involve thousands of volunteer participants, as the new drug is tested to evaluate its effectiveness and safety in many different population subsets.
Depending on the disease being targeted and the studies conducted to demonstrate safety and effectiveness, it may be possible to obtain FDA approval after completing a Phase 2 study(s).
Given the pharmacological activity and toxicity of some oncology products, often times it is unethical to subject healthy subjects to these types of products, or it is inappropriate to use a placebo control in the studies. Oncology studies may employ an approved drug as an active control or compare the investigational product to the standard of care for the particular cancer being targeted for treatment.
FDA has a number of programs to facilitate and expedite the development and availability of new therapies to patients with serious conditions while preserving standards for safety and effectiveness. The expedited programs include Fast Track designation or Breakthrough Therapy and approval may sometimes be based on surrogate endpoints (Accelerated Approval) and often be subject to a faster FDA review through the Priority Review designation.
During the clinical phase of drug development, the FDA may halt testing of a new drug, temporarily or permanently, if there are concerns for the safety of trial testing volunteers. Institutional Review Boards (“IRBs”) are also involved in reviewing the safety protocols for these trials and may halt testing of a new drug.
Once Phase III tests are concluded, a sponsor prepares and submits a New Drug Application (NDA) with the FDA. The FDA must approve the NDA before marketing of the new drug is permitted in the United States. The NDA contains the results of all new drug testing, including preclinical and clinical testing, along with other information about the new drug, including its chemical composition and manufacturing processes. The costs associated with preparation and submission of an NDA are significant, and the approval process can take years.
Given that the Magnetodrones involves the bacterium Bn1-S to be directed towards and stay contained with the tumor target where the drug is released, it is possible that CDER will request a consultative review with CBER to ensure the safety and disposition of the bacterium during the IND and ultimately the NDA review process.
After an NDA is submitted, the FDA has 60 days to assess if the NDA will be accepted for filing based whether the NDA is sufficiently complete. Upon acceptance of the filing by the FDA, a 180- day period review begins during which time the FDA conducts an in-depth review of the NDA’s contents to evaluate a sponsor’s research on the new drug’s safety and effectiveness. After the 180- day period ends, the FDA must either issue an approval letter for the NDA or a response letter to a sponsor addressing particular concerns. This 180-day period may be modified; the FDA and a sponsor may come to an agreement to alter the review period timeline.
The FDA subsequently reviews the labeling of the new drug to ensure relevant health information is present and inspects a sponsor’s manufacturing facility where a new drug is produced to confirm that the manufacturing of the new drug is compliant to standards.
After a sponsor’s new drug passes through these steps, the new drug approval process is complete. A sponsor is required to provide the FDA with periodic updates regarding the safety of the new drug.
The FDA review process – device component.
FDA will likely seek a consult from CDRH regarding the PolarTrak device component.
The FDA classifies medical devices into one of three classes based upon controls the FDA considers necessary to reasonably ensure their safety and effectiveness. Class I devices are subject to general controls such as labeling, adherence to good manufacturing practices and maintenance of product complaint records, but are usually exempt from premarket notification requirements. Class II devices are subject to the same general controls and also are subject to special controls such as performance standards and may also require clinical testing prior to approval. Class III devices are subject to the highest level of controls because they are life-sustaining or life- supporting devices.
Quality System Regulation and Good Manufacturing Practices for Medical Devices.
After FDA approval of the Starpax Magnetodrones, the PolarTrak device component of this combination product will be subject to FDA Quality Systems Regulation (“QS Regulation”) and Good Manufacturing Practices Manufacturers must establish and follow quality systems to help ensure that their medical device products consistently meet applicable requirements and specifications known as current good manufacturing practices. Continued Compliance with these QS Regulations and GMPs is an ongoing duty of the Company. On February 23, 2022, the FDA proposed a regulation which would incorporate the international standard specific for medical device quality management systems set by the International Organization for Standardization (ISO), ISO 13485:2016 Medical devices – Quality management systems – Requirements for regulatory purposes. The Company intends to use contract manufacturers. All of these contract manufacturers will be required by the Company to be ISO 13485:2016, and thus compliant with FDA GMP.
The Company does not anticipate that this proposed regulation will affect the operations of the Company, since the Company intends on complying with ISO 13485:2016 prior to the proposed rule change in development of its facilities.
Distribution of Company Products
The Company has assessed different business models to launch the Starpax Technology. The Company intends to develop its own cancer centers throughout the United States and later Europe and Canada (the “Starpax Cancer Centers”). These cancer centers will be the sole source of treatments using the Starpax Technology. The Company intends to own, staff, and operate these outpatient Starpax Cancer Centers. A Starpax Cancer Center will be comprised of a simple building with out-patient rooms, 10 PolarTrak devices, one CT Scanner and two MRI machines.
This go-to-market strategy offers faster market penetration along with the best financial returns for investors.
The United States market alone represents market opportunity of approximately 580 Starpax Cancer Centers, with a potential total of 1200 Starpax Cancer Centers when including Canada and Europe. A Starpax Cancer Center will be comprised of 10 PolarTrak, 2 MRIs and one computerized tomography scanner (“CT Scanner”). The conservative business plan is to build 25 Starpax Cancer Centers within a seven- year period. Serviceable Available Market (SAM) is significantly larger for Starpax Technology than for most new drugs or treatments because the Starpax Technology intends to address solid cancer tumors, which represent 90% of all types of cancer.
The Company will not engage in the development of the Starpax Cancer Centers using the Proceeds of this Offering. The Company has already completed the construction of its manufacturing plant for the Magnetodrones for the needs of the clinical trials described above. This manufacturing plant will also have the capacity to supply the first two Starpax Cancer Centers when they are eventually developed. The Proceeds of this Offering are primarily intended to be used to finance the Phase I and Phase II clinical trials. See “Use of Proceeds” section above. The Company intends to fund the development of the Starpax Cancer Centers through its own cash flows or possibly by one or more future offerings. However, different funding sources like mortgage loans on the real estate or equipment financings should be available for the Company, limiting the needs or size for future offerings.
Intellectual Property
The license for the 2 original patents for the virtual monopole magnetic field vector technology is under an exclusive license agreement, free of any debt, royalty. There is an option to acquire these two patents for $1.00, free of any future royalty, fee or other compensation, should the Company become publicly traded. The Company has expanded its patent portfolio to 31 issued patents and pending patents, with six issued patents and 25 pending applications in various jurisdictions including the United States Patent and Trademark Office (“USPTO”) the European Patent Office (“EPO”) and “Canadian Intellectual Property Office (“CIPO”) and other filings.
Company Facilities
The Company leases a 24,000 sq.ft. facility in Montréal, Québec, Canada. In January 2023, except for some specific areas, the Company took possession from a local general contractor specialized in GMP manufacturing plants and laboratory development, of a new GMP manufacturing plant and three laboratories for the production of the Starpax Magnetodrones and future R&D. The Company is in the process of completing the commissioning of its manufacturing plant and related equipment prior to initiating its clinical trials. The auditing process by Health Canada or FDA to obtain its GMP plant compliance certificate will follow. The Company does not own this property and is on a 5-year lease, with the option to renew at expiration. This space also includes office space.
Employees
The Company currently has 44 employees. 40 are full time employees, 4 are part time employees.
Legal Proceedings
There are no legal proceedings pending against the Company or its Management, including but not limited to bankruptcy or receivership proceedings.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion includes information from the audited financial statements for the year ended December 31, 2021, compared to the year ended December 31, 2020. All references to $ amounts are stated in thousands of US Dollars, except per Share data and otherwise noted. Furthermore, all historical amounts on a per Share basis and all figures related to a Share count reflect the subdivision of Shares that have taken place as at January 19, 2023. Use of “our” or “we” throughout this section refers to the Company.
The following discussion contains forward-looking statements that reflect our plans, estimates, and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements.
Overview
Starpax Biopharma Inc. (the “Company”) was incorporated in Canada on December 19, 2017, under the name Starpax Medical Inc. The legal name of the Company was modified on June 9, 2022, to Starpax Biopharma Inc.
The Company’s founder, Michel Gareau, an entrepreneur with 41 years of experience as Managing Director and founder of Ipax Capital which specialized in high technology investments through a Private Equity Co-investment Fund, Ipax 1 llp., came across, in 2016, the concept of a revolutionary technology: virtual monopole magnetic field vectors. This technology, then covered by two patents, had been in development for 17 years, involving approximately 180 professionals, multiple medical research universities and the department of Computer and Software Engineering of Montréal Polytechnique University. Following thorough analysis and due diligence, Mr. Gareau acquired, in 2017, the exclusive and perpetual license of the two patents through a new Canada-based limited liability partnership, Medpax Consortium LLP, which later became Ipax 2 llp. The MG Trust owns the vast majority of the units forming Ipax 2 llp’s capital.
Since the acquisition of the license by Mr. Gareau in 2017, more than 300 professionals (MDs, co- investigators, oncologists, pharmacokinetic and pharmacodynamic specialists, biochemists, microbiologists, artificial intelligence experts, electromagnetism and medical device engineers) have collaborated to transform a promising concept into a revolutionary multidisciplinary cancer treatment therapy with the potential of addressing solid cancer tumors and multiple non-cancer diseases.
The Company’s technology aims to treat solid cancer tumors without causing the side effects usually resulting from chemotherapies, radiation therapies and immunotherapies. Using its precision 3D guidance technology, which comprises of two major elements that cannot be dissociated (the PolarTrak and the Magnetodrones), the Company’s Technology is designed to deliver 50 times more drug inside the tumor than chemotherapy treatments, without circulating in the bloodstream, resulting in 800 times fewer toxic molecules inside a patient’s body. Preclinical trials on animals have shown a 100% remission rate in treated animal bearing HCT116 human tumors (one of the most aggressive cancer tumor types), with no sign of systemic inflammation or pathological changes to the organs. The Company is getting ready to proceed with clinical trials on humans in 2023.
Results of Operations
Year ended December 31, 2021, Compared to Year ended December 31, 2020
Revenues
The Company’s revenues for the year ended December 31, 2021, were $0 compared to $0 for the year ended December 31, 2020. During these periods, the Company was primarily focused on research and development.
Operating Expenses
The Company’s operating expenses for the year ended December 31, 2021, were $4,479 compared to $2,991 for the year ended December 31, 2020. The 50% increase was mainly due to the increase in salaries, resulting from new hires of personnel to pursue research and development and to support operations.
Operating expenses consist primarily of: (i) professional and consulting fees, (ii) salaries and benefits, (iii) research and development, and (iv) rent. For the year ended December 31, 2021, the Company’s professional and consulting fees amounted to $2,131 compared to $1,449 for the year ended December 31, 2020. The increase was due to higher legal and consulting fees in respect of research and development. The Company is eligible to Canadian and Québec’s tax credits on research and development activities, and these credits are recorded as a reduction to research and development expenses. These credits amounted to $1,463 for the year ended December 31, 2021, and $1,142 for the year ended December 31, 2020.
Net Loss
As a result of the foregoing, the Company realized a net loss of $4,552 for the year ended December 31, 2021, compared to net loss of $3,034 for the year ended December 31, 2020.
Six months ended June 30, 2022, Compared to six months ended June 30, 2021
Revenues
The Company’s revenues for the six months ended June 30, 2022, were $0 compared to $0 for the six months ended June 30, 2021. During these periods, the Company was primarily focused on research and development.
Operating Expenses
The Company’s operating expenses for the six months ended June 30, 2022, were $3,405 compared to $1,890 for the six months ended June 30, 2021. The 80% increase was mainly due to the increase in professional and consulting fees.
Operating expenses consist primarily of: (i) professional and consulting fees, (ii) salaries and benefits, and (iii) research and development. For the six months ended June 30, 2022, the
Company’s professional and consulting fees amounted to $1,727 compared to $909 for the six months ended June 30, 2021. The increase was due to higher legal and consulting fees in respect of administration and research and development. Federal and Québec’s tax credits on research and development activities, recorded as a reduction to research and development expenses, amounted to $1,007 for the six months ended June 30, 2022, and $737 for the six months ended June 30, 2021.
Net Loss
As a result of the foregoing, the Company realized a net loss of $3,520 for the six months ended June 30, 2022, compared to net loss of $1,925 for the six months ended June 30, 2021.
Liquidity and Capital Resources
As of the date of this Offering Circular, the Company has not generated any revenues from operations. As of December 31, 2021, the Company had cash of $7,892 compared to $5,573 for the year ended December 31, 2020. The increase can be attributed to cash proceeds from financing activities including equity capital raising and an increase in long-term debt. Over the next twelve months, the Company intends to fund its operations from drawings from long-term debt agreements and the proceeds from this Offering.
Historically, the Company has been funded primarily from equity through private placements. The Company has incurred an accumulated deficit of $10,528 as of December 31, 2021, and expects additional deficits in the future. We believe this Offering under Regulation A+ will provide sufficient funds for the Company to support its planned operations, including its Phase 1 clinical studies, in 2023.
Recent Offerings of Securities and Outstanding Debt Subsequent events (January 1, 2022, to February 7, 2023)
On February 15, 2022, the Company granted 75,000 options to purchase Class A common Shares to an officer at an exercise price of $0 per share with an expiry date of February 14, 2032. The fair value of the options was estimated at $5 per share option at the grant date for a total of $353 using a Black-Scholes option pricing model. 25,000 of those options were vested and exercised on October 18, 2022 for a cash consideration of $0.012 and 25,000 Class B common shares were repurchased and cancelled for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
From February 7, 2022, to December 31, 2022, the Company issued 829,915 Class A common shares for a total of $4,747 and 829,915 Class B common shares were repurchased and cancelled for a cash consideration of $13. 191,665 Class A common shares were issued at a price per share of approximately $5 (CAD $6) and 638,250 Class A common shares were issued at a price per share of approximately $6 (CAD $8). The Company used the proceeds for (i) investments in property and equipment assets, (ii) research and development expenses and (iii) general & administration expenses.
On March 25, 2022, an additional amount of $427 was drawn under the repayable contribution agreement with Economic Development Agency of Canada for the Regions of Québec. The difference between the fair value and the amount received by the Company is recorded as a grant totaling $202 in reduction of property and equipment assets.
On April 8, 2022, on May 16, 2022, on July 28, 2022, on August 11, 2022, and on November 15, 2022, amounts of respectively $2,193, $958, $530, $257, $1,094 have been drawn from the Investissement Quebec loan.
On October 14, 2022, the Company received an amendment from Economic Development Agency of Canada to push the completion of the financed project from March 31, 2023, to June 30, 2024. Therefore, the payment schedule was altered, and the fair value of the loan was recalculated using an effective interest rate of 10% to reflect this change. The impact of the change is a decrease of $85 of the fair value of the loan and an increase in grants.
On November 22, 2022, the Company granted 15,000 options to purchase Class A common Shares to an employee at an exercise price of $6 per share with an expiry date of November 21, 2032. The fair value of the options was estimated at $3 per share option at the grant date for a total of $43 using a Black-Scholes option pricing model.
On November 22, 2022, the Company granted 525,000 options to purchase Class A common Shares to directors at an exercise price of $0.016 per share all with an expiry date of November 21, 2032. The fair value of the options was estimated at $6 per share option at the grant date for a total of $3,130 using a Black-Scholes option pricing model.
On November 22, 2022, the Company granted 105,000 options to purchase Class A common Shares to a director at an exercise price of $6 per share with an expiry date of November 21, 2032. The fair value of the options was estimated at $3 per share option at the grant date for a total of $299 using a Black-Scholes option pricing model.
On December 13, 2022, the Company, and the medical entity where clinical trials will take place, have entered into an agreement whereby the Company will receive a payment of $2.3 million upon the commencement of its clinical trials. The receipt of these funds is contingent on the Phase I trials commencing.
On January 19, 2023, the Company amended its Articles (i) converting all outstanding Class A Common Shares and Class B Common Shares into shares of a single class called common shares (“Common Shares”) and (ii) executing a 5:1 stock split, subdividing all its issued and outstanding Common Shares into five (5) such Common Shares.
Year ended December 31, 2021
During the year ended December 31, 2021, the Company issued 3,272,370 Class A common shares for a cash consideration of $15,578 and repurchased and cancelled 3,272,370 Class B common shares for a cash consideration of $55.
During the year ended on December 31, 2021, an additional amount of $632 has been drawn from the repayable loan from Economic Development Agency of Canada.
During the year ended on December 31, 2021, the Company entered into an agreement with Investissement Québec (IQ) for a loan of a maximum amount of $5,522 (CAD $7,000) under the ESSOR program to finance the establishment of a pilot plant. The first draws were made subsequent to year end.
Year ended December 31, 2020
During the year ended December 31, 2020, the Company issued 1,268,330 Class A common shares for a cash consideration of $5,800 and repurchased and cancelled 1,268,330 Class B common shares for a cash consideration of $19.
On May 15, 2020, the Company entered into an interest-free repayable contribution agreement with Economic Development Agency of Canada for the Regions of Québec for a maximum amount of $1,578 (CAD $2,000) to finance the establishment of a pilot plant as well as the construction of an intervention room dedicated to oncological research, and an amount of $274 was drawn during the year ended December 31, 2020.
Indebtedness
On May 15, 2020, the Company entered into an interest-free repayable contribution agreement with Economic Development Agency of Canada for the Regions of Quebec for a maximum amount of $1,578 (CAD $2,000) to finance the establishment of a pilot plant as well as the construction of an intervention room dedicated to oncological research. This repayable contribution is secured by a movable hypothec in the amount of $1,578 (CAD $2,000) and an additional hypothec of $316 (CAD $400) on laboratory equipment with a net book value of $1,616 (CAD $2,049) as at December 31, 2021. As per the amended agreement, the project must be completed by June 30, 2024, and shall be repaid in 60 equal and consecutive monthly installments beginning 36 months after the completion date of the project. As of December 31, 2021, an additional amount of $632 ($274 as at December 31, 2020) has been drawn from the repayable contribution. The amount of $354 ($136 in the year ended December 31, 2020) was recognized as a grant due to the below-market interest rate has been recorded in reduction to the carrying value of property and equipment assets. The remaining loan balance of $473 is included in long-term debt.
On April 9, 2021, the Company entered into a financing agreement with Investissement Québec (IQ) to finance the establishment of a pilot plant through investment in Class A common stock for a total amount of $3,945 (CAD$5,000) and a loan for a maximum amount of $5,522 (CAD $7,000) under the ESSOR program. The loan will bear interest at a fixed rate of 10%. Interest will be capitalized monthly for a period of 36 months from the first drawing, and thereafter will be payable. The loan and capitalized interests must be repaid in 84 equal and consecutive monthly installments starting 36 months after the first drawing. The loan is secured by a first rank movable hypothec in the amount of $5,522 (CAD $7,000) and an additional hypothec in the amount of $1,104 (CAD $1,400) encumbering the universality of the Company’s equipment, with a net book value of $1,695 (CAD $2,149) as at December 31, 2021, except with regard to laboratory equipment, not exceeding $1,893 (CAD $2,400) which is first ranked in favor of Economic Development Agency of Canada for the Regions of Québec, and for which the hypothec in favor of IQ is second rank (net book value of laboratory equipment of $1,616 (CAD $2,049) as at December 31, 2021). In relation to this agreement, the Company incurred $89 of deferred financing costs which were recorded in deferred financing costs. The first draws were made subsequent to year end.
