UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2008 |
OR
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the transition period from ____________ to __________ | |
Commission file number 0-8527 |
DIALYSIS CORPORATION OF AMERICA
(Exact name of registrant as specified in its charter)
| FLORIDA | 59-1757642 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification No.) | |
| 1302 CONCOURSE DRIVE, SUITE 204, LINTHICUM, MARYLAND | 21090 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code (410) 694-0500
Securities registered under Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on Which Registered | |
| common stock, $.01 par value | The NASDAQ Stock Market, LLC | |
| (NASDAQ Global Market) |
Securities registered under Section 12(g) of the Exchange Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. (Check One)
Large accelerated filer o | Accelerated filer x | Non-accelerated filer o | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
As of June 30, 2008, the aggregate market value of the common stock held by non-affiliates computed by reference to the closing price of these shares of $7.28 on the Nasdaq Stock Market was approximately $54,002,000.
As of March 13, 2009, the Company had 9,594,368 shares of its common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Registrant’s Registration Statement on Form SB-2 dated December 22, 1995, as amended February 9, 1996, April 2, 1996 and April 15, 1996, Registration No. 33-80877-A Part II, Item 27, Exhibits, incorporated in Part IV of this Annual Report.
Registrant’s Annual Report, Form 10-K for the years ended December 31, 1999 and 2005, Part IV, Exhibits, incorporated in Part IV of this Annual Report.
Portions of Registrant’s Definitive Proxy Statement to be filed with the Securities and Exchange Commission in connection with its 2009 Annual Meeting of Shareholders are incorporated into Part III, Items 10-14 of this Annual Report.
DIALYSIS CORPORATION OF AMERICA
Index to Annual Report on Form 10-K
Year Ended December 31, 2008
| Page | ||
| PART I | ||
| Cautionary Notice Regarding Forward-Looking Information | 1 | |
| Item 1. | Business | 2 |
| Item 1A. | Risk Factors | 20 |
| Item 1B. | Unresolved Staff Comments | 29 |
| Item 2. | Properties | 29 |
| Item 3. | Legal Proceedings | 30 |
| Item 4. | Submission of Matters to a Vote of Security Holders | 30 |
| PART II | ||
| Item 5. | Market for the Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 31 |
| Item 6. | Selected Financial Data | 33 |
| Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | 34 |
| Item 7A. | Quantitative and Qualitative Disclosure About Market Risk | 46 |
| Item 8. | Financial Statements and Supplementary Data | 46 |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 46 |
| Item 9A. | Controls and Procedures | 47 |
| Item 9B. | Other Information | 48 |
i
| Page | ||
| PART III | ||
| Item 10. | Directors, Executive Officers and Corporate Governance | 48 |
| Item 11. | Executive Compensation | 49 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 49 |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 50 |
| Item 14. | Principal Accountant Fees and Services | 50 |
| PART IV | ||
| Item 15. | Exhibits and Financial Statement Schedules | 51 |
| Signatures | 56 | |
ii
Part I
Cautionary Notice Regarding Forward-Looking Information
The statements contained in this annual report on Form 10-K dated March 13, 2009 and the documents incorporated by reference in this annual report that are not historical are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”). In addition, from time to time, we or our representatives have made or may make forward looking statements, orally or in writing, and in press releases. The Private Securities Litigation Reform Act of 1995 contains certain safe harbors for forward-looking statements. Certain of the forward-looking statements include management’s expectations, intentions, beliefs and strategies regarding the growth of our company and our future operations, the character and development of the dialysis industry, anticipated revenues, our need for and sources of funding for expansion opportunities and construction, expenditures, costs and income, our business strategies and plans for future operations, potential business combinations, and similar expressions concerning matters that are not considered historical facts. Forward-looking statements also include our statements regarding liquidity, anticipated cash needs and availability, and anticipated expense levels in Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Words such as “anticipates,” “estimates,” “expects,” “projects,” “intends,” “plans” and “believes,” and words and terms of similar substance used in connection with any discussions of future operating or financial performance identify forward-looking statements. Such forward-looking statements, like all statements about expected future events, are based on assumptions and are subject to substantial risks and uncertainties that could cause actual results or transactions to materially differ from those expressed in the statements, including general economic and market conditions which substantially deteriorated in 2008, and continue in a global economic decline to date, business opportunities pursued or not pursued, competition, changes in federal and state laws or regulations affecting the company and our operations, and other factors discussed periodically in our filings. Many of the foregoing factors are beyond our control. Among the factors that could cause actual results to differ materially are the factors detailed in the risks discussed in Item 1A, “Risk Factors” section beginning on page 20 of this annual report on Form 10-K. If any of such events occur or circumstances arise that we have not assessed, they could have a material adverse effect upon our revenues, earnings, financial condition and business, as well as the trading price of our common stock, which could adversely affect your investment in our company. Accordingly, readers are cautioned not to place too much reliance on such forward-looking statements. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf, are expressly qualified in their entirety by the cautionary statements contained in this annual report. You should read this annual report on Form 10-K, the exhibits attached and the documents incorporated by reference completely and with the understanding that the company’s actual results may be materially different from what we expect.
The forward-looking statements speak only as of the date of this annual report on Form 10-K, and except as required by law, we undertake no obligation to rewrite or update such statements to reflect subsequent events.
| Item 1. | Business |
Historical
Dialysis Corporation of America, a Florida corporation organized in 1976, develops, owns, and operates outpatient kidney dialysis centers that provide quality dialysis and ancillary services to patients suffering from chronic kidney failure, generally referred to as end stage renal disease, or ESRD. We also provide acute inpatient dialysis treatments in hospitals, homecare services and dialysis center management services. We currently own 37 outpatient dialysis facilities. During 2008 we acquired the assets of two dialysis facilities located in Georgia and Maryland, the Georgia facility having previously been managed by us, and we opened a new facility in Pennsylvania. We also engage, on a limited basis, in medical product sales.
Our principal executive offices are located at 1302 Concourse Drive, Suite 204, Linthicum, Maryland 21090, and you may contact us as follows:
Telephone: (410) 694-0500
Fax: (410) 694-0596
Email: info@dialysiscorporation.com
Our internet website can be found at www.dialysiscorporation.com. You may obtain through our internet website, free of charge, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, amendments to those reports filed with the Securities and Exchange Commission, referred to as the SEC, press releases, corporate profiles and corporate governance materials. We also make those documents available to shareholders free of charge upon request. The SEC maintains a website at www.sec.gov where our reports and other information about us may be obtained
General
Our company distinguishes itself on the basis of quality patient care delivered by patient-focused, courteous, highly trained professional staff. In addition to outpatient facilities, we provide acute inpatient dialysis treatments that are conducted under contractual relationships with hospitals located in near proximity to certain of our outpatient facilities. Currently we have 11 such relationships. Our homecare services, primarily through the use of peritoneal dialysis, requires us to provide equipment and supplies, training, monitoring and follow-up assistance to patients who are able to perform their treatments at home.
Our medical services revenue is derived primarily from four sources: (i) outpatient hemodialysis services (ii) home and peritoneal dialysis services; (iii) inpatient hemodialysis services for acute patient care provided through agreements with hospitals and medical centers; and (iv) ancillary services associated with dialysis treatments, including the administration of erythropoietin (“EPO”), a bio-engineered protein that stimulates the production of red blood cells (a deteriorating kidney loses its ability to regulate red blood cell count, resulting in anemia). Dialysis is an ongoing and necessary therapy to sustain life for ESRD patients. ESRD patients typically receive up to 156 dialysis treatments each year.
2
Our medical services revenue distribution among these sources of revenue is as follows for 2008, 2007 and 2006:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Outpatient hemodialysis services | 54 | % | 55 | % | 54 | % | ||||||
| Home and peritoneal dialysis services | 5 | 5 | 6 | |||||||||
| Inpatient hemodialysis services | 4 | 4 | 3 | |||||||||
| Ancillary services* | 37 | 36 | 37 | |||||||||
| 100 | % | 100 | % | 100 | % | |||||||
* EPO represents 27% of medical services revenue for 2008 and 2007 and 28% for 2006.
Essential to our operations and income is Medicare reimbursement which is a fixed rate determined by the Center for Medicare and Medicaid Services (“CMS”) of the Department of Health and Human Services (“HHS”). The level of our revenues and profitability may be adversely affected by future legislation that could result in rate cuts. Further, our operating costs tend to increase over the years in excess of increases in the prescribed dialysis treatment rates. Congress approved a 1.6% composite rate increase for each of 2005 and 2006, and a 1.6% increase that was effective April 1, 2007. Also for 2007, the drug add-on adjustment to the composite rate increased 0.5%. In 2005, Medicare implemented an additional change in the manner it reimburses dialysis treatments, which includes a pricing revision to the current average wholesale price for separately billable drugs and biologicals. Effective January 1, 2006, payments for pharmaceuticals (including EPO) were set at the pharmaceutical’s average sales price as determined by the Inspector General of HHS, plus 6%, which remained the same for 2007 and 2008. Effective April, 2006, CMS implemented a policy for monitoring the dosage of EPO based upon the patient’s hematocrit level and for limiting the quantity of EPO that can be administered in any one month. Effective January 1, 2008, CMS further decreased the quantity of EPO that may be administered in any month. Medicare has implemented a case mix payment system, adjusting the composite rate for a limited number of patient characteristics. The increase in the composite rate is intended to offset reductions in pharmaceutical reimbursements. In July, 2008, Congress passed legislation aimed at establishing a broader “bundling” reimbursement system which is intended to take effect incrementally beginning in 2011. See under this Item 1, “Operations – Medicare Reimbursement.” Commercial third-party reimbursement rates, which have increased as a percentage of our revenues over the last two years, are also susceptible to reduction. The inpatient dialysis service agreements for treating acute kidney disease are not subject to government fixed rates, but rather are negotiated with hospitals. Typically these rates are higher than the government fixed rates on a per treatment basis.
Dialysis Industry
Kidneys act as filters removing harmful substances and excess water from the blood, enabling the body to maintain proper and healthy balances of chemicals and water. Chronic kidney failure, or ESRD, results from chemical imbalance and buildup of toxic chemicals, and is a state of kidney disease characterized by advanced irreversible renal impairment. ESRD is a likely consequence of complications resulting from diabetes, hypertension, advanced age, and specific hereditary, cystic and urological diseases. ESRD patients, in order to survive, must either obtain a kidney transplant, which procedure is limited due to lack of suitable kidney donors and the incidence of rejection of transplanted organs, or obtain dialysis treatments for the rest of their lives.
Based upon CMS information published in the 2008 United States Renal Data System, the number of ESRD patients requiring dialysis treatments in the United States in 2006 exceeded 350,000, with a growth rate of approximately 3% to 4% a year. This is thought to be attributable primarily to the aging of the population, greater patient longevity as a result of improved dialysis technology, and better treatment and survival rates for illnesses that lead to chronic kidney disease. The statistics further reflect over 5,067 dialysis facilities, with a current annual cost for treating ESRD patients in the United States of approximately $31 billion in 2006, of which Medicare accounted for approximately $23 billion.
3
ESRD Treatment Options
Treatment options for ESRD patients include: (1) hemodialysis, performed either at (i) an outpatient facility, (ii) inpatient hospital facility, or (iii) the patient’s home; (2) peritoneal dialysis, either continuous ambulatory peritoneal dialysis or continuous cycling peritoneal dialysis; or (3) kidney transplant. A significant portion of ESRD patients receive treatments at non-hospital owned outpatient dialysis facilities (according to CMS, approximately 82%) with most of the remaining patients treated at home through hemodialysis or peritoneal dialysis. Patients treated at home are monitored by a designated outpatient facility.
The most prevalent form of treatment for ESRD patients is hemodialysis, which involves the use of an artificial kidney, known as a dialyzer, to perform the function of removing toxins and excess fluids from the bloodstream. This is accomplished with a dialysis machine, a complex blood filtering device which takes the place of certain functions of the kidney, and also controls external blood flow and monitors the toxic and fluid removal process. The dialyzer has two separate chambers divided by a semi-permeable membrane. Simultaneously with the blood circulating through one chamber, dialyzer fluid is circulated through the other chamber. The toxins and excess fluid pass through the membrane into the dialysis fluid. On the average, patients usually receive three treatments per week with each treatment taking three to five hours. Dialysis treatments are performed by teams of licensed nurses and trained technicians pursuant to the physician’s instructions.
Home hemodialysis treatment requires the patient to be medically suitable and have a qualified assistant. Additionally, home hemodialysis requires training for both the patient and the patient’s assistant, which usually encompasses four to eight weeks.
A second home treatment option for ESRD patients is peritoneal dialysis. The two most common forms of peritoneal dialysis are continuous ambulatory peritoneal dialysis and continuous cycling peritoneal dialysis. All forms of peritoneal dialysis use the patient’s peritoneal (abdominal) cavity to eliminate fluid and toxins from the patient. Continuous ambulatory peritoneal dialysis utilizes dialysis solution infused manually into the patient’s peritoneal cavity through a surgically-placed catheter. The solution is allowed to remain in the abdominal cavity for a three to five hour period and is then drained. The process is then repeated. Continuous cycling peritoneal dialysis is performed in a manner similar to continuous ambulatory peritoneal dialysis, but utilizes a mechanical device to cycle the dialysis solution while the patient is sleeping. Peritoneal dialysis is the third most common form of ESRD therapy following center hemodialysis and renal transplant.
While kidney transplantation is typically the most desirable form of therapeutic intervention, the scarcity of suitable donors and possibility of donee rejection limits the availability of this surgical procedure as a treatment option.
Business Strategy
Dialysis Corporation of America has 33 years’ experience in developing and operating dialysis treatment facilities. Our priority is to provide quality patient care. We continue to establish alliances with physicians and hospitals and attempt to initiate dialysis service arrangements with nursing homes and managed care organizations.
4
We actively seek and negotiate with physicians and others to establish new outpatient dialysis facilities. In 2008, we acquired two centers and opened one new center. We are in different phases of negotiations with physicians for potential new facilities in a variety of states.
Same Center Growth
We endeavor to increase same center growth by adding quality staff and management and attracting new patients to our existing facilities. We seek to accomplish this objective by rendering high caliber patient care in convenient, safe and pleasant conditions. We believe that we have adequate space and stations within our facilities to accommodate greater patient volume and maximize our treatment potential. Same center growth relates to those centers that were operable during the entire prior year. During 2008, we experienced approximately 5% increase in dialysis treatments for our facilities that qualified to be included in same center growth compared to 6% during the preceding year.
Development and Acquisition of Facilities
One of the primary elements in developing or acquiring facilities is locating an area with an existing patient base under the current treatment of local nephrologists interested in working with a dialysis center, since the proposed facility would primarily be serving such patients. Other considerations in evaluating development of a dialysis facility or a proposed acquisition are the types of commercial insurance in the area and the ESRD patient census they serve, availability and cost of qualified and skilled personnel, particularly nursing and technical staff, the size and condition of the facility and its equipment, the atmosphere for the patients, the area’s demographics and population growth estimates, state regulation of dialysis and healthcare services, and the existence of competitive factors such as existing outpatient dialysis facilities within reasonable proximity to the proposed center.
Expansion is either through the development of new dialysis facilities, or acquisition of existing outpatient dialysis centers. While acquisition of existing outpatient dialysis centers is a faster means for achieving profitability, these transactions can be more costly, and therefore, the company takes a selective approach to its acquisition strategy. The primary reason for physicians selling or participating in the development of centers is the avoidance of administrative and financial responsibilities, freeing their time to devote to their professional practice. Other motivating forces are the physician’s desire to be part of a larger organization allowing for economies of scale and the ability to realize a return on their investment if they have an interest in the dialysis entity.
To construct and develop a new facility ready for operation takes an average of four to six months, and usually up to 12 months or longer to generate earnings, all of which are subject to variables based on location, size and competitive elements. Some of our centers are in the developmental stage, since they have not reached the point where the patient base is sufficient to generate and sustain earnings. See Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Construction of a 15 station facility, typically the size of our dialysis facilities, costs in a range of $1,000,000 to $1,500,000, including equipment and initial working capital requirements, and is dependent upon location, size and related services to be provided by the proposed facility. An acquisition of an existing facility is usually based primarily upon the patient base and earnings, and to a lesser extent, location and competition. As a result, the costs associated with an acquisition of a facility can be substantially greater than that of a development; therefore, we selectively identify potential acquisitions. Any significant expansion, whether through acquisition or development of new facilities, is dependent upon existing funds or financing from other sources. We have a revolving credit facility intended to provide available funds for the development and acquisition of new dialysis facilities and for other
5
general working capital and corporate purposes. See Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Inpatient Services
We also provide acute dialysis treatments through contracts with hospitals for inpatient dialysis services. These contracts are primarily sought with hospitals in areas serviced by our facilities. Hospitals are willing to enter into such inpatient care arrangements to eliminate the administrative burdens of providing dialysis services to their patients as well as the expense involved in maintaining dialysis equipment, supplies and personnel. We believe that these arrangements are beneficial to our operations, since the contract rates are individually negotiated with each hospital and are not fixed by government regulation as is the case with Medicare reimbursement fees for ESRD patient treatment.
There is no certainty as to when any additional centers or service contracts will be implemented, or, to the extent implemented, the number of dialysis stations or patient treatments these centers or service contracts may involve, or if they will ultimately be profitable. There is no assurance that we will be able to continue to enter into favorable relationships with physicians who would become medical directors of proposed dialysis facilities, or that we will be able to acquire or develop any new dialysis centers within a favorable geographic area. Newly established dialysis centers, although contributing to increased revenues, also adversely affect results of operations due to their start-up costs and expenses and to their having a smaller and slower developing patient base. See “Business Strategy,” “Operations” and “Competition” of this Item 1, and Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Operations
Location, Capacity and Use of Facilities
We currently own 37 outpatient dialysis facilities in Georgia, Maryland, New Jersey, Ohio, Pennsylvania, Virginia and South Carolina. These dialysis facilities have a total designed capacity 573 licensed stations.
Our 37 dialysis facilities are owned through subsidiaries as follows: 23 wholly-owned subsidiaries, one of which owns two facilities, and 13 majority-owned subsidiaries in conjunction with the medical directors of those centers who hold minority interests.
One of our Georgia subsidiaries exercised a call option which resulted in the acquisition of the assets (effective January 1, 2008) of the Georgia facility we previously managed. We hold an 80% interest in this facility and the former owner holds the remaining 20% interest.
The company provides acute care inpatient dialysis services to 11 hospitals in areas serviced by certain of our dialysis facilities. Most of our dialysis facilities have the capacity to provide training, supplies and on-call support services for home peritoneal patients. Dialysis Corporation of America provided approximately 272,000 hemodialysis treatments in 2008, an increase of approximately 20,000 treatments compared to fiscal 2007.
We estimate that on average our centers were operating at approximately 50% of capacity in December, 2008, based on the assumption that a dialysis center is able to provide up to three treatments a day per station, six days a week. We believe we can increase the number of dialysis treatments at most of our centers without making significant additional capital expenditures.
6
Operations of Dialysis Facilities
Our dialysis facilities are designed specifically for outpatient hemodialysis and generally contain, in addition to space for dialysis treatments, a nurses’ station, a patient weigh-in area, a supply room, water treatment space used to purify the water used in hemodialysis treatments, staff work area, offices, a staff lounge, and a dialyzer reprocessing room in select facilities (where, with both the patient’s and physician’s consent, the patient’s dialyzer is sterilized for reuse). Most of our facilities have a designated area for training patients in home dialysis. Each facility also offers amenities for the patients, such as a color television with headsets for each dialysis station, to ensure the patients are comfortable and relaxed.
Our facilities also offer home dialysis, primarily continuous ambulatory peritoneal dialysis and continuous cycling peritoneal dialysis. Training programs for continuous ambulatory peritoneal dialysis or continuous cycling peritoneal dialysis generally encompass two to three weeks at the dialysis facility, and such training is conducted by the facility’s home training nurse. After the patient completes training, they are able to perform treatment at home with equipment and supplies provided by the company.
We maintain a team of dialysis specialists to provide for the individual needs of each patient. In accordance with participation requirements under the Medicare ESRD program, each facility retains a physician medical director qualified and experienced in the practice of nephrology and the administration of a renal dialysis facility. See “Physician Relationships” below. Each facility is overseen by a governing body comprised of an administrator, the medical director of that facility and the company’s regional director for that area. The governing body supervises the facility’s daily operations, patient care, services necessary for the operation of the facility, and staff, which consists of registered nurses, licensed practical nurses, patient care technicians, a social worker to assist the patient and family to adjust to dialysis treatment and to provide help in financial assistance and planning, and a registered dietitian. See “Employees” below. In furtherance of our business strategy, we strive to attract and retain skilled nurses and other staff, for whom competition is intense.
We consider our dialysis equipment to be both modern and efficient, providing state of the art treatment in a safe and comfortable environment.
Inpatient Dialysis Services
We presently provide inpatient dialysis services to 11 hospitals in Georgia, Maryland, Ohio, Pennsylvania, and Virginia under agreements either with us or with one of our subsidiaries in the area. The agreements are for terms ranging from one to five years, some with automatic renewal terms. Inpatient services are typically necessary for patients with acute kidney failure resulting from trauma or similar causes and ESRD patients who require hospitalization for other reasons.
Ancillary Services
Our dialysis facilities provide ancillary services to ESRD patients including the administration of certain prescription drugs, such as EPO, upon a physician’s prescription. EPO is a bio-engineered protein which stimulates the production of red blood cells and is used in connection with dialysis to treat anemia, a medical complication frequently experienced by ESRD patients. EPO decreases the necessity for blood transfusions in ESRD patients. Amgen is the only manufacturer of EPO in the United States. Although we have a good relationship with this manufacturer and have not experienced any problems in receipt of our supply of EPO, any loss or limitation of supply of EPO could have a material adverse effect on patient treatments and our operating revenue and income.
7
Amgen has developed an additional product, darbepoetin alfa, known as Aranesp®. Roche has also developed a drug, Mircera®, used to treat anemia, although it is currently unavailable to patients in the United States due to Amgen’s current patent on EPO. These drugs can be administered to patients less frequently than EPO, which is generally administered to the patient with each dialysis treatment. Although we are unable to predict the impact of these products on our operations, a significant increase in the development and use of these alternatives to EPO could have an adverse effect on our revenues, earnings and cash flow.
Physician Relationships
An integral element to the success of a facility is its association with area nephrologists. A dialysis patient generally seeks treatment at a facility near the patient’s home and in proximity to where the patient’s nephrologist has an established practice. Consequently, we rely on our ability to develop affiliations with area nephrologists.
The conditions of a facility’s participation in the Medicare ESRD program mandate that treatment at a dialysis facility be under the general supervision of a medical director who is a physician. We retain, by written agreement, qualified physicians or groups of qualified physicians to serve as medical directors for each of our facilities. Generally, the medical directors are board eligible or board certified in internal medicine by a professional board specializing in nephrology and have had at least 12 months of experience in the care of dialysis patients at ESRD facilities. The medical directors are typically a source of patients treated at the particular center served. Our dialysis centers are operated through subsidiaries, either corporations or limited liability companies. The medical directors of 13 of our centers, through their affiliated entities, have a minority ownership interest in the center they service. We make every effort to comply with federal and state regulations concerning our relationship with the physicians and the medical directors treating patients at our facilities. See “Government Regulation” below.
Agreements with medical directors typically have terms ranging from five to ten years, with renewal provisions, usually two renewal options each for five years. Each agreement specifies the duties, responsibilities and compensation of the medical director. Under each agreement, the medical director or professional association maintains their own medical malpractice insurance. The agreements also typically provide for non-competition in a limited geographic area surrounding that particular dialysis center during the term of the agreement and upon termination for a limited period. These agreements, however, do not prohibit physicians providing services at our facilities from providing direct patient care services at other locations; and consistent with the federal and state law, such agreements do not require a physician to refer patients to our dialysis centers. Customarily, physician’s professional fees for services are billed directly to the patient or to government payment authorities by the treating physician and paid directly to the physician or the physician’s professional association.
Our ability to establish and operate a dialysis facility in a particular area is substantially dependent on the availability of a qualified nephrologist to serve as the medical director. The loss of a medical director who could not be readily replaced would have a material adverse effect on the operations of that facility. In our 33 years of operation, we have not been unable to provide for or replace a medical director. Compensation of medical directors is separately negotiated for each facility and generally depends on competitive factors, the size of the facility, and the fair market value of the services to be provided.
Quality Assurance
We have implemented a quality assurance program to maintain and improve the quality of dialysis treatment and care we provide to our patients in each facility. Quality assurance activities
8
involve the ongoing examination of care provided, the identification of therapy deficiencies, the need for any necessary improvements in the quality of care, and evaluation of improved technology. Specifically, this program requires each center’s staff, including its medical director and nurse administrator, to regularly review quality assurance data and initiate programs for improvement, including dialysis treatment services, equipment, technical and environmental improvements, and staff-patient and personnel relationships. These evaluations are in addition to assuring regulatory compliance with CMS and the Occupational Safety and Health Administration. Our Vice President of Clinical Services, a certified nephrology nurse, oversees this program in addition to ensuring that we meet federal and state compliance requirements for our dialysis centers. See “Government Regulation” below.
Quality Clinical Results
Our goal is to provide consistent quality clinical care to our patients from caring and qualified doctors, nurses, patient care technicians, social workers and dieticians. We have demonstrated an unwavering commitment to quality renal care through our continuous quality improvement initiatives. We strive to maintain a leadership position as a quality provider in the dialysis industry and often set our goals higher than the national average standards.
Kt/V is a formula that measures the amount of dialysis delivered to the patient, based on the removal of urea, an end product of protein metabolism. Kt/V provides a means to determine an individual dialysis prescription and to monitor the effectiveness or adequacy of the dialysis treatment as delivered to the patient. We believe it is critical to achieve a Kt/V level of greater than 1.2 for as many patients as possible. Approximately, 97% of our patients had a Kt/V level greater than 1.2 for the fourth quarter of 2008 compared to 95% for the fourth quarter of 2007.
