Exhibit 99.1

[ ], 2024
Dear 3M Company Shareholder:
On July 26, 2022, 3M Company (“3M”) announced its plan to separate its health care business into an independent public company. The separation will occur through a pro rata distribution by 3M of at least 80.1% of the outstanding shares of common stock of a newly formed company, Solventum Corporation (“Solventum”), which will hold 3M’s health care business.
The separation will result in two world-class public companies well-positioned to pursue their respective growth plans. As leading standalone companies, 3M and Solventum are each expected to benefit from (i) the ability to pursue tailored capital allocation strategies and make company-specific investment decisions to drive innovation and growth; (ii) enhanced management focus, with each public company having distinct boards and management teams with relevant expertise able to focus on strengthening its business; (iii) improved operational agility and focus, enabling each of 3M and Solventum to pursue its distinct operating priorities and strategies with increased flexibility to act based on its unique characteristics, better positioning each for long-term success; (iv) greater access to capital through the creation of distinct and compelling investment profiles appealing to different long-term investor bases; (v) independent equity currencies, enabling each company to use its own industry-focused stock to consummate future acquisitions or other transactions; and (vi) enhanced recruitment and retention, including by aligning employee, management, and board incentives with performance. We expect that 3M will remain a leading global material science innovator serving diverse end markets, with global science and technology and manufacturing capabilities, and a portfolio of iconic brands. 3M will continue to leverage its innovation to capitalize on customer opportunities aligned with key global megatrends, such as electronics, safety, mobility, digitization, home improvement, and sustainability.
Following the distribution, 3M will own up to 19.9% of the outstanding shares of Solventum common stock. Each 3M shareholder as of the close of business on March 18, 2024, the record date for the distribution, will receive one share of Solventum common stock for every four shares of 3M common stock held by such shareholder as of such time, with cash paid in lieu of fractional shares. Solventum common stock issued in the distribution will be issued in book-entry form only, which means that no physical share certificates will be issued.
For U.S. federal income tax purposes, the distribution is intended to be generally tax-free to 3M shareholders.
No vote of 3M shareholders is required for the distribution. You do not need to take any action to receive shares of Solventum common stock to which you are entitled as a 3M shareholder, and you do not need to pay any consideration or surrender or exchange your 3M common stock or take any other action to receive your shares of Solventum common stock.
Solventum’s common stock has been approved for listing on the New York Stock Exchange (the “NYSE”), subject to official notice of issuance, under the symbol “SOLV.” Following the distribution, 3M common stock will continue to trade on the New York Stock Exchange under the symbol “MMM.”
We encourage you to read the attached information statement, which is being made available to all 3M shareholders as of the record date for the distribution. The information statement describes the distribution in detail and contains important business and financial information about Solventum, including its historical financial statements.
We believe the separation provides tremendous opportunities for our businesses, as we work to continue to build long-term value. We appreciate your continuing support of 3M and look forward to your future support of 3M and Solventum.
| Sincerely, | ||
| Michael F. Roman | ||
| Chairman and Chief Executive Officer | ||
| 3M Company | ||
Dear Future Solventum Shareholder:
I am excited to welcome you as a shareholder of Solventum when we become an independent company after completion of the planned spin-off from 3M. Solventum is a leading global healthcare company with a broad and diverse portfolio across four operating segments. Our solutions are relied on every day within the global healthcare industry, and we believe they contribute to higher-quality patient care, more efficient processes and workflows, and improved standards of safety and accuracy. Our 70+ year history of discovering and innovating advanced solutions helps us solve our customers’ toughest challenges.
Our team is guided by our mission to deliver better, smarter, safer healthcare to improve lives. We are proud of the strengths and long heritage of innovation our team brings to Solventum from its history with 3M and plan to build on those strengths.
We believe Solventum is a proven global leader in large, diverse and growing markets with a wide portfolio of strong, reputable brands and long-standing customer relationships. We believe that our technology platforms and innovation expertise, our leading digital and data science capabilities and business models, supported by our global scale and reach and strong manufacturing expertise, will position us for continued growth and value creation. With our cash flow generation capability and attractive margins, we believe we will be able to reduce leverage, reinvest in our business, accelerate growth through M&A over time, and return capital to shareholders.
As we become an independent company, we will be better suited to recruit experienced talent from healthcare, operate with increased agility as a smaller, more nimble organization, enhance our focus within our portfolios, and allocate our capital and resources to drive our growth strategies.
Our common stock has been approved for listing on the NYSE, subject to official notice of issuance, under the symbol “SOLV.” I encourage you to learn more about Solventum by reading the attached information statement.
I am personally looking forward to this unique opportunity to introduce a leading healthcare company to you, and to earn your support and trust as a shareholder as we continue to work to deliver better, smarter, safer healthcare to improve lives and move healthcare forward.
Sincerely,
Bryan Hanson
Information contained herein is subject to completion or amendment. A Registration Statement on Form 10 relating to these securities has been filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended.
Preliminary and Subject to Completion, Dated March 11, 2024
INFORMATION STATEMENT
Solventum Corporation
This information statement is being furnished in connection with the distribution by 3M Company (“3M”) to its shareholders of at least 80.1% of the outstanding shares of common stock of Solventum Corporation (“Solventum”), a wholly owned subsidiary of 3M that will hold 3M’s health care business. 3M will distribute at least 80.1% of the shares of Solventum common stock on a pro rata basis to 3M shareholders in a distribution that is intended to qualify as generally tax-free to 3M shareholders for U.S. federal income tax purposes. Following the distribution, Solventum will be a separate publicly traded company, and 3M will initially own up to 19.9% of the outstanding shares of Solventum common stock.
For every four shares of 3M common stock held of record by you as of the close of business on March 18, 2024, which is the record date for the distribution, you will receive one share of Solventum common stock. You will receive cash in lieu of any fractional shares of Solventum common stock that you would have received after application of the above ratio. As discussed under “The Separation and Distribution—Trading Between the Record Date and Distribution Date,” if you sell your shares of 3M common stock in the “regular-way” market after the record date and before the distribution date, you also will be selling your right to receive shares of Solventum common stock in connection with the distribution. We expect the shares of Solventum common stock to be distributed by 3M to you at 3:30 a.m., Eastern Time, on April 1, 2024. We refer to the date of the distribution of the Solventum common stock as the “distribution date.”
Until the separation and distribution occur, Solventum will be a wholly owned subsidiary of 3M, and consequently, 3M will have the sole and absolute discretion to determine and change the terms of the separation (or to terminate the separation).
No vote of 3M shareholders is required for the distribution. Therefore, you are not being asked for a proxy, and you are requested not to send 3M a proxy, in connection with the distribution. You do not need to pay any consideration, exchange or surrender your existing shares of 3M common stock or take any other action to receive your shares of Solventum common stock.
There is no current trading market for Solventum common stock, although we expect that a limited market, commonly known as a “when-issued” trading market, will develop on or about March 26, 2024, and we expect “regular-way” trading of Solventum common stock to begin on the first trading day following the completion of the distribution. Solventum’s common stock has been approved for listing on the NYSE, subject to official notice of issuance, under the symbol “SOLV.” Following the distribution, 3M common stock will continue to trade on the New York Stock Exchange (“NYSE”) under the symbol “MMM.”
In reviewing this information statement, you should carefully consider the matters described under the section entitled “Risk Factors” beginning on page 40.
Neither the U.S. Securities and Exchange Commission nor any state securities commission has approved or disapproved these securities or determined if this information statement is truthful or complete. Any representation to the contrary is a criminal offense.
This information statement does not constitute an offer to sell or the solicitation of an offer to buy any securities.
The date of this information statement is [ ].
This information statement will be made publicly available on or about [ ]. Notice of this information statement’s availability will be first sent to 3M shareholders on or about [ ].
TABLE OF CONTENTS
| Page | |||||
Non-GAAP Financial Data
Except for the 3M financial information presented in the section entitled “Executive Compensation” and Appendix A to Executive Compensation, all financial information presented in this information statement is derived from the historical combined financial statements included elsewhere in this information statement. All financial information presented in this information statement has been prepared in U.S. Dollars in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”), except for the presentation of the following non-GAAP financial measures: Adjusted Operating Income, Adjusted Operating Income Margin, Free Cash Flow; and the following non-GAAP financial measures in the section entitled “Executive Compensation” (collectively, the “Executive Compensation Related Non-GAAP Metrics”): adjusted earnings per share, free cash flow, free cash flow growth, free cash flow conversion, return on invested capital, operating income and operating cash flow conversion.
We present Adjusted Operating Income, Adjusted Operating Income Margin, and Free Cash Flow in this information statement because we believe such measures provide investors with additional information to measure our performance and liquidity. Please refer to “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-GAAP Financial Measures” for an explanation on why we use these non-GAAP financial measures, their definitions, and their limitations.
i
We present the Executive Compensation Related Non-GAAP Metrics in this information statement because we believe such measures provide investors with additional information on the metrics 3M has used to determine the compensation of 3M’s named executive officers (who functioned as named executive officers of the Solventum business since the Health Care Business was conducted as part of the broader 3M Business in 2022). Please refer to Appendix A to Executive Compensation for a reconciliation of these non-GAAP financial measures with the most directly comparable GAAP financial measures.
Presentation of Information
Unless the context otherwise requires:
•Except as otherwise indicated or unless the context otherwise requires, the information included in this information statement about Solventum assumes the completion of all of the transactions referred to in this information statement in connection with the separation and distribution.
•References in this information statement to “Solventum,” “we,” “us,” “our,” “our company” and “the Company” refer to Solventum Corporation, a Delaware corporation, and its subsidiaries.
•References in this information statement to “3M” refer to 3M Company, a Delaware corporation, and its consolidated subsidiaries, including the Health Care Business prior to completion of the separation, unless the context otherwise requires or unless otherwise specified.
•References in this information statement to the “Health Care Business” refer to the health care business of 3M as defined in the historical combined financial statements included in this information statement.
•References in this information statement to the “3M Business” refer to 3M’s businesses other than the Health Care Business.
•References in this information statement to the “separation” refer to the separation of the Health Care Business from 3M’s other businesses and the creation, as a result of the distribution, of an independent, publicly traded company, Solventum, to hold the assets and liabilities associated with the Health Care Business after the distribution.
•References in this information statement to the “distribution” refer to the distribution by 3M of at least 80.1% of Solventum’s issued and outstanding shares of common stock to 3M shareholders as of the close of business on March 18, 2024, which is the record date for the distribution.
•References in this information statement to Solventum’s per share data assume a distribution ratio of one share of Solventum common stock for every four shares of 3M common stock.
•References in this information statement to Solventum’s historical assets, liabilities, products, businesses or activities generally refer to the historical assets, liabilities, products, businesses or activities of the Health Care Business as conducted by 3M prior to the completion of the separation.
•References in this information statement to the “separation and distribution agreement” refer to the separation and distribution agreement to be entered into between Solventum and 3M, that will, among other things, contain the key provisions relating to the separation of the Health Care Business from the remaining businesses of 3M and the distribution of at least 80.1% of the outstanding shares of Solventum’s common stock to holders of 3M common stock entitled to such distribution.
•References in this information statement to “PFAS” refer to any per- or poly-fluoroalkyl substance that contains at least one fully fluorinated methyl or methylene carbon atom (without any hydrogen, chlorine, bromine, or iodine atom attached to it).
ii
Trademarks, Trade Names and Service Marks
The trademarks, trade names and service marks of Solventum appearing in this information statement are, as applicable, our property, licensed to us or, prior to the completion of the distribution, the property of 3M. The name and mark, 3M, and other trademarks, trade names and service marks of 3M appearing in this information statement are the property of 3M. Solely for convenience, trademarks, trade names and service marks referred to in this information statement may appear without the “®”, “™” or “℠” symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent possible under applicable law, our rights or the rights of the applicable licensor to these trademarks, trade names and service marks. This information statement also contains additional trademarks, trade names and service marks belonging to other parties. We do not intend our use or display of these other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, such other parties.
Industry Information
Unless indicated otherwise, the information concerning the industries in which Solventum participates contained in this information statement is based on Solventum’s general knowledge of and expectations concerning the industry. Solventum’s competitive position and industry size are based on estimates using Solventum’s internal data and estimates, data from various industry analyses, our internal research and adjustments and assumptions that we believe to be reasonable. Further, Solventum’s estimates and assumptions involve risks and uncertainties and are subject to change based on various factors, including those discussed in the “Risk Factors” section. These and other factors could cause results to differ materially from those expressed in the estimates and assumptions.
iii
QUESTIONS AND ANSWERS ABOUT THE SEPARATION AND DISTRIBUTION
| What is Solventum and why is 3M separating the Health Care Business and distributing Solventum common stock? | Solventum, which is currently a wholly owned subsidiary of 3M, was formed to hold the Health Care Business. 3M intends to separate Solventum from the rest of 3M by distributing at least 80.1% of the outstanding Solventum common stock to 3M shareholders on a pro rata basis as of the record date for the distribution. The separation of Solventum from 3M is intended, among other things, to enable each company to pursue tailored capital allocation strategies and make company-specific investment decisions to drive innovation and growth, enhance management focus, and improve operational agility. 3M expects that the separation will result in enhanced long-term performance of the businesses held by both 3M and Solventum for the reasons discussed in the section entitled “The Separation and Distribution—Reasons for the Separation.” | ||||
| Why am I receiving this document? | 3M is delivering this document to you because you are a holder of shares of 3M common stock. If you are a holder of shares of 3M common stock as of the close of business on March 18, 2024, the record date of the distribution, you will be entitled to receive one share of Solventum common stock for every four shares of 3M common stock that you hold at such time. This document will help you understand how the separation and distribution will affect your post-separation ownership in 3M and Solventum. | ||||
| How will the separation of the Health Care Business from the 3M Business work? | As part of the separation, and prior to the completion of the distribution, 3M and its subsidiaries expect to complete an internal reorganization (which this information statement refers to as the “internal reorganization”) in order to transfer the Health Care Business to Solventum. To accomplish the separation, 3M will distribute at least 80.1% of the outstanding shares of Solventum common stock to 3M shareholders as of the record date on a pro rata basis, in a distribution intended to be generally tax-free to 3M shareholders for U.S. federal income tax purposes. The number of shares of 3M common stock you own will not change as a result of the separation. | ||||
| Why is the separation of Solventum structured as a distribution? | 3M believes that a distribution of shares of Solventum common stock to 3M shareholders, which is intended to be generally tax-free to 3M shareholders for U.S. federal income tax purposes, is an efficient way to separate the Health Care Business in a manner that will enhance the ability of each of 3M and Solventum to execute its long-term business strategies. | ||||
| What is the record date for the distribution? | The record date for the distribution will be the close of business on March 18, 2024. | ||||
| When will the distribution occur? | The distribution is subject to a number of conditions but, subject to the satisfaction or waiver of such conditions, it is expected that the distribution will occur at 3:30 a.m., Eastern Time, on April 1, 2024, to holders of record of shares of 3M common stock at the close of business on March 18, 2024, the record date for the distribution. | ||||
1
| What do shareholders need to do to participate in the distribution? | Shareholders of 3M as of the record date for the distribution are not required to take any action to receive Solventum common stock in the distribution, but you are urged to read this entire information statement carefully. No 3M shareholder approval is required for the distribution, and you are not being asked for a proxy. You do not need to pay any consideration, exchange or surrender your existing shares of 3M common stock, or take any other action to receive your shares of Solventum common stock. Please do not send in your 3M stock certificates. The distribution will not affect the number of outstanding shares of 3M common stock or any rights of 3M shareholders, although it will affect the market value of each outstanding share of 3M common stock. | ||||
| How will shares of Solventum common stock be issued? | You will receive shares of Solventum common stock through the same channels that you currently use to hold or trade shares of 3M common stock, whether through a brokerage account, 401(k) plan or other channels. Receipt of Solventum shares will be documented for you in the same manner that you typically receive shareholder updates, such as monthly broker statements or 401(k) statements. | ||||
| If you own shares of 3M common stock as of the close of business on the record date for the distribution, including shares owned in certificate form, 3M, with the assistance of Equiniti Trust Company, LLC, the distribution agent for the distribution (the “distribution agent” or “Equiniti”), will electronically distribute shares of Solventum common stock to you or to your brokerage firm on your behalf in book-entry form. Equiniti will mail you a book-entry account statement that reflects your shares of Solventum common stock or your bank or brokerage firm will credit your account for the shares. | |||||
| How many shares of Solventum common stock will I receive in the distribution? | You are entitled to receive one share of Solventum common stock for every four shares of 3M common stock held by you as of close of business on the record date for the distribution. Based on approximately 553,360,198 shares of 3M common stock outstanding as of March 6, 2024, a total of approximately 138,340,050 shares of Solventum common stock will be distributed to 3M’s shareholders and approximately 34,369,125 shares of Solventum common stock will continue to be owned by 3M. For additional information on the distribution, see “The Separation and Distribution.” | ||||
| Will fractional shares of Solventum common stock be distributed in the distribution? | No fractional shares will be distributed in the distribution. Fractional shares that 3M shareholders would otherwise have been entitled to receive will be aggregated and sold in the public market by the distribution agent. The net cash proceeds of these sales will be distributed pro rata (based on the fractional share such holder would otherwise be entitled to receive) to those stockholders who would otherwise have been entitled to receive fractional shares. Recipients of cash in lieu of fractional shares will not be entitled to any interest on the amounts paid in lieu of fractional shares. | ||||
2
| What are the conditions to the distribution? | The distribution is subject to the satisfaction (or waiver by 3M in its sole and absolute discretion) of the following conditions: •the U.S. Securities and Exchange Commission (the “SEC”) declaring effective the registration statement of which this information statement forms a part, there being no order suspending the effectiveness of the registration statement in effect, and there being no proceedings for such purposes having been instituted or threatened by the SEC; •this information statement having been made available to the holders of record of shares of 3M common stock at the close of business on March 18, 2024, the record date for the distribution; •(1) the private letter ruling received by 3M from the U.S. Internal Revenue Service (the “IRS”) regarding certain U.S. federal income tax matters relating to the separation and the distribution, including the qualification of the distribution, together with certain related transactions, as a transaction that is generally tax-free for U.S. federal income tax purposes pursuant to Sections 355 and 368(a)(1)(D) of the Internal Revenue Code of 1986, as amended (the “Code,” such qualification, “U.S. Tax-Free Status” and, such ruling, the “IRS Ruling”) continuing to be valid and satisfactory to the 3M Board of Directors in its sole and absolute discretion, and (2) the receipt by 3M and continuing validity of one or more opinions of 3M’s tax advisors, in each case satisfactory to the 3M Board of Directors in its sole and absolute discretion, regarding the qualification of the distribution, together with certain related transactions, as a transaction that is generally tax-free for U.S. federal income tax purposes pursuant to Sections 355 and 368(a)(1)(D) of the Code (each, a “Tax Opinion” and, collectively, the “Tax Opinion(s)”); | ||||
•the internal reorganization and the transfer of assets and liabilities from 3M to Solventum having been completed in accordance with the separation and distribution agreement; •the receipt of one or more opinions from an independent appraisal firm to the 3M Board of Directors as to the solvency of 3M and Solventum after the completion of the distribution, in each case, in a form and substance acceptable to the 3M Board of Directors in its sole and absolute discretion; | |||||
3
•all actions and filings necessary or appropriate under applicable U.S. federal, U.S. state or other securities or blue sky laws and the rules and regulations thereunder having been taken or made and, where applicable, having become effective or been accepted by the applicable governmental authority; •the execution of certain agreements contemplated by the separation and distribution agreement; •no order, injunction or decree issued by any government authority of competent jurisdiction or other legal restraint or prohibition preventing the consummation of the separation, the distribution or any of the related transactions being pending or being in effect; | |||||
•the shares of Solventum common stock to be distributed having been accepted for listing on the NYSE, subject to official notice of issuance; •3M having received certain proceeds from the financing arrangements described under “Description of Material Indebtedness” and being satisfied in its sole and absolute discretion that, as of the effective time of the distribution, 3M will have no liability under such arrangements; and •no other event or development existing or having occurred that, in the judgment of 3M’s Board of Directors, in its sole and absolute discretion, makes it inadvisable to effect the separation, the distribution and the other related transactions. 3M and Solventum cannot assure you that any or all of these conditions will be met, or that the separation or distribution will be consummated even if all of the conditions are met. 3M can decline at any time to go forward with the separation or distribution. In addition, 3M may waive any of the conditions to the distribution. For a complete discussion of all of the conditions to the distribution and the potential waiver of such conditions, see “The Separation and Distribution—Conditions to the Distribution.” | |||||
| What is the expected date of completion of the distribution? | The completion and timing of the distribution are dependent upon a number of conditions. It is currently expected that the shares of Solventum common stock will be distributed by 3M at 3:30 a.m., Eastern Time, on April 1, 2024, to the holders of record of shares of 3M common stock at the close of business on March 18, 2024, the record date for the distribution. However, no assurance can be provided as to the timing of the distribution or that all conditions to the distribution will be met. | ||||
4
| Can 3M decide to cancel the distribution of Solventum common stock even if all the conditions have been met, or proceed with the distribution of Solventum common stock even if any of the conditions have not been met? | Yes. Until the distribution has occurred, the 3M Board of Directors has the right to terminate the distribution, even if all of the conditions described in the section entitled “The Separation and Distribution—Conditions to the Distribution” are satisfied. Alternatively, 3M may waive any of the conditions to the distribution and proceed with the distribution even if such conditions have not been met. If the distribution is completed and the 3M Board of Directors waived any such condition, such waiver could have a material adverse effect on 3M’s and Solventum’s respective businesses, financial condition or results of operations, the trading price of Solventum’s or 3M’s common stock, or the ability of shareholders to sell their shares after the distribution. If 3M elects to proceed with the distribution notwithstanding that one or more of the conditions to the distribution has not been met, 3M will evaluate the applicable facts and circumstances at that time and make such additional disclosure and take such other actions as 3M determines to be necessary and appropriate in accordance with applicable law. | ||||
| What if I want to sell my 3M common stock or my Solventum common stock? | You should consult with your financial advisors, such as your stock broker, bank or tax advisor. If you sell your shares of 3M common stock in the “regular-way” market after the record date and before the distribution date, you also will be selling your right to receive shares of Solventum common stock in connection with the distribution. | ||||
| What is “regular-way” and “ex-distribution” trading of 3M common stock? | Beginning on or shortly before the record date for the distribution and continuing up to and through the distribution date, Solventum expects that there will be two markets in 3M common stock: a “regular-way” market and an “ex-distribution” market. 3M common stock that trades in the “regular-way” market will trade with an entitlement to shares of Solventum common stock distributed pursuant to the distribution. Shares that trade in the “ex-distribution” market will trade without an entitlement to Solventum common stock distributed pursuant to the distribution. If you are the registered holder of your shares and want to sell your shares, you should determine whether you want to sell your shares with or without an entitlement to shares of Solventum common stock in the distribution and make any trades in the “regular-way” or “ex-distribution” market accordingly. If you decide to sell any shares of 3M common stock before the distribution date and hold your shares in “street name,” you should make sure your stockbroker, bank or other nominee understands whether you want to sell your Solventum common stock with or without your entitlement to Solventum common stock pursuant to the distribution. | ||||
5
| Where will I be able to trade shares of Solventum common stock? | Solventum’s common stock has been approved for listing on the NYSE, subject to official notice of issuance, under the symbol “SOLV.” It is anticipated that trading in shares of Solventum common stock will begin on a “when-issued” basis on or about March 26, 2024 and will continue up to and through the distribution date, and that “regular-way” trading in Solventum common stock will begin on the first trading day following the completion of the distribution. If trading begins on a “when-issued” basis, you may purchase or sell Solventum common stock up to and through the distribution date, but your transaction will not settle until after the distribution date. Solventum cannot predict the trading prices for its common stock before, on or after the distribution date. | ||||
| What will happen to the listing of 3M common stock? | 3M common stock will continue to trade on the NYSE after the distribution under the symbol “MMM.” | ||||
| Will the number of shares of 3M common stock that I own change as a result of the distribution? | No. The number of shares of 3M common stock that you own will not change as a result of the distribution. | ||||
| Will the distribution affect the market price of my 3M common stock? | Yes. As a result of the distribution, it is expected that the trading price of shares of 3M common stock immediately following the distribution will be different from the “regular-way” trading price of such shares immediately prior to the distribution because the trading price of 3M common stock will no longer reflect the value of the Health Care Business. There can be no assurance whether the sum of the market value of the 3M common stock and the Solventum common stock following the separation will be higher or lower than the market value of 3M common stock if the separation did not occur. This means, for example, that the combined trading prices of one share of 3M common stock and one-quarter of a share of Solventum common stock after the distribution may be equal to, greater than or less than the trading price of one share of 3M common stock before the distribution. | ||||
6
| What are the material U.S. federal income tax consequences of the separation and the distribution? | It is a condition to the distribution (which condition 3M may waive in its sole discretion) that (1) the IRS Ruling regarding U.S. Tax-Free Status continues to be valid and (2) 3M receives the Tax Opinion(s) regarding U.S. Tax-Free Status. Accordingly, it is expected that you will not recognize any gain or loss, and no amount will be included in your income, upon your receipt of Solventum common stock pursuant to the distribution for U.S. federal income tax purposes. You will, however, recognize gain or loss for U.S. federal income tax purposes with respect to cash received in lieu of a fractional share of Solventum common stock. For more information regarding the material U.S. federal income tax consequences of the distribution, see the section entitled “Material U.S. Federal Income Tax Consequences.” You should consult your own tax advisor as to the particular tax consequences of the distribution to you, including the applicability and effect of any U.S. federal, state and local tax laws, as well as any non-U.S. tax laws. | ||||
7
| What will Solventum’s relationship be with 3M following the separation? | After the separation, 3M and Solventum will be separate companies with separate management teams and separate boards of directors. 3M and Solventum will enter into a separation and distribution agreement to effect the separation and to provide a framework for Solventum’s relationship with 3M after the separation, and they will enter into certain other agreements, including a transition services agreement, a transition distribution services agreement, a transition contract manufacturing agreement, research and development master services agreements, real estate license agreements, an intellectual property cross license agreement, a 3M mark use agreement, a transition trademark license agreement, master supply agreements, a tax matters agreement, an employee matters agreement, and a stockholder’s and registration rights agreement. See “Certain Relationships and Related Party Transactions.” These agreements will provide for the allocation between Solventum and 3M of the assets, employees, liabilities and obligations (including, among others, investments, property (including intellectual property) and employee benefits and tax-related assets and liabilities) of 3M and its subsidiaries attributable to periods prior to, at and after the separation and will govern the relationship between Solventum and 3M subsequent to the completion of the separation (including the relationship of 3M as a stockholder of Solventum). For additional information regarding the separation and distribution agreement and other transaction agreements, see the sections entitled “Risk Factors—Risks Related to the Separation and Distribution” and “Certain Relationships and Related Party Transactions.” Additionally, following the distribution, 3M will own up to 19.9% of the outstanding shares of Solventum common stock. See the questions below entitled “How will 3M vote any shares of Solventum common stock it retains?” and “What does 3M intend to do with any shares of Solventum common stock it retains?” | ||||
| How will 3M vote any shares of Solventum common stock it retains? | 3M will agree to vote any shares of common stock that it retains in proportion to the votes cast by Solventum’s other shareholders and is expected to grant Solventum a proxy to vote 3M’s shares of Solventum common stock in such proportion. For additional information on these voting arrangements, see “Certain Relationships and Related Party Transactions.” | ||||
| What does 3M intend to do with any shares of Solventum common stock it retains? | 3M currently plans to dispose of all of the Solventum common stock that it retains after the distribution as soon as a disposition is warranted consistent with the business reasons for the retention of those shares through one or more sales of such shares (not later than five years after the distribution). | ||||
| Who will manage Solventum after the separation? | Solventum’s management team will possess deep knowledge of the healthcare industry. For more information regarding Solventum’s management and directors, see “Management” and “Directors.” | ||||
8
| Are there risks associated with owning Solventum common stock? | Yes. Ownership of Solventum common stock is subject to both general and specific risks relating to its business, the industry in which it operates, its ongoing contractual relationships with 3M and its status as a separate, publicly traded company. Ownership of Solventum common stock is also subject to risks relating to the separation. Certain of these risks are described in the “Risk Factors” section of this information statement. We encourage you to read that section carefully. We also encourage you to read carefully the sections entitled “Information Statement Summary—Certain Risks Relating to Operating as a Standalone Entity” and “Information Statement Summary—Certain Risks Relating to Solventum’s Indebtedness.” | ||||
| Does Solventum plan to pay dividends? | After the separation and distribution, Solventum will evaluate whether to pay a regular cash dividend. The timing, declaration, amount of, and payment of any dividends following the separation and the distribution will be within the discretion of Solventum’s Board of Directors and will depend upon many factors, and there can be no assurances that Solventum will begin or continue to pay a dividend in the future. See “Dividend Policy.” There can also be no assurance that, after the separation and distribution, the combined annual dividends on the common stock of Solventum and 3M, if any, will be equal to the annual dividends on 3M common stock prior to the separation and distribution. | ||||
| Will Solventum incur any indebtedness prior to or at the time of the distribution? | Yes. In connection with the distribution, on February 27, 2024, Solventum issued $6.9 billion of senior unsecured notes in six series. Additionally, on February 16, 2024, Solventum entered into an 18-month senior unsecured term loan facility in an aggregate committed amount of $500 million and a three-year senior unsecured term loan facility in an aggregate committed amount of $1.0 billion. The proceeds of such financings are expected to be used to make cash payments to 3M, other than such amounts as need to be retained in order to cause Solventum to have $600 million of cash in the aggregate at the time of the distribution. The cash payments to 3M from the proceeds of the financings are expected to total approximately $7.7 billion, after taking into account fees, discounts and expenses, as well as the retention of $600 million. As a result of such transactions, Solventum anticipates having approximately $8.4 billion of outstanding indebtedness upon completion of the distribution. In addition, on February 16, 2024, Solventum entered into a revolving credit agreement in an aggregate committed amount of $2.0 billion, and Solventum also intends to enter into a $2.0 billion unsecured, unsubordinated commercial paper program prior to the distribution. Solventum does not currently expect to incur any borrowings under either of the revolving credit agreement or the commercial paper program prior to or at the time of the distribution. See “Description of Material Indebtedness” and “Risk Factors—Risks Related to Solventum’s Business.” | ||||
9
| Who will be the distribution agent for the distribution and transfer agent and registrar for Solventum common stock? | The distribution agent, transfer agent and registrar for the Solventum common stock will be Equiniti. For questions relating to the transfer or mechanics of the stock distribution, you should contact Equiniti toll free at (888) 666-0140 or non-toll free at (651) 450-4064. | ||||
| Do I have appraisal rights in connection with the distribution? | No. Holders of 3M common stock are not entitled to appraisal rights in connection with the distribution. | ||||
| Where can I find more information about 3M and Solventum? | Before the distribution, if you have any questions relating to 3M’s business performance, you should contact: 3M Investor Relations Department Bldg. 224-1W-02 St. Paul, MN 55144-1000 Tel: (651) 737-6523 After the distribution, Solventum shareholders who have any questions relating to Solventum’s business performance should contact Solventum at: Solventum Corporation 3M Center, Building 275-6W 2510 Conway Avenue East Maplewood, MN 55144 Attention: Investor Relations The Solventum investor website (www.solventum.com) will be operational on or around April 1, 2024. The Solventum website and the information contained therein or connected thereto are not incorporated into this information statement or the registration statement of which this information statement forms a part, or in any other filings with, or any information furnished or submitted to, the SEC. | ||||
10
INFORMATION STATEMENT SUMMARY
The following is a summary of selected information discussed in this information statement. This summary may not contain all of the details concerning the separation or other information that may be important to you. To better understand the separation and Solventum’s business and financial position, you should carefully review this entire information statement. Except as otherwise indicated or unless the context otherwise requires, the information included in this information statement about Solventum assumes the completion of all of the transactions referred to in this information statement in connection with the separation and distribution. Unless the context otherwise requires, or when otherwise specified, references in this information statement to “Solventum,” “we,” “us,” “our,” “our company,” and “the Company” refer to Solventum Corporation, a Delaware corporation, and its subsidiaries. Unless the context otherwise requires, references in this information statement to “3M” refer to 3M Company, a Delaware corporation, and its consolidated subsidiaries, including the Health Care Business prior to completion of the separation.
Unless the context otherwise requires, or when otherwise specified, references in this information statement to Solventum’s historical assets, liabilities, products, businesses or activities of Solventum’s businesses are generally intended to refer to the historical assets, liabilities, products, businesses, or activities of the Health Care Business of 3M as it was conducted as part of 3M prior to completion of the separation.
Our Company
Solventum is a leading global healthcare company developing, manufacturing, and commercializing a broad portfolio of solutions that leverages deep material science, data science, and digital capabilities to address critical customer and patient needs. We constantly seek to enable the improvement of standards of care and move healthcare forward with innovation powered by insights, clinical intelligence, technology, and manufacturing expertise. Our 70+ year history of discovering and innovating advanced solutions has helped us solve our customers’ toughest challenges.
In 2023, Solventum generated 56% of total revenues from the United States and 44% from international. Based on the breadth of our portfolio, Solventum serves a global addressable market that we estimate had approximately $93 billion of industry sales in 2022. We estimate that this addressable market will grow at an annual rate of 4-6% from 2024 through 2026. We participate in what we believe are large, stable global markets that have favorable market drivers, including changing demographics, optimization of workflows to improve quality of care, increasing digital technology and data-driven care delivery, shifting care from the hospital to lower-cost care sites and increasing demand for personalized care. See the sub-section below titled “Our Markets” for information about how we estimated the size and growth rate of our addressable market, and the risks to our ability to take advantage of these market opportunities.
We are organized into four operating business segments that are aligned with the markets we serve.
•MedSurg (56% of 2023 total sales), formerly Medical Solutions, is a provider of solutions including advanced wound care, I.V. site management, sterilization assurance, temperature management, surgical supplies, stethoscopes, and medical electrodes. These solutions are designed to accelerate healing, prevent complications, and lower the total cost of care. Specifically, our advanced wound care solutions follow the patient from hospital to home and support them through the recovery process. We are a leader in the advanced wound care market based on the market share data presented in a BCC Research report (BCC Publishing, Markets for Advanced Wound Management Technologies, July 2023) and, based on internal estimates, our products currently treat more than 1.6 million hard-to-heal wounds annually. Additionally, our comprehensive range of surgical solutions are designed to mitigate a patient’s risk of infection or complications.
•Dental Solutions (16% of 2023 total sales), formerly Oral Care Solutions, is a provider of a comprehensive suite of dental and orthodontic products including brackets, aligners, restorative cements, and bonding agents that span the “life of the tooth,” including products designed for preventative dental care, direct and indirect restoration, and broad orthodontic needs. We have a leading position in the dental and orthodontic bonding systems market based on published market share data from Key-Stone Network
11
(Key-Stone Fast Track Clinical Report — Clinical Ranking, 2022), SDM Northcoast (SDM Dental Products Market Share Study, 2022) and Orthodontic Manufacturers Association (OMA Sales Survey by Association Research, Inc., 2022). Additionally, we estimate our 3M™ Filtek™ branded products have been used in over two billion dental restoration procedures worldwide over the last twenty years.
•Health Information Systems (16% of 2023 total sales) provides healthcare systems with software solutions – including computer-assisted physician documentation, direct-to-bill and coding automation, classification methodologies, speech recognition, and data visualization platforms – that are designed to eliminate revenue cycle waste, create more time for patient care, and support value-based care. These solutions are designed to ensure accuracy of reimbursement and reduce the administrative burden that clinicians face. We have a leading market position in the United States for computer-assisted coding technology based on published market share data from Definitive HealthCare (Definitive Healthcare, HospitalView Database, Technology Search for “Computer Assisted Coding/NLP” technology, 2022) and, based on our internal estimates, more than 75% of U.S. hospitals currently use at least one of our software solutions.
•Purification and Filtration (12% of 2023 total sales), formerly Separation and Purification Sciences, is a provider of purification and filtration technologies including filters, purifiers, cartridges, and membranes. These solutions are designed to simplify purification processes, reduce debris and bioburden in fluids, and remove contaminants to enable the development and manufacturing of biopharmaceutical and medical technology treatments and provide cleaner water. Based on internal estimates, our membrane technology is currently used annually in more than 25 million life-saving dialysis treatments and approximately one million open heart surgeries are currently performed each year using oxygenators that are enabled by one of our membranes.
For a list of products, by business segment, that are regulated by the U.S. Food and Drug Administration (“FDA”) as medical devices or pharmaceuticals, see the section below titled “Product Regulation”.
We believe Solventum is an integral part of the global healthcare ecosystem. Our solutions are relied on every day within the global healthcare industry, and we believe they contribute to higher-quality patient care, more efficient processes and workflows, and improved standards of safety and accuracy. Additionally, our products and services are present along a patient’s journey through prevention, diagnosis, treatment, and recovery.
12
Our business possesses strong customer relationships, a broad, wide-ranging, and well-known portfolio of brands, differentiated technology, and manufacturing expertise. We serve a diverse customer base, ranging from multidisciplinary hospitals and local clinics/practices to biopharmaceutical manufacturers. Our long-tenured and collaborative customer relationships globally give us unique insights into their needs and preferences. These insights inform our innovation processes, drive stronger customer retention, and create multiple avenues for further customer engagement.
We serve customers in over 90 countries with a global team of approximately 22,000 employees and an established global manufacturing network. In each of the last three years, we have generated over $8 billion of revenue, $1.7 billion of operating income, and $2 billion of adjusted operating income. We believe Solventum will deliver growth at attractive margins with the mission of enabling better, smarter, safer healthcare to improve lives.
However, Solventum has no history of operating as an independent company, and its historical and pro forma financial information is not necessarily representative of the results that it would have achieved as a separate, publicly traded company and may not be a reliable indicator of its future results. In particular, Solventum currently benefits from 3M’s long operating history, reputation and well-known brand. Following the separation, Solventum will operate under its own brand, and accordingly may be negatively impacted due to the loss of benefits conferred by 3M’s brand recognition and reputation. Additionally, the debt obligations incurred by Solventum in connection with the separation will adversely affect its profitability and could affect its ability to use its cash flow for investing in the business, M&A and returning capital. See “Risk Factors - Risks Related to the Separation and Distribution” for a discussion of these risks, which you should consider carefully.
Our Markets
We believe Solventum operates in a large, diverse, and stable set of markets. Sustainable, long-term growth in our markets is driven by favorable global market drivers, including changing demographics, optimizing workflows to improve quality of care, increasing digital technology and data-driven care delivery, shifting care from the hospital to lower cost care sites, and increasing demand for personalized care.
•Changing demographics: An aging population, the prevalence and incidence rates of chronic conditions, and a rising middle class are driving the demand for improved access to quality care.
•Optimizing workflows to improve quality of care: Of the $4.5 trillion in annual U.S. healthcare spending, an estimated 25% represents administrative costs that do not contribute to health outcomes and which we believe to be potentially wasteful based on overall spending data reported by the Centers for Medicare & Medicaid Services in the NHE Fact Sheet (available on CMS.gov as of January 10, 2024) and administrative spending estimates published in JAMA (Shrank et. al., Waste in the US Health Care System: Estimated Costs and Potential for Savings, published October 7, 2019). Our solutions are designed to optimize workflows, enabling clinicians to be more productive by spending less time on administrative tasks and more time focused on improving the patient care experience. Our solutions also support reducing infections and complications that lead to an increase in avoidable administrative and clinical costs.
•Increasing digital technology and data-driven care delivery: Both clinicians and patients have shifted their preferences towards utilizing digitally enabled solutions to provide data-driven care. Whether it is interactions with patients through a digital interface or the use of data to make informed health decisions, the need for digital tools in the healthcare industry has grown over time. Our solutions integrate digital processes and data in multiple ways and across different parts of the healthcare industry and are intended to enable efficient and effective delivery of care.
•Shifting care from the hospital to lower-cost care sites: Although hospitals continue to be a core site for delivery of care, patients are increasingly looking for flexibility of care when and where they need it. Alternative care sites, such as ambulatory surgery centers, wound care clinics, retail pharmacies, and the home, are more affordable and accessible to patients. We believe our solutions enable clinicians to extend their care delivery from acute to ambulatory to home settings without compromising the quality of care and while reducing the total cost of care.
13
•Increasing demand for personalized care: Engaging patients in a personalized way allows clinicians to provide a better care experience while improving outcomes and reducing costs. This spans several areas of healthcare, including personalized biopharmaceutical treatments, customized orthodontic aligner treatments, and follow-up wound care at home. We believe our solutions deliver personalized care options in a way that is patient-centric, scalable, and cost-effective.
Our ability to take advantage of these market opportunities will be subject to various risks, including general economic, business and market dynamic risks, and specifically including effects of, and changes in, worldwide economic, political, regulatory, international trade and geopolitical conditions, natural disasters, wars and public health crises; operational execution risks; the highly competitive environment in which we operate; consolidation in the healthcare industry; reductions in customers’ research budgets or government funding; risks related to the timing and market acceptance of our new products and offerings; changes in reimbursement practices of third-party payers or other cost containment measures; vulnerability with respect to materials and fluctuations in the costs and availability of purchased components, compounds, raw materials, energy, and labor, the impact of our separation from 3M; and the substantial debt we will incur in connection with the separation. See “Information Statement Summary—Summary of Risk Factors”, “Information Statement Summary—Certain Risks Relating to Operating as a Standalone Entity”, “Information Statement Summary—Certain Risks Relating to Solventum’s Indebtedness”, and “Risk Factors” for a discussion of these risks, which you should consider carefully.
We estimate that the broader markets that Solventum participates in represented more than $205 billion of industry sales in the total global healthcare sector in 2022, based on the reports described below. Based on management estimates, we believe these markets in aggregate will grow at an annual rate of 4-6% from 2024 through 2026 (representing a weighted average (based on the relative market size) of the relevant management estimates).
Specifically, based on the summation described below, we estimated the size of the global addressable market in which we operate to have been approximately $93 billion in 2022, and based on management estimates, we believe this global addressable market is projected to grow at an annual rate of 4-6% from 2024 through 2026 (representing a weighted average (based on the relative market size) of the relevant management estimates for this global addressable market). Based on the recent performance of our business, we believe there is a significant market opportunity for future growth as we execute on our strategies.
14
| Segment | Addressable Market Size in 2022 | Addressable Market Annual Growth Rate (2024-2026) | ||||||
| MedSurg | ~$26 billion | 3-5% | ||||||
| Dental Solutions | ~$17 billion | 4-6% | ||||||
| Health Information Systems | ~$9 billion | 6-8% | ||||||
| Purification and Filtration | ~$41 billion | 4-6% | ||||||
| Total Solventum | ~$93 billion | 4-6% | ||||||
MedSurg Sources: Market size defined as the sum of total industry sales in advanced wound care (source: SmartTrak Advanced Wound Care Market Database, 2023), IV site management (sources: BCC Publishing Staff, Global Blood Transfusion and Intravenous Equipment Market Report, May 2022; BCC Publishing Staff, Advanced Medical Dressings: Global Market, July 2021), hospital supplies (source: BCC Research Staff, Hospital Supplies: Global Markets to 2023, February 2019), medical electrodes (source: Allied Market Research, Medical Electrodes Market: Global Opportunity Analysis and Industry Forecast, 2021-2031, May 2022), medical tapes and bandages (source: Grand View Research, Medical Tapes and Bandages: Market Analysis 2016-2027, 2020), skin antiseptics (source: Allied Market Research, Skin Antiseptic Market: Global Opportunity Analysis and Industry Forecast 2021-2031, March 2023), temperature management systems (source: Markets and Markets, Temperature Management Systems Market: Global Forecast to 2026, 2021), and surgical drapes and gowns (source: Grand View Research, Surgical Drapes: Market Analysis 2018-2030, 2021). Dental Solutions Sources: Market size defined as the sum of total industry sales in dental consumables and dental equipment; both sourced from Markets and Markets reports (Markets and Markets, Dental Consumables Market: Global Forecast to 2027, 2022; Markets and Markets, Dental Equipment Market: Global Forecast to 2027, 2023). Health Information Systems Sources: Market size defined as the total sum of industry sales in revenue cycle management (source: Markets and Markets, Revenue Cycle Management: Global Forecast to 2028, 2023), medical transcription (source: Fortune Business Insights, Medical Transcription Software: Global Market Analysis Insights and Forecast 2020-2026, 2020), and population health management (source: Frost & Sullivan, US Population Health Management Growth Opportunities, February 2022). Purification & Filtration Sources: Market size defined as the sum of total industry sales in industrial filtration (source: Markets and Markets, Industrial Filtration Global Forecast to 2027, 2022), separation membrane contractors (source: Markets and Markets, Membrane Contractor Market Global Forecast to 2025, 2020), residential water treatment systems (source: Baytel Associates, The Global Market for Home Water Treatment Products and Services, 2021 Edition), commercial water purifiers (source: Azoth Analytics Research, Global Commercial Water Purifier Market, June 2022 Edition), medical and industrial membranes (source: Markets and Markets, Medical Membranes Market: Global Forecast to 2022, May 2018; Markets and Markets, Membranes Market: Global Forecast to 2027, October 2022), and biopharmaceutical purification devices and equipment (source: Freedonia Custom Research, Global Biopharmaceutical Purification Device/Equipment Market Study, October 2021). For each segment, Solventum calculated the addressable market growth rate by estimating the growth rate for the relevant market size for the referenced components of such segment (based on the described reports, historical change, and internal forecasts and estimates) and computing a weighted average (based on the relative market size) of these growth rates.
In addition to the global market drivers described above, each of our segments benefits from segment-specific market drivers as outlined below:
•MedSurg: Growth in the MedSurg market is driven by increasing surgical procedure volumes and incidence rates of chronic wounds, shifting of care to out-of-hospital settings, and the increasing prevalence of digitally enabled solutions. Within MedSurg, a priority market is Advanced Wound Care, where we expect to see sustainable growth.
•Dental Solutions: Growth in the Dental Solutions market is driven by increasing oral care procedure volumes, evolving patient standards of preventative care, and shifting patient preferences that emphasize aesthetics. Furthermore, the changing industry service economics is enabled by innovation and growth of digital workflows to create custom solution offerings for all. For Dental Solutions, a priority is the
15
digitization across the market, as providers continue to adopt digital solutions to reduce chair time and improve patient outcomes.
•Health Information Systems: Growth in the Health Information Systems market is driven by hospital spending on information technology, increasing scrutiny of revenue leakage and healthcare information technology return on investment, care delivery shifting to lower-cost settings, digital technology driving healthcare efficiency, and a broad shift to value-based care. For Health Information Systems, a priority is the growing demand for conversational AI and ambient solutions to improve clinician productivity and reduce their administrative burden.
•Purification and Filtration: Growth in the Purification and Filtration market is driven by increasing biopharma innovation, expanding use of new modalities focused on personalized medicine, such as targeted antibodies and cell and gene therapies, growing efforts to reduce bioprocessing complexity, growing sustainability needs including water quality and preservation, and an increasingly complex global regulatory environment. For Purification and Filtration, a priority market is Bioprocessing Filtration.
The estimates of market opportunity and market growth may prove to be inaccurate, and even if the markets in which we operate achieve the expected growth, our business (or the applicable segments of our business) could fail to grow at similar rates, or at all. Our growth is subject to many factors, including our success in implementing our business strategy, which is subject to many risks and uncertainties. For the fiscal year ended 2023, sales in our Purification and Filtration segment declined relative to the fiscal year 2022. For the fiscal year ended 2022, sales in our MedSurg and Dental Solutions segments declined relative to the fiscal year ended 2021. For a further discussion of the historical performance of our segments, together with an overview of the key factors driving such performance, see the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Performance by Business Segment”.
Investment Highlights
Solventum has numerous competitive advantages in attractive markets that we expect to continue to drive our success over the long term, including those described below. We believe that our innovation expertise, digital capabilities and data science business model, global scale and manufacturing expertise, and cash flow generation capability position us for continued growth and value creation. Additionally, we are building new capabilities, including an incoming experienced executive team, that will support value creation as we become a standalone company.
Although we believe that these competitive strengths will contribute to the growth and success of our company, our business is subject to various risks that may prevent us from achieving our business objectives or otherwise adversely affect our business, results of operations or financial condition. In particular, following the distribution and separation, Solventum will be an independent company and will no longer have access to the competitive advantages that it has historically derived from being a part of 3M, such as 3M’s research capabilities, brand recognition and reputation. Additionally, the debt obligations incurred by Solventum in connection with the separation will adversely affect its profitability and could affect its ability to use its cash flow for investing in the business, M&A and returning capital. See “Information Statement Summary—Summary of Risk Factors”, “Information Statement Summary—Certain Risks Relating to Operating as a Standalone Entity”, “Information Statement Summary—Certain Risks Relating to Solventum’s Indebtedness”, and “Risk Factors” for a discussion of these risks, which you should consider carefully.
A Proven Global Leader in Large, Diverse and Growing Markets
•We believe Solventum operates in a global addressable market that we estimated to have been $93 billion in 2022 and we believe, based on management estimates, is projected to grow at an annual rate of 4-6% from 2024 through 2026. We expect there will be sustainable, long-term growth in our global addressable market because of favorable market drivers, including changing demographics, the increasing need to optimize workflows, deliver digitally enabled and data-driven care, shift to lower-cost alternative care sites and the increasing demand to provide personalized care. For further discussion about these factors, including estimated growth rates and factors by segment, see the sub-section titled “Markets” above. For a
16
discussion of the historical performance of our segments, together with an overview of the key factors driving such performance, see the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Performance by Business Segment”.
•We provide over 100,000 channel partners and customers across more than 90 countries with solutions that have contributed to leading market positions across our segments.
◦MedSurg segment: We are a leader in the advanced wound care market based on the market share data presented in a BCC Research report (BCC Publishing, Markets for Advanced Wound Management Technologies, July 2023) and, based on internal estimates, our products currently treat more than 1.6 million hard-to-heal wounds annually.
◦Dental Solutions segment: We have a leading position in the dental and orthodontic bonding systems market based on published market share data from Key-Stone Network (Key-Stone Fast Track Clinical Report — Clinical Ranking, 2022), SDM Northcoast (SDM Dental Products Market Share Study, 2022) and Orthodontic Manufacturers Association (OMA Sales Survey by Association Research, Inc., 2022). Additionally, we estimate our 3M™ Filtek™ branded products have been used in over two billion dental restoration procedures worldwide over the last twenty years.
◦Health Information Systems segment: We have a leading market position in the United States for computer-assisted coding technology based on published market share data from Definitive HealthCare (Definitive Healthcare, HospitalView Database, Technology Search for “Computer Assisted Coding/NLP” technology, 2022) and, based on our internal estimates, more than 75% of U.S. hospitals currently use at least one of our software solutions.
•Our ability to take advantage of these market opportunities is subject to various general economic, business and market dynamic risks. Our estimates of market opportunity and market growth may prove to be inaccurate, and even if the markets in which we operate achieve the expected growth, our business could fail to grow at similar rates, or at all.
Diverse Portfolio of Strong, Reputable Brands and Long-Standing Customer Relationships
•We believe Solventum is an integral part of the global healthcare ecosystem. Our solutions are relied on every day within the global healthcare industry, and we believe they contribute to higher-quality patient care, more efficient processes and workflows, and improved standards of safety and accuracy. Furthermore, the breadth and diversity of our portfolio enables us to have products and services present along a patient’s journey through prevention, diagnosis, treatment, and recovery.
•Across our businesses, we are a provider of what we believe are market-leading brands, such as 3M™ PREVENA™, 3M™ V.A.C.®, 3M™ Tegaderm™, 3M™ Littmann®, 3M™ Filtek™, 3M™ 360 Encompass™, and 3M™ Membrana™. Many of these brands carry a history of innovation and industry recognition, and today, we believe, are seen as among the best in class. Additionally, we believe our brand value is supported by strong economic evidence and the volume of published studies reviewing our brands.
•We attribute our strong market position and customer loyalty to the value of our brands. Globally, we sell our solutions to more than 100,000 channel partner and customers. We also have a long-standing history of partnering with national and local government agencies around the world.
•Our brands are critical to our success, and damage to our reputation or our brands could adversely affect our business, results of operations or financial condition. Solventum currently benefits from 3M’s long operating history, reputation, and well-known brand. Following the separation, Solventum will operate under its own brand, and accordingly may be negatively impacted due to the loss of benefits conferred by 3M’s brand recognition and reputation.
17
Technology Platforms and Expertise Powering Innovation
•For approximately 70 years, we have been innovating across the healthcare industry by leveraging our deep material science and process capabilities in areas such as adhesives, films, nonwovens, nanotechnology, advanced composites, biomaterials, surface filtration and separation membranes. By combining these technology platforms, we believe we have been successful at solving our customers’ problems with novel solutions where we have been the first-to-market and market disruptors.
◦MedSurg segment: We were first-to-market with Negative Pressure Wound Therapy (NPWT). 3M™ PREVENA™ Incision Management System is a negative pressure medical device intended to aid in the reduction of superficial surgical site infections (SSIs) for patients at a high risk for post-operative infections. It is the first and only disposable, powered NPWT system with reticulated open-cell foam (ROCF) dressings specifically designed to manage closed surgical incisions.
◦MedSurg segment: We were first-to-market with antimicrobial transparent film dressings. 3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Securement Dressing is an antimicrobial transparent film dressing that is designed to combine infection reduction, site visibility, consistent application, and catheter securement into one integrated product. It is the only transparent CHG dressing cleared by the Food and Drug Administration (FDA) and is designed to reduce vascular catheter colonization and catheter-related blood stream infections (CRBSI) in patients with central venous or arterial catheters.
◦Dental Solutions segment: We were first-to-market with tooth-colored dental composites used in the restoration process. 3M™ Filtek™ Matrix is a digital restorative workflow solution that is designed to simplify a traditional composite placement to a three-step process, with the goal of increasing procedure predictability for dentists and reducing chair time and improving aesthetics for the patient.
◦Health Information Systems segment: We were first-to-market with integrated computer-assisted coding and clinical documentation improvement. 3M™ M*Modal Fluency Direct combines proprietary speech recognition with natural language understanding with the goal of providing more accurate clinical documentation. This solution is compatible with more than 250 Electronic Health Record (EHR) Systems and has received a Best in KLAS Award for Speech Recognition: Front-End EMR.
◦Purification and Filtration segment: We were first-to-market with hybrid chromatographic clarification solutions. 3M™ Harvest RC is a new solution for the manufacturing of recombinant protein therapeutics that employs next-generation hybrid chromatographic technology to combine three processing steps into one.
•We plan to continue to bring novel, disruptive solutions to the healthcare industry by combining and leveraging elements of our broad material science, data science, and digital capabilities across our company. These capabilities are supported by proprietary intellectual property with over 7,300 patents issued globally and industry expertise from more than 2,100 members of our global R&D team.
•Our ability to deliver these novel, disruptive solutions depends on protecting our intellectual property rights. We cannot assure you that our means of obtaining, maintaining, and enforcing our intellectual property rights will be adequate to maintain a competitive advantage. New product and services development requires significant investment in research and development, clinical trials and regulatory approvals. The ability to bring new products and services to market is subject to difficulties or delays in product and service development, such as the inability to identify viable new products and services, obtain adequate intellectual property protection, regulatory approvals and reimbursement in the United States and abroad, and successfully complete clinical trials or gain market acceptance of new products and services. It is uncertain when or whether our products, services, or solutions currently under development will be launched or will be commercially successful. Additionally, new offerings may be quickly rendered obsolete by changing customer preferences, changing industry standards, or competitors’ innovations or reverse engineering efforts.
18
Leading Digital and Data Science Capabilities and Business Models
•We have added significant data science and digital capabilities through organic innovation and over a dozen acquisitions. We believe we are uniquely positioned to serve our customers in an increasingly digitizing healthcare landscape given the investments we have made and the expertise we have gained in our Health Information Systems segment.
•We believe our medical coding software is among the most widely used in the world with many health systems relying on 3M™ 360 Encompass™ to process their healthcare records each month. Furthermore, our 3M™ M*Modal speech solutions include innovative features designed to support clinical productivity.
•We have a deep understanding of the capabilities, resources, and management associated with operating and optimizing a software business. Our expertise encompasses the sales and marketing processes, subscription-based revenue model management, and software implementation and upgrade processes that are unique to a digital business. Our customer relationships are long tenured, diverse, and collaborative, and many of our customers have been with us longer than 30 years.
•As the healthcare industry increasingly looks to utilize digital and data-driven tools to increase efficiency and improve health outcomes, our expertise and track record in this space gives us a strong foundation to expand our capabilities and appropriate use of data to solve the industry’s toughest problems.
•The healthcare information technology (HCIT) industry is highly competitive and dynamic and characterized by the continual introduction of new products and technologies. Our ability to compete will be affected by various factors, including development of new products and innovative technologies; our ability to improve our existing portfolio of offerings; our ability to deliver return on investment for our customers; improving efficiency and productivity of healthcare workers; our ability to stay up-to-date on regulatory changes, reimbursement guidelines and other healthcare best practices; developing and selling our products cost effectively; meeting all relevant quality standards for our products and their markets; and protecting the proprietary technology of our products and development processes. Failure to accomplish these objectives may adversely affect our business, results of operations or financial condition.
Global Scale and Reach Supported by Strong Manufacturing Expertise
•We have an extensive global commercial footprint with customers in over 90 countries. In 2023, Solventum generated 56% of total revenues from the United States and 44% from international. To serve our diverse customer base across our prioritized geographies, we take a multi-model commercial approach, including direct-to-customer, distribution, key account management, inside sales, and e-commerce.
•This global commercial footprint is supported by a strong network of manufacturing expertise, which is a core driver of our ability to deliver high-quality and innovative solutions in a cost-efficient manner at scale. Our expertise areas include precision coating, inspecting films and nonwovens, specialty film and polymer processing and additive manufacturing. We have invested in smart, vertically integrated manufacturing capabilities powered by automation and data analytics.
•Across our global manufacturing network, we utilize an analytics-enabled framework to drive decision-making, manage costs, and optimize production. We believe that our global footprint and expertise drive our ability to produce better, smarter, and safer products at a consistent quality level.
•Our financial results depend on the successful execution of our business operating plans. The ability to adapt Solventum’s business model and respond to changes, including responding to evolving customer needs and service expectations, are important. Operational challenges, including those related to customer service, pace of change and productivity improvements, could have an adverse effect on our business.
19
Cash Flow Generation and Attractive Margins
•For each of the last three years, we have generated over $1.6 billion of cash from operating activities and over $1.4 billion of free cash flow. We have delivered greater than 20% operating income margin and greater than 25% adjusted operating income margin for each of the last three years.
•Given our cash generation capability, we believe we will be able to reduce leverage; reinvest in our business; accelerate growth through bolt-on M&A; and consider return of capital to shareholders.
•However, Solventum has no history of operating as an independent company, and its historical and pro forma financial information is not necessarily representative of the results that it would have achieved as a separate, publicly traded company and may not be a reliable indicator of its future results. The debt obligations incurred by Solventum in connection with the separation will adversely affect its profitability and could affect its ability to use its cash flow for investing in the business, M&A and returning capital.
Highly Engaged Workforce Led by an Experienced Executive Team
•Our executive team has extensive experience across the healthcare industry and will be invaluable as we embark on establishing Solventum as a standalone healthcare company. Many of these executive officers come to Solventum with significant public company and spin-off experience. Already, the new executive team is building a more focused, nimble, and empowered healthcare culture within Solventum.
•We have a long-tenured and diverse talent base with rich technical qualifications and deep healthcare industry experience. Our employee base consists of approximately 22,000 employees with over 40% having more than 10 years of tenure at the Health Care Business. Our strong culture of collaboration and partnership and focus on employee engagement programs enable us to create an integrated, equitable organization and are key drivers of employees spending a significant part of their career at Solventum.
•We believe our employees’ deep relationships with customers and expertise for developing and manufacturing our innovative solutions better helps us solve the industry’s toughest problems of today and tomorrow. We plan to continue to build on this momentum as a mission-driven workforce and make Solventum a preferred employer.
•There is substantial competition for key personnel, senior management, research and development personnel, and qualified employees in the healthcare industry and we may face increased competition for such a highly qualified scientific, technical, clinical, and management workforce in a highly competitive environment. The loss of one or more key employees, inability to attract or develop additional qualified employees, any delay in hiring key personnel, any deterioration of the relationships with its employees, or any material work stoppage, strike, or similar action could result in a material adverse effect on our business, results of operations, financial condition, and cash flows.
Business Strategies
Our business strategies include those set forth below. Our ability to implement these strategies and achieve the intended benefits are subject to numerous economic and business risks, as well as risks related to the fact that following the separation and distribution, we will operate as a standalone entity and will not be able to receive many of the benefits that we have historically received by operating as a part of 3M and that we will incur significant debt obligation in connection with the separation, which will adversely affect our profitability and could affect our ability to use our cash flow for investing in the business, M&A and returning capital. See “Information Statement Summary—Summary of Risk Factors”, “Information Statement Summary—Certain Risks Relating to Operating as a Standalone Entity”, “Information Statement Summary—Certain Risks Relating to Solventum’s Indebtedness”, and “Risk Factors” for a discussion of these risks, which you should consider carefully.
As we focus on our business strategy and setting Solventum up for success, we will take a measured and phased approach. We will start by embedding our new mission and culture throughout the business, bringing in new talent and capabilities, addressing execution challenges, and executing on our separation plan. Next, we will prioritize our
20
growth strategy, ensuring we have proper focus and resourcing in the right areas. And finally, we will look at opportunities further down the road to transform our portfolio.
To deliver on our financial commitments, we will focus on driving revenue growth through market selection and deploying capital to innovation, commercial execution, and M&A. Furthermore, we will continue to build on our capability to drive margin expansion and generate cash.
Invest in Markets with Highest Growth Potential
•We will be thoughtful about the markets we prioritize for resource allocation. We believe there is significant opportunity both within the markets we serve today and in strategic high-growth adjacent markets. As a part of our active portfolio management, we plan to focus our efforts on markets where we can both fulfill our mission to enable better, smarter, safer healthcare to improve lives and increase adoption of our innovative solutions to build on many of the leading market positions we have today. As we look to increase our presence in select high-growth markets where we operate, we believe we can leverage our geographic scale and innovation capabilities.
Continue to Deliver Customer-Centric Innovation
•We have a strong culture of purposeful innovation focused on solving our customers’ most critical problems. Our innovation engine has powered multiple key product launches across many years, including our original launch of surgical drapes in 1948, the 1964 launch of Addent™, the first tooth-colored filling material, and the 1982 launch of 3M™ Tegaderm™ transparent film dressings.
•From 2021 to 2023, we invested a cumulative $1.8 billion in R&D (excluding amortization), representing over 7% of sales in each year to power our innovation efforts. As a standalone company, we will have more flexibility to allocate resources to prioritize R&D investments.
•Our innovation will focus on key areas that enable us to not only leverage our existing capabilities and strengths, but also have a high impact in the markets we serve, including:
◦Setting new industry innovation standards: We have, in our view, launched multiple solutions that represented significant industry innovations when they were introduced, and set a new standard for healthcare. An example of this innovation is 3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Securement Dressing – the only transparent film dressing cleared by the FDA and designed to reduce catheter-related blood stream infections (CRBSIs) and vascular catheter colonization that aligns with evidence-based guidelines. These types of shifts are an area of focus for our ongoing innovation efforts.
◦Continuing to leverage our ability to share technology across our platforms to drive unique, differentiated solutions: We believe we have the unique capability to combine our deep heritage of material science innovation and existing digital and data science platforms to create unique solutions to solve our customers’ biggest challenges. In our ongoing innovation efforts, we plan to continue to harness these capabilities, while enhancing our focus to draw upon additional technology combinations across our portfolio of solutions.
◦Leverage data science expertise to increase digitization of healthcare: We believe our Health Information Systems segment puts us at the center of the digitization of the healthcare industry. We plan to use our knowledge and expertise from operating this business to increase our digital and analytics capabilities across our business. As the healthcare industry increasingly looks to utilize digital and data-driven tools to increase efficiency and improve health outcomes, our expertise and track record within the Health Information Systems segment gives us a strong foundation to expand our capabilities and use of data to solve the industry’s toughest problems.
21
Strengthen Commercial Model and Execution
•We plan to increase sales growth by growing our market share within our leading franchises through multiple strategies, including:
◦Expanding into alternative sites of care: Our current customers include hospitals and health systems, with whom we have long-lasting and collaborative relationships. As care increasingly shifts outside of traditional settings, we believe we have an opportunity to grow our customer relationships in these alternate sites of care and expand our market reach.
◦Evolving our commercial model to meet local expectations: As the need for high-quality healthcare solutions expands globally, we believe we are well-positioned to capitalize on our existing global footprint and expand our presence in key countries to broaden our customer reach. Our expansion strategy is designed to understand the specific needs of priority countries by tailoring solutions to focus on local and regional outcome measures. We plan to gather key insights and maintain relationships with regulatory authorities and clinical groups that inform our tailored commercial approach.
◦Increasing customer loyalty through our evidence-based approach: Our existing customer relationships are strong, partnership-oriented, and long-tenured. By integrating clinical evidence and customer education more deeply into our solution positioning with customers, we have an opportunity to strengthen our customer relationships and ground them in data, research, and best practices. To do this, we will rely on our relationships with the global clinical community and regulatory authorities, with whom we need to raise awareness of the evidence supporting our solutions.
Accelerate Growth Through Strategic M&A and Partnerships
•A key part of our strategy as a standalone company is to utilize targeted, strategic M&A and partnerships to augment our organic innovation and grow our healthcare solution offerings. As a standalone company, we will have the ability to more efficiently allocate resources to pursue these opportunities.
•Inorganic growth represents an opportunity for us to increase our innovation solutions and scale within our most attractive markets and submarkets, while also providing a vector into near adjacencies and strategic new white spaces over time. We will continue to seek attractive M&A opportunities that focus on differentiated, clinician-preferred solutions.
•We will also continue to seek opportunities to partner with institutions and companies to augment growth. A recent example is our ongoing collaboration with Amazon Web Services (“AWS”) to accelerate the innovation and advancement of 3M™ M*Modal ambient intelligence. We will look to continue to identify partnership opportunities that align with and accelerate our strategic goals.
Drive Margin Expansion and Free Cash Flow Generation
•Our continuous improvement focus and capabilities will enable us to improve our operating model as we become a stand-alone company. Furthermore, by focusing on improving the mix of our products, implementing manufacturing and supply chain efficiency initiatives and increasing commercial productivity, we will be able to drive future margins and cash flow. We expect to be able to reduce leverage; reinvest in our business; accelerate growth through bolt-on M&A; and consider return of capital to shareholders.
•However, the debt obligations incurred by Solventum in connection with the separation will adversely affect its profitability and could affect its ability to use its cash flow for investing in the business, M&A and returning capital.
22
Our Segments
MedSurg
Our MedSurg segment delivers a broad range of innovative solutions, including advanced wound care and surgical supplies that are intended to accelerate healing, prevent complications, and lower the total cost of care globally. Our solutions draw upon the deep heritage of material science and the technologies we have developed over decades, including adhesives, biomaterials, films, nonwovens, and specialty materials.
We are a pioneer across multiple markets, and we believe our innovative products have set the standard of care for healthcare professionals. In our view, uptake of our products is supported by patient outcomes, improved ease of use, and streamlined clinician and patient experience. We also have specialized personnel who work with clinicians and hospital staff to provide technical support and education.
Our global solutions are offered through our brands across:
•Advanced Wound Care Solutions: We are one of the leading providers of advanced wound care solutions in the world based on the market share data presented in a BCC Research report (BCC Publishing, Markets for Advanced Wound Management Technologies, July 2023). Our solutions are intended to deliver predictable and improved patient outcomes, streamline clinician and patient experiences, and enable faster healing. Our broad range of solutions can be used across clinical applications, care settings, and clinician specialties. We are focused on accelerating wound healing, reducing postoperative infections and complications, and lowering the total cost of care through Negative Pressure Wound Therapy (NPWT), Advanced Wound Dressings (AWD), and Advanced Skin Care products. A selection of our key brands and solutions include:
◦3M™ V.A.C.® Therapy, for chronic or acute open wounds
◦3M™ Prevena™ for surgical incisions, and as of 2023, received FDA approval for use on flaps, grafts, and open wounds
•Infection Prevention and Surgical Solutions: We offer solutions that are intended to address patient and staff safety by reducing preventable infections and complications while improving the efficiency and effectiveness of care. Our product range includes I.V. site management, temperature management, sterilization assurance, and other surgical solutions, as well as medical tapes and wraps, medical electrodes, and our well-known 3M™ Littmann® branded stethoscopes. Other key brands and solutions include:
◦3M™ Tegaderm™ I.V. dressings for I.V. site management
◦3M™ Bair Hugger™ solutions for perioperative temperature management
◦3M™ Attest™ solutions for sterilization assurance
We sell our products both direct and through distributors to hospitals for use in operating rooms, inpatient care units, and central sterilization, as well as the out-of-hospital setting, including ambulatory surgical centers, skilled nursing facilities, long-term care facilities, and patient homes.
Dental Solutions
Our Dental Solutions segment provides a comprehensive array of dental and orthodontic products that span the life of the tooth, and are intended to address clinical needs in prevention, restoration, replacement, and malocclusion correction. We believe the combination of our material science innovation and our dental market domain knowledge allow us to improve the efficiency and quality of treatment delivery.
Our solutions are used by most dental offices. We are responsible for several category inventions within the dental market, including the introduction of the first-ever tooth-colored composites.
23
Given our growing data science and digital capabilities, we believe we are well-positioned to take advantage of the movement toward digitization. We have launched solutions that combine our advanced material science expertise with data science and digital technologies to improve the workflows and processes associated with dental and orthodontic treatments. We are actively building our “custom smiles” solutions, which uses digital capabilities to enable our dental and orthodontic provider partners to deliver customized esthetic and restorative treatments to their patients.
Our solutions are designed to improve both the patient and practitioner experience, and offer differentiated value through our science, education, and service. We believe our brands, including 3M™ Filtek™, 3M™ Clinpro™, 3M™ Scotchbond™, and 3M™ Clarity™, are recognized in the global dental market. Our solutions serve both channel and end customers and include larger dental organizations, smaller practitioner groups, sole practitioner clinics and distributors.
Health Information Systems
Our Health Information Systems segment delivers a broad array of innovative software solutions and services that are designed to eliminate revenue cycle waste, create more time to care, and support the shift to value-based care. Our solutions operate across multiple areas of the healthcare system and reflect our deep and diverse knowledge of different aspects of healthcare.
Since this business began more than 35 years ago, we have developed a rich and unique understanding of the healthcare data landscape. We believe we are now an integral part of the day-to-day operations of the healthcare industry and we will be a key company in solving many of its largest future challenges. Based on internal estimates, many health systems worldwide — including over 75% of U.S. hospitals — use at least one of our software solutions. This includes our artificial intelligence (AI)-based clinical intelligence engine, which applies natural language processing (NLP) and natural language understanding (NLU) technology to significant amounts of structured and unstructured clinical documents each day. We have worked with federal government agencies, such as Centers for Medicare & Medicaid Services (CMS) to develop and update both public and proprietary algorithms, benchmarks, and classification systems. With many active industry and technology partners, including working with all the major electronic health record (EHR) companies, our solutions are present throughout the healthcare ecosystem.
Our solutions are typically delivered to our customers as an integrated workflow or set of workflows that involve both digital software-based solutions and related services. We have dedicated adoption specialists that manage physician experiences, and we often deploy client success managers that are physically “embedded” with our customers following the sale of a solution to ensure that their experience using our solutions is successful. These efforts result in uniquely collaborative customer relationships that drive a more robust customer experience and provide us with useful insights to inform our operations.
Our solutions, which include brands such as 3M™ 360™ Encompass and 3M™ M*Modal, are offered to customers across three key areas:
•Revenue Cycle Management: Our solutions are focused on eliminating unnecessary administrative inefficiencies in the health care reimbursement process and ensuring accurate and compliant reimbursement.
•Clinician Productivity: Our solutions are designed to reduce the administrative burden of clinical documentation and empower clinicians in all settings to easily document their full patient story.
•Performance Management: Our solutions and services help payers and provider organizations sort through data to find efficiencies and prioritize sustainable improvements.
Our solutions are utilized by a variety of customers across inpatient, outpatient, and ambulatory settings as well as payers and government agencies. Our representative channel and end customers span a broad range and include large healthcare providers, regional health systems, payers, and other third-party HCIT solution providers.
24
Purification and Filtration
Our Purification and Filtration segment is a provider of purification and filtration solutions that are designed and marketed for use in the manufacturing processes of biopharma and medical technologies, such as cell and gene therapies, vaccines, and hemodialysis, as well as in the manufacturing of microelectronics, food and beverage products, and water filtration for commercial and residential applications. We are dedicated to advancing membrane science to enable life-saving treatments and cleaner water by leveraging our technical expertise in functionalizing membranes and deep expertise in process filtration.
Every year, we estimate that our products touch the lives of millions of people. Based on internal estimates, each year, approximately one million open heart surgeries are currently performed using oxygenators that are enabled by one of our membranes, and currently more than 25 million life-saving dialysis treatments are enabled annually with one of our membranes.
Our products are offered globally under a wide variety of well-known brands, including 3M™ Zeta Plus™, 3M™ Aqua-Pure™, 3M™ Liqui-Cel™, 3M™ PUREMA™, 3M™ OXYPHAN™ and 3M™ OXYPLUS™. We sell our products directly to biopharmaceutical manufacturers, medical technology companies, integrators of manufacturing plants and water treatment systems, and through a broad range of distributors.
Research and Development Activities
Our R&D activities are focused on developing new solutions that are clinically supported and differentiated as well as improving on our marketed solutions to address evolving customer needs and enable better outcomes and access for patients. Our R&D capabilities include R&D organizations that operate within each of our business segments, as well as R&D capabilities spanning across our business segments.
Our business segment R&D organizations are responsible for the full product development life cycle, leveraging industry insights, domain-specific expertise in end-to-end product development, and a detailed understanding of customer applications and usability to innovate in both new and marketed products. Our cross-segment capabilities include building new technology platforms and advancing existing platforms. We believe that collaboration across our organization further enhances our R&D capabilities by encouraging the sharing of best practices, enabling collaborative development and issue resolution, promoting synergies in development and manufacturing, and creating a broad culture of exploration.
In 2023, our R&D team consisted of more than 2,100 employees, including research scientists, chemical engineers, data scientists, software engineers, application development engineers and product developers. They are supported by a team of accomplished clinicians from our medical affairs group. We partner with our medical affairs group to expand awareness of clinical studies regarding our solutions by increasing both the number of peer-reviewed publications and the visibility for existing publications that address our solutions.
Sales and Marketing
To serve our diverse customer base across our prioritized geographies, we take a multi-model commercial approach, including direct-to-customer, distribution, key account management, inside sales, and e-commerce. We augment our commercial model with both marketing and service support. Our service support teams include clinical specialists (licensed nurses or technicians), medical liaisons (clinical professionals such as surgeons and dentists), and application engineers (technical subject matter experts). These teams provide high-quality customer support serving as the clinical and/or technical expert for the customer.
To expand our market coverage into emerging geographies in the Latin America, Europe, Middle East, Africa, and Asia regions, we employ an export commercial model and leverage local partners to market and sell our products. Our export commercial team provides technical, clinical, and marketing support both directly to our customers, as well as to our third-party partners, to help increase customer satisfaction and support our ability to grow our global presence.
25
Global Supply Chain and Sourcing
We believe we have advanced manufacturing and assembly production capabilities across our global manufacturing network. Our manufacturing is supported by a global distribution network. Our distribution network is strategically designed as a “hub and spoke” model. This approach optimizes route planning and increases the speed of deliveries to our customers in all regions.
Product Regulation
The products Solventum develops, manufactures, and commercializes are regulated in most of the markets Solventum serves. Some of these products meet the definition of medical devices or pharmaceuticals and are regulated, as such, by various governmental bodies, globally. All products produced by the MedSurg and Dental Solutions segments, with very few exceptions, meet the definition of a medical device or pharmaceutical product. Accordingly, the development, manufacture, and commercialization of these products must comply with the regulations governing medical devices or pharmaceuticals in the markets we serve. Conversely, none of the products produced by the Health Information Systems and Purification and Filtration segments meet the definition of medical devices or pharmaceuticals. The products of each segment are dynamic and change with time, depending on the needs of the customers served. While the Health Information Systems and Purification and Filtration segments do not currently include medical devices or pharmaceutical products, this may change in the future.
The below table lists, by business segment, the products or product families named in this information statement that are regulated by the FDA as medical devices or pharmaceuticals.
Determinations of the safety and efficacy of our products are solely within the authority of the FDA or other applicable regulatory authorities in jurisdictions outside the United States.
26
| Business Segment | Products Regulated as Medical Devices | Products Regulated as Pharmaceuticals | ||||||||||||
MedSurg | 3M™ Littmann® family of products 3M™ Tegaderm™ family of products 3M™ V.A.C.®, 3M™ Veraflo™ and 3M™ PREVENA™ family of products 3M™ AbThera™ Therapies 3M™ Attest™ solutions 3M™ Granufoam™ 3M™ Cavilon™ No Sting Barrier Film 3M™ Prevena Restor™ BellaForm™ Incision Management System 3M™ Bair Hugger™ solutions 3M™ Littmann® stethoscopes 3M™ PREVENA™ Incision Management System 3M™ Scotchast™ 3M™ Tegaderm™ I.V. dressings 3M™ V.A.C.® Therapy Negative Pressure Wound Therapy (generally) 3M™ Cavilon™ family of products (excluding 3M™ Cavilon™ Durable Barrier Cream) 3M™ Promogran Prisma™ 3M™ Smart Instill™ 3M™ Veraflo™ Instillation Therapy 3M™ V.A.C.® Ulta Therapy Unit (Acute) 3M™ ActiV.A.C.™ Therapy System (Portable) 3M™ V.A.C.® Veraflo Cleanse Choice™ Dressing 3M™ Dermatac™ Drape 3M™ Snap™ Therapy System 3M™ iOn PROGRESS™ Remote Therapy monitoring 3M™ Prevena™ Therapy 3M™ Prevena Restor™ ArthroForm™ Dressing 3M™ AbThera™ SensaT.R.A.C.™ Open Abdomen Dressing 3M™ Steri-Strip™ Elastic Skin Closures 3M™ Cavilon™ Advanced Skin Protectant 3M™ Coban™ Compression Systems 3M™ Tegaderm™ transparent film dressings 3M™ Tegaderm™ CHG antimicrobial product family 3M™ Tegaderm™ CHG I.V. Dressings Family 3M™ PICC / CVC Securement Device with 3M™ Tegaderm™ I.V. Securement Dressings 3M™ Curos™ Disinfecting Caps 3M™ Ranger™ 3M™ Bair Hugger™ Warming Blanket System 3M™ Bair Hugger™ Temperature Monitoring System 3M™ Attest™ Chemical and Biological Indicators 3M™ Attest™ Reader 3M™ Ioban™ Antimicrobial Incise Drape 3M™ Red Dot™ 3M™ Red Dot™ ECG Monitoring Electrodes 3M™ Universal Electrosurgical Pads 3M™ Defib Pads 3M™ Micropore™ Surgical Tape 3M™ Coban™ Self-Adherent Wraps 3M™ Durapore™ Surgical Tape 3M™ Medipore™ H Soft Cloth Surgical Tape 3M™ Littmann® Monitoring Stethoscope 3M™ Littmann® CORE Digital Stethoscope | 3M™ Cavilon™ Durable Barrier Cream 3M™ Skin and Nasal Antiseptic | ||||||||||||
27
| Business Segment | Products Regulated as Medical Devices | Products Regulated as Pharmaceuticals | ||||||||||||
Dental Solutions | 3M™ Filtek™ Addent™ 3M™ Clinpro™ family of products (excluding the 3M™ Clinpro™ products listed under “Products Regulated as Pharmaceuticals”) 3M™ Scotchbond™ 3M™ Clarity™ 3M™ Clarity™ Aligners 3M™ Clinpro™ Sealant 3M™ Digital Bonding System 3M™ Filtek™ Matrix 3M™ Transbond™ family of products | 3M™ Clinpro™ Tooth Crème 3M™ Clinpro™ 5000 3M™ Clinpro™ Toothpaste | ||||||||||||
Health Information Systems | None | None | ||||||||||||
Purification and Filtration | None | None | ||||||||||||
Additionally, 3M™ Promogran™ Collagen Dressing and 3M™ Tegaderm™ CHG Chlorhexidine Gluconate IV Securement Dressing, which are products of the MedSurg segment, are regulated by the FDA’s Center for Device and Radiological Health as a combination product category.
Certain Risks Relating to Operating as a Standalone Entity
Solventum has no history of operating as an independent company. Following the separation and distribution, Solventum will operate as a separate standalone entity, which involves certain risks, including the following:
•The separation will result in Solventum being a smaller, less diversified company than 3M. As a result, Solventum may be more vulnerable to changing market conditions, which could have a material adverse effect on its business, financial condition, and results of operations. In addition, the diversification of Solventum’s revenues, costs, and cash flows will diminish as a standalone company, such that its results of operations, cash flows, working capital and financing requirements may be subject to increased volatility and its ability to fund capital expenditures and investments, pay dividends and service debt may be diminished. Following the separation, Solventum may also lose capital allocation efficiency and flexibility, as Solventum will no longer have access to cash flow from 3M to fund Solventum’s business. Solventum will also be more exposed to matters such as foreign currency exchange rates as a smaller, standalone company than it had been as a part of the larger 3M enterprise.
•Generally, Solventum’s working capital requirements and capital for its general corporate purposes, including capital expenditures and acquisitions, have historically been satisfied as part of the corporate-wide cash management policies of 3M. Following the completion of the distribution, Solventum’s results of operations and cash flows may be more volatile, and Solventum may need to obtain additional financing from banks, through public offerings or private placements of debt or equity securities, strategic relationships, or other arrangements, which may or may not be available and may be more costly. Please also read carefully the section entitled “Information Statement Summary—Certain Risks Relating to Solventum’s Indebtedness.”
•Prior to the distribution, Solventum’s business has been operated by 3M as part of its broader corporate organization, rather than as an independent company. 3M or one of its affiliates performed various corporate functions for the Health Care Business, such as information technology, legal, treasury, accounting, auditing, human resources, investor relations, and finance. The Health Care Business historical and pro forma financial results reflect allocations of corporate expenses from 3M for such functions, which may be less than the expenses the Health Care Business would have incurred had it operated as a separate publicly traded company. Solventum may also be unable to replicate corporate functions that will operate with the same degree of effectiveness as the equivalent 3M functions that the Health Care Business has historically benefited from.
28
•Currently, Solventum’s business is integrated with the other businesses of 3M. Historically, Solventum’s business benefited from 3M’s economies of scope and scale in costs, employees, vendor relationships and customer relationships. While Solventum has sought to minimize the impact on its business when separating these arrangements, there is no guarantee these arrangements will continue to capture these benefits in the future. Additionally, as a standalone company, Solventum may be unable to obtain similar arrangements to the same extent as 3M did, or on terms as favorable as those 3M obtained, prior to the distribution. Among other benefits, the Health Care Business currently has access to 3M’s extensive global research and development resources, which have historically enhanced the Health Care Business’s ability to innovate, develop new products and technologies, and improve and update existing products and technologies. Solventum’s lack of access to these research and development resources following the separation may negatively impact the Health Care Business.
•Solventum will engage in the process of creating its own, or engaging third parties separate from 3M to provide, systems and services to replace many of the systems and services that 3M currently provides to Solventum, including, for example, research and development support, information technology infrastructure and systems and accounting and reporting systems. Solventum may incur temporary interruptions in business operations if it cannot transition effectively from 3M’s existing operating systems, databases and programming languages that support these functions to its own systems. The process of implementing an information technology infrastructure, in particular, is expected to be expensive and time-consuming, and any difficulty or delay in developing such an infrastructure or transitioning from 3M’s information technology environment and systems could be disruptive to Solventum’s business operations and create risks to Solventum’s relationships with customers and other third parties. The failure to implement the new systems and transition data successfully and cost-effectively could disrupt Solventum’s business operations and have a material adverse effect on its profitability. In addition, Solventum’s costs for the operation of these systems may be higher than the amounts reflected in its historical combined financial statements.
•3M is the sole source of supply for certain chemical materials and inputs used in products of the Health Care Business (including transparent IV film dressings, biological indicators for sterilization assurance, medical securement tapes, and dental composites and cements) that accounted for approximately $3 billion of the revenue of the Health Care Business for fiscal year 2023, including a material with a manufacturing process proprietary to 3M that is used in products of the Health Care Business accounting for approximately $2 billion of revenue for fiscal year 2023. Our business will be harmed if 3M does not satisfy our requirements during the term of the supply agreement, and our failure to ensure a continuing supply of these materials or find acceptable substitutes, or the costs we incur in doing so, could have a material adverse effect on our business.
•Solventum and 3M will enter into various agreements that will provide for the performance of services or provision of goods by 3M for the benefit of Solventum. See “Certain Relationships and Related Party Transactions.” Solventum will rely on 3M to satisfy its obligations under these agreements not only for a successful transition but also for the success of its long-term operations. If 3M is unable to satisfy its obligations and fully perform under these agreements, Solventum could experience operational difficulties or losses. Following the expiration of the initial terms of these agreements and any automatic or required extensions, there is no guarantee that 3M will agree to renew these agreements or if 3M does agree to renew these agreements, that it will do so on substantially the same terms.
•Solventum and 3M will also enter into various agreements that will provide for the performance of services or provision of goods by Solventum for the benefit of 3M. If Solventum does not satisfactorily perform its obligations under these agreements, it may be held liable for any resulting losses suffered by 3M, subject to certain limits. Satisfaction by Solventum of its post-distribution indemnification obligations or 3M’s failure to satisfy its post-distribution indemnification obligations could have a material adverse effect on Solventum’s financial condition, results of operations and cash flows. In addition, during the transition support periods under the transition arrangements, Solventum’s management and employees may be required to divert their attention away from its business in order to provide services to 3M, which could adversely affect Solventum’s business.
29
•The agreements that Solventum will enter into with 3M in connection with the separation were prepared in the context of Solventum still being a wholly owned subsidiary of 3M. Accordingly, during the period in which the terms of those agreements were prepared, Solventum did not have an independent Board of Directors or a management team that was independent of 3M. As a result, the terms of those agreements may not reflect terms that would have resulted from arm’s-length negotiations between unaffiliated third parties and thus may be less beneficial to Solventum than the terms that may have otherwise been obtained from unaffiliated third parties.
•After the completion of the distribution, the cost of capital for Solventum’s business may be higher than 3M’s cost of capital prior to the distribution.
•As an independent public company, Solventum will separately become subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), the Sarbanes-Oxley Act and the Dodd-Frank Wall Street Reform and Consumer Protection Act and will be required to prepare its standalone financial statements according to the rules and regulations required by the SEC. These reporting and other obligations will place significant demands on Solventum’s management and administrative and operational resources. Moreover, to comply with these requirements, Solventum anticipates that it will need to migrate its systems, including information technology systems, implement additional financial and management controls, reporting systems and procedures, and hire additional accounting and finance staff. Solventum expects to incur additional annual expenses related to these steps, and those expenses may be significant. If Solventum is unable to implement appropriate financial and management controls, reporting systems, information technology and procedures in a timely and effective fashion, its ability to comply with financial reporting requirements and other rules that apply to reporting companies under the Exchange Act could be impaired.
•Because of the separation, Solventum itself will be required to pay costs that would otherwise have been paid on its behalf by 3M. These costs could be substantial and material to Solventum’s financial resources and may include accounting, tax, legal and other professional services costs, recruiting and relocation costs associated with hiring key senior management and personnel new to Solventum, tax costs and costs to separate information systems.
•Following the separation, Solventum will no longer benefit from 3M’s established brand and reputation.
•Under the terms of the tax matters agreement that Solventum will enter into with 3M, Solventum is expected to be restricted from taking certain actions that could cause the distribution or certain related transactions (or certain transactions undertaken as part of the internal reorganization) to fail to qualify as tax-free transactions and these restrictions may limit Solventum for a period of time from pursuing certain strategic transactions and equity issuances or engaging in other transactions that might increase the value of its business.
•Solventum may not be able to achieve the full strategic and financial benefits expected to result from the separation, or such benefits may be delayed or not occur at all.
Certain Risks Relating to Solventum’s Indebtedness
Solventum has completed a number of financing transactions in connection with the distribution. See “Description of Material Indebtedness.” The proceeds of such financings are expected to be used to make cash payments to 3M, other than such amounts as need to be retained in order to cause Solventum to have $600 million of cash in the aggregate at the time of the spin-off. The cash payments to 3M from the proceeds of the financings are expected to total approximately $7.7 billion, after taking into account fees, discounts and expenses, as well as the retention of $600 million. As a result of such transactions, Solventum anticipates having approximately $8.4 billion of outstanding indebtedness upon completion of the distribution. Solventum may also incur additional indebtedness in the future.
30
This significant amount of debt could potentially have important consequences to Solventum and its debt and equity investors, including:
•requiring a substantial portion of its cash flow from operations to make interest payments;
•making it more difficult to satisfy debt service and other obligations;
•increasing the risk of a future credit ratings downgrade of its debt, which could increase future debt costs and limit the future availability of debt financing;
•increasing its vulnerability to general adverse economic and industry conditions;
•reducing the cash flow available to fund capital expenditures and other corporate purposes and to grow its business;
•limiting Solventum’s flexibility in planning for, or reacting to, changes in its business and the industry;
•placing Solventum at a competitive disadvantage relative to its competitors that may not be as highly leveraged with debt;
•requiring Solventum to repatriate earnings to the United States, causing withholding taxes to be applied, which in turn could increase Solventum’s effective tax rate; and
•limiting Solventum’s ability to borrow additional funds as needed or take advantage of business opportunities as they arise, pay cash dividends or repurchase ordinary shares.
To the extent that Solventum incurs additional indebtedness, the foregoing risks could increase. In addition, Solventum’s actual cash requirements in the future may be greater than expected. Its cash flow from operations may not be sufficient to repay all of the outstanding debt as it becomes due, and Solventum may not be able to borrow money, sell assets or otherwise raise funds on acceptable terms, or at all, to refinance its debt.
Additionally, the Health Care Business has historically relied upon 3M to fund its working capital requirements and other cash requirements. After the separation and distribution, Solventum will not be able to rely on the earnings, assets, or cash flow of 3M, and 3M will not provide funds to finance Solventum’s working capital or other cash requirements. As a result, after the separation and distribution, Solventum will be responsible for servicing its own debt and obtaining and maintaining sufficient working capital and other funds to satisfy its cash requirements. After the separation and distribution, Solventum’s access to and cost of debt financing will be different from the historical access to and cost of debt financing under 3M. Differences in access to and cost of debt financing may result in differences in the interest rate charged to Solventum on financings, as well as the amount of indebtedness, types of financing structures and debt markets that may be available to Solventum. Solventum’s ability to make payments on and to refinance its indebtedness, including the debt incurred in connection with the separation and distribution, as well as any future debt that Solventum may incur, will depend on its ability to generate cash in the future from operations, financings, or asset sales. Solventum’s ability to generate cash is subject to general economic, financial, competitive, legislative, regulatory, and other factors that are beyond Solventum’s control.
Summary of Risk Factors
An investment in Solventum is subject to a number of risks, including risks relating to its business, risks related to Solventum’s separation from 3M, and risks related to Solventum common stock. Set forth below is a high-level summary of some, but not all, of these risks. Please read the information in the section entitled “Risk Factors” of this information statement for a more thorough description of these and other risks.
Risks Related to the Separation and Distribution and Solventum Common Stock
•Solventum has no history of operating as an independent company, and its historical and pro forma financial information is not necessarily representative of the results that it would have achieved as a separate, publicly traded company and may not be a reliable indicator of its future results.
31
•The separation will result in Solventum being a smaller, less diversified company than 3M and Solventum may be more vulnerable to changing market conditions. The diversification of Solventum’s revenues, costs, and cash flows will diminish as a standalone company and as a result its operations, cash flows, working capital and financing requirements may be subject to increased volatility.
•Solventum may not achieve some or all of the expected benefits of the separation for a variety of reasons, including diversion of management resources, susceptibility to market fluctuations, inability to obtain certain goods, services or technology on favorable terms or at all and higher than expected separation costs.
•Solventum may fail to have necessary systems and services in place when certain of the transaction agreements with 3M expire. Replacing these systems and services may also be more expensive or less efficient than the systems and services 3M is expected to provide during the transition period to Solventum.
•Solventum’s accounting and other management systems and resources may not be adequately prepared to meet the financial reporting and other requirements to which it will be subject as a standalone publicly traded company following the distribution.
•In connection with the distribution, Solventum expects to incur debt obligations, and Solventum may incur additional obligations in the future, which could adversely affect its business and profitability and its ability to meet other obligations.
•If the distribution, together with certain related transactions, were to fail to qualify as a transaction that is generally tax-free for U.S. federal income tax purposes, Solventum, as well as 3M and 3M’s shareholders, could be subject to significant tax liabilities. In addition, if certain internal restructuring transactions were to fail to qualify as transactions that are generally tax-free for U.S. federal or non-U.S. income tax purposes, Solventum, as well as 3M, could be subject to significant tax liabilities.
•Field of use allocations in the separation-related agreements may limit Solventum’s ability to fully exploit certain intellectual property, or may permit 3M to compete with Solventum using intellectual property owned by or licensed to Solventum. Solventum’s ability to compete may be further impacted by the non-competition provisions contained in the separation and distribution agreement.
•Following the distribution, the price of Solventum common stock may fluctuate significantly.
•A significant number of shares of Solventum common stock may be sold following the distribution, including the shares of Solventum common stock that will be retained by 3M after the distribution, which may cause the Solventum stock price to decline.
Risks Related to Solventum’s Business
•Solventum’s results may be impacted by the effects of, and changes in, worldwide economic, political, regulatory, international trade and geopolitical conditions, natural disasters, war, foreign currency exchange rates and fluctuation, changes in interest rates, and other events beyond its control.
•Solventum may not be able to access the capital and credit markets on terms that are favorable to Solventum, or at all. Solventum’s ability to issue additional debt or enter into other financing arrangements on acceptable terms could be adversely affected by its debt levels, unfavorable changes in economic conditions or uncertainties that affect the capital markets, including disruption caused by the COVID-19 pandemic.
•Solventum operates in highly competitive markets, competition may increase in the future, and the healthcare industry may be disrupted, causing Solventum to lower prices or resulting in a loss of market share.
•Consolidation in the healthcare industry may result in pricing pressures, decreased average selling prices, exclusion from important market segments, counterparties with greater negotiating power, and loss of customers.
32
•Solventum’s brands are critical to its success, and damage to its reputation or its brands could adversely affect its business, results of operations or financial condition.
•Solventum’s growth objectives are largely dependent on the timing and market acceptance of its new product and service offerings, including its ability to continually renew its pipeline of new products and services and to bring those products and services to market.
•The success of Solventum’s products could suffer if Solventum is unsuccessful in maintaining strong working relationships with healthcare professionals.
•Changes in reimbursement practices of third-party payers or other cost containment measures could affect the demand for Solventum’s products and the prices at which they are sold. Current or worsening economic conditions, including recessionary pressures, may result in decreased demand for Solventum’s products and services, declining cash flows, longer sales cycles, slower adoption of new technologies and increased price competition.
•Solventum’s future results are subject to vulnerability with respect to materials and fluctuations in the costs and availability of purchased components, compounds, raw materials, production capacity, energy, and labor due to shortages, increased demand and wages, logistics, supply chain interruptions, manufacturing site disruptions, regulatory developments, natural disasters and other disruptive factors.
•3M is the sole source of supply for certain chemical materials and inputs used in products of the Health Care Business (including transparent IV film dressings, biological indicators for sterilization assurance, medical securement tapes, and dental composites and cements)that accounted for approximately $3 billion of the revenue of the Health Care Business for fiscal year 2023, including a material with a manufacturing process proprietary to 3M that is used in products of the Health Care Business accounting for approximately $2 billion of revenue for fiscal year 2023. Our business will be harmed if 3M does not satisfy our requirements during the term of the supply agreement, and our failure to ensure a continuing supply of these materials or find acceptable substitutes, or the costs we incur in doing so, could have a material adverse effect on our business.
•Solventum operates in a strictly regulated industry and is subject to risks related to international, federal, state, and local treaties, laws, and regulations that are subject to change at any time, as well as compliance risks related to legal or regulatory requirements, contract requirements, policies and practices, or other matters that require or encourage Solventum or its suppliers, vendors, or channel partners to conduct business in a certain way. The failure to comply with or the outcome of legal and regulatory proceedings related to compliance with these requirements could have a material adverse effect on Solventum’s business.
•Solventum may face potential liabilities related to PFAS, which could adversely impact Solventum’s results. For additional details, please see the sections titled “Risk Factors — Legal and Compliance Risks — Solventum may face potential liabilities related to PFAS, which could adversely impact Solventum’s results” and “Certain Relationships and Related Party Transactions — Agreements with 3M — Separation and Distribution Agreement — PFAS Liabilities”.
•Solventum is exposed to risks associated with product liability claims, including existing claims and claims resulting from the actions or inactions of its customers or third parties that are outside of its control.
•Security and data breaches, cyberattacks, and other cybersecurity incidents involving Solventum’s information technology systems and infrastructure could disrupt or interfere with Solventum’s operations and expose Solventum to numerous expenses, liabilities, and other negative consequences.
•Solventum may be unable to obtain, maintain, protect, or effectively enforce its intellectual property rights. Protecting against the unauthorized use of proprietary technology is difficult and expensive and Solventum may need to litigate with third parties to enforce or defend its intellectual property rights.
33
The Separation and Distribution
On July 26, 2022, 3M announced its intention to separate its Health Care Business into an independent public company. The separation is expected to occur through a pro rata distribution to 3M shareholders of at least 80.1% of the shares of common stock of Solventum, a company formed to hold the Health Care Business.
On March 8, 2024, the 3M Board of Directors approved the distribution of at least 80.1% of Solventum’s issued and outstanding shares of common stock on the basis of one share of Solventum common stock for every four shares of 3M common stock held as of the close of business on March 18, 2024, the record date for the distribution.
Upon completion of the distribution, 3M will own up to 19.9% of the outstanding shares of Solventum common stock. 3M will agree to vote any shares of common stock that it retains in proportion to the votes cast by Solventum’s other shareholders, and 3M is expected to grant Solventum a proxy to vote 3M’s shares of Solventum common stock in such proportion. For additional information on these voting arrangements, see the section entitled “Certain Relationships and Related Party Transactions.” 3M will dispose of all of the retained shares of Solventum common stock as soon as a disposition is warranted consistent with the business reasons for the retention of those shares through one or more sales of such shares (not later than five years after the distribution).
Solventum’s Post-Separation Relationship with 3M
After the separation, 3M and Solventum will each be separate companies with separate management teams and separate boards of directors. Prior to the separation, 3M and Solventum will enter into the separation and distribution agreement. In connection with the separation, Solventum will also enter into various other agreements to effect the separation and to provide a framework for Solventum’s relationship with 3M after the separation, including a transition services agreement, a transition distribution services agreement, a transition contract manufacturing agreement, research and development master services agreements, real estate license agreements, an intellectual property cross license agreement, a 3M mark use agreement, a transition trademark license agreement, master supply agreements, a tax matters agreement, an employee matters agreement, and a stockholder’s and registration rights agreement. See “Certain Relationships and Related Party Transactions.” These agreements will provide for the allocation between Solventum and 3M of the assets, employees, liabilities, and obligations (including, among others, investments, property (including intellectual property), employee benefits, and tax-related assets and liabilities) of 3M and its subsidiaries attributable to periods prior to, at and after the separation and will govern the relationship between Solventum and 3M subsequent to the completion of the separation (including the relationship of 3M as a stockholder of Solventum). For additional information regarding the separation and distribution agreement and other transaction agreements, see the sections entitled “Risk Factors—Risks Related to the Separation and Distribution” and “Certain Relationships and Related Party Transactions.”
Reasons for the Separation
The 3M Board of Directors believes that the separation of the Health Care Business from 3M into an independent, publicly traded company is in the best interests of 3M and its shareholders for a number of reasons, including:
•Ability for each business to pursue tailored capital allocation strategies and make company-specific investment decisions to drive innovation and growth. The separation will permit each of 3M and Solventum to concentrate its financial resources to solely meet the unique needs of its own businesses, which will allow each company to invest capital at the time and in the manner most appropriate for its distinct strategic priorities and business needs. This will facilitate a more efficient allocation of capital between the Health Care Business and the 3M Business, based on the profitability, cash flow, and growth opportunities of each company. In addition, after the separation, the Health Care Business will no longer be required to compete internally with the 3M Business for capital and other corporate resources. As an independent entity, Solventum will be free to invest its financial resources in its own organic and inorganic opportunities at the pace of a standalone healthcare business, to accelerate growth and drive shareholder value. This will be facilitated by the fact that, in general, each party to the separation and distribution agreement will assume liability for all pending, threatened and unasserted legal matters related to its own business or its assumed
34
or retained liabilities and will indemnify the other party for any liability to the extent arising out of or resulting from such assumed or retained legal matters.
•Enhanced management focus through distinct boards and management teams with relevant expertise. The separation will permit each company to be led by a separate, dedicated board and management team with relevant and deep expertise in its industry, able to focus on strengthening such company’s core businesses, addressing its unique operating and other needs, and pursuing distinct and targeted opportunities. The board and management team of each company will be well-positioned to rapidly transform each company’s management systems and processes to those targeted at its specific growth and market strategies.
•Improved operational agility, better positioning each company for long-term success. The separation will permit each of 3M and Solventum to more effectively pursue its own distinct operating priorities and strategies in line with each company’s industry-specific focus. In particular, it will enable each of the two companies to maintain a sharper focus on strengthening its core business and growth opportunities, pursuing distinct paths to long-term growth and profitability, and addressing its unique operating and other needs. Each company will also have increased operational flexibility to design and implement corporate strategies based on the particular characteristics of the industry in which each business operates.
•Distinct and compelling investment profiles appealing to different long-term investor bases. The separation will allow each company to more effectively articulate a clear investment thesis and to enhance each company’s structural and operational transparency, enabling investors to separately value and invest in each company based on their distinct investment identities. This is expected to attract different, long-term investor bases for each company and facilitate each company’s access to capital by providing investors with two distinct and targeted investment opportunities. With investors better suited and aligned to its business, each company will be able to pursue its industry-specific business objectives consistent with the expectations of its distinct investor base.
•Creation of independent equity currencies. The separation will create independent equity securities for Solventum and 3M, aligned with each company’s respective industry, providing each company with more flexibility to capitalize on its unique strategic opportunities. This will afford Solventum direct access to the capital markets, and the opportunity to use its own industry-focused stock to consummate future acquisitions or other transactions that are more closely aligned with its strategic goals and expected growth opportunities.
•Enhanced employee recruitment and retention, including by aligning management incentives with performance. The separation will allow each of 3M and Solventum to more effectively recruit, retain and develop talent with the appropriate skill set and expertise directly applicable to each company’s needs. In addition, the separation will enable each of 3M and Solventum to offer equity-based and other incentive compensation arrangements that more closely reflect and align management and employee incentives with each company’s specific growth objectives, financial goals, and business performance.
The 3M Board of Directors also considered a number of potentially negative factors in evaluating the separation, including:
•Risk of Failure to Achieve Anticipated Benefits of the Separation. The anticipated benefits of the separation may not be achieved for a variety of reasons, including, among others: the separation will demand significant management resources and require significant amounts of management’s time and effort, which may divert management’s attention from operating the businesses of 3M and Solventum; and following the separation, each of 3M and Solventum may be more susceptible to market fluctuations and other adverse events than if they remained a combined company because the business of each entity will be less diversified than 3M’s business prior to the completion of the separation.
•Loss of Scale and Increased Administrative Costs. As part of 3M, Solventum currently takes advantage of 3M’s size and purchasing power in procuring certain goods and services. After the separation, as standalone companies, each of 3M and Solventum may be unable to obtain these goods, services and technologies at prices or on terms as favorable as those 3M obtained prior to completion of the separation.
35
In addition, as part of 3M, Solventum benefits from certain functions performed by 3M, such as accounting, tax, legal, human resources and other general and administrative functions. After the separation, 3M will not perform these functions for Solventum, other than certain functions that will be provided for a limited time pursuant to the transition arrangements, and, because of Solventum’s smaller scale as a standalone company, its cost of performing such functions could be higher than the amounts reflected in Solventum’s historical financial statements, which could cause Solventum’s profitability to decrease.
•Disruptions and Costs Related to the Separation. The actions required to separate Solventum from 3M could disrupt each company’s operations. In addition, 3M and Solventum will incur substantial costs in connection with the separation and the transition to Solventum becoming a standalone public company, which may include accounting, tax, legal and other professional services costs, recruiting and relocation costs associated with hiring key senior management personnel who are new to Solventum, tax costs and costs to separate information systems.
•Limitations on Strategic Transactions. Under the terms of the tax matters agreement that Solventum will enter into with 3M, Solventum is expected to be restricted from taking certain actions that could cause the distribution or certain related transactions (or certain transactions undertaken as part of the internal reorganization) to fail to qualify as tax-free transactions under applicable law. These restrictions may limit for a period of time Solventum’s ability to pursue certain strategic transactions and equity issuances or engage in other transactions that might increase the value of Solventum’s business.
•Uncertainty Regarding Stock Prices. Neither 3M nor Solventum can predict the effect of the separation on the trading prices of Solventum or 3M common stock or know with certainty whether the combined market value of one-quarter of a share of Solventum common stock and one share of 3M common stock will be less than, equal to or greater than the market value of one share of 3M common stock prior to the distribution.
In determining to pursue the separation, the 3M Board of Directors concluded the potential benefits of the separation outweighed the foregoing factors. See the sections entitled “The Separation and Distribution—Reasons for the Separation” and “Risk Factors” included elsewhere in this information statement.
Reasons for 3M’s Retention of Up to 19.9% of the Shares of Solventum Common Stock
In considering the appropriate structure for the separation, 3M determined that, immediately after the distribution becomes effective, 3M will retain up to 19.9% of the outstanding shares of Solventum common stock. 3M intends to responsibly dispose of such shares after the distribution as soon as a disposition is warranted consistent with the business reasons for the retention of those shares. Such dispositions will be effected through one or more sales of such shares of Solventum common stock (not later than five years after the distribution). 3M’s retention of shares of Solventum common stock is expected to support the establishment of optimal capital structures for each of 3M and Solventum by (i) providing 3M with a valuable asset that can be used to reduce its aggregate liabilities, allowing 3M the opportunity to capitalize on strategic opportunities and strengthen its balance sheet, and (ii) providing a means for 3M to increase its financial flexibility without increasing the amount of leverage that Solventum would incur, thereby providing Solventum with a stronger balance sheet and greater ability to fund growth.
Any sales of substantial amounts of Solventum common stock in the public market by 3M or the perception that such sales might occur, in connection with the distribution or otherwise, may cause the market price of Solventum common stock to decline. See the second risk factor in the section entitled “Risk Factors—Risks Related to Solventum Common Stock” for additional details.
Corporate Information
Solventum was incorporated in Delaware for the purpose of holding the Health Care Business in connection with the separation and distribution described in this information statement. Prior to the contribution of the Health Care Business to Solventum by 3M, which will occur prior to the distribution, Solventum will have no operations, and will have no assets or liabilities of any kind, other than those incidental to its formation and the separation. The
36
address of Solventum’s principal executive offices will be 3M Center, Building 275-6W, 2510 Conway Avenue East, Maplewood, MN 55144. Its telephone number after the distribution will be 1-800-228-3957. Solventum will maintain an Internet site at www.solventum.com. This website and the information contained therein or connected thereto are not incorporated into this information statement or the registration statement of which this information statement forms a part, or in any other filings with, or any information furnished or submitted to, the SEC.
Reason for Furnishing this Information Statement
This information statement is being furnished solely to provide information to 3M shareholders who will receive shares of Solventum common stock in the distribution. It is not to be construed as an inducement or encouragement to buy or sell any of Solventum’s securities. The information contained in this information statement is believed by Solventum to be accurate as of the date set forth on its cover. Changes may occur after that date, and neither 3M nor Solventum will update the information, except as may be required in the normal course of their respective disclosure obligations and practices.
37
SUMMARY HISTORICAL AND UNAUDITED PRO FORMA FINANCIAL INFORMATION
The following summary financial data reflects the combined operations of the Health Care Business. The summary historical and unaudited pro forma condensed combined financial data shown below should be read in conjunction with the sections herein entitled “Capitalization,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Unaudited Pro Forma Combined Financial Statements,” and “Certain Relationships and Related Party Transactions,” as well as our audited combined financial statements and the corresponding notes included elsewhere in this information statement. For factors that could cause actual results to differ materially from those presented in the summary historical and pro forma condensed combined financial data, see “Cautionary Note Regarding Forward-Looking Statements” and “Risk Factors” included elsewhere in this information statement.
We derived the summary historical combined financial information as of December 31, 2023 and 2022 and for each of the fiscal years ended December 31, 2023, 2022 and 2021 from our audited combined financial statements which are included elsewhere in this information statement.
The summary unaudited pro forma condensed combined financial information for the year ended December 31, 2023 has been derived from our unaudited pro forma condensed combined financial statements, which are included elsewhere in this information statement. The unaudited pro forma condensed combined financial data is based upon available information and assumptions that we believe are reasonable and supportable. The summary unaudited pro forma condensed combined financial data is for illustrative and informational purposes only. The summary unaudited pro forma condensed combined financial data may not necessarily reflect what our financial condition or results of operations would have been had we been a standalone company during the periods presented. In addition, the summary unaudited pro forma condensed combined financial data may not necessarily reflect what our financial condition, results of operations, and cash flows may be in the future.
| Pro Forma | Historical | |||||||||||||||||||||||||||||||
| Year ended December 31, | Year ended December 31, | |||||||||||||||||||||||||||||||
| ($ in millions) | 2023 | 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||
| Net sales | ||||||||||||||||||||||||||||||||
| Sales of product | $ | 6,340 | $ | 6,296 | $ | 6,300 | $ | 6,398 | ||||||||||||||||||||||||
| Sales of software and rentals | 1,901 | 1,901 | 1,830 | 1,773 | ||||||||||||||||||||||||||||
| Total net sales | 8,241 | 8,197 | 8,130 | 8,171 | ||||||||||||||||||||||||||||
| Operating expenses | ||||||||||||||||||||||||||||||||
| Costs of product | 3,200 | 3,023 | 2,953 | 2,773 | ||||||||||||||||||||||||||||
| Costs of software and rentals | 481 | 481 | 482 | 475 | ||||||||||||||||||||||||||||
| Selling, general and administrative | 2,321 | 2,243 | 2,235 | 2,278 | ||||||||||||||||||||||||||||
| Research and development | 759 | 758 | 767 | 766 | ||||||||||||||||||||||||||||
| Total operating expenses | 6,761 | $ | 6,505 | $ | 6,437 | $ | 6,292 | |||||||||||||||||||||||||
| Operating income | 1,480 | 1,692 | 1,693 | 1,879 | ||||||||||||||||||||||||||||
| Other expense (income) – net | 570 | 25 | 1 | (3) | ||||||||||||||||||||||||||||
| Income before income taxes | 910 | 1,667 | 1,692 | 1,882 | ||||||||||||||||||||||||||||
| Provision for income taxes | 218 | 321 | 349 | 422 | ||||||||||||||||||||||||||||
| Net income | $ | 692 | $ | 1,346 | $ | 1,343 | $ | 1,460 | ||||||||||||||||||||||||
| Cash from (used for) operating activities | $ | 1,915 | $ | 1,679 | $ | 2,202 | ||||||||||||||||||||||||||
Other data(a): | ||||||||||||||||||||||||||||||||
| Adjusted operating income* | $ | 1,990 | $ | 2,072 | $ | 2,080 | $ | 2,273 | ||||||||||||||||||||||||
| Free cash flow* | $ | 1,625 | $ | 1,428 | $ | 1,925 | ||||||||||||||||||||||||||
__________________
(a)In addition to reporting financial results in accordance with U.S. GAAP, the Health Care Business also provides non-GAAP measures that we use, and plan to continue using, when monitoring and evaluating operating performance and measuring cash available to invest in our
38
business. The adjusted measures are not in accordance with, nor are they a substitute for, GAAP measures. The non-GAAP financial measures presented in this information statement are supplemental measures of our performance and our liquidity that we believe help investors understand our underlying business performance and the Company uses these measures as an indication of the strength of the Company and its ability to generate cash.
*Non-GAAP financial measure.
| Pro Forma | Historical | |||||||||||||||||||||||||
As of December 31, | As of December 31, | |||||||||||||||||||||||||
| ($ in millions) | 2023 | 2023 | 2022 | |||||||||||||||||||||||
| Cash and cash equivalents | $ | 600 | $ | 194 | $ | 61 | ||||||||||||||||||||
| Total assets | $ | 14,377 | $ | 13,943 | $ | 13,594 | ||||||||||||||||||||
| Long-term debt | $ | 8,303 | $ | — | $ | — | ||||||||||||||||||||
| Defined benefit pension | $ | 431 | $ | 166 | $ | 91 | ||||||||||||||||||||
| Total liabilities | $ | 10,968 | $ | 2,277 | $ | 1,852 | ||||||||||||||||||||
| Total equity | $ | 3,409 | $ | 11,666 | $ | 11,742 | ||||||||||||||||||||
| Total liabilities and equity | $ | 14,377 | $ | 13,943 | $ | 13,594 | ||||||||||||||||||||
39
RISK FACTORS
You should carefully consider the following risks and other information in this information statement in evaluating Solventum and Solventum common stock. Any of the following risks and uncertainties could materially adversely affect Solventum’s business, financial condition or results of operations.
Risks Related to Solventum’s Business
General Economic Risks
Solventum’s results may be impacted by the effects of, and changes in, worldwide economic, political, regulatory, international trade and geopolitical conditions, natural disasters, war and other events beyond its control.
