Table of Contents
As filed with the Securities and Exchange Commission on December 15, 2003
Registration No. 333-
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-4
REGISTRATION STATEMENT
Under
THE SECURITIES ACT OF 1933
GENOME THERAPEUTICS CORP.
(Exact Name of Registrant as Specified in Its Certificate of Incorporation)
| Massachusetts | 2834 | 04-2297484 | ||
(State or Other Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
100 BEAVER STREET
WALTHAM, MASSACHUSETTS 02453
(781) 398-2300
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Stephen Cohen
Senior Vice President and Chief Financial Officer
Genome Therapeutics Corp.
100 Beaver Street
Waltham, Massachusetts 02453
(781) 398-2300
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
Copies to:
Patrick O’Brien, Esq. Ropes & Gray LLP One International Place Boston, Massachusetts 02109 (617) 951-7000 | Christopher Dillon, Esq. Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP 155 Constitution Drive Menlo Park, California 94025 (650) 321-2400 |
Approximate date of commencement of proposed sale to the public: As soon as possible after the effective date of this registration statement and the consummation of the merger described in this registration statement.
If the securities being registered on this form are being offered in connection with the formation of a holding company and there is compliance with General Instruction G, check the following box. ¨
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement number for the same offering. ¨
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
CALCULATION OF REGISTRATION FEE
Title of each Class of Securities to be Registered | Amount to be Registered | Proposed Maximum Offering Price Per Share | Proposed Maximum Aggregate Offering Price | Amount of Registration Fee | ||||
Common Stock, par value $.10 par value | 28,571,405(1) | N/A | $678(2) | $1(3) | ||||
| (1) | Represents the estimated maximum number of shares of common stock of the registrant that may be issued pursuant to Agreement and Plan of Merger and Reorganization, dated as of November 17, 2003, among registrant, Guardian Acquisition, Inc., a wholly-owned subsidiary of registrant, GeneSoft Pharmaceuticals, Inc., and Luke Evnin, as the representative of the stockholders of GeneSoft Pharmaceuticals, Inc. |
| (2) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(f)(2) under the Securities Act of 1933, as amended. Because there is no market for Genesoft’s securities and Genesoft has an accumulated capital deficit, the proposed maximum aggregate offering price was calculated as one-third of the par value per share of $0.0001 of (i) the 12,378,931 shares of Genesoft common stock to be exchanged in the merger, (ii) the 2,939,747 shares of Genesoft common stock issuable pursuant to options assumed in the merger and (iii) the 5,025,970 shares of Gensoft common stock issuable pursuant to warrants outstanding immediately prior to the merger, determined as of December 15, 2003. |
| (3) | As the registration fee calculated pursuant to Rule 457(f)(2) would be less than $1, the registrant is paying the minimum registration fee of $1. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
PRELIMINARY DRAFT DATED DECEMBER 15, 2003 – SUBJECT TO COMPLETION
| [GENOME LOGO] | [GENOME LOGO] |
The boards of directors of Genome Therapeutics Corp. and GeneSoft Pharmaceuticals, Inc. have agreed to combine the two companies through a business combination transaction pursuant to which (i) a wholly-owned subsidiary of Genome will first merge with and into Genesoft, with Genesoft surviving as a wholly-owned subsidiary of Genome and (ii) immediately following the foregoing step, the surviving entity will be merged with and into a second wholly-owned subsidiary of Genome. Both steps will occur as part of a single integrated plan.
The boards of directors of Genome and Genesoft believe that the merger between the two companies is advisable and in the best interests of their respective stockholders.
Genome recommends that its stockholders vote (i) to approve the issuance of a total of 28,571,405 shares of Genome common stock pursuant to the merger agreement and the issuance of shares of Genome common stock upon the potential conversion of the convertible notes of Genome, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger and (ii) to approve the Amendment to Genome’s Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000 shares of common stock.Since the merger is conditioned upon the approval of the matters set forth in each of these proposals, Genome recommends that its stockholders vote for each proposal.
If the merger is completed, each outstanding share of Genesoft common stock will be converted into a right to receive shares of Genome’s authorized common stock, unless Genesoft’s stockholders exercise appraisal or dissenters’ rights under the laws of Delaware or California, as applicable. Genome will issue a total of 28,571,405 shares of its common stock (i) in exchange for all shares of capital stock of Genesoft, (ii) as payment of certain interest and related amounts due to Genesoft’s note holders and (iii) upon the exercise of Genesoft options and warrants, which will be assumed by Genome. We will determine the number of shares of Genome common stock into which each share of Genesoft common stock will be converted immediately prior to completion of the merger in accordance with formulas specified in the merger agreement and described in the attached materials.
Genesoft recommends that its stockholders vote (i) to adopt and approve the merger agreement and (ii) to amend and restate Genesoft’s Seventh Amended and Restated Certificate of Incorporation to eliminate all authorized shares of Genesoft preferred stock if the merger is completed.
Genome common stock is listed in the Nasdaq National Market under the symbol “GENE.”
Your Vote is Very Important. Whether or not you plan to attend your company’s stockholders’ meeting, please take the time to vote on the proposal(s) submitted for your company’s meeting by completing and mailing the enclosed proxy card to us. If you sign, date and mail your proxy card without indicating how you wish to vote, your proxy will be counted as a vote in favor of the proposal(s) submitted at your meeting. Failure to return or sign your proxy card will have the effect of a vote against the merger and the related transactions, unless you attend your stockholders’ meeting and vote in person.
The dates, times and places of the stockholders meetings are as follows:
For Genome’s stockholders: , 2004 at 10:00 a.m. local time, at Ropes & Gray LLP, One International Place, 36th floor, Boston, Massachusetts.
For Genesoft’s stockholders: , 2004 at a.m. local time at .
Table of Contents
Following this letter you will find a formal notice of the special meeting of each company’s stockholders and a joint proxy statement/prospectus. The joint proxy statement/prospectus provides you with detailed information concerning the merger and the related transactions as well as the other proposals to be presented and voted upon at the special meetings of stockholders of Genome and Genesoft. You may also obtain more information about Genome from documents that it has filed with the Securities and Exchange Commission.
Sincerely,
Steven M. Rauscher | David B. Singer | |
Chairman, President and Chief Executive Officer of | Chairman and Chief Executive Officer of | |
Genome Therapeutics Corp. | GeneSoft Pharmaceuticals, Inc. |
Please give all of the information contained or incorporated by reference in the joint proxy statement/prospectus your careful attention. In particular, you should carefully consider the discussion in the section entitled “Risk Factors” beginning on page 24 of the joint proxy statement/prospectus. Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the shares to be issued under, or passed upon the adequacy of, this joint proxy statement/prospectus. Any representation to the contrary is a criminal offense.
The accompanying joint proxy statement/prospectus is dated , 2003 and was first mailed to stockholders of Genome and Genesoft on or about , 2003.
Table of Contents
GENOME THERAPEUTICS CORP.
100 Beaver Street
Waltham, Massachusetts 02453
(781) 398-2300
NOTICE OF SPECIAL MEETING OF STOCKHOLDERS
to be held on , 2004
To the Stockholders of Genome Therapeutics Corp.:
A special meeting of stockholders of Genome Therapeutics Corp. will be held on , 2004 at 10:00 a.m., local time, at Ropes & Gray, One International Place, 36th floor, Boston, Massachusetts, for the following purposes:
| 1. | To consider and vote on a proposal to approve (i) the issuance of 28,571,405 shares of Genome common stock pursuant to the Agreement and Plan of Merger and Reorganization, dated as of November 17, 2003, among Genome Therapeutics Corp., Guardian Acquisition, Inc., a wholly-owned subsidiary of Genome Therapeutics Corp., GeneSoft Pharmaceuticals, Inc., and Luke Evnin, as the representative of the Genesoft stockholders and (ii) the issuance of shares of Genome common stock upon the potential conversion of the convertible notes of Genome, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger. A copy of the merger agreement is attached as Annex A to the accompanying joint proxy statement/prospectus. |
| 2. | To consider and vote on a proposal to approve the Amendment to the Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000. |
| 3. | To transact any other business as may properly come before the special meeting and any adjournment or postponement of the special meeting. |
Only stockholders of record of Genome Therapeutics Corp. as of the close of business on , 2004 are entitled to notice of, and will be entitled to vote at, the special meeting or any adjournment or postponement thereof. Assuming a quorum is present at the special meeting, approval of proposal 1 described above will require a majority of the votes of Genome Therapeutics Corp. common stock properly cast upon the proposal at the special meeting. Under the by-laws of Genome Therapeutics Corp., a quorum consists of a majority in interest of all stock issued and outstanding and entitled to vote at the meeting. Approval of proposal 2 described above will require the affirmative vote of a majority of the shares of Genome Therapeutics Corp. common stock outstanding as of the record date and entitled to vote on the proposal at the special meeting.
We invite you to attend the special meeting because it is important that your shares be represented at the meeting. Whether or not you plan to attend the special meeting, please sign, date and return the enclosed proxy card in the accompanying postage-paid envelope. Please note that, by delivering a proxy to vote at the special meeting, you are also granting a proxy to vote at any adjournments or postponements of the special meeting of Genome Therapeutics Corp. If you attend the meeting, you may vote in person, which will revoke a signed proxy if you have already sent one in. You may also revoke your proxy at any time before the meeting in the manner described in the accompanying joint proxy statement/prospectus.
BY THE ORDER OF THE BOARD OF DIRECTORS,
Steven M. Rauscher
Chairman, President and Chief Executive Officer
Waltham, Massachusetts
, 2004
Table of Contents
THE MERGER
The merger is structured as a reverse triangular merger followed by a subsequent merger into a wholly-owned subsidiary of Genome as illustrated below. Both of these steps, which are intended to qualify as a tax-free reorganization under Section 368(a) of the Internal Revenue Code, will occur as part of a single integrated plan.
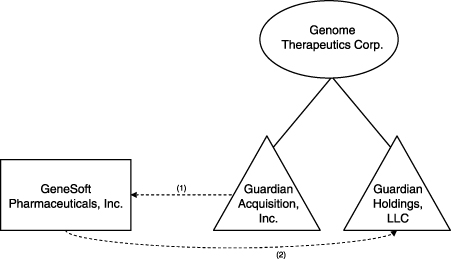
| (1) | Guardian Acquisition, Inc., a wholly-owned subsidiary of Genome Therapeutics Corp. merges with and into GeneSoft Pharmaceuticals, Inc., with GeneSoft Pharmaceuticals, Inc. surviving as a wholly-owned subsidiary of Genome Therapeutics Corp. |
| (2) | The surviving entity from step (1) merges with and into Guardian Holdings, LLC, a wholly-owned subsidiary of Genome Therapeutics Corp. |
Table of Contents
GENESOFT PHARMACEUTICALS, INC.
7300 Shoreline Court
South San Francisco, California 94080
650-837-1800
NOTICE OF SPECIAL MEETING OF STOCKHOLDERS
to be held on , 2004
To the Stockholders of GeneSoft Pharmaceuticals, Inc.:
A special meeting of stockholders of GeneSoft Pharmaceuticals, Inc. will be held on , 2004, at a.m., local time, at , for the following purposes:
| 1. | To consider and vote on a proposal to adopt and approve the Agreement and Plan of Merger and Reorganization, dated as of November 17, 2003, among Genome Therapeutics Corp., a Massachusetts corporation, Guardian Acquisition, Inc., a Delaware corporation and a wholly-owned subsidiary of Genome Therapeutics Corp., GeneSoft Pharmaceuticals, Inc., a Delaware corporation, and Luke Evnin, as the representative of the Genesoft stockholders. |
| 2. | To consider and vote on a proposal to amend and restate GeneSoft Pharmaceuticals, Inc.’s Seventh Amended and Restated Certificate of Incorporation to eliminate any authorized shares of GeneSoft Pharmaceuticals, Inc. preferred stock if the merger is completed. |
| 3. | To transact any other business as may properly come before the special meeting and any adjournment or postponement of the special meeting. |
Only stockholders of record of GeneSoft Pharmaceuticals, Inc. as of the close of business on , 2004 are entitled to notice of, and will be entitled to vote at, the special meeting or any adjournment or postponement thereof. Approval of the merger agreement and the transactions contemplated by the merger agreement requires the affirmative vote of the holders of a majority of the shares of GeneSoft Pharmaceuticals, Inc. common stock outstanding as of the record date. Approval of the amendment and restatement of the Seventh Amended and Restated Certificate of Incorporation of GeneSoft Pharmaceuticals, Inc. requires the affirmative vote of the holders of a majority of the shares of GeneSoft Pharmaceuticals, Inc. common stock outstanding as of the record date.
Your vote is important. To ensure that your shares are represented at the special meeting, you are urged to complete, date and sign the enclosed proxy card and mail it promptly in the postage-prepaid envelope provided, whether or not you plan to attend the special meeting in person. You may revoke your proxy in the manner described in the accompanying joint proxy statement/prospectus at any time before it has been voted at the special meeting of GeneSoft Pharmaceuticals, Inc. If you attend the special meeting, you may vote in person even if you returned a proxy.
BY ORDER OF THE BOARD OF DIRECTORS,
David B. Singer
Chairman and Chief Executive Officer
South San Francisco, California
, 2004
Please do not send your stock certificates at this time. If the merger is completed, you will be sent instructions regarding the surrender of your stock certificates.
Table of Contents
ADDITIONAL INFORMATION
This joint proxy statement/prospectus incorporates important business and financial information about Genome from other documents that are not included in or delivered with this document. We have listed the documents containing this information on page145. This information is available to you without charge upon your written or oral request. You can obtain those documents relating to Genome which are incorporated by reference in this joint proxy statement/prospectus by requesting them in writing or by telephone at the following address and telephone number:
GENOME THERAPEUTICS CORP.
100 Beaver Street
Waltham, Massachusetts 02453
(781) 398-2300
If you would like to request documents, you must do so by , 2004 in order to receive them before the special meeting of your company’s stockholders. You will not be charged for any of these documents that you request.
For additional information regarding where you can find information about Genome and Genesoft, please see the section entitled “Where You Can Find Additional Information” beginning on page145 of this joint proxy statement/prospectus. The information contained in this joint proxy statement/prospectus with respect to Genome was provided by Genome and the information contained in this joint proxy statement/prospectus with respect to Genesoft was provided by Genesoft.
SPECIAL NOTE REGARDING REFERENCES
References in this document towww.genomecorp.com, www.genesoft.com, any variations of the foregoing, or any other uniform resource locator, or URL, are inactive textual references only. The information on Genome’s web site, Genesoft’s web site or any other web site is not incorporated by reference into this document and should not be considered to be a part of this document.
NOTE ON TRADEMARKS
The following trademarks are the properties of the specified holders: FACTIVE® is the property of LG Life Sciences, Ltd., Nanobinder® is the property of Genesoft, Levaquin® is the property of Ortho-McNeil Pharmaceutical, Inc., Tequin® is the property of Bristol-Myers Squibb Company, Cipro® and Avelox® are both the property of Bayer Corporation, Biaxin® is the property of Abbott Laboratories, Zithromax® is the property of Pfizer Inc., Augmentin® is the property of GlaxoSmithKline, Ketek® is the property of Aventis Pharmaceuticals and Vanconin® is the property of Eli Lilly and Company. Unless otherwise indicated, trademarks or service marks appearing in this joint proxy statement/prospectus are the property of their respective holders.
Table of Contents
i
Table of Contents
| Page | ||
| 96 | ||
Regulatory Filings and Approvals Required to Complete the Merger | 96 | |
Restrictions on Sales of Genome Common Stock by Affiliates of Genesoft | 96 | |
Listing on the Nasdaq National Market of Genome Common Stock to be Issued in the Merger | 97 | |
| 97 | ||
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION | 100 | |
Unaudited Pro Forma Condensed Consolidated Statements of Operations | 101 | |
| 103 | ||
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION | 104 | |
| 108 | ||
| 108 | ||
| 114 | ||
| 114 | ||
GENESOFT MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 115 | |
| 115 | ||
| 118 | ||
| 120 | ||
| 122 | ||
| 126 | ||
| 127 | ||
| 129 | ||
| 130 | ||
| 130 | ||
| 130 | ||
| 130 | ||
| 130 | ||
| 131 | ||
COMPARISON OF RIGHTS OF HOLDERS OF GENOME COMMON STOCK AND HOLDERS OF GENESOFT CAPITAL STOCK | 132 | |
| 132 | ||
| 132 | ||
| 133 | ||
| 133 | ||
| 134 | ||
| 135 | ||
| 135 | ||
| 136 | ||
| 136 | ||
| 137 | ||
| 137 | ||
| 137 | ||
| 137 | ||
| 138 | ||
| 138 | ||
ii
Table of Contents
| Page | ||
| 138 | ||
| 138 | ||
| 139 | ||
| 139 | ||
| 140 | ||
| 140 | ||
| 141 | ||
| 141 | ||
| 142 | ||
| 143 | ||
| 143 | ||
| 143 | ||
| 143 | ||
| 144 | ||
| 144 | ||
| 144 | ||
| 145 | ||
| 147 | ||
ANNEX A—Agreement and Plan of Merger and Reorganization | ||||
ANNEX B—Escrow Agreement | ||||
ANNEX C—Opinion of Harris Nesbitt Corp. | ||||
ANNEX D—Opinion of Merrill Lynch, Pierce, Fenner & Smith Incorporated | ||||
ANNEX E—Section 262 of Delaware General Corporation Law | ||||
ANNEX F—Chapter 13 of the California Corporations Code |
iii
Table of Contents
QUESTIONS AND ANSWERS ABOUT THE MERGER
| Q: | Why are Genome and Genesoft proposing the merger? (See pages 61 and 69) |
| A: | Genome and Genesoft believe the merger will strengthen the outlook for both companies. Genome Therapeutics is a biopharmaceutical company focused on the discovery, development and commercialization of pharmaceutical products for specialty markets. Genesoft is a specialty pharmaceutical company focused on the discovery and development of novel anti-infective agents. |
Genome is proposing the merger because it will enable Genome to realize its goal of becoming a biopharmaceutical company with an FDA approved anti-infective product with the potential to significantly increase Genome’s revenue stream, permitting Genome to build a sales and marketing force that will benefit Ramoplanin and future product candidates of the combined company. Genesoft is proposing the merger because it will provide additional expertise and resources necessary to successfully launch FACTIVE in the U.S. and provide a broader portfolio of product candidates, including Ramoplanin.
Overall, both Genome and Genesoft believe the merger will provide added value to their respective stockholders. Achieving these anticipated benefits, however, is subject to risk and uncertainty, including the risks discussed in “Risk Factors” beginning on page 24.
| Q: | What will I, as a holder of Genesoft common stock or options or warrants to purchase Genesoft common stock, receive in the merger? (See page 81) |
| A: | If you are a holder of Genesoft common stock, you will receive a number of shares of Genome common stock equal to the common exchange ratio for each share of Genesoft common stock you own. The common exchange ratio is determined by: |
| • | deducting the shares of Genome common stock to be issued to the holders of Genesoft’s promissory notes as payment of accrued interest and related amounts from the total of 28,571,405 shares of Genome common stock issuable in the merger and |
| • | dividing that remaining number of Genome shares by the fully-diluted number of shares of Genesoft common stock outstanding on the closing date (assuming conversion or exercise of all Genesoft options and warrants). |
The exact exchange ratio between Genesoft and Genome common stock will depend on the closing date of the merger, which will determine how much interest has accrued on the Genesoft promissory notes, as well as the price at which the accrued interest and other related amounts due to the Genesoft promissory notes are converted into Genome common stock. The interest and other related amounts will be converted into Genome common stock at a price of $2.84 per share, unless the issuance price per share of Genome common stock expected to be issued in the capital raising transaction to raise a minimum of $32 million to finance the combined company, which is a condition to the merger agreement (unless waived by both parties), is less than $2.84, in which case that lesser per share price will become the conversion price.
Each holder of an option or warrant to purchase shares of Genesoft common stock that does not terminate by its terms prior to the merger will receive an option or warrant to purchase a number of shares of Genome common stock equal to the product of the number of Genesoft shares for which such option or warrant was exercisable multiplied by the common exchange ratio.
1
Table of Contents
The following chart shows the approximate common exchange ratio that would be used to determine the per share consideration to be received by Genesoft stockholders assuming different possible closing dates and prices of shares sold in the financing:
| Merger Closing Date | ||||||||
| February 28, 2004 | March 31, 2004 | April 30, 2004 | ||||||
| Closing Financing Price | Prices of $2.84 or higher | 1.188 | 1.186 | 1.184 | ||||
$2.66 | 1.171 | 1.170 | 1.168 | |||||
$2.48 | 1.153 | 1.151 | 1.149 | |||||
$2.30 | 1.131 | 1.129 | 1.127 | |||||
$2.12 | 1.106 | 1.103 | 1.101 | |||||
Each Genesoft stockholder will receive 80% of the merger consideration otherwise due to it upon closing of the merger. The remainder will be placed in escrow, pursuant to the escrow agreement attached to this joint proxy statement/prospectus as Annex B, to cover potential indemnity claims and any payments relating to those claims by Genome under the merger agreement. An additional 400,000 shares of Genome common stock issuable to the Genesoft stockholders in the merger will be placed in escrow to fund potential issuances of equity to a senior clinical development officer that may be hired by the combined company. The shares to be placed in escrow will be deducted from the shares of Genome common stock to be received by the Genesoft stockholders on a pro rata basis. If a holder of a Genesoft option assumed by Genome pursuant to the merger agreement exercises any portion of the holder’s option prior to the termination of the escrow fund, the holder will contribute a portion of the shares of Genome common stock issued upon exercise to the escrow fund in accordance with the terms of the escrow agreement. Subject to any claims made by Genome or its affiliates, officers, directors or employees, up to half of the indemnity escrow amount will be released from escrow one year after closing of the merger and the remainder will be released 18 months after closing. Adoption and approval of the merger agreement by Genesoft’s stockholders will constitute approval by such stockholders of the indemnification obligations set forth in the merger agreement, the terms of the escrow agreement and the authority of the stockholders’ representative named in the merger agreement.
| Q: | What will happen to Genesoft as a result of the merger? (See page 80) |
| A: | In the initial merger, Guardian Acquisition, Inc., a wholly-owned subsidiary of Genome, will be merged into Genesoft. Immediately thereafter, Genesoft will be merged into a second wholly-owned subsidiary of Genome in the second-step merger. As a result of the initial merger, the separate corporate existence of Guardian will cease, and Genesoft will continue as the surviving corporation of the initial merger and as a wholly-owned subsidiary of Genome. As a result of the second-step merger, the separate corporate existence of Genesoft will cease, and the second acquisition subsidiary, which is a Delaware limited liability company, will continue as the surviving company of the second-step merger and be wholly-owned by Genome. |
| Q: | Will Genesoft stockholders be able to trade the Genome common stock that they receive in the merger? (See page 96) |
| A: | Yes. The Genome common stock issued in the merger will be registered under the Securities Act and will be listed on the Nasdaq National Market under the symbol “GENE.” All shares of Genome common stock that you receive in the merger or upon exercise of Genesoft options or warrants assumed by Genome in the merger will be freely transferable unless you are deemed to be an affiliate of Genesoft at the time of the special meetings or your shares are subject to contractual transfer restrictions. Shares of Genome common stock received by persons deemed to be affiliates of Genesoft may only be sold in compliance with Rule 145 under the Securities Act or as otherwise permitted under the Securities Act. |
2
Table of Contents
| Q: | When do Genesoft and Genome expect to complete the merger? |
| A: | Genesoft and Genome expect to complete the merger when all of the conditions to completion of the merger contained in the merger agreement have been satisfied or waived. The stockholders of Genesoft must approve (i) the merger and the related transactions and (ii) the amendment and restatement of Genesoft’s Seventh Amended and Restated Certificate of Incorporation at their special stockholders meeting. The stockholders of Genome must vote for the proposals (i) to approve the issuance of a total of 28,571,405 shares of Genome common stock pursuant to the merger agreement and the issuance of shares of Genome common stock upon the potential conversion of the convertible notes of Genome, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger and (ii) to approve the Amendment to Genome’s Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000 shares of common stock. |
Genesoft and Genome are working toward satisfying these conditions and completing the merger as soon as practicable. Genesoft and Genome currently anticipate they will complete the merger in the first quarter of 2004 following the respective special meetings of Genesoft’s and Genome’s stockholders, assuming their stockholders approve the merger and related transactions, Genome is able to successfully raise a minimum of $32 million to fund the combined company, unless waived by both parties, and the other merger conditions are satisfied or waived. However, because the merger is subject to some conditions which are beyond Genesoft’s and Genome’s control, the exact timing cannot be predicted.
| Q: | What happens if the merger is not completed? (See page 93) |
| A: | If the merger is not completed, each of Genome and Genesoft will continue as independent companies. In addition, as a result of some termination events described in the merger agreement, Genome or Genesoft may be required to pay the other party a termination fee of $3,044,063.90 for certain termination events, and to reimburse the other for out-of-pocket expenses up to $1,000,000.00, including legal, accounting, investment banking, printing and other fees, related to this transaction if the merger is not completed. For a more complete discussion of requirements relating to payments of fees and expenses by each of Genome and Genesoft see the section entitled “The Merger and Related Transactions—The Merger Agreement—Termination Fees” in this joint proxy statement/prospectus. |
| Q: | What vote is required to approve the merger and the related transactions? (See pages 45 and 50) |
| A: | Approval of the merger agreement and the transactions contemplated by the merger agreement and approval of the amendment and restatement of the Seventh Amended and Restated Certificate of Incorporation of Genesoft each requires the affirmative vote of the holders of a majority of the shares of Genesoft common stock outstanding as of the record date. |
Approval of the issuance of Genome common stock to the Genesoft stockholders pursuant to the merger agreement and the issuance of Genome common stock upon the potential conversion of the Genome convertible notes to be exchanged for Genesoft promissory notes in connection with the merger will require a majority of the votes of Genome common stock properly cast upon this proposal at the special meeting. Approval of the increase in authorized Genome shares will require the affirmative vote of a majority of the shares of Genome common stock outstanding as of the record date and entitled to vote on the proposal at the special meeting.
The boards of directors of Genesoft and Genome have approved the merger agreement, the merger and the transactions contemplated by the merger agreement.
3
Table of Contents
| Q: | How do I vote on the merger? (See pages 47 and 51) |
| A: | First, please review the information contained or incorporated by reference in this joint proxy statement/prospectus, including the annexes, as it contains important information about Genome, Genesoft and the merger. It also contains important information about what each of the boards of directors of Genome and Genesoft, respectively, considered in evaluating the merger. Next, complete and sign the enclosed proxy card, and then mail it in the enclosed return envelope as soon as possible so that your shares can be voted at your company’s special meeting of stockholders at which, (a) in the case of Genesoft, the merger and the related transactions and the amendment and restatement of Genesoft’s Seventh Amended and Restated Certificate of Incorporation will be presented and voted upon or, (b) in the case of Genome, the increase in Genome authorized shares, the issuance of Genome common stock to the security holders of Genesoft in the merger and the issuance of Genome common stock upon the potential conversion of the Genome convertible notes to be exchanged for Genesoft promissory notes in connection with the merger. You may also attend the special meeting of your company in person and vote at the special meeting instead of submitting a proxy. |
| Q: | What happens if I don’t indicate how to vote my proxy? (See pages 47 and 51) |
| A: | If you sign and send in your proxy, but do not include instructions on how to vote your properly signed proxy card, your shares will be voted (a) FOR the adoption and approval of the merger and the related transactions and the amendment and restatement of Genesoft’s Seventh Amended and Restated Certificate of Incorporation, if you are a Genesoft stockholder, or (b) FOR the increase in Genome authorized shares and the issuance of Genome common stock to the security holders of Genesoft in the merger and the issuance of Genome common stock upon the potential conversion of the Genome convertible notes to be exchanged for Genesoft promissory notes in connection with the merger, if you are a Genome stockholder. |
| Q: | What happens if I don’t return a proxy card? (See pages 46 and 51) |
| A: | Not returning your proxy card will have the same effect as voting (a) against adoption and approval of the merger agreement and approval of the merger and the amendment and restatement of Genesoft’s Seventh Amended and Restated Certificate of Incorporation, if you are a Genesoft stockholder, and (b) against the increase in Genome authorized shares and the issuance of Genome common stock to the security holders of Genesoft in the merger, if you are Genome stockholder. |
| Q: | Can I change my vote after I have mailed my signed proxy card? (See pages 47 and 51) |
| A: | Yes. You can change your vote at any time before your proxy is voted at the special meeting of your company’s stockholders. You can do this in one of three ways: |
| • | first, you can send a written notice stating that you would like to revoke your proxy to the appropriate address below; |
| • | second, you can complete and submit a later-dated proxy card to the appropriate address below; or |
| • | third, you can attend the special meeting of Genome or Genesoft, as appropriate, and vote in person. Your attendance at the special meeting alone will not revoke your proxy. You must vote at the special meeting in order to revoke your previously submitted proxy. |
You should send any notice of revocation or your completed new proxy card, as the case may be, to:
For Genome Stockholders:
Genome Therapeutics Corp.
100 Beaver Street
Waltham, MA 02453
Attn: Stephen Cohen
Chief Financial Officer
4
Table of Contents
For Genesoft Stockholders:
GeneSoft Pharmaceuticals, Inc.
