UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| | |
| (Mark One) | | |
ý |
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2008 |
OR |
o |
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from N/A to N/A
|
Commission file number: 0-10961
QUIDEL CORPORATION
(Exact name of registrant as specified in its charter)
| | |
DELAWARE
(State or other jurisdiction of
incorporation or organization) | | 94-2573850
(I.R.S. Employer Identification No.) |
10165 McKellar Court
San Diego, California
(Address of principal executive offices) |
|
92121
(Zip Code) |
858-552-1100
(Registrant's telephone number, including area code)
Not Applicable
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:Common Stock,
$0.001 par value, and accompanying Preferred Shares Purchase Rights
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| | | | | | |
| Large accelerated filer o | | Accelerated filer ý | | Non-accelerated filer o
(Do not check if a smaller reporting company) | | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the registrant's common stock held by non-affiliates of the registrant was $479,352,634 based on the closing sale price at which the common stock was last sold, as of the last business day of the registrant's most recently completed second fiscal quarter.
As of February 25, 2009, 31,692,060 shares of the registrant's common stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
(To the Extent Indicated Herein)
Portions of the registrant's definitive proxy statement to be filed with the Securities and Exchange Commission in connection with the registrant's 2009 Annual Meeting of Stockholders (to be held on May 12, 2009) are incorporated by reference into Part III, Items 10, 11, 12, 13 and 14 of this Annual Report on Form 10-K.
A Warning About Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the federal securities laws that involve material risks, assumptions and uncertainties. Many possible events or factors could affect our future financial results and performance, such that our actual results and performance may differ materially from those currently expected. As such no forward-looking statement can be guaranteed. Differences in actual results and performance may arise as a result of a number of factors including, without limitation, seasonality, the timing of onset, length and severity of cold and flu seasons, the level of success in executing our strategic initiatives, uncertainty surrounding the detection of novel influenza viruses involving human specimens, adverse changes in the competitive and economic conditions in domestic and international markets, actions of our major distributors and the level of success in our recent distributor incentive programs, technological changes and uncertainty with research and technology development, including any future molecular-based technology, the reimbursement system currently in place and future changes to that system, manufacturing and production delays or difficulties, adverse actions or delays in product reviews by the U.S. Food and Drug Administration (the "FDA"), intellectual property, product liability, environmental or other litigation, required patent license fee payments not currently reflected in our costs, potential inadequacy of booked reserves and possible impairment of goodwill, and lower than anticipated sales or market penetration of our new products. Forward-looking statements typically are identified by the use of terms such as "may," "will," "should," "might," "expect," "anticipate," "estimate," and similar words, although some forward-looking statements are expressed differently. The risks described under "Risk Factors" in Item 1A of this Annual Report and elsewhere herein and in reports and registration statements that we file with the Securities and Exchange Commission (the "SEC") from time to time, should be carefully considered. You are cautioned not to place undue reliance on these forward-looking statements, which reflect management's analysis only as of the date of this Annual Report. The following should be read in conjunction with the audited Consolidated Financial Statements and Notes thereto beginning on page F-1 of this Annual Report. We undertake no obligation to publicly release the results of any revision or update of these forward-looking statements, except as required by law.
Part I
Item 1. Business
All references to "we," "our," and "us" in this Annual Report refer to Quidel Corporation and its subsidiaries.
Overview
We commenced our operations in 1979 and launched our first products, dipstick-based pregnancy tests, in 1984. Our product base and technology platforms have expanded through internal development and acquisitions of other products and technologies. We have a leadership position in the development, manufacturing and marketing of rapid diagnostic solutions for decentralized applications including professional point-of-care ("POC") in infectious diseases and reproductive and women's health. We focus on POC testing solutions specifically developed for the physician office lab ("POL") and acute care markets globally. We sell our products to professionals for use in physician offices, hospitals, clinical laboratories, retail clinics and wellness screening centers. Our POC testing solutions are designed to provide specialized results that meet two important value criteria that we have branded as Quidel Value Build™ ("QVB™"):
- •
- Clinical validation: the enabling of rapid patient management decisions leading to improved treatment and outcomes.
- •
- Economic validation: the reduction of overall costs associated with patient testing with emphasis upon critical reimbursement and payer performance criteria.
2
We market our POC products in the U.S. through a network of national and regional distributors, supported by a direct sales force. Internationally, we sell and market primarily in Japan, Europe and the Middle East through exclusive distributor arrangements.
In addition to our rapid, POC diagnostic business, we also develop research products through our Specialty Products Group (the "SPG"), with an emphasis on potential future rapid test applications. The SPG is currently responsible for more than 100 of our clinical and research products used worldwide in reference laboratories and in research applications at leading universities and biotechnology companies. The SPG revenues, income and assets are less than 10% of our overall operations.
In January 2009, we announced that Caren L. Mason, our President and Chief Executive Officer, has decided to retire from the Company on June 1, 2009. Ms. Mason will continue in her current capacity as President and CEO as well as a Board Director until March 1, 2009, and from March 1, 2009 through her retirement date, Ms. Mason will serve as a special advisor to the CEO. In January 2009, we also announced the appointment of Douglas C. Bryant as the Company's new President, CEO and member of the Board of Directors. Mr. Bryant began his employment in February 2009 and his role as President and CEO is effective March 1, 2009.
We are a corporation, incorporated in the State of Delaware. Our executive offices are located at 10165 McKellar Court, San Diego, California 92121, and our telephone number is (858) 552-1100. This Annual Report and each of our other periodic and current reports, including any amendments thereto, are available, free of charge, on our website,www.quidel.com, as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC. The information contained on our website is not incorporated by reference into this Annual Report and should not be considered part of this Annual Report. In addition, the SEC website contains reports, proxy and information statements, and other information about us atwww.sec.gov.
Business Strategy
We believe that the trend among healthcare providers to adopt POC testing continues to increase, and demographic changes, reimbursement policies, a shortage of skilled laboratory workers and the availability of clinically valuable tests will increase growth in this diagnostic category. More and more employers, health plans and payers are recognizing that POC testing is a cost-effective means for improving the quality of care and patient satisfaction. Continuous improvements in technologies are resulting in a growing number of new diagnostic tests that combine high levels of accuracy with rapid, easy-to-use product formats. It is our mission to further expand our leadership position in POC rapid diagnostics. In order to accomplish this mission, our strategy is to:
- •
- provide clinicians with validated, evidence-based studies which encompass the clinical efficacy and economic efficiency of our rapid POC tests for the professional market. In conjunction with our QVB™ commitment, we expect to present ongoing information that supports the adoption of rapid POC testing;
- •
- continue to focus on strengthening our market and brand leadership in infectious diseases and reproductive and women's health by acquiring, developing and introducing clinically superior diagnostic solutions;
- •
- drive growth by securing dedicated distributor partnerships and strengthening our sales organization to assure physician and laboratorian satisfaction through direct relationships with Integrated Delivery Networks and hospitals;
- •
- support payer evaluation of rapid tests and establishment of favorable reimbursement rates;
- •
- continue creation of strong global alliances to assure leadership in key markets;
3
- •
- drive profit through further refinement of our manufacturing efficiencies and productivity improvements, with continued focus on profitable products and markets and our effort to create exceptional competency in new product development;
- •
- continue to focus our research and development efforts on three areas: 1) new proprietary product platform development, 2) the creation of improved products and new products for existing markets and unmet clinical needs, and 3) products developed under collaborations with other companies for new and existing markets; and
- •
- identify and commercialize new markers, products and collaborations in bone health through the SPG.
Diagnostic Test Kit Industry Overview
The Overall Market forIn Vitro Diagnostics
The worldwide market forin vitro diagnostic, or IVD, products was estimated at approximately $38.4 billion in 2007, and is segmented by the particular test discipline. The largest market segments are immunodiagnostics testing and instrument-based clinical chemistry, which account for approximately 31% and 21% of the total IVD market, respectively. Geographically, in 2006, approximately 40% of total IVD revenues were generated in the U.S., while Europe and Asia accounted for approximately 37% and 18%, respectively.
Customers for IVD products are primarily large centralized laboratories, independent reference laboratories or hospital-based facilities. In the U.S., these central laboratories account for approximately 75% of the revenues generated by IVD products.
The centralized diagnostic testing process typically involves obtaining a specimen of blood, urine or other sample from the patient and sending the sample from the healthcare provider's office or hospital unit to a central laboratory. In a typical visit to the physician's office, after the patient's test specimen is collected, the patient is usually sent home and receives the results of the test several hours or days later. The result of this process is that the patient may leave the physician's office without confirmation of the diagnosis and the opportunity to begin more effective immediate care.
Hospitals in the U.S. have progressively sought to reduce the length of patient stays and, consequently, the proportion of cases seen as outpatients has increased. If the U.S. experience is representative of future trends, emergency departments and other critical care units such as intensive care units, operating rooms, trauma and cardiac centers are increasingly becoming the principal centers for the management of moderate and severe acute illness. In the U.S., there are between 110 and 120 million emergency room visits annually.
The broader IVD testing market is growing at 9%, while the professional POC segment is growing more than 11%. The molecular diagnostics segment, representing a smaller portion of IVD, is growing at an estimated 17%. Within both of those categories, infectious diseases represent a significant portion of testing.
The Professional POC Market
POC testing for certain diseases has become an accepted adjunct to central laboratory and self-testing. The professional POC market is comprised of two general segments: decentralized testing in non-institutional settings such as physicians' offices and hospital testing (emergency rooms and bedside). Hospital POC testing is accepted and growing and is generally an extension of the hospital's central laboratory.
Out-of-hospital testing sites consist of physicians' office laboratories, nursing homes, pharmacies, retail clinics and other non-institutional, ambulatory settings in which healthcare providers perform
4
diagnostic tests. This decentralized POC market encompasses a large variety of IVD products ranging from moderate-sized instrumented diagnostic systems serving larger group practices to single-use, disposable tests for smaller practice physicians' offices. We believe POC testing out-of-hospital is increasing due to its clinical benefit, cost-effectiveness and patient satisfaction.
Total revenues from the rapid, non-instrument-based professional POC market were estimated at approximately $4.3 billion world-wide. The growth in POC testing is in part due to evolving technological improvements creating high quality tests with laboratory accuracy and POC ease-of-use, which are capable of being granted a waiver under the Clinical Laboratory Improvement Amendments of 1988 ("CLIA"). A CLIA-waived test is defined as a simple laboratory test which employs methodologies that are so simple and accurate as to render the likelihood of erroneous results negligible and/or pose no reasonable risk of harm to the patient if the test is performed incorrectly. CLIA-waived tests may be used in physician office laboratories, as well as acute care, urgent care and hospital facilities. In 2006, an estimated 92,000, or 79%, of physician office laboratories had a CLIA waiver.
Products
We provide rapid POC and other diagnostic tests under the following brand names: QuickVue®, QuickVue+®, QuickVue Advance®, Metra®, Quidel® and MicroVue™. Our rapid POC diagnostic tests and our diagnostic and research markers participate in the following medical and wellness categories:
Influenza. Our influenza tests are rapid, qualitative tests for the detection of the viral antigens of influenza type A and B, the two most common types of the influenza virus. Our first influenza test received FDA clearance in September 1999, with commercialization beginning in December 1999. The FDA granted us the first CLIA waiver for an influenza test in October 2000. Our second generation test, the QuickVue® Influenza A+B test, which allows for the differential diagnosis of influenza type A and type B, received FDA clearance in September 2003 and a CLIA waiver in February 2004. In December 2005, we announced FDA clearance for several new claims for our QuickVue® Influenza A+B test, including 94% sensitivity for detecting type A influenza with nasal swabs versus culture and 90% specificity.
Group A Strep. Each year millions of people in the U.S. are tested for Group A Strep infections, commonly referred to as "strep throat." Group A Streptococci are bacteria that typically cause illnesses such as tonsillitis and pharyngitis which, if left untreated, can progress to secondary complications. Our initial Strep A test, the QuickVue In-line® Strep A test, was the first rapid Strep A test to be granted a CLIA waiver, and we launched additional product offerings with the QuickVue®+ Strep A and the QuickVue® Dipstick Strep A tests in 1996 and 2001, respectively. Our QuickVue® Strep A tests are intended for the rapid, qualitative detection of Group A Streptococcal antigen from throat swabs or confirmation of presumptive Group A Streptococcal colonies recovered from culture. The tests are to be used to aid in the diagnosis of Group A Streptococcal infection.
RSV Test. Our QuickVue® RSV test is a rapid immunoassay for Respiratory Syncitial Virus ("RSV"). The majority of upper respiratory tract infections in children are caused by viruses and RSV is generally recognized as a frequent agent responsible for these infections. We launched our RSV test during the fourth quarter of 2006, and we received CLIA waiver in February 2008.
Pregnancy. Our QuickVue® pregnancy tests are used in both the physicians' office lab and the acute care settings. The early detection of pregnancy enables the physician and patient to institute proper care, helping to promote the health of both the woman and the developing embryo. Our
5
QuickVue® pregnancy tests are sensitive immunoassay tests for the qualitative detection of human Chorionic Gonoadotropin ("hCG") in serum or urine for the early detection of pregnancy.
Chlamydia. Chlamydia trachomatis is responsible for the most widespread sexually transmitted disease in the U.S. Over one-half of infected women do not have symptoms and, if left untreated,Chlamydia trachomatis can cause sterility. Our QuickVue® Chlamydia test is a lateral flow immunoassay for the rapid, qualitative detection of Chlamydia from endocervical swab and cytology brush specimens. The test is intended for use as an aid in the presumptive diagnosis of Chlamydia.
Bacterial Vaginosis. Each year millions of women seek treatment of genital infections generally known as infectious vaginitis. One of the most common forms of infectious vaginitis is bacterial vaginosis ("BV"), a condition which, if left untreated, can lead to serious clinical complications, including pre-term births, pelvic inflammatory disease, infections following gynecological surgeries and an increased risk of contracting HIV. Our QuickVue® Advance G. Vaginalis test, launched in July 2002, is an enzyme activity test for use in the detection ofGardnerella vaginalis Proline IminoPeptidase ("PIP") activity in vaginal fluid specimens from patients suspected of having bacterial vaginosis.
Bone Health. Osteoporosis is a systemic skeletal disease characterized by low bone mass and deterioration of the microarchitecture of bone tissue, with a consequent increase in bone fragility and susceptibility to fractures. The risk for fracture increases exponentially with age. A key set of parameters in the monitoring of osteoporosis, both before and after therapy, are biochemical markers of bone metabolism. As a leader in the field of bone markers, we produce both clinical and research products for the assessment of osteoporosis and the evaluation of bone resorbtion/formation, which, including our metabolic bone markers, are used by physicians to monitor the effectiveness of therapy in pharmaceutical and related research.
Immunoassay fecal occult blood ("iFOB"). Our QuickVue® iFOB test is a rapid, fecal immunochemical test ("FIT") intended to detect the presence of blood in stool specimens. Blood in the stool is an indication of a number of gastrointestinal disorders, including colorectal cancer. We launched our iFOB test in late December 2005.
Helicobacter pylori ("H. pylori"). H. pylori is the bacterium associated with approximately 80% of patients diagnosed with peptic ulcers in the U.S.H. pylori is implicated in chronic gastritis and is recognized by the World Health Organization as a Class 1 carcinogen that may increase a person's risk of developing stomach cancer. Once anH. pylori infection is detected, antibiotic therapy is administered to eradicate the organism and effect a cure of the ulcer. Our rapid test is a serological test that measures antibodies circulating in the blood caused by the immune response to theH. pylori bacterium. Our initial test was the first rapid H. pylori test to be granted a CLIA waiver. We launched our second-generation CLIA-waived test, the QuickVue® H. Pylori gII™ test, in August 2000.
We have other products which include veterinary products as well as clinical laboratory and research tests used in the measurement of circulating immune complexes, complement deficiencies and complement activation.
Seasonality
Sales of our infectious disease products are subject to, and significantly affected by, the seasonal demands of the cold and flu seasons, prevalent during the fall and winter. As a result of these seasonal demands, we typically experience lower sales volume in the second quarter of the calendar year, and have higher sales in the first, third and fourth quarters of the calendar year. For the years ended December 31, 2008, 2007 and 2006, total revenue in the first, third and fourth quarters have combined for 83%, 84% and 84%, respectively. Historically, sales of our infectious disease products have varied
6
from year to year based in large part on the severity, length and timing of the onset of the cold and flu season. For the years ended December 31, 2008, 2007 and 2006, sales of our infectious disease products accounted for 72%, 64% and 65%, respectively, of total revenue. Sales of our products vary from year to year and quarter to quarter, and can be influenced significantly if distributors attempt to time the onset of an early cold and flu season, or if they initiate larger orders in anticipation of a more severe cold and flu season. Our influenza products have a two-year shelf life, which may also lead a distributor to initiate its purchases earlier in the flu season. While we believe that the severity, length and timing of the onset of the cold and flu season will continue to impact sales of our infectious disease products, there can be no assurance that our future sales of these products will necessarily follow historical patterns.
Research and Development
We continue to focus our research and development efforts on three areas: 1) new proprietary product platform development, 2) the creation of improved products and new products for existing markets and unmet clinical needs, and 3) products developed under collaborations with other companies for new and existing markets. Our immunoassay development program is evaluating a variety of leading technology and product licensing opportunities.
The SPG targets markers with potential downstream POC application in selected disease states. Several candidate tests have been developed on microwell platforms and are currently marketed and sold to clinicians and researchers. The SPG is strategically focused on developing clinical proof around these markers and demonstrating their utility in a variety of pathologies. We currently market and sell these products both directly and through select distributors throughout the world under the Metra®, Quidel® and MicroVue™ brands.
Research and development expenses were approximately $11.1 million, $12.9 million and $13.0 million for the years ended December 31, 2008, 2007 and 2006, respectively. We anticipate that we will continue to devote a significant amount of financial resources to product and technology research and development for the foreseeable future.
Marketing and Distribution
We focus on ensuring market leadership and providing points of differentiation by specializing in the diagnosis and monitoring of selected disease states. In order to support our value proposition as a company that markets the highest quality products in support of better medical outcomes, we are highlighting our QVB™ efforts through the development of new innovations and the communication of new solutions in the field of rapid diagnostic testing. Our QVB™ program includes significant work in understanding the needs of the end-use customer, building products that meet those needs, providing proof studies to validate rapid diagnostic testing at the point-of-care, and leveraging the work of researchers and key opinion leaders studying our tests and technology to help enhance the health and well being of people around the globe. Our marketing strategy includes ensuring that our key product portfolios are supported by economic and clinical validation that shows hospitals, laboratories, acute care facilities and POC clinicians that these tests deliver high quality results in a cost-effective manner.
In contrast to the central laboratory market, the U.S. POC market is highly fragmented, with many small or medium-sized customers. We have designed our business strategy around serving the needs of this market segment. To reach these customers, a network of national and regional distributors is utilized and supported by our sales force. We have developed priority status with several of the major distributors in the U.S., resulting in many of our products being the preferred products offered by these distributors.
Internationally, the use of professional rapid POC diagnostic tests, the acceptance of testing outside the central laboratory, the regulatory requirements to sell POC tests and consumer interest in
7
over-the-counter and self-test products, differ considerably from the U.S. Our international sales are significantly lower than domestic sales, largely due to the POC market being more developed in the U.S. relative to the overall IVD market in other countries.
During 2008, we continued to invest in several key areas: further validation of customer needs through voice of customer studies ("VOC"), expanding clinical research as part of our QVB™ program and expanding our communications through extensive advertising, direct mail, promotional campaigns and public relations. Our VOC emphasis enables us to better understand the customer's needs and requirements in both domestic and international markets in order to focus our product marketing and distribution partner plans. For example, annual post-season flu market research allows us to measure the success of our messaging to drive adoption as well as identify new product requirements for future application to the product line.
The essential aspect of QVB™ is building awareness about our products and their performance through the clinical validation value criterion. Among others, we sponsored a presentation on influenza testing at the annual meeting of the Society for Medical Decision Making (Philadelphia, PA), and we presented results of our internal research on fecal occult blood testing at the Colorectal Congress (St. Gallen, Switzerland). In February 2009, six other posters or oral presentations were made that are based on work sponsored in 2007 and 2008. These were presented at two important international meetings; the XI International Symposium on Respiratory Viral Infections and the 8th Asia Pacific Congress of Medical Virology.
We derive a significant portion of our total revenue from a relatively small number of distributors. Five of our distributors, which are considered to be among the market leaders, collectively accounted for approximately 60%, 61% and 68% of our total revenue for the years ended December 31, 2008, 2007 and 2006, respectively. Even though our distributor mix will likely change from period to period in the future, Cardinal Healthcare Corporation ("Cardinal"), DS Pharma Biomedical Co., Ltd ("DS Pharma") (formerly known as Sumitomo Seiyaku Biomedical Co., Ltd.), McKesson Corporation ("McKesson"), National Distribution Corporation ("NDC") and Physician Sales and Services Corporation ("PSS") have historically accounted for a significant portion of our total revenue. Our sales are affected by fluctuations in the buying patterns of these distributors and the corresponding changes in inventory levels maintained by them. Inventory levels held by these distributors may fluctuate significantly from quarter to quarter. If total revenue to our significant distributors were to decrease in any material amount in the future, our business, operating results and financial condition could be materially adversely affected.
See Note 7. "Industry and Geographic Information" in the Notes to Consolidated Financial Statements included in this Annual Report.
Manufacturing
We have manufacturing operations in San Diego, California and Santa Clara, California. The San Diego facility, our largest manufacturing operation, produces our lateral-flow, immunoassay products. The Santa Clara facility manufactures our microtiter plate products.
The San Diego facility consists of laboratories devoted to tissue culture, cell culture, protein purification and immunochemistry and production areas dedicated to manufacturing and assembly. In the manufacturing process, biological and chemical supplies and equipment are used. Since the year 2000, the San Diego and Santa Clara facilities have operated under a Quality Management System certified to the International Organization for Standardization ("ISO") 9001 certification. During 2005, we became certified to the ISO 13485:2003 Regulatory Standard as required for medical device manufacturers distributing product within the European Union and other countries. Many of the lateral-flow and immunoassay products manufactured in our San Diego, California facility are packaged and shipped by a third party located in Southern California.
8
We seek to conduct all of our manufacturing in compliance with the FDA Quality System Regulations ("QSR") (formerly Good Manufacturing Practices) governing the manufacture of medical devices. Our manufacturing facilities have been registered with the FDA and the Department of Health Services of the State of California (the "State FDA"), and have passed routine federal and state inspections confirming compliance with the QSR regulatory requirements.
In certain instances, we rely on a single source or a limited group of suppliers for certain components and raw materials for our products. Although we seek to reduce our dependence on sole or limited source suppliers, if available or practicable, the partial or complete loss of these sources could have a material adverse effect on our results of operations and damage customer relationships, due to the complexity of the products they supply and the significant amount of time required to qualify new suppliers.
The manufacture of medical diagnostic products is difficult, particularly with respect to the stability and consistency of complex biological components. Because of these complexities, manufacturing difficulties occasionally occur that delay the introduction or supply of products and result in unanticipated manufacturing costs.
Government Regulation
The testing, manufacture and commercialization of our products are subject to regulation by numerous governmental authorities, principally the FDA and corresponding state and foreign regulatory agencies. Pursuant to the U.S. Federal Food, Drug, and Cosmetic Act and the regulations promulgated thereunder, the FDA regulates the preclinical and clinical testing, manufacture, labeling, distribution and promotion of medical devices. Noncompliance with applicable requirements can result in, among other matters, fines, injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, failure of the FDA to grant premarket clearance or premarket approval for devices, withdrawal of marketing clearances or approvals and criminal prosecution. The FDA also has the authority to request a recall, repair, replacement or refund of the cost of any device manufactured or distributed in the U.S. if the device is deemed to be unsafe.
In the U.S., devices are classified into one of three classes (Class I, II or III) on the basis of the controls deemed necessary by the FDA to reasonably ensure their safety and effectiveness. Class I and II devices are subject to general controls including, but not limited to, performance standards, premarket notification ("510(k)") and postmarket surveillance. Class III devices generally pose the highest risk to the patient and are typically subject to premarket approval to ensure their safety and effectiveness. Our current products are all Class I or II.
