Exhibit 13
Management’s Discussion and Analysis of Financial Condition and Results of Operations
OVERVIEW
Our business is focused on the development, manufacture and distribution of cardiovascular medical devices for the global cardiac rhythm management, cardiology, cardiac surgery and atrial fibrillation therapy areas and implantable neurostimulation medical devices for the management of chronic pain. We sell our products in more than 100 countries around the world. Our largest geographic markets are the United States, Europe, Japan and Asia Pacific. Our four operating segments are Cardiac Rhythm Management (CRM), Cardiovascular (CV), Atrial Fibrillation (AF), and Neuromodulation (NMD). Our principal products in each operating segment are as follows: CRM – tachycardia implantable cardioverter defibrillator systems (ICDs) and bradycardia pacemaker systems (pacemakers); CV – vascular closure devices, heart valve replacement and repair products, pressure measurement guidewires, coronary imaging technology and structural heart defect and vascular abnormality devices; AF – electrophysiology introducers and catheters, advanced cardiac mapping, navigation and recording systems and ablation systems; and NMD – neurostimulation devices. References to “St. Jude Medical,” “St. Jude,” “the Company,” “we,” “us” and “our” are to St. Jude Medical, Inc. and its subsidiaries.
Our industry has undergone significant consolidation in the last decade and is highly competitive. Our strategy requires significant investment in research and development in order to introduce new products. We are focused on improving our operating margins through a variety of techniques, including the production of high quality products, the development of leading edge technology, the enhancement of our existing products and continuous improvement of our manufacturing processes. We expect competitive pressures in the industry, cost containment pressure on healthcare systems and the implementation of U.S. healthcare reform legislation to continue to place downward pressure on prices for our products, impact reimbursement for our products and potentially reduce medical procedure volumes.
In March 2010, significant U.S. healthcare reform legislation was enacted into law. As a U.S. headquartered company with significant sales in the United States, this health care reform legislation will materially impact us. Certain provisions of the legislation are not effective for a number of years and there are many programs and requirements for which the details have not yet been fully established or consequences not fully understood, and it is unclear what the full impacts will be from the legislation. The legislation does levy a 2.3% excise tax on all U.S. medical device sales beginning in 2013. This is a significant new tax that will materially and adversely affect our business and results of operations. The legislation also focuses on a number of Medicare provisions aimed at improving quality and decreasing costs. It is uncertain at this point what impacts these provisions will have on patient access to new technologies. The Medicare provisions also include value‐based payment programs, increased funding of comparative effectiveness research, reduced hospital payments for avoidable readmissions and hospital acquired conditions and pilot programs to evaluate alternative payment methodologies that promote care coordination (such as bundled physician and hospital payments). Additionally, the provisions include a reduction in the annual rate of inflation for hospitals starting in 2011 and the establishment of an independent payment advisory board to recommend ways of reducing the rate of growth in Medicare spending. We cannot predict what healthcare programs and regulations will be ultimately implemented at the federal or state level, or the effect of any future legislation or regulation. However, any changes that lower reimbursement for our products or reduce medical procedure volumes could adversely affect our business and results of operations.
We participate in several different medical device markets, each of which has its own expected growth rate. A significant portion of our net sales relate to CRM devices – ICDs and pacemakers. During early March 2010, a principal competitor in the CRM market, Boston Scientific, Inc. (Boston Scientific), suspended sales of its ICD products in the United States. Although Boston Scientific resumed sales in mid-April 2010, we experienced an incremental ICD net sales benefit in the range of approximately $35 million to $40 million. While the long-term impact on the CRM market is uncertain, management remains focused on increasing our worldwide CRM market share, as we are one of three principal manufacturers and suppliers in the global CRM market. We are also investing in our other three major growth platforms – cardiovascular, atrial fibrillation and neuromodulation – to increase our market share in these markets.
1
During 2010, we expanded our cardiovascular growth programs through the acquisitions of AGA Medical Holdings, Inc. (AGA Medical) and LightLab Imaging, Inc. (LightLab Imaging). Our AGA Medical acquisition strengthened our portfolio of products to treat structural heart defects and vascular abnormalities through minimally invasive transcatheter treatments. The LightLab Imaging acquisition provided us a new technology, Optical Coherence Tomography (OCT), for coronary imaging applications. OCT is a high resolution diagnostic coronary imaging technology that complements the Fractional Flow Reserve (FFR) technology acquired by the Company as part of the Radi Medical Systems AB (Radi Medical Systems) acquisition in December 2008.
We utilize a 52/53-week fiscal year ending on the Saturday nearest December 31st. Fiscal year 2010 and 2009 consisted of 52 weeks and ended on January 1, 2011 and January 2, 2010, respectively. Fiscal year 2008 consisted of 53 weeks and ended on January 3, 2009 with the additional week reflected in our fourth quarter 2008 results.
Net sales in 2010 increased 10% over 2009 net sales, led by sales growth of ICDs and products to treat atrial fibrillation. Our 2010 operational net sales growth (net sales changes excluding the impacts of foreign currency translation and acquisitions) increased 9% over 2009. Foreign currency translation comparisons increased our 2010 net sales by $23.3 million. Our 2010 CRM net sales increased 10% to $3,039.9 million, compared to 2009, driven by ICD operational net sales growth of 15%. Our 2010 AF net sales increased 13% to $707.9 million, compared to 2009, due to operational net sales growth of 12%. While 2010 Cardiovascular operational net sales growth was 3%, total net sales increased 9% to $1,036.7 million, compared to the prior year, driven by $25.2 million of incremental net sales from our AGA Medical acquisition. Our 2010 Neuromodulation net sales grew 15% to $380.3 million, compared to 2009, driven solely by operational net sales growth as a result of continued market acceptance and market penetration of our neurostimulation devices.
Our net sales in 2009 increased 7% over 2008 net sales driven by operational net sales growth of 7%. Unfavorable foreign currency translation comparisons decreased our 2009 net sales by $99.5 million compared to 2008. Our 2009 CRM net sales increased 3% to $2,769.0 million, compared to 2008, driven by ICD operational net sales growth of 5%. Our 2009 AF net sales increased 15% to $627.9 million, compared to 2008, due to operational net sales growth of 14%. Our 2009 Cardiovascular net sales increased 11% to $953.6 million, compared to the prior year, driven by incremental sales from our December 2008 Radi Medical Systems acquisition. Lastly, our 2009 Neuromodulation net sales grew 30% to $330.8 million, compared to 2008, driven by operational sales growth of 32%, as we continued to penetrate the Neuromodulation market. Refer to the Segment Performance section for a more detailed discussion of our net sales results by operating segment for both 2010 and 2009.
Net earnings in 2010 of $907.4 million and diluted net earnings per share of $2.75 increased compared to 2009 net earnings of $777.2 million and diluted net earnings per share of $2.26. These increases were due to incremental profits resulting from a 10% increase in 2010 net sales over 2009 as well as lower outstanding shares in 2010 resulting from $625.3 million of repurchases of our common stock. Our 2010 net earnings were impacted by after-tax charges of $50.2 million, or $0.15 per diluted share, and our 2009 net earnings were impacted by after-tax charges of $85.3 million, or $0.25 per diluted share. The charges incurred in both 2010 and 2009 included special charges, in-process research and development (IPR&D) charges and investment impairment charges. Refer to theResults of Operations section for a more detailed discussion of these charges. We also incurred $37.1 million of after-tax closing and other costs associated with our acquisitions of AGA Medical and LightLab Imaging.
Our 2009 net earnings of $777.2 million and diluted net earnings per share of $2.26 increased compared to 2008 net earnings of $353.0 million and diluted net earnings per share of $1.01. Our 2009 net earnings were impacted by after-tax charges of $85.3 million, or $0.25 per diluted share, and our fiscal year 2008 net earnings were impacted by after-tax charges of $400.1 million, or $1.15 per diluted share (refer to theResults of Operations section for a more detailed discussion of these charges). Compared to 2008, our 2009 net earnings and diluted net earnings per share benefited from 7% net sales growth and lower after-tax charges.
We generated $1,274.4 million of operating cash flows during 2010, compared to $868.9 million of operating cash flows during 2009. We ended the year with $500.3 million of cash and cash equivalents and $2,511.6 million of total debt. During 2010, we issued approximately $1.2 billion of long-term debt consisting of 3-year and 5-year senior notes in the U.S. and 7-year and 10-year Yen denominated notes in Japan. We also repaid approximately $0.9 billion of debt consisting of a term loan and maturing Yen-denominated notes in Japan. Additionally, we repurchased $625.3 million of our common stock resulting in the purchase of 15.4 million shares at an average repurchase price of $40.63 per share.
2
NEW ACCOUNTING PRONOUNCEMENTS
Certain new accounting standards will become effective for us in fiscal year 2011 and future periods. Information regarding new accounting pronouncements that impacted 2010 or our historical consolidated financial statements and related disclosures is included in Note 1 to the Consolidated Financial Statements.
In January 2010, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2010-6,Fair Value Measurements and Disclosures (ASC Topic 820):Improving Disclosures about Fair Value Measurements, which requires reporting entities to make new disclosures about recurring or nonrecurring fair value measurements including significant transfers into and out of Level 1 and Level 2 fair value measurements and information on purchases, sales, issuances, and settlements on a gross basis in the reconciliation of Level 3 fair value measurements. ASU 2010-6 is effective for annual reporting periods beginning after December 15, 2009, except for Level 3 reconciliation disclosures which are effective for annual periods beginning after December 15, 2010. We adopted the additional disclosures required for Level 1 and Level 2 fair value measurements in the first quarter of 2010 (see Note 13 to the Consolidated Financial Statements) and we will adopt Level 3 disclosure requirements beginning in the first quarter of 2011.
In December 2010, the FASB issued ASU 2010-29,Business Combinations (ASC Topic 805): Disclosure of Supplementary Pro Forma Information for Business Combinations, to address diversity in practice about the interpretation of the pro forma revenue and earnings disclosure requirements for business combinations that are material on an individual or aggregate basis. The disclosure requirements include pro forma revenue and earnings of the combined entity for the current reporting period and comparable prior reporting period as though the acquisition date for the business combination occurred at the beginning of the comparable prior annual reporting period. ASU 2010-29 is effective prospectively for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period on or after December 15, 2010. We will adopt ASU 2010-29 as of the beginning of our 2011 fiscal year.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Preparation of our consolidated financial statements in accordance with accounting principles generally accepted in the United States requires us to adopt various accounting policies and to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Our significant accounting policies are disclosed in Note 1 to the Consolidated Financial Statements.
On an ongoing basis, we evaluate our estimates and assumptions, including those related to our accounts receivable allowance for doubtful accounts; inventory reserves; valuation of IPR&D, other intangible assets and goodwill; income taxes; litigation reserves and insurance receivables; and stock-based compensation. We base our estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, and the results form the basis for making judgments about the reported values of assets, liabilities, and expenses. Actual results may differ from these estimates. We believe that the following represent our most critical accounting estimates:
Accounts Receivable Allowance for Doubtful Accounts: We grant credit to customers in the normal course of business, and generally do not require collateral or any other security to support our accounts receivable. We maintain an allowance for doubtful accounts for potential credit losses, which primarily consists of reserves for specific customer balances that we believe, may not be collectible. We determine the adequacy of this allowance by regularly reviewing the age of accounts receivable, customer financial conditions and credit histories, and current economic conditions. In some developed markets and in many emerging markets, payment of certain accounts receivable balances are made by the individual countries’ healthcare systems for which payment is dependent, to some extent, upon the political and economic environment within those countries. Although we consider our allowance for doubtful accounts to be adequate, if the financial condition of our customers or the individual countries’ healthcare systems were to deteriorate and impair their ability to make payments to us, additional allowances may be required in future periods. The allowance for doubtful accounts was $35.4 million at January 1, 2011 and $34.9 million at January 2, 2010.
Inventory Reserves: We value inventory at the lower of cost or market, with cost determined using the first-in, first-out method. We maintain reserves for excess and obsolete inventory based on forecasted product sales, new product introductions by us or our competitors, product expirations and historical experience. The inventory reserves we recognize are based on our estimates of how these factors are expected to impact the amount and value of inventory we expect to sell. The markets in which we operate are highly competitive and characterized by rapid product development and technological change putting our products at risk of losing market share and/or becoming obsolete. We monitor our inventory reserves on an ongoing basis, and although we consider our inventory reserves to be adequate, we may be required to recognize additional inventory reserves if future demand or market conditions are less favorable than we have estimated. In addition to our normal recurring inventory reserves, we have recognized special charge inventory write-downs of $27.9 million, $17.7 million and $3.0 million in 2010, 2009 and 2008, respectively.
3
Valuation of IPR&D, Other Intangible Assets and Goodwill: When we acquire a business, the purchase price is allocated, as applicable, between IPR&D, other identifiable intangible assets, tangible assets and goodwill. Determining the portion of the purchase price allocated to IPR&D and other intangible assets requires us to make significant estimates.
IPR&D is defined as the value assigned to those projects for which the related products have not yet reached technological feasibility and have no future alternative use. The primary basis for determining the technological feasibility of these projects at the time of acquisition is obtaining regulatory approval to market the underlying products in an applicable geographic region. Prior to 2009, we expensed the value attributed to any IPR&D projects acquired in a business acquisition in accordance with generally accepted accounting principles.
Beginning in fiscal year 2009, all IPR&D acquired in a business acquisition is subject to FASB’s ASC Topic 805,Business Combinations, which requires the fair value of IPR&D to be capitalized as an indefinite-lived intangible asset until completion of the IPR&D project or abandonment. Upon completion of the development project (generally when regulatory approval to market the product is obtained), acquired IPR&D assets are amortized over their estimated useful life. If the IPR&D projects are abandoned, the related IPR&D assets would likely be impaired and written down to fair value. During 2010, we capitalized $134.3 million of indefinite-lived IPR&D assets related to our AGA Medical and LightLab Imaging business acquisitions.
We use the income approach to establish the fair value of IPR&D and other identifiable intangible assets as of the acquisition date. This approach establishes fair value by estimating the after-tax cash flows attributable to a project or intangible asset over its estimated useful life and discounting these after-tax cash flows back to a present value. We base our revenue assumptions on estimates of relevant market sizes, expected market growth, and trends in technology as well as anticipated product introductions by competitors. In arriving at the value of an IPR&D project, we consider, among other factors, the stage of completion, the complexity of the work to complete, the costs incurred, the projected cost of completion, the contribution of core technologies and other acquired assets, the expected introduction date and the estimated useful life of the technology. The discount rate used is determined at the time of acquisition and includes consideration of the assessed risk of the project not being developed to commercial feasibility. In arriving at the value of an intangible asset we consider the underlying products and estimated useful life of the technology, projected future product sales, legal agreements, patent litigation and anticipated product introductions by competitors. The discount rate used is determined at the time of acquisition and includes consideration of the assessed risk of the underlying products future sales.
At the time of acquisition, we expect all acquired IPR&D will reach technological feasibility, but there can be no assurance that the commercial viability of these projects will actually be achieved. The nature of the efforts to develop the acquired technologies into commercially viable products consists principally of planning, designing and conducting clinical trials necessary to obtain regulatory approvals. The risks associated with achieving commercialization include, but are not limited to, delay or failure to obtain regulatory approvals to conduct clinical trials, failure of clinical trials, delay or failure to obtain required market clearances, and patent litigation. If commercial viability is not achieved, we would not realize the original estimated financial benefits expected for these projects. We fund all costs to complete IPR&D projects with internally generated cash flows.
Our other intangible assets consist of purchased technology and patents, customer lists and relationships, trademarks and tradenames, licenses and distribution agreements. Certain trademark assets related to our AGA Medical acquisition have been classified as indefinite-lived intangible assets. All other identifiable intangible assets are being amortized using the straight-line method over their estimated useful lives, ranging from three to 20 years. We review our other intangible assets for impairment as changes in circumstance or the occurrence of events suggest the carrying value may not be recoverable. Excluding IPR&D, other intangible assets, net of accumulated amortization, were $852.8 million at January 1, 2011 and $456.1 million at January 2, 2010.
4
In contrast to business combinations, generally accepted accounting principles requires that IPR&D in connection with asset purchases be expensed immediately. In many cases, the purchase of certain intellectual property assets or the rights to such intellectual property is considered a purchase of assets rather than the acquisition of a business. Accordingly, rather than being capitalized, any IPR&D acquired in such asset purchases is expensed. During 2010, 2009 and 2008, we expensed $12.2 million, $5.8 million and $319.4 million, respectively, related to IPR&D acquired in asset purchases.
Goodwill recognized in connection with a business acquisition represents the excess of the aggregate purchase price over the fair value of net assets acquired. Goodwill is tested for impairment annually or more frequently if changes in circumstance or the occurrence of events suggest impairment exists. The test for impairment requires us to make several estimates about fair value, most of which are based on projected future cash flows. Our estimates associated with the goodwill impairment tests are considered critical due to the amount of goodwill recorded on our consolidated balance sheets and the judgment required in determining fair value amounts, including projected future cash flows and the use of an appropriate risk-adjusted discount rate. Goodwill was $2,955.6 million at January 1, 2011 and $2,005.9 million at January 2, 2010.
Income Taxes: As part of the process of preparing our consolidated financial statements, we are required to estimate our income taxes in each of the jurisdictions in which we operate. This process involves estimating the actual current tax expense as well as assessing temporary differences in the treatment of items for tax and financial accounting purposes. These timing differences result in deferred tax assets and liabilities, which are included in our consolidated balance sheet. We also assess the likelihood that our deferred tax assets will be recovered from future taxable income, and to the extent that we believe that recovery is not likely, a valuation allowance is established. At January 1, 2011, we had $466.7 million of gross deferred tax assets, including net operating loss and tax credit carryforwards that will expire from 2013 to 2029 if not utilized. We believe that our deferred tax assets, including the net operating loss and tax credit carryforwards, will be fully realized based upon our estimates of future taxable income. As such, we have not recorded any valuation allowance for our deferred tax assets. If our estimates of future taxable income are not met, a valuation allowance for some of these deferred tax assets would be required.
We have not recorded U.S. deferred income taxes on certain of our non-U.S. subsidiaries’ undistributed earnings, as such amounts are intended to be reinvested outside the United States indefinitely. However, should we change our business and tax strategies in the future and decide to repatriate a portion of these earnings to one of our U.S. subsidiaries, including cash maintained by these non-U.S. subsidiaries, additional U.S. tax liabilities would be incurred. It is not practical to estimate the amount of additional U.S. tax liabilities we would incur.
We record our income tax provisions based on our knowledge of all relevant facts and circumstances, including the existing tax laws, our experience with previous settlement agreements, the status of current IRS examinations and our understanding of how the tax authorities view certain relevant industry and commercial matters. Although we have recorded all income tax accruals in accordance with ASC 740,Income Taxes, our accruals represent accounting estimates that are subject to the inherent uncertainties associated with the tax audit process, and therefore include certain contingencies.
The finalization of the tax audit process across the various tax authorities, including federal, state and foreign, often takes many years. We have substantially concluded all U.S. federal income tax matters for all tax years through 2001. Additionally, substantially all material foreign, state, and local income tax matters have been concluded for all tax years through 1999. The U.S. Internal Revenue Service (IRS) completed an audit of our 2002-2005 tax returns, and proposed adjustments in its audit report issued in November 2008. We are vigorously defending our positions and initiated defense of these adjustments at the IRS appellate level in January 2009. An unfavorable outcome could have a material negative impact on our effective income tax rate in future periods. At January 1, 2011, our liability for unrecognized tax benefits was $162.9 million and our accrual for interest and penalties was $33.8 million. We believe that any potential tax assessments from the various tax authorities that are not covered by our income tax accruals will not have a material adverse impact on our consolidated financial position or cash flows; however, they may be material to our consolidated earnings of a future period.
Litigation Reserves and Insurance Receivables: We operate in an industry that is susceptible to significant product liability and intellectual property claims. As a result, we are involved in a number of legal proceedings, the outcomes of which are not in our complete control and may not be known for extended periods of time. In accordance with ASC Topic 450,Contingencies, we record a liability in our consolidated financial statements for costs related to claims, including future legal costs, settlements and judgments where we have assessed that a loss is probable and an amount can be reasonably estimated. Product liability claims may be brought by individuals seeking relief for themselves or, increasingly, by groups seeking to represent a class. In addition, claims may be asserted against us in the future related to events that are not known to us at the present time. Our significant legal proceedings are discussed in detail in Note 5 to the Consolidated Financial Statements. While it is not possible to predict the outcome for most of the legal proceedings discussed in Note 5, the costs associated with such proceedings could have a material adverse effect on our consolidated earnings, financial position or cash flows of a future period.
5
We record a receivable from our legacy product liability insurance carriers for amounts expected to be recovered. This includes amounts for legal matters where we have incurred defense costs or where we have recognized a liability for probable and estimable future legal costs, settlements or judgments. We record a receivable for the amount of insurance we expect to recover based on our assessment of the specific insurance policies, the nature of the claim, our experience with similar claims and our assessment of collectability based on our insurers’ financial condition. To the extent our insurance carriers ultimately do not reimburse us, either because such costs are deemed to be outside the scope of our product liability insurance policies or because our insurers may not be able to meet their payment obligations to us, the related losses we incur relating to these unreimbursed costs could have a material adverse effect on our consolidated earnings or cash flows. Our receivable from legacy product liability insurance carriers was $12.8 million at January 1, 2011 and $42.5 million at January 2, 2010. During 2010, we did not record any losses on our legacy product liability insurance receivables and received payments of $57.5 million.
Stock-Based Compensation: Under the fair value recognition provisions of ASC Topic 718,Compensation – Stock Compensation(ASC Topic 718), we measure stock-based compensation cost at the grant date based on the fair value of the award and recognize the compensation expense over the requisite service period (vesting period) into cost of sales, research and development expense or selling, general and administrative expense in the Consolidated Statements of Earnings.
We use the Black-Scholes standard option pricing model (Black-Scholes model) to determine the grant date fair value of stock options and employee stock purchase rights. The awards’ grant date fair value using the Black-Scholes model is affected by our stock price as well as assumptions of other variables, including projected employee stock option exercise behaviors (expected option life), risk-free interest rate, expected dividend yield and expected volatility of our stock price in future periods. The grant date fair value of restricted stock units and restricted stock awards is based on the closing stock price on the grant date.
We analyze historical employee exercise and termination data to estimate the expected life assumption. We believe that historical data currently represents the best estimate of the expected life of a new employee option. We also stratify our employee population based upon distinctive exercise behavior patterns. The risk-free interest rate we use is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected life of the options. For all grants through fiscal year 2010, we have not anticipated paying cash dividends and therefore, we have used an expected dividend yield of zero. Since December 2008, we calculate our expected volatility assumption by equally weighting historical and implied volatility. Previously, we calculated the expected volatility assumption exclusively on the implied volatility of market-traded options. We changed the method of determining expected volatility to take into consideration how future volatility experience over the expected life of the option may differ from short-term volatility experience and thus provide a better estimate of expected volatility over the expected life of employee stock options.
The amount of stock-based compensation expense we recognize during a period is based on the portion of the awards that are ultimately expected to vest. We estimate pre-vesting award forfeitures at the time of grant by analyzing historical data and revising those estimates in subsequent periods if actual forfeitures differ from those estimates. Ultimately, the total expense recognized over the vesting period will equal the fair value of awards that actually vest.
If factors change and we employ different assumptions for estimating stock-based compensation expense in future periods or if we decide to use a different option pricing model, the expense in future periods may differ significantly from what we have recorded in the current period and could materially affect our net earnings and net earnings per share of a future period.
ACQUISITIONS AND MINORITY INVESTMENT
Acquisitions:On November 18, 2010, we completed our acquisition of AGA Medical (NASDAQ: AGAM), acquiring all of its outstanding shares for $20.80 per share in a cash and stock transaction valued at $1.1 billion (which consisted of $549.4 million in net cash consideration and 13.6 million shares of St. Jude Medical common stock). The transaction was consummated through an exchange offer followed by a merger. The AGA Medical acquisition expands our current cardiovascular product portfolio and future product pipeline to treat structural heart defects and vascular abnormalities through minimally invasive transcatheter treatments. AGA Medical is based in Plymouth, Minnesota and has become part of our Cardiovascular division.
6
On July 6, 2010, we completed our acquisition of LightLab Imaging for $92.8 million in net cash consideration. LightLab Imaging is based in Westford, Massachusetts and develops, manufactures and markets OCT for coronary imaging applications. The LightLab Imaging acquisition expands our current product portfolio and complements the FFR technology acquired as part of our Radi Medical Systems acquisition in December 2008. LightLab Imaging has become part of our Cardiovascular division.
On December 22, 2008, we completed the acquisition of MediGuide, Inc. (MediGuide) for $285.2 million in net consideration. MediGuide was a development-stage company based in Haifa, Israel and was focused on developing its Medical Positioning System (gMPSTM) technology for localization and tracking capability for interventional medical devices. We plan to expend additional research and development efforts to achieve technological feasibility for this technology. MediGuide has become part of our Atrial Fibrillation division.
On December 19, 2008, we completed the acquisition of Radi Medical Systems for $248.9 million in net cash consideration, including direct acquisition costs. Radi Medical Systems is based in Uppsala, Sweden and develops, manufactures and markets products that provide precise measurements of intravascular pressure during a cardiovascular procedure and manual compression systems that arrest bleeding of the femoral and radial arteries following an intravascular medical device procedure. We acquired Radi Medical Systems to accelerate our cardiovascular growth platform in these two segments of the cardiovascular medical device market in which we previously had not participated. Radi Medical Systems has become part of our Cardiovascular division.
