Building the Premier Women’s Health Company Q4 2021 Earnings March 10, 2022 Exhibit 99.2

Forward-Looking Statements This press release by TherapeuticsMD, Inc. may contain forward-looking statements. Forward-looking statements may include, but are not limited to, statements relating to TherapeuticsMD’s objectives, plans and strategies as well as statements, other than historical facts, that address activities, events or developments that the company intends, expects, projects, believes or anticipates will or may occur in the future. These statements are often characterized by terminology such as "believes," "hopes," "may," "anticipates," "should," "intends," "plans," "will," "expects," "estimates," "projects," "positioned," "strategy" and similar expressions and are based on assumptions and assessments made in light of management’s experience and perception of historical trends, current conditions, expected future developments and other factors believed to be appropriate. Forward-looking statements in this press release are made as of the date of this press release, and the company undertakes no duty to update or revise any such statements, whether as a result of new information, future events or otherwise. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties, many of which are outside of the company’s control. Important factors that could cause actual results, developments and business decisions to differ materially from forward-looking statements are described in the sections titled "Risk Factors" in the company’s filings with the Securities and Exchange Commission, including its most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, as well as reports on Form 8-K, and include the following: the effects of the COVID-19 pandemic; whether TherapeuticsMD will be able to successfully divest its vitaCare business and how the proceeds that may be generated by such divesture will be utilized; the company’s ability to maintain or increase sales of its products; the company’s ability to develop and commercialize IMVEXXY®, ANNOVERA®, and BIJUVA® and obtain additional financing necessary therefor; whether the company will be able to comply with the covenants and conditions under its term loan facility and the company’s ability to refinance such facility; the effects of supply chain issues on the supply of the company’s products; the potential of adverse side effects or other safety risks that could adversely affect the commercialization of the company’s current or future approved products or preclude the approval of the company’s future drug candidates; whether the FDA will approve the manufacturing supplement for ANNOVERA; the company’s ability to protect its intellectual property, including with respect to the Paragraph IV notice letters the company received regarding IMVEXXY and BIJUVA; the length, cost and uncertain results of future clinical trials; the company’s reliance on third parties to conduct its manufacturing, research and development and clinical trials; the ability of the company’s licensees to commercialize and distribute the company’s products; the ability of the company’s marketing contractors to market ANNOVERA; the availability of reimbursement from government authorities and health insurance companies for the company’s products; the impact of product liability lawsuits; the influence of extensive and costly government regulation; the impact of leadership transitions; the volatility of the trading price of the company’s common stock and the concentration of power in its stock ownership.

Drive top-line growth and overall operating performance Address capital structure to ease restrictive revenue and cash covenants currently in place Immediate Priorities Eliminate $60 million from our annual cost base, including the successful divestiture of vitaCare Achieve EBITDA breakeven in the second half of 2022 3 1 2 4
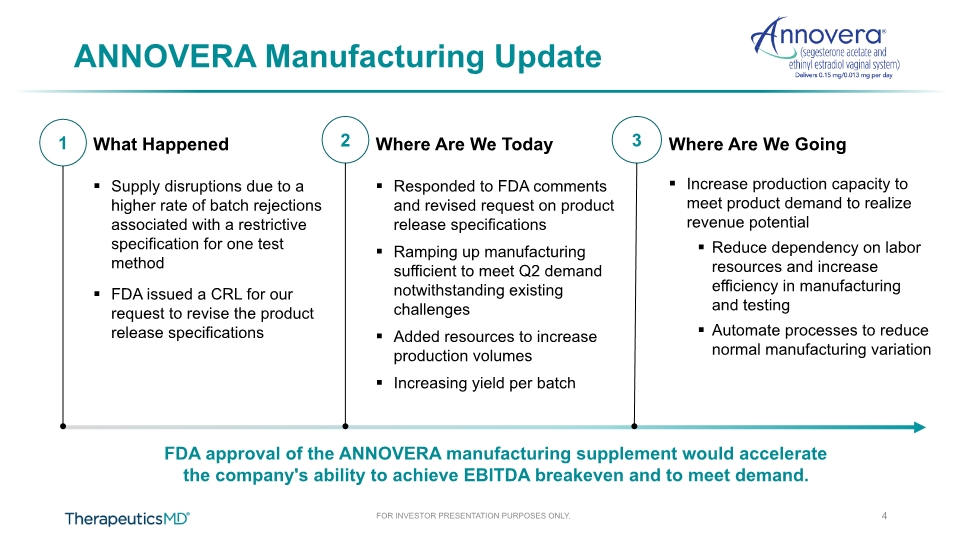
ANNOVERA Manufacturing Update 1 3 2 4 What Happened Supply disruptions due to a higher rate of batch rejections associated with a restrictive specification for one test method FDA issued a CRL for our request to revise the product release specifications Where Are We Today Responded to FDA comments and revised request on product release specifications Ramping up manufacturing sufficient to meet Q2 demand notwithstanding existing challenges Added resources to increase production volumes Increasing yield per batch Where Are We Going Increase production capacity to meet product demand to realize revenue potential Reduce dependency on labor resources and increase efficiency in manufacturing and testing Automate processes to reduce normal manufacturing variation FDA approval of the ANNOVERA manufacturing supplement would accelerate the company's ability to achieve EBITDA breakeven and to meet demand.

