Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended October 2, 2009
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 1-7598
VARIAN MEDICAL SYSTEMS, INC.
(Exact name of Registrant as specified in its charter)
| Delaware | 94-2359345 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) | |
| 3100 Hansen Way, Palo Alto, California | 94304-1030 | |
| (Address of principal executive offices) | (Zip Code) | |
(650) 493-4000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of each exchange on which registered | |
| Common Stock, $1 par value | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90
days. Yes x No ¨
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K x
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer x | Accelerated filer ¨ | |
Non-accelerated filer ¨ | Smaller reporting company ¨ | |
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
As of April 3, 2009, the last business day of Registrant’s most recently completed second fiscal quarter, the aggregate market value of shares of Registrant’s common stock held by non-affiliates of Registrant (based upon the closing sale price of such shares on the New York Stock Exchange on April 3, 2009) was approximately $3,713,775,200. Shares of Registrant’s common stock held by the Registrant’s executive officers and directors and by each entity that owns 5% or more of Registrant’s outstanding common stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
At November 19, 2009, the number of shares of the Registrant’s common stock outstanding was 124,451,760.
DOCUMENTS INCORPORATED BY REFERENCE
Definitive Proxy Statement for the Company’s 2010 Annual Meeting of Stockholders—Part III of this Form 10-K
Table of Contents
VARIAN MEDICAL SYSTEMS, INC.
| Page | ||||
| PART I | ||||
Item 1. | 3 | |||
| 24 | ||||
Item 1A. | 25 | |||
Item 1B | 49 | |||
Item 2. | 49 | |||
Item 3. | 49 | |||
Item 4. | 50 | |||
| PART II | ||||
Item 5. | 51 | |||
Item 6. | 53 | |||
Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 55 | ||
Item 7A. | 81 | |||
Item 8. | 84 | |||
Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 138 | ||
Item 9A. | 138 | |||
Item 9B. | 138 | |||
| PART III | ||||
Item 10. | 139 | |||
Item 11. | 139 | |||
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 140 | ||
Item 13. | Certain Relationships and Related Transactions, and Director Independence | 140 | ||
Item 14. | 140 | |||
| PART IV | ||||
Item 15. | 141 | |||
| 145 | ||||
2
Table of Contents
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, including the Management’s Discussion and Analysis of Financial Condition and Results of Operationsr (“MD&A”) contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, which provides a “safe harbor” for statements about future events, products and future financial performance that are based on the beliefs of, estimates made by and information currently available to the management of Varian Medical Systems, Inc. (“we,” “our” or the “Company”). The outcome of the events described in these forward-looking statements is subject to risks and uncertainties. Actual results and the outcome or timing of certain events may differ significantly from those projected in these forward-looking statements due to the factors listed under “Risk Factors,” and from time to time in our other filings with the Securities and Exchange Commissionr (“SEC”). For this purpose, statements concerning industry or market segment outlook; market acceptance of or transition to new products or technology such as fixed field intensity-modulated radiation therapy, image-guided radiation therapy, stereotactic radiosurgery, volumetric modulated arc therapy, brachytherapy, software, treatment techniques, proton therapy and advanced x-ray products; growth drivers; future orders, revenues, backlog, earnings or other financial results; and any statements using the terms “believe,” “expect,” “expectation,” “anticipate,” “can,” “should,” “would,” “could,” “estimate,” “appear,” “based on,” “may,” “intended,” “potential, “are emerging” and “possible” or similar statements are forward-looking statements that involve risks and uncertainties that could cause our actual results and the outcome and timing of certain events to differ materially from those projected or management’s current expectations. By making forward-looking statements, we have not assumed any obligation to, and you should not expect us to, update or revise those statements because of new information, future events or otherwise.
General
We, Varian Medical Systems, Inc., are a Delaware corporation and were originally incorporated in 1948 as Varian Associates, Inc. In 1999, we transferred our instruments business to Varian, Inc. (“VI”), a wholly owned subsidiary, and transferred our semiconductor equipment business to Varian Semiconductor Equipment Associates, Inc. (“VSEA”), a wholly owned subsidiary. We retained the medical systems business, principally the sales and service of oncology products and the sales of x-ray tubes and imaging subsystems. On April 2, 1999, we spun off VI and VSEA, which resulted in a non-cash dividend to our stockholders and which we refer to as the “Spin-offs” in this Annual Report on Form 10-K. Immediately after the Spin-offs, we changed our name to Varian Medical Systems, Inc. We have been involved in the medical systems business since 1959. An Amended and Restated Distribution Agreement dated as of January 14, 1999 and other associated agreements govern our ongoing relationships with VI and VSEA.
Overview
We are the world leader in the design, manufacture, sale and service of equipment and software products for treating cancer with radiotherapy, stereotactic radiosurgery and brachytherapy. We also design, manufacture, sell and service x-ray tubes for original equipment manufacturers (“OEMs”); replacement x-ray tubes; and flat panel digital image detectors for filmless x-ray imaging (commonly referred to as flat panel detectors or digital image detectors) in medical, dental, veterinary, scientific and industrial applications. We design, manufacture, sell and service linear accelerators, digital image detectors, image processing software and image detection products for security and inspection purposes. We also develop, design, manufacture, sell and service proton therapy products and systems for cancer treatment.
3
Table of Contents
Our mission is to explore and develop radiation technology that protects and saves lives and prevents harm. We seek to be a “Partner for Life” to our clients. We use our technology to suit the needs of our clients in a variety of fields: X-ray and proton cancer therapy and radiosurgery, flat panel imagers and X-ray tubes, and port and border cargo inspection and product quality assurance.
For all of our businesses, our goals are:
| · | for Oncology Systems, our largest business segment, and Varian Particle Therapy (previously known as ACCEL Proton Therapy), to be able to save another 100,000 lives per year by 2014 by applying new techniques to a variety of difficult to treat diseases; |
| · | for our X-Ray Products business, another of our business segments, to provide x-ray components with lower product cost, better product quality or superior technology or performance to enable our customers to supply reliable systems capable of instantly producing sharp, detailed images; |
| · | for our Security & Inspection Products (“SIP”) business, to provide innovative, high-value solutions to complex security, testing and quality assurance challenges by leveraging our expertise in focused energy. |
Oncology Systems designs, manufactures, sells and services hardware and software products for treating cancer. Our products include linear accelerators, brachytherapy afterloaders, treatment simulation and verification equipment and accessories; as well as information management, treatment planning and image processing software. Our products enable radiation oncology departments in hospitals and clinics to perform conventional radiotherapy treatments and offer advanced treatments such as fixed field intensity-modulated radiation therapy (“IMRT”), image-guided radiation therapy (“IGRT”), volumetric modulated arc therapy (“VMAT”), and stereotactic radiotherapy, as well as to treat patients using brachytherapy techniques, which involve temporarily implanting radioactive sources. Our products are also used by neurosurgeons to perform stereotactic radiosurgery. Our customers worldwide include university research and community hospitals, private and governmental institutions, healthcare agencies, doctors’ offices and cancer care clinics.
X-ray Products designs, manufactures and sells: (i) x-ray tubes for use in a range of applications including computed tomography (“CT”) scanning, radiographic or fluoroscopic imaging, mammography, special procedures and industrial applications; and (ii) flat panel digital image detectors for filmless x-ray imaging (commonly referred to as flat panel detectors or digital image detectors), which is for radiography an alternative to image intensifier tubes for fluoroscopy and x-ray film and computed radiography (“CR”) systems. Our x-ray tubes and flat panel detectors are sold to large imaging system OEM customers that incorporate them into their medical diagnostic, dental, veterinary, IGRT and industrial imaging systems. Our x-ray tubes are also sold directly to end-users for replacement purposes.
We have three other businesses that we report together under the “Other” category. Our SIP business designs, manufactures, sells and services Linatron® x-ray accelerators, imaging processing software and image detection products (including IntellXTM) for security and inspection purposes, such as cargo screening at ports and borders and nondestructive examination in a variety of applications. We generally sell SIP products to OEMs who incorporate our products into their inspection systems.
Our Varian Particle Therapy (previously known as ACCEL Proton Therapy) business develops, designs, manufactures and services products and systems for delivering proton therapy, another form of external beam radiation therapy using proton beams for the treatment of cancer. Our current focus is commercializing the proton therapy system and bringing our expertise in traditional radiation therapy to proton therapy to improve its clinical utility and to reduce its cost per patient.
The Ginzton Technology Center (“GTC”) develops technologies that enhance our current businesses or may lead to new business areas, including technology to improve radiation therapy and x-ray imaging and tubes, as well as other technology for a variety of applications, including security and cargo screening.
4
Table of Contents
In September 2008, we approved a plan to sell the scientific research instruments business (“Research Instruments”) that we acquired as part of our acquisition of ACCEL Instruments GmbH (“ACCEL,” which has since changed its name to Varian Medical Systems Particle Therapy GmbH) in order to focus our efforts on the development of the proton therapy systems portion of the business. The sale of Research Instruments was completed in the second quarter of fiscal year 2009. Research Instruments is classified as a discontinued operation. For additional information, see “Discontinued Operations” below.
Our business is subject to various risks and uncertainties. You should carefully consider the factors described in “Risk Factors” in conjunction with the description of our business set forth below and the other information included in this Annual Report on Form 10-K.
Radiation Therapy and the Cancer-Care Market
Radiation therapy, also referred to as radiotherapy, is the use of certain types of focused energy (radiation) to kill cancer cells and shrink tumors, with the goal of damaging as many cancer cells as possible, while limiting harm to nearby healthy tissue. Radiotherapy is commonly used either alone or in combination with surgery or chemotherapy. One important advantage is that radiation acts selectively on replicating cells. When a cell absorbs radiation, the radiation affects the cell’s genetic structure and inhibits its replication, leading to its gradual death. Cancerous cells must replicate in order to cause disease; therefore the radiation cancer cells absorb can disproportionately damage them compared to normal cells. The process for delivering radiotherapy typically consists of examining the patient, planning the treatment, simulating and verifying the treatment plan, providing quality assurance for the equipment and software, delivering the treatment, verifying that the treatment was delivered correctly, recording the history and results of the treatment and obtaining reimbursement for the radiotherapy services provided. The team responsible for delivering the radiotherapy treatment generally comprises a physician specializing in radiation oncology, a physicist for planning the treatment and a radiation therapist for operating the machines.
The most common form of radiotherapy involves delivering x-ray beams from outside of the patient’s body, a process sometimes referred to as external beam radiotherapy. A device called a linear accelerator generates the x-ray beams and administers the treatment by rotating around a patient lying on a treatment couch and delivering the x-ray beam to the tumor from different angles in order to concentrate radiation at the tumor while at the same time minimizing the dose delivered to the surrounding healthy tissue. Conventional radiotherapy typically involves multiple, or fractionated, treatments of a tumor in up to 50 radiation sessions. The linear accelerator may also deliver electron beams for the treatment of more diseases closer to the body surface.
IMRT is an advanced form of external beam radiotherapy in which the shape, intensity and angle of the radiation beams from a linear accelerator are varied, or modulated, across the target area while the patient is being treated. This form of radiotherapy conforms the radiation beams more closely to the shape of the tumor and allows physicians to deliver higher doses of radiation than conventional radiation, while more effectively limiting the amount of radiation delivered to nearby healthy tissue. In this way, clinicians can design and administer an individualized treatment plan for each patient, targeting the tumor as closely as a few millimeters. IMRT can be used to treat head and neck, breast, prostate, pancreatic, lung, liver, gynecological and central nervous system cancers. IMRT has become a well-accepted standard of treatment for cancer, and more treatment centers, from university hospitals to local community clinics, adopt IMRT for their treatments every year. We are a leading global provider of products that enable IMRT treatment of cancer.
IGRT is another advanced form of external beam radiotherapy complementing IMRT to enhance treatments. While IMRT helps physicians shape the beam to the tumor, IGRT goes further in allowing physicians to accommodate for a tumor moving or shrinking. This enables the delivery of even higher doses of radiation to tumors in a more effective manner, while sparing even more of the surrounding healthy tissue. IGRT technologies compensate for daily changes and movements in tumors and enable
5
Table of Contents
dynamic, real-time visualization and precise treatment of small, moving and changing tumors with greater intensity and accuracy. With the greater precision offered by IGRT, clinics and hospitals are potentially able to improve outcomes by concentrating even still higher doses of radiation at the tumors. We believe IGRT has become an accepted standard for treatment in the radiation oncology market.
Stereotactic radiosurgery (also referred to as stereotactic body radiotherapy) is an advanced radiation treatment procedure that employs linear accelerators and IMRT/IGRT technology to deliver very precisely placed, high dose beams of radiation to eradicate cancerous, non-cancerous and abnormal lesions anywhere in the body. Radition therapists and surgeons are recognizing stereotactic radiosurgery as a useful tool in curative radiation therapy.
VMAT is a significant advancement in IMRT that allows physicians to control three parameters simultaneously: (i) the rate at which the linear accelerator gantry rotates around the patient, (ii) the beam-shaping aperture and (iii) the rate at which the radiation dose is delivered to the patient. This creates a finely-shaped IMRT dose distribution that more closely matches the size and shape of the tumor. VMAT improves treatment precision by sparing more healthy tissue, makes treatments faster and offers the possibility of greater comfort for patients. Our RapidArcTM radiotherapy products plan and deliver VMAT treatments.
Physicians, hospitals and clinics place additional value on radiotherapy equipment and treatments, such as VMAT, that enable shorter treatment times and greater patient throughput. From the patients’ standpoint, shorter treatment times can mean greater comfort since treatments often involve the patient being immobilized on the treatment couch. Shorter treatment sessions decrease waiting times and, since treatments are delivered in fractions over the course of many days, can mean fewer disruptions to a patient’s daily routine. From the physicians’ and hospitals’ standpoint, shorter treatment times can lessen the chance of a tumor moving during treatment and can increase patient throughput. Shorter treatment times and increased patient throughput can increase the number of treatments per day (which is a particular concern in countries with lower numbers of treatment machines per capita), and, as a result, can decrease the cost per treatment which in turn can offer greater access to advanced care to more patients.
Dynamic Adaptive Radiotherapy (“DART”) is our vision of the future for radiotherapy treatments where better clinical practices and outcome are achieved through use of imaging, planning and delivery of radiation. Current image guidance technologies, including On-Board Imager® (“OBI”) and cone-beam computerized tomography (“CBCT”), allow for imaging the patient immediately prior to treatment, while in the treatment position. DART would enable imaging during treatment, allowing clinicians to take real-time account of patient and tumor motion, breathing, and anatomical and physiological changes that occur during the course of treatment. Product enhancements that allow for cost-efficient decision support, as well as data collection and analysis for the development of more broadly shared treatment standards, are expected to also be key aspects of DART. We expect that these guiding principles will contribute to continuing product development and business growth for our Oncology Systems business.
An alternative to external beam radiotherapy, brachytherapy involves the insertion of radioactive seeds, wires or ribbons directly into a tumor or into a body cavity close to the cancerous area. These techniques, unlike external beam radiation therapy, do not require the radiation to pass through surrounding healthy tissue in order to reach the tumor, and the physician can prescibe a higher total dose of radiation in a shorter time. Brachytherapy is often used for cancers of the head and neck, breast, uterus, cervix, soft tissue and prostate.
Proton therapy is another form of external beam radiotherapy that uses proton beams generated with a cyclotron rather than x-ray beams from a linear accelerator. A proton beam’s signature energy distribution curve, also known as the “Bragg peak,” allows for greater accuracy in targeting tumor cells with an even lower dose to nearby healthy tissue than may be delivered with x-ray beams from a linear accelerator. This makes proton therapy a preferred option for treating certain kinds of cancers,
6
Table of Contents
particularly tumors near critical structures such as the optic nerve and cancers in children. Although proton therapy has been in clinical use for more than four decades, it has not been widely deployed due to the cost of the technology and limited cost effectiveness. We have entered the proton therapy market because we believe we can apply our experience in traditional radiotherapy to proton therapy, reducing cost per patient for existing clinical applications and expanding the use of proton therapy into a broader array of cancer types. We believe that proton therapy will over time become a more widely accepted method of treatment.
The radiation oncology market is growing globally due to a number of factors. Annual cancer rates around the world are projected to increase by 50% to 15 million new cases in the year 2020, as indicated by the World Cancer Report issued by the International Agency for Research on Cancer in the World Health Organization. According to the World Cancer Report, the predicted increase in new cases will mainly be due to steadily aging populations in both developed and developing countries due to current trends in smoking prevalence and the growing adoption of unhealthy life styles. For example, the U.S. Census Report indicates that the population over 65 years of age in the United States is expected to increase by 41% to 48 million in 2015 from 34 million in 2000. The U.S. chart data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results program also indicates that the number of cancers diagnosed annually could double in the United States to 2.6 million by 2050.
The rise in cancer cases, together with the increase in sophistication of new treatment processes, have created demand for more automated products that can be integrated into clinically practical systems to make treatments more rapid and cost effective. Technology advances leading to improvements in patient care, the availability of more advanced, automated and efficient clinical tools in radiation therapy, the advent of more precise forms of radiotherapy treatment (such as IMRT, IGRT, VMAT, stereotactic radiotherapy, stereotactic radiosurgery, brachytherapy and, ultimately, proton therapy), and developing technology and equipment that enable treatments (such as VMAT) which reduce treatment times and increase patient throughput should drive the demand for our radiation therapy products and services.
International markets in particular are under-equipped to address the growing cancer incidence. Patients in many foreign countries must frequently endure long waits for radiotherapy, although many of these countries are now expanding and upgrading their radiotherapy services. This capacity shortfall, coupled with ever increasing incidences of cancer, represent additional drivers for our continued growth in international markets. As a U.S.-based company, the competitiveness of our product pricing is influenced by the fluctuation of the U.S. dollar against other currencies. In fiscal year 2009, the U.S. dollar was stronger against foreign currencies than in fiscal year 2008, which made our product pricing less competitive. However, the U.S. dollar has subsequently weakened, which could make our product pricing more competitive in the local currencies of our international customers. The fluctuation of the U.S dollar against foreign currencies also impacts our international revenues and net orders when measured in U.S. dollars.
Products
Oncology Systems
Our Oncology Systems business segment is the leading provider of advanced hardware and software products for radiation treatment of cancer with conventional radiation therapy, IMRT, IGRT, VMAT, stereotactic radiotherapy, stereotactic radiosurgery and brachytherapy. Oncology Systems products address each major aspect of the radiotherapy process, including linear accelerators and accessory products for positioning the patient and delivering the x-ray beam; brachytherapy afterloaders; treatment planning software; treatment simulation and verification equipment and accessories and quality assurance software for simulating and verifying the treatment plans before treatment and verifying that a treatment was delivered correctly afterwards; and information management software for recording the history and results of treatments and other patient treatment information and data, including patient x-ray images.
7
Table of Contents
The focus of our Oncology Systems business is addressing the key concerns of the market for advanced cancer care systems; improving efficiency, precision, cost-effectiveness, comfort to the patient and ease of delivery of these treatments; and providing greater access to advanced treatments. A core element of our business strategy is to provide our customers with highly versatile, clinically proven products that are interoperable and can be configured and integrated into automated systems that combine greater precision, shorter treatment times and greater cost effectiveness and that improve the entire process of treating a patient. Our products and accessories for IMRT and IGRT allow clinicians to track and treat tumors using very precisely shaped beams, targeting the tumor as closely as currently possible and allowing the delivery of higher doses to the tumor while limiting exposure of nearby healthy tissue. With our treatment planning, verification and information management software products, a patient’s treatment plans, treatment data and images are recorded and stored in a single database shared by each of our products, which enables effective communication among products. Additionally, the precision and versatility of our products and technology makes it possible to use radiotherapy to treat metastatic cancers. Our products also allow multiple medical specialties—radiation oncology, neurosurgery, radiographic imaging and medical oncology—to share equipment, resources and information in a more cost-effective manner. Furthermore, the ability of our products and technology to interoperate with each other and to interconnect into automated systems allows physicians to schedule and treat more patients within a set time period, which adds to the cost-effectiveness of our equipment.
Linear accelerators are the core device for delivering conventional external beam radiotherapy with IMRT, IGRT and VMAT treatments, and we produce versions of these devices to suit various requirements. Our Clinac® medical linear accelerators are used to treat cancer by producing therapeutic electrons and x-ray beams that target tumors and other abnormalities. The Clinac iX linear accelerators are designed for more streamlined and advanced treatment processes including IMRT and IGRT. We also produce the Trilogy™ linear accelerator, designed to be a versatile, cost-effective, ultra-precise device with a faster dose delivery rate and smaller isocenter compared to the Clinac iX. Trilogy was developed with IGRT and stereotactic radiotherapy in mind, but is also capable of delivering conventional, 3D conformal radiotherapy, IMRT and VMAT. Trilogy has the precision necessary to deliver stereotactic radiosurgery for neurosurgical treatments and is the accelerator that is at the core of the Novalis TxTM product offering, a combination of products from Varian and BrainLAB AG (“BrainLAB”), targeted to neurosurgeons. In the fourth quarter of fiscal year 2009, we introduced the UNIQUETM low-energy linear accelerator, which is capable of integrating our accessory products (including RapidArc) to deliver IMRT, IGRT and VMAT. The UNIQUE accelerator was developed to address more price sensitive markets in international regions.
We also manufacture and market linear accelerator accessories that enhance efficiency and enable delivery of advanced treatments such as IMRT, IGRT, stereotactic radiotherapy, stereotactic radiosurgery and VMAT. Our Millennium™ series of multi-leaf collimators and High Definition 120 (“HD 120”) multi-leaf collimators are used with a linear accelerator to define the size, shape and intensity of the generated beams. PortalVision™, our electronic portal-imager, is used to verify a patient’s position while on the treatment couch, which is critical for accurate treatments and simplifies quality assurance of individual treatment plans. We also offer an innovative real-time patient position monitoring product, the RPM™ respiratory gating system, which allows the linear accelerator to be synchronized with patient breathing to help compensate for tumor motion during the course of treatment.
Our IGRT accessories include the OBI hardware accessory affixed to the linear accelerator that allows dynamic, real-time imaging of tumors while the patient is on the treatment couch and a CBCT imaging software accessory that works with the OBI to allow patient positioning based on soft-tissue anatomy. Using sophisticated image analysis tools, the CBCT scan can be compared with a reference CT scan taken previously to determine how the treatment couch should be adjusted to fine-tune and verify the patient’s treatment setup and positioning prior to delivery of the radiation. Therefore, to deliver the most advanced forms of IGRT, our accelerators would typically have an OBI, CBCT, PortalVision and
8
Table of Contents
other IGRT-related hardware and software as accessories. We also have in our product portfolio the SonArray ultrasound imaging device for patient positioning and stereotactic treatment planning software for use in developing treatment plans for stereotactic radiosurgery.
Our RapidArc radiotherapy products enable the planning and delivery of image-guided IMRT in a single continuous rotation of up to 360 degrees rather than as a series of fixed fields. Our RapidArc products enable faster delivery of radiation treatment with the possibility of greater comfort to the patient, reduced opportunity for tumor movement during treatment and greater patient throughput, resulting in lower cost per patient for the hospital or clinic. RapidArc radiotherapy products are a proprietary implementation of VMAT that coordinates beam shaping, dose rate and gantry speed to deliver a highly conformal dose distribution to the target tumor. We believe RapidArc represents a significant advancement in IMRT cancer treatment and can help drive longer term demand for our linear accelerators and IMRT-related accessories.
Our treatment planning and information management software products enhance and enable the delivery of advanced radiotherapy treatments, from the initial treatment planning and plan quality assurance verification to the post-treatment recording of data and storing of patient information. Prior to any treatment, particularly IMRT, IGRT, stereotactic radiosurgery or one using RapidArc, physicians must plan the course of radiation delivery for the patient. We offer a range of treatment planning products that assist physicians in compiling this plan. Our Eclipse™ treatment planning system provides physicians with 3D image viewing, treatment simulation, radiation dosage calculation and verification and other tools for generating treatment delivery plans for the patient. The Eclipse software utilizes a sophisticated technique known as inverse planning to enable physicians to rapidly develop optimal treatment plans based on a desired radiation dose outcome to the tumor and surrounding tissue. In the third quarter of fiscal year 2009, we acquired certain assets of IKOEmed LLC and IKOEtech LLC (collectively referred to as “IKOE”) which are privately-owned suppliers of software used to enhance treatment planning. The acquired software, which is being incorporated into some of our Oncology Systems software products, complements the segmentation tools we currently supply to automate planning for prostate, breast and lung treatments.
Our Argus™ software manages the planning, recording and analysis of quality assurance data for linear accelerators. Finally, our ARIA™ Oncology Information Management System (“ARIA”) is a comprehensive real-time information management system and database that records and verifies radiotherapy treatments carried out on the linear accelerator, records and stores patient data relating to chemotherapy treatment which may be prescribed by a physician in addition to radiotherapy, performs patient charting and manages patient information and patient image data. This gives clinics and hospitals the ability to manage treatment and patient information across radiation oncology and medical oncology procedures. Also, because ARIA is an electronic medical record, it can enable users to operate filmless and paperless oncology departments and cancer clinics.
Our treatment simulators enable physicians to simulate radiation therapy treatments prior to delivery. We manufacture and sell Acuity™, a simulator that uses advanced amorphous silicon imaging technology and which has been designed to enhance IMRT treatments by integrating simulation more closely with treatment planning and by helping physicians better address tumor motion caused by breathing.
In addition to offering our own suite of equipment and software products for planning and delivering radiotherapy treatments, we have partnered with selected leaders in certain segments of the radiation therapy and radiosurgery market. With General Electric Medical Systems (“GE”) in North America, we have established the See and Treat Cancer Care™ program for radiation therapy that allows us to offer a suite of diagnostic and cancer treatment tools combining our comprehensive set of radiation therapy products with GE’s advanced diagnostic imaging systems. We have also established a strategic relationship with BrainLAB for the sale and marketing of the Novalis Tx, a radiosurgical suite of products targeted to neurosurgeons that integrates our Trilogy Tx linear accelerator and our HD 120
9
Table of Contents
multi-leaf collimator with specialty positioning and software products offered by BrainLAB. Novalis Tx works with a variety of our other accessory products, including our OBI, Eclipse treatment planning system and ARIA information management software. We have a 2.5% equity ownership in BrainLAB.
Our brachytherapy operations design, manufacture, sell and service advanced brachytherapy products, including VariSource™ HDR afterloaders and GammaMed™ HDR/PDR afterloaders, BrachyVision™ brachytherapy treatment planning system, applicators and accessories. Brachytherapy also develops and markets the VariSeed™ LDR prostate treatment planning system.
Revenues from our Oncology Systems business segment represented 81%, 81% and 82% of total revenues for fiscal years 2009, 2008 and 2007, respectively. Our Oncology Systems business segment revenues also include service revenues. See “—Customer Services and Support.” For a discussion of Oncology Systems business segment financial information, see Note 15, “Segment Information” of the Notes to the Consolidated Financial Statements.
X-ray Products
Our X-ray Products business segment is a world leader in designing and manufacturing x-ray tubes and flat panel detectors, which are key components of x-ray imaging systems. We sell our products to OEMs for new system configurations and replacement x-ray tubes for installed systems. We conduct an active research and development program to focus on new technology and applications in both the medical and industrial x-ray imaging markets.
We manufacture x-ray tubes for four primary medical diagnostic radiology applications: CT scanners, radiographic or fluoroscopic imaging, special procedures and mammography. We also offer a large line of industrial x-ray tubes, which consist of analytical x-ray tubes used for x-ray fluorescence and diffraction, as well as tubes used for non-destructive imaging and gauging and airport baggage inspection systems.
Our flat panel detectors, which are based on amorphous silicon imaging technologies, have found broad application as an alternative to image intensifier tubes and x-ray film. These flat panel detectors are being incorporated into next generation filmless medical diagnostic, dental, veterinary and industrial inspection imaging systems and also serve as a key component of our OBI, which helps enable IGRT. We believe that imaging equipment based on amorphous silicon technologies is more stable and reliable, needs fewer adjustments and suffers less degradation over time than image intensifier tubes and is more cost effective than x-ray film. In fiscal year 2009, we expanded our product offering of flat panel detectors with the addition of a family of radiographic panels. These panels may be used on digital radiography systems or may be used to convert film-based systems to digital systems.
Our radiographic flat panels were a key contributor to net order and revenue growth for X-ray Products in fiscal year 2009. Revenues from X-ray Products represented 15% of total revenues in each of fiscal years 2009, 2008 and 2007. For a discussion of the X-ray Products business segment financial information, see Note 15, “Segment Information” of the Notes to the Consolidated Financial Statements.
Other
Our SIP business designs, manufactures, sells and services Linatron® x-ray accelerators, imaging processing software and image detection products for security and inspection purposes, such as cargo screening at ports and borders and nondestructive examination in a variety of applications. The Linatron M-i is a dual energy accelerator that can perform non-intrusive inspection of cargo containers and aid in automatically detecting and alerting operators when high-density nuclear materials associated with dirty bombs or weapons of mass destruction are present during cargo screening. The Linatron K-15 is a high-energy accelerator for inspection of very large, dense objects, including, for example, the solid rocket boosters on NASA’s Space Shuttle. IntellX is an imaging product for cargo screening.
10
Table of Contents
Generally, we sell our SIP products to OEMs who incorporate our products into their inspection systems, which are then sold to customs and other government agencies who use them in overseas ports and borders to screen for contraband, weapons, stowaways, narcotics and explosives, as well as for manifest verification. We also sell our SIP products to commercial organizations in the casting, power, aerospace, chemical, petro-chemical and automotive industries for nondestructive product examination purposes, such as industrial inspection and manufacturing quality control.
Use of our SIP technology in security cargo screening and border protection is still in its early stages, but we believe demand for our SIP products will be driven by cargo screening and border protection needs, as well as by the needs of customs agencies to verify the content of shipments for assessing duties and taxes. As a result, this business is heavily influenced by U.S. and foreign governmental policies on national and homeland security, border protection and customs revenue activities and therefore depends on government budgets and appropriations and is subject to political change. In addition, this business depends on the success of our OEM customers. Orders and revenues for our SIP products have been and may continue to be unpredictable as governmental agencies may place large orders with us or with our OEM customers over a short period of time and then may not place any orders for a long time period thereafter.
Our Varian Particle Therapy business develops, designs, manufactures and services products and systems for delivering proton therapy, another form of external beam radiotherapy using proton beams for the treatment of cancer. Although proton therapy has been in clinical use for more than four decades, it has not been widely deployed due to the cost of the technology and limited cost effectiveness. We are investing substantial resources to commercialize this advanced proton technology and to build this new business. In the second quarter of fiscal year 2009, we met the Conformité Européenne (“CE”) mark requirements that permit us to market our proton therapy systems within the European Economic Area (“EEA”), and patient treatments started on our proton therapy system that is installed at the Rinecker Proton Therapy Center in Munich, Germany. Proton therapy facilities are large scale construction projects that can take three years or more to complete. With the cost of a multiple-gantry system in excess of $60 million and the total cost for each proton therapy center exceeding $100 million, significant customer investment and perhaps complex project financing will be required. Consequently, the customers’ decision-making cycle is very long and orders for proton therapy systems generally involve many contingencies.
GTC, our scientific research facility, continues to invest in developing technologies that enhance our current businesses or may lead to new business areas, including next generation digital x-ray imaging technology, volumetric and functional imaging, and improved x-ray sources and technology for security and cargo screening applications. In addition, GTC is developing technologies and products that are designed to improve disease management by more precise targeting of radiation, as well as by employing targeted energy and molecular agents to enhance the effectiveness and broaden the application of radiation therapy.
SIP, Varian Particle Therapy and GTC report their results from operations as part of the “Other” category. Combined revenues from these operations represented 4%, 4% and 3% of total revenues in fiscal years 2009, 2008 and 2007 respectively. For a discussion of segment financial information, see Note 15, “Segment Information” of the Notes to the Consolidated Financial Statements.
Customer Services and Support
We maintain service centers in Milpitas, California; Las Vegas, Nevada; Des Plaines, Illinois; Clark, New Jersey; Marietta, Georgia; Richardson, Texas; Corona, California; Buc, France; Crawley, United Kingdom; Zug, Switzerland; Copenhagen, Denmark; Brussels, Belgium; Darmstadt, Germany; Houten, The Netherlands; Madrid, Spain; Milan, Italy; Manama, Bahrain; Moscow, Russia; Mumbai, Delhi, and Chennai, India; Tokyo and Osaka, Japan; Beijing, Shanghai and Hong Kong, China; Kuala Lumpur, Malaysia; Singapore; Bangkok, Thailand; Belrose, Australia; and Sao Paulo, Brazil; as well as field
11
Table of Contents
service personnel throughout the world for Oncology Systems customer support services. Key Oncology Systems logistics and education operations are located in Las Vegas, Nevada, Beijing, China and Zug, Switzerland. Our network of service engineers and customer support specialists provide installation, warranty, repair, training and support services, and professional services. We generate service revenues by providing services to customers on a time-and-materials basis and through post-warranty equipment service contracts and software support contracts. Most of the field service engineers are our employees, but our products are serviced by employees of dealers and/or agents in a few foreign countries. Customers can access our extensive service network by calling any of our service centers.
We warrant most of our Oncology Systems products for parts and labor for 12 months, and we offer a variety of post-warranty equipment service contracts and software support contracts to suit customers’ requirements.
We believe customer service and support are an integral part of our Oncology Systems competitive strategy. Growth in our service revenues has resulted from the increasing customer adoption of service contracts as the sophistication and installed base of our products increase. We also believe superior service plays an important role in marketing and selling medical products and systems, particularly as the products become more complex. Nevertheless, some of our customers use their own internal service organizations and/or independent service organizations to service equipment after the warranty period expires and therefore do not enter into agreements with us for extended service.
We generally warrant our x-ray tubes and flat panel detector products in our X-ray Products business segment for 12 to 24 months, although for some x-ray tubes the warranty period is based on the number of times the product is used. We provide technical advice and consultation for x-ray tubes and imaging subsystems products to major OEM customers from our offices in Salt Lake City, Utah; Charleston, South Carolina; Tokyo, Japan; Beijing, China and Willich, Germany. Our applications specialists and engineers make recommendations to meet the customer’s technical requirements within the customer’s budgetary constraints. We often develop specifications for a unique product, which will be designed and manufactured to meet a specific customer’s requirements. We also maintain a technical customer support group in Charleston, South Carolina to meet the technical support requirements of independent tube installers that use our x-ray tube products.
We generally warrant our SIP products for 12 months. We provide technical support and service for these products to major OEM customers from our offices in Las Vegas, Nevada; Lincolnshire, Illinois; and Buc, France; Manama, Kingdom of Bahrain; Crawley, United Kingdom; Milano, Italy; and Brussels, Belgium. We use the Oncology Systems Customer Support Services organization in Asia, Australia and South America.
Marketing and Sales
We employ a combination of direct sales forces and independent distributors or resellers in North America, Europe, Australia and major parts of Asia and Latin America for the marketing and sales of our products worldwide. In fiscal years 2009, 2008 and 2007, we did not have a single customer that represented 10% or more of our total revenues.
For our Oncology Systems segment, we sell direct in North America and use a combination of direct sales and independent distributors in international regions. We have also launched direct-to-consumer advertising campaigns to increase consumer awareness of Oncology Systems’ products. We sell our Oncology Systems products primarily to university research and community hospitals, private and governmental institutions, healthcare agencies, physicians’ offices and cancer care clinics worldwide. These hospitals, institutes, agencies, physicians’ offices and clinics replace equipment and upgrade treatment capability as technology evolves. Sales cycles for our external beam radiotherapy products typically can be quite lengthy since many of them are considered capital equipment and are affected by budgeting cycles. Our customers frequently fix capital budgets one or more years in advance.
12
Table of Contents
In recent years, we have seen the purchasing cycle lengthen as a result of the more complex decision-making process associated with larger dollar value transactions for more sophisticated IGRT and surgical equipment, and other technical advances. With the current worldwide economic downturn, it has become and may continue to be more difficult to accurately forecast and plan future business activities. We have seen customers’ decision-making process further complicated and lengthened, especially in the United States, as the downturn causes hospitals, clinics and research institutions to more closely scrutinize and prioritize their capital spending in light of tightened capital budgets, tougher credit requirements and the general constriction in credit availability. In addition, we believe that the current economic downturn has caused customers to delay requested delivery dates. Because our product revenues are influenced by the timing of product shipments, which are tied to customer-requested delivery dates, these delivery delays have increased the average order to revenue conversion cycle in the United States. Historically, this conversion cycle has been longer when new products are introduced or when we sell more products internationally. The lengthening of order to revenue conversion cycle could reduce our revenues and margins in the current period and increase our deferred revenues. In addition, our receivables may take longer to collect.
Reimbursement rates in the United States have generally supported a favorable return on investment for the purchase of new radiotherapy equipment. While we believe that improved product functionality, greater cost-effectiveness and prospects for better clinical outcomes with new capabilities such as IMRT, IGRT and VMAT tend to drive demand for radiotherapy products, large changes in reimbursement rates or reimbursement structure can affect customer demand and cause market shifts. We have seen our customers’ decision-making process complicated by the uncertainty surrounding the recent proposed reduction in Medicare reimbursement rates for radiotherapy and radiosurgery at free-standing clinics in the United States and for physician reimbursement for radiation oncology. While the final enacted reimbursement rate reductions for 2010, which were announced by the U.S. Centers for Medicare and Medicaid Services (“CMS”) on October 30, 2009, were much more modest for radiotherapy than originally proposed, we believe that the confusion and uncertainty created by the proposal was one of the major factors negatively impacting our net orders in late fiscal year 2009, particularly from free-standing radiotherapy clinics (which we believe represents approximately 10% to 15% of Oncology Systems’ business). International reimbursement rates for radiation therapy tend to be low in national health systems, yet international markets continue to invest in better treatment capability, albeit often after it has been proven in the North American region or in other leading research centers worldwide.
Total Oncology Systems revenues, including service revenues, were $1.8 billion, $1.7 billion and $1.4 billion for fiscal years 2009, 2008 and 2007, respectively. We divide our market segments for Oncology Systems revenues into North America, Europe, Asia and rest of the world, and these regions constituted 54%, 29%, 14%, and 3%, respectively, of Oncology Systems revenues during fiscal year 2009; 52%, 31%, 12% and 5%, respectively, of Oncology Systems revenues during fiscal year 2008 and 52%, 32%, 11% and 5%, respectively, of Oncology Systems revenues during fiscal year 2007.
Our X-ray Products segment employs a combination of direct sales and independent distributors for sales in all of its regions and sells a high proportion of its products to a limited number of OEMs that incorporate our products into their imaging systems. The fundamental growth driver of this business segment is the on-going success of our key customers, and we expect that revenues from relatively few customers will continue to account for a high percentage of X-ray Products revenues in the foreseeable future. Our OEM customers include Toshiba Corporation, Hitachi Medical Corporation, Philips Medical Systems, GE Healthcare, Carestream Health, Inc., Sound Technologies, Inc. and Imaging Sciences International, Inc. The current economic downturn has made and may continue to make it difficult for these customers to accurately forecast and plan future business activities, and we saw our X-ray Products net orders and revenues negatively impacted in fiscal year 2009 as a result of inventory reduction efforts by some of these customers. These OEM customers represented 60%, 62% and 63% of our total X-ray Products segment revenues during fiscal years 2009, 2008 and 2007, respectively, with the remaining revenues coming from a large number of small OEMs and independent services companies. Total
13
Table of Contents
revenues for our X-ray Products segment were $331 million, $305 million and $258 million for fiscal years 2009, 2008 and 2007, respectively. We divide our market segments for X-ray Products revenues by region into North America, Europe, Asia and rest of the world, and these regions constituted 33%, 15%, 49% and 3%, respectively, of X-ray Products revenues during fiscal year 2009; 35%, 15%, 47% and 3%, respectively, of X-ray Products revenues during fiscal year 2008 and 37%, 14%, 46% and 3% respectively, of X-ray Products revenues during fiscal year 2007.
Our SIP business also uses a combination of direct sales and independent distributors and sells a high proportion of its products to a limited number of OEMs that incorporate our products into their systems. As with X-ray Products, this business depends on the success of our OEM customers, and we expect that revenues from relatively few customers will continue to account for a high percentage of SIP’s revenues in the foreseeable future. We supply Linatron linear accelerators and detector products to OEMs such as Smiths Detection, Rapiscan Systems, Inc., American Science & Engineering, Inc. and L-3 Communications. We also sell our SIP products to commercial organizations in the casting, power, aerospace, chemical, petro-chemical and automotive industries. In fiscal year 2009, we saw our SIP customers postpone some purchasing decisions as budgets were put on hold or reallocated during the current economic downturn.
In the Varian Particle Therapy business, we use direct sales specialist representatives who collaborate globally with our Oncology Systems sales group on projects. Potential customers are government-sponsored hospitals and research institutions and research universities, which typically purchase products through public tenders, and, to a lesser extent, private hospitals and clinics. We believe that growth in this business will initially develop in the major metropolitan areas in the United States and abroad, driven by institutions that wish to expand their clinical offerings and increase their profile in their respective communities. Proton therapy facilities, nevertheless, are large-scale construction projects and may involve complex project financing. Given the heavy reliance of customers of this business on credit and large-scale project financing, this business is the most vulnerable to the general worldwide economic downturn and contraction in the credit markets. Also, the customers’ decision-making cycle is very long, and orders for proton therapy systems generally involve many contingencies. Under our current practice, we only recognize orders with contingencies if we deem the contingencies perfunctory or if we publicly disclose the existence and nature of material contingencies. In the fourth quarter of fiscal year 2009, Skandion Kliniken awarded us, in a competitive bidding situation, an approximately $62 million contract to deliver and install a proton therapy system in Sweden, and we have included this contract in net orders. However, one of the unsuccessful bidders has filed a formal appeal of the award. Skandion Kliniken is actively opposing this appeal, and we believe it will be resolved in Skandion Kliniken’s favor. Additionally, the order is contingent on Skandion Kliniken’s execution of a contract for the construction of the proton therapy facility, which we also believe will occur. As this is the first proton therapy system to be delivered by Varian Particle Therapy since we acquired this business from ACCEL, the profitability of this contract is uncertain. We plan to start recognizing revenues in accordance with contract accounting when we commence working on this project. As Varian Particle Therapy is a relatively new business that offers highly customized proton therapy systems, we plan to evaluate revenue recognition for sales of proton therapy systems and related services on a contract by contract basis.
Competition
Rapidly evolving technology, intense competition and pricing pressure characterize the market for radiation therapy equipment and software products, including our Oncology Systems products. We compete with companies worldwide. Some of our competitors have greater financial, marketing and other resources than we have. These competitors could develop technologies and products that are more effective than those we currently use or produce or that could render our products obsolete or noncompetitive. Our smaller competitors could be acquired by companies with greater financial strength, which could enable them to compete more aggressively. Some of our suppliers or distributors could also be acquired by competitors, which could disrupt these supply or distribution arrangements
14
Table of Contents
and result in less predictable and reduced revenues. Furthermore, we believe that new competitors will enter our markets, just as we encounter new competitors as we move into new areas such as stereotactic radiosurgery, VMAT and proton therapy. We have directed substantial product development efforts into (i) greater interconnectivity of our products for more seamless operation within a system, (ii) enhancing the ease of use of our software products and (iii) reducing setup and treatment times and increasing patient throughput. We have emphasized maintaining an “open systems” approach that allows customers to “mix and match” individual products, incorporate products from other manufacturers, share information with other systems and use the equipment for offering various methods of radiation therapy. We anticipate that these efforts will increase the acceptance and adoption of IMRT, VMAT and IGRT and will stimulate demand for our products from new customers and upgrades from existing customers. We face competition though from “closed-ended” dedicated-use systems that place simplicity of use ahead of flexibility. If we have misjudged the importance to our customers of maintaining an “open systems” approach, or if we are unsuccessful in our efforts to enable greater interconnectivity, enhance ease-of-use and reduce setup and treatment times, our financial results could suffer.
Our Oncology Systems customers’ equipment purchase considerations typically include: reliability, servicing, patient throughput, precision, price, payment terms, connectivity, clinical features, the ability to track patient referral, long-term relationship with customers and capabilities of customers’ existing equipment. We believe we compete favorably with our competitors based upon our strategy of providing a complete package of products and services in the field of radiation oncology and our continued commitment to global distribution and customer service, value-added manufacturing, technological leadership and new product innovation. We strive to provide technically superior, clinically proven products for substantially all aspects of radiation therapy that deliver more precise, cost-effective, high quality clinical outcomes that meet or exceed customer quality and service expectations. However, our ability to compete may be adversely affected when purchase decisions are based solely upon price, since our products are generally sold on a basis of total value to the customer. This may occur if hospitals and clinics give purchasing decision authority to group purchasing organizations that focus solely on pricing as the primary determinant. If customers make purchase decisions based solely on price, our pricing, sales, revenues, market share and gross margins, as well as our ability to maintain or increase our operating margins, could be adversely impacted.
We are the leading provider of medical linear accelerators and related accessories. In radiotherapy and radiosurgery markets, we compete primarily with Elekta AB, Siemens Medical Solutions, Tomotherapy Incorporated and Accuray Incorporated. With our information and image management, simulation, treatment planning and radiosurgery products, we also compete with a variety of companies, such as Elekta AB, Philips Medical Systems, Best Theratronics, Ltd., Nucletron B.V. and Siemens Medical Solutions. We also encounter some competition from providers of hospital information systems. With respect to our brachytherapy operations, our primary competitor is Nucletron B.V. For the service and maintenance business for our Oncology Systems products, we compete with independent service organizations and our customers’ internal service organizations.
The market for x-ray imaging components and subsystems is extremely competitive, with our competitors frequently having greater financial, marketing and other resources than we have. Some of the major diagnostic imaging systems companies, which are the primary OEM customers for our x-ray tubes and panels, also manufacture x-ray components for use in their own imaging systems products. We must compete with these in-house manufacturing operations for business from their affiliated companies. As a result, we must have a competitive advantage in one or more significant areas, which may include lower product cost, better product quality or superior technology and/or performance. We sell a significant volume of our x-ray tubes to OEMs such as Toshiba Corporation, Hitachi Medical Corporation, Philips Medical Systems and GE Healthcare, all of which have in-house x-ray tube production capability. In addition, we compete against other stand-alone, independent x-ray tube manufacturers such as Comet AG and IAE Industria Applicazioni Elettroniche Spa. These companies compete with us for both the OEM business of major diagnostic imaging equipment manufacturers and
15
Table of Contents
the independent servicing business for x-ray tubes. The market for flat panel detectors is also very competitive. We incorporate our flat panel detectors into our equipment for IGRT within our Oncology Systems and also sell to a number of OEMs, which incorporate our flat panel detectors into their medical diagnostic, dental, veterinary and industrial imaging systems. Our amorphous silicon based flat panel detector technology competes with other detector technologies such as amorphous selenium, charge-coupled devices and variations of amorphous silicon scintillators. We believe that our product provides a competitive advantage due to lower product cost and better product quality and performance. For flat panel detectors, our significant customers include Toshiba Corporation, Sound Technologies, Inc., Carestream Health, Inc., and Imaging Sciences International Inc., and we primarily compete against Perkin-Elmer, Inc., Trixell S.A.S., Samsung Electronics and Canon, Inc.
We generally sell our SIP products to OEMs who incorporate our products into their inspection systems, which are then sold to customs and other government agencies, as well as to commercial organizations in the casting, power, aerospace, chemical, petro-chemical and automotive industries. We compete with other OEM suppliers primarily located outside of the United States in the security and inspection market, and our major competitor is Nuctech Company Limited. The market for our SIP products used for nondestructive testing in industrial applications is small and highly fractured and there is no single major competitor in this nondestructive testing market.
The market for proton therapy products is still developing and is characterized by rapidly evolving technology, high competition and pricing pressure. Our ability to compete successfully depends, in part, on our ability to complete the development of our commercial proton therapy system, lower our product costs, develop and provide technically superior, clinically proven products that deliver more precise, cost-effective, high quality clinical outcomes, including integration of IGRT technologies such as OBI. In the proton therapy market, we compete principally with Ion Beam Applications S.A., Hitachi Medical Corporation and Still River Systems, Inc.
Research and Development
Developing products, systems and services based on advanced technology is essential to our ability to compete effectively in the marketplace. We maintain a research and development and engineering staff responsible for product design and engineering. Research and development expenses totaled $147 million, $136 million and $117 million in fiscal years 2009, 2008 and 2007, respectively.
Our research and development are conducted both within the relevant product groups of our businesses and through GTC. GTC maintains technical expertise in x-ray technology, accelerator technology, imaging physics and applications, algorithms and software, electronic design, materials science and biosciences to prove feasibility of new product concepts and to improve current products. Present research topics include new imaging concepts, image-based radiotherapy treatment planning and delivery, real time accommodation of moving targets, functional imaging and combined modality therapy, manufacturing process improvements, improved x-ray tubes and large-area, high resolution digital x-ray sensor arrays for cone-beam CT and other applications. GTC is also pursuing the potential of combining advances in directed energy and imaging technology with the latest breakthroughs in biotechnology by employing targeted energy to enhance the effectiveness of biological and chemical therapeutic agents. GTC is also investigating the use of x-ray and high energy accelerator, detector, and image processing technology for security applications. GTC accepts some sponsored research contracts from external agencies such as the U.S. government or private sources.
Within Oncology Systems, our development efforts focus on enhancing the reliability and performance of existing products and developing new products. This development is conducted primarily in the United States, Switzerland, Canada, England, Finland and India. In addition, we support research and development programs at selected hospitals and clinics. Current areas for development within Oncology Systems include linear accelerator systems and accessories for medical applications, information systems, radiation treatment planning software, image processing software, imaging devices, simulation, patient
16
Table of Contents
positioning and equipment diagnosis and maintenance tools. Much of the Oncology Systems development efforts relate to our next generation linear accelerators; enhancements to IGRT and IMRT; our Monte Carlo and dose calculation algorithms for our treatment planning software products; and our new electronic health records within our information management software.
Within X-ray Products, development is conducted at our Salt Lake City, Utah and Mountain View, California facilities and is primarily focused on developing and improving x-ray imaging component and subsystem products. Current x-ray tube development areas include improvements to tube life and tube stability and reduction of tube noise. We are also working on x-ray tube designs which will operate at higher power loadings and at higher CT rotational speed to enhance the performance of next generation CT scanners as well as x-ray tubes to enhance the performance of our flat panel detectors. Research in flat panel imaging technology is aimed at developing new panel technologies for low cost radiographic imaging, flexible panel interfaces, cone beam CT, and high speed multi-slice CT detectors.
We expect that, in order to realize the full potential of the Varian Particle Therapy business, we will need to invest substantial resources to properly develop and commercialize proton therapy technology and build this new business.
Manufacturing and Supplies
We manufacture our medical linear accelerators in Palo Alto, California and in Beijing, China. Our treatment simulator systems and some accelerator subsystems are manufactured in Crawley, United Kingdom and some of our other accessory products in Baden, Switzerland; Helsinki, Finland; Toulouse, France and Winnipeg, Canada. We manufacture our high dose rate brachytherapy systems in Crawley, United Kingdom and Haan, Germany and our brachytherapy treatment planning products in Charlottesville, Virginia. Our SIP linear accelerators and certain radiographic products are manufactured in Las Vegas, Nevada, and Lincolnshire, Illinois. We manufacture components and sub-systems for our proton therapy products and systems in Bergisch Gladbach and Troisdorf, Germany, and we plan to develop additional manufacturing facilities as needed for this business. We manufacture our x-ray imaging component and subsystem and flat panel detector products in Salt Lake City, Utah; Charleston, South Carolina; and Willich, Germany. These facilities employ state-of-the-art manufacturing techniques, and several have been honored by the press, governments and trade organizations for their commitment to quality improvement. Except for the Lincolnshire, Illinois facility, these manufacturing facilities are certified by International Standards Organization (“ISO”) under ISO 9001 (for SIP) or ISO 13485 (for medical devices).
Manufacturing processes at our various facilities include machining, fabrication, subassembly, system assembly and final testing. We have invested in various automated and semi-automated equipment for the fabrication and machining of the parts and assemblies that we incorporate into our products. We may, from time to time, invest further in such equipment. Our quality assurance program includes various quality control measures from inspection of raw material, purchased parts and assemblies through on-line inspection. We also receive subassemblies from third-party suppliers and integrate them into a finished system. We outsource the manufacturing of many major subassemblies and perform system design, assembly and testing in-house. We believe outsourcing enables us to reduce fixed costs and capital expenditures, while also providing us with the flexibility to increase production capacity. We purchase material and components from various suppliers that are either standard products or customized to our specifications. We obtain some of the components included in our products from a limited group of suppliers or from a single-source supplier, such as the radioactive sources for high-dose afterloaders, klystrons for linear accelerators, array sensors for use in our imaging panels, cesium iodide coatings for the arrays, and specialized integrated circuits, x-ray tube targets, housings, glassframes and various other x-ray tube components. We require certain raw materials such as tungsten, lead and copper for Oncology Systems and SIP; copper, lead, tungsten, rhenium, molybdenum zirconium, and various high grades of steel alloy for X-ray Products, and high-grade steel and high-grade copper for the Varian
17
Table of Contents
Particle Therapy business. The rising costs and reduced supply of raw materials that we have seen over the last two years have abated with the recent worldwide economic downturn as global demand has decreased. Worldwide demand, availability and pricing of these raw materials have been volatile, and we expect that availability and pricing will continue to fluctuate in the future.
