UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| | FOR ANNUAL AND TRANSITION REPORTS PURSUANT TO SECTIONS 13 OR 15(d) OF |
| | THE SECURITIES EXCHANGE ACT OF 1934 |
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended August 2, 2008
OR
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ________________ to ________________
Commission file number 0-3319
DEL GLOBAL TECHNOLOGIES CORP.
(Exact Name of Registrant as Specified in Its Charter)
New York | | 13-1784308 |
| (State or Other Jurisdiction of | | (I.R.S. Employer Identification No.) |
| Incorporation or Organization) | | |
| | | |
| 11550 WEST KING STREET, FRANKLIN PARK, IL | | 60131 |
| (Address of Principal Executive Offices) | | (Zip Code) |
Registrant’s telephone number, including area code (847) 288-7000
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | | Name of Each Exchange on Which Registered |
| NONE | | NONE |
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $0.10 Par Value (“Common Stock”)
Rights to Purchase Common Stock, par value $0.10 per share, distributed pursuant to Rights Agreement dated January 22, 2007 (Common Stock Purchase Rights)
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes £ NoT
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes £ No T
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes T No £
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one).
| | Large accelerated filer £ | Accelerated filer £ |
| | | |
| | Non-accelerated filer £ (Do not check if a smaller reporting company) | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2). Yes £ NoT
The aggregate market value of the registrant’s Common Stock held by non-affiliates of the Registrant as of January 26, 2008 was $59,075,308. Solely for the purposes of this calculation, shares held by directors and executive officers of the Registrant have been excluded. Such exclusion should not be deemed a determination or an admission by the Registrant that such individuals are, in fact, affiliates of the Registrant
As of October 1, 2008, there were 24,246,165 shares of the registrant’s common stock outstanding.
| | |
| | 1 |
| | 10 |
| | 16 |
| | 16 |
| | 16 |
| | 19 |
| | | |
| | 19 |
| | 19 |
| | 21 |
| | 22 |
| | 33 |
| | 33 |
| | 33 |
| | 33 |
| | 34 |
| | | |
| | 34 |
| | 34 |
| | 36 |
| | 46 |
Del Global Technologies Corp., a New York corporation, was incorporated in 1954. Unless otherwise specifically indicated, “Del Global”, the “Company,” “we,” “our,” “ours,” and “us” refers to Del Global Technologies Corp. and its consolidated subsidiaries. We are a leader in developing, manufacturing and marketing medical and dental imaging systems and power conversion subsystems and components worldwide. Our products include stationary and portable medical and dental diagnostic imaging systems and electronic systems and components such as electronic filters, transformers and capacitors.
The Company is headquartered in Franklin Park, IL. The mailing address of our headquarters is 11550 West King Street, Franklin Park, IL. 60131 and our telephone number is 847-288-7000. Our Website is www.delglobal.com. Through the Investor Relations section of our Website, we make our filings with the Securities and Exchange Commission (“SEC”) available as soon as practicable after they are electronically filed with the SEC. These include our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K.
The sale of Del High Voltage Division (“DHV”), which was part of our Power Conversion Group, was consummated on October 1, 2004, as described in Note 1 of the Notes to Consolidated Financial Statements in Part II, Item 8 of this Annual Report. Accordingly, this business is presented as a discontinued operation in all fiscal years presented and throughout this Form 10-K.
EMPLOYEES
As of August 2, 2008, we had 310 employees. We believe that our employee relations are good. None of our approximately 180 U.S. based employees are represented by a labor union. Employment by functional area as of August 2, 2008 is as follows:
| Executive | 2 |
| Administration and finance | 29 |
| Manufacturing &# 160; | 218 |
| Engineering � 0; | 34 |
| Sales and Marketing | 27 |
| Total � 0; | 310 |
OPERATING SEGMENTS
The operating businesses that we report as segments consist of the Medical Systems Group and the Power Conversion Group. For fiscal 2008, the Medical Systems Group segment accounted for approximately 88% of our revenues and the Power Conversion Group segment accounted for approximately 12% of our revenues. Our consolidated financial statements include a non-operating segment which covers unallocated corporate costs. For the fiscal year ended August 2, 2008, one of our Medical Systems Group customers accounted for approximately 13% of consolidated revenues and 1% of gross accounts receivable at August 2, 2008. For the fiscal year ended July 28, 2007, one of our Medical Systems Group customers accounted for approximately 12% of consolidated revenues and 11% of gross accounts receivable at July 28, 2007. For the fiscal year ended July 29, 2006, no individual customer accounted for greater than 10% of total revenue or accounts receivable, nor was either segment dependent upon a single customer or a few customers, the loss of any one or more of which would have a material adverse effect on such segment. For further information concerning our operating segments, see Note 9 of the Notes to Consolidated Financial Statements in Part II, Item 8 of this Annual Report. Our operating segments and businesses are summarized in the following table:
| DIVISION | BRANDS | SUBSIDIARIES | FACILITIES |
| MEDICAL SYSTEMS GROUP: | | | |
| Medical Imaging | Del Medical, Villa, | Del Medical Imaging Corp | Franklin Park, IL |
| | UNIVERSAL, DynaRad | (“Del Medical”) | |
| | Apollo | | |
| | Strata-2000 | Villa Sistemi Medicali | Milan, Italy |
| | | S.p.A. (“Villa”) | |
| POWER CONVERSION GROUP: | | | |
| Electronic Systems & Components | RFI, Filtron, Sprague,
Stanley | RFI Corporation (“RFI”) | Bay Shore, NY |
MEDICAL SYSTEMS GROUP
Our Medical Systems Group designs, manufactures, markets and sells medical and dental diagnostic imaging systems consisting of stationary and portable imaging systems, radiographic/fluoroscopic systems, dental imaging systems and digital radiography systems. Approximately 71% of this segment’s revenues were attributed to Villa in fiscal 2008.
Prior to December 23, 2005, the Company owned 80% of the Villa subsidiary. On December 23, 2005, the Company acquired the remaining 20% of Villa for $2.6 million plus 904,762 restricted shares of Company common stock which were valued at $2.9 million. For further information concerning this acquisition, see Note 2 of the Notes to Consolidated Financial Statements in Part II, Item 8 of this Annual Report.
Medical imaging systems of the types we manufacture use x-ray technology to produce diagnostic images of matter beneath an opaque surface. An imaging system principally consists of a high voltage power supply, an x-ray tube, a patient positioning system, and an image recording system, which is either film or a digital detector. X-rays are generated as a result of high voltage passing through a filament, or cathode in an x-ray tube. The cathode emits energized electrons which collide with a positively charged tungsten anode within the tube. The collision of these energized electrons with the anode emits the x-ray photons or x-rays. The x-ray tube is surrounded by a lead shield which contains an opening and various filters to direct the x-rays towards the patient.
The performance of the x-ray system, including image resolution, is directly linked to the precision performance of the high voltage power supply. The object to be imaged is placed between the x-ray tube and the image recording system. X-rays, which are not reflected by opaque surfaces, pass through the object and expose the film or image recording system. However, if the object is comprised of areas of varying densities or chemical compositions, x-rays will be absorbed in proportion to the density or chemical composition of the matter. As a result, the film will be exposed to a varying degree, thereby producing an image of the density or chemical variation within the object. For example, because bone has a greater density than the surrounding tissue in the body, x-rays can be used to produce an image of a skeleton. X-ray systems are differentiated by a number of key characteristics such as application, image capture technology, image resolution, accuracy, portability, size and cost. The design of an x-ray system requires complex engineering, which determines the performance factors required of the various system components.
This segment designs, manufactures, markets and sells medical and dental diagnostic imaging systems worldwide in the following markets:
MEDICAL SYSTEMS GROUP MARKETS SERVED
| | Hospitals | Veterinary Clinics |
| | Teaching Institutions | Chiropractic Clinics |
| | Medical Clinics | Dental Offices |
| | Private Practitioners | Military/Government |
| | Orthopedic Facilities | Home Health Care Providers |
| | Imaging Centers | |
Our medical imaging systems are sold under the Del Medical, Villa, UNIVERSAL, and DynaRad brand names. The prices of our medical imaging systems range from approximately $9,000 to $300,000 per unit, depending on the complexity and flexibility of the system.
The following is a description of our product lines in this segment.
PRODUCTS
GENERAL RADIOGRAPHIC SYSTEMS - For more than 100 years, conventional projection radiography has used film to create x-ray images. Conventional technology requires that x-ray film be exposed and then chemically processed to create a visible image for diagnosis.
General Radiography represents approximately 60%-70% of the Medical Systems Group’s revenues depending on the product mix within each period. We produce a broad line of conventional radiographic products used in outpatient facilities, as well as more sophisticated and expensive x-ray systems typically used in hospitals and clinics. For example, our higher-end Advanced Radiography System (ARSII),U-ARC DRT Digital Radiographic Systems and TITAN Lines are designed to meet the broad requirements of a hospital or teaching university’s radiographic room, while our mid-range Del Medical and Villa Medical systems are suited more to the needs of medium sized hospitals, outpatient clinics and private practitioners.
The Moviplan product line manufactured under the Villa brand includes a variety of configurations that fit a wide range of markets, spanning from small private practices to hospitals, with a specific accent on emerging countries. A leading model is the tomographic version, which allows users to take images of multiple sections of the body. This type of system is manufactured by relatively few companies worldwide.
We also have a broad range of products serving medical practitioners, veterinarians and chiropractors through our UNIVERSAL and VetVision DRds brand product lines. These units are designed for durability, are space efficient, rugged and are priced more economically. Our UNIVERSAL medical products include a variety of configurations that can be constructed to best suit the needs of the desired work environment. Our UNIVERSAL AVchoice and DVChoice veterinary line of products is designed with many of the same attributes as the medical line. Our UNIVERSAL chiropractic line, consisting of our DCchoice, Raymaster and PreciseView product, combine precision alignment and positioning with a versatile Chiropractic imaging systems for both analog and digital applications. Our VetVision DRds is a digital high-resolution x-ray unit designed to give veterinarians greater flexibility as it produces high-resolution digital images and takes up less space than other comparable products.
During fiscal 2006 and 2008, we expanded our product portfolio with the introduction of the Del Medical U-ARC DRT Digital Radiographic System and TITAN product lines, respectively. These system enables radiologists to obtain better patient images within a fraction of the time and with lower overall costs than traditional film-based systems.
We also produce a full product line of high frequency medical x-ray generators which economically provide superior quality x-ray generation, resulting in lower patient dosage, extended tube life and less blurring due to patient motion when compared to single phase generators. We continue to investigate arrangements with generator suppliers on a global basis to further upgrade our medical x-ray generator offerings.
RADIOGRAPHIC/FLUOROSCOPIC SYSTEMS - We produce a wide range of radiographic/fluoroscopic, or R/F, systems that are able to perform complex x-ray examinations with contrast liquids for sequential and real time images. The Vision and Viromatic systems are based on the “classical approach” and require the operator to stay in close contact to the equipment and the patient. These systems are often used for diagnostic gastrointestinal procedures to image the progress of a radiopaque solution (typically barium) as it travels through the digestive tract. The Apollo Remote R/F system and Mercury systems are based on the more modern “Remote control” technology and allow the technologist and radiologist to operate the system and perform the entire examination from a separate room, being totally shielded from the x-ray source. The remote controlled system is also the most flexible x-ray imaging unit as it allows skeletal, gastro-intestinal, vascular, urological and gynaecological studies in the same room. Remote controlled systems (Apollo Remote R/F system and Mercury) are also widely used in connection with our digital acquisition system, DIVA, to perform digital image acquisition and real time angiographic examinations with a vast choice of image acquisition and post-processing tools. The DIVA system can also be equipped with DICOM functionalities that enable images to be sent to centralized archival units, image reviewing workstations, laser imagers, and in general allow the system to be fully integrated into PACS (Picture Archival and Communication Systems) networks within a hospital. As of today, the Apollo Remote R/F system table, with a DIVA-D digital acquisition system represents a sophisticated system and technology produced by the Medical Systems Group. Recently dynamic flat panels have become an available alternative to carry on fluoroscopy at 43 x 43 cm detector size. Villa already has released the Apollo version implementing this new technology. This product represents the most advanced and sophisticated system produced by the Medical Systems Group.
PORTABLE AND MOBILE MEDICAL X-RAY SYSTEMS - We sell portable x-ray equipment under our DynaRad brand including the HF-110A and PHANTOM systems, for the military and home health care provider markets. Both of these portable systems utilize high frequency, microprocessor-controlled technology to produce consistent quality x-rays with the added advantages of being smaller, lighter in weight and more cost-effective than stationary x-ray systems.
Larger and more powerful mobile units are also manufactured and distributed under the Villa brand and include the “Visitor” product line with high frequency generators up to 30 kilowatts that are typically used in hospitals to take radiographic images directly at the patient’s bed.
DENTAL SYSTEMS - We produce a broad range of DC and AC powered intra-oral (commonly known as bite wing) x-ray systems at our Villa facility. In addition, our Rotograph Plus and Strato-2000 systems are utilized to perform panoramic images for dental applications. The most recent addition to the dental product line are Direct Digital versions of Strato 2000 and Rotograph, which capture panoramic images directly in digital format and can be connected to a PC for image reviewing and post-examination processing. The relatively small price differential between digital and analog panoramic units has triggered a very quick shift to digital technology in the marketplace which accounts for approximately 12% of the volume of new units sold over the past two years. The dental products are sold both with our own brand (Villa), as well as private labelled units to selected OEM customers.
MAMMOGRAPHY SYSTEMS - We currently resell the Melody system outside of the U.S.. The Melody unit is manufactured by a European-based manufacturer and sold under the Villa brand. Although we have exclusive use of the “Melody” name, our supplier markets a similar product in several competing markets.
SURGICAL C-ARMS – We sell a mobile C-arm unit called “Arcovis 2000” under the Villa brand. The product is manufactured by a European company that also sells similar products to other customers.
MARKETING AND DISTRIBUTION
Our medical imaging systems are sold in the U.S. and internationally, principally by a network of over 300 distributors worldwide. This network has expanded during fiscal year 2008 as we have partnered with one of the largest dealer buying groups to distribute our digital and general radiographic imaging equipment for the medical, chiropractic and veterinary markets. Medical imaging system distributors are supported by our regional managers, area managers, marketing managers and technical support groups, who train distributor sales and service personnel and participate in customer calls. Due to the different markets and end use customers for dental as compared to medical imaging systems, dental products are distributed by a separate network of dental dealers who target the dental practitioners market. In addition, we do some private label manufacturing of dental product for certain OEM customers.
Technical support in the selection, use and maintenance of our products is provided to distributors and professionals by customer service representatives. We also maintain telephone hotlines to provide technical assistance to distributors during regular business hours. Additional product and distributor support is provided through participation in medical equipment exhibitions and trade specific advertising.
We typically exhibit our products at annual conferences, including the RSNA Conference in Chicago, the MEDICA Medical Conference in Dusseldorf, Germany, the European College of Radiology (ERC) Conference in Vienna, Austria, and the International Dental Show (IDS) in Cologne, Germany and other venues worldwide. Sales of the Company’s products in North America are typically on open account with 30 day terms. Our products sold outside of North America are usually secured by a letter of credit to mitigate any potential credit risk, with longer terms being given to non-U.S. customers as is customary in international business. Our Company also has the capacity to participate in and win large international tenders, which require careful assessment of the commercial aspects, regulatory requirements, production planning and financial exposure. Multi-million dollar tenders have been awarded to our Villa operation in the last two fiscal years in countries, including Mexico, Lithuania, Romania, Russia and Vietnam.
RAW MATERIALS AND PRINCIPAL SUPPLIERS
The Medical Systems Group in most cases uses two or more alternative sources of supply for each of its raw materials, which consist primarily of mechanical subassemblies, electronic components, x-ray tubes and x-ray generators. In certain instances, however, the Medical Systems Group will use a single source of supply when directed by a customer or by need. In order to ensure the consistent quality of the Medical System Group’s products, the Company follows strict supplier evaluation and qualification procedures, and where possible, enters into strategic relationships with its suppliers to assure a continuing supply of high quality critical components.
With respect to those items which are purchased from single sources, we believe that comparable items would be available in the event that there was a termination of our existing business relationships with any such supplier. Actual experience could differ materially from this belief as a result of a number of factors, including the time required to locate an alternate source for the material.
The majority of the Medical System Group’s raw materials are purchased on open account from vendors pursuant to various individual or blanket purchase orders. Procurement lead times are such that the Company is not required to hold significant amounts of inventory in order to meet customer demand. The Company believes its sources of supply for the Medical Systems Group are adequate to meet its needs.
COMPETITION
Based on industry data, we believe our Medical Systems Group is either the number one or number two supplier, measured by market share, to the independent distributors of radiographic equipment in North America. Our Medical Systems Group competes in two major segments of the highly competitive, world-wide conventional radiographic and R/F products marketplace. Our top-tier conventional radiographic products are sold through national, regional and independent distributors. In 2006, the Medical Systems Group extended its access to the U.S. market by entering into relationships with a national Group Purchasing Organization and several smaller multi-hospital networks. The three major competitors in this market segment are GE Healthcare Systems, a division of General Electric Company, Siemens Medical Solutions, a division of Siemens AG and Philips Medical Systems, a division of Philips Electronics N.V. They compete with us on customer support, features and breadth of product offerings. These larger competitors primarily sell directly to large hospitals and teaching institutions and sell a broader range of products designed to outfit a hospital’s entire imaging requirements. In Europe, Africa, the Middle East and the Far East, competition is also represented by other mid-tier European companies, as well as local manufacturers who mainly address the middle and low market tier.
Our lower-tier conventional radiographic products principally compete with several small companies based primarily in the U.S. and Europe. In some price-driven markets, we also find competition from Korean and Chinese products. Most of these companies sell through independent distributors and compete with us primarily on price, quality and performance. We believe that we can be differentiated from our competitors based on our combination of price, quality and performance, together with the strength and breadth of our independent distribution network, and the growth of our product portfolio.
The markets for our products are highly competitive and subject to technological change and evolving industry requirements and standards. Cost containment and pricing is also a critical driving factor, given the threat that is being posed by the aggressive policies of Korean and Chinese manufacturers attempting to capture market shares out of their boundaries. Price erosion is not only a factor in the low-end tier, but also at top level, where all companies, including the large multinationals such as GE, Philips and Siemens are driving down their prices.We believe that these trends will continue into the foreseeable future. Some of our current and potential competitors have substantially greater financial, marketing and other resources than we do. As a result, they may be able to adapt more quickly to new or emerging technologies and changes in customer requirements, or to devote greater resources to the promotion and sale of their products than we can. Competition could increase if new companies enter the market or if existing competitors expand their product lines or intensify efforts within existing product lines. Although we believe that our products are more cost-effective than those of our primary competitors, certain competing products may have other advantages which may limit our market. There can be no assurance that continuing improvements in current or new competing products will not make them technically equivalent or superior to our products in addition to providing cost or other advantages. There can be no assurance that our current products, products under development or ability to introduce new products will enable us to compete effectively.
PRODUCT DEVELOPMENT
It is generally accepted that digital radiography will continue to become the dominant technology used in hospitals and imaging clinics throughout the world over the next 10 to 15 years. Currently, there are a number of competing technologies available in connection with the digitization of x-ray images. In addition, there are substantial hurdles which need to be addressed in terms of transitioning radiology practices from the current analog environment to a digital environment. These ancillary issues include image storage and retrieval and record keeping. However, due to the high cost of this technology, many institutions have not yet adopted digital technology. In addition, there is uncertainty as to which technology will be accepted as the industry-standard for image capture, and communication and storage of digital image information.
For the medical imaging market, we currently have two digital radiographic solutions and are committed to expanding our selection to include a wider range of low-cost offerings for customers. While many of our competitors have invested heavily into developing a digital detector, we have chosen to align with technology leaders who have already made digital investments and could benefit from our x-ray platform design, our systems integration capabilities and our worldwide dealer network. This strategy also accelerates our time-to-market with new digital solutions and avoids the significant development costs being incurred by our competitors.
Consequently, our current research and development spending is focused on both enhancing our existing conventional radiographic products and continuing to enhance our digital radiographic solutions and explore partnerships with strategic vendors in the digital marketplace. The introduction of digital imaging is growing much faster in dental application where the cost difference between traditional and digital does not represent a significant barrier. In order to more fully participate in the digital dental market, Villa has initiated a strategic partnership with a French company, Owandy S.A.S., that provides the digital solutions for dental panoramic units and Villa is offering a full line of digital panoramic units.
Spending for research and development for our Medical Systems Group was approximately $2.5 million for fiscal year 2008, $2.0 million for fiscal year 2007 and $1.6 million for fiscal year 2006.
TRADEMARKS AND PATENTS
The majority of the Medical System Group’s products are based on technology that is not protected by patent or other rights. Within the Medical System Group, certain of our products and brand names are protected by trademarks, both in the U.S. and internationally. Because we do not have patent rights in our products, our technology may not preclude or inhibit competitors from producing products that have identical performance as our products. Our future success is dependent primarily on the technological expertise and management abilities of our employees and the strength of our relationship with our worldwide dealer network.
GOVERNMENT REGULATION
Our medical imaging systems are medical devices and, therefore, are subject to regulation by the U.S. Food and Drug Administration (the “FDA”) and to regulation by foreign governmental authorities. We also are subject to various state and local regulations. Regulatory requirements include registration as a manufacturer, compliance with established manufacturing practices, procedures and quality standards, strict requirements dealing with the safety, effectiveness and other properties of the products, conformance with applicable industry standards, product traceability, adverse event reporting, distribution, record keeping, reporting, compliance with advertising and packaging standards, labeling, and radiation emitting qualities of these products. Failure to comply can result in, among other things, the imposition of fines, criminal prosecution, recall and seizure of products, injunctions restricting or precluding production or distribution, the denial of new product approvals and the withdrawal of existing product approvals.
FDA’S PREMARKET CLEARANCE AND APPROVAL REQUIREMENTS
In the U.S., medical devices are classified into three different categories over which the FDA applies increasing levels of regulation: Class I, Class II, and Class III. Del Medical manufactures several Class I and Class II devices. Before a new Class II device can be introduced into the U.S. market, the manufacturer must obtain FDA clearance or approval through either premarket notification under Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or a premarket approval under Section 515 of that Act, unless the product is otherwise exempt from the requirements.
A Section 510(k) premarket notification must contain information supporting the claim of substantial equivalence, which may include laboratory results and product comparisons to existing devices. Following submission of a 510(k) application, a manufacturer may not market the device until the FDA finds the product is substantially equivalent for a specific or general intended use. FDA 510(k) clearance generally takes 90 days and may take longer if FDA requests additional information. There is no assurance the FDA will ultimately grant a clearance. The FDA may determine that a device is not substantially equivalent and may require submission and approval of a premarket approval application, or require further information before it is able to make a determination regarding substantial equivalence.
After a device receives 510(k) clearance, any modification made to the device requires the manufacturer to determine whether the modification could significantly affect its safety or effectiveness. If it does not, the manufacturer’s decision must be documented. If the modification could significantly affect the device’s safety and effectiveness, then the modification requires at least a new 510(k) clearance or, in some instances, could require a premarket approval. The FDA requires each manufacturer to make this determination, but the FDA can review any manufacturer’s decision. If the FDA disagrees with a manufacturer’s decision, the agency may retroactively require the manufacturer to seek 510(k) clearance or premarket approval. The FDA also can require the manufacturer to cease marketing the modified device or recall the modified device (or both) until 510(k) clearance or premarket approval is obtained. We have made minor modifications to our products and, using the guidelines established by the FDA, have determined that these modifications do not require us to file new 510(k) submissions. If the FDA disagrees with our determinations, we may not be able to sell one or more of our products until the FDA have cleared new 510(k) submissions for these modifications.
All of our products marketed in the U.S. have met the appropriate FDA requirements for marketing, either because they were exempt from submission or through 510(k) clearance. We continuously evaluate our products for any required new submission for changes or modifications.
PERVASIVE AND CONTINUING FDA REGULATION
Numerous FDA regulatory requirements apply to our products as well as to components manufactured by some of our suppliers. These requirements include:
| | - | The FDA’s quality system regulation which requires manufacturers to create, implement and follow numerous design, testing, control, documentation and other quality procedures; and |
| | - | Medical device reporting regulations, which require that manufacturers report to the FDA certain types of adverse and other events involving their products. |
Class II devices may also be subject to special controls, such as performance standards, post-market surveillance, patient registries and FDA guidelines that may not apply to Class I devices. Our products are currently subject to FDA guidelines for 510(k) cleared devices and are not subject to any other form of special controls. We believe we are in compliance with the applicable FDA guidelines, but we could be required to change our compliance activities or be subject to other special controls if the FDA changes its existing regulations or adopts new requirements.
We and some of our suppliers are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that either we or a supplier have failed to adequately comply, the agency can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as: fines, injunctions and civil penalties; recall or seizure of our products; the imposition of operating restrictions, partial suspension or total shutdown of production; the refusal of our requests for 510(k) clearance or premarket approval of new products; the withdrawal of 510(k) clearance or premarket approval already granted; and criminal prosecution.
The FDA also has the authority to require repair, replacement or refund of the cost of any medical device manufactured or distributed by us. Our failure to comply with applicable requirements could lead to an enforcement action that may have an adverse effect on our financial condition and results of operations.
OTHER FEDERAL AND STATE REGULATIONS
As a participant in the health care industry, we are subject to extensive and frequently changing regulation under many other laws administered by governmental entities at the federal, state and local levels, some of which are, and others of which may be, applicable to our business. For example, our Del Medical Imaging facility is also licensed as a medical product manufacturing site by the state of Illinois and is subject to periodic state regulatory inspections. Our health care service provider customers are also subject to a wide variety of laws and regulations that could affect the nature and scope of their relationships with us.
FOREIGN GOVERNMENT REGULATION
Our products are also regulated outside the U.S. as medical devices by foreign governmental agencies, similar to the FDA, and are subject to regulatory requirements, similar to the FDA’s, in the countries in which we plan to sell our products. We work with our foreign distributors to obtain the foreign regulatory approvals necessary to market our products outside of the U.S. In certain foreign markets, it is necessary to obtain ISO 9001 certification, which is analogous to compliance with the FDA’s Good Manufacturing Practices requirements. It is also necessary to obtain ISO 13485 certification, which specifies requirements for a quality system to be used for design and development, production, installation and servicing of medical devices. We have obtained ISO 9001 certification and ISO 13485 certification, for both of our medical systems manufacturing facilities. In many European Community and other international locations it is necessary or desirable to have a “CE” (Communities of Europe) mark on our products. This involves substantial testing by a third party such as Underwriters Laboratories or Electronics Testing Laboratories and for some devices, a certificate from a notified body declaring conformance to applicable directives and regulations. We have completed the necessary third party testing at both manufacturing locations, maintain the necessary certifications and are qualified to place the CE mark on all products intended for sale in such countries. The time and cost required obtaining market authorization from other countries and the requirements for licensing a product in another country may differ significantly from FDA requirements.
No assurance can be given that the FDA or foreign regulatory agencies will give the requisite approvals or clearances for any of our medical imaging systems and other products under development on a timely basis, if at all. Moreover, after clearance is given, both in the case of our existing products and any future products, these agencies can later withdraw the clearance or require us to change the system or our manufacturing process or labeling, to supply additional proof of its safety and effectiveness, or to withdraw, recall, repair, replace or refund the cost of the medical system, if it is shown to be hazardous or defective.
POWER CONVERSION GROUP
Our Power Conversion Group designs, manufactures, markets and sells high voltage precision components and sub-assemblies and electronic noise suppression components for a variety of applications. These products are utilized by original equipment manufacturers (“OEMs”) who build systems that are used in a broad range of markets. Our products are sold under the following industry brands: RFI, Filtron, Sprague and Stanley. This segment is comprised of Electronic Systems and Components.
This segment designs and manufactures key electronic components such as transformers, magnetics, noise suppression filters and high voltage capacitors for use in precision regulated high voltage applications. Noise suppression filters and components are used to help isolate and reduce the electromagnetic interference (commonly referred to as “noise”) among the different components in a system sharing the same power source. Examples of systems that use our noise suppression products include aviation electronics, mobile and land-based telecommunication systems and missile guidance systems.
The Power Conversion Group provides subsystems and components which are used in the manufacture of medical electronics, military and industrial applications as follows:
POWER CONVERSION GROUP MARKETS SERVED
| AEROSPACE, DEFENSE & HOMELAND SECURITY | INDUSTRIAL/COMMERCIAL |
| | Guidance & Weapons Systems | | Induction Heating |
| | Communications | | Meteorological |
| | Radar Systems Military Shelters Power Systems Aerospace Electronics Aircraft Lighting systems Telecommunication Electronics | | Power Systems Satellite Shielded Rooms/Enclosures Power Systems |
| TELECOMMUNICATIONS | MEDICAL |
| | Telecom Equipment for Voice & Data Transmission | | Radiation Oncology |
| | Telephone Switching Systems | | Magnetic Resonance Imaging (“MRI”) |
| | | | CT Imaging X-Ray |
PRODUCTS
MILITARY APPLICATIONS - Through our relationships with many of the federal government’s top defense suppliers, such as Raytheon, Boeing, Lockheed Martin and Northrop Grumman, we supply electronic components for various classified and unclassified programs including radar systems, guidance systems, weapons systems and communication electronics. On May 24, 2007, the Company's RFI subsidiary was served with a subpoena to testify before a grand jury of the United States District Court, Eastern District of New York to provide items and records from its Bay Shore, NY offices in connection with U.S. Department of Defense (“DOD”) contracts. A search warrant from the United States District Court, Eastern District of New York was issued and executed with respect to such offices. The Company believes that it is in full compliance with the quality standards that its customers require and is fully cooperating with investigators to assist them with their review. The Company's RFI subsidiary is continuing to ship products to the U.S. Government as well as to its commercial customers.
INDUSTRIAL APPLICATIONS - Our high voltage power components and EMI filters are used in many leading-edge high technology scientific and industrial applications by OEMs, universities and private research laboratories. Some industrial applications using high voltage subsystems include DNA sequencing, molecular analysis, printed circuit board inspection, structural inspection, food and mail sterilization and semiconductor capital equipment.
MARKETING, SALES AND DISTRIBUTION
We market our Power Conversion Group products through in-house sales personnel, independent sales representatives in the U.S., and international agents in Europe, Asia, the Middle East, Canada and Australia. Our sales representatives are compensated primarily on a commission basis and the international agents are compensated either on a commission basis or act as independent distributors. Our marketing efforts emphasize our ability to custom engineer products to optimal performance specifications. We emphasize team selling where our sales representatives, engineers and management personnel all work together to market our products. We also market our products through catalogs and trade journals and participation in industry shows. Sales of the Company’s products are typically on open account with 30 day terms. New accounts are established with cash on delivery or cash in advance terms.
RAW MATERIALS AND PRINCIPAL SUPPLIERS
The Power Conversion Group in most cases uses two or more alternative sources of supply for each of its raw materials, which consist primarily of electronic components and subassemblies, metal enclosures for its products and certain other materials. In certain instances, however, the Power Conversion Group will use a single source of supply when directed by a customer or by need. In order to ensure the consistent quality of the Power Conversion Group’s products, the Company performs certain supplier evaluation and qualification procedures, and where possible, enters into strategic partnerships with its suppliers to assure a continuing supply of high quality critical components.
With respect to those items which are purchased from single sources, we believe that comparable items would be available in the event that there was a termination of our existing business relationships with any such supplier. Actual experience could differ materially from this belief as a result of a number of factors, including the time required to locate an alternate source for the material.
The majority of the Power Conversion Group’s raw materials are purchased on open account from vendors pursuant to various individual or blanket purchase orders. Procurement lead times are such that the Company is not required to hold significant amounts of inventory in order to meet customer demand. The Company believes its sources of supply for the Power Conversion Group are adequate to meet its needs
COMPETITION
Our Power Conversion Group competes with several small, privately owned suppliers of electronic systems and components. From our perspective, competition is primarily based on each company’s design, service and technical capabilities, and secondarily on price. Excluding the OEMs that manufacture their own components, based on market intelligence we have gathered, we believe that we are among the top two or three in market share in supplying these products.
The markets for our products are subject to limited technological changes and gradually evolving industry requirements and standards. We believe that these trends will continue into the foreseeable future. Some of our current and potential competitors may have substantially greater financial, marketing and other resources than we do. As a result, they may be able to adapt more quickly to new or emerging technologies and changes in customer requirements, or to devote greater resources to the promotion and sale of their products than we can. Competition could increase if new companies enter the market or if existing competitors expand their product lines or intensify efforts within existing product lines. Although we believe that our products are more cost-effective than those of our primary competitors, certain competing products may have other advantages which may limit our market. There can be no assurance that continuing improvements in current or new products will not make them technically equivalent or superior to our products in addition to providing cost or other advantages. There can be no assurance that our current products, products under development or our ability to introduce new products will enable us to compete effectively.
PRODUCT DEVELOPMENT
We have a well developed engineering and technical staff in our Power Conversion Group. Our technical and scientific employees are generally employed in the engineering departments at our RFI business unit, and split their time, depending on business mix and their own technical background, between supporting existing production and development and research efforts for new product variations or new customer specifications. Our products include transformers, noise suppression filters and high voltage capacitors for use in precision regulated high voltage applications. Noise suppression filters and components are used to help isolate and reduce the electromagnetic interference (commonly referred to as “noise”) among the different components in a system sharing the same power source. Examples of systems that use our noise suppression products include aviation electronics, mobile and land-based telecommunication systems and missile guidance systems. No significant engineering related time was charged to research and development spending for the continuing operations of the Power Conversion Group in fiscal years 2008, 2007 or 2006. For 2009, an R&D budget has been established for growth in magnetics and material research to optimize the efficiency and performance of our products.
TRADEMARKS AND PATENTS
The majority of the Power Conversion Group’s products are based on technology that is not protected by patent or other rights. Within the Power Conversion Group, certain of our products and brand names are protected by trademarks, both in the U.S. and internationally. Our future success is dependent primarily on the technological expertise and management abilities of our employees.
GOVERNMENT REGULATION
We are subject to various U.S. government guidelines and regulations relating to the qualification of our non-medical products for inclusion in government-qualified product lists in order to be eligible to receive purchase orders from a government agency or for inclusion of a product in a system which will ultimately be used by a governmental agency. We have had many years of experience in designing, testing and qualifying our products for sale to governmental agencies. Certain government contracts are subject to cancellation rights at the Government’s election. We have experienced no material termination of any government contract and are not aware of any pending terminations of government contracts.
DISCONTINUED OPERATION
As of July 31, 2004, the DHV division was classified as a discontinued operation. This division manufactured and sold high voltage power systems, primarily for security, medical, scientific, military and industrial OEM applications. The results of this operation are segregated on the accompanying financial statements as income or loss from discontinued operation.
SEASONALITY
Revenue in both operating segments is typically lower during the first quarter of each fiscal year due to the shutdown of operations in our Milan, Italy (Medical Systems Group) and Bay Shore, New York (Power Conversion Group) facilities for part of August as a result of both vacation schedules and year-end physical inventories.
BACKLOG
Consolidated backlog at August 2, 2008 was $22.7 million versus backlog at July 28, 2007 of approximately $28.4 million. The backlog in the Power Conversion Group of $5.4 million decreased $1.2 million from levels at the beginning of the fiscal year while there was a $4.5 million decrease in the fiscal year end backlog of our Medical Systems Segment from July 28, 2007 reflecting weaker booking during the twelve month period in international markets. Substantially all of the backlog should result in shipments within the next 12 to 15 months.