Liquidity
The Company has incurred recurring losses from operations, and as at December 31, 2021, had an accumulated deficit of $10,528 (2020 - $5,921) and a working capital of $9,987 (2020 –$6,856). The working capital as at December 31, 2021 is sufficient to pay its operating expenses for a period of at least 12 months from the date of the financial statements. The Company’s continued existence is dependent upon its ability to continue to execute its operating plan and to obtain additional debt or equity financing. The Company has developed plans to raise funds and continues to pursue sources of funding that management believes, if successful, would be sufficient to support the Company’s operating plan. During the year ended December 31, 2021, the Company raised $15,578 through equity issuances and through the availability in its debt financing arrangements which was partially utilized subsequent to year end. The Company’s operating plan is predicated on a variety of assumptions including, but not limited to, success in research objectives, cost estimates, its ability to continue to raise additional financing and the state of the general economic environment in which the Company operates. There can be no assurance that these assumptions will prove accurate in all material respects, or that the Company will be able to successfully execute its operating plan. In the event that the Company is not able to raise capital from investors in a timely manner, the Company will explore available options. In the absence of additional appropriate financing, the Company may have to modify its plan or slow down the pace of research and development.
Plan of Operations
As part of its plan of operations, the Company intends to meet the following milestones over the course of the next 12 months:
- Construction of a GMP Manufacturing Plant
- In 2023, the Company expects to complete the commissioning of a new GMP manufacturing plant and related equipment for the production of Starpax Magnetodrones and three laboratories.
- In 2023, the Company expects to proceed with its Phase 1 clinical studies on humans for six Unmet Medical Needs cancer indications: 1) non operable rectum, 2) pancreas, 3) head & neck, 4) breast recurrence, 5) vulva, 6) prostate. Other indications, such as cervical, bladder, liver and kidney will be considered depending on the timing and availability of the medical resources.
Trend Information
In January 2023, except for some specific areas, the Company took possession from a local general contractor specialized in GMP manufacturing plants and laboratory development, of a new GMP manufacturing plant and three laboratories for the production of the Starpax Magnetodrones and future R&D. The Company shall complete the commissioning of its manufacturing plant and related equipment prior to initiating its clinical trials in 2023. Investments in property and equipment will therefore be significant.
The Company will also need to hire new production employees, and procure chemical products and consumables to supply the clinical studies, impacting primarily research and development expenses.
In 2023, the Company intends to proceed with its Phase 1 clinical studies, further impacting research and development expenses.
Relaxed Ongoing Reporting Requirements
If we become a public reporting company in the future, we will be required to publicly report on an ongoing basis as an “emerging growth company” (as defined in the Jumpstart Our Business Startups Act of 2012, which we refer to as the JOBS Act) under the reporting rules set forth under the Exchange Act. For so long as we remain an “emerging growth company”, we may take advantage of certain exemptions from various reporting requirements that are applicable to other Exchange Act reporting companies that are not “emerging growth companies”, including but not limited to:
| · | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| · | taking advantage of extensions of time to comply with certain new or revised financial accounting standards; |
| · | being permitted to comply with reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and |
| · | being exempt from the requirement to hold a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
If we become a public reporting company in the future, we expect to take advantage of these reporting exemptions until we are no longer an emerging growth company. We would remain an “emerging growth company” for up to five years, although if the market value of our Common Stock that is held by non-affiliates exceeds $700 million as of any June 30 before that time, we would cease to be an “emerging growth company” as of the following December 31.
If we do not become a public reporting company under the Exchange Act for any reason, we will be required to publicly report on an ongoing basis under the reporting rules set forth in Regulation A for Tier 2 issuers. The ongoing reporting requirements under Regulation A are more relaxed than for “emerging growth companies” under the Exchange Act. The differences include, but are not limited to, being required to file only annual and semiannual reports, rather than annual and quarterly reports. Annual reports are due within 120 calendar days after the end of the issuer’s fiscal year, and semiannual reports are due within 90 calendar days after the end of the first six months of the issuer’s fiscal year.
In either case, we will be subject to ongoing public reporting requirements that are less rigorous than Exchange Act rules for companies that are not “emerging growth companies”, and our stockholders could receive less information than they might expect to receive from more mature public companies.
DESCRIPTION OF PROPERTY
GMP Manufacturing Plant and Laboratories
The Company leases a 24,000 sq.ft. facility in Montréal, Québec, Canada. In January 2023, except for some specific areas, the Company took possession from a local general contractor specialized in GMP manufacturing plants and laboratory development, of a new GMP manufacturing plant and three laboratories for the production of the Starpax Magnetodrones and future R&D. The Company is in the process of completing the commissioning of its manufacturing plant and related equipment prior to initiating its clinical trials. The auditing process by Health Canada or FDA to obtain its GMP plant compliance certificate will follow. The Company does not own this property and is on a 5-year lease, with the option to renew at expiration. This space also includes office space.
DIRECTORS, OFFICERS, AND SIGNIFICANT EMPLOYEES
Directors
| NAME | | POSITION | | AGE | | TERM |
| Michael Gareau | | Chairman of the Board of Directors | | | 70 | | | | March 2018 - present | |
| André Monette | | Director | | | 82 | | | | March 2018 - present | |
| John Helou | | Director | | | 65 | | | | May 2022 - present | |
| Lisa Matar | | Director | | | 51 | | | | October 2022 - Present | |
| Berthe Latreille | | Director | | | 69 | | | | October 2022 - Present | |
| Dr. Jacques Jolivet | | Director | | | 69 | | | | October 2022 - Present | |
| Pierre Dozois | | Director | | | 76 | | | | December 2017 - present | |
Officers
| NAME | | POSITION | | AGE | | TERM | | APPROX HOURS PER WEEK |
| Michael Gareau | | President and CEO | | | 70 | | | | March 2018 - present | | | Full time |
| Thierry Pagé | | Executive VP and COO | | | 51 | | | | March 2018 - present | | | Full time |
Jean-François
Pruneau | | Executive VP
and CFO | | | 52 | | | | January 2022 - present | | | Full time |
| Dr. Sylvain Martel | | Vice President Technology | | | 63 | | | | October 2018 - present | | | Full time |
| Pierre Dozois | | Corporate Secretary | | | 76 | | | | December 2017 - present | | | Full time |
Significant Employees
| NAME | | POSITION | | AGE | | TERM | | APPROX HOURS PER WEEK |
| Dr. Marie Estelle Page Clisson | | Director Biopharmaceutical Development | | | 54 | | | | December 2021 - present | | | Full time |
| Dr. Mahmood Mohammadi | | Director Microbiology Research and Development | | | 59 | | | | March 2020 - present | | | Full time |
| Nathalie Rousseau | | Director of the Bn1-S Bacteria and Starpax Magnetodrones production plant | | | 55 | | | | March 2021 - present | | | Full time |
| Sebastien Naud | | Director Quality Assurance | | | 44 | | | | March 2019 - present | | | Full time |
| Dumitru Loghin | | Director Electromagnetic Engineering and
Medical Device | | | 40 | | | | April 2020 - present | | | Full time |
| Dr. Maxime Latulippe | | Director Artificial Intelligence and Algorithms | | | 37 | | | | July 2019 - present | | | Full time |
| Charles Tremblay | | Director Medical Technologies | | | 43 | | | | April 2020 - present | | | Full time |
Directors
Michael Gareau, Chairman of the Board of Directors, President, and CEO
Mr. Gareau has 41 years of experience as serial entrepreneur and CEO at various high-technology companies. He was also the founder and managing director of Ipax Capital with its Ipax One Private Equity Co-investment Fund, which was awarded “top 10 best-performing buyout fund’’ out of 20,500 private equity funds analyzed in the world by Preqin, a well-known London-based firm with 300 full time researchers and 14 global offices providing alternative assets data and analytics. Mr. Gareau has initiated over 32 acquisitions in eight different countries. He holds a P. Eng., M.Sc. Adm, MBA degrees from Sherbrooke University. He was awarded by the United States Government “Individual of Extraordinary Ability, O-1A’’ in 1999.
André Monette, Director
Mr. Monette was President of Johnson & Johnson France, where he executed a spectacular growth strategy during his presidency. Mr. Monette has a long experience in management at all levels of a company’s different departments, having worked his way up through the ranks and consecutively held the positions with Johnson & Johnson Canada as Production Engineer, Vice-President Manufacturing, Vice-President Finance, and then President of Johnson & Johnson France. He was later appointed President of Janssen Pharmaceuticals Canada, a company part of the Johnson & Johnson group. Mr. Monette graduated magna cum laude as a Chemical Engineer from Polytechnique Montréal University. He has received a diploma in management from McGill University and a diploma in advanced management from Harvard University.
He served as an independent board director in the audit committees and governance committees of large institutions over the years.
John Helou, Director
John Helou was President of Pfizer Canada for eight years until retirement in 2020. A seasoned biopharmaceuticals and biotech industry executive with over 37 years of experience. He was Chair of the Board of Directors for Pfizer Canada and the Canadian Hospira Healthcare Corporation (following the acquisition) until retirement. He has led a biotech commercial Startup, and managed multiple integrations, divestitures and reorganizations that have always focused on increased shareholder value, operational fluidity, and employee engagement. John has been a strong advocate for the life sciences sector and health care delivery with government and policy makers. He was actively involved in several industry, business, and community associations. He was Chair and later Vice Chair to the Board of Directors of Innovative Medicines Canada (IMC), member of the Board of Directors of BIOTECanada, a member of the Business Council of Canada, as well as Chair and Board member of the Advanced Coronary Treatment (ACT) Foundation. In 2014, he was inducted into the Canadian Marketing Hall of Fame.
Lisa Matar, Director
Lisa Matar is a Global Life Science executive with more than 25 years of experience leading organizations and driving significant growth, profitability, and launching excellence across the globe. During her 25-year career with Eli Lilly, she has held various senior roles in marketing, sales and General Management. Her extensive experience covers a variety of therapeutic areas such Oncology, Diabetes, Cardiology and Auto-Immunes in both Europe and Canada. She has most recently served as President and General Manager of Eli Lilly Canada from 2013 to February 2020 and was a member of the North American executive committee at Eli Lilly. She was a member of the 30% club Canada advocating growth through diversity. Lisa previously served on the board of directors of Innovative Medicine Canada (IMC) and is a past chair of the Ethical and Compliance committee at IMC. Lisa currently also serves on the board of Directors of Delphi Diagnostics, Inc. Lisa graduated with a Doctorate in Pharmacy from St Joseph University, Lebanon.
Berthe Latreille, Director
Berthe Latreille’s career at JP Morgan spans over 30 years. She was a Managing Director and served as COO of EMEA Investment Banking for over 10 years. Berthe has frontline Pan- European business experience across all Corporate Finance Products, as well as strong regulatory, compliance, risk and governance capability. As COO, Berthe oversaw JPM EMEA’s investment bank operations. She was a change maker in operational areas including a reorganisation of the management of the analysts and associates, the set-up of the Centralised Research Group in Mumbai, the creation of the Diversity and Inclusion Council. She helped shape and drive forward critical strategic initiatives in ESG and Digital Investment Bank. Berthe served on the Board of JPMorgan Ireland Plc and was member of the audit committee. She is presently on the Advisory Board of UK start-ups Greeenworkx and CrowdSurf. Berthe has a BAA (Finance) from the University of Montréal (HEC) and speaks French, English and Italian.
Dr. Jacques Jolivet, Director
Jacques Jolivet M.D. is a medical oncologist with a background in the preclinical and clinical pharmacology of anticancer agents. Following a fellowship at the National Cancer Institute in Bethesda, MD from 1980 to 1983, he held research grants from the Medical Research Council of Canada and the National Cancer Institute of Canada from 1983 to 1998. Dr. Jolivet then transferred to the pharmaceutical industry occupying various positions in oncology drug development at BioChem Pharma Inc., Shire Pharmaceutical Development Inc. and Aegera Therapeutics Inc. He was also active as a consultant for various biotechnology and pharmaceutical companies and has extensive experience in clinical oncology Dr Jolivet has authored 97 scientific publications. He is currently Chief Medical Officer at Alethia Biotherapeutics Inc.
Pierre Dozois, Director and Corporate Secretary
Pierre Dozois, LLL (University of Montréal), MBA (Harvard Business School), co-founded BCF llp in 1997 and lead its commercial and securities law practice and was instrumental in making BCF one the prominent business law firms in Québec. Having the benefit of more than 50 years of experience in several business law fields, his practice has been primarily focused on securities and mergers and acquisitions and on public and private financing. Mr. Dozois has, over the years, served, and still does, on several boards of directors of companies as well as non-profit and charitable organizations, both private and public, and still does.
Officers
Thierry Pagé, Executive Vice President and Chief Operating Officer
Thierry holds a Chemical Engineering degree from Polytechnique Montréal University (Canada) with graduate studies in applied sciences at the Université Catholique de Louvain (Belgium) and in Management at MIT Sloan, Cambridge (USA). Thierry is a seasoned executive with a successful track record in entrepreneurial management, technology development, commercialization and licensing, company operations, intellectual property, lean management and financing in several industries including high tech and health technologies. Before joining Starpax Biopharma, Mr. Pagé held a number of senior positions, including that of founding CEO at Odotech Inc. and Senior Director, Sciences & Engineering at Univalor (the Technology Transfer Office of Université de Montréal, Polytechnique Montréal, HEC and 6 affiliated research hospitals).
Jean-François Pruneau, Executive Vice President and Chief Financial Officer
Mr. Pruneau recently retired as President and CEO of Videotron (revenue of CAD $3.6 billion in 2021), a leading telecommunication and cable company in Canada and joined as Starpax partner with the founding president. He previously served as Senior VP and CFO of Québecor (TSX: QBR-B, CAD $7.5 billion market capitalization) and numerous senior positions including Director of Corporate Finance, Assistant Treasurer, Treasurer, and Vice President Finance of Québecor, Québecor Media, Videotron and Sun Media Corporation. He previously occupied treasury positions with BCE Media Inc. His honours and distinctions include the award in the “Financial Executive of a Large Corporation” category in the 2015 “Aces of Finance” competition, organized by the Québec section of Financial Executives Canada. Jean-François holds an M.Sc. in Finance from HEC Montréal and has been a member of the Montréal chapter of the CFA Institute since 2000.
Dr. Sylvain Martel, Vice President Technology
Dr. Martel holds a Post Doctorate from the Massachusetts Institute of Technology (MIT) and a Doctorate Ph.D. in Electrical/Biomedical Engineering from McGill University. He has over 35 years of experience in R&D including 10 years at the Massachusetts Institute of Technology (MIT). Dr. Martel is the inventor of the PolarTrak and is considered a world-renowned expert in medical nanorobotics. He authored over 300 scientific publications and his work has been recognized and received several times prestigious awards such as the Queen Elizabeth II Diamond Jubilee Medal for excellence in scientific contributions and realizations.
Significant Employees
Dr. Marie Estelle Page Clisson, Director Biopharmaceutical Development
Pharmacist by training, she completed her Ph.D. in Pharmaceutical Science at Paris XI University, in addition to 4 years of Hospital residency program. She began her career in France working for Sanofi, Merck Generics and Mylan, for a total of more than 18 years. She moved to Canada 6 years ago to join Pharmascience. Over the years, she has developed a specific expertise in the Pharmaceutical Development of sterile products including the technical and regulatory aspect for EU, Australasia, and US submissions. She held varied management positions in R&D, Technology Services (Life Cycle Management, Technology Transfer) and Global Operations.
Dr. Mahmood Mohammadi, Director Microbiology Research and Development
Dr Mohammadi holds a Ph.D in microbiology and molecular genetics from Université Laval. For the past 22 years, Dr. Mohammadi has been working on predecessors of the Bn1-S bacterial transporter owned by Starpax Biopharma. He developed in laboratory the only steerable, self- propelled, magnetotactic and aerotactic bacterial transporter intended for the human body. Dr. Mohammadi is a world expert on the integration of magnetotactic bacteria as bio-actuators and controllable nano-self-propulsion systems for critical applications such as tumor targeting for enhanced therapeutic effects in cancer therapy. He has been cited more than 100 times in specialized scientific journals, documentaries and TV interviews, and contributed to more than 50 scientific papers on the subject.
Nathalie Rousseau, Director of the Bn1-S Bacteria and Starpax Magnetodrones Production Plant
Nathalie Rousseau holds a M.Sc. in Microbiology-Immunology from Laval University. She has been working in the pharmaceutical industry mainly in manufacturing of aseptic products, biologicals, and medical devices for more than 20 years. After a few years in Quality Assurance, she held management positions in manufacturing at Sabex, Biomatrix, Draxis and Telesta Pharma, where she was involved in a BLA submission. At Prometic Bioproduction, she managed BLA submissions as Regulatory Affairs Manager. She has a lot of experience in Chemistry, Manufacturing and Controls and excellent knowledge of American, Canadian, and European GMPs and was involved in several routine audits as well as Pre license inspections by the FDA.
Sébastien Naud, Director of Quality Assurance
Sébastien Naud has been working in the pharmaceutical and medical device industry for more than
20 years. He started his career in operations, specializing in developing, validating and continuously improving manufacturing, cleaning and sterilization processes for sterile injectables & vaccines industries (eg. Sandoz Canada, GSK). His experience led him to work as a quality expert on developing new medication and maintaining GMP in short life-cycle radiopharmaceutical manufacturing at Jubilant DraxImage. Sébastien Naud acted as a quality representative alongside Canadian and American agencies in routine GMP inspections and PAI inspections. Sébastien Naud holds a B.Sc in biochemistry from McGill University.
Dumitru Loghin, Director Electromagnetic Engineering and Medical Device
Mr. Loghin obtained his master’s degree in Biomedical Engineering in 2016 from Polytechnique Montréal University, and his bachelor’s degree in Electronic Engineering in 2004 from the Technical University of Moldova. He received training in nuclear resonance sequence programming for Siemens Magnetom and Ethics of animal research. Prior to joining Starpax, he was a Research Assistant at the Nanorobotics Laboratory, Department of Computer and Software Engineering, and the Institute of Biomedical Engineering at Polytechnique Montréal University, where he was responsible for the hardware and software development with several associated algorithms of an experimental platform that was a precursor to the Starpax PolarTrak 1000. He was also involved in the preclinical tests and R&D of several experimental platforms, where he is co-author of six research publications.
Dr. Maxime Latulippe, Director Artificial Intelligence & Algorithms
Dr. Maxime Latulippe holds a Ph.D. from Polytechnique Montréal University in biomedical engineering, specializing in innovative applications of robotics and artificial intelligence in life sciences. He also holds a degree in computer engineering and a master's degree in computer science from Université Laval. Maxime has developed the Artificial Intelligence of the Starpax PolarTrak for the magnetic guidance of Starpax Magnetodrones for targeted cancer treatment. Maxime has received several grants of excellence and awards, including a FRQNT doctoral research grant and the mention of excellence for his thesis.