On July 3, 2008, we completed the acquisition of EP MedSystems, Inc. (EP MedSystems) for $95.7 million (consisting of $59.0 million in net cash consideration and direct acquisition costs and 0.9 million shares of St. Jude Medical common stock). EP MedSystems had been publicly traded on the NASDAQ Capital Market under the ticker symbol EPMD. EP MedSystems was based in West Berlin, New Jersey and developed, manufactured and marketed medical devices for the electrophysiology market which are used for visualization, diagnosis and treatment of heart rhythm disorders. We acquired EP MedSystems to strengthen our portfolio of products used to treat heart rhythm disorders. EP MedSystems has become part of our Atrial Fibrillation division.
Minority Investment:In September 2010, we made an equity investment of $60.0 million in CardioMEMS, Inc. (CardioMEMS), a privately-held company that is focused on the development of a wireless monitoring technology that can be placed directly into the pulmonary artery to assess cardiac performance via measurement of pulmonary artery pressure. The investment agreement resulted in a 19% ownership interest and provided us with the exclusive right, but not the obligation, to acquire CardioMEMS for an additional payment of $375 million during the period that extends through the completion of certain commercialization milestones.
SEGMENT PERFORMANCE
Our four operating segments are Cardiac Rhythm Management (CRM), Cardiovascular (CV), Atrial Fibrillation (AF), and Neuromodulation (NMD). The primary products produced by each operating segment are: CRM – ICDs and pacemakers; CV – vascular closure devices, heart valve replacement and repair products, pressure measurement guidewires, coronary imaging technology and structural heart defect and vascular abnormality devices; AF – electrophysiology introducers and catheters, advanced cardiac mapping, navigation and recording systems and ablation systems; and NMD – neurostimulation devices.
We aggregate our four operating segments into two reportable segments based upon their similar operational and economic characteristics: CRM/NMD and CV/AF. Net sales of our reportable segments include end-customer revenue from the sale of products they each develop and manufacture or distribute. The costs included in each of the reportable segments’ operating results include the direct costs of the products sold to customers and operating expenses managed by each of the reportable segments. Certain operating expenses managed by our selling and corporate functions, including all stock-based compensation expense, impairment charges, IPR&D charges and special charges have not been recorded in the individual reportable segments. As a result, reportable segment operating profit is not representative of the operating profit of the products in these reportable segments.
7
The following table presents net sales and operating profit by reportable segment (in thousands):
| | | | | | | | | | | | | |
| | CRM/NMD | | CV/AF | | Other | | Total | |
Fiscal Year 2010 | | | | | | | | | | | | | |
Net sales | | $ | 3,420,215 | | $ | 1,744,556 | | $ | — | | $ | 5,164,771 | |
Operating profit | | | 2,125,163 | | | 968,606 | | | (1,816,520 | ) | | 1,277,249 | |
Fiscal Year 2009 | | | | | | | | | | | | | |
Net sales | | $ | 3,099,800 | | $ | 1,581,473 | | $ | — | | $ | 4,681,273 | |
Operating profit | | | 1,931,929 | | | 829,966 | | | (1,648,849 | ) | | 1,113,046 | |
Fiscal Year 2008 | | | | | | | | | | | | | |
Net sales | | $ | 2,955,603 | | $ | 1,407,648 | | $ | — | | $ | 4,363,251 | |
Operating profit | | | 1,824,023 | | | 736,979 | | | (1,905,955 | ) | | 655,047 | |
The following discussion of the changes in our net sales is provided by class of similar products within our four operating segments, which is the primary focus of our sales activities.
| | | | | | | | | | | | | | | | |
Cardiac Rhythm Management | | | | | | | | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | | 2010 vs. 2009
% Change | | 2009 vs. 2008
% Change | |
ICD systems | | $ | 1,820,235 | | $ | 1,578,471 | | $ | 1,534,212 | | | 15.3 | % | | 2.9 | % |
Pacemaker systems | | | 1,219,718 | | | 1,190,563 | | | 1,167,251 | | | 2.4 | % | | 2.0 | % |
| | $ | 3,039,953 | | $ | 2,769,034 | | $ | 2,701,463 | | | 9.8 | % | | 2.5 | % |
Cardiac Rhythm Management 2010 net sales increased 10% to $3,039.9 million compared to 2009. CRM net sales growth was driven by operational net sales growth of 10%. Foreign currency translation had a $5.7 favorable impact on net sales during 2010 compared to 2009. ICD net sales in 2010 increased 15%, compared to the prior year, to $1,820.2 million due to operational net sales growth of 15%. The improved operational net sales growth was broad-based across both the U.S. and our international markets, reflecting our continued market penetration into new customer accounts and market demand for our cardiac resynchronization therapy ICD devices. During the second quarter of 2010, we launched a number of new ICD products, including the UnifyTM cardiac resynchronization therapy defibrillator (CRT-D) and FortifyTM ICD, which were both launched in the United States and European markets. The UnifyTM CRT-D and FortifyTM ICD are smaller, deliver more energy and have a longer battery life than comparable conventional devices. In the United States, 2010 ICD net sales of $1,137.3 million increased 14% compared to the prior year. The incremental benefit resulting from the suspension of U.S. ICD sales by a principal competitor in the CRM market during 2010 was approximately $35 million to $40 million. Internationally, 2010 ICD net sales of $682.9 million increased 18% compared to 2009 solely driven by operational net sales, as foreign currency translation had a minimal impact on international ICD net sales during 2010 compared to the prior year. Pacemaker systems 2010 net sales increased 2%, compared to the prior year, to $1,219.7 million due to operational net sales growth. In the United States, our 2010 pacemaker net sales of $525.4 million remained flat compared to 2009. Internationally, our 2010 pacemaker net sales of $694.3 million increased 3% compared to the prior year due to operational net sales growth of 2% and $7.1 million of favorable foreign currency translation compared to 2009.
Cardiac Rhythm Management 2009 net sales increased nearly 3% to $2,769.0 million compared to 2008. CRM net sales growth was driven by operational net sales growth of 5%, partially offset by unfavorable foreign currency translation comparisons of $64.0 million. 2009 ICD net sales increased 3%, compared to the prior year, to $1,578.5 million due to operational net sales growth of 5%, driven by our international markets. International ICD net sales in 2009 of $580.9 million increased 6% compared to 2008. Foreign currency translation had a $37.6 million unfavorable impact on international ICD net sales during 2009 compared to the prior year. 2009 U.S. ICD net sales of $997.6 million remained flat year over year. Pacemaker systems 2009 net sales increased 2%, compared to 2008, to $1,190.6 million, benefiting from operational net sales growth of 4%, due to our international growth. International pacemaker systems 2009 net sales increased 4% over 2008 to $671.6 million. Foreign currency translation had a $26.4 million unfavorable impact on international pacemaker net sales in 2009 compared to 2008. In the United States, pacemaker systems 2009 net sales of $518.9 million remained flat compared to 2008.
8
| | | | | | | | | | | | | | | | |
Cardiovascular | | | | | | | | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | | 2010 vs. 2009
% Change | | 2009 vs. 2008
% Change | |
Vascular closure devices | | $ | 374,769 | | $ | 380,965 | | $ | 367,893 | | | (1.6 | )% | | 3.6 | % |
Heart valve products | | | 337,455 | | | 323,202 | | | 321,534 | | | 4.4 | % | | 0.5 | % |
Other cardiovascular products | | | 324,459 | | | 249,453 | | | 172,709 | | | 30.1 | % | | 44.4 | % |
| | $ | 1,036,683 | | $ | 953,620 | | $ | 862,136 | | | 8.7 | % | | 10.6 | % |
Cardiovascular 2010 net sales increased 9% to $1,036.7 million compared to 2009 driven by $25.2 million of incremental net sales from our AGA Medical acquisition in November 2010. Total 2010 CV operational net sales growth was 3% over 2009. Our AGA Medical and LightLab Imaging acquisitions contributed to 5% of the net sales increase over the prior year. Foreign currency translation had a favorable impact on 2010 CV net sales of $11.4 million compared to 2009. Vascular closure device sales include sales of our Angio-Seal active closure devices and Radi Medical Systems’ compression assist products. Vascular closure devices’ 2010 net sales decreased 2% over 2009 due to a decline in operational net sales, with a minimal foreign currency translation impact year over year. 2010 heart valve net sales increased 4% compared to 2009, due to operational net sales growth of 3% and favorable foreign currency translation of $4.0 million. Other cardiovascular products 2010 net sales increased $75.0 million compared to 2009 due to our incremental net sales from our AGA Medical and LightLab Imaging acquisitions and favorable foreign currency translation impacts of $7.2 million.
Cardiovascular 2009 net sales increased 11% to $953.6 million compared to 2008 driven by incremental net sales of products from our Radi Medical Systems acquisition in December 2008. Our acquisitions contributed to 12% of the net sales increase over 2008. Foreign currency translation had an unfavorable impact on 2009 net sales of $18.6 million compared to 2008. Vascular closure devices’ 2009 net sales increased 4% compared to 2008 driven by incremental net sales of Radi Medical Systems’ compression assist products. Heart valve 2009 net sales remained flat year over year due to $8.8 million of unfavorable foreign currency translation, largely offsetting the 3% operational net sales increase. 2009 other cardiovascular products increased $76.7 million compared to 2008 due to incremental net sales of pressure measurement guidewires, a product line acquired from Radi Medical Systems.
| | | | | | | | | | | | | | | | |
Atrial Fibrillation | | | | | | | | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | | 2010 vs. 2009
% Change | | 2009 vs. 2008
% Change | |
Atrial fibrillation products | | $ | 707,873 | | $ | 627,853 | | $ | 545,512 | | | 12.7 | % | | 15.1 | % |
Atrial Fibrillation 2010 net sales increased 13% to $707.9 million compared to 2009 net sales. The increase in AF net sales was driven by operational net sales growth of 12%. Foreign currency translation had a favorable impact on AF net sales of $5.2 million compared to 2009. Our AF products include access, diagnosis, visualization, recording and ablation products that assist physicians in diagnosing and treating atrial fibrillation and other irregular heart rhythms.
Atrial Fibrillation 2009 net sales increased 15% to $627.9 million compared to 2008 net sales. The increase in AF net sales was driven by operational net sales growth of 14%. Our acquisitions contributed to 3% of the net sales increase over 2008. Foreign currency translation had an unfavorable impact on AF net sales of $12.8 million compared to 2008.
| | | | | | | | | | | | | | | | |
Neuromodulation | | | | | | | | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | | 2010 vs. 2009
% Change | | 2009 vs. 2008
% Change | |
Neurostimulation devices | | $ | 380,262 | | $ | 330,766 | | $ | 254,140 | | | 15.0 | % | | 30.2 | % |
Neuromodulation 2010 net sales increased 15% to $380.3 million compared to 2009 net sales. The increase in NMD net sales was driven solely by operational net sales growth of 15% due to continued market acceptance of our products and sales growth in our neurostimulation devices that help manage chronic pain. Foreign currency translation had a minimal impact on NMD 2010 net sales compared to 2009.
9
Neuromodulation 2009 net sales increased 30% to $330.8 million compared to 2008 net sales driven by operational net sales growth of 32%. Foreign currency translation had an unfavorable impact on NMD 2009 net sales of $4.1 million compared to 2008.
RESULTS OF OPERATIONS
Net Sales
| | | | | | | | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | | 2010 vs. 2009
% Change | | 2009 vs. 2008
% Change | |
Net sales | | $ | 5,164,771 | | $ | 4,681,273 | | $ | 4,363,251 | | | 10.3 | % | | 7.3 | % |
Overall, 2010 net sales increased 10% compared to 2009. Our total 2010 operational net sales growth increased 9% over 2009 operational net sales, led by sales growth of our ICDs and products to treat atrial fibrillation with total 2010 net sales volume growth of 11% being partially offset by global average selling price declines of 1%. Incremental sales from 2010 acquisitions accounted for 1% of the volume growth increase over 2009. Compared to 2009, foreign currency translation had a favorable impact on 2010 net sales of $23.3 million, due primarily to the weakening of the U.S. Dollar against the Yen and most other international currencies.
Total 2009 net sales increased 7% compared to 2008 driven by operational net sales growth of 7%, which was broad-based across all operating segments. Our total 2009 net sales volume growth of 12% was partially offset by global average selling price declines of 2%. Incremental sales from acquisitions accounted for 2% of the volume growth increase over 2009. Compared to 2008, foreign currency translation had an unfavorable impact on 2009 net sales of $99.5 million due primarily to the strengthening of the U.S. Dollar against the Euro.
Net sales by geographic location of the customer were as follows (in thousands):
| | | | | | | | | | |
Net Sales | | 2010 | | 2009 | | 2008 | |
United States | | $ | 2,655,034 | | $ | 2,468,191 | | $ | 2,319,645 | |
International | | | | | | | | | | |
Europe | | | 1,314,350 | | | 1,197,912 | | | 1,152,601 | |
Japan | | | 552,737 | | | 480,897 | | | 387,648 | |
Asia Pacific | | | 323,855 | | | 254,429 | | | 234,073 | |
Other (a) | | | 318,795 | | | 279,844 | | | 269,284 | |
| | | 2,509,737 | | | 2,213,082 | | | 2,043,606 | |
| | $ | 5,164,771 | | $ | 4,681,273 | | $ | 4,363,251 | |
| |
| (a) No one geographic market is greater than 5% of consolidated net sales. |
Foreign currency translation relating to our international operations can have a significant impact on our operating results from year to year. The two main currencies influencing our operating results are typically the Euro and the Japanese Yen. As discussed previously, foreign currency translation had a $23.3 million favorable impact on 2010 net sales, while the translation impact in 2009 had a $99.5 million unfavorable impact on net sales. These impacts to net sales are not indicative of the net earnings impact of foreign currency translation due to partially offsetting foreign currency translation impacts on cost of sales and operating expenses.
Gross Profit
| | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | |
Gross profit | | $ | 3,754,660 | | $ | 3,427,888 | | $ | 3,192,710 | |
Percentage of net sales | | | 72.7 | % | | 73.2 | % | | 73.2 | % |
10
Gross profit for 2010 totaled $3,754.7 million, or 72.7% of net sales, compared to $3,427.9 million, or 73.2% of net sales in 2009. Special charges in 2010 negatively impacted our gross profit by 0.5 percentage points due to inventory obsolescence charges primarily related to excess legacy ICD inventory that was not expected to be sold due to our recent launch of our UnifyTM CRT-D and FortifyTM ICD devices. Our market demand for these devices has resulted in a more rapid adoption than we expected or historically experienced. Additionally, generally accepted accounting principles requires inventory acquired in a business acquisition to be recorded at fair value, which closely approximates normal end-customer selling price. This resulted in higher cost of sales for AGA Medical and LightLab Imaging products sold in 2010, which negatively impacted our 2010 gross profit by approximately 0.2 percentage points. Special charges in 2009 negatively impacted our gross profit by approximately 0.7 percentage points related to inventory obsolescence charges for discontinued products, accelerated depreciation charges and write-offs for assets that will no longer be utilized and initiatives to streamline our production activities. The additional decrease in our gross profit percentage during 2010 compared to 2009 was primarily due to higher remote monitoring and wireless telemetry costs in our pacemaker product line. The unfavorable impacts on our gross profit percentage were partially offset by favorable foreign currency translation.
Gross profit for 2009 totaled $3,427.9 million, or 73.2% of net sales, compared to $3,192.7 million, or 73.2% of net sales in 2008. As discussed previously, 2009 special charges negatively impacted our gross profit by approximately 0.7 percentage points. 2008 special charges negatively impacted our gross profit by approximately 1.5 percentage points consisting primarily of charges related to the impairment of a technology license agreement, termination costs related to certain raw material purchase contracts, inventory obsolescence charges associated with a terminated distribution agreement and charges related to providing our new remote patient monitoring system to existing St. Jude Medical CRM patients at no charge. The remaining decrease in our 2009 gross profit percentage as a percent of net sales compared to 2008 was due to unfavorable foreign currency translation impacts, partially offset by productivity improvements.
Refer to Note 8 of the Consolidated Financial Statements for further details of the components of the special charges impacting our 2010, 2009 and 2008 gross profit.
Selling, General and Administrative (SG&A) Expense
| | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | |
Selling, general and administrative expense | | $ | 1,817,581 | | $ | 1,675,251 | | $ | 1,636,526 | |
Percentage of net sales | | | 35.2 | % | | 35.8 | % | | 37.5 | % |
SG&A expense for 2010 totaled $1,817.6 million, or 35.2% of net sales, compared with $1,675.3 million, or 35.8% of net sales in 2009. SG&A expense for 2010 decreased as a percent of net sales as a result of cost savings experienced from the restructuring activities initiated near the end of 2009. Refer to Note 8 of the Consolidated Financial Statements for further details of the 2009 special charges. These cost savings were partially offset by AGA Medical and LightLab Imaging acquisition closing costs and other associated costs, which negatively impacted our 2010 SG&A expense by 0.7 percentage points.
SG&A expense for 2009 totaled $1,675.3 million, or 35.8% of net sales, compared with $1,636.5 million, or 37.5% of net sales in 2008. SG&A expense for 2009 as a percent of net sales benefited from $26.0 million of lower discretionary company performance-based compensation costs. SG&A expense for 2008 as a percent of net sales was unfavorably impacted by 0.8 percentage points due to our $35.0 million contribution to non-profit organizations, including the St. Jude Medical Foundation.
Research and Development (R&D) Expense
| | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | |
Research and development expense | | $ | 631,086 | | $ | 559,766 | | $ | 531,799 | |
Percentage of net sales | | | 12.2 | % | | 12.0 | % | | 12.2 | % |
R&D expense in 2010 totaled $631.1 million, or 12.2% of net sales, compared with $559.8 million, or 12.0% of net sales in 2009 and $531.8 million, or 12.2% of net sales in 2008. While R&D expense as a percent of net sales has remained consistent from year to year, total R&D expense continues to increase each year, reflecting our continuing commitment to fund future long-term growth opportunities. We will continue to balance delivering short-term results with investments in long-term growth drivers.
11
Purchased In-Process Research and Development (IPR&D) Charges
| | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | |
Purchased in-process research and development charges | | $ | 12,244 | | $ | 5,842 | | $ | 319,354 | |
During 2010, we recorded IPR&D charges of $12.2 million in conjunction with the purchase of cardiovascular-related intellectual property. During 2009, we recorded IPR&D charges of $5.8 million in conjunction with the purchase of intellectual property in our CV and NMD segments. As the related technological feasibility had not yet been reached and such technology had no future alternative use, the purchases of these intellectual property assets were expensed as IPR&D.
In December 2008, we acquired MediGuide. As a development-stage company, the excess of the purchase price over the fair value of the net assets acquired was allocated on a pro-rata basis to the net assets acquired. Accordingly, the excess purchase price was allocated to IPR&D, the principal technology acquired. At the date of acquisition, $306.2 million of the purchase price was expensed as IPR&D since technological feasibility of the underlying projects had not yet been reached and such technology had no future alternative use.
In December 2008, we also made an additional minority investment in a development-stage company and in accordance with step-acquisition accounting treatment under the equity method of accounting, allocated the excess purchase price over the fair value of the investee’s net assets to IPR&D the principal technology acquired. At the December 2008 investment date, $11.6 million of IPR&D was expensed since technological feasibility of the underlying projects had not yet been reached and such technology had no future alternative use. Additionally, we recognized $1.6 million of IPR&D charges related to the purchase of regulatory pre-approved intellectual property in our CRM and CV segments.
Special Charges
| | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | |
Cost of sales special charges | | $ | 27,876 | | $ | 33,761 | | $ | 64,603 | |
Special charges | | | 16,500 | | | 73,983 | | | 49,984 | |
| | $ | 44,376 | | $ | 107,744 | | $ | 114,587 | |
Fiscal Year 2010
During 2010, we recorded $27.9 million of inventory obsolescence charges to cost of sales primarily related to excess legacy ICD inventory that was not expected to be sold due to our recent launch of our UnifyTM CRT-D and FortifyTM ICD devices. Our market demand for these devices has resulted in a more rapid adoption than we expected or historically experienced. In the U.S., these new devices have captured over 90% of our ICD product mix.
We also reached an agreement, without any admission of liability, to settle the previously disclosed Boston U.S. Department of Justice investigation initiated in 2005 related to an industry-wide review of post-market clinical studies and registries, resulting in a $16.5 million legal settlement charge. Refer to Note 5 of the Consolidated Financial Statements for further discussion of this legal matter.
Fiscal Year 2009
During 2009, we incurred charges totaling $107.7 million, of which $71.1 million related to severance and benefit costs for approximately 725 employees. These costs were recognized after our management determined that such severance and benefits were probable and estimable, in accordance with ASC Topic 712,Nonretirement Postemployment Benefits. Of the total $71.1 million severance and benefits charge, $6.6 million was recorded in cost of sales. We also recorded $17.7 million of inventory related charges to cost of sales associated with inventory that would be scrapped in connection with our decision to terminate certain product lines in our CRM and AF divisions that were redundant with other existing products lines. Additionally, we recorded $5.9 million of fixed asset related charges to cost of sales associated with the accelerated depreciation of phasing out older model diagnostic equipment and $6.1 million of asset write-offs related to the carrying value of assets that will no longer be utilized. Of the $6.1 million charge, $3.5 million was recorded in cost of sales. We also recorded charges of $1.8 million associated with contract terminations and $5.1 million of other unrelated costs.
12
Fiscal Year 2008
Impairment Charges: During 2008, we determined that technology under a license agreement covering certain CRM devices was no longer fully utilized and certain patents under the license were no longer valid based upon recent patent law developments. As a result, we recognized an impairment charge of $43.5 million to cost of sales to write our intangible asset for the technology license agreement down to its fair value. We also recognized a $37.0 million impairment charge to write down intangible assets relating to certain products lines acquired from Velocimed LLC to their fair value due to a reduction in future revenue and cash flow projections after termination of a clinical trial and unfavorable 2008 sales performance. We also recognized other impairment charges of $5.8 million related to assets in the Cardiovascular division that will no longer be utilized and discontinued the use of our Advanced Neuromodulation Systems, Inc. (ANS) tradename, resulting in a $1.7 million impairment charge to write-off of the ANS tradename intangible assets.
Inventory Charges: We enter into purchase contracts in the normal course of business for certain raw material commodities that are used in the manufacture of our products. Favorable decreases in commodity prices resulted in our election to terminate and exit some of our contracts resulting in a $10.7 million termination payment, which was recorded as a special charge in cost of sales. We also recognized additional inventory obsolescence charges related to inventory not expected to be sold due to the termination of a distribution agreement in Japan during 2007. We increased this estimate in 2008 and recorded an additional $3.0 million charge in cost of sales.
Other Charges: In 2008, we launched our MerlinTM@home wireless patient monitoring system and committed to provide this system without charge to existing St. Jude Medical CRM patients. In connection with the completion of this roll-out in the fourth quarter of 2008, we recorded a $7.4 million special charge in cost of sales to accrue for the related costs. We also recognized $5.5 million of other unrelated costs.
Other Income (Expense)
| | | | | | | | | | |
(in thousands) | | 2010 | | 2009 | | 2008 | |
Interest income | | $ | 2,076 | | $ | 2,057 | | $ | 16,315 | |
Interest expense | | | (67,372 | ) | | (45,603 | ) | | (72,554 | ) |
Other | | | (3,150 | ) | | (12,107 | ) | | (18,040 | ) |
Total other income (expense), net | | $ | (68,446 | ) | $ | (55,653 | ) | $ | (74,279 | ) |
The unfavorable change in other income (expense) during 2010 compared to 2009 was primarily the result of higher average interest rates and higher average outstanding debt balances (approximately $2.0 billion in 2010 and $1.5 billion in 2009). The partially offsetting change in other income (expense) during 2010 compared to 2009 was due to an $8.3 million investment impairment charge recognized in other expense during 2009 upon determining that the fair value of a cost method investment was below its carrying value and that the impairment was other-than-temporary. During 2010, we further determined that this cost method investment was fully impaired and recognized a $5.2 million investment impairment charge in other expense. The 2010 impairment charge was partially offset by a $4.9 million pre-tax realized gain associated with the sale of an available-for-sale common stock investment.
The favorable change in other income (expense) during 2009 compared to 2008 was primarily driven by lower average outstanding debt balances in 2009, resulting in less interest expense. Partially offsetting the favorable change in other income (expense) during 2009 was the recognition of our $8.3 million investment impairment charge to other expense discussed previously. During 2008, we also recognized $12.9 million of pre-tax impairment charges to other expense related to a decline in the fair values of certain investments that were deemed to be other-than-temporary.
Income Taxes
| | | | | | | | | | |
(as a percent of pre-tax income) | | 2010 | | 2009 | | 2008 | |
Effective tax rate | | | 24.9 | % | | 26.5 | % | | 39.2 | % |
13
Our effective tax rate differs from our U.S. federal statutory 35% tax rate due to certain operations that are subject to foreign taxes that are different from the U.S. federal statutory rate, state and local taxes and tax incentives. Our effective tax rate is also impacted by discrete factors or events such as IPR&D charges, special charges, impairment charges or the resolution of audits by tax authorities.
Our effective tax rate was 24.9% in 2010 compared to 26.5% in 2009 and 39.2% in 2008. Non-deductible IPR&D charges and legal settlement special charges unfavorably impacted the 2010 effective tax rate by 0.4 percentage points. Special charges, deductible IPR&D charges and an investment impairment charge favorably impacted the 2009 effective tax rate by 0.4 percentage points. In 2008, non-deductible IPR&D charges, special charges and investment impairment charges unfavorably impacted the 2008 effective tax rate by 12.2 percentage points. Refer toAcquisitions and Minority Investment, Purchased In-Process Research and Development (IPR&D) Charges,Special Charges andOther Income (Expense) sections for further details regarding these charges.