Prior to commercialization in the U.S. market, manufacturers must obtain FDA clearance through a premarket notification or premarket approval process, which can be lengthy, expensive and uncertain. The FDA has been requiring more rigorous demonstration of product performance as part of the 510(k) process, including submission of extensive clinical data. It generally takes from three to six months to obtain clearance but may take longer. For example, the FDA may determine that additional information is needed before a clearance determination can be made, which could prevent or delay the introduction of new products into the market. A premarket approval application must be supported by valid scientific evidence to demonstrate the safety and effectiveness of the device, typically including the results of clinical investigations, bench tests and reference laboratory studies. In addition, modifications or enhancements for existing products that could significantly affect safety or effectiveness, or constitute a major change in the intended use of the device, will require new submissions to the FDA, and there can be no assurance that the FDA will grant approval.
In September 2007, the FDA issued a draft guidance entitled "Draft Guidance for Industry and FDA Staff Recommendation for CLIA waiver applications." This guidance became effective on
9
January 30, 2008. The guidance sets forth new requirements for obtaining a CLIA waiver that are onerous and will increase the time and cost required to obtain a CLIA waiver.
We may not be able to obtain the necessary regulatory premarket approvals or clearances for our products on a timely basis, if at all. Delays in receipt of or failure to receive such approvals or clearances, or failure to comply with existing or future regulatory requirements, would have a material adverse effect on our business, financial condition and results of operations.
Any devices we manufacture or distribute pursuant to FDA clearance or approvals are subject to continuing regulation by the FDA and certain state agencies, including adherence to QSR relating to testing, control, documentation and other quality assurance requirements. We must also comply with Medical Device Reporting ("MDR") requirements mandating reporting to the FDA of any incident in which a product may have caused or contributed to a death or serious injury, or in which a product malfunctioned and, if the malfunction were to recur, would be likely to cause or contribute to a death or serious injury. Labeling and promotional activities are also subject to scrutiny by the FDA and, in certain circumstances, by the Federal Trade Commission. Current FDA enforcement policy prohibits the marketing of approved medical devices for unapproved uses.
We are subject to routine inspection by the FDA and other state agencies for compliance with applicable federal, state and local regulations. Changes in existing requirements or adoption of new requirements could have a material adverse effect on our business, financial condition and results of operations. We may also incur significant costs in complying with any applicable laws and regulations in the future, resulting in a material adverse effect on our business, financial condition and results of operations.
Our research and development and manufacturing activities involve the controlled use of hazardous materials, including but not limited to biological materials and chemicals such as dimethyl sulfate, sodium nitrite, acetaldehyde, acrylamide, potassium bromate and radionuclides. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of hazardous materials. These regulations include federal statutes popularly known as CERCLA, RCRA and the Clean Water Act. Compliance with these laws and regulations is expensive. If any governmental authorities were to impose new environmental regulations requiring compliance in addition to that required by existing regulations, these future environmental regulations could impose substantial costs on our business. In addition, because of the nature of the penalties provided for in some of these environmental regulations, we could be required to pay substantial fines, penalties or damages in the event of noncompliance with environmental laws or the exposure of individuals to hazardous materials. Any environmental violation or remediation requirement could also partially or completely shut down our research and manufacturing facilities and operations, which would have a material adverse effect on our business.
For marketing outside the U.S., we are subject to foreign regulatory requirements governing human clinical testing and marketing approval for our products. These requirements vary by jurisdiction, differ from those in the U.S., and may require us to perform additional preclinical or clinical testing regardless of whether FDA approval has been obtained. The amount of time required to obtain necessary approvals may be longer or shorter than that required for FDA approval. In many foreign countries, pricing and reimbursement approvals are also required.
Our initial focus for obtaining marketing approval outside the U.S. is typically the European Union (the "EU") and Japan. EU Regulations and Directives generally classify health care products either as medicinal products, medical devices orin vitro diagnostics. The European Conformity ("CE") mark certification requires us to receive ISO certification for the manufacture of our products. This certification comes only after the development of an all-inclusive quality system, which is reviewed for
10
compliance with ISO standards by a licensed body working within the EU. After certification is received, a technical file is developed which attests to the product's compliance with EU directive 98/79/EC forin vitro diagnostic medical devices. Only after this point is the product CE marked. The Japanese regulations require registration ofin vitro diagnostic products with the Japanese Ministry of Health, Labor and Welfare. Additional clinical trials are typically required for registration purposes. For products marketed in Canada, we have our independent party certification under the Canadian Medical Device Regulation.
Intellectual Property
The healthcare industry has traditionally placed considerable importance on obtaining and maintaining patent and trade secret protection for commercially relevant technologies, products and processes. We and other companies engaged in research and development of new diagnostic products actively pursue patents for technologies that are considered novel and patentable. However, important factors, many of which are not within our control, can affect whether and to what extent patent protection in the U.S. and in other important markets worldwide is obtained. By way of example, the speed, accuracy and consistency in application of the law in a patent office within any particular jurisdiction is beyond our control and can be unpredictable. The resolution of issues such as these and their effect upon our long-term success is likewise indeterminable. We have issued patents, both in the U.S. and internationally, with expiration dates ranging from the present through approximately 2029 and have patent applications pending throughout the world.
It has been our policy to file for patent protection in the U.S. and other countries with significant markets, such as Western European countries and Japan, if the economics are deemed to justify such filing and our patent counsel determines that relevant patent protection may be obtained. No assurance can be given that patents will be issued to us pursuant to our patent applications in the U.S. or abroad or that our patent portfolio will provide us with a meaningful level of commercial protection.
A large number of individuals and commercial enterprises seek patent protection for technologies, products and processes in fields in or related to our areas of product development. To the extent such efforts are successful, we may be required to obtain licenses and pay significant royalties in order to exploit certain of our product strategies and avoid a material adverse effect on our business. Licenses may not be available to us at all or, if so available, may not be available on acceptable terms.
We are aware of certain patents issued to various developers of diagnostic products with potential applicability to our diagnostic technology. We have licensed certain rights from certain companies to assist with the manufacturing of certain products. In the future, we expect that we will require or desire additional licenses from other parties in order to refine our products further and to allow us to develop, manufacture and market commercially viable or superior products effectively. There can be no assurance that such licenses will be obtainable on commercially reasonable terms, if at all, that any patents underlying such licenses will be valid and enforceable, or that the proprietary nature of any patented technology underlying such licenses will remain proprietary.
We seek to protect our trade secrets and technology by entering into confidentiality agreements with employees and third parties (such as potential licensees, customers, strategic partners and consultants). In addition, we have implemented certain security measures in our laboratories and offices. Despite such efforts, no assurance can be given that the confidentiality of our proprietary information can be maintained. Also, to the extent that consultants or contracting parties apply technical or scientific information independently developed by them to our projects, disputes may arise as to the proprietary rights to such data.
11
Under many of our distribution agreements, we have agreed to indemnify the distributors against costs and liabilities arising out of any patent infringement claims and other intellectual property claims asserted by a third party relating to products sold under those agreements.
Competition
Competition in the development and marketing of diagnostic products is intense, and diagnostic technologies have been subject to rapid change. We believe that some of the most significant competitive factors in the rapid diagnostic market include convenience, price and product performance as well as the distribution, advertising, promotion and brand name recognition of the marketer. Our success will depend on our ability to remain abreast of technological advances, to introduce technologically advanced products, to effectively market our differentiated value products, to maintain our brand strength and to attract and retain experienced personnel, who are in great demand. The majority of diagnostic tests requested by physicians and other healthcare providers are performed by independent clinical reference laboratories. We expect that these laboratories will continue to compete vigorously to maintain their dominance of the testing market. In order to achieve market acceptance for our products, we will be required to demonstrate that our products provide physicians cost-effective and time-saving alternatives to tests performed in the clinical reference laboratory. This requires that physicians change the way that they are used to handling diagnostic testing.
There has been a trend toward industry consolidation in our markets over the last few years. We may not be able to compete successfully in an increasingly consolidated industry and cannot predict with certainty how industry consolidation will affect our competitors or us. We expect this trend toward industry consolidation may continue as companies attempt to strengthen or hold their market positions in an evolving industry and as companies are acquired or are unable to continue operations. Many of our current and prospective competitors, including several large pharmaceutical and diversified healthcare companies, have substantially greater financial, marketing and other resources than we have. These competitors include, among others, Inverness Medical Innovations, Inc. ("IMA"), Beckman Coulter Primary Care Diagnostics ("Beckman"), Fisher Scientific Corporation ("Fisher"), Genzyme Diagnostics Corporation ("Genzyme"), and Becton Dickinson and Company ("Becton"). We also face competition from our distributors since some have created, and others may decide to create, their own products to compete with ours. Our competitors may succeed in developing or marketing technologies or products that are more effective or commercially attractive than our current or future products or that would render our technologies and products obsolete. Moreover, we may not have the financial resources, technical expertise or marketing, distribution or support capabilities to compete successfully in the future. In addition, many competitors have made substantial investments in competing technologies that may be more effective than our technologies, or that may prevent, limit or interfere with our ability to make, use or sell our products either in the U.S. or in international markets.
Human Resources
As of December 31, 2008, we had 322 employees, none of whom are represented by a labor union. We have experienced no work stoppages and believe that our employee relations are good.
Executive Officers of Quidel Corporation
The names, ages and positions of all executive officers as of December 31, 2008 are listed below, followed by a brief account of their business experience during the past five years or more. Officers are normally appointed annually by the Board of Directors at a meeting of the Board of Directors. There are no family relationships among these officers, nor any arrangements or understandings between any officer and any other person pursuant to which an officer was selected. None of these officers has been involved in any court or administrative proceeding within the past five years adversely reflecting on the officer's ability or integrity.
12
Caren L. Mason, 55, became our President and Chief Executive Officer on August 20, 2004. Ms. Mason announced her retirement in January 2009 and resigns as our President and Chief Executive Officer, effective March 1, 2009, although she will remain with us through June 1, 2009. Ms. Mason has more than 25 years experience in healthcare. Prior to joining us, Ms. Mason provided consultative services for Eastman Kodak Health Imaging as a result of the sale of MiraMedica, Inc., a digital technology, diagnostic imaging company, to Eastman Kodak. She served as President and CEO for MiraMedica, Inc. from April 2002 through September 2003. From January 2000 through June 2001, Ms. Mason served as CEO of eMed Technologies, Inc. of Lexington, Massachusetts, a digital technology, diagnostic imaging company. Prior to joining eMed Technologies, Ms. Mason served as General Manager of the Women's Healthcare business and as a General Manager in various capacities for the Services business of General Electric Medical Systems from July 1996 to January 2000. Ms. Mason's additional healthcare experience includes her tenure with Bayer AG/AGFA from October 1989 to July 1996 where she last served as Senior Vice President for the AGFA Technical Imaging Business Group. Ms. Mason began her career in healthcare with American Hospital Supply/Baxter Healthcare and served in sales, marketing and managerial roles from 1977 through 1988. Ms. Mason is a graduate of Indiana University. She has been a member of the Franciscan Sisters of the Poor Foundation Board of Governors and has also been a member of the Board of Directors for MediServ/GESCI, eMed Technologies, Inc. and MiraMedica, Inc., and currently serves as a member of the Board of Directors of AdvaMed. Ms. Mason announced her retirement on January 5, 2009.
Douglas C. Bryant, 51, was named President, Chief Executive Officer and a member of the Board of Directors in February 2009. Mr. Bryant's appointment as President and Chief Executive Office is effective on March 1, 2009. Prior to joining us, Mr. Bryant served as Executive Vice President and Chief Operating Officer at Luminex Corporation, managing its Bioscience Group, Luminex Molecular Diagnostics (Toronto), manufacturing, R&D, technical operations, and commercial operations. From 1983 to 2007, Mr. Bryant held various worldwide commercial operations positions with Abbott Laboratories including, among others: Vice President of Abbott Vascular for Asia/Japan, Vice President of Abbott Molecular Global Commercial Operations and Vice President of Abbott Diagnostics Global Commercial Operations. Earlier in his career with Abbott, Mr. Bryant was Vice President of Diagnostic Operations in Europe, the Middle East and Africa, and Vice President of Diagnostic Operations Asia Pacific. Mr. Bryant has over 25 years of industry experience in sales and marketing, product development, manufacturing and service and support in both the life sciences and diagnostics markets. Mr. Bryant holds a B.A. in Economics from the University of California at Davis.
John M. Radak, 48, became our Chief Financial Officer on February 1, 2007. Prior to joining us, Mr. Radak was Vice President of Finance and Chief Accounting Officer since January 2003 for Invitrogen Corporation, a leading provider of research tools for the life science industry. Mr. Radak also served as Vice President of Finance and Corporate Controller for Sunrise Medical Inc. from December 1994 to August 2001. Prior to joining Sunrise Medical Inc., Mr. Radak held a variety of senior financial management positions with manufacturing companies in the medical device and computer industries. After receiving his B.A. in Business Administration from California State University, Fullerton, Mr. Radak began his career with Deloitte Haskins and Sells. Mr. Radak holds an MBA from the University of Southern California and is a Certified Public Accountant.
Richard C. Tarbox, 57, became our Senior Vice President, Corporate Development Officer on July 16, 2007. Prior to joining us, Mr. Tarbox served as Vice President, Strategic Sourcing & Business Development for the Healthcare Market Division of Thermo Fisher Scientific Incorporated, a company focused on meeting diagnostic testing needs in healthcare facilities, since 2004. Prior to Thermo Fisher Scientific and from 1995 to 2003, Mr. Tarbox served as managing partner and founder of The Tarbox Group, L.P., providing management consulting services and investigation and evaluation of private equity investment opportunities. From 1992 to 1995, Mr. Tarbox served as Executive Vice President and Chief Operating Officer of Ostex International, Inc., a developer and manufacturer of diagnostic
13
products for osteoporosis and other bone health diseases. In addition, from 1981 to 1992, Mr. Tarbox held various senior business development, sales and operations positions with American Hospital Supply Corp/Baxter International, Inc., a global medical products and services company. Mr. Tarbox is a graduate of the University of Washington where he received his Bachelor's Degree in Clinical Psychology and the Kellogg School of Management at Northwestern University where he earned a Master's in Business Management.
Thomas J. Foley, Ph.D., 69, has been our Chief Technology Officer since November 2004. Dr. Foley was Senior Vice President of Research and Development and Regulatory Affairs at Lifepoint Inc., a clinical diagnostics company, from 1998 to 2004. Prior to 1998, he was Executive Vice President of Research and Development with HiChem/Elan Diagnostics from 1994 to 1997. From 1987 to 1994, Dr. Foley was Vice President of Research and Development at Hycor Biomedical, Inc., a company involved in developing reagents and controls for urinalysis, therapeutic drug monitoring and allergy and autoimmune disease states. Dr. Foley was Vice President of Research and Development at Gilford Instruments from 1983 to 1986 and Worthington Diagnostics from 1981 to 1983. In addition, Dr. Foley was Manager of Research and Development at Beckman Instruments from 1979 to 1981. Dr. Foley has a Bachelor of Science and a Ph.D. in Biochemistry from Trinity College, Dublin.
Robert J. Bujarski, J.D., 40, rejoined us as our Senior Vice President, General Counsel and Corporate Secretary in June 2008. Mr. Bujarski previously served as our Senior Vice President, General Counsel and Corporate Secretary from March 2007 through March 2008. From July 2005 to March 2007, he was our General Counsel and Vice President. Mr. Bujarski was an associate attorney with the law firm of Gibson, Dunn & Crutcher LLP in its transactions practice group from October 2001 to July 2005. Mr. Bujarski received his B.A. degree in 1991 and his law degree in 2001 from the University of Arizona.
Scot M. McLeod, 44, has been our Senior Vice President, Operations since July 2007. Mr. McLeod previously served as the Company's Vice-President, Operations from 2001 to July 2007. Mr. McLeod first joined the Company in 1997 as Director of Production and has held various management operations positions with the Company throughout his ten years of service. Mr. McLeod has over 20 years experience in operations, and a diverse manufacturing background in both domestic and international environments. Mr. McLeod spent five years in OUS / overseas manufacturing of computer peripherals. Prior to joining Quidel, Mr. McLeod held various positions in operations and quality with Medtronic Interventional Vascular, Hybritech Inc., ALCOA and Information Magnetics Corporation. Mr. McLeod has his B.S. in Chemical Engineering from the University of New Hampshire.
John D. Tamerius, Ph.D., 63, has been our Senior Vice President, Clinical/Regulatory Affairs since November 2008. Dr. Tamerius previously served as the Company's Vice President, Clinical/Regulatory Affairs from 2005 to November 2008. Dr. Tamerius has held a variety of positions with us including, among others: Vice President for Research and Development and General Manager of the Company's Special Products Group. Dr. Tamerius joined the Company in 1983 with the acquisition of Cytotech, Inc. where he served as President. Dr. Tamerius was previously a research associate at Scripps Clinic and Research Foundation. Dr. Tamerius performed postdoctoral research in tumor immunology at the Fred Hutchinson Cancer Center in Seattle and was awarded a Bachelor of Science, Master of Science, and Doctor of Philosophy in Microbiology and Immunology, all from the University of Washington.
14
Item 1A. Risk Factors
Risks Related to Our Business
Our operating results may fluctuate adversely as a result of many factors that are outside our control.
Fluctuations in our operating results, for any reason, could cause our growth or operating results to fall below the expectations of investors and securities analysts. For the year ended December 31, 2008, total revenue increased 9% to $128.1 million from $118.1 million for the year ended December 31, 2007. For further discussion of this increase, refer to Item 7. "Management's Discussion and Analysis of Financial Condition and Results of Operation" included in this Annual Report.
Our sales estimates for future periods are based on estimated end-user demand for our products. Sales to our distribution partners would fall short of expectations if distributor inventories increase because of less than estimated end-user consumption.
Other factors that are beyond our control and that could affect our operating results in the future include:
- •
- seasonal fluctuations in our sales of infectious disease tests, which are generally highest in fall and winter, thus resulting in generally lower operating results in the second calendar quarter and higher operating results in the first, third and fourth calendar quarters;
- •
- timing of the onset, length and severity of the cold and flu seasons;
- •
- government and media attention focused on a potential influenza pandemic and the related potential impact on humans from avian flu, including the uncertainty surrounding the detection of novel influenza viruses in human specimens and the U.S. Government's recent report which focused on vaccination solutions and called for the development of new rapid diagnostic tests, which are not commercially available at this time, that identify specific strains of influenza and have greater sensitivity and specificity;
- •
- changes in the level of competition, such as would occur if one of our larger and better financed competitors introduced a new or lower priced product to compete with one of our products;
- •
- changes in the reimbursement systems or reimbursement amounts that end-users rely upon in choosing to use our products;
- •
- changes in economic conditions in our domestic and international markets, such as economic downturns, decreased healthcare spending, reduced consumer demand, inflation and currency fluctuations;
- •
- changes in sales levels, since a significant portion of our costs are fixed costs with the result that relatively higher sales could likely increase profitability but relatively lower sales would not reduce costs by the same proportion, and hence could cause operating losses;
- •
- lower than anticipated market penetration of our new or more recently introduced products;
- •
- significant quantities of our product in our distributors' inventories or distribution channels; and
- •
- changes in distributor buying patterns.
To remain competitive, we must continue to develop or obtain proprietary technology rights; otherwise, other companies may increase their market share by selling technologically superior products that compete with our products.
Our competitive position is heavily dependent on obtaining and protecting our own proprietary technology or obtaining licenses from others. Our ability to compete successfully in the diagnostic market depends on continued development and introduction of new proprietary technology and the
15
improvement of existing technology. If we cannot continue to obtain and protect proprietary technology, our total revenue and gross profits could be adversely affected. Moreover, our current and future licenses may not be adequate for the operation of our business.
Our ability to obtain patents and licenses, and their benefits, is uncertain. We have issued patents both in the U.S. and internationally, with expiration dates ranging from the present through approximately 2029. Additionally, we have patent applications pending throughout the world. These pending patent applications may not result in the issuance of any patents, or if issued, may not have priority over others' applications or may not offer protection against competitors with similar technology. Moreover, any patents issued to us may be challenged, invalidated or circumvented in the future. In addition to the U.S., we have patents issued in various other countries including, for example, Australia, Canada, Japan and various European countries including France, Germany, Italy, Spain and the United Kingdom. Third parties can make, use and sell products covered by our patents in any country in which we do not have patent protection. We also license the right to use our products to our customers under label licenses that are for research purposes only. These licenses could be contested and, because we cannot monitor all potential unauthorized uses of our products around the world, we might not be aware of an unauthorized use and might not be able to enforce the license restrictions in a cost-effective manner. Also, we may not be able to obtain licenses for technology patented by others and required to produce our products on commercially reasonable terms.
In order to remain competitive and profitable, we must expend considerable resources to research new technologies and products and develop new markets. Our failure to successfully introduce new technologies and products and develop new markets could have a material adverse effect on our business and prospects.
We devote a significant amount of financial resources to researching and developing new technologies, new products and new markets. The development, manufacture and sale of diagnostic products require a significant investment of resources. Moreover, no assurances can be given that our efforts to develop new technologies or products will be successful or commercially viable.
The development of new markets also requires a substantial investment of resources, such as new employees, offices and manufacturing facilities. Accordingly, we are likely to incur increased operating expenses as a result of our increased investment in sales and marketing activities, manufacturing scale-up and new product development associated with our efforts to:
- •
- provide clinicians with validated, evidence-based studies which encompass the clinical efficacy and economic efficiency of our rapid POC tests for the professional market. In conjunction with our QVB™ commitment, we expect to present ongoing information that supports the adoption of rapid POC testing;
- •
- continue to focus on strengthening our market and brand leadership in infectious diseases and reproductive and women's health by acquiring, developing and introducing clinically superior diagnostic solutions;
- •
- drive growth by securing dedicated distributor partnerships and strengthening our sales organization to assure physician and laboratorian satisfaction through direct relationships with Integrated Delivery Networks and hospitals;
- •
- support payer evaluation of rapid tests and establishment of favorable reimbursement rates;
- •
- continue creation of strong global alliances to assure leadership in key markets;
- •
- drive profit through further refinement of our manufacturing efficiencies and productivity improvements, with continued focus on profitable products and markets and our effort to create exceptional competency in new product development;
16
- •
- continue to focus our research and development efforts on three areas: 1) new proprietary product platform development, 2) the creation of improved products and new products for existing markets and unmet clinical needs, and 3) products developed under collaborations with other companies for new and existing markets; and
- •
- identify and commercialize new markers, products and collaborations in bone health through the SPG.
As a result of any number of risk factors identified in this Annual Report, no assurance can be given that we will be successful in implementing our operational, growth and other strategic efforts. In addition, the funds for the foregoing projects have in the past come primarily from our business operations and a working capital line of credit. If our business slows and we become less profitable, and as a result have less money available to fund research and development, we will have to decide at that time which programs to cut, and by how much. Similarly, if adequate financial, personnel, equipment or other resources are not available, we may be required to delay or scale back our strategic efforts. Our operations will be adversely affected if our total revenue and gross profits do not correspondingly increase or if our technology, product and market development efforts are unsuccessful or delayed. Furthermore, our failure to successfully introduce new products and develop new markets could have a material adverse effect on our business and prospects.
We rely on a limited number of key distributors which account for a substantial majority of our total revenue. The loss of any key distributor or an unsuccessful effort to directly distribute our products could lead to reduced sales.
Although we have many distributor relationships in the U.S., the market is dominated by a small group of these distributors. Five of our distributors, which are considered to be among the market leaders, collectively accounted for approximately 60%, 61% and 68% of our total revenue for the years ended December 31, 2008, 2007 and 2006, respectively. Even though our distributor mix will likely change from period to period in the future, Cardinal, DS Pharma, McKesson, NDC and PSS have historically accounted for a significant portion of our total revenue. In addition, we rely on a few key distributors for a majority of our international sales, and will continue to do so for the foreseeable future. The loss or termination of our relationship with any of these key distributors could significantly disrupt our business unless suitable alternatives were timely found or lost sales to one distributor are absorbed by another distributor. Finding a suitable alternative may pose challenges in our industry's competitive environment, and another suitable distributor may not be found on satisfactory terms. For instance, some distributors already have exclusive arrangements with our competitors, and others do not have the same level of penetration into our target markets as our existing distributors. If total revenue to these or any of our other significant distributors were to decrease in any material amount in the future or we are not successful in timely transitioning business to new distributors, our business, operating results and financial condition could be materially and adversely affected.
Our operating results are heavily dependent on sales of our influenza diagnostic tests.
Revenues from the sale of our influenza tests represent a significant portion of our total revenues and are expected to remain so in at least the near future. In addition, the gross margins derived from sales of our influenza tests are significantly higher than the gross margins from our other core products. As a result, if sales of our influenza tests decline for any reason—whether as a result of market share loss or price pressure, obsolescence, a mild flu season, regulatory matters or any other reason—our operating results would be materially and adversely affected on a disproportionate basis.