7300 Shoreline Court
South San Francisco, CA 94080
Attn: Asha Rajagopal
Director of Finance
| Q: | If my broker holds my Genome shares in “street name,” will my broker vote these shares for me? (See page 47) |
| A: | No. Your broker will not be able to vote your shares without instructions from you. If you do not provide your broker with voting instructions, your shares may be considered present at the special meeting for purposes of determining a quorum, but will not be considered to have been voted in favor of the increase in Genome authorized shares and the issuance of Genome common stock to the security holders of Genesoft in the merger and the issuance of Genome common stock upon the potential conversion of the Genome convertible notes to be exchanged for Genesoft promissory notes in connection with the merger. As a result, failure to provide your broker with voting instructions will have the effect of a vote against the increase in Genome authorized shares, the issuance of Genome common stock to the security holders of Genesoft and the issuance of Genome common stock upon the potential conversion of the Genome convertible notes to be exchanged for Genesoft promissory notes in connection with the merger. If you have instructed a broker to vote your shares and wish to change your vote, you must follow the directions received from your broker to change those instructions. |
| Q: | Should I send my Genesoft stock certificates now? (See page 82) |
| A: | No. If the merger is completed, Genome will send you written instructions for exchanging your Genesoft stock certificates for Genome stock certificates. Please do not send in your stock certificates with your proxy. If you send your stock certificates to Genome, Genome assumes no risk of loss. |
| Q: | What do I need to do now? |
| A: | After carefully reading and considering the information contained in this document, please complete and sign your proxy card and return it in the enclosed, postage-paid envelope as soon as possible so that your shares may be represented at your special meeting. |
| Q: | Am I entitled to appraisal rights in connection with the merger? (See page 52 and 54) |
| A: | Appraisal and dissenters’ rights are available to Genesoft stockholders under the respective laws of Delaware and California, as applicable, in connection with the merger. Appraisal or dissenters’ rights are not available to Genome stockholders. |
| Q: | Are there any risks I should consider in deciding whether to vote for the merger? |
| A: | Yes. In the section entitled “Risk Factors” beginning on page24 of this joint proxy statement/prospectus, Genome and Genesoft have described a number of risk factors that you should consider. |
| Q: | What are the tax consequences to a Genesoft stockholder of the merger? (See page 94) |
| A: | It is the opinion of Genesoft and Genome’s respective legal counsel that, based on facts and representations provided to such counsel and assumptions stated in the opinions, the merger will qualify as a reorganization within the meaning of Section 368(a) of the Internal Revenue Code. As a consequence of the merger |
5
Table of Contents
qualifying as a reorganization within the meaning of Section 368(a) of the Internal Revenue Code, we expect that the exchange of shares of Genesoft common stock by the stockholders of Genesoft for shares of Genome common stock will not cause a Genesoft stockholder to recognize gain or loss for United States federal income tax purposes. A Genesoft stockholder will recognize gain or loss with respect to cash received upon proper exercise of its appraisal rights pursuant to the merger. These opinions will not bind the Internal Revenue Service, which could take a different view. The tax consequences to a Genesoft stockholder will depend on the facts of such holder’s particular situation. Therefore, we urge each Genesoft stockholder to consult with its own tax advisor to determine the particular tax consequences of the merger to it. To review the tax consequences in greater detail, see the section entitled “The Merger and Related Transactions—Material United States Federal Income Tax Consequences of the Merger” beginning on page 94of this joint proxy statement/prospectus. |
| Q: | Where can I find more information about the companies and who can help answer my questions about the proposals? |
| A: | You can find more information about Genome and Genesoft from various sources described under “Where You Can Find Additional Information” on page 145. |
If you have any questions about the proposals presented in this joint proxy statement/prospectus, you should contact:
For Genome Stockholders:
Genome Therapeutics Corp.
100 Beaver Street
Waltham, MA 02453
Attn: Christopher Taylor
Senior Director of Investor Relations
(781) 398-2300
For Genesoft Stockholders:
GeneSoft Pharmaceuticals, Inc.
7300 Shoreline Court
South San Francisco, CA 94080
Attn: Asha Rajagopal
Director of Finance
(650) 837-1800
6
Table of Contents
The following is a summary of the information contained in this joint proxy statement/prospectus. This summary may not contain all of the information that is important to you. You should carefully read this entire joint proxy statement/prospectus and the other documents referred to for a more complete understanding of the merger and related transactions. In particular, you should read the annexes attached to this joint proxy statement/prospectus, including the merger agreement, which is attached to this joint proxy statement/prospectus as Annex A. We have included page references in parentheses to direct you to a more complete description of the topics presented in this summary. In addition, important business and financial information concerning Genome is incorporated by reference into this joint proxy statement/prospectus. You may obtain the information incorporated by reference into this joint proxy statement/prospectus without charge by following the instructions in the section entitled “Where You Can Find Additional Information” beginning on page145 of this joint proxy statement/prospectus.
The Companies
GENOME THERAPEUTICS CORP.
100 Beaver Street
Waltham, Massachusetts 02453
www.genomecorp.com
Genome Therapeutics is a biopharmaceutical company focused on the discovery, development and commercialization of pharmaceutical products.
Genome has nine established product development programs. Genome is managing the development and commercialization of its lead product candidate, Ramoplanin, in the United States and Canada. This product is in a Phase III clinical trial for the prevention of bloodstream infections caused by vancomycin-resistant enterococci (VRE) and a Phase II trial for the treatment of patients withClostridium difficile-associated diarrhea (CDAD). The company has seven product discovery and development alliances with pharmaceutical companies including Amgen, AstraZeneca, bioMérieux, Schering-Plough and Wyeth. Genome’s biopharmaceutical product candidates are all currently in discovery or development phases and are neither approved by the FDA nor available for commercial sale.
Over the past two years, Genome’s primary business focus has evolved from providing basic research and genomic services for pharmaceutical companies to more downstream efforts emphasizing clinical development and commercialization of its own product candidates. The company continues to reduce its expenditures in the early-stage product discovery research areas, including genomics research, and to focus its resources on later stage drug discovery and development. This evolution in the company’s strategic focus reflects its goals of getting products to market more rapidly and generating more substantial revenues and, ultimately, profits for the company’s stockholders.
The address for Genome’s executive offices is 100 Beaver Street, Waltham, Massachusetts 02453 and its telephone number is (781) 398-2300.
For more information on the business of Genome, please refer to Genome’s Annual Report on Form 10-K for the fiscal year ended December 31, 2002 and Form 10-Q for the quarterly period ended September 27, 2003. Please refer to the section of this joint proxy statement/prospectus entitled “Where You Can Find Additional Information” on page 145to find out where you can obtain copies of Genome’s Annual Report as well as the other documents Genome files with the Securities and Exchange Commission.
7
Table of Contents
GENESOFT PHARMACEUTICALS, INC.
7300 Shoreline Court
South San Francisco, CA 94080
www.genesoft.com
Genesoft is an emerging pharmaceutical company based in South San Francisco, California, that has a FDA-approved anti-infective product and a portfolio of product development programs. Genesoft’s lead product is FACTIVE (gemifloxacin mesylate), an orally administered, broad-spectrum fluoroquinolone antibiotic to which the company has an exclusive license to develop and market in North America, France, Germany, the United Kingdom, Luxembourg, Ireland, Italy, Spain, Portugal, Belgium, the Netherlands, Austria, Greece, Sweden, Denmark, Finland, Norway, Iceland, Switzerland, Andorra, Monaco, San Marino and Vatican City. FACTIVE was approved for sale in the United States on April 4, 2003 by the FDA. Following the completion of the proposed merger with Genome, the combined company intends to launch the product in the second half of 2004.
The address for Genesoft’s executive offices is 7300 Shoreline Court, South San Francisco, California 94080 and its telephone number is (650) 837-1800.
For more information on the business of Genesoft, please refer to the section of this joint proxy statement/prospectus entitled “Information About Genesoft” beginning on page108.
Voting Requirements for the Merger and Other Matters (See pages 45 and 50)
Assuming a quorum is present at the special meeting, the affirmative vote of the holders of a majority of the votes of Genome common stock properly cast at the special meeting will be required to approve the issuance of a total of 28,571,405 shares of Genome common stock to the Genesoft security holders pursuant to the merger agreement and the issuance of Genome common stock upon the potential conversion of the Genome convertible notes, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger. The affirmative vote of a majority of the Genome shares outstanding as of the record date and entitled to vote on the proposal at the special meeting will be required to approve the Amendment to the Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000 shares. Holders of Genome common stock will be entitled to cast one vote per share of Genome common stock owned as of , 2004, the record date for the Genome special meeting of stockholders at which the proposals will be presented and voted upon.
The affirmative vote of the holders of a majority of the outstanding shares of Genesoft common stock as of the record date will be required (i) to approve the merger agreement and the transactions contemplated by the merger agreement and (ii) to approve the amendment and restatement of the Seventh Amended and Restated Certificate of Incorporation of Genesoft. Holders of Genesoft common stock will be entitled to cast one vote per share of Genesoft common stock owned as of , 2004, the record date for the Genesoft special meeting of stockholders at which the proposals will be presented and voted upon.
Share Ownership of Genome Directors and Officers (See page 46)
As of the close of business on the record date for the special meeting of Genome stockholders at which the proposals described in this joint proxy statement/prospectus will be presented and voted upon, directors and officers of Genome (and their respective affiliates) collectively owned approximately % of the outstanding shares of Genome common stock entitled to vote at the special meeting.
8
Table of Contents
This does not include shares of Genome common stock issuable upon the exercise of presently exercisable options which these directors and officers hold. If all of these stock options were exercised prior to the record date for the special meeting, the directors and executive officers of Genome (and their respective affiliates) would collectively beneficially own approximately % of the outstanding shares of Genome common stock entitled to vote at the special meeting.
The directors and executive officers of Genome have entered into a voting agreement with Genesoft, pursuant to which each such person has agreed to vote the shares owned beneficially by such person in favor of the merger and the related transactions. See “Voting Agreements” below.
Share Ownership of Genesoft Directors and Officers (See page 51)
As of the close of business on the record date for the special meeting of Genesoft stockholders at which the merger agreement will be presented and voted upon, directors and officers of Genesoft (and their respective affiliates) collectively owned approximately % of the outstanding shares of Genesoft common stock entitled to vote at the special meeting on the merger agreement.
The directors and executive officers of Genesoft have entered into a voting agreement with Genome, pursuant to which each such person has agreed to vote the shares owned beneficially by such person in favor of the merger and the related transactions. See “Voting Agreements” below.
Interests of Directors and Executive Officers of Genome in the Merger (See page 78)
Genome’s stockholders should be aware that some Genome executive officers and directors may have interests in the merger that may be different from, or in addition to, their interests as stockholders of Genome in considering the recommendation of the Genome board of directors that Genome’s stockholders vote in favor of the proposals (i) to approve the issuance of a total of 28,571,405 shares of Genome common stock pursuant to the merger agreement and the issuance of Genome common stock upon the potential conversion of the Genome convertible notes, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger and (ii) to approve the Amendment to the Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000 shares.
Genome’s board of directors was aware of, and considered, these interests among other matters during its deliberations on the merits of the proposals specified above and in making its recommendation to Genome’s stockholders that they vote for each of the proposals specified above.
Interests of Directors and Executive Officers of Genesoft in the Merger (See page 79)
In considering the recommendation of Genesoft’s board of directors that Genesoft stockholders vote in favor of approval of the merger agreement, Genesoft stockholders should be aware that some Genesoft executive officers and directors may have interests in the merger that may be different from, or in addition to, their interests as stockholders of Genesoft.
Genesoft’s board of directors was aware of, and considered, these interests among other matters during its deliberations on the merits of the merger and related transactions and in making its recommendation to Genesoft’s stockholders that they vote for the merger and related transactions.
9
Table of Contents
Board Recommendations to Stockholders and Reasons for the Merger
Recommendation of Genome’s Board of Directors (See page48)
After careful consideration, Genome’s board of directors determined that the merger is advisable, is in the best interests of Genome’s stockholders, and is on terms that are fair to the stockholders of Genome.Accordingly, Genome’s board of directors approved the merger and the related transactions and recommends that its stockholders vote FOR the proposals (i) to approve the issuance of a total of 28,571,405 shares of Genome common stock pursuant to the merger agreement and the issuance ofGenome common stock upon the potential conversion of the Genome convertible notes, in an aggregateprincipal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger and (ii) to approve the Amendment to the Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000 shares. Since the merger is conditioned upon the approval of the matters set forth in each of these proposals, Genome recommends that its stockholders vote for each proposal.
Genome’s Reasons for the Merger (See page 69)
In reaching its decision to approve the merger agreement and the transactions contemplated by the merger agreement, Genome’s board of directors consulted with management of Genome, as well as its financial and legal advisors, and considered a number of potential benefits and factors pertaining to the merger, including the following:
| • | the merger will enable Genome to realize its goal of becoming a biopharmaceutical company with an FDA approved anti-infective product; |
| • | potential revenue stream from FACTIVE will allow Genome to build a sales and marketing infrastructure that will benefit Ramoplanin and future product candidates; |
| • | the merger will help position Genome as a key participant in the commercialization of anti-infective therapeutics; |
| • | the merger will increase the visibility of Genome and provide a stronger portfolio of products to serve as the basis for raising additional capital for the company; and |
| • | the merger will significantly enhance Genome’s management expertise, clinical development staff, intellectual property and technical resources. |
Genome’s board of directors also identified a number of potentially negative factors, including the following:
| • | the risk that the potential benefits of the merger might not be realized; |
| • | the risk that FACTIVE might not launch in the second half of 2004, causing a delay in the realization of potential revenue; |
| • | the risk that FACTIVE may not attain commercial acceptance and generate the level of revenues expected; |
| • | the challenges and risks involved in combining the businesses of two geographically distant companies; |
| • | the effect of the announcement on Genome’s share price; |
| • | the risk of diverting management’s focus and resources from other strategic opportunities and from operational matters while working to complete and implement the merger; |
| • | the risk that Genome will not be able to raise a minimum of $32 million of capital, which is a condition to the closing unless both parties agree to waive this condition; |
10
Table of Contents
| • | the risk that LG Life Sciences will not be able to supply FACTIVE in a timely manner, or maintain its manufacturing facilities in accordance with regulatory requirements; |
| • | the risk that the merger would not be completed; and |
| • | the other risks described under “Risk Factors” beginning on page 24. |
Recommendation of Genesoft’s Board of Directors (See page 55)
After careful consideration, Genesoft’s board of directors has determined that the merger is advisable, in the best interests of Genesoft stockholders and on terms that are fair to the stockholders of Genesoft.Accordingly, Genesoft’s board of directors has approved the merger and the related transactions and recommends that stockholders vote FOR the proposals to (i) adopt and approve the merger agreement and (ii) to amend and restate Genesoft’s Seventh Amended and Restated Certificate of Incorporation to eliminate all authorized shares of Genesoft preferred stock if the merger is completed.
Genesoft’s Reasons for the Merger (See page 61)
In reaching its decision to approve the merger agreement and the transactions contemplated by the merger agreement, Genesoft’s board of directors consulted with management of Genesoft, as well as its financial and legal advisors, and considered a number of potential benefits and factors pertaining to the merger, including the following:
| • | the strategic fit of Genesoft’s and Genome’s respective core anti-infective product portfolios and the increased number of potential products of the combined company; |
| • | the proposed merger would substantially increase Genesoft’s immediate capital resources and the combined company is expected to have greater leverage in obtaining financing for its operations; |
| • | the difficulty in (and the related cost of) obtaining additional financing for Genesoft as a stand-alone enterprise and Genesoft’s management’s assessment of possible strategic alternatives; and |
| • | the amount and nature of the merger consideration to be received by the Genesoft stockholders. |
Genesoft’s board of directors also identified a number of potential negative factors pertaining to the merger, including the following:
| • | the risk that the transaction might not be completed in a timely matter or at all and, if not completed, the difficulty as a stand-alone company in being able to raise sufficient funds to meet its obligations; |
| • | the risk that the potential benefits of the merger may not be realized; |
| • | the risk of management and employee disruption associated with the merger, including the risk that key personnel may decide not to continue employment with Genome after the merger; |
| • | terms of the merger agreement and related agreements that limit the ability of Genesoft and its representatives to pursue alternative transactions; and |
| • | the other risks described above under “Risk Factors”. |
11
Table of Contents
Opinion of Genome’s Financial Advisor (See page 71)
In deciding to approve the merger, the Genome board of directors received an opinion from its financial advisor, Harris Nesbitt Corp. that, based upon and subject to the factors and assumptions set forth in the opinion, as of November 10, 2003, the aggregate stock consideration to be issued by Genome to Genesoft’s security holders in the merger and the related transactions was fair, from a financial point of view, to Genome.
The full text of the Harris Nesbitt Corp. written opinion which sets forth the assumptions made, matters considered and limitations on the review undertaken is attached to this joint proxy statement/prospectus as Annex C. We encourage you to read the opinion carefully in its entirety as well as the section of this joint proxy statement/prospectus entitled “The Merger and Related Transactions—Opinion of Genome’s Financial Advisor.” The opinion of Harris Nesbitt Corp. does not constitute a recommendation as to how any holder of Genome or Genesoft common stock should vote on the merger.
Opinion of Genesoft’s Financial Advisor (See page 63)
In deciding to approve the merger, the Genesoft board of directors considered the opinion of Merrill Lynch, Pierce, Fenner & Smith Incorporated, its financial advisor in connection with the merger, that, as of November 12, 2003 and subject to the factors and assumptions set forth in the opinion, the common exchange ratio was fair, from a financial point of view, to the holders of Genesoft shares, other than Genome and its affiliates. The Merrill Lynch opinion does not address any other aspect of the merger, including the merits of the underlying decision by Genesoft to engage in the merger, and does not constitute a recommendation to any stockholder as to how such stockholder should vote on the proposed merger or any matter related thereto.
The full text of the Merrill Lynch written opinion, which sets forth the assumptions made, matters considered, and qualifications and limitations on the review undertaken by Merrill Lynch, is included in this joint proxy statement/prospectus as Annex D. We encourage you to read the opinion, as well as the section of this joint proxy statement/prospectus entitled “The Merger and Related Transactions—Opinion of Genesoft’s Financial Advisor,” carefully and in its entirety.
Voting Agreements (See page 90)
The directors and executive officers of Genome, who collectively have voting control over approximately 0.5% of the outstanding shares of Genome, have entered into voting agreements with Genesoft, pursuant to which each such person has agreed to vote the shares of Genome common stock beneficially owned by such person in favor of the increase in Genome’s authorized shares and the issuance of Genome common stock to the Genesoft stockholders pursuant to the merger agreement, in favor of any other matter relating to consummation of the transactions contemplated by the merger agreement and against any other merger or similar transaction involving Genome.
Certain directors, executive officers and stockholders of Genesoft, who collectively have voting control over approximately 63% of the outstanding shares of Genesoft, have entered into similar voting agreements with Genome to vote the shares of Genesoft common stock beneficially owned by such person in favor of the merger agreement, in favor of any other matter relating to consummation of the transactions contemplated by the merger agreement and against any other merger or similar transaction involving Genesoft.
12
Table of Contents
Structure and Effects of the Merger (See page 80)
In the initial merger, Guardian Acquisition, a wholly-owned subsidiary of Genome, will be merged into Genesoft. Immediately thereafter, Genesoft will be merged into a second wholly-owned subsidiary of Genome in the second-step merger. As a result of the initial merger, the separate corporate existence of Guardian Acquisition will cease, and Genesoft will continue as the surviving corporation of the initial merger and as a wholly-owned subsidiary of Genome. As a result of the second-step merger, the separate corporate existence of Genesoft will cease, and the second acquisition subsidiary, which is a Delaware limited liability company, will continue as the surviving company of the second-step merger and be wholly-owned by Genome.
At the effective time of the merger, each share of Genesoft common stock will be cancelled and terminated and will be automatically converted into the right to receive the number of shares of Genome common stock equal to the common exchange ratio as described in “The Merger—The Merger Agreement—Conversion of Genesoft Stock.”
Indemnification (See page 90)
If the merger agreement is approved and the merger occurs, all holders of Genesoft capital stock who have not perfected dissenters’ rights under Delaware or California law will be deemed to have agreed, subject to the limitations described below, to indemnify Genome and its affiliates, officers, directors and employees against losses due to:
| • | any inaccuracy or breach of any representation or warranty of Genesoft contained in the merger agreement or any certificate required to be delivered in connection with the merger agreement; |
| • | any breach of, non-compliance with or non-fulfillment of any covenant or agreement made by Genesoft in the merger agreement or any certificate required to be delivered in connection with the merger agreement; |
| • | any fraudulent action, and any violation of any criminal law by Genesoft; or |
| • | any claim by a holder or former holder of Genesoft’s equity interests or any other person seeking to assert, or based upon: (i) ownership or rights of ownership to any shares of capital stock of Genesoft; (ii) any rights of a stockholder of Genesoft, including any option, preemptive rights, rights to notice or to vote or any appraisal rights under the applicable provisions of the DGCL; (iii) any rights under the organizational documents of Genesoft; (iv) any claim that his, her or its equity interests were wrongfully repurchased, canceled, terminated or otherwise limited by Genesoft; or (v) any claim in connection with the issuance of any equity interests or otherwise, regardless of whether an action, suit or proceeding can or has been made against Genesoft. |
Genome’s right to indemnification is limited to the merger consideration placed in escrow pursuant to the escrow agreement attached to this joint proxy statement/prospectus as Annex B (and described on page97), representing 20% of the Genome common stock that would otherwise be due to Genesoft stockholders in the merger. Genome is entitled to indemnification after all losses exceed $676,458.64 in the aggregate, and then for losses in excess of such amount. Luke Evnin will serve as Genesoft stockholders’ representative under the escrow agreement. Subject to any claims made by Genome or its affiliates, officers, directors or employees and any payments related to those claims, up to half of the escrow amount will be released from escrow one year after closing of the merger and the remainder will be released 18 months after closing.
Genome has agreed to indemnify Genesoft’s stockholders against losses due to:
| • | any inaccuracy or breach of any representation or warranty of Genome contained in the merger agreement or any certificate required to be delivered in connection with the merger agreement; |
13
Table of Contents
| • | any breach of, non-compliance with or non-fulfillment of any covenant or agreement made by Genome in the merger agreement or any certificate required to be delivered in connection with the merger agreement; or |
| • | any fraudulent action and any violation of any criminal law by Genome. |
The aggregate indemnification obligations of Genome will not exceed $13,529,172.87 and indemnification is available only when Genesoft losses exceed $676,458.64 in the aggregate, and then only for losses in excess of such amount.
Completion and Effectiveness of the Merger
��
The closing of the merger will take place as promptly as practicable following satisfaction or waiver of the closing conditions set forth in the merger agreement, including, without limitation, the condition that Genome raise a minimum of $32 million to finance the combined company (unless waived by both parties). Although Genome and Genesoft are working toward satisfying these conditions and completing the merger in the first quarter of 2004, the exact timing cannot be predicted as the merger is subject to specified conditions, some of which are beyond Genome’s and Genesoft’s control.
Assuming that the other conditions to completion of the merger have been satisfied or waived, the merger will become effective at the time specified in the certificates of merger filed with the Secretary of the State of Delaware with respect to the merger.
Management After the Merger (See page 130)
Upon consummation of the merger, the combined company will be led by a board of directors consisting of: Luke Evnin, Ph.D., Managing Director of MPM Asset Management, Robert J. Hennessey, former Chairman and CEO of Genome, Vernon R. Loucks, Jr., former Chairman and CEO of Baxter International, Steven Rauscher, President and CEO of the combined company, William S. Reardon, former partner at PricewaterhouseCoopers, Norbert G. Riedel, Ph.D., Corporate Vice President and Chief Scientific Officer at Baxter International, William Rutter, Ph.D., Professor Emeritus of Biochemistry at the University of California, San Francisco and Founder of Chiron, David B. Singer, Chairman of the Board of the combined company and David K. Stone, Managing Director of Flagship Ventures.
The management of the combined company will consist of the following: Steven Rauscher as Chief Executive Officer and President, Stephen Cohen as Senior Vice President and Chief Financial Officer and Martin Williams as Senior Vice President of Corporate Development and Marketing.
Genesoft and Genome are Prohibited from Soliciting Other Offers (See page 86)
Each of Genesoft and Genome has agreed that, while the merger is pending, it will not solicit, initiate or encourage, or, except with respect to an unsolicited superior offer as described in “The Merger—The Merger Agreement,” engage in discussions with any third parties regarding certain types of extraordinary transactions, such as a merger, consolidation or other similar transaction involving it.
Conditions to Completion of the Merger (See page 88 )
Genome’s and Genesoft’s obligations to complete the merger are subject, unless waived by Genome, Genesoft or both parties, as applicable, to specified conditions described under “The Merger and Related Transactions—The Merger Agreement—Conditions to Completion of the Merger.”
14
Table of Contents
Termination of the Merger Agreement and Payment of Termination Fee (See pages 92 and 93)
Genome and Genesoft may terminate the merger agreement by mutual agreement and under other circumstances specified in the merger agreement. Genome and Genesoft have agreed that if the merger agreement is terminated under certain circumstances described under “The Merger and Related Transactions—The Merger Agreement—Termination Fees,” Genome or Genesoft will pay the other party’s transaction expenses of up to $1,000,000 and an additional $3,044,063.90 if Genome or Genesoft, as the case may be, enters into (or announces its intention to enter into) an agreement to consummate a competing merger or acquisition transaction within 12 months of terminating the merger agreement.
Material United States Federal Income Tax Consequences of the Merger (See page 94)
Genome and Genesoft have structured the merger to qualify as a reorganization under the Internal Revenue Code. It is the intention of Genome and Genesoft that no gain or loss will generally be recognized by Genesoft stockholders for federal income tax purposes on the exchange of shares of Genesoft common stock solely for shares of Genome common stock.
Tax matters are very complicated, and the tax consequences of the merger to the Genesoft stockholders will depend on the facts of each Genesoft stockholder’s own situation. Each Genesoft stockholder should consult his, her or its tax advisor for a full understanding of the tax consequences of the merger.
Accounting Treatment of the Merger (See page 96)
The merger will be accounted for as a purchase by Genome under accounting principles generally accepted in the United States.
Regulatory Approvals Required to Complete the Merger (See page 96)
Neither Genome nor Genesoft is aware of any material governmental or regulatory approval required for completion of the merger, other than compliance with applicable corporate laws of the Commonwealth of Massachusetts and the State of Delaware and federal and state securities laws.
Appraisal Rights (See pages 52 and 54)
Appraisal rights are available to Genesoft stockholders under the respective laws of Delaware and California in connection with the merger. Appraisal rights are not available to Genome stockholders.
Restrictions on the Ability to Sell Genome Common Stock Received in the Merger (See page 96)
All shares of Genome common stock that Genesoft stockholders receive in the merger will be freely transferable unless a Genesoft stockholder is deemed to be an affiliate of Genesoft at the time of the special meetings or such shares are subject to contractual restrictions. Shares of Genome common stock received by persons deemed to be affiliates of Genesoft may only be sold in compliance with Rule 145 under the Securities Act or as otherwise permitted under the Securities Act.
Listing of Genome Common Stock (See page 143)
The shares of Genome common stock are listed on the Nasdaq National Market under the symbol “GENE.”
15
Table of Contents
GENOME SUMMARY SELECTED CONSOLIDATED FINANCIAL DATA
The following summary selected consolidated statement of operations data for each of the three fiscal years ended December 31, 2000, 2001 and 2002 and the summary selected consolidated balance sheet data as of December 31, 2001 and 2002 set forth below, are derived from the historical audited consolidated financial statements included in Genome’s Annual Report on Form 10-K for the year ended December 31, 2002. The financial statements for the year ended December 31, 2002 have been audited by Ernst & Young LLP, independent auditors. The financial statements for the two years ended December 31, 2001 have been audited by other independent auditors. The data should be read in conjunction with the consolidated financial statements, related notes, and other financial information incorporated by reference herein. The summary selected consolidated statement of operations data for the fiscal years ended December 31, 1998 and 1999, and the summary selected consolidated balance sheet data as of December 31, 1998, 1999 and 2000 are derived from Genome’s historical audited consolidated financial statements.
Genome derived the summary selected consolidated balance sheet and statement of operations data as of and for the nine months ended September 28, 2002 and September 27, 2003, respectively, from its unaudited condensed consolidated financial statements. These statements include, in the opinion of management, all normal and recurring adjustments that are necessary for a fair statement of results in accordance with generally accepted accounting principles. The operating results for the nine months ended September 27, 2003 are not necessarily indicative of the results that may be expected for the year ending December 31, 2003. It is important that you read the following summary historical data along with the historical consolidated financial statements and related notes in Genome’s Annual Report on Form 10-K for the year ended December 31, 2002 and Genome’s quarterly reports on Form 10-Q filed with the Securities and Exchange Commission, which are incorporated by reference into this joint proxy statement/prospectus, and other Genome documents to which we refer. See “Where You Can Find Additional Information” on page145.
| Year Ended December 31, | Nine Months Ended | |||||||||||||||||||||||||||
| 1998 | 1999 | 2000 | 2001 | 2002 | Sept. 28, 2002 | Sept. 27, 2003 | ||||||||||||||||||||||
| (in thousands, except per share amounts) | (Unaudited) | |||||||||||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||||||||||
Revenues: | ||||||||||||||||||||||||||||
Biopharmaceutical | $ | 18,135 | $ | 18,162 | $ | 11,851 | $ | 18,438 | $ | 7,716 | $ | 6,206 | $ | 5,519 | ||||||||||||||
Genomics services | 3,913 | 6,666 | 13,594 | 17,302 | 15,271 | 10,943 | 1,799 | |||||||||||||||||||||
Total revenues | 22,048 | 24,828 | 25,445 | 35,740 | 22,987 | 17,149 | 7,318 | |||||||||||||||||||||
Net loss | $ | (12,968 | ) | $ | (3,940 | ) | $ | (5,847 | ) | $ | (10,090 | ) | $ | (34,017 | ) | $ | (25,175 | ) | $ | (28,455 | ) | |||||||
Net loss per common share, basic and diluted | $ | (0.71 | ) | $ | (0.21 | ) | $ | (0.27 | ) | $ | (0.45 | ) | $ | (1.48 | ) | $ | (1.10 | ) | $ | (1.16 | ) | |||||||
Weighted average common shares outstanding, basic and diluted | 18,290 | 18,627 | 21,377 | 22,572 | 22,921 | 22,881 | 24,581 | |||||||||||||||||||||
| December 31, | Sept. 28, 2002 | Sept. 27, 2003 | |||||||||||||||||||
| 1998 | 1999 | 2000 | 2001 | 2002 | |||||||||||||||||
| (in thousands) | (Unaudited) | ||||||||||||||||||||
Balance Sheet Data: | |||||||||||||||||||||
Cash and cash equivalents, restricted cash, warrant and long and short-term marketable securities | $ | 30,819 | $ | 26,778 | $ | 73,010 | $ | 67,341 | $ | 50,866 | $ | 58,381 | $ | 25,786 | |||||||
Working capital | 19,750 | 19,447 | 51,601 | 44,156 | 36,511 | 43,607 | 15,174 | ||||||||||||||
Total assets | 48,921 | 45,443 | 90,251 | 82,740 | 65,845 | 72,936 | 30,739 | ||||||||||||||
Total liabilities | 21,364 | 16,596 | 17,564 | 16,008 | 30,428 | 28,863 | 11,393 | ||||||||||||||
Stockholders’ equity | 27,557 | 28,847 | 72,687 | 66,732 | 35,417 | 44,073 | 19,346 | ||||||||||||||
16
Table of Contents
GENESOFT SUMMARY SELECTED FINANCIAL DATA
The following summary financial data should be read in conjunction with the “Genesoft Management’s Discussion and Analysis of Financial Condition and Results of Operations” section included later in this joint proxy statement/prospectus, and Genesoft’s financial statements and related notes included in the back of this prospectus. Genesoft has derived the statements of operations data for the years ended December 31, 2000, 2001 and 2002 from its audited financial statements which are included in this joint proxy statement/prospectus. Genesoft has derived the statements of operations and balance sheet data as of and for the nine months ended September 30, 2002 and 2003 from its unaudited financial statements which are also included in this joint proxy statement/prospectus. These unaudited statements include, in the opinion of management, all normal and recurring adjustments that are necessary for a fair statement of results in accordance with generally accepted accounting principles.
| Year Ended December 31, | Nine Months Ended | |||||||||||||||||||||||||||
| 1998 | 1999 | 2000 | 2001 | 2002 | Sept. 30, 2002 | Sept. 30 2003 | ||||||||||||||||||||||
| (in thousands, except per share amounts) | (Unaudited) | |||||||||||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||||||||||
Total revenues | $ | — | $ | 2,520 | $ | 4,187 | $ | 2,059 | $ | 5,402 | $ | — | $ | 3,072 | ||||||||||||||
Net loss | (770 | ) | (2,987 | ) | (7,921 | ) | (18,321 | ) | (25,569 | ) | (18,368 | ) | (19,796 | ) | ||||||||||||||
Basic and diluted net loss per common share | $ | (1.03 | ) | $ | (2.92 | ) | $ | (8.27 | ) | $ | (15.69 | ) | $ | (12.81 | ) | $ | (14.04 | ) | $ | (1.69 | ) | |||||||
Weighted average shares used in computing basic and diluted net loss per common share | 745 | 1,024 | 957 | 1,168 | 1,996 | 1,308 | 11,729 | |||||||||||||||||||||
| December 31, | Sept. 30, 2002 | Sept. 30 2003 | |||||||||||||||||||||
| 1998 | 1999 | 2000 | 2001 | 2002 | |||||||||||||||||||
| (in thousands) | (Unaudited) | ||||||||||||||||||||||
Balance Sheet Data: | |||||||||||||||||||||||
Cash and cash equivalents, short-term investments and restricted cash | $ | 3,195 | $ | 12,405 | $ | 29,379 | $ | 24,714 | $ | 5,951 | $ | 7,225 | $ | 7,826 | |||||||||
Working capital (net capital deficiency) | 2,703 | 12,056 | 22,644 | 18,208 | (3,076 | ) | 715 | (20,993 | ) | ||||||||||||||
Total assets | 3,406 | 14,037 | 35,918 | 40,162 | 19,432 | 20,539 | 25,799 | ||||||||||||||||
Total liabilities | 548 | 1,200 | 6,202 | 7,498 | 11,983 | 6,270 | 32,924 | ||||||||||||||||
Stockholders’ equity (net capital deficiency) | 2,858 | 12,837 | 29,716 | 32,664 | 7,448 | 14,269 | (7,125 | ) | |||||||||||||||
17
Table of Contents
UNAUDITED PRO FORMA FINANCIAL INFORMATION
The following selected unaudited pro forma condensed combined financial information has been derived from and should be read in conjunction with the Unaudited Pro Forma Condensed Combined Financial Information and related notes included in this joint proxy statement/prospectus on page100. This information is based on the historical balance sheets and related historical statements of operations of Genome and the historical balance sheets and related historical statements of operations of Genesoft, using the purchase method of accounting for business combinations under accounting principles generally accepted in the United States. This information is for illustrative purposes only. The companies may have performed differently had they always been combined. You should not rely on the selected unaudited pro forma condensed combined financial information as being indicative of the historical results that would have been achieved had the companies always been combined or the future results that the combined company will experience after the merger. For the interim period, Genome’s nine months ended September 27, 2003 was combined with Genesoft’s nine months ended September 30, 2003.