5 Delivering on Our Mission Fulfill our commitment to reduce our annual cost base by $60 million Positioned to bring the Company to profitability and restructure our capitalization Successfully scale, manufacture and supply our flagship product, ANNOVERA Deliver results to our shareholders and provide a pathway to EBITDA breakeven Empowering women of all ages through better and affordable healthcare.

6 Q4 21 Financial Overview
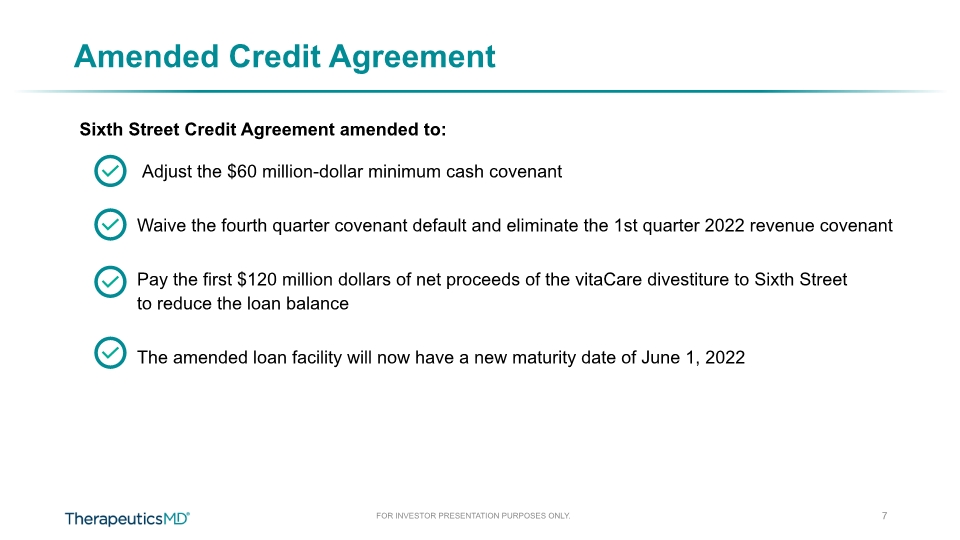
7 Amended Credit Agreement Sixth Street Credit Agreement amended to: Adjust the $60 million-dollar minimum cash covenant Waive the fourth quarter covenant default and eliminate the 1st quarter 2022 revenue covenant Pay the first $120 million dollars