GEOGRAPHIC AREAS
For further information about Geographic areas the Company operates in as well as other Segment related disclosures refer to Note 9 of the Notes to Consolidated Financial Statements in Part II, Item 8 of this Annual Report.
Prospective investors should carefully consider the following risk factors, together with the other information contained in this Annual Report, in evaluating the Company and its business before purchasing our securities. In particular, prospective investors should note that this Annual Report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”) and that actual results could differ materially from those contemplated by such statements. The factors listed below represent certain important factors which we believe could cause such results to differ. These factors are not intended to represent a complete list of the general or specific risks that may affect us. It should be recognized that other risks may be significant, presently or in the future, and the risks set forth below may affect us to a greater extent than indicated.
WE DO NOT INTEND TO PAY DIVIDENDS ON SHARES OF OUR COMMON STOCK IN THE
FORESEEABLE FUTURE.
We currently expect to retain our future earnings, if any, for use in the operation and expansion of our business. We do not anticipate paying any cash dividends on shares of our common stock in the foreseeable future. Our credit facility with our U.S. lender restricts our ability to pay dividends.
COMPLIANCE WITH CHANGING REGULATION OF CORPORATE GOVERNANCE AND PUBLIC
DISCLOSURE MAY RESULT IN ADDITIONAL EXPENSES.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002, are creating uncertainty for companies such as ours. We are committed to maintaining high standards of corporate governance and public disclosure. As a result, we intend to invest reasonably necessary resources to comply with evolving standards, and this investment may result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities, which could harm our business prospects.
OUR COMMON STOCK HAS BEEN DELISTED FROM THE NASDAQ NATIONAL MARKET AND WE CANNOT PREDICT WHEN OR IF IT EVER WILL BE LISTED ON ANY NATIONAL SECURITIES EXCHANGE.
Due to our past failure to comply with the United States Securities Laws, our common stock was suspended from trading on the NASDAQ National Market in December 2000. Current pricing information on our common stock has been available on the over the counter bulletin board (the “OTC”) published by National Quotation Bureau, LLC. The OTC is an over-the-counter market which generally provides significantly less liquidity than established stock exchanges or the NASDAQ National Market, and quotes for stocks included in the OTC are not listed in the financial sections of newspapers. Therefore, prices for securities traded solely in the OTC may be difficult to obtain, and shareholders may find it difficult to resell their shares. In order to be re-listed, we will need to meet certain listing requirements. There can be no assurance that we will be able to meet such listing requirements.
FAILURE BY US TO ADHERE TO OUR ADMINISTRATIVE AGREEMENT WITH THE DEFENSE LOGISTICS AGENCY COULD RESULT IN OUR DEBARMENT FROM DOING BUSINESS WITH THE U.S. GOVERNMENT.
On April 5, 2005, the Company announced that it had reached an administrative agreement with the U.S. Defense Logistics Agency (the “DLA”), a component of the U.S. Department of Defense (the “DOD”), which provides that RFI will not be debarred from doing business with the U.S. Government entities as long as RFI maintains its compliance program and adheres to the terms of the administrative agreement. If RFI fails to maintain its compliance program or RFI or the Company fails to adhere to the terms of the administrative agreement, the DLA could debar the Company from doing business with U.S. Government entities.
On May 24, 2007, the Company's RFI subsidiary was served with a subpoena to testify before a grand jury of the United States District Court, Eastern District of New York and to provide items and records from its Bay Shore, NY offices in connection with U.S. Department of Defense contracts. A search warrant from the United States District Court, Eastern District of New York was issued and executed with respect to such offices. The Company believes that it is in full compliance with the quality standards that its customers require and is fully cooperating with investigators to assist them with their review. The Company's RFI subsidiary is continuing to ship products to the U.S. Government as well as to its commercial customers.
OUR BUSINESS IS BASED ON TECHNOLOGY THAT IS NOT PROTECTED BY PATENT OR OTHER RIGHTS.
The technology and designs underlying our products are unprotected by patent rights. Our future success is dependent primarily on unpatented trade secrets and on the innovative skills, technological expertise and management abilities of our employees. Because we do not have patent rights in our products, our technology may not preclude or inhibit competitors from producing products that have identical performance as our products. In addition, we cannot guarantee that any protected trade secret could ultimately be proven valid if challenged. Any such challenge, with or without merit, could be time consuming to defend, result in costly litigation, divert management’s attention and resources and, if successful, require us to pay monetary damages.
WE MAY NOT BE ABLE TO COMPETE SUCCESSFULLY.
A number of foreign and domestic companies have developed, or are expected to develop, products that compete or will compete with our products. Many of these competitors offer a range of products in areas other than those in which we compete, which may make such competitors more attractive to hospitals, radiology clients, general purchasing organizations and other potential customers. In addition, many of our competitors and potential competitors are larger and have greater financial resources than we do and offer a range of products broader than our products. Some of the companies with which we now compete or may compete in the future have or may have more extensive research, marketing and manufacturing capabilities and significantly greater technical and personnel resources than we do, and may be better positioned to continue to improve their technology in order to compete in an evolving industry.
OUR DELAY OR INABILITY TO OBTAIN ANY NECESSARY U.S. OR FOREIGN REGULATORY CLEARANCES OR APPROVALS FOR OUR PRODUCTS COULD HARM OUR BUSINESS AND PROSPECTS.
Our medical imaging products, with the exception of certain veterinary lines, are the subject of a high level of regulatory oversight. Any delay in our obtaining or our inability to obtain any necessary U.S. or foreign regulatory approvals for new products could harm our business and prospects. There is a limited risk that any approvals or clearances, once obtained, may be withdrawn or modified which could create delays in shipping our product, pending re-approval. Medical devices cannot be marketed in the U.S. without clearance or approval by the FDA. Our Medical Systems Group businesses must be operated in compliance with FDA Good Manufacturing Practices, which regulate the design, manufacture, packing, storage and installation of medical devices. Our manufacturing facilities and business practices are subject to periodic regulatory audits and quality certifications and we do self audits to monitor our compliance. In general, corrective actions required as a result of these audits do not have a significant impact on our manufacturing operations; however there is a limited risk that delays caused by a potential response to extensive corrective actions could impact our operations. Virtually all of our products manufactured or sold overseas are also subject to approval and regulation by foreign regulatory and safety agencies. If we do not obtain these approvals, we could be precluded from selling our products or required to make modifications to our products which could delay bringing our products to market. Because our U.S. products lines are mature, new product changes are in general relatively minor, and accordingly regulatory approval is more streamlined.
WE MUST RAPIDLY DEVELOP NEW PRODUCTS IN ORDER TO COMPETE EFFECTIVELY.
Technology in our industry, particularly in the x-ray and medical imaging businesses, evolves rapidly, and making timely product innovations is essential to our success in the marketplace. The introduction by our competitors of products with improved technologies or features may render our existing products obsolete and unmarketable. If we cannot develop products in a timely manner in response to industry changes, or if our products do not perform well, our business and financial condition will be adversely affected. Also, our new products may contain defects or errors which give rise to product liability claims against us or cause the products to fail to gain market acceptance.
It is generally accepted that digital radiography will become the dominant technology used in hospitals and imaging clinics throughout the world over the next 10 to 15 years. Currently, there are a number of competing technologies available in connection with the digitization of x-ray images. However, due to the high cost of this technology, many institutions have not yet adopted digital technology. In addition, there is uncertainty as to which technology system will be accepted as the industry-leading protocol for image digitization and communication. Lack of an adequate digital capability could impact our business and result in a loss of market share.
A SHORTAGE OF AN ADEQUATE SUPPLY OF RAW MATERIALS COULD INCREASE OUR COSTS AND CAUSE A DELAY IN OUR ABILITY TO SHIP PRODUCT AND FULFILL ORDERS. A LARGE PORTION OF OUR MANUFACTURING COSTS CONSIST OF THE COST OF MATERIALS AND AN INCREASE IN THESE COSTS COULD ADVERSELY IMPACT OUR GROSS MARGINS.
We rely on external sources to supply raw materials, which consist primarily of mechanical subassemblies, electronic components, x-ray tubes and x-ray generators in the Medical Systems Group and electronic components and subassemblies and, metal enclosures for its products in the Power Conversion Group. Our ability to meet future demand and manufacture our product is dependent on these sources of supply. If disruptions in these sources of supply cause shortages of raw materials, our ability to ship products to customers will be impacted. In addition, due to the high material cost component of our manufactured goods, our gross margins would be adversely impacted by increases in raw material costs we may be unable to pass along to our customers due to market conditions.
DUE TO THE SIGNIFICANCE OF OUR INTERNATIONAL OPERATIONS, POLITICAL OR ECONOMIC CHANGES IN THE VARIOUS COUNTRIES OR REGIONS WE MANUFACTURE IN OR SELL OUR PRODUCTS TO COULD IMPACT OUR FINANCIAL CONDITION.
International sales, including product manufactured at our facility in Milan, Italy, as well as product manufactured in the US, comprised 64% and 66% of consolidated revenues for fiscal years 2008 and 2007, respectively. Our future results could be adversely affected by a variety of international risks, including unfavorable foreign currency exchange rates; difficulties in managing and staffing international operations, political or social unrest; economic instability or natural disasters; environmental or trade protection measures; changes in governmental or other entities buying patterns and tender order procedures; changes in other regulatory or certification requirements. In addition any changes in Italian tax laws including changes in withholding on dividends from our Italian subsidiary or other restrictions on transfers of funds to the U.S. could impact our financial condition.
THE COMPANY'S WORKING CAPITAL NEEDS ARE FINANCED IN PART BY CREDIT FACILITIES WITH U.S. AND ITALIAN BANKS. THE COMPANY HAS NEEDED TO OBTAIN WAIVERS FROM ITS U.S. LENDERS FOR COVENANT VIOLATIONS DUE TO LESS THAN ANTICIPATED OPERATING RESULTS.
On December 6, 2006, the Company and its U.S. lender signed an amendment to its U.S. revolving credit and term loan credit facility which waived covenant violations existing as of October 28, 2006 and adjusted the financial covenants for future periods based on a business plan the Company provided to its lender. Although the Company was in compliance with all covenants of its revolving credit and term loan facility as of August 2, 2008 and the Company had no outstanding borrowings under these agreements, should the Company’s results be less that anticipated in the business plan, the Company could have future covenant violations. If the Company and its lender were unable to cure the violations by signing a waiver agreement, or through other means, the Company could be in default under its foreign and domestic credit agreements and the banks would have the ability to stop revolving credit borrowings under the facility or accelerate the maturity of any outstanding balances. If additional sources of debt or equity capital were not available at that point, such acceleration could have a material adverse impact on the Company’s financial position.
WE MUST CONDUCT OUR BUSINESS OPERATIONS WITHOUT INFRINGING ON THE PROPRIETARY RIGHTS OF THIRD PARTIES.
Although we believe our products do not infringe on the intellectual property rights of others, there can be no assurance that infringement claims will not be asserted against us in the future or that, if asserted, any infringement claim will be successfully defended. A successful claim, or any claim, against us could distract our management’s attention from other business concerns and adversely affect our business, financial condition and results of operations.
POTENTIAL SETTLEMENTS REQUIRED UNDER LEGAL PROCEEDINGS WITH FORMER EMPLOYEES OF THE COMPANY COULD UNDULY BURDEN OUR COMPANY.
The Company had an employment agreement with Samuel Park, a previous Chief Executive Officer (“CEO”), for the period May 1, 2001 to April 30, 2004. The employment agreement provided for certain payments in the event of a change in the control of the Company. On October 10, 2003, the Company announced the appointment of Walter F. Schneider as President and CEO to replace Mr. Park, effective as of such date. As a result, the Company recorded a charge of $.2 million during the first quarter of fiscal 2004 to accrue the balance remaining under Mr. Park’s employment agreement.
The Company’s employment agreement with Mr. Park provided for payments upon certain changes of control. The Company’s Board of Directors elected at the Company’s Annual Meeting of Shareholders held on May 29, 2003, had reviewed the “change of control” provisions regarding payments totaling up to approximately $1.8 million under the employment agreement between the Company and Mr. Park. As a result of this review and based upon, among other things, the advice of special counsel, the Company’s Board of Directors has determined that no obligation to pay these amounts has been triggered. Prior to his departure from the Company on October 10, 2003, Mr. Park orally informed the Company that, after reviewing the matter with his counsel, he believed that the obligation to pay these amounts has been triggered. On October 27, 2003, the Company received a letter from Mr. Park’s counsel demanding payment of certain sums and other consideration pursuant to the Company’s employment agreement with Mr. Park, including these change of control payments. On November 17, 2003, the Company filed a complaint in the United States District Court, Southern District of New York, against Mr. Park seeking a declaratory judgment that no change in control payment was or is due to Mr. Park, and that an amendment to the employment contract with Mr. Park regarding advancement and reimbursement of legal fees is invalid and unenforceable. Mr. Park answered the complaint and asserted counterclaims seeking payment from the Company based on his position that a “change in control” occurred in June 2003. Mr. Park is also seeking other consideration he believes he is owed under his employment agreement. The Company filed a reply to Mr. Park’s counterclaims denying that he is entitled to any of these payments. Discovery in this matter was conducted and completed. Following discovery, the Company and Mr. Park filed motions for summary judgment on the issues related to the change in control and the amendment to the employment agreement, which motions have been fully submitted to the court for consideration. To date, no decision has been issued by the court on these motions. If Mr. Park prevails on his claims and the payments he seeks are required to be paid in a lump sum, these payments may have a material adverse effect on the Company’s liquidity. It is not possible to predict the outcome of these claims. However, the Company’s Board of Directors does not believe that such a claim is reasonably likely to result in a material decrease in the Company’s liquidity in the foreseeable future. The Company has not recorded an accrual for any potential settlements of this claim as it has no basis upon which to estimate either the outcome or amount of loss, if any.
On June 28, 2002, Jeffrey N. Moeller, the former Director of Quality Assurance and Regulatory Affairs of Del Medical, commenced an action in the Circuit Court of Cook County, Illinois, against the Company, Del Medical and Walter Schneider, the former President of Del Medical. In the most current iteration of his complaint, the third amended complaint, Mr. Moeller alleged four claims against the defendants in the action: for (1) retaliatory discharge from employment with Del Medical, allegedly in response to Mr. Moeller’s complaints to officers of Del Medical about purported prebilling and his stopping shipment of a product that allegedly did not meet regulatory standards, (2) defamation, (3) intentional interference with his employment relationship with Del Medical and prospective employers, and (4) to hold the Company liable for any misconduct of Del Medical under a theory of piercing the corporate veil. In their answer to the third amended complaint, the defendants denied the substantive allegations of each of these claims and denied that they have any liability to Mr. Moeller. By order dated September 15, 2006, the Court denied in part and granted in part defendants’ motion requesting summary judgment dismissing the third amended complaint. The court granted the motion only to the extent of dismissing that part of Mr. Moeller’s claim of interference with his employment relationship with Del Medical and his relationship with prospective employers.
In fiscal 2007, the Company recorded an accrual of $0.1 million relating to potential liability in the settlement of these claims. The parties appeared for mediation in January 2007 but the mediation did not result in a disposition of the action. A trial was held in April 2008 and on April 17, 2008, the jury returned a verdict in favor of Mr. Moeller for $1.8 million for lost earnings, back pay, front pay and benefits on the retaliatory discharge claim, and $0.2 million for emotional distress/reputation damages and $0.2 million in punitive damages on the defamation claim. On May 19, 2008, counsel for the defendants filed their motion for judgment in their favor notwithstanding the jury verdict, or, alternatively, for a new trial, on those claims on which the jury found the respective defendants liable. By order dated August 15, 2008, the Court denied that motion.
On August 25, 2008, the Company and the other defendants filed their notice of appeal by which they appeal to the Appellate Court of Illinois, First District, among other things, the judgment entered against the Company and the other defendants on the jury verdict. On September 12, 2008 the Company and the other defendants filed an amended notice of appeal. The Company intends to pursue the appeal vigorously, seeking thereby reversal of the judgment and to have a judgment entered in favor of the Company and the other defendants or to have a new trial. In lieu of a bond, the Company filed an irrevocable standby letter of credit in the amount of $2.6 million by which Mr. Moeller can collect the amount of the judgment entered by the trial court in the event the appellate court affirms that judgment.
On April 28, 2008, George Apergis (“Apergis”), the former General Manager of RFI, filed a charge with the Equal Employment Opportunity Commission (“EEOC”) alleging that RFI discriminated against him by terminating his employment with RFI on December 18, 2007. Apergis alleged three claims against RFI: (1) violation of Title VII of the Civil Rights Act; (2) violation of the Age Discrimination in Employment Act and (3) retaliation. RFI responded to the EEOC charge with a position statement filed with the EEOC on June 26, 2008 denying each allegation of the charge. As of September 16, 2008, RFI is waiting to hear for a response to their position statement from the EEOC. RFI intends to defend vigorously against Apergis.
THERE IS A RISK THAT OUR INSURANCE WILL NOT BE SUFFICIENT TO PROTECT US FROM PRODUCT LIABILITY CLAIMS, OR THAT IN THE FUTURE PRODUCT LIABILITY INSURANCE WILL NOT BE AVAILABLE TO US AT A REASONABLE COST, IF AT ALL.
Our business involves the risk of product liability claims inherent to the medical device business. We maintain product liability insurance subject to certain deductibles and exclusions. There is a risk that our insurance will not be sufficient to protect us from product liability claims, or that product liability insurance will not be available to us at a reasonable cost, if at all. An uninsured or underinsured claim could materially harm our operating results or financial condition.
WE FACE RISKS ASSOCIATED WITH HANDLING HAZARDOUS MATERIALS AND PRODUCTS.
Our research and development activity involves the controlled use of hazardous materials, such as toxic and carcinogenic chemicals. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by federal, state and local regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. In the event of an accident, we could be held liable for any resulting damages, and such liability could be extensive.
We are also subject to substantial regulation relating to occupational health and safety, environmental protection, hazardous substance control, and waste management and disposal. The failure to comply with such regulations could subject us to, among other things, fines and criminal liability.
OUR BUSINESS COULD BE HARMED IF OUR PRODUCTS CONTAIN UNDETECTED ERRORS OR DEFECTS OR DO NOT MEET CUSTOMER SPECIFICATIONS.
We are continuously developing new products and improving our existing products. Newly introduced or upgraded products can contain undetected errors or defects. In addition, these products may not meet their performance specifications under all conditions or for all applications. If, despite our internal testing and testing by our customers, any of our products contains errors or defects, or any of our products fails to meet customer specifications, we may be required to recall or retrofit these products. We may not be able to do so on a timely basis, if at all, and may only be able to do so at considerable expense. In addition, any significant reliability problems could result in adverse customer reaction and negative publicity and could harm our business and prospects.
THE SEASONALITY OF OUR REVENUE MAY ADVERSELY IMPACT THE MARKET PRICES FOR OUR SHARES.
Our revenue is typically lower during the first quarter of each fiscal year due to the shut-down of operations in our Milan, Italy and Bay Shore, New York facilities for part of August. This seasonality causes our operating results to vary from quarter to quarter and these fluctuations could adversely affect the market price of our common stock.
A SIGNIFICANT NUMBER OF OUR SHARES WILL BE AVAILABLE FOR FUTURE SALE AND COULD DEPRESS THE MARKET PRICE OF OUR STOCK.
As of October 1, 2008, an aggregate of 24,246,165 shares of our common stock were outstanding. In addition, as of October 1, 2008, there were outstanding warrants to purchase 500,321 shares of our common stock and options to purchase 2,094,815 shares of our common stock, 1,595,438 of which were fully vested. Sales of large amounts of our common stock in the market could adversely affect the market price of the common stock and could impair our future ability to raise capital through offerings of our equity securities. A large volume of sales by holders exercising the warrants or options could have a significant adverse impact on the market price of our common stock.
WE HAVE A LIMITED TRADING MARKET AND OUR STOCK PRICE MAY BE VOLATILE.
There is a limited public trading market for our common stock in the Over-the-Counter “OTC” Market. We cannot assure you that a regular trading market for our common stock will ever develop or that, if developed, it will be sustained.
The experiences of other small companies indicate that the market price for our common stock could be highly volatile. Many factors could cause the market price of our common stock to fluctuate substantially, including:
| | - | future announcements concerning us, our competitors or other companies with whom we have business relationships; |
| | - | changes in government regulations applicable to our business; |
| | - | overall volatility of the stock market and general economic conditions; |
| | - | changes in our earnings estimates or recommendations by analysts; and |
| | - | changes in our operating results from quarter to quarter. |
Accordingly, substantial fluctuations in the price of our common stock could limit the ability of our current shareholders to sell their shares at a favorable price.
THE COMPANY MAY SUBMIT, FROM TIME TO TIME, PROPOSALS TO SHAREHOLDERS TO AMEND THE COMPANY’S CERTIFICATE OF INCORPORATION OR TO INCREASE THE NUMBER OF COMMON SHARES AUTHORIZED.
At a special meeting of shareholders of the Company held on November 17, 2006, the Company’s shareholders approved an Amendment of the Certificate of Incorporation of the Company to increase the number of authorized shares of the Company’s common stock, par value $0.10 per share, from twenty million (20,000,000) shares to fifty million (50,000,000) shares in order to have a sufficient number of shares of Common Stock to provide a reserve of shares available for issuance to meet business needs as they may arise in the future. Such business needs may include, without limitation, rights offerings, financings, acquisitions, establishing strategic relationships with corporate partners, providing equity incentives to employees, officers or directors, stock splits or similar transactions. Issuances of any additional shares for these or other reasons could prove dilutive to current shareholders or deter changes in control of the Company, including transactions where the shareholders could otherwise receive a premium for there shares over then current market prices.
| UNRESOLVED STAFF COMMENTS |
Not applicable
The following is a list of our principal properties, classified by segment and subsidiary:
SEGMENT | LOCATION | APPROX. FLOOR AREA IN SQ. FT. | PRINCIPAL USES | OWNED/LEASED (EXPIRATION DATE IF LEASED) |
| MEDICAL SYSTEMS GROUP: | | | | |
| Del Medical Imaging Corp | Franklin Park, IL | 68,000 | Corporate headquarters, Design and manufacturing | Leased (2009) |
| Villa | Milan, Italy | 67,000 | Design and manufacturing | Leased (2011)(1) |
| | | | | |
| POWER CONVERSION GROUP: | | | | |
| RFI | Bay Shore, NY | 55,000 | Design and manufacturing | Owned |
| (1) | Villa has the option to purchase this property at the conclusion of this lease. |
We believe that our current facilities are sufficient for our present and anticipated future requirements. The Company’s manufacturing operations run on one shift and we have the ability to add a second shift, if needed. The Company’s domestic credit facilities are secured, in part, by a mortgage on RFI’s property.
DOD INVESTIGATION - On March 8, 2002, RFI, a subsidiary of the Company and the remaining part of the Power Conversion Group segment, was served with a subpoena by the US Attorney for the Eastern District of New York in connection with an investigation by the DOD. RFI supplies electro-magnetic interference filters for communications and defense applications. The DOD had investigated certain past practices at RFI which dated back to before 1996 and pertain to RFI's Military Specification testing, record keeping and general operating procedures. Management retained special counsel to represent the Company on this matter. The Company cooperated fully with this investigation, including voluntarily providing employees to be interviewed by the Defense Criminal Investigative Services division of the DOD.
In June 2003, the Company was advised that the U.S. Government was willing to enter into negotiations regarding a comprehensive settlement of this investigation. Prior to the preliminary discussions with the US Government in June 2003, the Company had no basis to estimate the financial impact of this investigation. Based on preliminary settlement discussions with the U.S. Government, discussions with the Company's advisors, consideration of settlements reached by other parties in investigations of this nature, and consideration of the Company's capital resources, management then developed an estimate of the low end of the potential range of the financial impact. Accordingly, during the third quarter of fiscal 2003, the Company recorded a charge of $2.3 million, which represented its estimate of the low end of a range of potential fines and legal and professional fees.
Following negotiations, the Company reached a global settlement in February 2004 with the U.S. Government that resolved the civil and criminal matters relating to the DOD's investigation. The settlement included the Company pleading guilty to one criminal count and agreeing to pay fines and restitution to the U.S. Government of $4.6 million if paid by June 30, 2004 and $5.0 million if paid by September 30, 2004. In connection with this settlement, the Company recognized an additional charge of approximately $3.2 million in the second quarter of fiscal 2004. This charge represents the difference between the $2.3 million charge taken during the third quarter of fiscal 2003, and up to $5.0 million in fines and restitution, plus estimated legal and professional fees related to this settlement.
On September 30, 2004, pursuant to the terms of the settlement, the Company fulfilled its obligation under this agreement by paying to the U.S. Government the sum of $5.0 million representing fines and restitution. On October 7, 2004, RFI entered a guilty plea to a single count conspiracy charge pursuant to the settlement and a criminal plea agreement. Sentencing occurred on March 15, 2005. At sentencing, the Court imposed an additional fine of $0.3 million to be paid within 30 days. The Company paid this additional fine on April 8, 2005.
The Company worked with the U.S. Defense Logistics Agency ("DLA"), a component of the DOD, to avoid any future limitations on the ability of the Company to do business with U.S. Government entities. Such limitations could have included the U.S. Government seeking a "debarment" or exclusion of the Company from doing business with U.S. Government entities for a period of time. On April 5, 2005, the Company announced that it had reached an administrative agreement with the DLA which provides that RFI will not be debarred from doing business with U.S. Government entities so long as RFI maintains its compliance program and adheres to the terms of the administrative agreement. This agreement with the DLA is the final component of the Company's previously announced settlement of an investigation by the DOD into practices at RFI.
On May 24, 2007, the Company's RFI subsidiary was served with a subpoena to testify before a grand jury of the United States District Court, Eastern District of New York and to provide items and records from its Bay Shore, NY offices in connection with U.S. DOD contracts. A search warrant from the United States District Court, Eastern District of New York was issued and executed with respect to such offices. The Company believes that it is in full compliance with the quality standards that its customers require and is fully cooperating with investigators to assist them with their review. The Company's RFI subsidiary is continuing to ship products to the U.S. Government as well as to its commercial customers.
EMPLOYMENT MATTERS
PARK - The Company had an employment agreement with Samuel Park, a previous CEO, for the period May 1, 2001 to April 30, 2004. The employment agreement provided for certain payments in the event of a change in the control of the Company. On October 10, 2003, the Company announced the appointment of Walter F. Schneider as President and CEO to replace Mr. Park, effective as of such date. As a result, the Company recorded a charge of $0.2 million during the first quarter of fiscal 2004 to accrue the balance remaining under Mr. Park’s employment agreement.
The Company’s employment agreement with Mr. Park provided for payments upon certain changes in control. The Company’s Board of Directors elected at the Company’s Annual Meeting of Shareholders held on May 29, 2003, had reviewed the “change of control” provisions regarding payments totaling up to approximately $1.8 million under the employment agreement between the Company and Mr. Park. As a result of this review and based upon, among other things, the advice of special counsel, the Company’s Board of Directors has determined that no obligation to pay these amounts has been triggered. Prior to his departure from the Company on October 10, 2003, Mr. Park orally informed the Company that, after reviewing the matter with his counsel, he believed that the obligation to pay these amounts has been triggered. On October 27, 2003, the Company received a letter from Mr. Park’s counsel demanding payment of certain sums and other consideration pursuant to the Company’s employment agreement with Mr. Park, including these change of control payments. On November 17, 2003, the Company filed a complaint in the United States District Court, Southern District of New York, against Mr. Park seeking a declaratory judgment that no change in control payment was or is due to Mr. Park, and that an amendment to the employment contract with Mr. Park regarding advancement and reimbursement of legal fees is invalid and unenforceable. Mr. Park answered the complaint and asserted counterclaims seeking payment from the Company based on his position that a “change in control” occurred in June 2003. Mr. Park is also seeking other consideration he believes he is owed under his employment agreement. The Company filed a reply to Mr. Park’s counterclaims denying that he is entitled to any of these payments. Discovery in this matter was conducted and completed. Following discovery, the Company and Mr. Park filed motions for summary judgment on the issues related to the change in control and the amendment to the employment agreement, which motions have been fully submitted to the court for consideration. To date, no decision has been issued by the court on these motions. If Mr. Park prevails on his claims and the payments he seeks are required to be paid in a lump sum, these payments may have a material adverse effect on the Company’s liquidity. It is not possible to predict the outcome of these claims. However, the Company’s Board of Directors does not believe that such a claim is reasonably likely to result in a material decrease in the Company’s liquidity in the foreseeable future. The Company has not recorded an accrual for any potential settlements of this claim as it has no basis upon which to estimate either the outcome or amount of loss, if any.
MOELLER - On June 28, 2002, Jeffrey N. Moeller, the former Director of Quality Assurance and Regulatory Affairs of Del Medical, commenced an action in the Circuit Court of Cook County, Illinois, against the Company, Del Medical and Walter Schneider, the former President of Del Medical. In the most current iteration of his complaint, the third amended complaint, Mr. Moeller alleged four claims against the defendants in the action: for (1) retaliatory discharge from employment with Del Medical, allegedly in response to Mr. Moeller’s complaints to officers of Del Medical about purported prebilling and his stopping shipment of a product that allegedly did not meet regulatory standards, (2) defamation, (3) intentional interference with his employment relationship with Del Medical and prospective employers, and (4) to hold the Company liable for any misconduct of Del Medical under a theory of piercing the corporate veil. In their answer to the third amended complaint, the defendants denied the substantive allegations of each of these claims and denied that they have any liability to Mr. Moeller. By order dated September 15, 2006, the Court denied in part and granted in part defendants’ motion requesting summary judgment dismissing the third amended complaint. The court granted the motion only to the extent of dismissing that part of Mr. Moeller’s claim of interference with his employment relationship with Del Medical and his relationship with prospective employers.
In fiscal 2007, the Company recorded an accrual of $0.1 million relating to potential liability in the settlement of these claims. The parties appeared for mediation in January 2007 but the mediation did not result in a disposition of the action. A trial was held in April 2008 and on April 17, 2008, the jury returned a verdict in favor of Mr. Moeller for $1.8 million for lost earnings, back pay, front pay and benefits on the retaliatory discharge claim, and $0.2 million for emotional distress/reputation damages and $0.2 million in punitive damages on the defamation claim. On May 19, 2008, counsel for the defendants filed their motion for judgment in their favor notwithstanding the jury verdict, or, alternatively, for a new trial, on those claims on which the jury found the respective defendants liable. By order dated August 15, 2008, the Court denied that motion.
On August 25, 2008, the Company and the other defendants filed their notice of appeal by which they appeal to the Appellate Court of Illinois, First District, among other things, the judgment entered against the Company and the other defendants on the jury verdict. On September 12, 2008 the Company and the other defendants filed an amended notice of appeal. The Company intends to pursue the appeal vigorously, seeking thereby reversal of the judgment and to have a judgment entered in favor of the Company and the other defendants or to have a new trial. In lieu of a bond, the Company filed an irrevocable standby letter of credit in the amount of $2.6 million by which Mr. Moeller can collect the amount of the judgment entered by the trial court in the event the appellate court affirms that judgment.
APERGIS - On April 28, 2008, Apergis, the former General Manager of RFI, filed a charge with the EEOC alleging that RFI discriminated against him by terminating his employment with RFI on December 18, 2007. Apergis alleged three claims against RFI: (1) violation of Title VII of the Civil Rights Act; (2) violation of the Age Discrimination in Employment Act and (3) retaliation. RFI responded to the EEOC charge with a position statement filed with the EEOC on June 26, 2008 denying each allegation of the charge. As of September 16, 2008, RFI is waiting to hear for a response to their position statement from the EEOC. RFI intends to defend vigorously against Apergis.
DIAMOND - Diamond v. Allied Diagnostic Imaging Resources, Inc., et al. (Superior Court, County of Los Angeles; Case No. BC362544). This action is brought against numerous defendants, including the Company. While the action commenced in late 2006, plaintiff first served the summons and complaint on the Company in December 2007. The plaintiff alleges that she is the wife of a chiropractor who died in June 2005. Plaintiff alleges that her husband was exposed to chemical products in developing x-ray films and cleaning film processing machines and worked with the x-ray film processing machines and x-ray machines, and that the defendants manufactured, supplied or serviced the chemical products and machines. The complaint further alleges that the decedent was exposed to toxic chemicals and radiation from the chemical products used on or in the machines, which caused “serious injuries to his internal organs, including acute myelogenous leukemia”, resulting in his death.
The complaint alleges the following six claims against the defendants: (1) negligence in manufacturing. and distributing the chemical products and machines, and servicing the machines, and failing adequately to warn decedent of the hazards of the chemical products and machines, (2) violation of a California statute and regulation by failing to determine whether the chemical products caused health hazards and failing to label or identify in material safety data sheets a health hazard relating to acute myelogenous leukemia, (3) strict products liability for failing to warn adequately of the chemical products’ and machines’ health hazards, (4) strict products liability for defects in the design of the chemical products and machines, (5) fraudulent concealment of the toxic and carcinogenic nature of the chemical products and the machines, and (6) breach of implied warranties as to the fitness of the chemical products and machines for intended uses, merchantability, and lack of defects.
While the complaint does not allege a total amount of damages sought, plaintiff alleges that she has suffered damages consisting of medical, funeral, and burial expenses, the decedent’s lost earnings prior to and lost wages after his death, lost benefits after his death, the value of his services in managing his family’s home, and loss of companionship and similar losses. The plaintiff also requests punitive damages. The Company has not recorded an accrual for any potential settlement of this claim as it has no basis upon which to estimate either the outcome or amount of loss, if any. In its answer to the complaint, the Company denied the substantive allegations of the complaint and denied that it has any liability to plaintiff. The Company intends to defend vigorously against plaintiff’s claims.
OTHER LEGAL MATTERS - The Company is a defendant in several other legal actions in various U.S. and foreign jurisdictions arising from the normal course of business. Management believes the Company has meritorious defenses to such actions and that the outcomes will not be material to the Company’s consolidated financial statements.
| SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
None
| MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Effective May 11, 2007, our common stock commenced trading on the Over the Counter “OTC” Bulletin Board under the symbol “DGTC.OB” and our warrants are traded under the symbol “DGTCW.OB.” Prior to that, they had been traded on the “pink sheets,”, under the symbol “DGTC.PK” and “DGTCW.PK”, respectively. The OTC is an over-the-counter market which provides significantly less liquidity than established stock exchanges or the NASDAQ National Market, and quotes for stocks included in the OTC are not listed in the financial sections of newspapers as are those for established stock exchanges and the NASDAQ National Market. Our securities had been suspended from trading on the NASDAQ National Market on December 19, 2000 because we had not filed an Annual Report for the year ended July 29, 2000 within the SEC’s prescribed time period.
At a special meeting of shareholders of the Company held on November 17, 2006, the Company’s shareholders approved an Amendment of the Certificate of Incorporation of the Company to increase the number of authorized shares of the Company’s common stock, par value $0.10 per share, from twenty million (20,000,000) shares to fifty million (50,000,000) shares in order to have a sufficient number of shares of common stock to provide a reserve of shares available for issuance to meet business needs as they may arise in the future. Such business needs may include, without limitation, rights offerings, financings, acquisitions, establishing strategic relationships with corporate partners, providing equity incentives to employees, officers or directors, stock splits or similar transactions. Issuances of any additional shares for these or other reasons could prove dilutive to current shareholders or deter changes in control of the Company, including transactions where the shareholders could otherwise receive a premium for there shares over then current market prices.