Solventum develops, manufactures, distributes and sells its products globally, and, accordingly, Solventum’s operations and the execution of its business strategies and plans are subject to global competition and economic and geopolitical risks that are beyond its control, such as, among other things, disruptions in financial markets, economic downturns, military conflicts, public health emergencies such as COVID-19, political changes and trends such as protectionism, economic nationalism resulting in government actions impacting international trade agreements, imposing trade restrictions such as tariffs, and retaliatory countermeasures, changes in regulatory regimes that could restrict Solventum’s ability to manufacture and sell its products (including healthcare regulatory regimes), diminished or insufficient protection of intellectual property and government deficit reduction and other austerity measures in locations or industries in which Solventum operates. Further escalation of specific trade tensions, including those between the United States and China, or more broadly of global trade conflict, could adversely impact Solventum’s business and operations around the world. Solventum’s business is also impacted by social, political and labor conditions in locations in which Solventum or its suppliers or customers operate; adverse changes in the availability and cost of capital; monetary policy; interest rates; inflation; recession; commodity prices; currency volatility or exchange control; ability to expatriate earnings; and other laws and regulations in the jurisdictions in which Solventum or its suppliers or customers operate. Both domestic and international markets experienced significant inflationary pressures in 2023, and inflation rates in the United States, as well as in other countries in which Solventum operates, may continue at elevated levels for the near term. Interest rate increases or other government actions taken to reduce inflation could also result in recessionary pressures in many parts of the world. Furthermore, currency exchange rates have been especially volatile in the recent past, and these currency fluctuations have affected, and may continue to affect, the reported value of Solventum’s assets and liabilities, as well as Solventum’s cash flows.
The global economy has been impacted by the military conflict between Russia and Ukraine. The United States and other governments have imposed export controls on certain products and financial and economic sanctions on certain industry sectors and parties in Russia. 3M suspended operations of its subsidiaries, including those of the Health Care Business, in Russia in March 2022 and, in September 2022, committed to a plan to exit the related net assets in Russia, including those of the Health Care Business, through a sale of 3M’s Russian subsidiaries that was consummated in June 2023. In the year ended December 31, 2022, Health Care recorded a charge of $8 million primarily related to impairment of net assets in Russia in connection with 3M management’s committed exit and disposal plan. In the fiscal year ended December 31, 2022, Health Care related sales in 3M’s Russian subsidiaries accounted for less than 1% of the combined net sales of the Health Care Business, approximately 2.4% of net sales for the fiscal year ended December 31, 2022 of the Dental Solutions segment, and less than 1% of net sales for all other business segments of the Health Care Business. Solventum did not experience any significant impact from this disposition. Solventum also has other operations that source certain raw materials from suppliers in Russia and has experienced related supply disruption due to the conflict. These geopolitical tensions could result in, among other things, cyberattacks, further supply chain disruptions impacting downstream customers, higher energy costs, lower consumer demand, and changes to foreign exchange rates and financial markets, any of which may adversely affect Solventum’s business and supply chain.
In addition, natural disasters, such as hurricanes, tornadoes, windstorms, earthquakes, wildfires and floods and other extreme weather events (including those caused by climate change) and actions taken by the United States and other governments in response to such events could cause significant economic disruption and political and social
40
instability in the United States and areas outside of the United States in which Solventum operates. These events could result in decreased demand for Solventum’s products, adversely affect its manufacturing and distribution capabilities, cause physical damage to its facilities as well as those of its suppliers, customers and other business partners, negatively impact the health and well-being of individuals and communities in which Solventum or its suppliers or customers operate or increase the costs for or cause interruptions in the availability of natural resources, sources and supply of energy.
Unexpected events, such as those related to the coronavirus (COVID-19) public health crisis, may increase Solventum’s cost of doing business and disrupt Solventum’s operations.
Due to Solventum’s global operations, Solventum’s business is and will be impacted by unexpected events, including war, acts of terrorism, public health crises (such as the COVID-19 pandemic), civil unrest, natural disasters, and severe weather in the locations in which Solventum or its suppliers or customers operate, and these events have adversely affected, and could in the future adversely affect, Solventum’s operations and financial performance.
For example, the global pandemic associated with COVID-19, including related governmental responses to the pandemic, has significantly increased economic and demand uncertainty and has impacted and will continue to impact Solventum’s operations, including its supply chain and its manufacturing and distribution capabilities. Some of Solventum’s products are particularly sensitive to reductions in elective medical procedures, which have previously been suspended or reduced in markets where Solventum’s products are marketed and sold in response to the COVID-19 pandemic. It is not possible to predict whether elective medical procedures will again be suspended or reduced in the future due to a resurgence of COVID-19 or other epidemic and, to the extent individuals and customers are required to continue to de-prioritize, delay or cancel elective procedures, Solventum’s business, cash flows, financial condition and results of operations could be negatively affected. In addition, the COVID-19 pandemic has adversely impacted the continued service and availability of skilled personnel necessary to run Solventum’s operations, including through increased absenteeism in connection with the rise of COVID-19 variants. Although Solventum may seek to mitigate these staffing challenges through overtime and enlisting contingent labor, staffing shortages could strain Solventum’s operations and increase its expenses. In addition, Solventum may be unable to retain employees who object to governmental vaccine mandates or heightened safety protocols. To the extent Solventum’s management or other personnel are impacted in significant numbers by COVID-19 and are not available to perform their professional duties, Solventum could experience disruptions in its manufacturing operations or disruptions in other activities and other functions. A future resurgence of COVID-19 or other public health crisis may also affect the ability of suppliers and vendors to provide products and services to Solventum. Some of these factors may increase demand for certain Solventum products, while others may decrease demand from certain end markets or make it more difficult for Solventum to serve customers. Solventum may also experience customer requests for potential payment deferrals or other contract modifications, supply chain under-liquidation, delays of deliveries and the achievement of other billing milestones, delays or cancellations of new projects and related down payments, and other factors related, directly and indirectly, to the resurgence of the COVID-19 pandemic’s or other public health crises’ effects on its customers that adversely impact Solventum’s businesses. The COVID-19 pandemic has also impacted and the resurgence of the COVID-19 pandemic or other public health crisis may further impact the broader economies of affected countries, including negatively impacting economic growth, the proper functioning of financial and capital markets, foreign currency exchange rates, and interest rates.
Solventum is not able to predict the impact of unexpected events, such as the COVID-19 pandemic, and unexpected events may have a material adverse effect on its business, cash flows, financial condition and results of operations.
General Business Risks
Solventum’s future results may be affected by its operational execution.
Solventum’s financial results depend on the successful execution of its business operating plans. Solventum intends to monitor the dynamics of the economy, the healthcare industry and the markets in which it competes and
41
assess opportunities for improved operational effectiveness, productivity and efficiency and to better align expenses with revenues, while preserving its ability to make investments in research and development projects, capital and its people. There can be no assurance that Solventum will realize the benefits of such activities, or that such activities will not result in unexpected consequences, such as a reduced ability to generate sales or provide the experience that Solventum’s customers, suppliers, vendors, and channel partners expect from it. Further, such improvements may be realized later than expected, and the ongoing costs of implementing these plans might be greater than expected. These measures could yield unintended consequences, such as distraction of Solventum’s management and employees, business disruption, inability to attract or retain key personnel and reduced employee productivity, any of which could negatively affect Solventum’s business, sales, financial condition and results of operations. If these measures are not successful or sustainable, Solventum might undertake additional realignment and cost reduction efforts, which could result in future charges.
In addition, the ability to adapt Solventum’s business model and other changes, including responding to evolving customer needs and service expectations, are important. If Solventum is unable to demonstrate these abilities, it could negatively impact Solventum’s ability to win new business, drive revenue and enhance its brand. Operational challenges, including those related to customer service, pace of change and productivity improvements, could result in a material adverse effect on Solventum’s business, results of operations, financial condition and cash flows.
Our brands are critical to our success, and damage to our reputation or our brands could adversely affect our business, results of operations or financial condition.
Our ability to compete successfully depends on the strength of our brands. Developing and maintaining the reputation of our brands is a critical component of our relationship with consumers, customers, manufacturers, suppliers, distributors and other third-party partners, including healthcare professionals, influencers and other individuals with whom we have relationships. We believe consumers, customers and third-party partners value the reputation and status of our brands. As a result, we devote significant time and resources to programs designed to grow, protect and preserve our brands. However, these efforts may not be successful, and failure to maintain the value of our brands could impact our brand loyalty with consumers, customers and third-party partners and otherwise adversely affect our business, results of operations or financial condition.
Our reputation and our brands could in the future be damaged by negative publicity, whether or not valid. Negative publicity could relate to our company, our brands, our products, our supply chain, our ingredients, our packaging, our ESG practices, our employees or any other aspect of our business. Our reputation or our brands could also be adversely affected by negative publicity related to our industry, our competitors, our competitors’ products, our customers or our third-party partners, including healthcare professionals, and other individuals with whom we have relationships, even if the publicity is not directly related to our company or our brands and even if the publicity is not accurate. In addition, widespread use of digital and social media platforms around the world has greatly increased the accessibility of information and the speed with which it is disseminated, which has made, and likely will continue to make, maintaining our reputation and our brands more challenging. Damage to our reputation or our brands could cause consumers, customers and third-party partners to lose trust in our products, require us to expend substantial resources to remedy the damage or otherwise adversely affect our business, results of operations or financial condition.
Acquisitions, strategic alliances, divestitures, and other strategic events resulting from portfolio management actions and other evolving business strategies, and possible organizational restructuring could affect future results.
Solventum intends to monitor its business portfolio and organizational structure and may make acquisitions, divestitures and changes to its organizational structure or enter into strategic alliances or joint ventures. These activities may result in substantial investment of Solventum’s resources. The success of any such activities will depend upon a number of factors, including Solventum’s ability to:
•identify suitable acquisition targets or assets, conduct due diligence, negotiate transactions on favorable terms, and ultimately complete such transactions;
42
•compete for acquisition targets and assets, which may lead to substantial increases in purchase price or terms that are less attractive to Solventum;
•finance any future acquisition, investment, alliance or other transaction on terms acceptable to Solventum, if at all (which may involve the use of Solventum’s shares for payment of the purchase price);
•comply with applicable laws and regulations, including foreign laws and regulations;
•obtain any legally required rulings by antitrust or other regulatory bodies;
•successfully and timely integrate and operate acquired businesses;
•protect intellectual property and prevail in litigation relating to newly acquired technologies;
•predict or realize expected growth opportunities, cost savings, synergies, and market acceptance of acquired companies’ products; and
•successfully identify and retain key target employees and customers.
In addition, acquisitions may expose Solventum to significant risks and uncertainties, including failure to identify significant non-compliant behaviors or practices by, or liabilities relating to, the acquisition target (or its agents) prior to acquisition; successor liability imposed by regulators for actions by the acquisition target (or its agents) prior to acquisition; and diversion of management’s attention from existing operations to the acquisition and integration process. Such transactions will be subject, in certain circumstances, to the consent of 3M under the Tax Matters Agreement, as discussed in “—Risks Related to the Separation and Distribution.” There can be no assurance that any future transactions of this type will be pursued or, if pursued, will be successful.
Solventum’s business dealings may involve third-party partners in various markets, and the actions or inactions of these third parties could adversely affect its business.
Solventum’s business dealings may involve third-party partners such as distributors, dealers, wholesalers, packagers, resellers, agents, and others. Such dealings expose Solventum to known and unknown risks, including risks related to economic, political, and regulatory environments, performance and quality control, difficulty in establishing additional or replacement suppliers in a timely or cost-effective manner in the event of termination, conflicts of interest, and legal and regulatory violations committed by these third parties, which may not be subject to Solventum’s control. These third parties may suffer or cause Solventum to suffer commercial, financial, or reputational harm or violate local laws or regulations, each of which may be outside of Solventum’s control and could jeopardize its ability to continue doing business in these markets or cause its relationships to deteriorate. A reduction or interruption in the supply of materials or components used in manufacturing Solventum’s products, due to factors such as one or more suppliers experiencing reductions in operations and/or worker absences due to the COVID-19 pandemic or other health epidemics, an inability to timely develop and validate alternative sources if required, or a significant increase in the price of such materials or components, could adversely affect Solventum’s business, results of operations, financial condition, cash flows or reputation.
Solventum may not be able to access the capital and credit markets on terms that are favorable to Solventum, or at all.
Solventum expects to access the capital markets to supplement its existing funds and cash generated from operations to satisfy its needs for working capital, to meet capital expenditure and debt service requirements, and for other business initiatives. Solventum’s ability to issue additional debt or enter into other financing arrangements on acceptable terms could be adversely affected by its debt levels, unfavorable changes in economic conditions or uncertainties that affect the capital markets, including disruption caused by the COVID-19 pandemic. In the event of adverse capital and credit market conditions, Solventum may be unable to obtain capital market financing on favorable terms, or at all.
43
Change in Solventum’s credit ratings could increase cost of funding.
Solventum’s credit ratings are important to its cost of capital. The major rating agencies will routinely evaluate Solventum’s credit profile and assign debt ratings to Solventum. This evaluation is based on a number of factors, which include financial strength, business and financial risk, as well as transparency with rating agencies and timeliness of financial reporting. As of February 27, 2024, Solventum has a credit rating of Baa3 from Moody’s Investors Service and a credit rating of BBB- from S&P Global Ratings. Changes in Solventum’s credit ratings could adversely affect its ability to obtain capital market financing and the cost of such financing. Moreover, a reduction in Solventum’s credit rating to below investment-grade could cause certain customers to reduce or cease to do business with Solventum, which would adversely impact its financial performance.
Foreign currency exchange rates and fluctuations in those rates may affect Solventum’s ability to realize projected growth rates in its sales and earnings.
Because Solventum’s financial statements are denominated in U.S. dollars and a material percentage of its revenues are derived from outside the United States, Solventum’s results of operations and its ability to realize projected growth rates in sales and earnings could be adversely affected if the value of the U.S. dollar strengthens significantly against foreign currencies.
Solventum cannot predict with any certainty changes in foreign currency exchange rates or its ability to mitigate these risks. Solventum may experience additional volatility because of increasing inflationary pressures and other macroeconomic factors, including in emerging market countries. Solventum may be unable to hedge the effects of foreign exchange rate changes in a cost-effective manner.
Changes in interest rates could adversely affect Solventum.
Solventum may be exposed to changes in interest rates, including through variable rate debt and due to the fact that increases in interest rates may adversely affect the financial condition of Solventum’s counterparties in a manner that may affect their ability to transact with Solventum or their demand for Solventum’s products and services. Any of the foregoing could adversely affect Solventum’s business, results of operations, financial condition and cash flows.
Market Dynamic Risks
Solventum operates in highly competitive markets, competition may increase in the future, and the healthcare industry may be disrupted, causing Solventum to lower prices or resulting in a loss of market share.
Healthcare markets are characterized by rapidly evolving technology, frequent introduction of new products, intense competition and pricing pressure. Solventum faces substantial competition from international and domestic companies of all sizes, including existing competitors, new market entrants and non-traditional entrants. Demand for Solventum’s products and services, which impacts revenue and profit margins, will be affected by, among other things, (i) the development and timing of the introduction of competitive products and services; (ii) Solventum’s pricing strategies; (iii) changes in customer order patterns, such as changes in the levels of inventory maintained by customers, vendors, or channel partners; (iv) changes in customers’ preferences for Solventum’s products and services, including the success of products and services offered by competitors; (v) changes in customer designs for their products and services that can affect the demand for Solventum’s products and services; (vi) changes in the business environment related to disruptive technologies, such as artificial intelligence, block-chain, expanded analytics and other enhanced learnings from increasing volume of available data; (vii) local market conditions, such as mandatory intellectual property transfers, protectionist measures and other government policies supporting increased local competition; (viii) costs of production or delivery, whether due to geographic location, currency fluctuations, taxes, duties, or otherwise; (ix) the perception of Solventum’s brand and image in the market; (x) changing regulatory standards, legal requirements, or enforcement rigor; (xi) failure to acquire or effectively integrate businesses and technologies that complement or expand Solventum’s existing businesses; and (xii) consolidation among customers, suppliers, channel partners or competitors.
44
In addition, Solventum’s inability to obtain and maintain regulatory authorizations for, and supply commercial quantities of, Solventum’s offerings as quickly and effectively as its competitors could limit market acceptance. Solventum’s competitors may also have greater financial, marketing and other resources, respond more quickly to new or emerging technologies, undertake more extensive marketing campaigns; adopt more aggressive pricing policies, or be more successful in attracting potential customers, employees and strategic partners.
Consolidation in the healthcare industry could have an adverse effect on Solventum’s revenues and results of operations.
Many healthcare industry companies, including healthcare systems, distributors, manufacturers, providers, and insurers, are consolidating or have formed strategic alliances. As the healthcare industry consolidates, competition to provide products and services is expected to continue to intensify, resulting in pricing pressures, decreased average selling prices and the exclusion of certain suppliers from important market segments. Consolidations create larger enterprises with greater negotiating power and may result in the loss of a Solventum customer where the combined enterprise selects one distributor from two incumbents. If consolidation trends continue, it could adversely affect Solventum’s business results, cash flows, financial condition, or prospects. If Solventum faces an increase in costs or reduces its prices because of industry consolidation, or if Solventum loses customers as a result of consolidation, its business, results of operations, financial condition and cash flows could be adversely affected.
Reductions in customers’ research budgets or government funding may adversely affect Solventum’s business.
Solventum’s customers include hospitals, universities, healthcare providers, government agencies and public and private research institutions; in particular, Solventum’s Health Information Systems segment sells products to ministries of health. Research and development spending of such customers can fluctuate based on spending priorities and general economic conditions. The level of government funding of research and development is unpredictable. The availability of governmental research funding may be adversely affected by many factors, including public spending priorities, available resources, economic conditions and governmental spending reductions, particularly during periods of economic uncertainty. Stalemates in national, regional, or local government budgeting decisions could also lead to substantial delays or reductions in governmental spending. Any reduction or delay in governmental funding could cause Solventum’s customers to delay or forgo purchases of its products.
Solventum’s growth objectives are largely dependent on the timing and market acceptance of its new product and service offerings, including its ability to continually renew its pipeline of new products and services and to bring those products and services to market.
A significant element of Solventum’s strategy is to increase revenue growth by focusing on innovation and new product and service development. New service and product development requires significant investment in research and development, clinical trials and regulatory approvals. The ability to bring new products and services to market is subject to difficulties or delays in product and service development, such as the inability to identify viable new products and services, obtain adequate intellectual property protection, regulatory approvals and reimbursement in the United States and abroad, and successfully complete clinical trials or gain market acceptance of new products and services. It is uncertain when or whether Solventum’s products, services, or solutions currently under development will be launched or will be commercially successful. Additionally, new offerings may be quickly rendered obsolete by changing customer preferences, changing industry standards, or competitors’ innovations or reverse engineering efforts. If Solventum cannot successfully introduce new offerings that address the needs of its customers, Solventum’s offerings may become obsolete, and its business results, cash flows, and financial condition could suffer.
The success of many of Solventum’s products depends upon certain key healthcare professionals.
Solventum works with leading global healthcare professionals who provide considerable knowledge and experience. The research, development, marketing and sales of many of Solventum’s products depend on maintaining working relationships with healthcare professionals. Solventum relies on these professionals for assistance in the development and marketing of its products. The resurgence of the COVID-19 pandemic or other public health crisis may limit access to these professionals, and resulting travel restrictions, shutdowns and similar
45
measures in response to any such public health crisis may impact Solventum’s ability to maintain these relationships, which in turn would adversely affect its ability to develop, market and sell new and improved products. If new laws, regulations, or other developments limit Solventum’s ability to appropriately engage these professionals or to continue to receive their advice and input or Solventum is otherwise unsuccessful in maintaining strong working relationships with these healthcare professionals, the success of Solventum’s products could suffer, which could result in a material adverse effect on its business, results of operations, financial condition and cash flows.
Changes in reimbursement practices of third-party payers or other cost containment measures could affect the demand for Solventum’s products and the prices at which they are sold.
Sales of many of Solventum’s products directly or indirectly depend on the availability of reimbursement and the amount of reimbursement that its customers may seek from various third-party payers, including government programs, authorities, or agencies (e.g., Medicare and Medicaid in the United States), and private health plans. The coverage policies and reimbursement levels of third-party payers, which can vary among public and private sources and by country, may affect which products customers purchase and the prices they are willing to pay for those products in a particular jurisdiction. Reimbursement rates can also affect the market acceptance rate of new technologies and products.
In general, employers and third-party payers, particularly in the United States, have become increasingly cost-conscious, with higher deductibles imposed in many medical plans. Additionally, austerity measures or other reforms by foreign governments may limit, reduce or eliminate payments for Solventum’s products and adversely affect both pricing flexibility and demand for Solventum’s products. Even if Solventum develops promising new products, it may find limited demand for the products unless reimbursement approval is obtained from third-party payers. Further legislative or administrative reforms that impact reimbursements or pricing could result in a material adverse effect on its business, results of operations, financial condition and cash flows.
In addition, current or worsening economic conditions, including recessionary pressures, may adversely affect the ability of Solventum’s customers to pay for its products and services, and the amount spent on healthcare generally, which could result in decreased demand for Solventum’s products and services, declining cash flows, longer sales cycles, slower adoption of new technologies and increased price competition.
Pricing pressure has also increased due to continued consolidation among healthcare providers, trends towards managed care, the shift towards governments becoming the primary payers of healthcare expenses, reduction in reimbursement levels and medical procedure volumes, and government laws and regulations relating to sales and promotion, reimbursement and pricing generally. As a result of these and other measures, including future measures or reforms that cannot be predicted, reimbursement may not be available or sufficient to allow Solventum to sell its products on a competitive basis. Legislation and regulations affecting reimbursement for Solventum’s products may change at any time. Solventum cannot predict the impact of these pressures and initiatives or any negative effects of any additional regulations that may affect its business.
Solventum’s future results are subject to vulnerability with respect to materials and fluctuations in the costs and availability of purchased components, compounds, raw materials, energy, production capacity and labor due to shortages, increased demand and wages, logistics, supply chain interruptions, manufacturing site disruptions, regulatory developments, natural disasters and other disruptive factors.
Solventum depends on various components, compounds, raw materials, and energy (including oil and natural gas and their derivatives) supplied by others for the manufacturing of its products. If suppliers fail to meet their delivery obligations, raise prices, or cease to supply to Solventum, it may continue to cause delays in delivery or significantly increase Solventum’s costs. If Solventum loses suppliers, if their operations are substantially interrupted, if their prices continue to increase significantly due to inflationary pressures, or if any of them fail to meet performance or quality specifications, Solventum may be required to identify and qualify one or more replacement suppliers. This also may require Solventum to redesign or modify its products to incorporate new components and obtain regulatory authorization, qualification or certification of these redesigned or modified products.
46
Supplier relationships could be interrupted due to supplier material shortages, climate impacts, natural and other disasters, equipment malfunctions, transportation delays, inflationary pricing pressures, work stoppages, labor shortages and other disruptive events such as military conflicts, or could be terminated. In addition, some of Solventum’s suppliers are limited- or sole-source suppliers, and Solventum’s ability to meet its obligations to customers depends on the performance, product quality, and stability of such suppliers and Solventum’s ability to source alternatives in a cost effective manner. Any sustained interruption in Solventum’s receipt of adequate supplies or the distribution of Solventum’s products, or disruption to key manufacturing sites’ operations due to natural and other disasters or events, such as government actions relating to discharge or emission permits or other legal or regulatory requirements, could result in a material adverse effect on its business, results of operations, financial condition and cash flows. In addition, there can be no assurance that Solventum’s processes to minimize volatility in component and material pricing will be successful or that future price fluctuations or shortages will not result in a material adverse effect on its business, results of operations, financial condition and cash flow and its ability to fulfill supply obligations to its customers. Solventum could incur contractual penalties, experience a deterioration in customer relationships, or suffer harm to its reputation if Solventum is unable to fulfill its obligations to customers, any of which could have a material adverse effect on Solventum’s business, results of operations, financial condition and cash flows. The risks of disruption, including from war, natural disasters, climate change-related physical and transitional risks, actual or threatened public health emergencies, or other business continuity events could adversely affect Solventum’s operations and limit Solventum’s ability to meet its commitments to customers or significantly impact its financial results and condition.
Solventum’s business also depends on having sufficient production capacity to meet the demand for Solventum’s products and to support Solventum’s future growth. Solventum is conducting capital expansion efforts to increase its manufacturing capacity in certain areas of its operations. These efforts may not be successful. If this is the case, it could adversely affect Solventum’s operations and limit Solventum’s ability to support future growth or significantly impact its financial results and condition.
In addition, many of Solventum’s products require sterilization prior to sale, and Solventum utilizes contract sterilizers to perform this service. To the extent Solventum’s contract sterilizers are unable to sterilize Solventum’s products, whether due to capacity, availability of materials for sterilization, regulatory or other constraints, including federal and state regulations on the use of ethylene oxide, Solventum may be unable to transition to alternative internal or external resources or methods in a timely or cost effective manner or at all, which could have a material impact on Solventum’s results of operations and financial condition.
3M is the sole source of supply for raw materials used in certain of our products and our business will be harmed if 3M does not satisfy our requirements.
3M is currently, and following the separation and distribution will continue to be, the sole source of supply for certain chemical materials and inputs used in products of the Health Care Business (including transparent IV film dressings, biological indicators for sterilization assurance, medical securement tapes, and dental composites and cements) that accounted for approximately $3 billion of the revenue of the Health Care Business for fiscal year 2023, including a material with a manufacturing process proprietary to 3M that is used in products of the Health Care Business accounting for approximately $2 billion of the revenue of the Health Care Business for fiscal year 2023. While 3M has agreed to supply these items to Solventum for a period of time following the separation and distribution (see the section titled “Certain Relationships and Related Party Transactions – Master Supply Agreements”), our business will be harmed if 3M does not satisfy our requirements during such period of time, and Solventum may subsequently need to either reach agreement with 3M for an extended supply arrangement, develop its own manufacturing capabilities for these materials or identify an appropriate substitution or product reformulation in order to continue manufacturing and selling the applicable products. There is no guarantee that 3M will agree to continue to supply these materials following the term of the supply agreement, on commercially reasonable terms or at all. At this time, the Health Care Business has not identified an appropriate substitute input to replace the materials supplied by 3M, and the Health Care Business does not currently have the capability to manufacture such materials itself. If 3M’s obligation to supply us with this material ends before we can develop or secure an alternative source of supply, the related product sales, which may be material, will be at risk. Any alternatives we pursue to mitigate this risk might also result in higher costs to source or produce the relevant products. Solventum’s failure to ensure a continuing supply of these materials or find acceptable substitutes, or the
47
costs incurred by Solventum in connection with securing such continuing supply or finding such substitutes, could have a material adverse effect on Solventum’s business, including potentially the loss of revenue from the relevant products.
The estimates of market opportunity and forecasts of market growth included in this information statement may prove to be inaccurate, and even if the markets in which we compete achieve the forecasted growth, our business could fail to grow at similar rates, or at all.
The estimates of market opportunity and forecasts of market growth included in this information statement may prove to be inaccurate. Market opportunity estimates and growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate, including as a result of any of the risks described in this information statement.
The variables that go into the calculation of our market opportunity are subject to change over time, and there is no guarantee that any particular number or percentage of addressable customers covered by our market opportunity estimates will purchase our products at all or generate any particular level of net revenues for us. In addition, our ability to expand in any of our target markets depends on a number of factors, including the cost, performance, quality and perceived value associated with our products and services. Even if the markets in which we compete meet the size estimates and growth forecasted in this information statement, our business could fail to grow at similar rates, or at all. Our growth is subject to many factors, including our success in implementing our business strategy, which is subject to many risks and uncertainties. Accordingly, the forecasts of market growth included in this information statement should not be taken as indicative of our future growth.
Legal and Compliance Risks
Solventum is subject to risks related to international, federal, state, and local treaties, laws, and regulations that are subject to change at any time, as well as compliance risks related to legal or regulatory requirements, contract requirements, policies and practices, or other matters that require or encourage Solventum or its suppliers, vendors, or channel partners to conduct business in a certain way. The outcome of legal and regulatory proceedings related to compliance with the treaties, laws, regulations, and requirements could have a material adverse effect on Solventum’s business, results of operations, financial condition and cash flows.
Solventum operates globally, including in some jurisdictions that pose potentially elevated risks of fraud or corruption or increased risk of internal control issues, and is subject to risks related to international, federal, state, and local treaties, laws, and regulations, including those involving product liability; antitrust; intellectual property; environmental, health, and safety; tax; the U.S. Foreign Corrupt Practices Act (“FCPA”) and other anti-bribery laws; international import and export requirements and trade sanctions compliance; regulations of the FDA and similar foreign agencies; privacy laws and information security policies and regulations; and U.S. federal healthcare program-related laws and regulations including the False Claims Act, anti-kickback laws and the Sunshine Act. Solventum is also subject to compliance risks related to legal or regulatory requirements, contract requirements, policies and practices, or other matters that require or encourage Solventum and its suppliers, vendors, or channel parties, to conduct business in a certain way. In particular, Solventum is subject to legal risks with respect to the below laws and regulations:
•Antitrust — Regulatory authorities may have authority in the event of alleged non-compliance with applicable law to impose fines and sanctions on Solventum or to require changes or impose conditions on the way Solventum conducts business. Under certain circumstances, violations of antitrust laws could result in suspension or debarment of Solventum’s ability to contract with certain parties or complete certain transactions. In addition, an increasing number of jurisdictions also provide private rights of action for competitors or consumers to seek damages asserting claims of anti-competitive conduct. An adverse outcome under any such investigation or audit could subject Solventum to fines or criminal or other penalties.
•FCPA and other anti-bribery laws — The failure to comply with the FCPA and similar anti-corruption and anti-bribery laws could result in significant civil fines and penalties or criminal sanctions against Solventum. Because of the predominance of government-sponsored healthcare systems around the world,
48
many customer relationships outside of the United States are with governmental entities, the employees of which may be considered government officials under such laws. Many anti-corruption laws also prohibit bribery of private sector individuals, and thus extend far beyond interactions with government officials. Solventum is subject to the FCPA’s accounting provisions, which require Solventum to keep accurate books and records and to maintain an adequate system of internal accounting controls sufficient to provide reasonable assurances of management’s control, authority, and responsibility over Solventum’s assets. Global enforcement of anti-corruption laws has increased substantially in recent years, with more frequent voluntary self-disclosure by companies, aggressive investigations (including coordinated investigations across countries and governmental authorities) and enforcement proceedings by U.S. and non-U.S. governmental agencies, and assessment of significant civil and criminal fines, penalties, and other sanctions against companies and individuals. From time to time, Solventum or its affiliates receive reports, internally and externally, via various reporting channels, about business and other activities that raise compliance or other legal or litigation issues. Solventum has been in the past, and in the future could be, required to investigate such reports and cooperate with U.S. and foreign regulatory authorities in such investigations, audit, monitor compliance or alter its practices as part of such investigations. While Solventum maintains and implements U.S. and international compliance programs, including policies and procedures, training, and internal controls designed to reduce the risk of noncompliance, Solventum’s employees, suppliers, vendors, channel partners or agents may violate such policies and procedures and engage in practices that contravene relevant laws and regulations. Any alleged or actual violations of these anti-corruption laws may subject Solventum to government scrutiny, criminal or civil sanctions and other liabilities, including exclusion from government contracting, and could disrupt its business, adversely affect its reputation and result in a material adverse effect on its business, results of operations, financial condition and cash flows.
•Environmental Laws — Solventum is subject to environmental, health, and safety laws, and regulations concerning, among other things, the generation, handling, transportation, and disposal of hazardous substances or wastes, the remediation of hazardous substances or materials at various sites, and emissions or discharges into the land, air or water. If Solventum or its suppliers violate these environmental laws and regulations, facilities could be shut down, and violators could be fined or otherwise sanctioned. New laws and regulations, violations of these laws or regulations, stricter enforcement of existing requirements, or the discovery of previously unknown contamination could require Solventum to incur costs or could become the basis for new or increased liabilities that could be material.
•Anti-Kickback and False Claims Laws — Solventum’s products are purchased by healthcare providers that typically bill various third-party payers, such as governmental healthcare programs (e.g., Medicare, Medicaid and comparable non-U.S. programs), private insurance plans and managed care plans, for the healthcare services provided to their patients. As a result, Solventum’s products are subject to regulation regarding quality and cost by the United States Department of Health and Human Services, including the Centers for Medicare & Medicaid Services (“CMS”), as well as comparable state and non-U.S. agencies responsible for reimbursement and regulation of healthcare goods and services, including laws and regulations related to kickbacks, false claims, self-referrals and healthcare fraud. Many states have similar laws that apply to reimbursement by state Medicaid and other funded programs as well as in some cases to all payers. As a manufacturer of products reimbursable by federal healthcare programs, Solventum is subject to the Physician Payments Sunshine Act, which requires it to annually report certain payments and other transfers of value it makes to U.S.-licensed physicians or U.S. teaching hospitals. Any failure to comply with these laws and regulations could subject Solventum or its officers and employees to criminal and civil financial penalties.
•Data Privacy and Cybersecurity Laws — Because Solventum is a business with a significant global footprint, compliance with evolving regulations and standards in data privacy and cybersecurity may result in increased costs, compliance challenges, and the threat of increased regulatory enforcement activity. Solventum’s business relies on the secure electronic transmission, storage and hosting of sensitive information, including personal information, protected health information, financial information, intellectual property and other sensitive information related to our customers and workforce. Solventum is required to comply with increasingly complex and changing legal and regulatory requirements that govern
49
the collection, use, storage, security, transfer, disclosure and other processing of personal data in the United States and in other countries, including, but not limited to, the Health Insurance Portability and Accountability Act of 1996 (HIPAA), as amended, the Health Information Technology for Economic and Clinical Health Act of 2009 (HITECH), the California Consumer Privacy Act (CCPA) and other similar state laws in the United States, the European Union’s Global Data Protection Regulation (GDPR), the United Kingdom’s Data Protection Act 2018 and General Data Protection Regulation, China’s Personal Information Protection Law, PRC Cybersecurity Law and Personal Data Cross Border Transfer Rule, and various other country-specific requirements at the state and federal level around the world. In addition, privacy laws and regulations are becoming stricter and may potentially impose additional requirements on Solventum’s business, and certain jurisdictions have implemented data localization laws which can be costly and operationally difficult to satisfy. Solventum cannot be sure how these laws and regulations will be interpreted, enforced, or applied to its operations. In addition to the risks associated with enforcement activities and potential contractual liabilities, Solventum’s ongoing efforts to comply with evolving laws and regulations may be costly and require ongoing modifications to its policies, procedures, and systems. If Solventum or third parties fail to adequately safeguard confidential personal data, or if such information or data are wrongfully used by Solventum or third parties or disclosed to unauthorized persons or entities, such an event could result in a material adverse effect on its business, results of operations, financial condition and cash flows.
Solventum’s results of operations could be adversely impacted if the costs to comply with these evolving treaties, laws, regulations, and requirements are greater than projected by Solventum. In addition, the outcome of legal and regulatory proceedings related to compliance with these treaties, laws, regulations, and requirements are difficult to reliably predict, may differ from Solventum’s expectations, and can result in, among other things, government scrutiny; criminal, civil or administrative sanctions, including fines; limitations on the extent to which Solventum can conduct business; employee and business partner terminations due to policy violations; reputational damage; private rights of action that result in litigation exposure, including expenses and costs incurred in connection with settlement or court proceedings, for Solventum; and other liabilities. In some instances, Solventum may make self-disclosures to relevant authorities that may pursue or decline to pursue enforcement proceedings against it. In addition, detecting, investigating and resolving actual or alleged violations of these acts is expensive and could consume significant time and attention of Solventum’s senior management. Although Solventum maintains general liability insurance to mitigate monetary exposure, the amount of liability that may result from certain of these risks may not always be covered by, or could exceed, the applicable insurance coverage. Various factors or developments can lead Solventum to change current estimates of liabilities and related insurance receivables where applicable or make such estimates for matters previously not susceptible of reasonable estimates, such as a significant judicial ruling or judgment, a significant settlement, significant regulatory developments or changes in applicable law. Conducting internal investigations or responding to audits or investigations by government agencies could be costly and time-consuming. A future adverse ruling, settlement or unfavorable development could result in future charges that could result in a material adverse effect on its business, results of operations, financial condition and cash flows in any particular period. In addition, negative publicity related to the matters noted above or other matters involving Solventum may negatively impact Solventum’s reputation.
Solventum may face potential liabilities related to PFAS, which could adversely impact Solventum’s results.
3M has agreed to assume, and indemnify and defend us against, certain liabilities relating to PFAS, generally including all such liabilities relating to the pre-separation period, as well as certain liabilities relating to Solventum products that contain PFAS and that continue to be sold on the same basis by Solventum following the separation through 2025. As an independent company, Solventum will generally be responsible for all PFAS-related liabilities resulting from the business, operations and activities of the Health Care Business as conducted at any time at or after the separation, subject to the foregoing indemnification by 3M of certain liabilities relating to certain Solventum products sold through 2025. See the sections titled “Certain Relationships and Related Party Transactions – Agreements with 3M – Separation and Distribution Agreement – PFAS Liabilities” and “—Risks Related to the Separation and Distribution—Satisfaction by Solventum of its post-distribution indemnification obligations or 3M’s failure to satisfy its post-distribution indemnification obligations could have a material adverse effect on Solventum’s financial condition, results of operations and cash flows.”
50
Certain products of the Solventum Business, like the products of other companies in Solventum’s industries, contain or are enabled by PFAS. 3M announced in December 2022 that it would work to discontinue the use of PFAS across its product portfolio by the end of 2025, and, as described above, 3M has agreed to indemnify Solventum for certain PFAS-related product claims related to sales of products through such date. The Health Care Business has also taken actions to meet this goal while owned by 3M, and, except as described below, Solventum intends to continue to work toward this goal following the distribution. In addition, Solventum understands that 3M intends to cease supplying Solventum with PFAS-related products or components by the end of 2025.
Solventum continues to evaluate the availability of third-party components and products that do not contain PFAS in connection with working towards the goal of discontinuing the use of PFAS in its products. Depending on the availability and feasibility of such third-party components and products not containing PFAS (including the ability to obtain any required regulatory approvals), Solventum expects there will be some circumstances in which the use of PFAS-containing materials manufactured by third parties and used in certain applications in Solventum’s product portfolio, such as o-rings, gaskets and seals, membranes, molded plastic parts, release liners, circuit boards, certain electronics and lithium ion batteries and printed circuit boards widely used in commerce across the industries in which Solventum operates, will continue beyond 2025, in which case the 3M indemnification described above would not be available with respect to sales of products containing such materials after 2025. For example, o-rings, gaskets and seals and are used in filters needed for high stress process conditions, molded plastic parts are used in the plastic housing of perioperative temperature management devices, medical grade membranes are used in negative pressure wound therapy fluid canisters, release liners are used with adhesive dressings, circuit boards are used in electronic hardgoods, such as negative pressure wound therapy devices, and lithium ion batteries are included in surgical clippers. In such instances, Solventum intends to continue to evaluate the adoption of third-party products and components that do not contain PFAS to the extent such products and components become available and such adoption is feasible. As noted above, many companies in Solventum’s industries use PFAS-containing products and components, and Solventum believes that its use of such products and components is of a nature and magnitude that is broadly consistent with that of other companies in these industries.
Solventum has noticed accelerating regulatory and legislative activities concerning PFAS in the United States, Europe and elsewhere, including increasingly strict restrictions on various uses of PFAS in products, as well as increased litigation relating to PFAS being filed against other parties. The potential options available to Solventum following the effective date of the distribution, and following 2025 (after which sales by Solventum of products containing or enabled by PFAS will no longer be subject to indemnification from 3M), regarding the use of PFAS in products will involve risks, which risks may be material, and could have a material adverse effect on Solventum’s results of operations, cash flows or consolidated financial position.
Climate change or legal, regulatory or market measures to address climate change may materially adversely affect Solventum’s business, results of operations, financial condition and cash flows.
The effects of global climate change present risks to Solventum’s business. The impacts of climate change may include physical risks (e.g., rising sea levels or frequency and severity of extreme weather conditions, including natural disasters), social and human effects (e.g., population dislocations or harm to health and well-being), compliance costs and transition risks (e.g., regulatory or technology changes), shifts in market trends (e.g., customers increasingly prioritize purchasing products that are sustainably made) and other adverse effects. Such impacts may disrupt Solventum’s supply chain and operations by adversely affecting its ability to procure goods or services required for the operation of its business at the quantities and levels it requires due to impairment of the availability and cost of certain products, materials, commodities and energy. These outcomes may in turn result in customers transitioning to competitive products, loss of market share, negative publicity, reputational damage, loss of customer confidence or other negative consequences (including a decline in stock price).
There has also been increased focus by federal, international, state and local regulatory and legislative bodies to combat and/or limit the effects of climate change through a variety of means, including regulating greenhouse gas emissions (and the establishment of enhanced internal processes or systems to track them), policies mandating or promoting the use of renewable or zero-carbon energy and sustainability initiatives, and additional taxes on fuel and energy. If legislation or regulations are enacted or promulgated in the United States or in any other jurisdiction in which Solventum does business that impose more stringent restrictions and requirements than its current legal or
51
regulatory obligations, Solventum and companies in its supply chain may experience increased compliance burdens and costs to meet the regulatory obligations, which could cause disruption in the sourcing, manufacturing and distribution of its products and adversely affect its business, results of operations, financial condition and cash flows.
Additionally, the impacts of climate change may further influence customer preferences and requirements, such as increased demand for products with lower environmental footprints, and for companies to produce and demonstrate progress against greenhouse gas reduction plans and targets. Failure to provide climate-friendly products or demonstrate greenhouse gas reductions could potentially result in loss of market share.
Solventum operates in a strictly regulated industry, and compliance with laws and regulations applicable to the commercialization of Solventum’s products is costly and failure to comply may result in significant penalties.
The products Solventum develops, manufactures, and commercializes are regulated in most of the markets Solventum serves. These regulations are promulgated and enforced by government bodies in individual countries. These regulations govern the methods and controls used for the design, manufacture, packaging, labeling, storage, safety, sales and distribution, marketing clearance or approval, advertising and promotion, sterilization, installation, servicing, performance and effectiveness of the products Solventum sells globally. These regulations apply to all facilities of Solventum’s business that conduct the activities previously described, regardless of where the facilities are located. These regulations apply to the activities performed by most of Solventum’s employees, including but not limited to, sales and marketing, research and development, regulatory affairs, quality assurance, medical affairs, and operations, both before and after a product is commercially distributed. Importantly, these regulations differ by country and/or region and are dynamic.
Solventum commits a significant amount of resources to maintain compliance with these regulations. Compliance with these regulations requires Solventum to create systems, processes and procedures that are aligned with the regulations in all markets Solventum serves. Compliance also requires Solventum to maintain knowledge of the current regulations that govern its activities. As these regulations change, Solventum must adapt its systems, processes, and procedures to comply with the new regulations.
Governing bodies monitor compliance, among other ways, by conducting regularly occurring and unexpected audits of Solventum’s facilities. These audits are conducted to determine if Solventum’s systems, processes, and procedures comply with the current regulations in the markets it serves. After each audit, the governing body typically provides a report of their findings. The report describes the observations made during the audit. Sometimes these observations describe minor non-compliance issues in Solventum’s systems, processes, and procedures. Often, these gaps require commensurate modifications but have no impact to Solventum’s ability to continue operations and commercialization of its products. In rare situations, the governing body may find significant or major non-compliance issues in Solventum’s systems, processes, and procedures. If a governing body concludes, through these audits or otherwise, that Solventum is not in compliance with applicable laws or regulations or that any of its products are defective, ineffective, or pose an unreasonable risk for patients, users, or others, the governing body may require Solventum to recall a product or products, retract promotional materials, and/or cease shipment of products, among other required actions; these requirements may remain in place until Solventum can demonstrate adequate compliance. Failure to demonstrate adequate modifications to Solventum’s systems, processes, and procedures and continued compliance or repeat findings may result in more significant enforcement actions including but not limited to: warning letters, revocation of product approvals and licenses, injunctions, product seizure, penalties and fines, consent decrees, and criminal prosecution, among other actions. These actions may have a negative impact to Solventum’s capital expenditures, earnings, and competitive position.
To market its products internationally in compliance with applicable medical device and pharmaceutical regulations, Solventum must obtain approvals for products and product modifications. The regulations promulgated by the governing bodies also require Solventum to submit data to demonstrate that its products meet the safety and effectiveness requirements to support the intended uses described in its labeling. This data is reviewed by the governing bodies to determine if Solventum has provided the necessary and sufficient information to demonstrate the safety and effectiveness of its products for the intended use described in its labeling. In many cases, the governing bodies request additional information to make this determination. The additional information requested by the governing bodies sometimes requires Solventum to conduct new testing, delaying the approval and
52
commercialization of the product. In rare instances, the governing body will disapprove the application, prohibiting Solventum’s ability to commercialize the product in that market. In these instances, Solventum may decide to cease commercialization efforts for the product in that (or those) market(s) or it may decide to modify the product or retest the products and resubmit the data to the governing bodies. Delays to products approvals or disapproval of Solventum’s applications may have a negative impact to Solventum’s capital expenditures, earnings, and competitive position.
This global regulatory environment will likely continue to evolve, which could impact Solventum’s ability, or increase the time and cost, to obtain future approvals for its products. The process of obtaining regulatory clearances and/or approvals to market and sell Solventum’s products can be rigorous, costly and time-consuming and the clearances and/or approvals might not be granted timely or result in limitations on the indicated uses of products.
Regulatory authorities, including the FDA, also strictly regulate the indications for use and associated promotional safety and effectiveness claims that may be made about approved or cleared products. If regulatory authorities determine that Solventum has promoted or marketed a product for off-label use, including through external-facing materials, oral statements, or physician training, Solventum could be subject to fines, injunctions or other penalties.
Solventum is subject to laws and regulations governing government contracts, public procurement, and government reimbursements in many jurisdictions, as to which the failure to comply could adversely affect Solventum’s business.
Solventum sells its products to government entities throughout the world and will be directly or indirectly subject to government policies governing reimbursement for healthcare procedures and services and various statutes and regulations in a variety of jurisdictions that apply to companies doing business with the government. The laws governing government contracts can differ from the laws governing private contracts and government contracts may contain terms and conditions that are not applicable to private contracts or that expose Solventum to higher levels of risk and potential liability than non-government contracts. Similarly, most jurisdictions have public procurement laws and reimbursement policies that set out rules and regulations for purchases and reimbursements by governmental entities. Solventum’s failure to comply with these laws could result in contract terminations, suspension or debarment from contracting with these entities, civil fines and damages, criminal prosecution and possible exclusion from participation in federal healthcare programs such as Medicare and Medicaid, as well as possible recoupment of any overpayments related to such violations. These jurisdictions may modify their laws, policies, rules or regulations, or impose new requirements that adversely affect Solventum’s business.
Additionally, some governmental entities, including the U.S. federal government, can terminate contracts for their convenience or for Solventum’s default. These governmental entities may also be subject to continued legislative funding approval. Early termination for convenience of one or more of Solventum’s contracts, or a change in a government customer’s funding levels, could impact Solventum’s expected revenues. Early termination for default of one or more of Solventum’s contracts could subject it to penalties and damages resulting from the default, including costs for the governmental entity to reprocure the items under contract, in addition to other penalties previously listed.
Solventum will also be subject to government audits, investigations, and oversight proceedings. Efforts to ensure its business arrangements comply with applicable laws will involve substantial costs. If any actions by governmental or enforcement authorities are instituted against Solventum, defense can be costly, time-consuming, and may require significant financial and personnel resources. If Solventum is not successful in defending itself or asserting its rights, those actions could have a significant impact on its business, including the imposition of civil, criminal, and administrative penalties, damages, disgorgement, monetary fines, individual imprisonment, possible exclusion from participation in certain government healthcare programs (including Medicare and Medicaid in the United States), contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of its operations. In addition, any of Solventum’s government contracts could be terminated or Solventum could be suspended or debarred from all government contract work.
53
Solventum is exposed to risks associated with product liability claims, including existing claims and claims resulting from the actions or inactions of its customers or third parties that are outside of its control.
Solventum is exposed to potential product liability risks that are inherent in the design, manufacture and marketing of medical technologies. Customers or their patients may bring product liability claims if Solventum’s products fail, or allegedly fail, to perform as expected or show a failure rate that is higher than expected, or the use of Solventum’s products results, or is alleged to result, in bodily injury, death, or property damage. Even if these or similar claims are without merit, they can result in costly and time-consuming litigation. Solventum may also be exposed to claims or regulatory action if its products do not conform or are alleged not to conform to applicable product or design specifications, labeling, or manufacturing requirements. Product and other liability actions, claims or injunctions are subject to significant uncertainty and may be expensive, time-consuming, and disruptive to Solventum’s operations. For these and other reasons, Solventum may choose to settle product liability claims and other liability actions, regardless of their actual merit. If any such action or injunction were finally determined adversely to Solventum, such decision could result in significant damages and reputational harm, including the possibility of punitive damages, and Solventum’s financial position could be adversely affected. Adverse publicity could result in additional regulation of Solventum’s products or the healthcare industry in general, delay regulatory approval of new products, cause reputational harm and adversely affect Solventum’s ability to promote, manufacture and sell its products, even if the claims against Solventum are later shown to be unfounded or unsubstantiated.
In addition, manufacturing or design defects, component failures, unapproved or improper use of Solventum’s products, or inadequate disclosure of risks or other information relating to the use of Solventum’s products could lead to injury or other serious adverse events. Such events could lead to recalls or safety alerts relating to Solventum’s products (either voluntary or as required by the FDA or similar governmental authorities in other countries), and could result, in certain cases, in the removal of a product from the market. A recall could result in significant costs and lost sales and customers, enforcement actions and/or investigations by state and federal governments or other enforcement bodies, as well as negative publicity and damage to Solventum’s reputation that could reduce future demand for its products.
Information Technology and Intellectual Property Risks
Solventum employs information technology systems to support its business and collect, store, and use proprietary and confidential information. Security and data breaches, cyberattacks, and other cybersecurity incidents involving Solventum’s information technology systems and infrastructure could disrupt or interfere with Solventum’s operations; result in the compromise and misappropriation of proprietary and confidential information belonging to Solventum or its customers, suppliers, and employees; and expose Solventum to numerous expenses, liabilities, and other negative consequences, including violations of applicable laws, any or all of which could adversely impact Solventum’s business, reputation, and results of operations.
In the ordinary course of business, Solventum relies on centralized and local information technology networks and systems, some of which are provided, hosted or managed by vendors and other third parties, to process, transmit, and store electronic information, and to manage or support a variety of businesses. That technology includes systems that could be used to process, transmit and store sensitive information, including personal information, protected health information, employee data, financial information, intellectual property, clinical data, and sales and marketing data. Third parties and threat actors, including organized criminals, nation-state, or nation-state supported actors who are increasingly well-resourced, regularly attempt to gain unauthorized access to the information technology networks and infrastructure, data, and other information used by or belonging to Solventum, and many such attempts are increasing in their frequency, sophistication and intensity and are not recognized until launched against a target. Despite Solventum’s cybersecurity and business continuity measures (including employee and third-party training, monitoring of networks and systems, patching, maintenance, and backup of systems and data), its information technology networks and infrastructure are still potentially susceptible to attack, compromise, damage, disruption, or shutdown, including as a result of the exploitation of known or unknown hardware or software vulnerabilities in its systems, the introduction of computer viruses or ransomware, service or cloud provider disruptions or security breaches, phishing attempts, employee error or malfeasance, power outages, telecommunication or utility failures, systems failures, natural disasters, or other catastrophic events. Furthermore, Solventum relies on third-party vendors to supply and/or support certain aspects of its information technology
54
systems and resulting products. These third-party systems could also become vulnerable to cyber-attack, malicious intrusions, breakdowns, interference, or other significant disruptions, and may contain defects in design or manufacture or other problems that could result in system disruption or compromise the information security of Solventum’s own systems. Solventum’s increased adoption of remote working, initially driven by the pandemic, also introduces additional threats and risk of disruptions to its information technology networks and infrastructure. Geopolitical conflict may increase cybersecurity risks on a global basis.
Despite its cybersecurity measures, it is possible for security vulnerabilities or a cyberattack to remain undetected for an extended time period, up to and including several years, and the prioritization of decisions with respect to security measures and remediation of known vulnerabilities that Solventum and the vendors and other third parties upon which Solventum relies may prove inadequate to protect against attacks. While Solventum may experience in the future cyberattacks on and disruptions of its information technology systems and infrastructure, Solventum is not aware of any such incidents to date having had a material impact.
If Solventum’s information technology systems, products or services or sensitive data are compromised, there are many consequences that could result. Consequences include, but are not limited to, patients or employees being exposed to financial or medical identity theft or suffering a loss of product functionality; losing existing customers or having difficulty attracting new customers; experiencing difficulty preventing, detecting, and controlling fraud; being exposed to the loss or misuse of confidential information; having disputes with customers, physicians, and other healthcare professionals; experiencing increases in operating expenses or an impairment in its ability to conduct its operations; incurring expenses or losing revenues as a result of a data privacy breach, product failure, information technology outages or disruptions; voluntary or forced recalls or modifications to Solventum’s products; or suffering other adverse consequences including time-consuming and expensive lawsuits or other legal action and damage to Solventum’s reputation.
As noted previously, Solventum is subject to numerous international, federal and state privacy and security laws. Security and data breaches, cyberattacks, and other cybersecurity incidents involving data protected by such laws, such as patient medical records and other health information, could subject Solventum to onerous governmental and regulatory investigations, fines and remediation actions, in addition to private litigation by affected individuals.
Solventum may be unable to obtain, maintain, protect, or effectively enforce its intellectual property rights.
Solventum is substantially dependent on patent and other proprietary rights and relies on a combination of patents, trademarks, tradenames, copyrights, trade secrets, and agreements (such as employee, non-disclosure and non-competition agreements) to protect its business and proprietary intellectual property. However, Solventum cannot assure that its means of obtaining, maintaining, and enforcing its intellectual property rights will be adequate to maintain a competitive advantage.
In addition, intellectual property laws differ in various jurisdictions in which Solventum operates and are subject to change at any time, which could further restrict Solventum’s ability to protect its intellectual property and proprietary rights. In particular, a portion of Solventum’s revenues is derived from jurisdictions where adequately protecting intellectual property rights may prove more challenging or impossible. In addition, the laws of many jurisdictions may not provide an adequate forum to effectively address situations where Solventum’s intellectual property rights have been compromised.
Furthermore, protecting against the unauthorized use of proprietary technology is difficult and expensive and Solventum may need to litigate with third parties to enforce or defend patents issued to it or to determine the enforceability and validity of its proprietary rights or those of others. Determining whether an offering infringes, misappropriates, or otherwise violates a third party’s intellectual property rights involves complex legal and factual issues, and the outcome of this type of litigation is often uncertain and inconsistent.
At any given time, Solventum may be involved as either plaintiff or defendant in a number of patent infringement actions, the outcomes of which may not be known for prolonged periods of time. While it is not possible to predict the outcome of patent and other intellectual property litigation, such litigation could in the future result in Solventum’s payment of significant monetary damages and/or royalty payments, negatively impact
55
Solventum’s ability to sell current or future products, or prohibit it from enforcing its patent and proprietary rights against others, which could result in a material adverse effect on its business, results of operations, financial condition and cash flows. Regardless of the merits or outcome, the resolution of any intellectual property dispute could require significant financial and management resources.
Solventum may not receive protection for pending or future applications relating to intellectual property rights owned by or licensed to it and the claims allowed under any issued intellectual property rights may not be sufficiently broad to protect Solventum’s products, services, solutions, and any associated trademarks. Products sold by Solventum’s competitors may infringe, misappropriate, or otherwise violate intellectual property rights owned or licensed by Solventum and such infringement, misappropriation or violation may be undetected by Solventum. In addition, as Solventum’s patents expire, Solventum may be unsuccessful in extending their protection through patent term extensions. Solventum’s inability to protect its intellectual property could result in a material adverse effect on its business, results of operations, financial condition and cash flows.
Additionally, Solventum licenses or will license certain intellectual property owned by 3M. As 3M is the owner of such intellectual property, 3M will have discretion to enforce such intellectual property rights, including whether or not to prosecute any infringement of the intellectual property. To the extent 3M does not prosecute infringement of the intellectual property licensed to Solventum, such intellectual property will not be protected and continued infringement of such intellectual property could result in a material adverse effect on Solventum’s business, results of operations, financial condition and cash flows.
Solventum intends to focus on and invest in cloud, edge, artificial intelligence, and software offerings. Solventum may not be successful in driving the successful global deployment and customer adoption of digital offerings.
Solventum intends to devote significant resources to develop and deploy cloud, edge and software solutions. Solventum will incur costs to develop these solutions and to build and maintain infrastructure to support cloud and edge computing offerings. It is uncertain whether Solventum’s strategies will succeed and Solventum’s success with these solutions depends on execution in many areas, including:
•establishing and maintaining the utility, compatibility, and performance of its cloud, edge, and software solutions (including the reliability of its third-party software vendors, network, and cloud providers) on a growing array of medical devices, software, and equipment;
•continuing to enhance the attractiveness of its solutions to its customers, while ensuring these solutions meet their reliability and security expectations; and
•ensuring these solutions meet regulatory requirements, including obtaining marketing authorizations when required.
Even where Solventum’s digital offerings satisfy applicable regulations and reimbursement policies, customers may not adopt them due to concerns about the security of personal data or the absence of digital infrastructure to support and effectively use the offerings, a hesitancy to embrace new technology, or for other reasons. Solventum also may not effectively execute organizational and technical changes to accelerate innovation and execution. In a number of countries, certain cloud, edge, and software solutions are restricted areas of foreign investment. Collaborating with a domestic qualified third party will increase the costs and may create uncertainties in such jurisdictions. The legality or validity of any collaboration may be challenged or subjected to scrutiny in such jurisdictions and the relevant governmental authorities have broad discretion in addressing such arrangements.
Cloud, edge, and software solutions in healthcare must comply with stringent regulations, including certification requirements, in many of the countries in which Solventum’s customers are located, particularly in relation to obtaining, using, storing, and transferring personal data. Solventum’s software solutions must be compliant with applicable regulations in the country in question before Solventum can launch its offerings. In some jurisdictions, Solventum must obtain marketing authorizations before commercializing software solutions. Ensuring such regulatory compliance may take longer or cost more than expected or require that design changes be incorporated into Solventum’s offerings. In addition, changes to reimbursement policies for digital healthcare offerings could potentially lead to delays and additional expense. The inability of customers to obtain adequate reimbursement from
56
private and governmental third-party payers could adversely affect purchasing decisions and prices and cause Solventum’s revenue and profitability to suffer.
Solventum intends to build artificial intelligence (“AI”) into many of its digital offerings, which presents risks and challenges that could affect its acceptance, including flawed AI algorithms, insufficient or biased datasets, unauthorized access to personal data, lack of acceptance from its customers, or failure to deliver positive outcomes. These deficiencies could undermine the decisions, predictions, or analyses that AI applications produce, as well as their adoption, subjecting Solventum to competitive harm, legal liability, regulatory actions, and reputational harm. In addition, some AI scenarios present ethical, privacy, or other social issues, risking reputational harm.
Tax Matters Risks
Changes in tax rates, laws or regulations could adversely impact Solventum’s financial results.
Solventum’s business is subject to tax-related external conditions, such as tax rates, laws and regulations in the United States and foreign jurisdictions. Changes in tax rates, laws or regulations, including further developments arising from tax reform legislation or regulation in the United States or foreign jurisdictions, could impact Solventum’s financial statements.
In particular, Solventum could be negatively impacted by the Base Erosion and Profit Shifting 2.0 initiative (“BEPS 2.0”) by the Organization for Economic Cooperation and Development (“OECD”) which, if enacted by OECD member countries, would likely impact the amount of tax that multinationals, such as Solventum, pay in the future.
Due to the uncertainty of any tax changes and other tax-related factors at this time, it is currently not possible to assess the ultimate impact these actions may have on Solventum’s financial statements. Solventum intends to monitor BEPS 2.0 and other tax-related developments in the United States and foreign jurisdictions, including rule changes and implementation timing, to evaluate the impact BEPS 2.0 and other tax legislation or regulation may have on Solventum’s financial results.
Solventum’s tax burden could increase as a result of ongoing or future tax audits and inquiries.
Solventum is subject to periodic tax audits and inquiries by tax authorities. Tax authorities may disagree with Solventum’s interpretation of applicable tax laws and regulations, which could result in examination from taxing authorities and additional taxes. Solventum regularly assesses the likely outcomes of these tax audits in order to determine the appropriateness of Solventum’s tax provision. However, Solventum may not accurately predict the outcomes of these tax audits and, as a result, the ultimate outcome of any of these examinations could have a retroactive or prospective impact on Solventum’s overall tax burden.
Solventum could be negatively impacted by future changes in the allocation of income to each of the income tax jurisdictions in which Solventum operates.
Solventum operates in multiple income tax jurisdictions both in the United States and internationally. Solventum has adopted transfer pricing policies that determine how income is allocated to each of the income tax jurisdictions in which Solventum operates, based on current interpretations of complex income tax regulations. The allocation of Solventum’s income could be impacted by different factors including tax law changes, tax audits, underlying business changes, organizational changes, or operating model changes, which could result in increases to Solventum’s overall tax burden.
Solventum may benefit from various global tax incentives intended to encourage investment or employment; if Solventum’s incentives are not renewed or Solventum cannot or does not wish to satisfy all or part of the tax incentive conditions, Solventum may lose the tax incentives and could be required to refund tax incentives previously realized.
Solventum benefits or may benefit in the future from various global tax incentives intended to encourage investment or employment. If Solventum’s incentives are not renewed or Solventum cannot or does not wish to satisfy all or part of the tax incentive conditions, Solventum may lose the tax incentives and could be required to
57
refund tax incentives previously realized or granted. As a result, Solventum’s tax burden could be higher than it would have been had Solventum maintained the benefits of the tax incentives.
Employee Matters Risks
If Solventum is unable to attract or retain key personnel and qualified employees, or maintain relations with its employees, unions, and other employee representatives, Solventum’s business would be adversely affected.
There is substantial competition for key personnel, senior management, research and development personnel, and qualified employees in the healthcare industry and Solventum may face increased competition for such a highly qualified scientific, technical, clinical, and management workforce in a highly competitive environment. Solventum’s ability to recruit and retain such talent will depend on a number of factors, including how its compensation, benefits, work location and work environment compare with those offered by its competitors and other local employers, and whether any changes in the employee benefits offered by Solventum relative to those previously offered to Solventum employees by 3M are favorably received by Solventum personnel. There can be no assurance that Solventum will be successful in retaining existing personnel or recruiting new personnel.
The loss of one or more key employees, inability to attract or develop additional qualified employees, any delay in hiring key personnel, any deterioration of the relationships with its employees, or any material work stoppage, strike, or similar action could result in a material adverse effect on its business, results of operations, financial condition and cash flows.
Risks Related to the Separation and Distribution
Solventum has no history of operating as an independent company, and its historical and pro forma financial information is not necessarily representative of the results that it would have achieved as a separate, publicly traded company and may not be a reliable indicator of its future results.
The historical information about Solventum in this information statement refers to the Health Care Business as operated by and integrated with 3M. The historical and pro forma financial information of Solventum included in this information statement is derived from the Consolidated Financial Statements and accounting records of 3M. Accordingly, the historical and pro forma financial information included in this information statement does not necessarily reflect the financial condition, results of operations or cash flows that the Health Care Business would have achieved as a separate, publicly traded company during the periods presented or those that Solventum will achieve in the future primarily as a result of the factors described below:
•Generally, Solventum’s working capital requirements and capital for its general corporate purposes, including capital expenditures and acquisitions, have historically been satisfied as part of the corporate-wide cash management policies of 3M. Following the completion of the distribution, Solventum’s results of operations and cash flows may be more volatile, and Solventum may need to obtain additional financing from banks, through public offerings or private placements of debt or equity securities, strategic relationships or other arrangements, which may or may not be available and may be more costly.
•Prior to the distribution, Solventum’s business has been operated by 3M as part of its broader corporate organization, rather than as an independent company. 3M or one of its affiliates performed various corporate functions for the Health Care Business, such as information technology, legal, treasury, accounting, auditing, human resources, investor relations, and finance. The Health Care Business (as defined in the historical combined financial statements included in this information statement) historical and pro forma financial results reflect allocations of corporate expenses from 3M for such functions, which may be less than the expenses the Health Care Business would have incurred had it operated as a separate publicly traded company. Solventum may also be unable to replicate corporate functions that will operate with the same degree of effectiveness as the equivalent 3M functions that the Health Care Business has historically benefited from.
•Currently, Solventum’s business is integrated with the other businesses of 3M. Historically, Solventum’s business benefited from 3M’s economies of scope and scale in costs, employees, vendor relationships and
58
customer relationships. While Solventum has sought to minimize the impact on its business when separating these arrangements, there is no guarantee these arrangements will continue to capture these benefits in the future. Additionally, as a standalone company, Solventum may be unable to obtain similar arrangements to the same extent as 3M did, or on terms as favorable as those 3M obtained, prior to the distribution. Among other benefits, the Health Care Business currently has access to 3M’s extensive global research and development resources, which have historically enhanced the Health Care Business’s ability to innovate, develop new products and technologies, and improve and update existing products and technologies. Solventum’s lack of access to these research and development resources following the separation may negatively impact on the Health Care Business.
•After the completion of the distribution, the cost of capital for Solventum’s business may be higher than 3M’s cost of capital prior to the distribution.
•Solventum’s historical financial information does not reflect the debt that Solventum will incur as part of the distribution.
•As an independent public company, Solventum will separately become subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act and the Dodd-Frank Wall Street Reform and Consumer Protection Act and will be required to prepare its standalone financial statements according to the rules and regulations required by the SEC. These reporting and other obligations will place significant demands on Solventum’s management and administrative and operational resources. Moreover, to comply with these requirements, Solventum anticipates that it will need to migrate its systems, including information technology systems, implement additional financial and management controls, reporting systems and procedures, and hire additional accounting and finance staff. Solventum expects to incur additional annual expenses related to these steps, and those expenses may be significant. If Solventum is unable to implement appropriate financial and management controls, reporting systems, information technology and procedures in a timely and effective fashion, its ability to comply with financial reporting requirements and other rules that apply to reporting companies under the Exchange Act could be impaired.
Other significant changes may occur in Solventum’s cost structure, management, financing and business operations as a result of operating as a company separate from 3M. For additional information about the past financial performance of its business and the basis of presentation of the historical combined financial statements and the unaudited pro forma condensed combined financial statements of its business, see “Unaudited Pro Forma Condensed Combined Financial Information,” “Summary Historical and Unaudited Pro Forma Financial Information,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the historical combined financial statements and accompanying notes included elsewhere in this information statement.
Following the separation, Solventum’s financial profile will change, and it will be a smaller, less diversified company than 3M prior to the separation.
The separation will result in Solventum being a smaller, less diversified company than 3M. As a result, Solventum may be more vulnerable to changing market conditions, which could have a material adverse effect on its business, financial condition and results of operations. In addition, the diversification of Solventum’s revenues, costs, and cash flows will diminish as a standalone company, such that its results of operations, cash flows, working capital and financing requirements may be subject to increased volatility and its ability to fund capital expenditures and investments, pay dividends and service debt may be diminished. Following the separation, Solventum may also lose capital allocation efficiency and flexibility, as Solventum will no longer have access to cash flow from 3M to fund Solventum’s business. Solventum will also be more exposed to matters such as foreign currency exchange rates as a smaller, standalone company than it had been as a part of the larger 3M enterprise.
Solventum may not achieve some or all of the expected benefits of the separation.
Solventum may not be able to achieve the full strategic and financial benefits expected to result from the separation, or such benefits may be delayed or not occur at all. The separation is expected to provide the following benefits, among others: (1) the ability to pursue tailored capital allocation strategies and make company-specific investment decisions to drive innovation and growth; (2) enhanced management focus, with each public company
59
having distinct boards and management teams with relevant expertise able to focus on strengthening its business; (3) improved operational agility and focus, enabling each of 3M and Solventum to pursue its distinct operating priorities and strategies with increased flexibility to act based on its unique characteristics, better positioning each for long-term success; (4) greater access to capital through the creation of distinct and compelling investment profiles appealing to different long-term investor bases; (5) independent equity currencies, enabling each company to use its own industry-focused stock to consummate future acquisitions or other transactions; and (6) enhanced recruitment and retention, including by aligning employee, management, and board incentives with performance.
Solventum may not achieve these and other anticipated benefits for a variety of reasons, including, among others: (1) the separation will demand significant management resources and require significant amounts of management’s time and effort, which may divert management’s attention from operating and growing Solventum’s business; (2) following the separation, Solventum may be more susceptible to market fluctuations and other adverse events than if it were still a part of 3M because Solventum’s business will be less diversified than 3M’s businesses prior to the completion of the separation; (3) after the separation, as a standalone company, Solventum may be unable to obtain certain goods, services and technologies at prices or on terms as favorable as those 3M obtained prior to completion of the separation; (4) the separation may require Solventum to pay costs that could be substantial and material to its financial resources, including accounting, tax, legal and other professional services costs, recruiting and relocation costs associated with hiring key senior management and personnel new to Solventum, tax costs and costs to separate information systems; (5) under the terms of the tax matters agreement that Solventum will enter into with 3M, Solventum is expected to be restricted from taking certain actions that could cause the distribution or certain related transactions (or certain transactions undertaken as part of the internal reorganization) to fail to qualify as tax-free transactions and these restrictions may limit Solventum for a period of time from pursuing certain strategic transactions and equity issuances or engaging in other transactions that might increase the value of its business; and (6) the contractual arrangements between Solventum and 3M may be on less favorable terms than the existing intercompany arrangements from which Solventum benefits, and such arrangements may be inadequate to provide for the ongoing operation and growth of Solventum’s business. If Solventum fails to achieve some or all of the benefits expected to result from the separation, or if such benefits are delayed, it could have a material adverse effect on its competitive position, business, financial condition, results of operations and cash flows.
Solventum may be held liable to 3M if it fails to perform under its agreements with 3M, and the performance of such services may negatively affect Solventum’s business and operations.
In connection with the separation, Solventum and 3M will enter into various agreements, including a transition services agreement, a transition distribution services agreement, a transition contract manufacturing agreement, research and development master services agreements and master supply agreements. See “Certain Relationships and Related Party Transactions.” These agreements will provide for the performance of certain services or provision of goods by each company for the benefit of the other for a period of time after the separation. If Solventum does not satisfactorily perform its obligations under these agreements, it may be held liable for any resulting losses suffered by 3M, subject to certain limits. In addition, during the transition support periods under the transition arrangements, Solventum’s management and employees may be required to divert their attention away from its business in order to provide services to 3M, which could adversely affect Solventum’s business.
If Solventum is unable to replace the services that 3M currently provides to it on terms that are at least as favorable to Solventum as the terms on which 3M is providing such services, Solventum’s business and results of operations could be adversely affected.
Solventum will engage in the process of creating its own, or engaging third parties separate from 3M to provide, systems and services to replace many of the systems and services that 3M currently provides to Solventum, including, for example, research and development support, information technology infrastructure and systems and accounting and reporting systems. Solventum may incur temporary interruptions in business operations if it cannot transition effectively from 3M’s existing operating systems, databases and programming languages that support these functions to its own systems. The process of implementing an information technology infrastructure, in particular, is expected to be expensive and time-consuming, and any difficulty or delay in developing such an infrastructure or transitioning from 3M’s information technology environment and systems could be disruptive to
60
Solventum’s business operations and create risks to Solventum’s relationships with customers and other third parties. The failure to implement the new systems and transition data successfully and cost-effectively could disrupt Solventum’s business operations and have a material adverse effect on its profitability. In addition, Solventum’s costs for the operation of these systems may be higher than the amounts reflected in its historical combined financial statements.
3M may not satisfy its obligations under various transaction agreements that have been or will be executed as part of the separation or Solventum may not have necessary systems and services in place when certain of the agreements with 3M expire.
Solventum and 3M will enter into various agreements that will provide for the performance of services or provision of goods by 3M for the benefit of Solventum. See “Certain Relationships and Related Party Transactions.” Under some of these agreements such as the research and development master services agreements and the master supply agreements, 3M will provide services or goods to Solventum for a long-term duration, while under other agreements such as the transition services agreement, 3M will provide the services for a shorter transition period. Solventum will rely on 3M to satisfy its obligations under these agreements not only for a successful transition but also for the success of its long-term operations. If 3M is unable to satisfy its obligations and fully perform under these agreements, Solventum could experience operational difficulties or losses.
With respect to the transition period agreements, if Solventum does not have its own systems and services in place, or if Solventum does not have agreements with other providers of these services when these agreements terminate, Solventum may not be able to operate its business effectively and its profitability may decline. Solventum is in the process of creating its own, or engaging third parties to provide, systems and services to replace many of the systems and services 3M currently provides to Solventum. Solventum may not be successful in effectively or efficiently implementing these systems and services or in transitioning data from 3M’s systems to Solventum’s. These systems and services may also be more expensive or less efficient than the systems and services 3M is expected to provide during the transition period to Solventum.
The longer term agreements with 3M are expected to provide research and development services and certain products to Solventum. There could be significant short-term and long-term disruptions to Solventum’s business and operations if 3M does not perform or does not perform adequately under these agreements. Following the expiration of the initial terms of these agreements and any automatic or required extensions, there is no guarantee that 3M will agree to renew these agreements or if 3M does agree to renew these agreements, that it will do so on substantially the same terms. If 3M and Solventum are unable to renew these agreements after they expire, Solventum may not be able to find replacement providers or replacement services or goods on substantially the same or better terms and quality.
Solventum’s accounting and other management systems and resources may not be adequately prepared to meet the financial reporting and other requirements to which it will be subject as a standalone publicly traded company following the distribution.
Solventum’s financial results previously were included within the consolidated results of 3M. Solventum was not directly subject to the reporting and other requirements of the Exchange Act. As a result of the distribution, Solventum will be directly subject to reporting and other obligations under the Exchange Act, including the requirements of Section 404 of Sarbanes-Oxley Act, which will require annual management assessments of the effectiveness of its internal control over financial reporting and a report by its independent registered public accounting firm. These reporting and other obligations will place significant demands on Solventum’s management and administrative and operational resources, including accounting resources.
Moreover, to comply with these requirements, Solventum anticipates that it will need to migrate its systems, including information technology systems, implement additional financial and management controls, reporting systems and procedures and hire additional accounting and finance staff. Solventum expects to incur additional annual expenses related to these steps, and those expenses may be significant. If Solventum is unable to implement appropriate financial and management controls, reporting systems, information technology and procedures in a timely and effective fashion, its ability to comply with its financial reporting requirements and other rules that apply
61
to reporting companies under the Exchange Act could be impaired. Any failure to achieve and maintain effective internal controls could have a material adverse effect on its business, financial condition, results of operations and cash flows.
In connection with the distribution, Solventum expects to incur debt obligations, and Solventum may incur additional obligations in the future, which could adversely affect its business and profitability and its ability to meet other obligations.
In connection with the distribution, we have completed certain financing transactions. As a result of such transactions, Solventum anticipates having approximately $8.4 billion of outstanding indebtedness upon completion of the distribution. Solventum may also incur additional indebtedness in the future.
This significant amount of debt could potentially have important consequences to Solventum and its debt and equity investors, including:
•requiring a substantial portion of its cash flow from operations to make interest payments;
•making it more difficult to satisfy debt service and other obligations;
•increasing the risk of a future credit ratings downgrade of its debt, which could increase future debt costs and limit the future availability of debt financing;
•increasing its vulnerability to general adverse economic and industry conditions;
•reducing the cash flow available to fund capital expenditures and other corporate purposes and to grow its business;
•limiting Solventum’s flexibility in planning for, or reacting to, changes in its business and the industry;
•placing Solventum at a competitive disadvantage relative to its competitors that may not be as highly leveraged with debt;
•requiring Solventum to repatriate earnings to the United States, causing withholding taxes to be applied, which in turn could increase Solventum’s effective tax rate; and
•limiting Solventum’s ability to borrow additional funds as needed or take advantage of business opportunities as they arise, pay cash dividends or repurchase ordinary shares.
To the extent that Solventum incurs additional indebtedness, the foregoing risks could increase. In addition, Solventum’s actual cash requirements in the future may be greater than expected. Its cash flow from operations may not be sufficient to repay all of the outstanding debt as it becomes due, and Solventum may not be able to borrow money, sell assets or otherwise raise funds on acceptable terms, or at all, to refinance its debt.
Additionally, the Health Care Business has historically relied upon 3M to fund its working capital requirements and other cash requirements. After the separation and distribution, Solventum will not be able to rely on the earnings, assets, or cash flow of 3M, and 3M will not provide funds to finance Solventum’s working capital or other cash requirements. As a result, after the separation and distribution, Solventum will be responsible for servicing its own debt and obtaining and maintaining sufficient working capital and other funds to satisfy its cash requirements. After the separation and distribution, Solventum’s access to and cost of debt financing will be different from the historical access to and cost of debt financing under 3M. Differences in access to and cost of debt financing may result in differences in the interest rate charged to Solventum on financings, as well as the amount of indebtedness, types of financing structures and debt markets that may be available to Solventum. Solventum’s ability to make payments on and to refinance its indebtedness, including the debt incurred in connection with the separation and distribution, as well as any future debt that Solventum may incur, will depend on its ability to generate cash in the future from operations, financings, or asset sales. Solventum’s ability to generate cash is subject to general economic, financial, competitive, legislative, regulatory, and other factors that are beyond Solventum’s control.
62
Solventum will no longer benefit from 3M’s established brand and reputation following the separation.
3M and its businesses, including the Health Care Business, currently benefit from 3M’s long operating history, reputation and well-known brand. 3M’s brand recognition may confer benefits to its various businesses, including an enhanced ability to win customer contracts, form brand partnerships, and attract employees, among others. Following the separation, Solventum will operate under its own brand, and accordingly may be negatively impacted due to the loss of benefits conferred by 3M’s brand recognition and reputation.
Solventum may not be able to engage in desirable capital-raising or strategic transactions following the separation.
Under current U.S. federal income tax law, a spin-off that otherwise qualifies for tax-free treatment can be rendered taxable to the parent corporation and its shareholders as a result of certain post-spin-off transactions, including certain acquisitions of shares or assets of the spun-off corporation. To preserve the tax-free treatment of the distribution and certain related transactions, and in addition to Solventum’s indemnity obligation described below, the tax matters agreement will restrict Solventum, for the two-year period following the distribution, except in specific circumstances, from (1) entering into any transaction pursuant to which all or a portion of the shares of Solventum stock would be acquired, whether by merger or otherwise; (2) issuing equity securities beyond certain thresholds; (3) repurchasing shares of Solventum stock other than in certain open-market transactions; and (4) ceasing to actively conduct certain of Solventum’s businesses. Further, the tax matters agreement is expected to impose similar restrictions on Solventum and its subsidiaries that are intended to prevent certain transactions undertaken as part of the internal reorganization from failing to qualify as transactions that are generally tax-free for U.S. federal income tax purposes under Sections 355 and 368(a)(1)(D) of the Code or for applicable non-U.S. income tax purposes. The tax matters agreement is also expected to prohibit Solventum from taking or failing to take any other action that would prevent the distribution and certain related transactions (or certain transactions undertaken as part of the internal reorganization) from qualifying as tax-free transactions under applicable law. These restrictions may limit Solventum’s ability to pursue certain equity issuances, strategic transactions or other transactions that it may otherwise believe to be in the best interests of its shareholders or that might increase the value of its business. For more information, see the section entitled “Certain Relationships and Related Party Transactions—Agreements with 3M—Tax Matters Agreement.”
Solventum’s agreements with 3M may be on terms that are less beneficial to Solventum than the terms may have otherwise been from unaffiliated third parties.
The agreements that Solventum will enter into with 3M in connection with the separation include the separation and distribution agreement, a transition services agreement, a transition distribution services agreement, a transition contract manufacturing agreement, research and development master services agreements, real estate license agreements, an intellectual property cross license agreement, a 3M mark use agreement, a transition trademark license agreement, master supply agreements, a tax matters agreement, an employee matters agreement, and a stockholder’s and registration rights agreement. See “Certain Relationships and Related Party Transactions.” These agreements were prepared in the context of the separation while Solventum was still a wholly owned subsidiary of 3M. Accordingly, during the period in which the terms of those agreements were prepared, Solventum did not have an independent Board of Directors or a management team that was independent of 3M. As a result, the terms of those agreements may not reflect terms that would have resulted from arm’s-length negotiations between unaffiliated third parties.
63
If the distribution, together with certain related transactions, were to fail to qualify as a transaction that is generally tax-free for U.S. federal income tax purposes, Solventum, as well as 3M and its shareholders, could be subject to significant tax liabilities. In addition, if certain internal restructuring transactions were to fail to qualify as transactions that are generally tax-free for U.S. federal or non-U.S. income tax purposes, Solventum, as well as 3M, could be subject to significant tax liabilities. In certain circumstances, Solventum could be required to indemnify 3M for material taxes and other related amounts pursuant to indemnification obligations under the tax matters agreement.
It is a condition to the distribution (which condition 3M may waive in its sole discretion) that (1) the IRS Ruling regarding U.S. Tax-Free Status continues to be valid and (2) 3M receives the Tax Opinion(s) regarding U.S. Tax-Free Status. The IRS Ruling is and any Tax Opinion will be based upon and rely on, among other things, various facts and assumptions, as well as certain representations, statements and undertakings of 3M and Solventum, including those relating to the past and future conduct of 3M and Solventum. If any of these representations, statements or undertakings are, or become, inaccurate or incomplete, or if any representations or covenants contained in any of the separation-related agreements and documents or in any documents relating to the IRS Ruling and/or any Tax Opinion are inaccurate or not complied with by 3M, Solventum or any of their respective subsidiaries, the IRS Ruling and/or such Tax Opinion may be invalid and the conclusions reached therein could be jeopardized.
Notwithstanding 3M’s receipt of the IRS Ruling and any Tax Opinion, in each case, regarding U.S. Tax-Free Status, the IRS could determine that the distribution and/or certain related transactions should be treated as taxable transactions for U.S. federal income tax purposes if it determines that any of the representations, assumptions, or undertakings upon which the IRS Ruling or such Tax Opinion was based are inaccurate or have not been complied with. In addition, the IRS Ruling does not address all of the issues that are relevant to determining whether the distribution, together with certain related transactions, qualifies as a transaction that is generally tax-free for U.S. federal income tax purposes, and any Tax Opinion will represent the judgment of such advisor and will not be binding on the IRS or any court and the IRS or a court may disagree with the conclusions in such Tax Opinion. Accordingly, notwithstanding 3M’s receipt of the IRS Ruling and the Tax Opinion(s), in each case, regarding U.S. Tax-Free Status, there can be no assurance that the IRS will not assert that the distribution and/or certain related transactions do not qualify for tax-free treatment for U.S. federal income tax purposes, or that a court would not sustain such a challenge. In the event the IRS were to prevail with such challenge, 3M, Solventum and 3M’s shareholders could be subject to significant U.S. federal income tax liability. For a discussion of the material U.S. federal income tax consequences of the distribution, see “Material U.S. Federal Income Tax Consequences.”
If the distribution and certain related transactions were to fail to qualify as a transaction that is generally tax-free for U.S. federal income tax purposes under Sections 355 and 368(a)(1)(D) of the Code, in general, for U.S. federal income tax purposes, 3M would recognize a taxable gain as if it had sold the Solventum common stock distributed to 3M shareholders in a taxable sale for its fair market value, and 3M shareholders who receive Solventum common stock in the distribution would be subject to tax as if they had received a taxable distribution equal to the fair market value of such shares. Even if the distribution and certain related transactions otherwise qualify as generally tax-free for U.S. federal income tax purposes under Section 355 and Section 368(a)(1)(D) of the Code, it may result in taxable gain to 3M (but not its shareholders) under Section 355(e) of the Code if the distribution were deemed to be part of a plan (or series of related transactions) pursuant to which one or more persons acquire, directly or indirectly, shares representing a 50 percent or greater interest (by vote or value) in 3M or Solventum. For this purpose, any acquisitions of 3M or Solventum shares within the period beginning two years before the distribution and ending two years after the distribution are presumed to be part of such a plan, although 3M or Solventum may be able to rebut that presumption. The process for determining whether an acquisition is part of a plan under these rules is complex, inherently factual in nature and subject to a comprehensive analysis of the facts and circumstances of the particular case. Notwithstanding the IRS Ruling and the Tax Opinion(s), a sufficient change in ownership of 3M or Solventum may occur that could result in a material tax liability to 3M.
In addition, as part of the separation, and prior to the distribution, 3M and its subsidiaries expect to complete the internal reorganization, and 3M, Solventum and their respective subsidiaries expect to incur certain tax costs in connection with the internal reorganization, including non-U.S. tax costs resulting from transactions in non-U.S. jurisdictions, which may be material. With respect to certain transactions undertaken as part of the internal
64
reorganization, 3M has requested and intends to obtain tax rulings in certain non-U.S. jurisdictions and/or opinions of external tax advisors, in each case, regarding the tax treatment of such transactions. Such tax rulings and opinions will be based upon and rely on, among other things, various facts and assumptions, as well as certain representations, statements and undertakings of 3M, Solventum or their respective subsidiaries. If any of these representations or statements is, or becomes, inaccurate or incomplete, or if 3M, Solventum or any of their respective subsidiaries do not fulfill or otherwise comply with any such undertakings or covenants, such tax rulings and/or opinions may be invalid or the conclusions reached therein could be jeopardized. Further, notwithstanding receipt of any such tax rulings and/or opinions, there can be no assurance that the relevant taxing authorities will not assert that the tax treatment of the relevant transactions differs from the conclusions reached in the relevant tax rulings and/or opinions. In the event any such tax rulings and/or opinions cannot be obtained or the relevant taxing authorities prevail with any challenge in respect of any relevant transaction, Solventum and 3M could be subject to significant tax liabilities.
Under the tax matters agreement that Solventum will enter into with 3M, Solventum generally would be required to indemnify 3M for any taxes resulting from the separation (and any related costs and other damages) to the extent such amounts resulted from (1) an acquisition of all or a portion of Solventum’s equity securities or assets, whether by merger or otherwise (and regardless of whether Solventum participated in or otherwise facilitated the acquisition), (2) other actions or failures to act by Solventum, or (3) certain of Solventum’s representations, covenants or undertakings being incorrect or violated. Any such indemnity obligations could be material. For a more detailed discussion, see “Certain Relationships and Related Party Transactions—Agreements with 3M—Tax Matters Agreement.” In addition, Solventum, 3M and each company’s respective subsidiaries may incur certain tax costs in connection with the separation, which may be material.
The transfer to Solventum of certain contracts, permits and other assets and rights may require the consents or approvals of, or provide other rights to, third parties and governmental authorities. If such consents or approvals are not obtained, Solventum may not be entitled to the full benefit of such contracts, permits and other assets and rights, which could increase its expenses or otherwise harm its business and financial performance.
The separation and distribution agreement will provide that certain contracts, permits and other assets and rights are to be transferred from 3M or its subsidiaries to Solventum or its subsidiaries in connection with the separation. The transfer of certain of these contracts, permits and other assets and rights may require consents or approvals of third parties or governmental authorities or provide other rights to third parties. In addition, in some circumstances, Solventum and 3M are joint beneficiaries of contracts, and Solventum and 3M may need the consents of third parties in order to split or separate the existing contracts or the relevant portion of the existing contracts to Solventum or 3M.
Some parties may use consent requirements or other rights to seek to terminate contracts or obtain more favorable contractual terms from Solventum, which, for example, could take the form of price increases. This could require Solventum to expend additional resources in order to obtain the services or assets previously provided under the contract or require Solventum to seek arrangements with new third parties or obtain letters of credit or other forms of credit support. If Solventum is unable to obtain required consents or approvals, it may be unable to obtain the benefits, permits, assets and contractual commitments that are intended to be allocated to Solventum as part of its separation from 3M, and Solventum may be required to seek alternative arrangements to obtain services and assets that may be more costly and/or of lower quality. The termination or modification of these contracts or permits or the failure to timely complete the transfer or separation of these contracts or permits could negatively affect Solventum’s business, financial condition, results of operations and cash flows.
The closing of the separation may be delayed in certain jurisdictions, or not occur at all, due to local regulatory requirements, which may adversely affect Solventum’s business, financial condition and results of operations.
The closing of the transfer of certain assets related to the Health Care Business in certain jurisdictions, as contemplated by the separation and distribution agreement may not occur at or prior to the distribution, if at all, due to local regulatory requirements. If Solventum is unable to obtain required approval of local regulators or otherwise comply with such local regulatory requirements to effect the separation in these jurisdictions, it may be unable to obtain the assets that are intended to be allocated to Solventum as part of its separation from 3M. The failure to
65
timely complete the transfer of these local assets could negatively affect Solventum’s business, financial condition, results of operations and cash flows.
Until the distribution occurs, the 3M Board of Directors has sole and absolute discretion to change the terms of the separation in ways that may be unfavorable to Solventum, including to determine not to effect the distribution at all.
On July 26, 2022, 3M announced a plan to separate the Health Care Business into an independent publicly traded company, Solventum. The separation is subject to the satisfaction (or waiver by 3M in its sole and absolute discretion) of certain conditions, including final approval by 3M’s Board of Directors of the separation and distribution. Furthermore, the separation is complex in nature, and unanticipated developments or changes, including changes in the law, the macroeconomic environment, competitive conditions of 3M’s markets, regulatory approvals or clearances, the uncertainty of the financial markets and challenges in executing the separation and distribution, could delay or prevent the completion of the proposed separation or distribution, or cause the separation or distribution to occur on terms or conditions that are different or less favorable than expected. Additionally, the 3M Board of Directors, in its sole and absolute discretion, may decide not to proceed with the distribution at any time prior to the distribution date.
No vote of 3M shareholders is required in connection with the distribution. As a result, if the distribution occurs and you do not want to receive Solventum common stock in the distribution, your sole recourse will be to divest yourself of your 3M common stock prior to the distribution date.
No vote of 3M shareholders is required in connection with the distribution. Accordingly, if the distribution occurs and you do not want to receive Solventum common stock in the distribution, your only recourse will be to divest your 3M common stock prior to the record date for the distribution or, following the record date, in the “regular-way” market for 3M common stock before the distribution date.
Satisfaction by Solventum of its post-distribution indemnification obligations or 3M’s failure to satisfy its post-distribution indemnification obligations could have a material adverse effect on Solventum’s financial condition, results of operations and cash flows.
Pursuant to the separation and distribution agreement and certain other agreements that Solventum expects to enter into with 3M in connection with the separation and distribution, 3M will agree to indemnify Solventum for certain liabilities, and Solventum will agree to indemnify 3M for certain liabilities as discussed further in “Certain Relationships and Related Party Transactions.” Solventum could be negatively affected if it is required to make material payments pursuant to its indemnification obligations to 3M.
The indemnity from 3M may not be sufficient to protect Solventum against the full amount of such liabilities if, for example, 3M fails to fully satisfy its indemnification obligations. Moreover, even if Solventum ultimately succeeds in recovering from 3M any amounts for which it is held liable, Solventum may be temporarily required to bear these losses itself, requiring Solventum to divert cash that would otherwise have been used in furtherance of its operating business. In addition, third parties could also seek to hold Solventum responsible for any of the liabilities that 3M has agreed to retain. Each of these risks could have a material adverse effect on Solventum.
3M may not complete the ultimate separation of the Health Care Business and may retain a significant ownership stake in Solventum for a period of time.
It is expected that 3M will ultimately dispose of its remaining ownership interest in Solventum, representing up to 19.9% of the outstanding Solventum common stock, as soon as a disposition is warranted consistent with the business reasons for the retention of such common stock, but in no event later than five years after the distribution, through one or more sales of such shares of Solventum common stock.
The disposition by 3M of its remaining ownership interest in Solventum may be subject to various conditions, including receipt of any necessary regulatory and other approvals and the existence of satisfactory market conditions. These conditions may not be satisfied or 3M may decide for any other reason not to consummate the disposition and instead retain a significant ownership interest in Solventum for a period of time, not exceeding five
66
years. Satisfying the conditions relating to such disposition may require actions that 3M has not anticipated. Any delay by 3M in completing the disposition could have a material adverse effect on the market price for Solventum common stock.
Following the distribution, certain of Solventum’s directors and employees may have actual or potential conflicts of interest because of their financial interests in 3M or because of their previous positions with 3M.
Because of former positions with 3M, certain of Solventum’s expected executive officers and directors own equity interests in both Solventum and 3M. Continuing ownership of 3M shares and equity awards could create, or appear to create, potential conflicts of interest if Solventum and 3M face decisions that could have implications for both Solventum and 3M. For example, potential conflicts of interest could arise in connection with the resolution of any dispute between Solventum and 3M regarding the terms of the agreements governing the separation and distribution and Solventum’s relationship with 3M following the separation and distribution. Potential conflicts of interest may also arise out of any commercial arrangements that Solventum or 3M may enter into in the future.
Field of use allocations in the separation-related agreements may limit Solventum’s ability to fully exploit certain intellectual property, or may permit 3M to compete with Solventum using intellectual property owned by or licensed to Solventum. Solventum’s ability to compete may be further impacted by the non-competition provisions contained in the separation and distribution agreement.
The transaction agreements that 3M and Solventum will enter into in connection with the separation will contain certain field of use restrictions on the licenses granted between the parties. See the section titled “Certain Relationships and Related Party Transactions – Intellectual Property Cross License Agreement.” As a result, each of 3M and Solventum will be limited in its ability to fully exploit certain of its intellectual property rights in the other party’s exclusive fields. 3M and Solventum may each also have the right to, and may elect to, use certain intellectual property of the other party to compete with the other party in certain fields other than the other party’s exclusive fields.
Pursuant to the separation and distribution agreement, and subject to specified exceptions, Solventum and 3M will agree not to compete in specified respects for a period of time after the distribution date. See the section titled “Certain Relationships and Related Party Transactions – Separation and Distribution Agreement – Non-Compete.” During the non-compete period, Solventum will be prohibited from manufacturing and/or selling certain products, or from selling its products to certain end users. This prohibition may negatively impact Solventum’s business during the non-compete period. The non-compete period is limited, and following the termination of such period, 3M could elect to compete with Solventum in the relevant fields or products.
Any of the foregoing may adversely impact demand for Solventum’s products or cause Solventum to lose market share, and could have an adverse effect on Solventum’s business, financial condition, results of operations and cash flows.
Risks Related to Solventum Common Stock
There is no assurance that an active trading market for Solventum common stock will develop or be sustained after the distribution and, following the distribution, the price of Solventum common stock may fluctuate significantly.
A public market for Solventum common stock does not currently exist. Solventum anticipates that on or about March 26, 2024, trading of shares of Solventum common stock will begin on a “when-issued” basis and will continue through the distribution date. However, Solventum cannot guarantee that an active trading market will develop or be sustained for Solventum common stock after the distribution, nor can Solventum predict the prices at which shares of Solventum common stock may trade after the distribution. Similarly, Solventum cannot predict the effect of the distribution on the trading prices of Solventum common stock or whether the combined market value of one-quarter of a share of Solventum common stock and one share of 3M common stock will be less than, equal to or greater than the market value of one share of 3M common stock prior to the distribution.
67
The prices at which shares of Solventum common stock trade may fluctuate more significantly than might otherwise be typical, even with other market conditions, including general volatility, held constant. The market price of Solventum common stock may fluctuate significantly due to a number of factors, some of which may be beyond our control, including:
•actual or anticipated fluctuations in Solventum’s operating results;
•changes in earnings estimated by securities analysts or Solventum’s ability to meet those estimates;
•the operating and stock price performance of comparable companies;
•changes to the regulatory and legal environment under which Solventum operates;
•actual or anticipated fluctuations in commodities prices;
•analyst research reports, recommendations and changes in recommendations, price targets, and withdrawals of coverage;
•whether Solventum common stock is included in stock market indices; and
•domestic and worldwide economic conditions, political and social conditions, including natural disasters or acts of nature, hostilities, acts of war, political upheaval, changes in government or administrations, sabotage or terrorism or military actions, disease outbreaks, epidemics or pandemics.
A significant number of shares of Solventum common stock may be sold by 3M or others following the distribution, which may cause the Solventum stock price to decline.
Any sales of substantial amounts of Solventum common stock in the public market or the perception that such sales might occur, in connection with the distribution or otherwise, may cause the market price of Solventum common stock to decline. Upon completion of the distribution, Solventum expects that it will have an aggregate of approximately 172,709,175 shares of common stock issued and outstanding. Shares distributed to 3M shareholders in the separation will generally be freely tradeable without restriction or further registration under the U.S. Securities Act of 1933, as amended (the “Securities Act”), except for shares owned by Solventum’s “affiliates,” as that term is defined in Rule 405 under the Securities Act.
Solventum cannot predict whether large amounts of Solventum common stock will be sold in the open market following the distribution. Solventum is also unable to predict whether a sufficient number of buyers of Solventum common stock to meet the demand to sell shares of Solventum common stock at attractive prices would exist at that time.
In addition, immediately after the completion of the distribution, 3M will hold up to 19.9% of Solventum’s outstanding common stock. 3M currently plans to dispose of all of the Solventum common stock that it retains after the distribution through one or more sales of such shares (not later than five years after the distribution). Solventum will agree that, upon the request of 3M and pursuant to the terms of the stockholder’s and registration rights agreement, it will use its reasonable best efforts to effect a registration under applicable federal and state securities laws of any shares of Solventum’s common stock retained by 3M to the extent that 3M wishes to sell the shares of our common stock it retains in a registered offering. These shares will be restricted securities within the meaning of Rule 144 under the Securities Act and will also be eligible for resale by 3M in the public market without registration subject to volume, manner of sale and holding period limitations under Rule 144 under the Securities Act. Any sales of substantial amounts of Solventum common stock in the public market by 3M or the perception that such sales might occur, in connection with the distribution or otherwise, may cause the market price of Solventum common stock to decline.
Your percentage of ownership in Solventum may be diluted in the future.
In the future, your percentage ownership in Solventum may be diluted because of equity issuances for acquisitions, capital market transactions or otherwise, including any equity awards that Solventum will grant to its
68
directors, officers and employees. Solventum employees will have stock-based awards that correspond to shares of Solventum common stock after the distribution as a result of conversion of their 3M stock-based awards. Such awards will have a dilutive effect on Solventum’s earnings per share, which could adversely affect the market price of Solventum common stock. From time to time, Solventum will issue additional stock-based awards to its employees under its employee benefits plans.
Solventum has not yet determined its dividend policy, and even if Solventum determines that its policy will be to pay a regular dividend, Solventum cannot guarantee the timing, declaration, amount or payment of dividends on its common stock.
Solventum has not yet determined whether it expects to pay a regular dividend after the separation and distribution. The timing, declaration, amount and payment of any dividends following the separation and distribution will be within the discretion of Solventum’s Board of Directors and will depend upon many factors, including Solventum’s financial condition, earnings, capital requirements of its operating subsidiaries, covenants associated with certain of Solventum’s debt service obligations, legal requirements, regulatory constraints, industry practice, ability to access capital markets, and other factors deemed relevant by Solventum’s Board of Directors. Moreover, if Solventum determines to pay any dividend in the future, there can be no assurance that it will continue to pay such dividends or the amount of such dividends. For more information, see the section entitled “Dividend Policy.”
Anti-takeover provisions could enable Solventum’s Board of Directors to resist a takeover attempt by a third party and limit the power of its shareholders.
Solventum’s amended and restated certificate of incorporation and amended and restated bylaws will contain, and Delaware law contains, provisions that are intended to deter coercive takeover practices and inadequate takeover bids by making such practices or bids unacceptably expensive to the bidder and to encourage prospective acquirers to negotiate with Solventum’s Board of Directors rather than to attempt a hostile takeover. These provisions are expected to include, among others:
•until the annual stockholder meeting in 2028, Solventum’s Board of Directors will be divided into classes, which could have the effect of making the replacement of incumbent directors more time consuming and difficult;
•as long as Solventum’s Board of Directors is classified, Solventum directors can be removed by stockholders only for cause;
•Solventum’s Board of Directors will have the sole authority to fix the size of Solventum’s Board of Directors;
•Solventum’s Board of Directors will have the authority to amend and repeal Solventum’s amended and restated bylaws without a stockholder vote;
•Solventum’s shareholders will not have a right to call a special meeting or act by written consent;
•Solventum’s amended and restated certificate of incorporation and amended and restated bylaws will not provide for cumulative voting in the election of directors;
•Solventum’s Board of Directors will have the power to designate and issue, without any further vote or action by the Solventum shareholders, shares of preferred stock from time to time in one or more series;
•Solventum’s shareholders will have to follow certain procedures and notice requirements in order to present certain proposals or nominate directors for election at shareholder meetings; and
•Solventum’s amended and restated certificate of incorporation will contain exclusive forum provisions (as described in more detail in the following risk factor).
In addition, Solventum will be subject to Section 203 of the Delaware General Corporation Law, which could have the effect of delaying or preventing a change of control that you may favor. Section 203 provides that, subject
69
to limited exceptions, persons that acquire, or are affiliated with persons that acquire, more than 15% of the outstanding voting stock of a Delaware corporation may not engage in a business combination with that corporation, including by merger, consolidation or acquisitions of additional shares, for a three-year period following the date on which that person or any of its affiliates becomes the holder of more than 15% of the corporation’s outstanding voting stock.
Solventum believes these provisions will protect Solventum shareholders from coercive or otherwise unfair takeover tactics by requiring potential acquirers to negotiate with Solventum’s Board of Directors and by providing Solventum’s Board of Directors with more time to assess any acquisition proposal. These provisions are not intended to make Solventum immune from takeovers; however, these provisions will apply even if the offer may be considered beneficial by some shareholders and could delay or prevent an acquisition that Solventum’s Board of Directors determines is not in the best interests of Solventum and its shareholders. These provisions may also prevent or discourage attempts to remove and replace incumbent directors. See “Description of Solventum Capital Stock—Anti-Takeover Effects of Governance Provisions.”
In addition, an acquisition or further issuance of Solventum stock could trigger the application of Section 355(e) of the Code, causing the distribution to be taxable to 3M. For a discussion of Section 355(e) of the Code, see “Material U.S. Federal Income Tax Consequences.” Under the tax matters agreement, Solventum would be required to indemnify 3M for the resulting tax, and this indemnity obligation might discourage, delay or prevent a change of control that Solventum shareholders may consider favorable.
Solventum’s amended and restated certificate of incorporation will designate the state courts within the State of Delaware or the federal district courts of the United States as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by Solventum shareholders, which could discourage lawsuits against Solventum and its directors and officers.
Solventum’s amended and restated certificate of incorporation will provide that, unless Solventum (through approval of Solventum’s Board of Directors) consents in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for (1) any derivative action brought on behalf of Solventum, (2) any action asserting a claim of breach of a fiduciary duty owed by any director or officer or other employee of Solventum to Solventum or Solventum’s shareholders, (3) any action asserting a claim against Solventum or any director or officer or other employee of Solventum arising pursuant to, or seeking to enforce any right, obligation or remedy under, any provision of the Delaware General Corporation Law (“DGCL”) or Solventum’s amended and restated certificate of incorporation or amended and restated bylaws (as either may be amended from time to time), (4) any action asserting a claim against Solventum or any director or officer or other employee of Solventum governed by the internal affairs doctrine, which is a conflict of laws principle that recognizes that only one state should have the authority to regulate a corporation’s internal affairs, or (5) any action as to which the DGCL (as it may be amended from time to time) confers jurisdiction on the Court of Chancery of the State of Delaware. If and only if the Court of Chancery of the State of Delaware dismisses any such action for lack of subject matter jurisdiction, such action may be brought in another state court sitting in the State of Delaware (or, if no state court located within the State of Delaware has jurisdiction, the federal district court for the District of Delaware). Solventum’s amended and restated certificate of incorporation will further provide that, unless Solventum (through approval of Solventum’s Board of Directors) consents in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the sole and exclusive forum for any action asserting a cause of action arising under the Securities Act. The exclusive forum provisions will be applicable to the fullest extent permitted by applicable law, subject to certain exceptions. Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As a result, the exclusive forum provisions will not apply to suits brought to enforce any duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. There is, however, uncertainty as to whether a court would enforce the exclusive forum provisions, and investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for state and federal courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder.
70
Although Solventum believes the exclusive forum provision benefits it by providing increased consistency in the application of law in the types of lawsuits to which it applies, the provision may limit the ability of Solventum shareholders to bring a claim in a judicial forum that such shareholders find favorable for disputes with Solventum or its directors or officers and it may be costlier for Solventum shareholders to bring a claim in the Court of Chancery of the State of Delaware than other judicial forums, each which may discourage such lawsuits against Solventum and its directors and officers.
Although Solventum’s amended and restated certificate of incorporation will include this exclusive forum provision, it is possible that a court could rule that this provision is inapplicable or unenforceable. Alternatively, if a court were to find this exclusive forum provision inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings described above, Solventum may incur additional costs associated with resolving such matters in other jurisdictions, which could negatively affect its business, results of operations and financial condition.
The combined post-separation value of one share of 3M common stock and one-quarter of a share of Solventum common stock may not equal or exceed the pre-distribution value of one share of 3M common stock.
As a result of the separation, the trading price of shares of 3M common stock immediately following the separation may be different from the “regular-way” trading price of such shares immediately prior to the separation because the trading price of 3M common stock will no longer reflect the value of the Health Care Business. There can be no assurance that the aggregate market value of a share of 3M common stock and one-quarter of a share of Solventum common stock following the separation will be higher than, lower than or the same as the market value of a share of 3M common stock if the separation did not occur.
71
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This information statement and other materials 3M and Solventum have filed or will file with the SEC (and oral communications that 3M or Solventum may make) contain or incorporate by reference statements that relate to future events and expectations and, as such, constitute forward-looking statements that involve risk and uncertainties. Forward-looking statements include those containing such words as “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts,” “goal,” “guidance,” “intends,” “may,” “outlook,” “plans,” “projects,” “seeks,” “sees,” “should,” “targets,” “will,” “would,” or other words of similar meaning. All statements that reflect 3M’s or Solventum’s expectations, assumptions or projections about the future, other than statements of historical fact, are forward-looking statements, including, without limitation, forecasts relating to discussions of future operations and financial performance (including volume growth, pricing, sales and earnings per share growth and cash flows) and statements regarding 3M’s or Solventum’s strategy for growth, future product development, regulatory clearances and approvals, competitive position and expenditures. Forward-looking statements are not guarantees of future performance and are subject to risks, uncertainties, and changes in circumstances that are difficult to predict. Although each of 3M and Solventum believes that the expectations reflected in any forward-looking statements it makes are based on reasonable assumptions, it can give no assurance that these expectations will be attained and it is possible that actual results may differ materially from those indicated by these forward-looking statements due to a variety of risks and uncertainties. Such risks and uncertainties include, but are not limited to:
•The effects of, and changes in, worldwide economic, political, regulatory, international, trade and geopolitical conditions, natural disasters, war, and other events beyond Solventum’s control.
•Unexpected events, such as those related to the COVID-19 public health crisis.
•Operational execution risks.
•Damage to our reputation or our brands.
•Risks from acquisitions, strategic alliances, divestitures and other strategic events.
•Solventum’s business dealings involving third-party partners in various markets.
•Solventum’s ability to access the capital and credit markets and changes in Solventum’s credit ratings.
•Exposure to interest rate and currency risks.
•The highly competitive environment in which Solventum operates and consolidation in the healthcare industry.
•Reduction in customers’ research budgets or government funding.
•The timing and market acceptance of Solventum’s new product and service offerings.
•Ongoing working relationships with certain key healthcare professionals.
•Changes in reimbursement practices of governments or private payers or other cost containment measures.
•Solventum’s ability to obtain components or raw materials supplied by third parties and other manufacturing and related supply chain difficulties, interruptions, and disruptive factors.
•Legal and regulatory proceedings and legal compliance risks (including third-party risks) with regards to antitrust, FCPA and other anti-bribery laws, environmental laws, anti-kickback and false claims laws, privacy laws, tax laws, and other laws and regulations in the United States and other countries in which Solventum operates.
•Potential liabilities related to PFAS.
•Risks related to the highly regulated environment in which Solventum operates.
72
•Risks associated with product liability claims.
•Climate change and measures to address climate change.
•Security breaches and other disruptions to information technology infrastructure.
•Solventum’s failure to obtain, maintain, protect, or effectively enforce its intellectual property rights.
•Pension and postretirement obligation liabilities.
•Any events that adversely affect the sale or profitability of one of Solventum’s key products or the revenue delivered from sales to its key customers.
•Any failure by 3M to perform any of its obligations under the various separation agreements to be entered into in connection with the separation and distribution.
•The expected benefits and timing of the separation and the risk that conditions to the separation will not be satisfied and/or that the separation will not be completed within the expected time frame, on the expected terms or at all.
•A determination by the IRS or other tax authorities that the distribution or certain related transactions should be treated as taxable transactions.
•The possibility that any consents or approvals required in connection with the separation will not be received or obtained within the expected time frame, on the expected terms or at all.
•Expected financing transactions undertaken in connection with the separation and risks associated with additional indebtedness.
•The risk that incremental costs of operating on a standalone basis (including the loss of synergies), costs of restructuring transactions and other costs incurred in connection with the separation will exceed Solventum’s estimates.
•The impact of the separation on its businesses and the risk that the separation may be more difficult, time-consuming or costly than expected, including the impact on its resources, systems, procedures and controls, diversion of management’s attention and the impact on relationships with customers, suppliers, employees and other business counterparties.
There can be no assurance that the separation, distribution or any other transaction described in this information statement will in fact be consummated in the manner described or at all. The above list of factors is not exhaustive or necessarily in order of importance. For additional information on identifying factors that may cause actual results to vary materially from those stated in forward-looking statements, see the discussions under “Risk Factors” in this information statement. Any forward-looking statement speaks only as of the date on which it is made, and each of 3M and Solventum assumes no obligation to update or revise such statement, whether as a result of new information, future events or otherwise, except as required by applicable law.
73
THE SEPARATION AND DISTRIBUTION
Background
On July 26, 2022, 3M announced a plan to separate its Health Care Business into an independent public company. 3M intends to effect the separation through a pro rata distribution to 3M shareholders of at least 80.1% of the common stock of Solventum, a new entity formed to hold the assets and liabilities associated with the Health Care Business. Upon completion of the distribution, 3M will own up to 19.9% of the outstanding shares of Solventum common stock and Solventum will be a separate public company. 3M will dispose of all of the retained shares of Solventum common stock as soon as a disposition is warranted consistent with the business reasons for the retention of those shares, but in no event later than five years after the distribution, through one or more sales of such shares of Solventum common stock.
In connection with the distribution, it is expected that:
•3M will complete the internal reorganization as a result of which Solventum will become the parent company of the 3M operations comprising, and the entities that will conduct, the Health Care Business;
•Solventum will incur approximately $8.4 billion of indebtedness, consisting of the Credit Facilities and $6.9 billion in principal amount of senior unsecured notes issued on February 27, 2024; and
•using a portion of the proceeds from one or more financing transactions before the distribution is completed, Solventum will make cash payments to 3M, in an amount calculated such that Solventum will have $600 million of cash in the aggregate at the time of the distribution (such cash payments to 3M from the proceeds of such financings are expected to total approximately $7.7 billion, taking into account fees, discounts and expenses).
On March 8, 2024, the 3M Board of Directors approved the distribution of at least 80.1% of Solventum’s issued and outstanding shares of common stock on the basis of one share of Solventum common stock for every four shares of 3M common stock held as of the close of business on March 18, 2024, the record date for the distribution.
At 3:30 a.m., Eastern Time, on April 1, 2024, the distribution date, each 3M shareholder as of the record date for the distribution will receive one share of Solventum common stock for every four shares of 3M common stock held at the close of business on the record date, as described below. 3M shareholders will receive cash in lieu of any fractional shares of Solventum common stock that they would have received after application of this ratio. Upon completion of the separation, each 3M shareholder as of the record date will continue to own shares of 3M and will receive a proportionate share of the outstanding common stock of Solventum to be distributed. You will not be required to make any payment, surrender or exchange your 3M common stock or take any other action to receive your shares of Solventum common stock in the distribution. The distribution of Solventum common stock as described in this information statement is subject to the satisfaction or waiver of certain conditions. For a more detailed description of these conditions and the potential waiver of such conditions, see “—Conditions to the Distribution.”
Reasons for the Separation
The 3M Board of Directors believes that the separation of the Health Care Business from 3M into an independent, publicly traded company is in the best interests of 3M and its shareholders for a number of reasons, including:
•Ability for each business to pursue tailored capital allocation strategies and make company-specific investment decisions to drive innovation and growth. The separation will permit each of 3M and Solventum to concentrate its financial resources to solely meet the unique needs of its own businesses, which will allow each company to invest capital at the time and in the manner most appropriate for its distinct strategic priorities and business needs. This will facilitate a more efficient allocation of capital between the Health Care Business and the 3M Business, based on the profitability, cash flow and growth opportunities of each company. In addition, after the separation, the Health Care Business will no longer be required to compete
74
internally with the 3M Business for capital and other corporate resources. As an independent entity, Solventum will be free to invest its financial resources in its own organic and inorganic opportunities at the pace of a standalone healthcare business, to accelerate growth and drive shareholder value. This will be facilitated by the fact that, in general, each party to the separation and distribution agreement will assume liability for all pending, threatened and unasserted legal matters related to its own business or its assumed or retained liabilities and will indemnify the other party for any liability to the extent arising out of or resulting from such assumed or retained legal matters.
•Enhanced management focus through distinct boards and management teams with relevant expertise. The separation will permit each company to be led by a separate, dedicated board and management team with relevant and deep expertise in its industry, able to focus on strengthening such company’s core businesses, addressing its unique operating and other needs, and pursuing distinct and targeted opportunities. The board and management team of each company will be well-positioned to rapidly transform each company’s management systems and processes to those targeted at its specific growth and market strategies.
