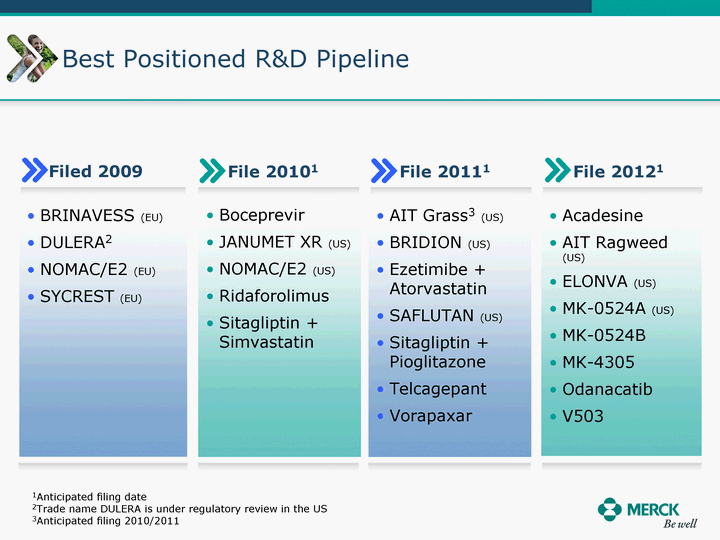
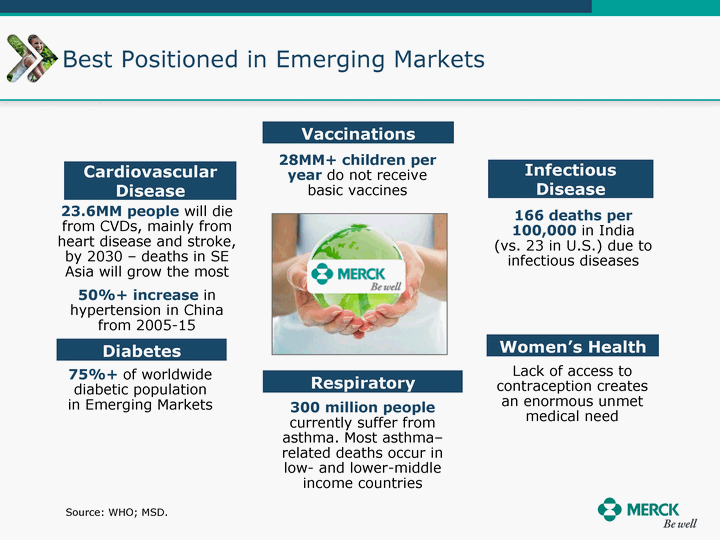
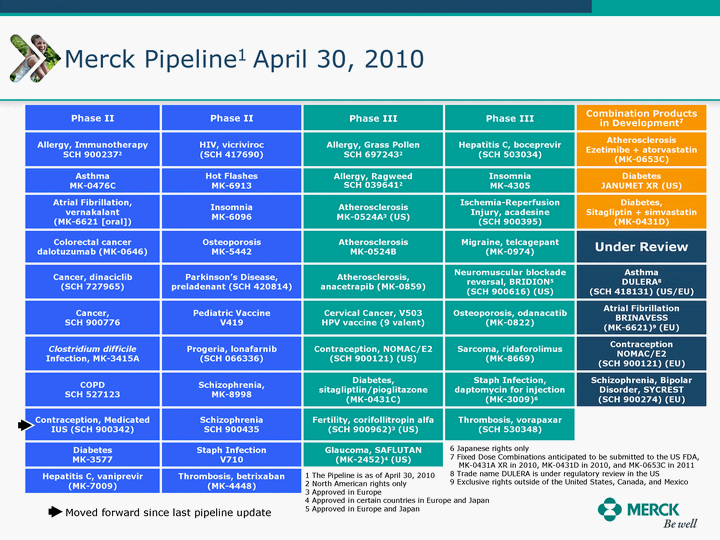
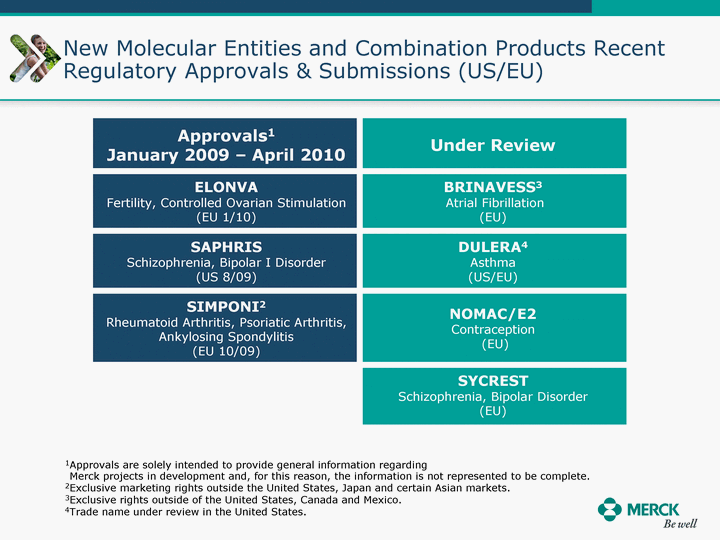
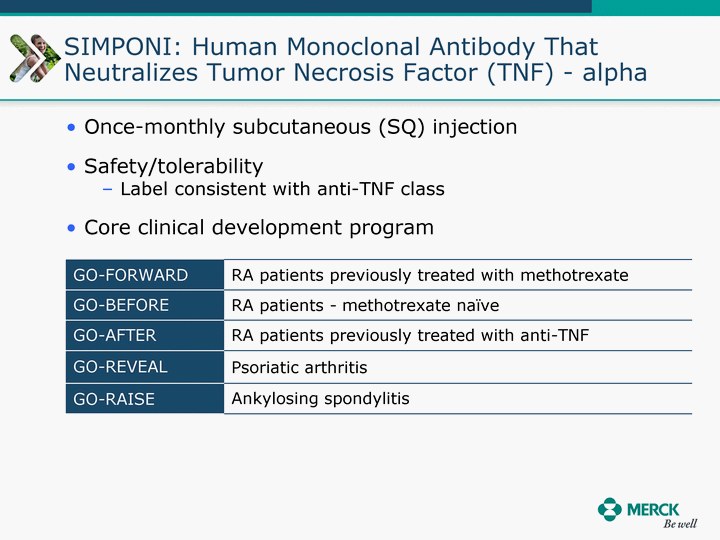
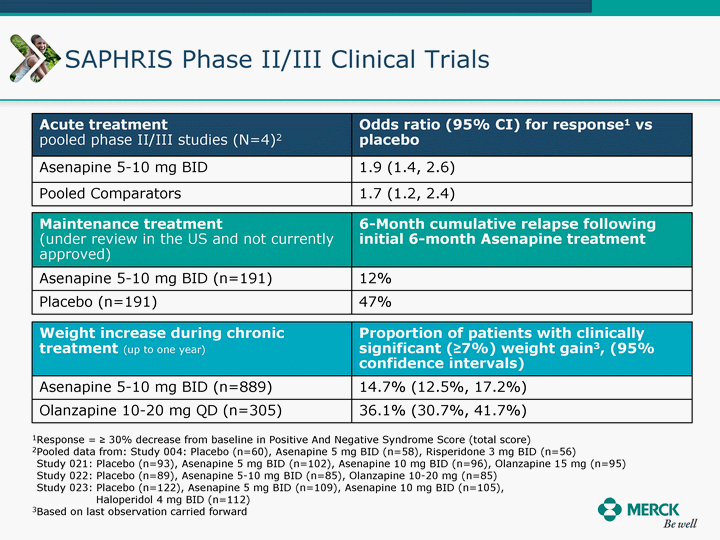
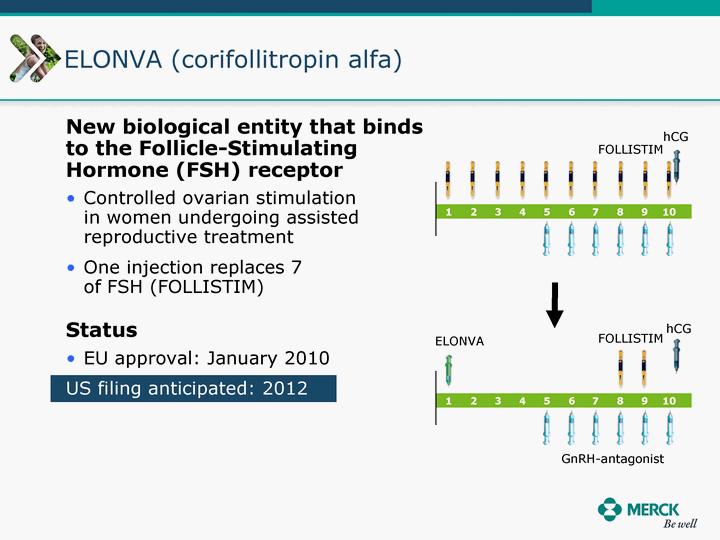
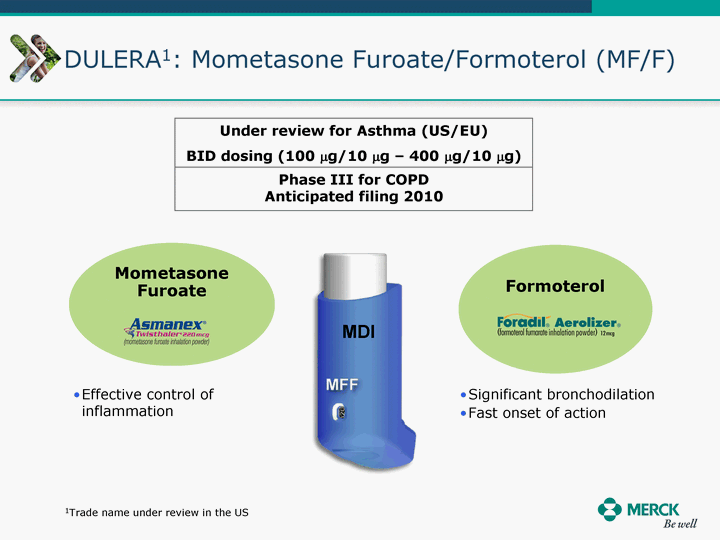
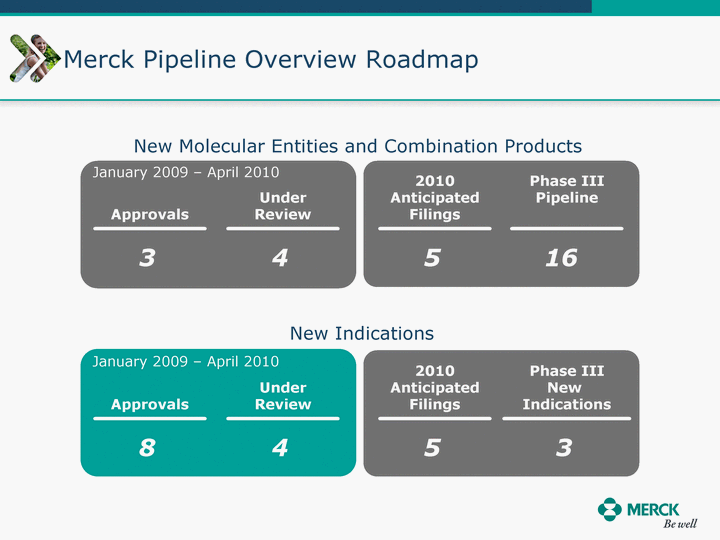
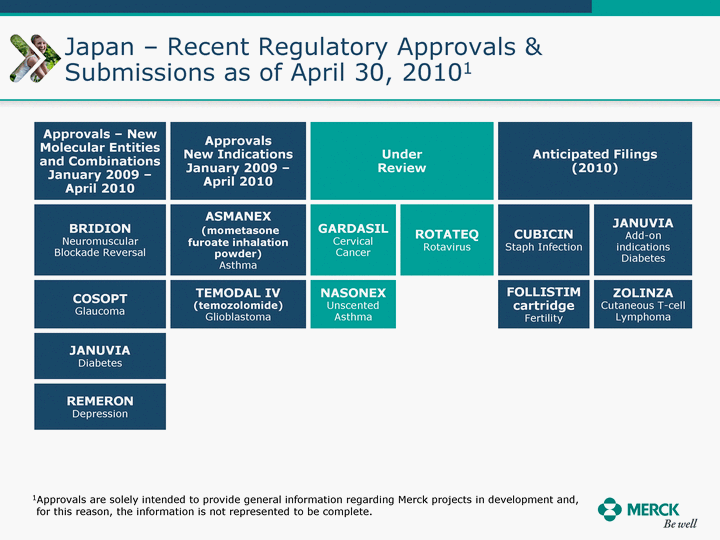
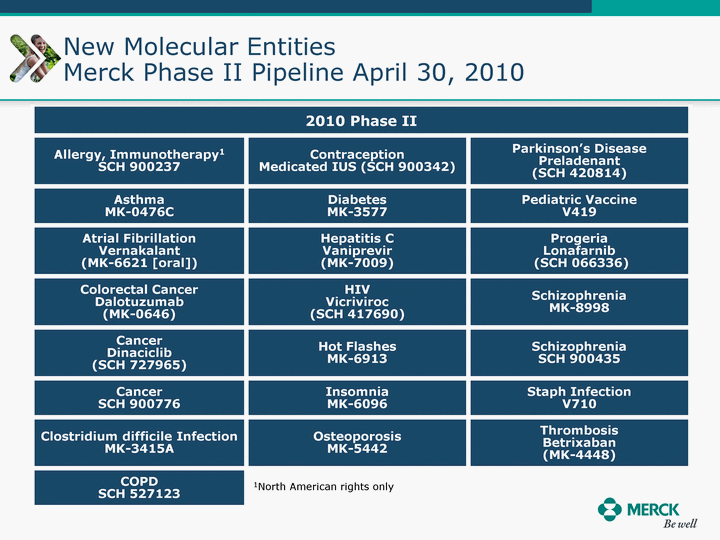
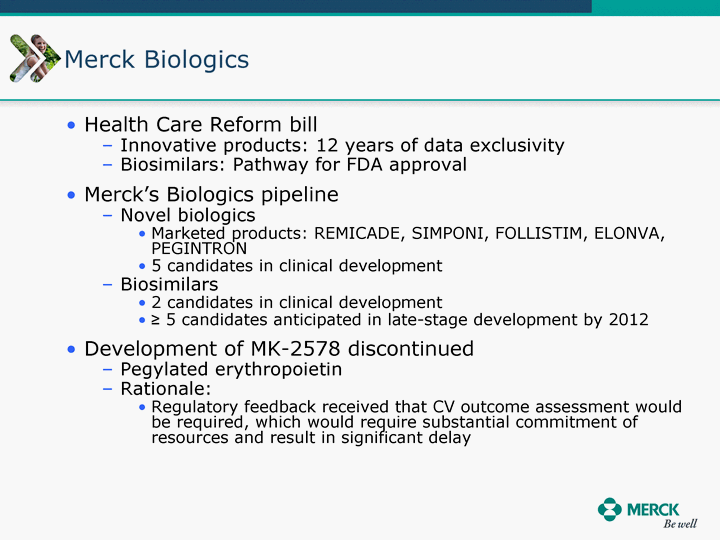
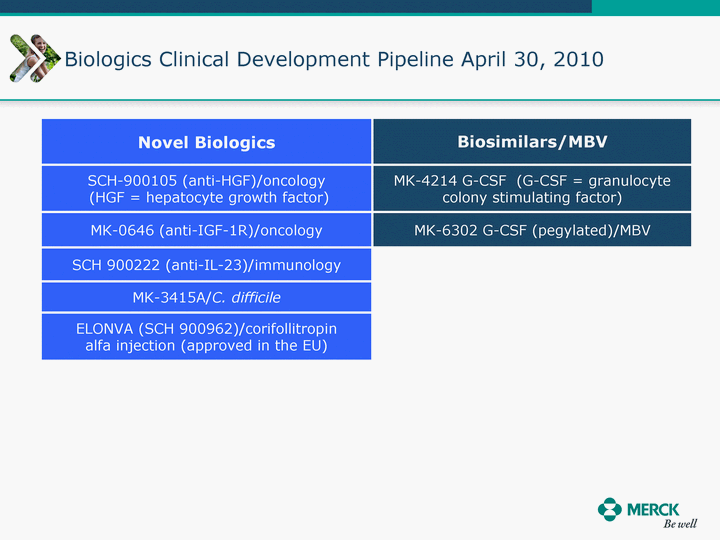
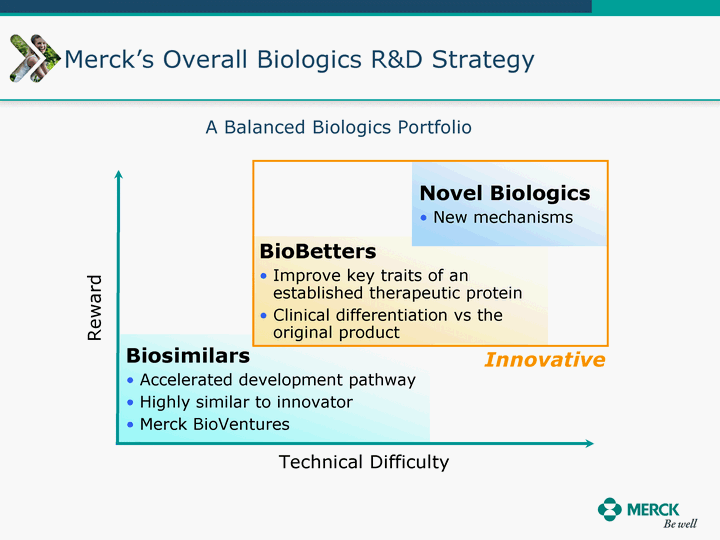