Charles Tremblay, Director Medical Technologies
Charles Tremblay graduated from Polytechnique Montréal University (Canada), in Physics Engineering in 2003 and obtained a certification in bioethics from the Université de Montréal in 2009. With 20 years of R&D experience, he co-authored more than 25 peer-reviewed scientific and engineering conference and journal papers. For nearly two decades he acted as laboratory manager at the Nanorobotics laboratory at Polytechnique Montréal University. He acted also as a coordinator for the implementation of various specialized research experimental platforms and infrastructures including two versions of a clinical magnetic resonance imaging facility, robotics systems, magnetic navigation platforms, a 3D nanofabrication facility, a cell culture laboratory, and bioanalysis units, to name only some examples.
COMPENSATION OF DIRECTORS AND OFFICERS
Directors
| Name | | Capacity of Compensation | | Cash Compensation | | Other Compensation | | Total Compensation |
| Michael Gareau | | Chairman of the Board of Directors | | | $0 | | | | $0 | | | | $0 | |
| André Monette | | Director | | | $0 | | | | $0* | | | | $0 | |
| John Helou | | Director | | | $0 | | | | $0* | | | | $0 | |
| Lisa Matar | | Director | | | $0 | | | | $0* | | | | $0 | |
| Berthe Latreille | | Director | | | $0 | | | | $0* | | | | $0 | |
| Dr. Jacques Jolivet | | Director | | | $0 | | | | $0* | | | | $0 | |
| Pierre Dozois | | Director | | | $0 | | | | $0* | | | | $0 | |
*See “Stock Options” below
Officers
| Name | | Capacity of Compensation | Cash Compensation | | Other Compensation | | Total Compensation |
| Michael Gareau | | Founding- President and CEO | | $382,961.00 | | | | $0 | | | | $382,961.00 | |
| Thierry Pagé | | Executive VP
and COO | | $169,356.00 | | | | $0 | | | | $169,356.00 | |
| Jean-François Pruneau | | Executive VP and CFO | | $0 | | | | $0* | | | | $0 | |
| Dr. Sylvain Martel | | Vice President Technology | | $88,068.00 | | | | $0 | | | | $88,068.00 | |
| Pierre Dozois | | Corporate Secretary | | $0 | | | | $0* | | | | $0 |
*See “Stock Options” below
Stock Options
The Company does not have a formal employee stock option program in place as of the date of this Offering Circular. However, the Company has granted stock options pursuant to individual stock option agreements entered into with certain Directors, Officers, and employees. The Anti- dilution Agreement applies in accordance with its terms to all such stock options when exercised into Common Shares. The following table describes the stock options granted to Directors and Officers as of the date of this Offering Circular. Note that the anti-dilution agreement applies to the exercise of the Company’s stock options by optionees.
| Name of Officer/Director | | Total Stock Option Grant | | Exercise Price Per Share (per Option) | | Vested (as of date of Offering Circular) | | Next Vesting Date | | # of Stock Options on Next Vesting Date |
| Jean-François Pruneau | | | 75,000 | | | | $0.0005 | | | | 25,000 | | | July 18,
2023 | | | 25,000 | |
| André Monette | | | 105,000 | | | | $0.016 | | | | 0 | | | October 23,
2023 | | | 35,000 | |
| John Helou | | | 105,000 | | | | $0.016 | | | | 0 | | | May 2,
2023 | | | 35,000 | |
| Lisa Matar | | | 105,000 | | | | $0.016 | | | | 0 | | | October 23,
2023 | | | 35,000 | |
| Berthe Latreille | | | 105,000 | | | | $0.016 | | | | 0 | | | October 23,
2023 | | | 35,000 | |
| Dr. Jacques Jolivet | | | 105,000 | | | | $0.016 | | | | 0 | | | October 23,
2023 | | | 35,000 | |
| Pierre Dozois | | | 105,000 | | | | $5.992 | | | | 0 | | | October 23,
2023 | | | 35,000 | |
Aggregate Compensation to Directors
$378,624.00 was the aggregate salary paid to Directors in 2021. This was paid to Michael Gareau in his capacity as an Officer of the Company (President and CEO) and not in his capacity as Director.
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
The following table encompasses the beneficial ownership of all Directors and Officers who own shares in the Company.
| Title of Class | Name and Address of Beneficial Owner | Number of Shares Owned | Amount and nature of beneficial ownership acquirable | Percent of Class*** |
| | Common Shares | | Ipax 2 llp* | | 54,175,110 | | | 0 | | | 70.82% | |
| | Common Shares | | Jean-François Pruneau | | 25,000 | | | 50,000 | | | 0.10% | |
| | Common Shares | | Pierre Dozois** | | 250,000 | | | 105,000 | | | 0.47% | |
*Ipax 2 llp owns 70.82% of all Common Shares outstanding. Ipax 2 llp is owned 92.54% by MG Trust. Michael Gareau is a beneficiary of MG Trust. Michael Gareau is therefore the beneficial owner of 65.53% of the Company. Thierry Pagé (Executive VP and COO) is a beneficiary of a trust that owns 1.11% of Ipax 2 llp.
**Pierre Dozois is the owner of 130,000 Shares as an individual. An additional 120,000 Shares are owned by 9058-3444 Quebec Inc., controlled and owned by Pierre Dozois.
*** This column incorporates the percentage beneficially owned by these persons on a fully diluted basis (with all stock options exercised) and taking into account the anti-dilution agreement (see “Dilution” section above)
Addresses:
Ipax 2 llp: 2500-1100 boul. René-Lévesque West, Montréal (Québec), Canada, H3B 5C9
Jean-François Pruneau: 6615 rue Abrams, Montréal, Québec, Canada H4S 1V9
Pierre Dozois: 2500-1100 boul. René-Lévesque West, Montréal (Québec), Canada, H3B 5C9
INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
Ipax 2 llp (formerly Medpax Consortium S.E.C) is a related party of the Company as it has a controlling interest in the Company. In 2022, in accordance with the anti-dilution agreement, the Company repurchased 170,983 of the Class B Common Shares for $13,000. As at December 31, 2022, the Company has $6,000 due to Ipax 2 llp (no amounts due as at December 31, 2021). As of December 31, 2022, Ipax 2 llp owned 71.8% of the Company (72.9% as of December 31, 2021). In 2021, in accordance with the anti-dilution agreement, the Company repurchased 654,474 of the Class B Common Shares for $55,000 and, in 2020, repurchased 253,666 shares for $19,000. Please see note 13 of the audited financial statements for further detail on the Class B Common Shares issued to Ipax 2 llp.
On November 23, 2022, the Company entered into an anti-dilution agreement between the Company and Ipax 2 llp. Subject to certain terms, this anti-dilution agreement avoids the dilution of the percentage of Shares that investors, excluding Ipax 2 llp, own in the Company when new Shares are issued from treasury.
This agreement includes a forced redemption option granted to the Company. Ipax 2 granted the Company an option (the "Forced Redemption Option") allowing the Company to redeem one share held by Ipax 2 in its share capital, for a price of $0.02 CAD per Share (approximately $0.014), upon each and every issue of a treasury share by the Corporation. Conversely, the Corporation undertakes to exercise the Forced Redemption Option upon each new issue of treasury shares, thus to buying back a number of shares held by Ipax 2 equal to the number of shares so issued.
This agreement will affect sales of the Shares through the Offering. This means that for every sale of a Share through this Offering, the Company will pay $0.02 CAD (approximately $0.014) to Ipax 2 llp.
BCF llp legal fees
BCF llp is a law firm located in Montreal, Quebec, Canada. Pierre Dozois is a partner in this law firm and has performed legal services for the Company since its inception. Over the past three completed fiscal years, BCF llp has billed the Company legal fees as follows:
| Fiscal Year | Total Billed CAD (USD*) |
| 2020 | $76,395.96 ($56,999.03) |
| 2021 | $143,191.64 ($114,266.92) |
| 2022 | $180,722.54 ($139,011.78) |
*based on the average exchange rate for that Fiscal Year.
DESCRIPTION OF THE SECURITIES
The Company has one class of authorized stock, e.g. Common Shares. Each Common Share is entitled to (i) receive all dividends declared by the Directors, (ii) attend and vote at all meetings of the shareholders and (iii) receive their proportionate share of the remaining property of the Company upon its dissolution (See Exhibit 2A “Articles of Consolidation and Other Corporate Documents”).
Dividends
Each Common Share confers the right to receive, form the profits or surplus available for the payment of dividends, any dividend declared by the Directors at their discretion.
Voting
Each Common Share confers upon its holder the right to vote one Share held and the right to attend Shareholder meetings.
Preemptive rights
There are no preemptive rights associated with the Shares.
Liquidation Rights
Each Common Share confers the right to share the remaining property of the Company upon voluntary or compulsory liquidation or dissolution.
Redemption Provisions
There are no redemption rights associated with the Shares.
Sinking Fund Provisions
There are no sinking fund provisions contained in the bylaws or the Amended Articles of Incorporation.
Liability to Further Calls
The Shareholders are not liable to further calls from the Company.
Restrictions on transferability
There are no restrictions on transferability associated with the Shares.
Drag Along Rights
There are no drag-along rights associated with the Shares.
PART F/S
Starpax Biopharma Inc.
Interim Condensed Statements of Financial Position
As at June 30, 2022 (unaudited) and December 31, 2021 (audited)
(Expressed in thousands of United States dollars, except per share data and where specified)
| | | June 30, 2022 | December 31, 2021 |
| | Notes | $ | $ |
| | | | |
| Assets | | | |
| Current assets | | | |
| Cash | | 4,780 | 7,892 |
| Grant receivables | | 3,340 | 3,308 |
| Other receivables | | 1,776 | 1,640 |
| Prepaid expenses and other assets | | 123 | 32 |
| Total current assets | | 10,019 | 12,872 |
| | | | |
| Non-current assets | | | |
| Property and equipment | | 16,169 | 10,851 |
| Intangible assets | | 963 | 939 |
| Deferred financing costs | 5 | 85 | 89 |
| Security deposits | | 17 | 24 |
| Total assets | | 27,253 | 24,775 |
| | | | |
| Liabilities | | | |
| Current liabilities | | | |
| Accounts payable and accrued liabilities | | 3,790 | 2,821 |
| Current portion of lease liabilities | | 63 | 64 |
| Total current liabilities | | 3,853 | 2,885 |
| | | | |
| Non-current liabilities | | | |
| Lease liabilities | | 562 | 525 |
| Long-term debt | 5 | 3,875 | 473 |
| Total liabilities | | 8,290 | 3,883 |
| | | | |
| Shareholders’ equity | | | |
| Share capital | 6 | 32,809 | 31,236 |
| Contributed surplus | | 178 | - |
| Accumulated deficit | | (14,053) | (10,528) |
| Accumulated other comprehensive income | | 29 | 184 |
| Total shareholders’ equity | | 18,963 | 20,892 |
| Total liabilities and shareholders’ equity | | 27,253 | 24,775 |
Subsequent Events (Note 7)
The accompanying notes are an integral part of these interim condensed financial statements.
Starpax Biopharma Inc.
Interim Condensed Statements of Loss and Comprehensive Loss (Unaudited)
For the six-month periods ended June 30, 2022 and 2021
(Expressed in thousands of United States dollars, except per share data and where specified)
| | June 30, 2022 | June 30, 2021 |
| | Notes | $ | $ |
| | | | |
| Expenses | | | |
| General and administrative | | (1,232) | (555) |
| Research and development | | (1,754) | (1,234) |
| Depreciation | | (419) | (95) |
| Loss on sale of property and equipment | | | (6) |
| Total expenses | | (3,405) | (1,890) |
| | | | |
| Net financial expenses | | (115) | (35) |
| Loss before income tax | | (3,520) | (1,925) |
| | | | |
| Income tax expense (recovery) | | — | — |
| Net loss | | (3,520) | (1,925) |
| | | | |
| Other comprehensive loss | | | |
Items that may be reclassified subsequently to
income or loss | | | |
| Foreign currency translation differences | | (155) | 542 |
| Comprehensive loss | | (3,675) | (1,383) |
| | | | |
| Loss per share | | | |
| Basic and diluted | | (0.05) | (0.03) |
| Weighted average number of common shares | | 75,500,000 | 75,500,000 |
| | | | |
The interim condensed statements of loss and comprehensive loss has been retroactively adjusted to account for the stock split of 5:1 that took place on January 19, 2023
The accompanying notes are an integral part of these interim condensed financial statements.
Starpax Biopharma Inc.
Interim Condensed Statements of Changes in Shareholders’ Equity (Unaudited)
For the six-month periods ended June 30, 2022 and 2021
(Expressed in thousands of United States dollars, except per share data and where specified)
| | | Share
capital | Contributed surplus | Accumulated
deficit | Other
comprehensive
income (loss) | Total
shareholders’
equity |
| | Notes | $ | $ | $ | $ | $ |
| | | | | | | |
Balance as at December 31,
2020 (audited) | | 16,476 | - | (5,921) | 131 | 10,686 |
| Net loss | | — | - | (1,925) | — | (1,925) |
Foreign currency
translation differences | | — | - | — | 542 | 542 |
| Share issuance (class A), net issuance costs | 6 | 13,980 | | — | — | 13,980 |
| Share repurchase (class B) | 6 | — | - | (49) | — | (49) |
| Balance as at June 30, 2021 | | 30,456 | - | (7,895) | 673 | 23,234 |
| | | | | | | |
| Balance as at December 31, 2021 (audited) | | 31,236 | - | (10,528) | 184 | 20,892 |
| Net loss | | - | - | (3,520) | - | (3,520) |
Foreign currency
translation differences | | - | - | - | (155) | (155) |
| Stock options granted | | - | 178 | - | - | 178 |
| Share issuance (class A), net issuance costs | 6 | 1,882 | - | - | - | 1,882 |
| Offering costs | | (309) | | | | (309) |
| Share repurchase (class B) | 6 | - | - | (5) | - | (5) |
| Balance as at June 30, 2022 | | 32,809 | | 178 | (14,053) | 29 | 18,963 |
The interim condensed statements of changes in stockholders' equity has been retroactively adjusted to account for the stock split of 5:1 that took place on January 19, 2023.
The accompanying notes are an integral part of these interim condensed financial statements.
Starpax Biopharma Inc.
Interim Condensed Statements of Cash Flows (Unaudited)
For the six-month periods ended June 30, 2022 and 2021
(Expressed in thousands of United States dollars, except per share data and where specified)
| | | June 30, 2022 | June 30, 2021 |
| | | $ | $ |
| | | | |
| Operating activities | | | |
| Net loss | | (3,520) | (1,925) |
| Items not affecting cash | | | |
| Depreciation | | 419 | 95 |
| Share-based compensation | | 180 | — |
| Interest income | | (9) | (19) |
| Interest expense | | 123 | 55 |
| Loss on disposal of property and equipment | | — | 6 |
| Changes in non-cash working capital items | | 947 | 838 |
| Interest paid | | 2 | (1) |
| Interest received | | 9 | 19 |
| Net cash flows used in operating activities | | (2,029) | (932) |
| | | | |
| Investing activities | | | |
| Acquisition of property and equipment | | (6,509) | (3,208) |
| Grants received | | 202 | 297 |
| Acquisition of intangible assets | | (66) | — |
| Net cash flows used in investing activities | | (6,373) | (2,911) |
| | | | |
| Financing activities | | | |
| Increase in long-term debt | 5 | 3,412 | 328 |
| Transaction costs paid | | — | (56) |
| Proceeds from issuance of class A common shares | 6 | 2,025 | 14,785 |
| Repurchase of class B common shares | 6 | (5) | (49) |
| Offerings costs | | (309) | - |
| Share issuance costs | 6 | (143) | (805) |
| Payments of principal portion of lease liabilities | | 13 | (98) |
| Net cash flows provided by financing activities | | 4,993 | 14,105 |
| | | | |
| Effect of foreign exchange rate changes on cash | | 297 | 447 |
| (Decrease) increase in cash during the period | | (3,112) | 10,709 |
| Cash, beginning of period | | 7,892 | 5,573 |
| Cash, end of period | | 4,780 | 16,282 |
The accompanying notes are an integral part of these interim condensed financial statements.
Starpax Biopharma Inc.
Notes to the interim condensed financial statements.
For the six-month periods ended June 30, 2022 and 2021
(Expressed in thousands of United States dollars, except per share data and where specified)
1. Corporate information
Starpax Biopharma Inc. (the “Company”), incorporated under the Business Corporations Act (Quebec) on December 19, 2017, conducts research into the eradication of solid cancerous tumors and started operations in March 2018.
2. Liquidity
The Company has incurred recurring losses from operations, and as at June 30, 2022, had an accumulated deficit of $14,053 (December 31, 2021 - $10,528) and a working capital of $6,166 (December 31, 2021 - $9,987). The working capital as at June 30, 2022 is sufficient to pay its operating expenses for a period of at least 12 months from the date of the financial statements. The Company’s continued existence is dependent upon its ability to continue to execute its operating plan and to obtain additional debt or equity financing. The Company has developed plans to raise funds and continues to pursue sources of funding that management believes, if successful, would be sufficient to support the Company’s operating plan. During the period ended June 30, 2022, the Company raised $2,025 through equity issuances and through the availability in its debt financing arrangements which was partially utilized subsequent to year end (Note 7). The Company’s operating plan is predicated on a variety of assumptions including, but not limited to, success in research objectives, cost estimates, its ability to continue to raise additional financing and the state of the general economic environment in which the Company operates. There can be no assurance that these assumptions will prove accurate in all material respects, or that the Company will be able to successfully execute its operating plan. In the event that the Company is not able to raise capital from investors in a timely manner, the Company will explore available options. In the absence of additional appropriate financing, the Company may have to modify its plan or slow down the pace of research and development.
3. Summary of significant accounting policies
Basis of presentation
The interim condensed financial statements of the Company have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB"). These interim condensed financial statements meet the requirements of International Accounting Standard (“IAS”) 34, “Interim Financial Reporting” and follow the same accounting policies as the financial statements for the years ended December 31, 2021 and 2020. These condensed interim financial statements should be read in conjunction with the December 31, 2021 and 2020 annual consolidated financial statements, which have been prepared in accordance with IFRS as issued by the IASB.
Basis of measurement
The interim condensed financial statements have been prepared on the historical cost basis.
Foreign currency
Functional and presentation currency
The Company’s functional currency is Canadian dollars. These interim condensed financial statements are presented in United States dollars which is the presentation currency of the Company, and all values are rounded to the nearest thousand ($000), except when otherwise indicated. The Company has adopted the United States dollar as its presentation currency as it is the most commonly used reporting currency in its industry.
Foreign currency transactions and balances
Monetary assets and liabilities denominated in foreign currencies are translated into the functional currency using the exchange rate in effect at year end. Other assets and liabilities are translated at the prevailing historical rates at the time of the transaction. Expenses arising from foreign currency transactions are translated at the exchange rates in effect on the transaction date. Exchange gains and losses are recorded in net financial expenses in the interim condensed statements of net loss and comprehensive loss.
Cash
Cash consists of cash on hand and balances with banks.
