expensive than products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market or make our development more complicated. The key competitive factors affecting the success of all of our programs are likely to be efficacy, safety and convenience.
These competitors may also vie for a similar pool of qualified scientific and management talent, sites and patient populations for clinical trials, as well as for technologies complementary to, or necessary for, our programs.
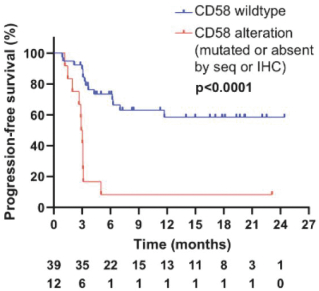
Certain competitor data
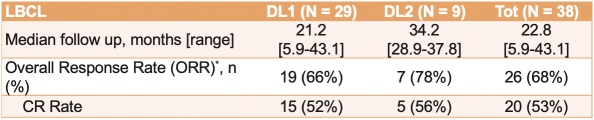
There are three currently approved CD19 CAR T-cell therapies for the treatment of LBCL. Select published clinical data from current FDA-approved CD19 CAR T-cell therapies in development for the treatment of LBCL are presented below.
Axicabtagene ciloleucel (Yescarta)
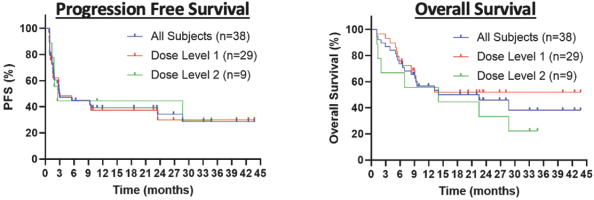
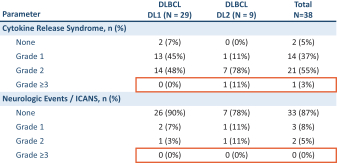
In a Phase 2 clinical trial of ZUMA-1, a single-arm, multi-center, registrational trial, Yescarta was administered to 101 patients. After 11.6 months of follow-up, the ORR and CR rate were 72% and 51%, respectively. At 18 months, the ORR and CR rate were 82% and 54%, respectively, and Grade 3 or higher CRS and neurologic events occurred in 13% and 28% of patients, respectively. After 2 years of follow-up, the ORR, CR rate and PFS were 83%, 54% and 39%, respectively, as compared to after 5 years of follow-up, where the ORR, CR rate and PFS were 83%, 58% and 32%, respectively.
In a Phase 3 clinical trial of ZUMA-7, a randomized, open-label, multi-center trial, Yescarta was administered to 180 patients and supported the initial treatment in adults with 2L R/R LBCL. After 14.7 months of follow-up, the ORR, CR rate and PFS were 83%, 65% and 50%, respectively, and Grade 3 or higher CRS and neurologic events occurred in 7% and 25% of patients, respectively. After 4 years of follow-up, the ORR, CR rate and PFS were 83%, 65% and 42%, respectively.
Tisagenlecleucel (Kymriah)
In a Phase 2 clinical trial of JULIET, an open-label, multi-center, single-arm trial, Kymriah was administered to 68 patients. At 9.4 months, the ORR and CR rate were 50% and 32%, respectively, and Grade 3 or higher CRS and neurologic events occurred in 22% and 12% of patients, respectively. At 24 months of follow-up, the ORR and CR rate were 52% and 38%, respectively, as compared to after 40.3 months of follow-up, where the ORR and CR rate were 53% and 39%, respectively. After 36 months of follow-up, the PFS was 31%.
Lisocabtagene maraleucel (Breyanzi)
In the pivotal TRANSCEND NHL 001 clinical trial, Breyanzi was administered to 192 patients. After 18.8 months of follow-up, the ORR and CR rate were 73% and 54%, respectively, and Grade 3 or higher CRS and neurologic events occurred in 2% and 10% of patients, respectively. After 2 years of follow-up, the ORR, CR rate and PFS were 73%, 53% and 41%, respectively.