Pro Forma Year Ended December 31, 2002 | Pro Forma Nine Months Ended September 27, 2003 | |||||||
Statement of Operations Data: | ||||||||
Total revenues | $ | 28,389 | $ | 10,390 | ||||
Net loss | (67,352 | ) | (54,076 | ) | ||||
Net loss per share, basic and diluted | (1.39 | ) | (1.08 | ) | ||||
Shares used in computing net loss per share, basic and diluted | 48,401 | 50,061 | ||||||
Balance Sheet Data: | ||||||||
Cash and cash equivalents, restricted cash and long and short-term marketable securities | $ | 23,915 | ||||||
Working capital (net capital deficiency) | (17,820 | ) | ||||||
Total assets | 139,944 | |||||||
Total liabilities | 46,620 | |||||||
Stockholders’ equity | 93,324 | |||||||
18
Table of Contents
COMPARATIVE HISTORICAL AND PRO FORMA PER SHARE DATA
The following table sets forth certain historical per share data of Genome and Genesoft and combined per share data on an unaudited pro forma basis after giving effect to the merger using the purchase method of accounting. For purposes of the table below, Genome has estimated that 25,479,517 shares of Genome common stock will be issued to existing Genesoft common stockholders and promissory note holders at the closing of the merger and the remainder of the 28,571,405 shares of Genome common stock to be issued in the merger will be reserved for issuance upon the exercise of Genesoft options and warrants assumed in the merger.
The following data should be read in connection with the separate historical financial statements of Genesoft included in this joint proxy statement/prospectus and the separate historical financial statements of Genome incorporated by reference in this joint proxy statement/prospectus, as well as the unaudited pro forma condensed combined financial information included in this joint proxy statement/prospectus. The unaudited pro forma combined per share data do not necessarily indicate the operating results that would have been achieved had the merger been completed as of the beginning of the earliest period presented and should not be taken as representative of future operations. The results may have been different if the companies had always been consolidated. For the interim period, Genome’s nine months ended September 27, 2003 was combined with Genesoft’s nine months ended September 30, 2003.
| Year Ended December 31, 2002 | Nine Months Ended September 27, 2003 | |||||||
Genome—Historical: | ||||||||
Basic and diluted net loss per share | $ | (1.48 | ) | $ | (1.16 | ) | ||
Book value per common share | 1.54 | .74 | ||||||
Genesoft—Historical: | ||||||||
Basic and diluted net loss per share | (12.81 | ) | (1.69 | ) | ||||
Book value per common share | 0.69 | (0.58 | ) | |||||
Pro Forma—Combined: | ||||||||
Basic and diluted net loss per share | (1.39 | ) | (1.08 | ) | ||||
Book value per common share | — | 1.81 | ||||||
Equivalent Pro Forma—Combined(1): | ||||||||
Basic and diluted net loss per share | (1.65 | ) | (1.28 | ) | ||||
Book value per common share | — | 2.15 | ||||||
| (1) | The equivalent pro forma—combined represents the pro forma—combined amount multiplied by the estimated exchange ratio of 1.188. See “Unaudited Pro Forma Combined Financial Statements of Genome and Genesoft.” |
No options or warrants outstanding are included in the calculation of diluted loss per share because their impact would have been anti-dilutive to loss per share for each period presented. Neither Genome nor Genesoft has paid a cash dividend to its stockholders.
19
Table of Contents
MARKET PRICE DATA AND DIVIDEND INFORMATION
| GENE* | ||||
Calendar Quarters | High | Low | ||
2001: | ||||
First Quarter | 11.120 | 5.500 | ||
Second Quarter | 16.850 | 4.940 | ||
Third Quarter | 13.850 | 4.690 | ||
Fourth Quarter | 8.270 | 5.520 | ||
2002: | ||||
First Quarter | 7.050 | 5.000 | ||
Second Quarter | 5.640 | 2.200 | ||
Third Quarter | 2.100 | 1.340 | ||
Fourth Quarter | 2.330 | 1.030 | ||
2003: | ||||
First Quarter | 2.070 | 1.280 | ||
Second Quarter | 3.750 | 1.450 | ||
Third Quarter | 3.430 | 2.410 | ||
Fourth Quarter (through December 12, 2003) | 3.320 | 2.630 | ||
| * | Based on closing price. |
Recent Closing Prices
The following table shows the closing prices per share of Genome common stock as reported on the Nasdaq National Market on (1) November 17, 2003, the business day preceding the public announcement that Genome and Genesoft had entered into the merger agreement, and (2) , 2004, the last full trading day for which closing prices were available at the time of the printing of this joint proxy statement/prospectus.
The following table also includes the equivalent price per share of Genesoft common stock on those dates. This equivalent per share price reflects the value of the Genome common stock Genesoft stockholders would receive for each share of Genesoft common stock if the merger was completed on either of these dates. These amounts are estimates based on the number of outstanding shares of Genesoft common stock on the date of this joint proxy statement/prospectus, and may change at the completion of the merger as a result of those factors described in the section of this joint proxy statement/ prospectus entitled “The Merger and Related Transactions—The Merger Agreement—Conversion of Genesoft Stock.”
| Genome Common Stock | Genesoft Equivalent Price Per Share | ||||||
November 17, 2003 | $ | 2.85 | $ | 3.39 | (1) | ||
, 2004 | $ | $ | (1 | ) | |||
| (1) | Assumes that the merger closes on February 28, 2004 and the price at which shares are sold in the transaction to fund the combined company is at least $2.84 per share. The actual number of shares of Genome common stock issued to the holders of Genesoft’s promissory notes will vary based on a variety of factors described in “The Merger and Related Transactions—The Merger Agreement—Conversion of Genesoft Stock.” |
Because the market price of Genome common stock may increase or decrease before the completion of the merger, you are urged to obtain current market quotations.
20
Table of Contents
As of the date of this joint proxy statement/prospectus, there were approximately stockholders of record of Genome who held an aggregate of shares of Genome common stock.
As of the date of this joint proxy statement/prospectus, there were approximately stockholders of record of Genesoft who held an aggregate of shares of Genesoft common stock.
Dividend Policy
Except as set forth below, neither Genome nor Genesoft has ever declared or paid dividends on its common stock in the past, and neither company intends to pay such dividends in the foreseeable future. Any determination to pay dividends after consummation of the merger will be at the discretion of the combined company’s board of directors and will depend on the combined company’s financial condition, results of operations, capital requirements and other factors the combined company’s board of directors deems relevant.
21
Table of Contents
REGARDING FORWARD-LOOKING STATEMENTS IN THIS
JOINT PROXY STATEMENT/PROSPECTUS
This joint proxy statement/prospectus and the documents incorporated by reference into this joint proxy statement/prospectus contain forward-looking statements about the merger, Genome and Genesoft within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements represent the judgment of the managements of Genome and Genesoft, as the case may be, regarding future events. Forward-looking statements typically are identified by use of terms such as “may,” “will,” “should,” “plan,” “expect,” “intend,” “anticipate,” “estimate,” and similar words, although some forward-looking statements are expressed differently. Genome, Genesoft or the combined company do not plan to update these forward-looking statements. You should be aware that actual results could differ materially from those contained in the forward-looking statements due to a number of risks affecting the business of Genome, Genesoft or the combined company.
Although each company believes that its plans, intentions and expectations as reflected in or suggested by these forward-looking statements are reasonable, it can give no assurance that these plans, intentions or expectations will be achieved. Genome stockholders and Genesoft stockholders are cautioned that all forward-looking statements involve risks and uncertainties and actual results may differ materially from those discussed as a result of various risk factors described in the section entitled “Risk Factors”. Listed below and discussed elsewhere in this joint proxy statement/prospectus are some important risks, uncertainties and contingencies which could cause each company’s actual results, performances or achievements to be materially different from the forward-looking statements made in this joint proxy statement/prospectus, particularly if the merger is not completed. These risks, uncertainties and contingencies include, but are not limited to, the following:
| • | the ability of the combined company to launch and successfully commercialize FACTIVE in the U.S.; |
| • | the ability of the combined company to obtain regulatory approval to commercialize FACTIVE in Europe and other foreign markets; |
| • | the ability of the combined company to successfully complete required clinical trials of Ramoplanin and to obtain regulatory approval to commercialize Ramoplanin; |
| • | the satisfaction or waiver of the conditions to closing of the merger, including receipt of stockholder approvals; |
| • | the ability of the combined company to raise sufficient capital to fund its programs following the merger; |
| • | the expected closing date of the merger; |
| • | the risk that the merger will not close; |
| • | the risk that the merger will not qualify as a reorganization under the provisions of Section 368(a) of the Internal Revenue Code for United States federal income tax purposes; |
| • | the risk that Genome will not integrate the acquired business successfully; |
| • | the risk that Genome will incur unanticipated costs to integrate and restructure the acquired business; |
| • | fluctuations in the trading price and volume of Genome common stock; |
| • | competitive factors, such as price competition from other products and competition from other comparable companies; and |
| • | the cost of complying with current and future governmental regulations. |
22
Table of Contents
In addition, events may occur in the future that Genome or Genesoft are not able to accurately predict or control and that may cause actual results to differ materially from the expectations described in these forward-looking statements.
In evaluating the merger and the related transactions, you should carefully consider the discussion of risks and uncertainties discussed in the section entitled “Risk Factors” and the risk factors set forth in Exhibit 99.1 to Genome’s Quarterly Report on Form 10-Q for the quarter ended September 27, 2003 and those set forth in other filings that the company may make with the Securities and Exchange Commission from time to time.
23
Table of Contents
In addition to the other information contained or incorporated by reference in this joint proxy statement/prospectus, (i) Genesoft stockholders should carefully consider the following factors in voting on whether to approve the merger and the related transactions and the consequent investment in Genome common stock and (ii) Genome stockholders should carefully consider the following factors in voting on whether to approve the issuance of shares of Genome common stock to Genesoft security holders, including issuances to the Genesoft note holders upon potential conversion of the Genome convertible notes that they will obtain in the merger, and the amendment to Genome’s articles of organization increasing Genome’s authorized common stock. In addition, you should keep the following risk factors in mind when you read forward-looking statements in this joint proxy statement/prospectus. Please refer to the section entitled “Cautionary Statements Regarding Forward-Looking Statements in this Joint Proxy Statement/Prospectus” beginning on page of this joint proxy statement/prospectus.
Risks Related to the Merger Transaction
The issuance of 28,571,405 shares of Genome common stock to Genesoft security holders in the merger will substantially reduce the percentage interests of Genome security holders.
If the merger is completed, 28,571,405 shares of Genome common stock will be issued to current Genesoft security holders, including shares of Genome common stock to be issued upon the exercise of Genesoft options and warrants that Genome will assume. As of November 30, 2003, Genome had 31,451,099 shares of its common stock outstanding, outstanding options exercisable into 4,086,634 shares of its common stock, outstanding warrants exercisable into 3,221,250 shares of common stock or a total of 38,758,983 shares of common stock on a fully diluted basis. The issuance of the 28,571,405 shares of Genome common stock to current Genesoft stockholders will cause a significant reduction in the relative percentage interests of current Genome stockholders in earnings, voting, liquidation value and book and market value.
The issuance of at least $32 million of securities of Genome to finance the combined company will substantially reduce the percentage interests of both Genome and Genesoft stockholders in the combined company.
As a condition to the closing of the merger, Genome must raise a minimum of $32 million of capital to finance the combined company, unless both parties agree to waive this condition. Genome intends to sell its securities in order to raise the capital. The type of security to be sold and price at which it will be sold are subject to market conditions and negotiations with investors. The securities may be sold at a discount to the market price at the time of sale. The issuance of the securities to finance the combined company will cause a significant reduction in the relative percentage interests of Genome stockholders and Genesoft stockholders in the earnings, voting power, liquidation value and book and market value of the combined company.
Because the number of shares of Genome common stock to be issued in the merger is fixed, Genome stockholders are exposed to the risk that the market price of Genome common stock could increase and Genesoft stockholders are exposed to the risk that the market price of Genome common stock could decrease.
Under the merger agreement, 28,571,405 shares of Genome common stock will be issued to current Genesoft security holders, including shares of Genome common stock to be issued upon the exercise of Genesoft options and warrants that Genome will assume and shares to be issued to the holders of Genesoft’s promissory notes as payment of accrued interest and related amounts. The number of shares to be issued is fixed and will not be adjusted if the price of Genome common stock increases or decreases prior to the completion of the merger. The price of Genome common stock at the closing of the merger might vary from its price on the date of this joint proxy statement/prospectus and on the dates of the Genome and Genesoft stockholders’ special meetings.
24
Table of Contents
These prices might vary because of changes in the business, operations or prospects of Genome or Genesoft, market assessments of the likelihood that the merger will be completed, the timing of the completion of the merger, the prospects of post-merger operations, regulatory considerations, general market and economic conditions and other factors. Because the date that the merger is completed will be later than the dates of the Genome and Genesoft stockholders’ special meeting, the price of Genome common stock on such dates might not be indicative of its price on the date the merger is completed. As a result, the market value of the shares of Genome common stock that Genome will be required to issue to former Genesoft security holders upon completion of the merger might be greater or lesser than the value attributed to Genesoft’s business and assets at the time the merger agreement was entered into and/or the date it is approved by Genome or Genesoft stockholders. We urge you to obtain current market quotations for Genome common stock, and to be aware that the price of Genome common stock might change dramatically after the Genome and Genesoft stockholders’ special meetings.
The assumption by Genome of approximately $24 million of debt of Genesoft at the closing will substantially increase its leverage and, to the extent that a portion of this debt is subsequently converted into Genome common stock, will substantially reduce the percentage interests of both Genome and Genesoft stockholders in the combined company.
Upon the closing of the merger, Genome will assume approximately $24 million of debt of Genesoft. Genome will pay approximately $1.7 million of this debt at closing. The remainder of the debt consists of promissory notes of Genesoft that Genome will exchange for convertible promissory notes of Genome, which will bear interest at 5% per annum and have a maturity date of five years from the closing date. If these notes are not converted into shares of Genome common stock during the five-year period, the subsequent payment of these notes at maturity may require Genome to expend a significant portion of its capital resources. Depending upon the combined company’s capital resources at the maturity date, the payment of these notes could impair the combined company’s working capital and prevent it from pursuing important clinical development and commercialization programs.
The $22,309,647 in aggregate original principal amount of convertible notes of Genome to be issued at the closing of the merger will be convertible into shares of Genome common stock at the holder’s election at any time after the closing of the merger at a price per share equal to one hundred and ten percent of the average closing price of Genome common stock for the five trading days preceding the closing date of the merger, subject to subsequent adjustment. In addition, following the one year anniversary of the closing of the merger, the combined company will have the right to force conversion if the price of Genome common stock closes above 150% of the then effective conversion price for 15 consecutive days. The conversion of all or a substantial portion of these convertible notes would cause a significant reduction in the relative percentage interests of Genome stockholders and Genesoft stockholders in the earnings, voting power, liquidation value and book and market value of the combined company.
Upon the consummation of the merger, Genome will be required to pay $8 million to LG Life Sciences, Ltd. under Genesoft’s License and Option Agreement with LG Life Sciences for FACTIVE, which will diminish the combined company’s financial resources.
Upon the closing of the merger, Genome will be required to pay $8 million to LG Life Sciences as a milestone payment under Genesoft’s License and Option Agreement with LG for FACTIVE. This payment will consume a substantial portion of the combined company’s available cash at closing and, depending upon how much capital has been raised at that point, may limit the combined company’s ability to pursue additional development or commercialization programs.
25
Table of Contents
The combined company may not realize all of the anticipated benefits of the merger.
The success of the merger will depend, in part, on the ability of the combined company to realize the anticipated synergies, cost savings and growth opportunities from integrating the business of Genome with the business of Genesoft. The combined company’s success in realizing these benefits and the timing of this realization depends upon the successful integration of the operations of Genesoft. The integration of two independent companies, especially when one company is located on the West Coast and the other on the East Coast, is a complex, costly and time-consuming process. The difficulties of combining the operations of the companies and realizing the expected benefits of the merger include, among others:
| • | coordinating commercial and clinical development initiatives and staffs for FACTIVE and Ramoplanin; |
| • | raising sufficient capital to fund the significant expenditures that are needed to launch and successfully commercialize FACTIVE and the further clinical development of Ramoplanin; |
| • | retaining key employees; |
| • | consolidating research and development operations; |
| • | consolidating corporate and administrative infrastructures and physical plant; |
| • | integrating and managing the technology of two companies; and |
| • | minimizing the diversion of management’s attention from ongoing business concerns. |
Genome and Genesoft cannot assure you that the integration of Genesoft with Genome will result in the realization of the full benefits anticipated by them to result from the merger.
Genome and Genesoft may suffer negative consequences if the merger is not completed.
If the merger is not completed for any reason, Genome and/or Genesoft will be subject to a number of material risks, including:
| • | the provision in the merger agreement which provides that under specified circumstances either Genome or Genesoft could be required to pay the other a termination fee of approximately $3 million, plus up to $1 million of expenses incurred in connection with the merger; |
| • | the market price of Genome common stock may decline to the extent that the current market price of such shares reflects a market assumption that the merger will be completed, or for other reasons; |
| • | costs related to the merger, such as legal and accounting fees, must be paid even if the merger is not completed; |
| • | benefits that Genome and Genesoft expect to realize from the merger would not be realized; |
| • | the diversion of management attention from the day-to-day business of the companies and the unavoidable disruption to their employees during the period before completion of the merger may make it difficult for Genome and Genesoft to regain their financial position and strategic focus if the merger does not occur; |
| • | if the merger is terminated and Genesoft’s board of directors seeks another merger or business combination, Genesoft’s stockholders cannot be certain that Genesoft will be able to (i) find a partner willing to pay an equivalent or more attractive price than the price to be paid by Genome in the merger or (ii) raise sufficient financing to pay its obligations and continue the operation of its business; |
| • | employees important to the success of either company as a stand-alone company may have left in anticipation of the merger; and |
| • | business opportunities important to either company as a stand-alone company may have been terminated or not pursued by either that company or third parties in anticipation of the merger. |
26
Table of Contents
If the merger does not close, Genome may not be able to obtain repayment of the $6.2 million bridge loan made to Genesoft.
Coincident with the signing of the merger agreement, Genome made a bridge loan of $6.2 million to Genesoft pursuant to a promissory note issued by Genesoft, which is repayable within 60 days of an event of default (as defined in the note) or termination of the merger agreement, unless the merger agreement is terminated by Genesoft due to a failure of Genome to obtain the stockholder vote necessary to approve the merger, in which case it is repayable within 180 days of termination. However, if the note becomes repayable, it is uncertain whether Genome will be able to obtain repayment due to Genesoft’s lack of liquid assets, and even if Genome is able to obtain repayment, Genome may be required to expend additional resources and time to foreclose on assets of Genesoft.
The cash resources of the combined company could be materially depleted if a substantial number of Genesoft stockholders exercise their dissenters’ rights under California law or appraisal rights under Delaware law.
Holders of Genesoft capital stock who dissent and do not consent to the approval and adoption of the merger agreement may be entitled to certain dissenters’ rights under the California Corporations Code and to appraisal rights under Delaware General Corporation Law, or DGCL, in connection with the merger. If the merger is consummated, a holder of record of Genesoft stock who complies with the statutory procedures will be entitled to have those shares appraised by the Delaware Court of Chancery under Section 262 of the DGCL and to receive payment for the “fair value” of those shares instead of the consideration provided for in the merger agreement. Similarly, under Chapter 13 of the California Corporations Code, a holder of Genesoft common stock who complies with the statutory procedures will be entitled to have its shares converted into the right to receive from Genesoft such consideration as may be determined to be due under the statute. These rights are described under “The Special Meeting of Genesoft Stockholders—Appraisal Rights Under Delaware General Corporation Law” and “The Special Meeting of Genesoft Stockholders—Dissenters’ Rights Under California Corporations Code” of this joint proxy statement/prospectus. If a substantial number of Genesoft stockholders exercise their dissenters’ rights under California law or appraisal rights under Delaware law, as the case may be, the combined company may be required to make substantial payments in cash to these stockholders, thereby materially depleting the cash resources of the combined company.
Directors and officers of Genome and Genesoft have potential conflicts of interest that may have influenced them to recommend the merger.
Some of the directors of Genome and Genesoft who recommend that you vote in favor of the merger and the officers of Genome and Genesoft who provided information to their board of directors relating to the merger have employment, indemnification and severance benefit arrangements and rights to acceleration of stock options that provide them with interests in the merger that may differ from yours. The receipt of compensation or other benefits in the merger may have influenced these directors in making their recommendation that you vote in favor of the transactions called for by the merger agreement and these officers in making recommendations to their board of directors relating to the merger. See “The Merger—Interests of Directors and Executive Officers of Genome in the Merger” and “—Interests of Directors and Executive Officers of Genesoft in the Merger.”
27
Table of Contents
Risks Related to the Business of our Combined Company
Both Genome and Genesoft have a history of significant operating losses and expect these losses to continue in the future.
Genome had a net loss of approximately $28,455,000 for the nine months ended September 27, 2003 and as of September 27, 2003, Genome had an accumulated deficit of approximately $154,231,000. Genome had a net loss of approximately $34,017,000 for the fiscal year ended December 31, 2002, and, as of December 31, 2002, Genome had an accumulated deficit of approximately $125,775,000. For the fiscal year ended December 31, 2001, Genome had a net loss of approximately $10,090,000, and for the fiscal year ended December 31, 2000, Genome had a net loss of approximately $5,847,000. The losses have resulted primarily from costs incurred in research and development, including Genome’s clinical trials, and from general and administrative costs associated with Genome’s operations. These costs have exceeded Genome’s revenues which to date have been generated principally from collaborations, government grants and sequencing services.
Genesoft had a net loss of approximately $19,796,000 for the nine months ended September 30, 2003 and as of September 30, 2003, Genesoft had an accumulated deficit of approximately $75,364,000. Genesoft had a net loss of approximately $25,569,000 for the fiscal year ended December 31, 2002, and, as of December 31, 2002, Genesoft had an accumulated deficit of approximately $55,568,000. For the fiscal year ended December 31, 2001, Genesoft had a net loss of approximately $18,321,000, and for the fiscal year ended December 31, 2000, Genesoft had a net loss of approximately $7,921,000. The losses have resulted primarily from costs incurred in research and development, including Genesoft’s clinical trials, and from general and administrative costs associated with our operations. These costs have exceeded Genesoft’s revenues which to date have been generated principally from funding from the U.S. government.
We anticipate that our combined company will incur additional losses this year and in future years and cannot predict when, if ever, our combined company will achieve profitability. These losses are expected to increase following the consummation of the merger as our combined company significantly increases its expenditures in the sales and marketing area to prepare for the commercial launch of FACTIVE. Our combined company also plans to continue to expand its research and development and clinical trial activities. In addition, our partners’ product development efforts which utilize our genomic discoveries are at an early stage and, accordingly, we do not expect our losses to be substantially mitigated by revenues from milestone payments or royalties under those agreements for a number of years, if ever.
We will need to raise additional funds in the future.
As described above, we need to raise a minimum of $32 million as a condition to the closing of the merger, unless this condition is waived by both parties. If we raise that money, we believe that those new funds along with our existing cash and marketable securities together with borrowings under equipment financing arrangements and anticipated cash flow from operations would be sufficient to support our current plans for the combined company for approximately 12 months following the closing of the merger. We expect to raise additional capital over the course of the twelve months following the closing of the merger for our combined company. In particular, we will need additional funds to support our sales and marketing activities, and fund clinical trials and other research and development activities of our combined company. We may seek funding through additional public or private equity offerings, debt financings or agreements with customers. Our ability to raise additional capital, however, will be heavily influenced by the investment market for biotechnology companies and the progress of the FACTIVE and Ramoplanin commercial and clinical development programs over that period. Additional financing may not be available when needed, or, if available, may not be available on favorable terms. If our combined company cannot obtain adequate financing on acceptable terms when such financing is required, its business will be adversely affected.
28
Table of Contents
Future fund raising could dilute the ownership interests of our stockholders.
In order to raise additional funds, our combined company may issue equity or convertible debt securities in the future. Depending upon the market price of the shares of our combined company at the time of any transaction, we may be required to sell a significant percentage of the outstanding shares of common stock of our combined company in order to fund its operating plans, potentially requiring a stockholder vote. In addition, our combined company may have to sell securities at a discount to the prevailing market price, resulting in further dilution to our stockholders.
The business of our combined company will be very dependent on the commercial success of FACTIVE.
FACTIVE will be the only commercial product of our combined company upon the closing of the merger and we expect it will account for substantially all of the revenues of our combined company for at least the next several years. FACTIVE has FDA marketing approval for the treatment of community-acquired pneumonia of mild to moderate severity, or CAP, and acute bacterial exacerbations of chronic bronchitis, or ABECB. The commercial success of FACTIVE will depend upon its acceptance by regulators, physicians, patients and other key decision-makers as a safe, therapeutic and cost-effective alternative to other anti-infectives and other products used, or currently being developed, to treat CAP and ABECB. If FACTIVE is not commercially successful, our combined company will have to find additional sources of funding or curtail or cease operations.
In December 2000, the FDA issued a non-approvable letter to the prior owner of rights to FACTIVE due, in part, to safety concerns arising out of an increased rate of rash relative to comparator drugs, especially in young women. While the FDA did approve FACTIVE for marketing in April 2003, it required, as a post-marketing study commitment, that Genesoft conduct a prospective, randomized study comparing FACTIVE (5,000 patients) to an active comparator (2,500 patients) in patients with CAP or ABECB. This study will include patients of different ethnicities, to gain safety information in populations not substantially represented in the existing clinical trial program, specifically as it relates to rash. Patients will be evaluated for clinical and laboratory safety. This Phase IV trial is in the design stage and the FDA required, as a condition to its approval, that the trial be initiated by March 2004. The results of this trial, as well as other safety information arising out of the marketing of the product, could restrict our ability to commercialize FACTIVE.
Our combined company will need to develop marketing and sales capabilities to successfully commercialize FACTIVE and our other product candidates.
FACTIVEwill be the first FDA approved product of our combined company. Accordingly, following the closing of the merger, our combined company will have very limited marketing and sales experience. Our combined company will need to develop a marketing and sales staff to successfully commercialize FACTIVE and our other product candidates, including Ramoplanin. In order to launch FACTIVE in the second half of 2004, our combined company will need to rapidly assemble a sales and marketing force. The development of these marketing and sales capabilities will require significant expenditures, management resources and time. Our combined company may be unable to build such a sales force, the cost of establishing such a sales force may exceed any product revenues, or the marketing and sales efforts of our combined company may be unsuccessful. Failure to successfully establish sales and marketing capabilities in a timely and regulatory compliant manner or to find suitable sales and marketing partners may prevent our combined company from successfully launching FACTIVE in 2004, which would materially adversely affect the business and results of operations of our combined company.
Our combined company will depend on third parties to manufacture our product candidates, including FACTIVE and Ramoplanin.
Our combined company will not have the internal capability to manufacture commercial quantities of pharmaceutical products under the FDA’s current Good Manufacturing Practices. Genesoft has entered into an agreement with LG Life Sciences to manufacture bulk quantities of FACTIVE. Genome has entered into an
29
Table of Contents
agreement with Biosearch (which merged with Versicor Inc. in March 2003 and subsequently changed its name to Vicuron Pharmaceuticals Inc.) to manufacture bulk quantities of Ramoplanin, and our combined company expects to enter into similar agreements with third parties for the manufacture of future product candidates. Although the LG Life Sciences facilities have previously been inspected by the FDA, they had not been actively manufacturing product for 32 months until their re-start of activity in October 2003. Future inspections may find deficiencies in the facilities or processes that may delay or prevent the manufacture or sale of FACTIVE.
