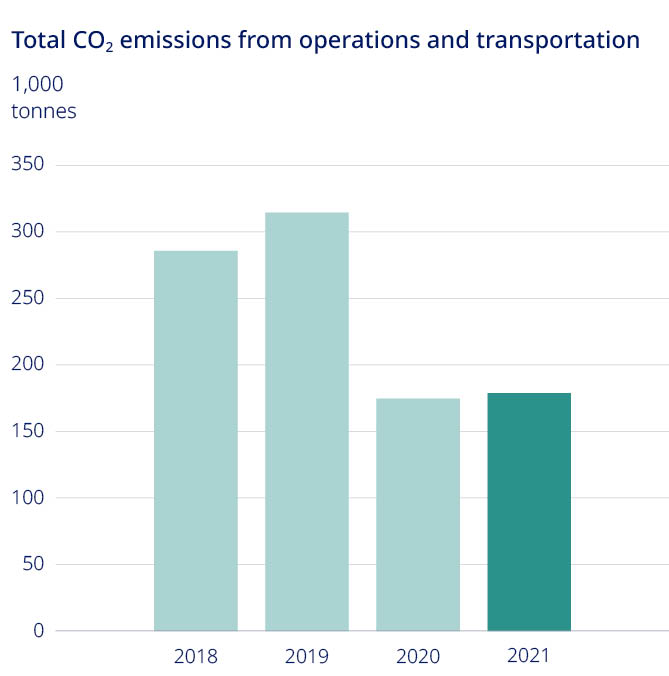
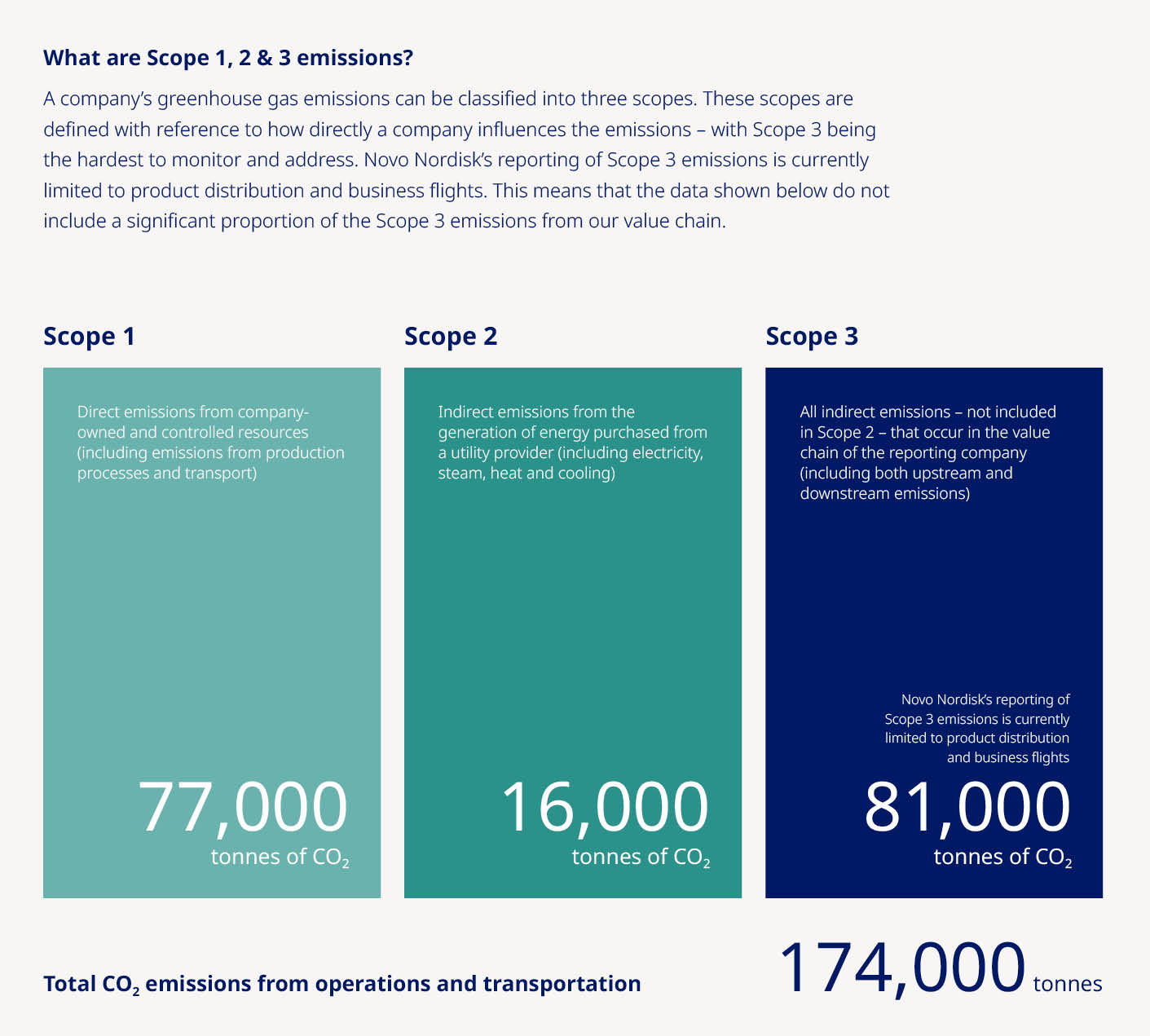
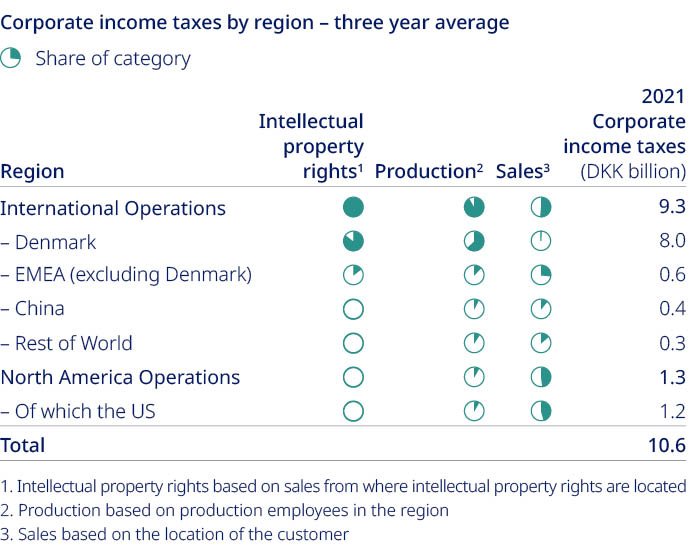
Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Content analysis
?| Positive | ||
| Negative | ||
| Uncertain | ||
| Constraining | ||
| Legalese | ||
| Litigous | ||
| Readability |
8th grade Avg
|
|
New words:
acid, adolescent, advice, alia, Alzheimer, Aurobindo, back, began, Beta, Boehringer, channel, CHMP, clarify, collaborative, comparison, counterpart, CSO, Deloitte, Department, Dicerna, direct, direction, disagreement, disclaimer, dismissed, DMCC, EMA, Endocrinology, endorsed, ESG, FDI, fermentation, forecasted, fourth, Germany, Glaxo, hand, Hospital, Hotline, Icosema, Ingelheim, interference, Lange, letter, longer, Mackay, mandatory, Martin, mathematical, methodology, MHRA, modestly, modified, newly, operationally, OSI, outpatient, overweight, overwhelming, Oxford, platform, Poulsen, preliminary, principle, procedure, proffered, Prosidion, purification, PwC, quantify, recovery, RESERVED, restore, reversal, reverse, ribonucleic, Rio, RNAi, rollout, rotation, royalty, satisfaction, Schindler, scope, screening, submission, Sun, Sweden, syringe, system, unbilled, uninsured, University, vaccination, valuation, Wegovy, whistleblowing, written, yearly
Removed:
affiliate, applied, Astra, billed, Brexit, CAD, carried, central, Chairmanship, CNY, creating, CV, delivered, DEVOTE, discontinued, EUR, exit, expert, GBP, hedge, Hewitt, hypoglycaemia, IDegLira, Inflation, interim, invest, JPY, Latin, lease, Liz, low, lower, modest, Nationalbank, notify, penetration, preference, recurring, showed, successful, TABULAR, withdrawal, writing, Zeneca
Filing tables
Filing exhibits
Associated NVO transcripts
NVO similar filings
Filing view
External links