Property and equipment and depreciation
Property and equipment are recorded at cost less accumulated depreciation. Cost includes expenditures that are directly attributable to the acquisition of the asset. Property and equipment are depreciated using the straight-line method over their estimated useful lives once available for use as follows:
| Furniture and office equipment | Straight-line | 5 years |
| Laboratory equipment | Straight-line | 10 years |
| Computer equipment | Straight-line | 3 to 4 years |
| Vehicles | Straight-line | 3 to 4 years |
| Leasehold improvements | Straight-line | Lease duration |
Depreciation methods, useful lives and the residual values of property, plant and equipment are reviewed at each reporting date for any change in circumstances and are adjusted if appropriate.
considered a plant-under-construction. All costs directly attributable to bring the plant to the condition necessary for it to be capable of operating in the manner intended by management are recorded at cost. Each significant part of the plant will be depreciated separately. Deprecation for each significant part of the plant is recorded over the useful life will begin when available for use.
Leases
The Company assesses at contract inception whether a contract is, or contains, a lease. That is, if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration.
The Company applies a single recognition and measurement approach for all leases, except for short-term leases and leases of low-value assets. The Company recognizes lease liabilities representing obligations to make lease payments and right-of-use assets representing the right to use the underlying assets.
The right-of-use assets are presented within property and equipment.
The Company recognizes right-of-use assets at the commencement date of the lease (i.e., the date the underlying asset is available for use). Right-of-use assets are measured at cost, less any accumulated depreciation and impairment losses, and adjusted for any remeasurement of lease liabilities. The cost of right-of-use assets includes the amount of lease liabilities recognized, initial direct costs incurred, and lease payments made at or before the commencement date less any lease incentives received. Right-of-use assets are depreciated using the straight-line method from the commencement date to the earlier of the end of the useful life of the right-of-use asset or the end date of the lease term.
3. Summary of significant accounting policies (continued)
Leases (continued)
The right-of-use assets are also subject to impairment.
At the commencement date of the lease, the Company recognizes lease liabilities measured at the present value of lease payments to be made over the lease term. The lease payments include fixed payments (including in-substance fixed payments) less any lease incentives receivable, variable lease payments that depend on an index or a rate, and amounts expected to be paid under residual value guarantees. The lease payments also include the exercise price of a purchase option reasonably certain to be exercised by the Company and payments of penalties for terminating the lease, if the lease term reflects the Company exercising the option to terminate.
Variable lease payments that do not depend on an index or a rate are recognized as expenses (unless they are incurred to produce inventories) in the period in which the event or condition that triggers the payment occurs.
In calculating the present value of lease payments, the Company uses its incremental borrowing rate at the lease commencement date when the interest rate implicit in the lease is not readily determinable. After the commencement date, the amount of lease liabilities is increased to reflect the accretion of interest and reduced for the lease payments made. In addition, the carrying amount of lease liabilities is remeasured if there is a modification, a change in the lease term, a change in the lease payments (e.g., changes to future payments resulting from a change in an index or rate used to determine such lease payments) or a change in the assessment of an option to purchase the underlying asset.
Intangible assets
| Licenses | | Indefinite |
| Intellectual properties | Straight-line | 20 years |
| Trademarks | | Indefinite |
Intangible assets with indefinite useful lives are not amortized, but are tested for impairment annually, either individually or at the cash-generating unit level. The assessment of indefinite life is reviewed annually to determine whether the indefinite life continues to be supportable. If not, the change in useful life from indefinite to finite is made on a prospective basis.
Management monitors the progress of each internal research and development project. Development costs will be recognized as an asset when the following criteria are met: (i) technical feasibility; (ii) management’s intention to complete the project; (iii) the ability to use or sell; and (iv) the ability to generate future economic benefits; (v) availability of technical and financial resources; (vi) ability to measure the expenditures reliably. During the period of development, the asset is tested for impairment annually. Research costs are expensed as incurred.
The Company made upfront payments to acquire licenses, intellectual properties and trademarks. The licenses, including the licenses for the use of intellectual properties, and trademarks have been granted for periods ranging between five and ten years by the relevant government agency with the option of renewal at the end of this period. These licenses and trademarks may be renewed at little or no cost to the Company. As a result, those licenses and trademarks are assessed as having an indefinite useful life.
3. Summary of significant accounting policies (continued)
Impairment of non-financial assets
At each reporting date, the Company reviews the carrying amounts of its non-financial assets to determine whether there is any indication of impairment. If any such indication exists, then the asset’s recoverable amount is estimated.
Property and equipment and intangible assets are reviewed for impairment when events or circumstances indicate that carrying value may not be recoverable. The Company assesses at each reporting date whether any such events or changes in circumstances exist.
For the purpose of measuring recoverable amounts, assets are grouped at the lowest levels for which there are separately identifiable cash flows (cash-generating units – CGUs). The recoverable amount is the higher of an asset's fair value less costs of disposal and value in use (being the present value of the expected future cash flows of the relevant assets of the CGU). An impairment loss is recognized for the amount by which the asset's carrying amount exceeds its recoverable amount.
The Company evaluates impairment losses for potential reversals when events or circumstances require such consideration.
Investment tax credits and other government grants
Government grants, consisting of grants and investment tax credits, are recorded as a reduction of the related expense or cost of the asset acquired. Government grants are recognized when there is reasonable assurance that the Company has met or will meet the requirements of the approved grant program and there is reasonable assurance that the grant will be received. Loans received at below market interest rate are treated in the same manner. The benefit of the below market interest rate is recorded as a reduction of the related expense or cost of the asset acquired.
Grants that compensate the Company for expenses incurred are recognized in reduction thereof on a systematic basis in the same years in which the expenses are recognized. Grants that compensate the Company for the cost of an asset are recognized in reduction of the cost of the asset and are depreciated on a systematic basis over the useful life of the asset.
The Company incurs research and development expenditures which are eligible for scientific research and experimental development (SR&ED) and investment and innovation tax credits. Refundable investment tax credits are recorded as SR&ED or investment and innovation tax credits in the interim condensed statements of net loss and comprehensive loss when there is reasonable assurance that the credits will be realized. Non-refundable SR&ED and investment and innovation tax credits, which are deductible against income taxes otherwise payable, are recorded as a reduction of the related expenses or cost of asset(s) when there is reasonable assurance that the credits will be realized.
The tax credits recorded are based on management’s best estimate of amounts expected to be recovered and are subject to audit by taxation authorities. To the extent that actual tax credits differ from the estimate, those differences are recorded in the period of assessment by taxation authorities as an adjustment of the items to which they relate.
Financial instruments
Recognition and initial measurement
Financial assets and financial liabilities are initially recognized when the Company becomes a party of the contractual provision of the instrument.
Financial assets and financial liabilities are initially measured at fair value. Transaction costs that are directly attributable to the acquisition or issue of financial assets and financial liabilities (other than financial assets and financial liabilities at fair value through profit or loss) are added to or
3. Summary of significant accounting policies (continued)
Financial instruments (continued)
Recognition and initial measurement (continued)
deducted from the fair value of the financial assets or financial liabilities, as appropriate, on initial recognition. Transaction costs directly attributable to the acquisition of financial assets or financial liabilities at fair value through profit or loss are recognized immediately in profit or loss.
Classification and subsequent measurement
Financial instruments are classified into the following specified categories: amortized cost, fair value through other comprehensive income (“FVOCI”) or fair value through profit or loss (“FVTPL”). The classification depends on the nature and purpose of the financial instrument and is determined at the time of initial recognition. The Company’s financial instruments have been classified as follows:
| Financial instrument | Classification |
| Financial assets | |
| Cash | Amortized cost |
| Other receivables | Amortized cost |
| | |
| Financial liabilities | |
Accounts payable and accrued
liabilities | Amortized cost |
| Lease liabilities | Amortized cost |
| Long-term debt | Amortized cost |
| | |
A financial asset is measured at amortized cost if it is held within a business model of holding financial assets and collecting contractual cash flows and those cash flows are comprised solely of payments of principal and interest. A financial asset is measured at FVTOCI if the financial asset is held within a business model of both collecting contractual cash flows and selling the financial assets or through an irrevocable election for equity instruments that are not held for trading. All other financial assets are measured at FVTPL.
Financial assets can only be reclassified when there is a change to the business model within which they are managed. Such reclassifications are applied on a prospective basis.
Financial assets are derecognized only when the contractual rights to the cash flows from the asset expire, or when the Company transfers the financial asset and substantially all the risks and rewards of ownership of the asset to another entity.
Share split
On January 19, 2023, the Company amended its Articles (i) converting all outstanding Class A Common Shares and Class B Common Shares into shares of a single class called common shares (“Common Shares”) and (ii) executing a 5:1 stock split, subdividing all its issued and outstanding Common Shares into five (5) such Common Shares.
Loss per share
Basic loss per share is calculated by dividing net loss attributable to the Class A and B common shareholders of the Company by the basic weighted-average number of outstanding common shares. Diluted loss per share is computed similar to basic loss per share except that the weighted
average shares outstanding are increased to include additional shares for the assumed exercise of stock options and warrants, if dilutive.
Income taxes
Income tax expense represents the sum of the tax currently payable and deferred tax.
The tax currently payable is based on taxable income for the year. Taxable income differs from “income before tax” as reported in the interim condensed statements of net loss and comprehensive loss because of
items of income or expense that are taxable or deductible in other years and items that are never taxable or deductible. The Company’s current tax is calculated using tax rates that have been enacted or substantively enacted by the end of the year.
Deferred tax is recognized on temporary differences between the carrying amounts of assets and liabilities in the interim condensed financial statements and the corresponding tax bases used in the computation of taxable income. Deferred tax liabilities are generally recognized for all taxable temporary differences. Deferred tax assets are generally recognized for all deductible temporary differences to the extent it is probable taxable income will be available against which those deductible temporary differences can be utilized. Such deferred tax assets and liabilities are not recognized if the temporary difference arises from the initial recognition of assets and liabilities in a transaction that affects neither the taxable income nor the accounting income.
The carrying amount of deferred tax assets is reviewed at the end of each year and reduced to the extent it is not probable sufficient taxable income will be available to allow all or part of the asset to be recovered. Deferred tax liabilities and assets are measured at the tax rates that are expected to apply in the year in which the liability is settled or the asset realized, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the year.
The measurement of deferred tax liabilities and assets reflects the tax consequences that would follow from the manner in which the Company expects, at the end of the year, to recover or settle the carrying amount of its assets and liabilities.
Current and deferred taxes are recognized in net loss, except when they relate to items that are recognized in other comprehensive loss or directly in equity, in which case the current and deferred taxes are also recognized in other comprehensive loss or directly in equity, respectively.
Deferred share issuance costs
The Company capitalizes certain legal, professional accounting and other third-party fees that are directly associated with in-process equity financings as deferred issuance costs until such financings are consummated. After consummation of the equity financing, these costs are recorded in stockholders’ equity as a reduction of proceeds generated as a result of the offering. Should the in-process equity financing be abandoned, the deferred share issuance costs will be expensed immediately as a charge to operating expenses in the interim condensed statements of loss and comprehensive loss.
Deferred financing costs
The Company capitalizes certain legal, professional accounting and other third-party fees that are directly associated with in-process debt financings as deferred financing costs until such financings are consummated. These costs are capitalized as a reduction to long-term debt and amortized using the effective interest rate method from the start date of the drawdown and until such date, the financing costs are included in other assets.
Employee Benefits
The Company has a post-retirement defined contribution plan. The Company pays contributions to a group RRSP on a mandatory, contractual basis. The Company has no further payment obligations once the contributions have been paid. The contributions are recognized as employee benefit expense when they are due.
3. Summary of significant accounting policies (continued)
Share based compensation
Share-based payments to employees are measured at the fair value of the instruments issued and amortized over their respective vesting periods. The fair value of options is measured at the date of grant using the Black-Scholes option pricing model and recorded as a compensation expense in the period the options are vested, or the performance is complete. The number of awards expected to vest is reviewed at least annually, with any impact being recognized immediately.
The increase in equity recognized in connection with a share-based payment transaction is presented in the interim condensed statements of financial position, as a separate component in equity.
Share capital
Share capital represents the amount received on the issue of shares, less issuance costs, net of any underlying income tax benefit from these issuance costs.
Other elements of equity:
Stock options accounts include unrealized charges related to stock options until they are exercised, if applicable.
Accumulated deficit includes all current and prior year retained losses.
Accumulated other comprehensive income includes all foreign currency translation adjustments.
4. Significant accounting judgments, estimates and assumptions
The preparation of the financial interim condensed statements in accordance with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, expenses and disclosure of contingent assets and liabilities. Actual results may differ from these estimates. Significant assumptions about the future and other sources of estimation and judgment uncertainty that management has made at the end of the reporting period, that could result in a material adjustment to the carrying amounts of assets and liabilities in the event that actual results differ from assumptions made, relate to:
Going concern
The assumption that the Company will be able to continue as a going concern (Note 2) is subject to estimates and judgement by management including the Company’s short and long-term operating budget, expected profitability, investing and financing activities, and management’s strategic planning.
Fair value measurement of non-interest-bearing debt
The Company has estimated, on initial recognition, the fair market value of certain debts that do not have a defined coupon rate, using a comparative interest rate for similar liabilities and a discounted cash flow to determine a reasonable present value. Additional details on debt are disclosed in Note 5.
Intangible assets
In applying its accounting policy for costs to be capitalized as intangible assets, the Company must determine whether the criteria for capitalization have been met. The most subjective judgement is whether a project will generate probable future
4. Significant accounting judgments, estimates and assumptions (continued)
Intangible assets (continued)
considers all appropriate facts and circumstances in making this assessment including expected market demand, cost and future economic conditions.
Income taxes
The recognition of deferred tax assets requires the Company to assess future taxable income available to utilize deferred tax assets related to deductible or taxable temporary differences. The Company considers the nature and carry-forward period of deferred tax assets, the Company's recent earnings history and forecast of future earnings in performing this assessment.
Leases
Leases requires lessees to discount lease payments using the rate implicit in the lease if that rate is readily available. If the rate cannot be readily determined, the lessee is required to use its incremental borrowing rate. The Company generally uses the incremental borrowing rate when initially recording real estate leases as the implicit rates are not readily available as information from the lessor regarding the fair value of underlying assets and initial direct costs incurred by the lessor related to the leases assets is not available. The Company determines the incremental borrowing rate as the interest rate of the Company would pay to borrow over a similar economic environment. Leases also requires lessees to estimate the lease term. In determining the period which the Company has the right to use an underlying asset, management considers the non-cancellable period along with all facts and circumstances that create an economic incentive to exercise an extension option, or not to exercise a termination option.
Investment tax credits recoverable
The recognition of investment tax credits recoverable requires the Company to assess future tax payable available to utilize the investment tax credits. The Company considers the carry-forward period of the investment tax credits, the Company's forecast of future earnings in performing this assessment. The Company determines the value of effort expended towards research and development projects that qualify for investment tax credits and calculates the estimated recoverable to be recognized. The allocation of direct salaries to qualifying projects is derived from time records and assessment by management. The actual investment tax credits claimed and realized may differ from the estimate based on the final tax returns and review by tax authorities.
Impairment of non-financial assets
At each reporting date, the Company reviews the carrying amounts of its non-financial assets to determine whether there is any indication of impairment. If any such indication exists, then the asset’s recoverable amount is estimated. See note 3 for more detail.
Share-based compensation
The estimation of the share-based compensation costs requires the selection of an appropriate valuation model and data and consideration as to the volatility of the Company’s own share, the probable life of share of share options and the time of exercise of those share options. The model used by the Company is a Black-Scholes valuation model.
5. Long-term debt
The terms and conditions of the Company’s loans and borrowings are as follows:
| | June 30, 2022 | December 31, 2021 |
| | $ | $ |
| | | |
| Repayable contribution agreement with the Economic Development Agency of Canada (Note 5 (a)) | 712 | 473 |
| Investissement Québec loan (Note 5 (b)) | 3,163 | — |
| Total long-term debt | 3,875 | 473 |
| | | |
| Current portion of long-term debt | — | — |
Loans and borrowings are presented net of unamortized transaction costs. Transaction costs relating to the issuance of loans and borrowings are amortized over the term of the debt using the effective interest rate method.
a) On May 15, 2020, the Company entered into an interest-free repayable contribution agreement with the Economic Development Agency of Canada for the Regions of Quebec for a maximum amount of $1,560 (CAD $2,000) to finance the establishment of a pilot plant as well as the construction of an intervention room dedicated to oncological research. A principal mortgage in the amount of $1,560 (CAD $2,000) and an additional mortgage of $312 (CAD $400) on laboratory equipment with a net book value of $1,486 as at June 30, 2022, will be given as security for this repayable contribution. As per the agreement, the project must be completed by March 31, 2023 (this was amended in 2021 from December 31, 2021) and is to be paid in 60 equal and consecutive monthly installments beginning 36 months after the project completion date. As a result of the amendment in 2021, the payment schedule was altered, and the fair value of the loan was recalculated using an effective interest rate of 10% to reflect this change. As at June 30, 2022, an additional amount of $427 ($632 as at December 31, 2021) has been drawn from the repayable contribution. The amount recognized as a grant due to the below-market interest rate of $202 ($354 in the year ended December 31, 2021) has been recognized as a reduction to the carrying value of property and equipment. The remaining loan balance of $712 is included in loans and borrowing.
b) On April 9, 2021, the Company entered into a financing agreement with Investissement Québec (IQ) to finance the establishment of a pilot plant through investment in Class A common stock for a total amount of $3,880 (CAD$5,000) and a loan for a maximum amount of $5,432 (CAD $7,000) under the ESSOR program. The loan will bear interest at a fixed rate of 10%. Interest will be capitalized monthly for a period of 36 months from the first loan disbursement, and thereafter will be payable. The loan must be repaid in 84 equal and consecutive monthly installments starting 36 months from the first disbursement of the loan. A movable hypothec in the amount of $5,459 (CAD $7,000) and an additional hypothec in the amount of $1,092 (CAD $1,400) encumbering the universality of the Company’s equipment, which will be first rank with a net book value of $2,791 as at June 30, 2022, except with regard to laboratory equipment, not exceeding $1,872 (CAD $2,400) which is first ranked in favor of the Economic Development Agency of Canada for the Regions of Quebec, for which the hypothec in favor of IQ will be second rank with a net book value of $1,486 as at June 30, 2022. As at June 30, 2022, an amount of $3,151 (nil in the year ended December 31, 2021) has been drawn from the repayable contribution. In relation to this agreement, the Company incurred $85 of deferred financing costs which were recorded in deferred financing costs. Additional claims were made subsequent to the period (Note 7).
6. Share capital
On January 19, 2023, the Company amended its Articles (i) converting all outstanding Class A Common Shares and Class B Common Shares into shares of a single class called common shares (“Common Shares”) and (ii) executing a 5:1 stock split, subdividing all its issued and outstanding Common Shares into five (5) such Common Shares.
