
Delivering Personalized Medicine Corporate Presentation NASDAQ: CLBS David J. Mazzo, PhD Chief Executive Officer August 2015

Forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements reflect management's current expectations, as of the date of this presentation, and involve certain risks and uncertainties. Forward-looking statements include statements herein with respect to the successful execution of the Company's business strategy, the Company's ability to develop and grow its business, the successful development of cellular therapies with respect to the Company's research and development and clinical evaluation efforts in connection with the Company's Immuno-oncology Program, Ischemic Repair Program, Immune Modulation Program and other cell therapies, the future of the regenerative medicine industry and the role of stem cells and cellular therapy in that industry, and the performance and planned expansion of the Company's wholly-owned subsidiary and its center of excellence for cell therapy process development, engineering and manufacturing, PCT. The Company's further development is highly dependent on future medical and research developments and market acceptance, which is outside of its control. The Company's actual results could differ materially from those anticipated in these forward-looking statements as a result of various factors. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the Company in general, see the factors described under the heading, “Item 1A. Risk Factors" in the Company's Annual Report on Form 10-K filed with the SEC on March 2, 2015 and those described in the Company's other periodic filings with the SEC. The Company undertakes no obligation to update or revise any forward-looking statements. 2
Transforming cells into therapies 3
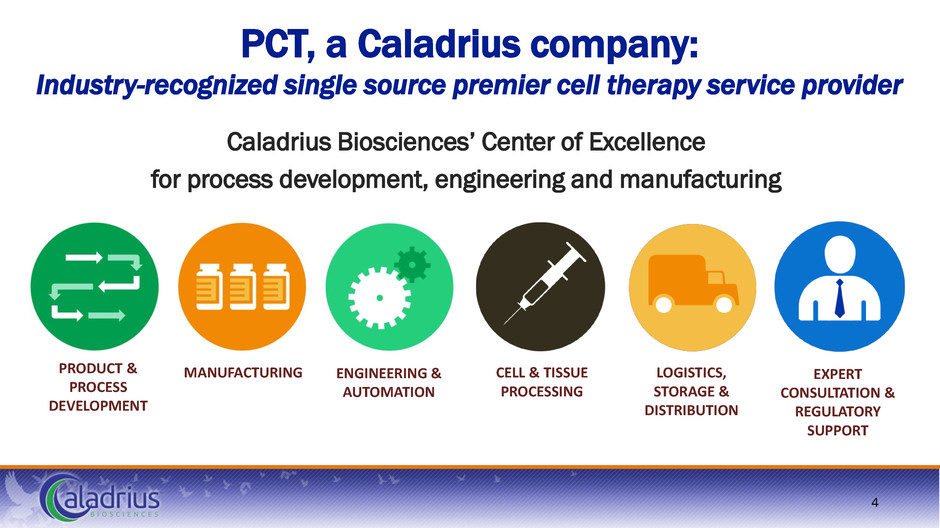
PCT, a Caladrius company: Industry-recognized single source premier cell therapy service provider Caladrius Biosciences’ Center of Excellence for process development, engineering and manufacturing PRODUCT & PROCESS DEVELOPMENT MANUFACTURING CELL & TISSUE PROCESSING LOGISTICS, STORAGE & DISTRIBUTION EXPERT CONSULTATION & REGULATORY SUPPORT ENGINEERING & AUTOMATION 4

Unmatched experience: >100 clients and 30,000 products over 15 years 5
At a glance Unifying platform approach yielding a robust, balanced pipeline targeting critical unmet medical needs Proven internal center of excellence (PCT) with bicoastal facilities innovating discovery, development, manufacturing and delivery of cell-based therapies Highly experienced management and scientific team 6

