
C o p y r i g h t © 2 0 2 3 L i s a t a T h e r a p e u t i c s , I n c . A l l r i g h t s r e s e r v e d . Targeted Therapy Delivered David J. Mazzo, Ph.D. Chief Executive Officer Corporate Presentation | March 30, 2023 Nasdaq: LSTA www.lisata.com Exhibit 99.1

Forward-looking Statements Notice This presentation contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this communication regarding strategy, future operations, future financial position, future revenue, projected expenses, prospects, plans and objectives of management are forward-looking statements. In addition, when or if used in this communication, the words “may,” “could,” “should,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “predict”, target and similar expressions and their variants, as they relate to Lisata or its management, may identify forward-looking statements. Examples of forward-looking statements include, but are not limited to, statements relating to the long-term success of Lisata’s recently completed merger (the “Merger”) with Cend Therapeutics, Inc. (“Cend”), including the ongoing integration of Cend’s operations; Lisata’s continued listing on the Nasdaq Capital Market; expectations regarding the capitalization, resources and ownership structure of Lisata; the approach Lisata is taking to discover, develop and commercialize novel therapeutics; the adequacy of Lisata’s capital to support its future operations and its ability to successfully initiate and complete clinical trials; and the difficulty in predicting the time and cost of development of Lisata’s product candidates. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: the impact of the ongoing COVID-19 pandemic on Lisata’s business, the safety and efficacy of Lisata’s product candidates, decisions of regulatory authorities and the timing thereof, the duration and impact of regulatory delays in Lisata’s clinical programs, Lisata’s ability to finance its operations, the likelihood and timing of the receipt of future milestone and licensing fees, the future success of Lisata’s scientific studies, Lisata’s ability to successfully develop and commercialize drug candidates, the timing for starting and completing clinical trials, rapid technological change in Lisata’s markets, the ability of Lisata to protect its intellectual property rights; unexpected costs, charges or expenses resulting from the Merger; potential adverse reactions or changes to business relationships resulting from the completion of the Merger; potential underperformance of Lisata’s business following the Merger as compared to management’s initial expectations; and legislative, regulatory, political and economic developments. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in Lisata’s Annual Report on Form 10-K filed with the SEC on March 30, 2023, and in other documents filed by Lisata with the Securities and Exchange Commission. Except as required by applicable law, Lisata undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise. 2

LISATA EUTICSTHERAP Nasdaq-listed clinical stage therapeutics development company with a novel solid tumor targeting and penetration technology to improve the efficacy of anti-cancer drugs 3 Lisata Therapeutics
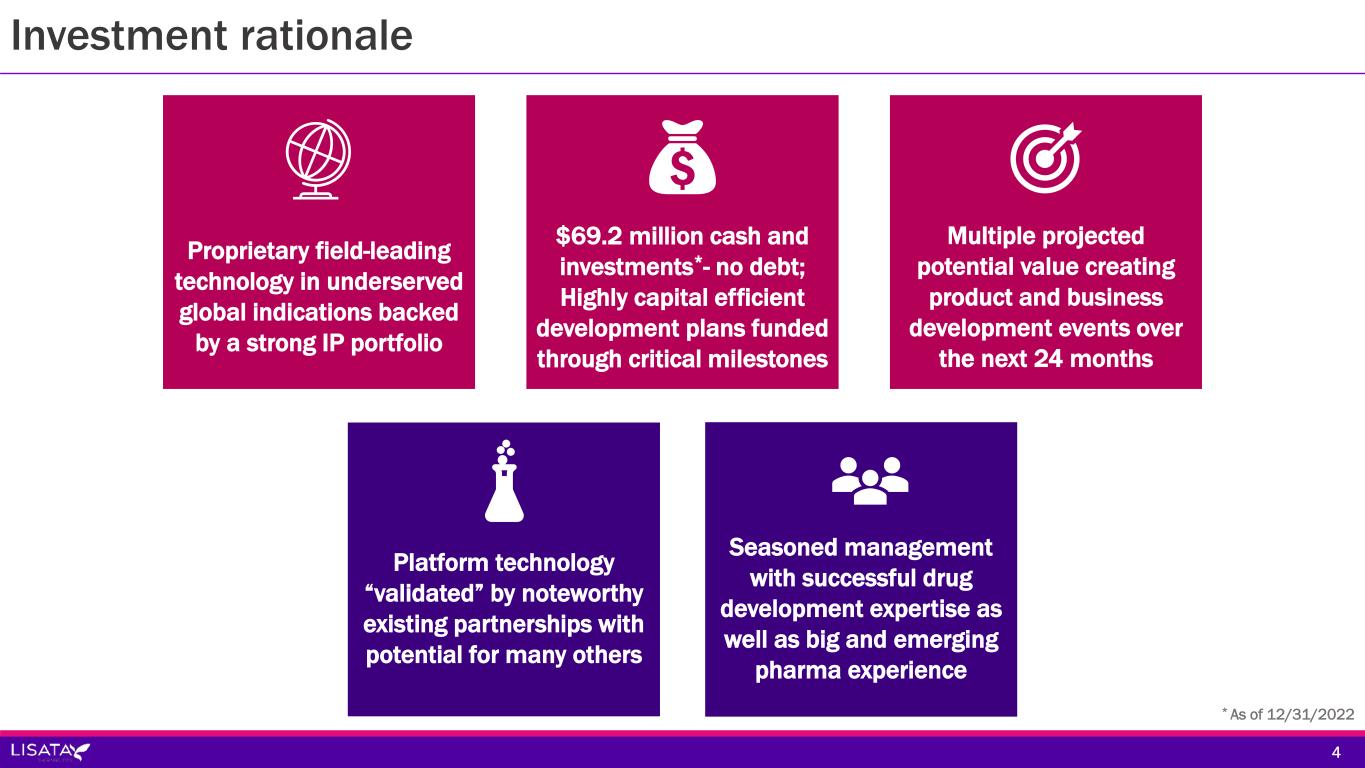
Investment rationale 4 * As of 12/31/2022 Proprietary field-leading technology in underserved global indications backed by a strong IP portfolio $69.2 million cash and investments*- no debt; Highly capital efficient development plans funded through critical milestones Multiple projected potential value creating product and business development events over the next 24 months Platform technology “validated” by noteworthy existing partnerships with potential for many others Seasoned management with successful drug development expertise as well as big and emerging pharma experience
Why Oncology? - Improved cancer treatment is a global need 5 Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths1 – World Health Organization 1 www.who.int/news-room/fact-sheets/detail/cancer
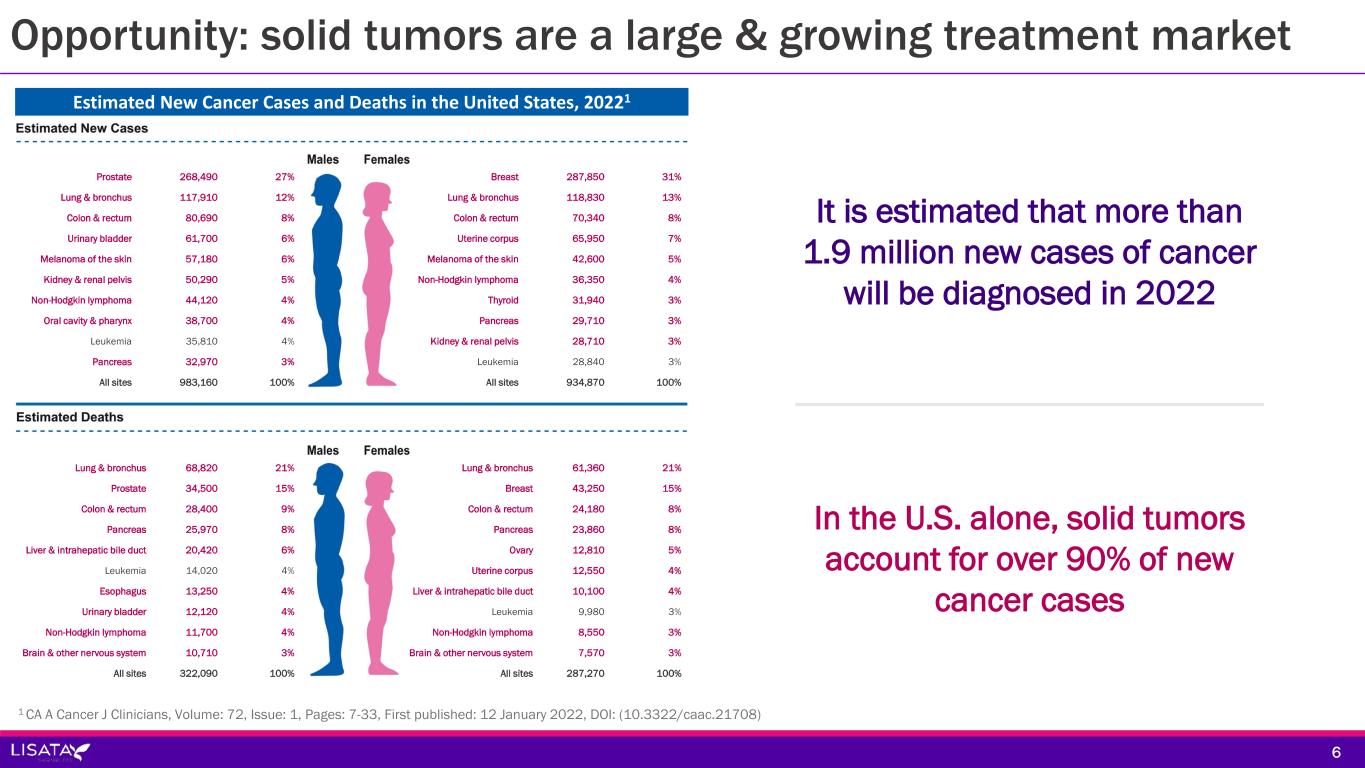
Opportunity: solid tumors are a large & growing treatment market 1 CA A Cancer J Clinicians, Volume: 72, Issue: 1, Pages: 7-33, First published: 12 January 2022, DOI: (10.3322/caac.21708) 6 It is estimated that more than 1.9 million new cases of cancer will be diagnosed in 2022 In the U.S. alone, solid tumors account for over 90% of new cancer cases Estimated New Cancer Cases and Deaths in the United States, 20221 Prostate 268,490 27% Lung & bronchus 117,910 12% Colon & rectum 80,690 8% Urinary bladder 61,700 6% Melanoma of the skin 57,180 6% Kidney & renal pelvis 50,290 5% Non-Hodgkin lymphoma 44,120 4% Oral cavity & pharynx 38,700 4% Leukemia 35,810 4% Pancreas 32,970 3% All sites 983,160 100% Breast 287,850 31% Lung & bronchus 118,830 13% Colon & rectum 70,340 8% Uterine corpus 65,950 7% Melanoma of the skin 42,600 5% Non-Hodgkin lymphoma 36,350 4% Thyroid 31,940 3% Pancreas 29,710 3% Kidney & renal pelvis 28,710 3% Leukemia 28,840 3% All sites 934,870 100% Lung & bronchus 68,820 21% Prostate 34,500 15% Colon & rectum 28,400 9% Pancreas 25,970 8% Liver & intrahepatic bile duct 20,420 6% Leukemia 14,020 4% Esophagus 13,250 4% Urinary bladder 12,120 4% Non-Hodgkin lymphoma 11,700 4% Brain & other nervous system 10,710 3% All sites 322,090 100% Lung & bronchus 61,360 21% Breast 43,250 15% Colon & rectum 24,180 8% Pancreas 23,860 8% Ovary 12,810 5% Uterine corpus 12,550 4% Liver & intrahepatic bile duct 10,100 4% Leukemia 9,980 3% Non-Hodgkin lymphoma 8,550 3% Brain & other nervous system 7,570 3% All sites 287,270 100%

Tumor targeting and intratumoral penetration are inadequate • Tumor stroma acts as an effective barrier to anti-cancer agent penetration • Tumor microenvironment immunosuppressive cells can lead to tumor resistance and/or metastases • Continued or escalating dosing of non-targeted anti-cancer therapy can lead to intolerable off-target side effects • Clinical response to many anti-cancer drugs is suboptimal 7 Challenge: tumor targeting & intratumoral penetration are inadequate

Solution: Lisata’s CendR Platform® technology Converts tumor stroma from barrier to conduit for penetration of anti-cancer treatments • Combination with many existing & emerging anti-cancer drugs possible in multiple indications • Mechanism effective with co-administered or tethered anti-cancer therapies • Co-administration presents a streamlined development path to registration • Tethering provides for prolonged compound exclusivity (NCE); product life cycle management Combats resistance by selectively depleting intratumoral immunosuppressive cells Platform extension possible to most drug modalities including nucleic acid-based drugs Targeted penetration technology to enhance drug delivery to solid tumors 8

LSTA1: lead clinical development candidate of the CendR Platform® Multiple Phase 1b to 2b studies in metastatic pancreatic ductal adenocarcinoma (mPDAC) combined with standards-of-care (SoC) chemotherapy [i.e., (gemcitabine + nab-paclitaxel) or FOLFIRINOX] • Granted Fast Track and Orphan Drug Designations by the U.S. FDA in PDAC • Studies in combination with SoC plus immunotherapies targeted to begin in 1H23 BOLSTER (basket) trial expanding development to cholangiocarcinoma, head and neck squamous cell carcinoma and esophageal squamous cell carcinoma with other anti-cancer drug combinations to initiate in 2Q23 Phase 1b/2a trial start in glioblastoma multiforme in combination with temozolomide planned for 3Q23 Phase 1/2a trial in peritoneal carcinomatosis targeted to begin in 3Q23 LSTA1 is being advanced in clinical trials in various difficult-to-treat solid tumor indications as part of a global registration strategy 9

Broad applicability: noteworthy existing partnerships and beyond Strategic partnership in China with Qilu Pharmaceutical Exclusive rights to LSTA1 in China, Taiwan, Hong Kong and Macau Qilu assumes all development and commercialization responsibilities/costs in licensed territories Potential for up to $220 million to Lisata for milestones & tiered double-digit royalties on sales Clinical development collaborations exploring combinations with immunotherapy LSTA1/gemcitabine/nab-paclitaxel treatment regimen ± durvalumab with WARPNINE (AUS) LSTA1/gemcitabine/nab-paclitaxel treatment regimen ± nivolumab with WARPNINE (AUS) LSTA1/gemcitabine/nab-paclitaxel treatment regimen ± atezolizumab with ROCHE 10 Additional partnership opportunities for the CendR Platform® in general and many combinations with LSTA1, specifically 10