Anemia is a shortage of oxygen-carrying red blood cells. Because red blood cells bring oxygen to all the cells in the body, untreated anemia can cause severe fatigue, heart disorders, difficulty concentrating, reduced immune function, and other problems. Anemia is common among renal patients, caused by insufficient erythropoietin, iron deficiency, repeated blood losses, and other factors. Anemia can be detected with a blood test for hemoglobin or hematocrit. It is ideal to have as many patients as possible with hemoglobin levels above 11. Approximately 82% of our patients had a hemoglobin level greater than 11 for the fourth quarter of 2008 compared to 79% for the fourth quarter of 2007.
Vascular access is the site on a patient’s body where blood is removed and returned during dialysis. CMS has indicated that fistulas are the “gold standard” for establishing access to a patient’s circulatory system in order to provide life-sustaining dialysis. Approximately 56% of our patients were dialyzed with a fistula during the fourth quarter of 2008 compared to 53% for the fourth quarter of 2007.
Patient Revenues
A substantial amount of the fees for outpatient dialysis treatments are funded under the ESRD Program established by the federal government under the Social Security Act, and administered in accordance with rates set by CMS. A majority of dialysis patients are covered under Medicare. The balance of the outpatient charges are paid by private payors including the patient’s medical insurance, private funds or state Medicaid plans. The states in which we operate provide Medicaid or comparable benefits to qualified recipients to supplement their Medicare coverage.
Under the ESRD Program, payments for dialysis services are determined pursuant to Part B of the Medicare Act which presently pays 80% of the allowable charges for each dialysis treatment furnished to patients. The maximum payments vary based on the geographic location of the center. The remaining 20% may be paid by Medicaid if the patient is eligible, from private insurance funds or the patient’s
9
personal funds. If there is no payor to cover the remaining 20%, Medicare may reimburse us for part of that balance as part of our annual cost report filings. Medicare and Medicaid programs are subject to regulatory changes, statutory limitations and government funding restrictions, which may adversely affect dialysis services payments and, consequently, our revenues. See “Medicare Reimbursement” below.
The inpatient dialysis services are paid for by each contracted hospital pursuant to contractual pre-determined fees for the different dialysis treatments.
Medicare Reimbursement
We are reimbursed primarily by Medicare under a prospective reimbursement system for chronic dialysis services, and by third party payors including Medicaid and commercial insurance companies. Our dialysis facilities are certified to participate in the Medicare program. Under the Medicare system, the reimbursement rates are fixed in advance and limit the allowable charge per treatment, but provide us with predictable and recurring per treatment revenues. An established composite rate set by CMS governs the Medicare reimbursement available for a designated group of dialysis services, including dialysis treatments, supplies used for such treatments, and certain laboratory tests and drugs.
Other ancillary services and items are eligible for separate reimbursement under Medicare and are not part of the composite rate, including certain drugs such as EPO, and certain physician-ordered tests provided to dialysis patients. Approximately 27% of our medical services revenue in 2008 was derived from providing dialysis patients with EPO. Effective April, 2006, and revised again in January, 2008, CMS implemented a policy for monitoring the dosage of EPO based upon the patient’s hematocrit level and for limiting the quantity of EPO that can be administered in any one month. This policy restricts payments based on EPO doses and hemoglobin levels for certain patients. Other ancillary services, mostly other drugs, account for approximately 10% of our medical services revenue. We submit claims monthly and are usually paid by Medicare within 21 days of the submission.
There have been a variety of proposals to Congress for Medicare reform. Congress approved a 1.6% composite rate increase for each of 2005 and 2006, a 1.6% increase which became effective April 1, 2007, and no increase for 2008. For 2008, the drug-add on adjustment to the composite rate increased 0.6%. Medicare reimburses dialysis providers for the ten most utilized ESRD drugs at an amount equal to the cost of such drugs, and for other ESRD drugs Medicare reimburses at an amount equal to the average sale price of the drug as determined by the Inspector General of HHS, plus 6%, and the composite rate has been increased by an amount estimated by HHS to be the dialysis provider’s average profit for these drugs. To make this change budget-neutral, a drug add-on composite was included. CMS reimburses providers using a case mix formula. CMS adjusted reimbursements based on predefined patient parameters such as patient height, weight and age. Congress has mandated a budget neutrality factor adjustment so that aggregate payments under the system equal payments that would have been made without the case mix adjustments and the add-on composite for reimbursement of the drugs. Management believes there has been minimal impact on its average Medicare revenue per treatment as a result of these changes in Medicare reimbursement. Our Medicare composite payment rate for 2008 was between $127 and $151 per dialysis treatment.
In July, 2008, Congress passed the Medicare Improvements for Patients and Providers Act, or MIPPA, which among other things, provided for a 1% increase in the composite rate in years 2009 and 2010, while also mandating the implementation of a “bundled” payment system for renal dialysis services effective January 1, 2011. The system also will take into account certain case mix adjustments, including patient weight, body mass index, comorbidities, length of time on dialysis, age, race, ethnicity and high cost outliers.
10
The estimated total amount of payments under the bundled payment system will be equal to 98% of the estimated total amount of payments for dialysis services that would have been made with respect to services furnished in 2011, using the lowest per patient utilization data from 2007, 2008 and 2009 for estimation purposes. Commencing in 2012, there will be an increase in bundled payment amounts by an ESRD market basket percentage increase factor that reflects changes over time in prices of an appropriate mix of goods and services included in the delivery of renal dialysis services, minus 1%.
In addition, providers will be required to meet certain quality measures that may include anemia management, patient satisfaction, iron management, bone mineral metabolism and vascular access. Commencing January 1, 2012, providers that fail to satisfy such measures will receive a reduction in the bundled payment rate up to 2%.
Medicaid Reimbursement
Medicaid programs are state administered programs partially funded by the federal government. These programs are intended to provide coverage for patients whose income and assets fall below state defined levels and who are otherwise uninsured. The programs also serve as supplemental insurance programs for the Medicare co-insurance portion and provide certain coverages (e.g., oral medications) that are not covered by Medicare. State reimbursements generally follow Medicare reimbursement levels and coverages without any co-insurance amounts. Certain states, however, require beneficiaries to pay a monthly share of the cost based upon levels of income or assets. Pennsylvania and New Jersey have Medical Assistance Programs comparable to Medicaid, with primary and secondary insurance coverage to those who qualify.
Sources of Medical Services Revenue
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Medicare | 46 | % | 49 | % | 52 | % | ||||||
| Medicaid and Comparable Programs | 8 | 8 | 9 | |||||||||
| Hospital inpatient dialysis services | 4 | 4 | 3 | |||||||||
| Commercial and private payors | 42 | 39 | 36 | |||||||||
| 100 | % | 100 | % | 100 | % | |||||||
Management Services
We have a management services agreement with each of our subsidiaries to provide them with administrative and management services, including, but not limited to, assisting in procuring capital equipment, preparing budgets, general accounting, data processing, and other corporate based information services, materials and human resource management, billing and collection, and accounts receivable and payable processing. These services are provided for a percentage of net revenues of each particular facility.
Compliance Program
We have a Compliance Program to assure we continue to achieve our goal of providing the highest level of care and service in a professional and ethical manner consistent with applicable federal and state laws and regulations. This program is intended to (i) reinforce our management’s, employees’ and professional affiliates’ awareness of their responsibilities to comply with applicable laws in the increased and complex regulatory environment relating to our operations, (ii) benefit the overall care and services for our dialysis patients, and (iii) assure our operations are in
11
compliance with the law, which, in turn, should assist us in operating in a cost-effective manner, and accordingly, benefit our shareholders.
The Compliance Program also assists us in compliance with fraud and abuse laws, enhance communication of information, and provide a mechanism to quickly identify and correct any problems that may arise. This Program supplements and enhances our existing policies, including those applicable to claims submission, cost report preparation, internal audit and human resources.
Our board of directors has established an audit committee consisting of three independent members of the board who oversee audits, accounting, financial reporting, and who have established procedures for receipt, retention and resolution of complaints relating to those areas, among other responsibilities. The audit committee operates under a charter providing for its detailed responsibilities.
We also maintain disclosure controls and procedures and internal controls over financial reporting to ensure the accuracy of our disclosures in our filings and financial reporting and financial statements filed under the federal securities laws. Management provides a report on, and our independent registered accountants audit, management’s assessment of internal control over financial reporting. See Item 9A, “Controls and Procedures” and “Report of Independent Registered Public Accounting Firm,” page F-2 of this annual report on Form 10-K.
Code of Ethics
As part of our Compliance Program, we have established a Code of Ethics and Business Conduct covering management and all employees to assure all persons affiliated with our company and our operations act in an ethical and lawful manner. The Code of Ethics and Business Conduct covers relationships among and between affiliated persons, patients, payors, and relates to information processing, compliance, workplace conduct, environmental practices, training, education and development, among other areas. In our commitment to delivering quality care to dialysis patients, we have mandated rigorous standards of ethics and integrity.
Our Code of Ethics and Business Conduct is designed to provide:
| ● | commitment to the best interests of the company by officers, directors and employees | |
| ● | ethical handling of actual or apparent conflicts of interest between personal and professional relationships | |
| ● | full, fair, accurate, timely, and understandable disclosure in reports and documents we file with the SEC and in our other public communications | |
| ● | compliance with applicable governmental laws, rules and regulations | |
| ● | prompt internal reporting of violations of the Code to an appropriate person identified in the Code | |
| ● | responsibilities of senior management with respect to related party transactions | |
| ● | accountability for adherence to the Code | |
| ● | non-waiver of policies and exception processes |
Our Code of Ethics and Business Conduct is posted on our website at www.dialysiscorporation.com under the caption “Investor Relations – Corporate Governance.” We will also provide the Code to any person without charge upon request to our corporate Secretary and counsel, Joshua M. Jaffe, Esq., Jaffe & Falk, LLC, at 777 Terrace Avenue, Hasbrouck Heights, New Jersey 07604, telephone (201) 288-8282, or email, jmj@jaffefalkllc.com.
12
The Compliance Program is reviewed and upgraded from time to time, to provide a highly professional work environment and lawful and efficient business operations to better serve our patients and our shareholders.
Potential Liability and Insurance
Participants in the health care industry are subject to lawsuits based upon alleged negligence, many of which involve large claims and significant defense costs. We currently have general and umbrella liability insurance, as well as professional and products liability. Our insurance policies provide coverage on an “occurrence” basis and are subject to annual renewal. A hypothetical successful claim against us in excess of our insurance coverage could have a material adverse effect upon our business and results of operations. The medical directors supervising our dialysis operations and other physicians practicing at the facilities are required to maintain their own professional malpractice insurance coverage.
Government Regulation
General
Regulation of healthcare facilities, including dialysis facilities, is extensive, with legislation continually proposed relating to safety, maintenance of equipment and records, quality assurance programs, government payment programs and reimbursement rates, confidentiality of medical records, licensing, patient care and other areas of operations. Each dialysis facility must be certified by CMS, and must comply with certain rules and regulations established by CMS. Each dialysis facility is also subject to periodic inspections by federal and state agencies to determine if their operations meet the appropriate regulatory standards. Our operations are also subject to the Occupational Safety and Health Administration, known as OSHA, relating to workplace safety and employee exposure to blood and other potentially infectious material.
Many states have eliminated the requirement to obtain a certificate of need prior to the establishment or expansion of a dialysis center. There are no certificate of need requirements in the states in which we are presently operating.
Our record of compliance with federal, state and local governmental laws and regulations remains excellent. Nevertheless, we are unable to predict the scope and effect of any changes in government regulations, particularly any modifications in the reimbursement rate for medical services or requirements to obtain certification from CMS. Enforcement, both privately and by the government, has become more stringent, particularly in attempts to combat fraud and waste. Since our inception in 1976, we continue to maintain our licenses, including our Medicare and Medicaid and equivalent certifications. The permanent loss of any licenses and certifications would have a material adverse effect on our results of operations, financial position and cash flows.
We regularly review legislative and regulatory changes and developments and will restructure a business arrangement if we determine such might place our operations in material noncompliance with applicable laws or regulations. See “Fraud and Abuse” and “Stark II” below. To date, none of our business arrangements have been the subject of investigation by any governmental authority. No assurance can be given, however, that our business arrangements will not be the subject of future investigation or prosecution by federal or state governmental authorities which could result in civil and/or criminal sanctions. Effective October, 2008, CMS promulgated new regulations revising the conditions of coverage for ESRD facilities like ours. The regulations established performance expectations for the dialysis facilities and staff, eliminate certain procedural requirements, and promote continuous quality
13
improvement and patient safety measures. We have established new Operating Policies and Procedures with auditing processes to monitor compliance.
Certification and Reimbursement
Our dialysis centers must meet certain requirements, including, among others, those relating to patient care, patient rights, medical records, the physical set-up of the center, and personnel, in order to be certified by CMS, to be covered under the Medicare program and to receive Medicare reimbursement. See above under “Operations – Medicare Reimbursement.” Our dialysis centers are certified under the Medicare program, and are certified under applicable state Medicaid programs.
Fraud and Abuse
Our dialysis operations are subject to federal and state laws governing financial relationships between health care providers and referral sources and the accuracy of information submitted in connection with reimbursement. These laws, collectively referred to as “fraud and abuse” laws, include the Anti-Kickback Statute, Stark II, other federal fraud laws, and similar state laws.
The fraud and abuse laws apply because our medical directors have financial relationships with the dialysis facilities and typically refer patients to those facilities for items and services reimbursed by federal and state health care programs. Financial relationships with patients who are federal program beneficiaries also involve the fraud and abuse laws. Other financial relationships which bear scrutiny under the fraud and abuse laws include relationships with hospitals, nursing homes, and various vendors.
Anti-Kickback Statutes
The federal Anti-Kickback Statute prohibits the knowing and willful solicitation, receipt, offer, or payment of any remuneration, directly or indirectly, in return for or to induce the referral of patients or the ordering or purchasing of items or services payable under the Medicare, Medicaid, or other federal health care program.
Sanctions for violations of the Anti-Kickback Statute include criminal penalties, such as imprisonment and fines of up to $25,000 per violation, and civil penalties of up to $50,000 per violation, as well as mandatory exclusion from Medicare, Medicaid, and other federal health care programs for a minimum of five years. Under U.S. Sentencing Guidelines, an individual may be fined up to $250,000 and an organization may be fined up to $500,000 upon conviction of offenses described in any federal statute.
The language of the Anti-kickback Statute has been construed broadly by the courts. Over the years, the federal government has published regulations that established “safe harbors” to the Anti-Kickback Statute. An arrangement that meets all of the elements of the safe harbor is immunized from prosecution under the Anti-Kickback Statute. The failure to satisfy all elements, however, does not necessarily mean the arrangement violates the Anti-Kickback Statute.
Some states have enacted laws similar to the Anti-Kickback Statute. These laws may apply regardless of payor source, may include criminal and civil penalties, and may contain exceptions that differ from the safe harbors to the Anti-Kickback Statute.
As required by Medicare regulations, each of our dialysis centers is supervised by a medical director, who is a licensed nephrologist or otherwise qualified physician. The compensation of each of our medical directors, who are independent contractors, is fixed by a medical director agreement and
14
reflects competitive factors in each respective location, the size of the center, the anticipated workload for that particular center, and the physician’s professional qualifications. The medical director’s fee is fixed in advance, typically for periods of one to five years and does not take into account the volume or value of any referrals to the dialysis center. Thirteen of our outpatient dialysis centers are owned jointly by us and physicians who hold a minority position through a professional association. These physicians act as the medical directors for those facilities. We attempt to structure our arrangements with our physicians to comply with the Anti-Kickback Statute with the understanding that many of these physicians’ patients are treated at our facilities. We believe that the fair market value of the minority interest in a subsidiary acquired by the physician has been consistent with the cash consideration paid and/or the assets transferred to the subsidiary, and there is no intent to induce referrals to any of our centers. See “Business – Physician Relationships” above. We lease space for six of our centers from entities in which physicians hold ownership interests. We sublease space to seven of our medical directors at eight of our dialysis centers. These arrangements must be in compliance with the Anti-Kickback Statute. We believe our arrangements with our medical directors are in compliance with applicable law. Several states in which we operate have laws prohibiting physicians from holding financial interests in various types of medical facilities. If these statutes are interpreted to apply to relationships we have with our medical directors who hold a percentage ownership in our dialysis facilities, we would restructure our relationship with these physicians but could be subject to penalties.
We believe that the Anti-Kickback Statute and other fraud and abuse laws are primarily directed at abusive practices that increase the utilization and cost of services covered by governmentally funded programs. The dialysis services we provide generally cannot, by their very nature, be over-utilized since dialysis treatment is not elective, and is only indicated when there is temporary or permanent kidney failure. Medical necessity is capable of being supported by objective documentation, drastically reducing the possibility of over-utilization. Additionally, there are safe harbors for certain arrangements. Nevertheless, while relationships created by medical director ownership of minority interests in our facilities satisfy many but not all of the criteria for the safe harbor, there can be no assurance that these relationships will not subject us to investigation or prosecution by enforcement agencies. In an effort to further our compliance with the law, we have adopted a corporate Compliance Program that addresses medical necessity and medical chart audits to confirm medical necessity of referrals.
With respect to our inpatient dialysis services, we provide hospitals with dialysis services, including qualified nursing and technical personnel, supplies, equipment and technical services. In certain instances, the medical director of our dialysis center who has a minority interest in that facility may refer patients to hospitals with which we have an inpatient dialysis services arrangement. We believe our acute inpatient hospital services are in compliance with the law. See “Stark II” below.
We endeavor in good faith to comply with all governmental regulations. However, there can be no assurance that we will not be required to change our practices or experience a material adverse effect as a result of any such potential challenge. We cannot predict the outcome of the rule-making process, enforcement procedures, or whether changes in the safe harbor rules will affect our position with respect to the Anti-Kickback Statute, but we will continue to make every effort to remain in compliance.
Stark II
The federal physician self referral law, commonly known as Stark II, prohibits a physician who has a financial relationship, or who has an immediate family member who has a financial relationship, with entities providing “designated health services” from referring Medicare or Medicaid patients to such entity for such services unless an exception applies. A “financial relationship” includes an ownership or investment interest in, or a compensation arrangement between the physician and the entity. For purposes of Stark II, “designated health services” include clinical laboratory services, durable medical equipment,
15
parenteral and enteral nutrients, home health services, and inpatient and outpatient hospital services. Dialysis treatments are not “designated health services.”
The self-referral prohibition has numerous exceptions, including exceptions for personal service arrangements, leases of space and equipment, employment relationships and referrals within group practices.
An entity is prohibited from submitting Medicare or Medicaid claims for services furnished pursuant to an unlawful referral and must refund amounts received pursuant to prohibited referrals. Violators are subject to civil monetary penalties of up to $15,000 for each improper claim and may be excluded from participation in the Medicare and Medicaid programs. Unlike the federal Anti-Kickback Statute, intent to violate the law is not required. Knowing violations of Stark II may also serve as the basis for liability under the False Claims Act. See “False Claims Act” below.
HHS regulations to Stark II became effective in January, 2002. CMS adopted Phase II of its regulations under Stark II in March, 2004. These regulations exclude from covered designated health services and referral prohibitions, services included in the ESRD composite rate and EPO and other drugs required as part of dialysis treatments under certain conditions. Also excluded from “inpatient hospital services” are dialysis services provided by a hospital not certified by CMS to provide outpatient dialysis services, which would exclude our inpatient hospital services agreements from Stark II. Equipment and supplies used in connection with home dialysis are excluded from the Stark II definition of “durable medical equipment.”
Stark II regulations and the legislative history of Stark II indicate that the purpose behind the Stark II prohibition on physician referral is to prevent Medicare and Medicaid program and patient abuse. Since dialysis is a necessary medical treatment for those with temporary or permanent kidney failure, it is not highly susceptible to that type of abuse. We believe, based upon current rules and the industry practice, Congress did not intend to include dialysis services and the services and items we provide that are incidental to dialysis services within the Stark II prohibitions.
If the provisions of Stark II were found to apply to our arrangements however, we believe that we would be in compliance. We compensate our medical directors pursuant to medical director agreements, which we believe meet the exception for personal service arrangements under Stark II. No other physicians who send their patients to or treat their patients at any of our facilities receive compensation from us.
Medical directors of our dialysis centers who hold a minority investment interest in the subsidiaries operating those centers may refer patients to hospitals with which we have an acute inpatient dialysis service arrangement. Although the regulations of Stark II may be interpreted to apply to these types of transactions, we believe that our contractual arrangements with hospitals for acute care inpatient dialysis services are in compliance with Stark II.
A limited number of dialysis facilities are leased from entities in which physicians hold ownership interests, and we sublease space to seven of our medical directors. Stark II provides an exception for these types of affiliated physician lease agreements if specific requirements are met. The company believes these leases satisfy the requirements for these exceptions.
If CMS or any other government entity otherwise interprets the Stark II regulations, we may be required to restructure certain existing compensation or investment agreements with our medical directors, or, in the alternative, refuse to accept referrals for designated health services from certain physicians. Stark II prohibits Medicare or Medicaid reimbursement of items or services provided
16
pursuant to a prohibited referral, and imposes substantial civil monetary penalties on facilities which submit claims for reimbursement for such prohibited referrals. If such were to be the case, we could be required to repay amounts reimbursed for drugs, equipment and services that CMS determines to have been furnished in violation of Stark II, in addition to substantial civil monetary penalties, which could adversely affect our operations and financial results. We believe that if Stark II is interpreted by CMS or any other governmental entity to apply to our arrangements, it is possible that we could be permitted to bring our financial relationships with referring physicians into material compliance with the provisions of Stark II on a prospective basis. However, prospective compliance may not eliminate the amounts or penalties, if any, that might be determined to be owed for past conduct, and there can be no assurance that the costs and expenses associated with such prospective compliance, if permissible, would not have a material adverse effect on our results of operation, financial position or cash flows.
False Claims Act
The False Claims Act is the federal government’s primary civil remedy for improper or fraudulent claims. It applies to all federal programs, including Medicare and Medicaid. The False Claims Act prohibits, among other things, (1) knowingly presenting or causing to be presented a false or fraudulent claim, (2) knowingly making or using, or causing to be made or used, a false record or statement in order to have a false or fraudulent claim paid, (3) conspiring to defraud the government by getting a false or fraudulent claim allowed or paid, and (4) knowingly making or using, or causing to be made or used, a false record or statement to conceal, avoid, or decrease an obligation to pay the government.
“Knowingly” means that a person, with respect to information: (1) has actual knowledge of the information; (2) acts in deliberate ignorance of the truth or falsity of the information; or (3) acts in reckless disregard of the truth or falsity of the information. No proof of specific intent to defraud is required.
Penalties for violation of the False Claims Act include fines of $5,500 to $11,000 per false claim, plus treble damages. Prosecution under the False Claims Act involves an extensive variety of alleged false claims and fraud allegedly perpetrated against Medicare and state healthcare programs, including billing for services not rendered, coding errors, the submission of false cost reports, and billing for services at a higher payment rate than appropriate. The federal government can use other criminal federal statutes to prosecute false claims.
Our Corporate Compliance Program is designed to address risks inherent in the submission of claims and other information in connection with the Medicare and Medicaid program and we are committed to ensuring the accuracy of all claims. There can be no assurance, however, that we will not be subject to an investigation or audit regarding the accuracy of any claim or other information submitted in connection with our operations.
HIPAA
The Health Insurance Portability and Accountability Act of 1996, known as HIPAA, provided for health insurance reforms which included a variety of provisions important to healthcare providers, such as significant changes to the Medicare and Medicaid fraud and abuse laws. HIPAA established two programs that coordinate federal, state and local healthcare fraud and abuse activities. Under these programs, these governmental entities undertake a variety of monitoring activities, including medical utilization and fraud review, cost report audits and secondary payor determinations.
17
As part of the administrative simplification provisions of HIPAA, there are regulations governing electronic transactions relating to healthcare information. These regulations require a party transmitting or receiving healthcare transactions electronically to send and receive data in single format. This regulation applies to our submissions and processing of healthcare claims and also applies to many of our payors. We believe that we are in compliance with the transactions standards rule.
HIPAA also includes provisions relating to the privacy of healthcare information. HHS’ privacy rules cover all individually identifiable healthcare information known as “protected health information” and apply to healthcare providers, health plans, and healthcare clearing houses, known as “covered entities.” The regulations are quite extensive and complex, but basically require companies to: (i) obtain patient acknowledgement of receipt of a notice of privacy practices; (ii) obtain patient authorization before certain uses and disclosures of protected health information; (iii) respond to patient requests for access to their healthcare information; and (iv) develop policies and procedures with respect to uses and disclosures of protected health information. We believe we are in compliance with the HIPAA privacy rules.
HIPAA has security regulations governing the security of health information that is maintained or transmitted electronically. These regulations generally require implementation of safeguards for ensuring the confidentiality of electronic health information. Management believes it is currently in compliance with the HIPAA security standards.
HIPAA expressly prohibits four practices, namely (1) submitting a claim that the person knows or has reason to know is for medical items or services that are not medically necessary, (2) transferring remuneration to Medicare and Medicaid beneficiaries that is likely to influence such beneficiary to order or receive items or services, (3) certifying the need for home health services knowing that all of the coverage requirements have not been met, and (4) engaging in a pattern or practice of upcoding claims in order to obtain greater reimbursement.
As for criminal penalties, HIPAA adds healthcare fraud, theft, embezzlement, obstruction of investigations and false statements to the general federal criminal code with respect to federally funded health programs, thus subjecting such acts to criminal penalties. Persons convicted of these crimes face up to 10 years imprisonment and/or fines. Moreover, a court imposing a sentence on a person convicted of federal healthcare offense may order the person to forfeit all real or personal property that is derived from the criminal offense. The Attorney General is also provided with a greatly expanded subpoena power under HIPAA to investigate fraudulent criminal activities, and federal prosecutors may utilize asset freezes, injunctive relief and forfeiture of proceeds to limit fraud during such an investigation.
Although we believe we substantially comply with currently applicable state and federal laws and regulations and to date have not had any difficulty in maintaining our licenses and Medicare and Medicaid authorizations, the healthcare service industry is and will continue to be subject to substantial and continually changing regulation at the federal and state levels, and the scope and effect of such and its impact on our operations cannot be predicted. No assurance can be given that our activities will not be reviewed or challenged by regulatory authorities. We continue to work with our healthcare counsel in reviewing our policies and procedures and make every effort to comply with HIPAA and other applicable federal and state laws and regulations.
