Seizing a World Class Opportunity
Company Updates
Safe Harbor Statement
The forward-looking statements made in this presentation and any subsequent
Q&A are subject to risks, uncertainties and certain assumptions. CHBP’s actual
results may differ materially from those currently anticipated due to a number of
risk factors, including, but not limited to, any comments relating to our financial
performance, the competitive nature of the marketplace, the condition of the
worldwide economy and other factors that have been or will be detailed in the
company’s form 8-K’s for 2003-2006 and subsequent 10KSB’s and 10QSB’s or
other documents filed with the Securities and Exchange Commission. For more
detailed information on the Company, please refer to the Company filings with
Securites and Exchange Commission, which are readily available at
http://www.sec.gov/cgi-bin/browse-edgar?action using the Company’s name or
ticker symbol.
1
Mission Statement
By 2010,
China Biopharmaceuticals Holdings, Inc. plans to be
among the top-tier of Chinese pharmaceutical companies
as measured by market share, profitability, new product
innovation and product quality
2
Investment Highlights
Focused on underpenetrated domestic Chinese market
Demonstrated track record of growing core base business
Accelerating revenue growth with expanding margins
Broad portfolio of currently marketed Rx and OTC products
Fully vertically-integrated R&D and GMP manufacturing capabilities
Multiple near-term new product launches
Strategic relationships with China Pharmaceutical University and China
New Drug Development Center
Industry consolidator with many opportunities for strategic acquisitions
3
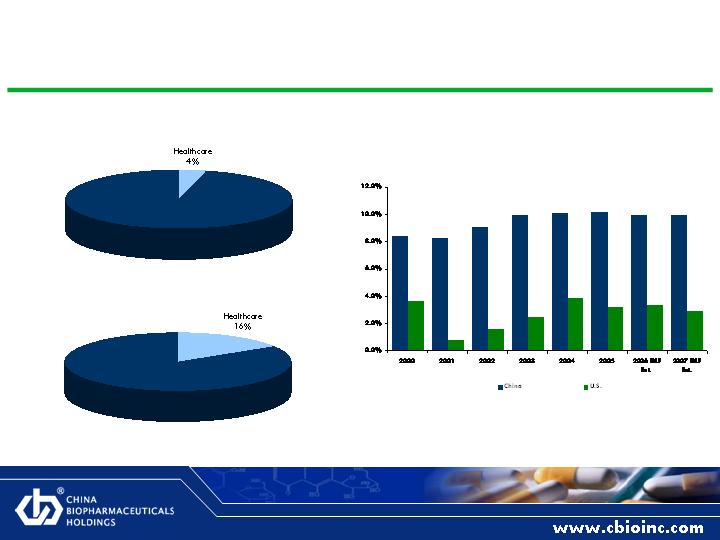
Chinese Pharmaceutical Industry: Poised for Growth
Healthcare as % of China GDP
China vs. U.S. GDP Growth
Healthcare as % of U.S. GDP
Source: The Wall Street Journal
4
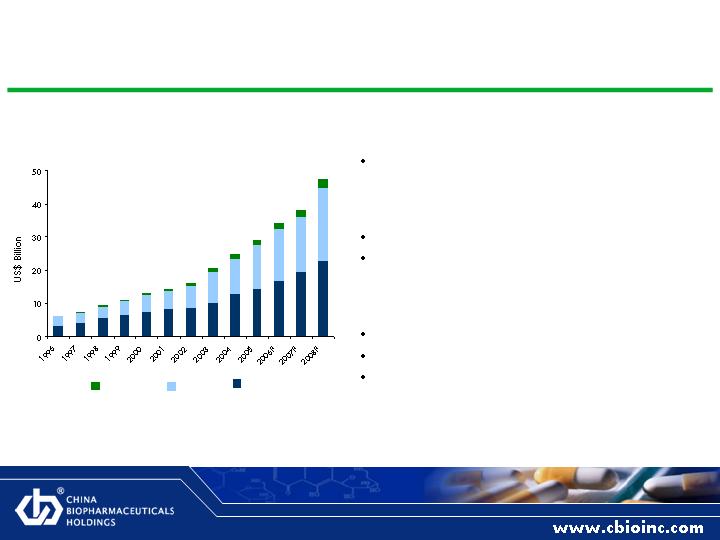
Chinese Pharmaceutical Market Overview
Market Size
2005 pharmaceutical end product sales at
wholesale: US$29.1 billion