On December 12, 2006, the Company filed a registration statement for a subscription rights offering with the SEC that became effective January 30, 2007. Under terms of this rights offering, the Company distributed to shareholders of record as of February 5, 2007, non-transferable subscription rights to purchase one share of the Company's common stock for each share owned at that date at a subscription price of $1.05 per share. On March 12, 2007, the Company completed the rights offering, selling 12,027,378 shares of its common stock at $1.05 per share. Total proceeds to the Company, net of $275,000 of expenses related to the rights offering, were $12,354,000.
The purpose of this rights offering was to raise equity capital in a cost-effective manner. Approximately $7,564,000 of the proceeds were used for debt repayment and the remainder invested in short-term money market securities for anticipated working capital needs and general corporate purposes. A portion of the net proceeds may also ultimately be used to acquire or invest in businesses, products and technologies that our management believes are complementary to the Company's business. Remaining unused proceeds are currently invested in short-term money market securities.
In addition, on January 22, 2007, the Company entered into a stockholders rights plan (the "Rights Plan"). The Rights Plan provides for a dividend distribution of one common stock purchase right for each outstanding share of the Company's common stock. The Company's Board of Directors adopted the Rights Plan to protect stockholder value by protecting the Company's ability to realize the benefits of its net operating losses ("NOLs") and capital loss carryforwards. The Company has experienced substantial operating and capital losses in previous years. Under the Internal Revenue Code and rules promulgated by the Internal Revenue Service, the Company may "carry forward" these losses in certain circumstances to offset current and future earnings and thus reduce its federal income tax liability, subject to certain requirements and restrictions. Assuming that the Company has future earnings, the Company may be able to realize the benefits of NOLs and capital loss carryforwards. These NOLs and capital loss carryforwards constitute a substantial asset to the Company. If the Company experiences an "Ownership Change," as defined in Section 382 of the Internal Revenue Code, its ability to use the NOLs and capital loss carryforwards could be substantially limited or lost altogether. The Rights Plan imposes a significant penalty upon any person or group that acquires more than a certain percentage of the Company's common stock by allowing other shareholders to acquire equity securities at half their fair values.
As of October 1, 2008, there were approximately 773 holders of record of our common stock. The following table shows the high and low sales prices per share of our common stock for the past eight quarters, as reported by the over the counter market. The over-the-counter market quotations listed below reflect inter-dealer prices, without retail mark-up, mark down or commission and may not represent actual transactions.
| FISCAL PERIOD | | HIGH | | | LOW | |
| FISCAL 2008 | | | | | | |
| First Quarter | | $ | 3.40 | | | $ | 2.25 | |
| Second Quarter | | | 3.25 | | | | 2.30 | |
| Third Quarter | | | 2.65 | | | | 1.99 | |
| Fourth Quarter | | | 2.45 | | | | 1.15 | |
| FISCAL 2007 | | | | | | | | |
| First Quarter | | $ | 2.05 | | | $ | 0.60 | |
| Second Quarter | | | 2.30 | | | | 1.55 | |
| Third Quarter | | | 2.40 | | | | 1.20 | |
| Fourth Quarter | | | 2.90 | | | | 1.35 | |
We have not paid any cash dividends, except for the payment of cash in lieu of fractional shares, since 1983. The payment of cash dividends is prohibited under our U.S. credit facility. We do not intend to pay any cash dividends in the foreseeable future.
The following table summarizes the securities authorized for issuance under equity compensation plans as of the end of Fiscal Year 2008
Equity Compensation Plan Information
PLAN CATEGORY | | NUMBER OF SECURITIES TO BE ISSUED UPON EXERCISE OF OUTSTANDING OPTIONS, WARRANTS AND RIGHTS | | | WEIGHTED-AVERAGE EXERCISE PRICE OF OUTSTANDING OPTIONS, WARRANTS AND RIGHTS | | | NUMBER OF SECURITIES REMAINING AVAILABLE FOR FUTURE ISSUANCE UNDER EQUITY COMPENSATION | |
| Equity compensation plans approved by security holders: | | | | | | | | | |
| Stock Option Plans | | | 2,094,815 | | | $ | 3.45 | | | | 604,500 | |
| Equity compensation plans not approved by security holders: | | | | | | | | | | | | |
Warrants issued in settlement of class action lawsuit (2) | | | 512,500 | | | $ | 1.50 | | | Not applicable |
(1) Excludes securities to be issued upon exercise of outstanding options, warrants and rights.
(2) Pursuant to our class action settlement with our shareholders concerning allegations that the Company had violated federal Securities laws, we issued 2.5 million shares of our common stock and one million warrants to purchase our common stock at $2.00 per share. The issuance of these securities was pursuant to a court order issued in connection with the settlement of this class action lawsuit in January 2002, and therefore was exempt from the registration requirements of the Securities Act of 1933 pursuant to Section 3(a) (10) thereof. These warrants were originally set to expire in March 2008.
In a motion filed in February 2004, a plaintiff class claimed damages due to Del Global’s failure to timely complete a registration statement for the shares of common stock issuable upon exercise of these warrants. The class sought damages of $1.25 million together with interest and costs, and a declaration that $2 million in subordinated notes issued as part of the 2002 class action settlement were immediately due and payable. In settlement of this matter, Del Global modified the exercise, or “strike,” price of the warrants issued in 2002 from $2.00 to $1.50 per share, and extended the expiration date of such warrants by one year to March 28, 2009.
PERFORMANCE GRAPH
The following graph compares the yearly percentage change in the cumulative shareholder return on the Common Stock with The Nasdaq Market Index and the peer group index for the Standard Industrial Classification Code ("SIC Code") 3844 for the period commencing August 2, 2003 and ending August 2, 2008. The peer group for SIC Code 3844 (X-Ray Apparatus and Tubes) consists of 3 companies and includes: American Science Engineering Inc., Hologic Inc., and Sirona Dental Systems, Inc. The graph assumes that $100 was invested on August 2, 2003 in the Common Stock and in each of the other indices and assumes monthly reinvestment of all dividends.
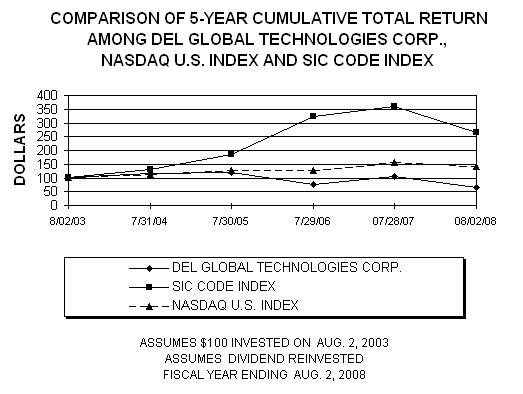
This stock price performance graph shall not be deemed to be "soliciting material" or "filed" or incorporated by reference in future filings with the Commission, or subject to the liabilities of Section 18 of the Exchange Act, except to the extent that the Company specifically incorporates it by reference into a document filed under the Securities Act or the Exchange Act.
The selected income statement data presented for the fiscal years ended August 2, 2008, July 28, 2007 and July 29, 2006 and the balance sheet data as of August 2, 2008 and July 28, 2007 have been derived from our audited consolidated financial statements included elsewhere in this Annual Report. The income statement data for the years ended July 30, 2005 and July 31, 2004 and the balance sheet data as of July 29, 2006, July 30, 2005 and July 31, 2004 have been derived from audited financial statements not included herein. This selected financial data should be read in conjunction with the Consolidated Financial Statements and related notes included in Part II, Item 8, “Financial Statements and Supplementary Data” thereto and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Part II, Item 7 of this Form 10-K.
| | | FISCAL YEARS ENDED | |
(IN THOUSANDS, EXCEPT SHARE AMOUNTS) | | AUGUST2, 2008 | | | JULY28, 2007 | | | JULY29, 2006 | | | JULY30, 2005 | | | JULY31, | |
| INCOME STATEMENT DATA: | | | | | | | | | | | | | | | |
| Net sales | | $ | 108,306 | | | $ | 104,167 | | | $ | 83,014 | | | $ | 84,872 | | | $ | 83,827 | |
| Gross margin | | | 26,787 | | | | 25,017 | | | | 19,358 | | | | 22,281 | | | | 21,315 | |
| Selling, general and administrative | | | 15,586 | | | | 14,590 | | | | 13,619 | | | | 16,452 | | | | 15,907 | |
| Research and development | | | 2,488 | | | | 2,013 | | | | 1,562 | | | | 1,636 | | | | 1,562 | |
| Goodwill impairment | | | 1,911 | | | | - | | | | - | | | | - | | | | - | |
| Litigation settlement costs | | | 450 | | | | - | | | | 697 | | | | 300 | | | | 3,652 | |
| Operating income | | | 6,352 | | | | 8,414 | | | | 3,480 | | | | 3,893 | | | | 194 | |
| Minority interest | | | - | | | | - | | | | 108 | | | | 393 | | | | 559 | |
| Provision for income taxes | | | 3,247 | | | | 3,553 | | | | 1,758 | | | | 2,054 | | | | 8,691 | |
| Income (loss) from continuing operation | | | 2,977 | | | | 3,816 | | | | 269 | | | | 193 | | | | (10,729 | ) |
| Discontinued operation | | | - | | | | - | | | | (175 | ) | | | 199 | | | | (5,095 | ) |
| Net income (loss) | | | 2,977 | | | | 3,816 | | | | 94 | | | | 392 | | | | (15,824 | ) |
| Net income (loss) per share – Basic | | | | | | | | | | | | | | | | | | | | |
| Continuing operations | | $ | 0.12 | | | $ | 0.24 | | | $ | 0.02 | | | $ | 0.02 | | | $ | (1.04 | ) |
| Discontinued operation | | | - | | | | - | | | | (0.01 | ) | | | 0.02 | | | | (0.49 | ) |
| Net income (loss) per basic share | | $ | 0.12 | | | $ | 0.24 | | | $ | 0.01 | | | $ | 0.04 | | | $ | (1.53 | ) |
| Net income (loss) per share – Diluted | | | | | | | | | | | | | | | |
| Continuing operations | | $ | 0.12 | | | $ | 0.23 | | | $ | 0.02 | | | $ | 0.01 | | | $ | (1.04 | ) |
| Discontinued operation | | | - | | | | - | | | | (0.01 | ) | | | 0.02 | | | | (0.49 | ) |
| Net income (loss) per diluted share | | $ | 0.12 | | | $ | 0.23 | | | $ | 0.01 | | | $ | 0.03 | | | $ | (1.53 | ) |
| Weighted average shares outstanding – Basic | | | 24,196 | | | | 16,155 | | | | 11,244 | | | | 10,490 | | | | 10,334 | |
| Weighted average shares outstanding – Diluted | | | 24,646 | | | | 16,455 | | | | 12,076 | | | | 11,465 | | | | 10,334 | |
| | | AS OF | |
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | | | JULY 29, 2006 | | | JULY 30, 2005 | | | JULY 31, | |
| BALANCE SHEET DATA: | | | | | | | | | | | | | | | |
| Working capital | | $ | 31,204 | | | $ | 24,978 | | | $ | 6,935 | | | $ | 10,122 | | | $ | 7,764 | |
| Total assets | | | 66,353 | | | | 66,339 | | | | 49,153 | | | | 40,776 | | | | 49,261 | |
| Long-term debt and subordinated note | | | 4,504 | | | | 5,393 | | | | 5,133 | | | | 6,454 | | | | 7,038 | |
| Shareholders’ equity | | | 36,163 | | | | 30,196 | | | | 12,814 | | | | 9,228 | | | | 7,775 | |
(1) Net loss for the year ended July 31, 2004 includes a $9,794 income tax provision related to the establishment of a deferred tax valuation allowance. In addition, the net loss reflects the accrual of a $3,199 charge related to the DOD investigation of our RFI subsidiary and $454 related to a motion filed in February 2004 related to the warrants to purchase common stock that were issued in fiscal year 2002. For more information about these legal charges, see Part I, Item 3 “Legal Proceedings” of this Annual Report.
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
FORWARD LOOKING STATEMENTS
In addition to other information in this Annual Report, this Management's Discussion and Analysis of Financial Condition and Results of Operations contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements are based on current expectations and the current economic environment. We caution that these statements are not guarantees of future performance. They involve a number of risks and uncertainties that are difficult to predict including, but not limited to, our ability to implement our business plan, retention of management, changing industry and competitive conditions, obtaining anticipated operating efficiencies, securing necessary capital facilities and favorable determinations in various legal and regulatory matters. Actual results could differ materially from those expressed or implied in the forward-looking statements. Important assumptions and other important factors that could cause actual results to differ materially from those in the forward-looking statements are specified in the Company's filings with the SEC including the Company’s Quarterly Reports on Form 10-Q and Current Reports on Form 8-K.
OVERVIEW
The Company is primarily engaged in the design, manufacture and marketing of cost-effective medical and dental diagnostic imaging systems consisting of stationary and portable imaging systems, radiographic/ fluoroscopic systems, dental imaging systems and digital radiography systems. The Company also manufactures electronic filters, high voltage capacitors, pulse modulators, transformers and reactors, and a variety of other products designed for industrial, medical, military and other commercial applications. We manage our business in two operating segments: our Medical Systems Group and our Power Conversion Group. In addition, we have a third reporting segment, other, comprised of certain unallocated corporate General and Administrative expenses. See Part I, Item 1, “Business-Operating Segments” of this Annual Report for discussions of the Company’s segments.
On October 1, 2004, we sold the Del High Voltage division, which manufactured proprietary high voltage power conversion subsystems, for a purchase price of $3.1 million, plus the assumption of approximately $0.8 million of liabilities. Accordingly, the results of operations have been restated to show this division as a discontinued operation.
At a special meeting of shareholders of the Company held on November 17, 2006, the Company’s shareholders approved an Amendment of the Certificate of Incorporation of the Company to increase the number of authorized shares of the Company’s common stock, par value $0.10 per share, from twenty million (20,000,000) shares to fifty million (50,000,000) shares in order to have a sufficient number of shares of common stock to provide a reserve of shares available for issuance to meet business needs as they may arise in the future. Such business needs may include, without limitation, rights offerings, financings, acquisitions, establishing strategic relationships with corporate partners, providing equity incentives to employees, officers or directors, stock splits or similar transactions. Issuances of any additional shares for these or other reasons could prove dilutive to current shareholders or deter changes in control of the Company, including transactions where the shareholders could otherwise receive a premium for there shares over then current market prices.
CRITICAL ACCOUNTING POLICIES
Complete descriptions of significant accounting policies are outlined in Note 1 of the Notes to Consolidated Financial Statements included in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report. Within these policies, we have identified the accounting for revenue recognition, deferred tax assets, the allowance for obsolete and excess inventory and goodwill as being critical accounting policies due to the significant amount of estimates involved. In addition, for interim periods, we have identified the valuation of finished goods inventory as being critical due to the amount of estimates involved.
REVENUE RECOGNITION
The Company recognizes revenue upon shipment, provided there is persuasive evidence of an arrangement, there are no uncertainties concerning acceptance, the sales price is fixed, collection of the receivable is probable and only perfunctory obligations related to the arrangement need to be completed. The Company maintains a sales return allowance, based upon historical patterns, to cover estimated normal course of business returns, including defective or out of specification product. The Company’s products are covered primarily by one year warranty plans and in some cases optional extended warranties for up to five years are offered. The Company establishes allowances for warranties on an aggregate basis for specifically identified, as well as anticipated, warranty claims based on contractual terms, product conditions and actual warranty experience by product line. The Company recognizes service revenue when repairs or out of warranty repairs are completed. The Company has an FDA obligation to continue to provide repair service for certain medical systems for up to seven years past the warranty period. These repairs are billed to the customers at market rates.
DEFERRED INCOME TAXES
The Company accounts for deferred income taxes in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 109 “Accounting for Income Taxes” whereby we recognize deferred income tax assets and liabilities for temporary differences between financial reporting basis and income tax reporting basis and for tax credit carry forwards.
The Company periodically assesses the realization of our net deferred income tax assets. This evaluation is primarily based upon current operating results and expectations of future operating results. A valuation allowance is recorded if the Company believes its net deferred income tax assets will not be realized. Our determination is based on what we believe will be the more likely than not result.
During fiscal years 2008, 2007 and 2006, the Company reported operating income on a consolidated basis. The Company’s foreign tax reporting entity was profitable and its U.S. tax reporting entities incurred a taxable loss in each fiscal year. Based primarily on these results, the Company concluded that it should maintain a 100% valuation allowance on its net U.S. deferred tax assets. As of August 2, 2008, the Company continues to carry a 100% valuation allowance on its net U.S. deferred income tax assets.
The Company recorded a tax expense with respect to its foreign subsidiary’s income in all periods presented and based on a more likely than not standard, believes that the foreign subsidiary’s net deferred income tax asset of $0.8 million at August 2, 2008 will be realized.
The Company’s foreign subsidiary operates in Italy. Italy recently enacted legislation that reduces tax rates effective for the Company’s fiscal year 2009. Fiscal 2008 income tax expense includes a charge that reduces the carrying value of the foreign subsidiary’s net deferred income tax asset resulting from the income tax rate reduction.
Additionally, the Company’s deferred income tax liabilities as of July 28, 2007 included the estimated tax obligation that would have been incurred upon a distribution of the foreign subsidiary’s earnings to its U.S. parent. This tax liability was recorded as the foreign subsidiary had routinely distributed monies to its U.S. parent. Based on current operating results, expectations of future results and available cash and credit in the U.S. the Company had determined it no longer intends to repatriate monies in the foreseeable future and has reversed this tax obligation, much of which was offset by having a lower valuation allowance. This reversal resulted in an adjustment to available net operating loss carryforwards and the related valuation allowance and in a reduction in tax expense for fiscal 2008.
The Company recorded a $0.1 million tax benefit resulting from the effect of the Italian tax rate change on deferred tax assets and the reversal of estimated tax obligation upon repatriation of non-U.S. earnings.
OBSOLETE AND EXCESS INVENTORY
We re-evaluate our allowance for obsolete inventory once a quarter, and this allowance comprises the most significant portion of our inventory reserves. The re-evaluation of reserves is based on a written policy, which requires at a minimum that reserves be established based on our analysis of historical actual usage on a part-by-part basis. In addition, if management learns of specific obsolescence in addition to this minimum formula, these additional reserves will be recognized as well. Specific obsolescence might arise due to a technological or market change, or based on cancellation of an order. As we typically do not purchase inventory substantially in advance of production requirements, we do not expect cancellation of an order to be a material risk. However, market or technology changes can occur.
VALUATION OF FINISHED GOODS INVENTORIES
In addition, we use certain estimates in determining interim operating results. The most significant estimates in interim reporting relate to the valuation of finished goods inventories. For certain subsidiaries, for interim periods, we estimate the amount of labor and overhead costs related to finished goods inventories. These estimates are revised based on actual results at year end. As of August 2, 2008, finished goods represented approximately 28.0% of the gross carrying value of our total gross inventory. We believe the estimation methodologies used are appropriate and are consistently applied.
GOODWILL
The Company’s goodwill is subject to, at a minimum, an annual fourth fiscal quarter impairment assessment of its carrying value. Goodwill impairment is deemed to exist if the net book value of a reporting unit exceeds its estimated fair value. Estimated fair values of the reporting units are estimated using an earnings model and a discounted cash flow valuation model. The discounted cash flow model incorporates the Company’s estimates of future cash flows, future growth rates and management’s judgment regarding the applicable discount rates used to discount those estimated cash flows.
Due primarily to continued operating results below planned levels and management’s resulting revaluation of its strategic plan for the Company’s domestic Medical Systems Group’s reporting unit, the Company completed a special assessment of that reporting unit’s goodwill realization in the third quarter of fiscal 2008. As part of its assessment, the Company estimated the fair value of the domestic reporting unit based on internal cash flows expected to be earned by the business and an appropriate risk-adjusted discount rate. While such estimates are subject to significant uncertainties and actual results could be materially different, the analysis resulted, pursuant to the implementation guidance of FASB No. 142, Accounting for Goodwill and Intangible Assets, in a complete impairment of the unit’s goodwill balance. Accordingly, the Company recorded a $1.9 million impairment charge during the third quarter of fiscal 2008.
CONSOLIDATED RESULTS OF OPERATIONS
FISCAL 2008 COMPARED TO FISCAL 2007
Consolidated net sales of $108.3 million for fiscal year 2008 increased by $4.1 million, or 4.0%, from fiscal 2007 net sales of $104.2 million, due primarily to increased sales in our Medical Systems Group. The Medical Systems Group’s sales for fiscal 2008 of $95.1 million increased $4.1 million, or 4.5 %, from the prior fiscal year. Net sales increases were primarily driven by increased international sales volume of several medical system product lines, particularly the Apollo line, offset by reduced sales of the domestic digital product line. The Power Conversion Group’s sales for fiscal 2008 of $13.3 million were consistent with sales in the prior fiscal year.
Consolidated backlog at August 2, 2008 was $22.7 million versus backlog at July 28, 2007 of approximately $28.4 million. The backlog in the Power Conversion Group of $5.4 million decreased $1.2 million from levels at the beginning of the fiscal year while there was a $4.5 million decrease in the fiscal year end backlog of our Medical Systems Segment from July 28, 2007 reflecting weaker booking during the twelve month period in international markets. Substantially all of the backlog should result in shipments within the next 12 to 15 months.
Gross margins as a percent of sales were 24.7% in fiscal 2008, compared to 24.0% in fiscal 2007. The Power Conversion Group margins were 37.9 % in fiscal 2008 as compared to 37.3% in fiscal 2007 reflecting increased margins in product mix and decreased production cost. For the Medical Systems Group, fiscal 2008 gross margins of 22.9% were higher than gross margins of 22.1% in the prior year due primarily to increased sales in the Del Medical legacy product lines which have lower selling prices but greater gross margins.
Research and Development expenses for fiscal 2008 were $2.5 million (2.3% of sales) compared to $2.0 million (1.9% of sales) in fiscal 2007 due primarily to higher international product development efforts in fiscal 2008.
Selling, General and Administrative expenses (“SG&A”) for fiscal 2008 were $15.6 million (14.4% of sales) compared to $14.6 million (14.0% of sales) in fiscal 2007. The increase is primarily due to higher stock based compensation expenses related to increased volume of stock options vesting during fiscal 2008, legal expenses related to the Moeller case discussed elsewhere in this document and increased expenses related to investigating potential business acquisitions.
As discussed above, during the third quarter of fiscal 2008, the Company recognized a goodwill impairment loss of $1.9 million on the carrying value of the goodwill of the Medical Systems Group’s U.S. medical business. No impairment was recorded in fiscal 2007.
As a result of the above, we recognized fiscal 2008 operating income of $6.4 million compared to operating income of $8.4 million in fiscal 2007. The Medical Systems Group had an operating profit of $5.0 million in fiscal 2008 and the Power Conversion Group achieved an operating profit of $2.5 million, offset by unallocated corporate costs of $1.1 million. The Medical Systems Group had an operating profit of $7.5 million in fiscal 2007 and the Power Conversion Group achieved an operating profit of $2.4 million, offset by unallocated corporate costs of $1.5 million.
Interest expense, net of $0.3 million for fiscal 2008, was $0.7 million lower than the prior year due to a reduction in borrowings resulting from the paydown of U.S. based debt with the proceeds of our March 2007 Right Offering, partially offset by additional borrowings in Italy to support it’s day to day operations.
On a consolidated basis, the Company recorded fiscal 2008, pretax income of $6.2 million, comprised of foreign pretax income of $8.3 million offset by a U.S. pretax loss of $2.1 million. During fiscal 2007, the Company recorded pretax income of $7.4 million which included foreign pretax income of $8.2 million, offset by a U.S. pretax loss of $0.8 million. The related fiscal 2008 and 2007 income tax expense of $3.2 million and $3.6 million, respectively, was primarily due to foreign taxes on the profits of Villa. The Company has not provided for any income tax benefits related to the U.S. pretax losses in either fiscal 2008 or fiscal 2007 due to uncertainty regarding the realizability of its U.S. net operating loss carry forwards as explained in Critical Accounting Policies above.
Reflecting the above, we recorded net income of $3.0 million, or $0.12 per basic share and diluted share, in fiscal 2008, as compared to a net income of $3.8 million, or $0.24 per basic share and $0.23 per diluted share, in fiscal 2007.
FISCAL 2007 COMPARED TO FISCAL 2006
Consolidated net sales of $104.2 million for fiscal year 2007 increased by $21.2 million, or 25.5%, from fiscal 2006 net sales of $83.0 million, with increases at both the Power Conversion Group and Medical Systems Group. The Medical Systems Group’s sales for fiscal 2007 of $91.0 million increased $20.7 million, or 29.5 %, from the prior fiscal year, with increases primarily due to increased international sales as well as stronger than expected dental sales and increased sales of higher priced digital products. The Power Conversion Group’s sales for fiscal 2007 of $13.2 million increased by $.5 million, or 3.6%, from the prior year’s levels, reflecting increased sales in the transformer business.
Consolidated backlog at July 28, 2007 was $28.4 million versus backlog at July 29, 2006 of approximately $22.4 million. The backlog in the Power Conversion Group of $16.6 million increased $0.3 million from levels at the beginning of the fiscal year while there was a $5.7 million increase in the fiscal year end backlog of our Medical Systems Segment from July 29, 2006 reflecting strong booking during the twelve month period in international markets. Substantially all of the backlog should result in shipments within the next 12 to 15 months.
Gross margins as a percent of sales were 24.0% in fiscal 2007, compared to 23.3% in fiscal 2006. The Power Conversion Group margins were 37.3 % in fiscal 2007 as compared to 35.5% in fiscal 2006 reflecting increased margins in product mix and decreased production cost. For the Medical Systems Group, fiscal 2007 gross margins of 22.1% were higher than gross margins of 21.1% in the prior year due primarily to the reversal of warranty reserves as the warranty period on several specific items expired. This impact was partially offset by lower margins associated with increased sales of digital products. Generally digital product have a higher selling price, as well as a higher cost than the non- digital product offerings, resulting in lower gross margin percentage
Research and Development expenses for fiscal 2007 were $2.0 million (1.9% of sales) compared to $1.6 million (1.9% of sales) in fiscal 2006 due primarily to higher international product development efforts in fiscal 2007.
Selling, General and Administrative expenses (“SG&A”) for fiscal 2007 were $14.6 million (14.0% of sales) compared to $13.6 million (16.4% of sales) in fiscal 2006. The increase is primarily due to increased corporate legal and bad debt expense offset by reduced selling cost in the Power Conversion Group.
Litigation settlement costs of $0.7 million recorded in fiscal 2006 include the accrual of $0.6 million based on a November 2005 settlement of litigation filed during fiscal 2005 by the potential buyers of the Company’s Medical Systems Group. The Company previously disclosed this litigation but had not recorded any affiliated expense during fiscal 2005, as it had no basis at that time upon which to estimate either the outcome or amount of loss. The fiscal 2006 cost also includes the settlement of two employment actions at our foreign subsidiary totaling $0.2 million net of the reversal of a $0.1 million accrual related to a previously settled litigation. No additional litigation settlement costs were incurred during 2007.
As a result of the above, we recognized fiscal 2007 operating income of $8.4 million compared to operating income of $3.5 million in fiscal 2006. The Medical Systems Group had an operating profit of $7.5 million in fiscal 2007 and the Power Conversion Group achieved an operating profit of $2.4 million, offset by unallocated corporate costs of $1.5 million. We recognized fiscal 2006 operating income of $3.5 million. The Medical Systems Group had an operating profit of $3.6 million in fiscal 2006 and the Power Conversion Group achieved an operating profit of $2.4 million, offset by unallocated corporate costs of $2.5 million.
Interest expense of $1.0 million for fiscal 2007 was $0.3 million lower than the prior year due to a reduction in borrowings resulting from the paydown of U.S. based debt with the proceeds of our March 2007 Right Offering partially offset by additional borrowings in Italy to support it’s day to day operations.
On a consolidated basis, the Company recorded fiscal 2007 pretax income of $7.4 million, comprised of foreign pretax income of $8.2 million offset by a U.S. pre-tax loss of $0.8 million. The related fiscal 2007 income tax expense of $3.6 million was due to foreign taxes on the profits of Villa. During fiscal 2006, the Company recorded pretax income of $2.1 million which included foreign pretax income of $3.7 million, offset by a U.S. pretax loss of $1.6 million. The Company has not provided for any income tax benefits related to the U.S. pretax losses in either fiscal 2007 or fiscal 2006 due to uncertainty regarding the realizability of its U.S. net operating loss carry forwards as explained in Critical Accounting Policies above.
Fiscal 2006 Discontinued Operations reflects the accrual of an estimated liability of $0.2 million related to a New York State Sales tax audit of the Company’s prior DHV business sold in October 2004.
Reflecting the above, we recorded net income of $3.8 million, or $0.24 per basic share and $0.23 per diluted share, in fiscal 2007, as compared to a net income of $0.1 million, or $0.01 per basic and diluted share, in fiscal 2006.
LIQUIDITY AND CAPITAL RESOURCES
We fund our investing and working capital needs through a combination of cash flow from operations and short-term credit facilities and the proceeds from our Right Offering discussed below.
Working Capital -- At August 2, 2008 and July 28, 2007, our working capital was approximately $31.2 million and $25.0 million, respectively. The increase in ending working capital for fiscal 2008 as compared to fiscal 2007 related primarily to increases in accounts receivable (due to higher sales) and decreases in trade payables offset partially by a decrease in ending inventories.
At August 2, 2008 and July 28, 2007, we had approximately $7.9 million in cash and cash equivalents. As of August 2, 2008, we had approximately $9.0 million of excess borrowing availability under our domestic revolving credit facility prior to consideration of $2.6 million of letters of credit issued thereunder.
In addition, as of August 2, 2008 and July 28, 2007, our Villa subsidiary had an aggregate of approximately $13.5 million and $11.0 million, respectively, of excess borrowing availability under its various short-term credit facilities. Terms of the Italian credit facilities do not permit the use of borrowing availability to directly finance operating activities at our U.S. subsidiaries.
FISCAL 2008 COMPARED TO FISCAL 2007
Cash Flows from Operating Activities -- For the year ended August 2, 2008, the Company generated approximately $1.7 million of cash for continuing operations, compared to $4.0 million in the prior fiscal year. The decrease is largely due to payment of accounts payable and income taxes payable in the 2008 period offset by the effect of reduced inventory levels.
Cash Flows from Investing Activities -- We made approximately $1.2 million in facility improvements and capital equipment expenditures for the fiscal year ended August 2, 2008. We made approximately $0.8 million in facility improvements and capital equipment expenditures for the comparable prior fiscal year period.
Cash Flows from Financing Activities – During the year ended August 2, 2008, we used $1.0 million in net cash from financing activities versus generating $4.2 million for the fiscal year ended July 28, 2007. This decrease is due to the impact of the net proceeds of our Rights Offering completed in March of 2007 in the prior fiscal year. The Rights Offering, discussed below, generated total proceeds to the Company, net of related expenses of $12.4 million. Approximately $7.6 million of the proceeds were used for debt repayment and the remainder invested in short-term money market securities. The Company also received $0.1 million in payment of the exercise price of stock options and warrants in the fiscal year ended August 2, 2008, $0.6 million less than the prior fiscal year. The Company made a total of $1.2 million in payments of long term debt during fiscal 2008 compared to payments of $5.9 million in the previous fiscal year.
FISCAL 2007 COMPARED TO FISCAL 2006
Cash Flows from Operating Activities -- For the year ended July 28, 2007, the Company generated approximately $4.0 million of cash for continuing operations, compared to $0.3 million in the prior fiscal year due primarily to an increase in net income and a larger increase in accounts and income taxes payable partially offset by a larger increase in inventories.
Cash Flows from Investing Activities -- We made approximately $0.8 million in facility improvements and capital equipment expenditures for the fiscal year ended July 28, 2007 and for the comparable prior fiscal year period. We also used approximately $2.6 million to fund the cash portion of the purchase of the minority interest in our Italian subsidiary in fiscal 2006.
Cash Flows from Financing Activities – During the year ended July 28, 2007, we generated $4.2 million in net cash from financing activities versus $2.1 million for the fiscal year ended July 29, 2006 due primarily to the net proceeds of our Rights Offering completed in March of 2007. The Rights Offering, discussed below, generated total proceeds to the Company, net of related expenses of $12.4 million. Approximately $7.6 million of the proceeds were used for debt repayment and the remainder invested in short-term money market securities. In addition, the Company received $0.7 million in payment of the exercise price of stock options and warrants. During the year ended July 29, 2006, the Company refinanced its domestic credit agreement and borrowed $2.0 million under a term loan. In addition, the Company borrowed a net $0.8 million under revolving credit facilities and received $0.2 million in payment of the exercise price of stock options. The Company made a total of $1.0 million in payments of long term debt during fiscal 2006.
The following table summarizes our contractual obligations, including debt and operating leases at August 2, 2008 (in thousands):
OBLIGATIONS | | | | | WITHIN 1 YEAR | | | 2-3 YEARS | | | 4-5 YEARS | | | AFTER 5 YEARS | |
Long-term debt obligations (1) | | $ | 3,662 | | | $ | 1,312 | | | $ | 2,229 | | | $ | 121 | | | $ | - | |
| Capital lease obligations | | | 2,639 | | | | 648 | | | | 1,991 | | | | - | | | | - | |
| Interest | | | 779 | | | | 292 | | | | 415 | | | | 72 | | | | - | |
| Operating lease obligations | | | 1,062 | | | | 605 | | | | 457 | | | | - | | | | - | |
| Total contractual cash obligations | | $ | 8,142 | | | $ | 2,857 | | | $ | 5,092 | | | $ | 193 | | | $ | - | |
| | (1) | As of August 2, 2008, we did not have any outstanding borrowings under our revolving credit facilities in the U.S. or in Italy. |
CREDIT FACILITY AND BORROWING–
On August 1, 2005, the Company entered into a three-year revolving credit and term loan facility with North Fork Business Capital (the "North Fork Facility") and repaid the prior facility. On June 1, 2007, the North Fork Facility was amended and restated, effective as of May 25, 2007. As restated, the North Fork Facility provides for a $7.5 million formula based revolving credit facility based on the Company’s eligible accounts receivable and inventory as defined in the credit agreement and a capital expenditure loan facility of up to $1.5 million, resulting in a total credit facility of $9.0 million.
Loans under the three-year North Fork Facility bear interest based on either LIBOR or the "base rate" which is defined as the higher of (i) the highest prime, base or equivalent rate of interest publicly announced from time to time by North Fork Bank, Citibank, N.A. or Bank of America or any successor to either of the foregoing banks (which may not be the lowest rate of interest charged by such bank) and (ii) the published annualized rate for ninety-day dealer commercial paper that appears in the "Money Rates" section of THE WALL STREET JOURNAL. Loans bear interest at the rate of either 0.50% plus the base rate or LIBOR plus 2.5%. The LIBOR rate option can cover up to 80% of the outstanding loans. Interest is payable monthly in arrears on the first business day of each month.
Other changes to the terms and conditions of the original loan agreement include the modification of covenants, removal of the Villa stock as loan collateral and the removal of daily collateral reporting which was part of the previous asset-based facility requirements. As of August 2, 2008 and July 28, 2007, no amounts were outstanding and the Company had approximately $6.4 million of availability under the North Fork Facility, as North Fork has reserved $2.6 million against possible litigation settlements in the form of letters of credit.
The North Fork Facility is subject to commitment fees of 0.5% per annum on the daily-unused portion of the facility, payable monthly. The Company granted a security interest to the lender on its U.S. credit facility in substantially all of its accounts receivable, inventory, property, plant and equipment, other assets and intellectual property in the U.S..