Experienced executive and scientific team David J. Mazzo, PhD Chief Executive Officer 30+ years’ experience - all aspects of large and emerging global biotech, biopharma company operations, successful international drug development Robert S. Vaters, MBA President and Chief Financial Officer 25+ years’ financial and management experience in a variety of healthcare, biotechnology, biologics, medical device and pharmaceutical companies Douglas W. Losordo, MD Senior VP, Clinical, Medical and Regulatory Affairs and Chief Medical Officer Leader in cell therapy research and development; renowned cardiologist with noteworthy academic and industry credentials Hans Keirstead, PhD Senior VP, Research and Chief Science Officer Internationally known stem cell expert; CEO of California Stem cell prior to acquisition by Caladrius; Founder of Stem Cell Research Center, University of California, Irvine Robert A. Preti, PhD Senior VP, Development and Technical Operations and Chief Technology Officer; President of PCT, a Caladrius company Leading authority on cell-based therapy engineering; unique development and commercialization experience 7
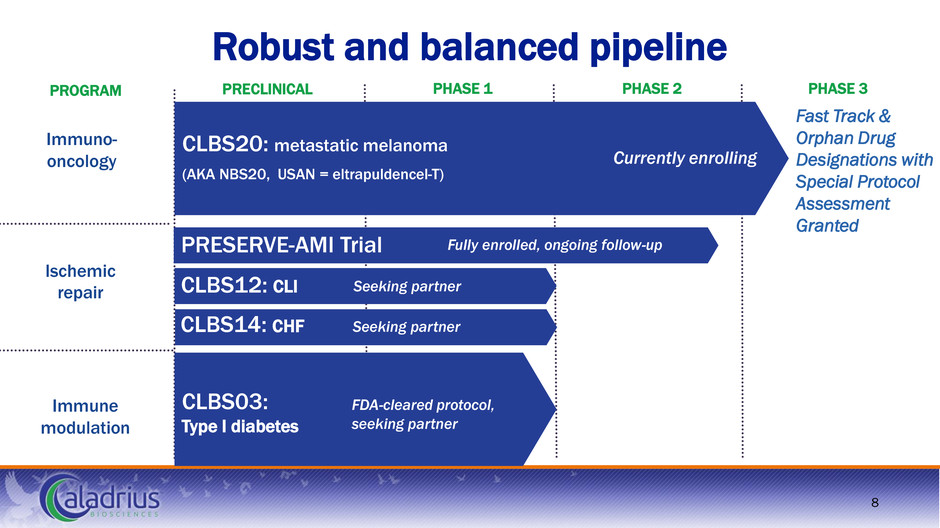
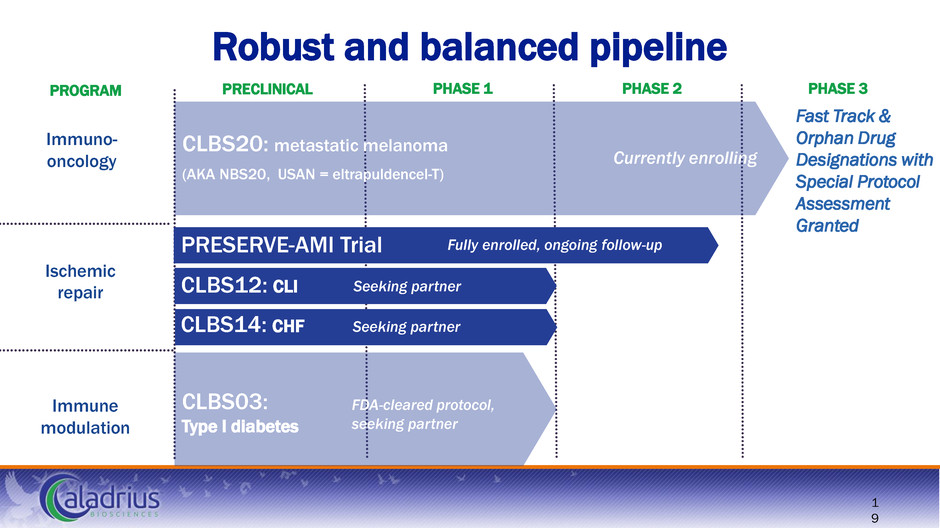
Immune modulation Ischemic repair Immuno- oncology Robust and balanced pipeline Fast Track & Orphan Drug Designations with Special Protocol Assessment Granted PRECLINICAL PHASE 1 PHASE 2 PHASE 3 8 PROGRAM CLBS20: metastatic melanoma (AKA NBS20, USAN = eltrapuldencel-T) CLBS14: CHF CLBS12: CLI PRESERVE-AMI Trial Currently enrolling Seeking partner FDA-cleared protocol, seeking partner CLBS03: Type I diabetes Seeking partner Fully enrolled, ongoing follow-up
Unifying root approach across platforms Ischemic repair Immuno- oncology Immune modulation 9
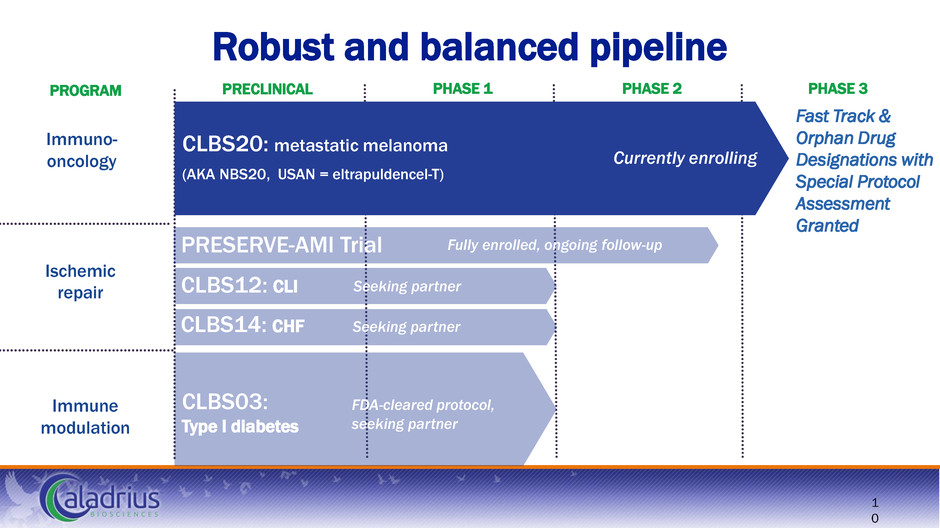
Immune modulation Ischemic repair Immuno- oncology Robust and balanced pipeline Fast Track & Orphan Drug Designations with Special Protocol Assessment Granted PRECLINICAL PHASE 1 PHASE 2 PHASE 3 1 0 PROGRAM CLBS20: metastatic melanoma (AKA NBS20, USAN = eltrapuldencel-T) CLBS14: CHF CLBS12: CLI PRESERVE-AMI Trial Currently enrolling Seeking partner FDA-cleared protocol, seeking partner CLBS03: Type I diabetes Seeking partner Fully enrolled, ongoing follow-up

