
GENE THERAPY
21st Century Solutions
for
Neurodegenerative
Diseases

FORWARD LOOKING STATEMENT
This presentation includes certain statements of the Company that may constitute
"forward-looking statements" within the meaning of Section 27A of the Securities Act of
1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended,
and which are made pursuant to the Private Securities Litigation Reform Act of 1995.
These forward-looking statements and other information relating to the Company are
based upon the beliefs of management and assumptions made by and information
currently available to the Company. Forward-looking statements include statements
concerning plans, objectives, goals, strategies, future events, or performance, as well as
underlying assumptions and statements that are other than statements of historical fact.
When used in this document, the words "expects," “promises,” "anticipates," "estimates,"
"plans," "intends," "projects," "predicts," "believes," "may" or "should," and similar
expressions, are intended to identify forward-looking statements. These statements
reflect the current view of the Company's management with respect to future events.
Many factors could cause the actual results, performance or achievements of the
Company to be materially different from any future results, performance or achievements
that may be expressed or implied by such forward-looking statements. Additional
information regarding factors that could cause results to differ materially from
management's expectations is found in the section entitled "Risk Factors" in the
Company's 2005 Annual Report on Form 10-KSB. Although the Company believes these
assumptions are reasonable, no assurance can be given that they will prove correct.
2

COMPANY HISTORY
2006
$12 Million Private Placement – Lead by GE and
DaimlerChrysler
2005
Presentation of Statistically Significant Safety and
Efficacy in “Controlled” Parkinson’s Disease Study
2004
Public via Merger with Shell (NRGX)
2003
Initiated First Ever Human Gene Therapy Trial for
Parkinson’s Disease “Phase I”
2002
Equity Investment by Medtronic
Private Equity by Palisade Capital Management
1999
Founded by Matthew J. During MD, D.Sc. & Michael G. Kaplitt MD, Ph.D
1999
Successful Preclinical Results with Epilepsy
Presented Successful Results of
1st Human Parkinson’s Trial
Second Equity Investment & Development Agreement
with Medtronic
3

Neurologix
Development Strategy
Diseases of the Central Nervous
System that:
Are Surgically Challenging
Represent Financial Burden to the
Healthcare System
Have No Good Therapeutic Solution
Are Economically Attractive: Large
Unmet Need
Have Established Reimbursement Policy
4

Neurologix
Current Research/Product Development
Activity
Parkinson's Disease
Temporal Lobe Epilepsy
Metabolic Syndrome
Diseases Characterized by Abnormal
Neurotransmission within the Brain
Platform
AAV…Genetic Delivery Mechanism
Therapeutic Gene…Unique to Each Indication
5

How the Platform Works
Employ Nonpathogenic Adeno-Associated Virus (AAV)
Unique Gene Required for Each Disease
Remove AAV Genetic Material
GAD Gene: Parkinson’s… Subthalamic Nucleus
NPY Gene: Epilepsy…Hippocampus
Metabolic Syndrome….Hypothalamus
Nonpathogenic Virus with New Gene Infused into Brain
Virus Locates and Enters Specifically Targeted Cells
Delivers New Genetic Material to Targeted Cells
New Genetic Material Reestablishes Normal Cell Function
6

Parkinson’s Disease Indication
Neurodegenerative Disease
Progressive
Interruption of Neurotransmitters
Symptoms:
Tremor
Rigidity
Difficulty in Initiating Movement
Impaired Balance
Difficulty Swallowing, Sexual Dysfunction, Depression
7

Parkinson’s Prevalence
1.5 Million Patients in US according to NPF
Expected to Double within Next 20 Years
60,000 New Patients Per Year – US
1.5 Million in Principal Countries Outside US
Cost to US Healthcare System: $25 Billion
8