of net proceeds of the vitaCare divestiture to Sixth Street to reduce the loan balance The amended loan facility will now have a new maturity date of June 1, 2022
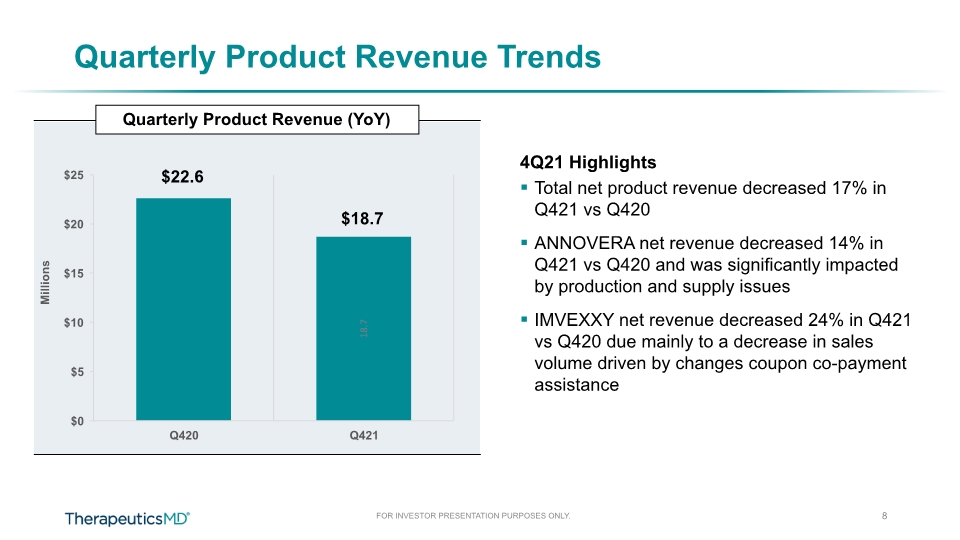
$18.7 Quarterly Product Revenue (YoY) Quarterly Product Revenue Trends Millions 4Q21 Highlights Total net product revenue decreased 17% in Q421 vs Q420 ANNOVERA net revenue decreased 14% in Q421 vs Q420 and was significantly impacted by production and supply issues IMVEXXY net revenue decreased 24% in Q421 vs Q420 due mainly to a decrease in sales volume driven by changes coupon co-payment assistance $22.6 8
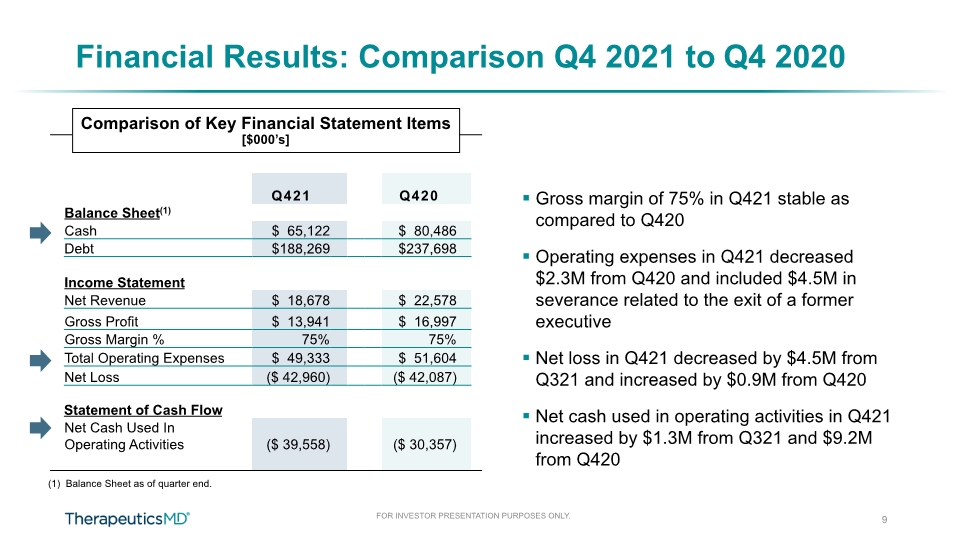