Net Earnings
| | | | | | | | | | | | | | | | |
(in thousands, except per share amounts) | | 2010 | | 2009 | | 2008 | | 2010 vs. 2009%
Change | | 2009 vs. 2008%
Change | |
Net earnings | | $ | 907,436 | | $ | 777,226 | | $ | 353,018 | | | 16.8 | % | | 120.2 | % |
Diluted net earnings per share | | $ | 2.75 | | $ | 2.26 | | $ | 1.01 | | | 21.7 | % | | 123.8 | % |
Our 2010 net earnings of $907.4 million and diluted net earnings per share of $2.75 increased compared to 2009 net earnings of $777.2 million and diluted net earnings per share of $2.26. These increases were due to incremental profits resulting from higher 2010 net sales, primarily driven by our ICDs and products to treat atrial fibrillation as well as lower outstanding shares in 2010 resulting from repurchases of our common stock. During 2010 we returned an additional $625.3 million to shareholders in the form of share repurchases. Net earnings for 2010 included after-tax special charges of $32.8 million, after-tax IPR&D charges of $12.2 million and an after-tax investment impairment charge of $5.2 million for a combined impact of $50.2 million, or $0.15 per diluted share. Net earnings for 2009 included after-tax special charges of $76.4 million, an after-tax investment impairment charge of $5.2 million and after-tax IPR&D charges of $3.7 million for a combined impact of $85.3 million, or $0.25 per diluted share.
Net earnings were $777.2 million in 2009, a 120.2% increase over 2008 net earnings of $353.0 million. Diluted net earnings per share were $2.26 in 2009, a 123.8% increase over 2008 diluted net earnings per share of $1.01. Compared to 2008, our net earnings and diluted net earnings per share benefited from continued net sales growth in all of our operating segments as well as lower outstanding shares in 2009 as a result of repurchases of our common stock. During 2009 we returned an additional $1.0 billion to shareholders in the form of share repurchases. Net earnings for 2009 included after-tax special charges of $76.4 million, an after-tax investment impairment charge of $5.2 million and after-tax IPR&D charges of $3.7 million for a combined impact of $85.3 million, or $0.25 per diluted share. Net earnings for 2008 included IPR&D charges of $319.4 million, after-tax special charges of $72.7 million and after-tax investment impairment charges of $8.0 million for a combined impact of $400.1 million, or $1.15 per diluted share.
LIQUIDITY
We believe that our existing cash balances, future cash generated from operations and available borrowing capacity under our $1.5 billion long-term committed credit facility (Credit Facility) and related commercial paper program, will be sufficient to fund our operating needs, working capital requirements, R&D opportunities, capital expenditures, debt service requirements and dividends (seeDividends section) over the next twelve months and in the foreseeable future thereafter. We do not have any significant debt maturities until 2013. The majority of our outstanding January 1, 2011 debt portfolio matures after January 14, 2016.
We believe that our earnings, cash flows and balance sheet position will permit us to obtain additional debt financing or equity capital should suitable investment and growth opportunities arise. Our credit ratings are investment grade. We monitor capital markets regularly and may raise additional capital when market conditions or interest rate environments are favorable.
At January 1, 2011, a portion of our cash and cash equivalents was held by our non-U.S. subsidiaries. These funds are only available for use by our U.S. operations if they are repatriated into the United States. The funds repatriated would be subject to additional U.S. taxes upon repatriation; however, it is not practical to estimate the amount of additional U.S. tax liabilities we would incur. We currently have no plans to repatriate funds held by our non-U.S. subsidiaries.
14
We use two primary measures that focus on accounts receivable and inventory – days sales outstanding (DSO) and days inventory on hand (DIOH). We use DSO as a measure that places emphasis on how quickly we collect our accounts receivable balances from customers. We use DIOH, which can also be expressed as a measure of the estimated number of days of cost of sales on hand, as a measure that places emphasis on how efficiently we are managing our inventory levels. These measures may not be computed the same as similarly titled measures used by other companies. Our DSO (ending net accounts receivable divided by average daily sales for the most recently completed quarter) increased from 89 days at January 2, 2010 to 90 days at January 1, 2011. Our DIOH (ending net inventory divided by average daily cost of sales for the most recently completed six months) decreased from 184 days at January 2, 2010 to 163 days at January 1, 2011. Special charges recognized in cost of sales in the second half of 2010 reduced our January 1, 2011 DIOH by 7 days; however, the impact of our acquisitions in the second half of 2010 offset the special charge impact, increasing our DIOH by 7 days. Special charges recognized in cost of sales in the second half of 2009 reduced our January 2, 2010 DIOH by 10 days.
A summary of our cash flows from operating, investing and financing activities is provided in the following table (in thousands):
| | | | | | | | | | |
| | | 2010 | | 2009 | | 2008 | |
Net cash provided by (used in): | | | | | | | | | | |
Operating activities | | $ | 1,274,372 | | $ | 868,875 | | $ | 945,592 | |
Investing activities | | | (1,080,384 | ) | | (490,585 | ) | | (871,073 | ) |
Financing activities | | | (86,553 | ) | | (130,696 | ) | | (322,493 | ) |
Effect of currency exchange rate changes on cash and cash equivalents | | | (26 | ) | | 8,890 | | | (4,677 | ) |
Net increase (decrease) in cash and cash equivalents | | $ | 107,409 | | $ | 256,484 | | $ | (252,651 | ) |
Cash Flows from Operating Activities
Cash provided by operating activities was $1,274.4 million for 2010 compared to $868.9 million for 2009 and $945.6 million for 2008. Operating cash flows can fluctuate significantly from period to period due to payment timing differences of working capital accounts such as accounts receivable, accounts payable, accrued liabilities and income taxes payable.
Cash Flows from Investing Activities
Cash used in investing activities was $1,080.4 million in 2010 compared to $490.6 million in 2009 and $871.1 million in 2008. Our purchases of property, plant and equipment, which totaled $304.9 million, $326.4 million and $343.9 million in 2010, 2009, and 2008, respectively, reflect our continued investment in our product growth platforms currently in place. During 2010, we acquired LightLab Imaging for $92.8 million in net cash consideration and AGA Medical for $549.4 million in net cash consideration and 13.6 million shares of St. Jude Medical common stock. We also made an equity investment of $60.0 million in CardioMEMS resulting in a 19% ownership interest. During 2009, we made a second scheduled acquisition payment of $113.8 million for MediGuide. During 2008, we spent $490.0 million of net cash consideration on acquisitions, with Radi Medical Systems, MediGuide and EP MedSystems being the most significant.
Cash Flows from Financing Activities
Cash used in financing activities was $86.6 million in 2010 compared to $130.7 million in 2009 and $322.5 million in 2008. Our financing cash flows can fluctuate significantly depending upon our liquidity needs and the amount of stock option exercises and the extent of our common stock repurchases. During 2010, we received net proceeds of $950.0 million principal amount of senior notes in the United States and 20.9 billion Yen senior notes in Japan. We used the proceeds to repay our 1.02% Yen-denominated notes due in May 2010 (1.02% Yen Notes) totaling 20.9 billion Yen and retire a 3-year unsecured term loan totaling $432.0 million. Additionally, we repurchased $625.3 million of our common stock, which was financed with the senior notes issued during 2010 and cash generated from operations.
15
During 2009, we issued $1.2 billion of senior notes, made borrowings of $180.0 million under a 3-year unsecured term loan and repaid all of our commercial paper borrowings (net $19.4 million) and outstanding borrowings of $500.0 million under our $1.0 billion long-term committed Credit Facility. Additionally, we repurchased $1.0 billion of our common stock, which was financed with both proceeds from the issuance of our senior notes and cash generated from operations. In December 2009, we voluntarily repaid 1.5 billion Japanese Yen under a 3-year unsecured Japan term loan (Japan Term Loan) totaling 8.0 billion Japanese Yen, resulting in an outstanding balance of 6.5 billion Japanese Yen at January 2, 2010 (the equivalent of $70.7 million at January 2, 2010).
During 2008, we borrowed $500.0 million from our $1.0 billion long-term committed Credit Facility to fund the repayment of our $1.2 billion 1.22% Convertible Senior Debentures. Additionally, we entered into a 3-year, unsecured term loan totaling $360.0 million and a Japan Term Loan totaling 8.0 billion Japanese Yen, which was the equivalent of $88.2 million at January 3, 2009. During 2008, we also used our outstanding cash balances to repurchase $300.0 million of our common stock.
DEBT AND CREDIT FACILITIES
Total debt increased to $2,511.6 million at January 1, 2011 from $1,922.4 million at January 2, 2010. During 2010, we issued approximately $1.2 billion of long-term debt consisting of 3-year and 5-year senior notes in the U.S. and 7-year and 10-year Yen denominated notes in Japan. We also repaid approximately $0.9 billion of debt consisting of a term loan and maturing Yen-denominated notes in Japan. The majority of our long-term debt maturities are after January 14, 2016 and our weighted average interest rate on outstanding long-term debt, inclusive of interest rate swaps, was 2.5% at January 1, 2011 compared to 3.7% at January 2, 2010.
In December 2010, we entered into a $1.5 billion unsecured committed Credit Facility used to support our commercial paper program and for general corporate purposes. The Credit Facility expires on February 28, 2015. Borrowings under this facility bear interest initially at LIBOR plus 0.875%, subject to adjustment in the event of a change in our credit ratings. Commitment fees under this facility are not material. The Credit Facility replaces our previous $1.0 billion credit facility that was scheduled to expire in December 2011. There were no outstanding borrowings under either credit facility as of January 1, 2011 or January 2, 2010.
Our commercial paper program provides for the issuance of short-term, unsecured commercial paper with maturities up to 270 days. We began issuing commercial paper during November 2010 and had an outstanding commercial paper balance of $25.5 million as of January 1, 2011. During 2010, our weighted average effective interest rate on our outstanding commercial paper borrowings was 0.27%. As of January 2, 2010, we had no outstanding commercial paper borrowings. Any future commercial paper borrowings would bear interest at the applicable then-current market rates. Our predominant historical practice has been to issue commercial paper (up to the amount backed by available borrowings capacity under the Credit Facility), as our commercial paper has historically been issued at lower interest rates.
In March 2010, we issued $450.0 million principal amount of 3-year, 2.20% unsecured senior notes (2013 Senior Notes). We used $432.0 million of the net proceeds to repay our 3-year unsecured term loan. Interest payments on the 2013 Senior Notes are required on a semi-annual basis. We may redeem the 2013 Senior Notes at any time at the applicable redemption price. The 2013 Senior Notes are senior unsecured obligations and rank equally with all of our existing and future senior unsecured indebtedness.
Concurrent with the issuance of the 2013 Senior Notes, we entered into a 3-year, $450.0 million notional amount interest rate swap designated as a fair value hedge of the changes in fair value of our fixed-rate 2013 Senior Notes. On November 8, 2010, we terminated the interest rate swap and received a cash payment of $19.3 million. The gain from terminating the interest rate swap agreement is being amortized as a reduction of interest expense over the remaining life of the 2013 Senior Notes.
In July 2009, we issued $700.0 million aggregate principal amount of 5-year, 3.75% Senior Notes (2014 Senior Notes) and $500.0 million aggregate principal amount of 10-year, 4.875% Senior Notes (2019 Senior Notes). In August 2009, we used $500.0 million of the net proceeds from the 2014 Senior Notes and 2019 Senior Notes to repay all amounts outstanding under our credit facility. We may redeem the 2014 Senior Notes or 2019 Senior Notes at any time at the applicable redemption prices. Both the 2014 Senior Notes and 2019 Senior Notes are senior unsecured obligations and rank equally with all of our existing and future senior unsecured indebtedness.
16
In December 2010, we issued our $500.0 million principal amount 5-year, 2.50% unsecured senior notes (2016 Senior Notes). The majority of the net proceeds from the issuance of the 2016 Senior Notes were used for general corporate purposes including the repurchase of our common stock. Interest payments are required on a semi-annual basis. We may redeem the 2016 Senior Notes at any time at the applicable redemption price. The 2016 Senior Notes are senior unsecured obligations and rank equally with all of our existing and future senior unsecured indebtedness.
Concurrent with the issuance of the 2016 Senior Notes, we entered into a 5-year, $500.0 million notional amount interest rate swap designated as a fair value hedge of the changes in fair value of our fixed-rate 2016 Senior Notes. As of January 1, 2011, the fair value of the swap was a $10.0 million liability which was classified as other liabilities on the consolidated balance sheet, with a corresponding adjustment to the carrying value of the 2016 Senior Notes. Refer to Note 13 of the Consolidated Financial Statements for additional information regarding the interest rate swap.
In April 2010, we issued 10-year, 2.04% unsecured senior notes in Japan (2.04% Yen Notes) totaling 12.8 billion Yen (the equivalent of $156.3 million at January 1, 2011) and 7-year, 1.58% unsecured senior notes in Japan (1.58% Yen Notes) totaling 8.1 billion Yen (the equivalent of $99.7 million at January 1, 2011). We used the net proceeds from these issuances to repay our 1.02% Yen Notes totaling 20.9 billion Yen. Interest payments on the 2.04% Yen Notes and 1.58% Yen Notes are required on a semi-annual basis and the principal amounts recorded on the balance sheet fluctuate based on the effects of foreign currency translation.
In December 2008, we entered into a Japan Term Loan. As of January 1, 2011, 6.5 billion Japanese Yen was outstanding (the equivalent of $79.6 million at January 1, 2011 and $70.7 million at January 2, 2010). We can initiate future borrowings up to the 8.0 billion Japan Term Loan amount. The borrowings bear interest at the Yen LIBOR plus 0.4%. Interest payments are required on a semi-annual basis and the entire principal balance is due in December 2011. The principal amount reflected on the consolidated balance sheet fluctuates based on the effects of foreign currency translation.
Our Credit Facility and Yen Notes contain certain operating and financial covenants. Specifically, the Credit Facility requires that we have a leverage ratio (defined as the ratio of total debt to EBITDA (net earnings before interest, income taxes, depreciation and amortization)) not exceeding 3.0 to 1.0. The Yen Notes require that we have a ratio of total debt to total capitalization not exceeding 60% and a ratio of consolidated EBIT (net earnings before interest and income taxes) to consolidated interest expense of at least 3.0 to 1.0. Under the Credit Facility, our senior notes and Yen Notes we also have certain limitations on how we conduct our business, including limitations on additional liens or indebtedness and limitations on certain acquisitions, mergers, investments and dispositions of assets. We were in compliance with all of our debt covenants as of January 1, 2011.
SHARE REPURCHASES
On October 15, 2010, our Board of Directors authorized a share repurchase program of up to $600.0 million of our outstanding common stock. On October 21, 2010, our Board of Directors authorized an additional $300.0 million of share repurchases as part of this share repurchase program. Through January 1, 2011, we had repurchased 15.4 million shares for $625.3 million at an average repurchase price of $40.63 per share. We continued repurchasing shares in 2011 and completed the repurchases under the program on January 20, 2011, repurchasing a program total of 22.0 million shares for $900.0 million at an average repurchase price of $40.87 per share.
In October 2009, our Board of Directors authorized a share repurchase program of up to $500.0 million of our outstanding common stock. We completed the repurchases under the program in December 2009, repurchasing 14.1 million shares for $500.0 million at an average repurchase price of $35.44 per share. In July 2009, our Board of Directors authorized a share repurchase program of up to $500.0 million of our outstanding common stock. We completed the repurchases under the program in September 2009, repurchasing 13.0 million shares for $500.0 million at an average repurchase price of $38.32 per share. For fiscal year 2009, we repurchased a total of 27.1 million shares for $1.0 billion at an average repurchase price of $36.83 per share.
In February 2008, our Board of Directors authorized a share repurchase program of up to $250.0 million of our outstanding common stock and in April 2008, our Board of Directors authorized an additional $50.0 million of share repurchases as part of this share repurchase program. We completed the repurchases under the program in May 2008. In total, we repurchased 6.7 million shares for $300.0 million at an average repurchase price of $44.51 per share.
17
DIVIDENDS
We did not declare or pay any cash dividends during 2010, 2009 or 2008. On February 26, 2011, our Board of Directors authorized a cash dividend of $0.21 per share payable on April 29, 2011 to holders of record as of March 31, 2011. We expect to pay quarterly cash dividends in the foreseeable future.
OFF-BALANCE SHEET ARRANGEMENTS AND CONTRACTUAL OBLIGATIONS
We believe that our off-balance sheet arrangements do not have a material current or anticipated future effect on our consolidated earnings, financial position or cash flows. Our off-balance sheet arrangements principally consist of operating leases for various facilities and equipment, purchase commitments and contingent acquisition commitments.
In the normal course of business, we periodically enter into agreements that require us to indemnify customers or suppliers for specific risks, such as claims for injury or property damage arising out of our products or the negligence of our personnel or claims alleging that our products infringe third-party patents or other intellectual property. In addition, under our bylaws and indemnification agreements we have entered into with our executive officers and directors, we may be required to indemnify our executive officers and directors for losses arising from their conduct in an official capacity on behalf of St. Jude Medical. We may also be required to indemnify officers and directors of certain companies that we have acquired for losses arising from their conduct on behalf of their companies prior to the closing of our acquisition. Our maximum exposure under these indemnification obligations cannot be estimated, and we have not accrued any liabilities within our consolidated financial statements or included any indemnification provisions in our commitments table. Historically, we have not experienced significant losses on these types of indemnification obligations.
In addition to the amounts shown in the following table, our noncurrent liability for unrecognized tax benefits was $162.9 million as of January 1, 2011, and we are uncertain as to if or when such amounts may be settled. Related to these unrecognized tax benefits, our liability for potential penalties and interest was $33.8 million as of January 1, 2011.
A summary of contractual obligations and other minimum commercial commitments as of January 1, 2011 is as follows (in thousands):
| | | | | | | | | | | | | | | | |
| | Payments Due by Period | |
| | | | | | | | | | | | | | | | |
| | Total | | Less than
1 Year | | 1-3
Years | | 3-5
Years | | More than
5 Years | |
Contractual obligations related to | | | | | | | | | | | | | | | | |
off-balance sheet arrangements: | | | | | | | | | | | | | | | | |
Operating leases | | $ | 150,541 | | $ | 41,046 | | $ | 62,379 | | $ | 38,729 | | $ | 8,387 | |
Purchase commitments (a) | | | 417,137 | | | 401,772 | | | 11,266 | | | 4,099 | | | — | |
Contingent consideration payments (b) | | | 38,472 | | | 26,877 | | | 9,709 | | | 830 | | | 1,056 | |
Total | | $ | 606,150 | | $ | 469,695 | | $ | 83,354 | | $ | 43,658 | | $ | 9,443 | |
| | | | | | | | | | | | | | | | |
Contractual obligations reflected | | | | | | | | | | | | | | | | |
in the balance sheet: | | | | | | | | | | | | | | | | |
Debt obligations (c) | | | 2,905,971 | | | 140,877 | | | 575,345 | | | 792,164 | | | 1,397,585 | |
Total | | $ | 3,512,121 | | $ | 610,572 | | $ | 658,699 | | $ | 835,822 | | $ | 1,407,028 | |
| | |
| (a) | These amounts include commitments for inventory purchases and capital expenditures that do not exceed our projected requirements and are in the normal course of business. The purchase commitment amounts do not represent the entire anticipated purchases and capital expenditures in the future, but only those for which we are contractually obligated. |
18
| | |
| (b) | These amounts include contingent commitments to acquire various businesses involved in the distribution of our products and other contingent acquisition consideration payments. In connection with certain acquisitions, we may agree to provide additional consideration payments upon the achievement of certain product development milestones, which may include but are not limited to: successful levels of achievement in clinical trials and certain product regulatory approvals. We may also provide for additional consideration payments to be made upon the achievement of certain levels of future product sales. While it is not certain if and/or when these payments will be made, we have included the payments in the table based on our best estimates of the dates when we expect the milestones and/or contingencies will be met. |
| (c) | Includes current debt obligations, scheduled maturities of long-term debt and scheduled interest payments. See Note 4 to the Consolidated Financial Statements for additional information on our debt obligations. |
MARKET RISK
We are exposed to foreign currency exchange rate fluctuations due to transactions denominated primarily in Euros, Japanese Yen, Canadian Dollars, Australian Dollars, Brazilian Reals, British Pounds, and Swedish Kronor. When the U.S. Dollar weakens against foreign currencies, the dollar value of sales denominated in foreign currencies increases. When the U.S. Dollar strengthens against foreign currencies, the dollar value of sales denominated in foreign currencies decreases. A hypothetical 10% change in the value of the U.S. Dollar in relation to our most significant foreign currency exposures would have had an impact of approximately $235.2 million on our 2010 net sales. This amount is not indicative of the hypothetical net earnings impact due to partially offsetting impacts on the related cost of sales and operating expenses in the applicable foreign currencies.
During 2010 and 2009, we hedged a portion of our foreign currency exchange rate risk through the use of forward exchange contracts. We use forward exchange contracts to manage foreign currency exposures related to intercompany receivables and payables arising from intercompany purchases of manufactured products. These forward contracts are not designated as qualifying hedging relationships under ASC Topic 815,Derivatives and Hedging (ASC Topic 815). We measure our foreign currency exchange rate contracts at fair value on a recurring basis. The fair value of all outstanding contracts was immaterial at both January 1, 2011 and January 2, 2010. During 2010 and 2009, we recorded a $0.2 million net loss and $6.7 million net loss, respectively, to other income (expense) for our forward currency exchange contracts not designated as hedging instruments under ASC Topic 815. The net losses were almost entirely offset by corresponding net gains on the foreign currency exposures being managed. We do not enter into contracts for trading or speculative purposes. Our policy is to enter into hedging contracts with major financial institutions that have at least an “A” (or equivalent) credit rating. Although we are exposed to credit loss in the event of nonperformance by counterparties on our outstanding derivative contracts, we do not anticipate nonperformance by any of the counterparties. We did not enter into any forward exchange contracts during 2008. We continue to evaluate our foreign currency exchange rate risk and the different mechanisms for use in managing such risk, including using derivative financial instruments and operational hedges, such as international manufacturing operations. Our derivative financial instruments accounting policy is discussed in detail in Note 1 to the Consolidated Financial Statements.
Although we have not entered into any derivative hedging contracts to hedge the net asset exposure of our foreign subsidiaries, we have elected to use natural hedging strategies in certain geographies. We have naturally hedged a portion of our Yen-denominated net asset exposure by issuing long-term Yen-denominated debt.
We are also exposed to fair value risk on our Senior Notes and Yen Notes. As of January 1, 2011, the aggregate fair value of our Senior Notes (measured using quoted prices in active markets) was $2,478.9 million compared to the aggregate carrying value of $2,406.5 million (inclusive of the interest rate swaps). Our 2014 Senior Notes have a fixed interest rate of 3.75%, our 2019 Senior Notes have a fixed rate of interest of 4.875%, our 2016 Senior Notes have a fixed rate of interest of 2.50% and our 2013 Senior Notes have a fixed rate of interest of 2.20%. A hypothetical one-percentage point change in the interest rates would have an aggregate impact of approximately $97 million on the fair value of our Senior Notes. As of January 1, 2011, the fair value of our yen-denominated notes (2.04% Yen Notes and 1.58% Yen Notes), both of which have a fixed interest rate, approximated their carrying value. A hypothetical one-percentage point change in the interest rates would have an aggregate impact of approximately $20 million on the fair value of the yen-denominated notes.
Our variable-rate debt consists of loans in the United States and Japan. Assuming average outstanding borrowings of $2.0 billion during 2010, a hypothetical one-percentage point change in the interest rates would have an impact of approximately $20.0 million on our 2011 interest expense.
19
We are also exposed to equity market risk on our marketable equity security investments. We hold certain marketable equity securities of publically-traded companies. Our investments in these companies had a fair value of $33.7 million at January 1, 2011, which are subject to the underlying price risk of the public equity markets.
