
1 Endo Investor Presentation September 2024

2 This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, any statements relating to listing, uplisting, or trading on a national securities exchange, business plans, business growth, growth strategies, cash flow generation, commercial or manufacturing capabilities, intellectual property, litigation, product development, product pipeline, product launches and any other statements that refer to expected, estimated or anticipated future results or that do not relate solely to historical facts. Statements including words such as "believes," "expects," "anticipates," "intends," "estimates," "plan," "will," "may," "look forward," "intends," "guidance," "future," "potential," "target" or similar expressions are forward-looking statements. Because these statements reflect Endo's current views, expectations and beliefs concerning future events, they involve risks and uncertainties, some of which Endo may not currently be able to predict. Although Endo believes that these forward-looking statements and other information are based upon reasonable assumptions and expectations, readers should not place undue reliance on these or any other forward-looking statements and information. Actual results may differ materially and adversely from current expectations based on a number of factors, including, among other things, the following: timing of receipt of required approvals and satisfaction of other customary conditions for listing on a national securities exchange; timing or results of any potential future litigation, investigations, claims, actual or contingent liabilities; changes in competitive, market or regulatory conditions; changes in legislation or regulations; the ability to obtain and maintain adequate protection for intellectual property rights; the impacts of competition such as those related to XIAFLEX®; the timing and uncertainty of the results of both the research and development and regulatory processes, including regulatory approvals; health care and cost containment reforms, including government pricing, tax and reimbursement policies litigation; the performance including the approval, introduction and consumer and physician acceptance of current and new products; the performance of third parties upon whom we rely for goods and services; issues associated with our supply chain; our ability to develop and expand our product pipeline and to continue to develop the market for XIAFLEX® and other branded, sterile injectable or unbranded products; the effectiveness of advertising and other promotional campaigns; the timely and successful implementation of any business development and/or strategic priorities; uncertainty associated with the identification of and successful consummation and execution of external corporate development initiatives and strategic partnering transactions; and our ability to obtain and successfully manufacture, maintain and distribute a sufficient supply of products to meet market demand in a timely manner. Endo assumes no obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise, except as may be required under applicable securities laws. Additional information concerning risk factors, including those referenced above, can be found in press releases issued by Endo and in Endo’s public filings with the U.S. Securities and Exchange Commission (the "SEC"), including the discussion under the heading “Risk Factors” in Endo’s most recent Form 10-Q and in Endo's final prospectus filed pursuant to Rule 424(b) under the Securities Act of 1933, as amended, in connection with Endo’s Form S-1/A. Cautionary Note Regarding Forward-Looking Statements ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial.

3 This presentation may refer to non-GAAP financial measures, including, among others, adjusted earnings before interest, taxes, depreciation and amortization (“adjusted EBITDA”), adjusted net income, adjusted gross profit, adjusted gross margin and adjusted operating expenses that are not prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) and that may be different from non-GAAP financial measures used by other companies. Endo utilizes these financial measures because ( i) they are used by Endo, along with financial measures in accordance with GAAP, to evaluate Endo’s operating performance; (ii) Endo believes that they will be used by certain investors to measure Endo’s operating results; (iii) the Compensation & Human Capital Committee of Endo’s Board of Directors uses adjusted EBITDA, or similar measures, in assessing the performance and compensation of substantially all of Endo’s employees, including executive officers. Endo believes that presenting these non-GAAP measures provides useful information about Endo’s per formance across reporting periods on a consistent basis by excluding certain items, which may be favorable or unfavorable, pursuant to certain specified procedures. These non-GAAP measures should be considered supplemental to and not a substitute for financial information prepared in accordance with GAAP. Endo’s definition of these non-GAAP measures may differ from similarly titled measures used by others. Refer to the Second Quarter Results slides for a reconciliation of these non-GAAP financial measures to the most directly comparable GAAP metric. Non-GAAP Financial Measures ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial.

4 Business Overview ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial.
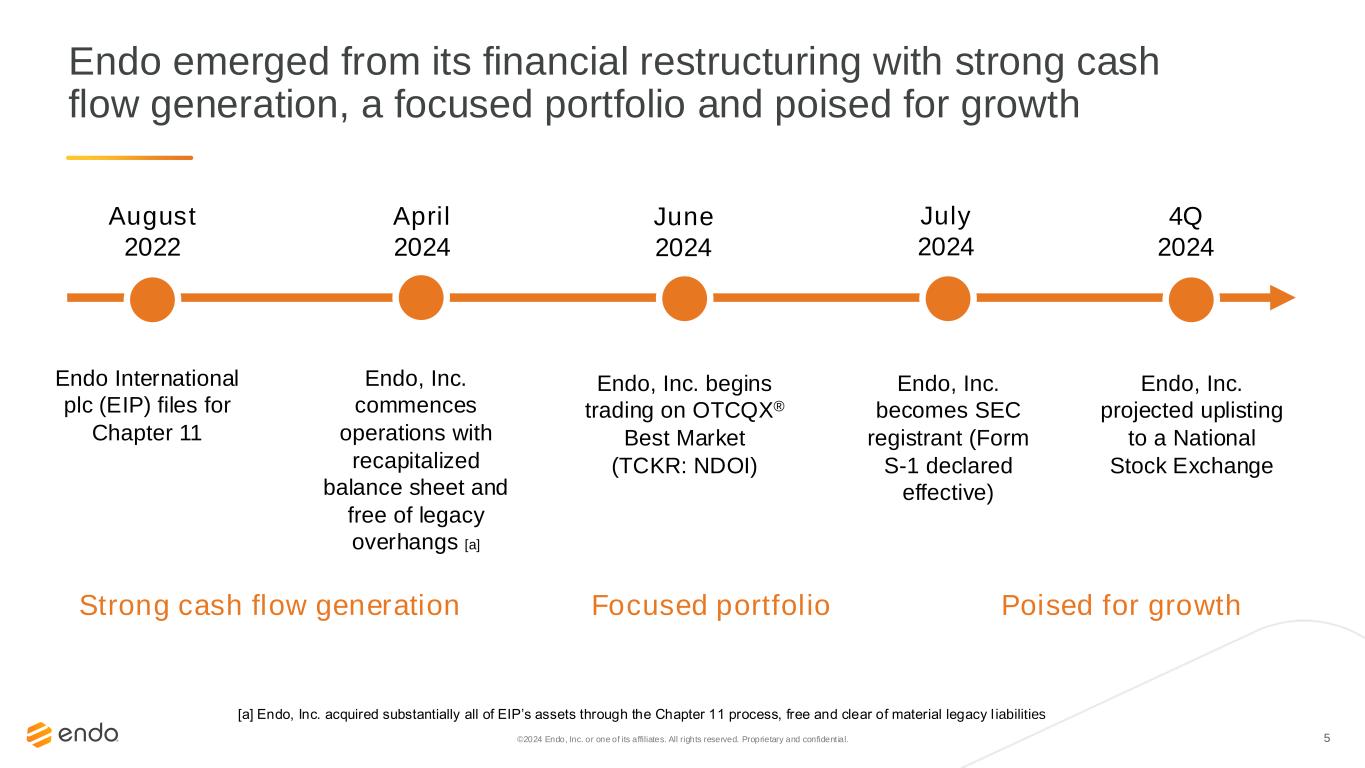
5©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Endo emerged from its financial restructuring with strong cash flow generation, a focused portfolio and poised for growth Strong cash flow generation Focused portfolio Poised for growth August 2022 April 2024 June 2024 July 2024 4Q 2024 Endo International plc (EIP) files for Chapter 11 Endo, Inc. commences operations with recapitalized balance sheet and free of legacy overhangs [a] Endo, Inc. begins trading on OTCQX® Best Market (TCKR: NDOI) Endo, Inc. becomes SEC registrant (Form S-1 declared effective) Endo, Inc. projected uplisting to a National Stock Exchange [a] Endo, Inc. acquired substantially all of EIP’s assets through the Chapter 11 process, free and clear of material legacy l iabilities
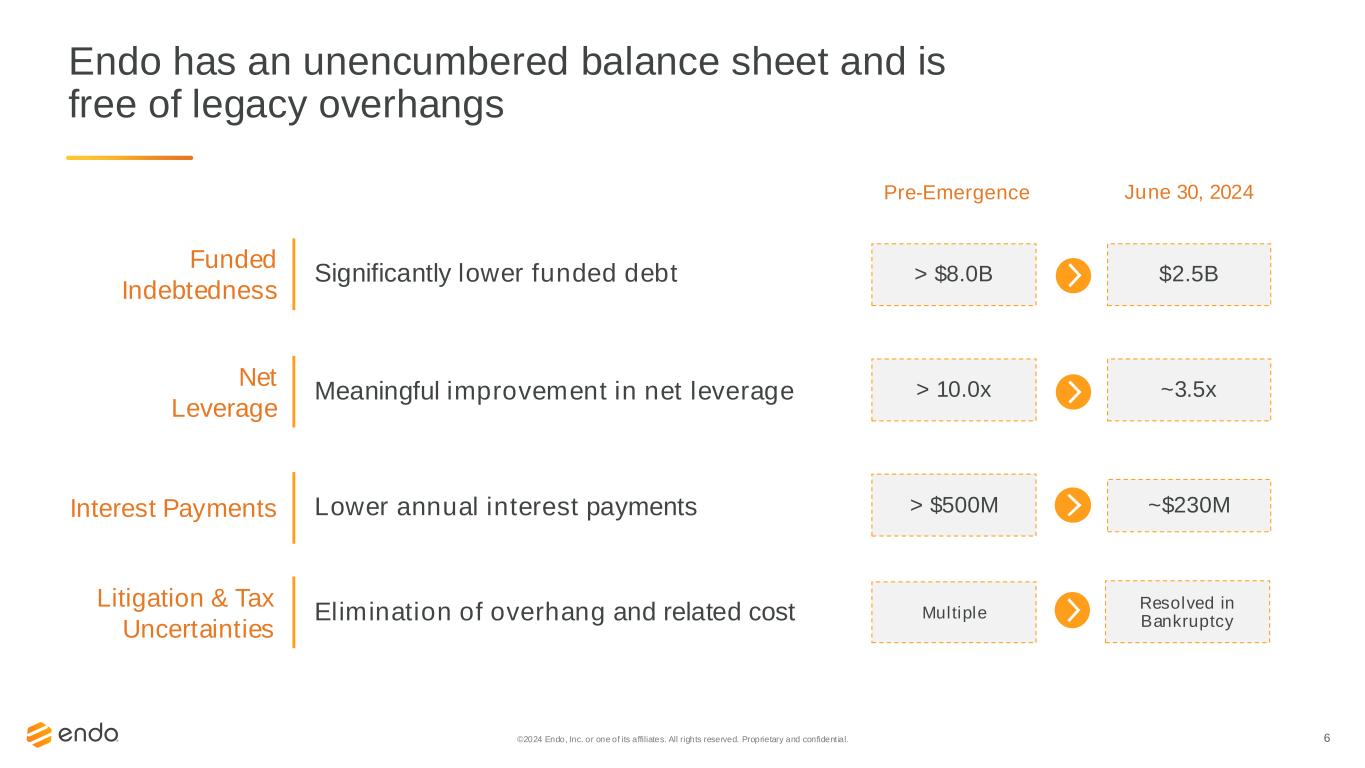
6 Significantly lower funded debt Funded Indebtedness Meaningful improvement in net leverage Net Leverage Lower annual interest paymentsInterest Payments > $8.0B $2.5B > 10.0x ~3.5x > $500M ~$230M Pre-Emergence June 30, 2024 Elimination of overhang and related cost Litigation & Tax Uncertainties Multiple Resolved in Bankruptcy Endo has an unencumbered balance sheet and is free of legacy overhangs ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial.
