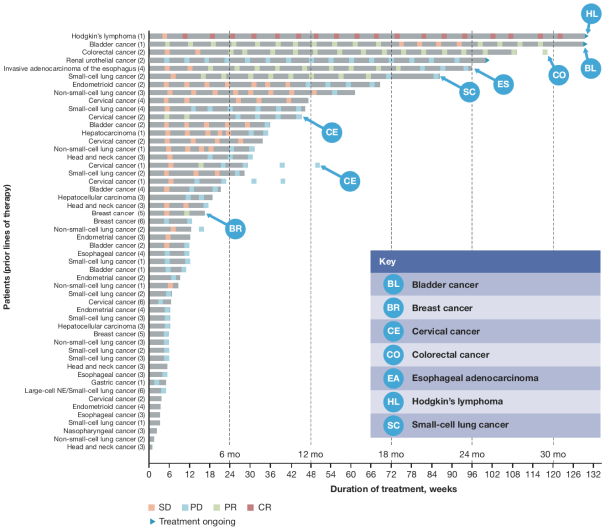
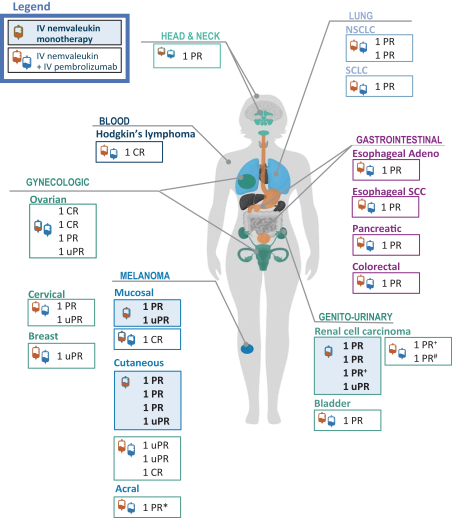
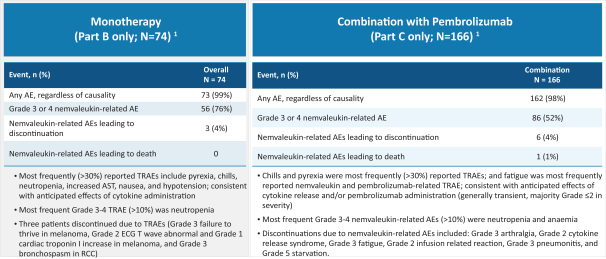
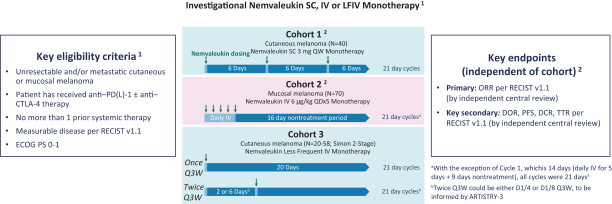
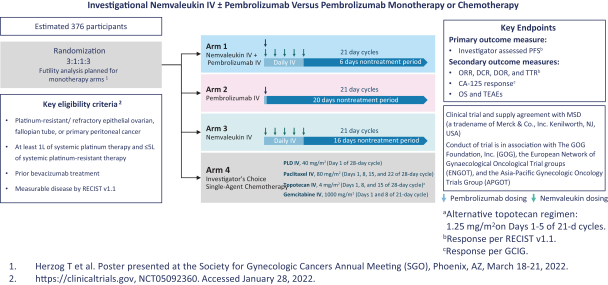
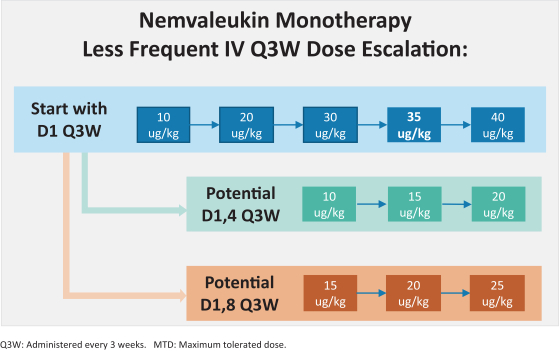
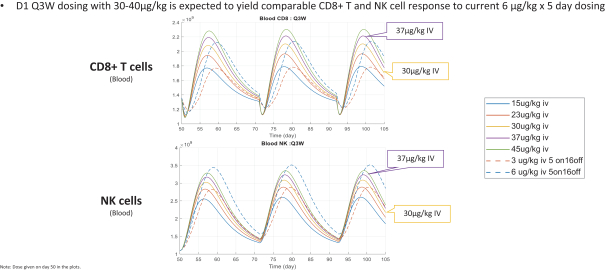
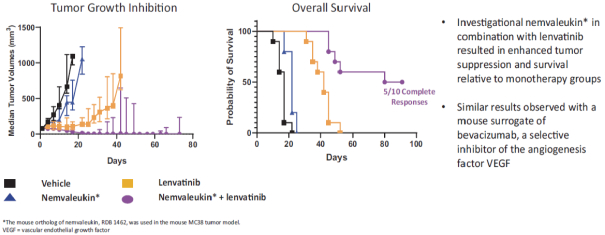
Many of our competitors, either independently or with strategic partners, have substantially greater financial, technical and human resources than we do. Accordingly, our competitors may be more successful than we are in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and achieving widespread market acceptance of approved products. Merger and acquisition activity in the biotechnology and biopharmaceutical industries may result in resources being concentrated among a smaller number of our competitors. These companies also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and clinical trial patient recruitment and acquiring intellectual property that may be complementary to, or necessary for, our programs. Smaller or earlier-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
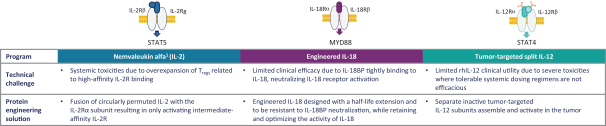
Our lead product candidate nemvaleukin, if approved, may face competition from other IL-2-based cancer therapies. For example, Proleukin® (aldesleukin), a synthetic protein similar to IL-2, is approved and marketed for the treatment of metastatic melanoma as well as metastatic RCC. In addition, we are aware of several companies that have IL-2-based programs in development for the treatment of different cancers, including Anaveon AG, Ascendis, Inc., Cue Biopharma, Inc., Cullinan Oncology Inc., Medicenna Therapeutics Corp., Roche AG, Sanofi, Xilio Therapeutics Inc., and Werewolf Therapeutics Inc.