Parkinson’s Treatment Today
Pharmacological: L-Dopa
After 5-10 Years Most Patients Become Resistant
More Frequent & Longer Off Periods
Dyskinesia
Post L-Dopa
Deep Brain Stimulation
High Complication Rates
Limited Patient Potential
Multiple Surgical Interventions
9
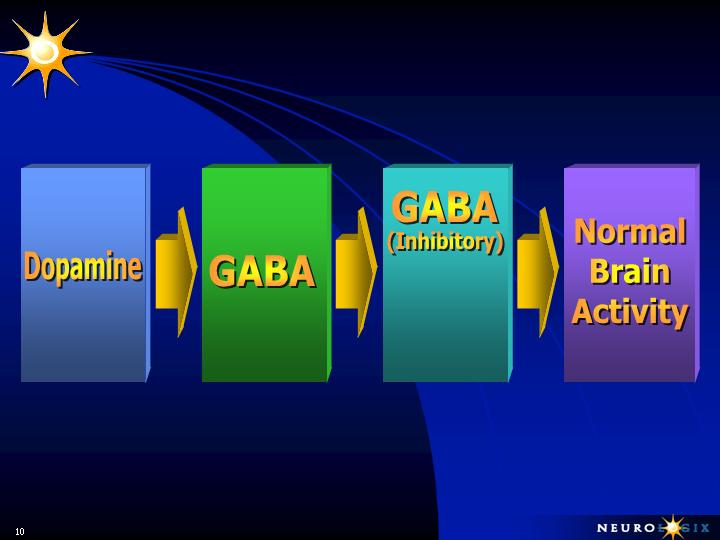
NORMAL BRAIN FUNCTION
Ignition
Power Brake
Balance Between
Accelerator and
Brake
Substantia Nigra
Globus Pallidus
Thalamus - Cortex
Sub-Thalamic
Nucleus
Glutamate
&
Acetylcholine
(Stimulatory)
10
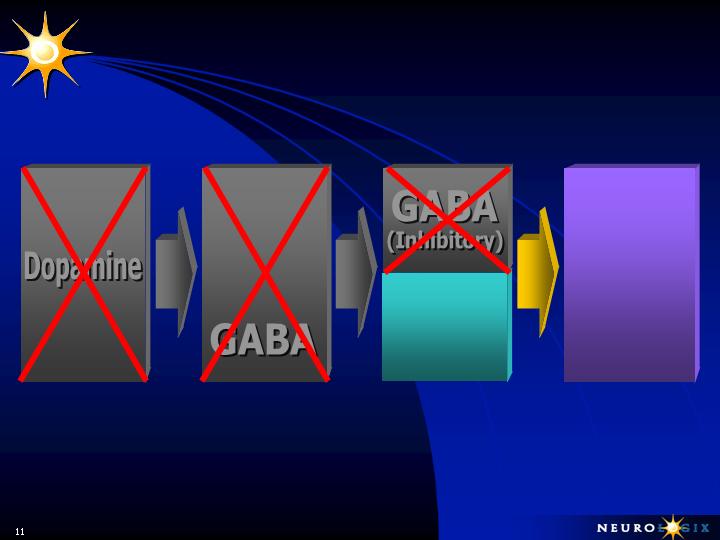
LATE STAGE PARKINSON’S DISEASE
Ignition
Power Brake
Unopposed
Accelerator/
No Brake
Substantia Nigra
Globus Pallidus
Thalamus - Cortex
Sub-Thalamic
Nucleus
L-DOPA
therapy
stimulates
production
of
Glutamate
&
Acetylcholine
(Stimulatory)
Tremors,
Rigidity,
difficulty in
initiating
movement
and
postural
instability
11
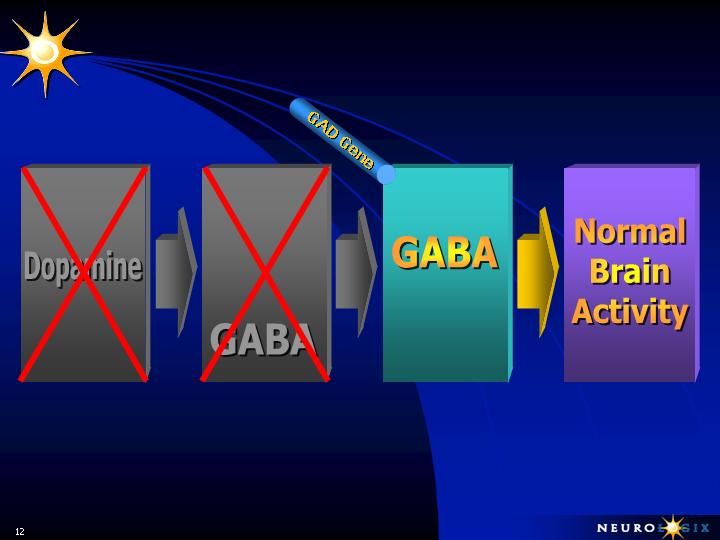
NEUROLOGIX APPROACH FOR
LATE STAGE PARKINSON’S DISEASE
Ignition
Power Brake
L-DOPA
therapy
stimulates
production
of
Reestablishes
Balance Between
Accelerator and
Brake
Thalamus - Cortex
Sub-Thalamic
Nucleus
Bypasses Dopamine
and Reestablishes
Normal Inhibition
Insert Gene
for
Glutamate
&
Acetylcholine
(Stimulatory)
12