Genesoft expects to purchase its requirements for the final drug product from LG Life Sciences for 2004, which final drug product will be tableted and packaged for LG Life Sciences by SB Pharmco at its manufacturing facility in Puerto Rico. This arrangement with SB Pharmco is expected to conclude by the end of 2004. Genesoft is in discussions with a new secondary manufacturer to assume these responsibilities for subsequent periods. Genesoft may be unable, however, to successfully complete these arrangements. If our combined company is unable to obtain an agreement with a qualified finish and fill contractor to provide services by the end of 2004, the commercialization of FACTIVE could be delayed and our business may be adversely affected. In addition, we cannot assure you that SB Pharmco or any new secondary manufacturer will be able to avoid batch failures or other production delays.
We cannot be certain that LG Life Sciences, Vicuron or future manufacturers will be able to deliver commercial quantities of product candidates to our combined company or that such deliveries will be made on a timely basis. Currently, the only source of supply for FACTIVE bulk drug product is LG Life Sciences’ facility in South Korea, and if such facility were damaged or otherwise unavailable, our combined company would incur substantial costs and delay in the commercialization of FACTIVE. If our combined company is forced to find an alternative source for Ramoplanin or other product candidates, we could also incur substantial costs and delays in the commercialization of such products. Our combined company may not be able to enter into alternative supply arrangements at commercially acceptable rates, if at all. Also, if our combined company changes the source or location of supply or modifies the manufacturing process, regulatory authorities will require us to demonstrate that the product produced by the new source or from the modified process is equivalent to the product used in any clinical trials that we had conducted.
Moreover, while our combined company may choose to manufacture products in the future, we have no experience in the manufacture of pharmaceutical products for clinical trials or commercial purposes. If our combined company decides to manufacture products, it would be subject to the regulatory requirements described above. In addition, our combined company would require substantial additional capital and would be subject to delays or difficulties encountered in manufacturing pharmaceutical products. No matter who manufactures the products, our combined company will be subject to continuing obligations regarding the submission of safety reports and other post-market information.
Our combined company cannot expand the indications for which it will market FACTIVE unless it receives FDA approval for each additional indication. Failure to expand these indications will limit the size of the commercial market for FACTIVE.
In April 2003, Genesoft received approval from the FDA for the use of FACTIVE to treat community-acquired pneumonia of mild to moderate severity and acute bacterial exacerbations of chronic bronchitis. One of the objectives of our combined company is to expand the indications for which FACTIVE is approved for marketing by the FDA, including for the indication of acute bacterial sinusitis. While clinical trials for acute bacterial sinusitis have previously been completed, our combined company may need to conduct additional clinical trials in order to market FACTIVE for this indication. In order to market FACTIVE for other indications, our combined company will need to conduct additional clinical trials, obtain positive results from those trials and obtain FDA approval for such proposed indications. If our combined company is unsuccessful in expanding the approved indications for the use of FACTIVE, the size of the commercial market for FACTIVE will be limited.
30
Table of Contents
Failure to obtain regulatory approval in foreign jurisdictions will prevent our combined company from marketing FACTIVE abroad.
In order to market FACTIVE in the European Union and other foreign jurisdictions for which we have rights to market the product, our combined company or its distribution partners must obtain separate regulatory approvals. Obtaining foreign approvals may require additional trials and expense. Our combined company may not be able to obtain approval or may be delayed in obtaining approval from any or all of the jurisdictions in which it seeks approval to market FACTIVE.
Sales of FACTIVE in European countries in which Genesoft does not have rights to market the product could adversely affect sales in the European countries in which Genesoft has exclusive rights to market the product.
Genesoft’s exclusive rights to market FACTIVE in Europe are limited to France, Germany, the United Kingdom, Luxembourg, Ireland, Italy, Spain, Portugal, Belgium, the Netherlands, Austria, Greece, Sweden, Denmark, Finland, Norway, Iceland, Switzerland, Andorra, Monaco, San Marino and Vatican City. These countries include the current members of the European Union. However, over the next several years, a number of additional European countries in which Genesoft does not have rights to market FACTIVE may be admitted as members of the European Union. If LG Life Sciences were to sell FACTIVE or license a third party to sell FACTIVE in such countries after they are admitted to the European Union, our combined company’s ability to maintain its projected profit margins based on sales in the territories covered by the LG Life Sciences license agreement may be adversely affected because customers in Genesoft’s territory may purchase FACTIVE from neighboring countries in the European Union and our combined company’s ability to prohibit such purchases may be limited under European Union antitrust restrictions.
Failure to secure distribution partners in foreign jurisdictions will prevent our combined company from marketing FACTIVE abroad.
Our combined company intends to market FACTIVE through distribution partners in most, if not all, of the international markets for which we have a license to market the product. This will include the European Union. Our combined company may not be able to secure distribution partners at all, or those that we do secure may not be successful in marketing and distributing FACTIVE. If we are not able to secure distribution partners or those partners are unsuccessful in their efforts, it would significantly limit the revenues that we expect to obtain from the sales of FACTIVE.
The development and commercialization of the products of our combined company may be terminated or delayed, and the costs of development and commercialization may increase, if third parties who our combined company relies on to manufacture and support the development and commercialization of its products do not fulfill their obligations.
The development and commercialization strategy of our combined company entails entering into arrangements with corporate and academic collaborators, contract research organizations, distributors, third-party manufacturers, licensors, licensees and others to conduct development work, manage its clinical trials, manufacture its products and market and sell its products outside of the United States. Our combined company will not have the expertise or the resources to conduct such activities on its own and, as a result, will be particularly dependent on third parties in these areas.
Our combined company may not be able to maintain its existing arrangements with respect to the commercialization of FACTIVE or establish and maintain arrangements to develop and commercialize Ramoplanin or any additional product candidates or products it may acquire on terms that are acceptable to it. Any current or future arrangements for development and commercialization may not be successful. If our combined company is not able to establish or maintain agreements relating to FACTIVE, Ramoplanin or any additional products it may acquire on terms which it deems favorable, its results of operations would be materially adversely affected.
31
Table of Contents
Third parties may not perform their obligations as expected. The amount and timing of resources that third parties devote to developing, manufacturing and commercializing the products of our combined company are not within our control. Furthermore, the interests of our combined company may differ from those of third parties that manufacture or commercialize the products of our combined company. Disagreements that may arise with these third parties could delay or lead to the termination of the development or commercialization of the product candidates of our combined company, or result in litigation or arbitration, which would be time consuming and expensive.
If any third party that manufactures or supports the development or commercialization of the products of our combined company breaches or terminates its agreement with our combined company, or fails to conduct its activities in a timely and regulatory compliant manner, such breach, termination or failure could:
| • | delay or otherwise adversely impact the development or commercialization of FACTIVE, Ramoplanin, our other product candidates or any additional product candidates that our combined company may acquire or develop; |
| • | require our combined company to undertake unforeseen additional responsibilities or devote unforeseen additional resources to the development or commercialization of its products; or |
| • | result in the termination of the development or commercialization of the products of our combined company. |
Clinical trials are costly, time consuming and unpredictable, and our combined company will have limited experience conducting and managing necessary preclinical and clinical trials for our product candidates.
Genesoft’s lead product, FACTIVE, will need to complete a Phase IV post-approval clinical trial in compliance with FDA requirements pursuant to the product’s approval. Additionally, clinical trials may be necessary to gain approval to market the product for the treatment of acute bacterial sinusitis. Additional clinical trials will be required to gain approval to market FACTIVE for other indications. Genome’s lead product candidate, Ramoplanin, is in a Phase III clinical trial for the prevention of bloodstream infections caused by vancomycin-resistant enterococci, also known as VRE, and a Phase II clinical trial to assess the safety and efficacy of Ramoplanin to treatClostridium difficile-associated diarrhea (CDAD). Prior clinical and preclinical trials for Ramoplanin were conducted by Biosearch Italia S.p.A. and its licensees, from whom we acquired our license to develop Ramoplanin.
Our combined company may not be able to demonstrate the safety and efficacy of Ramoplanin or of FACTIVE in indications other than those for which it has already been approved or our other products, in each case, to the satisfaction of the FDA, or other regulatory authorities. Our combined company may also be required to demonstrate that its proposed products represent an improved form of treatment over existing therapies and it may be unable to do so without conducting further clinical studies. Negative, inconclusive or inconsistent clinical trial results could prevent regulatory approval, increase the cost and timing of regulatory approval or require additional studies or a filing for a narrower indication.
The speed with which our combined company is able to complete its clinical trials and its applications for marketing approval will depend on several factors, including the following:
| • | the rate of patient enrollment, which is a function of many factors, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the study and the nature of the protocol; |
| • | fluctuations in the infection rates for patients enrolled in our trials; |
| • | compliance of patients and investigators with the protocol and applicable regulations; |
| • | prior regulatory agency review and approval of our applications and procedures; |
32
Table of Contents
| • | analysis of data obtained from preclinical and clinical activities which are susceptible to varying interpretations, which interpretations could delay, limit or prevent regulatory approval; |
| • | changes in the policies of regulatory authorities for drug approval during the period of product development; and |
| • | the availability of skilled and experienced staff to conduct and monitor clinical studies, to accurately collect data and to prepare the appropriate regulatory applications. |
In addition, the cost of human clinical trials varies dramatically based on a number of factors, including the order and timing of clinical indications pursued, the extent of development and financial support from alliance partners, the number of patients required for enrollment, the difficulty of obtaining clinical supplies of the product candidate, and the difficulty in obtaining sufficient patient populations and clinicians.
Our combined company will have limited experience in conducting and managing the preclinical and clinical trials necessary to obtain regulatory marketing approvals. Our combined company may not be able to obtain the approvals necessary to conduct clinical studies. Also, the results of the clinical trials of our combined company may not be consistent with the results obtained in preclinical studies or the results obtained in later phases of clinical trials may not be consistent with those obtained in earlier phases. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials, even after experiencing promising results in early animal and human testing. If regulatory approval of a drug is granted, such approval is likely to limit the indicated uses for which it may be marketed. Furthermore, even if a product of our combined company gains regulatory approval, the product and the manufacturer of the product will be subject to continuing regulatory review, including the requirement to conduct post-approval clinical studies. Our combined company may be restricted or prohibited from marketing or manufacturing a product, even after obtaining product approval, if previously unknown problems with the product or its manufacture are subsequently discovered.
The product candidates of our combined company will face significant competition in the marketplace.
FACTIVE is approved for the treatment of community-acquired pneumonia of mild to moderate severity and acute bacterial exacerbations of chronic bronchitis. There are several classes of antibiotics that are primary competitors for the treatment of these indications, including:
| • | other fluoroquinolones such as Levaquin® (levofloxacin), a product of Ortho-McNeil Pharmaceutical, Inc., Tequin® (gatifloxacin), a product of Bristol-Myers Squibb Company, and Cipro® (ciprofloxacin) and Avelox® (moxifloxacin), both products of Bayer Corporation; |
| • | macrolides such as Biaxin® (clarithromycin), a product of Abbott Laboratories and Zithromax® (azithromycin), a product of Pfizer Inc.; and |
| • | penicillins such as Augmentin® (amoxicillin/clavulanate potassium), a product of GlaxoSmithKline. |
In addition, a new drug application for Ketek®, a ketolide antibiotic from Aventis Pharmaceuticals, has been submitted to the FDA and Ketek is currently marketed in Europe. Many generic antibiotics are also currently prescribed to treat these infections.
Ramoplanin is currently in development for the prevention of bloodstream infections caused by vancomycin-resistant enterococci (VRE). We have no knowledge of any product currently approved by the FDA for this indication, nor are we aware of any product candidate currently in clinical trials for this indication. It is possible that competition exists without our knowledge and that current discovery and preclinical efforts are ongoing for this indication. Ramoplanin is also in clinical development for the treatment ofClostridium difficile-associated diarrhea (CDAD). We are aware of two products currently utilized in the marketplace— Vanconin® (vancomycin), a product of Eli Lilly, and metronidazole, a generic product —for treatment of this indication. We
33
Table of Contents
are also aware of at least three companies with products in development for the treatment of CDAD—Geltex/Genzyme in Phase II; ImmuCell in Phase I/II; and Acambis in Phase I/II. It is also possible that other companies are developing competitive products for this indication. Genesoft is aware that Vicuron and Novartis Pharma are jointly developing PDF inhibitor agents that may compete with any PDF products developed by our combined company.
All of our other internal product programs are in earlier stages and have not yet reached clinical development and are not yet indication specific. Our alliance-related product development programs are also all in preclinical stages, and it is therefore not possible to identify any product profiles or competitors for these product development programs at this time. Our industry is very competitive and it therefore is likely that if and when product candidates from our early stage internal programs or our alliance programs reach the clinical development stage or are commercialized for sale, these products will also face competition.
Many of the competitors of our combined company will have substantially greater capital resources, facilities and human resources than our combined company. Furthermore, many of those competitors are more experienced than our combined company in drug discovery, development and commercialization, and in obtaining regulatory approvals. As a result, those competitors may discover, develop and commercialize pharmaceutical products or services before our combined company. In addition, the competitors of our combined company may discover, develop and commercialize products or services that are more effective than, or otherwise render non-competitive or obsolete, the products or services that our combined company or its collaborators are seeking to develop and commercialize. Moreover, these competitors may obtain patent protection or other intellectual property rights that would limit the rights of our combined company or the ability of our collaborators to develop or commercialize pharmaceutical products or services.
Health care insurers and other payers may not pay for our combined company’s products or may impose limits on reimbursement.
The ability of our combined company to commercialize FACTIVE, Ramoplanin and its future products will depend, in part, on the extent to which reimbursement for such products will be available from third-party payers, such as Medicare, Medicaid, health maintenance organizations, health insurers and other public and private payers. If our combined company succeeds in bringing FACTIVE, Ramoplanin or other products in the future to market, we cannot assure you that third-party payers will pay for such products or will establish and maintain price levels sufficient for realization of an appropriate return on our investment in product development. If adequate coverage and reimbursement levels are not provided by government and private payers for use of the products of our combined company, our products may fail to achieve market acceptance and the results of operations of our combined company may be materially adversely affected.
Many health maintenance organizations and other third-party payers use formularies, or lists of drugs for which coverage is provided under a health care benefit plan, to control the costs of prescription drugs. Each payer that maintains a drug formulary makes its own determination as to whether a new drug will be added to the formulary and whether particular drugs in a therapeutic class will have preferred status over other drugs in the same class. This determination often involves an assessment of the clinical appropriateness of the drug and sometimes the cost of the drug in comparison to alternative products. We cannot assure you that FACTIVE, Ramoplanin or any of the future products of our combined company will be added to payers’ formularies, whether the products of our combined company will have preferred status to alternative therapies, nor whether the formulary decisions will be conducted in a timely manner. Our combined company may also decide to enter into discount or formulary fee arrangements with payers, which could result in our receiving lower or discounted prices for FACTIVE, Ramoplanin or future products.
34
Table of Contents
Our combined company will rely upon existing and prospective alliance partners, licensees and government grants and contracts as a source of revenue for its operations and as a means of developing and commercializing its products.
The strategy of our combined company for developing and commercializing therapeutic, vaccine and diagnostic products depends, in part, on strategic alliances and licensing arrangements with pharmaceutical and biotechnology partners. Genome currently has alliances with AstraZeneca, Amgen, bioMérieux, Schering-Plough and Wyeth. Genome has received a substantial portion of its revenue from these alliances, and the combined company expects to continue to earn revenues from them. Under these arrangements, the combined company will be entitled to receive payments and royalties based on the achievement by it and its partners of certain development milestones and the successful development of products arising from the collaborations. Although Genome has achieved many of the scientific milestones under its agreements, the combined company cannot assure you that it will continue these achievements in the future or that milestones dependent on its partners’ development and commercialization activities will be attained.
In order to maintain the collaboration agreements with each of Amgen and Wyeth, the combined company must fulfill certain obligations, including carrying out research programs agreed to by the parties, keeping records of its research activities, providing periodic reports on the status of each research program, devoting qualified personnel to each research program, and providing its collaborators with reasonable technical assistance in using the know-how or other information that it has licensed to them. Under Genome’s other collaboration agreements, Genome has fulfilled all of its research and development obligations and the combined company will have no material obligations going forward. Genome believes that it is currently in compliance with its obligations under its collaboration agreements, but there can be no assurance that the combined company will be able to successfully complete its obligations in the future due to, among other things, an inability to achieve scientific goals or a lack of qualified personnel.
If the partners of our combined company develop products using our discoveries, it will rely on these partners for product development, regulatory approval, manufacturing and marketing of those products before it can receive some of the milestone payments, royalties and other payments to which it may be entitled under the terms of some of its alliance agreements. Genome’s agreements with its partners typically allow the partners significant discretion in electing whether to pursue any of these activities. Our combined company will not be able to control the amount and timing of resources its partners may devote to its programs or potential products. As a result, there can be no assurance that the partners of our combined company will perform their obligations as expected.
The strategy of our combined company will include entering into multiple, concurrent alliances and business partnerships, including, but not limited to in-licensing and co-promotion agreements. There can be no assurance that our combined company will be able to manage multiple alliances and partnerships successfully. The risks our combined company will face in managing multiple alliances and partnerships include maintaining confidentiality among partners, avoiding conflicts between partners and avoiding conflicts between our combined company and its partners. If our combined company fails to manage its alliances and partnerships effectively, or if any of the problems described above arise, one or more of the following could occur which could have a material adverse effect on the business of our combined company:
| • | use of significant resources to resolve conflicts, |
| • | delay in, or an adverse effect on, sales and marketing efforts for the combined company’s products, |
| • | delay in development activities, |
| • | legal claims involving significant time, |
| • | expense, |
| • | loss of reputation, and |
| • | termination of one or more alliances, or loss of capital and loss of revenues. |
35
Table of Contents
Both Genome and Genesoft have applied for and received grants from the U.S. government in the past. The strategy of our combined company going forward will include the continued pursuit of government grants and contracts. We can not assure you that our combined company will obtain any additional grants or that our existing grants will continue to be funded. If our combined company is unable to obtain additional grants or maintain its existing grants, its revenues would be adversely affected.
Development of therapeutic, diagnostic and vaccine products by the strategic alliance partners of our combined company based on its discoveries will be subject to the high risks of failure inherent in the development or commercialization of biopharmaceutical products.
There can be no assurance of the successful development or commercialization of any products by the strategic alliance partners of our combined company. Successful development and commercialization will be subject to numerous risks at each stage. For example, there can be no assurance that the high-throughput screening or lead optimization processes for a given strategic alliance will identify any compounds suitable for clinical development. Even if product candidates based on discoveries of our combined company undergo clinical trials, there can be no assurance that those clinical trials will indicate that the product candidates are safe or effective. The pace at which the clinical trials proceed is also uncertain. Furthermore, after the completion of clinical trials, a product could fail to receive necessary regulatory approvals due to negative, inconclusive or insufficient clinical data or other reasons beyond the control of our combined company. Even if the necessary regulatory approvals for a product are obtained, it may be difficult or impossible to manufacture the product on a large scale, be uneconomical to market, fail to be developed prior to the successful marketing of similar products by competitors or infringe on proprietary rights of third parties.
The failure of our combined company to acquire and develop additional product candidates or approved products will impair its ability to grow.
As part of its growth strategy, our combined company intends to acquire and develop additional product candidates or approved products. The success of this strategy depends upon its ability to identify, select and acquire biopharmaceutical products that meet its criteria. Our combined company may not be able to acquire the rights to additional product candidates and approved products on terms that it finds acceptable, or at all.
New product candidates acquired or in-licensed by our combined company may require additional research and development efforts prior to commercial sale, including extensive preclinical and/or clinical testing and approval by the FDA and corresponding foreign regulatory authorities. All product candidates are prone to the risks of failure inherent in pharmaceutical product development, including the possibility that the product candidate will not be safe, non-toxic and effective or approved by regulatory authorities. In addition, it is uncertain whether any approved products that our combined company develops or acquires will be:
| • | manufactured or produced economically; |
| • | successfully commercialized; or |
| • | widely accepted in the marketplace. |
Future acquisitions may absorb significant resources and may be unsuccessful.
As part of its strategy, our combined company may pursue acquisitions of businesses or assets, investments and other relationships and alliances. Acquisitions may involve significant cash expenditures, debt incurrence, additional operating losses, dilutive issuances of equity securities, and expenses that could have a material adverse effect on the financial condition and results of operations of our combined company. For example, to the extent that our combined company elects to pay the purchase price for such acquisitions in shares of its stock, the issuance of additional shares of its stock will be dilutive to our stockholders. Acquisitions involve numerous other risks, including:
| • | difficulties integrating acquired technologies and personnel into the business of our combined company; |
| • | diversion of management from daily operations; |
36
Table of Contents
| • | inability to obtain required financing on favorable terms; |
| • | entering new markets in which our combined company has little or no previous experience; |
| • | potential loss of key employees or customers of acquired companies; |
| • | assumption of the liabilities and exposure to unforeseen liabilities of acquired companies; and |
| • | amortization of the intangible assets of acquired companies. |
It may be difficult for our combined company to complete these types of transactions quickly and to integrate the businesses efficiently into its business. Any acquisitions or investments by our combined company may ultimately have a negative impact on its business and financial condition.
Our combined company will depend on key personnel in a highly competitive market for skilled personnel.
Our combined company will be highly dependent on the principal members of our senior management and key scientific and technical personnel. The loss of any of its personnel could have a material adverse effect on its ability to achieve its goals. Our combined company will maintain the existing employment agreements with the following officers of Genome: Steven M. Rauscher, President and Chief Executive Officer; Stephen Cohen, Senior Vice President and Chief Financial Officer; and Martin D. Williams, Senior Vice President, Corporate Development & Marketing. The term of each employment agreement continues until it is terminated by the officer or the combined company. Genome does not currently maintain key person life insurance on any of its employees.
The future success of our combined company is dependent upon its ability to attract and retain additional qualified sales and marketing, clinical development, scientific and managerial personnel. The plan to launch the commercial sale of FACTIVE during the second half of 2004 will require the combined company to hire approximately 90 to 100 new employees, primarily with expertise in the areas of sales and marketing. Like others in our industry, our combined company may face, and in the past Genome and Genesoft have faced from time to time, difficulties in attracting and retaining certain employees with the requisite expertise and qualifications. Genome and Genesoft believe that their historical recruiting periods and employee turnover rates are similar to those of others in their industry, however, we cannot be certain that our combined company will not encounter greater difficulties in the future.
The intellectual property protection and other protections of our combined company may be inadequate to protect its products.
The success of our combined company will depend, in part, on its ability to obtain commercially valuable patent claims and protect its intellectual property. Genome currently has 15 issued U.S. patents, 86 pending U.S. patent applications, 10 issued foreign patents and 39 pending foreign patent applications. These patents and patent applications primarily relate to the field of human and pathogen genetics. Genome’s material patents are as follows:
| • | U.S. Patent No. 6,380,370 granted April 30, 2002, relating toStaphylococcus epidermidis;expiring August 13, 2018 |
| • | U.S. Patent No. 6,551,795 granted April 22, 2003, relating toPseudomonas aeruginosa;expiring February 18, 2019 |
| • | U.S. Patent No. 6,562,958 granted May 13, 2003, relating toAcinetobacter baumannii;expiring June 4, 2019 |
| • | U.S. Patent No. 6,583,275 granted June 24, 2003, relating toEnterococcus faecium;expiring June 30, 2018 |
37
Table of Contents
| • | U.S. Patent No. 6,583,266 granted June 24, 2003, relating toMycobacterium tuberculosis andleprae;expiring June 24, 2020 |
| • | U.S. Patent No. 6,605,709 granted August 12, 2003, relating toProteus mirabilis; expiring April 5, 2020 |
| • | U.S. Patent No. 6,6105,836 granted August 26, 2003, relating toKlebsiella pneumoniae; expiring January 27, 2020 |
| • | U.S. Patent No. 6,617,156 granted September 9, 2003, relating toEnterococcus faecalis; expiring August 13, 2018 |
Genesoft currently owns or licenses 34 issued U.S. patents, approximately 42 pending U.S. patent applications, approximately 40 issued foreign patents and approximately 104 pending foreign patent applications. These patents and patent applications primarily relate to the chemical composition, use, and method of manufacturing FACTIVE, to metalloenzyme inhibitors, their uses, and their targets, and to DNA-Nanobinder compounds and their use as anti-infective therapeutics. The following list of U.S. patents (along with their foreign counterparts) constitutes Genesoft’s material patents:
| • | U.S. Patent No. 5,633,262 filed June 15, 1995, entitled “Quinoline carboxylic acid derivatives having 7-(4-amino-methyl-3-oxime) pyrrolidine substituent and processes for preparing thereof,” licensed from LG Life Sciences; expiring June 15, 2015. |
| • | U.S. Patent No. 5,776,944 filed April 4, 1997, entitled “7-(4-aminomethyl-3-methyloxyiminopyrroplidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid and the process for the preparation thereof,” licensed from LG Life Sciences; expiring June 15, 2015. |
| • | U.S. Patent No. 5,869,670 filed March 27, 1998, entitled “7-(4-aminomethyl-3-methyloxyiminopyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid and the process for the preparation thereof,” licensed from LG Life Sciences; expiring June 15, 2015. |
| • | U.S. Patent No. 5,962,468 filed November 9, 1998, entitled “7-(4-aminomethyl-3-methyloxyiminopyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid and the process for the preparation thereof,” licensed from LG Life Sciences; expiring June 15, 2015. |
| • | U.S. Patent No. 6,423,690, entitled “Antibacterial agents,” licensed from Vernalis; expiring February 5, 2019. |
| • | U.S. Patent No. 6,441,042, entitled “Hydroxamic acid derivatives as antibacterials,” licensed from Vernalis; expiring May 14, 2019. |
While it is difficult to assess the value of our combined company’s intellectual property portfolio, we anticipate that the patents named above will provide a competitive advantage in certain instances in the pathogen and anti-infective field by requiring others to obtain a license from us if they wish to produce competing products.
Neither Genome nor Genesoft is currently involved in any litigation, settlement negotiations, or other legal action regarding patent issues and neither Genome nor Genesoft is aware of any patent litigation threatened against them. The patent position of both Genome and Genesoft involves complex legal and factual questions, and legal standards relating to the validity and scope of claims in the applicable technology fields are still evolving. Therefore, the degree of future protection for the proprietary rights of our combined company is uncertain.
The patents that Genome licenses to Ramoplanin under the License and Supply Agreement with Vicuron include claims relating to methods of manufacturing Ramoplanin as well as methods increasing the yield of the active compound. Genome also has applications pending relating to various novel uses of Ramoplanin. The
38
Table of Contents
patent covering the chemical composition of Ramoplanin has expired. To provide additional protection for Ramoplanin, Genome relies on proprietary know-how relating to maximizing yields in the manufacture of Ramoplanin, as well as the five year data exclusivity provisions under the Hatch-Waxman Act.
LG Life Sciences, as owner of U.S. Patent Nos. 5,776,944 and 5,962,468, submitted requests for reexamination to the U.S. Patent & Trademark Office, or PTO, in order to place additional references into the record of each patent. Both requests were granted by the PTO. Patent ‘468 has been reexamined with relatively minor modifications to the claims and confirmed patentable over the submitted references. The reexamination of Patent ‘944 is currently pending. If the PTO does not confirm the claims in this patent as patentable, our patent protection with respect to FACTIVE in the U.S. may be weakened.
The risks and uncertainties that our combined company will face with respect to its patents and other proprietary rights include the following:
| • | the pending patent applications that Genome and Genesoft have filed or to which they have exclusive rights may not result in issued patents or may take longer than expected to result in issued patents; |
| • | the claims of any patents which are issued may be limited from those in the patent applications and may not provide meaningful protection; |
| • | our combined company may not be able to develop additional proprietary technologies that are patentable; |
| • | the patents licensed or issued to our combined company or our partners may not provide a competitive advantage; |
| • | other companies may challenge patents licensed or issued to our combined company or our partners; |
| • | patents issued to other companies may harm the ability of our combined company to do business; |
| • | other companies may independently develop similar or alternative technologies or duplicate the technologies of our combined company; and |
| • | other companies may design around technologies our combined company has licensed or developed. |
In addition, Genome is aware that some companies have published patent applications relating to nucleic acids and proteins from various pathogenic organisms. If these companies receive issued patents, their patents may limit the ability of our combined company and the ability of its collaborators to practice under any patents that may be issued to our combined company or its collaborators. Because of this, our combined company or its collaborators may not be able to obtain patents with respect to the genes of infectious agents or the value of certain other patents issued to our combined company or its collaborators may be limited. Also, even if a patent were issued to our combined company, the scope of coverage or protection afforded to such patent may be limited.
Our combined company will bear substantial responsibilities under its license agreements for FACTIVE and Ramoplanin, and there can be no assurance that our combined company will successfully fulfill its responsibilities.
Under Genome’s agreement with Vicuron, its has obtained an exclusive license to develop and market oral Ramoplanin in the United States and Canada. Under this agreement, Genome is responsible, at its expense, for the clinical and non-clinical development of Ramoplanin in Genome’s field, the prevention and treatment of human disease, in the United States and Canada, including the conduct of clinical trials and the filing of drug approval applications with the FDA and other applicable regulatory authorities. Vicuron is responsible for providing us with all information in its possession relating to Ramoplanin in Genome’s licensed field, for cooperating with Genome in obtaining regulatory approvals of Ramoplanin and for using diligent efforts to provide us with bulk Ramoplanin sufficient to carry out Genome’s clinical development activities. Genome believes that it is currently in compliance with its obligations under the License and Supply Agreement, but there
39
Table of Contents
can be no assurance that our combined company will be able to remain in compliance due to the limitations on its resources and the many risks of conducting clinical trials, as described above in “Clinical trials are costly, time consuming and unpredictable, and our combined company has limited experience conducting and managing necessary preclinical and clinical trials for its product candidates”.
Under Genome’s agreement with Vicuron, Vicuron has the obligation to prosecute patents relating to Ramoplanin that are made by Vicuron personnel or conceived jointly by Genome personnel and Vicuron personnel. Genome has the obligation to prosecute patents relating to Ramoplanin that are made solely by Genome’s personnel. Genome has the right to control any suits brought by a third party alleging that the manufacture, use or sale of Ramoplanin in its licensed field in the United States or Canada infringes upon their rights. Our combined company will bear the costs of any such actions, which could be substantial; provided that if our combined company is obligated to pay any royalties or other payments to a third party to sell Ramoplanin as a result of this litigation, including any settlement reached with Vicuron’s consent, Vicuron is obligated to pay that expense.
