
FOR INVESTOR PRESENTATION PURPOSES ONLY. Building the Premier Women’s Health Company Q2 2022 Earnings August 15, 2022 Exhibit 99.2

Forward-Looking Statements FOR INVESTOR PRESENTATION PURPOSES ONLY. 2 This presentation by TherapeuticsMD, Inc. may contain forward-looking statements. Forward-looking statements may include, but are not limited to, statements relating to TherapeuticsMD’s objectives, plans and strategies as well as statements, other than historical facts, that address activities, events or developments that the company intends, expects, projects, believes or anticipates will or may occur in the future. These statements are often characterized by terminology such as "believes," "hopes," "may," "anticipates," "should," "intends," "plans," "will," "expects," "estimates," "projects," "positioned," "strategy" and similar expressions and are based on assumptions and assessments made in light of management’s experience and perception of historical trends, current conditions, expected future developments and other factors believed to be appropriate. Forward-looking statements in this press release are made as of the date of this press release, and the company undertakes no duty to update or revise any such statements, whether as a result of new information, future events or otherwise. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties, many of which are outside of the company’s control. Important factors that could cause actual results, developments and business decisions to differ materially from forward-looking statements are described in the sections titled "Risk Factors" in the company’s filings with the Securities and Exchange Commission, including its most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, as well as reports on Form 8-K, and include the following: the effects of the COVID-19 pandemic; how the proceeds from the divestiture of the company’s vitaCare business will be utilized; the company’s ability to maintain or increase sales of its products; the company’s ability to develop and commercialize IMVEXXY®, ANNOVERA®, and BIJUVA® and obtain additional financing necessary therefor; whether the company will be able to comply with the covenants and conditions under its term loan facility and the company’s ability to refinance such facility; the effects of supply chain issues on the supply of the company’s products; the potential of adverse side effects or other safety risks that could adversely affect the commercialization of the company’s current or future approved products or preclude the approval of the company’s future drug candidates; whether the FDA will approve the manufacturing supplement for ANNOVERA; the company’s ability to protect its intellectual property, including with respect to the Paragraph IV notice letters the company received regarding