
Non-Viral Genetic Medicine Preliminary Data from LEGEND Pivotal Cohort September 26, 2024 Exhibit 99.2

Cautionary Statement Regarding Forward-Looking Statements This Presentation contains certain forward-looking statements within the meaning of the federal securities laws and "forward-looking information" within the meaning of Canadian securities laws (collectively, "forward-looking statements"). Forward-looking statements may be identified by the use of the words such as “plan”, “forecast”, “intend”, “development”, “expect”, “anticipate”, “become”, “believe”, “continue”, “could”, “estimate”, “expect”, “intends”, “may”, “might”, “plan”, “possible”, “project”, “should”, “would”, “strategy”, “future”, “potential”, “opportunity”, “target”, “term”, “will”, “would”, “will be” or similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding the potential benefits of detalimogene, the anticipated market acceptance of detalimogene, plans for research and development, estimates and forecasts of financial and performance metrics, projections of market opportunity and market share, expectations and timing related to regulatory submissions and commercial product launches and the prospects for regulatory approval of detalimogene. These forward-looking statements are based on various estimates and assumptions, whether or not identified in this presentation, and on the current expectations of the management of enGene Holdings Inc. ("enGene"), are not predictions of annual performance, and are subject to risks and uncertainties. These forward-looking statements are subject to a number of risks and uncertainties, including but not limited to, those described in the “Risk Factors” sections of enGene’s Annual Report on Form 10-K for the fiscal year ended October 31, 2023, Quarterly Reports on Form 10-Q for the fiscal