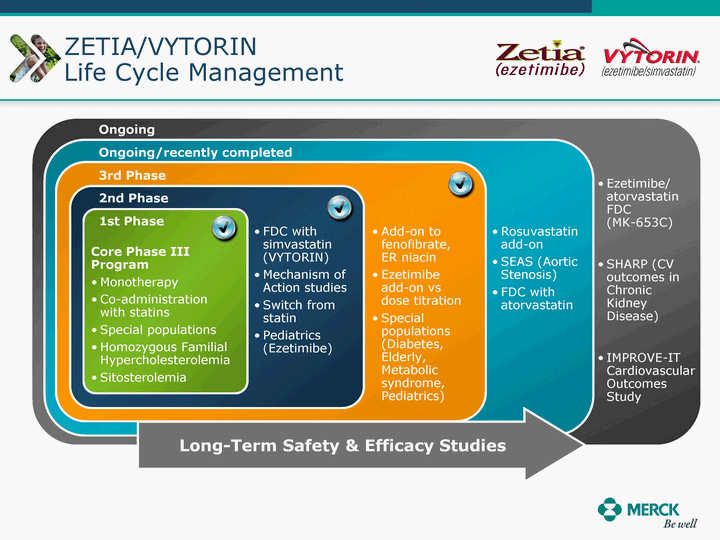
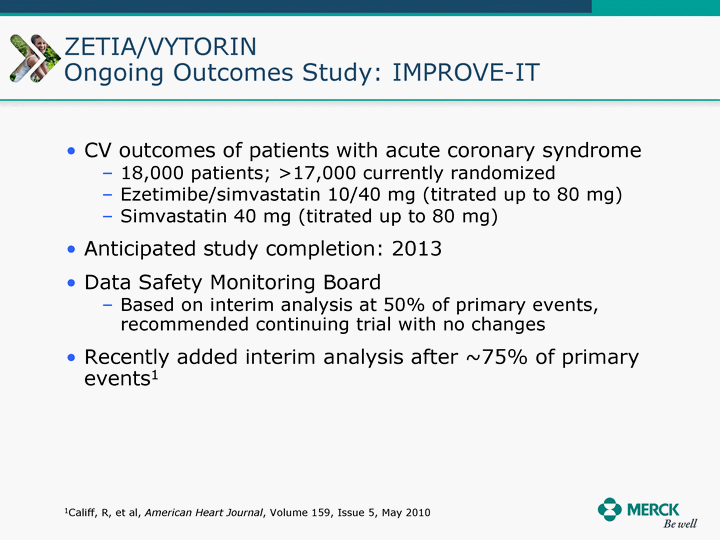
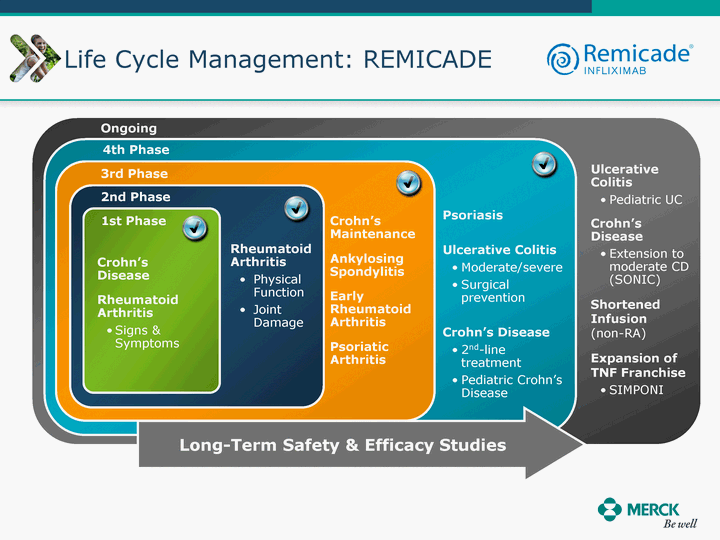
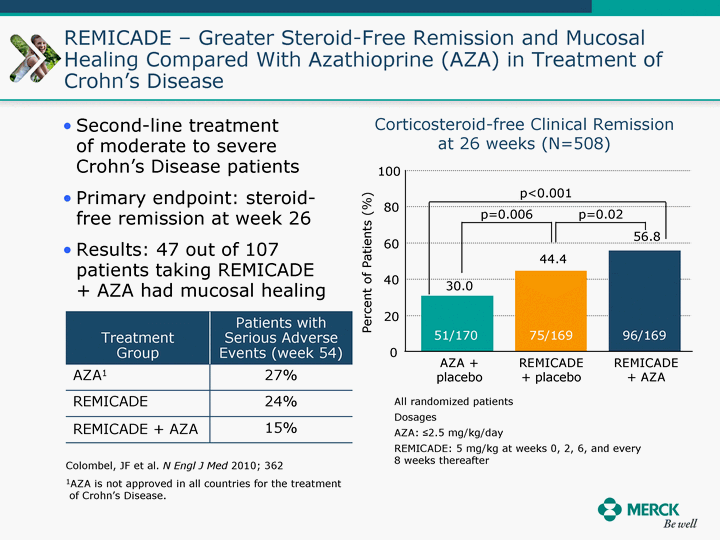
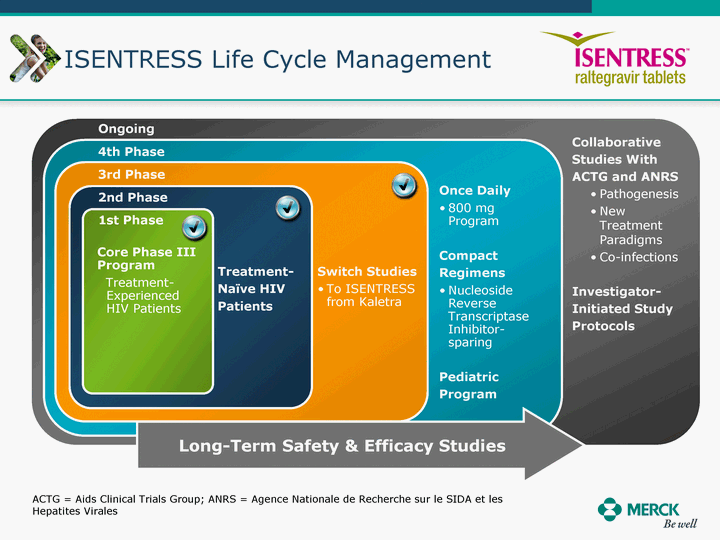
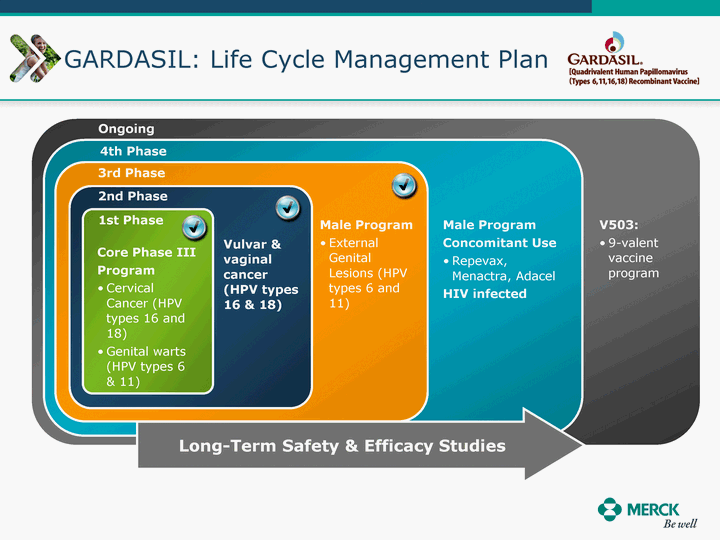
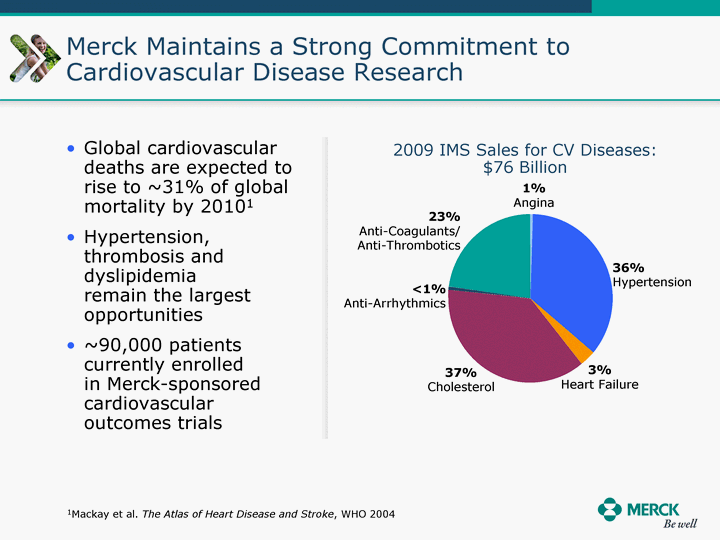
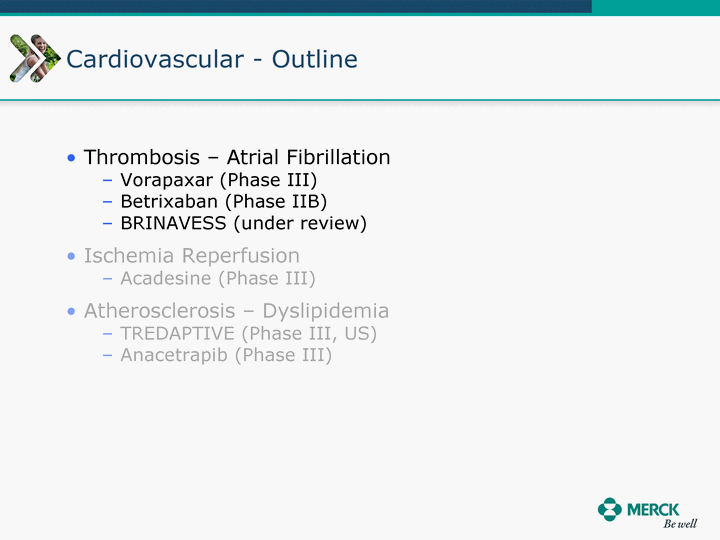
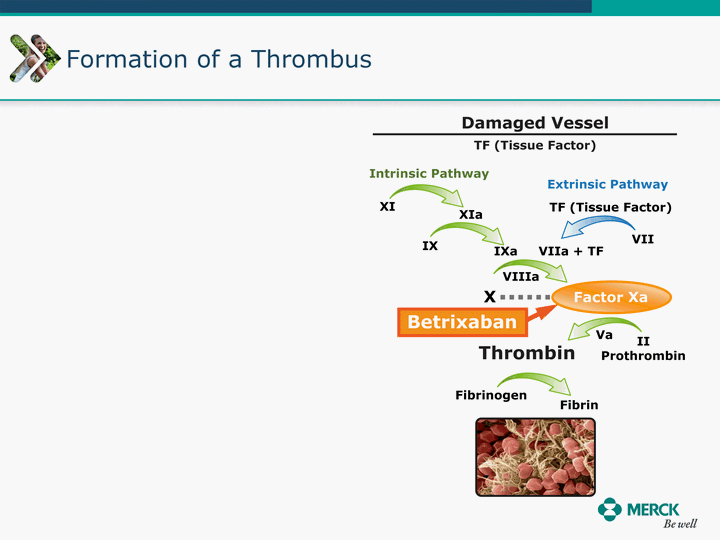
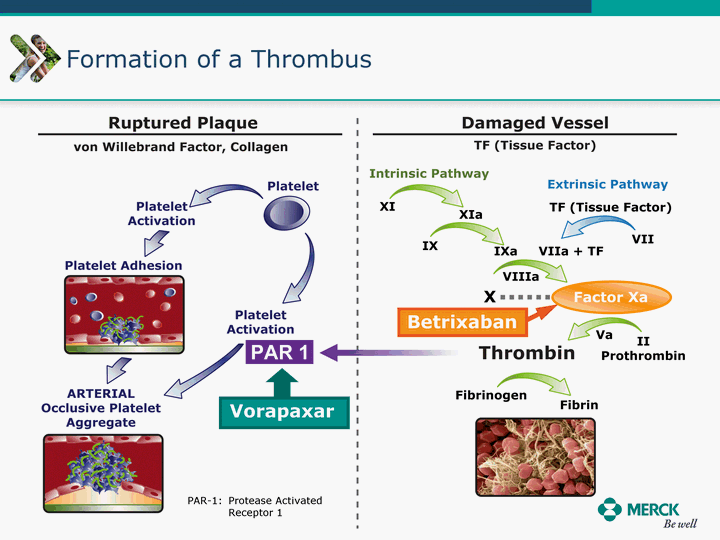
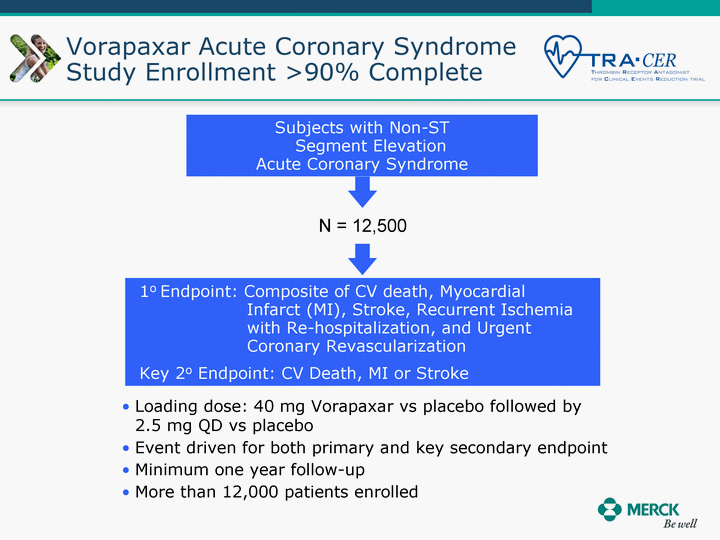
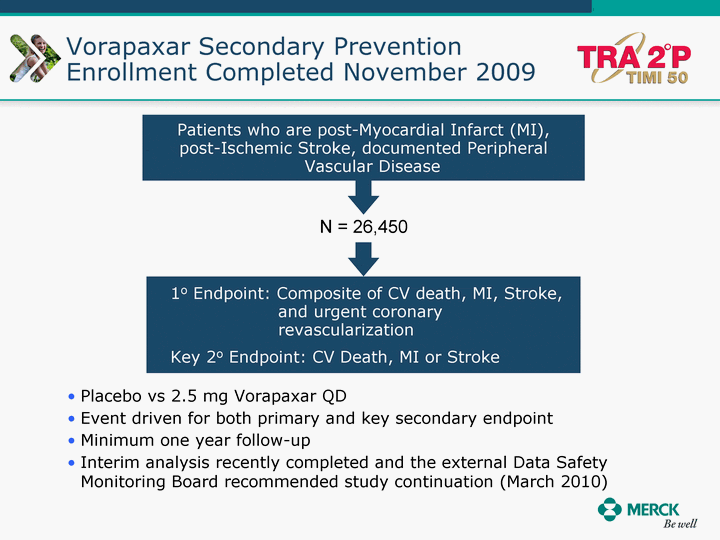
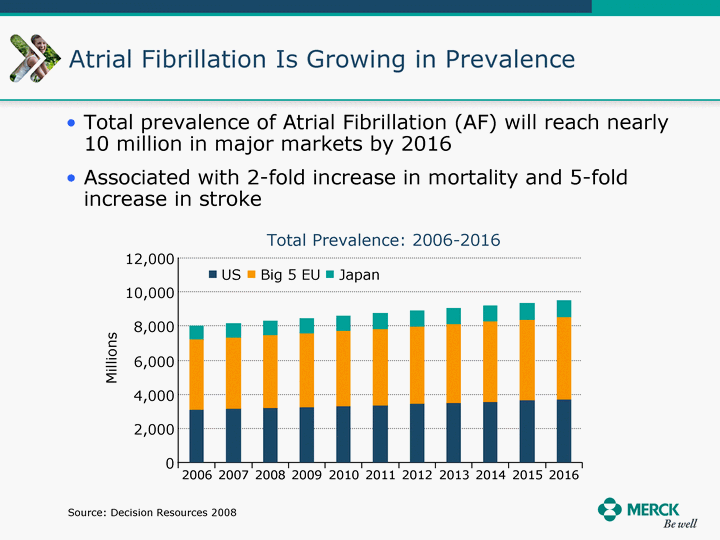
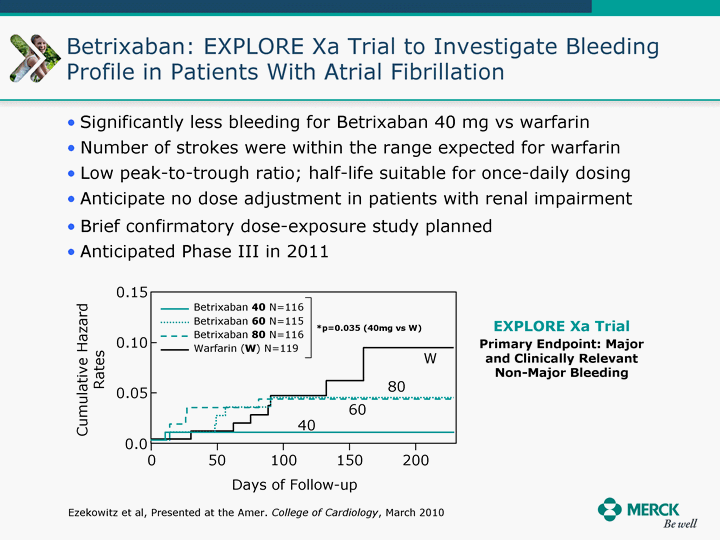