LSTA1 [formerly known as CEND-1] Well-characterized Mechanism of Action with Compelling Early Clinical Results
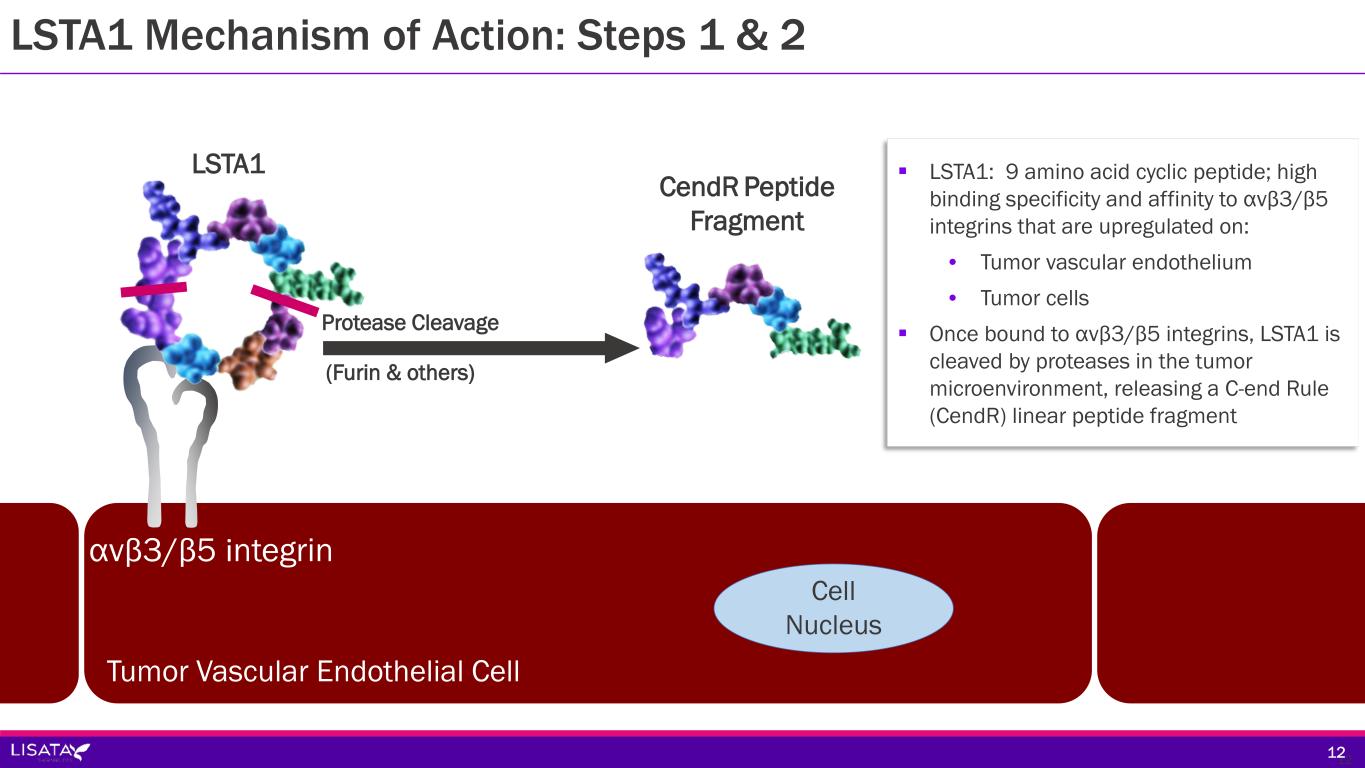
αvβ3/β5 integrin LSTA1 (Furin & others) Protease Cleavage CendR Peptide Fragment Tumor Vascular Endothelial Cell Cell Nucleus 12 LSTA1: 9 amino acid cyclic peptide; high binding specificity and affinity to αvβ3/β5 integrins that are upregulated on: • Tumor vascular endothelium • Tumor cells Once bound to αvβ3/β5 integrins, LSTA1 is cleaved by proteases in the tumor microenvironment, releasing a C-end Rule (CendR) linear peptide fragment 12 LSTA1 Mechanism of Action: Steps 1 & 2
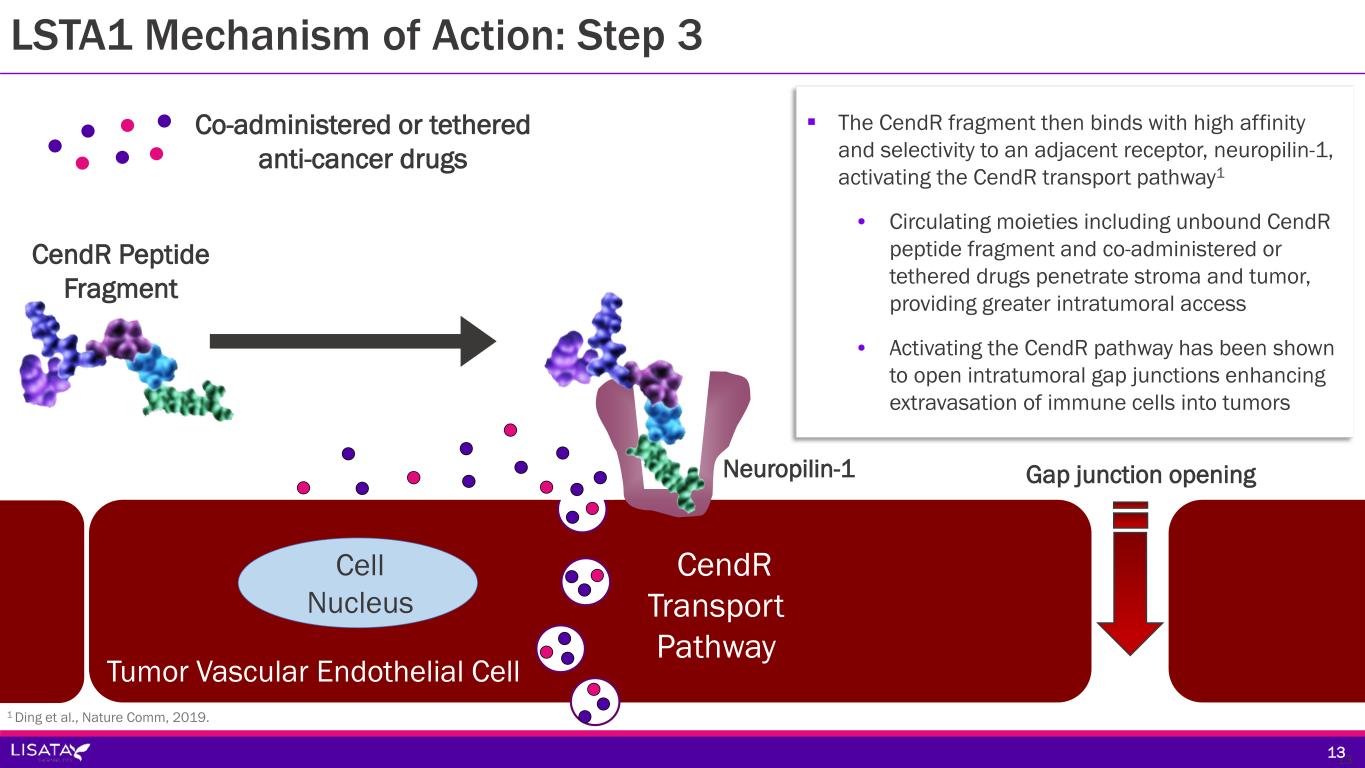
LSTA1 Mechanism of Action: Step 3 Neuropilin-1 CendR Peptide Fragment Co-administered or tethered anti-cancer drugs CendR Transport Pathway Tumor Vascular Endothelial Cell Cell Nucleus Gap junction opening 13 The CendR fragment then binds with high affinity and selectivity to an adjacent receptor, neuropilin-1, activating the CendR transport pathway1 • Circulating moieties including unbound CendR peptide fragment and co-administered or tethered drugs penetrate stroma and tumor, providing greater intratumoral access • Activating the CendR pathway has been shown to open intratumoral gap junctions enhancing extravasation of immune cells into tumors 13 1 Ding et al., Nature Comm, 2019.
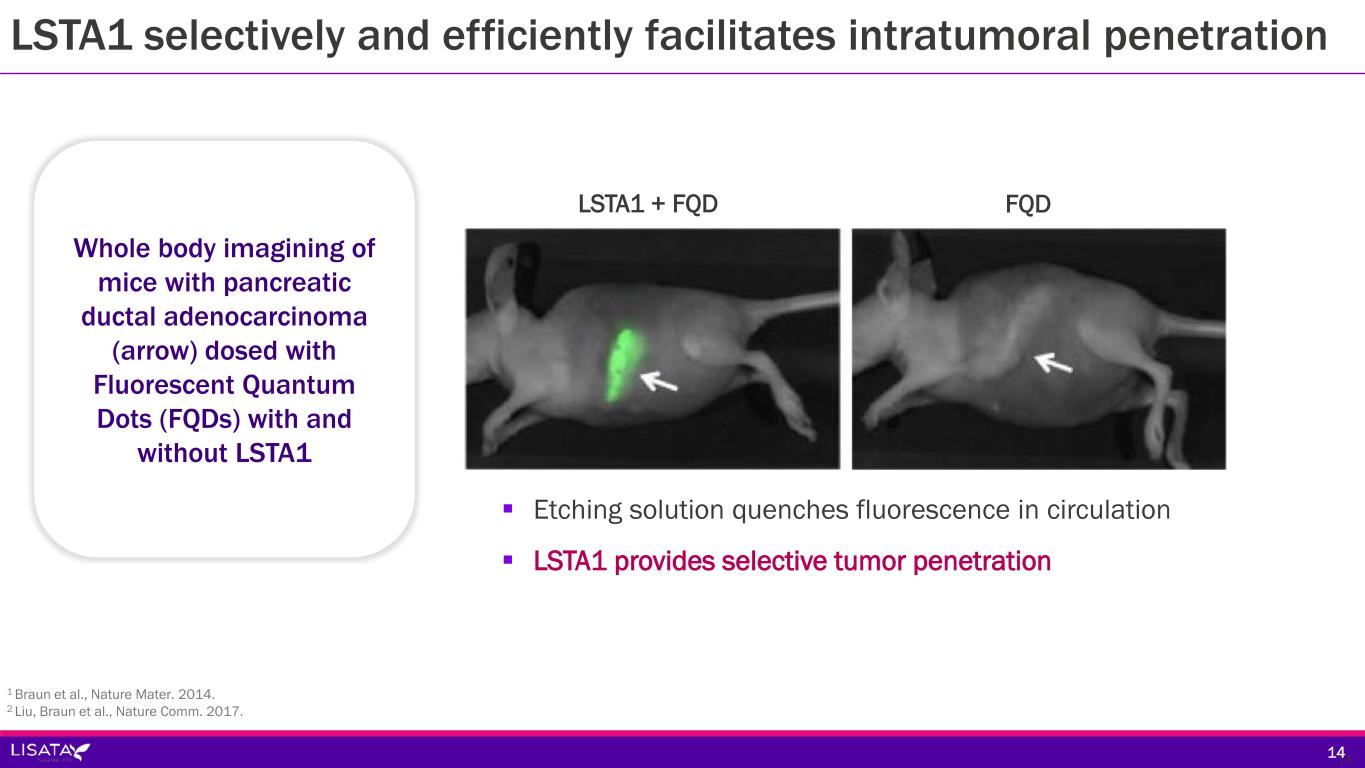
LSTA1 selectively and efficiently facilitates intratumoral penetration Whole body imagining of mice with pancreatic ductal adenocarcinoma (arrow) dosed with Fluorescent Quantum Dots (FQDs) with and without LSTA1 1 Braun et al., Nature Mater. 2014. 2 Liu, Braun et al., Nature Comm. 2017. 14 Etching solution quenches fluorescence in circulation LSTA1 provides selective tumor penetration 14 LSTA1 + FQD FQD
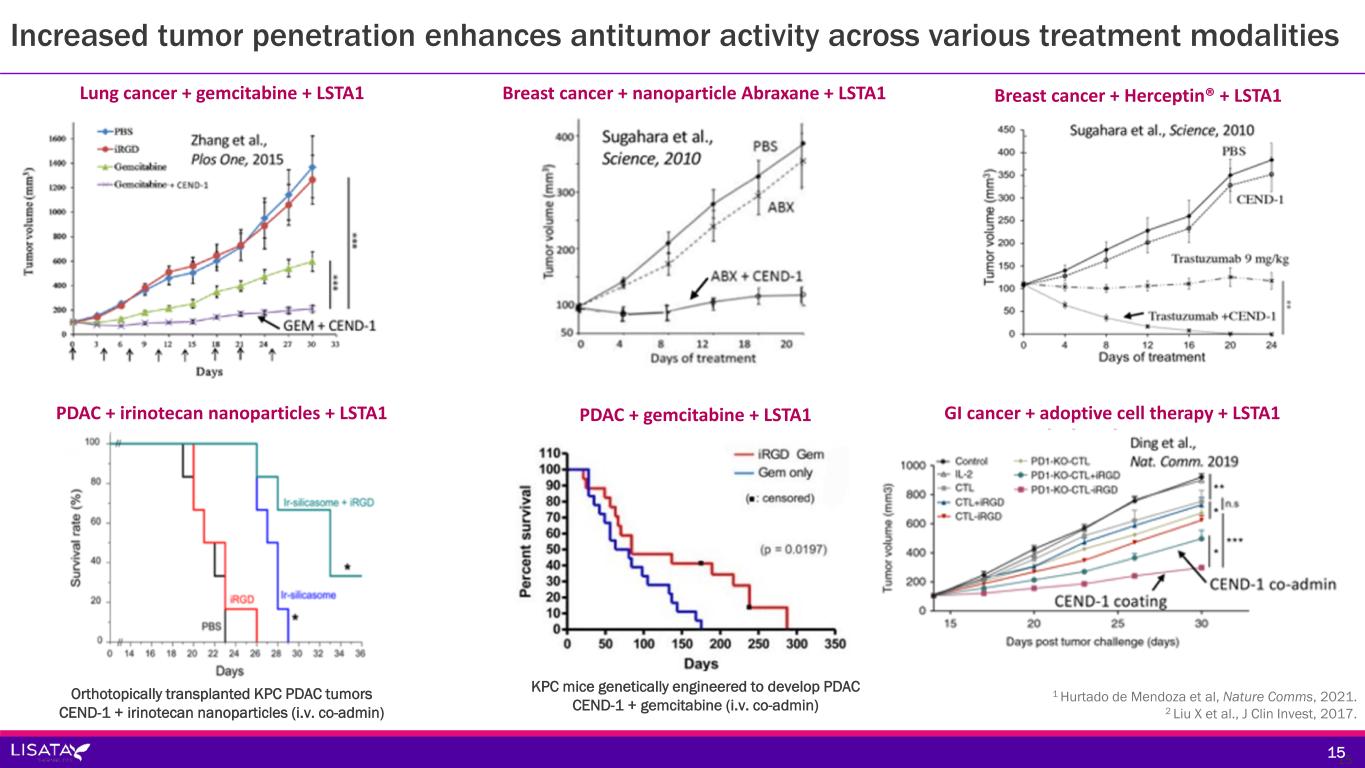
1 Hurtado de Mendoza et al, Nature Comms, 2021. 2 Liu X et al., J Clin Invest, 2017. 15 Lung cancer + gemcitabine + LSTA1 Breast cancer + nanoparticle Abraxane + LSTA1 GI cancer + adoptive cell therapy + LSTA1PDAC + irinotecan nanoparticles + LSTA1 Orthotopically transplanted KPC PDAC tumors CEND-1 + irinotecan nanoparticles (i.v. co-admin) PDAC + gemcitabine + LSTA1 KPC mice genetically engineered to develop PDAC CEND-1 + gemcitabine (i.v. co-admin) Increased tumor penetration enhances antitumor activity across various treatment modalities Breast cancer + Herceptin® + LSTA1 15
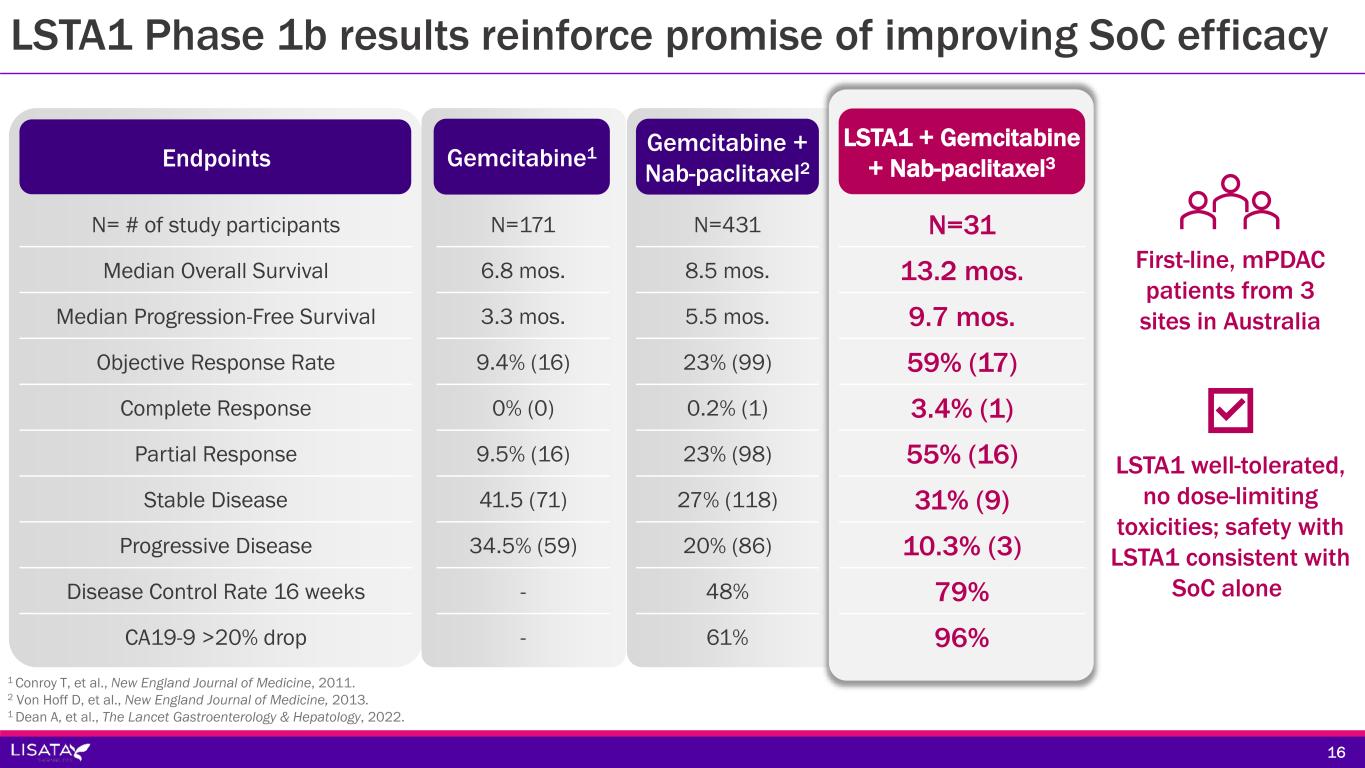
LSTA1 Phase 1b results reinforce promise of improving SoC efficacy N= # of study participants Median Overall Survival Median Progression-Free Survival Objective Response Rate Complete Response Partial Response Stable Disease Progressive Disease Disease Control Rate 16 weeks CA19-9 >20% drop N=171 6.8 mos. 3.3 mos. 9.4% (16) 0% (0) 9.5% (16) 41.5 (71) 34.5% (59) - - Gemcitabine + Nab-paclitaxel2 N=431 8.5 mos. 5.5 mos. 23% (99) 0.2% (1) 23% (98) 27% (118) 20% (86) 48% 61% Endpoints 1 Conroy T, et al., New England Journal of Medicine, 2011. 2 Von Hoff D, et al., New England Journal of Medicine, 2013. 1 Dean A, et al., The Lancet Gastroenterology & Hepatology, 2022. Gemcitabine1 LSTA1 well-tolerated, no dose-limiting toxicities; safety with LSTA1 consistent with SoC alone First-line, mPDAC patients from 3 sites in Australia N=31 13.2 mos. 9.7 mos. 59% (17) 3.4% (1) 55% (16) 31% (9) 10.3% (3) 79% 96% LSTA1 + Gemcitabine + Nab-paclitaxel3 16