•Improved operational agility, better positioning each company for long-term success. The separation will permit each of 3M and Solventum to more effectively pursue its own distinct operating priorities and strategies in line with each company’s industry-specific focus. In particular, it will enable each of the two companies to maintain a sharper focus on strengthening its core business and growth opportunities, pursuing distinct paths to long-term growth and profitability, and addressing its unique operating and other needs. Each company will also have increased operational flexibility to design and implement corporate strategies based on the particular characteristics of the industry in which each business operates.
•Distinct and compelling investment profiles appealing to different long-term investor bases. The separation will allow each company to more effectively articulate a clear investment thesis and to enhance each company’s structural and operational transparency, enabling investors to separately value and invest in each company based on their distinct investment identities. This is expected to attract different, long-term investor bases for each company and facilitate each company’s access to capital by providing investors with two distinct and targeted investment opportunities. With investors better suited and aligned to its business, each company will be able to pursue its industry-specific business objectives consistent with the expectations of its distinct investor base.
•Creation of independent equity currencies. The separation will create independent equity securities for Solventum and 3M, aligned with each company’s respective industry, providing each company with more flexibility to capitalize on its unique strategic opportunities. This will afford Solventum direct access to the capital markets, and the opportunity to use its own industry-focused stock to consummate future acquisitions or other transactions that are more closely aligned with its strategic goals and expected growth opportunities.
•Enhanced employee recruitment and retention, including by aligning management incentives with performance. The separation will allow each of 3M and Solventum to more effectively recruit, retain and develop talent with the appropriate skill set and expertise directly applicable to each company’s needs. In addition, the separation will enable each of 3M and Solventum to offer equity-based and other incentive compensation arrangements that more closely reflect and align management and employee incentives with each company’s specific growth objectives, financial goals, and business performance.
The 3M Board of Directors also considered a number of potentially negative factors in evaluating the separation, including:
•Risk of failure to achieve anticipated benefits of the separation. The anticipated benefits of the separation may not be achieved for a variety of reasons, including, among others: the separation will demand significant management resources and require significant amounts of management’s time and effort, which may divert management’s attention from operating the businesses of 3M and Solventum; and following the separation, each of 3M and Solventum may be more susceptible to market fluctuations and other adverse
75
events than if they remained a combined company because the business of each entity will be less diversified than 3M’s business prior to the completion of the separation.
•Loss of scale and increased administrative costs. As part of 3M, Solventum currently takes advantage of 3M’s size and purchasing power in procuring certain goods and services. After the separation, as standalone companies, each of 3M and Solventum may be unable to obtain these goods, services and technologies at prices or on terms as favorable as those 3M obtained prior to completion of the separation. In addition, as part of 3M, Solventum benefits from certain functions performed by 3M, such as accounting, tax, legal, human resources and other general and administrative functions. After the separation, 3M will not perform these functions for Solventum, other than certain functions that will be provided for a limited time pursuant to the transition arrangements, and, because of Solventum’s smaller scale as a standalone company, its cost of performing such functions could be higher than the amounts reflected in Solventum’s historical financial statements, which could cause Solventum’s profitability to decrease.
•Disruptions and costs related to the separation. The actions required to separate Solventum from 3M could disrupt each company’s operations. In addition, 3M and Solventum will incur substantial costs in connection with the separation and the transition to Solventum becoming a standalone public company, which may include accounting, tax, legal and other professional services costs, recruiting and relocation costs associated with hiring key senior management personnel who are new to Solventum, tax costs, and costs to separate information systems.
•Limitations on strategic transactions. Under the terms of the tax matters agreement that Solventum will enter into with 3M, Solventum is expected to be restricted from taking certain actions that could cause the distribution or certain related transactions (or certain transactions undertaken as part of the internal reorganization) to fail to qualify as tax-free transactions under applicable law. These restrictions may limit for a period of time Solventum’s ability to pursue certain strategic transactions and equity issuances or engage in other transactions that might increase the value of Solventum’s business.
•Uncertainty regarding stock prices. Neither 3M nor Solventum can predict the effect of the separation on the trading prices of Solventum or 3M common stock or know with certainty whether the combined market value of one-quarter of a share of Solventum common stock and one share of 3M common stock will be less than, equal to or greater than the market value of one share of 3M common stock prior to the distribution.
In determining to pursue the separation, the 3M Board of Directors concluded the potential benefits of the separation outweighed the foregoing factors. See the section entitled “Risk Factors” included elsewhere in this information statement.
Reasons for 3M’s Retention of Up to 19.9% of the Shares of Solventum Common Stock
In considering the appropriate structure for the separation, 3M determined that, immediately after the distribution becomes effective, 3M will retain up to 19.9% of the outstanding shares of Solventum common stock. 3M intends to responsibly dispose of such shares after the distribution as soon as a disposition is warranted consistent with the business reasons for the retention of those shares. Such dispositions will be effected through one or more sales of such shares of Solventum common stock (not later than five years after the distribution). 3M’s retention of shares of Solventum common stock is expected to support the establishment of optimal capital structures for each of 3M and Solventum by (i) providing 3M with a valuable asset that can be used to reduce its aggregate liabilities, allowing 3M the opportunity to capitalize on strategic opportunities and strengthen its balance sheet, and (ii) providing a means for 3M to increase its financial flexibility without increasing the amount of leverage that Solventum would incur, thereby providing Solventum with a stronger balance sheet and greater ability to fund growth.
Any sales of substantial amounts of Solventum common stock in the public market by 3M or the perception that such sales might occur, in connection with the distribution or otherwise, may cause the market price of Solventum common stock to decline. See the second risk factor in the section entitled “Risk Factors—Risks Related to Solventum Common Stock” for additional details.
76
Formation of Solventum
Solventum was formed in Delaware on January 24, 2023, for the purpose of holding the Health Care Business. As part of the plan to separate the Health Care Business from the remainder of 3M’s businesses, 3M plans to transfer the equity interests of certain entities that are expected to operate the Health Care Business and the assets and liabilities of the Health Care Business to Solventum prior to the distribution. For additional information, see “—Internal Reorganization.”
When and How You Will Receive the Distribution
Subject to the satisfaction or waiver of the conditions to the distribution, 3M expects to distribute at least 80.1% of the shares of Solventum common stock at 3:30 a.m., Eastern Time, on April 1, 2024, the distribution date, to all holders of outstanding 3M common stock as of the close of business on March 18, 2024, the record date for the distribution. Equiniti will serve as the settlement and distribution agent in connection with the distribution and the transfer agent and registrar for Solventum common stock.
If you own 3M common stock as of the close of business on the record date for the distribution, Solventum common stock that you are entitled to receive in the distribution will be issued electronically, as of the distribution date, to you in direct registration form or to your bank or brokerage firm on your behalf. If you are a registered holder, Equiniti will then mail you a direct registration account statement that reflects your shares of Solventum common stock. If you hold your 3M shares through a bank or brokerage firm, your bank or brokerage firm will credit your account for the Solventum shares. Direct registration form refers to a method of recording share ownership when no physical share certificates are issued to shareholders, as is the case in this distribution. If you sell 3M common stock in the “regular-way” market up to and including the distribution date, you will be selling your right to receive shares of Solventum common stock in the distribution.
Commencing on or shortly after the distribution date, if you hold physical share certificates that represent your 3M common stock and you are the registered holder of the shares represented by those certificates, the distribution agent will mail to you an account statement that indicates the number of shares of Solventum common stock that have been registered in book-entry form in your name.
Most 3M shareholders hold their common stock through a bank or brokerage firm. In such cases, the bank or brokerage firm is said to hold the shares in “street name” and ownership would be recorded on the bank or brokerage firm’s books. If you hold your 3M common stock through a bank or brokerage firm, your bank or brokerage firm will credit your account for the Solventum common stock that you are entitled to receive in the distribution. If you have any questions concerning the mechanics of having shares held in “street name,” please contact your bank or brokerage firm.
Transferability of Shares You Receive
Shares of Solventum common stock distributed to 3M shareholders in connection with the distribution will be transferable without registration under the Securities Act, except in certain cases for shares received by persons who may be deemed to be Solventum’s affiliates. Persons who may be deemed to be Solventum’s affiliates after the distribution generally include individuals or entities that control, are controlled by or are under common control with Solventum, which may include certain of its executive officers or directors. Securities held by Solventum’s affiliates will be subject to resale restrictions under the Securities Act. Solventum’s affiliates will be permitted to sell shares of Solventum common stock only pursuant to an effective registration statement or an exemption from the registration requirements of the Securities Act, such as the exemption afforded by Rule 144 under the Securities Act.
Number of Shares of Solventum Common Stock You Will Receive
For every four shares of 3M common stock that you own at the close of business on March 18, 2024, the record date for the distribution, you will receive one share of Solventum common stock on the distribution date. No fractional shares of Solventum common stock will be distributed. Instead, if you are a registered holder, Equiniti will aggregate fractional shares into whole shares, sell the whole shares in the open market at prevailing market prices and distribute the aggregate cash proceeds (net of discounts and commissions) of the sales pro rata (based on the
77
fractional share such holder would otherwise be entitled to receive) to each holder that otherwise would have been entitled to receive a fractional share in the distribution. The distribution agent, in its sole discretion, without any influence by 3M or Solventum, will determine when, how, and through which broker-dealer and at what price to sell the whole shares. Any broker-dealer used by the distribution agent will not be an affiliate of either 3M or Solventum and the distribution agent is not an affiliate of either 3M or Solventum. Neither Solventum nor 3M will be able to guarantee any minimum sale price in connection with the sale of these shares. Recipients of cash in lieu of fractional shares will not be entitled to any interest on the amounts paid in lieu of fractional shares.
If you hold physical certificates for shares of 3M common stock and are the registered holder, you will receive a check from the distribution agent in an amount equal to your pro rata share of the net cash proceeds of the sales. If you hold your shares of 3M common stock through a bank or brokerage firm, your bank or brokerage firm will receive, on your behalf, your pro rata share of the net cash proceeds of the sales and will electronically credit your account for your share of such proceeds.
The net cash proceeds of these sales of fractional shares will be taxable for U.S. federal income tax purposes. See “Material U.S. Federal Income Tax Consequences” for an explanation of certain material U.S. federal income tax consequences of the distribution.
Treatment of Equity-Based Compensation
Outstanding 3M equity awards held by Solventum employees will be converted into corresponding Solventum awards in a manner that substantially preserves the intrinsic value of the awards as of immediately prior to and immediately following the distribution (with 3M performance share awards converted into time-vesting Solventum restricted stock unit awards based on actual performance as determined by the 3M Compensation and Talent Committee in respect of 2022 and 2023 and target performance in respect of 2024).
Outstanding 3M equity awards held by current and former employees of 3M (other than Solventum employees) and former members of the 3M Board of Directors will be adjusted in a manner that substantially preserves the intrinsic value of the awards as of immediately prior to and immediately following the distribution (with applicable performance goals adjusted by the 3M Compensation and Talent Committee).
Outstanding 3M equity awards held by current members of the 3M Board of Directors will be converted into an adjusted 3M award and a corresponding Solventum award based on the distribution ratio.
Internal Reorganization
As part of the separation, and prior to the distribution, 3M and its subsidiaries expect to complete an internal reorganization in order to transfer the Health Care Business to Solventum. Among other things, the internal reorganization is expected to result in Solventum owning, directly or indirectly, the operations comprising, and the entities that conduct, the Health Care Business. The internal reorganization is expected to include various restructuring transactions pursuant to which (1) the operations, assets and liabilities of 3M and its subsidiaries used to conduct the Health Care Business will be separated from the operations, assets and liabilities of 3M and its subsidiaries used to conduct the 3M Business and (2) such Health Care Business operations, assets and liabilities will be contributed, transferred or otherwise allocated to Solventum or one of its direct or indirect subsidiaries. These restructuring transactions may take the form of asset or equity transfers, mergers, demergers, distributions, contributions and similar transactions, and may involve the formation of new subsidiaries in U.S. and non-U.S. jurisdictions to own and operate the Health Care Business or the 3M Business in such jurisdictions.
Following the completion of the internal reorganization and immediately prior to the distribution, Solventum will be the parent company of the entities that are expected to conduct the Health Care Business and 3M will remain the parent company of the entities that are expected to conduct the 3M Business.
Results of the Distribution
After the distribution, Solventum will be an independent, publicly traded company. The actual number of shares to be distributed will be determined at the close of business on March 18, 2024, the record date for the distribution,
78
and will reflect 3M shares issued under 3M equity compensation awards and 3M share repurchases between the date on which the 3M Board of Directors declares the distribution and the record date for the distribution. The distribution will not affect the number of outstanding shares of 3M common stock or any rights of 3M shareholders. No fractional shares of Solventum common stock will be distributed.
Solventum will enter into a separation and distribution agreement and other related agreements with 3M to effect the separation and to provide a framework for its relationship with 3M after the separation and will enter into certain other agreements, including a transition services agreement, a transition distribution services agreement, a transition contract manufacturing agreement, research and development master services agreements, real estate license agreements, an intellectual property cross license agreement, a 3M mark use agreement, a transition trademark license agreement, master supply agreements, a tax matters agreement, an employee matters agreement, and a stockholder’s and registration rights agreement. See “Certain Relationships and Related Party Transactions.” These agreements will provide for the allocation between Solventum and 3M of the assets, employees, liabilities and obligations (including, among others, investments, property (including intellectual property) and employee benefits and tax-related assets and liabilities) of 3M and its subsidiaries attributable to periods prior to, at and after Solventum’s separation from 3M and will govern the relationship between Solventum and 3M subsequent to the completion of the separation (including the relationship of 3M as a stockholder of Solventum). For additional information regarding the separation and distribution agreement and other transaction agreements, see the sections entitled “Risk Factors—Risks Related to the Separation and Distribution” and “Certain Relationships and Related Party Transactions.”
Market for Solventum Common Stock
As of the date of this information statement, Solventum is a wholly owned subsidiary of 3M. Accordingly, no public market for Solventum’s common stock currently exists, although a “when-issued” market in Solventum’s common stock may develop prior to the distribution. See “—Trading Between the Record Date and Distribution Date” below. Solventum’s common stock has been approved for listing on the NYSE, subject to official notice of issuance, under the symbol “SOLV.” Solventum has not and will not set the initial price of its common stock. The initial price will be established by the public markets.
Neither 3M nor Solventum can predict the price at which its common stock will trade after the distribution. In fact, the combined trading prices, after the distribution, of the shares of Solventum common stock that each 3M shareholder will receive in the distribution, together with the 3M common stock held at the record date for the distribution, may not equal the “regular-way” trading price of the 3M common stock immediately prior to the distribution. The price at which Solventum common stock trades may fluctuate significantly, particularly until an orderly public market develops. Trading prices for Solventum common stock will be determined in the public markets and may be influenced by many factors. See “Risk Factors—Risks Related to Solventum Common Stock.”
Incurrence of Debt
In connection with the distribution, on February 27, 2024, Solventum issued $6.9 billion of senior unsecured notes in six series. Additionally, on February 16, 2024, Solventum entered into an 18-month senior unsecured term loan facility in an aggregate committed amount of $500 million and a three-year senior unsecured term loan facility in an aggregate committed amount of $1.0 billion. The proceeds of such financings are expected to be used to make cash payments to 3M, other than such amounts as need to be retained in order to cause Solventum to have $600 million of cash in the aggregate at the time of the distribution. Such cash payments to 3M from the proceeds of such financings are expected to total approximately $7.7 billion, taking into account fees, discounts and expenses. As a result of such transactions, Solventum anticipates having approximately $8.4 billion of outstanding indebtedness upon completion of the distribution. In addition, on February 16, 2024, Solventum entered into a revolving credit agreement in an aggregate committed amount of $2.0 billion. Solventum also intends to enter into a $2 billion unsecured, unsubordinated commercial paper program prior to the distribution. Solventum does not currently expect to incur any borrowings under either of the revolving credit agreement or the commercial paper program prior to or at the time of the distribution. For more information, see “Description of Material Indebtedness.”
79
Trading Between the Record Date and Distribution Date
Beginning on or about March 26, 2024 and continuing up to and including through the distribution date, 3M expects that there will be two markets in 3M common stock: a “regular-way” market and an “ex-distribution” market. 3M common stock that trades on the “regular-way” market will trade with an entitlement to Solventum common stock distributed in the distribution. 3M common stock that trades on the “ex-distribution” market will trade without an entitlement to Solventum common stock distributed in the distribution. Therefore, if you sell shares of 3M common stock in the “regular-way” market up to and including through the distribution date, you will be selling your right to receive shares of Solventum common stock in the distribution. If you own 3M common stock at the close of business on the record date and sell those shares on the “ex-distribution” market up to and including through the distribution date, you will receive the shares of Solventum common stock that you are entitled to receive pursuant to your ownership of shares of 3M common stock as of the record date.
Furthermore, beginning on or shortly before the record date for the distribution and continuing up to and including the distribution date, Solventum expects that there will be a “when-issued” market in its common stock. “When-issued” trading refers to a sale or purchase made conditionally because the security has been authorized but not yet issued. The “when-issued” trading market will be a market for Solventum common stock that will be distributed to holders of 3M common stock on the distribution date. If you own 3M common stock at the close of business on the record date for the distribution, you will be entitled to Solventum common stock distributed pursuant to the distribution. You may trade this entitlement to shares of Solventum common stock, without trading the 3M common stock you own, on the “when-issued” market. On the first trading day following the distribution date, “when-issued” trading with respect to Solventum common stock will end, and “regular-way” trading with respect to Solventum common stock will begin.
Conditions to the Distribution
The distribution will be effective at 3:30 a.m., Eastern Time, on April 1, 2024, which is the distribution date, provided that the conditions set forth in the separation and distribution agreement have been satisfied (or waived by 3M in its sole and absolute discretion), including, among others:
•the SEC declaring effective the registration statement of which this information statement forms a part, there being no order suspending the effectiveness of the registration statement in effect, and no proceedings for such purposes having been instituted or threatened by the SEC;
•this information statement having been made available to the holders of record of shares of 3M common stock at the close of business on March 18, 2024, the record date for the distribution;
•(i) the continuing validity of the IRS Ruling regarding U.S. Tax-Free Status and (ii) the receipt by 3M and continuing validity of the Tax Opinion(s) regarding U.S. Tax-Free Status;
•the internal reorganization and the transfer of assets and liabilities of the Health Care Business from 3M to Solventum and the transfer of assets and liabilities of the 3M Business from Solventum to 3M having been completed in accordance with the separation and distribution agreement;
•the receipt of one or more opinions from an independent appraisal firm to the 3M Board of Directors as to the solvency of 3M and Solventum after the completion of the distribution, in each case in a form and substance acceptable to the 3M Board of Directors in its sole and absolute discretion;
•all actions and filings necessary or appropriate under applicable U.S. federal, U.S. state or other securities or blue sky laws and the rules and regulations thereunder having been taken or made and, where applicable, having become effective or been accepted by the applicable governmental authority;
•the execution of certain agreements contemplated by the separation and distribution agreement;
•no order, injunction or decree issued by any government authority of competent jurisdiction or other legal restraint or prohibition preventing the consummation of the separation, the distribution or any of the related transactions being pending or being in effect;
80
•the shares of Solventum common stock to be distributed having been accepted for listing on the NYSE, subject to official notice of distribution;
•the receipt by 3M of certain proceeds from the financing arrangements described under “Description of Material Indebtedness” and 3M’s satisfaction in its sole and absolute discretion that, as of the effective time of the distribution, 3M will have no liability under such arrangements; and
•no other event or development existing or having occurred that, in the judgment of 3M’s Board of Directors, in its sole and absolute discretion, makes it inadvisable to effect the separation, the distribution and the other related transactions.
3M will have the sole and absolute discretion to determine (and change) the terms of, and whether to proceed with, the distribution and, to the extent it determines to so proceed, to determine the record date for the distribution, the distribution date and the distribution ratio. 3M will also have sole and absolute discretion to waive any of the conditions to the distribution.
The IRS Ruling and the Tax Opinion(s) are intended to provide support that the intended tax treatment of the distribution, together with certain related transactions, as a transaction that is generally tax-free for U.S. federal income tax purposes, will be respected by the IRS. Were 3M to waive the condition with respect to continuing validity of the IRS Ruling or the receipt of the Tax Opinion(s), there would be less comfort that the intended tax treatment would be respected by the IRS. For more information regarding the material U.S. federal income tax consequences of the distribution (including in the event the distribution does not qualify for tax-free treatment for U.S. federal income tax purposes) see the section entitled “Material U.S. Federal Income Tax Consequences.”
3M does not intend to notify its shareholders of any modifications to the terms of the separation or distribution or waivers of conditions that, in the judgment of its Board of Directors, are not material. For example, the 3M Board of Directors might consider material such matters as significant changes to the distribution ratio and the assets to be contributed or the liabilities to be assumed in the separation or a waiver of the condition that 3M receive the Tax Opinion(s). To the extent that the 3M Board of Directors determines that any modifications by 3M materially change the material terms of the distribution or that any waivers of conditions by 3M are material to 3M shareholders (including any waiver of the conditions relating to the IRS Ruling and/or the Tax Opinion(s)), 3M will notify 3M shareholders in a manner reasonably calculated to inform them about the modification as may be required by law, by, for example, publishing a press release, filing a current report on Form 8-K or circulating a supplement to this information statement.
Reasons for Furnishing This Information Statement
We are furnishing this information statement solely to provide information to 3M shareholders that will receive shares of our common stock in the distribution. You should not construe this information statement as an inducement or encouragement to buy, hold, or sell any of our securities or any securities of 3M. No 3M shareholder approval is required for the distribution, and you are not being asked for a proxy. We believe that the information contained in this information statement is accurate as of the date set forth on the cover. Changes to the information contained in this information statement may occur after that date, and neither Solventum nor 3M undertakes any obligation to update the information except in the normal course of our and 3M’s public disclosure obligations and practices.
81
DIVIDEND POLICY
Solventum has not yet determined whether it expects to pay a regular dividend after the separation and distribution. The timing, declaration, amount of and payment of any dividends following the separation and the distribution will be within the discretion of Solventum’s Board of Directors and will depend upon many factors, including its financial condition, earnings, capital requirements of its operating subsidiaries, covenants associated with certain of its debt service obligations, legal requirements, regulatory constraints, industry practice, ability to access capital markets and other factors deemed relevant by Solventum’s Board of Directors. Moreover, if Solventum determines to pay any dividend in the future, there can be no assurance that Solventum will continue to pay such dividends or the amount of such dividends.
82
CAPITALIZATION
The following sets forth the capitalization of Solventum as of December 31, 2023, on a historical and a pro forma basis, which reflects the adjustments described in more detail in the notes to the unaudited pro forma financial information included elsewhere in this information statement. You should read this information in conjunction with those notes, as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” the “Unaudited Pro Forma Condensed Combined Financial Information,” and the audited annual combined financial statements and the related notes included elsewhere in this information statement.
| As of December 31, 2023 | ||||||||||||||
| (dollar amounts in millions) | Historical | Pro Forma | ||||||||||||
| Assets | ||||||||||||||
| Cash and cash equivalents | $ | 194 | $ | 600 | ||||||||||
| Liabilities | ||||||||||||||
| Total debt | $ | — | $ | 8,303 | ||||||||||
| Shareholders’ equity | ||||||||||||||
| Net parent investment | $ | 12,003 | $ | — | ||||||||||
| Common stock | — | 2 | ||||||||||||
| Additional paid-in capital | — | 4,229 | ||||||||||||
| Accumulated other comprehensive income (loss) – net | (337) | (822) | ||||||||||||
| Total shareholders’ equity | $ | 11,666 | $ | 3,409 | ||||||||||
| Total Capitalization | $ | 11,666 | $ | 11,712 | ||||||||||
83
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The following unaudited pro forma condensed combined financial statements consist of the unaudited pro forma condensed combined balance sheet as of December 31, 2023 and the unaudited pro forma condensed combined statements of income for the year ended December 31, 2023.
The unaudited pro forma condensed combined financial statements reflect adjustments to our historical audited combined balance sheet as of December 31, 2023, and our historical audited combined statement of income for the year ended December 31, 2023.
The unaudited pro forma condensed combined balance sheet gives effect to the separation and related transactions, described below, as if they occurred as of December 31, 2023, our latest balance sheet date. The unaudited pro forma condensed combined statements of income give effect to the separation and related transactions as if they had occurred on January 1, 2023, the beginning of our most recently completed fiscal year.
The unaudited pro forma condensed combined financial statements have been prepared to reflect transaction accounting and autonomous entity adjustments to present the financial condition and results of operations as if we were a separate standalone entity. In addition, we have provided a presentation of management adjustments that management believes are necessary to enhance the understanding of the pro forma effects of the separation. The unaudited pro forma condensed combined financial information has been adjusted to give effect to the following (collectively, the “Pro Forma Transactions”):
•the transfer of assets and liabilities that comprise the Health Care Business by 3M pursuant to the separation and distribution agreement;
•the expected transfer to Solventum, prior to or concurrent with the separation, of various 3M assets and liabilities not included in our historical combined balance sheets (including the transfer of certain pension and employee benefit obligations, net of any related assets, associated with our active, retired, and other former employees from 3M);
•the anticipated post-separation capital structure, including: (i) the issuance of approximately 172,466,022 shares of our common stock (based on the number of shares of 3M common stock outstanding as of December 31, 2023), at least 80.1% of which will be distributed to holders of 3M common stock in connection with the distribution and (ii) indebtedness of approximately $8.4 billion at an estimated weighted-average interest rate of 5.8% (additional details on debt issuance can be found in note (a));
•the impact of the tax matters agreement to be entered into with 3M in connection with the separation;
•the impact of the transition services agreement and other transaction agreements to be entered into with 3M in connection with the separation as described under “Certain Relationships and Related Party Transactions”;
•transactions and incremental income and costs expected to be incurred as an autonomous entity and specifically related to the separation;
•other adjustments described in the notes to the unaudited pro forma condensed combined financial statements; and
•management adjustments that consist of reasonably estimated transaction effects expected to occur.
The unaudited pro forma condensed combined financial statements were prepared in accordance with Article 11 of Regulation S-X, as amended. The unaudited pro forma condensed combined financial statements are presented for informational purposes only and do not purport to represent what our financial position and results of operations actually would have been had the Pro Forma Transactions occurred on the dates indicated, or to project our financial performance for any future period. The unaudited pro forma condensed combined financial statements are based on information and assumptions, which are described in the accompanying notes.
84
Our historical combined financial statements, which were the basis for the unaudited pro forma condensed combined financial statements, were prepared on a carve-out basis as we did not operate as a standalone entity for the periods presented. Accordingly, such financial information reflects an allocation of general corporate costs, such as information technology, finance and accounting, human resources, legal, and other expenses that are either specifically identifiable or clearly applicable to Solventum.
The unaudited pro forma condensed combined financial information has been prepared to include transaction accounting (including the impact of changes to our legal entity structure in anticipation of the separation) and autonomous entity adjustments to reflect the financial condition and results of operations as if we were a standalone entity. Transaction accounting adjustments have been presented to show the impact and associated cost as a result of the legal separation from 3M, including the incurrence of indebtedness, transfer of assets or assumption of liabilities not included in historical financial statements, and transfer of additional pension and employee benefit assets and liabilities.
As described in ‘Treatment of Equity-Based Compensation’, upon Spin-Off any outstanding 3M equity awards held by Solventum employees will be converted into corresponding Solventum awards in a manner that substantially preserves the intrinsic value of the awards as of immediately prior to and immediately following the distribution. Each converted award will generally be subject to the same terms, vesting conditions and other restrictions that applied to the original 3M award immediately before the Spin-Off, except that performance vesting conditions, as applicable, will be adjusted to reflect the Spin-Off. The modification of outstanding equity awards, including option awards with only time-based vesting conditions, may result in incremental compensation cost based on the fair value of the awards determined on the conversion date. Management is not able to estimate the incremental compensation cost for these awards as certain inputs to fair value are not reasonably determinable prior to the conversion date.
Autonomous entity adjustments have been presented to show the impact of items such as the transition services agreement and other transaction agreements with 3M, lease arrangements with third parties and 3M, and incremental costs expected to be incurred as an autonomous entity. Management has determined that certain transaction agreements, including the Tax Matters Agreement, Intellectual Property Cross License Agreement, 3M Mark Use Agreement and Transitional Trademark Cross License Agreement, are not expected to have a material financial impact and therefore no autonomous entity adjustment has been reflected. In addition, we have provided a presentation of management adjustments that management believes are necessary to enhance the understanding of the pro forma effects of the transaction. Actual future costs incurred may differ from these estimates.
The unaudited pro forma condensed combined financial information reported below should be read in conjunction with the section of this information statement entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the historical combined financial statements included elsewhere in this information statement.
85
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF INCOME
For the year ended December 31, 2023
(Dollars in millions, except per share amounts)
| Historical | Transaction Accounting Adjustments | Autonomous Entity Adjustments | Solventum Pro Forma | ||||||||||||||||||||||||||
| Net Sales | |||||||||||||||||||||||||||||
| Sales of product | $ | 6,296 | $ | (6) | (d), (e), (f) | $ | 50 | (k) | $ | 6,340 | |||||||||||||||||||
| Sales of software and rentals | 1,901 | 1,901 | |||||||||||||||||||||||||||
| Total net sales | 8,197 | (6) | 50 | 8,241 | |||||||||||||||||||||||||
| Operating expenses | |||||||||||||||||||||||||||||
| Costs of product | 3,023 | 18 | (d), (e), (f) | 159 | (k) | 3,200 | |||||||||||||||||||||||
| Costs of software and rentals | 481 | 481 | |||||||||||||||||||||||||||
| Selling, general and administrative | 2,243 | 47 | (d), (f) | 31 | (k) | 2,321 | |||||||||||||||||||||||
| Research and development | 758 | 1 | (k) | 759 | |||||||||||||||||||||||||
| Total operating expenses | 6,505 | 65 | 191 | 6,761 | |||||||||||||||||||||||||
| Operating income | 1,692 | (71) | (141) | 1,480 | |||||||||||||||||||||||||
| Other expense (income) - net | 25 | 545 | (a),(b),(g) | 570 | |||||||||||||||||||||||||
| Income before income taxes | 1,667 | (616) | (141) | 910 | |||||||||||||||||||||||||
| Provision for income taxes | 321 | (70) | (i) | (33) | (l) | 218 | |||||||||||||||||||||||
| Net income | $ | 1,346 | $ | (546) | $ | (108) | $ | 692 | |||||||||||||||||||||
| Weighted average common shares outstanding — basic | (n) | 172,466,022 | |||||||||||||||||||||||||||
| Earnings per share attributable to common shareholders — basic | 4.01 | ||||||||||||||||||||||||||||
| Weighted average common shares outstanding — diluted | (o) | 172,466,022 | |||||||||||||||||||||||||||
| Earnings per share attributable to common shareholders — diluted | 4.01 | ||||||||||||||||||||||||||||
See accompanying notes to unaudited pro forma condensed combined financial statements.
86
UNAUDITED PRO FORMA CONDENSED COMBINED BALANCE SHEET
As of December 31, 2023
(Dollars in millions)
| Historical | Transaction Accounting Adjustments | Autonomous Entity Adjustments | Solventum Pro Forma | ||||||||||||||||||||||||||
Assets | |||||||||||||||||||||||||||||
| Current assets | |||||||||||||||||||||||||||||
| Cash and cash equivalents | $ | 194 | $ | 406 | (a) | $ | 600 | ||||||||||||||||||||||
| Receivables - net of allowances | 1,313 | (200) | (d) | 1,113 | |||||||||||||||||||||||||
| Due from related party | — | 180 | (d) | 180 | |||||||||||||||||||||||||
| Inventories | 857 | 12 | (d) | 869 | |||||||||||||||||||||||||
| Other current assets | 155 | (1) | (d) | 154 | |||||||||||||||||||||||||
| Current assets | 2,519 | 397 | — | 2,916 | |||||||||||||||||||||||||
| Property, plant, and equipment – net | 1,457 | 9 | (d) | 1,466 | |||||||||||||||||||||||||
| Goodwill | 6,535 | 6,535 | |||||||||||||||||||||||||||
| Intangible assets - net | 2,902 | 2,902 | |||||||||||||||||||||||||||
| Other assets | 530 | (92) | (a),(b),(d),(i),(j) | 120 | (m) | 558 | |||||||||||||||||||||||
| Total assets | $ | 13,943 | $ | 314 | $ | 120 | $ | 14,377 | |||||||||||||||||||||
Liabilities | |||||||||||||||||||||||||||||
| Current liabilities | |||||||||||||||||||||||||||||
| Accounts payable | 477 | (9) | (d) | 468 | |||||||||||||||||||||||||
| Unearned revenue | 574 | 574 | |||||||||||||||||||||||||||
| Other current liabilities | 677 | 4 | (d) | 48 | (m) | 729 | |||||||||||||||||||||||
| Current liabilities | 1,728 | (5) | 48 | 1,771 | |||||||||||||||||||||||||
| Long-term debt | — | 8,303 | (a) | 8,303 | |||||||||||||||||||||||||
| Defined benefit pension | 166 | 265 | (b) | 431 | |||||||||||||||||||||||||
| Deferred income taxes | 231 | 8 | (i),(j) | 239 | |||||||||||||||||||||||||
| Other liabilities | 152 | 72 | (m) | 224 | |||||||||||||||||||||||||
| Total liabilities | 2,277 | 8,571 | 120 | 10,968 | |||||||||||||||||||||||||
Equity | |||||||||||||||||||||||||||||
| Net parent investment | 12,003 | (12,003) | (c) | — | |||||||||||||||||||||||||
| Common stock, $0.01 par value; 172,466,022 shares issued | — | 2 | (c) | 2 | |||||||||||||||||||||||||
| Additional paid-in capital | — | 4,229 | (h) | 4,229 | |||||||||||||||||||||||||
| Accumulated other comprehensive income (loss) - net | (337) | (485) | (b),(d), (g) | (822) | |||||||||||||||||||||||||
| Total equity | 11,666 | (8,257) | — | 3,409 | |||||||||||||||||||||||||
| Total liabilities and equity | $ | 13,943 | $ | 314 | $ | 120 | $ | 14,377 | |||||||||||||||||||||
See accompanying notes to unaudited pro forma condensed combined financial statements.
87
Note 1. Notes to Unaudited Pro Forma Condensed Combined Financial Statements
Transaction Accounting Adjustments
(a)Reflects approximately $8.4 billion of borrowings expected to be incurred in connection with the separation pursuant to financing transactions. The financing transactions include $6.9 billion of senior notes ranging in maturity from 3 years to 40 years, and approximately $1.5 billion of term loan facilities. The total borrowings are partially offset by original issuance discount and debt issuance costs of $77 million, which will be amortized over the lives of the respective borrowings. The borrowings have a weighted average interest rate of approximately 5.8%. We expect to pay 3M, as partial consideration for the Health Care Business that 3M is transferring to us in connection with the separation, one or more cash payments from the proceeds of such borrowings, other than such amounts as are required to cause us to have $600 million in cash and cash equivalents at the time of the distribution. Such cash payments to 3M from the proceeds of such distribution are expected to total approximately $7.7 billion, taking into account fees, discounts and expenses.
| (Millions) | For the year ended December 31, 2023 | |||||||
| Interest Expense on Total Debt at Estimated Weighted Average Rate | $ | 486 | ||||||
| Amortization of Debt Issuance Costs | 6 | |||||||
| Total Interest Expense from Debt | $ | 492 | ||||||
We have entered into a revolving credit facility in an amount of $2.0 billion which is expected to be used for working capital and the general corporate purposes of Solventum. We expect to capitalize an additional $4 million of issuance costs associated with the revolving credit facility, which will be amortized over the life of the facility agreement. The $4 million of issuance costs associated with the revolving credit facility has been included in Other assets in the unaudited pro forma condensed combined balance sheet. The pro forma financial information does not give effect to additional borrowings under this credit facility because no amount is expected to be drawn or used in connection with this offering or the separation.
(b)The historical financial statements only include assets and obligations for 3M retirement and non-pension postretirement benefit plans which have been historically dedicated to the Health Care Business. These dedicated plans have been accounted for as single employer plans under US GAAP. The majority of 3M’s retirement and non-pension postretirement benefit plan’s broadly include both 3M and Health Care Business active employees and retirees as participants. We have accounted for our participation in these retirement and non-pension postretirement benefit plans as participation in a multi-employer plan and as such the liability and assets for these plans is excluded from our historical combined financial statements. Under this method of accounting, we recognized our relevant portion of 3M’s service cost component of net periodic benefit cost within our historical combined financial statements with a corresponding increase in Net parent investment.
In connection with the separation, 3M will transfer to Solventum retirement and non-pension postretirement benefit plan assets and obligations which have been historically dedicated to the Health Care Business. 3M will also transfer to Solventum retirement and non-pension postretirement benefit plan obligations associated with Health Care Business active employees and retirees that were historically part of a commingled benefit plan for 3M. Assets from commingled plans will also be transferred to Solventum by 3M in an amount determined by the requirements of Section 414(l) of the U.S. Internal Revenue Code or other local regulations. Management has estimated the net benefit plan asset or obligation that will transfer for the commingled plans. The actual, assumed benefit plan obligation will be measured using an actuarial valuation at the date of legal transfer. The actual assets that transfer may change based on changes in the underlying fair value of the assets. As a result, the actual assets and obligations that transfer may change significantly from our estimates. The estimated pro forma adjustment for the pension and postretirement
88
benefit plans is reflected in the unaudited pro forma condensed combined balance sheet as of December 31, 2023 as follows:
| (Millions) | As of December 31, 2023 | |||||||
| Other assets | $ | 13 | ||||||
| Defined benefit pension | 265 | |||||||
| Accumulated other comprehensive income (loss) - net of tax impact of $177 | (543) | |||||||
We have also recognized an estimate of incremental pro forma non-operating costs of $5 million for the year ended December 31, 2023, related to the pension and postretirement benefit plans to be transferred to Solventum.
(c)Reflects the reclassification of 3M’s net investment in our Company to Additional paid-in capital as well as the issuance of 172,466,022 shares of our common stock with a par value of $0.01 per share pursuant to the Separation and Distribution Agreement. We have assumed the number of outstanding shares of our common stock based on 552,581,136 shares of 3M common stock outstanding on December 31, 2023, assuming a distribution of 80.1% of our common stock to 3M’s stockholders, and a distribution ratio of one share of our common stock for every four shares of 3M’s common stock. The actual number of shares issued will not be known until the record date for the distribution. We expect 19.9% of our common stock will continue to be owned by 3M.
(d)Reflects assets, liabilities, and certain operations that will be retained by 3M or transferred to Solventum in connection with the separation in the unaudited pro forma condensed combined balance sheet as of December 31, 2023 and pro forma condensed combined statements of income for the year ended December 31, 2023. Refer to the below tables for further details on specific adjustments.
| (Millions) | As of December 31, 2023 | |||||||
| Accounts receivable | $ | (200) | ||||||
| Inventory | 12 | |||||||
| Other current assets | (1) | |||||||
| Property, plant, and equipment | 9 | |||||||
| Other assets | 3 | |||||||
| Accounts payable | (9) | |||||||
| Other current liabilities | 4 | |||||||
| Accumulated other comprehensive income (loss) - net | 11 | |||||||
| (Millions) | For the year ended December 31, 2023 | |||||||
| Sales of product | $ | (37) | ||||||
| Costs of product | (8) | |||||||
| Selling, general and administrative | (9) | |||||||
Solventum will be entitled to the reimbursement of amounts resulting from settlement of transactions historically related to Solventum operations. As of December 31, 2023, Solventum is entitled to $180 million of receivables from 3M. This amount includes approximately $189 million of Accounts receivable, net of $9 million of Accounts payable that 3M will pay on Solventum’s behalf as of December 31, 2023. This amount has been reflected in the Due from related party line of the unaudited pro forma condensed combined balance sheet as of December 31, 2023.
89
Depreciation expense on additional transferred property, plant, and equipment is not expected to be material.
(e)Reflects the estimated revenue and cost of sales associated with Food Safety transition arrangements that will transfer to Solventum as part of the separation. The Food Safety transition arrangements are associated with the divestiture of 3M’s Food Safety business which occurred in September 2022. The estimate includes an additional $46 million in Sales of product as well as $39 million in Costs of product for the year ended December 31, 2023, respectively.
(f)Reflects the removal of the historical Sales of product, Costs of product, and gain on sale related to the dental local anesthetic business historically reported in the Dental Solutions segment. The sale closed on August 1, 2023 and the business will not be part of Solventum after the separation. The Sales of product included in the historical results was $15 million for the year ended December 31, 2023. The Costs of product included in the historical results was $9 million for the year ended December 31, 2023. The gain on sale of $56 million was included in Selling, general, and administrative expense. As part of the sale, Solventum entered into transition agreements, which are expected to yield an additional $4 million of income which has been reflected within Costs of product for the year ended December 31, 2023.
(g)Reflects expected charges that will be incurred as a result of changes to Solventum’s operating model that will be implemented in connection with the separation. As part of the change in operating model, Solventum will no longer have a physical presence in certain countries and will service customers in these countries through local distribution in an export model. These non-recurring changes in operating model are expected to be completed within a year of the separation. As a result of these operating model changes, cumulative translation adjustment balances of $47 million will be written-off and related charges have been reflected in the year-ended December 31, 2023.
(h)The additional paid-in capital adjustments are summarized below:
| (Millions) | As of December 31, 2023 | |||||||
Cash payments to 3M(a) | $ | (7,893) | ||||||
Retirement and non-pension postretirement benefit plans(b) | 291 | |||||||
Net parent investment(c) | 12,003 | |||||||
Solventum common stock issuance(c) | (2) | |||||||
Operations retained by 3M or transferred to Solventum(d) | (3) | |||||||
Substantial liquidation of certain foreign entities(g) | (47) | |||||||
Deferred taxes(i) | (129) | |||||||
Legal entity restructuring(j) | 9 | |||||||
| Total adjustment | $ | 4,229 | ||||||
(i)Reflects the tax impacts of the respective transaction accounting adjustments after applying the applicable statutory income tax rates to pre-tax pro forma adjustments in jurisdictions where the adjustments were taxable or deductible and further adjusting for certain expected tax impacts. For the year ended December 31, 2023, the Company anticipates that the estimated increase in interest expense from the Solventum financing will result in the need for an additional valuation allowance of $24 million, and an increase in the global intangible low-tax income inclusion of $23 million. These reconciling items decrease the tax benefit provided on the transaction accounting adjustments as compared to the Company’s statutory tax rate. The Company also reflected tax expense of $24 million associated with the deferred tax assets resulting from the anticipated adjustments to tax basis from legal entity restructuring in connection with the spin-off, as discussed in note (j). The applicable tax rates could be impacted (either higher or lower) depending on many factors subsequent to the separation including the profitability in local jurisdictions and the legal entity structure implemented subsequent to the separation and may be materially different from the pro forma results.
90
The Company increased Other assets and Deferred income taxes by $63 million and $3 million, respectively, in the proforma condensed combined balance sheet associated with the transfer of retirement and non-pension postretirement benefit obligations to Solventum discussed at note (b), reducing Accumulated other comprehensive loss - net by $177 million and Additional paid-in capital by $117 million in the pro forma condensed combined balance sheet as a result. The Company also decreased deferred tax assets by $181 million associated with tax attributes that will be retained by 3M, reducing Additional paid-in capital by $181 million. These two adjustments resulted in a change in the presentation of deferred tax assets and deferred tax liabilities presented in Other assets and Deferred income taxes in the pro forma condensed combined balance sheet due to netting deferred tax balances by jurisdiction as reflected.
(j)Reflects the addition of Other assets and Deferred income taxes of $14 million and $5 million, respectively, resulting from the anticipated adjustments to tax basis in certain of our assets and liabilities resulting from legal entity restructuring in connection with the spin-off. These tax basis adjustments and resulting additional deferred tax assets are based on our latest estimation of related assets and liabilities, which have not yet been completed. We expect to finalize such valuations at the completion of the separation.
Autonomous Entity Adjustments
(k)Reflects the effect of the transition services, transition contract manufacturing, transition distribution services, master supply and real estate license agreements we and 3M will enter into in connection with the separation. Included in the unaudited pro forma condensed combined statement of income for the year ended December 31, 2023 are adjustments to Sales of product, Costs of product, Research and development, and Selling, general, and administrative expenses that reduced operating income by $141 million as follows;
| (Millions) | For the year ended December 31, 2023 | |||||||
| Sales of product | $ | 50 | ||||||
| Costs of product | 159 | |||||||
| Selling, general and administrative | 31 | |||||||
| Research and development | 1 | |||||||
The adjustments for transition manufacturing, transition distribution and sales and supply agreements reflect the historical transfers between the Health Care Business and 3M under the pricing of each agreement. The adjustments for transition services and real estate license agreements are based on a monthly fixed fee over the period we expect services will be provided. All agreements are being drafted and will be completed prior to separation. The adjustments have been calculated based on the terms of the latest draft agreements and are subject to change.
(l)Reflects the tax impacts of the respective autonomous entity adjustments after applying the applicable statutory income tax rates to pre-tax pro forma adjustments in jurisdictions where valuation allowances are not necessary. This adjustment considers transfer pricing arrangements in place related to the jurisdictional source of supply. The applicable tax rates could be impacted (either higher or lower) depending on many factors subsequent to the separation including the profitability in local jurisdictions and the legal entity structure implemented subsequent to the separation and may be materially different from the pro forma results.
(m)Reflects the net impact of lease arrangements with third parties and sublease arrangements with 3M for facilities that will be entered into prior to the separation. These adjustments record the operating right-of-use assets and related operating lease liabilities based on the estimated present value of the lease payments over the lease term. The adjustment includes additional right-of-use assets of $120 million included in Other assets as well as $48 million and $72 million of operating lease liabilities in Other current liabilities and Other liabilities, respectively.
91
Pro Forma Earnings Per Share
(n)The weighted-average number of shares used to compute pro forma basic earnings per share for the year ended December 31, 2023 is 172,466,022 on the basis of one share of our common stock for every four shares of 3M common stock outstanding as of December 31, 2023 and the 19.9% interest in the outstanding shares of the Company’s common stock that are expected will be owned by 3M at the time of the Spin-Off.
(o)The weighted-average number of shares used to compute pro forma diluted earnings per share for the year ended December 31, 2023 is 172,466,022, which represents the number of shares we expect to be outstanding in connection with the Spin-Off. The actual dilutive effect following the completion of the Spin-Off will depend on various factors, including employees who may change employment between 3M and Solventum and the impact of 3M and Solventum equity-based compensation arrangements. We cannot estimate the dilutive effects at this time.
Management Adjustments
We have elected to present management adjustments to the pro forma financial information and included all adjustments necessary for a fair statement of such information. Following the separation, we expect to incur incremental one-time and non-recurring expenses associated with the stand-up of functions required to operate as a standalone company. These non-recurring costs primarily relate to IT system implementation, facility and manufacturing separation, licensing and permitting and development of our brands.
We estimated these additional costs by assessing the resources and associated one-time external costs each function (e.g., finance, IT, HR, etc.) will require to stand up Solventum as a stand-alone public company. Most one-time costs are expected to be incurred within a period of 12-24 months post-separation.
The additional expenses have been estimated based on assumptions that our management believes are reasonable. However, actual additional costs that will be incurred could be different from the estimates and would depend on several factors, including the economic environment, results of contractual negotiations with third party vendors, ability to execute on proposed separation plans, and strategic decisions made in areas such as manufacturing, selling and marketing, research and development, information technology, and infrastructures. In addition, adverse effects and limitations including those discussed in the section entitled “Risk Factors” to this document may impact actual costs incurred. The management adjustments also do not include any assumptions around synergies or dis-synergies that may result once we are operating as a standalone company. We may also decide to increase or reduce resources or invest more heavily in certain areas in the future, which may differentiate the management adjustments even further from actual costs incurred in the future.
The management adjustments presented below are incremental to the autonomous entity pro forma adjustments. Management believes the presentation of these adjustments is necessary to enhance an understanding of the pro
92
forma effects of the transaction. If we decide to increase or reduce resources or invest more heavily in certain areas in the future, that will be part of our future decisions and will not be included in the management adjustments below.
The tax effect has been determined by applying the applicable statutory tax rates to the aforementioned adjustments for the periods presented. These management adjustments include forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements.”
| (Millions, except per share amounts) | Year ended December 31, 2023 | |||||||
| Unaudited pro forma condensed combined net income | $ | 692 | ||||||
| Management adjustments | (148) | |||||||
| Provision for income taxes | 35 | |||||||
| Pro forma net income (loss) after Management adjustments | $ | 579 | ||||||
| Weighted average Solventum common shares outstanding — basic | 172,466,022 | |||||||
| Earnings per share attributable to Solventum common shareholders — basic | $ | 3.36 | ||||||
| Weighted average Solventum common shares outstanding — diluted | 172,466,022 | |||||||
| Earnings per share attributable to Solventum common shareholders — diluted | $ | 3.36 | ||||||
93
BUSINESS
All amounts discussed in this section are in millions of U.S. dollars, unless otherwise indicated. Except as otherwise indicated or unless the context otherwise requires, this section discusses Solventum’s business assuming the completion of all of the transactions described in this information statement, including the separation. References to “we,” “us,” and “our” refer to the Health Care Business to be held by Solventum and its subsidiaries.
Overview
Solventum is a leading global healthcare company developing, manufacturing, and commercializing a broad portfolio of solutions that leverages deep material science, data science, and digital capabilities to address critical customer and patient needs. We constantly seek to enable the improvement of standards of care and move healthcare forward with innovation powered by insights, clinical intelligence, technology, and manufacturing expertise. Our 70+ year history of discovering and innovating advanced solutions has helped us solve our customers’ toughest challenges.
In 2023, Solventum generated 56% of total revenues from the United States and 44% from international. Based on the breadth of our portfolio, Solventum serves a global addressable market that we estimate had approximately $93 billion of industry sales in 2022. We estimate that this addressable market will grow at an annual rate of 4-6% from 2024 through 2026. We participate in what we believe are large, stable global markets that have favorable market drivers, including changing demographics, optimization of workflows to improve quality of care, increasing digital technology and data-driven care delivery, shifting care from the hospital to lower-cost care sites and increasing demand for personalized care. See the sub-section below titled “Our Markets” for information about how we estimated the size and growth rate of our addressable market, and the risks to our ability to take advantage of these market opportunities.
We are organized into four operating business segments that are aligned with the markets we serve.
•MedSurg (56% of 2023 total sales), formerly Medical Solutions, is a provider of solutions including advanced wound care, I.V. site management, sterilization assurance, temperature management, surgical supplies, stethoscopes, and medical electrodes. These solutions are designed to accelerate healing, prevent complications, and lower the total cost of care. Specifically, our advanced wound care solutions follow the patient from hospital to home and support them through the recovery process. We are a leader in the advanced wound care market based on the market share data presented in a BCC Research report (BCC Publishing, Markets for Advanced Wound Management Technologies, July 2023) and, based on internal estimates, our products currently treat more than 1.6 million hard-to-heal wounds annually. Additionally, our comprehensive range of surgical solutions are designed to mitigate a patient’s risk of infection or complications.
•Dental Solutions (16% of 2023 total sales), formerly Oral Care Solutions, is a provider of a comprehensive suite of dental and orthodontic products including brackets, aligners, restorative cements, and bonding agents that span the “life of the tooth,” including products designed for preventative dental care, direct and indirect restoration, and broad orthodontic needs. We have a leading position in the dental and orthodontic bonding systems market based on published market share data from Key-Stone Network (Key-Stone Fast Track Clinical Report — Clinical Ranking, 2022), SDM Northcoast (SDM Dental Products Market Share Study, 2022) and Orthodontic Manufacturers Association (OMA Sales Survey by Association Research, Inc., 2022). Additionally, we estimate our 3M™ Filtek™ branded products have been used in over two billion dental restoration procedures worldwide over the last twenty years.
•Health Information Systems (16% of 2023 total sales) provides healthcare systems with software solutions – including computer-assisted physician documentation, direct-to-bill and coding automation, classification methodologies, speech recognition, and data visualization platforms – that are designed to eliminate revenue cycle waste, create more time for patient care, and support value-based care. These solutions are designed to ensure accuracy of reimbursement and reduce the administrative burden that clinicians face. We have a leading market position in the United States for computer-assisted coding
94
technology based on published market share data from Definitive HealthCare (Definitive Healthcare, HospitalView Database, Technology Search for “Computer Assisted Coding/NLP” technology, 2022) and, based on our internal estimates, more than 75% of U.S. hospitals currently use at least one of our software solutions.
•Purification and Filtration (12% of 2023 total sales), formerly Separation and Purification Sciences, is a provider of purification and filtration technologies including filters, purifiers, cartridges, and membranes. These solutions are designed to simplify purification processes, reduce debris and bioburden in fluids, and remove contaminants to enable the development and manufacturing of biopharmaceutical and medical technology treatments and provide cleaner water. Based on internal estimates, our membrane technology is currently used annually in more than 25 million life-saving dialysis treatments and approximately one million open heart surgeries are currently performed each year using oxygenators that are enabled by one of our membranes.
For a list of products, by business segment, that are regulated by the U.S. Food and Drug Administration (“FDA”) as medical devices or pharmaceuticals, see the section below titled “Product Regulation”.
We believe Solventum is an integral part of the global healthcare ecosystem. Our solutions are relied on every day within the global healthcare industry, and we believe they contribute to higher-quality patient care, more efficient processes and workflows, and improved standards of safety and accuracy. Additionally, our products and services are present along a patient’s journey through prevention, diagnosis, treatment, and recovery.

Our business possesses strong customer relationships, a broad, wide-ranging, and well-known portfolio of brands, differentiated technology, and manufacturing expertise. We serve a diverse customer base, ranging from multidisciplinary hospitals and local clinics/practices to biopharmaceutical manufacturers. Our long-tenured and collaborative customer relationships globally give us unique insights into their needs and preferences. These insights inform our innovation processes, drive stronger customer retention, and create multiple avenues for further customer engagement.
We serve customers in over 90 countries with a global team of approximately 22,000 employees and an established global manufacturing network. In each of the last three years, we have generated over $8 billion of revenue, $1.7 billion of operating income, and $2 billion of adjusted operating income. We believe Solventum will deliver growth at attractive margins with the mission of enabling better, smarter, safer healthcare to improve lives.
95
However, Solventum has no history of operating as an independent company, and its historical and pro forma financial information is not necessarily representative of the results that it would have achieved as a separate, publicly traded company and may not be a reliable indicator of its future results. In particular, Solventum currently benefits from 3M’s long operating history, reputation and well-known brand. Following the separation, Solventum will operate under its own brand, and accordingly may be negatively impacted due to the loss of benefits conferred by 3M’s brand recognition and reputation. Additionally, the debt obligations incurred by Solventum in connection with the separation will adversely affect its profitability and could affect its ability to use its cash flow for investing in the business, M&A and returning capital. See “Risk Factors - Risks Related to the Separation and Distribution” for a discussion of these risks, which you should consider carefully.
Our Markets
Introduction
We believe Solventum operates in a large, diverse, and stable set of markets. Sustainable, long-term growth in our markets is driven by favorable global market drivers, including changing demographics, optimizing workflows to improve quality of care, increasing digital technology and data-driven care delivery, shifting care from the hospital to lower cost care sites, and increasing demand for personalized care.
•Changing demographics: An aging population, the prevalence and incidence rates of chronic conditions, and a rising middle class are driving the demand for improved access to quality care.
•Optimizing workflows to improve quality of care: Of the $4.5 trillion in annual U.S. healthcare spending, an estimated 25% represents administrative costs that do not contribute to health outcomes and which we believe to be potentially wasteful based on overall spending data reported by the Centers for Medicare & Medicaid Services in the NHE Fact Sheet (available on CMS.gov as of January 10, 2024) and administrative spending estimates published in JAMA (Shrank et. al., Waste in the US Health Care System: Estimated Costs and Potential for Savings, published October 7, 2019). Our solutions are designed to optimize workflows, enabling clinicians to be more productive by spending less time on administrative tasks and more time focused on improving the patient care experience. Our solutions also support reducing infections and complications that lead to an increase in avoidable administrative and clinical costs.
•Increasing digital technology and data-driven care delivery: Both clinicians and patients have shifted their preferences towards utilizing digitally enabled solutions to provide data-driven care. Whether it is interactions with patients through a digital interface or the use of data to make informed health decisions, the need for digital tools in the healthcare industry has grown over time. Our solutions integrate digital processes and data in multiple ways and across different parts of the healthcare industry and are intended to enable efficient and effective delivery of care.
•Shifting care from the hospital to lower-cost care sites: Although hospitals continue to be a core site for delivery of care, patients are increasingly looking for flexibility of care when and where they need it. Alternative care sites, such as ambulatory surgery centers, wound care clinics, retail pharmacies, and the home, are more affordable and accessible to patients. We believe our solutions enable clinicians to extend their care delivery from acute to ambulatory to home settings without compromising the quality of care and while reducing the total cost of care.
•Increasing demand for personalized care: Engaging patients in a personalized way allows clinicians to provide a better care experience while improving outcomes and reducing costs. This spans several areas of healthcare, including personalized biopharmaceutical treatments, customized orthodontic aligner treatments, and follow-up wound care at home. We believe our solutions deliver personalized care options in a way that is patient-centric, scalable, and cost-effective.
Our ability to take advantage of these market opportunities will be subject to various risks, including general economic, business and market dynamic risks, and specifically including effects of, and changes in, worldwide economic, political, regulatory, international trade and geopolitical conditions, natural disasters, wars and public health crises; operational execution risks; the highly competitive environment in which we operate; consolidation in
96
the healthcare industry; reductions in customers’ research budgets or government funding; risks related to the timing and market acceptance of our new products and offerings; changes in reimbursement practices of third-party payers or other cost containment measures; vulnerability with respect to materials and fluctuations in the costs and availability of purchased components, compounds, raw materials, energy, and labor, the impact of our separation from 3M; and the substantial debt we will incur in connection with the separation. See “Information Statement Summary—Summary of Risk Factors”, “Information Statement Summary—Certain Risks Relating to Operating as a Standalone Entity”, “Information Statement Summary—Certain Risks Relating to Solventum’s Indebtedness”, and “Risk Factors” for a discussion of these risks, which you should consider carefully.
We estimate that the broader markets that Solventum participates in represented more than $205 billion of industry sales in the total global healthcare sector in 2022, based on the reports described below. Based on management estimates, we believe these markets in aggregate will grow at an annual rate of 4-6% from 2024 through 2026 (representing a weighted average (based on the relative market size) of the relevant management estimates).
Specifically, based on the summation described below, we estimated the size of the global addressable market in which we operate to have been approximately $93 billion in 2022, and based on management estimates, we believe this global addressable market is projected to grow at an annual rate of 4-6% from 2024 through 2026 (representing a weighted average (based on the relative market size) of the relevant management estimates for this global addressable market). Based on the recent performance of our business, we believe there is a significant market opportunity for future growth as we execute on our strategies.
| Segment | Addressable Market Size in 2022 | Addressable Market Annual Growth Rate (2024-2026) | ||||||
| MedSurg | ~$26 billion | 3-5% | ||||||
| Dental Solutions | ~$17 billion | 4-6% | ||||||
| Health Information Systems | ~$9 billion | 6-8% | ||||||
| Purification and Filtration | ~$41 billion | 4-6% | ||||||
| Total Solventum | ~$93 billion | 4-6% | ||||||
MedSurg Sources: Market size defined as the sum of total industry sales in advanced wound care (source: SmartTrak Advanced Wound Care Market Database, 2023), IV site management (sources: BCC Publishing Staff, Global Blood Transfusion and Intravenous Equipment Market Report, May 2022; BCC Publishing Staff, Advanced Medical Dressings: Global Market, July 2021), hospital supplies (source: BCC Research Staff, Hospital Supplies: Global Markets to 2023, February 2019), medical electrodes (source: Allied Market Research, Medical Electrodes Market: Global Opportunity Analysis and Industry Forecast, 2021-2031, May 2022), medical tapes and bandages (source: Grand View Research, Medical Tapes and Bandages: Market Analysis 2016-2027, 2020), skin antiseptics (source: Allied Market Research, Skin Antiseptic Market: Global Opportunity Analysis and Industry Forecast 2021-2031, March 2023), temperature management systems (source: Markets and Markets, Temperature Management Systems Market: Global Forecast to 2026, 2021), and surgical drapes and gowns (source: Grand View Research, Surgical Drapes: Market Analysis 2018-2030, 2021). Dental Solutions Sources: Market size defined as the sum of total industry sales in dental consumables and dental equipment; both sourced from Markets and Markets reports (Markets and Markets, Dental Consumables Market: Global Forecast to 2027, 2022; Markets and Markets, Dental Equipment Market: Global Forecast to 2027, 2023). Health Information Systems Sources: Market size defined as the total sum of industry sales in revenue cycle management (source: Markets and Markets, Revenue Cycle Management: Global Forecast to 2028, 2023), medical transcription (source: Fortune Business Insights, Medical Transcription Software: Global Market Analysis Insights and Forecast 2020-2026, 2020), and population health management (source: Frost & Sullivan, US Population Health Management Growth Opportunities, February 2022). Purification & Filtration Sources: Market size defined as the sum of total industry sales in industrial filtration (source: Markets and Markets, Industrial Filtration Global Forecast to 2027, 2022), separation membrane contractors (source: Markets and Markets, Membrane Contractor Market Global Forecast to 2025, 2020), residential water treatment systems (source: Baytel Associates, The Global Market for Home Water Treatment Products and Services, 2021 Edition), commercial water purifiers (source: Azoth Analytics Research, Global
97
Commercial Water Purifier Market, June 2022 Edition), medical and industrial membranes (source: Markets and Markets, Medical Membranes Market: Global Forecast to 2022, May 2018; Markets and Markets, Membranes Market: Global Forecast to 2027, October 2022), and biopharmaceutical purification devices and equipment (source: Freedonia Custom Research, Global Biopharmaceutical Purification Device/Equipment Market Study, October 2021). For each segment, Solventum calculated the addressable market growth rate by estimating the growth rate for the relevant market size for the referenced components of such segment (based on the described reports, historical change, and internal forecasts and estimates) and computing a weighted average (based on the relative market size) of these growth rates.
In addition to the global market drivers described above, each of our segments benefits from segment-specific market drivers as outlined below:
•MedSurg: Growth in the MedSurg market is driven by increasing surgical procedure volumes and incidence rates of chronic wounds, shifting of care to out-of-hospital settings, and the increasing prevalence of digitally enabled solutions. Within MedSurg, a priority market is Advanced Wound Care, where we expect to see sustainable growth
•Dental Solutions: Growth in the Dental Solutions market is driven by increasing oral care procedure volumes, evolving patient standards of preventative care, and shifting patient preferences that emphasize aesthetics. Furthermore, the changing industry service economics is enabled by innovation and growth of digital workflows to create custom solution offerings for all. For Dental Solutions, a priority is the digitization across the market, as providers continue to adopt digital solutions to reduce chair time and improve patient outcomes.
•Health Information Systems: Growth in the Health Information Systems market is driven by hospital spending on information technology, increasing scrutiny of revenue leakage and healthcare information technology return on investment, care delivery shifting to lower-cost settings, digital technology driving healthcare efficiency, and a broad shift to value-based care. For Health Information Systems, a priority is the growing demand for conversational AI and ambient solutions to improve clinician productivity and reduce their administrative burden.
•Purification and Filtration: Growth in the Purification and Filtration market is driven by increasing biopharma innovation, expanding use of new modalities focused on personalized medicine, such as targeted antibodies and cell and gene therapies, growing efforts to reduce bioprocessing complexity, growing sustainability needs including water quality and preservation, and an increasingly complex global regulatory environment. For Purification and Filtration, a priority market is Bioprocessing Filtration.
The estimates of market opportunity and market growth may prove to be inaccurate, and even if the markets in which we operate achieve the expected growth, our business (or the applicable segments of our business) could fail to grow at similar rates, or at all. Our growth is subject to many factors, including our success in implementing our business strategy, which is subject to many risks and uncertainties. For the fiscal year ended 2023, sales in our Purification and Filtration segment declined relative to the fiscal year 2022. For the fiscal year ended 2022, sales in our MedSurg and Dental Solutions segments declined relative to the fiscal year ended 2021. For a further discussion of the historical performance of our segments, together with an overview of the key factors driving such performance, see the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Performance by Business Segment”.
Investment Highlights
Solventum has numerous competitive advantages in attractive markets that we expect to continue to drive our success over the long term, including those described below. We believe that our innovation expertise, digital capabilities and data science business model, global scale and manufacturing expertise, and cash flow generation capability position us for continued growth and value creation. Additionally, we are building new capabilities, including an incoming experienced executive team, that will support value creation as we become a standalone company.
98
Although we believe that these competitive strengths will contribute to the growth and success of our company, our business is subject to various risks that may prevent us from achieving our business objectives or otherwise adversely affect our business, results of operations or financial condition. In particular, following the distribution and separation, Solventum will be an independent company and will no longer have access to the competitive advantages that it has historically derived from being a part of 3M, such as 3M’s research capabilities, brand recognition and reputation. Additionally, the debt obligations incurred by Solventum in connection with the separation will adversely affect its profitability and could affect its ability to use its cash flow for investing in the business, M&A and returning capital. See “Information Statement Summary—Summary of Risk Factors”, “Information Statement Summary—Certain Risks Relating to Operating as a Standalone Entity”, “Information Statement Summary—Certain Risks Relating to Solventum’s Indebtedness”, and “Risk Factors” for a discussion of these risks, which you should consider carefully.
A Proven Global Leader in Large, Diverse and Growing Markets
We believe Solventum operates in a global addressable market that we estimated to have been $93 billion in 2022 and we believe, based on management estimates, is projected to grow at an annual rate of 4-6% from 2024 through 2026. We expect there will be sustainable, long-term growth in our global addressable market because of favorable market drivers, including changing demographics, the increasing need to optimize workflows, deliver digitally enabled and data-driven care, shift to lower-cost alternative care sites and the increasing demand to provide personalized care. For further discussion about these factors, including estimated growth rates and factors by segment, see the sub-section titled “Markets” above. For a discussion of the historical performance of our segments, together with an overview of the key factors driving such performance, see the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Performance by Business Segment”.
We provide over 100,000 channel partners and customers across more than 90 countries with solutions that have contributed to leading market positions across our segments. In our MedSurg segment, we are a leader in the advanced wound care market based on the market share data presented in a BCC Research report (BCC Publishing, Markets for Advanced Wound Management Technologies, July 2023). Specifically, based on internal estimates, our products currently treat more than 1.6 million hard-to-heal wounds annually. In our Dental Solutions segment, we have a leading position in the dental and orthodontic bonding systems market (based on published market share data from Key-Stone Network (Key-Stone Fast Track Clinical Report — Clinical Ranking, 2022), SDM Northcoast (SDM Dental Products Market Share Study, 2022) and Orthodontic Manufacturers Association (OMA Sales Survey by Association Research, Inc., 2022). Additionally, we estimate our 3M™ Filtek™ branded products have been used in over two billion dental restoration procedures worldwide over the last twenty years. In our Health Information Systems segment, we have a leading market position in the United States for computer-assisted coding technology based on published market share data from Definitive HealthCare (Definitive Healthcare, HospitalView Database, Technology Search for “Computer Assisted Coding/NLP” technology, 2022) and, based on our internal estimates, more than 75% of U.S. hospitals currently use at least one of our software solutions.
We believe we have a track record of success because we consistently provide better, smarter, and safer solutions to our customers and patients.
Our ability to take advantage of these market opportunities is subject to various general economic, business and market dynamic risks. Our estimates of market opportunity and market growth may prove to be inaccurate, and even if the markets in which we operate achieve the expected growth, our business could fail to grow at similar rates, or at all.
Diverse Portfolio of Strong, Reputable Brands and Long-Standing Customer Relationships
We believe Solventum is an integral part of the global healthcare ecosystem. Our solutions are relied on every day within the global healthcare industry, and we believe they contribute to higher-quality patient care, more efficient processes and workflows, and improved standards of safety and accuracy. Furthermore, the breadth and diversity of our portfolio enables us to have products and services present along a patient’s journey through prevention, diagnosis, treatment, and recovery.
99
Across our businesses, we are a provider of what we believe are market-leading brands, such as 3M™ PREVENA™, 3M™ V.A.C.®, 3M™ Tegaderm™, 3M™ Littmann®, 3M™ Filtek™, 3M™ 360 Encompass™, and 3M™ Membrana™. Many of these brands carry a history of innovation and industry recognition, and today, we believe, are seen as among the best in class. Additionally, we believe our brand value is supported by strong economic evidence and the volume of published studies reviewing our brands. For instance, in October 2022, 3M™ V.A.C.® Therapy for negative pressure wound care surpassed a milestone of 2,000 published, peer-reviewed medical journal studies, making it reviewed, in our view, by more clinical data than any other negative pressure wound therapy brand in the world and accounting, by our estimates, for more than 70% of all published negative pressure wound therapy clinical studies.
We attribute our strong market position and customer loyalty to the value of our brands. Globally, we sell our solutions to more than 100,000 channel partner and customers. We also have a long-standing history of partnering with national and local government agencies around the world. For example, our Health Information Systems segment has been working with the Centers for Medicare and Medicaid Services (CMS) to develop and update both public and proprietary algorithms, benchmarks, and classification systems.
Our brands are critical to our success, and damage to our reputation or our brands could adversely affect our business, results of operations or financial condition. Solventum currently benefits from 3M’s long operating history, reputation and well-known brand. Following the separation, Solventum will operate under its own brand, and accordingly may be negatively impacted due to the loss of benefits conferred by 3M’s brand recognition and reputation.
Technology Platforms and Expertise Powering Innovation
For approximately 70 years, we have been innovating across the healthcare industry by leveraging our deep material science and process capabilities in areas such as adhesives, films, nonwovens, nanotechnology, advanced composites, biomaterials, surface filtration and separation membranes. By combining these technology platforms, we believe we have been successful at solving our customers’ problems with novel solutions where we have been the first-to-market and market disruptors. Some examples of where we have been first-to-market include Negative Pressure Wound Therapy (NPWT) and antimicrobial transparent film dressings in our MedSurg segment, tooth-colored dental composites in our Dental Solutions segment, integrated computer-assisted coding and clinical documentation improvement in the Health Information Systems segment, and hybrid chromatographic clarification solutions in our Purification and Filtration segment. In addition, we have a long history of being a market disruptor by introducing transformative solutions across our businesses:
•MedSurg segment: We were first-to-market with Negative Pressure Wound Therapy (NPWT). 3M™ PREVENA™ Incision Management System is a negative pressure medical device intended to aid in the reduction of superficial surgical site infections (SSIs) for patients at a high risk for post-operative infections. It is the first and only disposable, powered NPWT system with reticulated open-cell foam (ROCF) dressings specifically designed to manage closed surgical incisions.
•MedSurg segment: We were first-to-market with antimicrobial transparent film dressings. 3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Securement Dressing is an antimicrobial transparent film dressing that is designed to combine infection reduction, site visibility, consistent application, and catheter securement into one integrated product. It is the only transparent CHG dressing cleared by the FDA and is designed to reduce vascular catheter colonization and catheter-related blood stream infections (CRBSI) in patients with central venous or arterial catheters.
•Dental Solutions segment: We were first-to-market with tooth-colored dental composites used in the restoration process. 3M™ Filtek™ Matrix is a digital restorative workflow solution that is designed to simplify a traditional composite placement to a three-step process, with the goal of increasing procedure predictability for dentists and reducing chair time and improving aesthetics for the patient.
•Health Information Systems segment: We were first-to-market with integrated computer-assisted coding and clinical documentation improvement. 3M™ M*Modal Fluency Direct combines proprietary speech recognition with natural language understanding with the goal of providing more accurate clinical
100
documentation. This solution is compatible with more than 250 Electronic Health Record (EHR) Systems and has received a Best in KLAS Award for Speech Recognition: Front-End EMR.
•Purification and Filtration segment: We were first-to-market with hybrid chromatographic clarification solutions. 3M™ Harvest RC is a new solution for the manufacturing of recombinant protein therapeutics that employs next-generation hybrid chromatographic technology to combine three processing steps into one.
We plan to continue to bring novel, disruptive solutions to the healthcare industry by combining and leveraging elements of our broad material science, data science, and digital capabilities across our company. These capabilities are supported by proprietary intellectual property with over 7,300 patents issued globally and industry expertise from more than 2,100 members of our global R&D team.
Our ability to deliver these novel, disruptive solutions depends on protecting our intellectual property rights. We cannot assure you that our means of obtaining, maintaining, and enforcing our intellectual property rights will be adequate to maintain a competitive advantage. New product and services development requires significant investment in research and development, clinical trials and regulatory approvals. The ability to bring new products and services to market is subject to difficulties or delays in product and service development, such as the inability to identify viable new products and services, obtain adequate intellectual property protection, regulatory approvals and reimbursement in the United States and abroad, and successfully complete clinical trials or gain market acceptance of new products and services. It is uncertain when or whether our products, services, or solutions currently under development will be launched or will be commercially successful. Additionally, new offerings may be quickly rendered obsolete by changing customer preferences, changing industry standards, or competitors’ innovations or reverse engineering efforts.
Leading Digital and Data Science Capabilities and Business Models
We have added significant data science and digital capabilities through organic innovation and over a dozen acquisitions. We believe we are uniquely positioned to serve our customers in an increasingly digitizing healthcare landscape given the investments we have made and the expertise we have gained in our Health Information Systems segment. We believe our medical coding software is among the most widely used in the world with many health systems relying on 3M™ 360 Encompass™ to process their healthcare records each month. Furthermore, our 3M™ M*Modal speech solutions include innovative features designed to support clinical productivity.
We have a deep understanding of the capabilities, resources, and management associated with operating and optimizing a software business. Our expertise encompasses the sales and marketing processes, subscription-based revenue model management, and software implementation and upgrade processes that are unique to a digital business. Our customer relationships are long-tenured, diverse, and collaborative, and many of our customers have been with us longer than 30 years.
As the healthcare industry increasingly looks to utilize digital and data-driven tools to increase efficiency and improve health outcomes, our expertise and track record in this space gives us a strong foundation to expand our capabilities and appropriate use of data to solve the industry’s toughest problems.
The healthcare information technology (HCIT) industry is highly competitive and dynamic and characterized by the continual introduction of new products and technologies. Our ability to compete will be affected by various factors, including development of new products and innovative technologies; our ability to improve our existing portfolio of offerings; our ability to deliver return on investment for our customers; improving efficiency and productivity of healthcare workers; our ability to stay up-to-date on regulatory changes, reimbursement guidelines and other healthcare best practices; developing and selling our products cost effectively; meeting all relevant quality standards for our products and their markets; and protecting the proprietary technology of our products and development processes. Failure to accomplish these objectives may adversely affect our business, results of operations or financial condition.
101
Global Scale and Reach Supported by Strong Manufacturing Expertise
We have an extensive global commercial footprint with customers in over 90 countries. In 2023, Solventum generated 56% of total revenues from the United States and 44% from international. To serve our diverse customer base across our prioritized geographies, we take a multi-model commercial approach, including direct-to-customer, distribution, key account management, inside sales, and e-commerce.
This global commercial footprint is supported by a strong network of manufacturing expertise, which is a core driver of our ability to deliver high-quality and innovative solutions in a cost-efficient manner at scale. Our expertise areas include precision coating, inspecting films and nonwovens, specialty film and polymer processing and additive manufacturing. We deploy automation tools, including robotics, vision systems, unique high-speed mechanisms, and sophisticated machine controls to drive productivity and quality. Our specialized processes, including gamma sterilization and spore and API processing, further enhance our portfolios. We have invested in smart, vertically integrated manufacturing capabilities powered by automation and data analytics.
Our manufacturing team is comprised of over 6,000 employees supporting manufacturing operations and 1,100 technical service and engineer members. Across our global manufacturing network, we utilize an analytics-enabled framework to drive decision-making, manage costs, and optimize production. We believe that our global footprint and expertise drive our ability to produce better, smarter, and safer products at a consistent quality level.
Our financial results depend on the successful execution of our business operating plans. The ability to adapt Solventum’s business model and respond to changes, including responding to evolving customer needs and service expectations, are important. Operational challenges, including those related to customer service, pace of change and productivity improvements, could have an adverse effect on our business.
Cash Flow Generation and Attractive Margins
For each of the last three years, we have generated over $1.6 billion of cash from operating activities and over $1.4 billion of free cash flow. We also have delivered greater than 20% operating income margin and greater than 25% adjusted operating income margin for each of the last three years. Given our cash generation capability, we believe we will be able to reduce leverage; reinvest in our business; accelerate growth through bolt-on M&A; and consider return of capital to shareholders.
However, Solventum has no history of operating as an independent company, and its historical and pro forma financial information is not necessarily representative of the results that it would have achieved as a separate, publicly traded company and may not be a reliable indicator of its future results. The debt obligations incurred by Solventum in connection with the separation will adversely affect its profitability and could affect its ability to use its cash flow for investing in the business, M&A and returning capital.
Highly Engaged Workforce Led by an Experienced Executive Team
Our executive team has extensive experience across the healthcare industry and will be invaluable as we embark on establishing Solventum as a standalone healthcare company. Many of these executive officers come to Solventum with significant public company and spin-off experience. Already, the new executive team is building a more focused, nimble, and empowered healthcare culture within Solventum.
We have a long-tenured and diverse talent base with rich technical qualifications and deep healthcare industry experience. Our employee base consists of approximately 22,000 employees with over 40% having more than 10 years of tenure at the Health Care Business. Our strong culture of collaboration and partnership and focus on employee engagement programs enable us to create an integrated, equitable organization and are key drivers of employees spending a significant part of their career at Solventum. We believe our employees’ deep relationships with customers and expertise for developing and manufacturing our innovative solutions better helps us solve the industry’s toughest problems of today and tomorrow. We plan to continue to build on this momentum as a mission-driven workforce and make Solventum a preferred employer.
102
There is substantial competition for key personnel, senior management, research and development personnel, and qualified employees in the healthcare industry and we may face increased competition for such a highly qualified scientific, technical, clinical, and management workforce in a highly competitive environment. The loss of one or more key employees, inability to attract or develop additional qualified employees, any delay in hiring key personnel, any deterioration of the relationships with its employees, or any material work stoppage, strike, or similar action could result in a material adverse effect on our business, results of operations, financial condition, and cash flows.
Business Strategies
Our business strategies include those set forth below. Our ability to implement these strategies and achieve the intended benefits are subject to numerous economic and business risks, as well as risks related to the fact that following the separation and distribution, we will operate as a standalone entity and will not be able to receive many of the benefits that we have historically received by operating as a part of 3M and that we will incur significant debt obligation in connection with the separation, which will adversely affect our profitability and could adversely affect our ability to use our cash flow for investing in the business, M&A and returning capital. See “Information Statement Summary—Summary of Risk Factors”, “Information Statement Summary—Certain Risks Relating to Operating as a Standalone Entity”, “Information Statement Summary—Certain Risks Relating to Solventum’s Indebtedness”, and “Risk Factors” for a discussion of these risks, which you should consider carefully.
As we focus on our business strategy and setting Solventum up for success, we will take a measured and phased approach. We will start by embedding our new mission and culture throughout the business, bringing in new talent and capabilities, addressing execution challenges, and executing on our separation plan. Next, we will prioritize our growth strategy, ensuring we have proper focus and resourcing in the right areas. And finally, we will look at opportunities further down the road to transform our portfolio. The growth strategies below will be a roadmap for how we think about our portfolio – now and in the future.
To deliver on our financial commitments, we will focus on driving revenue growth through market selection and deploying capital to innovation, commercial execution, and M&A. Furthermore, we will continue to build on our capability to drive margin expansion and generate cash.
Invest in Markets with Highest Growth Potential
We will be thoughtful about the markets we prioritize for resource allocation. We believe there is significant opportunity both within the markets we serve today and in strategic high-growth adjacent markets. As a part of our active portfolio management, we plan to focus our efforts on markets where we can both fulfill our mission to enable better, smarter, safer healthcare to improve lives and increase adoption of our innovative solutions to build on many of the leading market positions we have today. As we look to increase our presence in select high-growth markets where we operate, we believe we can leverage our geographic scale and innovation capabilities.
Continue to Deliver Customer-Centric Innovation
We have a strong culture of purposeful innovation focused on solving our customers’ most critical problems. Our innovation engine has powered multiple key product launches across many years, including our original launch of surgical drapes in 1948, the 1964 launch of Addent™, the first tooth-colored filling material, and the 1982 launch of 3M™ Tegaderm™ transparent film dressings. Our R&D organization supports our innovation efforts and consists of more than 2,100 employees including research scientists, chemical engineers, data scientists, software engineers, application development engineers, and product developers. Our R&D team members have an average tenure of nine years, including advanced degrees in Chemistry, Bioengineering, Materials Science, Biological Science, Organic Chemistry, and Molecular Biology. From 2021 to 2023, we invested a cumulative $1.8 billion in R&D (excluding amortization), representing over 7% of sales in each year to power our innovation efforts.
As a standalone company, we will have more flexibility to allocate resources to prioritize R&D investments. We will continue to grow our solution offerings and expect to launch multiple new products in the coming years. Our innovation will focus on key areas that enable us to not only leverage our existing capabilities and strengths, but also have a high impact in the markets we serve, including:
103
•Setting new industry innovation standards: We have, in our view, launched multiple solutions that represented significant industry innovations when they were introduced, and set a new standard for healthcare. An example of this innovation is 3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Securement Dressing. It is the only transparent film dressing cleared by the Food and Drug Administration (FDA) and is designed to reduce catheter-related blood stream infections (CRBSIs) and vascular catheter colonization that aligns with evidence-based guidelines. These types of shifts are an area of focus for our ongoing innovation efforts.
•Continuing to leverage our ability to share technology across our platforms to drive unique, differentiated solutions: We believe we have the unique capability to combine our deep heritage of material science innovation and existing digital and data science platforms to create unique solutions to solve our customers’ biggest challenges. In our ongoing innovation efforts, we plan to continue to harness these capabilities, while enhancing our focus to draw upon additional technology combinations across our portfolio of solutions.
•Leverage data science expertise to increase digitization of healthcare: We believe our Health Information Systems segment puts us at the center of the digitization of the healthcare industry. We plan to use our knowledge and expertise from operating this business to increase our digital and analytics capabilities across our business. As the healthcare industry increasingly looks to utilize digital and data-driven tools to increase efficiency and improve health outcomes, our expertise and track record within the Health Information Systems segment gives us a strong foundation to expand our capabilities and use of data to solve the industry’s toughest problems.
Strengthen Commercial Model and Execution
We plan to increase sales growth by growing our market share within our leading franchises through multiple strategies, including expanding into alternative sites of care, evolving our commercial model to meet local expectations, and increasing customer loyalty through our evidence-based approach.
•Expanding into alternative sites of care: Our current customers include hospitals and health systems, with whom we have long-lasting and collaborative relationships. As care increasingly shifts outside of traditional settings, we believe we have an opportunity to grow our customer relationships in these alternate sites of care and expand our market reach. As an example, we will look to grow our remote-monitoring capabilities and enhance our sales coverage of alternate sites of care, including ambulatory surgery centers, long-term care, skilled nursing facilities, and patients’ homes.
•Evolving our commercial model to meet local expectations: As the need for high-quality healthcare solutions expands globally, we believe we are well-positioned to capitalize on our existing global footprint and expand our presence in key countries to broaden our customer reach. Our expansion strategy is designed to understand the specific needs of priority countries by tailoring solutions to focus on local and regional outcome measures. We plan to gather key insights and maintain relationships with regulatory authorities and clinical groups that inform our tailored commercial approach. We also have the opportunity to expand access to our solutions internationally. Given the importance of government policies and regulatory infrastructures in determining the success of healthcare business operations, we plan to conduct our international expansion with a strong focus on the regulatory environment and market access through frequent interactions with international regulatory bodies.
•Increasing customer loyalty through our evidence-based approach: Our existing customer relationships are strong, partnership-oriented, and long-tenured. By integrating clinical evidence and customer education more deeply into our solution positioning with customers, we have an opportunity to strengthen our customer relationships and ground them in data, research, and best practices. To do this, we will rely on our relationships with the global clinical community and regulatory authorities, with whom we need to raise awareness of the evidence supporting our solutions. We have a team of scientists, clinical specialists, and medical liaisons who play a critical role in the generation of clinical and economic evidence. We believe
104
these individuals are important thought leaders in the clinical community and can help further disseminate this evidence throughout the industry.
Accelerate Growth Through Strategic M&A and Partnerships
A key part of our strategy as a standalone company is to utilize targeted, strategic M&A and partnerships to augment our organic innovation and grow our healthcare solution offerings. As a standalone company, we will have the ability to more efficiently allocate resources to pursue these opportunities.
Inorganic growth represents an opportunity for us to increase our innovation solutions and scale within our most attractive markets and submarkets, while also providing a vector into near adjacencies and strategic new white spaces over time. We will continue to seek attractive M&A opportunities that focus on differentiated, clinician-preferred solutions. Identifying key areas of external innovation that are aligned to our broader strategic goals will be a focus area for Solventum, which we believe will have even more flexibility as a standalone company to allocate capital for strategic transactions that provide us with additional ways to deliver differentiated outcomes.
We will also continue to seek opportunities to partner with institutions and companies to augment growth. A recent example is our ongoing collaboration with Amazon Web Services (“AWS”) to accelerate the innovation and advancement of 3M™ M*Modal ambient intelligence. This collaboration expands on our early success in bringing conversational AI and ambient intelligence directly into clinical documentation workflows and includes the use of AWS Machine Learning and generative AI services to help expedite, refine, and scale the delivery of our ambient clinical documentation and virtual assistant solutions. We will look to continue to identify partnership opportunities that align with and accelerate our strategic goals.
Drive Margin Expansion and Free Cash Flow Generation
Our continuous improvement focus and capabilities will enable us to improve our operating model as we become a stand-alone company. Furthermore, by focusing on improving the mix of our products, implementing manufacturing and supply chain efficiency initiatives, and increasing commercial productivity, we will be able to drive future margins and cash flow. We expect to be able to reduce leverage; reinvest in our business; accelerate growth through bolt-on M&A; and consider return of capital to shareholders. However, the debt obligations incurred by Solventum in connection with the separation will adversely affect our profitability and could affect our ability to use our cash flow for investing in the business, M&A and returning capital.
OUR SEGMENTS
MedSurg
Our MedSurg segment delivers a broad range of innovative solutions , including advanced wound care and surgical supplies that are intended to accelerate healing, prevent complications, and lower the total cost of care globally. Our solutions draw upon the deep heritage of material science and the technologies we have developed over decades, including adhesives, biomaterials, films, nonwovens, and specialty materials.
Our products have helped treat wounds and are used daily by clinicians. We are a pioneer across multiple markets, and we believe our innovative products have set the standard of care for healthcare professionals. In our view, uptake of our products is supported by patient outcomes, improved ease of use, and streamlined clinician and patient experience. We also have specialized personnel who work with clinicians and hospital staff to provide technical support and education.
Our global solutions are offered through brands that we sell both direct and through distributors to hospitals for use in operating rooms, inpatient care units, and central sterilization, as well as the out-of-hospital setting, including ambulatory surgical centers, skilled nursing facilities, long-term care facilities, and patient homes.
Advanced Wound Care Solutions
We are one of the leading providers of advanced wound care solutions in the world based on the market share data presented in a BCC Research report (BCC Publishing, Markets for Advanced Wound Management
105
Technologies, July 2023). Our solutions are intended to deliver predictable and improved patient outcomes, streamline clinician and patient experiences, and enable faster healing. Our broad range of solutions can be used across clinical applications, care settings, and clinician specialties. We are focused on accelerating wound healing, reducing postoperative infections and complications, and lowering the total cost of care through Negative Pressure Wound Therapy (NPWT), Advanced Wound Dressings (AWD), and Advanced Skin Care products.
| Negative Pressure Wound Therapy (NPWT) | Advanced Wound Dressings (AWD) | Advanced Skin Care | ||||||
 |  |  | ||||||
3M™ V.A.C.® | 3M™ Promogran Prisma™ | 3M™ CavilonTM | ||||||
Negative Pressure Wound Therapy
The Health Care Business was the first-to-market in the NPWT market, launching our first NPWT product over 25 years ago, and we believe we remain a leading supplier today. Our comprehensive suite of NPWT products are designed to treat and manage a variety of wound types and conditions, including chronic wounds like venous leg ulcers, diabetic foot ulcers and pressure ulcers as well as acute wounds such as traumatic wounds, dehisced surgical wounds, skin grafts, and burns, as well as managed closed surgical incisions. Our recognized brands are 3M™ V.A.C.®, 3M™ Veraflo™, 3M™ Prevena™, and 3M™ Abthera™ therapies, and these solutions include reusable and disposable therapy units, as well as therapy-specific dressings. With a focused commitment to help clinicians heal many types of wounds and reduce the cost of care, our NPWT solutions are backed by over 2,000 peer-reviewed publications. Our NPWT home care model streamlines the delivery of equipment and supplies directly to healthcare providers and patients, monitors therapy remotely, and collects reimbursement from payors.
3M™ V.A.C.® Traditional-NPWT | 3M™ Veraflo™ NPWT with Instillation | 3M™ Prevena™ Single-Use NPWT | 3M™ AbThera™ Open Abdomen NPWT | |||||||||||
 |  |  |  |   | ||||||||||
3M™ V.A.C.® Ulta Therapy Unit (Acute) | 3M™ ActiV.A.C.™ Therapy Unit (Portable) | 3M™ Veraflo Cleanse Choice™ Dressing | 3M™ Prevena Restor™ BellaForm™ Incision Management System | 3M™ AbThera™ SensaT.R.A.C™ Open Abdomen Dressing | ||||||||||
Integrated wound management systems designed for acute and ambulatory care that provide multiple distinct wound treatment options in a single device | 3M™ Veraflo Cleanse Choice™ Dressing, in conjunction with 3M™ Veraflo™ Therapy, can be used to initiate hydromechanical removal of infections materials, non-viable tissue and debris | Portable and easy-to-use disposable therapy unit dressings designed for specific surgical procedures and wounds | 3M™ AbThera™ Therapy is used for temporary abdominal closure, helping surgeons take control early when managing a challenging open abdomen, helping to achieve primary fascial closure | |||||||||||
Our 3M™ V.A.C.® Therapy technology is designed to promote healing by delivering controlled and regulated negative pressure to the wound bed utilizing differentiated technologies such as 3M™ Granufoam™, which is an open-cell foam dressing that draws wound edges together, promotes granulation, and removes exudate. By removing contaminants and managing bioburden, 3M™ Veraflo™ Instillation Therapy is designed to add the instillation of fluids to NPWT to accelerate the healing of dirty or infected wounds. Our 3M™ Smart Instill™ technology is
106
designed to automate multiple time-consuming therapy steps to optimize workflow efficiencies, which is increasingly important amid ongoing hospital staffing shortages.
We continue to innovate in the NPWT space, focusing on launching easy-to-use solutions and expanding clinical usage. 3M™ Prevena™ and 3M™ AbThera™ Therapies support closed incisions and temporary abdominal closures. These products help manage the environment of closed surgical incisions and are designed to remove fluid away from the surgical incision, which reduces post-operative infection risk and accelerates healing. 3M™ AbThera™ manages the open abdomen in traumatic and emergency surgical procedures.
Our 3M™ Prevena™ Therapy is the first and only disposable, powered negative pressure system with reticulated open-cell foam (ROCF) dressings specifically designed to manage closed surgical incisions. It is designed to help protect, manage, and optimize the healing environment of closed incisions to help reduce the incidence of postoperative complications beyond the operating room. A multicenter randomized control trial (RCT), sponsored by the Health Care Business, demonstrated that 3M™ Prevena™ Therapy reduced surgical site complication incidence by 4x and readmission by 3x vs. standard postop dressings. A cost-effectiveness study of the RCT, sponsored by the Health Care Business, found that 3M™ Prevena™ Therapy reduced the patient cost of care by 1.9x vs. postop dressings. 3M™ Prevena™ Therapy is utilized by surgeons to help manage closed incisions for at-risk patients and procedures. In 2023, the FDA expanded the approved indications for 3M™ Prevena™ single-use therapy to include flaps, grafts, and open wounds.
Additionally, we have been a first mover in the integration of digital solutions in the NPWT space including our first-to-market 3M™ iOn PROGRESS™ Remote Therapy Monitoring module, which is designed to increase patient adherence and lower the total cost of wound care. Our digital service offers patient support resources and greater connectivity between patient and provider to support patient-managed care.
Advanced Wound Dressings
Our Advanced Wound Dressings (AWD) are designed to provide effective treatment of infection, management of chronic wound exudate, and delivery of therapeutic compression. We believe 3M™ Tegaderm™ exemplifies our material and skin science expertise and demonstrates our industry-leading capabilities with its versatility, ease of use, and performance.
| Infection & Inflammation Management | Manage Exudate | Provide Compression | ||||||
 |  |  | ||||||
| 3M™ Promogran™ Collagen Dressing | 3M™ Tegaderm™ Wound Dressings | 3M™ Coban™ Compression Systems | ||||||
| 3M™ Promogran™ Matrix Family is the only collagen dressing uniquely formulated with Oxidized Regenerated Cellulose (ORC). | Absorbent wound dressings that is designed to allow for wound and incision monitoring | Designed to be comfortable, consistent, and effective compression systems for venous leg ulcers and other conditions requiring compression | ||||||
107
Advanced Skin Care
Our highly durable and ultra-thin skin protectants create a protective environment that is designed to help repel irritants and support healing.
| 3M™ Cavilon™ Advanced Skin Protectant | 3M™ Cavilon™ No Sting Barrier Film | 3M™ Cavilon™ Durable Barrier Cream | ||||||
 |  |  | ||||||
| Revolutionary polymer-cyanoacrylate-based protectant that is designed to allow for superior performance and designed to prevent, stop and reverse the effects of incontinence-associated dermatitis | Versatile solution that is designed to guard skin from many skin problems including, medical adhesive-related skin injury (MARSI), periwound skin damage, peristomal skin damage and incontinence-associated dermatitis | Provides patient comfort and is designed to moisturize as it protects skin. Designed to resist wash-off and require fewer applications than typical moisture barriers, saving time and effort | ||||||
Infection Prevention and Surgical Solutions
We offer solutions that are intended to address patient and staff safety by reducing preventable infections and complications while improving the efficiency and effectiveness of care. Our product range includes I.V. site management, temperature management, sterilization assurance, and other surgical solutions, as well as medical tapes and wraps, medical electrodes and stethoscopes.
I.V. Site Management
Every intravenous (“I.V.”) site presents the potential for infection, dislodgment, skin damage, and other complications. These complications can potentially cause patient discomfort and pain, extended hospital stays, additional therapy, and surgical intervention — even increased patient mortality. Our material science expertise has enabled us to bring to market unique innovations that are designed to protect I.V. sites from insertion to removal. Our offerings include an array of products that are designed to improve patient outcomes through proper catheter securement, bacterial and viral barriers, and antimicrobial protection. We believe that our products have been relied upon by healthcare professionals for over 40 years and product range has transformed over time to offer comprehensive intra- and extraluminal protection for every line. Most recently, these innovations have focused on our 3M™ Tegaderm™ CHG antimicrobial product family, which is designed to reduce catheter-related bloodstream infections (“CRBSI”) and vascular catheter colonization.
 |  |  | ||||||
| 3M™ Tegaderm™ CHG I.V. Dressings Family | 3M™ PICC / CVC Securement Device with 3M™ Tegaderm™ I.V. Securement Dressings | 3M™ Curos™ Disinfecting Caps | ||||||
| 3M™ Tegaderm™ CHG I.V. Securement Dressing is intended to reduce vascular catheter colonization and catheter-related blood stream infections (“CRBSI”) in patients with central venous or arterial catheters. | An engineered stabilization device plus antimicrobial (CHG) dressing designed to provide immediate and continuous antimicrobial protection for up to 7 days | Next-generation solution for needleless connector disinfection that utilizes 70% isopropyl alcohol to disinfect all critical surfaces of intraluminal access points. | ||||||
108
Temperature Management
Our broad temperature management solutions are sold under the 3M™ Bair Hugger™ and 3M™ Ranger™ brands and are designed to help maintain normothermia before, during and after surgical procedures, to prevent hypothermia-related complications. We believe we are the therapy of choice; we estimate that our products are used in 9 out of 10 top hospitals in the U.S.
 |  | ||||
| 3M™ Bair Hugger™ Warming Blanket System | 3M™ Bair Hugger™ Temperature Monitoring System | ||||
| Range of blankets that are designed to facilitate maximum thermal transfer and hug the patient | A non-invasive, accurate core temperature monitoring system that continuously measures patient temperature with an affordable single-use sensor | ||||
Sterilization Assurance
Our solutions are designed to allow for rapid and complete sterilization assurance across steam, hydrogen peroxide, and ethylene oxide modalities. We believe that we, as the pioneer for rapid biological and multi-variable chemical indicators, are a global leader in sterilization assurance. For over 70 years, we believe our comprehensive approach, advanced education, and excellence in technical support have provided standardized and simplified workflows and protocols to promote patient safety. .
  |    | ||||
| 3M™ Attest™ Chemical and Biological Indicators | 3M™ Attest™ Reader | ||||
| Range of indicators and readers for biologics and chemicals | 3M™ Attest™ Auto-readers enable results for both steam and vaporized hydrogen peroxide (VH2O2) in one reader in just 24 minutes | ||||
Surgical Solutions
Our range of surgical solutions, including our antimicrobial incise drape, antiseptic skin preparation, and nasal decolonization products, are designed to help reduce the risk of surgical site infections through management of microbes and pathogens from the patient’s skin flora in the operating room. Our 3M™ Ioban™ Antimicrobial Incise Drape offers a combination of antiseptic impregnated adhesive, breathable film, and strong adhesion, which provides a sterile surface all the way to the wound edge. This solution provides continuous broad-spectrum antimicrobial activity throughout the surgical procedure while helping optimize the wound incision environment.
 |  | ||||
| 3M™ Ioban™ Antimicrobial Incise Drape | 3M™ Skin and Nasal Antiseptic | ||||
| Drapes designed to help prevent surgical site infection | Skin and nasal antiseptic designed to help improve patient safety without alcohol or antibiotics | ||||
109
Medical Tapes and Wraps
Our products address important securement clinical needs and includes material innovations designed to help improve patient experiences and outcomes. Leveraging our expertise in adhesive technology and manufacturing, we invented gentle-to-skin adhesives in the 1960s and have stewarded the growth of category-defining brands such as 3M™ Micropore™ Surgical Tape and 3M™ Coban™ Self-Adherent Wraps. We continue to advance clinical practice, recommending a simplified line-up with what we believe are four best-in-class products and formats designed to help reduce the risk of unnecessary complications from skin damage, cross-contamination, or poor product selection. We believe our skin performance products have the securement power to be effective but with less damage to skin than similar products.
| Self-Adhesive Securement | Multi-purpose Securement | High Strength Securement | Flexible Securement | ||||||||
 |  |  |  | ||||||||
| 3M™ Coban™ Self-Adherent Wrap | 3M™ Micropore™ S Surgical Tape | 3M™ Durapore™ Surgical Tape | 3M™ Medipore™ H Soft Cloth Surgical Tape | ||||||||
| A self-adherent wrap used to secure dressings and other devices, compress, or protect wound sites, immobilize injuries, and more | Multi-purpose surgical tape used in clinical applications such as blood draws, lightweight dressings, secondary securement of I.V. lines, and non-critical tubes | Silk-like cloth tape for clinical jobs such as securing catheters and tubes and patient positioning | Features a soft, conformable backing with bi-directional stretch, designed to accommodate swelling and movement, while minimizing the risk for skin injury | ||||||||
Additionally, our immobilization solutions include 3M™ Scotchcast™ branded tapes and splints. Our casting and splint solutions range in rigidity, strength, and stability to allow for comfortable, customized treatments for patients.
Medical Electrodes
Our medical electrodes solutions leverage our core strength in adhesive technology and a recognized brand known as 3M™ Red Dot™. As a pioneer of electrocardiogram (“ECG”) monitoring solutions for 49 years, our products are used for cardiac monitoring across acute (hospital) and out-of-hospital care settings. We offer products that are designed to do different ECG tests for clinicians, including products that can stay in place for up to five days with diaphoretic patient conditions and solutions for pediatrics and fragile skin. Through dedicated patient solutions, clinical support, and training, we help clinicians choose the appropriate electrode to maximize trace quality and reduce the risk of cross-contamination.
 |  |  | ||||||
| 3M™ Red Dot™ ECG Monitoring Electrodes | 3M™ Universal Electrosurgical Pads | 3M™ Defib Pads | ||||||
| Multi-purpose ECG monitoring electrodes with adhesion for a variety of patients, designed to provide consistent and reliable trace quality and ease of use | The green safety ring and advanced electrosurgical pad technology with 9100 series was invented by us. These pads are designed to be smaller in size, making them easy to place, while still meeting thermal performance safety standards | The ready-to-use pads (electro-conductive gel supported by a porous non-woven fabric) are designed to be faster to apply than gels and creams in multiple cardiac arrest situations and their bright orange color makes them easy to see | ||||||
110
Stethoscopes
Our solutions include 3M™ Littmann® branded traditional and digital stethoscopes. We believe our stethoscopes are seen as the industry standard for monitoring and assessing sounds and rhythms. We continue to innovate in this area by offering digitally enabled solutions such as the 3M™ Littmann® CORE Digital Stethoscope, our most advanced stethoscope yet, which is designed to provide up to 40x amplification, active noise cancellation, and in-app sound wave visualization.
 |  | ||||
| 3M™ Littmann® Monitoring Stethoscope | 3M™ Littmann® CORE Digital Stethoscope | ||||
| Compact and sensitive stethoscope manufactured with strong yet lightweight materials for clinicians | Digitally enabled stethoscope that pairs with the Eko app to see, record and share sounds | ||||
Medical Technologies OEM
We also have Medical Technologies OEM solutions that draw upon our deep heritage of adhesives and film technology to develop products including body worn adhesives, medical-grade adhesives, and transdermal components. Our product offering ranges from skin-friendly, long-wear adhesives to microfluidic films, products that get used in the construction of a variety of medical devices. A few examples incorporating our technologies include continuous glucose monitors, diabetic glucose test strips, and Covid-19 point of care diagnostic tests. Our technologies are designed to enable market-leading medical device manufacturers in many lifesaving applications.
Competitors
The advanced wound care market is highly competitive, particularly in the United States and Europe, with our principal competitors including Smith & Nephew, Medela, Mölnlycke, Coloplast, and Convatec. Our competitors look to provide comprehensive solutions across multiple care steps throughout debridement, advanced wound dressings, skin substitutes, and negative pressure technologies. It is a dynamic market, featuring ongoing commercialization of new wound care products, high levels of strategic mergers and acquisitions, and disruptive new entrants and emerging players specifically focused on cost-effective wound care solutions. Our advanced wound dressings and skincare customers typically purchase through bids and tenders. Competitors use distributors, direct contracts, and direct-to-patient channels in some regions and countries. We compete through product differentiation and, what we believe are, improved outcomes across different types of chronic wounds and acute incisions. Our ability to innovate and compete is driven by our technical capability in material science and skin science, supported with research, global reach, strong brand reputation, medical education, and manufacturing capability.
Our infection prevention and surgical supplies solutions are offered in highly competitive and fragmented end markets, especially in the United States and Europe. Our principal competitors in this segment include Becton Dickinson, Hartmann, ICU Medical, Medline, Cardinal Health, Fortive, Steris, MDF Instruments, BSN, Smith & Nephew, and Welch Allyn. Where companies offer services that support their products, their ability to successfully compete increases. Connection to patient portals, electronic medical records and software services all help to manage patient preparation and improve patient care pathways and outcomes. We see continued disruption in these markets, particularly with digital solutions as small start-up companies focus on tracking and analyzing, remote patient monitoring, and clinical decision support. Our customers purchase through bids and tenders and use channel distributors, direct contracts, and direct-to-patient channels in some regions and countries. We compete by leveraging our strong technical capability, research, and breadth and depth of product lines. By supporting best practices through medical education, we believe we create a compelling value proposition for our distributor and Group Purchasing Organization (GPO) partners, which helps to offset a fiercely competitive pricing environment.
111
Dental Solutions
Our Dental Solutions segment provides a comprehensive array of dental and orthodontic products that span the life of the tooth, and are intended to address clinical needs in prevention, restoration, replacement, and malocclusion correction. We believe the combination of our material science innovation and our dental market domain knowledge allow us to improve the efficiency and quality of treatment delivery.
Our solutions are used by most dental offices. We are responsible for several category inventions within the dental market, including the introduction of the first-ever tooth-colored composites.
We maintain a meaningful focus on innovation, which is informed by a long history of interactions with customers, where we can gather insights and apply them to our strategic processes. This innovation is also supported by our strong engagement with academic institutions as well as our significant material science experience, which allows us to use our unique chemistry and technology expertise to simplify and standardize procedures. We have consistently ranked highly on The Anaheim Group’s Innovation Index, which highlights innovative companies in the dental industry, and have been named the top-ranked company in multiple years. Our scientific approach to product development and innovation is central to our solutions, and our business is supported by proprietary intellectual property. In 2022, we were recognized with a Heroes of Chemistry award from the American Chemical Society highlighting our scientific innovation.
Given our growing data science and digital capabilities, we believe we are well-positioned to take advantage of the movement toward digitization. We have launched solutions that combine our advanced material science expertise with data science and digital technologies to improve the workflows and processes associated with dental and orthodontic treatments. We are actively building our “custom smiles” solutions, which uses digital capabilities to enable our dental and orthodontic provider partners to deliver customized esthetic and restorative treatments to their patients.
Our solutions are designed to improve both the patient and practitioner experience, and offer differentiated value through our science, education, and service. We believe our brands, including 3M™ Filtek™, 3M™ Clinpro™, 3M™ Scotchbond™, and 3M™ Clarity™, are recognized in the global dental market. Our solutions serve both channel and end customers and include larger dental organizations, smaller practitioner groups, sole practitioner clinics and distributors.
Dental Products
We believe our dental products deliver reliable and optimized patient outcomes and include solutions that are designed to for preventative dental care, to simplify tooth replacement procedures and to enable efficient and high-quality restorations. We believe that integration of digital design and planning through our Oral Care Portal (OCP) streamlines the ease of use to create exceptional outcomes.
112
Our prevention products include the Townie Choice Award-winning products for fluoride treatments, sealants, and take-home maintenance solutions, such as toothpaste.
 |  |  | ||||||
| 3M™ Vanish™ 5% Sodium Fluoride White Varnish | 3M™ Clinpro™ Sealant | 3M™ Clinpro™ 5000 1.1% Sodium Fluoride Anti Cavity Toothpaste | ||||||
| Optimized, clear varnish with an extended release of fluoride, calcium and phosphate, designed to stay in contact with teeth longer to provide protection | Offers smart color-change technology that goes on pink for easy-to-see application, and cures to a natural white. Has low viscosity to flow easily into pits and fissures and we believe is ideal for pediatric sealant applications | Prescription-strength 5000ppm fluoride toothpaste designed to strengthen enamel and help reverse white spot lesions | ||||||
Our indirect restoration products includes brands such as Dental Advisor Award winning 3M™ RelyX™, 3M™ Imprint™ and 3M™ Protemp™ and we operate across categories such as milled restorations, impression-taking, temporization, and cementation.
 |  |  | ||||||
| 3M™ RelyX™ Universal Resin Cement | 3M™ Impregum™ Penta™ Polyether Impression Material | 3M™ Protemp™ 4 Temporisation Material Refill | ||||||
| Eliminates the inconvenience of multiple resin cements, primers and adhesives, simplifying direct and indirect restorative workflows | Polyether impression material that is designed to provide superior initial hydrophilicity, allowing for accurate, void-free impressions even in moist conditions | Bis-acrylic composite designed for the fabrication of reliably strong temporaries with attractive aesthetics | ||||||
Our direct restoration products are used throughout the restorative procedure and are intended to enable efficient and high-quality restorations. These products are available under well-known brand names such as 3M™ Filtek™,
113
3M™ Scotchbond™, 3M™ Elipar™, 3M™ Sof-Lex™, and others across categories such as adhesives, composites, and finish and polish.
 |  |  | ||||||
| 3M™ Filtek™ Supreme Ultra Universal Restorative | 3M™ Filtek™ Supreme Flowable Restorative | 3M™ Scotchbond™ Universal Plus Adhesive | ||||||
| Versatile universal composite designed to blend aesthetics and strength for anterior and posterior restorations | A flowable composite featuring an innovative syringe design designed to virtually eliminate bubbles. | Radiopaque universal adhesive designed to minimize the risk of X-ray misdiagnosis and invasive overtreatment | ||||||
Our digital dental offering integrates digital design and planning with the restorative procedure to provide a streamlined option for conservative composite treatment. Our newly launched 3M™ Filtek™ Matrix is a digitally designed, customized anterior matrix system that streamlines composite treatment. The solutions focus on the ease of use in creating exceptional, consistent, custom smile outcomes through the OCP.
 | ||
| 3M™ Filtek™ Matrix | ||
| Digitally designed customized anterior matrix system that streamlines composite treatment and utilizes 3M™ Filtek™ restorative | ||
Orthodontics Products
We offer orthodontic products to address malocclusions of all types. These solutions combine our historical strengths in material science with data science and digital technology to transform how orthodontists improve
114
patient smiles. Our offerings range from traditional brackets to custom digital aligners and includes well-known brands such as 3M™ Clarity™, 3M™ Transbond™ and 3M™ APC™.
 |  |  | ||||||
| 3M™ Clarity™ Advanced Ceramic Brackets | 3M™ Transbond™ XT Light Cure Paste Adhesive | 3M™ APC™ Flash-Free Adhesive Coated Appliance System | ||||||
| Designed to deliver aesthetics, predictable debonding with proprietary Stress Concentrator, and enhanced patient comfort with advanced design features | Bonds metal and ceramic brackets to tooth surfaces, thereby providing additional working time for accurate bracket placement | Adhesive Pre-Coated Brackets designed to improve hygiene as well as practice efficiency, and “flash-free” technology. Eliminates the cumbersome process of flash (excess adhesive) removal on brackets that are individually sealed to prevent cross-contamination | ||||||
We provide a variety of “custom smile” solutions that digitize several steps of dental and orthodontic treatments to broaden access to ease of use in creating customized smile solutions. Many of the steps to use our “custom smile” solutions are completed through our OCP, a web-based oral care treatment management system. The OCP is an easy-to-use, intuitive “one stop shop” digital treatment management interface. Clinicians can use OCP to deposit patient records, including X-rays, scans, and other data points that influence their custom smile treatment plans, interact with Solventum team members to engage in guided iterative treatment planning, track their patient cases, and check in on product delivery times. OCP is also integrated with multiple intraoral scanners, a practice management solution, and our own patient treatment tracking app. OCP is the center point for our customized offerings.
 |  |  | ||||||
| 3M™ Clarity™ Aligners | 3M™ Digital Bonding Tray | 3M™ Oral Care Portal | ||||||
| Clear aligners that are used for custom esthetic treatment without the need for metal wires or braces | Digitally designed device that simultaneously bonds all brackets on one arch onto the patient’s teeth | Web-based digital platform designed to be the “one stop shop” and manage the oral care treatment process | ||||||
Competitors
The dental market is highly competitive, with players ranging from very large broad-based multinational companies to localized or specialized suppliers and start-ups. Principal multinational competitors within the oral care market include Dentsply Sirona, Envista, Straumann, and Align Technology, all of which compete with both dental and orthodontic solutions, and Ivoclar, which competes with only dental solution offerings.
Key competitive factors include new product innovation/intellectual property, evidenced-based clinical outcomes, product quality and reliability, practitioner education and support, brand awareness and reputation, service, business/coverage model and price. In recent years, there have been significant acquisitions and new
115
collaborations in the oral care market segment, especially in digital dentistry. These moves are indicative of a dynamic dental market, where new market entries in digital technologies, artificial intelligence and 3D printing are accelerating.
To remain competitive, we expect to need to continue innovating in dental and orthodontic materials, aligning our advanced materials with data science, enhancing our service and business/coverage models, partnering as needed to complement our materials offerings, and focusing on technology development, science, and healthcare practitioner education.
Health Information Systems
Our Health Information Systems segment delivers a broad array of innovative software solutions and services that are designed to eliminate revenue cycle waste, create more time to care, and support the shift to value-based care. Our solutions operate across multiple areas of the healthcare system and reflect our deep and diverse knowledge of different aspects of healthcare.
Since this business began more than 35 years ago, we have developed a rich and unique understanding of the healthcare data landscape. We believe we are now an integral part of the day-to-day operations of the healthcare industry and we will be a key company in solving many of its largest future challenges. Based on internal estimates, many health systems worldwide — including over 75% of U.S. hospitals — use at least one of our software solutions. This includes our artificial intelligence (AI)-based clinical intelligence engine, which applies natural language processing (NLP) and natural language understanding (NLU) technology to significant amounts of structured and unstructured clinical documents each day. We have worked with federal government agencies, such as CMS to develop and update both public and proprietary algorithms, benchmarks, and classification systems. With many active industry and technology partners, including working with all the major electronic health record (EHR) companies, our solutions are present throughout the healthcare ecosystem.
Our solutions are typically delivered to our customers as an integrated workflow or set of workflows that involve both digital software-based solutions and related services. We utilize our strong direct customer relationships and frequent customer interactions to gain unique insights that are incorporated into our development pipeline. In addition to regular customer interactions, we also host customer forums and an annual client experience summit. We have dedicated adoption specialists that manage physician experiences, and we often deploy client success managers that are physically “embedded” with our customers following the sale of a solution to ensure that their experience using our solutions is successful. These efforts result in uniquely collaborative customer relationships that drive a more robust customer experience and provide us with useful insights to inform our operations. Our solutions are utilized by a variety of customers across inpatient, outpatient, and ambulatory settings as well as payers and government agencies. Our representative channel and end customers span a broad range and include large healthcare providers, regional health systems, payers, and other third-party HCIT solution providers.
