
Izokibep Psoriatic Arthritis Global Phase 2b/3 Topline Results March 11, 2024 Exhibit 99.3

2 This presentation contains statements that are not of historical facts, considered forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements include, but are not limited to, statements about the therapeutic potential of our product candidate izokibep, including with respect to the potential for longer-term treatment with izokibep to provide for continued improvement over time; the long-term safety profile of izokibep; the timing and availability of data from clinical trials; the potential market size and size of the potential patient populations for certain indications we are pursuing, and our product candidates. These forward-looking statements are based on ACELYRIN’s current plans, objectives and projections, and are inherently subject to risks and uncertainties that may cause our actual results to differ materially and adversely from those anticipated in such forward-looking statements. Such risks and uncertainties include, without limitation, those associated with the successful completion of development and regulatory activities with respect to our product candidates; the timing and results of our clinical trials, including the potential that future results could differ adversely from prior results, where applicable; our ability to timely secure adequate supply of our product candidates; sufficient funding; legal proceedings and the outcome thereof; competitive risks; market volatility; macroeconomic conditions and other risks and uncertainties affecting ACELYRIN including those described from time to time under the heading “Risk Factors” and elsewhere in our current and future periodic and other reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Quarterly Report on Form 10-Q for the quarter ended September 30, 2023. These filings are available on the SEC’s website www.sec.gov. In addition, new risks may occur at any time, and we anticipate that subsequent developments could cause our views to change. Forward- looking statements herein are made of the date of this presentation, and ACELYRIN undertakes no duty to update them in the event of new information, future developments, or otherwise, except as required under applicable law. Any reader of this presentation is cautioned not to place undue reliance on these forward-looking statements. Izokibep is currently under clinical investigation, and no representation is made as of the safety or efficacy of our product candidates. In addition to our own internal research, this presentation contains information related to or based on studies and other data obtained from third-party sources. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness thereof. In addition, market data (e.g., market size) involves a number of projections, assumptions and estimates of our future performance and future performance of the markets in which we operate. There can be no guarantee of the accuracy or reliability thereof, as they are necessarily subject to a high degree of uncertainty and risk. The information in this presentation is as of the date of this presentation, and is subject to change without notice. TRADEMARKS: This presentation contains trademarks, service marks, trade names and copyrights of ACELYRIN and other companies which are the property of their respective owners. Forward Looking Statements And Disclaimer
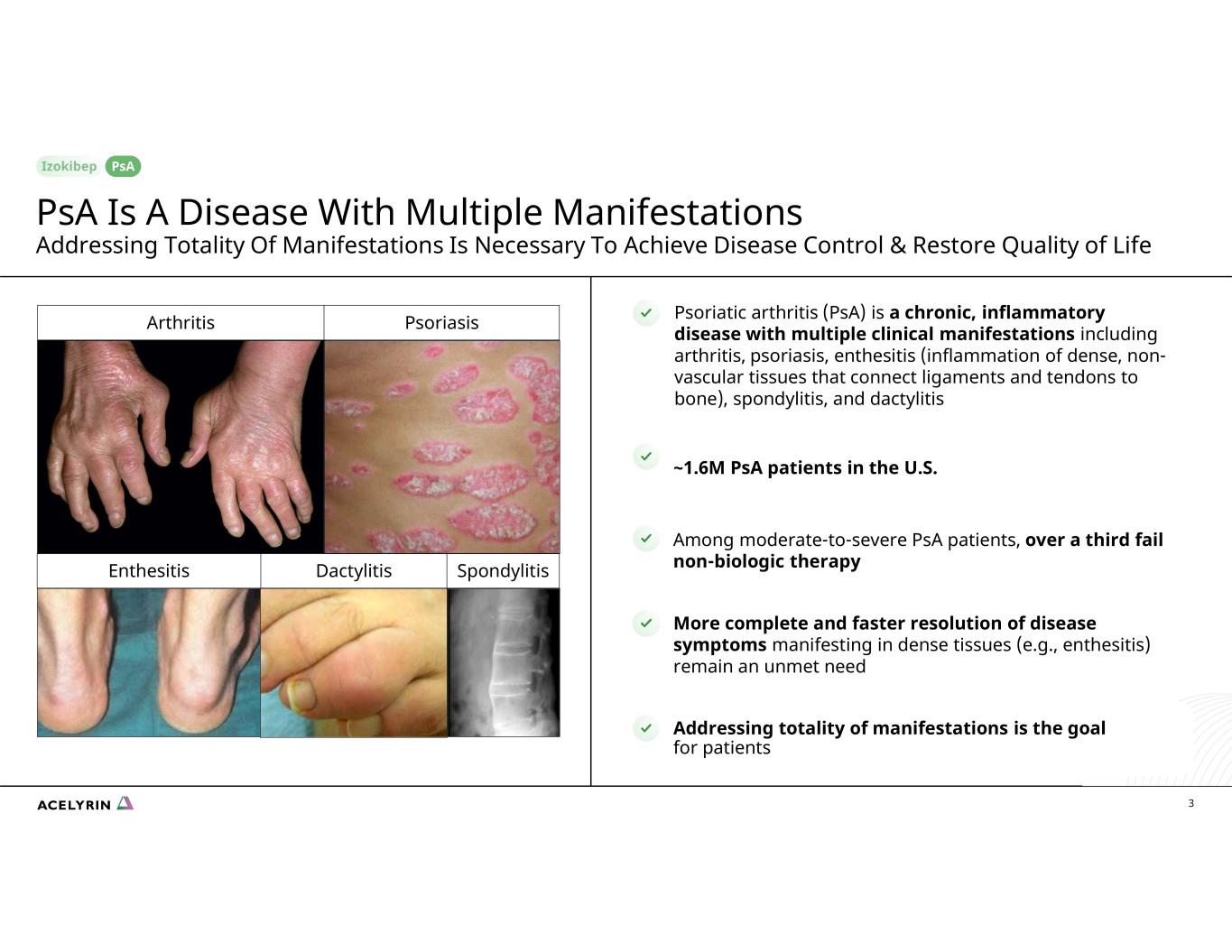
DactylitisEnthesitis Spondylitis Psoriatic arthritis (PsA) is a chronic, inflammatory disease with multiple clinical manifestations including arthritis, psoriasis, enthesitis (inflammation of dense, non- vascular tissues that connect ligaments and tendons to bone), spondylitis, and dactylitis ~1.6M PsA patients in the U.S. Among moderate-to-severe PsA patients, over a third fail non-biologic therapy More complete and faster resolution of disease symptoms manifesting in dense tissues (e.g., enthesitis) remain an unmet need 3 Addressing totality of manifestations is the goal for patients Arthritis Psoriasis PsA Is A Disease With Multiple Manifestations Addressing Totality Of Manifestations Is Necessary To Achieve Disease Control & Restore Quality of Life PsA
4 PsA • Izokibep IL-17A inhibition alone achieves rapid improvement in resolution across manifestations of disease • Pre-specified analyses support the potential for differentiation in enthesitis resolution • Higher clinical responses than reported by the IL-17A agents • Results comparable to those reported by the IL-17A&F agents but without the associated safety liabilities • Study met primary endpoint of ACR50 at 16 weeks with high statistical significance • Significant, multidomain responses achieved for the high hurdles of ACR70, PASI90, PASI100 and MDA • Improvement in magnitude of responses relative to Phase 2 notable given higher burden of disease in Phase 2b/3 • Expected to be the first of two registrational trials in psoriatic arthritis; 160mg Q2W appears to be optimal dose • Robust clinical responses in high hurdle composite endpoints (ACR50/PASI100 and MDA) • No safety limitation to long term treatment seen to date • Longer duration of therapy has previously demonstrated the potential for even further improvements over time Positive topline results Differentiated profile Deep and durable responses Positive Results For Izokibep Global Phase 2b/3 In PsA