Backlog
Including a $75 million backlog from Varian Particle Therapy, our backlog at the end of fiscal year 2009 was $2.1 billion, of which we expect to recognize approximately 50% to 55% as revenues in fiscal year 2010. Our backlog at the end of fiscal year 2008 was $1.9 billion, of which $1,024 million was recognized as revenues in fiscal year 2009. Our Oncology Systems backlog represented 87% and 90% of the total backlog at the end of fiscal years 2009 and 2008, respectively. Except for Varian Particle Therapy orders, we only recognize orders for products that are scheduled to be shipped within two years and only if any contingencies are deemed non-perfunctory. For our Varian Particle Therapy business, we recognize orders when construction of the related proton therapy treatment center is reasonably expected to start within two years. Also, we only recognize orders for Varian Particle Therapy products with contingencies if we deem the contingencies perfunctory or if we publicly disclose the existence and nature of material contingencies. Backlog also includes a small portion of service contracts when they become billable, as well as the amount of deferred revenue related to products that have been delivered but have outstanding contractual obligations or revenue related to acceptance. We perform a semi-annual review to verify that orders in our backlog remain valid. This review identifies aged orders and confirms these orders with our internal sales organization or our customers. Aged orders which are not expected to be converted to revenues during this backlog review are deemed dormant and are no longer included in the reported backlog. Orders may be revised or canceled, either according to their terms or as customers’ needs change; consequently, it is impossible to predict with certainty the amount of backlog that will result in revenues. In fiscal years 2009, 2008 and 2007, we reversed $71 million, $70 million and $62 million, respectively, of orders due to adjustments, revisions or cancellations. Our reported net orders included all backlog reversals.
Product and Other Liabilities
Our business exposes us to potential product liability claims that are inherent in the manufacture, sale, installation, servicing and support of medical devices and other devices that deliver radiation. Because our products are involved in the intentional delivery of radiation to the human body; other situations where people may come in contact with radiation (for example, when our SIP products are being used to scan cargo); the collection and storage of patient treatment data for medical analysis and treatment delivery; the planning of radiation treatment and diagnostic imaging of the human body; and the diagnosing of medical problems, the possibility for significant injury and/or death exists. As a result, we may face substantial liability to patients, our customers and others for damages resulting from the faulty, or allegedly faulty, design, manufacture, installation, servicing, support, testing or interoperability of our products, or their misuse or failure, as well as liability related to the loss or misuse of private patient data.
Additionally, in part because proton therapy is still developing and is not yet widely deployed, customers for proton therapy systems are requesting that the systems vendor, as the primary technology provider, provide guarantees for and suffer penalties in relation to the overall construction project. Since each proton center project may cost up to $100 million, the amount of potential liability may be higher than the levels historically assumed by us for our traditional radiation therapy business. If we cannot reasonably mitigate or eliminate these contingencies, our ability to competitively bid upon proton center projects will be negatively impacted and we may be required to assume material amounts of potential liability.
18
Table of Contents
Government Regulation
U.S. Regulation
As a manufacturer, seller and servicer of medical devices and devices emitting radiation or utilizing radioactive by-product material, we and some of our suppliers and distributors are subject to extensive regulation by federal governmental authorities, such as the Food and Drug Administration (“FDA”), Nuclear Regulatory Commission (“NRC”), and state and local regulatory agencies, such as the State of California, to ensure such devices are safe and effective and comply with laws governing products which emit, produce or control radiation. Such regulations, which include the U.S. Food, Drug and Cosmetic Act (the “FDC Act”) and regulations promulgated by the FDA, govern the design, development, testing, manufacturing, packaging, labeling, distribution, import/export, possession, marketing, disposal, clinical investigations involving humans, sale and marketing of medical devices, post-market surveillance, repairs, replacements, recalls and other matters relating to medical devices, radiation emitting devices and devices utilizing radioactive by-product material. State regulations are extensive and vary from state to state. Our Oncology Systems equipment and software, as well as proton therapy systems offered by our Varian Particle Therapy business, constitute medical devices subject to these regulations. Our x-ray tube products and flat panel detectors produced by X-ray Products are also considered medical devices. Future products in any of our business segments may constitute medical devices and be subject to regulation. These laws require that manufacturers adhere to certain standards designed to ensure that the medical devices are safe and effective. Under the FDC Act, each medical device manufacturer must comply with requirements applicable to good manufacturing practices.
Our manufacturing operations for medical devices are required to comply with the FDA’s Quality System Regulation (“QSR”), which addresses a company’s responsibility for quality systems, the requirements of good manufacturing practices and relate to product design, testing, and manufacturing quality assurance, and the maintenance of records and documentation. The QSR requires that each manufacturer establish a quality systems program by which the manufacturer monitors the manufacturing process and maintains records that show compliance with FDA regulations and the manufacturer’s written specifications and procedures relating to the devices. QSR compliance is necessary to receive FDA clearance or approval to market new and existing products. Among other things, these regulations require that manufacturers establish performance requirements before production. The FDA makes announced and unannounced inspections of medical device manufacturers and may issue reports, known as Form FDA 483 reports (listing instances where the manufacturer has failed to comply with applicable regulations and/or procedures), or Warning Letters citing failure to comply with applicable regulations or procedures which, if not adequately responded to, could result in the FDA bringing enforcement action against us, including criminal and civil fines and total shutdown of production facilities and criminal prosecution. Inspections usually occur every two years.
Unless an exception applies, the FDA requires that the manufacturer of a new medical device or a new indication for use of, or other significant change in, an existing medical device obtain either 510(k) pre market notification clearance or pre market approval application (“PMA”) before the manufacturer may take orders for and sell those products in the United States. For proton therapy systems, a 510(k) pre market notification clearance is required prior to the system being used for treating patients. The 510(k) clearance process is applicable when the new product being developed is substantially equivalent to a legally marketed device. The process of obtaining 510(k) clearance generally takes at least one to three months from the date the application is filed and generally requires submitting supporting design and testing data, which can be extensive and can lengthen the process considerably beyond three months. After a product receives 510(k) clearance, any modifications or enhancements that could significantly affect its safety or effectiveness, or that would constitute a major change in the intended use of the device, technology, materials, labeling, packaging, or manufacturing process may require a new 510(k) clearance. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with the manufacturer’s decision, it may
19
Table of Contents
retroactively require the manufacturer to submit a request for 510(k) pre-market notification clearance and can require the manufacturer to cease marketing and/or recall the product until 510(k) clearance is obtained. If we cannot establish that a proposed product is substantially equivalent to a legally marketed device, we must seek pre-market approval through a PMA application. Under the PMA process, the applicant must generally conduct at least one clinical protocol and submit extensive supporting data and clinical information in the PMA application to prove the safety and effectiveness of the product. This process typically takes at least one to two years from the date the pre-market approval is accepted for filing, but can take longer for the FDA to review. To date, we have produced Class 1 medical devices, which require no pre-market approvals or clearances, and Class 2 medical devices, which require only 510(k) clearance. Our x-ray tubes and flat panel detectors are Class 1 medical devices, while all of the products produced by our Oncology Systems segment and the proton therapy systems manufactured by our Varian Particle Therapy business are Class 2 medical devices.
The FDA and the Federal Trade Commission (“FTC”) also regulate the advertising of our products to ensure that the claims we make are consistent with our regulatory clearances, that there are scientific data to substantiate the claims and that our advertising is neither false nor misleading. In general, we may not promote or advertise our products for uses not within the scope of our intended use statement in our clearances or approvals or make unsupported safety and effectiveness claims.
It is also important that our products comply with electrical safety and environmental standards, such as those of Underwriters Laboratories (“UL”), the Canadian Standards Association (“CSA”), and the International Electrotechnical Commission (“IEC”). In addition, the manufacture and distribution of medical devices utilizing radioactive by-product material requires a specific radioactive material license. Manufacture and distribution of these radioactive sources and devices also must be in accordance with an approved NRC certificate, or an Agreement State registration certificate. Service of these products must be in accordance with a specific radioactive materials license. We are also subject to a variety of additional environmental laws regulating our manufacturing operations and the handling, storage, transport and disposal of hazardous materials, and which impose liability for the cleanup of any contamination from these materials. For a further discussion of these laws and regulations, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Environmental Remediation Liabilities.”
We, as a participant in the healthcare industry, are also subject to extensive federal, state and local laws and regulations on a broad array of additional subjects. Further, the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) sets national standards for some types of electronic health information transactions and the data elements used in those transactions and standards to ensure the integrity and confidentiality of patient health information.
The healthcare industry is also subject to a number of “fraud and abuse” laws and regulations, including physician self-referral prohibitions, anti-kickback laws, and false claims laws. See “—Medicare and Medicaid Reimbursement” for a description of these laws and regulations. We must also comply with numerous federal, state and local laws of more general applicability relating to such matters as safe working conditions, manufacturing practices and fire hazard control.
If we or any of our suppliers or distributors fail to comply with FDA and other applicable regulatory requirements or are perceived to potentially have failed to comply, we may face:
| · | adverse publicity affecting both us and our customers; |
| · | increased pressure from our competitors; |
| · | investigations or Warning Letters; |
| · | fines, injunctions, and civil penalties; |
| · | partial suspensions or total shutdown of production facilities, or the imposition of operating restrictions; |
20
Table of Contents
| · | losses of clearances or approvals already granted, or the refusal of future requests for clearance or approval; |
| · | seizures or recalls of our products or those of our customers; |
| · | the inability to sell our products; and |
| · | criminal prosecutions. |
Laws and regulations and their enforcement are constantly changing and new laws and regulations are continually being adopted, and we cannot predict what effect, if any, this may have on our business. There has been a trend in recent years, both in the United States and internationally, toward more stringent regulation and enforcement of requirements applicable to medical device manufacturers and requirements regarding protection and confidentiality of personal data.
Medicare and Medicaid Reimbursement
The federal and state governments of the U.S. establish guidelines and pay reimbursements to hospitals and free-standing clinics for diagnostic examinations and therapeutic procedures under Medicare at the federal level and Medicaid at the state level. Private insurers often establish payment levels and policies based on reimbursement rates and guidelines established by the government.
The federal government and the Congress review and adjust rates annually, and from time to time consider various Medicare and other healthcare reform proposals that could significantly affect both private and public reimbursement for healthcare services, including radiotherapy and radiosurgery, in hospitals and free-standing clinics. Recently, President Obama and members of Congress have proposed significant reforms to the U.S. healthcare system. Members of Congress have proposed legislation that would, among other things, reduce reimbursement rates to Medicare providers; introduce new patient care and payment models, including Medicare payment bundling and gain-sharing; and establish reimbursement policies and rates for different treatment technologies and methods based on their clinical outcomes, comparative effectiveness and costs. It is unclear whether the proposed healthcare legislation will be enacted, and, if there is legislation enacted, what will be contained in the final legislation as it relates to reimbursement. As a result, there is uncertainty among hospitals and free-standing clinics on how healthcare reform will impact their practices. In recent years, we have also seen dramatic reductions in Medicare reimbursements for diagnostic radiology. We believe reductions in these Medicare reimbursement rates have reduced demand for medical x-ray imaging equipment, such as CT scanners, which have negatively impacted demand for our x-ray tube products. State government reimbursement for services is determined pursuant to each state’s Medicaid plan, which is established by state law and regulations, subject to requirements of federal law and regulations. The Balanced Budget Act of 1997 revised the Medicaid program to give each state more control over coverage and payment issues. In addition, CMS has granted many states waivers to allow for greater control of the Medicaid program at the state level. The impact on our business of this greater state control on Medicaid payment for diagnostic services remains uncertain.
The sale of medical devices including radiotherapy products, the referral of patients for diagnostic examinations and treatments utilizing such devices, and the submission of claims to third-party payors (including Medicare and Medicaid) seeking reimbursement for such services, are subject to various federal and state laws pertaining to healthcare “fraud and abuse.” These laws include physician self- referral prohibitions, anti-kickback laws and false claims laws. Subject to enumerated exceptions, the federal physician self-referral law, also known as Stark II, prohibits a physician from referring Medicare or Medicaid patients to an entity with which the physician (or a family member) has a financial relationship, if the referral is for a “designated health service,” which is defined explicitly to include radiology and radiation therapy services. Anti-kickback laws make it illegal to solicit, induce, offer, receive or pay any remuneration in exchange for the referral of business, including the purchase of medical devices from a particular manufacturer or the referral of patients to a particular supplier of
21
Table of Contents
diagnostic services utilizing such devices. False claims laws prohibit anyone from knowingly and willfully presenting, or causing to be presented, claims for payment to third-party payors (including Medicare and Medicaid) that are false or fraudulent, for services not provided as claimed, or for medically unnecessary services. The Office of the Inspector General prosecutes violations of fraud and abuse laws and any violation may result in criminal and/or civil sanctions including, in some instances, imprisonment and exclusion from participation in federal healthcare programs such as Medicare and Medicaid.
Foreign Regulation
Our operations, sales and service of our products outside the United States are subject to regulatory requirements that vary from country to country and may differ significantly from those in the United States. In general, our products are regulated outside the United States as medical devices by foreign governmental agencies similar to the FDA. We are also subject to laws and regulations outside the United States applicable to manufacturers of radiation-producing devices and products utilizing radioactive materials, and laws and regulations of general applicability relating to matters such as environmental protection, safe working conditions, manufacturing practices and other matters, in each case that are often comparable to, if not more stringent than, regulations in the United States. In addition, our sales of products in foreign countries are also subject to regulation of matters such as product standards, packaging requirements, labeling requirements, import restrictions, environmental and product recycling requirements, tariff regulations, duties and tax requirements. We rely in some countries on our foreign distributors to assist us in complying with foreign regulatory requirements.
The European Union (“EU”) implemented a medical device directive that requires us to affix the Conformité Européene (“CE”) mark to our products in order to sell them in member countries of the EU. The CE mark is an international symbol of adherence to certain essential principles of safety and effectiveness, which once affixed, enables a product to be sold in member countries of the EU. The CE mark is also recognized in many countries outside the EU, such as Australia, and can assist in the clearance process. In order to receive permission to affix the CE mark to our products, we must obtain Quality System certification,e.g., ISO 13485, and must otherwise have a quality management system that complies with the EU medical device directives. The ISO promulgates standards for certification of quality assurance operations. We are certified as complying with the ISO 9001 for our Security Inspection Products and ISO 13485 for our medical devices. Several Asian countries, including Japan and China, have adopted regulatory schemes that are comparable, and in some cases more stringent, than the EU scheme. To import medical devices into Japan, the requirements of Japan’s New Medical Device Regulation must be met and a “shonin”, the approval to sell medical products in Japan, must be obtained. Similarly in China, a registration certification issued by the State Food and Drug Administration and a China Compulsory Certification (“CCC”) mark for certain products are required to sell medical devices in that country. Obtaining such certifications on our products can be time-consuming and can cause us to delay marketing or sales of certain products in such countries. Similarly, prior to selling a device in Canada, manufacturers of Class II, III and IV devices must obtain a medical device license. We sell Class II and Class III devices in Canada. Additionally, many countries have laws and regulations relating to radiation and radiation safety that also apply to our products. In most countries, radiological regulatory agencies require some form of licensing or registration by the facility prior to acquisition and operation of an x-ray generating device or a radiation source. The handling, transportation and the recycling of radioactive metals and source materials are also highly regulated.
A number of countries, including the members of the EU, have implemented or are implementing regulations that would require manufacturers to dispose, or bear certain disposal costs, of products at the end of a product’s useful life and restrict the use of some hazardous substances in certain products sold in those countries. For a further discussion of these regulations, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Estimates and Environmental Remediation Liabilities.” Also, many countries where we sell our products have legislation protecting the confidentiality of personal information and the circumstances under which such information may be released for inclusion in our databases, or released to third parties.
22
Table of Contents
Patent and Other Proprietary Rights
We place considerable importance on obtaining and maintaining patent, copyright and trade secret protection for significant new technologies, products and processes, because of the length of time and expense associated with bringing new products through the development process and to the marketplace.
We generally rely upon a combination of patents, copyrights, trademarks, trade secret and other laws, and contractual restrictions on disclosure, copying and transferring title, including confidentiality agreements with vendors, strategic partners, co-developers, employees, consultants and other third parties, to protect our proprietary rights in the developments, improvements and inventions that we have originated and which are incorporated in our products or that fall within our fields of interest. As of October 2, 2009, we owned 228 patents issued in the United States and 71 patents issued throughout the rest of the world and had 358 patent applications on file with various patent agencies worldwide. We intend to file additional patent applications as appropriate. We have trademarks, both registered and unregistered, that are maintained and enforced to provide customer recognition for our products in the marketplace. We also have agreements with third parties that provide for licensing of patented or proprietary technology, including royalty-bearing licenses and technology cross-licenses. For example, during fiscal year 2009, we licensed certain patents related to our flat panel detectors and certain technology related to our RapidArc treatment planning product.
Environmental Matters
For a discussion of environmental matters, see “Government Regulation—Foreign Regulation” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Environmental Remediation Liabilities,” which discussions are incorporated herein by reference.
Financial Information about Geographic Areas
We do business globally with manufacturing in the United States, Europe and China and with sales and service operations and customers throughout the world. Roughly half of our revenues are generated from our international regions. In addition to the potentially adverse impact of foreign regulations, see “Government Regulation—Foreign Regulation,” we also may be affected by other factors related to our international sales such as: lower average selling prices and profit margins; longer time periods from shipment to revenue recognition (which increases revenue recognition deferrals and time in backlog); and longer time periods from shipment to cash collection (which increases days sales outstanding (“DSO”). So to the extent that the geographic distribution of our sales continues to shift more towards international regions, our overall revenues and margins may suffer. We sell our products internationally predominantly in local currencies, but our cost structure is weighted towards the U.S. dollar. Accordingly, there may be adverse consequences from fluctuations in foreign currency exchange rates, which may affect both the affordability and competitiveness of our products and our profit margins. We do engage in currency hedging strategies to offset the effect of currency exchange fluctuations, but the protection offered by these hedges depends upon the timing of transactions, forecast volatility, effectiveness of such hedges and the extent of currency fluctuation.
We are also exposed to other economic, political and other risks inherent in doing business globally. For an additional discussion of these risks, see “Risk Factors” in Item 1A.
For a discussion of financial information about geographic areas, see Note 15, “Segment Information” of the Notes to the Consolidated Financial Statements.
Discontinued Operations
In September 2008, we approved a plan to sell Research Instruments, which develops, manufactures and services highly customized scientific instrument components and systems for fundamental and applied
23
Table of Contents
physics research primarily for national research laboratories worldwide. The sale of Research Instruments was completed in the second quarter of fiscal year 2009. We have classified Research Instruments as a discontinued operation in our Consolidated Statements of Earnings and Consolidated Balance Sheets for all periods presented. See Note 16, “Discontinued Operations” in Notes to Consolidated Financial Statements for detailed discussion. The operations of Research Instruments were conducted from Bergisch Gladbach, Germany. Research Instruments was previously included with the Varian Particle Therapy business, which is reported under the “Other” category in Note 15, “Segment Information” to our Consolidated Financial Statements. We decided to sell Research Instruments in order to focus exclusively on the development of our Varian Particle Therapy business.
Employees
We had approximately 5,100 full-time and part-time employees worldwide, 3,300 in the United States and 1,800 elsewhere at October 2, 2009. None of our employees based in the United States are unionized or subject to collective bargaining agreements. Employees based in some foreign countries may, from time to time, be subject to collective bargaining agreements. We currently consider our relations with our employees to be good.
Information Available to Investors
As soon as reasonably practicable after our filing or furnishing the information to the SEC we make the following available free of charge the Investors page of our websitehttp://www.varian.com: our annual reports on Form 10-K; quarterly reports on Form 10-Q; current reports on Form 8-K (including any amendments to those reports); and our proxy statements. Our Code of Business Ethics, Corporate Governance Guidelines and the charters of the Audit Committee, Compensation and Management Development Committee and Nominating and Corporate Governance Committee are also available on the Investors page of our website. Additionally, we will provide copies of our reports, proxy statements, Code of Business Ethics, Corporate Governance Guidelines and committee charters, without charge, to any stockholder upon written request to the Corporate Secretary at our principal executive offices. Please note that information on, or that can be accessed through, our website is not deemed “filed” with the SEC and is not to be incorporated by reference into any of our filings under the Securities Act of 1933, as amended (the “Securities Act”), or the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
Executive Officers of the Registrant
The biographical summaries of our executive officers as of are as follows:
Name | Age | Position | ||
Timothy E. Guertin | 60 | President and Chief Executive Officer | ||
Dow R. Wilson | 50 | Corporate Executive Vice President and President, Oncology Systems | ||
Elisha W. Finney | 48 | Corporate Senior Vice President, Finance and Chief Financial Officer | ||
Robert H. Kluge | 63 | Corporate Senior Vice President and President, X-ray Products | ||
Tai-yun Chen | 57 | Corporate Vice President, Finance and Corporate Controller | ||
John W. Kuo | 46 | Corporate Vice President, General Counsel and Corporate Secretary |
Timothy E. Guertinhas been Chief Executive Officer since February 2006 and President since August 2005. Previously, Mr. Guertin served as Chief Operating Officer from October 2004 to February 2006, and Corporate Executive Vice President from October 2002 to August 2005. Mr. Guertin also served as President of our Oncology Systems business unit from 1992 to January 2005. Mr. Guertin was Corporate Vice President from 1992 to 2002. Mr. Guertin has held various other positions in the medical systems business during his 33 years with the Company. Mr. Guertin holds a B.S. degree in electrical engineering and computer science from the University of California at Berkeley.
24
Table of Contents
Dow R. Wilson was appointed Corporate Executive Vice President and President, Oncology Systems in August 2005. Mr. Wilson served as Corporate Vice President and President, Oncology Systems from January 2005 to August 2005. Prior to joining the Company in January 2005, Mr. Wilson was Chief Executive Officer of the Healthcare-Information Technologies business in General Electric (a diversified technology and services company), from 2003 to 2005. Previously, Mr. Wilson served as General Manager, Surgical, x-ray and Interventional Businesses and General Manager, Functional Imaging of the Healthcare-Information Technologies business from 2002 to 2003, and was General Manager, Computed Tomography of the Healthcare-Information Technologies business from 2000 to 2002. During the previous 15 years, Mr. Wilson held various management positions within General Electric. Mr. Wilson holds a B.A. degree in English from Brigham Young University and an M.B.A. degree from Dartmouth’s Amos Tuck School of Business. Mr. Wilson also has served on the board of directors of Saba Software, Inc. (an e-learning software provider) since August 2006.
Elisha W. Finney was appointed Corporate Senior Vice President, Finance, in addition to being Chief Financial Officer, in January 2005. Ms. Finney was Corporate Vice President and Chief Financial Officer from April 1999 to January 2005. Ms. Finney has held various other positions during her 21 years with the Company including Treasurer. Ms. Finney holds a B.B.A. degree in risk management and insurance from the University of Georgia and an M.B.A. degree from Golden Gate University in San Francisco. Ms. Finney was appointed a director of Thoratec Corporation (a medical device manufacturer) in June 2007.
Robert H. Klugewas appointed Corporate Senior Vice President and President, X-ray Products of the Company in February 2008. Prior to that, Mr. Kluge served as Corporate Vice President and President, X-ray Products from December 1999 to February 2008 and as Vice President and General Manager of our X-ray Products business from 1993 to December 1999. Before joining the Company in 1993, Mr. Kluge held various positions with Picker International (an x-ray systems manufacturer). Mr. Kluge holds a B.A. degree in economics and an M.B.A. degree in finance from the University of Wisconsin.
Tai-yun Chenwas appointed Corporate Vice President, Finance and Corporate Controller in August 2006. From February 2006 to August 2006, Ms. Chen served as the Company’s Operations Controller. Prior to that, from January 2002 to February 2006, Ms. Chen was the Company’s Assistant Corporate Controller, and from 2000 to January 2002 Ms. Chen was the Company’s Director of Corporate Accounting. Ms. Chen has served in various accounting management positions throughout the Company during her 26 years with the Company. Ms. Chen holds a bachelor’s degree in economics from the National Chung Chi University in Taiwan and a master’s degree in managerial economics from the University of California at Santa Barbara.
John W. Kuo was appointed Corporate Vice President, General Counsel in July 2005 and Corporate Secretary in February 2005. Mr. Kuo joined the Company as Senior Corporate Counsel in March 2003 and became Associate General Counsel in March 2004. Prior to joining the Company, Mr. Kuo was General Counsel and Secretary at BroadVision, Inc. (an e-commerce software provider) in 2002 and held senior legal positions at 3Com Corporation (a networking equipment provider) from 1997 to 2002. Mr. Kuo has previously been with the law firms of Gray Cary Ware & Freidenrich (now DLA Piper) and Fulbright & Jaworski. Mr. Kuo holds a B.A. degree from Cornell University and a J.D. degree from Boalt Hall School of Law at the University of California at Berkeley.
The following risk factors and other information included in this Annual Report on Form 10-K should be carefully considered. Although the risk factors described below are the ones management deems significant, additional risks and uncertainties not presently known to us or that we presently deem less significant may also impair our business operations. If any of the following risks actually occur, our business, operating results, and financial condition could be adversely affected.
25
Table of Contents
IF WE ARE UNABLE TO ANTICIPATE OR KEEP PACE WITH CHANGES IN THE MARKETPLACE AND THE DIRECTION OF TECHNOLOGICAL INNOVATION AND CUSTOMER DEMANDS, OUR PRODUCTS MAY BECOME LESS USEFUL OR OBSOLETE AND OUR OPERATING RESULTS WILL SUFFER
Rapid change and technological innovation characterize the Oncology Systems market. Our products often have long development and government approval cycles, so we must anticipate changes in the marketplace, in technology and in customer demands. For example, most of our recent Oncology Systems product introductions have related to IMRT, IGRT, and VMAT, and enhancements of existing products through greater integration and simplification.
We believe that IMRT and IGRT have become accepted standards for treatment in the radiation oncology market. Demand for our IMRT products has been a historical driver for our net orders and revenues in Oncology Systems and, now, demand for our products for IGRT has been one of the main contributors to more recent net orders and revenue growth. However, if future studies call into question the effectiveness of our IMRT or IGRT products or show negative side effects, or if other more effective technologies are introduced, our revenues could suffer. Our success also depends on the continued acceptance and success of IMRT and IGRT in general and of our IMRT and IGRT products in particular. As more institutions buy or upgrade to achieve these capabilities, the market for IMRT and IGRT products may become saturated and we could face competition from newer technologies. For example, we have seen and continue to expect that the rate of growth for IMRT equipment will be lower than what we have experienced previously, particularly in the North American market where a majority of our customer sites have the products and accessories necessary to perform IMRT.
We believe that the acceptance of VMAT in general, and our RapidArc products in particular, is key to our future success. We believe that our RapidArc products for VMAT are a significant advance in IMRT treatments and can help drive longer term demand for our linear accelerators and IMRT-related products. Orders for our RapidArc technology have already contributed significantly to our recent net orders growth, even though VMAT and our RapidArc products are not yet widely-accepted as a treatment standard. Early adopters of VMAT and our RapidArc products continue to publish studies on VMAT treatments using our RapidArc products. If, however, future studies contradict current knowledge about VMAT or our RapidArc products, question the effectiveness of VMAT treatments or show negative side effects, or if other more effective technologies are introduced, our customers may not be willing to adopt VMAT or purchase our RapidArc products. In addition, if third party information systems do not support our VMAT technology, customers that have third party information systems may not purchase our RapidArc products, which could negatively impact our net orders and revenues.
As radiation oncology treatment becomes more complex, our customers are increasingly focusing on ease-of-use and interconnectivity. Our equipment and software is highly sophisticated and requires a high level of training and education to use them competently and safely, a requirement made even more important because they work together within integrated environments. We have directed substantial product development efforts into (i) greater interconnectivity of our products for more seamless operation within a system, (ii) enhancing the ease of use of our software products and (iii) reducing setup and treatment times and increasing patient throughput. We have emphasized maintaining an “open systems” approach that allows customers to “mix and match” individual products, incorporate products from other manufacturers, share information with other systems or products and use the equipment for offering various methods of radiation therapy treatment. We anticipate that these efforts will increase the acceptance and adoption of IMRT, VMAT and IGRT and will stimulate demand for our products from new customers and upgrades from existing customers. We face competition though from “closed-ended” dedicated-use systems that place simplicity of use ahead of flexibility. If we have misjudged the importance to our customers of maintaining an “open systems” approach, or if we are unsuccessful in our efforts to enable greater interconnectivity, enhance ease-of-use and reduce setup and treatment times, our revenues could suffer.
26
Table of Contents
The acquisition of Varian Particle Therapy should enable us to develop and offer products for delivering image-guided, intensity-modulated proton therapy for the treatment of cancer. While our investment in proton therapy product development will continue, this technology may not be accepted as quickly as others due to the relatively large scale, high costs and complex project financing associated with implementing a proton therapy system. Risks associated with this business could increase, given the heavy reliance of customers on credit and large-scale project financing, which is more difficult to obtain with the current general worldwide economic downturn and contraction in credit markets. Our future success will depend upon the wide-spread awareness, acceptance and adoption by the oncology market of proton therapy systems for the treatment of cancer. Our efforts to increase awareness and adoption of our proton therapy systems may not be successful, which could negatively impact this business.
Our X-ray Products business sells products primarily to a small number of imaging system OEM customers who use our products in their medical diagnostic and industrial imaging systems. Some of these companies also manufacture x-ray tubes or flat panel detectors for their own systems, which means that we compete with their in-house x-ray tube and flat panel detector manufacturing operations for business from their affiliated systems businesses. To succeed, we must provide x-ray tube and flat panel detector products that meet customer demands for lower cost, better product quality and superior technology and performance. If we are unable to continue to innovate our X-ray Products technology and anticipate our customers’ demands in the areas of cost, quality, technology and performance, then our customers may chose to purchase from their internal manufacturing operations or from other independent tube or panel manufacturers, which would negatively impact this business.
We may be unable to accurately anticipate changes in our markets and the direction of technological innovation and demands of our customers, our competitors may develop improved products or processes, or the marketplace may conclude that the tasks our products were designed to do is no longer an element of a generally accepted diagnostic or treatment regimen. If this occurs, the market for our products may be adversely affected and they may become less useful or obsolete. Any development adversely affecting the markets for our products would force us to reduce production volumes or to discontinue manufacturing one or more of our products or product lines and would reduce our revenues and earnings.
IF WE ARE UNABLE TO DEVELOP NEW PRODUCTS OR ENHANCE EXISTING PRODUCTS, WE MAY BE UNABLE TO ATTRACT OR RETAIN CUSTOMERS
Our success depends on the successful development, introduction and commercialization of new generations of products, treatment systems and enhancements to and/or simplification of existing products. Our Oncology Systems products are technologically complex and must keep pace with, among other things, those of our competitors. Our X-ray Products business must also continually develop improved and lower cost products. Accordingly, many of our products may require significant planning, design, development and testing. We are making significant investments in long-term growth initiatives, such as development of our SIP and Particle Therapy businesses, and expect that we will need more investment to develop and commercialize the products and technology for these businesses. These activities require significant capital commitments, involvement of senior management and other investments on our part, which we may be unable to recover. Our timeline for the development of new products or enhancements may not be achieved and price and profitability targets may not prove feasible. Commercialization of new products may prove challenging, and we may be required to invest more time and money than expected to successfully introduce them. Once introduced, new products may adversely impact orders and sales of our existing products, or make them less desirable or even obsolete. Compliance with regulations, competitive alternatives, and shifting market preferences, may also impact the successful implementation of new products or enhancements. A few of our research and development projects have been, and in the future may be, funded by government contracts, and changes in government priorities and our ability to attract similar funding may affect our overall research efforts.
27
Table of Contents
Our ability to successfully develop and introduce new products, treatment systems and product enhancements and simplifications, and the revenues and costs associated with these efforts, are affected by our ability to:
| · | properly identify customer needs; |
| · | prove feasibility of new products; |
| · | limit the time required from proof of feasibility to routine production; |
| · | comply with internal quality assurance systems and processes timely and efficiently; |
| · | limit the timing and cost of regulatory approvals; |
| · | accurately predict and control costs associated with inventory overruns caused by phase-in of new products and phase-out of old products; |
| · | price our products competitively; |
| · | manufacture and deliver our products in sufficient volumes on time, and accurately predict and control costs associated with manufacturing, installation, warranty and maintenance of the products; |
| · | manage customer acceptance and payment for products; |
| · | manage customer demands for retrofits of both new and old products; and |
| · | anticipate and compete successfully with competitors. |
Additionally, the budgeting cycles of hospitals and clinics for capital equipment purchases are frequently fixed well in advance and are experiencing increasing pressure in the current economic climate, which may lengthen sales and ordering timeframes. In addition, even if customers accept new products or product enhancements, the revenues from these products may not be sufficient to offset the significant costs associated with making them available to customers.
We cannot be sure that we will be able to successfully develop, manufacture or introduce new products, treatment systems or enhancements, the roll-out of which involves compliance with complex quality assurance processes, including the QSR of the FDA. Failure to complete these processes timely and efficiently could result in delays that could affect our ability to attract and retain customers, or could cause customers to delay or cancel orders, causing our revenues and operating results to suffer.
New products generally take longer to install than well-established products. Because a portion of a product’s revenue is generally tied to installation and acceptance of the product, our recognition of revenue associated with new products may be deferred longer than expected. While we will work to decrease the installation times for new products, such as we have done with installation times for OBI, these plans may not be successful or have a meaningful impact on reducing associated revenue recognition deferrals. Furthermore, even if these plans are successful, potential customers may not decide to upgrade their equipment, or customers may delay delivery of some of our more sophisticated products because of the longer preparation and renovation of treatment rooms required. As a result, our revenues and other financial results could be adversely affected.
ROUGHLY HALF OF OUR REVENUES ARE INTERNATIONAL, AND ECONOMIC, POLITICAL AND OTHER RISKS ASSOCIATED WITH INTERNATIONAL SALES AND OPERATIONS COULD ADVERSELY AFFECT OUR SALES OR MAKE THEM LESS PREDICTABLE
We conduct business globally. Our international revenues accounted for approximately 50%, 52% and 51% of revenues from continuing operations during fiscal years 2009, 2008 and 2007, respectively. As a result, we must provide significant service and support globally, and we have sales and service offices
28
Table of Contents
located in Europe, Asia, South America and Australia. We also have manufacturing and research operations in the United Kingdom, Germany, Switzerland, France, Finland, Canada and China. We also invested in the expansion of our China x-ray business through our acquisition of Pan-Pacific Enterprises, Inc. in fiscal year 2008. We have invested, and will continue to invest, substantial resources to meet the needs of our customers. We intend to continue to expand our presence in international markets, although we cannot be sure we will be able to compete successfully in the international markets, generate new business, or meet the service and support needs of our customers there. Accordingly, our future results could be harmed by a variety of factors, including:
| · | the difficulties in enforcing agreements and collecting receivables through many foreign country’s legal systems; |
| · | the longer payment cycles associated with many foreign customers; |
| · | the possibility that foreign countries may impose additional taxes, tariffs or other restrictions on foreign trade; |
| · | the fact that international regions typically have a lower gross margin on our products and a longer period from shipment to revenue recognition that generally results in greater revenue recognition deferrals and higher backlog; |
| · | our ability to obtain export licenses and other required export or import licenses or approvals; |
| · | failure to comply with export laws and requirements which may result in civil or criminal penalties and restrictions on our ability to export our products, particularly our industrial linear accelerator products; |
| · | failure to obtain proper business licenses or other documentation, or to otherwise comply with local laws and requirements regarding marketing, sales, service or any other business we conduct in a foreign jurisdiction, which may result in civil or criminal penalties and restrictions on our ability to conduct business in a foreign jurisdiction; |
| · | changes in the political, regulatory, safety or economic conditions in a country or region; and |
| · | the possibility that it may be more difficult to protect our intellectual property in foreign countries. |
Historically, our international sales have had lower average selling prices and gross margins. Although our orders and sales fluctuate from period to period, in recent years our international regions have represented a larger share of our business. As a result, our overall rate of orders growth (measured in U.S. dollars) could slow down and overall revenues and gross margins may be negatively affected.
In addition, we generally retain cash received through international operations in our local subsidiaries. As of October 2, 2009, 83% of our cash and cash equivalents were held abroad. If these funds were repatriated to the United States, they could be subject to additional taxation, and we would not receive the full benefit of the repatriation. If this happens, our overall tax rate and our results of operations could suffer.
Earnings from our international regions are generally taxed at rates lower than U.S. rates. Our effective tax rate is impacted by tax laws in both the United States and in the respective countries in which our international subsidiaries do business. A decrease in the percentage of our total earnings from international regions, or a change in the mix of international regions among particular tax jurisdictions, could increase our effective tax rate. Also, our current effective tax rate does not assume U.S. taxes on certain undistributed profits of certain foreign subsidiaries. These earnings could become subject to incremental foreign withholding or U.S. federal and state taxes should they either be deemed to be or are actually remitted to the United States, or if tax laws change, in which case our financial results could be adversely affected. In addition, recent proposals would make significant changes to U.S. taxation of
29
Table of Contents
U.S.-based multinational corporations. Although we cannot predict whether or in what form Congress would enact any such proposals, legislation of this type could have an adverse impact on our effective tax rate and financial results.
OUR RESULTS MAY BE HARMED BY THE WORLDWIDE ECONOMIC DOWNTURN
Since fiscal year 2008, the global economy has experienced a severe downturn due to the sequential effects of the subprime lending crisis, the credit market crisis, collateral effects on the finance and banking industries, volatile currency exchange rates and energy costs, concerns about inflation, slower economic activity, decreased consumer confidence, reduced corporate profits and capital spending, adverse business conditions and liquidity concerns. These economic conditions worsened in fiscal year 2009. These conditions have shrunk capital equipment budgets, slowed decision-making, made financing for large equipment purchases more expensive and more time consuming, and made it difficult for our customers and our vendors to accurately forecast and plan future business activities. This, in turn, has caused and may cause our customers to freeze or dramatically reduce purchases and capital project expenditures, and may result in consolidation of our customers. These conditions may also disrupt supply if vendors consolidate or go out of business. In such a climate, it has become and may continue to be more difficult for us to accurately forecast and plan our future business activities. We cannot predict the timing or duration of the economic downturn or when an economic recovery will occur, in general or specifically in the healthcare industry. A deteriorating healthcare market would inevitably adversely affect our business, financial conditions and results of operations.
HEALTHCARE REFORM LEGISLATION MAY AFFECT DEMAND FOR OUR PRODUCTS AND COULD ADVERSELY AFFECT OUR REVENUE AND FINANCIAL CONDITION
Healthcare costs have risen significantly over the past decade. There have been and continue to be proposals by legislators, regulators and third-party payors to keep these costs down. Certain proposals, if passed, may impose limitations on the amounts of reimbursement available for our products from governmental agencies or third-party payors, or additional taxes on medical devices. These proposals could have a negative impact on the demand for our products and services, and therefore on our financial position and results of operations.
Recently, President Obama and members of Congress have proposed significant reforms to the U.S. healthcare system. Both the U.S. Senate and House of Representatives have conducted hearings about U.S. healthcare reform. In the Obama administration’s fiscal year 2010 federal budget proposal, the administration emphasized maintaining patient choice, reducing inefficiencies and costs, increasing prevention programs, increasing coverage portability and universality, improving quality of care and maintaining fiscal sustainability. The Obama administration’s fiscal year 2010 budget included proposals to limit Medicare payments, reduce drug spending and increase taxes. In addition, members of Congress have proposed a significant tax on certain medical devices, a single-payer healthcare system, a government health insurance option to compete with private plans, and other expanded public healthcare measures. Various healthcare reform proposals have also emerged at the state level. We believe that the current uncertainty created by the prospects of healthcare reform in the United States has impacted our Oncology Systems business, and we expect that this uncertainty will persist until there is greater clarity on healthcare reform. We are unable to predict what healthcare reform legislation or regulations, if any, will be enacted in the United States or elsewhere; whether other healthcare legislation or regulations affecting our business may be proposed or enacted in the future; what effect any such legislation or regulation would have on our business; or the effect ongoing uncertainty surrounding these matters will have on our customer’s purchasing decisions. However, an expansion in government’s role in the U.S. healthcare industry may lower reimbursements for our products, reduce medical procedure volumes, and adversely affect our business, possibly materially.
30
Table of Contents
CHANGES TO RADIATION ONCOLOGY REIMBURSEMENTS MAY AFFECT DEMAND FOR OUR PRODUCTS
Sales of our healthcare products indirectly depend on whether adequate reimbursement is available to our customers from a variety of sources, such as government healthcare insurance programs, including the Medicare and Medicaid programs; private insurance plans; health maintenance organizations and preferred provider organizations. Once Medicare has made a decision to provide reimbursement for a given treatment, these reimbursement rates are generally reviewed and adjusted by Medicare annually. Private third-party payors, although independent from Medicare, sometimes use portions of Medicare reimbursement policies and payment amounts in making their own reimbursement decisions. As a result, decisions by CMS to reimburse for a treatment, or changes to Medicare’s reimbursement policies or reductions in payment amounts with respect to a treatment sometimes extend to third-party payor reimbursement policies and amounts for that treatment. We have seen our customers’ decision-making process complicated by the uncertainty surrounding the proposed reduction in Medicare reimbursement rates for radiotherapy and radiosurgery at free-standing clinics in the United States and for physician reimbursement for radiation oncology. While the final enacted reimbursement rate reductions for 2010, which were announced by CMS on October 30, 2009, were much more modest for radiotherapy than originally proposed, we believe that the confusion and uncertainty created by the proposal was one of the major factors impacting our net orders in late fiscal year 2009, particularly from free-standing radiotherapy clinics (which we believe represents approximately 10% to 15% of Oncology Systems’ business). Any significant cuts in these reimbursement rates, or other rates for radiotherapy, radiosurgery, proton therapy or brachytherapy, or concerns or proposals regarding further cuts, could further increase uncertainty, influence our customers’ decisions, reduce demand for our products, cause customers to cancel orders and have a material adverse effect on our revenues and stock price.
In general, third-party payors are increasingly cost-conscious, and we cannot be sure that they will reimburse our customers at levels sufficient to enable us to achieve or maintain sales and price levels for our products. Without adequate support from third-party payors, the market for our products may be limited. There is no uniform policy on reimbursement among third-party payors, nor can we be sure that procedures using our products will qualify for reimbursement from third-party payors. Likewise, foreign governments also have their own healthcare reimbursement systems and we cannot be sure that adequate reimbursement will be made available with respect to our products under any foreign reimbursement system.
OUR RESULTS MAY BE IMPACTED BY CHANGES IN FOREIGN CURRENCY EXCHANGE RATES
Because our business is global and payments are generally made in local currency, fluctuations in foreign currency exchange rates can impact our results by affecting product demand or our expenses and/or the profitability in U.S. dollars of products and services that we provide in foreign markets. We manage this risk through established policies and procedures that include the use of derivative financial instruments. We have historically entered into foreign currency forward exchange contracts, generally ranging from one to twelve months in maturity, to mitigate the effects of operational and balance sheet exposures to fluctuations in foreign currency exchange rates.
Although hedging strategies help to offset the effect of fluctuations in foreign currency exchange rates, the protection these strategies provide will be affected by the timing of transactions, and the effectiveness of those strategies, the number of transactions that are hedged, forecast volatility and the extent to which exchange rates have changed. In particular, foreign currency exchange rates have been extremely volatile over short periods of time since the beginning of 2008. If our hedging strategies do not offset these fluctuations, our revenues and other operating results may be harmed. In addition, movement in foreign currency exchange rates could impact our financial results positively or negatively in one period and not another, making it more difficult to compare our financial results from period to period.
31
Table of Contents
In addition, long-term movements in foreign currency exchange rates can also affect the competitiveness of our products in the local currencies of our international customers. Even though our international sales are mostly in local currencies, our cost structure is weighted towards the U.S. dollar, and some of our competitors may have cost structures based in other currencies. The volatility of the U.S. dollar that we have experienced over the last several years has affected the competitiveness of our pricing against our foreign competitors, either helping or hindering our international order and revenue growth, thereby affecting our overall financial performance and results. Changes in monetary or other policies here and abroad, including as a result of the current economic downturn or in reaction thereto, or in the United States as a result of a change in the U.S. laws or regulations that will likely affect foreign currency exchange rates.
WE FACE SIGNIFICANT COSTS IN ORDER TO COMPLY WITH LAWS AND REGULATIONS APPLICABLE TO THE MANUFACTURE AND DISTRIBUTION OF OUR PRODUCTS, AND FAILURE OR DELAYS IN OBTAINING REGULATORY CLEARANCES OR APPROVALS, OR FAILURE TO COMPLY WITH APPLICABLE LAWS AND REGULATIONSCOULD PREVENT US FROM DISTRIBUTING OUR PRODUCTS OR RESULT IN SIGNIFICANT PENALTIES
Our products and those of OEMs that incorporate our products are subject to extensive and rigorous government regulation, both in the United States and in foreign countries. Compliance with these laws and regulations is expensive and time-consuming, and failure to comply with these laws and regulations could adversely affect our business.
Marketing a medical device in the United States. In the United States, as a manufacturer and seller of medical devices and devices emitting radiation or utilizing radioactive by-product material, we and some of our suppliers and distributors are subject to extensive regulation by federal governmental authorities, such as the FDA, NRC and state and local regulatory agencies, such as the State of California, to ensure the devices are safe and effective and comply with laws governing products which emit, produce or control radiation. Similar international regulations apply overseas. These regulations govern, among other things, the design, development, testing, manufacturing, packaging, labeling, distribution, import/export, sale and marketing and disposal of our products.
Unless an exception applies, the FDA requires that the manufacturer of a new medical device or a new indication for use of, or other significant change in, an existing medical device obtain either 510(k) pre-market notification clearance or pre-market approval before we can market or sell those products in the United States. Modifications or enhancements to a product that could significantly affect its safety or effectiveness, or that would constitute a major change in the intended use of the device, technology, materials, labeling, packaging, or manufacturing process may also require a new 510(k) clearance. Obtaining clearances or approvals is time-consuming, expensive and uncertain. We may not be able to obtain the necessary clearances or approvals or may be unduly delayed in doing so, which could harm our business. Furthermore, even if we are granted regulatory clearances or approvals, they may include significant limitations on the indicated uses of the product, which may limit the market for those products. If we were unable to obtain required FDA clearance or approval for a product or unduly delayed in doing so, or the uses of that product were limited, our business would suffer. In the past, in the United States, our devices have generally been subject to 510(k) clearance or exempt from 510(k) clearance. The 510(k) clearance process is generally less time-consuming, expensive and uncertain than the PMA process. However, there are some in the regulatory field who believe that certain medical devices should be required to use the PMA approval process rather than the 510(k) clearance process. If we were required to use the PMA approval process for future products or product modifications, it could delay or prevent release of the proposed products or modifications, which could harm our business.
Marketing a medical device internationally.In order for us to market our products internationally, we must obtain clearances or approvals for products and product modifications. These processes (including for example in the EEA, China, Japan and Canada) can be time consuming, expensive, and uncertain,
32
Table of Contents
which can delay our ability to market products in those countries. If we do not obtain the clearance or approvals on one or more of our products, or are unduly delayed in doing so, or if a clearance or approval includes significant limitations on the indicated uses of the product, the market for the affected products would be negatively impacted.
Within the EEA, we must receive a CE mark, a European marking of conformity that indicates that a product meets the relevant regulatory requirements and, when used as intended, works properly and is acceptably safe. This conformity to the applicable directives is done through self-declaration and is verified by an independent certification body, called a Notified Body, before the CE mark can be granted. Once clearance is obtained and the CE mark is affixed to the device, the Notified Body will regularly audit us to ensure that we remain in compliance with the applicable European laws or directives. CE marking demonstrates that our products comply with the laws and regulations required by the EEA countries to allow free movement of trade within the EEA countries. If we cannot support our performance claims and demonstrate compliance with the applicable European laws and directives, we lose our CE mark, which would prevent us from selling our products within the EEA.
Quality systems, audits and failure to comply.Our manufacturing operations are required to comply with the FDA’s QSR, and other federal and state regulations for medical devices and radiation emitting products that address a company’s responsibility for complying with the quality systems regulations, which include the requirements for current good manufacturing practices. The FDA makes announced and unannounced inspections of medical device manufacturers to determine compliance with QSR and in connection with these inspections has issued, and in the future may issue, reports, known as Form FDA 483 reports (listing instances where the manufacturer has failed to comply with applicable regulations and/or procedures), or Warning Letters citing failure to comply with applicable regulations or procedures. If a Warning Letter were issued, we would be required to take prompt corrective action to come into compliance. Failure to respond timely to a Warning Letter or other notice of noncompliance and to come into compliance could result in the FDA bringing enforcement action against us, which could include the total shutdown of our production facilities and criminal and civil fines. Additionally, if a Warning Letter were issued, customers could delay purchasing decisions or cancel orders, and we could face increased pressure from our competitors, who could use the Warning Letter against us in competitive sales situations, either of which could adversely affect our reputation, business and stock price.
In addition, we are required to timely file various reports with the FDA and other international regulatory authorities, including reports required by the medical device reporting regulations, and similar international adverse event reporting regulations, that require that we report to regulatory authorities if our devices may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur. If these reports are not filed timely, regulators may impose sanctions and sales of our products may suffer, and we may be subject to product liability or regulatory enforcement actions, all of which could harm our business.
If we initiate a correction or removal of a device to reduce a risk to health posed by the device, we would be required to submit a Corrections and Removals report to the FDA and in many cases, similar reports to other regulatory agencies. This report could be classified by the FDA as a device recall which could lead to increased scrutiny by the FDA and other international regulatory agencies regarding the quality and safety of our devices.
Our medical devices utilizing radioactive material are subject to the NRC clearance and approval requirements, and the manufacture and sale of these products are subject to extensive international, federal and state regulation that varies from state to state and among countries or regions. Our manufacture, distribution installation and service of medical devices utilizing radioactive material or emitting radiation also requires us to obtain a number of licenses and certifications for these devices and materials. Service of these products must also be in accordance with a specific radioactive materials
33
Table of Contents
license. In addition, we are subject to a variety of environmental laws regulating our manufacturing operations and the handling, storage, transport and disposal of hazardous materials, and which impose liability for the cleanup of any contamination from these materials. In particular, the handling and disposal of radioactive materials resulting from the manufacture, use or disposal of our products may impose significant costs and requirements. Disposal sites for the lawful disposal of materials generated by the manufacture, use or decommissioning of our products may no longer accept these materials in the future, or may accept them on unfavorable terms.
The FDA and the FTC, also regulate advertising and promotion of our products to ensure that the claims we make are consistent with our regulatory clearances, that there are scientific data to substantiate the claims and that our advertising is neither false nor misleading. If the FDA or FTC determines that any of our advertising or promotional claims are not permissible, we may be subject to enforcement actions and may be required to revise our promotional claims or make other corrections or restitutions.
As a participant in the healthcare industry, we are also subject to extensive laws and regulations protecting the privacy and integrity of patient medical information, including HIPAA and similar laws and regulations in foreign countries covering data privacy and other protection of health and employee information, “fraud and abuse” laws and regulations, including, physician self-referral prohibitions, anti-kickback laws and false claims laws. We also must comply with numerous federal, state and local laws of more general applicability relating to such matters as safe working conditions, manufacturing practices and fire hazard control.
If we or any of our suppliers, distributors or customers fail to comply with FDA, FTC and other applicable U.S. and foreign country regulatory requirements or are perceived to potentially have failed to comply, we may face:
| · | adverse publicity affecting both us and our customers; |
| · | increased pressures from our competitors; |
| · | investigations by governmental authorities or Warning Letters; |
| · | fines, injunctions, and civil penalties; |
| · | partial suspensions or total shutdown of production facilities, or the imposition of operating restrictions; |
| · | increased difficulty in obtaining required FDA clearances or approvals, or the equivalent approvals in foreign countries; |
| · | losses of clearances or approvals already granted; |
| · | seizures or recalls of our products or those of our customers; |
| · | delays in purchasing decisions by customers or cancellation of existing orders; |
| · | the inability to sell our products, or, where we have failed to comply with foreign regulations, to import our products to such countries; and |
| · | criminal prosecutions. |
The laws and regulations and their enforcement are constantly undergoing change, and we cannot predict what effect, if any, changes to these laws and regulations may have on our business. There has been a trend in recent years, both in the United States and internationally, toward more stringent regulation and enforcement of requirements applicable to medical device manufacturers and requirements regarding protection and confidentiality of personal data.
Government regulation also may cause considerable delay or even prevent the marketing and full commercialization of future products or services that we may develop, and/or may impose costly requirements on our business. Insurance coverage is not commercially available for violations of law,
34
Table of Contents
including the fines, penalties or investigatory costs that may flow to us as the consequence of regulatory violations; consequently, we do not have insurance that would cover this type of liability.
COMPLIANCE WITH FOREIGN LAWS AND REGULATIONS APPLICABLE TO THE MANUFACTURE AND DISTRIBUTION OF OUR PRODUCTS MAY BE COSTLY, AND FAILURE TO COMPLY MAY RESULT IN PENALTIES
Regulatory requirements affecting our operations and sales outside the United States vary from country to country, often differing significantly from those in the United States. In general, outside the United States, our products are regulated as medical devices by foreign governmental agencies similar to the FDA. We are also subject to laws and regulations that apply to manufacturers of radiation emitting devices and products utilizing radioactive materials, as well as laws and regulations of general applicability relating to matters such as environmental protection, safe working conditions, manufacturing practices and other matters. These are often comparable, if not more stringent, than the equivalent regulations in the United States. Sales overseas are also affected by regulation of matters such as product standards, packaging, labeling, environmental and product recycling requirements, import and export restrictions, tariffs, duties and taxes. In some countries, we rely on our foreign distributors to assist us in complying with foreign regulatory requirements, and we cannot be sure that they will always do so. We may be required to incur significant time and expense in obtaining and maintaining regulatory approvals. Delays in receipt of or failure to receive regulatory approvals, the loss of previously obtained approvals or failure to comply with existing or future regulatory requirements could restrict or prevent us from doing business in a country or subject us to a variety of enforcement actions and civil or criminal penalties, which would adversely affect our business.
WE ARE SUBJECT TO FEDERAL, STATE AND FOREIGN LAWS GOVERNING OUR BUSINESS PRACTICES WHICH, IF VIOLATED, COULD RESULT IN SUBSTANTIAL PENALTIES. ADDITIONALLY, CHALLENGES TO OR INVESTIGATION INTO OUR PRACTICES COULD CAUSE ADVERSE PUBLICITY AND BE COSTLY TO RESPOND TO AND THUS COULD HARM OUR BUSINESS
The Medicare and Medicaid “anti-kickback” laws, and several similar state laws, prohibit payments or other remuneration that is intended to induce hospitals, physicians or others either to refer patients or to purchase, lease or order, or arrange for or recommend the purchase, lease or order of healthcare products or services for which payment may be made under federal and state healthcare programs, such as Medicare and Medicaid. These laws affect our sales, marketing and other promotional activities by limiting the kinds of financial arrangements we may have with hospitals, physicians or other potential purchasers of our products. They particularly impact how we structure our sales offerings, including discount practices, customer support, education and training programs, physician consulting, research grants and other service arrangements. These laws are broadly written, and it is often difficult to determine precisely how these laws will be applied to specific circumstances.