CAUTIONARY STATEMENTS
In this discussion and in other written or oral statements made from time to time, we have included and may include statements that constitute “forward-looking statements” with respect to the financial condition, results of operations, plans, objectives, new products, future performance and business of St. Jude Medical, Inc. and its subsidiaries. Statements preceded by, followed by or that include words such as “may,” “will,” “expect,” “anticipate,” “continue,” “estimate,” “forecast”, “project,” “believe” or similar expressions are intended to identify some of the forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are included, along with this statement, for purposes of complying with the safe harbor provisions of that Act. These forward-looking statements involve risks and uncertainties. By identifying these statements for you in this manner, we are alerting you to the possibility that actual results may differ, possibly materially, from the results indicated by these forward-looking statements. We undertake no obligation to update any forward-looking statements. Actual results may differ materially from those contemplated by the forward-looking statements due to, among others, the risks and uncertainties discussed in the previous section entitledOff-Balance Sheet Arrangements and Contractual Obligations, Market Riskand Competition and Other Considerations and in Part I, Item 1A, Risk Factors of our Annual Report on Form 10-K as well as the various factors described below. Since it is not possible to foresee all such factors, you should not consider these factors to be a complete list of all risks or uncertainties. We believe the most significant factors that could affect our future operations and results are set forth in the list below.
| | |
| 1. | Any legislative or administrative reform to the U.S. Medicare or Medicaid systems or international reimbursement systems that significantly reduces reimbursement for procedures using our medical devices or denies coverage for such procedures, as well as adverse decisions relating to our products by administrators of such systems on coverage or reimbursement issues. |
| 2. | Assertion, acquisition or grant of key patents by or to others that have the effect of excluding us from market segments or requiring us to pay royalties. |
| 3. | Economic factors, including inflation, contraction in capital markets, changes in interest rates, changes in tax laws and changes in foreign currency exchange rates. |
| 4. | Product introductions by competitors that have advanced technology, better features or lower pricing. |
| 5. | Price increases by suppliers of key components, some of which are sole-sourced. |
| 6. | A reduction in the number of procedures using our devices caused by cost-containment pressures or the development of or preferences for alternative therapies. |
| 7. | Safety, performance or efficacy concerns about our products, many of which are expected to be implanted for many years, some of which may lead to recalls and/or advisories with the attendant expenses and declining sales. |
| 8. | Declining industry-wide sales caused by product quality issues or recalls or advisories by our competitors that result in loss of physician and/or patient confidence in the safety, performance or efficacy of sophisticated medical devices in general and/or the types of medical devices recalled in particular. |
| 9. | Changes in laws, regulations or administrative practices affecting government regulation of our products, such as FDA regulations, including those that decrease the probability or increase the time and/or expense of obtaining approval for products or impose additional burdens on the manufacture and sale of medical devices. |
| 10. | Regulatory actions arising from concern over Bovine Spongiform Encephalopathy, sometimes referred to as “mad cow disease,” that have the effect of limiting our ability to market products using bovine collagen, such as Angio-Seal™, or products using bovine pericardial material, such as our Biocor®, Epic™ and Trifecta™ tissue heart valves, or that impose added costs on the procurement of bovine collagen or bovine pericardial material. |
| 11. | The intent and ability of our product liability insurers to meet their obligations to us, including losses related to our Silzone® litigation, and our ability to fund future product liability losses related to claims made subsequent to becoming self-insured. |
| 12. | Severe weather or other natural disasters that cause damage to the facilities of our critical suppliers or one or more of our facilities, such as an earthquake affecting our facilities in California or a hurricane affecting our facilities in Puerto Rico. |
| 13. | Healthcare industry changes leading to demands for price concessions and/or limitations on, or the elimination of, our ability to sell in significant market segments. |
20
| | |
| 14. | Adverse developments in investigations and governmental proceedings. |
| 15. | Adverse developments in litigation, including product liability litigation, patent or other intellectual property litigation, qui tam litigation or shareholder litigation. |
| 16. | Inability to successfully integrate the businesses that we have acquired in recent years and that we plan to acquire. |
| 17. | Failure to successfully complete or unfavorable data from clinical trials for our products or new indications for our products and/or failure to successfully develop markets for such new indications. |
| 18. | Changes in accounting rules that adversely affect the characterization of our results of operations, financial position or cash flows. |
| 19. | The disruptions in the financial markets and the economic downturn that adversely impact the availability and cost of credit and customer purchasing and payment patterns. |
| 20. | Conditions imposed in resolving, or any inability to timely resolve, any regulatory issues raised by the FDA, including Form 483 observations or warning letters, as well as risks generally associated with our regulatory compliance and quality systems. |
| 21. | Governmental legislation, including the recently enacted Patient Protection and Affordable Care Act and the Health Care and Educational Reconciliation Act, and/or regulation that significantly impacts the healthcare system in the United States and that results in lower reimbursement for procedures using our products, reduces medical procedure volumes or otherwise adversely affects our business and results of operations, including the recently enacted medical device excise tax. |
21
Report of Management
Management’s Report on the Financial Statements
We are responsible for the preparation, integrity and objectivity of the accompanying financial statements. The financial statements were prepared in accordance with accounting principles generally accepted in the United States and include amounts which reflect management’s best estimates based on its informed judgment and consideration given to materiality. We are also responsible for the accuracy of the related data in the annual report and its consistency with the financial statements.
Audit Committee Oversight
The adequacy of our internal accounting controls, the accounting principles employed in our financial reporting and the scope of independent and internal audits are reviewed by the Audit Committee of the Board of Directors, consisting solely of independent directors. The independent registered public accounting firm meets with, and has confidential access to, the Audit Committee to discuss the results of its audit work.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Under the supervision and with the participation of the Company’s management, including the CEO and the CFO, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework inInternal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, the CEO and CFO concluded that our internal control over financial reporting was effective as of January 1, 2011. Ernst & Young LLP, our independent registered public accounting firm, has also audited the effectiveness of the Company’s internal control over financial reporting as of January 1, 2011 as stated in its report which is included herein.
/s/ Daniel J. Starks
Daniel J. Starks
Chairman, President and Chief Executive Officer
/s/ John C. Heinmiller
John C. Heinmiller
Executive Vice President and Chief Financial Officer
22
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
of St. Jude Medical, Inc.
We have audited St. Jude Medical, Inc.’s internal control over financial reporting as of January 1, 2011, based on criteria established inInternal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). St. Jude Medical, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying report of management titled Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on St. Jude Medical, Inc.’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that: (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, St. Jude Medical, Inc. maintained, in all material respects, effective internal control over financial reporting as of January 1, 2011, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of St. Jude Medical, Inc. as of January 1, 2011 and January 2, 2010, and the related consolidated statements of earnings, shareholders’ equity, and cash flows for each of the three fiscal years in the period ended January 1, 2011, and our report dated March 2, 2011, expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Minneapolis, Minnesota
March 2, 2011
23
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
of St. Jude Medical, Inc.
We have audited the accompanying consolidated balance sheets of St. Jude Medical, Inc. as of January 1, 2011 and January 2, 2010, and the related consolidated statements of earnings, shareholders’ equity, and cash flows for each of the three fiscal years in the period ended January 1, 2011. These financial statements are the responsibility of St. Jude Medical, Inc.’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of St. Jude Medical, Inc. at January 1, 2011 and January 2, 2010, and the consolidated results of its operations and its cash flows for each of the three fiscal years in the period ended January 1, 2011, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), St. Jude Medical Inc.’s internal control over financial reporting as of January 1, 2011, based on criteria established inInternal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated March 2, 2011, expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Minneapolis, Minnesota
March 2, 2011
24
CONSOLIDATED STATEMENTS OF EARNINGS
(in thousands, except per share amounts)
| | | | | | | | | | |
Fiscal Year Ended | | January 1, 2011 | | January 2, 2010 | | January 3, 2009 | |
Net sales | | $ | 5,164,771 | | $ | 4,681,273 | | $ | 4,363,251 | |
Cost of sales: | | | | | | | | | | |
Cost of sales before special charges | | | 1,382,235 | | | 1,219,624 | | | 1,105,938 | |
Special charges | | | 27,876 | | | 33,761 | | | 64,603 | |
Total cost of sales | | | 1,410,111 | | | 1,253,385 | | | 1,170,541 | |
Gross profit | | | 3,754,660 | | | 3,427,888 | | | 3,192,710 | |
Selling, general and administrative expense | | | 1,817,581 | | | 1,675,251 | | | 1,636,526 | |
Research and development expense | | | 631,086 | | | 559,766 | | | 531,799 | |
Purchased in-process research and development charges | | | 12,244 | | | 5,842 | | | 319,354 | |
Special charges | | | 16,500 | | | 73,983 | | | 49,984 | |
Operating profit | | | 1,277,249 | | | 1,113,046 | | | 655,047 | |
Other income (expense), net | | | (68,446 | ) | | (55,653 | ) | | (74,279 | ) |
Earnings before income taxes | | | 1,208,803 | | | 1,057,393 | | | 580,768 | |
Income tax expense | | | 301,367 | | | 280,167 | | | 227,750 | |
Net earnings | | $ | 907,436 | | $ | 777,226 | | $ | 353,018 | |
| | | | | | | | | | |
Net earnings per share: | | | | | | | | | | |
Basic | | $ | 2.76 | | $ | 2.28 | | $ | 1.03 | |
Diluted | | $ | 2.75 | | $ | 2.26 | | $ | 1.01 | |
Weighted average shares outstanding: | | | | | | | | | | |
Basic | | | 328,191 | | | 340,880 | | | 342,888 | |
Diluted | | | 330,488 | | | 344,359 | | | 349,722 | |
See notes to the consolidated financial statements.
25
CONSOLIDATED BALANCE SHEETS
(in thousands, except share amounts)
| | | | | | | |
| | January 1, 2011 | | January 2, 2010 | |
ASSETS | | | | | | | |
Current Assets | | | | | | | |
Cash and cash equivalents | | $ | 500,336 | | $ | 392,927 | |
Accounts receivable, less allowances for doubtful accounts | | | 1,331,210 | | | 1,170,579 | |
Inventories | | | 667,545 | | | 659,960 | |
Deferred income taxes, net | | | 196,599 | | | 164,738 | |
Other current assets | | | 216,458 | | | 172,002 | |
Total current assets | | | 2,912,148 | | | 2,560,206 | |
Property, Plant and Equipment | | | | | | | |
Land, buildings and improvements | | | 493,992 | | | 424,310 | |
Machinery and equipment | | | 1,377,768 | | | 1,188,614 | |
Diagnostic equipment | | | 352,589 | | | 336,492 | |
Property, plant and equipment at cost | | | 2,224,349 | | | 1,949,416 | |
Less accumulated depreciation | | | (900,418 | ) | | (796,330 | ) |
Net property, plant and equipment | | | 1,323,931 | | | 1,153,086 | |
Goodwill | | | 2,955,602 | | | 2,005,851 | |
Other intangible assets, net | | | 987,060 | | | 456,142 | |
Other assets | | | 387,707 | | | 250,526 | |
TOTAL ASSETS | | $ | 8,566,448 | | $ | 6,425,811 | |
| | | | | | | |
LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | |
Current Liabilities | | | | | | | |
Current debt obligations | | $ | 79,637 | | $ | 334,787 | |
Accounts payable | | | 297,551 | | | 132,543 | |
Income taxes payable | | | — | | | 13,498 | |
Accrued expenses | | | | | | | |
Employee compensation and related benefits | | | 320,323 | | | 269,293 | |
Other | | | 319,739 | | | 317,192 | |
Total current liabilities | | | 1,017,250 | | | 1,067,313 | |
Long-term debt | | | 2,431,966 | | | 1,587,615 | |
Deferred income taxes, net | | | 310,503 | | | 132,392 | |
Other liabilities | | | 435,058 | | | 314,940 | |
Total liabilities | | | 4,194,777 | | | 3,102,260 | |
Commitments and Contingencies (Note 5) | | | — | | | — | |
Shareholders’ Equity | | | | | | | |
Preferred stock | | | — | | | — | |
Common stock (329,018,166 and 324,537,581 shares issued and outstanding at January 1, 2011 and January 2, 2010, respectively) | | | 32,902 | | | 32,454 | |
Additional paid-in capital | | | 156,126 | | | 5,860 | |
Retained earnings | | | 4,098,639 | | | 3,191,203 | |
Accumulated other comprehensive income: | | | | | | | |
Cumulative translation adjustment | | | 68,897 | | | 82,033 | |
Unrealized gain on available-for-sale securities | | | 15,107 | | | 12,001 | |
Total shareholders’ equity | | | 4,371,671 | | | 3,323,551 | |
TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | | $ | 8,566,448 | | $ | 6,425,811 | |
See notes to the consolidated financial statements.
26
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Additional
Paid-In
Capital | | Retained
Earnings | | Accumulated Other
Comprehensive
Income (Loss) | | Total
Shareholders’
Equity | |
Number of
Shares | | Amount | |
Balance at December 29, 2007 | | | 342,846,963 | | $ | 34,285 | | $ | 193,662 | | $ | 2,632,214 | | $ | 99,158 | | $ | 2,959,319 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | |
Net earnings | | | | | | | | | | | | 353,018 | | | | | | 353,018 | |
Other comprehensive loss: | | | | | | | | | | | | | | | | | | | |
Unrealized loss on available-for-sale securities, net of taxes of $(3,675) | | | | | | | | | | | | | | | (6,268 | ) | | (6,268 | ) |
Unrealized loss on derivative financial instruments, net of taxes of $(247) | | | | | | | | | | | | | | | (411 | ) | | (411 | ) |
Foreign currency translation adjustment, net of taxes of $(4,281) | | | | | | | | | | | | | | | (87,777 | ) | | (87,777 | ) |
Other comprehensive loss | | | | | | | | | | | | | | | | | | (94,456 | ) |
Comprehensive income | | | | | | | | | | | | | | | | | | 258,562 | |
Repurchases of common stock | | | (6,736,888 | ) | | (674 | ) | | (291,724 | ) | | (7,602 | ) | | | | | (300,000 | ) |
Stock-based compensation | | | | | | | | | 52,935 | | | | | | | | | 52,935 | |
Common stock issued under stock plans and other, net | | | 8,319,532 | | | 832 | | | 165,182 | | | | | | | | | 166,014 | |
Common stock issued in connection with acquisition | | | 902,665 | | | 90 | | | 36,621 | | | | | | | | | 36,711 | |
Tax benefit from stock plans | | | | | | | | | 62,365 | | | | | | | | | 62,365 | |
Balance at January 3, 2009 | | | 345,332,272 | | $ | 34,533 | | $ | 219,041 | | $ | 2,977,630 | | $ | 4,702 | | $ | 3,235,906 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | |
Net earnings | | | | | | | | | | | | 777,226 | | | | | | 777,226 | |
Other comprehensive gain: | | | | | | | | | | | | | | | | | | | |
Unrealized gain on available-for-sale securities, net of taxes of $3,369 | | | | | | | | | | | | | | | 5,865 | | | 5,865 | |
Reclassification of realized loss on derivative financial instruments to net earnings, net of taxes of $247 | | | | | | | | | | | | | | | 411 | | | 411 | |
Foreign currency translation adjustment, net of taxes of $(173) | | | | | | | | | | | | | | | 83,056 | | | 83,056 | |
Other comprehensive income | | | | | | | | | | | | | | | | | | 89,332 | |
Comprehensive income | | | | | | | | | | | | | | | | | | 866,558 | |
Repurchases of common stock | | | (27,154,078 | ) | | (2,715 | ) | | (433,632 | ) | | (563,653 | ) | | | | | (1,000,000 | ) |
Stock-based compensation | | | | | | | | | 59,795 | | | | | | | | | 59,795 | |
Common stock issued under stock plans and other, net | | | 6,359,387 | | | 636 | | | 125,620 | | | | | | | | | 126,256 | |
Tax benefit from stock plans | | | | | | | | | 35,036 | | | | | | | | | 35,036 | |
Balance at January 2, 2010 | | | 324,537,581 | | $ | 32,454 | | $ | 5,860 | | $ | 3,191,203 | | $ | 94,034 | | $ | 3,323,551 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | |
Net earnings | | | | | | | | | | | | 907,436 | | | | | | 907,436 | |
Other comprehensive gain: | | | | | | | | | | | | | | | | | | | |
Unrealized gain on available-for-sale securities, net of taxes of $1,893 | | | | | | | | | | | | | | | 6,187 | | | 6,187 | |
Reclassification of realized gain on available-for-sale securities, net of taxes of $1,848 | | | | | | | | | | | | | | | (3,081 | ) | | (3,081 | ) |
Foreign currency translation adjustment, net of taxes of $314 | | | | | | | | | | | | | | | (13,136 | ) | | (13,136 | ) |
Other comprehensive loss | | | | | | | | | | | | | | | | | | (10,030 | ) |
Comprehensive income | | | | | | | | | | | | | | | | | | 897,406 | |
Repurchases of common stock | | | (15,388,500 | ) | | (1,539 | ) | | (623,712 | ) | | | | | | | | (625,251 | ) |
Stock-based compensation | | | | | | | | | 69,586 | | | | | | | | | 69,586 | |
Common stock issued under stock plans and other, net | | | 6,293,732 | | | 629 | | | 151,144 | | | | | | | | | 151,773 | |
Common stock issued in connection with acquisition | | | 13,575,353 | | | 1,358 | | | 532,289 | | | | | | | | | 533,647 | |
Tax benefit from stock plans | | | | | | | | | 20,959 | | | | | | | | | 20,959 | |
Balance at January 1, 2011 | | | 329,018,166 | | $ | 32,902 | | $ | 156,126 | | $ | 4,098,639 | | $ | 84,004 | | $ | 4,371,671 | |
See notes to the consolidated financial statements
27
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | |
Fiscal Year Ended | | January 1, 2011 | | January 2, 2010 | | January 3, 2009 | |
OPERATING ACTIVITIES | | | | | | | | | | |
Net earnings | | $ | 907,436 | | $ | 777,226 | | $ | 353,018 | |
Adjustments to reconcile net earnings to net cash from operating activities: | | | | | | | | | | |
Depreciation and amortization | | | 244,015 | | | 213,465 | | | 202,428 | |
Amortization of debt discount | | | 1,262 | | | 370 | | | 49,973 | |
Inventory step-up amortization | | | 8,797 | | | — | | | — | |
Stock-based compensation | | | 69,586 | | | 59,795 | | | 52,935 | |
Excess tax benefits from stock-based compensation | | | (16,635 | ) | | (26,373 | ) | | (48,995 | ) |
Investment impairment charges | | | 5,222 | | | 8,300 | | | 12,902 | |
Gain on sale of investment | | | (4,929 | ) | | — | | | — | |
Purchased in-process research and development charges | | | 12,244 | | | 5,842 | | | 319,354 | |
Deferred income taxes | | | (33,629 | ) | | (14,058 | ) | | (50,362 | ) |
Other, net | | | 17,446 | | | 11,982 | | | 87,833 | |
Changes in operating assets and liabilities, net of business acquisitions: | | | | | | | | | | |
Accounts receivable | | | (123,300 | ) | | (39,090 | ) | | (92,301 | ) |
Inventories | | | 42,318 | | | (104,463 | ) | | (73,763 | ) |
Other current assets | | | (30,921 | ) | | 10,303 | | | (19,996 | ) |
Accounts payable and accrued expenses | | | 163,564 | | | (65,100 | ) | | 68,366 | |
Income taxes payable | | | 11,896 | | | 30,676 | | | 84,200 | |
Net cash provided by operating activities | | | 1,274,372 | | | 868,875 | | | 945,592 | |
INVESTING ACTIVITIES | | | | | | | | | | |
Purchases of property, plant and equipment | | | (304,901 | ) | | (326,408 | ) | | (343,912 | ) |
Business acquisition payments, net of cash acquired | | | (679,022 | ) | | (129,507 | ) | | (490,027 | ) |
Proceeds from sale of investments | | | 8,429 | | | — | | | — | |
Other investing activities, net | | | (104,890 | ) | | (34,670 | ) | | (37,134 | ) |
Net cash used in investing activities | | | (1,080,384 | ) | | (490,585 | ) | | (871,073 | ) |
FINANCING ACTIVITIES | | | | | | | | | | |
Proceeds from exercise of stock options and stock issued | | | 151,773 | | | 126,256 | | | 166,014 | |
Excess tax benefits from stock-based compensation | | | 16,635 | | | 26,373 | | | 48,995 | |
Common stock repurchased, including related costs | | | (590,793 | ) | | (1,000,000 | ) | | (300,000 | ) |
Borrowings under debt facilities | | | 1,188,518 | | | 11,151,754 | | | 967,622 | |
Payments under debt facilities | | | (852,686 | ) | | (10,435,079 | ) | | — | |
Repayment of convertible debentures | | | — | | | — | | | (1,205,124 | ) |
Net cash used in financing activities | | | (86,553 | ) | | (130,696 | ) | | (322,493 | ) |
Effect of currency exchange rate changes on cash and cash equivalents: | | | (26 | ) | | 8,890 | | | (4,677 | ) |
Net increase (decrease) in cash and cash equivalents | | | 107,409 | | | 256,484 | | | (252,651 | ) |
Cash and cash equivalents at beginning of year | | | 392,927 | | | 136,443 | | | 389,094 | |
Cash and cash equivalents at end of year | | $ | 500,336 | | $ | 392,927 | | $ | 136,443 | |
| | | | | | | | | | |
Supplemental Cash Flow Information | | | | | | | | | | |
Cash paid during the year for: | | | | | | | | | | |
Income taxes | | $ | 308,062 | | $ | 225,062 | | $ | 211,860 | |
Interest | | $ | 62,875 | | $ | 24,549 | | $ | 21,712 | |
Noncash investing activities: | | | | | | | | | | |
Issuance of stock in connection with acquisitions | | $ | 533,647 | | $ | — | | $ | 36,711 | |
See notes to the consolidated financial statements.
28
Notes to the Consolidated Financial Statements
NOTE 1 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Company Overview: St. Jude Medical, Inc., together with its subsidiaries (St. Jude Medical or the Company) develops, manufactures and distributes cardiovascular medical devices for the global cardiac rhythm management, cardiology, cardiac surgery and atrial fibrillation therapy areas and implantable neurostimulation devices for the management of chronic pain. The Company’s four operating segments are Cardiac Rhythm Management (CRM), Cardiovascular (CV), Atrial Fibrillation (AF) and Neuromodulation (NMD). The Company’s principal products in each operating segment are as follows: CRM – tachycardia implantable cardioverter defibrillator systems (ICDs) and bradycardia pacemaker systems (pacemakers); CV – vascular closure devices, heart valve replacement and repair products, pressure measurement guidewires, coronary imaging technology and structural heart defect and vascular abnormality devices; AF – electrophysiology (EP) introducers and catheters, advanced cardiac mapping, navigation and recording systems and ablation systems; and NMD – neurostimulation devices. The Company markets and sells its products primarily through a direct sales force. The principal geographic markets for the Company’s products are the United States, Europe, Japan and Asia Pacific.
Principles of Consolidation: The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. Intercompany transactions and balances have been eliminated in consolidation.
Fiscal Year: The Company utilizes a 52/53-week fiscal year ending on the Saturday nearest December 31st. Fiscal year 2010 and 2009 consisted of 52 weeks and ended on January 1, 2011 and January 2, 2010, respectively. Fiscal year 2008 consisted of 53 weeks and ended on January 3, 2009, with the additional week reflected in the Company’s fourth quarter 2008 results.
Use of Estimates: Preparation of the Company’s consolidated financial statements in conformity with accounting principles generally accepted in the United States (U.S. GAAP) requires management to make estimates and assumptions that affect the reported amounts in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
Cash Equivalents: The Company considers highly liquid investments with an original maturity of three months or less to be cash equivalents. Cash equivalents are stated at cost, which approximates fair value. The Company’s cash equivalents include bank certificates of deposit, money market funds and instruments and commercial paper investments. The Company performs periodic evaluations of the relative credit standing of the financial institutions and issuers of its cash equivalents and limits the amount of credit exposure with any one issuer.
Marketable Securities: Marketable securities consist of publicly-traded equity securities that are classified as available-for-sale securities and investments in mutual funds that are classified as trading securities. On the balance sheet, available-for-sale securities and trading securities are classified as other current assets and other assets, respectively.
The following table summarizes the components of the balance of the Company’s available-for-sale securities at January 1, 2011 and January 2, 2010 (in thousands):
| | | | | | | |
| | | January 1, 2011 | | | January 2, 2010 | |
Adjusted cost | | $ | 9,116 | | $ | 12,122 | |
Gross unrealized gains | | | 24,988 | | | 19,797 | |
Gross unrealized losses | | | (359 | ) | | (208 | ) |
Fair value | | $ | 33,745 | | $ | 31,711 | |
29
Available-for-sale securities are recorded at fair value based upon quoted market prices (see Note 12). Unrealized gains and losses, net of related incomes taxes, are recorded in accumulated other comprehensive income in shareholders’ equity. Upon the sale of an available-for-sale security, the unrealized gain (loss) is reclassified out of accumulated other comprehensive income and reflected as a realized gain (loss) in net earnings. Realized gains (losses) are computed using the specific identification method and recognized as other income (expense). During 2010, the Company sold an available-for-sale security, recognizing a realized after-tax gain of $3.1 million. The total pre-tax gain of $4.9 million was recognized as other income (see Note 9). There were no realized gains (losses) from the sale of available-for-sale securities recorded during fiscal years 2009 or 2008. Additionally, when the fair value of an available-for-sale security falls below its original cost and the Company determines that the corresponding unrealized loss is other-than-temporary, the Company recognizes an impairment loss to net earnings in the period the determination is made. In 2008, the Company recognized a pre-tax impairment charge of $0.7 million in other expense related to a decline in the fair value of an available-for-sale security that was deemed other-than-temporary. No available-for-sale security impairment losses were recognized during fiscal years 2010 or 2009.
The Company’s investments in mutual funds are recorded at fair market value based upon quoted market prices (see Note 12) and are held in a rabbi trust, which is not available for general corporate purposes and is subject to creditor claims in the event of insolvency. These investments are specifically designated as available to the Company solely for the purpose of paying benefits under the Company’s deferred compensation plan (see Note 11).
Accounts Receivable: The Company grants credit to customers in the normal course of business, but generally does not require collateral or any other security to support its receivables. The Company maintains an allowance for doubtful accounts for potential credit losses. The allowance for doubtful accounts was $35.4 million and $34.9 million at January 1, 2011 and January 2, 2010, respectively.