17
Intellectual property risks and third-party claims of infringement, misappropriation of proprietary rights or other claims against us could adversely affect our ability to market our products, require us to redesign our products or seek licenses from third parties, and materially adversely affect our operating results. In addition, the defense of such claims could result in significant costs and divert the attention of our management and other key employees.
Companies in or related to our industry often aggressively protect and pursue their intellectual property rights. There are often intellectual property risks associated with developing and producing new products and entering new markets, and we may not be able to obtain, at reasonable cost and upon commercially reasonable terms, licenses to intellectual property of others that is alleged to be part of such new or existing products. From time to time, we have received, and may continue to receive, notices that claim we have infringed upon, misappropriated or misused other parties' proprietary rights.
Moreover, in the past we have been engaged in litigation with parties that claim, among other matters, that we infringed their patents. We or our customers may be sued by other parties that claim that our products have infringed their patents or misappropriated their proprietary rights or which may seek to invalidate one or more of our patents. An adverse determination in any of these types of disputes could prevent us from manufacturing or selling some of our products, limit or restrict the type of work that employees involved in such litigation may perform for us, increase our costs of revenue and expose us to significant liability.
As a general matter, our involvement in litigation or in any claims to determine proprietary rights, as may arise from time to time, could materially and adversely affect our business, financial condition and results of operations for reasons such as:
- •
- the pendency of any litigation may of itself cause our distributors or end-users to reduce purchases of our products;
- •
- it may consume a substantial portion of our managerial and financial resources;
- •
- its outcome would be uncertain and a court may find any third-party patent claims valid and infringed by our products (issuing a preliminary or permanent injunction) that would require us to withdraw or recall such products from the market, redesign such products offered for sale or under development or restrict employees from performing work in their areas of expertise;
- •
- governmental agencies may commence investigations or criminal proceedings against our employees, former employees and us relating to claims of misappropriation or misuse of another party's proprietary rights;
- •
- an adverse outcome could subject us to significant liability in the form of past royalty payments, penalties, special and punitive damages and future royalty payments significantly affecting our future earnings; and
- •
- failure to obtain a necessary license (upon commercially reasonable terms, if at all) upon an adverse outcome could prevent us from selling our current products or other products we may develop.
In addition to the foregoing, we may also indemnify some customers, distributors and strategic partners under our agreements with such parties if a third party alleges or if a court finds that our products or activities have infringed upon, misappropriated or misused another party's proprietary rights. Further, our products may contain technology provided to us by other parties such as contractors, suppliers or customers. We may have little or no ability to determine in advance whether such technology infringes the intellectual property rights of a third party. Our contractors, suppliers and licensors may not be required or financially able to indemnify us in the event that a claim of
18
infringement is asserted against us, or they may be required to indemnify us only up to a maximum amount, above which we would be responsible for any further costs or damages.
Continued volatility and disruption to the global capital and credit markets may adversely affect our results of operations and financial condition, as well as our ability to access credit and the financial soundness of our customers and suppliers.
Recently, the global capital and credit markets have been experiencing a period of unprecedented turmoil and upheaval, characterized by the bankruptcy, failure, collapse or sale of various financial institutions and an unprecedented level of intervention from the United States federal government. These conditions could adversely affect the demand for our products and services and, therefore, reduce purchases by our customers, which would negatively affect our revenue growth and cause a decrease in our profitability. In addition, interest rate fluctuations, financial market volatility or credit market disruptions may limit our access to capital, and may also negatively affect our customers' and our suppliers' ability to obtain credit to finance their businesses on acceptable terms. As a result, our customers' needs and ability to purchase our products or services may decrease, and our suppliers may increase their prices, reduce their output or change their terms of sale. If our customers' or suppliers' operating and financial performance deteriorates, or if they are unable to make scheduled payments or obtain credit, our customers may not be able to pay, or may delay payment of, accounts receivable owed to us, and our suppliers may restrict credit or impose different payment terms. Any inability of customers to pay us for our products and services, or any demands by suppliers for different payment terms, may adversely affect our earnings and cash flow. Additionally, both state and federal government sponsored payers, as a result of budget deficits or reductions, may seek to reduce their health care expenditures by renegotiating their contracts with us. Any reduction in payments by such government sponsored payers may adversely affect our earnings and cash flow. Declining economic conditions may also increase our costs. If the economic conditions do not improve or continue to deteriorate, our results of operations or financial condition could be adversely affected.
We may not achieve market acceptance of our products among physicians and other healthcare providers, and this would have a negative effect on future sales growth.
A large part of our business is based on the sale of rapid POC diagnostic tests that physicians and other healthcare providers can administer in their own facilities without sending samples to central laboratories. Clinical reference laboratories and hospital-based laboratories are significant competitors of ours and provide a majority of the diagnostic tests used by physicians and other healthcare providers. Our future sales depend on, among other matters, capture of sales from these laboratories by achieving market acceptance of POC testing from physicians and other healthcare providers. If we do not capture sales at the levels we have budgeted for, our total revenue will not grow as much as we hope and the costs we have incurred will be disproportionate to our sales levels. We expect that clinical reference and hospital-based laboratories will continue to compete vigorously against our POC diagnostic products in order to maintain and expand their existing dominance of the overall diagnostic testing market. Moreover, even if we can demonstrate that our products are more cost-effective or save time, physicians and other healthcare providers may resist changing to POC tests. Our failure to achieve market acceptance from physicians and healthcare providers with respect to the use of our POC diagnostic products would have a negative effect on our future sales growth.
Intense competition with other providers of POC diagnostic products may reduce our sales.
In addition to competition from laboratories, our POC diagnostic tests compete with similar products made by our competitors. There are a large number of multinational and regional competitors making investments in competing technologies and products, including several large pharmaceutical and diversified healthcare companies. We also face competition from our distributors since some have
19
created, and others may decide to create, their own products to compete with ours. A number of our competitors have a potential competitive advantage because they have substantially greater financial, technical, research and other resources, and larger, more established marketing, sales, distribution and service organizations than we have. These competitors include, among others, IMA, Beckman, Fisher, Genzyme and Becton. Moreover, some competitors offer broader product lines and have greater name recognition than we have. If our competitors' products are more effective than ours or acquire market share from our products through more effective marketing or competitive pricing, our total revenue and profits could be materially and adversely affected. Competition also has the effect of limiting the prices we can charge for our products.
Our products are highly regulated by various governmental agencies. Any changes to the existing laws and regulations may adversely impact our ability to manufacture and market our products.
The testing, manufacture and sale of our products are subject to regulation by numerous governmental authorities in the U.S., principally the FDA and corresponding state and foreign regulatory agencies. The FDA regulates most of our products, which are currently all Class I or II devices. The U.S. Department of Agriculture regulates our veterinary products. Our future performance depends on, among other matters, our estimates as to when and at what cost we will receive regulatory approval for new products. In addition, certain of our foreign product registrations are owned or controlled by our international distribution partners that could result in the loss of or delay in transfer of any such product registrations, thereby interrupting our ability to sell our products in those markets. Regulatory approval can be a lengthy, expensive and uncertain process, making the timing and costs of approvals difficult to predict. Our total revenue would be negatively affected by failures or delays in the receipt of approvals or clearances, the loss of previously received approvals or clearances or the placement of limits on the marketing and use of our products.
Furthermore, in the ordinary course of business, we must frequently make subjective judgments with respect to compliance with applicable laws and regulations. If regulators subsequently disagree with the manner in which we have sought to comply with these regulations, we could be subjected to substantial civil and criminal penalties, as well as product recall, seizure or injunction with respect to the sale of our products. The assessment of any civil and criminal penalties against us could severely impair our reputation within the industry and any limitation on our ability to manufacture and market our products could have a material adverse effect on our business.
We are subject to numerous government regulations in addition to FDA regulation, and compliance with changes could increase our costs.
In addition to FDA and other regulations described previously, numerous laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances impact our business operations. If these laws change or laws regulating any of our businesses are added, the costs of compliance with these laws could substantially increase our costs. Compliance with any future modifications of these laws or laws regulating the manufacture and marketing of our products could result in substantial costs and loss of sales or customers. Because of the number and extent of the laws and regulations affecting our industry, and the number of governmental agencies whose actions could affect our operations, it is impossible to reliably predict the full nature and impact of future legislation or regulatory developments relating to our industry. To the extent the costs and procedures associated with meeting new requirements are substantial, our business and results of operations could be adversely affected.
20
We use hazardous materials in our business that may result in unexpected and substantial claims against us relating to handling, storage or disposal.
Our research and development and manufacturing activities involve the controlled use of hazardous materials, including but not limited to chemicals and biological materials such as dimethyl sulfate, sodium nitrite, acetaldehyde, acrylamide, potassium bromate and radionuclides. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of hazardous materials. These regulations include federal statutes popularly known as CERCLA, RCRA and the Clean Water Act. Compliance with these laws and regulations is already expensive. If any governmental authorities were to impose new environmental regulations requiring compliance in addition to that required by existing regulations, these future environmental regulations could impair our research, development or production efforts by imposing additional, and possibly substantial, costs on our business. In addition, because of the nature of the penalties provided for in some of these environmental regulations, we could be required to pay sizeable fines, penalties or damages in the event of noncompliance with environmental laws. Any environmental violation or remediation requirement could also partially or completely shut down our research and manufacturing facilities and operations, which would have a material adverse effect on our business. The risk of accidental contamination or injury from these hazardous materials cannot be completely eliminated and exposure of individuals to these materials could result in substantial fines, penalties or damages that are not covered by insurance.
Our total revenue could be affected by third-party reimbursement policies and potential cost constraints.
The end-users of our products are primarily physicians and other healthcare providers. Use of our products would be adversely impacted if physicians do not receive adequate reimbursement for the cost of our products by their patients' healthcare insurers or payers. Our total revenue could also be adversely affected by changes or trends in reimbursement policies of these governmental or private healthcare payers. In the U.S., healthcare providers such as hospitals and physicians who purchase diagnostic products generally rely on third-party payers, principally private health insurance plans, federal Medicare and state Medicaid, to reimburse all or part of the cost of the procedure. We believe that the overall escalating cost of medical products and services has led to, and will continue to lead to, increased pressures on the healthcare industry, both foreign and domestic, to reduce the cost of products and services. Given the efforts to control and reduce healthcare costs in the U.S. in recent years, currently available levels of reimbursement may not continue to be available in the future for our existing products or products under development. Third-party reimbursement and coverage may not be available or adequate in either U.S. or foreign markets, current reimbursement amounts may be decreased in the future and future legislation, regulation or reimbursement policies of third-party payers may reduce the demand for our products or adversely impact our ability to sell our products on a profitable basis.
Unexpected increases in, or inability to meet, current demand for our products could require us to spend considerable resources to meet the demand or harm our customer relationships if we are unable to meet demand.
If we experience unexpected increases in the demand for our products, we may be required to expend additional capital resources to meet these demands. These capital resources could involve the cost of new machinery or even the cost of new manufacturing facilities. This would increase our capital costs, which could adversely affect our earnings and cash resources. If we are unable to develop necessary manufacturing capabilities in a timely manner, our total revenue could be adversely affected. Failure to cost-effectively increase production volumes, if required, or lower than anticipated yields or production problems encountered as a result of changes that we may make in our manufacturing
21
processes to meet increased demand or changes in applicable laws and regulations, could result in shipment delays as well as increased manufacturing costs, which could also have a material adverse effect on our total revenue and profitability.
Unexpected increases in demand for our products could also require us to obtain additional raw materials in order to manufacture products to meet the demand. Some raw materials require significant ordering lead time and some are currently obtained from a sole supplier or a limited group of suppliers. We have long-term supply agreements with many of these suppliers, but these long-term agreements involve risks for us, such as our potential inability to obtain an adequate supply of raw materials and components and our reduced control over pricing, quality and timely delivery. It is also possible that one or more of these suppliers may become unwilling or unable to deliver materials to us. Any shortfall in our supply of raw materials and components, and our inability to quickly and cost-effectively obtain alternative sources for this supply, could have a material adverse effect on our total revenue or cost of sales and related profits.
Our inability to meet customer demand for our products, whether as a result of manufacturing problems or supply shortfalls, could harm our customer relationships and impair our reputation within the industry. This, in turn, could have a material adverse effect on our business.
If one or more of our products proves to be defective, we could be subject to claims of liability that could adversely affect our business.
A defect in the design or manufacture of our products could have a material adverse effect on our reputation in the industry and subject us to claims of liability for injuries and otherwise. Any substantial underinsured loss resulting from such a claim would have a material adverse effect on our profitability and the damage to our reputation in the industry could have a material adverse effect on our business.
We are exposed to business risk which, if not covered by insurance, could have an adverse effect on our profits.
Claims may be made against us for types of damages, or for amounts of damages, that are not covered by our insurance. For example, although we currently carry product liability insurance for liability losses, there is a risk that product liability or other claims may exceed the amount of our insurance coverage or may be excluded from coverage under the terms of our policy. Also, if we are held liable, our existing insurance may not be renewed at the same cost and level of coverage as currently in effect, or may not be renewed at all. Further, we do not currently have insurance against many environmental risks we confront in our business. If we are held liable for a claim against which we are not insured or for damages exceeding the limits of our insurance coverage, whether arising out of product liability matters or from some other matter, that claim could have a material adverse effect on our results of operations and profitability.
If we are not able to manage our growth strategy or if we experience difficulties integrating companies or technologies we may acquire after the acquisition, our earnings may be adversely affected.
Our business strategy contemplates further growth in the scope of operating and financial systems and the geographical area of our operations, including further expansion outside the U.S., as new products are developed and commercialized or new geographical markets are entered. We may experience difficulties integrating the operations of companies or technologies that we may acquire with our own operations, and as a result we may not realize our anticipated benefits and cost savings within our expected time frame, or at all. Because we have a relatively small executive staff, future growth may also divert management's attention from other aspects of our business, and will place a strain on existing management and our operational, financial and management information systems. Furthermore, we may expand into markets in which we have less experience or incur higher costs. Should we
22
encounter difficulties in managing these tasks, our growth strategy may suffer and our total revenue and gross profits could be adversely affected.
Our business could be negatively affected by the loss of or the inability to hire key personnel.
Our future success depends in part on our ability to retain our key technical, sales, marketing and executive personnel and our ability to identify and hire additional qualified personnel. Competition for these personnel is intense, both in the industry in which we operate and also in Santa Clara and San Diego, where our headquarters and the majority of our operations are located. Further, we expect to grow our operations, and our needs for additional management and other key personnel are expected to increase. If we are not able to retain existing key personnel, or identify and hire additional qualified personnel to meet expected growth, our business could be adversely impacted.
We face risks relating to our international sales, including inherent economic, political and regulatory risks, which could increase our costs, cause interruptions in our current business operations and stifle our growth opportunities.
Our products are sold internationally, with the majority of our international sales to our customers in Japan, Europe and the Middle East. We currently sell and market our products by channeling products through distributor organizations and sales agents. Sales to foreign customers accounted for 15%, 14%, and 20% of our total revenue for the years ended December 31, 2008, 2007 and 2006, respectively. Our international sales are subject to inherent economic, political and regulatory risks, which could increase our operating costs, cause interruptions in our current business operations and impede our international growth. These foreign risks include, among others:
- •
- compliance with new and changing registration requirements, our inability to benefit from registration for our products inasmuch as registrations may be controlled by a distributor, the difficulty in the transitioning of our product registrations, and tariffs or other barriers as we continue to expand into new countries and geographic regions;
- •
- exposure to currency exchange fluctuations, such as the 5% decrease and 2% increase in value of the Euro and Yen, respectively, against the U.S. dollar for the year ended December 31, 2008;
- •
- longer payment cycles, generally lower average selling prices and greater difficulty in accounts receivable collection;
- •
- reduced protection for, and enforcement of, intellectual property rights;
- •
- political and economic instability in some of the regions where we currently sell our products or that we may expand into in the future;
- •
- potentially adverse tax consequences; and
- •
- diversion of our products to the U.S. from products sold into international markets at lower prices.
Currently, all of our international sales are negotiated for and paid in U.S. dollars. Nonetheless, these sales are subject to currency risks, since changes in the values of foreign currencies relative to the value of the U.S. dollar can render our products comparatively more expensive. These exchange rate fluctuations could negatively impact international sales of our products and our anticipated foreign operations, as could changes in the general economic conditions in those markets. In order to maintain a competitive price for our products internationally, we may have to continue to provide discounts or otherwise effectively reduce our prices, resulting in a lower margin on products sold internationally. Continued change in the values of the Euro, the Japanese Yen and other foreign currencies could have a negative impact on our business, financial condition and results of operations. We do not currently
23
hedge against exchange rate fluctuations, which means that we are fully exposed to exchange rate changes.
Investor confidence and share value may be adversely impacted if we or our independent registered public accounting firm conclude that our internal controls over financial reporting are not effective.
As directed by Section 404 of the Sarbanes-Oxley Act of 2002, the SEC adopted rules requiring us, as a public company, to include a report of management on our internal controls over financial reporting in our Annual Reports on Form 10-K that contains an assessment by management of the effectiveness of our internal controls over financial reporting. In addition, our independent registered public accounting firm must attest to the effectiveness of our internal controls over financial reporting. How companies are implementing these requirements, including internal control reforms, if any, to comply with Section 404's requirements, and how independent registered public accounting firms are applying these requirements and testing companies' internal controls, remain subject to uncertainty. The requirements of Section 404 of the Sarbanes-Oxley Act of 2002 are ongoing. We expect that our internal controls will continue to evolve as our business activities change. Although we seek to diligently and vigorously review our internal controls over financial reporting in an effort to ensure compliance with the Section 404 requirements, any control system, regardless of how well designed, operated and evaluated, can provide only reasonable, not absolute, assurance that its objectives will be met. If, during any year, our independent registered public accounting firm is not satisfied with our internal controls over financial reporting or the level at which these controls are documented, designed, operated, tested or assessed, or if the independent registered public accounting firm interprets the requirements, rules or regulations differently than we do, then it may issue a report that is qualified. This could result in an adverse reaction in the financial marketplace due to a loss of investor confidence in the reliability of our financial statements and effectiveness of our internal controls, which ultimately could negatively impact the market price of our shares.
Risks Related to Our Common Stock
Our stock price has been highly volatile, and an investment in our stock could suffer a significant decline in value.
The market price of our common stock has been highly volatile and has fluctuated substantially in the past. For example, as of the end of each quarter period between December 31, 2006 and December 31, 2008, the closing price of our common stock, as reported by the Nasdaq Global Market, has ranged from a low of $10.20 to a high of $20.84. We expect our common stock to continue to be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
- •
- seasonal fluctuations in our sales of infectious disease tests, which are generally highest in fall and winter, thus resulting in generally lower operating results in the second and third calendar quarters and higher operating results in the first and fourth calendar quarters;
- •
- timing of onset, length and severity of the cold and flu seasons;
- •
- media attention focused on a potential influenza pandemic and the related potential impact on humans from avian flu, as well as the uncertainty surrounding the detection of novel influenza viruses in human specimens;
- •
- changes in the level of competition, such as would occur if one of our larger and better financed competitors introduced a new and superior technology or a lower priced product to compete with one of our products;
- •
- changes in economic conditions in our domestic and international markets, such as economic downturns, reduced consumer demand, inflation and currency fluctuations, particularly as we
24
In addition, the stock market in general, and the Nasdaq Global Market and the market for technology companies in particular, have experienced significant price and volume fluctuations that, at times, have been unrelated or disproportionate to the operating performance of the relevant companies. In the past, following periods of volatility in the market price of a company's securities, securities class action litigation has often been instituted. A securities class action suit against us could result in substantial costs, potential liabilities and the diversion of management's attention and resources.
Future sales by existing stockholders could depress the market price of our common stock.
Sales of our common stock in the public market, or the perception that such sales could occur, could negatively impact the market price of our common stock. As of December 31, 2008:
- •
- approximately 31.9 million shares of our common stock had been issued in registered offerings and 31.4 million are freely tradable in the public markets; and
- •
- approximately 1.8 million shares of our common stock were issuable upon exercise of outstanding stock options under our various equity incentive plans at a weighted average exercise price of $11.36.
We are unable to estimate the number of shares of our common stock that may actually be resold in the public market since this will depend on the market price for our common stock, the individual circumstances of the sellers and other factors. We also have a number of institutional stockholders that own significant blocks of our common stock. If one or more of these stockholders were to sell large portions of their holdings in a relatively short time, for liquidity or other reasons, the prevailing market price of our common stock could be negatively affected.
Anti-takeover devices may prevent a sale, or changes in the management, of the Company.
We have in place several anti-takeover devices, including a stockholder rights plan that may have the effect of delaying or preventing a sale, or changes in the management, of the Company. For example, our bylaws require stockholders to give written notice of any proposal or director nomination to us within a specified period of time prior to any stockholder meeting.
25
We may also issue shares of preferred stock without stockholder approval and on terms that our Board of Directors may determine in the future. The issuance of preferred stock could have the effect of making it more difficult for a third party to acquire a majority of our outstanding stock, and the holders of such preferred stock could have voting, dividend, liquidation and other rights superior to those of holders of our common stock.
We do not pay dividends and this may negatively affect the price of our stock.
We have not paid dividends on our common stock and do not anticipate paying dividends on our common stock in the foreseeable future. The future price of our common stock may be adversely impacted because we have not paid and do not anticipate paying dividends.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Our executive, administrative, manufacturing and research and development operation is located in San Diego, California where we lease a 78,000 square-foot facility. The San Diego lease expires in 2014 with options to extend the lease for two additional five-year periods. In addition, we lease approximately 24,000 square feet of manufacturing, laboratory and office space in Santa Clara, California. The Santa Clara lease expires in 2014 with an option to extend for one additional five-year period.
We believe that our facilities are adequate for our current needs, and we currently do not anticipate any material difficulty in renewing any of our leases as they expire or securing additional or replacement facilities, in each case, on commercially reasonable terms. However, in anticipation of our growth strategy, we may pursue alternative facilities.
Item 3. Legal Proceedings
None.
Item 4. Submission of Matters to a Vote of Security Holders
There were no matters submitted to a vote of security holders during the fourth quarter of 2008.
26
Part II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
COMMON STOCK PRICE RANGE
Our common stock is traded on the Nasdaq Global Market under the symbol "QDEL." The following table sets forth the range of high and low closing prices for our common stock for the periods indicated.
| | | | | | | |
Quarter Ended | | Low | | High | |
|---|
December 31, 2008 | | $ | 12.33 | | $ | 16.41 | |
September 30, 2008 | | | 16.00 | | | 20.81 | |
June 30, 2008 | | | 15.00 | | | 18.27 | |
March 31, 2008 | | | 14.03 | | | 19.47 | |
December 31, 2007 | |
$ |
17.71 | |
$ |
20.84 | |
September 30, 2007 | | | 13.57 | | | 19.56 | |
June 30, 2007 | | | 12.18 | | | 17.56 | |
March 31, 2007 | | | 10.20 | | | 13.96 | |
No cash dividends were declared for our common stock during the fiscal years ended in 2008 or 2007, and we do not anticipate paying any dividends in the foreseeable future. As of February 18, 2009, we had approximately 561 common stockholders of record.
Stock Repurchases
The table below sets forth information regarding repurchases of our common stock by us during the three months ended December 31, 2008.
| | | | | | | | | | | | | |
| | Total number
of shares
purchased | | Average
price paid
per share | | Total number of
shares purchased
as part of publicly
announced program | | Approximate dollar
value of shares that
may yet be purchased
under the program(1) | |
|---|
October 1 - October 31, 2008 | | | 44,114 | | $ | 15.01 | | | 44,114 | | $ | 14,080,000 | |
November 1 - November 30, 2008 | | | 522,082 | | | 15.40 | | | 522,082 | | | 6,038,000 | |
December 1 - December 31, 2008 | | | 289,750 | | | 14.14 | | | 289,750 | | | 26,941,000 | |
| | | | | | | | | | |
Ending Balance - December 31, 2008 | | | 855,946 | | $ | 14.95 | | | 855,946 | | $ | 26,941,000 | |
| | | | | | | | | | |
- (1)
- In June 2005, we announced that our Board of Directors authorized us to repurchase up to $25.0 million in shares of our common stock under our stock repurchase program. In March 2007, we announced that our Board of Directors authorized us to purchase up to an additional $25.0 million in shares of our common stock under our stock repurchase program. In December 2008, we announced that our Board of Directors authorized us to purchase up to an additional $25.0 million in shares of our common stock under our stock repurchase program. Any shares of common stock repurchased under this program will no longer be deemed outstanding upon repurchase and will be returned to the pool of authorized shares.