As of the end of the fourth quarter of fiscal 2006, the Company was non-compliant with the following covenants: the Adjusted U.S. Earnings, Adjusted Earnings, Senior U.S. Debt Ratio and Fixed Charge Coverage Ratio covenants under the North Fork Facility, due to the lower than anticipated performance during fiscal 2006. On October 25, 2006, the Company and North Fork signed an amendment to the facility that waived the non-compliance with these covenants for the fourth quarter of fiscal 2006 and adjusted the covenant levels going forward through the maturity of the credit facility. In addition, the amendment reversed $0.3 million of a sinking fund reserved for the March 2007 maturity of the subordinated shareholder notes and eliminated additional sinking fund reserves provisions related to the subordinated notes.
As of the end of the first quarter of fiscal 2007, the Company was non-compliant with the tangible net worth covenant under the North Fork Facility. On December 6, 2006, North Fork waived the non-compliance with this covenant for the first quarter of fiscal 2007 and adjusted the covenant levels going forward through the maturity of the credit facility. As of August 2, 2008 and July 28, 2007, the Company was in compliance with all covenants under the North Fork Facility.
The Company received a dividend from its Villa subsidiary in October 2006 of approximately $1.6 million which was used to pay down amounts outstanding under the North Fork Facility, in accordance with provisions of the facility.
Our Villa subsidiary maintains short term credit facilities which are renewed annually with Italian banks. Currently, these facilities are not being utilized and the balance due at August 2, 2008 is $0. Interest rates on these facilities are variable and currently range from 3.7% – 13.75%.
In October 2006, Villa entered into a 1.0 million Euro loan for financing of R&D projects, with an option for an additional 1 million Euro upon completion of 50% of the projects. In April, 2008, the Company declined the option for additional financing and demonstrated successful completion of the project triggering a more favorable interest rate. Interest, previously payable at Euribor 3 months plus 1.3 points, was reduced in the fourth fiscal quarter of 2008 to Euribor plus 1.04 points, currently at 5.998%. The note is repayable over a 7 year term, with reimbursement starting in September 2008. The note contains a financial covenant which provides that the net equity of Villa cannot fall below 5.0 million Euros. This covenant could limit Villa’s ability to pay dividends to the U.S. parent company in the event future losses, future dividends or other events should cause Villa’s equity to fall below the defined level.
In December 2006, Villa entered into a 1.0 million Euro loan with interest payable at Euribor 3 months plus 0.95 points, currently 5.897%. The loan is repayable in 4 years.
Villa was party to a 1.6 million Euro loan which was extinguished in March 2007. Two final installments for a total of 0.3 million Euro were repaid in fiscal year 2007.
Villa is also party to two Italian government long-term loans with a fixed interest rate of 3.425% with principal payable annually through maturity in February and September 2010. At the end of fiscal year 2008, total principal due is 0.6 million Euro. Villa manufacturing facility is subject to a capital lease obligation which matures in 2011 with an option to purchase .. Villa is in compliance with all related financial covenants under these short and long-term financings.
In connection with the settlement reached on January 29, 2002 with the plaintiffs in the class action litigation, the Company recorded the present value at 12% of the $2 million of subordinated notes that were issued in April 2002 and matured in March 2007. The subordinated notes did not pay interest currently, but accrued interest at 6% per annum, and were recorded at issuance at a discounted present value of $1.5 million. The balance was paid on March 29, 2007 with a portion of the proceeds from a Rights Offering described below.
EQUITY MATTERS
On December 12, 2006, the Company filed a registration statement for a subscription Rights Offering with the SEC that became effective January 30, 2007. Under the terms of this Rights Offering, the Company distributed to shareholders of record as of February 5, 2007, non-transferable subscription rights to purchase one share of the Company's common stock for each share owned at that date at a subscription price of $1.05 per share. On March 12, 2007, the Company completed the Rights Offering, selling 12,027,378 shares of its common stock at $1.05 per share. Total proceeds to the Company, net of expenses related to the Rights Offering, were $12.4 million.
The purpose of this Rights Offering was to raise equity capital in a cost-effective manner. Approximately $7.6 million of the proceeds were used for debt repayment and the remainder invested in short-term money market securities for anticipated working capital needs and general corporate purposes. A portion of the net proceeds may also ultimately be used to acquire or invest in businesses, products and technologies that Company management believes are complementary to the Company's business.
In addition, on January 22, 2007, the Company entered into a stockholders rights plan (the "Rights Plan"). The Rights Plan provides for a dividend distribution of one Common Stock purchase right for each outstanding share of the Company's Common Stock. The Company's Board of Directors adopted the Rights Plan to protect stockholder value by protecting the Company's ability to realize the benefits of its net operating losses ("NOLs") and capital loss carryforwards. The Company has experienced substantial operating and capital losses in previous years. Under the Internal Revenue Code and rules promulgated by the IRS, the Company may "carry forward" these losses in certain circumstances to offset current and future earnings and thus reduce its federal income tax liability, subject to certain requirements and restrictions. Assuming that the Company has future earnings, the Company may be able to realize the benefits of NOLs and capital loss carryforwards. These NOLs and capital loss carryforwards constitute a substantial asset to the Company. If the Company experiences an "Ownership Change," as defined in Section 382 of the Internal Revenue Code, its ability to use the NOLs and capital loss carryforwards could be substantially limited or lost altogether. In general terms, the Rights Plan imposes a significant penalty upon any person or group that acquires certain percentages of the Company's common stock by allowing other shareholders to acquire equity securities at half their fair values.
LITIGATION MATTERS
The Company had an employment agreement with Samuel Park, a previous CEO, for the period May 1, 2001 to April 30, 2004. The employment agreement provided for certain payments in the event of a change in the control of the Company. On October 10, 2003, the Company announced the appointment of Walter F. Schneider as President and CEO to replace Mr. Park, effective as of such date. As a result, the Company recorded a charge of $0.2 million during the first quarter of fiscal 2004 to accrue the balance remaining under Mr. Park’s employment agreement.
The Company’s employment agreement with Mr. Park provided for payments upon certain changes in control. The Company’s Board of Directors, elected at the Company’s Annual Meeting of Shareholders held on May 29, 2003, had reviewed the “change of control” provisions regarding payments totaling up to approximately $1.8 million under the employment agreement between the Company and Mr. Park. As a result of this review and based upon, among other things, the advice of special counsel, the Company’s Board of Directors has determined that no obligation to pay these amounts has been triggered. Prior to his departure from the Company on October 10, 2003, Mr. Park orally informed the Company that, after reviewing the matter with his counsel, he believed that the obligation to pay these amounts has been triggered. On October 27, 2003, the Company received a letter from Mr. Park’s counsel demanding payment of certain sums and other consideration pursuant to the Company’s employment agreement with Mr. Park, including these change of control payments. On November 17, 2003, the Company filed a complaint in the United States District Court, Southern District of New York, against Mr. Park seeking a declaratory judgment that no change in control payment was or is due to Mr. Park, and that an amendment to the employment contract with Mr. Park regarding advancement and reimbursement of legal fees is invalid and unenforceable. Mr. Park answered the complaint and asserted counterclaims seeking payment from the Company based on his position that a “change in control” occurred in June 2003. Mr. Park is also seeking other consideration he believes he is owed under his employment agreement. The Company filed a reply to Mr. Park’s counterclaims denying that he is entitled to any of these payments. Discovery in this matter was conducted and completed. Following discovery, the Company and Mr. Park filed motions for summary judgment on the issues related to the change in control and the amendment to the employment agreement, which motions have been fully submitted to the court for consideration. To date, no decision has been issued by the court on these motions. If Mr. Park prevails on his claims and the payments he seeks are required to be paid in a lump sum, these payments may have a material adverse effect on the Company’s liquidity. It is not possible to predict the outcome of these claims. However, the Company’s Board of Directors does not believe that such a claim is reasonably likely to result in a material decrease in the Company’s liquidity in the foreseeable future. The Company has not recorded an accrual for any potential settlements of this claim as it has no basis upon which to estimate either the outcome or amount of loss, if any.
On June 28, 2002, Jeffrey N. Moeller, the former Director of Quality Assurance and Regulatory Affairs of Del Medical, commenced an action in the Circuit Court of Cook County, Illinois, against the Company, Del Medical and Walter Schneider, the former President of Del Medical. In the most current iteration of his complaint, the third amended complaint, Mr. Moeller alleged four claims against the defendants in the action: for (1) retaliatory discharge from employment with Del Medical, allegedly in response to Mr. Moeller’s complaints to officers of Del Medical about purported prebilling and his stopping shipment of a product that allegedly did not meet regulatory standards, (2) defamation, (3) intentional interference with his employment relationship with Del Medical and prospective employers, and (4) to hold the Company liable for any misconduct of Del Medical under a theory of piercing the corporate veil. In their answer to the third amended complaint, the defendants denied the substantive allegations of each of these claims and denied that they have any liability to Mr. Moeller. By order dated September 15, 2006, the Court denied in part and granted in part defendants’ motion requesting summary judgment dismissing the third amended complaint. The court granted the motion only to the extent of dismissing that part of Mr. Moeller’s claim of interference with his employment relationship with Del Medical and his relationship with prospective employers.
In fiscal 2007, the Company recorded an accrual of $0.1 million relating to potential liability in the settlement of these claims. The parties appeared for mediation in January 2007 but the mediation did not result in a disposition of the action. A trial was held in April 2008 and on April 17, 2008, the jury returned a verdict in favor of Mr. Moeller for $1.8 million for lost earnings, back pay, front pay and benefits on the retaliatory discharge claim, and $0.2 million for emotional distress/reputation damages and $0.2 million in punitive damages on the defamation claim. On May 19, 2008, counsel for the defendants filed their motion for judgment in their favor notwithstanding the jury verdict, or, alternatively, for a new trial, on those claims on which the jury found the respective defendants liable. By order dated August 15, 2008, the Court denied that motion.
On August 25, 2008, the Company and the other defendants filed their notice of appeal by which they appeal to the Appellate Court of Illinois, First District, among other things, the judgment entered against the Company and the other defendants on the jury verdict. On September 12, 2008, the Company and the other defendants filed an amended notice of appeal. The Company intends to pursue the appeal vigorously, seeking thereby reversal of the judgment and to have a judgment entered in favor of the Company and the other defendants or to have a new trial. In lieu of a bond, the Company filed an irrevocable standby letter of credit in the amount of $2.6 million by which Mr. Moeller can collect the amount of the judgment entered by the trial court in the event the appellate court affirms that judgment.
On April 28, 2008, Apergis, the former General Manager of RFI, filed a charge with the EEOC alleging that RFI discriminated against him by terminating his employment with RFI on December 18, 2007. Apergis alleged three claims against RFI: (1) violation of Title VII of the Civil Rights Act; (2) violation of the Age Discrimination in Employment Act and (3) retaliation. RFI responded to the EEOC charge with a position statement filed with the EEOC on June 26, 2008 denying each allegation of the charge. As of September 16, 2008, RFI is waiting to hear for a response to their position statement from the EEOC. RFI intends to defend vigorously against Apergis.
Diamond v. Allied Diagnostic Imaging Resources, Inc., et al. (Superior Court, County of Los Angeles; Case No. BC362544). This action is brought against numerous defendants, including the Company. While the action commenced in late 2006, plaintiff first served the summons and complaint on the Company in December 2007. The plaintiff alleges that she is the wife of a chiropractor who died in June 2005. Plaintiff alleges that her husband was exposed to chemical products in developing x-ray films and cleaning film processing machines and worked with the x-ray film processing machines and x-ray machines, and that the defendants manufactured, supplied or serviced the chemical products and machines. The complaint further alleges that the decedent was exposed to toxic chemicals and radiation from the chemical products used on or in the machines, which caused “serious injuries to his internal organs, including acute myelogenous leukemia”, resulting in his death.
The complaint alleges the following six claims against the defendants: (1) negligence in manufacturing. and distributing the chemical products and machines, and servicing the machines, and failing adequately to warn decedent of the hazards of the chemical products and machines, (2) violation of a California statute and regulation by failing to determine whether the chemical products caused health hazards and failing to label or identify in material safety data sheets a health hazard relating to acute myelogenous leukemia, (3) strict products liability for failing to warn adequately of the chemical products’ and machines’ health hazards, (4) strict products liability for defects in the design of the chemical products and machines, (5) fraudulent concealment of the toxic and carcinogenic nature of the chemical products and the machines, and (6) breach of implied warranties as to the fitness of the chemical products and machines for intended uses, merchantability, and lack of defects.
While the complaint does not allege a total amount of damages sought, plaintiff alleges that she has suffered damages consisting of medical, funeral, and burial expenses, the decedent’s lost earnings prior to and lost wages after his death, lost benefits after his death, the value of his services in managing his family’s home, and loss of companionship and similar losses. The plaintiff also requests punitive damages. The Company has not recorded an accrual for any potential settlement of this claim as it has no basis upon which to estimate either the outcome or amount of loss, if any. In its answer to the complaint, the Company denied the substantive allegations of the complaint and denied that it has any liability to plaintiff. The Company intends to defend vigorously against plaintiff’s claims.
On May 24, 2007, RFI was served with a subpoena to testify before a grand jury of the United States District Court, Eastern District New York and to provide items and records from its Bay Shore NY offices in connection with U.S. Department of Defense contracts. A search warrant from the United States District Court, Eastern District of New York was issued and executed with respect to such offices. The Company believes that it is in full compliance with the quality standards that its customers require and is fully cooperating with investigators to assist them with their review. RFI continues to ship products to the U.S. Government as well as to its commercial customers.
The Company is a defendant in several other legal actions in various U.S. and foreign jurisdictions arising from the normal course of business. Management believes the Company has meritorious defenses to such actions and that the outcome will not be material to the Company’s consolidated financial statements.
The Company did not have any investments in unconsolidated variable interest entities or other off balance sheet arrangements during any of the periods presented in this Annual Report on Form 10K.
The Company anticipates that cash generated from the Rights Offering noted above, operations and amounts available from credit facilities will be sufficient to satisfy currently projected operating cash needs for at least the next twelve months.
EFFECTS OF NEW ACCOUNTING PRONOUNCEMENTS
In March 2008, the Financial Accounting Standards Board (the “FASB”) issued Statement of Financial Accounting Standards (“SFAS”) No. 161, Disclosures about Derivative Instruments and Hedging Activities, an amendment of SFAS No. 133. The Statement requires enhanced disclosures about an entity’s derivative and hedging activities. The Statement is effective for fiscal years and interim periods beginning after November 15, 2008. The Company is currently evaluating the requirements of SFAS 161, but does not expect it to have a material impact.
In December 2007, the FASB issued SFAS No. 160, Noncontrolling Interests in Consolidated Financial Statements--an amendment of ARB No. 51 (“SFAS 160”). SFAS 160 requires identification and presentation of ownership interests in subsidiaries held by parties other than the Company in the consolidated financial statements within the equity section but separate from the equity owned by Del Global. SFAS 160 also requires that (1) the amount of consolidated net income attributable to the parent and to the noncontrolling interest be clearly identified and presented on the face of the consolidated statement of operations, (2) changes in ownership interest be accounted for similarly, as equity transactions and (3) when a subsidiary is deconsolidated, any retained noncontrolling equity investment in the former subsidiary and the gain or loss on the deconsolidation of the subsidiary be measured at fair value. This statement is effective for the Company on August 2, 2009. The Company is currently evaluating the requirements of SFAS 160 but does not expect it to have a material impact.
In December 2007, the FASB issued SFAS No. 141R, Business Combinations (“SFAS 141R”). SFAS 141R states that acquisition-related costs are to be recognized separately from the acquisition and expensed as incurred with restructuring costs being expensed in periods after the acquisition date. SFAS 141R also states that business combinations will result in all assets and liabilities of the acquired business being recorded at their fair values. The Company is required to adopt SFAS No. 141R effective August 2, 2009. The impact of the adoption of SFAS No. 141R will depend on the nature and extent of business combinations occurring on or after the effective date.
In September 2006, the FASB issued SFAS No 157, “Fair Value Measurements,” (“SFAS 157”) which defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS No. 157 does not require any new fair value measurements, but provides guidance on how to measure fair value by providing a fair value hierarchy used to classify the source of the information. Portions of this statement are effective for the Company for fiscal years beginning after November 15, 2007, while others have been deferred until fiscal 2009. The Company has not evaluated the impact that the adoption of SFAS No. 157 will have on its financial statements at this time but does not expect it to have a material impact.
In February 2007, the FASB released SFAS No. 159, “Fair Value Option for Financial Assets and Financial Liabilities” (“SFAS 159”). This statement permits entities to choose to measure many financial instruments and certain other items at fair value. This statement is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those years. The Company has not evaluated the impact that the adoption of SFAS No. 159 will have on its financial statements at this time but does not expect it to have a material impact.
In June 2006, the FASB issued Interpretation No. 48 (“FIN 48”), “Accounting for Uncertainty in Income Taxes”— an interpretation of SFAS No. 109. FIN 48 requires that the Company recognize the financial statement effects of a tax position when it is more likely than not, based on the technical merits, that the position will be sustained upon examination. As used in this Interpretation, the term “more likely than not” means a likelihood of more than 50 percent. The terms “examined” and “upon examination” also include resolution of the related appeals or litigation processes, if any. The determination of whether or not a tax position has met the more-likely-than-not recognition threshold is to be determined based on the facts, circumstances, and information available at the reporting date. FIN 48 was adopted by the Company beginning July 29, 2007. The adoption of FIN 48 did not have any impact on the Company’s statement of financial position or on its results of operations.
| QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We do not ordinarily hold market risk sensitive instruments for trading purposes. We do, however, recognize market risk from interest rate and foreign currency exchange exposure.
INTEREST RATE RISK
Our U.S. revolving credit and Italian subsidiary’s long-term debt incur interest charges that fluctuate with changes in market interest rates. See Note 7 of Notes to Consolidated Financial Statements included in Part II, Item 8 of this Annual Report. Based on the balances as of August 2, 2008, an increase of 1/2 of 1% in interest rates would increase interest expense by approximately $32,000 annually. There is no assurance that interest rates will increase or decrease over the next fiscal year. Because we believe this risk is not material, we do not undertake any specific steps to reduce or eliminate this risk.
FOREIGN CURRENCY RISK
The financial statements of Villa are denominated in Euros. Based on our historical results and expected future results, Villa accounts for approximately 57% to 62% of our total revenues, based in part on the rate at which Villa’s Euro denominated financial statements have been or will be converted into U.S. dollars. In addition, over the last three years, Villa has contributed positive operating income, as compared to U.S. consolidated operating losses. Having a portion of our future income denominated in Euros exposes us to market risk with respect to fluctuations in the U.S. dollar value of future Euro earnings. A 5% decline in the value of the Euro in fiscal 2008, for example, would have reduced sales by approximately $3.4 million, and would have decreased our consolidated income from continuing operations by approximately $0.4 million (due to the reduction in the U.S. dollar value of Villa’s operating income.)
| FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The consolidated financial statements of the Company, including the notes to all such statements and other supplementary data are included in this report beginning on page F-1.
| CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
NONE
EVALUATION OF DISCLOSURE CONTROLS AND PROCEDURES
The Company, under the supervision and with the participation of the Company's management, including James A. Risher, Chief Executive Officer, and Mark A. Zorko, Chief Financial Officer, has evaluated the effectiveness of the design and operation of the Company's "disclosure controls and procedures", as such term is defined in Rules 13a-15e and 15d-15e promulgated under the Exchange Act, as of the end of the period covered by this Annual Report. Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that the Company's disclosure controls and procedures were effective as of the end of the period covered by this Annual Report to provide reasonable assurance that information required to be disclosed by the Company in reports that it files or submits under the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms.
A control system, no matter how well conceived and operated, can provide only reasonable, not absolute assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
REPORT OF MANAGEMENT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management of the Company is responsible for establishing and maintaining adequate “internal control over financial reporting”, as such term is defined in Rule 13a-15(f) of the Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s system of internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Management performed an assessment of the effectiveness of the Company’s internal control over financial reporting as of August 2, 2008 based upon criteria in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on our assessment, management determined that the Company’s internal control over financial reporting was effective as of August 2, 2008 based on the criteria in Internal Control—Integrated Framework issued by the COSO.
In the ordinary course of business, the Company routinely enhances its information systems by either upgrading its current systems or implementing new systems. There were no changes in the Company's internal controls or in other factors that could significantly affect these controls, during the Company's fourth quarter ended August 2, 2008, that have materially affected, or are reasonably likely to materially affect, the Company's internal control over financial reporting.
This Annual Report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit the Company to provide only management’s report in this Annual Report.
Not applicable.
| DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The names and ages of each director and executive officers of the Company, each of their principal occupations at present and for the past five (5) years and certain other information about each, are set forth below:
| | All Offices with the Company | |
| Gerald M. Czarnecki | 68 | Director | 2003 |
| | | | |
| James R. Henderson | 50 | Chairman of the Board and Director | 2003 |
| | | | |
| General Merrill A. McPeak | 72 | Director | 2005 |
| | | | |
| James A. Risher | 66 | Director, President and Chief Executive Officer | 2005 |
| | | | |
| | | | Officer Since |
| Mark A. Zorko | 56 | Chief Financial Officer | 2006 |
Gerald M. Czarnecki has been a member of the Company’s Board of Directors since June 3, 2003. He has served as the Chairman of The Deltennium Corporation, a privately held holding company (“Deltennium”), since November 1995. Mr. Czarnecki is also currently serving as President & CEO of Junior Achievement Worldwide, Inc. and is Managing Director of O2Media Inc. Mr. Czarnecki had a broad career as a corporate executive including serving as Chairman & CEO of Honfed Bank, a multi-billion dollar bank; President of UNC Inc., a manufacturing and services company in the aviation industry; and Senior Vice President of Human Resources and Administration of IBM, the world’s largest computer company. Mr. Czarnecki is a frequent speaker and seminar leader on a broad range of corporate governance issues and serves on a number of corporate boards. He has served as a member of the Board of Directors and Chairman of the Audit Committee of State Farm Insurance Companies since 1998; He is Chairman of the Board of Directors of the National Association of Corporate Directors, Florida Chapter and is Chairman of The National Leadership Institute, a non-profit organization committed to improving non-profit Leadership and Corporate Governance. Mr. Czarnecki has a B.S. in Economics from Temple University and an M.A. in Economics from Michigan State University and is a CPA.
James R. Henderson has been a member of the Company’s Board of Directors since November 20, 2003. Mr. Henderson is a Managing Director and operating partner of Steel Partners LLC (“Partners LLC”), a global investment management firm, which is the Investment Manager to Steel Partners II, L.P. (“Steel Partners”). He has been associated with Partners LLC and its affiliates since August 1999. Mr. Henderson has been the Executive Vice President of SP Acquisition Holdings, Inc., a company formed for the purpose of acquiring one or more businesses or assets, since February 2007. He has been a director of Point Blank Solutions, Inc., a designer and manufacturer of protective body armor, since August 2008. Mr. Henderson has been a director of GenCorp Inc., a major technology-based manufacturing company in the fields of aerospace and defense, since March 2008 and Chairman of the Board since May 2008. He has been a director of BNS Holding, Inc., a holding company that owns the majority of Collins Industries, Inc., a manufacturer of school buses, ambulances and terminal trucks, since June 2004. Mr. Henderson has been a director of SL Industries, Inc., a designer and manufacturer of power electronics, power motion equipment, power protection equipment, and teleprotection and specialized communication equipment, since January 2002. He was a director and Chief Executive Officer of WebFinancial Corporation, which through its operating subsidiaries, operates niche banking markets, from June 2005 to April 2008, President and Chief Operating Officer of WebFinancial from November 2003 to April 2008, and was the Vice President of Operations from September 2000 through December 2003. He was also the Chief Executive Officer of WebBank, a wholly-owned subsidiary of WebFinancial, from November 2004 to May 2005. He was a director of ECC International Corp. (“ECC”), a manufacturer and marketer of computer controlled simulators for training personnel to perform maintenance and operation procedures on military weapons, from December 1999 to September 2003 and was acting Chief Executive Officer from July 2002 to March 2003. Mr. Henderson has been the President of Gateway Industries, Inc., a provider of database development and web site design and development services, since December 2001. From January 2001 to August 2001, he was President of MDM Technologies, Inc., a direct mail and marketing company.
General Merrill A. McPeak has been a member of the Company’s Board of Directors since April 27, 2005. General McPeak is the President of McPeak and Associates, a management-consulting firm he founded in 1995. General McPeak was Chief of Staff of the Air Force from November 1990 to October 1994, when he retired from active military service. General McPeak was for several years Chairman of ECC. He has served as a director of several other public companies, including Tektronix, Inc. and TWA. Currently, General McPeak is Chairman of the Board of Ethicspoint, Inc., a company providing confidential corporate governance compliance and whistleblower reporting services. He is a director of Sensis Corp., a privately held manufacturer of military radars and civilian air traffic control systems. He is an investor in and director of several public and private companies in the early development stage, including: GigaBeam Corporation, a supplier of high performance, high availability fiber-speed wireless communications; MathStar, Inc., a designer and marketer of specialized semiconductor integrated circuits; and Quintessence Photonics, a subsidiary of QPC Lasers, Inc., a designer and manufacturer of high performance semiconductor laser diodes. General McPeak received a Bachelor of Arts degree in economics from San Diego State College and a Master of Science degree in international relations from George Washington University. He is a member of the Council on Foreign Relations, New York City.
James A. Risher has been a member of the Company’s Board of Directors since April 27, 2005. On July 22, 2006, Mr. Risher became the Interim President and CEO of Del Global. On August 31, 2006 Mr. Risher became the President and CEO of the Company. Mr. Risher has been the Managing Partner of Lumina Group, LLC, a private company engaged in the business of consulting and investing in small and mid-size companies, since 1998. From February 2001 to May 2002, Mr. Risher served as Chairman and Chief Executive Officer of BlueStar Battery Systems International, Inc., a Canadian public company that is an e-commerce distributor of electrical and electronic products to selected automotive aftermarket segments and targeted industrial markets. From 1986 to 1998, Mr. Risher served as a director, Chief Executive Officer and President of Exide Electronics Group, Inc. (“Exide”), a global leader in the uninterruptible power supply industry. He also served as Chairman of Exide from December 1997 to July 1998. Mr. Risher has also been a director of SL Industries, Inc. since May 2003 and a director of New Century Equity Holdings Corp., a holding company seeking to acquire a new business, since October 2004.
ADDITIONAL EXECUTIVE OFFICER OF THE COMPANY WHO IS NOT A DIRECTOR.
Mark A. Zorko, 56, has served as our Chief Financial Officer from August 30, 2006. He continues as a CFO Partner at Tatum, LLC, a professional services firm where he has held financial leadership positions with public and private client companies. From 1996 to 1999, Mr. Zorko was Chief Financial Officer and Chief Information Officer for Network Services Co., a privately held distribution company. His prior experience includes Vice President, Chief Financial Officer and Secretary of Comptronix Corporation, a publicly held electronic system manufacturing company, corporate controller for Zenith Data Systems Corporation, a computer manufacturing and retail electronics company, and finance manager positions with Honeywell, Inc. Mr. Zorko was a senior staff consultant with Arthur Andersen & Co. Mr. Zorko served in the Marine Corps. from 1970 to 1973. He has served as a director of Guardian Technologies International, Inc. Mr. Zorko is on the audit committee for Opportunity Int’l, a microenterprise development organization, and on the Board of Directors for St. Alexius Medical Center. Mr. Zorko earned a BS degree in Accounting from The Ohio State University, an MBA from the University of Minnesota, and completed the FEI's Chief Financial Officer program at Harvard University. He is a certified public accountant and a member of the National Association of Corporate Directors.
AUDIT COMMITTEE OF THE BOARD OF DIRECTORS; IDENTIFICATION OF AUDIT COMMITTEE FINANCIAL EXPERT.
The Board of Directors has a standing Audit Committee, the members of which are Gerald M. Czarnecki (chairman) and General Merrill McPeak. The Board of Directors has determined that Mr. Czarnecki is an "audit committee financial expert" as defined in Item 401(h) of Regulation S-K. Although the Company is currently not listed on any exchange, both Mr. Czarnecki and Gen. McPeak are considered to be an "independent director" as defined in Rule 4200 of the Marketplace Rules of the National Association of Securities Dealers, Inc.
CODE OF BUSINESS CONDUCT AND ETHICS.
The Company has adopted a Code of Business Conduct and Ethics that applies to the Company’s Chief Executive Officer and Principal Accounting Officer. The Company’s Code of Business Conduct and Ethics is posted on the Company’s website, http://www.delglobal.com.
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE.
Section 16(a) of the Exchange Act requires the Company's officers and directors, and persons who own more than ten percent of a registered class of the Company's equity securities, to file reports of ownership on Form 3 and changes in ownership on Form 4 or Form 5 with the Securities and Exchange Commission (the “Commission”). Such officers, directors and 10% stockholders are also required by Commission rules to furnish the Company with copies of all Section 16(a) forms they file. Based solely on its review of the copies of such forms received by it, or written representations from certain reporting persons, the Company believes that during the fiscal year ended August 2, 2008 there was compliance with all Section 16(a) filing requirements applicable to its officers, directors and 10% stockholders. The Company knows of no failures to file a required Section 16(a) form during the fiscal year ended August 2, 2008.
COMPENSATION DISCUSSION AND ANALYSIS
This compensation discussion and analysis describes the elements of compensation paid to each of the named executive officers who served in the fiscal year ended August 2, 2008. The discussion focuses primarily on the information contained in the following tables and related footnotes but may also describe compensation actions taken before or after the last completed fiscal year to the extent that such discussion enhances understanding of our compensation philosophy or policies. The Compensation Committee of the Board, which we refer to in this discussion as the “Committee”, oversees the design and administration of our executive compensation program.
THE ROLE OF THE COMPENSATION COMMITTEE
The Committee is responsible for ensuring that the Company’s executive compensation policies and programs are competitive within the markets in which the Company competes for talent and reflect the long-term investment interests of our shareholders. The Committee reviews and approves the executive compensation and benefits programs for all the Company’s executive officers annually, usually in first quarter of each fiscal year. Any options that are granted as a result of the Committee’s executive compensation review and approval process are only granted upon full Board approval of the option grant. The strike price of such options is set at the closing price of the Company’s stock on the day the options were granted.
With respect to the CEO, the Committee reviews and approves corporate goals and objectives, evaluates the CEO’s performance against these objectives, and makes recommendations to the Board regarding the CEO’s compensation level based on that evaluation.
| | The CEO participates, together with the Committee, in the executive compensation process by: |
| | · | approving perquisites valued at less than $10,000 per year (all perquisites valued at greater than this amount are still approved by the Compensation Committee); |
| | · | participating in informal discussions with the Compensation Committee regarding satisfaction of performance criteria by executive officers, other than the CEO; |
| | · | providing the Board with recommendations as to who should participate in the Del Global Incentive Stock Plan and the size of option grants to such participants; and |
| | · | assigning annual budget goals and other objectives that determine bonus awards for the CFO. |
COMPENSATION PHILOSOPHY AND OBJECTIVES
The Committee is responsible for ensuring that the Company’s executive compensation policies and programs are competitive within the markets in which the Company competes for talent and reflect the long-term investment interests of our shareholders. The goal of the executive compensation program is to (a) attract, retain and reward executive officers who contribute to the Company’s success and (b) align executive compensation with the achievement of the Company’s business objectives and the creation of longer-term value for shareholders. The Committee also strives to balance short and long-term incentive objectives by establishing goals, performance criteria, evaluating performance and determining actual incentive awards that are both effective and efficient. While the Committee believes that stock ownership by executive officers is an effective way of aligning the common interests of management and shareholders to enhance shareholder value, the Company has not established equity ownership guidelines for its executive officers.
RELATIONSHIP OF COMPANY PERFORMANCE TO EXECUTIVE COMPENSATION
When determining executive compensation, the Committee also takes into account the executives’ performance in special projects undertaken during the past fiscal year, contribution to improvements in our financial situation, development of new products, marketing strategies, manufacturing efficiencies and other factors. During the 2008 fiscal year, the Committee focused particularly on progress with respect to improvement in the Company's revenue growth, operating earnings and the development of a long-term strategic plan for the Company that provides a platform for growth and a return to shareholders.
Satisfaction of certain performance criteria (including initiative, contribution to overall corporate performance and managerial ability) is evaluated after informal discussions with other members of the Board and, for all of the executives other than the CEO, after discussions with the CEO. No specific weight or relative importance was assigned to the various qualitative factors and compensation information considered by the Committee. Accordingly, the Company's compensation policies and practices may be deemed subjective, within an overall published framework based on both the financial and non-financial factors.
ELEMENTS OF COMPENSATION
The Company’s compensation program is comprised primarily of four elements: base salary, annual cash bonuses, long-term equity incentives and perquisites. Together, these four elements are structured by the Committee to provide our named executive officers with cumulative total compensation consistent with our executive compensation philosophy described above. Each of these elements plays an important role in balancing executive rewards over short- and long-term periods, based on our program objectives.
1. BASE SALARY
Our base salary levels reflect a combination of factors, including competitive pay levels relative to our peers, the executive’s experience and tenure, our overall annual budget for merit increases and pre-tax profit, the executive’s individual performance, and changes in responsibility. Base salaries are reviewed annually by the Committee at the beginning of the year, but are not automatically increase annually. We do not target base salary at any particular percent of total compensation. The base salary for our CEO and CFO is set forth in their employment agreements, which are described in more detail below.
2. ANNUAL CASH BONUSES
The purpose of the annual cash bonus is to provide a competitive annual cash incentive opportunity that rewards both the Company’s performance toward corporate goals and objectives and also individual achievements. The annual bonus is a short-term annual incentive paid in cash pursuant to arrangements that cover all executive officers, including the CEO, and provide that a bonus will be paid upon achieving the Company’s annual budget goals. For fiscal year 2008, the Committee determined that bonuses would be paid out upon the achievement of improvement of revenue and operating income as compared to fiscal year 2008 business plan with targets set for the CEO and CFO of 70% and 45% of their annual base salary respectively. Incentive targets for fiscal year 2008 were not achieved and as a result, the CEO and CFO did not receive an annual bonus.
3. LONG TERM EQUITY INCENTIVES
A. DEL GLOBAL STOCK OPTION PLAN
The purpose of the Del Global Amended and Restated Stock Option Plan (the “DGTC Plan”), is to provide for the granting of incentive stock options and non-qualified stock options to the Company’s executive officers, directors, employees and consultants. The Committee administers the DGTC Plan. Among other things, the Committee: (i) determines participants to whom options may be granted and the number of shares to be granted pursuant to each option, based upon the recommendation of our chief executive officer; (ii) determines the terms and conditions of any option under the DGTC Plan, including whether options will be incentive stock options, within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended (the “Code”), or non-qualified stock options; (iii) may vary the vesting schedule of options; and (iv) may suspend, terminate or modify the DGTC Plan. Any Committee recommendations of awards, options or compensation levels for senior executive officers are approved by the entire Board, excluding any management directors.
Under the DGTC Plan, incentive stock options have an exercise price equal to the fair market value of the underlying stock as of the grant date and, unless earlier terminated, are exercisable for a period of ten (10) years from the grant date. Non-qualified stock options may have an exercise price that is less than, equal to or more than the fair market value on the grant date and, unless earlier terminated, are exercisable for a period of up to ten (10) years from their grant date.
For the fiscal year ended August 2, 2008, no options to purchase the Company’s common stock were granted under the terms of the DGTC Plan.
