
Johnson & Johnson Acquisition of Momenta Pharmaceuticals August 19, 2020 Exhibit 99.2

Cautions Concerning Forward-Looking Statements This presentation contains "forward-looking statements" regarding the potential acquisition of Momenta. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: the risk that the closing conditions for the acquisition will not be satisfied, including the risk that clearance under the Hart-Scott-Rodino Antitrust Improvements Act will not be obtained; uncertainty as to the percentage of Momenta stockholders that will support the proposed transaction and tender their shares in the offer; the possibility that the transaction will not be completed in the expected timeframe or at all; potential adverse effects to the businesses of Johnson & Johnson or Momenta during the pendency of the transaction, such as employee departures or distraction of management from business operations; the risk of stockholder litigation relating to the transaction, including resulting expense or delay; the potential that the expected benefits and opportunities of the acquisition, if completed, may not be realized or may take longer to realize than expected; challenges inherent in new product research and development, especially at an early stage of the development program, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new products; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; economic conditions, including currency exchange and interest rate fluctuations; the risks associated with global operations, including the impact of global public health crises and pandemics, such as the outbreak of the coronavirus (COVID-19), on Johnson & Johnson or Momenta and their customers and suppliers, including foreign governments in countries in which Johnson & Johnson or Momenta operates; competition, including technological advances, new products and patents attained by competitors; challenges to patents; changes to applicable laws and regulations, including tax laws and global health care reforms; adverse litigation or government action; changes in behavior and spending patterns or financial distress of purchasers of health care products and services; and trends toward health care cost containment. In addition, if and when the transaction is consummated, there will be risks and uncertainties related to the ability of the Johnson & Johnson family of companies to successfully integrate the products and employees/operations and clinical work of Momenta, as well as the ability to ensure continued performance or market growth of Momenta’s products. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson's Annual Report on Form 10-K for the fiscal year ended December 29, 2019, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in the company’s most recently filed Quarterly Report on Form 10-Q, and the company’s subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Johnson & Johnson does not undertake to update any forward-looking statement as a result of new information or future events or developments. Additional Information The tender offer described in this communication has not yet commenced, and this communication is neither an offer to purchase nor a solicitation of an offer to sell securities. At the time the tender offer is commenced, Johnson & Johnson will cause Merger Sub to file a tender offer statement on Schedule TO with the U.S. Securities and Exchange Commission (SEC). Investors and Momenta Pharmaceuticals, Inc. security holders are strongly advised to read the tender offer statement (including an offer to purchase, letter of transmittal and related tender offer documents) that will be filed by Johnson & Johnson with the SEC and the related solicitation/recommendation statement on Schedule 14D-9 that will be filed by Momenta Pharmaceuticals, Inc. with the SEC, when they become available, because they will contain important information. These documents will be available at no charge on the SEC’s website at www.sec.gov. In addition, a copy of the offer to purchase, letter of transmittal and certain other related tender offer documents (once they become available) may be obtained free of charge by directing a request to Johnson & Johnson, Office of the Corporate Secretary, One Johnson & Johnson Plaza, New Brunswick, NJ 08933, Attn: Corporate Secretary’s Office. A copy of the solicitation/recommendation statement on Schedule 14D-9 (once it becomes available) also may be obtained free of charge from Momenta under the “Investors & News” section of Momenta’s website at https://www.momentapharma.com/home/default.aspx.

Johnson & Johnson Leadership Perspective “This acquisition broadens Janssen’s leadership in autoimmune diseases and provides us with a major potential catalyst for sustained growth. Autoantibody-driven diseases are often serious, and patients are underserved by current treatment options. We’re excited by the opportunity to further advance patient care by combining Johnson & Johnson’s world-class R&D, commercial and supply chain capabilities with Momenta’s talented people, pipeline and deep expertise in this important area.” Jennifer Taubert Executive Vice President & Worldwide Chairman, Pharmaceuticals Johnson & Johnson Mathai Mammen, M.D., Ph.D. Global Head Janssen Research & Development Johnson & Johnson “Nipocalimab, and the rest of Momenta’s pipeline, built over many years by outstanding scientists who have turned important insights into actionable biology, expands and complements our portfolio by giving us clinical-stage and discovery-stage compounds in autoantibody biological pathways. Combining Momenta’s discoveries with our 20-year heritage in immunology, global scope, and scientific and medical expertise, we see a real opportunity to create an entire ‘pipeline in a pathway.’ We are excited about the significant potential to expand on Momenta’s excellent progress in rare diseases, and to increase our impact on patients both within and beyond our current focus areas.”