Equity Compensation Plan Information
Information regarding our equity compensation plans is set forth in the section titled "Equity Compensation Plan Information" in our 2009 Proxy Statement to be filed with the SEC no later than April 28, 2009.
27
STOCKHOLDER RETURN PERFORMANCE GRAPH
Set forth below is a line graph comparing the yearly percentage change in the cumulative total stockholder return on our common stock with the cumulative total return of the Nasdaq Composite Index and the Nasdaq Pharmaceutical Index for the period beginning December 31, 2003 and ending December 31, 2008. The graph assumes an initial investment of $100 on December 31, 2003 in our common stock, the Nasdaq Composite Index and the Nasdaq Pharmaceutical Index and reinvestment of dividends. The stock price performance of our common stock depicted in the graph represents past performance only and is not necessarily indicative of future performance.
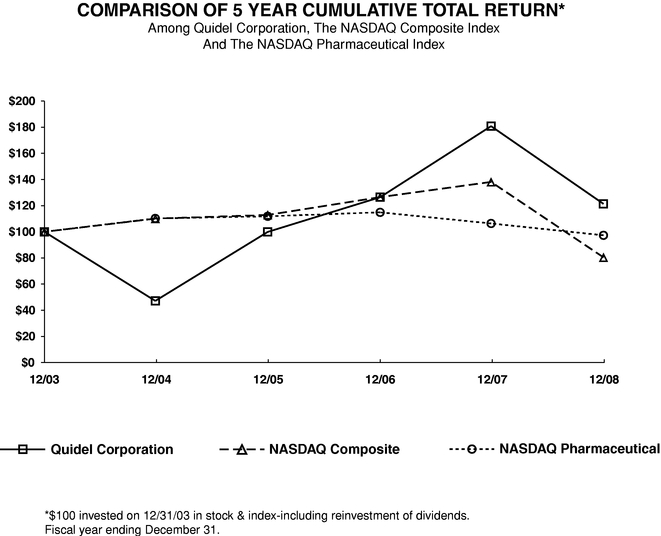
| | | | | | | | | | | | | | | | | | | |
Company/Index | | Base Period 12/31/03 | | 12/31/04 | | 12/31/05 | | 12/31/06 | | 12/31/07 | | 12/31/08 | |
|---|
Quidel Corporation | | $ | 100.00 | | $ | 47.17 | | $ | 99.91 | | $ | 126.46 | | $ | 180.78 | | $ | 121.36 | |
Nasdaq Composite | | | 100.00 | | | 110.08 | | | 112.88 | | | 126.51 | | | 138.13 | | | 80.47 | |
Nasdaq Pharmaceutical | | | 100.00 | | | 110.22 | | | 111.87 | | | 114.89 | | | 106.37 | | | 97.32 | |
28
Item 6. Selected Financial Data
The following table presents selected consolidated financial data of Quidel Corporation. This historical data should be read in conjunction with the Consolidated Financial Statements and related Notes thereto in Item 8 and "Management's Discussion and Analysis of Financial Condition and Results of Operation" in Item 7 in this Annual Report.
Consolidated Statements of Operations
| | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | | 2005(1) | | 2004 | |
|---|
| | (in thousands, except per share data)
| |
|---|
Total revenues | | $ | 128,132 | | $ | 118,065 | | $ | 106,015 | | $ | 92,299 | | $ | 78,691 | |
Costs and expenses | | | | | | | | | | | | | | | | |
| | Cost of sales (excludes amortization of intangible assets) | | | 50,206 | | | 48,573 | | | 44,818 | | | 37,101 | | | 35,234 | |
| | Research and development | | | 11,147 | | | 12,855 | | | 13,047 | | | 12,829 | | | 11,340 | |
| | Sales and marketing | | | 20,898 | | | 18,491 | | | 16,966 | | | 16,121 | | | 13,990 | |
| | General and administrative | | | 12,786 | | | 13,167 | | | 12,770 | | | 13,062 | | | 14,852 | |
| | Amortization of intangible assets | | | 4,476 | | | 5,493 | | | 4,580 | | | 1,476 | | | 1,459 | |
| | Patent litigation settlement | | | — | | | — | | | — | | | 17,000 | | | — | |
| | | | | | | | | | | | |
| | | Total costs and expenses | | | 99,513 | | | 98,579 | | | 92,181 | | | 97,589 | | | 76,875 | |
| | | | | | | | | | | | |
Operating income (loss) | | | 28,619 | | | 19,486 | | | 13,834 | | | (5,290 | ) | | 1,816 | |
Other income (expense) | | | | | | | | | | | | | | | | |
| | Interest income | | | 1,686 | | | 1,891 | | | 1,408 | | | 722 | | | 398 | |
| | Interest expense | | | (671 | ) | | (736 | ) | | (757 | ) | | (808 | ) | | (886 | ) |
| | Other income (expense) | | | 135 | | | (117 | ) | | 545 | | | 49 | | | 256 | |
| | | | | | | | | | | | |
| | | Total other income (expense) | | | 1,150 | | | 1,038 | | | 1,196 | | | (37 | ) | | (232 | ) |
| | | | | | | | | | | | |
Income (loss) from continuing operations before provision (benefit) for income taxes | | | 29,769 | | | 20,524 | | | 15,030 | | | (5,327 | ) | | 1,584 | |
Provision (benefit) for income taxes | | | 10,921 | | | 6,893 | | | (5,891 | ) | | 3,000 | | | — | |
| | | | | | | | | | | | |
Income (loss) from continuing operations | | | 18,848 | | | 13,631 | | | 20,921 | | | (8,327 | ) | | 1,584 | |
| | | | | | | | | | | | |
Gain (loss) from discontinued operations, net of taxes | | | — | | | — | | | 797 | | | (932 | ) | | (7,871 | ) |
| | | | | | | | | | | | |
Net income (loss) | | $ | 18,848 | | $ | 13,631 | | $ | 21,718 | | $ | (9,259 | ) | $ | (6,287 | ) |
| | | | | | | | | | | | |
Basic earnings (loss) per share: | | | | | | | | | | | | | | | | |
| | Continuing operations | | $ | 0.59 | | $ | 0.43 | | $ | 0.63 | | $ | (0.26 | ) | $ | 0.05 | |
| | Discontinued operations | | | — | | | — | | | 0.02 | | | (0.03 | ) | | (0.25 | ) |
| | Net income (loss) | | | 0.59 | | | 0.43 | | | 0.66 | | | (0.28 | ) | | (0.20 | ) |
Diluted earnings (loss) per share: | | | | | | | | | | | | | | | | |
| | Continuing operations | | $ | 0.58 | | $ | 0.41 | | $ | 0.61 | | $ | (0.26 | ) | $ | 0.05 | |
| | Discontinued operations | | | — | | | — | | | 0.02 | | | (0.03 | ) | | (0.25 | ) |
| | Net income (loss) | | | 0.58 | | | 0.41 | | | 0.63 | | | (0.28 | ) | | (0.20 | ) |
Shares used in basic per share calculation | | | 31,853 | | | 32,028 | | | 32,985 | | | 32,525 | | | 31,487 | |
Shares used in diluted per share calculation | | | 32,612 | | | 32,996 | | | 34,367 | | | 32,525 | | | 31,487 | |
Balance Sheet Data
| | | | | | | | | | | | | | | | |
| | December 31 | |
|---|
| | 2008 | | 2007 | | 2006 | | 2005(1) | | 2004 | |
|---|
| | (in thousands)
| |
|---|
Cash and cash equivalents | | $ | 57,908 | | $ | 45,489 | | $ | 36,625 | | $ | 34,930 | | $ | 36,322 | |
Working capital | | $ | 85,592 | | $ | 70,259 | | $ | 53,063 | | $ | 43,984 | | $ | 49,769 | |
Total assets | | $ | 142,808 | | $ | 133,838 | | $ | 127,048 | | $ | 113,848 | | $ | 112,691 | |
Long-term obligations | | $ | 8,138 | | $ | 9,161 | | $ | 9,166 | | $ | 9,986 | | $ | 10,780 | |
Stockholders' equity | | $ | 119,236 | | $ | 107,703 | | $ | 103,276 | | $ | 87,243 | | $ | 90,185 | |
Common shares outstanding | | | 31,894 | | | 32,706 | | | 33,530 | | | 33,778 | | | 31,848 | |
- (1)
- During the second quarter of 2005, we entered into an agreement to settle certain patent litigation. In conjunction with the settlement, we recorded a charge and paid cash of $17.0 million in 2005.
29
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operation
The following discussion of our financial condition and results of operations contains forward-looking statements within the meaning of the federal securities laws that involve material risks and uncertainties. This discussion should be read in conjunction with "A Warning About Forward-Looking Statements" on page 2 and "Risk Factors" under Item 1A of this Annual Report. In addition, our discussion of the financial condition and results of operations of Quidel Corporation in this Item 7 should be read in conjunction with our Consolidated Financial Statements and the related Notes included elsewhere in this Annual Report.
Executive Summary
We have a leadership position in the development, manufacturing and marketing of rapid diagnostic solutions for decentralized applications including point-of-care ("POC") in infectious diseases and reproductive and women's health. We focus on POC testing solutions specifically developed for the physician office lab ("POL") and acute care markets globally. We sell our products to professionals for use in physician offices, hospitals, clinical laboratories, retail clinics and wellness screening centers. We market our products in the U.S. through a network of national and regional distributors, supported by a direct sales force. Internationally, we sell and market primarily in Japan, Europe and the Middle East through exclusive distributor arrangements.
A majority of our total revenues relate to three product families. For the years ended December 31, 2008, 2007 and 2006, we derived approximately 84%, 81% and 82%, respectively, of our total revenues from sales of our influenza, Group A Strep and pregnancy tests. Additionally, a significant portion of our total revenue is from a relatively small number of distributors. Approximately 60%, 61% and 68% of our total revenue for the years ended December 31, 2008, 2007 and 2006, respectively, were related to sales through our five largest distributors in each of those periods.
We also develop research products through our Specialty Products Group (the "SPG") with an emphasis on potential future rapid test applications. The SPG is currently responsible for more than 100 of our clinical and research products used worldwide in reference laboratories and in research applications at leading universities and biotechnology companies. The SPG revenues, income and assets are less than 10% of our overall operations.
Our net revenue increased to $128.1 million for the year ended December 31, 2008 from $118.1 million for the year ended December 31, 2007. This was largely driven by increased domestic sales of our infectious disease products, partially offset by a decrease in our reproductive and women's health, and other products. We are continuing to focus our efforts to strengthen market and brand leadership in infectious disease and reproductive and women's health by delivering economic and clinical proof through our efforts with our Quidel Value Build™ ("QVB™") program. Our POC testing solutions are designed to provide specialized results that meet two important value criteria that we have branded as QVB™:
- •
- Clinical validation: the enabling of rapid patient management decisions leading to improved treatment and outcomes.
- •
- Economic validation: the reduction of overall costs associated with patient testing with emphasis upon critical reimbursement and payer performance criteria.
We focus on ensuring market leadership and providing points of differentiation by specializing in the diagnosis and monitoring of selected disease states. In order to support our value proposition as a company that markets the highest quality products in support of better medical outcomes, we are highlighting our QVB™ through the development of new innovations and the communication of new solutions in the field of rapid diagnostic testing. Our QVB™ includes significant work in understanding the needs of the end-use customer, building products that meet those needs, providing proof studies to
30
validate rapid diagnostic testing at the point-of-care and leveraging the work of researchers and key opinion leaders studying our tests and technology to help enhance the health and well being of people around the globe. Our marketing strategy includes ensuring that our key product portfolios are supported by economic and clinical validation that shows hospitals, acute care facilities and POC clinicians that these tests deliver high quality results in a cost-effective manner.
We believe that the trend among healthcare providers to adopt POC testing continues to increase, and demographic changes, reimbursement policies, a shortage of skilled laboratory workers and the availability of clinically valuable tests will increase growth in this diagnostic category. More and more employers, health plans and payers are recognizing that POC testing is a cost-effective means for improving the quality of care and patient satisfaction. Continuous improvements in technologies are resulting in a growing number of new diagnostic tests that combine high levels of accuracy with rapid, easy-to-use product formats. It is our mission to further establish our significant leadership position in POC rapid diagnostics. In order to accomplish this mission, our strategy is to:
- •
- provide clinicians with validated, evidence-based studies which encompass the clinical efficacy and economic efficiency of our rapid POC tests for the professional market. In conjunction with our QVB™ commitment, we expect to present ongoing information that supports the adoption of rapid POC testing;
- •
- continue to focus on strengthening our market and brand leadership in infectious diseases and reproductive and women's health by acquiring, developing and introducing clinically superior diagnostic solutions;
- •
- drive growth by securing dedicated distributor partnerships and strengthening our sales organization to assure physician and laboratorian satisfaction through direct relationships with Integrated Delivery Networks and hospitals;
- •
- support payer evaluation of rapid tests and establishment of favorable reimbursement rates;
- •
- continue creation of strong global alliances to assure leadership in key markets;
- •
- drive profit through further refinement of our manufacturing efficiencies and productivity improvements, with continued focus on profitable products and markets and our effort to create exceptional competency in new product development;
- •
- continue to focus our research and development efforts on three areas: 1) new proprietary product platform development, 2) the creation of improved products and new products for existing markets and unmet clinical needs, and 3) products developed under collaborations with other companies for new and existing markets; and
- •
- identify and commercialize new markers, products and collaborations in bone health through the SPG.
As a business in a highly regulated and competitive industry, we face many risks and challenges and we also have opportunities. There are many economic and industry factors that affect our business; some of the more important factors are outlined below:
- •
- sales of our infectious disease products, which have collectively accounted for approximately 72%, 64% and 65% of total revenue for the years ended December 31, 2008, 2007 and 2006, respectively, are subject to and significantly affected by the seasonal demands of the cold and flu seasons;
- •
- sales of our products can be affected significantly by many competitive factors, including convenience, price and product performance as well as the distribution, advertising, promotion and brand name recognition of the marketer;
31
- •
- intellectual property protection of our products is crucial to our business;
- •
- the testing, manufacture and commercialization of our products are subject to regulation by numerous governmental authorities, principally the FDA and corresponding state and foreign regulatory agencies;
- •
- the production processes for POC tests are complex, highly regulated and vary widely from product to product;
- •
- to successfully compete for business in our industry, we believe our POC testing solutions must be designed to provide specific results for clinical and economic validation; and
- •
- there has been a trend toward industry consolidation in our markets over the last several years.
In January 2009, we announced that Caren L. Mason, our President and Chief Executive Officer, has decided to retire from the Company on June 1, 2009. Ms. Mason will continue in her current capacity as President and CEO as well as a Board Director until March 1, 2009, and from March 1, 2009 through her retirement date, Ms. Mason will serve as a special advisor to the CEO. In January 2009, we also announced the appointment of Douglas C. Bryant as the Company's new President, CEO and member of the Board of Directors. Mr. Bryant began his employment in February 2009 and his role as President and CEO is effective March 1, 2009.
Outlook
For fiscal year 2009, we anticipate year-over-year revenue growth. We expect gross margins will be positively affected by a more favorable product mix, partially offset by lower average selling prices. We continue to expect a gradual conversion of the fecal occult blood test market from the current guaiac-based test to an immunochemical-based test. Successful conversion of this market requires changing physician behavior through education, focused in part on clinical and economic validation. Additionally, we expect continued growth from our QuickVue® RSV test for the qualitative detection of respiratory syncytial virus ("RSV") this season so that physicians are well prepared to diagnose and appropriately manage patients with influenza and/or RSV. We received Clinical Laboratory Improvement Amendments of 1988 ("CLIA") waiver on our RSV test in February 2008. Internationally, we expect continued growth as we increase the reach of our products to markets around the world. We expect an overall increase in operating expenses for fiscal 2009, including additional investments in research and development.
You should also refer to the discussion in Item 1A, "Risk Factors" in Part I of this Annual Report for further discussion of risks related to our business.
32
Results of Operations
Comparison of years ended December 31, 2008 and 2007
Total Revenues
The following table compares total revenues for the years ended December 31, 2008 and 2007 (in thousands, except percentages):
| | | | | | | | | | | | | |
| | For the year ended
December 31, | | Increase
(decrease) | |
|---|
| | 2008 | | 2007 | | $ | | % | |
|---|
Infectious disease net product sales | | $ | 92,426 | | $ | 75,896 | | $ | 16,530 | | | 22 | % |
Reproductive and women's health net product sales | | | 22,989 | | | 28,130 | | | (5,141 | ) | | (18 | )% |
Other net product sales | | | 11,427 | | | 12,864 | | | (1,437 | ) | | (11 | )% |
Royalty income and license fees | | | 1,290 | | | 1,175 | | | 115 | | | 10 | % |
| | | | | | | | | | | |
Total revenues | | $ | 128,132 | | $ | 118,065 | | $ | 10,067 | | | 9 | % |
| | | | | | | | | | | |
The increase in total revenues was primarily driven by increased sales of our infectious disease products, partially offset by a decrease in our reproductive and women's health and other product categories. We believe the increase in total revenue of our infectious disease products, for both our domestic and international markets, was largely driven by increased market penetration and increased utilization of our influenza test, while the decrease associated with our reproductive and women's health products was primarily driven by the timing of ordering patterns in the domestic market. Purchases by end-users of our non-seasonal products remained fairly constant for the twelve months ended December 31, 2008 as compared to the previous twelve months. Sales of our infectious disease and reproductive and women's health products accounted for 90% and 88% of our total revenue for the years ended December 31, 2008 and 2007, respectively.
The revenue from royalty income and license fees for all periods primarily relate to royalty payments earned on our patented technologies utilized by third parties.
Cost of Sales
Cost of sales increased 3% to $50.2 million, or 39% of total revenue, for the year ended December 31, 2008 compared to $48.6 million, or 41% of total revenues, for the year ended December 31, 2007. The absolute dollar increase is primarily related to the variable nature of direct costs (material and labor) associated with the 9% increase in total revenues. The percentage decrease in cost of sales as a percentage of total revenue was primarily due to a more favorable product mix, partially offset by lower average selling prices.
33
Operating Expenses
The following table compares operating expenses for the years ended December 31, 2008 and 2007 (in thousands, except percentages):
| | | | | | | | | | | | | | | | | | | |
| | For the year ended
December 31, | |
| |
| |
|---|
| | 2008 | | 2007 | |
| |
| |
|---|
| | Increase (decrease) | |
|---|
| | Operating expenses | | As a % of total revenues | | Operating expenses | | As a % of total revenues | |
|---|
| | $ | | % | |
|---|
Research and development | | $ | 11,147 | | | 9 | % | $ | 12,855 | | | 11 | % | $ | (1,708 | ) | | (13 | )% |
Sales and marketing | | | 20,898 | | | 16 | % | | 18,491 | | | 16 | % | | 2,407 | | | 13 | % |
General and administrative | | | 12,786 | | | 10 | % | | 13,167 | | | 11 | % | | (381 | ) | | (3 | )% |
Amortization of intangible assets | | | 4,476 | | | 4 | % | | 5,493 | | | 5 | % | | (1,017 | ) | | (19 | )% |
Research and Development Expense
The decrease in research and development expense was due primarily to the discontinuation of our layered thin film immunoassay program in the fourth quarter of 2007 and a decrease in overall incentive-based compensation for 2008, partially offset by increased investment in other strategic research and development efforts. The primary components of research and development expense are personnel and material costs associated with development of potential new technologies and processes and with products under development. In addition, we continue to incur substantial costs related to clinical trials as well as our overall efforts under our QVB programs.
Sales and Marketing Expense
The increase in sales and marketing expense was primarily related to an overall increase in sales personnel and related programs and expenses as well increased expenses associated with distribution events and trade shows, which support our leadership position and strategies to capitalize further on opportunities in POC diagnostics. This was partially offset by a decrease in overall incentive-based compensation for 2008. Other key components of this expense relate to continued investment in assessing future product extensions and enhancements, market research (including voice of customer surveys), reimbursement-related activities and product shipment costs.
General and Administrative Expense
The decrease in general and administrative expense was primarily due to a decrease in overall incentive-based compensation for 2008, partially offset by increased headcount added during late 2007.
Amortization of Intangible Assets
The amortization of intangible assets decreased primarily due to the full amortization of certain purchased technology in fiscal year 2007. In December 2008, amortization of $3.0 million associated with a license agreement became fully amortized. Unless the company acquires new intangible assets, amortization of intangibles will decrease in 2009.
Other Income (Expense)
The slight decrease in interest income to $1.7 million as of December 31, 2008 from $1.9 million as of December 31, 2007 was primarily related to the decrease in interest rates, partially offset by an increase in our average cash balance for the year ended December 31, 2008 as compared to the year ended December 31, 2007. Interest expense was relatively constant at $0.7 million for both of the years
34
ended December 31, 2008 and 2007 and relates to interest paid on obligations under capital leases, primarily associated with our San Diego facility.
Income Taxes
We recognized income tax expense of $11.0 million for the year ended December 31, 2008 as compared to $6.9 million for the year ended December 31, 2007, which was largely driven by the increase in taxable income from 2007 to 2008. Income tax expense for 2008 includes a net reduction primarily related to deduction associated with investments in foreign subsidiaries and a manufacturing tax deduction. Income tax expense for 2007 includes a reduction of $0.7 million for the completion of a research and development tax credit study for prior years.
Comparison of years ended December 31, 2007 and 2006
Total Revenues
The following table compares total revenues for the years ended December 31, 2007 and 2006 (in thousands, except percentages):
| | | | | | | | | | | | | |
| | For the year ended
December 31, | | Increase
(decrease) | |
|---|
| | 2007 | | 2006 | | $ | | % | |
|---|
Infectious disease net product sales | | $ | 75,896 | | $ | 68,565 | | $ | 7,331 | | | 11 | % |
Reproductive and women's health net product sales | | | 28,130 | | | 25,699 | | | 2,431 | | | 9 | % |
Other net product sales | | | 12,864 | | | 10,468 | | | 2,396 | | | 23 | % |
Royalty income and license fees | | | 1,175 | | | 1,283 | | | (108 | ) | | (8 | )% |
| | | | | | | | | | | |
Total revenues | | $ | 118,065 | | $ | 106,015 | | $ | 12,050 | | | 11 | % |
| | | | | | | | | | | |
The increase was largely driven by an increase in sales of our infectious disease and reproductive and women's health products of $7.3 million and $2.4 million, respectively. The overall increase was partially offset by an expected decrease of our influenza product revenues in our Japanese market. We believe the increase in total revenue from these product groups was due to successes related to our QVB™ programs, which have resulted in strengthened customer relationships and preferred partnership programs. We believe that sales of our influenza products continue to increase as a result of increased market awareness, greater utilization and the demonstrated quality of our test. We believe our average selling price in the U.S. has continued to increase largely as a result of our clinical proof claims and product quality, while we have experienced downward pressure in the Japanese market as a result of reimbursement changes and increased competition. Sales of our infectious disease and reproductive and women's health products accounted for 88% and 89% of our total revenue for the years ended December 31, 2007 and 2006, respectively.
The revenue from royalty income and license fees for all periods primarily relate to royalty payments earned on patented technologies of ours utilized by third parties.
Cost of Sales
Cost of sales increased 8% to $48.6 million, or 41% of total revenue, for the year ended December 31, 2007 compared to $44.8 million, or 42% of total revenues, for the year ended December 31, 2006. The absolute dollar increase was primarily related to the increase in direct costs (material and labor) associated with the 11% increase in total revenues. The percentage decrease in cost of sales to revenue was primarily due to a more favorable product and geographic mix and the leveraging of fixed costs associated with higher unit volume and increased average selling prices.
35
Operating Expenses
The following table compares operating expenses for the years ended December 31, 2007 and 2006 (in thousands, except percentages):
| | | | | | | | | | | | | | | | | | | |
| | For the year ended
December 31, | |
| |
| |
|---|
| | 2007 | | 2006 | | Increase
(decrease) | |
|---|
| | Operating expenses | | As a % of total revenues | | Operating expenses | | As a % of total revenues | |
|---|
| | $ | | % | |
|---|
Research and development | | $ | 12,855 | | | 11 | % | $ | 13,047 | | | 12 | % | $ | (192 | ) | | (1 | )% |
Sales and marketing | | | 18,491 | | | 16 | % | | 16,966 | | | 16 | % | | 1,525 | | | 9 | % |
General and administrative | | | 13,167 | | | 11 | % | | 12,770 | | | 12 | % | | 397 | | | 3 | % |
Amortization of intangible assets | | | 5,493 | | | 5 | % | | 4,580 | | | 4 | % | | 913 | | | 20 | % |
Research and Development Expense
Our research and development expenses were relatively constant. While we may experience some fluctuation in our research and development activities associated with the timing of certain projects, the primary components of research and development expense are personnel and material costs associated with development of potential new technologies and processes and with products under development. In addition, we continue to incur costs related to intellectual property, clinical activity as well as our overall efforts under our QVB™ programs.