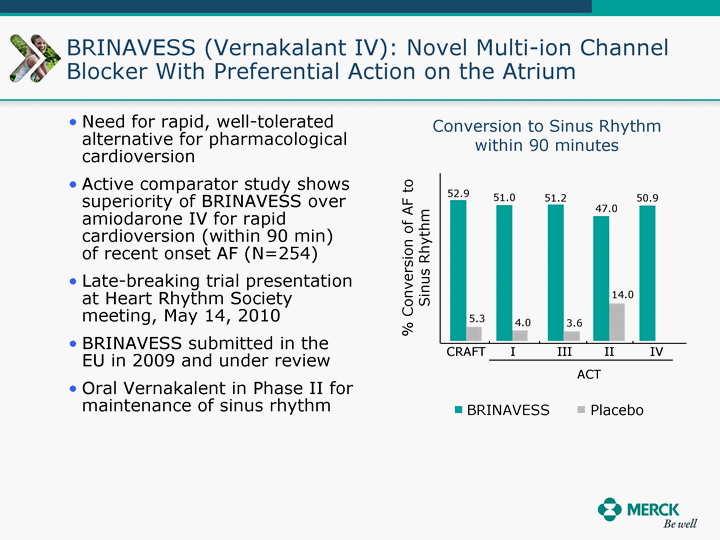
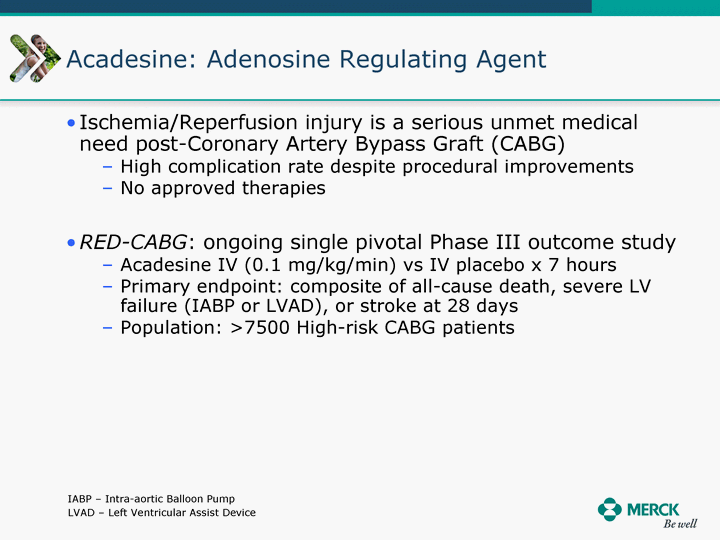
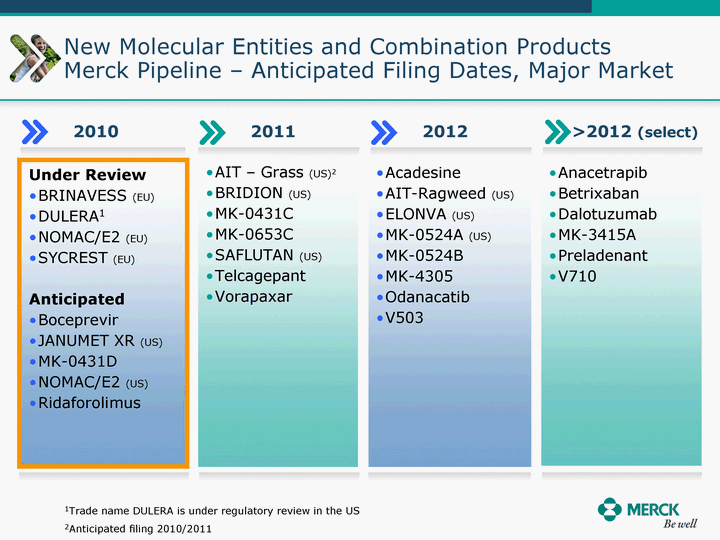
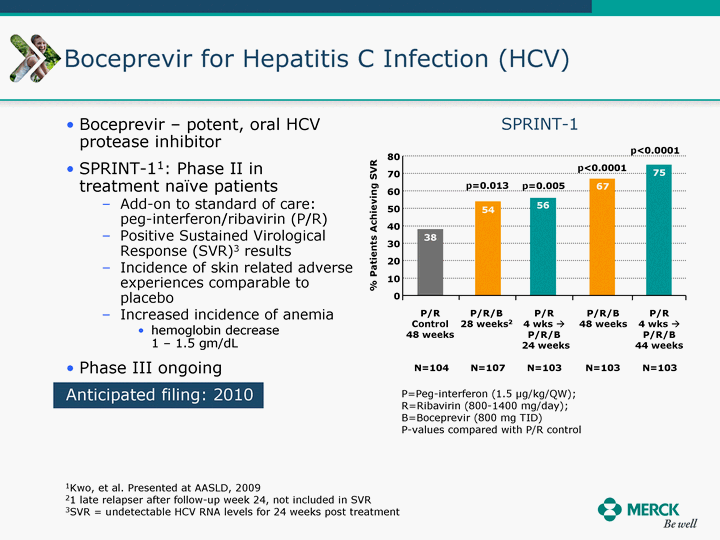
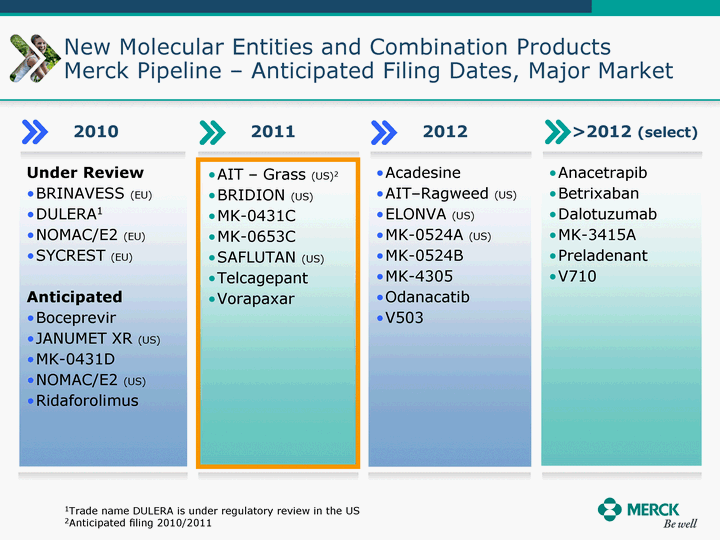
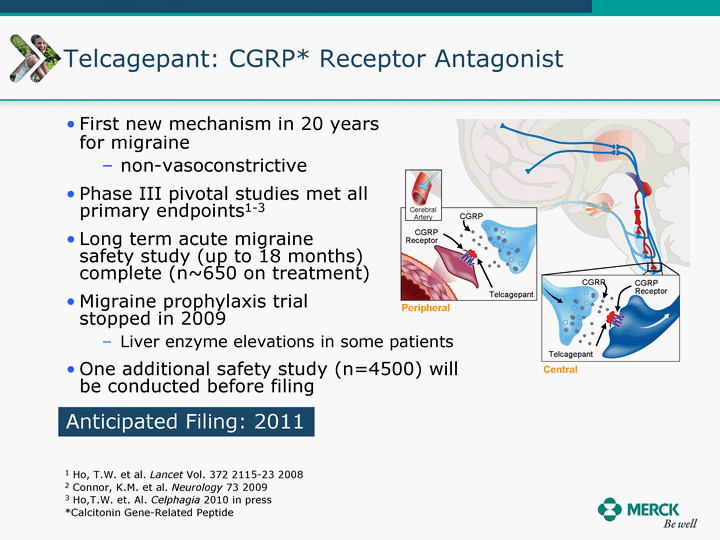
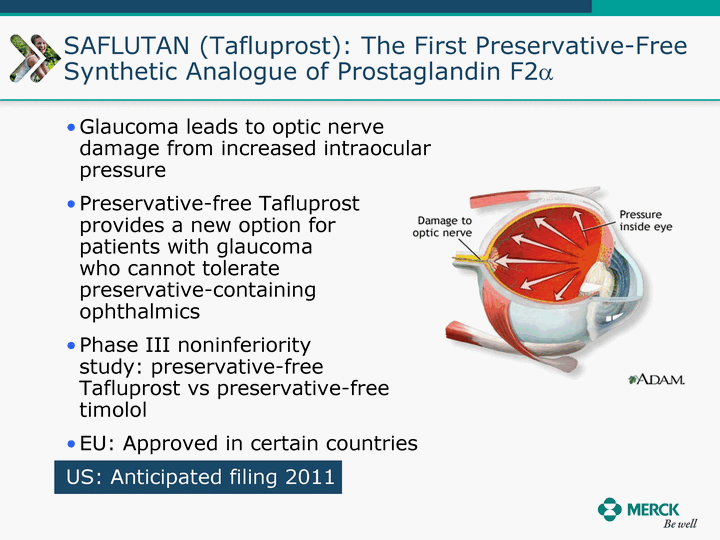
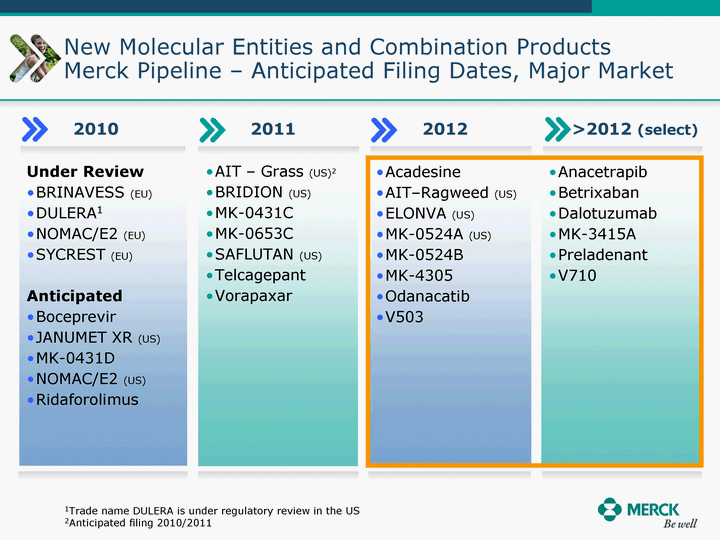
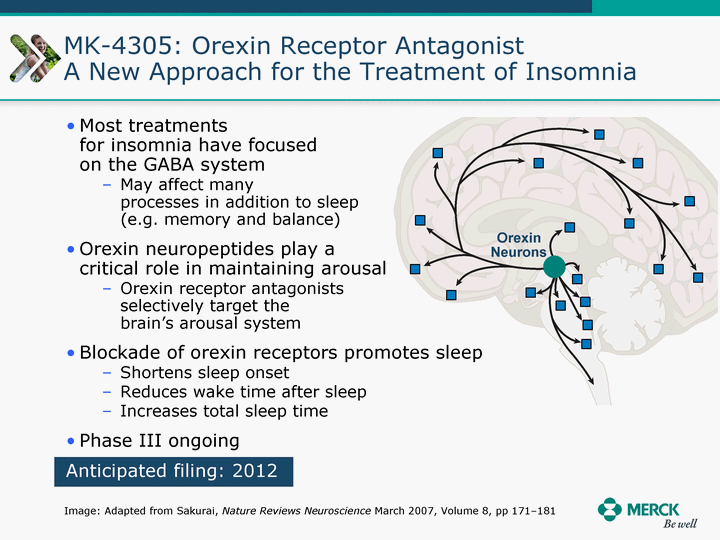
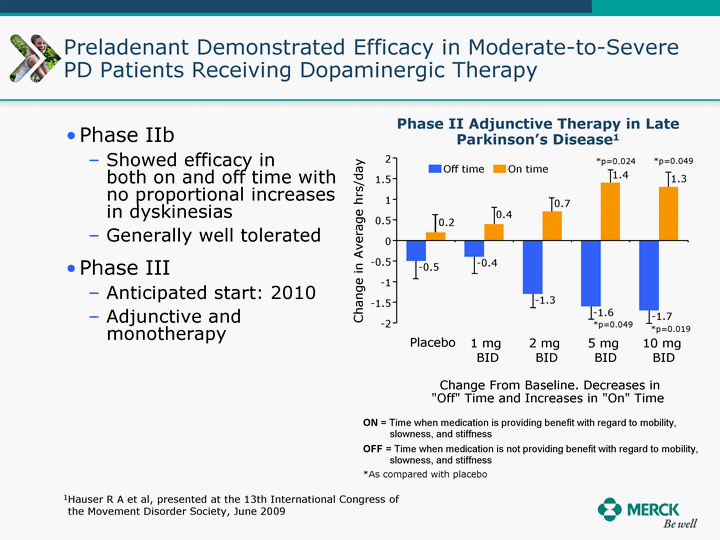
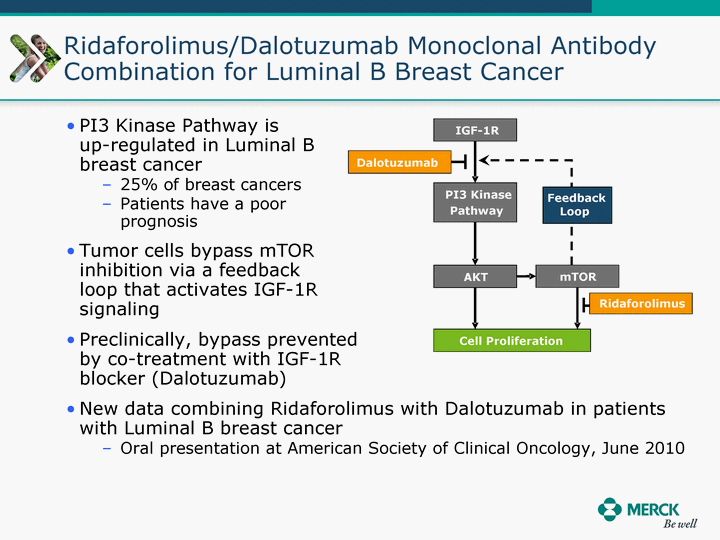
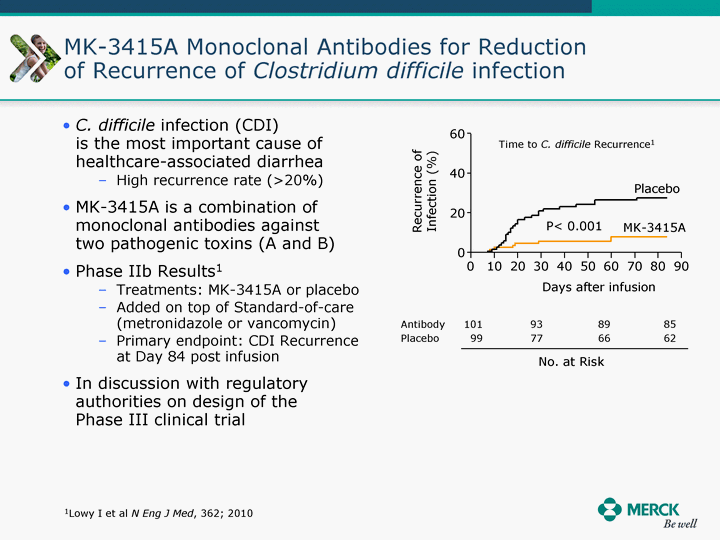
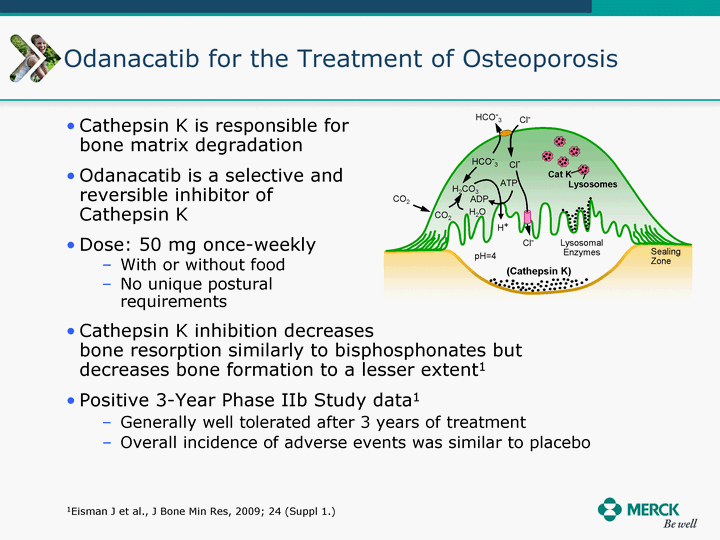
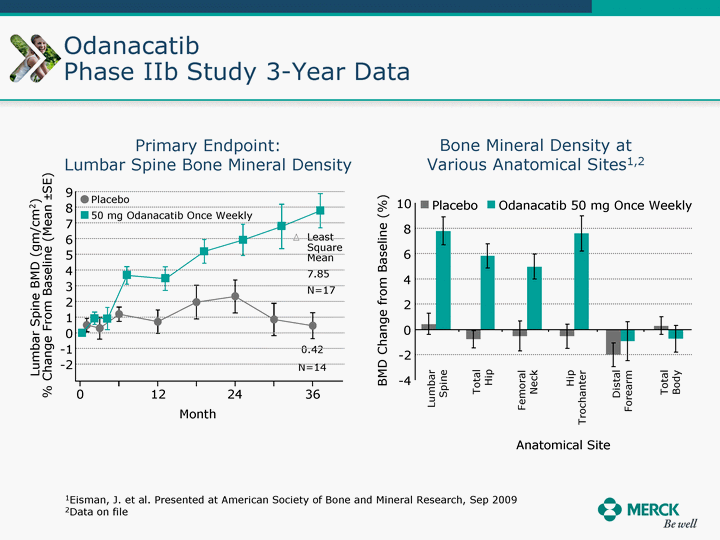