Federal and state “false claims” laws generally prohibit knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent, or for items or services that were not provided as claimed. Although we do not submit claims directly to payors, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by providing inaccurate billing or coding information to customers, or through certain other activities, including promoting products for uses not approved or cleared by the FDA, which is called off-label promotion. Violating “anti-kickback” and “false claims” laws can result in civil and criminal penalties, which can be substantial, and potential exclusion from healthcare programs for noncompliance. Even an unsuccessful challenge or investigation into our practices could cause adverse publicity, and be costly to defend, and thus could harm our business and results of operations. Additionally, several proposals and bills are being considered at both the state and federal levels expanding “anti-kickback” laws to require, among other things, extensive tracking, maintenance of data
35
Table of Contents
bases regarding and disclosures of relationships and payments to physicians and healthcare providers. If these proposals or bills were to become law, the implementation of the necessary infrastructure to comply with them could be quite costly.
We are subject to similar laws in foreign countries where we conduct business. For example, within the EU, the control of unlawful marketing activities is a matter of national law in each of the member states of the EU. The member states of the EU closely monitor perceived unlawful marketing activity by companies. We could face civil, criminal and administrative sanctions if any member state determines that we have breached our obligations under its national laws. Industry associations also closely monitor the activities of member companies. If these organizations or authorities name us as having breached our obligations under their regulations, rules or standards, our reputation would suffer and our business and financial condition could be adversely affected.
We are also subject to the U.S. Foreign Corrupt Practices Act, antitrust and anti-competition laws, and similar laws in foreign countries, any violation of which could create a substantial liability for us and also cause a loss of reputation in the market. From time to time, we may face audits or investigations by one or more domestic or foreign government agencies, compliance with which could be costly and time-consuming, and could divert our management and key personnel from our business operations. An adverse outcome under any such investigation or audit could subject us to fines or other penalties, which could adversely affect our business and financial results.
As we enter new businesses or pursue new business opportunities, we may become subject to laws, rules and regulations, such as FDA regulations applicable to clinical trials. Becoming familiar with and implementing the infrastructure necessary to comply with these laws, rules and regulations could be quite costly. In addition, failure to comply with these laws, rules and regulations could delay the introduction of new products and could adversely affect our business.
PRODUCT DEFECTS OR MISUSE MAY RESULT IN MATERIAL PRODUCT LIABILITY OR PROFESSIONAL ERRORS AND OMISSIONS CLAIMS, INVESTIGATION BY REGULATORY AUTHORITIES OR PRODUCT RECALLS THAT COULD HARM FUTURE REVENUES AND REQUIRE US TO PAY MATERIAL UNINSURED CLAIMS
Our business exposes us to potential product liability claims that are inherent in the manufacture, sale, installation, servicing and support of medical devices and other devices that deliver radiation. Because our products are involved in the intentional delivery of radiation to the human body, other situations where people may come in contact with radiation (for example, when our SIP products are being used to scan cargo), the collection and storage of patient treatment data for medical analysis and treatment delivery, the planning of radiation treatment and diagnostic imaging of the human body, and the diagnosing of medical problems, the possibility for significant injury and/or death exists. Our medical products operate within our customers’ facilities and network systems, and under QA procedures established by the facility that ultimately result in the delivery of radiation to patients. Human and other errors or accidents may arise from the fact that our products operate in complex environments with products from other vendors, where interoperability or data sharing protocol may not be optimized even though the equipment or system operate according to specifications. As a result, we may face substantial liability to patients, our customers and others for damages resulting from the faulty, or allegedly faulty, design, manufacture, installation, servicing, support, testing or interoperability of our products, or their misuse or failure, as well as liability related to the loss or misuse of private patient data. We may also be subject to claims for property damages or economic loss related to or resulting from any errors or defects in our products, or installation, servicing and support of our products. With any accident or mistreatment, we could be subject to legal costs, adverse publicity and damage to our reputation, whether or not our products or services were a factor. Adverse publicity could adversely impact our business by negatively affecting the reputation of radiation therapy in general, causing patients to question the efficacy of radiation therapy as a viable treatment for cancer and seek other methods of treatment.
36
Table of Contents
In addition, if a product we designed or manufactured were defective (whether due to design, labeling or manufacturing defects, improper use of the product or other reasons), we may be required to recall the product and notify regulatory authorities. The adverse publicity resulting from a recall could damage our reputation and cause customers to review and potentially terminate their relationships with us. A product recall could consume management time and have an adverse financial impact on our business, including incurring substantial costs, lost revenues and loss accruals under GAAP that may cause our quarterly results to fluctuate.
We maintain limited product liability insurance coverage and currently self-insure professional liability/errors and omissions liability. The product liability insurance policies that we maintain are expensive and have high deductible amounts and self-insured retentions. The insurance coverage we have obtained may prove to be inadequate, and future policies may not be available on acceptable terms or in sufficient amounts, if at all. A material claim successfully brought against us relating to a self-insured liability or a liability that is in excess of our insurance coverage, or for which insurance coverage is denied or limited would require us to pay damage amounts that could be substantial and have a material adverse effect on our financial position and results of operation.
WE COMPETE IN HIGHLY COMPETITIVE MARKETS, AND WE MAY LOSE MARKET SHARE TO COMPANIES WITH GREATER RESOURCES OR THE ABILITY TO DEVELOP MORE EFFECTIVE TECHNOLOGIES, OR WE COULD BE FORCED TO REDUCE OUR PRICES
Rapidly evolving technology, intense competition and pricing pressure characterize the markets for radiation therapy equipment and software. Some of our competitors have greater financial, marketing and other resources than we have. Also, we believe that new competitors will enter our markets, as we have encountered new competitors as we enter new markets such as stereotactic radiosurgery, VMAT and proton therapy. To compete successfully, we must provide technically superior, clinically proven products that deliver more precise, cost-effective, high quality clinical outcomes, together in a complete package of products and services, and to do so ahead of our competitors. As our Oncology Systems products are generally sold on a basis of total value to the customer, our business may suffer when purchase decisions are based solely upon price, which can if hospitals and clinics give purchasing decision authority to group purchasing organizations. In addition, additional competitors may delay customer purchasing decisions as customers evaluate the products of these competitors along with ours, potentially extending our sales cycle and adversely affect our net orders. In the radiotherapy and radiosurgery markets, we compete primarily with Elekta AB, Siemens Medical Solutions, Tomotherapy Incorporated and Accuray Incorporated. With our information and image management, simulation, treatment planning and radiosurgery products, we also compete with a variety of companies, such as Elekta, Philips Medical Systems, Best Theratronics, Ltd., Nucletron B.V. and Siemens Medical Solutions. We also encounter some competition from providers of hospital information systems. In our brachytherapy operations, our primary competitor is Nucletron B.V. For the service and maintenance business for our Oncology Systems products, we compete with independent service organizations and our customers’ internal service organizations.
In x-ray imaging components and subsystems, we often compete with companies that have greater financial, marketing and other resources than we have. Some of the major diagnostic imaging systems companies, which are the primary OEM customers for our x-ray components, also manufacture x-ray components for use in their own imaging systems products. We must compete with these in-house manufacturing operations for business from their affiliated companies. As a result, we must have a competitive advantage in one or more significant areas, which may include lower product cost, better product quality and/or superior technology and/or performance. We sell a significant volume of our x-ray tubes to OEMs such as Toshiba Corporation, Hitachi Medical Corporation, Philips Medical Systems and GE Healthcare, all of which have in-house x-ray tube production capability. In addition, we compete against other stand-alone, independent x-ray tube manufacturers such as Comet AG and IAE Industria
37
Table of Contents
Applicazioni Elettroniche Spa. These companies compete with us for both the OEM business of major diagnostic imaging equipment manufacturers and the independent servicing business for x-ray tubes. The market for flat panel detectors is also very competitive and we primarily compete against Perkin-Elmer, Inc., Trixell S.A.S., and Canon, Inc. in our flat panel detector product line.
In our SIP business, we compete with other OEM suppliers, primarily outside of the United States, and our major competitor in this market is Nuctech Company Limited. The market for our SIP products used for nondestructive testing in industrial applications is small and highly fractured, and there is no single major competitor.
The market for proton therapy products is still developing and is characterized by rapidly evolving technology, high competition and pricing pressure. Our ability to compete successfully depends, in part, on our ability to complete the development of our commercial proton therapy system, lower our product costs, develop and provide technically superior, clinically proven products that deliver more precise, cost-effective, high quality clinical outcomes, including integration of IGRT technologies such as OBI. In the proton therapy market, we compete principally with Ion Beam Applications S.A., Hitachi Medical Corporation, and Still River Systems, Inc.
In each of our business segments, existing competitors’ actions and new entrants may adversely affect our ability to compete. These competitors could develop technologies and products that are more effective than those we currently use or produce or that could render our products obsolete or noncompetitive. In addition, the timing of our competitors’ introduction of products into the market could affect the market acceptance and market share of our products. Some competitors offer specialized products that provide, or may be perceived by customers to provide, a marketing advantage over our mainstream cancer treatment products. Also, some of our competitors may not be subject to the same standards, regulatory and/or other legal requirements that we are, and therefore, they could have a competitive advantage in developing, manufacturing and marketing products and services. Any inability to develop, gain regulatory approval for and supply commercial quantities of competitive products to the market as quickly and effectively as our competitors could limit market acceptance of our products and reduce our sales. In addition, some of our smaller competitors could be acquired by larger companies that have greater financial strength, which could enable them to compete more aggressively. Our competitors could also acquire some of our suppliers or distributors, which could disrupt these supply or distribution arrangements and result in less predictable and reduced revenues in our businesses. Any of these competitive factors could negatively affect our pricing, sales, revenues, market share and gross margins and our ability to maintain or increase our operating margins.
OPEN ARCHITECTURE IS BECOMING INCREASINGLY IMPORTANT, AND SALES OF OUR PRODUCTS COULD FALL IF WE FAIL TO ACHIEVE THIS
As radiation therapy becomes more and more complex, interoperability and compatibility of the various products used in treating patients becomes more important. Our linear accelerators, treatment simulators, treatment verification products, treatment planning and information management software products are designed to interoperate with one another, and to be compatible with other widely used third-party radiation oncology products. Obtaining and maintaining this interoperability and compatibility is costly and time-consuming. When third parties modify the design or functionality of their products, we may need to modify our products to ensure compatibility. Conversely, when we implement design improvements to our products, customers may be reluctant to adopt our new technology due to interoperability issues. For example, a clinic may be unwilling to implement one of our new technologies because its third-party software network provider does not yet have available a proper software interface. Our ability to obtain compatibility with third-party products can depend on the third parties’ providing us with adequate information regarding their products. In many cases, these third parties are our competitors and may time their product changes, and their sharing of relevant information with us, to place us at a competitive disadvantage. Further, we could be required to obtain additional regulatory
38
Table of Contents
clearances for any modification of our products due to interoperability issues with the products of third parties. It is also possible that, despite our best efforts, we may not be able to make our products interoperable or compatible with widely used third-party products or may only be able to do so at a prohibitive expense, making our products less attractive or more costly to our customers.
PROTECTING OUR INTELLECTUAL PROPERTY CAN BE COSTLY, AND OUR COMPETITIVE POSITION WOULD BE HARMED IF WE ARE NOT ABLE TO DO SO
We file applications as appropriate for patents covering new products and manufacturing processes. We cannot be sure, however, that our current patents, the claims allowed under our current patents, or patents for technologies licensed to us will be sufficiently broad to protect our technology position against competitors. Issued patents owned by, or licensed to, us may be challenged, invalidated or circumvented, or the rights granted under the patents may not provide us with competitive advantages. We also cannot be sure that patents will be issued from any of our pending or future patent applications. Asserting our patent rights against others in litigation or other legal proceedings is costly and diverts managerial resources. An unfavorable outcome in any such litigation or proceeding could harm us. In addition, we may not be able to detect patent infringement by others or may lose our competitive position in the market before we are able to do so.
We also rely on a combination of copyright, trade secret and other laws, and contractual restrictions on disclosure, copying and transferring title (including confidentiality agreements with vendors, strategic partners, co-developers, employees, consultants and other third parties), to protect our proprietary rights. These protections may prove inadequate, since agreements may still be breached and we may not have adequate remedies for a breach, and our trade secrets may otherwise become known to or be independently developed by others. We have trademarks, both registered and unregistered, that are maintained and enforced to provide customer recognition for our products in the marketplace, but unauthorized third parties may still use them. We also have agreements with third parties that license to us certain patented or proprietary technologies. If we were to lose the rights to license these technologies, or our costs to license these technologies were to materially increase, our business would suffer.
THIRD PARTIES MAY CLAIM WE ARE INFRINGING THEIR INTELLECTUAL PROPERTY, AND WE COULD SUFFER SIGNIFICANT LITIGATION OR LICENSING EXPENSES OR BE PREVENTED FROM SELLING OUR PRODUCTS
The industries in which we compete are characterized by a substantial amount of litigation over patent and other intellectual property rights. Our competitors, like companies in many high technology businesses, continually review other companies’ products for possible conflicts with their own intellectual property rights. Determining whether a product infringes a third party’s intellectual property rights involves complex legal and factual issues, and the outcome of this type of litigation is often uncertain. Third parties may claim that we are infringing their intellectual property rights, and we may be found to infringe those intellectual property rights. We may not be aware of intellectual property rights of others that relate to our products, services or technologies. From time to time, we have received notices from third parties asserting infringement and we have been subject to lawsuits alleging infringement of third-party patent or other intellectual property rights. Any dispute regarding patents or other intellectual property could be costly and time-consuming, and could divert our management and key personnel from our business operations, and we may not prevail in a dispute. We do not maintain insurance for intellectual property infringement, so if we are unsuccessful in defending an infringement claim, we may be subject to significant damages or injunctions against development and sale of our products, or may be required to enter into costly royalty or license agreements. Required licenses may not be made available to us on acceptable terms or at all.
39
Table of Contents
THE LOSS OF A SUPPLIER OR ANY INABILITY TO OBTAIN SUPPLIES OF IMPORTANT COMPONENTS COULD RESTRICT OUR ABILITY TO MANUFACTURE PRODUCTS, CAUSE DELAYS IN OUR ABILITY TO DELIVER PRODUCTS, OR SIGNIFICANTLY INCREASE OUR COSTS
We obtain some of the components included in our products from a limited group of suppliers or from a single-source supplier, such as the radioactive sources for high-dose afterloaders, klystrons for linear accelerators, array sensors for use in our imaging panels, cesium iodide coatings for the arrays, and specialized integrated circuits, x-ray tube targets, housings, glassframes and various other x-ray tube components. If we lose any of these suppliers or if their operations were substantially interrupted, we would be required to obtain and qualify one or more replacement suppliers, which may then also require us to redesign or modify our products to incorporate new parts and/or further require us to obtain clearance, qualification or certification of such product by the FDA or other applicable regulatory approvals in other countries. Events like these could significantly increase costs for the affected product and likely cause material delays in delivery of that and other related products. We have limited insurance to protect against business interruption loss, although our coverage may not be adequate or continue to remain available on acceptable terms, if at all. Additionally, some of these suppliers, including our single-source suppliers, supply components for certain of our rapidly growing product lines. Manufacturing capacity limitations of any of these suppliers or other inability of these suppliers to meet increasing demand could adversely affect us, resulting in curtailed growth opportunities for any of our product lines. Shortage of, and greater demand for, components and subassemblies could also increase manufacturing costs by increasing prices. Disruptions or loss of any of our limited- or sole-source components or subassemblies or the capacity limitations of the suppliers for these components or subassemblies, including the ones referenced above, could adversely affect our business and financial results and could damage our customer relationships.
A SHORTAGE OF RAW MATERIALS COULD RESTRICT OUR ABILITY TO MANUFACTURE PRODUCTS, CAUSE DELAYS, OR SIGNIFICANTLY INCREASE OUR COST OF GOODS
We rely upon the supplies of certain raw materials such as tungsten, lead and copper for Oncology Systems and SIP; copper, lead, tungsten, rhenium, molybdenum zirconium, and various high grades of steel alloy for X-ray Products, and high-grade steel and high-grade copper for the Varian Particle Therapy business. Demand for these raw materials both within the United States and from foreign countries, such as China, has increased over the last few years, resulting in limited supplies and higher prices. Worldwide demand, availability and pricing of these raw materials have been volatile, and we expect that availability and pricing will continue to fluctuate in the future. If supplies are restricted and prices increase, this could constrain our manufacturing of affected products, reduce our profit margins or otherwise adversely affect our business.
CONSOLIDATION AMONG OUR ONCOLOGY SYSTEMS CUSTOMERS COULD ADVERSELY AFFECT OUR SALES OF ONCOLOGY PRODUCTS
We have seen and may continue to see some consolidation among our customers in our Oncology Systems business, as hospitals and clinics combine through mergers and acquisitions, and as they join group purchasing organizations or affiliated enterprises. As customers consolidate, the volume of product sales to these customers might decrease. Alternatively, order size may increase as what were previously more than one customer combine orders as one entity. As a result, the purchasing cycle for our Oncology Systems products could lengthen, as orders increase in size and require more approvals. Both increased order size and extended purchasing cycles could cause our net orders for these products to be more volatile and less predictable. In addition, group purchasing organizations often focus on pricing as the determinant in making purchase decisions. A reduction in net orders could affect the level
40
Table of Contents
of future revenues, which would adversely affect our operating results, financial condition, and the price of VMS common stock.
WE SELL OUR X-RAY TUBES TO A LIMITED NUMBER OF OEM CUSTOMERS, MANY OF WHICH ARE ALSO OUR COMPETITORS, AND A REDUCTION IN BUSINESS BY ONE OR MORE OF THESE CUSTOMERS OR CONSOLIDATION OF CUSTOMERS COULD REDUCE OUR SALES
There has been a consolidation of diagnostic imaging systems manufacturers over the past few years, including the consolidation of these customers into companies that already manufacture x-ray tubes. If this continues, we could experience less predictable and reduced sales of our x-ray tube products. In addition, the general worldwide economic downturn we have seen since 2008 has made and may continue to make it difficult for our OEM customers to accurately forecast and plan future business activities, and we saw our x-ray business impacted in fiscal year 2009 by inventory reduction efforts at some of these customers. The current market for new X-ray imaging equipment appears to be weak, and certain product lines, such as dental and veterinary, have been hit particularly hard in the recession. If the markets for our customers’ products significantly deteriorate due to the general economic downturn, our X-ray Products business may be adversely affected. In recent years, we have also seen dramatic reductions in Medicare reimbursements for diagnostic radiology. We believe reductions in these Medicare reimbursement rates have reduced demand for medical x-ray imaging equipment, such as CT scanners, which have negatively impacted demand for our x-ray tube products. Also, because we sell our x-ray products to a limited number of OEM customers and many of them are also our competitors with in-house x-ray tube manufacturing operations, we could experience the loss of, or reduction in purchasing volume by, one or more of these customers as they lower external sourcing costs in this economic downturn. Such a loss or reduction could have a material adverse effect on our X-ray Products business.
ORDERS FOR OUR SECURITY AND INSPECTION PRODUCTS COULD BE UNPREDICTABLE
Our SIP business designs, manufactures, sells and services Linatron x-ray accelerators, imaging processing software and image detection products for security and inspection, such as cargo screening at ports and borders and nondestructive examination for a variety of applications. We generally sell SIP products to OEMs who incorporate our products into their inspection systems, which are then sold to customs and other government agencies, as well as to commercial organizations in the casting, power, aerospace, chemical, petro-chemical and automotive industries. We believe growth in the SIP business will be driven by security cargo screening and border protection needs, as well as by the needs of customs agencies to verify shipments for assessing duties and taxes. However, use of linear accelerator and imaging technology in security cargo screening and border protection is in its early stages. Orders for our SIP products have been and may continue to be unpredictable and the actual timing of sales and revenue recognition will vary significantly, as it is difficult to predict our OEM customer delivery and acceptance schedules.
In addition, our SIP business is heavily influenced by U.S. and foreign governmental policies on national and homeland security, border protection and customs revenue activities, which depend upon government budgets and appropriations that are subject to political changes. We have begun to see customers freeze or dramatically reduce purchases and capital project expenditures, or act cautiously as governments around the world wrestle with spending priorities. As a result, this business is subject to unpredictability in the timing of orders, sales and revenue that could cause volatility in our revenues and earnings, and therefore the price of VMS common stock.
41
Table of Contents
IF WE ARE UNABLE TO PROVIDE THE SIGNIFICANT EDUCATION AND TRAINING REQUIRED FOR THE HEALTHCARE MARKET TO ACCEPT OUR PRODUCTS, OUR BUSINESS WILL SUFFER
In order to achieve market acceptance for our radiation therapy products, we often need to educate physicians about the use of a new treatment procedure such as IMRT, IGRT, VMAT, stereotactic radiosurgery or proton therapy, overcome physician objections to some of the effects of the product or its related treatment regimen, convince healthcare payors that the benefits of the product and its related treatment process outweigh its costs and help train qualified physicists in the skilled use of our products. For example, the complexity and dynamic nature of IMRT and IGRT requires significant education of hospital personnel and physicians regarding the benefits of IMRT and IGRT and the required departures from their customary practices. Further, the complexity and high cost of proton therapy requires similar significant education, as well as education regarding construction and facility requirements. We have expended and will continue to expend significant resources on marketing and educational efforts to create awareness of IMRT, IGRT, VMAT, stereotactic radiosurgery and proton therapy generally and to encourage the acceptance and adoption of our products for these technologies. We cannot be sure that any products we develop will gain significant market acceptance among physicians, patients and healthcare payors, even if we spend significant time and expense on their education.
OUR BUSINESS MAY SUFFER IF WE ARE NOT ABLE TO HIRE AND RETAIN QUALIFIED PERSONNEL
Our future success depends, to a significant extent, on our ability to attract, expand, integrate, train and retain our management team, qualified engineering personnel, technical personnel and sales and marketing staff. We compete for key personnel with other medical equipment and software manufacturers and technology companies, as well as universities and research institutions. Because this competition is intense, costs related to compensation could increase significantly if supply decreases or demand increases. If we are unable to hire, train or retain qualified personnel, we will not be able to maintain and expand our business, and our business would suffer.
IF WE ARE NOT ABLE TO MATCH OUR MANUFACTURING CAPACITY WITH DEMAND FOR OUR PRODUCTS, OUR FINANCIAL RESULTS MAY SUFFER
Our products have a long production cycle and we need to anticipate demand for our products in order to ensure adequate manufacturing or testing capacity. If we are unable to anticipate demand and our manufacturing or testing capacity does not keep pace with product demand, we will not be able to fulfill orders timely, which may negatively impact our financial results and overall business. Conversely, if demand for our products decreases, the fixed costs associated with excess manufacturing capacity may harm our financial results.
IF WE FAIL TO SUCCESSFULLY ACQUIRE OR INTEGRATE NEW BUSINESSES, PRODUCTS AND TECHNOLOGY, WE MAY NOT REALIZE EXPECTED BENEFITS OR MAY HARM OUR BUSINESS
We need to grow our businesses in response to changing technologies, customer demands and competitive pressures. In some circumstances, we may decide to grow our business through the acquisition of complementary businesses, products or technologies rather than through internal development. For example, in fiscal year 2008 we acquired Pan-Pacific, an independent distributor of medical x-ray tubes and other imaging components in China, and in fiscal year 2009 acquired certain assets of IKOE, a supplier of software used in the planning of radiotherapy and radiosurgery treatments. Identifying suitable acquisition candidates can be difficult, time-consuming and costly, and we may not be able to identify suitable candidates or successfully complete identified acquisitions. In addition,
42
Table of Contents
completing an acquisition can divert our management and key personnel from our business operations, which could harm our business and affect our financial results. Even if we complete an acquisition, we may not be able to successfully integrate newly acquired organizations, products, technologies or employees into our operations, or may not fully realize some of the expected synergies.
Integrating an acquisition can also be expensive and time-consuming, and may strain our resources. It may cost us more to commercialize new products than we originally anticipated, as we are experiencing with our proton therapy systems. These additional expenditures could be significant and could cause our results of operations to suffer. In many instances, integrating a new business will also involve implementing or improving internal controls appropriate for a public company at a business that lacks them. In addition, we may be unable to retain the employees of acquired companies, or the acquired company’s customers, suppliers, distributors or other partners for a variety of reasons, including the fact that these entities may be our competitors or may have close relationships with our competitors.
Further, we may find that we need to restructure or divest acquired businesses, or assets of those businesses. Even with restructuring activities or divestitures, an acquisition may not produce the full efficiencies and benefits we expect. Consequently, we may not achieve anticipated growth or other benefits from an acquisition, which could harm our existing business. If we decide to sell assets or a business, as we recently did with Research Instruments, it may be difficult to identify buyers or alternative exit strategies on acceptable terms, in a timely manner, or at all, which could delay the accomplishment of our strategic objectives, or we may dispose of a business at a price or on terms that are less than we had anticipated.
We account for our acquisitions under the purchase method of accounting. Under this method, we allocate the total purchase price to the acquired businesses’ tangible assets and liabilities, identifiable intangible assets and in-process research and development costs based on their fair values as of the date of the acquisition, and record the excess of the purchase price over those fair values as goodwill. If we fail to achieve the anticipated growth from an acquisition, or if we decide to sell assets or a business, we may be required to recognize an impairment loss on the write down of our assets and goodwill, which could adversely affect our business and financial operations. In addition, acquisitions can result in potentially dilutive issuances of equity securities or the incurrence of debt, contingent liabilities or expenses, or other charges, any of which could harm our business and affect our financial results.
WE MAY FACE ADDITIONAL RISKS FROM THE ACQUISITION OR DEVELOPMENT OF NEW LINES OF BUSINESS
From time to time, we may acquire or develop new lines of business, such as proton therapy. There are substantial risks and uncertainties associated with this, particularly in instances where the markets are not fully developed. Risks include developing knowledge of and experience in the new business, recruiting market professionals, increasing research and development expenditures, and developing and capitalizing on new relationships with experienced market participants. This may mean significant investment and involvement of our senior management to acquire or develop, then integrate, the business into our operations. Timelines for integration of new businesses may not be achieved and price and profitability targets may not prove feasible, as new products can carry lower gross margins. External factors, such as compliance with regulations, competitive alternatives, and shifting market preferences, may also impact whether implementation of a new business will be successful. Failure to manage these risks in the development and implementation of new businesses successfully could materially and adversely affect our business, results of operations and financial condition.
WE MAY NOT BE ABLE TO SUCCESSFULLY RESOLVE RESIDUAL ISSUES RELATED TO THE SALE OF OUR RESEARCH INSTRUMENTS BUSINESS
In the second quarter of fiscal year 2009, we completed the sale the Research Instruments business, but retained responsibility for certain contracts. We may incur additional costs beyond those expected with
43
Table of Contents
these remaining obligations which could adversely affect our financial condition. Continued efforts related to managing these remaining obligations may require a substantial amount of management, administrative and operational resources, particularly if unanticipated difficulties with the fulfillment of these contracts are encountered. These demands may distract our employees and management from the day-to-day operation of our other businesses.
WE WORK WITH DISTRIBUTORS FOR SALES IN SOME TERRITORIES, AND LOSING THEM COULD HARM OUR REVENUES IN THAT TERRITORY
We have strategic relationships with a number of key distributors for sales and service of our products, principally in Europe and Asia. If these strategic relationships end and are not replaced, our revenues and/or ability to service our products in the territories serviced by these distributors could be adversely affected.
FLUCTUATIONS IN OUR OPERATING RESULTS, INCLUDING QUARTERLY NET ORDERS, REVENUES, AND GROSS MARGINS, MAY CAUSE OUR STOCK PRICE TO BE VOLATILE, WHICH COULD CAUSE LOSSES FOR OUR STOCKHOLDERS
We have experienced and expect in the future to experience fluctuations in our operating results, including net orders, revenues and gross margins. Many of our products require significant capital expenditures by our customers. Accordingly, individual product orders can be quite large in dollar amounts, which can extend the customer purchasing cycle. We have experienced this with our IGRT products, and expect this to be even greater with our proton therapy products because of the high cost of the equipment and the complexity of project financing. With the current general worldwide economic downturn and contraction in credit markets, as well as the uncertainty surrounding healthcare reform and changes to reimbursement rates, the purchasing cycle has extended and may extend even further as potential customers more closely scrutinize and prioritize their capital spending budgets, and analyze appropriate financing alternatives. With larger projects, such as the purchase of a proton therapy system, the contraction in credit markets could cause customers to delay or cancel their projects, or request our participation in financing arrangements or payment concessions in their agreements with us, which could negatively impact our cash flows and results of operations. In addition, some of our more sophisticated equipment, such as IGRT and proton therapy products, requires greater site preparation and longer construction cycles, which can delay installation. For proton therapy products, this can delay the customer decision cycles even further. The timing of when individual orders are placed, installation is accomplished and the revenues recognized could have an effect our quarterly results.
Once orders are received, factors that may affect whether these orders become revenues and the timing include:
| · | delay in shipment due, for example, to longer construction projects or unanticipated construction delays at customer locations where our products are to be installed, cancellations or rescheduling by customers, extreme weather conditions, natural disasters, port strikes or manufacturing difficulties; |
| · | delay in the installation and/or acceptance of a product; |
| · | for proton therapy systems, failure to satisfy contingencies associated with an order; |
| · | the method of accounting used to recognize revenue; |
| · | a change in a customer’s financial condition or ability to obtain financing; or |
| · | appropriate regulatory approvals or authorizations. |
Our quarterly operating results may also be affected by a number of other factors, including:
| · | changes in our or our competitors’ pricing or discount levels; |
44
Table of Contents
| · | changes or anticipated changes in third-party reimbursement amounts or policies applicable to treatments using our products; |
| · | revenues becoming affected by seasonal influences; |
| · | timing of revenue recognition; |
| · | changes in foreign currency exchange rates; |
| · | changes in the relative portion of our revenues represented by our various products, including the relative mix between higher margin and lower margin products; |
| · | changes in the relative portion of our revenues represented by the international regions; |
| · | timing of the announcement, introduction and delivery of new products or product enhancements by us and by our competitors; |
| · | fluctuation in our effective tax rate resulting from various factors, which may or may not be known to us in advance; |
| · | disruptions in the supply or changes in the costs of raw materials, labor, product components or transportation services; |
| · | disruptions in our operations, including our ability to manufacture products, caused by events such as earthquakes, fires, floods, terrorist attacks or the outbreak of epidemic diseases; |
| · | changes in the general economic conditions or tightening of credit available to our customers in the regions in which we do business; |
| · | unexpected levels of cancellations; |
| · | the impact of changing levels of sales on sole purchasers of certain of our x-ray products; |
| · | the unfavorable outcome of any litigation or administrative proceeding or inquiry; |
| · | misleading information in the financial community; and |
| · | accounting changes, such as those relating to accounting reserves for product recalls, reserves for excess and obsolete inventories, share-based compensation expense, accounting for income taxes, and adoption of new accounting pronouncements. |
Because many of our operating expenses are based on anticipated capacity levels and a high percentage of these expenses are fixed for the short term, a small variation in the timing of revenue recognition can cause significant variations in operating results from quarter to quarter. Our overall gross margin may also be impacted by the gross margin of our proton therapy products, which are presently below the gross margins for our traditional radiotherapy products. If our gross margins fall below the expectation of securities analysts and investors, the trading price of VMS common stock would almost certainly decline.
We report on a quarterly and annual basis our net orders and backlog. It is important to understand that, unlike revenues, net orders and backlog are not governed by the rules of GAAP, and are not within the scope of the audit or reviews conducted by our independent registered public accounting firm; therefore, investors should not interpret our net orders or backlog in such a manner. Also, for the reasons set forth above, our net orders and backlog cannot necessarily be relied upon as accurate predictors of future revenues. Unexpected levels of cancellation of orders or delays in customer purchase decisions or delivery dates will reduce the quarterly net orders and backlog and also affect the level of future revenues. Accordingly, we cannot be sure if or when orders will mature into revenues. Our net orders, backlog, revenues and net earnings in one or more future periods may fall below the expectations of securities analysts and investors. In that event, the trading price of VMS common stock would almost certainly decline.
45
Table of Contents
THE FINANCIAL RESULTS OF OUR VARIAN PARTICLE THERAPY BUSINESS MAY FLUCTUATE AND BE UNPREDICTABLE
Our proton therapy projects are highly customized and vary in size and complexity. Planning for these projects will take more time and use more resources than those in the radiotherapy business conducted in our Oncology Systems segment. Due to its relatively large scale, the construction of a proton therapy facility requires significant capital investment and may involve complex project financing. If we are required to establish special purpose entities to finance and manage a proton therapy project, we may be required to consolidate these special purpose entities in our financial statements, or guarantee performance and assume liabilities that are in excess of the project value, which could negatively impact our financial results. Further, the current worldwide economic downturn and contraction in credit markets may make it more difficult for potential customers of this business to find appropriate financing for large proton therapy projects, which could cause them to delay or cancel their projects, or request participation in financing arrangements or payment concessions in their agreements with us. In addition, due to their size and complexity, the sales and customer decision cycles for proton therapy projects may take several years. As a result, the timing of these projects, and therefore our operating results for this business, may vary significantly from period to period.
In addition, many of the components used in proton therapy equipment require a long lead time, which may require an increase in our levels of inventory. This may cause fluctuations in the operating results of our Varian Particle Therapy business that may make it difficult to predict our operating results and to compare our financial results from period to period.
Moreover, entrance into the proton therapy business may subject us to increased risk and potential liability. For example, because proton therapy projects are large in scale and require detailed project planning, failure to deliver on our commitments could result in greater than expected liabilities, as we could be required to indemnify business partners and customers for losses suffered or incurred if we are unable to deliver our products in accordance with the terms of customer contracts. The greater size of proton projects means that the potential liability could similarly be greater. Additionally, customers are requesting that the systems vendor, as the primary technology provider, provide guarantees for and suffer penalties in relation to the overall construction project. Since the cost of each proton therapy center project may exceed $100 million, the amount of potential liability may be higher than the levels historically assumed by us for our traditional radiation therapy business. Insurance covering these contingencies may be unobtainable. If we cannot reasonably mitigate or eliminate these contingencies, our ability to competitively bid upon proton center projects will be negatively impacted and we may be required to assume material amounts of potential liability, all of which may have adverse consequences to our Varian Particle Therapy business. In addition, we have encountered and may encounter additional challenges in the commercialization of the proton therapy products, which may increase our research and development costs and delay the introduction of our products. This and other unanticipated events could adversely affect our business and make our results of operations unpredictable.
LOSS OF OUR FIRST PROTON THERAPY SYSTEM ORDER, OR FUTURE PROTON THERAPY SYSTEM CUSTOMERS, WOULD ADVERSELY AFFECT REVENUES AND EARNINGS FOR OUR PARTICLE THERAPY BUSINESS
In the fourth quarter of fiscal year 2009, Skandion Kliniken awarded us, in a competitive bidding situation, an approximately $62 million contract to deliver and install a proton therapy system in Sweden and we have included this contract in net orders. However, one of the unsuccessful bidders has filed a formal appeal of the award. Skandion Kliniken is actively opposing this appeal and we believe it will be resolved in Skandion Kliniken’s favor. Additionally, the order is contingent on Skandion Kliniken’s execution of a contract for the construction of the proton therapy facility. If the appeal is not resolved in Skandion Kliniken’s favor, or if they do not execute a construction contract, we would have to reverse the order and remove it from our backlog, which would have an adverse effect on our future revenues, earnings and other financial results.
46
Table of Contents
We expect that a limited number of customers will account for a substantial portion of our Particle Therapy business for the foreseeable future. If a customer cancels an order for a proton therapy system, we would lose product and services revenues, which would adversely affect our financial results.
WE HAVE ENTERED INTO A CREDIT FACILITY AGREEMENT THAT RESTRICTS CERTAIN ACTIVITIES, AND FAILURE TO COMPLY WITH THIS AGREEMENT MAY HAVE AN ADVERSE EFFECT ON OUR BUSINESS, LIQUIDITY AND FINANCIAL POSITION
We maintain a revolving credit facility that contains restrictive financial covenants, including financial covenants that require us to comply with specified financial ratios. We may have to curtail some of our operations to comply with these covenants. In addition, our revolving credit facility contains other affirmative and negative covenants that could restrict our operating and financing activities. These provisions limit our ability to, among other things, incur future indebtedness, contingent obligations or liens, guarantee indebtedness, make certain investments and capital expenditures, sell stock or assets and pay dividends, and consummate certain mergers or acquisitions. Because of the restrictions on our ability to create or assume liens, we may find it difficult to secure additional indebtedness if required. Furthermore, if we fail to comply with the credit facility requirements, we may be in default, and we may not be able to obtain the necessary amendments to the credit agreement or waivers of an event of default. Upon an event of default if the credit agreement is not amended or the event of default is not waived, the lender could declare all amounts outstanding, together with accrued interest, to be immediately due and payable. If this happens, we may not be able to make those payments or borrow sufficient funds from alternative sources to make those payments. Even if we were to obtain additional financing, that financing may be on unfavorable terms.
CHANGES IN INTERPRETATION OR APPLICATION OF GENERALLY ACCEPTED ACCOUNTING PRINCIPLES MAY ADVERSELY AFFECT OUR OPERATING RESULTS
We prepare our financial statements to conform with GAAP. These principles are subject to interpretation by the Financial Accounting Standards Board, American Institute of Certified Public Accountants, the Public Company Accounting Oversight Board, the Securities and Exchange Commission and various other regulatory or accounting bodies. A change in interpretations of, or our application of, these principles can have a significant effect on our reported results and may even affect our reporting of transactions completed before a change is announced. Additionally, as we are required to adopt new accounting standards, our methods of accounting for certain items may change, which could cause our results of operations to fluctuate from period to period. For example, as a result of our adoption of the provisions in Accounting Standards Codification (“ASC”) 740 related to accounting for uncertainty in income taxes, our effective tax rate and other related financial metrics have fluctuated and may in the future fluctuate more than they have in prior periods.
As our operations evolve over time, we may introduce new products or new technologies that require us to apply different accounting principles, including that regarding revenue recognition, than we have applied in past periods. Additionally, we recognize revenues for some of our proton therapy products and services and for certain highly customized image detection systems in our SIP business under the percentage-of-completion method or the completed-contract method, which affects the timing of revenue recognition. We could be required to apply these methods to other businesses in the future. The percentage-of-completion method involves considerable use of estimates in determining revenues, costs and profits and in assigning dollar amounts to relevant accounting periods which must be periodically reviewed and appropriately adjusted. If our estimates prove to be inaccurate or circumstances change over time, we would be required to adjust revenues or even record a contract loss in later periods, and our financial results could suffer. In addition, if a loss is expected on a contract under the percentage-of-completion method and completed contract method, the estimated loss would be charged to cost of sales in the period the loss is identified. The application of different types of accounting principles
47
Table of Contents
and related potential changes may make it more difficult to compare our financial results from quarter to quarter, and the trading price of VMS common stock could suffer or become more volatile as a result.
ENVIRONMENTAL LAWS IMPOSE COMPLIANCE COSTS ON OUR BUSINESS AND CAN ALSO RESULT IN LIABILITY
We are subject to environmental laws around the world. These laws regulate many aspects of our operations, including our handling, storage, transport and disposal of hazardous materials. They can also impose cleanup liabilities, including with respect to discontinued operations. Although we follow procedures intended to comply with existing environmental laws, we, like other businesses, can never completely eliminate the risk of contamination or injury from certain materials that we use in our business and, therefore, the prospect of resulting claims and damage payments. We may also be assessed fines or penalties for failure to comply with environmental laws and regulations. As a consequence of these various elements, we can incur significant environmental costs and liabilities, some recurring and reasonably predictable, and others not recurring or easily predicted. Although insurance has provided coverage for portions of cleanup costs resulting from historical occurrences, we maintain only limited insurance coverage for costs or claims that might result from any future contamination.
Future changes in environmental laws could also increase our costs of doing business, perhaps significantly. Several countries, including some in the EU, now require medical equipment manufacturers to bear certain disposal costs, of products at the end of a product’s useful life, thus creating increased costs for our operations. The EU has also adopted a directive that may lead to restrictions on the use of certain hazardous substances in some of our products sold there. These directives, along with another that requires material disclosure information to be provided upon request, could create increased costs for our operations. All of these costs, and any future violations or liabilities under environmental laws or regulations, could have a material adverse effect on our business.
AS A STRATEGY TO ASSIST OUR SALES EFFORTS, WE MAY OFFER EXTENDED PAYMENT TERMS, WHICH MAY POTENTIALLY RESULT IN HIGHER DSO AND GREATER PAYMENT DEFAULTS
We offer longer or extended payment terms for qualified customers in some circumstances. As of October 2, 2009, customer contracts with extended payment terms of more than one year amounted to less than 1% of our accounts receivable balance. While we qualify customers to whom we offer longer or extended payment terms, their financial positions may change adversely over the longer time period given for payment. This may result in an increase in payment defaults, which would affect our net earnings. Also, longer or extended payment terms have and may in the future result in an increase in our days sales outstanding.
DISRUPTION OF CRITICAL INFORMATION SYSTEMS COULD HARM OUR BUSINESS AND FINANCIAL CONDITION
Information technology helps us operate efficiently, interface with customers, maintain financial accuracy and efficiency, and accurately produce our financial statements. If we do not allocate and effectively manage the resources necessary to build and sustain the proper technology infrastructure, we could be subject to transaction errors, processing inefficiencies, the loss of customers, business disruptions, or the loss of or damage to intellectual property through security breach. If our data management systems do not effectively collect, store, process and report relevant data for the operation of our business, whether due to equipment malfunction or constraints, software deficiencies, or human error, our ability to effectively plan, forecast and execute our business plan and comply with applicable laws and regulations will be impaired, perhaps materially. Any such impairment could materially and adversely affect our financial condition, results of operations, cash flows and the timeliness with which we report our internal and external operating results.
48
Table of Contents
OUR OPERATIONS ARE VULNERABLE TO INTERRUPTION OR LOSS DUE TO NATURAL OR OTHER DISASTERS, POWER LOSS, STRIKES AND OTHER EVENTS BEYOND OUR CONTROL
We conduct a significant portion of our activities, including manufacturing, administration and data processing at facilities located in the State of California and other seismically active areas that have experienced major earthquakes in the past, as well as other disasters. We carry limited earthquake insurance that may not be adequate or continue to be available at commercially reasonable rates and terms. A major earthquake or other disaster affecting our facilities (such as a major fire, flood or terrorist attack), or those of our suppliers, could significantly disrupt our operations, and delay or prevent product manufacture and shipment during the time required to repair, rebuild or replace our or our suppliers’ damaged manufacturing facilities; these delays could be lengthy and costly. If any of our customers’ facilities are adversely affected by a disaster, shipments of our products could be delayed. In addition, our facilities, particularly those located in the western states of the United States, may be subject to a shortage of available electrical power and other energy supplies. Any shortages may increase our costs for power and energy supplies or could result in blackouts, which could disrupt the operations of our affected facilities and harm our business. Further, our products are typically shipped from a limited number of ports, and any disaster, strike or other event blocking shipment from these ports could delay or prevent shipments and harm our business.
THE EFFECT OF TERRORISM OR AN OUTBREAK OF EPIDEMIC DISEASES MAY NEGATIVELY AFFECT SALES AND HINDER OUR OPERATIONS
Concerns about terrorism, the effects of a terrorist attack or an outbreak of epidemic diseases, such as the swine flu, could have a negative effect on our business operations, those of our suppliers and customers, and the ability to travel, resulting in adverse consequences on our revenues and financial performance.
Item 1B. Unresolved Staff Comments
None.
As of October 2, 2009, we own and lease a total of approximately 1.9 million square feet of floor space for our office, manufacturing, research and development and other services worldwide. Our executive offices and our Oncology Systems management and some of our Oncology Systems manufacturing facilities are located in Palo Alto, California on 30 acres of land under leaseholds which expire in 2056. We own these facilities which contain 465,279 square feet of aggregate floor space. We also own 47,037 square feet of space and 2 acres of land in Crawley, England. In Beijing, China we own 138,618 square feet of space located on 5 acres of land under a leasehold that expires in 2056. Our X-Ray Products business segment is located in Salt Lake City, Utah, where we own 38 acres of land and 340,812 square feet of space. In Las Vegas, Nevada, we own 191,422 square feet of floor space and 12 acres of land where our SIP manufacturing, Oncology Systems customer services and support operations are located. Two Las Vegas buildings and the related land have been pledged as collateral against loans with a balance of $5.8 million as of October 2, 2009. The Ginzton Technology Center located in Mountain View, California is under a land and improvements lease that expires in 2012. The balance of our facilities are leased.
We are occupying substantially most of our currently available productive space to develop, manufacture, service and market our products. We believe that our facilities and equipment are generally well maintained, in good operating condition and adequate for present operations.
The following summarizes the current status of our previously reported legal proceedings.
49
Table of Contents
Under the Amended and Restated Distribution Agreement dated as of January 14, 1999 and other associated agreements that govern the Spin-offs, we retained the liabilities related to the medical systems business and agreed to manage and defend claims related to legal proceedings and environmental matters arising from corporate and discontinued operations. Generally, VI and VSEA is each obligated to indemnify us for one-third of these liabilities (after adjusting for any insurance proceeds we realize or tax benefits we receive), including certain environmental liabilities, and to indemnify us fully for liabilities arising from the operations of the business transferred to it as part of the Spin-offs. For a more detailed discussion of environmental costs and liabilities, see Note 9, “Commitments and Contingencies” to our Consolidated Financial Statements.
From time to time, we are involved in other legal proceedings arising in the ordinary course of our business and, from time-to-time, acquired as part of business acquisitions that we make. See “MD&A – Other Matters.” While we cannot assure you as to the ultimate outcome of any legal proceeding or other loss contingency involving us, management does not believe any pending matter will be resolved in a manner that would have a material adverse effect on our business.
Item 4. Submission of Matters to a Vote of Security Holders
None.
50
Table of Contents
Item 5. Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
VMS common stock is traded on the New York Stock Exchange (“NYSE”) under the symbol “VAR.” The following table sets forth the high and low sales prices for VMS common stock as reported in the consolidated transaction reporting system for the NYSE in fiscal years 2009 and 2008.
| High | Low | |||||
Fiscal Year 2009 | ||||||
First Quarter | $ | 61.10 | $ | 33.12 | ||
Second Quarter | $ | 39.77 | $ | 27.10 | ||
Third Quarter | $ | 38.31 | $ | 29.56 | ||
Fourth Quarter | $ | 44.53 | $ | 31.21 | ||
Fiscal Year 2008 | ||||||
First Quarter | $ | 53.22 | $ | 40.22 | ||
Second Quarter | $ | 54.71 | $ | 41.37 | ||
Third Quarter | $ | 53.29 | $ | 43.64 | ||
Fourth Quarter | $ | 65.84 | $ | 48.58 | ||
Since the Spin-offs and becoming Varian Medical Systems, Inc., we have not paid any cash dividends on VMS common stock. We have no current plan to pay cash dividends on VMS common stock, and will review that decision periodically. Further, our existing unsecured term loan agreement and revolving credit facility agreement contain provisions that limit our ability to pay cash dividends. Specifically, dividends would not be permitted if, when aggregated with other transactions, we would not be in compliance with our financial covenants. See Note 7, “Credit Facility” to the Consolidated Financial Statements for more information on our revolving credit facility.
As of November 19, 2009, there were approximately 3,587 holders of record of VMS common stock.
51
Table of Contents
PERFORMANCE GRAPH
This graph shows the total return on Varian Medical Systems, Inc. common stock and certain indices from October 1, 2004 until the last day of fiscal year 2009.
COMPARISON OF FIVE YEAR CUMULATIVE TOTAL RETURN*
AMONG VARIAN MEDICAL SYSTEMS, INC.,
THE S&P 500 INDEX AND
THE S & P HEALTHCARE EQUIPMENT INDEX
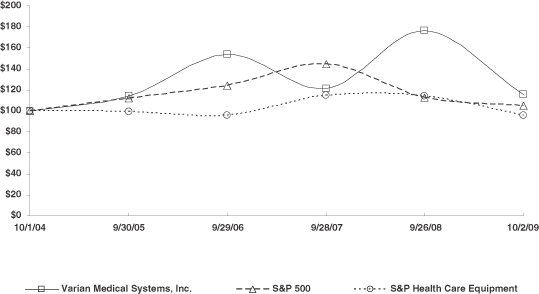
| * | $100 invested on 10/1/04 in stock or 9/30/04 in index, including reinvestment of dividends. Indexes calculated on month-end basis. |
| 10/1/04 | 9/30/05 | 9/29/06 | 9/28/07 | 9/26/08 | 10/2/09 | |||||||
Varian Medical Systems, Inc. | 100.00 | 114.06 | 154.13 | 120.93 | 176.62 | 115.50 | ||||||
S&P 500 | 100.00 | 112.25 | 124.37 | 144.81 | 112.99 | 105.18 | ||||||
S&P Health Care Equipment | 100.00 | 99.07 | 95.52 | 114.76 | 114.03 | 95.45 |
The performance graph and related information shall not be deemed to be soliciting material or to be “filed” with the SEC or to be deemed to be incorporated by reference to any filing under the Securities Act or the Exchange Act.
52
Table of Contents
Stock Repurchase Program
The following table provides information with respect to the shares of VMS common stock repurchased by VMS during the fourth quarter of fiscal year 2009.
Period | Total Number of Shares Purchased | Average Price Paid Per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Maximum Number of Shares that May Yet Be Purchased Under the Plans or Programs | |||||||
July 4, 2009—July 31, 2009 | — | $ | 0.00 | — | 8,000,000 | ||||||
August 1, 2009—August 28, 2009 | 211,687 | (1) | $ | 40.60 | (1) | 200,000 | 7,800,000 | ||||
August 29, 2009—October 2, 2009 | 500,000 | $ | 43.55 | 500,000 | 7,300,000 | ||||||
Total | 711,687 | $ | 42.67 | 700,000 | |||||||
On July 24, 2007, our Board of Directors approved the repurchase of 12,000,000 shares of VMS common stock over a period beginning on July 30, 2007 through December 31, 2008. The authorization expired on December 31, 2008 with 4,342,000 shares available for repurchase. On November 17, 2008, our Board of Directors authorized the repurchase of an additional 8,000,000 shares of VMS common stock from January 1, 2009 through December 31, 2009. As of October 2, 2009, 7,300,000 shares remained available for repurchase under the November 2008 authorization. On November 13, 2009, our Board of Directors authorized the repurchase of an additional 5,000,000 shares of VMS common stock from January 1, 2010 through December 31, 2010. We expect repurchases will be made in accordance with Rule 10b-18 and include plans designed to satisfy the Rule 10b5-1 safe harbor. Shares will be retired upon repurchase.
| (1) | Includes 11,687 shares of VMS common stock that were tendered to VMS in satisfaction of tax withholding obligations upon the vesting of restricted common stock granted under the Company’s employee stock plans. |
Item 6. Selected Financial Data
We derived the following selected financial data from our audited consolidated financial statements for the five fiscal years from October 1, 2004 to October 2, 2009. The following financial data should be read in conjunction with our consolidated financial statements and the accompanying notes and the MD&A included elsewhere herein.
53
Table of Contents
Summary of Operations:
| Fiscal Years | ||||||||||||||||||
| (In millions, except per share amounts) | 2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||||
Revenues | $ | 2,214.1 | $ | 2,069.7 | $ | 1,755.1 | $ | 1,597.8 | $ | 1,382.6 | ||||||||
Earnings from continuing operations before taxes | 474.6 | 426.0 | 346.0 | 318.7 | 308.3 | |||||||||||||
Taxes on earnings(1) | 143.1 | 130.7 | 103.1 | 75.1 | 101.7 | |||||||||||||
Earnings from continuing operations | 331.5 | 295.3 | 242.9 | 243.6 | 206.6 | |||||||||||||
Earnings (Loss) from discontinued operations, net of taxes(2) | (12.5 | ) | (15.8 | ) | (3.4 | ) | 1.5 | — | ||||||||||
Net earnings(1)(3) | $ | 319.0 | $ | 279.5 | $ | 239.5 | $ | 245.1 | $ | 206.6 | ||||||||
Net earnings (loss) per share—Basic(1)(3) | ||||||||||||||||||
Continuing operations | $ | 2.67 | $ | 2.37 | $ | 1.91 | $ | 1.86 | $ | 1.56 | ||||||||
Discontininued operations(2) | (0.10 | ) | (0.13 | ) | (0.03 | ) | 0.01 | — | ||||||||||
Net earnings per share | $ | 2.57 | $ | 2.24 | $ | 1.88 | $ | 1.87 | $ | 1.56 | ||||||||
Net earnings (loss) per share—Diluted(1)(3) | ||||||||||||||||||
Continuing operations | $ | 2.65 | $ | 2.31 | $ | 1.86 | $ | 1.80 | $ | 1.50 | ||||||||
Discontininued operations(2) | (0.10 | ) | (0.12 | ) | (0.03 | ) | 0.01 | — | ||||||||||
Net earnings per share | $ | 2.55 | $ | 2.19 | $ | 1.83 | $ | 1.81 | $ | 1.50 | ||||||||
Financial Position at Fiscal Year End: | |||||||||||||||
Working capital | $ | 830.1 | $ | 612.7 | $ | 378.5 | $ | 512.1 | $ | 473.0 | |||||
Total assets | 2,308.2 | 1,975.5 | 1,684.4 | 1,511.8 | 1,317.4 | ||||||||||
Long-term debt (including current maturities) | 32.4 | 40.4 | 49.4 | 57.3 | 60.0 | ||||||||||
Short-term borrowings | 4.4 | — | 41.0 | — | — | ||||||||||
Stockholders’ equity | 1,311.8 | 1,027.2 | 821.5 | 797.3 | 659.0 |
| (1) | During fiscal year 2006, we repatriated approximately $128 million in foreign earnings pursuant to the American Jobs Creation Act of 2004 and recorded a $12 million net tax benefit. We also recorded a net tax benefit of $7.2 million in fiscal year 2006 related to adjustments of certain prior years’ state and federal temporary differences. |
| (2) | In September 2008, we approved a plan to sell Research Instruments. The sale of Research Instruments was completed in the second quarter of fiscal year 2009. The Company classified the operating results as a discontinued operation in the Consolidated Statement of Earnings for all periods presented. The net loss of $12.5 million, $15.8 million and $3.4 million was reported in discontinued operations for fiscal years 2009, 2008 and 2007, respectively. |
| In fiscal year 1995, Varian Associates, Inc. completed the sale of its Electron Devices business segment. The transaction was accounted for as discontinued operations. In fiscal year 2006, we recognized a pre-tax gain from discontinued operations of $2.5 million and a related tax expense of $1.0 million. The net gain of $1.5 million resulted from the release of a reserve for certain contingencies associated with the Electron Devices business segment. Following release of that reserve, we no longer had any asset or liability related to this discontinued operation. |
| (3) | For fiscal years 2009, 2008, 2007 and 2006, net earnings included share-based compensation expense, net of taxes, of $28.8 million, $27.4 million, $29.7 million and $26.9 million, respectively, under ASC 718. For fiscal year 2005, net earnings included share-based compensation expense related to restricted stock, net of taxes, of $0.7 million, which was recorded in accordance with the prior authoritative guidance. See Note 12, “Employee Stock Plans” to the Consolidated Financial Statements. |
54
Table of Contents
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Overview
In fiscal year 2009, our net earnings per diluted share from continuing operations increased 15% from fiscal year 2008 to $2.65. Total revenues from continuing operations grew 7% and gross margin improved by one percentage point in fiscal year 2009 over fiscal year 2008 results. Including an approximately $62 million proton therapy system order from Skandion Kliniken in the fiscal fourth quarter, our net orders from continuing operations for fiscal year 2009 grew 3% over fiscal year 2008. The Company encountered a challenging business environment in North America and both of our business segments, as well as SIP, experienced declines in net orders in this region in fiscal year 2009. Backlog at the end of fiscal year 2009 increased 9% from the end of the prior fiscal year to $2.1 billion.
