Exhibit 99.1

Vreni Loewen and Tatiana Lashina OB Solutions October 2022 R emote Fetal Monitoring Company Overview July 2024

2 Disclaimer CAUTIONARY STATEMENT The information contained in this presentation (“Presentation”) has been prepared by Holdco Nuvo Group D.G Ltd., a limited li abi lity company organized under the laws of the State of Israel (“Nuvo”), and includes information pertaining to the business and operations of Nuvo. The information contained in the Presentation: (a) is pr ovided as at the date hereof, is subject to change without notice, and is based on publicly available information, internally developed data as well as third party information from other sources; and (b) i s f or informational purposes only. Where any opinion or belief is expressed in the Presentation, it is based on certain assumptions and limitations and is an expression of present opinion or belief only, ther e c an be no guarantee as to the accuracy and reliability of such assumptions. The Presentation also contains or references certain market, industry and peer group data, including statements regarding market siz e, which is based upon good faith estimates of Nuvo’s management team, which in turn are based upon Nuvo’s management’s review of internal surveys, independent industry surveys and publications an d o ther third - party research and publicly available information. Any third - party information has not been independently verified. While Nuvo’s management team may not have verified the third - party inform ation, they believe that it obtained the information from reliable sources and have no reason to believe it is not accurate in all material respects. No warranties or representations can be made as to th e origin, validity, accuracy, completeness, currency or reliability of the information. Nuvo disclaims and excludes all liability (to the maximum extent permitted by law), for losses, claims, damages, de mands, costs and expenses of whatever nature arising in any way out of or in connection with the information in the Presentation, its accuracy, completeness or by reason of reliance by any person on any of it. The Presentation should not be construed as legal, financial or tax advice to any individual, as each individual’s circumstances are different. Readers should consult with their own professional advisors re garding their particular circumstances. All financial information is in U.S. dollars, unless otherwise indicated. Copyright © 2024 Nuvo Group Ltd. All Rights Reserved.

3 FORWARD LOOKING STATEMENTS Certain information set forth in the Presentation contains “forward - looking statements” as that term is defined under the Private Securities Litigation Reform Act of 1995 . Except for statements of historical fact, certain information contained herein constitutes forward - looking statements which include but are not limited to statements related to activities, events or developments that Nuvo expects or anticipates will or may occur in the future, statements related to Nuvo’s business strategy objectives and goals, market opportunity, and Nuvo’s management’s assessment