
Investor Presentation NYSE MKT: NBS May 2013

Forward-Looking Statements This presentation includes “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995, as well as historical information. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements, or industry results, to be materially different from anticipated results, performance or achievements expressed or implied by such forward-looking statements. When used in this Annual Report on Form 10-K, statements that are not statements of current or historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, the words “plan,” “intend,” “may,” “will,” “expect,” “believe,” “could,” “anticipate,” “estimate,” or “continue” or similar expressions or other variations or comparable terminology are intended to identify such forward-looking statements, although some forward-looking statements are expressed differently. Additionally, statements regarding the future of the regenerative medicine industry and the role of stem cells and cellular therapy in that future, our ability to successfully develop and grow our business, including with regard to our research and development and clinical evaluation efforts and future marketing and sales in respect of AMR-001 and other cell therapies, the marketing and performance of our contract development and manufacturing business and our adult stem cell collection, processing and storage business are forward looking statements. Our future operating results are dependent upon many factors and our further development is highly dependent on future medical and research developments and market acceptance, which is outside our control. Forward-looking statements, including with respect to the successful execution of the Company's strategy, may not be realized due to a variety of factors and we cannot guarantee their accuracy or that our expectations about future events will prove to be correct. Such factors include, without limitation, (i) our ability to manage our business despite operating losses and cash outflows; (ii) our ability to obtain sufficient capital or strategic business arrangements to fund our operations and expansion plans, including meeting our financial obligations under various licensing and other strategic arrangements, the funding of our clinical trials for AMR-001, and the commercialization of the relevant technology; (iii) our ability to build the management and human resources and infrastructure necessary to support the growth of our business; (iv) our ability to integrate our acquired businesses successfully and grow such acquired businesses as anticipated, including expanding our PCT business into Europe; (v) whether a large global market is established for our cellular-based products and services and our ability to capture a share of this market; (vi) competitive factors and developments beyond our control; (vii) scientific and medical developments beyond our control; (viii) our ability to obtain appropriate governmental licenses, accreditations or certifications or comply with healthcare laws and regulations or any other adverse effect or limitations caused by government regulation of our business; (ix) whether any of our current or future patent applications result in issued patents, the scope of those patents and our ability to obtain and maintain other rights to technology required or desirable for the conduct of our business; (x) whether any potential strategic benefits of various licensing transactions will be realized and whether any potential benefits from the acquisition of these licensed technologies will be realized; (xi) the results of our development activities, including our current Phase 2 clinical trial of AMR-001; (xii) our ability to complete our Phase 2 clinical trial of AMR-001(or initiate future trials) in accordance with our estimated timeline due to delays associated with enrolling patients due to the novelty of the treatment, the size of the patient population and the need of patients to meet the inclusion criteria of the trial or otherwise; and (xiii) the other factors discussed in “Risk Factors” and elsewhere in this presentation and in the Company's other periodic filings with the Securities and Exchange Commission (the “SEC”) which are available for review at www.sec.gov under “Search for Company Filings.” All forward-looking statements attributable to us are expressly qualified in their entirety by these and other factors. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the Company undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. 2
Unsustainable Growth in US Health Care Costs 3 • $2.7 Trillion Dollars is spent annually on health care costs (currently 18% of US GDP)1 • 80% of health care costs are associated with chronic conditions2 o Cardiovascular disease costs over $445B today Projected to increase to over $1T by 20303 o Diabetes costs are over $174B today Projected to increase to over $300B by 20254 With an aging population, we need to move the paradigm from the treatment of chronic disease toward regenerative medicine and we believe NeoStem is part of that paradigm shift 1) Center for Medicare and Medicaid 2) “Chronic disease and medical innovation in an aging nation” www.silverbook.org 3) American Heart Association, Policy Statement January 24, 2011 4) American Diabetes Association

Regenerative Medicine • Repairing or replacing damaged tissue and restoring function • Novel regenerative therapies hold the promise of transforming clinical outcomes and reducing overall healthcare costs • The regenerative medicine market is estimated to grow to $88 billion by 20141 4 1) According to The Regenerative Medicine Report, MDB Capital Group, January 2011
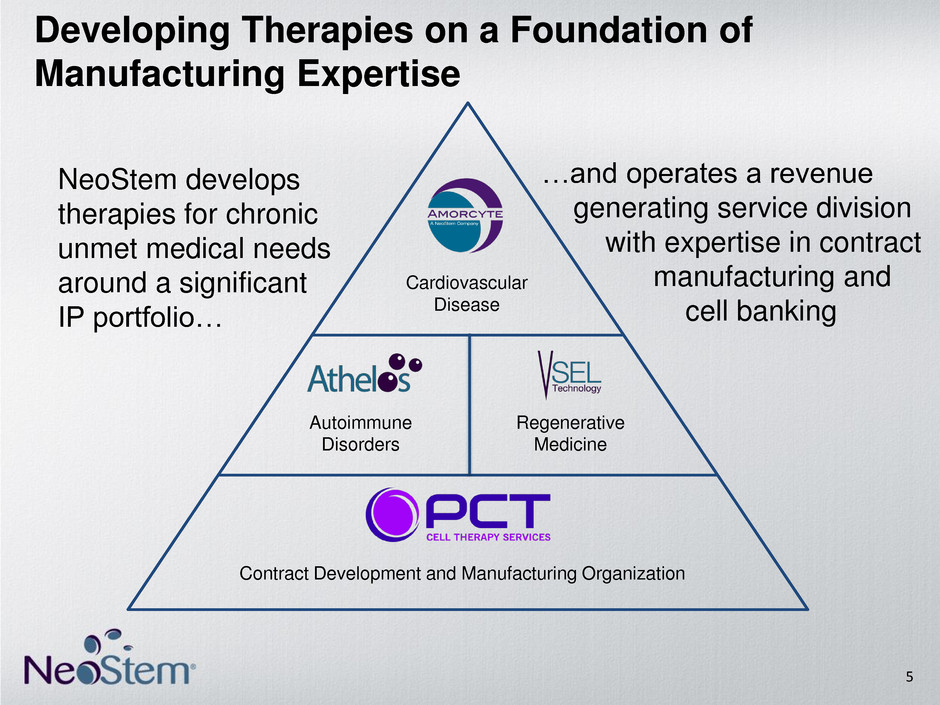
Autoimmune Disorders Regenerative Medicine NeoStem develops therapies for chronic unmet medical needs around a significant IP portfolio… Developing Therapies on a Foundation of Manufacturing Expertise Cardiovascular Disease …and operates a revenue generating service division with expertise in contract manufacturing and cell banking 5 Contract Development and Manufacturing Organization