Immuno-oncology: Turning cancer against itself Metastatic melanoma: CLBS20 (AKA NBS20, USAN = eltrapuldencel-T) • Fast Track & Orphan Drug designations • Special Protocol Assessment • ATMP (EMA) • $17.7 million CIRM grant award 11
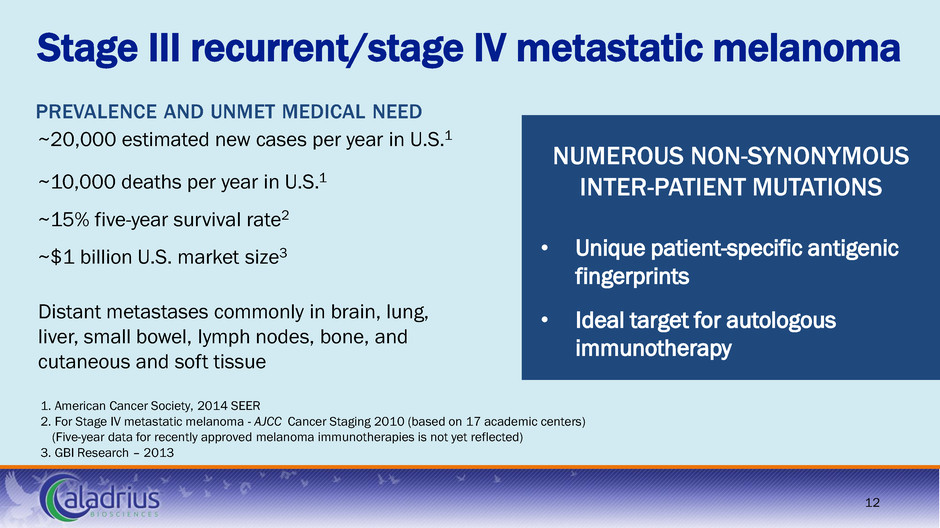
Stage III recurrent/stage lV metastatic melanoma 1. American Cancer Society, 2014 SEER 2. For Stage IV metastatic melanoma - AJCC Cancer Staging 2010 (based on 17 academic centers) (Five-year data for recently approved melanoma immunotherapies is not yet reflected) 3. GBI Research – 2013 PREVALENCE AND UNMET MEDICAL NEED ~20,000 estimated new cases per year in U.S.1 ~10,000 deaths per year in U.S.1 ~15% five-year survival rate2 ~$1 billion U.S. market size3 Distant metastases commonly in brain, lung, liver, small bowel, lymph nodes, bone, and cutaneous and soft tissue NUMEROUS NON-SYNONYMOUS INTER-PATIENT MUTATIONS • Unique patient-specific antigenic fingerprints • Ideal target for autologous immunotherapy 12
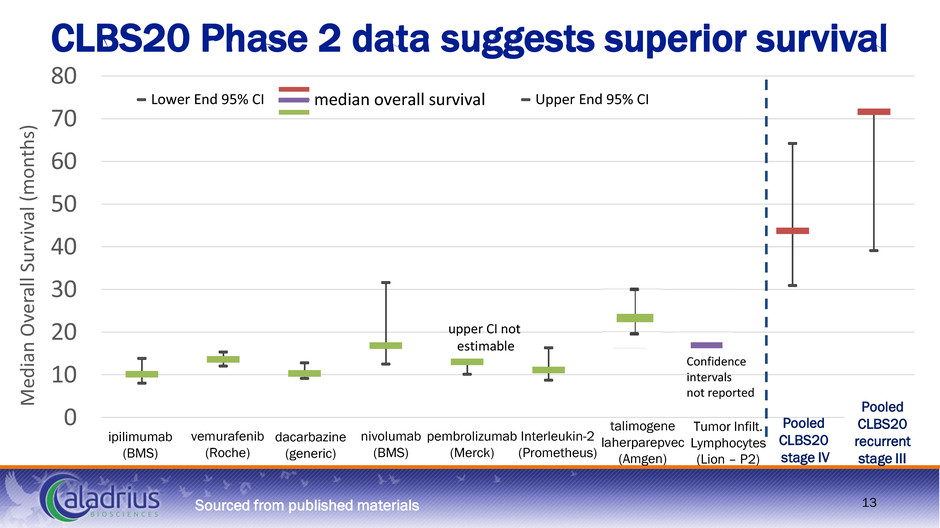
0 10 20 30 40 50 60 70 80 Medi an O ve ra ll Su rvi va l ( m o n th s) Lower End 95% CI median overall survival Upper End 95% CI upper CI not estimable Confidence intervals not reported CLBS20 Phase 2 data suggests superior survival ipilimumab (BMS) dacarbazine (generic) vemurafenib (Roche) pembrolizumab (Merck) nivolumab (BMS) Interleukin-2 (Prometheus) Tumor Infilt. Lymphocytes (Lion – P2) talimogene laherparepvec (Amgen) Pooled CLBS20 stage IV Pooled CLBS20 recurrent stage III Sourced from published materials 13
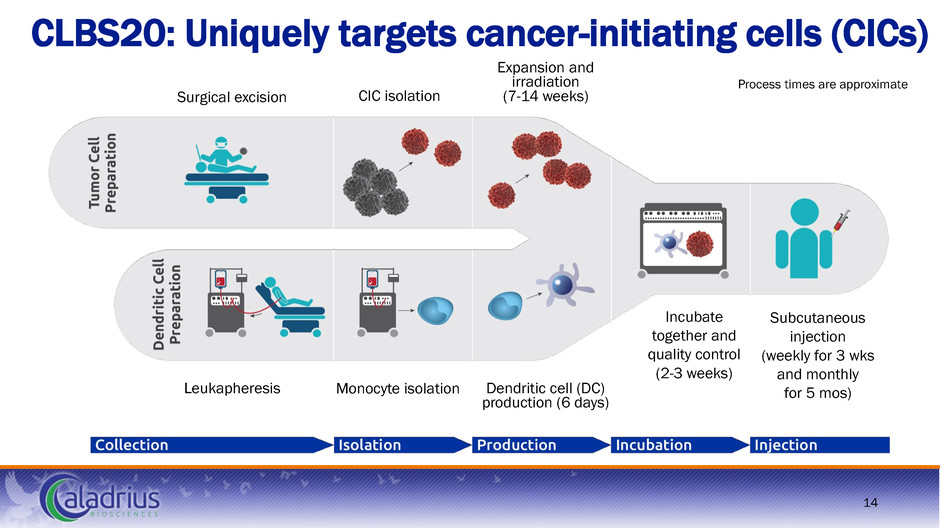
CLBS20: Uniquely targets cancer-initiating cells (CICs) Process times are approximate Surgical excision CIC isolation Expansion and irradiation (7-14 weeks) Incubate together and quality control (2-3 weeks) Subcutaneous injection (weekly for 3 wks and monthly for 5 mos) Leukapheresis Monocyte isolation Dendritic cell (DC) production (6 days) 14
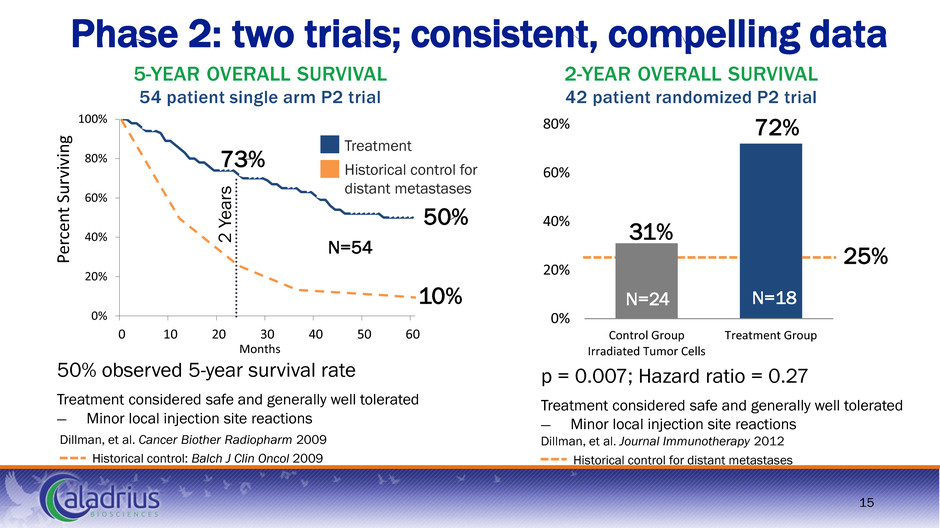
0% 20% 40% 60% 80% Control Group Irradiated Tumor Cells Treatment Group 0% 20% 40% 60% 80% 100% 0 10 20 30 40 50 60 Pe rc en t Su rviv in g Months Phase 2: two trials; consistent, compelling data Dillman, et al. Journal Immunotherapy 2012 Historical control for distant metastases 2-YEAR OVERALL SURVIVAL 42 patient randomized P2 trial p = 0.007; Hazard ratio = 0.27 Treatment considered safe and generally well tolerated ― Minor local injection site reactions 72% 31% N=24 N=18 50% observed 5-year survival rate Treatment considered safe and generally well tolerated ― Minor local injection site reactions Dillman, et al. Cancer Biother Radiopharm 2009 Historical control: Balch J Clin Oncol 2009 N=54 5-YEAR OVERALL SURVIVAL 54 patient single arm P2 trial 2 Y ea rs 73% Treatment 10% 25% Historical control for distant metastases 50% 15