Growth
18.6% annual growth rate over last decade
Expected to grow at 17.6% in the next 3
years
Market Segments
Western drugs: 46%
Traditional Chinese Medicine (TCM): 42%
Biological drugs: 12%
Source: China Pharmaceutical Yearbook, China Statistical Yearbook, IMS Health Webpage, China International Economy Consulting
Chinese Pharmaceutical Sales by Sector
Note: 1. Western drug refers to a drug that is produced through chemical synthesis or fermentation
2. Biological drug is produced through extraction from human or animal tissue or organ
TCM
Biological
Western
5
Chinese Pharmaceutical Market Drivers
Aging Population
Proportion of China’s aging population is increasing
Ages 65+ account for only 7.7% of population
Ages 65+ account for 35% of pharmaceutical expenditure
Retail Expansion
OTC channel of retail markets is growing at a 15% CAGR
Consumers have easier access to pharmaceutical products
Healthcare Reforms
Insurance reforms for urban areas
New cooperative healthcare plan for rural areas
Rising Living Standard
Urbanization
Increasing healthcare awareness
Pharmaceutical expenditures currently only 5.6% of disposable income
6
Key Ingredients for Success
Profitability: Focused on higher margin businesses
Growth: Organic growth enhanced through acquisitions
Distribution: Direct sales capability and distributor relationships
Manufacturing: Significant capacity to support growth
R&D: Numerous SFDA Approvals
Products: Rx and OTC; Western and TCM
Quality: Must meet new GMP standards
Status
Company Characteristic
7
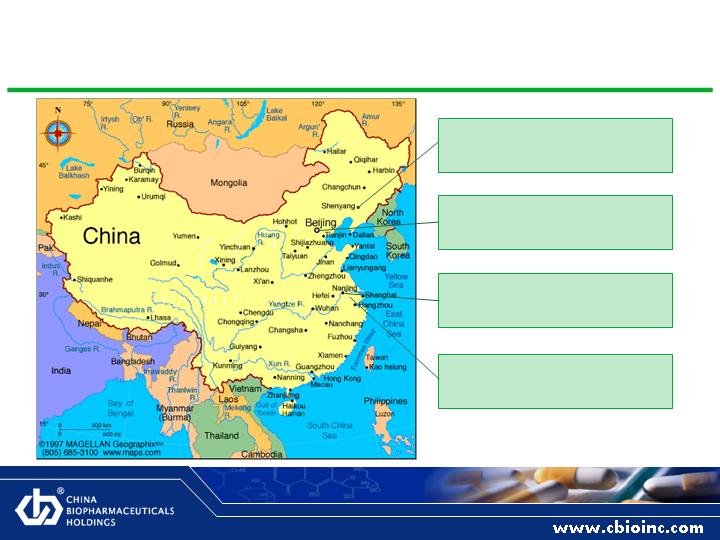
Strategic Footprint: High Growth Assets in Place
Nanjing Keyuan
R&D Facilities
Shenyang Enshi
TCM and Western Medicines
Beijing
Headquarters
Suzhou Erye
Antibiotic Focus
8
Operating Subsidiaries
CHBP
Shenyang Enshi Pharmaceutical Co. Ltd.
5 SFDA GMP lines
Over 120 prescription generic and TCM products
Suzhou Erye Pharmaceutical Co. Ltd.
7 SFDA GMP lines
Currently manufacturing 27 antibiotic drugs
Nanjing Keyuan Pharmaceutical R&D Co. Ltd.