Environmental and Health Regulations
Our dialysis centers are subject to various federal, state and local hazardous waste laws and non-hazardous medical waste disposal laws. Most of our waste is non-hazardous. We also follow OSHA’s Hazardous Waste Communications Policy, which requires all employees to be knowledgeable of the
18
presence of and familiar with the use and disposal of hazardous chemicals in the facility. Medical waste of each facility is handled by licensed local medical waste sanitation agencies who are responsible for compliance with such laws.
There are a variety of regulations promulgated under OSHA relating to employees exposed to blood and other potentially infectious materials requiring employers, including dialysis centers, to provide protection. We adhere to OSHA’s protective guidelines, including regularly testing employees and patients for exposure to hepatitis B and providing employees subject to such exposure with hepatitis B vaccinations on an as-needed basis, protective equipment, a written exposure control plan and training in infection control and waste disposal.
Other Regulation
The company complies with the employee education processes of the Deficit Reduction Act of 2005.
Certain states have Anti-Kickback legislation and laws dealing with self-referral provisions similar to the federal Anti-Kickback Statute and Stark II. We have no reason to believe that we are not in compliance with such state laws.
Our Compliance Program is designed to assure compliance with fraud and abuse laws and regulations. See above under the caption “Compliance Program.” The establishment and implementation of our Compliance Program, coupled with our existing policies and internal controls, could have the effect of mitigating any civil or criminal penalties for potential violations. We will continue to use our best efforts to fully comply with federal and state laws, regulations and requirements as applicable to our operations and business.
Medical Products
Subsequent to our merger in September, 2005, with our former parent company, Medicore, Inc., we became engaged in the distribution of medical products, primarily disposables and diabetic supplies, both domestically and internationally, to hospitals, blood banks, laboratories and retail pharmacies. We distribute blood lancets used to draw blood for testing under the names Producers of Quality Medical Disposables™, Lady Lite™, our brand name Lite Touch, or under a private label if requested by the customer. Medical devices are required by the FDA, as a condition of marketing, to secure a 510(k) premarket notification clearance or a Premarket Approval Application. A product will be cleared by the FDA under a 510(k) if it is found to be substantially equivalent in terms of safety, effectiveness and intended use to another legally marketed product. We received 510(k) clearance for our blood lancet line and insulin syringes. Our medical products are subject to continuing FDA oversight, including labeling, “good manufacturing practices,” as defined in FDA regulations, and adverse event reporting, none of which adverse events have occurred to date. Marketing of our medical products is conducted by our employees and independent manufacturer representatives.
Competition
The dialysis industry is highly competitive. There are numerous providers who have dialysis centers in the same areas as our centers. Many are owned by larger corporations, which operate dialysis centers regionally, nationally and internationally. Our operations are small in comparison with those corporations. Two of our major competitors are significantly larger public companies, Fresenius Medical Care, Inc., and DaVita, Inc. These companies have substantially greater financial resources, significantly more centers, patients and services than we do, and by virtue of such may have an advantage over us in
19
competing for nephrologists and acquisitions of dialysis facilities in areas and markets we target. Moreover, competition for acquisitions has increased the cost of acquiring existing dialysis centers. Fresenius also manufactures and sells dialysis equipment and supplies, which may provide it with a greater competitive edge. We also face competition from hospitals and physicians that operate their own dialysis facilities.
Competitive factors most important in dialysis treatment are quality of care and service, convenience of location and pleasantness of the environment. Another significant competitive factor is the ability to attract and retain qualified nephrologists. These physicians are required as medical directors of the dialysis center for it to participate in the Medicare ESRD program, and are responsible for the supervision of the medical operations of the center. Our medical directors usually are subject to non-compete restrictions within a limited geographic area from the center they administer. They can be a substantial source of patients for the centers. Additionally, there is always substantial competition for obtaining qualified, competent nurses and technical staff at reasonable labor costs. There can be no assurance that we will compete effectively.
The medical products industry is extremely competitive and our medical products operations are not a significant competitive factor in this area.
Employees
As of March 13, 2009, we had 550 full time employees, including administrators, licensed practical nurses, registered nurses, technical specialists, patient care technicians, clerical employees, social workers, dietitians, corporate staff and four employees in the medical products operations. We retain 38 part-time employees consisting of registered nurses, patient care technicians and clerical employees. We also utilize 149 per diem personnel to supplement staffing.
We retain nine independent contractors, including social workers and dietitians at various of our dialysis facilities. These contractors are in addition to the medical directors, who supervise patient treatment at each facility.
We believe our relationships with our employees are excellent and we have not suffered any strikes or work stoppages. None of our employees are represented by any labor union. We are an equal opportunity employer.
| Item 1A. | Risk Factors |
We have listed below certain of the risk factors relating to us and our securities. There may be other risks and uncertainties that we may face and of which we are currently unaware which could also adversely affect our business, operations and financial condition. If any of such risks or uncertainties arise, or the risks listed below occur, our operations, earnings and financial condition could be materially harmed, which, in turn, would most likely adversely affect the trading price of our common stock. Any such event could negatively impact a shareholder’s investment in the company.
Our dialysis operations are subject to extensive government regulation
Our dialysis operations are subject to extensive federal and state government regulations, which include:
| ● | licensing requirements for each dialysis center | |
| ● | government healthcare program participation requirements |
20
| ● | reimbursement for patient services | |
| ● | patient referral prohibitions; broad federal and state Anti-Kickback regulations | |
| ● | false claims prohibitions for health care reimbursement and other fraud and abuse regulations | |
| ● | record keeping requirements | |
| ● | health, safety and environmental compliance | |
| ● | expanded protection of the privacy and security of personal medical data | |
| ● | standards for the exchange of electronic health information; electronic transactions and code sets; unique identifiers for providers | |
| ● | medical waste disposal regulations |
Many of these laws and regulations are complex and open to further judicial and legislative interpretations. If we are forced to change our method of operations because of these regulations, our earnings, financial condition and business could be adversely affected. The imposition of additional licensing and other regulatory requirements may, among other things, increase our cost of doing business. In addition, any violation of these governmental regulations could involve substantial civil and criminal penalties and fines, revocation of our licenses, closure of one or more of our centers, and our exclusion from participating in Medicare and Medicaid programs. Any loss of federal or state certifications or licenses would materially adversely impact our business.
Our arrangements with our physician medical directors do not meet the safe harbor provisions of federal and state laws, and may subject us to greater governmental scrutiny
Neither our arrangements with the medical directors of our facilities, typically retained by us as independent contractors under a fixed fee medical director agreement, nor the minority ownership interests of physicians in certain of our dialysis facilities meet all of the requirements of safe harbors to the Anti-Kickback Statute and similar state laws. These laws impose civil and criminal sanctions on persons who receive or make payments for referring a patient for treatment that is paid for in whole or in part by Medicare, Medicaid or similar state programs. Transactions and arrangements involving physician minority ownership that do not fall within a safe harbor may be subject to greater scrutiny by enforcement agencies, and if determined to violate the Anti-Kickback Statute or Stark II law could subject us to repayment of certain reimbursement amounts, monetary penalties and exclusion from further participation in government programs. If we are unable to modify the arrangement to satisfy the applicable laws and safe-harbor provisions, we would not be allowed to accept patient referrals from the physician with whom we have the arrangement. In any of these circumstances, our financial position, results of operations and cash flows would be adversely affected.
Our operations are subject to Medicare and Medicaid audits with concurrent potential civil and criminal penalties for failure to comply
We are subject to periodic audits by the Medicare and Medicaid programs, which have various rights and remedies if they assert that we have overcharged the programs or failed to comply with program requirements. Rights and remedies available under these programs include repayment of any amounts alleged to be overpayments or in violation of program requirements, or making deductions from future amounts due to us. These programs may also impose fines, criminal penalties or program exclusions.
In the ordinary course of our business, we may receive notices of deficiencies for failure to comply with various regulatory requirements. We review such notices and take appropriate corrective action. In most cases, we and the reviewing agency will agree upon the measures that will bring the
21
center or services into compliance. In some cases or upon repeat violations, none of which we have experienced, the reviewing agency may take various adverse actions against a provider, including but not limited to:
| ● | the imposition of fines; | |
| ● | suspension of payments for new admissions to the center; and | |
| ● | in extreme circumstances, decertification from participation in the Medicare or Medicaid programs and revocation of a center’s license. |
Any such regulatory actions could adversely affect a center’s ability to continue to operate, to provide certain services, and/or its eligibility to participate in Medicare or Medicaid programs or to receive payments from other payors. Moreover, regulatory actions against one center may subject our other centers, which may be deemed under our common control or ownership, to similar adverse remedies.
There has been increased governmental focus and enforcement with respect to anti-fraud initiatives as they relate to healthcare providers
State and federal governments are devoting increased attention and resources to anti-fraud initiatives against healthcare providers. Legislation has expanded the penalties for healthcare fraud, including broader powers to exclude providers from the Medicare and Medicaid programs. We have established policies and procedures that we believe are sufficient to ensure that our facilities will operate in substantial compliance with these anti-fraud requirements. While we believe that our business practices are consistent with Medicare and Medicaid criteria, those criteria are often vague and subject to change and interpretation. Anti-fraud actions could have an adverse effect on our financial position, results of operations and cash flows.
Our revenues and financial stability are dependent on fixed reimbursement rates under Medicare and Medicaid
During 2006, 2007 and 2008, approximately 52%, 49% and 46%, respectively, of our patient revenues was derived from Medicare reimbursement and 9%, 8% and 8%, respectively, of our patient revenues was derived from Medicaid and equivalent programs. Federal and state governments seek to maintain, if not reduce, costs including through:
| ● | reductions in payments to us or government programs in which we participate | |
| ● | inclusion of those ancillary services which we currently bill separately in the flat composite rate for dialysis treatments |
Changes implemented by Congress in recent years, including modest percentage increases in the Medicare composite rate (the fixed payment rate for a dialysis treatment including related supplies, laboratory tests and pharmaceuticals) while at the same time modifying the bases by which CMS reimburses for separately billable services and pharmaceuticals, such as EPO, did not, in the aggregate, significantly impact our cash flows, results of operations or financial position. Nevertheless, the government has continued to review the processes for reimbursement of dialysis services and in July, 2008, Congress passed the Medicare Improvements for Patients and Providers Act which, while providing for marginal increases in the composite rate for 2009 and 2010, establishes “bundled” payments for all goods and services provided during the dialysis treatment beginning in 2011 at an initial rate set at 2% below the payment rate that providers historically received under prior methodologies. From and after 2012, although the new single bundled payment base rate will be adjusted annually for inflation
22
based upon a market basket index less 1% of such index, the bundled payment rate will be subject to the discretion of the Secretary of HHS based on the goods and services being provided. Although we have not been materially impacted by prior adjustments in reimbursement rates relative to our operating costs that are subject to inflation, such as labor and supply costs, because the bundled rates that will take effect in 2011 have not been set, we cannot predict the effect of those future rates on our operating costs and expenses. Changes in the Medicare program, including the bundling of payments under the composite rate for dialysis services, combined with possible negative adjustments in reimbursement could adversely affect our cash flows, results of operation and financial position.
If government agencies delay in providing certification and/or reimbursement for our facilities we could experience reduced cash flows and adverse effects on our earnings and operations
In light of the current economic and fiscal environments, the budgets of state governments are under significant constraints. Several states, albeit none in which we currently operate or are seeking to develop or acquire additional centers, have already advised that they are curtailing or delaying their ESRD certification processes for the remainder of 2009 and possibly longer. In order for a newly developed or acquired dialysis center of the company to submit bills for patients treated at that facility who are enrolled in a government based program, that facility must be certified to participate in such government based programs such as Medicare and Medicaid. To the extent that additional states, particularly ones in which the company operates or is seeking to develop or acquire additional centers, experience certification delays or seek to delay or reduce reimbursement payments, we could experience reduced cash flow as well as adverse impacts on our earnings and results of operations.
A decline in the reimbursement payments from third-party, non-government payors as well as a change in patient coverage from commercial to Medicare could adversely affect our earnings
The dialysis services industry is experiencing a growing number of commercial payors seeking to reduce payment rates as a result of several factors, including a greater focus by these payors on dialysis services and market conditions generally. Some of these commercial payors are increasingly aggressive in attempting to negotiate lower contracted payment rates and imposing limitations on out-of-network access and payment rates. A decline in the rates paid by commercial payors, hospitals and other non-governmental third-party organizations would adversely affect our business. Additionally, any change in patient coverage, such as Medicare eligibility as opposed to higher private insurance coverage, would result in a reduction of revenue. We estimate approximately 39%, 43% and 46% of our patient revenues for 2006, 2007 and 2008, respectively, was obtained from sources other than Medicare or Medicaid and equivalent programs. We generally charge non-governmental organizations for dialysis treatment rates which exceed the fixed Medicare and Medicaid and equivalent rates. If commercial payors reduce their payments or we experience a shift in revenue mix toward Medicare or Medicaid reimbursement, then our results of operations, financial position or cash flows would be adversely affected.
Our cash flow, earnings and financial condition could be adversely impacted to the extent that the current economic and financial crises affecting the United States continues for an extended period of time
Our revenues and earnings are based upon a payor mix balance that has reflected an increase in recent years of revenues from commercial payor reimbursement. To the extent, however, that the current economic slowdown and financial crisis affecting the United States persists for an extended period of time, we may face a number of potential effects that could materially impact our revenues, earnings and financial condition. In particular, continued increases in job losses could result in a decrease in the number of our patients covered by commercial insurance, from which reimbursement payments generally exceed that of government based programs. Furthermore, employers facing operating cost pressures may
23
seek out alternative insurance plans that offer reduced reimbursement rates. Finally, both commercial payors themselves as well as federal and state governments may engage in efforts to further reduce and/or delay reimbursement payments for our services to the extent that economic conditions continue to worsen.
Any decrease in the availability of or the reimbursement rate of EPO would reduce our revenues and earnings
EPO is currently available from a single manufacturer, Amgen, Inc. The available supply of EPO could be delayed or reduced, whether by Amgen itself, through unforeseen circumstances, or as a result of excessive demand. In addition, Amgen could increase the price of EPO. This could adversely impact our revenues and profitability, since approximately 28% of our medical services revenues in 2006, 27% in 2007 and 27% in 2008 were based upon the administration of EPO to our dialysis patients.
Most of our EPO reimbursement is from government programs. For 2008, 68% of total EPO revenue was derived from Medicare and Medicaid reimbursement. In recent years, CMS revised its rules for reimbursement of pharmaceuticals, including EPO, which resulted in a net reduction of average Medicare payment rates. In 2006, reimbursement for EPO was at the average sales price plus 6%, which resulted in lower reimbursement for pharmaceuticals, but was offset by the 1.6% composite rate increase. If government or commercial payors further reduce reimbursement for EPO, then our revenues and earnings per treatment may decline.
Further changes and rules intended to address EPO and other pharmaceuticals could adversely affect our revenues and income.
Changes in Medicare reimbursement criteria have impacted EPO revenue. CMS implemented a national monitoring policy for EPO claims in April, 2006, which aimed to reduce reimbursement for EPO utilization above a certain hemotacrit threshold in a patient. The policy also limited the quantity of EPO that could be administered in a month, regardless of hematocrit levels. We revised our protocols on anemia management to address this policy and did not incur an adverse impact on our results of operations. Nevertheless, since the latter part of 2006 a significant amount of government and media attention has been paid to the management of anemia generally in all fields of medicine resulting in congressional hearings and FDA required label changes to EPO and Aranesp® which have been and are continuing to be examined. In January, 2008, CMS put into effect additional changes to its EPO monitoring policy intended to further limit reimbursement. Commercial payors may also follow suit as they direct greater scrutiny to their policies for EPO use as part of their overall analysis of dialysis services reimbursement. Further changes in EPO administration policies that result in further restrictions on utilization could result in a decline in our revenues and earnings per treatment.
New drugs could affect use of EPO, adversely impacting our profitability.
Amgen is the sole manufacturer of EPO, which is administered in conjunction with dialysis treatments to address a patient’s anemia. Amgen has developed the drug Aranesp®, and Roche has developed Mircera®, each of which can be used to treat anemia, and which are indicated to be effective for longer periods than EPO. Based on their longer lasting capabilities, potential margins on these alternative pharmaceuticals could be significantly lower than on EPO, and furthermore, their potential for being administered by a dialysis patient’s physician, could further eliminate potential revenues from the treatment of anemia in our dialysis patients. The introduction and marketing of alternative pharmaceuticals as an anemia treatment for dialysis patients, could adversely impact our results of operations, financial position or cash flows. Currently, Mircera® is not available for use in the United States due to a patent held by Amgen.
24
Disruptions or inefficiencies in the supply chain, particularly with pharmaceuticals, could negatively impact our earnings and financial condition
Last year Baxter Healthcare Corporation engaged in a recall of heparin, a pharmaceutical used in the provision of dialysis services, and shortly thereafter ceased further sales of this pharmaceutical altogether, leaving one supplier of heparin in the United States. The price for heparin has since increased nearly four-fold and may continue to rise in the future. While we do not believe, given our historical utilization of heparin, that the current price will materially affect our earnings, continued increases in the price, or limitations on the availability of this pharmaceutical could negatively impact our operations and earnings. Similarly, while we rely on a number of vendors for our dialysis supplies and pharmaceuticals, to the extent that such supply were disrupted for an extended period of time, our inability to identify and establish a cost-effective replacement could significantly impact our operations, earnings and financial position.
Our ability to grow is subject to our resources and available locations
Over the past several years we have both built and acquired several dialysis centers, in some cases with partners holding a minority interest. We seek areas with qualified and cost-effective nursing and technical personnel and a sufficient population to sustain a dialysis center. These opportunities are limited and we compete with much larger dialysis companies for appropriate locations. Our growth strategy based on construction also involves the risks of our ability to identify suitable locations to develop additional centers. Those we do develop may never achieve profitability, and additional financing may not be available to finance future development.
Our inability to acquire or develop dialysis centers in a cost-effective manner would adversely affect our ability to expand our business and as a result, our profitability.
Growth places significant demands on our financial and management skills. Inability on our behalf to meet the challenges of expansion and to manage any such growth would have an adverse effect on our results of operations, financial position or cash flows.
Our attempt to expand through development or acquisition of dialysis centers which are not currently identified entails risks which shareholders and investors will not have a basis to evaluate
We expand generally by seeking an appropriate location for a dialysis center and by taking into consideration the potential geographic patient base, types of commercial insurance in the area, the availability of a physician nephrologist to be our medical director, and a skilled work force. Construction, equipment and initial working capital costs for a new dialysis center with 15 stations, typically the size of our dialysis facilities, range from $1,000,000 to $1,500,000. While and acquisition of existing outpatient dialysis centers is a faster means for achieving profitability, these transactions can be more costly. Therefore, the company takes a selective approach to its acquisition strategy. We cannot assure you that we will be successful in developing or acquiring dialysis facilities, or otherwise successfully expanding our operations. We are routinely negotiating with nephrologists and others to establish new dialysis centers, but we cannot assure you that these negotiations will result in the development of new centers. Furthermore, there is no basis for shareholders and investors to evaluate the specific merits or risks of any potential development or acquisition of dialysis facilities.
25
We depend on physician referrals, and the limitation or cessation of such referrals would adversely impact our revenues and earnings
Most dialysis facilities, including ours, are dependent upon referrals of ESRD patients for treatment by physicians, primarily those physicians specializing in nephrology. We retain by written agreement qualified physicians or groups of qualified physicians to serve as medical directors for each of our facilities. The medical directors are typically a source of patients treated at the particular facility served. There is no requirement for these physicians to refer their patients to us, and they are free to refer patients to any other dialysis facility. The loss of the patient base of the medical director or other physicians in the area of our facilities could result in a decline in our operations, revenues and earnings. We may not be able to renew or otherwise negotiate compensation under the medical director agreements with our medical director physicians which could terminate the relationship, and without a suitable medical director replacement could result in closure of the facility. Accordingly, the loss of these key physicians at a particular facility could have a material adverse effect on the operations of the facility and could adversely affect our revenues and earnings. Most of our medical director agreements range in terms of from five to ten years with renewals, and we have had no difficulty in renewing agreements as they have expired. All the medical director agreements provide for noncompetition restrictions. We have never had to attempt to enforce such restrictions, but there is no assurance that a particular jurisdiction in which the agreement is applicable would uphold such noncompetition agreement, which would increase the potential for competition with affiliated dialysis centers and could adversely impact our revenues and earnings.
Some of our medical directors or the medical groups with whom they are associated own minority interests in certain of our subsidiaries which operate dialysis centers. If these interests are deemed to violate applicable federal or state law, these physicians may be forced to dispose of their ownership interests.
Industry changes could adversely affect our business
Healthcare organizations, public and private, continue to change the manner in which they operate and pay for services. Our business is designed to function within the current healthcare financing and reimbursement system. In recent years, the healthcare industry has been subject to increasing levels of government regulation of reimbursement rates and capital expenditures, among other things. In addition, proposals to reform the healthcare system have been considered by Congress, and still remain a priority issue. Any new legislative initiatives, if enacted, may (i) further increase government regulation of or other involvement in healthcare, (ii) lower reimbursement rates, and (iii) otherwise change the operating environment for healthcare companies. We cannot predict the likelihood of those events or what impact they may have on our results of operation, financial position or cash flows.
Our business is subject to substantial competition, and we must compete effectively, otherwise our growth could slow
We are operating in a highly competitive environment in terms of the operation, development and acquisition of dialysis centers. Our competition comes from other dialysis centers, many of which are owned by much larger companies, and from hospitals. We also compete with physicians who open their own dialysis facilities. The dialysis industry is rapidly consolidating, resulting in several large dialysis companies competing for the acquisition of existing dialysis centers and the development of relationships with referring physicians. Some of our competitors have significantly greater financial resources, more dialysis facilities and a significantly larger patient base. Competition for acquiring existing centers has increased the costs of acquiring such facilities.
26
Competition is also intense for qualified nursing and technical staff as well as for nephrologists with an adequate patient base. Although we have exhibited growth over the last several years, we can provide no assurance that we will be able to compete effectively in the future. Our failure to do so could impair our continued growth and profitability.
Interest rate volatility could negatively impact our profitability and cash flows
We have successfully increased our borrowing capabilities in order to pursue our near term growth strategy. As a result, our borrowings under our principal credit facility have increased in recent periods. The majority of the loans under the credit facility are currently based on LIBOR-based variable rates. To the extent that these rates were to increase, our interest expense relating to the variable rates would increase, impacting our debt service and cash flows, and could negatively impact our profitability.
If the enhancements that we are currently making to our billing and clinical systems do not function effectively, we may experience adverse effects on our cash flow and operating results
We are in the process of establishing a new company-wide clinical and billing system that will significantly improve our efficiencies with respect to the compilation, processing and distribution of billing and collection information as well as our ability to monitor clinical information on our patients. In part, the system is intended to shift information gathering and processing to real time electronic transmission from all of the facilities and away from manual processing at the facility level. While we intend to perform all appropriate tests of the system as it comes online at each facility, the potential exists for malfunctions or integration issues to arise as the system becomes integrated with the operations of the company. To the extent that system malfunctions occur, we may be delayed in processing our billing and collections at one or more facilities. If these delays continue our revenues, cash flows and financial condition could be negatively impacted.
We could be subject to professional liability claims that may adversely affect us
Operation of dialysis centers and, in particular, the provision of dialysis treatments to ESRD patients, entails significant risks of liability, as is the case with most healthcare treatment services. Accordingly, we could be subject to various actions and claims of professional liability alleging negligence in the performance of our treatment and related services, as well as for the acts or omissions of our employees. As we grow and the number of our patients increases, so too does our exposure increase to potential malpractice, professional negligence, and other related legal theories and causes of action. These potential claims could seek substantial damages, possibly beyond our insurance coverage, and could subject us to the incurrence of significant fees and costs related to defending such potential claims. Such potential future claims for malpractice or professional liability, including any judgments, settlements or costs associated with such claims and actions, could have a material adverse effect on us.
Our insurance costs and deductibles have been substantially increasing over the last several years, and may not be sufficient to cover claims and losses
We maintain a program of insurance coverage against a broad range of risks in our business, including, and of primary importance, professional liability insurance, subject to certain deductibles. The premiums and deductibles under our insurance program have been increasing over the last several years as a result of general business rate increases coupled with our continued growth and development of dialysis centers. We are unable to predict further increases in premiums and deductibles, but based on experience we anticipate further increases in this area, which could adversely impact earnings. The liability exposure of operations in the healthcare services industry has increased, resulting not only in increased premiums, but in limited liability on behalf of the insurance carriers. Our ability to obtain the
27
necessary and sufficient insurance coverage for our operations upon expiration of our insurance policies may be limited, and sufficient insurance may not be available on favorable terms, if at all. Such insurance may not be sufficient to cover any judgments, settlements or costs relating to potential future claims, complaints or law suits. Our inability to obtain sufficient insurance for our operations, or if we obtain insurance which is limited, any future significant judgments, settlements and costs relating to future potential actions, suits or claims, could have an adverse effect on our company.
Shares eligible for future sale by restricted shareholders may adversely affect our stock price
Our officers and directors own approximately 2,165,000 shares of our common stock and vested options exercisable into an additional 40,000 shares of common stock, for an aggregate of 2,205,000 shares or approximately 23% of our outstanding common stock. All of the shares held by these officers and directors, other than the 40,000 shares obtainable upon exercise of their options, upon satisfying the conditions of Rule 144 under the Securities Act, may be sold without complying with the registration provisions of the Securities Act. Rule 144 conditions include:
| ● | holding the shares for six months from acquisition; | |
| ● | volume limits of selling every three months an amount of shares which does not exceed the greater of 1% of the outstanding common stock, or the average weekly volume of trading as reported by Nasdaq during the four calendar weeks prior to the sale; | |
| ● | filing Form 144 with the SEC; | |
| ● | the company continuing to timely file its reports under the Exchange Act; |
Our publicly tradable common stock, known as the float, is approximately 7,430,000 shares. Our common stock owned by our officers and directors represent approximately 29% of the float. Accordingly, the sale by such officers and directors under Rule 144 may have an adverse affect on the market price of our common stock, and may inhibit our ability to manage subsequent equity or debt financing.