The Intus study: Phase 3 with SPA and orphan drug and fast track designations DESIGN • Randomized (2:1), double blind, placebo controlled trial • Stage III recurrent or stage IV metastatic melanoma • Single trial for registration assuming positive outcome ENDPOINT • Overall survival POWERING • 80% power to detect 37.5% reduction in risk of death RELATION TO STANDARD THERAPIES • Adjunctive • Clinical practice based trial STUDY SIZE • Planned 250 eligible patients • Approximately 50 sites (U.S., Canada, Australia, New Zealand, considering E.U.) TREATMENT • CLBS20: Autologous DC loaded with antigens from autologous CICs, in GM-CSF CONTROL • Autologous monocytes in GM-CSF 16
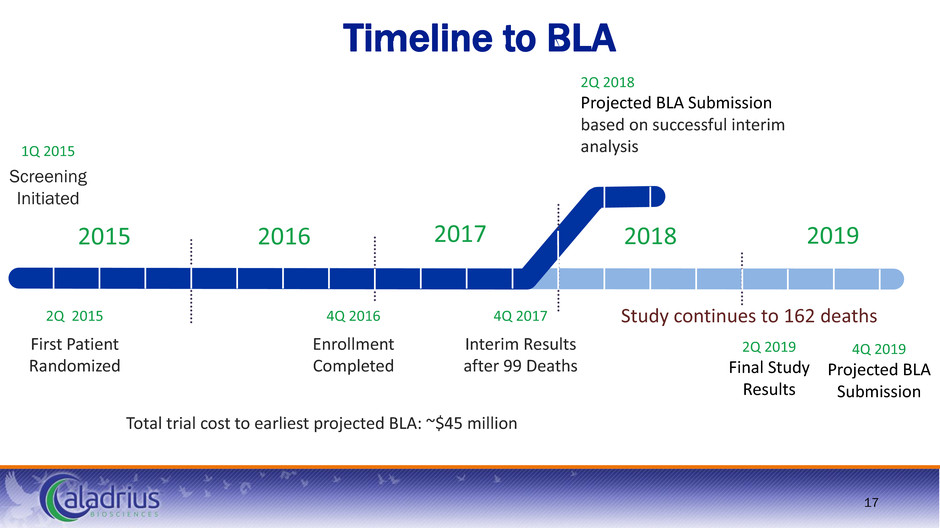
17 1Q 2015 Screening Initiated 4Q 2017 Interim Results after 99 Deaths 2Q 2015 First Patient Randomized 2Q 2018 Projected BLA Submission based on successful interim analysis 2Q 2019 Final Study Results 4Q 2019 Projected BLA Submission 4Q 2016 Enrollment Completed 2015 2016 2017 2018 2019 Study continues to 162 deaths Total trial cost to earliest projected BLA: ~$45 million Timeline to BLA
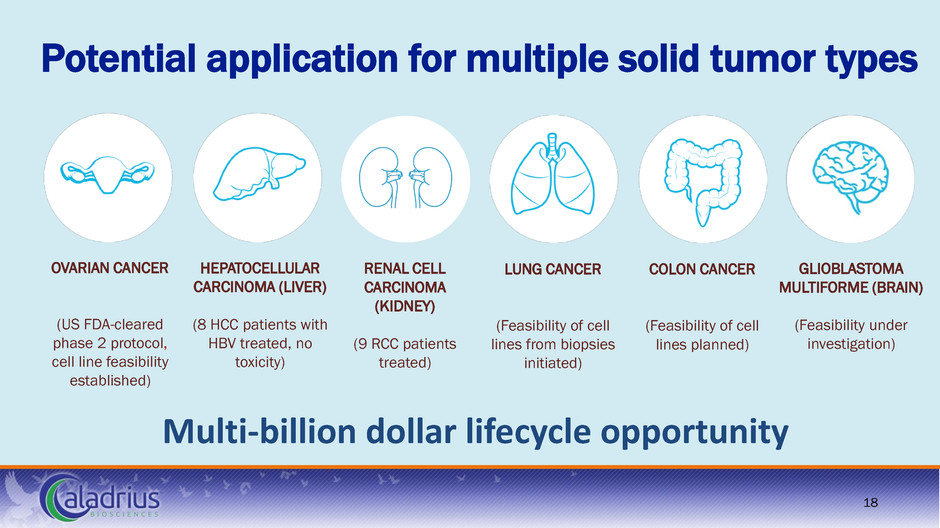
Potential application for multiple solid tumor types Multi-billion dollar lifecycle opportunity LUNG CANCER (Feasibility of cell lines from biopsies initiated) COLON CANCER (Feasibility of cell lines planned) OVARIAN CANCER (US FDA-cleared phase 2 protocol, cell line feasibility established) HEPATOCELLULAR CARCINOMA (LIVER) (8 HCC patients with HBV treated, no toxicity) GLIOBLASTOMA MULTIFORME (BRAIN) (Feasibility under investigation) 18 RENAL CELL CARCINOMA (KIDNEY) (9 RCC patients treated)
Immune modulation Ischemic repair Immuno- oncology Robust and balanced pipeline Fast Track & Orphan Drug Designations with Special Protocol Assessment Granted PRECLINICAL PHASE 1 PHASE 2 PHASE 3 1 9 PROGRAM CLBS20: metastatic melanoma (AKA NBS20, USAN = eltrapuldencel-T) CLBS14: CHF CLBS12: CLI PRESERVE-AMI Trial Currently enrolling Seeking partner FDA-cleared protocol, seeking partner CLBS03: Type I diabetes Seeking partner Fully enrolled, ongoing follow-up

Ischemic repair Critical Limb Ischemia: CLBS12 Chronic Heart Failure: CLBS14 20