of future plans and operations which are based on current internal expectations, estimates, projections, assumptions and beliefs, which m ay prove to be incorrect . Forward - looking statements can often be identified by the use of words such as “may”, “will”, “could”, “would”, “anticipate”, “believe”, “expect”, “intend”, “potential”, “estimate”, “budget”, "seek", "outlook", "target", "future”, “scheduled”, “plans”, “planned”, “forecasts”, “goals” and similar expressions or the negatives thereof . Such statements are based on Nuvo’s management’s belief or interpretation of information currently available . Forward - looking statements are neither historical facts nor assurances of future performance . Forward - looking statements in this business overview include statements regarding : Nuvo’s commercialization strategy and plans to grow its market share in existing and new markets ; Nuvo’s commercial collaborations, relationships with healthcare payers and strategic partnerships ; Nuvo’s investment in the INVU platform ; Nuvo’s supply chain and manufacturing capabilities ; the research and development in predictive analytics to improve health outcomes ; the future size of the pregnancy care market in the United States ; and Nuvo’s future financial performance . Forward - looking statements are based on a number of factors and assumptions made by management and considered reasonable at the time such information is provided, and forward - looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements to be materially different from those expressed or implied by the forward - looking statements . These forward - looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on as a guarantee, an assurance, a prediction or a definitive statement of fact or probability . Actual events and circumstances are difficult or impossible to predict and may differ from assumptions, and such differences may be material . Many actual events and circumstances are beyond the control of Nuvo . Risk factors that could cause actual results, performance or achievement to differ materially from those indicated in the forward - looking statements include, but are not limited to the following ( i ) the benefits of the recent business combination, (ii) Nuvo's financial performance following the business combination, (iii) the ability to maintain the listing of ordinary shares on the Nasdaq Global Market and the warrants on the Nasdaq Capital Market following the business combination, (iv) the projected financial information, anticipated growth rate, and market opportunity for Nuvo, and estimates of expenses and profitability, (v) the potential liquidity and trading of public securities of Nuvo, (vi) the ability to raise financing in the future, (vii) the effectiveness and profitability of Nuvo’s collaborations and partnerships, its ability to maintain current collaborations and partnerships and enter into new collaborations and partnerships, (viii) estimates related to future revenue, expenses, capital requirements and need for additional financing ; (ix) the impact of natural disasters or health