AMR-001 Brings Repair System to the Heart in Order to Preserve Function CD34⁺CXCR4+ Cells are a natural repair mechanism 6
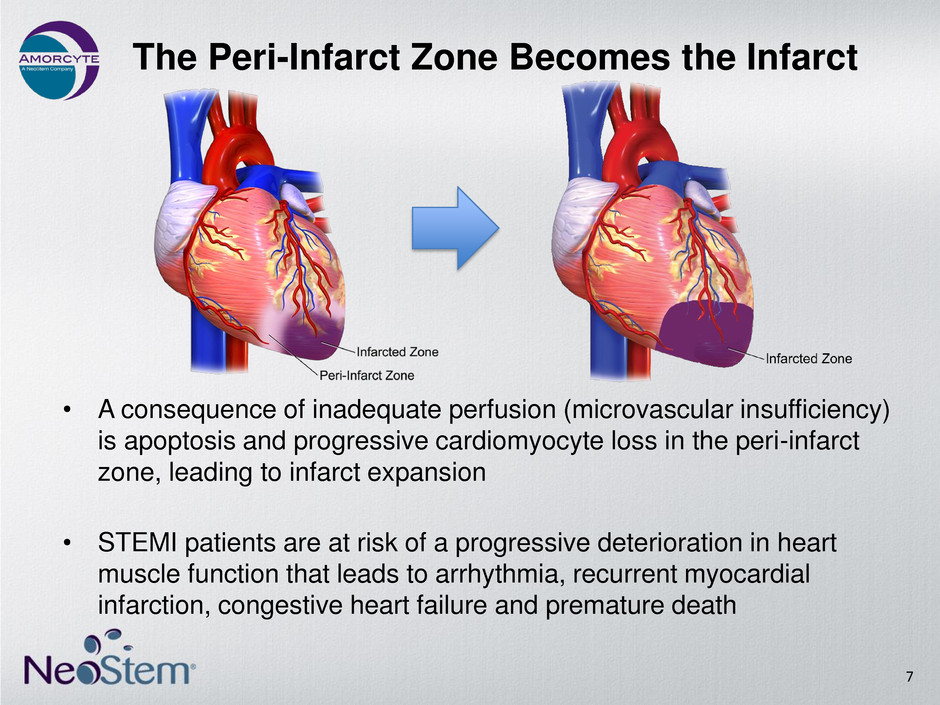
• A consequence of inadequate perfusion (microvascular insufficiency) is apoptosis and progressive cardiomyocyte loss in the peri-infarct zone, leading to infarct expansion • STEMI patients are at risk of a progressive deterioration in heart muscle function that leads to arrhythmia, recurrent myocardial infarction, congestive heart failure and premature death The Peri-Infarct Zone Becomes the Infarct 7

Cochrane Collaboration Review Bone Marrow Derived Cells: Likely Safe and Positive Impact on Mortality Clifford et al, Cochrane Library 2012 8