LSTA1 Clinical Development Portfolio Fast Track and Orphan Drug designated (PDAC) - USA
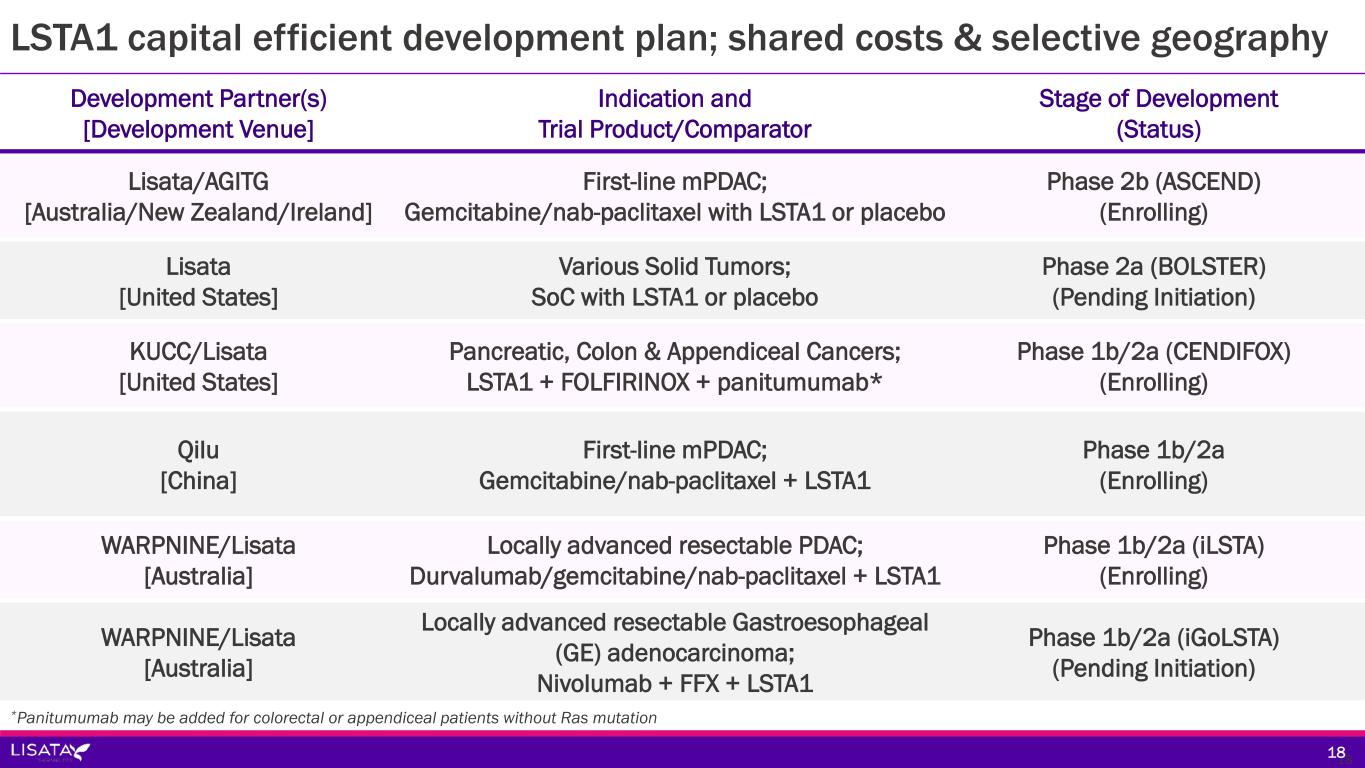
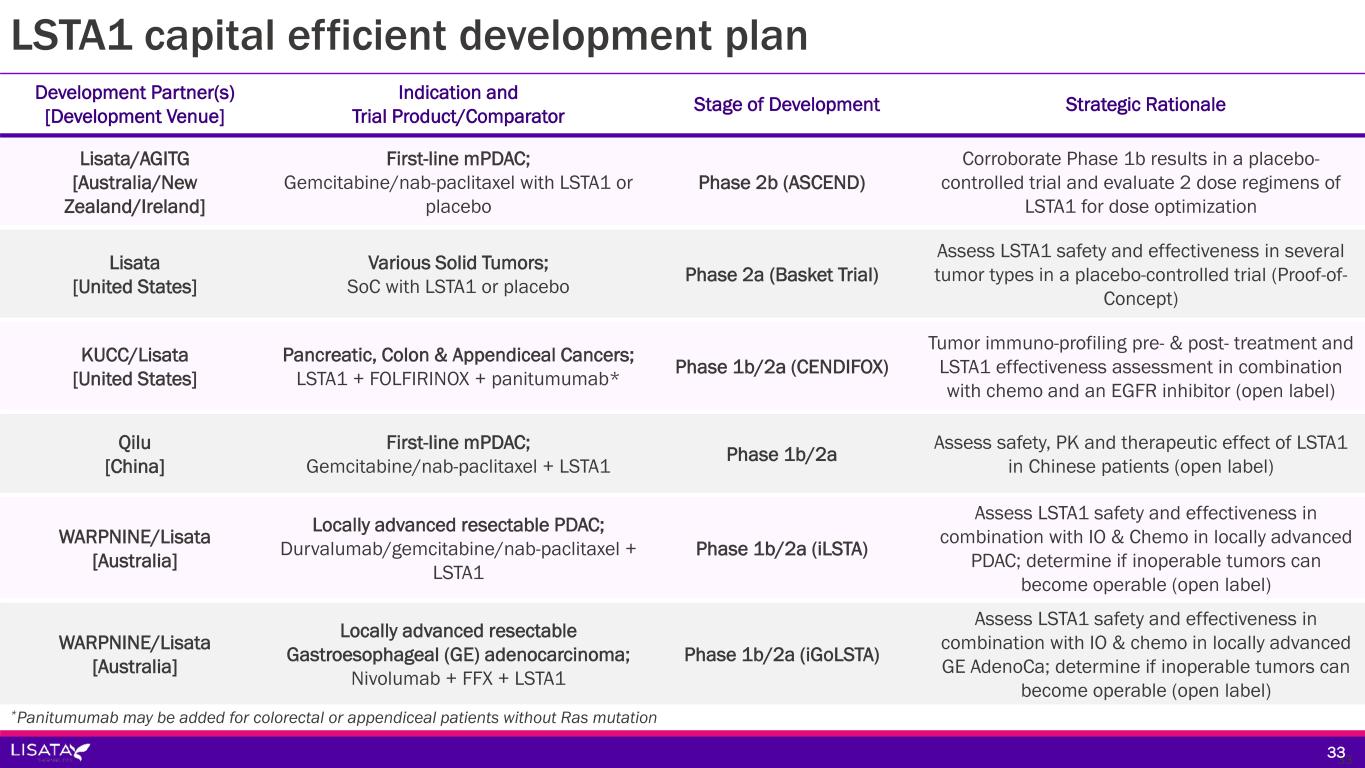
Development Partner(s) [Development Venue] Indication and Trial Product/Comparator Stage of Development (Status) Lisata/AGITG [Australia/New Zealand/Ireland] First-line mPDAC; Gemcitabine/nab-paclitaxel with LSTA1 or placebo Phase 2b (ASCEND) (Enrolling) Lisata [United States] Various Solid Tumors; SoC with LSTA1 or placebo Phase 2a (BOLSTER) (Pending Initiation) KUCC/Lisata [United States] Pancreatic, Colon & Appendiceal Cancers; LSTA1 + FOLFIRINOX + panitumumab* Phase 1b/2a (CENDIFOX) (Enrolling) Qilu [China] First-line mPDAC; Gemcitabine/nab-paclitaxel + LSTA1 Phase 1b/2a (Enrolling) WARPNINE/Lisata [Australia] Locally advanced resectable PDAC; Durvalumab/gemcitabine/nab-paclitaxel + LSTA1 Phase 1b/2a (iLSTA) (Enrolling) WARPNINE/Lisata [Australia] Locally advanced resectable Gastroesophageal (GE) adenocarcinoma; Nivolumab + FFX + LSTA1 Phase 1b/2a (iGoLSTA) (Pending Initiation) LSTA1 capital efficient development plan; shared costs & selective geography 1818 *Panitumumab may be added for colorectal or appendiceal patients without Ras mutation
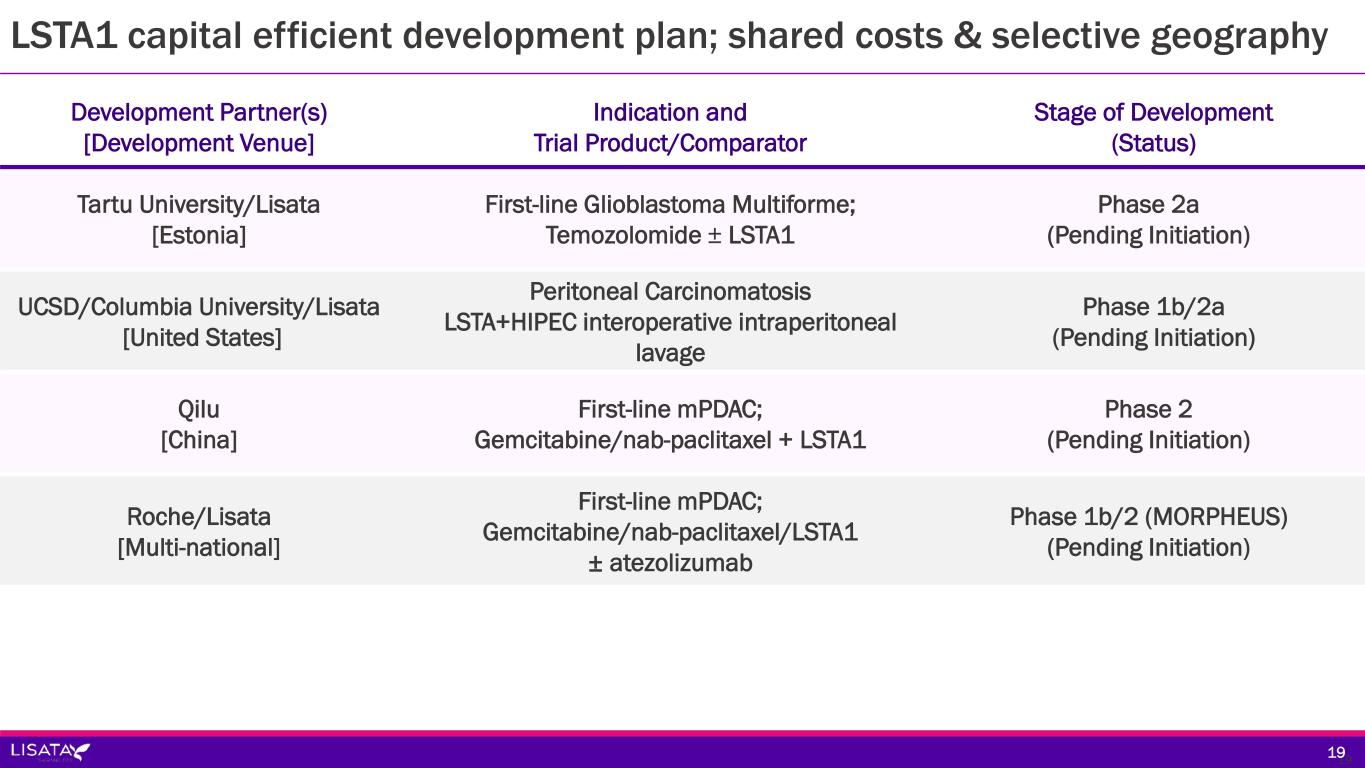
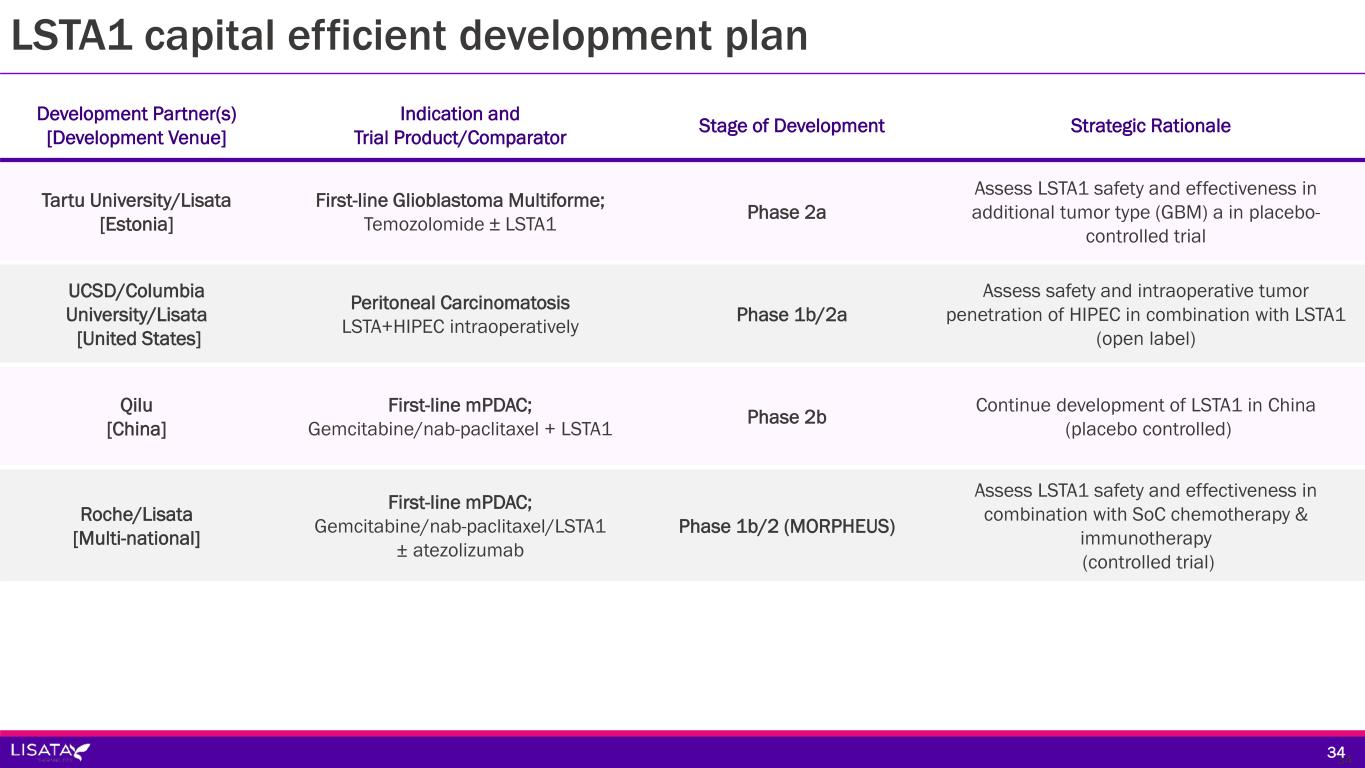
Development Partner(s) [Development Venue] Indication and Trial Product/Comparator Stage of Development (Status) Tartu University/Lisata [Estonia] First-line Glioblastoma Multiforme; Temozolomide ± LSTA1 Phase 2a (Pending Initiation) UCSD/Columbia University/Lisata [United States] Peritoneal Carcinomatosis LSTA+HIPEC interoperative intraperitoneal lavage Phase 1b/2a (Pending Initiation) Qilu [China] First-line mPDAC; Gemcitabine/nab-paclitaxel + LSTA1 Phase 2 (Pending Initiation) Roche/Lisata [Multi-national] First-line mPDAC; Gemcitabine/nab-paclitaxel/LSTA1 ± atezolizumab Phase 1b/2 (MORPHEUS) (Pending Initiation) LSTA1 capital efficient development plan; shared costs & selective geography 1919