B. 2007 INCENTIVE STOCK PLAN
The 2007 Incentive Stock Plan (the “2007 Plan”) is designed to provide an incentive to, and to retain in the employ of the Company and any Subsidiary of the Company, within the meaning of Section 424(f) of the United States Internal Revenue Code of 1986, as amended, directors, officers, consultants, advisors and employees with valuable training, experience and ability; to attract to the Company new directors, officers, consultants, advisors and employees whose services are considered valuable and to encourage the sense of proprietorship and to stimulate the active interest of such persons in the development and financial success of the Company and its Subsidiaries.
The 2007 Plan is administered by the Committee, which has full power and authority to designate recipients of options (as defined in the 2007 Plan) and restricted stock under the 2007 Plan and to determine the terms and conditions of the respective option and restricted stock agreements and to interpret the provisions and supervise the administration of the 2007 Plan. The Committee also has the authority to designate which options granted under the 2007 Plan will be incentive options and which shall be nonqualified options.
For the fiscal year ended August 2, 2008, under the terms of the 2007 Plan, the Company granted (a) James A. Risher an option to purchase 50,000 shares of the Company’s common stock and (b) Mark A. Zorko an option to purchase 20,000 shares of the Company’s common stock.
4. PERQUISITES
The Company’s compensation program also includes other benefits and perquisites. These benefits include annual matching contributions to certain executive officers’ 401(k) plan accounts, car allowances, living allowances and tax gross-ups to cover taxes on certain benefits. We are selective in our use of perquisites, attempting to utilize perquisites that are within range of modest to competitive within our industry. The Committee has delegated authority to the CEO to approve such perquisites for other executive officers, but the Committee must separately approve any perquisites that exceed $10,000 per year.
IMPACT OF TAX AND ACCOUNTING
As a general matter, the Committee always considers the various tax and accounting implications of compensation elements employed by the Company and attempts to structure such compensation in a tax efficient manner. When determining amounts of long-term incentive grants to executives and employees, the Committee examines the accounting cost associated with the grants.
Current compensation levels for our named executive officers are significantly lower than $1 million at which tax deductions are limited under Internal Revenue Code Section 162(m). In the event that future annual total compensation for any named executive officer exceeds the $1 million threshold, the Committee intends to balance tax deductibility of executive compensation with its responsibility to retain and motivate executives with competitive compensation programs. As a result, the Committee may take such actions as it deems in the best interests of shareholders, including: (i) provide non-deductible compensation above the $1 million threshold; (ii) require deferral of a portion of the bonus or other compensation to a time when payment may be deductible by the Company; and/or (iii) modify existing programs to qualify bonuses and other performance-based compensation to be exempt from the deduction limit.
FISCAL YEAR 2008 COMPENSATION DESICION
For fiscal year 2008, the Committee conducted an evaluation of the performance of the CEO and the CFO against per-established goals. Based upon these evaluations, decisions were made regarding salary increases and annual bonuses. The CEO received a salary increase of $20,000 for fiscal year 2008. Incentive targets for fiscal year 2008 were not achieved and as a result, the CEO and CFO did not receive an annual bonus.
In keeping with our philosophy of aligning management and the stockholder interests and consideration of the future contributions expected of the executive officers, the Committee granted long-term equity incentives to the CEO and CFO. See the “Grants of Plan-Based Awards Table” for equity granted to the Named Executive Officers in fiscal year 2008.
SUMMARY COMPENSATION TABLE
The following table sets forth all compensation awarded to, paid to or earned by the following type of executive officers for the fiscal years ended August 2, 2008 and July 28, 2007: (i) individuals who served as, or acted in the capacity of, the Company's principal executive officer for the fiscal years ended August 2, 2008 and July 28, 2007; (ii) individuals who served as, or acted in the capacity of, the Company's principal financial officer for the fiscal years ended August 2, 2008 and July 28, 2007; (iii) the Company's most highly compensated executive officers, other than the chief executive and chief financial officer, who were serving as executive officers at the end of the fiscal years ended August 2, 2008 and July 28, 2007 (of which there were none) and (iv) up to two additional individuals for whom disclosure would have been provided but for the fact that the individual was not serving as an executive officer of the Company at the end of the fiscal years ended August 2, 2008 and July 28, 2007 (of which there was one). We refer to these individuals collectively as our “named executive officers.”
SUMMARY COMPENSATION TABLE
| | | | | | | | | |
Name and Principal Position | | | | | | | | | | | | | | | | | | |
| James A. Risher, Chief Executive Officer | 2008 | | $ | 319,743 | | | $ | - | | | $ | - | | | $ | 75,092 | | | $ | 125,944 | | | $ | 520,779 | |
| | 2007 | | $ | 274,615 | | | $ | 224,324 | | | $ | - | | | $ | 51,171 | | | $ | 167,686 | | | $ | 717,796 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Mark A. Zorko, Chief Financial Officer | 2008 | | $ | 246,571 | | | $ | - | | | $ | - | | | $ | 37,356 | | | $ | 8,575 | | | $ | 292,502 | |
| | 2007 | | $ | 214,300 | | | $ | 131,291 | | | $ | - | | | $ | 23,526 | | | $ | 6,844 | | | $ | 375,961 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Mark A. Koch(3) Treasurer and Principal Accounting Officer | 2007 | | $ | 52,990 | | | $ | - | | | $ | - | | | $ | - | | | $ | 120,577 | (4) | | $ | 173,567 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (1) | The figures reported in the salary and bonus columns represent amounts earned and accrued for each year. |
| | |
| (2) | The amounts in this column include the following executive perquisites and other compensation for fiscal years 2008 and 2007: |
| Name | Benefit | | 2008 | | | 2007 | |
| James A. Risher | Living allowance | | $ | 72,720 | | | $ | 67,357 | |
| | Tax Gross-Ups | | | 53,224 | | | | 41,162 | |
| | Other | | | - | | | | 59,167 | (2)(e)) |
| | | | $ | 125,944 | | | $ | 167,686 | |
| | | | | | | | | | |
| Mark A. Zorko | Car Allowance | | $ | 6,900 | | | $ | 5,750 | |
| | 401 (k) Match | | | 1,675 | (2)(c)) | | | 1,094 | (2)(c)) |
| | | | $ | 8,575 | | | $ | 6,844 | |
| | | | | | | | | | |
| Mark A. Koch | Other | | $ | - | | | $ | 120,577 | (4) |
| 2(c) | Company-matching contribution of 50% of the first 4% of salary. Accelerated vesting schedule (100% vested in Company contributions after three (3) years of employment). |
| 2(e) | During fiscal year 2007, but prior to Mr. Risher’s appointment as CEO, Mr. Risher received $4,167 as compensation for his service to the Company as a Director and $55,000 for his service to the Company as a consultant. |
| (3) | Mr. Koch served as the Company’s Treasurer and Principal Accounting Officer until October 30, 2006. |
| (4) | Mr. Koch received separation payments totaling $120,577 in fiscal year 2007. |
| (5) | Refer to Footnote 10 Shareholder Equity in Item 8 “Stock Option Plan And Warrants” for details of stock option plan terms, SFAS 123R valuation techniques and assumptions and the fair value of stock options granted. |
Grants Of Plan-Based Awards :
| (a) | | (b) | | (i) | | (j) | | (k) | | (l) |
| Name | | Grant Date | | All Other Stock Awards: Number of Shares of Stock or Units (#) | | All Other Option Awards: Number of Securities Underlying Options (#) | | Exercise or Base Price of Option Awards ($/sh) | | Grant Date Fair Value of Stock and Option Awards ($) |
| James A. Risher, Chief Executive Officer | | 8/31/2006 | | - | | 120,000(1) | | 1.50 | | 118,800 |
| | | 9/17/2007 | | - | | 50,000(2) | | 2.70 | | 95,397 |
| | | | | | | | | | | |
| Mark A. Zorko, Chief Financial Officer | | 8/31/2006 | | - | | 60,000(1) | | 1.50 | | 59,400 |
| | 11/17/2006 | | - | | 20,000(1) | | 2.00 | | 26,200 |
| | | 9/17/2007 | | - | | 20,000(2) | | 2.70 | | 38,159 |
| | (1) | Granted pursuant to the Company's DGTC Plan. |
| | | |
| | | These stock options vest and become exercisable as to one-half of such shares on the first anniversary of the date of the grant and as to an additional 25% of such shares on the second and third anniversaries of the date of the grant, respectively. |
| | | |
| | (2) | Granted pursuant to the Company's 2007 Plan. |
| | | |
| | | These stock options vest and become exercisable as to 25% of such shares on the date of the option grant and 25% on each of the first, second and third anniversaries of the date of the grant. |
EMPLOYMENT AGREEMENTS
A. James A. Risher Employment Agreements
The Company and Mr. Risher entered into a Letter Agreement, dated August 31, 2006 (the “Risher Employment Agreement”), providing for Mr. Risher’s employment with the Company as its CEO and President. Pursuant to the Risher Employment Agreement, Mr. Risher was entitled to an annual salary of $300,000 and received an option grant to purchase 120,000 shares of the Company's common stock pursuant to and in accordance with the Company's DGTC Plan. Such stock options vest and become exercisable as to one-half of such shares on the first anniversary of the date of the grant and as to an additional 25% of such shares on the second and third anniversaries of the date of the grant, respectively. Under the terms of the Risher Employment Agreement, Mr. Risher also received a living allowance of $6,200 per month. In addition, Mr. Risher was eligible to receive an annual bonus with a target of 60% of his annual base salary based upon achieving the Company's annual budget and attaining specific objectives assigned by the Board. As a result of achieving these specific objectives in fiscal year 2007, Mr. Risher received a bonus of $224,324.
The Risher Employment Agreement was superseded by Letter Agreement between the Company and Mr. Risher, dated September 19, 2007 (the “2007 Risher Agreement”), setting forth the terms of Mr. Risher’s employment by the Company as its CEO and President. For fiscal year 2008, Mr. Risher received an annual base salary of $320,000 as well as a living allowance of $6,200 per month, which amount shall be “grossed up” for tax purposes. In addition, Mr. Risher was eligible to receive an annual bonus with a target of 70% of his annual base salary, based on achieving the Company’s annual budget and attaining specific objectives assigned by the Board. The annual bonus could have been anywhere from 0% to 150% of the target. As a result of the Company not achieving these specific objectives in fiscal year 2008, Mr. Risher did not receive a bonus for the 2008 fiscal year.
The 2007 Risher Agreement has been superseded by Letter Agreement between the Company and Mr. Risher, dated September 16, 2008 (the “2008 Risher Agreement”) setting forth the terms of Mr. Risher’s continued employment by the Company as its CEO and President, effective as of September 1, 2008. The 2008 Risher Agreement will terminate on August 31, 2009. The 2008 Risher Agreement provides for the same compensation as the 2007 Risher Agreement, including an annual base salary of $320,000 as well as a living allowance of $6,200 per month, which amount will be “grossed up” for tax purposes. In addition, Mr. Risher will be eligible to receive an annual bonus with a target of 70% of his annual base salary, based on achieving the Company’s annual budget and attaining specific objectives assigned by the Board. The annual bonus can be anywhere from 0% to 150% of the target. In addition to these terms, the 2008 Risher Agreement provides that in the event Mr. Risher terminates his employment with the Company for “Good Reason” or if the Company terminates his employment without “Cause” (and not, in each case, by reason of Mr. Risher’s death or disability), Mr. Risher will be entitled to certain payments and benefits more fully described below in the section entitled “Potential Payments Upon Termination Or A Change In Control.”
B. Mark A. Zorko Employment Agreement
The Company and Mr. Zorko entered into a Letter Agreement, dated August 30, 2006 (the “Zorko Employment Agreement), which remains in effect as of the date hereof, and provides for Mr. Zorko’s employment with the Company as its Chief Financial Officer. Pursuant to the Zorko Employment Agreement, Mr. Zorko is entitled to an annual salary of $245,000 and received an option grant to purchase 60,000 shares of the Company's common stock pursuant to and in accordance with the Company's DGTC Plan. Mr. Zorko is also entitled to receive an automobile allowance of $575 per month. In addition, Mr. Zorko was eligible to receive an annual bonus with a target of 45% of his annual base salary based upon achieving the Company's annual budget and attaining specific objectives assigned by the CEO of the Company. As a result of the Company not achieving these specific objectives in fiscal year 2008, Mr. Zorko did not receive a bonus for the fiscal year.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END:
| | |
| (a) | | (b) | | (c) | | (d) | | (e) | | (f) | | (g) | | (h) | | (i) | | (j) |
| | Number of Securities Underlying Unexercised Options (#) Exercisable | | Number of Securities Underlying Unexercised Options(#) Unexercisable | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options | | Option Exercise Price | | | | Number of Shares or Units of Stock That Have Not Vested | | Market Value of Shares or Units of Stock That Have Not Vested | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested | | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) |
| | | | | | | | | | | | | | | | | | | |
James A. Risher, Chief Executive Officer | | 12,500 (3) 25,000 (2) | | 37,500 (3) - (2) | | - | | 2.70 2.70 | | 9/17/17 4/26/15 | | | | | | | | |
| | 7,500 (2) | | 2,500 (2) | | - | | 2.26 | | 6/16/16 | | - | | - | | - | | - |
| | 60,000 (2) | | 60,000 (2) | | - | | 1.50 | | 8/31/16 | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Mark A. Zorko, Chief Financial Officer | | 5,000 (3) | | 15,000 (3) | | | | 2.70 | | 9/17/17 | | | | | | | | |
| | 30,000 (2) | | 30,000 (2) | | - | | 1.50 | | 8/31/16 | | - | | - | | - | | - |
| | 10,000 (2) | | 10,000 (2) | | - | | 2.00 | | 11/17/16 | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Mark A Koch, former Treasurer and Principal Accounting Officer | | 10,000 (2) | | - | | - | | - | | - | | - | | - | | - | | - |
| (1) | The exercise price per share of each option was equal to the closing price of the shares of Common Stock on the date of grant. |
| (2) | Granted pursuant to the Company's DGTC Plan. |
| (3) | Granted pursuant to the Company's 2007 Plan. |
POTENTIAL PAYMENTS UPON TERMINATION OR A CHANGE IN CONTROL
Separation Agreements with Current and Certain Former Named Executive Officers.
James A. Risher
Pursuant to the 2008 Risher Agreement, in the event Mr. Risher terminates his employment with the Company for “Good Reason” or if the Company terminates his employment without “Cause” (and not, in each case, by reason of Mr. Risher’s death or disability), Mr. Risher will be entitled to continue receiving his full salary, including the living allowance, subject to applicable withholding tax requirements, until August 31, 2009. The full value of these payments for the term of the 2008 Risher Agreement is $394,400. Pursuant to the Employment Agreement, Mr. Risher is not entitled to receive any other severance or compensation from the Company in the event his employment with the Company is terminated.
For the purposes of the 2008 Risher Agreement:
“Good Reason” means: (a) a material diminution in Mr. Risher’s duties as customarily performed by Mr. Risher for the Company, including but not limited to the assignment to Mr. Risher of duties inconsistent with the CEO position, duties or responsibilities as in effect after the date of execution of this Agreement and (b) the Company requires Mr. Risher to relocate 50 miles or more from his present place of work, provided, in each case, that Mr. Risher has given prompt notice to the Company of the existence of the condition (but in no event later than 90 days after its initial existence) and Mr. Risher has provided the Company with a minimum of 30 days following such notice to remedy such condition.
“Cause” means: (i) a material breach, by Mr. Risher, of any written agreement with the Company or its affiliates (after notice and, if capable of being cured, reasonable opportunity of not less than thirty (30) days to cure), (ii) a breach of Mr. Risher’s fiduciary duty to the Company (after notice and, if capable of being cured, reasonable opportunity of not less than thirty (30) days to cure) or any misappropriation, embezzlement or fraud with respect to the Company of affiliate of the Company, or any of their security holders, customers or suppliers, (iii) the commission by Mr. Risher of a felony, a crime involving dishonesty or moral turpitude or other engaging in material misconduct that has caused or is reasonably expected to cause injury to a the Company or an affiliate thereof, or their interests including, but not limited to, harm to the standing and reputation of, or which otherwise brings public disgrace or disrepute to the Company or any of its affiliates, (iv) Mr. Risher’s continued failure or refusal to perform any material duty to the Company or any of its affiliates, which is normally attached to his position (after notice and reasonable opportunity of not less than thirty (30) days to cure), (v) Mr. Risher’s gross negligence or willful misconduct in performing those duties which are normally attached to his position (after notice and reasonable opportunity of not less than thirty (30) to cure if capable of being cured), (vi) any breach of this Agreement, or (vii) a material breach by Mr. Risher of any written code of conduct or other material written policy of the Company or any of its affiliates.
Mark A. Zorko
Pursuant to the Zorko Employment Agreement, Mr. Zorko is entitled to a severance payment in the event his employment is terminated by the Company without cause. The severance payment is equal to one-year base salary (currently $245,000). The Company will make no such payment if employment is terminated for cause.
Mark A. Koch
Mr. Koch served as the Company’s Treasurer and Principal Accounting Officer until October 30, 2006.
The Company entered into a Separation Agreement and General Release dated as of September 7, 2006, (the "Koch Separation Agreement") with Mark A. Koch, the Company’s former Principal Accounting Officer. The Koch Separation Agreement was filed as Exhibit 99.01 to the Company’s Current Report on Form 8-K filed on September 7, 2006. The Koch Separation Agreement provided that Mr. Koch's last day of employment with the Company will be the first business day following the filing by the Company with the SEC of its Annual Report on Form 10-K for the fiscal year ending July 29, 2006, but in no event later than November 30, 2006, unless mutually agreed in writing by the parties (the "Termination Date"). The Separation Agreement also provided for a payment of one (1) year's base salary payable pro-rata over 12 months by the Company to Mr. Koch commencing with the first pay-day following the Termination Date; provided, however, that in the event the Company sells any of its assets or the assets of any of its U.S. Subsidiaries for cash and such sale results in net cash proceeds to the Company of at least $5.0 million, then the Company shall pay to Mr. Koch any balance outstanding of the severance payment within ten (10) days after receipt by the Company of such net cash proceeds from such asset sale. Mr. Koch agreed to release and discharges the Company, as more fully described in the Koch Separation Agreement. Pursuant to the Koch Separation Agreement, Mr. Koch’s last day of employment with the Company was October 30, 2006. The total amount to be paid to Mr. Koch in connection with the termination of his employment is $165,000, $120,577 of which was paid in fiscal 2007 and the remainder was paid in the first quarter of fiscal 2008.
The Koch Separation Agreement supersedes a certain former Severance Benefits Agreement, dated May 23, 2005, between the Company and Mr. Koch, except that the terms and conditions of Article IV of the former Severance Benefits Agreement which concern obligations with respect to Company confidential information and trade secrets, survive and remain in full force and effect.
DIRECTOR COMPENSATION
The Company seeks highly qualified individuals to serve as outside directors and compensates them with a combination of cash fees and stock option grants. The Company also reimburses Directors for, or pays, travel costs associated with meeting attendance. There is no retirement plan for outside directors, and no program of perquisites. The Committee periodically assesses whether its compensation structure is competitive in terms of attracting and retaining the type and quality of outside directors needed.
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
The Committee consists of Merrill A. McPeak as Chairman, Gerald M. Czarnecki and James R. Henderson. None of these individuals were at any time during the fiscal year ended August 2, 2008 or at any other time one of our officers or employees. Other than Mr. Risher, the Company’s CEO, none of our executive officers serve as a member of the Board or the Committee of any other entity which has one or more executive officers serving as a member of our Board or Committee.
DIRECTOR COMPENSATION:
| (a) | | (b) | | (d) | | (g) | | (h) |
| Name | | Fees Earned or Paid in Cash(1) ($) | | Option Awards(2) ($) | | All Other Compensation ($) | | Total ($) |
Gerald M. Czarnecki (4) | | 28,500 | | | 15,949 | | | - | | | 53,875 | |
| | | | | | | | | | | | | |
James R. Henderson (Chairman) (4) | | 33,000 | (3) | | 37,476 | | | - | | | 63,450 | |
| | | | | | | | | | | | | |
General Merrill A. McPeak (4) | | 28,000 | | | 22,731 | | | - | | | 51,345 | |
| | | | | | | | | | | | | |
James A. Risher(5) | | - | (5) | | - (5) | | | - (5) | | | - (5) | |
(1) Fees consist of:
| | · | Each non-employee director receives an annual retainer of $20,000; |
| | · | Each non-employee director receives an additional fee of $1,000 per each full length Board meeting attended (with lesser compensation for telephonic meetings, at the discretion of the chair of the Board or committee, as applicable); |
| | · | Each non-employee member of each standing committee receives a fee of $500 per each full-length committee meeting attended; and $250 for shorter duration committee meetings attended; and |
| | · | Chairs of the Board and the various standing committees, excepting the Audit Committee, receives double meeting fees. In lieu of the foregoing, the Chair of the Audit Committee receives an additional $1,000 per Audit Committee meeting. |
(2) During fiscal 2008, Mr. Czarnecki received a grant to purchase 12,500 shares of the Company’s common stock at an exercise price of $2.60 per share and an aggregate fair value of $21,861; Mr. Henderson received grants to purchase 15,000 shares of the Company’s common stock at an exercise price of $2.60 per share and aggregate fair values of $26,233 and General McPeak received a grant to purchase 11,500 shares of the Company’s common stock at an exercise price of $2.60 per share and an aggregate fair value of $20,112. Upon election to the Board, each non-employee member of the Board receives a one-time grant of 25,000 options to purchase the Company’s Common Stock, with an exercise price equal to the fair market value on the date of grant. Effective as of June 13, 2006, Directors also received annual grants of 10,000 options commencing after their first year of service as a director. The Chairman of the Audit Committee receives an additional annual grant of 2,500 options. The Chairman of the Committee receives an additional annual grant of 1,500 options. The Chairman of the Governance and Nominating Committee receives an additional annual grant of 1,000 options (as long as such person is not the Chair of any other committee of the Board). The Chairman of the Board receives an additional annual grant of 5,000 options. The annual grants of stock options to directors in fiscal year 2008 were made pursuant to the Company’s 2007 Plan. Directors are also eligible to receive restricted stock awards under the terms of the Company’s 2007 Plan. The dollar amounts in this column reflect the dollar amounts recognized for financial statement reporting purposes with respect to the fiscal year in accordance with FAS 123R. Refer to Footnote 10 Shareholder Equity in Item 8 “Stock Option Plan And Warrants” for details of stock option plan vesting terms, SFAS 123R valuation techniques and assumptions and the fair value of stock options granted.
(3) In addition to the above meeting fees, the Chairman of the Board receives $750 per each day other than Board meeting days where he or she spends more than half of such day working at the Company facilities. This amount is included in the amount reflected in Column (b).
(4) At August 2, 2008, Mr. Czarnecki held an aggregate of 62,500 options to purchase the Company’s Common Stock, of which 43,750 were exercisable; Mr. Henderson held an aggregate of 121,000 options to purchase the Company’s Common Stock, of which 73,250 exercisable; Mr. McPeak held an aggregate of 59,500 options to purchase the Company’s Common Stock, of which 42,250 were exercisable.
(5) As Mr. Risher is the Company's CEO, he is no longer eligible to receive any compensation for his service as a Director.
Restricted Stock and Option Awards
Upon election to the Board, each non-employee member of the Board receives a one-time grant of 25,000 options to purchase the Company’s Common Stock. The exercise price for such options is equal to the fair market price per share on the date of the grant, which is approved by the Committee. These options vest and become exercisable as to 25% of such shares on the date of the option grant, 25% on the first anniversary of the date of the grant and as to an additional 25% of such shares on the second and third anniversaries of the date of the grant, respectively, based on continued service through the applicable vesting date. Effective as of June 13, 2006, Directors also received annual grants of 10,000 options commencing after their first year of service as a director. The Chairman of the Audit Committee receives an additional annual grant of 2,500 options. The Chairman of the Stock Option and Compensation Committee receives an additional annual grant of 1,500 options. The Chairman of the Governance and Nominating Committee receives an additional annual grant of 1,000 options (as long as such person is not the Chair of any other committee of the Board). The Chairman of the Board receives an additional annual grant of 5,000 options. Directors are also eligible to receive restricted stock and option awards under the terms of the Company’s 2007 Plan. The annual grants of stock options to directors in fiscal year 2007 were made pursuant to the DGTC Plan and the annual grants of stock options to directors in fiscal year 2008 were made pursuant to the 2007 Plan.
COMPENSATION COMMITTEE REPORT
We have reviewed and discussed with management certain Executive Compensation and Compensation Discussion and Analysis provisions to be included in this Annual Report filed on Form 10K, filed pursuant the Exchange Act. Based on the reviews and discussions referred to above, we recommend to the Board of Directors that the Executive Compensation and Compensation Discussion and Analysis provisions referred to above be included in this Annual Report.
Submitted by the Committee of the Board of Directors
General Merrill A. McPeak, Chairman
Gerald M. Czarnecki
James R. Henderson
This Compensation Committee Report is not deemed incorporated by reference by any general statement incorporating by reference this Annual Report into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent that the Company specifically incorporates this information by reference, and shall not otherwise be deemed filed under either such Acts.
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
EQUITY COMPENSATION PLAN INFORMATION
The following table summarizes the securities authorized for issuance under equity compensation plans as of the end of Fiscal 2008:
| | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | | | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights | | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans(1) | |
| Equity compensation plans approved by security holders: | | | | | | | | | | | | |
| Stock Option Plan | | | 2,094,815 | | | $ | 3.45 | | | | 604,500 | |
| | | | | | | | | | | | | |
| Equity compensation plans not approved by security holders: | | | | | | | | | | | | |
Warrants issued in settlement of class action lawsuit(2) |
| | | 512,500 | | | $ | 1.50 | | | | Not applicable | |
| (1) | Excludes securities to be issued upon exercise of outstanding options, warrants and rights. |
| (2) | Pursuant to our class action settlement with our shareholders concerning allegations that the Company had violated federal Securities laws, we issued 2.5 million shares of our Common Stock and one million warrants to purchase our Common Stock at $2.00 per share. The issuance of these securities was pursuant to a court order issued in connection with the settlement of this class action lawsuit in January 2002, and therefore was exempt from the registration requirements of the Securities Act of 1933 pursuant to Section 3(a) (10) thereof. These warrants were originally set to expire in March 2008. In a motion filed in February 2004, a plaintiff class claimed damages due to Del Global’s failure to timely complete a registration statement for the shares of Common Stock issuable upon exercise of these warrants. The class sought damages of $1.25 million together with interest and costs, and a declaration that $2 million in subordinated notes issued as part of the 2002 class action settlement were immediately due and payable. In settlement of this matter, Del Global modified the exercise, or “strike,” price of the warrants issued in 2002 from $2.00 to $1.50 per share, and extended the expiration date of such warrants by one year to March 28, 2009. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS
The following table sets forth information concerning beneficial ownership of common stock of the Company outstanding at October 1, 2008 by each person or entity (including any "Group" as such term is used in Section 13(d) (3) of the Exchange Act, known by the Company to be the beneficial owner of more than five percent of its outstanding common stock. The percentage ownership of each beneficial owner is based upon 24,246,165 shares of common stock issued and outstanding as of October 1, 2008 plus shares issuable upon exercise of options, warrants or convertible securities (exercisable within 60 days after said date) that are held by such person or entity, but not those held by any other person or entity. The information presented in this table is based upon the most recent filings with the Commission by such persons or upon information otherwise provided by such persons to the Company.
Name and address of beneficial owner | | Amount and nature of beneficial ownership(1) | | |
Warren G. Lichtenstein c/o Steel Partners II, L.P. 590 Madison Avenue 32nd Floor New York, NY 10022 | | 5,037,943(2) | | | 20.78% | |
| | | | | | | |
Wellington Management Co. LLP 75 State Street Boston, MA 02109 | | 3,191,147(3) | | | 13.16% | |
| | | | | | | |
Wellington Trust Company NA c/o Wellington Management Co. LLP 75 State Street Boston, MA 02109 | | 1,761,241(4) | | | 7.26% | |
| | | | | | | |
Royce & Associates, LLC 1414 Avenue of the Americas New York, NY 10019 | | 1,521,085(5) | | | 6.27% | |
| | | | | | | |
| | (1) | | Unless otherwise noted, each beneficial owner has sole voting and investment power with respect to the shares shown as beneficially owned by him or it. | |
| | (2) | | According to information contained in Amendment No. 12 to Schedule 13D/A, dated December 28, 2007, and filed jointly on December 31, 2007 with the SEC by Mr. Lichtenstein, Steel Partners II, L.P., a Delaware limited partnership ("Steel Partners II"), Steel Partners II GP LLC, a Delaware limited liability company ("Steel GP LLC"), Steel Partners II Master Fund L.P., a Cayman Islands exempted limited partnership ("Steel Master") and Steel Partners LLC, a Delaware limited liability company ("Partners LLC"). Steel Partners II owns 5,037,943 shares of the Common Stock and Steel GP LLC, Steel Master, Partners LLC and Mr. Lichtenstein may be deemed to beneficially own such shares. Steel Master is the sole limited partner of Steel Partners II. Steel GP LLC is the general partner of Steel Partners II and Steel Master. Partners LLC is the investment manager of Steel Partners II and Steel Master. Mr. Lichtenstein is the manager of Partners LLC and the managing member of Steel GP LLC. By virtue of his positions with Steel GP LLC and Partners LLC, Mr. Lichtenstein may be deemed to have the sole power to vote and dispose of the 5,037,943 shares of our Common Stock owned by Steel Partners II. | |
| | (3) | | According to information contained in Amendment No. 8 to a Schedule 13G dated February 14, 2008, Wellington Management Company, LLP (“Wellington”), an investment advisor registered under the Investment Act, may be deemed the beneficial owner of 3,191,147 shares of Common Stock of the Company. Clients of Wellington are the owners of record of the shares held by Wellington. Accordingly, in its role as investment advisor, Wellington has shared power to vote as to 2,120,991 of our Common Stock and shared power to dispose of all 3,191,147 shares of our Common Stock beneficially owned by Wellington. | |
| | (4) | | According to information contained in a Schedule 13G dated February 14, 2008, Wellington Trust Company NA, a bank as defined by the Investment Act, may be deemed the beneficial owner of 1,761,241 shares of Common Stock of the Company. Clients of Wellington Trust are the owners of record of the shares held by Wellington Trust. Accordingly, in its role as investment advisor, Wellington Trust has shared power to vote and dispose 1,761,241 of our Common Stock beneficially owned by Wellington Trust. | |
| | (5) | | According to information contained in Amendment No. 5 to a Schedule 13G dated January 28, 2008, Royce & Associates, LLC, , an investment advisor registered under the Investment Act, may be deemed the beneficial owner of 1,521,085 shares of Common Stock of the Company. | |
SECURITY OWNERSHIP OF DIRECTORS AND MANAGEMENT
The following table sets forth information concerning beneficial ownership of Common Stock of the Company outstanding at October 1, 2008 by (i) each director; (ii) each executive officer of the Company and (iii) by all directors and executive officers of the Company as a group. The percentage ownership of each beneficial owner is based upon 24,246,165 shares of Common Stock issued and outstanding as of October 1, 2008, plus shares issuable upon exercise of options, warrants or convertible securities (exercisable within 60 days after said date) that are held by such person or entity, but not those held by any other person or entity. The information presented in this table is based upon the most recent filings with the Commission by such persons or upon information otherwise provided by such persons to the Company
Name and address of beneficial owner | | Amount and nature of beneficial ownership(1) | | | | |
Mark A Koch(4) | | | 10,000 | (2) | | | * | |
| Mark A. Zorko | | | 90,000 | (2) | | | * | |
| Gerald M. Czarnecki | | | 75,543 | (2) | | | * | |
| James A. Risher | | | 147,500 | (2) | | | * | |
James R. Henderson(3) | | | 85,750 | (2) | | | * | |
| Merrill A. McPeak | | | 83,366 | (2) | | | * | |
| All Directors and Named Executive Officers as a group (6 persons) | | | 492,159 | (2) | | | 2.00 | % |
| * | Represents less than 1% of the outstanding shares of our Common Stock |
| | |
| (1) | Unless otherwise noted, each director and executive officer has sole voting and investment power with respect to the shares shown as beneficially owned by him. |
| | |
| (2) | Includes shares of our common stock which may be acquired upon the exercise of stock options which are presently exercisable or will become exercisable within 60 days of October 1, 2008, in the following amounts: Mark A. Koch — 10,000, Mark A. Zorko — 70,000, Gerald M. Czarnecki — 43,750, James A. Risher — 147,500, James R. Henderson — 85,750 and Merrill A. McPeak 42,250. |
| | |
| (3) | Mr. Henderson is a Vice President of Steel Partners, Ltd., an entity of which Warren G. Lichtenstein is an affiliate by virtue of his ownership of Steel Partners, Ltd. directly and through Steel Partners II, L.P. (collectively, the “Group”). Mr. Henderson disclaims beneficial ownership of the 5,037,943 shares of our common stock collectively owned by the Group. |
| | |
| (4) | Mr. Koch resigned as Treasurer and Principal Accounting Officer, effective October 30, 2006. |
| CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
REVIEW, APPROVAL OR RATIFICATION OF TRANSACTIONS WITH RELATED PERSONS
During fiscal 2008, the Company had a policy for the review of transactions in which the Company was a participant, the amount involved exceeded the lesser of $120,000 or one percent of the average of the Company's total assets at year end for the last two completed fiscal years and in which any of the Company’s directors or executive officers, or their immediate family members, had a direct or indirect material interest. Any such related person transaction was to be for the benefit of the Company and upon terms no less favorable to the Company than if the related person transaction was with an unrelated party. While this policy was not in writing during fiscal 2008, the Company's Board of Directors was responsible for approving any such transactions and the CEO was responsible for maintaining a list of all existing related person transactions. The Company had no transactions, nor are there any currently proposed transactions, in which the Company was or is to be a participant, where the amount involved exceeded the lesser of $120,000 or one percent of the average of the Company's total assets at year end for the last two completed fiscal years, and any director, executive officer or any of their immediate family members had a material direct or indirect interest reportable under applicable SEC rules or that required approval of the Board of Directors under the Company’s Related Person Transaction Policy.
DIRECTOR INDEPENDENCE
Although the Company is currently not listed on any exchange, the Board of Directors has determined that three of the members of the Board of Directors, Mr. Czarnecki , Mr. Henderson and Gen. McPeak, are "independent" as defined in Rule 4200 of the Marketplace Rules of the National Association of Securities Dealers, Inc. Committee membership of the Company’s directors is as follows:
| Director | Audit Committee | Compensation Committee | Nominating and Governance Committee |
| Gerald M. Czarnecki* | Chair | X | X |
| James R. Henderson * | | X | Chair |
| General Merrill A. McPeak* | X | Chair | X |
*Independent
| PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Audit Fees- The aggregate fees billed by BDO Seidman, LLP for professional services rendered for the audit of our annual financial statements set forth in our Annual Report on Form 10-K for the fiscal years ended August 2, 2008 and July 28, 2007, for the reviews of the interim financial statements included in our Quarterly Reports on Form 10-Q for those fiscal years and for assistance with other registration statement filings made by the Company during those fiscal years were $323,755, and $290,650, respectively.
Audit-Related Fees- There were no fees billed by BDO Seidman, LLP for Audit-Related services for the fiscal year ended July 28, 2007.
Tax Fees -The aggregate fees billed by BDO Seidman, LLP for tax services for the fiscal years ended August 2, 2008 and July 28, 2007 were $0 and $71,665, respectively. These fees related to tax planning and consulting work.
All Other Fees - For the fiscal year ended August 2, 2008, fees billed by BDO Seidman, LLP for due diligence related services related to a potential business acquisition was approximately $99,600. There were no fees for other professional services rendered during the fiscal years ended August 2, 2008 and July 28, 2007.