IMVEXXY; the length, cost and uncertain results of future clinical trials; the company’s reliance on third parties to conduct its manufacturing, research and development and clinical trials; the ability of the company’s licensees to commercialize and distribute the company’s products; the ability of the company’s marketing contractors to market ANNOVERA; the availability of reimbursement from government authorities and health insurance companies for the company’s products; the impact of product liability lawsuits; the influence of extensive and costly government regulation; the impact of leadership transitions; the volatility of the trading price of the company’s common stock and the concentration of power in its stock ownership.

Hugh O’Dowd CEO FOR INVESTOR PRESENTATION PURPOSES ONLY. 3
Gained approval of our supplemental new drug application for ANNOVERA, which increases our current and future supply Successfully completed the divestiture of vitaCare, enabling the repayment of $120 million of debt Top-line growth and overall operating performance Q2 Accomplishments 1 FOR INVESTOR PRESENTATION PURPOSES ONLY. 4

Q2 2022 Financial Overview FOR INVESTOR PRESENTATION PURPOSES ONLY. 5
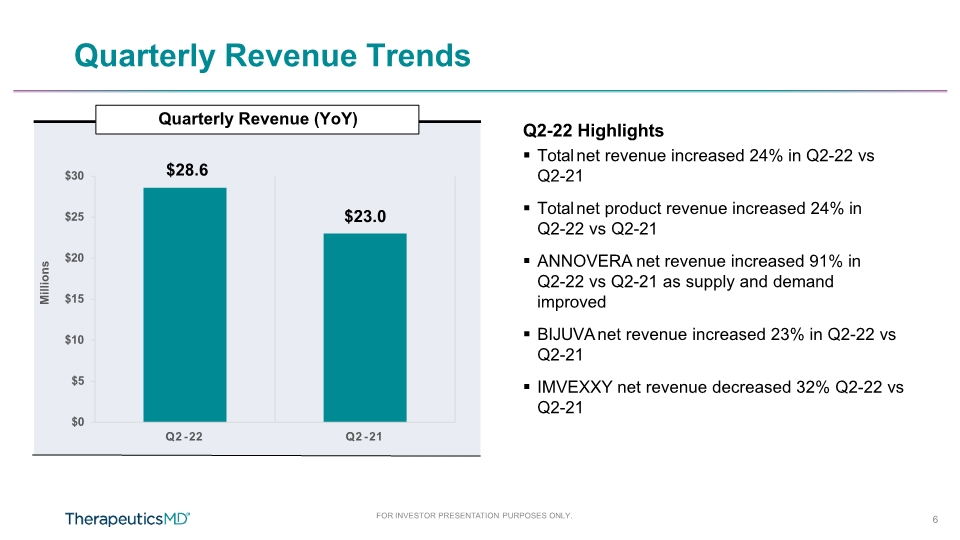