epidemics/pandemics, including a resurgence of the COVID - 19 pandemic ; (x) the effects of increased competition as well as innovations by new and existing competitors in Nuvo's industry ; (xi) geopolitical risk, including the impacts of the ongoing conflict between Russia and Ukraine, and the war between Israel and Hamas, (xii) Nuvo’s ability to demonstrate the feasibility of its INVU platform for commercial applications, (xiii) Nuvo’s ability to generate revenue in accordance with its business model, (xiv) Nuvo’s expectations regarding its ability to obtain and maintain intellectual property protection and not infringe on the rights of others, (xv) Nuvo’s ability to develop, market and sell its INVU platform, (xvi) Nuvo’s ability to develop its sales and marketing organization, (xvii) changes in applicable laws or regulations, (xviii) the outcome of any known and unknown litigation and regulatory proceedings, (xix) regulatory developments in the United States and foreign countries, among other factors, including all of the risks identified under the heading "Risk Factors" in our Annual Report on Form 20 - F and other filings with the Securities and Exchange Commission (the "SEC") . The foregoing list of important factors is not exhaustive . Nuvo undertakes no obligation to revise or update any forward - looking statement, or to make any other forward - looking statements, whether as a result of new information, future events or otherwise . Disclaimer
4 • De - risked platform - 2 FDA 510 (k) clearances 1 and CE mark pending • Commercial launch in March 2023 - now onboarding customers, monitoring patients and generating early revenue • Proven reimbursement - public and private payers • Philips Master Partnership Agreement signed August 2023 • Continued future innovation development - NIH Grants and International clinical partner s • High Patient Satisfaction and engagement Nuvo h ighlights 1. INVU is cleared by the FDA for fetal heart rate, maternal heart rate and uterine activity (contractions). “I’m confident there is a direct connection between my being hospitalized on a hybrid basis, performing all the necessary pregnancy monitoring tests remotely through your monitoring belt, and the fact that my test results improved.” – Nuvo patient from Sheba in Israel
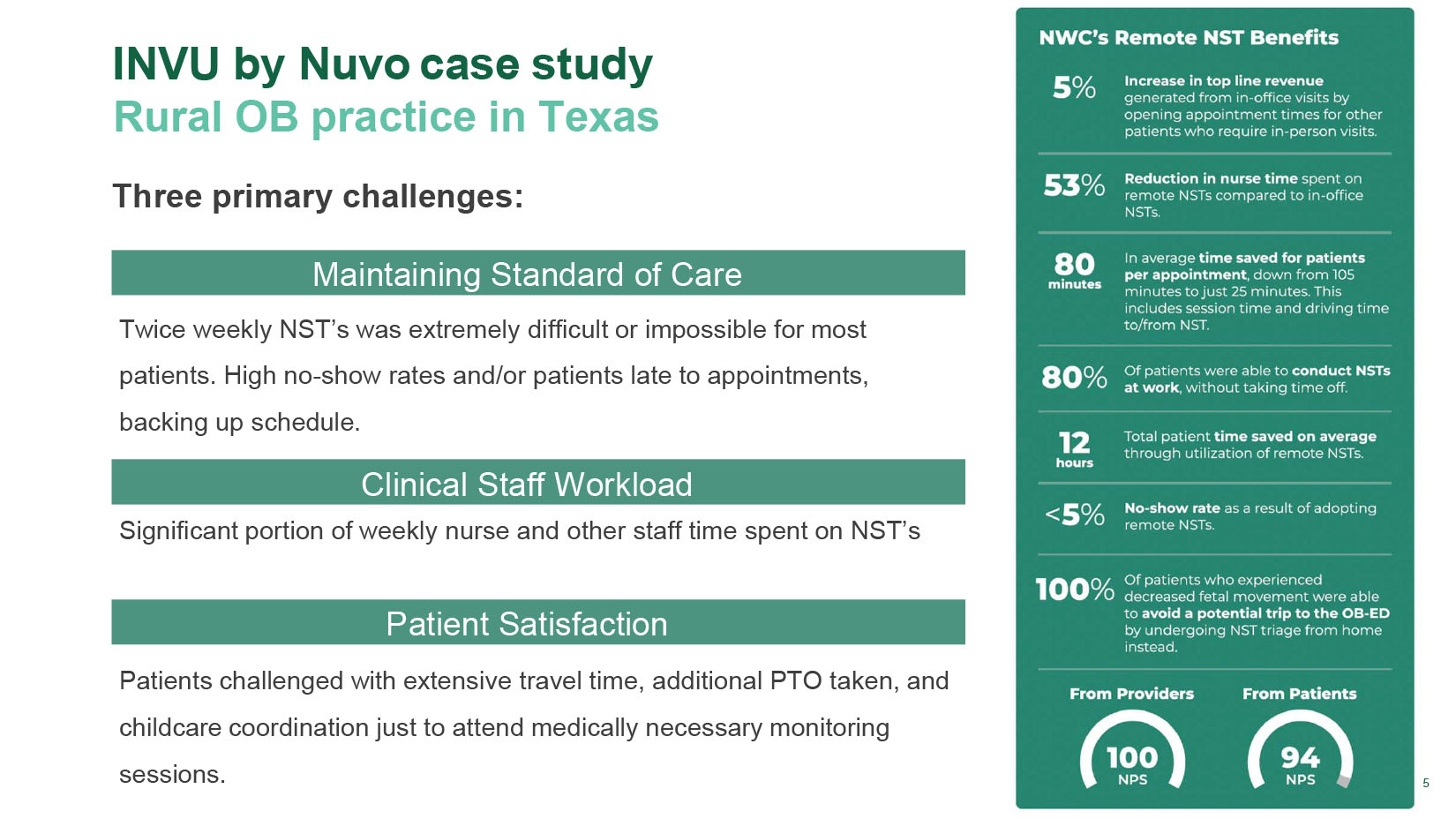
5 INVU by Nuvo c ase s tudy Rural OB practice in Texas Three primary challenges : Maintaining Standard of Care T wice weekly NST’s was extremely difficult or impossible for most patients. High no - show rates and/or patients late to appointments, backing up schedule. Clinical Staff Workload Significant portion of weekly nurse and other staff time spent on NST’s Patient Satisfaction Patients challenged with extensive travel time, additional PTO taken, and childcare coordination just to attend medically necessary monitoring sessions.

6 Pregnancy c are is broken Innovation in pregnancy care has lagged behind other healthcare practice areas M assive health equity issues in outcomes, access, and costs Contributing to Innovation has lagged Massive health equity issues