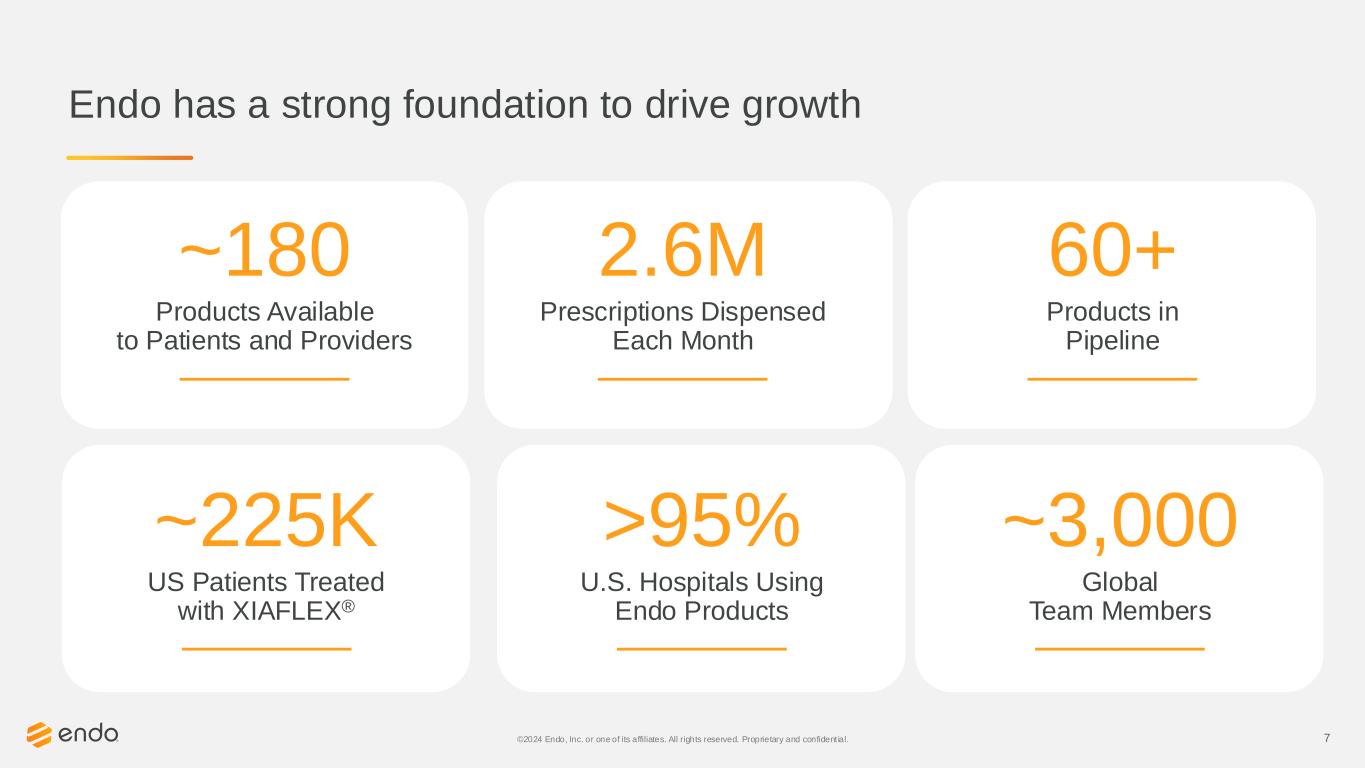
7 Endo has a strong foundation to drive growth ~180 Products Available to Patients and Providers 2.6M Prescriptions Dispensed Each Month 60+ Products in Pipeline ~225K US Patients Treated with XIAFLEX® >95% U.S. Hospitals Using Endo Products ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. ~3,000 Global Team Members
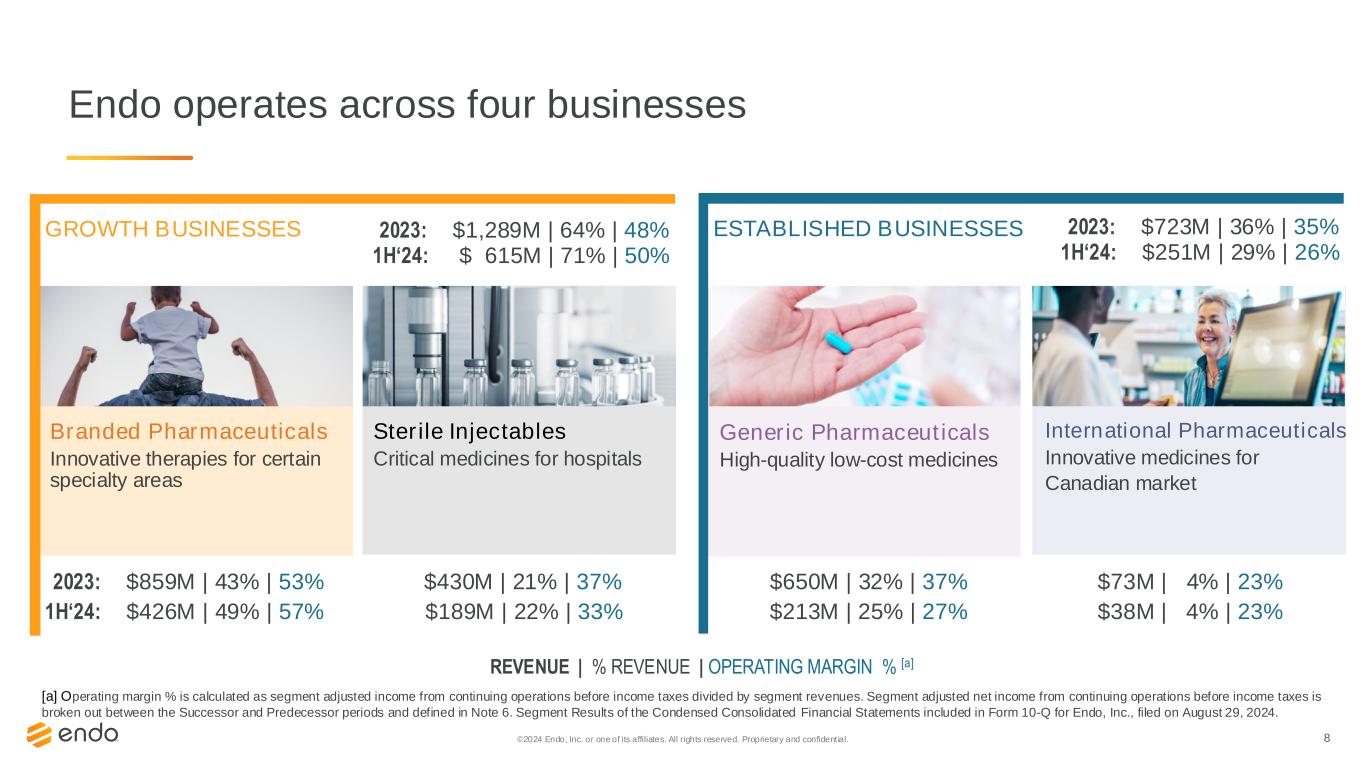
8 Endo operates across four businesses Branded Pharmaceuticals Innovative therapies for certain specialty areas Sterile Injectables Critical medicines for hospitals Generic Pharmaceuticals High-quality low-cost medicines International Pharmaceuticals Innovative medicines for Canadian market 2023: $859M | 43% | 53% $430M | 21% | 37% $650M | 32% | 37% $73M | 4% | 23% REVENUE | % REVENUE | OPERATING MARGIN % [a] GROWTH BUSINESSES ESTABLISHED BUSINESSES ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. [a] Operating margin % is calculated as segment adjusted income from continuing operations before income taxes divided by segment revenues. Segment adjusted net income from continuing operations before income taxes is broken out between the Successor and Predecessor periods and defined in Note 6. Segment Results of the Condensed Consolidated Financial Statements included in Form 10-Q for Endo, Inc., filed on August 29, 2024. 1H‘24: $426M | 49% | 57% $189M | 22% | 33% $213M | 25% | 27% $38M | 4% | 23% 1H‘24: $ 615M | 71% | 50% 2023: $1,289M | 64% | 48% 1H‘24: $251M | 29% | 26% 2023: $723M | 36% | 35%
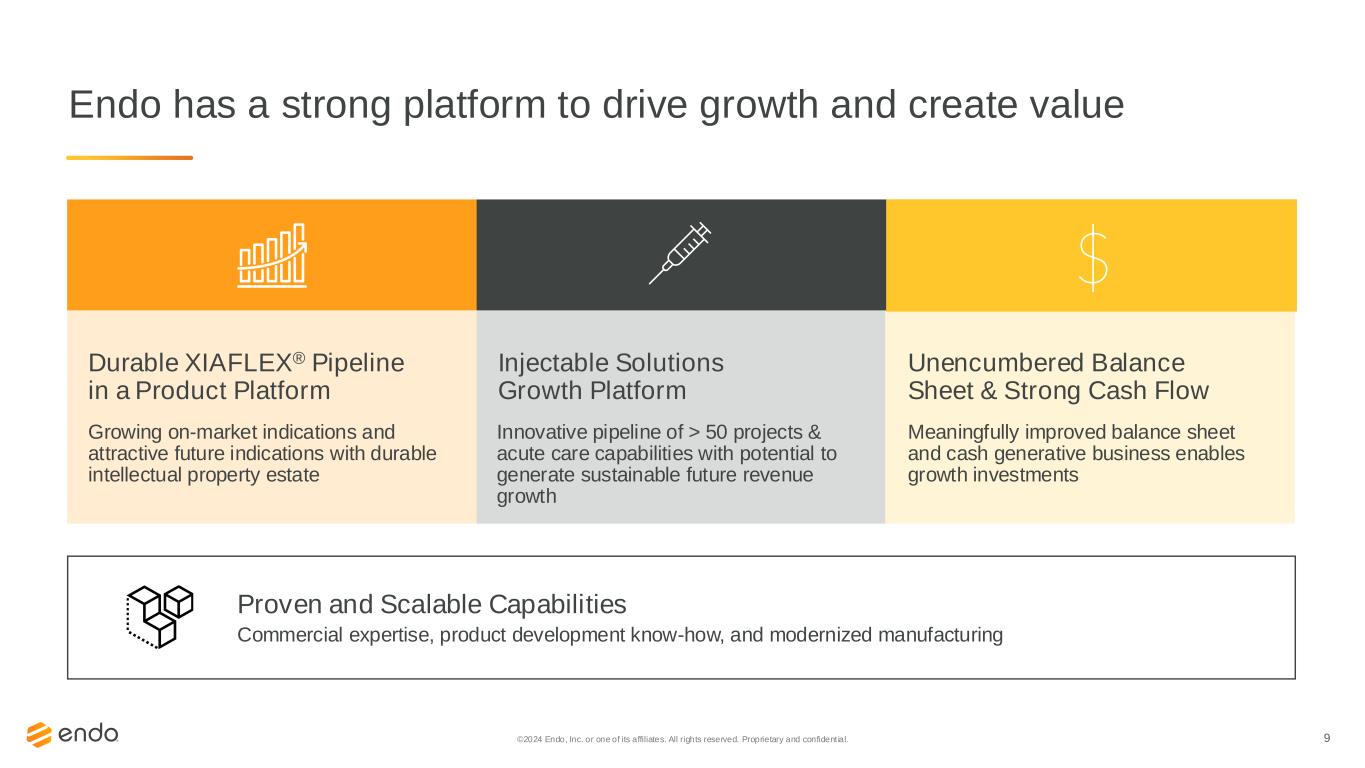
9 Endo has a strong platform to drive growth and create value Durable XIAFLEX® Pipeline in a Product Platform Injectable Solutions Growth Platform Proven and Scalable Capabilities Innovative pipeline of > 50 projects & acute care capabilities with potential to generate sustainable future revenue growth Commercial expertise, product development know-how, and modernized manufacturing Unencumbered Balance Sheet & Strong Cash Flow Meaningfully improved balance sheet and cash generative business enables growth investments Growing on-market indications and attractive future indications with durable intellectual property estate ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial.