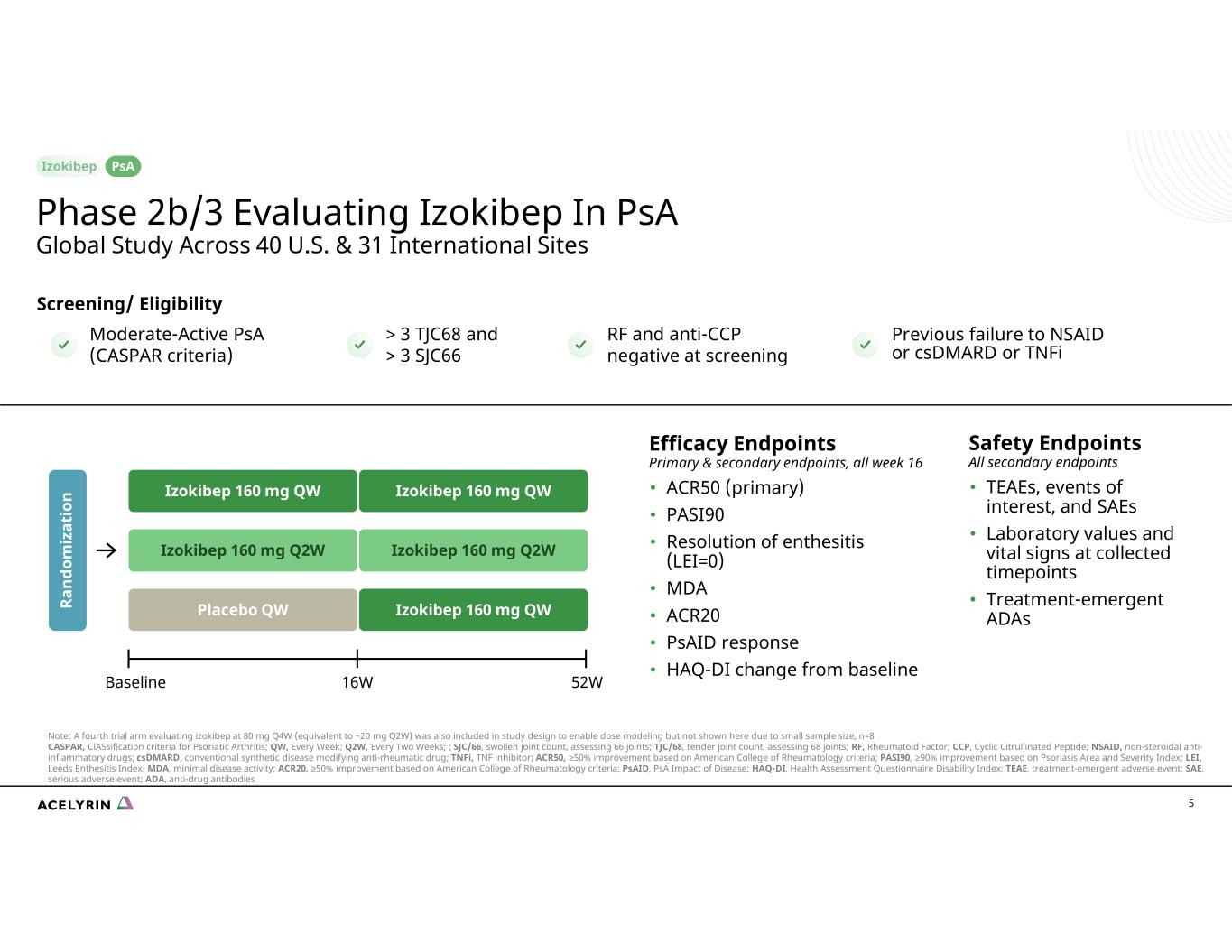
5 Efficacy Endpoints Primary & secondary endpoints, all week 16 • ACR50 (primary) • PASI90 • Resolution of enthesitis (LEI=0) • MDA • ACR20 • PsAID response • HAQ-DI change from baseline Moderate-Active PsA (CASPAR criteria) Screening/ Eligibility > 3 TJC68 and > 3 SJC66 RF and anti-CCP negative at screening Previous failure to NSAID or csDMARD or TNFi 16WBaseline 52W Izokibep 160 mg QW Ra nd om iz at io n Izokibep 160 mg Q2W Placebo QW Safety Endpoints All secondary endpoints • TEAEs, events of interest, and SAEs • Laboratory values and vital signs at collected timepoints • Treatment-emergent ADAs Izokibep 160 mg QW Izokibep 160 mg Q2W Izokibep 160 mg QW Phase 2b/3 Evaluating Izokibep In PsA Global Study Across 40 U.S. & 31 International Sites PsA Note: A fourth trial arm evaluating izokibep at 80 mg Q4W (equivalent to ~20 mg Q2W) was also included in study design to enable dose modeling but not shown here due to small sample size, n=8 CASPAR, ClASsification criteria for Psoriatic Arthritis; QW, Every Week; Q2W, Every Two Weeks; ; SJC/66, swollen joint count, assessing 66 joints; TJC/68, tender joint count, assessing 68 joints; RF, Rheumatoid Factor; CCP, Cyclic Citrullinated Peptide; NSAID, non-steroidal anti- inflammatory drugs; csDMARD, conventional synthetic disease modifying anti-rheumatic drug; TNFi, TNF inhibitor; ACR50, ≥50% improvement based on American College of Rheumatology criteria; PASI90, ≥90% improvement based on Psoriasis Area and Severity Index; LEI, Leeds Enthesitis Index; MDA, minimal disease activity; ACR20, ≥50% improvement based on American College of Rheumatology criteria; PsAID, PsA Impact of Disease; HAQ-DI, Health Assessment Questionnaire Disability Index; TEAE, treatment-emergent adverse event; SAE, serious adverse event; ADA, anti-drug antibodies
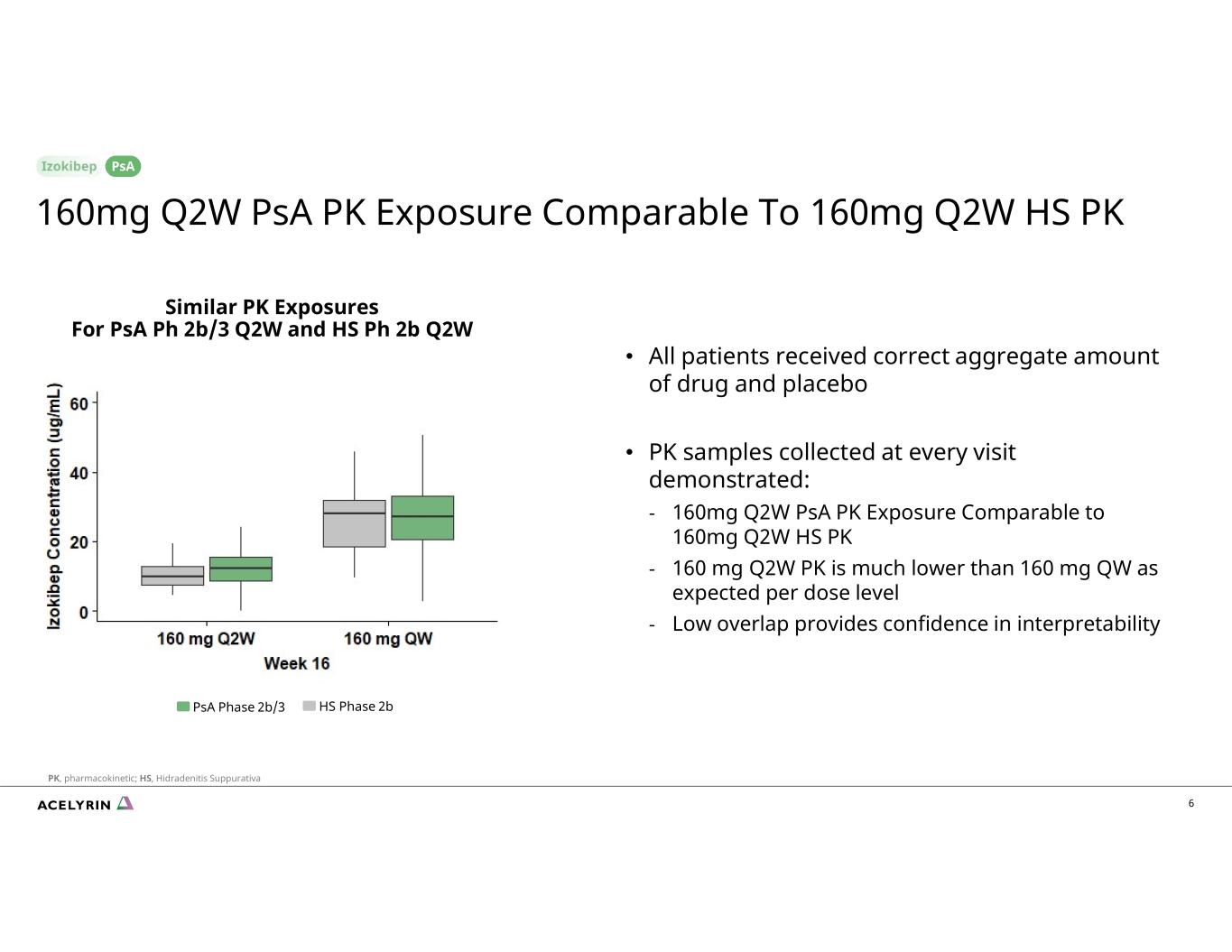
6 Similar PK Exposures For PsA Ph 2b/3 Q2W and HS Ph 2b Q2W • All patients received correct aggregate amount of drug and placebo • PK samples collected at every visit demonstrated: - 160mg Q2W PsA PK Exposure Comparable to 160mg Q2W HS PK - 160 mg Q2W PK is much lower than 160 mg QW as expected per dose level - Low overlap provides confidence in interpretability 160mg Q2W PsA PK Exposure Comparable To 160mg Q2W HS PK PsA PK, pharmacokinetic; HS, Hidradenitis Suppurativa PsA Phase 2b/3 HS Phase 2b
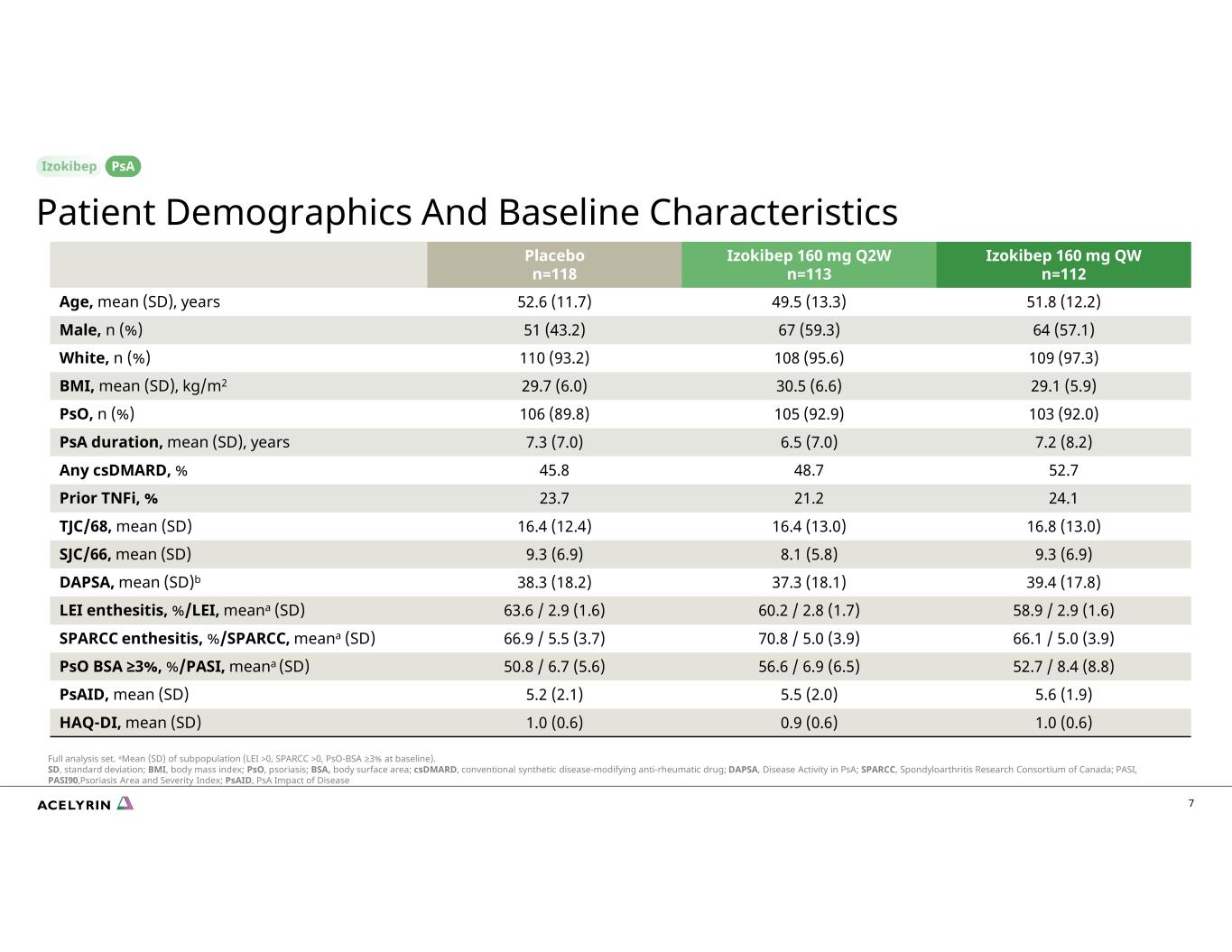
7 Izokibep 160 mg QW n=112 Izokibep 160 mg Q2W n=113 Placebo n=118 51.8 (12.2)49.5 (13.3)52.6 (11.7)Age, mean (SD), years 64 (57.1)67 (59.3)51 (43.2)Male, n (%) 109 (97.3)108 (95.6)110 (93.2)White, n (%) 29.1 (5.9)30.5 (6.6)29.7 (6.0)BMI, mean (SD), kg/m2 103 (92.0)105 (92.9)106 (89.8)PsO, n (%) 7.2 (8.2) 6.5 (7.0) 7.3 (7.0) PsA duration, mean (SD), years 52.748.745.8Any csDMARD, % 24.121.223.7Prior TNFi, % 16.8 (13.0)16.4 (13.0)16.4 (12.4)TJC/68, mean (SD) 9.3 (6.9)8.1 (5.8)9.3 (6.9)SJC/66, mean (SD) 39.4 (17.8)37.3 (18.1)38.3 (18.2)DAPSA, mean (SD)b 58.9 / 2.9 (1.6)60.2 / 2.8 (1.7)63.6 / 2.9 (1.6)LEI enthesitis, %/LEI, meana (SD) 66.1 / 5.0 (3.9)70.8 / 5.0 (3.9)66.9 / 5.5 (3.7)SPARCC enthesitis, %/SPARCC, meana (SD) 52.7 / 8.4 (8.8)56.6 / 6.9 (6.5)50.8 / 6.7 (5.6)PsO BSA ≥3%, %/PASI, meana (SD) 5.6 (1.9)5.5 (2.0)5.2 (2.1)PsAID, mean (SD) 1.0 (0.6)0.9 (0.6)1.0 (0.6)HAQ-DI, mean (SD) Patient Demographics And Baseline Characteristics PsA Full analysis set. aMean (SD) of subpopulation (LEI >0, SPARCC >0, PsO-BSA ≥3% at baseline). SD, standard deviation; BMI, body mass index; PsO, psoriasis; BSA, body surface area; csDMARD, conventional synthetic disease-modifying anti-rheumatic drug; DAPSA, Disease Activity in PsA; SPARCC, Spondyloarthritis Research Consortium of Canada; PASI, PASI90,Psoriasis Area and Severity Index; PsAID, PsA Impact of Disease
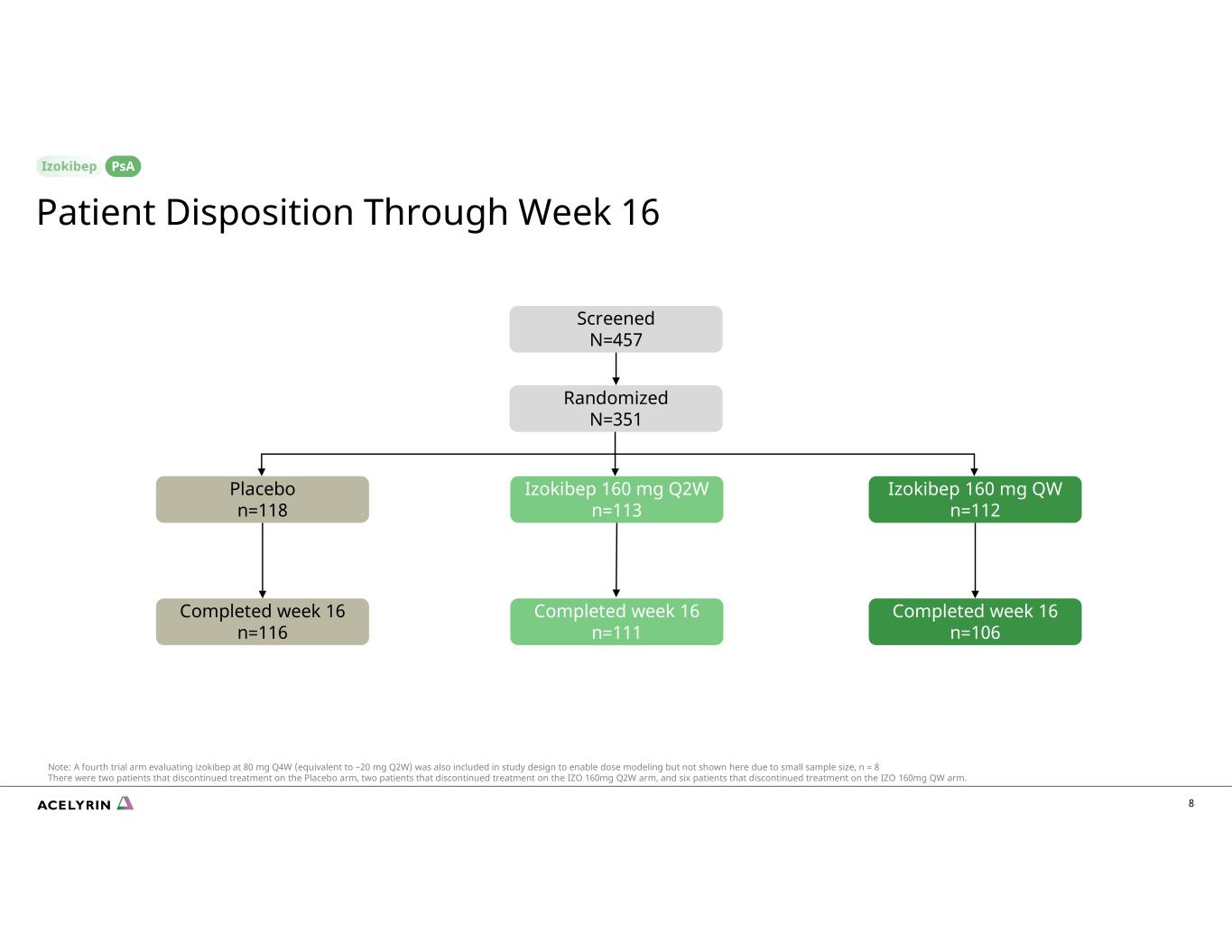
8 Screened N=457 Randomized N=351 Placebo n=118 Izokibep 160 mg Q2W n=113 Completed week 16 n=116 Completed week 16 n=106 PsA Patient Disposition Through Week 16 Izokibep 160 mg QW n=112 Completed week 16 n=111 Note: A fourth trial arm evaluating izokibep at 80 mg Q4W (equivalent to ~20 mg Q2W) was also included in study design to enable dose modeling but not shown here due to small sample size, n = 8 There were two patients that discontinued treatment on the Placebo arm, two patients that discontinued treatment on the IZO 160mg Q2W arm, and six patients that discontinued treatment on the IZO 160mg QW arm.

