
INVESTOR PRESENTATION NASDAQ: NBS MARCH 2014 TRANSFORMING MEDICINE

NASDAQ:NBS | www.neostem.com > 1 FORWARD-LOOKING STATEMENTS This presentation includes “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995, as well as historical information. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements, or industry results, to be materially different from anticipated results, performance or achievements expressed or implied by such forward-looking statements. When used in this presentation, statements that are not statements of current or historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, the words “plan,” “intend,” “may,” “will,” “expect,” “believe,” “could,” “anticipate,” “estimate,” or “continue” or similar expressions or other variations or comparable terminology are intended to identify such forward-looking statements, although some forward-looking statements are expressed differently. Additionally, statements regarding the future of the regenerative medicine industry and the role of stem cells and cellular therapy in that future, our ability to successfully develop and grow our business, including with regard to our research and development and clinical evaluation efforts and future marketing and sales in respect of product candidate in our CD34 Cell Program, our T-Regulatory Cell Program and other cell therapies and the marketing, performance and planned expansion of our contract development and manufacturing business are forward looking statements. Our future operating results are dependent upon many factors and our further development is highly dependent on future medical and research developments and market acceptance, which is outside our control. Forward-looking statements, including with respect to the successful execution of the Company's strategy, may not be realized due to a variety of factors and we cannot guarantee their accuracy or that our expectations about future events will prove to be correct. Such factors include, without limitation, (i) our ability to manage our business despite operating losses and cash outflows; (ii) our ability to obtain sufficient capital or strategic business arrangements to fund our operations and expansion plans, including meeting our financial obligations under various licensing and other strategic arrangements, the funding of our development programs for our CD34 Cell Program and our T Regulatory Cell Program, and the commercialization of the relevant technology; (iii) our ability to build and maintain the management and human resources infrastructure necessary to support the growth of our business; (iv) our ability to integrate our acquired businesses successfully and grow such acquired businesses as anticipated, including expanding our PCT business internationally; (v) whether a large global market is established for our cellular-based products and services and our ability to capture a meaningful share of this market; (vi)competitive factors and developments beyond our control; (vii) scientific and medical developments beyond our control; (viii) our ability to obtain and maintain, as applicable, appropriate governmental licenses, accreditations or certifications or comply with healthcare laws and regulations or any other adverse effect or limitations caused by government regulation of our business; (ix) whether any of our current or future patent applications result in issued patents, the scope of those patents and our ability to obtain and maintain other rights to technology required or desirable for the conduct of our business; (x) whether any potential strategic benefits of various licensing transactions will be realized and whether any potential benefits from the acquisition of these licensed technologies will be realized; (xi) the results of our development activities, including the results of our PreSERVE Phase 2 clinical trial of AMR-001 and planned clinical trials; (xii) our ability to complete our planned clinical trials (or initiate future trials) in accordance with our estimated timelines due to delays associated with enrolling patients due to the novelty of the treatment, the size of the patient population and the need of patients to meet the inclusion criteria of the trial or otherwise; (xiii) the prospects of entering into additional license agreements, or other forms of cooperation with other companies and/or medical institutions and (xiii) the other factors discussed in “Risk Factors” in our Annual Report on Form 10-K filed with the Securities and Exchange Commission (“the SEC”) on March 13, 2014 and elsewhere in this presentation and in the Company's other periodic filings with the SEC which are available for review at www.sec.gov under “Search for Company Filings.” All forward-looking statements attributable to us are expressly qualified in their entirety by these and other factors. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the Company undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
NASDAQ:NBS | www.neostem.com > 2 CELL THERAPY Using cells to prevent, treat, or cure disease by repairing and replacing damaged or aged tissue, cells, and organs in order to restore their normal function Holds the promise to dramatically transform the course of medicine: Improve clinical outcomes Reduce overall healthcare costs

NASDAQ:NBS | www.neostem.com > 3 ABOUT NEOSTEM Leader in the emerging cellular therapy industry developing novel proprietary cell therapy products as well as generating revenue through a contract development and manufacturing organization that we believe will benefit from the growth of this industry
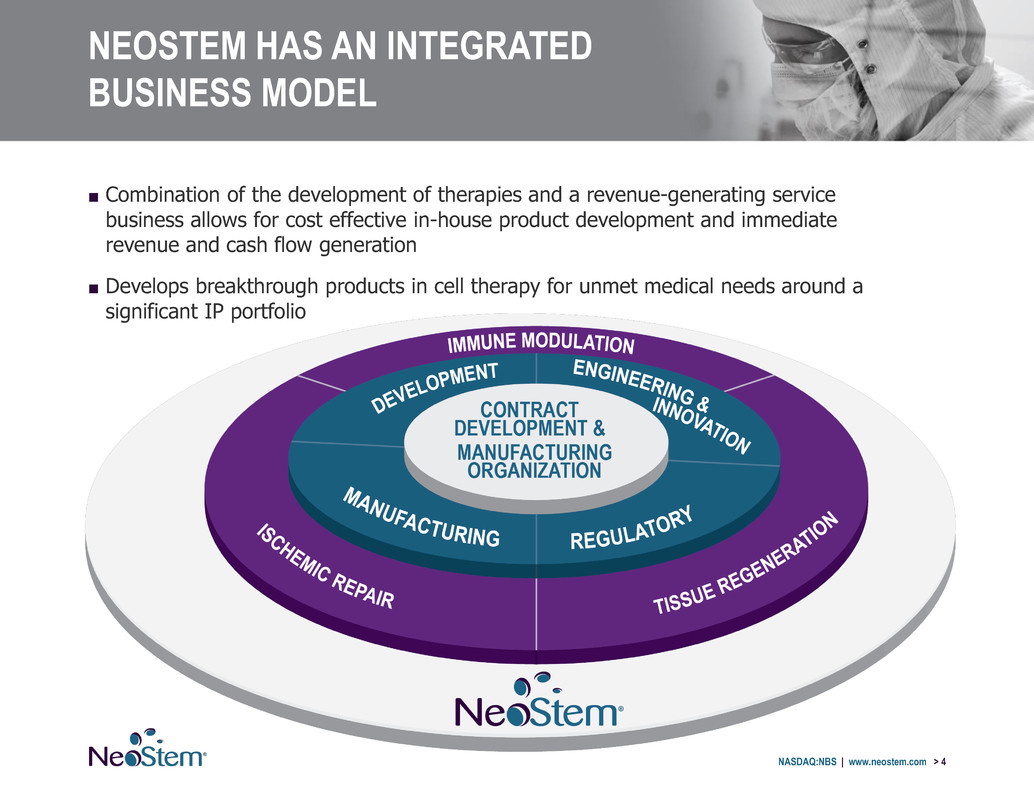
NASDAQ:NBS | www.neostem.com > 4 NEOSTEM HAS AN INTEGRATED BUSINESS MODEL ■ Combination of the development of therapies and a revenue-generating service business allows for cost effective in-house product development and immediate revenue and cash flow generation ■ Develops breakthrough products in cell therapy for unmet medical needs around a significant IP portfolio

COMPANY OVERVIEW NASDAQ:NBS | www.neostem.com > 5 ■ Leader in cell therapy developing potentially transformative treatments for patients in multiple indications ■ Founded in 2006 ■ Integrated entity with 3 pipeline technology platforms and revenue generating contract development and manufacturing service business ■ 28.5M common shares outstanding (36.9M fully diluted) as of March 11, 2014 ► 4.5M warrants that could generate $69.1M for the Company upon exercise ■ Market Capitalization: $199M as of February 28, 2014 ■ Over $46M in cash as of December 31, 2013 ■ Shares listed on NASDAQ, Ticker: NBS ■ Headquarters in New York City ■ GMP-compliant facilities in Allendale, NJ and Mountain View, CA ■ 108 employees as of December 31, 2013 ■ Management team with broad industry and academic experience ABOUT NEOSTEM We pursue the preservation and enhancement of human health globally through the development of cell-based therapeutics that prevent, treat or cure disease