The Audit Committee’s policy is to pre-approve services to be performed by the Company’s independent public accountants in the categories of audit services, audit-related services, tax services and other services. Additionally, the Audit Committee will consider on a case-by-case basis and, if appropriate, approve specific engagements that are not otherwise pre-approved. The Audit Committee has approved all fees and advised us that it has determined that the non-audit services rendered by BDO Seidman, LLP during our most recent fiscal year are compatible with maintaining the independence of such auditors.
| EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| | PAGE NUMBER |
| (a) 1. FINANCIAL STATEMENTS | |
| CONSOLIDATED FINANCIAL STATEMENTS OF DEL GLOBAL TECHNOLOGIES CORP. AND SUBSIDIARIES: | |
| Report of Independent Registered Public Accounting Firm | F-1 |
| Consolidated Balance Sheets as of August 2, 2008 and July 28, 2007 | F-2 |
| Consolidated Statements of Operations for the Fiscal Years Ended August 2, 2008, July 28, 2007 and July 29, 2006 | F-3 |
| Consolidated Statements of Cash Flows for the Fiscal Years Ended August 2, 2008, July 28, 2007 and July 29, 2006 | F-4 |
| Consolidated Statements of Shareholders’ Equity for the Fiscal Years Ended August 2, 2008, July 28, 2007 and July 29, 2006 | F-5 |
| Notes to Consolidated Financial Statements for the Fiscal Years Ended August 2, 2008, July 28, 2007 and July 29, 2006 | F-6 – F-22 |
3. EXHIBITS
EXHIBIT NUMBER | | DESCRIPTION OF DOCUMENT |
2.1 | | Stock Purchase Agreement (related to the acquisition of Villa Sistemi Medicali S.p.A.) dated as of December 28, 1999. Filed as Exhibit 2.1 to Del Global Technologies Corp. Current Report on Form 8-K dated May 4, 2000 and incorporated herein by reference. |
| | | |
2.2 | | Asset Purchase Agreement dated as of October 1, 2004 by and between Spellman High Voltage Electronics Corporation and Del Global Technologies Corp. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed October 7, 2004 and incorporated herein by reference. |
| | | |
3.1 | | Certificate of Incorporation dated October 25, 1954. Filed as Exhibit to Del Electronics Corp. Registration Statement on Form S-1 (No. 2-16839) and incorporated herein by reference. |
| | | |
3.2 | | Certificate of Amendment of Certificate of Incorporation dated January 26, 1957. Filed as Exhibit to Del Electronics Corp. Registration Statement on Form S-1 (No. 2-16839) and incorporated herein by reference. |
| | | |
3.3 | | Certificate of Amendment of Certificate of Incorporation dated July 12, 1960. Filed as Exhibit to Del Electronics Corp. Registration Statement on Form S-1 (No. 2-16839) and incorporated herein by reference. |
| | | |
3.4 | | Certificate of Amendment of Certificate of Incorporation dated March 18, 1985. Filed as Exhibit 3.5 to Del Electronics Corp. Form 10-K for the year ended August 2, 1989 and incorporated herein by reference. |
| | | |
3.5 | | Certificate of Amendment of Certificate of Incorporation dated January 19, 1989. Filed as Exhibit 4.5 to Del Electronics Corp. Form S-3 (No. 33-30446) filed August 10, 1989 and incorporated herein by reference. |
| | | |
3.6 | | Certificate of Amendment of the Certificate of Incorporation of Del Electronics Corp., dated February 5, 1991. Filed with Del Electronics Corp. Proxy Statement dated January 22, 1991 and incorporated herein by reference. |
| | | |
3.7 | | Certificate of Amendment of the Certificate of Incorporation of Del Electronics Corp. dated February 14, 1996. Filed as Exhibit 3.6 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 1, 1998 and incorporated herein by reference. |
| | | |
3.8 | | Certificate of Amendment of Certificate of Incorporation of Del Global Technologies Corp. dated February 13, 1997. Filed as Exhibit 3.1 to Quarterly Report on Form 10-Q for the quarter ended February 1, 1997 and incorporated herein by reference. |
3.9 | | Amended and Restated By-Laws of Del Global Technologies Corp. Filed as Exhibit 3.1 to Current Report on Form 8-K dated September 5, 2001 and incorporated herein by reference. |
| | | |
3.10 | | Amendment No. 1 to the Amended and Restated By-Laws of Del Global Technologies Corp. dated July 17, 2003. Filed as Exhibit 3.01 to Current Report on Form 8-K dated July 30, 2003 and incorporated herein by reference. |
| | | |
| 3.11 | | Certificate of Amendment at the Certificate of Incorporation of Del Global Technologies Corp. dated November 17, 2006. Filed as Exhibit 3.01 to Current Report on Form 8-K filed November 22, 2006 and incorporated herein by reference. |
| 3.12 | | Amendment No. 2 to the Amended and Restated By-Laws of Del Global Technologies Corp. dated December 5, 2007. Filed as Exhibit 3.1 to Current Report on Form 8-K dated December 6, 2007 and incorporated herein by reference. |
| 4.1 | | INTENTIONALLY OMITTED. |
| | | |
4.2 | | INTENTIONALLY OMITTED. |
| | | |
4.8 | | Warrant Certificate of Laurence Hirschhorn. Filed as Exhibit 4.1 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarter ended January 29, 2000 and incorporated herein by reference. |
| | | |
4.9 | | Warrant Certificate of Steven Anreder. Filed as Exhibit 4.2 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarter ended January 29, 2000 and incorporated herein by reference. |
| | | |
4.10 | | Warrant Certificate of UBS Capital S.p.A. dated as of December 28, 1999. Filed as Exhibit 4 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarter ended January 29, 2000 and incorporated herein by reference. |
| | | |
4.11* | | Del Global Technologies Corp. Amended and Restated Stock Option Plan (as adopted effective as of January 1, 1994 and as amended December 14, 2000). Filed as Exhibit 4.11 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 3, 2002 and incorporated herein by reference. |
| | | |
4.12* | | Stock Purchase Plan. Filed as Exhibit 4.9 to Del Electronics Corp. Annual Report on Form 10-K for the year ended July 29, 1989 and incorporated herein by reference. |
| | | |
4.13* | | Option Agreement, substantially in the form used in connection with options granted under the Plan. Filed as Exhibit 4.8 to Del Electronics Corp. Annual Report on Form 10-K for the year ended July 29, 1989 and incorporated herein by reference. |
| | | |
4.14* | | Option Agreement dated as of December 28, 1999. Filed as Exhibit 4.2 to Del Global Technologies Corp. Current Report on Form 8-K dated May 4, 2000 and incorporated herein by reference. |
| | | |
4.15 | | Warrant Agreement substantially in the form used for 1,000,000 warrants issued in connection with the settlement of the Class Action Lawsuit on January 29, 2002. Filed as Exhibit 10.12 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 3, 2002 and incorporated herein by reference. |
| | | |
4.16* | | Amendment No. 1 dated July 17, 2003 to the Del Global Technologies Corp. Amended and Restated Stock Option Plan (as adopted effective as of January 1, 1994 and as amended December 14, 2000). Filed as Exhibit 4.1 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarterly period ended November 1, 2003 and incorporated herein by reference. |
| | | |
4.17* | | Amendment No. 2 dated July 7, 2005 to the Del Global Technologies Corp. Amended and Restated Stock Option Plan (as adopted effective as of January 1, 1994 and as amended December 14, 2000 and July 17, 2003). Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K dated July 7, 2005 and incorporated herein by reference. |
| | | |
4.18 | | Stock Purchase Agreement dated as of December 22, 2005 by and among Del Global Technologies Corp. and Mr. Giuseppe Carmelo Ammendola, Mr. Emilio Bruschi, Mr. Roberto Daglio and Mr. Luigi Emmanuele Filed as Exhibit 10.1 to Del Global Technologies Corp. Current Report on Form 8-K filed December 28, 2005 and incorporated herein by reference. |
| 4.19 | | Rights Agreement, dated as of January 22, 2007, and between Del Global Technologies Corp. and Mellon investor Services LLC, as rights agent (including as Exhibit A the Form of Right Certificate and as Exhibit B the Summary of Rights to Purchase Common Stock). Filed as Exhibit 4.1 to Del Global Technologies Corp. Current Report on Form 8-K filed January 23, 2007 and incorporated herein by reference. |
| | | |
| 4.20 | | Joinder Agreement, dated June 27, 2007, between Del Global Technologies Corp. and Continental Stock Transfer & Trust Company. Filed as Exhibit 4.1 to Del Global Technologies Corp. Current Report on Form 8-K filed June 27, 2007 and incorporated herein by reference. |
| | | |
| 4.21* | | 2007 Incentive Stock Plan, dated February 22, 2007. Filed as Exhibit 4.21 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended July 28, 2007 and incorporated herein by reference. |
| | | |
10.2 | | INTENTIONALLY OMITTED. |
| | | |
10.3 | | INTENTIONALLY OMITTED. |
| | | |
10.4 | | INTENTIONALLY OMITTED. |
| | | |
10.5 | | INTENTIONALLY OMITTED. |
| | | |
10.6 | | INTENTIONALLY OMITTED. |
| | | |
10.7 | | Lease Agreement dated April 7, 1992 between Messenger Realty and Del Electronics Corp. Filed as Exhibit 6(a) to Del Electronics Corp. Quarterly Report on Form 10-Q for the quarter ended May 2, 1992 and incorporated herein by reference. |
| | | |
10.8 | | Lease and Guaranty of Lease dated May 25, 1994 between Leshow Enterprises and Bertan High Voltage Corp. Filed as Exhibit 2.5 to Del Electronics Corp. Current Report on Form 8-K dated June 10, 1994 and incorporated herein by reference. |
| | | |
10.9 | | Lease dated January 4, 1993 between Curto Reynolds Oelerich Inc. and Del Medical Imaging Corp. (formerly knows as Gendex-Del Medical Imaging Corp.). Filed as Exhibit 10.21 to the Del Global Technologies Corp. Registration Statement on Form S-2 (No. 333-2991) dated April 30, 1997 and incorporated herein by reference. |
| | | |
10.10 | | Loan and Security Agreement dated June 10, 2002, in the principal amount of $10,000,000, between Del Global Technologies Corp., Bertan High Voltage Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and Transamerica Business Capital Corporation. The Company agrees to furnish supplementally a copy of any omitted exhibits or schedules to the SEC upon request. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed on November 4, 2002 and incorporated herein by reference. |
| | | |
10.11 | | Subordinated Promissory Note substantially in the form used for a total principal amount of $2 million issued in connection with the settlement of the Class Action Lawsuit on January 29, 2002. Filed as Exhibit 10.11 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 3, 2002 and incorporated herein by reference. |
| | | |
10.12 | | INTENTIONALLY OMITTED. |
| | | |
10.13* | | Executive Employment Agreement dated May 1, 2001, by and between Del Global Technologies Corp. and Samuel E. Park. Filed as Exhibit 99.1 to Del Global Technologies Corp. Current Report on Form 8-K filed on August 1, 2001 and incorporated herein by reference. |
| | | |
10.14* | | Change of Control Agreement substantially in the form used by the Company for the current executive officers as named in Item 11, except for Samuel E. Park (see Exhibit 10.13). Filed as Exhibit 10.14 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 3, 2002 and incorporated herein by reference. |
| | | |
10.15 | | Extension and Modification Agreement (lease agreement) dated as of July 30, 2002 between Praedium II Valhalla LLC and Del Global Technologies Corp. Filed as Exhibit 10.15 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 3, 2002 and incorporated herein by reference. |
| | | |
10.16 | | Grant Decree No. 0213 between the Ministry of Industry, Trade and Handicrafts and Villa Sistemi Medicali S.p.A. dated September 6, 1995. Filed as Exhibit 10.16 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 3, 2002 and incorporated herein by reference. |
| | | |
10.17 | | Financial Property Lease Contract no. 21136 dated March 30, 2000 between ING Lease (Italia) S.p.A. and Villa Sistemi Medicali S.p.A. Filed as Exhibit 10.17 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 3, 2002 and incorporated herein by reference. |
| | | |
10.18 | | Declaration of Final Obligation between the Ministry of Productive Industry and Villa Sistemi Medicali S.p.A. dated May 6, 2002. Filed as Exhibit 10.18 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 3, 2002 and incorporated herein by reference. |
| | | |
10.19 | | Private Contract between Banca Mediocredito S.p.A and Villa Sistemi Medicali S.p.A. dated November 4, 1998 in the principal amount of 3 billion Lire. Filed as Exhibit 10.19 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 3, 2002 and incorporated herein by reference. |
| | | |
10.20* | | Change of Control Agreement as approved by the Board of Directors on October 24, 2002, substantially in the form used by its current executive officers (in the case of Walter F. Schneider, as amended pursuant to Exhibit 10.22 hereof). Filed as Exhibit 10.20 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 3, 2002 and incorporated herein by reference. |
| | | |
10.21 | | Waiver and First Amendment to Loan and Security Agreement dated as of November 1, 2002 among Del Global Technologies Corp., Bertan High Voltage Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and Transamerica Business Capital Corporation. Filed as Exhibit 99.02 to Del Global Technologies Corp. Current Report on Form 8-K filed on November 4, 2002 and incorporated herein by reference. |
| | | |
10.22 | | Second Amendment to the Loan and Security Agreement dated December 17, 2002 among Del Global Technologies Corp., Bertan High Voltage Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and Transamerica Business Capital Corporation. Filed as Exhibit 10.1 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarter ended November 2, 2002 and incorporated herein by reference. |
| | | |
10.23 | | Settlement Agreement and Release dated March 10, 2003 by and between Del Global Technologies Corp. and its affiliates, subsidiaries, present and former directors, officers, agents, accountants, attorneys, stockholders, predecessors and the agents and attorneys of its present and former directors, and Leonard A. Trugman and each of his heirs, administrators, liquidators, executors, successors, and assigns. Filed as Exhibit 10.22 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarter ended February 1, 2003 and incorporated herein by reference. |
| | | |
10.24 | | Separation Agreement and General Release of Claims dated April 9, 2003, by and between James M. Tiernan and Del Global Technologies Corp. Filed as Exhibit 99.01 to Del Global Technologies Corp. Amendment to Current Report on Form 8-K/A filed on April 23, 2003 and incorporated herein by reference. |
| | | |
10.25 | | Separation Agreement and General Release of Claims dated April 9, 2003, by and between David Michael, David Michael & Co., P.C. and Del Global Technologies Corp. Filed as Exhibit 99.02 to Del Global Technologies Corp. Amendment to Current Report on Form 8-K/A filed on April 23, 2003 and incorporated herein by reference. |
| | | |
10.26 | | Form of Indemnification Agreement. Filed as Exhibit 10.22 to Del Global Technologies Corp. Amendment #1 to Registration Statement on Form S-1/A, filed on May 1, 2003 and incorporated herein by reference. |
| | | |
10.27 | | Amendment to Executive Employment Agreement dated May 28, 2003 by and between Del Global Technologies Corp. and Samuel E. Park. Filed as Exhibit 10.23 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarterly period ended May 3, 2003 and incorporated herein by reference. |
| | | |
10.28 | | Amendment dated October 10, 2003 to Change of Control Agreement for Walter F. Schneider filed as Exhibit 10.28 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 2, 2003 and incorporated herein by reference. |
| | | |
10.29 | | Waiver and Third Amendment to the Loan and Security Agreement dated as of October 30, 2003, among Del Global Technologies Corp., Bertan High Voltage Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and Transamerica Business Capital Corporation filed as Exhibit 10.29 to Del Global Technologies Corp. Annual Report on Form 10-K for the year ended August 2, 2003 and incorporated herein by reference. |
| | | |
10.30 | | Waiver, Consent and Fourth Amendment to the Loan and Security Agreement dated as of March 12, 2004, by and among Del Global Technologies Corp. and General Electric Capital Corporation, as successor by assignment to Transamerica Business Corporation. Filed as Exhibit 10.30 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2004 and incorporated herein by reference. |
| | | |
10.31* | | Letter Agreement dated as of February 10, 2003 between Mark Koch and Del Global Technologies Corp. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed August 27, 2004 and incorporated herein by reference. |
| | | |
10.32 | | Non-Competition Agreement dated as of September 8, 2004 by and between Del Global Technologies Corp. and Walter F. Schneider. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed September 10, 2004 and incorporated herein by reference. |
| | | |
10.33 | | Separation Agreement and Release dated as of September 1, 2004 between Del Global Technologies Corp. and Thomas V. Gilboy. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed September 15, 2004 and incorporated herein by reference. |
| | | |
10.34 | | Amendment No. 1 dated as of September 15, 2004 to the Letter Agreement dated February 10, 2003 between Mark Koch and Del Global Technologies Corp. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed September 20, 2004 and incorporated herein by reference. |
| | | |
10.35 | | Loan Agreement dated as of September 23, 2004 between Del Global Technologies Corp. (“Del Global”) and Villa Sistemi Medicali S.p.A., a subsidiary of Del Global. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed September 28, 2004 and incorporated herein by reference. |
| | | |
10.36 | | Waiver, Consent and Fifth Amendment to the Loan and Security Agreement dated as of September 23, 2004, by and among Del Global Technologies Corp., Bertan High Voltage Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and General Electric Capital Corporation, as successor by assignment to Transamerica Business Capital Corporation. Filed as Exhibit 99.02 to Del Global Technologies Corp. Current Report on Form 8-K filed September 28, 2004 and incorporated herein by reference. |
| | | |
10.37 | | Settlement Agreement dated as of September 30, 2004, by and among the United States of America, on behalf of the Department of Defense, acting through the United States Attorney’s Office for the Eastern District of New York, Del Global Technologies Corp. and RFI Corporation. Current Report on Form 8-K filed October 5, 2004 and incorporated herein by reference. |
| | | |
10.38 | | Assignment, Assumption and Amendment of Lease dated as of October 1, 2004 among DP 16, LLC, Del Global Technologies Corp. and Spellman High Voltage Electronics Corporation. Filed as Exhibit 99.02 to Del Global Technologies Corp. Current Report on Form 8-K filed October 7, 2004 and incorporated herein by reference. |
| | | |
10.39 | | First Amendment to Villa Loan Agreement dated October 22, 2004 between Del Global Technologies Corp and Villa Sistemi Medicali, S.p.A filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed October 26, 2004 and incorporated herein by reference. |
| | | |
10.40 | | Sixth Amendment to the Loan and Security Agreement dated as of October 25, 2004 by and among Del Global Technologies Corp, Bertan High Voltage Corp, RFI Corporation and Del Medical Imaging Corp (Borrowers) and General Electric Capital Corporation as successor to Transamerica Business Capital Corporation filed as Exhibit 99.02 to Del Global Technologies Corp. Current Report on Form 8-K filed October 26, 2004 and incorporated herein by reference. |
| | | |
10.41 | | Consent and Seventh Amendment to the Loan and Security Agreement dated as of February 2, 2005, among Del Global Technologies Corp., Bertan High Voltage Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and GE Business Capital Corporation F/K/A Transamerica Business Capital Corporation filed as Exhibit 99.1 to Del Global Technologies Corp. Current Report on Form 8-K filed February 7, 2005 and incorporated herein by reference. |
| | | |
10.42 | | Administrative Agreement dated as of April 1, 2005 between Del Global Technologies Corp., RFI Corporation and the Defense Logistics Agency. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed April 5, 2005 and incorporated herein by reference. |
| | | |
10.43 | | Consent and Eighth Amendment to the Loan and Security Agreement dated as of April 5, 2005, among Del Global Technologies Corp., Bertan High Voltage Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and GE Business Capital Corporation F/K/A Transamerica Business Capital Corporation filed as Exhibit 99.02 to Del Global Technologies Corp. Current Report on Form 8-K filed April 5, 2005 and incorporated herein by reference. |
| | | |
10.44* | | Senior Management Incentive Plan filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed May 3, 2005 and incorporated herein by reference. |
| | | |
10.45* | | Severance Benefits Letter Agreement dated as of May 23, 2005 between Del Global Technologies Corp. and Walter F. Schneider. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed May 25, 2005 and incorporated herein by reference. |
| | | |
10.46* | | Severance Benefits Letter Agreement dated as of May 23, 2005 between Del Global Technologies Corp. and Mark A. Koch. Filed as Exhibit 99.02 to Del Global Technologies Corp. Current Report on Form 8-K filed May 25, 2005 and incorporated herein by reference. |
| | | |
10.47 | | Separation Agreement and Release dated as of April 1, 2005 between Del Global Technologies Corp. and Edward Ferris filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed June 6, 2005 and incorporated herein by reference. |
| | | |
10.48 | | Waiver and Ninth Amendment to the Loan and Security Agreement dated as of June 9, 2005, among Del Global Technologies Corp., Bertan High Voltage Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and GE Business Capital Corporation F/K/A Transamerica Business Capital Corporation filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed June 9, 2005 and incorporated herein by reference. |
| | | |
10.49 | | Loan and Security Agreement dated as of August 1, 2005 among Del Global Technologies Corp., RFI Corporation, Del Medical Imaging Corp. and North Fork Business Capital Corporation. Filed as Exhibit 10.01 to Del Global Technologies Corp. Current Report on Form 8-K filed August 3, 2005 and incorporated herein by reference. |
| | | |
10.50 | | Second Amendment to Villa Loan Agreement dated August 1, 2005 between Del Global Technologies Corp and Villa Sistemi Medicali, S.p.A filed as Exhibit 10.02 to Del Global Technologies Corp. Current Report on Form 8-K filed August 3, 2005 and incorporated herein by reference. |
| | | |
10.51 | | Waiver and First Amendment to the Loan and Security Agreement dated as of December 12, 2005 among Del Global Technologies Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and North Fork Business Capital Corporation. Filed as Exhibit 10.51 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarterly period ended October 29, 2005 and incorporated herein by reference. |
| | | |
10.52 | | Waiver to the Loan and Security Agreement dated as of March 14, 2006 among Del Global Technologies Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and North Fork Business Capital Corporation. Filed as Exhibit 10.52 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarterly period ended January 28, 2006 and incorporated herein by reference. |
| | | |
10.53* | | Separation Agreement and Release dated as of March 21, 2006 by and between Del Global Technologies Corp. and Christopher N. Japp. Filed as Exhibit 99.1 to Del Global Technologies Corp. Current Report on Form 8-K filed March 24, 2006 and incorporated herein by reference. |
| | | |
10.54 | | Waiver to the Loan and Security Agreement dated as of June 13, 2006 by and among Del Global Technologies Corp., Del Medical Imaging Corp., RFI Corporation (Borrowers) and North Fork Business Capital Corporation. Filed as Exhibit 10.53 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarterly period ended April 29, 2006 and incorporated herein by reference. |
| | | |
10.55 | | Consulting Agreement dated as of June 14, 2006 by and between Del Global Technologies Corp. and Lumina Group LLC. Filed as Exhibit 99.1 to Del Global Technologies Corp. Current Report on Form 8-K filed June 30, 2006 and incorporated herein by reference. |
| | | |
10.56 | | Second Amendment to the Loan and Security Agreement dated as of June 30, 2006 among Del Global Technologies Corp., RFI Corporation and Del Medical Imaging Corp. (Borrowers) and North Fork Business Capital Corporation. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed July 7, 2006 and incorporated herein by reference. |
| | | |
10.57* | | Separation Agreement and Release dated as of July 24, 2006 by and between Del Global Technologies Corp. and Walter F. Schneider. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed July 24, 2006 and incorporated herein by reference. |
| | | |
10.58* | | Letter Agreement dated as of August 31, 2006 between Del Global Technologies Corp. and James A. Risher. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed August 31, 2006 and incorporated herein by reference. |
| | | |
10.59* | | Letter Agreement dated as of August 30, 2006 between Del Global Technologies Corp. and Mark Zorko. Filed as Exhibit 99.02 to Del Global Technologies Corp. Current Report on Form 8-K filed August 31, 2006 and incorporated herein by reference. |
| | | |
10.60 | | Full-Time Permanent Engagement Resources Agreement dated as of August 21, 2006 between Del Global Technologies Corp. and Tatum, LLC. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed August 31, 2006 and incorporated herein by reference. |
| | | |
10.61* | | Separation Agreement and Release dated as of September 7, 2006 by and between Del Global Technologies Corp. and Mark A. Koch. Filed as Exhibit 99.01 to Del Global Technologies Corp. Current Report on Form 8-K filed September 7, 2006 and incorporated herein by reference. |
| | | |
10.62 | | Waiver and Third Amendment to the Loan and Security Agreement dated as of October 25, 2006 by and among Del Global Technologies Corp., Del Medical Imaging Corp., RFI Corporation (Borrowers) and North Fork Business Capital Corporation. Filed as Exhibit 10.62 to Del Global Technologies Corp. Annual Report on Form 10-K filed October 27, 2006 and incorporated herein by reference. |
| | | |
| 10.63 | | Waiver and Fourth Amendment to the Loan and Security Agreement dated as of December 6, 2006 by and among Del Global Technologies Corp., Del Medical Imaging Corp., RFI Corporation (Borrowers) and North Fork Business Capital Corporation. Filed as Exhibit 10.63 to Del Global Technologies Corp. Quarterly Report on Form 10-Q for the quarterly period ended October 28, 2006 and incorporated herein by reference. |
| | | |
| 10.64 | | Amendment No. 5 dated as of January 18, 2007 to the Loan and Security Agreement by and among the registrant, RFI Corporation, Del Medical Imaging Corp. and North Fork Business Capital Corporation, dated as of August 1, 2005. Filed as Exhibit 99.02 to Del Global Technologies Corp. Current Report on Form 8-K filed January 23, 2007 and incorporated herein by reference. |
| | | |
| 10.65 | | Amended and Restated Loan Agreement, dated as of May 25, 2007, among Del Global Technologies Corp., RFI Corporations, Del Medical Imaging Corp. and North Fork Business Capital Corporation. Filed as Exhibit 10.1 to Del Global Technologies Corp. Current Report on Form 8-K filed June 4, 2007 and incorporated herein by reference. |
| | | |
| 10.66* | | Letter Agreement dated as of September 19, 2007 between Del Global Technologies Corp. and James A. Risher. Filed as Exhibit 10.1 to Del Global Technologies Corp. Current Report on Form 8-K filed September 20, 2007 and incorporated herein by reference. |
| | | |
| 10.67 | | First Amendment dated September 3, 2008, to the Amended and Restated Loan and Security Agreement by and among the Del Global Technologies Corp., Del Medical Imaging Corp., RFI Corporation and North Fork Business Capital Corporation, now known as Capital One Leveraged Finance Corp., dated as of May 25, 2007. Filed as Exhibit 10.1 to Del Global Technologies Corp. Current Report on Form 8-K filed September 5, 2008 and incorporated herein by reference. |
| | | |
| 10.68* | | Letter Agreement dated as of September 16, 2008 between Del Global Technologies Corp. and James A. Risher. Filed as Exhibit 10.1 to Del Global Technologies Corp. Current Report on Form 8-K filed September 17, 2008 and incorporated herein by reference. |
| | | |
| 21.1** | | List of Subsidiaries |
| | | |
| 23.1** | | Consent of BDO Seidman, LLP. |
| | | |
| 31.1** | | Certification of Chief Executive Officer, James Risher, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 31.2** | | Certification of Principal Financial Officer, Mark Zorko, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
32.1** | | Certification of the Chief Executive Officer, James Risher, pursuant to 18 USC. Section 1350 adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | | |
32.2** | | Certification of the Principal Financial Officer, Mark Zorko, pursuant to 18 U.S.C. Section 1350 adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | | |
| | | |
| | | |
____________
* Represents a management contract or compensatory plan or arrangement.
** Filed herewith
Pursuant to the requirements of Section 13 or 15(d) of the Securities and Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | DEL GLOBAL TECHNOLOGIES CORP. |
| | |
| | | |
| October 6, 2008 | By: | /s/ James A. Risher |
| | | James A. Risher |
| | | President and Chief Executive Officer |
| | | |
| October 6, 2008 | By: | /s/ Mark A. Zorko |
| | | Mark A. Zorko |
| | | Chief Financial Officer |
Pursuant to the requirements of the Securities and Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| /s/ James R. Henderson | Director – Chairman | October 6, 2008 |
| James Henderson | | |
| | | |
| /s/ Merrill A. McPeak | Director | October 6, 2008 |
| Merrill McPeak | | |
| | | |
| /s/ Gerald M. Czarnecki | Director | October 6, 2008 |
| Gerald M. Czarnecki | | |
| | | |
| /s/ James A. Risher | Director | October 6, 2008 |
| James A. Risher | Chief Executive Officer | |
| | | |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Del Global Technologies Corp.
Franklin Park, Illinois
We have audited the accompanying consolidated balance sheets of Del Global Technologies Corp. and subsidiaries as of August 2, 2008 and July 28, 2007, and the related consolidated statements of operations, shareholders’ equity and cash flows for each of the three years in the period ended August 2, 2008. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Del Global Technologies Corp. and subsidiaries as of August 2, 2008 and July 28, 2007, and the results of their operations and their cash flows for each of the three years in the period ended August 2, 2008, in conformity with accounting principles generally accepted in the United States of America.
/s/ BDO SEIDMAN, LLP
Chicago, Illinois
September 29, 2008
DEL GLOBAL TECHNOLOGIES CORP. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(DOLLARS IN THOUSANDS EXCEPT PAR VALUE)
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | |
| ASSETS | | | | | | |
| CURRENT ASSETS: | | | | | | |
| Cash and cash equivalents | | $ | 7,828 | | | $ | 7,860 | |
| Trade receivables (net of allowance for doubtful accounts of $1,400 and $1,569 for 2008 and 2007, respectively) | | | 25,218 | | | | 21,221 | |
| Inventories (net of allowance for excess and obsolete of $4,435 and $3,869 for 2008 and 2007, respectively) | | | 18,439 | | | | 21,930 | |
| Prepaid expenses and other current assets | | | 2,085 | | | | 1,180 | |
| Total current assets | | | 53,570 | | | | 52,191 | |
| NON-CURRENT ASSETS: | | | | | | | | |
| Property plant and equipment, net | | | 7,377 | | | | 6,511 | |
| Deferred income taxes | | | 770 | | | | 1,011 | |
| Goodwill | | | 4,526 | | | | 6,437 | |
| Other assets | | | 110 | | | | 189 | |
| Total non-current assets | | | 12,783 | | | | 14,148 | |
| TOTAL ASSETS | | $ | 66,353 | | | $ | 66,339 | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | |
| CURRENT LIABILITIES: | | | | | | |
| Current portion of long-term debt | | $ | 1,797 | | | $ | 1,086 | |
| Accounts payable – trade | | | 12,191 | | | | 17,125 | |
| Accrued expenses | | | 8,378 | | | | 7,432 | |
| Income taxes payable | | | - | | | | 1,570 | |
| Total current liabilities | | | 22,366 | | | | 27,213 | |
| NON-CURRENT LIABILITIES: | | | | | | | | |
| Long-term debt, less current portion | | | 4,504 | | | | 5,398 | |
| Deferred income taxes………………………………………………………………………………………... | | | - | | | | 292 | |
| Other long-term liabilities | | | 3,320 | | | | 3,240 | |
| Total non-current liabilities | | | 7,824 | | | | 8,930 | |
| Total liabilities | | | 30,190 | | | | 36,143 | |
| COMMITMENTS AND CONTINGENCIES | | | | | | | | |
| SHAREHOLDERS’ EQUITY: | | | | | | | | |
| Common stock -- $.10 par value; authorized – 50,000,000 shares; issued – 24,897,723 and 24,753,526 shares at August 2, 2008 and July 28, 2007, respectively | | | 2,490 | | | | 2,475 | |
| Additional paid-in capital | | | 80,398 | | | | 79,726 | |
| Treasury shares – 654,464 and 622,770 shares at August 2, 2008 and July 28, 2007, respectively, at cost | | | (5,615 | ) | | | (5,546 | ) |
| Accumulated other comprehensive income | | | 4,252 | | | | 1,880 | |
| Accumulated deficit | | | (45,362 | ) | | | (48,339 | ) |
| Total shareholders’ equity | | | 36,163 | | | | 30,196 | |
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | | $ | 66,353 | | | $ | 66,339 | |
See notes to consolidated financial statements.
DEL GLOBAL TECHNOLOGIES CORP. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(IN THOUSANDS, EXCEPT PER SHARE AMOUNTS)
| | | FISCAL YEARS ENDED | |
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | | | JULY 29, 2006 | |
| NET SALES | | $ | 108,306 | | | $ | 104,167 | | | $ | 83,014 | |
| COST OF SALES | | | 81,519 | | | | 79,150 | | | | 63,656 | |
| GROSS MARGIN | | | 26,787 | | | | 25,017 | | | | 19,358 | |
| Selling, general and administrative | | | 15,586 | | | | 14,590 | | | | 13,619 | |
| Research and development | | | 2,488 | | | | 2,013 | | | | 1,562 | |
| Goodwill impairment | | | 1,911 | | | | - | | | | - | |
| Litigation settlement costs | | | 450 | | | | - | | | | 697 | |
| Total operating expenses | | | 20,435 | | | | 16,603 | | | | 15,878 | |
| OPERATING INCOME | | | 6,352 | | | | 8,414 | | | | 3,480 | |
| Interest expense (net of interest income of $141, $91 and $0 in 2008, 2007 and 2006, respectively) | | | (313 | ) | | | (991 | ) | | | (1,311 | ) |
| Other income (loss) | | | 185 | | | | (54 | ) | | | (34 | ) |
| INCOME FROM CONTINUING OPERATIONS BEFORE INCOME TAXES | | | | | | | | | | | | |
| AND MINORITY INTEREST | | | 6,224 | | | | 7,369 | | | | 2,135 | |
| INCOME TAX PROVISION | | | 3,247 | | | | 3,553 | | | | 1,758 | |
| INCOME FROM CONTINUING OPERATIONS BEFORE MINORITY INTEREST | | | 2,977 | | | | 3,816 | | | | 377 | |
| MINORITY INTEREST | | | - | | | | - | | | | 108 | |
| INCOME FROM CONTINUING OPERATIONS | | | 2,977 | | | | 3,816 | | | | 269 | |
| DISCONTINUED OPERATION | | | - | | | | - | | | | (175 | ) |
| NET INCOME | | $ | 2,977 | | | $ | 3,816 | | | $ | 94 | |
| | | | | | | | | | | | | |
| NET INCOME PER BASIC SHARE | | | | | | | | | | | | |
| Continuing operations | | $ | 0.12 | | | $ | 0.24 | | | $ | 0.02 | |
| Discontinued operation | | | - | | | | - | | | | (0.01 | ) |
| Net income per basic share | | $ | 0.12 | | | $ | 0.24 | | | $ | 0.01 | |
| Weighted average shares outstanding | | | 24,196 | | | | 16,155 | | | | 11,244 | |
| NET INCOME PER DILUTED SHARE | | | | | | | | | | | | |
| Continuing operations | | $ | 0.12 | | | $ | 0.23 | | | $ | 0.02 | |
| Discontinued operation | | | - | | | | - | | | | (0.01 | ) |
| Net income per diluted share | | $ | 0.12 | | | $ | 0.23 | | | $ | 0.01 | |
| Weighted average shares outstanding | | | 24,646 | | | | 16,455 | | | | 12,076 | |
| | | | | | | | | | | | | |
See notes to consolidated financial statements.