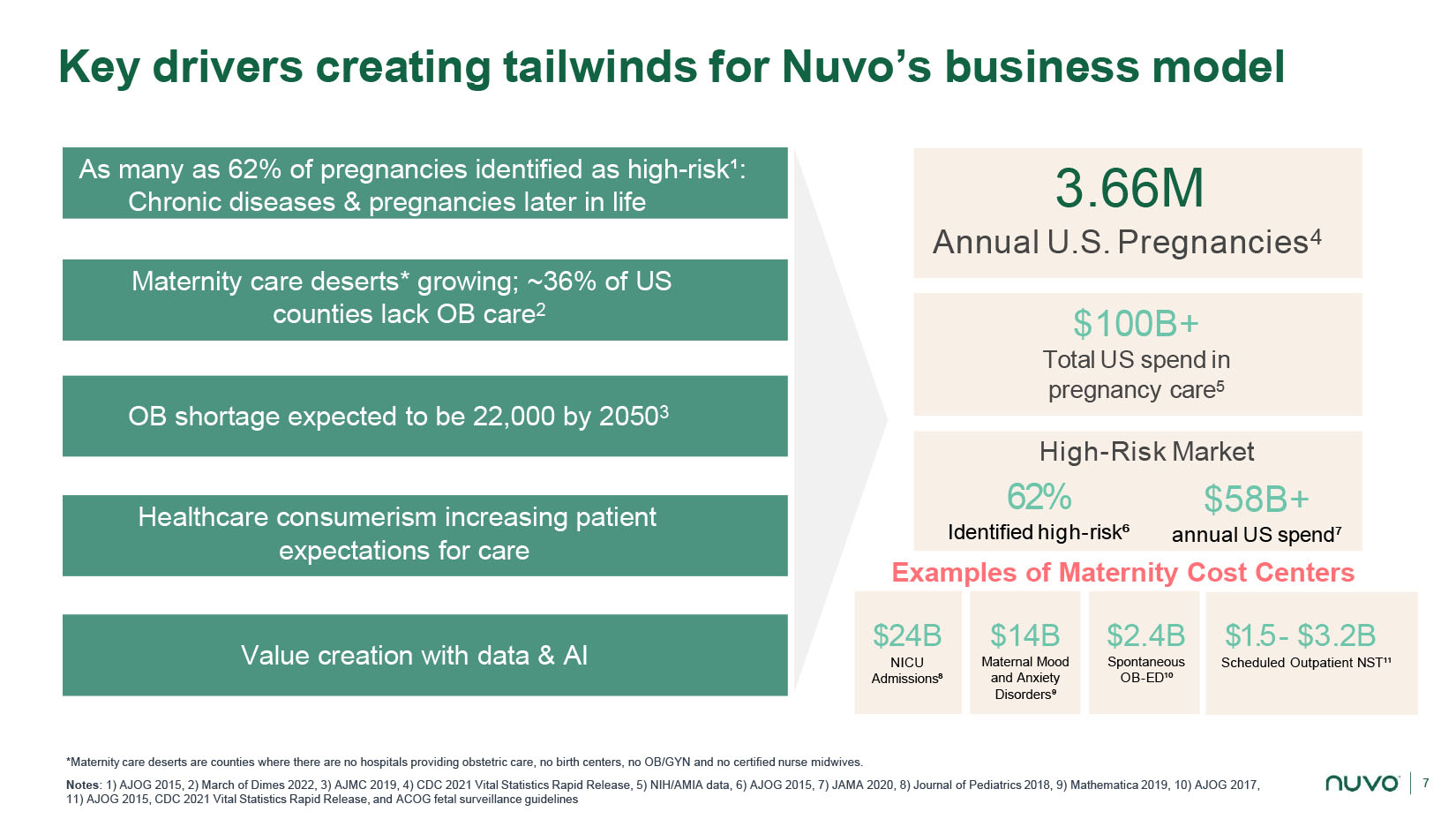
7 Key drivers creating tailwinds for Nuvo’s business model *Maternity care deserts are counties where there are no hospitals providing obstetric care, no birth centers, no OB/GYN and no certified nurse midwives. Notes : 1) AJOG 2015, 2) March of Dimes 2022, 3) AJMC 2019, 4) CDC 2021 Vital Statistics Rapid Release, 5) NIH/AMIA data, 6) AJOG 2015, 7) JAMA 2020, 8) Journal of Pediatrics 2018, 9) Mathematica 2019, 10) AJOG 2017, 11) AJOG 2015, CDC 2021 Vital Statistics Rapid Release, and ACOG fetal surveillance guidelines As many as 62% of pregnancies identified as high - risk¹: Chronic diseases & pregnancies later in life Healthcare consumerism increasing patient expectations for care Maternity care deserts* growing; ~36% of US counties lack OB care 2 OB shortage expected to be 22,000 by 2050 3 Value creation with data & AI 3.66M Annual U.S. Pregnancies 4 62% Identified high - risk⁶ $100B+ Total US spend in pregnancy care 5 $58B+ annual US spend⁷ High - Risk Market $24B NICU Admissions⁸ $14B Matern al Mood and Anxiety Disorders ⁹ $2.4B Spontaneous OB - ED¹⁰ $1.5 - $3.2B Scheduled Outpatient NST¹¹ Examples of Maternity Cost Centers

8 A paradigm shift advancing pregnancy care Antiquated technology in resource intensive clinical settings . Nuvo enables data - driven, actionable insights, anywhere , by everyone. Legacy Care Next Gen Care

9 Scalable pregnancy care for clinicians & expectant mothers Unique Data Capture Signal Processing & Analysis Digital Tools & Services Collect Prescribe Compute Visualize Clinician - driven, prescription initiated Advancing the standard of care today and setting the foundation for tomorrow’s innovation * INVU is cleared by the FDA for fetal heart rate, maternal heart rate, and uterine activity (contractions) *

10 Fetal non - stress tests create opportunities across the healthcare ecosystem x Convenience & quality of life x Care e mpowerment and reduced barriers of access to care x Reduced PTO, travel time & expense, etc x Improved staff efficiency x Increased revenue potential x Competitive differentiation x OB - ED avoidance x Reduced total cost of care x Competitive differentiation on social determinants of health & pregnancy outcomes x Employee satisfaction & productivity x Attracting & retaining talent x Reducing total cost of care Expectant Mothers Payers Clinicians Employers Fetal NST: • Medically necessary protocol to monitor fetal health • Guidelines call for twice weekly appointments for last 8 weeks of pregnancy 1 • Creates care equity challenges • 7 days PTO 2 • 3 3 hours of travel time 2 • ~ $ 120 travel expense 2 • ~$21K in nurse time/year 4 • Incremental annual rev potential/exam room = ~$475K 5 • US OB - ED avoidance alone = ~$2.4B system cost savings 3 • ~75% reduction in lost workplace productivity 2 • System cost savings 1) ACOG fetal surveillance guidelines, 2) Nuvo estimates and ACOG fetal surveillance guidelines, 3) CDC 2021 Vital Statistics Rapid Release, UHG 2019 report and AJOG 2017, 4) Nuvo estimates and Zippia nurse salary report, 5) Nuvo estimates and MDSave OBGYN average patient visit.