CD34 CELL PROGRAM: ENHANCING THE BODY’S NATURAL REPAIR MECHANISM ■ Following a heart attack, apoptosis and progressive cardiomyocyte loss leads to infarct expansion ■ ST segment Elevation MI (STEMI) patients are at a high risk of a progressive deterioration in heart muscle function that leads to worsening of heart function, morbidity and mortality ■ CD34/CXCR4 cells are a natural repair mechanism ■ This mechanism works the same for other areas of vascular insufficiency such as chronic heart failure and traumatic brain injury THE NATURAL PROGRESSION OF DISEASE POST-STEMI NASDAQ:NBS | www.neostem.com > 6 AMR-001 BRINGS REPAIR SYSTEM TO THE HEART TO PRESERVE FUNCTION AFTER A STEMI
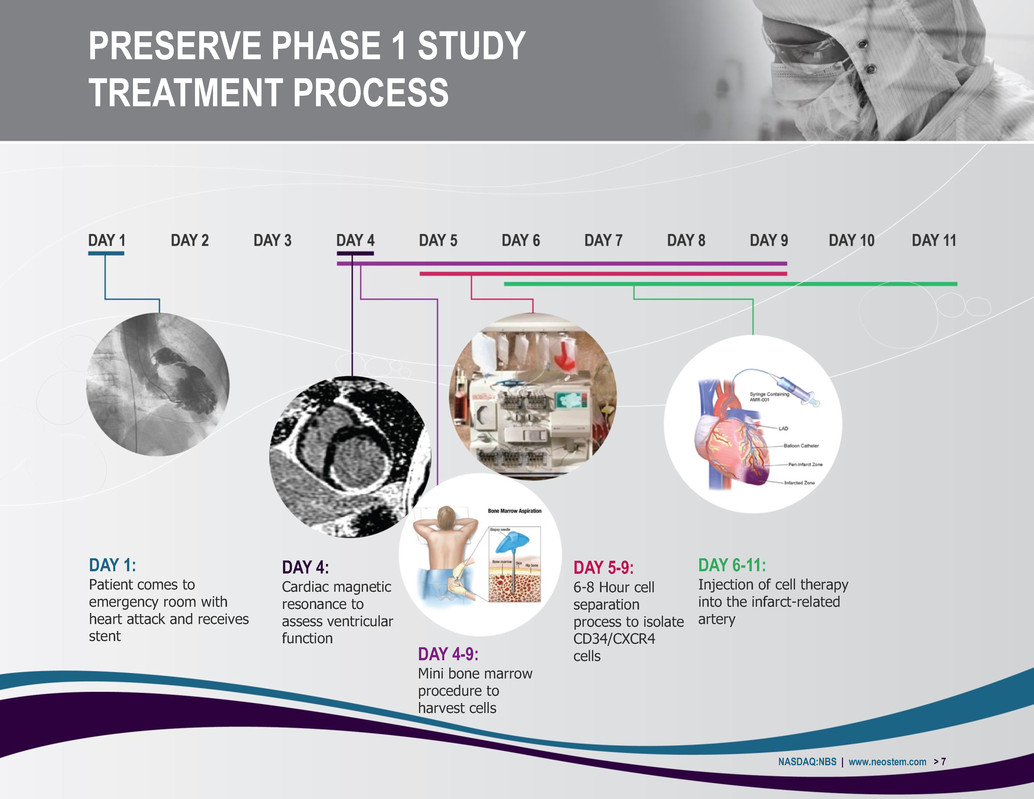
PRESERVE PHASE 1 STUDY TREATMENT PROCESS NASDAQ:NBS | www.neostem.com > 7 DAY 1: Patient comes to emergency room with heart attack and receives stent DAY 4: Cardiac magnetic resonance to assess ventricular function DAY 4-9: Mini bone marrow procedure to harvest cells DAY 5-9: 6-8 Hour cell separation process to isolate CD34/CXCR4 cells DAY 6-11: Injection of cell therapy into the infarct-related artery

FEATURES AND BENEFITS OF AMR-001 ■ CD34/CXCR4 cells home to the viable tissue surrounding the infarcted (dead) myocardium (peri-infarct zone) after administration and persist ■ Autologous cells take up residence in the peri-infarct zone, likely promoting angiogenesis (development and formation of new blood vessels) ■ Cell preparation has a 72 hour shelf life and is infused into patient 5 to 11 days following an AMI ► After the pro-inflammatory “hot phase” ► Prior to permanent scar formation NASDAQ:NBS | www.neostem.com > 8 FEATURES ■ Amplifies the body’s natural repair mechanism ■ Cells are not expanded – concern about abnormal growth once administered is not relevant ■ Cells are autologous – immune attack is not a concern ■ Delivery where cells are needed without having to inject into myocardium ► Safer and greater distribution BENEFITS

PHASE 1 RESULTS POINT TO AMR-001 POTENTIAL NASDAQ:NBS | www.neostem.com > 9 Patients dosed ≥ the threshold dose of 10 million cells showed significant improvement in perfusion Increasing doses of CD34/SDF-1 mobile cells reduced the size of the infarct region as measured by CMR DSMB DETERMINED THAT THERE WERE NO SAFETY CONCERNS THAT WARRANTED ANY ACTION Quyyumi AmHtJ 2011 and data on file Y = Δ Infarct % LV Mass, X = Dose of SDF1 mobile CD34 cells At threshold dose of 10 million cells or more, no individuals showed decrease in LVEF RTSS (HYPOPERFUSION) COHORT BASE LINE 6 MONTHS DELTA % CHANGE Control 259.0 273.5 +14.5 +5.6 5M Cells 714.2 722.0 +7.8 +1.1 10M Cells 998.6 635.8 -362.8 -36.4 15M Cells 584.0 462.0 -122.0 -20.9 % of individuals with decrease in left ventricular ejection fraction (LVEF) CD34 cells (millions) No individuals showed a decrease in LVEF Threshold DOSE RESPONSE CORRELATED WITH MOBILE CD34 CELLS
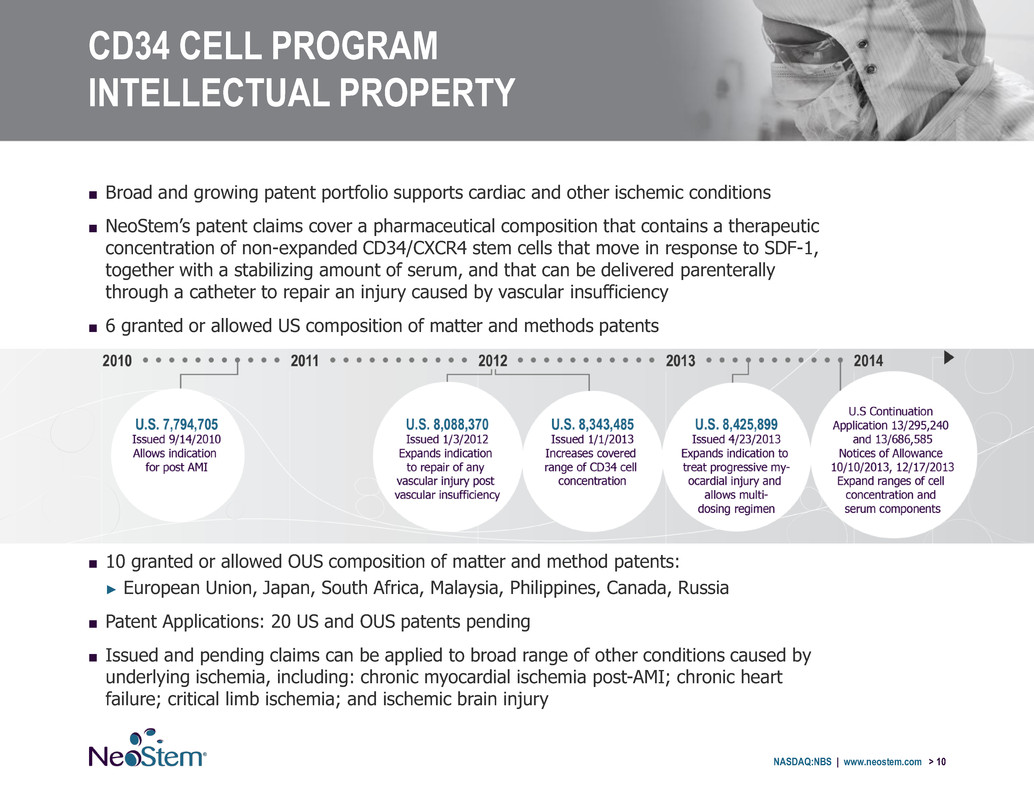
CD34 CELL PROGRAM INTELLECTUAL PROPERTY NASDAQ:NBS | www.neostem.com > 10 ■ Broad and growing patent portfolio supports cardiac and other ischemic conditions ■ NeoStem’s patent claims cover a pharmaceutical composition that contains a therapeutic concentration of non-expanded CD34/CXCR4 stem cells that move in response to SDF-1, together with a stabilizing amount of serum, and that can be delivered parenterally through a catheter to repair an injury caused by vascular insufficiency ■ 6 granted or allowed US composition of matter and methods patents ■ 10 granted or allowed OUS composition of matter and method patents: ► European Union, Japan, South Africa, Malaysia, Philippines, Canada, Russia ■ Patent Applications: 20 US and OUS patents pending ■ Issued and pending claims can be applied to broad range of other conditions caused by underlying ischemia, including: chronic myocardial ischemia post-AMI; chronic heart failure; critical limb ischemia; and ischemic brain injury