Ischemic repair: Leveraging the body’s natural repair mechanism to develop new blood vessels CD34/CXCR4 from peripheral blood • Ischemic events – caused by restriction of blood to tissue, e.g., stroke, acute myocardial infarction and claudication • Results of ischemia include chronic heart failure, critical limb ischemia and more • CD34+ cells have been shown to induce the development of new blood vessels, preventing tissue death by improving blood flow Critical limb ischemia 21 CD34 cell therapy promoting angiogenesis CD34/CXCR4 Post acute myocardial infarction CD34/CXCR4 from bone marrow
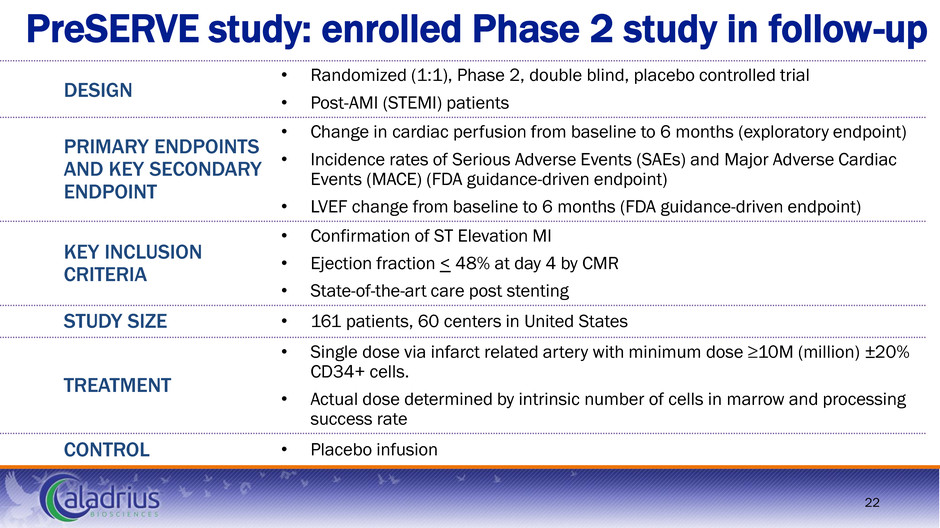
PreSERVE study: enrolled Phase 2 study in follow-up 22 DESIGN • Randomized (1:1), Phase 2, double blind, placebo controlled trial • Post-AMI (STEMI) patients PRIMARY ENDPOINTS AND KEY SECONDARY ENDPOINT • Change in cardiac perfusion from baseline to 6 months (exploratory endpoint) • Incidence rates of Serious Adverse Events (SAEs) and Major Adverse Cardiac Events (MACE) (FDA guidance-driven endpoint) • LVEF change from baseline to 6 months (FDA guidance-driven endpoint) KEY INCLUSION CRITERIA • Confirmation of ST Elevation MI • Ejection fraction < 48% at day 4 by CMR • State-of-the-art care post stenting STUDY SIZE • 161 patients, 60 centers in United States TREATMENT • Single dose via infarct related artery with minimum dose ≥10M (million) ±20% CD34+ cells. • Actual dose determined by intrinsic number of cells in marrow and processing success rate CONTROL • Placebo infusion
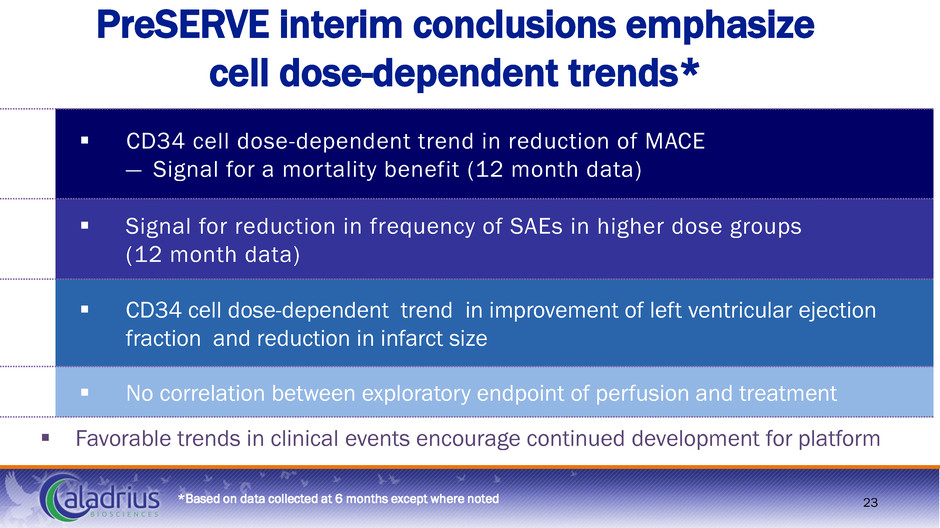
PreSERVE interim conclusions emphasize cell dose-dependent trends* CD34 cell dose-dependent trend in reduction of MACE — Signal for a mortality benefit (12 month data) Signal for reduction in frequency of SAEs in higher dose groups (12 month data) CD34 cell dose-dependent trend in improvement of left ventricular ejection fraction and reduction in infarct size No correlation between exploratory endpoint of perfusion and treatment Favorable trends in clinical events encourage continued development for platform *Based on data collected at 6 months except where noted 23
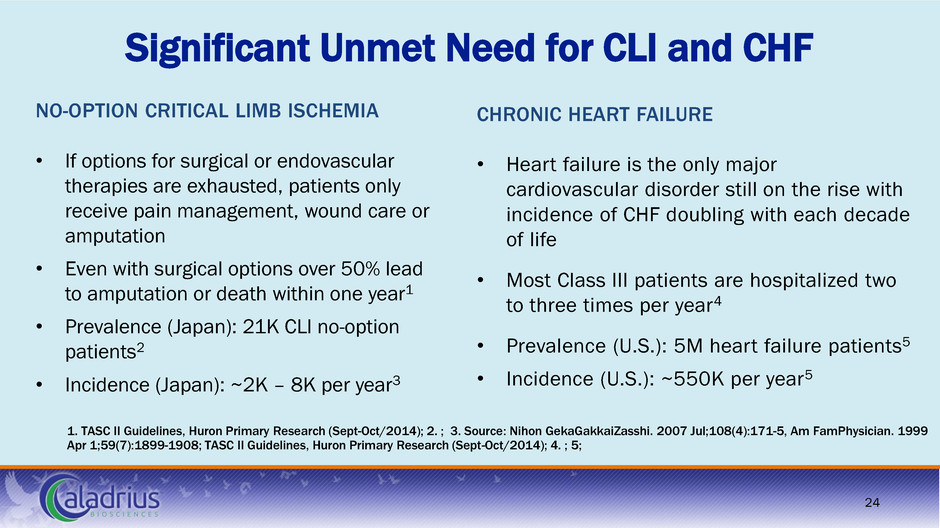