Over the last year, our stock price has exhibited volatility, and any investment in our common stock may, therefore, decline for reasons unrelated to our performance
Our common stock trades on the Nasdaq Global Market under the symbol “DCAI.” The market price of our common stock has exhibited volatility. For fiscal 2008, our per share price range was $5.20 to $9.46.
Other than the continued growth of the company, there was no information known to management that would cause significant fluctuation in the price of our common stock, or in the trading volume.
Other factors that could continue to cause fluctuation in our common stock include:
| ● | changes in government regulation, whether legislative, enforcement or reimbursement rates | |
| ● | third party reports relating to the dialysis industry and our company (unsolicited by management) | |
| ● | announcements by management relating to the company’s performance or other material events | |
| ● | actions and announcements by our competitors | |
| ● | the outlook for the healthcare industry, and the economic and market conditions generally |
28
Investors should understand that in general, stock prices may fluctuate for reasons unrelated to operating results. Any changes in the above discussed factors, or general economic, political, global and market conditions, could result in a decline in the market price and volume of trading in our common stock.
| Item 1B. | Unresolved Staff Comments |
None.
| Item 2. | Properties |
We own a property in Easton, Maryland which consists of approximately 7,500 square feet, most of which is leased to a competitor under a 10-year lease through June 30, 2009 with two renewals of five years each. The lease is guaranteed by the tenant’s parent company.
Our Easton, Maryland property has a mortgage to secure a development loan with an original principal balance of $700,000 for which interest is at the prime rate, maturing in May, 2026. This loan had a remaining principal balance of approximately $495,000 at December 31, 2008. See Item 7, “Management’s Discussions and Analysis of Financial Condition and Results of Operations.”
We own property in Lemoyne, Pennsylvania which consists of approximately 15,000 square feet and houses one of our dialysis centers, accounting for approximately 5,400 square feet, under a five year lease through December 22, 2013. The center is approved for 17 dialysis stations with space available for expansion. The remaining space is used for record storage and training sessions.
We acquired property in Valdosta, Georgia in 2000, subject to a five year $788,000 mortgage obtained in April, 2001, subsequently refinanced in April, 2006, with interest at the prime rate, with a minimum rate of 5.00% and a maximum rate of 7.50%, maturing in April, 2011. This mortgage had a remaining principal balance of approximately $555,000 at December 31, 2008. See item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” We constructed a dialysis center at this property comprising approximately 6,000 square feet which we have leased to one of our subsidiaries for $90,600 per year under a 10-year lease, with two additional renewal periods of five years each.
Our Barnwell, South Carolina dialysis center, which became ESRD certified in 2008, is located in an approximately 6,000 square foot building owned by our subsidiary that operates from that location.
Our Cincinnati, Ohio dialysis center is leased from a corporation owned by the medical director of that center who, together with his wife, holds a minority interest in the subsidiary operating that center. Our Calhoun, Georgia dialysis center is leased from a partnership comprised of local doctors who became the medical directors for that facility in 2007. Our Aiken, South Carolina dialysis center is leased from an entity owned by the medical director of that center. Our York, Pennsylvania dialysis center is leased from a limited partnership in which we have a 60% ownership interest with the remaining 40% owned by two doctors, one of whom serves as the medical director for that facility. These doctors are also affiliated with the entity that owns a 40% minority ownership in the subsidiary that operates that facility. Our Hawkinsville, Georgia dialysis center is leased from an entity owned by the medical director of the center, with the medical director owing a minority interest in the subsidiary operating that center. Our Bedford, Pennsylvania center is leased from a company owned by the medical director of that center.
29
We presently own 28 dialysis centers, that lease their respective facilities from unaffiliated third parties, most under five to ten year initial terms, usually with two additional renewal periods of five years each, for space ranging from approximately 3,000 to 7,000 square feet.
We sublet a minimal amount of space at eight of our dialysis centers to the physicians who are our medical directors at those centers for their medical offices. The subleases are on a commercially reasonable basis and are structured to comply with the safe harbor provisions of the “Anti-Kickback Statute.” See Item 1, “Business – Government Regulation – Fraud and Abuse.”
Pursuant to the merger with our parent, Medicore, Inc., in 2005, we acquired land and buildings in Hialeah, Florida. Two properties have buildings consisting of approximately 28,000 square feet and are leased through August 31, 2010 to a non-affiliated company. The remaining land, aggregating approximately 48,000 square feet, is comprised of two paved parking lots and three parcels of vacant land.
Most of our dialysis facilities do not operate at full capacity. See “Business – Operations – Location, Capacity and Use of Facilities” above. The existing dialysis facilities could accommodate greater patient volume, particularly if we increase hours and/or days of operation without adding additional dialysis stations or any additional capital expenditures. We also have the ability and space at most of our facilities to expand to increase patient volume subject to obtaining appropriate governmental approval.
We maintain executive offices at 1302 Concourse Drive, Suite 204, Linthicum, Maryland 21090, and 777 Terrace Avenue, Hasbrouck Heights, New Jersey 07604, and administrative offices at 214 Senate Avenue, Suite 300, Camp Hill, Pennsylvania 17011, and 2337 West 76th Street, Hialeah, Florida 33016. These offices aggregate approximately 25,000 square feet of space and are under leases expiring in 2009, except for the Camp Hill lease, which expires in 2012.
| Item 3. | Legal Proceedings |
None.
| Item 4. | Submission of Matters to a Vote of Security Holders |
No matter was submitted during the fourth quarter of our fiscal year ended December 31, 2008 to a vote of security holders through the solicitation of proxies or otherwise.
30
PART II
| Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Price Range
Our common stock trades on the Global Market of the Nasdaq Stock Market under the symbol “DCAI.” The following table indicates the high and low sales prices for our common stock for each of the four quarters for the years ended December 31, 2007 and 2008 as reported by Nasdaq.
| Sales Price | ||||||||
| 2007 | High | Low | ||||||
1st Quarter | $ | 14.16 | $ | 12.33 | ||||
2nd Quarter | 12.69 | 8.61 | ||||||
3rd Quarter | 11.00 | 9.02 | ||||||
4th Quarter | 9.83 | 7.70 | ||||||
| Sales Price | ||||||||
| 2008 | High | Low | ||||||
1st Quarter | $ | 9.46 | $ | 6.68 | ||||
2nd Quarter | 7.95 | 6.70 | ||||||
3rd Quarter | 8.34 | 6.91 | ||||||
4th Quarter | 7.99 | 5.20 | ||||||
As of March 12, 2009, the last sales price of our common stock was $5.29.
Stockholders
At March 11, 2009, we had 898 shareholders of record as reported by Continental Stock Transfer & Trust Company, our transfer agent. We have been advised by Broadridge Financial Solutions, Inc., which organization holds securities for banks, brokers and depositories, that there are approximately 2,450 beneficial owners of our common stock.
Dividend Policy
We have not declared or paid cash dividends to our shareholders, and we do not anticipate paying dividends in the foreseeable future. The board of directors intends to retain earnings to finance our growth strategy. Future dividend policy will be at the discretion of the board of directors, and will depend on our earnings, capital requirements, financial condition and other similar relevant factors. Any determination to pay a dividend is also subject to the covenants in the KeyBank National Association credit facility (see Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” which precludes the payment of any dividends (other than stock dividends), as does the mortgage on our Valdosta, Georgia property (see Item 2, “Properties”) which restricts the payment of dividends above 25% of our net worth.
31
Sale of Securities Not Registered Under the Securities Act
The only unregistered sales of our equity securities during 2008 were (i) the grant of 13,500 stock awards to key employees on January 10, 2008, and (ii) the vesting on December 31, 2008 of 6,500 shares of common stock pursuant to an aggregate grant of 64,000 stock awards to certain officers and key employees in the second quarter of 2006. The above transactions have been previously reported on a current report on Form 8-K dated June 29, 2006, and in our quarterly reports on Form 10-Q for the first three quarters of 2008. Each of the stock awards contains termination and acceleration provisions. See Item 11, “Executive Compensation.”
The sales of our securities were exempt from the registration requirements of Section 5 of the Securities Act under the private placement exemptions of Section 4(2) and/or Regulation D of the Securities Act, based on the limited number of persons, each of whom are officers, directors and/or key employees knowledgeable concerning the affairs of the company. See Item 11, “Executive Compensation” and Item 12, “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
Stock Repurchases
There were no repurchases of our common stock in 2008.
Equity Compensation Plan Information
Information relating to compensation plans under which equity securities of the company are authorized for issuance is set forth in Part III, Item 12, “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” of this annual report on Form 10-K, incorporated herein by reference.
PERFORMANCE GRAPH
The following graph shows a five-year comparison of cumulative total shareholder returns for the company, the Nasdaq Market Index and the Dialysis Center Industry Index from December 31, 2003 through December 31, 2008. The cumulative total shareholder returns on our common stock was measured by dividing the difference between our share price at the end and the beginning of the measurement period by the share price at the beginning of the measurement period. The total shareholder return assumes $100 invested at the beginning of the period in our common stock, in the Nasdaq Market Index and the Dialysis Center Industry Index. We did not pay dividends on our common stock during the measurement period and the calculations of cumulative total shareholders return on the common stock did not include dividends. Our share price was adjusted to reflect a two-for-one stock split on January 28, 2004. This graph is presented in accordance with SEC requirements. You are cautioned against drawing any conclusions from this information, as past results are not necessarily indicative of future performance. This graph in no way reflects a forecast of future financial performance.
32
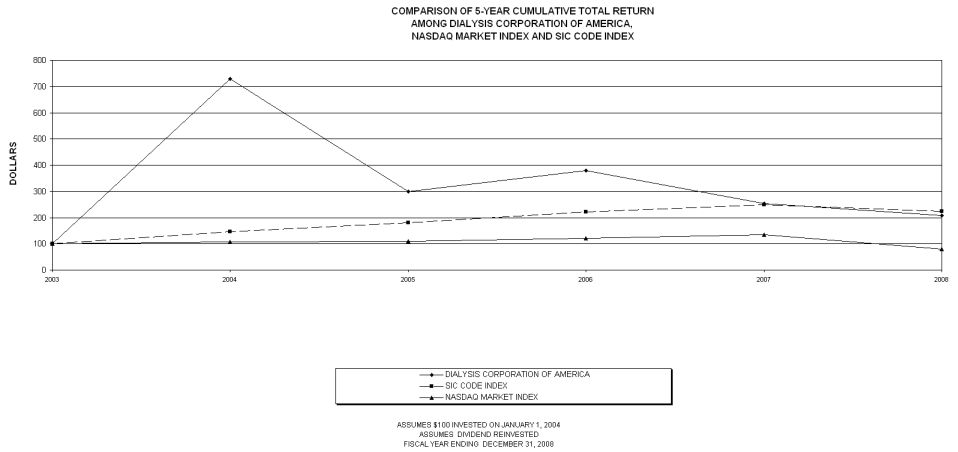
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | |||||||||||||||||||
| Dialysis Corporation of America | 100.00 | 729.25 | 299.40 | 379.40 | 253.73 | 208.96 | ||||||||||||||||||
| Dialysis Centers Industry | 100.00 | 145.72 | 181.62 | 221.23 | 250.22 | 224.60 | ||||||||||||||||||
| NASDAQ Market Index | 100.00 | 108.41 | 110.79 | 122.16 | 134.29 | 79.25 | ||||||||||||||||||
The current composition of SIC Code 8092 – Dialysis Centers Industry – is as follows:
DaVita Inc.*
Dialysis Corporation of America
Fresenius Medical Care AG&CO. KGaA.*
* These companies are significantly larger than our company.
The performance graph is furnished, not filed, and is not deemed to be soliciting material under the proxy rules or incorporated by reference into any company filing.
| Item 6. | Selected Financial Data |
The following selected financial data for the five years ended December 31, 2008 is derived from the audited consolidated financial statements of the company and its subsidiaries. The consolidated financial statements and related notes for the three years ended December 31, 2008, together with the related Reports of Independent Certified Public Accountants, are included elsewhere in this annual report on Form 10-K. The data should be read in conjunction with the consolidated financial statements, related notes and other financial information included herein, and Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| Consolidated Statements of Operations Data | ||||||||||||||||||||
| (in thousands except per share amounts) | ||||||||||||||||||||
| Years Ended December 31, | ||||||||||||||||||||
| 2008 | 2007 | 2006 | 2005 | 2004 | ||||||||||||||||
| Revenues* | $ | 86,837 | $ | 74,535 | $ | 62,460 | $ | 45,392 | $ | 40,986 | ||||||||||
| Net income | 2,844 | 3,086 | 3,049 | 1,900 | 2,214 | |||||||||||||||
| Earnings per share | ||||||||||||||||||||
| Basic | .30 | .32 | .32 | .22 | .27 | |||||||||||||||
| Diluted | .30 | .32 | .32 | .20 | .25 | |||||||||||||||
33
| Consolidated Balance Sheet Data | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| December 31, | ||||||||||||||||||||
| 2008 | 2007 | 2006 | 2005 | 2004 | ||||||||||||||||
| Working capital | $ | 20,266 | $ | 18,612 | $ | 17,098 | $ | 7,617 | $ | 3,644 | ||||||||||
| Total assets | 71,079 | 54,846 | 49,856 | 38,803 | 26,490 | |||||||||||||||
| Long term debt, net of current portion | 14,276 | 7,009 | 8,618 | 635 | 1,586 | |||||||||||||||
| Stockholders’ equity | 35,264 | 32,006 | 29,061 | 25,613 | 13,330 | |||||||||||||||
_________________________
* Prior year amounts have been reclassified to conform to current year presentation.
| Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations |
Management’s Discussion and Analysis of Financial Condition and Results of Operations, commonly known as MD&A, is our attempt to provide a narrative explanation of our financial statements, and to provide our shareholders and investors with the dynamics of our business as seen through our eyes as management. Generally, MD&A is intended to cover expected effects of known or reasonably expected uncertainties, expected effects of known trends on future operations, and prospective effects of events that have had a material effect on past operating results. Our discussion of MD&A should be read in conjunction with our consolidated financial statements, including the notes, included elsewhere in this annual report on Form 10-K. Please also review the Cautionary Notice Regarding Forward-Looking Information on page one of this annual report.
Overview
We provide dialysis services, primarily kidney dialysis treatments through 37 outpatient dialysis centers, including one center acquired on December 31, 2008, one center acquired effective January 1, 2008, and one new center opened during 2008, to patients with chronic kidney failure, also known as end-stage renal disease or ESRD. We provide dialysis treatments to dialysis patients of 11 hospitals and medical centers through acute inpatient dialysis services agreements with those entities. We provide homecare services, including home peritoneal dialysis. We engage in medical product sales, which is not a significant part of our business.
Quality Clinical Results
Our goal is to provide consistent quality clinical care to our patients from caring and qualified doctors, nurses, patient care technicians, social workers and dieticians. We have demonstrated an unwavering commitment to quality renal care through our continuous quality improvement initiatives. We strive to maintain a leadership position as a quality provider in the dialysis industry and often set our goals to exceed the national average standards. See Item 1, “Business – Operations – Quality Clinical Results.”
Patient Treatments
The following table shows the number of in-center, home peritoneal and acute inpatient treatments performed by us through the dialysis centers we operate, including one center we managed until January 1,
34
2008(1) and one in which we had a 40% ownership interest until August 1, 2006(2), and those hospitals and medical centers with which we have inpatient acute service agreements for the periods presented:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| In center | 248,232 | 228,456 | 197,628 | |||||||||
| Home and peritoneal | 15,980 | 15,940 | 16,115 | |||||||||
| Acute | 8,240 | 7,930 | 6,350 | |||||||||
| 272,452 | 252,326 | (3) | 220,093 | (3) | ||||||||
| (1) | At that date we acquired the 80% controlling interest. |
| (2) | At that date this center was consolidated, in which center we subsequently acquired the other 60% interest in December, 2006, and sold 30% to that facility’s medical director in 2007. |
| (3) | Includes treatments by the Georgia center managed through December 31, 2007, and acquired effective January 1, 2008: in-center treatments of 10,631 and 9,428, respectively, for 2007 and 2006, home and peritoneal treatments of 506 and 297, respectively for 2007 and 2006, and no acute treatments. |
New Business Development
Our future growth depends primarily on the availability of suitable dialysis centers for development or acquisition in appropriate and acceptable areas, and our ability to manage the development costs for these potential dialysis centers while competing with larger companies, some of which are public companies or divisions of public companies with greater numbers of personnel and financial resources available for acquiring and/or developing dialysis centers in areas targeted by us. Additionally, there is intense competition for qualified nephrologists who would serve as medical directors of dialysis facilities. There is no assurance as to when any new dialysis centers or inpatient service contracts with hospitals will be implemented, or the number of stations, or patient treatments such center or service contract may involve, or if such center or service contract will ultimately be profitable.
Start-up Losses
It has been our experience that newly established dialysis centers, although contributing to increased revenues, have adversely affected our results of operations in the short term due to start-up costs and expenses and a smaller patient base. These losses are typically a result of several months of pre-opening costs, and six to eighteen months of post opening costs, in excess of revenues. We consider new dialysis centers to be “start-up centers” through their initial 12 months of operations, or when they achieve consistent profitability, whichever is sooner. For the year ended December 31, 2008, we incurred an aggregate of approximately $641,000 in pre-tax losses for start-up centers compared to $608,000 for the preceding year.
EPO Utilization
We also provide ancillary services associated with dialysis treatments, including the administration of EPO for the treatment of anemia in our dialysis patients. EPO is currently available from only one manufacturer, and no alternative drug has been available to us for the treatment of anemia in our dialysis patients. If our available supply of EPO were reduced either by the manufacturer or due to excessive demand, our revenues and net income would be adversely affected. The manufacturer of EPO could implement price increases which would adversely affect our net income.
35
ESRD patients must either obtain a kidney transplant or obtain regular dialysis treatments for the rest of their lives. Due to a lack of suitable donors and the possibility of transplanted organ rejection, the most prevalent form of treatment for ESRD patients is hemodialysis through a kidney dialysis machine. Hemodialysis patients usually receive three treatments each week with each treatment lasting between three and five hours on an outpatient basis. Although not as common as hemodialysis in an outpatient facility, home peritoneal dialysis is an available treatment option, representing the third most common type of ESRD treatment after outpatient hemodialysis and kidney transplantation.
Reimbursement
Approximately 54% of our medical services revenue for 2008 was derived from Medicare and Medicaid reimbursement with rates established by CMS, and which rates are subject to legislative changes. Congress approved a 1.6% composite rate increase for each of 2006 and 2007. In 2007, Medicare changed the way it reimburses dialysis providers, which includes revision of pricing for separately billable drugs and biologics, with an add-on component to make the change budget-neutral. Medicare also implemented a case mix payment system as an adjustment to the composite rate. In 2008 Congress passed legislation establishing the bundling of reimbursement payments for dialysis services, intended to take effect in 2011. See Item 1, “Business – Operations – Medicare Reimbursement.” Dialysis is typically reimbursed at higher rates from private payors, such as a patient’s insurance carrier, as well as higher payments received under negotiated contracts with hospitals for acute inpatient dialysis services.
The following table shows the breakdown of our revenues by type of payor for the periods presented:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Medicare | 46 | % | 49 | % | 52 | % | ||||||
| Medicaid and comparable programs | 8 | 8 | 9 | |||||||||
| Hospital inpatient dialysis services | 4 | 4 | 3 | |||||||||
| Commercial insurers and other private payors | 42 | 39 | 36 | |||||||||
| 100 | % | 100 | % | 100 | % | |||||||
Our medical services revenue is derived primarily from four sources: outpatient hemodialysis services, home peritoneal dialysis services, inpatient hemodialysis services and ancillary services. The following table shows the breakdown of our medical services revenue (in thousands) derived from our primary revenue sources and the percentage of total medical service revenue represented by each source for the periods presented:
| Year Ended December 31, | ||||||||||||||||||||||||
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
| Outpatient hemodialysis services | $ | 46,348 | 54 | % | $ | 39,880 | 55 | % | $ | 32,856 | 54 | % | ||||||||||||
| Home and peritoneal dialysis services | 4,189 | 5 | 3,736 | 5 | 3,625 | 6 | ||||||||||||||||||
| Inpatient hemodialysis services | 3,109 | 4 | 2,897 | 4 | 2,191 | 3 | ||||||||||||||||||
| Ancillary services | 32,030 | 37 | 26,658 | 36 | 22,467 | 37 | ||||||||||||||||||
| $ | 85,676 | 100 | % | $ | 73,171 | 100 | % | $ | 61,139 | 100 | % | |||||||||||||
Compliance
The healthcare industry is subject to extensive regulation by federal and state authorities. There are a variety of fraud and abuse measures to combat waste, including Anti-Kickback regulations and extensive prohibitions relating to self-referrals, violations of which are punishable by criminal or civil penalties, including exclusion from Medicare and other governmental programs. Unanticipated changes in healthcare
36
programs or laws could require us to restructure our business practices which, in turn, could materially adversely affect our business, operations and financial condition. See Item 1, “Business – Government Regulation.” We have a Compliance Program to assure that we provide the highest level of patient care and services in a professional and ethical manner consistent with applicable federal and state laws and regulations. See Item 1, “Business – Compliance Program.”
Results of Operations
The following table shows our results of operations (in thousands):
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Medical services revenue | $ | 85,676 | $ | 73,171 | $ | 61,139 | ||||||
| Product sales | 1,161 | 1,078 | 891 | |||||||||
| Total sales | 86,837 | 74,249 | 62,030 | |||||||||
| Other income | --- | 286 | 430 | |||||||||
| Total operating revenues | 86,837 | 74,535 | 62,460 | |||||||||
| Cost of medical services | 51,452 | 44,248 | 36,969 | |||||||||
| Cost of product sales | 660 | 598 | 549 | |||||||||
| Total cost of sales | 52,112 | 44,846 | 37,518 | |||||||||
| Corporate selling, general and administrative expenses | 10,587 | 7,917 | 6,460 | |||||||||
| Facility selling, general and administrative expenses | 12,851 | 11,423 | 8,988 | |||||||||
| Total selling, general and administrative expense | 23,438 | 19,340 | 15,448 | |||||||||
| Stock compensation expense | 221 | 218 | 276 | |||||||||
| Depreciation and amortization | 2,784 | 2,619 | 2,319 | |||||||||
| Provision for doubtful accounts | 2,089 | 1,665 | 1,199 | |||||||||
| Total operating costs and expenses | 80,644 | 68,688 | 56,760 | |||||||||
| Operating income | 6,193 | 5,847 | 5,700 | |||||||||
| Other (expense) income | (49 | ) | (40 | ) | 101 | |||||||
| Income before income taxes, minority and other | ||||||||||||
| Equity interests and equity in affiliate earnings | 6,144 | 5,807 | 5,801 | |||||||||
| Income tax provision | 1,862 | 1,615 | 2,086 | |||||||||
| Income before minority and other equity interests | ||||||||||||
| and equity in affiliate earnings | 4,282 | 4,192 | 3,715 | |||||||||
| Minority and other equity interests in income of | ||||||||||||
| consolidated subsidiaries | (1,438 | ) | (1,106 | ) | (920 | ) | ||||||
| Equity in affiliate earnings | --- | --- | 254 | |||||||||
| Net income | $ | 2,844 | $ | 3,086 | $ | 3,049 | ||||||
37
2008 Compared to 2007
Medical services revenue increased approximately $12,505,000 (17%) for the year ended December 31, 2008, compared to the preceding year. Medical services revenue for 2008 includes approximately $553,000 of amounts previously included in excess insurance liability that was determined to be non-refundable compared to approximately $442,000 in the preceding year. Dialysis treatments performed increased from approximately 252,000 in 2007 to approximately 272,000 in 2008, an 8% increase which includes treatments at a previously managed facility, in which we acquired an 80% interest effective January 1, 2008. The increase in treatments includes a full year of treatments in 2008 at the two centers acquired in the first quarter of 2007, treatments at two centers opened at the end of 2007, and treatments at our new Pennsylvania center that opened in the third quarter of 2008. Some of our patients carry commercial insurance which may require an out of pocket co-pay by the patient, which is often uncollectible by us. This co-pay is typically limited, and therefore may lead to our under-recognition of revenue at the time of service. We routinely recognize these revenues as we become aware that these limits have been met.
We record contractual adjustments based on fee schedules for a patient’s insurance plan except in circumstances where the schedules are not readily determinable, in which case rates are estimated based on similar insurance plans and subsequently adjusted when actual rates are determined. Out-of-network providers generally do not provide fee schedules and coinsurance information and, consequently, represent the largest portion of contractual adjustment changes. Estimated contractual adjustments for 2007 and prior years made during 2008 resulted in an increase of approximately $1,480,000 in revenues in 2008. Based on historical data we do not anticipate that a change in estimates would have a significant impact on our financial conditions or results of operations.
Operating income increased approximately $347,000 (6%) for the year ended December 31, 2008, compared to the preceding year, including in start-up costs associated with our new centers of $641,000 in 2008 compared to $608,000 in the preceding year.
Management fee income in 2007 was pursuant to a management services agreement with a Georgia center in which we acquired an 80% interest effective January 1, 2008.
Cost of medical services sales as a percentage of sales was stable compared to the preceding year and amounted to 60% for the year ended December 31, 2008 and for the preceding year.
Approximately 27% of our medical services revenue for the year ended December 31, 2008 and for the preceding year derived from the administration of EPO to our dialysis patients. Beginning in 2006, Medicare reimburses dialysis providers for the top ten most utilized ESRD drugs at an amount equal to the cost of such drugs as determined by the Inspector General of HHS, with complementary increases in the composite rate. We believe these changes have had little impact on our average Medicare revenue per treatment. See Item 1, “Business – Operations – Medicare Reimbursement.”
Our medical products operation represents a minor portion of our operations with operating revenues of $1,161,000 during 2008, and $1,078,000 during 2007 (1.3% of total 2008 and 1.4% of total 2007 operating revenues). Operating income for the medical products operation was $179,000 for 2008 and $186,000 for 2007 (2.9% of total 2008 operating income and 3.1% of total 2007 operating income).
Cost of sales for our medical products operation amounted to 57% of sales for the year ended December 31, 2008 and 56% of sales for the year ended December 31, 2007. Cost of sales for this operation is largely related to product mix.