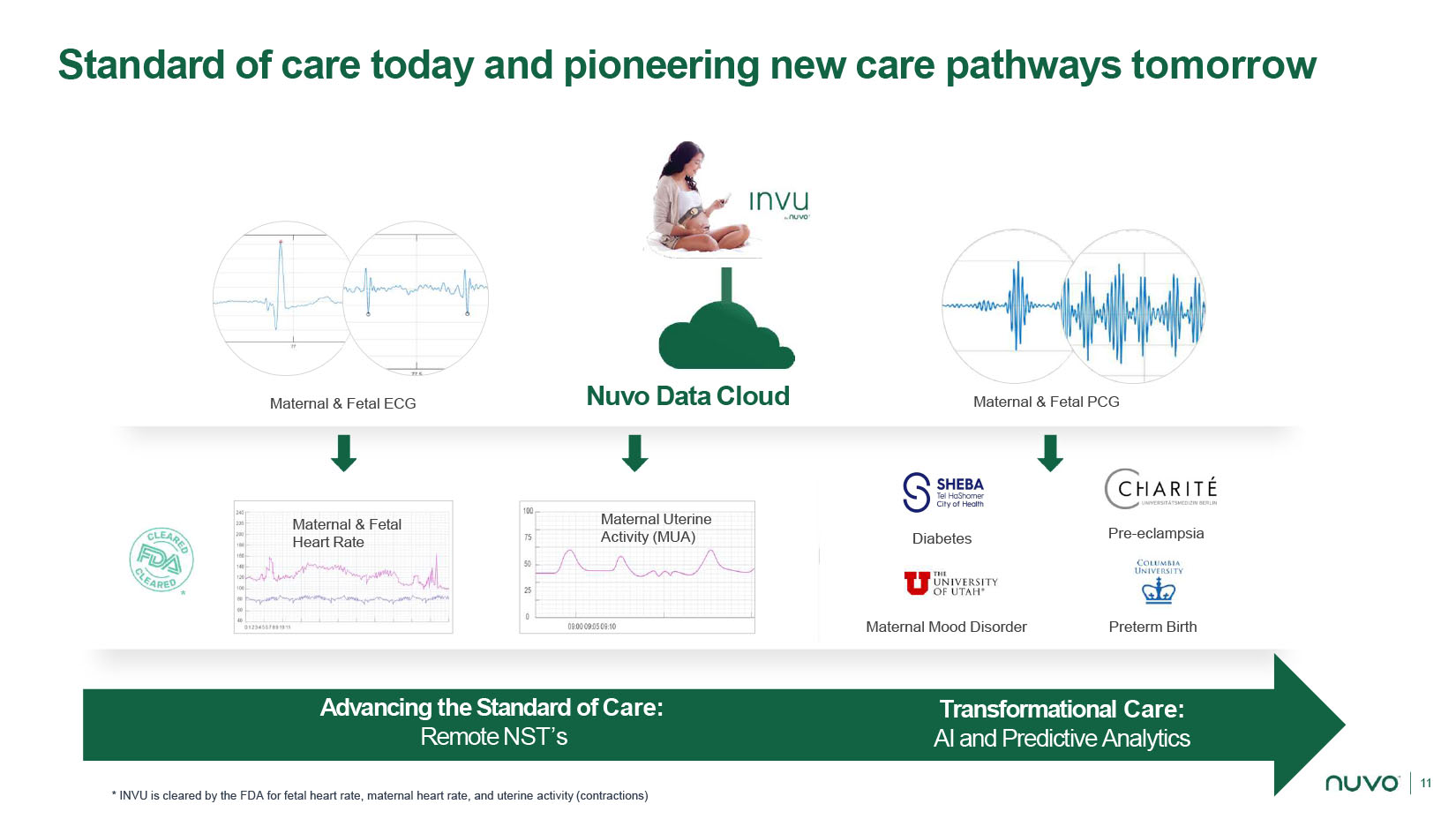
11 Pre - eclampsia Maternal Mood Disorder Preterm Birth Diabetes Nuvo Data Cloud Maternal & Fetal ECG Maternal & Fetal PCG Standard of care today and pioneering new care pathways tomorrow Maternal & Fetal Heart Rate Maternal Uterine Activity (MUA) Transformational Care: AI and Predictive Analytics Advancing the Standard of Care: Remote NST’s * * INVU is cleared by the FDA for fetal heart rate, maternal heart rate, and uterine activity (contractions)
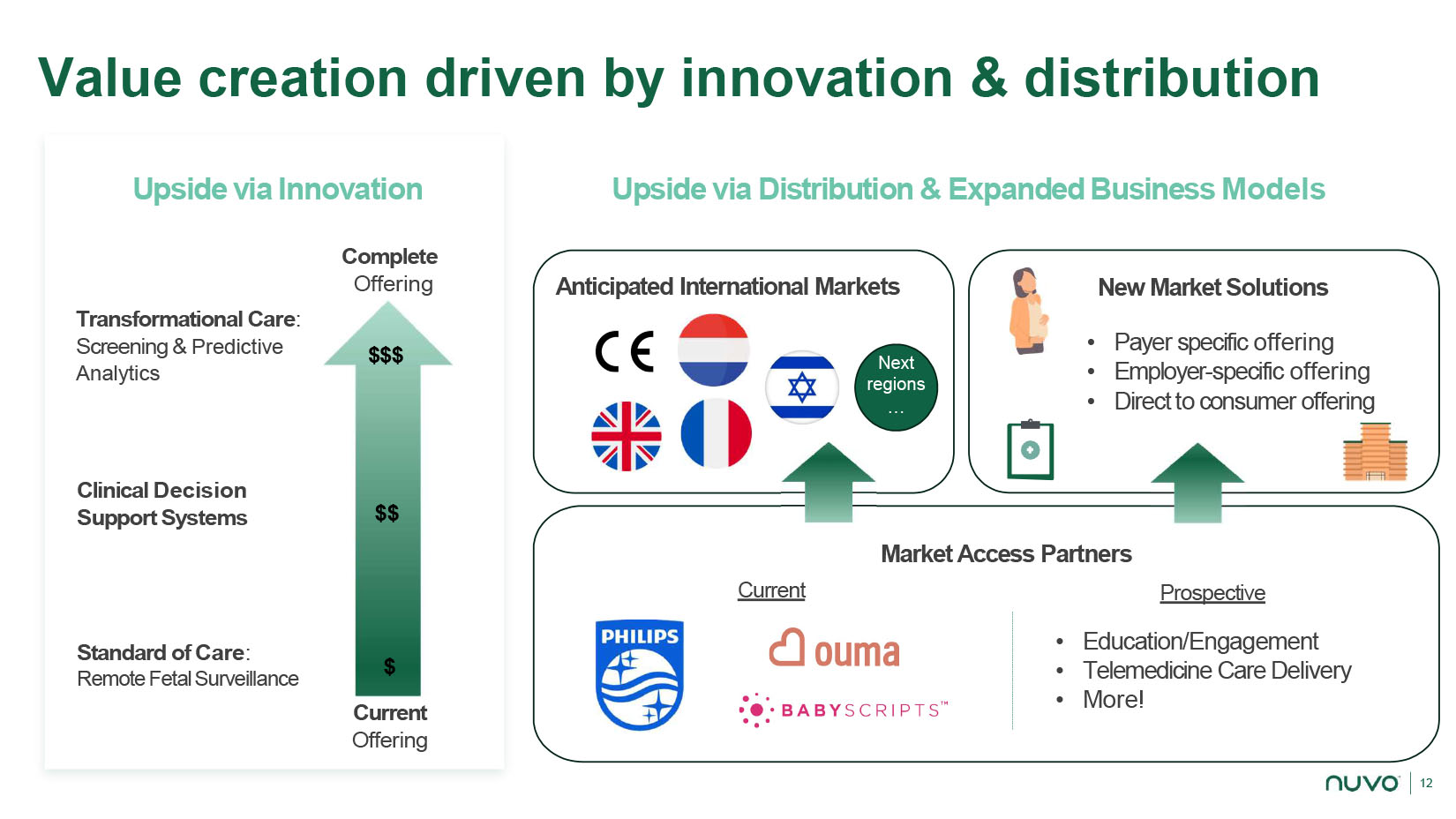
12 Value c reation d riven by i nnovation & distribution Standard of Care : Remote Fetal Surveillance Upside via Innovation $ Current Offering $$$ $$ Clinical Decision Support Systems Transformational Care : Screening & Predictive Analytics Market Access Partners Anticipated International Markets Upside via Distribution & Expanded Business