Phase 1 Trial Design for AMR-001 Indication Post-AMI with LVEF ≤50% and wall motion abnormality in the myocardium of the IRA Primary Endpoint Safety in post-AMI patients Other Endpoints RTSS* (Perfusion); LVEF; ESV; SDF mobility Key Inclusion Criteria Confirmation of ST Elevation MI; Ejection fraction ≤ 50% 96 hours post stenting Dosing Frequency Single dose Groups and Randomization 3 dose cohorts (5, 10, 15 million cells, randomized 1:1, open-label) Number of Subjects N=31 Number of Sites 4 (incl. Emory University, Texas Heart Institute, Vanderbilt, Cincinnati) Geography United States Trial Duration 6 months Day 4: CMR Day 1: Ventriculography Day 6-10: Injection into the IRA Day 5-8: 6-8 Hour Cell Separation Process 9
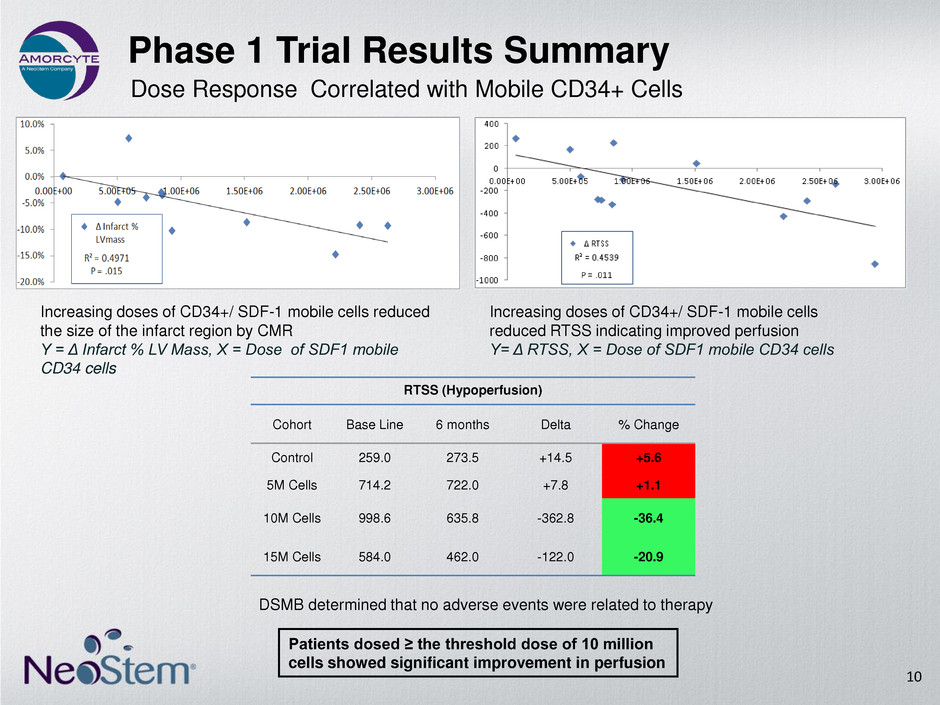
Phase 1 Trial Results Summary RTSS (Hypoperfusion) Cohort Base Line 6 months Delta % Change Control 259.0 273.5 +14.5 +5.6 5M Cells 714.2 722.0 +7.8 +1.1 10M Cells 998.6 635.8 -362.8 -36.4 15M Cells 584.0 462.0 -122.0 -20.9 DSMB determined that no adverse events were related to therapy 10 Patients dosed ≥ the threshold dose of 10 million cells showed significant improvement in perfusion Increasing doses of CD34+/ SDF-1 mobile cells reduced the size of the infarct region by CMR Y = Δ Infarct % LV Mass, X = Dose of SDF1 mobile CD34 cells Increasing doses of CD34+/ SDF-1 mobile cells reduced RTSS indicating improved perfusion Y= Δ RTSS, X = Dose of SDF1 mobile CD34 cells Dose Response Correlated with Mobile CD34+ Cells
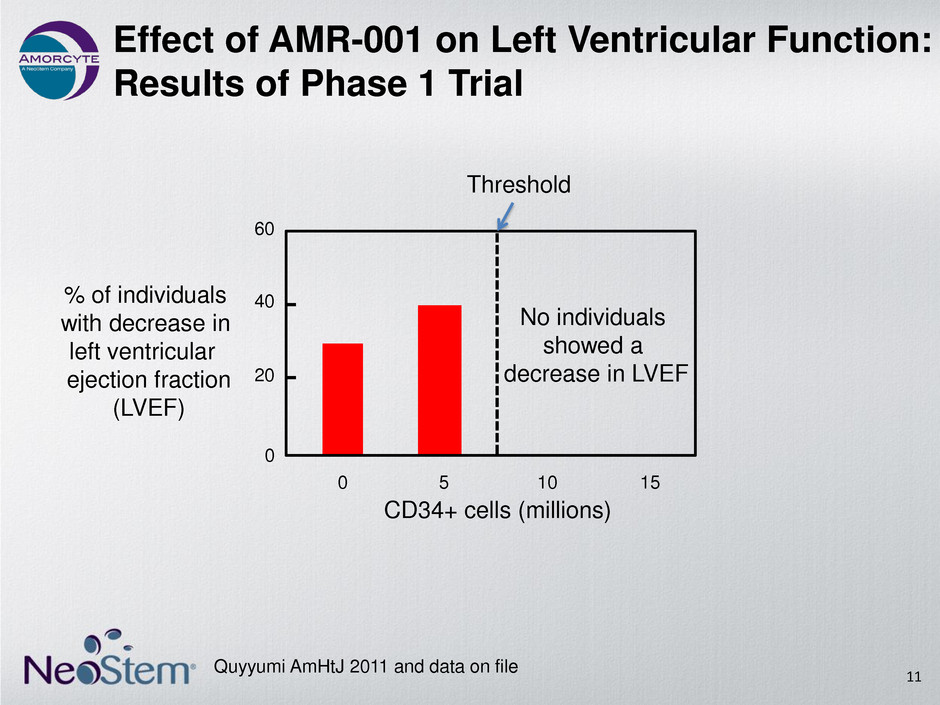
Effect of AMR-001 on Left Ventricular Function: Results of Phase 1 Trial Quyyumi AmHtJ 2011 and data on file 11 0 20 40 60 0 5 10 15 CD34+ cells (millions) % of individuals with decrease in left ventricular ejection fraction (LVEF) Threshold No individuals showed a decrease in LVEF
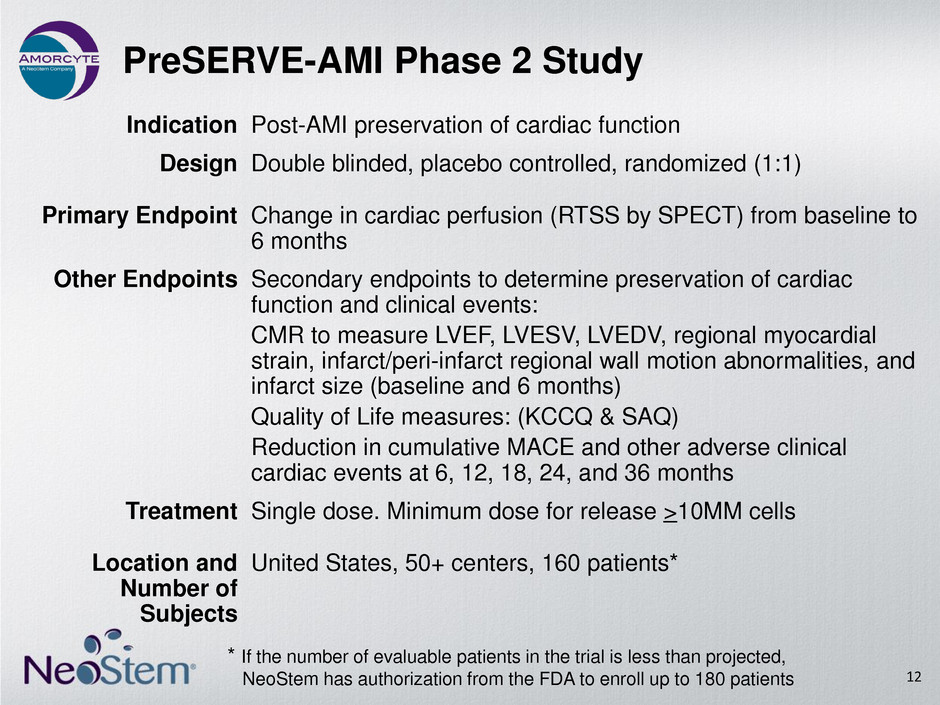
PreSERVE-AMI Phase 2 Study 12 Indication Post-AMI preservation of cardiac function Design Double blinded, placebo controlled, randomized (1:1) Primary Endpoint Change in cardiac perfusion (RTSS by SPECT) from baseline to 6 months Other Endpoints Secondary endpoints to determine preservation of cardiac function and clinical events: CMR to measure LVEF, LVESV, LVEDV, regional myocardial strain, infarct/peri-infarct regional wall motion abnormalities, and infarct size (baseline and 6 months) Quality of Life measures: (KCCQ & SAQ) Reduction in cumulative MACE and other adverse clinical cardiac events at 6, 12, 18, 24, and 36 months Treatment Single dose. Minimum dose for release >10MM cells Location and Number of Subjects United States, 50+ centers, 160 patients* * If the number of evaluable patients in the trial is less than projected, NeoStem has authorization from the FDA to enroll up to 180 patients
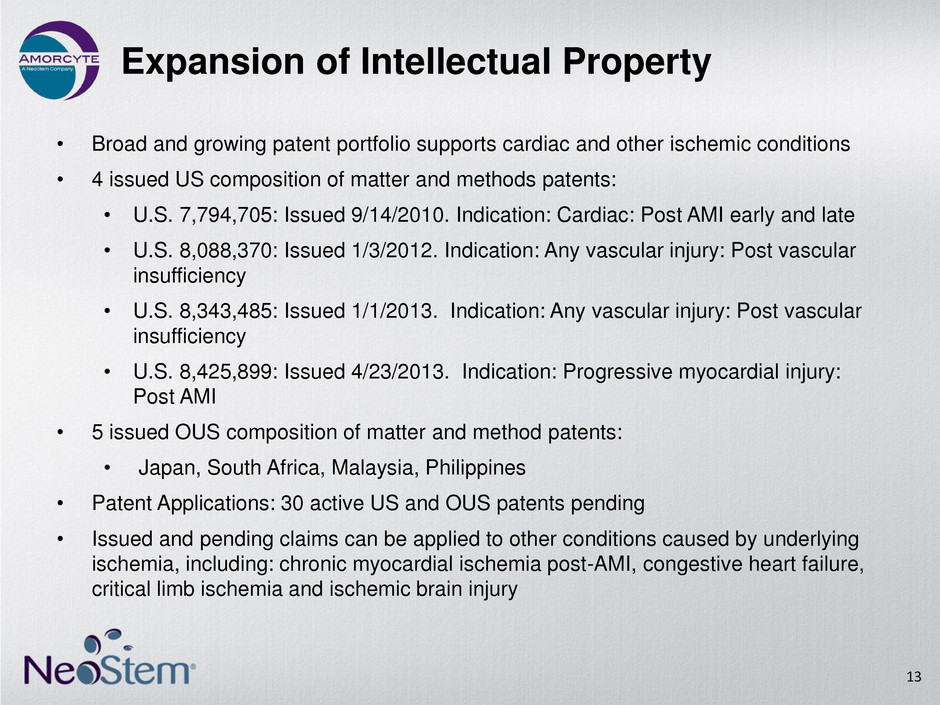
Expansion of Intellectual Property • Broad and growing patent portfolio supports cardiac and other ischemic conditions • 4 issued US composition of matter and methods patents: • U.S. 7,794,705: Issued 9/14/2010. Indication: Cardiac: Post AMI early and late • U.S. 8,088,370: Issued 1/3/2012. Indication: Any vascular injury: Post vascular insufficiency • U.S. 8,343,485: Issued 1/1/2013. Indication: Any vascular injury: Post vascular insufficiency • U.S. 8,425,899: Issued 4/23/2013. Indication: Progressive myocardial injury: Post AMI • 5 issued OUS composition of matter and method patents: • Japan, South Africa, Malaysia, Philippines • Patent Applications: 30 active US and OUS patents pending • Issued and pending claims can be applied to other conditions caused by underlying ischemia, including: chronic myocardial ischemia post-AMI, congestive heart failure, critical limb ischemia and ischemic brain injury 13
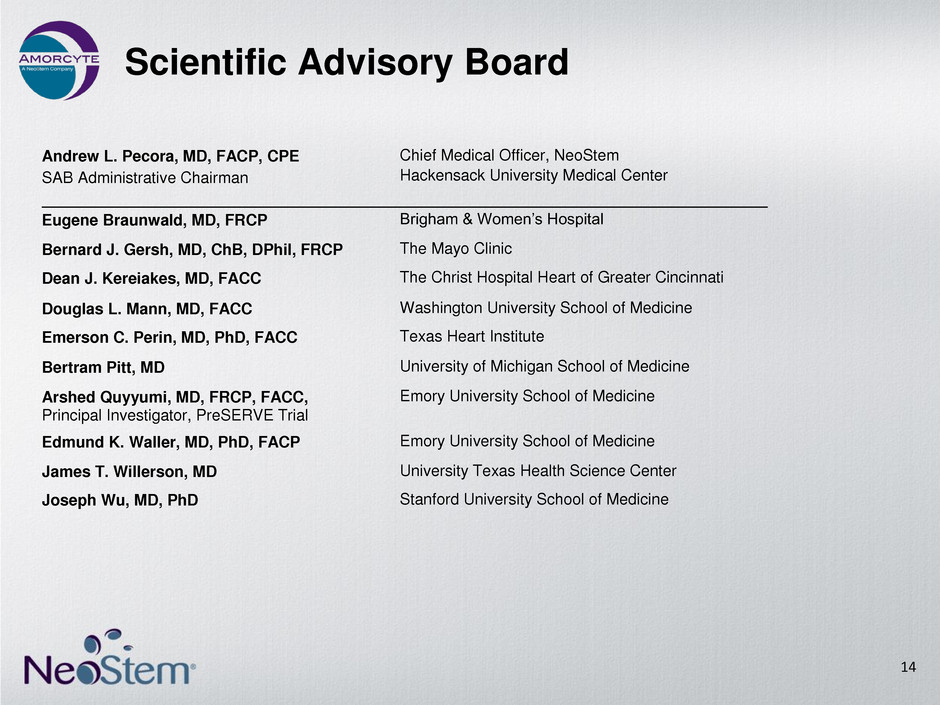
Scientific Advisory Board 14 Andrew L. Pecora, MD, FACP, CPE SAB Administrative Chairman Chief Medical Officer, NeoStem Hackensack University Medical Center Eugene Braunwald, MD, FRCP Brigham & Women’s Hospital Bernard J. Gersh, MD, ChB, DPhil, FRCP The Mayo Clinic Dean J. Kereiakes, MD, FACC The Christ Hospital Heart of Greater Cincinnati Douglas L. Mann, MD, FACC Washington University School of Medicine Emerson C. Perin, MD, PhD, FACC Texas Heart Institute Bertram Pitt, MD University of Michigan School of Medicine Arshed Quyyumi, MD, FRCP, FACC, Principal Investigator, PreSERVE Trial Emory University School of Medicine Edmund K. Waller, MD, PhD, FACP Emory University School of Medicine James T. Willerson, MD University Texas Health Science Center Joseph Wu, MD, PhD Stanford University School of Medicine