Revenue Cycle Management (RCM) Solutions
It has become increasingly important for hospitals, health systems and other providers to streamline their revenue cycle, automate coding and reduce burdens on clinical staff. Our RCM solutions are focused on eliminating unnecessary administrative inefficiencies in the health care reimbursement process and ensuring accurate and compliant reimbursement. We have worked with our clients and industry experts to develop solutions that improve the productivity of administrative and clinical staff with respect to the RCM workflow.
116
 |  |  | ||||||
| 3M™ 360 Encompass™ System | 3M™ 360 Encompass™ Professional System | 3M™ 360 Encompass™ in the Cloud | ||||||
| A collection of applications that are designed to work together to help hospitals streamline administrative processes, receive accurate reimbursement, promote compliance, and make data informed decisions | An encounter-based coding solution that classifies clinician services, including Evaluation and Management services, for accurate reimbursement | A solution that combines the power of the 3M™ 360 Encompass™ platform with the flexibility and efficiency of a cloud platform | ||||||
The 3M™ 360 Encompass™ platform uses our unique capabilities in applying natural language processing (NLP) to capture information and use it to automatically suggest the appropriate medical coding, classification, and documentation prior to the invoice being prepared. This system enables workflow visibility for coding, clinical documentation integrity (CDI) and quality teams so they can see each other’s notes, summaries, queries, follow-ups, and findings, all while the patient is in-house. It also allows customers to monitor and manage quality results in detail with many reporting options and to receive reports in real time from patient documentation on key quality indicators.
3M™ 360 Encompass™ is designed to decrease patient safety indicators (PSIs) and hospital acquired complications (HACs) and increase both hospital net revenues and coder productivity. As an example of a successful customer implementation, a Level 1 trauma center located in the Midwestern United States implemented 3M™ 360 Encompass™ and recognized gains in efficiency, case mix index (“CMI”), and coding productivity.
Clinician Productivity Solutions
Our clinician productivity solutions are committed to reducing the administrative burden on clinicians in all settings so that physicians, other providers, and specialists can focus on delivering care rather than documenting it. Through speech recognition and AI technologies, we believe we can improve clinician documentation processes, deliver proactive clinical insights within the EHR workflow, and enhance back-end CDI and care management efficiencies.
Our solutions and services are designed to help clinicians more efficiently capture the complete and accurate patient story. Our 3M™ M*Modal solutions bring conversational AI and ambient intelligence directly into EHR workflows and are designed to help improve clinician and patient satisfaction, and drive quality and productivity. We also offer 3M™ M*Modal imaging solutions that are designed to deliver speech driven reporting, proactive clinical insights, productivity-enhancing tools, and AI-powered analytics. In addition to revenue cycle management, our CDI solutions and their computerized assistance capabilities are designed to enable more efficient and accurate documentation for physicians, creating additional time to provide patient care.
117
 |  |  | ||||||
| 3M™ M*Modal Fluency Direct | 3M™ M*Modal CDI Engage One™ | 3M™ M*Modal HCC Management | ||||||
| All-in-one speech-to-text software solution which enables physicians to conversationally create, review, edit, and sign clinical notes directly in the EHR | A solution which is designed to enhance the clinician workflow through delivering proactive clinical insights to help with complete and compliant patient documentation and reducing retrospective queries | A comprehensive, technology-driven solution which is designed to deliver proactive, real-time nudges to clinicians to ensure appropriate risk adjustment for value-based payment models, such as Medicare Advantage | ||||||
The 3M™ M*Modal Fluency Direct solution leverages natural language understanding (NLU) technology for contextual understanding of the patient’s narrative and is designed to help improve documentation accuracy from the first word. With built-in computer-assisted physician documentation functionality, the technology continuously analyzes and monitors the clinical narrative. In real time, it nudges users for additional information or clarification and makes specific suggestions to improve the quality of care and clinical documentation. Its innovative features are designed to increase physician productivity.
3M™ M*Modal CDI Engage One™ operates across multiple areas of note-taking, including speech, templates, and typing. It identifies common documentation gaps and helps clarify documentation up front, as it is created. With proactive, real time AI “nudges,” physicians can capture complexity, acuity, and severity levels before saving notes in the EHR. This single workflow results in fewer errors being pushed downstream to CDI, fewer queries sent back to physicians, and improved CDI team productivity.
3M™ M*Modal HCC Management is a new, technology-driven solution that leverages AI to deliver frontline assistance to physicians so that risk adjustment opportunities are not missed while documenting patient encounters. Through a single-access web interface, 3M™ M*Modal HCC Management can help multiple stakeholders collaborate on the patient chart and monitor HCC coding. The solution is designed to help create complete, compliant documentation of chronic conditions and help identify diagnoses not yet captured on claims that would otherwise be missed.
Performance Management Solutions
The healthcare industry is shifting away from fee-for-service systems and toward value-based care systems. As this shift occurs, appropriate access to high quality, actionable data is paramount. Payers will need access to data that will help them improve quality and efficiency while reducing unnecessary care and improving outcomes, while organizations will need help reviewing terabytes of data to draw out actionable information that will help them identify and resolve issues faster. Our solutions and services help payers and provider organizations sort through data to find efficiencies and prioritize sustainable improvements.
118
 |  |  | ||||||
3MSM Informed Analytics Platform | 3M Grouper Methodologies | 3M Value-based Methodologies | ||||||
| A business intelligence platform that is designed to enable health care organizations to effectively move toward value-based care through the ability to develop and quickly share actionable insights | Methodologies for defining health care payment, benchmarking, reporting, and analytics in countries around the world | Methodologies for advanced payment models, including longitudinal clinical risk models, bundles, and episodic care, designed to drive improved outcomes and lower costs | ||||||
We believe we are the standard for innovative patient grouping and classification solutions, whether customers want to process claims for hospital reimbursement and reporting, edit patient records in real time, or leverage patient data for improved risk adjustment or risk stratification. Our new methodology, 3M™ Ambulatory Potentially Preventable Complications (AM-PPCs) software is the first comprehensive quality outcomes system for hospital outpatient departments or ambulatory surgery centers procedures. By addressing a comprehensive list of procedures and complications, AM-PPCs can provide a broad view, or a focused analysis as needed to pinpoint complication rates by procedure, facility or surgeon, and is thus designed to help the health system to improve patient safety and reduce costs.
Competitors
The health care software technology market in which we conduct our business, and the healthcare information technology (HCIT) industry in general, is highly competitive and dynamic, characterized by the continual introduction of new products and technologies. Principal competitors include R1 RCM, Ensemble, Optum/Change, Google, Amazon (AWS), Microsoft (Nuance), Epic, Cerner, Athena, and a host of start-up technologies actively working to disrupt the areas of revenue cycle management and clinician productivity. In the United States, the market for value-based care software solutions is highly fragmented and subject to continuous entry of new competitors. Today, we compete primarily with Optum, Cotiviti, Inovalon and Vizient, among others. The market is even more fragmented internationally. Outside the U.S., we compete primarily with local players in the respective country or region, some international players such as IQVIA, Cerner/Siemens, and Dedalus, and new market entrants.
The primary competitive factors we face include technological innovation and technical capability, price, breadth of solution line, consulting services capability, knowledge of health care industry trends, rules, and regulations, scale of operations, brand reputation, and customer service. In order to remain competitive in the future, we expect to be required to seek to continuously enhance our business. Our ability to compete will be affected by our ability to accomplish objectives, including the development of new products and innovative technologies; improving upon our existing offerings; delivering return on investment for our customers; improving efficiency and productivity of healthcare workers; staying up-to-date on regulatory changes, reimbursement guidelines and other healthcare best practices; developing and selling our products cost effectively; meeting all relevant quality standards for our products and their markets; and protecting the proprietary technology of our products and development processes.
Purification and Filtration
Our Purification and Filtration segment is a provider of purification and filtration solutions that are designed and marketed for use in the manufacturing processes of biopharma and medical technologies, such as cell and gene therapies, vaccines, and hemodialysis, as well as in the manufacturing of microelectronics, food and beverage products, and water filtration for commercial and residential applications. We are dedicated to advancing membrane science to enable life-saving treatments and cleaner water by leveraging our technical expertise in functionalizing membranes and deep expertise in process filtration.
119
Every year, we estimate that our products touch the lives of millions of people. Based on internal estimates, each year, approximately one million open heart surgeries are currently performed using oxygenators that are enabled by one of our membranes. The drinking water consumed by millions of households is purified by our treatment and filtration technologies, providing, by our estimation, over seven million units (cartridges) currently to households and commercial end users annually.
Our products are offered globally under a wide variety of well-known brands, including 3M™ Zeta Plus™, 3M™ Aqua-Pure™, 3M™ Liqui-Cel™, 3M™ PUREMA™, 3M™ OXYPHAN™ and 3M™ OXYPLUS™. We sell our products directly to biopharmaceutical manufacturers, medical technology companies, integrators of manufacturing plants and water treatment systems, and through a broad range of distributors.
Bioprocessing Filtration Solutions
Our bioprocessing filtration solutions include high-value purification products that enable the harvest, clarification, and sterile filtration of bioprocess fluids. Our filters are used by over 1,000 therapeutic developers and manufacturers and have a range of applications in both upstream and downstream processing across recombinant therapeutics, blood fractionation, vaccines, and gene therapies. Our products are designed to simplify purification processes and to provide predictable scaling from discovery to manufacturing, and to reduce production costs. The filters we sell are used to reduce cells, debris, and bioburden in process fluids during manufacturing of biologically derived therapeutics and vaccines. Additionally, our products are used to fractionate and purify medically important blood proteins and to remove contaminants in the production of viral vectors.
We believe we are at the forefront of high-impact, biopharma purification innovation: our products are transforming the manufacturing process for drug therapies. We benefit from direct exposure to ongoing trends, such as the increasing use of biopharma filtration technologies, the need for rapid innovation and lower-cost manufacturing, and the shift to cell and gene therapies. We also have a robust pipeline of innovative products focused on the harvest and clarification, sterile filtration, capture, polishing, and viral clearance phases of the purification process. For example, our launch of the 3M™ Emphaze™ AEX Hybrid Purifier brought hybrid chromatographic clarification solutions to the market. These products are used in the manufacturing of recombinant protein therapeutics and are intended to increase product recovery, improve product yields and lower the total cost of manufacturing.
 |  |  |  | ||||||||
| 3M™ Zeta Plus™ Encapsulated Filter Capsules | 3M™ Harvest RC Chromatographic Clarifier | 3M™ Polisher ST Product | 3M™ Emphaze™ AEX Hybrid Purifier | ||||||||
| A range of single-use depth filter capsules with a variety of media grades for clarification of biological and pharmaceutical fluid | Single-stage, single-use chromatographic clarification solution which is designed to remove cells, cell debris and DNA (<500 ppb) from the harvested cell culture fluid and result in high mAb product recovery (>90-95%) | Single use multi-mechanism membrane adsorber which removes residual turbidity, DNA and HCP and is designed to provide robust virus clearance for improved downstream/polishing purification | A novel and innovative single-use solution for customers looking for robustness and consistency in their purification process | ||||||||
Our products are primarily sold directly to biopharmaceutical manufacturers and are used throughout the drug development and approval process, including early-stage development, through clinical trials, and commercial production. Given the highly regulated and specialized nature of the biopharma industry, product life cycles are long, and our expertise has helped us create strong customer loyalty in the growing bioprocessing filtration market.
120
Once our products are incorporated in the early stages of our customers’ drug development processes, it is difficult and costly for them to switch to competitive products.
In addition to our differentiated material science technology, our customers value our global footprint, which allows us the ability to provide customer support through our application engineers and to manufacture in proximity to our customers as our customers move their manufacturing operations across geographies.
Drinking Water Filtration Solutions
Our drinking water solutions include high-efficiency filtration products that are designed to provide cleaner, clearer, and great-tasting water for the commercial and residential markets. Our commercial water treatment and filtration products deliver filtered water for small and medium commercial facilities and food service facilities. These solutions reduce impurities in the water to provide establishments with Recipe Quality Water™ that helps to improve the overall customer experience while helping equipment run more efficiently. Our point of use residential drinking water filtration products can be installed under sinks, on tabletops, and in other formats for dedicated drinking and cooking water use and are designed to reduce chlorine taste and odor as well as lead, microbial cysts, and other contaminants. Whole house filtration products are designed to service an entire home and are designed to improve all incoming water, which helps to provide better quality drinking water while reducing sediment buildup and increasing the life of hot water heaters and appliances.
We continue to develop innovative products, including a new tankless reverse osmosis system to dispense purified water under our High Flux Reverse Osmosis (RO) residential water product series. This novel and compact RO system is designed for global regulatory compliance.
 |  |  | ||||||
| 3M™ Aqua-Pure™ 900 Series Whole House Water Filtration Systems | 3M™ ScaleGard™ HP Reverse Osmosis System | 3M™ Dual Port 390 Series Water Filtration System | ||||||
| Residential whole house water filter systems designed to help improve all incoming water | High capacity commercial RO system designed for scale reduction and great tasting beverages | Designed to provide consistent quality water by reducing particulate, chloramines, chlorine taste and odor, and scale | ||||||
Our branded and private label drinking water products are sold direct and through distribution to both commercial and residential customers. In addition to our differentiated material science technology and vertically integrated structure, we offer the ability to manage the complex regulatory landscape of the drinking water industry. We believe this is particularly valuable for our key global accounts, which require filtration products that provide consistent quality water and meet regulatory requirements across multiple countries.
Industrial Filtration Solutions
We believe we improve customers’ product and process quality, efficiency, and safety by providing a contamination control platform and a dissolved gas control platform with applications across the microelectronics, food and beverage and industrial manufacturing industries.
Our next generation 3M™ Liqui-Cel™ Membrane Contactor will be the first product in the market using our patented Asymmetric Dry Stretch Membrane Technology, which we believe will offer improved membrane manufacturing efficiencies in a more environmentally friendly (solvent-free) process relative to our solvent-based
121
phase separation. This advanced technology is expected to enhance our position in membrane-based gas control technology.
 |  |  | ||||||
| 3M™ Liqui-Cel™ Membrane Contactors | 3M™ Betapure™ AU/AUL Series Filter Cartridges | 3M™ Betafine™ XL Series Filter Cartridges | ||||||
| Gas transfer devices that utilize hollow fiber membrane technology and are designed to provide efficient dissolved gas control in a compact design | Cartridges with a controlled pore size filter matrix, which is designed to allow for absolute distinction between cartridge grades to provide accurate and consistent filtration | Filters that contain pleated, absolute-rated, polypropylene filter media, designed to provide excellent retention of particles at fast flow rates | ||||||
Our products are sold to integrators and designers of manufacturing plants and water treatment systems. Following installation of manufacturing equipment, customers purchase replacement products for ongoing maintenance either direct or through distribution. We believe we offer high-performance solutions enabled by our differentiated filtration and purification technology. Additionally, our application engineers offer high-quality customer support in various geographies, which we view as a significant benefit.
Membrane OEM Solutions
We believe our membrane OEM solutions provide critical membrane media for medical and industrial customers, and drive performance and efficiency in devices such as dialyzers, oxygenators, blood filtration equipment, filter cartridges and I.V. filters. Our membrane media are used in dialyzers for treatment of end-state renal disease, in heart-lung oxygenators for open heart surgery and/or long-term lung assistance, and in therapeutic blood filtration and separation. Additionally, these products provide the foundational technology used across the segment’s other filtration solutions. For example, these membranes are the functional components of the 3M™ LifeASSURE™ and 3M™ Liqui-Cel™ product lines.
 |  |  |  | ||||||||
| 3M™ Membrana™ PUREMA™ Capillary Membrane | 3M™ Membrana™ OXYPHAN™ Capillary Membrane | 3M™ Membrana™ PLASMAPHAN™ Capillary Membrane | 3M™ Membrana™ MicroPES™ Capillary Membrane | ||||||||
| Membrane designed to combine enhanced clearance of small molecular weight uraemic toxins with stable clearance performance | Oxygenation membrane capillary with a highly branched, sponge-like pore structure designed to make it strongly resistant to entry of liquids and plasma breakthrough | Membrane that is produced through temperature-induced phase separation and is used in plasma separation | Membrane that is produced through solvent-induced phase separation and is used in plasma separation and IV-filtration | ||||||||
Our membrane products are primarily sold direct to medical technology companies. Given the specialized nature of the end products, our engineers work directly with our customers to develop customized solutions that meet their specifications. This direct working relationship creates loyalty with our customers as we collaborate
122
throughout the sales cycle, which can take several years. As our products are used in highly invasive medical devices, the stringent regulations have driven increasing quality and performance requirements from our customers. We believe we differentiate ourselves from our competitors by offering highly functional customer-specific membrane solutions, enabling optimal performance of our customers’ devices and filters, and hence improving patient outcomes and filtration processes.
Competitors
The Purification and Filtration segment competes against a diverse spectrum of competitors. Established global filtration competitors such as Danaher, Merck KGaA, Sartorius, Pentair, Repligen, and Entegris compete on a variety of factors across product lines, including breadth of offering, product performance, customer service, product availability, distribution capabilities, innovation, name recognition and price. Our ability to compete with these larger, established filtration competitors lies in our ability to consistently bring disruptive technology, such as 3M™ Harvest RC, to the market. Regional competitors appear with frequency. Our ability to compete with these smaller, regional competitors lies in our quality products, full range of solutions, and global presence.
We sell into markets where a meaningful volume of sales are specified for a particular process, or, if not specified, require a customer to make a change without disrupting an effective, established process. In these end markets, we rely on our sales, key account, and customer-facing technical teams to identify and engage closely with the customer to solve specific challenges unique to the application. Where markets are less process-intensive, we rely on consistent refreshing of our product set to offer attractive products that have a technical edge over competitors’ products.
Research and Development Activities
Our R&D activities are focused on developing new solutions that are clinically supported and differentiated as well as improving on our marketed solutions to address evolving customer needs and enable better outcomes and access for patients. Our R&D capabilities include R&D organizations that operate within each of our business segments, as well as R&D capabilities spanning across our business segments.
Our business segment R&D organizations drive effective and agile new product commercialization. These organizations are responsible for the full product development life cycle, leveraging industry insights, domain-specific expertise in end-to-end product development, and a detailed understanding of customer applications and usability to innovate in both new and marketed products. Our product developers and engineers are embedded and regularly engaged with our customers, which creates alignment and generates valuable insights that are leveraged throughout the new product development process.
In addition to our segment R&D capabilities, our cross-segment capabilities include building new technology platforms and advancing existing platforms. We bring together skills and capabilities that are present across our organization, including (i) R&D expertise in materials science, life science, and analytical research, (ii) process technologies, (iii) data science, and (iv) cloud software development platforms and data security. We believe that collaboration across our organization further enhances our R&D capabilities by encouraging the sharing of best practices, enabling collaborative development and issue resolution, promoting synergies in development and manufacturing, and creating a broad culture of exploration.
In 2023, our R&D team consisted of more than 2,100 employees, including research scientists, chemical engineers, data scientists, software engineers, application development engineers and product developers. They are supported by a team of accomplished clinicians from our medical affairs group. We partner with our medical affairs group to expand awareness of clinical studies regarding our solutions by increasing both the number of peer-reviewed publications and the visibility for existing publications that address our solutions.
We endeavor to drive disruptive innovation through our strategic investments in R&D. We conduct our R&D activities in both developed and developing markets and ensure our internal innovation efforts are informed by external partnerships with customers, universities, and government institutions.
123
Human Capital
We have a long-tenured and diverse talent base with significant work experience, technical qualifications, and healthcare industry expertise. Our employee base consists of approximately 22,000 employees, with approximately 40% having more than 10 years of tenure at 3M. We have approximately 11,000 employees in the United States and approximately 2,600 in Germany. Of our employees, approximately 6,000 are production employees working in plants across the globe. We have approximately 100 employees who are represented by a union in the U.S., all of whom are in one manufacturing facility and are covered by a three-year collective bargaining agreement that expires on October 31, 2024. Our relationship with employee-representative organizations outside the U.S. takes many forms, including in European Union countries where we engage with representative bodies for employees, such as employee forums, works councils and trade unions, in accordance with local law.
Our employees are united in our mission to provide better, smarter, safer healthcare to improve lives. Our culture highlights collaboration and teamwork, along with a focus on patient safety, quality, and integrity.
The ability to recruit, retain, develop, protect, and fairly compensate our global workforce will be a key driver of our success. Our key human capital priorities are designed to support those efforts, including those listed below.
•Health and Safety: Solventum is committed to the safety, health, and well-being of its employees. We continuously evaluate opportunities to raise safety and health standards, visiting sites to identify and manage environmental health and safety risks; evaluating compliance with regulatory requirements and company policies; and maintaining a global security operation for the protection of facilities and people on our sites. We also promote a culture of health and well-being through disease prevention programs, on-site clinical services, employee assistance programs, and comprehensive healthcare benefits.
•Development and Pipeline: We have a robust, continuous talent review process focused on succession planning and key talent development. We deploy functional and leadership development opportunities and support time to learn. In addition, we advance our talent pipeline by attracting competitive talent and accelerating leadership and key skill development. We are expanding our external talent pools and leveraging university and professional organizations to identify talent with critical skills.
•Diversity, Equity, and Inclusion: A diverse, global workforce and inclusive culture that provides fair and equitable opportunities will help us remain competitive, advance our innovation culture, and serve customers. We focus on attracting and advancing top talent and are committed to advancing global diversity in management across all dimensions. We will have multiple Employee Resource Networks that support our business goals and our local communities. These groups will increase engagement around select affinity groups, with many employees leading and participating in global programs through these networks. We are focused on advancing representation through hiring and development.
•Compensation and Benefits: Our total compensation for employees includes a variety of components that support sustainable employment and the ability to build a strong financial future, including competitive market-based pay and comprehensive benefits. In addition, we have a professional and flexible work environment that promotes innovation and well-being and rewards performance.
Sales and Marketing
We have an extensive global commercial footprint with sales in over 90 countries. To serve our diverse customer base across our prioritized geographies, we take a multi-model commercial approach, including direct-to-customer, distribution, key account management, inside sales, and e-commerce. In these geographies, we augment our commercial model with both marketing and service support. Key marketing activities include brand management, insights, price management, digital marketing, and integrated marketing communications. As our customers increasingly leverage both traditional and digital media in their path to purchase, we continue to optimize our own omnichannel execution to ensure the best customer experience possible. Our service support teams include clinical specialists (licensed nurses or technicians), medical liaisons (clinical professionals such as surgeons and dentists), and application engineers (technical subject matter experts). These teams provide high-quality customer support serving as the clinical and/or technical expert for the customer.
124
To expand our market coverage into emerging geographies in the LATAM, EMEA, and Asia regions, we employ an export commercial model and leverage local partners to market and sell our products. Our local third-party partners facilitate regulatory and cross-border import compliance and distribute directly to the customer. Our export commercial team provides technical, clinical, and marketing support both directly to our customers as well as to our third-party partners, to help increase customer satisfaction and support our ability to grow our global presence.
Global Supply Chain and Sourcing
Our sourcing, production, and distribution network is managed globally. We believe we have advanced manufacturing and assembly production capabilities across our global manufacturing network. Our manufacturing is supported by a global distribution network.
Our distribution network is strategically designed as a “hub and spoke” model. This approach optimizes route planning and increases the speed of deliveries to our customers in all regions. In addition, our distribution network footprint meets the needs of our customers in a cost-effective model.
Our supply chain resiliency program consisting of regional sources of supply, dual-source manufacturing capabilities and vertically integrated operations presents a competitive advantage by providing a reliable supply of products to our customers.
Intellectual Property
Development and protection of our proprietary technologies through intellectual property (“IP”) rights is a strategic priority for our business. To protect our proprietary technologies, Solventum will rely on a combination of patent, design, utility model, trademark, copyright, and trade secret protections as well as regulatory exclusivity periods and confidentiality agreements. Our IP team will collaborate with our R&D and product teams to develop product line focused IP strategies and secure IP rights as appropriate. We will continue our practice of generally filing patent applications in the United States and foreign countries that have strong technology patent protections. We will also continue to license from third parties IP that complements our internal R&D efforts and product offerings. While, in aggregate, our patents and other IP are vital to our operations, we do not consider any single IP asset or group of assets to be of material importance to any segment or to the business as a whole; rather, we believe understanding our customers’ needs, technology expertise, and manufacturing know-how are critical for our business.
125
The following chart illustrates the Solventum patent assets by jurisdiction as of March 5, 2024. Patent assets exist in approximately 30 jurisdictions. The three jurisdictions with the largest patent portfolios are the United States, European Union, and China.
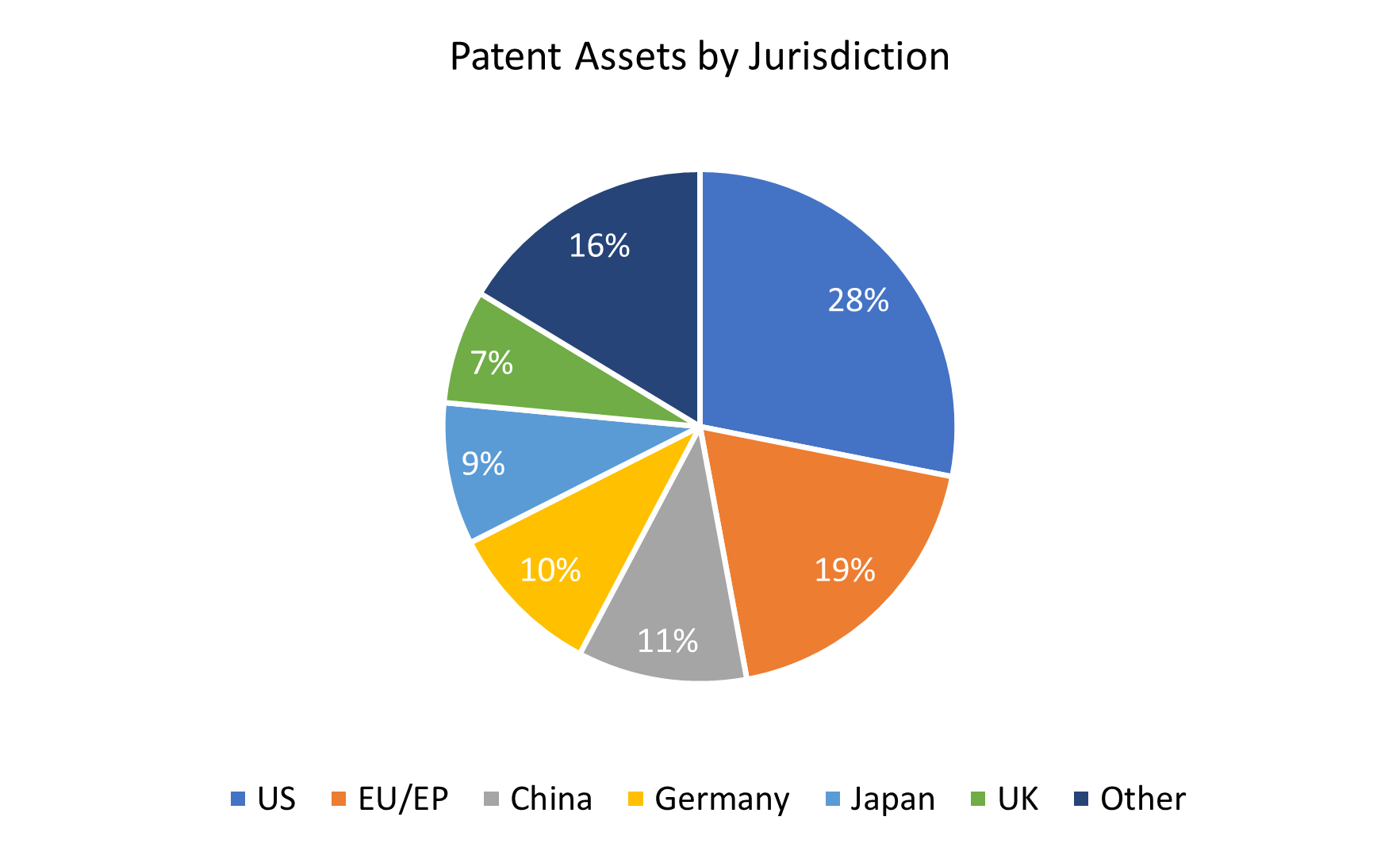
As of March 5, 2024, approximately 76% of Solventum’s patent portfolio consisted of issued patents; the remainder consisted of pending patent applications.
Of Solventum’s issued patents and pending patent applications, approximately 16% expire between 2024 and 2028, approximately 38% expire between 2029 and 2033, approximately 31% expire between 2034 and 2038 and approximately 15% expire after 2038.
126
The below chart illustrates the Solventum patent assets by segment as of March 5, 2024.
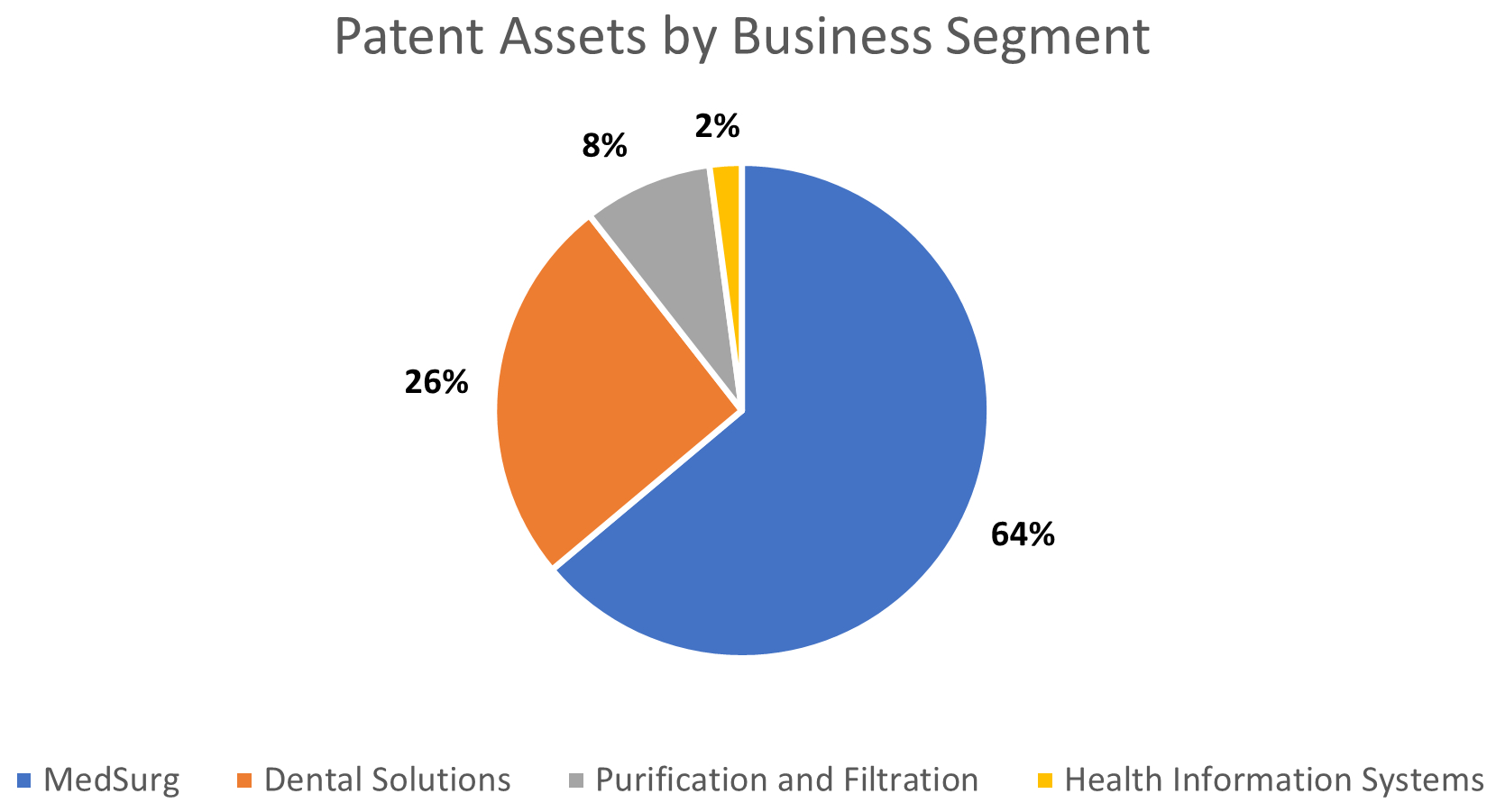
127
MedSurg Segment
The below table provides a summary of our patent assets within our MedSurg segment as of March 5, 2024.
| Portfolio | Proportion (and Number) of Patent Assets within Segment in Portfolio | Jurisdiction | Proportion (and Number) of Patent Assets in Portfolio by Jurisdiction | Proportion (and Number) of Patent Assets by Expiration Date Category and Jurisdiction(1) | Proportion (and Number) of Patent Assets by Form of Patent and Jurisdiction | ||||||||||||
| Wound Care | 76% (4587) | US | 27% (1,224) | 2024 – 2028: 14% (128) 2029 – 2033: 46% (420) 2034 – 2038: 29% (269) After 2038: 11% (102) | Issued: 71% (866) Pending: 29% (358) | ||||||||||||
| EU/EP | 18% (832) | 2024 – 2028: 7% (49) 2029 – 2033: 41% (272) 2034 – 2038: 35% (227) After 2038: 17% (108) | Issued: 71% (588) Pending: 29% (244) | ||||||||||||||
| China | 8% (366) | 2024 – 2028: 12% (36) 2029 – 2033: 57% (168) 2034 – 2038: 26% (77) After 2038: 5% (12) | Issued: 75% (274) Pending: 25% (92) | ||||||||||||||
| Others | 47% (2,165) | 2024 – 2028: 15% (315) 2029 – 2033: 52% (1,092) 2034 – 2038: 26% (541) After 2038: 7% (135) | Issued: 91% (1,980) Pending: 9% (185) | ||||||||||||||
128
| Portfolio | Proportion (and Number) of Patent Assets within Segment in Portfolio | Jurisdiction | Proportion (and Number) of Patent Assets in Portfolio by Jurisdiction | Proportion (and Number) of Patent Assets by Expiration Date Category and Jurisdiction(1) | Proportion (and Number) of Patent Assets by Form of Patent and Jurisdiction | ||||||||||||
| Infection Prevention Solutions, Surgical Solutions, and Medical Technologies OEM | 24% (1,484) | US | 31% (465) | 2024 – 2028: 23% (88) 2029 – 2033: 26% (98) 2034 – 2038: 27% (103) After 2038: 24% (92) | Issued: 62% (286) Pending: 38% (179) | ||||||||||||
| EU/EP | 18% (261) | 2024 – 2028: 4% (9) 2029 – 2033: 23% (50) 2034 – 2038: 30% (66) After 2038: 43% (92) | Issued: 62% (163) Pending: 38% (98) | ||||||||||||||
| China | 18% (263) | 2024 – 2028: 22% (49) 2029 – 2033: 28% (62) 2034 – 2038: 23% (51) After 2038: 27% (58) | Issued: 66% (173) Pending: 34% (90) | ||||||||||||||
| Others | 33% (495) | 2024 – 2028: 21% (96) 2029 – 2033: 30% (140) 2034 – 2038: 30% (137) After 2038: 19% (87) | Issued: 73% (359) Pending: 27% (136) | ||||||||||||||
__________________
(1)The expiration date data is provided only for issued patents and pending applications and does not include authorized but unfiled patent applications.
129
Dental Solutions Segment
The below table provides a summary of our patent assets within our Dental Solutions segment as of March 5, 2024.
| Portfolio | Proportion (and Number) of Patent Assets within Segment in Portfolio | Jurisdiction | Proportion (and Number) of Patent Assets in Portfolio by Jurisdiction | Proportion (and Number) of Patent Assets by Expiration Date Category and Jurisdiction(1) | Proportion (and Number) of Patent Assets by Form of Patent and Jurisdiction | ||||||||||||
| Dental Products | 81% (1,961) | US | 26% (510) | 2024 – 2028: 27% (122) 2029 – 2033: 27% (122) 2034 – 2038: 31% (139) After 2038: 15% (72) | Issued: 75% (384) Pending: 25% (126) | ||||||||||||
| EU/EP | 25% (496) | 2024 – 2028: 6% (29) 2029 – 2033: 20% (92) 2034 – 2038: 47% (211) After 2038: 26% (118) | Issued: 77% (382) Pending: 23% (114) | ||||||||||||||
| Germany | 11% (218) | 2024 – 2028: 13% (29) 2029 – 2033: 32% (69) 2034 – 2038: 48% (104) After 2038: 7% (16) | Issued: 98% (214) Pending: 2% (4) | ||||||||||||||
| Japan | 10% (200) | 2024 – 2028: 10% (17) 2029 – 2033: 18% (30) 2034 – 2038: 48% (82) After 2038: 24% (41) | Issued: 69% (137) Pending: 31% (63) | ||||||||||||||
| Others | 28% (537) | 2024 – 2028: 17% (86) 2029 – 2033: 23% (116) 2034 – 2038: 39% (197) After 2038: 21% (107) | Issued: 77% (415) Pending: 23% (122) | ||||||||||||||
130
| Portfolio | Proportion (and Number) of Patent Assets within Segment in Portfolio | Jurisdiction | Proportion (and Number) of Patent Assets in Portfolio by Jurisdiction | Proportion (and Number) of Patent Assets by Expiration Date Category and Jurisdiction(1) | Proportion (and Number) of Patent Assets by Form of Patent and Jurisdiction | ||||||||||||
| Orthodontic Products | 19% (468) | US | 31% (146) | 2024 – 2028: 26% (29) 2029 – 2033: 15% (17) 2034 – 2038: 25% (28) After 2038: 34% (36) | Issued: 62% (91) Pending: 38% (55) | ||||||||||||
| EU/EP | 22% (101) | 2024 – 2028: 9% (7) 2029 – 2033: 14% (10) 2034 – 2038: 36% (27) After 2038: 41% (30) | Issued: 44% (44) Pending: 56% (57) | ||||||||||||||
| Germany | 10% (45) | 2024 – 2028: 16% (7) 2029 – 2033: 23% (10) 2034 – 2038: 45% (20) After 2038: 16% (7) | Issued: 91% (41) Pending: 9% (4) | ||||||||||||||
| Japan | 14% (67) | 2024 – 2028: 7% (4) 2029 – 2033: 15% (8) 2034 – 2038: 39% (21) After 2038: 39% (21) | Issued: 58% (39) Pending: 42% (28) | ||||||||||||||
| Others | 23% (109) | 2024 – 2028: 19% (18) 2029 – 2033: 4% (4) 2034 – 2038: 39% (36) After 2038: 38% (35) | Issued: 60% (65) Pending: 40% (44) | ||||||||||||||
__________________
(1)The expiration date data is provided only for issued patents and pending applications and does not include authorized but unfiled patent applications.
131
Health Information Systems Segment
The below table provides a summary of our patent assets within our Health Information Systems segment as of March 5, 2024.
| Portfolio | Jurisdiction | Proportion (and Number) of Patent Assets in Portfolio by Jurisdiction | Proportion (and Number) of Patent Assets by Expiration Date Category and Jurisdiction(1) | Proportion (and Number) of Patent Assets by Form of Patent and Jurisdiction | ||||||||||
| Health Information Systems (200) | US | 57% (114) | 2024 – 2028: 27% (27) 2029 – 2033: 38% (38) 2034 – 2038: 28% (28) After 2038: 7% (6) | Issued: 78% (89) Pending: 22% (25) | ||||||||||
| EU/EP | 10% (19) | 2024 – 2028: 13% (2) 2029 – 2033: 27% (4) 2034 – 2038: 27% (4) After 2038: 33% (5) | Issued: 47% (9) Pending: 53% (10) | |||||||||||
| Others | 33% (67) | 2024 – 2028: 16% (10) 2029 – 2033: 42% (26) 2034 – 2038: 31% (19) After 2038: 11% (7) | Issued: 79% (53) Pending: 21% (14) | |||||||||||
__________________
(1)The expiration date data is provided only for issued patents and pending applications and does not include authorized but unfiled patent applications.
132
Purification and Filtration Segment
The below table provides a summary of our patent assets within our Purification and Filtration segment as of March 5, 2024.
| Portfolio | Proportion (and Number) of Patent Assets within Segment in Portfolio | Jurisdiction | Proportion (and Number) of Patent Assets in Portfolio by Jurisdiction | Proportion (and Number) of Patent Assets by Expiration Date Category and Jurisdiction(1) | Proportion (and Number) of Patent Assets by Form of Patent and Jurisdiction | ||||||||||||
| Bioprocessing Filtration, Industrial Filtration, Medical Filtration, Industrial Membrane Filtration | 70% (564) | US | 28% (159) | 2024 – 2028: 23% (31) 2029 – 2033: 41% (54) 2034 – 2038: 23% (30) After 2038: 13% (17) | Issued: 66% (105) Pending: 34% (54) | ||||||||||||
| EU/EP | 15% (82) | 2024 – 2028: 16% (10) 2029 – 2033: 45% (29) 2034 – 2038: 25% (16) After 2038: 14% (9) | Issued: 61% (50) Pending: 39% (32) | ||||||||||||||
| China | 16% (91) | 2024 – 2028: 15% (11) 2029 – 2033: 40% (29) 2034 – 2038: 28% (20) After 2038: 17% (12) | Issued: 70% (64) Pending: 30% (27) | ||||||||||||||
| Others | 41% (232) | 2024 – 2028: 32% (68) 2029 – 2033: 42% (91) 2034 – 2038: 20% (42) After 2038: 6% (14) | Issued: 81% (188) Pending: 19% (44) | ||||||||||||||
133
| Portfolio | Proportion (and Number) of Patent Assets within Segment in Portfolio | Jurisdiction | Proportion (and Number) of Patent Assets in Portfolio by Jurisdiction | Proportion (and Number) of Patent Assets by Expiration Date Category and Jurisdiction(1) | Proportion (and Number) of Patent Assets by Form of Patent and Jurisdiction | ||||||||||||
| Water Filtration | 30% (240) | US | 28% (66) | 2024 – 2028: 25% (15) 2029 – 2033: 32% (19) 2034 – 2038: 37% (22) After 2038: 6% (3) | Issued: 85% (56) Pending: 15% (10) | ||||||||||||
| EU/EP | 11% (26) | 2024 – 2028: 18% (4) 2029 – 2033: 27% (6) 2034 – 2038: 36% (8) After 2038: 19% (4) | Issued: 85% (22) Pending: 15% (4) | ||||||||||||||
| China | 32% (76) | 2024 – 2028: 28% (21) 2029 – 2033: 39% (29) 2034 – 2038: 22% (16) After 2038: 11% (8) | Issued: 92% (70) Pending: 8% (6) | ||||||||||||||
| Others | 29% (72) | 2024 – 2028: 16% (11) 2029 – 2033: 34% (23) 2034 – 2038: 45% (30) After 2038: 5% (3) | Issued: 89% (64) Pending: 11% (8) | ||||||||||||||
_________________
(1)The expiration date data is provided only for issued patents and pending applications and does not include authorized but unfiled patent applications.
General
Our Dental Solutions segment licenses rights to certain third party patents (other than 3M) relating to 3M™ Filtek™ Restoratives, 3M™ Clinpro™ Toothpaste, 3M™ Clinpro™ Tooth Crème, 3M™ Vanish™ NaFl Varnish, and 3M™ Clinpro™ NaFl Varnish. None of our other business segments license any material patents for any material product or product family from third parties (other than 3M).
We will continue to rely on confidentiality agreements with employees, contractors, consultants, and third parties to help protect our trade secrets, proprietary technology, and other confidential information. We will also continue to monitor development and commercialization activities of third parties so that our IP rights are not infringed upon. In addition, we will continue to assess the effectiveness of our IP protection efforts.
After the separation, Solventum will either own, or 3M will continue to own and provide Solventum a license to, all IP rights necessary to operate our business as of the separation. Solventum and 3M will enter into an intellectual property cross license agreement with respect to the licensing of IP rights that are relevant to both businesses to ensure that both Solventum and 3M will have all IP rights necessary to operate their respective businesses as of the separation.
134
Environmental, Health, and Safety Matters
We are subject to various laws, regulations, ordinances, customer requirements, and industry standards related to environment, health, and safety (“EHS”) matters. These include, but are not limited to, permitting, licensing, and authorization requirements, and regulatory obligations. These laws, regulations, ordinances, requirements and standards affect a significant portion of our activities globally across each of our segments and product lines and require compliance related to, among other things: (a) occupational health, safety, and well-being; (b) the protection of the environment; (c) emissions and discharges to air and water; (d) greenhouse gas management and climate change; (e) the use of natural resources; (f) the handling, use, storage, transportation, and disposal of toxic or hazardous materials, radioactive materials, and solid and hazardous wastes; and (g) the procurement and use of select materials and chemicals. EHS laws and regulations also vary widely by jurisdiction, may be established at supranational, international, national, state, and/or local levels, and are constantly evolving, often to become more stringent.
Compliance with EHS laws, regulations, customer requirements, and industry standards requires, among other things, that we maintain and operate our equipment safely; obtain and keep current environmental permits, radioactive material licenses, and radiation machine registrations; install pollution control technologies; and maintain certain records and submit specific reports. Failure to comply could lead to enforcement actions, such as the imposition of civil or criminal fines and penalties; the suspension or termination of our permits, licenses, authorizations, or operations; claims by third parties; remediation expenses or liabilities; or other sanctions.
Solventum also is subject to extensive and evolving regulations regarding the manufacturing, processing, distribution, importing, exporting, and labeling of its products and their raw materials. In the European Union, for instance, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulations came into effect in 2007, with implementation rolling out over time. Registered chemicals then can be subject to further evaluation and potential restrictions. Since the promulgation of REACH, other countries have enacted or are in the process of implementing similar comprehensive chemical regulations.
Some of Solventum’s products contain or are enabled by certain PFAS. Regulatory and legislative activities concerning PFAS are accelerating in the United States, Europe and elsewhere. These regulatory activities include gathering of exposure and use information, risk assessment activities, and increasingly strict restrictions on various uses of PFAS in products and on PFAS in manufacturing emissions and in water, soil and air, in some cases moving towards setting non-detectable limits for certain PFAS compounds. Governments, including in the United States, are also increasingly proposing changes to existing environmental regulations to specifically apply to PFAS. In February 2023, the European Chemicals Agency published a proposal under REACH, which has not yet been finalized, that aims to restrict the manufacture, placing on the market and use of PFAS, subject to certain exceptions.
Solventum’s operations are also subject to regulation under the federal Occupational Safety and Health Act (OSHA) and parallel state and local occupational health and safety standards, as well as occupational health and safety standards applicable to its operations in other jurisdictions. These standards establish certain employer responsibilities, including requirements to maintain a workplace free of recognized hazards likely to cause serious injury or death, certain medical and hygiene standards, licensing and permitting obligations and various recordkeeping, disclosure and procedural requirements. Solventum’s facilities and operations may be subject to periodic inspections by OSHA representatives and comparable authorities in other jurisdictions. Failure to comply with applicable occupational health and safety standards, even if no work-related serious injury or death occurs, could result in civil or criminal enforcement and substantial penalties, significant capital expenditures or suspension or limitation of Solventum’s operations.
In addition to REACH and OSHA, Solventum manufacturing facilities are also subject to additional environmental, health and safety statutes, regulations and permit requirements, including, but not limited to, applicable requirements under the Clean Air Act, the Clean Water Act, the Resource Conservation and Recovery Act, the Seveso-III Directive (Directive 2012/18/EU), the European Machinery Directive (Directive 2006/42/EC), the EU Industrial Emissions Directive (Directive 2010/75/EU), and the European Pollutant Release and Transfer Register.
135
Properties
Following the separation and distribution, we will license our U.S. headquarters located at 3M Center in St. Paul, Minnesota from 3M. Additionally, we will own, lease or otherwise have rights to use a number of facilities, including administration, research and development, manufacturing, warehousing, distribution and other facilities. We expect that we will own, lease or otherwise have rights to use approximately 273 facilities consisting of approximately 25 facilities that we will own and approximately 248 facilities that we will lease or otherwise have rights to use. These facilities cover approximately 7.6 million square feet, consisting of approximately 5.7 million square feet in facilities that we will own and approximately 1.9 million square feet in facilities that we will lease or otherwise have exclusive rights to use (excluding sites, such as our U.S. headquarters, where the occupancy will be non-exclusive in nature, such as transitional license agreements and co-working arrangements). These facilities are located throughout the United States and in many other countries around the world, including in Australia, Austria, Belgium, Brazil, Canada, Chile, China, Colombia, Costa Rica, Denmark, Finland, France, Germany, Hong Kong, India, Ireland, Israel, Italy, Japan, Malaysia, Malta, Mexico, Netherlands, New Zealand, Norway, Poland, Puerto Rico, Saudi Arabia, Singapore, South Africa, South Korea, Spain, Sweden, Switzerland, Taiwan, Thailand, Turkey, United Arab Emirates, United Kingdom, and Vietnam. Many of these facilities will serve more than one of our business segments and multiple functions across our business.
The table below sets forth our principal properties following the separation and distribution.
| Location | Principal segment(s) | Principal Use | Owned or Leased | Approximate Square Footage | ||||||||||||||||||||||
| Maplewood, Minnesota | All | Corporate Headquarters | Licensed from 3M | 1,335,432* | ||||||||||||||||||||||
| Brookings, South Dakota | MedSurg | Manufacturing | Owned | 604,000 | ||||||||||||||||||||||
| Forest City, Iowa | MedSurg | Distribution | Owned | 262,540 | ||||||||||||||||||||||
| Columbia, Missouri | MedSurg | Manufacturing | Owned | 380,000 | ||||||||||||||||||||||
| Monrovia, California | Dental Solutions | Manufacturing | Owned | 165,225 | ||||||||||||||||||||||
| Irvine, California | Dental Solutions | Manufacturing | Owned | 130,000 | ||||||||||||||||||||||
| Flemington, New Jersey | MedSurg | Manufacturing | Owned | 203,000 | ||||||||||||||||||||||
| Eden Prairie, Minnesota | MedSurg | Manufacturing | Leased | 110,789 | ||||||||||||||||||||||
| Meriden, Connecticut | Purification and Filtration | Manufacturing | Owned | 190,000 | ||||||||||||||||||||||
| Stafford Springs, Connecticut | Purification and Filtration | Manufacturing | Owned | 230,000 | ||||||||||||||||||||||
| Morden, Canada | MedSurg | Manufacturing | Owned | 104,276 | ||||||||||||||||||||||
| Guadalupe (Monterrey), Mexico | Purification and Filtration | Manufacturing | Leased | 165,263 | ||||||||||||||||||||||
| Athlone, Ireland | MedSurg | Manufacturing | Leased | 139,217 | ||||||||||||||||||||||
| Kamen, Germany | MedSurg | Manufacturing | Owned | 382,184 | ||||||||||||||||||||||
| Seefeld, Germany | Dental Solutions | Manufacturing | Owned | 592,000 | ||||||||||||||||||||||
| Wuppertal, Germany | Purification and Filtration | Manufacturing | Owned | 1,379,643 | ||||||||||||||||||||||
| Wrocław, Poland | MedSurg | Manufacturing | Owned | 333,143 | ||||||||||||||||||||||
| Mazeres sur Saiat, France | Purification and Filtration | Manufacturing | Owned | 107,639 | ||||||||||||||||||||||
| Shanghai, China | MedSurg | Manufacturing | Owned facility; land is leased | 171,601 | ||||||||||||||||||||||
__________________
*This location will be licensed from 3M. Figure represents square footage of the buildings primarily occupied by Solventum.
136
Legal Proceedings
Information on material pending legal proceedings is incorporated herein by reference to the information set forth in Note 11, “Commitments and Contingencies” to the audited combined financial statements included elsewhere in this Information Statement.
Regulations
General
The regulations applicable to Solventum are promulgated and enforced by government bodies in individual countries and govern the methods and controls used for the design, manufacture, packaging labeling, storage, safety, sales and distribution, marketing clearance or approval, advertising and promotion, sterilization, installation, servicing, performance and effectiveness of the products Solventum sells globally. These regulations apply to all facilities of Solventum’s business that conduct the foregoing activities, regardless of where the facilities are located. These regulations apply to the activities performed by most of Solventum’s employees, including but not limited to sales and marketing, research and development, regulatory affairs, quality assurance, medical affairs, and operations, both before and after a product is commercially distributed. These regulations differ by country and/or region and are dynamic.
Solventum commits a significant amount of resources to maintain compliance with these regulations. Compliance with these regulations requires Solventum to create systems, processes, and procedures that are aligned with the regulations in all markets Solventum serves. Compliance also requires Solventum to maintain knowledge of the current regulations that govern its activities. As these regulations change, Solventum must adapt its systems, processes, and procedures to comply with the new regulations.
Governing bodies monitor compliance, among other ways, by conducting regularly occurring and unexpected audits of Solventum’s facilities. These audits are conducted to determine if Solventum’s systems, processes, and procedures comply with the current regulations in the markets it serves. After each audit, the governing body typically provides a report of their findings. The report describes the observations made during the audit. Sometimes these observations describe minor non-compliance issues in Solventum’s systems, processes, and procedures. Often, these gaps require commensurate modifications but have no impact to Solventum’s ability to continue operations and commercialization of its products. In rare situations, the governing body may find significant or major non-compliance issues in Solventum’s systems, processes, and procedures. If a governing body concludes, through these audits or otherwise, that Solventum is not in compliance with applicable laws or regulations or that any of its products are defective, ineffective, or pose an unreasonable risk for patients, users, or others, the governing body may require Solventum to recall a product or products, retract promotional materials, and/or cease shipment of products, among other required actions; these requirements may remain in place until Solventum can demonstrate adequate compliance. Failure to demonstrate adequate modifications to Solventum’s systems, processes, and procedures and continued compliance or repeat findings may result in more significant enforcement actions, including but not limited to warning letters, revocation of product approvals and licenses, injunctions, product seizure, penalties and fines, consent decrees, and criminal prosecution, among other actions. These actions may have a negative impact on Solventum’s consolidated results of operations, financial condition or competitive position.
As discussed in more detail below under “Regulation of Medical Devices and Pharmaceutical Products”, to market its products internationally in compliance with applicable medical device and pharmaceutical regulations, Solventum must obtain approvals for products and product modifications. The regulations promulgated by the governing bodies also require Solventum to submit data to demonstrate that its products meet the safety and effectiveness requirements to support the intended uses described in its labeling. This data is reviewed by the governing bodies to determine if Solventum has provided the necessary and sufficient information to demonstrate the safety and effectiveness of its products for the intended use described in its labeling. In many cases, the governing bodies request additional information to make this determination. The additional information requested by the governing bodies sometimes requires Solventum to conduct new testing, delaying the approval and commercialization of the product. In rare instances, the governing body will disapprove the application, prohibiting Solventum’s ability to commercialize the product in that market. In these instances, Solventum may decide to cease
137
commercialization efforts for the product in that (or those) market(s) or it may decide to modify the product or retest the products and resubmit the data to the governing bodies. Delays to product approvals or disapproval of Solventum’s applications may have a negative impact on Solventum’s consolidated results of operations, financial condition or competitive position.
Failure to establish, follow or comply with any of the above requirements could have a negative impact on Solventum’s consolidated results of operations, financial condition or competitive position.
This global regulatory environment will likely continue to evolve, which could impact Solventum’s ability, or increase the time and cost, to obtain future approvals for its products. The process of obtaining regulatory clearances and/or approvals to market and sell Solventum’s products can be rigorous, costly and time-consuming and the clearances and/or approvals might not be granted timely or result in limitations on the indicated uses of products.
Regulation of Medical Devices and Pharmaceutical Products
The products Solventum develops, manufactures, and commercializes are regulated in most of the markets Solventum serves. Some of these products meet the definition of medical devices or pharmaceuticals and are regulated, as such, by various governmental bodies, globally. All products produced by the MedSurg and Dental Solutions segments, with very few exceptions, meet the definition of a medical device or pharmaceutical product. Accordingly, the development, manufacture, and commercialization of these products must comply with the regulations governing medical devices or pharmaceuticals in the markets we serve. Conversely, none of the products produced by the Health Information Systems and Purification and Filtration segments meet the definition of medical devices or pharmaceuticals. The product portfolio of each segment are dynamic and change with time, depending on the needs of the customers served. While the Health Information Systems and Purification and Filtration segments do not currently include medical devices or pharmaceutical products, this may change in the future.
The below table lists, by business segment, the products or product families named in this information statement that are regulated by the FDA as medical devices or pharmaceuticals.
Determinations of the safety and efficacy of our products are solely within the authority of the FDA or other applicable regulatory authorities in jurisdictions outside the United States.
138
| Business Segment | Products Regulated as Medical Devices | Products Regulated as Pharmaceuticals | ||||||||||||
MedSurg | 3M™ Littmann® family of products 3M™ Tegaderm™ family of products 3M™ V.A.C.®, 3M™ Veraflo™ and 3M™ PREVENA™ family of products 3M™ AbThera™ Therapies 3M™ Attest™ solutions 3M™ Granufoam™ 3M™ Cavilon™ No Sting Barrier Film 3M™ Prevena Restor™ BellaForm™ Incision Management System 3M™ Bair Hugger™ solutions 3M™ Littmann® stethoscopes 3M™ PREVENA™ Incision Management System 3M™ Scotchast™ 3M™ Tegaderm™ I.V. dressings 3M™ V.A.C.® Therapy Negative Pressure Wound Therapy (generally) 3M™ Cavilon™ family of products (excluding 3M™ Cavilon™ Durable Barrier Cream) 3M™ Promogran Prisma™ 3M™ Smart Instill™ 3M™ Veraflo™ Instillation Therapy 3M™ V.A.C.® Ulta Therapy Unit (Acute) 3M™ ActiV.A.C.™ Therapy System (Portable) 3M™ V.A.C.® Veraflo Cleanse Choice™ Dressing 3M™ Dermatac™ Drape 3M™ Snap™ Therapy System 3M™ iOn PROGRESS™ Remote Therapy monitoring 3M™ Prevena™ Therapy 3M™ Prevena Restor™ ArthroForm™ Dressing 3M™ AbThera™ SensaT.R.A.C.™ Open Abdomen Dressing 3M™ Steri-Strip™ Elastic Skin Closures 3M™ Cavilon™ Advanced Skin Protectant 3M™ Coban™ Compression Systems 3M™ Tegaderm™ transparent film dressings 3M™ Tegaderm™ CHG antimicrobial product family 3M™ Tegaderm™ CHG I.V. Dressings Family 3M™ PICC / CVC Securement Device with 3M™ Tegaderm™ I.V. Securement Dressings 3M™ Curos™ Disinfecting Caps 3M™ Ranger™ 3M™ Bair Hugger™ Warming Blanket System 3M™ Bair Hugger™ Temperature Monitoring System 3M™ Attest™ Chemical and Biological Indicators 3M™ Attest™ Reader 3M™ Ioban™ Antimicrobial Incise Drape 3M™ Red Dot™ 3M™ Red Dot™ ECG Monitoring Electrodes 3M™ Universal Electrosurgical Pads 3M™ Defib Pads 3M™ Micropore™ Surgical Tape 3M™ Coban™ Self-Adherent Wraps 3M™ Durapore™ Surgical Tape 3M™ Medipore™ H Soft Cloth Surgical Tape 3M™ Littmann® Monitoring Stethoscope 3M™ Littmann® CORE Digital Stethoscope | 3M™ Cavilon™ Durable Barrier Cream 3M™ Skin and Nasal Antiseptic | ||||||||||||
Dental Solutions | 3M™ Filtek™ Addent™ 3M™ Clinpro™ family of products (excluding the 3M™ Clinpro™ products listed under “Products Regulated as Pharmaceuticals”) 3M™ Scotchbond™ 3M™ Clarity™ 3M™ Clarity™ Aligners 3M™ Clinpro™ Sealant 3M™ Digital Bonding System 3M™ Filtek™ Matrix 3M™ Transbond™ family of products | 3M™ Clinpro™ Tooth Crème 3M™ Clinpro™ 5000 3M™ Clinpro™ Toothpaste | ||||||||||||
Health Information Systems | None | None | ||||||||||||
| Purification and Filtration | None | None | ||||||||||||
139
Additionally, 3M™ Promogran™ Collagen Dressing and 3M™ Tegaderm™ CHG Chlorhexidine Gluconate IV Securement Dressing, which are products of the MedSurg segment, are regulated by the FDA’s Center for Device and Radiological Health as a combination product category.
United States
In the United States, the Food, Drug, and Cosmetic Act authorizes the FDA to oversee and regulate the production, sale, and distribution of food, drugs, medical devices, and cosmetics.
Medical Devices
Solventum’s medical devices are regulated by the FDA’s Center for Devices and Radiological Health. The FDA classifies medical devices into one of three classes depending on the degree of risk associated with the medical device and the extent of manufacturer and regulatory control needed to ensure its safety and effectiveness: Class I (Low Risk), Class II (Moderate Risk), and Class III (High Risk).
•Class I: Class I medical devices are those where the General Controls for Medical Devices are sufficient to provide a reasonable assurance of safety and effectiveness.
•Class II: Class II medical devices are where the General Controls for Medical Devices alone are insufficient to provide reasonable assurance of its safety and effectiveness and there is sufficient information to establish special controls, including the promulgation of performance standards, post-market surveillance, patient registries, development and dissemination of guidelines, recommendations, and other appropriate actions as the FDA Commissioner deems necessary to provide such assurance.
•Class III: Class III medical devices are intended to be used in supporting or sustaining human life or preventing impairment of human health, or that may present a potential unreasonable risk of illness or injury for which the General Controls for Medical Devices and special controls are insufficient to provide reasonable assurance of the safety and effectiveness of a device, or for which there is insufficient information to make such a determination. Class III devices typically require pre-market approval.
Most medical devices are cleared by FDA through the Pre-Market Notification, or 510(k), process. This process requires medical device manufacturers to demonstrate that their devices are as safe and effective as (i.e., substantially equivalent to) a legally marketed medical device. Pre-market notifications are required for most Class II and some Class I medical devices. Due to the level of risk associated with Class III devices, the FDA has determined that these devices require a Pre-market approval (a “PMA”). A PMA application is the most stringent type of marketing application required by the FDA. Typically, PMA submissions require the submission of human clinical trials to achieve FDA approval. Currently, none of the medical devices in Solventum’s portfolio of products are Class III medical devices in the United States.
Pharmaceutical Products
Solventum’s pharmaceutical products are regulated by the FDA’s Center for Drug Evaluation and Research. Generally, drugs are brought to the market through the New Drug Application (“NDA”) process or an Over-the-Counter (“OTC”) Monograph. Solventum’s pharmaceutical products are brought to market through the NDA and OTC pathways.
The NDA process typically begins with the completion of extensive preclinical laboratory tests and preclinical animal studies, which may need to be performed in accordance with the Good Laboratory Practices regulations, followed by the submission to the FDA of an investigational new drug application which must become effective before human clinical trials may begin and must be updated annually.
Before each clinical study may be initiated, an independent Institutional Review Board or ethics committee representing each clinical site must approve such study. The clinical studies must be adequate, well-controlled and conducted in accordance with Good Clinical Practice (“GCP”) requirements.
140
After the completion of the clinical trials, an NDA is submitted to the FDA to demonstrate that a drug is safe and effective in its proposed use(s), the benefits of the drug outweigh the risks, the drug’s proposed labeling (package insert) is appropriate, and the methods used to manufacture the drug are adequate to preserve the drug’s identity, strength, quality, and purity. Following the completion of the clinical trials, the NDA process with the FDA generally involves the following:
•preparation of and submission to the FDA of an NDA;
•potential review of the product application by an FDA advisory committee, where appropriate and if applicable;
•a determination by the FDA within 60 days of its receipt of an NDA to file the application for review;
•satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities where the proposed product drug substance and drug product are produced to assess compliance with current cGMPs, and audits of selected clinical trial sites to ensure compliance with GCP; and
•FDA review and approval of an NDA prior to any commercial marketing or sale of the drug or biologic in the United States.
An OTC drug monograph is a type of “recipe book” covering acceptable ingredients, doses, formulations, labeling, and, in some cases, testing parameters. OTC drug monographs are continually updated to add additional ingredients and labeling as needed. Products conforming to a monograph may be marketed without FDA pre-approval. Under the OTC monograph system, selected OTC drugs are generally recognized as safe and effective and do not require the submission and approval of an NDA. The FDA OTC monographs include well-known ingredients and specific requirements for permitted indications, required warnings and precautions, allowable combinations of ingredients and dosage levels. Pharmaceutical products marketed under the OTC monograph system must conform to specific quality, formula and labeling requirements. Facilities where OTC drugs are manufactured, tested, packaged, stored or distributed must comply with cGMP regulations and/or regulations promulgated by the FDA or other competent authorities. OTC monograph products that do not comply with these standards can be deemed unapproved new drugs and can be required to be withdrawn from the market. The Over-the-Counter Monograph Safety, Innovation, and Reform Act, enacted in March 2020, is expected to introduce significant reform to the OTC monograph system, including by replacing the FDA’s existing rulemaking process with an administrative order process for issuing, revising and amending OTC monographs.
European Union
Medical Devices
All medical devices that are placed on the market or put into service in the European Union must meet the requirements of the Medical Device Regulation. Manufacturers must demonstrate compliance to the requirements of the Medical Device Regulation, prior to affixing the CE Mark on the products and commercializing in the European Union. A CE Mark is a symbol placed on a product that declares that the product is compliant with the essential requirements of applicable health, safety and environmental protection regulation. Compliance to the Medical Device Regulation requires a manufacturer to demonstrate that its products comply with minimum standards of performance, safety, and quality, through a conformity assessment procedure that depends on the product’s classification. The Medical Device Regulation describes four classes of medical devices: Class I (Lowest Risk), Class IIa, Class IIb, and Class III (Highest Risk). Classification is dependent on a variety of factors, including duration of use, whether the device is invasive or non-invasive, and whether the device is considered “active.” Notified bodies are responsible for ensuring that manufacturers comply with the requirements of the Medical Device Regulation. All medical devices in Solventum’s current portfolio are regulated as medical devices in the European Union.
141
Pharmaceutical Products
The European Medicines Agency is responsible for regulating pharmaceutical products in the European Union. The European Medicines Agency implements a system similar to the U.S. FDA’s Center for Drug Evaluation and Research and it is responsible for evaluating the quality, safety, and efficacy of drug products in the EU. If the European Medical Agency concludes that all requirements for efficacy, safety and quality are met, it issues a positive opinion that is forwarded to the European Commission and the European Commission makes the final decision on the granting of a marketing authorization.
China
The chief pharmaceutical product and medical device regulator in China is the National Medical Products Administration (“NMPA”), which enforces medical device and pharmaceutical product laws and regulations and standards and has the power to issue fines, seize products, withdraw or suspend an approval or a registration for serious non-compliances, and refer cases for criminal prosecution. These national laws and regulations are also supplemented by provincial and other local-level rules and enforcement policies.
Medical Devices
Locally manufactured medical devices gain market authorization through municipal authorities, while medical devices that are not manufactured in China are reviewed by the NMPA and must be accompanied by appropriate documentation showing that the device has been approved in its country of origin.
Medical devices are classified into three classes: Class I (Lowest Risk), Class II, and Class III (Highest Risk). Approved products are subject to post-market requirements for reporting adverse events and recalls, as well as regular risk assessments of devices and potentially re-evaluation reports of the safety and effectiveness of the device based on more significant safety signals.
In addition to product licenses, manufacturing and distribution facilities that handle Class II and III devices require licenses or notifications and must comply with Good Manufacturing Processes (“cGMP”) requirements and good supply practices. The NMPA regularly conducts inspections of manufacturing facilities in China (as part of a pre-market submission review, routine or for-cause inspections, or unannounced inspections) as well as periodic inspections of overseas manufacturers for compliance with China medical device cGMP requirements. The NMPA inspects distributors and user facilities and conducts annual national and provincial sampling inspections and testing to ensure compliance with labeling, licensing, mandatory standards, and other related requirements. In addition, the NMPA conducts regular and for-cause good clinical practice audits of clinical sites that provide data and clinical trial reports for product registration.
Pharmaceutical Products
Solventum’s pharmaceutical products are strictly regulated by the NMPA and various provincial, city, and county regulators. Some of Solventum’s pharmaceutical products require pre-market approval from the NMPA before they can be marketed in China, and those marketing applications must be supported by clinical data, which typically comes from a multi-phase study in China or by relying on clinical data generated abroad that meets the NMPA’s requirements.
Regulation on Advertising, Marketing, and Promotion
The advertising, marketing, and promotion of Solventum’s products must be truthful and non-misleading, consistent with applicable regulatory clearances and approvals, and supported by adequate and reasonable scientific data. Solventum typically is required to have a reasonable basis to support any factual marketing claims, and what constitutes a reasonable basis for substantiation can vary widely from market to market and from product to product.
With limited exceptions, Solventum may not market, promote, or sell regulated products prior to regulatory authority clearance or approval. Regulatory authorities, including the FDA, strictly regulate the indications for use and associated promotional safety and effectiveness claims that may be made about approved or cleared products. If regulatory authorities determine that Solventum has promoted or marketed a product for off-label use, including
142
through external-facing materials, oral statements, or physician training, Solventum could be subject to fines, injunctions or other penalties.
In addition, the National Advertising Division (“NAD”) of the Better Business Bureau administers a self-regulatory program of the advertising industry to ensure truth and accuracy in national advertising. NAD monitors national advertising and entertains inquiries and challenges from competitors and consumers. Solventum may also be subject to various state consumer protection laws, including California’s Proposition 65, which requires a specific warning on any product that contains a substance listed by California as having been found to cause cancer or birth defects, unless the level of such substance in the product is below a safe harbor level.
Solventum must also comply with advertising, marketing, and promotion rules in all countries in which it markets its products. In the European Union, advertising of products is subject both to general consumer advertising requirements pursuant to the Unfair Commercial Practices Directive (Directive 2005/29/EC), which imposes a general prohibition on misleading and aggressive advertising, as well as more specific regulations in respect of various product classifications. For example, pursuant to Directive 2001/83/EC, advertisements of Solventum’s OTC products must, among other requirements, (1) make clear that the message is an advertisement and that the product is clearly identified as a medicinal product, (2) not refer to claims of recovery in improper, alarming or misleading terms and (3) not suggest that the effects of taking the medicine are guaranteed, are unaccompanied by adverse reactions or are better than, or equivalent to, those of another treatment or medicinal product.
In China, advertisements of OTC products must, among other requirements, include an “OTC” marking and must not contain difficult or confusing medical or pharmaceutical terms that could mislead the public about a product’s efficacy or safety.
A failure to comply with these regulations could expose Solventum to legal liability, such as enforcement actions, investigations by a governmental authority, civil fines or criminal actions, lawsuits brought by competitors or company whistleblowers, or other actions.
Data Privacy Laws
Solventum is also subject to extensive laws and regulations protecting the privacy, security, and integrity of personal information, including patient medical information, that it receives, including, among others, the U.S. Health Insurance Portability and Accountability Act of 1996, as amended, (“HIPAA”), the California Consumer Privacy Act (the “CCPA”), and similar U.S. state laws, the European Union’s General Data Protection Regulation (the “E.U. GDPR”), the United Kingdom’s Data Protection Act 2018 (the “UK DPA”) and the General Data Protection Regulation (the “U.K. GDPR”), and China’s Personal Information Protection Law (“PIPL”), and Personal Data Cross Border Transfer Rule (“CBDT”). Federal health information privacy laws, such as HIPAA, and consumer protection laws impose requirements for the collection, use, storage, access, transfer and protection of health-related and other sensitive and personal information, and failure to comply may result, such as with respect to any CCPA violations, in civil penalties. The CCPA has been amended by the California Privacy Rights Act (“CPRA”), which came into effect, in most material respects, on January 1, 2023. The CPRA significantly modifies the CCPA, including by expanding consumers’ rights with respect to certain sensitive personal information. The E.U. GDPR and the U.K. GDPR and UK DPA, together with national legislation, regulations and guidelines of the E.U. Member States and the United Kingdom governing the processing of personal data, impose strict obligations and restrictions on the ability to collect, analyze, store, transfer and otherwise process personal data, including health data and adverse event reporting. The E.U. GDPR contemplates fines for certain violations of up to four percent of global annual revenue or €20 million (or GBP 17.5 million under the U.K. GDPR), whichever is greater, and enforcement can include limits on data transfers to countries outside the E.U. or U.K. In China, Solventum is subject to PIPL, which applies to the secure processing of personal information of natural persons within China, and Solventum is also subject to CBDT for the processing of personal information outside China where the purpose is to provide products and services within China and the analysis or assessment of the activities of individuals within China. Consequences of non-compliance may include monetary fines of up to five percent of the previous year’s revenue, termination of data transfers and personal liability imposed on those directly responsible. Solventum is also subject to similar privacy and data protection frameworks in other developed and emerging markets. While Solventum utilizes industry standard processes, including the National Institute of Standards and Technology (NIST)
143
privacy framework and third-party management processes, to assess the potential impact of emerging laws and enforcement trends on its business and to mitigate potential impacts on its business, data privacy laws and regulations and their scope and enforcement are constantly evolving, and Solventum cannot predict what effect, if any, changes to these laws and regulations and Solventum’s compliance with them may have on its business.
Global Healthcare Compliance
The marketing, promotion, and sale of medical devices, drugs, and services is regulated by the U.S. Department of Health and Human Services and equivalent U.S. state and non-U.S. agencies responsible for reimbursement and regulation of the delivery of healthcare items and services. These include laws and regulations related to kickbacks, false claims, self-referrals, and healthcare fraud and abuse. Similar regulations are imposed at the state level, as well as in many global markets in which we do business.
The U.S. FCPA, the U.K. Bribery Act of 2010, and similar anti-corruption and anti-bribery laws in other jurisdictions generally prohibit companies from promising to pay money or anything of value to any foreign official for the purpose of obtaining or retaining business. These laws apply to many of Solventum’s customer interactions, as healthcare professionals in other countries are or are often considered government officials, and in some cases lay out specific requirements of how to comply or demonstrate compliance with the legal requirements. Failure to comply with these laws may expose Solventum to monetary fines and penalties, criminal and civil enforcement actions and reputational damage.
Quality and Safety
The FDA and comparable authorities in other jurisdictions regulate the facilities and operational procedures that Solventum uses to manufacture its products. Solventum is required to register its facilities with these authorities. Solventum’s products are required to be manufactured in facilities that operate in accordance with current Good Manufacturing Processes (“cGMP”). Compliance with cGMPs requires Solventum to establish and follow quality systems that are designed to ensure that its products consistently meet applicable requirements and specifications. The scope of cGMP includes, but is not limited to, production and process controls, document controls, acceptance activities, labeling and packing controls, handling, storage, distribution, and installation activities, among others. The FDA and comparable authorities in other jurisdictions periodically inspect Solventum’s manufacturing facilities for compliance with cGMP or similar manufacturing standards in the applicable country. Failure to comply with cGMPs could disrupt Solventum’s ability to manufacture or supply its products.
Solventum is also required to establish and follow quality systems to comply with certain post-market surveillance requirements, specifically those pertaining to adverse event reporting for medical devices and pharmaceuticals. For medical devices, Solventum is required to report deaths and serious injuries that a device may have caused or to which a device may have contributed. Solventum is also required to report certain device malfunctions. For pharmaceuticals, Solventum is required to report any undesirable event that is associated with the use of a drug or biological product in humans whether or not the events are considered to be product related. For both medical devices and pharmaceuticals, Solventum is required to establish and implement quality systems to comply with regulations pertaining to post-market surveillance.
In addition, many of Solventum’s products are subject to regulation by the Consumer Product Safety Commission (the “CPSC”) under the Consumer Product Safety Act and other laws enforced by the CPSC. These statutes and related regulations establish safety standards and bans for consumer products. The CPSC monitors compliance of consumer products under its jurisdiction through market surveillance and has the authority to conduct product safety inspections of establishments where consumer products are manufactured, held or transported. The CPSC can require the recall of noncompliant products or products containing a defect that creates a substantial risk of injury to the public, and the CPSC may seek penalties for regulatory noncompliance under certain circumstances. CPSC regulations also require manufacturers of consumer products to report to the CPSC certain types of information regarding products that fail to comply with applicable regulations, contain a defect that could create a substantial product hazard or create an unreasonable risk of serious injury or death. Certain state laws also address the safety of consumer products and may mandate reporting or labeling requirements.
144
Pricing
Solventum’s activities are subject to a variety of price control laws, regulations and government mandates in some of the markets in which it operates. In certain markets the pricing for certain Solventum’s products may be subject to prior approval, including in jurisdictions where Solventum’s products are subject to government reimbursement, whereas in other markets Solventum may be able to set its own prices for its products subject to certain degrees of monitoring and control by the applicable governmental authority.
These price control mechanisms, and mechanisms in other markets, including long-term price commitments as the result of market dynamics, may restrict the amount Solventum is able to charge for its products, which may reduce its profits and otherwise adversely affect its business, results of operations or financial condition. In addition, price control laws, regulations or government mandates may be enacted or become more stringent during times of uncertain or unfavorable economic or market conditions, such as during times of economic slowdown, recession or inflation.
Environmental, Health, and Safety Laws
Solventum is subject to a broad range of federal, state, provincial and local environmental laws and regulations concerning environmental, health and safety matters. For a discussion of these laws and regulations, see the section titled “Environmental, Health, and Safety Matters.”
145
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our audited combined financial statements and corresponding notes and the unaudited pro forma condensed combined financial information and corresponding notes and other financial information included elsewhere in this information statement. This discussion contains forward-looking statements that are based upon current expectations and are subject to uncertainty and changes in circumstances. Our actual results could differ materially from the results contemplated by these forward-looking statements due to a number of factors, including those discussed below and elsewhere in this information statement, particularly in “Risk Factors.” Actual results may differ materially from these expectations. See “Cautionary Note Regarding Forward-Looking Statements.” All amounts discussed are in millions of U.S. dollars, unless otherwise indicated. Certain columns and rows within tables may not add up due to the use of rounded numbers.
Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A) is designed to provide a reader of the Health Care Business’s financial statements with a narrative from the perspective of management. The Health Care Business’s MD&A is presented in eight sections:
•Overview
•Results of Operations
•Performance by Business Segment
•Performance by Geographic Area
•Non-GAAP Financial Measures
•Financial Condition and Liquidity
•Critical Accounting Estimates
•New Accounting Pronouncements
OVERVIEW
Our Business
Solventum is a leading $8 billion global healthcare company developing, manufacturing, and commercializing a broad portfolio of solutions that leverages deep material science, data science, and digital capabilities to address critical customer and patient needs. We constantly seek to enable the improvement of standards of care and move healthcare forward with innovation powered by insights, clinical intelligence, technology, and manufacturing expertise. Our 70+ year history of discovering and innovating advanced solutions has helped us solve our customers’ toughest challenges and become a trusted partner.
In 2023, Solventum generated 56% of total revenues from the United States and 44% from international. Based on the breadth of our portfolio, Solventum serves a global addressable market that we estimate had approximately $93 billion of sales in 2022. We estimate that this market will grow at an annual rate of 4-6% from 2024 through 2026. We participate in what we believe are large, stable global markets that have favorable market drivers, including changing demographics, optimization of workflows to improve quality of care, increasing digital technology and data-driven care delivery, shifting care from the hospital to lower-cost care sites and increasing demand for personalized care. See “Information Statement Summary — Our Markets” and “Business — Our Markets” for information about how we estimated the size and growth rate of our addressable market, and the risks to our ability to take advantage of these market opportunities.
We serve customers in over 90 countries with a global team of approximately 22,000 employees and an established global manufacturing network. In each of the last three years, we have generated over $8 billion of
146
revenue, $1.7 billion of operating income, and $2 billion of adjusted operating income. We believe Solventum will deliver growth at attractive margins with the mission of enabling better, smarter, safer healthcare to improve lives.
However, Solventum has no history of operating as an independent company, and its historical and pro forma financial information is not necessarily representative of the results that it would have achieved as a separate, publicly traded company and may not be a reliable indicator of its future results. In particular, Solventum currently benefits from 3M’s long operating history, reputation and well-known brand. Following the separation, Solventum will operate under its own brand, and accordingly may be negatively impacted due to the loss of benefits conferred by 3M’s brand recognition and reputation. In addition, the debt obligations incurred by Solventum in connection with the separation will adversely affect its profitability and could affect its ability to use its cash flow for investing in the business, M&A and returning capital. See “Risk Factors - Risks Related to the Separation and Distribution” for a discussion of these risks, which you should consider carefully.
Healthcare Market Drivers
Changing demographics
An aging population, the prevalence and incidence rates of chronic conditions, and a rising middle class are driving the demand for improved access to quality care.
Optimizing workflows to improve quality of care
Of the $4.5 trillion in annual U.S. healthcare spending, an estimated 25% represents administrative costs that do not contribute to health outcomes and which we believe to be potentially wasteful based on overall spending data reported by the Centers for Medicare & Medicaid Services in the NHE Fact Sheet (available on CMS.gov as of January 10, 2024) and administrative spending estimates published in JAMA (Shrank et. al., Waste in the US Health Care System: Estimated Costs and Potential for Savings, published October 7, 2019). Our solutions are designed to optimize workflows, enabling clinicians to be more productive by spending less time on administrative tasks and more time focused on improving the patient care experience. Our solutions also support reducing infections and complications that lead to an increase in avoidable administrative and clinical costs.
Increasing digital technology and data-driven care delivery
Both clinicians and patients have shifted their preferences towards utilizing digitally enabled solutions to provide data-driven care. Whether it is interactions with patients through a digital interface or the use of data to make informed health decisions, the need for digital tools in the healthcare industry has grown over time. Our solutions integrate digital processes and data in multiple ways and across different parts of the healthcare industry and are intended to enable efficient and effective delivery of care.
Shifting care from the hospital to lower-cost care sites
Although hospitals continue to be a core site for delivery of care, patients are increasingly looking for flexibility of care when and where they need it. Alternative care sites, such as ambulatory surgery centers, wound care clinics, retail pharmacies, and the home, are more affordable and accessible to patients. We believe our solutions enable clinicians to extend their care delivery from acute to ambulatory to home settings without compromising the quality of care and while reducing the total cost of care.
Increasing demand for personalized care
Engaging patients in a personalized way allows clinicians to provide a better care experience while improving outcomes and reducing costs. This spans several areas of healthcare, including personalized biopharmaceutical treatments, customized orthodontic aligner treatments, and follow-up wound care at home. We believe our solutions deliver personalized care options in a way that is patient-centric, scalable, and cost-effective.
Our ability to take advantage of these market opportunities will be subject to various risks, including general economic, business and market dynamic risks, the impact of our separation from 3M; and the substantial debt we will incur in connection with the separation. See “Information Statement Summary—Summary of Risk Factors”,
147
“Information Statement Summary—Certain Risks Relating to Operating as a Standalone Entity”, “Information Statement Summary—Certain Risks Relating to Solventum’s Indebtedness”, and “Risk Factors” for a discussion of these risks, which you should consider carefully.
Other
Operating Segments and Sales Change Information
Solventum manages its operations in four business segments: MedSurg, Dental Solutions, Health Information Systems, and Purification and Filtration. Beginning in 2023, the Medical Solutions segment was renamed to MedSurg, the Oral Care Solutions segment was renamed to Dental Solutions, and the Separation and Purification Sciences segment was renamed to Purification and Filtration. Segment names in prior years have been conformed accordingly. There have been no changes to the composition of the segments or to financial information reported within each of the business segments.
References are made to organic sales change, which is defined as the change in net sales, absent the separate impacts on sales from foreign currency translation and acquisitions, net of divestitures. Acquisition and divestiture sales change impacts, if any, are measured separately for the first twelve months post-transaction. Solventum believes this information is useful to investors and management in understanding ongoing operations and in analysis of ongoing operating trends.
Sales and operating income by business segment for the years ended December 31, 2023, 2022, and 2021
The following tables contain sales and operating results by business segment for all periods presented. Refer to the section entitled “—Performance by Business Segment” below for discussion of sales change and operating performance. Refer to Note 14 to the audited combined financial statements for additional information on business segments.
Sales by Business Segment
| Years ended December 31, | ||||||||||||||||||||||||||||||||||||||
| 2023 | 2022 | Sales Change 2023 vs. 2022 | ||||||||||||||||||||||||||||||||||||
| (Dollars in millions) | Net Sales | Net Sales | Organic Sales | Acquisition /Divestiture | Translation | Total Sales Change | ||||||||||||||||||||||||||||||||
| Segment Sales | ||||||||||||||||||||||||||||||||||||||
| MedSurg | $ | 4,632 | $ | 4,585 | 1.6 | % | — | (0.6) | % | 1.0 | % | |||||||||||||||||||||||||||
| Dental Solutions | 1,329 | 1,327 | 1.6 | % | (1.0) | % | (0.4) | % | 0.2 | % | ||||||||||||||||||||||||||||
| Health Information Systems | 1,285 | 1,227 | 4.7 | % | — | — | 4.7 | % | ||||||||||||||||||||||||||||||
| Purification and Filtration | 951 | 991 | (3.6) | % | — | (0.4) | % | (4.0) | % | |||||||||||||||||||||||||||||
| Corporate and Unallocated | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||
| Total Company | $ | 8,197 | $ | 8,130 | 1.4 | % | (0.2) | % | (0.4) | % | 0.8 | % | ||||||||||||||||||||||||||
| Years ended December 31, | ||||||||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | Sales Change 2022 vs. 2021 | ||||||||||||||||||||||||||||||||||||||||||
| (Dollars in millions) | Net Sales | Net Sales | Organic Sales | Translation | Total Sales Change | |||||||||||||||||||||||||||||||||||||||
| Segment Sales | ||||||||||||||||||||||||||||||||||||||||||||
| MedSurg | $ | 4,585 | $ | 4,632 | 2.7 | % | (3.7) | % | (1.0) | % | ||||||||||||||||||||||||||||||||||
| Dental Solutions | 1,327 | 1,396 | (0.1) | % | (4.8) | % | (4.9) | % | ||||||||||||||||||||||||||||||||||||
| Health Information Systems | 1,227 | 1,160 | 6.6 | % | (0.8) | % | 5.8 | % | ||||||||||||||||||||||||||||||||||||
| Purification and Filtration | 991 | 983 | 7.1 | % | (6.3) | % | 0.8 | % | ||||||||||||||||||||||||||||||||||||
| Corporate and Unallocated | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||
| Total Company | $ | 8,130 | $ | 8,171 | 2.9 | % | (3.4) | % | (0.5) | % | ||||||||||||||||||||||||||||||||||
148
Operating Income by Business Segment
| Years ended December 31, | ||||||||||||||||||||||||||||||||||||||||||||
| (Dollars in millions) | 2023 | 2022 | 2021 | 2023 vs 2022 Change | 2022 vs 2021 Change | |||||||||||||||||||||||||||||||||||||||
| Segment Operating Income | ||||||||||||||||||||||||||||||||||||||||||||
| MedSurg | $ | 1,107 | $ | 1,061 | $ | 1,226 | 4.3 | % | (13.5) | % | ||||||||||||||||||||||||||||||||||
| Dental Solutions | 442 | 437 | 482 | 1.1 | % | (9.3) | % | |||||||||||||||||||||||||||||||||||||
| Health Information Systems | 423 | 359 | 354 | 17.8 | % | 1.4 | % | |||||||||||||||||||||||||||||||||||||
| Purification and Filtration | 162 | 177 | 229 | (8.5) | % | (22.7) | % | |||||||||||||||||||||||||||||||||||||
| Corporate and Unallocated | (442) | (341) | (412) | 29.6 | % | (17.2) | % | |||||||||||||||||||||||||||||||||||||
| Total Company | $ | 1,692 | $ | 1,693 | $ | 1,879 | (0.1) | % | (9.9) | % | ||||||||||||||||||||||||||||||||||
Sales by geographic area for the year ended 2023
Percent change information compares the year ended December 31, 2023 with the same period for the prior year, unless otherwise indicated.
| Year ended December 31, 2023 | ||||||||||||||||||||
| (Dollars in millions) | United States | International | Worldwide | |||||||||||||||||
| Net sales | $ | 4,603 | $ | 3,594 | $ | 8,197 | ||||||||||||||
| % of worldwide sales | 56.2 | % | 43.8 | % | 100.0 | % | ||||||||||||||
| Components of net sales change: | ||||||||||||||||||||
| Organic sales | 3.4 | % | (0.9) | % | 1.4 | % | ||||||||||||||
| Divestiture | — | (0.4) | (0.2) | |||||||||||||||||
| Translation | — | (1.0) | (0.4) | |||||||||||||||||
| Total sales change | 3.4 | % | (2.3) | % | 0.8 | % | ||||||||||||||
Additional information beyond what is included in the preceding table is as follows:
•In the United States geographic area, all business segments saw organic sales growth year on year, led by Health Information Systems and MedSurg.
•In the International geographic area, total sales growth and organic sales growth decreased. Organic growth decline in Purification and Filtration was partially offset by organic growth in MedSurg and Dental Solutions.
Sales by geographic area for the year ended 2022
Percent change information compares the year ended December 31, 2022 with the same period for the prior year, unless otherwise indicated.
| Year Ended December 31, 2022 | ||||||||||||||||||||
| (Dollars in millions) | United States | International | Worldwide | |||||||||||||||||
| Net sales | $ | 4,450 | $ | 3,680 | $ | 8,130 | ||||||||||||||
| % of worldwide sales | 54.7 | % | 45.3 | % | 100.0 | % | ||||||||||||||
| Components of net sales change: | ||||||||||||||||||||
| Organic sales | 1.3 | % | 5.6 | % | 2.9 | % | ||||||||||||||
| Translation | — | (8.2) | (3.4) | |||||||||||||||||
| Total sales change | 1.3 | % | (2.6) | % | (0.5) | % | ||||||||||||||
149
Additional information beyond what is included in the preceding table is as follows:
•In the United States geographic area, total and organic sales growth was led by Health Information Systems and MedSurg.
•In the International geographic area, total sales growth decreased, which included increased organic sales. All business segments saw organic sales growth year on year, led by MedSurg and Purification and Filtration.
Managing currency risks
Health Care indirectly participates in 3M’s centrally managed hedging program which utilizes a number of tools to manage currency risk including natural hedges such as pricing, productivity, hard currency, hard currency-indexed billings, and localizing source of supply. Parent also uses financial hedges to mitigate currency risk.
The stronger U.S. dollar had a negative impact on sales of less than 1 percent in the full year 2023 compared to the full year 2022. Net of 3M’s hedging strategy, foreign currency negatively impacted earnings for the full year 2023 compared to the same period last year.
The stronger U.S. dollar had a negative impact on sales of 3 percent in full year 2022 compared to the full year 2021. Net of 3M’s hedging strategy, foreign currency negatively impacted earnings for full year 2022 compared to the same period last year.
Russia-Ukraine Conflict
The global economy has been impacted by the military conflict between Russia and Ukraine. The U.S. and other governments have imposed export controls on certain products and financial and economic sanctions on certain industry sectors and parties in Russia. 3M suspended operations of its subsidiaries, including those of Health Care, in Russia in March 2022 and, in September 2022, committed to a plan to exit the related net assets in Russia, including those of Health Care, through a sale of 3M’s Russian subsidiaries that was consummated in June 2023. For the year ended December 31, 2022, Health Care recorded a charge of $8 million, primarily related to impairment of net assets in Russia in connection with 3M management’s committed exit and disposal plan. Health Care did not recognize a gain or loss upon the final sale of 3M’s Russian subsidiaries.
Solventum has other operations that source certain raw materials from suppliers in Russia and has experienced related supply disruption due to the conflict. These geopolitical tensions could result in, among other things, cyberattacks, further supply chain disruptions impacting downstream customers, higher energy costs, lower consumer demand, and changes to foreign exchange rates and financial markets, any of which may adversely affect Solventum’s business and supply chain.
Financial condition
Refer to the section entitled “—Financial Condition and Liquidity” below for a discussion of items impacting cash flows.
RESULTS OF OPERATIONS
COMPARISON OF RESULTS OF OPERATIONS FOR THE YEARS ENDED DECEMBER 31, 2023, 2022, AND 2021
Net Sales
Refer to the preceding “—Overview” section and the “—Performance by Business Segment” section later in MD&A for discussion of sales change.
150
Operating Expenses
| (Percent of corresponding net sales) | 2023 | 2022 | 2021 | 2023 versus 2022 | 2022 versus 2021 | |||||||||||||||||||||||||||||||||||||||
| Costs of product | 48.0 | % | 46.9 | % | 43.3 | % | 1.1 | % | 3.6 | % | ||||||||||||||||||||||||||||||||||
| Costs of software and rentals | 25.3 | % | 26.3 | % | 26.8 | % | (1.0) | % | (0.5) | % | ||||||||||||||||||||||||||||||||||
Costs of Product
Costs of product includes manufacturing, engineering and freight costs.
Costs of product, measured as a percent of sales of product, increased in 2023 when compared to 2022. Material and labor inflation, partially offset by benefits from both price and logistics costs, drove an increase of 0.7%. The material and labor inflation was primarily driven by a 1.4% impact from a higher cost of inventory produced in 2022 but sold in 2023.
Costs of product, measured as a percent of sales of product, increased in 2022 when compared to 2021. Product mix resulted in an increase of 1.9%, driven by lower organic sales growth in our Dental Solutions business. Material and labor inflation, partially offset by price, drove an increase of 1.1%. Labor inflation was driven by both worker shortages and wage inflation. Material inflation increased due to both higher material prices and logistics costs.
Costs of Software and Rentals
Costs of software and rentals includes compensation-related costs associated with installation, training and maintenance for our software products, and depreciation, maintenance and refurbishment cost and freight costs related to our hardware rental units.
Costs of software and rentals, measured as a percent of sales of software and rentals, decreased in 2023 as compared to 2022 due to product mix from higher software sales.
Costs of software and rentals, measured as a percent of sales of software and rentals, decreased in 2022 as compared to 2021 primarily due to product mix from higher software sales.
| (Percent of total net sales) | 2023 | 2022 | 2021 | 2023 versus 2022 | 2022 versus 2021 | |||||||||||||||||||||||||||||||||||||||
| Selling, general and administrative (SG&A) | 27.4 | % | 27.5 | % | 27.9 | % | (0.1) | % | (0.4) | % | ||||||||||||||||||||||||||||||||||
| Research and development (R&D) | 9.2 | % | 9.4 | % | 9.4 | % | (0.2) | % | — | % | ||||||||||||||||||||||||||||||||||
| Operating income | 20.6 | % | 20.8 | % | 23.0 | % | (0.2) | % | (2.2) | % | ||||||||||||||||||||||||||||||||||
Selling, General and Administrative
SG&A, measured as a percent of total net sales, decreased slightly in 2023 when compared to 2022. This decrease was driven by the impact of the gain related to the sale of the Company’s dental local anesthetic business of 0.7%, partially offset by higher expense due to restructuring charges of 0.5%.
SG&A, measured as a percent of total net sales, decreased in 2022 when compared to 2021. SG&A decreased compared to the prior year due to lower incentive compensation of 0.4% and spending discipline, partially offset by focused investments for key growth initiatives in Health Information Systems of 0.2%.
Research and Development
R&D, measured as a percent of total net sales, decreased slightly in 2023 when compared to 2022 as the Health Care Business prioritized investment initiatives.
151
R&D, measured as a percent of total net sales, was flat in 2022 when compared to 2021. The Health Care Business continued to prioritize investment initiatives to develop new products, build digital capabilities, and support sustainability projects and at the same time optimize cost controls.
Other Expense (Income) – Net
| (Dollars in millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||
| Other expense (income) – net | $ | 25 | $ | 1 | $ | (3) | ||||||||||||||||||||||||||
Other expense (income) – net primarily includes investment gains and losses and the non-service component of periodic pension cost.
Other expense (income) – net increased in 2023 as compared to 2022 due to investment losses and higher foreign currency transaction losses.
Other expense (income) – net increased in 2022 as compared to 2021 due to lower investment gains.
Provision for Income Taxes
| (Percent of pre-tax income) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||
| Effective tax rate | 19.3 | % | 20.6 | % | 22.4 | % | ||||||||||||||||||||||||||
The effective income tax rate for 2023 decreased by 1.3% as compared to 2022. The primary factors that decreased the tax rate were the recognition of a previously unrecognized tax benefit due to the expiration of the statute of limitations, partially offset by the tax impact of legal entity restructuring in connection with the spin-off.
The effective income tax rate for 2022 decreased by 1.8% as compared to 2021. The decrease in the effective tax rate for 2022 primarily resulted from unfavorable adjustments in 2021 related to the impact of U.S. international tax provisions.
PERFORMANCE BY BUSINESS SEGMENT
The section of this information statement titled “Business” provides an overview of the Health Care Business’s segments. In addition, disclosures relating to the Health Care Business’ segments are provided in Note 14 to the audited combined financial statements. We manage our operations in four business segments. The reportable segments are MedSurg, Dental Solutions, Health Information Systems, and Purification and Filtration. Beginning in 2023, the Medical Solutions segment was renamed to MedSurg, the Oral Care Solutions segment was renamed to Dental Solutions, and the Separation and Purification Sciences segment was renamed to Purification and Filtration. Segment names in prior years have been conformed accordingly. There have been no changes to financial information reported within each of the business segments. Our Chief Operating Decision Maker evaluates operating performance using revenue and segment operating income. Segment operating income excludes amortization of acquired intangible assets, restructuring related charges, benefits or costs related to the capitalization of manufacturing variances and other net costs that the Company chose not to allocate directly to its business segments and are reflected in Corporate and Unallocated.
Corporate and Unallocated
In addition to the four business segments, the Health Care Business assigns certain costs to “Corporate and Unallocated,” which is presented separately in the preceding business segments table and in Note 14 to the audited combined financial statements. Corporate and Unallocated includes gain or loss on the sale of a business, amortization of acquired intangible assets, restructuring related charges, benefits or costs related to capitalized manufacturing variances and other net costs that the Company chose not to allocate directly to its business segments. Because Corporate and Unallocated includes a variety of miscellaneous items, it is subject to fluctuation on a quarterly and annual basis.
152
Corporate and Unallocated net operating loss increased in 2023 when compared to 2022 driven primarily by impacts from capitalization of manufacturing variances and restructuring-related charges, partially offset by the gain on sale of the dental local anesthetic business.
Corporate and Unallocated net operating loss decreased in 2022 when compared to 2021 driven primarily by benefits from capitalization of manufacturing variances and decreased annual incentive compensation for centralized resources.
Operating Business Segments
Information related to the Health Care Business’ segments is presented in the tables that follow with additional context in the corresponding narrative below the tables.
Years ended December 31, 2023, 2022 and 2021
The following discusses total year results for 2023 compared to 2022 and 2022 compared to 2021 for each business segment.
MedSurg (56.5% of combined sales for the year ended December 31, 2023)
| 2023 | 2022 | 2021 | |||||||||||||||||||||||||||
| Sales (millions) | $ | 4,632 | $ | 4,585 | $ | 4,632 | |||||||||||||||||||||||
| Sales change analysis: | |||||||||||||||||||||||||||||
| Organic sales | 1.6 | % | 2.7 | % | 6.5 | % | |||||||||||||||||||||||
| Translation | (0.6) | (3.7) | 1.5 | ||||||||||||||||||||||||||
| Total sales change | 1.0 | % | (1.0) | % | 8.0 | % | |||||||||||||||||||||||
| Business segment operating income (millions) | $ | 1,107 | $ | 1,061 | $ | 1,226 | |||||||||||||||||||||||
| Percent change | 4.3 | % | (13.5) | % | 7.6 | % | |||||||||||||||||||||||
| Percent of sales | 23.9 | % | 23.1 | % | 26.5 | % | |||||||||||||||||||||||
Year 2023 results:
Sales in MedSurg were up 1.0 percent in U.S. dollars.
On an organic basis:
•Growth of 1.6% was driven by price partially offset by lower volume. Volume declines from our microfluidics and hand hygiene product lines, which benefited from higher sales during the pandemic, negatively impacted growth by 1.1%.
Business segment operating income margin increased when compared to the same period last year. The increase was driven by spending discipline and price partially offset by material inflation.
Year 2022 results:
Sales in MedSurg were down 1.0 percent in U.S. dollars.
On an organic basis:
•Growth of 2.7% benefited from a continued modest recovery in total surgical procedure volumes. Surgical procedure recovery to pre-pandemic levels continued to be impacted by hospital system challenges related to staffing, labor and budget constraints.
•Declines in our medical technology OEM, immobilization solutions and advanced skin care products negatively impacted growth by 0.2% as compared to the prior year.
153
Business segment operating income margin decreased year-on-year. Material, labor inflation, and lower productivity drove higher costs of product. Higher costs were partially offset by increased price and spending discipline.
Dental Solutions (16.2% of combined sales for the year ended December 31, 2023)
| 2023 | 2022 | 2021 | |||||||||||||||||||||||||||
| Sales (millions) | $ | 1,329 | $ | 1,327 | $ | 1,396 | |||||||||||||||||||||||
| Sales change analysis: | |||||||||||||||||||||||||||||
| Organic sales | 1.6 | % | (0.1) | % | 30.4 | % | |||||||||||||||||||||||
| Divestitures | (1.0) | — | — | ||||||||||||||||||||||||||
| Translation | (0.4) | (4.8) | 2.6 | ||||||||||||||||||||||||||
| Total sales change | 0.2 | % | (4.9) | % | 33.0 | % | |||||||||||||||||||||||
| Business segment operating income (millions) | $ | 442 | $ | 437 | $ | 482 | |||||||||||||||||||||||
| Percent change | 1.1 | % | (9.3) | % | 106.0 | % | |||||||||||||||||||||||
| Percent of sales | 33.3 | % | 32.9 | % | 34.5 | % | |||||||||||||||||||||||
Year 2023 results:
Sales in Dental Solutions were up 0.2 percent in U.S. dollars.
On an organic basis:
•Positive volume and price growth of 3.5% from dental products, partially offset by declines in traditional orthodontic products.
•Sales growth was negatively impacted by 1.1% due to the exit of Health Care operations in Russia.
Business segment operating income margin increased slightly when compared to the same period last year as price increases offset the cost of material inflation.
Year 2022 results:
Sales in Dental Solutions were down 4.9 percent in U.S. dollars.
On an organic basis:
•Growth was driven by a price increase of 3.9% as volumes declined due to COVID related shutdowns within China and distributor inventory rebalancing.
•Sales growth was negatively impacted by 2.4% due to the exit of Health Care operations in Russia.
Business segment operating income margin decreased year-on-year, driven by volume headwinds, material inflation and lower productivity due to labor shortages offsetting benefits from pricing, product sales mix, and spending discipline.
154
Health Information Systems (15.7% of combined sales for the year ended December 31, 2023)
| 2023 | 2022 | 2021 | |||||||||||||||||||||
| Sales (millions) | $ | 1,285 | $ | 1,227 | $ | 1,160 | |||||||||||||||||
| Sales change analysis: | |||||||||||||||||||||||
| Organic sales | 4.7 | % | 6.6 | % | 7.9 | % | |||||||||||||||||
| Translation | — | (0.8) | 0.4 | ||||||||||||||||||||
| Total sales change | 4.7 | % | 5.8 | % | 8.3 | % | |||||||||||||||||
| Business segment operating income (millions) | $ | 423 | $ | 359 | $ | 354 | |||||||||||||||||
| Percent change | 17.8 | % | 1.4 | % | 18.0 | % | |||||||||||||||||
| Percent of sales | 32.9 | % | 29.3 | % | 30.5 | % | |||||||||||||||||
Year 2023 results:
Sales in Health Information Systems were up 4.7 percent in U.S. dollars.
On an organic sales basis:
•Sales growth was broadly driven across the portfolio, including revenue cycle management, performance management and clinician productivity solutions. Growth was driven by both new customers and product upgrades at existing customers.
•Sales growth was negatively impacted by delays to customers’ investments in IT which were driven by ongoing stress on hospital budgets.
Business segment operating income margin increased when compared to the same period last year driven by both price increases and lower spending, partially offset by wage inflation. Volume growth into higher margin products drove mix benefit.
Year 2022 results:
Sales in Health Information Systems were up 5.8 percent in U.S. dollars.
On an organic sales basis:
•Sales growth was driven by new customers in our hospital accounts, U.S. federal markets and sales of additional solutions, primarily outpatient and professional computer-assisted coding modules, to our existing customer accounts.
•Sales growth was negatively impacted by delays to customer’s investments in IT which were driven by pressure on hospital margins due to higher labor and supply chain costs.
Business segment operating income margin decreased year-on-year primarily due to focused investments which helped drive a pipeline of new product launches, partially offset by product mix benefits driven by product upgrades at existing customers.
155
Purification and Filtration (11.6% of combined sales for the year ended December 31,)
| 2023 | 2022 | 2021 | |||||||||||||||||||||||||||
| Sales (millions) | $ | 951 | $ | 991 | $ | 983 | |||||||||||||||||||||||
| Sales change analysis: | |||||||||||||||||||||||||||||
| Organic sales | (3.6) | % | 7.1 | % | 10.7 | % | |||||||||||||||||||||||
| Translation | (0.4) | (6.3) | 2.2 | ||||||||||||||||||||||||||
| Total sales change | (4.0) | % | 0.8 | % | 12.9 | % | |||||||||||||||||||||||
| Business segment operating income (millions) | $ | 162 | $ | 177 | $ | 229 | |||||||||||||||||||||||
| Percent change | (8.5) | % | (22.7) | % | 8.0 | % | |||||||||||||||||||||||
| Percent of sales | 17.0 | % | 17.9 | % | 23.3 | % | |||||||||||||||||||||||
Year 2023 results:
Sales in Purification and Filtration were down 4.0 percent in U.S. dollars.
On an organic basis:
•Sales growth was primarily impacted by inventory rebalancing at our bioprocessing filtration customers, which reduced sales by 5.4%. This decline was partially offset by growth in our separation products.
Business segment operating income margin decreased due primarily to the negative impact of product mix from lower bioprocessing filtration sales.
Year 2022 results:
Sales in Purification and Filtration were up 0.8 percent in U.S. dollars.
On an organic basis:
•Sales increased in products focused within our priority market, bioprocessing filtration, by 7.9%; however, growth moderated as compared to the prior year.
•Sales of our membrane OEM products grew 18.6%, driven by strong growth in demand within several end markets including electronics, food & beverage, dialysis and recovering oxygenation.
Business segment operating income margin decreased compared to 2021. The decrease was driven by material inflation, raw material shortages and lower productivity driven by labor constraints.
PERFORMANCE BY GEOGRAPHIC AREA
While the Health Care Business manages its businesses globally and believes its business segment results are the most relevant measure of performance, the Health Care Business also utilizes geographic area data as a secondary performance measure. Sales are generally reported within the geographic area that originated the invoice to the Health Care Business customer. Additional geographic financial information related to Health Care Business’s operations is provided in Note 14 to the audited combined financial statements.
Refer to the “Overview” section for a summary of net sales by geographic area and business segment.
156
Geographic Area Supplemental Information
| Employees as of December 31, | Capital Spending | Property, Plant and Equipment - net as of December 31, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (Millions, except Employees) | 2023 | 2022 | 2021 | 2023 | 2022 | 2021 | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| United States | 10,906 | 9,850 | 10,003 | $ | 160 | $ | 144 | $ | 142 | $ | 770 | $ | 718 | $ | 702 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| International | 11,101 | 10,248 | 10,012 | 130 | 107 | 135 | 687 | 601 | 604 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total Company | 22,007 | 20,098 | 20,015 | $ | 290 | $ | 251 | $ | 277 | $ | 1,457 | $ | 1,319 | $ | 1,306 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Employment:
Employment increased in 2023 when compared to 2022 and remained consistent in 2022 when compared to 2021. The above table includes the impact of acquisitions and other actions.
Capital Spending/Net Property, Plant and Equipment:
Investments in property, plant and equipment enable growth across many diverse markets, helping to meet product demand and increasing manufacturing efficiency. The Health Care Business is increasing its investment in manufacturing and sourcing capability in order to more closely align its product capability with its sales in major geographic areas in order to best serve its customers throughout the world with proprietary, automated, efficient, safe and sustainable processes. Capital spending is discussed in more detail below in the section entitled “—Cash Flows from Investing Activities.”
NON-GAAP FINANCIAL MEASURES
We use non-GAAP financial measures to supplement the financial measures prepared in accordance with U.S. GAAP. These include (1) Adjusted operating income, and Adjusted operating income margin, and (2) Free cash flow. Management believes that these non-GAAP financial measures, together with the measures used by management, reflect how we measure our business internally and set operational goals. In particular, we believe that these non-GAAP financial measures are useful in evaluating current performance and focusing management on our underlying operational results.
There are limitations to the use of the non-GAAP financial measures presented in this information statement. These non-GAAP financial measures are not prepared in accordance with U.S. GAAP nor do they have any standardized meaning under U.S. GAAP. In addition, other companies may use similarly titled non-GAAP financial measures that are calculated differently from the way we calculate such measures. Accordingly, our non-GAAP financial measures may not be comparable to such similarly titled non-GAAP financial measures used by other companies. We caution you not to place undue reliance on these non-GAAP financial measures, but instead to consider them with the most directly comparable U.S. GAAP measure. These non-GAAP financial measures have limitations as analytical tools and should not be considered in isolation. These non-GAAP financial measures should
157
be considered supplements to, not substitutes for, or superior to, the corresponding financial measures calculated in accordance with U.S. GAAP.
| Pro Forma | Historical | |||||||||||||||||||||||||||||||||||||||||||||||||
Year ended December 31, | Years ended December 31, | |||||||||||||||||||||||||||||||||||||||||||||||||
| ($ in millions) | 2023 | 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||||||||||||||||||
| Net sales | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Sales of product | $ | 6,340 | $ | 6,296 | $ | 6,300 | $ | 6,398 | ||||||||||||||||||||||||||||||||||||||||||
| Sales of software and rentals | 1,901 | 1,901 | 1,830 | 1,773 | ||||||||||||||||||||||||||||||||||||||||||||||
| Total net sales | 8,241 | 8,197 | 8,130 | 8,171 | ||||||||||||||||||||||||||||||||||||||||||||||
| Operating expenses | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs of product | 3,200 | 3,023 | 2,953 | 2,773 | ||||||||||||||||||||||||||||||||||||||||||||||
| Costs of software and rentals | 481 | 481 | 482 | 475 | ||||||||||||||||||||||||||||||||||||||||||||||
| Selling, general and administrative | 2,321 | 2,243 | 2,235 | 2,278 | ||||||||||||||||||||||||||||||||||||||||||||||
| Research and development | 759 | 758 | 767 | 766 | ||||||||||||||||||||||||||||||||||||||||||||||
| Total operating expenses | $ | 6,761 | $ | 6,505 | $ | 6,437 | $ | 6,292 | ||||||||||||||||||||||||||||||||||||||||||
| Operating income | 1,480 | 1,692 | 1,693 | 1,879 | ||||||||||||||||||||||||||||||||||||||||||||||
| Other expense (income) – net | 570 | 25 | 1 | (3) | ||||||||||||||||||||||||||||||||||||||||||||||
| Income before income taxes | 910 | 1,667 | 1,692 | 1,882 | ||||||||||||||||||||||||||||||||||||||||||||||
| Provision for income taxes | 218 | 321 | 349 | 422 | ||||||||||||||||||||||||||||||||||||||||||||||
| Net income | $ | 692 | $ | 1,346 | $ | 1,343 | $ | 1,460 | ||||||||||||||||||||||||||||||||||||||||||
| Cash from (used for) operating activities | $ | 1,915 | $ | 1,679 | $ | 2,202 | ||||||||||||||||||||||||||||||||||||||||||||
Other data(a): | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Adjusted operating income* | $ | 1,990 | $ | 2,072 | $ | 2,080 | $ | 2,273 | ||||||||||||||||||||||||||||||||||||||||||
| Free cash flow* | $ | 1,625 | $ | 1,428 | $ | 1,925 | ||||||||||||||||||||||||||||||||||||||||||||
__________________
(a)In addition to reporting financial results in accordance with U.S. GAAP, the Health Care Business also provides non-GAAP measures that we use, and plan to continue using, when monitoring and evaluating operating performance and measuring cash available to invest in our business. The adjusted measures are not in accordance with, nor are they a substitute for, GAAP measures. The non-GAAP financial measures presented in this information statement are supplemental measures of our performance and our liquidity that we believe help investors understand our underlying business performance and the Company uses these measures as an indication of the strength of the Company and its ability to generate cash.
*Non-GAAP financial measure.
The tables below reconcile our non-GAAP financial measures to the nearest financial measure that is in accordance with U.S. GAAP for the periods presented
Adjusted Operating Income, and Adjusted Operating Income Margin*
Adjusted operating income and Adjusted operating income margin are not defined under U.S. GAAP. Therefore, they should not be considered a substitute for earnings data prepared in accordance with U.S. GAAP and may not be comparable to similarly titled measures used by other companies. The Health Care Business defines adjusted operating income as operating income excluding the effects of amortization, restructuring costs, spin-off and separation-related costs, Russia exit costs, and gains related to the sale of businesses. Adjusted operating income margin is Adjusted operating income divided by the U.S GAAP measure total net sales for the same period. The Company believes Adjusted operating income and Adjusted operating income margin provide investors with visibility into the Company's unleveraged, pre-tax operating results and reflects underlying financial performance. However, Adjusted operating income should not be construed as inferring that the Company’s future results will be unaffected by the items for which the measure adjusts.
158
Refer to the preceding “Results of Operations” section for discussion of items that impacted the operating income component of the calculation of Adjusted operating income.
| Pro Forma | Historical | |||||||||||||||||||||||||||||||||||||||||||
| Year ended December 31 | Years ended December 31 | |||||||||||||||||||||||||||||||||||||||||||
| ($ in millions) | 2023 | 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||||||||||||
| Operating income | 1,480 | 1,692 | 1,693 | 1,879 | ||||||||||||||||||||||||||||||||||||||||
| Non-GAAP adjustments: | ||||||||||||||||||||||||||||||||||||||||||||
| Add: Amortization of acquisition-related intangible assets | 365 | 365 | 373 | 381 | ||||||||||||||||||||||||||||||||||||||||
Add: Restructuring costs(a) | 51 | 51 | 6 | 13 | ||||||||||||||||||||||||||||||||||||||||
Add: Spin-off and separation-related costs(b) | 94 | 20 | — | — | ||||||||||||||||||||||||||||||||||||||||
Add: Russia exit costs(c) | — | — | 8 | — | ||||||||||||||||||||||||||||||||||||||||
Less: Gain on business divestitures(d) | — | (56) | — | — | ||||||||||||||||||||||||||||||||||||||||
| Adjusted operating income* | $ | 1,990 | $ | 2,072 | $ | 2,080 | $ | 2,273 | ||||||||||||||||||||||||||||||||||||
| Operating income margin (U.S. GAAP) | 18.0 | % | 20.6 | % | 20.8 | % | 23.0 | % | ||||||||||||||||||||||||||||||||||||
| Adjusted operating income margin* | 24.1 | % | 25.3 | % | 25.6 | % | 27.8 | % | ||||||||||||||||||||||||||||||||||||
__________________
(a)Consists of severance associated with restructuring programs.