Inventories: Inventories are stated at the lower of cost or market with cost determined using the first-in, first-out method. Inventories consisted of the following (in thousands):
| | | | | | | |
| | | January 1, 2011 | | | January 2, 2010 | |
Finished goods | | $ | 466,191 | | $ | 460,600 | |
Work in process | | | 62,607 | | | 60,702 | |
Raw materials | | | 138,747 | | | 138,658 | |
| | $ | 667,545 | | $ | 659,960 | |
Property, Plant and Equipment: Property, plant and equipment are recorded at cost and are depreciated using the straight-line method over their estimated useful lives, ranging from 15 to 39 years for buildings and improvements, three to seven years for machinery and equipment and three to five years for diagnostic equipment. Diagnostic equipment primarily consists of programmers that are used by physicians and healthcare professionals to program and analyze data from ICDs and pacemakers. The estimated useful lives of this equipment are based on anticipated usage by physicians and healthcare professionals and the timing and impact of expected new technology platforms and rollouts by the Company. Property, plant and equipment are depreciated using accelerated methods for income tax purposes.
Goodwill: Goodwill represents the excess of cost over the fair value of identifiable net assets of a business acquired. Goodwill for each reporting unit is reviewed for impairment at least annually. The Company has four reporting units as of January 1, 2011, consisting of its four operating segments (see Note 14). The Company tests goodwill for impairment using a two-step process. In the first step, the Company compares the fair value of each reporting unit, as computed primarily by present value cash flow calculations, to its book carrying value, including goodwill. If the carrying value exceeds the fair value, the goodwill of the reporting unit is potentially impaired and the Company would complete step 2 in order to measure the potential impairment loss. In step 2, the Company calculates the implied fair value of goodwill by deducting the fair value of all tangible and intangible net assets (including unrecognized intangible assets) of the reporting unit from the fair value of the reporting unit (as determined in step 1). If the implied fair value of goodwill is less than the carrying value of goodwill, the Company would recognize an impairment loss equal to the difference. During the fourth quarters of 2010, 2009 and 2008, the Company completed its annual goodwill impairment test and identified no impairment associated with the carrying values of goodwill.
30
Other Intangible Assets: Other intangible assets consist of purchased technology and patents, in-process research and development (IPR&D) acquired in a business acquisition, customer lists and relationships, trademarks and tradenames, licenses and distribution agreements. Definite-lived intangible assets are amortized on a straight-line basis over the estimated useful life ranging from 3 to 20 years. Certain trademark assets are considered indefinite-lived intangible assets and are not amortized.
The Company’s policy defines IPR&D as the value of technology acquired for which the related products have not yet reached technological feasibility and have no future alternative use. The primary basis for determining the technological feasibility of these projects is obtaining regulatory approval to market the underlying products in an applicable geographic region. Prior to 2009, the Company expensed the value attributed to any IPR&D acquired in a business acquisition. Effective in fiscal year 2009, all IPR&D acquired in a business acquisition is subject to ASC Topic 805, Business Combinations, which requires the fair value of IPR&D to be capitalized as an indefinite-lived intangible asset until completion of the IPR&D project or abandonment. Upon completion of the development project (generally when regulatory approval to market the product is obtained), the IPR&D is amortized over its estimated useful life. If the IPR&D projects are abandoned, the related IPR&D assets would likely be impaired and written down to fair value. The Company’s 2009 adoption of ASC Topic 805 did not change the Company’s accounting policy with respect to asset purchases. In many cases, the purchase of certain intellectual property assets or the rights to such intellectual property is considered a purchase of assets rather than the acquisition of a business. Accordingly, rather than being capitalized, any IPR&D acquired in such asset purchases is expensed.
The Company also reviews other intangible assets for impairment at least annually to determine if any adverse conditions exist that would indicate impairment. If the carrying value of other intangible assets exceeds the related undiscounted future cash flows, the carrying value is written down to fair value in the period identified. In assessing fair value, the Company generally utilizes present value cash flow calculations using an appropriate risk-adjusted discount rate. In 2008, the Company recorded a $37.0 million impairment charge to write down purchased technology intangible assets associated with its 2005 Velocimed LLC (Velocimed) acquisition and a $1.7 million impairment charge to write off Advanced Neuromodulation Systems, Inc. (ANS) tradename intangible assets. There was no impairment of intangible assets during fiscal year 2010 or 2009. Refer to Note 8 for further detail regarding these impairment charges.
Product Warranties: The Company offers a warranty on various products; the most significant of which relate to pacemaker and ICD systems. The Company estimates the costs that may be incurred under its warranties and records a liability in the amount of such costs at the time the product is sold. Factors that affect the Company’s warranty liability include the number of units sold, historical and anticipated rates of warranty claims and cost per claim. The Company periodically assesses the adequacy of its recorded warranty liabilities and adjusts the amounts as necessary.
Changes in the Company’s product warranty liability during fiscal years 2010 and 2009 were as follows (in thousands):
| | | | | | | |
| | 2010 | | 2009 | |
Balance at beginning of year | | $ | 19,911 | | $ | 15,724 | |
Warranty expense recognized | | | 7,442 | | | 6,627 | |
Warranty credits issued | | | (2,226 | ) | | (2,440 | ) |
Balance at end of year | | $ | 25,127 | | $ | 19,911 | |
Product Liability: As a result of higher costs and increasing coverage limitations, effective June 16, 2009, the Company ceased purchasing product liability insurance. Based on historical loss trends, the Company accrues for product liability claims through its self-insurance program in effort to adequately cover future losses. Additionally, the Company accrues for product liability claims when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated. Receivables for insurance recoveries from prior product liability insurance coverage are recorded when it is probable that a recovery will be realized.
31
Litigation: The Company accrues a liability for costs related to claims, including future legal costs, settlements and judgments where it has assessed that a loss is probable and an amount can be reasonably estimated.
Revenue Recognition: The Company sells its products to hospitals primarily through a direct sales force. In certain international markets, the Company sells its products through independent distributors. The Company recognizes revenue when persuasive evidence of a sales arrangement exists, delivery of goods occurs through the transfer of title and risks and rewards of ownership, the selling price is fixed or determinable and collectability is reasonably assured. A portion of the Company’s inventory is held by field sales representatives or consigned at hospitals. Revenue is recognized at the time the Company is notified that the inventory has been implanted or used by the customer. For products that are not consigned, revenue recognition occurs upon shipment to the hospital or, in the case of distributors, when title transfers under the contract. The Company offers sales rebates and discounts to certain customers. The Company records such rebates and discounts as a reduction of net sales in the same period revenue is recognized. The Company estimates rebates based on sales terms and historical experience.
Research and Development: Research and development costs are expensed as incurred. Research and development costs include product development costs, pre-approval regulatory costs and clinical research expenses.
Stock-Based Compensation: The Company accounts for stock-based compensation in accordance with ASC Topic 718,Compensation – Stock Compensation(ASC Topic 718). Under the fair value recognition provisions of ASC Topic 718, the Company measures stock-based compensation cost at the grant date fair value and recognizes the compensation expense over the requisite service period, which is the vesting period, using a straight-line attribution method.
The amount of stock-based compensation expense recognized during a period is based on the portion of the awards that are ultimately expected to vest. The Company estimates pre-vesting award forfeitures at the time of grant by analyzing historical data and revises those estimates in subsequent periods if actual forfeitures differ from those estimates. Ultimately, the total expense recognized over the vesting period will only be for those awards that vest. The Company’s awards are not eligible to vest early in the event of retirement, however, the majority of the Company’s awards vest early in the event of a change in control.
Net Earnings Per Share: Basic net earnings per share is computed by dividing net earnings by the weighted average number of outstanding common shares during the period, exclusive of restricted stock awards. Diluted net earnings per share is computed by dividing net earnings by the weighted average number of outstanding common shares and dilutive securities.
32
The following table sets forth the computation of basic and diluted net earnings per share for fiscal years 2010, 2009, and 2008 (in thousands, except per share amounts):
| | | | | | | | | | |
| | 2010 | | 2009 | | 2008 | |
Numerator: | | | | | | | | | | |
Net earnings | | $ | 907,436 | | $ | 777,226 | | $ | 353,018 | |
| | | | | | | | | | |
Denominator: | | | | | | | | | | |
Basic weighted average shares outstanding | | | 328,191 | | | 340,880 | | | 342,888 | |
Dilutive options and restricted stock | | | 2,297 | | | 3,479 | | | 6,834 | |
Diluted weighted average shares outstanding | | | 330,488 | | | 344,359 | | | 349,722 | |
Basic net earnings per share | | $ | 2.76 | | $ | 2.28 | | $ | 1.03 | |
Diluted net earnings per share | | $ | 2.75 | | $ | 2.26 | | $ | 1.01 | |
| | | | | | | | | | |
Approximately 18.3 million, 22.8 million and 15.0 million shares of common stock subject to employee stock options, restricted stock awards and restricted stock units were excluded from the diluted net earnings per share computation because they were not dilutive during fiscal years 2010, 2009 and 2008, respectively.
Foreign Currency Translation: Sales and expenses denominated in foreign currencies are translated at average exchange rates in effect throughout the year. Assets and liabilities of foreign operations are translated at period-end exchange rates. Gains and losses from translation of net assets of foreign operations, net of related income taxes, are recorded in accumulated other comprehensive income (loss). Foreign currency transaction gains and losses are included in other income (expense).
Derivative Financial Instruments: The Company follows the provisions of ASC Topic 815,Derivatives and Hedging (ASC Topic 815) to account for its derivative instruments and hedging activities. ASC Topic 815 requires all derivative financial instruments to be recognized on the balance sheet at fair value. Changes in the fair value of derivatives are recognized in net earnings or other comprehensive income depending on whether the derivative is designated as part of a qualifying hedging transaction.
The Company uses forward contracts to manage foreign currency exposures primarily related to intercompany receivables and payables arising from intercompany purchases of manufactured products. These forward contracts are not designated as qualifying hedges and therefore, the changes in the fair values of these derivatives are recognized in net earnings and classified in other income (expense). The gains and losses on these forward contracts largely offset the losses or gains on the foreign currency exposures being managed.
The Company has periodically entered into interest rate swap contracts to hedge the risk to net earnings associated with movements in interest rates by converting variable-rate borrowings into fixed-rate borrowings. As designated cash flow hedges, the fair value of the swap contract is recorded to other current assets, other assets, other accrued expenses or other liabilities, as applicable, with the related unrealized gain (loss) recorded to other comprehensive income. Payments made or received under the swap contract are recorded to interest expense. The Company has also periodically entered into interest rate swap contracts to hedge the risk of the change in the fair value of fixed-rate borrowings due to changes in the benchmark interest rate. As designated fair value hedges, the change in the fair value of the interest rate swap is recorded to interest expense and the equivalent amount is reflected as a change in the carrying value of the fixed-rate borrowings, offsetting interest expense.
New Accounting Pronouncements: The Company adopted new accounting standards in fiscal year 2010, the impacts of which have been reflected in the 2010 consolidated financial statements and historical consolidated financial statements, as applicable.
33
In October 2009, the Financial Accounting Standards Board (FASB) updated the revenue recognition accounting guidance of FASB Accounting Standards Codification (ASC) Topic 605,Revenue Recognition, relating to the accounting for revenue arrangements that involve more than one deliverable or unit of accounting. The updated guidance allows companies to allocate arrangement consideration in multiple deliverable arrangements in a manner that better reflects the economics of the transaction by revising certain thresholds for separation, and providing criteria for allocation of revenue among deliverables. The FASB also updated the scope of the revenue recognition accounting guidance of FASB ASC Topic 985,Software, removing both non-software components and certain software components of tangible products from the scope of existing software revenue guidance, resulting in the recognition of revenue similar to that for other tangible products. The updated accounting guidance was effective for annual periods beginning after June 15, 2010. Early adoption was permitted and may be prospective or retrospective. In the first quarter of 2010, the Company elected to adopt both accounting guidance updates prospectively, effective January 3, 2010. The Company’s adoption did not have a material impact during 2010.
In December 2009, the FASB issued Accounting Standards Update (ASU) 2009-17,Consolidations (ASC Topic 810): Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities. ASU 2009-17 requires a qualitative approach to identifying a controlling financial interest in a variable interest entity (VIE), and requires ongoing assessment of whether an entity is a VIE and whether an interest in a VIE makes the holder the primary beneficiary of the VIE. The adoption of ASU 2009-17 in January 2010 did not have a material impact on the Company’s consolidated financial statements.
In January 2010, the FASB issued ASU 2010-6,Fair Value Measurements and Disclosures (ASC Topic 820): Improving Disclosures about Fair Value Measurements, which requires reporting entities to make new disclosures about recurring or nonrecurring fair value measurements including (i) significant transfers into and out of Level 1 and Level 2 fair value measurements and (ii) information on purchases, sales, issuances and settlements on a gross basis in the reconciliation of Level 3 fair value measurements. ASU 2010-6 was effective for interim and annual reporting periods beginning after December 15, 2009, except for Level 3 reconciliation disclosures which are effective for interim and annual periods beginning after December 15, 2010. The Company adopted the additional disclosures required for Level 1 and Level 2 fair value measurements in the first quarter of 2010 (see Note 13). The Company adopted Level 3 disclosures beginning in the first quarter of 2011.
NOTE 2 – ACQUISITIONS AND MINORITY INVESTMENT
The Company made acquisitions during 2010, 2009 and 2008; the more significant acquisitions are described below. The results of operations of businesses acquired have been included in the Company’s consolidated results of operations since the dates of acquisition. Pro forma results of operations have not been presented for these acquisitions since the effects of these business acquisitions were not material to the Company either individually or in the aggregate.
Fiscal Year 2010
LightLab Imaging, Inc: On July 6, 2010, the Company completed its acquisition of LightLab Imaging, Inc. (LightLab Imaging) for $92.8 million in net cash consideration. The Company recorded direct transaction costs of $1.4 million. LightLab Imaging is based in Westford, Massachusetts and develops, manufactures and markets Optical Coherence Tomography (OCT) for coronary imaging applications. OCT is a high resolution diagnostic coronary imaging technology that complements the Fractional Flow Reserve (FFR) technology acquired by the Company as part of the Radi Medical Systems AB (Radi Medical Systems) acquisition in December 2008.
The goodwill recorded as a result of the LightLab Imaging acquisition is deductible for income tax purposes and was entirely allocated to the Cardiovascular operating segment. The goodwill represents the strategic benefits of growing our Cardiovascular product portfolio and the expected revenue growth from increased market penetration from future products and customers. In connection with the acquisition of LightLab Imaging, the Company recorded $39.6 million of developed and core technology intangible assets that have an estimated useful life of 15 years and capitalized acquired IPR&D of $14.3 million. The acquired IPR&D was recorded at fair value and capitalized as an indefinite-lived intangible asset. The aggregate LightLab Imaging purchase price was allocated on a preliminary basis to the assets acquired and liabilities assumed based on their estimated fair values at the date of acquisition.
34
AGA Medical, Inc: On November 18, 2010 the Company completed its acquisition of AGA Medical Holdings, Inc. (AGA Medical), acquiring all of the outstanding shares of AGA Medical (NASDAQ: AGAM) for $20.80 per share in a cash and stock transaction valued at $1.1 billion (which consisted of $549.4 million in net cash consideration and 13.6 million shares of St. Jude Medical common stock). The transaction was consummated through an exchange offer followed by a merger. The Company recorded direct transaction costs of $15.0 million and assumed debt of $197.0 million that was paid off at closing. The AGA Medical acquisition will expand the Company’s current cardiovascular product portfolio and future product pipeline to treat structural heart defects and vascular abnormalities through minimally invasive transcatheter treatments. AGA Medical is based in Plymouth, Minnesota.
The goodwill recorded as a result of the AGA Medical acquisition is not deductible for income tax purposes and was allocated entirely to the Company’s Cardiovascular operating segment. The goodwill represents the strategic benefits of growing our Cardiovascular product portfolio and the expected revenue growth from increased market penetration from future products and customers. In connection with the acquisition of AGA Medical, the Company capitalized $372.0 million of developed and core technology intangible assets, $120.0 million of IPR&D and $48.8 million of trademark intangible assets. The estimated useful lives of the developed and core technology intangible assets range from 12 to15 years. Both the IPR&D and trademark assets have been recorded as indefinite-lived intangible assets. The aggregate AGA Medical purchase price was allocated on a preliminary basis to the assets acquired and liabilities assumed based on their estimated fair values at the date of acquisition.
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed as a result of the significant business acquisitions made by the Company in fiscal year 2010 (in thousands):
| | | | | | | | | | |
| | LightLab Imaging | | AGA Medical | | Total | |
Current assets | | $ | 15,424 | | $ | 96,936 | | $ | 112,360 | |
Deferred income taxes, net | | | 4,240 | | | 13,038 | | | 17,278 | |
Goodwill | | | 40,543 | | | 880,679 | | | 921,222 | |
Other intangible assets | | | 39,640 | | | 420,800 | | | 460,440 | |
Acquired IPR&D | | | 14,270 | | | 120,000 | | | 134,270 | |
Other long-term assets | | | 2,219 | | | 45,007 | | | 47,226 | |
Total assets acquired | | | 116,336 | | | 1,576,460 | | | 1,692,796 | |
| | | | | | | | | | |
Current liabilities | | | 23,555 | | | 62,154 | | | 85,709 | |
Deferred income taxes, net | | | — | | | 195,477 | | | 195,477 | |
Other long-term liabilities | | | — | | | 235,756 | | | 235,756 | |
Net assets acquired | | $ | 92,781 | | $ | 1,083,073 | | $ | 1,175,854 | |
| | | | | | | | | | |
| | | | | | | | | | |
Cash paid, net of cash acquired | | | 92,781 | | | 549,426 | | | 642,207 | |
Non-cash (SJM shares at fair value) | | | — | | | 533,647 | | | 533,647 | |
Net assets acquired | | $ | 92,781 | | $ | 1,083,073 | | $ | 1,175,854 | |
| | | | | | | | | | |
Fiscal Year 2008
EP MedSystems, Inc.: On July 3, 2008, the Company completed the acquisition of EP MedSystems, Inc. (EP MedSystems) for $95.7 million (consisting of $59.0 million in net cash consideration and direct acquisition costs and 0.9 million shares of St. Jude Medical common stock). EP MedSystems had been publicly traded on the NASDAQ Capital Market under the ticker symbol EPMD. EP MedSystems was based in West Berlin, New Jersey and developed, manufactured and marketed medical devices for the electrophysiology market which are used for visualization, diagnosis and treatment of heart rhythm disorders. The Company acquired EP MedSystems to strengthen its portfolio of products used to treat heart rhythm disorders.
35
The goodwill recorded as a result of the EP MedSystems acquisition is not deductible for income tax purposes and was allocated entirely to the Company’s Atrial Fibrillation operating segment. The goodwill represents the strategic benefits of growing our Atrial Fibrillation product portfolio and the expected revenue growth from increased market penetration from future products and customers. In connection with the acquisition of EP MedSystems, the Company recorded $17.0 million of developed and core technology intangible assets and $3.3 million of customer relationship intangible assets that both have estimated useful lives of 7 to 10 years. The aggregate EP MedSystems purchase price was allocated on a preliminary basis to the assets acquired and liabilities assumed based on their estimated fair values at the date of acquisition. During 2009, the Company finalized the EP MedSystems purchase price allocation and recorded a $3.3 million net decrease to goodwill. The impacts of finalizing the purchase price allocation were not material.
Radi Medical Systems AB: On December 19, 2008, the Company completed the acquisition of Radi Medical Systems for $248.9 million in net cash consideration, including direct acquisition costs. Radi Medical Systems is based in Uppsala, Sweden and develops, manufactures and markets products that provide precise measurements of intravascular pressure during a cardiovascular procedure and compression systems that arrest bleeding of the femoral and radial arteries following an intravascular medical device procedure. The Company acquired Radi Medical Systems to accelerate its cardiovascular growth platform in these two segments of the cardiovascular medical device market in which the Company previously had not participated.
The goodwill recognized as a result of the Radi Medical Systems acquisition is not deductible for income tax purposes and was allocated entirely to the Company’s Cardiovascular operating segment. The goodwill represents the strategic benefits of growing our Cardiovascular product portfolio and the expected revenue growth from increased market penetration from future products and customers. In connection with the acquisition of Radi Medical Systems, the Company recorded $46.0 million of developed and core technology intangible assets that have estimated useful lives of 8 to 10 years. During 2009, the Company finalized the Radi Medical Systems purchase price allocation and recorded a $3.3 million net decrease to goodwill. The impacts of finalizing the purchase price allocation were not material.
MediGuide, Inc.: On December 22, 2008, the Company completed the acquisition of MediGuide, Inc. (MediGuide), a development stage company, for $285.2 million in net cash consideration, which included additional cash consideration payments of approximately $145.1 million and direct acquisition costs. The additional cash consideration payments consisted of a $113.8 million payment paid in November 2009 and a $31.3 million payment paid in April 2010. The final cash payment was held as security for potential indemnification obligations of MediGuide. MediGuide was a development-stage company based in Haifa, Israel and has been focused on developing a Medical Positioning System (gMPSTM) technology that provides localization and tracking capability for interventional medical devices. As MediGuide was a development-stage company, the excess of the purchase price over the fair value of the net assets acquired was allocated to IPR&D, the principal technology acquired.
36
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed as a result of the significant business acquisitions (EP MedSystems and Radi Medical Systems) and asset acquisition (MediGuide) made by the Company in fiscal year 2008 (in thousands):
| | | | | | | | | | | | | |
| | EP MedSystems | | Radi
Medical Systems | | MediGuide | | Total | |
Current assets | | $ | 8,506 | | $ | 21,224 | | $ | 132 | | $ | 29,862 | |
Goodwill | | | 69,719 | | | 219,428 | | | — | | | 289,147 | |
Other intangible assets | | | 20,250 | | | 46,000 | | | — | | | 66,250 | |
IPR&D | | | — | | | — | | | 306,202 | | | 306,202 | |
Deferred income taxes, net | | | 17,213 | | | — | | | — | | | 17,213 | |
Other long-term assets | | | 1,101 | | | 6,629 | | | 408 | | | 8,138 | |
Total assets acquired | | $ | 116,789 | | $ | 293,281 | | $ | 306,742 | | $ | 716,812 | |
| | | | | | | | | | | | | |
Current liabilities | | | 21,084 | | $ | 31,405 | | | 21,580 | | | 74,069 | |
Deferred income taxes, net | | | — | | | 12,930 | | | — | | | 12,930 | |
Net assets acquired | | $ | 95,705 | | $ | 248,946 | | $ | 285,162 | | $ | 629,813 | |
| | | | | | | | | | | | | |
Cash paid, net of cash acquired | | $ | 58,994 | | $ | 248,946 | | $ | 140,104 | | $ | 448,044 | |
Non-cash (SJM shares at fair value) | | | 36,711 | | | — | | | — | | | 36,711 | |
Future cash consideration | | | — | | | — | | | 145,058 | | | 145,058 | |
Net assets acquired | | $ | 95,705 | | $ | 248,946 | | $ | 285,162 | | $ | 629,813 | |
Minority Investment:During 2010, the Company made an equity investment of $60.0 million in CardioMEMS, Inc. (CardioMEMS), a privately-held company that is focused on the development of a wireless monitoring technology that can be placed directly into the pulmonary artery to assess cardiac performance via measurement of pulmonary artery pressure. The investment agreement resulted in a 19% ownership interest and provided the Company with the exclusive right, but not the obligation, to acquire CardioMEMS for an additional payment of $375 million during the period that extends through the completion of certain commercialization milestones. The equity investment and allocated value of the fixed price purchase option are being carried at cost.
NOTE 3 – GOODWILL AND OTHER INTANGIBLE ASSETS
The changes in the carrying amount of goodwill for each of the Company’s reportable segments for the fiscal years ended January 1, 2011 and January 2, 2010 were as follows (in thousands):
| | | | | | | | | | |
| | CRM/NMD | | CV/AF | | Total | |
Balance at January 3, 2009 | | $ | 1,190,851 | | $ | 793,715 | | $ | 1,984,566 | |
EP Medsystems | | | — | | | (3,261 | ) | | (3,261 | ) |
Radi Medical Systems | | | — | | | (3,265 | ) | | (3,265 | ) |
Foreign currency translation and other | | | 27,478 | | | 333 | | | 27,811 | |
Balance at January 2, 2010 | | $ | 1,218,329 | | $ | 787,522 | | $ | 2,005,851 | |
AGA Medical | | | — | | | 880,679 | | | 880,679 | |
LightLab Imaging | | | — | | | 40,543 | | | 40,543 | |
Foreign currency translation and other | | | 12,791 | | | 15,738 | | | 28,529 | |
Balance at January 1, 2011 | | $ | 1,231,120 | | $ | 1,724,482 | | $ | 2,955,602 | |
37
The following table provides the gross carrying amount of other intangible assets and related accumulated amortization (in thousands):
| | | | | | | | | | | | | |
| | January 1, 2011 | | January 2, 2010 | |
| | Gross
carrying
amount | | Accumulated
amortization | | Gross
carrying
amount | | Accumulated
amortization | |
Definite-lived intangible assets: | | | | | | | | | | | | | |
Purchased technology and patents | | $ | 910,035 | | $ | 208,362 | | $ | 506,893 | | $ | 171,760 | |
Customer lists and relationships | | | 184,327 | | | 100,608 | | | 182,368 | | | 81,129 | |
Trademarks and tradenames | | | 24,370 | | | 7,431 | | | 24,286 | | | 6,336 | |
Licenses, distribution agreements and other | | | 6,170 | | | 4,511 | | | 5,693 | | | 3,873 | |
| | $ | 1,124,902 | | $ | 320,912 | | $ | 719,240 | | $ | 263,098 | |
| | | | | | | | | | | | | |
Indefinite-lived intangible assets: | | | | | | | | | | | | | |
Acquired IPR&D | | $ | 134,270 | | | | | $ | — | | | | |
Trademarks and tradenames | | | 48,800 | | | | | | — | | | | |
| | $ | 183,070 | | | | | $ | — | | | | |
Amortization expense of other intangible assets was $63.3 million, $58.5 million and $53.4 million for fiscal years 2010, 2009 and 2008, respectively. In 2008, the Company recorded a $37.0 million impairment charge to write down purchased technology intangible assets associated with its 2005 Velocimed acquisition and a $1.7 million impairment charge to write off its ANS tradename intangible assets (see Note 8). There was no impairment charges recognized during fiscal years 2010 or 2009.