Genome also has the primary right to pursue actions for infringement of any patent licensed from Vicuron under the License and Supply Agreement within the United States and Canada within its licensed field. Vicuron has the primary right to pursue actions for infringement of any patents that it licenses to Genome under the License and Supply Agreement outside of Genome’s licensed field within the United States and Canada and for all purposes outside of the United States and Canada. If the party with the primary right to pursue the infringement actions elects not to pursue it, the other party generally has the right to pursue it. The costs of any infringement actions are first paid out of any damages recovered and are then allocated to the parties depending upon their interest in the suit. The costs of pursuing any such action could substantially diminish the resources of our combined company.
Under Genesoft’s License and Option Agreement with LG Life Sciences, its has obtained an exclusive license to develop and market FACTIVE in North America and France, Germany, the United Kingdom, Luxembourg, Ireland, Italy, Spain, Portugal, Belgium, the Netherlands, Austria, Greece, Sweden, Denmark, Finland, Norway, Iceland, Switzerland, Andorra, Monaco, San Marino and Vatican City. Under this agreement, Genesoft is responsible, at its expense and through consultation with LG Life Sciences, for the clinical and commercial development of FACTIVE in the countries covered by the license, including the conduct of clinical trials, the filing of drug approval applications with the FDA and other applicable regulatory authorities and the marketing, distribution and sale of FACTIVE in its territory. The agreement also requires a minimum sales commitment over a period of time, which if not met, could result in the technology being returned to LG Life Sciences. Genesoft believes that it is currently in compliance with its obligations under its agreement with LG Life Sciences, but there can be no assurance that our combined company will be able to remain in compliance due to the limitations on its resources and the many risks of conducting clinical trials, as described above in “Clinical trials are costly, time consuming and unpredictable, and our combined company has limited experience conducting and managing necessary preclinical and clinical trials for its product candidates” and the challenges inherent in the commercialization of new products as described above in “The product candidates of our combined company will face significant competition in the marketplace”.
Under Genesoft’s License and Option Agreement with LG Life Sciences, LG Life Sciences has the obligation to diligently maintain its patents and the patents of third parties to which it has rights that, in each case, relate to FACTIVE. Genesoft has the right to control any litigation relating to suits brought by a third party alleging that the manufacture, use or sale of FACTIVE in its licensed field in the territories covered by the license infringes upon their rights. Our combined company will bear the costs of any such actions, which could be substantial.
Genesoft also has the primary right to pursue actions for infringement of any patent licensed from LG Life Sciences under the License and Option Agreement within the territories covered by the license. If Genesoft elects not to pursue any infringement action, LG Life Sciences has the right to pursue it. The costs of any infringement actions are first paid out of any damages recovered. If Genesoft is the plaintiff, the remainder of the damages are
40
Table of Contents
retained by Genesoft, subject to its royalty obligations to LG Life Sciences. If LG Life Sciences is the plaintiff, the remainder of the damages are divided evenly between the parties, subject to Genesoft’s royalty obligations to LG Life Sciences. The costs of pursuing any such action could substantially diminish the resources of our combined company.
The proprietary position of our combined company may depend on its ability to protect trade secrets.
Our combined company will rely on trade secret protection for its confidential and proprietary information and procedures. Genome and Genesoft currently protect such information and procedures as trade secrets. Our combined company will continue to protect our trade secrets through recognized practices, including access control, confidentiality agreements with employees, consultants, collaborators, and customers, and other security measures. These confidentiality agreements may be breached, however, and our combined company may not have adequate remedies for any such breach. In addition, the trade secrets of our combined company may otherwise become known or be independently developed by competition.
Our combined company may infringe the intellectual property rights of third parties and may become involved in expensive intellectual property litigation.
The intellectual property rights of biotechnology companies, including our combined company, are generally uncertain and involve complex legal, scientific and factual questions. The success of our combined company in developing and commercializing biopharmaceutical products may depend, in part, on its ability to operate without infringing on the intellectual property rights of others and to prevent others from infringing on its intellectual property rights.
There has been substantial litigation regarding patents and other intellectual property rights in the biopharmaceutical industry. Our combined company may become party to patent litigation or proceedings at the U.S. Patent and Trademark Office or a foreign patent office to determine its patent rights with respect to third parties which may include competitors in the biopharmaceutical industry. Interference proceedings in the U.S. Patent and Trademark Office or opposition proceedings in a foreign patent office may be necessary to establish which party was the first to discover such intellectual property. Our combined company may become involved in patent litigation against third parties to enforce its patent rights, to invalidate patents held by such third parties, or to defend against such claims. The cost to our combined company of any patent litigation or similar proceeding could be substantial, and it may absorb significant management time. Our combined company does not expect to maintain separate insurance to cover intellectual property infringement. The general liability insurance policy of our combined company may not cover infringement by it of the intellectual property rights of others, depending upon the circumstances. The aggregate coverage provided under Genome’s existing general liability insurance policy is $10,000,000. We do not currently intend to increase the amount of this insurance following completion of the merger, though our combined company will continue to evaluate the sufficiency of its coverage levels periodically. If an infringement litigation against our combined company is resolved unfavorably, our combined company may be enjoined from manufacturing or selling certain of its products or services without a license from a third party. Our combined company may not be able to obtain such a license on commercially acceptable terms, or at all.
Our combined company may not be able to obtain meaningful patent protection for discoveries under its government contracts.
Under the government grants and contracts of our combined company, the government will have a statutory right to practice or have practiced any inventions developed under the government research contracts. In addition, under certain circumstances, such as inaction on the part of our combined company or its licensees to achieve practical application of the invention or a need to alleviate public health or safety concerns not reasonably satisfied by our combined company or its licensees, the government will have the right to grant to
41
Table of Contents
other parties licenses to any inventions first reduced to practice under the government grants and contracts. If the government grants such a license to a third party, the patent position of our combined company may be jeopardized. In addition, the government will have ownership rights in the data and discoveries derived from any materials furnished to our combined company by the government.
International patent protection is uncertain.
Patent law outside the United States is uncertain and is currently undergoing review and revision in many countries. Further, the laws of some foreign countries may not protect the intellectual property rights of our combined company to the same extent as U.S. laws. Our combined company may participate in opposition proceedings to determine the validity of its or its competitors’ foreign patents, which could result in substantial costs and diversion of its efforts.
The activities of our combined company will involve hazardous materials and may subject it to environmental liability.
The research and development activities of our combined company will involve the controlled use of hazardous and radioactive materials and biological waste. Our combined company will be subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these materials and certain waste products. Although Genome and Genesoft believe that their existing safety procedures for handling and disposing of these materials comply with legally prescribed standards, our combined company will not be able to completely eliminate the risk of accidental contamination or injury from these materials. In the event of an accident, our combined company could be held liable for damages or penalized with fines, and this liability could exceed its resources. We do not expect our combined company to maintain separate insurance to cover contamination or injuries relating to hazardous materials. Such liabilities may not be covered by Genome’s existing general liability insurance coverage, depending upon the circumstances. The aggregate coverage provided under our general liability insurance policy is $10,000,000. We do not currently intend to increase the amount of this insurance following completion of the merger, though our combined company will continue to evaluate the sufficiency of its coverage levels periodically.
If a successful product liability claim or series of claims is brought against our combined company for uninsured liabilities or in excess of insured liabilities, our combined company could be forced to pay substantial damage awards.
The use of any of our combined company’s product candidates in clinical trials, and the sale of any approved products, might expose our combined company to product liability claims. Genome currently maintains, and we expect that our combined company will continue to maintain, product liability insurance coverage in the amount of $10 million per occurrence and $10 million in the aggregate. Such insurance coverage might not protect our combined company against all of the claims to which our combined company might become subject. Our combined company might not be able to maintain adequate insurance coverage at a reasonable cost or in sufficient amounts or scope to protect us against potential losses. In the event a claim is brought against us, our combined company might be required to pay legal and other expenses to defend the claim, as well as uncovered damage awards resulting from a claim brought successfully against us. Furthermore, whether or not we are ultimately successful in defending any such claims, our combined company might be required to direct financial and managerial resources to such defense and adverse publicity could result, all of which could harm our combined company’s business.
42
Table of Contents
Risks Related to The Securities Market
Genome’s stock price is highly volatile and we expect the stock price of our combined company to remain highly volatile.
The market price of Genome stock has been highly volatile and we expect the stock price of our combined company to remain highly volatile due to the risks and uncertainties described in this section of the prospectus, as well as other factors, including:
| • | our ability to successfully launch and commercialize FACTIVE; |
| • | the revenues that we may derive from the sale of FACTIVE, as compared to analyst estimates; |
| • | the results of our clinical trials for Ramoplanin and additional indications for FACTIVE and the pace of our progress in those clinical trials; |
| • | our ability to license or develop other compounds for clinical development; |
| • | the timing of achievement of our development milestones and other payments under our strategic alliance agreements; |
| • | termination of, or an adverse development in, our strategic alliances; |
| • | conditions and publicity regarding the biopharmaceutical industry generally; |
| • | price and volume fluctuations in the stock market at large which do not relate to our operating performance; and |
| • | comments by securities analysts, or our failure to meet market expectations. |
Over the two-year period ended December 12, 2003, the closing price of Genome common stock as reported on the Nasdaq National Market ranged from a high of $7.18 to a low of $1.03. The stock market has from time to time experienced extreme price and volume fluctuations that are unrelated to the operating performance of particular companies. In the past, companies that have experienced volatility have sometimes been the subject of securities class action litigation. If litigation were instituted on this basis, it could result in substantial costs and a diversion of management’s attention and resources.
Genome has issued warrants to purchase 3,221,250 shares of common stock and the re-sale of the shares underlying these warrants could cause a dilution of the stockholders of our combined company.
On June 4, 2003, as part of the Amendment, Redemption and Exchange Agreement pertaining to Genome’s convertible notes held by two institutional investors, Genome issued warrants that are exercisable to purchase 511,250 shares of common stock at an exercise price of $3.53 per share (subject to future weighted average anti-dilution adjustments for issuances of securities and other adjustments), which are exercisable and expire on June 4, 2008. In connection with the issuance of these convertible notes, Genome is also obligated to issue to the placement agent warrants that are exercisable to purchase up to 100,000 shares of common stock at an exercise price of $15.00 per share, which warrants expire on March 5, 2005. On September 29, 2003 and October 15, 2003, Genome had closings of a private placement transaction in which Genome issued warrants to purchase 1,910,000 and 700,000 shares of common stock, respectively, at an exercise price of $3.48. The September 2003 warrants become exercisable on March 29, 2004 and remain exercisable until September 29, 2008. The October 2003 warrants become exercisable on April 15, 2004 and remain exercisable until October 15, 2008. The shares underlying all of these warrants are registered for re-sale. If any of the warrants are exercised, these shares could be sold into the market creating dilution of the ownership of the stockholders of our combined company at that time.
43
Table of Contents
Multiple factors beyond our control may cause fluctuations in the operating results of our combined company and may cause its business to suffer.
The revenues and results of operations of our combined company may fluctuate significantly, depending on a variety of factors, including the following:
| • | the pace of our commercial launch of FACTIVE; |
| • | the level of acceptance by physicians and third party payors of FACTIVE; |
| • | the progress of our clinical trials for FACTIVE, Ramoplanin and our other product candidates; |
| • | our success in concluding deals to acquire additional approved products and product candidates; |
| • | the introduction of new products and services by our competitors; |
| • | regulatory actions; and |
| • | expenses related to, and the results of, litigation and other proceedings relating to intellectual property rights. |
Our combined company will not be able to control many of these factors. In addition, if the revenues of our combined company in a particular period do not meet expectations, it may not be able to adjust its expenditures in that period, which could cause its business to suffer. We believe that period-to-period comparisons of the financial results of our combined company will not necessarily be meaningful. You should not rely on these comparisons as an indication of our future performance. If the operating results of our combined company in any future period fall below the expectations of securities analysts and investors, our stock price may fall, possibly by a significant amount.
Certain of the financial statements of Genome have been audited by Arthur Andersen LLP, and the ability to recover damages from Arthur Andersen may be limited.
Prior to June 24, 2002, Arthur Andersen LLP served as Genome’s independent public accountants. Genome’s inability to obtain the consent of Arthur Andersen to include its report on certain financial statements audited by Arthur Andersen and incorporated by reference in this prospectus may limit your recovery against Arthur Andersen. SEC rules require Genome to include or incorporate by reference in this prospectus certain historical financial statements for the years ended December 31, 2001 and 2000 that were audited by Arthur Andersen. As a result of the well-publicized events concerning Arthur Andersen, Genome has not been able to obtain the consent of Arthur Andersen to the inclusion of its audit report in this prospectus and will not be able to obtain Arthur Andersen’s consent in the future. The absence of this consent may limit any recovery to which you might be entitled against Arthur Andersen. It is also likely that these events concerning Arthur Andersen would adversely affect its ability to satisfy any claims Genome might have arising from its provision of auditing and other services to Genome.
44
Table of Contents
THE SPECIAL MEETING OF GENOME STOCKHOLDERS
Genome is furnishing this joint proxy statement/prospectus to all stockholders of record of Genome common stock in connection with the solicitation of proxies by the Genome board of directors for use at the special meeting of Genome stockholders to be held on , 2004, and at any adjournment or postponement of the special meeting.
Date, Time and Place of Meeting
The special meeting will be held at Ropes & Gray LLP, One International Place, 36th floor, Boston, Massachusetts on , 2004, at 10:00 a.m., local time.
Purpose of the Special Meeting
At the special meeting, and any adjournment or postponement thereof, Genome stockholders will be asked:
| 1. | To consider and vote on a proposal to approve (i) the issuance of 28,571,405 shares of Genome common stock pursuant to the Agreement and Plan of Merger and Reorganization, dated as of November 17, 2003, among Genome Therapeutics Corp., Guardian Acquisition, Inc., a wholly-owned subsidiary of Genome Therapeutics Corp., GeneSoft Pharmaceuticals, Inc., and Luke Evnin, as the representative of the Genesoft stockholders and (ii) the issuance of shares of Genome common stock upon the potential conversion of the convertible notes of Genome, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger. |
| 2. | To consider and vote on a proposal to approve the Amendment to the Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000. |
| 3. | To transact any other business as may properly come before the special meeting and any adjournment or postponement of the special meeting. |
A copy of the merger agreement is attached to this joint proxy statement/prospectus as Annex A. Genome stockholders are encouraged to read the merger agreement in its entirety and the other information contained in this joint proxy statement/prospectus carefully before deciding how to vote.
The Genome board of directors has fixed the close of business on , 2004 as the record date for determination of Genome stockholders entitled to notice of and to vote at the special meeting.
Votes Required for the Merger and Other Matters
Assuming a quorum is present at the special meeting, the affirmative vote of the holders of a majority of the votes of Genome common stock properly cast at the special meeting will be required to approve the issuance of a total of 28,571,405 shares of Genome common stock to the Genesoft security holders pursuant to the merger agreement and the issuance of Genome common stock upon the potential conversion of the Genome convertible notes, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger. The affirmative vote of a majority of the Genome shares outstanding as of the record date and entitled to vote on the proposal at the special meeting will be required to approve the Amendment to the Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000 shares. Holders of Genome common stock will be entitled to cast one vote per share of Genome common stock owned as of , 2004, the record date for the Genome special meeting of stockholders at which the proposals will be presented and voted upon.
45
Table of Contents
As of the close of business on the record date for the special meeting of Genome stockholders at which the proposals described in this joint proxy statement/prospectus will be presented and voted upon, directors and officers of Genome (and their respective affiliates) collectively owned approximately % of the outstanding shares of Genome common stock entitled to vote at the special meeting.
This does not include shares of Genome common stock issuable upon the exercise of presently exercisable options which these directors and officers beneficially own. If all of these stock options were exercised prior to the record date for the special meeting, the directors and executive officers of Genome (and their respective affiliates) would collectively beneficially own approximately % of the outstanding shares of Genome common stock entitled to vote at the special meeting.
Quorum, Abstentions and Broker Non-Votes
Consistent with Massachusetts law and under the Genome’s by-laws, a majority of the shares entitled to be cast on a particular matter, present in person or represented by proxy, constitutes a quorum as to such matter. Votes cast by proxy or in person at the special meeting will be counted by persons appointed by the company to act as inspector of elections for the special meeting. Genome has appointed EquiServe Trust Company N.A. to function as the inspector of elections of the special meeting. The inspector of elections will ascertain whether a quorum is present, tabulate votes and determine the voting results on all matters presented to Genome stockholders at the special meeting. If less than a quorum is present at the special meeting, the holders of Genome common stock representing a majority of the voting power present at the special meeting or the inspector of elections may adjourn the meeting. At any subsequent reconvening of the special meeting, all proxies will be voted in the same manner as the proxies would have been voted at the original convening of the special meeting, except for any proxies that have been effectively revoked or withdrawn prior to the subsequent reconvening of the special meeting.
Approval of proposal 1 described above will require a majority of the votes of Genome common stock properly cast upon this proposal at the special meeting. Approval of proposal 2 described above will require the affirmative vote of a majority of the shares of Genome common stock outstanding as of the record date and entitled to vote on the proposal at the special meeting. The election inspectors will count shares represented by proxies that reflect abstentions or “broker non-votes” (i.e., shares represented at the special meeting held by brokers or nominees as to which (i) instructions have not been received from the beneficial owners or persons entitled to vote and (ii) the broker or nominee does not have or does not exercise the discretionary voting power on a particular matter) only as shares that are present and entitled to vote on the matter for purposes of determining the presence of a quorum, but abstentions, broker non-votes and proxies that withhold authority to vote will not be counted as votes properly cast for purposes of determining the outcome of voting on any matter. In addition, the failure of a Genome stockholder to return a proxy will have the same effect as a vote against each of the proposals described above.Consequently, Genome stockholders are urged to return the enclosed proxy card marked to indicate their vote.
Solicitation of Proxies and Expenses
Genome will bear its own expenses in connection with the solicitation of proxies for the special meeting.
In addition to solicitation by mail, directors, officers and employees of Genome may solicit proxies from stockholders by telephone, facsimile, e-mail or in person. No additional compensation will be paid to these individuals for those services. Genome may retain outside agencies for the purpose of soliciting proxies, in which case Genome will pay the fees and expenses of those agencies. Record holders such as brokerage houses, nominees, fiduciaries and other custodians will be requested to forward soliciting materials to beneficial owners and to request authority for the exercise of proxies, and, upon the request of such record holders, they will be reimbursed for their reasonable expenses incurred in sending proxy materials to beneficial owners.
46
Table of Contents
Voting of Proxies at the Special Meeting and Revocation of Proxies
Genome requests that all holders of Genome common stock on the record date complete, date and sign the accompanying proxy card and promptly return it in the accompanying envelope or otherwise mail it to Genome. Brokers holding voting shares in “street name” may vote the shares only if the beneficial owner provides instructions on how to vote. Brokers will provide directions to beneficial owners on how to instruct your broker to vote the shares. Please note, however, that if the holder of record of your shares is your broker, bank or other nominee and you wish to vote at the special meeting, you must bring a letter from the broker, bank or other nominee confirming that you are the beneficial owner of the shares. All properly executed proxies that Genome receives prior to the vote at the special meeting, and that are not revoked, will be voted in accordance with the instructions indicated on the proxy card. If no direction is indicated on such proxies, such proxies will be voted in favor of the proposals (i) to approve the issuance of a total of 28,571,405 shares of Genome common stock pursuant to the merger agreement and the issuance of shares of Genome common stock upon the potential conversion of the convertible notes of Genome, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger and (ii) to approve the Amendment to Genome’s Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000 shares of common stock.
A Genome stockholder may revoke a previously submitted proxy at any time prior to its use by:
| • | delivering to the Secretary of Genome a later-dated signed notice of revocation; |
| • | delivering to the Secretary of Genome a later-dated, signed proxy (which will automatically replace any earlier dated proxy card that you returned); or |
| • | attending the special meeting and voting in person. |
Attendance at the special meeting does not in itself constitute the revocation of a previously submitted proxy.
If your shares are held in “street name,” your broker or nominee may permit you to vote by telephone or electronically. Please check your proxy card or contact your broker or nominee to determine whether either of these methods of voting is available to you.
Holders of Genome common stock do not have appraisal or dissenters’ rights under Massachusetts law in connection with the merger or any other matter presented to Genome stockholders at the special meeting.
Genome knows of no matters that will be presented for consideration at the special meeting other than those stated in this joint proxy statement/prospectus. However, if any other matters do properly come before the special meeting, the proxy holders will vote proxies in accordance with their best judgment regarding such matters.
47
Table of Contents
Recommendation of Genome’s Board of Directors
Since the merger is conditioned upon the approval of the matters set forth in each of the proposals described below, Genome recommends that its stockholders vote for each proposal.
Proposal 1. To consider and vote on a proposal to approve (i) the issuance of 28,571,405 shares of Genome common stock pursuant to the Agreement and Plan of Merger and Reorganization, dated as of November 17, 2003, among Genome Therapeutics Corp., Guardian Acquisition, Inc., a wholly-owned subsidiary of Genome Therapeutics Corp., GeneSoft Pharmaceuticals, Inc., and Luke Evnin, as the representative of the Genesoft stockholders and (ii) the issuance of shares of Genome common stock upon the potential conversion of the convertible notes of Genome, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger.
On November 10, 2003, Genome’s board of directors authorized Genome management to execute the merger agreement and consummate the transactions contemplated by the merger agreement and the related documents. For information about the factors considered by the Genome board of directors to approve the merger, see the section entitled “Consideration of the Merger by Genome’s Board of Directors” beginning on page69 of this joint proxy statement/prospectus.
In connection with the merger, Genome, Genesoft, and holders of Genesoft promissory notes issued during financing rounds in December 2002-January 2003 and April-May 2003 have entered into a note amendment and exchange agreement that restructures $22,309,647 of Genesoft notes. Upon the closing of the merger, these notes will be converted into new convertible promissory notes to be issued by Genome. The new notes will be convertible at any time prior to the maturity, at the option of the holder, into shares of Genome common stock at a 10% premium to the average trading price of Genome common stock for the five trading days immediately preceding the date of the closing of the merger. For a description of the note amendment and exchange agreement, see “The Merger—Other Material Agreements Relating to the Merger—Note Amendment and Exchange Agreement.”
After careful consideration, Genome’s board of directors determined that the merger is advisable and in the best interests of Genome’s stockholders. Accordingly, Genome’s board of directors approved the merger and the related transactions, and recommends that its stockholders vote FOR (i) the issuance of 28,571,405 shares of Genome common stock pursuant to the Agreement and Plan of Merger and Reorganization, dated as of November 17, 2003, among Genome Therapeutics Corp., Guardian Acquisition, Inc., a wholly-owned subsidiary of Genome Therapeutics Corp., GeneSoft Pharmaceuticals, Inc., and Luke Evnin, as the representative of the Genesoft stockholders and (ii) the issuance of shares of Genome common stock upon the potential conversion of the convertible notes of Genome, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger.
Proposal 2. To consider and vote on a proposal to approve the Amendment to the Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000.
On November 10, 2003, Genome’s board of directors voted, subject to approval by the stockholders, to amend Article 4 of the company’s Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000.
On , 2004, Genome had shares of its common stock outstanding, shares of common stock reserved for issuance upon exercise of outstanding stock options or for future awards under its equity incentive plans and shares of common stock reserved for issuance upon exercise of outstanding warrants.
48
Table of Contents
Genome needs additional shares of authorized common stock in order to issue 28,571,405 shares of its common stock pursuant to the merger agreement and to complete the sale of at least $32 million of its securities as a condition to the closing of the merger and to have sufficient shares reserved for potential conversion of the convertible notes of Genome, in an aggregate principal amount of $22,309,647, to be issued to the holders of Genesoft promissory notes in connection with the merger. In addition, Genome’s board of directors believes that it is desirable to have available a substantial additional number of authorized but unissued shares of Genome common stock which may be issued from time to time, without further action by the stockholders, to provide for stock splits or stock dividends, to be able to take advantage of acquisition opportunities, to meet future capital needs and for other general corporate purposes.
The issuance of additional authorized shares of Genome common stock may dilute the voting power and equity interest of present stockholders. It is not possible to predict in advance whether the issuance of additional shares will have a dilutive effect on earnings per share as it depends on the specific events associated with a particular transaction. Shares of authorized but unissued Genome common stock may be issued from time to time by Genome’s board of directors without further stockholder action unless such action is required by the law of The Commonwealth of Massachusetts, under which the company is incorporated, the company’s Articles of Organization, or the rules of the Nasdaq. Additional authorized but unissued shares of Genome common stock might be used in the context of a defense against or response to possible or threatened hostile takeovers. For example, such additional shares could be used to dilute the stock ownership of parties seeking to obtain control of the company, to increase the total amount of consideration necessary for a party to obtain control, or to increase the voting power of friendly third parties. These uses could have the effect of making it more difficult for a third party to remove incumbent management or to accomplish a given transaction, even if such actions would generally be beneficial to stockholders. Genome’s board of directors has concluded, however, that the advantages of the additional authorized shares outweigh any potential disadvantages.
Genome’s board of directors recommends that its stockholders vote FOR the approval of the Amendment to the Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000.
49
Table of Contents
THE SPECIAL MEETING OF GENESOFT STOCKHOLDERS
Genesoft is furnishing this joint proxy statement/prospectus to all stockholders of record of Genesoft common stock in connection with the solicitation of proxies by Genesoft’s board of directors for use at the special meeting of Genesoft stockholders to be held on , 2004, and at any adjournment or postponement of the special meeting. This joint proxy statement/prospectus also is being furnished to Genesoft stockholders as a prospectus for Genome Therapeutics common stock to be issued in connection with the merger.
Date, Time and Place of Meeting
The special meeting will be held at on , 2004, at a.m., local time. This joint proxy statement/prospectus and accompanying form of proxy card are first being mailed to Genesoft stockholders on or about , 2004.
Purpose of the Special Meeting
At the special meeting, and any adjournment or postponement thereof, Genesoft stockholders will be asked:
| 1. | To consider and vote on a proposal to adopt and approve the Agreement and Plan of Merger and Reorganization, dated as of November 17, 2003, among Genome Therapeutics Corp., a Massachusetts corporation, Guardian Acquisition, Inc., a Delaware corporation and a wholly-owned subsidiary of Genome Therapeutics Corp., GeneSoft Pharmaceuticals, Inc., a Delaware corporation, and Luke Evnin, as the representative of the Genesoft stockholders. |
| 2. | To consider and vote on a proposal to amend and restate Genesoft’s Seventh Amended and Restated Certificate of Incorporation to eliminate any authorized shares of Genesoft preferred stock if the merger is completed. |
| 3. | To transact any other business as may properly come before the special meeting and any adjournment or postponement of the special meeting. |
A copy of the merger agreement is attached to this joint proxy statement/prospectus as Annex A. Genesoft stockholders are encouraged to read the merger agreement in its entirety and the other information contained in this joint proxy statement/prospectus carefully before deciding how to vote.
Genesoft’s board of directors has fixed the close of business on , 2004 as the record date for determination of Genesoft stockholders entitled to notice of and to vote at the special meeting.
Approval of the merger agreement and the transactions contemplated by the merger agreement requires the affirmative vote of the holders of a majority of the shares of Genesoft common stock outstanding as of the record date. Approval of the amendment and restatement of the Seventh Amended and Restated Certificate of Incorporation of Genesoft requires the affirmative vote of the holders of a majority of the shares of Genesoft common stock outstanding as of the record date.
As of the close of business on the record date for the special meeting, shares of Genesoft common stock were outstanding, and held by approximately stockholders of record.
50
Table of Contents
As of the close of business on the record date for the special meeting of Genesoft stockholders at which the merger and the related transactions and the amendment and restatement of the Seventh Amended and Restated Certificate of Incorporation will be presented and voted upon, directors and officers of Genesoft (and their respective affiliates) collectively owned an aggregate of shares of Genesoft common stock (exclusive of any shares issuable upon the exercise of options or warrants or conversion of notes), or approximately �� % of the outstanding shares of Genesoft common stock entitled to vote at the special meeting on the merger and the related transactions and the restatement of the Seventh Amended and Restated Certificate of Incorporation. Genome has entered into voting agreements with the holders of approximately 7,881,334 shares of Genesoft common stock (exclusive of any shares issuable upon the exercise of options or warrants or conversion of notes), or approximately % of the outstanding shares of Genesoft common stock requiring such persons to vote FOR approval and adoption of the merger agreement.
A majority of all voting shares of Genesoft common stock issued and outstanding as of the record date, represented in person or by proxy, constitutes a quorum for the transaction of business at the special meeting. If a quorum is not present at the special meeting, it is expected that the meeting will be adjourned or postponed to solicit additional proxies. At any subsequent reconvening of the special meeting, all proxies will be voted in the same manner as the proxies would have been voted at the original convening of the special meeting, except for any proxies that have been effectively revoked or withdrawn prior to the subsequent reconvening of the special meeting.
If you submit a proxy that indicates an abstention from voting on all matters presented at the special meeting, your shares will be counted as present for the purpose of determining the existence of a quorum at the special meeting, but will not be voted on any matter presented at the special meeting. Consequently, your abstention will have the same effect as a vote against the proposal to adopt and approve the merger agreement. In addition, the failure of a Genesoft stockholder to return a proxy will have the effect of a vote against the proposal to adopt and approve the merger agreement.Consequently, Genesoft stockholders are urged to return the enclosed proxy card marked to indicate their vote.
Solicitation of Proxies and Expenses
Genesoft will bear its own expenses in connection with the solicitation of proxies for the special meeting.
Voting of Proxies at the Special Meeting and Revocation of Proxies
Genesoft requests that all holders of voting common stock on the record date complete, date and sign the accompanying proxy card and promptly return it in the accompanying envelope or otherwise mail it to Genesoft. All properly executed proxies that Genesoft receives prior to the vote at the special meeting, and that are not revoked, will be voted in accordance with the instructions indicated on the proxy card. If no direction is indicated on such proxies, such proxies will be voted in favor of adoption and approval of the merger agreement.
A Genesoft stockholder may revoke a previously submitted proxy at any time prior to its use by:
| • | delivering to the Secretary of Genesoft a later-dated signed notice of revocation; |
| • | delivering to the Secretary of Genesoft a later-dated, signed proxy (which will automatically replace any earlier dated proxy card that you returned); or |
| • | attending the special meeting and voting in person. |
Attendance at the special meeting does not in itself constitute the revocation of a previously submitted proxy.
51
Table of Contents
Any written notice of revocation should be sent to GeneSoft Pharmaceuticals, Inc., 7300 Shoreline Court, South San Francisco, California 94080, Attention: Secretary, or hand delivered to the Secretary of Genesoft at or before the taking of the vote at the special meeting.