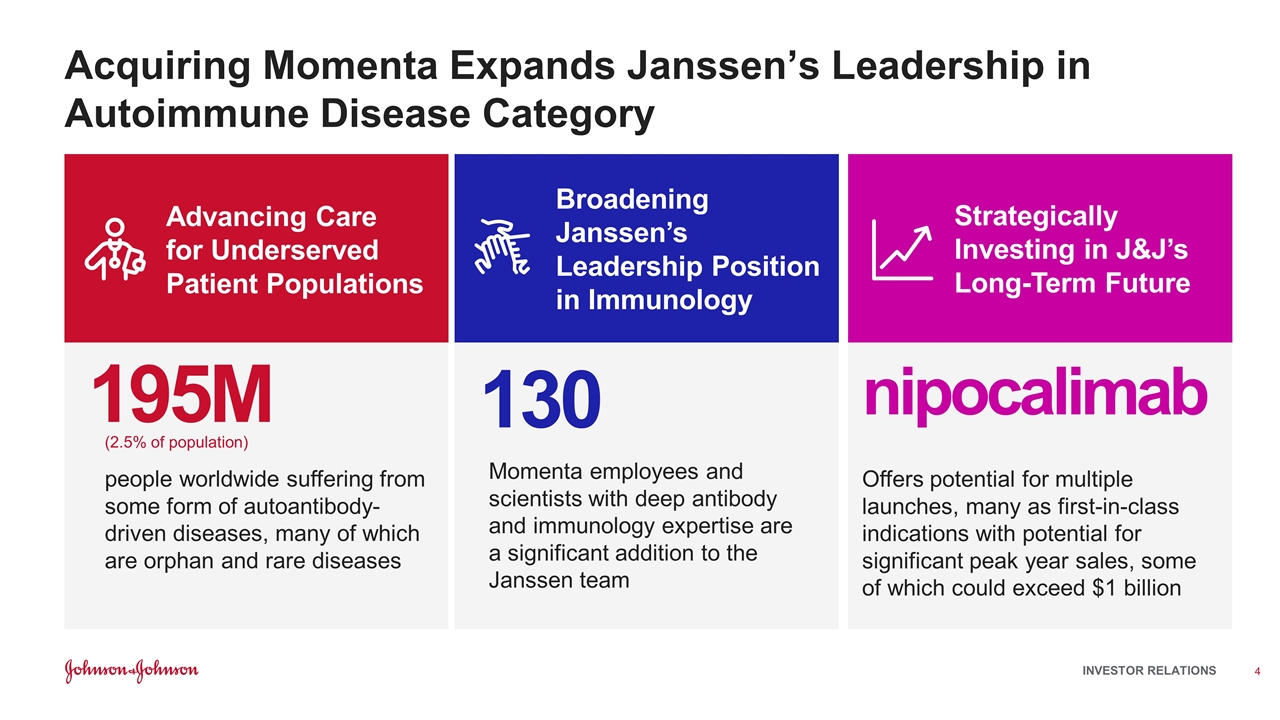
Acquiring Momenta Expands Janssen’s Leadership in Autoimmune Disease Category Advancing Care for Underserved Patient Populations Broadening Janssen’s Leadership Position in Immunology Strategically Investing in J&J’s Long-Term Future 195M (2.5% of population) people worldwide suffering from some form of autoantibody-driven diseases, many of which are orphan and rare diseases 130 Momenta employees and scientists with deep antibody and immunology expertise are a significant addition to the Janssen team nipocalimab Offers potential for multiple launches, many as first-in-class indications with potential for significant peak year sales, some of which could exceed $1 billion
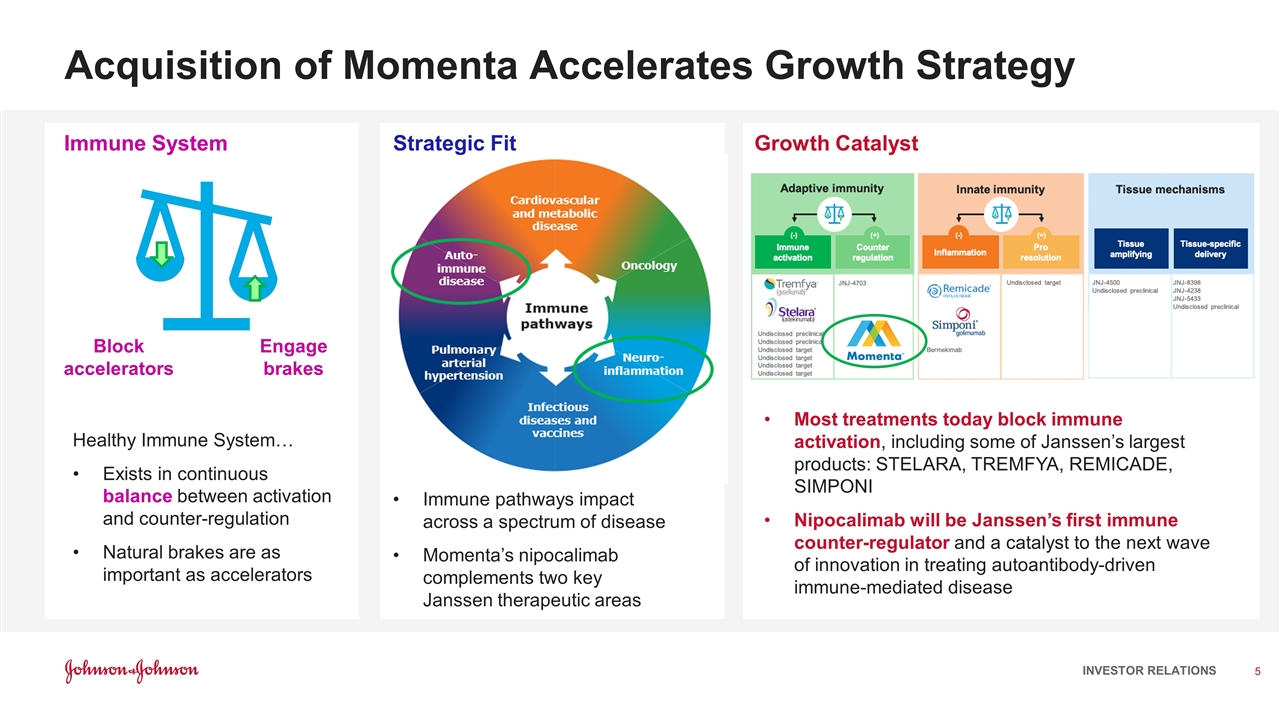
Acquisition of Momenta Accelerates Growth Strategy Immune pathways impact across a spectrum of disease Momenta’s nipocalimab complements two key Janssen therapeutic areas Block accelerators Engage brakes Healthy Immune System… Exists in continuous balance between activation and counter-regulation Natural brakes are as important as accelerators Most treatments today block immune activation, including some of Janssen’s largest products: STELARA, TREMFYA, REMICADE, SIMPONI Nipocalimab will be Janssen’s first immune counter-regulator and a catalyst to the next wave of innovation in treating autoantibody-driven immune-mediated disease Strategic Fit Immune System Growth Catalyst