(b)Costs incurred in the spin-off and separation from 3M including transition support, advisory fees, and other transaction-related costs.
(c)Charge related to impairment of net assets in Russia in connection with 3M Company's committed exit and disposal plan.
(d)Gains related to the sale of businesses.
*Non-GAAP financial measure.
Free Cash Flow*
Free cash flow is not defined under U.S. GAAP. Therefore, it should not be considered a substitute for income or cash flow data prepared in accordance with U.S. GAAP and may not be comparable to similarly titled measures used by other companies. The Company defines free cash flow as net cash provided by operating activities less purchases of property, plant and equipment. It should not be inferred that the entire free cash flow amount is available for discretionary expenditures. The Company believes free cash flow is meaningful to investors as it is a useful measure of liquidity and the Company uses these measures as an indication of the strength of the Company and its ability to generate cash. Free cash flow varies across quarters throughout the year. Below find a recap of free cash flow.
Refer to the subsequent “Cash Flows from Operating Activities” and “Cash Flows from Investing Activities” sections for discussion of items that impacted the operating cash flow and purchases of property, plant and equipment components of the calculation of free cash flow.
| Years ended December 31, (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||
| Major GAAP Cash Flow Categories | ||||||||||||||||||||||||||||||||
| Net cash provided by operating activities | $ | 1,915 | $ | 1,679 | $ | 2,202 | ||||||||||||||||||||||||||
| Net cash provided by (used in) investing activities | (230) | (253) | (278) | |||||||||||||||||||||||||||||
| Net cash used in financing activities | (1,552) | (1,460) | (1,960) | |||||||||||||||||||||||||||||
| Free Cash Flow* | ||||||||||||||||||||||||||||||||
| Net cash provided by operating activities | $ | 1,915 | $ | 1,679 | $ | 2,202 | ||||||||||||||||||||||||||
| Purchases of property, plant and equipment | (290) | (251) | (277) | |||||||||||||||||||||||||||||
| Free cash flow* | $ | 1,625 | $ | 1,428 | $ | 1,925 | ||||||||||||||||||||||||||
__________________
*Non-GAAP financial measure.
159
FINANCIAL CONDITION AND LIQUIDITY
The strength and stability of Health Care Business’s operating model and strong free cash flow capability, provides financial flexibility and enables the Company to invest through business cycles. Investing in the Health Care business to drive organic growth and operating margins and deliver strong cash flow remains a top priority for the business. The Company is also focused on accelerating growth through strategic acquisitions.
Historically, the Health Care Business has generated positive operating cash flows. A significant majority of such cash flows were transferred to 3M Company as part of 3M’s cash pooling arrangements, the effect of which is presented as net parent investment in our audited combined financial statements included elsewhere in this information statement.
Upon completion of this separation and distribution, we will cease participation in 3M cash pooling arrangement and our cash and cash equivalents will be held and used solely for our own operations. Our capital structure, long-term commitments and sources of liquidity will change significantly from historical practices. For additional detail regarding changes to our capital structure, see section entitled “Description of Material Indebtedness” below. Our cash balance on the date of completion of this separation and distribution is expected to be approximately $600 million.
On February 16, 2024, the Company entered into a five-year $2,000 million unsecured revolving credit facility expiring in 2029, an 18-month unsecured term loan facility of $500 million and a three-year unsecured term loan facility of $1,000 million (together the “Facilities”). The Company intends to use the Facilities for general corporate purposes. There are no amounts outstanding under the Facilities.
Cash Flows
Cash flows from operating, investing and financing activities are provided in the tables that follow. Individual amounts in the audited combined statements of cash flows exclude the effect of exchange rate impacts on cash and cash equivalents, which are presented separately in the cash flows. Thus, the amounts presented in the following operating, investing and financing activities tables reflect changes in balances from period to period adjusted for these effects.
Cash Flows from Operating Activities:
| Years ended December 31, (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||||||||
| Net income | $ | 1,346 | $ | 1,343 | $ | 1,460 | ||||||||||||||||||||||||||||||||
| Depreciation and amortization | 561 | 578 | 597 | |||||||||||||||||||||||||||||||||||
| Postretirement benefit plan expense | 41 | 64 | 74 | |||||||||||||||||||||||||||||||||||
| Stock-based compensation expense | 39 | 37 | 38 | |||||||||||||||||||||||||||||||||||
| Gain on business divestitures | (56) | — | — | |||||||||||||||||||||||||||||||||||
| Deferred income taxes | (142) | (141) | (34) | |||||||||||||||||||||||||||||||||||
| Accounts receivable | (129) | (32) | (1) | |||||||||||||||||||||||||||||||||||
| Inventories | 23 | (82) | (136) | |||||||||||||||||||||||||||||||||||
| Accounts payable | 105 | 25 | 68 | |||||||||||||||||||||||||||||||||||
| Accrued expenses and other current liabilities | 137 | (101) | 73 | |||||||||||||||||||||||||||||||||||
| Other — net | (10) | (12) | 63 | |||||||||||||||||||||||||||||||||||
| Net cash provided by operating activities | $ | 1,915 | $ | 1,679 | $ | 2,202 | ||||||||||||||||||||||||||||||||
In 2023, cash flows provided by operating activities increased compared to 2022 primarily due to decreases in inventories, increases in accounts payable, and higher year over year accrued compensation, partially offset by increases in accounts receivables. The lower cash outflows from inventory is driven by supply chain stabilization.
In 2022, cash flows provided by operating activities decreased compared to 2021, with this decrease primarily due to lower net income and year over year changes in deferred income taxes, inventories and accrued expenses and
160
other current liabilities. Net working capital, the combination of accounts receivable, inventories and accounts payable, decreased operating cash flow by $89 million in 2022, primarily driven by higher inventory as the Company's business continued to recover from supply chain disruptions caused by the global pandemic.
Cash Flows from Investing Activities:
| Years ended December 31, (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||||||||||||||
| Purchases of property, plant and equipment (PP&E) | $ | (290) | $ | (251) | $ | (277) | ||||||||||||||||||||||||||||||||||||||
| Proceeds from sale of businesses | 60 | — | — | |||||||||||||||||||||||||||||||||||||||||
| Other — net | — | (2) | (1) | |||||||||||||||||||||||||||||||||||||||||
| Net cash provided by (used in) investing activities | $ | (230) | $ | (253) | $ | (278) | ||||||||||||||||||||||||||||||||||||||
Overall PP&E spending increased in 2023 as compared to 2022 as the Company continues to invest in growth, productivity and sustainability. Proceeds from sale of businesses include the sale of assets associated with the Company’s dental local anesthetic business.
Investments in property, plant and equipment enable growth across many diverse markets, helping to meet product demand and increasing manufacturing efficiency. Overall spending in PP&E decreased slightly from 2021 to 2022 as the Company reduced investments in response to slowing growth.
The Health Care Business invests in renewal and maintenance programs, which pertain to cost reduction, cycle time, maintaining and renewing current capacity, eliminating pollution, and compliance. Costs related to maintenance, ordinary repairs, and certain other items are expensed. The Company also invests in growth, which adds to capacity, driven by new products, both through expansion of current facilities and new facilities. Finally, the Company also invests in other initiatives, such as information technology, laboratory facilities, and a continued focus on investments in sustainability.
Cash Flows from Financing Activities:
| Years ended December 31, (Millions) | 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||||
| Net transfers to 3M | $ | (1,553) | $ | (1,456) | $ | (1,947) | |||||||||||||||||||||||||||||
| Other — net | 1 | (4) | (13) | ||||||||||||||||||||||||||||||||
| Net cash used in financing activities | $ | (1,552) | $ | (1,460) | $ | (1,960) | |||||||||||||||||||||||||||||
Financing cash outflows increased in 2023 due to higher net transfers to 3M.
Financing cash outflows decreased in 2022 due to lower net transfers to 3M.
Material Cash Requirement from Known Contractual and Other Obligations:
Health Care Business’s material cash requirements from known contractual and other obligations primarily relate to following, for which information on both a short-term and long-term basis is provided in the indicated notes to the audited combined financial statements:
•Tax obligations—Refer to Note 9 to the audited combined financial statements.
•Commitments and contingencies—Refer to Note 11 to the audited combined financial statements.
•Operating leases—Refer to Note 12 to the audited combined financial statements.
The Health Care Business purchases the majority of its materials and services as needed, with no unconditional commitments. In limited circumstances, in the normal course of business, the Company enters into unconditional purchase obligations with various vendors that may take the form of, for example, take or pay contracts in which the
161
Company guarantees payment to ensure availability of certain materials or services or to ensure ongoing efforts on capital projects. The Company expects to receive underlying materials or services for these purchase obligations. To the extent the limited amount of these purchase obligations fluctuates, it largely trends with normal-course changes in regular operating activities. Additionally, contractual capital commitments represent a small part of the Company’s expected capital spending.
162
CRITICAL ACCOUNTING ESTIMATES
Information regarding significant accounting policies is included in Note 2 to the audited combined financial statements. As stated in Note 2 to the audited combined financial statements, the preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make certain estimates and assumptions. Such estimates and assumptions are subject to inherent uncertainties which may result in actual amounts differing from these estimates.
The Company considers the items below to be critical accounting estimates. Critical accounting estimates are those estimates made in accordance with generally accepted accounting principles that involve a significant level of estimation uncertainty and have had or are reasonably likely to have a material impact on the financial condition or results of operations of the Company.
Legal Proceedings:
Assessments of lawsuits and claims can involve a series of complex judgments about future events, the outcomes of which are inherently uncertain, and can rely heavily on estimates and assumptions. The Company accrues an estimated liability for legal proceeding claims that are both probable and reasonably estimable in accordance with Accounting Standard Codification (ASC) 450, Contingencies. Please refer to the section entitled “Process for Disclosure and Recording of Liabilities Related to Legal Proceedings” (contained in “Legal Proceedings” in Note 11 to the audited combined financial statements) for additional information about such estimates.
Goodwill:
The Company makes certain estimates and judgments in impairment assessments of goodwill. As of December 31, 2023, the Health Care Business goodwill totaled approximately $6.5 billion. Goodwill is tested for impairment annually in the fourth quarter of each year, as further discussed below, and is tested between annual tests if an event occurs or circumstances change that would indicate the carrying amount may be impaired. If future non-cash asset impairment charges are taken, the Health Care Business would expect that only a portion of the goodwill would be impaired.
Impairment testing for goodwill is done at a reporting unit level, with all goodwill assigned to a reporting unit. The Health Care Business reporting units correspond to a business segment as this represents the lowest level of discrete financial information below sales that is available. The Health Care Business did not combine any of its reporting units for impairment testing. An impairment loss would be recognized when the carrying amount of the reporting unit’s net assets exceeds the estimated fair value of the reporting unit, and the loss would equal that difference. The estimated fair value of a reporting unit is determined based on a market approach using comparable company information such as EBITDA (earnings before interest, taxes, depreciation and amortization) multiples. The Company also performs a discounted cash flow analysis for certain reporting units where the market approach indicates additional review is warranted. A discounted cash flow analysis involves key assumptions including projected sales, EBITDA margins, capital expenditures, and discount rates. Changes in reporting unit earnings, comparable company information, and expected future cash flows, as well as underlying market and overall economic conditions, among other factors, make these estimates subject to uncertainty.
Based on the annual tests in the fourth quarter of 2023, 2022, and 2021, no goodwill impairment was indicated for any of the reporting units. Further, there were no events or changes in circumstances during the year to date period ending December 31, 2023 that would indicate the carrying amount may be impaired. As of December 31, 2023, the Company had four reporting units, with the MedSurg reporting unit accounting for approximately 56 percent of the goodwill. The Health Care Business will continue to monitor its reporting units and asset groups for any triggering events or other indicators of impairment.
Uncertainty in Income Tax Positions:
The extent of the Health Care Business’s operations involves dealing with uncertainties and judgments in the application of complex tax regulations in a multitude of jurisdictions. The final taxes paid are dependent upon many
163
factors, including negotiations with taxing authorities in various jurisdictions and resolution of disputes arising from federal, state, and international tax audits. The Company recognizes potential liabilities and records tax liabilities for anticipated tax audit issues in the United States and other tax jurisdictions based on its estimate of whether, and the extent to which, additional taxes will be due. The Company follows the guidance provided by ASC 740, Income Taxes to record these liabilities (refer to Note 9 to the audited combined financial statements for additional information). The accrual for tax positions for which the Company is not legally liable is charged to “Net parent investment” on the audited combined balance sheets.
The Company adjusts these reserves in light of changing facts and circumstances. Due to the complexity of some of these uncertainties, the ultimate resolution may result in a payment that is materially different from the Company’s current estimate of the tax liabilities. If the Company’s estimates of the tax liabilities prove to be less than the ultimate assessments, an additional charge to income tax expense would result. If payments of these amounts ultimately prove to be less than the recorded amounts, the reversal of the liabilities would result in tax benefits being recognized in the period when the Company determines the liabilities are no longer necessary.
NEW ACCOUNTING PRONOUNCEMENTS
Information regarding new accounting pronouncements is included in Note 3 to the Health Care Business's audited combined annual financial statements included elsewhere in this information statement.
Quantitative and Qualitative Disclosures About Market Risk
Foreign Currency Exchange Rate Risks:
The Health Care Business faces transactional exchange rate risk from transactions with customers in countries outside the United States and from intercompany transactions between 3M affiliates. Foreign currency exchange rates and fluctuations in those rates may cause fluctuations in cash flows related to foreign denominated transactions. The Health Care Business is also exposed to the translation of foreign currency earnings to the U.S. dollar.
3M enters into foreign exchange forward and option contracts to hedge against the effect of exchange rate fluctuations on cash flows denominated in foreign currencies. As part of 3M, the Health Care Business indirectly benefits from the Parent’s hedging activities and has been allocated a proportion of the realized gains or losses on these contracts. However, the benefits and costs realized from these contracts may not reflect the benefits and costs the Health Care Business would have incurred as a standalone company for the periods presented.
Concentration of Credit Risk:
The Health Care Business’s sales are not materially dependent on any single customer. For the annual periods presented, no one individual customer or group of affiliated customers represented more than 10 percent of the Health Care Business's total sales. At December 31, 2023, one customer accounted for approximately 10 percent of the Health Care Business’s total receivables, while no customers represented more than 10 percent at December 31, 2022. Credit risk associated with the Health Care Business’s receivables is representative of the geographic, industry, and customer diversity associated with the global businesses.
Commodity Prices Risk:
The Health Care Business manages commodity price risks through negotiated supply contracts and price protection agreements. The Health Care Business does not participate in material hedging activity.
164
MANAGEMENT
Executive Officers Following the Distribution
The following table sets forth information (as of the date of this information statement) regarding the individuals who are currently expected to serve as executive officers of Solventum following the distribution.
| Name | Age | Position | ||||||||||||
| Bryan Hanson | 57 | Chief Executive Officer and Director Nominee | ||||||||||||
| Wayde McMillan | 54 | Chief Financial Officer | ||||||||||||
| Marcela Kirberger | 57 | Chief Legal Affairs Officer | ||||||||||||
The following are brief biographies describing the backgrounds of our executive officers following the distribution.
Bryan Hanson. Mr. Hanson has been the Chief Executive Officer of 3M’s Health Care Business Group since September 2023 and will be appointed as our Chief Executive Officer in connection with the distribution. Previously, Mr. Hanson served as Chairman of the Board of Directors of Zimmer Biomet, a global medical technology company with annual revenues over $7 billion, from May 2021 to August 2023, and as President and Chief Executive Officer and a member of the Board of Zimmer Biomet from December 2017 to August 2023. Previously, Mr. Hanson served as Executive Vice President and President, Minimally Invasive Therapies Group of Medtronic plc, a medical device company, from January 2015 until joining Zimmer Biomet in December 2017. Prior to that, he was Senior Vice President and Group President of Covidien plc, a medical device company, from October 2014 to January 2015; Senior Vice President and Group President, Medical Devices and United States of Covidien from October 2013 to September 2014; Senior Vice President and Group President of Covidien for the Surgical Solutions business from July 2011 to October 2013; and President of Covidien’s Energy-based Devices business from July 2006 to June 2011. Mr. Hanson held several other positions of increasing responsibility in sales, marketing and general management with Covidien from October 1992 to July 2006. Mr. Hanson holds a Bachelor of Science degree in Finance from Florida State University. He also completed the Kellogg School of Management Finance for Executives program in 2010 and the Harvard Executive Education in Leadership program in 2013.
Wayde McMillan. Mr. McMillan has been the Chief Financial Officer of 3M’s Health Care Business Group since November 2023 and will be appointed as our Chief Financial Officer in connection with the distribution. Previously, Mr. McMillan served as Executive Vice President, Chief Financial Officer and Treasurer of Insulet, a medical device company, from March 2019 to November 2023. From January 2015 to February 2019, he was Chief Financial Officer and Vice President of Finance of the Minimally Invasive Therapies Group at Medtronic plc., a medical device company. From November 2006 to January 2015, prior to Medtronic’s acquisition of Covidien plc, a medical device company, Mr. McMillan held a variety of leadership positions at Covidien, including Chief Financial Officer and Vice President of Finance of the Medical Devices Group & U.S., Chief Financial Officer and Vice President of Finance of the Surgical Solutions Business Unit, and Vice President Finance and Controller of the Respiratory and Monitoring Solutions Business Unit. Mr. McMillan started his career in accounting, audit, financial analysis and investor relations positions at various institutions. Mr. McMillan earned his Bachelor of Science in Business Administration from Merrimack College and an MBA from Bentley University McCallum Graduate School of Business.
Marcela Kirberger. Ms. Kirberger has been Chief Legal Affairs Officer of 3M’s Health Care Business Group since November 2023 and will be appointed as our Chief Legal Affairs Officer in connection with the distribution. Previously, Ms. Kirberger was Executive Vice President, General Counsel and Corporate Secretary at Elanco, a pharmaceutical company in the animal health field, from June 2021 to November 2023, where she was responsible for the global strategy and operations of the legal function and had responsibility for Corporate Affairs, Ethics & Compliance and ESG. Prior to joining Elanco, Ms. Kirberger held U.S., regional and global leadership roles within Fortune 500 life sciences companies, including as General Counsel and Corporate Secretary at Roche Diagnostics NA, a diagnostics company, from 2019 to 2021; and General Counsel and Chief Compliance Officer at Leica Microsystems GmbH, a global medical device manufacturer and subsidiary of Danaher Corporation, from 2017 to 2019. She also worked for the Novartis Group of Companies from 2006 to 2017 in different Novartis companies,
165
including Gerber Products, Novartis Consumer Health, Novartis Pharmaceuticals and Sandoz International, where she was Global Head of Legal for Biopharma and before that, Global Chief Compliance Officer. Ms. Kirberger began her career as a securities litigator at Lowenstein Sandler in New Jersey. A native of Argentina, she earned her law degrees from Rutgers School of Law in Newark, New Jersey and the Catholic University of Argentina in Buenos Aires.
166
DIRECTORS
Board of Directors Following the Distribution
The following table sets forth information regarding those persons who are expected, as of the date of this information statement, to serve on Solventum’s Board of Directors following completion of the distribution and until their respective successors are duly elected and qualified. The nominees will be presented to Solventum’s sole stockholder, 3M, for election prior to the distribution.
| Name | Age | Position | ||||||||||||
Carlos Albán | 61 | Director Nominee | ||||||||||||
Carrie S. Cox | 66 | Director Nominee and Chairman of the Board of Directors | ||||||||||||
Susan D. DeVore | 65 | Director Nominee | ||||||||||||
Glenn A. Eisenberg | 62 | Director Nominee | ||||||||||||
Bryan Hanson | 57 | Chief Executive Officer and Director Nominee | ||||||||||||
Dr. Bernard A. Harris Jr. | 67 | Director Nominee | ||||||||||||
Karen J. May . | 65 | Director Nominee | ||||||||||||
Elizabeth A. Mily | 56 | Director Nominee | ||||||||||||
John H. Weiland | 68 | Director Nominee | ||||||||||||
Amy A. Wendell | 62 | Director Nominee | ||||||||||||
Darryl L. Wilson | 60 | Director Nominee | ||||||||||||
Carlos Albán. Mr. Albán will be appointed to our board of directors in connection with the distribution. Mr. Albán is a seasoned pharmaceutical executive with over 30 years of experience in global commercial strategy and operations. From December 2018 until March 2021, Mr. Albán served as Vice Chairman, Chief Commercial Officer of AbbVie, Inc., responsible for global commercial operations, including the Pharmacyclics commercial functions. From January 2013 until December 2018, he served as AbbVie’s Executive Vice President, Commercial Operations. From February 2011 until December 2012, Mr. Albán served as Senior Vice President, Proprietary Pharmaceutical Products, Global Commercial Operations for Abbott Laboratories, Inc., and previously served at Abbott as Senior Vice President, International Pharmaceuticals from 2009 to 2011, as Vice President, Pharmaceuticals, Western Europe and Canada Operations from 2007 to 2009, as Vice President, Pharmaceuticals, European Operations from 2006 to 2007, as Regional Director for the Northern Europe area of the international pharmaceutical business from 2004 to 2006, and General Manager, Portugal from 2002 to 2004. Mr. Albán currently serves on the board of SpringWorks Therapeutics, Inc., a commercial-stage biopharmaceutical company. Mr. Albán received a degree in economics from Pontificia Universidad Javeriana in Bogotá, Colombia. We believe Mr. Albán is well-qualified to serve on our board of directors because of his significant leadership experience and business expertise.
Carrie S. Cox. Ms. Cox will be appointed our chairman of the board of directors in connection with the distribution. Ms. Cox most recently served as the Chief Executive Officer of Humacyte, Inc., a then privately-held regenerative medicine company, between 2010 and 2018; in addition, she served as Chairman of the Humacyte’s Board of Directors between 2013 and 2019, and she remained a member of its board until 2021, prior to its public offering. Ms. Cox served as Executive Vice President of Schering-Plough Corporation and President of Schering-Plough’s global pharmaceutical business between 2003 and 2009, when Schering-Plough merged with Merck. Prior to joining Schering-Plough, Ms. Cox served as President of Pharmacia Corporation’s global pharmaceutical business from 1997 until its merger with Pfizer Inc. in 2003. She currently serves as Chairman of Organon & Co. and Cartesian Therapeutics, Inc. and as a director of Texas Instruments Inc. She served on the Board of Directors of Cardinal Health Inc. from 2009 to 2023, on the Board of Directors of Celgene Corporation from 2009 until its acquisition by Bristol-Myers Squibb Company in 2019, as Chairperson of the Board of Directors of electroCore, Inc. between 2018 and 2020, and as Chairman of the Board of Directors of Array BioPharma Inc. from 2018 until its acquisition by Pfizer Inc. in 2019. Ms. Cox is a graduate of the Massachusetts College of Pharmacy. We believe Ms. Cox is well-qualified to serve on our board of directors because she is an experienced leader with a deep understanding of health care across different sectors of the industry.
167
Susan D. DeVore. Ms. DeVore will be appointed to our board of directors in connection with the distribution. She previously served as Chief Executive Officer and a member of the Board of Directors of Premier Inc., a health care improvement company, from 2013 to May 2021, and also served as President of Premier from 2013 to April 2019. She has served in a consulting capacity for Premier since her retirement in June 2021. Ms. DeVore was President and CEO of a predecessor company, Premier Healthcare Solutions, Inc. from 2009 to 2013 prior to its reorganization, and before that was the Chief Operating Officer for a number of affiliated Premier entities from 2006 to 2009. Prior to joining Premier, Ms. DeVore had two decades of finance, strategy and healthcare consulting experience. Ms. DeVore is also a director of Elevance Health, Inc., serving since August 2021. Ms. DeVore holds an M.M. degree from McGill University and B.A. degree from the University of North Carolina at Charlotte. We believe Ms. DeVore is well-qualified to serve on our board of directors because of her extensive healthcare expertise and experience as a chief executive officer.
Glenn A. Eisenberg. Mr. Eisenberg will be appointed to our board of directors in connection with the distribution. He has served as Executive Vice President and Chief Financial Officer of Laboratory Corporation of America Holdings, a healthcare company, since June 2014. From 2002 until joining Laboratory Corporation of America Holdings, he served as the Executive Vice President of Finance and Administration and Chief Financial Officer at The Timken Company, a global manufacturer of highly engineered bearings and alloy steels and related products and services. Previously, he served as President and Chief Operating Officer of United Dominion Industries, a diversified industrial manufacturer, now a subsidiary of SPX Corporation, after working in several roles in finance, including Executive Vice President and Chief Financial Officer. Mr. Eisenberg served on the Board of Directors of US Ecology, Inc. until May 2022, where he chaired the Audit Committee; Family Dollar Stores Inc. until July 2015, where he chaired the Audit Committee; Alpha Natural Resources Inc. until May 2015, where he was the lead independent director and chaired the Nominating and Corporate Governance Committee, and Perspecta Inc. until May 2021, where he served on the Audit Committee. Mr. Eisenberg holds a Bachelor of Arts degree from Tulane University and a Master of Business Administration from Georgia State University. We believe Mr. Eisenberg is well-qualified to serve on our board of directors because of his long history as a public company executive and his strong financial and audit committee expertise.
Bryan Hanson. The biographical information of Mr. Hanson is set forth above in the section captioned “Management—Executive Officers Following the Distribution.”
Dr. Bernard A. Harris Jr. Dr. Harris will be appointed to our board of directors in connection with the distribution. He is currently chief executive officer and managing partner of Vesalius Ventures, Inc., a venture capital firm. He served as a trustee/director for Salient and Barings investment funds. He currently serves on the boards of directors of RTX Corporation, U.S. Physical Therapy and MassMutual. He also served as a board member for the National Academies of Science, Engineering and Medicine - Board on Health Sciences Policy, and is currently on the boards of the Texas Medical Center, and the Harris Foundation, which he founded. Dr. Harris is a former NASA astronaut, has logged more than 438 hours and traveled over 7.2 million miles in space. He was a mission specialist on the space shuttle Columbia in 1993 and a payload commander on space shuttle Discovery in 1995, where he became the first African American to walk in space. He earned a Bachelor of Science in Biology from the University of Houston, a Master of Medical Science from the University of Texas Medical Branch at Galveston, a Master of Business Administration from the University of Houston – Clear Lake, and a Doctor of Medicine from Texas Tech University Health Sciences Center School of Medicine. He completed a residency in internal medicine at the Mayo Clinic, a National Research Council Fellowship in endocrinology at the NASA Ames Research Center and trained as a flight surgeon at the United States Air Force School of Aerospace Medicine. He is a fellow of the American College of Physicians, and a member of the American Academy of Arts and Sciences. We believe Dr. Harris is well-qualified to serve on our board of directors because of his deep science and technology background and executive leadership experience.
Karen J. May. Ms. May will be appointed to our board of directors in connection with the distribution. She has been a member of the board of directors of Ace Hardware Corporation, where she is Chair of the Audit and Finance Committee, since 2017. Since 2019, Ms. May is also a member of the board of directors of Alcon, Inc., where she is chair of the Compensation Committee. Previously, Ms. May was on the board of directors of MB Financial, Inc., where she served as Chair of the Compensation Committee until 2019. From 2012 to 2018, she was the Executive Vice President and Chief Human Resources Officer at Mondelez International, Inc. From 2005 to
168
2012, Ms. May was the Executive Vice President and Chief Human Resources Officer of Kraft Foods, Inc. Between 1990 and 2005, she held various positions in Human Resources and Finance at Baxter International Inc., including Corporate Vice President and Chief Human Resources Officer, Vice President, International Finance, and Vice President, Division Controller. Prior to Baxter International Inc., Ms. May was a Certified Public Accountant in the audit practice of Price Waterhouse. Ms. May holds a Bachelor of Science in Accounting from the University of Illinois. We believe Ms. May is well-qualified to serve on our board of directors because of her extensive operational and human capital expertise.
Elizabeth A. Mily. Ms. Mily will be appointed to our board of directors in connection with the distribution. She has over 30 years of healthcare and financial industry experience, serving most recently as the Executive Vice President, Strategy & Business Development, at Bristol-Myers Squibb. In this role, she oversaw the company strategy and approach to sourcing external innovation, including all business development activities, strategic partnerships, alliance management, mergers and acquisitions, and the company’s broad equity investing portfolio. Elizabeth was appointed to the position in March 2020. Prior to joining Bristol-Myers Squibb, Elizabeth was a senior member of the Global Healthcare Group at Barclays, where she led its Lifesciences business, including the BioPharma, Life Science Tools and Diagnostics sectors. Prior to joining Barclays, Elizabeth served as Senior Vice President, Corporate Strategy and Development at Thermo Fisher Scientific. Before Thermo Fisher, Elizabeth spent 16 years at Goldman, Sachs & Co., where she was a Managing Director and senior coverage officer within the Healthcare Department of the Investment Banking Division. Elizabeth holds a Master of Science in Foreign Service from Georgetown University and a Bachelor of Arts in German Literature and European History from the Ohio State University. She was also a Fulbright Scholar to the Universities of Cologne and Hamburg, Germany. Prior to attending University, Elizabeth completed a Congress-Bundestag scholarship to Germany. Elizabeth previously served on the Dean’s Advisory Council of the Ohio State University - Fisher College of Business and previously served as a Trustee of the Stephen Gaynor School. We believe Ms. Mily is well-qualified to serve on our board of directors because of her extensive healthcare and financial industry background.
John H. Weiland. Mr. Weiland will be appointed to our board of directors in connection with the distribution. He has served as President and Chief Operating Officer of medical device company C. R. Bard, Inc. from 2003 until 2017, when Bard was acquired by Becton, Dickinson and Company. He also served on Bard’s board of directors from 2005 to 2017, becoming Vice Chairman of the Board in 2016. Mr. Weiland joined Bard in 1996 and held the position of Group President, with global responsibility for several Bard divisions and its worldwide manufacturing operations prior to becoming President and Chief Operating Officer. Prior to Bard, he held senior management positions at Dentsply International, American Hospital Supply, Baxter Healthcare, and Pharmacia AB. Mr. Weiland served on the board of Cardinal Health, Inc. from 2019 to 2022, where he chaired the Risk Oversight Committee. He has also served on the boards of directors of Celgene Corporation, including service on its Audit Committee, and West Pharmaceutical Services’ board of directors, including chairing its Compensation and Finance Committees. We believe Mr. Weiland is well-qualified to serve on our board of directors because of his international and healthcare business experience.
Amy A. Wendell. Ms. Wendell will be appointed to our board of directors in connection with the distribution. She was a Senior Advisor for Perella Weinberg Partner’s Healthcare Investment Banking Practice from 2016 to 2019. From 2015 to September 2018, Ms. Wendell served as a Senior Advisor for McKinsey’s Strategy and Corporate Finance Practice and also served as a member of McKinsey’s Transactions Advisory Board to help define trends in mergers and acquisitions, as well as help shape McKinsey’s knowledge agenda. From 1986 until January 2015, Ms. Wendell held various roles of increasing responsibility at Covidien plc (including its predecessors, Tyco Healthcare and Kendall Healthcare Products), including engineering, product management and business development. From December 2006 until Covidien’s acquisition by Medtronic plc in January 2015, she served as Senior Vice President of Strategy and Business Development, where she led the company’s strategy and portfolio management initiatives and managed all business development, including acquisitions, equity investments, divestitures and licensing/distribution. Ms. Wendell serves as Lead Director of AxoGen, Inc., where she is a member of the Nominating and Governance Committee and the Audit Committee; on the board of directors of Baxter International, Inc., where she is a member of the Quality, Compliance and Technology Committee and Compensation and Human Capital Committee; and on the board of directors of Hologic, Inc., where she is a member of the Audit and Finance Committee. She also serves on the board of Por Cristo, a non-profit charitable medical
169
service organization involved in health care work for at-risk women and children in Latin America. Ms. Wendell holds a Bachelor of Science in mechanical engineering from Lawrence Technological University and a Master of Science degree in biomedical engineering from the University of Illinois. We believe Ms. Wendell is well-qualified to serve on our board of directors because of her extensive business development and strategy experience in the healthcare industry.
Darryl L. Wilson. Mr. Wilson will be appointed to our board of directors in connection with the distribution. He is a strategic business executive with more than 35 years of experience in the General Electric (GE) and British Petroleum businesses around the world. Mr. Wilson was a Vice President and Corporate Officer of the General Electric Company. After leaving GE in 2017, Darryl founded The Wilson Collective, an investment and business advisory firm that advises, consults and invests in a portfolio of businesses. He serves on the NextEra Energy board of directors, audit and compensation committees and also serves on the Eaton Corporation board of directors, audit and governance Committees. He was recently appointed to the Primerica board of directors. He has served as Chairman of the Board of the Dallas Federal Reserve - Houston Branch; Houston Endowment - Finance and Investment Committees; Texas Children's Hospital - Finance and Public Policy Committees; The Kinkaid School Board of Trustees - Finance and Endowment Committees. Mr. Wilson also served on the Dallas Federal Reserve Board, Energy Advisory Committee. He held P&L leadership roles based internationally in Canada; Budapest, Hungary; London, England and Shanghai, China. He held CEO roles in Asia Pacific, Europe, Middle East and Africa for GE Consumer and Industrial. He served as President & CEO of GE Aero Derivatives Energy, a global aircraft engine technology business and also served as President & CEO of GE Consumer Products EMEA. In his final position at GE, Mr. Wilson was Vice President, Commercial in the GE Power division, leading global sales and marketing for a provider of power generation, transmission, distribution and services. Mr. Wilson joined GE in 1992, after earning an MBA in Marketing from Indiana University. He has a BA in Business Administration from Baldwin Wallace College in Ohio and spent the first six years of his career in the Executive Development Program at British Petroleum North America in Cleveland, Ohio. He has been active in many other community organizations; he is a former board member and executive committee member of the Greater Houston Partnership, The Houston Food Bank, The American Heart Association, The Dream Foundation, The East End Neighborhood Center and The Greater Cleveland Urban League. He has also served on several corporate boards representing GE affiliates, and joint ventures internationally. He was appointed by GE and the US Government to the US and Mexico Energy council. We believe Mr. Wilson is well-qualified to serve on our Board because of his advisory expertise and significant executive leadership experience.
Upon the completion of the distribution, Solventum’s amended and restated certificate of incorporation will provide that, until the annual stockholder meeting in 2028, Solventum’s Board of Directors will be divided into three classes, with each class consisting, as nearly as may be possible, of one-third of the total number of directors. The directors designated as Class I directors will have terms expiring at the first annual meeting of stockholders following the distribution, which Solventum expects to hold in 2025, and will be up for re-election at that meeting for a three-year term to expire at the 2028 annual meeting of stockholders. The directors designated as Class II directors will have terms expiring at the 2026 annual meeting of stockholders and will be up for re-election at that meeting for a two-year term to expire at the 2028 annual meeting of stockholders. The directors designated as Class III directors will have terms expiring at the 2027 annual meeting of stockholders and will be up for re-election at that meeting for a one-year term to expire at the 2028 annual meeting of stockholders. Commencing with the 2028 annual meeting of stockholders, directors will be elected annually and for a term of office to expire at the next annual meeting of stockholders, and Solventum’s Board of Directors will thereafter no longer be divided into classes. Before Solventum’s Board of Directors is declassified, it would take at least two annual meetings of stockholders to be held for any individual or group to gain control of Solventum’s Board of Directors.
Director Independence
Providing objective, independent judgment will be at the core of Solventum’s Board of Directors’ oversight function. Solventum’s “Director Independence Guidelines” will set forth certain criteria to assess the independence of directors of Solventum. Under the Director Independence Guidelines, which will conform to the corporate governance listing standards of the NYSE, a director will not be considered “independent” unless Solventum’s Board of Directors affirmatively determines that the director has no material relationship with Solventum directly or as a partner, shareholder or officer of an organization that has a relationship with Solventum. Solventum’s Board of
170
Directors is expected to affirmatively determine that a majority of the directors of Solventum will be independent under the Director Independence Guidelines.
Committees of the Board of Directors
Effective upon the completion of the distribution, Solventum’s Board of Directors will have the following committees, each of which will operate under a written charter that will be posted to Solventum’s website concurrently with, or immediately after, the distribution: the Audit Committee, the Talent Committee, the Governance Committee and the Science, Technology and Quality Committee.
Audit Committee
The Audit Committee will be established in accordance with Rule 10A-3 under the Exchange Act and the listing rules of the NYSE. The responsibilities of the Audit Committee will be more fully described in the Audit Committee charter. Solventum anticipates that these responsibilities will include:
•reviewing Solventum’s annual audited and unaudited quarterly financial statements;
•reviewing Solventum’s financial reporting process, including internal controls over financial reporting and any significant issues regarding the application of accounting principles and financial statement presentation, and critical accounting policies to be used in the consolidated financial statements;
•reviewing and discussing with management and Solventum’s registered public accounting firm (the “Independent Accounting Firm”) Solventum’s report on internal controls over financial reporting and the Independent Accounting Firm’s audit of internal controls over financial reporting;
•reviewing earnings press releases prior to issuance;
•appointing, overseeing, and approving compensation of the Independent Accounting Firm;
•reviewing with the Independent Accounting Firm the scope of the annual audit, including fees and staffing, and approving all audit and permissible non-audit services provided by the Independent Accounting Firm;
•reviewing findings and recommendations of the Independent Accounting Firm and management’s response to the recommendations of the Independent Accounting Firm;
•reviewing and approving any transaction between Solventum and any related person, which is required to be disclosed under the rules of the Securities and Exchange Commission;
•periodically reviewing Solventum’s capital allocation and capital structure strategies, insurance coverage, funding for pension and other post-retirement benefit plans, and global tax planning;
•periodically reviewing Solventum’s global treasury activities, including risks associated with cash investments, counterparties, and use of derivatives and other financial instruments for risk management purposes;
•periodically reviewing and approving Solventum’s use of the swaps exemption pursuant to the Dodd-Frank derivatives clearing policy;
•periodically reviewing the guidelines and policies that govern the process by which information and cyber security assessment and risk management are undertaken, including discussing with management any significant cybersecurity and data privacy risk exposures and management's plan to monitor and mitigate or remediate any such exposures;
•periodically obtaining reports from Solventum’s senior internal auditing executive, who will have direct reporting obligations to the Audit Committee, on the annual audit plan, scope of work, and the results of internal audits and management’s response thereto;
171
•periodically obtaining reports from Solventum’s Chief Compliance Officer, who will have direct reporting obligations to the Audit Committee, on compliance with Solventum’s Code of Conduct, and at least annually, on the implementation and effectiveness of the Solventum compliance and ethics program;
•reviewing with Solventum’s General Counsel legal matters that may have a material impact on the consolidated financial statements and any material reports or inquiries received from regulators or government agencies regarding compliance;
•providing general oversight of Solventum’s sustainability and stewardship activities and Solventum’s environmental, health and safety matters;
•reviewing Solventum’s policies and programs on sustainability; environmental and product stewardship; and environmental, health, and safety, including for compliance with all applicable laws and regulations;
•assisting Solventum’s Board of Directors in identifying and analyzing significant emerging science and technology, disruptive innovations, sustainability, materials vulnerability, and geopolitical issues that may impact Solvetum’s overall business strategy, global business continuity, and financial results;
•annually reviewing Solventum’s sustainability report;
•establishing procedures for (i) the receipt, retention, and treatment of complaints received by Solventum regarding accounting, internal accounting controls, or auditing matters; and (ii) the confidential, anonymous submission by Solventum employees of concerns regarding questionable accounting or auditing matters and periodically review with the Chief Compliance Officer and Solventum senior internal auditing executive these procedures and any significant complaints received; and
•periodically obtaining reports from senior management regarding Solventum’s performance of its obligations under the separation and distribution agreement and other spin-off related agreements, monitoring the implementation of such agreements and reviewing Solventum’s progress under such agreements.
Glenn Eisenberg, Darryl Wilson and Elizabeth Mily are expected to be the members of the Audit Committee. Mr. Eisenberg is expected to be the Audit Committee Chair. Each member of the Audit Committee is expected to be financially literate, and Solventum’s Board of Directors is expected to determine that at least one member of the Audit Committee is an “audit committee financial expert” for purposes of the rules of the SEC. In addition, Solventum expects that its Board of Directors will determine that each of the members of the Audit Committee will be independent, as defined by the rules of the NYSE, Section 10A(m)(3) of the Exchange Act, and in accordance with Solventum’s Director Independence Guidelines.
Talent Committee
The Talent Committee will have the responsibilities set forth in the charter of such committee. Solventum anticipates that these responsibilities will include:
•reviewing disclosures in Solventum’s proxy statement regarding advisory votes on executive compensation;
•approving the adoption, amendment, and termination of incentive compensation and deferred compensation programs for employees of Solventum;
•approving the adoption, amendment, or termination of equity compensation programs or, if shareholder approval would be required, recommending such actions to Solventum’s Board of Directors;
•approving, subject to ratification by the independent directors of the Solventum Board of Directors, employment agreements and severance arrangements for the Chief Executive Officer, as appropriate;
172
•approving employment agreements and severance arrangements for the senior executives of Solventum (other than the CEO), as appropriate;
•overseeing the administration of Solventum’s stock and long-term incentive compensation programs and determining the employees who receive awards and the size of their awards under such programs;
•approving the adoption and amendment of Solventum guidelines covering ownership of Solventum common stock by executives and annually reviewing compliance with these guidelines;
•reviewing and making recommendations to Solventum’s Board of Directors concerning any amendment to a retirement benefit plan that would require its approval;
•annually reviewing a risk assessment of Solventum’s compensation policies and practices for its employees;
•reviewing shareholder proposals relating to executive compensation matters and making recommendations to Solventum’s Board of Directors regarding responses;
•periodically reviewing and discussing with management matters relating to talent sourcing, diversity, and retention strategies; talent development; internal pay equity; and equal employment opportunities; and
•as needed, retaining compensation consultants, counsel, or other advisors and approving such advisors’ fees and retention terms.
Executive officers will not determine the amount or form of executive or director compensation, although Solventum’s Chief Executive Officer will provide recommendations to the Talent Committee regarding compensation changes and incentive compensation for executive officers other than himself/herself.
Karen May, Carlos Albán, Carrie Cox and Darryl Wilson are expected to be the members of the Talent Committee. Ms. May is expected to be the Chair of such committee. Solventum’s Board of Directors is expected to determine that each member of the Talent Committee will be independent, as defined by the rules of the NYSE and in accordance with Solventum’s Director Independence Guidelines. In addition, Solventum expects that the members of the Talent Committee will qualify as “non-employee directors” for purposes of Rule 16b-3 under the Exchange Act.
Governance Committee
The Governance Committee will have the responsibilities set forth in the charter of such committee. Solventum anticipates that these responsibilities will include:
•selecting and recommending director candidates to Solventum’s Board of Directors, in light of the board membership criteria to be adopted by Solventum’s Board of Directors, either to be submitted for election at the Annual Meeting or to fill any vacancies on the Board of Directors, including consideration of any shareholder nominees for director (submitted in accordance with Solventum’s amended and restated bylaws);
•reviewing and making recommendations to Solventum’s Board of Directors concerning the composition and size of Solventum’s Board of Directors and its committees, the Board Membership Criteria (see below), frequency of meetings, and changes in compensation for non-employee directors;
•reviewing Solventum’s Corporate Governance Guidelines at least annually, and recommending any proposed changes to Solventum’s Board of Directors for approval;
•developing and recommending to Solventum’s Board of Directors standards to be applied in making determinations on the types of relationships that constitute material relationships between Solventum and a director for purposes of determining director independence;
173
•discussing policies with respect to enterprise risk assessment and enterprise risk management, Solventum’s major risk exposures, and the steps management has taken to monitor and mitigate such exposures;
•developing and recommending to Solventum’s Board of Directors for its approval an annual self-assessment process of Solventum’s Board of Directors and its committees and overseeing the process;
•reviewing periodically with Solventum’s Chairman/Chief Executive Officer succession plans relating to positions held by elected corporate officers, and making recommendations to Solventum’s Board of Directors with respect to the selection of individuals to occupy these positions; and
•periodically reviewing Solventum’s positions and engagement on important public policy, social responsibility and corporate governance issues affecting its business and shareholder engagement.
Susan DeVore, Bernard Harris, Carlos Albán and Amy Wendell are expected to be the members of the Governance Committee. Ms. DeVore is expected to be the Chair of such committee. Solventum’s Board of Directors is expected to determine that each member of the Governance Committee will be independent, as defined by the rules of the NYSE and in accordance with the Director Independence Guidelines.
Science, Technology and Quality Committee
The Science, Technology and Quality Committee will have the responsibilities set forth in the charter of such committee. Solventum anticipates that these responsibilities will include:
•monitoring and reviewing the overall strategy, direction, and effectiveness of Solventum’s research and development and business development activities;
•reviewing management’s strategy and allocation of resources for research and development and business development activities, including product line extensions and new product platforms;
•overseeing risk management in the area of product quality and safety;
•overseeing the quality and regulatory aspects of Solventum’s research and development programs; and
•overseeing Solventum’s policies, programs and performance related to medical affairs.
John Weiland, Carrie Cox, Amy Wendell, Elizabeth Mily and Bernard Harris are expected to be the members of the Science, Technology and Quality Committee. Mr. Weiland is expected to be the Chair of such committee.
Compensation Committee Interlocks and Insider Participation
During the fiscal year ended December 31, 2023, Solventum was not an independent company and did not have a compensation committee or any other committee serving a similar function. Decisions as to the compensation of those who currently serve as Solventum's executive officers were made by 3M.
Corporate Governance
Solventum believes that good corporate governance practices serve the long-term interests of shareholders, strengthen Solventum’s Board of Directors and management, and will further enhance the public trust 3M has earned from more than a century of operating with uncompromising integrity and doing business the right way. The following sections provide an overview of Solventum’s expected corporate governance practices, which will be published on Solventum's website, including the Corporate Governance Guidelines, the Codes of Conduct for directors and employees and other important governance-related policies.
Corporate Governance Guidelines
Solventum’s Board of Directors will adopt the Corporate Governance Guidelines, which will provide a framework for the effective governance of Solventum. The guidelines will address matters such as the respective roles and responsibilities of Solventum’s Board of Directors and management, Solventum’s Board of Directors’
174
leadership structure, the responsibilities of the independent Lead Director, director independence, the Board Membership Criteria, Board committees, and Board and management evaluation. Solventum’s Board of Directors’ Governance Committee will be responsible for overseeing and reviewing the Guidelines at least annually and recommending any proposed changes to Solventum’s Board of Directors for approval. Some expected key governance guidelines are set forth below. The Corporate Governance Guidelines, the amended and restated certificate of incorporation and amended and restated bylaws, the charters of Solventum’s Board of Directors’ committees, the Director Independence Guidelines, and the Codes of Conduct will provide the framework for the governance of Solventum and will be available on Solventum’s website at www.solventum.com.
Mandatory Retirement Age
The retirement age of a non-employee director will be 75. A director elected to Solventum’s Board of Directors prior to their 75th birthday will be permitted to continue to serve until the annual shareholder meeting coincident with or following their 75th birthday. Absent special circumstances, directors will not be nominated for election after their 75th birthday.
Outside Board Policy
Independent directors who also serve as CEOs of publicly traded companies or in equivalent positions will not be permitted to serve on more than two boards of public companies in addition to Solventum’s Board of Directors, and other independent directors will not be permitted to serve on more than four other boards of public companies in addition to Solventum’s Board of Directors. Independent directors will be required to advise the Chairman/Chief Executive Officer before accepting an invitation to serve on another for-profit board.
Access to Employees and Outside Advisors
Solventum’s Board of Directors members will have complete access to all members of Solventum management and its employees, as well as outside advisors.
Communication with Directors
Solventum’s Board of Directors will adopt a process for shareholders and other interested parties to send communications to members of the Board. The process is expected to involved shareholders and other interested parties being able to communicate with the Chairman, the chairs of the Audit, Talent, Governance and Science, Technology and Quality Committees of the Board, or with any of Solventum’s other independent directors, or all of them as a group, by sending a letter to the following address: Corporate Secretary, Solventum Corporation, 3M Center, Building 275-6W, 2510 Conway Avenue East, Maplewood, MN 55144. The Corporate Secretary will review communications to the independent directors and forward those communications to the independent directors as discussed below. Communications involving substantive accounting or auditing matters will be immediately forwarded to the Chair of the Audit Committee and Solventum’s Chief Compliance Officer consistent with time frames established by the Audit Committee for the receipt of communications dealing with these matters. Communications that pertain to non-financial matters will be forwarded promptly. Items that are unrelated to the duties and responsibilities of Solventum’s Board of Directors will not be forwarded, such as: business solicitation or advertisements; product-related inquiries; mass mailings; resumes or other job-related inquiries; and unsolicited commercial e-mails.
Solventum’s Codes of Conduct
All of Solventum's employees, including its Chief Executive Officer, Chief Financial Officer, and Chief Accounting Officer, will be required to abide by Solventum’s Code of Conduct to ensure that its business is conducted in a consistently legal and ethical manner. The Code of Conduct will form the foundation of a comprehensive process that includes compliance with corporate policies and procedures and a company-wide focus on uncompromising integrity in every aspect of Solventum’s operations. Solventum’s Code of Conduct will cover many topics, including antitrust and competition law, conflicts of interest, financial reporting, protection of confidential information, and compliance with all laws and regulations applicable to the conduct of its business.
175
Employees will be required to report any conduct that they believe in good faith to be an actual or apparent violation of the Code of Conduct. The Audit Committee is expected to adopt procedures to receive, retain, and treat complaints received regarding accounting, internal accounting controls, or auditing matters, and to allow for the confidential and anonymous submission by employees or others of concerns regarding questionable accounting or auditing matters. Information on how to submit any such communications can be found on Solventum’s Investor Relations website, under “Contact Us.” Solventum’s Chief Compliance Officer will have direct reporting obligations to the Audit Committee and will periodically report to the Audit Committee on compliance with Solventum's Code of Conduct, including the effectiveness of Solventum's compliance program.
Solventum’s Board of Directors will also adopt a Code of Business Conduct and Ethics for Directors of Solventum. This Code will incorporate principles of conduct Solventum and Solventum’s Board of Directors will follow to ensure Solventum’s business and the activities of Solventum’s Board of Directors are conducted with integrity and adherence to the highest ethical standards, and in compliance with the law. Solventum’s Code of Conduct for employees and the Code of Business Conduct and Ethics for Directors will be available on Solventum’s website at www.solventum.com.
Board’s Role in Risk Oversight
Solventum’s Board of Directors will be tasked with: overseeing Solventum’s risk profile and management’s processes for assessing and managing risk, both as a whole Board and through its committees, providing general oversight of Solventum’s overall ESG and human resources strategies, goals and results; reviewing enterprise risks at least annually (including environmental, health and safety (EHS) compliance, human capital management, ESG, cybersecurity and information security risks); and assigning other important categories of risk and specific ESG elements to designated Board committees and receiving reports from them.
176
In addition, we expect that the following bodies will be tasked with risk oversight as follows:
Audit Committee | Will have the oversight responsibilities over information and cyber security assessment and risk management. Will also have the oversight responsibilities over risks associated with Solventum’s capital structure, credit ratings and cost of capital, long-term benefit obligations, and use of or investment in financial products, such as derivatives, to manage risk related to foreign currencies, commodities, and interest rates. | ||||
Talent Committee | Will oversee risks associated with Solventum’s compensation practices, including by performing an annual review of Solventum’s risk assessment of its compensation policies and practices for its employees, including talent sourcing, diversity and retention strategies; talent development; internal pay equity, and equal employment opportunities. | ||||
Governance Committee | Will have the primary responsibility for the oversight of enterprise risks facing Solventum and will oversee risks associated with Solventum’s overall governance (e.g., diversity of Solventum’s Board of Directors, public policy, social responsibility, political activities and contributions) and its succession planning process to ensure that Solventum has a slate of future, qualified candidates for key management positions. | ||||
Auditor | The Senior Vice President, Head of Global Internal Audit of Solventum (the “Auditor”), whose appointment and performance will be reviewed and evaluated by the Audit Committee and who will have direct reporting obligations to the Audit Committee, will be responsible for leading the formal risk assessment and management process within Solventum. The Auditor, through consultation with Solventum’s senior management, will periodically assess the major risks facing Solventum and will work with those executives responsible for managing each specific risk. The Auditor will periodically review with the Audit Committee the major risks facing Solventum and the steps management has taken to monitor and mitigate those risks. The Auditor’s risk management report, which will be provided in advance of the meeting, will be reviewed with the entire Solventum Board by either the chair of the Audit Committee or the Auditor. | ||||
Management | Will provide consultation to the Auditor when he or she assesses the major risks facing Solventum. Will manage and mitigate risks. Will report, as needed, to the full Solventum Board on how a particular risk is being managed and mitigated. | ||||
Solventum believes that its Board of Directors’ oversight of risks, primarily through delegation to the Audit Committee, but also through delegation to other committees to oversee specific risks within their areas of responsibility and expertise, and the sharing of information with the full Board, will be appropriate for Solventum. The chair of each committee that oversees risk will provide a summary of the matters discussed with the committee to the full Board following each committee meeting. The minutes of each committee meeting will also be provided to all Board members.
Board Membership Criteria
Solventum’s Corporate Governance Guidelines will contain Board Membership Criteria that will include a list of key skills and characteristics deemed critical to serve Solventum’s long-term business strategy and expected to be represented on Solventum’s Board. The Governance Committee will periodically review with Solventum’s Board of Directors the appropriate skills and characteristics required of Board members given the current Board composition. It is the intent of Solventum’s Board of Directors that the Board itself will be a high-performance organization creating a competitive advantage for Solventum. To perform as such, the Board will be composed of individuals who have distinguished records of leadership and success in their arena of activity and who will make substantial contributions to Board operations and effectively represent the interests of all shareholders. The Committee’s and
177
Solventum’s Board of Directors’ assessment of Board candidates will include, but will not be limited to, consideration of:
•roles in and contributions valuable to the business community;
•personal qualities of leadership, character, judgment, and whether the candidate possesses and maintains throughout service on the Board a reputation in the community at large of integrity, trust, respect, competence, and adherence to the highest ethical standards;
•relevant knowledge and diversity of background and experience in business, manufacturing, technology, finance and accounting, marketing, international business, government, and other areas; and
•whether the candidate is free of conflicts and has the time required for preparation, participation, and attendance at all meetings.
In addition to these minimum requirements, the Governance Committee will also evaluate whether the nominee’s skills are complementary to the existing Board members’ skills, the Board’s needs for particular expertise in certain areas, and the nominee’s impact on Board dynamics, effectiveness, and diversity of experience and perspectives.
Board Self-evaluation Process
Solventum’s Board of Directors will conduct a multi-step annual self-evaluation to determine whether it, its committees and its directors are functioning effectively and consider opportunities for continual enhancement. While this formal self-evaluation will be conducted on an annual basis, directors will be encouraged to share perspectives, feedback, and suggestions year-round.
Director Nomination Process
In addition to its annual assessment and nomination of incumbent directors, the Governance Committee will oversee the process for selecting new director candidates.
The Committee will also focus on overall Board-level succession planning at the director level, periodically review the appropriate size and composition of Solventum’s Board of Directors and anticipate future vacancies and needs of Solventum’s Board of Directors. In the event the Committee recommends an increase in the size of Solventum’s Board of Directors or a vacancy occurs, the Committee may consider qualified nominees recommended from several sources, including current Board members, shareholders, and other persons.
The Committee will be authorized to retain a director search firm from time to time to help the Committee identify qualified director nominees for consideration by the Committee.
Shareholder Nominations
The Governance Committee will adopt a policy to consider properly submitted shareholder recommendations for candidates for membership on Solventum’s Board of Directors. Shareholders proposing individuals for consideration by the Committee are expected to be required to include at least the following information about the proposed nominee: the proposed nominee’s name, age, business, or residence address, principal occupation or employment, and whether such person has given written consent to being named in the Proxy Statement as a nominee and to serving as a director if elected. Shareholders will be required to send the required information about the proposed nominee to: Corporate Secretary, Solventum Corporation, 3M Center, Building 275-6W, 2510 Conway Avenue East, Maplewood, MN 55144.
Such proposals must be sent via registered, certified, or express mail (or other means that allows the shareholder to determine when the proposal was received by Solventum). The Corporate Secretary will refer properly submitted shareholder proposed nominations to the Chair of the Governance Committee for consideration at a future Committee meeting. Individuals proposed by shareholders in accordance with these procedures will receive the same consideration received by individuals identified to the Committee through other means.
178
Advance Notice Bylaws
In addition, Solventum’s amended and restated bylaws will permit shareholders to nominate directors at an annual meeting of shareholders or at a special meeting at which directors are to be elected in accordance with the notice of meeting. Shareholders intending to nominate a person for election as a director will be required to comply with the requirements set forth in Solventum’s amended and restated bylaws. The notice must contain the information required by the amended and restated bylaws, a copy of which will be available on Solventum’s website at www.solventum.com.
Proxy Access Nominations
Further, pursuant to the proxy access bylaw, a shareholder, or a group of up to 20 shareholders, continuously owning for three years at least three percent of Solventum’s outstanding common shares will be allowed to nominate and include in Solventum’s proxy materials up to the greater of two directors and 20 percent of the number of directors currently serving, if the shareholder(s) and nominee(s) satisfy the bylaw requirements. The notice must contain the information required by Solventum’s amended and restated bylaws.
Procedures for Approval of Related Persons Transactions
Solventum’s Board of Directors will adopt a written Related Person Transaction Policy and Procedures that will be administered by the Audit Committee. This policy will apply to any transaction or series of transactions in which Solventum or a subsidiary is a participant, the amount involved exceeds $120,000, and a Related Person (as that term is defined in the policy) has a direct or indirect material interest and which is required to be disclosed under Item 404(a) of Regulation S-K. Transactions that fall within this definition will be referred to the Audit Committee for approval or other action. Based on its consideration of all of the relevant facts and circumstances, the Audit Committee will decide whether or not to approve a transaction and will approve only those transactions that are in the best interests of Solventum and its shareholders. In the course of its review and approval or ratification of a transaction, the Audit Committee will consider:
•the nature of the Related Person’s interest in the transaction;
•the material terms of the transaction, including whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances;
•the significance of the transaction to the Related Person;
•the significance of the transaction to Solventum;
•whether the related person is involved in the negotiation of the terms of the transaction or receives any special benefit as a result of the transaction;
•whether the transaction would impair the judgment of a director or executive officer to act in the best interest of Solventum and its shareholders; and
•any other matters the Audit Committee deems appropriate.
Any Audit Committee member who is a Related Person with respect to a transaction under review will not participate in the deliberations or vote respecting such approval, except that such a director may be counted in determining the presence of a quorum at a meeting at which the Audit Committee considers the transaction.
179
Non-employee Director Compensation
During 2022 and 2023, Solventum was not an independent public company and did not pay any director compensation. Following the distribution, the Solventum director compensation program will be subject to review and modification by the Solventum board of directors or a committee thereof.
Stock Retention Requirement
It is expected that Solventum will consider the adoption of a stock retention requirement for its non-employee directors following the distribution in consultation with its compensation consultant.
Prohibition of Hedging, Pledging, and Other Actions
It is expected that Solventum will adopt stock trading policies effective as of the distribution that prohibit its directors and executive officers from (1) purchasing any financial instrument that is designed to hedge or offset any decrease in the market value of Solventum’s common stock, including prepaid variable forward contracts, equity swaps, collars, and exchange funds; (2) engaging in short sales related to Solventum’s common stock; (3) placing standing orders; (4) maintaining margin accounts; and (5) pledging Solventum securities as collateral for a loan.
180
EXECUTIVE COMPENSATION
Solventum is currently a subsidiary of 3M and not yet an independent public company. Following the distribution, the executive compensation plans and programs for our named executive officers will be determined by a compensation committee of the board of directors of Solventum, which will be formed in connection with the distribution. For a description of certain Solventum executive compensation plans and programs that will be in effect following the distribution, see the section entitled “—Expected Solventum Compensation Programs.”
Since the Solventum business was conducted as part of the broader 3M business in 2023, 3M’s named executive officers functioned as named executive officers of the Solventum business, and the Compensation Discussion and Analysis and Executive Compensation Tables describe the compensation of the 3M executives listed below (referred to as the “Named Executive Officers” or “NEOs” for purposes of this information statement):
•Michael E. Roman – Chairman of the 3M Board and Chief Executive Officer
•Monish Patolawala – President and Chief Financial Officer
•Peter D. Gibbons – Group President, Enterprise Supply Chain
•Bryan C. Hanson – Group President and Chief Executive Officer, Health Care
•Kevin H. Rhodes – Executive Vice President and Chief Legal Affairs Officer
The titles shown above reflect the position of each Named Executive Officer with 3M as of March 1, 2024.
For a list of the individuals who will be Solventum executive officers following the distribution and biographical information for each such executive, see the section entitled “Management.”
181
Compensation Discussion and Analysis
This Compensation Discussion and Analysis describes 3M’s executive compensation program, explains how the Compensation and Talent Committee (the “3M C&T Committee”) of the 3M Board of Directors (the “3M Board”) oversees and implements this program, and reviews the 2023 compensation for the Named Executive Officers.
See Appendix B for the meaning of certain capitalized terms used throughout this Compensation Discussion and Analysis.
Section I: Executive overview
3M is building momentum and a foundation for future growth
In 2023, 3M focused on building momentum and improved operational performance to position itself for a bright future. The 3M team executed strategic priorities, while it delivered for customers, exceeded earnings and cash flow expectations, and exited the year stronger, leaner, and more focused. 3M managed dynamic external environments as it prioritized investments in attractive markets, applying its material science expertise to meet customer needs across its core and new platforms, including automotive electrification, climate technology, and industrial automation. 3M also made significant progress simplifying its supply chain, restructuring its organization, advancing the spin-off of its Health Care business, and reducing risk and uncertainty by proactively and effectively managing litigation. 3M has clear strategic priorities to capitalize on its strong cash flow generation as it works to unlock value for customers and shareholders, both today and into the future.
3M is preparing to spin off its Health Care business to create two world-class public companies
The spin-off of the Health Care business is on track to be completed on April 1, 2024. As a standalone health care business with a diverse portfolio of trusted brands, the spin off Health Care business that is expected to be called Solventum will be better positioned to deliver industry-leading innovation for millions of patients worldwide. As 3M progressed the spin-off, it appointed Bryan Hanson as CEO of the Health Care Business Group in September 2023. With his unique qualifications and proven executive track record of successfully leading, growing and transforming global medical device businesses, 3M is confident Mr. Hanson is the right leader for the new company to ensure its success for customers, patients, and shareholders.
Compensation program supports talent and value creation strategy
To enable progress and continued momentum, 3M is deliberately prioritizing its talent and compensation strategy to encourage the contributions of a high-caliber executive leadership team. In 2023, 3M set the target compensation levels for the majority of its executive officers at or near the peer group median. To align pay outcomes with the long-term interests of its shareholders, over 91 percent of its CEO’s target pay and on average 84 percent of other NEOs’ target opportunities were provided in the form of at-risk variable incentives that deliver value only if 3M achieves pre-set performance goals or increase or decrease in value consistent with 3M’s total shareholder return or, in the case of stock options, the value of 3M’s common stock. Only in select circumstances did compensation packages reflect expanded pay benchmarks and did 3M utilize special incentives to support its ability to attract and retain individual skillsets and incentivize critical contributions during a pivotal transformation period. 3M’s short- and long-term performance metrics reflect its growth drivers and were further refined for 2024 to incorporate shareholder feedback and to enhance focus on cash flow, a key driver of value for 3M, and comprehensive sustainability priorities that are important for its future.
Executive compensation program aligned with shareholders
3M’s total shareholder return for the year reflected significant external uncertainties, including rapid declines in consumer-facing markets such as electronics and consumer retail, slowing growth in China, and mixed demand across industrial markets. Consistent with its shareholder experience, the realizable compensation in 2023 for the CEO was 66 percent of target pay, underscoring the overall alignment of pay outcomes with outcomes for our
182
shareholders. Three-year average realizable compensation for the CEO was 51 percent of target, consistent with longer-term shareholder returns.
•The short-term incentive program paid out at 104.0 percent of target for the CEO and between 85.5 percent and 124.8 percent of target for the other Named Executive Officers. These payouts reflected particularly strong performance on Free Cash Flow Conversion driven by 3M’s actions to streamline its supply chains and its ongoing focus on working capital management, especially inventory, and above target performance on Operating Income, which was partially offset by below target performance on Local Currency Sales; and
•The long-term performance shares for the 2021-2023 performance period were earned at 83.8 percent of target, which represented 59.7 percent of the initial target grant value after considering the change in market value of 3M’s common stock over the performance period and accounting for the dividend equivalents associated with earned performance shares.
3M continues to act with urgency as it supports its mission to innovate, reimagine what is possible, and deliver value to its shareholders and the broader communities that it serves.
_________________ See Appendix A for a reconciliation of the non-GAAP financial measures used in this Compensation Discussion and Analysis to the most directly comparable GAAP financial measures. As explained in Appendix A, all non-GAAP financial measures presented in this Compensation Discussion and Analysis are used for compensation purposes and include the adjustment of certain special items that the 3M C&T Committee believes are outside the control of management and are not reflective of ongoing operations. The non-GAAP financial measures used herein may not be comparable to similarly titled measures used by other companies and the adjusted amounts used for compensation purposes may differ from the adjusted amounts used by 3M elsewhere or included in 3M’s Form 10-K. | ||||||||
Elements of 2023 target total direct compensation
The table below shows how the 2023 target Total Direct Compensation of the Named Executive Officers was apportioned among base salary, annual incentives, performance share awards, stock options, and restricted stock units (RSUs), summarizes the rationale for providing and key characteristics of each such element, and lists the performance metrics, weightings, and modifiers used for annual and long-term incentives granted in 2023.
CEO(1) | Other NEOs(1) | Why it is provided | Performance metrics, weightings, and modifiers(2) | ||||||||||||||||||||||||||||||||||||||
| Key characteristics | |||||||||||||||||||||||||||||||||||||||||
 |  | 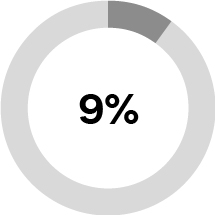 | 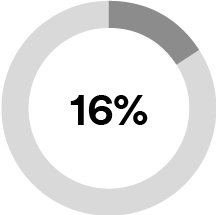 | •Compensate executives for their normal day-to-day responsibilities | •Only component of compensation that is considered to be fixed rather than variable in nature | ||||||||||||||||||||||||||||||||||||
183
 |  | 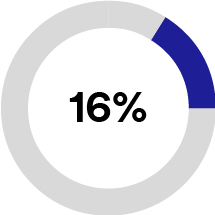 | 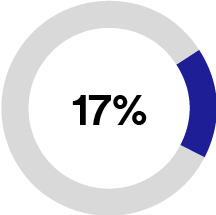 | •Motivate executives to stay focused on day-to-day operations by aligning a significant portion of Total Cash Compensation with the near-term financial performance of 3M and its business units | •Performance metrics and goals approved by the 3M C&T Committee, which is comprised entirely of independent directors •Payouts based on performance against preestablished business objectives over a 12-month period •Payouts adjusted or left unchanged based on individual performance against preestablished goals and objectives and the 3M C&T Committee’s determination of 3M’s holistic performance against a set of preestablished objective ESG metrics •Payouts cannot exceed 200% of an executive’s weighted-average target annual incentive amount | •Local Currency Sales (of 3M or a business unit, as applicable) vs. Plan (50%) •Operating Income (of 3M or a business unit, as applicable) vs. Plan (30%) •3M Operating Cash Flow Conversion vs. Plan (20%) •Individual performance multiplier (± 20%) •ESG modifier (± 10% of target) | |||||||||||||||||||||||||||||||||||
184
| Performance Shares | •Motivate executives to focus on continuously improving performance in key financial metrics believed to drive long-term shareholder value •Retain executive talent | •Performance metrics and goals approved by the Committee, which is comprised entirely of independent directors •Payouts based on performance against preestablished goals over three years •Maximum payout equal to 200% of the target number of performance shares •Cash-settled dividend equivalent rights that are payable only if the underlying shares are earned | •Adjusted Earnings per Share Growth (33.3%) •Free Cash Flow Growth (33.3%) •Relative Organic Sales Growth (33.3%) | ||||||||||||||||||||||||||||||||||||||
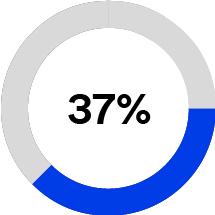 | 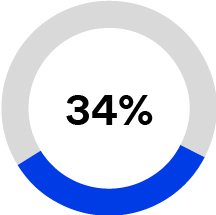 | ||||||||||||||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||||||||||||||
185
Stock Options(3) | •Motivate executives to build long-term shareholder value •Retain executive talent | •Provide value only if stock price increases •Exercise price equal to the grant date closing price for a share of 3M common stock •Ratable three-year vesting schedule •Maximum term of 10 years | •Vesting is based on continued service, while value of the options is based on stock price appreciation (100%) | |||||||||||||||||||||||||||||||||||||||||
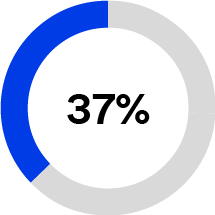 | 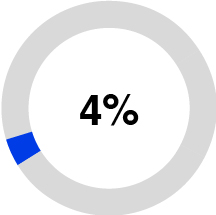 | |||||||||||||||||||||||||||||||||||||||||||
Restricted Stock Units(3) | •Motivate executives to build long-term shareholder value •Retain executive talent | •Three-year “cliff” vesting schedule •Cash-settled dividend equivalent rights that are payable only if the underlying shares are earned | •Vesting is based on continued service, while value of the RSUs is based on total shareholder return (100%) | |||||||||||||||||||||||||||||||||||||||||
 | 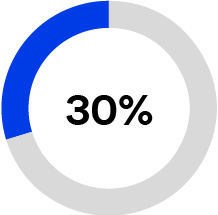 | |||||||||||||||||||||||||||||||||||||||||||
__________________
(1)Percentages shown reflect the apportionment (or, in the case of the percentages shown for the Other NEOs, the average apportionment) of the components of target total direct compensation that are expected to be recurring. Such amounts do not reflect special items such as hiring bonuses, one-time make-whole and inducement awards granted in connection with the commencement of employment, or special grants.
(2)In determining the level of achievement of the performance goals established under the AIP and the performance share awards for any given period, the costs, sales and impact on assets and liabilities from acquisitions are excluded in the year that the acquisition is completed. The 3M C&T Committee also makes other adjustments from time to time for special items that it believes are unrelated to the operational performance of 3M for the relevant measurement period (e.g., changes in tax laws or accounting principles, asset write-downs, the impact of restructurings, divestitures, or asset sales, unusual tax transactions, litigation or claim judgments and settlements, and other special items described in management’s discussion and analysis of financial condition and results of operations appearing in 3M’s annual/quarterly report to shareholders for the applicable period). These adjustments can have either a positive or negative impact on award payouts.
(3)For the CEO, the 3M C&T Committee chose to deliver 50 percent of the target grant value of his 2023 annual long-term incentive awards in the form of performance shares and the remaining 50 percent in the form of stock options. Each of 3M’s other executives was given an opportunity to indicate a preference to receive 50 percent of the target grant value of their annual long-term incentive awards in the form of RSUs, stock options, or an equal split of both stock options and RSUs. Regardless of an executive’s indicated preference, the remaining 50 percent of the target grant value of his or her 2023 annual long-term incentive awards was delivered in the form of performance shares. The percentages shown reflect the apportionment of stock options and RSUs based on the Named Executive Officers’ 2023 elections (other than the CEO, who was not offered an opportunity to make an election).
186
Paying for performance
A primary objective of 3M’s incentive compensation program is to align the Named Executive Officers’ real pay delivery with performance. 3M’s performance directly impacted incentive compensation pay outcomes for its Named Executive Officers as discussed below.
Consistent with the 3M C&T Committee’s pre-established exclusions policy(1), which is aligned with 3M’s non-GAAP adjusted operating income disclosure in its Form 10-K, 3M’s 2023 operating income goals and performance results for AIP excluded special items or one-time events that the 3M C&T Committee believes are unrelated to the operational performance of 3M for the relevant measurement period, including the impact of net costs for significant litigation. In approving the incentive program payouts based on 2023 performance, the 3M C&T Committee considered the impact of the litigation charges related to 3M's respirator mask/asbestos, PFAS-related other environmental, and Combat Arms Earplugs matters, recognizing that, as anticipated, they were significantly larger than in prior years. Even while litigation-related actions and expenses may be anticipated, the 3M C&T Committee views such adjustments as appropriate because (as in this case), such expenses do not relate to 3M’s core operating performance in 2023 or ongoing business operations, but rather were the result of multi-year lawsuits that stem from events taking place many years, even decades prior, unrelated to decision-making of the current management team. In addition, failure to exclude litigation settlement charges could disincentivize management from settling litigation when it is in the best interests of shareholders but would adversely impact their incentive compensation payouts. The 3M C&T Committee also took into account its belief that the litigation charges largely were already reflected in 3M’s stock price performance, which has significantly impacted the NEOs’ realizable compensation for multiple years (for example, realizable compensation for the CEO was 66 percent and 51 percent of his total target compensation on a one- and three-year average basis, respectively), as well as the importance of continuing to appropriately incentivize and retain the executive leadership and broader senior management team to drive 3M’s transformation strategy. The 3M C&T Committee believes this approach is balanced, aligned with its shareholder experience, in line with market practice related to adjustment policies for litigation settlement charges, and essential to supporting 3M’s value creation strategy and efforts to reduce risk and uncertainty. | |||||||||||
__________________
(1)Under a pre-established exclusions policy, the 3M C&T Committee may adjust financial performance for purposes of goal-setting and/or performance measurement in 3M’s incentive plans to exclude the impact of special items or one-time events that it believes are unrelated to the operational performance of 3M for the relevant measurement period (e.g., changes in tax laws or accounting principles, asset write-downs, the impact of restructurings, divestitures, or asset sales, unusual tax transactions, litigation or claim judgments and settlements, and other special items described in management’s discussion and analysis of financial conditions and results of operations appearing in 3M’s annual/quarterly report to shareholders for the applicable period.) These adjustments can have a positive or negative impact on award payouts.
2023 annual incentive compensation
For the Named Executive Officers whose 2023 annual incentive compensation payout was calculated based on 3M’s overall performance, the payout (before any adjustment for individual performance) was 104.0 percent of the target amount. The payouts reflect the 3M C&T Committee’s view of 3M’s performance against the financial goals established for 2023, as shown below, and its decision not to increase or decrease the payouts using the ESG modifier.
187
After considering 3M’s 2023 operating plan, the 3M C&T Committee, in consultation with its independent compensation consultant and following discussions with management of 3M, approved the 2023 financial performance targets for the AIP, as shown below. •The targets established for the Local Currency Sales and Operating Income metrics were lower than 2022 actual results for these two metrics, reflecting 3M’s portfolio management actions, including 3M’s decision to exit PFAS manufacturing, the divestiture of 3M’s Food Safety business, the deconsolidation of its Aearo subsidiary, the discontinuation of 3M’s business in Russia, and the anticipated spin-off of 3M’s Health Care business, and the post-COVID reduction in demand for disposable respirators. •The targets were informed by 3M’s operational plan for the year and were intended to be challenging and maintain a similar level of rigor as those established for past years. | |||||||||||
Dollar Amounts in Millions*
| Performance metric | Threshold ($) | Target ($) | Maximum ($) | Actual vs. target | Payout % | Weighting | Weighted payout % | |||||||||||||||||||||||||||||||||||||
| Local Currency Sales vs. Plan | 31,514 | 98 | % | 81.3 | % | 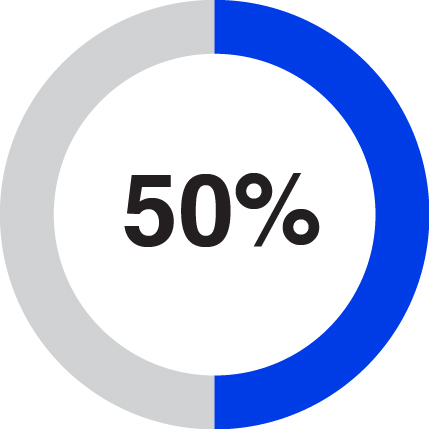 | 40.7 | % | ||||||||||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||||||||||||||||
| 29,527 | 32,095 | 34,663 | ||||||||||||||||||||||||||||||||||||||||||
| Operating Income vs. Plan | 6,374 | 104 | % | 126.7 | % | 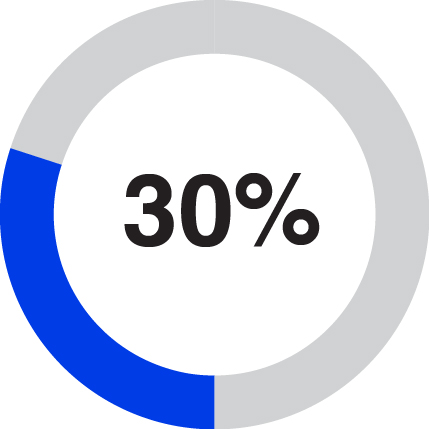 | 38.0 | % | ||||||||||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||||||||||||||||
| 5,198 | 6,115 | 7,032 | ||||||||||||||||||||||||||||||||||||||||||
| Operating Cash Flow Conversion vs. Plan | 145% | 104 | % | 126.7 | % | 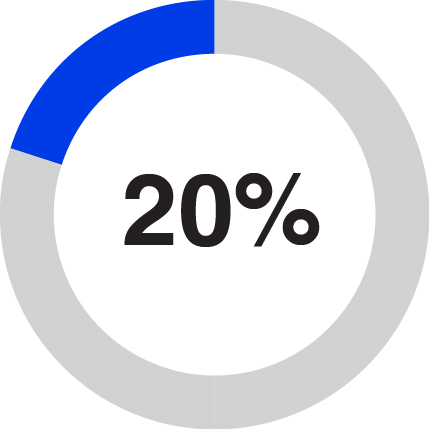 | 25.3 | % | ||||||||||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||||||||||||||||
| 119% | 140% | 161% | ||||||||||||||||||||||||||||||||||||||||||
Weighted-average payout percent for total 3M performance (before adjustment for individual performance) | 104.0 | % | ||||||||||||||||||||||||||||||||||||||||||
__________________
*Results reflect certain adjustments that the 3M C&T Committee believed were appropriate to better reflect 3M’s 2023 performance. See Appendix A for a reconciliation of Local Currency Sales, Operating Income and Operating Cash Flow Conversion used for compensation purposes to 3M’s results for the most directly comparable financial measures as reported under GAAP.
For more information concerning the calculation of the 2023 annual incentive payout for each Named Executive Officer, including the threshold, target, and maximum goals and attainments used to calculate the annual incentive payouts of the Named Executive Officers who are paid, in part, based on the performance of a business group, see “2023 AIP attainments and payouts.”
Performance share award payouts and accruals (long-term incentive compensation)
The three-year performance period for the 2021 performance share awards issued to the Named Executive Officers ended on December 31, 2023. Based on the financial results achieved during 2021 – 2023, the Named Executive Officers received 83.8 percent of the target performance shares subject to their 2021 performance share
188
awards. After considering the change in the market value of 3M’s common stock over the three-year performance period and the additional cash delivered pursuant to the dividend equivalent rights granted as part of the 2021 performance share awards, the value delivered to the Named Executive Officers in settlement of these awards (determined using the closing price of a share of 3M common stock on the NYSE for December 29, 2023) equaled 59.7 percent of the initial target grant value approved by the 3M C&T Committee.
When evaluating the payouts for these awards against 3M’s performance, it is important to keep in mind the weightings applied to each year (2021 — 50 percent; 2022 — 30 percent; and 2023 — 20 percent) and each metric (Relative Organic Volume Growth — 40 percent; Return on Invested Capital — 20 percent; Adjusted Earnings per Share Growth — 20 percent; and Free Cash Flow Conversion — 20 percent). As illustrated in the charts below, the payout of 2021 performance share awards reflects the mixed results achieved during the performance period.
2021 performance share award results (2021-2023 performance period)
| Performance levels | Payout level (% of target) | Performance year and weighting | Actual result* | Actual payout (% of target) | |||||||||||||||||||||||||||||||||||||
| Adjusted Earnings per Share Growth | 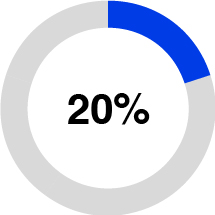 | Threshold** | 4.0 | % | 4.0 | % | Year 1 – 50% | 14.4 | % | 20.0 | % | ||||||||||||||||||||||||||||||
| Target | 8.0 | % | 20.0 | % | Year 2 – 30% | -0.2 | % | — | % | ||||||||||||||||||||||||||||||||
| Maximum | 12.0 | % | 40.0 | % | Year 3 – 20% | -6.5 | % | — | % | ||||||||||||||||||||||||||||||||
| Relative Organic Volume Growth |  | Threshold** | -1.0 | % | 8.0 | % | Year 1 – 50% | 0.5 | % | 20.0 | % | ||||||||||||||||||||||||||||||
| Target | 0.5 | % | 40.0 | % | Year 2 – 30% | -7.1 | % | — | % | ||||||||||||||||||||||||||||||||
| Maximum | 2.0 | % | 80.0 | % | Year 3 – 20% | -7.3 | % | — | % | ||||||||||||||||||||||||||||||||
| Return on Invested Capital |  | Threshold** | 16.0 | % | 4.0 | % | Year 1 – 50% | 19.5 | % | 15.0 | % | ||||||||||||||||||||||||||||||
| Target | 18.0 | % | 20.0 | % | Year 2 – 30% | 18.4 | % | 6.8 | % | ||||||||||||||||||||||||||||||||
| Maximum | 21.0 | % | 40.0 | % | Year 3 – 20% | 16.9 | % | 2.2 | % | ||||||||||||||||||||||||||||||||
| Free Cash Flow Conversion |  | Threshold** | 95.0 | % | 4.0 | % | Year 1 – 50% | 100.9 | % | 11.8 | % | ||||||||||||||||||||||||||||||
| Target | 100.0 | % | 20.0 | % | Year 2 – 30% | 81.8 | % | — | % | ||||||||||||||||||||||||||||||||
| Maximum | 105.0 | % | 40.0 | % | Year 3 – 20% | 122.9 | % | 8.0 | % | ||||||||||||||||||||||||||||||||
| Total | 83.8 | % | |||||||||||||||||||||||||||||||||||||||
__________________
*Results reflect certain adjustments that the 3M C&T Committee believed were appropriate to better reflect 3M’s performance during the performance period. See Appendix A for a reconciliation of Adjusted Earnings per Share, Return on Invested Capital, Free Cash Flow, and Free Cash Flow Conversion used for compensation purposes to 3M’s results for the most directly comparable financial measures as reported under GAAP.
** No payout is provided for below threshold performance.
3M’s 2023 performance will also impact the payouts of the 2022 performance share awards and 2023 performance share awards, where the weighting of 2023 performance is 30 percent and 50 percent, respectively. To illustrate this point, the charts below show the percent of target performance shares accrued each year during the relevant performance period based on 3M’s performance.
189
Percent of target performance shares accrued by year*
| 2021 performance share awards | 2022 performance share awards | 2023 performance share awards | ||||||||||||
 |  |  | ||||||||||||
__________________
*Amounts shown reflect the percent of target shares accrued based on the performance results for the specified year. The sum of the percentages accrued for each year during the performance period may differ slightly from the final total payout reported due to rounding.
| The final payout percentage for each performance share award equals the sum of the payout percentages for each year during the performance period based on 3M’s performance against the financial goals approved by the 3M C&T Committee at the beginning of the performance period. | ||||||||
For more information on the performance share awards that were outstanding on December 31, 2023, and the impact that 3M’s 2023 performance had on such awards, see “Status of outstanding performance share awards” and “Performance share accruals based on 2023 performance.”
Impact of changes in stock price
The performance of 3M’s stock has a material impact on the amount of compensation realized by the Named Executive Officers. 3M’s stock ownership guidelines also require covered executives, including the Named Executive Officers, to own amounts of 3M stock having a value exceeding a specified multiple of their base salary. If the market price of 3M’s stock declines, so does the value of the stock they own. Similarly, all long-term incentives awarded to the Named Executive Officers are equity-based and, therefore, increase or decrease in value consistent with 3M’s total shareholder return or, in the case of stock options, the value of 3M’s common stock.
The stock and stock options held by the Named Executive Officers throughout 2023 decreased in value during the year as the closing price for a share of 3M’s common stock on the NYSE decreased from $119.92 on December 30, 2022, to $109.32 on December 29, 2023, at which time all outstanding stock options held by the Named Executive Offers were underwater. Likewise, the value of the performance shares and restricted stock units held by the Named Executive Officers throughout the year decreased consistent with the one-year total shareholder return for 3M stock.
The chart below shows how the 3M CEO’s target Total Direct Compensation for each of the last three fiscal years compared to his realizable pay as of the end of each such year and demonstrates that 3M’s compensation programs have been working as the 3M C&T Committee intended to align 3M CEO’s realizable pay to 3M’s performance. Realizable pay provides a retrospective look at pay versus performance that reflects the intrinsic value of equity awards granted during the year.
190
Target total direct compensation vs. realizable CEO compensation ($ millions)
| 2021 realizable pay is 54% of target | 2022 realizable pay is 31% of target | 2023 realizable pay is 66% of target | Average 2021 – 2023 realizable pay is 51% of target | |||||||||||||||||||||||
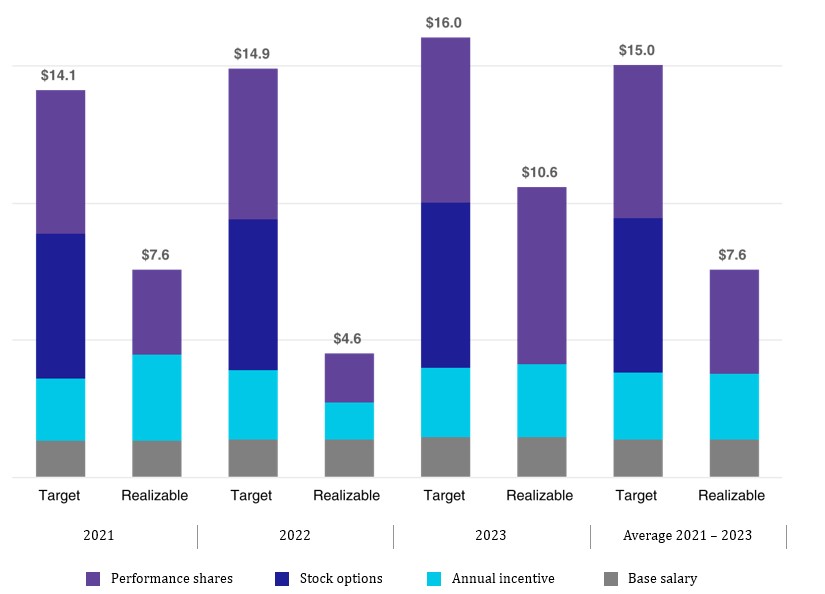
Amounts reflected in the realizable compensation figures shown in the chart above reflect (a) base salary earned during the year specified; (b) annual incentive compensation earned during the year specified; (c) the intrinsic value of all stock options granted to the 3M CEO during the year specified, as determined using the closing price for a share of 3M common stock on the NYSE for December 29, 2023 ($109.32); and (d) the intrinsic value of performance share awards (including the related dividend equivalents) granted to the 3M CEO during the year specified, as determined based on actual performance results for years 2021 through 2023, assuming target performance for years after 2023, and using the closing price for a share of 3M common stock on the NYSE for December 29, 2023 ($109.32). These amounts do not correspond to amounts reported in the Summary Compensation Table for the applicable fiscal year.
Say-on-pay results
3M has a history of strong say-on-pay results. In 2023, approximately 88 percent of the votes cast on the say-on-pay proposal approved the compensation of 3M’s named executive officers as disclosed in 3M’s annual proxy statement for the last year. Based on this level of support and the generally positive feedback received from shareholders during its 2023 investor outreach and engagement efforts, 3M did not make significant changes to its executive compensation program in 2023. As it has in past years, the 3M C&T Committee will consider the results of this year’s say-on-pay proposal, as well as feedback from 3M’s shareholders, when making future executive compensation decisions.
191
Recent noteworthy compensation program actions
Since January 1, 2023, the 3M Board and the 3M C&T Committee took the following noteworthy actions:
•Appointed Bryan C. Hanson as Group President and Chief Executive Officer, Health Care and approved his initial compensation arrangements. For more information, see “Section V: 2023 compensation decisions and performance highlights — Bryan C. Hanson — Compensation Decisions.”
•Amended the 3M Executive Severance Plan to provide for pro rata vesting of inducement restricted stock unit awards based on whole years of completed service.
•Significantly expanded the population of employees subject to 3M’s clawback policy with approximately 350 employees at the Vice President level and above now subject to recoupment based on the issuance of noncompliant financial reports, significant misconduct, or a significant risk-management failure. Made other revisions in line with new regulatory guidance implemented by the NYSE, including the expanded definition of an accounting restatement to cover so-called “little r” restatements. For more information, see “Clawback policy and other remedial actions.”
•Effective for the 2024 annual incentive compensation program offered to eligible employees, updated the metrics and weightings with the intent of enhancing focus on cash flow growth, a key driver of value for 3M.
•Approved various actions related to 3M’s U.S. retirement plans, including a future pension “freeze” for non-union employees, effective December 31, 2028, and a supplemental three percent annual company retirement contribution to the 401(k) plan accounts of eligible employees impacted by the future pension plan freeze, effective January 1, 2029.
192
Section II: How 3M determines executive compensation
Principles
3M maintains global compensation principles that are intended to ensure that its compensation practices are fair and reasonable as applied to both executive and non-executive employees. These principles align with 3M’s vision and strategies, balance both individual and enterprise performance, and seek to provide wages and benefits that are competitive in the most-relevant markets to employees based on roles, responsibilities, skills, and performance.
The core principles of 3M’s executive compensation program support its pay-for-performance philosophy, as follows:
•Total Direct Compensation should be competitive to attract the best talent to 3M, motivate executives to perform at their highest levels, reward individual contributions that improve 3M’s ability to deliver outstanding performance, and retain those executives with the leadership abilities and skills necessary for building long-term shareholder value;
•The portion of Total Direct Compensation that is at-risk and performance-based should increase with the level of an individual’s responsibility;
•The program should balance incentives for delivering outstanding long-term, sustainable performance against the potential to encourage inappropriate risk-taking;
•The metrics and targets for earning performance-based incentives should be consistent with, and aligned to, increasing shareholder value over the long term; and
•A significant portion of each executive’s personal net worth should be tied to the value of 3M common stock as further motivation to build long-term shareholder value and mitigate the risk of inappropriate risk-taking.
To monitor and support the effectiveness of this program, the 3M C&T Committee periodically reviews the compensation principles used for setting target annual Total Cash Compensation for 3M’s global workforce and approves the methodology for determining annual long-term incentive target grant values for employees eligible to receive such awards. 3M also periodically compares its pay components to those of other premier companies and adjusts them as necessary to stay competitive and attract, retain, and motivate a highly qualified, diverse workforce at all levels throughout the organization, not just for its executives.
Roles and responsibilities
3M believes that a collaborative process best ensures that compensation decisions reflect the principles of its executive compensation program. Set forth below is a summary of the roles and responsibilities of the key
193
participants that were involved in making decisions relating to the compensation that the Named Executive Officers earned in 2023.
| Responsible party | Primary roles and responsibilities relating to compensation decisions | |||||||
Compensation and Talent Committee (Composed solely of independent, non-employee directors and reports to the 3M Board) | •Reviews the design of, and risks associated with, 3M’s compensation policies and practices; •Approves the compensation of the Chief Executive Officer, subject to ratification by the independent members of the 3M Board; •Approves the compensation of the other Named Executive Officers; •Approves the performance metrics, goals, modifiers, payout slopes, and other elements used in the performance-based long-term and short-term incentive compensation arrangements of the 3M executive officers; •Approves annual performance goals and objectives for the Chief Executive Officer; •Conducts an annual evaluation of the Chief Executive Officer’s performance and reviews such evaluation with the independent members of the 3M Board of Directors; and •Approves all changes to the composition of the executive compensation peer group. | |||||||
| Independent non-employee members of the 3M Board of Directors | •Considers the 3M C&T Committee’s annual evaluation of the Chief Executive Officer’s performance; and •Considers the 3M C&T Committee’s actions regarding the compensation of the Chief Executive Officer and, if deemed appropriate, ratifies such actions. | |||||||
194
| Responsible party | Primary roles and responsibilities relating to compensation decisions | |||||||
Independent consultant to the Compensation and Talent Committee* (FW Cook) | •Provides the 3M C&T Committee with advice regarding the design of all elements of 3M’s executive compensation program; •Reviews 3M’s compensation policies and practices and, based on its review and expertise, provides an assessment as to whether such policies and practices are reasonably likely to have a material adverse effect on 3M; •Reviews and provides an independent assessment of materials provided to the 3M C&T Committee by management of 3M; •Provides advice and recommendations to the 3M C&T Committee regarding the composition of compensation peer groups; •Provides expert knowledge of regulatory developments, marketplace trends, and best practices relating to executive compensation and competitive pay levels; •Makes recommendations regarding the compensation of the Named Executive Officers (including the Chief Executive Officer); and •Regularly attends and actively participates in meetings of the 3M C&T Committee, including executive sessions. | |||||||
Chief Executive Officer (Assisted by the Executive Vice President and Chief Human Resources Officer and other 3M employees) | •Approves annual performance goals and objectives for the Named Executive Officers (other than himself); •Conducts an annual performance evaluation for each of the Named Executive Officers (other than himself) and presents the results to the 3M C&T Committee; and •Makes recommendations to the 3M C&T Committee with respect to the compensation of the Named Executive Officers (other than himself) based on the final assessment of their performance. | |||||||
__________________
*During 2023, the 3M C&T Committee was assisted by its independent compensation consultant, FW Cook. Other than the support that it provided to the 3M C&T Committee, FW Cook provided no other services to 3M or 3M management, except for independent advisory support to the Nominating and Governance Committee on the compensation of 3M’s non-employee directors so that valuation methodologies and peer groups are consistent with those used for executives and other employees. During the year, the 3M C&T Committee considered an evaluation of the independence of FW Cook based on the relevant regulations of the Securities and Exchange Commission and the NYSE listing standards. The 3M C&T Committee concluded that the services performed by FW Cook did not raise any noteworthy conflicts of interest.
Use of market data
3M competes for executive talent in a global market. To ensure that 3M is providing Total Direct Compensation that is competitive, the 3M C&T Committee annually considers the available pay data of two peer groups: an executive compensation peer group and a survey peer group.
Executive compensation peer group
For setting 2023 target compensation levels, the executive compensation peer group consisted of the companies identified below (which remained the same as in the previous year), as recommended by the 3M C&T Committee’s independent compensation consultant and approved by the 3M C&T Committee. The companies in this executive compensation peer group were selected because (1) their performance was monitored regularly by the same market analysts who monitor the performance of 3M (investment peers) and they are considered major business-segment competitors used internally for performance comparisons, or (2) they met certain criteria based on similarity of their
195
business, market capitalization (based on an eight-quarter rolling average), annual revenues, and/or Midwest corporate headquarters, and compete with 3M for capital or talent.
| (Dollars in millions) | ||||||||||||||||||||
Latest four quarters revenues(1) | Trailing eight-quarter average market capitalization(1) | |||||||||||||||||||
| Johnson & Johnson | $85,159 | Johnson & Johnson | $420,567 | |||||||||||||||||
| The Procter & Gamble Company | $83,933 | The Procter & Gamble Company | $347,111 | |||||||||||||||||
| The Boeing Company | $77,794 | Abbott Laboratories | $185,212 | |||||||||||||||||
| General Electric Company | $67,954 | Danaher Corporation | $183,105 | |||||||||||||||||
| Caterpillar Inc. | $67,060 | Honeywell International Inc. | $128,897 | |||||||||||||||||
| Deere & Company | $60,755 | Caterpillar Inc. | $126,020 | |||||||||||||||||
| Abbott Laboratories | $40,109 | The Boeing Company | $114,780 | |||||||||||||||||
| Honeywell International Inc. | $36,662 | Deere & Company | $110,461 | |||||||||||||||||
| 3M Company | $32,681 | General Electric Company | $110,342 | |||||||||||||||||
| Medtronic plc | $32,320 | Medtronic plc | $109,864 | |||||||||||||||||
| Johnson Controls International plc | $26,819 | Eaton Corporation plc | $76,370 | |||||||||||||||||
| Danaher Corporation | $23,890 | Illinois Tool Works Inc. | $69,673 | |||||||||||||||||
| Eaton Corporation plc | $23,196 | 3M Company | $60,012 | |||||||||||||||||
| Kimberly-Clark Corporation | $20,431 | Emerson Electric Co. | $52,587 | |||||||||||||||||
| Parker-Hannifin Corporation | $19,826 | Parker-Hannifin Corporation | $46,404 | |||||||||||||||||
| Illinois Tool Works Inc. | $16,107 | Kimberly-Clark Corporation | $42,996 | |||||||||||||||||
| TE Connectivity Ltd. | $16,024 | TE Connectivity Ltd. | $40,080 | |||||||||||||||||
| Emerson Electric Co. | $15,909 | Johnson Controls International plc | $39,390 | |||||||||||||||||
| Corning Incorporated | $12,588 | DuPont de Nemours, Inc. | $31,189 | |||||||||||||||||
| DuPont de Nemours, Inc. | $12,068 | Corning Incorporated | $27,166 | |||||||||||||||||
| 75th Percentile | $63,908 | 75th Percentile | $127,458 | |||||||||||||||||
| Mean | $38,874 | Mean | $119,064 | |||||||||||||||||
| Median | $26,819 | Median | $109,864 | |||||||||||||||||
| 25th Percentile | $17,967 | 25th Percentile | $44,700 | |||||||||||||||||
| 3M Percentile Rank | 56 | % | 3M Percentile Rank | 36 | % | |||||||||||||||
__________________
(1)All data shown was obtained from Standard & Poor's Capital IQ. Revenues are stated in millions for the latest four quarters disclosed as of February 29, 2024. Market capitalizations are stated in millions as of February 29, 2024.
The 3M C&T Committee, with assistance from its independent compensation consultant, periodically reviews the composition of the executive compensation peer group to determine whether any changes are appropriate. Following its review in August 2023, FW Cook recommended, and the 3M C&T Committee approved, the changes below to ensure that there is sufficient overlap between 3M’s businesses and the businesses of the members of the peer group following completion of the spin-off of its Health Care business. These changes did not affect 2023 compensation decisions made by the 3M C&T Committee.
| Entities removed from the executive peer group | Entities added to the executive peer group | |||||||||||||||||||
 | •Danaher Corporation •Medtronic plc |  | •None | |||||||||||||||||
3M receives market surveys with pay data and information on the executive compensation practices at the companies in 3M’s executive compensation peer group from Aon plc.
196
To provide relevant competitive market information for Mr. Hanson, the 3M C&T Committee used a separate executive compensation peer group of health care equipment, supplies, and technology companies (1) who were health care segment competitors used internally for relevant performance comparisons, and/or (2) of similar size to Solventum in terms of pro forma annual revenues. The companies in the peer group used were: Align Technology Inc., Baxter International Inc., Becton Dickinson & Co., Boston Scientific Corporation, Danaher Corporation, DENTSPLY Sirona Inc., Edwards Lifesciences Corporation, Hologic Inc., Intuitive Surgical Inc., IQVIA Holdings Inc., Medtronic plc, Resmed Inc., STERIS plc, Stryker Corporation, and Zimmer Biomet Holdings Inc.
Survey peer group
For 2023, there were approximately 400 comparator companies in the survey peer group. Although the number and identity of the companies varies from year to year and from survey to survey, each of the companies included in the survey peer group had annual revenue exceeding $10 billion. All companies in the survey peer group also participated in one or more executive compensation surveys that 3M obtained from three consulting firms: Aon plc, FW Cook, and Willis Towers Watson plc. Pay data for the survey peer group is statistically regressed (based on annual revenues) to recognize the different sizes of the comparator companies as compared to the size of 3M. The pay data for the survey peer group is then used to assess the reasonableness of the executive compensation peer group data received, helping to ensure that 3M’s compensation objectives are being met. The 3M C&T Committee does not review the identity of the companies in the survey peer group.
How the 3M C&T Committee establishes target compensation levels
The 3M C&T Committee considers pay data from the executive compensation peer group as one of several reference points it uses to inform its decisions about overall compensation opportunities and specific compensation elements. The 3M C&T Committee does not benchmark specific compensation elements or total compensation to any specific percentile relative to the Peer Groups or the broader United States market. The 3M C&T Committee instead applies informed judgment in establishing targeted pay levels for the Named Executive Officers, considering pay data from the executive compensation peer group and other factors, such as:
•the breadth and complexity of the executive’s duties and responsibilities;
•the quality of the executive’s leadership;
•the financial and operational performance of the business activities for which the executive is responsible;
•the executive’s ability to successfully achieve assigned goals related to company culture;
•the annual performance evaluation that the 3M Chief Executive Officer, assisted by 3M’s Executive Vice President and Chief Human Resources Officer and other 3M employees, completes for each Named Executive Officer (other than himself) and the annual performance evaluation that the 3M C&T Committee completes for the 3M Chief Executive Officer;
•the executive’s ability to successfully achieve assigned goals related to environmental, social, and governance matters, including sustainability goals;
•the executive’s performance rating for the prior year;
•experience and time in their current position (or other positions with comparable duties and responsibilities); and
•internal pay equity.
The 3M C&T Committee also uses information on the executive compensation practices at companies in the executive compensation peer group when considering design changes to 3M’s executive compensation program. Overall, 3M believes that use of this information from the peer groups enables the 3M C&T Committee to create better alignment between executive pay and performance and to help ensure that 3M can attract and retain high-performing executive leaders.
197
Section III: Overview of compensation program design
Target total direct compensation mix for 2023
The illustrations below show how the 2023 target Total Direct Compensation of the 3M CEO and other Named Executive Officers was apportioned among base salary, annual incentives, performance share awards, restricted stock units, and stock options. To provide a better representation of the intended mix of annual compensation provided to the Named Executive Officers, the percentages shown below do not take into consideration non-recurring special items such as one-time make-whole and inducement awards granted in connection with the commencement of employment or retention awards.
| CEO | Other NEOs (average)* | |||||||
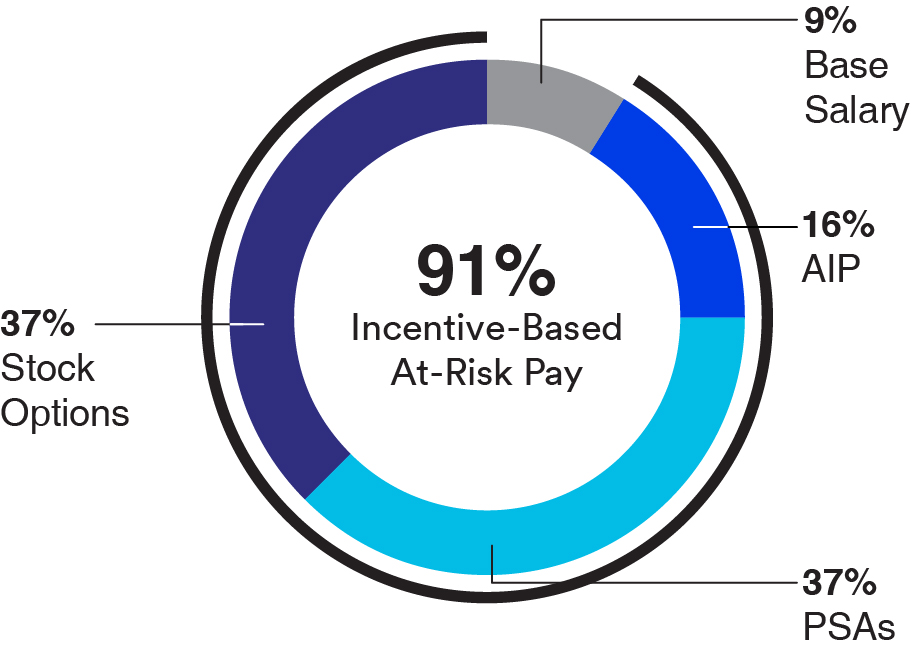 | 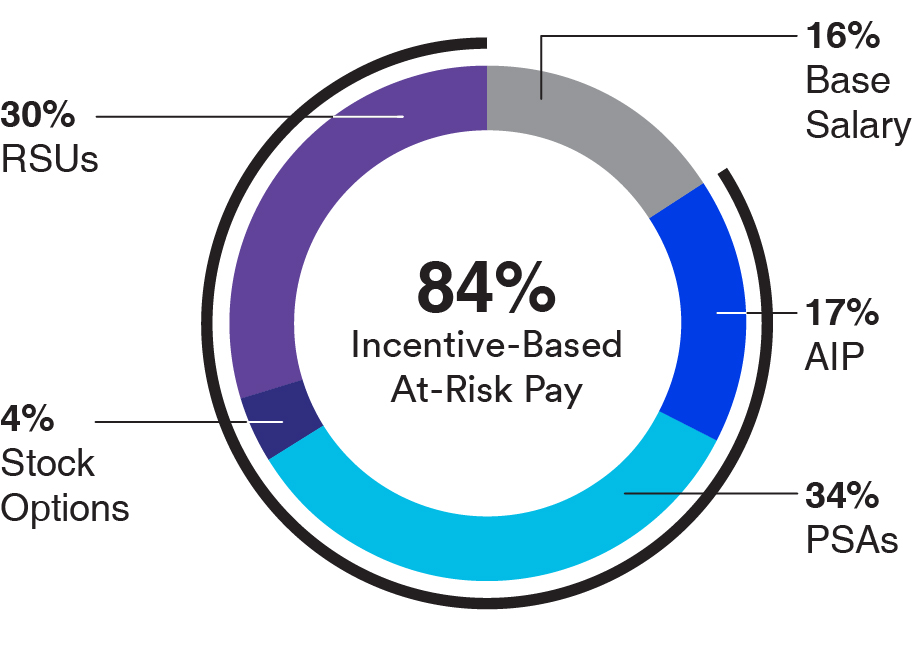 | |||||||
Abbreviations: AIP = annual incentive pay; PSAs = performance share awards; RSUs = restricted stock units.
__________________
*Amounts shown reflect the average apportionment for all Named Executive Officers other than Mr. Roman. Numbers may not add to 100 percent due to rounding.
Annual incentive
3M provides its executives with an opportunity to earn annual incentive compensation under the 3M Annual Incentive Plan, which 3M refers to as the “AIP.” Participation in the AIP is intended to align a significant portion of participants’ Total Cash Compensation with the near-term performance of 3M and its business units. Each executive is assigned a target amount of annual incentive compensation as part of his or her target Total Cash Compensation, but the actual amount paid under the AIP depends on the performance of 3M and its relevant business units and the executive’s individual performance, in each case, measured against preestablished goals and objectives.
198
Basic calculation. For 2023, the amount each Named Executive Officer earned under the AIP was calculated using the formula shown below.
| Total Weighted-Average Target AIP Payout ($) | Business Performance Multiplier (%) | Individual Performance Multiplier (%) | ESG Modifier (if any) ($) | Annual Incentive Payment ($) | ||||||||||||||||||||||
| X | X | ± | = | |||||||||||||||||||||||
| Calculated amount that reflects mid-year changes in the participant’s target annual incentive compensation opportunity | Corporate and business unit results adjust annual incentive pay based on performance against preestablished goals | Payouts adjusted or left unchanged based on individual performance against preestablished goals and objectives, which can be both quantitative and qualitative | Amounts earned may be adjusted by ±10 percent of the participant’s total weighted-average target AIP payout, based on the 3M C&T Committee’s assessment of 3M’s holistic performance against a set of preestablished, objective ESG metrics | Final payment amount may range from 0 percent to 200 percent of an individual’s total weighted-average target AIP payout | ||||||||||||||||||||||
Business performance factor. The business performance factor is determined based on the performance of 3M and, in some cases, the business unit(s) for which each Named Executive Officer had responsibility throughout the year against the goals established for the three metrics specified in the table below.
| Performance metric | Local currency sales vs. plan | Operating income vs. plan | Operating cash flow conversion vs. plan | |||||||||||||||||||||||
Weighting |  |  |  | |||||||||||||||||||||||
Business unit used to calculate business performance factor | Mr. Roman | 3M Worldwide | 3M Worldwide | 3M Worldwide | ||||||||||||||||||||||
| Mr. Patolawala | 3M Worldwide | 3M Worldwide | 3M Worldwide | |||||||||||||||||||||||
| Mr. Gibbons | 3M Worldwide | 3M Worldwide | 3M Worldwide | |||||||||||||||||||||||
| Mr. Hanson | Health Care | Health Care | 3M Worldwide | |||||||||||||||||||||||
| Mr. Rhodes | 3M Worldwide | 3M Worldwide | 3M Worldwide | |||||||||||||||||||||||
Individual performance multiplier. The amount of annual incentive compensation paid to an eligible employee may be increased, decreased, or left unchanged depending on his or her performance during the year. When determining the individual performance multipliers to be used for the Named Executive Officers, the 3M C&T Committee considers the individual performance of the Named Executive Officers using the performance evaluations described under “How the 3M C&T Committee establishes target compensation levels.”
ESG Modifier. Amounts earned by senior executives may be increased by 10 percent of target, decreased by 10 percent of target, or left unchanged based on the 3M C&T Committee’s determination of 3M’s holistic performance against a set of preestablished, objective ESG metrics (referred to as the “ESG Scorecard”). For 2023, the metrics included on the ESG Scorecard related to 3M’s carbon and water reduction commitments; operational improvements in the area of environment, health, and safety; progress on key social measures; and on-time completion of required ethics and compliance training.
199
Long-term incentives
3M provides its executives with long-term incentives to motivate executives to drive long-term shareholder value creation and incentivize executives to remain with 3M. Each of 3M’s executives (other than the CEO) may ask to receive 50 percent of the target grant value of their annual long-term incentive awards in the form of RSUs, stock options, or an equal split of both stock options and RSUs. Regardless of an executive’s indicated preference, the remaining 50 percent of the target grant value of his or her 2023 annual long-term incentive awards was delivered in the form of performance shares. For 3M’s CEO, the 3M C&T Committee chose to deliver 50 percent of the target grant value of his 2023 annual long-term incentive awards in the form of performance shares and the remaining 50 percent in the form of stock options. The terms of the 2023 performance share awards, 2023 stock options, and 2023 restricted stock units are described in detail below.
In limited circumstances, the Named Executive Officers also may receive other equity awards on an ad hoc basis as new hires or for recognition and retention, promotions, or other purposes. For additional information about a special performance-based RSU award granted to Mr. Patolawala in June 2023 and a special make-whole RSU award granted to Mr. Hanson in September 2023, see “2023 special long-term equity incentive awards.”
2023 performance share awards
Performance shares awarded in 2023 will result in the issuance of actual shares of 3M common stock to 3M’s Named Executive Officers if 3M achieves certain financial goals over the years 2023, 2024, and 2025. The number of shares of 3M common stock that may be issued is linked to 3M’s performance as measured by the equally weighted criteria of Adjusted Earnings per Share Growth, Relative Organic Sales Growth, and Free Cash Flow Growth. These performance criteria were selected because they are aligned with 3M’s operating plan and the financial objectives communicated to shareholders, and the 3M C&T Committee believes that they are important drivers of long-term shareholder value. Attainment of these three independent performance criteria is measured separately for each calendar year during the three-year measurement period, with each year weighted as follows: 2023 — 50 percent; 2024 — 30 percent; and 2025 — 20 percent. However, the targets against which 3M’s performance is measured over the course of the three-year performance period are fixed at the time the grant is awarded, subject to subsequent adjustments only in limited circumstances (such as a stock split, spin-off, etc.).
The actual number of shares of 3M common stock that will be delivered at the end of the three-year performance period ending on December 31, 2025, may be anywhere from 0 percent to 200 percent of the target number of shares awarded, depending on 3M’s performance over such time period. However, an executive may forfeit all or a portion of such shares if he or she does not remain employed by 3M throughout the performance period. Each performance share award also includes cash-settled dividend equivalent rights that are payable only on the final number of shares earned.
For awards tied to the achievement of performance goals over the years 2023, 2024, and 2025, the 3M C&T Committee approved the targets shown below, with the total number of shares actually delivered being the sum of the number of earned shares, based on 3M’s financial goal achievement. If 3M’s performance falls between any of the percentages listed below, the number of shares of 3M common stock earned will be determined by linear interpolation.
200
| 2023 Performance share award targets | 2023 Performance levels | 2024-2025 Performance levels | Payout level (% of target) | |||||||||||||||||||||||||||||
| Adjusted Earnings per Share Growth |  | Threshold* | –6% | 2 | % | 6 2/3% | ||||||||||||||||||||||||||
| Target | –4% – 0% | 5 | % | 33 1/3% | ||||||||||||||||||||||||||||
| Maximum | 1 | % | 8 | % | 66 2/3% | |||||||||||||||||||||||||||
| Relative Organic Sales Growth |  | Threshold* | –2.9% | –1.5% | 6 2/3% | |||||||||||||||||||||||||||
| Target | –1.4% – 0% | 0 | % | 33 1/3% | ||||||||||||||||||||||||||||
| Maximum | 0.5 | % | 1.5 | % | 66 2/3% | |||||||||||||||||||||||||||
| Free Cash Flow Growth |  | Threshold* | –2% | 2 | % | 6 2/3% | ||||||||||||||||||||||||||
| Target | 3 | % | 5 | % | 33 1/3% | |||||||||||||||||||||||||||
| Maximum | 8 | % | 8 | % | 66 2/3% | |||||||||||||||||||||||||||
__________________
*No payout is provided for below threshold performance.