In September 2008, we approved a plan to sell Research Instruments in order to focus the business acquired from ACCEL exclusively on the development of our proton therapy business, now operating as “Varian Particle Therapy.” The sale of Research Instruments was completed in the second quarter of fiscal year 2009. Research Instruments is classified as a discontinued operation for all periods presented and we have segregated the net assets and operating results of Research Instruments from continuing operations in our Consolidated Balance Sheets and in our Consolidated Statements of Earnings. Unless otherwise stated, the discussion below pertains to our continuing operations. Research Instruments was previously included in the “Other” category. The Research Instruments business reduced total net earnings per diluted share for fiscal year 2009 by $0.10 to $2.55.
Oncology Systems. Our largest business segment is Oncology Systems, which designs, manufacturers, sells and services hardware and software products for radiation treatment of cancer with conventional radiotherapy, IMRT, IGRT, VMAT (a special form of IMRT), stereotactic radiotherapy, stereotactic radiosurgery and brachytherapy.
In fiscal year 2009, Oncology Systems experienced the effects of a recession that has shrunk customer capital equipment budgets, slowed decision making and made financing more expensive and time consuming. This business was also negatively impacted by the uncertainty created by the prospects of healthcare reform in the United States; as well as the added uncertainty in late fiscal year 2009 about the proposed reduction in Medicare reimbursement rates for radiotherapy and radiosurgery at free-standing clinics in the United States, and for physician reimbursement for radiation oncology. While the final enacted reimbursement rate reductions for 2010, which were announced by CMS on October 30, 2009, were much more modest for radiotherapy than originally proposed, we believe that the confusion and uncertainty created by the proposal was one of the major factors impacting our net orders in late fiscal year 2009, particularly from free-standing radiotherapy clinics (which we believe currently represents approximately 10% to 15% of Oncology Systems’ business). While we benefited from pre-recession capital equipment budgets in the first half of fiscal year 2009, we experienced the effects of smaller capital budgets established by many health care providers in the second half of the fiscal year. We expect that uncertainty in North American radiation therapy capital spending will persist so long as the economic downturn continues and until that there is greater clarity on healthcare reform and that the North American market is likely to remain very challenging at least for the first half of fiscal year 2010.
In fiscal year 2009, Oncology Systems net orders increased 1% compared to fiscal year 2008, with North American net orders decreasing 7% and international net orders, which were negatively affected by the strengthening of the U.S. dollar, growing 11%. Oncology Systems revenues increased 8% in fiscal year 2009 over fiscal year 2008 with North American revenues increasing 12% and international revenues, which were negatively affected by the strengthening of the U.S. dollar, growing by 3%. A product mix shift toward higher margin software products, as well as an improvement in service contract gross margin, contributed to a 1.6 percentage-point improvement in Oncology Systems gross margin in fiscal year 2009 compared to fiscal year 2008. As of the end of fiscal year 2009, we had more than 270 installations of our RapidArcTM products since we introduced these products in the second quarter of fiscal year 2008.
55
Table of Contents
Our success in Oncology Systems largely depends upon our ability to retain leadership in technological innovation, the reliability and cost effectiveness of our products, the efficacy of our treatment technology and external influences. Factors affecting the adoption rate of new technologies (such as VMAT) could include their more-widely demonstrated efficacy and our ability to efficiently introduce and install our new technologies and products. Additional factors could include customer training on the use of our new technologies or related products and our ability to educate customers about the cost effectiveness of our new technologies. External influences could include the current economic environment, the current state of healthcare reform, significant changes to Medicare and Medicaid reimbursement rates for radiotherapy and brachytherapy procedures and radiosurgery in the United States, the financial strength of our customers, consolidation among our customers, currency exchange rates, and government budgeting and tendering cycles.
In recent years, we have seen the purchasing cycle lengthening as a result of the more complex decision-making process associated with larger dollar value transactions for more sophisticated IGRT and surgical equipment, and other technical advances. With the current worldwide economic downturn, it has become and may continue to be more difficult to accurately forecast and plan future business activities. We have seen customers’ decision-making process further complicated and lengthened, especially in the United States, as the downturn causes hospitals, clinics and research institutions to more closely scrutinize and prioritize their capital spending in light of tightened capital budgets, tougher credit requirements and the general constriction in credit availability. In addition, we believe that the current economic downturn has caused customers to delay requested delivery dates. Because our product revenues are influenced by the timing of product shipments, which are tied to customer-requested delivery dates, these delivery delays have increased the average order to revenue conversion cycle in the United States. We cannot predict the duration of the economic slowdown or the timing or strength of a subsequent economic recovery, in general or specifically in the healthcare industry. If the healthcare market significantly deteriorates due to the general economic downturn, our business, financial condition and results of operations will likely be materially and adversely affected. We have also seen our customers’ decision-making process complicated by the uncertainty surrounding the recent proposed reduction in Medicare reimbursement rates, as described above, and uncertainty about the shape and scope of U.S. healthcare reform legislation. Any significant cuts in these reimbursement rates, or other rates for radiotherapy, radiosurgery, brachytherapy or proton therapy, could have a material adverse effect on our revenues and results of operations.
Overall, the U.S. dollar was stronger against foreign currencies in fiscal year 2009 than it was in fiscal year 2008, which made our pricing less competitive and contributed to slower growth in our international net orders and revenues. However, the U.S. dollar has subsequently weakened, which could make our product pricing more competitive in the local currencies of our international customers. The fluctuation of the U.S dollar against foreign currencies also impacts our international revenues and net orders when measured in U.S. dollars. Fluctuation in the strength of the U.S. dollar against foreign currencies may result in greater fluctuation in our Oncology Systems net orders and revenues.
X-Ray Products. Our X-ray Products business segment, designs, manufactures and sells: (i) x-ray tubes for use in a range of applications including CT scanning, radiographic or fluoroscopic imaging, mammography, special procedures and industrial applications; and (ii) flat panel digital image detectors for filmless x-ray imaging, which is for radiography an alternative to image intensifier tubes for fluoroscopy and x-ray film and CR systems.
Impacted by customer inventory reduction efforts in response to a slowdown in the imaging equipment market, X-ray Products saw net order declines in the second and third quarters of fiscal year 2009 versus the same periods in the previous year. However, this business segment reported a 1% net order growth for the full fiscal year 2009 over fiscal year 2008. Even with weak net orders, X-ray Products revenues increased 9% in fiscal year 2009 over fiscal year 2008. Our radiographic flat panels were a key contributor to net order and revenue growth for X-ray Products in fiscal year 2009. Compared to the
56
Table of Contents
prior fiscal year, the flat panel product line represented a higher percentage of total X-ray Products net orders and revenues in fiscal year 2009.
Our success in our X-ray Products business depends upon our ability to anticipate changes in our markets, the direction of technological innovation and the demands of our customers. The general worldwide economic downturn has made and may continue to make it difficult for our OEM customers, our vendors and us to accurately forecast and plan future business activities. The current market for new X-ray imaging equipment appears to be weak, and certain product lines, such as dental and veterinary, have been hit particularly hard in the recession. If the markets for our customers’ products significantly deteriorate due to the general economic downturn, our business and results of operations may be adversely affected. In recent years, we have also seen dramatic reductions in Medicare reimbursements for diagnostic radiology. We believe reductions in Medicare reimbursement rates have reduced demand for medical x-ray imaging equipment, such as CT scanners, which have negatively impacted demand for our x-ray tube products in the United States.
Other. The “Other” category is comprised of SIP, the Varian Particle Therapy business, and the operations of the GTC.
SIP designs, manufactures, sells and services Linatron x-ray accelerators, imaging processing software and image detection products (including IntellX) for security and inspection purposes, such as cargo screening at ports and borders and nondestructive examination in a variety of applications. Generally, we sell our SIP products to OEMs who incorporate them into their inspection systems. In fiscal year 2009, we saw our SIP customers postpone some purchasing decisions as budgets were put on hold or reallocated during the current economic downturn.
Use of our SIP technology in security cargo screening and border protection is still in its early stages but we believe demand for SIP products will be driven by cargo screening and border protection needs, as well as by the needs of customs agencies to verify the content of shipments for assessing duties and taxes. As a result, this business is heavily influenced by U.S. and foreign governmental policies on national and homeland security, border protection and customs revenue activities and therefore depends on government budgets and appropriations and is subject to political change. In addition, this business depends on the success of our OEM customers. Orders and revenues for our SIP products have been and may continue to be unpredictable as governmental agencies may place large orders with us or our OEM customers over a short period of time and then may not place any orders for a long time period thereafter.
Our Varian Particle Therapy business develops, designs, manufactures and services products and systems for delivering proton therapy, another form of external beam radiotherapy using proton beams for the treatment of cancer. Although proton therapy has been in clinical use for more than four decades, it has not been widely deployed due to the cost of the technology and limited cost effectiveness. We are investing substantial resources to commercialize this advanced proton technology and to build this new business. In the second quarter of fiscal year 2009, we met the CE mark requirements that permit us to market our proton therapy systems within the EEA and patient treatments started on our proton therapy system that is installed at the Rinecker Proton Therapy Center in Munich, Germany.
Proton therapy facilities are large scale construction projects that are time consuming and involve significant customer investment and perhaps complex project financing. Consequently, this business is the most vulnerable to the general worldwide economic downturn and contraction in the credit markets. In addition, the customers’ decision-making cycle is very long and orders for proton therapy systems generally involve many contingencies. Under our current practice, we will only recognize proton therapy system orders with contingencies if we deem the contingencies perfunctory or if we publicly disclose the existence and nature of material contingencies.
57
Table of Contents
In the fourth quarter of fiscal year 2009, Skandion Kliniken awarded us, in a competitive bidding situation, an approximately $62 million contract to deliver and install a proton therapy system in Sweden and we have included this contract in net orders. However, one of the unsuccessful bidders has filed a formal appeal of the award. Skandion Kliniken is actively opposing this appeal and we believe it will be resolved in Skandion Kliniken’s favor. Additionally, the order is contingent on Skandion Kliniken’s execution of a contract for the construction of the proton therapy facility, which we also believe will occur. As this is the first proton therapy system to be delivered by Varian Particle Therapy we acquired ACCEL, the profitability of this contract is uncertain. We plan to start recognizing revenues in accordance with contract accounting when we commence working on this project. As Varian Particle Therapy is a relatively new business that offers highly customized proton therapy systems, we plan to evaluate revenue recognition for sales of proton therapy systems and related services on a contract by contract basis.
GTC, our scientific research facility, continues to invest in developing technologies that enhance our current businesses or may lead to new business areas, including next generation digital x-ray imaging technology, volumetric and functional imaging, and improved x-ray sources and technology for security and cargo screening applications. In addition, GTC is developing technologies and products that are designed to improve disease management by more precise targeting of radiation, as well as by employing targeted energy and molecular agents to enhance the effectiveness and broaden the application of radiation therapy.
Including the large Skandion Kliniken order to deliver and install a proton therapy system, net orders in the “Other” category increased 59% in fiscal year 2009 over the prior fiscal year. Revenues in the “Other” category decreased 9% in fiscal year 2009 over fiscal year 2008. In fiscal year 2009, SIP net orders and revenues decreased over fiscal year 2008 as government postponed purchasing decisions for and delayed deployments of products for security and inspection systems.
This discussion and analysis of our financial condition and results of operations is based upon and should be read in conjunction with the Consolidated Financial Statements and the notes included elsewhere in this Annual Report on Form 10-K, as well as the information contained under “Risk Factors contained in Item 1A. We discuss our results of operations below.
Critical Accounting Estimates
The preparation of our financial statements and related disclosures in conformity with accounting principles generally accepted in the United States (“GAAP”) requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses. These estimates and assumptions are based on historical experience and on various other factors that we believe are reasonable under the circumstances. We periodically review our accounting policies, estimates and assumptions and make adjustments when facts and circumstances dictate. In addition to the accounting policies that are more fully described in the Notes to the Consolidated Financial Statements included in this Annual Report on Form 10-K, we consider the critical accounting policies described below to be affected by critical accounting estimates. Our critical accounting policies that are affected by accounting estimates include share-based compensation expense, revenue recognition, valuation of allowance for doubtful accounts, valuation of inventories, assessment of recoverability of goodwill and intangible assets, valuation of warranty obligations, assessment of environmental remediation liabilities, valuation of defined benefit pension and post-retirement benefit plans, valuation of derivative instruments and taxes on earnings. Such accounting policies require us to use judgments, often as a result of the need to make estimates and assumptions regarding matters that are inherently uncertain; and actual results could differ materially from these estimates. For a discussion of how these estimates and other factors may affect our business, also refer to the Risk Factors in Item 1A.
58
Table of Contents
Revenue Recognition
We frequently enter into sales arrangements with customers that contain multiple elements or deliverables such as hardware, software and services. Judgments as to the allocation of the proceeds received from an arrangement to the multiple elements of the arrangement, the determination of whether any undelivered elements are essential to the functionality of the delivered elements and the appropriate timing of revenue recognition are critical with respect to these arrangements to ensure compliance with GAAP. In addition, the amount of product revenues we recognize is affected by our judgments as to whether objective and reliable evidence of fair value exists for hardware products and vendor-specific objective evidence of the fair value for software products in arrangements with multiple elements. Changes to the elements in an arrangement and the ability to establish objective and reliable evidence of fair value or vendor-specific objective evidence of the fair value for those elements could affect the timing of revenue recognition. Revenue recognition also depends on the timing of shipment and is subject to customer acceptance and the readiness of customers’ facilities. If shipments are not made on scheduled timelines or if the products are not accepted by the customer in a timely manner, our reported revenues may differ materially from expectations. In addition, revenues related to our highly customized image detection systems are recognized under the percentage-of-completion method. Under the percentage-of-completion method of accounting, sales and gross profit are recognized as work is performed, based on the relationship between actual costs incurred and total estimated costs at the completion of the contract. Because the percentage-of-completion method involves considerable use of estimates in determining revenues, costs and profits and in assigning the dollar amounts to relevant accounting periods, and because the estimates must be periodically reviewed and appropriately adjusted, if our estimates prove to be inaccurate or circumstances change over time, we may be forced to adjust revenues or even record a contract loss in later periods. If a loss is expected on a contract under the percentage-of-completion method or completed contract method, the estimated loss would be charged to cost of sales in the period the loss is identified.
Share-based Compensation Expense
We value our share-based payment awards granted using the Black-Scholes option-pricing model. The determination of fair value of share-based payment awards on the date of grant using the Black-Scholes option-pricing model is affected by VMS’s stock price, as well as the input of other subjective assumptions, including the expected term of stock awards and the expected price volatility of VMS stock over the expected term of the awards.
The expected term is based on the observed and expected time to post-vesting exercise and post-vesting cancellations of stock options by our employees. We determined the expected term of stock options based on the demographic grouping of employees and retirement eligibility. We used a combination of historical and implied volatility, or blended volatility, in deriving the expected volatility assumption. Blended volatility represents the weighted average of implied volatility and historical volatility. Implied volatility was derived based on six-month traded options on VMS common stock. Implied volatility is weighted in the calculation of blended volatility based on the ratio of the six-month term of the exchange-traded options to the expected lives of the employee stock options. Historical volatility represents the remainder of the weighting. Our decision to incorporate implied volatility was based on our assessment that implied volatility of publicly traded options on VMS common stock is reflective of market conditions and is generally reflective of both historical volatility and expectations of how future volatility will differ from historical volatility. In determining the extent of use of implied volatility, we considered: (i) the volume of market activity of traded options; (ii) the ability to reasonably match the input variables of traded options to those of stock options granted by us, including the date of grant; (iii) the similarity of the exercise prices; and (iv) the length of term of traded options. After considering the above factors, we determined that we could rely exclusively on implied volatility based on the fact that the term of VMS six-month exchange-traded options is less than one year and that it is
59
Table of Contents
different from the expected lives of the stock options we grant. Therefore, we believe a combination of the historical volatility over the expected lives of the stock options we granted and the implied volatility of six-month exchange-traded options best reflects the expected volatility of VMS common stock going forward. The risk-free interest rate assumption is based upon observed interest rates appropriate for the term of our stock options. The dividend yield assumption is based on our history and expectation of no dividend payouts. If factors change and we employ different assumptions in future periods, the compensation expense that we record may differ significantly from what we have recorded in the current period. In addition, we are required to estimate the expected forfeiture rate and recognize expense only for those shares expected to vest. If our actual forfeiture rate is materially different from our estimate, the stock-based compensation expense could be significantly different from what we have recorded in the current period.
Allowance for Doubtful Accounts
We evaluate the creditworthiness of our customers prior to authorizing shipment for all major sale transactions. Our payment terms usually require payment of: a small portion of the total amount due when the customer signs the purchase order, a significant amount upon transfer of risk of loss to the customer, and the remaining amount upon completion of the installation. On a quarterly basis, we evaluate aged items in the accounts receivable aging report and provide an allowance in an amount we deem adequate for doubtful accounts. If our evaluation of our customers’ financial conditions does not reflect our future ability to collect outstanding receivables, additional provisions may be needed and our operating results could be negatively affected.
Inventories
Our inventories include high technology parts and components that are highly specialized in nature and that are subject to rapid technological obsolescence. We have programs to minimize the required inventories on hand and we regularly review inventory quantities on hand and adjust for excess and obsolete inventory based primarily on historical usage rates and our estimates of product demand and production. Actual demand may differ from our estimates, in which case we may have understated or overstated the provision required for obsolete and excess inventory, which would have an impact on our operating results.
Goodwill and Intangible Assets
Goodwill is initially recorded when the purchase price paid for a business acquisition exceeds the estimated fair value of the net identified tangible and intangible assets acquired. The majority of businesses that we have acquired have not had significant identified tangible assets and, as a result, we have typically allocated a significant portion of the purchase price to intangible assets and goodwill. Our future operating performance will be impacted by the future amortization of these acquired intangible assets and potential impairment charges related to these intangibles or to goodwill if indicators of impairment exist. The allocation of the purchase price from business acquisitions to goodwill and intangible assets could have a significant impact on our future operating results. In addition, the allocation of the purchase price of the acquired businesses to goodwill and intangible assets requires us to make significant estimates and assumptions, including estimates of future cash flows expected to be generated by the acquired assets and the appropriate discount rate for these cash flows. Should conditions differ from management’s estimates at the time of the acquisition, material write-downs of intangible assets and/or goodwill may be required, which would adversely affect our operating results.
In accordance with ASC 350, we evaluate goodwill for impairment at least annually or whenever an event occurs or circumstances changes that would more likely than not reduce the fair value of a reporting unit below its carrying amount. The impairment test for goodwill is a two-step process. Step one consists of a comparison of the fair value of a reporting unit against its carrying amount, including
60
Table of Contents
the goodwill allocated to each reporting unit. We determine the fair value of our reporting units based on the present value of estimated future cash flows of the reporting units. If the carrying amount of the reporting unit is in excess of its fair value, step two requires the comparison of the implied fair value of the reporting unit’s goodwill against the carrying amount of the reporting unit’s goodwill. Any excess of the carrying value of the reporting unit’s goodwill over the implied fair value of the reporting unit’s goodwill is recorded as an impairment loss. The impairment test for intangible assets with indefinite useful lives, if any, consists of a comparison of fair value to carrying value, with any excess of carrying value over fair value being recorded as an impairment loss. We will continue to make assessments of impairment on an annual basis in the fourth quarter of our fiscal years or more frequently if indicators of potential impairment arise.
Warranty Obligations
We warrant most of our products for a specific period of time, usually twelve months, against material defects. We provide for the estimated future costs of warranty obligations in cost of revenues when the related revenues are recognized. The accrued warranty costs represent our best estimate at the time of sale of the total costs that we will incur to repair or replace product parts that fail while still under warranty. The amount of accrued estimated warranty costs obligation for established products is primarily based on historical experience as to product failures adjusted for current information on repair costs. For new products, estimates will include historical experience of similar products, as well as reasonable allowance for start-up expenses. Actual warranty costs could differ from the estimated amounts. On a quarterly basis, we review the accrued balances of our warranty obligations and update the historical warranty cost trends, if required. If we were required to accrue additional warranty costs in the future, it would have a negative effect on our operating results.
Environmental Matters
We are subject to a variety of environmental laws around the world. Those laws regulate multiple aspects of our operations, including the handling, storage, transport and disposal of hazardous substances. They impose costs on our operations and in connection with past operations. In connection with past operations, we record environmental remediation liabilities when we conclude that environmental assessments or remediation efforts are probable and we believe we can reasonably estimate the costs of those efforts. Our accrued environmental costs represent our best estimate of the total costs of assessments and remediation and the time period over which we expect to incur those costs. We review these accrued balances quarterly. Were we required to increase or decrease the accrued environmental costs in the future, it would adversely or favorably impact our operating results.
Defined Benefit Pension and Post-Retirement Benefit Plans
We sponsor six defined benefit pension plans in Germany, Japan, Switzerland and the United Kingdom covering employees who meet the applicable eligibility requirements in these countries. In fiscal year 2009, we terminated one pension plan in Germany as a result of the sale of Research Instruments. In July 2007, we made changes to the defined benefit pension plan in the United Kingdom by terminating the accrual of additional benefits for existing participants and suspending the enrollment of new participants. We also sponsor a post-retirement benefit plan that provides healthcare benefits to certain eligible retirees in the United States. We do not have any defined benefit pension plans in the United States. Several statistical and other factors that attempt to anticipate future events are used in calculating the expense and liability related to those plans for which the benefit is actuarially determined, such as our defined benefit pension and post-retirement benefit plans. These factors include assumptions about: the discount rate; expected return on plan assets; rate of future compensation increases; and rate of healthcare cost increases, all of which we determine within certain guidelines. In addition, we also use assumptions, such as withdrawal and mortality rates, to calculate the expense and liability. The actuarial
61
Table of Contents
assumptions we use are long-term assumptions and may differ materially from actual experience particularly in the short term due to changing market and economic conditions and changing participant demographics. These differences may have a significant impact on the amount of defined benefit pension and post-retirement benefit plan expense we record.
The expected rates of return on the various defined benefit pension plans’ assets are based on the asset allocation of each plan and the long-term projected return on those assets. The discount rate enables us to state expected future cash flows at a present value on the measurement date. The discount rates used for defined benefit plans in all countries are based primarily on the yields of a universe of high quality corporate bonds in each country or the spot rate of high quality AA-rated corporate bonds, with durations corresponding to the expected durations of the benefit obligations. In countries where the corporate bond market is not sufficiently representative of the time period at longer durations, the discount rate also takes into account the yield of long-term government bonds corresponding to the duration of the benefit obligations and the difference between the yield curve on high quality corporate fixed-income investments and government fixed-income investment. A change in the discount rate will cause the present value of benefit obligations to change in the opposite direction.
Valuation of Derivative Instruments
We use foreign currency forward contracts to reduce the effects of currency fluctuations on sales transactions denominated in foreign currencies and on assets and liabilities denominated in foreign currencies. These foreign currency forward contracts are derivative instruments and are measured at fair value. ASC 820 establishes three levels of inputs that may be used to measure fair value (see Note 3, “Fair Value Measurements” to Consolidated Financial Statements). Each level of input has different levels of subjectivity and difficulty involved in determining fair value. The fair value of foreign currency forward contracts are calculated primarily using Level 2 inputs, which include currency spot and forward rates, interest rate and credit or non-performance risk. The spot rate for each currency is the same spot rate used for all balance sheet translations at the measurement date and sourced from our major trading banks. The following values are interpolated from commonly quoted broker services: forward point values for each currency and the London Interbank Offered Rate (“LIBOR”) to discount assets and liabilities. One year credit default swap spreads of the counterparty at the measurement date are used to adjust derivative assets, all of which have maturity terms less than 12 months, for non-performance risk. We are required to adjust derivative liabilities to reflect the potential non-performance risk to lenders based on our incremental borrowing rate. Each contract is individually adjusted using the counterparty (for net asset) or our discount rate (for net liability). The use of Level 2 inputs in determining fair values requires certain management judgment and subjectivity. Changes to these Level 2 inputs could have a material impact to the valuation of our derivative instruments, as well as on our result of operations.
Taxes on Earnings
We are subject to taxes on earnings in both the United States and numerous foreign jurisdictions. As a global taxpayer, significant judgments and estimates are required in evaluating our tax positions and determining our provision for taxes on earnings.
Effective as of the beginning of fiscal year 2008, we adopted the provisions in ASC 740 related to accounting for uncertainty in income taxes, which contains a two-step approach to recognizing, derecognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining whether the weight of available evidence indicates that it is more likely than not that, based on the technical merits, the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon settlement. Recognition, derecognition, and measurement are based on management’s best judgment given the facts, circumstances and information available at the end of the accounting period. A tax benefit should be recognized in the first period in
62
Table of Contents
which it meets the more likely than not recognition threshold, and conversely, a tax benefit previously recognized should be derecognized in the first period in which new information results in a change in judgment in which the position fails to meet the recognition threshold. A benefit not previously recognized would be recognized when the tax position is effectively settled through examination, negotiation or litigation with tax authorities, or when the statute of limitations for the relevant taxing authority to examine and challenge the position has expired. Our policy is to include interest and penalties related to unrecognized tax benefits within the provision for taxes on earnings.
In addition, the carrying value of our net deferred tax assets assumes that we will be able to generate sufficient future taxable earnings in certain tax jurisdictions to utilize these deferred tax assets. Should we conclude it is more likely than not that we will be unable to recover our net deferred tax assets in these tax jurisdictions, we would increase our valuation allowance and our tax provision would increase in the period in which we make such a determination.
Earnings derived from our international regions are generally taxed at rates lower than U.S. rates. Our effective tax rate is impacted by existing tax laws in both the United States and in the respective countries in which our international subsidiaries do business. In addition, a decrease in the percentage of our total earnings from our international regions, or a change in the mix of international regions among particular tax jurisdictions, could increase our effective tax rate. Also, our current effective tax rate does not assume U.S. taxes on certain undistributed profits of certain foreign subsidiaries. These earnings could become subject to incremental foreign withholding or U.S. federal and state taxes should they either be deemed or actually remitted to the United States.
Results of Operations
Fiscal Year
Our fiscal year is the 52- or 53-week period ending on the Friday nearest September 30. Fiscal year 2009 comprised the 53-week period ended on October 2, 2009. Fiscal year 2008 comprised the 52-week period ended on September 26, 2008 and fiscal year 2007 was the 52-week period ended on September 28, 2007. Set forth below is a discussion of our results of operations for the fiscal years 2009, 2008 and 2007. As indicated above, the operating results of Research Instruments have been segregated and presented as a discontinued operation for all periods.
63
Table of Contents
Discussion of Results of Operations for Fiscal Years 2009, 2008 and 2007
Total Revenues
Revenues by sales classification | Fiscal Years | |||||||||||||||||
| (Dollars in millions) | 2009 | % Change | 2008 | % Change | 2007 | |||||||||||||
Product | $ | 1,767 | 5 | % | $ | 1,690 | 17 | % | $ | 1,448 | ||||||||
Service Contracts and Other | 447 | 18 | % | 380 | 24 | % | 307 | |||||||||||
Total Revenues | $ | 2,214 | 7 | % | $ | 2,070 | 18 | % | $ | 1,755 | ||||||||
Product as a percentage of total revenues | 80 | % | 82 | % | 82 | % | ||||||||||||
Service Contracts and Other as a percentage of total revenues | 20 | % | 18 | % | 18 | % | ||||||||||||
Revenues by region | ||||||||||||||||||
North America | $ | 1,111 | 11 | % | $ | 1,003 | 16 | % | $ | 865 | ||||||||
Europe | 620 | 0 | % | 619 | 17 | % | 529 | |||||||||||
Asia | 412 | 18 | % | 349 | 24 | % | 281 | |||||||||||
Rest of world | 71 | (28 | )% | 99 | 24 | % | 80 | |||||||||||
Total International(1) | 1,103 | 3 | % | 1,067 | 20 | % | 890 | |||||||||||
Total | $ | 2,214 | 7 | % | $ | 2,070 | 18 | % | $ | 1,755 | ||||||||
North America as a percentage of total revenues | 50 | % | 48 | % | 49 | % | ||||||||||||
International as a percentage of total revenues | 50 | % | 52 | % | 51 | % | ||||||||||||
| (1) | We consider international revenues to be revenues outside of North America. |
Total revenues, as well as product revenues, increased in fiscal year 2009 over fiscal year 2008 primarily due to the revenue growth in both of our Oncology Systems and X-ray Products business segments partially offset by a decline in SIP revenues. Both of these business segments, as well as SIP, contributed to the growth in total revenues and product revenues in fiscal year 2008 over fiscal year 2007. Product revenues grew more slowly in fiscal year 2009 over fiscal year 2008 than fiscal year 2008 grew over fiscal year 2007, primarily due to the slower growth in Oncology Systems product revenues. Oncology Systems service contracts revenues were the primary contributor to the growth in service contracts and other revenues in fiscal year 2009 over fiscal year 2008 and in fiscal year 2008 over fiscal year 2007. Service contracts and other revenues grew more slowly in fiscal years 2009 over 2008 compared to fiscal year 2008 over fiscal year 2007 primarily due to the slower growth in Oncology Systems service contract revenues.
In North America, both of our business segments and SIP contributed to the revenue growth in fiscal year 2009 over fiscal year 2008 and in fiscal year 2008 over fiscal year 2007. In the international region, the growth in Oncology Systems and X-ray Products international revenues in fiscal year 2009 over fiscal year 2008 was partially offset by the decrease in SIP international revenues. For fiscal year 2009 over fiscal year 2008, both of our business segments and SIP saw revenue growth in Asia and both of our business segments experienced revenue decline in the rest of the world region. During fiscal year 2009, revenue growth in Europe in both of our business segments was offset by the decline in SIP revenues. The overall stronger U.S. dollar in fiscal year 2009 against foreign currencies compared to fiscal year 2008 negatively affected our international revenues in fiscal year 2009 when measured in U.S. dollars. In fiscal year 2008, both business segments and SIP contributed to the revenue growth over fiscal year 2007 in all international regions. The overall weaker U.S. dollar against foreign currencies in fiscal year 2008 compared to fiscal year 2007 benefited our international revenues in fiscal year 2008 when measured in U.S. dollars. Since fiscal year 2007, international revenues have represented half or more of our worldwide revenues.
64
Table of Contents
Oncology Systems Revenues
Revenues by sales classification | Fiscal Years | |||||||||||||||||
| (Dollars in millions) | 2009 | % Change | 2008 | % Change | 2007 | |||||||||||||
Product | $ | 1,363 | 5 | % | $ | 1,302 | 14 | % | $ | 1,145 | ||||||||
Service Contracts(1) | 435 | 17 | % | 370 | 26 | % | 295 | |||||||||||
Total Oncology Systems | $ | 1,798 | 8 | % | $ | 1,672 | 16 | % | $ | 1,440 | ||||||||
Product as a percentage of Oncology Systems revenues | 76 | % | 78 | % | 80 | % | ||||||||||||
Service Contracts as a percentage of Oncology Systems revenues | 24 | % | 22 | % | 20 | % | ||||||||||||
Oncology Systems revenues as a percentage of total revenues | 81 | % | 81 | % | 82 | % | ||||||||||||
| (1) | Revenues from service contracts represent revenues from fixed-term service contracts and labor cost services. This excludes revenues from spare parts sold by our service department. |
The increase in revenues from sales of our software products (including our RapidArc products) was the primary contributor to the increase in Oncology Systems product revenues for fiscal year 2009 over fiscal year 2008. In fiscal year 2008, the increase in Oncology Systems product revenues over fiscal year 2007 was primarily driven by increased sales of our high energy linear accelerators, our software products (including our RapidArc products) and our accessory products that enable IGRT (including our OBI), partially offset by a decline in sales of IMRT-upgrades that reflected the slowdown in demand for IMRT-upgrade products after several years of rapid adoption of IMRT technology.
The increases in service contract revenues in fiscal year 2009 over fiscal year 2008 and in fiscal year 2008 over fiscal year 2007 were primarily driven by increased customer adoption of service contracts as the sophistication of our products and the installed base of our products continue to increase. Since service contract revenues grew faster than product revenues from fiscal year 2007 to fiscal year 2008 and from fiscal year 2008 to fiscal year 2009, service contract revenues also increased as a percentage of total Oncology Systems revenues in the same time periods.
In fiscal year 2009, the stronger U.S. dollar against foreign currencies compared to fiscal year 2008 negatively affected Oncology Systems international revenues when measured in U.S. dollars, contributing to the slower growth in product and service revenues in fiscal year 2009 over fiscal year 2008 compared to fiscal year 2008 over fiscal year 2007. In fiscal year 2008, the weaker U.S. dollar against foreign currencies compared to fiscal year 2007 favorably affected Oncology Systems international revenues when measured in U.S. dollars.
Revenues by region | Fiscal Years | |||||||||||||||||
| (Dollars in millions) | 2009 | % Change | 2008 | % Change | 2007 | |||||||||||||
North America | $ | 970 | 12 | % | $ | 866 | 15 | % | $ | 754 | ||||||||
Europe | 524 | 1 | % | 517 | 14 | % | 454 | |||||||||||
Asia | 242 | 21 | % | 200 | 25 | % | 160 | |||||||||||
Rest of world | 62 | (31 | )% | 89 | 24 | % | 72 | |||||||||||
Total International | 828 | 3 | % | 806 | 18 | % | 686 | |||||||||||
Total Oncology Systems | $ | 1,798 | 8 | % | $ | 1,672 | 16 | % | $ | 1,440 | ||||||||
North America as a percentage of Oncology Systems revenues | 54 | % | 52 | % | 52 | % | ||||||||||||
International as a percentage of Oncology Systems revenues | 46 | % | 48 | % | 48 | % | ||||||||||||
65
Table of Contents
The slower Oncology Systems revenue growth rate in fiscal year 2009 over fiscal year 2008, as compared with the growth rate in fiscal year 2008 over fiscal year 2007, was primarily due to the weaker net order growth in fiscal year 2009 compared to fiscal year 2008 and, to a lesser extent, delays in customer requested delivery dates in fiscal year 2009, which we believe were caused primarily by the current economic downturn.
Except for the rest of the world region, all of our geographic regions contributed to the Oncology Systems revenues growth in fiscal year 2009 over fiscal year 2008. For fiscal year 2009, North American revenues grew by 12% and international revenues increased 3% over fiscal year 2008. The increase in North American revenues was primarily due to an increase in sales of our software products (including our RapidArc products) and our linear accelerators, as well as an increase in service contract revenues. The increase in sales of our software products (including our RapidArc products) and the increase in service contract revenues in Asia and Europe were significantly offset by the decline in sales of our linear accelerators in the rest of the world region and Europe. The stronger U.S. dollar against foreign currencies in fiscal year 2009 compared to fiscal year 2008 also negatively affected our international revenues when measured in U.S. dollars.
All of our geographic regions contributed to the Oncology Systems revenues growth in fiscal year 2008 over fiscal year 2007. For fiscal year 2008, the growth in international and North American revenues over fiscal year 2007 were due to increases in sales of our high energy linear accelerators, our accessory products that enable IGRT (including our OBI) and our software products (including our RapidArc products) in all regions, as well as an increase in service contracts revenues. The weaker U.S. dollar against foreign currencies in fiscal year 2008 compared to fiscal year 2007 favorably affected our international revenues in fiscal year 2008 when measured in U.S. dollars. These increases in both international revenues and North American revenues were partially offset by decreases in sales of IMRT-upgrades, reflecting the slowdown in demand for IMRT-upgrade products after several years of rapid adoption of IMRT technology.
Varying cycles of higher and lower revenues between the international and North American regions is a historical pattern reflecting different technology adoption cycles and demand cycles that are consistent with the net order patterns discussed more fully under “Net Orders.” Oncology Systems revenues also continue to be influenced by the timing of product shipments in accordance with planned customer-requested delivery dates, and have been impacted by the effects of the recession, uncertainty created by the prospects of healthcare reform in the United States, and added uncertainty in late fiscal year 2009 about the proposed reduction in Medicare reimbursement rates for radiotherapy and radiosurgery at free-standing clinics in the United States and for physician reimbursement for radiation oncology.
X-ray Products Revenues
Revenues by region | Fiscal Years | |||||||||||||||||
| (Dollars in millions) | 2009 | % Change | 2008 | % Change | 2007 | |||||||||||||
North America | $ | 110 | 3 | % | $ | 107 | 12 | % | $ | 96 | ||||||||
Europe | 49 | 9 | % | 45 | 28 | % | 35 | |||||||||||
Asia | 162 | 14 | % | 143 | 21 | % | 119 | |||||||||||
Rest of world | 10 | (2 | )% | 10 | 21 | % | 8 | |||||||||||
Total International | 221 | 12 | % | 198 | 22 | % | 162 | |||||||||||
Total X-ray Products | $ | 331 | 9 | % | $ | 305 | 19 | % | $ | 258 | ||||||||
North America as a percentage of X-ray Products revenues | 33 | % | 35 | % | 37 | % | ||||||||||||
International as a percentage of X-ray Products revenues | 67 | % | 65 | % | 63 | % | ||||||||||||
X-ray Products revenues as a percentage of total revenues | 15 | % | 15 | % | 15 | % | ||||||||||||
66
Table of Contents
X-ray Products revenues grew more slowly in fiscal year 2009 over fiscal year 2008 as compared to the growth in fiscal year 2008 over fiscal year 2007 primarily as a result of our customers reducing inventory levels in response to a slowdown in the imaging equipment market, particularly in the dental and veterinary markets.
For fiscal year 2009, X-ray Products revenue grew 9% over fiscal year 2008 due primarily to the growth in international revenues and, to a lesser extent, the growth in North American revenues. The increase in international revenues in fiscal year 2009 over fiscal year 2008 was primarily due to increased sales of our flat panel detectors, including our radiographic flat panels, in Europe and Asia, as well as increased sales of x-ray tubes in Asia compared to fiscal year 2008. Revenue growth in North America in fiscal year 2009 over fiscal year 2008 was largely due to the growth in sales of our x-ray tubes, while sales of our flat panel products decreased slightly notwithstanding growth from our radiographic flat panels.
All of our geographic regions contributed to the increase in X-ray Products revenues in fiscal year 2008 over fiscal year 2007. The growth in X-ray Products revenues in both the international and in North American regions in fiscal year 2008 over fiscal year 2007 reflects increased sales of our flat panel detectors and, to a lesser extent, increased sales of our x-ray tubes in international regions.
The general worldwide economic downturn we have seen since 2008 has made and may continue to make it difficult for our OEM customers to accurately forecast and plan future business activities, and we saw our x-ray business impacted in fiscal year 2009 by inventory reduction efforts at some of these customers. The current market for new X-ray imaging equipment appears to be weak, and certain product lines, such as dental and veterinary, have been hit particularly hard in the recession. If the markets for our customers’ products significantly deteriorate due to the general economic downturn, our X-ray Products business may be adversely affected. In recent years, we have also seen dramatic reductions in Medicare reimbursements for diagnostic radiology. We believe reductions in these Medicare reimbursement rates have reduced demand for medical x-ray imaging equipment, such as CT scanners, which have negatively impacted demand for our x-ray tube products. Also, because we sell our x-ray products to a limited number of OEM customers and many of them are also our competitors with in-house x-ray tube manufacturing operations, we could experience the loss of, or reduction in purchasing volume by, one or more of these customers as they lower external sourcing costs in this economic downturn. Such a loss or reduction could have a material adverse effect on our X-ray Products business.
Other Revenues
Revenues by sales classification | Fiscal Years | |||||||||||||||||
| (Dollars in millions) | 2009 | % Change | 2008 | % Change | 2007 | |||||||||||||
Product | $ | 73 | (13 | )% | $ | 83 | 84 | % | $ | 45 | ||||||||
Service Contracts and Other | 12 | 24 | % | 10 | (21 | )% | 12 | |||||||||||
Total Other | $ | 85 | (9 | )% | $ | 93 | 62 | % | $ | 57 | ||||||||
Other revenues as a percentage of total revenues | 4 | % | 4 | % | 3 | % | ||||||||||||
Revenues in our “Other” category, which is comprised of SIP, Varian Particle Therapy and GTC, decreased in fiscal year 2009 over fiscal year 2008 primarily due to declines in product revenues in our SIP business as a result of slower deployment of products for security and inspection systems. Revenues in the “Other” category in fiscal year 2008 increased over fiscal year 2007 primarily due to growth in product revenues in our SIP business. The higher product revenues from SIP were attributable to increased sales of our Linatron x-ray accelerators and image detection products to OEM customers for cargo screening and border protection.
67
Table of Contents
Gross Margin
| Fiscal Years | ||||||||||||||||||
| (Dollars in millions) | 2009 | % Change | 2008 | % Change | 2007 | |||||||||||||
Dollar by segment | ||||||||||||||||||
Oncology Systems | $ | 806 | 12 | % | $ | 723 | 19 | % | $ | 609 | ||||||||
X-ray Products | 130 | 9 | % | 120 | 16 | % | 104 | |||||||||||
Other | 25 | (30 | )% | 35 | 71 | % | 20 | |||||||||||
Gross margin | $ | 961 | 9 | % | $ | 878 | 20 | % | $ | 733 | ||||||||
Percentage by segment | ||||||||||||||||||
Oncology Systems | 44.8 | % | 43.2 | % | 42.3 | % | ||||||||||||
X-ray Products | 39.3 | % | 39.3 | % | 40.2 | % | ||||||||||||
Total Company | 43.4 | % | 42.4 | % | 41.8 | % | ||||||||||||
The increase in total company gross margin percentage for fiscal year 2009 over fiscal year 2008 was primarily due to the improvement in Oncology System gross margin, which was partially offset by the decreases in gross margins in the “Other” category while X-ray Product gross margin remained flat. The decrease in gross margin in the “Other” category in fiscal year 2009 was primarily due to the decrease in Varian Particle Therapy gross margin resulting from higher estimated costs for completion of contractual commitments associated with the acquisition of ACCEL, which is discussed in more details in “Acquisition-Related Commitments/Obligations.” In fiscal year 2008, total gross margin improved by 0.6 percentage points compared with fiscal year 2007, primarily due to the increase in gross margin for Oncology Systems and SIP, partially offset by the decline in gross margin for X-ray Products. Total product gross margin was 42.6% in fiscal year 2009, compared to 41.7% in fiscal year 2008 and 41.1% in fiscal year 2007. Total service contracts and other gross margin was 46.4% in fiscal year 2009, compared to 45.5% in fiscal year 2008 and 44.9% in fiscal years 2007.
Improvements in both product gross margin and service contract gross margin contributed to the increases in Oncology Systems gross margins in fiscal year 2009 over fiscal year 2008 and in fiscal year 2008 over fiscal year 2007. Oncology Systems product gross margin was 43.7% in fiscal year 2009, compared to 42.4% in fiscal year 2008 and 41.4% in fiscal year 2007. The increase in Oncology Systems product gross margin in fiscal year 2009 over fiscal year 2008 was primarily due to product mix shift toward higher margin software products. The increase in Oncology Systems product gross margin in fiscal year 2008 over fiscal year 2007 was primarily due to higher sales volume and product mix shift toward higher margin products. Service contract gross margin was 48.3% in fiscal year 2009, compared to 46.1% in fiscal year 2008 and 45.7% in fiscal year 2007. The increase in Oncology Systems service contract gross margin in fiscal year 2009 over fiscal year 2008 was primarily due to cost control initiatives and higher volume in fiscal year 2009. The increase in Oncology Systems service contract gross margin in fiscal year 2008 over fiscal year 2007 was primarily due to higher contract volumes and growth in higher margin software maintenance contracts.
X-ray Products gross margin remained relatively flat in fiscal year 2009 compared to fiscal year 2008, with a gross margin improvement in x-ray tubes, offset by a decrease in flat panel gross margin due to higher start up costs and quality costs for the new radiographic flat panels. The X-ray Products gross margin decrease of 0.9 percentage points in fiscal year 2008 from fiscal year 2007 was primarily due to increased raw material costs and quality costs for x-ray tube products and increased raw material costs for flat panel products, although these increases were partially offset by the product mix shift toward a greater proportion of flat panel detectors which generally carry higher margin than x-ray tube products.
68
Table of Contents
Research and Development
| Fiscal Years | ||||||||||||||||||
| (Dollars in millions) | 2009 | % Change | 2008 | % Change | 2007 | |||||||||||||
Research and development | $ | 147 | 9 | % | $ | 136 | 16 | % | $ | 117 | ||||||||
As a percentage of total revenues | 7 | % | 7 | % | 7 | % | ||||||||||||
The $11 million increase in research and development expense for fiscal year 2009 over fiscal year 2008 was driven by increased expenses of $6 million in Oncology Systems, $4 million in the “Other” category and $1 million in X-ray Products. The $6 million increase in Oncology Systems was attributable primarily to an increase in employee headcount, material costs and consulting expenses for product development, although these expenses were partially offset by a $4 million favorable currency translation impact, due to the relatively strong U.S. dollar against foreign currencies in fiscal year 2009 compared to fiscal year 2008, when foreign currency denominated research and development expenses for Oncology Systems were translated into U.S. dollars. The $4 million increase in the “Other” category was primarily due to higher expense for development projects in Varian Particle Therapy and SIP. The $1 million increase in X-ray Products was mainly for development projects for both x-ray tubes and flat panel products.
The $19 million increase in research and development expense for fiscal year 2008 over fiscal year 2007 was driven by a $14 million increase in Oncology Systems and a $5 million increase in the “Other” category. The $14 million increase in research and development expenses in Oncology Systems for fiscal year 2008 compared to fiscal year 2007 was attributable primarily to a $15 million increase in employee headcount, materials costs and consulting expenses for development of our next generation linear accelerator products. Because of the relative weakness of the U.S. dollar in fiscal year 2008 compared to fiscal year 2007, research and development expenses in our foreign Oncology Systems operations were impacted by a $5 million unfavorable currency impact as they were translated into U.S. dollars. A reduction in $5 million in expenses related to other product development projects partially offset these effects. The $5 million increase in the “Other” category primarily reflected a $3 million increase in research and development expenses for x-ray accelerator products in SIP.
Selling, General and Administrative
| Fiscal Years | ||||||||||||||||||
| (Dollars in millions) | 2009 | % Change | 2008 | % Change | 2007 | |||||||||||||
Selling, general and administrative | $ | 339 | 5 | % | $ | 323 | 16 | % | $ | 277 | ||||||||
As a percentage of total revenues | 15 | % | 16 | % | 16 | % | ||||||||||||
The $16 million increase in selling, general and administrative expenses for fiscal year 2009 compared to fiscal year 2008 was primarily attributable to: (a) a $7 million increase in fees for certain commission arrangements and product promotions which were primarily tied to growth in Oncology Systems revenues; (b) a $5 million increase in depreciation expenses for our enterprise resource planning system that was placed in service in the second quarter of fiscal year 2009; (c) a $4 million increase in expenses primarily related to accruals for contingent liabilities in the ordinary course of business and (d) a $3 million net increase in employee-related costs due to an increase in headcount to support our growing business activities, was partially offset by a reduction in accrued bonuses and other cost control measures. These items were partially offset by favorable foreign currency impact of $5 million as the foreign currency denominated selling, general and administrative expenses of our foreign operations were translated into stronger U.S. dollars.
The $46 million increase in selling, general and administrative expenses for fiscal year 2008 compared to the same period in fiscal year 2007 was primarily attributable to: (a) a $16 million increase in expenses resulting from an increase in employee- related costs and headcount to support our growing business activities; (b) a $7 million unfavorable foreign currency impact as the selling, general and administrative
69
Table of Contents
expenses of our foreign operations were translated into U.S. dollars; (c) a $7 million increase in fees for certain commission arrangements and product promotions which were tied to growth in Oncology Systems revenues; (d) a $7 million increase in expenses primarily related to accruals for contingent liabilities in the ordinary course of business and (e) a $6 million increase in operating expenses associated with Varian Particle Therapy, Bio-Imaging Research, Inc. (“BIR”) and Pan-Pacific, Inc. (each of which were acquired through acquisition) and (f) a loss of $1 million from hedging balance sheet exposures from our various foreign subsidiaries and business units compared to a gain of $4 million in fiscal year 2007. These increases were partially offset by the receipt of a $5 million payment related to resolution of a gain contingency.
Interest Income, Net
| Fiscal Years | |||||||||||||||
| (Dollars in millions) | 2009 | % Change | 2008 | % Change | 2007 | ||||||||||
Interest income, net | $ | 0.5 | (92 | )% | $ | 6.6 | (10 | )% | $ | 7.4 | |||||
The decrease in interest income, net, in fiscal year 2009, compared to fiscal year 2008, was attributable to the lower average interest rates earned on our cash and cash equivalents. The decrease in interest income, net, in fiscal year 2008 over fiscal year 2007 was attributable to increased borrowings in fiscal year 2008 and lower average interest rate earned on our cash and cash equivalents in fiscal year 2008 than in fiscal year 2007.
Taxes on Earnings
| Fiscal Years | |||||||||||||||
| 2009 | Change | 2008 | Change | 2007 | |||||||||||
Effective tax rate | 30 | % | (1 | )% | 31 | % | 1 | % | 30 | % | |||||
The decrease in our effective tax rate in fiscal year 2009 from the prior fiscal year was primarily due to a net benefit from discrete items, primarily the release of certain liabilities for uncertain tax positions, including the expiration of the statutes of limitation in various jurisdictions and the favorable resolution of several income tax audits, partially offset by a decrease in the benefit of the foreign tax rate differential.
The increase in the effective tax rate in fiscal year 2008 compared to fiscal year 2007 was primarily because the earlier period included a greater tax benefit realized from the federal research and development credit. The effective tax rate for fiscal year 2007 included the benefit of the federal research and development credit for the full year plus the benefit of a retroactive reinstatement of the credit, which had previously expired on December 31, 2005. By comparison, the federal research and development credit was in effect for only one quarter during fiscal year 2008.
In general, our effective income tax rate differs from the U.S. federal statutory rate primarily because our foreign earnings are taxed at rates that are, on average, lower than the U.S. federal rate, and our domestic earnings are subject to state income taxes. Our future effective tax rate could be adversely affected by having lower earnings than anticipated in countries where we have lower statutory rates and higher earnings than anticipated in countries where we have higher statutory rates, by changes in the valuation of our deferred tax assets or liabilities, and by changes in tax laws or interpretations of those laws. For example, recent proposals would make significant changes to U.S. taxation of U.S.-based multinational corporations. Although we cannot predict whether or in what form Congress would enact any such proposals, legislation of this type could have an adverse impact on our effective tax rate. We also expect that our effective tax rate may experience increased fluctuation from period to period under the provisions in ASC 740 related to accounting for uncertainty in income taxes. Please refer to further discussion in Note 13 “Income Taxes” of the Notes to the Consolidated Financial Statements.
70
Table of Contents
Net Earnings Per Diluted Share
| Fiscal Years | |||||||||||||||
| 2009 | % Change | 2008 | % Change | 2007 | |||||||||||
Net earnings per diluted share | $ | 2.65 | 15 | % | $ | 2.31 | 24 | % | $ | 1.86 | |||||
The increase in earnings per diluted share in fiscal year 2009 over fiscal year 2008 resulted from (i) an increase in total revenues; (ii) an improvement in gross margin, (iii) leverage in our operating expenses, (iv) a reduction in our effective tax rate and (v) a reduction in the number of diluted shares of common stock due to stock repurchases and lower stock prices compared to fiscal year 2008.
The increase in earnings per diluted share in fiscal year 2008 over fiscal year 2007 resulted from (i) an increase in total revenues, (ii) an improvement in our gross margin (iii) leverage in our operating expenses and (iv) a reduction in outstanding shares of common stock due to stock repurchases.