Sales and Marketing Expense
The increase in sales and marketing expense was primarily related to an overall increase in sales personnel and related programs and expenses, which support our leadership position and strategies to capitalize further on opportunities in POC diagnostics. Other key components of this expense relate to continued investment in assessing future product extensions and enhancements, market research (including voice of customer surveys), programs aimed at distribution partners and end-user customers and reimbursement-related activities and product shipment costs.
General and Administrative Expense
The increase in general and administrative expenses was primary driven by increased stock compensation expense and costs associated with the departure of our former Chief Financial Officer and hiring a new Chief Financial Officer.
Amortization of Intangible Assets
The increase in the amortization of intangible assets was primarily due to a license agreement entered into during late 2006 and an additional license agreement entered into during 2007.
Other Income (Expense)
Interest income was $1.9 million and $1.4 million for the years ended December 31, 2007 and 2006, respectively. The increase in interest income was largely related to the increase in our average cash balance as well as more favorable interest rates for the year ended December 31, 2007 as compared to the prior year. Interest expense was relatively constant at $0.7 million for both of the years ended December 31, 2007 and 2006. Interest expense relates to interest paid on obligations under capital leases, primarily associated with our San Diego facility.
36
Income Taxes
We recognized income tax expense of $6.9 million for the year ended December 31, 2007 versus a tax benefit of $5.9 million for the year ended December 31, 2006. Income tax expense for 2007 includes a reduction of $0.7 million for the completion of a research and development tax credit study for prior years. For 2006, we recorded a tax benefit which was primarily related to a decrease in the deferred tax valuation allowance during the fourth quarter ended December 31, 2006 and recognizes the deferred tax asset amount considered by management, more likely than not, to be realized.
Liquidity and Capital Resources
As of December 31, 2008, our principal sources of liquidity consisted of $57.9 million in cash and cash equivalents, as well as $120.0 million available to us under our senior secured syndicated credit facility (the "Senior Credit Facility"). Our working capital as of December 31, 2008 was $85.6 million.
Cash provided by our operating activities was $28.9 million for the year ended December 31, 2008. We had net income of $18.8 million, including non-cash charges of $8.7 million of depreciation and amortization of intangible assets and property and equipment. Other changes in operating assets and liabilities included increases in accounts receivable and inventory of $2.2 million and $0.7 million, respectively, and is due to the timing of sales during the fourth quarter of 2008, and a decrease of $0.5 million and $1.6 million for accounts payable and accrued payroll and related expenses, respectively. The decrease in accounts payable was largely due to seasonal demand fluctuations of our influenza product and amounts included in accounts payable for capital expenditures at the end of fiscal 2007, while the decrease in payroll and related expenses was largely related to payments made during 2008 under our 2007 employee compensation programs as well as a reduction of incentive-based compensation in 2008. The increase in other accrued liabilities of $2.2 million was primarily due to an increase in the allowance for volume discounts related to the increase in total revenues.
Our investing activities used $4.4 million during the year ended December 31, 2008, which was primarily for the acquisition of production and scientific equipment and building improvements. We had investments in property, plant and equipment of $0.3 million which had not been paid as of December 31, 2008.
We are currently planning approximately $6.0 million in capital expenditures over the next 12 months. The primary purpose for our capital expenditures is to acquire manufacturing equipment, implement facility improvements, and for information technology. We plan to fund these capital expenditures with cash flow from operations. We do not have any material firm purchase commitments with respect to such planned capital expenditures as of the date of filing this report.
Our financing activities used $12.1 million of cash during the year ended December 31, 2008 which was primarily related to the repurchase of approximately 1.3 million shares of our common stock in the open market at a cost of $19.8 million, partially offset by the benefit of $6.5 million from excess taxes from shared-based compensation, and proceeds of $3.3 million received from the issuance of common stock under our equity incentive and our employee stock purchase plans.
On October 8, 2008, we entered into our new $120.0 million Senior Credit Facility, which matures on October 8, 2013. The new credit facility replaced the Company's $30.0 million credit facility. The Senior Credit Facility bears interest at a rate ranging from 0.50% to 1.75% plus the lender's prime rate or, at the Company's option, a rate ranging from 1.50% to 2.75% plus the London InterBank Offering Rate. The agreement governing the Senior Credit Facility is subject to certain customary limitations, including among others: limitation on liens; limitation on mergers, consolidations and sales of assets; limitation on debt; limitation on dividends, stock redemptions and the redemption and/or prepayment of other debt; limitation on investments (including loans and advances) and acquisitions; limitation on transactions with affiliates; and limitation on annual capital expenditures. We are also subject to
37
financial covenants which include a funded debt to earnings before interest, taxes, depreciation and amortization (EBITDA) ratio, and an interest coverage ratio. The Senior Credit Facility is secured by substantially all present and future assets and properties of the Company. As of December 31, 2008, we had no amounts outstanding and $120.0 million of availability under the Senior Credit Facility and we were in compliance with all financial covenants. See Note 2 in the Notes to the Consolidated Financial Statements included in this Annual Report.
We also intend to continue evaluation of acquisition and technology licensing candidates. As such, we may need to incur additional debt, or issue additional equity, to successfully complete these transactions. Cash requirements fluctuate as a result of numerous factors, such as the extent to which we generate cash from operations, progress in research and development projects, competition and technological developments and the time and expenditures required to obtain governmental approval of our products. Based on our current cash position and the current assessment of future operating results, we believe that our existing sources of liquidity will be adequate to meet operating needs during the next 12 months and the foreseeable future.
Off-Balance Sheet Arrangements
At December 31, 2008 and 2007, we did not have any other relationships with unconsolidated entities or financial partners, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. As such, we are not materially exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in such relationships.
Contractual Obligations
Our facilities and certain equipment are leased under noncancelable capital and operating leases. The following is a summary of our contractual obligations (in thousands):
| | | | | | | | | | | | | | | | |
| | Payment due by period | |
|---|
| | Total | | Less than 1 year | | 1-3 Years | | 3-5 Years | | More than 5 years | |
|---|
Capital lease obligations(1) | | $ | 9,035 | | $ | 1,432 | | $ | 2,951 | | $ | 3,070 | | $ | 1,582 | |
Operating lease obligations(2) | | | 5,078 | | | 834 | | | 1,729 | | | 1,712 | | | 803 | |
| | | | | | | | | | | | |
Total | | $ | 14,113 | | $ | 2,266 | | $ | 4,680 | | $ | 4,782 | | $ | 2,385 | |
| | | | | | | | | | | | |
- (1)
- Reflects obligations on facilities and equipment under capital leases, including current maturities, in place as of December 31, 2008. Future minimum lease payments are included in the table above.
- (2)
- Reflects obligations on facilities and equipment under operating leases in place as of December 31, 2008. In the fourth quarter of 2007, we entered into a new operating lease at our Santa Clara location, including extending the term of the lease through 2014. Future minimum lease payments are included in the table above.
We have entered into various licensing agreements, which require royalty payments based on specified product sales. These agreements, which have anticipated expiration dates through 2014, encompass the majority of our products. Royalty expenses under these licensing agreements, which are charged to cost of sales, collectively totaled $10.5 million, $9.4 million and $9.6 million for the years ended December 31, 2008, 2007 and 2006, respectively. We believe we will continue to incur substantial royalty expenses relating to future sales of our products.
38
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to customer programs and incentives, bad debts, inventories, intangible assets, income taxes, restructuring and contingencies and litigation. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our consolidated financial statements:
We record revenues primarily from product sales. These revenues are recorded net of rebates and other discounts which are estimated at the time of sale. The rebates and other discounts are largely driven by various customer program offerings, including special pricing agreements, promotions and other volume-based incentives. Revenue from product sales is recorded upon passage of title and risk of loss to the customer. Change in title to the product and recognition of revenue occur upon delivery to the customer when sales terms are free on board ("FOB") destination and at the time of shipment when the sales terms are FOB shipping point and there is no right of return. We also earn income from the licensing of technology. Royalty income from the grant of license rights is recognized during the period in which the revenue is earned and the amount is determinable from the licensee. Income earned from licensing activities is a component of total revenues in the accompanying Consolidated Statements of Income.
Effective January 1, 2006, we adopted the fair value recognition provisions of SFAS No. 123(R), "Share-Based Payment," using the modified prospective transition method. Under that transition method, compensation expense that we recognize beginning on that date includes: (a) compensation expense for all share-based awards granted prior to, but not yet vested as of December 31, 2005, based on the grant-date fair value estimated in accordance with the original provisions of SFAS No. 123, and (b) compensation expense for all share-based awards granted on or after January 1, 2006 based on the grant-date fair value estimated in accordance with the provisions of SFAS No. 123(R). Results for prior periods have not been restated.
The computation of the expected option life is based on a weighted-average calculation combining the average life of options that have already been exercised and post-vest cancellations with the estimated life of the remaining vested and unexercised options. The expected volatility is based on the historical volatility of our stock. The volatility of our stock has decreased in recent fiscal periods, and as a result we changed our look-back period in determining volatility to the third quarter of 2005. This change did not have a material impact on our financial statements for fiscal year 2008. The risk-free interest rate is based on the U.S Treasury yield curve over the expected term of the option. We have never paid any cash dividends on our common stock, and we do not anticipate paying any cash dividends in the foreseeable future. Consequently, we use an expected dividend yield of zero in the Black-Scholes option valuation model. The estimated forfeiture rate is based on our historical experience and future expectations.
39
Compensation expense related to stock options granted is recognized ratably over the service vesting period for the entire option award. The total number of stock options expected to vest is adjusted by estimated forfeiture rates. The estimated fair value of each stock option was determined on the date of grant using the Black-Scholes option valuation model. Compensation expense for restricted stock awards ("stock awards") is measured at the grant date and recognized ratably over the vesting period. The fair value of stock awards is determined based on the closing market price of our common stock on the grant date. For stock awards granted prior to December 31, 2005, vesting is service-based with performance goals that allow for acceleration of a portion of the stock awards. A majority of the stock awards granted in 2006 and 2007 were performance-based and vesting was tied to achievement of predetermined revenue and/or EBITDA goals. For purposes of measuring compensation expense, the amount of shares ultimately expected to vest is estimated at each reporting date based on management's expectations regarding the relevant performance criteria. The recognition of compensation expense associated with performance-based grants requires judgment in assessing the probability of meeting the performance goals, as well as defined criteria for assessing achievement of the performance related goals. This may result in significant expense recognition or reversal in the period in which the performance goals are met or when achievement of the goals is deemed probable or may result in the reversal of previously recognized stock-based compensation expense if the performance criteria are deemed not probable of being met. The grant date of the performance-based stock grants takes place when the grant is authorized and the specific achievement goals are communicated. The communication date of the performance goals can impact the valuation and associated expense of the stock awards.
We maintain an allowance for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. Our allowance for doubtful accounts is based on our assessment of the collectibility of specific customer accounts, the aging of accounts receivable, our history of bad debts, and the general condition of the industry. If a major customer's credit worthiness deteriorates, or our customers' actual defaults exceed our historical experience, our estimates could change and adversely impact our reported results.
Our policy is to value inventories at the lower of cost or market on a part-by-part basis. This policy requires us to make estimates regarding the market value of our inventories, including an assessment of excess or obsolete inventories. We determine excess and obsolete inventories based on an estimate of the future demand for our products within a specified time horizon, generally 12 months. The estimates we use for demand are also used for near-term capacity planning and inventory purchasing and are consistent with our revenue forecasts. If our demand forecast is greater than our actual demand, we may be required to take additional excess inventory charges, which would decrease gross margin and adversely impact net operating results in the future.
Intangible assets with definite lives are amortized over their estimated useful lives. Useful lives are based on the expected number of years the asset will generate revenue or otherwise be used by us. SFAS No. 142 "Goodwill and Other Intangible Assets," requires that goodwill and other intangible assets that have indefinite lives not be amortized but instead be tested at least annually for impairment, or more frequently when events or changes in circumstances indicate that the asset might be impaired. Examples of such events or circumstances include:
- •
- the asset's ability to continue to generate income from operations and positive cash flow in future periods;
40
- •
- any volatility or significant decline in our stock price and market capitalization compared to our net book value;
- •
- loss of legal ownership or title to an asset;
- •
- significant changes in our strategic business objectives and utilization of our assets; and
- •
- the impact of significant negative industry or economic trends.
If a change were to occur in any of the above-mentioned factors or estimates, the likelihood of a material change in our reported results would increase.
For goodwill, a two-step test is used to identify the potential impairment and to measure the amount of impairment, if any. The first step is to compare the fair value of a reporting unit with the carrying amount, including goodwill. If the fair value of a reporting unit exceeds its carrying amount, goodwill is considered not impaired; otherwise, goodwill is impaired and the loss is measured by performing step two. Under step two, the impairment loss is measured by comparing the implied fair value of the reporting unit with the carrying amount of goodwill. SFAS No. 142 requires periodic evaluations for impairment of goodwill balances. We completed our annual evaluation for impairment of goodwill as of December 31, 2008 and determined that no impairment of goodwill existed.
We account for income taxes in accordance with SFAS No. 109, "Accounting for Income Taxes." Significant judgment is required in determining our provision for income taxes, current tax assets and liabilities, deferred tax assets and liabilities, and our future taxable income for purposes of assessing our ability to realize future benefit from our deferred tax assets. A valuation allowance is established to reduce our deferred tax assets to the amount that is considered more likely than not to be realized through the generation of future taxable income and other tax planning opportunities. To the extent that a determination is made to establish or adjust a valuation allowance, the expense or benefit is recorded in the period in which the determination is made.
Effective January 1, 2007, we adopted the provisions of FASB No. 48, "Accounting for Uncertainty in Income Taxes" ("FIN 48"). FIN 48 provides guidance for the recognition threshold and measurement attribute for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. In accordance with FIN 48, we recognized a cumulative-effect adjustment of $0.7 million, increasing the January 1, 2007 balance of retained earnings. See Note 3 in the Notes to the Consolidated Financial Statements included in this Annual Report for more information on income taxes.
As a result of the adoption of SFAS No. 123(R), we recognize excess tax benefits associated with the exercise of stock options directly to stockholders' equity only when realized. Accordingly, deferred tax assets are not recognized for net operating loss carryforwards resulting from excess tax benefits. As of December 31, 2008 and 2007, deferred tax assets do not include $2.7 million and $7.1 million, respectively, of these excess tax benefits from employee stock option exercises that are a component of our net operating loss carryforwards. Additional paid-in capital will be increased up to $2.7 million if such excess tax benefits are realized.
New Accounting Pronouncements
In December 2007, the Financial Accounting Standards Board ("FASB") revised Statement No. 141, "Business Combinations" ("FAS 141R"), which establishes principles and requirements for how the acquirer in a business combination (i) recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed and any noncontrolling interest in the acquiree, (ii) recognizes and measures the goodwill acquired in the business combination or a gain from a
41
bargain purchase and (iii) determines what information to disclose to enable readers of the financial statements to evaluate the nature and financial effects of the business combination. FAS 141R will be effective for any business combination that occurs beginning in fiscal year 2009.
In April 2008, the FASB issued FASB Staff Position 142-3, "Determination of the Useful Life of Intangible Assets" ("FSP 142-3"). FSP 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB Statement No. 142, "Goodwill and Other Intangible Assets" ("FAS 142"). The objective of FSP 142-3 is to improve the consistency between the useful life of a recognized intangible asset under FAS 142 and the period of expected cash flows used to measure the fair value of the asset under FAS 141R and other U.S. generally accepted accounting principles. FSP 142-3 will be effective beginning in fiscal year 2009. We are currently evaluating the impact that this pronouncement will have on our consolidated financial statements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
The fair market value of our floating interest rate debt is subject to interest rate risk. Generally, the fair market value of floating interest rate debt will vary as interest rates increase or decrease. A hypothetical 100 basis point adverse move in interest rates along the entire interest rate yield curve would not materially affect the fair value of our interest sensitive financial instruments at December 31, 2008. Based on our market risk sensitive instruments outstanding at December 31, 2008 and 2007, we have determined that there was no material market risk exposure to our consolidated financial position, results of operations or cash flows as of such dates.
Our current investment policy with respect to our cash and cash equivalents focuses on maintaining acceptable levels of interest rate risk and liquidity. Although we continually evaluate our placement of investments, as of December 31, 2008, our cash and cash equivalents were placed in money market or overnight funds that are highly liquid and which we believe are not subject to material market fluctuation risk.
All of our international sales are negotiated for and paid in U.S. dollars. Nonetheless, these sales are subject to currency risks, since changes in the values of foreign currencies relative to the value of the U.S. dollar can render our products comparatively more expensive. These exchange rate fluctuations could negatively impact international sales of our products and our anticipated foreign operations, as could changes in the general economic conditions in those markets. Continued change in the values of the Euro, the Japanese Yen and other foreign currencies could have a negative impact on our business, financial condition and results of operations. We do not currently hedge against exchange rate fluctuations, which means that we are fully exposed to exchange rate changes.
Item 8. Financial Statements and Supplementary Data
The consolidated financial statements and supplementary data required by this item are set forth at the pages indicated in Item 15(a)(1) and are incorporated herein.
42
Part III
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of disclosure controls and procedures: We have performed an evaluation under the supervision and with the participation of our management, including our Chief Executive Officer ("CEO") and Chief Financial Officer ("CFO"), of the effectiveness of our disclosure controls and procedures, as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934 (the "Exchange Act"). Based on that evaluation, our CEO and CFO concluded that our disclosure controls and procedures were effective as of December 31, 2008 to provide reasonable assurance that information required to be disclosed by us in the reports filed or submitted by us under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms.
Changes in internal control over financial reporting: There was no change in our internal controls over financial reporting during the three months ended December 31, 2008 that materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management's Report on Internal Control Over Financial Reporting: Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Under the supervision and with the participation of our management, including our CEO and CFO, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework inInternal Control—Integrated Framework, issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework inInternal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2008.
The effectiveness of our internal control over financial reporting as of December 31, 2008 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report which is included in this Item 9A.
43
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and
Stockholders of Quidel Corporation
We have audited Quidel Corporation's internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission ("the COSO criteria"). Quidel Corporation's management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Quidel Corporation maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Quidel Corporation as of December 31, 2008 and 2007, and the related consolidated statements of income, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2008 of Quidel Corporation and our report dated February 26, 2009 expressed an unqualified opinion thereon.
San Diego, California
February 26, 2009
44
Item 9B. Other Information
2009 Annual Meeting of Stockholders
The Company's 2009 Annual Meeting of Stockholders will be held on Tuesday, May 12, 2009, beginning at 8:30 a.m. (local time) at Hyatt Regency, La Jolla at Aventine, 3777 La Jolla Village Drive, San Diego, California, 92122.
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this item (with respect to directors) is incorporated by reference from the information under the caption "Election of Directors" to be contained in our 2009 Proxy Statement, which will be filed with the SEC no later than April 28, 2009. Information with respect to executive officers is included under Item 1 on pages 12-14 of this Annual Report.
The information required by Items 405, 406 and 407 of Regulation S-K is incorporated by reference from the information under the captions "Corporate Governance," "Code of Business Conduct and Ethics" and "Section 16(a) Beneficial Ownership Reporting Compliance," to be contained in our 2009 Proxy Statement, which will be filed with the SEC no later than April 28, 2009.
Item 11. Executive Compensation
The information required by this item is incorporated by reference from the information under the captions "Director Compensation" and "Executive Compensation" to be contained in our 2009 Proxy Statement, which will to be filed with the SEC no later than April 28, 2009.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by Items 201(d) and 403 of Regulation S-K is incorporated by reference from the information under the captions "Equity Compensation Plan Information" and "Security Ownership of Certain Beneficial Owners and Management" to be contained in our 2009 Proxy Statement, which will be filed with the SEC no later than April 28, 2009.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item is incorporated by reference from the information under the captions "Compensation Committee Interlocks and Insider Participation," "Certain Relationships and Related Transactions" and "Director Independence" to be contained in our 2009 Proxy Statement, which will be filed with the SEC no later than April 28, 2009.
Item 14. Principal Accounting Fees and Services
The information required by this item is incorporated by reference from the information under the caption "Independent Registered Public Accounting Firm" to be contained in our 2009 Proxy Statement, which will be filed with the SEC no later than April 28, 2009.
45
Table of Contents
Part IV
Item 15. Exhibits and Financial Statement Schedules
The following documents are filed as part of this Form 10-K:
- (a)
- (1) Financial Statements
The Consolidated Financial Statements required by this Item are submitted in a separate section beginning on page F-1 of this Annual Report and incorporated herein by reference.
Consolidated Financial Statements of Quidel Corporation
| | |
Report of Independent Registered Public Accounting Firm | | F-1 |
Consolidated Balance Sheets as of December 31, 2008 and 2007 | | F-2 |
Consolidated Statements of Income for the years ended December 31, 2008, 2007 and 2006 | | F-3 |
Consolidated Statements of Stockholders' Equity for the years ended December 31, 2008, 2007 and 2006 | | F-4 |
Consolidated Statements of Cash Flows for the years ended December 31, 2008, 2007 and 2006 | | F-5 |
Notes to Consolidated Financial Statements | | F-6 |
- (2) Financial Statement Schedules
The following Financial Statement Schedule of Quidel Corporation for the years ended December 31, 2008, 2007 and 2006 is filed as part of this Annual Report and should be read in conjunction with the Consolidated Financial Statements of Quidel Corporation:
Schedule II. Consolidated Valuation and Qualifying Accounts.
Financial Statement Schedules not listed above have been omitted because of the absence of conditions under which they are required or because the required information is included in the Consolidated Financial Statements or the Notes thereto.
- (3) Exhibits. See Paragraph 15(b) below.
- (b)
- Exhibits
The exhibits listed on the accompanying Exhibit Index immediately following the Financial Statement Schedule are filed as part of, and incorporated by reference into, this Annual Report on Form 10-K.
- (c)
- Financial Statements required by Regulation S-X which are excluded from this Annual Report on Form 10-K by Rule 14(a)-3(b).
Not applicable.
46
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | |
| | | QUIDEL CORPORATION |
|
|
|
|
|
| Date: February 27, 2009 | | By | | /s/ CAREN L. MASON
Caren L. Mason
President, Chief Executive Officer
(Principal Executive Officer) and Director |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | | | |
Signature | | Title | | Date | |
|---|
|
|
|
|
|
|
|
/s/ CAREN L. MASON
Caren L. Mason | | President, Chief Executive Officer (Principal Executive Officer), and Director | | | February 27, 2009 | |
/s/ JOHN M. RADAK
John M. Radak |
|
Chief Financial Officer, (Principal Financial Officer and Accounting Officer) |
|
|
February 27, 2009 |
|
/s/ MARK A. PULIDO
Mark A. Pulido |
|
Chairman of the Board |
|
|
February 27, 2009 |
|
/s/ THOMAS D. BROWN
Thomas D. Brown |
|
Director |
|
|
February 27, 2009 |
|
/s/ KENNETH F. BUECHLER
Kenneth F. Buechler |
|
Director |
|
|
February 27, 2009 |
|
/s/ RODNEY F. DAMMEYER
Rodney F. Dammeyer |
|
Director |
|
|
February 27, 2009 |
|
/s/ MARY LAKE POLAN
Mary Lake Polan |
|
Director |
|
|
February 27, 2009 |
|
/s/ JACK W. SCHULER
Jack W. Schuler |
|
Director |
|
|
February 27, 2009 |
|
/s/ DOUGLAS C. BRYANT
Douglas C. Bryant |
|
Director |
|
|
February 27, 2009 |
|
47
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and
Stockholders of Quidel Corporation
We have audited the accompanying consolidated balance sheets of Quidel Corporation as of December 31, 2008 and 2007, and the related consolidated statements of income, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2008. Our audits also included the financial statement schedule listed in the Index at Item 15(a)(2). These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Quidel Corporation at December 31, 2008 and 2007, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2008, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Quidel Corporation's internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 26, 2009 expressed an unqualified opinion thereon.