DEL GLOBAL TECHNOLOGIES CORP. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(DOLLARS IN THOUSANDS)
| | | FISCAL YEARS ENDED | |
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | | | JULY 29, 2006 | |
| CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | | | | |
| Net income | | $ | 2,977 | | | $ | 3,816 | | | $ | 94 | |
| Loss on discontinued operation | | | - | | | | - | | | | 175 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
| Depreciation and amortization | | | 1,076 | | | | 899 | | | | 1,026 | |
| Deferred income tax provision (benefit) | | | 384 | | | | 229 | | | | (277 | ) |
| Loss on sale of property plant and equipment | | | 3 | | | | 65 | | | | 161 | |
| Non cash litigation settlement costs | | | 450 | | | | - | | | | 455 | |
| Imputed interest - subordinated note | | | - | | | | 185 | | | | 257 | |
| Minority interest | | | - | | | | - | | | | 108 | |
| Stock based compensation expense | | | 481 | | | | 220 | | | | 142 | |
| Goodwill impairment | | | 1,911 | | | | - | | | | - | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Trade receivables | | | (1,832 | ) | | | (2,814 | ) | | | (2,707 | ) |
| Inventories | | | 5,667 | | | | (4,519 | ) | | | (1,180 | ) |
| Prepaid expenses and other current assets | | | (333 | ) | | | (328 | ) | | | (68 | ) |
| Other assets | | | 85 | | | | 50 | | | | 11 | |
| Accounts payable – trade | | | (6,563 | ) | | | 5,331 | | | | 1,486 | |
| Accrued expenses | | | 241 | | | | (654 | ) | | | 1,038 | |
| Payment of accrued litigation settlement costs | | | (390 | ) | | | (200 | ) | | | (311 | ) |
| Income taxes payable | | | (2,003 | ) | | | 1,573 | | | | (177 | ) |
| Other long-term liabilities | | | (428 | ) | | | 142 | | | | 96 | |
| Net cash provided by operating activities | | | | | | | | | | | | |
| of continuing operations | | | 1,726 | | | | 3,995 | | | | 329 | |
| Net cash used in operating activities of discontinued operation | | | - | | | | - | | | | (175 | ) |
| Net cash provided by all operating activities | | | 1,726 | | | | 3,995 | | | | 154 | |
CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | | | | | |
| Property plant and equipment purchases | | | (1,208 | ) | | | (779 | ) | | | (765 | ) |
| Acquisition of minority interest | | | - | | | | - | | | | (2,612 | ) |
| Net cash used in investing activities | | | (1,208 | ) | | | (779 | ) | | | (3,377 | ) |
| | | | | | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | | | | | |
| Borrowing under short-term credit facilities | | | - | | | | 37,193 | | | | 39,112 | |
| Repayment under short-term credit facilities | | | - | | | | (43,247 | ) | | | (38,303 | ) |
| Borrowings of long-term debt | | | - | | | | 3,113 | | | | 2,000 | |
| Repayment of long-term debt | | | (1,184 | ) | | | (5,900 | ) | | | (972 | ) |
| Proceeds from rights offering, net of related costs | | | - | | | | 12,354 | | | | - | |
| Proceeds from warrant exercises | | | 91 | | | | 551 | | | | 2 | |
| Proceeds of stock option exercises | | | 46 | | | | 170 | | | | 238 | |
| Dividend paid to minority shareholders | | | - | | | | (16 | ) | | | - | |
| Net cash (used in) provided by financing activities of continuing operations | | | (1,047 | ) | | | 4,218 | | | | 2,077 | |
| EFFECT OF EXCHANGE RATE CHANGES ON CASH | | | 497 | | | | 93 | | | | 13 | |
| CASH AND CASH EQUIVALENTS (DECREASE) INCREASE FOR THE YEAR | | | (32 | ) | | | 7,527 | | | | (1,133 | ) |
| CASH AND CASH EQUIVALENTS, BEGINNING OF THE YEAR | | | 7,860 | | | | 333 | | | | 1,466 | |
| CASH AND CASH EQUIVALENTS, END OF THE YEAR | | $ | 7,828 | | | $ | 7,860 | | | $ | 333 | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | | | | | | | | | | | | |
| Cash paid during the period for interest | | $ | 454 | | | $ | 744 | | | $ | 1,054 | |
| Cash paid during the period for income taxes | | | 4,817 | | | | 837 | | | | 1,443 | |
| NON-CASH TRANSACTIONS | | | | | | | | | | | | |
| Financing Activities | | | | | | | | | | | | |
Acquisition of minority interest | | $ | - | | | $ | - | | | $ | (2,950 | ) |
| Investing Activities | | | | | | | | | | | | |
| Stock issued for purchase of minority interest | | | - | | | | - | | | | 2,950 | |
See notes to consolidated financial statements.
DEL GLOBAL TECHNOLOGIES CORP. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(DOLLARS IN THOUSANDS)
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| | | COMMON | | | ADDITIONAL | | | | | | | | | | | | | |
| | | STOCK ISSUED | | | PAID-IN | | | | | | ACCUMULATED | | | TREASURY STOCK | | | | |
| | | SHARES | | | AMOUNT | | | CAPITAL | | | INCOME | | | DEFICIT | | | SHARES | | | AMOUNT | | | TOTAL | |
| BALANCE, JULY 30, 2005 | | | 11,252,958 | | | $ | 1,125 | | | $ | 64,448 | | | $ | 1,450 | | | $ | (52,249 | ) | | | 622,770 | | | $ | (5,546 | ) | | $ | 9,228 | |
| Stock option exercises | | | 99,000 | | | | 10 | | | | 228 | | | | - | | | | - | | | | - | | | | - | | | | 238 | |
| Stock warrant exercises | | | 1,574 | | | | 1 | | | | 1 | | | | - | | | | - | | | | - | | | | - | | | | 2 | |
| Stock compensation | | | - | | | | - | | | | 142 | | | | - | | | | - | | | | - | | | | - | | | | 142 | |
| Stock issued in minority interest acquisition | | | 904,762 | | | | 90 | | | | 2,860 | | | | - | | | | - | | | | - | | | | - | | | | 2,950 | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | - | | | | - | | | | - | | | | - | | | | 94 | | | | - | | | | - | | | | 94 | |
| Foreign currency adjustments | | | - | | | | - | | | | - | | | | 160 | | | | - | | | | - | | | | - | | | | 160 | |
| Total comprehensive income | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 254 | |
| BALANCE, JULY 29, 2006 | | | 12,258,294 | | | | 1,226 | | | | 67,679 | | | | 1,610 | | | | (52,155 | ) | | | 622,770 | | | | (5,546 | ) | | | 12,814 | |
| Stock option exercises | | | 101,000 | | | | 10 | | | | 161 | | | | - | | | | - | | | | - | | | | - | | | | 171 | |
| Stock warrant exercises | | | 366,854 | | | | 37 | | | | 514 | | | | - | | | | - | | | | - | | | | - | | | | 551 | |
| Stock compensation | | | - | | | | - | | | | 220 | | | | - | | | | - | | | | - | | | | - | | | | 220 | |
| Issuance of stock for Rights Offering | | | 12,027,378 | | | | 1,202 | | | | 11,152 | | | | - | | | | - | | | | - | | | | - | | | | 12,354 | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | - | | | | - | | | | - | | | | - | | | | 3,816 | | | | - | | | | - | | | | 3,816 | |
| Foreign currency adjustments | | | - | | | | - | | | | - | | | | 270 | | | | - | | | | - | | | | - | | | | 270 | |
| Total comprehensive income | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 4,086 | |
| BALANCE, JULY 28, 2007 | | | 24,753,526 | | | | 2,475 | | | | 79,726 | | | | 1,880 | | | | (48,339 | ) | | | 622,770 | | | | (5,546 | ) | | | 30,196 | |
| Stock option exercises | | | 83,181 | | | | 9 | | | | 106 | | | | - | | | | - | | | | 31,694 | | | | (69 | ) | | | 46 | |
| Stock warrant exercises | | | 61,016 | | | | 6 | | | | 85 | | | | - | | | | - | | | | - | | | | - | | | | 91 | |
| Stock compensation | | | - | | | | - | | | | 481 | | | | - | | | | - | | | | - | | | | - | | | | 481 | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | - | | | | - | | | | - | | | | - | | | | 2,977 | | | | - | | | | - | | | | 2,977 | |
| Foreign currency adjustments | | | - | | | | - | | | | - | | | | 2,372 | | | | - | | | | - | | | | - | | | | 2,372 | |
| Total comprehensive income | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 5,349 | |
| BALANCE, AUGUST 2, 2008 | | | 24,897,723 | | | $ | 2,490 | | | $ | 80,398 | | | $ | 4,252 | | | $ | (45,362 | ) | | | 654,464 | | | $ | (5,615 | ) | | $ | 36,163 | |
See notes to consolidated financial statements.
DEL GLOBAL TECHNOLOGIES CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(DOLLARS IN THOUSANDS, EXCEPT PER SHARE DATA)
| 1. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
DESCRIPTION OF BUSINESS ACTIVITIES - Del Global Technologies Corp. (“Del Global”) together with its subsidiaries (collectively, the “Company”), is engaged in two major lines of business: Medical Systems Group and Power Conversion Group. The Medical Systems Group segment designs, manufactures and markets imaging and diagnostic systems consisting of stationary and portable x-ray imaging systems, radiographic/fluoroscopic systems, mammography systems and dental systems. The Power Conversion Group segment designs, manufactures and markets key electronic components such as transformers, noise suppression filters and high voltage capacitors for use in precision regulated high voltage applications.
On October 1, 2004, the Company completed the sale of its Del High Voltage Division (“DHV”) for a purchase price of $3,100, plus the assumption of approximately $800 of liabilities. This division was formerly part of the Power Conversion Group and designed, manufactured and marketed proprietary precision power conversion subsystems for medical as well as critical industrial applications. The results of operations of this division are shown as a discontinued operation in the accompanying financial statements.
Loss from discontinued operations for fiscal 2006 reflects the accrual of an estimated liability of $175 related to a New York State Sales tax audit of its Valhalla location, including the DHV business.
PRINCIPLES OF CONSOLIDATION - The consolidated financial statements are prepared on the accrual basis of accounting, which conforms to accounting principles generally accepted in the United States of America, (“U.S. GAAP”) and include the accounts of Del Global and its subsidiaries. All material intercompany accounts and transactions have been eliminated.
USE OF ESTIMATES - The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated balance sheets, as well as reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Significant estimates underlying the accompanying consolidated financial statements include the allowance for doubtful accounts, allowance for obsolete and excess inventory, realizability of deferred income tax assets, recoverability of intangibles and other long-lived assets, and future obligations associated with the Company’s litigation.
Certain reclassifications have been made to prior years' amounts to conform to the current year's presentation.
ACCOUNTING PERIOD - The Company’s fiscal year-end is based on a 52/53-week cycle ending on the Saturday nearest to July 31. Results of the Company’s subsidiary, Villa Sistemi Medicali S.p.A. (“Villa”) are consolidated into Del Global’s consolidated financial statements based on a fiscal year that ends on June 30 and are reported on a one-month lag.
CASH EQUIVALENTS - The Company considers highly liquid instruments readily convertible to known amounts of cash with original maturities of three months or less (measured from their acquisition date) to be cash equivalents.
FOREIGN CURRENCY TRANSLATION - The financial statements of Villa are recorded in “Euro” and translated into U.S. dollars. Villa’s balance sheet accounts are translated at the current exchange rate and income statement items are translated at the average exchange rate for the period. Gains and losses resulting from translation are accumulated in a separate component of shareholders’ equity.
INVENTORIES - - Inventories are stated at the lower of cost or market value. Cost is comprised of direct materials and, where applicable, direct labor costs and overhead that has been incurred in getting the inventories to their present location and condition. Engineering costs incurred to set up products to be manufactured for a customer purchase order are capitalized when the scope of the purchase order indicates that such costs are recoverable. Such costs are included in work-in-process inventory and amortized on a units shipped basis over the life of the customer order from the date of first shipment. Cost is calculated using the first in, first out method. Market value represents the estimated selling price less all estimated costs to completion and costs to be incurred in marketing, selling and distribution.
PROPERTY PLANT AND EQUIPMENT, NET – Property, plant and equipment, net are stated at cost less accumulated depreciation and amortization. Replacements and major improvements are capitalized; maintenance and repairs are expensed as incurred. Gains or losses on asset dispositions are included in the determination of net income or loss. Depreciation is computed utilizing the straight-line method. The cost of leasehold improvements is amortized over the shorter of the useful life or the term of the lease.
Depreciable lives are generally as follows:
| DESCRIPTION | | USEFUL LIVES | |
| Buildings | | | 25-33 | |
| Machinery and equipment | | | 5-15 | |
| Furniture and fixtures | | | 5-10 | |
| Transportation equipment | | | 3-4 | |
| Computer and other equipment | | | 3-7 | |
DEFERRED FINANCING COSTS, NET - Financing costs, including fees, commission and legal expenses, are capitalized as other non-current assets and amortized on a straight line basis, which approximates the interest method, over the term or expected term of the relevant loan. Amortization of deferred financing costs is included in interest expense.
GOODWILL - - Goodwill represents the excess of the cost of acquisitions over the fair value of the identifiable assets acquired and liabilities assumed. The Company evaluates goodwill for impairment on an annual basis by comparing the fair value to the carrying value for reporting units within the Medical Systems Group. Fair value is primarily determined using a discounted cash flow method.
RECOVERABILITY OF LONG-LIVED ASSETS - The Company evaluates the carrying amounts of long-lived assets (including goodwill) whenever events have occurred (and at least annually for goodwill) which might require modification to the carrying values. In evaluating carrying values of long-lived assets, the Company reviews certain indicators of potential impairment, such as undiscounted projected cash flows and business plans. In the event that impairment has occurred, the fair estimated value of the related asset is determined and the Company records a charge to operations calculated by comparing the asset’s carrying value to the estimated fair value. The Company estimates fair value based on the best information available making whatever estimates, judgments and projections are considered necessary.
REVENUE RECOGNITION – The Company recognizes revenue upon shipment, provided there is persuasive evidence of an arrangement, there are no uncertainties concerning acceptance, the sales price is fixed, collection of the receivable is probable and only perfunctory obligations related to the arrangement need to be completed. The Company maintains a sales return allowance, based upon historical patterns, to cover estimated normal course of business returns, including defective or out of specification product. The Company’s products are covered primarily by one year warranty plans and in some cases optional extended warranties for up to five years are offered. The Company establishes allowances for warranties on an aggregate basis for specifically identified, as well as anticipated, warranty claims based on contractual terms, product conditions and actual warranty experience by product line. The Company recognizes service revenue when repairs or out of warranty repairs are completed. The Company has a Food and Drug Administration (“FDA”) obligation to continue to provide repair service for certain medical systems for up to seven years past the warranty period. These repairs are billed to the customers at market rates.
RESEARCH AND DEVELOPMENT COSTS - Research and development costs are recognized as an expense in the period in which they are incurred.
INCOME TAXES - Deferred income tax assets and liabilities represents the effects of the differences between the income tax basis and financial reporting basis of assets and liabilities and tax credit carryforwards at the tax rates expected at the time the deferred income tax liability or asset is expected to be settled or realized. Management provides valuation allowances on deferred income tax assets for which realization does not meet a “more likely than not” standard.
NET INCOME (LOSS) PER SHARE - Net income (loss) per share is computed by dividing net income (loss) by the weighted average number of common shares outstanding during the year. The effect of the assumed exercise of options and warrants to purchase common stock are excluded from the calculation of earnings (loss) per share when their inclusion would be anti-dilutive.
CONCENTRATION OF CREDIT RISK - Financial instruments which potentially subject the Company to concentrations of credit risk are cash equivalents, investments in marketable securities, trade receivables and lines of credit. With respect to accounts receivable, the Company limits its credit risk by performing ongoing credit evaluations and, when necessary, requiring letters of credit, guarantees or collateral. Management does not believe significant risk exists in connection with the Company’s concentrations of credit at August 2, 2008.
The activity in allowances for doubtful accounts is as follows:
| | | BALANCE AT BEGINNING OF YEAR | | | CHARGED TO COSTS AND EXPENSE | | | | | | BALANCE AT END OF YEAR | |
| YEAR ENDED AUGUST 2, 2008 | | | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | 1,569 | | | $ | 250 | | | $ | 419 | | | $ | 1,400 | |
| YEAR ENDED JULY 28, 2007 | | | | | | | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | 1,095 | | | $ | 646 | | | $ | 172 | | | $ | 1,569 | |
| YEAR ENDED JULY 29, 2006 | | | | | | | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | 1,028 | | | $ | 338 | | | $ | 271 | | | $ | 1,095 | |
(1) Write-off of accounts receivable previously charged to costs and expenses.
STOCK-BASED COMPENSATION – In December 2004, the Financial Accounting Standards Board (“FASB”) issued Statement of Financial Accounting Standards (“SFAS”) No. 123 (R), “Share-Based Payments,” which established standards for transactions in which an entity exchanges its equity instruments for goods and services. The standard requires a public entity to measure the equity instruments award based on the grant-date fair value. This eliminates the exception to account for such awards using the intrinsic method previously allowed under Accounting Principles Board (“APB”) Opinion No. 25, “Accounting for Stock Issued to Employees.” SFAS No. 123 (R) was adopted beginning in fiscal year 2006. The adoption did not require restatement of previously issued statements and is being applied on a prospective basis. See Note 11, Shareholders’ Equity.
EFFECTS OF NEW ACCOUNTING PRONOUNCEMENTS - In March 2008, the Financial Accounting Standards Board (the “FASB”) issued Statement of Financial Accounting Standards (“SFAS”) No. 161, Disclosures about Derivative Instruments and Hedging Activities, an amendment of SFAS No. 133. The Statement requires enhanced disclosures about an entity’s derivative and hedging activities. The Statement is effective for fiscal years and interim periods beginning after November 15, 2008. The Company is currently evaluating the requirements of SFAS 161, but does not expect it to have a material impact.
In December 2007, the FASB issued SFAS No. 160, Noncontrolling Interests in Consolidated Financial Statements--an amendment of ARB No. 51 (“SFAS 160”). SFAS 160 requires identification and presentation of ownership interests in subsidiaries held by parties other than the Company in the consolidated financial statements within the equity section but separate from the equity owned by Del Global. SFAS 160 also requires that (1) the amount of consolidated net income attributable to the parent and to the noncontrolling interest be clearly identified and presented on the face of the consolidated statement of operations, (2) changes in ownership interest be accounted for similarly, as equity transactions and (3) when a subsidiary is deconsolidated, any retained noncontrolling equity investment in the former subsidiary and the gain or loss on the deconsolidation of the subsidiary be measured at fair value. This statement is effective for the Company on August 2, 2009. The Company is currently evaluating the requirements of SFAS 160 but does not expect it to have a material impact.
In December 2007, the FASB issued SFAS No. 141R, Business Combinations (“SFAS 141R”). SFAS 141R states that acquisition-related costs are to be recognized separately from the acquisition and expensed as incurred with restructuring costs being expensed in periods after the acquisition date. SFAS 141R also states that business combinations will result in all assets and liabilities of the acquired business being recorded at their fair values. The Company is required to adopt SFAS No. 141R effective August 2, 2009. The impact of the adoption of SFAS No. 141R will depend on the nature and extent of business combinations occurring on or after the effective date.
In September 2006, the FASB issued SFAS No 157, “Fair Value Measurements,” (“SFAS 157”) which defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS No. 157 does not require any new fair value measurements, but provides guidance on how to measure fair value by providing a fair value hierarchy used to classify the source of the information. Portions of this statement are effective for the Company for fiscal years beginning after November 15, 2007, while others have been deferred until fiscal 2009. The Company has not evaluated the impact that the adoption of SFAS No. 157 will have on its financial statements at this time.
In February 2007, the FASB released SFAS No. 159, “Fair Value Option for Financial Assets and Financial Liabilities” (“SFAS 159”). This statement permits entities to choose to measure many financial instruments and certain other items at fair value. This statement is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those years. The Company has not evaluated the impact that the adoption of SFAS No. 159 will have on its financial statements at this time.
In June 2006, the FASB issued Interpretation No. 48 (“FIN 48”), “Accounting for Uncertainty in Income Taxes”— an interpretation of SFAS No. 109. FIN 48 requires that the Company recognize the financial statement effects of a tax position when it is more likely than not, based on the technical merits, that the position will be sustained upon examination. As used in this Interpretation, the term “more likely than not” means a likelihood of more than 50 percent. The terms “examined” and “upon examination” also include resolution of the related appeals or litigation processes, if any. The determination of whether or not a tax position has met the more-likely-than-not recognition threshold is to be determined based on the facts, circumstances, and information available at the reporting date. FIN 48 was adopted by the Company beginning July 29, 2007. The adoption of FIN 48 did not have any impact on the Company’s statement of financial position or on its results of operations.
The Company’s primary income tax jurisdictions are in the United States and Italy. The Company is currently not under audit in either jurisdiction. Tax years since 2003 are open pursuant to statutes in Italy and tax years since 2004 are open pursuant to statutes in the Unites States.
It is the Company’s practice to recognize interest and/or penalties related to income tax matters in tax expense. As of August 2, 2008, there were no material interest or penalty amounts to accrue.
| 2. | ACQUISITION OF MINORITY INTEREST IN VILLA |
On December 23, 2005, the Company acquired the remaining 20% of Villa for $2,612 plus 904,762 restricted shares of Company common stock. These shares were valued at $3.26 a share, or $2,950, and were subject to SEC Rule 144 limitations as to holding periods and trading volume limitations. Goodwill in the amount of $4,526 was recorded and $934 of minority interest was reversed after recognition of a $388 dividend. Due to the previous 80% ownership interest existing at the time of the original acquisition, the assets and liabilities of the Villa subsidiary were fully consolidated before the transaction and considered to be at fair market value with no additional adjustments necessary.
| Inventories consists of the following: | | | | | | |
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | |
| Raw materials and purchased parts | | $ | 13,920 | | | $ | 15,237 | |
| Work-in-process | | | 2,526 | | | | 3,910 | |
| Finished goods | | | 6,428 | | | | 6,652 | |
| | | | 22,874 | | | | 25,799 | |
| Less: allowance for obsolete and excess inventories | | | (4,435 | ) | | | (3,869 | ) |
| Total inventories net | | $ | 18,439 | | | $ | 21,930 | |
The activity in the allowance for obsolete and excess inventories accounts is as follows:
| | | BALANCE AT BEGINNING OF YEAR | | | CHARGED TO COSTS AND EXPENSE | | | | | | BALANCE AT END OF YEAR | |
| YEAR ENDED AUGUST 2, 2008 | | | | | | | | | | | | |
| Allowance for obsolete and excess inventories | | $ | 3,869 | | | $ | 1,554 | | | $ | 988 | | | $ | 4,435 | |
| YEAR ENDED JULY 28, 2007 | | | | | | | | | | | | | | | | |
| Allowance for obsolete and excess inventories | | | 3,703 | | | | 651 | | | | 485 | | | | 3,869 | |
| YEAR ENDED JULY 29, 2006 | | | | | | | | | | | | | | | | |
| Allowance for obsolete and excess inventories | | | 3,017 | | | | 1,050 | | | | 364 | | | | 3,703 | |
(1) Write-off of inventories previously charged to costs and expenses.
The Company has pledged all of its inventories in the U.S. having a net carrying amount of approximately $6,154 and $6,608 at August 2, 2008 and July 28, 2007, respectively, to secure its credit facility with its U.S. lender.
| 4. | PROPERTY PLANT AND EQUIPMENT |
Property plant and equipment consist of the following:
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | |
| Land | | $ | 694 | | | $ | 694 | |
| Buildings | | | 7,249 | | | | 6,549 | |
| Machinery and equipment | | | 7,153 | | | | 6,757 | |
| Furniture and fixtures | | | 694 | | | | 817 | |
| Leasehold improvements | | | 1,810 | | | | 1,754 | |
| Transportation equipment | | | 106 | | | | 109 | |
| Computers and other equipment | | | 2,870 | | | | 2,552 | |
| | | | 20,576 | | | | 19,232 | |
| Less: accumulated depreciation and amortization | | | (13,199 | ) | | | (12,721 | ) |
| Property plant and equipment, net | | $ | 7,377 | | | $ | 6,511 | |
The Company has pledged all of its property, plant and equipment in the U.S. having a net carrying amount of approximately $2,045 and $1,985 at August 2, 2008 and July 28, 2007, respectively, to secure its credit facility with its U.S. lender. Included in the table above are assets held under capital leases, including the Villa building, in the net amount of $2,639 and $3,099 at August 2, 2008 and July 28, 2007, respectively. Accumulated amortization relating to capital leases was $983 and $845 at August 2, 2008 and July 28, 2007, respectfully. Amortization expense relating to capital leases was $125, $116 and $113 for fiscal 2008, 2007 and 2006, respectively.
Depreciation expense, including amortization of capital leased assets, for fiscal years 2008, 2007 and 2006 was $1,050, $872 and $998, respectively.
Goodwill consists of the following:
| | | |
| Balance at July 29, 2006 and July 28, 2007 | | $6,437 |
Impairment of goodwill | | |
| Balance at August 2, 2008 | | |
As described in Note 2, during fiscal year 2006, the Company completed the acquisition of the remaining 20% minority interest in its Villa subsidiary and recorded additional goodwill of $4,526.
Due primarily to continued operating results below planned levels and management’s resulting revaluation of its strategic plan for the Company’s domestic Medical Systems Group’s reporting unit, the Company completed a special assessment of that reporting unit’s goodwill realization during the third quarter of 2008. The Company’s scheduled assessment of goodwill is during the fourth quarter of each fiscal year.
As part of its assessment, the Company estimated the fair value of the domestic reporting unit based on internal cash flows expected to be earned by the business and an appropriate risk-adjusted discount rate. While such estimates are subject to significant uncertainties and actual results could be materially different, the analysis resulted, pursuant to the implementation guidance of FASB No. 142, Accounting for Goodwill and Intangible Assets, in a complete impairment of the unit’s goodwill balance. Accordingly, the Company recorded a $1,911 impairment charge during the fiscal 2008. The Company’s annual fourth quarter assessments of Villa’s goodwill has not indicated any impairment.
The Company’s products are covered primarily by one-year warranty plans and in some cases optional extended contracts may be offered covering products for periods up to five years, depending upon the product and contractual terms of sale. The Company establishes allowances for warranties on an aggregate basis for specifically identified, as well as anticipated, warranty claims based on contractual terms, product conditions and actual warranty experience by product line.
The activity in the warranty reserve accounts is as follows:
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | |
| Balance at beginning of year | | $ | 1,065 | | | $ | 971 | |
| Provision for anticipated warranty claims | | | 526 | | | | 879 | |
| Costs incurred related to warranty claims | | | (514 | ) | | | (785 | ) |
| Balance at end of year | | $ | 1,077 | | | $ | 1,065 | |
| 7. | SHORT-TERM CREDIT FACILITIES, LONG-TERM DEBT AND SUBORDINATED NOTE |
The Company did not have any outstanding borrowings under its short term credit facilities at August 2, 2008 or July 28, 2007.
Long-term debt was comprised of the following:
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | | | INTEREST RATE AT AUGUST 2, 2008 |
| Foreign capital lease obligation | | $ | 2,639 | | | $ | 2,650 | | | | 5.0 | % |
| Foreign credit facilities | | | 2,703 | | | | 2,699 | | | Euribor + 1.00 | % |
| Foreign Italian Government loans | | | 959 | | | | 1,135 | | | | 3.4 | % |
| Total long term debt | | | 6,301 | | | | 6,484 | | | | | |
| Less current portion of long-term bank debt | | | (1,797 | ) | | | (1,086 | ) | | | | |
| Long-term debt, less current portion | | $ | 4,504 | | | $ | 5,398 | | | | | |
On August 1, 2005, the Company entered into a three-year revolving credit and term loan facility with North Fork Business Capital (the “North Fork Facility”) and repaid the prior facility. During the first nine months of 2007, an average of $1,400 was outstanding under this facility. In March 2007, the Company used a portion of the proceeds from the Rights Offering described below to pay all outstanding balances under this facility as well as $2,505 of subordinated notes then outstanding and $146 in related interest.
On June 1, 2007, the North Fork Facility was amended and restated. As restated, the North Fork Facility provides for a $7,500 formula based revolving credit facility based on the Company’s eligible accounts receivable and inventory as defined in the credit agreement and a capital expenditure loan facility up to $1,500. Interest on the revolving credit and capital expenditure borrowings is payable at prime plus 0.5% or alternatively at a LIBOR rate plus 2.5%. Other changes to the terms and conditions of the original loan agreement include the modification of covenants, removal of the Villa stock as loan collateral and the removal of daily collateral reporting which was part of the previous asset-based facility requirements. As of August 2, 2008 and July 28, 2007, no amounts were outstanding and the Company had approximately $6,400 of availability under the North Fork Facility, as North Fork has reserved $2,600 against possible litigation settlements in the form of letters of credit..
The North Fork Facility is subject to commitment fees of 0.5% per annum on the daily-unused portion of the facility, payable monthly. The Company granted a security interest to the lender on its U.S. credit facility in substantially all of its accounts receivable, inventory, property, plant and equipment, other assets and intellectual property in the U.S..
As of the end of the first quarter of fiscal 2007, the Company was non-compliant with the tangible net worth covenant under the North Fork Facility. On December 6, 2006, North Fork waived the non-compliance with this covenant for the first quarter of fiscal 2007 and adjusted the covenant levels going forward through the maturity of the credit facility. As of August 2, 2008 and July 28, 2007, the Company was in compliance with all covenants under the North Fork Facility.
The Company’s Villa subsidiary maintains short term credit facilities which are renewed annually with Italian banks. Currently, these facilities are not being utilized and the balance due at August 2, 2008 is $0. Interest rates on these facilities are variable and currently range from 3.7% – 13.75%.
In October 2006, Villa entered into a 1.0 million Euro loan for financing of R&D projects, with an option for an additional 1 million Euro upon completion of 50% of the projects. In April, 2008, the Company declined the option for additional financing and demonstrated successful completion of the project triggering a more favorable interest rate. Interest, previously payable at Euribor 3 months plus 1.3 points, was reduced in the fourth fiscal quarter of 2008 to Euribor plus 1.04 points, currently at 5.998%. The note is repayable over a 7 year term, with reimbursement starting in September 2008. The note contains a financial covenant which provides that the net equity of Villa cannot fall below 5.0 million Euros. This covenant could limit Villa’s ability to pay dividends to the U.S. parent company in the event future losses, future dividends or other events should cause Villa’s equity to fall below the defined level.
Villa was party to a 1.6 million Euro loan which was extinguished in March 2007. Two final installments for a total of 0.3 million Euro were repaid in fiscal year 2007.
Villa is also party to two Italian government long-term loans with a fixed interest rate of 3.425% with principal payable annually through maturity in February and September 2010. At the end of fiscal year 2008, total principal due is 0.6 million Euro. Villa manufacturing facility is subject to a capital lease obligation which matures in 2011 with an option to purchase. Villa is in compliance with all related financial covenants under these short and long-term financings.
SUBORDINATED NOTE - In connection with the settlement reached on January 29, 2002, with the plaintiffs in the class action litigation, the Company recorded the present value at 12% of the $2,000 of subordinated notes that were issued in April 2002 and matured in March 2007. The subordinated notes did not pay interest currently, but accrued interest at 6% per annum, and were recorded at issuance at a discounted present value of $1,500. The balance was paid on March 29, 2007 with a portion of the proceeds from a Rights Offering described below.
The Company is obligated to make principal payments under its long-term debt and capital lease obligation as follows:
FISCAL YEARS | | DEBT | | | CAPITAL LEASE | | | TOTAL | |
| 2009 | | $ | 1,312 | | | $ | 648 | | | $ | 1,960 | |
| 2010 | | | 1,325 | | | | 648 | | | | 1,973 | |
| 2011 | | | 904 | | | | 487 | | | | 1,391 | |
| 2012 | | | 121 | | | | - | | | | 121 | |
| Purchase option | | | -- | | | | 1,221 | | | | 1,221 | |
| Total payments | | | 3,662 | | | | 3,004 | | | | 6,666 | |
| Less: amount representing interest | | | -- | | | | (365 | ) | | | (365 | ) |
| Total | | $ | 3,662 | | | $ | 2,639 | | | $ | 6,301 | |
The Company has a Profit Sharing Plan that provides for contributions as determined by the Board of Directors. The contributions can be paid to the Plan in cash or common stock of the Company. No contributions were authorized for fiscal years 2008, 2007 or 2006.
The Profit Sharing Plan also incorporates a 401(k) Retirement Plan that is available to substantially all domestic employees, allowing them to defer a portion of their salary. The Company matches employee contributions at a 50% rate up to a maximum of 4% of annual salary, and recorded a related expense of $102, $20 and $106 for fiscal years 2008, 2007 and 2006, respectively.
The Company also had a defined benefit plan, which was frozen effective February 1, 1986. During fiscal 2005, the Company applied to the Pension Benefit Guaranty Corp and to the IRS for a determination letter and approval to terminate this plan. The Company received the IRS determination letter approving the final settlement during the second quarter of fiscal 2006 and the plan fully paid out all of the plan participants in March 2006.
In addition, the Company’s Villa subsidiary provides for employee termination indemnities. Villa has established a reserve, representing the liability for indemnities payable upon termination of employment, accrued in accordance with labor laws and labor agreements in force. This liability is subject to annual revaluation using the officially-established indices. The liability for these indemnities is included in other long-term liabilities on the accompanying Consolidated Balance Sheets and was $3,172, and $3,127 at August 2, 2008 and July 28, 2007, respectively. Provisions for employee termination indemnities were $729, $357 and $401 for fiscal years 2008, 2007 and 2006, respectively.
The Company has three reportable segments; the Medical Systems Group, the Power Conversion Group and Other. The Other segment includes unallocated corporate costs. For each fiscal year presented, corporate costs (which include certain shared services) were allocated to domestic subsidiaries on the basis of a percentage of each unit’s annual sales. Corporate costs were allocated at a fixed dollar amount to the international subsidiary based upon an intercompany management services agreement. The percentages and the dollar amounts used to allocate actual corporate costs are based on management’s estimate of the benefits received by each reporting segment from corporate activities and shared services.
Operating segments are defined as components of an enterprise, about which separate financial information is available which is evaluated regularly by the chief decision maker, or decision making group, in deciding how to allocate resources and in assessing performance. The Company’s chief operating decision making group is comprised of the Chief Executive Officer and the senior executives of the Company’s operating segments. The Company evaluates its reporting segments based on operating income or loss. The accounting policies of the segments are the same as those described in the summary of significant accounting policies.
Selected financial data of these segments are as follows:
FISCAL YEAR ENDED AUGUST 2, 2008 | | MEDICAL SYSTEMS GROUP | | | POWER CONVERSION GROUP | | | OTHER | | | TOTAL | |
| Net sales to external customers | | $ | 95,052 | | | $ | 13,254 | | | $ | -- | | | $ | 108,306 | |
| Cost of sales | | | 73,317 | | | | 8,202 | | | | -- | | | | 81,519 | |
| Gross margin | | | 21,735 | | | | 5,052 | | | | -- | | | | 26,787 | |
| Selling, general and administrative | | | 11,840 | | | | 2,522 | | | | 1,224 | | | | 15,586 | |
| Research and development | | | 2,488 | | | | -- | | | | -- | | | | 2,488 | |
| Goodwill impairment | | | 1,911 | | | | -- | | | | -- | | | | 1,911 | |
| Litigation settlement costs | | | 450 | | | | -- | | | | -- | | | | 450 | |
| Total operating expenses | | | 16,689 | | | | 2,522 | | | | 1,224 | | | | 20,435 | |
| Operating income (loss) | | $ | 5,046 | | | $ | 2,530 | | | $ | (1,224 | ) | | | 6,352 | |
| Interest expense | | | | | | | | | | | | | | | (313 | ) |
| Other income | | | | | | | | | | | | | | | 185 | |
| Income from continuing operations, before income taxes and minority interest | | | | | | | | | | | | | | $ | 6,224 | |
| Depreciation | | $ | 891 | | | $ | 163 | | | $ | -- | | | $ | 1,054 | |
| Amortization | | | 22 | | | | -- | | | | -- | | | | 22 | |
| Segment assets | | | 53,133 | | | | 4,651 | | | | 8,569 | | | | 66,353 | |
| Capital expenditures | | | 1,140 | | | | 68 | | | | -- | | | | 1,208 | |
Inter-segment sales were $83 for the fiscal year ended August 2, 2008. Approximately $50,678 of Medical Systems Group assets are located in Italy, including $10,656 of long-lived assets.