3M C&T Committee Consideration of 2023 Long-Term Performance Incentive Targets After considering 3M’s 2023 operating plan and its long-term strategic plan, the 3M C&T Committee, in consultation with its independent compensation consultant and following discussions with management of 3M, set the threshold, target, and maximum goals as shown above based on its expectations for each year, with certain goals being set higher for 2024 and 2025. •The targets reflect 3M’s decision to exit PFAS manufacturing and, for the 2023 fiscal year, performance will be adjusted to neutralize any impact (positive or negative) associated with respirator sales, the discontinuation of 3M’s business in Russia, and changes in foreign currency exchange rates, which the 3M C&T Committee believed would more accurately reflect the management team’s performance results. •The 3M C&T Committee also added a “flat spot” or a target range to the payout curves for the Relative Organic Sales Growth and Adjusted Earnings per Share Growth metrics in order to ensure that the final payout for each metric in any given year would not exceed target unless 3M achieves positive growth on such metric. •The 3M C&T Committee believes all of the goals were set consistent with, and aligned to, 3M’s strategic priorities, significant transformation initiatives, internal operational plan, business outlook, objective of increasing long-term shareholder value, and pay-for-performance philosophy. | ||||||||
The above targets are not a prediction of how 3M will perform during the years 2023 through 2025 or any other period in the future. The sole purpose of these formulas, which were approved by the 3M C&T Committee at the time the awards were granted, is to establish a method for determining the number of shares of 3M common stock to
201
be delivered for the performance share awards described above. 3M is not providing any guidance, nor updating any prior guidance, of its future performance with the disclosure of these formulas, and you are cautioned not to rely on these formulas as a prediction of 3M’s future performance.
2023 stock options
Stock options granted to the Named Executive Officers in 2023 as part of their annual long-term incentive compensation have the following features:
•an exercise price equal to the closing price of a share of 3M common stock on the NYSE for the date of grant;
•a ratable three-year vesting schedule; and
•a maximum term of 10 years.
2023 restricted stock units
Restricted stock unit awards granted to the Named Executive Officers in 2023 as part of their annual long-term incentive compensation have the following features:
•a three-year “cliff” vesting schedule; and
•cash-settled dividend equivalent rights that are payable only if the underlying shares are earned.
Benefits and perquisites
The Named Executive Officers participate in the same health care, disability, life insurance, pension, and 401(k) benefit plans available to most of 3M’s U.S. employees. They also are eligible to receive certain additional benefits and perquisites that are provided for the executives’ convenience (relocation assistance for moves required by 3M, financial planning assistance, and meals when attending to 3M business, for example), financial security (nonqualified deferred compensation plans and additional group term life insurance coverage, for example), personal security (home security equipment/monitoring, for example) or personal health (on-site exercise facilities and physical exams, for example). The Named Executive Officers and other employees also may receive 3M tickets for sporting or other events. 3M believes that the benefits and perquisites offered generally are similar to those of our peers and assist in attracting and retaining executives. In some cases, there is no incremental cost to 3M associated with providing these additional benefits and perquisites (physical exams and certain tickets to events, for example) or the executives pay all or a substantial portion of the incremental costs incurred by 3M (on-site exercise facilities, for example).
These additional benefits and perquisites generally are provided on a consistent basis only to a limited group of 3M’s most senior U.S. employees (including all of the Named Executive Officers), although enhanced personal security equipment and monitoring is provided only to the Chief Executive Officer.
3M also operates aircraft that are used by its senior officers and other employees to conduct company business. For personal security reasons, the 3M Board requires the 3M Chief Executive Officer to use private aircraft chartered, leased, or owned by 3M for all air travel, both business and personal. As part of the benefits negotiated in connection with Mr. Hanson’s commencement of employment, 3M agreed to allow Mr. Hanson reasonable personal use of private aircraft chartered, leased, or owned by 3M, subject to compliance with 3M’s policy on personal use of corporate aircraft. When the 3M Chief Executive Officer or Mr. Hanson travel private aircraft chartered, leased, or owned by 3M, his or her spouse and other guests also may accompany him or her.
The incremental cost to 3M of providing these additional benefits to the Named Executive Officers is reflected in the All Other Compensation Table. No tax gross ups are provided on any of these additional benefits and perquisites other than taxable relocation benefits.
202
Section IV: Incentive compensation attainments and awards
2023 AIP attainments and payouts
During 2023, the 3M C&T Committee provided the Named Executive Officers with the opportunity to earn short-term incentive compensation under the AIP. Each Named Executive Officer’s target annual incentive for the year equaled the difference between his or her target Total Cash Compensation and annual base salary (weighted to reflect mid-year adjustments, if appropriate).
Business performance factor. For purposes of measuring business performance against the targets and converting that performance into a business performance factor in accordance with the AIP, each Named Executive Officer was assigned an appropriate business unit for each metric (the entire company, in some cases). The metrics, relevant business unit, goals, and attainments used to calculate the business performance factor for each Named Executive Officer are shown below.
The targets established for the Local Currency Sales and Operating Income metrics reflected 3M’s portfolio management actions, including 3M’s decision to exit PFAS manufacturing, the divestiture of 3M’s Food Safety business, the deconsolidation of its Aearo subsidiary, the discontinuation of 3M’s business in Russia, and the anticipated spin-off of 3M’s Health Care business, and the post-COVID reduction in demand for disposable respirators. The targets were informed by 3M’s operational plan for the year and were intended to be challenging and maintain a similar level of rigor as those established for past years.
Business performance factor calculation for Mr. Roman, Mr. Patolawala, Mr. Gibbons, and Mr. Rhodes*
Dollar amounts in millions
| Performance metric | Business unit | Threshold ($) | Target ($) | Maximum ($) | Actual vs. target | Payout % | Weighting | Weighted payout % | ||||||||||||||||||||||||||||||||||||||||||
| Local Currency Sales vs. Plan | 3M Worldwide | 31,514 | 98 | % | 81.3 | % |  | 40.7 | % | |||||||||||||||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 29,527 | 32,095 | 34,663 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Operating Income vs. Plan | 3M Worldwide | 6,374 | 104 | % | 126.7 | % |  | 38.0 | % | |||||||||||||||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 5,198 | 6,115 | 7,032 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Operating Cash Flow Conversion vs. Plan | 3M Worldwide | 145% | 104 | % | 126.7 | % |  | 25.3 | % | |||||||||||||||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 119 | % | 140 | % | 161 | % | |||||||||||||||||||||||||||||||||||||||||||||
| Business Performance Factor | 104.0 | % | ||||||||||||||||||||||||||||||||||||||||||||||||
203
Business performance factor calculation for Mr. Hanson*
Dollar amounts in millions
| Performance metric | Business unit | Threshold ($) | Target ($) | Maximum ($) | Actual vs. target | Payout % | Weighting | Weighted payout % | ||||||||||||||||||||||||||||||||||||||||||
Local Currency Sales vs. Plan(1) | Health Care | 8,243 | 98 | % | 81.3 | % |  | 40.7 | % | |||||||||||||||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 7,747 | 8,421 | 9,095 | ||||||||||||||||||||||||||||||||||||||||||||||||
Operating Income vs. Plan(1) | Health Care | 1,605 | 93 | % | 65.0 | % |  | 19.5 | % | |||||||||||||||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 1,471 | 1,730 | 1,990 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Operating Cash Flow Conversion vs. Plan | 3M Worldwide | 145% | 104 | % | 126.7 | % |  | 25.3 | % | |||||||||||||||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 119 | % | 140 | % | 161 | % | |||||||||||||||||||||||||||||||||||||||||||||
Business Performance Factor | 85.5 | % | ||||||||||||||||||||||||||||||||||||||||||||||||
__________________
*Results reflect certain adjustments that the 3M C&T Committee believed were appropriate to better reflect 3M’s 2023 performance. See Appendix A for a reconciliation of 3M Worldwide Local Currency Sales, Operating Income and Operating Cash Flow Conversion used for compensation purposes to 3M’s results for the most directly comparable financial measures as reported under GAAP.
(1)In light of the challenging goal-setting environment in 2023 given macroeconomic conditions and uncertainty in the markets in which we do business, the 3M C&T Committee set stretch targets for each business unit’s Local Currency Sales and Operating Income metrics that, in the aggregate, were above the targets for 3M Worldwide (such excess amounts over the 3M Worldwide targets, the “corporate allocation” for each metric). Portions of the corporate allocation were then applied to the business unit targets throughout the year as the 2023 market dynamics and their impacts on each business unit were better understood, so that the sum of the final business unit targets equaled the 3M Worldwide target set in early 2023 for each metric. The Health Care business unit goals in the table above include $104 million of corporate allocation to the preliminary Local Currency Sales target for the Health Care business unit and $287 million of corporate allocation to the preliminary Operating Income target for the Health Care business unit.
Individual performance multiplier. The amount of annual incentive compensation paid to an eligible employee may be increased, decreased, or left unchanged depending on his or her performance during the year. The 3M C&T Committee determined the individual performance multiplier for each Named Executive Officer based upon each officer’s performance evaluation, as shown in the table under “Final 2023 AIP payouts” below. For a listing of selected individual 2023 performance highlights, see “Section V: 2023 compensation decisions and performance highlights.”
ESG Modifier. Amounts earned by 3M’s senior executives may be increased by 10 percent of target, decreased by 10 percent of target, or left unchanged based on the 3M C&T Committee’s assessment of 3M’s overall performance against a collection of pre-set objective ESG goals. For 2023, the ESG goals approved by the 3M C&T Committee related to 3M’s carbon and water commitments, operational improvements in the area of environment, health, and safety, progress on key social measures, and on-time completion of required ethics and compliance training for our global workforce. 3M successfully achieved most of its 2023 ESG goals. After reviewing 3M’s performance, the 3M C&T Committee decided not to increase or decrease the senior executives’ 2023 annual incentive payouts using the ESG modifier.
204
Final 2023 AIP payouts. At its meeting in February 2024, the 3M C&T Committee approved (and with respect to Mr. Roman, the independent members of the 3M Board ratified) AIP payments as shown below.
| (a) | (b) | (c) | (d) | (e) = (a) × (b) × (c) + (d) | |||||||||||||||||||||||||||||||
| Named Executive Officer | Total weighted- average target AIP payout* ($) | Business performance factor | Individual performance multiplier** | ESG modifier ($) | Approved 2023 AIP payout ($) | ||||||||||||||||||||||||||||||
| Michael F. Roman | 2,562,952 | 104.0 | % | 100 | % | — | 2,665,470 | ||||||||||||||||||||||||||||
| Monish Patolawala | 1,485,901 | 104.0 | % | 100 | % | — | 1,545,337 | ||||||||||||||||||||||||||||
| Peter D. Gibbons | 832,500 | 104.0 | % | 120 | % | — | 1,038,960 | ||||||||||||||||||||||||||||
| Bryan C. Hanson | 676,755 | 85.5 | % | 100 | % | — | 578,558 | ||||||||||||||||||||||||||||
| Kevin H. Rhodes | 785,508 | 104.0 | % | 100 | % | — | 816,928 | ||||||||||||||||||||||||||||
__________________
*Amounts shown reflect mid-year adjustments to target Total Cash Compensation. The amounts shown for Mr. Hanson are prorated to reflect the portion of the year worked for 3M.
** For a listing of selected 2023 performance highlights of each Named Executive Officer, see “Section V: 2023 compensation decisions and performance highlights.”
2023 annual long-term incentive awards
After considering the most recent compensation data available from companies in the peer groups and 2022 individual performance, the 3M C&T Committee approved (and in the case of Mr. Roman, the independent members of the 3M Board ratified) the 2023 annual target grant values for the Named Executive Officers’ long-term incentive compensation awards.
| Name | Target grant value of 2023 annual performance share awards ($) | Target grant value of 2023 annual stock option awards ($) | Target grant value of 2023 annual RSU awards ($) | Aggregate target grant value of all 2023 annual awards ($) | |||||||||||||||||||||||||
| Michael F. Roman | 6,000,000 | 6,000,000 | — | 12,000,000 | |||||||||||||||||||||||||
| Monish Patolawala | 2,931,500 | 1,465,750 | 1,465,750 | 5,863,000 | * | ||||||||||||||||||||||||
| Peter D. Gibbons | 1,570,000 | — | 1,570,000 | 3,140,000 | |||||||||||||||||||||||||
| Bryan C. Hanson (joined 3M eff. September 1, 2023) | — | — | — | — | ** | ||||||||||||||||||||||||
| Kevin H. Rhodes | 1,904,500 | — | 1,904,500 | 3,809,000 | |||||||||||||||||||||||||
__________________
*Excludes the target grant value of a special award granted to Mr. Patolawala. For additional information, see “2023 special long-term equity incentive awards.”
** Excludes the target grant value of a special one-time make-whole award granted to Mr. Hanson in connection with his commencement of employment. For additional information, see “2023 special long-term equity incentive awards.”
Each of 3M’s executives (other than its CEO) was given an opportunity to indicate a preference to receive 50 percent of the target grant value of their annual long-term incentive awards in the form of RSUs, stock options, or an equal split of both stock options and RSUs. Regardless of an executive’s indicated preference, the remaining 50 percent of the target grant value was delivered in the form of performance shares. For the 3M CEO, the 3M C&T Committee chose to deliver 50 percent of the target grant value of his annual long-term incentive awards in the form of performance shares and the remaining 50 percent in the form of stock options.
Status of outstanding performance share awards
3M’s annual award cycle and three-year performance periods result in an overlap of awards. For example, the performance goals for 2023 performance share awards relate to the years 2023, 2024, and 2025. Similarly, the performance goals for 2022 performance share awards relate to the years 2022, 2023, and 2024, and so on, as shown
205
below. Performance against the goals established for each award are measured separately for each calendar year during the measurement period, with each year weighted as shown below in parenthesis. The 3M C&T Committee believes this structure reduces motivation to maximize performance in any one period by providing the highest-level rewards only by building sustainable long-term results.
| Award | 2021 | 2022 | 2023 | 2024 | 2025 | |||||||||||||||||||||||||||
| 2021 PSA | Year 1 (50%) | Year 2 (30%) | Year 3 (20%) | |||||||||||||||||||||||||||||
| 2022 PSA | Year 1 (50%) | Year 2 (30%) | Year 3 (20%) | |||||||||||||||||||||||||||||
| 2023 PSA | Year 1 (50%) | Year 2 (30%) | Year 3 (20%) | |||||||||||||||||||||||||||||
The 3M C&T Committee periodically reviews 3M’s performance against the goals established for each performance share award throughout the duration of its measurement period. The tables below summarize the status of the different performance share awards held by the Named Executive Officers as of December 31, 2023.
2023 PSA (2023-2025 measurement period)
Three-year performance period – actual performance level achieved(1) | ||||||||||||||||||||||||||||||||||||||
| Performance measures and weighting | Performance levels | 2023 (Year 1; weighted at 50%) | 2024 (Year 2; weighted at 30%) | 2025 (Year 3; weighted at 20%) | ||||||||||||||||||||||||||||||||||
| Threshold | Target | Maximum | ||||||||||||||||||||||||||||||||||||
Adjusted Earnings per Share Growth (33 1/3%)(2) | 2023: -6.0% 2024/25: 2.0% | 2023: -4.0 - 0.0% 2024/25: 5.0% | 2023: 1:0% 2024/25: 8.0% | -0.4 | % | |||||||||||||||||||||||||||||||||
Relative Organic Sales Growth (33 1/3%)(3) | 2023: -2.9% 2024/25: -1.5% | 2023: -1.4 - 0.0% 2024/25: 0.0% | 2023: 0.5% 2024/25: 1.5% | -2.8 | % | |||||||||||||||||||||||||||||||||
| Free Cash Flow Growth (33 1/3%) | 2023: -2.0% 2024/25: 2.0% | 2023: 3.0% 2024/25: 5.0% | 2023: 8.0% 2024/25: 8.0% | 30.3 | % | |||||||||||||||||||||||||||||||||
2022 PSA (2022-2024 measurement period)
Three-year performance period – actual performance level achieved(1) | ||||||||||||||||||||||||||||||||||||||
| Performance measures and weighting | Performance levels | 2022 (Year 1; weighted at 50%) | 2023 (Year 2; weighted at 30%) | 2024 (Year 3; weighted at 20%) | ||||||||||||||||||||||||||||||||||
| Threshold | Target | Maximum | ||||||||||||||||||||||||||||||||||||
Adjusted Earnings per Share Growth (33 1/3%)(2) | 2022: 1:0% 2023/24: 3.0% | 2022: 4:0% 2023/24: 6.0% | 2022: 7:0% 2023/24: 9.0% | -0.2 | % | -6.5 | % | |||||||||||||||||||||||||||||||
Relative Organic Sales Growth (33 1/3%)(3) | -1.5% | 0.0% | 1.5% | -1.7 | % | -4.7 | % | |||||||||||||||||||||||||||||||
| Free Cash Flow Growth (33 1/3%) | 2022: -7:0% 2023/24: 3.0% | 2022: -4:0% 2023/24: 6.0% | 2022: -1:0% 2023/24: 9.0% | -21.5 | % | 30.3 | % | |||||||||||||||||||||||||||||||
206
2021 PSA (2021-2023 measurement period)
Three-year performance period – actual performance level achieved(1) | ||||||||||||||||||||||||||||||||||||||
| Performance levels | 2021 (Year 1; weighted at 50%) | 2022 (Year 2; weighted at 30%) | 2023 (Year 3; weighted at 20%) | |||||||||||||||||||||||||||||||||||
| Performance measures and weighting | Threshold | Target | Maximum | |||||||||||||||||||||||||||||||||||
Adjusted Earnings per Share Growth (20%)(2) | 4.0 | % | 8.0 | % | 12.0 | % | 14.4 | % | -0.2 | % | -6.5 | % | ||||||||||||||||||||||||||
Relative Organic Volume Growth (40%)(4) | -1.0 | % | 0.5 | % | 2.0 | % | 0.5 | % | -7.1 | % | -7.3 | % | ||||||||||||||||||||||||||
| Return on Invested Capital (20%) | 16.0 | % | 18.0 | % | 21.0 | % | 19.5 | % | 18.4 | % | 16.9 | % | ||||||||||||||||||||||||||
| Free Cash Flow Conversion (20%) | 95.0 | % | 100.0 | % | 105.0 | % | 100.9 | % | 81.8 | % | 122.9 | % | ||||||||||||||||||||||||||
__________________
(1)Results reflect certain adjustments that the 3M C&T Committee believed were appropriate to better reflect 3M’s performance during the performance period. See Appendix A for a reconciliation of Adjusted Earnings per Share, Return on Invested Capital, Free Cash Flow, and Free Cash Flow Conversion used for compensation purposes to our results for the most directly comparable financial measures as reported under GAAP.
(2)For purposes of calculating Adjusted Earnings per Share Growth for any given fiscal year, the baseline Adjusted Earnings per Share figure is set equal to the final Adjusted Earnings per Share figure used to calculate the Adjusted Earnings per Share Growth attainment for the preceding year. As a result, any increase in Adjusted Earnings per Share attributable to adjustments in one fiscal year necessarily will make it more difficult for 3M to achieve its Adjusted Earnings per Share Growth target in the following year.
(3)The reported level of performance for Relative Organic Sales Growth has been determined, in part, using a weighted blend of Worldwide IPI and Worldwide GDP, as reported by S&P Global Market Intelligence on January 15, 2024.
(4)The reported level of performance achieved for Relative Organic Volume Growth has been determined, in part, using the Worldwide IPI for each relevant period, as reported by S&P Global Market Intelligence on January 15, 2024. The final performance level achieved may vary based on changes in reported Worldwide IPI for the relevant period.
Performance share accruals based on 2023 performance
The table below shows the number of shares of 3M common stock that were accrued for the outstanding performance share awards held by each Named Executive Officer (other than Mr. Hanson) based on 3M’sperformance during 2023. As Mr. Hanson joined 3M on September 1, 2023, he did not receive any performance share awards based on 3M’s performance during 2023.
| Name | Performance share award | Target number of performance shares | Fraction of each target performance share accrued based on 2023 performance | Total number of shares accrued based on 2023 performance(1) | Market value of shares accrued based on 2023 performance(2)($) | |||||||||||||||||||||||||||
| Michael F. Roman | 2023 PSA | 54,442 | 0.542 | 29,508 | 3,225,767 | |||||||||||||||||||||||||||
| 2022 PSA | 37,997 | 0.200 | 7,603 | 831,174 | ||||||||||||||||||||||||||||
| 2021 PSA | 29,761 | 0.102 | 3,048 | 333,200 | ||||||||||||||||||||||||||||
| Total | 4,390,141 | |||||||||||||||||||||||||||||||
| Monish Patolawala | 2023 PSA | 26,600 | 0.542 | 14,417 | 1,576,088 | |||||||||||||||||||||||||||
| 2022 PSA | 19,310 | 0.200 | 3,864 | 422,405 | ||||||||||||||||||||||||||||
| 2021 PSA | 12,160 | 0.102 | 1,245 | 136,124 | ||||||||||||||||||||||||||||
| Total | 2,134,617 | |||||||||||||||||||||||||||||||
Peter D. Gibbons(3) | 2023 PSA | 14,246 | 0.542 | 7,721 | 844,096 | |||||||||||||||||||||||||||
| 2022 PSA | 10,432 | 0.200 | 2,087 | 228,156 | ||||||||||||||||||||||||||||
| Total | 1,072,252 | |||||||||||||||||||||||||||||||
| Kevin H. Rhodes | 2023 PSA | 17,282 | 0.542 | 9,366 | 1,023,865 | |||||||||||||||||||||||||||
| 2022 PSA | 9,328 | 0.200 | 1,866 | 204,020 | ||||||||||||||||||||||||||||
| 2021 PSA | 852 | 0.102 | 88 | 9,627 | ||||||||||||||||||||||||||||
| Total | 1,237,512 | |||||||||||||||||||||||||||||||
__________________
(1)The amounts in this column reflect the number of shares accrued based on, among other things, Worldwide IPI for the 2023 calendar year, as reported by S&P Global Market Intelligence on January 15, 2024. The final number of shares accrued may vary in the event of changes
207
in Worldwide IPI reported by S&P Global Market Intelligence. Due to rounding, the numbers shown in this column may not equal the result obtained by multiplying the Target Number of Performance Shares by the Shares Accrued Per Target Performance Share Based on 2023 performance.
(2)Represents the closing price of a share of 3M common stock on the NYSE for December 29, 2023 ($109.32), multiplied by the total number of shares accrued (before rounding) based on 3M’s 2023 performance. Amounts shown do not include the value of the cash-settled divided equivalents that will be paid based on the final number of shares earned.
(3)Mr. Gibbons joined 3M and was appointed its Group President, Enterprise Operations, effective November 29, 2021.
Although shares of 3M common stock are accrued annually for each outstanding performance share award, an executive may forfeit all or a portion of the shares otherwise issuable pursuant to his or her award if he or she does not remain employed by 3M throughout the entire three-year performance period.
For additional information concerning the manner in which the compensation of the Named Executive Officers is determined and the role of the 3M C&T Committee and its advisors, see “Section II: How 3M determines executive compensation — Roles and responsibilities.”
2023 special long-term equity incentive awards
Although the 3M C&T Committee typically does not grant off-cycle equity awards, it may do so to attract, retain, and motivate talented leaders in extraordinary circumstances.
Special performance-based RSU award for Mr. Patolawala.
Mr. Patolawala, who was named President and Chief Financial Officer of 3M, effective September 1, 2023, has played, continues to play, and is expected to continue playing a key role in the design, implementation, and oversight of multiple projects that are vital to the successful transformation of 3M, including the separation of the Health Care business; the creation of a future roadmap for 3M, aligned to strong end-markets where 3M wins; and the expansion of 3M’s Global Service Center. Mr. Patolawala also plays a pivotal role in 3M’s ongoing efforts to deliver financial results that meet investor expectations through tight operating rigor and a strong cash position. The 3M C&T Committee believes that retaining Mr. Patolawala’s leadership is essential at this time given his expertise in critical areas of need for 3M, the role he plays within the cohesive executive leadership team, including his new role as President of 3M, and his significant contributions to 3M’s transformation efforts, all of which requires above and beyond effort and commitment, in addition to his day-to-day duties and responsibilities as Chief Financial Officer.
Recognizing the pivotal role and contributions of Mr. Patolawala’s near-term efforts to 3M’s long-term success, the 3M C&T Committee approved a special performance-based RSU award for Mr. Patolawala, effective June 1, 2023, with a target grant value of $2.5 million (referred to as the “Performance-based RSU Award”). In alignment with 3M’s pay-for-performance philosophy, the award is exclusively performance-based and is eligible to vest on June 30, 2024, but only if four critical strategic initiatives related to 3M’s transformation are achieved. If any one of the four performance goals is not achieved, no portion of the award will vest, underscoring the rigor of the incentive opportunity. The performance conditions were carefully calibrated by the 3M C&T Committee with input from its independent compensation consultant to incentivize delivery of exceptional strategic and operational results during a pivotal period for 3M’s future. Vesting is also subject to continued employment, except for limited circumstances in accordance with 3M standard provisions (e.g. vesting upon termination due to death, disability, a qualifying termination following a change in control, or in the event of a termination by 3M other than due to misconduct, provided in such case that all of the performance goals are still timely achieved).
| Realignment of Global Services Center and Strategic Planning | Provide satisfactory change leadership for the Global Service Center and Strategic Planning teams and ensure the successful transition and integration of such teams into their new functional alignment | |||||||
| Transformation Roadmap and Execution | Develop a transformation plan for the Global Service Centers and deliver satisfactory progress against the plan of record established for such transformation efforts | |||||||
| 2023 Strategic Plan | Successfully complete the 2023 strategic planning cycle | |||||||
| Solventum Spin-off | Execute against key performance milestones related to the Health Care business spin-off | |||||||
208
Considering the critical role Mr. Patolawala plays in the future success of 3M, and upon review of his total compensation package, the 3M C&T Committee determined that the award was reasonable, appropriate, and in the best interests of 3M C&T . The 3M C&T Committee strongly believes that 3M and its shareholders will benefit from Mr. Patolawala’s continued leadership, including in his new role as President.
Special make-whole RSU award for Mr. Hanson
Upon Mr. Hanson’s commencement of employment with 3M, he entered into an agreement that protects 3M’s confidential information and includes noncompetition and non-solicitation covenants. In consideration for such agreement and in connection with his appointment, Mr. Hanson received a make-whole RSU award with a target grant value of $13,000,000, vesting in equal installments on each of the first three anniversaries of the grant date. The make-whole RSU award was intended to partially offset the value of certain unvested equity awards that Mr. Hanson forfeited when he left his prior employer to join 3M. The 3M C&T Committee believes the make-whole RSU award and overall compensation package offered to Mr. Hanson were necessary to incentivize Mr. Hanson to leave his former position to accept the job offer with 3M and appropriate in light of his unique qualifications and proven executive track record of successfully leading, growing and transforming global medical device businesses. For additional information regarding Mr. Hanson’s initial compensation package, see “Section V: 2023 compensation decisions and performance highlights — Bryan C. Hanson — Compensation Decisions.”
Section V: 2023 compensation decisions and performance highlights
Michael F. Roman
Chairman of the 3M Board and Chief Executive Officer
Selected 2023 performance highlights
•Took decisive action to simplify 3M, initiating restructuring to streamline and optimize the organization, which drove strong benefits in 2023 and continues into 2024, improving adjusted operating margins used for compensation purposes to 20.3 percent.*
•Drove portfolio optimization and positioned 3M for future growth by streamlining go-to-market structures, investing in attractive markets (such as automotive electrification, climate technology, and industrial automation), and executing on geographic prioritization.
•Continued strong operational execution throughout 2023 with a focus on working capital improvement, advancing supply chain performance to improve service, driving inventory levels down, and delivering productivity and improved yields. Through this focus, delivered Free Cash Flow Conversion used for compensation purposes of 123 percent alongside strong underlying financial returns.*
•Continued to advance and execute the planned Health Care spin-off, including building a strong leadership team, which remains on track for first-half 2024.
•Deepened 3M’s commitment to reducing risk and uncertainty, advancing the execution towards the 2025 PFAS manufacturing exit, and discontinuation of PFAS use across 3M’s product portfolio, and making substantial progress towards Public Water Systems and Combat Arms Earplug settlements.
•Continued driving progress towards the bold, public goals established to achieve carbon neutrality, reduce water use, and improve water quality.
•Advanced 3M’s leadership and impact in the communities we serve, including delivering on-pace against the $50M investment commitment made in 2020 to address racial opportunity gaps, delivering over 1.2 million learning experiences for underrepresented individuals, providing over 40,000 work hours in skill-based services, and increasing diverse supplier spending in 2023.
•Continued developing 3M’s senior leadership team, including the appointment of a new EVP and Chief Public Affairs Officer, and several internal promotions. Continued to deliver leadership development
209
experiences and retention and recognition programs, retaining and advancing talent in a competitive talent market.
*See Appendix A for a reconciliation of adjusted operating margins and Free Cash Flow Conversion used for compensation purposes to 3M’s results for the most directly comparable financial measures as reported under GAAP.
Compensation decisions
In February 2023, the 3M C&T Committee approved, and the independent members of the 3M Board ratified, the following compensation actions with respect to Mr. Roman:
•a 3.7 percent increase, effective April 1, 2023, to target Total Cash Compensation (from $3,918,750 to $4,063,744), which included a base salary increase (from $1,425,000 to $1,477,725); and
•the issuance of long-term incentive awards with an aggregate target grant value of $12 million, which was split equally between performance shares and stock options.
The increase to Mr. Roman’s target Total Cash Compensation was intended to progress his compensation closer to the market median.
In February 2024, the 3M C&T Committee approved, and the independent members of the 3M Board ratified, a 2023 AIP payout for Mr. Roman in the amount of $2,665,470, which represented 104.0 percent of his target. See “Section IV: Incentive compensation attainments and awards — 2023 AIP attainments and payouts” for more information.
Monish Patolawala
President and Chief Financial Officer
Selected 2023 performance highlights
•Advanced portfolio optimization, through leadership of the carve out and operational execution of the planned Health Care spin-off and driving restructuring execution rigor including geographic portfolio execution.
•Continued to drive improvements in operating rigor and deliver sustainable returns from the 3M model resulting in expanded margins and strong cash generation. Championed working capital council, delivering strong Free Cash Flow Conversion, and reducing risk and uncertainty through litigation settlements and investments in cybersecurity.
•Advanced organic capital allocation to prioritize investments in higher growth priorities, infrastructure, and environmental, health and safety.
•Expanded role with new leadership responsibilities for strategy, global service centers and country governance, while still advancing transformation and agility through daily management, predictive visualization and tighter root cause analysis.
•Active participation with and mentoring of diverse talent, including as executive sponsor of the 3M Asian employee affinity group. Continued visible leadership as a strong and frequent communicator inside and outside of 3M.
210
Compensation decisions
In February 2023, the 3M C&T Committee approved the following compensation actions with respect to Mr. Patolawala in recognition of his exceptional performance in 2022:
•a 5.9 percent increase, effective April 1, 2023, to target Total Cash Compensation (from $2,378,354 to $2,519,308), which included a base salary increase (from $1,106,211 to $1,171,771); and
•the issuance of long-term incentive awards with an aggregate target grant value of $5,863,000, which was split 50 percent performance shares, 25 percent restricted stock units, and 25 percent stock options.
In light of the significant contributions expected of Mr. Patolawala (including leadership of 3M’s Global Service Center expansion, oversight of critical aspects of the anticipated spin-off of 3M’s Health Care business, and 3M’s transformation) and to encourage the retention of Mr. Patolawala’s unique skillset during a critical time for 3M, the 3M C&T Committee approved the following additional compensation actions with respect to Mr. Patolawala in May 2023:
•a 20.0 percent increase, effective June 1, 2023, to target Total Cash Compensation (from $2,519,308 to $3,023,170), which included a base salary increase (from $1,171,771 to $1,406,125); and
•the issuance of a performance-based retention award with a target grant value of $2,500,000, which will vest in June 2024 upon the achievement of four rigorous qualitative performance objectives. See “2023 special long-term equity incentive awards” for more information.
Following the 3M C&T Committee’s actions in May 2023, Mr. Patolawala’s target Total Cash Compensation and target total direct compensation exceeded market median. The Committee determined these levels to be appropriate given Mr. Patolawala’s skills and contributions and the role he plays within the cohesive executive leadership team.
In February 2024, the Committee approved a 2023 AIP payout for Mr. Patolawala in the amount of $1,545,337, which represented 104.0 percent of his target. See “Section IV: Incentive compensation attainments and awards — 2023 AIP attainments and payouts” for more information.
Peter D. Gibbons
Group President, Enterprise Supply Chain
Selected 2023 performance highlights
•Navigated new supply chain demands with agility, driving restructuring aligned to a new Supply Chain organization model designed to deliver efficiency and cost improvement, with lower overhead.
•Drove substantial reduction in backlog and improved service to our customers, while reducing $550M inventory, lowering our spending in logistics and sourcing, and improving manufacturing performance.
•Identified and prioritized actions in safety performance, including focused safety campaigns, leadership training and expectation reinforcement, establishing incident reduction goals, and focused root cause efforts in key plants.
•Actively delivered on portfolio optimization actions, including portfolio value chain analysis, support for the planned spin-off of the Health Care business, and progressing the PFAS manufacturing exit.
•Integrated five key new senior vice presidents and realigned the organization to be focused on transformation and outcomes. Advanced hiring and internal promotions at all levels consistent with key priorities. Actively coached and mentored across 3M leadership, including executive sponsorship of the 3M Pride enterprise resource network.
211
Compensation decisions
In February 2023, the 3M C&T Committee approved the following compensation actions with respect to Mr. Gibbons:
•a 3.7 percent increase, effective April 1, 2023, to target Total Cash Compensation (from $1,620,000 to $1,680,000), which included a base salary increase (from $810,000 to $840,000); and
•the issuance of long-term incentive awards with an aggregate target grant value of $3,140,000, which was split equally between performance shares and restricted stock units.
The increase to Mr. Gibbons’ target Total Cash Compensation was intended to progress his compensation closer to the market median.
In February 2024, the 3M C&T Committee approved a 2023 AIP payout for Mr. Gibbons in the amount of $1,038,960, which represented 124.8 percent of his target. See “Section IV: Incentive compensation attainments and awards — 2023 AIP attainments and payouts” for more information.
Bryan C. Hanson
Group President and Chief Executive Officer, Health Care
Selected 2023 performance highlights
•Joined 3M in September 2023 as Group President and Chief Executive Officer, Health Care and onboarded in preparation for leading the planned independent health care company when it is spun off.
•Assembled a strong leadership team with deep functional expertise, experience in the health care and med tech industries, and relevant experience in spin-offs and other transactions.
•Guided Health Care’s business and operational performance through the final quarter, continuing to drive organic growth, operational efficiencies, and geographic prioritization.
Compensation decisions
On September 1, 2023, 3M appointed Mr. Hanson as Group President and Chief Executive Officer of the Health Care business and, upon the completion of the spin-off of the Health Care business, as Chief Executive Officer of Solventum. Mr. Hanson’s initial target Total Cash Compensation was set at $3,375,000 consisting of an annual base salary of $1,350,000 and a target annual incentive compensation opportunity of $2,025,000.
Upon Mr. Hanson’s commencement of employment with 3M, he entered into an agreement that protects 3M’s confidential information and includes non-competition and non-solicitation covenants. In consideration for such agreement and in connection with his appointment, Mr. Hanson received (a) a hiring bonus of $2,700,000, subject to repayment if his employment is terminated for any reason prior to September 1, 2025; (b) a make-whole cash award in the amount of $13,000,000, 50 percent of which vested on September 1, 2023 and the remaining 50 percent of which will vest on the first anniversary thereof, subject to his continued employment; and (c) a make-whole RSU award with a target grant value of $13,000,000, vesting in equal installments on each of the first three anniversaries of the grant date. Mr. Hanson is also eligible to receive following the spin-off, (x) a Solventum inducement performance share award with a target grant date value of $16,000,000 that will be subject to goals to be established by the compensation committee or the board of directors of Solventum, and (y) as his “annual” long-term incentive compensation in 2024, a Solventum RSU award and a Solventum performance share award, each with a target grant date value of $6,500,000.
The sign-on awards were intended to incentivize Mr. Hanson to leave his former position to join 3M, offset the value of certain unvested equity awards and annual cash incentive that Mr. Hanson forfeited when he left his former employer to join 3M, and incentivize the successful launch of the Heath Care business unit as a standalone world-class public company.
212
In determining the sign-on package and its structure, the 3M C&T Committee considered Mr. Hanson’s unique qualifications and proven executive track record of successfully leading, growing and transforming global medical device businesses and evaluated the value and structure of incentive opportunities Mr. Hanson was forfeiting at his former employer, which included portions of performance-based equity awards covering three separate performance cycles as well as portions of four annual options grants, in addition to an annual cash incentive. With the assistance of its compensation consultant, the 3M C&T Committee estimated that Mr. Hanson was forfeiting approximately $28 million in total incentive value that he otherwise would have earned at his prior employer. The 3M C&T Committee believes the sign-on cash and RSU awards and overall compensation package offered to Mr. Hanson were necessary to secure his commitment to, and leadership of, the Health Care business at this pivotal time of 3M’s strategic transformation.
If Mr. Hanson’s employment is terminated without misconduct or for good reason, then, subject to his execution and nonrevocation of a release of claims: (a) any unvested portion of the make-whole RSU award will vest; (b) any unpaid portion of the make-whole cash award will be paid; and (c) if the basis for Mr. Hanson’s termination of employment is 3M’s breach of its covenant to appoint him, prior to January 1, 2024, as Chief Executive Officer of an independent, publicly traded company established to hold 3M’s Health Care business, Mr. Hanson also will be entitled to (i) continued payment of his annual base salary and annual incentive plan payments for 24 months following such termination in accordance with the 3M Executive Severance Plan, and (ii) a $16,000,000 lump sum cash payment (in lieu of the inducement performance share award that otherwise would be granted following the spin-off).
In February 2024, the 3M C&T Committee approved a 2023 AIP payout for Mr. Hanson in the amount of $578,558, which represented 85.5 percent of his prorated target. See “Section IV: Incentive compensation attainments and awards — 2023 AIP attainments and payouts” for more information.
Kevin H. Rhodes
Executive Vice President and Chief Legal Affairs Officer
Selected 2023 performance highlights
•Led in-house and external legal efforts in support and implementation of settlement agreements related to US Public Water Systems PFAS matters and Combat Arms Earplugs litigation.
•Drove actions to reduce risk and uncertainty through reduced exposure to litigation and regulatory risks, aligning and driving priorities with teams focused on litigation, information, digital and data privacy, in compliance with emerging regulations globally, and in the management of ethics and compliance matters and investigations.
•Led Legal Affairs’ strategies in support of the Health Care spin-off, including design of legal agreements, governance documents, filings, and other workstreams involved in creation of two public companies.
•Provided legal support to the 3M Board in connection with significant corporate actions, including settlement agreements, exit of PFAS manufacturing, the spin-off of the Health Care business, and other matters.
•Delivered on spending commitments within the Legal Affairs department.
•Provided visible senior leadership on compliance and ethical business conduct issues, and on legal settlements. Continued to actively mentor and coach, including advancing external leadership in organizations promoting diversity in the legal profession and internally as executive sponsor of 3M Black Leadership Advancement Coalition.
213
Compensation decisions
In February 2023, the 3M C&T Committee approved the following compensation actions with respect to Mr. Rhodes in recognition of his exceptional performance in 2022:
•a 7.1 percent increase, effective April 1, 2023, to target Total Cash Compensation (from $1,575,000 to $1,686,060), which included a base salary increase (from $828,947 to $887,400); and
•the issuance of long-term incentive awards with an aggregate target grant value of $3,809,000, which was split equally between performance shares and restricted stock units.
The increase to Mr. Rhodes’s target Total Cash Compensation was intended to progress his compensation closer to the market median.
In February 2024, the 3M C&T Committee approved a 2023 AIP payout for Mr. Rhodes in the amount of $816,928, which represented 104.0 percent of his target. See “Section IV: Incentive compensation attainments and awards — 2023 AIP attainments and payouts” for more information.
Section VI: Ways in which 3M addresses risk and governance
Stock ownership guidelines
3M maintains robust stock ownership guidelines that are intended to align the financial interests of 3M’s Section 16 officers with those of its shareholders. The table below shows the stock ownership guideline for each Named Executive Officer and their compliance status as of December 31, 2023.
| Name | Multiple of measurement date base salary required | Compliance status as of December 31, 2023(1) | Percentage of Named Executive Officers in compliance with 3M’s stock ownership guidelines as of December 31, 2023: 100% | ||||||||||||||||||||||||||
| Mr. Roman | 6x | In compliance | |||||||||||||||||||||||||||
| Mr. Patolawala | 3x | In compliance | |||||||||||||||||||||||||||
| Mr. Gibbons | 3x | In compliance | |||||||||||||||||||||||||||
| Mr. Hanson | 3x | In compliance | |||||||||||||||||||||||||||
| Mr. Rhodes | 3x | In compliance | |||||||||||||||||||||||||||
__________________
(1)In accordance with the terms of the stock ownership guidelines, the number of shares required to be beneficially owned by each Named Executive Officer in order to maintain compliance was most recently recalculated as of December 31, 2022, using the closing price of a share of 3M common stock on the NYSE for December 30, 2022, except that the number of shares required to be beneficially owned by Mr. Hanson was calculated as of September 1, 2023, using the closing price of a share of 3M common stock on the NYSE for the same date. Each such Named Executive Officer has until December 31, 2025 (November 29, 2026, for Mr. Gibbons, January 1, 2027, for Mr. Rhodes and September 1, 2028, for Mr. Hanson) to acquire beneficial ownership of any additional shares required as a result of the recalculation.
Calculation of Required Ownership. The number of shares required to be beneficially owned in order to comply with the guidelines is determined by dividing the specified multiple of the executive’s annual base salary on the calculation date by the closing price of 3M common stock on such date.
Calculation Dates. The number of shares required to comply with the guidelines is calculated (or recalculated) on each of the following:
•the date an executive first becomes subject to the guidelines;
•the date an executive’s target ownership multiple increases or decreases due to a change in position; and
•every third anniversary of December 31, 2022.
214
Grace Period. Each covered executive is expected to attain beneficial ownership of the required number of shares of 3M stock by the later of the fifth anniversary of his or her appointment to the position triggering the calculation date or, if an executive’s required ownership level increases as a result of a triennial recalculation, the third anniversary of the calculation date. If a covered executive who is within the five-year period described above becomes subject to an increased stock ownership requirement as a result of a triennial recalculation, the covered executive will have until the later of (a) the expiration of the five-year compliance window or (b) the third anniversary of the recalculation date to attain the higher level of ownership.
Shares Counted. For purposes of determining compliance with the stock ownership guidelines, the following shares are considered to be beneficially owned by the covered executive:
•shares owned directly by a covered executive or by members of the covered executive’s immediate family;
•shares owned indirectly through a covered executive’s account in 3M’s 401(k) plan or another deferred compensation plan;
•outstanding shares of restricted stock owned by a covered executive; and
•shares underlying outstanding restricted stock units held by a covered executive.
Stock Holding Requirements. If a covered executive is not making adequate progress to meet the specified level of ownership by the end of the grace period, the guidelines provide that he or she must hold a sufficient number of the after-tax 3M shares received upon the payout of his or her performance shares to be on track to satisfy the required ownership level.
For more information concerning the 3M stock ownership of the Named Executive Officers, see “Security ownership of management.”
Prohibition of hedging, pledging, and other actions
| 3M’s stock trading policies prohibit 3M’s directors and executive officers from (1) purchasing any financial instrument that is designed to hedge or offset any decrease in the market value of 3M common stock, including prepaid variable forward contracts, equity swaps, collars, and exchange funds; (2) engaging in short sales related to 3M common stock; (3) placing standing orders; (4) maintaining margin accounts; and (5) pledging 3M securities as collateral for a loan. All discretionary transactions in 3M securities by directors and executive officers must be pre-cleared with 3M’s Legal Affairs department and conducted during approved trading windows. | •No hedging •No short sales •No standing orders •No margin accounts •No pledging | ||||||||||
Severance benefits
The 3M Executive Severance Plan provides separation payments and benefits to certain U.S. executives, including the Named Executive Officers, in the event of a qualifying termination of their employment. Among other things, the plan is intended to support talent recruitment and retention objectives (especially at times when there are uncertainties around restructurings and reductions in force) and to provide a consistent approach to executive departures. Additional information concerning the benefits made available under the Severance Plan and the circumstances under which benefits will be made available can be found under “Potential payments upon termination or change in control.”
215
Clawback policy and other remedial actions
Clawback policy. The 3M Board has adopted a policy under which it is authorized to require reimbursement of certain amounts provided to 3M’s executive officers, as described in the table below.
| Potential clawback triggering events | Amounts the 3M board is authorized to recoup | |||||||
Issuance of noncompliant financial reports. 3M’s issuance of a financial report that, due to the covered executive’s misconduct, is materially noncompliant with Federal securities laws | All profits realized by the covered executive on the sale of 3M securities during the 12-month period following the issuance of the noncompliant financial report | |||||||
Accounting Restatement. 3M’s filing of an accounting restatement of 3M’s financial statements with the Securities and Exchange Commission to correct an error that is material to the previously issued financial statements, or that would result in a material restatement if the error were corrected in the current period or left uncorrected in the current period (regardless of whether the restatement is due to a covered executive’s misconduct or failure of risk management) | All annual and long-term incentive compensation that is granted, earned or vested (on a pre-tax basis) based wholly or in part upon the attainment of a financial reporting measure or 3M’s stock price (e.g., annual cash incentive and performance-based equity awards) in excess of amounts that would have been provided based on the restated financial results. The trigger applies to incentive compensation that is received by current and former executive officers during the three completed fiscal years preceding the date that 3M concludes, or reasonably should have concluded, that 3M is required to prepare an accounting restatement | |||||||
Significant misconduct. An act of misconduct by the covered executive that has or might reasonably be expected to cause significant financial or reputational harm to 3M | Annual incentive payments, long-term incentive awards and other amounts paid or provided to the covered executive that would not have been awarded or earned if the circumstances surrounding the triggering event had been known to the 3M C&T Committee | |||||||
Significant risk-management failure. Improper or grossly negligent failure of a covered executive, including in a supervisory capacity, to identify, escalate, monitor or manage, in a timely manner and as reasonably expected, risks material to 3M, which has or might reasonably be expected to cause significant financial or reputational harm to 3M | Annual incentive payments, long-term incentive awards and other amounts paid or provided to the covered executive that would not have been awarded or earned if the circumstances surrounding the triggering event had been known to the 3M C&T Committee | |||||||
In 2023, 3M made significant revisions to its clawback policy in line with new regulatory requirements of the NYSE. Under the new policy, 3M must recover erroneously awarded incentive-based compensation from executive officers reasonably promptly after an accounting restatement, regardless of the fault or conduct of the executive or the application of discretion on behalf of the 3M C&T Committee (subject to certain limited exceptions under the NYSE rules), and the definition of an accounting restatement that requires recovery was expanded to include so-called “little r” restatements.
In addition, 3M expanded the population of employees subject to the clawback policy. While only executive officers are subject to recoupment based solely on an accounting restatement, approximately 350 employees at the Vice President level and above are now subject to recoupment based on the issuance of noncompliant financial reports, significant misconduct, or a significant risk-management failure. These changes were intended to promote 3M’s culture of compliance, further develop a compensation scheme that rewards integrity and accountability, and reinforce 3M’s pay-for-performance and compliance compensation philosophy across the entire organization, while furthering 3M’s efforts to mitigate compensation risk.
During the period beginning on January 1, 2023, through the date of this filing, 3M was not required to prepare an accounting restatement that required recovery of erroneously-awarded compensation pursuant to 3M’s clawback policy.
Other remedial actions. Whether or not an executive’s actions trigger a potential clawback, 3M also may take other remedial actions to appropriately address employee conduct that is considered detrimental to 3M. Depending on the circumstances, such other actions may include, without limitation, reducing the executive’s duties and responsibilities, limiting the progression of the executive’s career with 3M, reducing the executive’s base salary or
216
target Total Cash Compensation, reducing the target grant value of the executive’s future long-term incentive compensation awards, causing the forfeiture of the executive’s outstanding long-term incentive awards, or terminating the executive’s employment with 3M.
Assessment of risk related to compensation programs
Following completion of a recent compensation risk assessment, 3M concluded that none of its compensation policies and practices is reasonably likely to have a material adverse effect on 3M. In connection with this assessment, 3M completed an inventory of its executive and non-executive compensation programs globally, with particular emphasis on incentive compensation plans or programs. The 3M C&T Committee’s independent compensation consultant, FW Cook, conducted a risk assessment of 3M’s executive compensation policies and practices and reviewed the inventory of non-executive compensation programs compiled by management. The scope of the fiscal 2023 risk assessment generally was consistent with that conducted in recent years, through which 3M evaluated the primary components of its compensation plans and practices to identify whether those components, either alone or in combination, properly balanced compensation opportunities and risk.
3M believes that its overall cash versus equity pay mix, balance of shorter-term versus longer-term performance focus, balance of revenue- versus profit-focused performance measures, stock ownership guidelines, forfeiture provisions, and “clawback” policy all work together to provide its employees and executives with incentives to deliver outstanding performance to build long-term shareholder value, while taking only necessary and prudent risks. In this regard, 3M’s strong ethics and its corporate compliance systems, which are overseen by the 3M Audit Committee, further mitigate against excessive or inappropriate risk taking. In addition, 3M’s employee sales plans are designed under global guidelines, where oversight of plan terms, administration, and operation is strong and governance roles are segregated.
Based on its consideration of these assessments, the 3M C&T Committee concurred with 3M’s determination that none of 3M’s compensation policies and practices is reasonably likely to have a material adverse effect on 3M.
Tally sheets
The 3M C&T Committee periodically reviews a report comparing the amounts of compensation actually received by 3M’s Named Executive Officers to the amounts reported in its annual proxy statement and summarizing the compensation that would be owed to such individuals in the event of the termination of their employment under certain circumstances. Reviewing this report helps the 3M C&T Committee better understand 3M’s potential obligations to the Named Executive Officers following the termination of their employment. It also helps the 3M C&T Committee better assess the risk of any of the Named Executive Officers leaving 3M prematurely because 3M is not providing sufficient retention incentives.
Tax considerations
Section 162(m) of the Internal Revenue Code disallows a tax deduction to public companies for compensation paid in any given year in excess of $1 million to certain current and former executive officers of 3M. As a result, 3M expects that compensation paid per year to the Named Executive Officers and certain other current and former executive officers in excess of $1 million generally will not be deductible, subject to limited exceptions. Interpretations of and changes in applicable tax laws and regulations as well as other factors beyond the control of the 3M C&T Committee can affect deductibility of compensation, and there can be no assurance that compensation paid to 3M’s executive officers will be tax deductible. The 3M C&T Committee reserves the right to make changes or amendments to existing compensation programs and arrangements, including changes or amendments that may result in the loss of tax deductions, if the 3M C&T Committee believes it is in the best interests of 3M and its shareholders to do so.
217
Executive Compensation Tables
2023 summary compensation table
The following table shows the compensation earned or received during 2023, 2022, and 2021 by each of the Named Executive Officers (as determined pursuant to the Securities and Exchange Commission’s disclosure requirements for executive compensation in Item 402 of Regulation S-K).
| Name and principal position | Year | Salary ($) | Bonus ($)(1) | Stock awards ($)(2) | Option awards ($)(3) | Non-equity incentive plan compensation ($)(4) | Change in pension value and nonqualified deferred compensation earnings ($)(5) | All other compensation ($)(6) | Total ($) | |||||||||||||||||||||||||||||||||||||||||||||||
| Michael F. Roman | 2023 | 1,464,544 | — | 6,000,053 | 5,999,289 | 2,665,470 | — | 295,210 | 16,424,566 | |||||||||||||||||||||||||||||||||||||||||||||||
| Chairman of the Board and Chief Executive Officer | 2022 | 1,406,250 | — | 5,500,066 | 5,500,883 | 1,327,676 | — | 296,512 | 14,031,387 | |||||||||||||||||||||||||||||||||||||||||||||||
| 2021 | 1,337,487 | — | 5,250,138 | 5,250,960 | 3,131,774 | 2,978,538 | 251,687 | 18,200,584 | ||||||||||||||||||||||||||||||||||||||||||||||||
Monish Patolawala(7) | 2023 | 1,292,088 | — | 4,397,395 | 1,465,587 | 1,545,337 | — | 254,530 | 8,954,937 | |||||||||||||||||||||||||||||||||||||||||||||||
| President and Chief Financial Officer | 2022 | 1,100,469 | — | 4,192,661 | 1,397,729 | 793,011 | — | 171,641 | 7,655,511 | |||||||||||||||||||||||||||||||||||||||||||||||
| 2021 | 934,096 | — | 5,717,782 | 1,072,700 | 1,514,739 | — | 211,589 | 9,450,906 | ||||||||||||||||||||||||||||||||||||||||||||||||
Peter D. Gibbons(8)(9) | 2023 | 832,500 | — | 3,140,136 | — | 1,038,960 | — | 173,477 | 5,185,073 | |||||||||||||||||||||||||||||||||||||||||||||||
| Group President, Enterprise Supply Chain | 2022 | 803,702 | — | 3,020,120 | — | 417,734 | — | 122,621 | 4,364,177 | |||||||||||||||||||||||||||||||||||||||||||||||
Bryan C. Hanson(9)(10) | 2023 | 450,000 | 9,200,000 | 13,556,340 | — | 578,558 | — | 111,333 | 23,896,231 | |||||||||||||||||||||||||||||||||||||||||||||||
| Group President and Chief Executive Officer, Health Care | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Kevin H. Rhodes(9) | 2023 | 872,787 | — | 3,809,184 | — | 816,928 | 1,121,163 | 87,951 | 6,708,013 | |||||||||||||||||||||||||||||||||||||||||||||||
| Executive Vice President and Chief Legal Affairs Officer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
__________________
FOOTNOTES TO 2023 SUMMARY COMPENSATION TABLE
(1)The amount in the Bonus column reflects the hiring bonus and fifty percent of the cash make-whole award negotiated in connection with Mr. Hanson’s commencement of employment with 3M.
(2)The amounts in the Stock Awards column reflect the aggregate grant date fair value of such awards computed in accordance with ASC Topic 718, excluding the effect of estimated forfeitures. Assumptions made in the calculation of these amounts are included in Note 20 to 3M’s audited financial statements for the fiscal year ended December 31, 2023, included in 3M’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 7, 2024. The amounts included in this column for the performance share awards made during 2023 are calculated based on the probable satisfaction of the performance conditions for such awards, determined as of the grant date. If the highest level of performance is achieved for the most recent performance share awards, the maximum value of the awards at the grant date would be as follows: Mr. Roman — $12,000,106; Mr. Patolawala — $5,863,172; Mr. Gibbons — $3,140,103; and Mr. Rhodes — $3,809,298.
(3)The amounts in the Option Awards column reflect the aggregate grant date fair value of such awards computed in accordance with ASC Topic 718, excluding the effect of estimated forfeitures. Assumptions made in the calculation of these amounts are included in Note 20 to 3M’s audited financial statements for the fiscal year ended December 31, 2023, included in 3M’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 7, 2024.
(4)The amounts in the Non-Equity Incentive Plan Compensation column reflect the annual incentive compensation earned by each individual during the year specified pursuant to 3M’s Annual Incentive Plan.
(5)The amounts in the Change in Pension Value and Nonqualified Deferred Compensation Earnings column reflect the actuarial increase in the present value (if any) of each individual’s pension benefits under all defined benefit pension plans of 3M, determined using the same interest rate and mortality assumptions as those used for financial statement reporting purposes. See Note 14 to 3M’s audited financial statements for the fiscal year ended December 31, 2023, included in 3M’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 7, 2024. Consistent with the reporting requirements of the Securities and Exchange Commission, the change in Mr. Roman’s pension value reported for 2023 is shown as zero because the actuarial change in the present value of his pension benefits, calculated as described in the preceding sentence, decreased by $320,105. There were no above-market or preferential earnings on deferred compensation under 3M’s nonqualified deferred compensation programs.
(6)See the All Other Compensation table below for details of the amounts reported for 2023. For eligible employees hired on or after January 1, 2009, the amounts reported in the All Other Compensation column for each fiscal year include 3M retirement income contributions with respect to the portion (if any) of each Named Executive Officer’s annual incentive payment earned for the year shown, even though the matching contribution was not actually made until after the end of such year. In 3M’s 2023 proxy statement, such company retirement income contributions were reflected in the All Other Compensation column for the year in which the contribution was made. As a result, the amounts reported in the All Other Compensation column for Mr. Patolawala for the years 2021 and 2022, and Mr. Gibbons for the year 2022, differ from the corresponding amounts reported herein.
218
(7)As discussed in “2023 special long-term equity incentive awards — Special performance-based RSU award for Mr. Patolawala,” Mr. Patolawala also received a special performance-based RSU grant in June 2023 with a target value of $2.5 million that will vest on June 30, 2024, based upon achievement of certain qualitative performance criteria. Under ASC Topic 718, due to the subjective nature of the performance vesting criteria, the special award is generally not considered “granted” for financial reporting purposes until the applicable performance year and therefore no value is reported in the table above for the special award in 2023. 3M will report the grant date fair value for Mr. Patolawala’s special award in the Stock Awards column of the Summary Compensation Table in future years as and when such grant date fair value is determined in accordance with ASC Topic 718.
(8)Mr. Gibbons joined 3M and was appointed Group President, Enterprise Operations, effective November 29, 2021.
(9)No amounts are reported for Mr. Gibbons for the year 2021, or Mr. Hanson or Mr. Rhodes for the years 2021 and 2022, since each first became a Named Executive Officer after those years.
(10)Mr. Hanson joined 3M and was appointed Group President and Chief Executive Officer, Health Care, effective September 1, 2023.
2023 all other compensation table
| Name | 401(k) company contributions ($)(1) | VIP Excess Plan company contributions ($)(2) | Executive life insurance ($)(3) | Financial planning ($)(4) | Personal aircraft use ($)(5) | Security systems/ services ($)(6) | Total ($) | |||||||||||||||||||||||||||||||||||||
| Michael F. Roman | 5,344 | — | 23,760 | 13,500 | 251,557 | 1,049 | 295,210 | |||||||||||||||||||||||||||||||||||||
| Monish Patolawala | 26,400 | 200,594 | 14,036 | 13,500 | — | — | 254,530 | |||||||||||||||||||||||||||||||||||||
| Peter D. Gibbons | 26,400 | 123,317 | 23,760 | — | — | — | 173,477 | |||||||||||||||||||||||||||||||||||||
| Bryan C. Hanson | 21,150 | 20,957 | — | — | 69,226 | — | 111,333 | |||||||||||||||||||||||||||||||||||||
| Kevin H. Rhodes | 9,355 | 41,336 | 23,760 | 13,500 | — | — | 87,951 | |||||||||||||||||||||||||||||||||||||
__________________
FOOTNOTES TO 2023 ALL OTHER COMPENSATION TABLE
(1)The amounts shown reflect 3M matching and additional automatic contributions under the tax-qualified 3M Voluntary Investment Plan and Employee Stock Ownership Plan. All eligible employees under this plan may receive 3M matching contributions on their pre-tax or Roth 401(k) contributions to the plan on up to five percent of their eligible pay. Eligible employees hired on or after January 1, 2009, also receive additional automatic 3M retirement income contributions equal to three percent of their eligible pay.
(2)The amounts shown reflect 3M contributions under the VIP Excess Plan, a nonqualified defined contribution plan. Eligibility for this plan and 3M contributions is limited to employees whose compensation exceeds a limit established by Federal income tax laws for tax-qualified defined contribution plans. The plan permits eligible employees to save additional amounts from their current cash compensation beyond the contribution limits established by Federal tax laws, and to receive company contributions similar to those provided under the tax-qualified 3M Voluntary Investment Plan and Employee Stock Ownership Plan.
(3)The amounts shown reflect the amount of premiums paid by 3M on behalf of each individual for additional group term life insurance coverage obtained for them under the Executive Life Insurance Plan.
(4)These amounts reflect fees for personal financial planning and tax return preparation services paid by 3M on behalf of each individual.
(5)This amount reflects the aggregate incremental cost to 3M for Mr. Roman's and Mr. Hanson's personal use of aircraft chartered, leased, or operated by 3M during 2023. This aggregate incremental cost was calculated by combining the variable operating costs of such travel, including the incremental costs incurred (if any) to charter or lease the aircraft and the incremental costs incurred for fuel, landing fees, parking fees, trip preparation fees, en route communication charges, en route navigation charges, on-board catering, and crew travel expenses. The 3M C&T Committee required Mr. Roman to use the corporate aircraft for all business and personal travel. As part of the benefits negotiated in connection with Mr. Hanson’s commencement of employment, 3M agreed to allow Mr. Hanson reasonable personal use of private aircraft chartered, leased, or owned by 3M, subject to compliance with 3M’s policy on personal use of corporate aircraft.
(6)This amount reflects the expenses incurred by 3M during 2023 for home security equipment and monitoring services at the personal residence of Mr. Roman.
Grants of plan-based awards
The following table reflects the various equity and non-equity plan awards granted to the Named Executive Officers during 2023. Except for the annual incentive compensation earned by such Named Executive Officers under the Annual Incentive Plan, all of the equity incentive plan awards referred to in this table were granted under the 2016 Long-Term Incentive Plan.
219
2023 grants of plan-based awards table
Estimated future payouts under non-equity incentive plan awards(2) | Estimated future payouts under equity incentive plan awards(3) | All other stock awards: number of shares of stock or units (#)(4) | All other option awards: number of securities underlying options (#)(5) | Exercise or base price of option awards ($/Sh)(6) | Grant date fair value of stock and option awards ($)(7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Name/ Award type(1) | Grant Date | Approval Date | Threshold ($) | Target ($) | Maximum ($) | Threshold (#) | Target (#) | Maximum (#) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Michael F. Roman | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | 03/01/23 | 02/06/23 | 10,888 | 54,442 | 108,884 | 6,000,053 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock option | 02/07/23 | 02/06/23 | 269,995 | 116.90 | 5,999,289 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AIP | n/a | n/a | 128,148 | 2,562,952 | 5,125,903 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Monish Patolawala(8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | 03/01/23 | 02/06/23 | 5,320 | 26,600 | 53,200 | 2,931,586 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock option | 02/07/23 | 02/06/23 | 65,958 | 116.90 | 1,465,587 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RSU | 02/07/23 | 02/06/23 | 12,539 | 1,465,809 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AIP | n/a | n/a | 74,295 | 1,485,901 | 2,971,802 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Peter D. Gibbons | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | 03/01/23 | 02/06/23 | 2,849 | 14,246 | 28,492 | 1,570,052 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RSU | 02/07/23 | 02/06/23 | 13,431 | 1,570,084 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AIP | n/a | n/a | 41,625 | 832,500 | 1,665,000 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bryan C. Hanson | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Special RSU(9) | 09/01/23 | 08/09/23 | 126,754 | 13,556,340 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AIP | n/a | n/a | 33,838 | 676,755 | 1,353,510 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kevin H. Rhodes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | 03/01/23 | 02/06/23 | 3,456 | 17,282 | 34,564 | 1,904,649 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RSU | 02/07/23 | 02/06/23 | 16,292 | 1,904,535 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AIP | n/a | n/a | 39,275 | 785,508 | 1,571,016 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
__________________
FOOTNOTES TO 2023 GRANTS OF PLAN-BASED AWARDS TABLE
(1)Abbreviations: PSA = performance share award; RSU = restricted stock unit award; AIP = annual incentive pay.
(2)The amounts shown under “Estimated future payouts under non-equity incentive plan awards” reflect the threshold, target, and maximum amounts that may be earned by each individual during 2023 under the Annual Incentive Plan assuming continued employment through the end of the year. For more information, see “Annual incentive.”
(3)The amounts shown under “Estimated future payouts under equity incentive plan awards” with respect to performance share awards reflect the threshold, target, and maximum number of shares of 3M common stock that may be earned by each individual pursuant to their 2023 performance share awards. The actual number of shares of 3M common stock to be delivered as a result of these performance shares will be determined by the performance of 3M during the three-year performance period of 2023, 2024, and 2025, as measured against three performance criteria selected by the 3M C&T Committee (Relative Organic Sales Growth, Adjusted Earnings per Share Growth, and Free Cash Flow Growth). For more information on the performance criteria and formulas used to determine the final number of shares of 3M common stock payable pursuant to these performance shares, see “2023 performance share awards.”
(4)The amounts shown in this column reflect the number of shares of 3M common stock subject to restricted stock unit awards granted to each individual during 2023. The restricted stock unit awards granted on February 7, 2023, were part of 3M’s annual equity grants made to approximately 5,700 employees, and they will vest in full on the third anniversary of the grant date.
(5)The amounts shown under “All other option awards” reflect the number of shares of 3M common stock subject to nonqualified stock options granted to each individual during 2023. The options granted on February 7, 2023, were part of 3M’s annual equity grants made to approximately 5,700 employees, and they vest in installments of one-third on each of the first three anniversaries of the grant date.
(6)The exercise price for all stock options granted to the Named Executive Officers is set at the closing price at which 3M common stock traded on the NYSE on the option grant date.
(7)The amounts shown under “Grant date fair value of stock and option awards” were determined in accordance with ASC Topic 718, excluding the effect of estimated forfeitures, and, in the case of performance share awards, are based upon the probable outcome of the applicable performance conditions at the time of grant. Assumptions made in the calculation of these amounts are included in Note 20 to 3M’s audited financial statements for the fiscal year ended December 31, 2023, included in 3M’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 7, 2024.
(8)As discussed in footnote 7 to the Summary Compensation Table, the awards listed for Mr. Patolawala exclude his special performance-based RSUs because a grant date was not established for such award under ASC Topic 718. For additional information, see “2023 special long-term equity incentive awards — Special performance-based RSU award for Mr. Patolawala.”
(9)These restricted stock units will vest in installments of one-third on September 1, 2024, September 1, 2025, and September 1, 2026, or immediately in the event Mr. Hanson resigns from employment for good reason or 3M terminates his employment for any reason other than misconduct. For additional information see “2023 special long-term equity incentive awards — Special make-whole RSU award for Mr. Hanson.”
220
2023 outstanding equity awards at fiscal year-end table
| Option Awards | Stock Awards | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable | Option exercise price ($) | Option expiration date | Number of shares or units of stock that have not vested (#) | Market value of shares or units of stock that have not vested ($)(1) | Equity incentive plan awards: number of unearned shares, units or other rights that have not vested (#) | Equity incentive plan awards: market or payout value of unearned shares, units or other rights that have not vested ($)(1) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Michael F. Roman | 37,997 | (11) | 4,551,661 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 108,884 | (12) | 12,393,177 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25,867 | 126.72 | 02/03/24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 39,846 | 165.94 | 02/02/25 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48,206 | 147.87 | 02/01/26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 52,249 | 175.76 | 02/06/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 56,750 | 233.63 | 02/05/28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36,284 | 195.52 | 07/01/28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 146,240 | 201.12 | 02/04/29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 231,768 | 157.24 | 02/03/30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 138,201 | 69,101 | (2) | 175.02 | 02/01/31 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 72,361 | 144,722 | (3) | 162.41 | 02/07/32 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| — | 269,995 | (4) | 116.90 | 02/06/33 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monish Patolawala | 19,310 | (11) | 2,313,145 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 53,200 | (12) | 6,055,224 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 26,517 | (13) | 2,978,389 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36,027 | 155.43 | 06/30/30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 28,232 | 14,117 | (2) | 175.02 | 02/01/31 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18,386 | 36,773 | (3) | 162.41 | 02/07/32 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| — | 65,958 | (4) | 116.90 | 02/06/33 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6,524 | (5) | 849,033 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6,128 | (6) | 770,412 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13,922 | (7) | 1,709,065 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8,605 | (8) | 1,043,614 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12,539 | (9) | 1,445,997 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Peter D. Gibbons | 10,432 | (11) | 1,249,649 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 28,492 | (12) | 3,242,959 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9,298 | (8) | 1,127,661 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13,431 | (9) | 1,548,863 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bryan C. Hanson | 126,754 | (10) | 14,046,878 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kevin H. Rhodes | 9,328 | (11) | 1,117,401 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 34,564 | (12) | 3,934,074 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 414 | (6) | 52,048 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6,270 | 126.72 | 02/03/24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6,028 | 165.94 | 02/02/25 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7,019 | 147.87 | 02/01/26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6,749 | 175.76 | 02/06/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5,721 | 233.63 | 02/05/28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4,783 | 201.12 | 02/04/29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3,790 | 157.24 | 02/03/30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1,974 | 988 | (2) | 175.02 | 02/01/31 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8,040 | (8) | 975,091 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15,757 | (9) | 1,817,097 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
_________________
FOOTNOTES TO 2023 OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END TABLE
(1)The amounts shown in this column represent (a) the number of unearned restricted stock units or performance shares shown, as applicable, multiplied by (b) the sum of (i) the closing price of a share of 3M common stock on the NYSE for December 29, 2023 ($109.32) and (ii) the aggregate amount of cash-settled dividend equivalents accrued through December 31, 2023, on each restricted stock unit or performance share, as applicable.
(2)These stock options vested in full on February 2, 2024.
(3)These stock options vested or will vest in installments of one-half on each of February 8, 2024, and February 8, 2025.
(4)These stock options vested or will vest in installments of one-third on each of February 7, 2024, February 7, 2025, and February 7, 2026.
(5)These restricted stock units will vest in full on July 1, 2025, or immediately in the event of Mr. Patolawala’s termination of employment by 3M for reasons other than misconduct or his resignation for good reason.
(6)These restricted stock units will vest in full on February 2, 2024.
(7)These restricted stock units will vest in full on November 1, 2024.
221
(8)These restricted stock units will vest in full on February 8, 2025.
(9)These restricted stock units will vest in full on February 7, 2026.
(10)These restricted stock units will vest in installments of one-third on each of September 1, 2024, September 1, 2025, and September 1, 2026, or immediately in the event of Mr. Hanson’s employment is terminated without misconduct or for good reason.
(11)The shares of 3M common stock to be delivered as a result of 3M’s performance over the three-year performance period ending December 31, 2024, will not vest until December 31, 2024. Under the terms of the awards, these shares of 3M common stock will be delivered no later than March 15, 2025, unless deferred by the executive. In accordance with the Securities and Exchange Commission’s regulations, the number of shares and payout value for these performance shares reflect the target payout under the formula for this grant since 3M’s performance during the first two years of the three-year performance period exceeded the threshold levels for this grant.
(12)The shares of 3M common stock to be delivered as a result of 3M’s performance over the three-year performance period ending December 31, 2025, will not vest until December 31, 2025. Under the terms of the awards, these shares of 3M common stock will be delivered no later than March 15, 2026, unless deferred by the executive. In accordance with the Securities and Exchange Commission’s regulations, the number of shares and payout value for these performance shares reflect the maximum payout under the formula for this grant since 3M’s performance during the first year of the three-year performance period exceeded the target level for this grant.
(13)Reflects all of the performance-based RSUs for which a grant date was not established under ASC Topic 718. The RSUs are eligible to vest in full on June 30, 2024, subject to continued employment, and provided that the 3M C&T Committee certifies that Mr. Patolawala has successfully delivered all four performance conditions related to 3M’s transformation and the spin-off of the Health Care business. The performance-based RSUs also vest upon termination due to death, disability, a qualifying termination of employment following a change in control, or in the event of Mr. Patolawala’s termination of employment by 3M for reasons other than misconduct, provided in such case that all of the performance goals are still timely achieved. For additional information, see “2023 special long-term equity incentive awards — Special performance-based RSU award for Mr. Patolawala.”
2023 option exercises and stock vested table
| Option exercises and stock vested | ||||||||||||||||||||||||||||||||
| Option awards | Stock awards | |||||||||||||||||||||||||||||||
| Name | Number of shares acquired on exercise (#) | Value realized on exercise ($) | Number of shares acquired on vesting (#) | Value realized on vesting ($)(1) | ||||||||||||||||||||||||||||
| Michael F. Roman | — | — | 24,954 | (2) | 2,748,963 | |||||||||||||||||||||||||||
| Monish Patolawala | — | — | 16,209 | (3) | 1,832,202 | |||||||||||||||||||||||||||
| Peter D. Gibbons | — | — | 3,222 | (4) | 360,252 | |||||||||||||||||||||||||||
| Bryan C. Hanson | — | — | — | — | ||||||||||||||||||||||||||||
| Kevin H. Rhodes | — | — | 1,418 | (5) | 148,461 | |||||||||||||||||||||||||||
___________________
FOOTNOTES TO 2023 OPTION EXERCISES AND STOCK VESTED TABLE
(1)The amounts shown in this column represent (a) the number of shares acquired on vesting, multiplied by (b) the sum of (i) the closing price of a share of 3M common stock on the NYSE for the vesting date and (ii) the aggregate amount of cash-settled dividend equivalents payable on the each share issuable pursuant to the restricted stock units or performance shares that were earned.
(2)Reflects the number of shares earned by Mr. Roman upon the vesting of performance shares granted to him under the 2016 Long-Term Incentive Plan. All 24,954 of these shares were attributable to his 2021 performance shares for which the three-year performance period ended on December 31, 2023. Mr. Roman previously elected to defer receipt of all of the shares issuable pursuant to his 2021 performance shares until following his termination of employment.
(3)Reflects the number of shares earned by Mr. Patolawala upon the vesting of restricted stock units and performance shares granted to him under the 2016 Long-Term Incentive Plan. Of this total number of shares, 6,013 were attributable to restricted stock units granted on July 1, 2020, and 10,196 were attributable to his 2021 performance shares for which the three-year performance period ended on December 31, 2023.
(4)Reflects the number of shares earned by Mr. Gibbons upon the vesting of restricted stock units granted to him on December 1, 2021, under the 2016 Long-term Incentive Plan.
(5)Reflects the number of shares earned by Mr. Rhodes upon the vesting of restricted stock units and performance shares granted to him under the 2016 Long-term Incentive Plan. Of this total number of shares, 168 were attributable to restricted stock units granted on February 4, 2020, 535 were attributable to restricted stock units granted on February 7, 2023, and 715 were attributable to his 2021 performance shares for which the three-year performance period was completed on December 31, 2023. Mr. Rhodes previously elected to defer receipt of 50 percent of the shares issuable pursuant to his 2021 performance shares until following his termination of employment.
Pension benefits
The following table shows the present value of the accumulated benefits payable to each of the Named Executive Officers, as well as the number of years of service credited to each individual, under each of 3M’s defined benefit pension plans determined using the same interest rate and mortality assumptions as those used for financial statement reporting purposes. See Note 14 to 3M’s audited financial statements for the fiscal year ended December
222
31, 2023, included in 3M’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 7, 2024.
2023 pension benefits table
| Name | Plan name | Number of years credited service (#) | Present value of accumulated benefits ($) | Payments during last fiscal year ($) | ||||||||||||||||||||||
| Michael F. Roman | Employee retirement income plan | 35 | 1,906,433 | — | ||||||||||||||||||||||
| Nonqualified pension plan | 35 | 23,583,985 | — | |||||||||||||||||||||||
| Monish Patolawala | None | — | — | — | ||||||||||||||||||||||
| Peter D. Gibbons | None | — | — | — | ||||||||||||||||||||||
| Bryan C. Hanson | None | — | — | — | ||||||||||||||||||||||
| Kevin H. Rhodes | Employee retirement income plan | 22 | 1,075,762 | — | ||||||||||||||||||||||
| Nonqualified pension plan | 22 | 2,871,075 | — | |||||||||||||||||||||||
The Employee Retirement Income Plan (ERIP) is a tax-qualified defined benefit pension plan maintained by 3M for its eligible employees in the United States. Effective January 1, 2001, 3M amended the ERIP to include a pension equity formula for (1) employees hired or rehired on or after January 1, 2001, and (2) employees who voluntarily elected the pension equity formula during the one-time choice election period in 2001. The ERIP was closed to new participants effective January 1, 2009, meaning that employees hired or rehired on or after January 1, 2009 (including Mr. Patolawala, Mr. Gibbons, and Mr. Hanson), do not participate in the plan. Of the Named Executive Officers, Mr. Roman participates under the non-pension equity formula of the ERIP (referred to as the Portfolio I Plan), while Mr. Rhodes participates under the pension equity formula of the ERIP (referred to as the Portfolio II Plan). Retirement benefits under the ERIP are based on an employee’s years of service and average annual earnings during the employee’s four highest-paid consecutive years of service. As applied to the Named Executive Officers, earnings for purposes of the ERIP include base salary and target annual incentive compensation. All benefits earned under the ERIP by the Named Executive Officers will be payable in the form of life annuities, unless an individual elects at the time their employment ends to receive the entire amount of his or her earned pension benefits in the form of a single lump-sum cash payment.
Under the Portfolio I Plan, employees earn annual benefits payable at retirement generally equal to 1.15 percent of their high-four average annual earnings multiplied by their years of service plus 0.35 percent of their high-four average annual earnings in excess of a Social Security breakpoint multiplied by their years of service (up to a maximum of 35 years). The Social Security breakpoint is an average of the Social Security taxable wage bases for each of the 35 years ending with the year each employee qualifies to receive unreduced Social Security retirement benefits. Under the Portfolio I Plan, an employee may retire with an unreduced pension at age 60 (61 or 62 for employees born after 1942 and 1959, respectively). If the employee’s age and service at the time of retirement total at least 90 (91 or 92 for employees born after 1942 and 1959, respectively) the employee also would receive a Social Security bridge payment until age 62. Mr. Roman is eligible to retire with unreduced retirement benefits.
Under the Portfolio II Plan, employees earn pension credits (from 3 percent to 12 percent) for each year of employment based on their age and accumulated years of service under the plan. Once their employment ends, these accumulated pension credits are multiplied by their high-four average annual earnings and added to an amount determined by multiplying one-half of these accumulated pension credits by their high-four average annual earnings in excess of a Social Security integration level (70 percent of the Social Security taxable wage base in the year employment ends). The sum of these two amounts is then converted into an annuity payable over the lifetime of the employee using fixed conversion factors. The Portfolio II Plan does not provide any subsidies for early retirement.
As a tax-qualified plan, the ERIP is subject to a variety of limits that apply to both the amount of any employee’s earnings that may be considered when determining the benefits earned under the plan as well as the maximum amount of benefits that any employee may earn. The Nonqualified Pension Plan is designed to provide additional benefits to employees, including the Named Executive Officers, affected by these limits. The amount of
223
benefits earned under this Nonqualified Pension Plan generally equals the amount of benefits an employee was not able to earn under the ERIP as a result of the limits imposed by Federal tax laws. The benefits earned under this Nonqualified Pension Plan generally are paid in the form of a single lump-sum cash payment following the termination of their employment (subject to any applicable delay under Federal tax laws). Each of the Named Executive Officers who participates in the Nonqualified Pension Plan was given a one-time opportunity during 2008 to elect to receive their benefits earned under such plan in the form of a life annuity following their retirement, and none of them elected to receive their benefits in the form of a life annuity.
As of December 31, 2028, benefit accruals for non-union employees under 3M’s U.S. defined benefit pension plans, including the ERIP and the Nonqualified Pension Plans, will cease.
Nonqualified deferred compensation
The following table reflects the participation during 2023 by the Named Executive Officers in three nonqualified deferred compensation plans offered by 3M. The Deferred Compensation Excess Plan allows eligible employees to defer for a number of years or until retirement from 3M the receipt of a portion of their base salary and annual incentive compensation. The Performance Awards Deferred Compensation Plan allows eligible employees to defer for a number of years or until retirement from 3M the payout of their performance share awards under the 2016 Long-Term Incentive Plan. The VIP Excess Plan allows eligible employees to defer until retirement from 3M the receipt of a portion of their base salary and annual incentive compensation. All three plans generally allow the eligible employees to elect to receive payment of their account balances in the form of either a single lump sum payment or in up to ten annual installments. With the exception of deferrals of performance shares under the Performance Awards Deferred Compensation Plan, earnings are credited to the amounts deferred under all three plans based on the returns paid on the investment funds available to participants in 3M’s qualified 401(k) plan or a fixed rate of return based on corporate bond yields (as selected by each participant). Earnings are credited to the deferrals of performance shares under the Performance Awards Deferred Compensation Plan based on the return on shares of 3M common stock, including reinvested dividends.
224
2023 nonqualified deferred compensation table
| Name | Executive contributions in last FY ($)(1) | Registrant contributions in last FY ($)(2) | Aggregate earnings in last FY ($)(3) | Aggregate withdrawals/ distributions ($) | Aggregate balance at Last FYE ($)(4) | |||||||||||||||||||||||||||
| Michael F. Roman | ||||||||||||||||||||||||||||||||
| VIP excess plan | 132,768 | 29,873 | 116,499 | — | 2,747,895 | |||||||||||||||||||||||||||
| Deferred compensation excess plan | — | — | 11,452 | — | 254,601 | |||||||||||||||||||||||||||
| Performance awards deferred compensation plan | 3,991,124 | — | (322,889) | — | 12,166,170 | |||||||||||||||||||||||||||
| Monish Patolawala | ||||||||||||||||||||||||||||||||
| VIP excess plan | 123,457 | 140,408 | 110,009 | — | 810,972 | |||||||||||||||||||||||||||
| Deferred compensation excess plan | — | — | — | — | — | |||||||||||||||||||||||||||
| Performance awards deferred compensation plan | — | — | — | — | — | |||||||||||||||||||||||||||
| Peter D. Gibbons | ||||||||||||||||||||||||||||||||
| VIP excess plan | 65,016 | 73,619 | 25,560 | — | 251,296 | |||||||||||||||||||||||||||
| Deferred compensation excess plan | 56,848 | — | 8,160 | — | 79,972 | |||||||||||||||||||||||||||
| Performance awards deferred compensation plan | — | — | — | — | — | |||||||||||||||||||||||||||
| Bryan C. Hanson | ||||||||||||||||||||||||||||||||
| VIP excess plan | — | 3,600 | 10 | — | 3,610 | |||||||||||||||||||||||||||
| Deferred compensation excess plan | — | — | — | — | — | |||||||||||||||||||||||||||
| Performance awards deferred compensation plan | — | — | — | — | — | |||||||||||||||||||||||||||
| Kevin H. Rhodes | ||||||||||||||||||||||||||||||||
| VIP excess plan | 105,900 | 29,165 | 34,397 | — | 831,523 | |||||||||||||||||||||||||||
| Deferred compensation excess plan | — | — | 2,223 | — | 49,416 | |||||||||||||||||||||||||||
| Performance awards deferred compensation plan | 64,017 | — | (5,853) | — | 214,388 | |||||||||||||||||||||||||||
__________________
FOOTNOTES TO 2023 NONQUALIFIED DEFERRED COMPENSATION TABLE
(1)With the exception of the amounts contributed by Mr. Roman and Mr. Rhodes from the payout of their performance share awards for the 2020-2022 performance period, all amounts contributed by these individuals during 2023 have been included in the Summary Compensation Table as Salary, Stock Awards, or Non-Equity Incentive Plan Compensation earned in 2021, 2022, or 2023. The Summary Compensation Table does not reflect any portion of the contributions from the payout of Mr. Roman's and Mr. Rhodes's performance share awards for the 2020-2022 performance period because those awards were granted prior to the earliest year covered by the Summary Compensation Table.
(2)The amounts shown reflect 3M contributions under the VIP Excess Plan, a nonqualified defined contribution plan. Eligibility for this plan and the company contributions is limited to employees whose compensation exceeds a limit established by Federal income tax laws for tax-qualified defined contribution plans. The plan permits eligible employees to save additional amounts from their current cash compensation beyond the contribution limits established by Federal tax laws, and to receive company contributions similar to the company contributions provided under the tax-qualified 3M Voluntary Investment Plan and Employee Stock Ownership Plan. With the exception of 3M contributions to Mr. Rhodes’s VIP Excess Plan account in respect of his 2022 annual incentive payment (paid in 2023), all amounts contributed by 3M on behalf of these individuals during 2023 are included in the “All Other Compensation” column of the Summary Compensation Table. The Summary Compensation Table does not reflect any portion of the 3M contributions made to Mr. Rhodes’s VIP Excess Plan account in respect of his 2022 annual incentive payment because that amount was earned prior to the first year he became a Named Executive Officer.
(3)None of these amounts are included in the Summary Compensation Table as compensation earned in 2023, since none of 3M’s nonqualified deferred compensation plans provide above-market or preferential earnings.
(4)Includes the following amounts reported as compensation for 2021 and 2022 in the Summary Compensation Table: Mr. Roman — $817,906; Mr. Patolawala — $529,829; Mr. Gibbons — $139,050; Mr. Hanson — $0; and Mr. Rhodes — $0. Due to errors in the data received from 3M’s third-party administrator, certain of the 2022 contributions and earnings under the VIP Excess Plan and Deferred Compensation Excess Plan were underreported in 3M’s 2023 proxy statement. The aggregate amounts underreported in 2022 were as follows: VIP Excess Plan (Mr. Roman — $13,714; Mr. Patolawala — $12,214; and Mr. Gibbons — $4,768) and Deferred Compensation
225
Excess Plan (Mr. Roman — $12; and Mr. Gibbons — $962). The amounts reported in this column have been adjusted, as necessary, to reflect the correct balance as of December 31, 2023.
Potential payments upon termination or change in control
As reflected in the Compensation Discussion and Analysis section of this information statement, 3M has no fixed-term employment agreements with any of the Named Executive Officers nor does it have any change in control plans or arrangements that would provide benefits to any of the Named Executive Officers solely in the event of a change in control of 3M. 3M also does not have any agreements that would provide automatic “single-trigger” accelerated vesting of equity compensation or excise tax gross-up payments to any of the Named Executive Officers in the event of a change in control of 3M. 3M does maintain a severance plan that provides separation benefits to eligible U.S. employees (including the Named Executive Officers) who incur a qualifying termination. In addition, certain of 3M’s executive compensation and benefit plans provide all participants (including the Named Executive Officers) with certain rights or the right to receive payments in the event of the termination of their employment or upon a change in control of 3M. The terms applicable to these potential rights or payments in various situations are described below. Payments or benefits under other plans and arrangements that generally are provided on a non-discriminatory basis to all similarly situated employees of 3M upon the termination of their employment are not described, including:
•accrued base salary;
•annual incentive earned with respect to completed performance periods;
•retiree welfare benefits provided to substantially all of 3M’s U.S. employees, including retiree medical benefits;
•distribution of vested account balances under 3M’s qualified 401(k) plan;
•accrued pension benefits under 3M’s defined benefit pension plans payable following an employee’s retirement or other termination of employment (the amounts of these benefits earned by the Named Executive Officers are reported in the 2023 Pension Benefits Table);
•life insurance benefits generally available to all salaried employees; and
•distribution of account balances under 3M’s nonqualified deferred compensation plans (the account balances of the Named Executive Officers are reported in the 2023 Nonqualified Deferred Compensation Table).
Rights and payments upon retirement
Following retirement (as described below), the Named Executive Officers are entitled to receive:
•continued vesting of stock options previously granted under 3M’s stock plans, and the opportunity to exercise vested stock options previously granted under such plans during the remainder of the original term (up to 10 years) of such options;
•an annual incentive plan payment for the year of retirement, prorated based on the number of days worked prior to the date of separation;
•for those Named Executive Officers initially appointed to a 3M executive position before January 1, 2006, or after December 31, 2017, payment for all previously granted performance shares upon completion of the respective three-year performance period (prorated to reflect the portion of the year worked only with respect to the performance shares granted in the year of retirement) and based on actual performance; and
•for those Named Executive Officers initially appointed to a 3M executive position on or after January 1, 2006, and on or before December 31, 2017, payment for all previously granted performance shares upon completion of the respective three-year performance period (prorated to reflect the portion of the three-year
226
performance period completed prior to the date of the Named Executive Officer’s retirement) and based on actual performance.
For this purpose, the term “Retirement” means a termination of employment with 3M after attaining age 55 with at least 10 years of service (age 55 with at least five years of service for stock options granted before January 1, 2016).
Rights and payments upon termination due to death
In the event of the termination of their employment due to death, the Named Executive Officers are entitled to receive:
•immediate vesting of all unvested stock options and restricted stock units previously granted under 3M’s stock plans, and the opportunity for the Named Executive Officer’s estate or beneficiaries to exercise all vested stock options during the two-year period following the date of death (but not beyond the original expiration date of any such stock option);
•payment to the Named Executive Officer’s estate or beneficiaries, no later than March 15 of the year following the year in which the Named Executive Officer died, for all previously granted performance shares (in the same amount as paid for the performance shares granted to other Named Executive Officers if the date of death occurs after the end of the three-year performance period for such shares, and at the lesser of the target value or such other amount as determined by the 3M C&T Committee in its discretion if the date of death occurs before the end of the three-year performance period for such shares); and
•payment to the Named Executive Officer’s beneficiaries of the proceeds from the life insurance policies provided for such Named Executive Officer pursuant to 3M’s Executive Life Insurance Plan.
Rights and payments upon termination due to disability
In the event of the termination of their employment due to disability, the Named Executive Officers are entitled to receive:
•continued vesting of stock options previously granted under 3M’s stock plans, and the opportunity to exercise vested stock options previously granted under such plans during the remainder of the original term (up to 10 years) of such options;
•immediate vesting of all restricted stock units previously granted under 3M’s stock plans; and
•payment for all previously granted performance shares upon completion of the respective three-year performance period, based on actual performance.
Rights and payments upon a qualifying termination not in connection with a change in control
3M maintains the 3M Executive Severance Plan (which 3M refers to as the “Severance Plan”) to provide separation benefits for certain U.S. executives (including 3M’s Named Executive Officers) in the event an eligible employee’s employment is terminated by 3M other than for Misconduct or an eligible employee resigns for Good Reason. An eligible employee who becomes entitled to benefits under the Severance Plan receives the following:
•continued payment of his or her annual base salary for a number of months (24 months for the Chief Executive Officer, and 18 months for all other Named Executive Officers);
•continued payment of annual incentive payments that would have been paid if the participant remained employed through the end of the severance payment period, calculated based on actual results for the relevant year, using the participant’s final performance rating as of the end of the applicable year (or if none, a performance rating of “fully meets expectations” or equivalent), and prorated based on the number of days in the year preceding the end of the severance period (if the severance payment period ends prior to the end of a year); provided, however, that any such annual incentive payment related to the period
227
following the participant’s termination of employment will not exceed 100 percent of the prorated target annual incentive compensation opportunity that otherwise would have been in effect for such period if he or she remained employed with 3M;
•an opportunity to exercise all vested stock options and stock appreciation rights following the participant’s termination and prior to the expiration date of such award;
•accelerated vesting and payment of a prorated portion of the participant’s outstanding “annual” restricted stock unit awards and “inducement” restricted stock unit awards, with proration based on the number of full years of the participant’s completed service over the number of years required to vest under the original vesting schedule;
•outstanding performance share awards will not be forfeited solely as a result of the participant’s termination, and the target number of shares under each award will be prorated based on the number of months the participant worked during the applicable performance period;
•full vesting of the participant’s VIP company contribution account; and
•outplacement services in accordance with 3M’s policy.
In addition, if Mr. Patolawala voluntarily resigns from employment with 3M for Good Reason or his employment is terminated by 3M other than for Misconduct, he also will be entitled to receive accelerated vesting of an inducement equity award granted in connection with his commencement of employment, subject to Mr. Patolawala’s execution of an effective and irrevocable general release of all claims against 3M and its affiliates and without duplication of any benefits to which he may become entitled under the Severance Plan. These additional benefits are not part of the Severance Plan but were negotiated in connection with Mr. Patolawala’s commencement of employment. Mr. Patolawala’s special 2023 performance-based RSUs also would remain outstanding and be eligible to vest if 3M terminates Mr. Patolawala’s employment for any reason other than Misconduct (as defined in the 2016 Long-Term Incentive Plan) and he timely executes (and does not revoke) a general release of claims against 3M and its affiliates, but none of the restricted stock units will vest unless and until the 3M C&T Committee certifies that all of the performance conditions were achieved no later than June 30, 2024.
Any severance payments under the Severance Plan will reduce on a dollar-for-dollar basis (but not below $0) any amounts the participant may otherwise be eligible to receive under his or her non-competition agreement with 3M. In addition, severance payments will cease in the event the participant becomes reemployed by 3M.
The Severance Plan does not provide severance benefits to employees who terminate pursuant to a mandatory retirement policy adopted by the 3M Board. Employees who terminate in connection with a spin-off, divestiture or similar transaction involving the employee’s business unit may be eligible to participate at the discretion of 3M. To be eligible to receive benefits under the Severance Plan, an employee must execute and not revoke a general release of claims in favor of 3M in a form approved by 3M.
In the event a Named Executive Officer’s payments under the Severance Plan would be subject to an excise tax on “parachute payments” in connection with a change in control of 3M due to the application of Section 280G of the Internal Revenue Code, the Named Executive Officer will not be entitled to a “gross-up.” Instead, the Named Executive Officer’s “parachute payments” may be reduced if such reduction would provide the Named Executive Officer with a greater post-tax benefit than he or she would receive if the excise taxes were to apply.
For purposes of the Severance Plan and Mr. Patolawala’s special equity benefits, the terms “Misconduct” and “Good Reason” mean the following:
“Misconduct” means (i) the employee’s willful failure to substantially perform his or her duties (other than a failure resulting from his or her disability); (ii) the employee’s willful failure to carry out, or comply with any lawful and reasonable directive of the 3M Board or his or her immediate supervisor; (iii) the occurrence of any act or omission by the employee that could reasonably be expected to result in (or has resulted in) his or her conviction, plea of no contest, plea of nolo contendere, or imposition of unadjudicated probation for any felony or indictable
228
offense or crime involving moral turpitude; (iv) the employee’s commission of an act of fraud, embezzlement, misappropriation, misconduct, or breach of fiduciary duty against 3M or any of its subsidiaries or affiliates or any of their officers, directors, employees, customers, suppliers, insurers or agents; (v) for purposes of the Severance Plan only, the employee’s material breach of any material provision of any written agreement with 3M or any subsidiary; (vi) any action (or inaction) by the employee that 3M reasonably determines constitutes gross negligence or misconduct in the performance of his or her duties and responsibilities; or (vii) any other intentional misconduct by the Named Executive Officer that significantly affects the business or affairs of 3M or any subsidiary in an adverse manner.
“Good Reason” means, with respect to a voluntary termination occurring within 18 months following a change in control of 3M, (i) a material diminution in the employee’s position, authority, duties or responsibilities as in effect immediately prior to the change in control, (ii) a material diminution in the employee’s base salary or annual planned cash compensation, or (iii) a material change in the geographic location at which the employee is required to perform services for his or her employer. If the voluntary termination does not occur within 18 months following a change in control, “Good Reason” means (i) a material diminution in the employee’s base salary or annual planned cash compensation, other than an across-the-board reduction that applies to all comparable positions, or (ii) a change in excess of 100 miles in the primary work location at which the employee is required to perform services for his or her employer. For terminations that do not occur within 18 months following a change in control, the employee will be required to provide written notice to 3M of the grounds for a Good Reason termination within 30 days of the initial existence of such grounds, and 3M has 30 days from the date of such notice to cure such circumstances. An employee must terminate employment for Good Reason within 60 days after the first occurrence of the applicable grounds.
If Mr. Hanson’s employment is terminated without Misconduct (as defined above) or for Good Reason (as defined below), then, subject to his execution and nonrevocation of a release of claims: (a) any unvested portion of the make-whole 3M RSU award will vest; and (b) any unpaid portion of the make-whole cash award will be paid. For additional information regarding the make-whole cash award, see “Section V: 2023 compensation decisions and performance highlights — Bryan C. Hanson — Compensation Decisions.”
For purposes of Mr. Hanson’s severance benefits described above, “Good Reason” generally means (i) a material diminution in his base salary or annual planned cash compensation, other than an across-the-board reduction that applies to all comparable positions; or (ii) a change in excess of one hundred (100) miles in the primary work location at which he is required to perform services.
Rights and payments upon a qualifying termination in connection with a change in control
If 3M terminates a Named Executive Officer’s employment for reasons other than Misconduct (or for awards granted prior to May 10, 2016, reasons other than Cause) or if a Named Executive Officer resigns for Good Reason, in any such case within 18 months following a “change in control event” of 3M (as defined for purposes of Section 409A of the Internal Revenue Code), then in addition to any benefits provided under the Severance Plan and the special benefits provided to Mr. Patolawala and Mr. Hanson described above, all of the Named Executive Officer’s outstanding unvested stock options and restricted stock units granted under 3M’s stock plans will be immediately vested and all of such Named Executive Officer’s outstanding performance shares will be prorated and settled in accordance with the terms of the plan.
For purposes of 3M’s outstanding long-term incentive awards, the terms “Misconduct,” “Cause,” and “Good Reason” mean the following:
“Misconduct” means (i) the Named Executive Officer’s willful failure to substantially perform his or her duties (other than a failure resulting from his or her disability); (ii) the Named Executive Officer’s willful failure to carry out, or comply with any lawful and reasonable directive of the 3M Board or his or her immediate supervisor; (iii) the occurrence of any act or omission by the Named Executive Officer that could reasonably be expected to result in (or has resulted in) his or her conviction, plea of no contest, plea of nolo contendere, or imposition of unadjudicated probation for any felony or indictable offense or crime involving moral turpitude; (iv) the Named Executive Officer’s commission of an act of fraud, embezzlement, misappropriation, misconduct, or breach of fiduciary duty
229
against 3M or any of its subsidiaries or affiliates or any of their officers, directors, employees, customers, suppliers, insurers or agents; (v) the Named Executive Officer’s material breach of any material provision of any written agreement with 3M or any subsidiary; or (vi) any other intentional misconduct by the Named Executive Officer that significantly affects the business or affairs of 3M or any subsidiary in an adverse manner.
“Cause” means a material violation of any policy of 3M, or embezzlement or theft of property belonging to 3M.
“Good Reason” means (i) a material diminution in the Named Executive Officer’s position, authority, duties, or responsibilities as in effect immediately prior to the change in control; (ii) a material diminution in the Named Executive Officer’s base salary or annual planned cash compensation; or (iii) a material change in the geographic location at which the Named Executive Officer is required to perform services for 3M.
Rights and payments upon termination for any other reason
In the event of the termination of a Named Executive Officer’s employment for any reason that does not fit into one of the categories described above:
•the Named Executive Officers will have the opportunity to exercise vested stock options granted under 3M’s stock plans within the first 90 days following the termination date (but not beyond the original expiration date of any such stock option), at which time any remaining vested stock options will be forfeited; and
•all unvested stock options, restricted stock units, and performance shares granted to the Named Executive Officers will be forfeited immediately.
The amounts payable to or on behalf of each of the Named Executive Officers in each of the above situations (other than amounts relating to payments or benefits generally provided on a non-discriminatory basis to all similarly situated employees) is reflected in the table below, assuming that each individual’s employment had terminated and/or a change in control of 3M had occurred on December 31, 2023. As of December 31, 2023, Mr. Roman and Mr. Rhodes were eligible to retire (as that term is defined for purposes of 3M’s stock plans).
2023 potential payments upon termination or change in control table
| All amounts in U.S. dollars | Termination of employment due to ... | ||||||||||||||||||||||||||||||||||
| Name | Death | Disability | Qualifying termination not in connection with a change in control | Qualifying termination in connection with a change in control(1) | Retirement/ other reason | ||||||||||||||||||||||||||||||
| Michael F. Roman | |||||||||||||||||||||||||||||||||||
| Cash severance | — | — | 8,127,488 | 8,127,488 | — | ||||||||||||||||||||||||||||||
Outstanding PSAs(2) | 10,748,249 | — | — | 1,073,469 | — | ||||||||||||||||||||||||||||||
Unvested RSUs(3) | — | — | — | — | — | ||||||||||||||||||||||||||||||
Unvested options(4) | — | — | — | — | — | ||||||||||||||||||||||||||||||
Life insurance proceeds(5) | 3,000,000 | — | — | — | — | ||||||||||||||||||||||||||||||
| Outplacement services | — | — | 3,500 | 3,500 | — | ||||||||||||||||||||||||||||||
| Total | 13,748,249 | — | 8,130,988 | 9,204,457 | — | ||||||||||||||||||||||||||||||
| Monish Patolawala | |||||||||||||||||||||||||||||||||||
| Cash severance | — | — | 4,534,754 | 4,534,754 | — | ||||||||||||||||||||||||||||||
Outstanding PSAs(2) | 5,340,757 | — | 1,668,429 | 528,855 | — | ||||||||||||||||||||||||||||||
Unvested RSUs(3) | 8,805,581 | 8,805,581 | 867,517 | 8,805,581 | — | ||||||||||||||||||||||||||||||
Unvested options(4) | — | — | — | — | |||||||||||||||||||||||||||||||
Life insurance proceeds(5) | 3,000,000 | — | — | — | — | ||||||||||||||||||||||||||||||
| Outplacement services | — | — | 3,500 | 3,500 | — | ||||||||||||||||||||||||||||||
230
| All amounts in U.S. dollars | Termination of employment due to ... | ||||||||||||||||||||||||||||||||||
| Name | Death | Disability | Qualifying termination not in connection with a change in control | Qualifying termination in connection with a change in control(1) | Retirement/ other reason | ||||||||||||||||||||||||||||||
| Total | 17,146,338 | 8,805,581 | 7,074,200 | 13,872,690 | — | ||||||||||||||||||||||||||||||
| Peter D. Gibbons | |||||||||||||||||||||||||||||||||||
| Cash severance | — | — | 2,520,000 | 2,520,000 | — | ||||||||||||||||||||||||||||||
Outstanding PSAs(2) | 2,871,129 | — | 896,434 | 283,755 | — | ||||||||||||||||||||||||||||||
Unvested RSUs(3) | 2,676,524 | 2,676,524 | 375,850 | 2,676,524 | — | ||||||||||||||||||||||||||||||
Unvested options(4) | — | — | — | — | — | ||||||||||||||||||||||||||||||
Life insurance proceeds(5) | 3,000,000 | — | — | — | — | ||||||||||||||||||||||||||||||
| 401(k) Plan vesting | — | — | 17,944 | 17,944 | — | ||||||||||||||||||||||||||||||
| Outplacement services | — | — | 3,500 | 3,500 | — | ||||||||||||||||||||||||||||||
| Total | 8,547,653 | 2,676,524 | 3,813,727 | 5,501,722 | — | ||||||||||||||||||||||||||||||
| Bryan C. Hanson | |||||||||||||||||||||||||||||||||||
Cash severance(6) | — | — | 11,562,500 | 11,562,500 | — | ||||||||||||||||||||||||||||||
Outstanding PSAs(2) | — | — | — | — | — | ||||||||||||||||||||||||||||||
Unvested RSUs(3) | 14,046,878 | 14,046,878 | 14,046,878 | 14,046,878 | — | ||||||||||||||||||||||||||||||
Unvested options(4) | — | — | — | — | — | ||||||||||||||||||||||||||||||
Life insurance proceeds(5) | — | — | — | — | — | ||||||||||||||||||||||||||||||
| 401(k) Plan vesting | — | — | 22,767 | 22,767 | — | ||||||||||||||||||||||||||||||
| Outplacement services | — | — | 3,500 | 3,500 | — | ||||||||||||||||||||||||||||||
| Total | 14,046,878 | 14,046,878 | 25,635,645 | 25,635,645 | — | ||||||||||||||||||||||||||||||
| Kevin H. Rhodes | |||||||||||||||||||||||||||||||||||
| Cash severance | — | — | 2,529,090 | 2,529,090 | — | ||||||||||||||||||||||||||||||
Outstanding PSAs(2) | 3,084,438 | — | — | 324,724 | — | ||||||||||||||||||||||||||||||
Unvested RSUs(3) | 2,844,849 | 2,844,849 | — | 2,844,849 | — | ||||||||||||||||||||||||||||||
Unvested options(4) | — | — | — | — | — | ||||||||||||||||||||||||||||||
Life insurance proceeds(5) | 3,000,000 | — | — | — | — | ||||||||||||||||||||||||||||||
| Outplacement services | — | — | 3,500 | 3,500 | — | ||||||||||||||||||||||||||||||
| Total | 8,929,287 | 2,844,849 | 2,532,590 | 5,702,163 | — | ||||||||||||||||||||||||||||||
__________________
FOOTNOTES TO 2023 POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL TABLE
(1)Pursuant to the terms of the Severance Plan, certain payments and benefits may be subject to reduction if they otherwise would cause the recipient to incur an excise tax imposed under section 4999 of the Internal Revenue Code and the reduction would place the participant in a better after-tax position than if the participant received such payments and benefits. The amounts set forth in this column do not reflect the effect of any such reduction.
(2)Amounts shown reflect the value of performance share awards (PSAs) under the 2016 Long-Term Incentive Plan for which the three-year performance period has not been completed (adjusted to reflect the closing price of a share of 3M common stock on the NYSE for December 29, 2023 ($109.32), and which would be paid upon the occurrence of the respective triggering events in accordance with the terms of the awards.
(3)Amounts shown reflect the value of the shares underlying the unvested 3M restricted stock units that would vest upon the occurrence of the respective triggering events in accordance with the terms of the awards. For Mr. Patolawala, the amounts also include the value of the shares underlying his special 2023 performance-based RSUs that would vest upon the occurrence of the respective triggering events. For Mr. Hanson, the amounts also include the value of the shares underlying his make-whole RSU award that would vest upon the occurrence of the respective triggering events. Share values are based on the closing price of a share of 3M common stock on the NYSE for December 29, 2023 ($109.32).
(4)Amounts shown reflect the intrinsic value on December 31, 2023, of unvested, in-the-money 3M stock options that will vest upon the occurrence of the respective triggering events in accordance with the existing contractual terms of the stock options. Intrinsic values are based on the closing price of a share of 3M common stock on the NYSE for December 29, 2023 ($109.32).
231
(5)Amounts shown reflect the group term life insurance proceeds that would be payable to each individual’s beneficiary or beneficiaries pursuant to the policies obtained for them under the Executive Life Insurance Plan.
(6)Amounts shown reflect the sum of the estimated amount Mr. Hanson would be eligible to receive under the Severance Plan for the continued payment of his annual base salary and annual incentive compensation and the unpaid amount of Mr. Hanson’s make-whole cash award.
Pay ratio
Presented below is the ratio of annual total compensation of the 3M CEO to the annual total compensation of 3M’s median employee (excluding the 3M CEO) for 2023. The ratio reflects a reasonable estimate calculated in a manner consistent with Item 402(u) of Regulation S-K under the Exchange Act.
In identifying 3M’s median employee (a full-time production employee in the Midwestern United States), 3M calculated the target annual Total Cash Compensation of each employee as of December 31, 2023. For these purposes, annual Total Cash Compensation included base salary or hourly wages, cash incentives, commissions, and comparable cash elements of compensation in non-U.S. jurisdictions and was calculated using internal human resources records. All amounts were annualized for permanent employees who did not work for the entire year, such as new hires, employees on paid or unpaid leave of absence and employees called for active military duty. 3M did not apply any cost-of-living adjustments as part of the calculation.
The median employee was selected from among 86,464 full-time, part-time, temporary, and seasonal workers who were employed as of December 31, 2023. We did not exclude any employees (whether pursuant to the de minimis exemption for foreign employees or any other permitted exclusion) when making this determination.
As determined in accordance with Item 402 of Regulation S-K, the 2023 annual total compensation was $16,424,566 for the 3M CEO and $61,664 for 3M’s median employee. Among other things, these amounts include base salary, overtime, incentive payments, stock-based compensation (based on the grant date fair value of awards granted during 2023), changes in pension values and retirement plan contributions. As calculated in this manner, the ratio of the 3M CEO’s total compensation to the 3M median employee’s total compensation for fiscal year 2023 is 266 to 1.
Compensation Arrangements to be Adopted in Connection with the Separation
Solventum 2024 Long-Term Incentive Plan
Solventum intends to adopt the Solventum 2024 Long-Term Incentive Plan (the “Solventum 2024 LTIP”) prior to completion of the separation. This section describes the material terms of the Solventum 2024 LTIP.
The purpose of the Solventum 2024 LTIP is to enhance Solventum’s ability to attract, retain and motivate persons who make (or are expected to make) important contributions to Solventum by providing these individuals with equity ownership opportunities. In addition to shares of Solventum common stock covered by awards granted after the completion of the spinoff, the Solventum 2024 LTIP will cover any awards relating to 3M common stock that are converted into awards relating to Solventum common stock in connection with the spinoff. For purposes of this summary, we refer to these awards as “Adjusted Awards.”
Authorized Shares. The Solventum 2024 LTIP will authorize the issuance of a number of shares of Solventum common stock equal to the sum of 13,000,000 and the number of shares that may be issuable upon exercise, vesting or settlement of Adjusted Awards. Shares issued under the Solventum 2024 LTIP may be authorized but unissued shares, shares purchased on the open market, or treasury shares. In no event will more than 13,000,000 shares of common stock be issuable pursuant to the exercise of incentive stock options (“ISOs”) under the Solventum 2024 LTIP during its ten-year term.
Share Counting Provisions. If, following the effective date of the Solventum 2024 LTIP, an award under the Solventum 2024 LTIP expires, lapses or is terminated, exchanged for cash, surrendered, repurchased, canceled without having been fully exercised or forfeited, in any case, in a manner that results in Solventum acquiring shares covered by the award at a price not greater than the price (as adjusted to reflect any equity restructuring) paid by the participant for the shares or not issuing one or more shares covered by the award, the unused shares covered by the
232
award will, as applicable, become or again be available for award grants under the Solventum 2024 LTIP. Further, shares delivered (either by actual delivery or attestation) to Solventum by a participant or withheld by Solventum to satisfy any applicable tax withholding obligation (including shares retained by Solventum from the award being exercised or settled and/or creating the tax obligation) after the effective date of the Solventum 2024 LTIP will, as applicable, become or again be available for award grants under the Solventum 2024 LTIP. Notwithstanding the foregoing, following the effective date of the Solventum 2024 LTIP, the following shares shall not become or again be available for award grants under the Solventum 2024 LTIP: (a) shares delivered to Solventum or withheld by Solventum to satisfy the applicable exercise price of an option under the Solventum 2024 LTIP or to satisfy any tax withholding obligation with respect to an option or a stock appreciation right under the Solventum 2024 LTIP (including shares retained by Solventum from the award being exercised and/or creating the tax obligation), (b) shares subject to a stock appreciation right that are not issued in connection with the settlement or exercise, as applicable, of the stock appreciation right, and (c) shares purchased on the open market with the cash proceeds from the exercise of options. Dividend equivalents paid in cash will not be counted against the number of shares reserved under the Solventum 2024 LTIP.
Administration. The Solventum 2024 LTIP will be administered by the compensation committee or a subcommittee thereof (or by the Solventum Board of Directors (the “Solventum Board”) or another Solventum Board committee as may be determined by the Solventum Board from time to time). The administrator of the Solventum 2024 LTIP (the “Administrator”) or its delegate will have the authority to determine which service providers receive awards and set the terms and conditions applicable to the award within the confines of the Solventum 2024 LTIP’s terms. The Administrator will have the authority to make all determinations and interpretations under, prescribe all forms for use with, and adopt rules for the administration of, the Solventum 2024 LTIP.
Compensation Limit for Non-Employee Directors. The sum of all cash or other compensation and the value (determined as of the grant date in accordance with FASB ASC Topic 718 (or any successor thereto)) of all awards granted to a non-employee director during any calendar year for services as a member of the Board may not exceed $750,000. This limit applies to all compensation provided as a non-employee director, whether or not such compensation is provided under the Solventum 2024 LTIP.
Eligibility. In addition to any individuals who hold Adjusted Awards at any time, employees and non-employee directors of Solventum or any of its subsidiaries are eligible to receive awards under the Solventum 2024 LTIP. Service providers other than employees and non-employee directors are not eligible to participate in the Solventum 2024 LTIP.
Types of Awards. The Solventum 2024 LTIP provides for the grant of stock options (including ISOs and nonqualified stock options), stock appreciation rights, restricted stock, restricted stock units, performance bonus awards, performance shares and other stock- or cash-based awards. Awards to eligible individuals will be subject to the terms of an individual award agreement between Solventum and the individual. A brief description of each award type follows.
•Stock Options. Stock options may be granted under the Solventum 2024 LTIP, including both ISOs and non-qualified stock options, which provide the holder a right to purchase shares of common stock at a specified exercise price. The exercise price per share for each stock option will be set by the Administrator, but will not be less than the fair market value on the date of grant (or 110 percent of the grant date fair market value in the case of an individual who, on the date of grant, owns or is deemed to own shares representing more than 10 percent of the stock of Solventum or any “parent corporation” or “subsidiary corporation” within the meaning of Section 424 of the Code); provided, that the Administrator may grant stock options with an exercise price that is less than the fair market value on the date of grant in the event stock options are assumed or substituted in connection with certain corporate transactions. The term of any option award may not be longer than ten years (or five years in the case of an ISO granted to a 10 percent shareholder of Solventum). The Administrator will determine the time period for exercise of each award, including the time period for exercise following a termination of service by the recipient, subject to the maximum ten-year term.
233
•Stock Appreciation Rights. The Administrator is authorized to grant stock appreciation rights to eligible recipients in its discretion, on such terms and conditions as it may determine, consistent with the Solventum 2024 LTIP. A stock appreciation right entitles the holder to exercise the stock appreciation right to acquire shares of Solventum’s common stock upon exercise within a specified time period from the date of grant. Subject to the provisions of the stock appreciation right award agreement, the recipient may receive from Solventum an amount determined by multiplying the difference between the price per share of the stock appreciation right and the value of the share on the date of exercise by the number of shares of common stock subject to the award. The maximum term for which stock appreciation rights may be exercisable under the Solventum 2024 LTIP is ten years.