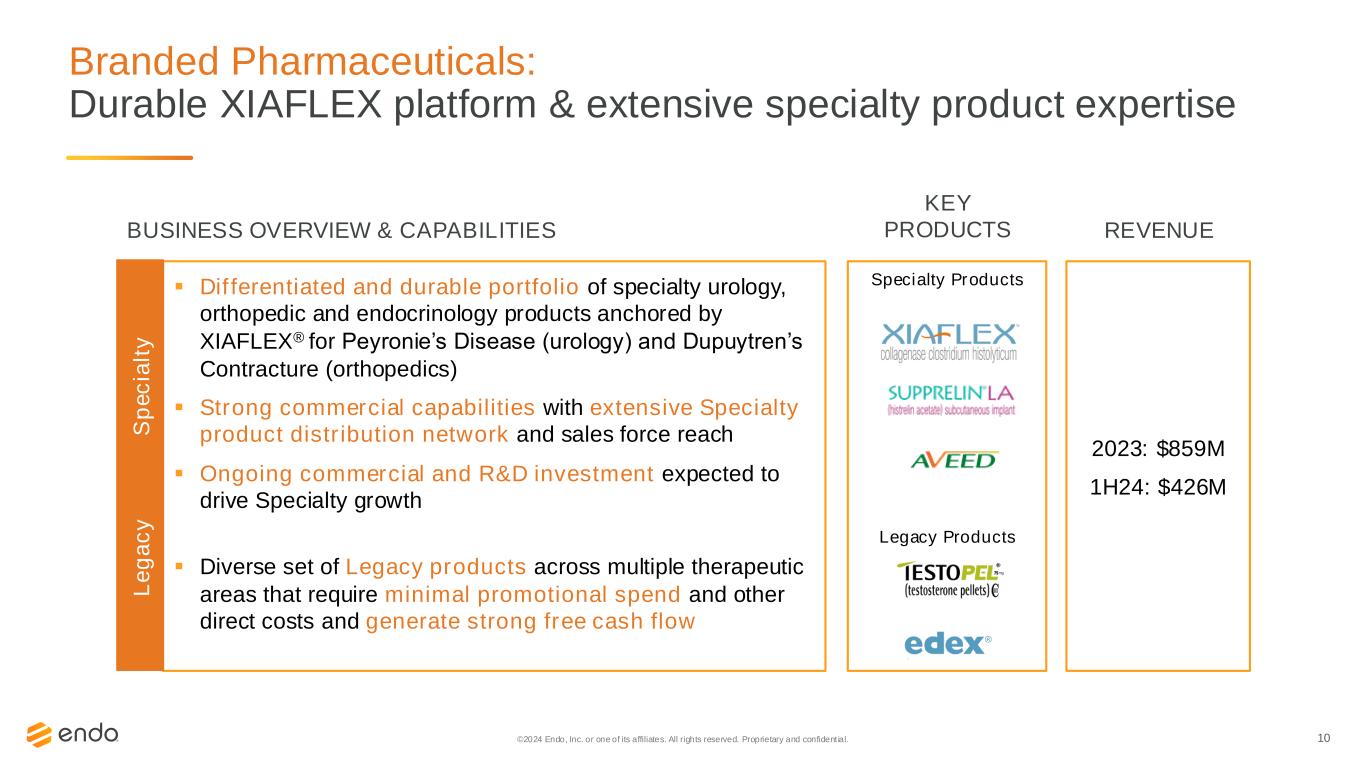
10 Branded Pharmaceuticals: Durable XIAFLEX platform & extensive specialty product expertise ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Specialty Products Legacy Products 2023: $859M 1H24: $426M ▪ Differentiated and durable portfolio of specialty urology, orthopedic and endocrinology products anchored by XIAFLEX® for Peyronie’s Disease (urology) and Dupuytren’s Contracture (orthopedics) ▪ Strong commercial capabilities with extensive Specialty product distribution network and sales force reach ▪ Ongoing commercial and R&D investment expected to drive Specialty growth ▪ Diverse set of Legacy products across multiple therapeutic areas that require minimal promotional spend and other direct costs and generate strong free cash flow BUSINESS OVERVIEW & CAPABILITIES REVENUE KEY PRODUCTS L e g a c y S p e c ia lt y
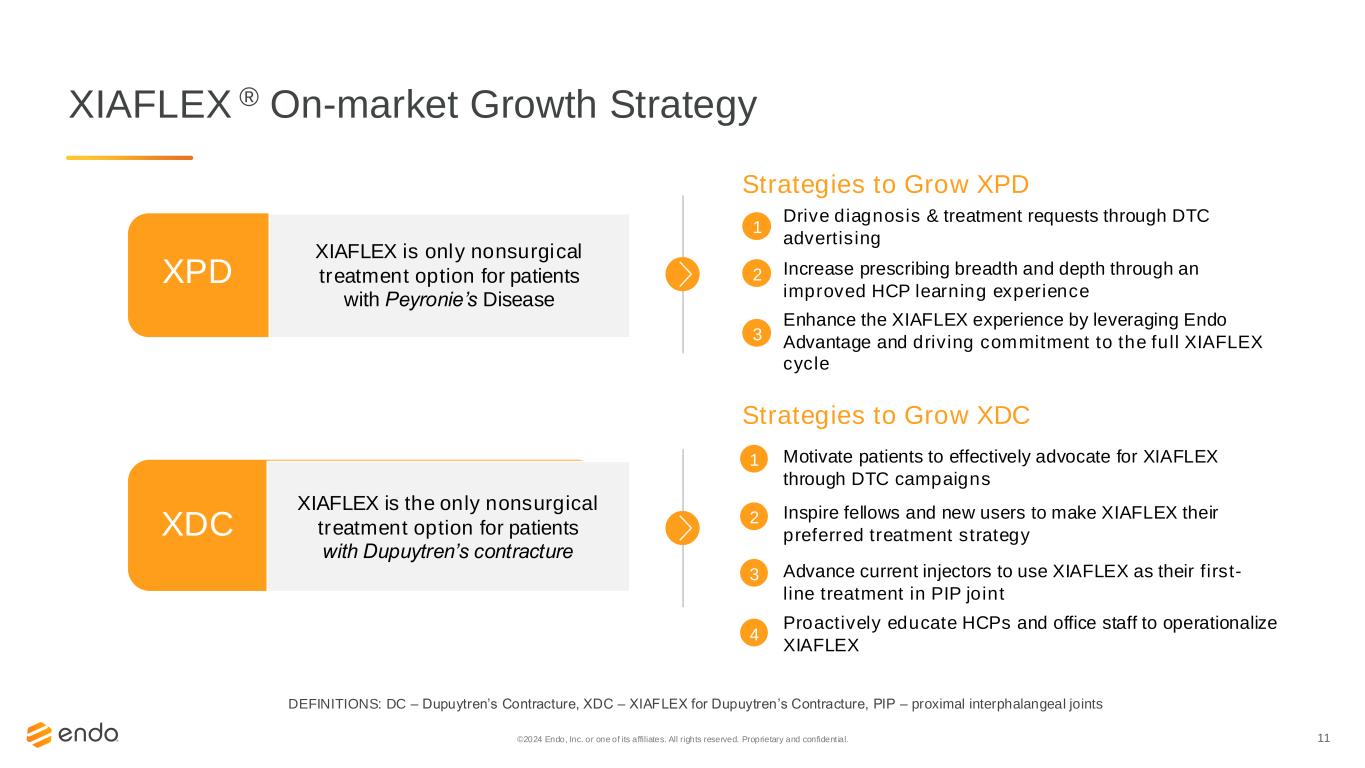
11©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. XIAFLEX ® On-market Growth Strategy XDC Strategies to Grow XDC Motivate patients to effectively advocate for XIAFLEX through DTC campaigns Inspire fellows and new users to make XIAFLEX their preferred treatment strategy Advance current injectors to use XIAFLEX as their first- line treatment in PIP joint Proactively educate HCPs and office staff to operationalize XIAFLEX 1 2 3 4 XIAFLEX is the only nonsurgical treatment option for patients with Dupuytren’s contracture DEFINITIONS: DC – Dupuytren’s Contracture, XDC – XIAFLEX for Dupuytren’s Contracture, PIP – proximal interphalangeal joints XIAFLEX Peyronie’s Disease Indication Represents Endo’s Single Largest Branded Product XPD XPD Strategies to Grow XPD Drive diagnosis & treatment requests through DTC advertising Enhance the XIAFLEX experience by leveraging Endo Advantage and driving commitment to the full XIAFLEX cycle Increase prescribing breadth and depth through an improved HCP learning experience 1 2 3 XIAFLEX is only nonsurgical treatment option for patients with Peyronie’s Disease XPD
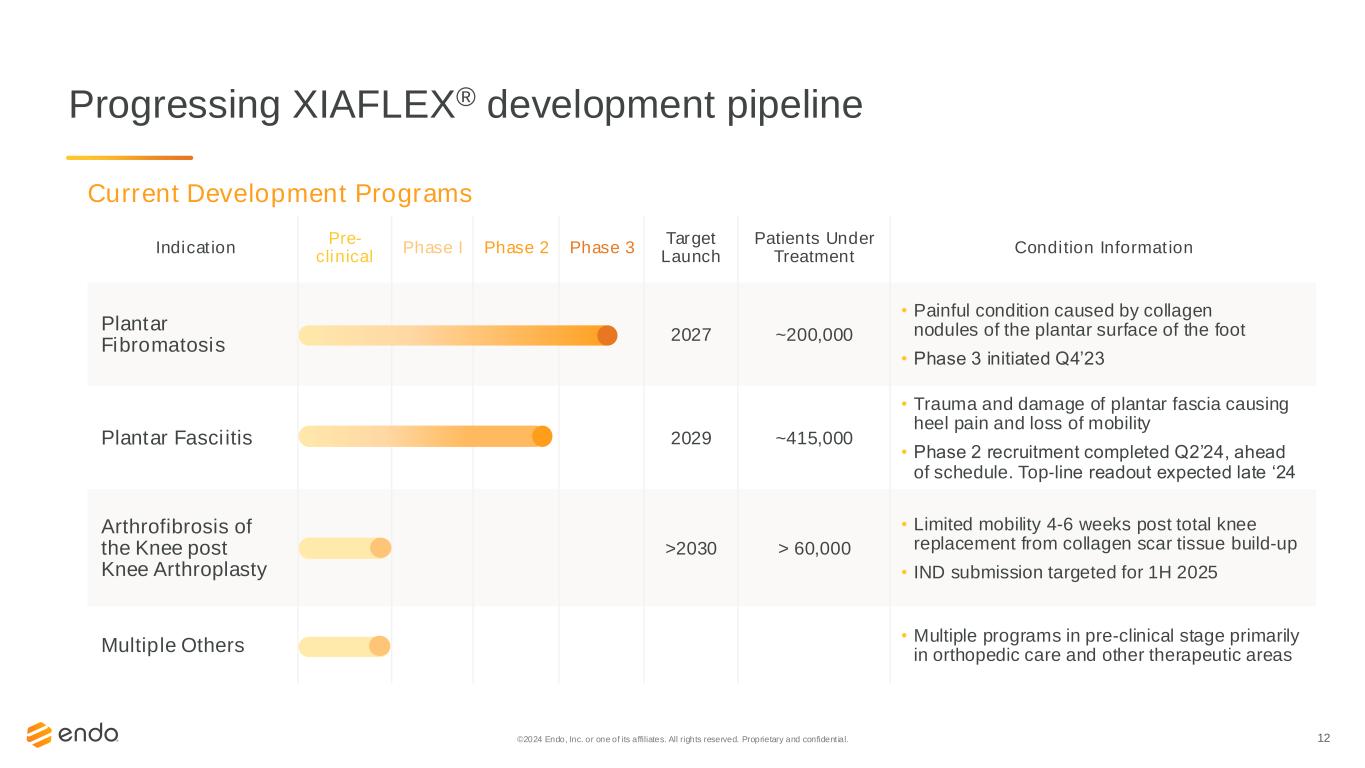
12 Progressing XIAFLEX® development pipeline Indication Pre- clinical Phase I Phase 2 Phase 3 Target Launch Patients Under Treatment Condition Information Plantar Fibromatosis 2027 ~200,000 • Painful condition caused by collagen nodules of the plantar surface of the foot • Phase 3 initiated Q4’23 Plantar Fasciitis 2029 ~415,000 • Trauma and damage of plantar fascia causing heel pain and loss of mobility • Phase 2 recruitment completed Q2’24, ahead of schedule. Top-line readout expected late ‘24 Arthrofibrosis of the Knee post Knee Arthroplasty >2030 > 60,000 • Limited mobility 4-6 weeks post total knee replacement from collagen scar tissue build-up • IND submission targeted for 1H 2025 Multiple Others • Multiple programs in pre-clinical stage primarily in orthopedic care and other therapeutic areas Current Development Programs ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial.