quarters ended January 31, 2024, April 30, 2024 and July 31, 2024, each of which has been filed with the Securities and Exchange Commission (“SEC”) and Canadian Securities Regulators (copies of which may be obtained at www.sedarplus.ca or www.sec.gov). You should carefully consider the risks and uncertainties described in the “Risk Factors” section of such Annual Report and Quarterly Reports, as well as other documents if and when filed by enGene from time to time with the SEC and Canadian securities regulators. If any of these risks materialize or our assumptions prove incorrect, actual events and results could differ materially from those contained in the forward-looking statements. There may be additional risks that enGene presently knows or that enGene currently believes are immaterial that could also cause actual events and results to differ. In addition, forward-looking statements reflect enGene’s expectations, plans, or forecasts of future events and views as of the date of this presentation. enGene anticipates that subsequent events and developments will cause enGene’s assessments to change. While enGene may elect to update these forward-looking statements at some point in the future, enGene specifically disclaim any obligation to do so, unless required by applicable law. These forward-looking statements should not be relied upon as representing enGene’s assessments as of any date subsequent to the date of this presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements contained herein Intellectual Property This Presentation contains trademarks, service marks, trade names, copyrights, and products of enGene and other companies, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade names, copyrights, or products in this Presentation is not intended to, and does not, imply a relationship with