Significant Unmet Need for CLI and CHF NO-OPTION CRITICAL LIMB ISCHEMIA • If options for surgical or endovascular therapies are exhausted, patients only receive pain management, wound care or amputation • Even with surgical options over 50% lead to amputation or death within one year1 • Prevalence (Japan): 21K CLI no-option patients2 • Incidence (Japan): ~2K – 8K per year3 24 CHRONIC HEART FAILURE • Heart failure is the only major cardiovascular disorder still on the rise with incidence of CHF doubling with each decade of life • Most Class III patients are hospitalized two to three times per year4 • Prevalence (U.S.): 5M heart failure patients5 • Incidence (U.S.): ~550K per year5 1. TASC II Guidelines, Huron Primary Research (Sept-Oct/2014); 2. ; 3. Source: Nihon GekaGakkaiZasshi. 2007 Jul;108(4):171-5, Am FamPhysician. 1999 Apr 1;59(7):1899-1908; TASC II Guidelines, Huron Primary Research (Sept-Oct/2014); 4. ; 5;

25 CLBS12 CLI Japan Program Update Objective: file J-IND approval and consummate partnership to allow for clinical study execution Could take advantage of new Japanese regulatory path to early conditional approval Clinical background: Previous studies of autologous CD34+ cells in no-option CLI patients in Japan and U.S. (combined total, N=56) • Conclusions from 2 previous studies in Japan: • Study 1: CD34 cell injection was safe and led to improvement in all clinical parameters • Study 2: CD34 cell injection was safe and led to improvement in CLI-free status as well as other clinical parameters • Conclusion from previous study in U.S.: • CD34 cell injection was safe and led to improved amputation free survival Study 1: Kawamoto A et al. Stem Cells 2009; Study 2: Fujita Y et al. Circ J 2014; Study 3: Losordo et al. Circ Cardiovasc Interv 2012.
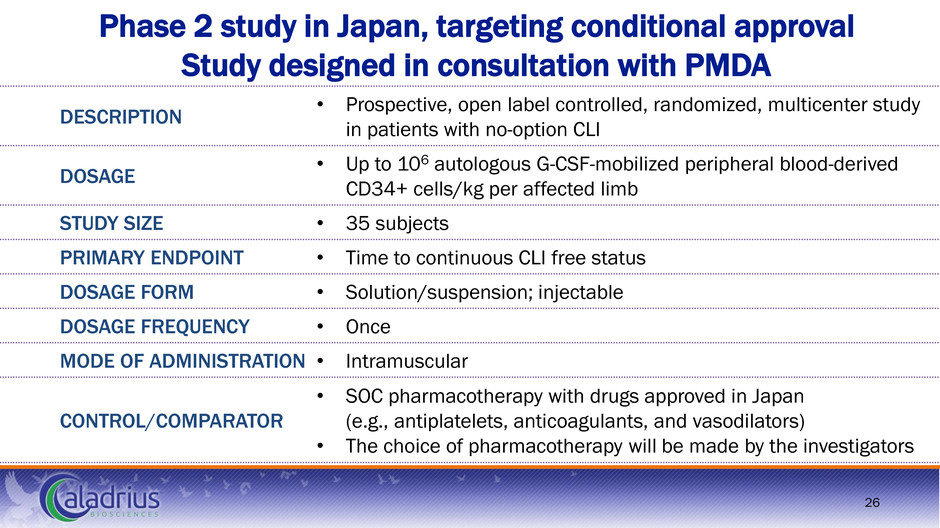
Phase 2 study in Japan, targeting conditional approval Study designed in consultation with PMDA 26 DESCRIPTION • Prospective, open label controlled, randomized, multicenter study in patients with no-option CLI DOSAGE • Up to 106 autologous G-CSF-mobilized peripheral blood-derived CD34+ cells/kg per affected limb STUDY SIZE • 35 subjects PRIMARY ENDPOINT • Time to continuous CLI free status DOSAGE FORM • Solution/suspension; injectable DOSAGE FREQUENCY • Once MODE OF ADMINISTRATION • Intramuscular CONTROL/COMPARATOR • SOC pharmacotherapy with drugs approved in Japan (e.g., antiplatelets, anticoagulants, and vasodilators) • The choice of pharmacotherapy will be made by the investigators
Potential application across several indications Multi-billion dollar lifecycle opportunity STROKE CLAUDICATION 27 N-STEMI REFRACTORY ANGINA SYNDROME X Platform supported by independent preclinical and early clinical data, as well as positive suggestion of safety and therapeutic activity seen to date in the PreSERVE-AMI trial