The following table presents expected future amortization expense for amortizable intangible assets. Actual amounts of amortization expense may differ due to additional intangible assets acquired and foreign currency translation impacts (in thousands):
| | | | | | | | | | | | | | | | | | | |
| | | 2011 | | | 2012 | | | 2013 | | | 2014 | | | 2015 | | | After
2015 | |
Amortization expense | | | $93,829 | | | $91,431 | | | $89,820 | | | $86,865 | | | $76,774 | | | $365,271 | |
NOTE 4 – DEBT
The Company’s debt consisted of the following (in thousands):
| | | | | | | |
| | January 1, 2011 | | January 2, 2010 | |
2.20% senior notes due 2013 | | $ | 467,168 | | $ | — | |
3.75% senior notes due 2014 | | | 699,248 | | | 699,036 | |
2.50% senior notes due 2016 | | | 489,496 | | | — | |
4.875% senior notes due 2019 | | | 494,563 | | | 493,927 | |
1.58% Yen-denominated senior notes due 2017 | | | 99,737 | | | — | |
2.04% Yen-denominated senior notes due 2020 | | | 156,254 | | | — | |
1.02% Yen-denominated notes due 2010 | | | — | | | 226,787 | |
Yen-denominated term loan due 2011 | | | 79,637 | | | 70,652 | |
3-year unsecured term loan | | | — | | | 432,000 | |
Commercial paper borrowings | | | 25,500 | | | — | |
Total debt | | | 2,511,603 | | | 1,922,402 | |
Less: current debt obligations | | | 79,637 | | | 334,787 | |
Long-term debt | | $ | 2,431,966 | | $ | 1,587,615 | |
| | | | | | | |
38
Expected future minimum principal payments under the Company’s debt obligations are as follows: $79.6 million in 2011; $450.0 million in 2013; $700.0 million in 2014; and $1,281.5 million in years thereafter.
Senior notes due 2013: On March 10, 2010, the Company issued $450.0 million principal amount of 3-year, 2.20% unsecured senior notes (2013 Senior Notes) that mature in September 2013. The majority of the net proceeds from the issuance of the 2013 Senior Notes was used to retire the Company’s 3-year unsecured term loan due 2011. Interest payments are required on a semi-annual basis. The 2013 Senior Notes were issued at a discount, yielding an effective interest rate of 2.23% at issuance. The Company may redeem the 2013 Senior Notes at any time at the applicable redemption price. The debt discount is being amortized as interest expense through maturity.
Concurrent with the issuance of the 2013 Senior Notes, the Company entered into a 3-year, $450.0 million notional amount interest rate swap designated as a fair value hedge of the changes in fair value of the Company’s fixed-rate 2013 Senior Notes. On November 8, 2010, the Company terminated the interest rate swap and received a cash payment of $19.3 million. The gain from terminating the interest rate swap agreement is being amortized as a reduction of interest expense over the remaining life of the 2013 Senior Notes resulting in a net average interest rate of 0.8% that will be recognized over the remaining term of the 2013 Senior Notes. The cash receipt from the termination of the interest rate swap has been classified as an operating cash flow in the Consolidated Statements of Cash Flows.
Senior notes due 2014: On July 28, 2009, the Company issued $700.0 million principal amount, 5-year, 3.75% unsecured senior notes (2014 Senior Notes) that mature in July 2014. Interest payments are required on a semi-annual basis. The 2014 Senior Notes were issued at a discount, yielding an effective interest rate of 3.78% at issuance. The debt discount is being amortized as interest expense through maturity. The Company may redeem the 2014 Senior Notes at any time at the applicable redemption price.
Senior notes due 2016: On December 1, 2010, the Company issued $500.0 million principal amount of 5-year, 2.50% unsecured senior notes (2016 Senior Notes) that mature in January 2016. The majority of the net proceeds from the issuance of the 2016 Senior Notes was used for general corporate purposes including the repurchase of the Company’s common stock. Interest payments are required on a semi-annual basis. The 2016 Senior Notes were issued at a discount, yielding an effective interest rate of 2.54% at issuance. The debt discount is being amortized as interest expense through maturity. The Company may redeem the 2016 Senior Notes at any time at the applicable redemption price.
Concurrent with the issuance of the 2016 Senior Notes, the Company entered into a 5-year, $500.0 million notional amount interest rate swap designated as a fair value hedge of the changes in fair value of the Company’s fixed-rate 2016 Senior Notes. As of January 1, 2011, the fair value of the swap was a $10.0 million liability which was classified as other liabilities on the consolidated balance sheet, with a corresponding adjustment to the carrying value of the 2016 Senior Notes. Refer to Note 13 for additional information regarding the interest rate swap.
Senior notes due 2019: On July 28, 2009, the Company issued $500.0 million principal amount, 10-year, 4.875% unsecured senior notes (2019 Senior Notes) that mature in July 2019. Interest payments are required on a semi-annual basis. The 2019 Senior Notes were issued at a discount, yielding an effective interest rate of 5.04% at issuance. The debt discount is being amortized as interest expense through maturity. The Company may redeem the 2019 Senior Notes at any time at the applicable redemption price.
1.58% Yen-denominated senior notes due 2017: On April 28, 2010, the Company issued 7-year, 1.58% unsecured senior notes in Japan (1.58% Yen Notes) totaling 8.1 billion Yen (the equivalent of $99.7 million at January 1, 2011). The net proceeds from the issuance of the 1.58% Yen Notes were used to repay the 1.02% Yen Notes. The principal amount of the 1.58% Yen Notes recorded on the balance sheet fluctuates based on the effects of foreign currency translation. Interest payments are required on a semi-annual basis and the entire principal balance is due on April 28, 2017.
2.04% Yen-denominated senior notes due 2020: On April 28, 2010, the Company issued 10-year, 2.04% unsecured senior notes in Japan (2.04% Yen Notes) totaling 12.8 billion Yen (the equivalent of $156.3 million at January 1, 2011). The net proceeds from the issuance of the 2.04% Yen Notes were used to repay the 1.02% Yen-denominated notes that matured May 7, 2010 (1.02% Yen Notes). The principal amount of the 2.04% Yen Notes recorded on the balance sheet fluctuates based on the effects of foreign currency translation. Interest payments are required on a semi-annual basis and the entire principal balance is due on April 28, 2020.
39
Yen-denominated term loan due 2011: In December 2008, the Company entered into a 3-year, Yen-denominated unsecured term loan in Japan (Japan Term Loan) totaling 8.0 billion Japanese Yen. In December 2009, the Company voluntarily repaid 1.5 billion Japanese Yen, resulting in an outstanding balance of 6.5 billion Japanese Yen (the equivalent of $79.6 million at January 1, 2011 and $70.7 million at January 2, 2010). The Company can initiate future borrowings up to the 8.0 billion Japan Term Loan amount. The principal amount reflected on the consolidated balance sheet fluctuates based on the effects of foreign currency translation. The borrowings bear interest at the Yen LIBOR plus 0.4%. Interest payments are required on a semi-annual basis and the entire principal balance is due in December 2011.
Other available borrowings: In December 2010, the Company entered into a 4-year, $1.5 billion unsecured committed credit facility (Credit Facility) that it may draw on for general corporate purposes and to support its commercial paper program. The Credit Facility expires on February 28, 2015. Borrowings under the Credit Facility bear interest initially at LIBOR plus 0.875%, subject to adjustment in the event of a change in the Company’s credit ratings. The Credit Facility replaces the Company’s previous $1.0 billion credit facility that was scheduled to expire in December 2011. As of January 1, 2011 and January 2, 2010, the Company had no outstanding borrowings under either credit facility.
The Company’s commercial paper program provides for the issuance of short-term, unsecured commercial paper with maturities up to 270 days. The Company began issuing commercial paper during November 2010 and had an outstanding commercial paper balance of $25.5 million as of January 1, 2011. During 2010, the Company’s weighted average effective interest rate on our outstanding commercial paper borrowings was 0.27%. The Company had no commercial paper borrowings outstanding as of January 2, 2010. Any future commercial paper borrowings would bear interest at the applicable then-current market rates. The Company classifies all of its commercial paper borrowings as long-term debt, as the Company has the ability to repay any short-term maturity with available cash from its existing long-term, committed Credit Facility.
NOTE 5 – COMMITMENTS AND CONTINGENCIES
Leases
The Company leases various facilities and equipment under non-cancelable operating lease arrangements. Future minimum lease payments under these leases are as follows: $41.0 million in 2011; $34.3 million in 2012; $28.1 million in 2013; $21.7 million in 2014; $17.0 million in 2015; and $8.4 million in years thereafter. Rent expense under all operating leases was $36.3 million, $33.5 million and $28.6 million in fiscal years 2010, 2009 and 2008, respectively.
Litigation
Silzone® Litigation and Insurance Receivables: The Company has been sued in various jurisdictions beginning in March 2000 by some patients who received a heart valve product with Silzone® coating, which we stopped selling in January 2000. Some of these claimants allege bodily injuries as a result of an explant or other complications, which they attribute to these products. Others, who have not had their Silzone-coated heart valve explanted, seek compensation for past and future costs of special monitoring they allege they need over and above the medical monitoring of all other replacement heart valve patients. Some of the lawsuits seeking the cost of monitoring have been initiated by patients who are asymptomatic and who have no apparent clinical injury to date. The Company has vigorously defended against the claims that have been asserted and expects to continue to do so with respect to any remaining claims. While the Company has a small number of individual Silzone claims outstanding, the Company’s historical experience with similar cases and the Company’s expectations for these specific claims are that it will be able to resolve them at minimal, if any, cost to the Company.
40
The Company has resolved class action complaints in British Columbia and Quebec. As part of the British Columbia settlement, the Company made a $2.1 million payment in March 2010. As part of the Quebec settlement, the Company made a $5.7 million payment in April 2010. The Quebec settlement also resolved the claim raised by the Quebec Provincial health insurer seeking to recover the cost of insured services furnished or to be furnished to class members in the Quebec class action. These settlement payments were reimbursed to the Company by its legacy product liability insurance carriers.
The Company has two outstanding class action cases in Ontario and one individual case in British Columbia by the Provincial health insurer. In Ontario, a class action case involving Silzone patients has been certified, and the trial began in February 2010. A second case seeking class action status in Ontario has been stayed pending resolution of the ongoing Ontario class action. The complaints in the Ontario cases request damages up to 2.0 billion Canadian Dollars (the equivalent of $1.9 billion at January 1, 2011). Based on the Company’s historical experience, the amount ultimately paid, if any, often does not bear any relationship to the amount claimed. The British Columbia Provincial health insurer has a lawsuit seeking to recover the cost of insured services furnished or to be furnished to class members in the British Columbia class action resolved in 2010, and that lawsuit remains pending in the British Columbia court.
The Company has recorded an accrual for probable legal costs, settlements and judgments for Silzone related litigation. The Company is not aware of any unasserted claims related to Silzone-coated products. For all Silzone legal costs incurred, the Company records insurance receivables for the amounts that it expects to recover. Any costs (the material components of which are settlements, judgments, legal fees and other related defense costs) not covered by the Company’s product liability insurance policies or existing reserves could be material to the Company’s consolidated earnings, financial position and cash flows. The following table summarizes the Company’s Silzone legal accrual and related insurance receivable at January 1, 2011 and January 2, 2010 (in thousands):
| | | | | | | |
| | January 1, 2011 | | January 2, 2010 | |
Silzone legal accrual | | $ | 24,032 | | $ | 23,326 | |
Silzone insurance receivable | | $ | 12,799 | | $ | 42,538 | |
Part of the Company’s legacy product liability insurance for Silzone claims consisted of a $50.0 million layer of insurance covered by American Insurance Company (AIC). In December 2007, AIC had initiated a lawsuit in Minnesota Federal District Court seeking a court order declaring that it is not required to provide coverage for a portion of the Silzone litigation defense and indemnity expenses incurred by the Company. This matter was resolved in December 2010 when AIC remitted a $50.0 million payment in settlement of all of the then pending litigation and as payment for full exhaustion of its $50.0 million layer of insurance. The payment was applied to the $50.0 million receivable from AIC the Company had recorded for the amount it expected to recover.
Part of the Company’s final layer of insurance was covered by Lumberman’s Mutual Casualty Insurance, a unit of the Kemper Insurance Companies (collectively referred to as Kemper). Kemper is currently in “run off,” which means it is no longer issuing new policies, and therefore, is not generating any new revenue that could be used to cover claims made under previously-issued policies. In September 2010, Kemper agreed to settle its future insurance obligations with the Company, the amount of which was not material.
The Company’s remaining insurance for Silzone claims consists of $30 million of coverage with other insurance carriers. To the extent that the Company’s future Silzone costs and expenses exceed our remaining insurance coverage, the Company would be responsible for such costs. The Company has not accrued for any potential losses relating to future costs as they are not probable or reasonably estimable at this time.
Volcano Corporation & LightLab Imaging Litigation:The Company’s recently acquired subsidiary, LightLab Imaging, has pending litigation with Volcano Corporation (Volcano) and Axsun Technologies, Inc. (Axsun), a subsidiary of Volcano, in the Superior Court of Massachusetts and in state court in Delaware. LightLab Imaging makes and sells optical coherence tomography (OCT) imaging systems. Volcano is a LightLab Imaging competitor in medical imaging. Axsun makes and sells lasers and is a supplier of lasers to LightLab Imaging for use in OCT imaging systems. The lawsuits arise out of Volcano’s acquisition of Axsun in December 2008. Before Volcano acquired Axsun, LightLab Imaging and Axsun had worked together to develop a tunable laser for use in OCT imaging systems. While the laser was in development, LightLab Imaging and Axsun entered into an agreement pursuant to which Axsun agreed to sell its tunable lasers exclusively to LightLab in the field of human coronary artery imaging for a period of years.
41
After Volcano acquired Axsun in December 2008, LightLab Imaging sued Axsun and Volcano in Massachusetts, asserting a number of claims arising out of Volcano’s acquisition of Axsun. In January 2011, the court ruled that Axsun’s and Volcano’s conduct constituted knowing and willful violations of a statute that prohibits unfair or deceptive acts or practices or acts of unfair competition, entitling LightLab Imaging to double damages, and furthermore, that LightLab Imaging was entitled to recover attorneys’ fees. In February 2011, Volcano and Axsun were ordered to pay the Company $4.5 million as reimbursement of its attorneys’ fees, and the Court assessed double damages against Volcano and Asxun making the total award to LightLab Imaging $5.1 million. The Court also issued certain injunctions against Volcano and Axsun when it entered its final judgment.
In Delaware, Axsun and Volcano commenced an action in February 2010 against LightLab Imaging, seeking a declaration as to whether Axsun may supply a certain light source for use in OCT imaging systems to Volcano. Axsun’s and Volcano’s position is that this light source is not a tunable laser and hence falls outside Axsun’s exclusivity obligations to Volcano. LightLab Imaging’s position, among other things, is that this light source is a tunable laser. The parties are presently involved in expedited discovery, and a trial is presently expected to commence in the second quarter of 2011.
Volcano Corporation & St. Jude Medical Patent Litigation:In July 2010, the Company filed a lawsuit in federal district court in Delaware against Volcano for patent infringement. The suit involves five patents and seeks injunctive relief and monetary damages. The infringed patents are used for the St. Jude Medical PressureWire® technology platform, which was acquired as part of St. Jude Medical’s purchase of Radi Medical Systems in December 2008. In September 2010, Volcano filed counterclaims against the Company, alleging certain St. Jude Medical patent claims are unenforceable and that certain St. Jude Medical products infringe three Volcano patents. The Company believes the assertions and claims made by Volcano are without merit. Trial in this case is scheduled for October 2012.
Securities Class Action Litigation: On March 18, 2010, a securities class action lawsuit was filed in federal district court in Minnesota against the Company and certain officers on behalf of purchasers of St. Jude Medical common stock between April 22, 2009 and October 6, 2009. The lawsuit relates to the Company’s earnings announcements for the first, second and third quarters of 2009, as well as a preliminary earnings release dated October 6, 2009. The complaint, which seeks unspecified damages and other relief as well as attorneys’ fees, alleges that the Company failed to disclose that it was experiencing a slowdown in demand for its products and was not receiving anticipated orders for CRM devices. Class members allege that the Company’s failure to disclose the above information resulted in the class purchasing St. Jude Medical stock at an artificially inflated price. The Company intends to vigorously defend against the claims asserted in this lawsuit. In October 2010, the Company filed a motion to dismiss the lawsuit, which is scheduled to be heard by the District Court in early April 2011.
42
Derivative Litigation: In September 2010, two separate derivative actions involving the Company were filed in the United States District Court for the District of Minnesota. In both of these matters, the defendants consist of members (or former members) of St. Jude Medical’s Board of Directors (the Board) as well as various officers and former officers of the Company. The plaintiffs in these actions are asserting breach of fiduciary duty claims against the named defendants for their purported failure to stop the alleged underlying conduct (which relates to the contents of qui tam actions filed in Ohio and Massachusetts). In October 2010, the plaintiffs filed a motion before the Judicial Panel on MultiDistrict Litigation requesting that the two cases be transferred to the District of Massachusetts and consolidated with what they claim are related actions there. St. Jude Medical intends to oppose the transfer request and to vigorously defend against the claims asserted in these two derivative lawsuits. Under the existing schedule, the plaintiffs are to file their consolidated complaint in March 2011.
Regulatory Matters
The FDA inspected the Company’s manufacturing facility in Minnetonka, Minnesota at various times between December 8 and December 19, 2008. On December 19, 2008, the FDA issued a Form 483 identifying certain observed non-conformity with current Good Manufacturing Practice (cGMP) primarily related to the manufacture and assembly of the SafireTM ablation catheter with a 4 mm or 5 mm non-irrigated tip. Following the receipt of the Form 483, the Company’s AF division provided written responses to the FDA detailing proposed corrective actions and immediately initiated efforts to address the FDA’s observations of non-conformity. The Company subsequently received a warning letter dated April 17, 2009 from the FDA relating to these non-conformities with respect to this facility.
The FDA inspected the Company’s Plano, Texas manufacturing facility at various times between March 5 and April 6, 2009. On April 6, 2009, the FDA issued a Form 483 identifying certain observed nonconformities with cGMP. Following the receipt of the Form 483, the Company’s Neuromodulation division provided written responses to the FDA detailing proposed corrective actions and immediately initiated efforts to address FDA’s observations of nonconformity. The Company subsequently received a warning letter dated June 26, 2009 from the FDA relating to these non-conformities with respect to its Neuromodulation division’s Plano, Texas and Hackettstown, New Jersey facilities.
With respect to each of these warning letters, the FDA notes that it will not grant requests for exportation certificates to foreign governments or approve pre-market approval applications for Class III devices to which the quality system regulation deviations are reasonably related until the violations have been corrected. The Company is working cooperatively with the FDA to resolve all of its concerns.
On April 23, 2010, the FDA issued a warning letter based upon a July 29, 2009 inspection of our Sunnyvale, California facility and a review of our website. The warning letter cites the Company for its promotion and marketing of the Epicor™ LP Cardiac Ablation System and the Epicor UltraCinch LP Ablation Device based on certain statements made in the Company’s marketing materials. The Company is working cooperatively with the FDA to resolve all of its concerns. The warning letter is not expected to have any material impact on the Company’s business.
Customer orders have not been and are not expected to be impacted while the Company works to resolve the FDA’s concerns. The Company is working diligently to respond timely and fully to the FDA’s requests. While the Company believes the issues raised by the FDA can be resolved without a material impact on the Company’s financial results, the FDA has recently been increasing its scrutiny of the medical device industry and raising the threshold for compliance. The government is expected to continue to scrutinize the industry closely with inspections, and possibly enforcement actions, by the FDA or other agencies. The Company is regularly monitoring, assessing and improving its internal compliance systems and procedures to ensure that its activities are consistent with applicable laws, regulations and requirements, including those of the FDA.
43
Other Matters
Boston U.S. Attorney Investigation: In October 2005, the U.S. Department of Justice (DOJ), acting through the U.S. Attorney’s office in Boston, commenced an industry-wide investigation into whether the provision of payments and/or services by makers of ICDs and pacemakers to doctors or other persons constitutes improper inducements under the federal health care program anti-kickback law. In January 2011, without an admission of liability, the Company agreed to settle this matter and paid $16.5 million.
In December 2008, the U.S. Attorney’s Office in Boston delivered a subpoena issued by the U.S. Department of Health and Human Services, Office of the Inspector General (OIG) requesting the production of documents relating to implantable cardiac rhythm device and pacemaker warranty claims. The Company has been cooperating with the investigation.
U.S. Department of Justice - Civil Investigative Demand: In March 2010, the Company received a Civil Investigative Demand (CID) from the Civil Division of the U.S. Department of Justice. The CID requests documents and sets forth interrogatories related to communications by and within the Company on various indications for ICDs and a National Coverage Decision issued by Centers for Medicare and Medicaid Services. Similar requests were made of our major competitors. The Company is cooperating with the investigation and is continuing to work with the U.S. Department of Justice in responding to the CID.
AGA Securities Class Action: In connection with the acquisition of AGA Medical, the Company, in addition to AGA Medical and other defendants, has been named as a defendant in two putative stockholder class action complaints, one filed in the Fourth Judicial District Court of Minnesota on October 27, 2010 and the other filed in the Delaware Court of Chancery of October 28, 2010. The plaintiffs in the complaints allege, among other claims, that AGA Medical’s directors breached their fiduciary duties to AGA Medical’s stockholders by accepting an inadequate price, failing to make full disclosure and utilizing unreasonable deal protection devices and further alleges that AGA Medical and the Company aided and abetted the purported breaches of fiduciary duty. The complaints seek injunctive relief, including to enjoin the transaction, in addition to unspecified compensatory damages, attorneys’ fees, other fees and costs and other relief. On November 8, 2010, the parties to this action entered into a memorandum of understanding (MOU) to settle the litigation, the amount of which was not material. The settlement contemplated by the MOU is subject to several conditions, including the negotiation and execution of a stipulation of settlement and the approval of the Delaware Court of Chancery.
The Company is also involved in various other lawsuits, claims and proceedings that arise in the ordinary course of business.
NOTE 6 – SHAREHOLDERS’ EQUITY
Capital Stock: The Company’s authorized capital consists of 25 million shares of $1.00 per share par value preferred stock and 500 million shares of $0.10 per share par value common stock. There were no shares of preferred stock issued or outstanding during 2010, 2009 or 2008.
Share Repurchases: On October 15, 2010, the Company’s Board of Directors authorized a share repurchase program of up to $600.0 million of the Company’s outstanding common stock. On October 21, 2010, the Company’s Board of Directors authorized an additional $300.0 million of share repurchases as part of this share repurchase program. Through January 1, 2011, the Company had repurchased 15.4 million shares for $625.3 million at an average repurchase price of $40.63 per share. The Company continued repurchasing shares in 2011 and completed the repurchases under the program on January 20, 2011, repurchasing a program total of 22.0 million shares for $900.0 million at an average repurchase price of $40.87 per share.
In October 2009, the Company’s Board of Directors authorized a share repurchase program of up to $500.0 million of the Company’s outstanding common stock. The Company completed the repurchases under the program in December 2009, repurchasing 14.1 million shares for $500.0 million at an average repurchase price of $35.44 per share. In July 2009, the Company’s Board of Directors authorized a share repurchase program of up to $500.0 million of the Company’s outstanding common stock. The Company completed the repurchases under the program in September 2009, repurchasing 13.0 million shares for $500.0 million at an average repurchase price of $38.32 per share. For fiscal year 2009, the Company repurchased a total of 27.1 million shares for $1.0 billion at an average repurchase price of $36.83 per share.
44
In February 2008, the Company’s Board of Directors authorized a share repurchase program of up to $250.0 million of the Company’s outstanding common stock. In April 2008, the Company’s Board of Directors authorized an additional $50.0 million of share repurchases as part of this share repurchase program. The Company completed the repurchases under the program in May 2008. In total, the Company repurchased 6.7 million shares for $300.0 million at an average repurchase price of $44.51 per share.
NOTE 7 – STOCK-BASED COMPENSATION
Stock Compensation Plans
The Company’s stock compensation plans provide for the issuance of stock-based awards, such as stock options, restricted stock units and restricted stock awards, to directors, officers, employees and consultants. Stock option awards under these plans have an exercise price equal to the fair market value on the date of grant, and generally, have an eight-year contractual life and four-year vesting term. Since 2000, all stock option awards have been granted with an eight-year contractual term regardless of the maximum allowable under the plan. Restricted stock units and restricted stock awards under these plans generally vest over a four-year period. During the vesting period, ownership of the shares or units cannot be transferred. Restricted stock awards are considered issued and outstanding at the grant date and have the same dividend and voting rights as other common stock. Directors can elect to receive half or their entire annual retainer in the form of a restricted stock award with a six-month vesting term. Restricted stock units are not issued and outstanding at the grant date; instead, upon vesting the recipient receives one share of the Company’s common stock for each vested restricted stock unit. At January 1, 2011, the Company had 7.6 million shares of common stock available for stock option grants under its stock compensation plans. The Company has the ability to grant a portion of the available shares in the form of restricted stock. Specifically, in lieu of granting up to 6.8 million stock options under these plans, the Company may grant up to 3.0 million restricted stock awards or units (for certain grants of restricted stock units or awards, the number of shares available are reduced by 2.25 shares). Additionally, in lieu of granting up to 0.1 million stock options under these plans, the Company may grant up to 0.1 million restricted stock awards (for certain grants of restricted stock awards, the number of shares available are reduced by one share). The remaining 0.7 million shares of common stock are available only for stock option grants. At January 1, 2011, there was $160.2 million of total unrecognized stock-based compensation expense, adjusted for estimated forfeitures, which is expected to be recognized over a weighted average period of 3.0 years and will be adjusted for any future changes in estimated forfeitures.