Positive Phase 2b/3 Topline Results 9
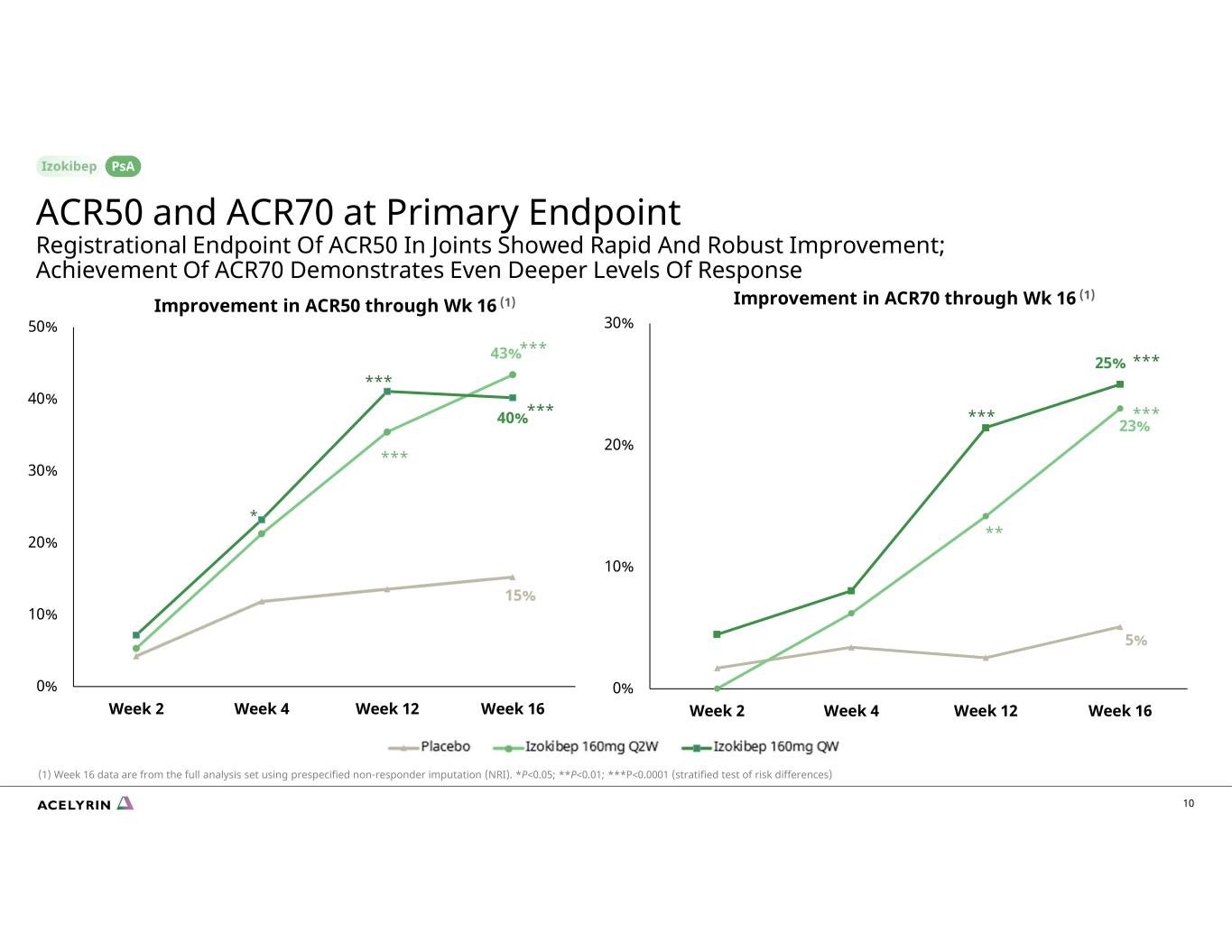
5% 23% 25% 0% 10% 20% 30% Week 2 Week 4 Week 12 Week 16 15% 43% 40% 0% 10% 20% 30% 40% 50% Week 2 Week 4 Week 12 Week 16 Improvement in ACR50 through Wk 16 (1) Improvement in ACR70 through Wk 16 (1) * *** *** *** *** *** *** *** ** (1) Week 16 data are from the full analysis set using prespecified non-responder imputation (NRI). *P<0.05; **P<0.01; ***P<0.0001 (stratified test of risk differences) 10 ACR50 and ACR70 at Primary Endpoint Registrational Endpoint Of ACR50 In Joints Showed Rapid And Robust Improvement; Achievement Of ACR70 Demonstrates Even Deeper Levels Of Response PsA
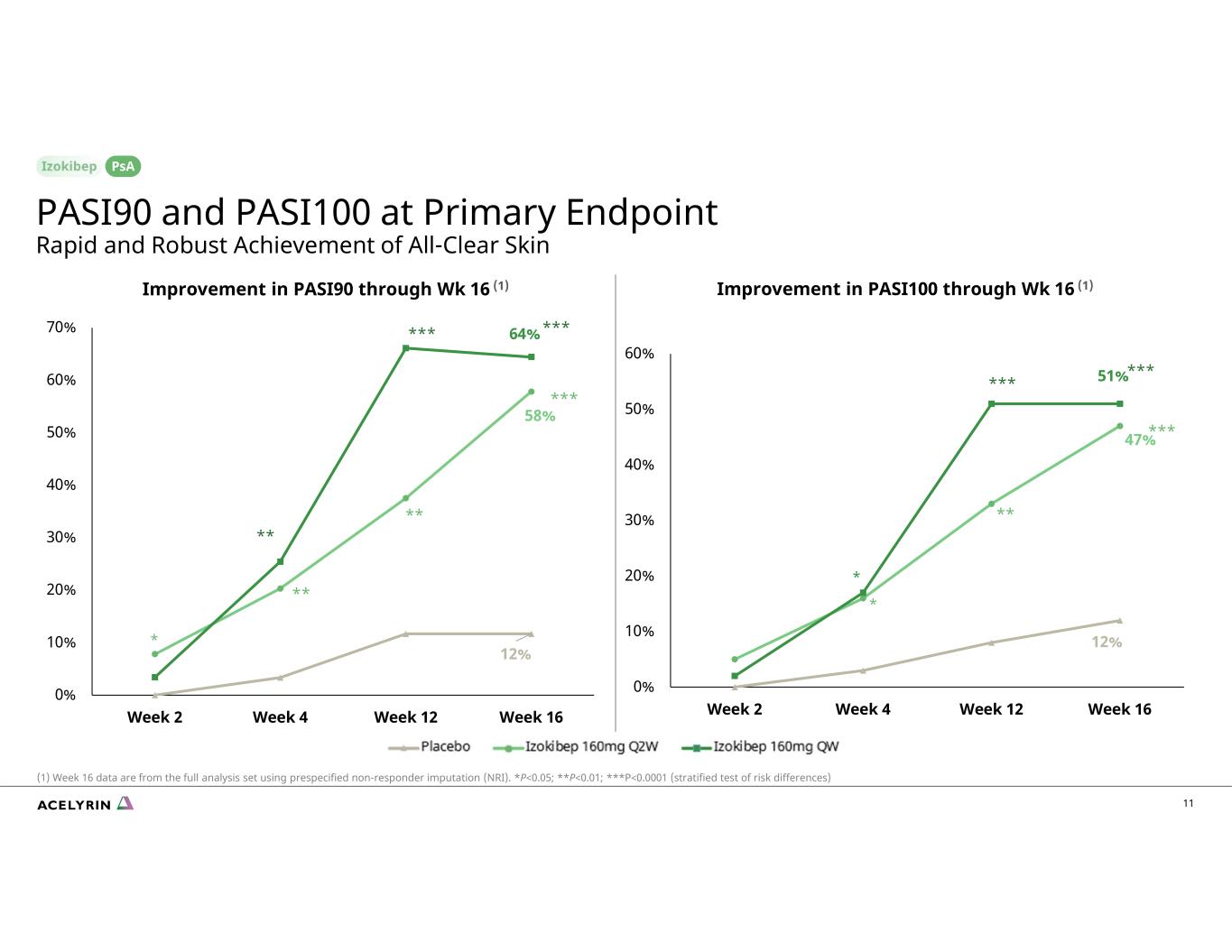
12% 47% 51% 0% 10% 20% 30% 40% 50% 60% Week 2 Week 4 Week 12 Week 16 12% 58% 64% 0% 10% 20% 30% 40% 50% 60% 70% Week 2 Week 4 Week 12 Week 16 * * * *** *** *** ** ** ** ** *** *** *** Improvement in PASI90 through Wk 16 (1) Improvement in PASI100 through Wk 16 (1) 11 PsA PASI90 and PASI100 at Primary Endpoint Rapid and Robust Achievement of All-Clear Skin (1) Week 16 data are from the full analysis set using prespecified non-responder imputation (NRI). *P<0.05; **P<0.01; ***P<0.0001 (stratified test of risk differences)
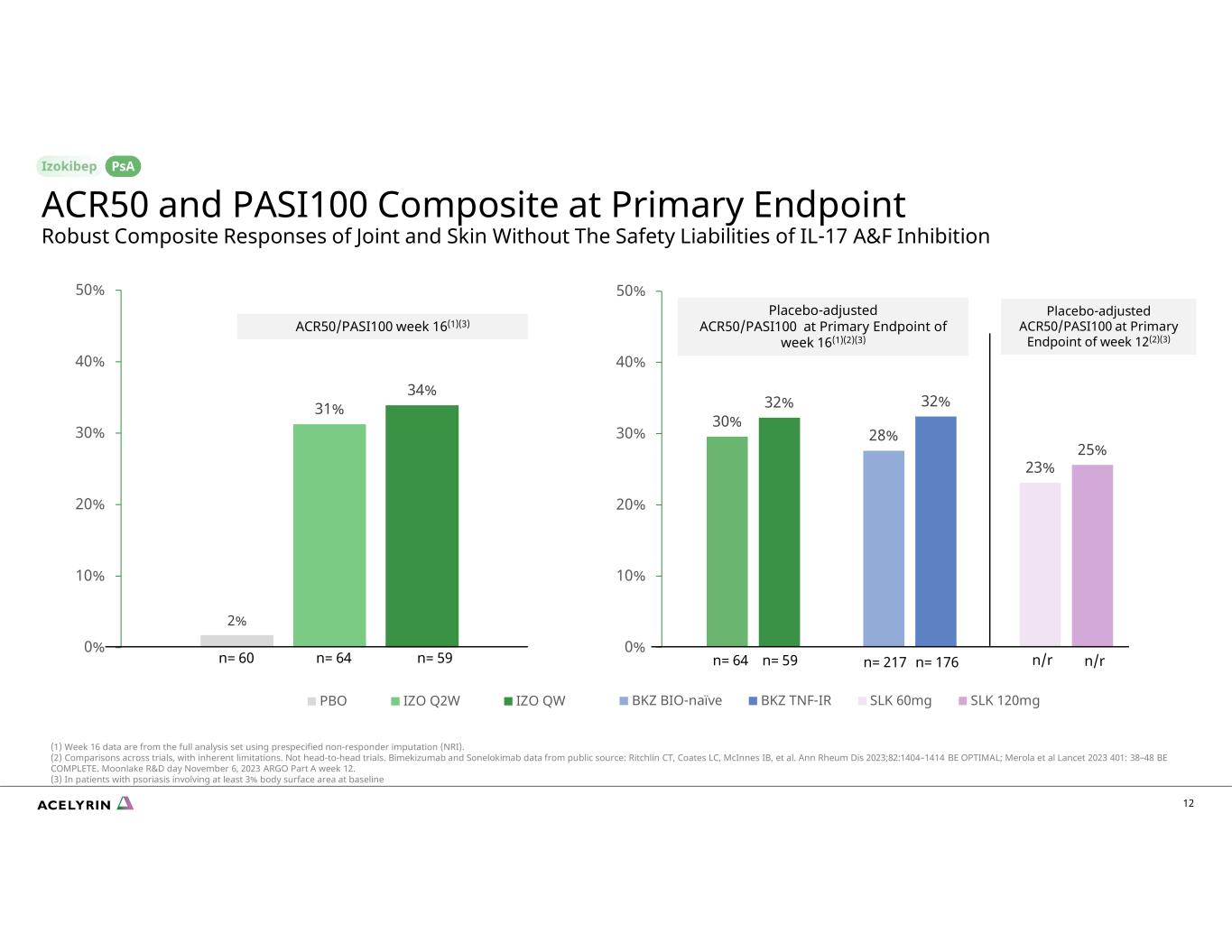
30% 32% 28% 32% 23% 25% 0% 10% 20% 30% 40% 50% BKZ BIO-naïve BKZ TNF-IR SLK 60mg SLK 120mg 12 ACR50 and PASI100 Composite at Primary Endpoint Robust Composite Responses of Joint and Skin Without The Safety Liabilities of IL-17 A&F Inhibition PsA 2% 31% 34% 0% 10% 20% 30% 40% 50% PBO IZO Q2W IZO QW Placebo-adjusted ACR50/PASI100 at Primary Endpoint of week 16(1)(2)(3) ACR50/PASI100 week 16(1)(3) n= 217 n= 176n= 60 n= 64 n= 59 n= 64 n= 59 (1) Week 16 data are from the full analysis set using prespecified non-responder imputation (NRI). (2) Comparisons across trials, with inherent limitations. Not head-to-head trials. Bimekizumab and Sonelokimab data from public source: Ritchlin CT, Coates LC, McInnes IB, et al. Ann Rheum Dis 2023;82:1404–1414 BE OPTIMAL; Merola et al Lancet 2023 401: 38–48 BE COMPLETE. Moonlake R&D day November 6, 2023 ARGO Part A week 12. (3) In patients with psoriasis involving at least 3% body surface area at baseline n/r n/r Placebo-adjusted ACR50/PASI100 at Primary Endpoint of week 12(2)(3)
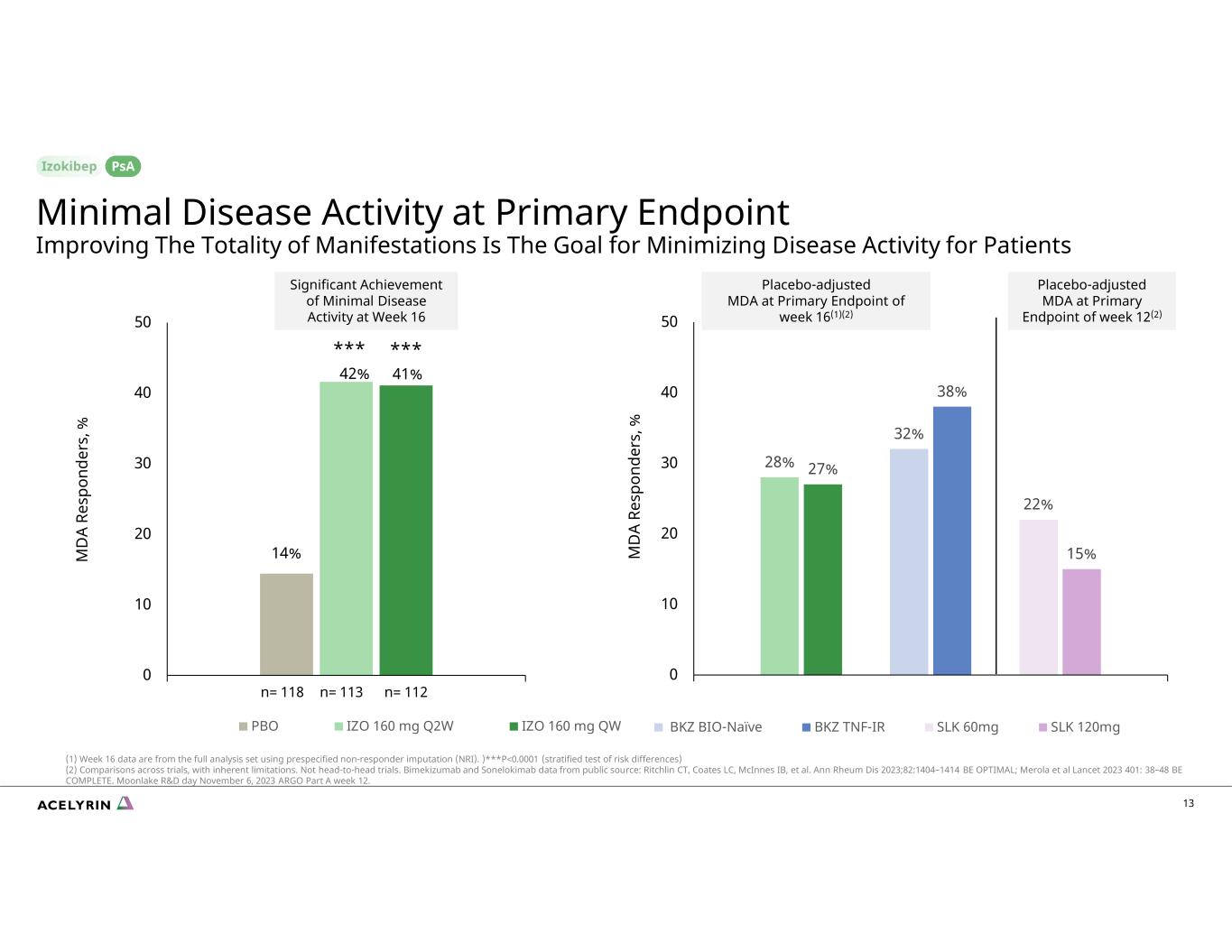
14% 42% 41% 0 10 20 30 40 50 Week 16 M D A Re sp on de rs , % PBO IZO 160 mg Q2W IZO 160 mg QW 13 28% 27% 32% 38% 22% 15% 0 10 20 30 40 50 Week 16 M D A Re sp on de rs , % BKZ BIO-Naïve BKZ TNF-IR SLK 60mg SLK 120mg *** *** Significant Achievement of Minimal Disease Activity at Week 16 Minimal Disease Activity at Primary Endpoint Improving The Totality of Manifestations Is The Goal for Minimizing Disease Activity for Patients PsA n= 118 n= 113 n= 112 Placebo-adjusted MDA at Primary Endpoint of week 12(2) Placebo-adjusted MDA at Primary Endpoint of week 16(1)(2) (1) Week 16 data are from the full analysis set using prespecified non-responder imputation (NRI). )***P<0.0001 (stratified test of risk differences) (2) Comparisons across trials, with inherent limitations. Not head-to-head trials. Bimekizumab and Sonelokimab data from public source: Ritchlin CT, Coates LC, McInnes IB, et al. Ann Rheum Dis 2023;82:1404–1414 BE OPTIMAL; Merola et al Lancet 2023 401: 38–48 BE COMPLETE. Moonlake R&D day November 6, 2023 ARGO Part A week 12.