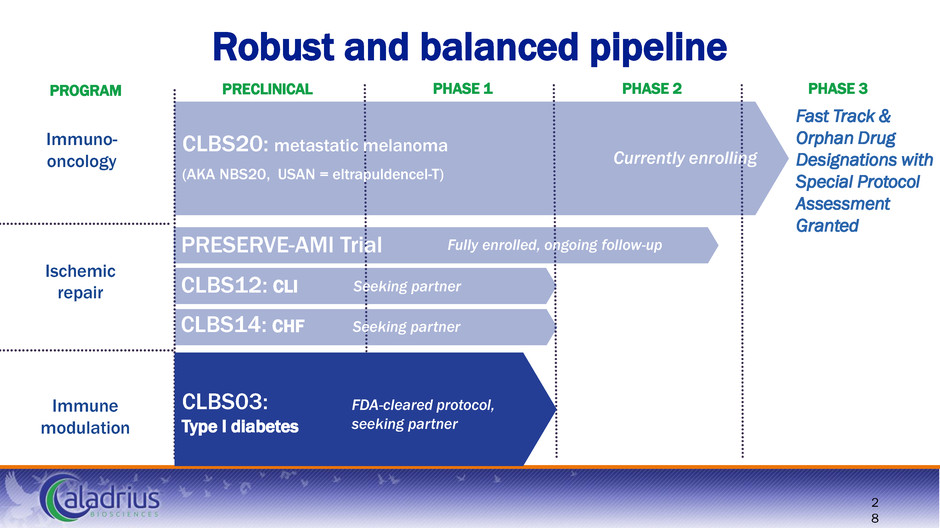
Immune modulation Ischemic repair Immuno- oncology Robust and balanced pipeline Fast Track & Orphan Drug Designations with Special Protocol Assessment Granted PRECLINICAL PHASE 1 PHASE 2 PHASE 3 2 8 PROGRAM CLBS20: metastatic melanoma (AKA NBS20, USAN = eltrapuldencel-T) CLBS14: CHF CLBS12: CLI PRESERVE-AMI Trial Currently enrolling Seeking partner FDA-cleared protocol, seeking partner CLBS03: Type I diabetes Seeking partner Fully enrolled, ongoing follow-up

Immune Modulation Diabetes Mellitus Type-1 (T1D): CLBS03 29
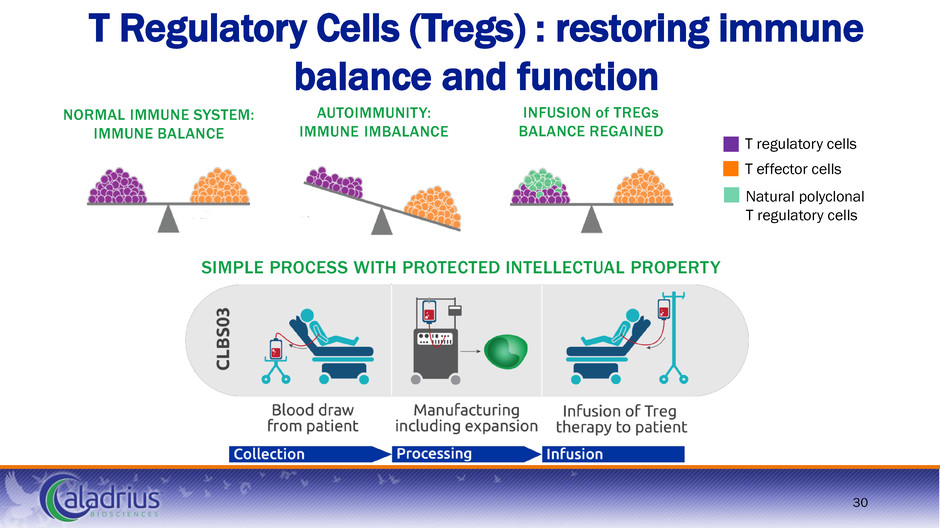
30 T Regulatory Cells (Tregs) : restoring immune balance and function NORMAL IMMUNE SYSTEM: IMMUNE BALANCE INFUSION of TREGs BALANCE REGAINED AUTOIMMUNITY: IMMUNE IMBALANCE T regulatory cells T effector cells Natural polyclonal T regulatory cells SIMPLE PROCESS WITH PROTECTED INTELLECTUAL PROPERTY
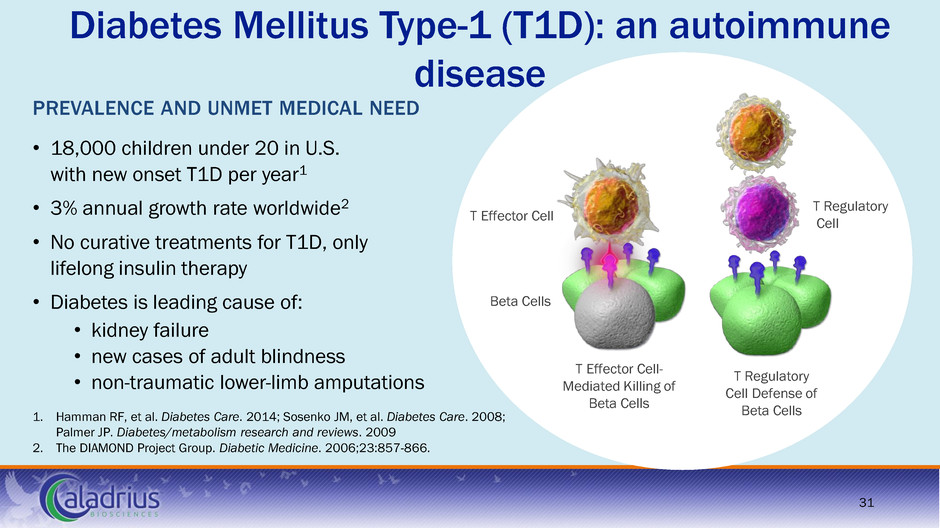
Diabetes Mellitus Type-1 (T1D): an autoimmune disease 1. Hamman RF, et al. Diabetes Care. 2014; Sosenko JM, et al. Diabetes Care. 2008; Palmer JP. Diabetes/metabolism research and reviews. 2009 2. The DIAMOND Project Group. Diabetic Medicine. 2006;23:857-866. PREVALENCE AND UNMET MEDICAL NEED • 18,000 children under 20 in U.S. with new onset T1D per year1 • 3% annual growth rate worldwide2 • No curative treatments for T1D, only lifelong insulin therapy • Diabetes is leading cause of: • kidney failure • new cases of adult blindness • non-traumatic lower-limb amputations Beta Cells T Regulatory Cell T Effector Cell- Mediated Killing of Beta Cells T Effector Cell T Regulatory Cell Defense of Beta Cells 31
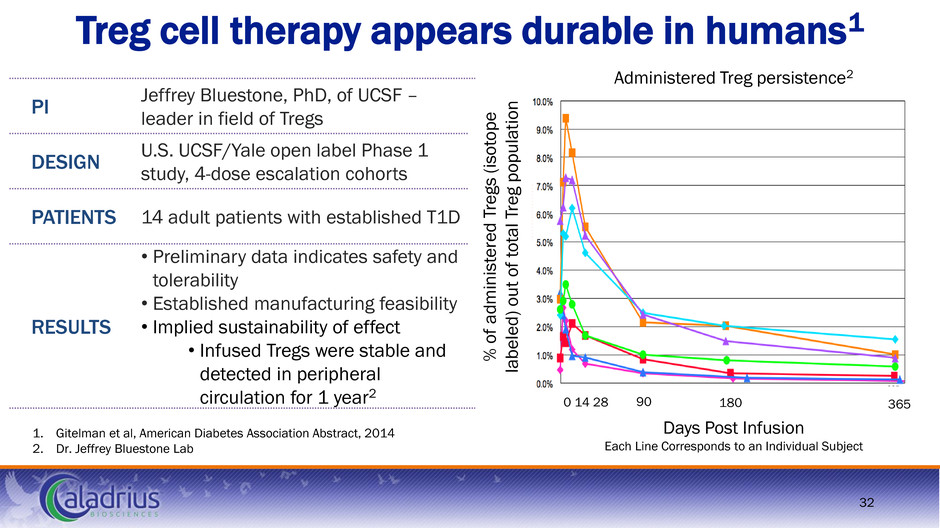
Treg cell therapy appears durable in humans1 1. Gitelman et al, American Diabetes Association Abstract, 2014 2. Dr. Jeffrey Bluestone Lab PI Jeffrey Bluestone, PhD, of UCSF – leader in field of Tregs DESIGN U.S. UCSF/Yale open label Phase 1 study, 4-dose escalation cohorts PATIENTS 14 adult patients with established T1D RESULTS • Preliminary data indicates safety and tolerability • Established manufacturing feasibility • Implied sustainability of effect • Infused Tregs were stable and detected in peripheral circulation for 1 year2 % of ad m inis te re d T re g s (i so tope la b eled) out of to tal T re g p op u lat io n Days Post Infusion Each Line Corresponds to an Individual Subject 180 90 0 14 28 365 Administered Treg persistence2 32
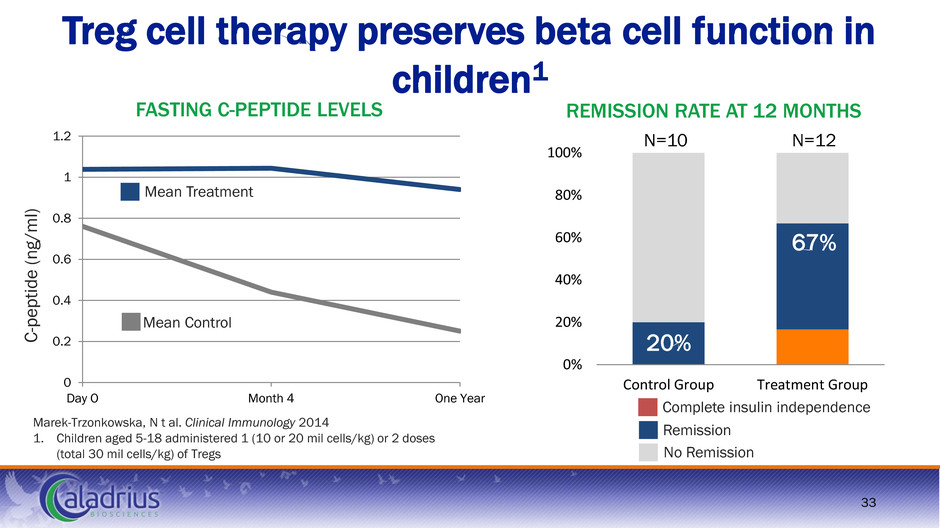