•Restricted Stock. The Administrator may make awards of restricted stock to eligible individuals in such amounts and at purchase prices (if any) to be established by the Administrator in connection with each award. Such awards will be subject to restrictions and other terms and conditions as are established by the Administrator. Upon issuance of restricted stock, recipients generally have the rights of a shareholder with respect to such shares, subject to the limitations and restrictions established by the Administrator in the individual award agreement. Such rights generally include the right to receive dividends and other distributions in relation to the award; however, dividends may be paid with respect to restricted stock subject to vesting only to the extent vesting conditions have been satisfied and the restricted stock vests.
•Restricted Stock Units. The Solventum 2024 LTIP authorizes awards of restricted stock units to eligible individuals in amounts and at purchase prices (if any) and upon such other terms and conditions as are established by the Administrator for each award. Restricted stock unit awards entitle recipients to acquire shares of Solventum’s common stock in the future under certain conditions. Holders of restricted stock units generally have no rights of ownership or as shareholders in relation to the award, unless and until the restrictions lapse and the restricted stock unit award vests in accordance with the terms of the grant. Restricted stock units may be accompanied by the right to receive the equivalent value of dividends paid on shares of Solventum’s common stock prior to the delivery of the underlying shares (i.e., dividend equivalent rights); however, dividend equivalents with respect to an award subject to vesting that are based on dividends paid prior to the vesting of such award will be paid to the holder only to the extent that the vesting conditions are subsequently satisfied and the award vests. The Administrator may provide that settlement of restricted stock units will occur upon or as soon as reasonably practicable after the restricted stock units vest or will instead be deferred, on a mandatory basis or at the participant’s election.
•Performance Shares. The Administrator is authorized to grant performance shares under the Solventum 2024 LTIP. Performance shares will be denominated in shares of common stock, unit equivalents and/or units of value (including a dollar value of shares of common stock) and may be linked to any one or more of the performance criteria listed below, or other specific criteria determined by the Administrator, in each case on a specified date or dates or over any period or periods determined by the Administrator.
•Performance Bonus Awards. Performance bonus awards will be denominated in cash and will be initially payable in cash, but may be paid in cash, shares or a combination of cash and shares in the discretion of the Administrator. Performance bonus awards will be payable upon the attainment of performance goals that are established by the Administrator and relate to any one or more of the performance criteria listed below, or other specific criteria determined by the Administrator, in each case on a specified date or dates or over any period or periods determined by the Administrator.
•Other Stock- or Cash-based Awards. Other stock- or cash-based awards are awards of cash, fully vested shares of Solventum’s common stock and other awards valued wholly or partially by referring to, or otherwise based on, shares of Solventum’s common stock. Other stock- or cash-based awards may be granted to participants and may also be available as a payment form in the settlement of other awards, as standalone payments and as payment in lieu of compensation otherwise payable to any individual who is eligible to receive awards. The Administrator will determine the terms and conditions of other stock- or cash-based awards, including any purchase price, performance goals (which may be based on performance criteria or otherwise), transfer restrictions and vesting conditions.
234
•Performance-Based Awards. Any awards granted pursuant to the Solventum 2024 LTIP may be subject to performance-based vesting conditions. The value of performance awards may be linked to any one or more of the performance criteria listed below, or other specific criteria determined by the Administrator, in each case on a specified date or dates or over any period or periods determined by the Administrator. The goals are established and evaluated by the Administrator and may relate to performance over any periods as determined by the Administrator.
For purposes of the Solventum 2024 LTIP, the pre-established performance goals may be based on one or more performance criteria or other specific criteria determined to be appropriate by the Administrator.
Prohibition on Repricing. Under the Solventum 2024 LTIP, the Administrator may not, without the approval of Solventum’s shareholders, authorize the repricing of any outstanding option or stock appreciation right to reduce its price per share, cancel any option or stock appreciation right in exchange for cash or another award when the price per share exceeds the fair market value of the underlying shares, or take any other action with respect to an option or stock appreciation right that Solventum determines would be treated as a repricing under the rules and regulations of the principal U.S. national securities exchange on which the shares of common stock are listed.
Certain Transactions. The Administrator has broad discretion to take action under the Solventum 2024 LTIP, as well as make adjustments to the terms and conditions of existing and future awards, to prevent the dilution or enlargement of intended benefits and facilitate necessary or desirable changes in the event of certain transactions and events affecting Solventum’s common stock, such as dividends or other distributions (whether in the form of cash, common stock, other securities, or other property), reorganizations, mergers, consolidations, change in control events and other corporate transactions. In addition, in the event of certain non-reciprocal transactions with Solventum’s shareholders known as “equity restructurings,” the Administrator will make equitable adjustments to outstanding awards and/or with respect to which awards may be granted under the Solventum 2024 LTIP and the individual award limits under the Solventum 2024 LTIP. No automatic “single-trigger” vesting acceleration applies under the Solventum 2024 LTIP in connection with a change in control event.
Amendment and Termination. The Board or the Compensation Committee of the Board may amend, suspend or terminate the Solventum 2024 LTIP at any time and from time to time. No amendment, other than an amendment that increases the number of shares available under the Solventum 2024 LTIP, may materially and adversely affect the economic benefits to be delivered under an outstanding award as of the date of such amendment without the consent of the affected participant. Shareholder approval is required for any amendment to the Solventum 2024 LTIP to the extent necessary to comply with applicable laws. The Solventum 2024 LTIP provides that in no event may an award be granted pursuant to the Solventum 2024 LTIP after ten years from the date of the Board’s approval of the Solventum 2024 LTIP.
Forfeiture and Clawbacks. All awards (including the gross amount of any proceeds, gains or other economic benefit obtained in connection with any award) made under the Solventum 2024 LTIP are subject to recoupment by Solventum to the extent required to comply with applicable laws or any policy of Solventum providing for the reimbursement of incentive compensation.
Securities Laws. The Solventum 2024 LTIP is intended to conform to all provisions of the Securities Act of 1933, as amended, and the Securities and Exchange Act of 1934, as amended, and any and all regulations and rules promulgated by the SEC thereunder, including, without limitation, Rule 16b-3. The Solventum 2024 LTIP will be administered, and awards will be granted and may be exercised, only in such a manner as to conform to such laws, rules and regulations.
United States federal income tax consequences
The following is a general summary under current law of the material federal income tax consequences to an employee or non-employee director granted an award under the Solventum 2024 LTIP. This summary deals with the general federal income tax principles that apply and is provided only for general information. Some kinds of taxes, such as state, local and foreign income taxes and federal employment taxes, are not discussed. Tax laws are complex and subject to change and may vary depending on individual circumstances and from locality to locality. The summary does not discuss all aspects of federal income taxation that may be relevant in light of a holder’s personal
235
circumstances. This summarized tax information is not tax advice and a holder of an award should rely on the advice of his or her legal and tax advisors.
ISOs. No income will be recognized by a participant upon the grant or exercise of an ISO. The basis of shares transferred to a participant upon exercise of an ISO is the price paid for the shares. If the participant holds the shares for at least one year after the transfer of the shares to the participant and two years after the grant of the option, the participant will recognize capital gain or loss upon sale of the shares received upon exercise equal to the difference between the amount realized on the sale and the basis in the shares. Generally, if the shares are not held for that period, the participant will recognize ordinary income upon disposition in an amount equal to the excess of the fair market value of the shares on the date of exercise over the amount paid for the shares, or if less (and if the disposition is a transaction in which loss, if any, will be recognized), the gain on disposition. Any additional gain realized by the participant upon the disposition will be a capital gain. The excess of the fair market value of shares received upon the exercise of an ISO over the option price for the shares is an item of adjustment for the participant for purposes of the alternative minimum tax. Therefore, although no income is recognized upon exercise of an ISO, a participant may be subject to alternative minimum tax as a result of the exercise.
Non-Qualified Stock Options. No income is expected to be recognized by a participant upon the grant of a non-qualified stock option. Upon exercise of a non-qualified stock option, the participant will recognize ordinary income in an amount equal to the excess of the fair market value of the shares on the date of exercise over the amount paid for the shares. Income recognized upon the exercise of a non-qualified stock option will be considered compensation subject to withholding at the time the income is recognized, and, therefore, the participant’s employer must make the necessary arrangements with the participant to ensure that the amount of the tax required to be withheld is available for payment. Non-qualified stock options generally provide the employer with a deduction equal to the amount of ordinary income recognized by the participant at the time of the recognition by the participant, subject to the deduction limitations described below.
Stock Appreciation Rights. Participants are not expected to recognize income upon receiving a grant of stock appreciation rights. Generally, the participant will recognize ordinary income subject to withholding upon the receipt of payment pursuant to stock appreciation rights in an amount equal to the aggregate amount of cash and the fair market value of any common stock received. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the amount includible in the participant’s income.
Restricted Stock. If the restrictions on an award of shares of restricted stock are of a nature that the shares are both subject to a “substantial risk of forfeiture” and are not freely transferable (within the meaning of Section 83 of the Code), the participant will not recognize income at the time of the award unless the participant affirmatively elects to include the fair market value of the shares of restricted stock on the date of the award, less any amount paid for the shares, in gross income for the year of the award pursuant to Section 83(b) of the Code. In the absence of this election, the participant will be required to recognize income on the date the shares either become freely transferable or are no longer subject to a substantial risk of forfeiture (within the meaning of Section 83 of the Code), the fair market value of the shares of restricted stock on such date, less any amount paid for the shares. The employer will be entitled to a deduction at the time of income recognition to the participant in an amount equal to the amount the participant is required to include in income with respect to the shares, subject to the deduction limitations described below.
If a Section 83(b) election is made within 30 days after the date the restricted stock is received, the participant will recognize ordinary income at the time of the receipt of the restricted stock, and the employer will be entitled to a corresponding deduction, equal to the fair market value of the shares at the time, less the amount paid, if any, by the participant for the restricted stock. If a Section 83(b) election is made, no additional income will be recognized by the participant upon the lapse of restrictions on the restricted stock, but, if the restricted stock is subsequently forfeited, the participant may not deduct the income that was recognized pursuant to the Section 83(b) election at the time of the receipt of the restricted stock.
Dividends paid to a participant holding restricted stock before the expiration of the restriction period will constitute additional compensation taxable as ordinary income to the participant subject to withholding, unless the
236
participant made an election under Section 83(b). Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the dividends includible in the participant’s income as compensation. If the participant has made a Section 83(b) election, the dividends will be dividend income, rather than additional compensation, to the participant.
If the restrictions on an award of restricted stock are not of a nature that the shares are both subject to a substantial risk of forfeiture and not freely transferable, within the meaning of Section 83 of the Code, the participant will recognize ordinary income at the time of the transfer of the shares in an amount equal to the fair market value of the shares of restricted stock on the date of the transfer, less any amount paid therefore.
The employer will be entitled to a deduction at that time in an amount equal to the amount the participant is required to include in income with respect to the restricted shares, subject to the deduction limitations described below.
Restricted Stock Units and Deferred Stock Units. A recipient of restricted stock units or deferred stock units generally should not recognize ordinary income at the time of grant. Generally, the participant will recognize ordinary income subject to withholding upon the receipt of cash and/or transfer of shares of common stock in payment of the restricted stock units or deferred stock units in an amount equal to the aggregate of the cash received and the fair market value of the common stock so transferred. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the amount includible in the participant’s income.
A participant generally will recognize ordinary income subject to withholding upon the payment of any dividend equivalents paid with respect to an award in an amount equal to the cash and the fair market value of any common stock the participant receives. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the amount includible in the participant’s income.
Performance Shares and Performance Bonus Awards. Participants are not expected to recognize income upon the grant of performance shares or performance bonus awards. Generally, the participant will recognize ordinary income subject to withholding at the time of payment, vesting or settlement of the award based on the fair market value of the award on that date. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the amount includible in the participant’s income.
Limitations on the Employer’s Compensation Deduction. In general, under Section 162(m), income tax deductions of publicly-held corporations may be limited to the extent total compensation (including base salary, annual bonus, stock option exercises and non-qualified benefits paid) for certain executive officers exceeds $1 million (less the amount of any “excess parachute payments” as defined in Section 280G of the Code) in any one year. Prior to the TCJA, covered employees generally consisted of our Chief Executive Officer and each of the next three highest compensated officers serving at the end of the taxable year other than our Chief Financial Officer, and compensation that qualified as “performance-based” under Section 162(m) was exempt from this $1 million deduction limitation. As part of the TCJA, the ability to rely on this exemption was, with certain limited exceptions, eliminated; in addition, the definition of covered employees was expanded to generally include all named executive officers. Certain Adjusted Awards granted prior to November 2, 2017 may be grandfathered from the changes made by the TCJA under certain limited transition relief, however, for grants after that date and any grants which are not grandfathered, we will not be able to take a deduction for any compensation in excess of $1 million that is paid to a covered employee. There is no guarantee that we will be able to take a deduction for any compensation in excess of $1 million that is paid to a covered employee pursuant to the Adjusted Awards or under the Solventum 2024 LTIP.
Excess Parachute Payments. Section 280G of the Code limits the deduction that an employer may take for otherwise deductible compensation payable to certain individuals if the compensation constitutes an “excess parachute payment.” Excess parachute payments arise from payments made to disqualified individuals that are in the nature of compensation and are contingent on changes in ownership or control of the employer or certain affiliates. Among other things, excess parachute payments could result from grants made during the 12-month period preceding a change in ownership or control of the employer or its affiliates and accelerated vesting or payment of awards under the Solventum 2024 LTIP upon a change in ownership or control of the employer or its affiliates. In
237
addition to the deduction limitation applicable to the employer, a disqualified individual receiving an excess parachute payment is subject to a 20 percent excise tax on the amount thereof.
Application of Section 409A of the Code. Section 409A of the Code imposes an additional 20 percent tax and interest on an individual receiving non-qualified deferred compensation under a plan that fails to satisfy certain requirements. For purposes of Section 409A, “non-qualified deferred compensation” includes equity-based incentive arrangements, including some stock options, stock appreciation rights, restricted stock unit awards, performance share awards and other awards that may be granted under the Solventum 2024 LTIP. Generally speaking, Section 409A does not apply to ISOs, non-discounted non-qualified stock options and stock appreciation rights if no deferral is provided beyond exercise, or restricted stock.
The awards made pursuant to the Solventum 2024 LTIP are expected to be designed in a manner intended to comply with the requirements of Section 409A of the Code to the extent the awards granted under the Solventum 2024 LTIP are not exempt from coverage. However, if the Solventum 2024 LTIP fails to comply with Section 409A in operation, a participant could be subject to the additional taxes and interest.
State, local and foreign tax consequences may in some cases differ from the United States federal income tax consequences described above. The foregoing summary of the United States federal income tax consequences in respect of the Solventum 2024 LTIP is for general information only. Interested parties should consult their own advisors as to specific tax consequences of their awards.
The Solventum 2024 LTIP is not subject to the Employee Retirement Income Security Act of 1974, as amended, and is not intended to be qualified under Section 401(a) of the Code.
Solventum Employee Stock Purchase Plan
Solventum intends to adopt the Solventum 2024 Employee Stock Purchase Plan (the “Solventum ESPP”) prior to completion of the separation. This section describes the material terms of the Solventum ESPP.
Interpretation
The ESPP and the options granted under the ESPP are intended to satisfy the requirements for an “employee stock purchase plan” under Section 423 of the Internal Revenue Code (the “Code”). Notwithstanding the foregoing, Solventum is not obligated to, and is not promising that it will, maintain the qualified status of the Solventum ESPP or any options granted thereunder. Options that do not satisfy the requirements for an “employee stock purchase plan” under Section 423 of the Code may be granted under the Solventum ESPP pursuant to the rules, procedures, or sub-plans adopted by the Administrator (as defined below) for certain eligible employees.
Share Reserve
Subject to adjustment in connection with certain corporate transactions, the maximum number of shares of Solventum common stock that may be purchased under the Solventum ESPP, consisting of authorized but unissued shares, treasury shares, or shares purchased on the open market, will be 4,000,000 shares.
Administration
The Solventum ESPP will be administered, at Solventum’s expense, under the direction of the compensation committee of the Solventum Board or other committee(s) thereof designated by the Solventum Board or the compensation committee thereof from time to time (any such entity, the “Administrator”). The Administrator will have the authority to take any actions it deems necessary or advisable for the administration of the Solventum ESPP, which includes establishing the timing and length of offering periods and purchase periods, establishing minimum and maximum contribution rates and adopting such rules, procedures, or sub-plans as may be deemed advisable or necessary to comply with the laws of countries other than the United States, to allow for tax-preferred treatment of the options or otherwise to provide for the participation by eligible employees who reside outside of the United States. The Administrator’s decisions will be final, conclusive, and binding upon all persons.
238
Eligibility
Employees (including officers) of Solventum or any of Solventum’s direct or indirect subsidiaries designated by the Administrator from time to time (a “participating affiliate”) may be eligible to participate in the Solventum ESPP; provided, however, that the Administrator may specify a minimum continuous service requirement (which period must be less than two years) that must be satisfied before an employee becomes eligible to participate. The following employees are ineligible to participate in the Solventum ESPP: (i) employees who, after exercising their options to purchase Solventum common stock under the Solventum ESPP, would own, directly or indirectly, shares of Solventum common stock (including shares that may be acquired under any outstanding options under the Solventum ESPP) representing 5% or more of the total combined voting power of all classes of Solventum capital stock; (ii) employees who are citizens or residents of a non-U.S. jurisdiction (without regard to whether such employees are also U.S. citizens or resident aliens), if the grant of an option under the Solventum ESPP or an offering period to such employee is prohibited under the laws of such non-U.S. jurisdiction or compliance with the laws of such non-U.S. jurisdiction would cause the Solventum ESPP or an offering period to violate the requirements of Section 423 of the Code (unless the option is granted pursuant to a “Non-423(b) Offering” as defined below); and (iii) any other employee who the Administrator determines to exclude from an offering designed to satisfy the requirements of Section 423 of the Code but only if such exclusion is permitted by Section 423 of the Code and the regulations issued thereunder and, to the extent applicable, the laws of a non-U.S. jurisdiction. Notwithstanding the foregoing, for purposes of an offering under the Solventum ESPP that is not intended to satisfy the requirements of Section 423 of the Code (a “Non-423(b) Offering”), the Administrator will have the authority to establish a different definition of eligible employee as it may deem advisable or necessary. In addition, the Administrator may determine that highly compensated employees (within the meaning of Section 414(q) of the Code) will not be eligible to participate in an offering period.
Participation Election
An eligible employee may participate in an offering period under the Solventum ESPP by submitting an enrollment form to Solventum or its designee, which will authorize Solventum to make after-tax payroll deductions up to a designated percentage or a maximum dollar amount as designated by the Administrator (but in any event not to exceed 15% of the participant’s eligible compensation) on each pay day following enrollment, or if authorized by the Administrator, will indicate the amount of other cash contributions that a participant will make to the Solventum ESPP. The Administrator will credit the deductions or contributions to the participant’s account under the Solventum ESPP. Subject to certain exceptions, a participant may cease their payroll deductions or cash contributions during an offering period, by submitting a new enrollment form, at any time prior to the last day of such offering period (or purchase period). A participant may increase or decrease their payroll deductions or cash contributions to take effect on the first trading day of the next offering period, by submitting a new enrollment form. Once an eligible employee becomes a participant in the Solventum ESPP, the participant will automatically participate in each successive offering period until the participant ceases their payroll deductions or is no longer eligible to participate in the Solventum ESPP or a specific offering period under the Solventum ESPP.
Offering Periods and Purchase Periods
The Administrator will determine the periods during which payroll deductions or other cash contributions will accumulate to purchase shares of Solventum common stock, which periods will not exceed 27 months. Each of these periods is known as an “offering period.” The Administrator may, but is not required to, permit more than one purchase of Solventum common stock within a single offering period. The periods during which payroll deductions or other cash contributions will accumulate for these purchases are referred to as “purchase periods.”
Purchase Price
On the last trading day of the offering period (or purchase period, if the offering period has multiple purchase periods), a participant is deemed to purchase the number of whole shares of Solventum common stock determined by dividing the total amount of payroll deductions withheld from the participant’s paychecks during the offering period (or purchase period) or the total value of all cash contributions made to the Solventum ESPP during the
239
offering period (or purchase period) by the purchase price. Any cash not applied to the purchase of shares will be refunded to the participant.
Purchase of Shares
On the last trading day of the offering period (or purchase period, if the offering period has multiple purchase periods), a participant is deemed to purchase the number of whole shares of Solventum common stock determined by dividing the total amount of payroll deductions withheld from the participant’s paychecks during the offering period (or purchase period) or the total value of all cash contributions made to the Solventum ESPP during the offering period (or purchase period) by the purchase price. Any cash not applied to the purchase of fractional shares will be transferred to the participants’ brokerage account.
Purchase Limitations
No participant may purchase shares of Solventum common stock in any calendar year under the Solventum ESPP and under all other “employee stock purchase plans” of Solventum and its subsidiaries having an aggregate fair market value in excess of $25,000, determined as of the first trading day of the offering period. The fair market value for this purpose will be equal to the closing sales prices of Solventum common stock as reported on the NYSE. If the Administrator determines that the total number of shares remaining available under the Solventum ESPP is insufficient to permit all participants to exercise their options to purchase shares, the Administrator will make a participation adjustment and proportionately reduce the number of shares purchasable by all participants.
Termination of Participation
A participant may voluntarily withdraw from an offering period under the Solventum ESPP. A participant will automatically be withdrawn by Solventum from an offering period under the Solventum ESPP (i) upon a termination of employment with Solventum or a participating affiliate, (ii) in certain cases, following a leave of absence or a temporary period of ineligibility, and (iii) upon cessation of eligibility to participate in the Solventum ESPP for any reason.
Stockholder Rights
A participant shall not be a stockholder or have any rights as a stockholder with respect to shares of Solventum common stock subject to the participant’s options under the Solventum ESPP until the shares are purchased pursuant to the options and transferred into the participant’s name on Solventum’s books and records. Shares purchased under the Solventum ESPP will be held by the custodian designated under the Solventum ESPP. Following the purchase and transfer of the shares, a participant will become a stockholder with respect to the shares purchased and will then have all dividend, voting, and other ownership rights incident thereto. Notwithstanding the foregoing, the Administrator has the right to (i) limit the reissuance of the shares into the participant’s own name and delivery until two years from the first trading day of the offering period in which the shares were purchased and until one year from the last trading day of the offering period (or purchase period) in which the shares were purchased (the “holding period”), (ii) require that any sales during the holding period be performed through a licensed broker acceptable to Solventum, and (iii) limit sales or other transfers of shares of Solventum common stock for up to two years from the date the participant purchases shares under the Solventum ESPP.
Transferability
A participant’s options to purchase shares under the Solventum ESPP may not be sold, pledged, assigned, or transferred in any manner, whether voluntarily, by operation of law, or otherwise. Any payment of cash or issuance of shares under the Solventum ESPP may be made only to the participant (or, in the event of the participant’s death, to the participant’s estate or beneficiary). During a participant’s lifetime, only the participant may exercise their options to purchase shares under the Solventum ESPP.
Corporate Transactions
If certain changes occur to Solventum common stock by reason of a recapitalization, reclassification, stock split, reverse stock split, spin-off, combination of shares, exchange of shares, stock dividend, other distribution payable in
240
capital stock or other increase or decrease in the outstanding shares of Solventum common stock without receipt of consideration by the Company occurring after the effective date of the Solventum ESPP, the number and kinds of shares of Solventum common stock for which options may be made under the Solventum ESPP will be adjusted proportionately by the Administrator. In addition, the number and kind of shares for which options are outstanding will be similarly adjusted so that the proportionate interest of a participant immediately following such event will, to the extent practicable, be the same as immediately prior to such event. Upon dissolution or liquidation of Solventum, upon a merger, consolidation, or reorganization of Solventum with one or more other corporations in which Solventum is not the surviving entity, upon a sale of all or substantially all of Solventum’s assets, or upon consummation of any other transaction approved by the Solventum Board resulting in any person or entity owning more than 50% of the combined voting power of all classes of Solventum’s capital stock, the Solventum ESPP and all options outstanding thereunder will terminate, except to the extent the continuation or assumption of the Solventum ESPP, or the substitution of the options under the Solventum ESPP with new options covering the capital stock of the successor entity, with corresponding appropriate adjustments to the number and kinds of shares and purchase prices, are made in writing in connection with the transaction. Upon termination of the Solventum ESPP in this circumstance, the offering period will end on the last trading day prior to such termination unless the Administrator determines, in its sole discretion, that such ending date is impractical for administrative purposes and sets a different last day of the offering period, and the options of each participant will automatically be exercised on such last trading day. Subject to the foregoing, if Solventum is the surviving corporation in any reorganization, merger, or consolidation of Solventum with one or more other corporations, all outstanding options under the Solventum ESPP will pertain to and apply to the securities to which a holder of the number of shares of Solventum common stock subject to such options would have been entitled immediately following such transaction, with corresponding appropriate adjustments to the number and kinds of shares and purchase prices. Furthermore, notwithstanding anything to the contrary in the Solventum ESPP and in accordance with applicable law, in the event of a change in stock or a corporate transaction described in the Solventum ESPP, the Administrator will have the right to shorten any offering period then in progress and establish a new last trading day of the purchase period for such offering period, which will occur before the effective date of the applicable change in stock or corporate transaction. In addition, the Administrator will notify participants in writing, at least five (5) business days in advance, of such new earlier purchase date and that the participant’s option will be exercised automatically on that date (unless the participant withdraws prior to such date or has ceased to be eligible to participate in the Solventum ESPP).
Term
The Solventum ESPP will terminate on the earliest of (i) the date on which all shares of Solventum common stock reserved for issuance under the Solventum ESPP have been issued, (ii) the date the Solventum ESPP is terminated in connection with certain corporate transactions, and (iii) the date the Administrator terminates the Solventum ESPP.
Amendment, Suspension, or Termination
The Administrator may, at any time and from time to time, amend, suspend, or terminate the Solventum ESPP or an offering period under the Solventum ESPP; provided, however, that no amendment, suspension, or termination will, without the consent of the participant, impair any vested rights of a participant. Without stockholder approval, the Administrator may not (i) increase the number of shares reserved for issuance under the Solventum ESPP or (ii) change the eligibility requirements for participating in the Solventum ESPP.
Solventum Executive Severance Plan
Solventum intends to adopt the Solventum Executive Severance Plan (the “Solventum Executive Severance Plan”) prior to completion of the separation. This section describes the material terms of the plan.
The Solventum Executive Severance Plan will provide separation benefits for certain U.S. executives (including our Named Executive Officers) in the event an eligible employee’s employment is terminated by Solventum other
241
than for Misconduct or an eligible employee resigns for Good Reason. An eligible employee who becomes entitled to benefits under the Solventum Severance Plan will receive the following:
•continued payment of his or her annual base salary for a number of months (24 months for the Chief Executive Officer, and 18 months for all other Named Executive Officers);
•continued payment of annual incentive payments that would have been paid if the participant remained employed through the end of the severance payment period, calculated based on actual results for the relevant year, using the participant’s final performance rating as of the end of the applicable year (or if none, a performance rating of “fully meets expectations” or equivalent), and prorated based on the number of days in the year preceding the end of the severance period (if the severance payment period ends prior to the end of a year); provided, however, that any such annual incentive payment related to the period following the participant’s termination of employment will not exceed 100 percent of the prorated target annual incentive compensation opportunity that otherwise would have been in effect for such period if he or she remained employed with Solventum;
•an opportunity to exercise all vested stock options and stock appreciation rights following the participant’s termination and prior to the expiration date of such award;
•accelerated vesting and payment of a prorated portion of the participant’s outstanding “annual” restricted stock unit awards and “inducement” restricted stock unit awards, with proration based on the number of full years of the participant’s completed service over the number of years required to vest under the original vesting schedule;retention of target number of shares under each performance share award with vesting based on actual performance through the end of the performance period, and the number of shares that vest pro-rated based on the portion of the performance period that elapsed through the employment termination date;
•full vesting of the participant’s VIP company contribution account; and
•outplacement services in accordance with Solventum’s policy.
Any severance payments under the Severance Plan will reduce on a dollar-for-dollar basis (but not below $0) any amounts the participant may otherwise be eligible to receive under his or her non-competition agreement with Solventum. In addition, severance payments will cease in the event the participant becomes reemployed by Solventum.
The Solventum Severance Plan does not provide severance benefits to employees who terminate pursuant to a mandatory retirement policy adopted by the Board. Employees who terminate in connection with a spinoff, divestiture or similar transaction involving the employee’s business unit may be eligible to participate at the discretion of Solventum. To be eligible to receive benefits under the Solventum Severance Plan, an employee must execute and not revoke a general release of claims in favor of Solventum in a form approved by Solventum.
In the event a Named Executive Officer’s payments under the Solventum Severance Plan would be subject to an excise tax on “parachute payments” in connection with a change in control of Solventum due to the application of Section 280G of the Internal Revenue Code, the Named Executive Officer will not be entitled to a “gross-up.” Instead, the Named Executive Officer’s “parachute payments” may be reduced if such reduction would provide the Named Executive Officer with a greater post-tax benefit than he or she would receive if the excise taxes were to apply.
For purposes of the Severance Plan, the terms “Misconduct” and “Good Reason” mean the following:
“Misconduct” means (i) the employee’s willful failure to substantially perform his or her duties (other than a failure resulting from his or her disability); (ii) the employee’s willful failure to carry out, or comply with any lawful and reasonable directive of the Board or his or her immediate supervisor; (iii) the occurrence of any act or omission by the employee that could reasonably be expected to result in (or has resulted in) his or her conviction, plea of no contest, plea of nolo contendere, or imposition of unadjudicated probation for any felony or indictable offense or
242
crime involving moral turpitude; (iv) the employee’s commission of an act of fraud, embezzlement, misappropriation, misconduct, or breach of fiduciary duty against Solventum or any of its subsidiaries or affiliates or any of their officers, directors, employees, customers, suppliers, insurers or agents; (v) for purposes of the Solventum Severance Plan only, the employee’s material breach of any material provision of any written agreement with Solventum or any subsidiary; (vi) any action (or inaction) by the employee that Solventum reasonably determines constitutes gross negligence or misconduct in the performance of his or her duties and responsibilities; or (vii) any other intentional misconduct by the Named Executive Officer that significantly affects the business or affairs of Solventum or any subsidiary in an adverse manner.
“Good Reason” means, with respect to a voluntary termination occurring within 18 months following a change in control of Solventum, (i) a material diminution in the employee’s position, authority, duties or responsibilities as in effect immediately prior to the change in control, (ii) a material diminution in the employee’s base salary or annual planned cash compensation, or (iii) a material change in the geographic location at which the employee is required to perform services for his or her employer. If the voluntary termination does not occur within 18 months following a change in control, “Good Reason” means (i) a material diminution in the employee’s base salary or annual planned cash compensation, other than an across-the-board reduction that applies to all comparable positions, or (ii) a change in excess of 100 miles in the primary work location at which the employee is required to perform services for his or her employer. For terminations that do not occur within 18 months following a change in control, the employee will be required to provide written notice to Solventum of the grounds for a Good Reason termination within 30 days of the initial existence of such grounds, and Solventum has 30 days from the date of such notice to cure such circumstances. An employee must terminate employment for Good Reason within 60 days after the first occurrence of the applicable grounds.
Solventum Clawback Policy
Prior to the distribution, Solventum will adopt a compensation clawback policy that meets the requirements of the NYSE.
243
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Agreements with 3M
Following the separation and distribution, Solventum and 3M will operate separately, each as an independent public company. Prior to the distribution, Solventum and 3M will enter into a separation and distribution agreement and other agreements that will outline the terms and conditions of the separation and distribution and provide a framework for Solventum’s relationship with 3M after the separation and distribution.
Forms of the material agreements described below, other than those agreements described under “—Other Agreements,” are filed as exhibits to the registration statement on Form 10 of which this information statement is a part and are incorporated by reference into this information statement. The summaries of each of these agreements set forth below are qualified in their entireties by reference to the full text of the applicable agreements. The agreements described below will be entered into following the effectiveness of the registration statement on Form 10 of which this information statement is a part, shortly prior to the completion of the distribution.
Separation and Distribution Agreement
The separation and distribution agreement will contain the key provisions relating to the separation of the Health Care Business from the remaining businesses of 3M and the distribution of at least 80.1% of the outstanding shares of Solventum’s common stock to holders of 3M common stock entitled to such distribution, as described under “The Separation and Distribution.”
Transfer of Assets and Assumption of Liabilities
The separation and distribution agreement will identify the assets to be transferred, the liabilities to be assumed and the contracts to be transferred to each of Solventum and 3M as part of the separation of the Health Care Business from 3M into an independent, publicly traded company. In particular, the separation and distribution agreement will provide that, among other things, subject to the terms and conditions contained therein:
•certain assets related to the Health Care Business, which this information statement refers to as the “Solventum Assets,” will be retained by or transferred to Solventum or one of its subsidiaries. Generally, assets that are primarily related to the Health Care Business at the time of the distribution will be Solventum Assets;
•certain liabilities related to the Health Care Business or the Solventum Assets, which this information statement refers to as the “Solventum Liabilities,” will be retained by or transferred to Solventum. Except as set forth below with respect to PFAS related liabilities, generally liabilities will be Solventum Liabilities to the extent they relate to the business, operations and activities of the Health Care business or any Solventum Assets; and
•all of the assets and liabilities (whether accrued, contingent or otherwise) of 3M and its subsidiaries (including for this purpose Solventum and its subsidiaries) other than the Solventum Assets and the Solventum Liabilities (referred to in this information statement as the “3M Assets” and “3M Liabilities,” respectively) will be retained by or transferred to 3M or a subsidiary of 3M (other than Solventum and its subsidiaries).
Except as expressly set forth in the separation and distribution agreement or any ancillary agreement entered into in connection with the separation and distribution agreement (referred to in this information statement as an “ancillary agreement”), neither 3M nor Solventum will make any representation or warranty as to the assets, business or liabilities transferred or assumed as part of the separation, as to any approvals or notifications required in connection with the transfers, as to the value of or the freedom from any security interests of any of the assets transferred, as to the absence or presence of any defense or right of setoff or freedom from counterclaim with respect to any claim or other asset of either of Solventum or 3M, or as to the legal sufficiency of any document or instrument delivered to convey title to any asset or thing of value to be transferred in connection with the separation. All assets will be transferred on an “as is,” “where is” basis, and the respective transferees will bear the economic
244
and legal risks that any conveyance will prove to be insufficient to vest in the transferee good and marketable title, free and clear of all security interests, that any necessary consents or governmental approvals are not obtained, or that any requirements of law, agreements, security interests, or judgments are not complied with.
Unless the context otherwise requires, information in this information statement with respect to the assets and liabilities of the parties following the distribution is presented based on the allocation of such assets and liabilities pursuant to the separation and distribution agreement. The separation and distribution agreement will generally provide that in the event that the transfer of certain assets and liabilities to Solventum or 3M, as applicable, does not occur prior to the separation (other than as needed to allow 3M or Solventum to fulfill their obligations under certain transition related agreements), then until such assets or liabilities are able to be transferred, Solventum or 3M, as applicable, will hold such assets and liabilities on behalf of the transferee for the use and benefit of the member of the transferee (at the expense of the transferee, and to the extent permitted by applicable law).
PFAS Liabilities
The separation and distribution agreement will also govern the allocation of liabilities related to PFAS between the 3M and Solventum groups, which liabilities will not be subject to the general allocation principles otherwise set forth in the separation and distribution agreement. The 3M group will retain all PFAS-related liabilities resulting from the business, operations and activities of (x) the 3M Business and (y) the Health Care Business prior to the effective time of the distribution (except as described below, including for site-based PFAS liabilities resulting from Solventum’s post-spin conduct of its business, operations and activities as an independent company). Solventum will retain liability for all PFAS-related liabilities resulting from the business, operations and activities of the Health Care Business at or after the effective time of the distribution, other than liabilities from product claims alleging harm from the presence of PFAS in certain products of the Solventum Business sold at or after the effective time of the distribution and prior to January 1, 2026 (subject to exceptions described in further detail below). The 3M group will retain liabilities related to site-based PFAS contamination at any real property owned, leased or operated by any member of the Parent group and liabilities for site-based PFAS contamination arising from third-party claims at sites allocated to the Solventum group in the separation to the extent such liabilities relate to PFAS contamination existing at or prior to the effective time of the distribution, other than PFAS liabilities from the Solventum sites to the extent resulting from an action taken by any member of the Solventum group following the effective time of the distribution or from any failure by a member of the Solventum group following the effective time of the distribution to use commercially reasonable efforts that are consistent with then-current industry standards to avoid contamination. The 3M group will also retain PFAS liabilities for product claims (x) arising from products of the 3M Business, (y) arising from products of the Health Care Business sold prior to the effective time of the distribution, and (z) arising from certain products of the Solventum Business sold at or after the effective time of the distribution and prior to January 1, 2026 (subject to the exceptions described below). Clause (z) in the immediately preceding sentence will not extend to PFAS liabilities for product claims resulting from (i) new products introduced by the Solventum group following the effective time of the distribution that contain or are enabled by PFAS that is not supplied by the 3M group, (ii) products that are modified by Solventum after the effective time of the distribution to add, contain or become enabled by PFAS that is not supplied by the 3M group, or with respect to which any modification is made after the effective time of the distribution in the formulation or production of the product that changes the amount or type of PFAS contained in the product or the amount or type of PFAS enabling the product, in each case from and after the date of such modification, (iii) PFAS that is added to a product of the Solventum group after it is sold by the Solventum group and (iv) PFAS that has accumulated in or on a product of the Solventum group as a result of the use of the product (whether or not the product is being used as directed), including through filtration, purification or similar application.
In addition, and consistent with the allocation described above, the 3M group will retain specifically identified PFAS-related liabilities, including those resulting from specified PFAS-related litigation matters and liabilities under 3M’s publicly announced settlement agreement with public water systems in the United States.
The Solventum group will be responsible for the maintenance of certain PFAS containment measures at its properties after the effective time of the distribution.
245
Disputes between 3M and Solventum relating to the PFAS liability allocation will be subject to mediation and, if not resolved, confidential binding arbitration.
The Distribution
The separation and distribution agreement will also govern the rights and obligations of the parties regarding the distribution following the completion of the separation. On the distribution date, 3M will distribute to its shareholders that hold 3M common stock as of the record date for the distribution at least 80.1% of the issued and outstanding shares of Solventum common stock on a pro rata basis. Shareholders will receive cash in lieu of any fractional shares.
Conditions to the Distribution
The separation and distribution agreement will provide that the distribution is subject to satisfaction (or waiver by 3M in its sole and absolute discretion) of certain conditions. These conditions are described under “The Separation and Distribution—Conditions to the Distribution.” 3M will have the sole and absolute discretion to determine (and change) the terms of, and to determine whether to proceed with, the distribution and, to the extent that it determines to so proceed, to determine the record date for the distribution, the distribution date and the distribution ratio.
Claims
In general, and except as described under “—PFAS Liabilities,” each of Parent and Solventum will assume liability for all pending, threatened and unasserted legal matters related to its own business or its assumed or retained liabilities and will indemnify the other party for any liability to the extent arising out of or resulting from such assumed or retained legal matters.
Releases
The separation and distribution agreement will provide that Solventum and its affiliates will release and discharge 3M and its affiliates from all liabilities assumed by Solventum as part of the separation, from all actions, inactions, events, omissions, conditions, facts or circumstances occurring or existing prior to the effective time of the distribution to the extent arising out of or resulting from the Health Care Business, the Solventum Assets or the Solventum Liabilities and from all liabilities arising from or in connection with the transactions and all other activities to implement the separation and distribution (including all decisions as to any allocation of assets and liabilities between the 3M and Solventum Groups), except as expressly set forth in the separation and distribution agreement. 3M and its affiliates will release and discharge Solventum and its affiliates from all liabilities retained by 3M and its affiliates as part of the separation, from all actions, inactions, events, omissions, conditions, facts or circumstances occurring or existing prior to the effective time of the distribution to the extent arising out of or resulting from the 3M Business, the Parent Assets and the Parent Liabilities, and from all liabilities arising from or in connection with the transactions and all other activities to implement the separation and distribution, except as expressly set forth in the separation and distribution agreement.
These releases will not extend to obligations or liabilities under any agreements between the parties that remain in effect following the separation, which agreements include the separation and distribution agreement and the other agreements described in this section.
Indemnification
In the separation and distribution agreement, Solventum will agree to indemnify, defend and hold harmless 3M, each of 3M’s affiliates, and 3M’s and its affiliates’ respective directors, officers, employees, and agents, from and against all liabilities arising out of or resulting from:
•the Solventum Liabilities, which includes any untrue statement or alleged untrue statement or omission or alleged omission of a material fact in the Form 10 or in this information statement or other related disclosure document (as amended or supplemented), except for any such statements or omissions made explicitly in 3M’s name;
246
•Solventum’s failure or the failure of any other person to pay, perform or otherwise promptly discharge any of the Solventum Liabilities in accordance with their respective terms, whether prior to, at or after the distribution;
•except to the extent arising from a 3M Liability, any guarantee, indemnification or contribution obligation, surety bond or other credit support agreement, arrangement, commitment, or understanding for the benefit of Solventum by 3M that survives the distribution; and
•any breach by Solventum of the separation and distribution agreement or any of the ancillary agreements.
3M will agree to indemnify, defend and hold harmless Solventum, each of Solventum’s affiliates and Solventum’s and its affiliates’ respective directors, officers, employees, and agents from and against all liabilities arising out of or resulting from:
•the 3M Liabilities, which includes any untrue statement or alleged untrue statement or omission or alleged omission of a material fact made explicitly in 3M’s name in the Form 10 or in this information statement or other related disclosure document (as amended or supplemented);
•the failure of 3M or any other person to pay, perform or otherwise promptly discharge any of the 3M Liabilities in accordance with their respective terms, whether prior to, at or after the distribution;
•except to the extent arising from a Solventum Liability, any guarantee, indemnification or contribution obligation, surety bond or other credit support agreement, arrangement, commitment or understanding for the benefit of 3M by Solventum that survives the distribution; and
•any breach by 3M of the separation and distribution agreement or any of the ancillary agreements.
The separation and distribution agreement will also establish procedures with respect to claims subject to indemnification and related matters.
Indemnification with respect to taxes, and the procedures related thereto, will be governed by the tax matters agreement.
Insurance
The separation and distribution agreement will provide for the allocation between the parties of rights and obligations under existing insurance policies with respect to occurrences prior to the distribution and set forth procedures for the administration of insured claims and related matters.
Non-Compete
The separation and distribution agreement will provide that until the third anniversary of the distribution date:
•Solventum will be prohibited from marketing or selling hollow fiber membrane products in certain specified fields relating to 3M’s business and from making, marketing or selling industrial adhesives for inclusion in third party products, provided that Solventum can make, market or sell (x) medical device assembly products that include an industrial adhesive sourced from the 3M group (or, with respect to new products commercialized following the distribution date, from any third party or internally)and (y) specified products incorporating industrial adhesives sourced from 3M; and
•3M will be prohibited from marketing or selling hollow fiber membrane products in certain specified fields relating to Solventum’s business and from making, marketing or selling medical grade adhesives or medical grade films, provided that 3M can make, market or sell (x) medical grade adhesives or medical grade films for incorporation into finished goods that 3M’s consumer health business supplies to third parties or (y) finished goods that incorporate medical grade adhesives or medical grade films,
247
in each case as further specified in the separation and distribution agreement. The restrictions described above will be subject to exceptions relating to specified merger and acquisitions activity, ownership of less than 5% of the outstanding securities of any person, passive investments and performance under any spin-off related agreements.
Non-Solicit
The separation and distribution agreement will provide that for a period of twelve (12) months following the distribution, neither Solventum nor 3M shall solicit for employment any employees of the other party’s group with a title of “director” or above, subject to exceptions for, among other things, general solicitations (including via a search firm or employment agency) not directly targeted at restricted employees.
Further Assurances
In addition to the actions specifically provided for in the separation and distribution agreement, except as otherwise set forth therein or in any ancillary agreement, Solventum and 3M will agree in the separation and distribution agreement to use reasonable best efforts, prior to, on and after the distribution date, to take, or cause to be taken, all actions, and to do, or cause to be done, all things reasonably necessary, proper or advisable under applicable laws, regulations and agreements to consummate and make effective the transactions contemplated by the separation and distribution agreement and the ancillary agreements.
Dispute Resolution
The separation and distribution agreement will contain provisions that govern, except as otherwise provided in any ancillary agreement, the resolution of disputes, controversies or claims that may arise between Solventum and 3M related to the separation or distribution and that are unable to be resolved through good faith discussions between Solventum and 3M.
Expenses
Except as expressly set forth in the separation and distribution agreement or in any ancillary agreement, the party incurring the expense will be responsible for all fees, costs, and expenses incurred in connection with the separation prior to the distribution date.
Other Matters
Other matters governed by the separation and distribution agreement will include, among others, approvals and notifications of transfer, termination of intercompany agreements, release of guarantees, treatment of bank accounts, the allocation of cash (including a post-closing adjustment based on the amount of cash held by Solventum and its subsidiaries at the time of the distribution), shared contracts, financial information certifications, transition committee provisions, confidentiality, access to and provision of information, production of witnesses, privileged matters, and financing arrangements.
Amendment and Termination
The separation and distribution agreement will provide that it may be terminated, and the separation and distribution agreement may be amended, modified or abandoned, at any time prior to the distribution date in the sole and absolute discretion of the 3M Board of Directors without the approval of any person, including Solventum or 3M shareholders.
The separation and distribution agreement will provide that no provision of the separation and distribution agreement or any ancillary agreement may be waived, amended, supplemented or modified by a party without the written consent of the party against whom it is sought to enforce such waiver, amendment, supplement or modification.
After the distribution date, the separation and distribution agreement may not be terminated, except by an agreement in writing signed by both Solventum and 3M.
248
In the event of a termination of the separation and distribution agreement, no party, nor any of its directors, officers or employees, will have any liability of any kind to the other parties or any other person.
Transition Services Agreement
Solventum and 3M will enter into a transition services agreement in connection with the separation pursuant to which 3M and its affiliates will provide to Solventum and its affiliates, and Solventum and its affiliates will provide to 3M and its affiliates, on an interim, transitional basis, various services, including, but not limited to, information technology support and access, logistics services, certain finance support functions, compliance reporting, human resources and toxicology laboratory support services. The service recipient will generally be required to pay a fixed monthly service fee per service, with the total fees in any month depending on the services provided in that month, and will be required to reimburse the service provider for third party expenses incurred by the service provider that are not included in the fixed service fee, except in cases where service provider decides to outsource services only after the effective date of the agreement in which case service provider will generally bear the additional costs.
The service provider will be required to perform the services in accordance with specified key performance indicators (“KPIs”) or if no KPIs are specified, with the same degree of care and in the same manner as it provides similar services to its internal organization at the time such services are performed.
The transition services agreement will have an overall term of two years following the distribution date, with shorter terms for individual services. Individual service durations may be extended by the relevant schedules to the transition services agreement or by mutual written consent subject to service fee escalations of 10% of the existing fee for each six-month extension period, but no service may be extended beyond the two-year term of the transition services agreement. Either party will be able to terminate the transition services agreement for uncured material breaches, and the service recipient will be able to generally terminate a service for convenience with ninety days’ advance notice. Additionally, 3M will be permitted to terminate the transition services agreement if Solventum undergoes a change of control. In cases of termination for convenience by the service recipient or termination for cause by the service provider, the service recipient will be required to reimburse the service provider for all costs arising in connection with the termination. If single transition services are terminated, all entangled services will automatically and concurrently terminate as well.
If, at or prior to the expiration or termination of the transition services agreement, a party is unable to operate independently from the rights or services provided under the transition services agreement due to circumstances not caused by such party’s action or inaction, the parties are required to discuss in good faith commercially reasonable alternatives (up to and including a one-year extension of the transition services agreement beyond the initial term on a country-by-country basis, as needed) to avoid a business disruption for the requesting party. A request for such one-year extension may not be unreasonably withheld, so long as such extensions are subject to the escalated services fees described in the following sentence. The transition services agreement does not specify the applicable fees for any such extension term, but states that the applicable fees for any such extension must be at least as much as would be calculated pursuant to the service fee escalation provision described in the previous paragraph for extensions within the initial two-year term.
Tax Matters Agreement
In connection with the separation, Solventum and 3M will enter into a tax matters agreement that will govern the parties’ respective rights, responsibilities and obligations with respect to taxes (including responsibility for taxes, entitlement to refunds, allocation of tax attributes, preparation of tax returns, control of tax contests and other tax matters).
In addition, the tax matters agreement will impose certain restrictions on Solventum and its subsidiaries (including restrictions on share issuances, business combinations, sales of assets and similar transactions) that will be designed to preserve the tax-free status of the distribution and certain related transactions. The tax matters agreement will provide special rules that allocate tax liabilities in the event the distribution, together with certain related transactions, is not tax-free. In general, under the tax matters agreement, each party is expected to be responsible for any taxes, whether imposed on Solventum or 3M, that arise from (1) the failure of the distribution, together with certain related transactions, to qualify for tax-free treatment under the Code or (2) the failure of certain
249
related transactions to qualify for their intended tax treatment, in each case, to the extent that the failure to so qualify is attributable to post-distribution actions by such party or transactions with respect to such party’s stock, or to a breach of certain representations or covenants made by that party in the tax matters agreement.
Employee Matters Agreement
Solventum and 3M will enter into an employee matters agreement in connection with the separation to allocate liabilities and responsibilities relating to employment matters, employee compensation and benefits plans and programs, and other related matters. The employee matters agreement will govern certain compensation and employee benefit obligations with respect to the current and former employees and non-employee directors of each company.
The employee matters agreement will provide that, unless otherwise specified, each party will be responsible for liabilities associated with current and former employees of such party and its subsidiaries. For the U.S. qualified and non-qualified defined benefit pension plans, Solventum will establish plans that mirror the corresponding 3M plans and the mirror Solventum plans will assume all obligations under 3M’s plans for the accrued benefits of current and former Solventum employees. In the case of 3M’s U.S. qualified defined benefit pension plan, assets in an amount determined in accordance with applicable law will be transferred from the 3M trust to the corresponding Solventum trust. In addition, as of December 31, 2028, benefit accruals under 3M’s and Solventum’s U.S. defined benefit pension plans will cease.
The employee matters agreement will also govern the terms of equity-based awards granted by 3M prior to the separation. See “The Separation and Distribution—Treatment of Equity‑Based Compensation.”
Transition Distribution Services Agreement
Solventum and 3M will enter into a transition distribution services agreement in connection with the separation pursuant to which 3M and its affiliates will retain inventories of and purchase certain Solventum products from Solventum and its subsidiaries and sell those products to Solventum’s customers, on an interim, transitional basis.
3M and service providers will provide the transition distribution services in a manner consistent with 3M's internal practices for similar activities at the time the relevant services are provided and substantially with the same degree of care, skill and diligence used by 3M or its affiliates, as applicable.
The transition distribution services agreement provides that Solventum and 3M will determine and agree on a mark-up factor to be factored into the price for the Solventum products to achieve compensation for 3M in every month of a low single-digit percentage of net sales (as calculated pursuant to the transition distribution services agreement) in exchange for the transition distribution services. 3M shall remit the compensation received from the sales by 3M and its affiliates of Solventum products to Solventum, other than the agreed compensation to 3M. There is also a true-up procedure in the event that the mark-up factor does not result in the payment of the agreed compensation to 3M. Solventum will bear all costs for obtaining licenses or consents attributable to the activities under the transition distribution services agreement.
Solventum will maintain the risk of loss for all products subject to the transition distribution services agreement, even if 3M has legal title to such products or stores such products in its facilities. Solventum will reimburse 3M for any amounts paid to customers for returned products and will be responsible for costs related to a recall or a similar event.
The transition distribution services agreement will have an overall term of two years following the distribution date, with shorter terms for individual countries. Either party will be able to terminate the transition distribution services agreement for uncured material breaches and Solventum (or, in some cases, 3M) will be able to generally terminate services with respect to an individual country for convenience. Additionally, 3M will be permitted to terminate the transition distribution services agreement if Solventum undergoes a change of control.
If, at or prior to the expiration or termination of the transition distribution services agreement, a party is unable to operate independently from the rights or services provided under the transition distribution services agreement
250
due to circumstances not caused by such party’s action or inaction, the parties are required to discuss in good faith commercially reasonable alternatives (up to and including a one-year extension of the transition distribution services agreement beyond the initial term on a country-by-country basis, as needed) to avoid a business disruption for the requesting party. A request for such one-year extension may not be unreasonably withheld. No changes to the fee structure for an extension term are specified in the transition distribution services agreement.
Transition Contract Manufacturing Agreement
Solventum and 3M will enter into a transition contract manufacturing agreement in connection with the separation pursuant to which each of 3M and Solventum, as suppliers, will manufacture certain products of the other party at specified manufacturing sites and will supply such products to the other party, in each case on a transitional basis to allow for the orderly exit of production at the supplier site and relocation as determined by recipient.
As compensation for contract manufacturing services, the recipient will be required to pay the supplier a specified price per product. Prices under the transition contract manufacturing agreement will be determined on a per product basis taking into account certain matters agreed between the parties, with discretionary adjustments permitted to be made by the supplier from time to time.
The supplier will be required to manufacture each product in accordance with its applicable specification. The contract manufacturing services must be provided in accordance with specified service levels and applicable quality agreements. If service standards are not specified, the supplier must use at least the same degree of care, skill, quality and manner of performance as used by the supplier in providing substantially similar services to its own internal organization at the time the contract manufacturing services are performed.
The transition contract manufacturing agreement will have an overall term of three years after the distribution date, with the terms of individual services ranging from eighteen to thirty-six months. Individual service durations may be extended by the relevant schedules to the transition contract manufacturing agreement or by mutual written consent, but no service may be extended beyond the three-year term of the transition contract manufacturing agreement. Either party will be permitted to terminate the transition contract manufacturing agreement, in whole or in part, for a material uncured breach by the other party. Additionally, 3M may terminate the transition contract manufacturing agreement if Solventum undergoes a change of control.
If, at or prior to the expiration or termination of the transition contract manufacturing agreement, a party is unable to operate independently from the rights or services provided under the transition contract manufacturing agreement, the parties are required to discuss in good faith commercially reasonable alternatives (up to and including an extension of the transition contract manufacturing agreement) to avoid a business disruption for the requesting party; and the other party may not unreasonably withhold consent to a request for a reasonable extension of the transition contract manufacturing agreement for a period of time no longer than reasonably necessary to allow the requesting party to operate independently. The parties are required to discuss in good faith the applicable financial terms of such extension to ensure that the terms are commercially reasonable.
The supplier may, without liability, suspend manufacturing services and/or the supply of products containing or manufactured with the aid of PFAS at any time. The supplier may also substitute any products containing PFAS with reformulated products to remove the use of PFAS.
Research and Development Master Services Agreements
In connection with the separation, Solventum and 3M will enter into two research and development master services agreements pursuant to which each of the parties will provide the other with certain research and development services. The services to be provided by 3M to Solventum will include, without limitation, certain analytical, design, development, consulting, research and development line, pilot line and prototype services. The services to be provided by Solventum to 3M will include, without limitation, certain analytical, consulting, research and development line, pilot line, testing and adhesive development services. The specified services, required service levels and pricing for such services will be set forth in mutually agreed statements of work (SOW).
251
The research and development master services agreements will each have an overall term of three years after the distribution date, with the option to renew for one year upon mutual agreement. Additionally, the term may be automatically extended to the extent the parties agree that existing SOWs need to extend past three years. The receiving party may terminate any services by giving thirty days’ prior written notice (or such longer period provided in an SOW) to the provider of such services. Additionally, either party may terminate the research and development master services agreements, in whole or in part, under certain circumstances, including material uncured breach by the other party.
Real Estate License Agreements
In connection with the separation, Solventum or one of its affiliates, as licensee, will enter into certain real estate license agreements with 3M or one of its affiliates, as licensor, pursuant to which the Solventum group will be able to continue to use certain premises owned or leased by 3M (or its applicable affiliate) for a limited period of time following the distribution date. Pursuant to the real estate license agreements, the licensor will provide customary building services to the licensee consistent with the property’s use prior to the separation, including, without limitation, basic utilities, janitorial and trash removal services, maintenance services and employee amenities. The licensee at each site will pay to the licensor a monthly, fixed license fee (inclusive of all utilities and building service costs), which fee will generally be determined according to licensee’s anticipated prorated portion of annual occupancy costs (including utilities and services).
The real estate license agreements will contain customary confidentiality and non-disclosure provisions to mitigate potential security and intellectual property risks with the shared usage of space.
The terms of the real estate license agreements will generally be two years or less. The duration of specific real estate license agreements will vary by location and may be dictated by availability of alternate locations, lead time to build out new space and complexity of relocation.
The licensee will be required to carry liability insurance, and the parties to the real estate license agreements will be subject to customary mutual indemnification obligations.
Intellectual Property Cross License Agreement
In connection with the separation, certain members of the 3M and Solventum groups will enter into an intellectual property cross license agreement pursuant to which each group will grant to the other group the following licenses: (a) an irrevocable, worldwide, royalty-free, exclusive, sublicenseable, and fully transferrable license to patents and know-how in the other group’s field to make, have made, have, use, sell, offer for sale, import, or otherwise transfer products and services; (b) an irrevocable, royalty-free, full paid-up, global, sole and non-sublicenseable license to patents and know-how in the joint field to make, have made, have, use, sell, offer for sale, import, or otherwise transfer products and services; (c) an irrevocable, worldwide, royalty-free, non-exclusive, sublicenseable, and fully transferrable license to patents and know-how in the open field to make, have made, have, use, sell, offer for sale, import, or otherwise transfer products and services; (d) an irrevocable, worldwide, royalty-free, exclusive, and non-sublicenseable, license to trade secrets used as of the time of separation or at the time of exit under the transition contract manufacturing agreement in the other group’s field to make, have, use, sell, offer for sale, import, or otherwise transfer products and services; (e) an irrevocable, royalty-free, full paid-up, global, sole and non-sublicenseable license to trade secrets used as of the time of separation or at the time of exit under the transition contract manufacturing agreement in the joint field to make, have, use, sell, offer for sale, import, or otherwise transfer products and services; and (f) an irrevocable, worldwide, royalty-free, non-exclusive, and non-sublicenseable, license to trade secrets used as of the time of separation or at the time of exit under the transition contract manufacturing agreement in the open field to make, have, use, sell, offer for sale, import, or otherwise transfer products and services. For purposes of the foregoing licenses, a “group’s field” is the Health Care Business (in the case of the Solventum group) and the 3M Business (in the case of the 3M group). The “joint field” activities that the 3M Business and Health Care Business are jointly or individually engaged in at the time of separation. The “open field” includes activities that neither group was engaged in at the time of separation.
For confidential manufacturing information (CMI) related to certain products that the parties are supplying each other under a supply agreement (“Company Specialty Products” and “SpinCo Specialty Products”, as applicable),
252
3M will grant to Solventum: (g) worldwide, royalty-free, fully-paid up, exclusive, non-sublicenseable license to CMI in Solventum’s field to have, use, import, offer to sell, or otherwise transfer the Company Specialty Products; (h) a ten (10) year option for worldwide, royalty-free, fully-paid up, exclusive, non-sublicenseable license to CMI in Solventum’s field to make Company Specialty Products upon its showing of a capital allocation plan, and (i) a worldwide, royalty-free, fully-paid up, exclusive, non-sublicenseable license to CMI in Solventum’s field to have made Company Specialty Products by a qualified supplier (as specified in the intellectual property cross license agreement). Additionally, each group will grant to the other group the following licenses in the joint field: (j) a worldwide, royalty free, fully-paid up, sole non-sublicensable license to the CMI in the other group’s field to have, use, sell, offer for sale, import, or otherwise transfer Company Specialty Products or SpinCo Specialty Products, as applicable; (k) a ten (10) year option for worldwide, royalty-free, fully-paid up, sole, non-sublicenseable license to CMI in the other group’s field to make Company Specialty Products or SpinCo Specialty Products, as applicable, upon its showing of a capital allocation plan, and (l) a worldwide, royalty-free, fully-paid up, sole, non-sublicenseable license to CMI in Solventum’s field to have made Company Specialty Products by a qualified supplier (as specified in the intellectual property cross license agreement) , where the supplied products for sections (j), (k), and (l) herein are for use in bandages, wraps, tapes, and dressings in the consumer health care channel and in animal care.
The licenses recited in sections (a) through (l) inclusive are transferable to a qualified purchaser (as specified in the intellectual property cross license agreement).
A qualified supplier, as defined in the intellectual property cross license agreement, is a third party supplier that (a) has their business headquarters in, and will manufacture Company Specialty Products or SpinCo Specialty Products, as applicable, only in, the United States, Canada, Australia, the United Kingdom, Japan or European Union, (b) is not a competitor of the licensor, (c) agrees to the application of Delaware law, jurisdiction, and venue, and (d) has established and ongoing operations in the relevant manufacturing field, including having protection standards for confidential information.
The licensed patents will include patents issued on patent applications filed within 24 months of the separation, if such patent application was based on an invention submission submitted prior to the separation.
The term of the agreement expires on the last-to-expire of the intellectual property rights licensed under the agreement. Specifically, the license of the patents and copyrights will expire upon the last to expire of the licensed patents and copyrights, and the license of the know-how and the trade secrets will be perpetual. There are no additional termination provisions of the agreement.
The licensee will have the right to bring enforcement actions with the reasonable assistance of the licensor if such licensee has an exclusive license.
3M Mark Use Agreement
In connection with the separation, certain members of the 3M group will enter into a 3M mark use agreement with certain members of the Solventum group pursuant to which the 3M group will grant a worldwide, fee-based, non-exclusive license to the Solventum group to use the 3M mark for legal entity names, and certain internal and external technology-related uses and systems including, for example, public email addresses of employees. The license may not be sublicensed, except to affiliates of Solventum, and may not be assigned. The 3M mark use agreement will prohibit the Solventum group from using the 3M mark standing alone or as a singular element. The license will also be limited to the territories where the Health Care Business was using the 3M mark immediately prior to the distribution date.
The term of the license will vary from two to five years, with extensions available for up to an additional five years, depending on the context in which the 3M mark is used. Solventum will be required to reimburse 3M for costs associated with monitoring the quality of the uses of the 3M mark, which costs may not exceed $50,000 annually. There are no other fees payable for the use of the 3M mark by Solventum under this agreement.
The 3M mark use agreement may be terminated (a) by either party due to a material uncured breach by the other party, if the license grant is no longer permitted by law, or due to insolvency of the other party, (b) by Solventum for
253
convenience upon notice of documented cessation of use of the 3M mark, or (c) by 3M upon a material breach by Solventum that 3M determines has or will materially harm the value of the 3M mark.
Solventum will agree to use the licensed trademark in compliance with certain standards provided by 3M. 3M will retain the sole right to file, prosecute until registration, maintain and renew the 3M mark. 3M will also retain the sole right to defend, enforce and litigate the 3M mark and control any related legal proceeding.
Transitional Trademark Cross License Agreement
In connection with the separation, certain members of the 3M and Solventum groups will enter into a transitional trademark cross license agreement pursuant to which each group will grant to the other group a worldwide, royalty-free, fee-based, non-exclusive license to use certain trademarks and 3M will grant to Solventum a worldwide, royalty-free, exclusive license to use Scotchbond and Scotchcast trademarks solely in connection with the manufacturing, marketing, distribution, importation, exportation, packaging, display, promotion, delivery, performance, and sale of specified products or in connection with their business during the term of the license. The licenses may not be sublicensed or assigned, subject to specified exceptions. The specified products covered by the license are limited to products in inventory as of the distribution date or manufactured thereafter in accordance with quality standards and sold in a manner substantially consistent with past practice, except that for the Scotchbond and Scotchcast trademarks, products sold before the distribution date and bona fide natural evolutions thereof developed before the distribution date as scheduled in such agreement are licensed.
The term of the licenses will vary from two to ten years depending on the licensed trademark and the context in which the licensed trademark is used. The license to Scotchbond and Scotchcast trademarks may be extended upon agreement between 3M and Solventum after the first 10-year term with payment of a reasonable royalty (to be agreed at the time of renewal) by Solventum. Solventum will be required to pay 3M an annual fee in the amount of $250,000 for costs associated with the quality monitoring of Solventum’s use of the 3M family of marks, which fee reflects an offset for Solventum’s costs associated with the quality monitoring of 3M’s use of the licensed Solventum trademarks. Solventum will reimburse 3M for expenses incurred for registration and maintenance of Scotchbond, and Scotchcast trademarks. There are no other fees payable for the use of the licensed trademarks by the licensee under this agreement.
The transitional trademark cross license agreement may be terminated by either party due to a material uncured breach by the other party, if the license grant is no longer permitted by law, or due to insolvency of the other party.
The licensee will agree to use the licensed trademarks in compliance with certain brand guidelines. The licensor will retain the sole right to file, prosecute until registration, maintain and renew the licensed trademarks. 3M is required to use commercially reasonable efforts to file, prosecute until registration, maintain and renew all Scotchbond and Scotchcast trademarks. The licensor will also retain the sole right to defend, enforce and litigate the licensed trademarks and control any related legal proceeding except for Scotchbond and Scotchcast trademarks which Solventum will have the right to enforce, but shall indemnify 3M for any liabilities or payments as a result of the enforcement.
Master Supply Agreements
3M and Solventum will enter into two master supply agreements in connection with the separation under which each will agree to supply the other with certain products in accordance with applicable specifications for the term of the applicable master supply agreement. The initial pricing for the products will be set forth in the applicable master supply agreement. During the first three years, the supplier may change the price of any product to fully reflect any changes in production costs in order to maintain the supplier’s gross margin rate in effect as of the distribution date, provided that such price changes may only occur on an annual basis, or by giving prior thirty days’ written notice if certain production costs have increased above specified amounts. Beginning on the third anniversary of the distribution date, the supplier may change the price of any product at any time by giving prior thirty days’ written notice, provided that any such change would not increase the supplier’s gross margin rate to more than a specified rate during the term of the agreement (approximately 15 to 20 percentage points above the supplier’s gross margin rate as of the distribution date).
254
Under the terms of each master supply agreement, the purchaser will be required to furnish a forecast on a 36-month rolling basis to the supplier, and the supplier will be subject to certain obligations to prioritize supplying the purchaser and to maintain adequate inventory to meet the lead time for products. Certain products will be supplied to the purchaser on an exclusive basis in designated territories.
The term of the master supply agreements will initially be three years following the distribution date. The term will automatically be extended as described in more detail below. For any product for which either party identifies a third-party supplier, and such third-party supplier provides validated production samples (as defined in the master supply agreements), the extension term will end either five years or seven years following the delivery of the validated production samples, depending on the product and on the party that identified such third-party supplier. For products where neither party identifies a third-party supplier able to produce validated production samples, the agreement may not extend beyond an outside date of either ten years or twelve years following the distribution date, depending on the product.
The master supply agreements may be terminated earlier upon the occurrence of certain specified events (including bankruptcy, material uncured default, failure to implement anti-bribery compliance requirements, and delinquent payments exceeding ten percent of the yearly purchase volume).
Stockholder’s and Registration Rights Agreement
Solventum will enter into a stockholder and registration rights agreement with 3M pursuant to which it will agree that, upon the request of 3M, Solventum will use its reasonable best efforts to effect the registration under applicable federal and state securities laws of any shares of Solventum common stock retained by 3M. In addition, 3M will agree to vote any shares of Solventum common stock that it retains immediately after the separation in proportion to the votes cast by Solventum’s other shareholders. In connection with such agreement, 3M will grant Solventum a proxy to vote its shares of Solventum common stock in such proportion. This proxy, however, will be automatically revoked as to any particular share upon any sale or transfer of such share from 3M to a person other than 3M, and neither the voting agreement nor proxy will limit or prohibit any such sale or transfer.
Other Agreements
Global Regulatory Agreement. Solventum and 3M will enter into a global regulatory agreement in connection with the separation pursuant to which 3M will transfer to Solventum with respect to Solventum products, and Solventum will transfer to 3M with respect to 3M products, marketing authorizations on a country-by-country basis. The global regulatory agreement will define the regulatory responsibilities applicable to 3M, Solventum and their respective affiliates while the transfers of such marketing authorizations are ongoing.
Local Arrangements. In order to comply with applicable local law requirements, members of the Solventum and 3M groups will enter into local agreements in certain jurisdictions with respect to transitional contract manufacturing, transitional distribution arrangements and transitional supply arrangements. These local agreements will contain provisions that are substantially similar to those of the transition contract manufacturing agreement and transition distribution services agreement described above.
In connection with 3M’s retention of certain portions of the Health Care Business in India and Nepal, members of the Solventum and 3M groups will enter into agreements pursuant to which, among other things, the 3M group will be the exclusive distributor for Solventum in India and Nepal for specified products and will be granted an exclusive license for the manufacture and sale of certain Solventum products in India and Nepal.
255
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
The following is a discussion of the material U.S. federal income tax consequences of the distribution to “U.S. holders” (as defined below) of 3M common stock. This discussion is based on the Code, U.S. Treasury Regulations promulgated thereunder, rulings and other administrative pronouncements issued by the IRS, and judicial decisions, in each case as in effect and available on the date of this information statement and all of which are subject to differing interpretations and change at any time, possibly with retroactive effect. Any such change could affect the accuracy of the statements and conclusions set forth in this document.
This discussion applies only to U.S. holders of shares of 3M common stock who hold such shares as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion is based upon the assumption that the separation and the distribution, together with certain related transactions, will be consummated in accordance with the separation and distribution agreement and the other agreements related to the separation and as described in this information statement. This discussion does not address all aspects of U.S. federal income taxation that may be relevant to particular holders of 3M common stock in light of their particular circumstances nor does it address tax considerations applicable to holders that are or may be subject to special treatment under the U.S. federal income tax laws, including, without limitation:
•broker-dealers;
•tax-exempt organizations;
•financial institutions, mutual funds, regulated investment companies or insurance companies;
•certain former U.S. citizens or long-term residents of the United States;
•partnerships (or entities or arrangements treated as partnerships for U.S. federal income tax purposes) or other pass-through entities or the owners thereof;
•traders in securities who elect a mark-to-market method of accounting;
•holders who acquired 3M common stock or Solventum common stock upon the exercise of employee stock options or otherwise as compensation;
•holders who hold their 3M common stock as part of a “hedge,” “straddle,” “conversion,” “synthetic security,” “integrated investment,” or “constructive sale transaction”;
•holders required to accelerate the recognition of any item of gross income as a result of such income being recognized on an applicable financial statement; or
•holders whose functional currency is not the U.S. Dollar.
This discussion also does not address any tax consequences arising under any alternative minimum tax, the unearned Medicare contribution tax pursuant to the Health Care and Education Reconciliation Act of 2010 or the Foreign Account Tax Compliance Act (including the Treasury Regulations promulgated thereunder and intergovernmental agreements entered into pursuant thereto or in connection therewith). In addition, no information is provided with respect to any tax considerations under state, local or non-U.S. laws or U.S. federal laws other than those pertaining to the U.S. federal income tax. This discussion does not address the tax consequences to any person who actually or constructively owns 5 percent or more of 3M common stock.
If a partnership (or any other entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds 3M common stock, the tax treatment of a partner in such partnership will generally depend upon the status of the partner and the activities of the partnership. Holders of 3M common stock that are partnerships and partners in such partnerships should consult their own tax advisors as to the consequences of the distribution.
256
For purposes of this discussion, a “U.S. holder” is a beneficial owner of 3M common stock that is, for U.S. federal income tax purposes:
•an individual citizen or resident of the United States;
•a corporation (or any other entity treated as a corporation) created or organized in or under the laws of the United States, any state thereof or the District of Columbia;
•an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or
•a trust, if (1) a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all of the substantial decisions of such trust or (2) it has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person.
THE FOLLOWING DISCUSSION IS A SUMMARY OF MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE DISTRIBUTION UNDER CURRENT LAW. ALL HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE PARTICULAR TAX CONSEQUENCES OF THE DISTRIBUTION TO THEM, INCLUDING THE APPLICATION AND EFFECT OF U.S. FEDERAL, STATE, LOCAL, NON-U.S. AND OTHER TAX LAWS, IN LIGHT OF THEIR PARTICULAR CIRCUMSTANCES.
It is a condition to the distribution (which condition 3M may waive in its sole discretion) that (1) the IRS Ruling regarding U.S. Tax-Free Status continues to be valid and (2) 3M receives the Tax Opinion(s) regarding U.S. Tax-Free Status. The IRS Ruling and any Tax Opinion will be based upon and rely on, among other things, various facts and assumptions, as well as certain representations, statements and undertakings of 3M and Solventum, including those relating to the past and future conduct of 3M and Solventum. If any of these representations, statements or undertakings are, or become, inaccurate or incomplete, or if any representations or covenants contained in any of the separation-related agreements and documents or in any documents relating to any ruling and/or opinion of a tax advisor are inaccurate or not complied with by 3M, Solventum, or any of their respective subsidiaries, such ruling and/or opinion may be invalid and the conclusions reached therein could be jeopardized.
Notwithstanding 3M’s receipt of the IRS Ruling and any Tax Opinion, in each case, regarding U.S. Tax-Free Status, the IRS could determine that the distribution and/or certain related transactions should be treated as taxable transactions for U.S. federal income tax purposes if it determines that any of the representations, assumptions, or undertakings upon which the IRS Ruling or such Tax Opinion was based are inaccurate or have not been complied with. In addition, the IRS Ruling does not address all of the issues that are relevant to determining whether the distribution, together with certain related transactions, qualifies as a transaction that is generally tax-free for U.S. federal income tax purposes, and any Tax Opinion will represent the judgment of such advisor and will not be binding on the IRS or any court and the IRS or a court may disagree with the conclusions in any Tax Opinion. Accordingly, notwithstanding 3M’s receipt of the IRS Ruling and the Tax Opinion(s), in each case, regarding U.S. Tax-Free Status, there can be no assurance that the IRS will not assert that the distribution and/or certain related transactions do not qualify for tax-free treatment for U.S. federal income tax purposes, or that a court would not sustain such a challenge. In the event the IRS were to prevail with such challenge, 3M, Solventum, and 3M shareholders could be subject to significant U.S. federal income tax liability. Please refer to “—Material U.S. Federal Income Tax Consequences if the Distribution Is Taxable.”
Material U.S. Federal Income Tax Consequences if the Distribution, Together with Certain Related Transactions, Qualifies as a Transaction That is Generally Tax-Free Under Sections 355 and 368(a)(1)(D) of the Code.
If the distribution, together with certain related transactions, qualifies as a transaction that is generally tax-free for U.S. federal income tax purposes under Sections 355 and 368(a)(1)(D) of the Code, the U.S. federal income tax consequences of the distribution are as follows:
•no gain or loss will be recognized by, and no amount will be includible in the income of 3M as a result of the distribution, other than gain or income arising in connection with certain internal restructurings
257
undertaken in connection with the separation and distribution (including with respect to any portion of the borrowing proceeds transferred to 3M from Solventum that is not used for qualifying purposes) and with respect to any “excess loss account” or “intercompany transaction” required to be taken into account by 3M under Treasury Regulations relating to consolidated federal income tax returns;
•no gain or loss will be recognized by (and no amount will be included in the income of) U.S. holders of 3M common stock upon the receipt of Solventum common stock in the distribution for U.S. federal income tax purposes, except with respect to any cash received in lieu of fractional shares of Solventum common stock (as described below);
•the aggregate tax basis of the 3M common stock and the Solventum common stock received in the distribution (including any fractional share interest in Solventum common stock for which cash is received) in the hands of each U.S. holder of 3M common stock immediately after the distribution will equal the aggregate basis of 3M common stock held by the U.S. holder immediately before the distribution, allocated between the 3M common stock and the Solventum common stock (including any fractional share interest in Solventum common stock for which cash is received) in proportion to the relative fair market value of each on the date of the distribution; and
•the holding period of the Solventum common stock received by each U.S. holder of 3M common stock in the distribution (including any fractional share interest in Solventum common stock for which cash is received) will generally include the holding period at the time of the distribution for the 3M common stock with respect to which the distribution is made.
A U.S. holder who receives cash in lieu of a fractional share of Solventum common stock in the distribution will be treated as having sold such fractional share for cash, and will recognize capital gain or loss in an amount equal to the difference between the amount of cash received and such U.S. holder’s adjusted tax basis in such fractional share. Such gain or loss will be long-term capital gain or loss if the U.S. holder’s holding period for its 3M common stock exceeds one year at the time of the distribution.
If a U.S. holder of 3M common stock holds different blocks of 3M common stock (generally shares of 3M common stock purchased or acquired on different dates or at different prices), such holder should consult its tax advisor regarding the determination of the basis and holding period of shares of Solventum common stock received in the distribution in respect of particular blocks of 3M common stock.
Material U.S. Federal Income Tax Consequences if the Distribution Is Taxable.
As discussed above, notwithstanding receipt by 3M of the IRS Ruling and the Tax Opinion(s), in each case, regarding U.S. Tax-Free Status, the IRS could assert that the distribution does not qualify for tax-free treatment for U.S. federal income tax purposes. If the IRS were successful in taking this position, some or all of the consequences described above would not apply, and 3M, Solventum and 3M shareholders could be subject to significant U.S. federal income tax liability. In addition, certain events that may or may not be within the control of 3M or Solventum could cause the distribution and certain related transactions to not qualify for tax-free treatment for U.S. federal income tax purposes. Depending on these circumstances, Solventum may be required to indemnify 3M for taxes (and certain related losses) resulting from the distribution and certain related transactions not qualifying as tax-free.
If the distribution were to fail to qualify as a transaction that is generally tax-free for U.S. federal income tax purposes under Sections 355 and 368(a)(1)(D) of the Code, in general, for U.S. federal income tax purposes, 3M would recognize taxable gain as if it had sold the Solventum common stock in a taxable sale for its fair market value (unless 3M and Solventum jointly make an election under Section 336(e) of the Code with respect to the distribution, in which case, in general, (1) the 3M group would recognize taxable gain as if Solventum had sold all of its assets in a taxable sale in exchange for an amount equal to the fair market value of the Solventum common stock and the assumption of all of Solventum’s liabilities and (2) Solventum would obtain a related step up in the basis of its assets), and 3M shareholders who receive Solventum common stock in the distribution would be subject to tax as if they had received a taxable distribution equal to the fair market value of such shares.
258
Even if the distribution were to otherwise qualify as a tax-free transaction under Sections 355 and 368(a)(1)(D) of the Code, it may result in taxable gain to 3M (but not its shareholders) under Section 355(e) of the Code if the distribution were deemed to be part of a plan (or series of related transactions) pursuant to which one or more persons acquire, directly or indirectly, shares representing a 50% or greater interest (by vote or value) in 3M or Solventum. For this purpose, any acquisitions of 3M or Solventum shares within the period beginning two years before the distribution and ending two years after the distribution are presumed to be part of such a plan, although 3M or Solventum may be able to rebut that presumption depending on the circumstances.
In connection with the distribution, Solventum and 3M will enter into a tax matters agreement pursuant to which Solventum will be responsible for certain liabilities and obligations following the distribution. In general, under the terms of the tax matters agreement, if the distribution, together with certain related transactions, were to fail to qualify as a transaction that is generally tax-free for U.S. federal income tax purposes under Sections 355 and 368(a)(1)(D) of the Code (including as a result of Section 355(e) of the Code) or if certain related transactions were to fail to qualify as tax-free under applicable law, and if such failure were the result of actions taken after the distribution by 3M or Solventum, then the party responsible for such failure will be responsible for all taxes imposed on 3M or Solventum to the extent such taxes result from such actions. However, if such failure was the result of any acquisition of Solventum shares, or of certain of Solventum’s representations, statements or undertakings being incorrect, incomplete or breached, then Solventum generally will be responsible for all taxes imposed a result of such acquisition or breach. For a discussion of the tax matters agreement, see “Certain Relationships and Related Party Transactions—Agreements with 3M—Tax Matters Agreement.” Solventum’s indemnification obligations to 3M under the tax matters agreement are not expected to be limited in amount or subject to any cap. If Solventum is required to pay any taxes or indemnify 3M and its subsidiaries and officers and directors under the circumstances set forth in the tax matters agreement, Solventum may be subject to substantial liabilities.
Backup Withholding and Information Reporting
Payments of cash to U.S. holders of 3M common stock in lieu of fractional shares of Solventum common stock may be subject to information reporting and backup withholding (currently at a rate of 24%), unless such U.S. holder delivers a properly completed IRS Form W-9 certifying such U.S. holder’s correct taxpayer identification number and certain other information, or otherwise establishes an exemption from backup withholding. Backup withholding is not an additional tax. Amounts withheld under the backup withholding rules may be refunded or credited against a U.S. holder’s U.S. federal income tax liability, provided that the required information is timely furnished to the IRS.
THE FOREGOING DISCUSSION IS A SUMMARY OF MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE DISTRIBUTION UNDER CURRENT LAW. ALL HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE PARTICULAR TAX CONSEQUENCES OF THE DISTRIBUTION TO THEM, INCLUDING THE APPLICATION AND EFFECT OF U.S. FEDERAL, STATE, LOCAL, NON-U.S., AND OTHER TAX LAWS, IN LIGHT OF THEIR PARTICULAR CIRCUMSTANCES.
259
DESCRIPTION OF MATERIAL INDEBTEDNESS
The material agreements described below are filed as exhibits to the registration statement on Form 10 of which this information statement is a part and are incorporated by reference into this information statement. The summaries of each of these agreements set forth below are qualified in their entireties by reference to the full text of the applicable agreements.
General
The proceeds of the Term Facilities and the Notes described below are expected to be used to make cash payments to 3M, other than such amounts as need to be retained in order to cause Solventum to have $600 million of cash in the aggregate at the time of the distribution. The cash payments to 3M from the proceeds of the financings are expected to total approximately $7.7 billion, after taking into account fees, discounts and expenses, as well as the retention of $600 million of cash and cash equivalents by Solventum. As a result of these transactions, Solventum anticipates having approximately $8.4 billion of outstanding indebtedness upon completion of the distribution. Solventum does not currently expect to incur any borrowings under the 5-Year Revolving Credit Facility (described below) prior to or at the time of the distribution.
Credit Facilities
On February 16, 2024, Solventum entered into (i) a five-year senior unsecured revolving credit facility (the “5-Year Revolving Credit Facility”) in an aggregate committed amount of $2.0 billion, (ii) an 18-month senior unsecured term loan facility (the “18-Month Term Facility”) in an aggregate committed amount of $500 million and (iii) a three-year senior unsecured term loan facility (the “3-Year Term Facility” and, together with the 18-Month Term Facility, the “Term Facilities”; the Term Facilities, together with the 5-year Revolving Credit Facility, the “Credit Facilities”) in an aggregate committed amount of $1.0 billion, in each case, with the respective syndicates of lenders named therein. JPMorgan Chase Bank, N.A. is the administrative agent under the 5-Year Revolving Credit Facility and Bank of America, N.A., is the administrative agent under the Term Facilities.
Borrowings under the 5-Year Revolving Credit Facility will be available in Euros and U.S. Dollars.
The obligations of Solventum under each Credit Facility will be guaranteed on a senior unsecured basis by 3M; provided, 3M’s guarantee shall automatically, irrevocably and unconditionally terminate, without any further action from the applicable administrative agent, banks or any other person, (i) upon the occurrence of the consummation of the distribution or (ii) otherwise in accordance with the provisions of the Credit Facilities.
Maturity
The 5-Year Revolving Credit Facility will mature five years after the effective date of the Credit Facilities. The 18-Month Term Facility will mature on the date that is eighteen months following the funding thereof. The 3-Year Term Facility will mature on the date that is three years after the funding thereof.
There will be no amortization with respect to the borrowings under any of the 5-Year Revolving Credit Facility, the 18-Month Term Facility and the 3-Year Term Facility.
Interest Rate and Fees
The interest rate applicable to loans under our Credit Facilities is (x) with respect to borrowings in U.S. dollars, at our option, equal to either a term SOFR rate for a one-, three- or six-month interest period plus 0.10% or an alternate base rate and (y) with respect to borrowings in Euro under the 5-Year Revolving Credit Facility, the EURIBO rate for a one-, three- or six-month interest period, in each case, plus in each case an applicable margin. The applicable margin payable on borrowings will be determined by reference to a pricing schedule based on our long-term senior unsecured non-credit-enhanced debt ratings. In addition, we will pay customary commitment fees based on the unused portion of the respective commitments of the lenders under the 5-Year Revolving Credit Facility and customary ticking fees based on the undrawn portion of the commitments in respect of the applicable Term Facility from and including the date which is 90 days after the effective date of each such Term Facility.
260
Prepayments
We may voluntarily prepay borrowings under the Credit Facilities without premium or penalty, subject to customary “breakage” costs with respect to loans bearing interest by reference to a term SOFR rate or the EURIBO rate, as applicable. We may also reduce or terminate the commitments under any of the Credit Facilities, in whole or in part, in each case, subject to certain minimum amounts.
All term loans under the Term Facilities shall be required to be prepaid in full (or, if applicable, commitments related to the Term Facilities shall be terminated) in the event that the Spin-Off shall not have been consummated on or prior to June 28, 2024.
In the event that the Spin-Off shall not have been consummated on or prior to June 28, 2024, all commitments under the 5-Year Revolving Credit Facility shall be automatically reduced to zero and all revolving loans (if any) thereunder shall be required to be prepaid in full, in each case, on such date.
Certain Covenants
The Credit Facilities include various customary covenants that limit, among other things, the incurrence of liens and the entry into certain fundamental change transactions by Solventum. In addition, the Credit Facilities require us to maintain a maximum leverage ratio commencing with the first fiscal quarter ended after the consummation of the distribution.
Events of Default
The Credit Facilities include customary events of default, including with respect to a failure to make timely payments under the Credit Facilities, violation of covenants, inaccuracy of representations and warranties, cross-acceleration and certain bankruptcy and insolvency events.
Notes
On February 27, 2024, Solventum issued $6,900,000,000 in aggregate principal amount of unsecured senior notes in six series: $1,000,000,000 aggregate principal amount of 5.450% senior notes due 2027, $1,500,000,000 aggregate principal amount of 5.400% senior notes due 2029, $1,000,000,000 aggregate principal amount of 5.450% senior notes due 2031, $1,650,000,000 aggregate principal amount of 5.600% senior notes due 2034, $1,250,000,000 aggregate principal amount of 5.900% senior notes due 2054 and $500,000,000 aggregate principal amount of 6.000% senior notes due 2064. The notes were issued pursuant to an indenture, dated as of February 27, 2024, as supplemented by a first supplemental indenture, dated as of February 27, 2024, each by and between Solventum and U.S. Bank Trust Company, National Association, as trustee. The notes were sold in private placements to qualified institutional buyers in accordance with Rule 144A under the Securities Act, and outside the United States to non-U.S. persons in reliance on Regulation S under the Securities Act. The notes are subject to customary affirmative covenants, negative covenants and events of default for financing of this type and are redeemable at Solventum’s option in a customary manner.
In connection with the issuance of these notes, Solventum entered into a registration rights agreement with representatives of the initial purchasers of the notes, pursuant to which Solventum has agreed to use commercially reasonable efforts to file a registration statement with respect to a registered offer to exchange each series of notes for new notes with substantially identical terms in all material respects to such series of notes, to cause such registration statement to be declared effective under the Securities Act on or before February 26, 2025, and to complete the exchange offer within sixty days after such registration statement is declared effective. Under certain circumstances, Solventum has agreed to use commercially reasonable efforts to file and have declared effective a resale shelf registration statement relating to the notes. In the event of certain failures by Solventum to complete these actions on the contemplated timelines with respect to some or all of the notes, the interest rate on the affected notes may be increased by up to 0.50% per annum.
261
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Before the separation and distribution, all of the outstanding shares of Solventum’s common stock will be owned beneficially and of record by 3M. Following the separation and distribution, Solventum expects to have outstanding an aggregate of approximately 172,709,175 shares of common stock based upon approximately 553,360,198 shares of 3M common stock issued and outstanding on March 6, 2024, excluding treasury shares, assuming no exercise of any shares issued under 3M equity compensation awards and applying the distribution ratio. Following the distribution, 3M will retain up to 19.9% of Solventum’s common stock.
Securities Owned by Certain Beneficial Owners
The following table sets forth information concerning those persons known to Solventum that are expected to be the beneficial owner of more than 5% of Solventum’s outstanding common stock immediately following the completion of the distribution. The below table is based on information available as of March 6, 2024 and based upon the assumption that, for every four shares of 3M common stock held by such persons, they will receive one share of Solventum common stock. In general, “beneficial ownership” includes those shares that a person has the sole or shared power to vote or dispose of, including shares that the person has the right to acquire within 60 days.
| Name and Address of Beneficial Owner | Amount and Nature of Beneficial Ownership | Percent of Class | ||||||||||||
| 3M Corporation 3M Center St. Paul, MN 55144 | 34,369,125 | 19.9% | ||||||||||||
The Vanguard Group(1) 100 Vanguard Blvd. Malvern, PA 1935 | 12,270,052 | 7.1% | ||||||||||||
BlackRock, Inc.(2) 55 East 52nd Street New York, NY 1005 | 10,466,752 | 6.1% | ||||||||||||
State Street Corporation(3) State Street Financial Center One Lincoln Street Boston, MA 0211 | 8,959,974 | 5.2% | ||||||||||||
__________________
(1)Based on the Schedule 13G/A filed with the SEC on February 13, 2024 by the Vanguard Group (“Vanguard”) with respect to 3M common stock. Vanguard reported that, as of December 29, 2023, it had shared voting power with respect to 683,607 shares of 3M common stock, sole dispositive power with respect to 46,729,293 shares of 3M common stock, and shared dispositive power with respect to 2,350,916 shares of 3M common stock, and that it beneficially owned in the aggregate 49,080,209 shares of 3M common stock.
(2)Based on the Schedule 13G/A filed with the SEC on January 26, 2024 by BlackRock, Inc. (“BlackRock”) with respect to 3M common stock. BlackRock reported that, as of December 31, 2023, it had sole voting power with respect to 37,610,493 shares of 3M common stock and sole dispositive power with respect to 41,867,008 shares of 3M common stock, and that it beneficially owned in the aggregate 41,867,008 shares of 3M common stock.
(3)Based on the Schedule 13G/A filed with the SEC on January 29, 2024 by State Street Corporation (“State Street”) with respect to 3M common stock. State Street reported that, as of December 31, 2023, it had shared voting power with respect to 20,586,992 shares of 3M common stock and shared dispositive power with respect to 35,820,369 shares of 3M common stock, and that it beneficially owned in the aggregate 35,839,898 shares of 3M common stock.
Stock Ownership of Directors and Executive Officers
The following table sets forth information concerning the expected beneficial ownership of Solventum common stock immediately following the completion of the distribution by each person expected to be a director or executive officer of Solventum as of such time and all such Solventum directors and executive officers as a group, based on information available as of March 6, 2024 and based on the assumption that, for every four shares of 3M common stock held by such persons, they will receive one share of Solventum common stock. Each person has the sole power to vote and dispose of the shares he or she beneficially owns. None of these individuals, or the group as a whole,
262
would be expected to beneficially own more than 1% of Solventum’s common stock immediately following the completion of the distribution.
| Name | Beneficial Ownership | Percent of Class | ||||||||||||
| Carlos Albán | — | — | ||||||||||||
| Carrie S. Cox | — | — | ||||||||||||
| Susan D. DeVore | — | — | ||||||||||||
| Glenn A. Eisenberg | — | — | ||||||||||||
| Bryan Hanson | — | |||||||||||||
| Bernard A. Harris, Jr. | — | — | ||||||||||||
| Marcela Kirberger | — | — | ||||||||||||
| Karen J. May | — | — | ||||||||||||
| Wayde McMillan | 5 | * | ||||||||||||
| Elizabeth A. Mily | 62 | * | ||||||||||||
| John H. Weiland | — | — | ||||||||||||
| Amy A. Wendell | — | — | ||||||||||||
| Darryl L. Wilson | — | — | ||||||||||||
| Directors and Executive Officers as a Group (13 Persons) | 67 | * | ||||||||||||
_________________
* Less than one percent.
263
DESCRIPTION OF SOLVENTUM CAPITAL STOCK
Solventum’s certificate of incorporation and bylaws will be amended and restated prior to the distribution. The following briefly summarizes the material terms of Solventum capital stock that will be contained in its amended and restated certificate of incorporation and amended and restated bylaws. These summaries do not describe every aspect of these securities and documents and are subject to all the provisions of Solventum’s amended and restated certificate of incorporation or amended and restated bylaws that will be in effect at the time of the distribution, and are qualified in their entirety by reference to these documents, which you should read (along with the applicable provisions of Delaware law) for complete information on its capital stock as of the time of the distribution. The amended and restated certificate of incorporation and amended and restated bylaws, each in a form expected to be in effect at the time of the distribution, will be included as exhibits to Solventum’s registration statement on Form 10, of which this information statement forms a part. Solventum will include its amended and restated certificate of incorporation and amended and restated bylaws, as in effect at the time of the distribution, in a current report on Form 8-K filed with the SEC. The following also summarizes certain relevant provisions of the Delaware General Corporation Law, or the DGCL. Since the terms of the DGCL are more detailed than the general information provided below, you should read the actual provisions of the DGCL for complete information.
General
Solventum’s authorized capital stock will consist of 750,000,000 shares of common stock, par value $0.01 per share, and 50,000,000 shares of preferred stock without par value. Solventum’s Board of Directors may establish the rights and preferences of the preferred stock from time to time. Immediately following the distribution, Solventum expects that approximately 172,709,175 shares of its common stock will be issued and outstanding (based on the number of shares of 3M common stock outstanding on March 6, 2024), and that no shares of its preferred stock will be issued and outstanding.
Voting Rights
Each holder of Solventum’s common stock will be entitled to one vote per share on all matters to be voted upon by the shareholders.
Dividends
Subject to preferences that may be applicable to any outstanding preferred stock, the holders of Solventum common stock will be entitled to receive ratably such dividends, if any, as may be declared from time to time by Solventum’s Board of Directors out of funds legally available for that purpose.
Rights Upon Liquidation
In the event of Solventum’s liquidation, dissolution or winding up, the holders of Solventum common stock will be entitled to share ratably in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding.
Preemptive or Conversion Rights
The holders of Solventum common stock will have no preemptive or conversion rights or other subscription rights. There will be no redemption or sinking fund provisions applicable to Solventum common stock.
Preferred Stock
Solventum’s Board of Directors will have the authority, without action by the shareholders, to designate and issue preferred stock in one or more series and to designate the designations, powers, rights, preferences, qualifications, limitations and privileges of each series, which may be greater than the rights of the common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock upon the rights of holders of
264
the common stock until Solventum’s Board of Directors determines the specific rights of the holders of such preferred stock. However, the effects might include, among other things:
•restricting dividends on the common stock;
•diluting the voting power of the common stock;
•impairing the liquidation rights of the common stock; or
•delaying or preventing a change in control of Solventum without further action by the shareholders.
Anti-Takeover Effects of Governance Provisions
Some provisions of Delaware law and Solventum’s amended and restated certificate of incorporation and amended and restated bylaws, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of Solventum to first negotiate with Solventum’s Board of Directors. Solventum believes that these provisions will give its Board of Directors the flexibility to exercise its fiduciary duties in a manner consistent with the interests of its shareholders.
•Classified Board. Solventum’s amended and restated certificate of incorporation will provide that, until the annual stockholder meeting in 2028, Solventum’s Board of Directors will be divided into three classes, with each class consisting, as nearly as reasonably possible, of one-third of the total number of directors. The first term of office for the Class I directors will expire at the 2025 annual meeting of stockholders. The first term of office for the Class II directors will expire at the 2026 annual meeting of stockholders. The first term of office for the Class III directors will expire at the 2027 annual meeting of stockholders. At the 2025 annual meeting of stockholders, the Class I directors will be elected for a term of office to expire at the 2028 annual meeting of stockholders. At the 2026 annual meeting of stockholders, the Class II directors will be elected for a term of office to expire at the 2028 annual meeting of stockholders. At the 2027 annual meeting of stockholders, the Class III directors will be elected for a term of office to expire at the 2028 annual meeting of stockholders. Commencing with the 2028 annual meeting of stockholders, all directors will be elected annually and for a term of office to expire at the next annual meeting of stockholders, and Solventum’s Board of Directors will thereafter no longer be divided into classes. Before Solventum’s Board of Directors is declassified, it would take at least two annual stockholders meetings to occur for any individual or group to gain control of Solventum’s Board of Directors. Accordingly, while the Board of Directors is divided into classes, these provisions could discourage a third-party from initiating a proxy contest, making a tender offer or otherwise attempting to control Solventum.
•Removal and Vacancies. Solventum’s amended and restated certificate of incorporation and bylaws will provide that (i) until the 2028 annual meeting of stockholders (or such other time as Solventum’s Board of Directors is no longer classified under the DGCL), Solventum stockholders may remove directors only for cause and (ii) from and including the 2028 annual meeting of stockholders (or such other time as Solventum’s Board of Directors is no longer classified under the DGCL), Solventum shareholders may remove directors with or without cause. Removal will require the affirmative vote of holders of at least a majority of the voting power of Solventum stock outstanding and entitled to vote on such removal. Vacancies occurring on Solventum’s Board of Directors, whether due to death, resignation, removal, retirement, disqualification or for any other reason, and newly created directorships resulting from an increase in the authorized number of directors, shall be filled solely by a majority of the remaining members of Solventum’s Board of Directors or by a sole remaining director.
•Shareholder Meetings. Under Solventum’s amended and restated bylaws, only (x) Solventum’s Board of Directors or (y) with the concurrence of a majority of Solventum’s Board of Directors, the chairman of Solventum’s Board of Directors, the chief executive officer or the secretary will be allowed to call special meetings of shareholders. Solventum’s shareholders shall not be entitled to call special meetings of shareholders.
265
•Requirements for Advance Notification of Shareholder Nominations and Proposals. Solventum’s amended and restated bylaws will establish advance notice procedures with respect to shareholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of Solventum’s Board of Directors or a committee thereof.
•Delaware Law. Solventum will be subject to Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years following the date the person became an interested stockholder, unless the “business combination” or the transaction in which the person became an interested stockholder is approved in a prescribed manner. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. Generally, an “interested stockholder” is a person who, together with affiliates and associates, owns or within three years prior to the determination of interested stockholder status, did own, 15% or more of a corporation’s voting stock. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by Solventum’s Board of Directors, including discouraging attempts that might result in a premium over the market price for the shares of Solventum common stock held by shareholders.
•Elimination of Shareholder Action by Written Consent. Solventum’s amended and restated certificate of incorporation will eliminate the right of shareholders to act by written consent without a meeting. Stockholder action must therefore take place at an annual meeting or at a special meeting of Solventum’s stockholders.
•No Cumulative Voting. Solventum’s amended and restated certificate of incorporation and amended and restated bylaws will not provide for cumulative voting in the election of directors.
•Undesignated Preferred Stock. The authorization of undesignated preferred stock will make it possible for Solventum’s Board of Directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of Solventum. These and other provisions may have the effect of deferring hostile takeovers or delaying changes in control or management of Solventum.
•Amendments to Bylaws. Solventum’s amended and restated certificate of incorporation and amended and restated bylaws will provide that Solventum’s Board of Directors will have the authority to amend and repeal Solventum’s amended and restated bylaws without a stockholder vote.
•Exclusive Forum. Solventum’s amended and restated certificate of incorporation will provide that, unless Solventum (through approval of Solventum’s Board of Directors) consents in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for (1) any derivative action or proceeding brought on behalf of Solventum, (2) any action or proceeding asserting a claim for or based on a breach of a fiduciary duty owed by any current or former director or officer or other employee of Solventum to Solventum or Solventum’s shareholders, including any claim alleging aiding and abetting of such a breach of fiduciary duty, (3) any action or proceeding asserting a claim against Solventum or any current or former director or officer or other employee of Solventum arising pursuant to, or seeking to enforce any right, obligation or remedy under, any provision of the DGCL or Solventum’s amended and restated certificate of incorporation or amended and restated bylaws (as either may be amended from time to time), (4) any action or proceeding asserting a claim related to or involving Solventum or any current or former director or officer or other employee of Solventum governed by the internal affairs doctrine, or (5) any action or proceeding as to which the DGCL (as it may be amended from time to time) confers jurisdiction on the Court of Chancery of the State of Delaware. If and only if the Court of Chancery of the State of Delaware dismisses any such action or proceeding for lack of subject matter jurisdiction, such action or proceeding may be brought in another state court located within the State of Delaware (or, if no state court located within the State of Delaware has jurisdiction, the federal district court for the District of Delaware). Solventum’s amended and restated certificate of incorporation will further provide that, unless Solventum (through approval of Solventum’s Board of Directors) consents in writing to the selection of an alternative forum, the federal district courts of the United States of America
266
shall be the sole and exclusive forum for any action asserting a cause of action arising under the Securities Act. The exclusive forum provisions will be applicable to the fullest extent permitted by applicable law, subject to certain exceptions. Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As a result, the exclusive forum provisions will not apply to suits brought to enforce any duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. There is, however, uncertainty as to whether a court would enforce the exclusive forum provisions, and investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for state and federal courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Although Solventum believes the exclusive forum provisions benefit it by providing increased consistency in the application of law in the types of lawsuits to which they apply, the provisions may have the effect of discouraging lawsuits against Solventum’s directors and officers.
Limitation on Liability and Indemnification of Directors and Officers
Delaware law authorizes corporations, subject to certain limitations, to limit or eliminate the personal liability of directors and officers to corporations and their shareholders for monetary damages for breaches of directors’ and officers’ fiduciary duties as directors or officers, as applicable. Solventum’s amended and restated certificate of incorporation will include such an exculpation provision limiting or eliminating such liability to fullest extent permitted under Delaware law.
Solventum’s amended and restated certificate of incorporation and amended and restated bylaws will generally require Solventum to provide indemnification and advancement of expenses for its directors and officers to the fullest extent permitted by the DGCL. Prior to the completion of the distribution, Solventum also intends to enter into indemnification agreements with each of its directors and executive officers that may, in some cases, be broader than the specific indemnification and advancement of expenses provisions contained under Delaware law.
Listing
Solventum’s common stock has been approved for listing on the NYSE, subject to official notice of issuance, under the symbol “SOLV.”
Sale of Unregistered Securities
Pursuant to Section 4(a)(2) of the Securities Act, Solventum issued shares of its common stock to 3M in the following amounts: 100 shares on January 24, 2023; 139,567,723 shares on February 16, 2024; and 34,674,153 shares on February 17, 2024. Solventum did not register these issuances of the issued shares under the Securities Act because such issuances did not constitute a public offering.
Transfer Agent and Registrar
After the distribution, the transfer agent and registrar for Solventum common stock will be Equiniti.
Stockholder’s and Registration Rights Agreement
3M will have the right to require Solventum to register shares of common stock for resale in some circumstances. See “Certain Relationships and Related Party Transactions – Agreements with 3M—Stockholder’s and Registration Rights Agreement.”
267
WHERE YOU CAN FIND MORE INFORMATION
Solventum has filed a registration statement on Form 10 with the SEC with respect to the shares of its common stock being distributed as contemplated by this information statement. This information statement is a part of, and does not contain all of the information set forth in, the registration statement and the exhibits and schedules to the registration statement. For further information with respect to Solventum and its common stock, please refer to the registration statement, including its exhibits and schedules. Statements made in this information statement relating to any contract or other document filed as an exhibit to the registration statement are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contract or document. You may review a copy of the registration statement, including its exhibits and schedules, on the Internet website maintained by the SEC at www.sec.gov. Information contained on or connected to any website referenced in this information statement is not incorporated into this information statement or the registration statement of which this information statement forms a part, or in any other filings with, or any information furnished or submitted to, the SEC.
As a result of the distribution, Solventum will become subject to the information and reporting requirements of the Exchange Act and, in accordance with the Exchange Act, will file periodic reports, proxy statements, and other information with the SEC.
Solventum intends to furnish holders of its common stock with annual reports containing consolidated financial statements prepared in accordance with U.S. generally accepted accounting principles and audited and reported on, with an opinion expressed, by an independent registered public accounting firm.
You should rely only on the information contained in this information statement or to which this information statement has referred you. Solventum has not authorized any person to provide you with different information or to make any representation not contained in this information statement.
268
INDEX TO THE FINANCIAL STATEMENTS
| Page | |||||
| Audited Combined Financial Statements | |||||
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of 3M Company
Opinion on the Financial Statements
We have audited the accompanying combined balance sheets of the Health Care Business of 3M Company (the “Company”) as of December 31, 2023 and 2022, and the related combined statements of income, of comprehensive income, of changes in equity and of cash flows for each of the three years in the period ended December 31, 2023, including the related notes (collectively referred to as the “combined financial statements”). In our opinion, the combined financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023 in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These combined financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s combined financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these combined financial statements in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the combined financial statements are free of material misstatement, whether due to error or fraud.
Our audits included performing procedures to assess the risks of material misstatement of the combined financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the combined financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the combined financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the combined financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the combined financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the combined financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Legal Proceedings
As described in Note 11 to the combined financial statements, management records liabilities for legal proceedings in those instances where it can reasonably estimate the amount of the loss and when the loss is probable. Where the reasonable estimate of the probable loss is a range, management records as an accrual in the Company’s financial statements the most likely estimate of the loss, or the low end of the range if there is no one best estimate. Management either discloses the amount of a possible loss or range of loss in excess of established accruals if estimable, or states that such an estimate cannot be made. Management discloses significant legal proceedings even where liability is not probable or the amount of the liability is not estimable, or both, if management believes there is at least a reasonable possibility that a loss may be incurred.
F-2
The principal considerations for our determination that performing procedures relating to legal proceedings is a critical audit matter are (i) the significant judgment by management when determining the likelihood of a loss being incurred and when estimating the loss or range of loss for each claim, and (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s assessment of the liabilities and disclosures related to legal proceedings.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the combined financial statements. These procedures included testing the effectiveness of controls relating to management’s assessment of the liabilities related to legal proceedings, including controls over determining the likelihood of a loss and whether the amount of loss can be reasonably estimated, as well as financial statement disclosures. These procedures also included, among others, obtaining and evaluating the letters of audit inquiry with internal and external legal counsel, evaluating the reasonableness of management’s assessment regarding whether an unfavorable outcome is reasonably possible or probable and reasonably estimable, and evaluating the sufficiency of the Company’s disclosures related to legal proceedings.
| /s/ PricewaterhouseCoopers LLP | ||
| Minneapolis, Minnesota | ||
| February 20, 2024 | ||
We have served as the Company’s auditor since 2022.
F-3
Health Care Business of 3M Company
Combined Statements of Income
| Years ended December 31, | ||||||||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||
| Net sales | ||||||||||||||||||||||||||||||||
| Sales of product | $ | 6,296 | $ | 6,300 | $ | 6,398 | ||||||||||||||||||||||||||
| Sales of software and rentals | 1,901 | 1,830 | 1,773 | |||||||||||||||||||||||||||||
| Total net sales | 8,197 | 8,130 | 8,171 | |||||||||||||||||||||||||||||
| Operating expenses | ||||||||||||||||||||||||||||||||
| Costs of product | 3,023 | 2,953 | 2,773 | |||||||||||||||||||||||||||||
| Costs of software and rentals | 481 | 482 | 475 | |||||||||||||||||||||||||||||
| Selling, general and administrative | 2,243 | 2,235 | 2,278 | |||||||||||||||||||||||||||||
| Research and development | 758 | 767 | 766 | |||||||||||||||||||||||||||||
| Total operating expenses | 6,505 | 6,437 | 6,292 | |||||||||||||||||||||||||||||
| Operating income | 1,692 | 1,693 | 1,879 | |||||||||||||||||||||||||||||
| Other expense (income) – net | 25 | 1 | (3) | |||||||||||||||||||||||||||||
| Income before income taxes | 1,667 | 1,692 | 1,882 | |||||||||||||||||||||||||||||
| Provision for income taxes | 321 | 349 | 422 | |||||||||||||||||||||||||||||
| Net income | $ | 1,346 | $ | 1,343 | $ | 1,460 | ||||||||||||||||||||||||||
The accompanying notes are an integral part of these combined financial statements.
F-4
Health Care Business of 3M Company
Combined Statements of Comprehensive Income
| Years ended December 31, | ||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||
| Net income | $ | 1,346 | $ | 1,343 | $ | 1,460 | ||||||||||||||||||||
| Other comprehensive income (loss) – net of tax: | ||||||||||||||||||||||||||
| Cumulative translation adjustment | 157 | (331) | (280) | |||||||||||||||||||||||
| Defined benefit pension adjustment | (33) | 34 | 10 | |||||||||||||||||||||||
| Total other comprehensive income (loss) – net of tax | 124 | (297) | (270) | |||||||||||||||||||||||
| Comprehensive income | $ | 1,470 | $ | 1,046 | $ | 1,190 | ||||||||||||||||||||
The accompanying notes are an integral part of these combined financial statements.
F-5
Health Care Business of 3M Company
Combined Balance Sheets
| December 31, | ||||||||||||||||||||
| (Millions) | 2023 | 2022 | ||||||||||||||||||
| Assets | ||||||||||||||||||||
| Current assets | ||||||||||||||||||||
| Cash and cash equivalents | $ | 194 | $ | 61 | ||||||||||||||||
| Receivables – net of allowances of $82 and $93 | 1,313 | 1,171 | ||||||||||||||||||
| Inventories | ||||||||||||||||||||
| Finished goods | 453 | 468 | ||||||||||||||||||
| Work in process | 171 | 173 | ||||||||||||||||||
| Raw materials and supplies | 233 | 232 | ||||||||||||||||||
| Total inventories | 857 | 873 | ||||||||||||||||||
| Other current assets | 155 | 126 | ||||||||||||||||||
| Current assets | 2,519 | 2,231 | ||||||||||||||||||
| Property, plant, and equipment – net | 1,457 | 1,319 | ||||||||||||||||||
| Goodwill | 6,535 | 6,434 | ||||||||||||||||||
| Intangible assets – net | 2,902 | 3,252 | ||||||||||||||||||
| Other assets | 530 | 358 | ||||||||||||||||||
| Total assets | $ | 13,943 | $ | 13,594 | ||||||||||||||||
| Liabilities | ||||||||||||||||||||
| Current liabilities | ||||||||||||||||||||
| Accounts payable | $ | 477 | $ | 348 | ||||||||||||||||
| Unearned revenue | 574 | 559 | ||||||||||||||||||
| Other current liabilities | 677 | 404 | ||||||||||||||||||
| Current liabilities | 1,728 | 1,311 | ||||||||||||||||||
| Defined benefit pension | 166 | 91 | ||||||||||||||||||
| Deferred income taxes | 231 | 215 | ||||||||||||||||||
| Other liabilities | 152 | 235 | ||||||||||||||||||
| Total liabilities | 2,277 | 1,852 | ||||||||||||||||||
| Commitments and contingencies (Note 11) | ||||||||||||||||||||
| Equity | ||||||||||||||||||||
| Net parent investment | 12,003 | 12,239 | ||||||||||||||||||
| Accumulated other comprehensive income (loss) – net | (337) | (497) | ||||||||||||||||||
| Total equity | 11,666 | 11,742 | ||||||||||||||||||
| Total liabilities and equity | $ | 13,943 | $ | 13,594 | ||||||||||||||||
The accompanying notes are an integral part of these combined financial statements.
F-6
Health Care Business of 3M Company
Combined Statements of Changes in Equity
| (Millions) | Net Parent Investment | Accumulated Other Comprehensive Income (Loss) | Total | |||||||||||||||||
Balance at January 1, 2021 | $ | 12,644 | $ | 70 | $ | 12,714 | ||||||||||||||
| Net income | 1,460 | — | 1,460 | |||||||||||||||||
| Other comprehensive income (loss) – net of tax | ||||||||||||||||||||
| Cumulative translation adjustment | — | (280) | (280) | |||||||||||||||||
| Defined benefit pension adjustment | — | 10 | 10 | |||||||||||||||||
| Total other comprehensive income (loss) – net of tax | — | (270) | (270) | |||||||||||||||||
| Net transfers to 3M | (1,845) | — | (1,845) | |||||||||||||||||
Balance at December 31, 2021 | $ | 12,259 | $ | (200) | $ | 12,059 | ||||||||||||||
| Net income | 1,343 | 1,343 | ||||||||||||||||||
| Other comprehensive income (loss) – net of tax | ||||||||||||||||||||
| Cumulative translation adjustment | — | (331) | (331) | |||||||||||||||||
| Defined benefit pension adjustment | — | 34 | 34 | |||||||||||||||||
| Total other comprehensive income (loss) – net of tax | — | (297) | (297) | |||||||||||||||||
| Net transfers to 3M | (1,363) | — | (1,363) | |||||||||||||||||
Balance at December 31, 2022 | $ | 12,239 | $ | (497) | $ | 11,742 | ||||||||||||||
| Net income | 1,346 | — | 1,346 | |||||||||||||||||
| Other comprehensive income (loss) – net of tax | ||||||||||||||||||||
| Cumulative translation adjustment | — | 157 | 157 | |||||||||||||||||
| Defined benefit pension adjustment | — | (33) | (33) | |||||||||||||||||
| Total other comprehensive income (loss) – net of tax | — | 124 | 124 | |||||||||||||||||
| Net transfers to 3M | (1,582) | 36 | (1,546) | |||||||||||||||||
Balance at December 31, 2023 | $ | 12,003 | $ | (337) | $ | 11,666 | ||||||||||||||
The accompanying notes are an integral part of these combined financial statements.