San Diego, California
February 26, 2009
F-1
Table of Contents
QUIDEL CORPORATION
CONSOLIDATED BALANCE SHEETS
(in thousands, except par value)
| | | | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
ASSETS | | | | | | | |
Current assets: | | | | | | | |
| | Cash and cash equivalents | | $ | 57,908 | | $ | 45,489 | |
| | Accounts receivable, net | | | 25,320 | | | 23,163 | |
| | Inventories | | | 11,702 | | | 11,037 | |
| | Deferred tax asset—current | | | 5,043 | | | 5,955 | |
| | Prepaid expenses and other current assets | | | 1,053 | | | 1,589 | |
| | | | | | |
| | | Total current assets | | | 101,026 | | | 87,233 | |
Property, plant and equipment, net | | | 19,081 | | | 19,926 | |
Intangible assets, net | | | 9,833 | | | 14,320 | |
Deferred tax asset—non-current | | | 11,240 | | | 11,923 | |
Other non-current assets | | | 1,628 | | | 436 | |
| | | | | | |
Total assets | | $ | 142,808 | | $ | 133,838 | |
| | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | |
Current liabilities: | | | | | | | |
| | Accounts payable | | $ | 4,317 | | $ | 5,618 | |
| | Accrued payroll and related expenses | | | 2,719 | | | 4,341 | |
| | Accrued royalties | | | 2,659 | | | 3,289 | |
| | Current portion of obligations under capital leases | | | 862 | | | 764 | |
| | Other current liabilities | | | 4,877 | | | 2,962 | |
| | | | | | |
| | | Total current liabilities | | | 15,434 | | | 16,974 | |
Capital leases, net of current portion | | | 6,137 | | | 7,000 | |
Deferred rent | | | 948 | | | 1,108 | |
Income taxes payable | | | 1,053 | | | 1,053 | |
Commitments and contingencies | | | | | | | |
Stockholders' equity: | | | | | | | |
Preferred stock, $.001 par value per share; 5,000 shares authorized, none issued or outstanding at December 31, 2008 and 2007 | | | — | | | — | |
Common stock, $.001 par value per share; 50,000 shares authorized, 31,894 and 32,706 shares issued and outstanding at December 31, 2008 and 2007, respectively | | | 32 | | | 33 | |
Additional paid-in capital | | | 138,126 | | | 145,440 | |
Accumulated deficit | | | (18,922 | ) | | (37,770 | ) |
| | | | | | |
| | | Total stockholders' equity | | | 119,236 | | | 107,703 | |
| | | | | | |
Total liabilities and stockholders' equity | | $ | 142,808 | | $ | 133,838 | |
| | | | | | |
See accompanying Notes.
F-2
Table of Contents
QUIDEL CORPORATION
CONSOLIDATED STATEMENTS OF INCOME
(in thousands, except per share data)
| | | | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Total revenues | | $ | 128,132 | | $ | 118,065 | | $ | 106,015 | |
Costs and expenses | | | | | | | | | | |
| | Cost of sales (excludes amortization of intangible assets) | | | 50,206 | | | 48,573 | | | 44,818 | |
| | Research and development | | | 11,147 | | | 12,855 | | | 13,047 | |
| | Sales and marketing | | | 20,898 | | | 18,491 | | | 16,966 | |
| | General and administrative | | | 12,786 | | | 13,167 | | | 12,770 | |
| | Amortization of intangible assets | | | 4,476 | | | 5,493 | | | 4,580 | |
| | | | | | | | |
| | | Total costs and expenses | | | 99,513 | | | 98,579 | | | 92,181 | |
| | | | | | | | |
Operating income | | | 28,619 | | | 19,486 | | | 13,834 | |
Other income (expense) | | | | | | | | | | |
| | Interest income | | | 1,686 | | | 1,891 | | | 1,408 | |
| | Interest expense | | | (671 | ) | | (736 | ) | | (757 | ) |
| | Other income (expense) | | | 135 | | | (117 | ) | | 545 | |
| | | | | | | | |
| | | Total other income | | | 1,150 | | | 1,038 | | | 1,196 | |
| | | | | | | | |
Income from continuing operations before provision (benefit) for income taxes | | | 29,769 | | | 20,524 | | | 15,030 | |
Provision (benefit) for income taxes | | | 10,921 | | | 6,893 | | | (5,891 | ) |
| | | | | | | | |
Income from continuing operations | | | 18,848 | | | 13,631 | | | 20,921 | |
Gain from discontinued operations, net of taxes | | | — | | | — | | | 797 | |
| | | | | | | | |
Net income | | $ | 18,848 | | $ | 13,631 | | $ | 21,718 | |
| | | | | | | | |
Basic earnings per share: | | | | | | | | | | |
| | Continuing operations | | $ | 0.59 | | $ | 0.43 | | $ | 0.63 | |
| | Discontinued operations | | | — | | | — | | | 0.02 | |
| | Net income | | | 0.59 | | | 0.43 | | | 0.66 | |
Diluted earnings per share: | | | | | | | | | | |
| | Continuing operations | | $ | 0.58 | | $ | 0.41 | | $ | 0.61 | |
| | Discontinued operations | | | — | | | — | | | 0.02 | |
| | Net income | | | 0.58 | | | 0.41 | | | 0.63 | |
Shares used in basic per share calculations | | | 31,853 | | | 32,028 | | | 32,985 | |
Shares used in diluted per share calculations | | | 32,612 | | | 32,996 | | | 34,367 | |
See accompanying Notes.
F-3
Table of Contents
QUIDEL CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | |
| |
| |
| |
| |
| |
|---|
| | Shares | | Amount | | Additional paid-in capital | | Deferred stock compensation | | Accumulated other comprehensive income | | Accumulated deficit | | Total stockholders' equity | | Total comprehensive income | |
|---|
| | (in thousands)
| |
|---|
Balance at December 31, 2005 | | | 33,778 | | $ | 34 | | $ | 161,662 | | $ | (1,947 | ) | $ | 1,326 | | $ | (73,832 | ) | $ | 87,243 | | $ | (9,340 | ) |
Issuance of common stock under equity compensation plans | | | 998 | | | 1 | | | 3,697 | | | — | | | — | | | — | | | 3,698 | | | | |
Income tax benefit due to exercise/disposition of employee stock options | | | — | | | — | | | 111 | | | — | | | — | | | — | | | 111 | | | | |
Cancellation of common stock under equity compensation plans | | | (24 | ) | | — | | | — | | | — | | | — | | | — | | | — | | | | |
Reclassification due to adoption of SFAS 123R | | | — | | | — | | | (1,947 | ) | | 1,947 | | | — | | | — | | | — | | | | |
Stock-based compensation expense | | | — | | | — | | | 3,398 | | | — | | | — | | | — | | | 3,398 | | | | |
Translation adjustment | | | — | | | — | | | — | | | — | | | (1,326 | ) | | — | | | (1,326 | ) | | (1,326 | ) |
Purchase of common stock | | | (1,222 | ) | | (2 | ) | | (11,564 | ) | | — | | | — | | | — | | | (11,566 | ) | | | |
Net income | | | — | | | — | | | — | | | — | | | — | | | 21,718 | | | 21,718 | | | 21,718 | |
| | | | | | | | | | | | | | | | | | |
Balance at December 31, 2006 | | | 33,530 | | | 33 | | | 155,357 | | | — | | | — | | | (52,114 | ) | | 103,276 | | $ | 20,392 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of common stock under equity compensation plans | | | 912 | | | 1 | | | 2,792 | | | — | | | — | | | — | | | 2,792 | | | | |
Cancellation of common stock under equity compensation plans | | | (147 | ) | | — | | | (1 | ) | | — | | | — | | | — | | | (1 | ) | | | |
Income tax benefit due to exercise/disposition of employee stock options | | | — | | | — | | | 1,033 | | | — | | | — | | | — | | | 1,033 | | | | |
Stock-based compensation expense | | | — | | | — | | | 4,117 | | | — | | | — | | | — | | | 4,117 | | | | |
Purchase of common stock | | | (1,589 | ) | | (1 | ) | | (17,858 | ) | | — | | | — | | | — | | | (17,858 | ) | | | |
Cumulative effect to prior year accumulated deficit related to the adoption of FIN 48 | | | — | | | — | | | — | | | — | | | — | | | 713 | | | 713 | | | | |
Net income | | | — | | | — | | | — | | | — | | | — | | | 13,631 | | | 13,631 | | | 13,631 | |
| | | | | | | | | | | | | | | | | | |
Balance at December 31, 2007 | | | 32,706 | | | 33 | | | 145,440 | | | — | | | — | | | (37,770 | ) | | 107,703 | | $ | 13,631 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of common stock under equity compensation plans | | | 661 | | | — | | | 3,346 | | | — | | | — | | | — | | | 3,346 | | | | |
Cancellation of common stock under equity compensation plans | | | (131 | ) | | — | | | (1 | ) | | — | | | — | | | — | | | (1 | ) | | | |
Income tax benefit due to exercise/disposition of employee stock options | | | — | | | — | | | 6,476 | | | — | | | — | | | — | | | 6,476 | | | | |
Stock-based compensation expense | | | — | | | — | | | 2,677 | | | — | | | — | | | — | | | 2,677 | | | | |
Purchase of common stock | | | (1,342 | ) | | (1 | ) | | (19,812 | ) | | — | | | — | | | — | | | (19,813 | ) | | | |
Net income | | | — | | | — | | | — | | | — | | | — | | | 18,848 | | | 18,848 | | | 18,848 | |
| | | | | | | | | | | | | | | | | | |
Balance at December 31, 2008 | | | 31,894 | | $ | 32 | | $ | 138,126 | | $ | — | | $ | — | | $ | (18,922 | ) | $ | 119,236 | | $ | 18,848 | |
| | | | | | | | | | | | | | | | | | |
See accompanying Notes.
F-4
Table of Contents
QUIDEL CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
| | (in thousands)
| |
|---|
OPERATING ACTIVITIES | | | | | | | | | | |
Net income | | $ | 18,848 | | $ | 13,631 | | $ | 21,718 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | |
| | Depreciation, amortization and other | | | 8,681 | | | 9,684 | | | 8,980 | |
| | Gain on discontinued operations | | | — | | | — | | | (797 | ) |
| | Foreign currency translation | | | — | | | — | | | (558 | ) |
| | Stock-based compensation expense | | | 2,677 | | | 4,117 | | | 3,398 | |
| | Deferred tax asset | | | 8,071 | | | 5,523 | | | (6,078 | ) |
| | Excess tax benefit from share-based compensation | | | (6,476 | ) | | (1,033 | ) | | (111 | ) |
| | Changes in assets and liabilities: | | | | | | | | | | |
| | | Accounts receivable | | | (2,157 | ) | | (5,024 | ) | | (2,320 | ) |
| | | Inventories | | | (665 | ) | | (1,412 | ) | | (1,125 | ) |
| | | Prepaid expenses and other current assets | | | 536 | | | 101 | | | (336 | ) |
| | | Accounts payable | | | (547 | ) | | 769 | | | (1,302 | ) |
| | | Accrued payroll and related expenses | | | (1,622 | ) | | (527 | ) | | 963 | |
| | | Accrued royalties | | | (630 | ) | | (270 | ) | | 192 | |
| | | Accrued income taxes payable | | | — | | | 1,053 | | | — | |
| | | Other current liabilities | | | 2,225 | | | 730 | | | (1,893 | ) |
| | | | | | | | |
| | Net cash provided by operating activities | | | 28,941 | | | 27,342 | | | 20,731 | |
| | | | | | | | |
INVESTING ACTIVITIES | | | | | | | | | | |
| | Acquisition of property and equipment | | | (4,118 | ) | | (3,109 | ) | | (4,469 | ) |
| | Acquisition of intangibles | | | (360 | ) | | (640 | ) | | (6,500 | ) |
| | Other assets | | | 49 | | | (21 | ) | | 308 | |
| | | | | | | | |
| | Net cash used for investing activities | | | (4,429 | ) | | (3,770 | ) | | (10,661 | ) |
| | | | | | | | |
FINANCING ACTIVITIES | | | | | | | | | | |
| | Proceeds from issuance of common stock, net of cancellations | | | 3,345 | | | 2,792 | | | 3,697 | |
| | Excess tax benefit from share-based compensation | | | 6,476 | | | 1,033 | | | 111 | |
| | Payments on capital lease obligations | | | (765 | ) | | (675 | ) | | (648 | ) |
| | Purchase of common stock | | | (19,813 | ) | | (17,858 | ) | | (11,564 | ) |
| | Fees paid to establish line of credit | | | (1,336 | ) | | — | | | — | |
| | | | | | | | |
| | Net cash used for financing activities | | | (12,093 | ) | | (14,708 | ) | | (8,404 | ) |
| | | | | | | | |
Effect of exchange rate changes on cash | | | — | | | — | | | 29 | |
| | | | | | | | |
Net increase in cash and cash equivalents | | | 12,419 | | | 8,864 | | | 1,695 | |
Cash and cash equivalents at beginning of year | | | 45,489 | | | 36,625 | | | 34,930 | |
| | | | | | | | |
Cash and cash equivalents at end of year | | $ | 57,908 | | $ | 45,489 | | $ | 36,625 | |
| | | | | | | | |
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION | | | | | | | | | | |
| | Cash paid for interest | | $ | 671 | | $ | 736 | | $ | 757 | |
| | | | | | | | |
| | Cash paid for income taxes | | $ | 3,425 | | $ | 362 | | $ | — | |
| | | | | | | | |
NON-CASH INVESTING ACTIVITIES | | | | | | | | | | |
Purchase of license agreements by incurring current liabilities | | $ | — | | $ | 560 | | $ | — | |
| | | | | | | | |
Purchase of capital equipment by incurring current liabilities | | $ | 263 | | $ | 1,017 | | $ | — | |
| | | | | | | | |
See accompanying Notes.
F-5
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Company Operations and Summary of Significant Accounting Policies
Quidel Corporation (the "Company") commenced operations in 1979. The Company operates in one business segment, which develops, manufactures and markets point-of-care ("POC") rapid diagnostics for detection and management of a variety of medical conditions and illnesses. The majority of the Company's products are specifically developed for the physician office lab and acute care market and are substantially focused on infectious diseases and reproductive and women's health. The Company's products are sold to professionals for use in physician offices, hospitals, clinical laboratories, retail clinics and wellness screening centers through a domestic network of national and regional distributors, supported by a national sales force. Internationally, the Company sells and markets primarily in Japan, Europe, and the Middle East through exclusive distributor arrangements.
Consolidation—The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All significant intercompany accounts and transactions have been eliminated.
Cash and Cash Equivalents—The Company considers cash equivalents to be highly liquid investments with a maturity at the date of purchase of three months or less.
Accounts Receivable—The Company sells its products primarily to distributors in the U.S., Europe, the Middle East and Japan. The Company periodically assesses the financial strength of these customers and establishes reserves for anticipated losses when necessary, which historically have not been material. The Company's reserves primarily consist of amounts related to cash discounts and contract rebates, and to a lesser extent returned good allowances and bad debts. The balance of accounts receivable is net of reserves of $1.5 million and $0.9 million at December 31, 2008 and 2007, respectively.
Inventories—Inventories are stated at the lower of cost (first-in, first-out method) or market. The Company reviews the components of its inventory on a quarterly basis for excess, obsolete and impaired inventory and makes appropriate dispositions as obsolete stock is identified. Inventories consisted of the following, net of reserves of $0.3 million for year ended December 31, 2008 and $0.5 million for year ended December 31, 2007, (in thousands):
| | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
Raw materials | | $ | 4,956 | | $ | 5,361 | |
Work-in-process (materials, labor and overhead) | | | 3,108 | | | 2,896 | |
Finished goods (materials, labor and overhead) | | | 3,638 | | | 2,780 | |
| | | | | | |
| | $ | 11,702 | | $ | 11,037 | |
| | | | | | |
Property, Plant and Equipment—Property, plant and equipment is recorded at cost and depreciated over the estimated useful lives of the assets (three to 15 years) using the straight-line method. Amortization of leasehold improvements is computed on the straight-line method over the shorter of the lease term or the estimated useful lives of the assets. The total expense for depreciation of fixed assets and amortization of leasehold improvements was $3.8 million, $4.1 million and $4.0 million for the years ended December 31, 2008, 2007 and 2006, respectively. The portion of this expense related to capital leases is $0.8 million, $0.7 million and $0.6 million for the years ended December 31, 2008, 2007 and 2006, respectively. Maintenance and minor repairs are charged to operations as incurred. When assets are sold, or otherwise disposed of, the cost and related accumulated depreciation are removed
F-6
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Company Operations and Summary of Significant Accounting Policies (Continued)
from the accounts and any gain or loss is included in Other Income (Expense) in the Consolidated Statements of Income.
Property, plant and equipment consisted of the following (in thousands):
| | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
Equipment, furniture and fixtures | | $ | 36,050 | | $ | 34,733 | |
Building and improvements | | | 19,535 | | | 18,997 | |
Land | | | 1,080 | | | 1,080 | |
| | | | | | |
| | | 56,665 | | | 54,810 | |
Less: Accumulated depreciation and amortization | | | (37,584 | ) | | (34,884 | ) |
| | | | | | |
| | $ | 19,081 | | $ | 19,926 | |
| | | | | | |
Intangible Assets—Intangible assets are recorded at cost and amortized, except for indefinite-lived intangibles such as goodwill, on a straight-line basis over their estimated useful lives. Intangible assets consisted of the following (dollar amounts in thousands):
| | | | | | | | | | | | | | | | | | | | | | |
| |
| | December 31, 2008 | | December 31, 2007 | |
|---|
| | Weighted-
Average
Life
(years) | |
|---|
Description | | Gross
Assets | | Accumulated
Amortization | | Net | | Gross
Assets | | Accumulated
Amortization | | Net | |
|---|
Goodwill | | | N/A | | $ | 9,918 | | | (3,448 | ) | $ | 6,470 | | $ | 9,918 | | | (3,448 | ) | $ | 6,470 | |
Purchased technology | | | 7.0 | | | 6,100 | | | (6,100 | ) | | — | | | 6,100 | | | (6,100 | ) | | — | |
License agreements | | | 4.0 | | | 17,490 | | | (14,895 | ) | | 2,595 | | | 17,500 | | | (10,633 | ) | | 6,867 | |
Patent and trademark costs | | | 3.0 | | | 3,623 | | | (3,181 | ) | | 442 | | | 3,623 | | | (3,020 | ) | | 603 | |
Favorable lease | | | 16.0 | | | 1,700 | | | (1,374 | ) | | 326 | | | 1,700 | | | (1,320 | ) | | 380 | |
| | | | | | | | | | | | | | | | | |
| | | | | $ | 38,831 | | $ | (28,998 | ) | $ | 9,833 | | $ | 38,841 | | $ | (24,521 | ) | $ | 14,320 | |
| | | | | | | | | | | | | | | | | |
Amortization expense was $4.5 million, $5.5 million and $4.6 million for the years ended December 31, 2008, 2007 and 2006, respectively.
The expected future annual amortization expense of the Company's intangible assets is as follows (in thousands):
| | | | |
Years Ended December 31, | | Amortization Expense | |
|---|
2009 | | $ | 1,419 | |
2010 | | | 1,355 | |
2011 | | | 342 | |
2012 | | | 141 | |
2013 | | | 55 | |
Thereafter | | | 51 | |
| | | | |
Total | | $ | 3,363 | |
| | | | |
F-7
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Company Operations and Summary of Significant Accounting Policies (Continued)
The Company completed its annual evaluation for impairment of goodwill as of December 31, 2008 and determined that no impairment of goodwill existed. A significant decline in the Company's projected revenue or earnings growth or cash flows, a significant decline in the Company's stock price or the stock price of comparable companies, loss of legal ownership or title to an asset, and any significant change in the Company's strategic business objectives and utilization of assets are among many factors that could result in an impairment charge that could have a material negative impact on the Company's operating results.
Impairment of Long-Lived Assets—The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the total book value of an asset may not be recoverable. An impairment loss is recognized when estimated undiscounted future cash flows expected to result from the use of the asset and the eventual disposition are less than its carrying amount. An impairment loss is equal to the excess of the book value of an asset over its determined fair value. For the year ended December 31, 2008, the Company recorded $0.4 million of impairment charges related to certain production equipment.
Other current liabilities—Other current liabilities consisted of the following (in thousands):
| | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
Volume discounts | | $ | 3,593 | | $ | 1,200 | |
State and foreign taxes | | | — | | | 701 | |
Amounts due on technology and license acquisitions | | | 250 | | | 560 | |
Accrued professional fees | | | 337 | | | 285 | |
Other | | | 697 | | | 216 | |
| | | | | | |
| | $ | 4,877 | | $ | 2,962 | |
| | | | | | |
Revenue Recognition—The Company records revenues primarily from product sales. These revenues are recorded net of rebates and other discounts which are estimated at the time of sale, and are largely driven by various customer program offerings, including special pricing agreements, promotions and other volume-based incentives. Revenue from product sales are recorded upon passage of title and risk of loss to the customer. Change in title to the product and recognition of revenue occurs upon delivery to the customer when sales terms are free on board ("FOB") destination and at the time of shipment when the sales terms are FOB shipping point and there is no right of return. The Company also earns income from the licensing of technology. Royalty income from the grant of license rights is recognized during the period in which the revenue is earned and the amount is determinable from the licensee. Income earned from licensing activities is a component of total revenues in the accompanying Consolidated Statements of Income.
Research and Development Costs—All research and development costs are charged to operations as incurred.
Product Shipment Costs—Product shipment costs are included in sales and marketing expense in the accompanying Consolidated Statements of Income. Shipping and handling costs were $1.5 million, $1.7 million and $1.5 million for the years ended December 31, 2008, 2007 and 2006, respectively.
F-8
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Company Operations and Summary of Significant Accounting Policies (Continued)
Advertising Costs—Advertising costs are expensed as incurred. Advertising costs were $0.8 million, $0.7 million and $0.5 million for the years ended December 31, 2008, 2007 and 2006, respectively.
Deferred Rent—Rent expense is recorded on a straight-line basis over the term of the lease. The difference between rent expense and amounts paid under the lease agreement is recorded as deferred rent.
Income Taxes—Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes, using enacted tax rates in effect for the year in which the differences are expected to reverse. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
Foreign Currency Translation—The financial statements of the Company's subsidiaries outside the U.S. are measured using the local currency as the functional currency. Assets and liabilities of these subsidiaries are translated at the rates of exchange at the balance sheet date and revenue and expense accounts are translated using average exchange rates during the periods. The resulting translation adjustments are presented as a separate component of stockholders' equity. Exchange gains and losses arising from transactions denominated in foreign currencies are recorded in operations and have historically not been significant. These foreign subsidiaries are dormant and any transaction adjustments are included in the Statement of Stockholders' Equity.
Fair Value of Financial Instruments—The carrying amounts of the Company's financial instruments, including cash, receivables, accounts payable, accrued liabilities and outstanding balances owed under the line of credit, if any, approximate their fair values due to their short-term nature. Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of trade accounts receivable. The Company establishes reserves for estimated uncollectible accounts and believes its reserves are adequate.
Product Warranty—The Company generally sells products with a limited product warranty and certain limited indemnifications. The accrual and the related expense for known issues were not significant as of and for the fiscal years presented. Due to product testing, the short time between product shipment and the detection and correction of product failures and a low historical rate of payments on indemnification claims, the accrual based on historical activity and the related expense were not significant as of and for the fiscal years presented.
Stock-Based Compensation—Effective January 1, 2006, the Company adopted the fair value recognition provisions of SFAS No. 123(R), "Share-Based Payment," using the modified prospective transition method. Under that transition method, compensation expense that the Company recognizes beginning on that date includes: (a) compensation expense for all share-based awards granted prior to, but not yet vested as of December 31, 2005, based on the grant-date fair value estimated in accordance with the original provisions of SFAS No. 123, and (b) compensation expense for all share-based awards granted on or after January 1, 2006 based on the grant-date fair value estimated in accordance with the provisions of SFAS No. 123(R).