0 0.2 0.4 0.6 0.8 1 1.2 Day O Month 4 One Year 0% 20% 40% 60% 80% 100% Control Group Treatment Group Treg cell therapy preserves beta cell function in children1 33 Marek-Trzonkowska, N t al. Clinical Immunology 2014 1. Children aged 5-18 administered 1 (10 or 20 mil cells/kg) or 2 doses (total 30 mil cells/kg) of Tregs REMISSION RATE AT 12 MONTHS FASTING C-PEPTIDE LEVELS C -p e p tide ( n g /m l) 67% 20% Mean Treatment Mean Control Complete insulin independence Remission No Remission N=12 N=10

The Trutina study: Phase 2 in adolescents with T1D1 34 DESIGN • Double blind, placebo controlled, randomized (1:1:1) • Adolescent patients with recent onset T1D ages 12 to 18 PRIMARY ENDPOINT • Preservation of C-peptide at 52 weeks in comparison to placebo POWERING • 80% power to detect 50% attenuation in fasting c-peptide levels STUDY SIZE • 18 patient cohort with early interim safety analysis, total of 111 patients to be enrolled • ~11 U.S. sites TREATMENT • CLBS03: Dose cohorts of 10 or 20 million cells/kg CONTROL • Placebo infusion 1. Study cleared by FDA to proceed based on efficacy data in children establishing prospect of direct benefit

Trutina study: Efficient asset de-risking study design 35 18 51 Initial 18 patients evaluated for safety through 3-6 months (~$3 million cost to these results) PATIENT ENROLLMENT Interim blinded analysis when ~50% of subjects complete 12-month follow up Expected cost of trial: ~$22.5 million 111
Potential application across multiple autoimmune and allergic diseases Multi-billion dollar lifecycle opportunity 36 STEROID RESISTANT ASTHMA MULTIPLE SCLEROSIS (MS) CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) INFLAMMATORY BOWEL DISEASE GRAFT VS. HOST DISEASE RHEUMATOID ARTHRITIS LUPUS
Investment summary Lead immuno-oncology program (Phase 3 study in metastatic melanoma) with Fast Track and Orphan Drug designations and a SPA Additional platforms for autoimmune disorders (FDA-cleared Phase 2 study in adolescents with type I diabetes) and multiple cardiovascular indications Internal center of excellence (PCT) with bicoastal facilities and proven capabilities innovating discovery, development, manufacturing and delivery of cell-based therapies Highly experienced management and scientific team 37

Delivering Personalized Medicine NASDAQ: CLBS Contact: Eric Powers, Manager of Communications & Marketing Phone: 212.584.4173 Email: epowers@caladrius.com Web: www.caladrius.com





