Net Orders
Total Net Orders (by segment and region) | Fiscal Years | ||||||||||||||
| (Dollars in millions) | 2009 | % Change | 2008 | % Change | 2007 | ||||||||||
Oncology Systems: | |||||||||||||||
North America | $ | 949 | (7 | )% | $ | 1,020 | 13 | % | $ | 905 | |||||
Total International | 942 | 11 | % | 851 | 16 | % | 731 | ||||||||
Total Oncology Systems | $ | 1,891 | 1 | % | $ | 1,871 | 14 | % | $ | 1,636 | |||||
X-ray Products: | |||||||||||||||
North America | $ | 111 | (15 | )% | $ | 131 | 28 | % | $ | 102 | |||||
Total International | 228 | 11 | % | 206 | 21 | % | 171 | ||||||||
Total X-ray Products | $ | 339 | 1 | % | $ | 337 | 24 | % | $ | 273 | |||||
Other: | $ | 151 | 59 | % | $ | 94 | (7 | )% | $ | 101 | |||||
Total Net Orders | $ | 2,381 | 3 | % | $ | 2,302 | 15 | % | $ | 2,010 | |||||
Including an approximately $62 million order from Skandion Kliniken to deliver and install a proton therapy system, total net orders for fiscal year 2009 increased 3% over fiscal year 2008, with slight increases in Oncology Systems and X-ray Products net orders and a decline in SIP net orders. Total net orders grew in fiscal year 2008 from fiscal year 2007 primarily due to the net order growth in Oncology Systems and, to a lesser extent, net order growth in X-ray Products and SIP, partially offset by a decline in Varian Particle Therapy net orders.
Net order growth in Oncology Systems slowed to 1% in fiscal year 2009 over fiscal year 2008, compared to 14% growth in fiscal year 2008 over fiscal year 2007. For fiscal year 2009, the growth in Oncology Systems international net orders over fiscal year 2008 was significantly offset by the net order decrease in North America as this region was impacted by the recession, the uncertainty created by the prospects of healthcare reform and the added uncertainty in late fiscal year 2009 about the proposed reduction in Medicare reimbursement rates for radiotherapy and radiosurgery at free-standing clinics and for physician reimbursement for radiation oncology. In North America, Oncology Systems experienced net order declines in most of its product lines in fiscal year 2009 over fiscal year 2008 while this region continued to experience growth in demand for service contracts. All international regions contributed to the growth in international Oncology Systems net orders in fiscal year 2009 over fiscal year 2008. Growth in our service contracts, as well as growth in demand for our software products in all international regions and our high energy linear accelerators in Europe and Asia, contributed to the fiscal year 2009 growth in international Oncology Systems net orders over fiscal year 2008. The stronger U.S. dollar
71
Table of Contents
against foreign currencies in fiscal year 2009 compared to fiscal year 2008 negatively impacted Oncology Systems international net orders when measured in U.S. dollars. When measured in constant currency, international Oncology Systems net orders grew 16% in fiscal year 2009 over fiscal year 2008.
North American Oncology Systems net orders grew 13% in fiscal year 2008 over fiscal year 2007 primarily driven by the increase in demand for our software products (including our RapidArc products which was introduced in the second quarter of fiscal year 2008) and our high energy linear accelerators, as well as growth in demand for our service contracts. All international regions contributed to the 16% net order growth in the international region in fiscal year 2008 over fiscal year 2007. The increase in international net orders in fiscal year 2008 over the prior fiscal year was primarily due to growth in demand for our software products (including our RapidArc products which was introduced in the second quarter of fiscal year 2008) and our high energy linear accelerators, as well as growth in demand for our accessory products that enable IGRT (including our OBI). Growth in demand for our service contracts also contributed to Oncology Systems net order growth in the international region. In addition, international Oncology Systems net orders in fiscal year 2008 were favorably impacted by the weaker U.S. dollar against foreign currencies compared to most of fiscal year 2007. When measured in constant currency, the international Oncology Systems growth rate for fiscal year 2008 was 8%, with increases in all regions.
The trailing 12 months growth in net orders for Oncology Systems for the last three fiscal quarters were: an 8% total increase, with a 5% increase in North America and a 13% increase for international regions, as of July 3, 2009; a 12% total increase, with a 10% increase in North America and a 14% increase for international regions, as of April 3, 2009; and a 13% total increase, with a 12% increase in North America and a 14% increase for international regions, as of January 2, 2009. Consistent with the historical pattern, we expect that Oncology Systems net orders will continue to experience regional fluctuations.
Impacted by customer inventory reduction efforts in response to a slowdown in the imaging equipment market, X-ray Products saw net order declines in the second and third quarter of fiscal year 2009 versus the same periods in the previous year. However, this business segment reported 1% net order growth for the full fiscal year 2009 over fiscal year 2008, compared to 24% growth in fiscal year 2008 over fiscal year 2007. In fiscal year 2009, the increase in net orders over fiscal year 2008 for our flat panel detectors (including our radiographic imaging panels) was significantly offset by the decrease in net orders for x-ray tubes. The increase in X-ray Products net orders in fiscal year 2008 over fiscal year 2007 was primarily due to increased demand for our flat panel detectors and, to a lesser extent, increased demand for our x-ray tube products.
Net orders in the “Other” category, comprised of SIP, Varian Particle Therapy and GTC, increased significantly in fiscal year 2009 over fiscal year 2008 primarily due to a significant order in Varian Particle Therapy partially offset by a decrease in SIP net orders. In fiscal year 2009, Skandion Kliniken awarded us, in a competitive bidding situation, an approximately $62 million contract to deliver and install a proton therapy system in Sweden and we have included this contract in net orders. However, one of the unsuccessful bidders has filed a formal appeal of the award. Skandion Kliniken is actively opposing this appeal and we believe it will be resolved in Skandion Kliniken’s favor. Additionally, the order is contingent on Skandion Kliniken’s execution of a contract for the construction of the proton therapy facility, which we also believe will occur. The decline in SIP net orders in fiscal year 2009 over fiscal year 2008 is largely due to government postponements of several large proposals for port and border security systems. Net orders in the “Other” category decreased 7% in fiscal year 2008 over fiscal year 2007 primarily due to a decrease in net orders for Varian Particle Therapy services partially offset by an increase in SIP net orders.
As previously stated, orders for our SIP products may be unpredictable as governmental agencies may place large orders over a short period of time and then may not place any orders for a long time
72
Table of Contents
thereafter. However, as budgets are put on hold or reallocated during the economic downturn, customers have postponed their purchasing decisions. Also, proton therapy has not been widely deployed due to the cost of the technology and limited cost effectiveness. Due to the large scale of the related construction projects, the complexity of project financing and the resulting longer customer decision cycles when compared with our Oncology Systems business, we expect great variability in the demand for proton therapy products. This business may also be the most vulnerable to general economic turmoil and contraction in credit markets.
In any given period, orders growth in either North America or international regions, or both, could fluctuate because of the high dollar amount of individual orders. In addition, our net orders have been and may continue to be impacted by the current general economic downturn, which has shrunk capital equipment budgets, slowed decision-making, made financing for large equipment purchases more expensive and more time consuming, and made it difficult for our customers to accurately forecast and plan future business activities; as well as by uncertainty created by the prospects of healthcare reform in the United States. As the economy recovers and there is greater clarity on the impact of healthcare reform, we could experience a temporary increase in orders due to pent-up demand of customers, which in turn could increase the volatility of our orders and revenues. Orders in any quarter or period may not be directly correlated to the level of revenues in any particular future quarter or period since the timing of revenue recognition will vary significantly based on: the delivery requirements of individual orders, acceptance schedules; and the readiness of individual customer sites for installation of our products. Moreover, certain types of orders, such as software products or newly introduced products in our Oncology Systems segment, typically take more time from order to completion of installation and acceptance. Thus, as the overall mix of net orders includes a greater proportion of these types of products, the average time period within which orders convert into revenues could lengthen and our revenue in a specific period could be lower as a result.
Discontinued Operations
In the fourth quarter of fiscal year 2008, we approved a plan to sell Research Instruments to focus the business that we acquired from ACCEL exclusively on the development of our Varian Particle Therapy business. The sale of Research Instruments was completed in the second quarter of fiscal year 2009. Research Instruments has been classified as a discontinued operation and we have segregated the net assets and operating results of Research Instruments from continuing operations in our Consolidated Balance Sheets and in our Consolidated Statements of Earnings for all periods presented. Research Instruments was previously included in the “Other” category. For fiscal year 2009, revenues from Research Instruments were $9.8 million, compared to revenues of $35.2 million in fiscal year 2008 and $21.6 million in fiscal year 2007. Net loss from Research Instruments in fiscal year 2009 was $12.5 million (including a loss on the disposal of Research Instruments of $8.1 million), compared to a net loss of $15.8 million in fiscal year 2008 and a net loss of $3.4 million in fiscal year 2007. See Note 16, “Discontinued Operations” to the Consolidated Financial Statements for a detailed discussion.
Backlog
At October 2, 2009, including the $62 million proton therapy system order, our backlog was $2.1 billion, which is an increase of 9% over the backlog at September 26, 2008. With respect to our Oncology Systems segment, our backlog at October 2, 2009 increased 5% over the backlog at September 26, 2008, which reflects a 15% increase for the international regions and no increase for North America.
Liquidity and Capital Resources
Liquidity is the measurement of our ability to meet potential cash requirements, including ongoing commitments to repay borrowings, acquire businesses and fund continuing operations. Our sources of cash have included operations, borrowings, stock option exercises and employee stock purchases and
73
Table of Contents
interest income. Our cash usage is actively managed on a daily basis to ensure the maintenance of sufficient funds to meet our needs. Because Research Instruments’ cash flows were not material for any period presented, we have not segregated them from continuing operations on our Consolidated Statements of Cash Flows and the discussion herein.
Cash and Cash Equivalents
The following table summarizes our cash and cash equivalents:
| (In millions) | October 2, 2009 | September 26, 2008 | Increase | ||||||
Cash and cash equivalents | $ | 554 | $ | 397 | $ | 157 | |||
Our cash and cash equivalents increased $157 million from $397 million at September 26, 2008 to $554 million at October 2, 2009. The increase in cash and cash equivalents in fiscal year 2009 was due primarily to: $305 million of cash generated from operating activities, $28 million of cash provided by stock option exercises and employee stock purchases, $10 million of cash provided by the excess tax benefits from share-based compensation and $4 million of cash provided by net borrowings under our credit facilities. These increases were partially offset by $101 million used for the repurchase of VMS common stock, $63 million of capital expenditures, $8 million used for the repayment of bank borrowings, $6 million used for the net loan advance to dpiX LLC (“dpiX”), $3 million used for a business acquisition and $3 million used to satisfy employee tax withholding obligations upon vesting of our restricted common stock. In addition, exchange rate changes in fiscal year 2009 increased cash and cash equivalents by $1 million.
At October 2, 2009, we had approximately $92 million or 17%, of total cash and cash equivalents in the United States. Approximately $462 million, or 83%, of total cash and cash equivalents was held abroad and could be subject to additional taxation if it were repatriated to the United States. As of October 2, 2009, most of our cash and cash equivalents that were held abroad were in U.S. dollars. Because our cash levels in the United States are relatively low, we have used our credit facilities to meet our cash needs from time to time and expect to continue to do so in the future. Borrowings under our credit facilities may be used for working capital, capital expenditures, acquisitions and other corporate purposes.
Cash Flows
| Fiscal Years | ||||||||||||
| (In millions) | 2009 | 2008 | 2007 | |||||||||
Net cash flow provided by (used in): | ||||||||||||
Operating activities | $ | 305 | $ | 372 | $ | 300 | ||||||
Investing activities | (78 | ) | (88 | ) | (56 | ) | ||||||
Financing activities | (71 | ) | (142 | ) | (240 | ) | ||||||
Effects of exchange rate changes on cash and cash equivalents | 1 | (8 | ) | (13 | ) | |||||||
Net increase (decrease) in cash and cash equivalents | $ | 157 | $ | 134 | $ | (9 | ) | |||||
Our primary cash inflows and outflows for fiscal years 2009, 2008 and 2007 were as follows:
| · | We generated net cash from operating activities of $305 million in fiscal year 2009, compared to $372 million and $300 million in fiscal years 2008 and 2007, respectively. |
The $67 million decrease in net cash from operating activities during fiscal year 2009 compared to fiscal year 2008 was driven primarily by a net change of $93 million in operating assets and liabilities (working capital items) and a decrease in non-cash items of $14 million, partially offset by an increase of $40 million in net earnings.
74
Table of Contents
The major contributors to the net change in working capital items in fiscal year 2009 were accounts receivable, inventories, other long-term liabilities, accrued expenses and advance payments from customers as follows:
| ¡ | Accounts receivable increased $86 million due to higher revenues and an increase in DSO from the end of fiscal year 2008. |
| ¡ | Inventories increased by $40 million due to anticipated customer demands for products in fiscal year 2010 in all of our businesses. |
| ¡ | Other long-term liabilities decreased by $22 million primarily due to a decrease in long-term income taxes payable as a result of the expiration of the statutes of limitation in various jurisdictions and the favorable resolution of several income tax audits. |
| ¡ | Accrued expenses increased $47 million primarily due to an increase in income taxes payable. |
| ¡ | Advance payments from customers increased by $22 million due to an increase in volume of our Oncology Systems’ service contracts. |
The $72 million increase in net cash from operating activities during fiscal year 2008 compared to fiscal year 2007 was primarily driven by an increase of $40 million in net earnings, a net change of $22 million in operating assets and liabilities (working capital items) and an increase in non-cash items of $10 million.
The major contributors to the net change in working capital items in fiscal year 2008 were deferred revenues, advance payments from customers, accounts receivable, inventories and prepaid expenses and other current assets as follows:
| ¡ | Deferred revenues increased by $40 million primarily due to timing of revenue recognized based on customer acceptance of our Oncology Systems products and the increase in Oncology Systems product revenues. |
| ¡ | Advance payments from customers increased $23 million due to increased orders. |
| ¡ | Accounts receivables decreased by $22 million due to strong collection performance in fiscal year 2008. |
| ¡ | Inventories increased by $56 million due to anticipated customer demands for products in fiscal year 2009 in all of our businesses. |
| ¡ | Prepaid expenses and other current assets increased by $37 million primarily due to estimated tax payments made during fiscal year 2008 and the overall growth of our business operations. |
We expect that cash provided by operating activities may fluctuate in future periods as a result of a number of factors, including fluctuations in our operating results, timing of product shipments and customer acceptance, accounts receivable collections, inventory management, and the timing and amount of tax and other payments. For additional discussion, please refer to the “Risk Factors” in Item 1A.
| · | Investing activities used $78 million of net cash in fiscal year 2009, $88 million in fiscal year 2008 and $56 million in fiscal year 2007. Cash used for purchases of property, plant and equipment was $63 million in fiscal year 2009, compared to $81 million and $64 million in fiscal years 2008 and 2007, respectively. In fiscal year 2009, we made an additional net loan advance of $6 million to dpiX. In fiscal year 2008, we also invested $8 million in a privately held company. In fiscal year 2007, we used cash of $27 million to acquire ACCEL and $21 million to acquire BIR. We also made a $4 million “earn-out” payment to Mitsubishi Electric Co. (“MELCO”) in fiscal year 2007. In fiscal years 2007, we invested $25 million in dpiX Holding for the construction of a manufacturing facility in Colorado. Our net proceeds from maturities of marketable securities were $94 million during fiscal year 2007. We did not hold any marketable securities in fiscal years 2008 and 2009. |
75
Table of Contents
| · | Financing activities used net cash of $71 million in fiscal year 2009 compared to $142 million and $240 million in fiscal years 2008 and 2007, respectively. In fiscal year 2009, we used $101 million for the repurchases of common stock, compared to $262 million in fiscal year 2008 and $319 million in fiscal year 2007. In fiscal years 2009, 2008 and 2007, we used $8 million, $9 million and $15 million, respectively, to repay bank borrowings. We also used $41 million to repay borrowings under our credit facilities in fiscal year 2008. In fiscal year 2007, we also used $12 million to repurchase the 35% ownership interest in our Japanese subsidiary from MELCO. Cash used for financing activities in fiscal years 2009 and 2008 also includes $3 million and $1 million (the value of withheld shares), respectively, to satisfy employee tax withholding obligations when employees tendered VMS common stock in payment when their restricted common stock vested. These uses were partially offset by cash proceeds from employee stock option exercises and employee stock purchases of $28 million, $129 million and $45 million in fiscal years 2009, 2008 and 2007 respectively, as well as cash provided by excess tax benefits from share-based compensation of $10 million in fiscal year 2009, $42 million in fiscal year 2008 and $20 million in fiscal year 2007. In fiscal years 2009 and 2007, we also borrowed $4 million and $41 million, respectively, in net cash from our credit facilities. |
We expect our capital expenditures, which typically represent construction and/or purchases of facilities, manufacturing equipment, office equipment and furniture and fixtures, as well as capitalized costs related to the implementation of software applications, will be approximately 3.8% of revenues in fiscal year 2010.
We have a $150 million credit facility with Bank of America, N.A. (“BofA”), which was amended and restated in November 2008 and in July 2009. This credit facility, as amended and restated, is referred to as the “Amended BofA Credit Facility”. The July 2009 amendment to the Amended BofA Credit Facility (the “Japanese Line of Credit”) enabled VMS’s Japanese subsidiary (“VMS KK”) to borrow up to 2.7 billion Japanese Yen. At any time amounts are outstanding under the Japanese Line of Credit, the full borrowing capacity is deemed committed for use in Japan and therefore the maximum amount VMS can otherwise borrow under the Amended BofA Credit Facility will be reduced by $30 million to $120 million. VMS guarantees the payment of the outstanding balance under the Japanese Line of Credit. We collateralized a portion of the Amended BofA Credit Facility with a pledge of stock of certain present and future subsidiaries that are deemed to be material subsidiaries under its terms. As of October 2, 2009, we have pledged to BofA 65% of the voting shares that we hold in Varian Medical Systems Nederland B.V., a wholly-owned subsidiary.
The Amended BofA Credit Facility may be used for: working capital; capital expenditures; permitted acquisitions; and other lawful corporate purposes. Borrowings under the Japanese Line of Credit can be used by VMS KK for refinancing certain intercompany debts, working capital, capital expenditures and other lawful corporate purposes. Borrowings under the Amended BofA Credit Facility (outside of the Japanese Line of Credit) accrue interest either: (i) based on LIBOR plus a margin of 1.25% to 1.50% based on a leverage ratio involving funded indebtedness and earnings before interest, taxes, depreciation and amortization (“EBITDA”); or (ii) based upon a base rate of either the federal funds rate plus 0.5% or BofA’s announced prime rate, whichever is greater, minus a margin of 0.5% to 0% based on a leverage ratio involving funded indebtedness and EBITDA (depending upon our instructions to BofA). We may select borrowing periods of one, two, three or six months for advances based on the LIBOR rate. Interest rates on advances based on the base rate are adjustable daily. Borrowings under the Japanese Line of Credit accrue interest at the basic loan rate announced by the Bank of Japan plus a margin of 1.25% to 1.50% based on a leverage ratio involving funded indebtedness and EBITDA. The Amended BofA Credit Facility will expire, if not extended by mutual agreement of VMS and BofA, on November 10, 2011. The Japanese Line of Credit will expire on November 10, 2010.
As of October 2, 2009, there was no outstanding balance under the Amended BofA Credit Facility other than $4.4 million outstanding under the Japanese Line of Credit with a weighted average interest rate of
76
Table of Contents
1.55%. The Amended BofA Credit Facility contains customary affirmative and negative covenants for facilities of this type. We have also agreed to maintain certain financial covenants relating to: (i) leverage ratios involving funded indebtedness and EBITDA; (ii) liquidity; and (iii) consolidated assets. As of October 2, 2009, we were in compliance with all covenants. For further discussion regarding the credit facilities, please refer to Note 7 “Credit Facility” to the Consolidated Financial Statements.
Our liquidity is affected by many factors, some of which result from the normal ongoing operations of our business and some of which arise from uncertainties and conditions in the United States and global economies. Although our cash requirements will fluctuate as a result of the shifting influences of these factors, we believe that existing cash and cash equivalents and cash to be generated from operations and current or future credit facilities will be sufficient to satisfy anticipated commitments for capital expenditures and other cash requirements for the next 12 months. We currently anticipate that we will continue to utilize our available liquidity and cash flows from operations, as well as borrowed funds, to make strategic acquisitions, invest in the growth of our business, invest in advancing our systems and processes and repurchase VMS common stock.
Total debt as a percentage of total capital decreased to 2.7% at October 2, 2009 from 3.8% at September 26, 2008. The ratio of current assets to current liabilities increased to 1.99 to 1 at October 2, 2009 from 1.81 to 1 at September 26, 2008.
Days Sales Outstanding
Trade accounts receivable DSO were 81 days at October 2, 2009 compared to 74 days at September 26, 2008. Our accounts receivable and DSO are primarily impacted by a number of factors: the timing of product shipments; collections performance; payment terms; and the mix of revenues from different regions. As of October 2, 2009, less than 1% of our accounts receivable balance was related to customer contracts with extended payment terms of more than one year.
Stock Repurchase Program
During fiscal years 2009, 2008 and 2007, we paid $101 million, $262 million and $319 million, respectively, to repurchase 2,248,000 shares, 5,110,000 shares and 7,000,000 shares, respectively, of VMS common stock under various authorizations by our Board of Directors. All shares that have been repurchased have been retired. As of October 2, 2009, 7,300,000 shares of VMS common stock remained available for repurchase under an authorization that expires on December 31, 2009. On November 13, 2009, our Board of Directors authorized the repurchase of an additional 5,000,000 shares of VMS common stock from January 1, 2010 through December 31, 2010.
Contractual Obligations
The following summarizes our contractual obligations as of October 2, 2009 and the effect such obligations are expected to have on our liquidity and cash flows in future periods:
| Payments Due By Period | |||||||||||||||
| (In millions) | Fiscal Year 2010 | Fiscal Years 2011 - 2012 | Fiscal Years 2013 - 2014 | Beyond | Total | ||||||||||
Short-term borrowings(1) | $ | 4.4 | $ | — | $ | — | $ | — | $ | 4.4 | |||||
Long term debt(2) | 9.0 | 17.1 | 6.3 | — | 32.4 | ||||||||||
Interest obligation on long term debt | 2.1 | 2.4 | 0.7 | — | 5.2 | ||||||||||
Operating Leases(3) | 15.6 | 17.9 | 9.2 | 4.7 | 47.4 | ||||||||||
Defined benefit pension plans(4) | 4.2 | — | — | — | 4.2 | ||||||||||
Post-retirement benefit plan(5) | 0.5 | 1.1 | 1.2 | 2.7 | 5.5 | ||||||||||
Other liabilities(6) | 16.0 | — | — | — | 16.0 | ||||||||||
Loan agreement with dpiX(7) | 7.2 | — | — | — | 7.2 | ||||||||||
Total(8) | $ | 59.0 | $ | 38.5 | $ | 17.4 | $ | 7.4 | $ | 122.3 | |||||
77
Table of Contents
| (1) | Short-term borrowings were outstanding under the Japanese Line of Credit of the Amended BofA Credit Facility with a weighted average interest rate of 1.55%. See a detailed discussion of our credit facilities in Note 7, “Credit Facilities” to the Consolidated Financial Statements. |
| (2) | Long-term debt, including current maturities, decreased $8 million from September 26, 2008 due to principal repayments. The fixed interest rates on the outstanding debt on this date ranged from 6.70% to 7.58% with a weighted average interest rate of 6.88%. As of October 2, 2009, land and buildings with a carrying amount of $13.7 million were pledged as collateral against certain loans we assumed related to purchases of land and buildings in Las Vegas. For further discussion regarding long-term debt, see Note 6, “Long-term Debt” to the Consolidated Financial Statements. |
| (3) | Operating leases include future minimum lease payments under all our noncancelable operating leases as of October 2, 2009. |
| (4) | As further described in Note 10, “Retirement Plans” to the Consolidated Financial Statements, as of October 2, 2009, the Company’s defined benefit pension plans were underfunded by $21.2 million. Due to the impact of future plan asset performance, changes in interest rates and other economic and demographic assumptions the potential for changes in legislation in the United States and other foreign jurisdictions, the Company is not able to reasonably estimate the timing and amount of contributions to fund its defined benefit pension plans beyond the next fiscal year. |
| (5) | As further described in Note 10, “Retirement Plans” to the Consolidated Financial Statements, as of October 2, 2009, the Company’s post-retirement benefit plan had an estimated total benefit obligation of $6.2 million. Due to changes in health care cost trend rates, mortality rates of plan participants, and the potential for the Company to change the type of health care plans offered or the level of contributions from plan participants, the Company is not able to reasonably estimate the timing and amount of contributions to fund its post-retirement benefit plan beyond fiscal year 2019. |
| (6) | In October 2008, we consummated an agreement with VI under which VI will surrender its sublease of a building containing approximately 210,000 square feet of floor space and the related leasehold interest for the land and we agreed to pay VI an aggregate of $21 million in cash and assume the obligations of sublessor under a below-market rate sublease to a third party for a portion of the building. As of October 2, 2009, $5 million had been paid to VI pursuant to our agreement, and the remaining $16 million will be payable in June 2010. |
| (7) | In February 2009, we agreed to loan $14 million to dpiX in four separate installments over a period through December 2009. As of October, 2009, we had loaned $6.8 million to dpiX under this loan agreement and we expect to loan the remaining $7.2 million through December 2009. See detailed discussion in Note 5, “Related Party Transactions” to the Consolidated Financial Statements. |
| (8) | Long-term income taxes payable includes the liability for uncertain tax positions, including interest and penalties, and may also include other long-term tax liabilities. As of October 2, 2009, our liability for uncertain tax positions was $67.8 million and we do not anticipate payment of these amounts in the next 12 months. We are unable to reliably estimate the timing of future payments related to uncertain tax positions; therefore, the liability for uncertain tax positions has been excluded from the table above. See a detailed discussion in Note 13, “Taxes on Earnings” to the Consolidated Financial Statements. |
Contingencies
Environmental Remediation Liabilities
For a discussion of environmental remediation liabilities, see Note 9, “Commitments and Contingencies—Environmental Remediation Liabilities” to the Consolidated Financial Statements, which discussion is incorporated herein by reference.
78
Table of Contents
Acquisition-Related Commitments/Obligations
When we acquired ACCEL in January 2007, the company was involved in a contract-related lawsuit. Subsequent to the acquisition, we settled this lawsuit and agreed to perform certain services under a new contract for a fixed price. From January to September 2007, we gathered information related to the expected cost of satisfying our contractual commitments and completed our assessment as of September 28, 2007. As a result, the final purchase price allocation of ACCEL included a loss accrual related to this contingency of €28.3 million. In the third quarter of fiscal year 2009, we increased the cost estimate to complete our contractual commitments by €4.9 million, which was recorded in the Consolidated Statement of Earnings. If the actual costs related to the contingency exceed the estimated amount, or if the estimated loss increases, the variances will be recognized in the Consolidated Statement of Earnings in the periods these variances arise. As of October 2, 2009, the balance of the loss accrual related to this contingency was €7.6 million.
Other Matters
We are involved, from time to time, in legal proceedings, claims and government inspections or investigations, arising in the ordinary course of our business. Such matters are subject to many uncertainties and outcomes are not predictable with assurance. We accrue amounts, to the extent they can be reasonably estimated, that we believe are adequate to address any liabilities related to legal proceedings and other loss contingencies that we believe will result in a probable loss. While we cannot assure you as to the ultimate outcome of any legal proceeding or other loss contingency involving us, management does not believe any pending matter will be resolved in a manner that would have a material adverse effect on our consolidated financial position, results of operations or cash flows. However, it is possible that a legal or other proceeding brought against us could have an impact of this nature.
Off-Balance Sheet Arrangements
In conjunction with the sale of our products in the ordinary course of business, we provide standard indemnification of business partners and customers for losses suffered or incurred for property damages, death and injury and for patent, copyright or any other intellectual property infringement claims by any third parties with respect to our products. The term of these indemnification arrangements is generally perpetual. Except for losses related to property damages, the maximum potential amount of future payments we could be required to make under these agreements is unlimited. As of October 2, 2009, we have not incurred any significant costs to defend lawsuits or settle claims related to these indemnification arrangements since we spun off VI and VSEA in 1999.
We have entered into indemnification agreements with our directors and officers and certain of our employees that serve as officers or directors of our foreign subsidiaries that may require us to indemnify our directors and officers and those certain employees against liabilities that may arise by reason of their status or service as directors or officers, and to advance their expenses incurred as a result of any legal proceeding against them as to which they could be indemnified. Generally, the maximum obligation under these indemnifications is not explicitly stated and, as a result, the overall amount of these obligations cannot be reasonably estimated.
Recent Accounting Pronouncements
On July 1, 2009, the Financial Accounting Standards Board (“FASB”) released the authoritative version of its new ASC as the single source for GAAP, which replaces all previous GAAP accounting standards. While not intended to change GAAP, ASC significantly changes the way in which the accounting literature is organized. In the fourth quarter of fiscal year 2009, we adopted ASC to reference GAAP accounting standards in our consolidated financial statements.
79
Table of Contents
In December 2007, the FASB issued new accounting standards for business combinations under ASC 805, which establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, any noncontrolling interest in the acquiree and the goodwill acquired. The new standards also establish disclosure requirements to enable the evaluation of the nature and financial effects of the business combination. In April 2009, the FASB issued additional standards under ASC 805-20 to clarify initial recognition and measurement, subsequent measurement and accounting, and disclosure of assets and liabilities arising from contingencies in a business combination. The new standards related to business combinations under ASC 805 will be effective for us in the first quarter of fiscal year 2010. The impact of the adoption of these new standards will depend on the nature and extent of business combinations occurring on or after the beginning of fiscal year 2010.
In December 2007, the FASB established new accounting and reporting standards under ASC 810 for ownership interests in subsidiaries held by parties other than the parent, the amount of consolidated net income attributable to the parent and to the noncontrolling interest, changes in a parent’s ownership interest, and the valuation of retained noncontrolling equity investments when a subsidiary is deconsolidated. The new accounting standards under ASC 810 also establish disclosure requirements that clearly identify and distinguish between the interests of the parent and the interests of the noncontrolling owners. The new standards under ASC 810 will be effective for us in the first quarter of fiscal year 2010. We do not expect that the adoption of these new standards under ASC 810 will have a material impact on our consolidated financial position, results of operations or cash flows.
In November 2008, the FASB ratified an Emerging Issues Task Force (“EITF”) Issue, which clarifies the accounting for certain transactions and impairment considerations involving equity method investments. The new standards, which will be included in ASC 323-10, will be effective for us in the first quarter of fiscal year 2010, with early adoption prohibited. We do not expect that the adoption of this new standard will have a material impact on our consolidated financial position, results of operations and cash flows.
In December 2008, the FASB issued new standards under ASC 715-20, which provides guidance on an employer’s disclosure about plan assets of a defined benefit pension or other post-retirement plan and requires employers to disclose information about fair value measurements of plan assets. The new standards under ASC 715-20 will be effective for us as of the end of fiscal year 2010.
In June 2009, the FASB issued the consolidation guidance for variable-interest entities to replace the quantitative-based risks and rewards calculation for determining which enterprise, if any, has a controlling financial interest in a variable interest entity with an approach focused on identifying which enterprise has the power to direct the activities of a variable interest entity that most significantly impact the entity’s economic performance. These new standards will be effective for us in the first quarter of fiscal year 2011. We are currently assessing the potential impact, if any, these new standards may have on our consolidated financial position, results of operations and cash flows.
In August 2009, the FASB issued an update to ASC 820. This Accounting Standards Update (“ASU”) No. 2009-5,Measuring Liabilities at Fair Value (“ASU 2009-5”) amends the provisions in ASC 820 related to the fair value measurement of liabilities and clarifies for circumstances in which a quoted price in an active market for the identical liability is not available. ASU 2009-5 is intended to reduce potential ambiguity in financial reporting when measuring the fair value of liabilities. ASU 2009-5 is effective for us in the first quarter of fiscal year 2010. ASU 2009-5 concerns disclosure only and will not have an impact on our consolidated financial position or results of operations.
In October 2009, the FASB issued an update to ASC 605. This ASU No. 2009-13,Multiple Deliverable Revenue Arrangements (“ASU 2009-13”) provides guidance on whether multiple deliverables in a revenue arrangement exist, how the arrangement should be separated, and the consideration allocated. ASU 2009-13 eliminates the requirement to establish the fair value of undelivered products and services and instead provides for separate revenue recognition based upon management’s estimate of the selling
80
Table of Contents
price for an undelivered item when there is no other means to determine the fair value of that undelivered item. ASU 2009-13 is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted if we elect to adopt ASU No. 2009-14,Certain Revenue Arrangements That Include Software Elements(“ASU 2009-14”) concurrently. We are currently evaluating the potential impact of ASU 2009-13 on our consolidated financial position, results of operations and cash flows.
In October 2009, the FASB issued an update to ASC 985-605. This ASU 2009-14, amends the scope of the software revenue guidance in ASC 985-605 to exclude tangible products containing software components and non-software components that function together to deliver the tangible product’s essential functionality. ASU 2009-14 is effectively prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted if we elect to adopt ASU 2009-13 concurrently. We are currently evaluating the potential impact of ASU 2009-14 on our consolidated financial position, results of operations and cash flows.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
We are exposed to three primary types of market risks: credit risk, foreign currency exchange rate risk and interest rate risk.
Credit Risk
There has been significant deterioration and instability in the financial markets beginning in fiscal year 2008. This period of extraordinary disruption and readjustment in the financial markets exposes us to additional credit risk. We are exposed to credit loss in the event of nonperformance by counterparties on the foreign exchange contracts used in hedging activities. These counterparties are large international financial institutions and to date, no such counterparty has failed to meet its financial obligation under such contracts. In addition, cash and cash equivalents held with financial institutions may exceed the Federal Deposit Insurance Corporation insurance limits or similar limits in foreign jurisdictions. We also have the credit facility described below. Our access to our cash and cash equivalents or ability to borrow could be reduced if one or more financial institutions with which we have deposits or from which we borrow should fail or otherwise be adversely impacted by conditions in the financial or credit markets.
Foreign Currency Exchange Rate Risk
As a global entity, we are exposed to movements in foreign currency exchange rates. These exposures may change over time as business practices evolve. Adverse movements could have a material negative impact on our financial results. Our primary exposures related to foreign currency denominated sales and purchases are in Europe, Asia, Australia and Canada.
We have many transactions denominated in foreign currencies and address certain of those financial exposures through a program of risk management that includes the use of derivative financial instruments. We sell products throughout the world, often in the currency of the customer’s country, and typically hedge certain of these larger foreign currency transactions when they are not in the subsidiaries’ functional currency. The foreign currency sales transactions that fit our risk management policy criteria are hedged with forward contracts. We may use other derivative instruments in the future. We enter into foreign currency forward contracts primarily to reduce the effects of fluctuating foreign currency exchange rates. We do not enter into forward contracts for speculative or trading purposes. The forward contracts range from one to twelve months in maturity.
We also hedge the balance sheet exposures from our various foreign subsidiaries and business units. We enter into foreign currency forward contracts to minimize the short-term impact of currency fluctuations on assets and liabilities denominated in currencies other than the U.S. dollar functional currency.
81
Table of Contents
The notional amounts of forward contracts are not a measure of our exposure. The fair value of forward contracts generally reflects the estimated amounts that we would receive or pay to terminate the contracts at the reporting date, thereby taking into account and approximating the current unrealized and realized gains or losses of the open contracts. A move in foreign currency exchange rates would change the fair value of the contracts, and the fair value of the underlying exposures hedged by the contracts would change in a similar offsetting manner.
The notional values and the weighted average contractual foreign currency exchange rates of our sold and purchased forward exchange contracts outstanding at October 2, 2009 were as follows:
| (In millions) | Notional Value Sold | Notional Value Purchased | Weighted Average Contract Rate (Foreign Currency Units per USD) | |||||
Australian dollar | $ | 14.8 | $ | — | 1.1594 | |||
British pound | — | 8.9 | 0.6295 | |||||
Canadian dollar | 6.2 | — | 1.0855 | |||||
Danish krone | — | 3.0 | 5.1053 | |||||
Euro | 136.1 | 0.3 | 0.6856 | |||||
Indian rupee | 2.1 | — | 47.9500 | |||||
Japanese yen | 15.8 | — | 89.6057 | |||||
New Zealand dollar | 0.4 | — | 1.3943 | |||||
Norwegian kron | 0.3 | — | 5.8117 | |||||
Swedish krona | 3.6 | 3.9 | 7.0336 | |||||
Swiss franc | — | 33.9 | 1.0343 | |||||
Totals | $ | 179.3 | $ | 50.0 | ||||
Interest Rate Risk
Our market risk exposure to changes in interest rates depends primarily on our investment portfolio and short-term borrowings. Our investment portfolio consisted of cash and cash equivalents as of October 2, 2009. The principal amount of cash and cash equivalents at October 2, 2009 totaled $554 million with a weighted average interest rate of 0.16%.
The Amended BofA Credit Facility (including the Japanese Line of Credit) allows us to borrow up to a maximum amount of $150 million. We collateralized a portion of the Amended BofA Credit Facility with a pledge of 65% of the voting shares that we hold in Varian Medical Systems Nederland B.V., a wholly-owned subsidiary. Borrowings under the Amended BofA Credit Facility (outside of the Japanese Line of Credit) accrue interest based on the LIBOR, the federal funds rate, or the BofA’s prime rate plus a margin. Borrowings under the Japanese Line of Credit accrue interest at the basic loan rate announced by the Bank of Japan plus a margin.
We are affected by market risk exposure primarily through the effect of changes in interest rates on amounts payable under the Amended BofA Credit Facility (including the Japanese Line of Credit). As of October 2, 2009, the amount outstanding under the Amended BofA Credit Facility was the $4.4 million in principal under the Japanese Line of Credit, with interest being accrued on the basic loan rate plus a margin. If the amount outstanding under the Japanese Line of Credit remained at this level for an entire year and the basic loan rate increased or decreased, respectively, by 1%, our interest expense would increase or decrease, respectively, by an additional $44,000. See a detailed discussion of our credit facility under “Liquidity and Capital Resources” section in Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
In addition, we had $32.4 million of long-term debt (including the current maturities of long term debt) outstanding at October 2, 2009 that carried at a weighted average fixed interest rate of 6.9% with
82
Table of Contents
principal payments due in various installments over a five-year period. To date, we have not used derivative financial instruments to hedge the interest rate of our investment portfolio, short-term borrowings or long-term debt, but may consider the use of derivative instruments in the future.
The table below presents principal amounts and related weighted average interest rates by year for our cash and cash equivalents, short-term borrowings and long term debt.
| Fiscal Years | ||||||||||||||||||||||||||
| (Dollars in millions) | 2010 | 2011 | 2012 | 2013 | 2014 | Thereafter | Total | |||||||||||||||||||
Assets: | ||||||||||||||||||||||||||
Cash and cash equivalents | $ | 553.5 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 553.5 | ||||||||||||
Average interest rate | 0.16 | % | — | — | — | — | — | 0.16 | % | |||||||||||||||||
Liabilities: | ||||||||||||||||||||||||||
Long-term debt | $ | 9.0 | $ | 5.5 | $ | 11.6 | $ | — | $ | 6.3 | $ | — | $ | 32.4 | ||||||||||||
Average interest rate | 6.85 | % | 6.80 | % | 7.03 | % | — | 6.70 | % | — | 6.88 | % | ||||||||||||||
Short-term borrowing under credit facilities | $ | 4.4 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 4.4 | ||||||||||||
Average interest rate | 1.55 | % | — | — | — | — | — | 1.55 | % | |||||||||||||||||
The estimated fair value of our cash and cash equivalents (83% of which was held abroad at October 2, 2009 and could be subject to additional taxation if it were repatriated to the United States) and the estimated fair value of our short-term borrowings under the credit facility approximated the principal amounts reflected above based on the maturities of these financial instruments.
The fair value of our long-term debt was estimated based on the current rates available to us for debt of similar terms and remaining maturities. Under this method, the fair value of our debt was estimated to be $34.8 million at October 2, 2009. We determined the estimated fair value amount by using available market information and commonly accepted valuation methodologies. However, considerable judgment is required in interpreting market data to develop estimates of fair value. Accordingly, the fair value estimate presented herein is not necessarily indicative of the amount that we or holders of the instruments could realize in a current market exchange. The use of different assumptions and/or estimation methodologies may have a material effect on the estimated fair value.
Although payments under certain of our operating leases for our facilities are tied to market indices, these operating leases do not expose us to material interest rate risk.
83
Table of Contents
Item 8. Financial Statements and Supplementary Data
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EARNINGS
| Fiscal Years Ended | ||||||||||||
| (In thousands, except per share amounts) | 2009 | 2008 | 2007 | |||||||||
Revenues: | ||||||||||||
Product | $ | 1,766,929 | $ | 1,689,724 | $ | 1,447,746 | ||||||
Service contracts and other | 447,131 | 380,006 | 307,326 | |||||||||
Total revenues | 2,214,060 | 2,069,730 | 1,755,072 | |||||||||
Cost of revenues: | ||||||||||||
Product | 1,013,973 | 985,133 | 852,980 | |||||||||
Service contracts and other | 239,582 | 207,065 | 169,229 | |||||||||
Total cost of revenues | 1,253,555 | 1,192,198 | 1,022,209 | |||||||||
Gross margin | 960,505 | 877,532 | 732,863 | |||||||||
Operating expenses: | ||||||||||||
Research and development | 147,375 | 135,599 | 117,320 | |||||||||
Selling, general and administrative | 338,984 | 322,529 | 276,918 | |||||||||
Total operating expenses | 486,359 | 458,128 | 394,238 | |||||||||
Operating earnings | 474,146 | 419,404 | 338,625 | |||||||||
Interest income | 4,594 | 11,498 | 12,165 | |||||||||
Interest expense | (4,097 | ) | (4,879 | ) | (4,791 | ) | ||||||
Earnings from continuing operations before taxes | 474,643 | 426,023 | 345,999 | |||||||||
Taxes on earnings | 143,167 | 130,767 | 103,083 | |||||||||
Earnings from continuing operations | 331,476 | 295,256 | 242,916 | |||||||||
Loss from discontinued operations, net of taxes | (12,454 | ) | (15,772 | ) | (3,460 | ) | ||||||
Net Earnings | $ | 319,022 | $ | 279,484 | $ | 239,456 | ||||||
Net earnings (loss) per share—basic: | ||||||||||||
Continuing operations | $ | 2.67 | $ | 2.37 | $ | 1.91 | ||||||
Discontinued operations | (0.10 | ) | (0.13 | ) | (0.03 | ) | ||||||
Net earnings per share | $ | 2.57 | $ | 2.24 | $ | 1.88 | ||||||
Net earnings (loss) per share—diluted: | ||||||||||||
Continuing operations | $ | 2.65 | $ | 2.31 | $ | 1.86 | ||||||
Discontinued operations | (0.10 | ) | (0.12 | ) | (0.03 | ) | ||||||
Net earnings per share | $ | 2.55 | $ | 2.19 | $ | 1.83 | ||||||
Shares used in the calculation of net earnings per share: | ||||||||||||
Weighted average shares outstanding—Basic | 124,034 | 124,800 | 127,407 | |||||||||
Weighted average shares outstanding—Diluted | 124,995 | 127,604 | 130,622 | |||||||||
See accompanying notes to the consolidated financial statements.
84
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| (In thousands, except par values) | October 2, 2009 | September 26, 2008 | ||||||
| Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 553,529 | $ | 397,306 | ||||
Accounts receivable, net of allowance for doubtful accounts of $4,347 at October 2, 2009 and $3,110 at September 26, 2008 | 580,918 | 486,310 | ||||||
Inventories | 321,861 | 282,980 | ||||||
Prepaid expenses and other current assets | 71,751 | 78,018 | ||||||
Deferred tax assets | 144,392 | 130,988 | ||||||
Current assets of discontinued operations | — | 18,799 | ||||||
Total current assets | 1,672,451 | 1,394,401 | ||||||
Property, plant and equipment, net | 264,060 | 218,183 | ||||||
Goodwill | 210,346 | 209,146 | ||||||
Other assets | 161,391 | 150,694 | ||||||
Long-term assets of discontinued operations | — | 3,088 | ||||||
Total assets | $ | 2,308,248 | $ | 1,975,512 | ||||
| Liabilities and Stockholders’ Equity | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 116,093 | $ | 105,281 | ||||
Accrued expenses | 304,402 | 252,915 | ||||||
Product warranty | 50,823 | 51,141 | ||||||
Deferred revenues | 130,588 | 141,368 | ||||||
Advance payments from customers | 226,964 | 201,783 | ||||||
Short-term borrowings | 4,445 | — | ||||||
Current maturities of long-term debt | 9,005 | 7,987 | ||||||
Current liabilities of discontinued operations | — | 21,202 | ||||||
Total current liabilities | 842,320 | 781,677 | ||||||
Long-term debt | 23,394 | 32,399 | ||||||
Other long-term liabilities | 130,751 | 134,251 | ||||||
Total liabilities | 996,465 | 948,327 | ||||||
Commitments and contingencies (Note 9) | ||||||||
Stockholders’ equity: | ||||||||
Preferred stock of $1 par value: 1,000 shares authorized; none issued and outstanding | — | — | ||||||
Common stock of $1 par value: 189,000 shares authorized; 125,281 and 125,590 shares issued and outstanding at October 2, 2009 and at September 26, 2008, respectively | 125,281 | 125,590 | ||||||
Capital in excess of par value | 516,478 | 468,384 | ||||||
Retained earnings | 696,409 | 451,439 | ||||||
Accumulated other comprehensive loss | (26,385 | ) | (18,228 | ) | ||||
Total stockholders’ equity | 1,311,783 | 1,027,185 | ||||||
Total liabilities and stockholders’ equity | $ | 2,308,248 | $ | 1,975,512 | ||||
See accompanying notes to the consolidated financial statements.
85
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Fiscal Years Ended | ||||||||||||
| (In thousands) | 2009 | 2008 | 2007 | |||||||||
Cash flows from operating activities: | ||||||||||||
Net earnings | $ | 319,022 | $ | 279,484 | $ | 239,456 | ||||||
Adjustments to reconcile net earnings to net cash provided by operating activities: | ||||||||||||
Share-based compensation expense | 42,577 | 40,994 | 44,882 | |||||||||
Tax benefits from exercises of share-based payment awards | 8,270 | 45,656 | 21,144 | |||||||||
Excess tax benefits from share-based compensation | (9,639 | ) | (42,020 | ) | (19,678 | ) | ||||||
Depreciation | 41,008 | 32,247 | 26,957 | |||||||||
Amortization of intangible assets | 3,601 | 4,462 | 5,249 | |||||||||
Deferred taxes | (22,008 | ) | 3,097 | 2,609 | ||||||||
Provision for doubtful accounts receivable | 2,038 | 250 | 1,086 | |||||||||
Net change in fair value of derivatives and underlying commitments | 1,920 | 2,200 | (3,509 | ) | ||||||||
(Income) loss on equity investment in affiliate | 905 | (286 | ) | 301 | ||||||||
Impairment loss on long-lived assets and goodwill | — | 3,324 | — | |||||||||
Loss on sale of Research Instruments | 8,062 | — | — | |||||||||
Other | (3,334 | ) | (2,391 | ) | (1,180 | ) | ||||||
Changes in assets and liabilities: | ||||||||||||
Accounts receivable | (86,012 | ) | 21,978 | (4,697 | ) | |||||||
Inventories | (39,575 | ) | (56,062 | ) | (30,066 | ) | ||||||
Prepaid expenses and other current assets | (3,495 | ) | (36,806 | ) | (12,771 | ) | ||||||
Accounts payable | 6,042 | 10,462 | 5,281 | |||||||||
Accrued expenses | 47,139 | 3,045 | (1,969 | ) | ||||||||
Product warranty | (1,492 | ) | 14 | 6,706 | ||||||||
Deferred revenues | (10,819 | ) | 39,529 | (15,974 | ) | |||||||
Advance payments from customers | 22,349 | 23,038 | 35,485 | |||||||||
Other long-term liabilities | (22,126 | ) | 12 | 881 | ||||||||
Net cash provided by operating activities | 304,433 | 372,227 | 300,193 | |||||||||
Cash flows from investing activities: | ||||||||||||
Proceeds from maturities or sale of marketable securities | — | — | 193,470 | |||||||||
Purchases of marketable securities | — | — | (99,900 | ) | ||||||||
Purchases of property, plant and equipment | (62,562 | ) | (81,424 | ) | (64,135 | ) | ||||||
Equity and cost investments | — | (7,783 | ) | (24,504 | ) | |||||||
(Increase) decrease in cash surrender value of life insurance | (2,505 | ) | 4,330 | (6,407 | ) | |||||||
Acquisition of businesses, net of cash acquired | (2,550 | ) | (2,092 | ) | (52,374 | ) | ||||||
Notes repayment (receivable) from affiliate and other | (5,662 | ) | (315 | ) | 1,242 | |||||||
Other, net | (4,627 | ) | (301 | ) | (3,050 | ) | ||||||
Net cash used in investing activities | (77,906 | ) | (87,585 | ) | (55,658 | ) | ||||||
Cash flows from financing activities: | ||||||||||||
Repurchases of common stock | (101,485 | ) | (261,558 | ) | (319,300 | ) | ||||||
Proceeds from issuance of common stock to employees | 27,825 | 128,743 | 44,504 | |||||||||
Excess tax benefits from share-based compensation | 9,639 | 42,020 | 19,678 | |||||||||
Employees’ tax withheld and paid for restricted performance shares | (3,193 | ) | (1,134 | ) | (84 | ) | ||||||
Repayments on bank borrowings | (7,987 | ) | (8,971 | ) | (14,547 | ) | ||||||
Net borrowings (repayments) under line of credit agreements | 4,171 | (41,000 | ) | 41,000 | ||||||||
Payment of mandatorily redeemable financial instrument | — | — | (11,771 | ) | ||||||||
Other | (251 | ) | (176 | ) | — | |||||||
Net cash used in financing activities | (71,281 | ) | (142,076 | ) | (240,520 | ) | ||||||
Effects of exchange rate changes on cash and cash equivalents | 977 | (8,506 | ) | (13,277 | ) | |||||||
Net increase (decrease) in cash and cash equivalents | 156,223 | 134,060 | (9,262 | ) | ||||||||
Cash and cash equivalents at beginning of fiscal year | 397,306 | 263,246 | 272,508 | |||||||||
Cash and cash equivalents at end of fiscal year | $ | 553,529 | $ | 397,306 | $ | 263,246 | ||||||
See accompanying notes to the consolidated financial statements.
86
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
AND COMPREHENSIVE EARNINGS
| Common Stock | Capital in Excess of Par Value | Retained Earnings | Accumulated Other Comprehensive Loss | Total | |||||||||||||||||||
| (In thousands) | Shares | Amount | |||||||||||||||||||||
Balances at September 29, 2006 | 129,721 | $ | 129,721 | $ | 265,214 | $ | 406,849 | $ | (4,531 | ) | $ | 797,253 | |||||||||||
Net earnings | — | — | — | 239,456 | — | 239,456 | |||||||||||||||||
Currency translation adjustment | — | — | — | — | 2,615 | 2,615 | |||||||||||||||||
Minimum pension liability adjustment, net of taxes of $1,968 | — | — | — | — | 4,531 | 4,531 | |||||||||||||||||
Comprehensive earnings | — | — | — | — | — | 246,602 | |||||||||||||||||
Adjustment to initially apply the recognition and disclosure provisions of ASC 715 | (13,528 | ) | (13,528 | ) | |||||||||||||||||||
Issuance of common stock | 2,226 | 2,226 | 42,278 | — | — | 44,504 | |||||||||||||||||
Tax benefits from exercises of share-based payment awards | — | — | 21,144 | — | — | 21,144 | |||||||||||||||||
Issuance of common stock in settlement of deferred stock units and restricted stock, net of shares withheld for employee taxes and cancellation | 268 | 268 | (352 | ) | — | — | (84 | ) | |||||||||||||||
Share-based compensation expense | — | — | 44,864 | — | — | 44,864 | |||||||||||||||||
Repurchases of common stock | (7,000 | ) | (7,000 | ) | (61,737 | ) | (250,563 | ) | — | (319,300 | ) | ||||||||||||
Balances at September 28, 2007 | 125,215 | 125,215 | 311,411 | 395,742 | (10,913 | ) | 821,455 | ||||||||||||||||
Net earnings | — | — | — | 279,484 | — | 279,484 | |||||||||||||||||
Currency translation adjustment | — | — | — | — | 29 | 29 | |||||||||||||||||
Unrealized loss on derivatives, net of taxes of $307 | — | — | — | — | (487 | ) | (487 | ) | |||||||||||||||
Defined benefit pension and post-retirement benefit plans: | |||||||||||||||||||||||
Net loss arising during the year, net of taxes of $2,675 | — | — | — | — | (7,473 | ) | (7,473 | ) | |||||||||||||||
Amortization of transition obligation, net of taxes of $191 | — | — | — | — | 304 | 304 | |||||||||||||||||
Amortization of prior service cost, net of taxes of $19 | — | — | — | — | 127 | 127 | |||||||||||||||||
Amortization and settlement of net actuarial loss, net of taxes of $144 | — | — | — | — | 185 | 185 | |||||||||||||||||
Comprehensive earnings | — | — | — | — | — | 272,169 | |||||||||||||||||
Adoption of the provisions in ASC 740 relating to accounting for uncertainty in income taxes | — | — | — | (19,064 | ) | — | (19,064 | ) | |||||||||||||||
Issuance of common stock | 4,973 | 4,973 | 123,770 | — | — | 128,743 | |||||||||||||||||
Tax benefits from exercises of share-based payment awards | — | — | 45,656 | — | — | 45,656 | |||||||||||||||||
Issuance of common stock in settlement of deferred stock units and restricted stock, net of shares withheld for employee taxes and cancellation | 512 | 512 | (1,646 | ) | — | — | (1,134 | ) | |||||||||||||||
Share-based compensation expense | 40,918 | — | — | 40,918 | |||||||||||||||||||
Repurchases of common stock | (5,110 | ) | (5,110 | ) | (51,725 | ) | (204,723 | ) | — | (261,558 | ) | ||||||||||||
Balances at September 26, 2008 | 125,590 | 125,590 | 468,384 | 451,439 | (18,228 | ) | 1,027,185 | ||||||||||||||||
Net earnings | — | — | — | 319,022 | — | 319,022 | |||||||||||||||||
Currency translation adjustment | — | — | — | — | 2,362 | 2,362 | |||||||||||||||||
Reclassification of foreign currency translation resulting from the sale of Research Instruments | (778 | ) | (778 | ) | |||||||||||||||||||
Unrealized gain on derivatives: | |||||||||||||||||||||||
Increase in unrealized gain, net of taxes of $2,616 | 4,164 | 4,164 | |||||||||||||||||||||
Reclassification adjustments, net of taxes of $2,310 | (3,677 | ) | (3,677 | ) | |||||||||||||||||||
Defined benefit pension and post-retirement benefit plans: | |||||||||||||||||||||||
Net loss arising during the year, net of taxes of $2,352 | — | — | — | — | (11,265 | ) | (11,265 | ) | |||||||||||||||
Amortization of transition obligation, net of taxes of $191 | — | — | — | — | 301 | 301 | |||||||||||||||||
Amortization of prior service cost, net of taxes of $19 | — | — | — | — | 132 | 132 | |||||||||||||||||
Amortization and settlement of net actuarial loss, net of taxes of $287 | — | — | — | — | 535 | 535 | |||||||||||||||||
Comprehensive earnings | — | — | — | — | — | 310,796 | |||||||||||||||||
Adoption of measurement date provision of ASC 715 | (122 | ) | 69 | (53 | ) | ||||||||||||||||||
Issuance of common stock | 1,500 | 1,500 | 26,325 | — | — | 27,825 | |||||||||||||||||
Tax benefits from exercises of share-based payment awards | — | — | 8,270 | — | — | 8,270 | |||||||||||||||||
Issuance of common stock in settlement of deferred stock units and restricted stock, net of shares withheld for employee taxes and cancellation | 439 | 439 | (3,631 | ) | — | — | (3,192 | ) | |||||||||||||||
Share-based compensation expense | — | — | 42,437 | — | — | 42,437 | |||||||||||||||||
Repurchases of common stock | (2,248 | ) | (2,248 | ) | (25,307 | ) | (73,930 | ) | — | (101,485 | ) | ||||||||||||
Balances at October 2, 2009 | 125,281 | $ | 125,281 | $ | 516,478 | $ | 696,409 | $ | (26,385 | ) | $ | 1,311,783 | |||||||||||
See accompanying notes to the consolidated financial statements.