Appraisal Rights Under Delaware General Corporation Law
If the merger is consummated, a holder of record of Genesoft stock who properly makes a demand for appraisal, as described below, will be entitled to have those shares appraised by the Delaware Court of Chancery under Section 262 of the Delaware corporation statute and to receive payment for the “fair value” of those shares instead of the consideration provided for in the merger agreement. In order to be eligible to receive this payment, however, a Genesoft stockholder must (1) continue to hold his or her shares through the time of the merger; (2) strictly comply with the procedures discussed under Section 262; and (3) not vote in favor of the merger agreement. This joint proxy statement/prospectus is being sent to all holders of record of Genesoft stock on the record date for the Genesoft special meeting and constitutes notice of the appraisal rights available to those holders under Section 262.
THE STATUTORY RIGHT OF APPRAISAL GRANTED BY SECTION 262 REQUIRES STRICT COMPLIANCE WITH THE PROCEDURES IN SECTION 262. FAILURE TO FOLLOW ANY OF THESE PROCEDURES MAY RESULT IN A TERMINATION OR WAIVER OF APPRAISAL RIGHTS UNDER SECTION 262. THE FOLLOWING IS A SUMMARY OF THE PRINCIPAL PROVISIONS OF SECTION 262.
The following summary is not a complete statement of Section 262 of the Delaware corporation statute, and is qualified in its entirety by reference to Section 262 which is incorporated herein by reference, together with any amendments to the laws that may be adopted after the date of this joint proxy statement/prospectus. A copy of Section 262 is attached as Annex E to this joint proxy statement/prospectus.
A holder of Genesoft stock who elects to exercise appraisal rights under Section 262 must deliver a written demand for appraisal of its shares of Genesoft prior to the vote on the merger agreement. A vote against the merger agreement does not constitute a demand for appraisal. The written demand must identify the stockholder of record and state the stockholder’s intention to demand appraisal of his or her shares. All demands should be delivered to Asha Rajagopal of Genesoft, 7300 Shoreline Court, South San Francisco, California 94080.
Only a holder of shares of Genesoft stock on the date of making a written demand for appraisal who continuously holds those shares through the time of the merger is entitled to seek appraisal. Demand for appraisal must be executed by or for the holder of record, fully and correctly, as that holder’s name appears on the holder’s stock certificates representing shares of Genesoft stock. If Genesoft stock is owned of record in a fiduciary capacity by a trustee, guardian or custodian, the demand should be made in that capacity. If Genesoft stock is owned of record by more than one person, as in a joint tenancy or tenancy in common, the demand should be made by or for all owners of record. An authorized agent, including one or more joint owners, may execute the demand for appraisal for a holder of record; that agent, however, must identify the record owner or owners and expressly disclose in the demand that the agent is acting as agent for the record owner or owners of the shares.
A record holder such as a broker who holds shares of Genesoft stock as a nominee for beneficial owners, some of whom desire to demand appraisal, must exercise appraisal rights on behalf of those beneficial owners with respect to the shares of Genesoft stock held for those beneficial owners. In that case, the written demand for appraisal should state the number of shares of Genesoft stock covered by it. Unless a demand for appraisal specifies a number of shares, the demand will be presumed to cover all shares of Genesoft stock held in the name of the record owner.
52
Table of Contents
BENEFICIAL OWNERS WHO ARE NOT RECORD OWNERS AND WHO INTEND TO EXERCISE APPRAISAL RIGHTS SHOULD INSTRUCT THE RECORD OWNER TO COMPLY WITH THE STATUTORY REQUIREMENTS WITH RESPECT TO THE EXERCISE OF APPRAISAL RIGHTS BEFORE THE DATE OF THE GENESOFT SPECIAL MEETING.
Within 10 days after the effective date of the merger, the surviving corporation to the merger is required to send notice of the approval of the merger agreement to all stockholders who are entitled to appraisal rights.
Within 120 days after the effective date of the merger, the surviving corporation or any stockholder who has complied with the requirements of Section 262 may file a petition in the Delaware Court of Chancery demanding a determination of the fair value of the shares of Genesoft stock held by all stockholders seeking appraisal. A dissenting stockholder must serve a copy of the petition on the surviving corporation. If no petition is filed by either the surviving corporation or any dissenting stockholder within the 120-day period, the rights of all dissenting stockholders to appraisal will cease. Stockholders seeking to exercise appraisal rights should not assume that the surviving corporation will file a petition with respect to the appraisal of the fair value of their shares or that the surviving corporation will initiate any negotiations with respect to the fair value of those shares. The surviving corporation is under no obligation to and has no present intention to take any action in this regard. Accordingly, stockholders who wish to seek appraisal of their shares should initiate all necessary action with respect to the perfection of their appraisal rights within the time periods and in the manner prescribed in Section 262.FAILURE TO FILE THE PETITION ON A TIMELY BASIS WILL CAUSE THE STOCKHOLDER’S RIGHT TO AN APPRAISAL TO CEASE.
Within 120 days after the effective date of the merger, any stockholder who has complied with subsections (a) and (d) of Section 262 is entitled, upon written request, to receive from the surviving corporation a statement setting forth the total number of shares of Genesoft stock not voted in favor of the merger agreement with respect to which demands for appraisal have been received by the surviving corporation and the number of holders of those shares. The statement must be mailed within 10 days after the surviving corporation has received the written request or within 10 days after the time for delivery of demands for appraisal under subsection (d) of Section 262 has expired, whichever is later.
If a petition for an appraisal is filed in a timely manner, at the hearing on the petition, the Delaware Court of Chancery will determine which stockholders are entitled to appraisal rights and will appraise the shares of Genesoft stock owned by those stockholders. The court will determine the fair value of those shares, exclusive of any element of value arising from the accomplishment or expectation of the merger, together with a fair rate of interest, to be paid, if any, upon the fair value.
Stockholders who consider seeking appraisal should consider that the fair value of their shares under Section 262 could be more than, the same as, or less than, the value of the consideration provided for in the merger agreement without the exercise of appraisal rights. The Court of Chancery may determine the cost of the appraisal proceeding and assess it against the parties as the Court deems equitable. Upon application of a dissenting stockholder, the Court may order that all or a portion of the expenses incurred by any dissenting stockholder in connection with the appraisal proceeding (including, without limitation, reasonable attorney’s fees and the fees and expenses of experts) be charged pro rata against the value of all shares of Genesoft stock entitled to appraisal. In the absence of a court determination or assessment, each party bears its own expenses.
Any stockholder who has demanded appraisal in compliance with Section 262 will not, after the merger, be entitled to vote his or its Genesoft stock for any purpose or receive payment of dividends or other distributions, if any, on the Genesoft stock, except for dividends or distributions, if any, payable to stockholders of record at a date prior to the merger.
A stockholder may withdraw a demand for appraisal and accept the consideration payable pursuant to the merger agreement at any time within 60 days after the merger. If an appraisal proceeding is properly instituted, it may not be dismissed as to any stockholder without the approval of the Delaware Court of Chancery, and any
53
Table of Contents
such approval may be conditioned on the Court of Chancery’s deeming the terms to be just. If, after the merger, a holder of Genesoft stock who had demanded appraisal for his shares fails to perfect or loses his right to appraisal, those shares will be treated under the merger agreement as if they were converted into the consideration payable pursuant to the merger agreement at the time of the merger.
IN VIEW OF THE COMPLEXITY OF THESE PROVISIONS OF THE DELAWARE CORPORATE LAW, ANY GENESOFT STOCKHOLDER WHO IS CONSIDERING EXERCISING APPRAISAL RIGHTS SHOULD CONSULT A LEGAL ADVISOR.
Dissenters’ Rights Under California Corporations Code
Holders of Genesoft capital stock who dissent and do not consent to the approval and adoption of the merger agreement may be entitled to certain dissenters’ rights under the California Corporations Code (“California Law”) in connection with the merger. Such holders who perfect their dissenters’ rights and follow certain procedures in the manner prescribed by Chapter 13 of the California Law will be entitled to have their shares converted into the right to receive from Genesoft such consideration as may be determined to be due pursuant to the California Law. Any stockholder who wishes to dissent and to seek the payment in cash of the “fair market value” of his, her or its shares, or who wishes to preserve his, her or its right to do so, should review this section carefully, since failure to comply with the procedures set forth in Chapter 13 of the California Law may result in the loss of such rights. The term “fair market value” will be determined as of November 17, 2003, the day before the announcement of the terms of the proposed merger, excluding any appreciation or depreciation resulting from the proposed merger, but adjusted for any stock split, or share dividend that becomes effective thereafter.
DISSENTERS’ RIGHTS GRANTED BY CHAPTER 13 OF THE CALIFORNIA LAW REQUIRE STRICT COMPLIANCE WITH THE PROCEDURES IN CHAPTER 13. FAILURE TO FOLLOW ANY OF THESE PROCEDURES MAY RESULT IN A TERMINATION OR WAIVER OF DISSENTERS’ RIGHTS UNDER CHAPTER 13. THE FOLLOWING IS A SUMMARY OF THE PRINCIPAL PROVISIONS OF CHAPTER 13.
The following summary is not a complete statement of Chapter 13 of the California Law, and is qualified in its entirety by reference to Chapter 13 which is incorporated herein by reference, together with any amendments to the laws that may be adopted after the date of this joint proxy statement/prospectus. A copy of Chapter 13 is attached as Annex F to this joint proxy statement/prospectus.
Each stockholder who elects to exercise dissenters’ rights must make written demand upon Genesoft for the purchase of such shares. The demand must be made no later than the date of the special meeting. The stockholder’s demand must state the number and class of shares held of record by the stockholder that the stockholder demands that Genesoft purchase, as well as a statement by the stockholder as to what such holder claims the fair market value of such share was as of November 17, 2003, the day prior to the announcement of the merger. The statement of fair market value constitutes an offer by the Genesoft stockholder to sell the shares at such price. Neither voting against, abstaining from voting nor failing to vote on the merger agreement constitutes such written demand. All demands should be delivered to Asha Rajagopal of Genesoft, 7300 Shoreline Court, South San Francisco, California 94080.
Within 10 days after stockholder approval of the merger agreement, Genesoft is required to send notice of the approval of the merger agreement to all stockholders who have demanded dissenters’ rights. The notice must be accompanied by a copy of Sections 1300-1304 of the California Law. The notice shall also state the price determined by Genesoft to be the fair market value of the Genesoft stock with respect to which dissenters’ rights are properly exercised under Chapter 13 of the California Law and a brief description of the procedure to be followed by a stockholder who elects to dissent.
Within the same 30-day period following the mailing of the notice, the dissenting stockholder must submit to Genesoft for endorsement certificates for any shares that the Genesoft stockholder demands Genesoft
54
Table of Contents
purchase. If Genesoft and the stockholder agree upon the price of the dissenting shares, the dissenting stockholder is entitled to the agreed price with interest at the legal rate on judgments from the date of such agreement. Payment must be made within 30 days of the later of the date of the agreement between the Genesoft stockholder and Genesoft or the date the contractual conditions to the merger are satisfied or waived.
If Genesoft and the stockholder cannot agree as to the fair market value or as to the fact that such shares are dissenting shares, such stockholder may file within six months of the date of mailing of the notice a complaint with the superior court of the proper county demanding judicial determination of such matters. Genesoft will then be required to make any payments in accordance with such judicial determination. If the complaint is not filed within the specified six-month period, the stockholder’s rights as a dissenter are lost.
Genesoft dissenting shares lose their status as such if (i) Genesoft abandons the merger, (ii) the stockholder and Genesoft do not agree as to the fair market value of such shares and a complaint is not filed within six months of the date the Genesoft notice was mailed; or (iii) the dissenting stockholder withdraws, with the consent of Genesoft, his or her demand for purchase of such shares.
Genesoft knows of no matters that will be presented for consideration at the special meeting other than those stated in this joint proxy statement/prospectus. However, if any other matters do properly come before the special meeting, the proxy holders will vote proxies in accordance with their best judgment regarding such matters.
Recommendation of Genesoft’s Board of Directors
Genesoft’s board of directors has determined that the merger is advisable, in the best interests of Genesoft stockholders and on terms that are fair to the stockholders of Genesoft.Accordingly, Genesoft’s board of directors has approved the merger and the related transactions and recommends that stockholders vote FOR adoption and approval of the merger agreement and related transactions. In considering such recommendation, Genesoft stockholders should be aware that some Genesoft directors and officers have interests in the merger that are different from, or in addition to, those of Genesoft stockholders. For more information about these interests, see the section entitled “The Merger and Related Transactions—Interests of Directors and Executive Officers of Genesoft in the Merger” beginning on page79 of this joint proxy statement/prospectus.
Genesoft’s board of directors also recommends that Genesoft stockholders vote FOR the approval of the amendment and restatement of the Seventh Amended and Restated Certificate of Incorporation to eliminate any authorized shares of Genesoft preferred stock if the merger is completed.
The matters to be considered at the special meeting are of great importance to the stockholders of Genesoft. Accordingly, Genesoft stockholders are urged to read and carefully consider the information presented in this joint proxy statement/prospectus, and to complete, date, sign and promptly return the enclosed proxy in the enclosed postage-prepaid envelope.
Genesoft stockholders should not send any stock certificates with their proxy cards. A transmittal form with instructions for the surrender of Genesoft common stock certificates will be mailed to you promptly following completion of the merger. For more information regarding the procedures for exchanging Genesoft stock certificates for Genome Therapeutics stock certificates, see the section entitled “The Merger and Related Transactions—The Merger Agreement—Exchange of Genesoft Certificates” beginning on page 82 of this joint proxy statement/prospectus.
55
Table of Contents
THE MERGER AND RELATED TRANSACTIONS
The following is a description of the material aspects of the merger and related transactions, including the merger agreement and certain other agreements entered into in connection with the merger agreement. While we believe that the following description covers the material terms of the merger, the merger and the related transactions and agreements, the description may not contain all of the information that is important to you. You should carefully read this entire document and the other documents we refer to for a more complete understanding of the merger and the related transactions. In particular, the following summary of the merger agreement is not complete and is qualified in its entirety by reference to the copy of the merger agreement attached to this joint proxy statement/prospectus as Annex A and incorporated by reference herein. You should read the merger agreement carefully and in its entirety for a complete understanding of the terms of the merger and related transactions.
Since 2001, one of Genome’s principal strategies has been the in-licensing and/or acquisition of late stage development product candidates, approved products or marketed products. During the period from early 2001 through the present, numerous potential opportunities have been identified and evaluated by Genome. In October 2001, Genome successfully in-licensed Ramoplanin, a novel antibiotic, to supplement its product portfolio. During 2002, one of the product candidates Genome evaluated was an anti-infective, FACTIVE, discovered by LG Life Sciences, Ltd., a South Korean company. Genome investigated this opportunity, but did not obtain rights to the product.
Genesoft was founded in 1997 and is focused on the discovery and development of anti-infective products. During 2002, Genesoft identified an opportunity to license FACTIVE from LG Life Sciences and it subsequently entered into an agreement with LG Life Sciences to license FACTIVE on October 22, 2002.
In December 2002, Genesoft raised capital through the sale of promissory notes in order to fund its operations and manage the continued clinical development of FACTIVE.
At a regular meeting of Genesoft’s board of directors on January 21, 2003, the board discussed a variety of strategic options available to Genesoft. The board also discussed and approved engaging a financial advisor to explore and advise on various strategic alternatives.
On February 21, 2003, Genesoft retained Merrill Lynch, Pierce, Fenner & Smith Incorporated to act as its financial advisor in connection with any strategic transaction. In the spring of 2003, Merrill Lynch, on behalf of Genesoft, began a process of soliciting interest from numerous specialty pharmaceutical, major pharmaceutical and biotechnology companies in entering into a strategic transaction with Genesoft. As part of this process, which continued through the fall of 2003, Merrill Lynch contacted a total of nearly 60 companies.
On March 27, 2003, Genesoft’s board of directors expanded the scope and authority of a previously constituted transaction committee, composed of David Singer, the Chairman and CEO of Genesoft, and directors William Rutter and Vernon Loucks, to include the authority to review and advise on business combination transactions.
After continued clinical development, the FDA approved FACTIVE for sale in the U.S. on April 4, 2003.
In April 2003, Genesoft raised additional funds through the sale of promissory notes to replenish its depleted cash assets and to provide for Genesoft’s continued operations.
During the summer of 2003, Genesoft entered into preliminary discussions with various companies, including Genome, regarding a possible business combination transaction, asset sale or co-promotion arrangement involving FACTIVE. Genesoft’s board of directors met several times during July and August for
56
Table of Contents
updates on the status of discussions with other parties and to consider alternative strategies for Genesoft, including whether to raise capital in order to commercialize FACTIVE as an independent company. The transaction committee of Genesoft’s board of directors, now composed of Messrs. Singer, Rutter, Loucks and Dr. Luke Evnin of MPM Capital and a director of Genesoft, also met regularly, together with legal and financial advisors, to discuss the merits of possible combinations or other transactions with third parties.
On July 27, 2003, Steven Rauscher, President, CEO, and Chairman of Genome, was contacted via telephone by Dr. Evnin regarding a potential strategic transaction with Genesoft.
On July 29, 2003, Mr. Rauscher held a further discussion via telephone with Dr. Evnin, concerning a potential strategic transaction between Genome and Genesoft. Dr. Evnin advised Mr. Rauscher that Merrill Lynch had been retained to conduct a process to explore Genesoft’s strategic alternatives.
On July 30, 2003, Genome and Genesoft entered into a mutual non-disclosure agreement providing for the safeguarding of confidential information that the parties might share with one another during their discussions. Also on July 30, 2003, Mr. Rauscher and Mr. Singer conducted a phone conversation and agreed that a logical next step in the initial review processes of both parties was to conduct a joint meeting during which each party would describe its existing programs and potential synergies between the two organizations would be explored.
On August 6, 2003, a team from Genesoft consisting of Dr. Evnin, Gary Patou, President, Tom Feldman, Vice President Sales and Marketing, Glenn Tillotson, Director, Scientific Affairs, and Christine Nash, FACTIVE Product Manager met with members of Genome’s senior management team, including Mr. Rauscher, Stephen Cohen, Senior Vice President and Chief Financial Officer, and Martin Williams, Senior Vice President, Corporate Development and Marketing, at its Waltham, Massachusetts headquarters. Genesoft’s team presented an overview of their company, as well as descriptions of their research and development programs, including the development and commercialization program for FACTIVE. The Genome team also provided an overview of their company.
On August 12, 2003, Mr. Rauscher and Dr. Evnin conducted a follow-up call and reviewed the preliminary discussions that had occurred between the parties. Mr. Rauscher informed Dr. Evnin that he planned to discuss Genome’s preliminary review of a strategic transaction with Genesoft at Genome’s next board meeting, scheduled for August 21, 2003.
On August 21, 2003, Genome held its regularly scheduled board of directors meeting. As part of the planned agenda, Mr. Rauscher presented an update on overall corporate development activities, including management’s preliminary findings on a potential strategic transaction with Genesoft. The board voted to authorize Mr. Rauscher and other senior executives of Genome to pursue further discussions and negotiations with Genesoft regarding a potential transaction, subject to the board’s approval of any terms or agreements. The board also voted to appoint a transaction committee consisting of directors Robert Hennessey, David Stone and Steven Rauscher, which would be authorized and empowered to direct and assist Genome in its evaluation of a possible transaction with Genesoft.
On August 21, 2003, Mr. Rauscher called Mr. Singer to inform him that Genome’s board had authorized further discussions regarding a potential strategic transaction between the two companies. Mr. Rauscher and Mr. Singer agreed that a logical next step would be for Genome to begin the due diligence review of Genesoft’s documentation relating to its FACTIVE and other product programs and to review Genesoft’s finances.
On August 25, 2003, Genome sent a team comprised of Messrs. Rauscher, Cohen, Williams and other members of Genome’s business development staff to California to visit Genesoft’s facility in South San Francisco and to commence the due diligence review of documentation regarding Genesoft’s product programs and finances at the offices of Merrill Lynch. The members of the Genome team were assisted by members of
57
Table of Contents
Ropes & Gray LLP, Genome’s outside counsel, in their due diligence activities. The Genome team along with their legal advisors continued their due diligence review at the offices of Merrill Lynch on August 26, focusing on documents necessary to provide a preliminary overview of Genesoft’s contractual commitments, financial statements and condition, capital structure and funding requirements.
On August 27, 2003, Genome met with members of Genesoft management led by Messrs. Singer and Patou at the Genesoft facility in South San Francisco. Messrs. Rauscher, Cohen and Williams made presentations focusing primarily on Genome’s Ramoplanin program, as well as the finances of Genome. Messrs. Singer, Patou and other members of Genesoft management made presentations regarding the status of their FACTIVE program and their other ongoing discovery programs and assets.
On August 28, 2003, the Genome and Genesoft teams reconvened at the Genesoft facility, with Dr. Evnin and representatives of Merrill Lynch also present. Both the Genome and Genesoft teams gave further presentations regarding Ramoplanin and FACTIVE, respectively, and the parties engaged in preliminary discussions regarding the synergies of a combined entity and its preliminary financial profile.
On August 29 and 30, 2003, Mr. Rauscher engaged in several telephone discussions with Messrs. Evnin, Singer and Patou summarizing the meetings of the previous three days and discussing potential synergies and areas where both parties needed to engage in further diligence.
Through the month of August, Genesoft, with the assistance of Merrill Lynch, continued to explore other possible business combinations, asset sales or co-promotion arrangements involving FACTIVE with various other parties.
On September 9, 2003, Mr. Rauscher held a conference telephone call with Messrs. Evnin, Singer and Patou. The parties discussed potential synergies that could be realized by a combined entity and identified open diligence issues that required further review by each party. Based on the discussion, Mr. Rauscher suggested that the next step would be to update the transaction committee of his board of directors and get further direction.
On September 11, 2003, a special meeting of the transaction committee of Genome was held by telephone conference call. The participants in the call included Messrs. Cohen, Williams and other members of management and Messrs. Hennessey, Stone and Rauscher, the members of the transaction committee. Mr. Rauscher outlined the discussions that had taken place between the parties, the potential synergies presented by a potential merger of the two companies, the potential competition for the opportunity and the numerous diligence and other issues that needed further review. The transaction committee recommended continuation of discussions with Genesoft and authorized management to submit a letter setting forth Genome’s interest in a potential merger transaction with Genesoft. Following the meeting, Genome submitted this letter to Genesoft.
On September 12, 2003, Mr. Rauscher and Mr. Singer discussed the letter submitted by Genome and the open diligence issues on both sides.
On September 17, 2003, the board of directors of Genome held an informal information conference call during which a number of topics were discussed, including the status of discussions with Genesoft.
On September 17, 2003, the board of directors of Genesoft met with and was updated by its management team and Merrill Lynch on the status of preliminary discussions with various parties. The board also reviewed Genesoft’s existing operations and financial results and condition.
On September 18, 2003, Mr. Rauscher and Mr. Singer had discussions by telephone regarding Genome’s due diligence on the manufacturing of the active pharmaceutical ingredient for FACTIVE.
58
Table of Contents
On September 19, 2003, Mr. Rauscher had further due diligence discussions with Dr. Patou concerning the manufacturing plan for FACTIVE and related FDA matters. Genome’s legal advisors sent preliminary comments on a form of merger agreement to the legal advisors of Genesoft.
On September 22, 2003 and September 23, 2004, Mr. Rauscher had telephone conversations with both Mr. Singer and Dr. Patou concerning potential structures of a combined entity, including the valuation of Genesoft, board composition and future financing needs of a combined company.
Between September 22, 2003 and October 1, 2003, each of the parties continued their due diligence reviews of the other party.
Through the month of September, Genesoft, with the assistance of Merrill Lynch, continued to explore possible other business combinations, asset sales or co-promotion arrangements involving FACTIVE with various other parties.
On October 1, 2003, Mr. Singer and representatives of Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP, Genesoft’s outside counsel, and Merrill Lynch met with Messrs. Rauscher, Cohen and Williams, other members of Genome management and Genome’s legal advisor at Genome’s headquarters in Waltham, Massachusetts. The parties conducted preliminary discussions concerning open due diligence matters for both parties, the valuations of Genesoft and Genome and the financial needs of the combined company.
On October 6, 2003, Messrs. Rauscher, Cohen and Williams and representatives from their legal advisor traveled to Genesoft’s headquarters in South San Francisco to conduct additional due diligence. From October 6 through October 10, the Genome team conducted due diligence review of documents and discussions with Genesoft management at Genesoft’s headquarters. In addition, during this period the parties held multiple discussions regarding the restructuring of Genesoft’s outstanding promissory notes, the manufacturing of FACTIVE and the cash needs of a combined company moving forward. The parties and their respective legal advisors also continued negotiation of the terms of a merger agreement and related ancillary documents.
From October 7 through October 14, 2003, representatives of each of Genome and Genesoft and each of the parties’ legal advisors continued their due diligence reviews and conducted numerous telephone conferences to discuss diligence matters and the terms and conditions of the merger agreement and related matters.
On October 15, 2003, the Genome board of directors held a regularly scheduled informational call, and among other topics, Mr. Rauscher updated the board on the status of ongoing discussion with Genesoft regarding a potential merger transaction. While numerous outstanding diligence and economic issues remained to be discussed and resolved, the board concurred that discussions should continue.
On October 15, 2003, Mr. Rauscher called Mr. Singer to inform him that, following Genome’s board call, Genome intended to continue to move forward with discussions regarding a potential merger transaction.
On October 15, 2003, representatives of Genesoft’s legal advisor visited the headquarters of Genome in Waltham, Massachusetts to conduct a due diligence review of Genome documents.
On October 16 and 17, 2003, members of Genesoft management joined Genesoft’s legal advisors in their due diligence review of documents, product programs and finances at Genome’s headquarters.
From October 18, 2003 through October 28, 2003, representatives of each of Genome and Genesoft and each of the parties’ legal advisors continued their due diligence reviews and conducted numerous telephone conferences to discuss diligence matters, the terms and conditions of the merger agreement, the valuation of Genesoft, the restructuring of Genesoft’s convertible notes, Genesoft’s cash needs between the signing of a merger agreement and the potential closing date, the budget of a combined company moving forward and other related matters.
59
Table of Contents
On October 29, 2003, Dr. Patou visited Genome’s headquarters and met with a number of company employees, including Mr. Rauscher, Mr. Cohen, Mr. Williams, Dr. Richard Labaudiniere, Senior Vice President of Research and Development, and members of Genome’s clinical development team. The parties discussed the roles and responsibilities for a combined management team, as well as clinical development matters.
On October 30, 2003, Mr. Rauscher and Mr. Singer discussed the proposed valuations of Genesoft and Genome and the status of the potential transaction in preparation for their respective scheduled board of directors meetings. They also engaged in discussions regarding the potential financing required to fund a combined company going forward.
During the month of October, Genesoft and Merrill Lynch continued to explore possible other business combinations, asset sales or co-promotion arrangements involving FACTIVE with various other parties.
On November 3, 2003, Mr. Rauscher and Mr. Singer discussed via telephone potential employment agreements for members of Genesoft management and initial terms for restructuring of Genesoft’s debt, as well as other terms and conditions for a potential merger transaction.
On November 6, 2003, Genome held a special meeting of its board of directors. During this meeting, Mr. Rauscher, Mr. Cohen and Mr. Williams outlined the status of discussions with Genesoft, outstanding diligence matters and the financial projections of a combined company. A representative from Ropes & Gray LLP summarized for the board the terms and conditions of the proposed merger agreement and related documents and the remaining outstanding issues. Mr. Rauscher discussed the potential synergies of combining the two companies along with the risks of combining the two companies. The board authorized Genome’s management to continue discussions and negotiations with Genesoft.
On November 7, 2003, Genesoft held a special meeting of its board of directors. During this meeting, Mr. Singer provided an update of current developments at Genesoft. Messrs. Singer and Patou presented a description of Genome and its product programs, as well as a summary of a combined company. Representatives from Merrill Lynch reviewed for the board their preliminary analysis of the proposed merger and related transactions. A representative from Genesoft’s legal counsel summarized for the board in detail the preliminary terms and conditions of the proposed merger agreement and related documents, the remaining outstanding issues and a tentative timeline to signing and closing the transaction.
On November 8 and 9, 2003, Messrs. Rauscher, Cohen and Williams and representatives of Ropes & Gray LLP held telephone conference calls with Mr. Singer and representatives of Gunderson Dettmer to discuss the terms and conditions of the merger agreement, the documents relating to the refinancing of Genesoft’s promissory notes, the documents relating to the proposed bridge loan to Genesoft as well as other open diligence and economic issues.
On November 10, 2003, Genome held a special meeting of its board of directors at which all of its directors were present either in person or by telephone. Harris Nesbitt Corp., Genome’s financial advisor, reviewed with the board its financial analysis of the proposed merger and related transactions with Genesoft and delivered to the board its opinion, which was subsequently confirmed in writing, that based upon and subject to the factors and assumptions set forth in the opinion, as of November 10, 2003, the aggregate stock consideration to be issued by Genome to Genesoft’s security holders in the merger and the related transactions was fair, from a financial point of view, to Genome. Mr. Rauscher and a representative of Ropes & Gray LLP updated the board on the latest negotiations regarding the terms and conditions of the merger agreement and the related open business issues. The board held an executive session and full discussion of the proposed transaction followed. Subject to resolution of the outstanding issues in the merger agreement and related documents in a manner consistent with the provisions explained to the board and the receipt of required third party consents, the board authorized Genome management to execute the merger agreement and the related documents.
60
Table of Contents
On November 10, 2003, Genesoft held a special meeting of its board of directors. During this meeting, Mr. Singer and legal counsel of Genesoft provided an update of the proposed merger and related transactions and the remaining outstanding issues. Mr. Singer and legal counsel also responded to a number of questions and comments raised by members of the board regarding the transaction.
On November 11, 2003, at a special meeting of Genesoft’s board of directors, Mr. Singer and legal counsel to Genesoft provided a further update on the proposed merger and related transactions, including a summary of unresolved issues. Representatives from Merrill Lynch gave a detailed presentation on the economic and financial terms and structure of the proposed transaction and a financial analysis of a combined company. A full discussion among the board of the proposed transactions ensued.
From November 11 through November 15, 2003, numerous phone calls between Genome and Genesoft and their respective legal advisors took place to negotiate the remaining issues in the merger agreement and to obtain certain required third party consents to the execution of the merger agreement.
On November 12, 2003, Genesoft held a special meeting of its board of directors. Merrill Lynch reviewed with the board its financial analysis of the proposed merger and related transactions with Genome and delivered to the board its opinion, which was subsequently confirmed in writing, that as of November 12, 2003 and subject to the factors and assumptions set forth in the opinion, the common exchange ratio was fair, from a financial point of view, to holders of shares of Genesoft common stock, other than Genome and its affiliates. A full discussion among the board of the proposed transactions followed. Subject to the appropriate resolution of the outstanding issues and the obtaining of third party consents, the board approved the merger and authorized Genesoft’s management to execute the merger agreement and the related documents.