38
Selling, general and administrative expenses, those corporate and facility costs not directly related to the care of patients, including, among others, administration, accounting and billing, increased by approximately $4,098,000 (21%) for the year ended December 31, 2008, compared to the preceding year. This increase reflects operations of our new dialysis centers and increased support activities resulting from expanded operations. Included are expenses of new centers incurred prior to Medicare approval for which there were no corresponding medical services revenue. Selling, general and administrative expenses as a percentage of medical services revenue amounted to approximately 27% for the year ended December 31, 2008 compared to 26% for the preceding year.
Provision for doubtful accounts increased approximately $423,000 for year ended December 31, 2008, compared to the preceding year. The provision amounted to 2% of sales for the year ended December 31, 2008 and for the preceding year. Medicare bad debt recoveries of $549,000 were recorded during the year ended December 31, 2008, compared to approximately $253,000 for the preceding year. Without the effect of the Medicare bad debt recoveries, the provision would have amounted to 3% of sales for the year ended December 31, 2008 and for the preceding year. The provision for doubtful accounts reflects our collection experience with the impact of that experience included in accounts receivable presently reserved, plus recovery of accounts previously considered uncollectible from our Medicare cost report filings. The provision for doubtful accounts is determined under a variety of criteria, primarily aging of the receivables and payor mix. Accounts receivable are estimated to be uncollectible based upon various criteria including the age of the receivable, historical collection trends and our understanding of the nature and collectibility of the receivables, and are reserved for in the allowance for doubtful accounts until they are written off.
Days sales outstanding were 84 as of December 31, 2008 compared to 93 as of December 31, 2007. Days sales outstanding are impacted by the expected and typical slower receivable turnover at our new centers opened or acquired and by payor mix. Based on our collection experience with the different payor groups comprising our accounts receivable, our analysis indicates that our allowance for doubtful accounts reasonably estimates the amount of accounts receivable that we will ultimately not collect.
After a patient’s insurer has paid the applicable coverage for the patient, the patient is billed for the applicable co-payment or balance due. If payment is not received from the patient for its applicable portion, collection letters and billings are sent to that patient until such time as the patient’s account is determined to be uncollectible, at which time the account will be charged against the allowance for doubtful accounts. Patient accounts that remain outstanding four months after initial collection efforts are generally considered uncollectible.
Other non-operating expense increased approximately $9,000 for the year ended December 31, 2008, compared to the preceding year. This includes a decrease in interest income of $135,000, largely resulting from lower interest rates earned on invested funds, an increase in rental income of $15,000, a decrease in miscellaneous other income of $57,010 and a decrease in interest expense to unrelated parties of $168,000 resulting from reduced average borrowings and lower interest rates on borrowed funds.
Although operations of additional centers have resulted in additional revenues, certain of these centers are still in the start-up stage and, accordingly, their operating results will adversely impact our overall results of operations until they achieve a patient count sufficient to sustain profitable operations.
Minority and other equity interests represents the proportionate equity interests of minority owners of our subsidiaries whose financial results are included in our consolidated results. Equity in affiliate earnings represents our proportionate interest in the earnings of our Toledo, Ohio subsidiary until its consolidation effective August 1, 2006, prior to which it was accounted for on the equity method.
39
2007 Compared to 2006
Medical services revenue increased approximately $12,032,000 (20%) for the year ended December 31, 2007, compared to the preceding year. Medical services revenue for 2007 includes approximately $442,000 of amounts previously included in excess insurance liability that was determined to be non-refundable compared to approximately $688,000 in the preceding year. Dialysis treatments performed increased from approximately 220,000 in 2006 to approximately 252,000 in 2007, a 15% increase which includes treatments at managed facilities, one for which we acquired the 60% interest not already owned by the company in December, 2006. The increase in treatments includes treatments at the three centers acquired in the first quarter of 2006, three new centers opened during 2006, and two centers acquired in the first quarter of 2007. Some of our patients carry commercial insurance which may require an out of pocket co-pay by the patient, which is often uncollectible by us. This co-pay is typically limited, and therefore may lead to our under-recognition of revenue at the time of service. We routinely recognize these revenues as we become aware that these limits have been met.
We record contractual adjustments based on fee schedules for a patient’s insurance plan except in circumstances where the schedules are not readily determinable, in which case rates are estimated based on similar insurance plans and subsequently adjusted when actual rates are determined. Out-of-network providers generally do not provide fee schedules and coinsurance information and, consequently, represent the largest portion of contractual adjustment changes. Estimated contractual adjustments for 2006 and prior years made during 2007 resulted in approximately a $381,000 reduction in revenues in 2007. Based on historical data we do not anticipate that a change in estimates would have a significant impact on our financial conditions or results of operations.
Operating income increased approximately $147,000 (3%) for the year ended December 31, 2007, compared to the preceding year, including in start-up costs associated with our new centers of $608,000 in 2007 compared to $1,423,000 in the preceding year.
Other operating income, representing management fee income pursuant to management services agreements with our Toledo, Ohio facility (until its consolidation effective August 1, 2006) and a Georgia center in which we acquired an 80% interest effective January 1, 2008, decreased approximately $144,000 during 2007 compared to the preceding year. This decrease largely resulted from the consolidation of our Toledo facility effective August 1, 2006 whereas that facility had previously been accounted for on the equity method with no revenue elimination in our consolidated financial statements.
Cost of medical services sales as a percentage of sales was stable and amounted to 60% for the year ended December 31, 2007 and for the preceding year.
Approximately 27% of our medical services revenue for the year ended December 31, 2007 derived from the administration of EPO to our dialysis patients compared to 28% for the preceding year. Beginning in 2006, Medicare reimburses dialysis providers for the top ten most utilized ESRD drugs at an amount equal to the cost of such drugs as determined by the Inspector General of HHS, with complementary increases in the composite rate. We believe these changes have had little impact on our average Medicare revenue per treatment. See Item 1, “Business – Operations – Medicare Reimbursement.”
Our medical products operation was acquired pursuant to our merger with our former parent company in September, 2005. The medical products operation is included in our operating results subsequent to the merger. This operation represents a minor portion of our operations with operating revenues of $1,078,000 during 2007, and $891,000 during 2006 (1.4% of total 2007 and 2006 operating
40
revenues). Operating income for the medical products operation was $186,000 for 2007 and $69,000 for 2006 (3.1% of total 2007 operating income and 1.2% of total 2006 operating income).
Cost of sales for our medical products operation amounted to 56% of sales for the year ended December 31, 2007 and 62% of sales for the year ended December 31, 2006. Cost of sales for this operation is largely related to product mix.
Selling, general and administrative expenses, those corporate and facility costs not directly related to the care of patients, including, among others, administration, accounting and billing, increased by approximately $3,892,000 (25%) for the year ended December 31, 2007, compared to the preceding year. This increase reflects operations of our new dialysis centers and increased support activities resulting from expanded operations. Included are expenses of new centers incurred prior to Medicare approval for which there were no corresponding medical services revenue. Selling, general and administrative expenses as a percentage of medical services revenue amounted to approximately 26% for the year ended December 31, 2007 compared to 25% for the preceding year.
Provision for doubtful accounts increased approximately $466,000 for year ended December 31, 2007, compared to the preceding year. The provision amounted to 2% of sales for the year ended December 31, 2007 and for the preceding year. Medicare bad debt recoveries of $253,000 were recorded during the year ended December 31, 2007, compared to approximately $537,000 for the preceding year. Without the effect of the Medicare bad debt recoveries, the provision would have amounted to 3% of sales for the year ended December 31, 2007 and for the preceding year. The provision for doubtful accounts reflects our collection experience with the impact of that experience included in accounts receivable presently reserved, plus recovery of accounts previously considered uncollectible from our Medicare cost report filings. The provision for doubtful accounts is determined under a variety of criteria, primarily aging of the receivables and payor mix. Accounts receivable are estimated to be uncollectible based upon various criteria including the age of the receivable, historical collection trends and our understanding of the nature and collectibility of the receivables, and are reserved for in the allowance for doubtful accounts until they are written off.
Days sales outstanding were 93 as of December 31, 2007 compared to 82 as of December 31, 2006. Days sales outstanding are impacted by the expected and typical slower receivable turnover at our new centers opened and by payor mix. Based on our collection experience with the different payor groups comprising our accounts receivable, our analysis indicates that our allowance for doubtful accounts reasonably estimates the amount of accounts receivable that we will ultimately not collect.
After a patient’s insurer has paid the applicable coverage for the patient, the patient is billed for the applicable co-payment or balance due. If payment is not received from the patient for its applicable portion, collection letters and billings are sent to that patient until such time as the patient’s account is determined to be uncollectible, at which time the account will be charged against the allowance for doubtful accounts. Patient accounts that remain outstanding four months after initial collection efforts are generally considered uncollectible.
Other non-operating income decreased approximately $141,000 for the year ended December 31, 2007, compared to the preceding year. This includes an increase in interest income of $29,000, an increase in rental income of $19,000, an increase in miscellaneous other income of $73,000 and an increase in interest expense to unrelated parties of $347,000 due to increased average non-inter-company borrowings and net litigation settlement expense of $85,000 in 2006. The prime rate was 7.25% at December 31, 2007, and 8.25% at December 31, 2006.
41
Although operations of additional centers have resulted in additional revenues, certain of these centers are still in the start-up stage and, accordingly, their operating results will adversely impact our overall results of operations until they achieve a patient count sufficient to sustain profitable operations.
Minority and other equity interests represent the proportionate equity interests of minority owners of our subsidiaries whose financial results are included in our consolidated results. Equity in affiliate earnings represents our proportionate interest in the earnings of our Toledo, Ohio subsidiary until its consolidation effective August 1, 2006, prior to which it was accounted for on the equity method.
Liquidity and Capital Resources
Working capital totaled approximately $20,266,000 at December 31, 2008, which reflected an increase of $1,654,000 (9%) during the current year. Included in the changes in components of working capital was an increase in cash and cash equivalents of $4,095,000, which included net cash provided by operating activities of $11,321,000; net cash used in investing activities of $13,162,000 (including additions to property and equipment of $4,333,000; and payments of $9,193,000 on dialysis center acquisitions), and net cash provided by financing activities of $5,937,000 (including borrowings of $12,000,000 under our line of credit, repayments of $4,650,000 on our line of credit, other debt repayments of $66,000, distributions to subsidiary minority members of $1,798,000, and capital contributions of $450,000 by subsidiary minority members).
Net cash provided by operating activities consists of net income before non-cash items consisting of depreciation and amortization of $2,784,000, bad debt expense of $2,088,000, deferred income tax expense of $514,000, income applicable to minority interest of $1,438,000, and non-cash stock and stock option compensation expense of $221,000, as adjusted for changes in components of working capital. Significant changes in components of working capital, in addition to the $4,095,000 increase in cash, included an increase in accounts receivable of $1,334,000, an increase in inventories of $912,000, a decrease of $335,000 in prepaid expenses and other current assets including application of the $400,000 deposit on our self-insured health plan liability, and increases in accounts payable of $3,170,000 and accrued expenses of $1,324,000. The major source of cash from operating activities is medical service revenue. The major uses of cash in operating activities are supply costs, payroll, independent contractor costs, and costs for our leased facilities.
Our Easton, Maryland building has a mortgage to secure a subsidiary development loan. This loan had a remaining principal balance of $495,000 at December 31, 2008, and $526,000 at December 31, 2007. In April, 2001, we obtained a $788,000 five-year mortgage on our building in Valdosta, Georgia which we refinanced upon maturity in April, 2006 for an additional five years with a new maturity of April, 2011. This loan had an outstanding principal balance of approximately $555,000 at December 31, 2008, and $589,000 at December 31, 2007.
We acquired an 80% interest in a Georgia center effective January 1, 2008, opened a new Pennsylvania center during 2008, and acquired a Maryland center on December 31, 2008.
Capital is needed primarily for the development of outpatient dialysis centers. The construction of a 15 station facility, typically the size of our dialysis facilities, costs in the range of $1,000,000 to $1,500,000, depending on location, size and related services to be provided, which includes equipment and initial working capital requirements. Acquisition of an existing dialysis facility is more expensive than construction, although acquisition would provide us with an immediate ongoing operation, which most likely would be generating income. Although our expansion strategy focuses primarily on construction of new centers, we have expanded through acquisition of dialysis facilities and continue to
review potential acquisitions. Development of a dialysis facility to initiate operations takes four to six months and usually up to 12 months or longer to generate earnings. We consider some of our centers to be in the developmental stage since they have not developed a patient base sufficient to generate and sustain earnings.
42
We are seeking to expand our outpatient dialysis treatment facilities and inpatient dialysis care and are presently in different phases of negotiations with physicians and others for the development or acquisition of additional outpatient centers. Such expansion requires capital. We have been funding our expansion through internally generated cash flow and a revolving line of credit with KeyBank National Association. To assist with our future expansion we entered into a $15,000,000, three year credit agreement for a revolving line of credit with KeyBank National Association in October, 2005, that we extended for one additional year. On November 5, 2008, we increased the line of credit to $25,000,000 and extended the line to November 4, 2011. We have outstanding borrowings of $13,300,000 under this credit facility as of December 31, 2008. No assurance can be given that we will be successful in implementing our growth strategy or that available financing will be adequate to support our expansion. See Item 1, “Business-Business Strategy.”
Aggregate Contractual Obligations
As of December 31, 2008, our contractual obligations (in thousands), including payments due by period, are as follows:
| Payments due by period | ||||||||||||||||||||
| Less than | More than | |||||||||||||||||||
| Total | 1 year | 1-3 years | 3-5 years | 5 years | ||||||||||||||||
| Long-term debt | $ | 14,350 | $ | 74 | $ | 13,456 | $ | 165 | $ | 655 | ||||||||||
| Operating leases | 13,519 | 2,722 | 4,569 | 3,253 | 2,975 | |||||||||||||||
| Expected interest payments: | ||||||||||||||||||||
| Long-term debt | 3,021 | 1,000 | 1,852 | 61 | 108 | |||||||||||||||
| Purchase obligations: | ||||||||||||||||||||
| Medical services | 8,976 | 1,944 | 2,726 | 2,118 | 2,188 | |||||||||||||||
| Miscellaneous purchase obligations | 83 | 83 | --- | --- | --- | |||||||||||||||
| Total purchase obligations | 9,059 | 2,027 | 2,726 | 2,118 | 2,188 | |||||||||||||||
| Total contractual obligations | $ | 39,949 | $ | 5,823 | $ | 22,603 | $ | 5,597 | $ | 5,926 | ||||||||||
New Accounting Pronouncements
In September, 2006, the FASB issued Statement of Financial Accounting Standards No. 157 (“SFAS 157”), “Fair Value Measurements”. SFAS 157 defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. We adopted SFAS 157 effective January 1, 2008 except for the nonfinancial assets and liabilities that are subject to a one year deferral allowed by FASB Staff Position FAS157-2 (“FSP FAS 157-2”) Effective Date of FASB Statement No. 157 issued February 12, 2008. FSP FAS157-2 delays the effective date of SFAS No. 157 until fiscal years beginning after November 15, 2008 for nonfinancial assets and liabilities that are not recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually). The
43
adoption of SFAS 157 did not have a material effect on our financial statements. We are evaluating the impact SFAS 157 will have on our financial statements beginning in fiscal 2009 as it relates to the items subject to the one-year deferral allowed by FSP FAS 157-2.
In December, 2007, the FASB issued Statement of Financial Accounting Standards No. 141 (revised 2007) (“SFAS 141R”), “Business Combinations.” SFAS 141R expands the definitions of a business and a business combination and requires all assets and liabilities of an acquired business (for full, partial and step acquisitions) to be recorded at fair values, with limited exceptions. SFAS will be effective for us in 2009. We are evaluating the impact on our financial statements of adopting SFAS 141R.
In December, 2007, the FASB issued Statement of Financial Accounting Standards No. 160 (“SFAS 160”), “Noncontrolling Interests in Consolidated Financial Statements,” an amendment of ARB 51. SFAS 160 requires noncontrolling interests to be reported in the equity section of consolidated financial statements and requires that consolidated net income include the amounts attributable to both the parent and noncontrolling interests with these amounts disclosed on the face of the consolidated income statement and requires any losses attributable to the noncontrolling interests in excess of noncontrolling interests in equity to be allocated to the noncontrolling interests. SFAS 160 will be effective for us in 2009. Other than presentation differences, we do not expect SFAS160 to have a significant impact on our financial statements.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make complex judgments and estimates. On an on-going basis, we evaluate our estimates, the most significant of which include establishing allowances for doubtful accounts, a valuation allowance for our deferred tax assets and determining the recoverability of our long-lived assets. The basis for our estimates are historical experience and various assumptions that are believed to be reasonable under the circumstances, given the available information at the time of the estimate, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily available from other sources. Actual results may differ from the amounts estimated and recorded in our financial statements.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition: Revenues are recognized net of contractual provisions at the expected collectable amount. We receive payments through reimbursement from Medicare and Medicaid for our outpatient dialysis treatments coupled with patients’ private payments, individually and through private third-party insurers. A substantial portion of our revenues are derived from the Medicare ESRD program, which outpatient reimbursement rates are fixed under a composite rate structure, which includes the dialysis services and certain supplies, drugs and laboratory tests. Certain of these ancillary services are reimbursable outside of the composite rate. Medicaid reimbursement is similar and supplemental to the Medicare program. Our acute inpatient dialysis operations are paid under contractual arrangements, usually at higher contractually established rates, as are certain of the private pay insurers for outpatient dialysis. We have developed a sophisticated information and computerized coding system, but due to the complexity of the payor mix and regulations, we sometimes receive more or less than the amount expected when the services are provided. We reconcile any differences at least quarterly.
44
In those situations where a patient’s insurance fee schedule cannot be readily determined, which typically occurs with out of network providers, we estimate fees based on our knowledge base of historical data for patients with similar insurance plans. Our internal controls, including an ongoing review and follow-up on estimated fees, allows us to make necessary changes to estimated fees on a timely basis. When the actual fee schedule is determined, we adjust the amounts originally estimated, and then use the actual fees to estimate fees for similar future situations. We adhere to the guidelines of Staff Accounting Bulletin Topic 13 (“SAB 104”) in regard to recording reasonable estimates of revenue based on our historical experience and identifying on a timely basis necessary changes to estimates.
Allowance for Doubtful Accounts: We maintain an allowance for doubtful accounts for estimated losses resulting from the inability of our patients or their insurance carriers to make required payments. Based on historical information, we believe that our allowance is adequate. Changes in general economic, business and market conditions could result in an impairment in the ability of our patients and the insurance companies to make their required payments, which would have an adverse effect on cash flows and our results of operations. The allowance for doubtful accounts is reviewed monthly and changes to the allowance are updated based on actual collection experience. We use a combination of percentage of sales and the aging of accounts receivable to establish an allowance for losses on accounts receivable. We adhere to the guidelines of Statement of Financial Accounting Standards No. 5 (“SFAS 5”), “Accounting for Contingencies” in determining reasonable estimates of accounts for which uncollectibility is possible.
Valuation Allowance for Deferred Tax Assets: The carrying value of deferred tax assets assumes that we will be able to generate sufficient future taxable income to realize the deferred tax assets based on estimates and assumptions. If these estimates and assumptions change in the future, we may be required to adjust our valuation allowance for deferred tax assets which could result in additional income tax expense.
Goodwill and Intangible Asset Impairment: In assessing the recoverability of our goodwill and other intangibles we must make assumptions regarding estimated future cash flows and other factors to determine the fair value of the respective assets. This impairment test requires the determination of the fair value of the intangible asset. If the fair value of the intangible asset is less than its carrying value, an impairment loss will be recognized in an amount equal to the difference. If these estimates or their related assumptions change in the future, we may be required to record impairment charges for these assets. We analyze goodwill and indefinite lived intangible assets for impairment on at least an annual basis according to Statement of Financial Accounting Standards No. 142 (“SFAS 142”), “Goodwill and Other Intangible Assets.”
Long-Lived Assets: We state our property and equipment at acquisition cost and compute depreciation for book purposes by the straight-line method over estimated useful lives of the assets. In accordance with Statement of Financial Accounting Standards No. 144 (“SFAS No. 144”), “Accounting for the Impairment or Disposal of Long-Lived Assets,” long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate the carrying amount of the asset may not be recoverable. Recoverability of assets to be held and used is measured by comparison of the carrying amount of an asset to the future cash flows expected to be generated by the asset. If the carrying amount of the asset exceeds its estimated future cash flows, an impairment charge is recognized to the extent the carrying amount of the asset exceeds the fair value of the asset. These computations are complex and subjective.
45
Impact of Inflation
Inflationary factors have not had a significant effect on our operations. A substantial portion of our revenue is subject to reimbursement rates established and regulated by the federal government. These rates do not automatically adjust for inflation. Any rate adjustments relate to legislation and executive and Congressional budget demands, and have little to do with the actual cost of doing business. Therefore, dialysis services revenues cannot be voluntarily increased to keep pace with increases in nursing and other patient care costs. Increased operating costs without a corresponding increase in reimbursement rates may adversely affect our earnings in the future.
| Item 7A. | Quantitative and Qualitative Disclosure About Market Risk |
We do not consider our exposure to market risks, principally changes in interest rates, to be significant.
Sensitivity of results of operations to interest rate risks on our investments is managed by conservatively investing funds in liquid interest bearing accounts of which we held approximately
$1,890,000 at December 31, 2008.
Interest rate risk on debt is managed by negotiation of appropriate rates for equipment financing and other fixed rate obligations based on current market rates. There is an interest rate risk associated with our variable rate debt obligations, which totaled approximately $14,350,000 at December 31, 2008.
We have exposure to both rising and falling interest rates. Due to the global financial crisis and the corresponding exceedingly low interest rates on invested funds, there was no substantial risk of a decrease in interest income as a result of decreased yields at December 31, 2008. Assuming a relative 15% increase in rates on our period-end variable rate debt would have resulted in a negative impact of approximately $54,000 on our results of operations for the year ended December 31, 2008.
We do not utilize financial instruments for trading or speculative purposes and do not currently use interest rate derivatives.
| Item 8. | Financial Statements and Supplementary Data |
The response to this item is submitted as a separate section to this annual report, specifically, Part IV, Item 15, “Exhibits, Financial Statement Schedules and Reports on Form 8-K,” subpart (a)1, “All Financial Statements – See Index to Consolidated Financial Statements,” and subpart (a)2, “Financial Statement Schedules – See Index to Consolidated Financial Statements,” and begins on page F-1 of this annual report on Form 10-K.
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
None
46
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures.
As of the end of the period covered by this annual report on Form 10-K, we carried out an evaluation, under the supervision and with the participation of our management, including our President and CEO, and the Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Rule 13a-15(e) of the Exchange Act. These disclosure controls and procedures are designed to provide reasonable assurance that, among other things, information is accumulated and communicated to our management, including our President and CEO, and our Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. Based upon such evaluation, our President and CEO, and our Chief Financial Officer have concluded that, as of the end of such period, our disclosure controls and procedures are effective in providing reasonable assurance that information required to be disclosed by our company in the reports that it files under the Exchange Act is recorded, processed, summarized and reported within required time periods specified by the SEC’s rules and forms.
Management’s Report on Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during our fourth quarter ended December 31, 2008 that have materially affected, or are reasonably likely to materially affect, our control over financial reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is a process designed by, or under the supervision of, our President and CEO and Chief Financial Officer and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes polices and procedures that:
| ● | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; | |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and | |
| ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the consolidated financial statements |
All internal control systems, no matter how well designed, have inherent limitations and can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations to all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, and none of which we are aware, within our company have been detected. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
47
In connection with the preparation of our Form 10-K, our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2008. In making that assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in “Internal Control-Integrated Framework.” Management’s assessment included an examination of the design of the company’s internal control over financial reporting and testing of the operating effectiveness of its internal control over financial reporting. Based on management’s assessment, management believes that, as of December 31, 2008, our internal control over financial reporting was effective based on those criteria. Management’s assessment of the effectiveness of the company’s internal control over financial reporting as of December 31, 2008, has been audited by MSPC Certified Public Accountants and Advisors, P.C., an independent registered public accounting firm, as stated in their report attached to this annual report on Form 10-K, which expresses unqualified opinions with respect to management’s assessment and on the effectiveness of the company’s internal control over financial reporting as of December 31, 2008.
| Item 9B. | Other Information |
To the best of our knowledge, we have reported all information required to be disclosed in our current reports on Form 8-K during the fourth quarter of 2008.
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
Directors and Executive Officers
Information concerning the company’s directors and executive officers is incorporated herein by reference to the company’s 2009 proxy statement* under the captions “Proposal No. 1: Election of Directors” and “Information About Directors and Executive Officers.”
Information relating to compliance with Section 16(a) of the Exchange Act is incorporated herein by reference to the company’s 2009 proxy statement* under the caption “Section 16(a) Beneficial Ownership Reporting Compliance.”
The company adopted a Code of Ethics and Business Conduct, referred to as the Code of Ethics, that applies to our Chief Executive Officer, Chief Financial Officer, Principal Accounting Officer or Controller, and persons performing similar functions, as well as other employees. See Item 1, “Business – Corporate Integrity Program – Code of Ethics.” See also “Code of Ethics” in the company’s 2009 proxy statement* incorporated herein by reference. The Code of Ethics is available on our website at www.dialysiscorporation.com under the caption, “Investor Relations.” A copy of the Code of Ethics is also available without charge upon request from the Chief Financial Officer at Dialysis Corporation of America, 1302 Concourse Drive, Suite 304, Linthicum, MD 21090. If we make substantive amendments to our Code of Ethics or grant any waiver from a provision of it, which has not occurred to date, we will disclose the nature of such amendment or waiver on our website or in a current report on Form 8-K.