87
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Description of Business
Varian Medical Systems, Inc. (“VMS”) and subsidiaries (collectively, the “Company”) designs, manufactures, sells and services equipment and software products for treating cancer with radiotherapy, stereotactic radiosurgery and brachytherapy. The Company also designs, manufactures, sells and services x-ray tubes for original equipment manufacturers; replacement x-ray tubes; and flat panel digital image detectors for filmless x-rays imaging in medical, dental, veterinary, scientific and industrial applications. It designs, manufactures, sells and services linear accelerators, digital image detectors, image processing software and image detection products for security and inspection purposes. The Company also develops, designs, manufacturers and services proton therapy products and systems for cancer treatment.
Basis of Presentation
The consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States (“GAAP”). On July 1, 2009, the Financial Accounting Standards Board (“FASB”) released the authoritative version of its new Accounting Standards Codification (“ASC”) as the single source for GAAP, which replaces all previous GAAP accounting standards. While not intended to change GAAP, ASC significantly changes the way in which the accounting literature is organized. In the fourth quarter of fiscal year 2009, the Company adopted ASC to reference GAAP accounting standards in its consolidated financial statements. The adoption of ASC did not have an effect on the Company’s consolidated financial position, results of operations or cash flows.
Fiscal Year
The fiscal years of the Company as reported are the 52- or 53- week periods ending on the Friday nearest September 30. Fiscal year 2009 was the 53-week period that ended on October 2, 2009. Fiscal year 2008 was the 52-week period that ended on September 26, 2008 and fiscal year 2007 was the 52-week period that ended on September 28, 2007.
Distribution
On April 2, 1999, Varian Associates, Inc. reorganized into three separate publicly traded companies by spinning off, through a tax-free distribution, two of its businesses to stockholders (the “Spin-offs”). The Spin-offs resulted in the following three companies: 1) the Company (renamed from Varian Associates, Inc. to Varian Medical Systems, Inc. following the Spin-offs); 2) Varian, Inc. (“VI”); and 3) Varian Semiconductor Equipment Associates, Inc. (“VSEA”). The Spin-offs resulted in a non-cash dividend to stockholders.
In connection with the Spin-offs, the Company, VI and VSEA also entered into various agreements that set forth the principles to be applied in separating the companies and allocating certain related costs and specified portions of contingent liabilities (see Note 9).
Reclassifications
Certain financial statement items have been reclassified to conform to the current fiscal year’s format. As discussed in Note 16 “Discontinued Operations,” the Company has classified the assets and liabilities of the scientific research instruments business (“Research Instruments”) of ACCEL Instruments GmbH (“ACCEL,” which has since changed its name to Varian Medical Systems Particle Therapy GmbH) as
88
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
discontinued operations in the Consolidated Balance Sheets and presented its operating results as a discontinued operation in the Consolidated Statements of Earnings for all periods presented. Because amounts related to Research Instruments in the Consolidated Statements of Cash Flows and in the Consolidated Statements of Stockholders’ Equity and Comprehensive Earnings were not material for any period presented, the Company has not segregated them from continuing operations. Unless noted otherwise, discussion in these notes pertains to the Company’s continuing operations. These reclassifications had no impact on previously reported total net earnings.
Subsequent Event
The Company has evaluated subsequent events through November 25, 2009, the date the Company filed its Annual Report on Form 10-K for the fiscal year ended October 2, 2009.
Principles of Consolidation
The consolidated financial statements include those of VMS and its subsidiaries. Intercompany balances, transactions, and stock holdings have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
Fair Value of Financial Instruments
The carrying amounts of the Company’s financial instruments, including cash and cash equivalents, accounts receivable, net of allowance for doubtful accounts, accounts payable and short-term borrowings, approximate fair value due to their short maturities.
Foreign Currency Translation
For foreign subsidiaries where the U.S. dollar is the functional currency, gains and losses from remeasurement of foreign currency financial statements into U.S. dollars are included in the Consolidated Statements of Earnings. The aggregate foreign exchange net gains were $7.4 million, $0.4 million and $4.2 million in fiscal years 2009, 2008 and 2007, respectively. For foreign subsidiaries where the local currency is the functional currency, translation adjustments of foreign currency financial statements into U.S. dollars are recorded to a separate component of accumulated other comprehensive income. Cumulative currency translation adjustments were a gain of $4.2 million as of October 2, 2009, a gain of $2.6 million at September 26, 2008 and a gain of $2.6 million as of September 28, 2007.
Cash and Cash Equivalents
The Company considers currency on hand, demand deposits, time deposits, and all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash and cash equivalents. Cash and cash equivalents are held in various financial institutions in the United States and internationally.
89
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Investments
Marketable securities with an original maturity of more than three months and less than one year at the date of purchase are considered to be short-term. Auction rate securities are classified as short-term available-for-sale securities. Marketable securities are classified as held-to-maturity because the Company has the intent and ability to hold these securities to maturity. The held-to-maturity securities are carried at amortized cost using the specific identification method. Interest income is recorded using an effective interest rate, with the associated premium or discount amortized to interest income. Additionally, the Company assesses whether an other-than-temporary impairment loss on the investments has occurred due to declines in fair value or other market conditions. Declines in fair value that are considered other than temporary, if any, are recorded as charges in the Consolidated Statements of Earnings. The Company also invests in privately held companies. These investments are included in other assets in the Consolidated Balance Sheets and are carried at cost. The Company monitors these investments for impairment and makes appropriate reductions in carrying values if the Company determines that an impairment charge is required based primarily on the financial condition and near-term prospects of these companies. The Company did not have any impairment loss on marketable securities or investments in privately held companies for fiscal years 2009, 2008 and 2007.
Concentration of Credit Risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist principally of cash, cash equivalents, trade accounts receivable and derivative financial instruments used in hedging activities. The Company is exposed to credit loss in the event of nonperformance by counterparties on the foreign exchange contracts used in hedging activities. These counterparties are large international financial institutions and, to date, no such counterparty has failed to meet its financial obligations under such contracts. Cash and cash equivalents held with financial institutions may exceed the Federal Deposit Insurance Corporation insurance limits or similar limits in foreign jurisdictions. The Company has not experienced any losses on its deposits of cash and cash equivalents. Concentrations of credit risk with respect to trade accounts receivable are limited due to the large number of customers comprising the Company’s customer base and their geographic dispersion. The Company performs ongoing credit evaluations of its customers and, other than a down payment typically required before shipments of products, it generally does not require collateral from its customers. The Company maintains an allowance for doubtful accounts based upon the expected collectability of all accounts receivable. No single customer represented more than 10% of the accounts receivable amount for any period presented.
Inventories
Inventories are valued at the lower of cost or market (realizable value). Excess and obsolete inventories are determined primarily based on future demand forecasts and write-downs of excess and obsolete inventories are recorded as a component of cost of revenues. Cost is computed using standard cost (which approximates actual cost) and actual cost on a first-in-first-out or average basis.
Property, Plant and Equipment
Property, plant and equipment are stated at cost, net of accumulated depreciation. Major improvements are capitalized, while repairs and maintenance are expensed as incurred. Costs incurred for internally developed software during the application development stage are capitalized in accordance with
90
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
ASC 350-40. Internally developed software primarily includes enterprise-level business software that the Company customizes to meet its specific operational needs. Depreciation and amortization are computed using the straight-line method over the estimated useful lives of the assets. Land is not subject to depreciation, but land improvements are depreciated over fifteen years. Land leasehold rights and leasehold improvements are amortized over the lesser of estimated useful lives or remaining lease terms. Buildings are depreciated over twenty years. Machinery and equipment are depreciated over their estimated useful lives, which range from three to seven years. Assets subject to lease are amortized over the lesser of estimated useful lives or lease terms. When assets are retired or otherwise disposed of, the assets and related accumulated depreciation are removed from the accounts. Gains or losses resulting from retirements or disposals are included in operating earnings.
Goodwill and Intangible Assets
Goodwill is recorded when the purchase price of an acquisition exceeds the fair value of the net identified tangible and intangible assets acquired. Purchased intangible assets are carried at cost, net of accumulated amortization. Intangible assets with finite lives are amortized over their estimated useful lives of approximately one to twenty years using the straight-line method.
Impairment of Long-Lived Assets, Goodwill and Intangible Assets
The Company reviews long-lived assets and identifiable intangible assets with finite lives for impairment whenever events or changes in circumstances indicate that the carrying amount of these assets may not be recoverable. The Company assesses these assets for impairment based on estimated undiscounted future cash flows from these assets. If the carrying value of the assets exceeds the estimated future undiscounted cash flows, the Company recognizes an impairment loss based on the excess of the carrying amount over the fair value of the assets. For assets held for sale, the Company assesses these assets for impairment based on their fair value less cost to sell. If the carrying value of the assets held for sale exceeds the fair value less cost to sell, the Company recognizes an impairment loss based on the excess of the carrying amount over the fair value of the assets less cost to sell. In fiscal year 2008, the Company recognized an impairment charge of $2.7 million for the impairment of long-lived assets of Research Instruments, which was sold in the second quarter of fiscal year 2009. See Note 16 “Discontinued Operations” for a detailed discussion. The Company did not recognize any impairment charge in fiscal years 2009 and 2007.
In accordance with ASC 350, the Company evaluates goodwill for impairment annually or whenever an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. The impairment test for goodwill is a two-step process. Step one consists of a comparison of the fair value of a reporting unit with its carrying amount, including the goodwill allocated to each reporting unit. The Company determines the fair value of its reporting units based on the present value of estimated future cash flows of the reporting units. The Company determines the fair value of businesses held for sale based on the expected selling price of the businesses. If the carrying amount is in excess of the fair value, step two requires the comparison of the implied fair value of the reporting unit with the carrying amount of the reporting unit’s goodwill. Any excess of the carrying value of the reporting unit’s goodwill over the implied fair value of the reporting unit’s goodwill is recorded as an impairment loss.
In the fourth quarter of fiscal year 2007, the Company performed evaluations for the four reporting units that carried goodwill, Oncology Systems, X-ray Products, Security and Inspections Products (“SIP”) and
91
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
ACCEL, and found no impairment. In the fourth quarter of fiscal year 2008, the Company performed a goodwill impairment test for Research Instruments, which became a business held for sale in the fourth quarter of fiscal year 2008, and recognized a goodwill impairment charge of $0.6 million. In the fourth quarter of fiscal years 2008 and 2009, the Company also performed the annual goodwill impairment testing for the four remaining reporting units that carried goodwill, Oncology Systems, X-ray Products, SIP and Varian Particle Therapy (the business of ACCEL that remained after the sale of Research Instruments), and found no impairment.
Environmental Remediation Liabilities
Environmental remediation liabilities are recorded when environmental assessments and/or remediation efforts are probable, and the costs of these assessments or remediation efforts can be reasonably estimated. The Company records these liabilities in accordance with ASC 410-30.
Revenue Recognition
The Company’s revenues are derived primarily from the sale of hardware and software products, and related services and contracts from the Company’s Oncology Systems, X-ray Products, SIP and Varian Particle Therapy businesses. The Company recognizes its revenues net of any value added or sales tax and net of sales discounts.
Hardware Products
Except as described below under “Service Contracts and Other,” the Company recognizes revenues for hardware products in accordance with ASC 605, when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable and collectability is reasonably assured. For an arrangement with multiple deliverables, the Company recognizes product revenues in accordance with ASC 605-25 and 985-605, with revenues allocated among the different elements. Except for government tenders, group purchases and orders with letters of credit, the Company typically requires its customers to provide a down payment prior to transfer of risk of loss of ordered products or prior to performance under service contracts. These down payments are recorded as “Advance payments from customers” in the Consolidated Balance Sheets.
For Oncology Systems and SIP hardware products with installation obligations, the Company recognizes as revenues a portion of the product purchase price upon transfer of risk of loss and defers revenue recognition on the portion associated with product installation until “acceptance,” provided that all other criteria for revenue recognition are met. The portion deferred is the greater of the fair market value of the installation services for such products or the amount of payment contractually linked to the “acceptance.” However, when (a) all of the purchase price for the hardware product is conditioned upon “acceptance,” (b) the hardware product does not have value to the customer on a standalone basis or (c) there is no objective and reliable evidence of the fair value of the undelivered item, then the Company defers all revenues until “acceptance” in accordance with the treatment for “delivered items” under ASC 605-25.
Installation of Oncology Systems and SIP hardware products involves the Company’s testing of each product at its factory prior to the product’s delivery to ensure that the product meets the Company’s published specifications. Once these tests establish that the specifications have been met, the product is then disassembled and shipped to the customer’s site as specified in the customer contract. Risk of loss is transferred to the customer either at the time of shipment or delivery, depending upon the shipping
92
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
terms of the contract. At the customer’s site, the product is reassembled, installed and retested in accordance with the Company’s installation procedures to ensure and demonstrate compliance with the Company’s published specifications for that product.
Under the terms of the Company’s hardware sales contract, “acceptance” of a hardware product with installation obligations is deemed to have occurred upon the earliest of (i) completion of product installation and testing in accordance with the Company’s standard installation procedures showing compliance with the Company’s published specifications for that product, (ii) receipt by the Company of an acceptance form executed by the customer acknowledging installation and compliance with the Company’s published specifications for that product, (iii) use by the customer of the product for any purpose after its delivery or (iv) six months after the delivery of the product to the customer by the Company. The contract allows for cancellation only by mutual agreement, thus the customer does not have a unilateral right to return the delivered hardware product.
The Company does not have installation obligations for x-ray tubes, digital image detectors, spare parts and certain hardware products in Oncology Systems and the SIP business. For the products that do not include installation obligations, the Company recognizes revenues upon the transfer of risk of loss, which is either at the time of shipment or delivery, depending upon the shipping terms of the contract, provided that all other revenue recognition criteria under ASC 605-10 and ASC 605-25 have been met.
Software Products
Except as described below under “Service Contracts and Other,” the Company recognizes revenues for software products in accordance with ASC 985-605. The Company recognizes license revenues when all of the following criteria are met: persuasive evidence of an arrangement exists, the vendor’s fee is fixed or determinable, collection of the related receivable is probable, delivery of the product has occurred and the Company has received from the customer an acceptance form acknowledging installation and substantial conformance with the Company’s specifications (as set forth in the user manual) for such product, or upon verification of installation when customer acceptance is not required to be received, or upon the expiration of an acceptance period, provided that all other criteria for revenue recognition under ASC 985-605 have been met. Revenues earned on software arrangements involving multiple elements are allocated to each element based on vendor-specific objective evidence of the fair value (“VSOE”), which is based on the price charged when the same element is sold separately. In instances when evidence of VSOE of all undelivered elements exists, but evidence does not exist for one or more delivered elements, revenues are recognized using the residual method. Under the residual method, the fair value of the undelivered elements is deferred and the remaining portion of the arrangement fee is recognized as revenue. Revenue allocated to maintenance and support is recognized ratably over the maintenance term (typically one year).
Installation of the Company’s software products may involve a certain amount of customer-specific implementation to enable the software product to function within the customer’s operating environment (i.e., with the customer’s information technology network and other hardware, with the customer’s data interfaces and with the customer’s administrative processes) and substantially in conformance with the Company’s specifications (as set forth in the user manual) for such product. With these software products, customers do not have full use of the software (i.e., functionality) until the software is installed as described above and functioning within the customer’s operating environment. Therefore, the Company recognizes 100% of such software revenues upon receipt from the customer of the Company’s acceptance form acknowledging installation and such substantial conformance, or upon verification of
93
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
installation when the Company is not required to receive customer acceptance, or upon the expiration of an acceptance period, provided that all other criteria for revenue recognition under ASC 985-605 have been met.
The Company does not have installation obligations for certain brachytherapy and SIP software products. For software products that do not include installation obligations, the Company recognizes revenues upon the transfer of risk of loss, which is either at the time of shipment or delivery, depending upon the shipping terms of the contract, provided that all other criteria under ASC 985-605 are met.
Service Contracts and Other
Revenues related to service contracts are recognized ratably over the period of the related contracts. Revenues related to services performed on a time-and-materials basis are recognized when they are earned and billable.
Revenues related to certain proton therapy commissioning service contracts and highly customized image detection systems are recognized under the percentage-of-completion method or the completed-contract method in accordance with ASC 605-35. Revenues recognized under the percentage-of-completion method are primarily based on contract costs incurred to date compared with total estimated contract costs. Estimated losses on contracts are charged to cost of sales in the period when the loss is identified.
Deferred revenue includes (i) the amount equal to the greater of the fair value of the installation services for hardware products or the amount of the payment that is contractually linked to acceptance and (ii) the entire sale price applicable to products shipped but for which installation and/or final acceptance have not been completed.
Share-Based Compensation Expense
The Company measures and recognizes compensation expense for all share-based payment awards made to employees and directors, including stock options, employee stock purchases related to the Varian Medical Systems, Inc. Employee Stock Purchase Plan (the “Employee Stock Purchase Plan”), deferred stock units, restricted stock and restricted stock units based on their fair values in accordance with ASC 718. Share-based compensation expense is based on the value of the portion of share-based payment awards that is ultimately expected to vest. Share-based compensation expense recognized in the Consolidated Statements of Earnings included compensation expense for share-based payment awards granted prior to, but not yet vested as of, September 30, 2005 based on the grant date fair value estimated in accordance with prior authoritative guidance and compensation expense for the share-based payment awards granted subsequent to September 30, 2005 based on the grant date fair value estimated in accordance with ASC 718. The Company attributes the value of share-based compensation to expense using the straight-line method.
The Company has valued its share-based payment awards using the Black-Scholes option-pricing model, which was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. The Black-Scholes model requires the input of certain assumptions. VMS’s stock options and the option component of the Employee Stock Purchase Plan shares have characteristics significantly different from those of traded options, and changes in the assumptions can materially affect the fair value estimates.
94
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
The Company uses the “short-cut” method to establish the beginning balance of the additional paid-in capital pool (“APIC pool”) related to the tax effects of share-based compensation, and to determine the subsequent impact on the APIC pool and the Consolidated Statements of Cash Flows of the tax effects of share-based compensation awards that were outstanding upon adoption ASC 718. The Company considers only the direct tax impacts of share-based compensation awards when calculating the amount of tax windfalls or shortfalls.
For fiscal years 2009, 2008 and 2007, total share-based compensation expenses, before taxes, were $42.6 million, $41.0 million and $44.9 million, respectively. See Note 12, “Employee Stock Plans” for a detailed discussion.
Earnings per Share
Basic net earnings per share is computed by dividing net earnings by the weighted average number of shares of common stock outstanding for the period. Diluted net earnings per share is computed by dividing net earnings by the sum of the weighted average number of common shares outstanding and dilutive common shares under the treasury method.
The following table sets forth the computation of net basic and diluted earnings per share:
| Fiscal Years | ||||||||||||
(In thousands, except per share amounts) | 2009 | 2008 | 2007 | |||||||||
Earnings from continuing operations | $ | 331,476 | $ | 295,256 | $ | 242,916 | ||||||
Loss from discontinued operations, net of taxes | (12,454 | ) | (15,772 | ) | (3,460 | ) | ||||||
Net earnings | $ | 319,022 | $ | 279,484 | $ | 239,456 | ||||||
Basic weighted average shares outstanding | 124,034 | 124,800 | 127,407 | |||||||||
Dilutive effect of potential common shares | 961 | 2,804 | 3,215 | |||||||||
Diluted weighted average shares outstanding | 124,995 | 127,604 | 130,622 | |||||||||
Net earnings (loss) per share—basic: | ||||||||||||
Continuing operations | $ | 2.67 | $ | 2.37 | $ | 1.91 | ||||||
Discontinued operations | (0.10 | ) | (0.13 | ) | (0.03 | ) | ||||||
Net earnings per share | $ | 2.57 | $ | 2.24 | $ | 1.88 | ||||||
Net earnings (loss) per share—diluted: | ||||||||||||
Continuing operations | $ | 2.65 | $ | 2.31 | $ | 1.86 | ||||||
Discontinued operations | (0.10 | ) | (0.12 | ) | (0.03 | ) | ||||||
Net earnings per share | $ | 2.55 | $ | 2.19 | $ | 1.83 | ||||||
The Company excluded stock options from the computation of diluted weighted average shares outstanding if the per share value, including the sum of (a) the exercise price of the options and (b) the amount of the compensation cost attributed to future services and not yet recognized, was greater than the average market price of the shares, because the inclusion of these stock options would be antidilutive to earnings per share. Accordingly, stock options to purchase 8,245,887 shares, 4,744,873 shares and 5,093,330 shares at weighted average exercise prices of $46.82, $51.08 and $50.39, respectively, were excluded from the computation of diluted weighted average shares outstanding during fiscal years 2009, 2008 and 2007, respectively.
95
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Shipping and Handling Costs
Shipping and handling costs are included as a component of cost of revenues.
Research and Development
To date, research and development costs have been expensed as incurred. These costs primarily include employees’ compensation, consulting fees and material costs.
Software Development Costs
Costs for the development of new software products and substantial enhancements to existing software products are expensed as incurred until technological feasibility has been established, at which time any additional costs would be capitalized in accordance with ASC 985-20. No costs associated with the development of software have been capitalized as the Company believes its current software development process is essentially completed concurrent with the establishment of technological feasibility.
Comprehensive Earnings
Comprehensive earnings include all changes in equity (net assets) during a period from non-owner sources. Comprehensive earnings include currency translation adjustments, reclassification of foreign currency translation resulting from the sale of Research Instruments, change in unrealized gain or loss on derivative instruments designated as cash flow hedges, net of taxes (see Note 8, “Derivative Instruments and Hedging Activities”), minimum pension liability adjustments, net of taxes, and adjustments to and amortization of unrecognized actuarial gain or loss, unrecognized transition obligation and unrecognized prior service cost of our defined benefit pension and post-retirement benefit plans. (See Note 10, “Retirement Plans”).
Taxes on Earnings
Taxes on earnings are based on pretax financial accounting income. Deferred tax assets and liabilities are recorded based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse.
Recent Accounting Pronouncements
In December 2007, the FASB issued new accounting standards for business combinations under ASC 805, which establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, any noncontrolling interest in the acquiree and the goodwill acquired. The new standards also establish disclosure requirements to enable the evaluation of the nature and financial effects of the business combination. In April 2009, the FASB issued additional standards under ASC 805-20 to clarify initial recognition and measurement, subsequent measurement and accounting, and disclosure of assets and liabilities arising from contingencies in a business combination. The new standards related to business combinations under ASC 805 will be effective for the Company in the first quarter of fiscal year 2010. The impact of the adoption of these new standards will depend on the nature and extent of business combinations occurring on or after the beginning of fiscal year 2010.
96
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
In December 2007, the FASB established new accounting and reporting standards under ASC 810 for ownership interests in subsidiaries held by parties other than the parent, the amount of consolidated net income attributable to the parent and to the noncontrolling interest, changes in a parent’s ownership interest, and the valuation of retained noncontrolling equity investments when a subsidiary is deconsolidated. The new accounting standards under ASC 810 also establish disclosure requirements that clearly identify and distinguish between the interests of the parent and the interests of the noncontrolling owners. The new standards under ASC 810 will be effective for the Company in the first quarter of fiscal year 2010. The Company does not expect that the adoption of these new standards under ASC 810 will have a material impact on its consolidated financial position, results of operations or cash flows.
In November 2008, the FASB ratified an EITF Issue, which clarifies the accounting for certain transactions and impairment considerations involving equity method investments. The new standards, which will be included in ASC 323-10, will be effective for the Company in the first quarter of fiscal year 2010, with early adoption prohibited. The Company does not expect that the adoption of this new standard will have a material impact on its consolidated financial position, results of operations and cash flows.
In December 2008, the FASB issued new standards under ASC 715-20, which provides guidance on an employer’s disclosure about plan assets of a defined benefit pension or other post-retirement plan and requires employers to disclose information about fair value measurements of plan assets. The new standards under ASC 715-20 will be effective for the Company as of the end of fiscal year 2010.
In June 2009, the FASB issued the consolidation guidance for variable-interest entities to replace the quantitative-based risks and rewards calculation for determining which enterprise, if any, has a controlling financial interest in a variable interest entity with an approach focused on identifying which enterprise has the power to direct the activities of a variable interest entity that most significantly impact the entity’s economic performance. These new standards will be effective for the Company in the first quarter of fiscal year 2011. The Company is currently assessing the potential impact, if any, these new standards may have on its consolidated financial position, results of operations and cash flows.
In August 2009, the FASB issued an update to ASC 820.This Accounting Standards Update (“ASU”) No. 2009-5,Measuring Liabilities at Fair Value (“ASU 2009-5”) amends the provisions in ASC 820 related to the fair value measurement of liabilities and clarifies for circumstances in which a quoted price in an active market for the identical liability is not available. ASU 2009-5 is intended to reduce potential ambiguity in financial reporting when measuring the fair value of liabilities. ASU 2009-5 is effective for the Company in the first quarter of fiscal year 2010. ASU 2009-5 concerns disclosure only and will not have an impact on the Company’s consolidated financial position or results of operations.
In October 2009, the FASB issued an update to ASC 605. This ASU No. 2009-13,Multiple Deliverable Revenue Arrangements (“ASU 2009-13”), provides guidance on whether multiple deliverables in a revenue arrangement exist, how the arrangement should be separated, and the consideration allocated. ASU 2009-13 eliminates the requirement to establish the fair value of undelivered products and services and instead provides for separate revenue recognition based upon management’s estimate of the selling price for an undelivered item when there is no other means to determine the fair value of that undelivered item. ASU 2009-13 is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted if the Company elects to adopt ASU No. 2009-14,Certain Revenue Arrangements That Include Software Elements(“ASU 2009-14”) concurrently. The Company is currently evaluating the potential impact of ASU 2009-13 on its consolidated financial position, results of operations and cash flows.
97
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
In October 2009, the FASB issued an update to ASC 985-605. This ASU 2009-14, amends the scope of the software revenue guidance in ASC 985-605 to exclude tangible products containing software components and non-software components that function together to deliver the tangible product’s essential functionality. ASU 2009-14 is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted if the Company elects to adopt ASU 2009-13 concurrently. The Company is currently evaluating the potential impact of ASU 2009-14 on its consolidated financial position, results of operations and cash flows.
2. BALANCE SHEET COMPONENTS
| (In millions) | October 2, 2009 | September 26, 2008 | ||||||
Inventories: | ||||||||
Raw materials and parts | $ | 183.1 | $ | 156.8 | ||||
Work-in-progress | 54.7 | 36.6 | ||||||
Finished goods | 84.1 | 89.6 | ||||||
Total inventories | $ | 321.9 | $ | 283.0 | ||||
Property, plant and equipment: | ||||||||
Land and land improvements | $ | 42.5 | $ | 11.4 | ||||
Buildings and leasedhold improvements | 185.8 | 167.6 | ||||||
Machinery and equipment | 280.0 | 226.3 | ||||||
Construction in progress | 18.1 | 46.5 | ||||||
Assets subject to lease | 0.8 | 0.8 | ||||||
| 527.2 | 452.6 | |||||||
Accumulated depreciation and amortization | (263.1 | ) | (234.4 | ) | ||||
Property, plant and equipment, net | $ | 264.1 | $ | 218.2 | ||||
Accrued expenses: | ||||||||
Accrued compensation and benefits | $ | 125.0 | $ | 128.8 | ||||
Income taxes payable | 48.0 | 20.4 | ||||||
Current deferred tax liabilities | 1.9 | 8.6 | ||||||
Other | 129.5 | 95.1 | ||||||
Total accrued expenses | $ | 304.4 | $ | 252.9 | ||||
Other long-term liabilities: | ||||||||
Long-term income taxes payable | $ | 67.8 | $ | 89.5 | ||||
Other | 63.0 | 44.8 | ||||||
Total other long-term liabilities | $ | 130.8 | $ | 134.3 | ||||
As of October 2, 2009, the “Other” category of other long-term liabilities primarily consisted of accruals for environmental costs, accrued pension and post-retirement benefits, deferred income tax liabilities and deferred rental income. As of September 26, 2008, the “Other” category of other long-term liabilities primarily consisted of accruals for environmental costs, accrued pension and post-retirement benefits and deferred income tax liabilities. Accruals for environmental costs, accrued pension and post-retirement benefits and deferred rental income that are included in other long-term liabilities are not
98
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
expected to be recognized in the following fiscal year. The current portion of the accruals for environmental costs, accrued pension and post-retirement benefits and deferred rental income are included within “Accrued expenses.”
3. FAIR VALUE
Effective September 27, 2008, the Company adopted the provisions of ASC 820, which defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. ASC 820-10 establishes a three-level fair value hierarchy that prioritizes the inputs used to measure fair value. This hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The Company’s financial assets and liabilities are valued using Level 1 and Level 2 inputs. Level 1 instrument valuations are obtained from quotes for transactions in active exchange markets involving identical assets. Level 2 instruments include valuations obtained from quoted prices for identical assets in markets that are not active. In addition, the Company has elected to use the income approach to value its derivative instruments using standard valuation techniques and Level 2 inputs, such as currency spot rates, forward points and credit default swap spreads. The Company’s derivative instruments are short-term in nature, typically one month to twelve months in duration. As of October 2, 2009, the Company did not have any financial assets or liabilities without observable market values that would require a high level of judgment to determine fair value (Level 3 instruments).
The Company’s adoption of the provisions of ASC 820-10 did not have a material impact on its consolidated financial statements. The Company has segregated all financial assets and liabilities that are measured at fair value on a recurring basis (at least annually) into the most appropriate level within the fair value hierarchy based on the inputs used to determine the fair value at the measurement date in the table below. The Company is not required to apply the provisions of ASC 820-10 for nonfinancial assets and liabilities until the first quarter of fiscal year 2010, except those that are recognized or disclosed at fair value in the financial statements on a recurring basis.
Effective September 27, 2008, the Company adopted the provisions of ASC 825-10-25, which provides entities the option to measure many financial instruments and certain other items at fair value. The Company has currently chosen not to elect the fair value option for any items that are not already required to be measured at fair value in accordance with GAAP.
99
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Assets/Liabilities Measured at Fair Value on a Recurring Basis
The following tables present the Company’s financial assets as of October 2, 2009 that are measured at fair value on a recurring basis. There were no financial liabilities that were measured at fair value as of October 2, 2009.
| Fair Value Measurement Using | ||||||||||||
Type of Instruments | Quoted Prices in Active Markets for Identical Instruments (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total Balance | ||||||||
| (In millions) | ||||||||||||
Assets: | ||||||||||||
Money market funds | $ | 85.0 | $ | — | $ | — | $ | 85.0 | ||||
Total assets measured at fair value | $ | 85.0 | $ | — | $ | — | $ | 85.0 | ||||
| Fair Value Measurement Using | ||||||||||||
Line Item in Condensed Consolidated Balance Sheet | Quoted Prices in Active Markets for Identical Instruments (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total Balance | ||||||||
| (In millions) | ||||||||||||
Assets: | ||||||||||||
Cash and cash equivalents | $ | 84.0 | $ | — | $ | — | $ | 84.0 | ||||
Other assets | 1.0 | — | — | 1.0 | ||||||||
Total assets measured at fair value | $ | 85.0 | $ | — | $ | — | $ | 85.0 | ||||
4. GOODWILL AND INTANGIBLE ASSETS
The following table reflects the gross carrying amount and accumulated amortization of the Company’s intangible assets included in “Other assets” on the Consolidated Balance Sheets as follows:
| (In millions) | October 2, 2009 | September 26, 2008 | ||||||
Intangible Assets: | ||||||||
Acquired existing technology | $ | 20.8 | $ | 19.7 | ||||
Patents, licenses and other | 15.2 | 14.5 | ||||||
Customer contracts and supplier relationship | 10.4 | 10.5 | ||||||
Accumulated amortization | (37.2 | ) | (33.6 | ) | ||||
Net carrying amount | $ | 9.2 | $ | 11.1 | ||||
Amortization expense for intangible assets was $3.6 million, $4.3 million and $5.1 million for fiscal years 2009, 2008 and 2007, respectively. The Company estimates amortization expense on a straight-line basis for fiscal years 2010 through 2014 and thereafter, to be as follows (in millions): $3.2, $2.5, $1.6, $1.2 and $0.7.
100
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
The following table reflects the allocation of goodwill:
| (In millions) | October 2, 2009 | September 26, 2008 | ||||
Oncology Systems | $ | 126.7 | $ | 125.4 | ||
X-ray Products | 2.7 | 2.7 | ||||
Other | 80.9 | 81.0 | ||||
Total | $ | 210.3 | $ | 209.1 | ||
The decrease in goodwill balance in the “Other” category reflects the impact of foreign currency translation adjustments and the sale of Research Instruments. The increase in goodwill balance in Oncology Systems was due to an acquisition.
5. RELATED PARTY TRANSACTIONS
In fiscal years 1999 and 2000, VMS invested a total of $5 million in a three member consortium for a 20% ownership interest in dpiX Holding LLC (“dpiX Holding”), which in turn invested $25 million for an 80.1% ownership interest in dpiX LLC (“dpiX”), a supplier of amorphous silicon based thin-film transistor arrays (“flat panels”) for the Company’s X-ray Products’ digital image detectors and for its Oncology Systems’ On-Board Imager®, or OBI, and PortalVisionTM imaging products. VMS had the right to appoint one manager of the five person board of managers. In accordance with the dpiX Holding agreement, net losses were to be allocated to the three members, in succession, until their capital accounts equaled zero, then to the three members in accordance with their ownership interests. The dpiX Holding agreement also provided that net profits were to be allocated to the three members, in succession, until their capital accounts equaled the net losses previously allocated, then to the three members in accordance with their ownership interests.
In September 2004, VMS acquired another member’s 20% ownership interest in dpiX Holding for $1 million. As a result, VMS has the right to appoint two managers of the five person board of managers and its ownership interest in dpiX Holding increased to 40% with the remaining 60% being held by the other original member. When VMS acquired this additional 20% ownership interest, the capital account of the selling member was nearly zero because it was the first in the consortium to be allocated losses. As a result, when dpiX Holding recorded net profits after VMS acquired the additional 20% ownership interest, VMS was the first to be allocated net profits to recover previously allocated losses.
The investment in dpiX Holding is accounted for under the equity method of accounting. When VMS recognizes its share of net profits or losses of dpiX Holding, profits in inventory purchased from dpiX are eliminated until realized by VMS. In fiscal year 2009, VMS recorded a loss on the equity investment in dpiX Holding of $0.9 million. In fiscal year 2008, VMS recorded income on the equity investment in dpiX Holding of $0.3 million. VMS recorded a loss on the equity investment in dpiX Holding of $0.3 million in fiscal year 2007. Incomes and losses on the equity investment in dpiX Holding are included in “Selling, general and administrative” expenses in the Consolidated Statements of Earnings.
The member that owned the other 19.9% ownership interest in dpiX had the right to sell back to dpiX on dpiX’s last business day in December 2004, 2005 and 2006, cumulatively all of that member’s ownership interest for $5 million if dpiX had not become a publicly traded company as of the last business day in December 2004. In December 2004, that member exercised its right to sell back to dpiX its 19.9% ownership interest. On each of December 22, 2005 and December 24, 2004, dpiX repurchased from that member a 7.96% ownership interest for a payment of $2 million (in aggregate, a 15.92% interest for $4
101
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
million). On December 22, 2006, dpiX repurchased the remaining 3.98% ownership interest for $1 million and VMS’s indirect ownership interest in dpiX increased to 40%.
In December 2004, VMS agreed to loan $2 million to dpiX in four separate installments, with the loan bearing interest at prime plus 1% per annum. The principal balance was due and payable to VMS in twelve equal quarterly installments that began in October 2006; interest was payable in full according to a quarterly schedule, which began in April 2005; and the entire principal balance, together with accrued and unpaid interest thereon and all other related amounts payable thereunder, was due and payable on July 10, 2009. The note receivable of $0.7 million from dpiX at September 26, 2008 was included in “Prepaid expense and other current assets” in the Consolidated Balance Sheets, and has been paid as of October 2, 2009.
In February 2008, VMS agreed to loan an additional $1.6 million to dpiX, with the loan bearing interest at prime plus 1% per annum. The principal balance is due and payable to VMS in twelve equal quarterly installments beginning in January 2010; interest is payable in full according to a quarterly schedule which began in April 2008; and the entire principal balance, together with accrued and unpaid interest thereon and all other related amounts payable thereunder, is due and payable on October 10, 2012. The additional note receivable from dpiX was $1.6 million at both October 2, 2009 and September 26, 2008. The current portion of the note receivable was included in “Prepaid expense and other current assets” and the long-term portion was included in “Other Assets” in the Consolidated Balance Sheets.
In February 2009, VMS agreed to loan a further $14 million to dpiX in four separate installments over a period through December 2009. The loan bears interest at prime plus 1% per annum. The principal balance is due and payable to VMS in four installments beginning in December 2011; interest is payable in full according to a quarterly schedule which began in April 2009; and the entire principal balance, together with accrued and unpaid interest thereon and all other related amounts payable thereunder, is due and payable on September 10, 2012. As of October 2, 2009, VMS had loaned $6.8 million to dpiX under this loan agreement, which was included in “Other assets” in the Consolidated Balance Sheet.
In March 2006, VMS and the other member of dpiX Holding agreed to invest an aggregate $92 million in dpiX Holding, with each member’s contribution based on its percentage ownership interest in dpiX Holding, for dpiX to acquire and construct a manufacturing facility in Colorado to increase its production capacity. As of October 2, 2009 and September 26, 2008, VMS’s contribution of $36.8 million to dpiX Holding for the Colorado manufacturing facility was included in “Other assets” in the Consolidated Balance Sheets as of October 2, 2009 and September 26, 2008.
During fiscal years 2009, 2008 and 2007, the Company purchased glass transistor arrays from dpiX totaling approximately $26.4 million, $25.4 million and $21.0 million, respectively. These purchases of flat panels are included as a component of “Inventory” in the Consolidated Balance Sheets and “Cost of revenues—product” in the Consolidated Statements of Earnings for these periods.
102
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
6. LONG-TERM DEBT
Long-term debt outstanding at October 2, 2009 and September 26, 2008 is summarized as follows:
| (Dollars in millions) | October 2, 2009 | September 26, 2008 | ||||
Unsecured term loan, 6.70% due in installments of $6.25 payable in fiscal years 2010, 2012, and 2014 | $ | 18.8 | $ | 18.8 | ||
Unsecured term loan, 6.76% due in installments of $5.25 payable in fiscal year 2011 | 5.3 | 10.5 | ||||
Unsecured term loan, 7.15% due in installments of $2.5 payable in fiscal years 2010 | 2.5 | 5.0 | ||||
Loans assumed through purchases of land and buildings, 7.34% and 7.58% due in monthly installments (including principal and interest) of $0.7 payable in fiscal years 2010—2011 and balloon payments of $5.5 in fiscal year 2012(1) | 5.8 | 6.1 | ||||
| 32.4 | 40.4 | |||||
Less: current maturities of long-term debt | 9.0 | 8.0 | ||||
Long-term debt | $ | 23.4 | $ | 32.4 | ||
| (1) | As of October 2, 2009, land and buildings with a carrying amount of $13.7 million were pledged as collateral against these loans. |
The term loan agreements contain a covenant that requires the Company to pay prepayment penalties if the Company elects to pay off this debt before the maturity dates and the market interest rate is lower than the fixed interest rates of the debt at the time of repayment. They also contain covenants that limit future borrowings and cash dividend payments and require the Company to maintain specified levels of working capital and operating results. For all fiscal years presented within these consolidated financial statements, the Company was in compliance with all restrictive covenants of the unsecured term loan agreements.
Interest paid on long-term debt was $2.6 million for fiscal year 2009, $3.2 million for fiscal year 2008 and $3.8 million for fiscal year 2007. At October 2, 2009, aggregate debt maturities for fiscal years 2010, 2011, 2012, 2013, 2014 and thereafter are as follows (in millions): $9.0, $5.5, $11.6, $0.0, $6.3 and $0.0, respectively.
The fair value of the Company’s long-term debt was estimated to be $34.8 million at October 2, 2009 and $42.2 million at September 26, 2008. The fair value of long-term debt was estimated based on the then-current rates available to the Company for debt of similar terms and remaining maturities. The Company determined the estimated fair value amount by using available market information and commonly accepted valuation methodologies. However, considerable judgment is required in interpreting market data to develop estimates of fair value. Accordingly, the fair value estimate presented herein is not necessarily indicative of the amount that the Company or holders of the instruments could realize in a current market exchange. The use of different assumptions and/or estimation methodologies may have a material effect on the estimated fair value.
103
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
7. CREDIT FACILITY
In July 2007, VMS entered into a credit agreement with Bank of America, N.A. (“BofA”) providing for an unsecured revolving credit facility that enabled the Company to borrow and have outstanding at any given time a maximum of $100 million (the “BofA Credit Facility”). On November 10, 2008, VMS amended and restated the BofA Credit Facility to increase the line of credit to $150 million and collateralize a portion of the credit facility with a pledge of stock of certain of the VMS’s present and future subsidiaries that are deemed to be material subsidiaries. As of October 2, 2009, VMS has pledged to BofA 65% of the voting shares that it holds in Varian Medical Systems Nederland B.V., a wholly-owned subsidiary.
On July 14, 2009, the Company further amended and restated the credit facility (the “Amended BofA Credit Facility”) to enable VMS’s Japanese subsidiary (“VMS KK”) to borrow up to 2.7 billion Japanese Yen as part of the overall credit facility (the “Japanese Line of Credit”). At any time amounts are outstanding under the Japanese Line of Credit, the full borrowing capacity is deemed committed for use in Japan and therefore the maximum amount VMS can otherwise borrow under the Amended BofA Credit Facility will be reduced by $30 million to $120 million. VMS guarantees the payment of the outstanding balance under the Japanese Line of Credit.
The Amended BofA Credit Facility may be used for working capital, capital expenditures, permitted acquisitions and other lawful corporate purposes. Borrowings under the Japanese Line of Credit can be used by VMS KK for refinancing certain intercompany debts, working capital, capital expenditures and other lawful corporate purposes. Borrowings under the Amended BofA Credit Facility (outside of the Japanese Line of Credit) accrue interest either (i) based on the London Inter Bank Offered Rate (“LIBOR”) plus a margin of 1.25% to 1.50% based on a leverage ratio involving funded indebtedness and earnings before interest, taxes, depreciation and amortization (“EBITDA”), or (ii) based upon a base rate of either the federal funds rate plus 0.5% or BofA’s announced prime rate, whichever is greater, minus a margin of 0.5% to 0% based on a leverage ratio involving funded indebtedness and EBITDA, depending upon the Company’s instructions to BofA. The Company may select borrowing periods of one, two, three or six months for advances based on the LIBOR rate. Interest rates on advances based on the base rate are adjustable daily. Under the Amended BofA Credit Facility, the Company paid commitment fees at an annual rate of 0.2% to 0.3% based on a leverage ratio involving funded indebtedness and EBITDA. Borrowings under the Japanese Line of Credit accrue interest at the basic loan rate announced by the Bank of Japan plus a margin of 1.25% to 1.50% based on a leverage ratio involving funded indebtedness and EBITDA. The Amended BofA Credit Facility will expire, if not extended by mutual agreement of VMS and BofA, on November 10, 2011. The Japanese Line of Credit will expire on November 10, 2010.
As of October 2, 2009, there was no outstanding balance under the Amended BofA Credit Facility other than $4.4 million outstanding under the Japanese Line of Credit with a weighted average interest rate of 1.55%. There were no outstanding balances under the BofA Credit Facility as of September 26, 2008. For fiscal years 2009, 2008 and 2007, the Company paid commitment fees of $256,000, $82,000 and $11,000, respectively. Up to $25 million of these facilities could also be used to support letters of credit issued on behalf of the Company, of which none were outstanding as of October 2, 2009 or September 26, 2008.
The Amended BofA Credit Facility contains customary affirmative and negative covenants for facilities of this type. The Company has also agreed to maintain certain financial covenants relating to (i) leverage ratios involving funded indebtedness and EBITDA, (ii) liquidity and (iii) consolidated assets. For all
104
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
fiscal years presented within these consolidated financial statements, the Company was in compliance with all covenants.
Interest paid for credit facilities were $0.2 million, $1.0 million and $0.3 million in fiscal years 2009, 2008 and 2007, respectively.
8. DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
Pursuant to ASC 815, the Company measures all derivatives at fair value on the Consolidated Balance Sheets. The accounting for gains or losses resulting from changes in the fair value of those derivatives depends upon the use of the derivative and whether it qualifies for hedge accounting. Changes in the fair value of derivatives that do not qualify for hedge accounting treatment must be recognized in earnings, together with elements excluded from effectiveness testing and the ineffective portion of a particular hedge. The Company’s derivative instruments are recorded at their fair value in “Prepaid expenses and other current assets” and “Accrued expenses” on the Company’s Consolidated Balance Sheets.
As of October 2, 2009, the fair value of derivative instruments reported on the Company’s Consolidated Balance Sheet was zero. See Note 3, “Fair Value” and “Valuation of Derivative Instruments” under Critical Accounting Estimates in Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” regarding valuation of the Company’s derivative instruments. Also see Note 1, “Significant Accounting Policies” to the Consolidated Financial Statements regarding credit risk associated with the Company’s derivative instruments.
Cash Flow Hedging Activities
The Company has many transactions denominated in foreign currencies and addresses certain of those financial exposures through a program of risk management that includes the use of derivative financial instruments. The Company sells products throughout the world, often in the local currency of the customer’s country, and typically hedges certain of these larger foreign currency transactions when they are not in the subsidiaries’ functional currency. These foreign currency sales transactions are hedged using forward exchange contracts. The Company may use other derivative instruments in the future. The Company enters into foreign currency forward exchange contracts primarily to reduce the effects of fluctuating foreign currency exchange rates. The Company does not enter into forward exchange contracts for speculative or trading purposes. The forward exchange contracts range from one to twelve months in maturity. As of October 2, 2009, the Company did not have any forward exchange contracts with an original maturity greater than 12 months.
The hedges of foreign currency denominated forecasted revenues are accounted for in accordance with ASC 815, pursuant to which the Company has designated its hedges of forecasted foreign currency revenues as cash flow hedges. For derivative instruments that are designated and qualify as cash flow hedges under ASC 815, the Company formally documents for each derivative contract at the hedge’s inception the relationship between the hedging instrument (forward contract) and hedged item (forecasted foreign currency revenues), the nature of the risk being hedged, as well as its risk management objective and strategy for undertaking the hedge. The Company records the effective portion of the gain or loss on the derivative instrument in “Accumulated other comprehensive income (loss)” and reclassifies these amounts into “Revenues” in the period during which the hedged transaction is recognized in earnings. The Company assesses hedge effectiveness both at the onset of the hedge and on an ongoing basis using regression analysis. The Company measures hedge ineffectiveness by comparing the cumulative change in the fair value of the hedge contract with the cumulative change in
105
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
the fair value of the hedged item. The Company recognizes any ineffective portion of the hedge in “Revenues,” and amounts not included in the assessment of effectiveness in “Cost of revenues” in the Consolidated Statements of Earnings. During fiscal years 2009 and 2008, there were no material gains or losses due to hedge ineffectiveness of cash flow hedges and the Company did not discontinue any cash flow hedges that had a material impact on the Company’s results of operations. At the inception of the hedge, the Company assesses whether the likelihood of meeting the forecasted cash flow is highly probable. As of October 2, 2009, all forecasted cash flows are still probable to occur. The Company did not have any cash flow hedges in fiscal year 2007. As of September 26, 2008, net unrealized loss on derivative instruments of $0.8 million, before tax, was included in “Accumulated other comprehensive income (loss).” As of October 2, 2009, there was no net unrealized gain or loss on derivative instruments included in “Accumulated other comprehensive income (loss).” As of October 2, 2009, the Company did not have any outstanding foreign exchange forward contracts that were entered into to hedge forecasted revenues.
The following table presents the amounts, before tax, recognized in accumulated other comprehensive income (loss) and in the Consolidated Statements of Earnings that are related to the effective portion of the foreign exchange forward contracts designated as cash flow hedges:
| Gain (Loss) Recognized in Other Comprehensive Income (Effective Portion) | Location of Gain (Loss) Reclassified from Accumulated Other Comprehensive Income into Net Earnings (Effective Portion) | Gain (Loss) Reclassified from Accumulated Other Comprehensive Income into Net Earnings (Effective Portion) | ||||||
| (in millions) | Fiscal Year 2009 | Fiscal Year 2009 | ||||||
Foreign exchange contracts | $ | 6.8 | Revenues | $ | 6.0 | |||
The following table presents the amounts recognized in the Consolidated Statements of Earnings that are related to (i) the ineffective portion of the cash flow hedges and (ii) the amount excluded from effectiveness testing of the cash flow hedges:
| (in millions) | Location of gain (loss) recognized | Fiscal Year 2009 | ||||
Ineffective portion of cash flow hedges—Gain (Loss) | Revenues | $ | — | |||
Amount excluded from assessment of effectiveness of cash flow hedges—Gain (Loss) | Cost of Revenues | $ | (0.1 | ) | ||
Fair Value Hedging Activities
The Company has in the past used forward exchange contracts that were designated and qualified as fair value hedges under ASC 815 for hedging certain of its foreign currency sales orders. During fiscal years 2009, 2008 and 2007, there were no material gains or losses due to hedge ineffectiveness of fair value hedges and there were no material gains or losses recognized when hedged firm commitments no longer qualified as fair value hedges. At October 2, 2009, the Company had no outstanding foreign exchange forward contracts designated as fair value hedges. In fiscal year 2009, the Company did not enter into any forward exchange contracts that were designated as fair value hedges.
106
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Balance Sheet Hedging Activities
The Company also hedges balance sheet exposures from its various subsidiaries and business units, where the U.S. dollar is the functional currency. The Company enters into foreign currency forward exchange contracts to minimize the short-term impact of foreign currency fluctuations on monetary assets and liabilities denominated in currencies other than the U.S. dollar functional currency. The foreign currency forward exchange contracts are short term in nature, typically with maturity of approximately one month, and are based on the net forecasted balance sheet exposure. These hedges of foreign-currency-denominated assets and liabilities do not qualify for hedge accounting treatment under ASC 815. For derivative instruments not designated as hedging instruments, changes in their fair values are recognized in “Selling, general and administrative expenses” in the Consolidated Statements of Earnings. Changes in the values of these hedging instruments are offset by changes in the values of foreign currency denominated assets and liabilities.Variations from the forecasted foreign currency assets or liabilities, coupled with a significant currency movement, may result in a material gain or loss if the hedges are not effectively offsetting the change in value of the foreign currency asset or liability. Other than foreign exchange hedging activities, the Company has no other free-standing or embedded derivative instruments.
As of October 2, 2009, the Company had the following outstanding foreign exchange forward contracts that were entered into to hedge balance sheet exposures from its various foreign subsidiaries and business units:
| (In millions) | Notional Value Sold | Notional Value Purchased | ||||
Australian dollar | $ | 14.8 | $ | — | ||
British pound | — | 8.9 | ||||
Canadian dollar | 6.2 | — | ||||
Danish krone | — | 3.0 | ||||
Euro | 136.1 | 0.3 | ||||
Indian rupee | 2.1 | — | ||||
Japanese yen | 15.8 | — | ||||
New Zealand dollar | 0.4 | — | ||||
Norwegian krone | 0.3 | — | ||||
Swedish krona | 3.6 | 3.9 | ||||
Swiss franc | — | 33.9 | ||||
Totals | $ | 179.3 | $ | 50.0 | ||
The following table presents the gains (losses) recognized in the Consolidated Statements of Earnings related to the foreign currency forward exchange contracts that do not qualify for hedge accounting treatment under ASC 815.
Location of Gain or (Loss) Recognized in Income on Derivative | Amount of Gain or (Loss) Recognized in Net Earnings on Derivative | |||
| (In millions) | Fiscal Year 2009 | |||
Selling, general and administrative expenses | $ | (2.0 | ) | |
The gains (losses) on these derivative instruments were significantly offset by the gains (losses) resulting from the remeasurement of monetary assets and liabilities denominated in certain currencies other than the U.S. dollar functional currency.