What’s Next? Congestive Heart Failure US: Incidence – 660,000, Prevalence 5.8 million1 Worldwide: Prevalence – 20-23 million2 15 1) American Heart Association 2) Study by Case Western Reserve University
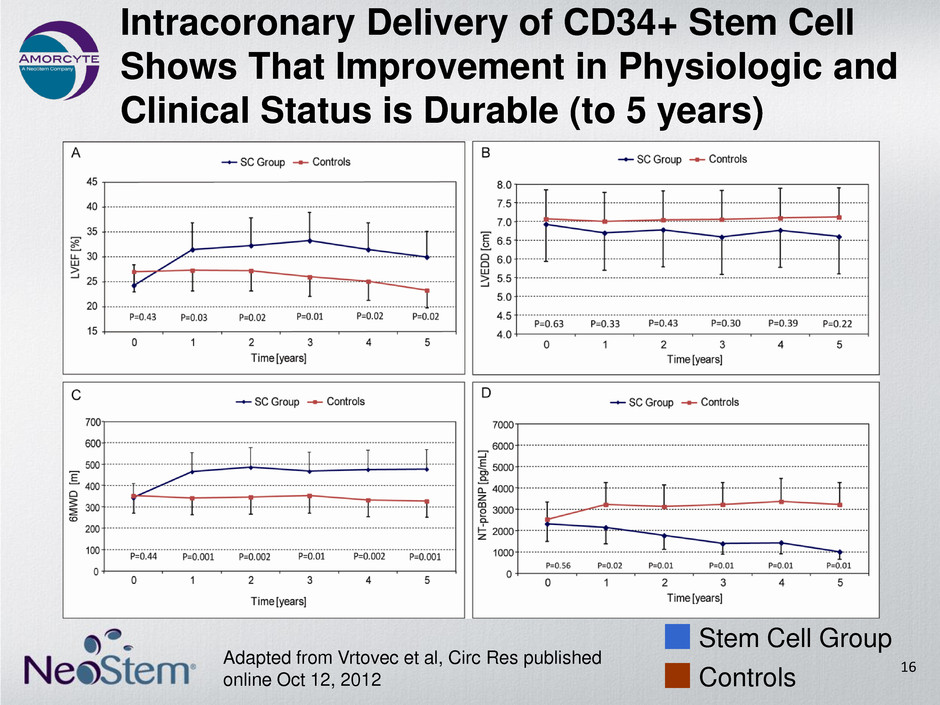
Intracoronary Delivery of CD34+ Stem Cell Shows That Improvement in Physiologic and Clinical Status is Durable (to 5 years) Adapted from Vrtovec et al, Circ Res published online Oct 12, 2012 Stem Cell Group Controls 16