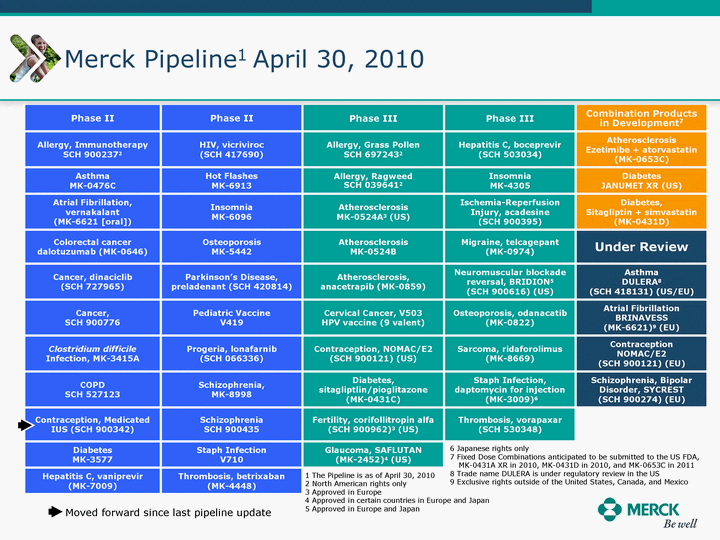
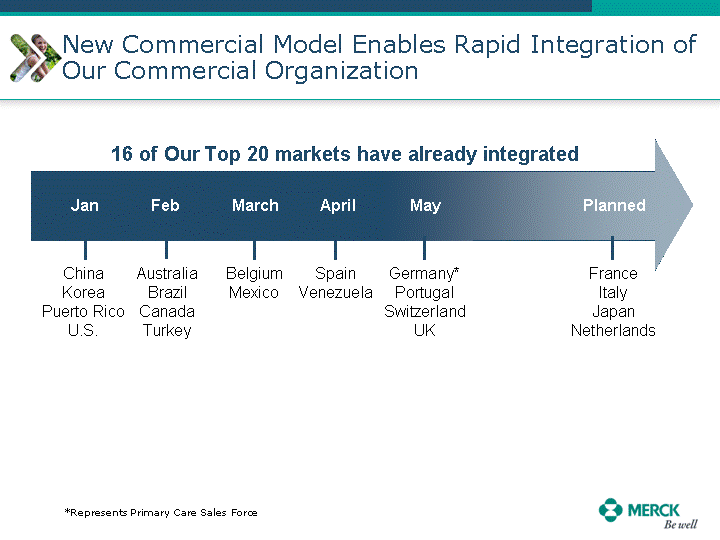
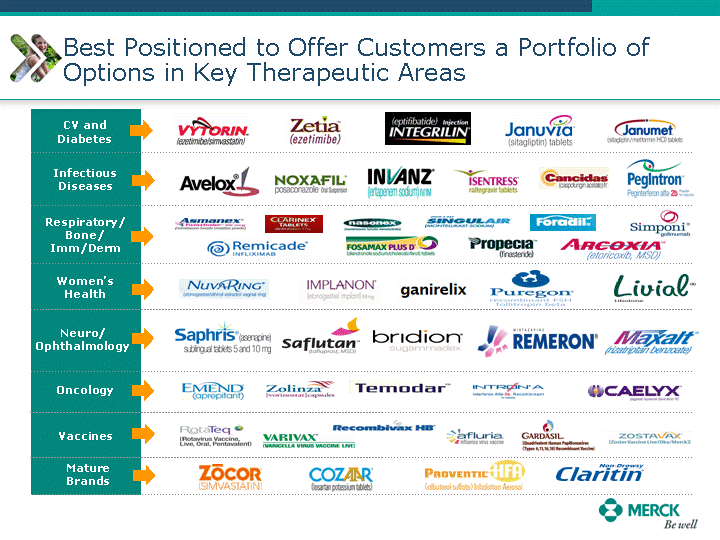
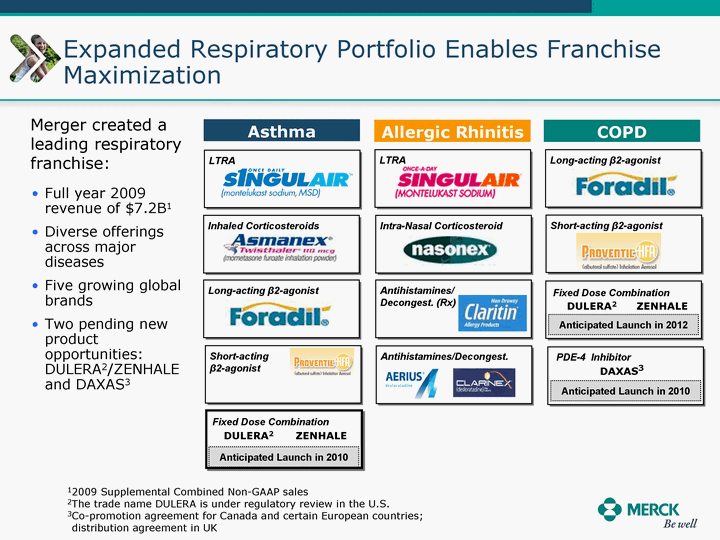
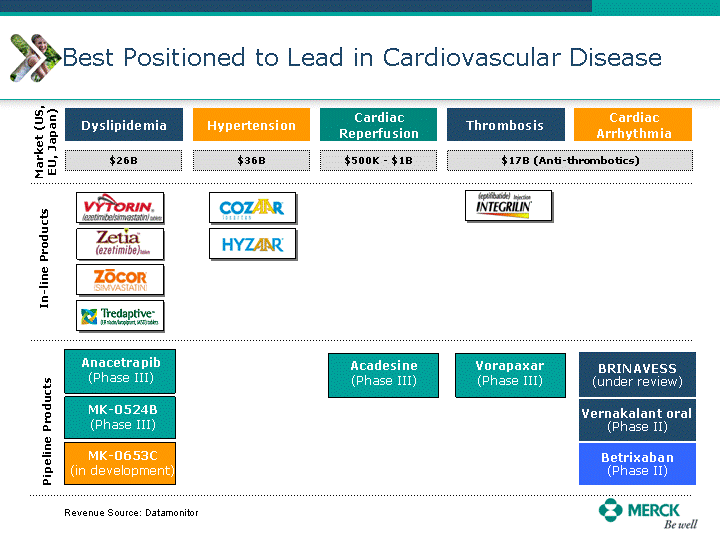
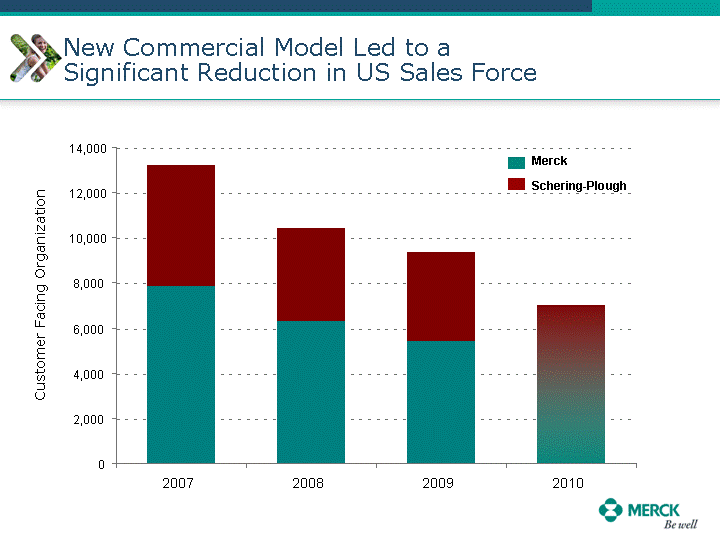
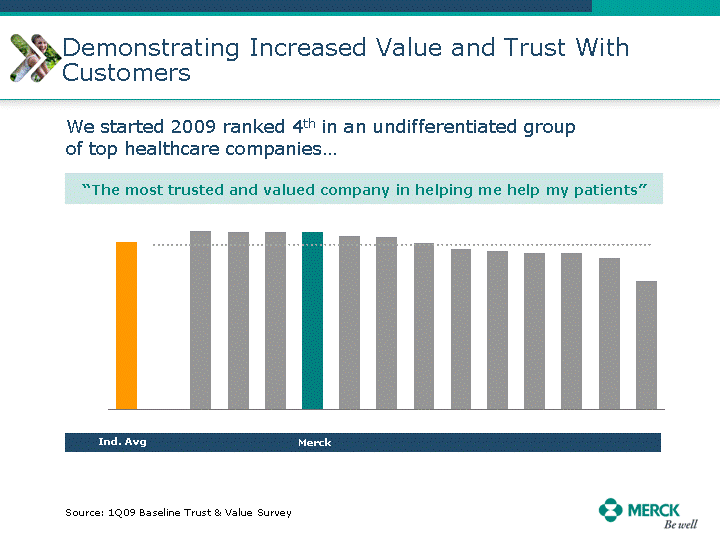
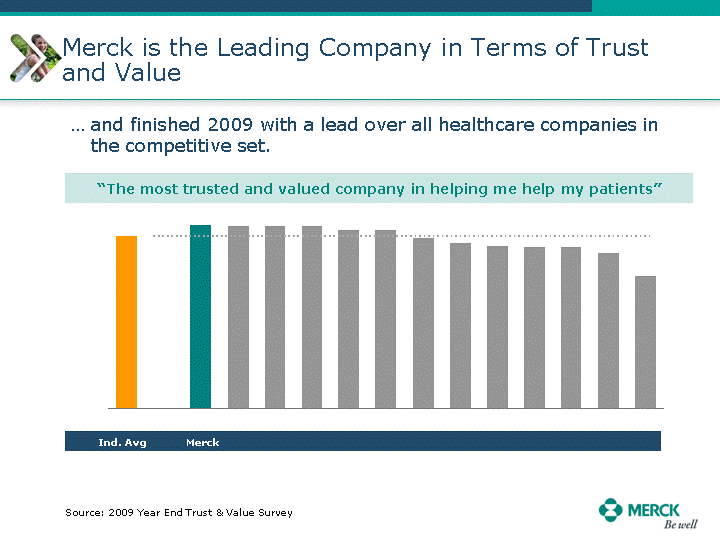
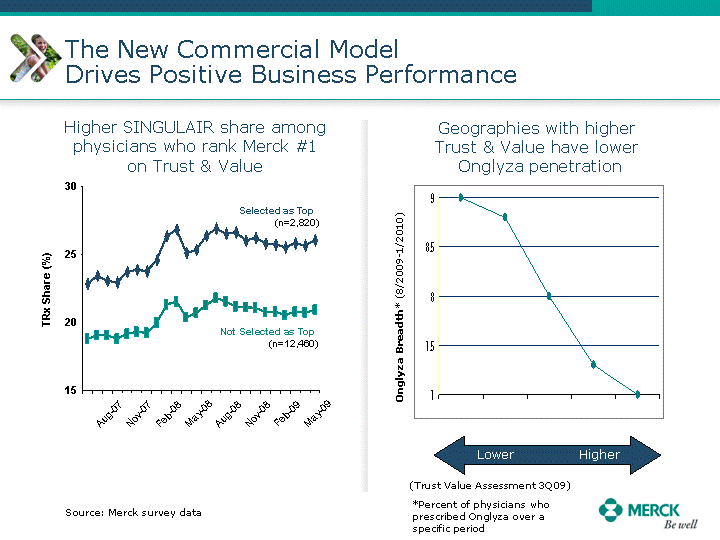
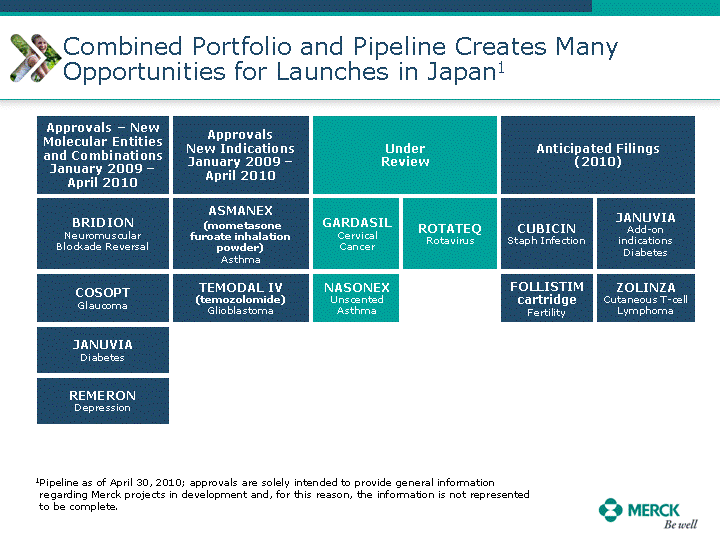
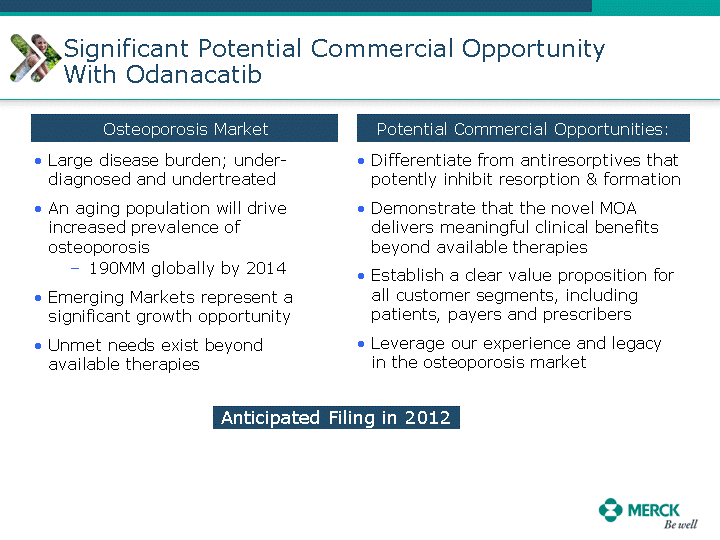
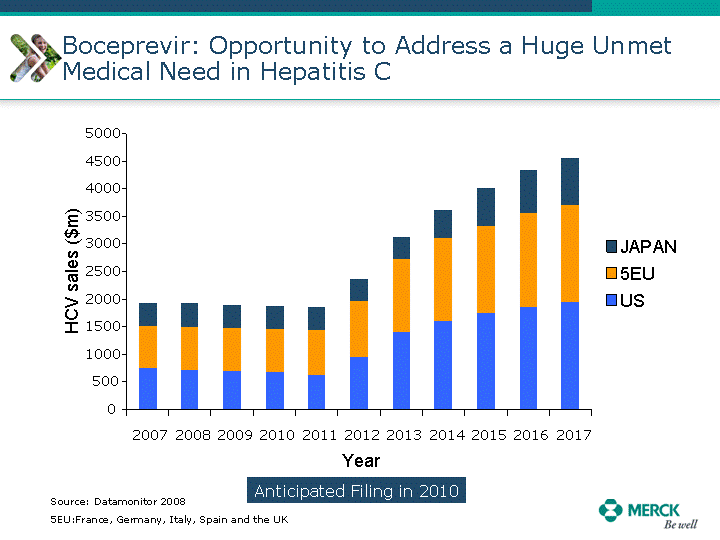
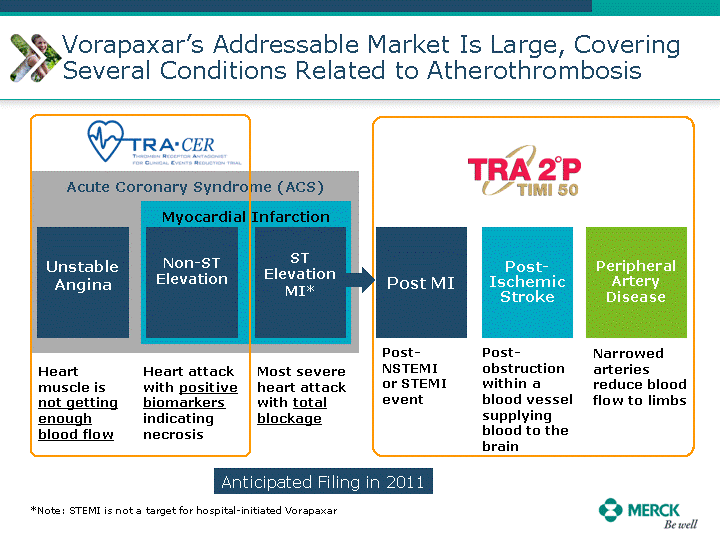