Financial Results: Comparison Q4 2021 to Q4 2020 Gross margin of 75% in Q421 stable as compared to Q420 Operating expenses in Q421 decreased $2.3M from Q420 and included $4.5M in severance related to the exit of a former executive Net loss in Q421 decreased by $4.5M from Q321 and increased by $0.9M from Q420 Net cash used in operating activities in Q421 increased by $1.3M from Q321 and $9.2M from Q420 9 (1) Balance Sheet as of quarter end. Comparison of Key Financial Statement Items [$000’s]

10 Q4 2021 Commercial Overview

ANNOVERA Quarterly TRx +11% Source: Symphony Metys – December 2021 Performance
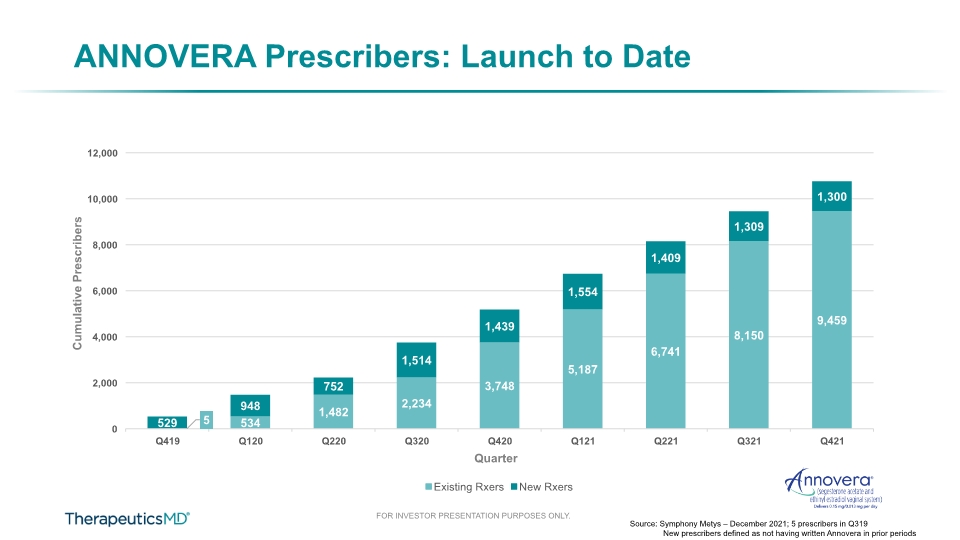
ANNOVERA Prescribers: Launch to Date Source: Symphony Metys – December 2021; 5 prescribers in Q319 New prescribers defined as not having written Annovera in prior periods