107
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Contingent Features
Certain of the Company’s derivative instruments are subject to a master netting agreement which contains provisions that require the Company, in the event of a default, to settle the outstanding contracts in net liability positions by making settlement payments in cash or by setting off amounts owed to the counterparty against any credit support or collateral held by the counterparty. The counterparty’s right of set-off is not limited to the derivative instruments and applies to other rights held by the counterparty. Pursuant to the master netting agreement, an event of default includes the Company’s failure to pay the counterparty under the derivative instruments, voluntary or involuntary bankruptcy, the Company’s failure to repay an aggregate of $25 million or more in debts, and deterioration of creditworthiness of the surviving entity when the Company merges or transfers its assets or liabilities to another entity. As of October 2, 2009, the Company did not have significant outstanding derivative instruments with credit-risk-related contingent features that were in a net liability position.
9. COMMITMENTS AND CONTINGENCIES
Indemnification Agreements
In conjunction with the sale of the Company’s products in the ordinary course of business, the Company provides standard indemnification of business partners and customers for losses suffered or incurred for property damages, death and injury and for patent, copyright or any other intellectual property infringement claims by any third parties with respect to its products. The terms of these indemnification arrangements are generally perpetual. Except for losses related to property damages, the maximum potential amount of future payments the Company could be required to make under these arrangements is unlimited. As of October 2, 2009, the Company had not incurred any significant costs since the Spin-offs to defend lawsuits or settle claims related to these indemnification arrangements. As a result, the Company believes the estimated fair value of these arrangements is minimal.
VMS has entered into indemnification agreements with its directors and officers and certain of its employees that serve as officers or directors of its foreign subsidiaries that may require VMS to indemnify its directors and officers and those certain employees against liabilities that may arise by reason of their status or service as directors or officers, and to advance their expenses incurred as a result of any legal proceeding against them as to which they could be indemnified.
Product Warranty
The Company discloses estimated future costs of warranty obligations in accordance with ASC 460-10, which requires an entity to disclose and recognize a liability for the fair value of the obligation it assumes upon issuance of a guarantee. The Company warrants most of its products for a specific period of time, usually 12 months, against material defects. The Company provides for the estimated future costs of warranty obligations in cost of revenues when the related revenues are recognized. The accrued warranty costs represent the best estimate at the time of sale of the total costs that the Company will incur to repair or replace product parts that fail while still under warranty. The amount of the accrued estimated warranty costs obligation for established products is primarily based on historical experience as to product failures adjusted for current information on repair costs. For new products, estimates include the historical experience of similar products, as well as reasonable allowance for warranty expenses associated with new products. On a quarterly basis, the Company reviews the accrued warranty costs and updates the historical warranty cost trends, if required.
108
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
The following table reflects the changes in the Company’s accrued product warranty during fiscal years 2009 and 2008:
| Fiscal Years | ||||||||
| (In millions) | 2009 | 2008 | ||||||
Accrued product warranty, beginning of fiscal year | $ | 51.1 | $ | 51.3 | ||||
Charged to cost of revenues | 54.9 | 50.8 | ||||||
Actual product warranty expenditures | (55.2 | ) | (51.0 | ) | ||||
Accrued product warranty, end of fiscal year | $ | 50.8 | $ | 51.1 | ||||
Lease Commitments
At October 2, 2009, the Company was committed to minimum rentals under noncancelable operating leases (including rent escalation clauses) for fiscal years 2010, 2011, 2012, 2013, 2014 and thereafter, as follows (in millions): $15.6, $10.9, $7.0, $5.4, $3.8 and $4.7, respectively. Rental expenses for fiscal years 2009, 2008 and 2007 (in millions) were $22.3, $21.9 and $20.6, respectively.
Other Commitments
In October 2008, VMS consummated an agreement with VI under which VI would surrender its sublease of a building containing approximately 210,000 square feet of floor space and the related leasehold interest for the land. The term of this sublease expires in the year 2056. This building, which is located adjacent to the Company’s corporate headquarters in Palo Alto, California, is intended to support the growth of the Company’s operations and its longer term objective of co-locating certain of its operations. Pursuant to this agreement, VI agreed to surrender the space in the building to the Company over a period which began in October 2008 and which ends in June 2010 and the Company agreed to pay VI an aggregate of $21 million in cash and assume the obligations of sublessor under a below-market rate sublease to a third party for a portion of the building. As of October 2, 2009, $5 million had been paid to VI pursuant to this agreement and the remaining $16 million will be payable in June 2010.
Following a decision by Mitsubishi Electric Co. (“MELCO”) to exit the radiotherapy equipment and service business and its desire to do so in a nondisruptive manner with an established radiotherapy equipment service provider, the Company entered into two separate transactions with MELCO contemporaneously whereby (i) the Company purchased MELCO’s radiotherapy equipment service business in Japan and certain other Asian and Latin American countries (the “MELCO Service Business”) to service MELCO’s existing customers and (ii) the Company formed a joint venture (“JVA”) in Japan with MELCO that became effective as of February 3, 2004.
On February 2, 2004, VMS KK purchased the MELCO Service Business for 2.0 billion Japanese Yen, or US$19.1 million, plus a contingent “earn out” payable to MELCO at the end of the three-year JVA period. The Company accounted for the purchase of the MELCO Service Business as an acquisition and 100% of the profits and losses from VMS KK are reflected in the Company’s consolidated results. In fiscal year 2007, the Company made the “earn out” payment of $4.1 million to MELCO, which was recorded as an adjustment to goodwill.
The JVA was accomplished through MELCO’s purchase on February 3, 2004 of a 35% ownership interest in VMS KK for 1.4 billion Japanese Yen, or US$13.5 million. During the three-year JVA period, MELCO was not entitled to any profits or losses generated by VMS KK. However, MELCO was entitled
109
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
to elect one of the five members of VMS KK’s board of directors. At the end of the three-year JVA period, MELCO was required to unconditionally sell and the Company was required to unconditionally repurchase MELCO’s 35% ownership interest in VMS KK at the original sale price (1.4 billion Japanese Yen) and there were no settlement alternatives to such a repurchase obligation. The Company accounted for MELCO’s 35% ownership interest as a mandatorily redeemable financial instrument. On February 2, 2007, the Company repurchased the 35% ownership interest in the JVA from MELCO for 1.4 billion Japanese Yen, or US$11.8 million at the then-current exchange rate.
Contingencies
Environmental Remediation Liabilities
The Company’s operations and facilities, past and present, are subject to environmental laws, including laws that regulate the handling, storage, transport and disposal of hazardous substances. Certain of those laws impose cleanup liabilities under certain circumstances. In connection with those laws and certain of the Company’s past and present operations and facilities, the Company oversees various environmental cleanup projects and also reimburses certain third parties for cleanup activities. Those include facilities sold as part of the Company’s electron devices business in 1995 and thin film systems business in 1997. In addition, the U.S. Environmental Protection Agency (“EPA”) or third parties have named the Company as a potentially responsible party under the amended Comprehensive Environmental Response Compensation and Liability Act of 1980 (“CERCLA”), at sites to which the Company or the facilities of the sold businesses was alleged to have shipped waste for recycling or disposal (the “CERCLA sites”). In connection with the CERCLA sites, the Company to date has been required to pay only modest amounts as its contributions to cleanup efforts. Under the agreement that governs the Spin-offs, VI and VSEA are each obligated to indemnify the Company for one-third of the environmental cleanup costs associated with corporate, discontinued or sold operations prior to the Spin-offs (after adjusting for any insurance proceeds or tax benefits received by the Company), as well as fully indemnify the Company for other liabilities arising from the operations of the business transferred to it as part of the Spin-offs.
The Company spent $1.0 million, $1.0 million and $0.9 million (net of amounts borne by VI and VSEA) during fiscal years 2009, 2008 and 2007, respectively, on environmental cleanup costs, third-party claim costs, project management costs and legal costs.
Inherent uncertainties make it difficult to estimate the likelihood of the cost of future cleanup, third-party claims, project management and legal services for the CERCLA sites and one of the Company’s past facilities. Nonetheless, as of October 2, 2009, the Company estimated that, net of VI’s and VSEA’s indemnification obligations, future costs associated with the CERCLA sites and this facility would range in total from $2.9 million to $7.2 million. The time frames over which these cleanup project costs are estimated vary, ranging from 1 year up to 30 years as of October 2, 2009. Management believes that no amount in that range is more probable of being incurred than any other amount and therefore accrued $2.9 million for these cleanup projects as of October 2, 2009. The accrued amount has not been discounted to present value due to the uncertainties that make it difficult to develop a single best estimate.
The Company believes it has gained sufficient knowledge to better estimate the scope and cost of monitoring, cleanup and management activities for its other past and present facilities. This, in part, is based on agreements with other parties and also cleanup plans approved by or completed in accordance with the requirements of the governmental agencies having jurisdiction. As of October 2, 2009, the Company estimated that the Company’s future exposure, net of VI’s and VSEA’s indemnification
110
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
obligations, for the costs at these facilities, and reimbursements of third party’s claims for these facilities, ranged in total from $7.4 million to $36.2 million. The time frames over which these costs are estimated to be incurred vary, ranging from 1 year to 30 years as of October 2, 2009. As to each of these facilities, management determined that a particular amount within the range of estimated costs was a better estimate than any other amount within the range, and that the amount and timing of these future costs were reliably determinable. The best estimate within that range was $15.7 million at October 2, 2009. Accordingly, the Company has accrued $11.3 million for these costs, which represents the best estimate discounted at 4%, net of inflation. This accrual is in addition to the $2.9 million described in the preceding paragraph.
The table that follows presents information about the Company’s reserve for future environmental costs at October 2, 2009, based on estimates as of that date.
| (In millions) | Recurring Costs | Non-Recurring Costs | Total Anticipated Future Costs | |||||||
Fiscal Years: | ||||||||||
2010 | $ | 1.0 | $ | 1.1 | $ | 2.1 | ||||
2011 | 0.6 | 1.0 | 1.6 | |||||||
2012 | 0.7 | 0.6 | 1.3 | |||||||
2013 | 0.7 | 0.6 | 1.3 | |||||||
2014 | 0.7 | 0.6 | 1.3 | |||||||
Thereafter | 9.1 | 1.9 | 11.0 | |||||||
Total costs | $ | 12.8 | $ | 5.8 | $ | 18.6 | ||||
Less imputed interest | (4.4 | ) | ||||||||
Reserve amount | $ | 14.2 | ||||||||
Recurring costs include expenses for such tasks as the ongoing operation, maintenance and monitoring of cleanup. Non-recurring costs include expenses for such tasks as soil excavation and treatment, installation of injection and monitoring wells, other costs for soil and groundwater treatment by injection, construction of ground and surface water treatment systems, soil and groundwater investigation, governmental agency costs required to be reimbursed by the Company, removal and closure of treatment systems and monitoring wells, and the defense and settlement of pending and anticipated third-party claims.
These amounts are only estimates of anticipated future costs. The amounts the Company will actually spend may be greater or less than these estimates, even as the Company believes the degree of uncertainty will narrow as cleanup activities progress. While the Company believes its reserve is adequate, as the scope of the Company’s obligations becomes more clearly defined, the Company may modify the reserves, and charge or credit future earnings accordingly. Nevertheless, based on information currently known to management, and assuming VI and VSEA satisfy their indemnification obligations, management believes the costs of these environmental-related matters are not reasonably likely to have a material adverse effect on the consolidated financial statements of the Company in any one fiscal year.
The Company evaluates its liability for investigation and cleanup costs in light of the obligations and apparent financial strength of potentially responsible parties and insurance companies with respect to which the Company believes it has rights to indemnity or reimbursement. The Company has asserted
111
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
claims for recovery of environmental investigation and cleanup costs already incurred, and to be incurred in the future against various insurance companies and other third parties. The Company receives certain cash payments in the form of settlements and judgments from defendants, insurers and other third parties from time to time. The Company has also reached an agreement with an insurance company under which that insurer has agreed to pay a portion of the Company’s past and future environmental-related expenditures. The Company recorded receivables, from that insurer, of $2.8 million at October 2, 2009 and $2.9 million at September 26, 2008, which were included in “Other assets” in the Consolidated Balance Sheets. The Company believes that this receivable is recoverable because it is based on a binding, written settlement agreement with what appears to be a financially viable insurance company, and the insurance company has paid the Company’s claims in the past.
The availability of the indemnities of VI and VSEA will depend upon the future financial strength of VI and VSEA. Given the long-term nature of some of the liabilities, VI and VSEA may be unable to fund the indemnities in the future. It is also possible that a court would disregard this contractual allocation among the parties and require the Company to assume responsibility for obligations allocated to another party, particularly if the other party were to refuse or was unable to pay any of its allocated share. The agreement governing the Spin-offs generally provides that if a court prohibits a company from satisfying its shared indemnification obligations, the indemnification obligations will be shared equally by the two other companies.
Acquisition-Related Commitments/Obligations
When the Company acquired ACCEL in January 2007, the company was involved in a contract-related lawsuit. Subsequent to the acquisition, the Company settled this lawsuit and agreed to perform certain services under a new contract for a fixed price. Based on the expected cost of satisfying its contractual commitments as of September 28, 2007, the final purchase price allocation of ACCEL included a loss accrual related to this contingency of €28.3 million. In the third quarter of fiscal year 2009, the Company increased the cost estimate to complete its contractual commitments by €4.9 million, which was recorded in the Consolidated Statement of Earnings. If the actual costs related to the contingency exceed the estimated amount or if the estimated loss increases, the variances will be recognized in the Consolidated Statement of Earnings in the periods these variances arise. As of October 2, 2009, the balance of the loss accrual related to this contingency was €7.6 million.
Other Matters
The Company is involved, from time to time, in legal proceedings, claims and government inspections or investigations both in and outside the United States, arising in the ordinary course of its business. Such matters are subject to many uncertainties and outcomes are not predictable with assurance. The Company accrues amounts, to the extent they can be reasonably estimated, that it believes are adequate to address any liabilities related to legal proceedings and other loss contingencies that the Company believes will result in a probable loss. While there can be no assurances as to the ultimate outcome of any legal proceeding or other loss contingency involving the Company, management does not believe any pending matter will be resolved in a manner that would have a material adverse effect on the Company’s consolidated financial position, results of operations or cash flows.
10. RETIREMENT PLANS
The Company sponsors the Varian Medical Systems, Inc. Retirement Plan (the “Retirement Plan”)—a defined contribution plan that is available to substantially all of its employees in the United States.
112
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Under Section 401(k) of the Internal Revenue Code, the Retirement Plan allows for tax-deferred salary contributions by eligible employees.
Participants can contribute from 1% to 40% of their eligible base compensation to the Retirement Plan (up to 25% on a pre-tax basis and an additional 15% on an after-tax basis. However, participant contributions are limited to a maximum annual amount as determined periodically by the Internal Revenue Service. The Company matches eligible participant contributions dollar for dollar for the first 6% of eligible base compensation (for those employees with one or more years of service with the Company). In addition, should a participant elect to contribute his or her bonus under the Employee Incentive Plan to the Retirement Plan, the Company matches 6% of this contribution. All matching contributions vest immediately. The Retirement Plan allows participants to invest up to 25% of their contributions in shares of VMS common stock as an investment option.
The Company also sponsors six defined benefit pension plans for regular full-time employees in Germany, Japan, Switzerland and the United Kingdom. In fiscal year 2009, the Company terminated one pension plan in Germany as a result of the sale of Research Instruments. In July 2007, the Company (i) terminated the accrual of additional benefits for existing participants and (ii) suspended the enrollment of new participants under the defined benefit pension plan in the United Kingdom (the “U.K. Pension Plan”). The Company did not make any changes to the participants’ accrued retirement pensions, including the continuing linkage to future salary growth. At the same time, the Company established a defined contribution plan that is available to regular full-time employees in the United Kingdom (the “U.K. Savings Plan”). Participants can contribute from 1% to 100% of their eligible base compensation to the U.K. Savings Plan. The Company matches participant contributions up to 6% of participants’ eligible base compensation, based on the participants’ level of contributions under this UK Savings Plan. For the first and second years after the establishment of the U.K. Savings Plan, the Company also matched an additional 2% and 1%, respectively, of eligible base compensation when the participants contributed 6% or more of their eligible base compensation. All matching contributions vest immediately. The Company also sponsors a post-retirement benefit plan that provides healthcare benefits to certain eligible retirees in the United States.
In fiscal year 2009, the Company adopted the measurement date provisions pursuant to ASC 715, which requires the Company to measure the assets and obligations of its defined benefit pension and post-retirement benefit plans to determine their funded status as of the end of the Company’s fiscal year. As a result of the adoption of the measurement date provisions, the Company recorded a charge to retained earnings of $122,000, net of tax, and a benefit to accumulated other comprehensive income (loss) of $69,000, net of tax, in fiscal year 2009.
Beginning on September 28, 2007, the Company recognizes the funded status of its defined benefit pension and post-retirement benefit plans on its Consolidated Balance Sheet. Each overfunded plan is recognized as an asset, and each underfunded plan is recognized as a liability. Unrecognized prior service costs or credits and net actuarial gains or losses, as well as subsequent changes in the funded status are recognized as a component of Accumulated other comprehensive income (loss) within Stockholders’ Equity.
Total retirement and defined benefit plan expense for all retirement plans amounted to $18.8 million, $16.9 million and $16.6 million for fiscal years 2009, 2008 and 2007, respectively.
113
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Obligations and Funded Status
The funded status of the defined benefit pension and post-retirement benefit plans as of October 2, 2009 and September 26, 2008 was as follows:
| Defined Benefit Plans | Post- Retirement Benefit Plans | |||||||||||||||
| (In millions) | 2009 | 2008 | 2009 | 2008 | ||||||||||||
Change in benefit obligation: | ||||||||||||||||
Benefit obligation—beginning of fiscal year | $ | 116.4 | $ | 113.0 | $ | 5.7 | $ | 6.3 | ||||||||
Adoption of measurement date provision | — | — | (0.1 | ) | — | |||||||||||
Service cost | 2.0 | 2.0 | — | — | ||||||||||||
Interest cost | 4.9 | 5.2 | 0.4 | 0.4 | ||||||||||||
Plan participants’ contributions | 4.6 | 5.1 | — | — | ||||||||||||
Plan settlements | (1.4 | ) | (1.1 | ) | — | — | ||||||||||
Actuarial (gain) loss | 8.7 | (0.3 | ) | 0.7 | (0.4 | ) | ||||||||||
Foreign currency changes | (3.3 | ) | (0.9 | ) | — | — | ||||||||||
Benefit and expense payments | (10.9 | ) | (6.6 | ) | (0.5 | ) | (0.6 | ) | ||||||||
Transfers in | — | — | — | — | ||||||||||||
Benefit obligation—end of fiscal year | $ | 121.0 | $ | 116.4 | $ | 6.2 | $ | 5.7 | ||||||||
Change in plan assets: | ||||||||||||||||
Plan assets—beginning of fiscal year | $ | 104.0 | $ | 106.9 | $ | — | $ | — | ||||||||
Employer contributions | 5.3 | 4.9 | 0.5 | 0.6 | ||||||||||||
Actual return on plan assets/Adjustments | 0.9 | (4.8 | ) | — | — | |||||||||||
Plan participants’ contributions | 4.6 | 5.1 | — | — | ||||||||||||
Plan settlements | (0.8 | ) | (0.7 | ) | — | — | ||||||||||
Foreign currency changes | (3.3 | ) | (0.8 | ) | — | — | ||||||||||
Benefit and expense payments | (10.9 | ) | (6.6 | ) | (0.5 | ) | (0.6 | ) | ||||||||
Plan assets—end of fiscal year | $ | 99.8 | $ | 104.0 | $ | — | $ | — | ||||||||
Funded status | $ | (21.2 | ) | $ | (12.4 | ) | $ | (6.2 | ) | $ | (5.7 | ) | ||||
Distributions | — | — | — | 0.1 | ||||||||||||
Net amount recognized | $ | (21.2 | ) | $ | (12.4 | ) | $ | (6.2 | ) | $ | (5.6 | ) | ||||
Amounts recognized within the consolidated balance sheet: | ||||||||||||||||
Noncurrent assets | $ | — | $ | 1.0 | $ | — | $ | — | ||||||||
Current liabilities | (0.1 | ) | (0.1 | ) | (0.5 | ) | (0.5 | ) | ||||||||
Noncurrent liabilities | (21.1 | ) | (13.3 | ) | (5.7 | ) | (5.1 | ) | ||||||||
Net amount recognized | $ | (21.2 | ) | $ | (12.4 | ) | $ | (6.2 | ) | $ | (5.6 | ) | ||||
114
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
The amounts recognized in accumulated other comprehensive loss (before tax) as of October 2, 2009 were as follows:
| Defined Benefit Plans | Post- Retirement Benefit Plans | |||||||||||||||
| (In millions) | 2009 | 2008 | 2009 | 2008 | ||||||||||||
Transition obligation | $ | — | $ | — | $ | (0.1 | ) | $ | (0.7 | ) | ||||||
Prior service cost | (0.9 | ) | (1.1 | ) | — | — | ||||||||||
Net gain (loss) | (38.3 | ) | (26.3 | ) | (0.5 | ) | 0.3 | |||||||||
Accumulated other comprehensive loss | $ | (39.2 | ) | $ | (27.4 | ) | $ | (0.6 | ) | $ | (0.4 | ) | ||||
The total fair value of plan assets, projected benefit obligation and accumulated benefit obligation for those defined benefit pension plans where accumulated benefit obligation exceeded the fair value of plan assets as of the end of the fiscal years were as follows:
| Defined Benefit Plans | ||||||
| (In millions) | 2009 | 2008 | ||||
Projected benefit obligation | $ | 60.5 | $ | 64.7 | ||
Accumulated benefit obligation | $ | 54.9 | $ | 58.2 | ||
Fair value of plan assets | $ | 48.0 | $ | 51.9 | ||
The accumulated benefit obligation for all defined benefit pension plans was $100.6 million and $102.5 million at October 2, 2009 and September 26, 2008, respectively.
115
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Components of Net Periodic Benefit Cost and Other Amounts Recognized in Other Comprehensive (Income) Loss
The following table shows the components of the Company’s net periodic benefit costs and the other amounts recognized in other comprehensive (income) loss, before tax, related to the Company’s defined benefit pension plans and the Company’s post-retirement benefit plan:
| Defined Benefit Plans | Post-Retirement Benefit Plans | ||||||||||||||||||||||
| (In millions) | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | |||||||||||||||||
Net Periodic Benefit Costs: | |||||||||||||||||||||||
Service cost | $ | 2.0 | $ | 2.0 | $ | 4.3 | $ | — | $ | — | $ | — | |||||||||||
Interest cost | 4.9 | 5.2 | 4.5 | 0.4 | 0.4 | 0.4 | |||||||||||||||||
Settlement gain | (0.7 | ) | (0.6 | ) | — | — | — | — | |||||||||||||||
Expected return on assets | (5.1 | ) | (6.2 | ) | (5.0 | ) | — | — | — | ||||||||||||||
Amortization of transition obligation | — | — | — | 0.4 | 0.5 | 0.5 | |||||||||||||||||
Amortization of prior service cost | 0.1 | 0.2 | 0.1 | — | — | — | |||||||||||||||||
Recognized actuarial loss | 1.1 | 0.5 | 0.9 | — | — | — | |||||||||||||||||
Net periodic benefit cost | 2.3 | 1.1 | 4.8 | 0.8 | 0.9 | 0.9 | |||||||||||||||||
Other Amounts Recognized in Other Comprehensive (Income) Loss: | |||||||||||||||||||||||
Net (gain) loss arising during the year | 12.9 | 10.6 | * | 0.8 | (0.4 | ) | * | ||||||||||||||||
Amortization of transition obligation | — | — | * | (0.4 | ) | (0.5 | ) | * | |||||||||||||||
Amortization of prior service cost | (0.1 | ) | (0.2 | ) | * | — | — | * | |||||||||||||||
Amortization and settlement of net actuarial loss | (0.9 | ) | (0.3 | ) | * | — | — | * | |||||||||||||||
Total recognized in other comprehensive (income) loss | 11.9 | 10.1 | * | 0.4 | (0.9 | ) | * | ||||||||||||||||
Total recognized in net periodic benefit cost and other comprehensive loss | $ | 14.2 | $ | 11.2 | * | $ | 1.2 | $ | — | * | |||||||||||||
| * | Certain information was not applicable prior to the adoption of the recognition and related disclosure provisions of ASC 715. |
The amounts in accumulated other comprehensive loss that are expected to be recognized as components of net periodic benefit cost during fiscal year 2010 are as follows:
| (In millions) | Defined Benefit Plans | Post-Retirement Benefit Plans | Total | |||||||||
Transition obligation | $ | — | $ | (0.1 | ) | $ | (0.1 | ) | ||||
Prior service cost | (0.1 | ) | — | (0.1 | ) | |||||||
Net loss | (1.7 | ) | (0.1 | ) | (1.8 | ) | ||||||
| $ | (1.8 | ) | $ | (0.2 | ) | $ | (2.0 | ) | ||||
116
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Assumptions
The assumptions used to determine net periodic benefit cost and to compute the expected long-term return on assets for the Company’s defined benefit pension and post-retirement benefit plans were as follows:
| Fiscal Years Ended | |||||||||
Net Periodic Benefit Cost | 2009 | 2008 | 2007 | ||||||
Defined benefit plans: | |||||||||
Discount rates | 4.73 | % | 4.64 | % | 3.99 | % | |||
Rates of compensation increase | 3.29 | % | 3.24 | % | 3.11 | % | |||
Expected long-term return on assets | 5.42 | % | 5.68 | % | 5.22 | % | |||
Post-retirement benefit plans: | |||||||||
Discount rate | 6.70 | % | 6.00 | % | 6.00 | % | |||
The assumptions used to measure the benefit obligations for the Company’s defined benefit pension and post-retirement benefit plans were as follows:
Benefit Obligations | October 2, 2009 | September 26, 2008 | ||||
Defined benefit plans: | ||||||
Discount rates | 4.17 | % | 4.73 | % | ||
Rates of compensation increase | 2.99 | % | 3.29 | % | ||
Post-retirement benefit plans: | ||||||
Discount rate | 5.30 | % | 6.70 | % | ||
The benefit obligations of defined benefit pension plans and post-retirement benefit plans were measured as of October 2, 2009. For defined benefit pension plans, the discount rate was adjusted as of October 2, 2009 to the range of 2.00% to 5.70% primarily based on the yields of a universe of high quality corporate bonds in each applicable country or the spot rates on high quality AA-rated corporate bonds, with durations corresponding to the expected duration of the benefit obligations. In countries where the corporate bond market is not sufficiently representative at longer durations, the discount rate also took into account the yield of long-term government bonds corresponding to the duration of the benefit obligation and the difference between the yield curve on high quality corporate fixed-income investments and government fixed-income investment. Additionally, the rate of projected compensation increase was adjusted as of October 2, 2009 to the range of 1.75% to 4.75% reflecting expected inflation levels and future outlook. For post-retirement benefit plans, the discount rate as of October 2, 2009 decreased to 5.30%. This discount rate was determined based on the yields of high quality zero-coupon corporate bonds with maturities that match the expected durations of the benefit obligations.
The Company reviewed the expected long-term rate of return on defined benefit pension plan assets. This review consisted of forward-looking projections for a risk-free rate of return, inflation rate and implied equity risk premiums for particular asset classes. Historical returns were not used. The results of this review were applied to the target asset allocation in accordance with the Company’s planned investment strategies, which are implemented by outside investment managers. The expected long-term rate of return on plan assets was determined based on the weighted average of projected returns on each asset class.
117
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
The assumed healthcare cost trend rates for post-retirement benefit plans are as follows:
| Fiscal Years Ended | |||||||||
Assumed Healthcare Cost Trend Rates | 2009 | 2008 | 2007 | ||||||
Post-retirement benefit plans: | |||||||||
Current medical cost trend rate | 10.5 | % | 10.5 | % | 12.0 | % | |||
Ultimate medical cost trend rate | 4.5 | % | 5.0 | % | 5.0 | % | |||
Current medical cost trend rates represent expected increases in healthcare costs in the short term and are based on assessments and surveys from health plan providers. While the current medical cost trend rate is based on market conditions, the ultimate trend rate reflects a long-term view of expected increases in healthcare costs in the U.S., which is assumed to be consistent with the long-term expected nominal gross domestic product growth rates. Assumed healthcare cost trend rates could have an effect on the amounts reported for healthcare plans. A 1.0 percentage point increase in the assumed healthcare cost trend rates would have increased the total service cost and interest cost components reported in fiscal year 2009 by $26,000 and would have increased the post-retirement benefit obligation reported in fiscal year 2009 by $449,000. A 1.0 percentage point decrease in the assumed healthcare cost trend rates would have decreased the total service cost and interest cost components reported in fiscal year 2009 by $23,000 and would have decreased the post-retirement benefit obligation in fiscal year 2009 by $403,000.
Plan Assets
The Company’s defined benefit pension plans’ weighted average asset allocations at October 2, 2009 and September 26, 2008 and target allocations for fiscal year-end 2009, by asset category, were as follows:
| October 2, 2009 Target Allocations | Defined Benefit Plans | ||||||||
| October 2, 2009 | September 26, 2008 | ||||||||
Equity securities | 35.0 | % | 32.4 | % | 31.1 | % | |||
Debt securities | 58.0 | 59.6 | 61.9 | ||||||
Real estate | 0.0 | 2.2 | 1.7 | ||||||
Other(1) | 7.0 | 5.8 | 5.3 | ||||||
Total | 100.0 | % | 100.0 | % | 100.0 | % | |||
| (1) | The other category primarily consists of investments in general accounts and other investment funds offered by insurance companies. |
The investment objectives of the Company for the defined benefit plans are to generate returns that will enable the defined benefit pension plans to meet their future obligations. The precise amount of these obligations depends on future events, including the life expectancy of the benefit plans’ members and the level of salary increases. The obligations are estimated using actuarial assumptions, based on the current economic environment. The investment strategy depends on the country to which the defined benefit pension plan applies. The investment objectives of some defined benefit plans are more conservative than others. In general, the investment strategy of the defined benefit pension plans is to balance the requirement to generate return using higher-returning assets such as equity securities, with the need to control risk with less volatile assets, such as fixed-income securities. Risks include, among others, the likelihood of the defined benefit pension plans becoming underfunded, thereby increasing their dependence on contributions from the Company. Within each asset class, consideration is given by
118
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
investment managers to balance the portfolio among industry sectors, geographies, interest rate sensitivity, dependence on economic growth, currency and other factors that affect investment returns.
The Company contributes to post-retirement benefit plans on a cash basis as benefits are paid. No assets have been segregated and restricted to provide post-retirement benefits.
Medicare Prescription Drug Act
The Medicare Prescription Drug, Improvement and Modernization Act (the “Prescription Drug Act”) provides a prescription drug benefit under Medicare (Medicare Part D) as well as a federal subsidy to sponsors of retiree healthcare benefit plans that provide a benefit that is at least actuarially equivalent to Medicare Part D. Since it sponsors post-retirement benefit plans that provide prescription drug benefits, the Company enrolled all Medicare eligible retirees in fiscal years 2009, 2008 and 2007 in either Medicare Advantage plans or in health plans where prescription drug benefits are supplied via fully insured Prescription Drug Plans.
Estimated Contributions and Future Benefit Payments
The Company made contributions of $5.3 million to the defined benefit pension plans during fiscal year 2009, compared to $4.9 million in fiscal year 2008. The Company made contributions of $0.5 million to the post-retirement benefit plans for fiscal year 2009. The Company expects total contribution to the defined benefit pension plans and the post-retirement benefit plans for fiscal year 2010 will be approximately $4.2 million and approximately $0.5 million, respectively.
Estimated future benefit payments at October 2, 2009 are as follows:
| (In millions) | Defined Benefit Plans | Post- Retirement Benefit Plans | Total | ||||||
Fiscal Years: | |||||||||
2010 | $ | 2.7 | $ | 0.5 | $ | 3.2 | |||
2011 | 2.9 | 0.5 | 3.4 | ||||||
2012 | 3.1 | 0.6 | 3.7 | ||||||
2013 | 3.6 | 0.6 | 4.2 | ||||||
2014 | 4.0 | 0.6 | 4.6 | ||||||
2015-2019 | 21.4 | 2.7 | 24.1 | ||||||
| $ | 37.7 | $ | 5.5 | $ | 43.2 | ||||
Because amounts related to retirement plans of Research Instruments were not material for any period presented, the Company has not segregated them from continuing operations in this note. See Note 16, “Discontinued Operations” for a detailed discussion.
11. STOCKHOLDERS’ EQUITY
Stockholder Rights Plan
Until December 2008, the Company had a stockholder rights plan. Under the plan, a dividend distribution of one preferred stock purchase right (a “Right”) for each outstanding share of VMS common stock was made to stockholders of record on December 4, 1998 and one Right was issued in connection with each share of VMS common stock issued thereafter. The Rights were exercisable only if
119
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
a person or group acquired 15% or more of VMS common stock (an “Acquiring Person”) or announced a tender offer for 15% or more of VMS common stock. Each Right entitled stockholders to buy one one-thousandth of a share of VMS’s Participating Preferred Stock, par value $1.00 per share, at an exercise price of $105 per Right, subject to adjustment from time to time. However, if any person became an Acquiring Person, each Right could have entitled its holder (other than the Acquiring Person) to purchase at the exercise price VMS common stock (or, in certain circumstances, VMS participating preferred stock) having a market value at that time of twice the Right’s exercise price. The Rights would also have entitled holders (other than the Acquiring Person) to purchase at the exercise price common stock of the Acquiring Person having a market value at that time of twice the Right’s exercise price if the Acquiring Person were to control VMS’s Board of Directors and cause VMS to enter into certain mergers or other transactions. In addition, if an Acquiring Person acquired between 15% and 50% of VMS’s voting stock, VMS’s Board of Directors could have, at its option, exchanged one share of VMS common stock for each Right held (other than Rights held by the Acquiring Person). The Rights expired on December 4, 2008.
Stock Repurchase Program
During fiscal years 2009, 2008 and 2007, the Company paid $101 million, $262 million and $319 million, respectively, to repurchase 2,248,000 shares, 5,110,000 shares and 7,000,000 shares, respectively, of VMS common stock under various authorizations by VMS’s Board of Directors. All shares that have been repurchased have been retired. As of October 2, 2009, 7,300,000 shares of VMS common stock remained available for repurchase under an authorization that expires on December 31, 2009.
12. EMPLOYEE STOCK PLANS
Employee Stock Plans
During fiscal year 1991, VMS adopted the stockholder-approved Omnibus Stock Plan (the “Omnibus Plan”) under which shares of common stock could be issued to officers, directors, key employees and consultants. The Omnibus Plan was amended and restated as of the Spin-offs. The maximum number of shares that could have been issued was limited to 20,000,000 shares. Stock options granted under the Omnibus Plan have an exercise price equal to the closing market price of the underlying stock on the grant date (unless the stock market was closed on the grant date, in which case the exercise price was equal to the average of the highest and lowest quoted selling prices on the stock market on the day before and the day after the grant date) and expire no later than ten years from the grant date. Options granted under the Omnibus Plan before November 2000 were generally exercisable in cumulative installments of one third each year, commencing one year following the date of grant. Options granted after November 2000 were exercisable in the following manner: the first one-third one year from the date of grant, with the remainder vesting monthly during the following two-year period. No further awards may be made under the Omnibus Plan.
In November 2000, VMS adopted the 2000 Stock Option Plan (the “2000 Plan”), which was intended to supplement the Omnibus Plan. The maximum number of shares that could have been issued was limited to 12,000,000 shares. The 2000 Plan is similar to the Omnibus Plan in all material respects, with the exception that shares available for awards under the 2000 Plan could not be issued to directors or officers of VMS. Stock options granted under the 2000 Plan are exercisable for the first one-third of the option shares one year from the date of grant, with the remainder vesting monthly during the following two-year period. Other terms of the 2000 Plan mirror the Omnibus Plan. No further awards may be made under the 2000 Plan.
120
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
In February 2005, VMS’s stockholders approved the 2005 Omnibus Stock Plan (the “2005 Plan”), which was amended and restated in February 2006 and February 2007 and further amended in 2008 and 2009. The 2005 Plan, as amended and restated to date, is referred to as the “Second Amended 2005 Plan.” The Second Amended 2005 Plan provides for the grant of equity incentive awards, including stock options, restricted stock, stock appreciation rights, performance units, restricted stock units and performance shares to officers, directors, key employees and consultants. The Second Amended 2005 Plan also provides for the grant of deferred stock units to non-employee directors. Including the 4,200,000 shares added to the number of shares available for grant under the Second Amended 2005 Plan upon VMS stockholder approval in February 2009, the maximum number of shares issuable under the Second Amended 2005 Plan is (a) 13,450,000, plus (b) the number of shares authorized for issuance, but never issued, under the Omnibus Plan and the 2000 Plan, plus (c) the number of shares subject to awards previously granted under the Omnibus Plan and 2000 Plan that terminate, expire, or lapse, plus (d) amounts granted in substitution of options in connection with certain transactions.
For purposes of the total number of shares available for grant under the Second Amended 2005 Plan, any shares subject to awards of stock options or stock appreciation rights are counted against the available-for-grant limit as one share for every one share subject to the award. Awards other than stock options and stock appreciation rights are counted against the available-for-grant limit as three shares for every one share awarded before February 16, 2007 and as 2.5 shares for every one share awarded on or after February 16, 2007. All awards may be subject to restrictions on transferability and continued employment as determined by the Compensation and Management Development Committee.
Stock options granted under the Second Amended 2005 Plan generally have an exercise price equal to the closing market price of the underlying stock on the grant date. Stock options granted under the Second Amended 2005 Plan generally are exercisable in the following manner: the first one-third one year from the date of grant, with the remainder vesting monthly during the following two-year period. For grants of non-qualified stock options made on or after November 17, 2005 under the Second Amended 2005 Plan to employees who retire from the Company within one year of the grant date, the number of shares subject to the stock option are reduced proportionally by the time during such one-year period that the employee ceased to be an employee of the Company (based upon a 365 day year). The revised number of shares subject to the stock option would continue to vest in accordance with the original vesting schedule, and the remaining shares would be cancelled as of the date of retirement. For grants of non-qualified stock options prior to November 17, 2005, if an employee retired within one year of the grant date, all shares subject to the option grant would continue to vest in accordance with the original vesting schedule. Restricted stock awards generally vest over a period of one to five years from the date of grant. For restricted stock awards granted after February 16, 2007, any unvested restricted stock awards are forfeited at the time of termination. For restricted stock awards granted on or before February 16, 2007, any unvested restricted stock awards are forfeited in the event that the Company terminates the employee’s service prior to the end of the vesting period or the employee retires more than three years prior to the date such vesting occurs. Deferred stock units to non-employee directors vest over a period of not less than one year from the date of grant, unless otherwise provided in the grant agreement as determined by VMS’s Board of Directors, and vesting may be pro rata during the vesting period. Each deferred stock unit is deemed to be the equivalent of one share of VMS common stock. Payment of deferred stock units generally will be made in shares of VMS common stock upon the earlier of the third anniversary of the grant date or the director’s termination. Under the Second Amended 2005 Plan, stock options granted on or prior to February 16, 2007 generally have a term of ten years and stock options granted after February 16, 2007 generally have a term of
121
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
seven years. The Second Amended 2005 Plan prohibits the repricing of stock options and stock appreciation rights without the approval of VMS’s stockholders.
The fair value of options granted and the option component of the shares purchased under the Employee Stock Purchase Plan (which is described further below) shares were estimated at the date of grant using the Black-Scholes model with the following weighted average assumptions:
| Employee Stock Plans | Employee Stock Purchase Plan | |||||||||||||||||||||||
| 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | |||||||||||||||||||
Expected term (in years) | 4.57 | 4.31 | 4.32 | 0.50 | 0.50 | 0.50 | ||||||||||||||||||
Risk-free interest rate | 1.8 | % | 2.6 | % | 4.6 | % | 0.3 | % | 2.1 | % | 4.8 | % | ||||||||||||
Expected volatility | 39.2 | % | 29.7 | % | 29.3 | % | 41.9 | % | 21.3 | % | 19.3 | % | ||||||||||||
Expected dividend yield | — | — | — | — | — | — | ||||||||||||||||||
Weighted average fair value at grant date | $ | 13.00 | $ | 15.39 | $ | 15.96 | $ | 8.97 | $ | 9.51 | $ | 9.94 | ||||||||||||
The expected term of stock options represents the weighted average period the stock options are expected to remain outstanding. The expected term is based on the observed and expected time to post-vesting exercise and post-vesting cancellations of stock options by our employees. The Company determined the expected term of stock options based on the demographic grouping of employees and retirement eligibility. The Company used a combination of historical and implied volatility, or blended volatility, in deriving the expected volatility assumption. Blended volatility represents the weighted average of implied volatility and historical volatility. Implied volatility was derived based on six-month traded options on VMS common stock. Implied volatility is weighted in the calculation of blended volatility based on the ratio of the six-month term of the exchange-traded options to the expected lives of the employee stock options. Historical volatility represents the remainder of the weighting. The decision to incorporate implied volatility was based on the Company’s assessment that implied volatility of publicly traded options in VMS common stock is reflective of market conditions and is generally reflective of both historical volatility and expectations of how future volatility will differ from historical volatility. In determining the extent of use of implied volatility, the Company considered: (i) the volume of market activity of traded options; (ii) the ability to reasonably match the input variables of traded options to those of stock options granted by the Company, including the date of grant; (iii) the similarity of the exercise prices; and (iv) the length of term of traded options. After considering the above factors, the Company determined that it cannot rely exclusively on implied volatility based on the fact that the term of VMS six-month exchange-traded options is less than one year and that it is different from the expected lives of the stock options granted by the Company. Therefore, the Company believes a combination of the historical volatility over the expected lives of the stock options granted by the Company and the implied volatility of six-month exchange-traded options best reflects the expected volatility of VMS common stock going forward. The risk-free interest rate assumption is based upon observed interest rates appropriate for the term of VMS’s stock options. The dividend yield assumption is based on the Company’s history and expectation of no dividend payouts.
As share-based compensation expense recognized in the Consolidated Statements of Earnings is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures are estimated based on historical experience. In fiscal years 2009, 2008 and 2007, the Company adjusted share-based compensation expense based on its actual forfeitures.
122
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
The table below summarizes the effect of recording share-based compensation expense:
| Fiscal Years | ||||||||||||
| (In thousands, except per share amounts) | 2009 | 2008 | 2007 | |||||||||
Cost of revenues—Product | $ | 4,285 | $ | 4,128 | $ | 4,496 | ||||||
Cost of revenues—Service contracts and other | 4,068 | 3,638 | 3,466 | |||||||||
Research and development | 5,239 | 4,701 | 4,958 | |||||||||
Selling, general and administrative | 28,985 | 28,527 | 31,967 | |||||||||
Taxes on earnings | (13,796 | ) | (13,565 | ) | (15,177 | ) | ||||||
Net decrease in net earnings | $ | 28,781 | $ | 27,429 | $ | 29,710 | ||||||
Increase (decrease) on: | ||||||||||||
Cash flows from operating activities | $ | (9,639 | ) | $ | (42,020 | ) | $ | (19,678 | ) | |||
Cash flows from financing activities | $ | 9,639 | $ | 42,020 | $ | 19,678 | ||||||
During fiscal years 2009, 2008 and 2007, total share-based compensation expense recognized in earnings before taxes was $42.6 million, 41.0 million and $44.9 million, respectively, and the total related recognized tax benefit was $13.8 million, $13.6 million and $15.2 million, respectively. Total share-based compensation expense capitalized as part of inventory as of October 2, 2009, September 26, 2008 and September 28, 2007 was $1.5 million, $2.0 million and $2.5 million, respectively.
Activity under the Company’s employee stock plans is presented below:
| Shares Available for Grant | Options Outstanding | ||||||||
| (In thousands, except per share amounts) | Number of Shares | Weighted Average Exercise Price | |||||||
Balance at September 29, 2006 (11,455 options exercisable at a weighted average exercise price of $23.26) | 3,816 | 15,111 | $ | 28.90 | |||||
Authorized | 2,650 | — | — | ||||||
Granted(1) | (3,371 | ) | 2,624 | 50.38 | |||||
Canceled, expired or forfeited(2) | 209 | (199 | ) | 44.74 | |||||
Exercised | — | (1,951 | ) | 17.47 | |||||
Balance at September 28, 2007 (11,995 options exercisable at a weighted average exercise price of $28.92) | 3,304 | 15,585 | $ | 33.75 | |||||
Authorized | 2,600 | — | — | ||||||
Granted(1) | (2,556 | ) | 1,175 | 52.51 | |||||
Canceled, expired or forfeited(2) | 175 | (126 | ) | 49.97 | |||||
Exercised | — | (4,677 | ) | 25.13 | |||||
Balance at September 26, 2008 (9,734 options exercisable at a weighted average exercise price of $35.91) | 3,523 | 11,957 | $ | 38.79 | |||||
Authorized | 4,200 | — | — | ||||||
Granted(1) | (2,575 | ) | 1,070 | 37.17 | |||||
Canceled, expired or forfeited(2) | 204 | (146 | ) | 46.45 | |||||
Exercised | — | (1,028 | ) | 15.34 | |||||
Balance at October 2, 2009 | 5,352 | 11,853 | $ | 40.59 | |||||
123
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
| (1) | The difference between the number of shares subject to options outstanding and the number of shares available for grant under the Company’s employee stock plans represents the award of shares of restricted common stock, restricted stock units and deferred stock units. Awards other than stock options were counted against the shares available-for-grant limit as three shares for every one share awarded before February 16, 2007 and as 2.5 shares for every one awarded on February 16, 2007 and thereafter. |
| (2) | The difference between the number of shares subject to options outstanding and the number of shares available for grant under the Company’s employee stock plans represents: (i) the cancellation of shares of restricted common stock that were tendered to VMS to satisfy employee tax withholding obligations upon vesting of restricted common stock, (ii) the cancellation of shares of restricted common stock due to employee termination and (iii) the cancellation of restricted stock units due to employee termination. |
For fiscal year 2009, the total pre-tax intrinsic value of options exercised was $25 million. The following table summarizes information related to options outstanding and exercisable under the Company’s employee stock plans at October 2, 2009:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
Range of Exercise Prices | Number of Shares | Weighted Average Remaining Contractual Term (in years) | Weighted Average Exercise Price | Aggregate Intrinsic Value (1) | Number of Shares | Weighted Average Remaining Contractual Term (in years) | Weighted Average Exercise Price | Aggregate Intrinsic Value (1) | ||||||||||||
| (In thousands, except years and per-share amounts) | ||||||||||||||||||||
$3.88 – $13.89 | 3 | 1.1 | $ | 12.80 | $ | 82 | 3 | 1.1 | $ | 12.80 | $ | 82 | ||||||||
$13.95 – $14.72 | 179 | 1.1 | 13.95 | 4,655 | 179 | 1.1 | 13.95 | 4,655 | ||||||||||||
$14.73 – $21.27 | 594 | 2.0 | 17.92 | 13,122 | 594 | 2.0 | 17.92 | 13,122 | ||||||||||||
$21.50 – $29.19 | 1,203 | 3.0 | 24.39 | 18,784 | 1,203 | 3.0 | 24.39 | 18,784 | ||||||||||||
$32.10 – $39.85 | 4,271 | 4.9 | 36.25 | 16,039 | 3,239 | 4.4 | 35.96 | 13,107 | ||||||||||||
$40.21 – $52.07 | 4,361 | 6.2 | 49.91 | — | 4,200 | 6.2 | 49.94 | — | ||||||||||||
$52.08 – $65.84 | 1,242 | 5.4 | 53.18 | — | 722 | 5.4 | 53.60 | — | ||||||||||||
Total | 11,853 | 5.0 | $ | 40.59 | $ | 52,682 | 10,140 | 4.9 | $ | 40.18 | $ | 49,750 | ||||||||
| (1) | The aggregate intrinsic value represents the total pre-tax intrinsic value, based on VMS’s closing stock price of $40.01 as of October 2, 2009, which would have been received by the option holders had all option holders exercised their options as of that date. |
As of October 2, 2009, there was $14.3 million of total unrecognized compensation expense related to stock options granted under the Company’s employee stock plans. This unrecognized compensation expense is expected to be recognized over a weighted average period of 1.6 years.
124
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
The activity for restricted stock, restricted stock units and deferred stock units is summarized as follows:
| (In thousands, except per share amounts) | Shares | Weighted Average Grant-Date Fair Value | ||||
Balance at September 29, 2006 | 66 | $ | 46.05 | |||
Granted | 288 | 44.19 | ||||
Vested | (5 | ) | 54.19 | |||
Cancelled or expired | (1 | ) | 50.66 | |||
Balance at September 28, 2007 | 348 | $ | 44.38 | |||
Granted | 552 | 52.58 | ||||
Vested | (61 | ) | 46.04 | |||
Cancelled or expired | (11 | ) | 52.36 | |||
Balance at September 26, 2008 | 828 | $ | 49.62 | |||
Granted | 602 | 37.15 | ||||
Vested | (243 | ) | 51.33 | |||
Cancelled or expired | (15 | ) | 47.27 | |||
Balance at October 2, 2009 | 1,172 | $ | 42.89 | |||
Stock compensation for restricted common stock, restricted stock units and deferred stock units is measured at the stock’s fair value on the date of grant and is amortized over each award’s respective vesting period. For fiscal years 2009, 2008 and 2007, the Company recognized total stock based compensation expense related to restricted stock, and restricted stock units of $15.9 million, $8.0 million and $1.8 million, respectively. In addition, the Company recognized $0.7 million, $0.9 million and $0.9 million of compensation expense related to deferred stock units in fiscal years 2009, 2008 and 2007, respectively.
As of October 2, 2009, unrecognized compensation expense totaling $36.7 million was related to restricted stock, restricted stock units and deferred stock units granted under the Company’s employee stock plans. This unrecognized compensation expense is expected to be recognized over a weighted average period of 2.4 years. The 242,597 shares that vested during the year ended October 2, 2009 represented deferred stock units and restricted stock, and the total fair value of these shares upon vesting was $8.8 million. The Company withheld 88,154 shares (fair value of approximately $3.2 million) for employees’ minimum withholding taxes at vesting.
Because amounts related to employee stock plans of Research Instruments, which is classified as a discontinued operation, were not material for any period presented, the Company has not segregated them from continuing operations in this note. See Note 16, “Discontinued Operations” for a detailed discussion.
Employee Stock Purchase Plan
VMS has an Employee Stock Purchase Plan (the “ESPP”) under which VMS common stock can be issued to substantially all employees in the United States. The participants’ purchase price for VMS common stock under the ESPP is the lower of 85% of the closing market price on the first trading day of each six-month period in the fiscal year or the last trading day of the same six-month period. VMS issued approximately 472,000 shares for $12.1 million in fiscal year 2009, 296,000 shares for $11.2 million in
125
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
fiscal year 2008 and 275,000 shares for $10.4 million in fiscal year 2007 under the ESPP. At October 2, 2009, 3,997,971 shares were available for issuance under the ESPP. In May 2009, as part of a broader set of cost control measures, VMS’s Board of Directors authorized the suspension of the ESPP beginning in October 2009.
13. TAXES ON EARNINGS
The Company accounts for income taxes in accordance with ASC 740. ASC 740 provides for an asset and liability approach under which deferred income taxes are based upon enacted tax laws and rates applicable to the periods in which the taxes become payable.
Taxes on earnings from continuing operations were as follows:
| Fiscal Years Ended | ||||||||||||
| (In millions) | 2009 | 2008 | 2007 | |||||||||
Current provision: | ||||||||||||
Federal | $ | 104.1 | $ | 75.6 | $ | 67.6 | ||||||
State and local | 19.7 | 10.1 | 9.5 | |||||||||
Foreign | 41.4 | 42.0 | 23.4 | |||||||||
Total current | 165.2 | 127.7 | 100.5 | |||||||||
Deferred provision (benefit): | ||||||||||||
Federal | (14.5 | ) | (0.7 | ) | (12.9 | ) | ||||||
State and local | (1.8 | ) | (1.0 | ) | 0.3 | |||||||
Foreign | (5.7 | ) | 4.8 | 15.2 | ||||||||
Total deferred | (22.0 | ) | 3.1 | 2.6 | ||||||||
Taxes on earnings | $ | 143.2 | $ | 130.8 | $ | 103.1 | ||||||
Earnings from continuing operations before taxes are generated from the following geographic areas:
| Fiscal Years Ended | |||||||||
| (In millions) | 2009 | 2008 | 2007 | ||||||
United States | $ | 261.0 | $ | 195.6 | $ | 165.0 | |||
Foreign | 213.6 | 230.4 | 181.0 | ||||||
| $ | 474.6 | $ | 426.0 | $ | 346.0 | ||||
The effective tax rate differs from the U.S. federal statutory tax rate as a result of the following:
| Fiscal Years Ended | |||||||||
| 2009 | 2008 | 2007 | |||||||
Federal statutory income tax rate | 35.0 | % | 35.0 | % | 35.0 | % | |||
State and local taxes, net of federal tax benefit | 2.1 | 1.6 | 1.6 | ||||||
Non-U.S. income taxed at different rates, net | (3.0 | ) | (4.7 | ) | (5.5 | ) | |||
Resolution of tax contingencies due to lapses of statute of limitations | (3.5 | ) | (0.9 | ) | (0.7 | ) | |||
Other | (0.4 | ) | (0.3 | ) | (0.6 | ) | |||
Effective tax rate | 30.2 | % | 30.7 | % | 29.8 | % | |||
126
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
During fiscal years 2009, 2008, and 2007, the Company’s effective tax rate was lower than the U.S. federal statutory rate primarily because the Company’s foreign earnings are taxed at rates that, on average, are lower than the U.S. federal rate. This reduction is partly offset by the fact that the Company’s domestic earnings are also subject to state income taxes.