Information concerning the audit, nominating and compensation committees, their composition, charters and policies, the audit committee financial expert (as defined in Item 407(5)(ii) and (iii) of Regulation S-K) and the audit and compensation committee reports are incorporated herein by reference to the company’s 2009 proxy statement* under the captions “Corporate Governance,” “Compensation Committee Report” and “Audit Committee Report.” The audit, nominating and compensation committee
48
charters outline those committees’ purposes, membership requirements, administration and responsibilities. Each committee member is independent as defined under the market rules of the Nasdaq Stock Market. Each committee reviews and updates its charter at least annually, which charters are approved by the board of directors. All the charters are posted on our website at www.dialysiscorporation.com under the caption “Investor Relations.” The charter information is also available in print without charge to any shareholder who requests it from our Secretary, Joshua M. Jaffe, Esq., at Jaffe & Falk, LLC, 777 Terrace Avenue, Hasbrouck Heights, NJ 07604.
| Item 11. | Executive Compensation |
Information concerning Compensation Discussion and Analysis, the Compensation Committee Report and executive management and director compensation is incorporated herein by reference to the company’s 2009 proxy statement* under the captions “Corporate Governance – Compensation of Directors,” “Compensation Discussion and Analysis,” “Compensation Committee Report,” “Summary Compensation Table,” “Grants of Plan-Based Awards – 2008,” “Outstanding Equity Awards at Fiscal Year End – 2008” and “Option Exercises and Stock Vested in 2008.”
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Information concerning security ownership of certain beneficial owners and management is incorporated herein by reference to the company’s 2009 proxy statement* under the caption “Beneficial Ownership of the Company’s Securities.”
Securities Authorized For Issuance Under Equity Compensation Plans
The following table provides information with respect to equity compensation plans approved by shareholders in effect at December 31, 2008. Options and stock awards are only granted under the Dialysis Corporation of America 1999 Incentive Plan, referred to as the DCA Incentive Plan, which has been approved by shareholders.
Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||
| Plan Category | (a) | (b) | (c) | ||||||
Equity compensation plans approved by security holders: | 31,250(1) | $7.28 | 704,154(2) | ||||||
Equity compensation plans not approved by security holders:(3) | N/A | N/A |
_______________
(1) Includes only vested portion of outstanding options as follows: (i) 18,750 five year options exercisable through June 6, 2009 at $4.02 per share; (ii) 12,500 option shares of a five year option for 50,000 shares exercisable through April 15, 2012 at $12.18 per share, vesting in equal increments of 12,500 shares each April 15, 2008 through 2011; does not include 37,500 non-vested option shares; and
49
(iii) does not include two five year options for an aggregate of 60,000 shares, exercisable through February 28, 2013 at $12.18 per share, vesting in the aggregate in equal increments of 15,000 shares each February 28, 2009 through February 28, 2012; no option shares vested at December 31, 2008; 15,000 option shares vested on February 28, 2009. Each of the options provide for adjustments under limited circumstances, and have termination and acceleration provisions.
(2) Includes: (i) 97,500 option shares not yet vested and not included in column (a); and (ii) 15,875 non-vested outstanding stock awards. On February 27, 2009, the board authorized the issuance of 5,000 stock awards, 1,000 shares each to the independent board members and 2,000 shares to the Chairman of the Board of Directors. Accordingly, there are 699,114 shares currently available for issuance under the DCA Incentive Plan, including subparagraphs (i) and (ii) above.
(3) There are employment agreements with five members of executive management, none of which specifically provides for the issuance of equity securities of the company. Stephen W. Everett, President and CEO of the company, has an employment agreement which provided for the issuance of securities, which equity issuance portions of the agreement have expired. Executive officers may be entitled to cash and equity bonuses from time to time within the guidelines of the company’s compensation policies, and as may be recommended by the Compensation Committee for approval by the board of directors.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
Information concerning certain relationships and related transactions and director independence is incorporated herein by reference to the company’s 2009 proxy statement* under the captions “Proposal No. 1: Election of Directors,” “Information About Directors and Executive Officers” and “Corporate Governance.”
| Item 14. | Principal Accountant Fees and Services |
Information concerning fees and services of the company’s independent auditors and the audit committee’s pre-approved policies and procedures is incorporated herein by reference to the company’s 2009 proxy statement* under the caption “Proposal No. 3: Ratification of the Appointment of the Independent Auditors.”
| * | The company’s definitive proxy statement relating to the upcoming annual meeting of shareholders will be filed within 120 days of the end of the company’s fiscal year, which is no later than April 30, 2009. |
50
PART IV
| Item 15. | Exhibits and Financial Statement Schedules |
| (a) | The following is a list of documents filed as part of this report. | |
| 1. | All financial statements – See Index to Consolidated Financial Statements. | |
| 2. | Financial statement schedules – See Index to Consolidated Financial Statements. | |
| (b) | Exhibits + | |
| 3.1 | Articles of Incorporation ‡ | |
| 3.2 | By-Laws of the Company, as amended October 30, 2007 (incorporated by reference to the Company’s Current Report on Form 8-K dated November 5, 2007, Exhibit (3)(ii)). ‡ | |
| 4 | Instruments defining the rights of equity holders, including indentures | |
| 4.1 | Form of Common Stock Certificate of the Company ‡ | |
| 4.2 | Form of Stock Option Certificate under the 1999 Stock Incentive Plan (May 21, 1999) (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 1999, Part IV, Item 14(c)(10)(xxiv)). | |
| 4.3 | Form of Restricted Stock Agreement (incorporated by reference to the Company’s Current Report on Form 8-K dated June 30, 2006, Item 9.01(d)(10) 10.1). | |
| 10 | Material Contracts | |
| 10.1 | Indemnity Deed of Trust from the Company to Trustees for the benefit of St. Michaels Bank [now PNC Bank] dated December 3, 1999 (incorporated by reference to the Company’s Current Report on Form 8-K dated December 13, 1999 (“December Form 8-K”), Item 7(c)(99)(i)). | |
| 10.2 | Guaranty Agreement from the Company to St. Michaels Bank [now PNC Bank] dated December 3, 1999 (incorporated by reference to the Company’s December Form 8-K, Item 7(c)(99)(ii)). | |
| 10.3 | The Company’s Section 125 Plan effective September 1, 2002 (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2002, Part II, Item 6(99)). | |
| 10.4 | Agreement of Lease by and between Copt Concourse, LLC and the Company dated March 31, 2004 (incorporated by reference to the Company’s Current Report on Form 8-K dated March 31, 2004, item 7(c)(10((i)). | |
51
| 10.5 | Credit Agreement between the Company and KeyBank National Association dated October 24, 2005 (incorporated by reference to the Company’s Current Report on Form 8-K dated October 27, 2005 (“October 2005 Form 8-K”), Item 9.01(d)(10)(i)). | |
| 10.6 | Revolving Credit Note dated October 24, 2005 (incorporated by reference to the Company’s October 2005 Form 8-K, Item 9.01(d)(10)(ii)). | |
| 10.7 | Guaranty of Payment by the Company’s Wholly-Owned Subsidiaries in favor of KeyBank National Association dated October 24, 2005 (incorporated by reference to the Company’s October 2005 Form 8-K, Item 9.01(d)(10)(iii)). | |
| 10.8 | Pledge Agreement by the Company in favor of KeyBank National Association dated October 24, 2005 (incorporated by reference to the Company’s October 2005 Form 8-K, Item 9.01(d)(10)(iv). | |
| 10.9 | Amendment No. 1 to Credit Agreement between the Company and KeyBank National Association dated December 15, 2005 (incorporated by reference to the Company’s Current Report on Form 8-K dated December 16, 2005, Item 9.01(d)(10)(i)). | |
| 10.10 | Amendment No. 2 to Credit Agreement between the Company and KeyBank National Association dated as of February 14, 2006 (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2005, Part IV, Item 15, (10.19)). | |
| 10.11 | Employment Agreement between the Company and Stephen W. Everett dated February 22, 2006 (incorporated by reference to the Company’s Current Report on Form 8-K dated February 28, 2006, Item 9.01(d)(10)10.1).* | |
| 10.12 | Promissory Note by the Company to American Banking Company, dated April 3, 2006 (incorporated by reference to the Company’s Current Report on Form 8-K dated April 4, 2006, Item 9.01(d)(99) 99.1). | |
| 10.13 | Amendment No. 3 to Credit Agreement between the Company and KeyBank National Association dated July 27, 2006 (incorporated by reference to the Company’s Current Report on Form 8-K dated August 2, 2006, Item 9.01(d)(10)(i)). | |
| 10.14 | Debt Modification Agreement by DCA of Vineland, LLC(1) dated May 25, 2006 (incorporated by reference to the Company’s Current Report on Form 8-K, dated May 26, 2006 (“May 2006 Form 8-K”), Item 9.01(d)(99) 99.1). | |
| 10.15 | Notice and Consent to Modification by Guarantor [the Company] dated May 25, 2006 (incorporated by reference to the Company’s May 2006 Form 8-K, Item 9.01 (d)(99) 99.2) | |
| 10.16 | Amendment No. 4 to Credit Agreement between the Company and KeyBank National Association dated December 7, 2007 (incorporated by reference to the Company’s Current Report on Form 8-K dated December 17, 2007, Item 9.01(d)(10(i)). | |
| 10.17 | 1999 Stock Incentive Plan of the Company, as amended (April, 2006) (incorporated by reference to the Company’s Current Report on Form 8-K dated March 5, 2008, Item 9.01(d)(99)(ii). |
52
| 10.18 | Amendment No. 5 to Credit Agreement between the Company and KeyBank National Association dated November 5, 2008 (incorporated by reference to the Company’s Current Report on Form 8-K dated November 6, 2008 (“November 2008 Form 8-K”), Item 9.01(d)(10)(i)). | |
| 10.19 | Revolving Credit Note dated November 5, 2008 (incorporated by reference to the Company’s November 2008 Form 8-K, Item 9.01(d)(10)(ii)). | |
| 10.20 | Guarantor Acknowledgment and Agreement dated November 5, 2008 (incorporated by reference to the Company’s November 2008 Form 8-K, Item 9.01(d)(10)(iii)). | |
| 10.21 | Asset Purchase Agreement dated December 31, 2008 between DCA of Hyattsville(3) and St. Thomas More Dialysis Center, LLC (incorporated by reference to the Company’s Current Report on Form 8-K dated January 7, 2009 (“January 2009 Form 8-K”), Item 9.01(d)2.1). | |
| 10.22 | Escrow Agreement dated October 15, 2008 by and among St. Thomas More Dialysis Center, LLC, Dialysis Corporation of America and Sack, Harris & Martin, P.C. (incorporated by reference to the Company’s January 2009 Form 8-K, Item 9.01(d)10.1). | |
| 10.23 | Bill of Sale and Assignment and Assumption Agreement dated December 31, 2008 between St. Thomas More Dialysis Center, LLC and DCA of Hyattsville, LLC(3) (incorporated by reference to the Company’s January 2009 Form 8-K, Item 9.01(d)10.2). | |
| 10.24 | Guaranty by St. Thomas More, LLC dated December 31, 2008 (incorporated by reference to the Company’s January 2009 Form 8-K, Item 9.01(d)10.3). | |
| 10.25 | Amendment No. 6 to Credit Agreement between the Company and KeyBank National Association dated February 27, 2009 (incorporated by reference to the Company’s Current Report on Form 8-K, dated February 27, 2009 (“February 2009 Form 8-K”), Item 9.01(d)(10)(i)). | |
| 10.26 | Guarantor Acknowledgment and Agreement dated February 27, 2009 (incorporated by reference to the Company’s February 2009 Form 8-K, Item 9.01(d)(10)(ii)). | |
| 10.27 | Employment Agreement, effective January 1, 2009, between the Company and Thomas P. Carey (incorporated by reference to the Company’s Current Report on Form 8-K, dated March 2, 2009 (“March 2009 Form 8-K”) Item 9.01 (d)10.1).* | |
| 10.28 | Employment Agreement, effective January 1, 2009, between the Company and Andrew Jeanneret (incorporated by reference to the Company’s March 2009 Form 8-K, Item 9.01(d)10.2).* | |
| 10.29 | Employment Agreement, effective January 1, 2009, between the Company and Daniel R. Ouzts (incorporated by reference to the Company’s March 2009 Form 8-K, Item 9.01(d)10.3).* | |
| 10.30 | Employment Agreement, effective January 1, 2009, between the Company and Joanne Zimmerman (incorporated by reference to the Company’s March 2009 Form 8-K, Item 9.01(d)10.4).* |
53
| 10.31 | Form of Indemnification Agreement for officers and directors of the Company (incorporated by reference to the Company’s March 2009 Form 8-K, Item 9.01(d)10.5). | |
| 14 | Code of Ethics | |
| 14.1 | Code of Ethics and Business Conduct, as amended, September 6, 2006 and April, 2007 (incorporated by reference to the Company’s Current Report on Form 8-K dated September 12, 2006, Item 14.1). | |
| 21 | Subsidiaries of the Company | |
| 23 | Consent of experts and counsel | |
| 23.1 | Consent of MSPC Certified Public Accountants and Advisors, P.C. | |
| 31 | Rule 13a-14(a)/15d-14(a) Certifications. | |
| 31.1 | Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934. | |
| 31.2 | Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934. | |
| 32 | Section 1350 Certifications | |
| 32.1 | Certification of the Chief Executive Officer and the Chief Financial Officer pursuant to Rule 13a-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350(2). |
_________________________
| + | Documents incorporated by reference not included in Exhibit Volume. | |
| ‡ | Incorporated by reference to the company’s registration statement on Form SB-2 dated December 22, 1995 as amended February 9, 1996, April 2, 1996 and April 15, 1996, registration no. 33-80877-A, Part II, Item 27. | |
| * | Management contract or compensatory plan or arrangement. | |
| (1) | 51% owned subsidiary. | |
| (2) | In accordance with Release No. 34-47551, this exhibit is furnished to the SEC as an accompanying document and is not deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 or otherwise subject to the liabilities of that Section, and it shall not be deemed incorporated by reference into any filing under the Securities Act of 1933. | |
| (3) | Wholly owned subsidiary. |
54
| (c) | Financial Statement Schedules Required By Regulation S-X, Excluded From This Annual Report | |
| None |
55
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| DIALYSIS CORPORATION OF AMERICA | |||
| By: | /s/ STEPHEN W. EVERETT | ||
| Stephen W. Everett | |||
| President and Chief Executive Officer | |||
March 13, 2009
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | |
| /s/ THOMAS K. LANGBEIN | Chairman of the Board of Directors | March 13, 2009 | |
| Thomas K. Langbein | |||
| /s/ STEPHEN W. EVERETT | President, Chief Executive Officer | ||
| Stephen W. Everett | and Director | March 13, 2009 | |
| /s/ ANDREW JEANNERET | Vice President of Finance and Chief | ||
| Andrew Jeanneret | Financial Officer | March 13, 2009 | |
| /s/ DANIEL R OUZTS | Vice President of Finance | March 13, 2009 | |
| Daniel R. Ouzts | and Treasurer | ||
| /s/ PETER D. FISCHBEIN | Director | March 13, 2009 | |
| Peter D. Fischbein | |||
| /s/ ROBERT W. TRAUSE | Director | March 13, 2009 | |
| Robert W. Trause | |||
| /s/ ALEXANDER BIENENSTOCK | Director | March 13, 2009 | |
| Alexander Bienenstock | |||
56
ANNUAL REPORT ON FORM 10-K
ITEM 8, ITEM 15(a) (1) and (2)
LIST OF FINANCIAL STATEMENTS AND FINANCIAL STATEMENT SCHEDULES
FINANCIAL STATEMENTS AND SUPPLEMENTAL DATA
FINANCIAL STATEMENT SCHEDULES
YEAR ENDED DECEMBER 31, 2008
DIALYSIS CORPORATION OF AMERICA
LINTHICUM, MARYLAND
FORM 10-K—ITEM 15(a)(1) and (2)
DIALYSIS CORPORATION OF AMERICA
LIST OF FINANCIAL STATEMENTS
The following consolidated financial statements of Dialysis Corporation of America and subsidiaries are included in Item 8 of the Annual Report on Form 10-K:
Page
| Report of Independent Registered Public Accounting Firm | F-2 |
| Consolidated Balance Sheets – December 31, 2008 and 2007 | F-4 |
| Consolidated Statements of Income – Years Ended December 31, 2008, 2007 and 2006 | F-5 |
| Consolidated Statements of Stockholders’ Equity – Years Ended December 31, 2008, | |
| 2007 and 2006 | F-6 |
| Consolidated Statements of Cash Flows – Years Ended December 31, 2008, | |
| 2007 and 2006 | F-7 |
| Notes to Consolidated Financial Statements | F-8 |
The following financial statement schedule of Dialysis Corporation of America and subsidiaries is included in Item 15(a)(2):
| Schedule II – Valuation and qualifying accounts | F-28 |
All other schedules for which provision is made in the applicable accounting regulation of the Securities and Exchange Commission are not required under the related instructions or are inapplicable, and therefore have been omitted.
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders
Dialysis Corporation of America
We have audited the accompanying consolidated balance sheets of Dialysis Corporation of America as of December 31, 2008 and 2007, and the related consolidated statements of income, stockholders' equity, and cash flows for each of the years in the three-year period ended December 31, 2008. We also have audited Dialysis Corporation of America's internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control— Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Dialysis Corporation of America's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on these consolidated financial statements and an opinion on the company's internal control over financial reporting based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the consolidated financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
F-2
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Dialysis Corporation of America as of December 31, 2008 and 2007, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2008, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, Dialysis Corporation of America maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
MSPC
Certified Public Accountants and Advisors, P.C.
Cranford, New Jersey
February 27, 2009
F-3
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31, | December 31, | |||||||
| 2008 | 2007 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 6,543,000 | $ | 2,447,820 | ||||
| Accounts receivable, less allowance | ||||||||
| of $2,540,000 at December 31, 2008; | ||||||||
| $2,114,000 at December 31, 2007 | 21,493,780 | 20,159,926 | ||||||
| Inventories, less allowance for obsolescence | ||||||||
| of $15,000 at December 31, 2008; $25,000 at December 31, 2007 | 2,918,578 | 2,006,661 | ||||||
| Deferred income tax asset | 1,185,000 | 998,000 | ||||||
| Prepaid expenses and other current assets | 2,978,049 | 3,313,406 | ||||||
| Total current assets | 35,118,407 | 28,925,813 | ||||||
| Property and equipment: | ||||||||
| Land | 1,333,191 | 1,333,191 | ||||||
| Buildings and improvements | 5,721,879 | 5,716,904 | ||||||
| Machinery and equipment | 15,143,283 | 12,359,797 | ||||||
| Leasehold improvements | 10,789,122 | 9,356,531 | ||||||
| 32,987,475 | 28,766,423 | |||||||
| Less accumulated depreciation and amortization | 14,452,018 | 12,264,029 | ||||||
| 18,535,457 | 16,502,394 | |||||||
| Goodwill | 16,492,401 | 8,576,893 | ||||||
| Other assets | 932,930 | 841,092 | ||||||
| Total other assets | 17,425,331 | 9,417,985 | ||||||
| $ | 71,079,195 | $ | 54,846,192 | |||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 7,232,288 | $ | 4,062,611 | ||||
| Accrued expenses | 7,485,404 | 6,161,588 | ||||||
| Income taxes payable | 60,470 | 33,297 | ||||||
| Current portion of long-term debt | 74,000 | 56,000 | ||||||
| Total current liabilities | 14,852,162 | 10,313,496 | ||||||
| Long-term debt, less current portion | 14,275,798 | 7,009,419 | ||||||
| Deferred income tax liability | 1,275,000 | 574,000 | ||||||
| Total liabilities | 30,402,960 | 17,896,915 | ||||||
| Minority interest in subsidiaries | 5,412,208 | 4,942,797 | ||||||
| Commitments and Contingencies | ||||||||
| Stockholders' equity: | ||||||||
Common stock, $.01 par value, authorized 20,000,000 shares: 9,579,743 shares issued and outstanding at December 31, 2008; 9,573,596 shares issued and outstanding at December 31, 2007 | 95,797 | 95,736 | ||||||
| Additional paid-in capital | 16,001,153 | 15,587,782 | ||||||
| Retained earnings | 19,167,077 | 16,322,962 | ||||||
| Total stockholders' equity | 35,264,027 | 32,006,480 | ||||||
| $ | 71,079,195 | $ | 54,846,192 | |||||
See notes to consolidated financial statements.
F-4
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Operating revenues: | ||||||||||||
| Sales: | ||||||||||||
| Medical services revenue | $ | 85,675,687 | $ | 73,170,573 | $ | 61,138,963 | ||||||
| Product sales | 1,161,206 | 1,077,988 | 891,242 | |||||||||
| Total sales revenues | 86,836,893 | 74,248,561 | 62,030,205 | |||||||||
| Management fee income | --- | 286,174 | 430,033 | |||||||||
| 86,836,893 | 74,534,735 | 62,460,238 | ||||||||||
| Cost and expenses: | ||||||||||||
| Cost of sales revenues: | ||||||||||||
| Cost of medical services | 51,452,299 | 44,248,309 | 36,968,682 | |||||||||
| Cost of product sales | 659,766 | 598,086 | 549,590 | |||||||||
| Total cost of sales revenues | 52,112,065 | 44,846,395 | 37,518,272 | |||||||||
| Selling, general and administrative expenses | ||||||||||||
| Corporate | 10,586,797 | 7,917,038 | 6,460,309 | |||||||||
| Facility | 12,851,367 | 11,423,072 | 8,988,078 | |||||||||
| Total | 23,438,164 | 19,340,110 | 15,448,387 | |||||||||
| Stock compensation expense | 221,166 | 217,474 | 275,438 | |||||||||
| Depreciation and amortization | 2,783,826 | 2,619,049 | 2,319,070 | |||||||||
| Provision for doubtful accounts | 2,088,248 | 1,665,120 | 1,198,995 | |||||||||
| 80,643,469 | 68,688,148 | 56,760,162 | ||||||||||
| Operating income | 6,193,424 | 5,846,587 | 5,700,076 | |||||||||
| Other (expense) income, net | (48,605 | ) | (39,637 | ) | 100,968 | |||||||
| Income before income taxes, minority and other | ||||||||||||
| equity interests and equity in affiliate earnings | 6,144,819 | 5,806,950 | 5,801,044 | |||||||||
| Income tax provision | 1,862,479 | 1,614,892 | 2,085,874 | |||||||||
| Income before minority and other equity interests | ||||||||||||
| and equity in affiliate earnings | 4,282,340 | 4,192,058 | 3,715,170 | |||||||||
| Minority and other equity interests in income | ||||||||||||
| of consolidated subsidiaries | (1,438,225 | ) | (1,105,736 | ) | (919,739 | ) | ||||||
| Equity in affiliate earnings | --- | --- | 253,765 | |||||||||
| Net income | $ | 2,844,115 | $ | 3,086,322 | $ | 3,049,196 | ||||||
| Earning per share: | ||||||||||||
| Basic | $ | .30 | $ | .32 | $ | .32 | ||||||
| Diluted | $ | .30 | $ | .32 | $ | .32 | ||||||
| Weighted average shares outstanding: | ||||||||||||
| Basic | 9,579,837 | 9,572,893 | 9,484,926 | |||||||||
| Diluted | 9,613,866 | 9,607,672 | 9,575,733 | |||||||||
See notes to consolidated financial statements.
F-5
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| Capital in | ||||||||||||||||
| Common | Excess of | Retained | ||||||||||||||
| Stock | Par Value | Earnings | Total | |||||||||||||
| Balance January 1, 2006 | $ | 92,655 | $ | 15,332,975 | $ | 10,187,444 | $ | 25,613,074 | ||||||||
| Exercise of stock options for 303,750 | ||||||||||||||||
| shares of common stock, net of 27,205 | ||||||||||||||||
| shares tendered in payment; includes | ||||||||||||||||
| tax effect of $31,000 | 2,765 | 411,484 | --- | 414,249 | ||||||||||||
| Share compensation issued (22,250 shares) | 223 | 258,847 | --- | 259,070 | ||||||||||||
| Payment to dissenting merger shareholders | --- | (2,100 | ) | --- | (2,100 | ) | ||||||||||
| Merger deferred tax adjustment | --- | (272,000 | ) | --- | (272,000 | ) | ||||||||||
| Net income | --- | --- | 3,049,196 | 3,049,196 | ||||||||||||
| Balance at December 31, 2006 | 95,643 | 15,729,206 | 13,236,640 | 29,061,489 | ||||||||||||
| Share compensation issued (9,250 shares) | 93 | 103,476 | --- | 103,569 | ||||||||||||
| Stock option compensation vesting | --- | 58,259 | --- | 58,259 | ||||||||||||
| Sale of minority interest by subsidiary | --- | (303,159 | ) | --- | (303,159 | ) | ||||||||||
| Net income | --- | --- | 3,086,322 | 3,086,322 | ||||||||||||
| Balance at December 31, 2007 | 95,736 | 15,587,782 | 16,322,962 | 32,006,480 | ||||||||||||
| Share compensation issued (6,500 shares) | 65 | 71,950 | --- | 72,015 | ||||||||||||
| �� | ||||||||||||||||
| Stock related compensation expense | --- | 221,166 | --- | 221,166 | ||||||||||||
| Share cancellation due to escheatment | (4 | ) | 4 | |||||||||||||
| Sale/acquisition of subsidiary minority interest | --- | 120,251 | --- | 120,251 | ||||||||||||
| Net income | --- | --- | 2,844,115 | 2,844,115 | ||||||||||||
| Balance at December 31, 2008 | $ | 95,797 | $ | 16,001,153 | $ | 19,167,077 | $ | 35,264,027 | ||||||||
See notes to consolidated financial statements.