PRESERVE PHASE 2 STUDY: ENROLLMENT COMPLETED, AWAITING DATA RELEASE NASDAQ:NBS | www.neostem.com > 11 TARGET Post-AMI preservation of cardiac function KEY INCLUSION CRITERIA Confirmation of ST Elevation MI (STEMI); ejection fraction < 48% at day 4; state of the art care post stenting LOCATION AND NUMBER OF SUBJECTS United States, 60 centers, 160 patients (enrollment completed) DESIGN Double blind, placebo controlled, randomized (1:1) PRIMARY ENDPOINT Change in cardiac perfusion (RTSS by SPECT) from baseline to 6 months OTHER ENDPOINTS Secondary endpoints to determine preservation of cardiac function and clinical events: ■ CMR to measure LVEF, LVESV, LVEDV, regional myocardial strain, infarct/peri-infarct regional wall motion abnormalities, and infarct size (baseline and 6 months) ■ Quality of Life measures: (KCCQ & SAQ) ■ Reduction in cumulative MACE and other adverse clinical cardiac events at 6, 12, 18, 24, and 36 months TREATMENT Single dose via infarct related artery with minimum dose for release >10MM CD34+ cells

WHAT’S NEXT? NASDAQ:NBS | www.neostem.com > 12 CHRONIC HEART FAILURE ■ Significant need - prevalence of over 23 million worldwide, 5.7 million US ■ Therapy would enable larger distribution (not limited to mapping systems) ■ Expect to initiate Phase 2 clinical trial in 2014 in Europe ■ Dilated cardiomyopathy is one cause of chronic heart failure TRAUMATIC BRAIN INJURY ■ 1.7 million people sustain TBI annually ■ Expect data in 2014 from preclinical studies on effect of CD34 product in animal model ■ Plan to seek partnerships if studies are positive

RECENT DATA SUPPORTS CD34 USE IN CHRONIC HEART FAILURE NASDAQ:NBS | www.neostem.com > 13 Adapted from Vrtovec et al, Circ Res published online October 12, 201 Note: 110 patients (open label, 55 treated with cells and 55 standard of care) CD34 STEM CELL THERAPY SIGNIFICANTLY IMPROVES EVENT FREE SURVIVIAL AT 5 YEARS IN PATIENTS WITH DILATED CARDIOMYOPATHY
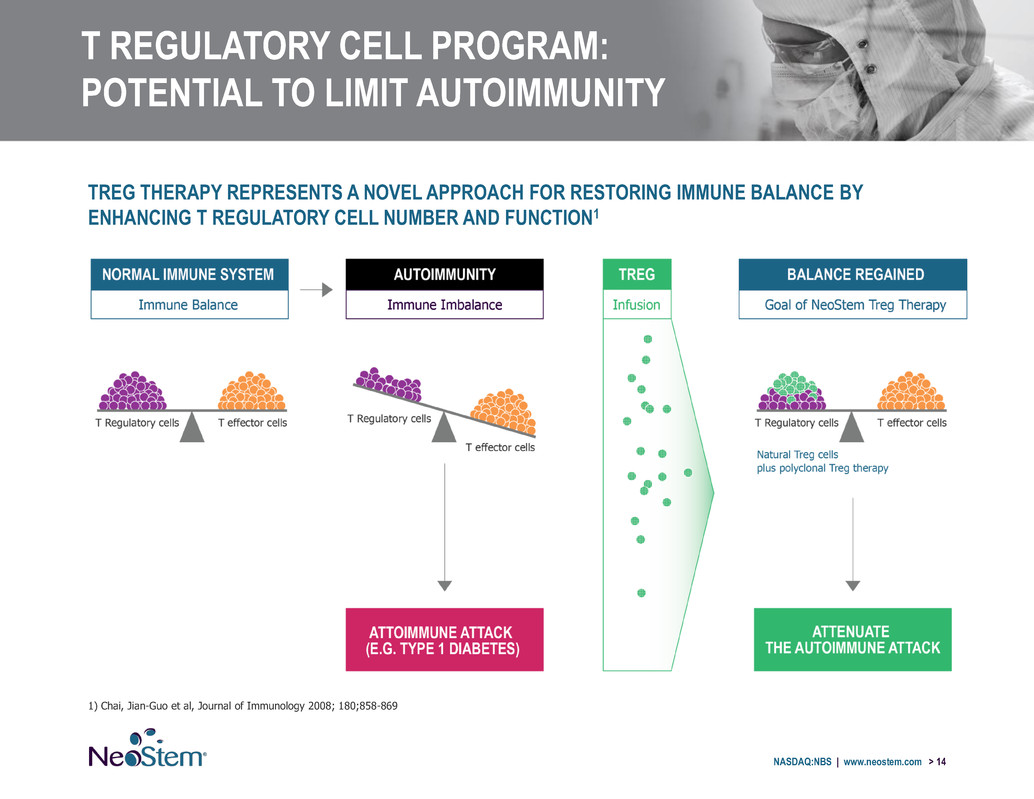
T REGULATORY CELL PROGRAM: POTENTIAL TO LIMIT AUTOIMMUNITY NASDAQ:NBS | www.neostem.com > 14 TREG THERAPY REPRESENTS A NOVEL APPROACH FOR RESTORING IMMUNE BALANCE BY ENHANCING T REGULATORY CELL NUMBER AND FUNCTION1 1) Chai, Jian-Guo et al, Journal of Immunology 2008; 180;858-869
FEATURES OF THE TREG PROGRAM NASDAQ:NBS | www.neostem.com > 15 FEATURES OF TREGS: ■ Natural part of immune system ■ Regulate activity of T effector cells (responsible for protection from viruses and foreign antigens) ■ In autoimmune disease it is thought that deficient Treg activity permits the T effector cells to attack the bodies’ own tissues SIGNIFICANT COLLABORATIONS: ■ Partnership with Becton Dickinson (11.5% program ownership) ■ Accelerated development through collaboration with University of California, San Francisco and laboratory of Dr. Jeffrey Bluestone INTELLECTUAL PROPERTY: ■ Exclusive rights to 22 issue patents covering isolation, activation, expansion and methods of treating or preventing certain conditions and/or diseases using Tregs in US and major international markets

SIGNIFICANT MARKET OPPORTUNITY IN INITIAL INDICATIONS NASDAQ:NBS | www.neostem.com > 16 TYPE 1 DIABETES ■ Also referred to as insulin dependent diabetes or juvenile diabetes ■ Affects >34 million worldwide or 1 in 300 children ■ Diabetes is leading cause of kidney failure, new cases of adult blindness and non-traumatic lower-limb amputations ■ Type 1 diabetes accounts for $14.9 billion in annual healthcare costs in US ASTHMA ■ Affects 25 million in US and 300 million worldwide ■ Asthma accounts for $56 billion in annual direct and indirect health care costs in US ■ Steroid resistant asthma afflicts <5% of the total asthma population, but accounts for up to 50% of healthcare spending on asthma