On November 13, 2003, Genome held a special meeting of its board of directors to update them on the negotiations regarding the remaining issues in the merger and the related transactional documents. Mr. Rauscher updated the board on issues regarding employment matters for executives of Genesoft. A representative of Ropes & Gray LLP reviewed the matters still subject to negotiation in the merger agreement with the board and discussed the status of third party consents required for the execution of the merger agreement. The board held an executive session and full discussion of the proposed transaction followed. The board confirmed its authorization for Genome’s management to execute the merger agreement and the related documents, subject to the resolution of the outstanding issues in a manner consistent with the terms outlined to the board.
On November 16, 2003, Mr. Singer traveled to Genome’s headquarters in Waltham to work directly with Messrs. Rauscher, Cohen and Williams to resolve the remaining outstanding issues and to help obtain the required third party consents to the signing of the merger agreement. These discussions and efforts continued through the 17th of November.
On November 17, 2003, after the close of business, Genome and Genesoft executed the merger agreement and related agreements.
On November 18, 2003, before the opening of business, Genome and Genesoft issued a joint press release announcing the execution of the merger agreement.
Consideration of the Merger by Genesoft’s Board of Directors
In reaching its decision to approve the merger agreement and the transactions contemplated by the merger agreement, Genesoft’s board of directors consulted with management of Genesoft, as well as its financial and legal advisors, and considered a number of potential benefits and factors pertaining to the merger, including the following:
| • | the judgment, advice and analysis of Genesoft’s management with respect to the potential strategic, financial and operational benefits of the proposed transaction, including management’s favorable recommendation of the transaction based in part on the business, product, financial and legal due diligence investigation performed with respect to Genome; |
61
Table of Contents
| • | the strategic fit of Genesoft’s and Genome’s respective core anti-infective product portfolios; |
| • | the increased number of potential products of the combined company increases the opportunity for commercial success while decreasing the potential adverse impact that could result from failure of individual products to perform successfully; |
| • | the proposed merger would substantially increase Genesoft’s capital resources by providing Genesoft with immediate access to Genome’s existing cash assets as well as the proceeds of a financing to raise a minimum of $32 million, which is a condition to the closing of the merger, unless waived by both parties; |
| • | the difficulty in (and the related cost of) obtaining additional financing for Genesoft as a stand-alone enterprise given the current position of the company and the existing financing environment for private companies similar to Genesoft; |
| • | the combined company is expected to have greater leverage in obtaining financing for its operations; |
| • | the proposed merger will exchange illiquid shares of privately held Genesoft for shares in an entity that is publicly traded on Nasdaq; |
| • | the fact that the proposed transaction provides for the restructuring of Genesoft’s convertible notes into shares of Genome common stock and new convertible notes of Genome and that, without the proposed transaction, certain of the notes would have been due in December 2003; |
| • | the fact that Genome was willing to provide Genesoft with the interim financing described under “The Merger and Related Transactions – Other Material Agreements Relating to the Merger – Bridge Loan”; |
| • | the exchange ratio in the proposed merger and the resulting ownership interest of Genome by former stockholders of Genesoft (including the ownership interest after giving effect to a financing to raise a minimum of $32 million, which is a condition to the closing of the merger, unless waived by both parties); |
| • | Genesoft’s management’s assessment of possible strategic alternatives, including remaining a separate company, entering into joint ventures, co-promotion arrangements or combining with a third party; |
| • | the fact that Genesoft’s management believed that a more attractive offer would not be available based on previous discussions with other companies and the solicitation of interest from numerous third parties by Genesoft’s financial advisor over the prior months; |
| • | the opinion of Merrill Lynch to the effect that, as of November 12, 2003, and subject to the factors and assumptions set forth in the opinion, the common exchange ratio was fair, from a financial point of view, to the holders of Genesoft common stock, other than Genome and its affiliates; and |
| • | the other terms and attributes of the merger, including its expected tax treatment as a tax-free reorganization for United States federal income tax purposes. |
Genesoft’s board of directors also identified a number of potential negative factors pertaining to the merger, including the following:
| • | the risk that the transaction might not be completed in a timely matter or at all and, if not completed, the difficulty as a stand-alone company in being able to raise sufficient funds to meet its obligations; |
| • | the risk that the potential benefits of the merger may not be realized; |
| • | the risk of management and employee disruption associated with the merger, including the risk that key personnel may decide not to continue employment with Genome after the merger; |
| • | terms of the merger agreement and related agreements that limit the ability of Genesoft and its representatives to pursue alternative transactions; and |
| • | the other risks described above under “Risk Factors”. |
62
Table of Contents
Opinion of Financial Advisor to Genesoft
Genesoft retained Merrill Lynch, Pierce, Fenner & Smith Incorporated to act as its financial advisor in connection with the merger. As part of its engagement, Genesoft requested that Merrill Lynch evaluate the fairness of the common exchange ratio, from a financial point of view, to the holders of Genesoft shares (other than Genome and its affiliates). On November 12, 2003, Merrill Lynch delivered its oral opinion to the Genesoft board of directors, subsequently confirmed in writing, that, as of that date and subject to the factors and assumptions set forth in the opinion, the common exchange ratio was fair, from a financial point of view, to the holders of Genesoft shares, other than Genome and its affiliates.
The full text of Merrill Lynch’s opinion, dated November 12, 2003, which sets forth the assumptions made, matters considered, and qualifications and limitations on the review undertaken by Merrill Lynch, is included in this joint proxy statement/prospectus as Annex D and is incorporated into this joint proxy statement/prospectus by reference. The summary of the Merrill Lynch fairness opinion set forth in this joint proxy statement/prospectus is qualified in its entirety by reference to the full text of the opinion. Genesoft stockholders are urged to read such opinion carefully and in its entirety.
Merrill Lynch’s opinion was prepared for, and addressed to, the Genesoft board of directors for the information of such directors and is directed only to the fairness, from a financial point of view, of the common exchange ratio to the holders of Genesoft shares (other than Genome and its affiliates). The Merrill Lynch opinion does not address any other aspect of the merger, including the merits of the underlying decision by Genesoft to engage in the merger, and does not constitute a recommendation to any stockholder as to how such stockholder should vote on the proposed merger or any matter related thereto. In addition, the Merrill Lynch opinion does not address, the fairness to, or any other consideration of, the holders of any class of securities, creditors or other constituencies of Genesoft, other than holders of Genesoft shares, and the Merrill Lynch opinion does not address the fairness to any person of the terms of the Note Amendment and Exchange Agreement, substantially in the form of the draft dated November 12, 2003, among Genesoft, Genome and the holders listed on the signature page thereto or any other financing arrangement to be entered into by Genesoft or Genome in connection with the merger. Merrill Lynch did not express any opinion as to the prices at which Genome shares will trade following the announcement or consummation of the merger.
In arriving at its opinion, Merrill Lynch, among other things:
| (1) | Reviewed certain publicly available business and financial information relating to Genesoft and Genome that Merrill Lynch deemed to be relevant; |
| (2) | Reviewed certain information, including financial forecasts, relating to the business, earnings, cash flow, cash and other resource requirements, assets, liabilities and financing and other prospects of Genesoft and Genome furnished to Merrill Lynch by Genesoft and Genome, respectively; |
| (3) | Conducted discussions with certain members of senior management and representatives of Genesoft made available to Merrill Lynch concerning the matters described in clauses (1) and (2) above; |
| (4) | Reviewed the market price of Genome shares and compared them with those of certain publicly traded companies that Merrill Lynch deemed to be relevant; |
| (5) | Reviewed the valuation multiples for Genesoft shares and Genome shares and compared them with those of certain publicly traded companies that Merrill Lynch deemed to be relevant; |
| (6) | Compared the proposed financial terms of the merger with the financial terms of certain other transactions that Merrill Lynch deemed to be relevant; |
| (7) | Participated in certain discussions and negotiations among representatives of Genesoft and Genome and their financial and legal advisors; |
| (8) | Reviewed a draft dated November 12, 2003 of the merger agreement; and |
63
Table of Contents
| (9) | Reviewed such other financial studies and analyses and took into account such other matters as Merrill Lynch deemed necessary, including Merrill Lynch’s assessment of general economic, market and monetary conditions. |
In preparing its opinion, Merrill Lynch has assumed and relied on the accuracy and completeness of all information supplied or otherwise made available to Merrill Lynch, discussed with or reviewed by or for Merrill Lynch, or publicly available, and has not assumed any responsibility for independently verifying such information or undertaken an independent evaluation or appraisal of any of the assets or liabilities of Genesoft or Genome or been furnished with any such evaluation or appraisal, nor has Merrill Lynch evaluated the solvency or fair value of Genesoft or Genome under any state or federal laws relating to bankruptcy, insolvency or similar matters. In addition, Merrill Lynch has not assumed any obligation to conduct any physical inspection of the properties or facilities of Genesoft or Genome. With respect to the financial forecast information furnished to or discussed with Merrill Lynch by Genesoft or Genome, Merrill Lynch has assumed that they have been reasonably prepared and reflect the best currently available estimates and judgment of Genesoft’s or Genome’s management as to the expected future financial performance of Genesoft or Genome, as the case may be. Merrill Lynch also assumed that the final form of the merger agreement would be substantially similar to the last draft reviewed by Merrill Lynch and that the merger will be consummated in accordance with the terms of the merger agreement without waiver of any of the conditions precedent to the merger contained in the merger agreement. Merrill Lynch further assumed that the merger will qualify as a tax-free reorganization for U.S. federal income tax purposes.
Merrill Lynch’s opinion is necessarily based on market, economic and other conditions as they existed and can be evaluated on, and on the information made available to Merrill Lynch, as of the date of the opinion. Merrill Lynch assumed that in the course of obtaining the necessary regulatory or other consents or approvals (contractual or otherwise) for the merger, no restrictions, including any divestiture requirement or amendments or modifications, will be imposed that will have a material adverse effect on the contemplated benefits of the merger. Merrill Lynch also assumed that the consideration to be received by the holders of Genesoft shares pursuant to the merger will not be reduced as a result of hold harmless or indemnification provisions of the merger agreement. In connection with Merrill Lynch’s engagement, Merrill Lynch was requested to solicit indications of interest from, and held discussions with, a substantial number of third parties regarding the possible acquisition of all or a part of the company.
The following is a summary of the material analyses that Merrill Lynch performed in arriving at its opinion and presented to the Genesoft board of directors dated November 12, 2003. The Merrill Lynch opinion is based upon Merrill Lynch’s consideration of the collective results of all such analysis, together with other factors referenced in its written opinion. Some of the financial analyses summarized below include information presented in tabular format. In order to understand fully Merrill Lynch’s financial analysis, the tables must be read together with the text of the summary. The tables alone do not constitute a complete description of the financial analyses. Considering the data set forth below without considering the full narrative description of the financial analyses, including the methodologies and assumptions underlying the analyses, could create a misleading or incomplete view of Merrill Lynch’s financial analyses.
Genesoft Financial Analysis
The implied equity values set forth in this “Genesoft Financial Analysis” section compare to the merger’s implied offer value of $72 million, based on the issuance of 28,571,405 Genome shares, the assumption of $22 million of net debt and based on the per share closing price of Genome common stock on November 11, 2003, after deducting the number of Genome shares to be issued to Genesoft’s bridge debt holders in connection with the merger.
Discounted Cash Flow Analysis.Merrill Lynch performed a discounted cash flow analysis of the projected unlevered free cash flows that Genesoft could produce over the years 2004 through 2015, the expected useful
64
Table of Contents
patent life of Genesoft’s lead product, based on projections from Genesoft management, without giving effect to the merger. Merrill Lynch calculated the following implied equity values for Genesoft by utilizing discount rates ranging from 15% to 18%, and assuming approximately $22 million of debt:
| Low | High | |||||
| (in millions) | ||||||
Implied Valuation Range | $ | 70 | $ | 100 | ||
Selected Comparable Public Companies Analysis. Using publicly available information, Merrill Lynch compared selected financial and other data of Genesoft to corresponding data for selected publicly traded companies with operations that for purposes of this analysis may be considered reasonably comparable to the operations of Genesoft. Merrill Lynch compared Genesoft with the biotechnology and specialty pharmaceutical companies listed below.
Biotechnology Companies: | Specialty Pharmaceutical Companies: | |
• Amgen Inc. • Chiron Corp. • Genzyme Corp. • Gilead Sciences Inc. • IDEC Pharmaceuticals Corp. • MedImmune Inc. • Serono | • Altana AG • Biovail Corp. • Enzon Pharmaceuticals Inc. • King Pharmaceuticals, Inc. • Medicis Pharmaceutical Corp. • Novo Nordisk A/S • Shire Pharmaceuticals Group • Teva Pharmaceutical Industries • Watson Pharmaceuticals Inc |
For each of these comparable companies, Merrill Lynch calculated the price per share of common stock as a multiple of estimated 2004 earnings per share, or the forward P/E multiple. All multiples were based on closing stock prices on November 7, 2003. Estimated financial data for the selected companies were based on publicly available research analysts’ estimates. This analysis yielded a range of forward P/E multiples for the selected companies of 18.0x to 22.0x. Merrill Lynch then applied this range of multiples to Genesoft’s estimated 2007 net income, which Merrill Lynch assumed would be Genesoft’s first full fiscal year of profitability based on Genesoft management’s projections, to derive an estimated 2006 equity value for Genesoft. Utilizing a discount rate of 17% to discount to present value Genesoft’s estimated 2006 equity value, Merrill Lynch calculated the following range of implied equity values for Genesoft:
| Low | High | |||||
| (in millions) | ||||||
Implied Valuation Range | $ | 75 | $ | 95 | ||
65
Table of Contents
Selected Comparable Transactions Analysis. Using publicly available information, Merrill Lynch reviewed and analyzed certain financial and operating data relating to selected biotechnology and specialty pharmaceutical product acquisition transactions with respect to Genesoft, including, but not limited to, the following transactions:
Comparable Product Acquisition Transactions
| Announcement Date | Acquiror | Product (Seller) | ||
3/18/03 | Novartis AG | Enablex (Pfizer Inc.) | ||
1/29/03 | King Pharmaceuticals, Inc. | Skelaxin, Sonata (Elan Corp.) | ||
10/02/02 | Enzon Pharmaceuticals Inc. | Abelcet (Elan Corp.) | ||
5/3/02 | Schering AG | Leukine (Immunex) | ||
10/29/01 | Biovail Corp. | Zovirax (GlaxoSmithKline PLC) | ||
9/26/01 | IVAX Corp. | Naserel, Nasalide (Elan Corp.) | ||
8/9/01 | King Pharmaceuticals, Inc. | Corizide, Corgard, Delestrogen, Florinef (Bristol-Myers Squibb Co.) | ||
7/10/01 | Andrx Corp. | Entrex (Elan Corp.) | ||
7/2/01 | Galen Holdings PLC | Estrace Tablets (Bristol-Myers Squibb Co.) | ||
2/5/01 | Alza | Flexeril (Merck & Co Inc.) | ||
1/2/01 | Biovail Corp. | Cardizem (Aventis SA) |
Comparable Biotechnology Acquisition Transactions
| Announcement Date | Acquiror | Target | ||
6/17/03 | Cell Therapeutics Inc. | Novuspharma SpA | ||
5/19/03 | Chiron Corp. | PowderJect | ||
2/10/03 | Johnson & Johnson Inc. | Scios | ||
1/23/03 | C.A.T. | Oxford Glycosciences | ||
1/16/03 | Johnson & Johnson Inc. | 3 Dimensional | ||
12/4/02 | Gilead Sciences Inc. | Triangle | ||
11/21/02 | H. Lundbeck A/S | Synaptic | ||
11/13/02 | Johnson & Johnson Inc. | OraPharma | ||
12/13/01 | Amgen Inc. | Immunex | ||
12/5/01 | Millennium | COR | ||
12/3/01 | MedImmune Inc. | Aviron | ||
4/30/01 | Vertex Pharmaceuticals Inc. | Aurora Biosciences |
Merrill Lynch examined the multiples of transaction value to last twelve months’ revenue for the selected transactions and derived a reference range of 3.0x to 4.0x. Merrill Lynch then applied this range to Genesoft’s 2007 revenue, which Merrill Lynch assumed would be Genesoft’s first full fiscal year of profitability, to derive an estimated 2007 transaction value for Genesoft. Merrill Lynch calculated the following range of implied equity values for Genesoft, by discounting to present value, utilizing a 17% discount rate, the estimated 2007 transaction value for Genesoft and subtracting net debt of approximately $22 million:
| Low | High | |||||
| (in millions) | ||||||
Implied Valuation Range | $ | 90 | $ | 125 | ||
66
Table of Contents
Genome Financial Analysis
Discounted Cash Flow Analysis. Merrill Lynch performed a discounted cash flow analysis of the projected unlevered free cash flows that Genome could produce over the years 2004 through 2017, the expected useful patent life of Genome’s lead product, based on projections from Genome management, without giving effect to the merger. Merrill Lynch calculated the following implied equity values for Genome, by utilizing discount rates ranging from 22% to 27% and assuming approximately $35 million of net cash:
| Low | High | |||||
| (in millions) | ||||||
Implied Valuation Range | $ | 80 | $ | 105 | ||
Selected Comparable Public Companies Analysis.Merrill Lynch compared selected financial and stock market data of Genome to corresponding data for the biotechnology companies listed under “—Genesoft Financial Analysis—Selected Comparable Public Companies Analysis.” Merrill Lynch applied a forward P/E multiple range of 20.0x to 25.0x to Genome’s estimated 2008 net income, which Merrill Lynch assumed would be Genome’s first full fiscal year of profitability based on Genome management’s projections, to derive an estimated 2007 equity value for Genome. Utilizing a discount rate of 25% to discount to present value Genome’s estimated 2007 equity value, Merrill Lynch calculated the following range of implied equity values for Genome:
| Low | High | |||||
| (in millions) | ||||||
Implied Valuation Range | $ | 140 | $ | 180 | ||
Selected Comparable Transaction Analysis. Merrill Lynch reviewed and analyzed certain financial and operating data relating to selected biotechnology company acquisition transactions with respect to Genome, including the transactions listed under “—Genesoft Financial Analysis— Selected Comparable Transactions Analysis.” Merrill Lynch derived a reference range of multiples of transaction value to last twelve months’ revenue for the selected transactions of 5.0x to 7.0x. Merrill Lynch then applied this range to Genome’s 2008 revenue, which Merrill Lynch assumed would be Genome’s first full year of profitability, to derive an estimated 2008 transaction value for Genome. Utilizing a 25% discount rate, Merrill Lynch then discounted to present value Genome’s estimated 2008 transaction value and then added net cash of approximately $35 million to derive the following range of implied equity values:
| Low | High | |||||
| (in millions) | ||||||
Implied Valuation Range | $ | 145 | $ | 190 | ||
Implied Genesoft Ownership Analysis
Based on the implied equity values for Genesoft and Genome derived from the Discounted Cash Flow, Selected Comparable Public Companies and Selected Comparable Transactions analyses described above, Merrill Lynch derived ranges of the implied equity value that Genesoft would represent of the combined company on a percentage basis and hence the implied relative ownership of Genesoft stockholders in the combined company. The implied ranges are summarized below:
| Low | High | |||||
Analysis | ||||||
Discounted Cash Flow Analysis | 40 | % | 56 | % | ||
Selected Comparable Public Companies Analysis | 29 | % | 40 | % | ||
Selected Comparable Transactions Analysis | 32 | % | 46 | % | ||
67
Table of Contents
Merrill Lynch noted that, as of November 11, 2003, based on the implied equity value of Genesoft in the merger and the then current fully-diluted market value of Genome, Genesoft would represent approximately 43% of the combined company and that Genesoft stockholders would own approximately 36% of the combined company.
Other Factors
In the course of preparing its opinion, Merrill Lynch also reviewed and considered other information and data, including the following:
| • | the trading characteristics of Genome shares; and |
| • | historical market prices for Genome shares. |
The summary set forth above does not purport to be a complete description of all the analyses performed by Merrill Lynch in arriving at its opinion. The preparation of a fairness opinion is a complex analytic process involving various determinations as to the most appropriate and relevant methods of financial analysis and the application of those methods to the particular circumstances and, therefore, a fairness opinion is not readily susceptible to partial analysis or summary description. No company, business or transaction used in such analysis as a comparison is identical to Genesoft, Genome or the merger, nor is an evaluation of the results of each analysis entirely mathematical. Rather, it involves complex considerations and judgments concerning financial and operating characteristics and other factors that could affect the merger, public trading or other values of the companies, business segments or transactions being analyzed. In arriving at its opinion, Merrill Lynch did not attribute any particular weight to any analysis or factor considered by it, but rather made qualitative judgments as to the significance and relevance of each analysis and factor. Accordingly, Merrill Lynch believes that its analysis must be considered as a whole and that selecting portions of its analysis and factors, or focusing on information presented in tabular format, without considering all of the analyses and factors of the narrative description of the analyses, would create a misleading or incomplete view of the process underlying its opinion.
In performing its analyses, Merrill Lynch made numerous assumptions with respect to industry performance, general business, economic, market and financial conditions and other matters, many of which are beyond the control of Genesoft and Genome. The estimates contained in the analysis performed by Merrill Lynch and the ranges of valuation resulting from any particular analysis are not necessarily indicative of actual values or predictive future results or values, which may be significantly more or less favorable than those suggested by such analyses. In addition, estimates of the value of businesses or securities do not purport to be appraisals or to reflect the prices at which businesses or securities may actually be sold. Accordingly, such analyses and estimates are inherently subject to uncertainty, being based upon numerous factors or events beyond the control of the parties or their respective advisors, and none of Genesoft, Genome, Merrill Lynch or any other person assumes responsibility if future results are materially different from those projected. As described above, the Merrill Lynch fairness opinion and analyses were among the factors taken into consideration by the Genesoft board of directors in making its determination to approve the merger agreement and the merger. Consequently, Merrill Lynch’s fairness opinion and analyses should not be viewed as determinative of the decision of the Genesoft board of directors or Genesoft’s management with respect to the fairness of the common exchange ratio.
Genesoft retained Merrill Lynch based upon Merrill Lynch’s experience and expertise. Merrill Lynch is a nationally recognized investment banking firm. Merrill Lynch, as part of its investment banking business, is regularly engaged in the valuation of businesses and securities in connection with mergers and acquisitions, negotiated underwritings, competitive biddings, secondary distributions of listed and unlisted securities and valuations for corporate and other purposes.
Merrill Lynch has, in the past, provided financial advisory and financing services to Genesoft and Genome and/or its affiliates and may continue to do so and has received, and may receive, fees for the rendering of such
68
Table of Contents
services. In the ordinary course of its business, Merrill Lynch and its affiliates may actively trade Genome shares and other securities of Genome for its own account and for the account of customers and, accordingly, may at any time hold a long or short position in such securities.
Pursuant to the terms of a letter agreement between Genesoft and Merrill Lynch, Genesoft has agreed to pay Merrill Lynch a customary fee in connection with the merger, substantially all of which is contingent upon the consummation of the merger. Genesoft has agreed to reimburse Merrill Lynch for all reasonable expenses, including reasonable fees and disbursements of Genesoft’s counsel, up to a specified maximum, and has agreed to indemnify Merrill Lynch and related persons and entities against certain liabilities, including liabilities under the federal securities laws, arising out of Merrill Lynch’s engagement.
Consideration of the Merger by Genome’s Board of Directors
The decision of Genome’s Board of Directors to approve the merger was based on several potential benefits of the merger that it believes will contribute to Genome’s success. Beginning in 2001, the primary strategic focus for Genome has been to become a product-focused biopharmaceutical company. Genome believes that the merger with Genesoft will:
| • | enable Genome to realize its goal of becoming a biopharmaceutical company with an FDA approved anti-infective product; |
| • | significantly increase Genome’s revenue stream, permitting Genome to build a sales and marketing force that will benefit Ramoplanin and future product candidates; |
| • | position Genome as a key participant in the commercialization of anti-infective products; |
| • | increase the visibility of Genome and provide a stronger portfolio of products to serve as a basis for raising additional capital for the company; and |
| • | significantly enhance Genome’s management expertise, clinical development staff, intellectual property and technical resources. |
Since 2001, Genome has had ongoing efforts to identify and evaluate product candidates in clinical development and approved products for potential in-licensing or acquisition. One such opportunity that came to fruition was the in-licensing of the anti-infective product candidate Ramoplanin from Biosearch Italia (now merged with Versicor, Inc. to become Vicuron Pharmaceuticals, Inc.) in October of 2001. Since the time of that transaction, Genome has been seeking additional product candidates and approved products, particularly in the anti-infective area, to strengthen Genome’s product portfolio.
Through the merger with Genesoft, Genome will obtain the rights to FACTIVE, which was approved by the FDA for the treatment of community-acquired pneumonia of mild to moderate severity and acute bacterial exacerbations of chronic bronchitis in April 2003. Subject to raising at least $32 million of capital, which is a condition to the closing of the merger (unless waived by both parties), Genome expects the commercial launch of FACTIVE to occur in the second half of 2004. Genome expects that revenues from the launch of FACTIVE, an orally administered, broad-spectrum fluoroquinolone antibiotic, will drive the combined company’s near-term growth as a biopharmaceutical company. Genome believes that these revenues will also support the growth of its sales and marketing expertise, which will benefit other product candidates in its pipeline, such as Ramoplanin. Strengthened by a broader portfolio of products and product candidates, including FACTIVE, Ramoplanin and a number of earlier stage product opportunities, Genome believes that the combined company will be a more prominent participant in the commercialization of anti-infectives and will have a broader foundation upon which to raise future capital.
In connection with its approval of the merger and recommendation that the stockholders approve the issuance of the Genome common stock in connection with the merger, the board of directors of Genome
69
Table of Contents
consulted with its legal advisors concerning the duties of the members of the board, as well as with members of management and Genome’s financial advisors. The board of directors of Genome also considered the following information and factors in reaching its decision to approve the merger:
| • | the benefits described above; |
| • | the amount of Genome common stock to be issued in the merger and the resulting ownership interest of Genome by former security holders of Genesoft; |
| • | presentations by senior members of Genome’s management regarding the strategic advantages of merging with Genesoft, as well as the results of management’s operational and legal due diligence review; |
| • | historical information concerning Genome’s and Genesoft’s respective businesses, financial performance and condition, management, competitive position and overall operations; |
| • | Genome’s management’s view as to the financial condition of Genesoft based on management’s due diligence and other available information; |
| • | the characteristics of FACTIVE, including: |
| • | FDA approved labeling and indications; |
| • | therapeutic advantages of the product, including its effectiveness in treating infections caused by organisms resistant to other antibiotics; |
| • | once-daily dosing; and |
| • | patent life; |
| • | the strategic fit of Genome and Genesoft, including the belief that the merger has the potential to enhance stockholder value; |
| • | the management team for the combined company; |
| • | the financial analysis of Harris Nesbitt Corp., and its written opinion to the effect that, as of November 10, 2003, and based upon and subject to the factors and assumptions set forth in the opinion, the aggregate stock consideration to be issued by Genome in the merger and related transactions was fair, from a financial point of view, to Genome; |
| • | the terms and conditions of the merger agreement, the note amendment and exchange agreement, the bridge loan to Genesoft and the other related documents; |
| • | the likelihood that the merger will be completed; |
| • | the expected tax treatment of the merger as a tax-free reorganization for United States federal income tax purposes; |
| • | the impact of the merger on Genome’s stockholders and employees. |
Genome’s board of directors also identified and considered the potential adverse consequences of other factors on the proposed merger, including;
| • | the risk that the potential benefits of the merger might not be realized; |
| • | the risk that FACTIVE might not launch in the second half of 2004, causing a delay in the realization of revenue; |
| • | the risk that FACTIVE may not attain commercial acceptance and generate the level of revenues expected; |
| • | the challenges and risks involved in combining the businesses of two geographically distant companies; |
| • | the effect of the announcement on Genome’s share price; |
70
Table of Contents
| • | the risk of diverting management’s focus and resources from other strategic opportunities and from operational matters while working to complete and implement the merger; |
| • | the risk that Genome will not be able to raise a minimum of $32 million of capital, which is a condition to the closing, unless waived by both parties; |
| • | the risk that LG Life Sciences will not be able to supply FACTIVE in a timely manner, or maintain its manufacturing facilities in accordance with regulatory requirements; and |
| • | the risk that the merger would not be completed. |
This discussion of the information and factors considered by the Genome board of directors is not intended to be exhaustive, but includes the material factors considered. The Genome board of directors did not assign particular weight to the factors it considered in approving the merger. In considering all the information above, the individual members of the board of directors may have considered one factor more important than another. However, the Genome board of directors considered all of these factors as a whole, and overall considered them to be favorable to support its determination to approve the merger.
Opinion of Financial Advisor to Genome
On November 10, 2003, Harris Nesbitt Corp., or Harris Nesbitt, delivered its oral opinion, which it subsequently confirmed in writing, to Genome’s board of directors to the effect that, as of such date, and based upon and subject to the factors and assumptions set forth therein, the aggregate stock consideration to be issued by Genome to the security holders of Genesoft in the merger and the related transactions under the note amendment and exchange agreement described in the section of this joint proxy statement/prospectus entitled “Other Material Agreements Relating to the Merger” is fair to Genome from a financial point of view.
The full text of the opinion of Harris Nesbitt dated November 10, 2003, which sets forth assumptions made, matters considered and limitations on the review undertaken in connection with the opinion, is attached as Annex C to this proxy statement/prospectus and is incorporated by reference. The opinion of Harris Nesbitt was provided for the information and assistance of Genome’s board of directors in connection with its consideration of the merger and related transactions. The opinion of Harris Nesbitt addresses only the fairness, from a financial point of view, of the aggregate merger consideration to be issued by Genome and does not constitute a recommendation as to how any stockholder should vote at the special meeting. This summary of the Harris Nesbitt opinion is qualified by the full text of such opinion, and Genome’s stockholders are urged to read the Harris Nesbitt opinion in its entirety.