NLX P-101 Phase I Design
12 Patients in 3 Cohorts
All PD >5 Years and Resistant to L-DOPA
Single Injection of AAV-GAD One Side of Brain
Highest Dose 10 X Lowest
Untreated Side Acted as Control
Efficacy:
Universal Parkinson’s Disease Rating System (UPDRS)
Activities Daily Living (ADL)
Clinical Data at 1, 3, 6 and 12 Months
PET Scans at Baseline 6 and 12 months
Monitor Safety and Adverse Events
13

Phase I Results
Total Improvement UPDRS
38.6 ± 8.4 to 28.7 ± 13.2 at 6 mos. (P<0.01)
29.7 ± 13.6 at 1 Year (P<0.01)
23.0% Avg. Improvement on all Patients
9 of 12 Patients Improved 37%
5 of 12 Patients Improved 40% - 60%
No Suggestion of Placebo Effect
Improvements Appear to be Dose Related
14

UPDRS Changes Over Time
* P < 0.05; ** P < 0.01; *** P < 0.005; Post-hoc Bonferroni tests
0
1
6
3
Months
12
***
**
***
Treated Side
P < 0.005
0
1
6
3
Months
12
*
***
**
15

PET Scan Data
NLX-P101 Treated Side
Improvement Demonstrated by:
Declines in Pallidal & Thalamic Metabolism
At 6 mos.
At 1 Year
Untreated Side:
Progressive Increases in Pallidal & Thalamic
Metabolism
At 6 mos.
At 1 Year
16
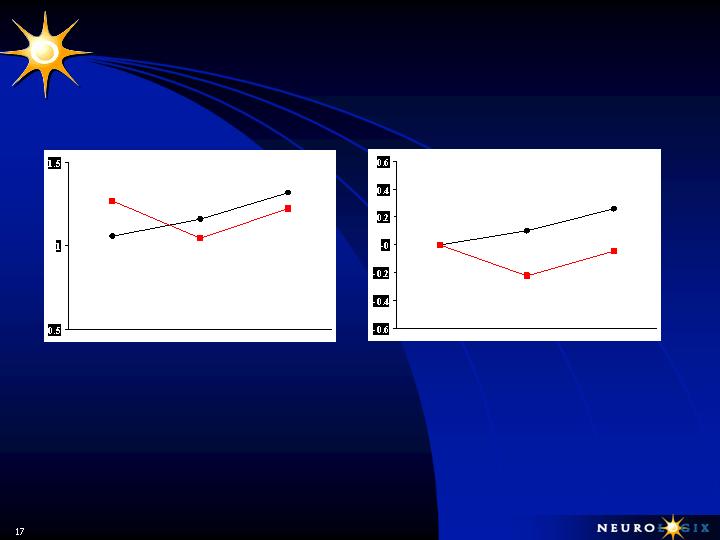
Changes in hemispheric PDRP
Baseline
OP (red) vs. UNOP (black)
6
Months
Months
12
Baseline
6
12
Relative to Baseline
A
B
***
***
*** p < 0.005
***
***
17