TREG PROGRAM CLINICAL STATUS NASDAQ:NBS | www.neostem.com > 17 ■ Phase 1 study for type 1 diabetes completed by Dr. Bluestone and Dr. Kevan Herold (Yale University) with results to be presented at American Diabetes Association Scientific Sessions in June 2014 ■ Advancing to initiate type 1 diabetes Phase 2 study in 2014 through collaboration with Drs. Jeffrey Bluestone and Qizhi Tang (UCSF) ■ Data published in 2012 of a study1 of autologous Treg therapy for type 1 diabetes revealed significant preservation of pancreatic function in treated patients compared to controls, with higher c-peptide levels and lower insulin requirements, including 20% of patients being able to come off of insulin after 4 months after treatment ■ Evaluating second indication for Treg program; steroid resistant asthma proof- of-concept study planned to initiate in 2014 1) Marek-Trzonkowska N et al. Diabetes Care 2012;35:1817-1820
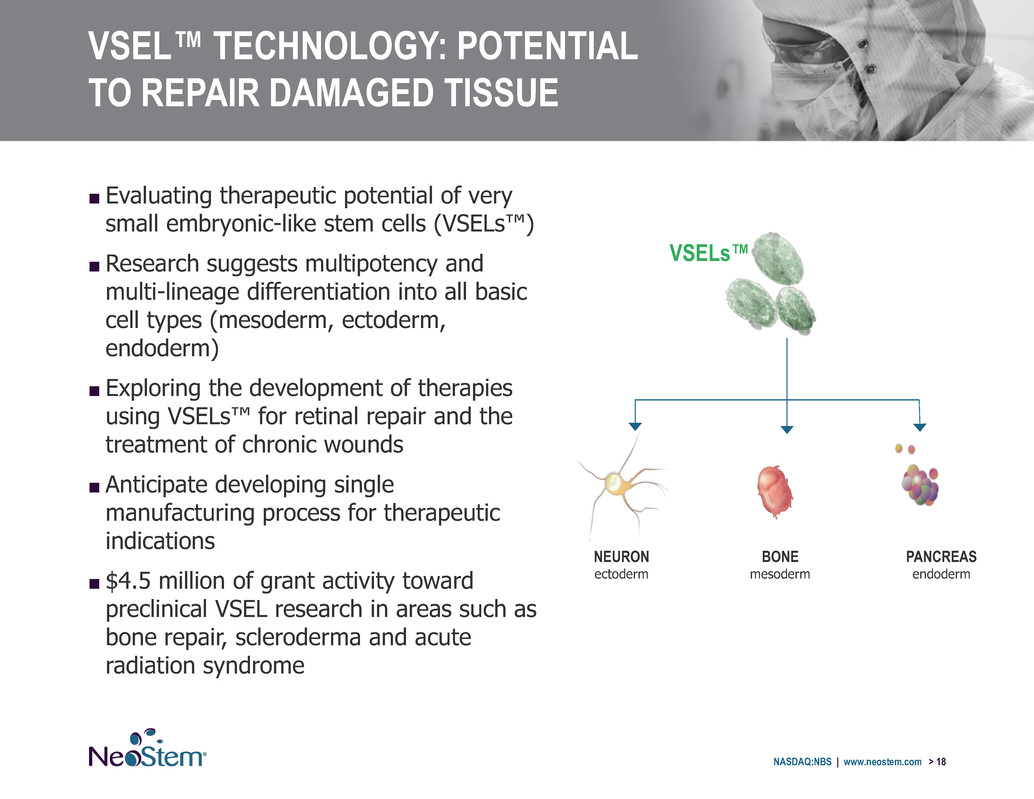
VSEL™ TECHNOLOGY: POTENTIAL TO REPAIR DAMAGED TISSUE NASDAQ:NBS | www.neostem.com > 18 ■ Evaluating therapeutic potential of very small embryonic-like stem cells (VSELs™) ■ Research suggests multipotency and multi-lineage differentiation into all basic cell types (mesoderm, ectoderm, endoderm) ■ Exploring the development of therapies using VSELs™ for retinal repair and the treatment of chronic wounds ■ Anticipate developing single manufacturing process for therapeutic indications ■ $4.5 million of grant activity toward preclinical VSEL research in areas such as bone repair, scleroderma and acute radiation syndrome BONE mesoderm NEURON ectoderm PANCREAS endoderm VSELs™
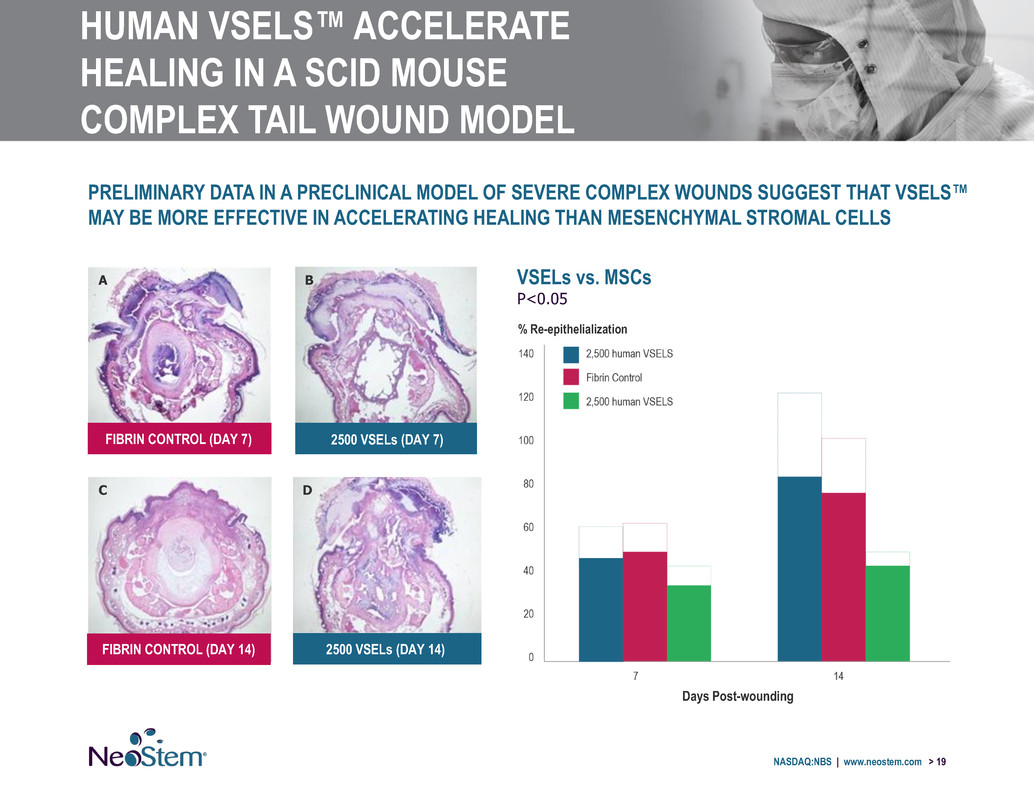
HUMAN VSELS™ ACCELERATE HEALING IN A SCID MOUSE COMPLEX TAIL WOUND MODEL NASDAQ:NBS | www.neostem.com > 19 PRELIMINARY DATA IN A PRECLINICAL MODEL OF SEVERE COMPLEX WOUNDS SUGGEST THAT VSELS™ MAY BE MORE EFFECTIVE IN ACCELERATING HEALING THAN MESENCHYMAL STROMAL CELLS FIBRIN CONTROL (DAY 7) FIBRIN CONTROL (DAY 14) 2500 VSELs (DAY 7) 2500 VSELs (DAY 14) A B C D VSELs vs. MSCs P<0.05 % Re-epithelialization Days Post-wounding
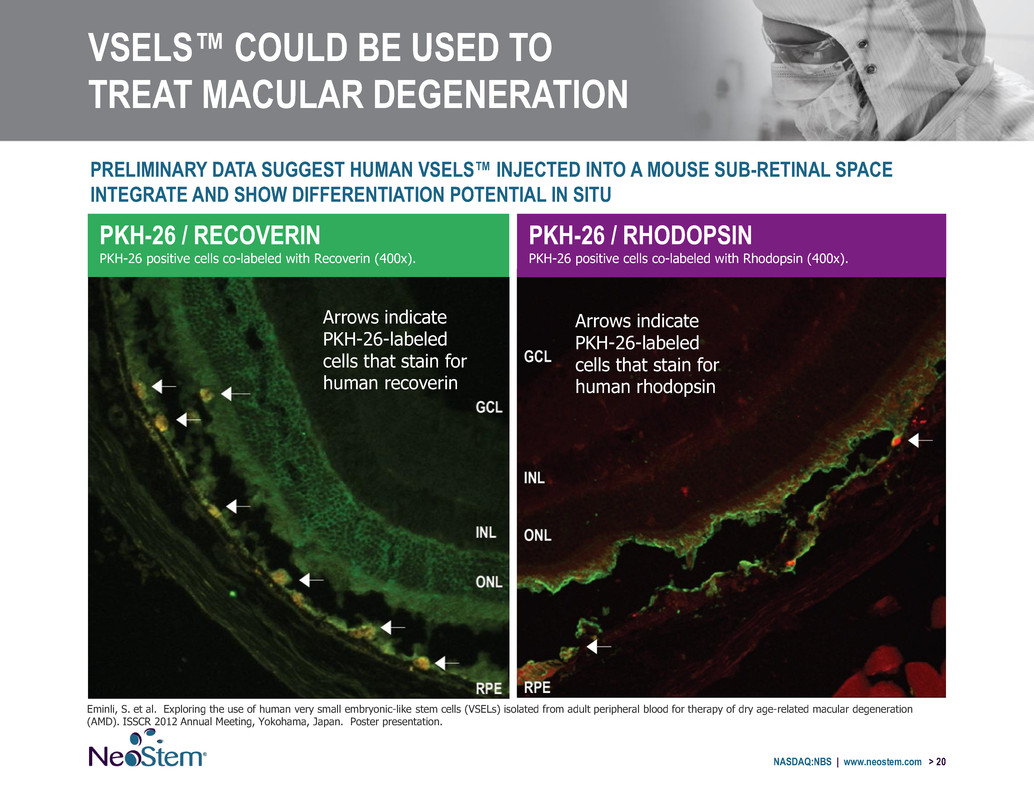
VSELS™ COULD BE USED TO TREAT MACULAR DEGENERATION NASDAQ:NBS | www.neostem.com > 20 Eminli, S. et al. Exploring the use of human very small embryonic-like stem cells (VSELs) isolated from adult peripheral blood for therapy of dry age-related macular degeneration (AMD). ISSCR 2012 Annual Meeting, Yokohama, Japan. Poster presentation. PKH-26 / RECOVERIN PKH-26 positive cells co-labeled with Recoverin (400x). PKH-26 / RHODOPSIN PKH-26 positive cells co-labeled with Rhodopsin (400x). Arrows indicate PKH-26-labeled cells that stain for human recoverin Arrows indicate PKH-26-labeled cells that stain for human rhodopsin PRELIMINARY DATA SUGGEST HUMAN VSELS™ INJECTED INTO A MOUSE SUB-RETINAL SPACE INTEGRATE AND SHOW DIFFERENTIATION POTENTIAL IN SITU