The Company also has an Employee Stock Purchase Plan (ESPP) that allows participating employees to purchase newly issued shares of the Company’s common stock at a discount through payroll deductions. The ESPP consists of a 12-month offering period whereby employees can purchase shares at 85% of the market value at either the beginning of the offering period or the end of the offering period, whichever price is lower. Employees purchased 0.9 million, 0.8 million and 0.7 million shares in 2010, 2009, and 2008, respectively. At January 1, 2011, 2.6 million shares of common stock were available for future purchases under the ESPP.
Valuation Assumptions
The Company uses the Black-Scholes standard option pricing model (Black-Scholes model) to determine the fair value of stock options and ESPP purchase rights. The determination of the fair value of the awards on the date of grant using the Black-Scholes model is affected by the Company’s stock price as well as assumptions of other variables, including projected employee stock option exercise behaviors, risk-free interest rate, expected volatility of the Company’s stock price in future periods and expected dividend yield. The fair value of both restricted stock and restricted stock units is based on the Company’s closing stock price on the date of grant. The weighted average fair values of restricted stock awards granted during fiscal years 2010, 2009 and 2008 were $37.08, $39.83 and $40.52, respectively. Fiscal year 2010 was the first year the Company issued restricted stock units. The weighted average fair value of the restricted units granted during fiscal year 2010 was $41.65. The weighted average fair values of ESPP purchase rights granted to employees during fiscal years 2010, 2009 and 2008 were $9.70, $10.49 and $13.12, respectively.
45
The following table provides the weighted average fair value of stock options granted to employees during fiscal years 2010, 2009 and 2008 and the related weighted average assumptions used in the Black-Scholes model:
| | | | | | | | | | |
| | 2010 | | 2009 | | 2008 | |
Fair value of options granted | | $ | 11.79 | | $ | 12.17 | | $ | 9.99 | |
| | | | | | | | | | |
Assumptions: | | | | | | | | | | |
Expected life (years) | | | 4.8 | | | 4.7 | | | 4.2 | |
Risk-free interest rate | | | 2.2 | % | | 2.3 | % | | 1.8 | % |
Volatility | | | 31.7 | % | | 32.8 | % | | 37.3 | % |
Dividend yield | | | 0 | % | | 0 | % | | 0 | % |
Expected life: The Company analyzes historical employee exercise and termination data to estimate the expected life assumption. Annually, the Company updates these assumptions unless circumstances would indicate a more frequent update is necessary. The Company uses different expected lives for the general employee population compared to the officer and director population, as the Company’s expected life analysis continues to show that officers and directors hold their stock options for a longer period of time before exercising compared to the rest of the employee population. As a result, the Company continues to use two different populations for estimating its expected life assumptions in determining the fair value of its stock options.
Risk-free interest rate: The rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity equal to or approximating the expected life of the options.
Volatility: Effective in the fourth quarter of 2008, the Company calculates its expected volatility assumption by blending the historical and implied volatility. The historical volatility is based on the daily closing prices of the Company’s common stock over a period equal to the expected term of the option. Market-based implied volatility is based on utilizing market data of actively traded options on the Company’s stock, from options at- or near-the-money, at a point in time as close to the grant date of the employee options as reasonably practical and with similar terms to the employee share option, or a remaining maturity of at least six months if no similar terms are available. The historical volatility of the Company’s common stock price over the expected term of the option is a strong indicator of the expected future volatility. In addition, implied volatility takes into consideration market expectations of how future volatility will differ from historical volatility. The Company does not believe that one estimate is more reliable than the other, and as a result, the Company uses an equal weighting of historical volatility and market-based implied volatility. Prior to the fourth quarter of 2008, the Company calculated the expected volatility assumption exclusively on market-based implied volatility. The impact of changing the method of determining expected volatility was not material to fiscal year 2010, 2009 or 2008 stock compensation expense. The Company changed the method of determining expected volatility to take into consideration how future volatility experience over the expected life of the option may differ from short-term volatility experience and thus provide a better estimate of expected volatility over the expected life of employee stock options.
Dividend yield: For all grants through fiscal year 2010, the Company had not anticipated paying cash dividends and therefore assumed a dividend yield of zero.
46
Stock Compensation Activity
The following table summarizes stock option activity under all stock compensation plans during the fiscal year ended January 1, 2011:
| | | | | | | | | | | | | |
| | Options
(in thousands) | | Weighted
Average
Exercise
Price | | Weighted
Average Remaining
Contractual
Term (years) | | Aggregate
Instrinsic
Value
(in thousands) | |
Outstanding at January 2, 2010 | | | 36,728 | | $ | 35.73 | | | | | | | |
Granted | | | 4,087 | | | 41.42 | | | | | | | |
Canceled | | | (1,835 | ) | | 37.91 | | | | | | | |
Exercised | | | (5,466 | ) | | 24.52 | | | | | | | |
Outstanding at January 1, 2011 | | | 33,514 | | $ | 38.13 | | | 4.9 | | $ | 180,954 | |
Vested and expected to vest | | | 31,479 | | $ | 38.14 | | | 4.7 | | $ | 171,239 | |
Exercisable at January 1, 2011 | | | 20,575 | | $ | 38.49 | | | 3.7 | | $ | 113,728 | |
The aggregate intrinsic value of options outstanding and options exercisable is based on the Company’s closing stock price on the last trading day of the fiscal year for in-the-money options. The total intrinsic value of options exercised during fiscal years 2010, 2009 and 2008 was $83.0 million, $106.6 million, and $182.6 million, respectively. The aggregate intrinsic value represents the cumulative difference between the fair market value of the underlying common stock and the option exercise prices.
The following table summarizes restricted stock activity under all stock compensation plans during the fiscal year ended January 1, 2011:
| | | | | | | |
| | Restricted Stock
(in thousands) | | Weighted Average
Grant Price | |
Unvested balance at January 2, 2010 | | | 8 | | $ | 39.89 | |
Granted | | | 851 | | | 41.59 | |
Vested | | | (12 | ) | | 37.19 | |
Canceled | | | (2 | ) | | 40.81 | |
Unvested balance at January 1, 2011 | | | 845 | | $ | 41.63 | |
The total fair value of restricted stock vested during fiscal years 2010, 2009 and 2008 was $0.5 million, $2.5 million and $3.1 million, respectively. In December 2010, the Company granted 0.8 million restricted stock units at a fair value of $35.0 million, which represented the closing stock price of $41.65 on the date of grant.
NOTE 8 – PURCHASED IN-PROCESS RESEARCH AND DEVELOPMENT (IPR&D) AND SPECIAL CHARGES
IPR&D Charges
During 2010, the Company recorded IPR&D charges of $12.2 million in conjunction with the purchase of cardiovascular-related intellectual property. During 2009, the Company recorded IPR&D charges of $5.8 million in conjunction with the purchase of intellectual property in its CV and NMD segments. As the related technological feasibility had not yet been reached and such technology had no future alternative use, these intellectual property purchases were expensed as IPR&D.
In December 2008, the Company acquired MediGuide. As a development-stage company, the excess of the purchase price over the fair value of the net assets acquired was allocated on a pro-rata basis to the net assets acquired. Accordingly, the excess purchase price was allocated to IPR&D, the principal technology acquired. At the date of acquisition, $306.2 million of the purchase price was expensed as IPR&D since technological feasibility of the underlying projects had not yet been reached and such technology had no future alternative use. Through January 1, 2011, the Company has incurred costs of approximately $20 million related to these projects. The Company expects to incur an additional $10 million to bring the technology to commercial viability on a worldwide basis within the next twelve to eighteen months.
47
In December 2008, the Company also made an additional minority investment in a development-stage company and in accordance with step-acquisition accounting treatment under the equity method of accounting, allocated the excess purchase price over the fair value of the investee’s net assets to IPR&D the principal technology acquired. At the December 2008 investment date, $11.6 million of IPR&D was expensed since technological feasibility of the underlying projects had not yet been reached and such technology had no future alternative use. Additionally, the Company recognized $1.6 million of IPR&D charges related to the purchase of intellectual property in its CRM and CV segments.
Special Charges
Fiscal Year 2010
During 2010, the Company recorded $27.9 million of inventory obsolescence charges to cost of sales primarily related to excess legacy ICD inventory that was not expected to be sold due to the Company’s recent launch of its UnifyTM CRT-D and FortifyTM ICD devices. The Company’s market demand for these devices has resulted in a more rapid adoption than expected or historically experienced from other ICD product launches.
The Company also reached an agreement with the Boston U.S. Department of Justice to settle the previously disclosed investigation initiated in 2005 related to an industry-wide review of post-market clinical studies and registries, resulting in a $16.5 million legal settlement charge (see Note 5).
In order to enhance segment comparability and reflect management’s focus on the ongoing operations of the Company, the 2010 special charges have not been recorded in the individual reportable segments.
Fiscal Year 2009
During 2009, the Company incurred charges totaling $107.7 million, of which $71.1 million related to severance and benefit costs for approximately 725 employees. These costs were recognized after management determined that such severance and benefits were probable and estimable, in accordance with ASC Topic 712,Nonretirement Postemployment Benefits. Of the total $71.1 million severance and benefits charge, $6.6 million was recorded in cost of sales. The Company also recorded $17.7 million of inventory related charges to cost of sales associated with inventory that would be scrapped in connection with the Company’s decision to terminate certain product lines in its CRM and AF divisions that were redundant with other existing products lines. Additionally, the Company recorded $5.9 million of fixed asset related charges to cost of sales associated with the accelerated depreciation of phasing out older model diagnostic equipment and $6.1 million of asset write-offs related to the carrying value of assets that will no longer be utilized. Of the $6.1 million charge, $3.5 million was recorded in cost of sales. The Company also recorded charges of $1.8 million associated with contract terminations and $5.1 million of other unrelated costs.
48
A summary of the activity related to the 2009 special charge accrual is as follows (in thousands):
| | | | | | | | | | | | | | | | |
| | Employee
termination
costs | | Inventory
charges | | Fixed asset
charges | | Other | | Total | |
Balance at January 3, 2009 | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | |
| | | | | | | | | | | | | | | | |
Special charges | | | 71,158 | | | 17,735 | | | 11,982 | | | 6,869 | | | 107,744 | |
Non-cash charges used | | | — | | | (17,735 | ) | | (11,982 | ) | | — | | | (29,717 | ) |
Cash payments | | | (22,560 | ) | | — | | | — | | | (349 | ) | | (22,909 | ) |
Foreign exchange rate impact | | | (758 | ) | | — | | | — | | | — | | | (758 | ) |
| | | | | | | | | | | | | | | | |
Balance at January 2, 2010 | | $ | 47,840 | | $ | — | | $ | — | | $ | 6,520 | | $ | 54,360 | |
| | | | | | | | | | | | | | | | |
Cash payments | | | (37,532 | ) | | — | | | — | | | (5,857 | ) | | (43,389 | ) |
Foreign exchange rate impact | | | (1,156 | ) | | — | | | — | | | (118 | ) | | (1,274 | ) |
| | | | | | | | | | | | | | | | |
Balance at January 1, 2011 | | $ | 9,152 | | $ | — | | $ | — | | $ | 545 | | $ | 9,697 | |
In order to enhance segment comparability and reflect management’s focus on the ongoing operations of the Company, the 2009 special charges have not been recorded in the individual reportable segments.
Fiscal Year 2008
Impairment Charges: During 2008, the Company determined that technology under a license agreement covering certain CRM devices was no longer fully utilized and certain patents under the license were no longer valid based upon recent patent law developments. As a result, the Company recognized an impairment charge of $43.5 million to cost of sales to write its intangible asset for the technology license agreement down to its fair value. The Company also recognized a $37.0 million impairment charge to write down intangible assets relating to certain products lines acquired from Velocimed to their fair value due to a reduction in future revenue and cash flow projections after termination of a clinical trial and unfavorable 2008 sales performance. The Company also recognized other impairment charges of $5.8 million related to assets in the Cardiovascular division that will no longer be utilized and discontinued the use of its ANS tradename, resulting in a $1.7 million impairment charge to write-off of the ANS tradename intangible assets.
Inventory Charges: The Company enters into purchase contracts in the normal course of business for certain raw material commodities that are used in the manufacture of its products. Favorable decreases in commodity prices resulted in the Company’s election to terminate and exit some of its contracts resulting in a $10.7 million termination payment, which was recorded as a special charge in cost of sales. The Company also recognized additional inventory obsolescence charges related to inventory not expected to be sold due to the termination of a distribution agreement in Japan during 2007. The Company increased this estimate in 2008 and recorded an additional $3.0 million charge in cost of sales.
Other Charges: In 2008, the Company launched its MerlinTM@home wireless patient monitoring system and committed to provide this system without charge to existing St. Jude Medical CRM patients. In connection with the completion of this roll-out in the fourth quarter of 2008, the Company recorded a $7.4 million special charge in cost of sales to accrue for the related costs. The Company also recognized $5.5 million of other unrelated costs.
In order to enhance segment comparability and reflect management’s focus on the ongoing operations of the Company, the 2008 special charges have not been recorded in the individual reportable segments.
49
NOTE 9 – OTHER INCOME (EXPENSE), NET
The Company’s other income (expense) consisted of the following (in thousands):
| | | | | | | | | | |
| | 2010 | | 2009 | | 2008 | |
Interest income | | $ | 2,076 | | $ | 2,057 | | $ | 16,315 | |
Interest expense | | | (67,372 | ) | | (45,603 | ) | | (72,554 | ) |
Other | | | (3,150 | ) | | (12,107 | ) | | (18,040 | ) |
Total other income (expense), net | | $ | (68,446 | ) | $ | (55,653 | ) | $ | (74,279 | ) |
The Company classifies realized gains or losses from the sale of investments and investment impairment charges as other income (expense). The Company recorded a $4.9 million realized gain in other income associated with the sale of an available-for-sale investment in 2010. During 2010, 2009 and 2008, the Company recognized investment impairment charges of $5.2 million, $8.3 million and $12.9 million, respectively, in other expense (see Note 12).
NOTE 10 – INCOME TAXES
The Company’s earnings before income taxes were generated from its U.S. and international operations as follows (in thousands):
| | | | | | | | | | |
| | 2010 | | 2009 | | 2008 | |
U.S. | | $ | 553,090 | | $ | 559,868 | | $ | 530,843 | |
International | | | 655,713 | | | 497,525 | | | 49,925 | |
Earnings before income taxes | | $ | 1,208,803 | | $ | 1,057,393 | | $ | 580,768 | |
| | | | | | | | | | |
Income tax expense consisted of the following (in thousands): | | | | | | | | | | |
| | | | | | | | | | |
| | 2010 | | 2009 | | 2008 | |
Current: | | | | | | | | | | |
U.S. federal | | $ | 263,743 | | $ | 212,721 | | $ | 198,179 | |
U.S. state and other | | | 14,498 | | | 23,292 | | | 26,863 | |
International | | | 56,755 | | | 58,212 | | | 53,070 | |
Total current | | | 334,996 | | | 294,225 | | | 278,112 | |
|
Deferred | | | (33,629 | ) | | (14,058 | ) | | (50,362 | ) |
Income tax expense | | $ | 301,367 | | $ | 280,167 | | $ | 227,750 | |
The tax effects of the cumulative temporary differences between the tax bases of assets and liabilities and their respective carrying amounts for financial statement purposes were as follows (in thousands):
| | | | | | | |
| | 2010 | | 2009 | |
Deferred income tax assets: | | | | | | | |
Net operating loss carryforwards | | $ | 23,759 | | $ | 22,057 | |
Tax credit carryforwards | | | 66,437 | | | 59,623 | |
Inventories | | | 145,239 | | | 115,247 | |
Stock-based compensation | | | 68,854 | | | 56,837 | |
Accrued liabilities and other | | | 162,453 | | | 148,607 | |
Deferred income tax assets | | | 466,742 | | | 402,371 | |
Deferred income tax liabilities: | | | | | | | |
Unrealized gain on available-for-sale securities | | | (9,360 | ) | | (7,584 | ) |
Property, plant and equipment | | | (190,236 | ) | | (168,173 | ) |
Intangible assets | | | (381,050 | ) | | (194,268 | ) |
Deferred income tax liabilities | | | (580,646 | ) | | (370,025 | ) |
Net deferred income tax assets (liabilities) | | $ | (113,904 | ) | $ | 32,346 | |
50
The Company has not recorded any valuation allowance for its deferred tax assets as of January 1, 2011 or January 2, 2010 as the Company believes that its deferred tax assets, including the net operating and capital loss carryforwards, will be fully realized based upon its estimates of future taxable income.
A reconciliation of the U.S. federal statutory income tax rate to the Company’s effective income tax rate is as follows:
| | | | | | | | | | |
| | 2010 | | 2009 | | 2008 | |
U.S. federal statutory tax rate | | | 35.0 | % | | 35.0 | % | | 35.0 | % |
Increase (decrease) in tax rate resulting from: | | | | | | | | | | |
U.S. state income taxes, net of federal tax benefit | | | 2.2 | | | 1.6 | | | 3.3 | |
International taxes at lower rates | | | (10.0 | ) | | (6.4 | ) | | (9.9 | ) |
Tax benefits from domestic manufacturer’s deduction | | | (1.1 | ) | | (0.9 | ) | | (1.7 | ) |
Research and development credits | | | (2.4 | ) | | (2.9 | ) | | (6.0 | ) |
Non-deductible IPR&D charges | | | 0.4 | | | — | | | 19.2 | |
Other | | | 0.8 | | | 0.1 | | | (0.7 | ) |
Effective income tax rate | | | 24.9 | % | | 26.5 | % | | 39.2 | % |
The Company’s effective income tax rate is favorably impacted by Puerto Rican tax exemption grants, which result in Puerto Rico earnings being partially tax exempt through the year 2023.
At January 1, 2011, the Company had $78.7 million of U.S. federal net operating and capital loss carryforwards and $0.6 million of U.S. tax credit carryforwards that will expire from 2013 through 2029 if not utilized. The Company also has state net operating loss carryforwards of $22.6 million that will expire from 2012 through 2016 and tax credit carryforwards of $91.6 million that have an unlimited carryforward period. These amounts are subject to annual usage limitations. The Company’s net operating loss carryforwards arose primarily from acquisitions.
The Company has not recorded U.S. deferred income taxes on $1,819.9 million of its non-U.S. subsidiaries’ undistributed earnings because such amounts are intended to be reinvested outside the United States indefinitely. If these earnings were repatriated to the United States, the Company would be required to accrue and pay U.S. Federal income taxes and foreign withholding taxes, as adjusted for foreign tax credits. Determination of the amount of any unrecognized deferred income tax liability on these earnings is not practicable.
The Company records all income tax accruals in accordance with ASC Topic 740,Income Taxes. At January 1, 2011, the liability for unrecognized tax benefits was $162.9 million, and the accrual for interest and penalties was $33.8 million. At January 2, 2010, the Company had $120.5 million accrued for unrecognized tax benefits and $28.3 million accrued for interest and penalties. The Company recognizes interest and penalties related to income tax matters in income tax expense. The Company recognized interest and penalties, net of tax benefit, of $3.5 million, $4.3 million and $2.8 million during fiscal years 2010, 2009 and 2008, respectively. The Company does not expect its unrecognized tax benefits to change significantly over the next 12 months.
The following table summarizes the activity related to the Company’s unrecognized tax benefits (in thousands):
| | | | | | | |
| | 2010 | | 2009 | |
Balance at beginning of year | | $ | 120,517 | | $ | 82,692 | |
Increases related to current year tax positions | | | 32,721 | | | 36,327 | |
Increases related to prior year tax positions | | | 19,029 | | | 5,303 | |
Reductions related to prior year tax positions | | | (8,648 | ) | | (586 | ) |
Reductions related to settlements / payments | | | — | | | (50 | ) |
Expiration of the statute of limitations for the assessment of taxes | | | (715 | ) | | (3,169 | ) |
Balance at end of year | | $ | 162,904 | | $ | 120,517 | |
51
The Company is subject to U.S. federal income tax as well as income tax of multiple state and foreign jurisdictions. The Company has substantially concluded all U.S. federal income tax matters for all tax years through 2001. Additionally, substantially all material foreign, state, and local income tax matters have been concluded for all tax years through 1999. The U.S. Internal Revenue Service (IRS) completed an audit of the Company’s 2002 through 2005 tax returns and proposed adjustments in its audit report issued in November 2008. The Company is vigorously defending its positions and initiated defense of these adjustments at the IRS appellate level in January 2009. An unfavorable outcome could have a material negative impact on the Company’s effective income tax rate in future periods.
NOTE 11 – RETIREMENT PLANS
Defined Contribution Plans: The Company has a 401(k) profit sharing plan that provides retirement benefits to substantially all full-time U.S. employees. Eligible employees may contribute a percentage of their annual compensation, subject to IRS limitations, with the Company matching a portion of the employees’ contributions. The Company also may contribute a portion of its earnings to the plan based upon Company performance. The Company’s matching and profit sharing contributions are at the discretion of the Company’s Board of Directors. In addition, the Company has defined contribution programs for employees in certain countries outside the United States. Company contributions under all defined contribution plans totaled $21.1 million, $22.2 million and $63.2 million in 2010, 2009 and 2008, respectively.
The Company also has a non-qualified deferred compensation plan that provides certain officers and employees the ability to defer a portion of their compensation until a later date. The deferred amounts and earnings thereon are payable to participants, or designated beneficiaries, at specified future dates upon retirement, death or termination from the Company. The deferred compensation liability, which is classified as other liabilities, was approximately $190 million and $160 million at January 1, 2011 and January 2, 2010, respectively.
Defined Benefit Plans: The Company has funded and unfunded defined benefit plans for employees in certain countries outside the United States. The Company had an accrued liability totaling $42.0 million and $30.2 million at January 1, 2011 and January 2, 2010, respectively, which approximated the actuarial calculated unfunded liability. The amount of funded plan assets and the amount of pension expense was not material.
NOTE 12 – FAIR VALUE MEASUREMENTS AND FINANCIAL INSTRUMENTS
The fair value measurement accounting standard, codified in ASC Topic 820, provides a framework for measuring fair value and defines fair value as the price that would be received to sell an asset or paid to transfer a liability. Fair value is a market-based measurement that should be determined using assumptions that market participants would use in pricing an asset or liability. The standard establishes a valuation hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability developed based on independent market data sources. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available. The valuation hierarchy is composed of three categories. The categorization within the valuation hierarchy is based on the lowest level of input that is significant to the fair value measurement.
The categories within the valuation hierarchy are described as follows:
| | |
| • | Level 1 – Inputs to the fair value measurement are quoted prices in active markets for identical assets or liabilities. |
| | |
| • | Level 2 – Inputs to the fair value measurement include quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly. |
| | |
| • | Level 3 – Inputs to the fair value measurement are unobservable inputs or valuation techniques. |
52
Assets and Liabilities that are Measured at Fair Value on a Recurring Basis
The fair value measurement standard applies to certain financial assets and liabilities that are measured at fair value on a recurring basis (each reporting period). These financial assets and liabilities include money-market securities, trading marketable securities, available-for-sale marketable securities and derivative instruments. The Company continues to record these items at fair value on a recurring basis and the fair value measurements are applied using ASC Topic 820. The Company does not have any material nonfinancial assets or liabilities that are measured at fair value on a recurring basis. A summary of the valuation methodologies used for the respective financial assets and liabilities measured at fair value on a recurring basis is as follows:
Money-market securities: The Company’s money-market securities include funds that are traded in active markets and are recorded at fair value based upon the quoted market prices. The Company classifies these securities as level 1.
Trading securities: The Company’s trading securities include publicly-traded mutual funds that are traded in active markets and are recorded at fair value based upon the net asset values of the shares. The Company classifies these securities as level 1.
Available-for-sale securities: The Company’s available-for-sale securities include publicly-traded equity securities that are traded in active markets and are recorded at fair value based upon the closing stock prices. The Company classifies these securities as level 1.
Derivative instruments: The Company’s derivative instruments consist of foreign currency exchange contracts and interest rate swap contracts. The Company classifies these instruments as level 2 as the fair value is determined using inputs other than observable quoted market prices. These inputs include spot and forward foreign currency exchange rates and interest rates that the Company obtains from standard market data providers. The fair value of the Company’s foreign currency exchange contracts was not material at January 1, 2011 or January 2, 2010.