NLX-P101 NEXT STEPS
Company Has Begun Pilot Plant Operations
in Preparation for Next Study
Will Begin Phase II Bilateral, Multi-Center,
Double-Blind, Placebo-Controlled, Dose
Ranging Study
Expect to Start Q4-07
12 Month Study
18

Advantages of Neurologix
NLX-P101 Solution
Bypasses Dopamine Completely
Single Dose Application
Local Anesthesia
Short O.R. Time: Same Day Surgery
No Post Op. Maintenance Visits: No Hardware
Programming or Complications
Non-Immunogenic
No Treatment Related Adverse Events
Patients Treated as far Back as 3+ Years
19

Epilepsy
Group of Diseases Associated with Recurrent
Seizures
Abnormal Electrical Impulses Within the Brain
2.5 Million People in US have the Disease
Cost to US Healthcare System $2.5 B
50% Continue to have Seizures Despite Drug
Therapy
Approximately 150,000 are Candidates for
Temporal Lobe Surgery
20

Epilepsy Program NLX- E201
Temporal Lobe Epilepsy
Leverage Existing Vector Platform
Modulate NPY Receptors (Inhibitory Response)
Expect to Initiate Trial Q2/Q3-07
Phase I, 12 Month, Two Centers, Two Dose, Open Label
Approved by NIH Recombinant Advisory Committee
Preclinical Non Human Primate Work Extremely Positive
21

Metabolic Syndrome
Hypothalamic Dysfunction
Severe Obesity
High Blood Pressure
Increased Risk of Heart Disease
Undiagnosed
Preclinical Stage
22

Company’s Current Projects
Parkinson’s Disease
Phase II Expected to Begin Q4-07
Expected Completion 2009
Temporal Lobe Epilepsy
Submitted IND
Non Human Primate Study Completed
No Safety Issues
No Adverse Effects
Metabolic Syndrome
Preclinical Phase
23

INTELLECTUAL PROPERTY
Propriety GAD Based Delivery System
US and Foreign
Proprietary Payloads/Field of Use
US and Foreign
Brain Infusion Device and Method
US and Foreign
Manufacturing:
Technology
Processes
14 US & Foreign Patents Pending
24

MEDTRONIC PARTNERSHIP
2 Equity Investments
Medtronic Will
Commercialize Catheters
for Therapy Infusion
Royalty on Catheter
Sales
Medtronic right-of-first
offer on Parkinson’s and
Epilepsy drugs
25
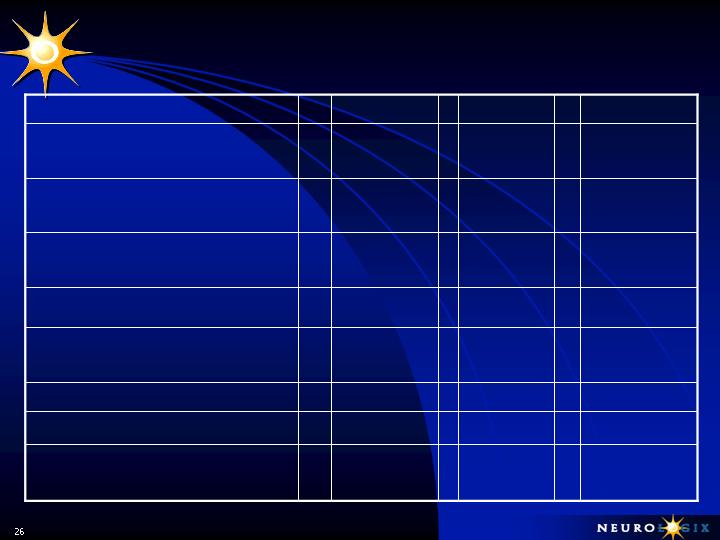
Financial Results – Balance Sheet
4,522
14,022
$
12,019
$
Stockholder’s Equity
896
1,201
$
944
$
Current Liabilities
5,418
15,278
$
12,963
$
Total Assets
592
$
677
$
746
$
Other Assets, Net
3,930
$
13,400
$
11,273
$
Working Capital
4,826
$
14,546
$
12,217
$
Current Assets
4,050
$
13,654
$
11,656
$
Cash & S-T Investments
12/31/05
6/30/06
9/30/06
000s
26