CD34+ Stem Cell Therapy Yields Meaningful Clinical Benefits in DCM Adapted from Vrtovec et al, Circ Res published online Oct 12, 2012 Stem Cell Group Controls 17

Treg Cells to Restore Immune Balance • Partnership with Becton Dickinson, which owns 20% of Athelos • Immune-mediated diseases, such as graft-versus-host-disease (GVHD), autoimmune disorders, such as type 1 diabetes and multiple sclerosis, and allergic conditions, are a result of an imbalance between T-effector cells and T-regulatory cells (Treg) • Treg therapy represents a novel approach for restoring immune balance by enhancing T-regulatory cell number and function1 • Phase 1 work is ongoing globally under several independent physician INDs, including Dr. P. Trzonkowski, Dr. Jeffrey Bluestone and Dr. Rob Negrin, results of which will inform NeoStem’s future clinical direction • Exclusive rights to 21 issued patents and 3 patents pending related primarily to methods of isolating, purifying and expanding Tregs 1) Chai, Jian-Guo et al, Journal of Immunology 2008; 180;858-869 18
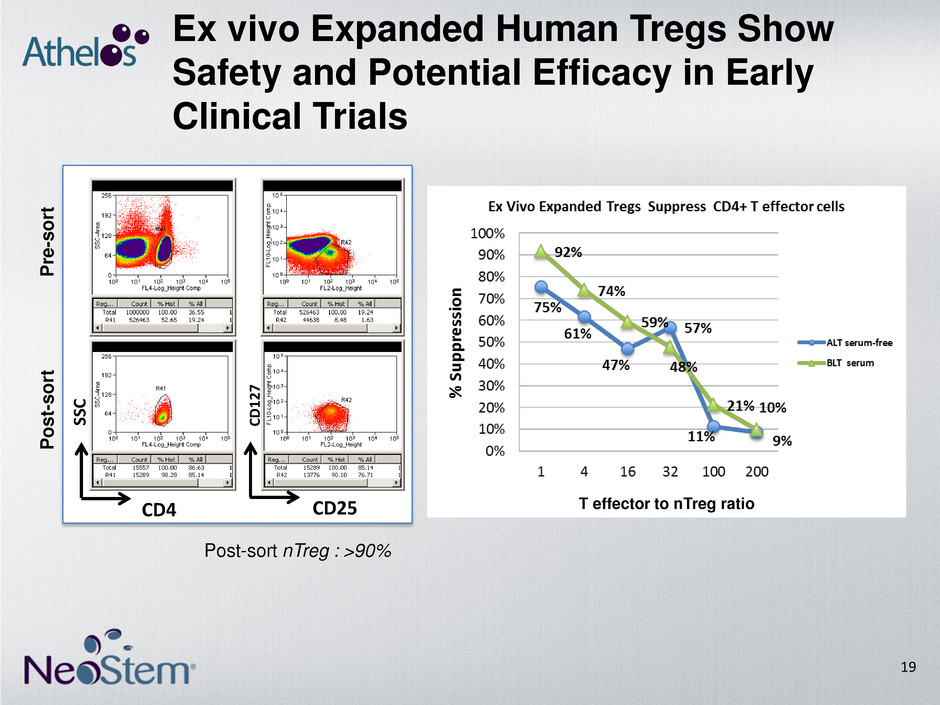
Ex vivo Expanded Human Tregs Show Safety and Potential Efficacy in Early Clinical Trials CD4 SS C CD25 CD 1 2 7 Post-sort nTreg : >90% T effector to nTreg ratio P re -s o rt P o s t- s o rt 19

Published Treg Clinical Trials • Trzonkowski et al., First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells . Clin. Immunol. 2009 • Di Ianni et al., Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 2011 • Brunstein et al., Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011 • Marek-Trzonkowski et al., Administration of CD4+CD25highCD127− Regulatory T Cells Preserves β-Cell Function in Type 1 Diabetes in Children. Diabetes Care, 2012 20

Open Treg Trials • Laport and Negrin, Stanford (NCT01660607) Phase I/II MAHCT w/ TCell Depleted Graft w/ Simultaneous Infusion Conventional and Regulatory T Cell (unpublished pers comm) • Gitelman and Bluestone, UCSF (NCT01210664) T1DM Immunotherapy Using CD4+CD127lo/-CD25+ Polyclonal Tregs (unpublished pers comm) • Bykovskaia, Russian State Medical University (NCT01446484) Treatment of Children With Kidney Transplants by Injection of CD4+CD25+FoxP3+ T Cells to Prevent Organ Rejection • Brunstein, UMinn (NCT00602693 and NCT01163201)T-Regulatory Cell and CD3 Depleted Double Umbilical Cord Blood Transplantation in Hematologic Malignancies • Lu, Nanjing Medical University, China, and Blazar, UMinn, USA (NCT01624077) Safety Study of Using Regulatory T Cells Induce Liver Transplantation Tolerance (Treg) Source: Clinicaltrials.gov database 21

Scientific Advisory Board 22 Robert A. Preti, PhD, SAB Administrative Chairman Progenitor Cell Therapy Jeffrey Bluestone, PhD University of California, San Francisco, Diabetes Center David A. Horwitz, MD University of Southern California Robert Korngold, PhD Hackensack University Medical Center Robert S. Negrin, MD Stanford University David Peritt, PhD Hospira Noel L. Warner, PhD BD Biosciences
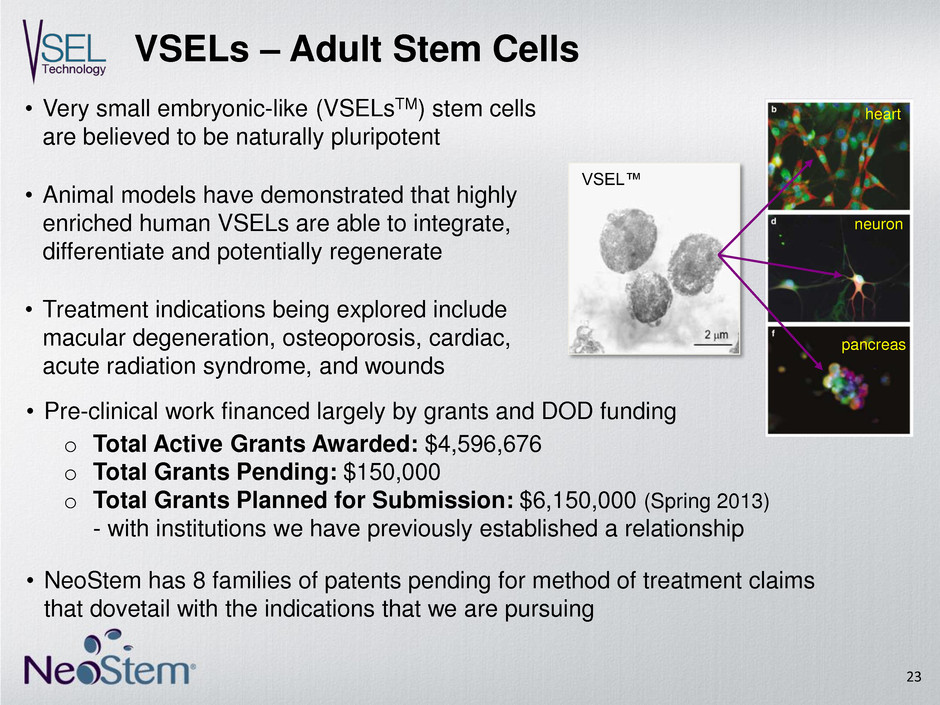
VSELs – Adult Stem Cells heart neuron pancreas VSEL™ • Very small embryonic-like (VSELsTM) stem cells are believed to be naturally pluripotent • Animal models have demonstrated that highly enriched human VSELs are able to integrate, differentiate and potentially regenerate • Treatment indications being explored include macular degeneration, osteoporosis, cardiac, acute radiation syndrome, and wounds 23 o Total Active Grants Awarded: $4,596,676 o Total Grants Pending: $150,000 o Total Grants Planned for Submission: $6,150,000 (Spring 2013) - with institutions we have previously established a relationship • Pre-clinical work financed largely by grants and DOD funding • NeoStem has 8 families of patents pending for method of treatment claims that dovetail with the indications that we are pursuing