F-6
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Operating activities: | ||||||||||||
| Net income | $ | 2,844,115 | $ | 3,086,322 | $ | 3,049,196 | ||||||
| Adjustments to reconcile net income to net cash | ||||||||||||
| provided by operating activities: | ||||||||||||
| Depreciation | 2,727,253 | 2,583,506 | 2,303,795 | |||||||||
| Amortization | 56,573 | 35,543 | 15,275 | |||||||||
| Bad debt expense | 2,088,248 | 1,665,120 | 1,198,995 | |||||||||
| Inventory obsolescence adjustment | --- | (52,836 | ) | --- | ||||||||
| Deferred income tax provision (benefit) | 514,000 | 132,343 | (331,413 | ) | ||||||||
| Deferred tax asset utilized | --- | 907,952 | 1,190,000 | |||||||||
| Stock and stock option compensation expense | 221,166 | 217,474 | 275,438 | |||||||||
| Minority and other equity interests | 1,438,225 | 1,105,736 | 919,739 | |||||||||
| Equity in affiliate earnings | --- | --- | (253,765 | ) | ||||||||
| Increase (decrease) relating to operating activities from: | ||||||||||||
| Accounts receivable | (2,812,206 | ) | (6,004,744 | ) | (5,945,256 | ) | ||||||
| Inventories | (723,337 | ) | 54,376 | (411,557 | ) | |||||||
| Prepaid expenses and other current assets | 374,148 | (47,644 | ) | 336,311 | ||||||||
| Accounts payable | 3,169,677 | 2,064,486 | 161,266 | |||||||||
| Accrued expenses | 1,395,830 | 448,794 | (115,824 | ) | ||||||||
| Income taxes payable | 27,173 | (626,795 | ) | 614,444 | ||||||||
| Net cash provided by operating activities | 11,320,865 | 5,569,633 | 3,006,644 | |||||||||
| Investing activities: | ||||||||||||
| Payments received on physician affiliate loans | 283,450 | 171,025 | 16,468 | |||||||||
| Additions to property and equipment, net of minor disposals | (4,333,087 | ) | (2,520,735 | ) | (5,196,967 | ) | ||||||
| Payment of employment contract liability | --- | --- | (1,960,000 | ) | ||||||||
| Payment dissenting merger shareholders | --- | --- | (2,100 | ) | ||||||||
| Acquisition of dialysis centers | (9,167,568 | ) | (2,173,134 | ) | (4,161,304 | ) | ||||||
| Distributions from affiliate | --- | --- | 163,821 | |||||||||
| Purchase of minority interests in subsidiaries | (25,000 | ) | (345,000 | ) | --- | |||||||
| Consolidation of former affiliate | --- | --- | 149,225 | |||||||||
| Other assets | 80,001 | (161,528 | ) | (1,264 | ) | |||||||
| Net cash used in investing activities | (13,162,204 | ) | (5,029,372 | ) | (10,992,121 | ) | ||||||
| Financing activities: | ||||||||||||
| Line of credit borrowings | 12,000,000 | 2,550,000 | 8,000,000 | |||||||||
| Line of credit repayments | (4,650,000 | ) | (4,100,000 | ) | (500,000 | ) | ||||||
| Payments on other long-term debt | (65,621 | ) | (132,906 | ) | (339,582 | ) | ||||||
| Exercise of stock options | --- | --- | 383,249 | |||||||||
| Minority investment in Toledo subsidiary | --- | 750,000 | --- | |||||||||
| Capital contributions by subsidiaries’ minority members | 450,000 | 78,107 | 1,301,884 | |||||||||
| Distribution to subsidiary minority members | (1,797,860 | ) | (729,211 | ) | (306,062 | ) | ||||||
| Net cash provided by (used in) financing activities | 5,936,519 | (1,584,010 | ) | 8,539,489 | ||||||||
| Increase (decrease) in cash and cash equivalents | 4,095,180 | (1,043,749 | ) | 554,012 | ||||||||
| Cash and cash equivalents at beginning of year | 2,447,820 | 3,491,569 | 2,937,557 | |||||||||
| Cash and cash equivalents at end of year | $ | 6,543,000 | $ | 2,447,820 | $ | 3,491,569 | ||||||
| Supplemental disclosure of cash flow information: | ||||||||||||
| Interest paid | $ | 460,000 | $ | 743,000 | $ | 395,000 | ||||||
| Income taxes paid (refunded) | 1,541,000 | 1,496,000 | (675,000 | ) | ||||||||
| Share payment (87,500 options exercised; 27,205 shares paid) | ||||||||||||
| for stock option exercises | --- | --- | 351,000 | |||||||||
| Increase in additional paid-in capital from exercise of non- | ||||||||||||
| qualified stock options | -- | --- | 31,000 | |||||||||
See notes to consolidated financial statements.
F-7
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 1--SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business
We are primarily engaged in kidney dialysis operations which include outpatient hemodialysis services, home dialysis services, inpatient dialysis services and ancillary services associated with dialysis treatments. We own 37 operating dialysis centers located in Georgia, Maryland, New Jersey, Ohio, Pennsylvania, South Carolina and Virginia and have agreements to provide inpatient dialysis treatments to 11 hospitals. Our medical products operations are not a significant component of our operations with operating revenues of $1,161,000 in 2008, $1,078,000 in 2007 and $891,000 in 2006 (1.3% for 2008 and 1.4% for 2007 and 2006 of operating revenues) and operating income of $179,000 in 2008, $186,000 in 2007 and $69,000 in 2006 (2.9%, 3.1% and 1.2% respectively of operating income).
Medical Service Revenue
Our revenues by payor are as follows:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Medicare | 46 | % | 49 | % | 52 | % | ||||||
| Medicaid and comparable programs | 8 | 8 | 9 | |||||||||
| Hospital inpatient dialysis services | 4 | 4 | 3 | |||||||||
| Commercial insurers and other private payors | 42 | 39 | 36 | |||||||||
| 100 | % | 100 | % | 100 | % | |||||||
Our sources of revenue (in thousands) are as follows:
| Year Ended December 31, | ||||||||||||||||||||||||
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
| Outpatient hemodialysis services | $ | 46,348 | 54 | % | $ | 39,880 | 55 | % | $ | 32,856 | 54 | % | ||||||||||||
| Home and peritoneal dialysis services | 4,189 | 5 | 3,736 | 5 | 3,625 | 6 | ||||||||||||||||||
| Inpatient hemodialysis services | 3,109 | 4 | 2,897 | 4 | 2,191 | 3 | ||||||||||||||||||
| Ancillary services | 32,030 | 37 | 26,658 | 36 | 22,467 | 37 | ||||||||||||||||||
| $ | 85,676 | 100 | % | $ | 73,171 | 100 | % | $ | 61,139 | 100 | % | |||||||||||||
Consolidation
The consolidated financial statements include the accounts of Dialysis Corporation of America and its subsidiaries, collectively referred to as the “company.” Intercompany accounts and transactions have been eliminated in consolidation.
F-8
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 1--SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES--Continued
Our Toledo, Ohio subsidiary, which was 40% owned until December, 2006, has been consolidated since August, 2006 due to our taking control of this facility. This subsidiary was previously accounted for using the equity method of accounting. In April, 2007, this subsidiary sold a 30% minority interest to the medical directors of the facility for total consideration of $750,000 and non-compete agreements. This transaction resulted in a charge against capital of approximately $123,000 due to a decrease in our equity position in that subsidiary.
Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Our principal estimates are for estimated uncollectible accounts receivable as provided for in our allowance for doubtful accounts, estimated useful lives of depreciable assets, and estimates for patient revenues from non-contracted payors. Our estimates are based on historical experience and assumptions believed to be reasonable given the available evidence at the time of the estimates. Actual results could differ from those estimates.
Vendor Volume Discounts
We have contractual arrangements with certain vendors pursuant to which we receives discounts based on volume of purchases. These discounts are recorded in accordance with paragraph 4 of Emerging Issues Task Force Issue No. 02-16 (“EITF 02-16”) “Accounting by a Customer for Certain Consideration Received from a Vendor” as a reduction in inventory costs resulting in reduced costs of sales as the related inventory is consumed.
Government Regulation
A substantial portion of our revenues are attributable to payments received under Medicare, which is supplemented by Medicaid or comparable benefits in the states in which we operate.
Reimbursement rates under these programs are subject to regulatory changes and governmental funding restrictions. Laws and regulations governing the Medicare and Medicaid programs are complex and subject to interpretation. We believe we are in compliance with all applicable laws and regulations and are not aware of any pending or threatened investigations involving allegations of potential wrongdoing. While no such regulatory inquiries have been made, compliance with such laws and regulations can be subject to future government review and interpretation as well as significant regulatory action including fines, penalties, and exclusions from the Medicare and Medicaid programs.
F-9
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 1--SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES--Continued
Cash and Cash Equivalents
We consider all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. The carrying amounts reported in the balance sheet for cash and cash equivalents approximate their fair values. Although cash and cash equivalents are largely not federally insured, the credit risk associated with these deposits that typically may be redeemed upon demand is considered low due to the high quality of the financial institutions in which they are deposited.
Credit Risk
Our primary concentration of credit risk is with accounts receivable, which consist of amounts owed by governmental agencies, insurance companies and private patients. Combined receivables from Medicare and Medicaid comprised 47% of receivables at December 31, 2008 and 49% December 31, 2007, respectively.
Inventories
Inventories are valued at the lower of cost (first-in, first-out method) or market value and consist of supplies used in dialysis treatments and the finished goods inventory of our medical products division.
Accrued Expenses
Accrued expenses are comprised as follows:
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| Accrued compensation | $ | 1,486,042 | $ | 1,342,122 | ||||
| Excess insurance liability | 4,687,512 | 2,789,055 | ||||||
| Health insurance premiums payable | 340,851 | 687,031 | ||||||
| Other | 970,999 | 1,343,380 | ||||||
| $ | 7,485,404 | $ | 6,161,588 | |||||
Excess insurance liability represents amounts paid by insurance companies in excess of the amounts we believe that we earned from the associated treatments. We communicate with the payors regarding these amounts, which can result from duplicate payments, payments in excess of contractual agreements, payments as primary when payor is secondary, and underbillings by us based on estimated fee schedules. These amounts remain in excess insurance liability until resolution. We identified approximately $1,113,000 of the excess insurance liability as of December 31, 2008 and $726,000 as of December 31, 2007, as relating to duplicate payments that will be refunded to the payers. Approximately $553,000 for 2008 and $442,000 for 2007 of amounts determined to be nonrefundable that had been included in excess insurance liability were recorded in medical services revenues.
We have a self-insured medical and dental insurance plan administered by a third party administrator pursuant to which we are responsible for claims and administrative fees. There was an estimated obligation payable of approximately $341,000 at December 31, 2008 and $687,000 at
F-10
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 1--SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES--Continued
December 31, 2007 included in accrued expenses. We had a deposit toward our plan obligations of approximately $400,000 at December 31, 2007 included in prepaid expenses and other current assets that was applied toward our plan obligations during the first quarter of 2008. There was no such deposit at December 31, 2008.
Vendor Concentration
There is only one supplier of erythropoietin (EPO) in the United States. This supplier and another manufacturer received FDA approval for two alternative products available for dialysis patients, which is indicated to be effective for a longer period than EPO. The alternative drugs also could be administered by the patient’s physician. Accordingly, the use of these drugs could reduce our revenues from our current treatment of anemia, thereby adversely impacting our revenues and profitability. There are no other suppliers of any similar drugs available to dialysis treatment providers. Revenues from the administration of EPO, which amounted to approximately $23,553,000 in 2008, $19,662,000 in 2007 and $16,830,000 in 2006, comprised 27% of medical services revenues in each of 2008 and 2007 and 28% in 2006.
Property and Equipment
Property and equipment is stated on the basis of cost. Depreciation is computed for book purposes by the straight-line method over the estimated useful lives of the assets, which range from 5 to 34 years for buildings and improvements; 3 to 10 years for machinery, computer and office equipment, and furniture; and 5 to 10 years for leasehold improvements based on the shorter of the lease term or estimated useful life of the property. Replacements and betterments that extend the lives of assets are capitalized. Maintenance and repairs are expensed as incurred. Upon the sale or retirement of assets, the related cost and accumulated depreciation are removed and any gain or loss is recognized.
Revenue Recognition
Net revenue is recognized as services are rendered at the net realizable amount from Medicare, Medicaid, commercial insurers and other third party payors. We occasionally provide dialysis treatments on a charity basis to patients who cannot afford to pay. The amount is not significant, and we do not record revenues related to these charitable treatments. Product sales are recorded pursuant to stated shipping terms.
Goodwill
Goodwill represents cost in excess of net assets acquired. Pursuant to Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets” (FAS 142), goodwill and intangible assets with indefinite lives are not amortized but are reviewed annually (or more frequently if impairment indicators are present) for impairment after determination that no other long-lived assets have indicators of impairment, which testing as of December 31, 2008, has indicated no impairment for goodwill.
F-11
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 1--SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES--Continued
Deferred Expenses
Deferred expenses, except for deferred loan costs, are amortized on the straight-line method over their estimated benefit period with deferred loan costs amortized over the lives of the respective loans. Deferred expenses of approximately $663,000 at December 31, 2008 and $207,000 at December 31, 2007 are included in other assets. Amortization expense was approximately $57,000 for 2008, $36,000 for 2007 and $15,000 for 2006.
Income Taxes
Deferred income taxes are determined by applying enacted tax rates applicable to future periods in which the taxes are expected to be paid or recovered to differences between financial accounting and tax basis of assets and liabilities.
Stock-Based Compensation
Pursuant to Statement of Financial Accounting Standards No. 123 (revised), “Share-Based Payment” (SFAS 123R), we measure compensation cost for stock award compensation arrangements based on grant date fair value to be expensed ratably over the requisite vesting period. Stock compensation expense was approximately $97,000, $159,000 and $275,000 2008, 2007 and 2006, respectively, with income tax benefits for shares vesting during these periods of approximately $17,000, $21,000 and $41,000, respectively.
During February, 2008, the company issued two incentive stock options with a total grant date value of $199,200. These stock options are being expensed over the four-year vesting period with the expense relating to these options amounting to approximately $42,000 for the year ended December 31, 2008, with no such expense for the preceding year. The fair value of these options was estimated at the date of grant using a Black-Scholes option pricing model with the following assumptions: risk free interest rate of 2.73%; no dividend yield; volatility factor of the expected market price of our common stock of 0.626 based on historical volatility for a period coinciding with the expected option life; and an expected life of four years. During April, 2007, the company issued an incentive stock option with a total grant date value of $329,000. This stock option is being expensed over the four-year vesting period with the expense relating to this option amounting to approximately $82,000 for the year ended December 31, 2008 and $58,000 for the preceding year. The fair value of this option was estimated at the date of grant using a Black-Scholes option pricing model with the following assumptions: risk free interest rate of 4.61%; no dividend yield; volatility factor of the expected market price of our common stock of 0.666 based on historical volatility for a period coinciding with the expected option life; and an expected life of four years. As the above options are incentive stock options, the related expense is not deductible for tax purposes.
F-12
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 1--SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES--Continued
The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options which have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective input assumptions including the expected stock price volatility. Because our employee stock options have characteristics significantly different from those of traded options, and because changes in the subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing models do not necessarily provide a reliable measure of the fair value of our employee stock options.
Earnings per Share
Diluted earnings per share gives effect to potential common shares that would be dilutive, such as stock options and warrants, calculated using the treasury stock method and average market price.
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Net income | $ | 2,844,115 | $ | 3,086,322 | $ | 3,049,196 | ||||||
| Weighted average shares outstanding | 9,579,837 | 9,572,893 | 9,484,926 | |||||||||
| Weighted average shares outstanding | 9,579,837 | 9,572,893 | 9,484,926 | |||||||||
| Shares issuable for employee stock awards and director fees | 25,492 | 23,178 | 17,701 | |||||||||
| Weighted average shares diluted computation | 9,605,328 | 9,596,071 | 9,502,627 | |||||||||
| Effect of dilutive stock options | 8,537 | 11,601 | 73,106 | |||||||||
| Weighted average shares, as adjusted diluted computation | 9,613,866 | 9,607,672 | 9,575,733 | |||||||||
| Earnings per share: | ||||||||||||
| Basic | $ | .30 | $ | .32 | $ | .32 | ||||||
| Diluted | $ | .30 | $ | .32 | $ | .32 | ||||||
Other (Expense) Income
Non-operating:
Other non-operating (expense) income is comprised as follows:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Rental income | $ | 404,694 | $ | 389,324 | $ | 369,888 | ||||||
| Interest income | 87,078 | 222,163 | 193,429 | |||||||||
| Interest expense | (589,081 | ) | (756,671 | ) | (409,676 | ) | ||||||
| Other | 48,704 | 105,547 | 32,327 | |||||||||
| Litigation settlement, net | --- | --- | (85,000 | ) | ||||||||
| Other (expense)income, net | $ | (48,605 | ) | $ | (39,637 | ) | $ | 100,968 | ||||
F-13
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 1--SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES—Continued
Estimated Fair Value of Financial Instruments
The carrying value of cash and cash equivalents, accounts receivable, prepaid expenses and other current assets and accounts payable and accrued expenses in the accompanying financial statements approximate their fair value because of the short-term maturity of these instruments. Debt approximates fair value since such instruments either bear variable interest rates which approximate market or have interest rates approximating those currently available to us for loans with similar terms and maturities.
Reclassification
Certain prior year amounts have been reclassified to conform with the current year’s presentation.
New Pronouncements
In September, 2006, the Financial Accounting Standards Board (FASB) issued Statement of Financial Accounting Standards No. 157 (SFAS 157), “Fair Value Measurements.” SFAS 157 defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS 157 applies under other accounting pronouncements that require fair value measurement in which the FASB concluded that fair value was the relevant measurement, but does not require any new fair value measurements. We adopted SFAS 157 effective January 1, 2008, except for the nonfinancial assets and liabilities that are subject to a one-year deferral allowed by FASB Staff Position (FSP) FAS 157-2 (FSP FAS157-2), “Effective Date of FASB Statement No. 157” issued February 12, 2008. The standard applies to assets and liabilities that are carried at fair value on a recurring basis. FSP FAS157-2 delays the effective date of SFAS 157 until fiscal years beginning after November 15, 2008 for nonfinancial assets and liabilities that are not recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually). The adoption of SFAS 157 did not have a material effect on our financial statements. We are evaluating the impact SFAS 157 will have on our financial statements beginning in fiscal 2009 as it relates to the items subject to the one-year deferral allowed by FSP FAS 157-2.
In December, 2007, the FASB issued Statement of Financial Accounting Standards No. 141 (revised 2007) (SFAS 141R), “Business Combinations.” SFAS 141R expands the definitions of a business and business combination and requires all assets and liabilities of an acquired business (for full, partial and step acquisitions) to be recorded at fair values, with limited exceptions. SFAS 141R requires earn-outs and other contingent consideration to be recorded at fair value on acquisition date and contingencies to be recorded at fair value on acquisition date with provision for subsequent remeasurement. SFAS 141R requires acquisitions costs to be expensed as incurred and generally requires restructuring costs to be expensed in periods after the acquisition date. SFAS 141R requires amounts previously called “negative goodwill” which result from a bargain purchase in which acquisition date fair value of identifiable net assets acquired exceeds the fair value of consideration transferred plus any noncontrolling interest in the acquirer to be recognized in earnings as a gain attributable to the acquirer. SFAS 141R is effective with the first annual reporting period beginning on or after December 15, 2008. We are evaluating the impact on our financial statements of adopting SFAS 141R.
F-14
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 1--SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES--Continued
In December, 2007, the FASB issued Statement of Financial Accounting Standards No. 160 (SFAS 160), “Noncontrolling Interests in Consolidated Financial Statements,” an amendment of ARB 51. SFAS 160 requires noncontrolling interests to be reported in the equity section of consolidated financial statements and requires that consolidated net income include the amounts attributable to both the parent and the noncontrolling interests with disclosure on the face of the consolidated income statement of net income attributable to the parent and to the noncontrolling interests, with any losses attributable to the noncontrolling interests in excess of the noncontrolling interests’ equity to be allocated to the noncontrolling interest. Calculation of earnings per share amounts in the consolidated financial statements will continue to be based on amounts attributable to the parent. SFAS 160 is effective with the first annual reporting period beginning on or after December 15, 2008. Other than presentation differences, we do not expect SFAS160 to have a significant impact on our financial statements.
NOTE 2--LONG-TERM DEBT
Long-term debt is as follows:
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| Revolving line of credit | $ | 13,300,000 | $ | 5,950,000 | ||||
| Development loan secured by land and building | 495,292 | 526,304 | ||||||
| Mortgage note secured by land and building | 554,506 | 589,115 | ||||||
| 14,349,798 | 7,065,419 | |||||||
| Less current portion | 74,000 | 56,000 | ||||||
| $ | 14,275,798 | $ | 7,009,419 | |||||
Pursuant to a December 3, 1999 loan agreement through our subsidiary, DCA of Vineland, LLC, we obtained a $700,000 development loan currently secured by a mortgage on our real property in Easton, Maryland. In May 2006, the loan was modified to extend the maturity date to May 2, 2026. Monthly payments are $2,402 plus interest at prime. This loan had an outstanding principal balance of approximately $495,000 at December 31, 2008 and $526,000 at December 31, 2007.
In April, 2001, we obtained a $788,000 five-year mortgage through April, 2006, on our building in Valdosta, Georgia. We refinanced this mortgage in April, 2006 with the new mortgage having an April, 2011, maturity. Interest was at prime with a rate floor of 5.75% and a rate ceiling of 8.00% until September 15, 2008 when the rate floor was revised to 5.00% and the rate ceiling was revised to 7.50%. As of May, 2006, payments are $6,000 including principal and interest, with a final payment consisting of a balloon payment and any unpaid interest due April, 2011. The remaining principal balance under this mortgage amounted to approximately $555,000 at December 31, 2008 and $589,000 at December 31, 2007.
The prime rate was 3.50% at December 31, 2008 and 7.25% at December 31, 2007.
F-15
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 2--LONG-TERM DEBT--Continued
Scheduled maturities of long-term debt outstanding at December 31, 2008 are approximately:
| 2009 | $ | 74,000 | ||
| 2010 | 77,000 | |||
| 2011 | 13,379,000 | |||
| 2012 | 81,000 | |||
| 2013 | 84,000 | |||
| Thereafter | 655,000 | |||
| $ | 14,350,000 |
On October 24, 2005, we entered into a three year, $15,000,000 revolving line of credit with a maturity date of October 24, 2008 which was extended for one year to October 24, 2009. On November 5, 2008, the line of credit was increased to $25,000,000 and extended to November 4, 2011. Each of our wholly-owned subsidiaries has guaranteed this credit facility, as will any future wholly-owned subsidiaries. Further, the obligation under the revolving line of credit is secured by our pledge of our ownership in our subsidiaries. The credit facility, which has provisions for both base rate and LIBOR loans, is intended to provide funds for the development and acquisition of new dialysis facilities, to meet general working capital requirements, and for other general corporate purposes. Up to $3,000,000 has been allocated for the repurchase of company stock should management determine to establish a stock repurchase program, provided the company continues to be in compliance with the financial covenants of the credit facility after giving effect to any such stock repurchase. Borrowings under the revolving line of credit accrue interest at the base rate for base rate loans and the LIBOR rate for LIBOR loans plus an applicable margin, as those terms are defined in the agreement. The LIBOR rate applicable to a LIBOR loan is determined by the interest period selected by the company for that particular loan, which represents the duration of the loan. We have the right to convert a base rate loan to a LIBOR loan, and vice versa. The agreement contains customary reporting and financial covenant requirements for this type of credit facility. We are in compliance with the requirements of this credit facility at December 31, 2008 and December 31, 2007. We have $13,300,000 in outstanding borrowings under our line of credit at December 31, 2008, consisting of three LIBOR loans with one month terms. The interest rate on the individual LIBOR loans ranges from 3.50% to 4.9375% and includes LIBOR at issuance plus an applicable margin of 3.0%. The individual LIBOR loans outstanding at December 31, 2008 are renewable at the end of their respective one month terms.
Our two mortgage agreements contain certain restrictive covenants that, among other things, limit the payment of dividends, require lenders’ approval for a merger, sale of substantially all of our assets, or other business combination to which we are a party, and require maintenance of certain financial ratios. The company was in compliance with the debt covenants at December 31, 2008 and December 31, 2007.
Effective January 1, 2007, we adopted the provisions of FIN 48. FIN 48 clarifies the accounting for uncertain tax positions in accordance with FASB Statement No. 109, Accounting for Income Taxes and prescribes a recognition threshold and measurement attribute criteria for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The interpretation also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. We evaluated our tax position in tax returns filed, as well as
F-16
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 3--INCOME TAXES
tax positions to be taken in future returns and the amounts comprising our deferred tax assets. The adoption of FIN 48 did not have a material impact on our financial condition, results of operations or cash flows.
We file income tax returns in the U.S. federal jurisdiction and various state and local jurisdictions. Our federal income tax returns for 2004 through 2007 are open tax years. State and local jurisdictions that remain subject to examination range from 2003 to 2007.
We may from time to time be assessed interest or penalties by major tax jurisdictions, although such assessments historically have been minimal and immaterial to our financial results. Our policy is to recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense.
The income tax provision consists of the following income tax expense (benefit) components:
| 2008 | 2007 | 2006 | ||||||||||
| Current: | ||||||||||||
| Federal | $ | 931,000 | $ | 1,238,000 | $ | 1,970,000 | ||||||
| State | 378,000 | 207,000 | 410,000 | |||||||||
| City | 39,000 | 38,000 | 37,000 | |||||||||
| 1,348,000 | 1,483,000 | 2,417,000 | ||||||||||
| Deferred: | ||||||||||||
| Federal | 540,000 | 72,000 | (317,000 | ) | ||||||||
| State | (26,000 | ) | 60,000 | (14,000 | ) | |||||||
| 514,000 | 132,000 | (331,000 | ) | |||||||||
| $ | 1,862,000 | $ | 1,615,000 | $ | 2,086,000 | |||||||
Deferred income taxes reflect the net tax effect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax liabilities and assets are as follows:
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| Deferred tax liabilities: | ||||||||
| Depreciation and amortization | $ | 1,305,000 | $ | 619,000 | ||||
| Total deferred tax liabilities | 1,305,000 | 619,000 | ||||||
| Deferred tax assets: | ||||||||
| Accrued expenses | 317,000 | 256,000 | ||||||
| Bad debt allowance | 832,000 | 700,000 | ||||||
| Startup costs and other intangibles | 30,000 | 45,000 | ||||||
| Inventory costs capitalization | 36,000 | 42,000 | ||||||
| Total deferred tax assets | 1,215,000 | 1,043,000 | ||||||
| Net deferred tax liability | $ | 90,000 | $ | 424,000 | ||||
F-17
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 3--INCOME TAXES--Continued
No valuation allowance was recorded for deferred tax assets at December 31, 2008 or December 31, 2007, due to our anticipated prospects for future taxable income in an amount sufficient to realize the deferred tax assets.
As a result of our merger with our former parent, we acquired a deferred tax asset of approximately $3,300,000, representing tax benefits from our former parent’s net operating loss carryforwards that we were able to utilize to satisfy income tax liabilities. We fully utilized the available net operating loss carryforwards as of December 31, 2007.