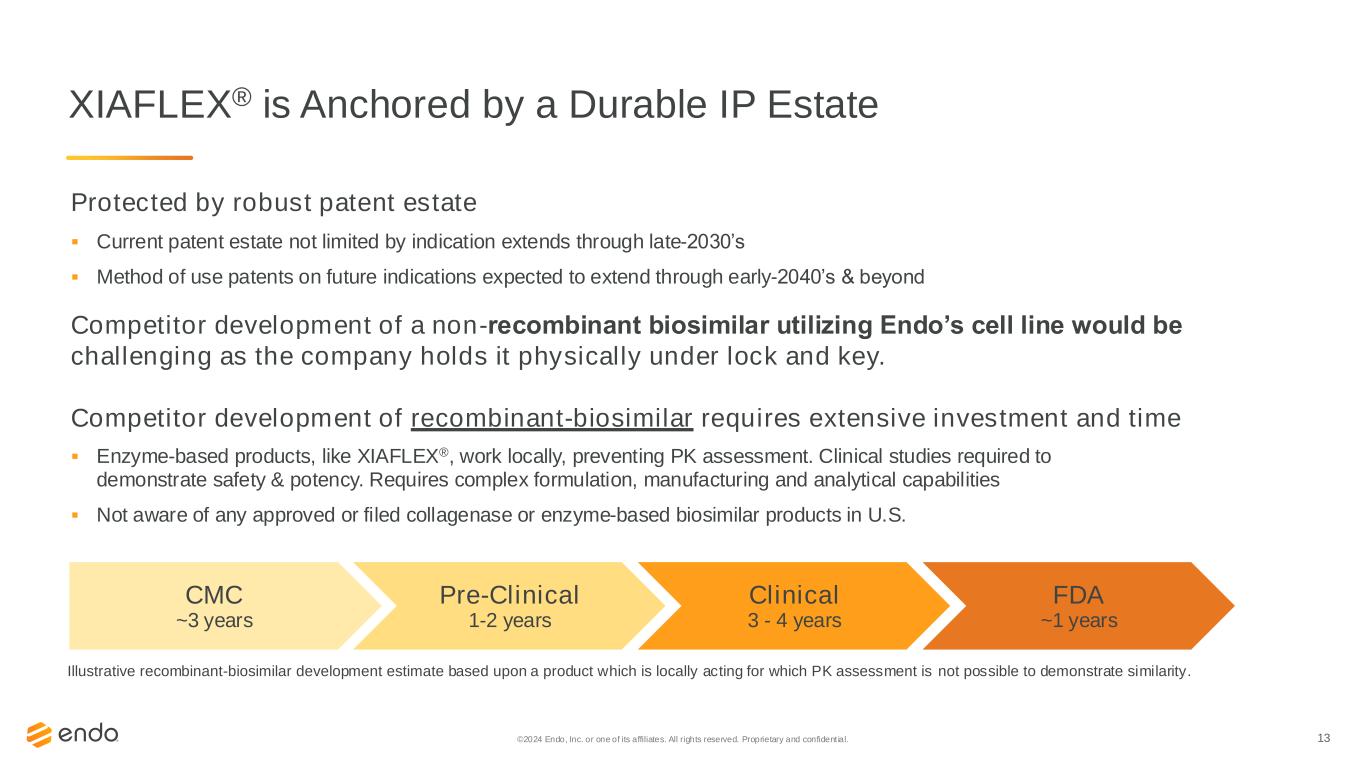
13©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. XIAFLEX® is Anchored by a Durable IP Estate Protected by robust patent estate ▪ Current patent estate not limited by indication extends through late-2030’s ▪ Method of use patents on future indications expected to extend through early-2040’s & beyond Competitor development of a non-recombinant biosimilar utilizing Endo’s cell line would be challenging as the company holds it physically under lock and key. Competitor development of recombinant-biosimilar requires extensive investment and time ▪ Enzyme-based products, like XIAFLEX®, work locally, preventing PK assessment. Clinical studies required to demonstrate safety & potency. Requires complex formulation, manufacturing and analytical capabilities ▪ Not aware of any approved or filed collagenase or enzyme-based biosimilar products in U.S. Illustrative recombinant-biosimilar development estimate based upon a product which is locally acting for which PK assessment is not possible to demonstrate similarity. CMC ~3 years Pre-Clinical 1-2 years Clinical 3 - 4 years FDA ~1 years
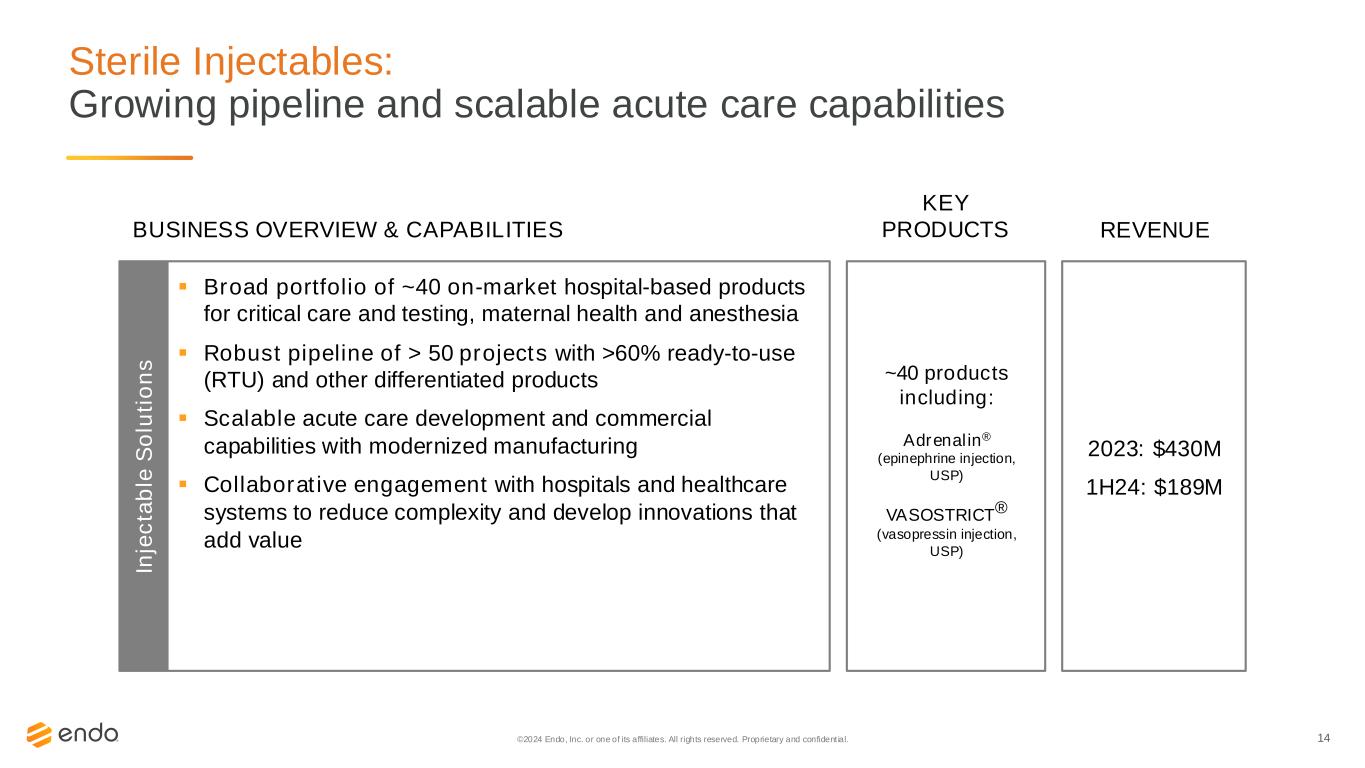
14 Sterile Injectables: Growing pipeline and scalable acute care capabilities ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. ~40 products including: Adrenalin® (epinephrine injection, USP) VASOSTRICT® (vasopressin injection, USP) 2023: $430M 1H24: $189M ▪ Broad portfolio of ~40 on-market hospital-based products for critical care and testing, maternal health and anesthesia ▪ Robust pipeline of > 50 projects with >60% ready-to-use (RTU) and other differentiated products ▪ Scalable acute care development and commercial capabilities with modernized manufacturing ▪ Collaborative engagement with hospitals and healthcare systems to reduce complexity and develop innovations that add value BUSINESS OVERVIEW & CAPABILITIES REVENUE KEY PRODUCTS In je c ta b le S o lu ti o n s
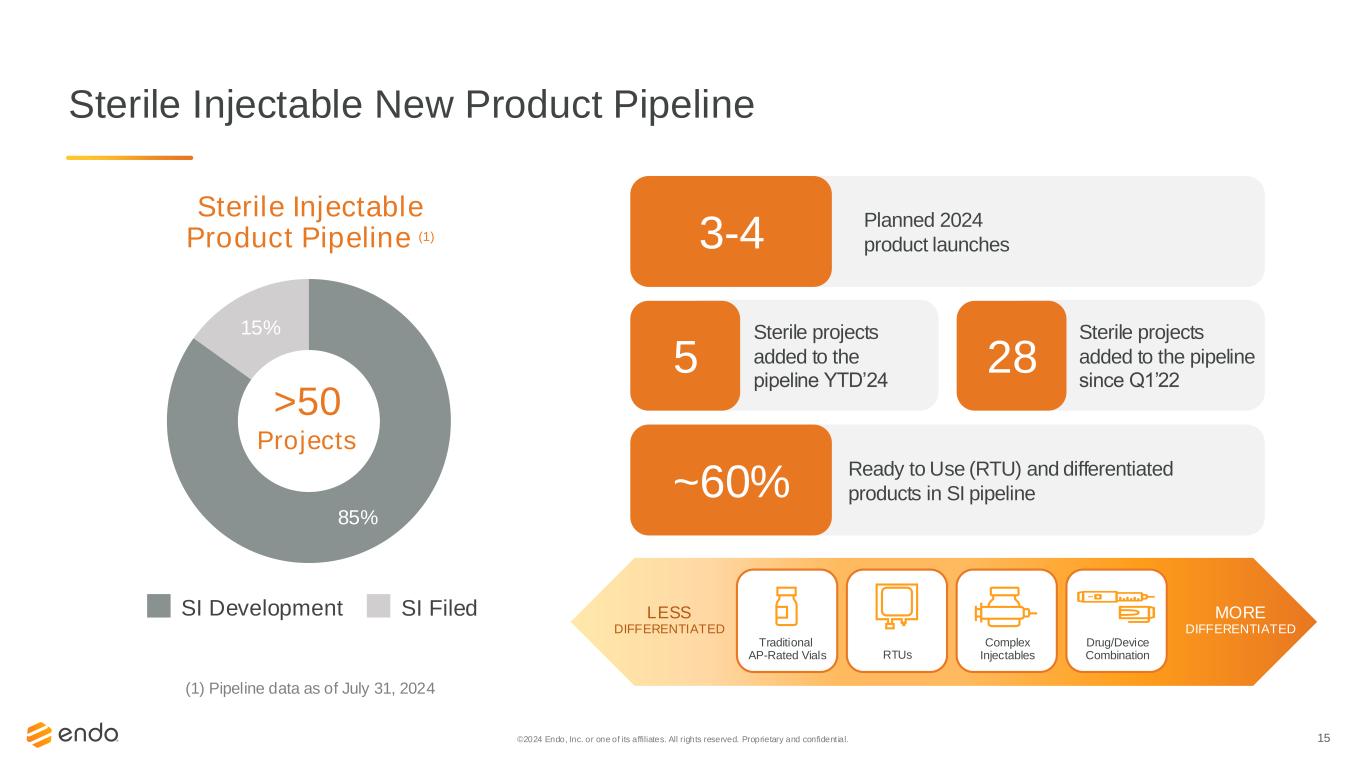