enGene, or an endorsement of or sponsorship by enGene. Solely for convenience, the trademarks, service marks, and trade names referred to in this Presentation may appear without the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that enGene will not assert, to the fullest extent permitted under applicable law, their rights or the right of the applicable licensor in such trademarks, service marks and trade names. Industry and Market Data This Presentation relies on and refers to certain information and statistics based on estimates by enGene’s management and/or obtained from third party sources which enGene believes to be reliable. enGene has not independently verified the accuracy or completeness of any such third party information, which involves elements of subjective judgment and analysis that may or may not prove to be accurate. None enGene, or its affiliates or any third parties that provide information to enGene or its affiliates, such as market research firms, guarantees the accuracy, completeness, timeliness, or availability of any information. None enGene, or its affiliates, or any third parties that provide information to enGene, and its affiliates, such as market research firms, is responsible for any errors or omissions (negligent or otherwise), regardless of the cause, or the results obtained from the use of such content. enGene may have supplemented such information where necessary, taking into account publicly available information about other industry participants and enGene management’s best view as to information that is not publicly available. Neither enGene nor its affiliates give any express or implied warranties with respect to the information included herein, including, but not limited to, any warranties regarding its accuracy or of merchantability or fitness for a particular purpose or use, and they expressly disclaim any responsibility or liability for direct, indirect, incidental, exemplary, compensatory, punitive, special, or consequential damages, costs, expenses, legal fees, or losses (including lost income or profits and opportunity costs) in connection with the use of the information herein. Lead Program (detalimogene voraplasmid) The lead program described herein is an investigational drug therapy, which has not been approved for marketing by the U.S. Food and Drug Administration or any other regulatory agency and that has not been subject to testing designed to demonstrate that the therapy is effective in humans or to provide a basis to predict in advance whether an adequate level of efficacy in humans will be demonstrated in further testing. Although deemed sufficient to permit further testing, the limited, early Phase 1 testing to date is not a sufficient basis on which to predict efficacy or safety and no representation is made as to detalimogene’s efficacy or safety. Although the FDA has indicated that the Phase 2 portion of the current LEGEND study may potentially support BLA approval, that outcome will depend entirely on the results of Phase 2 clinical testing, which are not expected to be available until 2026. Disclaimers
Pivotal Study Currently Enrolling Patients: BCG-Unresponsive High-risk NMIBC with CIS Design: Global, single-arm, open label N ≈ 100 Dosing: 800μg/ml intravesical at weeks 1,2,5,6 Q3M Endpoints: 1° - CR rate at 12-months; 2° - safety and durability 3 To date, LEGEND protocol has not included surgical biopsy/resection or re-induction of HG Ta patients at 3m
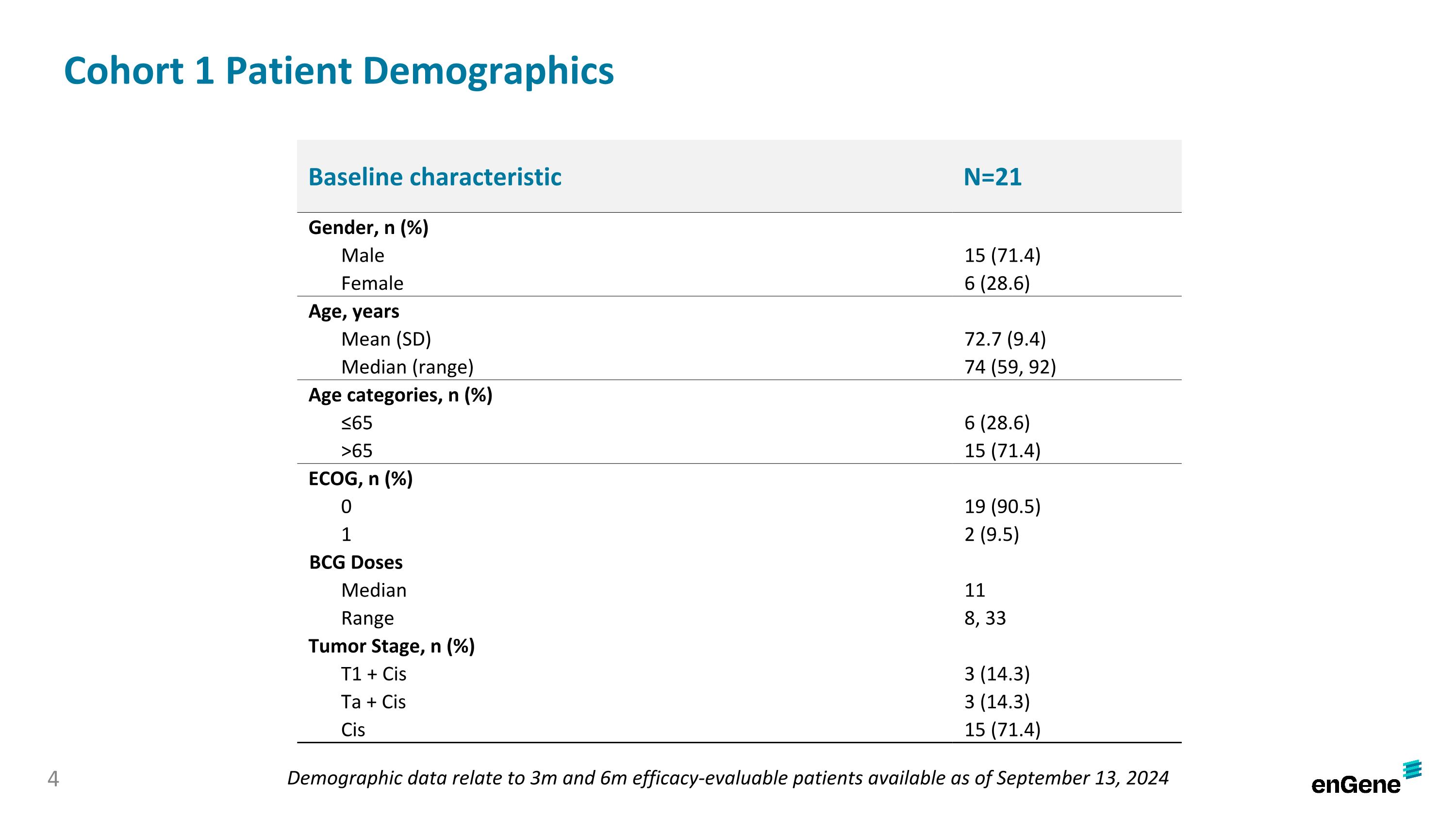