2014 OUTLOOK: CLINICAL MILESTONES NASDAQ:NBS | www.neostem.com > 21 1. The last patient primary endpoint follow-up for this study is expected in June followed by data lock and analysis with data available in 2H 2014. 2. It is expected that this study will be presented at the American Diabetes Association’s Scientific Sessions, to be held June 13 – 17, 2014, by Study Director Dr. Jeffrey Bluestone (University of California, San Francisco) and Dr. Kevan Herold (Yale University), the Study Principal Investigator. The data from the study has been licensed by the Company from The University of California, San Francisco, and is expected to serve as the basis for initiation of a Phase 2 study by the Company.

MOUNTAIN VIEW, CALIFORNIA (25,000 ft2) ISO Class 7 / Class 10,000 suites Recent expansion of clean room space ALLENDALE, NEW JERSEY (30,000 ft2) ISO Class 7 / Class 10,000 suites ISO Class 6 / Class 1,000 suite Recent expansion of clean room space PCT PROVIDES OUTSOURCED MANUFACTURING CAPABILITIES TO CELL THERAPY INDUSTRY NASDAQ:NBS | www.neostem.com > 22 ■ High quality manufacturing capabilities with 15-year track record of success ■ Proven efficiencies and reduced capital investment for customers through outsourcing ■ Demonstrated regulatory expertise: ► 50+ EU and US regulatory filings; ►All clinical trial phases including BLA submission and product approval by FDA ■ Significant focus on innovation, engineering and automation ■ EU product distribution requirement compliant ■ Continuing to expand commercial capabilities in the US and internationally (expect Europe in 2014)
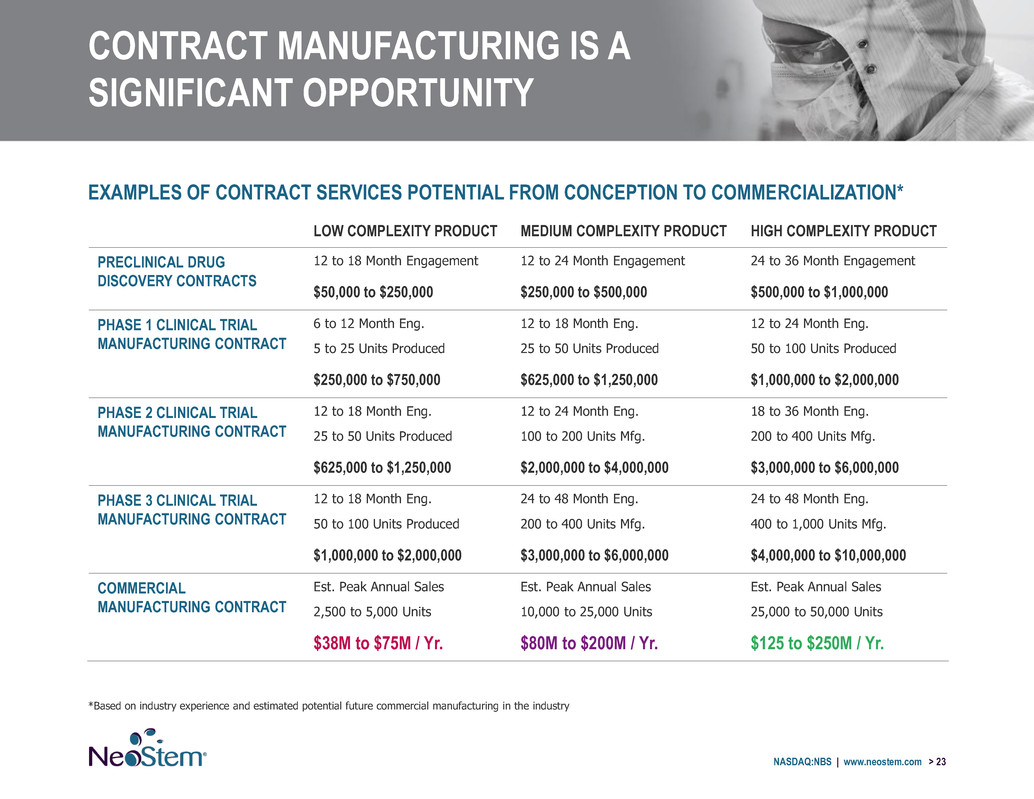
CONTRACT MANUFACTURING IS A SIGNIFICANT OPPORTUNITY NASDAQ:NBS | www.neostem.com > 23 EXAMPLES OF CONTRACT SERVICES POTENTIAL FROM CONCEPTION TO COMMERCIALIZATION* *Based on industry experience and estimated potential future commercial manufacturing in the industry LOW COMPLEXITY PRODUCT MEDIUM COMPLEXITY PRODUCT HIGH COMPLEXITY PRODUCT PRECLINICAL DRUG DISCOVERY CONTRACTS 12 to 18 Month Engagement 12 to 24 Month Engagement 24 to 36 Month Engagement $50,000 to $250,000 $250,000 to $500,000 $500,000 to $1,000,000 PHASE 1 CLINICAL TRIAL MANUFACTURING CONTRACT 6 to 12 Month Eng. 12 to 18 Month Eng. 12 to 24 Month Eng. 5 to 25 Units Produced 25 to 50 Units Produced 50 to 100 Units Produced $250,000 to $750,000 $625,000 to $1,250,000 $1,000,000 to $2,000,000 PHASE 2 CLINICAL TRIAL MANUFACTURING CONTRACT 12 to 18 Month Eng. 12 to 24 Month Eng. 18 to 36 Month Eng. 25 to 50 Units Produced 100 to 200 Units Mfg. 200 to 400 Units Mfg. $625,000 to $1,250,000 $2,000,000 to $4,000,000 $3,000,000 to $6,000,000 PHASE 3 CLINICAL TRIAL MANUFACTURING CONTRACT 12 to 18 Month Eng. 24 to 48 Month Eng. 24 to 48 Month Eng. 50 to 100 Units Produced 200 to 400 Units Mfg. 400 to 1,000 Units Mfg. $1,000,000 to $2,000,000 $3,000,000 to $6,000,000 $4,000,000 to $10,000,000 COMMERCIAL MANUFACTURING CONTRACT Est. Peak Annual Sales Est. Peak Annual Sales Est. Peak Annual Sales 2,500 to 5,000 Units 10,000 to 25,000 Units 25,000 to 50,000 Units $38M to $75M / Yr. $80M to $200M / Yr. $125 to $250M / Yr.