The reconciliation of income tax attributable to income before income taxes computed at the U.S. federal statutory rate is:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Statutory tax rate (34%) applied to income | ||||||||||||
before income taxes, minority and other equity interest and equity in affiliate earnings | $ | 2,089,000 | $ | 1,974,000 | $ | 1,972,000 | ||||||
| Adjustments due to: | ||||||||||||
| State and city taxes, net of federal benefit | 275,000 | 162,000 | 295,000 | |||||||||
| Previously unrecognized NOL carryforwards | --- | (239,000 | ) | --- | ||||||||
| Tax return accrual adjustment | --- | (84,000 | ) | --- | ||||||||
| Non-deductible items | 18,000 | 14,000 | 13,000 | |||||||||
| Subsidiary minority ownership | (584,000 | ) | (254,000 | ) | (271,000 | ) | ||||||
| Ownership in affiliate | --- | --- | 72,000 | |||||||||
| Other | 64,000 | 42,000 | 5,000 | |||||||||
| $ | 1,862,000 | $ | 1,615,000 | $ | 2,086,000 | |||||||
We have equity positions in 31 limited liability companies (“LLC’s”), and one limited partnership (“LP”) each possessing a finite life, as well as ownership in 6 corporate subsidiaries. Based on their limited liability status, members are not liable for the LLC’s and LP’s debts, liabilities, or obligations. Each LLC and LP has complied with the criteria for tax treatment as a partnership. As a result, taxable income or loss is to be reported on each member’s respective tax returns. Income and losses attributable to our equity position in subsidiary LLC’s and LP’s are included in our Consolidated Statements of Income with the minority interest in the results of operations of subsidiary LLC’s and LP’s shown separately on the Consolidated Statements of Income. Income attributable to our equity position in our 40% owned affiliate prior to the affiliate’s consolidation as a subsidiary effective August 1, 2006, is shown separately on the company’s Consolidated Statements of Income.
The exercise of 10,000 non-qualified stock options during 2006 resulted in a tax deduction of $84,000, corresponding to the difference in the market value of the shares obtained on exercise and the exercise price of the options. The reduction in income taxes payable of approximately $31,000 resulted in a corresponding increase in additional paid-in capital.
F-18
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 4--OTHER RELATED PARTY TRANSACTIONS
We have constructed dialysis facilities which we sold upon completion to entities in which medical directors of the related facilities have an interest. Our subsidiaries that operate those dialysis facilities lease the facilities from the purchasers on terms which are as favorable as could be obtained from unaffiliated parties. The cost of the land and construction costs for any such facilities were included in prepaid expenses and other current assets until the sale of the facilities.
NOTE 5--STOCK OPTIONS AND STOCK AWARDS
In September, 2001, the board of directors granted five-year options exercisable at $.75 per share through September 5, 2006, to certain officers, directors and key employees. 100,000 options were exercised in March, 2006 and the balance of 5,000 options were exercised in April, 2006 with the company receiving cash payments totaling $78,750 for the exercise price.
In June, 2003, the board of directors granted to an officer a five-year option for 50,000 shares exercisable at $1.80 per share through June 3, 2008. These options were exercised in March, 2006 with the company receiving a $90,000 cash payment for the exercise price.
In August, 2003, the board of directors granted a three-year option to a director for 10,000 shares exercisable at $2.25 per share through August 18, 2006. These options were exercised in June, 2006 with the company receiving a $22,500 cash payment.
In January, 2004, the board of directors granted a five year option to an employee exercisable at $3.09 per share through January 12, 2009. 5,000 options were exercised in January, 2006 with the company receiving a cash payment of $15,425 for the exercise price, and 10,000 options were exercised in June, 2006 with the company receiving a $30,850 cash payment.
In June, 2004, the board of directors granted 160,000 stock options to officers, directors and a key employee exercisable at $4.02 per share through June 6, 2009. 25,000 options were exercised in March, 2006 with the company receiving a $100,500 cash payment for the exercise price. An additional 87,500 options were exercised in March, 2006 with an exercise price of $351,750 that was satisfied through payment of 27,205 shares of our stock. 7,500 options were exercised in May and June, 2006 and 3,750 options were exercised in August, 2006 with the company receiving a total of $45,225 cash payments. There are 18,750 options outstanding under this grant.
On June 8, 2006, our shareholders approved an amendment to our stock option plan to allow for the grant of stock awards in addition to options. The employment agreement of Stephen W. Everett, our President, CEO and a director, contains provisions for the receipt of up to 40,000 shares of our common stock, pursuant to which 10,000 shares were issued during 2006 resulting in approximately $100,000 stock compensation expense, with an additional up to 10,000 performance based shares for each of 2006, 2007 and 2008 not earned.
We granted stock awards of 1,000 shares to each of our independent directors in February, 2006 with the shares vesting in 250 share increments for each director at the end of each quarter of 2006, resulting in approximately $38,000 stock compensation expense.
F-19
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 5--STOCK OPTIONS AND STOCK AWARDS--Continued
On June 27, 2006, we granted stock awards of 64,000 shares to officers and key employees with the awards vesting in equal yearly increments over four years commencing December 31, 2006. One of the June, 2006 stock awards for 30,000 shares, which contained performance criteria, was cancelled in November, 2006 upon resignation of the officer. 8,500 of the June, 2006 stock awards vested during 2006 resulting in $94,180 stock compensation expense with the related shares being issued during the first quarter of 2007. One of the June, 2006 stock awards for 8,000 shares was cancelled in July, 2007 upon resignation of the employee and the remaining 6,000 shares did not vest. 6,500 of the June, 2006 stock awards vested during each of 2007 and 2008 resulting in approximately $72,000 stock compensation expense each year with the related shares being issued during the first quarter of 2008 and the first quarter of 2009, respectively.
In November, 2006, we entered a six-month contract with an individual to serve as our Chief Operating Officer for which the compensation was 10,000 shares of our common stock to be earned over the six month period, with 3,333 shares earned during 2006, resulting in approximately $44,000 stock compensation expense and the remaining 6,667 shares earned during 2007 with approximately $87,000 stock compensation expense.
In April, 2007, the board of directors granted a five-year 50,000 share option to our newly retained Vice President of Operations, exercisable at $12.18 per share through April 15, 2012. The option vests in equal increments of 12,500 shares every 12 months commencing April 15, 2008. The grant date fair value of $329,000 is being expensed over the five-year vesting period with approximately $58,000 expense recorded during 2007 and $82,000 expense recorded during 2008.
On January 10, 2008, the board of directors granted stock awards for 13,500 shares to non-executive management personnel with the awards to vest over four years in 25% equal increments commencing on January 9, 2009. One award for 1,000 shares was cancelled due to resignation of an employee, leaving awards for 12,500 shares outstanding for which approximately $25,000 stock compensation expense was recorded during 2008.
On February 29, 2008, the board of directors granted a five year option to our newly appointed Chief Financial Officer for 50,000 shares and a five year option to a key employee for 10,000 shares, each option exercisable at $12.18 through February 28, 2013. Each of the options vests in equal increments totaling 15,000 shares every 12 months commencing February 28, 2009. The grant date fair value of $199,200 is being expensed over the four-year vesting period with approximately $42,000 expense recorded during 2008.
F-20
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 5--STOCK OPTIONS AND STOCK AWARDS--Continued
On September 23, 2008, the company filed a registration statement on Form S-8 covering 15,000 shares issuable under the June, 2004 options, and all the shares issuable under the April, 2007 and February, 2008 options.
A summary of our stock option activity and related information for the years ended December 31 follows:
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
Weighted Average | Weighted Average | Weighted Average | ||||||||||||||||||||||
| Options | Exercise Price | Options | Exercise Price | Options | Exercise Price | |||||||||||||||||||
| Outstanding-beginning of year | 68,750 | $ | 9.95 | 18,750 | $ | 4.02 | 322,500 | $ | 2.51 | |||||||||||||||
| Granted | 60,000 | $ | 12.18 | 50,000 | $ | 12.18 | --- | |||||||||||||||||
| Cancellations | --- | --- | --- | |||||||||||||||||||||
| Exercised | --- | --- | (303,750 | ) | $ | 2.42 | ||||||||||||||||||
| Expired | --- | --- | --- | |||||||||||||||||||||
| Outstanding-end of year | 128,750 | 68,750 | 18,750 | |||||||||||||||||||||
| Outstanding: | ||||||||||||||||||||||||
| February 2008 options | 60,000 | $ | 12.18 | --- | --- | |||||||||||||||||||
| April 2007 options | 50,000 | $ | 12.18 | 50,000 | $ | 12.18 | --- | |||||||||||||||||
| June 2004 options | 18,750 | $ | 4.02 | 18,750 | $ | 4.02 | 18,750 | $ | 4.02 | |||||||||||||||
| 128,750 | 68,750 | 18,750 | ||||||||||||||||||||||
Outstanding and exercisable end of year: | ||||||||||||||||||||||||
| April 2007 options | 12,500 | $ | 12.18 | --- | --- | |||||||||||||||||||
| June 2004 options | 18,750 | $ | 4.02 | 18,750 | $ | 4.02 | 18,750 | $ | 4.02 | |||||||||||||||
| 31,250 | 18,750 | 18,750 | ||||||||||||||||||||||
Weighted-average fair value of options granted during the year | $ | 3.32 | $ | 6.58 | ||||||||||||||||||||
The remaining contractual life at December 31, 2008 is 0.4 years for the June, 2004 options, 3.3 years for the April, 2007 options, and 4.2 years for the February, 2008 options.
The total exercise price for stock options exercised during 2006 was approximately $735,000 compared to a total stock value on the dates of exercises of approximately $3,923,000, resulting in an intrinsic value on these exercises of $3,188,000. There were no options exercised during 2007 or 2008.
F-21
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 5--STOCK OPTIONS AND STOCK AWARDS--Continued
A summary of information on our stock compensation and nonvested shares for the years ended December 31 follows:
| 2008 | 2007 | 2006 | ||||||||||||||||||||||||||||||||||
Total | Weighted Average | Total | Weighted Average | Total | Weighted Average | |||||||||||||||||||||||||||||||
| Shares | Fair Value | Fair Value | Shares | Fair Value | Fair Value | Shares | Fair Value | Fair Value | ||||||||||||||||||||||||||||
| Nonvested-beginning of year: | ||||||||||||||||||||||||||||||||||||
| Issued | --- | $ | --- | $ | --- | 6,667 | $ | 87,204 | $ | 13.08 | --- | $ | --- | $ | --- | |||||||||||||||||||||
| Unissued | 23,000 | 244,140 | $ | 10.61 | 45,500 | 482,740 | $ | 10.61 | --- | --- | --- | |||||||||||||||||||||||||
| 23,000 | $ | 244,140 | $ | 10.61 | 52,167 | $ | 569,944 | $ | 10.93 | --- | $ | --- | $ | --- | ||||||||||||||||||||||
| Shares granted: | ||||||||||||||||||||||||||||||||||||
| Issued | --- | $ | --- | --- | $ | --- | 22,250 | $ | 259,070 | $ | 11.64 | |||||||||||||||||||||||||
| Unissued | 13,500 | 109,755 | $ | 8.13 | --- | --- | 94,750 | 1,018,810 | $ | 10.75 | ||||||||||||||||||||||||||
| 13,500 | $ | 109,755 | $ | 8.13 | --- | $ | --- | 117,000 | $ | 1,277,880 | $ | 10.92 | ||||||||||||||||||||||||
| Shares vesting: | ||||||||||||||||||||||||||||||||||||
| Issued | --- | $ | --- | (6,667 | ) | $ | (87,204 | ) | $ | 13.08 | (15,583 | ) | $ | (171,866 | ) | $ | 11.03 | |||||||||||||||||||
| Unissued | (6,500 | ) | (72,020 | ) | $ | 11.08 | (6,500 | ) | (72,020 | ) | $ | 11.08 | (9,250 | ) | (103,570 | ) | $ | 11.20 | ||||||||||||||||||
| (6,500 | ) | $ | (72,020 | ) | $ | 11.08 | (13,167 | ) | $ | (159,224 | ) | $ | 12.09 | (24,833 | ) | $ | (275,436 | ) | $ | 11.09 | ||||||||||||||||
| Shares forfeited: | ||||||||||||||||||||||||||||||||||||
| Issued | --- | $ | --- | --- | --- | --- | --- | --- | ||||||||||||||||||||||||||||
| Unissued | (11,000 | ) | (108,230 | ) | $ | 9.84 | (16,000 | ) | (166,580 | ) | $ | 10.41 | (40,000 | ) | (432,500 | ) | $ | 10.81 | ||||||||||||||||||
| (11,000 | ) | $ | (108,230 | ) | $ | 9.94 | (16,000 | ) | (166,580 | ) | $ | 10.41 | (40,000 | ) | (432,500 | ) | $ | 10.81 | ||||||||||||||||||
| Nonvested-end of year: | ||||||||||||||||||||||||||||||||||||
| Issued | --- | $ | --- | --- | $ | --- | 6,667 | $ | 87,204 | $ | 13.08 | |||||||||||||||||||||||||
| Unissued | 19,000 | 173,645 | $ | 10.61 | 23,000 | 244,140 | $ | 10.61 | 45,500 | 482,740 | $ | 10.61 | ||||||||||||||||||||||||
| 19,000 | $ | 173,645 | $ | 10.61 | 23,000 | $ | 244,140 | $ | 10.61 | 52,167 | $ | 569,944 | $ | 10.93 | ||||||||||||||||||||||
| Weighted average period over which nonvested | ||||||||||||||||||||||||||||||||||||
| compensation cost expected to be recognized: | ||||||||||||||||||||||||||||||||||||
| Issued shares | 0.33 years | |||||||||||||||||||||||||||||||||||
| Unissued shares: | ||||||||||||||||||||||||||||||||||||
| Performance based | 1.00 years | 2.00 years | ||||||||||||||||||||||||||||||||||
| Nonperformance based | 1.01 years | 2.00 years | 3.00 years | |||||||||||||||||||||||||||||||||
| Overall | 1.01 years | 1.09 years | 1.64 years | |||||||||||||||||||||||||||||||||
F-22
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 5--STOCK OPTIONS AND STOCK AWARDS--Continued
The total grant date fair value of stock awards vesting during 2008, 2007 and 2006 was approximately $72,000, $159,000 and $275,000, respectively compared to a total stock value on the dates of vesting of approximately $46,000, $140,000 and $289,000, resulting in fair value differentials of $(26,000), ($19,000) and $14,000.
As of December 31, 2008, we have 755,404 shares reserved for issuance, including 60,000 shares for the February, 2008 options. 50,000 shares for the April, 2007 options, 18,750 shares for the June, 2004 options, and 30,000 for outstanding non-vested and performance-based stock awards. All options were issued at fair market value on date of grant.
NOTE 6--COMMITMENTS
We have leases on facilities housing our dialysis, administrative and medical operations, as well as equipment leases. Total rent expense was approximately $2,676,000, $2,315,000 and $1,727,000 for the years ended December 31, 2008, 2007, and 2006, respectively. The aggregate lease commitments at December 31, 2008 for our non-cancelable operating leases with a term of one year or more are approximately:
| 2009 | $ | 2,722,000 | ||
| 2010 | 2,441,000 | |||
| 2011 | 2,128,000 | |||
| 2012 | 1,892,000 | |||
| 2013 | 1,361,000 | |||
| Thereafter | 2,975,000 | |||
| $ | 13,519,000 |
We have a 401(k) savings plan (salary deferral plan) with eligibility requirements of one year of service and an age requirement of at least 21 years. Our 401(k) plan allows employees, in addition to regular employee contributions, to elect to have a portion of bonus payments contributed. As an incentive to save for retirement, we will match 10% of an employee’s contribution resulting from any bonus paid during the year and may make a discretionary contribution with the percentage of any discretionary contribution to be determined each year with only employee contributions up to 6% of annual compensation considered when determining employer matching. To date, employer matching expense has been minimal.
NOTE 7--ACQUISITIONS
Our various acquisitions were made either on the basis of existing profitability or expectation of future profitability for the interest acquired based on our analysis of the potential for each acquisition, and the value of the relationship with the physician affiliated with the selling entity. Each acquisition was intended to either strengthen our market share within a geographic area or provide us with the opportunity to enter a new geographic area and market. Management also reviews the purchase price and
F-23
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 7--ACQUISITIONS--Continued
any resulting goodwill based on established current per patient valuations for dialysis centers. Also considered are the synergistic effects of a potential acquisition, including potential costs integration and the effect of the acquisition on our overall valuation. Certain of the acquisition transactions were of minority interests held by medical directors of certain of our dialysis facilities.
These transactions resulted in an aggregate of approximately $16,492,000 of goodwill, representing the excess of the purchase price over the fair value of the net assets acquired, including net goodwill of $7,916,000 from 2008 acquisitions as further described below. The goodwill is being amortized for tax purposes over a 15-year period with the exception of $1,358,000 goodwill from a stock acquisition which is not amortizable for tax purposes.
During the first quarter of 2006, we acquired a Virginia dialysis center and a Maryland company with two dialysis centers. These transactions resulted in approximately $326,000 of goodwill amortizable over 15 years for tax purposes. We determined there is no impairment of goodwill. In December, 2006, we acquired the remaining 60% interest in our 40% owned Toledo, Ohio dialysis facility pursuant to a put option valued at $3,200,000 resulting in goodwill of approximately $2,707,000 amortizable over 15 years for tax purposes. We determined there is no impairment of goodwill.
During the first quarter of 2007 we acquired the assets of an Ohio dialysis center and a Pennsylvania dialysis center. These transactions resulted in approximately $1,705,000 of goodwill amortizable over 15 years for tax purposes. We determined there is no impairment of goodwill.
Effective December 1, 2007, we increased our ownership in DCA of West Baltimore, LLC from 60% to 75% by acquiring a portion of the existing minority interest for $345,000 resulting in goodwill of approximately $190,000 amortizable over 15 years for tax purposes. We determined there is no impairment of goodwill.
Pursuant to a call option which was exercised on January 11, 2007, one of our subsidiaries acquired, effective January 1, 2008, the assets of a Georgia dialysis facility that we had been managing pursuant to a management services agreement. Effective January 1, 2008, we have an 80% interest in the facility, which is operated through our subsidiary with the former owner having a 20% interest. The purchase price included the 20% minority interest and approximately $2,541,000, one half of which was paid at closing with the remaining portion subject to a one year promissory note payable to the seller with interest at prime plus 1% which was paid in December, 2008. This transaction resulted in approximately $2,311,000 of goodwill amortizable over 15 years for tax purposes. We determined there is no impairment of goodwill.
F-24
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 7--ACQUISITIONS--Continued
Effective March 1, 2008, we acquired the 20% minority interest in DCA of Chesapeake, LLC and DCA of North Baltimore, LLC for $25,000. This transaction resulted in a reduction of $159,000 in the existing goodwill on these subsidiaries.
On December 31, 2008, we acquired a Maryland dialysis center for approximately $6,627,000, including acquisition costs resulting in approximately $5,715,000 of goodwill, the excess of the net purchase price over the estimated fair value of net assets acquired, including the valuation of a ten year non-competition agreement that will be amortized over the life of the agreement. The goodwill is amortizable for tax purposes over 15 years. We determined there is no impairment of goodwill. The initial allocation of purchase cost at fair value was based upon available information and will be finalized as any contingent purchase amounts are resolved and estimated fair values of assets are finalized. We will begin recording the results of operations for the acquired company as of January 1, 2009.
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed at the date of acquisition for the December, 2008 Maryland acquisition:
| Inventory and other current assets | $ | 134,000 | ||
| Property, plant and equipment, | 365,000 | |||
| Intangible assets | 413,000 | |||
| Goodwill | 5,715,000 | |||
| Net assets acquired | $ | 6,627,000 |
Pro forma results of operations as if the Maryland acquisition had completed as of January 1, 2006 are as follows (in thousands except per share data):
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Operating revenues | $ | 91,998 | $ | 78,967 | $ | 66,067 | ||||||
| Net income | $ | 2,932 | $ | 3,116 | $ | 2,999 | ||||||
| Earnings per share: | ||||||||||||
| Basic | $ | .31 | $ | .33 | $ | .32 | ||||||
| Diluted | $ | .30 | $ | .32 | $ | .31 | ||||||
NOTE 8--LOAN TRANSACTIONS
The company has and may continue to provide funds in excess of capital contributions subject to financing agreements with our subsidiaries to meet working capital requirements of our dialysis facility subsidiaries, usually until they become self-sufficient. The operating agreements for the subsidiaries provide for cash flow and other proceeds to first pay any such financing, exclusive of any tax payment distributions. Minority members of the subsidiaries would be responsible for their respective portion of the financing according to their ownership interests.
F-25
DIALYSIS CORPORATION OF AMERICA AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2008
NOTE 9--QUARTERLY FINANCIAL INFORMATION (Unaudited)
The following summarizes certain quarterly operating data (in thousands except per share data):
| Year Ended December 31, 2008 | ||||||||||||||||||||
| March 31 | June 30 | Sept. 30 | Dec.31 | Total | ||||||||||||||||
| Net sales | $ | 20,485 | $ | 20,836 | $ | 21,860 | $ | 23,656 | $ | 86,837 | ||||||||||
| Gross profit | 7,967 | 8,000 | 8,896 | 9,862 | 34,725 | |||||||||||||||
| Net income | 449 | 663 | 875 | 857 | 2,844 | |||||||||||||||
| Earnings per shares: | ||||||||||||||||||||
| Basic | $ | .05 | $ | .07 | $ | .09 | $ | .09 | $ | .30 | ||||||||||
| Diluted | $ | 05 | $ | .07 | $ | .09 | $ | .09 | $ | .30 | ||||||||||
| Year Ended December 31, 2007 | ||||||||||||||||||||
| March 31 | June 30 | Sept. 30 | Dec.31 | Total | ||||||||||||||||
| Net Sales | $ | 16,828 | $ | 18,418 | $ | 19,092 | $ | 19,911 | $ | 74,249 | ||||||||||
| Gross profit | 6,282 | 7,342 | 7,762 | 8,017 | 29,403 | |||||||||||||||
| Net income | 383 | 753 | 927 | 1,023 | 3,086 | |||||||||||||||
| Earnings per shares: | ||||||||||||||||||||
| Basic | $ | .04 | $ | .08 | $ | .10 | $ | .11 | $ | .32 | ||||||||||
| Diluted | $ | .04 | $ | .08 | $ | .10 | $ | .11 | $ | .32 | ||||||||||
Since the computation of earnings per share is made independently for each quarter using the treasury stock method, the total of four quarters’ earnings do not necessarily equal earnings per share for the year.
F-26
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To The Board of Directors and Shareholders of
Dialysis Corporation of America
Our report on our audit of the basic consolidated financial statements of Dialysis Corporation of America and subsidiaries appears on page F-2. That audit was conducted for the purpose of forming an opinion on the basic financial statements taken as a whole. The supplemental schedule II is presented for purposes of complying with the Securities and Exchange Commission’s Rules and Regulations under the Securities Exchange Act of 1934 and is not otherwise a required part of the basic consolidated financial statements. Such information has been subjected to the auditing procedures applied in the audit of the basic consolidated financial statements, and in our opinion, is fairly stated in all material respects in relation to the basic consolidated financial statements taken as a whole.
| MSPC | ||
| Certified Public Accountants and Advisors, P.C. | ||
Cranford, New Jersey
February 27, 2009
F-27
Schedule II - Valuation and Qualifying Accounts
Dialysis Corporation of America, Inc. and Subsidiaries
December 31, 2008
| COL. A | COL. B | COL. C | COL. D | COL. E | |||||||||||||
| Classification | Balance at Beginning Of Period | Additions (Deductions) Charged (Credited) to Cost and Expenses | Additions Charged to Other Accounts Describe | Other Changes Add (Deduct) Describe | Balance at End of Period | ||||||||||||
| YEAR ENDED DECEMBER 31, 2008: | |||||||||||||||||
| Reserves and allowances deducted | |||||||||||||||||
| from asset accounts: | |||||||||||||||||
| Allowance for uncollectible accounts | $ | 2,114,000 | $ | 2,088,000 | $ | (1,662,000 | )(1) | $ | 2,540,000 | ||||||||
| Reserve for inventory obsolescence | 25,000 | --- | (10,000 | )(2) | 15,000 | ||||||||||||
| $ | 2,139,000 | $ | 2,088,000 | $ | 1,672,000 | $ | 2,555,000 | ||||||||||
| YEAR ENDED DECEMBER 31, 2007: | |||||||||||||||||
| Reserves and allowances deducted | |||||||||||||||||
| from asset accounts: | |||||||||||||||||
| Allowance for uncollectible accounts | $ | 2,512,000 | $ | 1,665,000 | $ | (2,063,00 | )(1) | $ | 2,114,000 | ||||||||
| Reserve for inventory obsolescence | 78,000 | (53,000 | ) | -- | 25,000 | ||||||||||||
| $ | 2,590,000 | $ | 1,612,000 | $ | (2,063,000 | ) | $ | 2,139,000 | |||||||||
| YEAR ENDED DECEMBER 31, 2006: | |||||||||||||||||
| Reserves and allowances deducted | |||||||||||||||||
| from asset accounts: | |||||||||||||||||
| Allowance for uncollectible accounts | $ | 1,886,000 | $ | 1,199,000 | $ | (573,000 | )(1) | $ | 2,512,000 | ||||||||
| Reserve for inventory obsolescence | 77,000 | 1,000 | 78,000 | ||||||||||||||
| $ | 1,963,000 | $ | 1,200,000 | $ | (573,000 | ) | $ | 2,590,000 | |||||||||
| (1) | Uncollectible accounts written off, net of recoveries. |
| (2) | Net write-offs against inventory reserves. |
F-28
Exhibit Index
DIALYSIS CORPORATION OF AMERICA
Annual Report on Form 10-K for the Fiscal Year Ended December 31, 2008
| 21 | Subsidiaries of the Company |
| 23.1 | Consent of MSPC, Certified Public Accountant and Advisors, P.C. |
| 31.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934 |
| 31.2 | Certification of Chief Financial Officer Pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934 |
| 32.1 | Certification of Chief Executive Officer and Chief Financial Officer Pursuant to Rule 13a-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350 |