Tissue-Penetrating Nanocomplex (TPN) Platform™ Applying the CendR Platform® to nucleic acid-based drugs
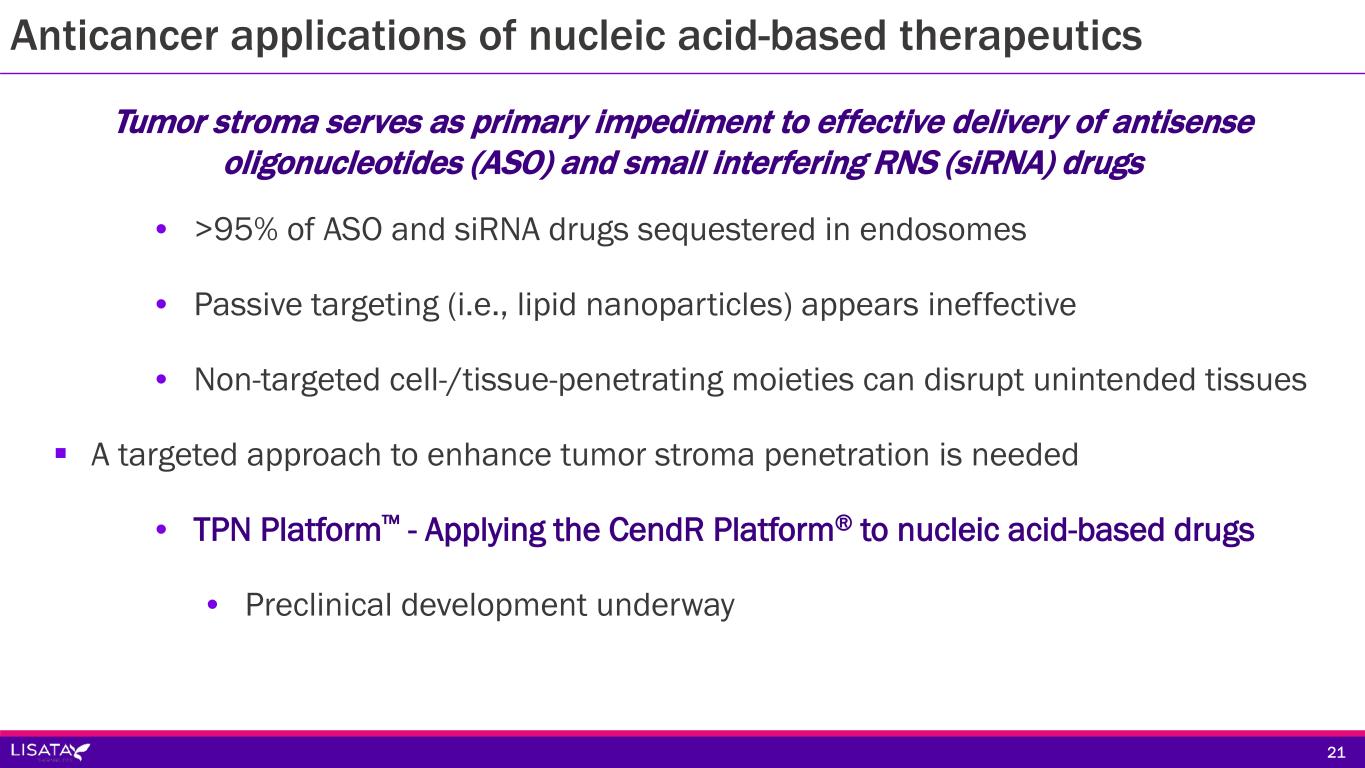
Anticancer applications of nucleic acid-based therapeutics Tumor stroma serves as primary impediment to effective delivery of antisense oligonucleotides (ASO) and small interfering RNS (siRNA) drugs • >95% of ASO and siRNA drugs sequestered in endosomes • Passive targeting (i.e., lipid nanoparticles) appears ineffective • Non-targeted cell-/tissue-penetrating moieties can disrupt unintended tissues A targeted approach to enhance tumor stroma penetration is needed • TPN Platform™ - Applying the CendR Platform® to nucleic acid-based drugs • Preclinical development underway 21
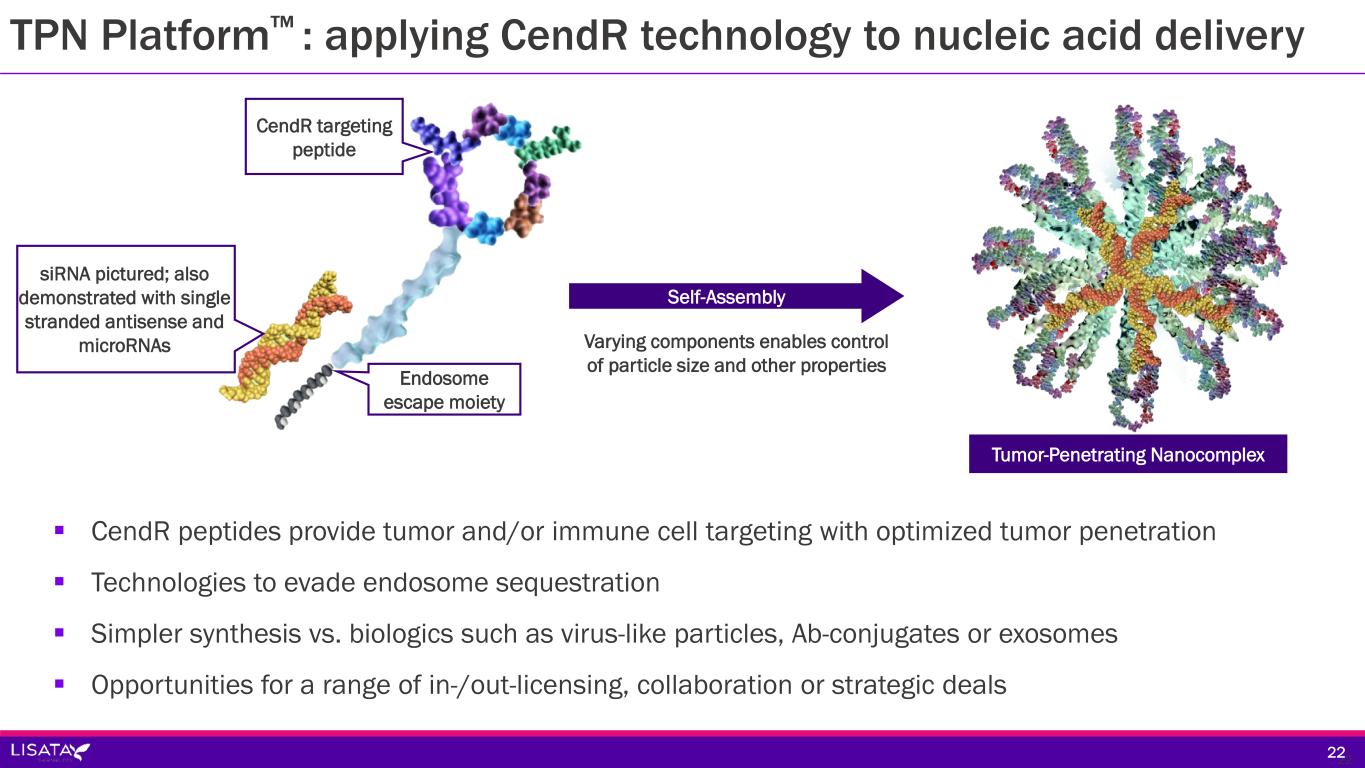
TPN Platform™ : applying CendR technology to nucleic acid delivery 2222 CendR peptides provide tumor and/or immune cell targeting with optimized tumor penetration Technologies to evade endosome sequestration Simpler synthesis vs. biologics such as virus-like particles, Ab-conjugates or exosomes Opportunities for a range of in-/out-licensing, collaboration or strategic deals Self-Assembly Varying components enables control of particle size and other properties Tumor-Penetrating Nanocomplex CendR targeting peptide siRNA pictured; also demonstrated with single stranded antisense and microRNAs Endosome escape moiety

CD34+ Cell Therapy Platform Technology

2424 Sponsor [Development Venue] Indication and Trial Product/Comparator Stage of Development Lisata [Japan] Critical Limb Ischemia & Buerger's Disease; HONEDRA® (LSTA12) Registration Eligible CD34+ cell therapy current clinical trials Legacy development programs provide potential value upside with no further capital outlay