15 Sterile Injectable New Product Pipeline 85% 15% >50 Projects RTUs Complex Injectables Traditional AP-Rated Vials Drug/Device Combination LESS DIFFERENTIATED MORE DIFFERENTIATED Sterile Injectable Product Pipeline (1) Planned 2024 product launches3-4 (1) Pipeline data as of July 31, 2024 SI Development SI Filed Sterile projects added to the pipeline YTD’24 5 Sterile projects added to the pipeline since Q1’22 28 Ready to Use (RTU) and differentiated products in SI pipeline~60% ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial.
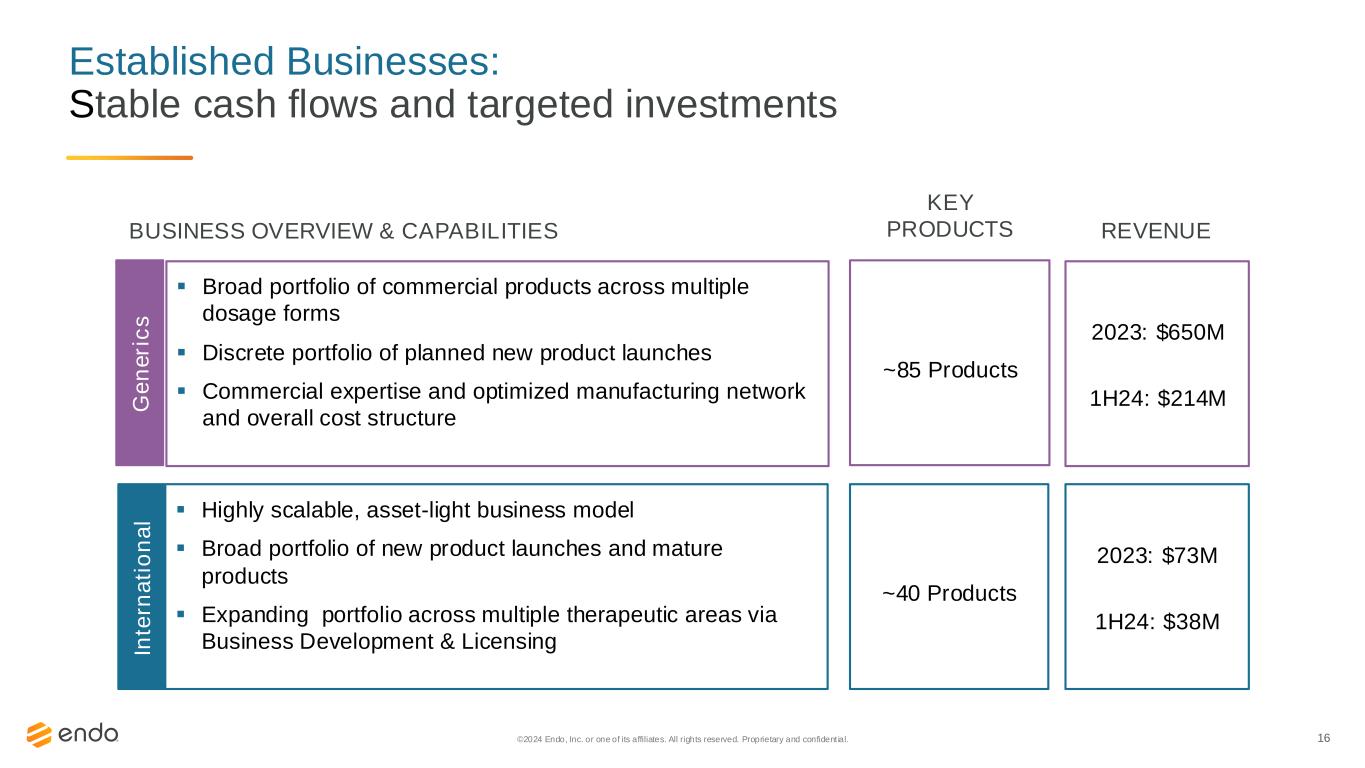
16 Established Businesses: Stable cash flows and targeted investments ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. ~85 Products 2023: $650M 1H24: $214M ▪ Broad portfolio of commercial products across multiple dosage forms ▪ Discrete portfolio of planned new product launches ▪ Commercial expertise and optimized manufacturing network and overall cost structure BUSINESS OVERVIEW & CAPABILITIES REVENUE KEY PRODUCTS ~40 Products 2023: $73M 1H24: $38M ▪ Highly scalable, asset-light business model ▪ Broad portfolio of new product launches and mature products ▪ Expanding portfolio across multiple therapeutic areas via Business Development & Licensing G e n e ri c s In te rn a ti o n a l
17 Endo has a strong platform to drive growth and create value Durable XIAFLEX® Pipeline in a Product Platform Injectable Solutions Growth Platform Proven and Scalable Capabilities Innovative pipeline of > 50 projects & acute care capabilities with potential to generate sustainable future revenue growth Commercial expertise, product development know-how, and modernized manufacturing Unencumbered Balance Sheet & Strong Cash Flow Meaningfully improved balance sheet and cash generative business enables growth investments Growing on-market indications and attractive future indications with durable intellectual property estate ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial.