Significant components of deferred tax assets and liabilities are as follows:
| (In millions) | October 2, 2009 | September 26, 2008 | ||||||
Deferred Tax Assets: | ||||||||
Deferred revenues | $ | 70.6 | $ | 56.4 | ||||
Deferred compensation | 25.6 | 26.5 | ||||||
Product Warranty | 14.2 | 14.0 | ||||||
Inventory adjustments | 18.4 | 24.4 | ||||||
Equity-based compensation | 44.4 | 35.9 | ||||||
Environmental Reserve | 8.0 | 8.5 | ||||||
Net operating loss carryforwards | 28.6 | 17.7 | ||||||
Contingent loss reserve | 7.4 | 8.5 | ||||||
Other | 38.5 | 16.0 | ||||||
| 255.7 | 207.9 | |||||||
Valuation allowance | (35.4 | ) | (20.8 | ) | ||||
Total deferred tax assets | 220.3 | 187.1 | ||||||
Deferred Tax Liabilities: | ||||||||
Goodwill amortization | (22.0 | ) | (19.0 | ) | ||||
Accelerated depreciation | (15.3 | ) | (7.8 | ) | ||||
Other | (11.7 | ) | (5.8 | ) | ||||
Total deferred tax liabilities | (49.0 | ) | (32.6 | ) | ||||
Net deferred tax assets | $ | 171.3 | $ | 154.5 | ||||
Reported As: | ||||||||
Net current deferred tax assets | 144.4 | 131.0 | ||||||
Net long-term deferred tax assets (included in “Other Assets”) | 42.4 | 43.1 | ||||||
Net current deferred tax liabilities (included in “Accrued Expenses”) | (1.9 | ) | (8.6 | ) | ||||
Net long-term deferred tax liabilities (included in “Other long-term liabilities”) | (13.6 | ) | �� | (11.0 | ) | |||
Net deferred tax assets | $ | 171.3 | $ | 154.5 | ||||
The Company has not provided for U.S. federal income and foreign withholding taxes on $620.7 million of cumulative undistributed earnings of non-U.S. subsidiaries. Such earnings are intended to be reinvested in the non-U.S. subsidiaries for an indefinite period of time. If such earnings were not considered to be reinvested indefinitely, additional deferred taxes of approximately $137.1 million would be provided.
The Company has federal net operating loss carryforwards of approximately $4.2 million expiring between 2012 and 2027. The federal net operating loss carryforwards are subject to an annual limitation of approximately $0.2 million per year. The Company has state net operating loss carryforwards of $4.0
127
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
million expiring between 2011 and 2028. The Company has foreign net operating loss carryforwards of $86.2 million with an indefinite life. Of this amount, $38.6 million is unavailable to the Company under local loss utilization rules.
The valuation allowance increased by $14.6 million during fiscal 2009. Of the ending valuation allowance of $35.4 million, $15.3 million is attributable to ACCEL’s deferred tax assets as of the acquisition date which, if recognized, will be allocated to reduce goodwill; and $0.1 million is attributable to the tax benefit of share-based compensation which, if recognized, will be allocated directly to paid-in-capital.
Income taxes paid were as follows:
| Fiscal Years Ended | |||||||||
| (In millions) | 2009 | 2008 | 2007 | ||||||
Federal income taxes paid, net | $ | 83.5 | $ | 75.7 | $ | 49.7 | |||
State income taxes paid, net | 17.1 | 8.5 | 4.9 | ||||||
Foreign income taxes paid, net | 35.0 | 33.6 | 43.4 | ||||||
Total | $ | 135.6 | $ | 117.8 | $ | 98.0 | |||
Effective as of the beginning of fiscal year 2008, the Company adopted the provisions in ASC 740 related to accounting for uncertainty in income taxes, which contain a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining whether the weight of available evidence indicates that it is more likely than not that, based on the technical merits, the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon settlement.
The following table reflects the changes in the Company’s unrecognized tax benefits during the year:
| (In millions) | Fiscal Years Ended 2009 | |||
Unrecognized tax benefits balance at September 26, 2008 | $ | 78.4 | ||
Additions based on tax positions related to a prior year | 3.0 | |||
Reductions based on tax positions related to a prior year | (8.6 | ) | ||
Additions based on tax positions related to the current year | 9.8 | |||
Reductions based on tax positions related to the current year | (4.2 | ) | ||
Settlements | (6.0 | ) | ||
Reductions resulting from the expiration of the applicable statute of limitations | (13.5 | ) | ||
Unrecognized tax benefits balance at October 2, 2009 | $ | 58.9 | ||
As of October 2, 2009, the total amount of gross unrecognized tax benefits was $58.9 million. Of this amount, $42.3 million would affect the effective tax rate if recognized. The difference would be offset by changes to deferred tax assets and liabilities.
It is reasonably possible that the Company’s unrecognized tax benefits will decrease within the next 12 months. Unrecognized tax benefits of approximately $14.1 million related to the tax treatment of certain timing differences may be reduced if the IRS consents to a tax accounting method change that the Company has requested.
128
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
The Company historically classified unrecognized tax benefits in current taxes payable, which is included in “Accrued expenses.” As a result of adoption of the provisions related to accounting for uncertainty in income taxes in ASC 740, the Company reclassified unrecognized tax benefits to “Other long-term liabilities.”
The Company’s policy to include interest and penalties related to unrecognized tax benefits within “Taxes on earnings” on the Consolidated Statements of Earnings did not change as a result of adopting the provisions related to accounting for uncertainty in income taxes in ASC 740. As of September 26, 2008, the Company had accrued $11.4 million for the payment of interest and penalties related to unrecognized tax benefits. A net benefit of $1.3 million related to interest and penalties was included in “Taxes on Earnings.” As of October 2, 2009, the Company had accrued $8.9 million for the payment of interest and penalties related to unrecognized tax benefits. A net benefit of $1.8 million related to interest and penalties was included in “Taxes on Earnings.”
The Company files U.S. federal, U.S. state, and foreign tax returns. The Company’s U.S. federal tax returns are generally no longer subject to tax examinations for years prior to 2005. The Company has significant operations in Switzerland. The Company’s Swiss tax returns are generally no longer subject to tax examinations for years prior to 2005. For U.S. states and other foreign tax returns, the Company is generally no longer subject to tax examinations for years prior to 2003.
14. BUSINESS COMBINATIONS
In January 2007, the Company acquired all of the outstanding equity of ACCEL, a German privately-held supplier of scientific research instruments and proton therapy systems for cancer treatment. The acquisition of ACCEL leverages the Company’s existing technology in treatment planning, image guidance and cancer informatics and it enables Varian to offer all the products needed for delivering proton therapy.
In the quarter ended March 30, 2007, the Company recorded the preliminary purchase price allocation for this acquisition. In September 2007, the Company completed its purchase price allocation related to a contingency that was associated with an unresolved lawsuit, existing at the time of the acquisition. As part of the settlement of this lawsuit, the Company agreed to perform under a contract for a fixed price. From January to September 2007, the Company was gathering information related to the expected cost of satisfying this contract commitment and completed its assessment as of September 28, 2007. As a result, the Company recorded an additional loss related to this contingency of €25.6 million, or approximately $36.1 million, based on the exchange rate as of September 28, 2007, in “Accrued Liabilities” and a reduction to net deferred tax liabilities of $2.7 million, with a corresponding net increase in goodwill of approximately $33.4 million. The final purchase price allocation includes a total contingent loss accrual of €28.3 million, or approximately $40 million, based on the exchange rate as of September 28, 2007. See Note 9, “Commitments and Contingencies” for a detail discussion of this contingency.
In May 2007, the Company acquired all of the outstanding equity of Bio-Imaging Research, Inc. (“BIR”), a privately-held supplier of x-ray imaging products for security and inspection, for $21.9 million. The acquisition enables the Company to offer security and inspection customers x-ray imaging detectors and image processing software in addition to its existing line of specialized linear accelerators for cargo screening, inspection and non-destructive testing. BIR operates under the Company’s SIP business.
129
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
The following is the final allocation of the purchase considerations for these acquisitions:
| (In millions) | Consideration | Net Assets (Liabilities) Acquired | Identifiable Intangible Assets | Goodwill | |||||||||
ACCEL | $ | 20.5 | $ | (46.4 | ) | $ | 4.9 | $ | 62.0 | ||||
BIR | 21.9 | 3.5 | 2.2 | 16.2 | |||||||||
Total | $ | 42.4 | $ | (42.9 | ) | $ | 7.1 | $ | 78.2 | ||||
The Company’s methodology for allocating the purchase price to intangible assets is determined using commonly accepted valuation techniques in the high-technology industry. The valuation method used by the Company included the income approach which established the fair value of the assets based on the value of the cash flows that the assets can be expected to generate in the future using the discounted cash flow method. The purchase prices were allocated to the acquired assets and liabilities based on their estimated fair values as of the date of acquisition, including identifiable intangible assets, with the remaining amount being classified as goodwill.
The consolidated financial statements include the operating results of ACCEL from January 1, 2007, as specified in the purchase agreement, and include the operating results of BIR from May 23, 2007, the closing date for the acquisition. As discussed in Note 1, “Summary of Significant Accounting Policies,” the Company has classified the Research Instruments business of ACCEL as a discontinued operation. See further discussion of the sale of Research Instruments in Note 16, “Discontinued Operations.” Pro forma results of operations have not been presented because the acquisitions were not significant.
15. SEGMENT INFORMATION
Description of Segments
The Company’s operations are grouped into two reportable operating segments: Oncology Systems and X-ray Products. These reportable operating segments were determined based on how the Company’s Chief Executive Officer, its Chief Operating Decision Maker (“CODM”), views and evaluates the Company’s operations. The Company’s Ginzton Technology Center (“GTC”), SIP business and Varian Particle Therapy (previously known as ACCEL Proton Therapy) are reflected in the “Other” category because these operations do not meet the criteria of a reportable operating segment as defined under ASC 280. The CODM allocates resources to and evaluates the financial performance of each operating segment primarily based on operating earnings.
The Oncology Systems business segment designs, manufacturers, sells and services hardware and software products for treating cancer. Products include linear accelerators, brachytherapy afterloaders, treatment simulation and verification equipment and accessories; as well as information management, treatment planning and image processing software. Oncology Systems’ products enable radiation oncology departments in hospitals and clinics to perform conventional radiotherapy treatments and offer advanced treatments such as fixed field intensity-modulated radiation therapy (“IMRT”), image-guided radiation therapy (“IGRT”), volumetric modulated arc therapy (“VMAT”), and stereotactic radiotherapy, as well as to treat patients using brachytherapy techniques, which involve temporarily implanting radioactive sources. Our Oncology Systems products are also used by neurosurgeons to perform stereotactic radiosurgery. Oncology Systems’ customers worldwide include university research and community hospitals, private and governmental institutions, healthcare agencies, physicians’ offices and cancer care clinics.
130
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
The X-ray Products business segment, designs, manufactures and sells: (i) x-ray tubes for use in a range of applications including computed tomography (“CT”), scanning, radiographic or fluoroscopic imaging, mammography, special procedures and industrial applications; and (ii) flat panel digital image detectors for filmless x-ray imaging (commonly referred to as flat panel detectors or digital image detectors), which are for radiography an alternative to image intensifier tubes for fluoroscopy and x-ray film and computed radiography (“CR”) systems. X-ray tubes and flat panel detectors are sold to large imaging systems original equipment manufacturers (“OEMs”) that incorporate our X-ray tube products and flat panel detectors into their medical diagnostic, dental, veterinary, IGRT and industrial imaging systems. X-ray tubes are also sold directly to end-users for replacement purposes.
The Company has three other businesses that are reported together under the “Other” category. SIP designs, manufactures, sells and services Linatron® x-ray accelerators, imaging processing software and image detection products (including IntellXTM) for security and inspection purposes, such as cargo screening at ports and borders and nondestructive examination in a variety of applications. The Company generally sells SIP products to OEMs who incorporate its products into their inspection systems, which are then sold to customs and other government agencies, as well as to commercial private parties in the casting, power, aerospace, chemical, petro-chemical and automotive industries for nondestructive product examination purposes.
The Varian Particle Therapy business develops, designs, manufactures and services products and systems for delivering proton therapy, another form of external beam radiation therapy using proton beams for the treatment of cancer.
In the second quarter of fiscal year 2009, the Company completed the sale of Research Instruments in order to focus that business exclusively on the development of the Varian Particle Therapy business. Research Instruments develops, manufactures and services highly customized scientific instrument components and systems for fundamental and applied physics research primarily for national research laboratories worldwide. Research Instruments is classified as a discontinued operation for all periods presented and the Company has segregated the assets and liabilities and operating results of Research Instruments from continuing operations on the Consolidated Balance Sheets and on the Consolidated Statement of Earnings. Segment data does not include amounts for discontinued operations. Research Instruments was previously included in the “Other” category. See Note 16, “Discontinued Operations” for a more detailed discussion.
GTC develops technologies that enhance the Company’s current businesses or may lead to new business areas, including technology to improve radiation therapy and x-ray imaging and tubes, as well as other technology for a variety of applications, including security and cargo screening.
Corporate includes shared costs of legal, tax, accounting, human resources, real estate, insurance, information technology, treasury, finance and other management costs. A portion of the indirect and common costs has been allocated through the use of estimates. Accordingly, the following information is provided for purposes of achieving an understanding of operations, but may not be indicative of the financial results of the reported segments were they independent organizations. In addition, comparisons of the Company’s operations to similar operations of other companies may not be meaningful.
131
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Segment Data
| Revenues | Operating Earnings | ||||||||||||||||||||
| (In millions) | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | |||||||||||||||
Oncology Systems | $ | 1,798 | $ | 1,672 | $ | 1,440 | $ | 482 | $ | 412 | $ | 340 | |||||||||
X-ray Products | 331 | 305 | 258 | 82 | 73 | 61 | |||||||||||||||
Total reportable segments | 2,129 | 1,977 | 1,698 | 564 | 485 | 401 | |||||||||||||||
Other | 85 | 93 | 57 | (19 | ) | 2 | (7 | ) | |||||||||||||
Corporate | — | — | — | (71 | ) | (68 | ) | (55 | ) | ||||||||||||
Total company | $ | 2,214 | $ | 2,070 | $ | 1,755 | $ | 474 | $ | 419 | $ | 339 | |||||||||
| (In millions) | Depreciation & Amortization | Capital Additions | |||||||||||||||||||
| 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | ||||||||||||||||
Oncology Systems | $ | 18 | $ | 16 | $ | 15 | $ | 20 | $ | 22 | $ | 36 | |||||||||
X-ray Products | 7 | 6 | 5 | 5 | 7 | 6 | |||||||||||||||
Total reportable segments | 25 | 22 | 20 | 25 | 29 | 42 | |||||||||||||||
Other | 3 | 3 | 2 | 3 | 16 | 2 | |||||||||||||||
Corporate | 16 | 11 | 10 | 58 | 37 | 19 | |||||||||||||||
Total company | $ | 44 | $ | 36 | $ | 32 | $ | 86 | $ | 82 | $ | 63 | |||||||||
| Total Assets | Goodwill | ||||||||||||||||||||
| (In millions) | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | |||||||||||||||
Oncology Systems | $ | 986 | $ | 897 | $ | 863 | $ | 126 | $ | 125 | $ | 125 | |||||||||
X-ray Products | 154 | 143 | 119 | 3 | 3 | 1 | |||||||||||||||
Total reportable segments | 1,140 | 1,040 | 982 | 129 | 128 | 126 | |||||||||||||||
Other | 200 | 155 | 120 | 81 | 81 | 80 | |||||||||||||||
Corporate | 968 | 759 | 547 | — | — | — | |||||||||||||||
Total company | $ | 2,308 | $ | 1,954 | $ | 1,649 | $ | 210 | $ | 209 | $ | 206 | |||||||||
The reconciliation of segment operating results information to the Company’s earnings from continuing operations before taxes was as follows:
| (In millions) | 2009 | 2008 | 2007 | |||||||||
Earnings from operations before taxes: | ||||||||||||
Oncology Systems | $ | 482 | $ | 412 | $ | 340 | ||||||
X-ray Products | 82 | 73 | 61 | |||||||||
Total reportable segments | 564 | 485 | 401 | |||||||||
Other | (19 | ) | 2 | (7 | ) | |||||||
Corporate | (71 | ) | (68 | ) | (55 | ) | ||||||
Interest income, net | 1 | 7 | 7 | |||||||||
Total company | $ | 475 | $ | 426 | $ | 346 | ||||||
132
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
Geographic Information
| Revenues | Long-Lived Assets | |||||||||||||||||
| (In millions) | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | ||||||||||||
United States | $ | 1,068 | $ | 964 | $ | 836 | $ | 214 | $ | 172 | $ | 126 | ||||||
International | 1,146 | 1,106 | 919 | 50 | 46 | 42 | ||||||||||||
Total company | $ | 2,214 | $ | 2,070 | $ | 1,755 | $ | 264 | $ | 218 | $ | 168 | ||||||
The Company operates various manufacturing and marketing operations outside the United States. Allocation between domestic and foreign revenues is based on final destination of products sold. No single foreign country represented 10% or more of the Company’s total revenues for fiscal years 2009, 2008 and 2007. Revenues between geographic areas are accounted for at cost plus prevailing markups arrived at through negotiations between profit centers. Intercompany and intracompany profits are eliminated in consolidation.
16. DISCONTINUED OPERATIONS
In September 2008, the Company approved a plan to sell Research Instruments, which develops, manufactures and services highly customized scientific instrument components and systems for fundamental and applied physics research primarily for national research laboratories worldwide. Research Instruments was part of the January 2007 ACCEL acquisition and was previously included in the “Other” category in the Company’s Consolidated Financial Statements. The Company decided to sell Research Instruments in order to focus exclusively on the development of its Varian Particle Therapy business. In the second quarter of fiscal year 2009, the Company completed the sale of Research Instruments for total cash proceeds of $0.4 million. In connection with the sale of Research Instruments, the Company entered into a non-binding supply agreement with the buyer to supply certain inventory parts for the Varian Particle Therapy business. The supply agreement can be terminated by either party upon a six months’ notice after December 31, 2011. The inventory purchases under this supply agreement are not expected to have a significant impact on the cash flows of Research Instruments.
The Company classified the assets and liabilities of Research Instruments as “assets of discontinued operations” and “liabilities of discontinued operations” in the Consolidated Balance Sheets and classified its operating results as a discontinued operation in the Consolidated Statements of Earnings for all periods presented. Because the amounts related to Research Instruments are not material in the Consolidated Statements of Cash Flows and in the Consolidated Statements of Stockholders’ Equity and Comprehensive Earnings for all periods presented, the Company has not segregated them from continuing operations.
Total revenues of Research Instruments, reported in discontinued operations, for fiscal years 2009, 2008 and 2007 were $9.8 million, $35.2 million and $21.6 million, respectively. Loss reported in discontinued operations for fiscal years 2009, 2008 and 2007 was $12.5 million, $15.8 million and $3.4 million, respectively. In fiscal year 2009, loss in discontinued operations included a loss of $8.1 million on the disposal of Research Instruments. In fiscal year 2008, loss from discontinued operations included goodwill impairment and impairment of long-lived assets related to Research Instruments business.
17. SUBSEQUENT EVENTS
On November 13, 2009, the Company’s Board of Directors authorized the repurchase of an additional 5,000,000 shares of VMS common stock from January 1, 2010 through December 31, 2010.
133
Table of Contents
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
18. QUARTERLY FINANCIAL DATA (UNAUDITED)
| Fiscal Year 2009 | ||||||||||||||||||||
| (In millions, except per share amounts) | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | Total Year | |||||||||||||||
Revenue | $ | 508.7 | $ | 553.6 | $ | 509.8 | $ | 642.0 | $ | 2,214.1 | ||||||||||
Gross margin | $ | 219.0 | $ | 240.4 | $ | 216.2 | $ | 284.9 | $ | 960.5 | ||||||||||
Net earnings from continuing operations | $ | 69.6 | $ | 79.3 | $ | 85.4 | $ | 97.2 | $ | 331.5 | ||||||||||
Net loss from discontinued operations | $ | (0.8 | ) | $ | (11.5 | ) | $ | — | $ | (0.2 | ) | $ | (12.5 | ) | ||||||
Net earnings | $ | 68.8 | $ | 67.8 | $ | 85.4 | $ | 97.0 | $ | 319.0 | ||||||||||
Net earnings (loss) per share—basic: | ||||||||||||||||||||
Continuing operations | $ | 0.56 | $ | 0.64 | $ | 0.69 | $ | 0.78 | $ | 2.67 | ||||||||||
Discontinued operations | $ | — | $ | (0.09 | ) | $ | — | $ | — | $ | (0.10 | ) | ||||||||
Net earnings per share | $ | 0.56 | $ | 0.55 | $ | 0.69 | $ | 0.78 | $ | 2.57 | ||||||||||
Net earnings (loss) per share—diluted: | ||||||||||||||||||||
Continuing operations | $ | 0.56 | $ | 0.64 | $ | 0.68 | $ | 0.78 | $ | 2.65 | ||||||||||
Discontinued operations | $ | (0.01 | ) | $ | (0.10 | ) | $ | — | $ | (0.01 | ) | $ | (0.10 | ) | ||||||
Net earnings per share | $ | 0.55 | $ | 0.54 | $ | 0.68 | $ | 0.77 | $ | 2.55 | ||||||||||
| Fiscal Year 2008 | ||||||||||||||||||||
| (In millions, except per share amounts) | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | Total Year | |||||||||||||||
Revenue | $ | 451.2 | $ | 518.4 | $ | 507.4 | $ | 592.7 | $ | 2,069.7 | ||||||||||
Gross margin | $ | 191.1 | $ | 211.6 | $ | 212.3 | $ | 262.5 | $ | 877.5 | ||||||||||
Net earnings from continuing operations | $ | 58.2 | $ | 72.9 | $ | 77.1 | $ | 87.1 | $ | 295.3 | ||||||||||
Net loss from discontinued operations | $ | (2.7 | ) | $ | (1.6 | ) | $ | (2.9 | ) | $ | (8.6 | ) | $ | (15.8 | ) | |||||
Net earnings | $ | 55.5 | $ | 71.3 | $ | 74.2 | $ | 78.5 | $ | 279.5 | ||||||||||
Net earnings (loss) per share—basic: | ||||||||||||||||||||
Continuing operations | $ | 0.47 | $ | 0.58 | $ | 0.62 | $ | 0.70 | $ | 2.37 | ||||||||||
Discontinued operations | $ | (0.03 | ) | $ | (0.01 | ) | $ | (0.02 | ) | $ | (0.07 | ) | $ | (0.13 | ) | |||||
Net earnings per share | $ | 0.44 | $ | 0.57 | $ | 0.60 | $ | 0.63 | $ | 2.24 | ||||||||||
Net earnings (loss) per share—diluted: | ||||||||||||||||||||
Continuing operations | $ | 0.46 | $ | 0.57 | $ | 0.61 | $ | 0.68 | $ | 2.31 | ||||||||||
Discontinued operations | $ | (0.03 | ) | $ | (0.01 | ) | $ | (0.03 | ) | $ | (0.06 | ) | $ | (0.12 | ) | |||||
Net earnings per share | $ | 0.43 | $ | 0.56 | $ | 0.58 | $ | 0.62 | $ | 2.19 | ||||||||||
The operating results of Research Instruments are presented as a discontinued operation for all periods. See Note 16, “Discontinued Operations” for detailed discussion.
The four quarters for net earnings per share may not add to the total year because of differences in the weighted average numbers of shares outstanding during the quarters and the year.
134
Table of Contents
REPORT OF MANAGEMENT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management of Varian Medical Systems, Inc. and its subsidiaries (the “Company”) is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles in the United States of America, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions and that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of the Company’s internal control over financial reporting as of October 2, 2009. In making this assessment, management used the criteria set forth inInternal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on its assessment and those criteria, management concluded that the Company maintained effective internal control over financial reporting as of October 2, 2009. PricewaterhouseCoopers LLP has issued an attestation report on the Company’s internal control over financial reporting as of October 2, 2009, which appears immediately after this report.
135
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Varian Medical Systems, Inc.:
In our opinion, the consolidated financial statements listed in the index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of Varian Medical Systems, Inc. and its subsidiaries at October 2, 2009 and September 26, 2008, and the results of their operations and their cash flows for each of the three years in the period ended October 2, 2009 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the index appearing under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of October 2, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Report of Management on Internal Control over Financial Reporting. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in Note 10 to the consolidated financial statements, effective September 28, 2007, the Company changed its method of accounting for certain defined benefit plans. As discussed in Note 13 to the consolidated financial statements, effective September 28, 2007, the Company changed its method of accounting for uncertainty in income taxes.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
136
Table of Contents
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/S/ PRICEWATERHOUSECOOPERS LLP
San Jose, California
November 25, 2009
137
Table of Contents
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
| (a) | Evaluation of disclosure controls and procedures. Based on the evaluation of our disclosure controls and procedures (as defined in the Rules 13a-15(e) and 15d-15(e) under the Exchange Act required by Exchange Act Rules 13a-15(b) or 15d-15(b), our principal executive officer and principal financial officer have concluded that as of the end of the period covered by this report, our disclosure controls and procedures were effective to ensure that information required to be disclosed by the Company in reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission rules and forms, and include controls and procedures designed to ensure that information required to be disclosed by us in such reports is accumulated and communicated to our management, including the principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure. |
| (b) | Report of management on internal control over financial reporting. The information required to be furnished pursuant to this item is set forth under the caption “Report of Management on Internal Control over Financial Reporting” on page 135 of this Annual Report on Form 10-K, and is incorporated here by reference. |
| (c) | Changes in internal control over financial reporting. There were no changes in our internal control over financial reporting that occurred during our fourth fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. |
| (d) | Certificates. Certificates with respect to disclosure controls and procedures and internal control over financial reporting under Rule 13a-14(a) of the Exchange Act are attached as Exhibits 31.1 and 31.2 to this Annual Report on Form 10-K. |
None.
138
Table of Contents
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this item with respect to our executive officers is set forth in Part I of this Annual Report on Form 10-K. The information required by this item with respect to our directors, our Audit Committee and its members, and audit committee financial expert is incorporated by reference from our definitive proxy statement for the 2010 Annual Meeting of Stockholders under the caption “Proposal One—Election of Directors.” The information required by this item with respect to compliance with Section 16(a) of the Exchange Act is incorporated by reference from our definitive proxy statement for the 2010 Annual Meeting of Stockholders under the caption “Stock Ownership—Section 16(a) Beneficial Ownership Reporting Compliance.”
We have adopted a Code of Business Ethics that applies to all of our executive officers and directors. The Code of Business Ethics is posted on our website. The Internet address for our website ishttp://www.varian.com, and the Code of Business Ethics may be found as follows:
| 1. | From our main web page, first click “Investors.” |
| 2. | Next click on “Corporate Governance” in the left hand navigation bar. |
| 3. | Finally, click on “Code of Ethics.” |
Additionally, copies of our Code of Business Ethics may also be obtained without charge by sending a written request to our Secretary at our executive offices.
We intend to satisfy the disclosure requirements under Item 5.05 of Form 8-K regarding an amendment to, or waiver from, a provision of the Code of Business Ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions by posting such information on our website, at the address and location specified above.
Furthermore, since VMS common stock is listed on the NYSE, our Chief Executive Officer is required to make, and he has made as of March 16, 2009, an Annual Certification to the NYSE in accordance with Section 303A of the NYSE Listed Company Manual stating that he was not aware of any violations by us of the NYSE corporate governance listing standards.
Item 11. Executive Compensation
The information required by this item is incorporated by reference from our definitive proxy statement for the 2010 Annual Meeting of Stockholders under the caption “Compensation of the Named Executive Officers and Directors.”
139
Table of Contents
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Equity Compensation Plan Information
The following table provides information as of October 2, 2009 with respect to the shares of VMS common stock that may be issued under existing equity compensation plans.
| A | B | C | |||||||
Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column A) | ||||||
Equity compensation plans approved by security holders | 8,793,002 | (1) | $ | 44.46 | 9,350,428 | (2) | |||
Equity compensation plans not approved by security holders(3) | 3,059,717 | $ | 29.47 | — | |||||
Total | 11,852,719 | $ | 40.59 | 9,350,428 | |||||
| (1) | Consists of awards granted under the Omnibus Stock Plan, the 2005 Omnibus Stock Plan, the Amended and Restated 2005 Omnibus Stock Plan and the Second Amended and Restated 2005 Omnibus Stock Plan, as amended. Effective February 17, 2005, no further grants can be made under the Omnibus Stock Plan. |
| (2) | Includes 3,997,971 shares available for future issuance under the Employee Stock Purchase Plan. |
| (3) | Consists of awards granted under the 2000 Stock Option Plan. Effective February 17, 2005, no further grants can be made under the 2000 Stock Option Plan. |
The 2000 Stock Option Plan was intended to supplement the Omnibus Stock Plan. The 2000 Stock Option Plan is similar to the Omnibus Stock Plan in all material respects, with the exception that awards under the 2000 Stock Option Plan could not be made to directors or officers of the Company. For a description of the material features of the Omnibus Stock Plan and the 2000 Stock Option Plan, see Note 12, “Employee Stock Plans” of the Notes to the Consolidated Financial Statements, which description is incorporated by reference.
The information required by this item with respect to the security ownership of certain beneficial owners and the security ownership of directors and executive officers is incorporated by reference from our definitive proxy statement for the 2010 Annual Meeting of Stockholders under the caption “Stock Ownership—Beneficial Ownership of Certain Stockholders, Directors and Executive Officers.”
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item with respect to certain relationships and related transactions is incorporated by reference from our definitive proxy statement for the 2010 Annual Meeting of Stockholders under the caption “Certain Relationships and Related Transactions.” The information required by this item with respect to director and committee member independence is incorporated by reference from our definitive proxy statement for the 2010 Annual Meeting of Stockholders under the caption “Proposal One—Election of Directors.”
Item 14. Principal Accountant Fees and Services
The information required by this item is incorporated by reference from our definitive proxy statement for the 2010 Annual Meeting of Stockholders under the caption “Proposal Four—Ratification of the Appointment of Our Independent Registered Public Accounting Firm.”
140
Table of Contents
Item 15. Exhibits and Financial Statement Schedules
| (a) | The following documents are filed as part of this report: |
| (1) | Consolidated Financial Statements: |
| · | Consolidated Statements of Earnings |
| · | Consolidated Balance Sheets |
| · | Consolidated Statements of Cash Flows |
| · | Consolidated Statements of Stockholders’ Equity and Comprehensive Earnings |
| · | Notes to the Consolidated Financial Statements |
| · | Report of Independent Registered Public Accounting Firm |
| (2) | Consolidated Financial Statement Schedule: |
The following financial statement schedule of the Registrant and its subsidiaries for fiscal years 2009, 2008 and 2007 is filed as a part of this report and should be read in conjunction with the Consolidated Financial Statements of the Registrant and its subsidiaries.
Schedule | ||
| II | Valuation and Qualifying Accounts |
All other schedules are omitted because of the absence of conditions under which they are required or because the required information is given in the financial statements or the notes thereto.
| (3) | Exhibits: |
Exhibit | Description | |
| 2 | Amended and Restated Distribution Agreement, dated as of January 14, 1999, by and among Varian Associates, Inc. (which has been renamed Varian Medical Systems, Inc.), Varian, Inc. and Varian Semiconductor Equipment Associates, Inc. (incorporated by reference to Exhibit No. 2 to the Registrant’s Form 8-K Current Report dated as of April 2, 1999, File No. 1-7598). | |
| 3.1 | Registrant’s Amended and Restated Certificate of Incorporation, as amended (incorporated by reference to Exhibit No. 3.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended July 2, 2004, File No. 1-7598). | |
| 3.2 | Registrant’s By-Laws, as amended, effective November 17, 2005 (incorporated by reference to Exhibit 99.4 to the Company’s Current Report on Form 8-K filed on November 23, 2005). | |
| 4.1 | Specimen Common Stock Certificate (incorporated by reference to Exhibit No. 4.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 2, 1999, File No. 1-7598). | |
| 10.1† | Registrant’s Amended and Restated Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended July 2, 2004, File No. 1-7598). | |
| 10.2† | Registrant’s Amended and Restated 2000 Stock Option Plan (incorporated by reference to Exhibit No. 10.2 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended July 2, 2004, File No. 1-7598). | |
| 10.3† | Form of Registrant’s Indemnity Agreement with the directors and executive officers (incorporated by reference to Exhibit No. 10.3 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 2, 1999, File No. 1-7598). |
141
Table of Contents
Exhibit | Description | |
| 10.4† | Form of Registrant’s Change in Control Agreement for Chief Executive Officer (incorporated by reference to Exhibit No. 10.4 to the Registrant’s Form 10-K Annual Report for the fiscal year ended September 29, 2006, File No. 1-7598). | |
| 10.5† | Form of Registrant’s Change in Control Agreement for Senior Executives (Chief Financial Officer and General Counsel) (incorporated by reference to Exhibit No. 10.5 to the Registrant’s Form 10-K Annual Report for the fiscal year ended September 29, 2006, File No. 1-7598). | |
| 10.6† | Form of Registrant’s Change in Control Agreement for Senior Executives (other than the Chief Executive Officer, the Chief Financial Officer, and the General Counsel) (incorporated by reference to Exhibit No. 10.6 to the Registrant’s Form 10-K Annual Report for the fiscal year ended September 29, 2006, File No. 1-7598). | |
| 10.7† | Form of Registrant’s Change in Control Agreement for Key Employees (incorporated by reference to Exhibit No. 10.7 to the Registrant’s Form 10-K Annual Report for the fiscal year ended September 29, 2006, File No. 1-7598). | |
| 10.8† | Form of Amendment to the Change in Control Agreement for Chief Executive Officer, Senior Executives (Chief Financial Officer and General Counsel), Senior Executives (other than the Chief Executive Officer, the Chief Financial Officer, and the General Counsel) and Key Employees (incorporated by reference to Exhibit No. 10.8 to the Registrant’s Form 10-K Annual Report for the year ended September 26, 2008, File No. 1-7598). | |
| 10.9 | Amended and Restated Note Purchase and Private Shelf Agreement, dated as of April 2, 1999, between the Registrant and Prudential Insurance Company of America (certain exhibits and schedules omitted) (incorporated by reference to Exhibit No. 10.7 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 2, 1999, File No. 1-7598). | |
| 10.10 | Employee Benefits Allocation Agreement, dated April 2, 1999, by and among Varian Associates, Inc. (which has been renamed Varian Medical Systems, Inc.), Varian, Inc. and Varian Semiconductor Equipment Associates, Inc. (incorporated by reference to Exhibit No. 99.1 to the Registrant’s Form 8-K Current Report dated as of April 2, 1999, File No. 1-7598). | |
| 10.11 | Intellectual Property Agreement, dated April 2, 1999, by and among Varian Associates, Inc. (which has been renamed Varian Medical Systems, Inc.), Varian, Inc. and Varian Semiconductor Equipment Associates, Inc. (incorporated by reference to Exhibit No. 99.2 to the Registrant’s Form 8-K Current Report dated as of April 2, 1999, File No. 1-7598). | |
| 10.12 | Tax Sharing Agreement, dated April 2, 1999, by and among Varian Associates, Inc. (which has been renamed Varian Medical Systems, Inc.), Varian, Inc. and Varian Semiconductor Equipment Associates, Inc. (incorporated by reference to Exhibit No. 99.3 to the Registrant’s Form 8-K Current Report dated as of April 2, 1999, File No. 1-7598). | |
| 10.13† | Registrant’s Frozen Deferred Compensation Plan (incorporated by reference to Exhibit No. 10.17 to the Registrant’s Form 10-K Annual Report for the fiscal year ended September 29, 2000, File No. 1-7598). | |
| 10.14† | Registrant’s Amended and Restated 2005 Deferred Compensation Plan (incorporated by reference to Exhibit No. 10.2 of the Registrant’s Form 10-Q Quarterly Report for the quarter ended January 2, 2009, File No. 1-7598). | |
| 10.15† | Registrant’s Management Incentive Plan (incorporated by reference to Exhibit No. 10.5 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 3, 2009, File No. 1-7598). | |
| 10.16† | Registrant’s Retirement Plan (incorporated by reference to Exhibit No. 99.1 to the Registrant’s Registration Statement on Form S-8 filed on March 14, 2001, and amended June 20, 2001, Registration No. 333-57012). |
142
Table of Contents
Exhibit | Description | |
| 10.17† | Registrant’s Amended and Restated Employee Stock Purchase Plan (incorporated by reference to Exhibit No. 10.3 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended July 2, 2004, File No. 1-7598). | |
| 10.18† | Registrant’s Employment Letter dated September 17, 2004 with Dow R. Wilson as Corporate Vice President and President, Oncology Systems, effective January 10, 2005 (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended December 31, 2004, File No. 1-7598). | |
| 10.19† | Amendment to the Registrant’s Employment Letter dated August 5, 2005 with Dow R. Wilson (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended July 1, 2005, File No. 1-7598) | |
| 10.20†* | Description of Certain Compensatory Arrangements between the Registrant and its Executive Officers and Directors as of November 14, 2008. | |
| 10.21† | Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended March 30, 2007, File No. 1-7598). | |
| 10.22† | Amendment No. 1 to Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended June 27, 2008, File No. 1-7598). | |
| 10.23† | Amendment No. 2 to Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.23 to the Registrant’s Form 10-K Annual Report for the year ended September 26, 2008, File No. 1-7598). | |
| 10.24† | Amendment No. 3 to Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.4 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 3, 2009, File No. 1-7598). | |
| 10.25† | Form of Registrant’s Restricted Stock Agreement under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.3 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 3, 2009, File No. 1-7598). | |
| 10.26† | Form of Registrant’s Nonqualified Stock Option Agreement under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.22 to the Registrant’s Form 10-K Annual Report for the year ended September 28, 2007, File No. 1-7598). | |
| 10.27† | Form of Registrant’s Nonqualified Stock Option Agreement for Officers under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.23 to the Registrant’s Form 10-K Annual Report for the year ended September 28, 2007, File No. 1-7598). | |
| 10.28† | Form of Registrant’s Nonqualified Stock Option Agreement for Directors under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Option Plan (incorporated by reference to Exhibit No. 10.24 to the Registrant’s Form 10-K Annual Report for the year ended September 28, 2007, File No. 1-7598). | |
| 10.29† | Form of Registrant’s Non-Employee Director NonQualified Stock Option Agreement (for use outside the United States) under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit 99.1 of the registrant’s Current Report on Form 8-K filed on February 18, 2009, File No. 1-7598). | |
| 10.30† | Form of Registrant’s Grant Agreement for Deferred Stock Units under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.28 to the Registrant’s Form 10-K Annual Report for the year ended September 26, 2008, File No. 1-7598). |
143
Table of Contents
Exhibit | Description | |
| 10.31† | Form of Registrant’s Non-Employee Director Deferred Stock Unit Agreement (for use outside the United States) under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit 99.2 of the registrant’s Current Report on Form 8-K filed on February 18, 2009, File No. 1-7598). | |
| 10.32++ | Amended and Restated Credit Agreement entered into as of November 10, 2008 by and between the Registrant and Bank of America, N.A. (incorporated by reference to Exhibit No. 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended January 2, 2009, File No. 1-7598). | |
| 10.33* | Amendment to Amended and Restated Credit Agreement entered into as of July 14, 2009 by and between the Registrant and Bank of America, N.A. | |
| 21* | List of Subsidiaries. | |
| 23* | Consent of Independent Registered Public Accounting Firm. | |
| 31.1* | Chief Executive Officer Certification Pursuant to Rule 13a-14(a) of the Securities Exchange Act. | |
| 31.2* | Chief Financial Officer Certification Pursuant to Rule 13a-14(a) of the Securities Exchange Act. | |
| 32.1* | Certification pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 32.2* | Certification pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 101.INS** | XBRL Instance Document | |
| 101.SCH** | XBRL Taxonomy Extension Schema Document | |
| 101.CAL** | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF** | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB** | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE** | XBRL Taxonomy Extension Presentation Linkbase Document |
| † | Management contract or compensatory arrangement. |
| * | Filed herewith. |
| ++ | Confidential treatment has been requested as to certain portions of this exhibit pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| ** | Attached as Exhibit 101 to this Annual Report on Form 10-K are the following formatted in XBRL (Extensible Business Reporting Language): (i) Consolidated Statements of Earnings for the fiscal years ended October 2, 2009, September 26, 2008 and September 28, 2007; (ii) Consolidated Balance Sheets at October 2, 2009 and September 26, 2008; (iii) Consolidated Statements of Cash Flows for the fiscal years ended October 2, 2009, September 26, 2008 and September 28, 2007; (iv) Consolidated Statements of Stockholders’ Equity and Comprehensive Earnings for the fiscal years ended October 2, 2009, September 26, 2008 and September 28, 2007; and (iv) Notes to Consolidated Financial Statements for fiscal year ended October 2, 2009. |
In accordance with Rule 406T of Regulation S-T, the XBRL related information in Exhibit 101 to this Annual Report on Form 10-K shall not be deemed to be “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that section, and shall not be part of any registration statement or other document filed under the Securities Act or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
144
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: November 25, 2009
| VARIAN MEDICAL SYSTEMS, INC. | ||
By: | /S/ ELISHA W. FINNEY | |
Elisha W. Finney Senior Vice President, Finance and Chief Financial Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated.
Signature | Capacity | Date | ||
/S/ TIMOTHY E. GUERTIN Timothy E. Guertin | President and Chief Executive Officer and Director (Principal Executive Officer) | November 25, 2009 | ||
/S/ ELISHA W. FINNEY Elisha W. Finney | Senior Vice President, Finance and Chief Financial Officer (Principal Financial Officer) | November 25, 2009 | ||
/S/ TAI-YUN CHEN Tai-yun Chen | Corporate Vice President, Finance and Corporate Controller (Principal Accounting Officer) | November 25, 2009 | ||
/S/ RICHARD M. LEVY Richard M. Levy | Chairman of the Board | November 25, 2009 | ||
/S/ SUSAN L. BOSTROM Susan L. Bostrom | Director | November 25, 2009 | ||
/S/ JOHN SEELY BROWN John Seely Brown | Director | November 25, 2009 | ||
/S/ R. ANDREW ECKERT R. Andrew Eckert | Director | November 25, 2009 | ||
/S/ MARK R. LARET Mark R. Laret | Director | November 25, 2009 | ||
/S/ DAVID W. MARTIN, JR. David W. Martin, Jr. | Director | November 25, 2009 | ||
145
Table of Contents
Signature | Capacity | Date | ||
/S/ RUEDIGER NAUMANN-ETIENNE Ruediger Naumann-Etienne | Director | November 25, 2009 | ||
/S/ VENKATRAMAN THYAGARAJAN Venkatraman Thyagarajan | Director | November 25, 2009 | ||
146
Table of Contents
Schedule II
VARIAN MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
VALUATION AND QUALIFYING ACCOUNTS
Fiscal Year | Description | Balance at Beginning of Period | Charged to Bad Debt Expense | Write-Offs/ Adjustments Charged to Allowance | Balance at End of Period | |||||||||
| (In thousands) | ||||||||||||||
2009 | Allowance for doubtful accounts receivable | $ | 3,110 | $ | 2,038 | $ | 801 | $ | 4,347 | |||||
2008 | Allowance for doubtful accounts receivable | $ | 3,859 | $ | 250 | $ | 999 | $ | 3,110 | |||||
2007 | Allowance for doubtful accounts receivable | $ | 4,473 | $ | 1,086 | $ | 1,700 | $ | 3,859 | |||||
Fiscal Year | Description | Balance at Beginning of Period | Increases | Deductions | Balance at End of Period | |||||||||
| (In thousands) | ||||||||||||||
2009 | Valuation allowance for deferred tax assets | $ | 20,757 | $ | 15,450 | $ | 778 | $ | 35,429 | |||||
2008 | Valuation allowance for deferred tax assets | $ | 17,951 | $ | 3,783 | $ | 977 | $ | 20,757 | |||||
2007 | Valuation allowance for deferred tax assets | $ | 1,608 | $ | 16,435 | $ | 92 | $ | 17,951 | |||||
147
Table of Contents
EXHIBIT INDEX
Exhibit | Description | |
| 2 | Amended and Restated Distribution Agreement, dated as of January 14, 1999, by and among Varian Associates, Inc. (which has been renamed Varian Medical Systems, Inc.), Varian, Inc. and Varian Semiconductor Equipment Associates, Inc. (incorporated by reference to Exhibit No. 2 to the Registrant’s Form 8-K Current Report dated as of April 2, 1999, File No. 1-7598). | |
| 3.1 | Registrant’s Amended and Restated Certificate of Incorporation, as amended (incorporated by reference to Exhibit No. 3.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended July 2, 2004, File No. 1-7598). | |
| 3.2 | Registrant’s By-Laws, as amended, effective November 17, 2005 (incorporated by reference to Exhibit 99.4 to the Company’s Current Report on Form 8-K filed on November 23, 2005). | |
| 4.1 | Specimen Common Stock Certificate (incorporated by reference to Exhibit No. 4.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 2, 1999, File No. 1-7598). | |
| 10.1† | Registrant’s Amended and Restated Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended July 2, 2004, File No. 1-7598). | |
| 10.2† | Registrant’s Amended and Restated 2000 Stock Option Plan (incorporated by reference to Exhibit No. 10.2 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended July 2, 2004, File No. 1-7598). | |
| 10.3† | Form of Registrant’s Indemnity Agreement with the directors and executive officers (incorporated by reference to Exhibit No. 10.3 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 2, 1999, File No. 1-7598). | |
| 10.4† | Form of Registrant’s Change in Control Agreement for Chief Executive Officer (incorporated by reference to Exhibit No. 10.4 to the Registrant’s Form 10-K Annual Report for the fiscal year ended September 29, 2006, File No. 1-7598). | |
| 10.5† | Form of Registrant’s Change in Control Agreement for Senior Executives (Chief Financial Officer and General Counsel) (incorporated by reference to Exhibit No. 10.5 to the Registrant’s Form 10-K Annual Report for the fiscal year ended September 29, 2006, File No. 1-7598). | |
| 10.6† | Form of Registrant’s Change in Control Agreement for Senior Executives (other than the Chief Executive Officer, the Chief Financial Officer, and the General Counsel) (incorporated by reference to Exhibit No. 10.6 to the Registrant’s Form 10-K Annual Report for the fiscal year ended September 29, 2006, File No. 1-7598). | |
| 10.7† | Form of Registrant’s Change in Control Agreement for Key Employees (incorporated by reference to Exhibit No. 10.7 to the Registrant’s Form 10-K Annual Report for the fiscal year ended September 29, 2006, File No. 1-7598). | |
| 10.8† | Form of Amendment to the Change in Control Agreement for Chief Executive Officer, Senior Executives (Chief Financial Officer and General Counsel), Senior Executives (other than the Chief Executive Officer, the Chief Financial Officer, and the General Counsel) and Key Employees (incorporated by reference to Exhibit No. 10.8 to the Registrant’s Form 10-K Annual Report for the year ended September 26, 2008, File No. 1-7598). | |
| 10.9 | Amended and Restated Note Purchase and Private Shelf Agreement, dated as of April 2, 1999, between the Registrant and Prudential Insurance Company of America (certain exhibits and schedules omitted) (incorporated by reference to Exhibit No. 10.7 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 2, 1999, File No. 1-7598). |
148
Table of Contents
Exhibit | Description | |
| 10.10 | Employee Benefits Allocation Agreement, dated April 2, 1999, by and among Varian Associates, Inc. (which has been renamed Varian Medical Systems, Inc.), Varian, Inc. and Varian Semiconductor Equipment Associates, Inc. (incorporated by reference to Exhibit No. 99.1 to the Registrant’s Form 8-K Current Report dated as of April 2, 1999, File No. 1-7598). | |
| 10.11 | Intellectual Property Agreement, dated April 2, 1999, by and among Varian Associates, Inc. (which has been renamed Varian Medical Systems, Inc.), Varian, Inc. and Varian Semiconductor Equipment Associates, Inc. (incorporated by reference to Exhibit No. 99.2 to the Registrant’s Form 8-K Current Report dated as of April 2, 1999, File No. 1-7598). | |
| 10.12 | Tax Sharing Agreement, dated April 2, 1999, by and among Varian Associates, Inc. (which has been renamed Varian Medical Systems, Inc.), Varian, Inc. and Varian Semiconductor Equipment Associates, Inc. (incorporated by reference to Exhibit No. 99.3 to the Registrant’s Form 8-K Current Report dated as of April 2, 1999, File No. 1-7598). | |
| 10.13† | Registrant’s Frozen Deferred Compensation Plan (incorporated by reference to Exhibit No. 10.17 to the Registrant’s Form 10-K Annual Report for the fiscal year ended September 29, 2000, File No. 1-7598). | |
| 10.14† | Registrant’s Amended and Restated 2005 Deferred Compensation Plan (incorporated by reference to Exhibit No. 10.2 of the Registrant’s Form 10-Q Quarterly Report for the quarter ended January 2, 2009, File No. 1-7598). | |
| 10.15† | Registrant’s Management Incentive Plan (incorporated by reference to Exhibit No. 10.5 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 3, 2009, File No. 1-7598). | |
| 10.16† | Registrant’s Retirement Plan (incorporated by reference to Exhibit No. 99.1 to the Registrant’s Registration Statement on Form S-8 filed on March 14, 2001, and amended June 20, 2001, Registration No. 333-57012). | |
| 10.17† | Registrant’s Amended and Restated Employee Stock Purchase Plan (incorporated by reference to Exhibit No. 10.3 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended July 2, 2004, File No. 1-7598). | |
| 10.18† | Registrant’s Employment Letter dated September 17, 2004 with Dow R. Wilson as Corporate Vice President and President, Oncology Systems, effective January 10, 2005 (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended December 31, 2004, File No. 1-7598). | |
| 10.19† | Amendment to the Registrant’s Employment Letter dated August 5, 2005 with Dow R. Wilson (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended July 1, 2005, File No. 1-7598) | |
| 10.20†* | Description of Certain Compensatory Arrangements between the Registrant and its Executive Officers and Directors as of November 14, 2008. | |
| 10.21† | Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended March 30, 2007, File No. 1-7598). | |
| 10.22† | Amendment No. 1 to Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended June 27, 2008, File No. 1-7598). | |
| 10.23† | Amendment No. 2 to Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.23 to the Registrant’s Form 10-K Annual Report for the year ended September 26, 2008, File No. 1-7598). | |
| 10.24† | Amendment No. 3 to Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.4 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 3, 2009, File No. 1-7598). |
149
Table of Contents
Exhibit | Description | |
| 10.25† | Form of Registrant’s Restricted Stock Agreement under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.3 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended April 3, 2009, File No. 1-7598). | |
| 10.26† | Form of Registrant’s Nonqualified Stock Option Agreement under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.22 to the Registrant’s Form 10-K Annual Report for the year ended September 28, 2007, File No. 1-7598). | |
| 10.27† | Form of Registrant’s Nonqualified Stock Option Agreement for Officers under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.23 to the Registrant’s Form 10-K Annual Report for the year ended September 28, 2007, File No. 1-7598). | |
| 10.28† | Form of Registrant’s Nonqualified Stock Option Agreement for Directors under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Option Plan (incorporated by reference to Exhibit No. 10.24 to the Registrant’s Form 10-K Annual Report for the year ended September 28, 2007, File No. 1-7598). | |
| 10.29† | Form of Registrant’s Non-Employee Director NonQualified Stock Option Agreement (for use outside the United States) under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit 99.1 of the registrant’s Current Report on Form 8-K filed on February 18, 2009, File No. 1-7598). | |
| 10.30† | Form of Registrant’s Grant Agreement for Deferred Stock Units under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit No. 10.28 to the Registrant’s Form 10-K Annual Report for the year ended September 26, 2008, File No. 1-7598). | |
| 10.31† | Form of Registrant’s Non-Employee Director Deferred Stock Unit Agreement (for use outside the United States) under the Registrant’s Second Amended and Restated 2005 Omnibus Stock Plan (incorporated by reference to Exhibit 99.2 of the registrant’s Current Report on Form 8-K filed on February 18, 2009, File No. 1-7598). | |
| 10.32++ | Amended and Restated Credit Agreement entered into as of November 10, 2008 by and between the Registrant and Bank of America, N.A. (incorporated by reference to Exhibit No. 10.1 to the Registrant’s Form 10-Q Quarterly Report for the quarter ended January 2, 2009, File No. 1-7598). | |
| 10.33* | Amendment to Amended and Restated Credit Agreement entered into as of July 14, 2009 by and between the Registrant and Bank of America, N.A. | |
| 21* | List of Subsidiaries. | |
| 23* | Consent of Independent Registered Public Accounting Firm. | |
| 31.1* | Chief Executive Officer Certification Pursuant to Rule 13a-14(a) of the Securities Exchange Act. | |
| 31.2* | Chief Financial Officer Certification Pursuant to Rule 13a-14(a) of the Securities Exchange Act. | |
| 32.1* | Certification pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 32.2* | Certification pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 101.INS** | XBRL Instance Document | |
| 101.SCH** | XBRL Taxonomy Extension Schema Document | |
| 101.CAL** | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF** | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB** | XBRL Taxonomy Extension Label Linkbase Document |
150
Table of Contents
Exhibit | Description | |
| 101.PRE** | XBRL Taxonomy Extension Presentation Linkbase Document |
| † | Management contract or compensatory arrangement. |
| * | Filed herewith. |
| ++ | Confidential treatment has been requested as to certain portions of this exhibit pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| ** | Attached as Exhibit 101 to this Annual Report on Form 10-K are the following formatted in XBRL (Extensible Business Reporting Language): (i) Consolidated Statements of Earnings for the fiscal years ended October 2, 2009, September 26, 2008 and September 28, 2007; (ii) Consolidated Balance Sheets at October 2, 2009 and September 26, 2008; (iii) Consolidated Statements of Cash Flows for the fiscal years ended October 2, 2009, September 26, 2008 and September 28, 2007; (iv) Consolidated Statements of Stockholders’ Equity and Comprehensive Earnings for the fiscal years ended October 2, 2009, September 26, 2008 and September 28, 2007; and (iv) Notes to Consolidated Financial Statements for fiscal year ended October 2, 2009. |
In accordance with Rule 406T of Regulation S-T, the XBRL related information in Exhibit 101 to this Annual Report on Form 10-K shall not be deemed to be “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that section, and shall not be part of any registration statement or other document filed under the Securities Act or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
151