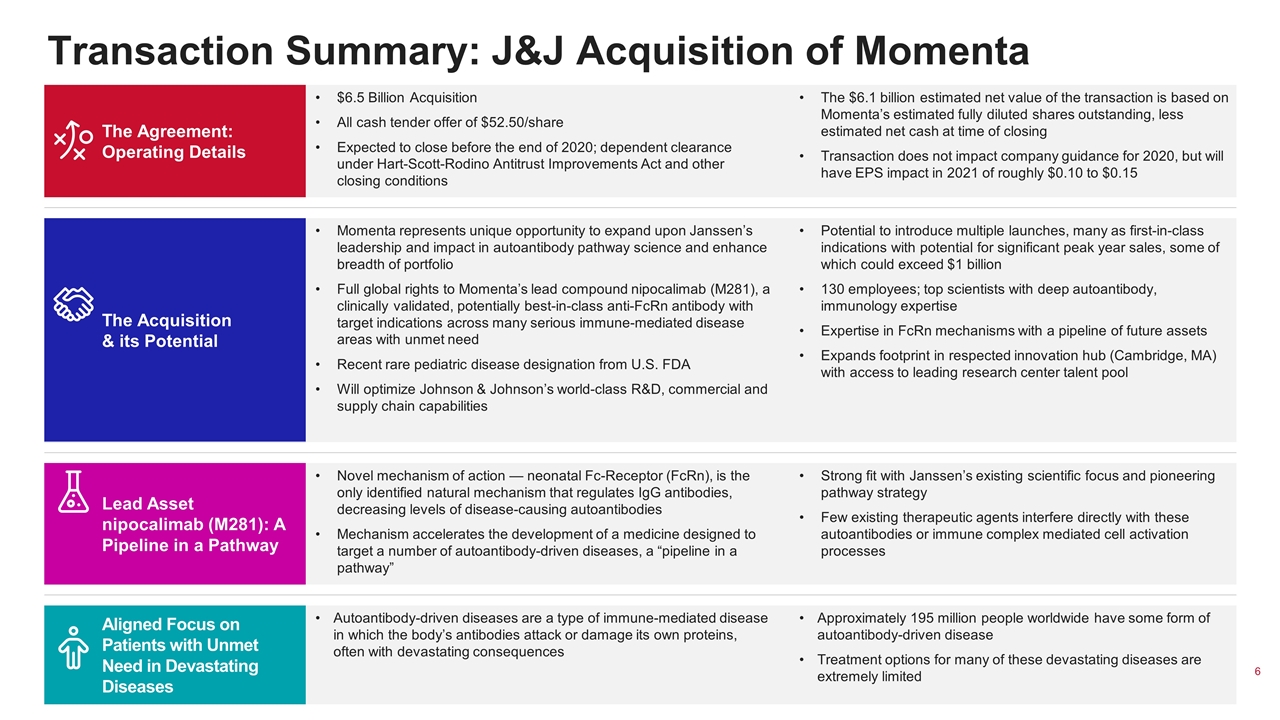
Transaction Summary: J&J Acquisition of Momenta The Agreement: Operating Details $6.5 Billion Acquisition All cash tender offer of $52.50/share Expected to close before the end of 2020; dependent clearance under Hart-Scott-Rodino Antitrust Improvements Act and other closing conditions The $6.1 billion estimated net value of the transaction is based on Momenta’s estimated fully diluted shares outstanding, less estimated net cash at time of closing Transaction does not impact company guidance for 2020, but will have EPS impact in 2021 of roughly $0.10 to $0.15 The Acquisition & its Potential Momenta represents unique opportunity to expand upon Janssen’s leadership and impact in autoantibody pathway science and enhance breadth of portfolio Full global rights to Momenta’s lead compound nipocalimab (M281), a clinically validated, potentially best-in-class anti-FcRn antibody with target indications across many serious immune-mediated disease areas with unmet need Recent rare pediatric disease designation from U.S. FDA Will optimize Johnson & Johnson’s world-class R&D, commercial and supply chain capabilities Potential to introduce multiple launches, many as first-in-class indications with potential for significant peak year sales, some of which could exceed $1 billion 130 employees; top scientists with deep autoantibody, immunology expertise Expertise in FcRn mechanisms with a pipeline of future assets Expands footprint in respected innovation hub (Cambridge, MA) with access to leading research center talent pool Lead Asset nipocalimab (M281): A Pipeline in a Pathway Novel mechanism of action — neonatal Fc-Receptor (FcRn), is the only identified natural mechanism that regulates IgG antibodies, decreasing levels of disease-causing autoantibodies Mechanism accelerates the development of a medicine designed to target a number of autoantibody-driven diseases, a “pipeline in a pathway” Strong fit with Janssen’s existing scientific focus and pioneering pathway strategy Few existing therapeutic agents interfere directly with these autoantibodies or immune complex mediated cell activation processes Aligned Focus on Patients with Unmet Need in Devastating Diseases Autoantibody-driven diseases are a type of immune-mediated disease in which the body’s antibodies attack or damage its own proteins, often with devastating consequences Approximately 195 million people worldwide have some form of autoantibody-driven disease Treatment options for many of these devastating diseases are extremely limited