Financial Results – P & L (con’t)
5,345
$
3,149
$
5,643
$
Net Loss
(177)
$
(126)
$
(290)
$
Other Expense
(Income)
3,008
$
1,746
$
3,141
$
G & A Expense
2,514
$
1,529
$
2,792
$
R & D Expense
Year Ended
12/31/05
6 Months
6/30/06
9 Months
9/30/06
000s
27

Michael G. Kaplitt,
MD, PhD
Matthew J. During, MD,
DSc
SCIENTIFIC FOUNDERS
Expert and innovator in gene
therapy, among first scientists to
publish on the use of viruses for
direct gene delivery in the living
brain
Assistant Professor of
Neurosurgery, Director of
Stereotactic and Functional
Neurosurgery, Director of the
Laboratory of Molecular
Neurosurgery at Weill Medical
College of Cornell University
Internationally recognized leader and
pioneer in gene therapy of neurological
diseases and human brain microdialysis
Currently Professor Ohio State University
Professor Weill-Cornell Medical Center
Director of the CNS Gene Therapy
Center at Jefferson Medical College
(1998-2002)
Faculty at Yale University (1988-1998);
directed program on the molecular basis
of learning and memory, headed Yale’s
first gene therapy protocol
28

SCIENTIFIC ADVISORY BOARD
Paul Greengard, Ph.D., Chairman
2000 Nobel Prize winner - dopamine interactions and a
number of other transmitters with the nervous system
Andres Lozano, M.D., Ph.D
World leader in surgery for Parkinson’s Disease
Published 100+ papers and recently edited Book on
Epilepsy
Eric Nestler, M.D., Ph.D, University of Texas
Chairman Dept of Psychiatry, UT Southwest Medical Ctr
Thought Leader - Drug Addiction Alcoholism-Depression
Daniel Lowenstein, M.D.
UCSF-Director of Epilepsy Program
29

Neurologix Management
John E Mordock – CEO
30 Years Life Science Management Experience
President – Teleflex, Inc. Instruments & Surgical
Services Group (NYSC)
President – Cabot Medical Corp. (NASDQ)
Industry Representative – USFDA OB/GYN Device
Approval Panel
30

Neurologix Management
Marc L. Panoff – Chief Financial Officer &
Treasurer
More than a Decade of Experience in Financial
Management
CFO - Nephros, Inc., Publicly Traded Medical
Device Company
Corporate Controller at Medicis Pharmaceutical
Corporation, Publicly Traded Specialty
Pharmaceutical Company
31

Neurologix Management
Christine V Sapan, PhD – Executive Vice
President and Chief Development Officer
More than 30 Years Regulatory & Clinical
Development Experience
Nabi Pharmaceuticals – Vice President
Regulatory, Manufacturing & Clinical
Development
Beckman-Coulter, Inc. – Manager Hematology
Research & Development
Post-Doctoral Fellow – Howard Hughes Research
Institute, Duke University
32

Medical Revolution of the 20th Century:
Recombinant DNA, Cell Fusion, and
the new Bio-processing Techniques of
Biotechnology Change the Course of
Medicine, Specifically in the Area of
Cancer Treatment.
33

Neurologix
Medical Revolution of the 21st Century:
Gene Transfer Therapy will
Change the Course and Landscape
of Medicine in the Near Future,
Beginning with Parkinson’s
Disease Treatment.
34

WHY INVEST IN NEUROLOGIX
Strong Scientific Foundation
Established Method of Action
Large Market Potential
Underserved Patient Population
Strong Value Proposition
Large Pipeline Potential
Low Valuation Relative to Progress & Potential
35

Ticker: NRGX
36

37




