Computation of Earnings (Loss) Per Share—Basic earnings per share were computed by dividing net earnings by the weighted-average number of common shares outstanding, including vested restricted stock awards, during the period. Diluted earnings per share reflects the potential dilution that could occur if the earnings were divided by the weighted-average number of common shares and potentially
F-9
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Company Operations and Summary of Significant Accounting Policies (Continued)
dilutive common shares from outstanding stock options as well as unvested restricted stock awards. Potential dilutive common shares were calculated using the treasury stock method and represent incremental shares issuable upon exercise of the Company's outstanding stock options and unvested restricted stock awards. The Company has awarded restricted stock with both service-based as well as performance-based vesting provisions. Stock awards based on performance only are not included in the calculation of basic earnings per share until the performance criteria are met. For periods in which the Company incurs losses, potentially dilutive shares are not considered in the calculation of net loss per share as their impact would be anti-dilutive. For periods in which the Company has earnings, out-of-the-money stock options (i.e., the average stock price during the period is below the exercise price of the stock option) are not included in diluted earnings per common share as their effect would be anti-dilutive.
During the years ended December 31, 2008, 2007 and 2006, the Company had income from continuing operations. Accordingly, 0.8 million, 0.3 million and 0.4 million shares of outstanding stock options were not included in the computation of diluted earnings per common share for 2008, 2007 and 2006, respectively, because the option exercise price was greater than the average market price of the common stock, and therefore, the effect on diluted earnings per common share would be anti-dilutive.
The following table reconciles the weighted-average shares used in computing basic and diluted earnings per share in the respective periods (in thousands):
| | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Shares used in basic earnings per share (weighted-average common shares outstanding) | | | 31,853 | | | 32,028 | | | 32,985 | |
Effect of dilutive stock options and restricted stock awards | | | 759 | | | 968 | | | 1,382 | |
| | | | | | | | |
Shares used in diluted earnings per share calculation | | | 32,612 | | | 32,996 | | | 34,367 | |
| | | | | | | | |
Comprehensive Income—Comprehensive income includes unrealized gains and losses excluded from the Company's Consolidated Statements of Income. The unrealized losses include foreign currency translation adjustments. The Company has presented the required information in the Consolidated Statements of Stockholders' Equity.
Use of Estimates—The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
New Accounting Pronouncements—In December 2007, the Financial Accounting Standards Board ("FASB") revised Statement No. 141, "Business Combinations" ("FAS 141R"), which establishes principles and requirements for how the acquirer in a business combination (i) recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed and any noncontrolling interest in the acquiree, (ii) recognizes and measures the goodwill acquired in the business combination or a gain from a bargain purchase and (iii) determines what information to disclose to enable readers
F-10
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 1. Company Operations and Summary of Significant Accounting Policies (Continued)
of the financial statements to evaluate the nature and financial effects of the business combination. FAS 141R will be effective for any business combination that occurs beginning in fiscal year 2009.
In April 2008, the FASB issued FASB Staff Position 142-3, "Determination of the Useful Life of Intangible Assets" ("FSP 142-3"). FSP 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB Statement No. 142, "Goodwill and Other Intangible Assets" ("FAS 142"). The objective of FSP 142-3 is to improve the consistency between the useful life of a recognized intangible asset under FAS 142 and the period of expected cash flows used to measure the fair value of the asset under FAS 141R and other U.S. generally accepted accounting principles. FSP 142-3 will be effective beginning in fiscal year 2009. The Company is currently evaluating the impact that this pronouncement will have on its consolidated financial statements.
Accounting Periods—Each of the Company's fiscal quarters end on the Sunday closest to the end of the calendar quarter. The Company's fiscal year end is December 28, 2008. For ease of reference, the calendar quarter end dates are used herein.
Note 2. Line of Credit
On October 8, 2008, the Company entered into a new $120.0 million senior secured syndicated credit facility (the "Senior Credit Facility"), which matures on October 8, 2013. The new credit facility replaces the Company's $30 million credit facility. The Senior Credit Facility bears interest at a rate ranging from 0.50% to 1.75% plus the lender's prime rate or, at the Company's option, a rate ranging from 1.50% to 2.75% plus the London InterBank Offering Rate. The agreement governing the Senior Credit Facility is subject to certain customary limitations, including among others: limitation on liens; limitation on mergers, consolidations and sales of assets; limitation on debt; limitation on dividends, stock redemptions and the redemption and/or prepayment of other debt; limitation on investments (including loans and advances) and acquisitions; limitation on transactions with affiliates; and limitation on annual capital expenditures. The Company is also subject to financial covenants which include a funded debt to earnings before interest, taxes, depreciation and amortization (EBITDA) ratio, and an interest coverage ratio. The Senior Credit Facility is secured by substantially all present and future assets and properties of the Company. As of December 31, 2008, the Company had no amounts outstanding under the line of credit and $120.0 million of availability under the Senior Credit Facility and the Company was in compliance with all financial covenants.
Note 3. Income Taxes
The Company's income from continuing operations before provision (benefit) for income taxes were subject to taxes in the following jurisdictions for the following periods (in thousands):
| | | | | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
United States | | $ | 29,671 | | $ | 20,628 | | $ | 14,524 | |
Foreign | | | 98 | | | (104 | ) | | 506 | |
| | | | | | | | |
| | $ | 29,769 | | $ | 20,524 | | $ | 15,030 | |
| | | | | | | | |
F-11
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 3. Income Taxes (Continued)
Significant components of the provision (benefit) for income taxes from continuing operations are as follows (in thousands):
| | | | | | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Current: | | | | | | | | | | |
| | Federal | | $ | 7,677 | | $ | 310 | | $ | 181 | |
| | State | | | 1,650 | | | 1,017 | | | 113 | |
| | | | | | | | |
| | Total current provision | | | 9,327 | | | 1,327 | | | 294 | |
Deferred: | | | | | | | | | | |
| | Federal | | | 1,287 | | | 6,325 | | | (5,072 | ) |
| | State | | | 307 | | | (759 | ) | | (1,113 | ) |
| | | | | | | | |
| | Total deferred provision (benefit) | | | 1,594 | | | 5,566 | | | (6,185 | ) |
| | | | | | | | |
Provision (benefit) for income taxes | | $ | 10,921 | | $ | 6,893 | | $ | (5,891 | ) |
| | | | | | | | |
Significant components of the Company's deferred tax assets as of December 31, 2008 and 2007 are shown below (in thousands).
| | | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
Deferred tax assets: | | | | | | | |
| | Net operating loss carryforwards | | $ | — | | $ | 1,288 | |
| | Acquired intangibles | | | 2,854 | | | 1,522 | |
| | Sale-leaseback, net | | | 2,851 | | | 2,859 | |
| | Capitalized research and development costs | | | 2,750 | | | 4,320 | |
| | Allowance for returns and discounts | | | 1,989 | | | 680 | |
| | Stock compensation | | | 1,424 | | | 1,092 | |
| | Tax credit carryforwards | | | 1,333 | | | 3,216 | |
| | Depreciation | | | 582 | | | 1,017 | |
| | Other, net | | | 2,500 | | | 1,884 | |
| | | | | | |
Total deferred tax assets | | $ | 16,283 | | $ | 17,878 | |
| | Valuation allowance for deferred tax assets | | | — | | | — | |
| | | | | | |
Deferred tax assets, net of valuation allowance | | | 16,283 | | | 17,878 | |
Deferred tax liabilities | | | — | | | — | |
| | | | | | |
Net deferred tax assets | | $ | 16,283 | | $ | 17,878 | |
| | | | | | |
A valuation allowance of $18.2 million had been established against a portion of the Company's deferred tax assets, "DTA's", at December 31, 2005. As of December 31, 2006, the Company believed it was more likely than not that it would be able to realize its deferred tax asset through expected future taxable profits, and released a valuation allowance of approximately $18.2 million of which $11.6 million was recognized as an income tax benefit and $6.6 million reduced the existing value of goodwill. The amount of the net deferred tax assets considered realizable, however, could be reduced
F-12
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 3. Income Taxes (Continued)
in the near term if actual future income or income tax rates are lower than estimated, or if there are differences in the timing or amount of future reversals of existing taxable or deductible temporary differences.
The Company will continue to assess the assumptions used to determine the valuation allowance. Should the Company determine that it would not be able to realize all or part of its other components of the deferred tax asset in the future, an adjustment to the deferred tax asset would be charged to income in the period such determination were made.
As a result of the adoption of SFAS No. 123(R), the Company will recognize excess tax benefits associated with the exercise of stock options directly to stockholders' equity only when realized. Accordingly, deferred tax assets are not recognized for net operating loss ("NOL") carryforwards resulting from excess tax benefits. As of December 31, 2008 and 2007, deferred tax assets do not include $2.7 million and $7.1 million, respectively, of these excess tax benefits from employee stock option exercises that are a component of the Company's NOL carryforwards. Additional paid-in capital will be increased up to an additional $2.7 million if such excess tax benefits are realized.
As of December 31, 2008, the Company had federal NOL carryforwards of approximately $7.6 million which will expire at various dates through December 31, 2025, unless previously utilized. The Company has gross federal and state research credits of $0.3 million and $3.5 million, respectively. The federal credits begin to expire in 2012 and the state credits do not expire. The Company also has an alternative minimum tax credit of $1.2 million that does not expire.
Pursuant to Internal Revenue Code ("IRC") Sections 382 and 383, the Company's use of its NOL and research credit carryforwards may be limited as a result of cumulative changes in ownership of more than 50% over a three-year period.
The reconciliation of income tax computed at the federal statutory rate to the provision (benefit) for income taxes from continuing operations is as follows (in thousands):
| | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Tax expense at statutory tax rate | | $ | 10,417 | | $ | 6,978 | | $ | 5,110 | |
State taxes, net of federal tax | | | 1,588 | | | 993 | | | 735 | |
Permanent differences | | | (153 | ) | | 291 | | | 462 | |
Federal and state research credits—current year | | | (569 | ) | | (655 | ) | | (458 | ) |
Federal and state research credits—prior year true-up | | | — | | | (765 | ) | | 1,235 | |
FIN 48 accrual | | | 152 | | | 288 | | | — | |
Foreign taxes and foreign (income) losses not benefited (taxed) | | | (34 | ) | | 35 | | | (194 | ) |
Federal and state NOL change related to IRC Section 382 | | | — | | | — | | | (1,103 | ) |
Impact of change in federal and state tax rate on revaluing DTAs | | | (594 | ) | | — | | | — | |
Change in valuation allowance | | | — | | | — | | | (11,612 | ) |
Other | | | 114 | | | (272 | ) | | (66 | ) |
| | | | | | | | |
| | $ | 10,921 | | $ | 6,893 | | $ | (5,891 | ) |
| | | | | | | | |
F-13
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 3. Income Taxes (Continued)
The Company adopted FIN 48 on January 1, 2007 and recognized a cumulative-effect adjustment of $0.7 million, increasing retained earnings.
The following table summarizes the activity related to the Company's unrecognized tax benefits (in thousands):
| | | | | | | |
| | 2008 | | 2007 | |
|---|
Beginning balance | | $ | 6,165 | | $ | 6,006 | |
Increases related to prior year tax positions | | | — | | | 31 | |
Increases related to current year tax positions | | | 269 | | | 310 | |
Decreases due to settlements | | | (64 | ) | | (182 | ) |
Expiration of the statute of limitations for the assessment of taxes | | | — | | | — | |
Other | | | 140 | | | — | |
| | | | | | |
Ending balance | | $ | 6,510 | | $ | 6,165 | |
| | | | | | |
Included in the unrecognized tax benefits of $6.5 million as of December 31, 2008 was $5.2 million of tax benefits that, if recognized, would reduce the Company's annual effective tax rate. The Company does not expect the unrecognized tax benefits to change significantly over the next 12 months. The Company's policy is to recognize the interest expense and penalties related to income tax matters as a component of income tax expense. The Company has accrued an immaterial amount of interest and penalties associated with uncertain tax positions as of December 31, 2008.
The Company is subject to periodic audits by domestic and foreign tax authorities. The Company's tax years for 1993 and forward are subject to examination by the U.S. authorities due to the carryforward of unutilized net operating losses and research and development credits. With few exceptions, the Company's tax years 1999 and forward are subject to examination by state and foreign authorities. The Company believes it has appropriate support for the income tax positions taken on its tax returns and that its accruals for tax liabilities are adequate for all open years based on an assessment of many factors, including past experience and interpretations of tax law applied to the facts of each matter.
Note 4. Stockholders' Equity
Preferred Stock. The Company's certificate of incorporation, as amended, authorizes the issuance of up to five million preferred shares. The Board of Directors is authorized to fix the number of shares of any series of preferred stock and to determine the designation of such shares. However, the amended certificate of incorporation specifies the initial series and the rights of that series. No shares of preferred stock were outstanding as of December 31, 2008 and 2007.
F-14
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 4. Stockholders' Equity (Continued)
Stockholder Rights Plan. The Board of Directors of the Company adopted a Stockholder Rights Plan, effective December 31, 1996 and as amended and restated, effective May 24, 2002 and then again on December 29, 2006 (the "Rights Plan"), which provides for a dividend of one right (a "Right") to purchase fractions of shares of the Company's Series C Junior Participating Preferred Stock for each share of the Company's common stock. Under certain conditions involving an acquisition by any person or group of 15% or more of the Company's common stock, the Rights permit the holders (other than the 15% holder) to purchase the Company's common stock at a 50% discount upon payment of an exercise price of $24 per Right. In addition, in the event of certain business combinations, the Rights permit the purchase of the common stock of an acquirer at a 50% discount. Under certain conditions, the Rights may be redeemed by the Board of Directors in whole, but not in part, at a price of $.005 per Right. The Rights have no voting privileges and are attached to and automatically trade with the Company's common stock. The Rights shall expire on December 31, 2011, unless earlier triggered, redeemed or exchanged.
Restricted Stock. For the year ended December 31, 2008, the Company granted approximately 0.1 million shares of restricted common stock to a newly hired officer which vest over a three year period. For the year ended December 31, 2007, the Company granted approximately 0.4 million shares of restricted common stock to officers and management, including 0.1 million shares for which restrictions lapse 25% each year over a four-year period and 0.3 million shares are performance-based and are tied to the achievement of three-year performance goals; restrictions lapse at the end of the three-year period depending upon the Company's achievement of predetermined revenue and EBITDA goals. For the year ended December 31, 2006, the Company granted approximately 0.3 million shares of restricted common stock to officers and management. The shares of restricted stock awarded to officers during 2006 are all performance-based and remain subject to forfeiture until the restrictions covering such restricted shares lapse. The lapse of restrictions covering two-thirds of the total number of shares of these restricted shares (the "2006 Annual Shares") is tied to the achievement of annual performance targets for the Company over a three-year period. Assuming the officer remains employed by the Company on the relevant date, restrictions lapse on one-third of these 2006 Annual Shares each year on the anniversary of the grant date in 2006, 2007 and 2008 upon the Company's achievement of the annual goals set by the Company's Board of Directors with respect to revenue, EBITDA and a key strategic imperative for the Company in each of the fiscal years ending in 2006, 2007 and 2008, respectively. The lapse of restrictions on the remaining one-third of the total number of shares of these restricted shares is tied to the Company's achievement of a three-year EBITDA goal, as determined by the Board of Directors, with restrictions lapsing on the third anniversary of the grant date if such EBITDA goal is achieved. The remaining shares granted to management were service-based and vest over four years.
Until the restrictions lapse, ownership of the affected shares of restricted stock granted to the Company's officers is conditional upon continuous employment with the Company. During the restricted period, holders of restricted stock have full voting rights with respect to their shares of restricted stock, even though the restricted stock remains subject to transfer restrictions and generally is subject to forfeiture upon termination of employment or service. If an officer or director terminates service before the restrictions lapse, the restricted stock may be repurchased by the Company from the individual and any compensation expense previously recognized would be reversed, thereby reducing the amount of stock-based compensation expense during that period.
F-15
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 4. Stockholders' Equity (Continued)
Restricted Stock Units. During the year ended December 31, 2008, restricted stock units were granted to certain members of the Board of Directors in lieu of cash compensation as a part of the Company's non-employee director's deferred compensation program. The compensation expense associated with the grant of restricted stock units was $0.2 million for the year ended December 31, 2008.
Stock Options. The Company grants options to employees and non-employee directors under its Amended and Restated 2001 Equity Incentive Plan (the "2001 Plan") and previously granted options under the 1998 Stock Incentive Plan and the 1996 Non-Employee Directors Stock Option Plan. The 1998 and 1996 Plans were terminated at the time of adoption of the 2001 Plan, but the terminated Plans continue to govern outstanding options granted thereunder. The Company has stock options outstanding which were issued under those various equity incentive plans to certain employees and directors, which have terms ranging up to ten years, have exercise prices ranging from $2.25 to $19.48, and generally vest over four years. As of December 31, 2008, approximately 1.8 million shares remained available for grant under the 2001 Plan.
Employee Stock Purchase Plan. Under the Company's 1983 Employee Stock Purchase Plan (the "ESPP"), full-time employees are allowed to purchase common stock through payroll deductions (which cannot exceed 10% of the employee's compensation) at the lower of 85% of fair market value at the beginning or end of each six-month purchase period. As of December 31, 2008, 865,308 shares had been sold under the Plan, leaving 134,536 shares available for future issuance.
Share Repurchase Program. In May 2005, the Company's Board of Directors authorized the Company to repurchase up to $25.0 million in shares of its common stock. In March 2007, the Company's Board of Directors authorized the Company to repurchase up to an additional $25.0 million in shares of the Company's common stock under this program. In December 2008, the Company's Board of Directors authorized the Company to repurchase up to an additional $25.0 million in shares of the Company's common stock under this program. Shares of the Company's common stock repurchased under this program will no longer be deemed outstanding upon repurchase and will be returned to the pool of authorized shares. As of December 31, 2008, the Company had repurchased approximately 4.1 million shares under this program, at a cost of approximately $48.1 million.
Shares Reserved for Future Issuance. At December 31, 2008, approximately 3.6 million shares of common stock were reserved under the Company's equity incentive plans, and 0.1 million were reserved for purchases under the ESPP.
Note 5. Stock-Based Compensation
Effective January 1, 2006, the Company began recording compensation expense associated with stock options in accordance with SFAS No. 123(R). The Company has adopted the modified prospective transition method provided under SFAS No. 123(R). Under this transition method, compensation expense associated with stock options recognized in the fiscal years 2008, 2007 and 2006 includes: 1) expense related to the remaining unvested portion of all stock option awards granted prior to January 1, 2006, based on the grant-date fair value estimated in accordance with the original provisions of SFAS No. 123; and 2) expense related to all stock option awards granted subsequent to January 1, 2006 based on the grant-date fair value estimated in accordance with the provisions of SFAS No. 123(R).
F-16
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 5. Stock-Based Compensation (Continued)
Compensation expense related to the Company's share-based awards for the years ended December 31, 2008, 2007 and 2006 was $2.7 million, $4.1 million and $3.4 million, respectively, of which $2.3 million, $2.4 million and $2.1 million, respectively, related to stock options and $0.4 million, $1.7 million and $1.3 million, respectively, related to restricted stock awards ("stock awards").
Total estimated share-based compensation expense, related to all of the Company's share-based awards, was comprised as follows (in millions):
| | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Cost of Sales | | $ | 0.3 | | $ | 0.4 | | $ | 0.3 | |
Research and Development | | | 0.4 | | | 0.6 | | | 0.6 | |
Sales and Marketing | | | 0.1 | | | 0.2 | | | 0.5 | |
General and Administrative | | | 1.9 | | | 2.9 | | | 2.0 | |
| | | | | | | | |
| | $ | 2.7 | | $ | 4.1 | | $ | 3.4 | |
| | | | | | | | |
Compensation costs capitalized to inventory and compensation expense related to the Company's ESPP were not material for the year ended December 31, 2008.
Compensation expense related to stock options granted is recognized ratably over the service vesting period for the entire option award. The total number of stock option awards expected to vest is adjusted by estimated forfeiture rates. The estimated fair value of each stock option award was determined on the date of grant using the Black-Scholes option valuation model with the following weighted-average assumptions for the option grants:
| | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Risk-free interest rate | | | 2.58 | % | | 4.54 | % | | 4.64 | % |
Expected option life (in years) | | | 4.54 | | | 4.72 | | | 4.55 | |
Volatility | | | 0.50 | | | 0.67 | | | 0.75 | |
Dividend Rate | | | 0 | % | | 0 | % | | 0 | % |
The computation of the expected option life is based on a weighted-average calculation combining the average life of options that have already been exercised and post-vest cancellations with the estimated life of the remaining vested and unexercised options. The expected volatility rate is based on the historic volatility of the Company's stock. Prior to 2008, the historic volatility rate was determined using the length of the expected option life. The volatility of our stock has decreased in recent fiscal periods, and as a result we changed our look- back period in determining volatility to the third quarter of 2005. This change did not have a material impact on our financial statements for the fiscal year 2008. The risk-free interest rate is based on the U.S. Treasury yield curve over the expected term of the option. The Company has never paid cash dividends on its common stock and does not anticipate paying cash dividends in the foreseeable future. Consequently, the Company uses an expected dividend
F-17
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 5. Stock-Based Compensation (Continued)
yield of zero in the Black-Scholes option valuation model. The Company's estimated forfeiture rate is based on its historical experience.
The Company's determination of fair value is affected by the Company's stock price as well as a number of assumptions that require judgment. The weighted-average fair value per share was $7.37, $8.82 and $6.76 for options granted during the years ended December 31, 2008, 2007 and 2006, respectively. The total intrinsic value was $5.6 million, $5.4 million and $5.0 million for options exercised during the years ended December 31, 2008, 2007 and 2006, respectively. As of December 31, 2008, total unrecognized compensation cost related to stock options was approximately $4.1 million and the related weighted-average period over which it is expected to be recognized is approximately 2.7 years. The maximum contractual term of the Company's stock options is ten years.
A summary of the status of stock option activity for the years ended December 31, 2006, 2007 and 2008 is as follows (in thousands, except price data and years):
| | | | | | | | | | | | | |
| | Number
of Shares | | Weighted-
average
exercise
price per
share | | Weighted-
average
remaining
contractual
term (in
years) | | Aggregate
intrinsic
value | |
|---|
Outstanding at January 1, 2006 | | | 2,453 | | $ | 5.09 | | | | | | | |
Granted | | | 301 | | | 10.95 | | | | | | | |
Exercised | | | (666 | ) | | 5.13 | | | | | | | |
Cancelled | | | (54 | ) | | 7.81 | | | | | | | |
| | | | | | | | | | | | |
Outstanding at December 31, 2006 | | | 2,034 | | | 5.87 | | | | | | | |
Granted | | | 289 | | | 15.23 | | | | | | | |
Exercised | | | (495 | ) | | 5.19 | | | | | | | |
Cancelled | | | (99 | ) | | 7.43 | | | | | | | |
| | | | | | | | | | | | |
Outstanding at December 31, 2007 | | | 1,729 | | | 7.55 | | | | | | | |
Granted | | | 632 | | | 16.61 | | | | | | | |
Exercised | | | (554 | ) | | 5.61 | | | | | | | |
Cancelled | | | (41 | ) | | 9.41 | | | | | | | |
| | | | | | | | | | | | |
Outstanding at December 31, 2008 | | | 1,766 | | $ | 11.36 | | | 7.20 | | $ | 5,220 | |
| | | | | | | | | | |
Vested and expected to vest at December 31, 2008 | | | 1,518 | | $ | 10.58 | | | 6.89 | | $ | 5,197 | |
| | | | | | | | | | |
Exercisable at December 31, 2008 | | | 920 | | $ | 7.26 | | | 5.55 | | $ | 5,020 | |
| | | | | | | | | | |
Available for future grant at December 31, 2008 | | | 1,763 | | | | | | | | | | |
| | | | | | | | | | | | | |
The fair value of stock awards is determined based on the closing market price of the Company's common stock on the grant date. Compensation expense for stock awards is measured at the grant date and recognized ratably over the vesting period. For stock awards granted in 2005, vesting is based on both the service period as well as the achievement of the Company's performance goals. Meeting the performance goals for these awards allows for acceleration of a portion of the stock awards. A majority
F-18
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 5. Stock-Based Compensation (Continued)
of the stock awards granted in 2006 and 2007 were performance-based and vesting is tied to achievement of the Company's goals. For purposes of measuring compensation expense, the amount of shares ultimately expected to vest is estimated at each reporting date based on management's expectations regarding the relevant performance criteria. The recognition of compensation expense associated with performance-based stock awards requires judgment in assessing the probability of meeting the performance goals, as well as defined criteria for assessing achievement of the performance-related goals. The measurement date of the performance-based stock grants takes place when the grant is authorized and the specific achievement goals are communicated. The communication date of the performance goals can impact the valuation and associated expense of the stock grant. In 2008, it was determined that it was not probable that the performance goals would be met for a majority of the performance-based awards, and therefore, approximately $0.5 million in previously recognized stock compensation expense was reversed in 2008.