In the pivotal Phase 3 TRANSFORM clinical trial, Breyanzi was administered to 92 patients and supported the initial treatment in adults with 2L R/R LBCL. At 6.2 months, the ORR and CR rate were 84% and 66%, respectively, and Grade 3 or higher CRS and neurologic events occurred in 1% and 7% of patients, respectively. After 17.5 months of follow-up, the ORR, CR rate and PFS were 87%, 74% and 58%, respectively.
146

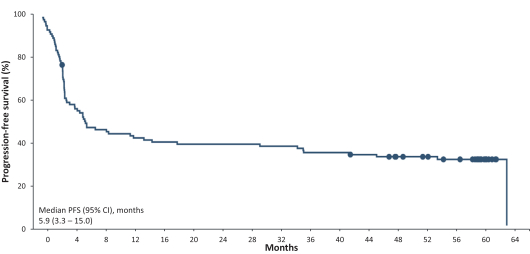
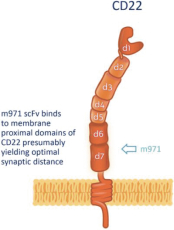
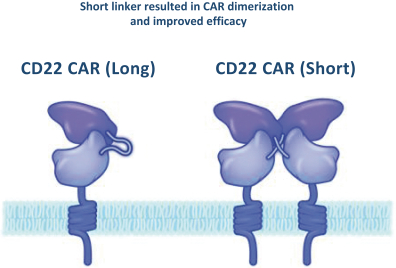
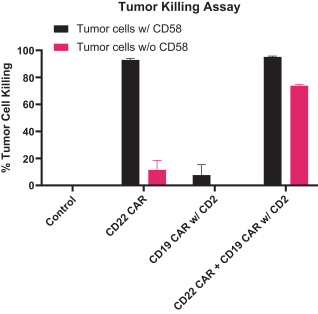
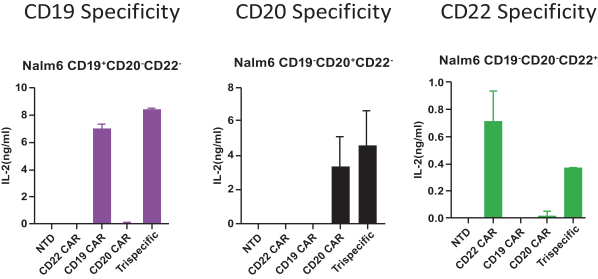
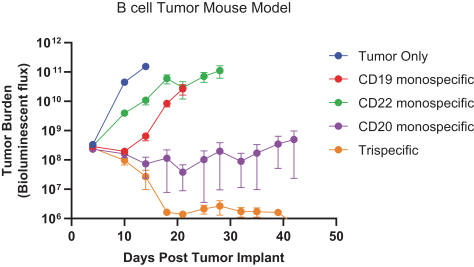
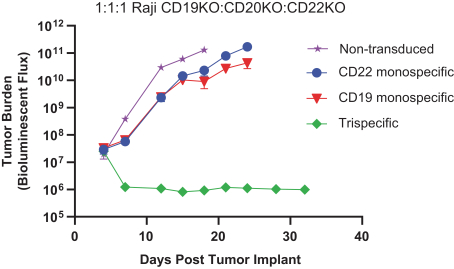
 .. It has been shown in a third-party study that the choice of costimulatory domain influences the persistence and memory phenotype of CAR T Cells. The inclusion of a 4-1BB costimulatory domain has been associated with reduced frequencies of serious adverse events and improved clinical outcomes in tumor models. The CD22 CAR used to create CRG-022 contains a 4-1BB costimulatory domain.
.. It has been shown in a third-party study that the choice of costimulatory domain influences the persistence and memory phenotype of CAR T Cells. The inclusion of a 4-1BB costimulatory domain has been associated with reduced frequencies of serious adverse events and improved clinical outcomes in tumor models. The CD22 CAR used to create CRG-022 contains a 4-1BB costimulatory domain.