18 Second Quarter Results ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial.
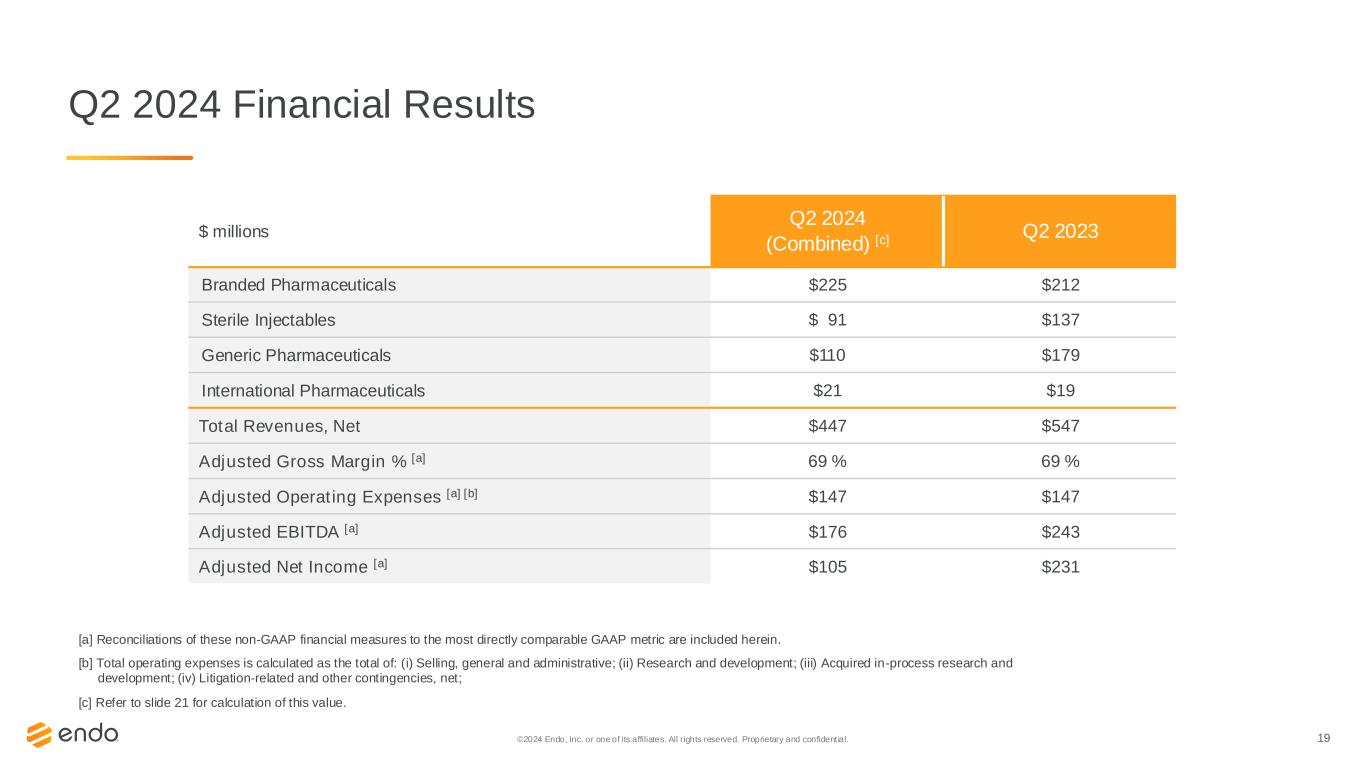
19©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Q2 2024 Financial Results [a] Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP metric are included herein. [b] Total operating expenses is calculated as the total of: (i) Selling, general and administrative; (ii) Research and development; (iii) Acquired in-process research and development; (iv) Litigation-related and other contingencies, net; [c] Refer to slide 21 for calculation of this value. $ millions Q2 2024 (Combined) [c] Q2 2023 Branded Pharmaceuticals $225 $212 Sterile Injectables $ 91 $137 Generic Pharmaceuticals $110 $179 International Pharmaceuticals $21 $19 Total Revenues, Net $447 $547 Adjusted Gross Margin % [a] 69 % 69 % Adjusted Operating Expenses [a] [b] $147 $147 Adjusted EBITDA [a] $176 $243 Adjusted Net Income [a] $105 $231
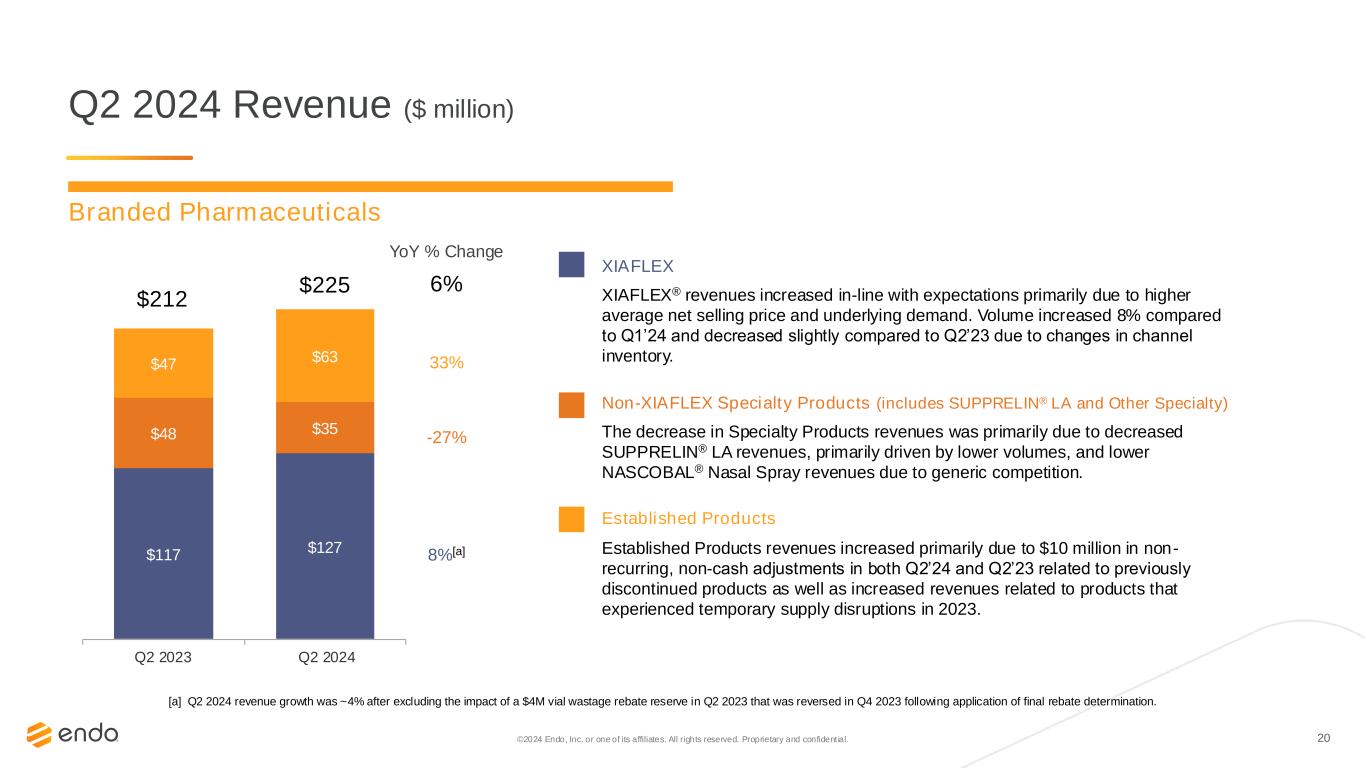
20©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Q2 2024 Revenue ($ million) $117 $127 $48 $35 $47 $63 $212 $225 Q2 2023 Q2 2024 YoY % Change 6% 33% -27% 8%[a] XIAFLEX XIAFLEX® revenues increased in-line with expectations primarily due to higher average net selling price and underlying demand. Volume increased 8% compared to Q1’24 and decreased slightly compared to Q2’23 due to changes in channel inventory. Non-XIAFLEX Specialty Products (includes SUPPRELIN® LA and Other Specialty) The decrease in Specialty Products revenues was primarily due to decreased SUPPRELIN® LA revenues, primarily driven by lower volumes, and lower NASCOBAL® Nasal Spray revenues due to generic competition. Established Products Established Products revenues increased primarily due to $10 million in non- recurring, non-cash adjustments in both Q2’24 and Q2’23 related to previously discontinued products as well as increased revenues related to products that experienced temporary supply disruptions in 2023. [a] Q2 2024 revenue growth was ~4% after excluding the impact of a $4M vial wastage rebate reserve in Q2 2023 that was reversed in Q4 2023 following application of final rebate determination. Branded Pharmaceuticals
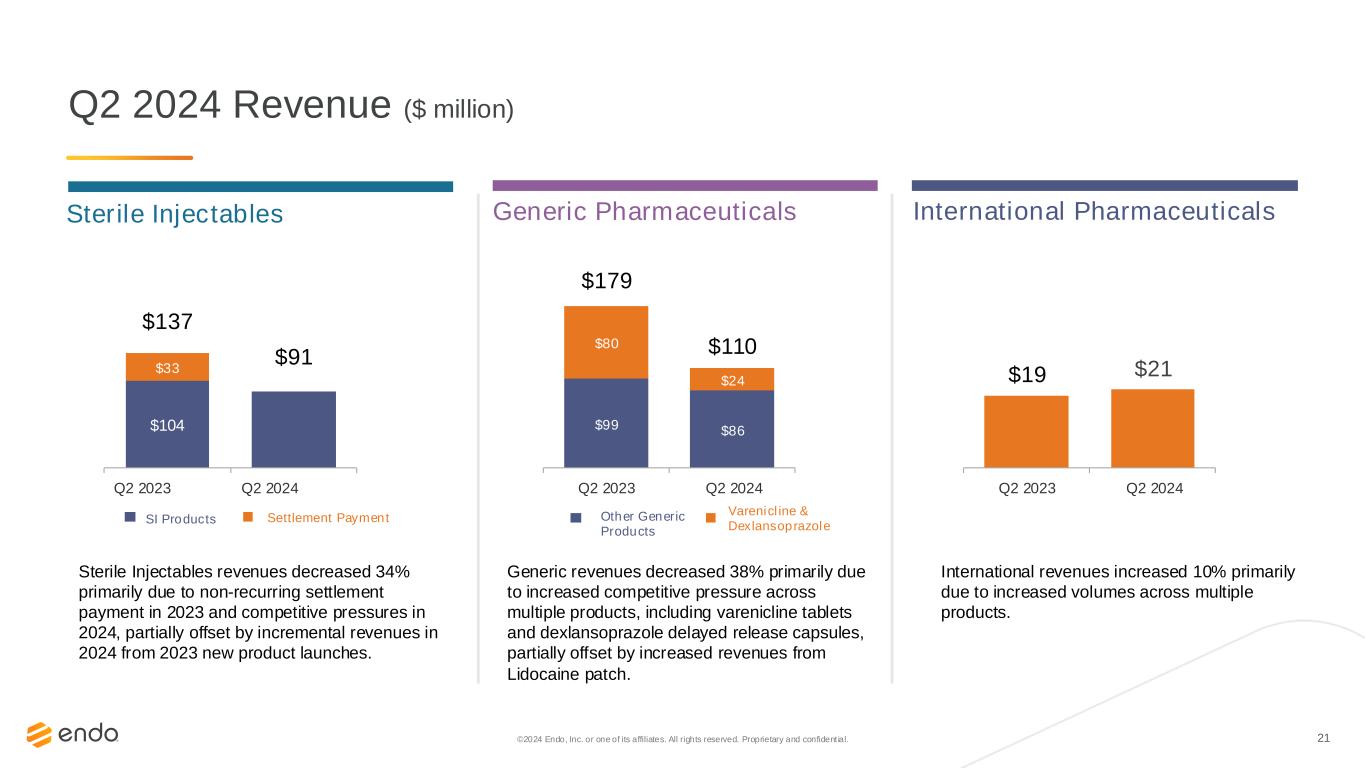
21 Sterile Injectables $21$19 $99 $86 $80 $24 $179 $110 $104 $91 $33 $137 Q2 2023 Q2 2024 ©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Q2 2024 Revenue ($ million) Sterile Injectables revenues decreased 34% primarily due to non-recurring settlement payment in 2023 and competitive pressures in 2024, partially offset by incremental revenues in 2024 from 2023 new product launches. Q2 2023 Q2 2024 Generic revenues decreased 38% primarily due to increased competitive pressure across multiple products, including varenicline tablets and dexlansoprazole delayed release capsules, partially offset by increased revenues from Lidocaine patch. Q2 2023 Q2 2024 International revenues increased 10% primarily due to increased volumes across multiple products. Other Generic Products Varenicline & Dexlansoprazole SI Products Settlement Payment Generic Pharmaceuticals International Pharmaceuticals
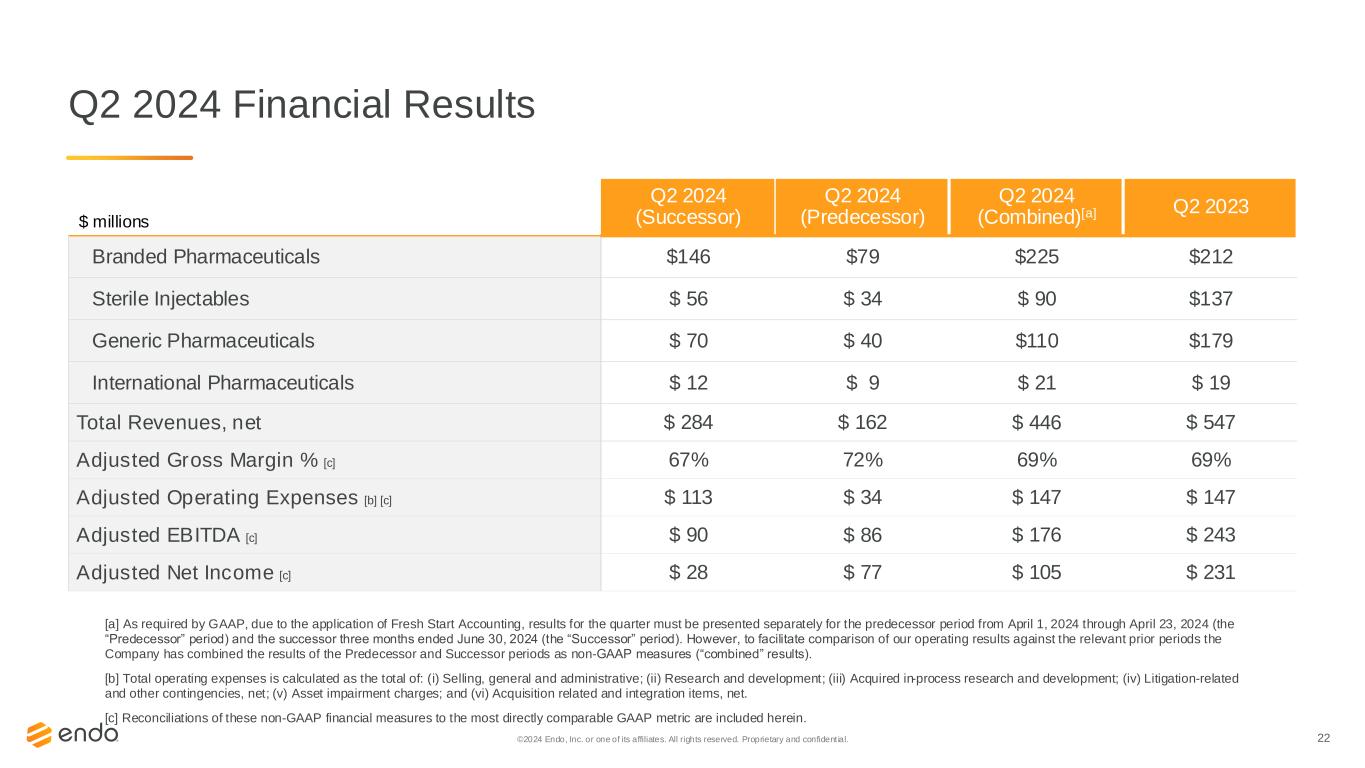