The Company has authorized the following classes of share capital:
Authorized
Unlimited Class A common shares, voting, participating, no par value
Unlimited Class B common shares, voting, participating, no par value. The Company has the option (forced repurchase option) to repurchase a Class B common share held in its share capital for CAD $0.02 per share, concurrently with each issuance of a share by the Company and up to the number of Class B common shares in the Company’s share capital. Similarly, it undertakes to exercise the forced repurchase option upon each issuance of shares and repurchase a number of Class B common shares held equal to the number of shares issued, no more and no less. In no event shall the number of issued and outstanding shares of the Company exceed 75,500,000 while the forced repurchase option is in effect. The forced repurchase option will cease to be in effect upon the earlier of the following two events:
· The cumulative amount of subscription proceeds for shares of the Company, donations from private sources, profits made, and dividends paid combined have reached CAD $88 million.
· The Company will have achieved commercialization of its developed treatment technology.
| | June 30, 2022 | December 31, 2021 |
| | | Carrying value | | Carrying value |
| Type of share | Quantity | $ | Quantity | $ |
| | | | | |
Company’s share capital Class A common shares Balance, beginning of period Issuance of shares | | | | |
| 20,469,975 | 31,235 | 17,197,605 | 16,475 |
| 394,540 | 1,573 | 3,272,370 | 14,760 |
| Balance, end of period | 20,864,515 | 32,808 | 20,469,975 | 31,235 |
| | | June 30, 2022 | | December 31, 2021 |
| | | Carrying value | | Carrying value |
| Type of share | Quantity | $ | Quantity | $ |
| | | | | |
| Company’s share capital Class B common shares | | | | |
| Balance, beginning of period | 55,030,025 | 1 | 58,302,395 | 1 |
| Repurchase of shares | (394,540) | — | (3,272,370) | — |
| Balance, end of period | 54,635,485 | 1 | 55,030,025 | 1 |
6. Share capital (continued)
Year ended December 31, 2021
During the year ended December 31, 2021, the Company issued 3,287,370 Class A common shares for a cash consideration of $15,578 and repurchased 3,287,370 Class B common shares for a cash consideration of $55. The Company then cancelled these Class B Common shares.
The Company incurred $818 in costs directly relating to the issuance. These issuance costs were adjusted against share capital in the interim condensed statements of changes in shareholders’ equity.
Period ended June 30, 2022
During the period ended June 30, 2022, the Company issued 394,540 Class A common shares for a cash consideration of $2,025 and repurchased 394,540 Class B common shares for a cash consideration of $5. The Company then cancelled these Class B Common shares. The Company incurred $143 in costs directly relating to the issuance. These issuance costs were adjusted against share capital in the interim condensed statements of changes in shareholders’ equity.
On February 15, 2022, the Company granted 75,000 options to purchase Class A common Shares to an officer at an exercise price of $0 per share with an expiry date of February 14, 2032. The fair value of the options was estimated at $5 per share option at the grant date for a total of $353 using a Black-Scholes option pricing model. 25,000 of those options were vested and exercised on December 20, 2022 for a cash consideration of $0.012 and 25,000 Class B common shares were repurchased and cancelled for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
The Company recorded share-based compensation expense during the six months period ended June 30, 2022 of $180 (June 30, 2021 - $Nil), in relation to the stock options issued.
7. Subsequent events
On July 28, 2022, on August 11, 2022 and on November 15, 2022, amounts of $530, $257 and $1,094 respectively, have been drawn from the Investissement Quebec loan.
On August 3, 2022, the Company issued 62,500 Class A common shares for a cash consideration of $389 and repurchased 62,500 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On August 3, 2022, the Company issued 25,000 Class A common shares for a cash consideration of $156 and repurchased 25,000 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On August 3, 2022, the Company issued 16,625 Class A common shares for a cash consideration of $103 and repurchased 16,625 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On October 14, 2022, the Company received an amendment from Economic Development Agency of Canada to push the completion of the project from March 31, 2023 to June 30, 2024. Therefore, the payment schedule was altered and the fair value of the loan was recalculated using an effective interest rate of 10% to reflect this change. The impact of the change is a decrease of $85 of the fair value of the loan.
On October 18, 2022, an officer exercised 25,000 options. The Company then issued 25,000 Class A common shares for a cash consideration of $0.012 and repurchased 25,000 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On November 22, 2022, the Company granted 15,000 options to purchase Class A common Shares to an employee at an exercise price of $6 per share with an expiry date of November 21, 2032. The fair value of the options was estimated at $3 per share option at the grant date for a total of $43 using a Black-Scholes option pricing model.
On November 22, 2022, the Company granted 525,000 options to purchase Class A common Shares to directors at an exercise price of $0.016 per share all with an expiry date of November 21, 2032. The fair value of the options was estimated at $6 per share option at the grant date for a total of $3,130 using a Black-Scholes option pricing model.
7. Subsequent events (continued)
On November 22, 2022, the Company granted 105,000 options to purchase Class A common Shares to a director at an exercise price of $6 per share with an expiry date of November 21, 2032. The fair value of the options was estimated at $3 per share option at the grant date for a total of $299 using a Black-Scholes option pricing model.
On November 23, 2022, the Company issued 6,250 Class A common shares for a cash consideration of $37 and repurchased 6,250 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On December 13, 2022, the Company, and the medical entity where clinical trials will take place, have entered into an agreement whereby the Company would receive a payment of $2.3 million upon the commencement of its clinical trials. The receipt of these funds is contingent on the Phase I trials commencing.
On December 14, 2022, the Company issued 162,500 Class A common shares for a cash consideration of $959 and repurchased 162,500 Class B common shares for a cash consideration of $2. The Company then proceeded with the cancellation of these same Class B common shares.
On December 20, 2022, the Company issued 187,500 Class A common shares for a cash consideration of $1,101 and repurchased 187,500 Class B common shares for a cash consideration of $3. The Company then proceeded with the cancellation of these same Class B common shares.
On December 31, 2022, the Company issued 37,500 Class A common shares for a cash consideration of $221 and repurchased 37,500 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On January 19, 2023, the Company amended its Articles (i) converting all outstanding Class A Common Shares and Class B Common Shares into shares of a single class called common shares (“Common Shares”) and (ii) executing a 5:1 stock split, subdividing all its issued and outstanding Common Shares into five (5) such Common Shares.
After the six-months period ending June 30, 2022, the Company had issued capital commitments totaling $4,187 to various suppliers in connection with its operation and the establishment of a pilot plant of which $1,486 has already been paid.
Financial statements Starpax Biopharma Inc.
(formerly Starpax Medical)
December 31, 2021 and 2020
(In thousands of United States dollars)
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Starpax Biopharma Inc. (formerly Starpax Medical)
Opinion on the Financial Statements
We have audited the accompanying statements of financial position of Biopharma Inc. (formerly Starpax Medical) (the “Company”) as of December 31, 2021, December 31, 2020 and January 1, 2020, the related statements of loss and comprehensive loss, changes in shareholders’ equity, and cash flows for each of the two years in the period ended December 31, 2021, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021, December 31, 2020 and January 1, 2020, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2021, in conformity with the International Financial Reporting Standards.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Company’s auditor since 2022 Richmond Hill, Ontario, Canada February 7, 2023 | /s/ SRCO Professional Corporation CHARTERED PROFESSIONAL ACCOUNTANTS Authorized to practice public accounting by the Chartered Professional Accountants of Ontario |
Starpax Biopharma Inc.
(formerly Starpax Medical Inc.)
Statements of financial position
As at December 31, 2021 and 2020 and January 1, 2020
(Expressed in thousands of United States dollars, except per share data and where specified)
| | | 2021 | 2020 | January 1, 2020 |
| | Notes | $ | $ | $ |
| | | | | |
| Assets | | | | |
| Current assets | | | | |
| Cash | | 7,892 | 5,573 | 6,051 |
| Grant recoverable | 7 | 3,308 | 1,689 | 1,007 |
| Other receivables | 6 | 1,640 | 340 | 373 |
| Prepaid expenses and other assets | | 32 | 11 | 13 |
| Total current assets | | 12,872 | 7,613 | 7,444 |
| | | | | |
| Non-current assets | | | | |
| Property and equipment | 8 | 10,851 | 3,692 | 1,287 |
| Intangible assets | 10 | 939 | 856 | 827 |
| Deferred financing costs | 12 | 89 | - | - |
| Security deposits | | 24 | 13 | 13 |
| Total assets | | 24,775 | 12,174 | 9,571 |
| | | | | |
| Liabilities | | | | |
| Current liabilities | | | | |
| Accounts payable and accrued liabilities | 11 | 2,821 | 697 | 523 |
| Current portion of lease liabilities | 9 | 64 | 60 | 53 |
| Total current liabilities | | 2,885 | 757 | 576 |
| | | | | |
| Non-current liabilities | | | | |
| Lease liabilities | 9 | 525 | 580 | 627 |
| Long-term debt | 12 | 473 | 151 | - |
| Total liabilities | | 3,883 | 1,488 | 1,203 |
| | | | | |
| Shareholders’ equity | | | | |
| Share capital | 13 | 31,236 | 16,476 | 11,236 |
| Accumulated deficit | | (10,528) | (5,921) | (2,868) |
| Accumulated other comprehensive income | | 184 | 131 | - |
| Total shareholders’ equity | | 20,892 | 10,686 | 8,368 |
| Total liabilities and shareholders’ equity | | 24,775 | 12,174 | 9,571 |
Commitments (Note 21)
First-time adoption of IFRS and restatement (Note 23)
Subsequent events (Note 24)
The accompanying notes are an integral part of these financial statements.
Starpax Biopharma Inc.
(formerly Starpax Medical Inc.)
Statements of loss and comprehensive loss
Years ended December 31, 2021 and 2020
(Expressed in thousands of United States dollars, except per share data and where specified)
| | | 2021 | 2020 |
|---|
| | Notes | $ | $ |
|---|
| Expenses | | | |
| General and Administrative | | (1,245) | (459) |
| Research and Development | | (3,010) | (2,408) |
| Depreciation | 8 | (218) | (124) |
| Loss on sale of property, plant and equipment | | (6) | - |
| Total Expenses | | (4,479) | (2,991) |
| | | | |
| Net financial expenses | | (73) | (43) |
| Loss before income tax | | (4,552) | (3,034) |
| | | | |
| Income tax (recovery) | 16 | - | - |
| Net loss | | (4,552) | (3,034) |
| | | | |
| Other Comprehensive loss | | | |
| Items that may be reclassified subsequently to income or loss | | | |
| Foreign currency translation differences | | 53 | 131 |
| Comprehensive loss | | (4,499) | (2,903) |
| | | | |
| Loss per share | 17 | | |
| Basic and diluted | | (0.06) | (0.04) |
| Weighted average number of common shares | | 75,500,000 | 75,500,000 |
The statements of loss and comprehensive loss has been retroactively adjusted to account for the stock split of 5:1 that took place on January 19, 2023
The accompanying notes are an integral part of these financial statements.
Starpax Biopharma Inc.
(formerly Starpax Medical Inc.)
Statements of changes in shareholders’ equity
Years ended December 31, 2021 and 2020
(Expressed in thousands of United States dollars, except per share data and where specified)
| | Share
capital | Accumulated
deficit | Other
comprehensive
income | Total
shareholders’
equity |
| | Notes | $ | $ | $ | $ |
| | | | | | |
| | | | | | |
| Balance as at December 31, 2019 | | 11,236 | (2,899) | — | 8,337 |
| Impact of first-time adoption of IFRS (Note 23) | | — | 31 | — | 31 |
| Balance as at January 1, 2020 | | 11,236 | (2,868) | — | 8,368 |
| Net loss | | — | (3,034) | — | (3,034) |
Foreign currency
translation differences | | — | — | 131 | 131 |
| Share issuance (class A), net of issuance costs | 13 | 5,240 | — | — | 5,240 |
| Share repurchase (class B) | 13 | — | (19) | — | (19) |
Balance as at December 31,
2020 | | 16,476 | (5,921) | 131 | 10,686 |
| Net loss | | — | (4,552) | — | (4,552) |
Foreign currency
translation differences | | — | — | 53 | 53 |
| Share issuance (class A), net of issuance costs | 13 | 14,760 | — | — | 14,760 |
| Share repurchase (class B) | 13 | — | (55) | — | (55) |
Balance as at December 31,
2021 | | 31,236 | (10,528) | 184 | 20,892 |
The statements of changes in stockholders' equity has been retroactively adjusted to account for the stock split of 5:1 that took place on January 19, 2023.
The accompanying notes are an integral part of these financial statements.
Starpax Biopharma Inc.
(formerly Starpax Medical Inc.)
Statements of cash flows
For the years ended December 31, 2021 and 2020
(Expressed in thousands of United States dollars, except per share data and where specified)
| | 2021 | 2020 |
| | Notes | $ | $ |
| | | | |
| Operating activities | | | |
| Net loss | | (4,552) | (3,034) |
| Items not affecting cash | | | |
| Depreciation | 8 | 218 | 124 |
| Interest income | | (41) | (32) |
| Interest expense | | 114 | 74 |
| Loss on disposal of property and equipment | | 6 | — |
| Changes in non-cash working capital items | 18 | (2,169) | (350) |
| Interest paid | | (2) | (4) |
| Interest received | | 41 | 32 |
| Net cash flows used in operating activities | | (6,385) | (3,190) |
| | | | |
| Investing activities | | | |
| Purchase of property and equipment | 8 | (6,554) | (2,595) |
| Grants received | | 358 | 129 |
| Acquisition of intangible assets | 10 | (56) | (13) |
| Net cash flows used in investing activities | | (6,252) | (2,479) |
| | | | |
| Financing activities | | | |
| Proceeds from long-term debt, net | 12 | 239 | 143 |
| Transaction costs paid | | (89) | — |
| Proceeds from issuance of class A common shares | 13 | 15,578 | 5,800 |
| Repurchase of class B common shares | 13 | (55) | (19) |
| Share issuance costs | | (818) | (560) |
| Payment of principal portion of lease liabilities | 9 | (164) | (121) |
| Net cash flows from financing activities | | 14,691 | 5,247 |
| | | | |
| Effect of foreign exchange rate changes on cash | | 265 | (56) |
| Increase (decrease) in cash during the period | | 2,319 | (478 |
| Cash, beginning of year | | 5,573 | 6,051 |
| Cash, end of year | | 7,892 | 5,573 |
The accompanying notes are an integral part of these financial statements.
Starpax Biopharma Inc.
(formerly Starpax Medical Inc.)
Notes to the financial statements
December 31, 2021 and 2020
(Expressed in thousands of United States dollars, except per share data and where specified)
1. Corporate information
Starpax Biopharma Inc. (the “Company”), incorporated under the Business Corporations Act (Quebec) on December 19, 2017, conducts research into the eradication of solid cancerous tumors and started operations in March 2018.
The financial statements of Starpax Biopharma Inc. were authorized for issue in accordance with a resolution of the directors on February 7, 2022. The registered office is located at 2500-1100 René-Lévesque Boulevard, Montreal, Quebec, Canada.
The Company performed an assessment of the impact of the uncertainties around the pandemic of the novel strain of the coronavirus, specifically identified as COVID-19, on the carrying amounts of its assets and liabilities. This assessment, which required the use of significant judgments and estimates, had no material impact on the Company’s financial statements. The Company assessed that the uncertainties around the impact of COVID-19 could generate, in future reporting periods, a risk of material adjustment to the carrying amounts of its intangible assets. In the recent months, there has been an ease of restrictions related to COVID-19, however, the risk, duration and full financial effect of the COVID-19 pandemic (including future variants) are unknown, and accordingly, estimates of the extent to which the COVID-19 pandemic may materially and adversely affect the Company are subject to significant uncertainties.
2. Liquidity
The Company has incurred recurring losses from operations, and as at December 31, 2021, had an accumulated deficit of $10,528 (2020 - $5,921) and a working capital of $9,987 (2020 –$6,856). The working capital as at December 31, 2021 is sufficient to pay its operating expenses for a period of at least 12 months from the date of the financial statements. The Company’s continued existence is dependent upon its ability to continue to execute its operating plan and to obtain additional debt or equity financing The Company has developed plans to raise funds and continues to pursue sources of funding that management believes, if successful, would be sufficient to support the Company’s operating plan. During the year ended December 31, 2021, the Company raised $15,578 through equity issuances and through the availability in its debt financing arrangements which was partially utilized subsequent to year end (Note 24). The Company’s operating plan is predicated on a variety of assumptions including, but not limited to, success in research objectives, cost estimates, its ability to continue to raise additional financing and the state of the general economic environment in which the Company operates. There can be no assurance that these assumptions will prove accurate in all material respects, or that the Company will be able to successfully execute its operating plan. In the event that the Company is not able to raise capital from investors in a timely manner, the Company will explore available options. In the absence of additional appropriate financing, the Company may have to modify its plan or slow down the pace of research and development.
3. Summary of significant accounting policies
Overall considerations and first-time adoption of IFRS
The financial statements have been prepared using accounting policies specified by those IFRS that are in effect at December 31, 2021.
The significant accounting policies that have been applied in the preparation of the financial statements are summarized below.
These accounting policies have been used throughout all periods presented in the financial statements.
Basis of presentation
The financial statements of the Company have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB").
For all periods up to and including the year ended December 31, 2019, the Company prepared its financial statements in accordance with the Canadian Accounting Standards for Private Enterprises. These financial statements for the years ended December 31, 2021 and 2020 are the first the Company has prepared in accordance with IFRS. In accordance with IFRS 1, the Company presents three statements of financial position in its first IFRS financial statements.
The preparation of the financial statements in conformity with IFRS requires the use of certain critical accounting estimates. Additionally, it requires management to exercise judgment in the process of applying the Company’s accounting policies. The areas involving a higher degree of judgment or complexity, or areas where judgments and estimates are significant to the financial statements are disclosed in note 4.
Basis of measurement
The financial statements have been prepared on the historical cost basis.
Foreign currency
Functional and presentation currency
The Company’s functional currency is Canadian dollars. These financial statements are presented in United States dollars which is the presentation currency of the Company, and all values are rounded to the nearest thousand ($000), except when otherwise indicated. The Company has adopted the United States dollar as its presentation currency as it is the most commonly used reporting currency in its industry.
Foreign currency transactions and balances
Monetary assets and liabilities are translated into the functional currency using the exchange rate in effect at year end. Other assets and liabilities are translated at the prevailing historical rates at the time of the transaction. Expenses are translated at the exchange rates in effect on the transaction date. Exchange gains and losses are recorded in net financial expenses in the statement of net loss and comprehensive loss.
3. Summary of significant accounting policies (continued)
Segments
The Company has determined that its chief executive officer is the chief operating decision maker (CODM). The Company operates and manages the business as one reporting and one operating segment, which is the business of developing a non-systemic cancer treatment technology. The Company’s CODM reviews financial information on an aggregate basis for purposes of allocating resources and evaluating financial performance. All the Company’s assets are located in Canada.
Cash
Cash consists solely of cash on hand and balances with banks.