Robert Dickey IV Chief Financial Officer Over 15 years management experience at life sciences companies, following a career as an investment banker Andrew L. Pecora, MD, FACP Chief Visionary Officer, CMO of PCT, CSO of Amorcyte Chief Innovations Officer at John Theurer Cancer Center at Hackensack University Medical Center Co-founder of PCT with significant experience in design and conduct of clinical trials, IRB practices, and payor relationships Douglas W. Losordo, MD, FACC, FAHA Chief Medical Officer Leader in cell therapy research and renowned cardiologist Obtained over $35 million in NIH funding during career-long efforts to develop novel therapeutics Robert A. Preti, PhD Chief Scientific Officer, President of PCT One of the country’s leading authorities on cell engineering and co-founder of PCT 10 years experience as Director of Hematopoietic Stem Cell Processing & Research Laboratory Stephen W. Potter, MBA Executive Vice President Biotech and pharma experience: Osiris Therapeutics (approval of Prochymal®, first-ever stem cell drug therapy), Genzyme, DuPont Pharmaceuticals, Booz Allen & Hamilton Jonathan Sackner-Bernstein, MD, FACC Vice President, Clinical Development and New Technologies Formerly FDA Assoc. Center Director for Innovation and Technology At FDA launched innovation initiative; Established inter-agency relationship between FDA & DARPA Robin Smith, MD, MBA Chief Executive Officer Leading NeoStem since 2006, completing five acquisitions & one divestiture, raising over $180 million Extensive and diversified experience in executive and board level capacities for medical enterprises and healthcare-based entities David Altarac, MD, MPA Vice President, Regulatory Affairs Extensive experience in U.S. and global regulatory affairs, including strategy, operations, labeling and departmental leadership 13 year tenure at Merck, most recently VP, Regulatory Affairs Emerging Markets R&D MANAGEMENT HIGHLIGHTS NASDAQ:NBS | www.neostem.com > 24

BOARD OF DIRECTORS Robin Smith, MD, MBA Chairman of the Board MD – Yale; MBA – The Wharton School Formerly President & CEO IP2M, EVP & CMO HealthHelp Experience - Trustee of NYU Medical Center; Chairman of the Board of NYU Hospital for Joint Diseases (through November 2009) and Stem for Life Foundation Richard Berman Independent Director Over 35 years of venture capital, management, M&A experience Experience – Current Board of Directors of Apricus Biosciences, Easylink Services International, Inc., Advaxis, Inc., Broadcaster, Inc., National Investment Managers Drew Bernstein, CPA Independent Director BS – University of Maryland Business School Licensed in State of New York; member AICPA, NYSSCPA and NSA Experience – Bernstein & Pinchuk LLP (member of BDO Seidman Alliance); PRC auditing; 200+ real estate transactions with $3B+ aggregate value; accountant and business advisor Martyn Greenacre, MBA Independent Director BA – Harvard College; MBA – Harvard Business School Experience – Board and executive positions for multiple biopharmaceutical companies; Former CEO of Delsys Pharmaceutical Corporation and Zynaxis Inc; Chairman of the Board of BMP Sunstone Corporation Steven Myers Independent Lead Director BS Mathematics – Stanford University Experience – Founder/Chairman/CEO SM&A (competition management services); career in aerospace and defense sectors supporting DoD & NASA programs Andrew Pecora, MD, FACP Director MD — University of Medicine and Dentistry of New Jersey Experience – Chief Innovations Officer, Professor and Vice President of Cancer Services at John Theurer Cancer Center at Hackensack University Medical Center, and Managing Partner of the Northern New Jersey Cancer Center Eric Wei Director BS – Mathematics & Economics – Amherst College; MBA – The Wharton School Experience – Founder/Managing Partner of RimAsia Capital partners (private equity); Peregrine Capital, Prudential Securities, Lazard Freres, Citibank; Gilbert Global Equity Partners Crimson Asia Capital Partners NASDAQ:NBS | www.neostem.com > 25
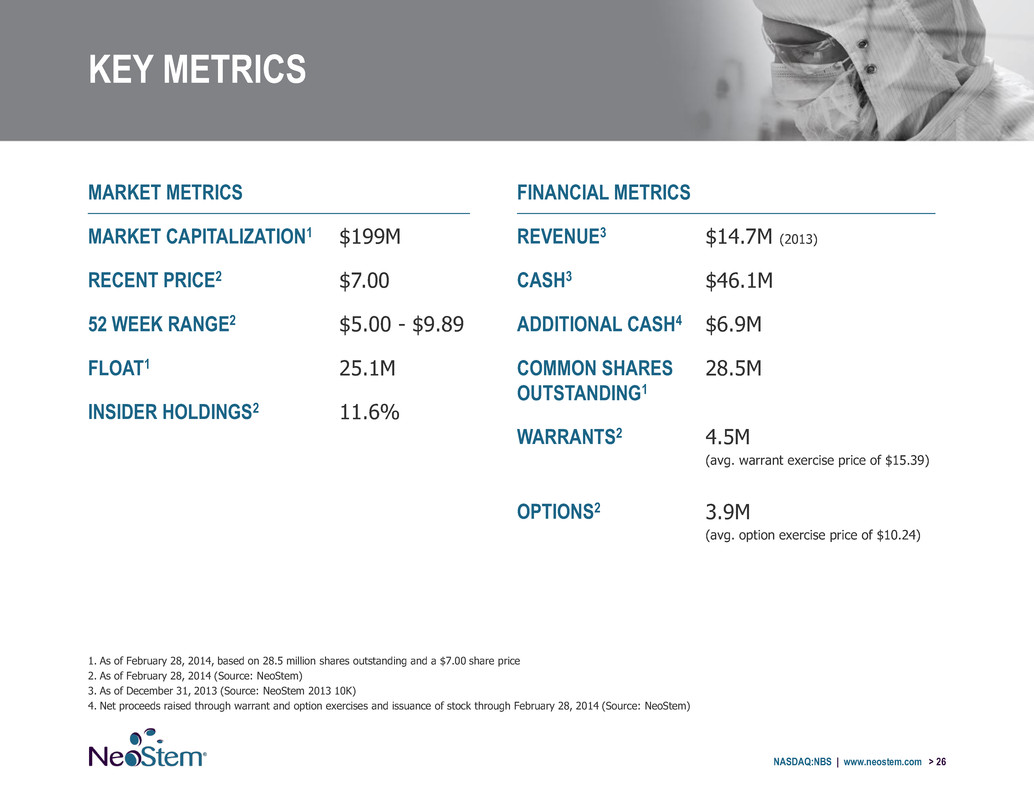
KEY METRICS NASDAQ:NBS | www.neostem.com > 26 MARKET METRICS MARKET CAPITALIZATION1 $199M RECENT PRICE2 $7.00 52 WEEK RANGE2 $5.00 - $9.89 FLOAT1 25.1M INSIDER HOLDINGS2 11.6% FINANCIAL METRICS REVENUE3 $14.7M (2013) CASH3 $46.1M ADDITIONAL CASH4 $6.9M COMMON SHARES OUTSTANDING1 28.5M WARRANTS2 4.5M (avg. warrant exercise price of $15.39) OPTIONS2 3.9M (avg. option exercise price of $10.24) 1. As of February 28, 2014, based on 28.5 million shares outstanding and a $7.00 share price 2. As of February 28, 2014 (Source: NeoStem) 3. As of December 31, 2013 (Source: NeoStem 2013 10K) 4. Net proceeds raised through warrant and option exercises and issuance of stock through February 28, 2014 (Source: NeoStem)