HONEDRA® [LSTA12 (formerly known as CLBS12)] Critical Limb Ischemia (Japan) SAKIGAKE designated – Japan Orphan Drug designated (Buerger’s disease) - USA Advanced Therapeutic Medicinal Product (ATMP) designated – EU
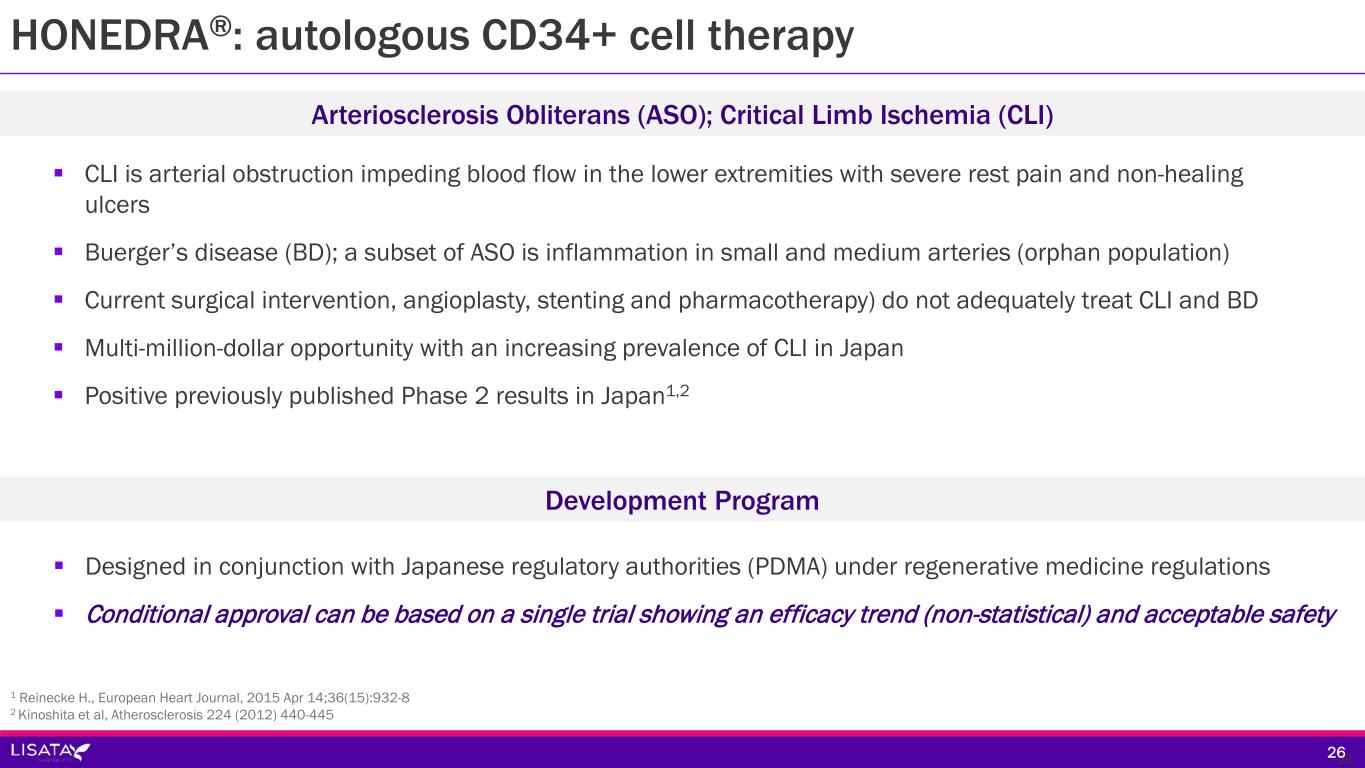
HONEDRA®: autologous CD34+ cell therapy 2626 1 Reinecke H., European Heart Journal, 2015 Apr 14;36(15):932-8 2 Kinoshita et al, Atherosclerosis 224 (2012) 440-445 CLI is arterial obstruction impeding blood flow in the lower extremities with severe rest pain and non-healing ulcers Buerger’s disease (BD); a subset of ASO is inflammation in small and medium arteries (orphan population) Current surgical intervention, angioplasty, stenting and pharmacotherapy) do not adequately treat CLI and BD Multi-million-dollar opportunity with an increasing prevalence of CLI in Japan Positive previously published Phase 2 results in Japan1,2 Arteriosclerosis Obliterans (ASO); Critical Limb Ischemia (CLI) Designed in conjunction with Japanese regulatory authorities (PDMA) under regenerative medicine regulations Conditional approval can be based on a single trial showing an efficacy trend (non-statistical) and acceptable safety Development Program
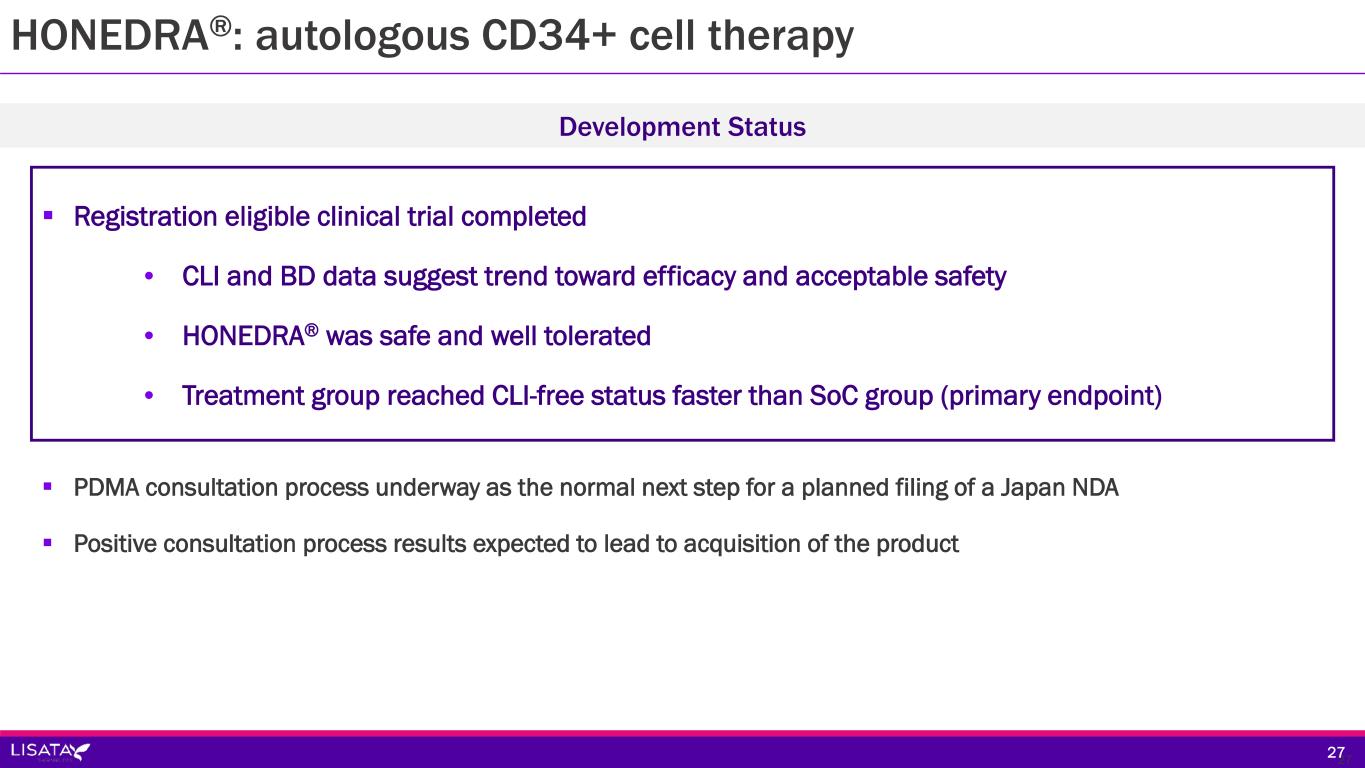
HONEDRA®: autologous CD34+ cell therapy Registration eligible clinical trial completed • CLI and BD data suggest trend toward efficacy and acceptable safety • HONEDRA® was safe and well tolerated • Treatment group reached CLI-free status faster than SoC group (primary endpoint) Development Status 2727 PDMA consultation process underway as the normal next step for a planned filing of a Japan NDA Positive consultation process results expected to lead to acquisition of the product
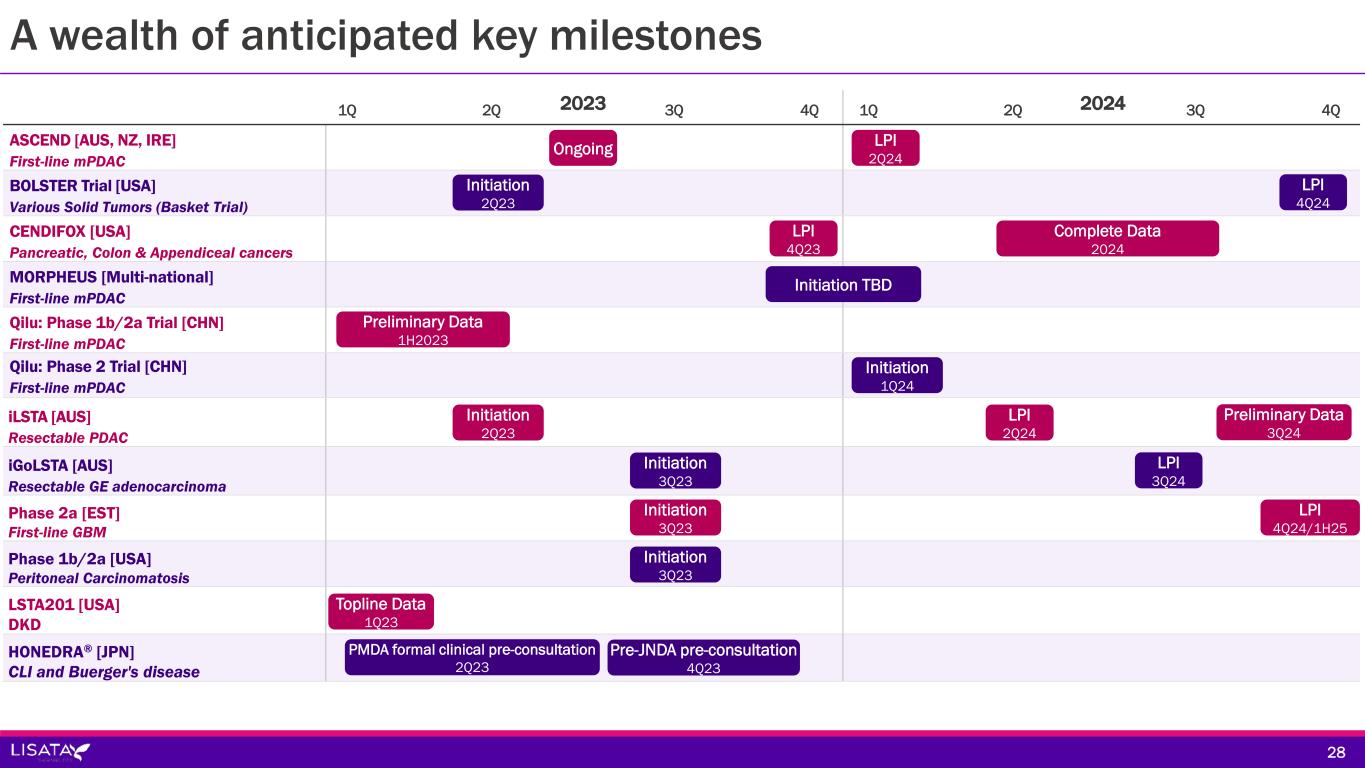
2023 2024 ASCEND [AUS, NZ, IRE] First-line mPDAC BOLSTER Trial [USA] Various Solid Tumors (Basket Trial) CENDIFOX [USA] Pancreatic, Colon & Appendiceal cancers MORPHEUS [Multi-national] First-line mPDAC Qilu: Phase 1b/2a Trial [CHN] First-line mPDAC Qilu: Phase 2 Trial [CHN] First-line mPDAC iLSTA [AUS] Resectable PDAC iGoLSTA [AUS] Resectable GE adenocarcinoma Phase 2a [EST] First-line GBM Phase 1b/2a [USA] Peritoneal Carcinomatosis LSTA201 [USA] DKD HONEDRA® [JPN] CLI and Buerger's disease A wealth of anticipated key milestones 1Q 2Q 3Q 4Q 28 LPI 2Q24 Initiation 2Q23 LPI 4Q24 LPI 4Q23 Complete Data 2024 Initiation TBD Preliminary Data 1H2023 Initiation 1Q24 Initiation 2Q23 LPI 2Q24 Preliminary Data 3Q24 Initiation 3Q23 LPI 3Q24 Initiation 3Q23 LPI 4Q24/1H25 Initiation 3Q23 Topline Data 1Q23 PMDA formal clinical pre-consultation 2Q23 Pre-JNDA pre-consultation 4Q23 1Q 2Q 3Q 4Q Ongoing
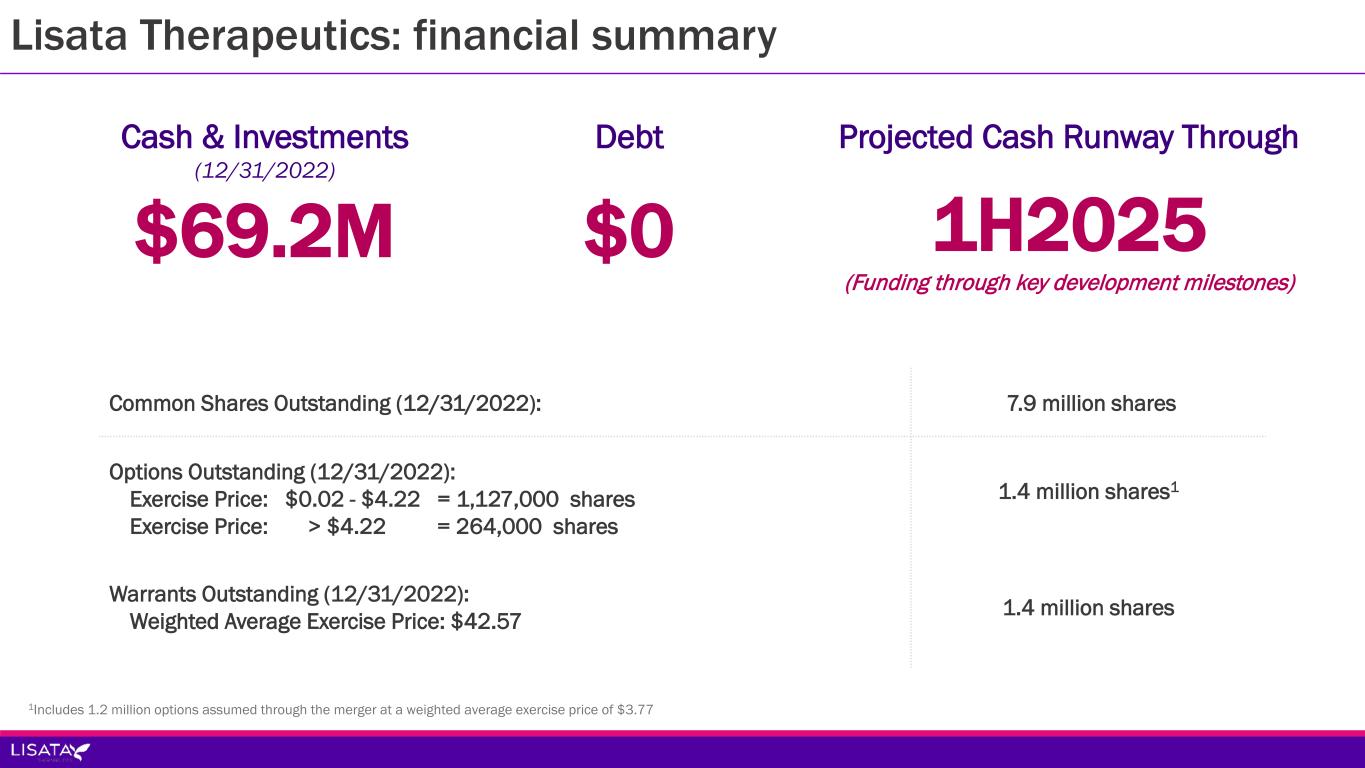
Lisata Therapeutics: financial summary $69.2M Cash & Investments (12/31/2022) $0 Debt 1H2025 (Funding through key development milestones) Projected Cash Runway Through Common Shares Outstanding (12/31/2022): 7.9 million shares Options Outstanding (12/31/2022): Exercise Price: $0.02 - $4.22 = 1,127,000 shares Exercise Price: > $4.22 = 264,000 shares 1.4 million shares1 Warrants Outstanding (12/31/2022): Weighted Average Exercise Price: $42.57 1.4 million shares 1Includes 1.2 million options assumed through the merger at a weighted average exercise price of $3.77
Investment rationale 30 * As of 12/31/2022 Proprietary field-leading technology in underserved global indications backed by a strong IP portfolio $69.2 million cash and investments*- no debt; Highly capital efficient development plans funded through critical milestones Multiple projected potential value creating product and business development events over the next 24 months Platform technology “validated” by noteworthy existing partnerships with potential for many others Seasoned management with successful drug development expertise as well as big and emerging pharma experience