Property and equipment and depreciation
Property and equipment are recorded at cost less accumulated depreciation. Cost includes expenditures that are directly attributable to the acquisition of the asset. Property and equipment are depreciated using the straight-line method over their estimated useful lives once available for use as follows:
| Furniture and office equipment | Straight-line | 5 years |
| Laboratory equipment | Straight-line | 10 years |
| Computer equipment | Straight-line | 3 to 4 years |
| Vehicles | Straight-line | 3 to 4 years |
| Leasehold improvements | Straight-line | Lease duration |
Depreciation methods, useful lives and the residual values of property and equipment are reviewed at each reporting date for any change in circumstances and are adjusted if appropriate.
The Company is currently constructing a manufacturing plant which is considered a plant-under-construction. All costs directly attributable to bring the plant to the condition necessary for it to be capable of operating in the manner intended by management are recorded at cost. Each significant part of the plant will be depreciated separately. Deprecation for each significant part of the plant is recorded over the useful life will begin when available for use.
Leases
The Company assesses at contract inception whether a contract is, or contains, a lease. That is, if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration.
The Company applies a single recognition and measurement approach for all leases, except for short-term leases and leases of low-value assets. The Company recognizes lease liabilities representing obligations to make lease payments and right-of-use assets representing the right to use the underlying assets.
The right-of-use assets are presented within property, plant and equipment.
The Company recognizes right-of-use assets at the commencement date of the lease (i.e., the date the underlying asset is available for use). Right-of-use assets are measured at cost, less any accumulated depreciation and impairment losses, and adjusted for any remeasurement of lease liabilities. The cost of right-of-use assets includes the amount of lease liabilities recognized, initial direct costs incurred, and lease payments made at or before the commencement date less any lease incentives received. Right-of-use assets are depreciated using the straight-line method from the commencement date to the earlier of the end of the useful life of the right-of-use asset or the end date of the lease term.
The right-of-use assets are also subject to impairment.
3. Summary of significant accounting policies (continued)
Leases (continued)
At the commencement date of the lease, the Company recognizes lease liabilities measured at the present value of lease payments to be made over the lease term. The lease payments include fixed payments (including in-substance fixed payments) less any lease incentives receivable, variable lease payments that depend on an index or a rate, and amounts expected to be paid under residual value guarantees. The lease payments also include the exercise price of a purchase option reasonably certain to be exercised by the Company and payments of penalties for terminating the lease, if the lease term reflects the Company exercising the option to terminate.
Variable lease payments that do not depend on an index or a rate are recognized as expenses (unless they are incurred to produce inventories) in the period in which the event or condition that triggers the payment occurs.
In calculating the present value of lease payments, the Company uses its incremental borrowing rate at the lease commencement date when the interest rate implicit in the lease is not readily determinable. After the commencement date, the amount of lease liabilities is increased to reflect the accretion of interest and reduced for the lease payments made. In addition, the carrying amount of lease liabilities is remeasured if there is a modification, a change in the lease term, a change in the lease payments (e.g., changes to future payments resulting from a change in an index or rate used to determine such lease payments) or a change in the assessment of an option to purchase the underlying asset.
Intangible assets
| License | | Indefinite |
| Intellectual properties | Straight-line | 20 years |
| Trademark | | Indefinite |
Intangible assets with indefinite useful lives are not amortized, but are tested for impairment annually, either individually or at the cash-generating unit level. The assessment of indefinite life is reviewed annually to determine whether the indefinite life continues to be supportable. If not, the change in useful life from indefinite to finite is made on a prospective basis.
Management monitors the progress of each internal research and development project. Development costs will be recognized as an asset when the following criteria are met: (i) technical feasibility; (ii) management’s intention to complete the project; (iii) the ability to use or sell; and (iv) the ability to generate future economic benefits; (v) availability of technical and financial resources; (vi) ability to measure the expenditures reliably. During the period of development, the asset is tested for impairment annually. Research costs are expensed as incurred.
The Company made upfront payments to acquire licenses, intellectual properties and trademarks. The licenses, including the licenses for the use of intellectual properties, and trademarks have been granted for periods ranging between five and ten years by the relevant government agency with the option of renewal at the end of this period. These licenses and trademarks may be renewed at little or no cost to the Company. As a result, those licenses and trademarks are assessed as having an indefinite useful life.
3. Summary of significant accounting policies (continued)
Impairment of non-financial assets
At each reporting date, the Company reviews the carrying amounts of its non-financial assets to determine whether there is any indication of impairment. If any such indication exists, then the asset’s recoverable amount is estimated. Property, plant and equipment and intangible assets are reviewed for impairment when events or circumstances indicate that carrying value may not be recoverable. The Company assesses at each reporting date whether any such events or changes in circumstances exist.
For the purpose of measuring recoverable amounts, assets are grouped at the lowest levels for which there are separately identifiable cash flows (cash-generating units – CGUs). The recoverable amount is the higher of an asset's fair value less costs of disposal and value in use (being the present value of the expected future cash flows of the relevant assets of the CGU). An impairment loss is recognized for the amount by which the asset's carrying amount exceeds its recoverable amount.
The Company evaluates impairment losses for potential reversals when events or circumstances require such consideration.
Investment tax credits and other government grants
Government grants, consisting of grants and investment tax credits, are recorded as a reduction of the related expense or cost of the asset acquired. Government grants are recognized when there is reasonable assurance that the Company has met or will meet the requirements of the approved grant program and there is reasonable assurance that the grant will be received. Loans received at below market interest rate are treated in the same manner. The benefit of the below market interest rate is recorded as a reduction of the related expense or cost of the asset acquired.
Grants that compensate the Company for expenses incurred are recognized in net loss in reduction thereof on a systematic basis in the same years in which the expenses are recognized. Grants that compensate the Company for the cost of an asset are recognized in net loss on a systematic basis over the useful life of the asset.
The Company incurs research and development expenditures which are eligible for scientific research and experimental development (SR&ED) and investment and innovation tax credits. Refundable investment tax credits are recorded as SR&ED or investment and innovation tax credits in the statements of net loss and comprehensive loss when there is reasonable assurance that the credits will be realized. Non-refundable SR&ED and investment and innovation tax credits, which are deductible against income taxes otherwise payable, are recorded as a reduction of the related expenses or cost of asset(s) when there is reasonable assurance that the credits will be realized.
The tax credits recorded are based on management’s best estimate of amounts expected to be recovered and are subject to audit by taxation authorities. To the extent that actual tax credits differ from the estimate, those differences are recorded in the period of assessment by taxation authorities as an adjustment of the items to which they relate.
Financial instruments
Recognition and initial measurement
Financial assets and financial liabilities are initially recognized when the Company becomes a party of the contractual provision of the instrument. Financial assets and financial liabilities are initially measured at fair value. Transaction costs that are directly attributable to the acquisition or issue of financial assets and financial liabilities (other than financial assets and financial liabilities at fair value through profit or loss) are added to or deducted from the fair value of the financial assets or financial liabilities, as appropriate, on initial recognition. Transaction costs directly attributable to the acquisition of financial assets or financial liabilities at fair value through profit or loss are recognized immediately in profit or loss.
3. Summary of significant accounting policies (continued)
Financial instruments (continued)
Classification and subsequent measurement
Financial instruments are classified into the following specified categories: amortized cost, fair value through other comprehensive income (“FVOCI”) or fair value through profit or loss (“FVTPL”). The classification depends on the nature and purpose of the financial instrument and is determined at the time of initial recognition. The Company’s financial instruments have been classified as follows:
| Financial instrument | Classification |
| Financial assets | |
| Cash | Amortized cost |
| Other receivables | Amortized cost |
| | |
| Financial liabilities | |
Accounts payable and accrued
liabilities | Amortized cost |
| Lease liabilities | Amortized cost |
| Long-term debt | Amortized cost |
| | |
A financial asset is measured at amortized cost if it is held within a business model of holding financial assets and collecting contractual cash flows and those cash flows are comprised solely of payments of principal and interest. A financial asset is measured at FVTOCI if the financial asset is held within a business model of both collecting contractual cash flows and selling the financial assets or through an irrevocable election for equity instruments that are not held for trading. All other financial assets are measured at FVTPL.
Financial assets can only be reclassified when there is a change to the business model within which they are managed. Such reclassifications are applied on a prospective basis. Financial assets are derecognized only when the contractual rights to the cash flows from the asset expire, or when the Company transfers the financial asset and substantially all the risks and rewards of ownership of the asset to another entity.
Share split
On January 19, 2023, the Company amended its Articles (i) converting all outstanding Class A Common Shares and Class B Common Shares into shares of a single class called common shares (“Common Shares”) and (ii) executing a 5:1 stock split, subdividing all its issued and outstanding Common Shares into five (5) such Common Shares.
Loss per share
Basic loss per share is calculated by dividing net loss attributable to the Class A and B common shareholders of the Company by the basic weighted-average number of outstanding common shares. Except for the repurchase option, the Company's Class A and Class B common shares have the same rights, are equal in all respects, and are otherwise treated as if they were one class of shares, including the treatment for the loss per share calculations. Diluted loss per share is computed similar to basic loss per share except that the weighted average shares outstanding are increased to include additional shares for the assumed exercise of stock options and warrants, if dilutive. As at December 31, 2021 and 2020, there were no outstanding options or warrants.
3. Summary of significant accounting policies (continued)
Income taxes
Income tax expense represents the sum of the tax currently payable and deferred tax.
The tax currently payable is based on taxable income for the year. Taxable income differs from “income before tax” as reported in the statement of net loss and comprehensive loss because of items of income or expense that are taxable or deductible in other years and items that are never taxable or deductible. The Company’s current tax is calculated using tax rates that have been enacted or substantively enacted by the end of the year.
Deferred tax is recognized on temporary differences between the carrying amounts of assets and liabilities in the financial statements and the corresponding tax bases used in the computation of taxable income. Deferred tax liabilities are generally recognized for all taxable temporary differences. Deferred tax assets are generally recognized for all deductible temporary differences to the extent it is probable taxable income will be available against which those deductible temporary differences can be utilized. Such deferred tax assets and liabilities are not recognized if the temporary difference arises from the initial recognition of assets and liabilities in a transaction that affects neither the taxable income nor the accounting income.
The carrying amount of deferred tax assets is reviewed at the end of each year and reduced to the extent it is not probable sufficient taxable income will be available to allow all or part of the asset to be recovered. Deferred tax liabilities and assets are measured at the tax rates that are expected to apply in the year in which the liability is settled or the asset realized, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the year.
The measurement of deferred tax liabilities and assets reflects the tax consequences that would follow from the manner in which the Company expects, at the end of the year, to recover or settle the carrying amount of its assets and liabilities.
Current and deferred taxes are recognized in net loss, except when they relate to items that are recognized in other comprehensive loss or directly in equity, in which case the current and deferred taxes are also recognized in other comprehensive loss or directly in equity, respectively.
Deferred share issuance costs
The Company capitalizes certain legal, professional accounting and other third-party fees that are directly associated with in-process equity financings as deferred issuance costs until such financings are consummated. After consummation of the equity financing, these costs are recorded in stockholders’ equity as a reduction of proceeds generated as a result of the offering. Should the in-process equity financing be abandoned, the deferred share issuance costs will be expensed immediately as a charge to operating expenses in the statements of loss and comprehensive loss.
Deferred financing costs
The Company capitalizes certain legal, professional accounting and other third-party fees that are directly associated with in-process debt financings as deferred financing costs until such financings are consummated. These costs are included in deferred financing costs.
Employee Benefits
The Company has a post-retirement defined contribution plan. The Company pays contributions to a group RRSP on a mandatory, contractual basis. The Company has no further payment obligations once the contributions have been paid. The contributions are recognized as employee benefit expense when they are due.
4. Significant accounting judgments, estimates and assumptions
The preparation of the financial statements in accordance with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, expenses and disclosure of contingent assets and liabilities. Actual results may differ from these estimates. Significant assumptions about the future and other sources of estimation and judgment uncertainty that management has made at the end of the reporting period, that could result in a material adjustment to the carrying amounts of assets and liabilities in the event that actual results differ from assumptions made, relate to:
Going concern
The assumption that the Company will be able to continue as a going concern is subject to estimates and judgement by management including the Company’s short and long-term operating budget, expected profitability, investing and financing activities, and management’s strategic planning.
Fair value measurement of non-interest-bearing debt
The Company has estimated, on initial recognition, the fair market value of certain debts that do not have a defined coupon rate, using a comparative interest rate for similar liabilities and a discounted cash flow to determine a reasonable present value. Additional details on debt are disclosed in Note 12.
Intangible assets
In applying its accounting policy for costs to be capitalized as intangible assets, the Company must determine whether the criteria for capitalization have been met. The most subjective judgement is whether a project will generate probable future economic benefits. Management considers all appropriate facts and circumstances in making this assessment including expected market demand, cost and future economic conditions.
Income taxes
The recognition of deferred tax assets requires the Company to assess future taxable income available to utilize deferred tax assets related to deductible or taxable temporary differences. The Company considers the nature and carry-forward period of deferred tax assets, the Company's recent earnings history and forecast of future earnings in performing this assessment.
Leases
Leases requires lessees to discount lease payments using the rate implicit in the lease if that rate is readily available. If the rate cannot be readily determined, the lessee is required to use its incremental borrowing rate. The Company generally uses the incremental borrowing rate when initially recording real estate leases as the implicit rates are not readily available as information from the lessor regarding the fair value of underlying assets and initial direct costs incurred by the lessor related to the leases assets is not available. The Company determines the incremental borrowing rate as the interest rate of the Company would pay to borrow over a similar economic environment. Leases also requires lessees to estimate the lease term. In determining the period which the Company has the right to use an underlying asset, management considers the non-cancellable period along with all facts and circumstances that create an economic incentive to exercise an extension option, or not to exercise a termination option.
Investment tax credits recoverable
The recognition of investment tax credits recoverable requires the Company to assess future tax payable available to utilize the investment tax credits. The Company considers the carry-forward period of the investment tax credits, the Company's forecast of future earnings in performing this assessment. The Company determines the value of effort expended towards research and development projects that qualify for investment tax credits and calculates the estimated recoverable to be recognized. The allocation of direct salaries to qualifying projects is derived from time records and assessment by management. The actual investment tax credits claimed and realized may differ from the estimate based on the final tax returns and review by tax authorities.
Impairment of non-financial assets
At each reporting date, the Company reviews the carrying amounts of its non-financial assets to determine whether there is any indication of impairment. If any such indication exists, then the asset’s recoverable amount is estimated. See note 3 for more detail.
5. Future accounting standard changes
The IASB has issued several new standards and amendments that will be effective on various dates. The listing below is of standards, interpretation and amendments issued which the Company reasonably expects to be applicable at a future date. The Company intends to adopt those standards when they become effective. The impact on the Company is currently being assessed.
Amendments to IAS 1 – Classification of Liabilities as Current or Non-current
The amendments to IAS 1 affect only the presentation of liabilities as current or non-current in the statement of financial position and not the amount or timing of recognition of any asset, liability, income or expenses, or the information disclosed about those items.
The amendments clarify that the classification of liabilities as current or non-current is based on rights that are in existence at the end of the reporting period, specify that classification is unaffected by expectations about whether an entity will exercise its right to defer settlement of a liability, explain that rights are in existence if covenants are complied with at the end of the reporting period, and introduce a definition of 'settlement' to make clear that settlement refers to the transfer to the counterparty of cash, equity instruments, other assets or services.
The amendments are applied retrospectively for annual periods beginning on or after January 1, 2023, with early application permitted. The amendment is not expected to have a material impact on the Company.
Annual Improvements to IFRS Standards 2018–2020 - IFRS 9 Financial Instruments
The amendment clarifies that in applying the '10 per cent' test to assess whether to derecognize a financial liability, an entity includes only fees paid or received between the entity (the borrower) and the lender, including fees paid or received by either the entity or the lender on the other's behalf.
The amendment is applied prospectively to modifications and exchanges that occur on or after the date the entity first applies the amendment.
The amendment is effective for annual periods beginning on or after January 1, 2022, with early application permitted. The amendment is not expected to have a material impact on the Company.
Disclosure of Accounting Policies (Amendments to IAS 1 and IFRS Practice Statement 2)
In February 2021, the IASB issued amendments to IAS 1 relating to an entity’s accounting policies disclosure requirements. Entities will be required to disclose material accounting policies instead of significant accounting policies for annual periods starting on or after January 1, 2023, with early adoption permitted. Entities will use a four-step materiality process in order to determine which accounting policies should be disclosed going forward. Several examples and guidance have been developed to demonstrate the application of the amendment.
The amendment is not expected to have a material impact on the Company.
5. Future accounting standard changes (continued)
Definition of Accounting Estimates (Amendments to IAS 8)
The amendments replace the definition of a change in accounting estimates with a definition of accounting estimates. Under the new definition, accounting estimates are “monetary amounts in financial statements that are subject to measurement uncertainty”. Entities develop accounting estimates if accounting policies require items in financial statements to be measured in a way that involves measurement uncertainty. The amendments clarify that a change in accounting estimate that results from new information or new developments is not the correction of an error.
The amendment is effective for annual reporting periods beginning on or after January 1, 2023. The amendment is not expected to have a material impact on the Company.
| | 2021 | 2020 |
| | $ | $ |
Other receivable | 46 | — |
| Sales tax recoverable | 1,594 | 340 |
| | 1,640 | 340 |
| | 2021 | 2020 |
| | $ | $ |
Research and development tax credits receivable | 2,941 | 1,516 |
| Investment and innovation tax credits receivable | 357 | 167 |
| Other grant receivables (Note 22) | 10 | 6 |
| | 3,308 | 1,689 |
The following table presents the property and equipment for the Company:
The following table presents the property and equipment for the Company:
| | Furniture and office equipment | Computer equipment | Leasehold Improvements | Vehicles | Right-of use-assets - Buildings | Laboratory Equipment | Right-of-use assets – Vehicles | Total |
| $ | $ | $ | $ | $ | | $ | $ |
| Cost | | | | | | | | |
Balance as at
December 31, 2019 | 22 | 8 | 265 | — | 713 | 311 | — | 1,319 |
| Additions | 4 | 15 | 1,356 | 44 | — | 965 | — | 2,384 |
| Dispositions | — | — | — | — | — | — | — | — |
| Foreign exchange | 1 | 1 | 77 | 2 | 14 | 57 | — | 152 |
Balance as at
December 31, 2020 | 27 | 24 | 1,698 | 46 | 727 | 1,333 | — | 3,855 |
| Accumulated depreciation | | | | | | | | |
Balance as at
December 31, 2019 | 5 | 1 | — | — | 18 | 8 | — | 32 |
| Depreciation | 4 | 4 | — | 7 | 69 | 40 | — | 124 |
| Dispositions | — | — | — | — | — | — | — | — |
| Foreign exchange | 1 | — | — | — | 4 | 2 | — | 7 |
Balance as at
December 31, 2020 | 10 | 5 | — | 7 | 91 | 50 | — | 163 |
Net carrying value
as at December 31, 2020 | 17 | 19 | 1,698 | 39 | 636 | 1,283 | — | 3,962 |
|
| 8. | Property and equipment (continued) |
| | Furniture and office equipment | Computer equipment | Leasehold Improvements | Vehicles | Right-of use-assets - Buildings | Laboratory Equipment | Right-of-use assets – Vehicles | Total |
| $ | $ | $ | $ | $ | | $ | $ |
| | | | | | | | | |
| Cost | | | | | | | | |
Balance as at
December 31, 2020 | 27 | 24 | 1,698 | 46 | 727 | 1,333 | — | 3,855 |
| Additions | 54 | 8 | 6,071 | — | — | 1,225 | 41 | 7,479 |
| Dispositions | — | — | — | (46) | — | — | — | (46) |
| Foreign exchange | — | (1) | (63) | — | 3 | (8) | — | (69) |
Balance as at
December 31, 2021 | 81 | 111 | 7,706 | — | 730 | 2,550 | 41 | 11,219 |
| | | | | | | | | |
| Accumulated depreciation | | | | | | | | |
Balance as at
December 31, 2020 | 10 | 5 | — | 7 | 91 | 50 | — | 163 |
| Depreciation | 10 | 18 | — | 2 | 74 | 103 | 11 | 218 |
| Dispositions | — | — | — | (9) | — | — | — | (9) |
| Foreign exchange | (2) | — | — | — | (1) | — | (1) | (4) |
Balance as at
December 31, 2021 | 18 | 23 | — | — | 164 | 153 | 10 | 368 |
Net carrying value
as at December 31, 2020 | 63 | 88 | 7,706 | — | 566 | 2,397 | 31 | 10,851 |
|
During the year ended December 31, 2021, the Company acquired property and equipment in the amount of $8,057 ($2,667 in 2020), of which an amount of $1,681 was unpaid as at December 31, 2021 ($229 in 2020). The leasehold improvements are under construction as at December 31, 2021.
