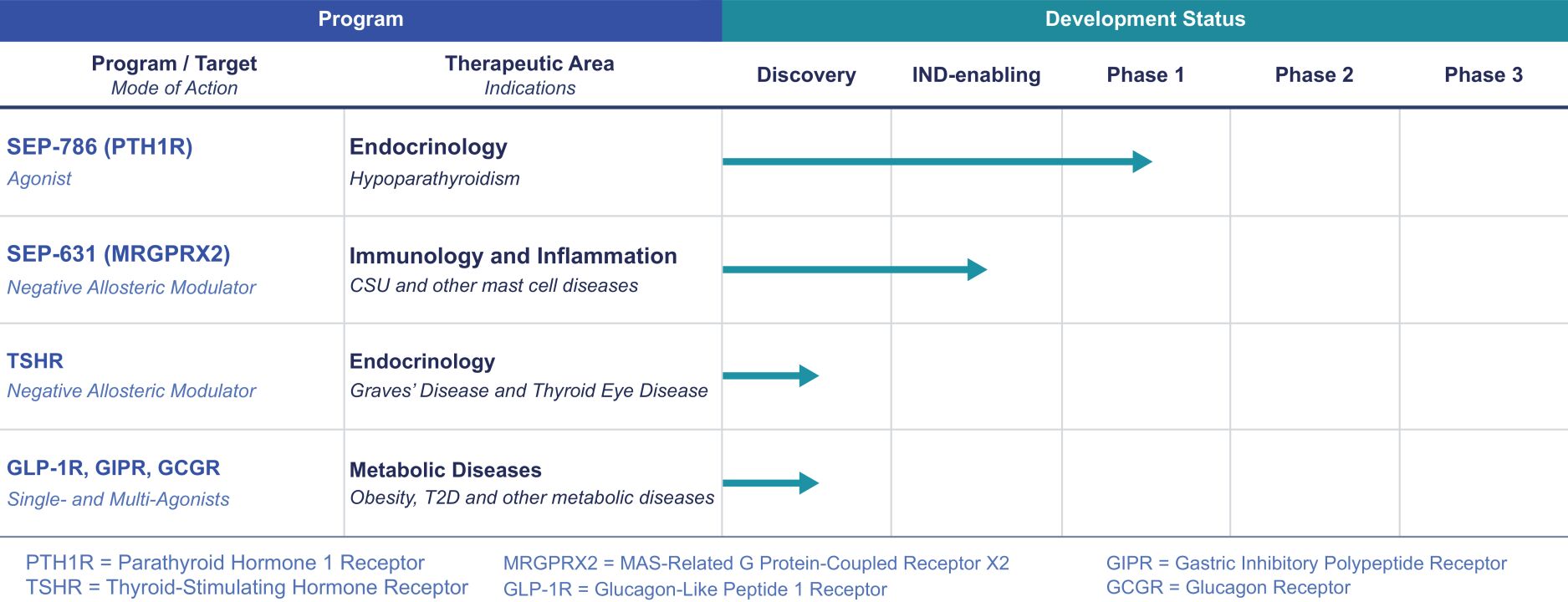
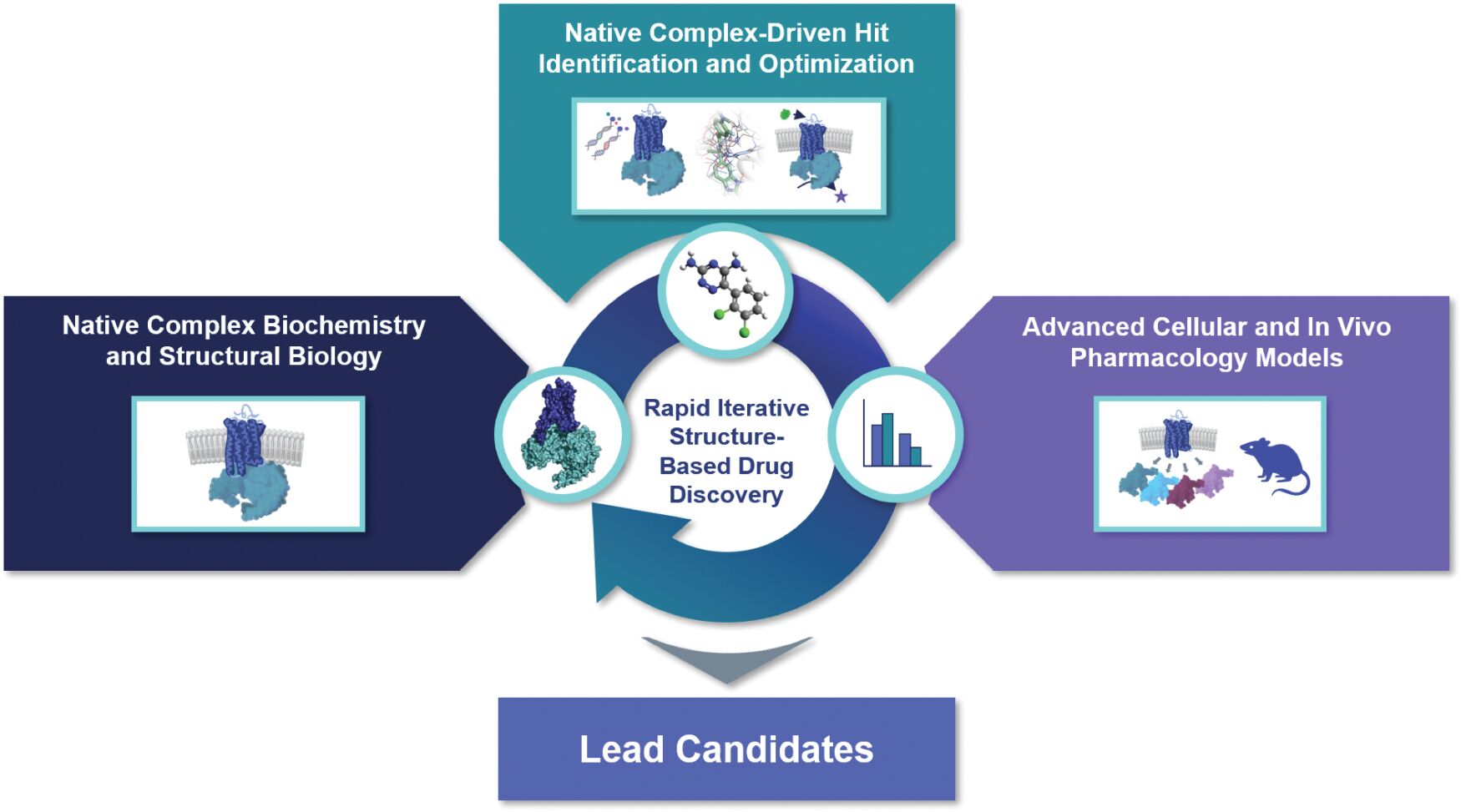
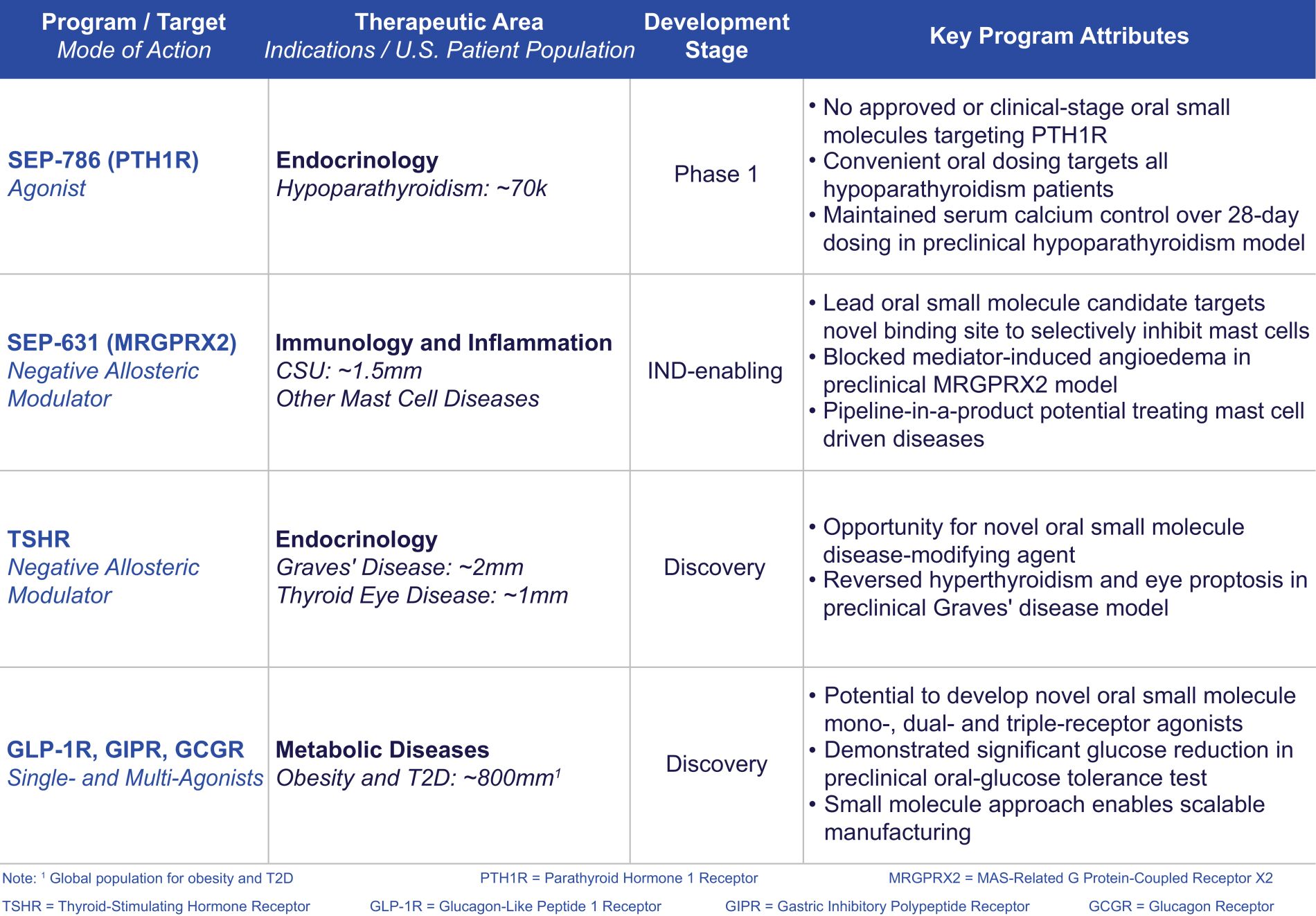
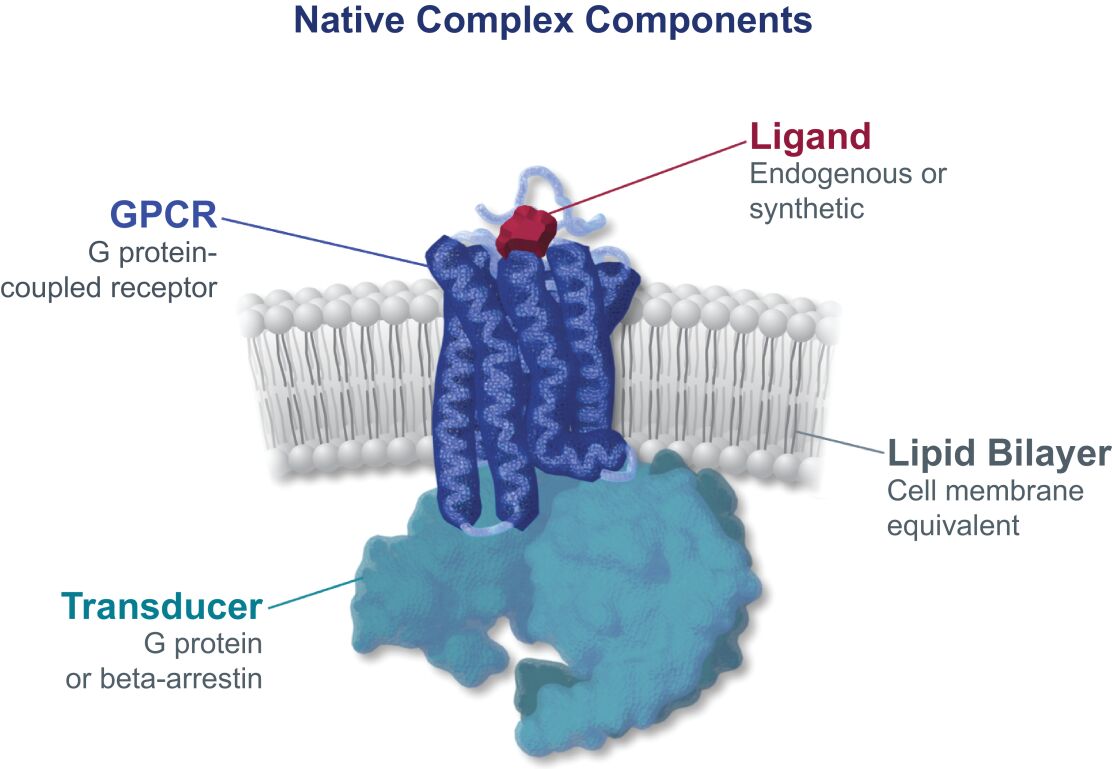
may not be a direct therapeutic advantage but is a clinically important improvement from a patient and public health perspective. If granted, accelerated approval is usually contingent on the sponsor’s agreement to conduct, in a diligent manner, additional post-approval confirmatory studies to verify and describe the drug’s clinical benefit. Under FDORA, the FDA is permitted to require, as appropriate, that a post-approval confirmatory study or studies be underway prior to approval or within a specified time period after the date of approval for a product granted accelerated approval. FDORA also gives the FDA increased authority to withdraw approval of a drug or biologic granted accelerated approval on an expedited basis if the sponsor fails to conduct such studies in a timely manner, send status updates on such studies to the FDA every 180 days to be publicly posted by the agency, or if such post-approval studies fail to verify the drug’s predicted clinical benefit. The FDA is empowered to take action, such as issuing fines, against companies that fail to conduct with due diligence any post-approval confirmatory study or submit timely reports to the agency on their progress.