A summary of financial assets measured at fair value on a recurring basis at January 1, 2011 and January 2, 2010 is as follows (in thousands):
| | | | | | | | | | | | | |
| | January 1, 2011 | | Quoted Prices
In Active
Markets
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | |
Assets | | | | | | | | | | | | | |
Money-market securities | | $ | 364,418 | | $ | 364,418 | | $ | — | | $ | — | |
Trading marketable securities | | | 190,438 | | | 190,438 | | | — | | | — | |
Available-for-sale marketable securities | | | 33,745 | | | 33,745 | | | — | | | — | |
Total assets | | $ | 588,601 | | $ | 588,601 | | $ | — | | $ | — | |
|
Liabilities | | | | | | | | | | | | | |
Interest rate swap | | $ | 10,046 | | $ | — | | $ | 10,046 | | $ | — | |
Total liablities | | $ | 10,046 | | $ | — | | $ | 10,046 | | $ | — | |
53
| | | | | | | | | | | | | |
| | January 2, 2010 | | Quoted Prices
In Active
Markets
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | |
Assets | | | | | | | | | | | | | |
Money-market securities | | $ | 258,936 | | $ | 258,936 | | $ | — | | $ | — | |
Trading marketable securities | | | 160,285 | | | 160,285 | | | — | | | — | |
Available-for-sale marketable securities | | | 31,711 | | | 31,711 | | | — | | | — | |
Total | | $ | 450,932 | | $ | 450,932 | | $ | — | | $ | — | |
The Company’s money-market securities are classified as cash equivalents as the funds are highly liquid investments readily convertible to cash. The Company also had $135.9 million and $134.0 million of cash equivalents invested in short-term time deposits and interest and non-interest bearing bank accounts at January 1, 2011 and January 2, 2010, respectively. The Company’s marketable securities consist of publicly-traded equity securities that are classified as available-for-sale marketable securities and investments in mutual funds that are classified as trading marketable securities. On the balance sheet, available-for-sale marketable securities and trading marketable securities are classified as other current assets and other assets, respectively. The interest rate swap is currently classified in other liabilities, given the long-term nature of the instrument.
Assets and Liabilities that are Measured at Fair Value on a Nonrecurring Basis
At the beginning of fiscal year 2009, the fair value measurement standard also applied to certain nonfinancial assets and liabilities that are measured at fair value on a nonrecurring basis. For example, certain long-lived assets such as goodwill, intangible assets and property, plant and equipment are measured at fair value in connection with business combinations or when an impairment is recognized and the related assets are written down to fair value.
The following table provides information by level for assets and liabilities that were measured at fair value on a nonrecurring basis. This table provides the fair value of net identifiable tangible and intangible assets and liabilities (excluding goodwill) for business combinations that closed during 2010. The Company used inputs other than quoted prices that are observable, such as interest rates, cost of capital and market comparable royalty rates, which are applied to income and market valuation approaches to value its business combinations. A summary of the nonfinancial assets and liabilities measured at fair value in conjunction with the business combinations is as follows (in thousands):
| | | | | | | | | | | | | |
| | Fair Value | | Quoted Prices
In Active
Markets
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | |
Description | | | | | | | | | | | | | |
Business Combination | | $ | 254,632 | | $ | — | | $ | 254,632 | | $ | — | |
The Company did not make any material business combinations during 2009. Additionally, no material impairments of the Company’s long-lived assets were recognized during 2010 or 2009.
The Company also holds investments in equity securities that are accounted for as cost method investments, which are classified as other assets and measured at fair value on a nonrecurring basis. The carrying value of these investments approximated $124 million at January 1, 2011 and $57 million at January 2, 2010. The fair value of the Company’s cost method investments is not estimated if there are no identified events or changes in circumstances that may have a significant adverse effect on the fair value of these investments. When measured on a nonrecurring basis, the Company’s cost method investments are considered Level 3 in the fair value hierarchy, due to the use of unobservable inputs to measure fair value. During 2009, the Company determined that the fair value of a cost method investment was below its carrying value and that the carrying value of the investment would not be recoverable within a reasonable period of time. As a result, the Company measured the fair value of the investment using market participant valuations from recent and proposed equity offerings for this company (Level 3) and recognized an $8.3 million impairment charge in other expense (see Note 9), reducing the $13.5 million carrying value of the investment to $5.2 million. During 2010, the Company further determined that this cost method investment was fully impaired as it did not believe that any of the investment carrying value would be recovered due to the company’s substantial inability to operate as a going concern given its financial condition. As a result, the Company recognized a $5.2 million impairment charge in other expense during 2010.
54
Prior to adopting the fair value measurement accounting guidance of ASC Topic 820, the Company recorded other cost method investment impairment charges in 2008 of $12.2 million. The Company evaluated the fair values of the related investments and determined that the impairments were other-than-temporary based upon the magnitude and length of time that the investments’ fair values had declined.
Fair Value of Other Financial Instruments
The aggregate fair value of the Company’s fixed-rate senior notes at January 1, 2011 (measured using quoted prices in active markets) was $2,478.9 million compared to the aggregate carrying value of $2,406.5 million (inclusive of the interest rate swaps). The fair value of the Company’s other debt obligations at January 1, 2011 approximated their aggregate $105.1 million carrying value due to the variable interest rate and short-term nature of these instruments.
NOTE 13 – DERIVATIVE FINANCIAL INSTRUMENTS
The Company follows the provisions of ASC Topic 815 in accounting for and disclosing derivative instruments and hedging activities. All derivative financial instruments are recognized on the balance sheet at fair value. Changes in the fair value of derivatives are recognized in net earnings or other comprehensive income depending on whether the derivative is designated as part of a qualifying hedging transaction. Derivative assets and derivative liabilities are classified as other current assets, other assets, other current liabilities or other liabilities, as appropriate.
Foreign Currency Forward Contracts
The Company hedges a portion of its foreign currency exchange rate risk through the use of forward exchange contracts. The Company uses forward exchange contracts to manage foreign currency exposures related to intercompany receivables and payables arising from intercompany purchases of manufactured products. These forward contracts are not designated as qualifying hedging relationships under ASC Topic 815. The Company measures its foreign currency exchange contracts at fair value on a recurring basis. The fair value of outstanding contracts was immaterial as of January 1, 2011 and January 2, 2010. During fiscal years 2010, 2009 and 2008, the net amount of gains (losses) the Company recorded to other income (expense) for its forward currency exchange contracts not designated as hedging instruments under ASC Topic 815 were net losses of $(0.2) million, $(6.7) million and $(7.5) million, respectively. These net losses were almost entirely offset by corresponding net gains on the foreign currency exposures being managed. The Company does not enter into contracts for trading or speculative purposes. The Company’s policy is to enter into hedging contracts with major financial institutions that have at least an “A” (or equivalent) credit rating.
Interest Rate Swap
The Company hedges the fair value of certain debt obligations through the use of interest rate swap contracts. For interest rate swap contracts that are designated and qualify as fair value hedges, the gain or loss on the swap and the offsetting gain or loss on the hedged debt instrument attributable to the hedged risk are recognized in net earnings. Changes in the value of the fair value hedge are recognized in interest expense, offsetting the changes in the fair value of the hedged debt instrument. Additionally, any payments made or received under the swap contracts are accrued and recognized as interest expense. The Company’s current interest rate swap is designed to manage the exposure to changes in the fair value of its 2016 Senior Notes. The swap is designated as a fair value hedge of the variability of the fair value of the fixed-rate 2016 Senior Notes due to changes in the long-term benchmark interest rates. Under the swap agreement, the Company agrees to exchange, at specified intervals, fixed and floating interest amounts calculated by reference to an agreed-upon notional principal amount. As of January 1, 2011, the fair value of the interest rate swap was a $10.0 million liability which was classified as other liabilities on the consolidated balance sheet.
55
In March 2010, the Company entered into a 3-year, $450.0 million notional amount interest rate swap designated as a fair value hedge of the changes in fair value of the Company’s fixed-rate 2013 Senior Notes. On November 8, 2010, the Company terminated the interest rate swap and received a cash payment of $19.3 million. The gain from terminating the interest rate swap is being amortized as a reduction of interest expense over the remaining life of the 2013 Senior Notes.
In November 2008, the Company entered into an interest rate swap contract to convert $400.0 million of variable-rate borrowings under the Company’s Credit Facility into fixed-rate borrowings (see Note 4). The Company designated this interest rate swap as a cash flow hedge under ASC Topic 815. This contract terminated in February 2009. The ineffective portion of the amount of gains (losses) recognized in net earnings was immaterial. The Company recorded the $(0.4) million after-tax loss on the settlement of the interest rate swap contract to interest expense.
NOTE 14 – SEGMENT AND GEOGRAPHIC INFORMATION
Segment Information: The Company’s four operating segments are Cardiac Rhythm Management (CRM), Cardiovascular (CV), Atrial Fibrillation (AF), and Neuromodulation (NMD). The primary products produced by each operating segment are: CRM – ICDs and pacemakers; CV – vascular closure devices, heart valve replacement and repair products, pressure measurement guidewires, coronary imaging technology and structural heart defect and vascular abnormality devices; AF – EP introducers and catheters, advanced cardiac mapping, navigation and recording systems and ablation systems; and NMD – neurostimulation devices.
The Company has aggregated the four operating segments into two reportable segments based upon their similar operational and economic characteristics: CRM/NMD and CV/AF. Net sales of the Company’s reportable segments include end-customer revenues from the sale of products they each develop and manufacture or distribute. The costs included in each of the reportable segments’ operating results include the direct costs of the products sold to customers and operating expenses managed by each of the reportable segments. Certain operating expenses managed by the Company’s selling and corporate functions, including all stock-based compensation expense, impairment charges, certain acquisition-related charges, purchased IPR&D charges and special charges have not been recorded in the individual reportable segments. As a result, reportable segment operating profit is not representative of the operating profit of the products in these reportable segments. Additionally, certain assets are managed by the Company’s selling and corporate functions, principally including trade receivables, inventory, corporate cash and cash equivalents and deferred income taxes. For management reporting purposes, the Company does not compile capital expenditures by reportable segment; therefore, this information has not been presented as it is impracticable to do so.
56
The following table presents certain financial information by reportable segment (in thousands):
| | | | | | | | | | | | | |
| | CRM/NMD | | CV/AF | | Other | | Total | |
| | | | | | | | | | | | | |
Fiscal Year 2010 | | | | | | | | | | | | | |
Net sales | | $ | 3,420,215 | | $ | 1,744,556 | | $ | — | | $ | 5,164,771 | |
Operating profit | | | 2,125,163 | | | 968,606 | | | (1,816,520 | ) | | 1,277,249 | |
Depreciation and amortization expense | | | 91,387 | | | 52,184 | | | 100,444 | | | 244,015 | |
Total assets | | | 2,150,359 | | | 3,097,190 | | | 3,318,899 | | | 8,566,448 | |
| | | | | | | | | | | | | |
Fiscal Year 2009 | | | | | | | | | | | | | |
Net sales | | $ | 3,099,800 | | $ | 1,581,473 | | $ | — | | $ | 4,681,273 | |
Operating profit | | | 1,931,929 | | | 829,966 | | | (1,648,849 | ) | | 1,113,046 | |
Depreciation and amortization expense | | | 83,506 | | | 45,765 | | | 84,194 | | | 213,465 | |
Total assets | | | 2,124,534 | | | 1,294,009 | | | 3,007,268 | | | 6,425,811 | |
| | | | | | | | | | | | | |
Fiscal Year 2008 | | | | | | | | | | | | | |
Net sales | | $ | 2,955,603 | | $ | 1,407,648 | | $ | — | | $ | 4,363,251 | |
Operating profit | | | 1,824,023 | | | 736,979 | | | (1,905,955 | ) | | 655,047 | |
Depreciation and amortization expense | | | 93,397 | | | 38,743 | | | 70,288 | | | 202,428 | |
Total assets | | | 2,018,478 | | | 1,267,290 | | | 2,436,736 | | | 5,722,504 | |
Net sales by class of similar products for the respective fiscal years were as follows (in thousands):
| | | | | | | | | | |
Net Sales | | 2010 | | 2009 | | 2008 | |
Cardiac rhythm management | | $ | 3,039,953 | | $ | 2,769,034 | | $ | 2,701,463 | |
Cardiovascular | | | 1,036,683 | | | 953,620 | | | 862,136 | |
Atrial fibrillation | | | 707,873 | | | 627,853 | | | 545,512 | |
Neuromodulation | | | 380,262 | | | 330,766 | | | 254,140 | |
| | $ | 5,164,771 | | $ | 4,681,273 | | $ | 4,363,251 | |
| | | | | | | | | | |
Geographic Information: The Company markets and sells its products primarily through a direct sales force. The principal geographic markets for the Company’s products are the United States, Europe, Japan and Asia Pacific. The Company attributes net sales to geographic markets based on the location of the customer. Other than the United States, Europe, Japan and Asia Pacific no one geographic market is greater than 5% of consolidated net sales. Net sales by significant geographic market based on customer location for the respective fiscal years were as follows (in thousands):
| | | | | | | | | | |
Net Sales | | 2010 | | 2009 | | 2008 | |
United States | | $ | 2,655,034 | | $ | 2,468,191 | | $ | 2,319,645 | |
International | | | | | | | | | | |
Europe | | | 1,314,350 | | | 1,197,912 | | | 1,152,601 | |
Japan | | | 552,737 | | | 480,897 | | | 387,648 | |
Asia Pacific | | | 323,855 | | | 254,429 | | | 234,073 | |
Other | | | 318,795 | | | 279,844 | | | 269,284 | |
| | | 2,509,737 | | | 2,213,082 | | | 2,043,606 | |
| | $ | 5,164,771 | | $ | 4,681,273 | | $ | 4,363,251 | |
57
The amounts for long-lived assets by significant geographic market include net property, plant and equipment by physical location of the asset as follows (in thousands):
| | | | | | | | | | |
Long-Lived Assets | | January 1, 2011 | | January 2, 2010 | | January 3, 2009 | |
United States | | $ | 965,936 | | $ | 876,462 | | $ | 775,205 | |
International | | | | | | | | | | |
Europe | | | 85,961 | | | 77,790 | | | 84,266 | |
Japan | | | 25,583 | | | 18,756 | | | 16,001 | |
Asia Pacific | | | 74,537 | | | 39,946 | | | 17,087 | |
Other | | | 171,914 | | | 140,132 | | | 87,617 | |
| | | 357,995 | | | 276,624 | | | 204,971 | |
| | $ | 1,323,931 | | $ | 1,153,086 | | $ | 980,176 | |
NOTE 15 – QUARTERLY FINANCIAL DATA (UNAUDITED)
| | | | | | | | | | | | | |
(in thousands, except per share amounts) | | First
Quarter | | Second
Quarter | | Third
Quarter | | Fourth
Quarter | |
|
Fiscal Year 2010: | | | | | | | | | | | | | |
Net sales | | $ | 1,261,696 | | $ | 1,312,769 | | $ | 1,239,905 | | $ | 1,350,401 | |
Gross profit | | | 940,527 | | | 967,467 | | | 900,086 | | $ | 946,580 | (b) |
Net earnings | | | 238,569 | | | 254,038 | | | 208,385 | (a) | $ | 206,444 | (c) |
Basic net earnings per share | | $ | 0.73 | | $ | 0.78 | | $ | 0.63 | | $ | 0.62 | |
Diluted net earnings per share | | $ | 0.73 | | $ | 0.77 | | $ | 0.63 | | $ | 0.62 | |
Fiscal Year 2009: | | | | | | | | | | | | | |
Net sales | | $ | 1,133,793 | | $ | 1,184,412 | | $ | 1,159,606 | | $ | 1,203,462 | |
Gross profit | | | 839,298 | | | 878,868 | | | 853,875 | (d) | | 855,847 | (f) |
Net earnings | | | 201,271 | | | 219,370 | | | 166,935 | (e) | | 189,650 | (g) |
Basic net earnings per share | | $ | 0.58 | | $ | 0.63 | | $ | 0.49 | | $ | 0.57 | |
Diluted net earnings per share | | $ | 0.58 | | $ | 0.63 | | $ | 0.48 | | $ | 0.57 | |
| | |
| (a) | Includes after-tax IPR&D charges of $12.2 million related to the Company’s purchase of certain pre-development technology assets. |
| (b) | Includes pre-tax special charges of $27.9 million primarily related to inventory obsolescence charges resulting from excess ICD inventory. |
| (c) | Includes after-tax special charges of $17.4 million primarily related to inventory obsolescence charges resulting from excess ICD inventory; after-tax special charges of $15.3 million in connection with the settlement of a U.S. Department of Justice investigation; and an after-tax impairment charge of $5.2 million related to a cost method investment deemed to be other-than-temporarily impaired. Partially offsetting these after-tax charges is a $19.7 million income tax benefit related to the federal research and development tax credit extended in the fourth quarter of 2010 retroactive to the beginning of the year. |
| (d) | Includes pre-tax special charges of $6.1 million related to initiatives to streamline the Company’s production activities. |
| (e) | Includes after-tax special charges of $29.4 million related to initiatives to enhance the efficiency and effectiveness of the sales, marketing and customer service operations and to streamline the Company’s production activities; and $2.5 million associated with other unrelated costs. The Company also recorded an after-tax impairment charge of $5.2 million related to a cost method investment deemed to be other-than-temporarily impaired. |
58
| | |
| (f) | Includes pre-tax special charges of $0.5 million related to initiatives to streamline the Company’s production activities; $17.7 million of inventory obsolescence charges for discontinued products; and $9.4 million of accelerated depreciation charges and write-offs for assets that will no longer be utilized. |
| (g) | Includes after-tax special charges of $44.5 million, which consist of the following: $22.3 million related to initiatives to enhance the efficiency and effectiveness of the sales, marketing and customer service operations and to streamline the Company’s production activities; $11.3 million of inventory obsolescence charges for discontinued products; $8.7 million of accelerated depreciation charges and write-offs for assets that will no longer be utilized; and $2.2 million associated with contract terminations and other unrelated costs. The Company also recorded after-tax IPR&D charges of $3.7 million related to the Company’s purchase of certain pre-development technology assets. |
NOTE 16 – SUBSEQUENT EVENT
On February 26, 2011, the Company’s Board of Directors authorized a cash dividend of $0.21 per share payable on April 29, 2011 to holders of record as of March 31, 2011. The Company expects to pay quarterly cash dividends in the foreseeable future.
59
Five-Year Summary Financial Data
(in thousands, except per share amounts)
| | | | | | | | | | | | | | | | |
| | 2010 (a) | | 2009 (b) | | 2008 (c) | | 2007 (d) | | 2006 (e) | |
SUMMARY OF OPERATIONS FOR THE FISCAL YEAR: | | | | | | | | |
Net sales | | $ | 5,164,771 | | $ | 4,681,273 | | $ | 4,363,251 | | $ | 3,779,277 | | $ | 3,302,447 | |
Gross profit | | $ | 3,754,660 | | $ | 3,427,888 | | $ | 3,192,710 | | $ | 2,737,683 | | $ | 2,388,934 | |
Percent of net sales | | | 72.7 | % | | 73.2 | % | | 73.2 | % | | 72.4 | % | | 72.3 | % |
Operating profit | | $ | 1,277,249 | | $ | 1,113,046 | | $ | 655,047 | | $ | 793,503 | | $ | 743,083 | |
Percent of net sales | | | 24.7 | % | | 23.8 | % | | 15.0 | % | | 21.0 | % | | 22.5 | % |
Net earnings | | $ | 907,436 | | $ | 777,226 | | $ | 353,018 | | $ | 537,756 | | $ | 539,042 | |
Percent of net sales | | | 17.6 | % | | 16.6 | % | | 8.1 | % | | 14.2 | % | | 16.3 | % |
Diluted net earnings per share | | $ | 2.75 | | $ | 2.26 | | $ | 1.01 | | $ | 1.53 | | $ | 1.45 | |
FINANCIAL POSITION AT YEAR END: | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 500,336 | | $ | 392,927 | | $ | 136,443 | | $ | 389,094 | | $ | 79,888 | |
Working capital (f) | | | 1,894,898 | | | 1,492,893 | | | 1,051,539 | | | 278,954 | | | 1,013,958 | |
Total assets | | | 8,566,448 | | | 6,425,811 | | | 5,722,504 | | | 5,329,404 | | | 4,789,794 | |
Total debt (g) | | | 2,511,603 | | | 1,922,402 | | | 1,201,602 | | | 1,338,018 | | | 859,137 | |
Shareholders’ equity | | $ | 4,371,671 | | $ | 3,323,551 | | $ | 3,235,906 | | $ | 2,959,319 | | $ | 2,969,226 | |
OTHER DATA: | | | | | | | | | | | | | | | | |
Diluted weighted average | | | | | | | | | | | | | | | | |
shares outstanding | | | 330,488 | | | 344,359 | | | 349,722 | | | 352,444 | | | 372,830 | |
Fiscal year 2008 consisted of 53 weeks. All other fiscal years noted above consisted of 52 weeks. The Company did not declare or pay any cash dividends during 2006 through 2010.
| | |
| (a) | Results for 2010 include after-tax special charges of $32.8 million, after-tax IPR&D charges of $12.2 million and an after-tax investment impairment charge of $5.2 million. The impact of these items on 2010 net earnings was $50.2 million, or $0.15 per diluted share. See Notes to the Consolidated Financial Statements in the Financial Report for further detail. |
| (b) | Results for 2009 include after-tax special charges of $76.4 million, an after-tax investment impairment charge of $5.2 million and after-tax IPR&D charges of $3.7 million. The impact of these items on 2009 net earnings was $85.3 million, or $0.25 per diluted share. See Notes to the Consolidated Financial Statements in the Financial Report for further detail. |
| (c) | Results for 2008 include $319.4 million of after-tax IPR&D charges, after-tax special charges of $72.7 million and after-tax investment impairment charges of $8.0 million. The impact of these items on 2008 net earnings was $400.1 million, or $1.15 per diluted share. See Notes to the Consolidated Financial Statements in the Financial Report for further detail. |
| (d) | Results for 2007 include after-tax special charges of $77.2 million and an after-tax investment impairment charge of $15.7 million. The impact of these items on 2007 net earnings was $92.9 million, or $0.26 per diluted share. |
| (e) | Results for 2006 include after-tax special charges of $22.0 million, or $0.06 per diluted share, related to restructuring activities in the Company’s former Cardiac Surgery and Cardiology divisions and international selling organization. |
| (f) | Total current assets less total current liabilities. Working capital fluctuations can be significant based on the maturity dates of the Company’s debt obligations. |
| (g) | Total debt consists of current debt obligations and long-term debt. |
60
Cumulative Total Shareholder Returns
(in dollars)
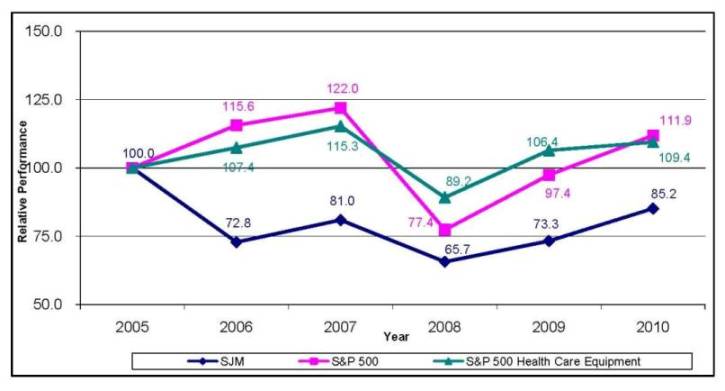
The graph compares the cumulative total shareholder returns for St. Jude Medical common stock for the last five fiscal years with the Standard & Poor’s 500 Health Care Equipment Index and the Standard & Poor’s 500 Index weighted by market value at each measurement point. The comparison assumes that $100 was invested on December 31, 2005, in St. Jude Medical common stock and in each of these Standard & Poor’s indexes and assumes the reinvestment of any dividends.
61
Stock Transfer Agent
Requests concerning the transfer or exchange of shares, lost stock certificates, duplicate mailings or change of address should be directed to the Company’s Transfer Agent at:
Wells Fargo Shareowner Services
P.O. Box 64874
St. Paul, MN 55164-0874
1.800.468.9716
www.shareowneronline.com
Hearing Impaired #TDD: +1 651 450 4144
Annual Meeting of Shareholders
The annual meeting of shareholders will be held at 8:30 a.m. Central time on Thursday, May 12, 2011, at the Minnesota History Center, 345 Kellogg Boulevard West, St. Paul, Minnesota, 55102.
Investor Contact
To obtain information about the Company, call the Investor Relations (IR) Department at +1 800 328 9634, visit St. Jude Medical’s Web site,sjm.com, or write to:
Investor Relations
St. Jude Medical, Inc.
One St. Jude Medical Drive
St. Paul, Minnesota 55117
The IR section on St. Jude Medical’s website includes all SEC filings, a list of analysts who cover the Company, webcasts and presentations, financial information and a calendar of upcoming earnings announcements and IR events.
Trademarks
All product names appearing in this document are trademarks owned by, or licensed to, St. Jude Medical Inc.
Company Stock Splits
2:1 on 6/15/79, 3/12/80, 9/30/86, 3/15/89, 4/30/90, 6/28/02 and 11/22/04.
3:2 on 11/16/95
Stock Exchange Listings
New York Stock Exchange
Symbol: STJ
The range of high and low prices per share for the Company’s common stock for fiscal years 2010 and 2009 is set forth below. As of February 23, 2011, the Company had 2,206 shareholders of record.
| | | |
| | Fiscal Year | |
| | 2010 | | 2009 | |
Quarter | | High | | Low | | High | | Low | |
First | | $ | 41.76 | | $ | 36.73 | | $ | 39.55 | | $ | 28.86 | |
Second | | $ | 42.87 | | $ | 34.00 | | $ | 41.96 | | $ | 32.57 | |
Third | | $ | 39.64 | | $ | 34.25 | | $ | 40.16 | | $ | 35.73 | |
Fourth | | $ | 42.98 | | $ | 37.38 | | $ | 38.82 | | $ | 31.66 | |
62