In addition to IL-2-based therapies, we may also face competition from other therapies targeting our current and future target indications for nemvaleukin. For example, we may face competition in melanoma from companies with approved and marketed therapies, and/or programs in development, including, but not limited to, Bristol-Myers Squibb Co., Iovance Biotherapeutics, Inc., Merck Inc., and Pfizer, Inc. In ovarian cancer, we may face competition from companies with approved and marketed therapies, and/or programs in development including, but not limited to, Immunogen Inc., Merck, Inc., and Mersana, Inc.
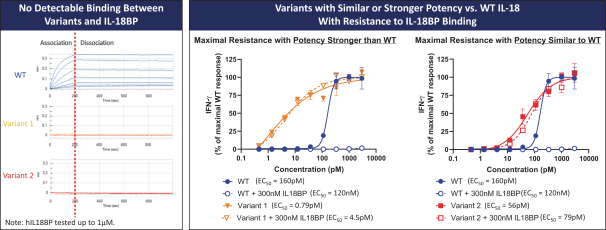
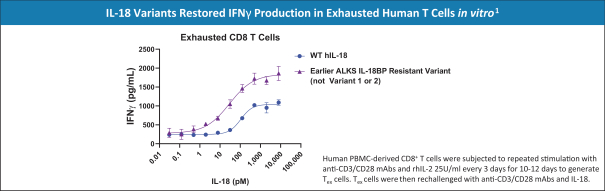
With respect to our IL-18 program, while there are no approved IL-18 therapies currently on the market for the treatment of cancer, we are aware of several other companies that have IL-18-based cancer therapies that are in development, including, but not limited to, Bright Peak Therapeutics, Inc. and Simcha Therapeutics, Inc.
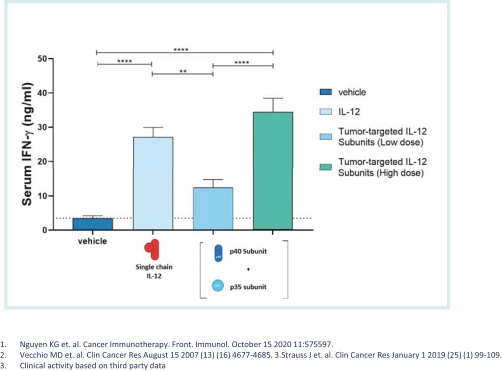
With respect to our IL-12 program, while there are no approved IL-12 therapies currently on the market for the treatment of cancer, we are aware of several other companies that have modified IL-12 or intra-tumoral IL-12 delivery programs in development for the treatment of cancer, including, but not limited to, Amunix, Inc., a subsidiary of Sanofi, Cullinan Oncology Inc., DragonFly Therapeutics, Inc., Moderna Inc., Merck KGaA, Werewolf Therapeutics Inc., Xencor, Inc. and Xilio Therapeutics, Inc.
License Agreements
Mural and Alkermes will enter into a separation agreement in connection with the separation, which will contain intellectual property licenses pursuant to which each party will grant a license to certain intellectual property and/or technologies to the other company. Under the terms of the separation agreement, Alkermes will grant Mural a perpetual, worldwide, non-exclusive, royalty-free, fully paid-up license (or, as the case may be, sublicense) to intellectual property controlled by Alkermes as of the distribution date to allow Mural to use such intellectual property for the oncology business, and Mural will grant Alkermes a perpetual, worldwide, non-exclusive, royalty-free, fully paid-up license (or, as the case may be, sublicense) to intellectual property transferred to Mural as part of the separation for use outside of the oncology business.
Manufacturing
We do not own or operate, and currently have no plans to establish, any manufacturing facilities. We rely substantially on third parties for the manufacture of our product candidates for preclinical and clinical development, and expect to continue to rely on third parties for commercial manufacturing of any of our products that receive marketing approval. For example, we have entered into certain agreements with external partners in
138