Human VSELs Accelerate Healing in a SCID Mouse Complex Tail Wound Model Days Post-wounding VSELs vs. MSCs % R e -e p it h e li a li z a ti o n p < 0.05 2,500 human VSELS 500,000 human MSCs Fibrin Control 24
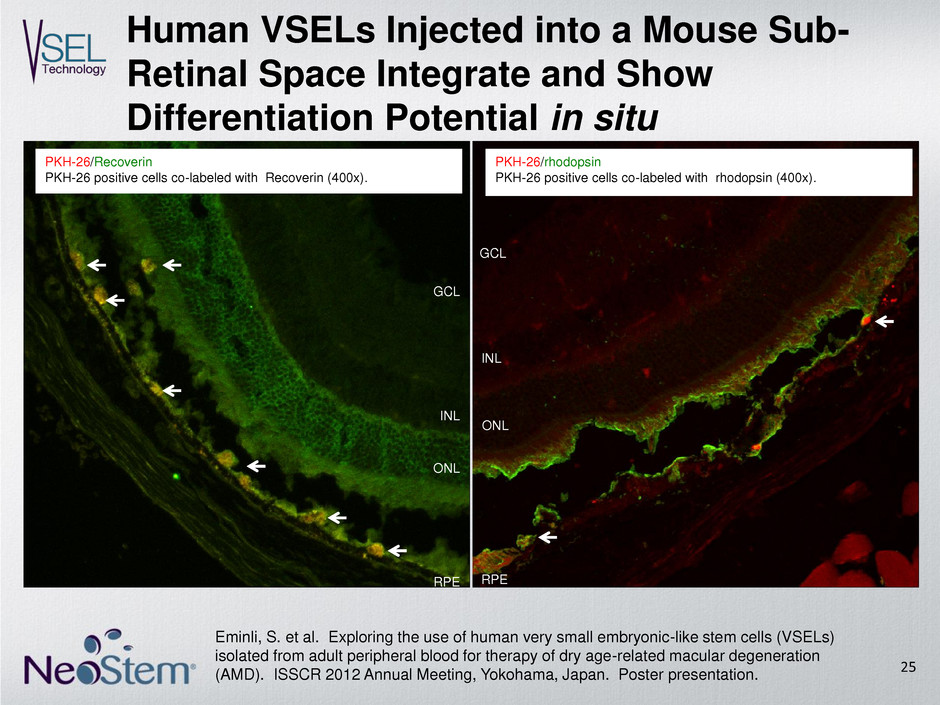
PKH-26/Recoverin PKH-26 positive cells co-labeled with Recoverin (400x). ONL INL GCL RPE PKH-26/rhodopsin PKH-26 positive cells co-labeled with rhodopsin (400x). ONL INL RPE GCL Human VSELs Injected into a Mouse Sub- Retinal Space Integrate and Show Differentiation Potential in situ 25 Eminli, S. et al. Exploring the use of human very small embryonic-like stem cells (VSELs) isolated from adult peripheral blood for therapy of dry age-related macular degeneration (AMD). ISSCR 2012 Annual Meeting, Yokohama, Japan. Poster presentation.

Academic Collaborators 26 Vincent Falanga, MD Roger Williams Medical Center Kameran Lashkari, MD Schepens Eye Institute, Harvard Medical School Song Li, PhD University of California, Berkeley Mariusz Ratajczak, MD, PhD, DSci University of Louisville Russel Tacihman, DMD, DMSc University of Michigan

27 Progenitor Cell Therapy Development and delivery of high quality, cost-efficient, and effective therapeutics can be leveraged by state-of-the-art manufacturing and regulatory expertise

Mountain View, California (25,000 ft2) ISO Class 7 / Class 10,000 suites Allendale, New Jersey (30,000 ft2) ISO Class 7 / Class 10,000 suites ISO Class 6 / Class 1,000 suite 28 • cGMP/GLP Accredited and Certified Facilities • Experience with over • 100 Clients Served and Growing • 30,000 Products Manufactured • 18,000 Products Stored • 14,000 Products Shipped for Clinical Use • 50 US and EU Regulatory Filings Successfully Completed PCT Manufactured for Phase 1, 2 and 3 for Dendreon’s FDA Approved Provenge® Product 15 Year Track Record of Success