C o p y r i g h t © 2 0 2 3 L i s a t a T h e r a p e u t i c s , I n c . A l l r i g h t s r e s e r v e d . Targeted Therapy Delivered Investor Relations Contact: John D. Menditto VP, IR & Corporate Communications o: (908) 842-0084 | e: jmenditto@lisata.com Nasdaq: LSTA | www.lisata.com

Appendix
Development Partner(s) [Development Venue] Indication and Trial Product/Comparator Stage of Development Strategic Rationale Lisata/AGITG [Australia/New Zealand/Ireland] First-line mPDAC; Gemcitabine/nab-paclitaxel with LSTA1 or placebo Phase 2b (ASCEND) Corroborate Phase 1b results in a placebo- controlled trial and evaluate 2 dose regimens of LSTA1 for dose optimization Lisata [United States] Various Solid Tumors; SoC with LSTA1 or placebo Phase 2a (Basket Trial) Assess LSTA1 safety and effectiveness in several tumor types in a placebo-controlled trial (Proof-of- Concept) KUCC/Lisata [United States] Pancreatic, Colon & Appendiceal Cancers; LSTA1 + FOLFIRINOX + panitumumab* Phase 1b/2a (CENDIFOX) Tumor immuno-profiling pre- & post- treatment and LSTA1 effectiveness assessment in combination with chemo and an EGFR inhibitor (open label) Qilu [China] First-line mPDAC; Gemcitabine/nab-paclitaxel + LSTA1 Phase 1b/2a Assess safety, PK and therapeutic effect of LSTA1 in Chinese patients (open label) WARPNINE/Lisata [Australia] Locally advanced resectable PDAC; Durvalumab/gemcitabine/nab-paclitaxel + LSTA1 Phase 1b/2a (iLSTA) Assess LSTA1 safety and effectiveness in combination with IO & Chemo in locally advanced PDAC; determine if inoperable tumors can become operable (open label) WARPNINE/Lisata [Australia] Locally advanced resectable Gastroesophageal (GE) adenocarcinoma; Nivolumab + FFX + LSTA1 Phase 1b/2a (iGoLSTA) Assess LSTA1 safety and effectiveness in combination with IO & chemo in locally advanced GE AdenoCa; determine if inoperable tumors can become operable (open label) LSTA1 capital efficient development plan 3333 *Panitumumab may be added for colorectal or appendiceal patients without Ras mutation
Development Partner(s) [Development Venue] Indication and Trial Product/Comparator Stage of Development Strategic Rationale Tartu University/Lisata [Estonia] First-line Glioblastoma Multiforme; Temozolomide ± LSTA1 Phase 2a Assess LSTA1 safety and effectiveness in additional tumor type (GBM) a in placebo- controlled trial UCSD/Columbia University/Lisata [United States] Peritoneal Carcinomatosis LSTA+HIPEC intraoperatively Phase 1b/2a Assess safety and intraoperative tumor penetration of HIPEC in combination with LSTA1 (open label) Qilu [China] First-line mPDAC; Gemcitabine/nab-paclitaxel + LSTA1 Phase 2b Continue development of LSTA1 in China (placebo controlled) Roche/Lisata [Multi-national] First-line mPDAC; Gemcitabine/nab-paclitaxel/LSTA1 ± atezolizumab Phase 1b/2 (MORPHEUS) Assess LSTA1 safety and effectiveness in combination with SoC chemotherapy & immunotherapy (controlled trial) LSTA1 capital efficient development plan 3434

3535 Sponsor [Development Venue] Indication and Trial Product/Comparator Stage of Development Strategic Rationale Lisata [Japan] Critical Limb Ischemia & Buerger's Disease; HONEDRA® (LSTA12) Registration Eligible Assess safety and efficacy of LSTA12 in a controlled trial vs. SoC alone in the context of qualifying for approval in Japan under the accelerated regulatory pathway applicable to regenerative medicines CD34+ cell therapy current clinical trials Legacy development programs provide potential value upside with no further capital outlay

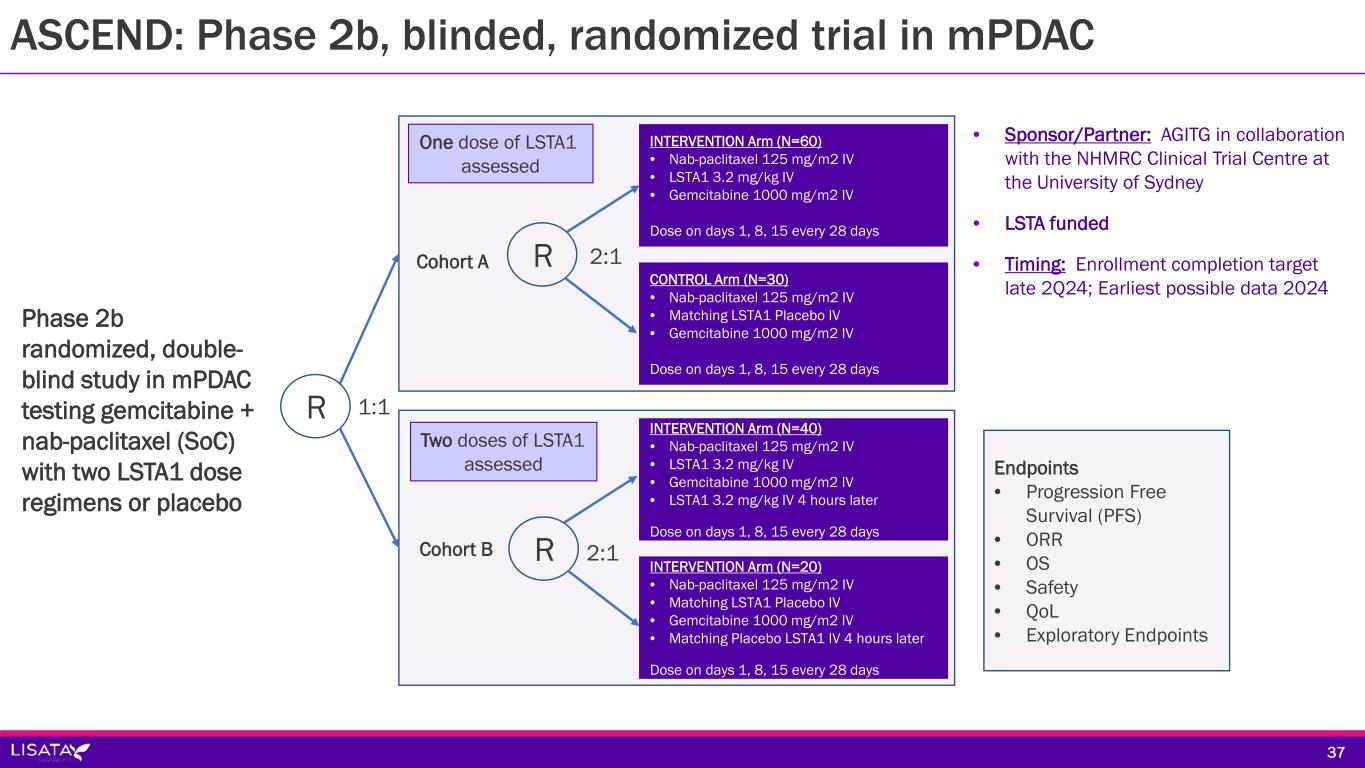
ASCEND: Phase 2b, blinded, randomized trial in mPDAC Sponsor/Partner Australasian Gastro-Intestinal Trials Group (AGITG) in collaboration with the NHMRC Clinical Trials Centre at the University of Sydney Lisata funded (LSTA eligible for ~43% rebate on all qualified R&D expenses in AUS) Objective Corroborate Phase 1b results in a placebo-controlled study Determine if a second dose of LSTA1 further improves patient outcomes Design Phase 2b randomized, double-blind study in mPDAC testing gemcitabine + nab-paclitaxel SoC with one of two LSTA1 dose regimens or placebo Study Size ~150 subjects (~40 sites planned in Australia, New Zealand and Ireland) Endpoints Primary: Progression Free Survival Secondary: AEs, SAEs, Overall Survival, Objective Tumor Response Rate Timing Enrollment completion target late 2Q24 Earliest possible data 2024 3636
R INTERVENTION Arm (N=60) • Nab-paclitaxel 125 mg/m2 IV • LSTA1 3.2 mg/kg IV • Gemcitabine 1000 mg/m2 IV Dose on days 1, 8, 15 every 28 days CONTROL Arm (N=30) • Nab-paclitaxel 125 mg/m2 IV • Matching LSTA1 Placebo IV • Gemcitabine 1000 mg/m2 IV Dose on days 1, 8, 15 every 28 days INTERVENTION Arm (N=40) • Nab-paclitaxel 125 mg/m2 IV • LSTA1 3.2 mg/kg IV • Gemcitabine 1000 mg/m2 IV • LSTA1 3.2 mg/kg IV 4 hours later Dose on days 1, 8, 15 every 28 days INTERVENTION Arm (N=20) • Nab-paclitaxel 125 mg/m2 IV • Matching LSTA1 Placebo IV • Gemcitabine 1000 mg/m2 IV • Matching Placebo LSTA1 IV 4 hours later Dose on days 1, 8, 15 every 28 days R R Cohort A Cohort B 1:1 2:1 2:1 One dose of LSTA1 assessed Two doses of LSTA1 assessed Endpoints • Progression Free Survival (PFS) • ORR • OS • Safety • QoL • Exploratory Endpoints • Sponsor/Partner: AGITG in collaboration with the NHMRC Clinical Trial Centre at the University of Sydney • LSTA funded • Timing: Enrollment completion target late 2Q24; Earliest possible data 2024 37 ASCEND: Phase 2b, blinded, randomized trial in mPDAC Phase 2b randomized, double- blind study in mPDAC testing gemcitabine + nab-paclitaxel (SoC) with two LSTA1 dose regimens or placebo

Sponsor/Partner Qilu Pharmaceutical (funds all development in China) Objective Evaluate safety, pharmacokinetics and preliminary efficacy of LSTA1 added to SoC in Chinese patients with mPDAC Design Phase 1b/2a open-label study in advanced mPDAC patients of Chinese ethnicity testing SoC chemotherapy (gemcitabine + Qilu-produced nab-paclitaxel) in combination with LSTA1 Study Size 50 subjects (~15 sites) Endpoints Primary: AEs, SAEs, Objective Response Rate, Duration of Response, Disease Control Rate, Overall Survival, and Progression Free Survival Secondary: Pharmacokinetic parameters Timing Preliminary data expected 1H23 3838 Phase 1b/2a open-label trial in mPDAC in China
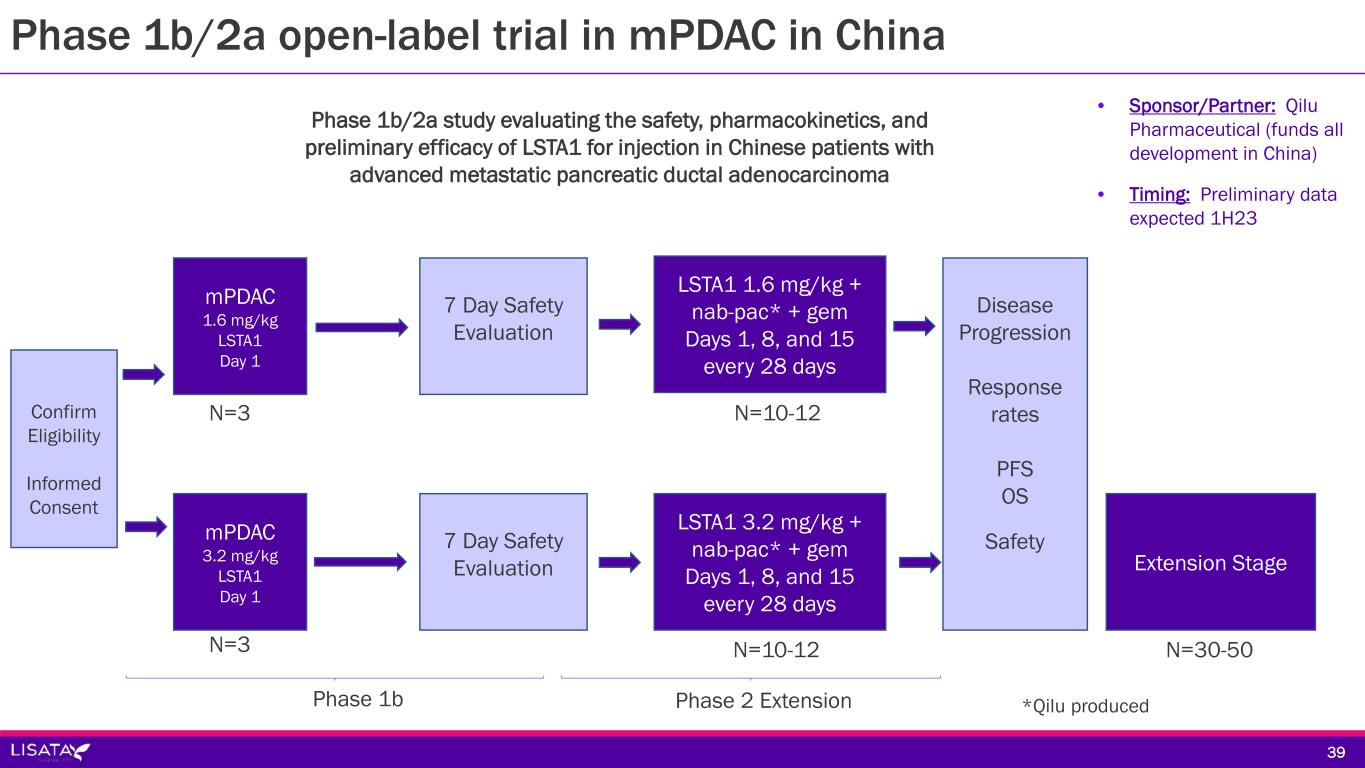
7 Day Safety Evaluation LSTA1 1.6 mg/kg + nab-pac* + gem Days 1, 8, and 15 every 28 days Confirm Eligibility Informed Consent Extension Stage mPDAC 1.6 mg/kg LSTA1 Day 1 Phase 1b/2a study evaluating the safety, pharmacokinetics, and preliminary efficacy of LSTA1 for injection in Chinese patients with advanced metastatic pancreatic ductal adenocarcinoma mPDAC 3.2 mg/kg LSTA1 Day 1 7 Day Safety Evaluation LSTA1 3.2 mg/kg + nab-pac* + gem Days 1, 8, and 15 every 28 days Phase 1b N=3 N=3 Phase 2 Extension N=10-12 N=10-12 N=30-50 Disease Progression Response rates PFS OS Safety • Sponsor/Partner: Qilu Pharmaceutical (funds all development in China) • Timing: Preliminary data expected 1H23 39 Phase 1b/2a open-label trial in mPDAC in China *Qilu produced
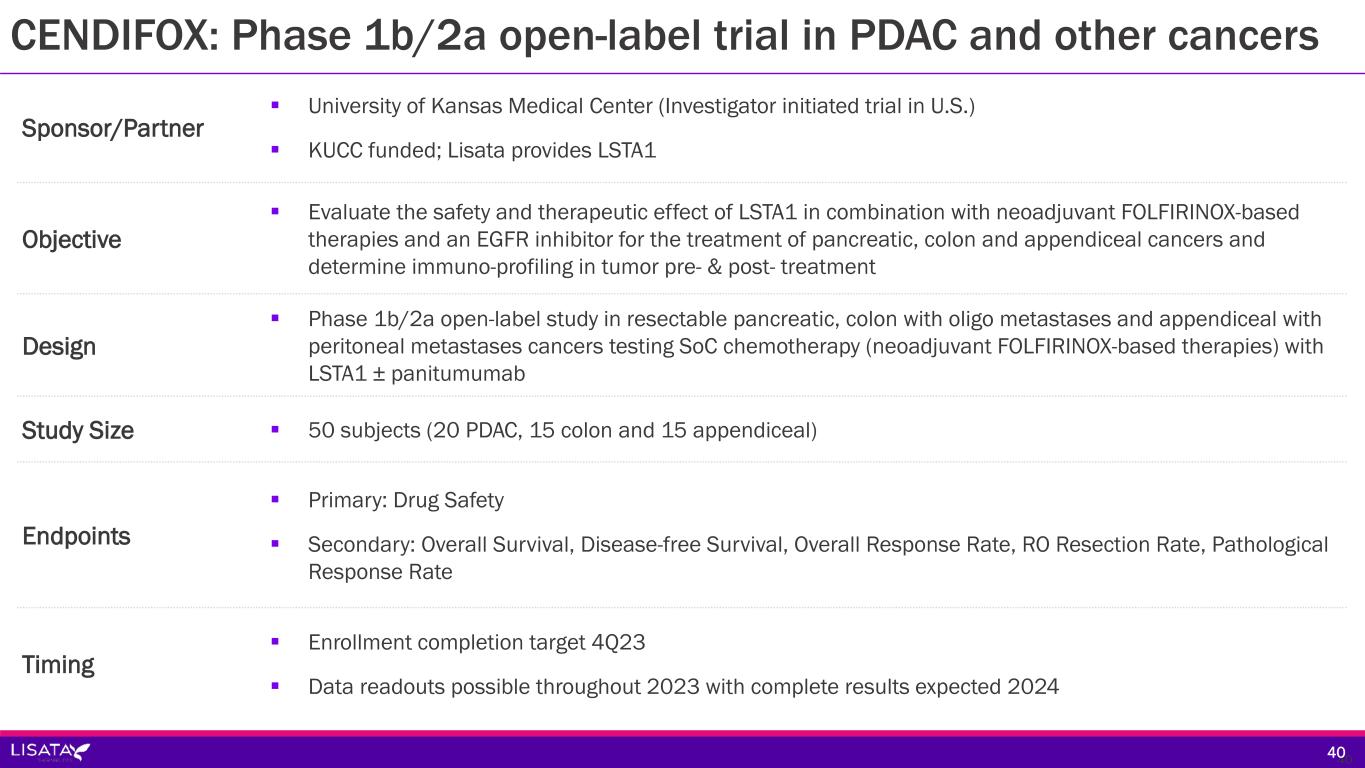
Sponsor/Partner University of Kansas Medical Center (Investigator initiated trial in U.S.) KUCC funded; Lisata provides LSTA1 Objective Evaluate the safety and therapeutic effect of LSTA1 in combination with neoadjuvant FOLFIRINOX-based therapies and an EGFR inhibitor for the treatment of pancreatic, colon and appendiceal cancers and determine immuno-profiling in tumor pre- & post- treatment Design Phase 1b/2a open-label study in resectable pancreatic, colon with oligo metastases and appendiceal with peritoneal metastases cancers testing SoC chemotherapy (neoadjuvant FOLFIRINOX-based therapies) with LSTA1 ± panitumumab Study Size 50 subjects (20 PDAC, 15 colon and 15 appendiceal) Endpoints Primary: Drug Safety Secondary: Overall Survival, Disease-free Survival, Overall Response Rate, RO Resection Rate, Pathological Response Rate Timing Enrollment completion target 4Q23 Data readouts possible throughout 2023 with complete results expected 2024 4040 CENDIFOX: Phase 1b/2a open-label trial in PDAC and other cancers