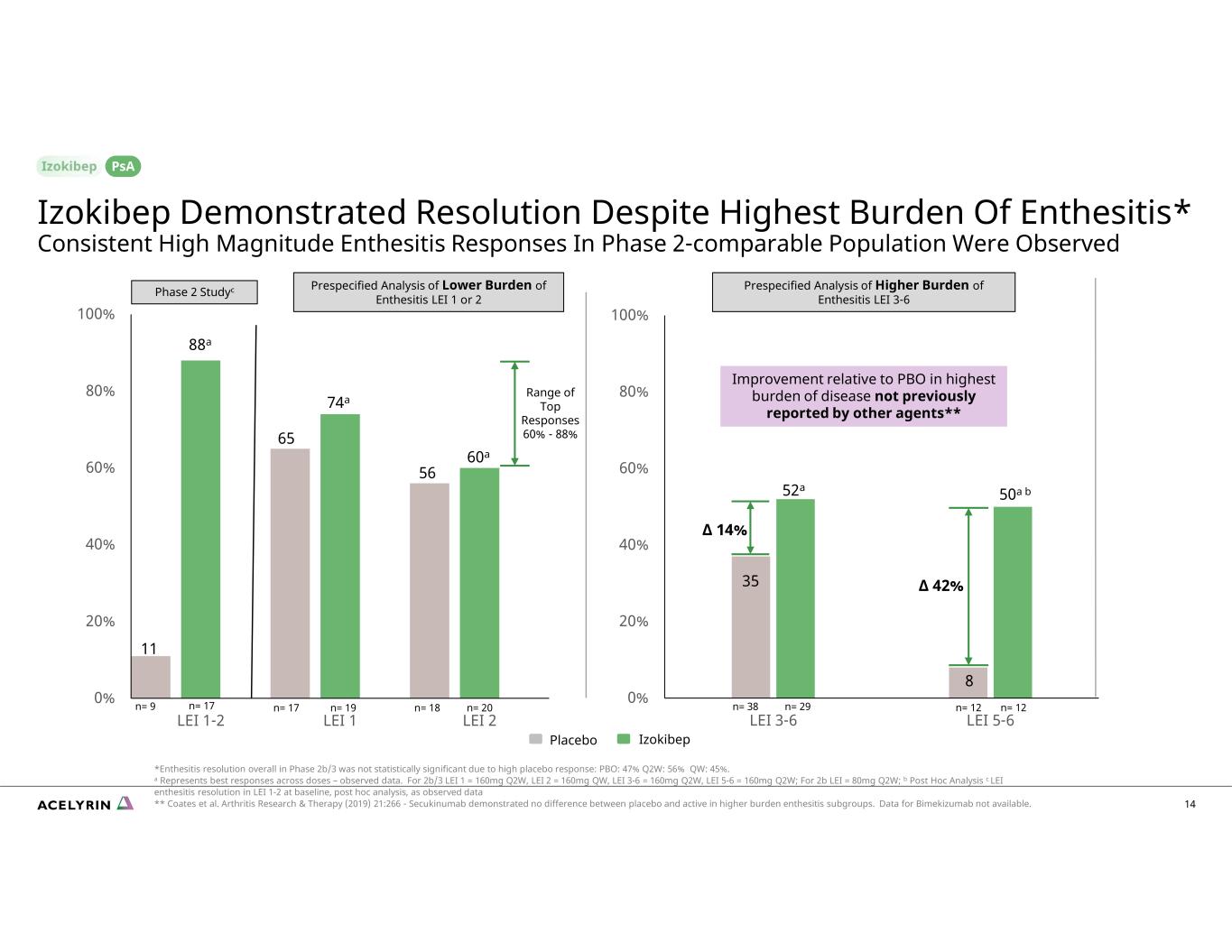
0% 20% 40% 60% 80% 100% LEI 3-6 LEI 5-6 0% 20% 40% 60% 80% 100% LEI 1-2 LEI 1 LEI 2 Range of Top Responses 60% - 88% 35 88a 11 65 74a 56 60a 52a ∆ 14% IzokibepPlacebo n= 12n= 12 8 50a b n= 17 n= 19 n= 18 n= 20 n= 38 n= 29n= 9 n= 17 ∆ 42% Improvement relative to PBO in highest burden of disease not previously reported by other agents** 14 Izokibep Demonstrated Resolution Despite Highest Burden Of Enthesitis* Consistent High Magnitude Enthesitis Responses In Phase 2-comparable Population Were Observed *Enthesitis resolution overall in Phase 2b/3 was not statistically significant due to high placebo response: PBO: 47% Q2W: 56% QW: 45%. a Represents best responses across doses – observed data. For 2b/3 LEI 1 = 160mg Q2W, LEI 2 = 160mg QW, LEI 3-6 = 160mg Q2W, LEI 5-6 = 160mg Q2W; For 2b LEI = 80mg Q2W; b Post Hoc Analysis c LEI enthesitis resolution in LEI 1-2 at baseline, post hoc analysis, as observed data ** Coates et al. Arthritis Research & Therapy (2019) 21:266 - Secukinumab demonstrated no difference between placebo and active in higher burden enthesitis subgroups. Data for Bimekizumab not available. PsA Prespecified Analysis of Higher Burden of Enthesitis LEI 3-6Phase 2 Studyc Prespecified Analysis of Lower Burden of Enthesitis LEI 1 or 2
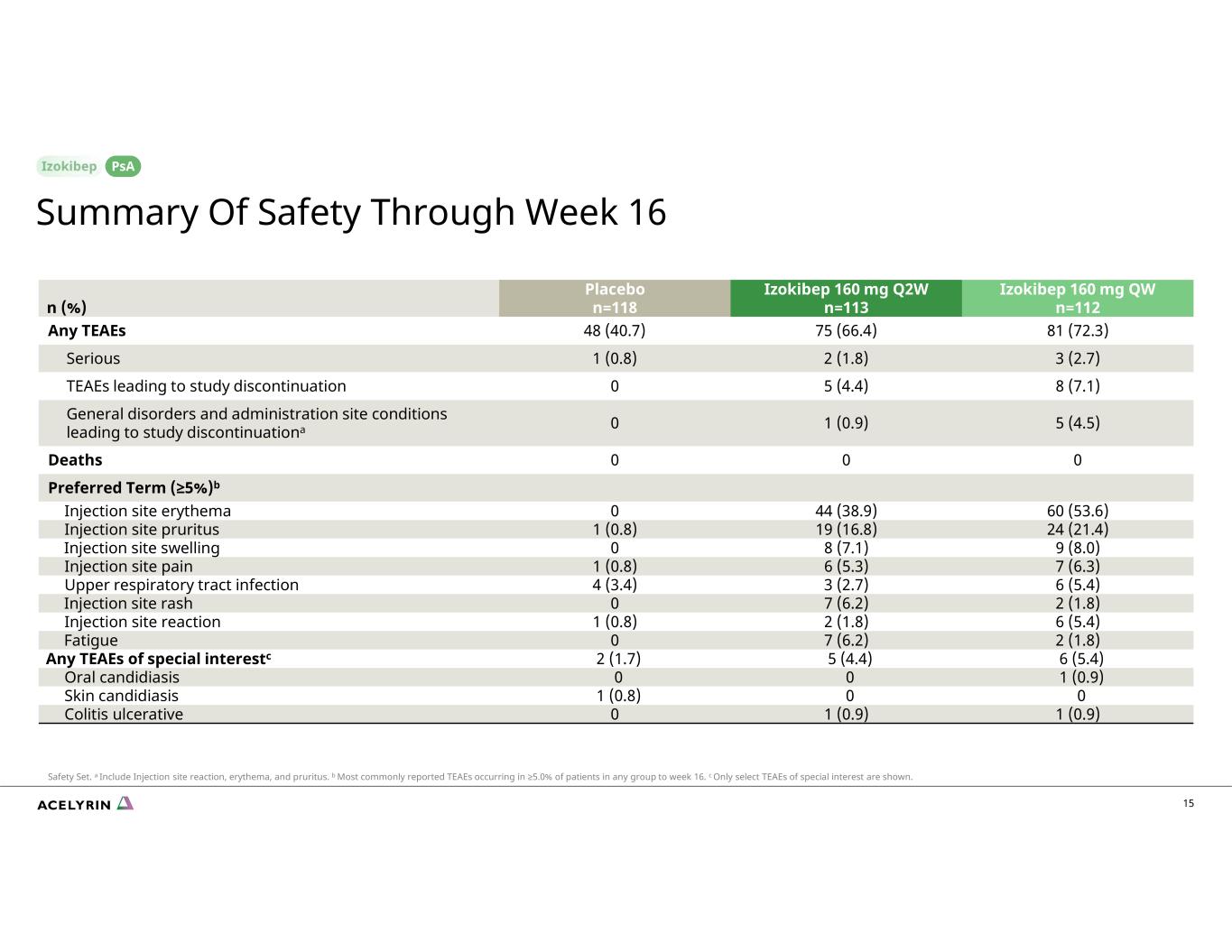
15 Izokibep 160 mg QW n=112 Izokibep 160 mg Q2W n=113 Placebo n=118n (%) 81 (72.3)75 (66.4)48 (40.7)Any TEAEs 3 (2.7)2 (1.8)1 (0.8)Serious 8 (7.1)5 (4.4)0TEAEs leading to study discontinuation 5 (4.5)1 (0.9)0General disorders and administration site conditions leading to study discontinuationa 000Deaths Preferred Term (≥5%)b 60 (53.6)44 (38.9)0Injection site erythema 24 (21.4)19 (16.8)1 (0.8)Injection site pruritus 9 (8.0)8 (7.1)0Injection site swelling 7 (6.3)6 (5.3)1 (0.8)Injection site pain 6 (5.4)3 (2.7)4 (3.4)Upper respiratory tract infection 2 (1.8)7 (6.2)0Injection site rash 6 (5.4)2 (1.8)1 (0.8)Injection site reaction 2 (1.8)7 (6.2)0Fatigue 6 (5.4)5 (4.4)2 (1.7)Any TEAEs of special interestc 1 (0.9)00Oral candidiasis 001 (0.8)Skin candidiasis 1 (0.9)1 (0.9)0Colitis ulcerative Summary Of Safety Through Week 16 PsA Safety Set. a Include Injection site reaction, erythema, and pruritus. b Most commonly reported TEAEs occurring in ≥5.0% of patients in any group to week 16. c Only select TEAEs of special interest are shown.