9. Leases
The Company entered lease arrangements for the use of facility property and vehicle used in operations. The facility property lease commenced on October 1, 2019, with a five-year term and an option to extend for a maximum of 5 years, while the vehicle lease commenced March 23, 2021, for a three-year term. The Company have determined that the extension option are reasonably certain to be exercised.
The Company also has certain leases of machinery with lease terms of 12 months or less and leases of office equipment with low value. The Company applies the ‘short-term lease’ and ‘lease of low-value assets’ recognition exemptions for these leases.
Amounts recognized in the statements of loss and comprehensive loss:
| | 2021 | 2020 |
| | $ | $ |
| | | |
| Depreciation expense of right-of use assets | 85 | 69 |
| Interest expense on lease liability | 69 | 70 |
| Variable rent expense | 90 | 78 |
| Expenses relating to short-term leases | 62 | 65 |
| Expenses related to low-value leases | — | — |
| | 306 | 282 |
9. Leases (continued)
Change in the lease liability for period ended December 31, 2021 and 2020 is as follows:
| | 2021 | 2020 |
| | $ | $ |
| | | |
| Beginning balance, as at January 1 | 640 | 680 |
| Additions | 41 | — |
| Interest expense | 69 | 70 |
| Lease payments | (164) | (121) |
| Foreign exchange | 3 | 11 |
| Ending balance, as at December 31 | 589 | 640 |
| Current | 64 | 60 |
| Non-current | 525 | 580 |
The maturity analysis of lease liabilities is disclosed in Note 19.
When measuring lease liabilities, the Company discounted lease payments using its incremental borrowing rate ranging between 4.9% to 11.2% (2020 – 11.2%).
10. Intangible assets
The following tables presents the intangible assets for the Company:
| | License and intellectual properties | Trademarks | Total |
| | $ | $ | $ |
| Cost | | | |
| Balance as at January 1, 2020 | 816 | 11 | 827 |
| Additions | 10 | — | 10 |
| Foreign exchange | 18 | 1 | 19 |
| Balance as at December 31, 2020 | 844 | 12 | 856 |
| | License and intellectual properties | Trademarks | Total |
| | $ | $ | $ |
| Cost | | | |
| Balance as at December 31, 2020 | 844 | 12 | 856 |
| Additions | 72 | 9 | 81 |
| Foreign exchange | 2 | — | 2 |
| Balance as at December 31, 2021 | 918 | 21 | 939 |
During the year ended December 31, 2021, the Company acquired intangibles in the amount of $81 ($10 in 2020), of which an amount of $25 was unpaid as at December 31, 2021 ($nil in 2020).
11. Accounts payable and accrued liabilities
| | 2021 | 2020 |
| | $ | $ |
| | | |
| Accounts payable and accrued liabilities | 2,698 | 644 |
| Wages and vacation payable | 123 | 45 |
| Other | - | 8 |
| | 2,821 | 697 |
Information about the Company’s exposure to currency and liquidity risk is included in Note 19.
12. Long-term debt
The terms and conditions of the Company’s loans and borrowings are as follows:
| | 2021 | 2020 |
| | $ | $ |
| | | |
Repayable contribution agreement with
the Economic Development Agency of
Canada (Note 12 (a)) | 473 | 151 |
| Investissement Québec loan (Note 12 (b)) | — | — |
| Total long-term debt | 473 | 151 |
| | | |
| Current portion of long-term debt | — | — |
Loans and borrowings are presented net of unamortized transaction costs. Transaction costs relating to the issuance of loans and borrowings are amortized over the term of the debt using the effective interest rate method.
a) On May 15, 2020, the Company entered into an interest-free repayable contribution agreement with the Economic Development Agency of Canada for the Regions of Quebec for a maximum amount of $1,578 (CAD $2,000) to finance the establishment of a pilot plant as well as the construction of an intervention room dedicated to oncological research. A principal mortgage in the amount of $1,578 (CAD $2,000) and an additional mortgage of $316 (CAD $400) on laboratory equipment with a net book value of $1,616 (CAD $2,049) as at December 31, 2021, will be given as security for this repayable contribution. As per the agreement, the project must be completed by March 31, 2023 (this was amended in 2021 from December 31, 2021) and is to be paid in 60 equal and consecutive monthly installments beginning 36 months after the project completion date. As a result of the amendment in 2021, the payment schedule was altered and the fair value of the loan was recalculated using an effective interest rate of 10% to reflect this change. As at December 31, 2021, an additional amount of $632 ($274 as at December 31, 2020) has been drawn from the repayable contribution. The amount recognized as a grant due to the below-market interest rate of $354 ($136 in the year ended December 31, 2020) has been recognized as a reduction to the carrying value of property, plant and equipment. The remaining loan balance of $473 is included in loans and borrowing.
12. Long-term debt (continued)
b) On April 9, 2021, the Company entered into a financing agreement with Investissement Québec (IQ) to finance the establishment of a pilot plant through investment in Class A common stock for a total amount of $3,945 (CAD$5,000) and a loan for a maximum amount of $5,522 (CAD $7,000) under the ESSOR program. The loan will bear interest at a fixed rate of 10%. Interest will be capitalized monthly for a period of 36 months from the first loan disbursement, and thereafter will be payable. The loan must be repaid in 84 equal and consecutive monthly installments starting 36 months from the first disbursement of the loan. A movable hypothec in the amount of $5,522 (CAD $7,000) and an additional hypothec in the amount of $1,104 (CAD $1,400) encumbering the universality of the Company’s equipment, which will be first rank with a net book value of $1,695 (CAD $2,149) as at December 31, 2021, except with regard to laboratory equipment, not exceeding $1,893 (CAD $2,400) which is first ranked in favor of the Economic Development Agency of Canada for the Regions of Quebec, for which the hypothec in favor of IQ will be second rank with a net book value of $1,616 (CAD $2,049) as at December 31,2021. In relation to this agreement, the Company incurred $89 of deferred financing costs which were recorded in deferred financing costs. A first claim was made subsequently to the year end. (Note 24)
13. Share capital
On January 19, 2023, the Company amended its Articles (i) converting all outstanding Class A Common Shares and Class B Common Shares into shares of a single class called common shares (“Common Shares”) and (ii) executing a 5:1 stock split, subdividing all its issued and outstanding Common Shares into five (5) such Common Shares.
The Company has authorized the following classes of share capital:
Authorized
Unlimited Class A common shares, voting, participating, no par value
Unlimited Class B common shares, voting, participating, no par value. The Company has the option (forced repurchase option) to repurchase a Class B common share held in its share capital for CAD$0.02 per share, concurrently with each issuance of a share by the Company and up to the number of Class B common shares in the Company’s share capital. Similarly, it undertakes to exercise the forced repurchase option upon each issuance of shares and repurchase a number of Class B common shares held equal to the number of shares issued, no more and no less. In no event shall the number of issued and outstanding shares of the Company exceed 75,500,000 while the forced repurchase option is in effect. The forced repurchase option will cease to be in effect upon the earlier of the following two events:
| · | The cumulative amount of subscription proceeds for shares of the Company, donations from private sources, profits made and dividends paid combined have reached CAD $88 million. |
| · | The Company will have achieved commercialization of its developed treatment technology. |
| | | 2021 | | 2020 |
| | | Carrying value | | Carrying Value |
| Type of share | Quantity | $ | Quantity | $ |
| | | | | |
Company’s share capital Class A common shares Balance, beginning of period Issuance of shares | | | | |
| 17,197,605 | 16,475 | 15,929,275 | 11,235 |
| 3,272,370 | 14,760 | 1,268,330 | 5,240 |
| Balance, end of period | 20,469,975 | 31,235 | 17,197,605 | 16,475 |
13. Share capital (continued)
| | | 2021 | | 2020 |
| | | Carrying value | | Carrying value |
| Type of share | Quantity | $ | Quantity | $ |
| | | | | |
| Company’s share capital Class B common shares | | | | |
| Balance, beginning of period | 58,302,395 | 1 | 59,570,725 | 1 |
| Repurchase of shares | (3,272,370) | — | (1,268,330) | — |
| Balance, end of period | 55,030,025 | 1 | 58,302,395 | 1 |
During the year ended December 31, 2021, the Company issued 3,272,370 Class A common shares for a cash consideration of $15,578 and repurchased 3,272,370 Class B common shares for a cash consideration of $55. The Company then cancelled these Class B Common shares. The Company incurred $818 in costs directly relating to the issuance. These issuance costs were adjusted against share capital in the statements of changes in shareholders’ equity.
During the year ended December 31, 2020, the Company issued 1,268,330 Class A common shares for a cash consideration of $5,800 and repurchased 1,268,330 Class B common shares for a cash consideration of $19. The Company then cancelled these Class B Common shares. The Company incurred $560 in costs directly relating to the issuance. These issuance costs were adjusted against share capital in the statements of changes in shareholders’ equity.
14. Capital management
The Company’s objectives in managing capital are to ensure sufficient liquidity to meet financial obligations when due and to execute its operating and strategic plans and to provide returns to its shareholders. The capital structure of the Company consists of net borrowings and lease liabilities (disclosed in Note 12 and 9), equity of the Company comprising of issued capital (disclosed in Note 13), accumulated deficit and other comprehensive income. The Company is not subject to any externally imposed capital requirements.
The Company manages its capital with the intent of maintaining a flexible capital structure that optimizes the cost of capital at an acceptable risk. To maintain or adjust the capital structure, the Company may attempt to issue new shares, issue new debt, and acquire or dispose of assets, all of which are subject to market conditions and the terms of the underlying third-party agreements. The Company monitors its capital by analyzing its monthly cash consumption and short-term commitments related to its financial liabilities. The total capital as at December 31, 2021 and 2020 is calculated as follows:
| | 2021 | 2020 |
| | $ | $ |
| | | |
Non-current liabilities comprising lease Liabilities and debt | 998 | 731 |
| Current portion of lease liability | 64 | 60 |
| Shareholders’ equity | 20,892 | 10,686 |
| | 21,954 | 11,477 |
15. Expenses by nature
The following table presents expenses of the Company for the years ended December 31, 2021 and 2020.
| | 2021 | 2020 |
| | $ | $ |
| | | |
| Employee compensation | 2,079 | 1,321 |
| Employee benefits | 16 | 10 |
Note, the employee benefits are the amount related to the defined contribution plan described in Note 3.
16. Income tax expense
The reconciliation of income taxes at Canadian statutory rate with the reported loss is as follows:
| | 2021 | 2020 |
| | $ | $ |
| | | |
| Loss before income tax | (4,552) | (3,034) |
| Income tax recovery at statutory rate of 26.5% (26.5% in 2020) | (1,206) | (804) |
| | Change resulting from the tax effect of: | | |
| | Share issue costs | (81) | (38) |
| | Other differences | 52 | (5) |
| | Effect of unused tax losses not recognized as deferred tax asset | 1,235 | 847 |
| Income tax expense (recovery) | — | — |
Deferred income taxes reflect the net tax effects of differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets and liabilities are as follows:
| | 2021 | 2020 |
| | $ | $ |
| Non capital losses carry forward | 4,783 | 3,792 |
| Research and development expenses | 1,231 | 833 |
| Property, plant and equipment | (538) | (159) |
| Right of use assets | (158) | (169) |
| Lease liabilities | 170 | 175 |
| Intangible assets | (249) | (227) |
| Share issuance costs | 213 | 106 |
| Investment tax credits taxable in a future year | (204) | (222) |
| Deferred tax assets not recognized | (5,248) | (4,129) |
| | — | — |
17. Loss per share
The calculation of basic and diluted earnings per share has been based on the following loss attributable to ordinary shareholders and weighted average number of shares outstanding. In the years ended December 31, 2021 and 2020 all the basic loss per share is equal to the diluted loss per share as there were no potentially dilutive instruments in these periods:
Basic and dilutive loss per share
Loss attributable to Class A and Class B common shares
| | 2021 | 2020 |
| | $ | $ |
| | | |
| Loss attributable to ordinary shareholders | (4,552) | (3,034) |
| Weighted-average number of Class A and Class B common shares | 75,500,000 | 75,500,000 |
| Basic and dilutive loss per share | (0.06) | (0.04) |
18. Supplemental cash flow information
The table below disclose the changes in non-cash working capital balances related to operations activities:
| | 2021 | 2020 |
| | $ | $ |
| | | |
| Decrease (increase) in | | |
| Grant receivables | (1,439) | (475) |
| Other receivables | (1,342) | 39 |
| Prepaid expenses | (22) | 1 |
| Other assets | (11) | — |
| Increase (decrease) in | | |
| Accounts payable and accrued liabilities | 645 | 85 |
| Total | (2,169) | (350) |
19. Financial instruments and risk management
The Company is exposed to various financial risks through transactions in financial instruments
a) Liquidity risk
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they fall due. The Company is therefore exposed to liquidity risk with respect to all of the financial liabilities recognized on the statements of financial position.
The Company manages its liquidity by monitoring it operating requirements. The Company prepares budget and cash forecasts to ensure it has sufficient funds to fulfill its obligations.
The following are the undiscounted contractual maturities of financial liabilities, including estimated interest payments, as at December 31, 2021:
19. Financial instruments and risk management (continued)
| | Carrying amount
$ | Contractual cash flows
$ | Less than a year $ | Between
1 and 2 years
$ | More than
2 years
$ |
| | | | | | |
Accounts payable and
accrued liabilities | 2,821 | 2,821 | 2,821 | — | — |
| Long-term debt | 473 | 906 | — | — | 906 |
| Lease liabilities | 589 | 978 | 131 | 260 | 587 |
As at December 31, 2020:
| | Carrying amount
$ | Contractual
cash flows
$ | Less than a year $ | Between
1 and 2 years
$ | More than
2 years
$ |
| | | | | | |
Accounts payable and
accrued liabilities | 697 | 697 | 697 | — | — |
| Long-term debt | 151 | 274 | — | — | 274 |
| Lease liabilities | 640 | 1,016 | 49 | 255 | 712 |
b) Credit risk
Credit risk is the risk that one party to a financial instrument will cause a financial loss for the other party by failing to discharge an obligation. Credit risk arises principally from the Company’s cash, and long-term deposits. The carrying amounts of these financial assets represent the maximum credit exposure.
The credit risk associated with cash is limited because it is maintained with two large financial institutions.
c) Market risks
Market risk is the risk that the Company will incur losses arising from adverse changes in underlying market factors, including interest and foreign currency exchange rates.
(i) Interest rate risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes is the market interest rate. The Company’s exposure to interest rate risk as at December 31, 2021, and 2020 is as follows:
| Accounts payable and accrued liabilities | | | Non-interest bearing |
| Long-term debt | Non-interest bearing | |
The Company does not account for any financial assets or financial liabilities at FVTPL.
(ii) Foreign currency risk
The risk around foreign currency exchange rates is not considered significant for the years ended December 31, 2021 and 2020 as the foreign currency exposure is minimal.
19. Financial instruments and risk management (continued)
Fair value
Estimated fair values for financial instruments are designed to approximate amounts at which the instruments could be exchanged in a current arm's-length transaction between knowledgeable willing parties.
The Company classifies and discloses fair value measurements using a fair value hierarchy that reflects the significance of the inputs used in making the measurements. The three levels of the fair value hierarchy are:
Level 1 – Valuation based on quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 – Valuation techniques based on inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices);
Level 3 – Valuation techniques using inputs for the asset or liability that are not based on observable market data (unobservable inputs).
The fair value hierarchy requires the use of observable market inputs whenever such inputs exist. A financial instrument is classified to the lowest level of the hierarchy for which a significant input has been considered in measuring fair value.
The Company has determined that the carrying amounts of its financial assets and financial liabilities measured at amortized cost approximate their fair value given their short-term nature with the exception of long-term debt. Long term debt received at below market interest rate is initially measured at fair value with the benefit of the below market interest rate recorded as a reduction of the related expense or cost of the asset acquired and subsequently measured at amortized cost.
20. Related party transactions
Medpax Consortium S.E.C (“Medpax”) is a related party of the Company as it has a controlling interest in the Company. In 2021, the Company repurchased 3,272,370 of these shares for $55, in 2020 repurchased 1,268,330 shares for $19. As at December 31, 2021, the Company has no due to Medpax ($6 as at December 31, 2020). As at December 31, 2021, Medpax owned 72.9% of the Company. Please see note 13 for further detail on the Class B Common shares issued to Medpax.
Remuneration of key management personnel
The Company's key management personnel are members of senior management, executive and non-executive and board of directors, who have the power and responsibility to plan, manage and control the Company's operations.
The remuneration of key management personnel of the Company is as follows:
| | 2021 | 2020 |
| | $ | $ |
| | | |
| Salary and other short-term benefits | 640 | 556 |
21. Commitments
As at December 31, 2021, the Company had issued capital commitments totaling $6,070 to various suppliers in connection with its operation and the establishment of a pilot plant.
22. Government grants
Temporary Wage Subsidy Program
On March 25, 2020, the Temporary Wage Subsidy ("TWS") program was introduced by the Government of Canada, reimbursing eligible employers who for a portion of wages paid to employees during the COVID-19 pandemic. The claims submitted or expected to be submitted under the TWS program was nil for the year ended December 2021 and $8 for the period ended December 31, 2020. The TWS has been recognized as an offset to wage expense on the Company's statements of loss and comprehensive loss.
Repayable Contribution Agreement
On May 15, 2020, the Company entered into an interest-free repayable contribution agreement with the Economic Development Agency of Canada for the Regions of Quebec. The benefit of the below-market interest rate is accounted for as a grant. See note 12 for more detail.