Research and development
Average of 3-5 SFDA approvals annually
Successful history of licensing programs
9
Our Presence
Nanjing Keyuan
Shenyang Enshi
Suzhou Erye
Shenyang Enshi
10
Business Strategy
Accelerate growth and profitability of core business
Maximize R&D platform and research alliances
Expand footprint via select, accretive acquisitions
Lever capabilities throughout value chain, including manufacturing and
distribution
11
Accelerating Core Business Growth
Relaunch Enshi OTC brands: powerful new marketing mix
Hired top marketing team from Sanzhu Pharmaceuticals
Raise prices to be in-line with other leading Chinese brands
Target wealthier (top 25%) 300MM consumers on east coast of China
Use direct marketing to maximize sales of high margin products
Assign lower margin products to distributors
Drive growth in Erye prescription products
Multiple near-term product launches
12
Maximizing R&D Potential
Focus on new, high growth, high margin Rx drugs
Launch recently approved Rx drugs, 7 new products anticipated in 2007
Target 30 new product launches through 2010
Acquire new products with strong growth characteristics
Fully utilize development and regulatory relationships
Exclusive agreement with China Pharmaceutical University
Rapid drug approvals from SFDA based on experience and relationships
13
Multiple Near-Term Product Launches
2007
1.4 million
Disseminated Intravascular
Coagulation
Nafamostat (injection)
2007
22.0 million
Acute Heart Failure, Renal Function
Failure
Torasemide (injection)
2007
30.0 million
Allergic Rhinitis
Desloratadine (tablet)
2007
30.0 million
Diabetes
Gliclazide SR (tablet)
2007
100.0 million
Hepatitis B
Adefovir Dipivoxil (tablet)
2007
140.0 million
Digestive System Disease
Nizatidine (injection)
2007
165.0 million
Hypertension, Heart Failure
Torasemide (tablet)
Expected
Launch
Chinese Patient
Population1
Indication
Product
(1) Source: CHBP Estimates
14
Expanding Our Strategic Footprint
Act as an industry consolidator by applying disciplined approach
Proprietary access and negotiated transactions only
Attractive entry valuation and adjustment based on future performance