$0 $5 $10 $15 $20 $25 $30 Q2 - 22 Q2 - 21 Quarterly Revenue (YoY) FOR INVESTOR PRESENTATION PURPOSES ONLY. 6 Quarterly Revenue Trends Millions Q2-22 Highlights Total net revenue increased 24% in Q2-22 vs Q2-21 Total net product revenue increased 24% in Q2-22 vs Q2-21 ANNOVERA net revenue increased 91% in Q2-22 vs Q2-21 as supply and demand improved BIJUVA net revenue increased 23% in Q2-22 vs Q2-21 IMVEXXY net revenue decreased 32% Q2-22 vs Q2-21 $28.6 $23.0
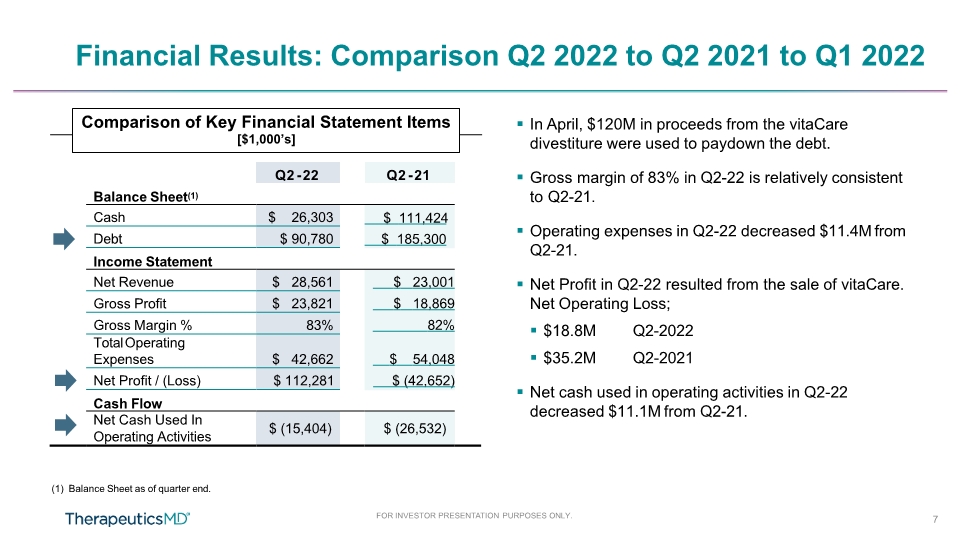
Financial Results: Comparison Q2 2022 to Q2 2021 to Q1 2022 In April, $120M in proceeds from the vitaCare divestiture were used to paydown the debt. Gross margin of 83% in Q2-22 is relatively consistent to Q2-21. Operating expenses in Q2-22 decreased $11.4M from Q2-21. Net Profit in Q2-22 resulted from the sale of vitaCare. Net Operating Loss; $18.8M $35.2M Q2-2022 Q2-2021 Net cash used in operating activities in Q2-22 decreased $11.1M from Q2-21. Q2 - 22 Q2 - 21 Balance Sheet(1) Income Statement Cash Flow (1) Balance Sheet as of quarter end. Comparison of Key Financial Statement Items [$1,000’s] FOR INVESTOR PRESENTATION PURPOSES ONLY. 7

Q2 2022 Commercial Overview FOR INVESTOR PRESENTATION PURPOSES ONLY. 8

9 FOR INVESTOR PRESENTATION PURPOSES ONLY. Inventory Impact Sources: TRx: Symphony Metys - 7/1/22 Shipments: CORD 0 500 1,000 1,500 2,000 2,500 3,000 3,500 4,000 4,500 5,000 400 500 600 700 800 900 1,000 Shipments TRx Shipments Week Ending TRx Count Weeks without Shipments New Initiatives Resonating
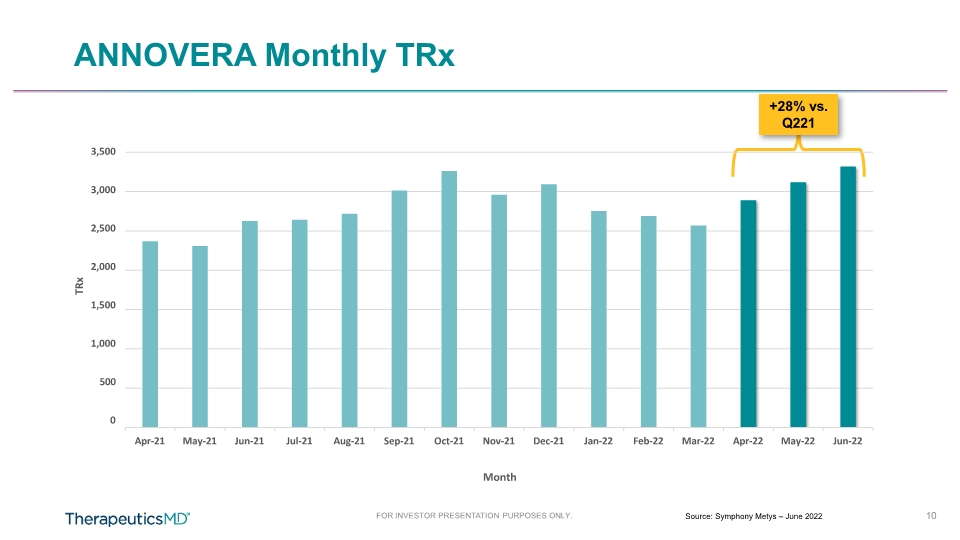