Surgery COHORT 1 Resectable and borderline resectable PDAC Key Objectives: • Pathological response • Immune response pre- & post- treatment • PFS, OS FOLFIRINOX X 3 Cycles (± Panitumumab if RAS/BRAF wildtype - Cohorts 2, 3) Tissue immune profiling Biopsy if archival tissue not available COHORT 2 Colon and appendiceal cancer with peritoneal mets COHORT 3 Colon cancer with oligo metastatic disease Repeat Biopsy ~72 hours after C3D1 tx Tissue immune profiling FOLFIRINOX (± Panitumumab if RAS/BRAF wildtype - Cohorts 2, 3) + LSTA1 X 3, 6, or 9 Cycles Resume Standard of Care Phase 1b/2a open-label trial of LSTA1 in combination with neoadjuvant FOLFIRINOX based therapies in pancreatic, colon and appendiceal cancers (CENDIFOX) • Sponsor/Partner: University of Kansas Medical Center (ITT) • KUCC funded: Lisata provides LSTA1 • Timing: Enrollment completion target 4Q23; data readouts possible throughout 2023; complete results expected 2024 41 CENDIFOX: Phase 1b/2a open-label trial in PDAC and other cancers
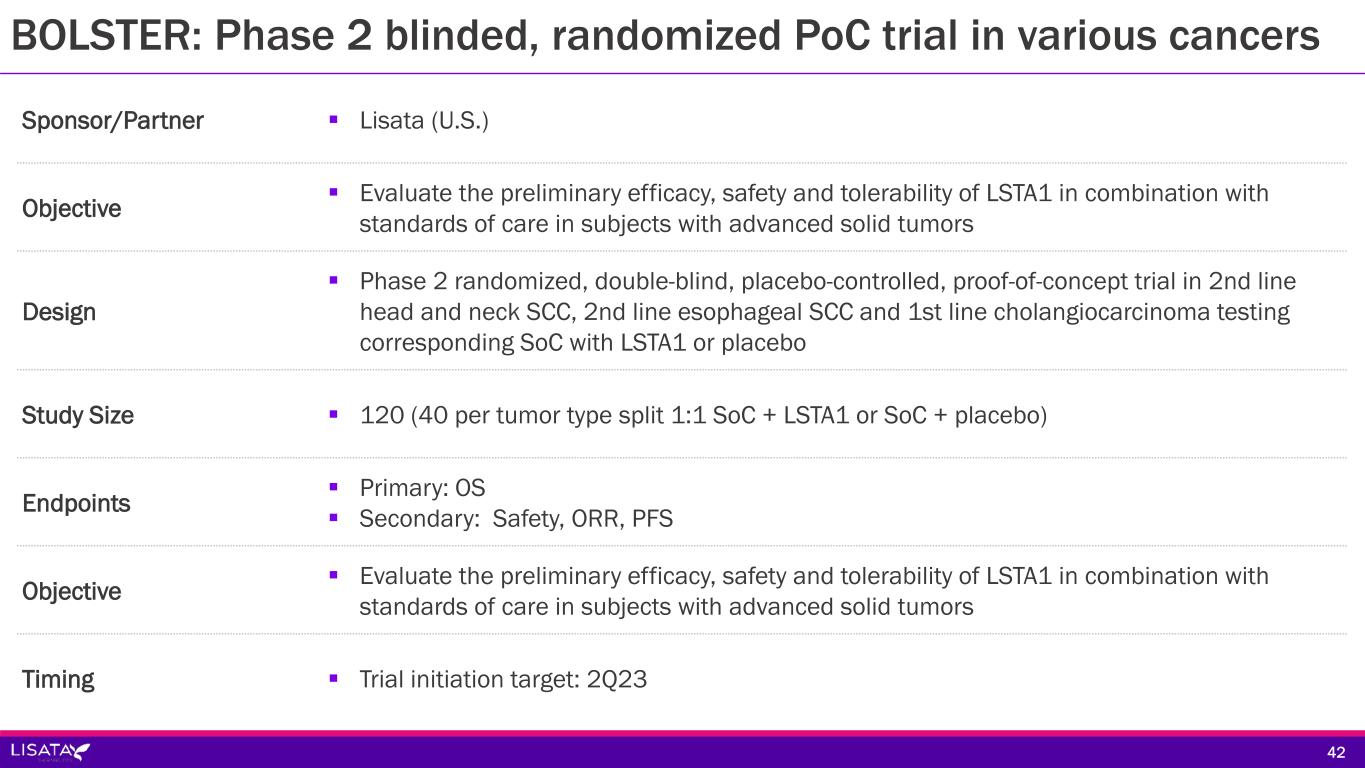
Sponsor/Partner Lisata (U.S.) Objective Evaluate the preliminary efficacy, safety and tolerability of LSTA1 in combination with standards of care in subjects with advanced solid tumors Design Phase 2 randomized, double-blind, placebo-controlled, proof-of-concept trial in 2nd line head and neck SCC, 2nd line esophageal SCC and 1st line cholangiocarcinoma testing corresponding SoC with LSTA1 or placebo Study Size 120 (40 per tumor type split 1:1 SoC + LSTA1 or SoC + placebo) Endpoints Primary: OS Secondary: Safety, ORR, PFS Objective Evaluate the preliminary efficacy, safety and tolerability of LSTA1 in combination with standards of care in subjects with advanced solid tumors Timing Trial initiation target: 2Q23 42 BOLSTER: Phase 2 blinded, randomized PoC trial in various cancers
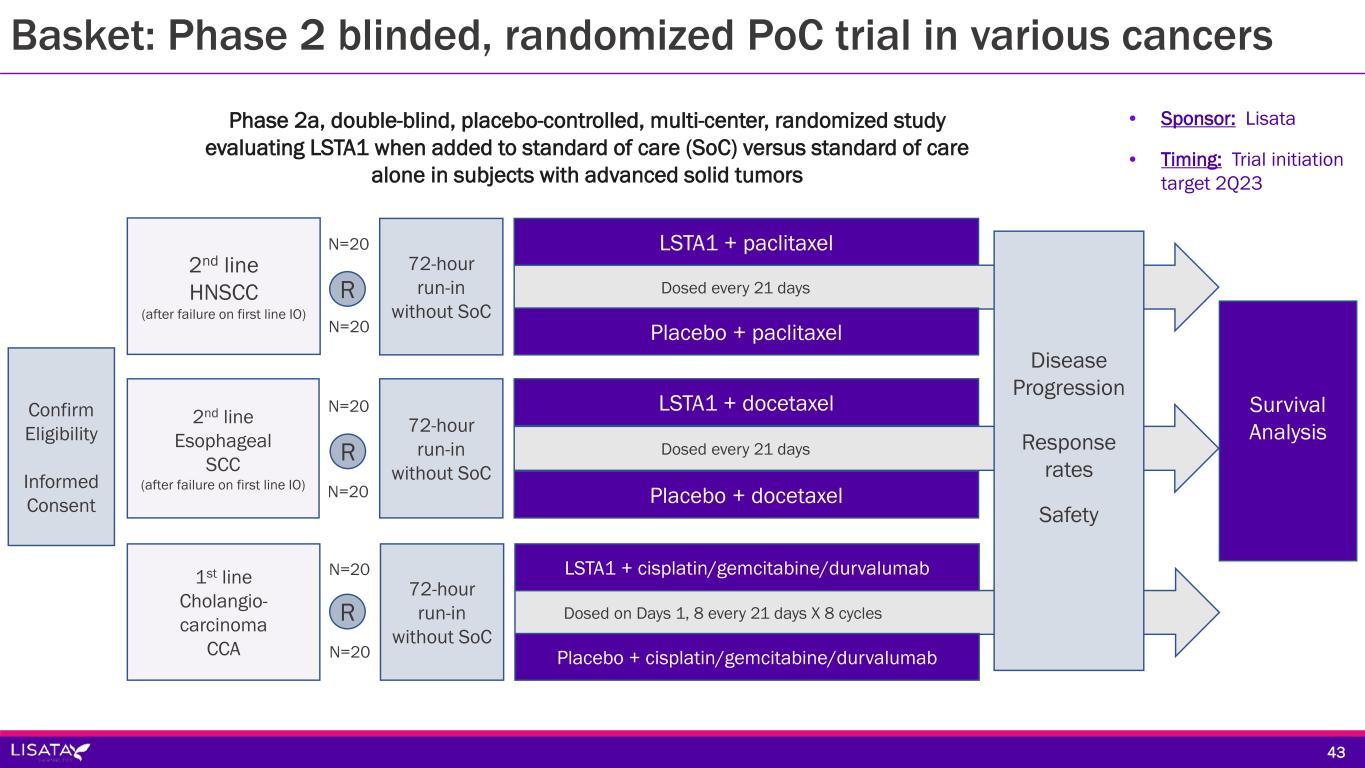
Dosed on Days 1, 8 every 21 days X 8 cycles Dosed every 21 days Dosed every 21 days Disease Progression Response rates Safety LSTA1 + paclitaxel Confirm Eligibility Informed Consent 2nd line Esophageal SCC (after failure on first line IO) Survival Analysis 72-hour run-in without SoC 72-hour run-in without SoC 2nd line HNSCC (after failure on first line IO) R Placebo + paclitaxel LSTA1 + docetaxel Placebo + docetaxel N=20 N=20 N=20 N=20 LSTA1 + cisplatin/gemcitabine/durvalumab 72-hour run-in without SoC 1st line Cholangio- carcinoma CCA Placebo + cisplatin/gemcitabine/durvalumab N=20 N=20 R R Phase 2a, double-blind, placebo-controlled, multi-center, randomized study evaluating LSTA1 when added to standard of care (SoC) versus standard of care alone in subjects with advanced solid tumors • Sponsor: Lisata • Timing: Trial initiation target 2Q23 43 Basket: Phase 2 blinded, randomized PoC trial in various cancers
Sponsor/Partner Qilu Pharmaceutical (funds all development in China) Objective Further evaluate safety and therapeutic efficacy of LSTA1 when added to SoC in Chinese patients with mPDAC Design Phase 2b, double-blind, placebo-controlled, randomized study evaluating LSTA1 + SoC (Qilu-produced nab-paclitaxel and gemcitabine) vs. placebo + SoC Study Size TBD Endpoints Objective response rate, progression free survival, overall survival Safety Timing Trial initiation target 1Q24 44 Phase 2 blinded, placebo-controlled trial in mPDAC in China
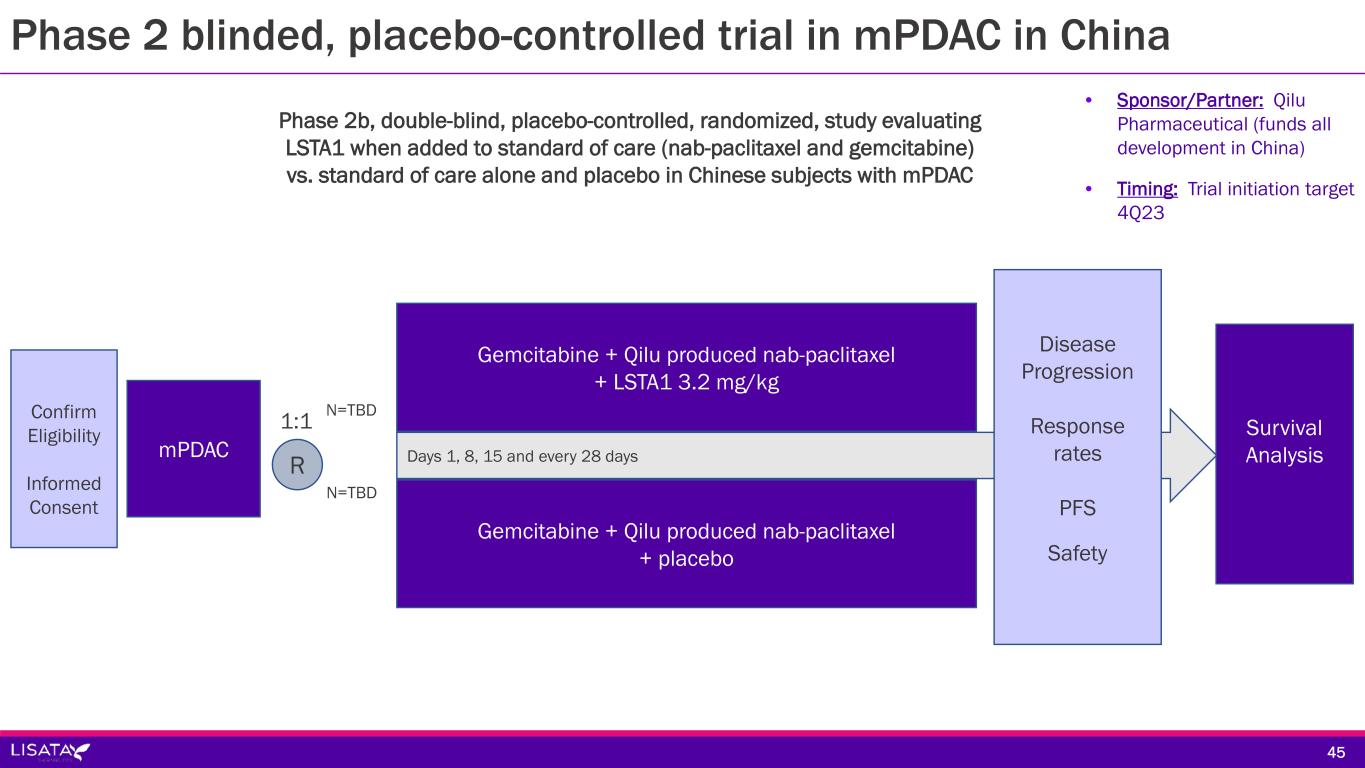
Days 1, 8, 15 and every 28 days Disease Progression Response rates PFS Safety Gemcitabine + Qilu produced nab-paclitaxel + LSTA1 3.2 mg/kg Confirm Eligibility Informed Consent 1:1 Survival AnalysismPDAC R Gemcitabine + Qilu produced nab-paclitaxel + placebo N=TBD N=TBD Phase 2b, double-blind, placebo-controlled, randomized, study evaluating LSTA1 when added to standard of care (nab-paclitaxel and gemcitabine) vs. standard of care alone and placebo in Chinese subjects with mPDAC • Sponsor/Partner: Qilu Pharmaceutical (funds all development in China) • Timing: Trial initiation target 4Q23 45 Phase 2 blinded, placebo-controlled trial in mPDAC in China