Accretive to product portfolio, sales channels or R&D capability
Strategic fit with existing development and operational strategy
Significant areas of potential operational and financial synergies
Rigorous due diligence process
Retain strong management with complementary industry expertise
15
Proven Acquisition Success
Purchases 100% of
Shenyang Enshi Pharmaceutical
Co., Ltd.
Purchases 51% of
SuzhouErye Pharmaceutical
Limited Company
Merges with 90% owner of
Nanjing Keyuan
Pharmaceutical R&D Co., Ltd.
Traditional Chinese
Medicine and Western
medicine manufacturing
capability
Antibiotic product portfolio
Leading R&D capability
June
2006
June
2005
August
2004
16
Optimizing the Value Chain
Increase penetration across China
Expand existing distributor relationships
Form alliances with new distributors in other provinces
Utilize own pharmacy channels to drive acceptance and usage of marketed
products
Drive manufacturing efficiency
Combine assets and reduce duplication in acquired entities
17
CHBP Competitive Advantages
Diversified product portfolio
260 SFDA approved drugs
Products in all segments: OTC, Patented, TCM and Western
~230 OTC drugs and new drugs under production
OTC portfolio represents ~25% of all OTC drugs sold through drug stores
Average gross margin ~40%, with significant room for expansion
Strong R&D platform
Nanjing Keyuan developed ~40 new drugs in 4 years
Represents 80% of all new drug applications in our province
~30 drugs to be introduced in next 5 years
Exclusive agreement with China Pharmaceutical University’s R&D
Laboratory provides additional projects and experts
Selective product out-licensing provides fee and royalty income
18
CHBP Competitive Advantages (Continued)
Vertically-integrated operation
Over 15 SFDA certified GMP lines
Sales and marketing expertise
Proprietary pharmacy network and distribution channel
Access to national distribution channels
Proven management team
Significant PRC sector experience
Demonstrated M&A track record
Management team with excellent OTC experience
19
Case Study:
Synergy of Nanjing Keyuan and Suzhou Erye