16 PsA • Izokibep IL-17A inhibition alone achieves rapid improvement in resolution across manifestations of disease • Pre-specified analyses support the potential for differentiation in enthesitis resolution • Higher clinical responses than reported by the IL-17A agents • Results comparable to those reported by the IL-17A&F agents but without the associated safety liabilities • Study met primary endpoint of ACR50 at 16 weeks with high statistical significance • Significant, multidomain responses achieved for the high hurdles of ACR70, PASI90, PASI100 and MDA • Improvement in magnitude of responses relative to Phase 2 notable given higher burden of disease in Phase 2b/3 • Expected to be the first of two registrational trials in psoriatic arthritis; 160mg Q2W appears to be optimal dose • Robust clinical responses in high hurdle composite endpoints (ACR50/PASI100 and MDA) • No safety limitation to long term treatment seen to date • Longer duration of therapy has previously demonstrated the potential for even further improvements over time Positive topline results Differentiated profile Deep and durable responses Positive Results For Izokibep Global Phase 2b/3 In PsA

Izokibep Hidradenitis Suppurativa Phase 2b Long Term 32-Week Data March 11, 2024

18 This presentation contains statements that are not of historical facts, considered forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements include, but are not limited to, statements about the therapeutic potential of our product candidate izokibep, including with respect to izokibep’s ability to offer clinically meaningful, differentiated benefits for patients, and that longer-term treatment with izokibep may provide for deepening of response over time without the important safety considerations emerging for agents targeting sub-units beyond IL-17A; the long-term safety profile of izokibep; the timing and availability of data from clinical trials; the potential market size and size of the potential patient populations for certain indications we are pursuing, and other statements that are not historical fact. These forward-looking statements are based on ACELYRIN’s current plans, objectives and projections, and are inherently subject to risks and uncertainties that may cause our actual results to differ materially and adversely from those anticipated in such forward-looking statements. Such risks and uncertainties include, without limitation, those associated with the successful completion of development and regulatory activities with respect to our product candidates; the timing and results of our clinical trials, including the potential that future results could differ adversely from prior results, where applicable; our ability to timely secure adequate supply of our product candidates; sufficient funding; legal proceedings and the outcome thereof; competitive risks; market volatility; macroeconomic conditions and other risks and uncertainties affecting ACELYRIN including those described from time to time under the heading “Risk Factors” and elsewhere in our current and future periodic and other reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Quarterly Report on Form 10-Q for the quarter ended September 30, 2023. These filings are available on the SEC’s website www.sec.gov. In addition, new risks may occur at any time, and we anticipate that subsequent developments could cause our views to change. Forward-looking statements herein are made of the date of this presentation, and ACELYRIN undertakes no duty to update them in the event of new information, future developments, or otherwise, except as required under applicable law. Any reader of this presentation is cautioned not to place undue reliance on these forward-looking statements. Izokibep is currently under clinical investigation, and no representation is made as of the safety or efficacy of our product candidates. In addition to our own internal research, this presentation contains information related to or based on studies and other data obtained from third-party sources. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness thereof. In addition, market data (e.g., market size) involves a number of projections, assumptions and estimates of our future performance and future performance of the markets in which we operate. There can be no guarantee of the accuracy or reliability thereof, as they are necessarily subject to a high degree of uncertainty and risk. The information in this presentation is as of the date of this presentation, and is subject to change without notice. TRADEMARKS This presentation contains trademarks, service marks, trade names and copyrights of ACELYRIN and other companies which are the property of their respective owners. Forward Looking Statements And Disclaimer