FOR INVESTOR PRESENTATION PURPOSES ONLY. 10 ANNOVERA Monthly TRx Source: Symphony Metys – June 2022 +28% vs. Q221 3,500 3,000 2,500 2,000 1,500 1,000 500 0 Apr-21 May-21 Jun-21 Jul-21 Aug-21 Sep-21 Oct-21 Nov-21 Dec-21 Jan-22 Feb-22 Mar-22 Apr-22 May-22 Jun-22 TRx Month
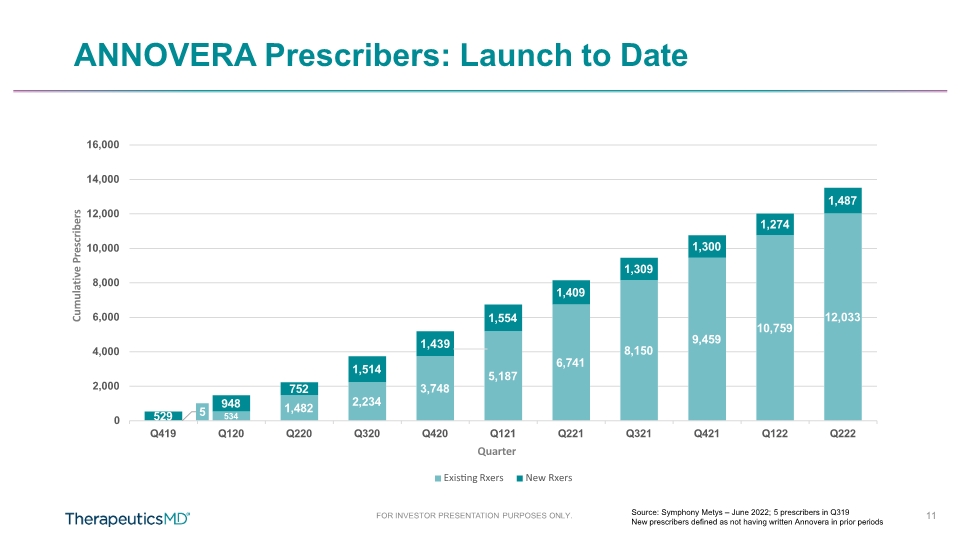
FOR INVESTOR PRESENTATION PURPOSES ONLY. ANNOVERA Prescribers: Launch to Date Source: Symphony Metys – June 2022; 5 prescribers in Q319 New prescribers defined as not having written Annovera in prior periods 5 534 1,482 2,234 3,748 5,187 6,741 8,150 9,459 10,759 12,033 529 948 752 1,514 1,439 1,554 1,409 1,309 1,300 1,274 1,487 0 2,000 4,000 6,000 8,000 10,000 12,000 16,000 14,000 Q419 Q120 Q220 Q320 Q420 Q221 Q321 Q421 Q122 Q222 Cumulative Prescribers Q121 Quarter Existing Rxers New Rxers 11
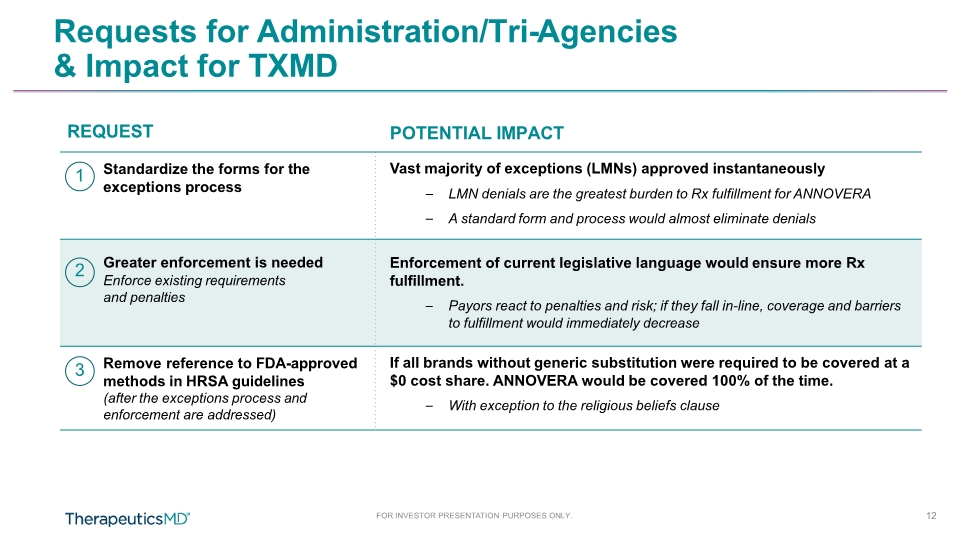
FOR INVESTOR PRESENTATION PURPOSES ONLY. 12 Requests for Administration/Tri-Agencies & Impact for TXMD REQUEST POTENTIAL IMPACT
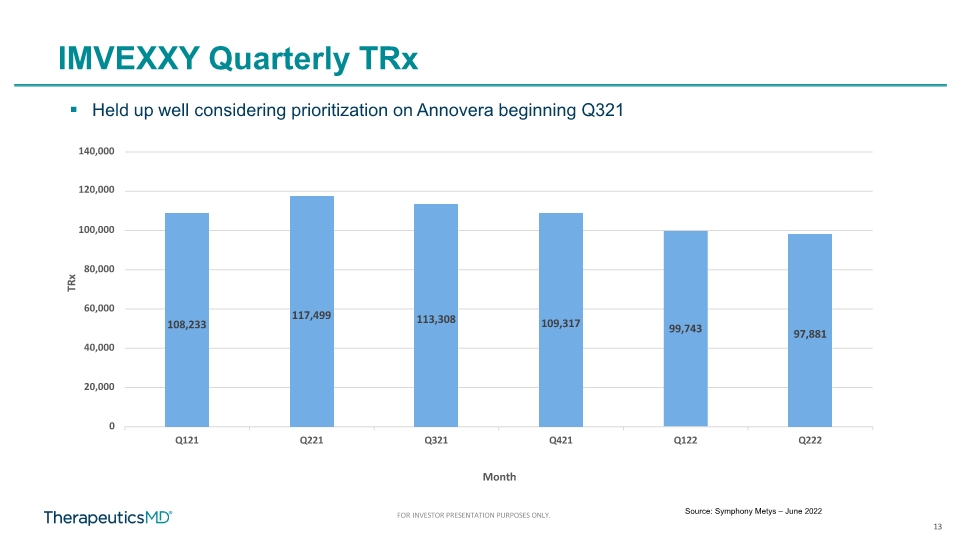
13 FOR INVESTOR PRESENTATION PURPOSES ONLY. IMVEXXY Quarterly TRx Source: Symphony Metys – June 2022 Held up well considering prioritization on Annovera beginning Q321 108,233 117,499 113,308 109,317 99,743 97,881 0 20,000 40,000 60,000 80,000 100,000 140,000 120,000 Q121 Q221 Q321 Q421 Q122 Q222 TRx Month
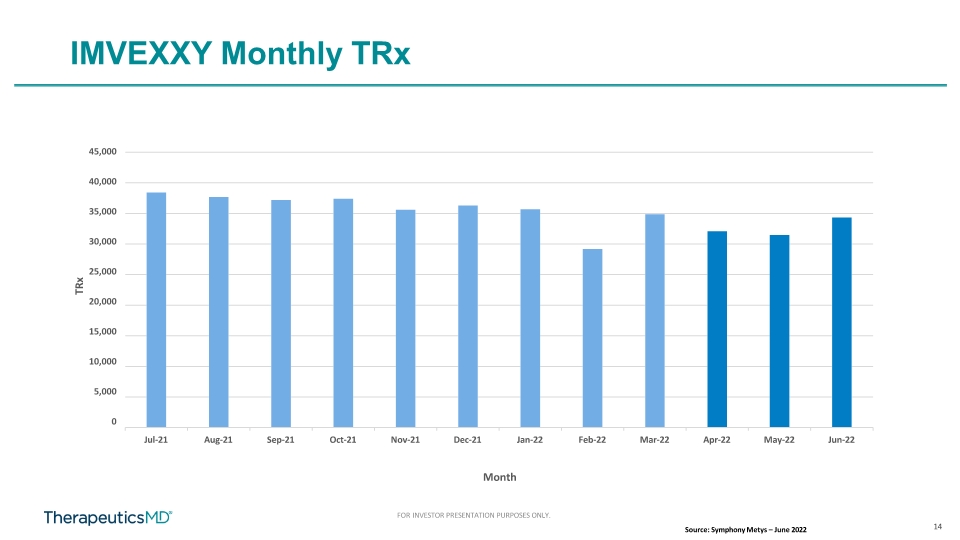