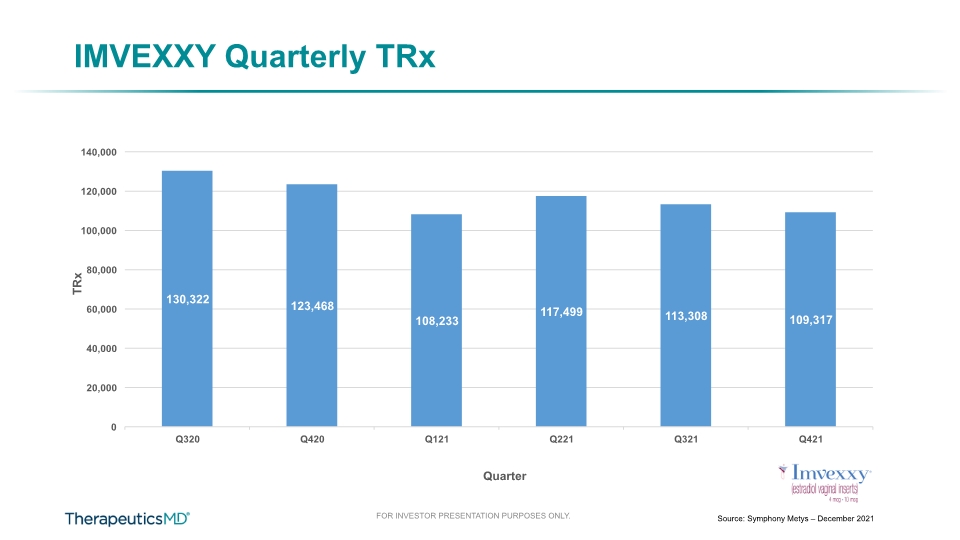
IMVEXXY Quarterly TRx Source: Symphony Metys – December 2021
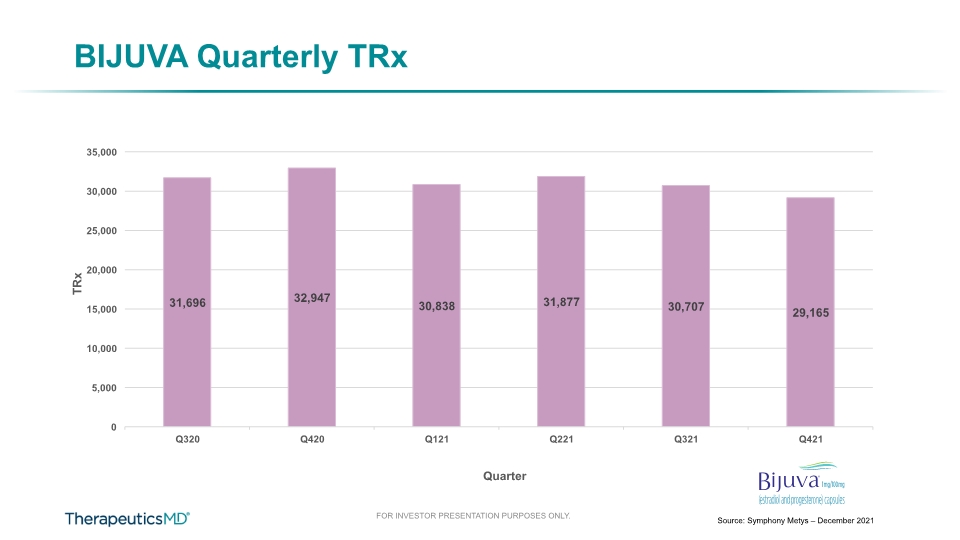
BIJUVA Quarterly TRx Source: Symphony Metys – December 2021
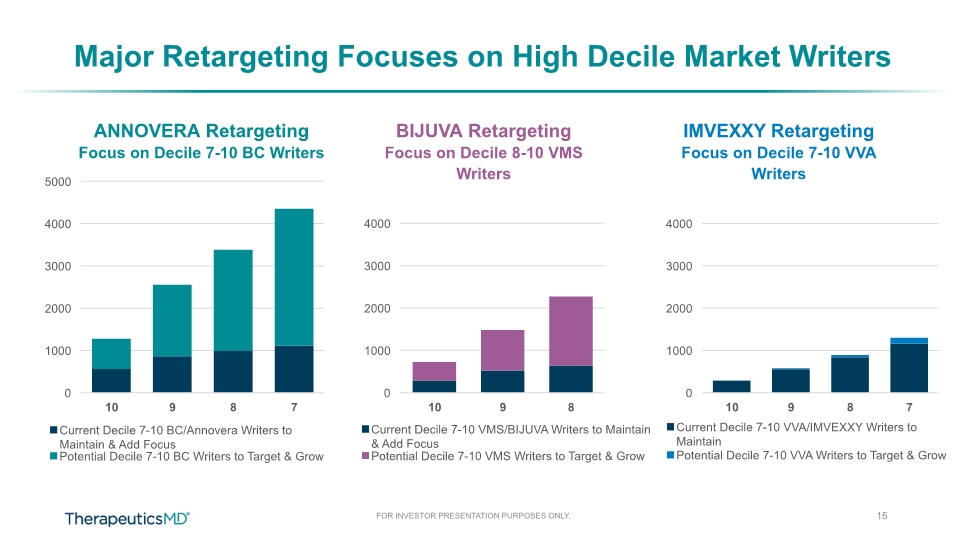
Major Retargeting Focuses on High Decile Market Writers 15
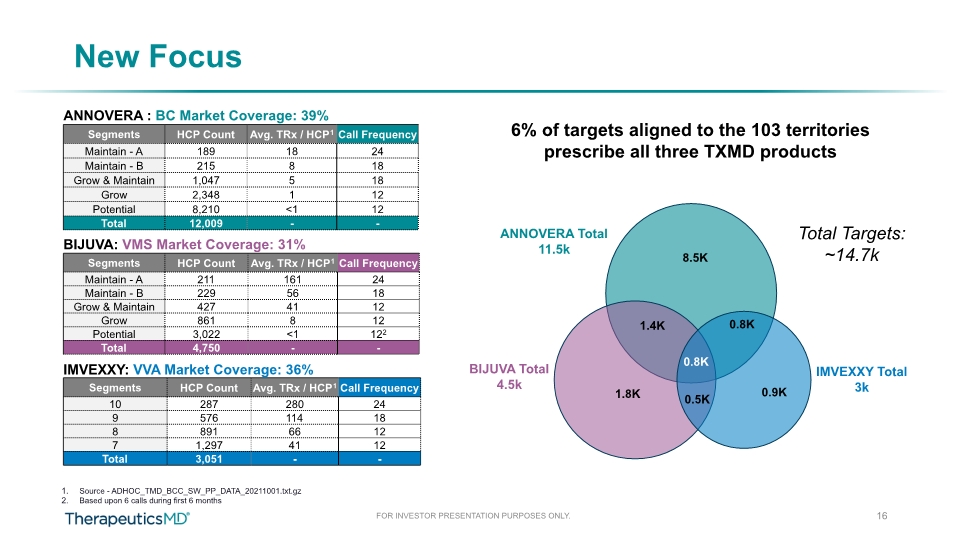
New Focus 16 Total Targets: ~14.7k 6% of targets aligned to the 103 territories prescribe all three TXMD products ANNOVERA : BC Market Coverage: 39% BIJUVA: VMS Market Coverage: 31% IMVEXXY: VVA Market Coverage: 36% Source - ADHOC_TMD_BCC_SW_PP_DATA_20211001.txt.gz Based upon 6 calls during first 6 months
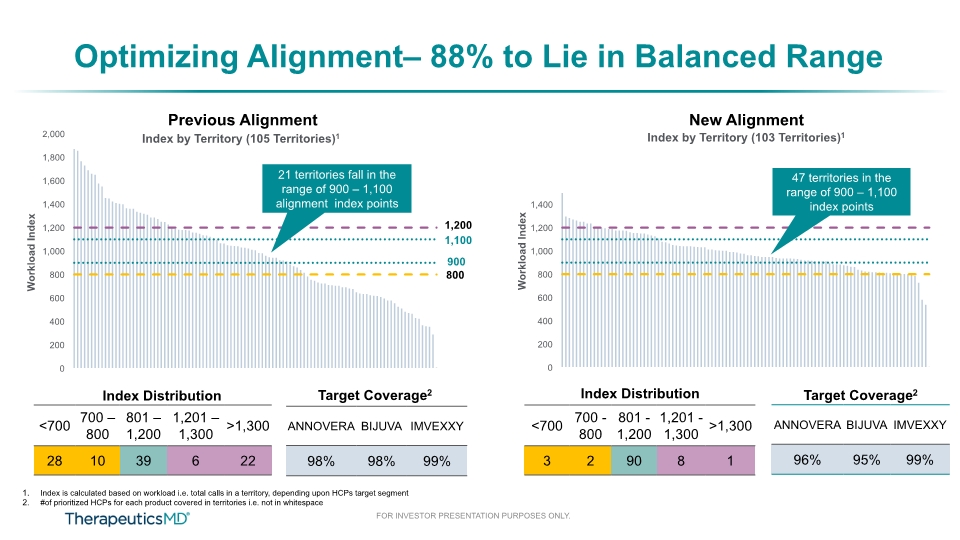