INVESTMENT HIGHLIGHTS NASDAQ:NBS | www.neostem.com > 27 DEVELOPING A PORTFOLIO OF CELL THERAPY PRODUCTS THAT LEVERAGES THE BODY’S NATURAL ABILITY TO HEAL AND FIGHT DISEASE. ISCHEMIC REPAIR – CD34 CELL PROGRAM* ■ Acute myocardial infarction – PreSERVE Phase 2 study (data avaibale 2H 2014) ■ Chronic heart failure – Preparing for Phase 2 study in Europe ■ Traumatic brain injury – Preclinical IMMUNE MODULATION – T REGULATORY CELL PROGRAM ■ Type 1 diabetes – Preparing for Phase 2 study ► Phase 1 data readout presented at ADA June 2014 ■ Steroid resistant asthma – Preparing for Phase 1 study in Canada TISSUE REGENERATION – VSEL™ TECHNOLOGY ■ Macular degeneration, wound healing, bone regeneration – Preclinical REVENUE-GENERATING COMMERCIAL OPERATIONS ■ Product and service expansion transaction(s) ■ Cell therapy automation to lower cost and improve efficiency ■ Manufacturing expansion in US and internationally ■ Expand service activities into Europe during 2014 * These cells are autologous and not expanded

CONTACT INFORMATION NASDAQ:NBS | www.neostem.com > 28 NEOSTEM, INC. NASDAQ: NBS WWW.NEOSTEM.COM ROBIN SMITH, MD, MBA CHAIRMAN & CEO PHONE: (212) 584-4174 EMAIL: RSMITH@NEOSTEM.COM

NASDAQ:NBS | www.neostem.com > 29 APPENDIX
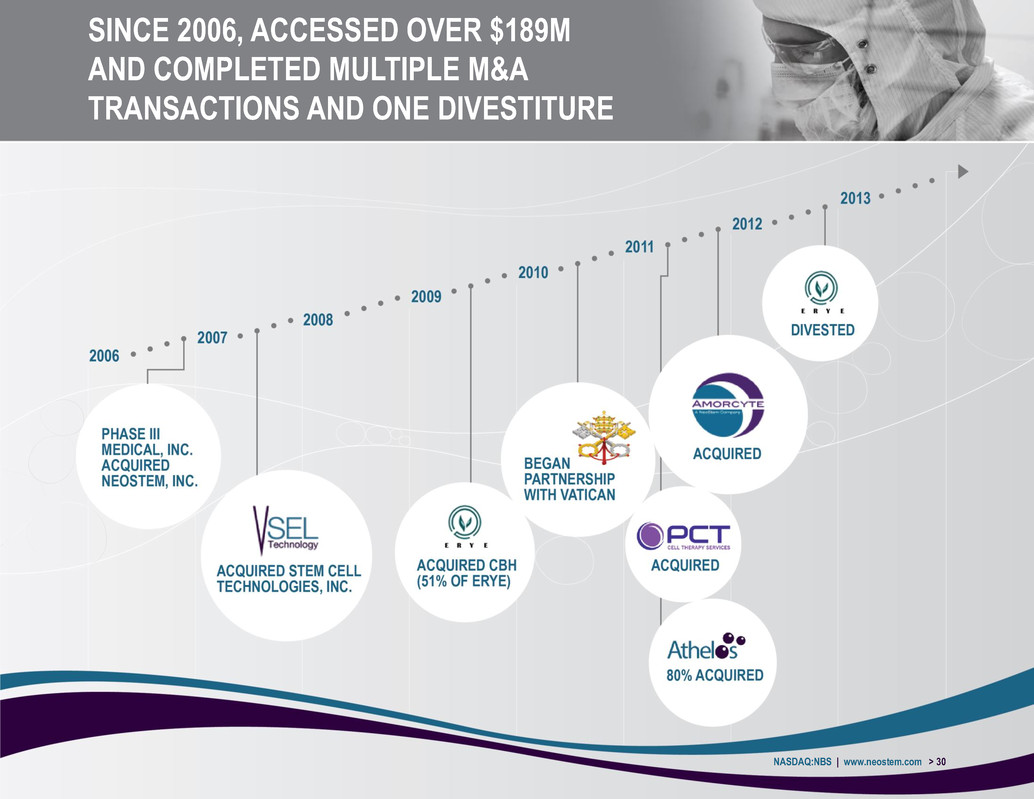
SINCE 2006, ACCESSED OVER $189M AND COMPLETED MULTIPLE M&A TRANSACTIONS AND ONE DIVESTITURE NASDAQ:NBS | www.neostem.com > 30

ATHELOS SCIENTIFIC ADVISORY BOARD NASDAQ:NBS | www.neostem.com > 31 Robert A. Preti, PhD SAB Administrative Chairman CSO of NeoStem and President of PCT Jeffrey Bluestone, PhD University of California, San Francisco, Diabetes Center David A. Horwitz, MD University of Southern California Robert Korngold, PhD Hackensack University Medical Center Robert S. Negrin, MD Stanford University David Peritt, PhD Hospira Noel L. Warner, PhD BD Biosciences