Models New Market Solutions • Payer specific offering • Employer - specific offering • Direct to consumer offering Next regions … Current Prospective • Education/Engagement • Telemedicine Care Delivery • More! Complete Offering

13 Reimbursement & public policy accelerators In r emote p regnancy monitoring Public policy is funding improvements that address care equity Existing codes being utilized to reimburse remote NST’s by both public and private payers Remote NSTs reimbursed & endorsed Important Notice Regarding At - Home Fetal Non - Stress Tests “In the interest of expanding access to care and improving member outcomes , we are writing today to confirm that CPT Code 59025 is reimbursable…” GA initiative, lobbied by Philips, kickstarts new maternity care initiatives including remote NSTs
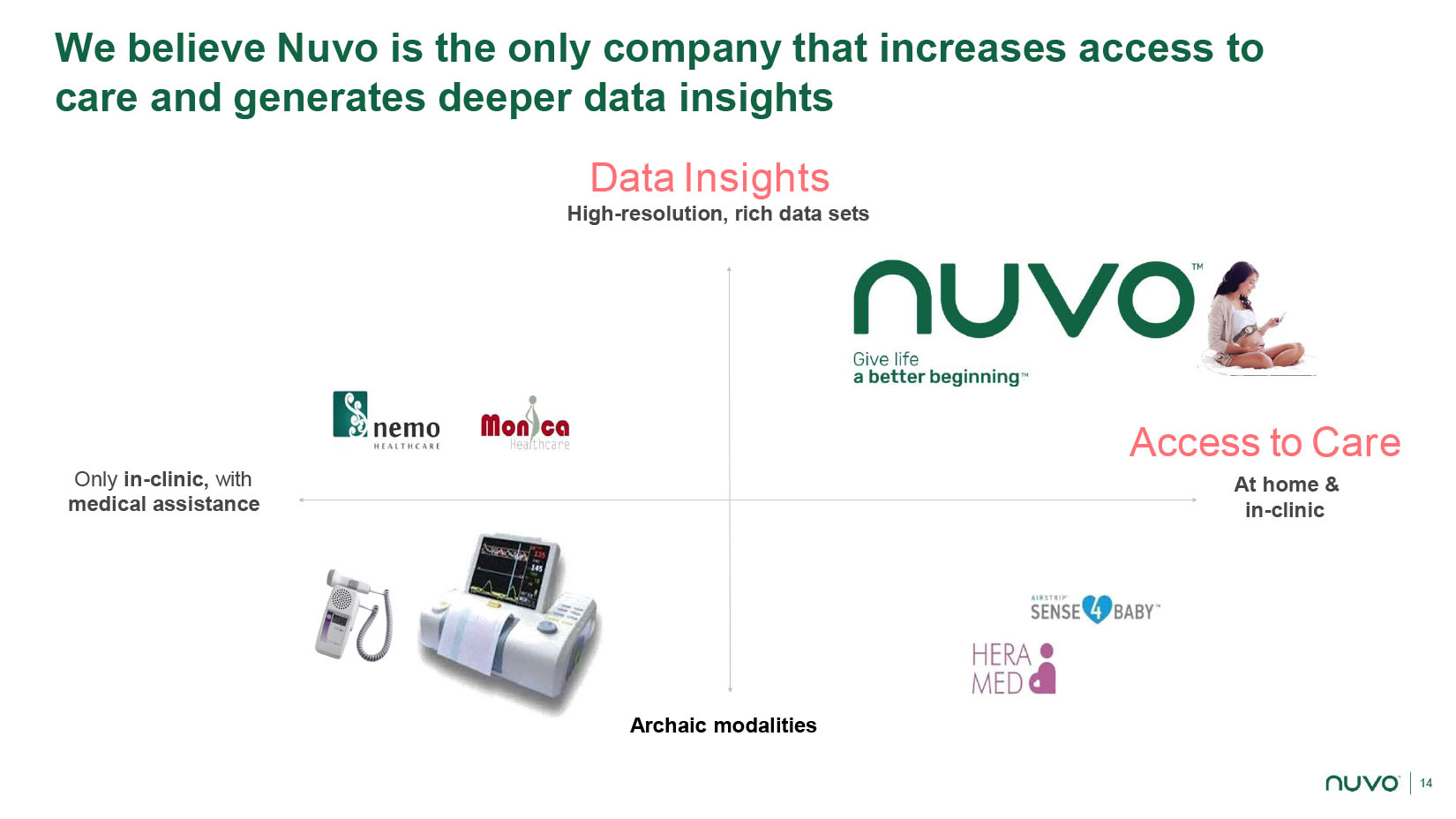