14 FOR INVESTOR PRESENTATION PURPOSES ONLY. 45,000 40,000 35,000 30,000 25,000 20,000 15,000 10,000 5,000 0 Jul-21 Aug-21 Sep-21 Oct-21 Nov-21 Dec-21 Jan-22 Feb-22 Mar-22 Apr-22 May-22 Jun-22 TRx Month IMVEXXY Monthly TRx Source: Symphony Metys – June 2022
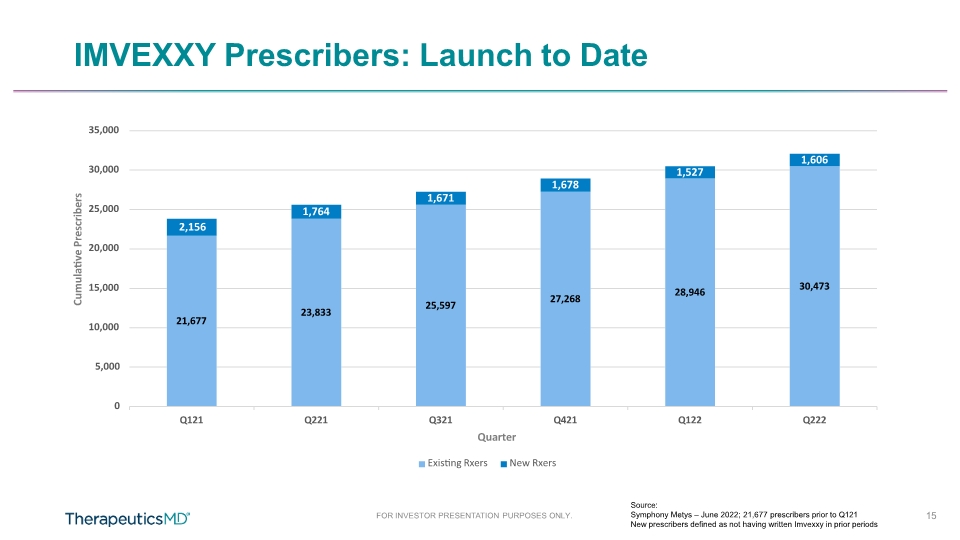
FOR INVESTOR PRESENTATION PURPOSES ONLY. IMVEXXY Prescribers: Launch to Date Source: Symphony Metys – June 2022; 21,677 prescribers prior to Q121 New prescribers defined as not having written Imvexxy in prior periods 21,677 23,833 25,597 27,268 28,946 30,473 1,764 1,671 1,678 1,527 1,606 5,000 0 10,000 15,000 2,156 20,000 25,000 30,000 35,000 Q121 Q221 Q321 Q421 Q122 Q222 Cumulative Prescribers Quarter Existing Rxers New Rxers 15
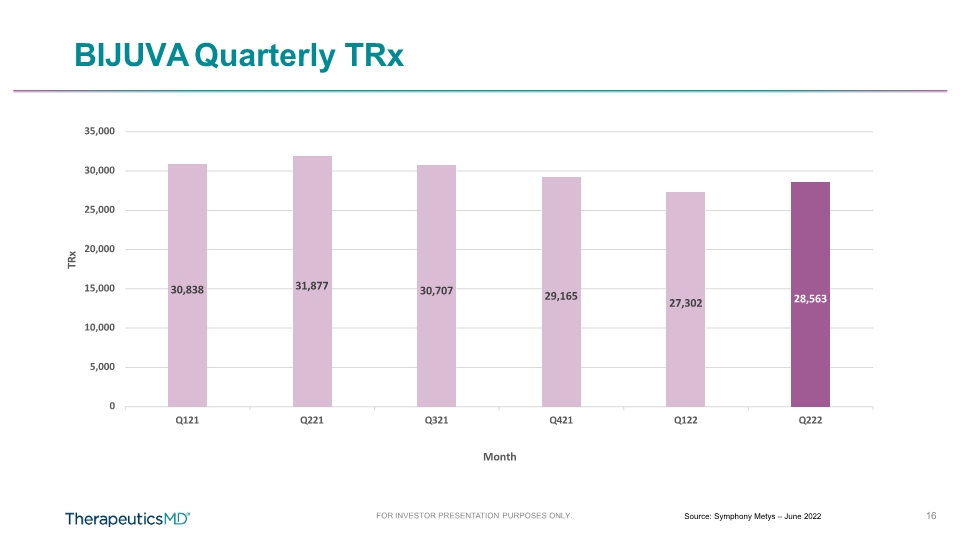
BIJUVA Quarterly TRx 30,838 16 FOR INVESTOR PRESENTATION PURPOSES ONLY. Source: Symphony Metys – June 2022 31,877 30,707 29,165 27,302 28,563 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 Q121 Q221 Q321 Q421 Q122 Q222 TRx Month
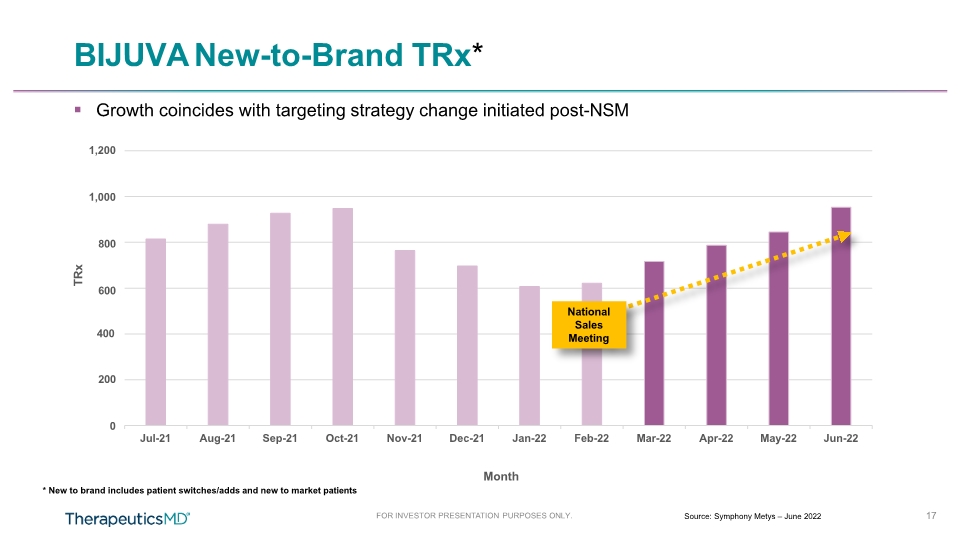
BIJUVA New-to-Brand TRx* * New to brand includes patient switches/adds and new to market patients 200 0 17 FOR INVESTOR PRESENTATION PURPOSES ONLY. Source: Symphony Metys – June 2022 400 1,200 1,000 800 600 Jul-21 Aug-21 Sep-21 Oct-21 Nov-21 Dec-21 Jan-22 Feb-22 Mar-22 Apr-22 May-22 Jun-22 TRx Month National Sales Meeting Growth coincides with targeting strategy change initiated post-NSM

FOR INVESTOR PRESENTATION PURPOSES ONLY. 18 Q&A

