Optimizing Alignment– 88% to Lie in Balanced Range Index Distribution Target Coverage2 Previous Alignment 47 territories in the range of 900 – 1,100 index points 3 Index Distribution Target Coverage2 Index is calculated based on workload i.e. total calls in a territory, depending upon HCPs target segment #of prioritized HCPs for each product covered in territories i.e. not in whitespace New Alignment
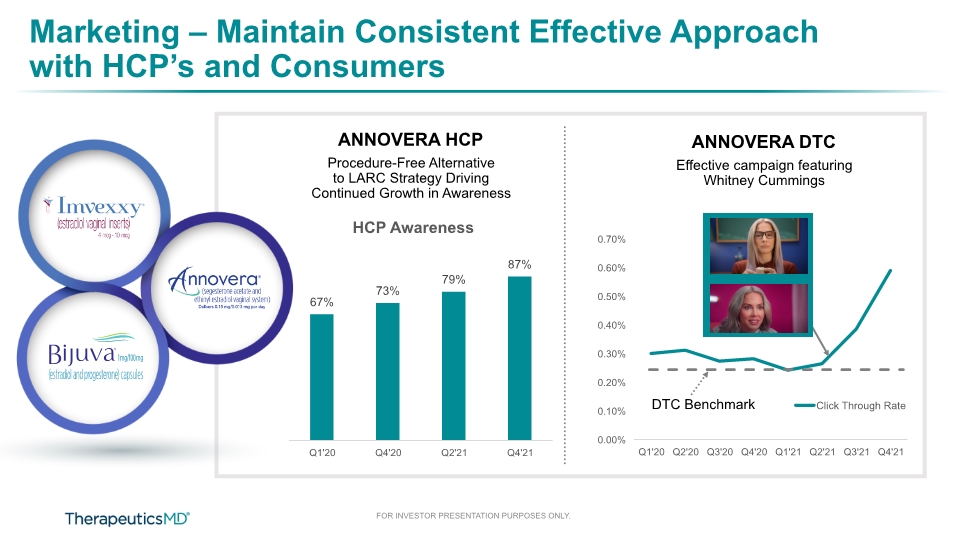
Marketing – Maintain Consistent Effective Approach with HCP’s and Consumers DTC Benchmark ANNOVERA DTC Effective campaign featuring Whitney Cummings ANNOVERA HCP Procedure-Free Alternative to LARC Strategy Driving Continued Growth in Awareness

Q&A


