Prior to seeking accelerated approval, we would seek feedback from the FDA, EMA, or other comparable foreign regulatory authorities and would otherwise evaluate our ability to seek and receive such accelerated approval. There can be no assurance that after our evaluation of the feedback and other factors we will decide to pursue or submit an NDA or BLA for accelerated approval or any other form of expedited development, review or approval. Similarly, there can be no assurance that after subsequent feedback from the FDA, EMA, or other comparable foreign regulatory authorities, we will continue to pursue or apply for accelerated approval or any other form of expedited development, review or approval, even if we initially decide to do so. Furthermore, if we decide to submit an application for accelerated approval, there can be no assurance that such application will be accepted or that any approval will be granted on a timely basis, or at all. The FDA, EMA, or other comparable foreign regulatory authorities could also require us to conduct further studies prior to considering our application or granting approval of any type, including, for example, if other products are approved via the accelerated pathway and subsequently converted by FDA to full approval. A failure to obtain accelerated approval or any other form of expedited development, review or approval for our product candidate would result in a longer time period to commercialization of such product candidate, could increase the cost of development of such product candidate and could harm our competitive position in the marketplace.
We are subject to stringent and evolving U.S. and foreign laws, rules, regulations, policies, industry standards, contractual requirements, and other obligations related to data protection, privacy, and security. Our actual or perceived failure to comply with such obligations could adversely affect our business.
We are subject to various data protection, privacy, and security laws, rules, regulations, policies, industry standards, contractual requirements, and other obligations that apply to our collection, transmission, storage, use, disclosure, transfer, maintenance and other processing of sensitive information, including personal data. The legislative and regulatory landscape for data protection, privacy, and security continues to evolve across jurisdictions worldwide. For example, in the United States, federal, state, and local governments have enacted numerous data protection, privacy, and security laws, including data breach notification laws, personal data privacy laws, consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), and other similar laws (e.g., wiretapping laws).
In particular, regulations promulgated pursuant to the Health Insurance Portability and Accountability Act of 1996 (HIPAA), as amended by the Health Information Technology for Economic and Clinical Health Act (HITECH), and implementing regulations, establish stringent privacy and security standards that limit the use and disclosure of individually identifiable health information, or protected health information, and require the implementation of administrative, physical and technological safeguards to protect the privacy of protected health information and ensure the confidentiality, integrity and availability of electronic protected health information.
Data protection, privacy, and security obligations remain an evolving landscape at both the domestic and foreign level, with new laws, rules and regulations coming into effect, posing continued legal and compliance challenges. For example, in the past few years, numerous U.S. states—including California, Virginia, Colorado,
52