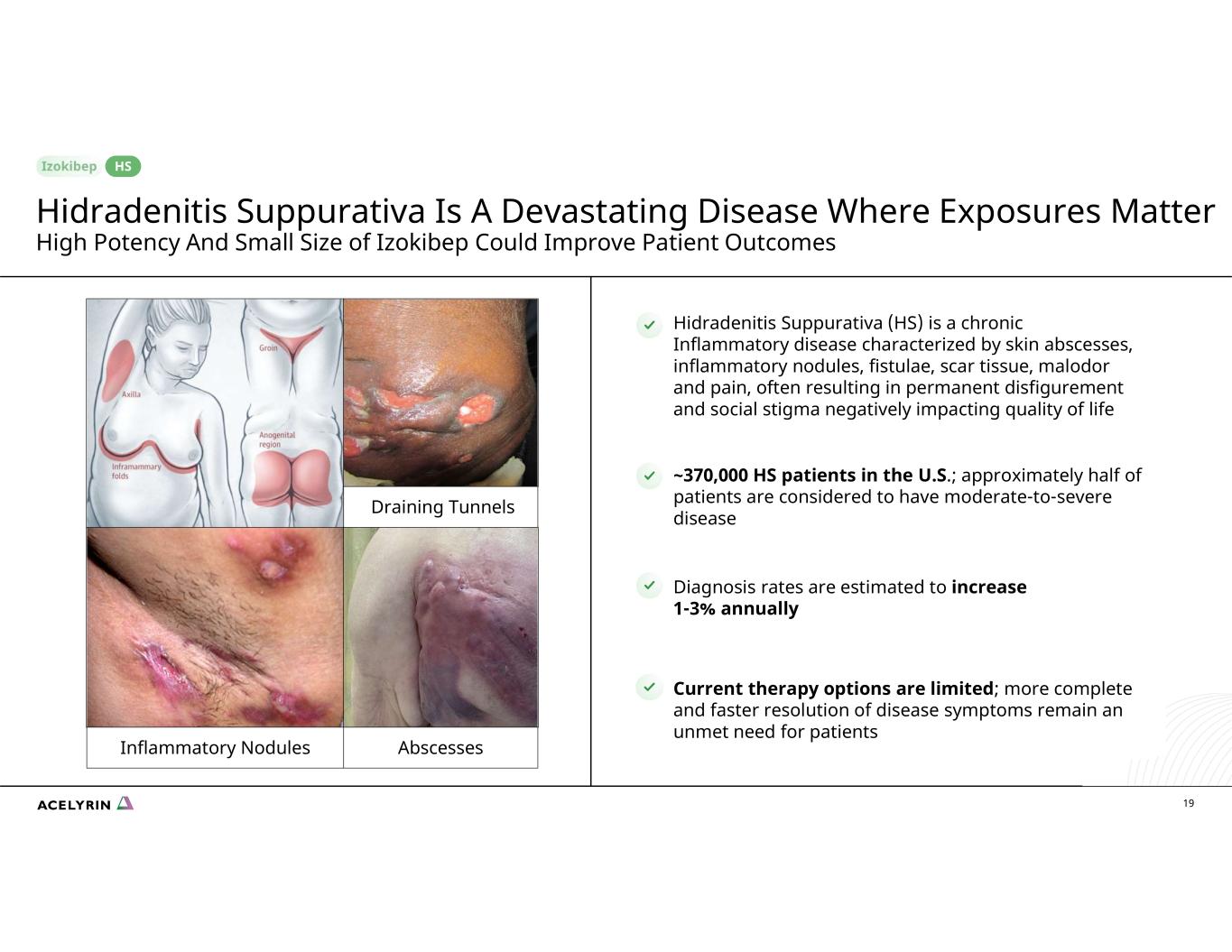
19 ~370,000 HS patients in the U.S.; approximately half of patients are considered to have moderate-to-severe disease Diagnosis rates are estimated to increase 1-3% annually Current therapy options are limited; more complete and faster resolution of disease symptoms remain an unmet need for patients Hidradenitis Suppurativa (HS) is a chronic Inflammatory disease characterized by skin abscesses, inflammatory nodules, fistulae, scar tissue, malodor and pain, often resulting in permanent disfigurement and social stigma negatively impacting quality of life Inflammatory Nodules Draining Tunnels Abscesses HS Hidradenitis Suppurativa Is A Devastating Disease Where Exposures Matter High Potency And Small Size of Izokibep Could Improve Patient Outcomes
20 • Magnitude and depth of responses support hypothesis that the characteristics of izokibep – including small size and highly potent inhibition of IL-17A alone – could deliver differentiated clinical benefit • Resolution of abscesses and nodules (HiSCR100) achieved more rapidly than the other IL-17A agents and than the IL-17A&F agents without the associated safety liabilities such as dose-dependent increased risk of fungal infection, for which HS patients are predisposed • Rapid, dose ordered improvement across manifestations through week 32 • HiSCR100 consistently achieved in about 1/3 of patients on 160mg QW including in pbo switch from week 16 • Consistent improvement in resolution of abscesses, nodules, and draining tunnels • Robust reduction in skin pain and remarkable improvement in overall quality of life • A phase 3 trial in HS is ongoing and topline data is expected by end of 2024 • We are planning a confirmatory phase 3 trial of approximately 400 patients to address FDA guidance HS Improvements across manifestations of disease Differentiated profile Path forward HS 32-Week Data Demonstrate Sustained & Deepening Responses
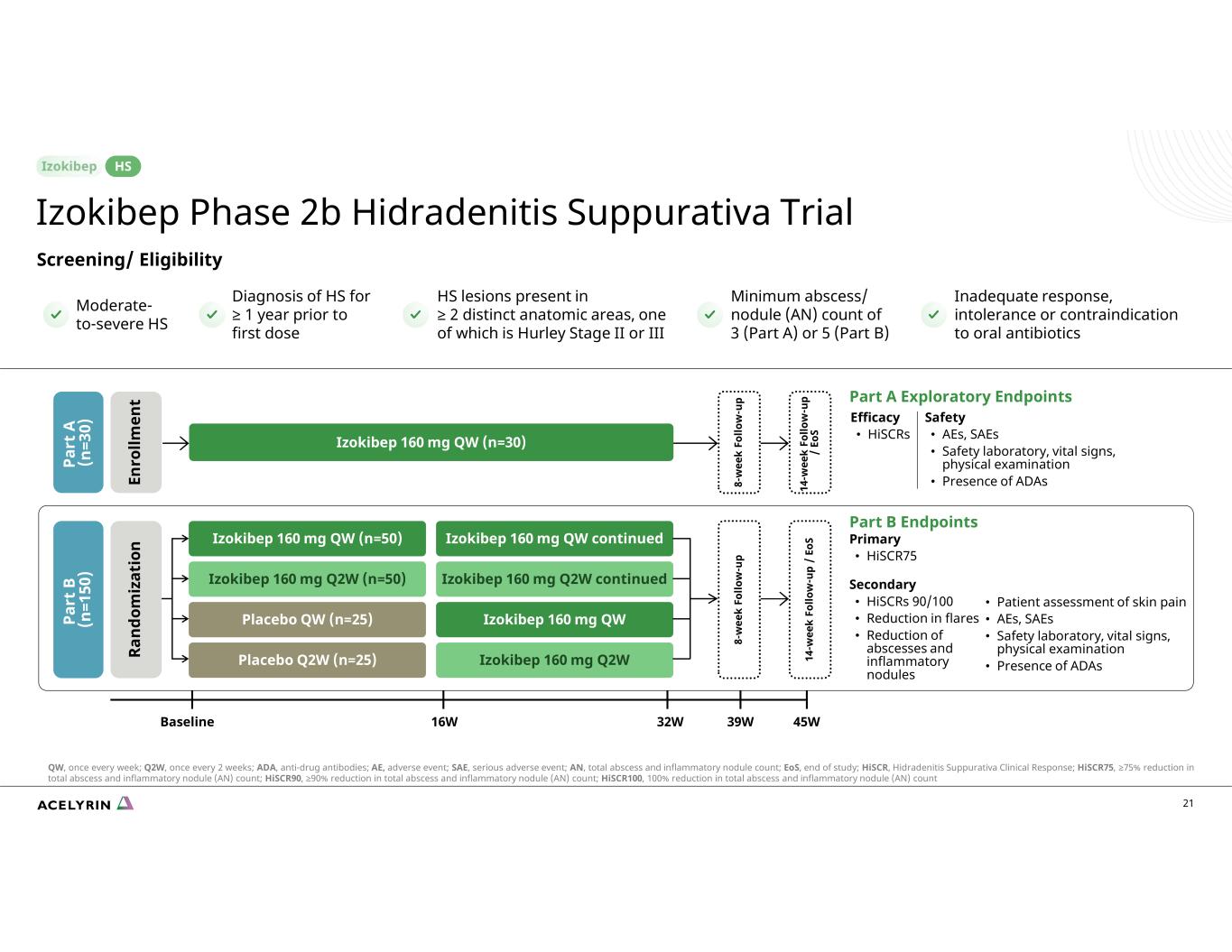
21 Screening/ Eligibility Part A Exploratory Endpoints Efficacy • HiSCRs 16WBaseline 32W 45W39W 8- w ee k Fo llo w -u p 14 -w ee k Fo llo w -u p / Eo S Izokibep 160 mg QW (n=30) Safety • AEs, SAEs • Safety laboratory, vital signs, physical examination • Presence of ADAs Part B Endpoints Primary • HiSCR75 Secondary • HiSCRs 90/100 • Reduction in flares • Reduction of abscesses and inflammatory nodules 8- w ee k Fo llo w -u p 14 -w ee k Fo llo w -u p / Eo SIzokibep 160 mg QW (n=50) Izokibep 160 mg QW continued Izokibep 160 mg Q2W (n=50) Izokibep 160 mg Q2W continued Placebo QW (n=25) Izokibep 160 mg QW Placebo Q2W (n=25) Izokibep 160 mg Q2W • Patient assessment of skin pain • AEs, SAEs • Safety laboratory, vital signs, physical examination • Presence of ADAs Pa rt A (n =3 0) En ro llm en t Pa rt B (n =1 50 ) Ra nd om iz at io n Inadequate response, intolerance or contraindication to oral antibiotics Minimum abscess/ nodule (AN) count of 3 (Part A) or 5 (Part B) HS lesions present in ≥ 2 distinct anatomic areas, one of which is Hurley Stage II or III Diagnosis of HS for ≥ 1 year prior to first dose Moderate- to-severe HS Izokibep Phase 2b Hidradenitis Suppurativa Trial HS QW, once every week; Q2W, once every 2 weeks; ADA, anti-drug antibodies; AE, adverse event; SAE, serious adverse event; AN, total abscess and inflammatory nodule count; EoS, end of study; HiSCR, Hidradenitis Suppurativa Clinical Response; HiSCR75, ≥75% reduction in total abscess and inflammatory nodule (AN) count; HiSCR90, ≥90% reduction in total abscess and inflammatory nodule (AN) count; HiSCR100, 100% reduction in total abscess and inflammatory nodule (AN) count