22©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Q2 2024 Financial Results [a] As required by GAAP, due to the application of Fresh Start Accounting, results for the quarter must be presented separately for the predecessor period from April 1, 2024 through April 23, 2024 (the “Predecessor” period) and the successor three months ended June 30, 2024 (the “Successor” period). However, to facilitate comparison of our operating results against the relevant prior periods the Company has combined the results of the Predecessor and Successor periods as non-GAAP measures (“combined” results). [b] Total operating expenses is calculated as the total of: (i) Selling, general and administrative; (ii) Research and development; (iii) Acquired in-process research and development; (iv) Litigation-related and other contingencies, net; (v) Asset impairment charges; and (vi) Acquisition related and integration items, net. [c] Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP metric are included herein. $ millions Q2 2024 (Successor) Q2 2024 (Predecessor) Q2 2024 (Combined)[a] Q2 2023 Branded Pharmaceuticals $146 $79 $225 $212 Sterile Injectables $ 56 $ 34 $ 90 $137 Generic Pharmaceuticals $ 70 $ 40 $110 $179 International Pharmaceuticals $ 12 $ 9 $ 21 $ 19 Total Revenues, net $ 284 $ 162 $ 446 $ 547 Adjusted Gross Margin % [c] 67% 72% 69% 69% Adjusted Operating Expenses [b] [c] $ 113 $ 34 $ 147 $ 147 Adjusted EBITDA [c] $ 90 $ 86 $ 176 $ 243 Adjusted Net Income [c] $ 28 $ 77 $ 105 $ 231
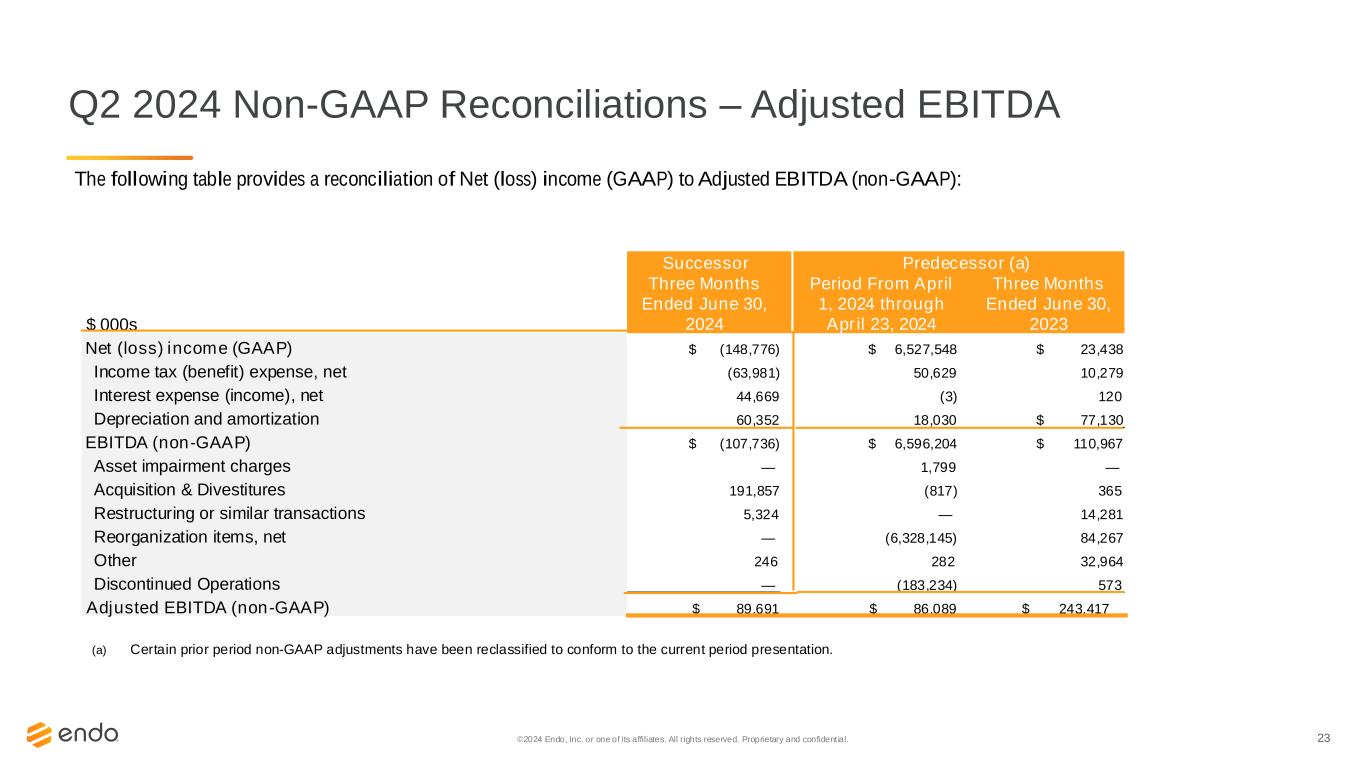
23©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Q2 2024 Non-GAAP Reconciliations – Adjusted EBITDA The following table provides a reconciliation of Net (loss) income (GAAP) to Adjusted EBITDA (non-GAAP): Successor Predecessor (a) $ 000s Three Months Ended June 30, 2024 Period From April 1, 2024 through April 23, 2024 Three Months Ended June 30, 2023 Net (loss) income (GAAP) $ (148,776) $ 6,527,548 $ 23,438 Income tax (benefit) expense, net (63,981) 50,629 10,279 Interest expense (income), net 44,669 (3) 120 Depreciation and amortization 60,352 18,030 $ 77,130 EBITDA (non-GAAP) $ (107,736) $ 6,596,204 $ 110,967 Asset impairment charges — 1,799 — Acquisition & Divestitures 191,857 (817) 365 Restructuring or similar transactions 5,324 — 14,281 Reorganization items, net — (6,328,145) 84,267 Other 246 282 32,964 Discontinued Operations — (183,234) 573 Adjusted EBITDA (non-GAAP) $ 89,691 $ 86,089 $ 243,417 (a) Certain prior period non-GAAP adjustments have been reclassified to conform to the current period presentation.
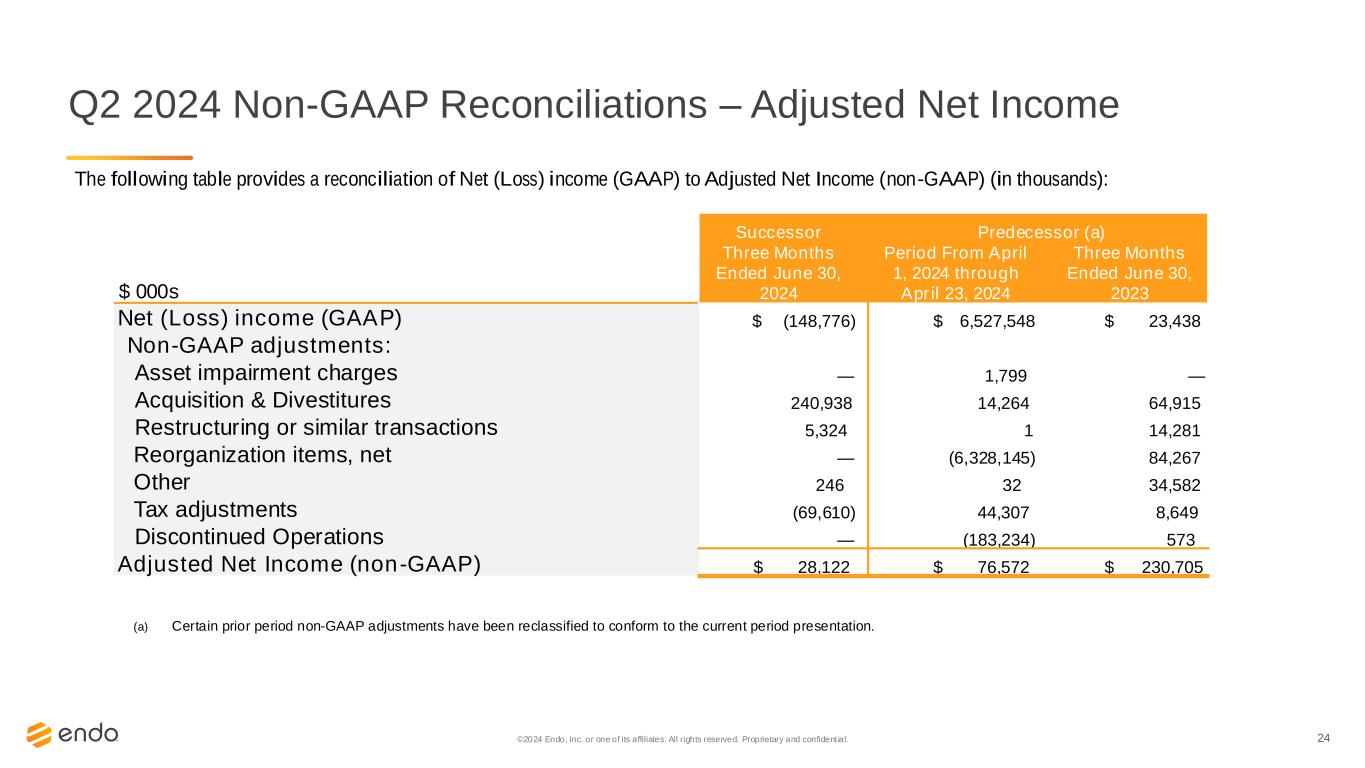
24©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Q2 2024 Non-GAAP Reconciliations – Adjusted Net Income The following table provides a reconciliation of Net (Loss) income (GAAP) to Adjusted Net Income (non-GAAP) (in thousands): Successor Predecessor (a) $ 000s Three Months Ended June 30, 2024 Period From April 1, 2024 through April 23, 2024 Three Months Ended June 30, 2023 Net (Loss) income (GAAP) $ (148,776) $ 6,527,548 $ 23,438 Non-GAAP adjustments: Asset impairment charges — 1,799 — Acquisition & Divestitures 240,938 14,264 64,915 Restructuring or similar transactions 5,324 1 14,281 Reorganization items, net — (6,328,145) 84,267 Other 246 32 34,582 Tax adjustments (69,610) 44,307 8,649 Discontinued Operations — (183,234) 573 Adjusted Net Income (non-GAAP) $ 28,122 $ 76,572 $ 230,705 (a) Certain prior period non-GAAP adjustments have been reclassified to conform to the current period presentation.