14 Only in - clinic, with medical assistance Access to Care At home & in - clinic Data Insights High - resolution, rich data sets We believe Nuvo is the only company that increases access to care and generates deeper data insights Archaic modalities
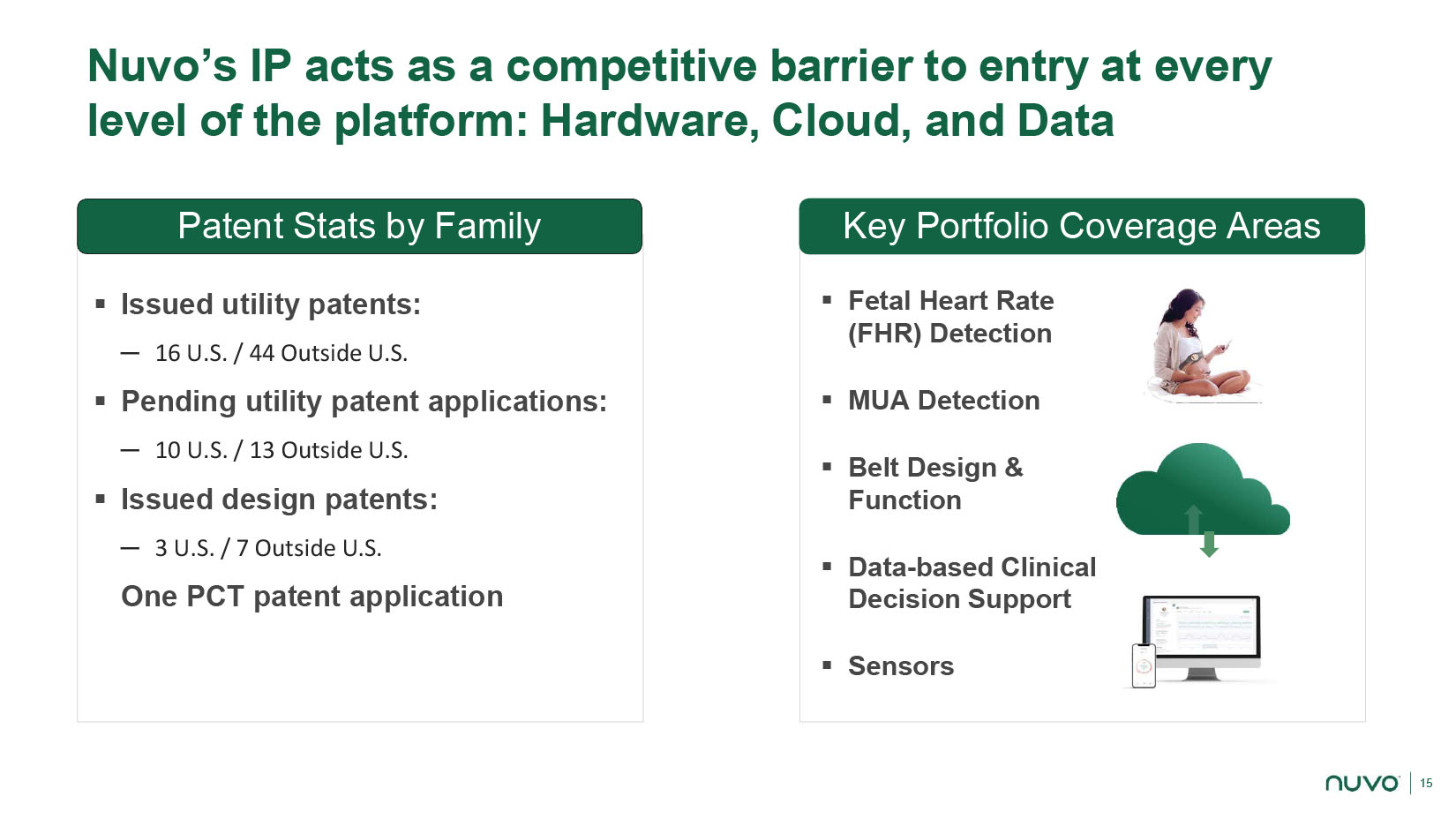
15 Patent Stats by Family ▪ Issued utility patents: ─ 16 U.S. / 44 Outside U.S. ▪ Pending utility patent applications: ─ 10 U.S. / 13 Outside U.S. ▪ Issued design patents: ─ 3 U.S. / 7 Outside U.S. One PCT patent application Nuvo’s IP a cts as a competitive b arrier to e ntry at every level of the p latform: Hardware, Cloud, and Data Key Portfolio Coverage Areas ▪ Fetal Heart Rate (FHR) Detection ▪ MUA Detection ▪ Belt Design & Function ▪ Data - based Clinical Decision Support ▪ Sensors Patent Stats by Family

16 Laurence Klein Founding Investor / Private Equity Fund Owner/Manager Board of Directors Christina Spade Former CEO of AMC Gerald Ostrov Board Chairman Former Chairman & CEO of Bausch + Lomb, Former Group Chairman J&J Nuvo Public Company Leadership Douglas Blankenship Chief Financial Officer Rice Powell CEO and Board Member Former CEO, Chairman, Fresenius Medical Care Adriana Machado Former CEO of GE Brazil Strategic Advisory Council Chair Deb Henretta Independent Board Director Former President P&G

17 Large & growing market in need of innovation We believe Nuvo is one of the leading disruptors of status quo with 2 FDA 510(k) clearances 1 Go - to - market de - risked with commercial partnerships & reimbursed by certain existing codes Notes : 1) I NVU is cleared by the FDA for fetal heart rate, maternal heart rate, and uterine activity (contractions) S ummary

The future of pregnancy care is in our hands. Join the movement at nuvocares.com

