Government assistance
The Company incurred research and development expenditures which are eligible for tax credits. The tax credits recorded are based on management’s estimate of amounts expected to be recovered and are subject to audit by the taxation authorities and, accordingly, these amounts may vary. For the year ended December 31, 2021 the Company recorded provision for refundable tax credits of $1,463 (December 31, 2020 - $1,142). This amount has been recorded as a reduction of research and development for the year. Total research and development costs before tax credits applied for the period ended December 31, 2021 - $4,473 (December 31, 2020- $3,550). The Company also received, $191 of innovation tax credits, which were recorded as a reduction of laboratory equipment costs ($154 in the year ended 2020).
23. First-time adoption of IFRS and restatement
These are the Company’s first financial statements prepared in accordance with International Financial Reporting Standards (IFRS). The date of transition to IFRS is January 1, 2020.
The Company’s IFRS accounting policies presented in Note 3 have been applied in preparing the financial statements for the year ended December 31, 2021, the comparative information, and the opening statement of financial position at the date of transition.
The Company’s has applied IFRS 1 First-time Adoption of International Financial Reporting Standards in preparing these first IFRS financial statements. The effects of the transition to IFRS on equity, total comprehensive income and reported cash flows are presented in this section and are further explained in the notes accompany the tables.
23. First-time adoption of IFRS and restatement (continued)
Reconciliation of the statement of financial position including equity as at January 1, 2020:
| | Previous GAAP in CAD | IFRS Adjustments | IFRS in CAD | IFRS in USD |
| Assets | | | | |
| Current assets | | | | |
| Cash | 7,859 | - | 7,859 | 6,051 |
| Other receivables | 485 | - | 485 | 373 |
| Prepaid expenses | 31 | (14) | 17 | 13 |
| Grant receivables | 1,307 | - | 1,307 | 1,007 |
| Total current assets | 9,682 | (14) | 9,668 | 7,444 |
| | | | | |
| Non-current assets | | | | |
| Property and equipment | 769 | 903 | 1,672 | 1,287 |
| Intangible assets | 1,074 | - | 1,074 | 827 |
| Deferred financing costs | - | - | - | - |
| Other assets | 30 | (14) | 16 | 13 |
| Total assets | 11,555 | 875 | 12,430 | 9,571 |
| | | | | |
| Liabilities | | | | |
| Current liabilities | | | | |
| Accounts payable and accrued liabilities | 679 | - | 679 | 523 |
| Current portion of the lease liabilities | - | 68 | 68 | 53 |
| Total current liabilities | 679 | 68 | 747 | 576 |
| | | | | |
| Non-current liabilities | | | | |
| Lease liabilities | - | 814 | 814 | 627 |
| Long-term debt | - | - | - | - |
| Total liabilities | 679 | 882 | 1,561 | 1,203 |
| | | | | |
| Shareholders’ Equity | | | | |
| Share capital | 14,708 | - | 14,708 | 11,236 |
| Accumulated deficit | (3,832) | (7) | (3,839) | (2,868) |
| Accumulated other comprehensive income | - | - | - | - |
| Total Shareholders’ Equity | 10,876 | (7) | 10,869 | 8,368 |
| Total Liabilities and Shareholders’ Equity | 11,555 | 875 | 12,430 | 9,571 |
23. First-time adoption of IFRS and restatement (continued)
Reconciliation of the statement of financial position including equity as at December 31, 2020:
| | Previous GAAP in CAD | IFRS Adjustments | IFRS in CAD | IFRS in USD |
| Assets | | | | |
| Current assets | | | | |
| Cash | 7,095 | - | 7,095 | 5,573 |
| Other receivables | 432 | - | 432 | 340 |
| Prepaid expenses | 29 | (14) | 15 | 11 |
| Grant receivables | 2,150 | - | 2,150 | 1,689 |
| Total current assets | 9,706 | (14) | 9,692 | 7,613 |
| | | | | |
| Non-current assets | | | | |
| Property and equipment | 3,891 | 810 | 4,701 | 3,692 |
| Intangible assets | 1,089 | - | 1,089 | 856 |
| Deferred financing costs | - | - | - | - |
| Other assets | 30 | (14) | 16 | 13 |
| Total assets | 14,716 | 782 | 15,498 | 12,174 |
| | | | | |
| Liabilities | | | | |
| Current liabilities | | | | |
| Accounts payable and accrued liabilities | 888 | - | 888 | 697 |
| Current portion of lease liabilities | - | 77 | 77 | 60 |
| Total current liabilities | 888 | 77 | 965 | 757 |
| | | | | |
| Non-current liabilities | | | | |
| Lease liabilities | - | 736 | 736 | 580 |
| Long-term debt | 192 | - | 192 | 151 |
| Total liabilities | 1,080 | 813 | 1,893 | 1,488 |
| | | | | |
| Shareholders’ Equity | | | | |
| Share capital | 21,572 | - | 21,572 | 16,476 |
| Accumulated deficit | (7,936) | (31) | (7,967) | (5,921) |
| Accumulated other comprehensive income | - | - | - | 131 |
| Total Shareholders’ Equity | 13,636 | (31) | 13,605 | 10,686 |
| Total Liabilities and Shareholders’ Equity | 14,716 | 782 | 15,498 | 12,174 |
23. First-time adoption of IFRS and restatement (continued)
Reconciliation of the statement of financial position including equity as at December 31, 2021:
| | Previous GAAP in CAD | IFRS Adjustments | Restatement in CAD | IFRS in CAD | IFRS in USD |
| Assets | | | | | |
| Current assets | | | | | |
| Cash | 10,005 | - | - | 10,005 | 7,892 |
| Other receivables | 2,179 | (100) | - | 2,079 | 1,640 |
| Prepaid expenses | 84 | (44) | - | 40 | 32 |
| Grant receivables | 4,194 | - | - | 4,194 | 3,308 |
| Total current assets | 16,462 | (144) | - | 16,318 | 12,872 |
| | | | | | |
| Non-current assets | | | | | |
| Property and equipment | 12,905 | 755 | 97 | 13,757 | 10,851 |
| Intangible assets | 1,190 | - | - | 1,190 | 939 |
| Deferred financing costs | 112 | - | - | 112 | 89 |
| Other assets | 44 | (14) | - | 30 | 24 |
| Total assets | 30,713 | 597 | 97 | 31,407 | 24,775 |
| | | | | | |
| Liabilities | | | | | |
| Current liabilities | | | | | |
| Accounts payable and accrued liabilities | 3,480 | - | 97 | 3,577 | 2,821 |
| Current portion of lease liabilities | - | 81 | - | 81 | 64 |
| Current portion of the deferred lease incentive | 12 | (12) | - | - | - |
| Total current liabilities | 3,492 | 69 | 97 | 3,658 | 2,885 |
| | | | | | |
| Non-current liabilities | | | | | |
| Lease liabilities | - | 665 | - | 665 | 525 |
| Deferred lease incentive | 88 | (88) | - | - | - |
| Long-term debt | 599 | - | - | 599 | 473 |
| Total liabilities | 4,179 | 646 | 97 | 4,922 | 3,883 |
| | | | | | |
| Shareholders’ Equity | | | | | |
| Share capital | 40,193 | - | - | 40,193 | 31,236 |
| Accumulated deficit | (13,659) | (49) | - | (13,708) | (10,528) |
| Accumulated other comprehensive income | - | - | - | - | 184 |
| Total Shareholders’ Equity | 26,534 | (49) | - | 26,485 | 20,892 |
| Total Liabilities and Shareholders’ Equity | 30,713 | 597 | 97 | 31,407 | 24,775 |
23. First-time adoption of IFRS and restatement (continued)
Reconciliation of total comprehensive income for the year ended December 31, 2020:
| | Previous GAAP in CAD | IFRS Adjustments | IFRS in CAD | IFRS in USD |
| Expenses | | | | |
| General and administrative | 890 | (162) | 728 | 459 |
| Research and development | 3,152 | - | 3,152 | 2,408 |
| Depreciation of property and equipment | 74 | 93 | 167 | 124 |
| Foreign exchange gain (loss) | - | - | - | - |
| Loss on sale of property and equipment | - | - | - | - |
| Total expenses | 4,116 | (69) | 4,047 | 2,991 |
| | | | | |
| Net financial expenses | (37) | 94 | 57 | 43 |
| | | | | |
| Loss before income tax | 4,079 | 25 | 4,104 | 3,034 |
| | | | | |
| Income tax expense (recovery) | - | - | - | - |
| | | | | |
| Net loss | 4,079 | 25 | 4,104 | 3,034 |
| | | | | |
| Other comprehensive loss | | | | |
| Items that may be reclassified subsequently to income or loss | | | | |
| Foreign currency translation differences | - | - | - | 131 |
| Comprehensive loss | 4,079 | 25 | 4,104 | 2,903 |
23. First-time adoption of IFRS and restatement (continued)
Reconciliation of total comprehensive income for the year ended December 31, 2021:
| | Previous GAAP in CAD | IFRS Adjustments | IFRS in CAD | IFRS in USD |
| Expenses | | | | |
| General and administrative | 1,576 | (176) | 1,400 | 1,245 |
| Research and development | 3,903 | - | 3,903 | 3,010 |
| Depreciation of property and equipment | 166 | 107 | 273 | 218 |
| Foreign exchange gain (loss) | - | - | - | - |
| Loss on sale of property and equipment | 7 | - | 7 | 6 |
| Total expenses | 5,652 | (69) | 5,583 | 4,479 |
| Net financial expenses | 5 | 86 | 91 | 73 |
| Loss before income tax | 5,657 | 17 | 5,674 | 4,552 |
| Income tax expense (recovery) | - | - | - | - |
| Net loss | 5,657 | 17 | 5,674 | 4,552 |
| Other comprehensive loss | | | | |
| Foreign currency translation differences | - | - | - | 53 |
| Comprehensive loss | 5,657 | 17 | 5,674 | 4,499 |
Notes to the reconciliation of equity as at January 1, 2020, December 31, 2020 and December 31, 2021 and total comprehensive loss for the years ended December 31, 2021 and December 31, 2020
a. Leases
Under Canadian Accounting Standards for Private Enterprises (ASPE), a lease is classified as a finance lease or an operating lease. Operating lease payments are recognized as an operating expense in the statement of loss on a straight-line basis over the lease term. Under IFRS, as explained in Note 4, a lessee applies a single recognition and measurement approach for all leases, except for short-term leases and leases of low-value assets and recognizes lease liabilities to make lease payments and right-of-use assets representing the right to use the underlying assets.
At the date of transition to IFRS, the Company applied the transitional provision and measured lease liabilities at the present value of the remaining lease payments, discounted using the lessee’s incremental borrowing rate at the date of transition to IFRS. Right-of-use assets were measured at the amount equal to the lease liabilities adjusted by the amount of any prepaid or accrued lease payments. As a result, the Company recognized an increase $589 (December 31, 2020 - $640) (January 1, 2020 - $680) of lease liabilities included under lease liabilities and $595 (December 31, 2020 - $636) (January 1, 2020 - $695) of right-of-use assets. The difference between lease liabilities and right-of-use assets has been recognized in accumulated deficit.
b. Statement of cash flows
Under ASPE, a lease is classified as a finance lease or an operating lease. Cash flows arising from operating lease payments are classified as operating activities. Under IFRS, a lessee generally applies a single recognition and measurement approach for all leases and recognizes lease liabilities. Cash flows arising from payments of principal portion of lease liabilities are classified as financing activities. Therefore, cash outflows from operating activities decreased by $132 (December 31, 2020 - $121) and cash outflows from financing activities increased by the same amount for the years ended December 31, 2021 and 2020.
23. First-time adoption of IFRS and restatement (continued)
c. Restatement
After the ASPE financial statement were finalized, the Company has discovered that it should have recognized $76 of holdback payables for some leasehold improvements done through the year ended December 31, 2021.
24. Subsequent events
On February 7, 2022, the Company issued 33,330 Class A common shares for a cash consideration of $158 and repurchased 33,330 Class B common shares for a cash consideration of $1. The Company then cancelled these Class B Common shares.
On February 11, 2022, the Company issued 41,665 Class A common shares for a cash consideration of $197 and repurchased 41,665 Class B common shares for a cash consideration of $1. The Company then cancelled these Class B Common shares.
On February 15, 2022, the Company granted 75,000 options to purchase Class A common Shares to an officer at an exercise price of $0 per share with an expiry date of February 14, 2032. The fair value of the options was estimated at $5 per share option at the grant date for a total of $353 using a Black-Scholes option pricing model. 25,000 of those options were vested and exercised on December 20, 2022 for a cash consideration of $0.012 and 25,000 Class B common shares were repurchased and cancelled for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On March 25, 2022, an amount of $427 has been drawn from the repayable contribution. The amount recognized as a grant due to the below-market interest rate of $202 has been recognized as a reduction to the carrying value of property and equipment.
On April 8, 2022, on May 16, 2022, on July 28, 2022, on August 11, 2022 and on November 15, 2022, amounts of respectively $2,193, $958, $530, $257, $1,094 have been drawn from the Investissement Quebec loan.
On April 27, 2022, the Company issued 75,000 Class A common shares for a cash consideration of $351 and repurchased 75,000 Class B common shares for a cash consideration of $1. The Company then cancelled these Class B common shares.
On May 2, 2022, the Company issued 41,670 Class A common shares for a cash consideration of $194 and repurchased 41,670 Class B common shares for a cash consideration of $1. The Company then cancelled these Class B common shares.
On May 30, 2022, the Company issued 140,375 Class A common shares for a cash consideration of $887 and repurchased 140,375 Class B common shares for a cash consideration of $2. The Company then proceeded with the cancellation of these same Class B common shares.
On August 3, 2022, the Company issued 62,500 Class A common shares for a cash consideration of $389 and repurchased 62,500 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On August 3, 2022, the Company issued 25,000 Class A common shares for a cash consideration of $156 and repurchased 25,000 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On August 3, 2022, the Company issued 16,625 Class A common shares for a cash consideration of $103 and repurchased 16,625 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
24. Subsequent events (continued)
On October 14, 2022, the Company received an amendment from Economic Development Agency of Canada to push the completion of the project from March 31, 2023 to June 30, 2024. Therefore, the payment schedule was altered and the fair value of the loan was recalculated using an effective interest rate of 10% to reflect this change. The impact of the change is a decrease of $85 of the fair value of the loan.
On November 22, 2022, the Company granted 15,000 options to purchase Class A common Shares to an employee at an exercise price of $6 per share with an expiry date of November 21, 2032. The fair value of the options was estimated at $3 per share option at the grant date for a total of $43 using a Black-Scholes option pricing model.
On November 22, 2022, the Company granted 525,000 options to purchase Class A common Shares to directors at an exercise price of $0.016 per share all with an expiry date of November 21, 2032. The fair value of the options was estimated at $6 per share option at the grant date for a total of $3,130 using a Black-Scholes option pricing model.
On November 22, 2022, the Company granted 105,000 options to purchase Class A common Shares to a director at an exercise price of $6 per share with an expiry date of November 21, 2032. The fair value of the options was estimated at $3 per share option at the grant date for a total of $299 using a Black-Scholes option pricing model.
On November 23, 2022, the Company issued 6,250 Class A common shares for a cash consideration of $37 and repurchased 6,250 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On December 13, 2022, the Company, and the medical entity where clinical trials will take place, have entered into an agreement whereby the Company would receive a payment of $2.3 million upon the commencement of its clinical trials. The receipt of these funds is contingent on the Phase I trials commencing.
On December 14, 2022, the Company issued 162,500 Class A common shares for a cash consideration of $959 and repurchased 162,500 Class B common shares for a cash consideration of $2. The Company then proceeded with the cancellation of these same Class B common shares.
On December 20, 2022, the Company issued 187,500 Class A common shares for a cash consideration of $1,101 and repurchased 187,500 Class B common shares for a cash consideration of $3. The Company then proceeded with the cancellation of these same Class B common shares.
On December 31, 2022, the Company issued 37,500 Class A common shares for a cash consideration of $221 and repurchased 37,500 Class B common shares for a cash consideration of $1. The Company then proceeded with the cancellation of these same Class B common shares.
On January 19, 2023, the Company amended its Articles (i) converting all outstanding Class A Common Shares and Class B Common Shares into shares of a single class called common shares (“Common Shares”) and (ii) executing a 5:1 stock split, subdividing all its issued and outstanding Common Shares into five (5) such Common Shares.
After the year end, the Company had issued capital commitments totaling $8,523 to various suppliers in connection with its operation and the establishment of a pilot plant of which $4,972 has already been paid.
EXHIBIT LIST
Exhibit 2A: Certificate of Constitution and Articles of Amendment
Exhibit 2B: Bylaws
Exhibit 3: Anti-Dilution Agreement
Exhibit 4. Subscription Agreement
Exhibit 8. Escrow Agreement
Exhibit 10: Power of Attorney
Exhibit 11. Written Expert Consent Letter of Accountant
Exhibit 12. Legal Opinion Letter
Signature Page
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this Offering Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Montréal, Québec, Canada; London, United Kingdom; and Val-Morin, Québec, Canada on February 7, 2023.
(Exact name of the Issuer as specified in its Charter)
Starpax Biopharma Inc.
2500-1000 boul. René-Lévesque West Montréal, Québec, Canada H3B 5C9
(514) 427-3004
By:
s/ Michael Gareau
Chairman of the Board of Directors, CEO of Starpax Biopharma Inc. (Date): February 7, 2023
Location Signed: Montréal, Québec, Canada
This Offering Statement has been signed by the following Officers in the capacities and on the dates indicated.
s/ Michael Gareau
Director of StarpaxBiopharma Inc.
(Date): Febraury 7, 2023
Location Signed: Montréal, Québec, Canada
s/
Pierre Dozois
Corporate Secretary of Starpax Biopharma Inc.
(Date): February 7, 2023
Location Signed: Montréal, Québec, Canada
s/ Jean-François Pruneau
Chief Financial Officer of Starpax Biopharma Inc.
(Date): February 7, 2023
Location Signed: Montréal, Québec, Canada
This Offering Statement has been signed by the following Directors in the capacities and on the dates indicated.
s/Michael Gareau
Director of Starpax Biopharma Inc.
(Date): February 7, 2023
Location Signed: Montréal, Québec, Canada
s/ André Monette
Director of Starpax Biopharma Inc.
(Date): February 7, 2023
Location Signed: Montréal, Québec, Canada
s/ John Helou
Director of Starpax Biopharma Inc.
(Date): February 7, 2023
Location Signed: Montréal, Québec, Canada
s/ Lisa Matar
Director of Starpax Biopharma Inc.
(Date): February 7, 2023
Location Signed: Montréal, Québec, Canada
s/Berthe Latreille
Director of Starpax Biopharma Inc.
(Date): February 7, 2023
Location Signed: Montréal, Québec, Canada
s/ Dr. Jacques Jolivet
Director of Starpax Biopharma Inc.
(Date): February 7, 2023
Location Signed: Montréal, Québec, Canada
s/ Pierre Dozois
Director of Starpax Biopharma Inc.
(Date): February 7, 2023
Location Signed: Montréal, Québec, Canada