iLSTA: Phase 1b/2a trial in locally advanced PDAC with chemo & IO Sponsor/Partner WARPNINE, Inc. (registered charity in Australia) is funding trial Lisata providing study drug Objective Evaluate safety and therapeutic effect of LSTA1 in combination with IO & Chemo in locally advanced PDAC; determine if inoperable tumors can become operable Design Phase 1b/2a proof-of-concept safety and early efficacy study of LSTA1 in combination with durvalumab, gemcitabine and nab-paclitaxel, as first-line treatment in locally advanced pancreatic adenocarcinoma Study Size N=30 Endpoints Safety and tolerability; 28-day DLTs Objective response rate, PFS, OS, duration of response, immune cell infiltration Timing Trial initiation target 2Q23 46
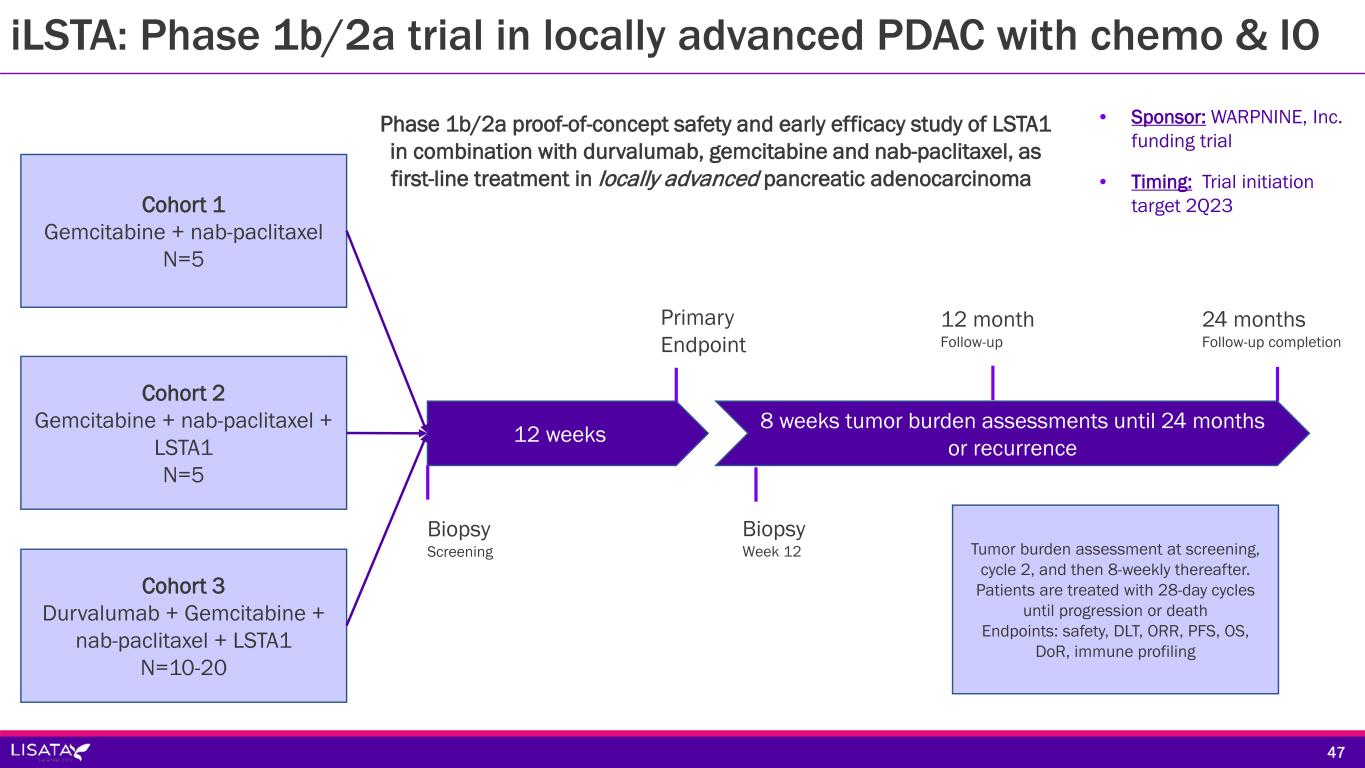
iLSTA: Phase 1b/2a trial in locally advanced PDAC with chemo & IO Cohort 2 Gemcitabine + nab-paclitaxel + LSTA1 N=5 Cohort 1 Gemcitabine + nab-paclitaxel N=5 Cohort 3 Durvalumab + Gemcitabine + nab-paclitaxel + LSTA1 N=10-20 12 weeks 8 weeks tumor burden assessments until 24 months or recurrence 12 month Follow-up Primary Endpoint 24 months Follow-up completion Biopsy Screening Biopsy Week 12 Tumor burden assessment at screening, cycle 2, and then 8-weekly thereafter. Patients are treated with 28-day cycles until progression or death Endpoints: safety, DLT, ORR, PFS, OS, DoR, immune profiling Phase 1b/2a proof-of-concept safety and early efficacy study of LSTA1 in combination with durvalumab, gemcitabine and nab-paclitaxel, as first-line treatment in locally advanced pancreatic adenocarcinoma • Sponsor: WARPNINE, Inc. funding trial • Timing: Trial initiation target 2Q23 47
Sponsor/Partner WARPNINE, Inc. (registered charity in Australia) is funding trial Lisata providing study drug Objective Evaluate safety and therapeutic effect of LSTA1 in combination with IO & Chemo in locally advanced GE AdenoCa; determine if inoperable tumors can become operable Design Phase 1b/2a proof-of-concept, safety and early efficacy study of LSTA1 in combination with nivolumab and FOLFIRINOX, as first-line treatment in locally advanced gastroesophageal adenocarcinoma Study Size N=30 Endpoints Safety and tolerability; 28-day DLTs Objective response rate, PFS, OS, duration of response, immune cell infiltration Timing Trial initiation target 3Q23 48 iGoLSTA: Phase 1b/2a trial in locally advanced GEAC with chemo & IO
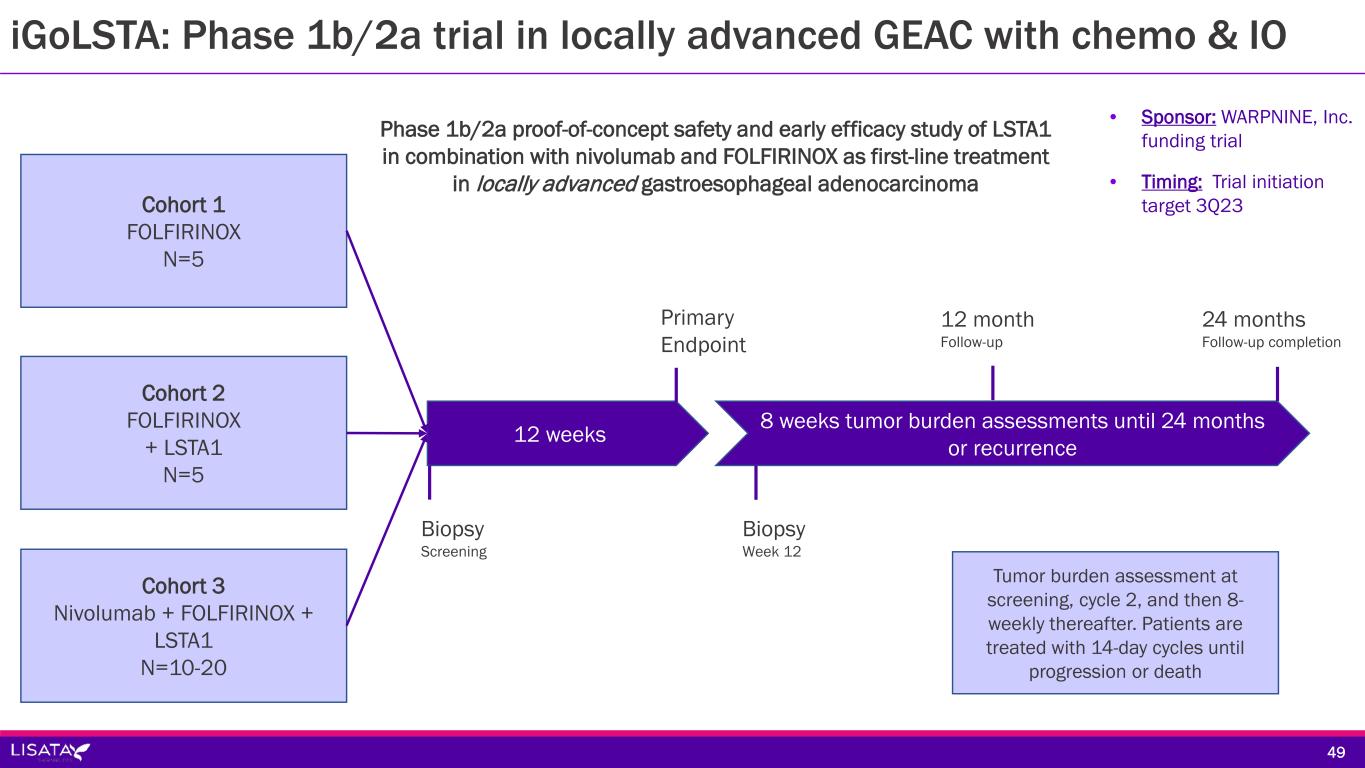
Cohort 2 FOLFIRINOX + LSTA1 N=5 Cohort 1 FOLFIRINOX N=5 Cohort 3 Nivolumab + FOLFIRINOX + LSTA1 N=10-20 12 weeks 8 weeks tumor burden assessments until 24 months or recurrence 12 month Follow-up Primary Endpoint 24 months Follow-up completion Biopsy Screening Biopsy Week 12 Tumor burden assessment at screening, cycle 2, and then 8- weekly thereafter. Patients are treated with 14-day cycles until progression or death Phase 1b/2a proof-of-concept safety and early efficacy study of LSTA1 in combination with nivolumab and FOLFIRINOX as first-line treatment in locally advanced gastroesophageal adenocarcinoma • Sponsor: WARPNINE, Inc. funding trial • Timing: Trial initiation target 3Q23 iGoLSTA: Phase 1b/2a trial in locally advanced GEAC with chemo & IO 49
Sponsor/Partner Tartu University Hospital (Investigator initiated trial in Estonia) Lisata providing study drug and funding trial Objective Evaluate safety, tolerability, and therapeutic effect of LSTA1 in combination with standard- of-care (temozolomide) in patients with previously untreated Glioblastoma Multiforme Design Phase 2a proof-of-concept, double-blind, placebo-controlled, randomized study evaluating LSTA1 when added to standard of care (temozolomide) versus SoC and placebo in subjects with newly diagnosed Glioblastoma Multiforme (GBM) Study Size N=40 Endpoints Safety, tolerability ORR, PFS, OS, disease control rate Timing Trial initiation target 3Q23 50 Phase 2a trial of LSTA1 with SOC in first-line GBM

Days 1, 2, 3, 4, 5 and every 28 days for 6 cycles Disease Progression Response rates Safety Temodar® + LSTA1Confirm Eligibility Informed Consent 1:1 Survival Analysis 72-hour Run-in without SoC Newly Diagnosed GBM R Temodar® + LSTA1 matching placebo N=20 N=20 Phase 2a proof-of-concept double-blind, placebo-controlled, randomized, proof-of- concept study evaluating LSTA1 when added to standard of care (temozolomide) versus temozolomide and matching LSTA1 placebo in subjects with newly diagnosed GBM • Sponsor: Tartu University Hospital; Estonia • Funding: Lisata • Timing: Trial initiation target 3Q23 Phase 2a trial of LSTA1 with SOC in first-line in GBM 51


















