F-7
Health Care Business of 3M Company
Combined Statements of Cash Flows
| Years ended December 31, | ||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||
| Cash Flows from Operating Activities | ||||||||||||||||||||||||||
| Net income | $ | 1,346 | $ | 1,343 | $ | 1,460 | ||||||||||||||||||||
| Adjustments to reconcile net income to net cash provided by operating activities | ||||||||||||||||||||||||||
| Depreciation and amortization | 561 | 578 | 597 | |||||||||||||||||||||||
| Postretirement benefit plan expense | 41 | 64 | 74 | |||||||||||||||||||||||
| Stock-based compensation expense | 39 | 37 | 38 | |||||||||||||||||||||||
| Gain on business divestitures | (56) | — | — | |||||||||||||||||||||||
| Deferred income taxes | (142) | (141) | (34) | |||||||||||||||||||||||
| Changes in assets and liabilities | ||||||||||||||||||||||||||
| Accounts receivable | (129) | (32) | (1) | |||||||||||||||||||||||
| Inventories | 23 | (82) | (136) | |||||||||||||||||||||||
| Accounts payable | 105 | 25 | 68 | |||||||||||||||||||||||
| Other current liabilities | 137 | (101) | 73 | |||||||||||||||||||||||
| Other — net | (10) | (12) | 63 | |||||||||||||||||||||||
| Net cash provided by operating activities | 1,915 | 1,679 | 2,202 | |||||||||||||||||||||||
| Cash Flows from Investing Activities | ||||||||||||||||||||||||||
| Purchases of property, plant and equipment (PP&E) | (290) | (251) | (277) | |||||||||||||||||||||||
| Proceeds from sale of business | 60 | — | — | |||||||||||||||||||||||
| Other — net | — | (2) | (1) | |||||||||||||||||||||||
| Net cash used in investing activities | (230) | (253) | (278) | |||||||||||||||||||||||
| Cash Flows from Financing Activities | ||||||||||||||||||||||||||
| Net transfers to 3M | (1,553) | (1,456) | (1,947) | |||||||||||||||||||||||
| Other — net | 1 | (4) | (13) | |||||||||||||||||||||||
| Net cash used in financing activities | (1,552) | (1,460) | (1,960) | |||||||||||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | — | 4 | 3 | |||||||||||||||||||||||
| Net increase (decrease) in cash and cash equivalents | 133 | (30) | (33) | |||||||||||||||||||||||
| Cash and cash equivalents at beginning of year | 61 | 91 | 124 | |||||||||||||||||||||||
| Cash and cash equivalents at end of period | $ | 194 | $ | 61 | $ | 91 | ||||||||||||||||||||
The accompanying notes are an integral part of these combined financial statements.
F-8
Health Care Business of 3M Company
Notes to Combined Financial Statements
Note 1 - Description of the business and basis of presentation
Organization and Description of Business
The Health Care Business (the “Company”) is a carve-out business of 3M Company (“3M” or “Parent”). On July 26, 2022, Parent announced a plan to spin off the Health Care Business. In connection with the spin-off, Parent will transfer certain assets and liabilities associated with the Health Care Business to 3M Health Care Company, a newly formed wholly owned subsidiary of Parent, and will effect a pro rata distribution of at least 80.1% of the common stock of 3M Health Care Company to 3M’s shareholders. The completion of the spin-off is subject to certain conditions, including the effectiveness of a registration statement for the common stock of 3M Health Care Company, the continuing validity of a private letter ruling received from the Internal Revenue Service and receipt by 3M of one or more tax opinions, and approval by 3M’s Board of Directors. 3M Health Care Company had no assets, liabilities, operations, or commitments and contingencies during the periods presented in these combined financial statements and will not have any assets, liabilities, operations or commitments and contingencies in respect of the Health Care Business until such business is transferred to 3M Health Care Company.
The Health Care Business is a leading global healthcare company with a broad portfolio of trusted solutions that leverage deep material science, data science, and digital capabilities to address critical customer needs.
The Health Care Business is organized into four operating business segments that are aligned with the end markets that the Company serves:
•MedSurg: Solutions including active wound care and incision management, vascular access, sterilization, temperature management, surgical supplies, auscultation, and monitoring, designed to accelerate healing, prevent complications, and lower the total cost of care.
•Dental Solutions: Comprehensive suite of dental and orthodontic solutions including brackets, aligners, restorative cement, and bonding agents that address oral care across the “life of the tooth” including disease prevention, direct and indirect restoration, and broad orthodontic needs, while maximizing practitioner effectiveness through digitally enabled workflows and tools.
•Health Information Systems: Software and consulting services including computer-assisted physician documentation, direct-to-bill and coding automation, classification methodologies, speech recognition, and data visualization platforms that eliminate revenue cycle waste, create more time for patient care, and drive value-based care.
•Purification and Filtration: Filtration and purification technologies including filters, purifiers, cartridges, and membranes that simplify purification processes, reduce debris and bioburden in fluids, and remove contaminants to enable the development and manufacturing of biopharmaceutical and medical technology treatments and provide cleaner water.
Basis of Presentation
The Health Care Business is a carve-out business of 3M Company. The combined financial statements have been derived from the consolidated financial statements and accounting records of 3M, including the historical cost basis of assets and liabilities comprising the Company, as well as the historical revenues, direct costs, and allocations of indirect costs attributable to the operations of the Company, using the historical accounting policies applied by 3M. These combined financial statements do not purport to reflect what the results of operations, comprehensive income, financial position, or cash flows would have been had the Company operated as a separate, standalone entity during the periods presented.
The combined financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) and pursuant to the rules and regulations of the U.S. Securities and Exchange Commission and present the historical results of operations, comprehensive income, and cash flows for the years ended December 31, 2023, 2022 and 2021, and the financial position as of December 31, 2023 and 2022.
F-9
Health Care Business of 3M Company
Notes to Combined Financial Statements
These combined financial statements include general corporate expenses and shared expenses of 3M that were historically incurred by or charged to the Health Care Business for certain support functions that are provided on a centralized basis, such as expenses related to information technology, finance and accounting, human resources, legal, and use of shared assets. Additional information is included in Note 13. Direct usage has been used to attribute expenses that are specifically identifiable to the business, where practicable. In certain instances these expenses have been allocated to the Health Care Business primarily based on a pro rata proportion of revenue. The attribution methodologies of corporate and shared expenses reasonably reflect the utilization of services by, or the benefits provided to the Health Care Business, in the aggregate. The combined financial statements reflect all the costs of doing business, the allocations may not, however, reflect the expenses the Health Care Business would have incurred as a standalone company for the periods presented. All intercompany transactions and balances within the Health Care Business have been eliminated.
The Health Care Business’s combined balance sheets include 3M assets and liabilities that are specifically identifiable or otherwise attributable to the Health Care Business. 3M manages cash and other treasury operations at a centralized level. As such, cash and cash equivalents in the combined balance sheets primarily represent cash managed by Health Care Business subsidiaries directly and cash that has not yet been swept to 3M’s central accounts. Cash transfers to and from the cash management accounts of 3M are reflected in the combined statements of cash flows as “Net transfers to 3M.” 3M’s third party debt, including any related interest expense, which is not directly attributable to the Health Care Business has been excluded from the combined financial statements as the Company is not the legal obligor nor a guarantor of the debt. These arrangements may not be reflective of the way the Company would have financed its operations had it been a separate, standalone entity during the periods presented.
The operations of the Health Care Business are included in the consolidated U.S. federal, and certain state, local and foreign income tax returns filed by 3M, where applicable. Income tax expense and other income tax related information contained in these combined financial statements are presented following the separate return methodology as if the Health Care Business filed its own income tax returns. The income tax results of the Health Care Business as presented in the combined financial statements may not be reflective of the results the Health Care Business would generate in the future. In jurisdictions where the Health Care Business has been included in income tax returns filed by Parent, any income taxes payable resulting from the related income tax provision have been reflected within “Net parent investment” on the combined balance sheets.
Note 2 - Summary of Significant Accounting Policies
Foreign currency translation: Local currencies generally are considered the functional currencies outside the United States, and accordingly, the financial statements of these subsidiaries are remeasured as if their functional currency is that of their parent. Assets and liabilities for operations in local-currency environments are translated at month-end exchange rates of the period reported. Income and expense items are translated at average monthly currency exchange rates in effect during the period. Cumulative translation adjustments are recorded as a component of accumulated other comprehensive income (loss) in the combined balance sheets.
Use of estimates: The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Such estimates and assumptions are subject to inherent uncertainties which may result in actual amounts differing from these estimates.
Investments: All equity securities that do not result in consolidation are measured at fair value with changes therein reflected in net income. The Health Care Business utilizes the measurement alternative for equity investments that do not have readily determinable fair values and measures these investments at cost less impairment plus or minus observable price changes in orderly transactions.
Other assets: Other assets primarily includes long-lived medical equipment in rental arrangements utilized primarily by hospitals and other medical clinics, and other long-term assets. Depreciation expense incurred on the
F-10
Health Care Business of 3M Company
Notes to Combined Financial Statements
equipment used in rental arrangements was $32 million, $28 million, and $32 million for the years ended December 31, 2023, 2022, and 2021, respectively.
Inventories: Inventories are stated at the lower of cost or net realizable value (NRV), which is defined as estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. Cost is generally determined on a first-in, first-out basis.
Property, plant and equipment: Property, plant and equipment, including capitalized interest and internal direct engineering costs, are recorded at cost. Depreciation of property, plant and equipment generally is computed using the straight-line method based on the estimated useful lives of the assets. The estimated useful lives of buildings and improvements primarily range from ten to forty years, with the majority in the range of twenty to forty years. The estimated useful lives of machinery and equipment primarily range from three to fifteen years, with the majority in the range of five to ten years. Fully depreciated assets are retained in property, plant and equipment and accumulated depreciation accounts until disposal. Upon disposal, assets and related accumulated depreciation are removed from the accounts and the net amount, less proceeds from disposal, is charged or credited to operations. Property, plant and equipment amounts are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset (asset group) may not be recoverable. An impairment loss would be recognized when the carrying amount of an asset exceeds the estimated undiscounted future cash flows expected to result from the use of the asset and its eventual disposition. The amount of the impairment loss recorded is calculated by the excess of the asset’s carrying value over its fair value. Fair value is generally determined using a discounted cash flow analysis.
Goodwill: Goodwill is the excess of cost of an acquired entity over the amounts assigned to assets acquired and liabilities assumed in a business combination. Goodwill is not amortized. Goodwill is tested for impairment annually in the fourth quarter of each year, and is tested for impairment between annual tests if an event occurs or circumstances change that would indicate the carrying amount may be impaired. Impairment testing for goodwill is done at a reporting unit level, with all goodwill assigned to a reporting unit. The Health Care Business reporting units correspond to a business segment as this represents the lowest level of discrete financial information below sales that is available. The Health Care Business did not combine any of its reporting units for impairment testing. The impairment loss is measured as the amount by which the carrying value of the reporting unit’s net assets exceeds its estimated fair value, not to exceed the carrying value of the reporting unit’s goodwill. The estimated fair value of a reporting unit is determined based on a market approach using comparable company information such as EBITDA (earnings before interest, taxes, depreciation and amortization) multiples or, in some cases, based on a discounted cash flow analysis.
Intangible assets: Intangible asset types include customer-related, patents and other technology-based, tradenames and other intangible assets acquired from an independent party. Intangible assets with a definite life are amortized on a systematic and rational basis (generally straight line) that is representative of the asset’s use. The estimated useful lives vary by category, with customer-related between twelve to nineteen years, patents and other technology-based between six to ten years, and definite lived tradenames and other between eleven and sixteen years. Intangible assets are removed from their respective gross asset and accumulated amortization accounts when they are no longer in use. Refer to Note 6 for additional details on the gross amount and accumulated amortization of the Company’s intangible assets.
Intangible assets with a definite life are tested for impairment whenever events or circumstances indicate that the carrying amount of an asset (asset group) may not be recoverable. An impairment loss is recognized when the carrying amount exceeds the estimated undiscounted cash flows from the asset’s or asset group’s ongoing use and eventual disposition. If an impairment is identified, the amount of the impairment loss recorded is calculated by the excess of the asset’s carrying value over its fair value. Fair value is generally determined using a discounted cash flow analysis.
Intangible assets with an indefinite life, namely certain tradenames, are not amortized. Indefinite-lived intangible assets are tested for impairment annually in the third quarter of each year, and are tested for impairment between annual tests if an event occurs or circumstances change that would indicate that the carrying amount may be
F-11
Health Care Business of 3M Company
Notes to Combined Financial Statements
impaired. An impairment loss would be recognized when the fair value is less than the carrying value of the indefinite-lived intangible asset.
Restructuring actions: Restructuring actions generally include significant actions involving employee-related severance charges, contract termination costs, and impairment or accelerated depreciation/amortization of assets associated with such actions. Employee-related severance charges are largely based upon distributed employment policies and substantive severance plans. These charges are reflected in the quarter when the actions are probable and the amounts are estimable, which typically is when management approves the associated actions. Severance amounts for which affected employees in certain circumstances are required to render service in order to receive benefits at their termination dates are measured at the date such benefits were communicated to the applicable employees and recognized as expense over the employees’ remaining service periods. Contract termination and other charges primarily reflect costs to terminate a contract before the end of its term (measured at fair value at the time the Company provided notice to the counterparty) or costs that will continue to be incurred under the contract for its remaining term without economic benefit to the Company.
Revenue recognition: The Company sells a wide range of products to a diversified base of customers around the world and has no material concentration of credit risk or significant payment terms extended to customers. The majority of the Health Care Business’s customer arrangements contain a single performance obligation. The Health Care Business also enters into customer arrangements that involve multiple performance obligations (such as rental of equipment and related consumables), software with coterminous post-contract support, and software-as-a-service.
The Company recognizes revenue in light of the guidance of Accounting Standards Codification (ASC) 606, Revenue from Contracts with Customers. Revenue is recognized when control of goods has transferred to customers. For the majority of the Company’s customer arrangements, control transfers to customers at a point-in-time when goods/services have been delivered as that is generally when legal title, physical possession and risks and rewards of goods/services transfer to the customer. For certain arrangements, specifically software sold with coterminous post-contract support that is integral to maintaining the utility of the software license to the customer and software-as-a-service, control transfers over time as the customer simultaneously receives and consumes the benefits as the Health Care Business completes the performance obligation(s).
Revenue is recognized at the transaction price which the Company expects to be entitled. When determining the transaction price, the Health Care Business estimates variable consideration applying the portfolio approach practical expedient under ASC 606. The main sources of variable consideration for the Health Care Business are customer rebates, trade promotion funds, and cash discounts. These sales incentives are recorded as a reduction to revenue at the time of the initial sale using the most-likely amount estimation method. The most-likely amount method is based on the single most likely outcome from a range of possible consideration outcomes. The range of possible consideration outcomes are primarily derived from the following inputs: sales terms, historical experience, trend analysis, and projected market conditions in the various markets served. Because the Health Care Business serves numerous markets, the sales incentive programs offered vary across businesses, but the most common incentive relates to amounts paid or credited to customers for achieving defined volume levels or growth objectives. There are no material instances where variable consideration is constrained and not recorded at the initial time of sale. Free goods are accounted for as an expense and recorded in costs of product. Product returns are recorded as a reduction to revenue based on anticipated sales returns that occur in the normal course of business. The Health Care Business primarily has assurance-type warranties that do not result in separate performance obligations. Sales, use, value-added, and other excise taxes are not recognized in revenue. The Company has elected to present revenue net of sales taxes and other similar taxes.
For contracts with multiple performance obligations, the Company allocates the contract’s transaction price to each performance obligation using the Health Care Business’s best estimate of the standalone selling price of each distinct good or service in the contract. For customers purchasing software from the Health Care Business, these performance obligations include providing software licenses with ongoing customer support, installation and training. For customers renting medical devices from the Health Care Business, this includes the Company furnishing a rental unit to a customer as well as delivery of consumables for the rented medical device.
F-12
Health Care Business of 3M Company
Notes to Combined Financial Statements
The Company did not recognize any material revenue in the current reporting period for performance obligations that were fully satisfied in previous periods.
The Company does not have material unfulfilled performance obligation balances for contracts with an original length greater than one year in any years presented. Additionally, the Company does not have material costs related to obtaining a contract with amortization periods greater than one year for any year presented.
The Health Care Business applies ASC 606 utilizing the following allowable exemptions or practical expedients:
•Exemption to not disclose the unfulfilled performance obligation balance for contracts with an original length of one year or less.
•Practical expedient relative to costs of obtaining a contract by expensing sales commissions when incurred because the amortization period would have been one year or less.
•Portfolio approach practical expedient relative to estimation of variable consideration.
•“Right to invoice” practical expedient based on the Health Care Business’s right to invoice the customer at an amount that reasonably represents the value to the customer of the Health Care Business’s performance completed to date.
•Election to present revenue net of sales taxes and other similar taxes.
•Sales-based royalty exemption permitting future intellectual property out-licensing royalty payments to be excluded from the otherwise required remaining performance obligations disclosure.
The Company recognizes revenue from the rental of durable medical devices in accordance with the guidance of ASC 842, Leases. The Company recognizes rental revenue based on the length of time a device is used by the patient/organization, (i) at the contracted rental rate for contracted customers and (ii) generally, retail price for non-contracted customers. The leases are short-term in nature, generally providing for daily or monthly pricing, and are all classified as operating leases.
Accounts receivable and allowances: Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The Company maintains allowances for bad debts, cash discounts, and various other items. The allowances for bad debts and cash discounts are based on the best estimate of the amount of expected credit losses in existing accounts receivable and anticipated cash discounts. The Company determines the allowances based on historical write-off experience informed by industry and regional economic data, current expectations of future credit losses, and historical cash discounts. The Company reviews the allowances monthly. The allowances for bad debts as well as the provision for credit losses, write-off activity and recoveries for the periods presented are not material. The Company does not have any significant off-balance-sheet credit exposure related to its customers.
Research and development: Research and development includes costs related to basic scientific research and the application of scientific advances in the development of new and improved products and their uses; technical support; internally developed patent costs, which include costs and fees incurred to prepare, file, secure and maintain patents; amortization of externally acquired patents and externally acquired in-process research and development. Research and development costs are expensed as incurred.
Internal-use software: The Company capitalizes direct costs of services used in the development of, and external software acquired for use as, internal-use software. Amounts capitalized are amortized over a period of three to seven years, generally on a straight-line basis, unless another systematic and rational basis is more representative of the software’s use.
Income taxes: The income tax provision of the Health Care Business was prepared using the separate return method. The calculation of income taxes on a separate return basis requires a considerable amount of judgment and use of both estimates and allocations. As a result, transactions included in the consolidated financial statements of 3M may not be included in the combined financial statements. Similarly, the tax treatment of certain items reflected
F-13
Health Care Business of 3M Company
Notes to Combined Financial Statements
in the combined financial statements may not be reflected in the consolidated financial statements and tax returns of 3M. Therefore, items such as net operating losses, credit carryforwards, and valuation allowances may exist in the standalone financial statements that may or may not exist in 3M’s consolidated financial statements. In the future, as a standalone entity, the Health Care Business will file tax returns on its own behalf and its deferred taxes and actual income tax rate may differ from those in the historical periods.
In jurisdictions where the Health Care Business has been included in income tax returns filed by Parent, income taxes currently payable will be deemed to have been remitted to the Parent, in cash, in the period the liability arose and income taxes currently receivable are deemed to have been received from the Parent in the period that a refund could have been recognized. Adjustments to the recorded payable that derive from the Health Care Business’s current year activity are recorded through current tax expenses and the ending adjusted payable/receivable is settled through “Net parent investment” on the combined balance sheets.
Current obligations for tax in jurisdictions where the Company does not file a consolidated tax return with 3M, including certain foreign and certain U.S. state tax jurisdictions, are recorded as accrued liabilities within “Other current liabilities” on the combined balance sheets. The effects of tax adjustments and settlements with taxing authorities are presented in our combined financial statements in the period to which they relate.
Uncertain tax positions that meet the more likely than not recognition threshold are measured to determine the amount of tax benefit to recognize in the combined financial statements. An uncertain tax position is measured at the largest amount of benefit that the Company believes has a greater than 50% likelihood of realization upon settlement. Tax benefits not meeting the measurement or realization criteria represent unrecognized tax benefits. The Company recognizes interest and penalties related to income tax matters as a component of “Provision for income taxes” in the combined statements of income.
Deferred income tax balances reflect the effects of temporary differences between the carrying amounts of assets and liabilities and their respective tax bases, as well as from net operating loss and tax credit carryforwards. The deferred income tax balances are stated at enacted tax rates expected to be in effect when those taxes are paid or recovered. Deferred income tax assets represent amounts available to reduce income taxes payable on taxable income in future years. We evaluate the recoverability of these future tax deductions and tax credits by evaluating all available positive and negative evidence, specifically assessing the adequacy of future expected taxable income from all sources, including reversal of existing taxable temporary differences, forecasted operating earnings, and available tax planning strategies. To the extent we consider it more likely than not that a deferred tax asset will not be recovered, a valuation allowance is established.
Stock-based compensation: Certain employees participate in the stock-based compensation plans sponsored by 3M. The awards to these employees are reflected in “Net parent investment” within the combined statements of changes in equity at the time they are expensed. The Company recognizes all stock-based payments to employees as compensation cost over the service period based on their estimated fair value on the date of grant. The Company’s annual stock option and restricted stock unit grant is made in February to provide a strong and immediate link between the performance of individuals during the preceding year and the size of their annual stock compensation grants. The grant to eligible employees uses the closing stock price on the grant date. Accounting rules require recognition of expense under a non-substantive vesting period approach, requiring compensation expense recognition when an employee is eligible to retire. Employees are considered eligible to retire at age 55 and after having completed ten years of service.
Comprehensive income: Total comprehensive income and the components of accumulated other comprehensive income (loss) are presented in the combined statements of comprehensive income and the combined statements of changes in equity. Accumulated other comprehensive income (loss) is composed of foreign currency translation effects and defined benefit pension adjustments. The Company uses the portfolio approach for releasing income tax effects from accumulated other comprehensive income.
Fair value measurements: The Health Care Business follows ASC 820, Fair Value Measurements and Disclosures, with respect to assets and liabilities that are measured at fair value on a recurring basis and nonrecurring basis. Under the standard, fair value is defined as the exit price, or the amount that would be received
F-14
Health Care Business of 3M Company
Notes to Combined Financial Statements
to sell an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. The standard also establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available in the circumstances. The hierarchy is broken down into three levels. Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities. Level 2 inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs (other than quoted prices) that are observable for the asset or liability, either directly or indirectly. Level 3 inputs are unobservable inputs for the asset or liability. Categorization within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The carrying value of the Health Care Business's accounts receivable, accounts payable, and accrued expenses approximate their fair value due to the short period of time to maturity or repayment.
Leases: The Health Care Business determines if an arrangement is a lease upon inception. A contract is or contains a lease if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. The right to control the use of an asset includes the right to obtain substantially all of the economic benefits of the underlying asset and the right to direct how and for what purpose the asset is used.
Operating lease right-of-use assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As the Health Care Business’ leases typically do not provide an implicit rate, the present value of our lease liability is determined using 3M’s incremental borrowing rate at lease commencement. 3M determines the incremental borrowing rate for leases using a portfolio approach based primarily on the lease term and the economic environment of the applicable country or region.
As a lessee, the Company leases distribution centers, office space, land, and equipment. Certain Health Care Business lease agreements include rental payments adjusted annually based on changes in an inflation index. The Health Care Business’s leases do not contain material residual value guarantees or material restrictive covenants. Lease expense is recognized on a straight-line basis over the lease term.
Certain leases include one or more options to renew, with terms that can extend the lease term up to five years. The Health Care Business includes options to renew the lease as part of the right of use lease asset and liability when it is reasonably certain the Company will exercise the option. In addition, certain leases contain fair value purchase and termination options with an associated penalty. In general, the Health Care Business is not reasonably certain to exercise such options.
For the measurement and classification of its lease agreements, the Health Care Business groups lease and non-lease components into a single lease component for all underlying asset classes. Variable lease payments primarily include payments for non-lease components, such as maintenance costs, payments for leased assets used beyond their noncancellable lease term as adjusted for contractual options to terminate or renew, additional payments related to a subsequent adjustment in an inflation index, and payments for non-components such as sales tax. Certain Health Care leases contain immaterial variable lease payments based on number of units produced.
F-15
Health Care Business of 3M Company
Notes to Combined Financial Statements
Note 3 - New Accounting Pronouncements
The table below provides summaries of applicable new accounting pronouncements issued, but not yet adopted by Health Care.
| Standard | Relevant Description | Effective Date for the Health Care Business | Impact and Other Matters | ||||||||
ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures | Issued in November 2023. Requires public entities to expand on segment disclosure requirements, primarily through enhanced disclosures about significant segment expenses. | Year-end December 31, 2024. | As this ASU relates to disclosures only, there will be no impact to the Health Care Business’s combined results of operations and financial condition. | ||||||||
ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures | Issued in December 2023. Requires disaggregated information about a Company's effective tax rate reconciliation as well as information on income taxes paid. | Year-end December 31, 2025 | As this ASU relates to disclosures only, there will be no impact to the Health Care Business’s combined results of operations and financial condition. | ||||||||
Note 4 - Revenue recognition
Contract Balances
Unearned revenue primarily relates to revenue that is recognized over time for one-year software license contracts. Approximately $540 million of the December 31, 2022 balance was recognized as revenue during the year ended December 31, 2023, while approximately $540 million of the December 31, 2021 balance was recognized as revenue during the year ended December 31, 2022.
Operating Lease Revenue
Sales of software and rental includes rental revenue from durable medical devices as part of operating lease arrangements (reported within the MedSurg segment), which was $616 million, $603 million, and $613 million for the years ended December 31, 2023, 2022 and 2021, respectively.
No customer accounted for more than 10% of the Company’s revenues for the years ended December 31, 2023, 2022 and 2021. Additionally, one customer accounted for approximately 10% of accounts receivable as of December 31, 2023 and none accounted for more than 10% of accounts receivable as of December 31, 2022.
F-16
Health Care Business of 3M Company
Notes to Combined Financial Statements
Note 5 - Property, Plant, and Equipment - Net
Property, plant and equipment, net consisted of the following:
| December 31, | |||||||||||||||||||||||
| (Millions) | 2023 | 2022 | |||||||||||||||||||||
Property, plant and equipment - at cost | |||||||||||||||||||||||
| Buildings and leasehold improvements | $ | 937 | $ | 856 | |||||||||||||||||||
| Machinery and equipment | 2,057 | 1,965 | |||||||||||||||||||||
| Construction in progress | 320 | 240 | |||||||||||||||||||||
| Other fixed assets | 24 | 29 | |||||||||||||||||||||
Gross property, plant and equipment | 3,338 | 3,090 | |||||||||||||||||||||
| Accumulated depreciation | (1,881) | (1,771) | |||||||||||||||||||||
Property, plant and equipment – net | $ | 1,457 | $ | 1,319 | |||||||||||||||||||
Depreciation expense consisted of the following:
| For the years ended December 31, | ||||||||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||
| Depreciation expense | $ | 164 | $ | 177 | $ | 184 | ||||||||||||||||||||||||||
Note 6 - Goodwill and Intangible assets
Goodwill
There was no goodwill recorded from acquisitions during 2023 or 2022.
In August 2023, 3M completed the sale of assets associated with its dental local anesthetic business (part of the Dental Solutions segment) to Pierrel S.p.A for approximately $60 million in cash. This transaction resulted in a net pre-tax gain of $56 million. In connection with this transaction, goodwill was reduced by approximately $4 million.
The goodwill balance by business segment follows:
| (Millions) | MedSurg | Dental Solutions | Health Information Systems | Purification and Filtration | Total | |||||||||||||||||||||||||||
Balance at December 31, 2021 | $ | 3,763 | $ | 472 | $ | 873 | $ | 1,558 | $ | 6,666 | ||||||||||||||||||||||
| Translation impact | (157) | (20) | (2) | (53) | (232) | |||||||||||||||||||||||||||
Balance at December 31, 2022 | $ | 3,606 | $ | 452 | $ | 871 | $ | 1,505 | $ | 6,434 | ||||||||||||||||||||||
| Divestitures | — | (4) | — | — | (4) | |||||||||||||||||||||||||||
| Translation impact | 79 | 10 | 2 | 14 | 105 | |||||||||||||||||||||||||||
Balance at December 31, 2023 | $ | 3,685 | $ | 458 | $ | 873 | $ | 1,519 | $ | 6,535 | ||||||||||||||||||||||
F-17
Health Care Business of 3M Company
Notes to Combined Financial Statements
Acquired Intangible Assets
The carrying amount and accumulated amortization of acquired intangible assets follow:
| December 31, | ||||||||||||||||||||
| (Millions) | 2023 | 2022 | ||||||||||||||||||
| Customer related intangible assets | $ | 2,734 | $ | 2,715 | ||||||||||||||||
| Patents and other technology-based intangible assets | 1,897 | 1,893 | ||||||||||||||||||
| Tradenames and other amortizable intangible assets | 705 | 706 | ||||||||||||||||||
Total gross carrying amount | 5,336 | 5,314 | ||||||||||||||||||
| Accumulated amortization — customer related | (1,081) | (924) | ||||||||||||||||||
| Accumulated amortization — patents and other technology-based | (1,055) | (880) | ||||||||||||||||||
| Accumulated amortization — tradenames and other | (323) | (283) | ||||||||||||||||||
Total accumulated amortization | (2,459) | (2,087) | ||||||||||||||||||
Total finite-lived intangible assets — net | 2,877 | 3,227 | ||||||||||||||||||
| Indefinite-lived tradenames | 25 | 25 | ||||||||||||||||||
Total intangible assets — net | $ | 2,902 | $ | 3,252 | ||||||||||||||||
Amortization expense consisted of the following:
| For the years ended December 31, | ||||||||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||
| Amortization expense | $ | 365 | $ | 373 | $ | 381 | ||||||||||||||||||||||||||
Expected amortization expense for acquired amortizable intangible assets recorded as of December 31, 2023 follows:
| (Millions) | 2024 | 2025 | 2026 | 2027 | 2028 | After 2028 | ||||||||||||||||||||||||||||||||
| Amortization expense | $ | 346 | $ | 318 | $ | 313 | $ | 308 | $ | 307 | $ | 1,285 | ||||||||||||||||||||||||||
The preceding expected amortization expense is an estimate. Actual amounts of amortization expense may differ from estimated amounts due to additional intangible asset acquisitions, impairment of intangible assets, accelerated amortization of intangible assets and other events.
Note 7 - Other Current Liabilities
Other current liabilities included in the combined balance sheets consists of the following;
| December 31, | ||||||||||||||||||||
| (Millions) | 2023 | 2022 | ||||||||||||||||||
| Accrued compensation | $ | 209 | $ | 65 | ||||||||||||||||
| Accrued rebates | 206 | 168 | ||||||||||||||||||
| Other | 262 | 171 | ||||||||||||||||||
| Total other current liabilities | $ | 677 | $ | 404 | ||||||||||||||||
F-18
Health Care Business of 3M Company
Notes to Combined Financial Statements
Note 8 - Accumulated Other Comprehensive Income (Loss)
Changes in Accumulated Other Comprehensive Income (Loss) by Component
| (Millions) | Cumulative Translation Adjustment | Defined Benefit Pension Plans Adjustment | Total Accumulated Other Comprehensive Income (Loss) | |||||||||||||||||
Balance at January 1, 2021, net of tax | $ | 107 | $ | (37) | $ | 70 | ||||||||||||||
| Other comprehensive income (loss), before tax | ||||||||||||||||||||
| Amounts before reclassification | (280) | 10 | (270) | |||||||||||||||||
| Amounts reclassified out | — | 3 | 3 | |||||||||||||||||
| Total other comprehensive income (loss), before tax | (280) | 13 | (267) | |||||||||||||||||
| Tax effect | — | (3) | (3) | |||||||||||||||||
| Total other comprehensive income (loss), net of tax | (280) | 10 | (270) | |||||||||||||||||
Balance at December 31, 2021, net of tax | $ | (173) | $ | (27) | $ | (200) | ||||||||||||||
| Other comprehensive income (loss), before tax | ||||||||||||||||||||
| Amounts before reclassification | (331) | 45 | (286) | |||||||||||||||||
| Amounts reclassified out | — | 2 | 2 | |||||||||||||||||
| Total other comprehensive income (loss), before tax | (331) | 47 | (284) | |||||||||||||||||
| Tax effect | — | (13) | (13) | |||||||||||||||||
| Total other comprehensive income (loss), net of tax | (331) | 34 | (297) | |||||||||||||||||
Balance at December 31, 2022, net of tax | $ | (504) | $ | 7 | $ | (497) | ||||||||||||||
| Other comprehensive income (loss), before tax | ||||||||||||||||||||
| Amounts before reclassification | 157 | (50) | 107 | |||||||||||||||||
| Amounts reclassified out | — | — | — | |||||||||||||||||
| Total other comprehensive income (loss), before tax | 157 | (50) | 107 | |||||||||||||||||
| Tax effect | — | 17 | 17 | |||||||||||||||||
| Total other comprehensive income (loss), net of tax | 157 | (33) | 124 | |||||||||||||||||
| Transfers from 3M, net of tax | — | 36 | 36 | |||||||||||||||||
Balance at December 31, 2023, net of tax | $ | (347) | $ | 10 | $ | (337) | ||||||||||||||
Income taxes are not provided for foreign translation relating to permanent investments in international subsidiaries. Reclassification adjustments are made to avoid double counting in comprehensive income items that are subsequently recorded as part of net income.
Defined Benefit Pension Reclassification out of Accumulated Other Comprehensive Income (Loss)
| For the years ended December 31, | ||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||
| Gains (losses) associated with defined benefit pension plans amortization, before tax | ||||||||||||||||||||||||||
| Transition asset | $ | — | $ | 1 | $ | 1 | ||||||||||||||||||||
| Net actuarial loss | (1) | 1 | 2 | |||||||||||||||||||||||
| Settlements | 1 | — | — | |||||||||||||||||||||||
| Total reclassification, before tax | — | 2 | 3 | |||||||||||||||||||||||
| Tax effect | — | — | (1) | |||||||||||||||||||||||
Total, net of tax | $ | — | $ | 2 | $ | 2 | ||||||||||||||||||||
F-19
Health Care Business of 3M Company
Notes to Combined Financial Statements
Note 9 - Income taxes
See Note 2 “Summary of Significant Accounting Policies” for a description of the Company’s accounting policies and separate return method of preparation for income taxes. The components of income before income taxes were:
| For the years ended December 31, | ||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||
| United States | $ | 1,221 | $ | 1,276 | $ | 1,262 | ||||||||||||||||||||
| International | 446 | 416 | 620 | |||||||||||||||||||||||
Total | $ | 1,667 | $ | 1,692 | $ | 1,882 | ||||||||||||||||||||
The provision for income taxes consists of the following:
| For the years ended December 31, | ||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||
| Currently payable | ||||||||||||||||||||||||||
| Federal | $ | 148 | $ | 355 | $ | 310 | ||||||||||||||||||||
| State | 44 | 53 | 36 | |||||||||||||||||||||||
| International | 271 | 82 | 114 | |||||||||||||||||||||||
| Deferred | ||||||||||||||||||||||||||
| Federal | (8) | (127) | (52) | |||||||||||||||||||||||
| State | (9) | (8) | (2) | |||||||||||||||||||||||
| International | (125) | (6) | 16 | |||||||||||||||||||||||
Total | $ | 321 | $ | 349 | $ | 422 | ||||||||||||||||||||
The Tax Cuts and Jobs Act (“TCJA”) imposes tax on U.S. shareholders for Global Intangible Low-Taxed Income (“GILTI”) earned by certain non-U.S. subsidiaries. The Company has elected to account for the income tax effects associated with the GILTI U.S. income inclusion as a period cost.
F-20
Health Care Business of 3M Company
Notes to Combined Financial Statements
The deferred tax assets and deferred tax liabilities included in the combined balance sheets consist of the following:
| December 31, | ||||||||||||||||||||
| (Millions) | 2023 | 2022 | ||||||||||||||||||
| Deferred tax assets: | ||||||||||||||||||||
| Miscellaneous accruals | $ | 68 | $ | 52 | ||||||||||||||||
| Accrued compensation | 102 | 35 | ||||||||||||||||||
| Net operating loss carryforward | 95 | 95 | ||||||||||||||||||
| Foreign tax credits | 52 | 50 | ||||||||||||||||||
| Advanced royalties | 26 | 48 | ||||||||||||||||||
| Research and experimentation capitalization | 213 | 181 | ||||||||||||||||||
| Other deferred tax assets | 37 | 51 | ||||||||||||||||||
| Gross deferred tax assets | 593 | 512 | ||||||||||||||||||
| Valuation allowance | (56) | (54) | ||||||||||||||||||
| Total deferred tax assets | 537 | 458 | ||||||||||||||||||
| Deferred tax liabilities: | ||||||||||||||||||||
| Property, plant, and equipment | (124) | (128) | ||||||||||||||||||
| Intangible assets | (324) | (392) | ||||||||||||||||||
| Total deferred tax liabilities | (448) | (520) | ||||||||||||||||||
| Net deferred tax asset (liability) | $ | 89 | $ | (62) | ||||||||||||||||
A reconciliation of the net deferred tax asset (liability) in the combined balance sheets is as follows:
| December 31, | ||||||||||||||
| (Millions) | 2023 | 2022 | ||||||||||||
| Other assets | $ | 320 | $ | 153 | ||||||||||
| Deferred income taxes | (231) | (215) | ||||||||||||
| Net deferred tax asset (liability) | $ | 89 | $ | (62) | ||||||||||
As of December 31, 2023, the Company had tax-effected operating loss carryforwards of $95 million and tax credit carryforwards of $52 million for federal, state, and international jurisdictions, with all amounts before limitation impacts and valuation allowances. Federal tax attributes will expire after one to ten years and international tax attributes after one to an indefinite carryover period. As of December 31, 2023, the Company has provided $56 million of valuation allowances against certain of those deferred tax assets based upon management’s determination that it is more-likely-than-not that the tax benefits related to these assets will not be realized.
F-21
Health Care Business of 3M Company
Notes to Combined Financial Statements
The difference between the U.S. statutory income tax rate and the effective income tax rate is as follows:
| For the years ended December 31, | ||||||||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||||||||
| U.S. Statutory income tax rate | 21.0 | % | 21.0 | % | 21.0 | % | ||||||||||||||||||||
| State income taxes - net of federal benefit | 1.7 | % | 2.1 | % | 1.4 | % | ||||||||||||||||||||
| International income taxes - net | (1.3) | % | (1.7) | % | (2.0) | % | ||||||||||||||||||||
| Global Intangible Low Taxed Income (GILTI) | 0.8 | % | 0.7 | % | 3.3 | % | ||||||||||||||||||||
| Foreign derived intangible income (FDII) | (2.2) | % | (2.5) | % | (2.6) | % | ||||||||||||||||||||
| U.S. research and development credit | (1.2) | % | (1.8) | % | (0.9) | % | ||||||||||||||||||||
| Reserves for tax contingencies | (4.1) | % | 0.6 | % | 0.4 | % | ||||||||||||||||||||
| Tax impact of legal entity restructuring | 2.3 | % | — | % | — | % | ||||||||||||||||||||
| Changes in valuation allowance | 1.4 | % | 0.7 | % | 1.1 | % | ||||||||||||||||||||
| Deferred rate change | 0.1 | % | (0.3) | % | 0.6 | % | ||||||||||||||||||||
| All other - net | 0.8 | % | 1.8 | % | 0.1 | % | ||||||||||||||||||||
| Effective income tax rate | 19.3 | % | 20.6 | % | 22.4 | % | ||||||||||||||||||||
The effective income tax rate for 2023 was 19.3%, compared to 20.6% in 2022, a decrease of 1.3%. The primary factors that decreased the tax rate were the recognition of a previously unrecognized tax benefit due to the expiration of the statute of limitations, partially offset by the tax impact of legal entity restructuring in connection with the spin-off.
The Company recognizes the amount of income tax benefit that has a greater than 50% likelihood of being ultimately realized upon settlement. Changes in unrecognized tax benefits impacting the provision for income taxes of the Company have been reflected in the combined statements of income. Interest and penalties are also recognized in the provision for income taxes in the combined statements of income. For uncertain tax positions that the Company expects to be legally liable for, the unrecognized tax benefits and interest and penalties of $40 million and $138 million have been recorded to “Other liabilities” as of December 31, 2023 and 2022, respectively on the combined balance sheets. For uncertain tax positions where the Company is not legally and directly liable for, unrecognized tax benefits and interest and penalties are charged to “Net parent investment” on the combined balance sheets. A rollforward of unrecognized tax benefits (UTB) is as follows:
| December 31, | ||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||
| Gross UTB Balance at January 1 | $ | 307 | $ | 308 | $ | 314 | ||||||||||||||||||||
| Additions based on tax positions related to the current year | 22 | 18 | 23 | |||||||||||||||||||||||
| Additions for tax positions of prior years | 17 | 17 | 2 | |||||||||||||||||||||||
| Additions related to recent acquisitions | — | — | — | |||||||||||||||||||||||
| Reductions for tax positions of prior years | (35) | (19) | (11) | |||||||||||||||||||||||
| Settlements | — | — | — | |||||||||||||||||||||||
| Reductions due to lapse of applicable statute of limitations | (74) | (10) | (10) | |||||||||||||||||||||||
| Reductions for amounts recorded to Net parent investment | (32) | (7) | (10) | |||||||||||||||||||||||
| Gross UTB Balance at December 31 | $ | 205 | $ | 307 | $ | 308 | ||||||||||||||||||||
The total amount of UTB, if recognized, would affect the effective tax rate by $142 million as of December 31, 2023. It is reasonably possible that the amount of unrecognized tax benefits could significantly change within the next 12 months. The Company has ongoing federal, state and international income tax audits in various jurisdictions and evaluates uncertain tax positions that may be challenged by local tax authorities and not fully sustained. These
F-22
Health Care Business of 3M Company
Notes to Combined Financial Statements
uncertain tax positions are reviewed on an ongoing basis and adjusted in light of facts and circumstances including progression of tax audits, developments in case law and closing statutes of limitation. At this time, the Company is not able to estimate the range by which these potential events could impact the Company’s unrecognized tax benefits within the next 12 months.
The IRS has completed its field examination of 3M’s U.S. federal income tax returns through 2018, but the years 2005 through 2018 have not closed, as 3M is in the process of resolving issues identified during those examinations. Currently, 3M is under examination by the IRS for its U.S. federal income tax returns for the years ended 2019 and 2020. As noted previously, for uncertain tax positions that the Company is not legally and directly liable for, unrecognized tax benefits and interest and penalties are charged to Net parent investment. In addition to the U.S. federal examination, there is also audit activity in several U.S. state and foreign jurisdictions where the Company is subject to ongoing tax examinations and governmental assessments, which could be impacted by evolving political environments in those jurisdictions. As of December 31, 2023, no taxing authority proposed significant adjustments to the Company’s tax positions for which the Company is not adequately reserved.
The Company recognizes interest and penalties accrued related to unrecognized tax benefits in tax expense. The Company recognized in the combined statements of income on a gross basis approximately $7.5 million of benefit in 2023 and $3.9 million and $1.1 million of expense in 2022 and 2021, respectively. The amount of interest and penalties recognized may be an expense or benefit due to new or remeasured unrecognized tax benefit accruals. At December 31, 2023 and December 31, 2022, accrued interest and penalties in the combined balance sheets on a gross basis were $4.5 million and $12.1 million, respectively.
As of December 31, 2023, the Company has approximately $1,830 million of undistributed earnings in its foreign subsidiaries. Approximately $1,296 million of these earnings are no longer considered permanently reinvested. The incremental tax cost to repatriate these earnings to the U.S. was immaterial. The Company has not provided deferred taxes on approximately $534 million of undistributed earnings from non-U.S. subsidiaries as of December 31, 2023, which are indefinitely reinvested in operations. Because of the multiple avenues by which to repatriate the earnings to minimize tax cost, and because a large portion of these earnings are not liquid, it is not practical to determine the income tax liability that would be payable if such earnings were not reinvested indefinitely.
F-23
Health Care Business of 3M Company
Notes to Combined Financial Statements
Note 10 - Postretirement Benefit Plans
3M Company Sponsored Pension and Postretirement Benefit Plans
Certain employees of the Health Care Business participate in U.S. and non-U.S. retirement plans sponsored by 3M. For purposes of the combined financial statements, these plans are accounted for as multiemployer plans as they are not sponsored by the Health Care Business. Therefore, the related assets and liabilities are not reflected in the combined balance sheets. The combined statements of income reflects a proportionate allocation of service costs for the multiemployer plans associated with the Health Care Business’s employees. Expenses associated with employee's participation in 3M Company sponsored pension plans were $32 million, $56 million, and $64 million for the years ended December 31, 2023, 2022, and 2021, respectively.
International Health Care Business Sponsored Defined Benefit Plans
During 2023, certain non-U.S. defined benefit plan obligations and assets were legally transferred to the Health Care Business from 3M Company, these amounts are disclosed as “Transfers from 3M” in the following tables. As of December 31, 2023, the Health Care Business has 18 company-sponsored retirement plans covering certain employees in ten countries. As these plans are sponsored by the Health Care Business, they are accounted for as single employer plans. Therefore, the funded status is reflected in the combined balance sheets, and the net periodic benefit costs are included in the combined statements of income.
The following tables include a reconciliation of the beginning and ending balances of the benefit obligation and the fair value of plan assets as well as a summary of the related amounts recognized in the Health Care Business’s combined balance sheets as of December 31, 2023 and 2022. The Health Care Business also has certain non-qualified pension and postretirement benefit plans that individually and in the aggregate are not significant and which are not included in the tables that follow.
| December 31, | ||||||||||||||||||||
| (Millions) | 2023 | 2022 | ||||||||||||||||||
| Change in benefit obligation | ||||||||||||||||||||
| Benefit obligation at beginning of year | $ | 105 | $ | 159 | ||||||||||||||||
| Service cost | 5 | 6 | ||||||||||||||||||
| Interest cost | 7 | 2 | ||||||||||||||||||
| Actuarial (gain) loss | 70 | (45) | ||||||||||||||||||
| Benefit payments | (8) | (3) | ||||||||||||||||||
| Foreign exchange rate changes | 19 | (9) | ||||||||||||||||||
| Transfers from 3M | 321 | (5) | ||||||||||||||||||
| Benefit obligation at end of year | $ | 519 | $ | 105 | ||||||||||||||||
| Change in plan assets | ||||||||||||||||||||
| Fair value of plan assets at beginning of year | 9 | 9 | ||||||||||||||||||
| Actual return on plan assets | 21 | (1) | ||||||||||||||||||
| Company contributions | 11 | 4 | ||||||||||||||||||
| Foreign exchange rate changes | 16 | — | ||||||||||||||||||
| Benefit payments | (8) | (3) | ||||||||||||||||||
| Transfer from 3M | 318 | — | ||||||||||||||||||
| Fair value of plan assets at end of year | $ | 367 | $ | 9 | ||||||||||||||||
| Funded status at end of year | $ | (152) | $ | (96) | ||||||||||||||||
F-24
Health Care Business of 3M Company
Notes to Combined Financial Statements
| December 31, | ||||||||||||||||||||
| Amounts recognized in the combined balance sheets (Millions) | 2023 | 2022 | ||||||||||||||||||
| Other assets | $ | 9 | $ | — | ||||||||||||||||
| Accrued benefit cost | ||||||||||||||||||||
| Current liabilities | (1) | (2) | ||||||||||||||||||
| Non-current liabilities | (160) | (94) | ||||||||||||||||||
| Ending balance | $ | (152) | $ | (96) | ||||||||||||||||
| December 31, | ||||||||||||||||||||
| Amounts recognized in accumulated other comprehensive income (Millions) | 2023 | 2022 | ||||||||||||||||||
| Net transition obligation (asset) | $ | — | $ | — | ||||||||||||||||
| Net actuarial loss (gain) | (11) | (10) | ||||||||||||||||||
| Prior service cost (credit) | — | — | ||||||||||||||||||
| Ending balance | $ | (11) | $ | (10) | ||||||||||||||||
The balance of amounts recognized for non-U.S. plans in accumulated other comprehensive income (loss) as of December 31 in the preceding table are presented based on the foreign currency exchange rates on that date.
The pension accumulated benefit obligation represents the actuarial present value of benefits based on employee service and compensation as of the measurement date and does not include an assumption about future compensation levels. The following table summarizes the total accumulated benefit obligations, the accumulated benefit obligations and fair value of plan assets for defined benefit pension plans with accumulated benefit obligations in excess of plan assets, and the projected benefit obligation and fair value of plan assets for defined benefit pension plans with projected benefit obligation in excess of plan assets as of December 31:
| December 31, | ||||||||||||||||||||
| (Millions) | 2023 | 2022 | ||||||||||||||||||
| Accumulated benefit obligation | $ | 460 | $ | 96 | ||||||||||||||||
| Plans with accumulated benefit obligation in excess of plan assets | ||||||||||||||||||||
| Accumulated benefit obligation | $ | 398 | $ | 90 | ||||||||||||||||
| Fair value of plan assets | 294 | 3 | ||||||||||||||||||
| Plans with projected benefit obligation in excess of plan assets | ||||||||||||||||||||
| Projected benefit obligation | $ | 476 | $ | 106 | ||||||||||||||||
| Fair value of plan assets | 316 | 9 | ||||||||||||||||||
F-25
Health Care Business of 3M Company
Notes to Combined Financial Statements
Components of net periodic cost and other amounts recognized in other comprehensive income
The service cost component of defined benefit net periodic benefit cost is recorded in costs of product; costs of software and rentals; selling, general and administrative; and research and development. Components of net periodic benefit cost and other supplemental information for the years ended December 31 follow:
| For the years ended December 31, | ||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||
| Net periodic benefit cost (benefit) | ||||||||||||||||||||||||||
| Operating expense | ||||||||||||||||||||||||||
| Service cost | $ | 5 | $ | 6 | $ | 6 | ||||||||||||||||||||
| Non-operating expense | ||||||||||||||||||||||||||
| Interest cost | 7 | 2 | 2 | |||||||||||||||||||||||
| Expected return on plan assets | (3) | (1) | (1) | |||||||||||||||||||||||
| Other | — | 2 | 3 | |||||||||||||||||||||||
| Total non-operating expense (benefit) | 4 | 3 | 4 | |||||||||||||||||||||||
| Total net periodic benefit cost (benefit) | $ | 9 | $ | 9 | $ | 10 | ||||||||||||||||||||
| Other changes in plan assets and benefit obligations recognized in other comprehensive (income) loss | ||||||||||||||||||||||||||
| Amortization of transition asset | — | (1) | (1) | |||||||||||||||||||||||
| Prior service cost (benefit) | — | — | 1 | |||||||||||||||||||||||
| Net actuarial (gain) loss | 52 | (43) | (8) | |||||||||||||||||||||||
| Amortization of net actuarial income (loss) | 1 | (1) | (2) | |||||||||||||||||||||||
| Settlements | (1) | — | — | |||||||||||||||||||||||
| Foreign currency | (2) | (2) | (3) | |||||||||||||||||||||||
| Total recognized in other comprehensive (income) loss | $ | 50 | $ | (47) | $ | (13) | ||||||||||||||||||||
| Total recognized in net periodic benefit cost (benefit) and other comprehensive (income) loss | $ | 59 | $ | (38) | $ | (3) | ||||||||||||||||||||
Weighted-average assumptions used to determine benefit obligations as of December 31
| 2023 | 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Germany | All Others | Germany | All Others | |||||||||||||||||||||||||||||||||||||||||||||||
| Discount rate | 3.26 | % | 3.52 | % | 3.81 | % | 10.12 | % | ||||||||||||||||||||||||||||||||||||||||||
| Compensation rate increase | 3.00 | % | 2.40 | % | 3.00 | % | 4.79 | % | ||||||||||||||||||||||||||||||||||||||||||
Weighted-average assumptions used to determine net cost for years ended December 31
| Germany | All Others | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2023 | 2022 | 2021 | 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discount rate - service cost | 4.01 | % | 1.33 | % | 0.90 | % | 7.64 | % | 8.57 | % | 7.51 | % | |||||||||||||||||||||||||||||||||||||||||||||||
| Discount rate - interest cost | 4.06 | % | 0.97 | % | 0.53 | % | 9.02 | % | 9.28 | % | 8.22 | % | |||||||||||||||||||||||||||||||||||||||||||||||
| Expected return on assets | 4.79 | % | 2.00 | % | 2.00 | % | 5.86 | % | 7.16 | % | 7.87 | % | |||||||||||||||||||||||||||||||||||||||||||||||
| Compensation rate increase | 3.00 | % | 3.00 | % | 3.00 | % | 2.40 | % | 4.23 | % | 4.22 | % | |||||||||||||||||||||||||||||||||||||||||||||||
The Company determines the discount rate used to measure plan liabilities as of the December 31 measurement date for the defined benefit plans, which is also the date used for the related annual measurement assumptions. The discount rate reflects the current rate at which the associated liabilities could be effectively settled at the end of the year. The Company sets its rate to reflect the yield of a portfolio of high quality, fixed-income debt instruments that would produce cash flows sufficient in timing and amount to settle projected future benefits. A decrease in the discount rate increases the
F-26
Health Care Business of 3M Company
Notes to Combined Financial Statements
Projected Benefit Obligation (PBO). The annual remeasurement of the PBO as of December 31, 2023, resulted in a $70 million increase in the PBO, primarily driven from lower discount rates as of December 31, 2023. The annual remeasurement of the PBO as of December 31, 2022 resulted in a $45 million decrease in the PBO, primarily driven from higher discount rates as of December 31, 2022.
The Company measures service cost and interest cost separately using the spot yield curve approach applied to each corresponding obligation. Service costs are determined based on duration-specific spot rates applied to the service cost cash flows. The interest cost calculation is determined by applying duration-specific spot rates to the year-by-year projected benefit payments. The spot yield curve approach does not affect the measurement of the total benefit obligations as the change in service and interest costs offset in the actuarial gains and losses recorded in other comprehensive income.
During 2023, the Company contributed $11 million to its defined benefit pension plans. In 2024, the Company expects to contribute approximately $10 million to $15 million of cash to its defined benefit pension plans.
Future Pension Benefit Payments
The following table provides the estimated pension and postretirement benefit payments that are payable from the plans to participants.
| (Millions) | 2024 | 2025 | 2026 | 2027 | 2028 | 2029-2033 | ||||||||||||||||||||||||||||||||
| Estimated future benefit payments | $ | 16 | $ | 19 | $ | 18 | $ | 20 | $ | 21 | $ | 126 | ||||||||||||||||||||||||||
Plan Asset Management
The Company’s investment strategy for its pension and postretirement plans is to manage the funds on a going-concern basis. The primary goal of the trust funds is to meet the obligations as required. The secondary goal is to earn the highest rate of return possible, without jeopardizing its primary goal, and without subjecting the Company to an undue amount of contribution risk. Fund returns are used to help finance present and future obligations to the extent possible within actuarially determined funding limits and tax-determined asset limits, thus reducing the potential need for additional contributions from the Company. The investment strategy has used long duration cash bonds and derivative instruments to offset a significant portion of the interest rate sensitivity of pension liabilities.
Normally, the Company does not buy or sell any Parent securities as a direct investment for its pension and other postretirement benefit funds. However, due to external investment management of the funds, the plans may indirectly buy, sell or hold 3M securities. The aggregate amount of 3M securities are not considered to be material relative to the aggregate fund percentages.
The discussion that follows references the fair value measurements of certain assets in terms of levels 1, 2 and 3. See Note 2 for descriptions of these levels. While the company believes the valuation methods are appropriate and consistent with other market participants, the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different estimate of fair value at the reporting date.
Outside the U.S., pension plan assets are typically managed by decentralized fiduciary committees. The disclosure below of asset categories is presented in aggregate for the six defined benefit plans in five countries, which have plan assets; however, there is significant variation in asset allocation policy from country to country. Local regulations, local funding rules, and local financial and tax considerations are part of the funding and investment allocation process in each country. The Company provides standard funding and investment guidance to all international plans with more focused guidance to the larger plans.
Each plan has its own strategic asset allocation. The asset allocations are reviewed periodically and rebalanced when necessary.
F-27
Health Care Business of 3M Company
Notes to Combined Financial Statements
The fair values of the assets held by the international pension plans by asset class are as follows:
| Fair Value Measurements Using Inputs Considered as | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Investments Measured at Net Asset Value * | Fair Value at December 31, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Asset Class | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Equities | $ | 7 | $ | 4 | $ | 23 | $ | — | $ | — | $ | — | $ | 24 | $ | — | $ | 54 | $ | 4 | ||||||||||||||||||||||||||||||||||||||||||
| Fixed Income | 6 | 4 | 188 | — | — | — | 31 | — | 225 | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Private Equity | — | — | — | — | — | — | 47 | — | 47 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Absolute Return | — | — | — | — | 28 | — | 10 | — | 38 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cash and Cash Equivalents | 2 | 1 | — | — | — | — | — | — | 2 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | $ | 15 | $ | 9 | $ | 211 | $ | — | $ | 28 | $ | — | $ | 112 | $ | — | $ | 366 | $ | 9 | ||||||||||||||||||||||||||||||||||||||||||
| Other items to reconcile to fair value of plan assets | 1 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fair Value of Plan Assets | $ | 367 | $ | 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
____________
* In accordance with ASC 820-10, certain investments that are measured at fair value using the NAV per share (or its equivalent) as a practical expedient have not been classified in the fair value hierarchy. The NAV is based on the fair value of the underlying assets owned by the fund, minus its liabilities then divided by the number of units outstanding and is determined by the investment manager or custodian of the fund. The fair value amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the amounts presented in the fair value of plan assets.
Equities consist primarily of mandates in public equity securities managed to various public equity indices. Publicly traded equities are valued at the closing price reported in the active market in which the individual securities are traded.
Fixed Income investments include domestic and foreign government, and corporate debt securities. The debt securities are valued at the closing price reported if traded on an active market or at yields currently available on comparable securities of issuers with similar credit ratings or valued under a discounted cash flow approach that utilizes observable inputs, such as current yields of similar instruments, but includes adjustments for certain risks that may not be observable such as credit and liquidity risks.
Private equity funds consist of partnership interests in a variety of funds which are valued at NAV as described above. Real estate consists of property funds and REITS (Real Estate Investment Trusts). REITS are valued at NAV with published prices provided by the custodians.
Absolute return consists primarily of private partnership interests in hedge funds, insurance contracts and derivative instruments. Partnerships and hedge funds are valued at NAV as described above. Insurance consists of insurance contracts, which are valued using cash surrender values which is the amount the plan would receive if the contract was cashed out at year end. Derivative instruments consist of various swaps and bond futures that are used to help manage risks and are valued by the custodian using closing market swap curves and market derived inputs.
Other items to reconcile to fair value of plan assets include the net of interest receivables, amounts due for securities sold, amounts payable for securities purchased and interest payable.
The balances of and changes in the fair values of the international pension plans’ level 3 assets consist primarily of insurance contracts under the absolute return asset class. In 2023 these balances were transferred from the 3M plans near the end of the year and had few transactions. In 2022 there were no level 3 assets.
F-28
Health Care Business of 3M Company
Notes to Combined Financial Statements
Note 11 – Commitments and Contingencies
Legal Proceedings: 3M is involved in numerous claims and lawsuits, principally in the United States, and regulatory proceedings worldwide. These claims, lawsuits and proceedings relate to matters including, but not limited to, products liability (involving products that the Company now or formerly manufactured and sold), intellectual property, commercial, antitrust, federal healthcare program related laws and regulations, such as the False Claims Act and anti-kickback laws, securities, and environmental laws in the United States and other jurisdictions. Unless otherwise stated, 3M is vigorously defending all such litigation and proceedings. From time to time, 3M also receives subpoenas, investigative demands or requests for information from various government agencies in the United States and foreign countries. 3M generally responds in a cooperative, thorough and timely manner. These responses sometimes require time and effort and can result in considerable costs being incurred by the Company. Such requests can also lead to the assertion of claims or the commencement of administrative, civil, or criminal legal proceedings against 3M and others, as well as to settlements. The outcomes of legal proceedings and regulatory matters are often difficult to predict. Any determination that the Company’s operations or activities are not, or were not, in compliance with applicable laws or regulations could result in the imposition of fines, civil or criminal penalties, and equitable remedies, including disgorgement, suspension or debarment or injunctive relief.
Process for Disclosure and Recording of Liabilities Related to Legal Proceedings
Many lawsuits and claims involve highly complex issues relating to causation, scientific evidence, and alleged actual damages, all of which are otherwise subject to substantial uncertainties. Assessments of lawsuits and claims can involve a series of complex judgments about future events and can rely heavily on estimates and assumptions. The categories of legal proceedings in which the Company is involved may include multiple lawsuits and claims, may be spread across multiple jurisdictions and courts which may handle the lawsuits and claims differently, may involve numerous and different types of plaintiffs, raising claims and legal theories based on specific allegations that may not apply to other matters, and may seek substantial compensatory and, in some cases, punitive, damages. These and other factors contribute to the complexity of these lawsuits and claims and make it difficult for the Company to predict outcomes and make reasonable estimates of any resulting losses. The Company's ability to predict outcomes and make reasonable estimates of potential losses is further influenced by the fact that a resolution of one or more matters within a category of legal proceedings may impact the resolution of other matters in that category in terms of timing, amount of liability, or both.
When making determinations about recording liabilities related to legal proceedings, the Company complies with the requirements of ASC 450, Contingencies, and related guidance, and records liabilities in those instances where it can reasonably estimate the amount of the loss and when the loss is probable. Where the reasonable estimate of the probable loss is a range, the Company records as an accrual in its financial statements the most likely estimate of the loss, or the low end of the range if there is no one best estimate. The Company either discloses the amount of a possible loss or range of loss in excess of established accruals if estimable, or states that such an estimate cannot be made. The Company discloses significant legal proceedings even where liability is not probable or the amount of the liability is not estimable, or both, if the Company believes there is at least a reasonable possibility that a loss may be incurred. Based on experience and developments, the Company reexamines its estimates of probable liabilities and associated expenses and receivables each period, and whether a loss previously determined to not be reasonably estimable and/or not probable is now able to be reasonably estimated or has become probable. Where appropriate, the Company makes additions to or adjustments of its reasonably estimated losses and/or accruals. As a result, the current accruals and/or estimates of loss and the estimates of the potential impact on the Company’s combined financial position, results of operations and cash flows for the legal proceedings and claims pending against the Company will likely change over time.
Because litigation is subject to inherent uncertainties, and unfavorable rulings or developments could occur, the Company may ultimately incur charges substantially in excess of presently recorded liabilities, including with respect to matters for which no accruals are currently recorded because losses are not currently probable and reasonably estimable. Many of the matters described herein are at varying stages, seek an indeterminate amount of damages or seek damages in amounts that the Company believes are not indicative of the ultimate losses that may be incurred. It is not uncommon for claims to be resolved over many years. As a matter progresses, the Company may receive information, through plaintiff demands, through discovery, in the form of reports of purported experts, or in
F-29
Health Care Business of 3M Company
Notes to Combined Financial Statements
the context of settlement or mediation discussions that purport to quantify an amount of alleged damages, but with which the Company may not agree. Such information may or may not lead the Company to determine that it is able to make a reasonable estimate as to a probable loss or range of loss in connection with a matter. However, even when a loss or range of loss is not probable and reasonably estimable, developments in, or the ultimate resolution of, a matter could be material to the Company and could have a material adverse effect on the Company, its combined financial position, results of operations and cash flows. In addition, future adverse rulings or developments, or settlements in, one or more matters could result in future changes to determinations of probable and reasonably estimable losses in other matters.
Process for Disclosure and Recording of Insurance Receivables Related to Legal Proceedings
The Company estimates insurance receivables based on an analysis of the terms of its numerous policies, including their exclusions, pertinent case law interpreting comparable policies, its experience with similar claims, and assessment of the nature of the claim and remaining coverage, and records an amount it has concluded is recognizable and expects to receive in light of the loss recovery and/or gain contingency models under ASC 450, ASC 610-30, and related guidance. For those insured legal proceedings where the Company has recorded an accrued liability in its financial statements, the Company also records receivables for the amount of insurance that it concludes as recognizable from the Company’s insurance program. For those insured matters where the Company has not recorded an accrued liability because the liability is not probable or the amount of the liability is not estimable, or both, but where the Company has incurred an expense in defending itself, the Company records receivables for the amount of insurance that it concludes as recognizable for the expense incurred.
Product Liability Litigation
The following sections first describe the significant legal proceedings in which the Company is involved, and then describe the liabilities the Company has accrued relating to its significant legal proceedings.
3M is a named defendant in over 6,200 lawsuits in the United States and one Canadian putative class action with a single named plaintiff, alleging that they underwent various joint arthroplasty, cardiovascular, and other surgeries and later developed surgical site infections due to the use of the Bair Hugger patient warming system.
The plaintiffs seek damages and other relief based on theories of strict liability, negligence, breach of express and implied warranties, failure to warn, design and manufacturing defect, fraudulent and/or negligent misrepresentation/concealment, unjust enrichment, and violations of various state consumer fraud, deceptive or unlawful trade practices and/or false advertising acts.
The U.S. Judicial Panel on Multidistrict Litigation (“JPML”) consolidated all cases pending in federal courts to the U.S. District Court for the District of Minnesota to be managed in a multi-district litigation (“MDL”) proceeding. In July 2019, the court excluded several of the plaintiffs’ causation experts, and granted summary judgment for 3M in all cases pending at that time in the MDL. Plaintiffs appealed that decision to the U.S. Court of Appeals for the Eighth Circuit. Plaintiffs also appealed a 2018 jury verdict in favor of 3M in the first bellwether trial in the MDL and appealed the dismissal of another bellwether case. A panel of the appellate court in August 2021 reversed the district court’s exclusion of the plaintiffs’ causation experts and the grant of summary judgment for 3M. 3M sought further appellate en banc review by the full Eighth Circuit court. In November 2021, the Eighth Circuit court denied 3M’s petition for rehearing en banc. In May 2022, the U.S. Supreme Court declined 3M's February 2022 request to review the Eighth Circuit court's decision. Separately, in August 2021, the Eighth Circuit court affirmed the 2018 jury verdict in 3M’s favor in the only bellwether trial in the MDL.
In February 2022, the MDL court ordered the parties to engage in any mediation sessions that a court-appointed mediator deems appropriate. Mediation sessions took place in May and August 2022 without success in resolving the litigation. The MDL court in 2023 assigned a new mediator to facilitate discussions of the litigation and possible resolution. The MDL court denied plaintiffs’ April 2023 motion to disqualify the judge and magistrate judge overseeing the MDL. The parties, working with the new mediator, agreed on the beginning of a bellwether process, selecting 34 cases, with federal court trials to potentially begin in 2024. The MDL court recommended remand of the non-Minnesota bellwether cases; the JPML will consider that recommendation during the first quarter of 2024.
F-30
Health Care Business of 3M Company
Notes to Combined Financial Statements
In addition to the federal MDL cases, there are five state court cases relating to the Bair Hugger patient warming systems. Two are pending in Missouri state court and combine Bair Hugger product liability claims with medical malpractice claims. One of the Missouri cases was tried in September and October of 2022; the jury returned a verdict in 3M’s favor on all the claims. The trial court denied plaintiff’s motion for a new trial, and plaintiffs have filed a notice of appeal. The other Missouri case is scheduled for trial in September 2024. There is one case in Etowah County, Alabama that combines Bair Hugger product liability claims with medical malpractice claims. It is also set for trial in November 2024. A Texas case that 3M removed to federal court was remanded to state court in January 2024. Finally, a putative class action has been filed in Ramsey County, Minnesota, seeking economic damages for the use of the Bair Hugger system in orthopedic surgeries of medically obese people in Minnesota from May 2017 to the present. The Ramsey County court denied a motion to dismiss in August 2023. Three other state court cases have been resolved in 2023, including a Missouri state court case that was voluntarily dismissed in June 2023 and a Texas state court case that was voluntarily dismissed in September 2023. Three cases (two in Montana, and one in Pennsylvania) have been removed to federal court; plaintiffs’ motions to remand are pending.
3M had been named a defendant in 61 cases in Minnesota state court. In January 2018, the Minnesota state court excluded plaintiffs’ experts and granted 3M’s motion for summary judgment on general causation. The Minnesota Court of Appeals affirmed the state court orders in their entirety and the Minnesota Supreme Court denied plaintiffs’ petition for review and entered the final dismissal in 2019, effectively ending the Minnesota state court cases.
In June 2016, 3M was served with a putative class action filed in the Ontario Superior Court of Justice for all Canadian residents who underwent various joint arthroplasty, cardiovascular, and other surgeries and later developed surgical site infections that the representative plaintiff claims were due to the use of the Bair Hugger patient warming system. The representative plaintiff seeks relief (including punitive damages) under Canadian law based on theories similar to those asserted in the MDL.
For product liability litigation matters described in this section for which a liability has been recorded, the amount recorded is not material to the Company’s results of operations or financial condition. In addition, the Company is not able to estimate a possible loss or range of possible loss in excess of the recorded liability at this time.
Federal False Claims Act/Qui Tam Litigation
In October 2019, 3M acquired Acelity, Inc. and its KCI subsidiaries, including Kinetic Concepts, Inc. and KCI USA, Inc. As previously disclosed in the SEC filings by the KCI entities, in 2009, Kinetic Concepts, Inc. received a subpoena from the U.S. Department of Health and Human Services Office of Inspector General. In 2011, following the completion of the government’s review and its decision declining to intervene in two qui tam actions described further below, the qui tam relator-plaintiffs’ pleadings were unsealed.
The government inquiry followed two qui tam actions filed in 2008 by two former employees against Kinetic Concepts, Inc. and KCI USA, Inc. (collectively, the “KCI Defendants”) under seal in the U.S. District Court for the Central District of California. One qui tam action (the Godecke case) was dismissed in January 2022. In the remaining action (the Hartpence case), the complaint contains allegations that the KCI Defendants violated the federal False Claims Act by submitting false or fraudulent claims to federal healthcare programs by billing for 3M V.A.C. Therapy in a manner that was not consistent with the Local Coverage Determinations issued by the Durable Medical Equipment Medicare Administrative Contractors and seeks monetary damages.
In June 2019, the district court entered summary judgment in the KCI Defendants’ favor on all of the relator-plaintiff’s claims. The relator-plaintiff then filed an appeal in the U.S. Court of Appeals for the Ninth Circuit. Oral argument in the Hartpence case was held in July 2020. The appellate court issued an opinion in August 2022 reversing the decision of the district court and remanding the case for further proceedings. The district court held a status conference in January 2023 where no case deadlines were set; the litigation remains in a pre-trial stage. The KCI Defendants filed a renewed motion for summary judgment in March 2023. In July 2023, the parties filed a joint status report notifying the court of the parties’ agreement to mediate the matter in November 2023.
F-31
Health Care Business of 3M Company
Notes to Combined Financial Statements
As a result of a mediation held in November 2023, the relator-plaintiff and KCI reached an agreement in principle to settle the case and resolve all the remaining claims in this action, including the dismissal of the relator-plaintiff’s complaint with prejudice, subject to the agreement of the government and the parties’ negotiation and agreement of all remaining terms of the settlement. The KCI Defendants and relator-plaintiff have jointly requested that the court continue to hold in abeyance any hearing on the KCI Defendants’ pending Renewed Motion for Summary Judgment and any further proceedings in this case, to allow the parties to confer with counsel for the government and negotiate the remaining terms of the settlement agreement. The KCI Defendants and the relator-plaintiff submitted an updated status report to the court during January 2024.
For the matters described in this section for which a liability has been recorded, the amount recorded is not material to the Company’s combined results of operations or financial condition. The Company is not able to estimate a possible loss or range of possible loss in excess of the recorded liability at this time.
Compliance Matter
The Company, through its internal processes, discovered certain travel activities and related funding and record keeping issues raising concerns, arising from marketing efforts by certain business groups based in China. The Company initiated an internal investigation to determine whether the expenditures may have violated the U.S. Foreign Corrupt Practices Act (FCPA) or other potentially applicable anti-corruption laws. In July 2019, the Company voluntarily disclosed this investigation to both the Department of Justice (“DOJ”) and Securities and Exchange Commission (“SEC”) and cooperated with both agencies. In August 2023, the Company resolved the investigation with both above agencies. The DOJ closed its investigation with no action taken against the Company. Without admitting or denying the findings, the Company entered into a voluntary settlement with the SEC which found violations of the books and records and internal accounting controls provisions of Sections 13(b)(2)(A) and 13(b)(2)(B) of the Securities Exchange Act of 1934. The resolution includes an agreement to cease and desist from committing any violations of these provisions and a payment of approximately $6.5 million.
Warranties/Guarantees: The Company has not issued any material financial guarantees of loans with third parties or other guarantee arrangements. Furthermore, the Company does not disclose information on its product warranties, as management considers the balance immaterial to its combined results of operations and financial condition.
Note 12 – Leases
Operating lease costs for the years ended December 31, 2023, 2022 and 2021, were $28 million, $32 million, and $31 million, respectively. Finance lease, variable lease costs, short-term lease cost and income related to sub-lease activity is immaterial for the Company.
Supplemental balance sheet information related to leases is as follows:
| December 31, | ||||||||||||||||||||||||||
| (Millions unless noted) | Location on face of balance sheets | 2023 | 2022 | |||||||||||||||||||||||
| Operating leases: | ||||||||||||||||||||||||||
| Operating lease right of use assets | Other assets | $ | 98 | $ | 94 | |||||||||||||||||||||
| Current operating lease liabilities | Other current liabilities | $ | 29 | $ | 27 | |||||||||||||||||||||
| Noncurrent operating lease liabilities | Other liabilities | 69 | 68 | |||||||||||||||||||||||
| Total operating lease liabilities | $ | 98 | $ | 95 | ||||||||||||||||||||||
| Weighted average remaining lease term (in years) | 4.3 | 5.2 | ||||||||||||||||||||||||
| Weighted average discount rate | 2.9 | % | 2.2 | % | ||||||||||||||||||||||
F-32
Health Care Business of 3M Company
Notes to Combined Financial Statements
Supplemental cash flow and other information related to leases is as follows:
| For the years ended December 31, | ||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||
| Cash paid for amounts included in the measurement of lease liabilities | $ | 29 | $ | 31 | $ | 33 | ||||||||||||||||||||
| Right of use assets obtained in exchange for lease liabilities | 37 | 14 | 57 | |||||||||||||||||||||||
Maturities of operating lease liabilities were as follows:
| December 31, | ||||||||||||||
| (Millions) | 2023 | |||||||||||||
| 2024 | $ | 30 | ||||||||||||
| 2025 | 25 | |||||||||||||
| 2026 | 18 | |||||||||||||
| 2027 | 12 | |||||||||||||
| 2028 | 5 | |||||||||||||
| After 2028 | 11 | |||||||||||||
| Total | 101 | |||||||||||||
| Less: Amounts representing interest | (3) | |||||||||||||
| Present value of future minimum lease payments | 98 | |||||||||||||
| Less: Current obligations | 29 | |||||||||||||
| Long-term obligations | $ | 69 | ||||||||||||
Note 13 - Related Party Transactions and Corporate Allocations
Related Party Transactions
The Health Care Business has not entered into any related party transactions apart from those described below related to 3M. The Health Care Business has not historically entered into material arrangements with other businesses of 3M.
The Company participates in centralized 3M Treasury programs. This arrangement is not reflective of the manner in which the Company would have financed its operations had it been a stand-alone business separate from 3M during the periods presented. Centralized cash management arrangements are used to fund expansion and certain working capital needs. All adjustments relating to certain transactions among the Company and 3M, which include the transfer of the balance of cash to and from 3M, transfer of the balance of cash held in centralized cash management arrangements to and from 3M, and pushdown of all costs of doing business that were paid on behalf of the Company by 3M, are excluded from the asset and liability balances in the combined balance sheets and have instead been reported within Net parent investment as a component of equity.
Corporate Allocations
The combined statements of income include general corporate expenses of 3M for services provided by 3M for certain corporate and shared service functions that are provided on a centralized basis, including the use of shared assets. As stated in the “Basis of Presentation” section of Note 1, these expenses have been included on a direct usage basis where costs are specifically identifiable to the Health Care Business or allocated primarily based on the Health Care Business’s pro rata proportion of revenue.
Management believes that the expense allocations have been determined on a basis that is a reasonable reflection of the utilization of services provided for or the benefit received by the Company during each of the periods presented. The amounts that would have been, or will be incurred, on a stand-alone basis could materially differ from the amounts allocated due to economies of scale, a requirement for more or fewer employees, or other
F-33
Health Care Business of 3M Company
Notes to Combined Financial Statements
factors. Management does not believe, however, that it is practicable to estimate what these expenses would have been had the Company operated as an independent entity, including any expenses associated with obtaining any of these services from unaffiliated entities.
3M expense allocations were recorded in the combined statements of income within the following captions:
| For the years ended December 31, | ||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||
| Costs of product | $ | 89 | $ | 74 | $ | 106 | ||||||||||||||||||||
| Costs of software and rentals | — | — | — | |||||||||||||||||||||||
| Selling, general and administrative | 678 | 617 | 576 | |||||||||||||||||||||||
| Research and development | 86 | 87 | 96 | |||||||||||||||||||||||
| Total | $ | 853 | $ | 778 | $ | 778 | ||||||||||||||||||||
Net Parent investment
Net transfers to 3M are included within Net Parent investment from the combined statements of changes in equity and within financing activities in the combined statements of cash flows and represent the net effect of transactions between the Company and 3M. The reconciliation of net transfers to 3M between the combined statements of changes in equity and the combined statements of cash flows are as follows:
| For the years ended December 31, | ||||||||||||||||||||||||||
| (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||
| Net transfers to 3M per the combined statements of changes in equity | $ | (1,582) | $ | (1,363) | $ | (1,845) | ||||||||||||||||||||
| Stock compensation expense | (39) | (37) | (38) | |||||||||||||||||||||||
| Multiemployer pension expense | (32) | (56) | (64) | |||||||||||||||||||||||
| Net liabilities transferred from Parent | 100 | — | — | |||||||||||||||||||||||
| Net transfers to 3M per the combined statements of cash flows | $ | (1,553) | $ | (1,456) | $ | (1,947) | ||||||||||||||||||||
Note 14 - Segment and geographical information
Operating segments include components of an enterprise where separate financial information is available that is evaluated regularly by the Company’s Chief Operating Decision Maker (“CODM”) for the purpose of assessing performance and allocating resources. The Company’s CODM is the 3M Health Care Business Group President. Our operating activities are managed through four operating segments: MedSurg, Dental Solutions, Health Information Systems, and Purification and Filtration. Beginning in 2023, the Medical Solutions segment was renamed to MedSurg, the Oral Care Solutions segment was renamed to Dental Solutions, and the Separation and Purification Sciences segment was renamed to Purification and Filtration. There have been no changes to the composition of the segments or to financial information reported within each of the business segments. These segments have been identified based on the nature of the products sold and how the Company manages its operations. Transactions among reportable segments are recorded at cost. No operating segments have been aggregated to form reportable segments.
Corporate and Unallocated includes amortization of acquired intangible assets, restructuring related charges, costs or benefits from the capitalization of manufacturing variances and other net costs that the Company chose not to allocate directly to its business segments. Because Corporate and Unallocated includes a variety of miscellaneous items, it is subject to fluctuation on a quarterly and annual basis. Business segment operating income is reconciled to total operating income and pre-tax income below.
Consistent accounting policies have been applied by all segments for all reporting periods. A description of our reportable segments as of and for the years ended December 31, 2023, 2022 and 2021, has been provided in Note 1.
F-34
Health Care Business of 3M Company
Notes to Combined Financial Statements
Business Segment Offerings:
| Business Segment | Representative revenue-generating activities, products or services | |||||||||||||
| MedSurg | ◦Provider of solutions including advanced wound care, I.V. site management, sterilization assurance, temperature management, surgical supplies, stethoscopes, and medical electrodes | |||||||||||||
| Dental Solutions | ◦Provider of a comprehensive suite of dental and orthodontic products including brackets, aligners, restorative cements, and bonding agents | |||||||||||||
| Health Information Systems | ◦Provider of software solutions – including computer-assisted physician documentation, direct-to-bill and coding automation, classification methodologies, speech recognition, and data visualization platforms | |||||||||||||
| Purification and Filtration | ◦Provider of purification and filtration technologies including filters, purifiers, cartridges, and membranes | |||||||||||||
Business Segment Information
| For the years ended December 31, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Net Sales (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| MedSurg | $ | 4,632 | $ | 4,585 | $ | 4,632 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Dental Solutions | 1,329 | 1,327 | 1,396 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Information Systems | 1,285 | 1,227 | 1,160 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Purification and Filtration | 951 | 991 | 983 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Corporate and Unallocated | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total Company | $ | 8,197 | $ | 8,130 | $ | 8,171 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| For the years ended December 31, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Operating Income (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| MedSurg | $ | 1,107 | $ | 1,061 | $ | 1,226 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Dental Solutions | 442 | 437 | 482 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heath Information Systems | 423 | 359 | 354 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Purification and Filtration | 162 | 177 | 229 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Total business segment operating income | 2,134 | 2,034 | 2,291 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Corporate and Unallocated: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amortization expense | (365) | (373) | (381) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Corporate and Unallocated | (77) | 32 | (31) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total Corporate and Unallocated | $ | (442) | $ | (341) | $ | (412) | ||||||||||||||||||||||||||||||||||||||||||||||||||
Total Company operating income | 1,692 | 1,693 | 1,879 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other expense (income), net | 25 | 1 | (3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Income before income taxes | $ | 1,667 | $ | 1,692 | $ | 1,882 | ||||||||||||||||||||||||||||||||||||||||||||||||||
The Company does not report total assets by segment for internal or external reporting purposes as the Company’s CODM does not assess performance, make strategic decisions or allocate resources based on assets.
F-35
Health Care Business of 3M Company
Notes to Combined Financial Statements
Sales are generally reported within the geographic area that originated the invoice to the Health Care Business customer.
| For the years ended December 31, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Net Sales (Millions) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| United States | $ | 4,603 | $ | 4,450 | $ | 4,392 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| International | 3,594 | 3,680 | 3,779 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Worldwide | $ | 8,197 | $ | 8,130 | $ | 8,171 | ||||||||||||||||||||||||||||||||||||||||||||||||||
Long-lived assets include property, plant, and equipment, equipment rented to customers, as well as operating lease right-of-use assets. The following table presents long-lived assets based on the physical location of those assets.
| December 31, | ||||||||||||||||||||
| Long-lived assets (Millions) | 2023 | 2022 | ||||||||||||||||||
| United States | $ | 875 | $ | 836 | ||||||||||||||||
| Germany | 477 | 412 | ||||||||||||||||||
| Other countries | 268 | 236 | ||||||||||||||||||
| Worldwide | $ | 1,620 | $ | 1,484 | ||||||||||||||||
Note 15 – Subsequent Events
These combined financial statements are derived from the consolidated financial statements of 3M Company, which issued its annual financial statements for the fiscal year ended December 31, 2023 on February 7, 2024. Accordingly, the Company has evaluated recognizable subsequent events through the date of February 7, 2024 and non-recognizable subsequent events through February 20, 2024, the date these financial statements were available for issuance.
On February 16, 2024, the Company entered into a five-year $2,000 million unsecured revolving credit facility expiring in 2029, an 18-month unsecured term loan facility of $500 million and a three-year unsecured term loan facility of $1,000 million (together the “Facilities”). The Company intends to use the Facilities for general corporate purposes. There are no amounts outstanding under the Facilities.
F-36
Appendix A to Executive Compensation: Supplemental Consolidated Statement of Income Information
Reconciliation of GAAP to non-GAAP financial measures (dollars in millions, except per-share amounts) (unaudited)
In addition to reporting financial results in accordance with GAAP, 3M also periodically excludes special items to calculate financial metrics utilized for compensation plans. Some of the metrics are non-GAAP measures. Operating income, net income attributable to 3M, and diluted earnings per share attributable to 3M common shareholders (hereafter referred to as “earnings per diluted share”) are all measures for which 3M provides the reported GAAP measure and an adjusted measure (excluding special items). Adjusted Earnings per Share, Free Cash Flow, Free Cash Flow Growth (Free Cash Flow percentage change), Free Cash Flow Conversion, Return on Invested Capital, Operating Income (used for compensation purposes), and Operating Cash Flow Conversion, each adjusted for special items are non-GAAP measures that 3M believes are meaningful in measuring the payouts for its compensation plans. These measures are not in accordance with, nor are they a substitute for, GAAP measures. Special items represent significant charges or credits that are important to an understanding of 3M’s ongoing operations. The non-GAAP financial measures used herein may not be comparable to similarly titled measures used by other companies, and the adjusted amounts used for compensation purposes may differ from the adjusted amounts used by 3M elsewhere or included in 3M’s Form 10-K. Each reference to a non-GAAP financial measure used in the Compensation Discussion & Analysis section refers to the applicable measure as used for compensation purposes, after application of the adjustments of special items set forth in the tables below.
The reconciliations provided below reconcile the non-GAAP financial measures with the most directly comparable GAAP financial measures for the years ended December 31, 2023, 2022 and 2021.
Performance share award metrics:
Adjusted Earnings per Share used for compensation purposes (non-GAAP measure)
| 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reported measure | Net Costs for Significant Litigation (a) | Gain on Business Divestitures (b) | Divestiture Costs (c) | Russia Exit Charges (Benefits) (d) | Manufactured PFAS Products (e) | Adjusted non-GAAP measure used for 2021 and 2022 PSAs | Adjustments Approved for 2023 PSA (f) | Adjusted non-GAAP measure used for 2023 PSAs | ||||||||||||||||||||||||||||||||||||||||||||||||
| Net income (loss) attributable to 3M | $ | (6,995) | $ | 11,630 | $ | (25) | $ | 378 | $ | (21) | $ | 155 | $ | 5,122 | $ | 340 | $ | 5,462 | ||||||||||||||||||||||||||||||||||||||
| Earnings (loss) per diluted share | $ | (12.63) | $ | 21.00 | $ | (0.05) | $ | 0.68 | $ | (0.04) | $ | 0.28 | $ | 9.24 | $ | 0.60 | $ | 9.84 | ||||||||||||||||||||||||||||||||||||||
| Earnings (loss) per diluted share percent change | (224.1 | %) | -6.5 | % | -0.4 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 2022 | ||||||||||||||||||||||||||||||||||||||||||||
| Reported measure | Net Costs for Significant Litigation (a) | Food Safety Divestiture, Net of Restructuring Items (b) | Divestiture Costs (c) | Russia Exit Charges (d) | PFAS Manufacturing Exit (e) | Adjusted non-GAAP measure used for compensation purposes | ||||||||||||||||||||||||||||||||||||||
| Net income attributable to 3M | $ | 5,777 | $ | 1,815 | $ | (2,648) | $ | 42 | $ | 111 | $ | 638 | $ | 5,735 | ||||||||||||||||||||||||||||||
| Earnings per diluted share | $ | 10.18 | $ | 3.20 | $ | (4.67) | $ | 0.07 | $ | 0.20 | $ | 1.12 | $ | 10.10 | ||||||||||||||||||||||||||||||
| Earnings per diluted share percent change | 0.6 | % | -0.2 | % | ||||||||||||||||||||||||||||||||||||||||
A-1
| 2021 | ||||||||
Reported measure (g) | ||||||||
| Net income attributable to 3M | $ | 5,921 | ||||||
| Earnings per diluted share | $ | 10.12 | ||||||
| Earnings per diluted share percent change | 14.4 | % | ||||||
Free Cash Flow used for compensation purposes (non-GAAP measure)
| 2023 | ||||||||||||||||||||||||||||||||||||||||||||
| Reported measure | Net Costs for Significant Litigation and TCJA (a) | Gain on Business Divestitures, Net of Divestiture-Related Restructuring Items (b) | Divestiture Costs (c) | Russia Exit Charges (d) | Manufactured PFAS Products (e) | Adjusted non-GAAP measure used for compensation purposes | ||||||||||||||||||||||||||||||||||||||
Major GAAP cash flow categories | ||||||||||||||||||||||||||||||||||||||||||||
| Net cash provided by operating activities | $ | 6,680 | ||||||||||||||||||||||||||||||||||||||||||
| Net cash provided by (used in) investing activities | (1,207) | |||||||||||||||||||||||||||||||||||||||||||
| Net cash used in financing activities | (3,147) | |||||||||||||||||||||||||||||||||||||||||||
Free Cash Flow (non-GAAP measure) | ||||||||||||||||||||||||||||||||||||||||||||
| Net cash provided by operating activities | $ | 6,680 | $ | 895 | $ | 11 | $ | 313 | $ | — | $ | (157) | $ | 7,742 | ||||||||||||||||||||||||||||||
| Purchases of property, plant and equipment | (1,615) | 167 | (1,448) | |||||||||||||||||||||||||||||||||||||||||
Free cash flow(h) | $ | 5,065 | $ | 895 | $ | 11 | $ | 313 | $ | — | $ | 10 | $ | 6,294 | ||||||||||||||||||||||||||||||
| Free cash flow percent change | 31.8 | % | 30.3 | % | ||||||||||||||||||||||||||||||||||||||||
| Net income attributable to 3M | $ | (6,995) | $ | 11,630 | $ | (25) | $ | 378 | $ | (21) | $ | 155 | $ | 5,122 | ||||||||||||||||||||||||||||||
Free cash flow conversion(h) | (72 | %) | 123 | % | ||||||||||||||||||||||||||||||||||||||||
| 2022 | ||||||||||||||||||||||||||||||||||||||||||||
| Reported measure | Significant Litigation Related Charges and TCJA (a) | Food Safety Divestiture, Net of Restructuring Items (b) | Divestiture Costs (c) | Russia Exit Charges (d) | PFAS Manufacturing Exit (e) | Adjusted non-GAAP measure used for compensation purposes | ||||||||||||||||||||||||||||||||||||||
Major GAAP cash flow categories | ||||||||||||||||||||||||||||||||||||||||||||
| Net cash provided by operating activities | $ | 5,591 | ||||||||||||||||||||||||||||||||||||||||||
| Net cash provided by (used in) investing activities | (1,046) | |||||||||||||||||||||||||||||||||||||||||||
| Net cash used in financing activities | (5,350) | |||||||||||||||||||||||||||||||||||||||||||
Free Cash Flow (non-GAAP measure) | ||||||||||||||||||||||||||||||||||||||||||||
| Net cash provided by operating activities | $ | 5,591 | $ | 784 | $ | 55 | $ | 8 | $ | 2 | $ | — | $ | 6,440 | ||||||||||||||||||||||||||||||
| Purchases of property, plant and equipment | (1,749) | (1,749) | ||||||||||||||||||||||||||||||||||||||||||
Free cash flow(h) | $ | 3,842 | $ | 784 | $ | 55 | $ | 8 | $ | 2 | $ | — | $ | 4,691 | ||||||||||||||||||||||||||||||
| Free cash flow percent change | -34.3 | % | -21.5 | % | ||||||||||||||||||||||||||||||||||||||||
| Net income attributable to 3M | $ | 5,777 | $ | 1,815 | $ | (2,648) | $ | 42 | $ | 111 | $ | 638 | $ | 5,735 | ||||||||||||||||||||||||||||||
Free cash flow conversion(h) | 66 | % | 82 | % | ||||||||||||||||||||||||||||||||||||||||
A-2
| 2021 | ||||||||||||||||||||
| Reported measure | Adjustment for payment of previous excluded items(a) | Adjusted non-GAAP measure used for compensation purposes | ||||||||||||||||||
Major GAAP cash flow categories | ||||||||||||||||||||
| Net cash provided by operating activities | $ | 7,454 | ||||||||||||||||||
| Net cash provided by (used in) investing activities | (1,317) | |||||||||||||||||||
| Net cash used in financing activities | (6,145) | |||||||||||||||||||
Free cash flow (non-GAAP measure) | ||||||||||||||||||||
| Net cash provided by operating activities | $ | 7,454 | $ | 123 | $ | 7,577 | ||||||||||||||
| Purchases of property, plant and equipment | (1,603) | $ | (1,603) | |||||||||||||||||
Free cash flow(h) | $ | 5,851 | $ | 123 | $ | 5,973 | ||||||||||||||
| Net income attributable to 3M | $ | 5,921 | $ | — | $ | 5,921 | ||||||||||||||
Free cash flow conversion(h) | 99 | % | 101 | % | ||||||||||||||||
Return on invested capital (non-GAAP measure)
| 2023 | ||||||||||||||||||||||||||||||||||||||||||||
| Reported measure | Net Costs for Significant Litigation and TCJA (a) | Gain on Business Divestitures (b) | Divestiture Costs (c) | Russia Exit Charges (Benefits) (d) | Manufactured PFAS Products (e) | Adjusted non-GAAP measure used for compensation purposes | ||||||||||||||||||||||||||||||||||||||
Net income including non-controlling interest | $ | (6,979) | $ | 11,630 | $ | (25) | $ | 378 | $ | (21) | $ | 155 | $ | 5,138 | ||||||||||||||||||||||||||||||
| Interest expense (after-tax) (1) | 680 | (271) | — | — | — | — | 409 | |||||||||||||||||||||||||||||||||||||
| Adjusted net income (Return) | $ | (6,299) | $ | 11,359 | $ | (25) | $ | 378 | $ | (21) | $ | 155 | $ | 5,547 | ||||||||||||||||||||||||||||||
| Average shareholders’ equity (including non-controlling interest) (2) | $ | 8,202 | $ | 9,058 | $ | — | $ | 87 | $ | — | $ | (525) | $ | 16,822 | ||||||||||||||||||||||||||||||
| Average short-term and long-term debt (3) | 15,999 | — | — | — | — | — | 15,999 | |||||||||||||||||||||||||||||||||||||
| Average invested capital | $ | 24,201 | $ | 9,058 | $ | — | $ | 87 | $ | — | $ | (525) | $ | 32,821 | ||||||||||||||||||||||||||||||
Return on Invested Capital(i) | (26.0 | %) | 16.9 | % | ||||||||||||||||||||||||||||||||||||||||
| (1) Effective income tax rate used for interest expense | 27.8 | % | 27.8 | % | ||||||||||||||||||||||||||||||||||||||||
| (2) Calculation of average equity (includes non-controlling interest) | ||||||||||||||||||||||||||||||||||||||||||||
| Ending total equity as of: | ||||||||||||||||||||||||||||||||||||||||||||
| March 31 | $ | 15,351 | $ | 1,713 | $ | — | $ | 68 | $ | — | $ | (510) | $ | 16,622 | ||||||||||||||||||||||||||||||
| June 30 | 7,857 | 9,477 | — | 95 | — | (550) | 16,879 | |||||||||||||||||||||||||||||||||||||
| September 30 | 4,731 | 12,556 | — | 100 | — | (545) | 16,842 | |||||||||||||||||||||||||||||||||||||
| December 31 | 4,868 | 12,486 | — | 85 | — | (494) | 16,945 | |||||||||||||||||||||||||||||||||||||
| Average total equity | $ | 8,202 | $ | 9,058 | $ | — | $ | 87 | $ | — | $ | (525) | $ | 16,822 | ||||||||||||||||||||||||||||||
| (3) Calculation of average debt | ||||||||||||||||||||||||||||||||||||||||||||
| Ending short-term and long-term debt as of: | ||||||||||||||||||||||||||||||||||||||||||||
| March 31 | $ | 15,960 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 15,960 | ||||||||||||||||||||||||||||||
| June 30 | 15,987 | — | — | — | — | — | 15,987 | |||||||||||||||||||||||||||||||||||||
| September 30 | 16,013 | — | — | — | — | — | 16,013 | |||||||||||||||||||||||||||||||||||||
| December 31 | 16,035 | — | — | — | — | — | 16,035 | |||||||||||||||||||||||||||||||||||||
| Average short-term and long-term debt | $ | 15,999 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 15,999 | ||||||||||||||||||||||||||||||
A-3
| 2022 | ||||||||||||||||||||||||||||||||||||||||||||
| Reported measure | Significant Litigation Related Charges and TCJA (a) | Food safety divestiture, net of restructuring items (b) | Divestiture Costs (c) | Russia Exit Charges (d) | PFAS manufacturing exit (e) | Adjusted non-GAAP measure used for compensation purposes | ||||||||||||||||||||||||||||||||||||||
Net income including non-controlling interest | $ | 5,791 | $ | 1,497 | $ | (2,648) | $ | 42 | $ | 111 | $ | 638 | $ | 5,431 | ||||||||||||||||||||||||||||||
| Interest expense (after-tax) (1) | 418 | — | — | — | — | — | 418 | |||||||||||||||||||||||||||||||||||||
| Adjusted net income (Return) | $ | 6,209 | $ | 1,497 | $ | (2,648) | $ | 42 | $ | 111 | $ | 638 | $ | 5,849 | ||||||||||||||||||||||||||||||
| Average shareholders’ equity (including non-controlling interest) (2) | $ | 14,437 | $ | 1,036 | $ | 17 | $ | 8 | $ | 54 | $ | 160 | $ | 15,711 | ||||||||||||||||||||||||||||||
| Average short-term and long-term debt (3) | 16,149 | — | — | — | — | — | 16,149 | |||||||||||||||||||||||||||||||||||||
| Average invested capital | $ | 30,586 | $ | 1,036 | $ | 17 | $ | 8 | $ | 54 | $ | 160 | $ | 31,860 | ||||||||||||||||||||||||||||||
Return on Invested Capital(i) | 20.3 | % | 18.4 | % | ||||||||||||||||||||||||||||||||||||||||
| (1) Effective income tax rate used for interest expense | 9.6 | % | 9.6 | % | ||||||||||||||||||||||||||||||||||||||||
| (2) Calculation of average equity (includes non-controlling interest) | ||||||||||||||||||||||||||||||||||||||||||||
| Ending total equity as of: | ||||||||||||||||||||||||||||||||||||||||||||
| March 31 | $ | 15,004 | $ | 173 | $ | — | $ | — | $ | — | $ | — | $ | 15,177 | ||||||||||||||||||||||||||||||
| June 30 | 13,816 | 1,412 | — | — | — | — | 15,228 | |||||||||||||||||||||||||||||||||||||
| September 30 | 14,156 | 1,356 | 66 | 1 | 109 | — | 15,688 | |||||||||||||||||||||||||||||||||||||
| December 31 | 14,770 | 1,202 | 2 | 31 | 108 | 638 | 16,751 | |||||||||||||||||||||||||||||||||||||
| Average total equity | $ | 14,437 | $ | 1,036 | $ | 17 | $ | 8 | $ | 54 | $ | 160 | $ | 15,711 | ||||||||||||||||||||||||||||||
| (3) Calculation of average debt | ||||||||||||||||||||||||||||||||||||||||||||
| Ending short-term and long-term debt as of: | ||||||||||||||||||||||||||||||||||||||||||||
| March 31 | $ | 16,678 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 16,678 | ||||||||||||||||||||||||||||||
| June 30 | 16,276 | — | — | — | — | — | 16,276 | |||||||||||||||||||||||||||||||||||||
| September 30 | 15,705 | — | — | — | — | — | 15,705 | |||||||||||||||||||||||||||||||||||||
| December 31 | 15,939 | — | — | — | — | — | 15,939 | |||||||||||||||||||||||||||||||||||||
| Average short-term and long-term debt | $ | 16,149 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 16,149 | ||||||||||||||||||||||||||||||
A-4
| 2021 | ||||||||
| Reported measure | ||||||||
Net income including non-controlling interest | $ | 5,929 | ||||||
| Interest expense (after-tax) (1) | 400 | |||||||
| Net income (Return) | $ | 6,329 | ||||||
| Average shareholders’ equity (including non-controlling interest) (2) | $ | 14,497 | ||||||
| Average short-term and long-term debt (3) | 17,991 | |||||||
| Average invested capital | $ | 32,488 | ||||||
Return on Invested Capital(i) | 19.5 | % | ||||||
| (1) Effective income tax rate used for interest expense | 17.8 | % | ||||||
| (2) Calculation of average equity (includes non-controlling interest) | ||||||||
| Ending total equity as of: | ||||||||
| March 31 | $ | 13,828 | ||||||
| June 30 | 14,516 | |||||||
| September 30 | 14,530 | |||||||
| December 31 | 15,117 | |||||||
| Average total equity | $ | 14,497 | ||||||
| (3) Calculation of average debt | ||||||||
| Ending short-term and long-term debt as of: | ||||||||
| March 31 | $ | 18,187 | ||||||
| June 30 | 18,248 | |||||||
| September 30 | 18,165 | |||||||
| December 31 | 17,363 | |||||||
| Average short-term and long-term debt | $ | 17,991 | ||||||
AIP metrics:
| 2023 | |||||||||||||||||||||||||||||||||||||||||||||||
| Reported Measure | Net Costs for Significant Litigation and TCJA(a) | Gain on Business Divestitures, Net of Divestiture-Related Restructuring Items(b) | Divestiture Costs(c) | Russia Exit Charges (Benefits)(d) | Manufactured PFAS Products(e) | Adjustments for Business Results not in the Plan(j) | Adjusted non-GAAP measure used for compensation purposes | ||||||||||||||||||||||||||||||||||||||||
Income Statement | |||||||||||||||||||||||||||||||||||||||||||||||
| Net Sales | $ | 32,681 | $ | (1,289) | $ | (62) | $ | 31,330 | |||||||||||||||||||||||||||||||||||||||
| Net Sales-Local Currency | $ | 32,865 | $ | (1,289) | $ | (62) | $ | 31,514 | |||||||||||||||||||||||||||||||||||||||
| Operating Income | (9,128) | $ | 14,869 | $ | (36) | $ | 496 | $ | (18) | $ | 205 | $ | (14) | 6,374 | |||||||||||||||||||||||||||||||||
Operating Income Margin | (27.9) | % | 20.3 | % | |||||||||||||||||||||||||||||||||||||||||||
Operating Cash Flow Conversion (non-GAAP measure) | |||||||||||||||||||||||||||||||||||||||||||||||
| Net cash provided by operating activities | $ | 6,680 | $ | 895 | $ | 11 | $ | 313 | $ | — | $ | (157) | $ | 222 | $ | 7,964 | |||||||||||||||||||||||||||||||
| Net Income Attributable to 3M | $ | (6,995) | $ | 11,630 | $ | (25) | $ | 378 | $ | (21) | $ | 155 | $ | 364 | $ | 5,486 | |||||||||||||||||||||||||||||||
Operating Cash Flow Conversion(k) | (95 | %) | 145 | % | |||||||||||||||||||||||||||||||||||||||||||
__________________
(a)Net costs for significant litigation relate to 3M's respirator mask/asbestos (which include Aearo and non-Aearo items), PFAS-related other environmental, and Combat Arms Earplugs matters (as discussed in Note 18 to 3M Company’s Form 10-K). Net costs include the impacts of changes in accrued liabilities (including interest imputation on applicable settlement obligations), external legal fees, and insurance recoveries, along with the associated tax impacts. 3M does not consider the elements of the net costs associated with these matters to be normal operating expenses related to 3M’s ongoing operations, revenue generating activities, business strategy, industry, and regulatory
A-5
environment. Cash Flow and ROIC balance sheet adjustments also include the impact from payments related to the Tax Cuts and Jobs Act (TCJA).
(b)In 2023, 3M recorded a gain related to the sale of its dental local anesthetic business partially offset by a loss associated with a previously contingent indemnification obligation from a 2020 divestiture. In 2022, 3M recorded a gain related to the split-off and combination of its Food Safety business with Neogen Corporation. Refer to Note 3 to 3M Company’s Form 10-K for further details. Also in 2022, following the split-off of the Food Safety business, management approved and committed to undertake certain restructuring actions addressing corporate functional costs across 3M in relation to the magnitude of amounts previously allocated to the divested businesses. Refer to Note 5 to 3M Company’s Form 10-K for further details.
(c)Divestiture costs related to separating and divesting substantially an entire business segment of 3M following public announcement of its intended divestiture.
(d)In 2023, 3M recorded a gain on final disposal of net assets in Russia. Previously, in 2022, 3M recorded a charge primarily related to impairment of these assets in connection with management's committed exit and disposal plan. Refer to Note 17 to 3M Company’s Form 10-K for further details.
(e)For 2023, these relate to sales and estimates of income (loss) regarding manufactured PFAS products that 3M plans to exit by the end of 2025. Estimated income does not contemplate impacts on non-operating items such as net interest income/expense and the non-service cost components portion of defined benefit plan net periodic benefit costs. Relative to the impact of the activity of manufactured PFAS products on cash provided by (used in) operating activities, amounts are based on estimates of associated income, depreciation/amortization, certain changes in working capital and accruals, and timing of associated payments. For 2022, these relate just to costs associated with 3M's 2022 commitment to a plan to exit PFAS manufacturing; charges relate to asset impairments.
(f)When the 3M Compensation and Talent Committee approved the targets for the 2023 Performance Share Plan, it provided an exclusion to the Organic Sales Growth and Adjusted Earnings per Share Growth metrics from the impact from the loss of the Russia sales and net income from 2022, lower sales and net income from the lower 2023 disposable respirator sales and net income and the impact of foreign exchange to net income.
(g)In the first quarter of 2021, 3M changed the method it uses to calculate the market-related value of fixed income securities included in its pension and other post-retirement plan assets. This change was applied retrospectively to all periods presented within 3M’s financial statements. The change did not impact consolidated operating income or net cash provided by operating activities but did impact the previously reported portion of pension and post-retirement net periodic benefit cost (benefit) that was included within non-operating other expense (income) along with related consolidated income items such as net income and earnings per share. The 2020 restatement increased 2020 net income by $65 million and diluted earnings per share by $0.11. This increases the 2020 adjusted diluted earnings per share from $8.74 to $8.85 on a restated basis. The diluted earnings per share of $8.85 is the base amount for calculating the 14.4 percent earnings per diluted share percent increase in 2021.
(h)Free Cash Flow and Free Cash Flow Conversion are not defined under GAAP. Therefore, they should not be considered a substitute for income or cash flow data prepared in accordance with GAAP and may not be comparable to similarly titled measures used by other companies. 3M defines Free Cash Flow as net cash provided by operating activities less purchases of property, plant and equipment. 3M defines Free Cash Flow Conversion as Free Cash Flow divided by net income attributable to 3M. 3M defines adjusted net cash provided by (used in) operating activities as net cash provided by operating activities, adjusted for special items. 3M defines adjusted purchases of property, plant and equipment (also referred to as adjusted capital expenditures) as purchases of property, plant and equipment (PPE) adjusted for the estimated impact of such purchases associated with manufactured PFAS products activity. 3M defines Free Cash Flow used for compensation purposes as adjusted net cash provided by (used in) operating activities less adjusted purchases of PPE. Cash payments or receipts associated with special items in the determination of adjusted net cash provided by (used in) operating activities are reflected net of applicable tax. For most special items, the cash tax impact is estimated using the U.S. statutory corporate tax rate during the period of payment or receipt. However, the cash tax impact for the portion of payments of costs for significant litigation under the 2023 settlement agreements relative to Combat Arms Earplugs and relative to public water systems regarding PFAS is based on the timing and amount of the actual cash tax deduction (which differs from the timing of the pre-tax settlement payments). 3M defines Free Cash Flow Conversion for compensation purposes as Free Cash Flow used for compensation purposes divided by net income (loss) attributable to 3M, adjusted for special items. 3M believes Free Cash Flow and Free Cash Flow Conversion (including the versions of those metrics used for compensation purposes) are meaningful to investors as they are useful measures of performance and 3M uses these measures as an indication of the strength of 3M and its ability to generate cash. The terms “Free Cash Flow” and “Free Cash Flow Conversion” used in the Compensation Discussion and Analysis section in each instance refer to Free Cash Flow and Free Cash Flow Conversion used for compensation purposes.
(i)Return on Invested Capital (ROIC) is not defined under GAAP. Therefore, ROIC should not be considered a substitute for other measures prepared in accordance with GAAP and may not be comparable to similarly titled measures used by other companies. 3M defines ROIC as adjusted net income (net income including non-controlling interest plus after-tax interest expense) divided by average invested capital (equity plus debt). 3M believes ROIC is meaningful to investors as it focuses on shareholder value creation.
(j)The AIP is measured versus the plan. Results are adjusted for acquisitions, divestitures, and certain transactions such as restructurings not included in the plan.
(k)Operating Cash Flow Conversion and Operating Cash Flow Conversion used for compensation purposes are not defined under GAAP. Therefore, they should not be considered a substitute for income or cash flow data prepared in accordance with GAAP and may not be comparable to similarly titled measures used by other companies. 3M defines Operating Cash Flow Conversion as operating cash flow divided by net income attributable to 3M. 3M defines Operating Cash Flow Conversion used for compensation purposes as operating cash flow, adjusted for special items, divided by net income attributable to 3M, adjusted for special items. For most special items, the cash tax impact is estimated using the U.S. statutory corporate tax rate during the period of payment or receipt. However, the cash tax impact for the portion of payments of costs for significant litigation under the 2023 settlement agreements relative to Combat Arms Earplugs and relative to public water systems regarding PFAS is based on the timing and amount of the actual cash tax deduction (which differs from the timing of the pre-tax settlement payments). The term “Operating Cash Flow Conversion” used in the Compensation Discussion and Analysis section refers to Operating Cash Flow Conversion used for compensation purposes.
A-6
Appendix B to Executive Compensation: Certain Defined Terms
Except as otherwise noted, capitalized terms used in the Compensation Discussion and Analysis section have the meaning specified below.
| Adjusted Net Income | means the net income of 3M as reported in its Consolidated Statement of Income, as adjusted to exclude special items. | |||||||
| AIP | means the broad-based Annual Incentive Plan by which 3M provides annual incentive compensation to approximately 32,000 eligible employees. | |||||||
| Adjusted Earnings per Share (EPS) Growth | means the percentage increase or decrease in 3M’s diluted earnings per share attributable to 3M common shareholders (as reported in its Consolidated Statement of Income) for a year as compared to the previous year, in each case, as adjusted to exclude certain special items. | |||||||
| Free Cash Flow | means the sum of 3M’s operating cash flows minus capital expenditures, as adjusted to exclude certain special items. | |||||||
| Free Cash Flow Conversion | means the sum of 3M’s operating cash flows minus capital expenditures, divided by net income, as adjusted to exclude certain special items. | |||||||
| Free Cash Flow Growth | means the percentage increase or decrease in 3M’s Free Cash Flow for a year as compared to the previous year. | |||||||
| GAAP | means generally accepted accounting principles in the United States. | |||||||
| Local Currency Sales | means the net sales of 3M (as reported in its Consolidated Statement of Income) or a business unit, in local currency, adjusted to exclude the impact of acquisitions or divestitures in the year each acquisition or divestiture is completed (unless such acquisition or divestiture is included in the operating plan for the business unit). | |||||||
| Net Cash Provided by Operating Activities | means the amount of cash generated by the regular operating activities of 3M, calculated as Net Income less non-cash expenses and adjusted for changes in working capital. The amount of cash generated by the regular operating activities of 3M will not include cash generated from activities of the type included in the investing (such as capital expenditures, sales of equipment, investment activity, acquisition, and divestitures) or financing sections (such as debt and equity transactions) of 3M’s cash flow statement. | |||||||
| Net Income | means Net Sales minus (a) all Operating Expenses and (b) all interest, taxes, and other non-operating expenses, in each case, of 3M or a business unit. | |||||||
| Net Sales | means gross sales of the relevant business unit minus returns, allowances, customer rebates, trade promotion funds, cash discounts, and other sources of variable consideration, as reflected in the consolidated financial statements and related notes set forth in 3M’s Annual Report on Form 10-K for the year ended December 31, 2023. | |||||||
| Operating Cash Flow | means the Net Cash Provided by Operating Activities. | |||||||
| Operating Cash Flow Conversion | means the Net Cash Provided by Operating Activities divided by Net Income. | |||||||
| Operating Expenses | means all costs and expenses that are related in any way to the operating activities of 3M or a business unit, including all costs and expenses that are part of cost of goods sold; selling, general and administrative expenses; research and development expenses; depreciation and amortization; and gains or losses on sales of businesses or equipment. | |||||||
| Operating Income | means Net Sales minus Operating Expenses. | |||||||
B-1
| Organic Sales Growth | means the percentage amount by which 3M’s net sales (as reported in its Consolidated Statement of Income) for a year increase or decrease as compared to the previous year. For this purpose, 3M’s net sales will be adjusted to exclude currency effects and neutralize sales attributable to acquisitions or divestitures for the 12-month period following the date each acquisition is completed. | |||||||
| Peer Groups | means both 3M’s executive compensation peer group and the survey peer group, each as described under “Use of market data” in the Compensation Discussion and Analysis section. | |||||||
| Relative Organic Volume Growth | means the amount by which the percentage increase or decrease in 3M’s net sales (as reported in its Consolidated Statement of Income) for a year as compared to the previous year exceeds the percentage increase or decrease in worldwide real sales growth over the same period, as reflected in the Worldwide Industrial Production Index published by S&P Global Market Intelligence. For this purpose, 3M’s net sales are adjusted to neutralize price and currency effects and, during the 12-month period following the date of each acquisition or divestiture, the sales attributable to such acquired or divested business or products. | |||||||
| Relative Organic Sales Growth | means the amount by which 3M’s Organic Sales Growth for a year exceeds the percentage increase or decrease in a market benchmark measured over the same period. For this purpose, the market benchmark is a blend of Worldwide Industrial Production Index and Global Domestic Product, in each case, as published by S&P Global Market Intelligence no later than 30 days following completion of the relevant year. | |||||||
| Return on Invested Capital (ROIC) | means the operating income of 3M (as reported in its Consolidated Statement of Income), plus interest income and minus income taxes, adjusted to exclude certain special items and the impact of acquisitions in the year each acquisition is completed, divided by the average invested capital (equity plus debt, as reported in its Consolidated Balance Sheet). | |||||||
| Total Cash Compensation | means the total of an individual’s base salary and annual incentive compensation. | |||||||
| Total Direct Compensation | means the total of an individual’s Total Cash Compensation plus the compensation value of their annual long-term incentive compensation awards (which is based on their grant date fair value as measured under accounting standards). | |||||||
| Worldwide GDP | means the Worldwide Gross Domestic Product for a specified period, as reported by S&P Global Market Intelligence. | |||||||
| Worldwide IPI | means the Worldwide Industrial Production Index for a specified period, as reported by S&P Global Market Intelligence. | |||||||
B-2