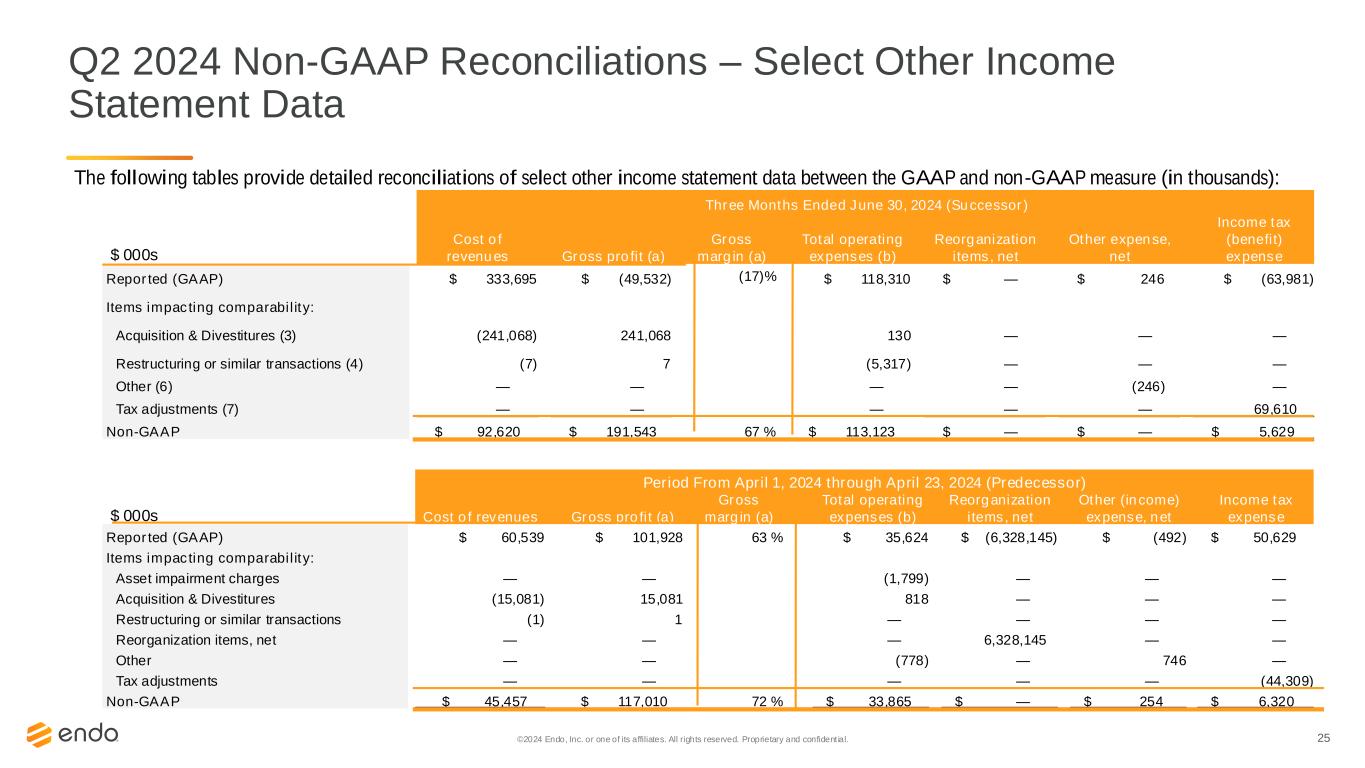
25©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Q2 2024 Non-GAAP Reconciliations – Select Other Income Statement Data The following tables provide detailed reconciliations of select other income statement data between the GAAP and non-GAAP measure (in thousands): Period From April 1, 2024 through April 23, 2024 (Predecessor) $ 000s Cost of revenues Gross profit (a) Gross margin (a) Total operating expenses (b) Reorganization items, net Other (income) expense, net Income tax expense Reported (GAAP) $ 60,539 $ 101,928 63 % $ 35,624 $ (6,328,145) $ (492) $ 50,629 Items impacting comparability: Asset impairment charges — — (1,799) — — — Acquisition & Divestitures (15,081) 15,081 818 — — — Restructuring or similar transactions (1) 1 — — — — Reorganization items, net — — — 6,328,145 — — Other — — (778) — 746 — Tax adjustments — — — — — (44,309) Non-GAAP $ 45,457 $ 117,010 72 % $ 33,865 $ — $ 254 $ 6,320 Three Months Ended June 30, 2024 (Successor) $ 000s Cost of revenues Gross profit (a) Gross margin (a) Total operating expenses (b) Reorganization items, net Other expense, net Income tax (benefit) expense Reported (GAAP) $ 333,695 $ (49,532) (17)% $ 118,310 $ — $ 246 $ (63,981) Items impacting comparability: Acquisition & Divestitures (3) (241,068) 241,068 130 — — — Restructuring or similar transactions (4) (7) 7 (5,317) — — — Other (6) — — — — (246) — Tax adjustments (7) — — — — — 69,610 Non-GAAP $ 92,620 $ 191,543 67 % $ 113,123 $ — $ — $ 5,629
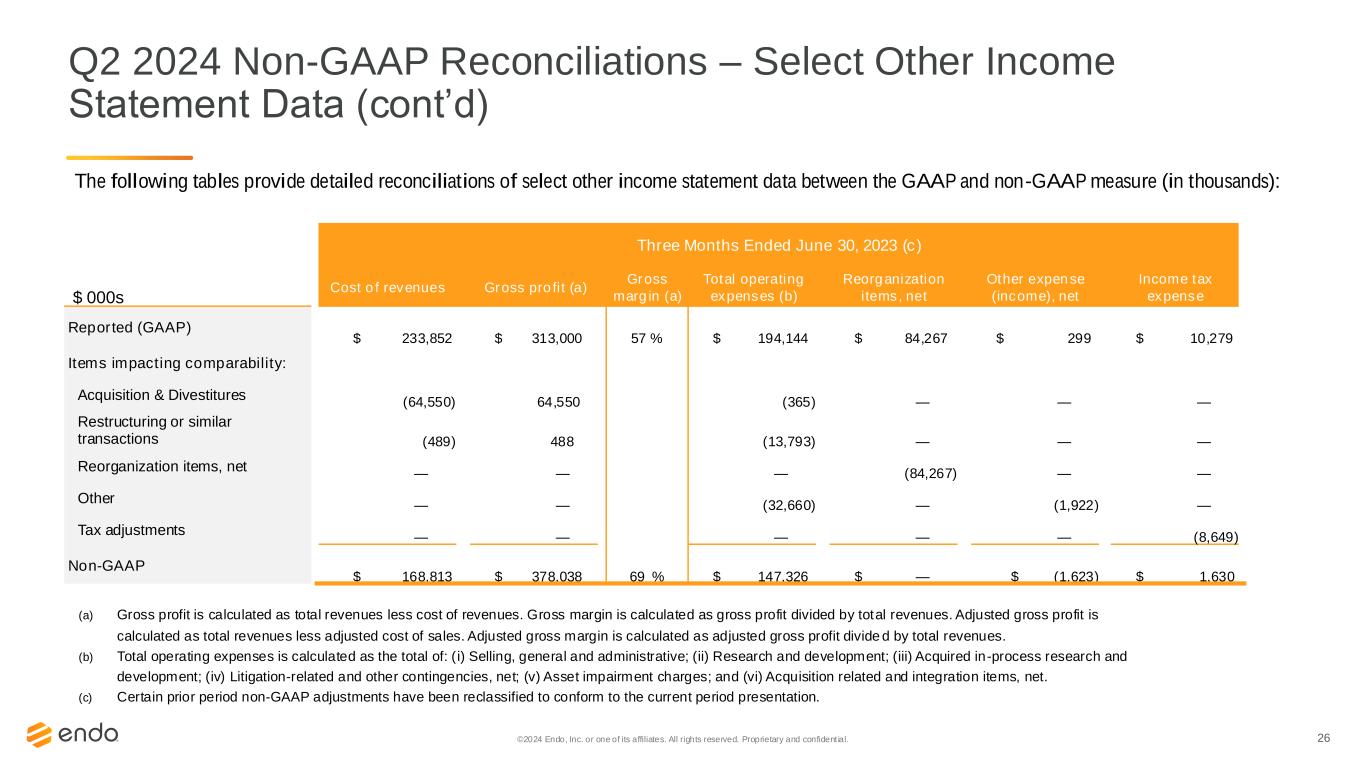
26©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Q2 2024 Non-GAAP Reconciliations – Select Other Income Statement Data (cont’d) The following tables provide detailed reconciliations of select other income statement data between the GAAP and non-GAAP measure (in thousands): Three Months Ended June 30, 2023 (c) $ 000s Cost of revenues Gross profit (a) Gross margin (a) Total operating expenses (b) Reorganization items, net Other expense (income), net Income tax expense Reported (GAAP) $ 233,852 $ 313,000 57 % $ 194,144 $ 84,267 $ 299 $ 10,279 Items impacting comparability: Acquisition & Divestitures (64,550) 64,550 (365) — — — Restructuring or similar transactions (489) 488 (13,793) — — — Reorganization items, net — — — (84,267) — — Other — — (32,660) — (1,922) — Tax adjustments — — — — — (8,649) Non-GAAP $ 168,813 $ 378,038 69 % $ 147,326 $ — $ (1,623) $ 1,630 (a) Gross profit is calculated as total revenues less cost of revenues. Gross margin is calculated as gross profit divided by total revenues. Adjusted gross profit is calculated as total revenues less adjusted cost of sales. Adjusted gross margin is calculated as adjusted gross profit divide d by total revenues. (b) Total operating expenses is calculated as the total of: ( i) Selling, general and administrative; (ii) Research and development; (iii) Acquired in-process research and development; (iv) Litigation-related and other contingencies, net; (v) Asset impairment charges; and (vi) Acquisition related and integration items, net. (c) Certain prior period non-GAAP adjustments have been reclassified to conform to the current period presentation.

27 Appendix

28©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial. Recently Launched DTC Campaign Emboldens DC Patients Be informed. Assert yourself. Don’t settle. Launched June 2024 Helping Patients Take Charge of Their DC Treatment Options It’s in your hands Call to Action: 5 Simple Reminders I don’t want surgery for my contracture I don’t want to wait for my contracture to get worse I want treatment with minimal downtime I want a non-surgical treatment If non-surgical treatment isn’t offered, I will get a second opinion • TV – National broadcast, streaming services, and online • Full digital media ecosystem – social media, digital, and search advertising 1 2 3 4 5

32©2024 Endo, Inc. or one of its affiliates. All rights reserved. Proprietary and confident ial.




