VSEL™ TECHNOLOGY ACADEMIC COLLABORATORS NASDAQ:NBS | www.neostem.com > 32 Mariusz Ratajczak, MD, PhD, Dsci University of Louisville Russell Taichman, DMD, DMSc University of Michigan Vincent Falanga, MD Boston University Michael Young, PhD Schepens Eye Institute, Harvard Medical School Kameran Lashkari, MD Schepens Eye Institute, Harvard Medical School Song Li, PhD University of California, Berkeley
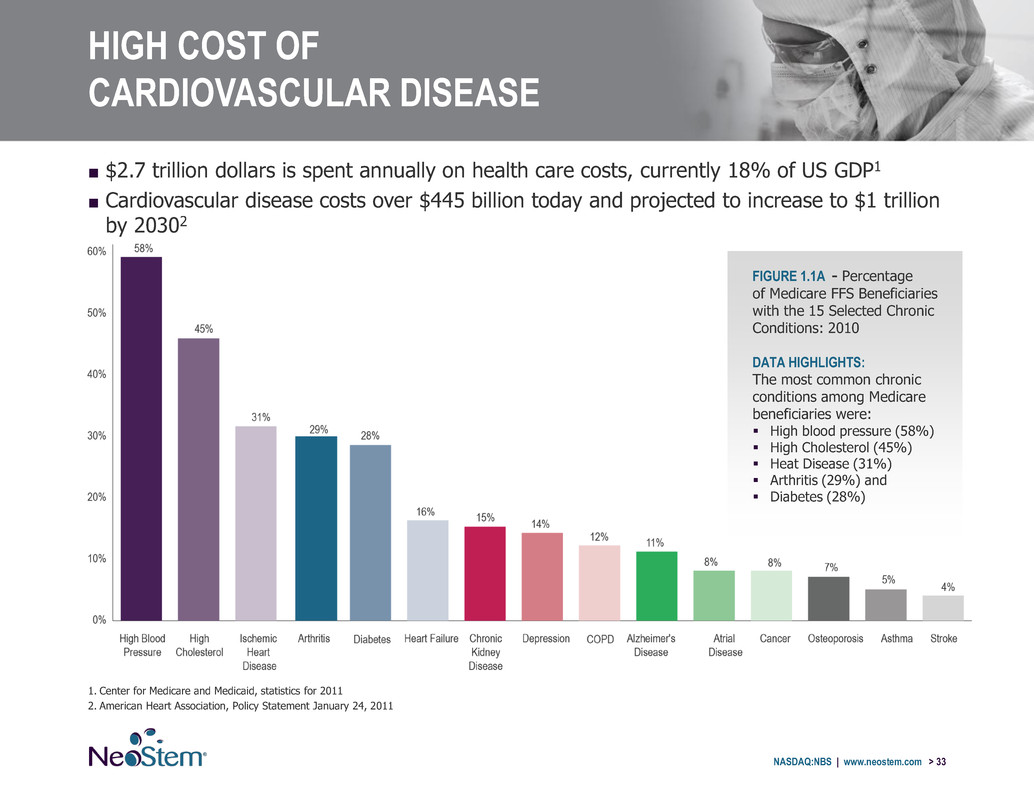
HIGH COST OF CARDIOVASCULAR DISEASE ■ $2.7 trillion dollars is spent annually on health care costs, currently 18% of US GDP1 ■ Cardiovascular disease costs over $445 billion today and projected to increase to $1 trillion by 20302 1. Center for Medicare and Medicaid, statistics for 2011 2. American Heart Association, Policy Statement January 24, 2011 NASDAQ:NBS | www.neostem.com > 33 FIGURE 1.1A - Percentage of Medicare FFS Beneficiaries with the 15 Selected Chronic Conditions: 2010 DATA HIGHLIGHTS: The most common chronic conditions among Medicare beneficiaries were: High blood pressure (58%) High Cholesterol (45%) Heat Disease (31%) Arthritis (29%) and Diabetes (28%)
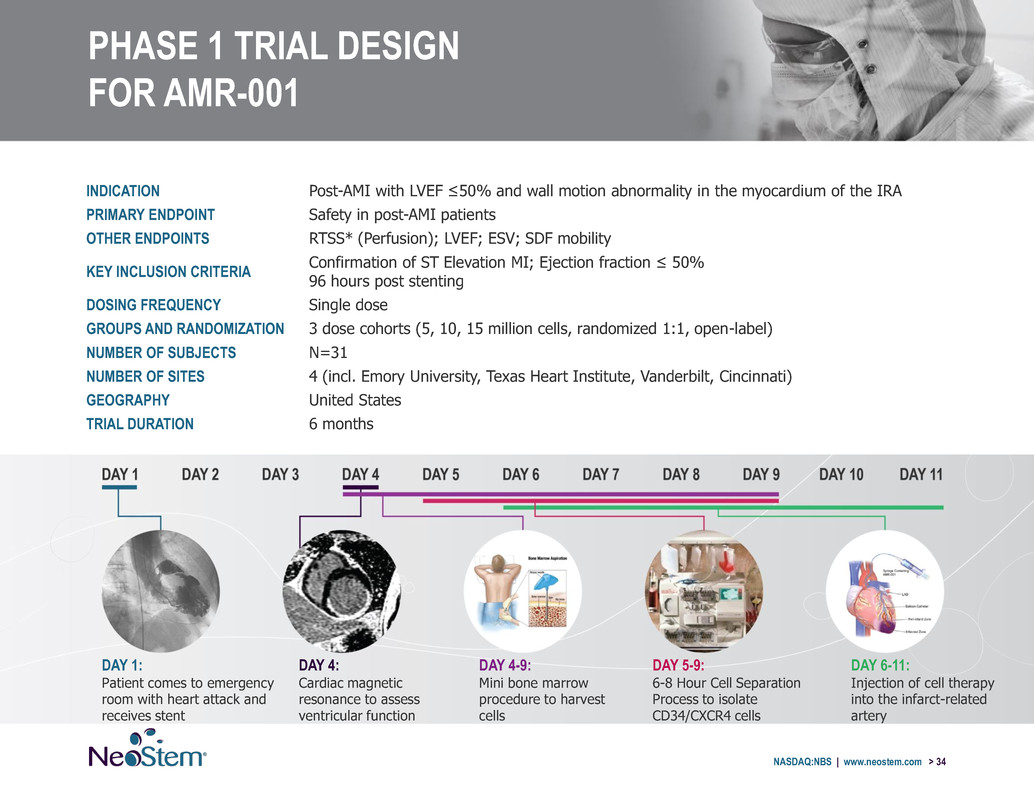
PHASE 1 TRIAL DESIGN FOR AMR-001 INDICATION Post-AMI with LVEF ≤50% and wall motion abnormality in the myocardium of the IRA PRIMARY ENDPOINT Safety in post-AMI patients OTHER ENDPOINTS RTSS* (Perfusion); LVEF; ESV; SDF mobility KEY INCLUSION CRITERIA Confirmation of ST Elevation MI; Ejection fraction ≤ 50% 96 hours post stenting DOSING FREQUENCY Single dose GROUPS AND RANDOMIZATION 3 dose cohorts (5, 10, 15 million cells, randomized 1:1, open-label) NUMBER OF SUBJECTS N=31 NUMBER OF SITES 4 (incl. Emory University, Texas Heart Institute, Vanderbilt, Cincinnati) GEOGRAPHY United States TRIAL DURATION 6 months NASDAQ:NBS | www.neostem.com > 34 DAY 1: Patient comes to emergency room with heart attack and receives stent DAY 4: Cardiac magnetic resonance to assess ventricular function DAY 4-9: Mini bone marrow procedure to harvest cells DAY 5-9: 6-8 Hour Cell Separation Process to isolate CD34/CXCR4 cells DAY 6-11: Injection of cell therapy into the infarct-related artery
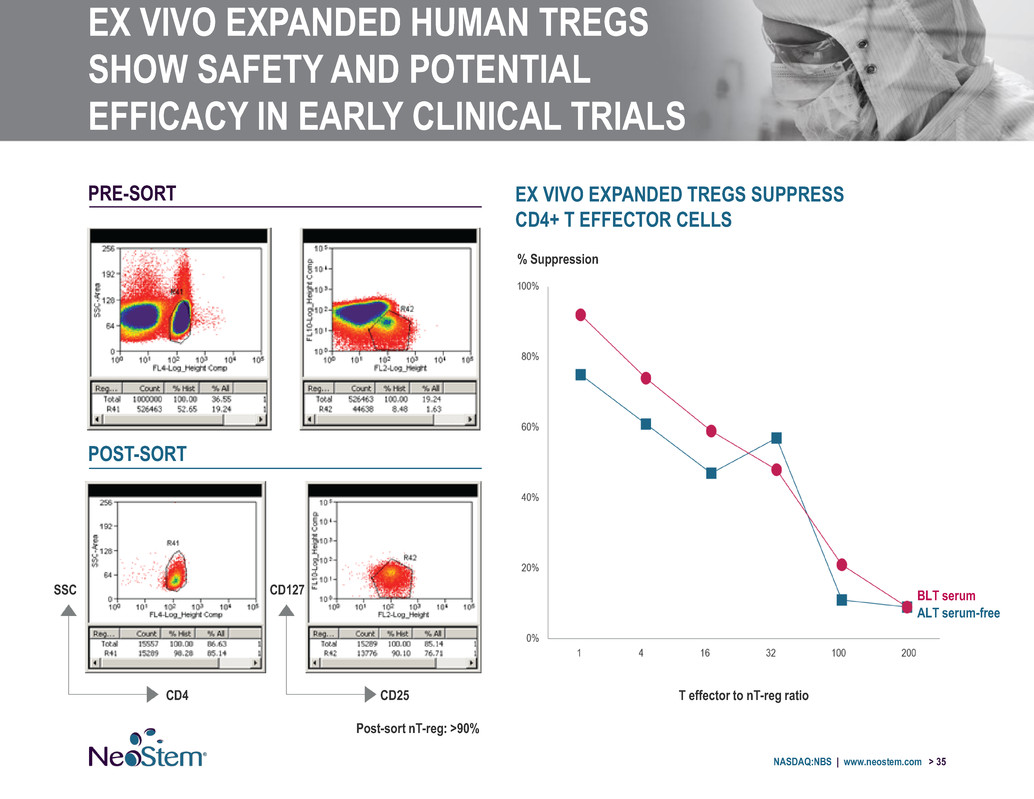
EX VIVO EXPANDED HUMAN TREGS SHOW SAFETY AND POTENTIAL EFFICACY IN EARLY CLINICAL TRIALS NASDAQ:NBS | www.neostem.com > 35 EX VIVO EXPANDED TREGS SUPPRESS CD4+ T EFFECTOR CELLS % Suppression T effector to nT-reg ratio PRE-SORT POST-SORT Post-sort nT-reg: >90% BLT serum ALT serum-free CD25 CD127 CD4 SSC



