• Large and small companies in the cell therapy space outsource services for all or part of their manufacturing needs to improve efficiencies and profitability and to reduce capital investment: • PCT supports NeoStem’s cell therapy development programs with o Lower costs for internal cell therapy development o Cash flow that can be reinvested toward growth and internal development activities • Establish opportunities for early partnering relationships with goals of commercial manufacturing, equity participation and back-end royalties • Automation initiatives focused on lowering cost of goods and increasing gross profits • Initiatives being pursued to expand commercial manufacturing in the US and Europe Business Model & Capabilities 29
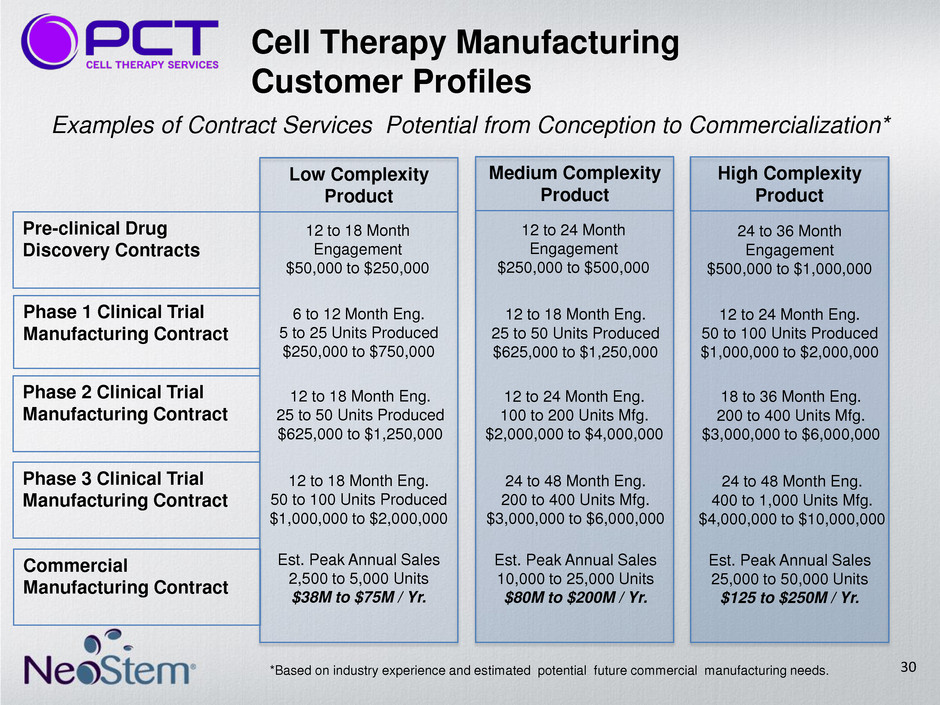
Low Complexity Product Medium Complexity Product High Complexity Product Pre-clinical Drug Discovery Contracts Phase 2 Clinical Trial Manufacturing Contract Phase 1 Clinical Trial Manufacturing Contract Phase 3 Clinical Trial Manufacturing Contract Commercial Manufacturing Contract 12 to 18 Month Engagement $50,000 to $250,000 12 to 24 Month Engagement $250,000 to $500,000 24 to 36 Month Engagement $500,000 to $1,000,000 6 to 12 Month Eng. 5 to 25 Units Produced $250,000 to $750,000 12 to 18 Month Eng. 25 to 50 Units Produced $625,000 to $1,250,000 12 to 24 Month Eng. 50 to 100 Units Produced $1,000,000 to $2,000,000 12 to 18 Month Eng. 25 to 50 Units Produced $625,000 to $1,250,000 12 to 24 Month Eng. 100 to 200 Units Mfg. $2,000,000 to $4,000,000 18 to 36 Month Eng. 200 to 400 Units Mfg. $3,000,000 to $6,000,000 12 to 18 Month Eng. 50 to 100 Units Produced $1,000,000 to $2,000,000 24 to 48 Month Eng. 200 to 400 Units Mfg. $3,000,000 to $6,000,000 24 to 48 Month Eng. 400 to 1,000 Units Mfg. $4,000,000 to $10,000,000 Est. Peak Annual Sales 2,500 to 5,000 Units $38M to $75M / Yr. Est. Peak Annual Sales 10,000 to 25,000 Units $80M to $200M / Yr. Est. Peak Annual Sales 25,000 to 50,000 Units $125 to $250M / Yr. *Based on industry experience and estimated potential future commercial manufacturing needs. Examples of Contract Services Potential from Conception to Commercialization* Cell Therapy Manufacturing Customer Profiles 30

NeoStem Key Executives Robin Smith, MD, MBA CEO & Chairman of the Board MD – Yale; MBA – Wharton Formerly President & CEO IP2M (HC multimedia), EVP & CMO HealthHelp (radiology management) Trustee of NYU Medical Center; Chairman of the Board of NYU Hospital for Joint Diseases (through November 2009) and Stem for Life Foundation Larry May Chief Financial Officer BS Business Administration – University of Missouri Formerly Treasurer & Controller at Amgen; SVP Finance & CFO at BioSource Intl Extensive experience building accounting, finance and IT operations Andrew Pecora, MD, FACP Chief Medical Officer MD – University of Medicine and Dentistry of New Jersey Chief Innovations Officer, Professor and Vice President of Cancer Services at John Theurer Cancer Center at Hackensack University Medical Center Robert Preti, PhD President and Chief Scientific Officer of PCT PhD and MS in Cellular Biology / Hematology - New York University One of the country’s leading authorities on cell engineering and the principal investigator for a number of clinical trials relating to stem cell transplantation 10 years experience as Director of Hematopoietic Stem Cell Processing & Research Laboratory Timothy C. Fong, PhD, MBA VP, Technology & Product Development of PCT PhD in Immunology – UCLA, MBA – Saint Mary’s College Recently Technical Director Cell Therapy at BD Biosciences Over 18 years experience in drug development; Has led R&D groups in cell and gene therapies from discovery research to clinical trials Jonathan Sackner-Bernstein, MD, FACC VP of Clinical Development and Regulatory Affairs MD – Jefferson Medical College Internationally recognized clinical researcher in cardiology 20 years experience in clinical practice, medical research and healthcare management FDA background as past Associate Director for Technology and Innovation; Former CMO at Clinilabs, a clinical research organization Martin E. Schmieg VP, Corporate Development BA – LaSalle University Expertise in bus dev for health care product and med tech companies Formerly President of Nuvilex, Inc., President and CEO of Freedom2, Inc. Selected transactions include multi-billion dollar sale of Advanced Bionics Corp. to Boston Scientific & development and market launch of the Cytoscan instrument 31
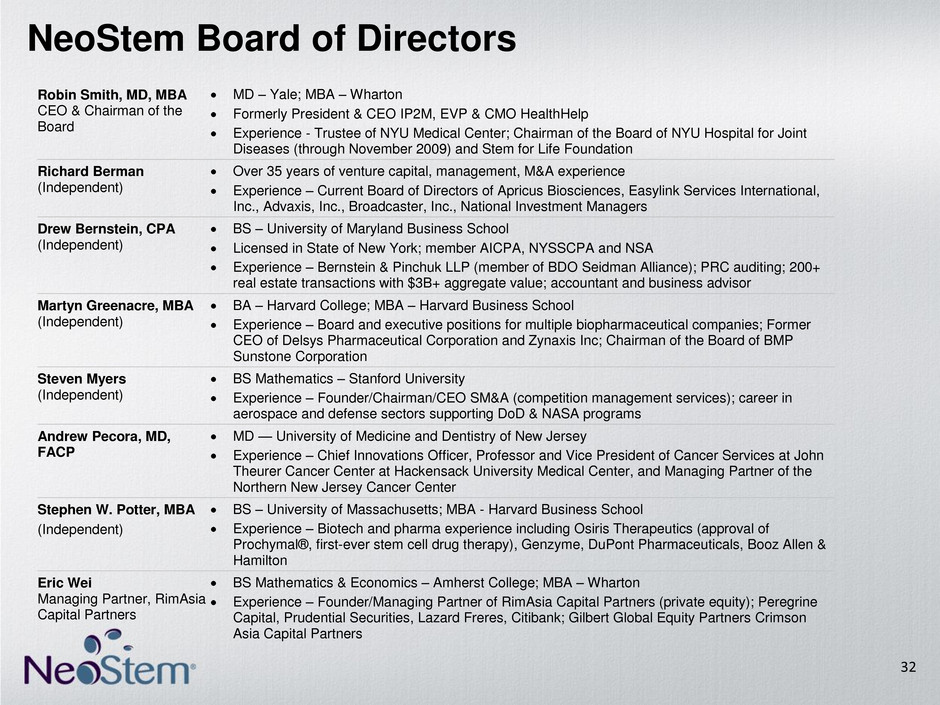
NeoStem Board of Directors Robin Smith, MD, MBA CEO & Chairman of the Board MD – Yale; MBA – Wharton Formerly President & CEO IP2M, EVP & CMO HealthHelp Experience - Trustee of NYU Medical Center; Chairman of the Board of NYU Hospital for Joint Diseases (through November 2009) and Stem for Life Foundation Richard Berman (Independent) Over 35 years of venture capital, management, M&A experience Experience – Current Board of Directors of Apricus Biosciences, Easylink Services International, Inc., Advaxis, Inc., Broadcaster, Inc., National Investment Managers Drew Bernstein, CPA (Independent) BS – University of Maryland Business School Licensed in State of New York; member AICPA, NYSSCPA and NSA Experience – Bernstein & Pinchuk LLP (member of BDO Seidman Alliance); PRC auditing; 200+ real estate transactions with $3B+ aggregate value; accountant and business advisor Martyn Greenacre, MBA (Independent) BA – Harvard College; MBA – Harvard Business School Experience – Board and executive positions for multiple biopharmaceutical companies; Former CEO of Delsys Pharmaceutical Corporation and Zynaxis Inc; Chairman of the Board of BMP Sunstone Corporation Steven Myers (Independent) BS Mathematics – Stanford University Experience – Founder/Chairman/CEO SM&A (competition management services); career in aerospace and defense sectors supporting DoD & NASA programs Andrew Pecora, MD, FACP MD — University of Medicine and Dentistry of New Jersey Experience – Chief Innovations Officer, Professor and Vice President of Cancer Services at John Theurer Cancer Center at Hackensack University Medical Center, and Managing Partner of the Northern New Jersey Cancer Center Stephen W. Potter, MBA (Independent) BS – University of Massachusetts; MBA - Harvard Business School Experience – Biotech and pharma experience including Osiris Therapeutics (approval of Prochymal®, first-ever stem cell drug therapy), Genzyme, DuPont Pharmaceuticals, Booz Allen & Hamilton Eric Wei Managing Partner, RimAsia Capital Partners BS Mathematics & Economics – Amherst College; MBA – Wharton Experience – Founder/Managing Partner of RimAsia Capital Partners (private equity); Peregrine Capital, Prudential Securities, Lazard Freres, Citibank; Gilbert Global Equity Partners Crimson Asia Capital Partners 32