Cohort 1 Patient Demographics Baseline characteristic N=21 Gender, n (%) Male 15 (71.4) Female 6 (28.6) Age, years Mean (SD) 72.7 (9.4) Median (range) 74 (59, 92) Age categories, n (%) ≤65 6 (28.6) >65 15 (71.4) ECOG, n (%) 0 19 (90.5) 1 2 (9.5) BCG Doses Median 11 Range 8, 33 Tumor Stage, n (%) T1 + Cis 3 (14.3) Ta + Cis 3 (14.3) Cis 15 (71.4) Demographic data relate to 3m and 6m efficacy-evaluable patients available as of September 13, 2024 4
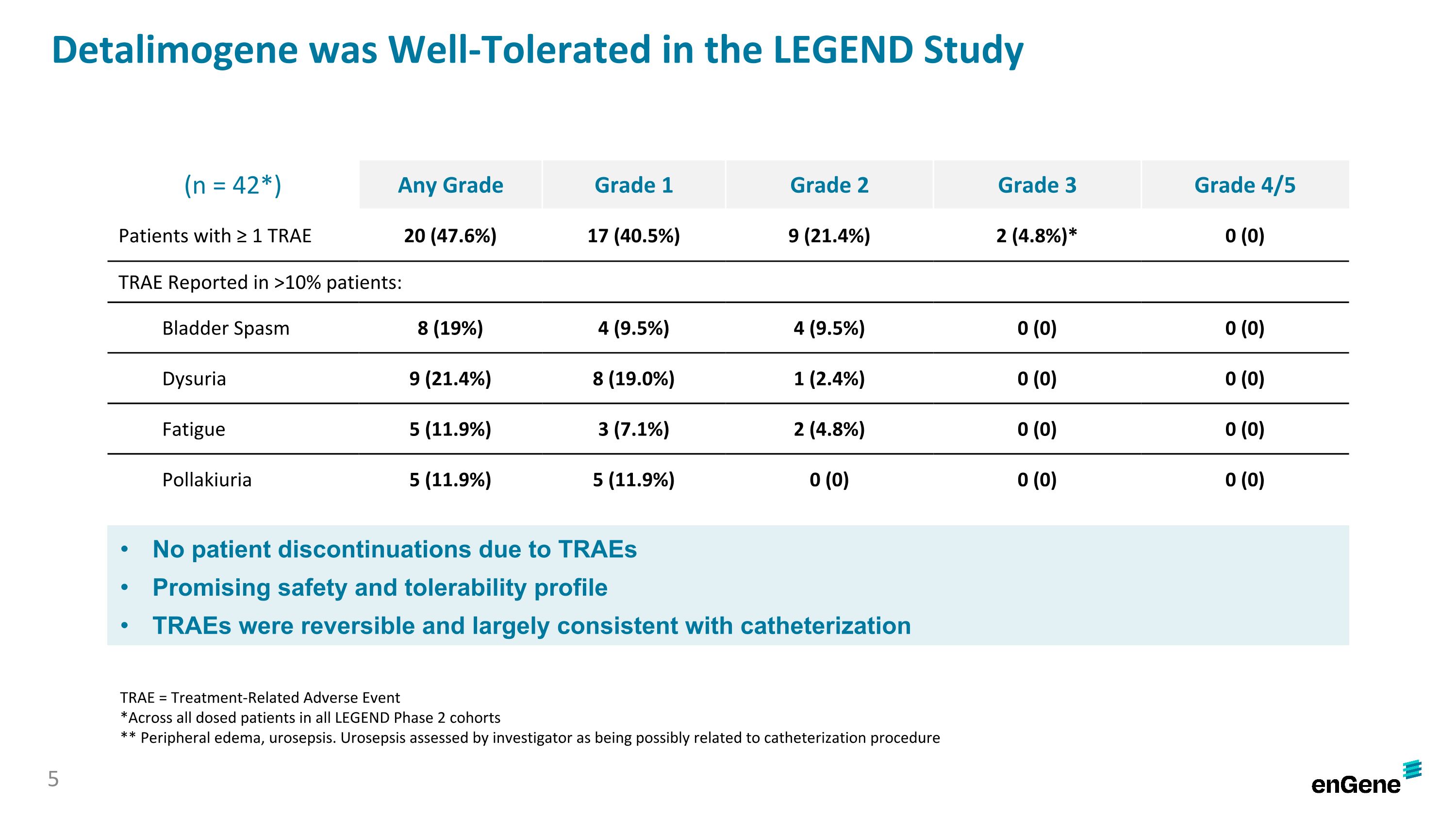
Detalimogene was Well-Tolerated in the LEGEND Study (n = 42*) Any Grade Grade 1 Grade 2 Grade 3 Grade 4/5 Patients with ≥ 1 TRAE 20 (47.6%) 17 (40.5%) 9 (21.4%) 2 (4.8%)* 0 (0) TRAE Reported in >10% patients: Bladder Spasm 8 (19%) 4 (9.5%) 4 (9.5%) 0 (0) 0 (0) Dysuria 9 (21.4%) 8 (19.0%) 1 (2.4%) 0 (0) 0 (0) Fatigue 5 (11.9%) 3 (7.1%) 2 (4.8%) 0 (0) 0 (0) Pollakiuria 5 (11.9%) 5 (11.9%) 0 (0) 0 (0) 0 (0) No patient discontinuations due to TRAEs Promising safety and tolerability profile TRAEs were reversible and largely consistent with catheterization TRAE = Treatment-Related Adverse Event *Across all dosed patients in all LEGEND Phase 2 cohorts ** Peripheral edema, urosepsis. Urosepsis assessed by investigator as being possibly related to catheterization procedure
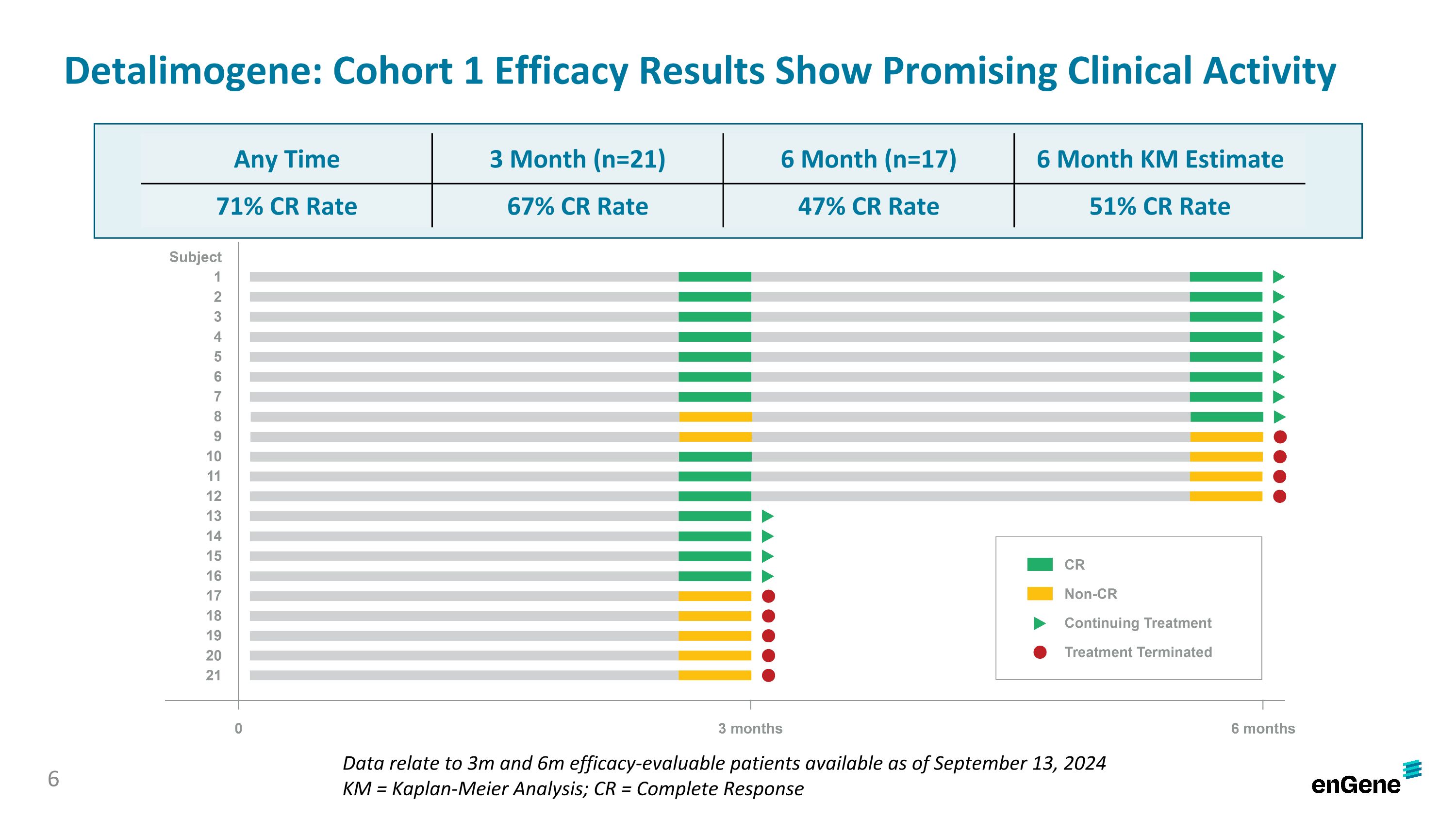
Detalimogene: Cohort 1 Efficacy Results Show Promising Clinical Activity Data relate to 3m and 6m efficacy-evaluable patients available as of September 13, 2024 KM = Kaplan-Meier Analysis; CR = Complete Response Any Time 3 Month (n=21) 6 Month (n=17) 6 Month KM Estimate 71% CR Rate 67% CR Rate 47% CR Rate 51% CR Rate 6

Detalimogene: Designed To Be the First-Choice Therapy 71% CR rate at any time highlights promising activity Promising safety profile observed; no disconnections due to tolerability Convenient product attributes support community use Evolving care paradigm Global expansion expected to further enrollment (US, Canada, EU, Asia Pacific) + + + 7