| Moved forward since last pipeline update Merck Pipeline1 April 30, 2010 Phase II Phase II Phase III Phase III Combination Products in Development7 Allergy, Immunotherapy SCH 9002372 HIV, vicriviroc (SCH 417690) Allergy, Grass Pollen SCH 6972432 Hepatitis C, boceprevir (SCH 503034) Atherosclerosis Ezetimibe + atorvastatin (MK-0653C) Asthma MK-0476C Hot Flashes MK-6913 Allergy, Ragweed SCH 0396412 Insomnia MK-4305 Diabetes JANUMET XR (US) Atrial Fibrillation, vernakalant (MK-6621 [oral]) Insomnia MK-6096 Atherosclerosis MK-0524A3 (US) Ischemia-Reperfusion Injury, acadesine (SCH 900395) Diabetes, Sitagliptin + simvastatin (MK-0431D) Colorectal cancer dalotuzumab (MK-0646) Osteoporosis MK-5442 Atherosclerosis MK-0524B Migraine, telcagepant (MK-0974) Under Review Cancer, dinaciclib (SCH 727965) Parkinson's Disease, preladenant (SCH 420814) Atherosclerosis, anacetrapib (MK-0859) Neuromuscular blockade reversal, BRIDION5 (SCH 900616) (US) Asthma DULERA8 (SCH 418131) (US/EU) Cancer, SCH 900776 Pediatric Vaccine V419 Cervical Cancer, V503 HPV vaccine (9 valent) Osteoporosis, odanacatib (MK-0822) Atrial Fibrillation BRINAVESS (MK-6621)9 (EU) Clostridium difficile Infection, MK-3415A Progeria, lonafarnib (SCH 066336) Contraception, NOMAC/E2 (SCH 900121) (US) Sarcoma, ridaforolimus (MK-8669) Contraception NOMAC/E2 (SCH 900121) (EU) COPD SCH 527123 Schizophrenia, MK-8998 Diabetes, sitagliptlin/pioglitazone (MK-0431C) Staph Infection, daptomycin for injection (MK-3009)6 Schizophrenia, Bipolar Disorder, SYCREST (SCH 900274) (EU) Contraception, Medicated IUS (SCH 900342) Schizophrenia SCH 900435 Fertility, corifollitropin alfa (SCH 900962)3 (US) Thrombosis, vorapaxar (SCH 530348) Diabetes MK-3577 Staph Infection V710 Glaucoma, SAFLUTAN (MK-2452)4 (US) Hepatitis C, vaniprevir (MK-7009) Thrombosis, betrixaban (MK-4448) 6 Japanese rights only 7 Fixed Dose Combinations anticipated to be submitted to the US FDA, MK-0431A XR in 2010, MK-0431D in 2010, and MK-0653C in 2011 8 Trade name DULERA is under regulatory review in the US 9 Exclusive rights outside of the United States, Canada, and Mexico 1 The Pipeline is as of April 30, 2010 2 North American rights only 3 Approved in Europe 4 Approved in certain countries in Europe and Japan 5 Approved in Europe and Japan |