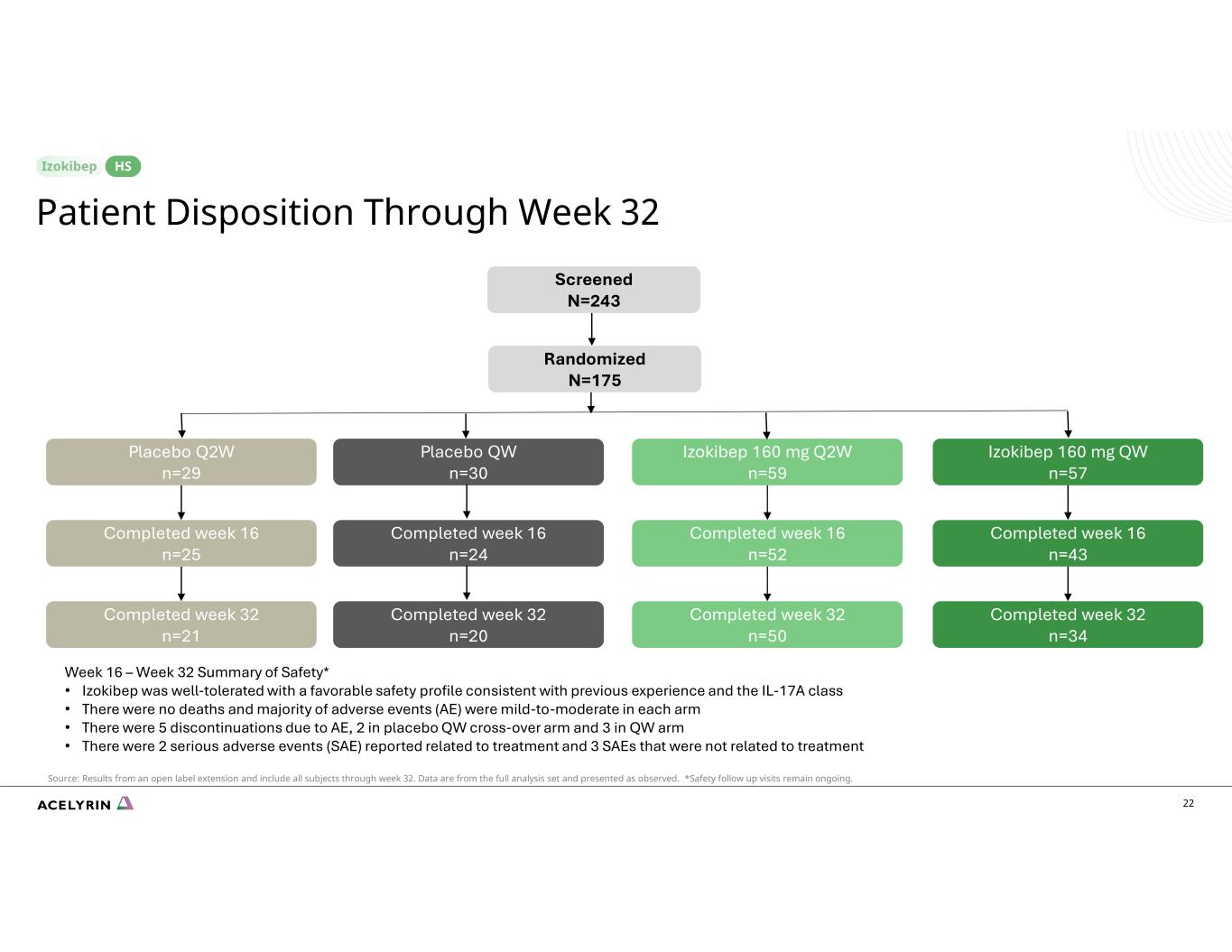
22 Patient Disposition Through Week 32 HS Screened N=243 Randomized N=175 Placebo Q2W n=29 Izokibep 160 mg Q2W n=59 Izokibep 160 mg QW n=57 Completed week 16 n=25 Completed week 16 n=52 Completed week 16 n=43 Completed week 32 n=21 Completed week 32 n=50 Completed week 32 n=34 Placebo QW n=30 Completed week 16 n=24 Completed week 32 n=20 Source: Results from an open label extension and include all subjects through week 32. Data are from the full analysis set and presented as observed. *Safety follow up visits remain ongoing. Week 16 – Week 32 Summary of Safety* • Izokibep was well-tolerated with a favorable safety profile consistent with previous experience and the IL-17A class • There were no deaths and majority of adverse events (AE) were mild-to-moderate in each arm • There were 5 discontinuations due to AE, 2 in placebo QW cross-over arm and 3 in QW arm • There were 2 serious adverse events (SAE) reported related to treatment and 3 SAEs that were not related to treatment
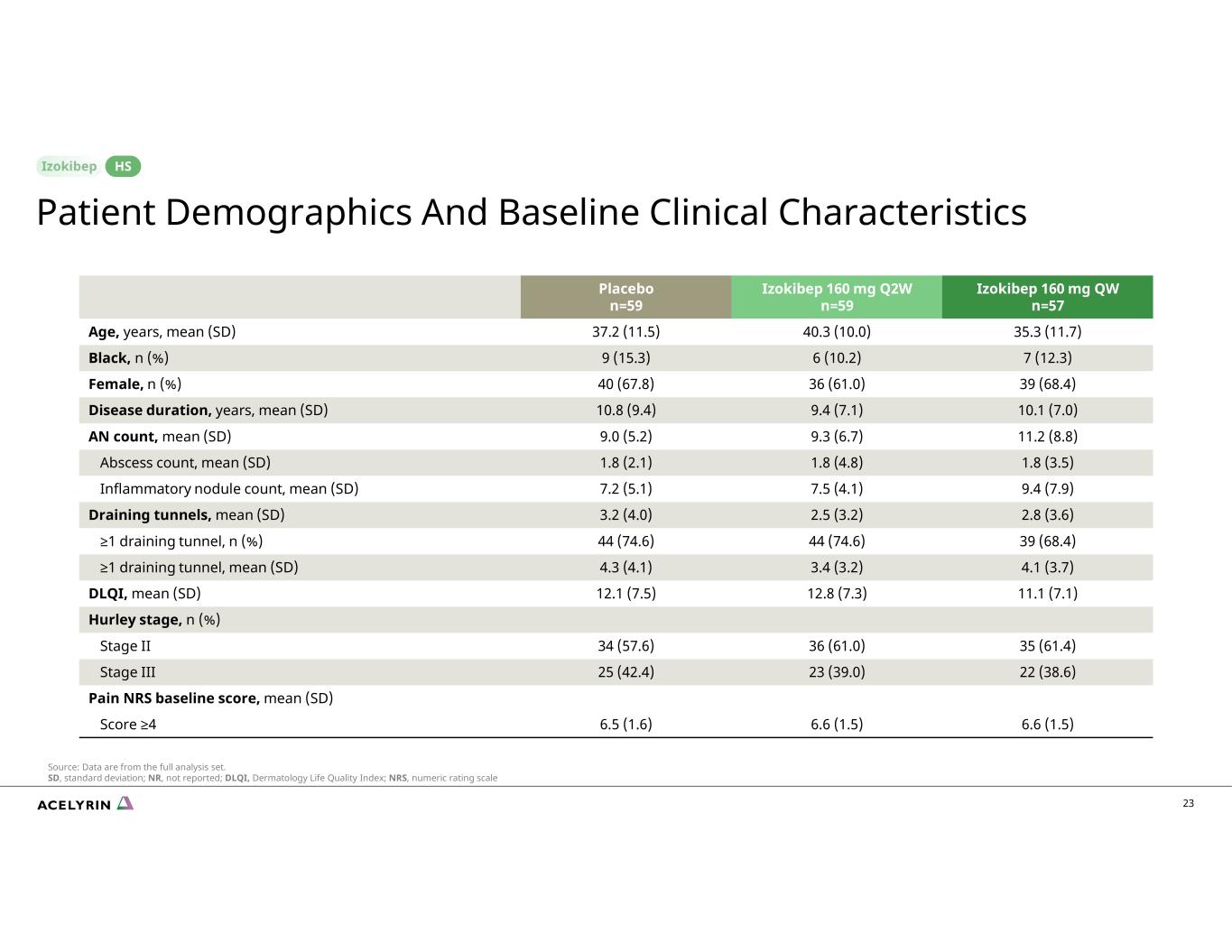
23 Izokibep 160 mg QW n=57 Izokibep 160 mg Q2W n=59 Placebo n=59 35.3 (11.7)40.3 (10.0)37.2 (11.5)Age, years, mean (SD) 7 (12.3)6 (10.2)9 (15.3)Black, n (%) 39 (68.4)36 (61.0)40 (67.8)Female, n (%) 10.1 (7.0)9.4 (7.1)10.8 (9.4)Disease duration, years, mean (SD) 11.2 (8.8)9.3 (6.7)9.0 (5.2)AN count, mean (SD) 1.8 (3.5)1.8 (4.8)1.8 (2.1)Abscess count, mean (SD) 9.4 (7.9)7.5 (4.1)7.2 (5.1)Inflammatory nodule count, mean (SD) 2.8 (3.6)2.5 (3.2)3.2 (4.0)Draining tunnels, mean (SD) 39 (68.4)44 (74.6)44 (74.6)≥1 draining tunnel, n (%) 4.1 (3.7)3.4 (3.2)4.3 (4.1)≥1 draining tunnel, mean (SD) 11.1 (7.1)12.8 (7.3)12.1 (7.5)DLQI, mean (SD) Hurley stage, n (%) 35 (61.4)36 (61.0)34 (57.6)Stage II 22 (38.6)23 (39.0)25 (42.4)Stage III Pain NRS baseline score, mean (SD) 6.6 (1.5)6.6 (1.5)6.5 (1.6) Score ≥4 Patient Demographics And Baseline Clinical Characteristics HS Source: Data are from the full analysis set. SD, standard deviation; NR, not reported; DLQI, Dermatology Life Quality Index; NRS, numeric rating scale
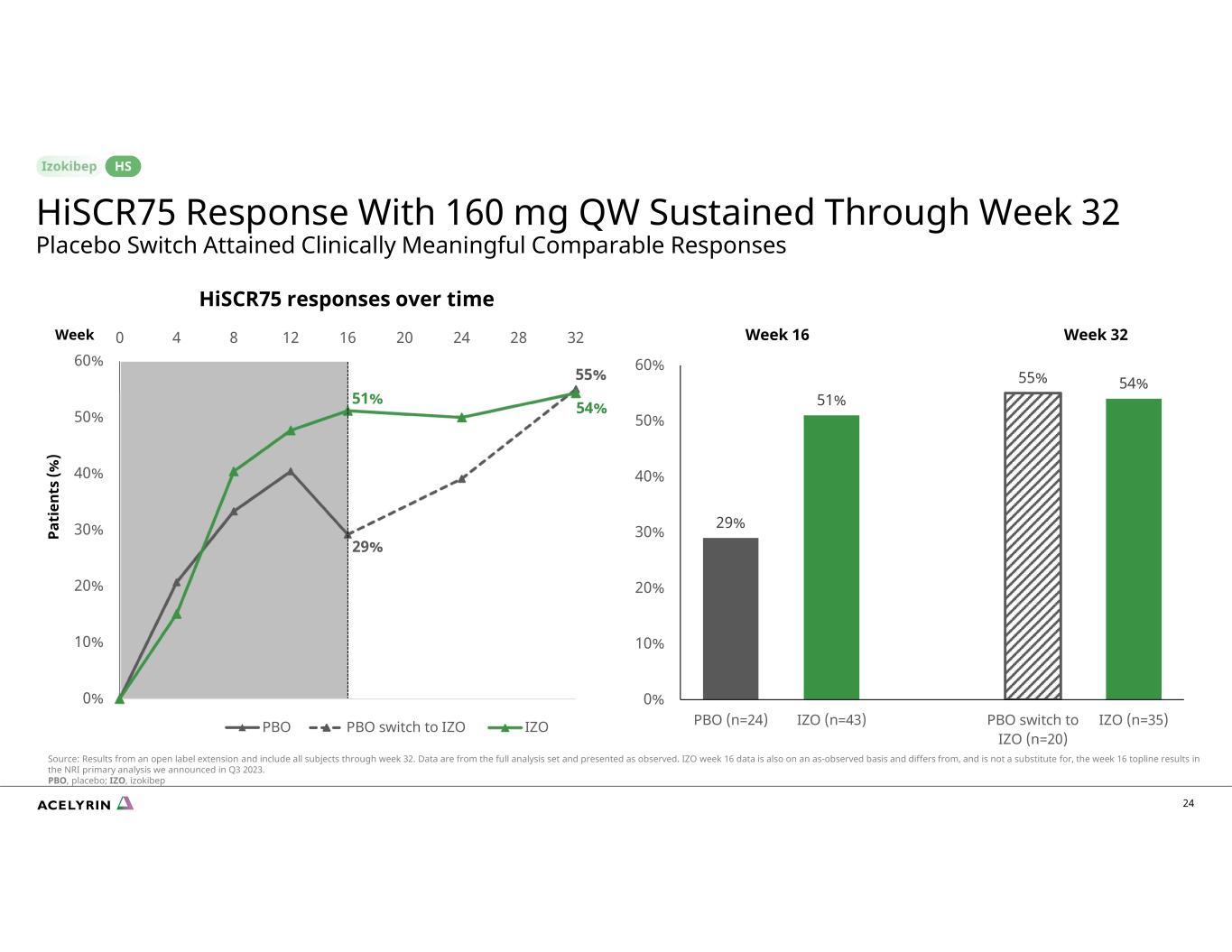
29% 55% 51% 54% 0% 10% 20% 30% 40% 50% 60% 0 4 8 12 16 20 24 28 32 HiSCR75 responses over time PBO PBO switch to IZO IZO 24 29% 51% 55% 54% 0% 10% 20% 30% 40% 50% 60% PBO (n=24) IZO (n=43) PBO switch to IZO (n=20) IZO (n=35) Week 32 Pa ti en ts (% ) Week 16Week HiSCR75 Response With 160 mg QW Sustained Through Week 32 Placebo Switch Attained Clinically Meaningful Comparable Responses HS Source: Results from an open label extension and include all subjects through week 32. Data are from the full analysis set and presented as observed. IZO week 16 data is also on an as-observed basis and differs from, and is not a substitute for, the week 16 topline results in the NRI primary analysis we announced in Q3 2023. PBO, placebo; IZO, izokibep
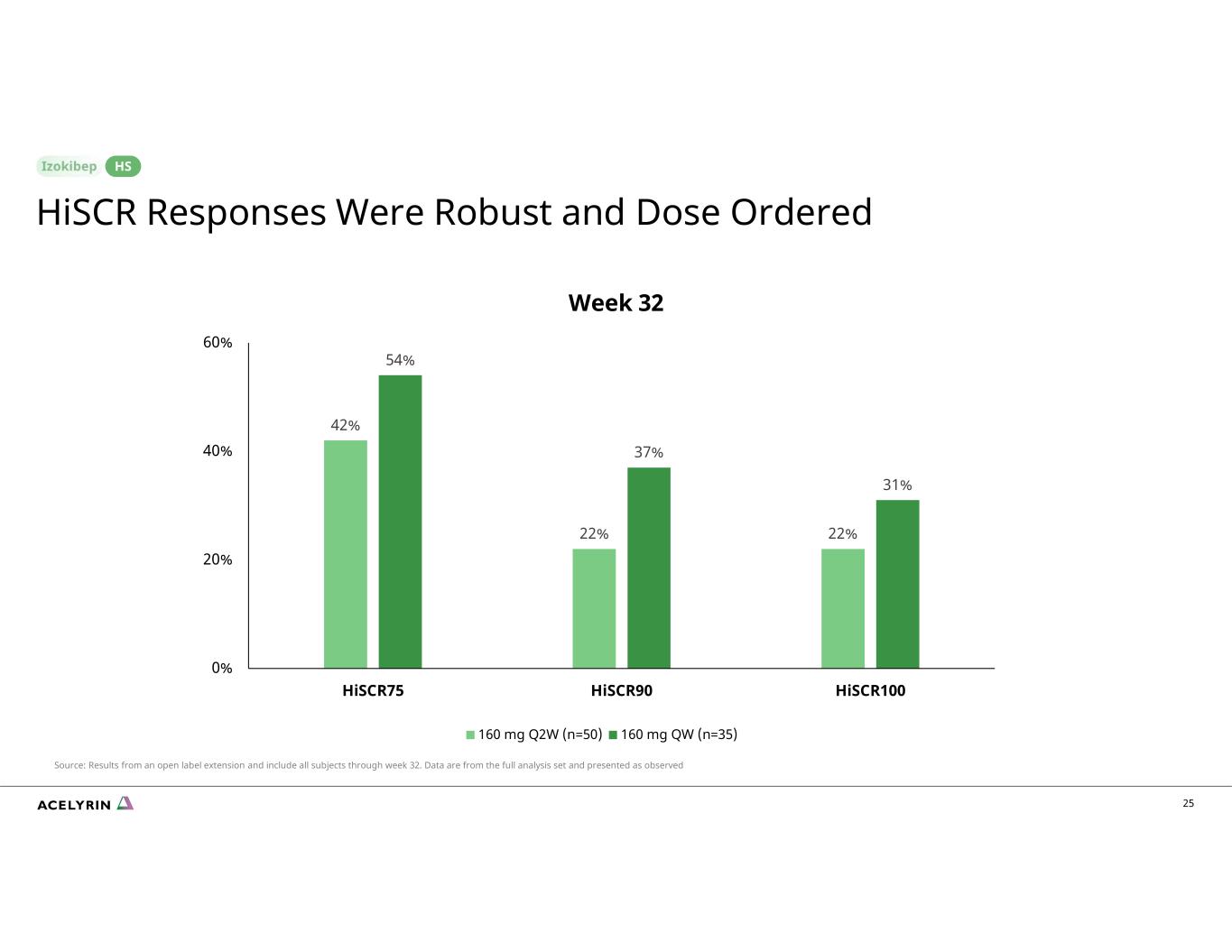
25 42% 22% 22% 54% 37% 31% 0% 20% 40% 60% HiSCR75 HiSCR90 HiSCR100 160 mg Q2W (n=50) 160 mg QW (n=35) Week 32 HiSCR Responses Were Robust and Dose Ordered HS Source: Results from an open label extension and include all subjects through week 32. Data are from the full analysis set and presented as observed
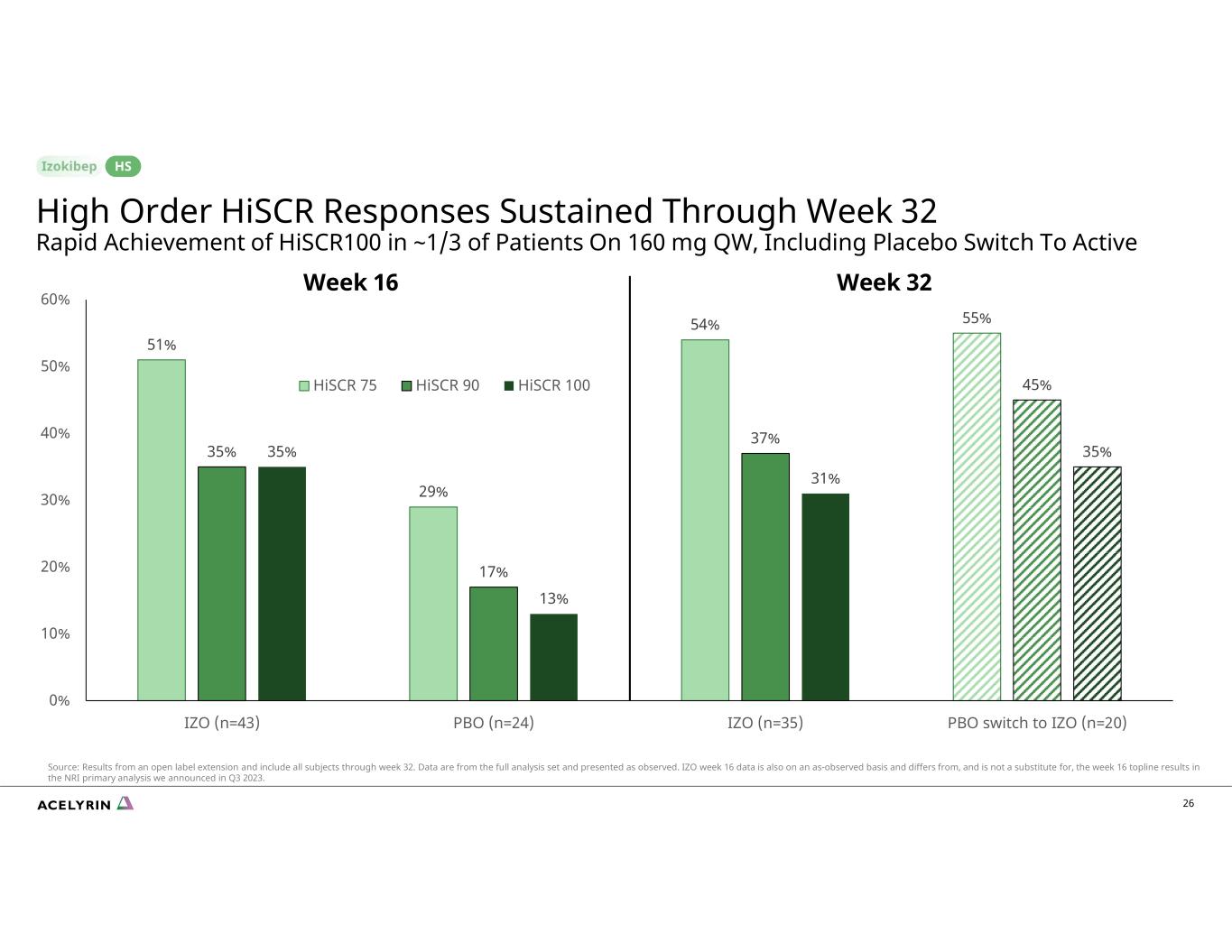
51% 29% 54% 55% 35% 17% 37% 45% 35% 13% 31% 35% 0% 10% 20% 30% 40% 50% 60% IZO (n=43) PBO (n=24) IZO (n=35) PBO switch to IZO (n=20) HiSCR 75 HiSCR 90 HiSCR 100 26 Week 16 Week 32 High Order HiSCR Responses Sustained Through Week 32 Rapid Achievement of HiSCR100 in ~1/3 of Patients On 160 mg QW, Including Placebo Switch To Active HS Source: Results from an open label extension and include all subjects through week 32. Data are from the full analysis set and presented as observed. IZO week 16 data is also on an as-observed basis and differs from, and is not a substitute for, the week 16 topline results in the NRI primary analysis we announced in Q3 2023.
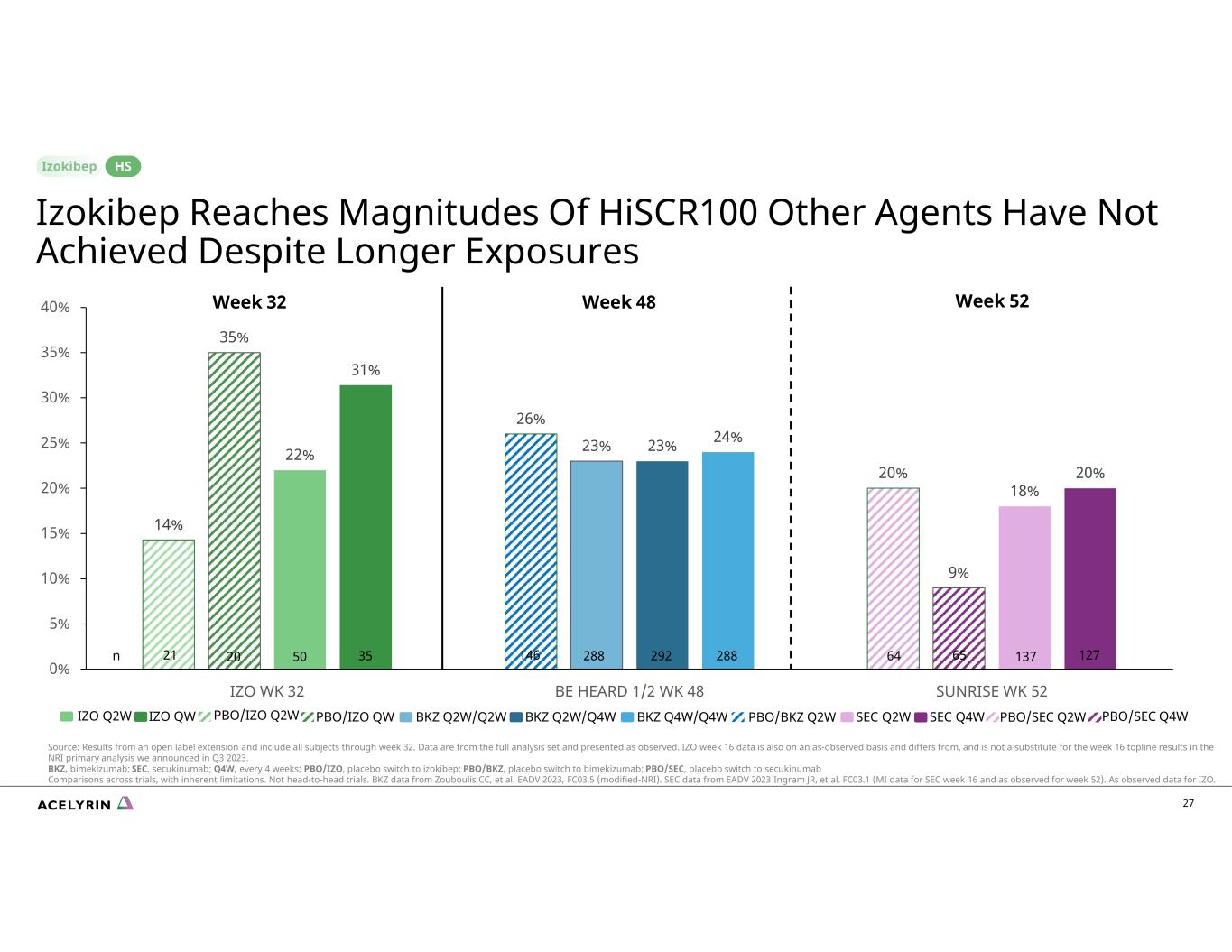