FISCAL YEAR ENDED JULY 28, 2007 | | MEDICAL SYSTEMS GROUP | | | POWER CONVERSION GROUP | | | OTHER | | | TOTAL | |
| Net sales to external customers | | $ | 90,979 | | | $ | 13,188 | | | $ | -- | | | $ | 104,167 | |
| Cost of sales | | | 70,879 | | | | 8,271 | | | | -- | | | | 79,150 | |
| Gross margin | | | 20,100 | | | | 4,917 | | | | -- | | | | 25,017 | |
| Selling, general and administrative | | | 10,635 | | | | 2,476 | | | | 1,479 | | | | 14,590 | |
| Research and development | | | 2,013 | | | | -- | | | | -- | | | | 2,013 | |
| Litigation settlement costs | | | -- | | | | -- | | | | -- | | | | -- | |
| Total operating expenses | | | 12,648 | | | | 2,476 | | | | 1,479 | | | | 16,603 | |
| Operating income (loss) | | $ | 7,452 | | | $ | 2,441 | | | $ | (1,479 | ) | | | 8,414 | |
| Interest expense | | | | | | | | | | | | | | | (991 | ) |
| Other income (expense) | | | | | | | | | | | | | | | (54 | ) |
| Income from continuing operations, before income taxes and minority interest | | | | | | | | | | | | | | $ | 7,369 | |
| Depreciation | | $ | 704 | | | $ | 165 | | | $ | 3 | | | $ | 872 | |
| Amortization | | | 27 | | | | -- | | | | -- | | | | 27 | |
| Segment assets | | | 60,658 | | | | 5,014 | | | | 667 | | | | 66,339 | |
| Capital expenditures | | | 703 | | | | 76 | | | | -- | | | | 779 | |
Inter-segment sales were $86 for the fiscal year ended July 28, 2007. Approximately $43,720 of Medical Systems Group assets are located in Italy, including $10,110 of long-lived assets.
FISCAL YEAR ENDED JULY 29, 2006 | | MEDICAL SYSTEMS GROUP | | | POWER CONVERSION GROUP | | | OTHER | | | TOTAL | |
| Net sales to external customers | | $ | 70,287 | | | $ | 12,727 | | | $ | -- | | | $ | 83,014 | |
| Cost of sales | | | 55,453 | | | | 8,203 | | | | -- | | | | 63,656 | |
| Gross margin | | | 14,834 | | | | 4,524 | | | | -- | | | | 19,358 | |
| Selling, general and administrative | | | 9,467 | | | | 2,148 | | | | 2,004 | | | | 13,619 | |
| Research and development | | | 1,562 | | | | -- | | | | -- | | | | 1,562 | |
| Litigation settlement costs | | | 252 | | | | (55 | ) | | | 500 | | | | 697 | |
| Total operating expenses | | | 11,281 | | | | 2,093 | | | | 2,504 | | | | 15,878 | |
| Operating income (loss) | | $ | 3,553 | | | $ | 2,431 | | | $ | (2,504 | ) | | | 3,480 | |
| Interest expense | | | | | | | | | | | | | | | (1,311 | ) |
| Other expense | | | | | | | | | | | | | | | (34 | ) |
| Income from continuing operations, before income taxes and minority interest | | | | | | | | | | | | | | $ | 2,135 | |
| Depreciation | | $ | 793 | | | $ | 193 | | | $ | 2 | | | $ | 988 | |
| Amortization | | | 38 | | | | -- | | | | -- | | | | 38 | |
| Segment assets | | | 43,630 | | | | 5,055 | | | | 468 | | | | 49,153 | |
| Capital expenditures | | | 695 | | | | 66 | | | | 4 | | | | 765 | |
Inter-segment sales were $149 for the fiscal year ended July 29, 2006. Approximately $35,481 of Medical Systems Group assets are located in Italy, including $10,249 of long-lived assets.
MAJOR CUSTOMERS AND EXPORT SALES - For the fiscal year ended August 2, 2008 one of our Medical Systems Group customers accounted for approximately 13% of consolidated revenues and 1% of gross accounts receivable at August 2, 2008. For the fiscal years ended July 28, 2007, one of our Medical Systems Group customers accounted for approximately 12% of consolidated revenues and 11% of gross accounts receivable at July 28, 2007. For fiscal year ended July 29, 2006, no individual customer accounted for greater than 10% of total revenue or accounts receivable.
Foreign sales were 64%, 66% and 64% of the Company’s consolidated net sales in fiscal years ended August 2, 2008, July 28, 2007 and July 29, 2006, respectively. Net sales by geographic areas were:
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | | | JULY 29 2006 | |
| United States | | $ | 40,402 | | | | 37 | % | | $ | 38,397 | | | | 37 | % | | $ | 29,979 | | | | 36 | % |
| Canada | | | 2,045 | | | | 2 | % | | | 869 | | | | 1 | % | | | 158 | | | | - | % |
| Europe | | | 55,428 | | | | 51 | % | | | 48,129 | | | | 46 | % | | | 37,078 | | | | 45 | % |
| Far East | | | 7,354 | | | | 7 | % | | | 7,603 | | | | 7 | % | | | 6,298 | | | | 8 | % |
| Mexico, Central and South America | | | 514 | | | | 1 | % | | | 3,117 | | | | 3 | % | | | 6,750 | | | | 8 | % |
| Africa, Middle East and Australia | | | 2,563 | | | | 2 | % | | | 6,052 | | | | 6 | % | | | 2,751 | | | | 3 | % |
| | | $ | 108,306 | | | | 100 | % | | $ | 104,167 | | | | 100 | % | | $ | 83,014 | | | | 100 | % |
Revenues are attributable to geographic areas based on the location of the customers.
RIGHTS OFFERING AND STOCKHOLDERS’ RIGHTS PLAN – On December 12, 2006, the Company filed a registration statement for a subscription rights offering with the SEC that became effective January 30, 2007. Under terms of this rights offering, the Company distributed to shareholders of record as of February 5, 2007, non-transferable subscription rights to purchase one share of the Company’s common stock for each share owned at that date at a subscription price of $1.05 per share. On March 12, 2007, the Company completed the rights offering, selling 12,027,378 shares of its common stock at $1.05 per share. Total proceeds to the Company, net of $275 of expenses related to the rights offering, were $12,354.
The purpose of this rights offering was to raise equity capital in a cost-effective manner. Approximately $7,564 of the proceeds were used for debt repayment and the remainder invested in short-term money market securities for anticipated working capital needs and general corporate purposes. A portion of the net proceeds may also ultimately be used to acquire or invest in businesses, products and technologies that Company management believes are complementary to the Company's business.
In addition, on January 22, 2007, the Company entered into a stockholders’ rights plan (the “Rights Plan”). The Rights Plan provides for a dividend distribution of one common stock purchase right for each outstanding share of the Company’s common stock. The Company’s Board of Directors adopted the Rights Plan to protect stockholder value by protecting the Company’s ability to realize the benefits of its net operating losses (“NOLs”) and capital loss carryforwards. The Company has experienced substantial operating and capital losses in previous years. Under the Internal Revenue Code and rules promulgated by the Internal Revenue Service, the Company may “carry forward” these losses in certain circumstances to offset current and future earnings and thus reduce its federal income tax liability, subject to certain requirements and restrictions. Assuming that the Company has future earnings, the Company may be able to realize the benefits of NOLs and capital loss carryforwards. These NOLs and capital loss carryforwards constitute a substantial asset to the Company. If the Company experiences an “Ownership Change,” as defined in Section 382 of the Internal Revenue Code, its ability to use the NOLs and capital loss carryforwards could be substantially limited or lost altogether. In general terms, the Rights Plan imposes a significant penalty upon any person or group that acquires certain percentages of the Company’s common stock by allowing other shareholders to acquire equity securities at half their fair values.
STOCK BUY-BACK PROGRAM - In September 2000, the Board of Directors approved an additional repurchase of $3,000 of the Company’s common stock bringing the total authorized to $7,500. The Company has not purchased any shares under this program since fiscal 2001, when 11,500 shares were purchased for $108. As of August 2, 2008, 489,806 shares had been purchased by the Company for $4,502 under this Stock Buy-Back Program. These shares are included in Treasury Shares on the accompanying Balance Sheet.
INCREASE OF AUTHORIZED SHARES - At a special meeting of shareholders of the Company held on November 17, 2006, the Company’s shareholders approved an Amendment of the Certificate of Incorporations of the Company to increase the number of authorized shares of the Company’s common stock, par value $0.10 per share, from twenty million (20,000,000) shares to fifty million (50,000,000) shares in order to have a sufficient number of shares of Common Stock to provide a reserve of shares available for issuance to meet business needs as they may arise in the future. Such business needs may include, without limitation, rights offerings, financings, acquisitions, establishing strategic relationships with corporate partners, providing equity incentives to employees, officers or directors, stock splits or similar transactions.
STOCK OPTION PLAN AND WARRANTS – Effective July 31, 2005, the Company adopted SFAS No. 123 (R). This standard requires that the Company measure the cost of employee services received in exchange for an award of equity instruments based on the grant date fair value of the award. That cost will be recognized over the period in which the employee is required to provide the services – the requisite service period (usually the vesting period) – in exchange for the award. The grant date fair value for options and similar instruments will be estimated using option pricing models. Under SFAS 123 (R), the Company is required to select a valuation technique or option pricing model that meets the criteria as stated in the standard, which includes a binomial model and the Black-Scholes model. At the present time, the Company is continuing to use the Black-Scholes model. The adoption of SFAS 123 (R), applying the “modified prospective method,” as elected by the Company, requires the Company to value stock options granted prior to its adoption of SFAS 123 (R) under the fair value method and expense the related unvested amounts over the remaining vesting period of the stock options.
Details regarding the fair value of stock options granted in fiscal 2008, 2007 and 2006 are as follows:
| | 2008 | | 2007 | | 2006 |
| Estimated life (in years) | 7 | | 7 | | 7 |
| Volatility rate | 64%-72% | | 63%-74% | | 62% |
| Risk free interest rate | 3.60%-4.20% | | 4.44%-5.16% | | 4.74%-4.93% |
| Dividend rate | 0% | | 0% | | 0% |
| Forfeiture rate | 2% | | 2% | | 0% |
| Weighted average fair value | 1.82 | | 1.16 | | 1.58 |
| Recorded compensation expense | $ 481 | | $ 220 | | $ 142 |
The Black Scholes Option Pricing Model requires the use of various assumptions. The key assumptions are summarized as follows:
Estimated life: The Company derives its estimated life based on historical experience.
Volatility rate: The Company estimates the volatility of its common stock at the date of grant based on historical volatility of its common stock.
Risk free interest rate: The Company derives its risk-free interest rate on the Barron’s zero coupon bond rate for a term equivalent to the expected life of the option.
Dividend rate: The Company estimates the dividend yield assumption based on the Company’s historical and projected dividend payouts.
Forfeiture rate: The Company estimates the annual forfeiture rate based on historical experience.
On March 20, 2007, shareholders approved the 2007 Incentive Stock Plan. A total of 1,000,000 shares of the Company’s common stock may be granted under the Plan, of which, 604,500 shares are still available for grant as of August 2, 2008. No additional options will be granted under the former stock option plan. Substantially all of the options granted under this Plan and the prior plan provide for graded vesting and vest generally at a rate of 25% per year beginning with the date of grant, expiring ten to fifteen years from the date they are granted. The option price per share is approved by the Board of Directors. All options to date have been granted at the fair market value of the Company’s stock at the date of grant. No options can be granted under this plan subsequent to February 21, 2017.
In December 2000, the Board of Directors approved an extension of time to exercise for all stock option holders. The extension covers all options whose term would have expired during the period from the stock de-listing date (December 19, 2000) up to the date that the shares become re-listed on a national exchange. This extension grants those stock option holders a period of six months from the date of re-listing to exercise vested options which may have otherwise expired without the extension. Options that otherwise expired due to termination of employment for cause were not effected by this extension. During fiscal 2005, the plan was modified to remove this extension provision from options granted after January 2005. The majority of the Company’s stock options have a 10 year term, however, due to uncertainty regarding the duration of this extension, the Company cannot calculate the weighted average remaining contractual term of outstanding or vested options. The extension provision does not impact the 2007 Incentive Stock Plan.
OPTION ACTIVITY
The following stock option information is as of:
| | | August 2, 2008 | | | JULY 28, 2007 | | | JULY 29, 2006 | |
| | | SHARES OUTSTANDING | | | WEIGHTED AVERAGE EXERCISE PRICE | | | SHARES OUTSTANDING | | | WEIGHTED AVERAGE EXERCISE PRICE | | | SHARES OUTSTANDING | | | WEIGHTED AVERAGE EXERCISE PRICE | |
| Granted and outstanding, beginning of year | | | 1,913,996 | | | $ | 3.51 | | | | 1,545,996 | | | $ | 3.93 | | | | 1,662,494 | | | $ | 3.81 | |
| Granted | | | 336,500 | | | | 2.62 | | | | 469,000 | | | | 1.73 | | | | 100,000 | | | | 2.66 | |
| Exercised | | | (83,181 | ) | | | 1.37 | | | | (101,000 | ) | | | 1.69 | | | | (99,000 | ) | | | 2.09 | |
| Cancelled and forfeited | | | (72,500 | ) | | | 3.58 | | | | - | | | | - | | | | (117,498 | ) | | | 2.56 | |
| Outstanding at end of year | | | 2,094,815 | | | | 3.45 | | | | 1,913,996 | | | | 3.51 | | | | 1,545,996 | | | | 3.93 | |
| Exercisable at end of year | | | 1,595,438 | | | | 3.85 | | | | 1,494,743 | | | | 4.00 | | | | 1,477,243 | | | | 4.00 | |
As mentioned above, due to an extension of exercise time granted to option holders that has an uncertain term, the Company is unable to calculate the weighted average contractual term of the above options.
As of August 2, 2008 the distribution of stock option exercise prices is as follows:
| | | | OPTIONS OUTSTANDING | | | OPTIONS EXERCISABLE | |
| | | | | | | | | | | | | | |
EXERCISE PRICE RANGE | | | NUMBER OF OPTION SHARES | | | WEIGHTED AVERAGE EXERCISE PRICE | | | SHARES EXERCISABLE | | | WEIGHTED AVERAGE EXERCISE PRICE | |
| $ | 1.00 - $3.34 | | | | 1,414,107 | | | $ | 1.94 | | | | 914,729 | | | $ | 1.80 | |
| $ | 4.00 - $6.60 | | | | 313,256 | | | | 4.85 | | | | 313,258 | | | | 4.85 | |
| $ | 7.00 - $7.94 | | | | 220,775 | | | | 7.51 | | | | 220,774 | | | | 7.51 | |
| $ | 8.00 - $10.00 | | | | 146,677 | | | | 8.93 | | | | 146,667 | | | | 8.93 | |
| | | | | | 2,094,815 | | | $ | 3.45 | | | | 1,595,438 | | | $ | 3.85 | |
| | | | | | | | | | | | | | | | | | | |
At August 2, 2008, the aggregate intrinsic value of options outstanding and options exercisable was $127 and $127, respectively. The intrinsic value is the amount by which the market value of the underlying stock exceeds the exercise price of the option at the measurement date for all-in-the money options.
Future compensation expense related to the vesting of employee options granted by August 2, 2008 is expected to be $344 in 2009, $186 in 2010 and $45 in 2011.
Cash proceeds and intrinsic value related to total stock options exercised are provided in the following table:
| YEAR ENDED | | AUGUST 2, 2008 | | | JULY 28, 2007 | | | JULY 29, 2006 | |
| Proceeds from stock options exercised | | $ | 46 | (a) | | $ | 170 | | | $ | 238 | |
| Intrinsic Value | | | 71 | | | | 57 | | | | 137 | |
(a) In addition to these proceeds, the Company accepted 31,694 shares of common stock held by the option holder and valued at $69 into treasury.
WARRANTS
On February 6, 2004, a motion was filed for summary judgment to enforce a January 2002 class action settlement agreement entered into by the Company. The motion sought damages in the amount of $1,250 together with interest, costs and disbursements, and a declaration that $2,000 in promissory notes issued as part of the class action settlement are immediately due and payable, as the value of damages due to the Company’s failure to timely complete a registration statement related to the common shares underlying certain warrants granted in the class action settlement. The Company filed opposition to this matter on March 5, 2004. Plaintiffs filed reply papers on March 19, 2004. In addition, the Company filed a registration statement related to the warrant shares on March 23, 2004, and it was declared effective by the SEC on May 7, 2004. In July 2004, in settlement of this matter, Del Global modified the exercise, or “strike,” price of the 1,000,000 warrants issued in 2002 from $2.00 to $1.50 per share, and extended the expiration date of such warrants by one year to March 28, 2009. During fiscal 2008, 2007 and 2006, 61,016, 366,854 and 1,574, respectively, of these warrants were exercised for cash proceeds to the Company of $91, $551 and $2, respectively. As of August 2, 2008, 512,500 of these warrants were outstanding.
| 11. | INCOME (LOSS) PER SHARE |
| | | FOR FISCAL YEARS ENDED | |
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | | | JULY 29, 2006 | |
| Numerator: | | | | | | | | | |
| Net income | | $ | 2,977 | | | $ | 3,816 | | | $ | 94 | |
| Denominator: | | | | | | | | | | | | |
| Weighted average shares outstanding for basic income per share | | | 24,195,498 | | | | 16,154,552 | | | | 11,244,421 | |
| Effect of dilutive securities | | | 450,911 | | | | 300,673 | | | | 832,075 | |
| Weighted average shares outstanding for diluted income per share | | | 24,646,409 | | | | 16,455,225 | | | | 12,076,496 | |
| Income per basic common share | | $ | 0.12 | | | $ | 0.24 | | | $ | 0.01 | |
| Income per diluted common share | | $ | 0.12 | | | $ | 0.23 | | | $ | 0.01 | |
Common shares outstanding for the fiscal years ended August 2, 2008, July 28, 2007 and July 29, 2006, were reduced by 654,464, 622,770 and 622,770 shares of treasury stock, respectively.
The computation of diluted shares outstanding does not include the effect of the assumed exercise of 1,295,612, 1,121,684 and 1,180,389 for employee stock options outstanding as of August 2, 2008, July 28, 2007 and July 29, 2006, respectively, and 0, 0 and 474,113 warrants to purchase Company common stock for those years because the effect of their assumed exercise would be anti-dilutive.
The Company’s consolidated income from continuing operations before income tax benefit and minority interest for fiscal years 2008, 2007 and 2006 of $6,224, $7,369 and $2,135 reflects foreign pre-tax net income of $8,294, $8,180 and $3,620 for fiscal years 2008, 2007 and 2006, respectively, and a U.S. pre-tax loss of $2,070, $811 and $1,485, respectively.
Provision for income taxes consists of the following:
| | | FOR FISCAL YEARS ENDED | |
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | | | JULY 29, 2006 | |
| CURRENT TAX EXPENSE: | | | | | | | | | |
| Foreign | | $ | 3,297 | | | $ | 3,410 | | | $ | 1,739 | |
| State and local | | | - | | | | 5 | | | | 35 | |
| DEFERRED PROVISION (BENEFIT): | | | | | | | | | | | | |
| Federal | | | - | | | | - | | | | - | |
| State and local | | | -- | | | | -- | | | | -- | |
| Foreign | | | (50 | ) | | | 138 | | | | (16 | ) |
| NET PROVISION | | $ | 3,247 | | | $ | 3,553 | | | $ | 1,758 | |
The following is a reconciliation of the statutory Federal and effective income tax rates:
| | | FOR FISCAL YEARS ENDED | |
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | | | JULY 29, 2006 |
| Statutory Federal income tax rate | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % |
| State tax, less Federal tax effect | | | 0.0. | % | | | 0.1. | % | | | 1.6 | % |
| Foreign taxes | | | 12.7 | % | | | 9.2 | % | | | 8.2 | % |
| Valuation allowance adjustment | | | 41.9 | % | | | (1.9 | )% | | | (98.5 | )% |
Provision (reversal) for undistributed earnings of foreign subsidiary | | | (36.7 | )% | | | 7.2 | % | | | 138.8 | % |
| Other | | | 0.3 | % | | | (0.4 | )% | | | (1.7 | )% |
| Effective tax rate | | | 52.2 | % | | | 48.2 | % | | | 82.4 | % |
Deferred income tax assets (liabilities) are comprised of the following:
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | |
| Deferred income tax assets: | | | | | | |
| Federal net operating loss carry forward | | $ | 15,375 | | | $ | 15,420 | |
| State tax credits and operating loss carry forwards | | | 2,071 | | | | 2,223 | |
| Reserve for inventory obsolescence | | | 1,545 | | | | 1,417 | |
| Allowances and reserves not currently deductible | | | 1,076 | | | | 933 | |
| Amortization | | | 295 | | | | 197 | |
| Stock based compensation | | | 599 | | | | 430 | |
| Fixed assets | | | 133 | | | | - | |
| Other | | | - | | | | 55 | |
| Gross deferred income tax assets | | | 21,094 | | | | 20,675 | |
| Deferred income tax liabilities: | | | | | | | | |
| Undistributed earnings of foreign subsidiary | | | - | | | | (2,278 | ) |
| Fixed assets | | | - | | | | (383 | ) |
| Other | | | (15 | ) | | | - | |
| Gross deferred income tax liabilities | | | (15 | ) | | | (2,661 | ) |
| Less: valuation allowance | | | (20,309 | ) | | | (17,295 | ) |
| Net deferred income tax assets | | $ | 770 | | | $ | 719 | |
Deferred income tax assets and liabilities are recorded in the consolidated balance sheets as follows:
| | | AUGUST 2, 2008 | | | JULY 28, 2007 | |
| Deferred income tax assets – non-current | | $ | 770 | | | $ | 1,011 | |
| Deferred income tax liabilities -- non-current | | | -- | | | | (292 | ) |
| | | $ | 770 | | | $ | 719 | |
The Company accounts for deferred income taxes in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 109 “Accounting for Income Taxes” whereby it recognizes deferred tax assets and liabilities for temporary differences between financial
reporting basis and income tax reporting basis and for tax credit carry forwards.
The Company periodically assesses the realization of its net deferred income tax assets. This evaluation is primarily based upon current operating results and expectations of future operating results. A valuation allowance is recorded if the Company believes its net deferred income tax assets will not be realized. Its determination is based on what it believes will be the more likely than not result.
During fiscal years 2008, 2007 and 2006, the Company reported operating income on a consolidated basis. The Company’s foreign tax reporting entity was profitable, and its U.S. tax reporting entities incurred a loss in each fiscal year. Based primarily on these results, the Company concluded that it should maintain a 100% valuation allowance on its net U.S. deferred tax assets as of August 2, 2008.
The Company recorded a tax expense with respect to its foreign subsidiary’s income in all periods presented and based on a more likely than not standard, believes that the foreign subsidiary’s net deferred income tax asset of $770 at August 2, 2008 will be realized.
The Company’s foreign subsidiary operates in Italy. Italy recently enacted legislation that reduces tax rates effective for the Company’s fiscal year 2009. Fiscal 2008 income tax expense includes a charge that reduces the carrying value of the foreign subsidiary’s net deferred income tax asset resulting from the income tax rate reduction.
Additionally, the Company’s deferred income tax liabilities as of July 28,2007 included the estimated tax obligation that would have been incurred upon a distribution of the foreign subsidiary’s earnings to its U.S. parent. This tax liability was recorded as the foreign subsidiary had routinely distributed monies to its U.S. parent. Based on current operating results, expectations of future results and available cash and credit in the U.S., the Company has determined it no longer intends to repatriate monies in the foreseeable future and has reversed this tax obligation. The reversal resulted in an adjustment to available net operating loss carryforwards and the related valuation allowance and in a reduction in tax expense for Fiscal 2008.
The Company recorded a $100 tax benefit resulting from the effect of the Italian tax rate change on deferred tax assets and the reversal of the estimated tax obligation upon repatriation of non-U.S. earnings..
At August 2, 2008, the Company has federal net operating loss carry forwards of $15,375 that expire at various times between July, 2020 and July, 2026.
It is the Company’s practice to recognize interest and/or penalties related to income tax matters in tax expense. As of August 2, 2008, there were no material interest or penalty amounts to accrue.
| 13. | COMMITMENTS AND CONTINGENCIES |
a. The Company had an employment agreement with Samuel Park, a previous Chief Executive Officer (“CEO”), for the period May 1, 2001 to April 30, 2004. The employment agreement provided for certain payments in the event of a change in the control of the Company. On October 10, 2003, the Company announced the appointment of Walter F. Schneider as President and CEO to replace Mr. Park, effective as of such date. As a result, the Company recorded a charge of approximately $200 during the first quarter of fiscal 2004 to accrue the balance remaining under Mr. Park’s employment agreement.
The Company’s employment agreement with Mr. Park provided for payments upon certain changes in control. The Company’s Board of Directors elected at the Company’s Annual Meeting of Shareholders held on May 29, 2003, had reviewed the “change of control” provisions regarding payments totaling up to approximately $1,800 under the employment agreement between the Company and Mr. Park. As a result of this review and based upon, among other things, the advice of special counsel, the Company’s Board of Directors has determined that no obligation to pay these amounts has been triggered. Prior to his departure from the Company on October 10, 2003, Mr. Park orally informed the Company that, after reviewing the matter with his counsel, he believed that the obligation to pay these amounts has been triggered. On October 27, 2003, the Company received a letter from Mr. Park’s counsel demanding payment of certain sums and other consideration pursuant to the Company’s employment agreement with Mr. Park, including these change of control payments. On November 17, 2003, the Company filed a complaint in the United States District Court, Southern District of New York, against Mr. Park seeking a declaratory judgment that no change in control payment was or is due to Mr. Park, and that an amendment to the employment contract with Mr. Park regarding advancement and reimbursement of legal fees is invalid and unenforceable. Mr. Park answered the complaint and asserted counterclaims seeking payment from the Company based on his position that a “change in control” occurred in June 2003. Mr. Park is also seeking other consideration he believes he is owed under his employment agreement. The Company filed a reply to Mr. Park’s counterclaims denying that he is entitled to any of these payments. Discovery in this matter was conducted and completed. Following discovery, the Company and Mr. Park filed motions for summary judgment on the issues related to the change in control and the amendment to the employment agreement, which motions have been fully submitted to the court for consideration. To date, no decision has been issued by the court on these motions. If Mr. Park prevails on his claims and the payments he seeks are required to be paid in a lump sum, these payments may have a material adverse effect on the Company’s liquidity. It is not possible to predict the outcome of these claims. However, the Company’s Board of Directors does not believe that such a claim is reasonably likely to result in a material decrease in the Company’s liquidity in the foreseeable future. The Company has not recorded an accrual for any potential settlements of this claim as it has no basis upon which to estimate either the outcome or amount of loss, if any.
b. On May 24, 2007, the Company’s Power Conversion subsidiary (“RFI”) was served with a subpoena to testify before a grand jury of the United States District Court of New York and to provide items and records from its Bay Shore NY offices in connection with U.S. Department of Defense contracts. A search warrant from the United States District Court, Eastern District of New York was issued and executed with respect to such offices. The Company believes that it is in full compliance with the quality standards that its customers require and is fully cooperating with investigators to assist them with their review. RFI continues to ship products to the U.S. Government as well as to its commercial customers.
c. On June 28, 2002, Jeffrey N. Moeller, the former Director of Quality Assurance and Regulatory Affairs of Del Medical, commenced an action in the Circuit Court of Cook County, Illinois, against the Company, Del Medical and Walter Schneider, the former President of Del Medical. In the most current iteration of his complaint, the third amended complaint, Mr. Moeller alleged four claims against the defendants in the action: for (1) retaliatory discharge from employment with Del Medical, allegedly in response to Mr. Moeller’s complaints to officers of Del Medical about purported prebilling and his stopping shipment of a product that allegedly did not meet regulatory standards, (2) defamation, (3) intentional interference with his employment relationship with Del Medical and prospective employers, and (4) to hold the Company liable for any misconduct of Del Medical under a theory of piercing the corporate veil. In their answer to the third amended complaint, the defendants denied the substantive allegations of each of these claims and denied that they have any liability to Mr. Moeller. By order dated September 15, 2006, the Court denied in part and granted in part defendants’ motion requesting summary judgment dismissing the third amended complaint. The court granted the motion only to the extent of dismissing that part of Mr. Moeller’s claim of interference with his employment relationship with Del Medical and his relationship with prospective employers.
In fiscal 2007, the Company recorded an accrual of $100 relating to potential liability in the settlement of these claims. The parties appeared for mediation in January 2007 but the mediation did not result in a disposition of the action. A trial was held in April 2008 and on April 17, 2008, the jury returned a verdict in favor of Mr. Moeller for $1,800 million for lost earnings, back pay, front pay and benefits on the retaliatory discharge claim, and $200 for emotional distress/reputation damages and $200 in punitive damages on the defamation claim. On May 19, 2008, counsel for the defendants filed their motion for judgment in their favor notwithstanding the jury verdict, or, alternatively, for a new trial, on those claims on which the jury found the respective defendants liable. By order dated August 15, 2008, the Court denied that motion.
On August 25, 2008, the Company and the other defendants filed their notice of appeal by which they appeal to the Appellate Court of Illinois, First District, among other things, the judgment entered against the Company and the other defendants on the jury verdict. On September 12, 2008, the Company and the other defendants filed an amended notice of appeal. The Company intends to pursue the appeal vigorously, seeking thereby reversal of the judgment and to have a judgment entered in favor of the Company and the other defendants or to have a new trial. In lieu of a bond, the Company filed an irrevocable standby letter of credit in the amount of $2,600 by which Mr. Moeller can collect the amount of the judgment entered by the trial court in the event the appellate court affirms that judgment.
d. On April 28, 2008, Apergis, the former General Manager of RFI, filed a charge with the EEOC alleging that RFI discriminated against him by terminating his employment with RFI on December 18, 2007. Apergis alleged three claims against RFI: (1) violation of Title VII of the Civil Rights Act; (2) violation of the Age Discrimination in Employment Act and (3) retaliation. RFI responded to the EEOC charge with a position statement filed with the EEOC on June 26, 2008 denying each allegation of the charge. As of September 16, 2008, RFI is waiting to hear for a response to their position statement from the EEOC. RFI intends to defend vigorously against Apergis.
e. In December 2007, the Company was added to an action brought against several other defendants in which the plaintiff alleges that she is the wife of a chiropractor who died in June 2005. Plaintiff alleges that her husband was exposed to chemical products in developing x-ray films and cleaning film processing machines and worked with the x-ray film processing machines and x-ray machines, and that the defendants manufactured, supplied or serviced the chemical products and machines. The complaint further alleges that the decedent was exposed to toxic chemicals and radiation from the chemical products used on or in the machines, which caused “serious injuries to his internal organs, including acute myelogenous leukemia”, resulting in his death.
The complaint alleges the following six claims against the defendants: (1) negligence in manufacturing. and distributing the chemical products and machines, and servicing the machines, and failing adequately to warn decedent of the hazards of the chemical products and machines, (2) violation of a California statute and regulation by failing to determine whether the chemical products caused health hazards and failing to label or identify in material safety data sheets a health hazard relating to acute myelogenous leukemia, (3) strict products liability for failing to warn adequately of the chemical products’ and machines’ health hazards, (4) strict products liability for defects in the design of the chemical products and machines, (5) fraudulent concealment of the toxic and carcinogenic nature of the chemical products and the machines, and (6) breach of implied warranties as to the fitness of the chemical products and machines for intended uses, merchantability, and lack of defects.
While the complaint does not allege a total amount of damages sought, plaintiff alleges that she has suffered damages consisting of medical, funeral, and burial expenses, the decedent’s lost earnings prior to and lost wages after his death, lost benefits after his death, the value of his services in managing his family’s home, and loss of companionship and similar losses. The plaintiff also requests punitive damages. The Company has not recorded an accrual for any potential settlement of this claim as it has no basis upon which to estimate either the outcome or amount of loss, if any. In its answer to the complaint, the Company denied the substantive allegations of the complaint and denied that it has any liability to plaintiff. The Company intends to defend vigorously against plaintiff’s claims.
f. In addition, the Company is a defendant in several other legal actions in various U.S. and foreign jurisdictions arising from normal course of business. Management believes the Company has meritorious defenses to such actions and that the outcomes will not be material to the Company’s consolidated financial statements
LEASE COMMITMENTS - The Company leases facilities for its manufacturing operations with expiration dates ranging from 2007 through 2012. In addition, the Company has various office equipment and auto leases accounted for as operating leases. The future minimum annual lease commitments as of August 2, 2008 are as follows:
| 2009 | $605 | |
| 2010 | 244 | |
| 2011 | 120 | |
| 2012-2014 | 93 | |
| Total | $1,062 | |
Rent expense for fiscal years 2008, 2007 and 2006 was $416, $396 and $300, respectively.
| 14. | SUPPLEMENTAL QUARTERLY FINANCIAL INFORMATION (UNAUDITED) |
YEAR ENDED AUGUST 2, 2008:
| | | | | | QUARTER | | | | |
| | | FIRST | | | SECOND | | | THIRD | | | FOURTH | |
| Net sales | | $ | 26,716 | | | $ | 29,894 | | | $ | 24,450 | | | $ | 27,246 | |
| Gross margin | | $ | 6,431 | | | $ | 7,490 | | | $ | 5,715 | | | $ | 7,151 | |
| Net income (loss) | | $ | 1,107 | | | $ | 1,427 | | | $ | (1,638 | ) | | $ | 2,081 | |
| Net income (loss) per basic share | | $ | 0.05 | | | $ | 0.06 | | | $ | (0.07 | ) | | $ | 0.08 | |
| Net income (loss) per diluted share | | $ | 0.04 | | | $ | 0.06 | | | $ | (0.07 | ) | | $ | 0.09 | |
YEAR ENDED JULY 28, 2007:
| | | | | | QUARTER | | | | |
| | | FIRST | | | SECOND | | | THIRD | | | FOURTH | |
| Net sales | | $ | 19,286 | | | $ | 26,771 | | | $ | 27,122 | | | $ | 30,988 | |
| Gross margin | | $ | 4,011 | | | $ | 6,749 | | | $ | 6,025 | | | $ | 8,232 | |
| Income (loss) from continuing operations | | $ | (487 | ) | | $ | 1,082 | | | $ | 1,056 | | | $ | 2,165 | |
| Net income (loss) | | $ | (487 | ) | | $ | 1,082 | | | $ | 1,056 | | | $ | 2,165 | |
| Net income (loss) per basic share | | $ | (0.04 | ) | | $ | 0.09 | | | $ | 0.06 | | | $ | 0.09 | |
| Net income (loss) per diluted share | | $ | (0.04 | ) | | $ | 0.09 | | | $ | 0.06 | | | $ | 0.09 | |