A summary of the status of stock awards activity for the year ended December 31, 2008 is as follows (in thousands, except price data):
| | | | | | | |
| | Shares | | Weighted-
Average
Grant Date
Fair Value | |
|---|
Nonvested at January 1, 2006 | | | 553 | | $ | 4.44 | |
Granted | | | 160 | | | 12.05 | |
Vested | | | (198 | ) | | 4.55 | |
Forfeited | | | (24 | ) | | 6.90 | |
| | | | | | |
Nonvested at December 31, 2006 | | | 491 | | | 6.76 | |
Granted | | | 438 | | | 11.97 | |
Vested | | | (182 | ) | | 7.01 | |
Forfeited | | | (192 | ) | | 9.62 | |
| | | | | | |
Nonvested at December 31, 2007 | | | 555 | | | 7.75 | |
Granted | | | 125 | | | 17.11 | |
Vested | | | (57 | ) | | 6.18 | |
Forfeited | | | (129 | ) | | 11.47 | |
| | | | | | |
Nonvested at December 31, 2008 | | | 494 | | $ | 9.33 | |
| | | | | | |
The total amount of unrecognized compensation cost related to nonvested stock awards as of December 31, 2008 was approximately $1.1 million, which is expected to be recognized over a weighted-average period of approximately 2.1 years, if all performance-based criteria are met.
F-19
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 6. Commitments and Contingencies
Leases
The Company leases its facilities and certain equipment. Commitments for minimum rentals under non-cancelable leases at the end of 2008 are as follows (in thousands):
| | | | | | | |
Years ending December 31, | | Operating Leases | | Capital Leases | |
|---|
2009 | | $ | 834 | | $ | 1,432 | |
2010 | | | 865 | | | 1,461 | |
2011 | | | 864 | | | 1,490 | |
2012 | | | 852 | | | 1,520 | |
2013 | | | 860 | | | 1,550 | |
Thereafter | | | 803 | | | 1,582 | |
| | | | | | |
Total minimum lease payments | | $ | 5,078 | | | 9,035 | |
| | | | | | | |
Less amount representing interest | | | | | | (2,036 | ) |
| | | | | | | |
Present value of capital lease payments | | | | | | 6,999 | |
Less current portion | | | | | | (862 | ) |
| | | | | | | |
Long-term obligations under capital leases | | | | | $ | 6,137 | |
| | | | | | | |
At December 31, 2008, assets under capital leases included in property and equipment totaled $12.9 million with accumulated amortization of $9.5 million.
Rent expense under operating leases totaled approximately $0.8 million for the year ended December 31, 2008, $1.4 million for the year ended December 31, 2007 and $1.4 million for the year ended December 31, 2006. In the fourth quarter of 2007, the Company entered into a new operating lease at its Santa Clara location, including extending the term of the lease through 2014.
During 1999, the Company completed a sale and leaseback transaction of its San Diego facility. The facility was sold for $15.0 million, of which $3.8 million was capital contributed by the Company. The Company's lease for its 78,000 square foot facility in San Diego, CA is for 15 years, with options to extend the lease for up to two additional five-year periods. The sale was an all cash transaction, netting the Company approximately $7.0 million. The Company is a 25% limited partner in the partnership that acquired the facility. The transaction was deemed a financing transaction under SFAS No. 98 "Accounting for Sales of Real Estate." As such, the assets sold remain on the books of the Company and will continue to be depreciated over the estimated useful life. The Company made lease payments of approximately $1.4 million for the year ended December 31, 2008, $1.4 million for the year ended December 31, 2007 and $1.3 million for the year ended December 31, 2006.
Contracts
The Company has entered into various licensing agreements which require royalty payments based on specified product sales. These agreements encompass the majority of the Company's products, and range in expiration through 2014. The Company also has a royalty agreement with Inverness Medical Innovations, Inc., which requires ongoing royalty payments of 8.5% on the majority of the Company's current products. Royalty expenses, which are charged to cost of sales under these licensing
F-20
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 6. Commitments and Contingencies (Continued)
agreements, totaled $10.5 million, $9.4 million and $9.6 million for the years ended December 31, 2008, 2007 and 2006, respectively. As of December 31, 2008 and 2007, $2.7 million and $3.3 million, respectively, were recorded as accrued royalties in the accompanying Consolidated Balance Sheets. During the fourth quarter of 2006, the Company entered into a cross-licensing agreement with another company and paid $6.5 million, which became fully amortized as of December 31, 2008. The Company believes it will continue to incur substantial royalty expenses relating to future sales of its products.
Legal
The Company is involved in litigation matters from time to time in the ordinary course of business. Management believes that all such current legal actions, in the aggregate, will not have a material adverse effect on the Company. The Company also maintains insurance, including coverage for product liability claims, in amounts which management believes appropriate given the nature of its business.
Note 7. Industry and Geographic Information
The Company operates in one reportable segment. Sales to customers outside the U.S. totaled 15%, 14%, and 20% of total revenue for the years ended December 31, 2008, 2007 and 2006, respectively. As of December 31, 2008 and 2007, balances due from foreign customers, in U.S. dollars, were $4.7 million and $4.0 million, respectively.
The Company had sales to individual customers in excess of 10% of total revenue, as follows:
| | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Customer: | | | | | | | | | | |
A | | | 19 | % | | 19 | % | | 17 | % |
B | | | 18 | % | | 21 | % | | 18 | % |
C | | | 13 | % | | 9 | % | | 9 | % |
D | | | 6 | % | | 6 | % | | 11 | % |
E | | | 4 | % | | 6 | % | | 13 | % |
As of December 31, 2008 and 2007, accounts receivable from individual customers with balances due in excess of 10% of total accounts receivable totaled $17.9 million and $17.8 million, respectively.
The following presents long-lived assets (excluding intangible assets) and total revenue by geographic territory (in thousands):
| | | | | | | | | | | | | | | | | |
| | Long-lived assets
December 31, | | Total revenue year ended
December 31, | |
|---|
| | 2008 | | 2007 | | 2008 | | 2007 | | 2006 | |
|---|
United States operations | | | | | | | | | | | | | | | | |
| | Domestic | | $ | 19,081 | | $ | 19,926 | | $ | 109,081 | | $ | 102,075 | | $ | 84,975 | |
| | Foreign | | | — | | | — | | | 19,051 | | | 15,990 | | | 21,040 | |
| | | | | | | | | | | | |
Total | | $ | 19,081 | | $ | 19,926 | | $ | 128,132 | | $ | 118,065 | | $ | 106,015 | |
| | | | | | | | | | | | |
F-21
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 7. Industry and Geographic Information (Continued)
Consolidated product revenues by disease state are as follows (in thousands):
| | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Infectious disease | | $ | 92,426 | | $ | 75,896 | | $ | 68,565 | |
Reproductive and women's health | | | 22,989 | | | 28,130 | | | 25,699 | |
Other | | | 11,427 | | | 12,864 | | | 10,468 | |
| | | | | | | | |
| | $ | 126,842 | | $ | 116,890 | | $ | 104,732 | |
| | | | | | | | |
Note 8. Fair Value Measurement
Effective January 1, 2008, the Company adopted the provisions of SFAS No. 157, Fair Value Measurements. SFAS No. 157 defines fair value, establishes a framework for measuring fair value in accordance with GAAP, and expands disclosures about fair value measurements, but does not require any new fair value measurements. SFAS No. 157's valuation techniques are based on observable and unobservable inputs. Observable inputs reflect readily obtainable data from independent sources, while unobservable inputs reflect market assumptions. Pursuant to SFAS No. 157, the fair value of our cash equivalents is determined based on Level 1 inputs, which consist of quoted prices in active markets for identical assets.
Note 9. Employee Benefit Plan
The Company has a defined contribution 401(k) plan (the "401(k) Plan") covering all employees who are eligible to join the 401(k) Plan upon employment. Employee contributions are subject to a maximum limit by federal law. This Plan includes an employer match of 50% on the first 6% of pay contributed by the employee. The Company contributed approximately $0.6 million to the 401(k) Plan during the year ended December 31, 2008 and $0.5 million for each of the years ended December 31, 2007 and 2006.
Note 10. Discontinued Operations
In the accompanying financial statements, the Company's urinalysis and ultrasonometer businesses are reported as discontinued operations under SFAS No. 144. The Company discontinued all operations of its ultrasonometer business during the fourth quarter of 2004, and during the second quarter of 2005, the Company sold certain assets of the urinalysis business for $0.5 million. Accordingly, the operations of both businesses have been classified as discontinued operations in the statements of income for all periods presented. During the fourth quarter of 2006, the Company finalized its open tax audits in Germany and recognized a non-cash gain on discontinued operations of approximately $0.8 million associated with certain remaining balance sheet credits of the urinalysis operation sold during 2005.
During the fourth quarter of 2006, the Company recognized non-cash income of approximately $1.3 million associated with certain remaining balance sheet credits of its foreign entities. These amounts were recorded as a gain on discontinued operations of $0.8 million related to the prior divestiture of the Company's urinalysis business, and other income of $0.5 million related to the previous closure of other foreign operations.
F-22
Table of Contents
QUIDEL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Note 11. Quarterly Financial Information (unaudited)
| | | | | | | | | | | | | | | | |
| | First Quarter | | Second Quarter | | Third Quarter | | Fourth Quarter | | Total Year | |
|---|
| | (in thousands, except per share data)
| |
|---|
2008 | | | | | | | | | | | | | | | | |
Total revenues | | $ | 40,865 | | $ | 21,916 | | $ | 31,868 | | $ | 33,483 | | $ | 128,132 | |
Cost of sales | | | 14,127 | | | 10,242 | | | 12,070 | | | 13,767 | | | 50,206 | |
Total cost and expenses | | | 27,304 | | | 23,009 | | | 24,516 | | | 24,684 | | | 99,513 | |
Net income (loss) | | | 8,550 | | | (513 | ) | | 4,741 | | | 6,070 | | | 18,848 | |
Basic net earnings (loss) per share | | | 0.27 | | | (0.02 | ) | | 0.15 | | | 0.19 | | | 0.59 | |
Diluted net earnings (loss) per share | | | 0.26 | | | (0.02 | ) | | 0.15 | | | 0.19 | | | 0.58 | |
2007 | | | | | | | | | | | | | | | | |
Total revenues | | $ | 33,934 | | $ | 18,580 | | $ | 27,570 | | $ | 37,981 | | $ | 118,065 | |
Cost of sales | | | 12,952 | | | 9,316 | | | 11,159 | | | 15,146 | | | 48,573 | |
Total cost and expenses | | | 26,191 | | | 21,742 | | | 23,829 | | | 26,817 | | | 98,579 | |
Net income (loss) | | | 4,817 | | | (1,712 | ) | | 2,420 | | | 8,106 | | | 13,631 | |
Basic net earnings (loss) per share | | | 0.15 | | | (0.05 | ) | | 0.08 | | | 0.25 | | | 0.43 | |
Diluted net earnings (loss) per share | | | 0.14 | | | (0.05 | ) | | 0.07 | | | 0.25 | | | 0.41 | |
F-23
Table of Contents
SCHEDULE II
QUIDEL CORPORATION
CONSOLIDATED VALUATION AND QUALIFYING ACCOUNTS
(in thousands)
| | | | | | | | | | | | | | | | | |
| | Additions | |
| |
| |
|---|
Description | | Balance at
beginning
of period | | Charges to
costs and
expenses(1) | | Charges to
other
accounts | | Deductions(2) | | Balance at
end of
period | |
|---|
Year ended December 31, 2008: | | | | | | | | | | | | | | | | |
| | Accounts Receivable Allowance | | $ | 929 | | $ | 1,608 | | $ | — | | $ | 1,025 | | $ | 1,512 | |
Year ended December 31, 2007: | | | | | | | | | | | | | | | | |
| | Accounts Receivable Allowance | | $ | 791 | | $ | 855 | | $ | — | | $ | 717 | | $ | 929 | |
Year ended December 31, 2006: | | | | | | | | | | | | | | | | |
| | Accounts Receivable Allowance | | $ | 1,082 | | $ | 1,167 | | $ | — | | $ | 1,458 | | $ | 791 | |
- (1)
- Represent charges associated primarily to accruals for early payment discounts and bad debt.
- (2)
- The deductions represent actual charges against the accrual described above.
Table of Contents
EXHIBIT INDEX
| | |
Exhibit Number | | Description |
|---|
| 3.1 | | Certificate of Incorporation, as amended. (Incorporated by reference to Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on February 26, 1991.) |
3.2 |
|
Amended and Restated Bylaws. (Incorporated by reference to Exhibit 3.2 to the Registrant's Form 8-K dated November 8, 2000.) |
4.1 |
|
Certificate of Designations of Series C Junior Participating Preferred Stock as filed with the State of Delaware on December 31, 1996 (Incorporated by reference to Exhibit 1(A) to the Registrant's Registration Statement on Form 8-A filed on January 14, 1997.) |
4.2 |
|
Amended and Restated Rights Agreement dated as of December 29, 2006 between Registrant and American Stock Transfer and Trust Company, as Rights Agent. (Incorporated by reference to Exhibit 4.1 to the Registrant's Current Report on Form 8-K filed on January 5, 2007.) |
10.1(1) |
|
Registrant's 1983 Employee Stock Purchase Plan, as amended. (Incorporated by reference to Exhibit 10.6 to the Registrant's Form 10-Q filed on July 27, 2007.) |
10.2(1) |
|
Registrant's 1990 Employee Stock Option Plan. (Incorporated by reference to Exhibit 10.3 to the Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 1990.) |
10.3(1) |
|
Registrant's 1996 Non-Employee's Director Plan. (Incorporated by reference to Registrant's Proxy Statement filed on September 27, 1996.) |
10.4(1) |
|
Registrant's 1998 Stock Incentive Plan. (Incorporated by reference to Exhibit 10.7 to the Registrant's Form 10-Q filed on July 27, 2007.) |
10.5(1) |
|
Registrant's Amended and Restated 2001 Equity Incentive Plan. (Incorporated by reference to Exhibit 10.1 to Registrant's Form 8-K filed on May 10, 2007.) |
10.6(1) |
|
Form of Restricted Stock/Stock Option Agreement used in connection with the Registrant's Amended and Restated 2001 Equity Incentive Plan. (Incorporated by reference to Exhibit 10.6 to the Registrant's Form 10-Q for the quarter ended September 30, 2004.) |
10.7 |
|
Settlement Agreement effective April 1, 1997 between the Registrant and Becton, Dickinson and Company. (Incorporated by reference to Exhibit 10.18 to the Registrant's Form 10-K for the year ended March 31, 1997.) |
10.8 |
|
Rosenstein License Agreement effective April 1, 1997 between the Registrant and Becton, Dickinson and Company. (Incorporated by reference to Exhibit 10.20 to the Registrant's Form 10-K for the year ended March 31, 1997.) |
10.9 |
|
Settlement Agreement dated April 27, 2005 between the Registrant and Inverness Medical Innovations, Inc. (Incorporated by reference to Exhibit 10.1 to the Registrant's Form 8-K filed on May 3, 2005.) |
10.10 |
|
Form of Purchase and Sale Agreement and Escrow Instructions. (Incorporated by reference to Exhibit 10.6 to the Registrant's Form 8-K filed on January 4, 2000.) |
10.11 |
|
Form of Single Tenant Absolute Net Lease. (Incorporated by reference to Exhibit 10.7 to the Registrant's Form 8-K filed on January 4, 2000.) |
10.12 |
|
Form of Indemnification Agreement—Corporate Officer and/or Director. (Incorporated by reference to Exhibit 10.1 to the Registrant's Form 8-K filed August 23, 2005.) |
10.13(1) |
|
Employment Agreement, dated as of January 16, 2009, between Registrant and Douglas C. Bryant. (Incorporated by reference to Exhibit 10.1 to the Registrant's Form 8-K filed on January 20, 2009.) |
Table of Contents
| | |
Exhibit Number | | Description |
|---|
| 10.14(1) | | Stock Option Agreement, dated as of January 16, 2009, between Registrant and Douglas C. Bryant. (Incorporated by reference to Exhibit 10.2 to the Registrant's Form 8-K filed on January 20, 2009.) |
10.15(1) |
|
Restricted Stock Agreement, dated as of January 16, 2009, between Registrant and Douglas C. Bryant. (Incorporated by reference to Exhibit 10.3 to the Registrant's Form 8-K filed on January 20, 2009.) |
10.16(1) |
|
Agreement Re: Change in Control, dated as of January 16, 2009, between Registrant and Douglas C. Bryant. (Incorporated by reference to Exhibit 10.4 to the Registrant's Form 8-K filed on January 20, 2009.) |
10.17(1) |
|
Stock Option Agreement effective August 20, 2004 between the Registrant and Caren L. Mason. (Incorporated by reference to Exhibit 10.1 to the Registrant's Form 8-K filed on August 26, 2004.) |
10.18(1) |
|
Employment Agreement dated as of August 20, 2004 between the Registrant and Caren L. Mason. (Incorporated by reference to Exhibit 10.2 to the Registrant's Form 8-K filed on August 26, 2004.) |
10.19(1) |
|
Amendment of Employment Agreement, dated December 31, 2007, between Registrant and Caren L. Mason. (Incorporated by reference to Exhibit 10.1 to Registrant's Form 8-K filed on January 3, 2008.) |
10.20(1) |
|
Change in Control Agreement dated August 20, 2004, between Registrant and Caren L. Mason. (Incorporated by reference to Exhibit 10.3 to Registrant's Form 8-K filed on August 26, 2004.) |
10.21(1) |
|
Amendment of Agreement Re: Change in Control, dated December 31, 2007, between Registrant and Caren L. Mason. (Incorporated by reference to Exhibit 10.5 to Registrant's Form 8-K filed on January 3, 2008.) |
10.22(1) |
|
Retirement Agreement, dated as of January 16, 2009, between Registrant and Caren L. Mason. (Incorporated by reference to Exhibit 10.5 to Registrant's Form 8-K filed on January 20, 2009.) |
10.23(1) |
|
Employment Offer Letter dated as of October 26, 2004 between Registrant and Thomas J. Foley, Ph.D. (Incorporated by reference to Exhibit 10.1 to Registrant's Form 8-K filed on November 2, 2004.) |
10.24(1) |
|
Change in Control Agreement effective as of November 8, 2004 between Registrant and Thomas J. Foley, Ph.D. (Incorporated by reference to Exhibit 10.2 to Registrant's Form 8-K filed on November 2, 2004.) |
10.25(1) |
|
Amendment of Agreement Re: Change in Control, dated December 31, 2007, between Registrant and Thomas J. Foley. (Incorporated by reference to Exhibit 10.8 to Registrant's Form 8-K filed on January 3, 2008.) |
10.26(1) |
|
Employment Offer Letter, entered into on June 5, 2008, between Registrant and Robert J. Bujarski. (Incorporated by reference to Exhibit 10.1 to Registrant's Form 8-K filed on June 6, 2008.) |
10.27(1) |
|
Agreement Re: Change in Control, entered into on June 5, 2008, between Registrant and Robert J. Bujarski. (Incorporated by reference to Exhibit 10.2 to Registrant's Form 8-K filed on June 6, 2008.) |
10.28(1) |
|
Employment Offer Letter, entered into on December 18, 2006, between Registrant and John M. Radak. (Incorporated by reference to Exhibit 10.1 to Registrant's Form 8-K filed on January 3, 2007.) |
Table of Contents
| | |
Exhibit Number | | Description |
|---|
| 10.29(1) | | Amendment of Employment Offer Letter, dated December 31, 2007, between Registrant and John M. Radak. (Incorporated by reference to Exhibit 10.3 to Registrant's Form 8-K filed on January 3, 2008.) |
10.30(1) |
|
Agreement Re: Change in Control, entered into on December 18, 2006, between Registrant and John M. Radak. (Incorporated by reference to Exhibit 10.2 to Registrant's Form 8-K filed on January 3, 2007.) |
10.31(1) |
|
Amendment of Agreement Re: Change in Control, dated December 31, 2007, between Registrant and John M. Radak. (Incorporated by reference to Exhibit 10.10 to Registrant's Form 8-K filed on January 3, 2008.) |
10.32(1) |
|
Employment Offer Letter, dated June 19, 2007, between Registrant and Richard C. Tarbox III. (Incorporated by reference to Exhibit 10.1 to Registrant's Form 8-K filed on June 26, 2007.) |
10.33(1) |
|
Amendment of Employment Offer Letter, dated December 31, 2007, between Registrant and Richard C. Tarbox III. (Incorporated by reference to Exhibit 10.4 to Registrant's Form 8-K filed on January 3, 2008.) |
10.34(1) |
|
Agreement Re: Change in Control, entered into on June 25, 2007, between Registrant and Richard C. Tarbox III. (Incorporated by reference to Exhibit 10.2 to Registrant's Form 8-K filed on June 26, 2007.) |
10.35(1) |
|
Amendment of Agreement Re: Change in Control, dated December 31, 2007, between Registrant and Richard C. Tarbox III. (Incorporated by reference to Exhibit 10.11 to Registrant's Form 8-K filed on January 3, 2008.) |
10.36(1) |
|
Agreement Re: Change in Control, entered into on June 25, 2007, between Registrant and Scot M. McLeod. (Incorporated by reference to Exhibit 10.3 to Registrant's Form 8-K filed on June 26, 2007.) |
10.37(1) |
|
Amendment of Agreement Re: Change in Control, dated December 31, 2007, between Registrant and Scot M. McLeod. (Incorporated by reference to Exhibit 10.9 to Registrant's Form 8-K filed on January 3, 2008.) |
10.38(1) |
|
Change in Control Agreement dated July 19, 2004 between Registrant and Michael J. Beck. (Incorporated by reference to Exhibit 10.35 to Registrant's Form 10-Q for the quarter ended June 30, 2004.) |
10.39(1) |
|
Amendment of Agreement Re: Change in Control, dated December 31, 2007, between Registrant and Michael J. Beck. (Incorporated by reference to Exhibit 10.6 to Registrant's Form 8-K filed on January 3, 2008.) |
10.40(1) |
|
Agreement Re: Change in Control, entered into on November 7, 2008, between Registrant and John D. Tamerius, Ph.D. (Incorporated by reference to Exhibit 10.1 to Registrant's Form 8-K filed on November 7, 2008.) |
10.41(1) |
|
Annual Base Salary for the Company's Executive Officers effective as of March 3, 2008. (Incorporated by reference to Exhibit 10.1 to Registrant's Form 8-K filed on February 21, 2008.) |
10.42(1) |
|
2008 Compensation for President and Chief Executive Officer. (Incorporated by reference to Exhibit 10.3 to Registrant's Form 8-K filed on April 25, 2008.) |
10.43(1) |
|
2008 Short-Term Cash Incentive Program for the Company's Executive Officers, effective as of April 7, 2008. (Incorporated by reference to Exhibit 10.2 to Registrant's Form 8-K filed on April 10, 2008.) |
Table of Contents
| | |
Exhibit Number | | Description |
|---|
| 10.44(1) | | Registrant's 2008 Equity Incentive Program for the Company's Executive Officers, effective as of April 7, 2008. (Incorporated by reference to Exhibit 10.1 to Registrant's Form 8-K filed on April 10, 2008.) |
10.45 |
|
Credit Agreement, by and among Registrant, as Borrower, each lender from time to time party thereto (collectively, "Lenders" and individually, a "Lender") and Bank of America, N.A., as Agent, Swing Line Lender and L/C Issuer, dated as of October 8, 2008. (Incorporated by reference to Exhibit 10.1 to Registrant's Form 8-K filed on October 10, 2008.) |
10.46 |
|
Security Agreement by and among Registrant, as Borrower, direct and indirect domestic subsidiaries of Borrower, each additional grantor that may become a party thereto and Bank of America, N.A., as Agent, dated as of October 8, 2008. (Incorporated by reference to Exhibit 10.2 to Registrant's Form 8-K filed on October 10, 2008.) |
21.1* |
|
Subsidiaries of the Registrant. |
23.1* |
|
Consent of Independent Registered Public Accounting Firm. |
31.1* |
|
Certification by Principal Executive Officer of Registrant pursuant to Rules 13a-14 and 15d-14, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
31.2* |
|
Certification by Principal Financial and Accounting Officer of Registrant pursuant to Rules 13a-14 and 15d-14, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
32.1* |
|
Certifications by Principal Executive Officer and Principal Financial and Accounting Officer of Registrant pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
- *
- Filed herewith
- (1)
- Indicates a management plan or compensatory plan or arrangement.