Key Financial Metrics 33 Revenue $2.5m (1Q 2013) Cash $9.3m (as of March 31, 2013) Additional Cash $10.7m (net proceeds from common stock offering in May 2013) Total Stock and Equivalent Shares Outstanding (as of May 9, 2013) Common Shares 193.8m Warrants 55.0m (avg. warrant exercise price of $1.56) Options 26.5m (avg. option exercise price of $1.16) 33
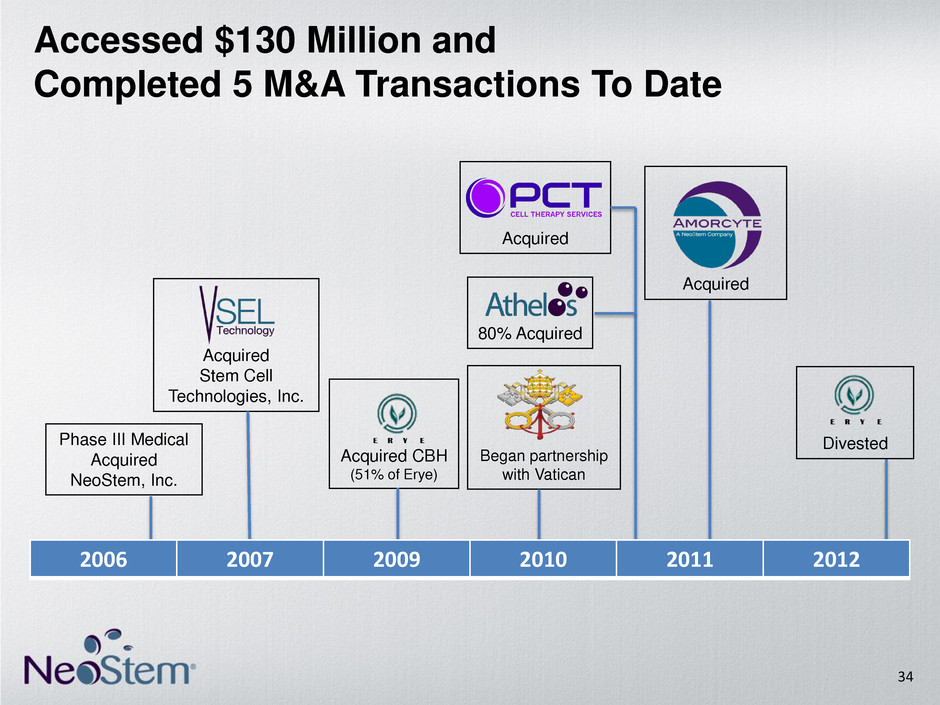
Acquired Stem Cell Technologies, Inc. Acquired CBH (51% of Erye) Acquired Acquired Divested Began partnership with Vatican Phase III Medical Acquired NeoStem, Inc. Accessed $130 Million and Completed 5 M&A Transactions To Date 80% Acquired 34 2006 2007 2009 2010 2011 2012

NeoStem Milestones 35 • Therapeutic Pipeline o Complete enrollment PreSERVE-AMI Phase 2 trial o 1st data readout 6-8 months after last patient enrolled o Progress towards Phase 1b/2a AMR-001 CHF trial o Progress towards VSELTM human bone growth trials o Progress Treg cell program towards Phase 2 trial in type 1 diabetes • Commercial Operations o Cell therapy automation project o Manufacturing expansion into Europe o US commercial manufacturing expansion o Product/service expansion transaction(s)

• Dynamic, experienced and nimble management with a proven track record of success with leadership that can execute • Clinical and preclinical pipeline of cell therapies around a strong IP portfolio o Cardiovascular disease o Autoimmune disorders o Regenerative medicine • Recognized, state-of-the art contract development and manufacturing organization (East and West coast operations) NeoStem is Built for Success in Regenerative Medicine 36

Contact Information NeoStem, Inc. NYSE MKT: NBS www.neostem.com Robin Smith, MD, MBA Chairman & CEO Phone: (212) 584-4174 Email: rsmith@neostem.com v7 37




