27 14% 26% 20% 35% 23% 9% 22% 23% 18% 31% 24% 20% 0% 5% 10% 15% 20% 25% 30% 35% 40% IZO WK 32 BE HEARD 1/2 WK 48 SUNRISE WK 52 Week 32 Week 52 IZO Q2W IZO QW BKZ Q2W/Q2W BKZ Q2W/Q4W BKZ Q4W/Q4W SEC Q2W SEC Q4WPBO/IZO Q2W PBO/IZO QW Week 48 PBO/BKZ Q2W PBO/SEC Q2W PBO/SEC Q4W Izokibep Reaches Magnitudes Of HiSCR100 Other Agents Have Not Achieved Despite Longer Exposures HS Source: Results from an open label extension and include all subjects through week 32. Data are from the full analysis set and presented as observed. IZO week 16 data is also on an as-observed basis and differs from, and is not a substitute for the week 16 topline results in the NRI primary analysis we announced in Q3 2023. BKZ, bimekizumab; SEC, secukinumab; Q4W, every 4 weeks; PBO/IZO, placebo switch to izokibep; PBO/BKZ, placebo switch to bimekizumab; PBO/SEC, placebo switch to secukinumab Comparisons across trials, with inherent limitations. Not head-to-head trials. BKZ data from Zouboulis CC, et al. EADV 2023, FC03.5 (modified-NRI). SEC data from EADV 2023 Ingram JR, et al. FC03.1 (MI data for SEC week 16 and as observed for week 52). As observed data for IZO. 64 65 137 127146 288 292 28821 20 50 35n
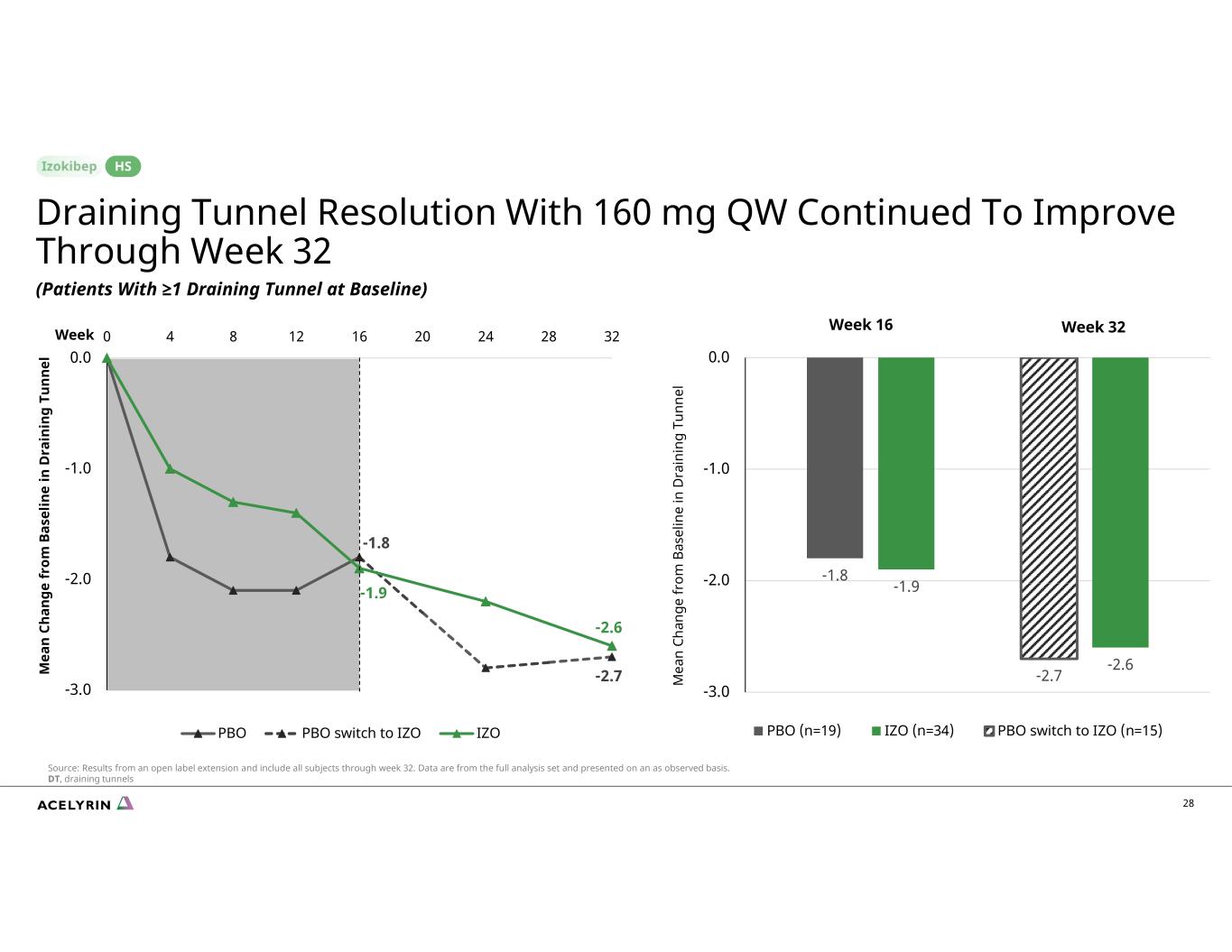
-1.8 -2.7 -1.9 -2.6 -3.0 -2.0 -1.0 0.0 0 4 8 12 16 20 24 28 32 PBO PBO switch to IZO IZO -1.8 -1.9 -2.7 -2.6 -3.0 -2.0 -1.0 0.0 PBO (n=19) IZO (n=34) PBO switch to IZO (n=15) 28 Week 16 Week 32 M ea n Ch an ge fr om B as el in e in D ra in in g Tu nn el M ea n Ch an ge fr om B as el in e in D ra in in g Tu nn el Draining Tunnel Resolution With 160 mg QW Continued To Improve Through Week 32 (Patients With ≥1 Draining Tunnel at Baseline) HS Source: Results from an open label extension and include all subjects through week 32. Data are from the full analysis set and presented on an as observed basis. DT, draining tunnels Week
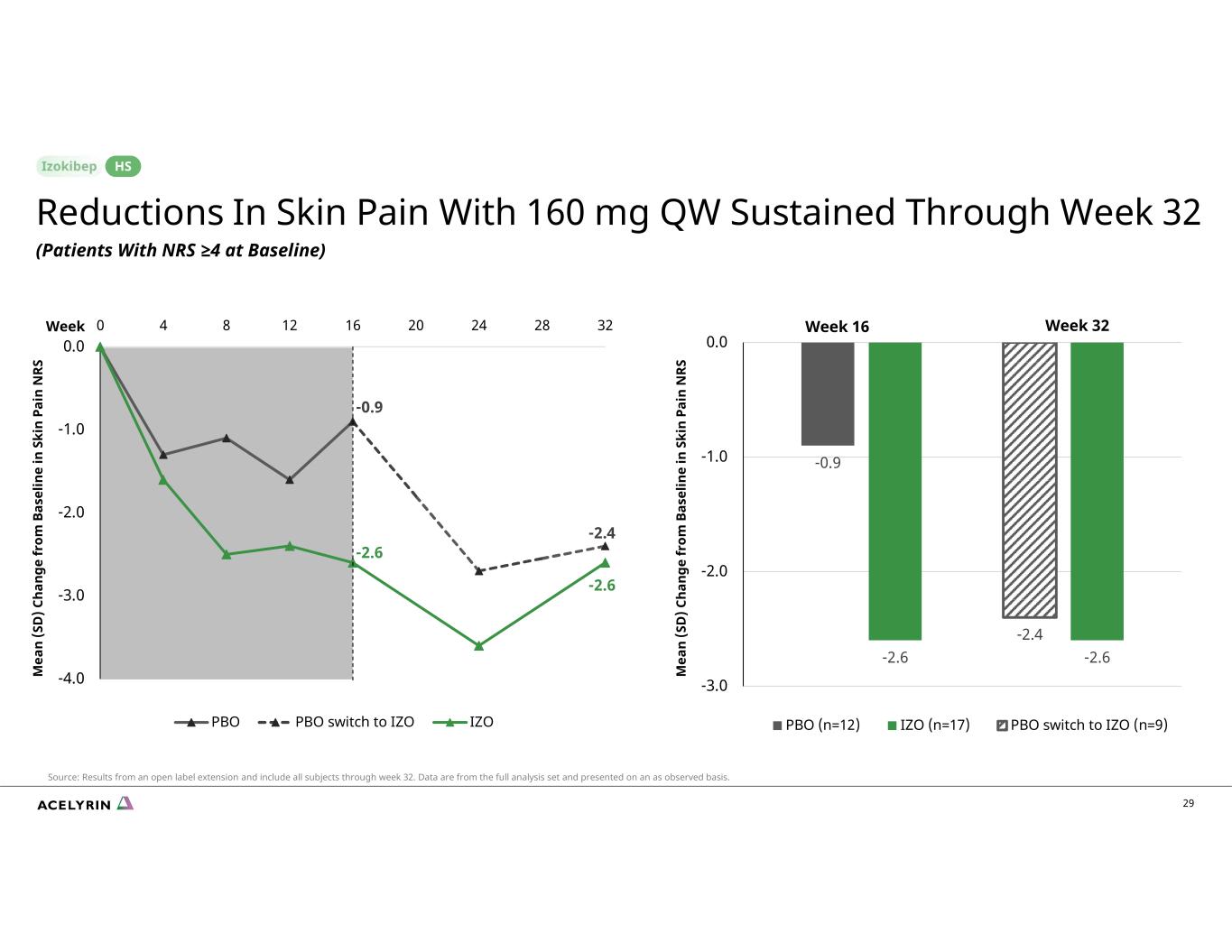
29 Week 16 Week 32 -0.9 -2.6 -2.4 -2.6 -3.0 -2.0 -1.0 0.0 PBO (n=12) IZO (n=17) PBO switch to IZO (n=9) M ea n (S D ) C ha ng e fr om B as el in e in S ki n Pa in N RS M ea n (S D ) C ha ng e fr om B as el in e in S ki n Pa in N RS -0.9 -2.4 -2.6 -2.6 -4.0 -3.0 -2.0 -1.0 0.0 0 4 8 12 16 20 24 28 32 PBO PBO switch to IZO IZO Reductions In Skin Pain With 160 mg QW Sustained Through Week 32 (Patients With NRS ≥4 at Baseline) HS Source: Results from an open label extension and include all subjects through week 32. Data are from the full analysis set and presented on an as observed basis. Week
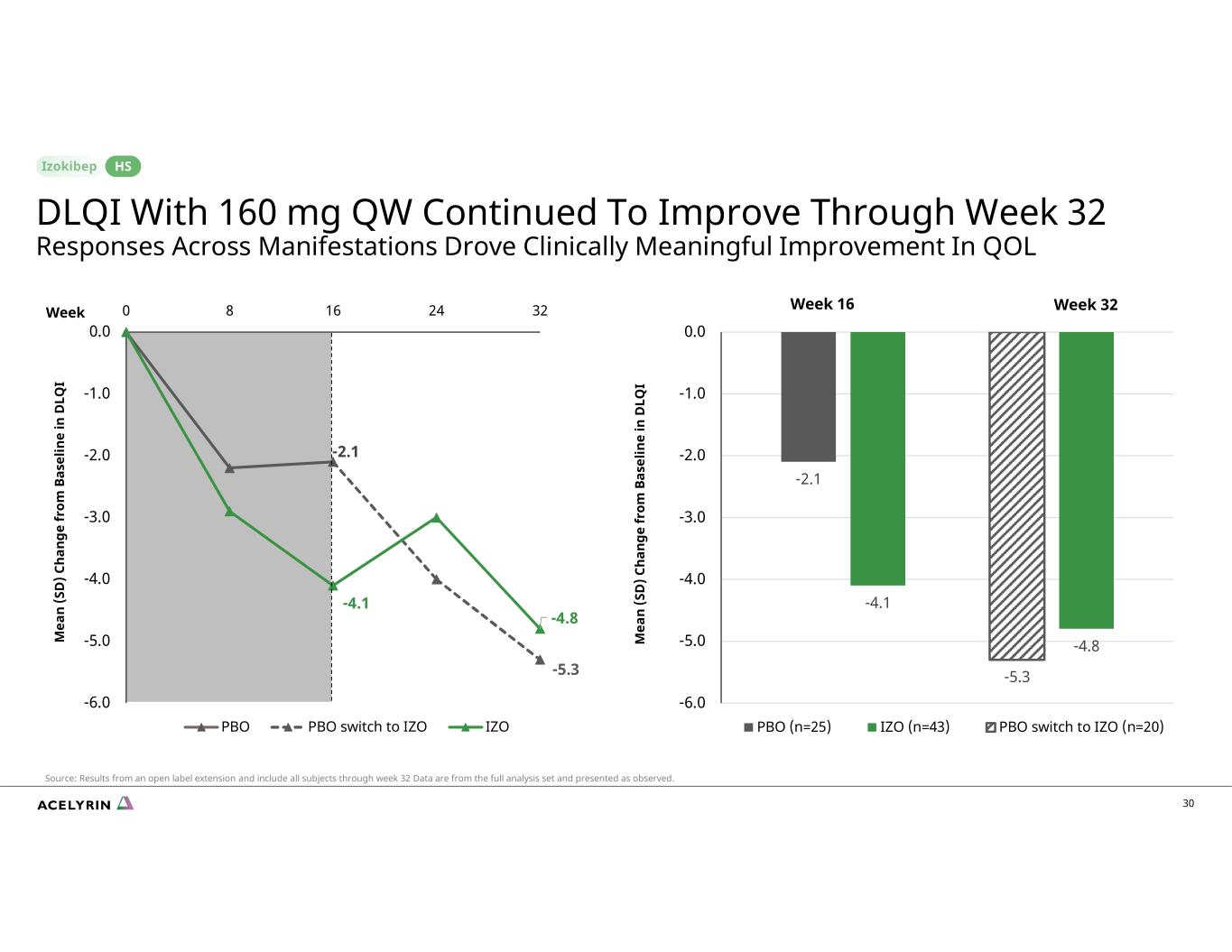
30 -2.1 -5.3 -4.1 -4.8 -6.0 -5.0 -4.0 -3.0 -2.0 -1.0 0.0 0 8 16 24 32 PBO PBO switch to IZO IZO Week 16 Week 32 M ea n (S D ) C ha ng e fr om B as el in e in D LQ I M ea n (S D ) C ha ng e fr om B as el in e in D LQ I -2.1 -4.1 -5.3 -4.8 -6.0 -5.0 -4.0 -3.0 -2.0 -1.0 0.0 PBO (n=25) IZO (n=43) PBO switch to IZO (n=20) DLQI With 160 mg QW Continued To Improve Through Week 32 Responses Across Manifestations Drove Clinically Meaningful Improvement In QOL HS Source: Results from an open label extension and include all subjects through week 32 Data are from the full analysis set and presented as observed. Week
31 • Magnitude and depth of responses support hypothesis that the characteristics of izokibep – including small size and highly potent inhibition of IL-17A alone – could deliver differentiated clinical benefit • Resolution of abscesses and nodules (HiSCR100) achieved more rapidly than the other IL-17A agents and than the IL-17A&F agents without the associated safety liabilities such as dose-dependent increased risk of fungal infection, for which HS patients are predisposed • Rapid, dose ordered improvement across manifestations through week 32 • HiSCR100 consistently achieved in about 1/3 of patients on 160mg QW including in pbo switch from week 16 • Consistent improvement in resolution of abscesses, nodules, and draining tunnels • Robust reduction in skin pain and remarkable improvement in overall quality of life • A phase 3 trial in HS is ongoing and topline data is expected by end of 2024 • We are planning a confirmatory phase 3 trial of approximately 400 patients to address FDA guidance HS Improvements across manifestations of disease Differentiated profile Path forward HS 32-Week Data Demonstrate Sustained & Deepening Responses






