In June 2005 acquired 51% of Suzhou Erye by issuance of 3.3 million
shares of CHBP stock and injection of $2.2 million cash as working capital
into company
Core business is production of antibiotics and APIs
2006: Erye showed 13% sales growth and 213% net profit growth despite
2 rounds of price cuts by government
Driven by 2 new antibiotics developed by Keyuan R&D arm
2007: Erye to launch 2 new high margin Rx products
Anticoagulant and Hepatitis B antiviral
Both developed by Keyuan R&D arm
Result: Continued significant growth in both sales and net profit
20
Case Study:
New Enshi Management Team
Enshi acquired June 2006; 80% of its sales are OTC products
CHBP hired new management team to run Enshi
Previously the top management of Sanzhu Pharmaceuticals
Sanzhu generated US$1 billion in annual sales in China
Enshi plans to launch new national commercial campaign
Promote major OTC TCM cardiovascular and Hepatitis B drugs
Increase Enshi’s sales and build brand images
Result: Enshi anticipates significant growth in OTC drug sales in 2007
21
Management
Infogroup Investment Corporation
CFO
Floyd Huang
Shanghai Fuxing Pharmaceutical
SVP, Sales
Xiaohao Liu
China Pharmaceutical University, State
Food and Drug Administration
CTO
Luyong Zhang
Sandong Tungtai Pharmaceutical, China
Pharmaceutical University
President and COO;
CEO, Nanjing Keyuan
Lufan An
China Pharmaceuticals Investment Fund,
Infogroup Investment Corporation,
Shandong China Life S.T. Research Institute
Chairman and CEO
Chris Peng Mao
Experience
Title
Name
22
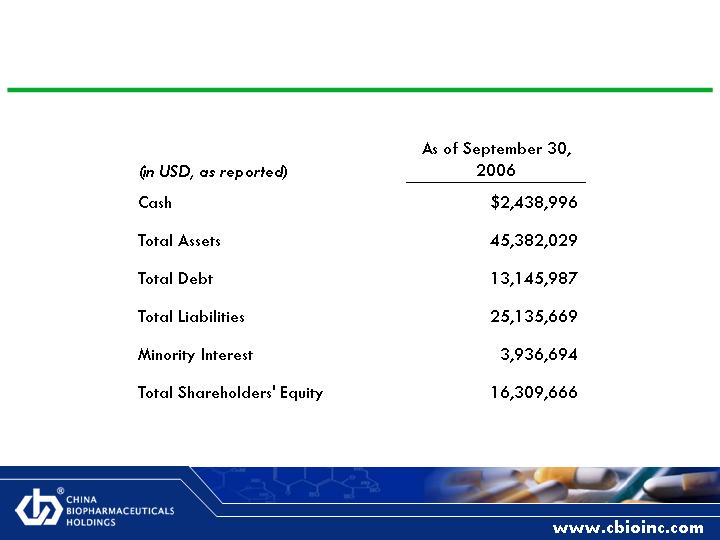
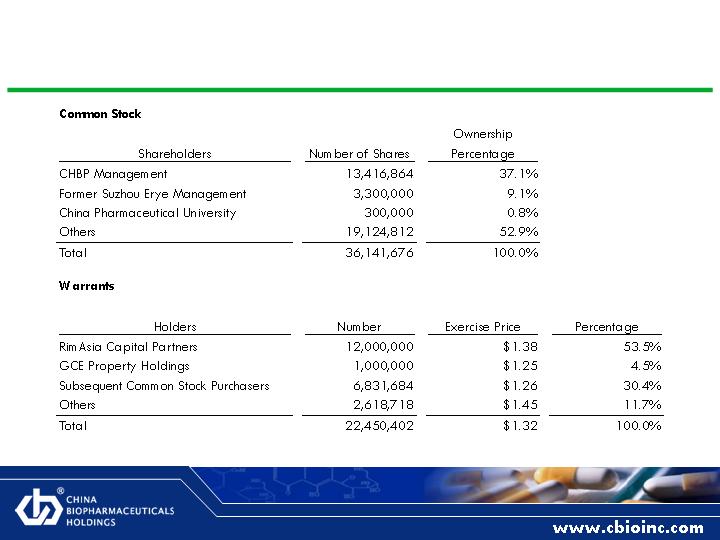
Financial Highlights
Significant revenue and income growth
Acquisition and organic
Cash flow positive
Scaleable model
Ability to make accretive acquisitions
23
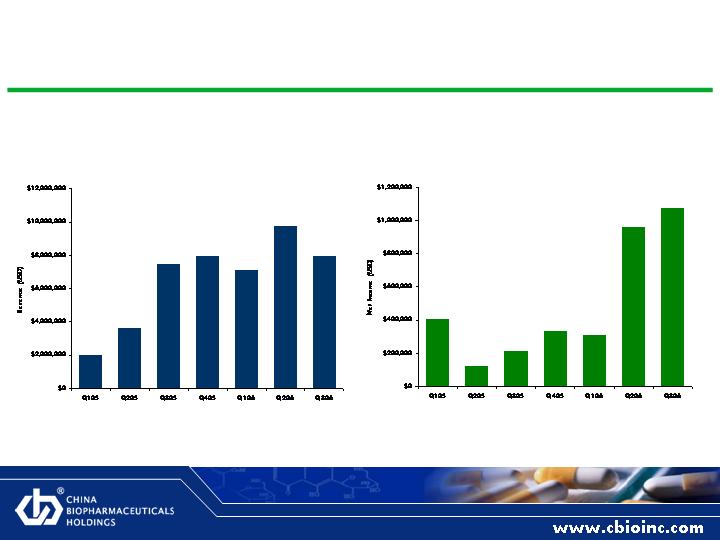
As Reported Revenue and Net Income Growth
Quarterly Revenue, As Reported
Quarterly Net Income, As Reported
24
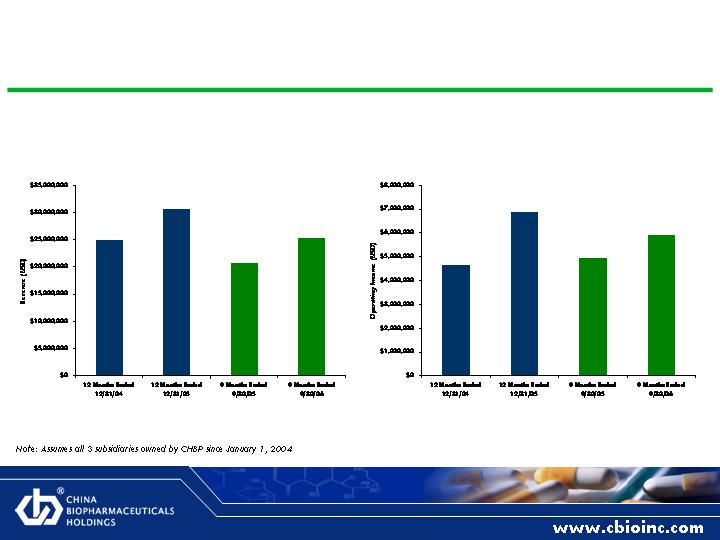
Organic Revenue and Operating Income Growth
Pro Forma Revenue
Pro Forma Operating Income
25
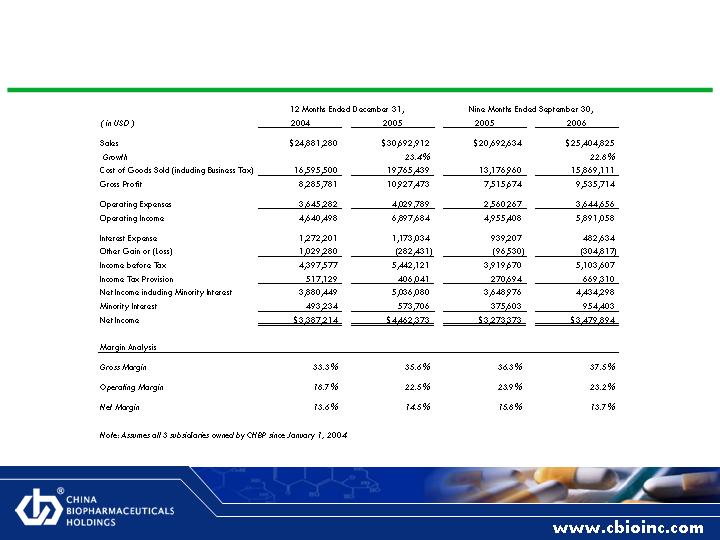
Pro-Forma Income Statement
26