For purposes of its opinion, Harris Nesbitt has:
| • | Reviewed Genome’s Annual Reports on Form 10-K and related publicly-available financial information for the three fiscal years ended December 31, 2000, 2001, 2002 and Genome’s Form 10-Q and the related unaudited financial information for the nine months ended September 27, 2003; |
| • | Reviewed certain financial and operating information relating to the business, earnings, cash flow, assets and prospects of Genesoft and Genome, furnished to it by Genesoft and Genome, as the case may be; |
| • | Reviewed certain financial projections prepared by the management of Genesoft and Genome; |
| • | Conducted discussions with members of senior management of Genesoft and Genome concerning their respective operations, financial condition and prospects; |
| • | Reviewed the historical market prices and trading activity for the shares of the common stock of Genesoft and Genome and compared them with those of certain publicly-traded companies which it deemed to be reasonably similar to Genesoft and Genome; |
| • | Compared the financial performance of Genesoft and Genome with that of certain companies which it deemed to be reasonably similar to Genesoft and Genome, respectively; |
71
Table of Contents
| • | Compared the proposed financial terms to Genome of the merger and related transaction with the financial terms of certain other mergers and acquisitions which it deemed to be relevant; |
| • | Reviewed a draft of the merger agreement dated November 10, 2003; |
| • | Reviewed drafts, each dated November 10, 2003, of the note amendment and exchange agreement, and forms of the voting agreements, registration rights agreement and escrow agreement to be executed in connection with the merger; and |
| • | Reviewed such other financial studies, performed such other analyses and investigations and took into account such other matters as it deemed appropriate. |
Harris Nesbitt assumed and relied upon the accuracy and completeness of all information reviewed by it, and did not independently verify such information or undertake an independent valuation or appraisal of the assets of Genesoft, nor was Harris Nesbitt furnished with any such valuation or appraisal. Harris Nesbitt noted that it understood that in connection with the merger, Genome would lend $6,200,000 to Genesoft; however Harris Nesbitt did not review the documents providing for this loan and expressed no opinion on its terms.
With respect to the financial projections, Harris Nesbitt assumed that they were reasonably prepared on bases reflecting the best currently available estimates and good faith judgments of senior management of Genesoft and Genome of their respective future competitive, operating and regulatory environments and related financial performance of Genesoft and Genome.
Harris Nesbitt did not review the books and records of Genesoft or Genome and did not conduct a physical inspection of the properties or facilities of Genesoft or Genome. Harris Nesbitt assumed that the executed versions of the merger agreement and related agreements identified above as reviewed by Harris Nesbitt would not differ in any material respect from the last draft it reviewed and that the merger and related transaction would be consummated on the terms set forth therein without waiver or modification of any material terms. The Harris Nesbitt opinion is necessarily based on economic, market and other conditions and circumstances as they exist and can be evaluated on, and the information made available to us as of, November 10, 2003.
The following is a summary of the financial analyses used by Harris Nesbitt in connection with providing its opinion. Some of these summaries of financial analyses include information presented in tabular format. In order to understand fully the financial analyses used by Harris Nesbitt, the tables must be read together with the text of each summary. The tables alone do not constitute a complete description of the financial analyses. Considering the data set forth below in the tables without considering the full narrative description of the financial analyses, including the methodologies and assumptions underlying the analyses, could create a misleading or incomplete view of Harris Nesbitt’s financial analyses.
Discounted Cash Flow Analysis of Genesoft. Harris Nesbitt performed a discounted cash flow analysis of Genesoft to calculate the present value of the stand-alone unlevered free cash flows that Genesoft would generate from January 1, 2004 through December 31, 2010, assuming that Genesoft’s operating performance would be as reflected in the projections of Genesoft’s management as to the potential future financial performance of Genesoft.
Harris Nesbitt discounted the estimated unlevered free cash flows provided by the model using discount rates ranging from 12.5% to 27.5%. Harris Nesbitt calculated terminal values based on multiples of projected 2008 net income, 2010 net income and 2007 revenue, and then discounted these terminal values. The implied range of values calculated by Harris Nesbitt for Genesoft derived from the discounted cash flow analysis is presented below:
| 20% Discount Rate | |||||
| ($ in millions) | |||||
2008 | Net Income | $ | 60-120 | ||
2010 | Net Income | $ | 235-365 | ||
2007 | Revenue | $ | 400-570 | ||
72
Table of Contents
Selected Comparable Public Companies Analysis of Genesoft
Profitable Biotechnology Companies. Using publicly available information, Harris Nesbitt compared certain financial and other information for Genesoft with the corresponding financial and other information for the following publicly-traded profitable biotechnology companies: Amgen Inc., Biogen Inc., Chiron Corp., Genentech Inc., Genzyme Corp., Gilead Sciences Inc., IDEC Pharmaceuticals Corp. and MedImmune Inc. Each of these companies was selected for comparison because it was a profitable publicly-traded biotechnology company.
The financial information compared included (1) equity value, (2) enterprise value, (3) ratio of enterprise value to revenue, (4) ratio of enterprise value to gross profit, (5) ratio of enterprise value to EBITDA, which is earnings before interest, taxes, depreciation and amortization, (6) ratio of share price to earnings, (7) ratio of equity value to book value, (8) long-term projected growth rate and (9) ratio of share price to earnings to long-term projected growth rate.
Developmental Stage Companies. Using publicly available information, Harris Nesbitt compared certain financial and other information for Genesoft with the corresponding financial and other information for the following publicly-traded developmental stage biotechnology companies: Cubist Pharmaceuticals Inc., InterMune Pharmaceuticals Inc., Nabi Biopharmaceuticals Inc., Sciclone Pharmaceuticals Inc., The Medicines Company, Vicuron Pharmaceuticals, Inc., and XOMA Limited. Each of these was selected because it is comparable based on either its phase of development, the therapeutic indications for which it is developing products, or both.
The financial information compared included (1) equity value, (2) enterprise value, (3) ratio of enterprise value to revenue, (4) ratio of enterprise value to gross profit, (5) ratio of enterprise value to EBITDA, which is earnings before interest, taxes, depreciation and amortization, (6) ratio of share price to earnings, (7) ratio of equity value to book value, (8) long-term projected growth rate and (9) ratio of share price to earnings to long-term projected growth rate.
Harris Nesbitt calculated the enterprise value, defined as the total market value of equity on a diluted basis plus the estimated market value of debt and minority interest minus cash, of Genesoft and each of the companies identified above in the paragraphs entitled “Profitable Biotechnology Companies” and “Developmental Stage Companies,” respectively, as a multiple of its respective 2004 and 2005 projected revenue, gross profit and EBITDA. The following table presents the enterprise valuation multiples of Genesoft compared to the corresponding multiples for the selected comparable companies:
Profitable Median Multiple | Developmental Median Multiple | Genesoft at Deal Price Multiples1 2 | Implied Equity Value of Median | |||||||||
| Profitable | Developmental | |||||||||||
| ($ in millions) | ||||||||||||
Enterprise Value/ | ||||||||||||
2004 Revenue | 6.7x | 9.4x | 38.6x | NM4 | $ | 3.8 | ||||||
2004 Gross Profit | 7.9x | 11.7x | 43.3x | NM4 | $ | 6.9 | ||||||
2004 EBITDA | 16.2x | 46.5x | NM4 | NM4 | NM4 | |||||||
2005 Revenue | 5.9x | 6.8x | 3.8x | $ | 143.1 | $ | 170.5 | |||||
2005 Gross Profit | 7.0x | 9.3x | 7.7x | $ | 76.2 | $ | 107.8 | |||||
2005 EBITDA | 14.6x | 35.4x | NM4 | NM4 | NM4 | |||||||
| 1 | Assumes Genesoft enterprise value of $108.2 million ($85.7 million equity value + $22.5 of Genesoft net debt). |
| 2 | Assumes Genesoft equity value (paid by Genome of $85.7 million). |
| 3 | Equity value = enterprise value less Genesoft net debt of $22.5 million. |
| 4 | Not measurable. |
The implied range of values calculated by Harris Nesbitt for Genesoft derived from the comparable public companies analysis is $75–170 million.
73
Table of Contents
Precedent M&A Transactions Valuation Analysis of Genesoft
Harris Nesbitt compared certain financial information of Genesoft with the same publicly available information with respect to 17 acquisition transactions identified below that Harris Nesbitt believed to be appropriate for comparison. These transactions were selected because the acquired businesses are biotechnology and pharmaceuticals companies. The following table presents the selected transactions used in Harris Nesbitt’s analysis:
Announcement Date | Target | Acquiror | ||
11/3/03 | CIMA Labs | Cephalon Inc. | ||
10/14/03 | Oculex Pharmaceuticals | Allergan Inc. | ||
8/8/03 | Nostrum Pharmaceuticals | Elite Pharmaceuticals | ||
8/4/03 | SangStat Medical | Genzyme General | ||
7/17/03 | Cyclis Pharmaceuticals | Arqule Inc. | ||
6/20/03 | Biogen, Inc. | IDEC Pharmaceuticals | ||
4/15/03 | Diacrin, Inc. | GenVec Inc. | ||
2/25/03 | Corvas International | Dendreon Corporation | ||
2/10/03 | Cell Pathways | OSI Pharmaceuticals | ||
2/10/03 | Scios | Johnson & Johnson | ||
2/4/03 | Eos Biotechnology | Protein Design Labs | ||
12/4/02 | Triangle Pharmaceuticals | Gilead Sciences Inc. | ||
11/21/02 | Synaptic Pharmaceutical | H. Lunbeck A/S | ||
11/13/02 | Anthrogenesis | Celegene | ||
11/12/02 | Maxia Pharmaceuticals | Incyte Genomics | ||
11/11/02 | Variagenics | Hyseq Pharmaceuticals | ||
10/21/02 | Meridian Medical Technologies | King Pharmaceuticals |
The following table summarizes the range of ratio of equity value to book value and ratios of enterprise value to revenue, gross profit and EBITDA for the latest twelve months for Genesoft and each of the comparable acquired businesses prior to acquisition:
Multiple based on: | Genesoft at Transaction Value | Reference Range | Median Multiple | Mean Multiple | ||||||
| Low | High | |||||||||
Equity Value / Book Value | NM1 | 0.7x | 10.1x | 2.7x | 3.5x | |||||
Enterprise Value / Revenue | NA | 1.3x | 30.8x | 7.9x | 13.1x | |||||
Enterprise Value / Gross Profit | NA | 1.6x | 34.2x | 12.2x | 16.3x | |||||
Enterprise Value / EBITDA | NM1 | 10.0x | 45.6x | 22.7x | 25.2x | |||||
| 1 | Not measurable. |
No transaction included in the comparable transaction analysis is identical to the contemplated merger between Genesoft and Genome. Harris Nesbitt made judgments and assumptions with regard to industry performance, size of the businesses acquired, timing of the transactions, current business, economic, market and financial conditions and other matters. This analysis did not lead to specific conclusions regarding the implied merger consideration, in the aggregate, but rather was part of Harris Nesbitt’s evaluation of the relevancy of the analysis of the precedent transactions with regard to the particular circumstances of the contemplated merger.
The average implied range of values calculated by Harris Nesbitt for Genesoft derived from the analyses performed is $192.5–306.3 million.
74
Table of Contents
Discounted Cash Flow Analysis of Genome. Harris Nesbitt performed a discounted cash flow analysis of Genome to calculate the present value of the stand-alone unlevered free cash flows that Genome would generate from January 1, 2004 through December 31, 2010, assuming that Genome’s operating performance would be as reflected in the projections of Genome’s management as to the potential future financial performance of Genome.
Harris Nesbitt discounted the estimated unlevered free cash flows provided by the model using discount rates ranging from 22.5% to 37.5%. Harris Nesbitt calculated terminal values based on multiples of projected 2008 net income, 2010 net income and 2007 revenue, and then discounted these terminal values. The implied range of values calculated by Harris Nesbitt for the Genome derived from the discounted cash flow analysis is presented below.
30% Discount Rate | ||||
| ($ in millions) | ||||
2008 | Net Income | $75 – 130 | ||
2010 | Net Income | $210 – 315 | ||
2007 | Revenue | $85 – 115 |
Selected Comparable Public Companies Analysis of Genome
Profitable Biotechnology Companies. Using publicly available information, Harris Nesbitt compared certain financial and other information for Genome with the corresponding financial and other information for the following publicly-traded profitable biotechnology companies: Amgen, Biogen, Chiron Corp., Genentech, Genzyme Corp., Gilead Sciences, IDEC Pharmaceuticals Corp. and Medimmune. Each of these companies was selected for comparison because it was a profitable publicly-traded biotechnology company.
The financial information compared included (1) equity value, (2) enterprise value, (3) ratio of enterprise value to revenue, (4) ratio of enterprise value to gross profit, (5) ratio of enterprise value to EBITDA, which is earnings before interest, taxes, depreciation and amortization, (6) ratio of share price to earnings, (7) ratio of equity value to book value, (8) long-term projected growth rate and (9) ratio of share price to earnings to long-term projected growth rate.
Developmental Stage Companies. Using publicly available information, Harris Nesbitt compared certain financial and other information for Genome with the corresponding financial and other information for and with the following publicly-traded developmental stage biotechnology companies: Cubist Pharmaceuticals, InterMune, Nabi Biopharmaceuticals, Sciclone Pharmaceuticals, The Medicines Company, Vicuron Pharmaceuticals and Xoma. Each of these companies was selected for comparison because it was a developmental stage publicly-traded biotechnology company.
The financial information compared included (1) equity value, (2) enterprise value, (3) ratio of enterprise value to revenue, (4) ratio of enterprise value to gross profit, (5) ratio of enterprise value to EBITDA, which is earnings before interest, taxes, depreciation and amortization, (6) ratio of share price to earnings, (7) ratio of equity value to book value, (8) long-term projected growth rate and (9) ratio of share price to earnings to long-term projected growth rate.
75
Table of Contents
Harris Nesbitt calculated the enterprise value, defined as the total market value of equity on a diluted basis plus the estimated market value of debt and minority interest minus cash, of Genome and each of the companies listed in the above paragraphs entitled “Profitable Biotechnology Companies” and “Developmental Stage Companies” as a multiple of its respective 2004 and 2005 projected revenue, gross profit and EBITDA. The following table presents the enterprise valuation multiples of Genome compared to the corresponding multiples for the selected comparable companies:
Extended Equity Value of Genome Based on:1 | ||||||||||||
Profitable
Median | Developmental
Median | Genome at Current Price of $3.00 Multiples | Median | |||||||||
| Profitable2 | Developmental3 | |||||||||||
| ($ in millions) | ||||||||||||
Enterprise Value/ | ||||||||||||
LTM Revenue LTM Gross Profit LTM EBITDA | 8.8x 10.2x 20.1x | 19.5x 19.7x 43.7x | 5.4x 11.1x NM4 | $ $
| 156.8 106.7 NM | $ $
| 297.9 168.1 NM | |||||
2004 Revenue 2004 Gross Profit 2004 EBITDA | 6.7x 7.9x 16.2x | 9.4x 11.7x 46.5x | 15.8x 15.8x NM | $ $
| 71.6 76.9 NM | $ $
| 83.8 94.3 NM | |||||
2005 Revenue 2005 Gross Profit 2005 EBITDA | 5.9x 7.0x 14.6x | 6.8x 9.3x 35.4x | 54.6x 54.6x NM | $ $
| 49.2 50.7 NM | $ $
| 50.4 53.6 NM | |||||
Equity Value/ Book Value | 4.3x | 5.8x | 5.8x | $ | 82.3 | $ | 112.8 | |||||
| 1 | Equity value = enterprise value less Genome net debt of ($41.5) million. |
| 2 | Enterprise value was derived by multiplying Profitable Biotechnology Companies Median Multiples (Revenue, Gross Profit, EBITDA) by Genome’s corresponding financial data for each time period (i.e. LTM, 2004, 2005). Equity value was then calculated by subtracting out net debt of ($41.5) million. In the case of extended equity value based on a book value median multiple, equity value was derived by multiplying the Profitable Biotechnology Companies Median book value multiple by Genome’s book value. |
| 3 | Enterprise value was derived by multiplying Developmental Biotechnology Companies Median Multiples (Revenue, Gross Profit, EBITDA) by Genome’s corresponding financial data for each time period (i.e. LTM, 2004, 2005). Equity value was then calculated by subtracting out net debt of ($41.5) million. In the case of extended equity value based on a book value median multiple, equity value was derived by multiplying the Developmental Biotechnology Companies Median book value multiple by Genome’s book value. |
| 4 | Not Measurable |
The implied range of values calculated by Harris Nesbitt for Genome derived from the comparable public companies analysis is $50–150 million.
76
Table of Contents
Precedent M&A Transactions Valuation Analysis of Genome
Harris Nesbitt compared certain financial information of Genome with the same publicly available information with respect to the same 17 acquisition transactions used for comparison to Genome above. These transactions were selected because the acquired businesses are biotechnology and pharmaceuticals companies.
The following table summarizes the range of ratio of equity value to book value and ratios of enterprise value to revenue, gross profit and EBITDA for the latest twelve months for Genome and each of the comparable acquired businesses prior to acquisition:
| Reference Range | ||||||||||
| Multiple based on: | Genome at Price of $3.00 | Low | High | Median Multiple | Mean Multiple | |||||
Equity Value / Book Value | 5.8x | 0.7x | 10.1x | 2.7x | 3.5x | |||||
Enterprise Value / Revenue | 5.4x | 1.3x | 30.8x | 7.9x | 13.1x | |||||
Enterprise Value / Gross Profit | 11.1x | 1.6x | 34.2x | 12.2x | 16.3x | |||||
Enterprise Value / EBITDA | NM | 10.0x | 45.6x | 22.7x | 25.2x | |||||
The implied range of values calculated by Harris Nesbitt for Genome derived from the precedent M&A transactions analysis is $50–145 million.
No transaction included in the comparable transaction analysis is identical to the proposed merger between Genome and Genesoft. Harris Nesbitt made judgments and assumptions with regard to industry performance, size of the businesses acquired, timing of the transactions, current business, economic, market and financial conditions and other matters. This analysis did not lead to specific conclusions regarding the implied merger consideration, in the aggregate, but rather was part of Harris Nesbitt’s evaluation of the relevancy of the analysis of the precedent transactions with regard to the particular circumstances of the contemplated merger.
The average implied range of values calculated by Harris Nesbitt for Genome derived from the analyses performed is $94–171 million.
The preparation of a fairness opinion is a complex process involving subjective judgments, and is not necessarily susceptible to partial analysis or summary description. In arriving at its opinion, Harris Nesbitt considered the results of all of its analyses and all of the factors it considered as a whole and did not assign specific weights to particular analyses or factors considered, but, rather, made qualitative judgments as to the significance and relevance of all the analyses and factors considered. Accordingly, Harris Nesbitt believes that its analyses, and the summary set forth above, must be considered as a whole, and that selecting portions of the analyses and of the factors considered by Harris Nesbitt, without considering all of the analyses and factors, could create a misleading or incomplete view of the processes underlying the opinion of Harris Nesbitt.
With regard to the precedent M&A transactions analysis and the comparable public companies analysis summarized above, Harris Nesbitt selected such precedent transactions and such comparable companies on the bases noted above; however, no transaction or company utilized as a comparison in these analyses summarized above is identical to Genesoft or Genome. As a result, these analyses are not purely mathematical, but also take into account significant differences in financial, operating and other characteristics of the transactions and the subject companies and other factors that could affect the value of the subject companies to which Genesoft and Genome are being compared.
In its analyses, Harris Nesbitt made numerous assumptions with respect to Genesoft and Genome, industry performance, general business, economic, market and financial conditions, and other matters, many of which are beyond the control of Genesoft and Genome. Any estimates contained in Harris Nesbitt’s analyses are not necessarily indicative of actual values or predictive of future results or values, which may be significantly more or less favorable than those suggested by these analyses. Estimates of values of companies do not purport to be
77
Table of Contents
appraisals or necessarily to reflect the prices at which companies may actually be sold. Because these estimates are inherently subject to uncertainty, neither Genome, Genesoft, Harris Nesbitt or any other person assumes responsibility if future results or actual values differ materially from the estimates.
Genome retained Harris Nesbitt based upon its experience and expertise. Harris Nesbitt is a nationally recognized investment banking and advisory firm. Harris Nesbitt, as part of its investment banking business, is continuously engaged in the valuation of businesses and securities in connection with mergers and acquisitions, negotiated underwritings, competitive biddings, secondary distributions of listed and unlisted securities, private placements and valuations for corporate and other purposes.
Harris Nesbitt is a full service securities firm engaged in securities trading and brokerage activities, financing and financial advisory services in addition to its investment banking activities. In the ordinary course of its trading, brokerage, and financing activities, Harris Nesbitt or its affiliates may at any time hold long or short positions, and may trade or otherwise effect transactions, for its own account or the accounts of its customers, in debt or equity securities of Genesoft or its affiliates.
Pursuant to Harris Nesbitt’s engagement letter, Genome agreed to pay Harris Nesbitt a fee of $250,000 when Harris Nesbitt rendered its opinion. None of the consideration to be paid to Harris Nesbitt is contingent on the completion of the merger or the related transactions. In addition, Genome also has agreed to reimburse Harris Nesbitt for its reasonable travel and other expenses incurred in connection with its engagement and to indemnify Harris Nesbitt and certain related persons against certain liabilities, including certain liabilities under the federal securities laws, and expenses relating to or arising out of its engagement.
Interests of Directors and Executive Officers of Genome in the Merger
Genome’s stockholders should be aware that some Genome executive officers and directors may have interests in the merger that may be different from, or in addition to, their interests as stockholders of Genome in considering the recommendation of the Genome board of directors that Genome’s stockholders vote in favor of the proposals (i) to approve the issuance of a total of 28,571,405 shares of Genome common stock pursuant to the merger agreement and the issuance of shares of Genome common stock upon the potential conversion of the convertible notes of Genome, in an aggregate principal amount of $22,309,647, to be exchanged for Genesoft promissory notes in connection with the merger and (ii) to approve the Amendment to Genome’s Articles of Organization to increase the number of shares of Genome common stock the company is authorized to issue from 50,000,000 to 175,000,000 shares of common stock.
Governance Structure and Management Positions
The merger agreement provides for the initial composition of the board of directors of the combined company and the executive officer positions for the combined company, and specified members of Genome’s existing board of directors and its executive officers will retain their positions in the combined company. See “Management of the Combined Company After the Merger.”
Severance and Other Arrangements
Genome has amended the employment agreements with Steven Rauscher, Stephen Cohen and Martin Williams, its executive officers.
As amended, the employment agreements with Messrs. Rauscher, Cohen and Williams provide that, in the event employment is terminated by Genome other than for cause, or by the executive for good reason, within twenty-four months following the consummation of the merger, then the executive will receive continuation of base salary and benefits coverage for 18 months, in the case of Mr. Rauscher, and 12 months, in the case of Messrs. Cohen and Williams. In such event, all of the executive’s unvested options and non-exercisable restricted
78
Table of Contents
shares will vest and become exercisable. All of the executive’s options will remain exercisable until the earlier of two years from the date of termination of the executive’s employment and the final exercise date of the option.
For purposes of the employment agreements, termination for “cause” means the executive’s termination by Genome as a result of executive’s (i) material failure to perform (other than by reason of disability), or material negligence in the performance of, the executive’s duties and responsibilities to Genome; (ii) material breach of the executive’s employment agreement or any other agreement between the executive and Genome; (iii) commission of a felony or other crime involving an act of moral turpitude; or (iv) material act of dishonesty or breach of trust resulting or intended to result, directly or indirectly, in a personal gain or enrichment at the expense of Genome.
For purposes of the employment agreements, an executive may terminate his employment with Genome for “good reason” following the occurrence, after the consummation of the merger, of any one or more of the following events without his consent: any change in the executive’s position with Genome that results in a material diminution in the executive’s position, authority or duties as such position, authority or duties existed immediately prior to the merger or Genome takes any action that would require the executive to have his principal place of work changed to any location outside a thirty-five mile radius of the City of Boston.
Genome has also amended the terms of the stock options granted to its directors. For those directors of Genome that will not be continuing as directors following the merger, all of such directors’ unvested options will become exercisable upon the consummation of the merger and all of his options will remain exercisable until the earlier of two years from the date of the closing of the merger and the final exercise date of the option. With respect to the non-employee directors of Genome that will continue to be directors following the merger, if, within two years following the merger, a director is either not nominated to serve as a director or is not elected by the shareholders to serve as a director, all of such director’s unvested options will become exercisable upon such director ceasing to be a director of Genome and all of the director’s options will remain exercisable until the earlier of two years from the date such director ceases to be a director of Genome and the final exercise date of the option.
Interests of Directors and Executive Officers of Genesoft in the Merger
In considering the recommendation of Genesoft’s board of directors that Genesoft’s stockholders vote in favor of approval of the merger agreement, Genesoft stockholders should be aware that some Genesoft executive officers and directors may have interests in the merger that may be different from, or in addition to, their interests as stockholders of Genesoft. Genesoft’s board of directors was aware of these interests during its deliberations on the merits of the merger and in making its recommendation to Genesoft’s stockholders that they vote for the merger.
Governance Structure and Management Positions
The merger agreement provides for the initial composition of the board of directors of the combined company and the executive officer positions for the combined company, and specified members of Genesoft’s board of directors will serve on the board of directors of the combined company. See “Management of the Combined Company After the Merger.”
Indemnification; Directors and Officers’ Insurance
Under the merger agreement, Genome has agreed to indemnify all directors and officers of Genesoft to the same extent such persons are indemnified by Genesoft prior to the merger for all acts or omissions occurring at or prior to the merger by such individuals in such capacities. Genome has also agreed to provide, for six years after the merger, directors’ and officers’ liability insurance in respect of acts or omissions occurring prior to the merger covering each person currently covered by the directors’ and officers’ liability insurance policy of
79
Table of Contents
Genesoft on terms and in amounts no less favorable than those of the policies of Genome, provided that Genome will not be required to pay an annual premium for the insurance in excess of approximately $54,000. Genome has agreed to maintain charter and by-law provisions with respect to indemnification and advancement of expenses that are at least as favorable to the intended beneficiaries as those contained in the charter and by-laws of Genesoft as in effect on the date the merger agreement was signed.
Severance and Other Arrangements
In January 2003, the board of directors of Genesoft approved a severance plan for, and the grant of options to, employees and officers of Genesoft in anticipation of a possible merger or other sale of Genesoft.
Under the terms of Mr. Singer’s agreements with Genesoft, he will be entitled to receive severance payments and to have the vesting of his options accelerated. Due to the fact that Mr. Singer will not be offered a position as an employee of the combined company following the merger, immediately prior to the merger, Genesoft will pay to Mr. Singer a cash severance payment equal to $472,500. In addition, upon consummation of the merger, options to purchase approximately 531,000 shares of Genesoft common stock held by Mr. Singer will become vested and exercisable.
Following the merger, in connection with Mr. Singer’s service as chairman of board of directors of Genome, Genome has agreed to provide Mr. Singer an office and the services of an assistant that is an employee of Genome until December 31, 2004.
Amendment and Exchange of Genesoft Promissory Notes
As described more fully in this document under the caption “The Merger and Related Transactions—Other Material Contracts Relating to the Merger—Note Amendment and Exchange Agreement,” Mr. Singer, Mr. Rutter (including trusts and family members of Mr. Rutter) and certain investment funds affiliated with Dr. Evnin and MPM Capital Management each hold promissory notes of Genesoft, the principal amount of which will be converted into convertible promissory notes of Genome at the time of the merger. The interest and other amounts payable under the Genesoft notes will be converted into shares of Genome common stock at the time of the merger. Mr. Singer holds $100,000 of these Genesoft promissory notes, Mr. Rutter (including trusts and family members of Mr. Rutter) holds $1,300,000 of these Genesoft promissory notes and investment funds affiliated with Dr. Evnin and MPM Capital Management hold $5,750,000 of these Genesoft promissory notes. Each of Messrs. Singer, Rutter and Evnin are directors of Genesoft and are anticipated to serve as directors of Genome following the merger.
The following is a summary of the material provisions of the Agreement and Plan of Merger and Reorganization, dated as of November 17, 2003 by and among Genome Therapeutics Corp., a Massachusetts corporation, Guardian Acquisition, Inc., a Delaware corporation and a wholly-owned subsidiary of Genome, GeneSoft Pharmaceuticals, Inc., a Delaware corporation, and Luke Evnin, as representative of the Genesoft stockholders, a copy of which is attached hereto as Annex A, and incorporated herein by reference. The following summary is qualified in its entirety by reference to the text of the merger agreement.
The Merger
In the initial merger, Guardian Acquisition, Inc. will be merged into Genesoft. Immediately thereafter, Genesoft will be merged into a second wholly-owned subsidiary of Genome, which is a Delaware limited liability company, in the second-step merger. As a result of the initial merger, the separate corporate existence of Guardian will cease, and Genesoft will continue as the surviving corporation of the initial merger and as a wholly-owned subsidiary of Genome. As a result of the second-step merger, the separate corporate existence of Genesoft will cease, and the second acquisition subsidiary will continue as the surviving company of the second-step merger and be wholly-owned by Genome.
80
Table of Contents
The closing of the merger will take place as promptly as practicable following satisfaction or waiver of the conditions to closing set forth in the merger agreement. The merger will become effective at the time specified in the certificates of merger filed with the Secretary of the State of Delaware with respect to the merger.
Conversion of Genesoft Stock
At the effective time of the merger, each share of Genesoft common stock will be cancelled and terminated and will be automatically converted into the right to receive the number of shares of Genome common stock equal to the common exchange ratio (as defined below). No fractional shares of Genome common stock will be issued in connection with the merger. Any fractional shares of Genome common stock that would be issuable upon the conversion or exchange of a share of Genesoft common stock will be rounded up or down to the nearest whole share (with 0.5 being rounded up).
At the effective time of the merger, Genome will assume all options to purchase Genesoft common stock. Immediately after the effective time of the merger, each such assumed option will be deemed to constitute an option to acquire, on the same terms and conditions as were applicable under such option at the effective time of the merger, such number of shares of Genome common stock that is equal to the number of shares of Genesoft common stock subject to the unexercised portion of such option multiplied by the common exchange ratio (rounded down to the nearest whole number). The per share exercise price for the shares of Genome common stock issuable upon exercise of such assumed option will be equal to the exercise price per share of such option in effect immediately prior to the effective time of the merger divided by the common exchange ratio (rounded up to the nearest whole cent).
At the effective time of the merger, each warrant to acquire shares of Genesoft common stock that does not terminate by its terms at or prior to the effective time of the merger will be converted into a warrant to acquire shares of Genome common stock. Each warrant so converted will continue to have, and be subject to, the same terms and conditions set forth in such warrant immediately prior to the effective time of the merger, except that (i) such warrant will be exercisable for that number of whole shares of Genome common stock (rounded down to the nearest whole number) equal to the product of (x) the number of shares of Genesoft for which such warrant was exercisable immediately prior to the effective time of the merger multiplied by (y) the common exchange ratio, and (ii) the per share exercise price for the shares of Genome common stock issuable upon exercise of such assumed warrant will be equal to the exercise price per share of Genesoft common stock at which such warrant was exercisable immediately prior to the effective time of the merger divided by the common exchange ratio (rounded up to the nearest whole cent).
The term “common exchange ratio” means the quotient obtained by dividing (x) the aggregate merger consideration (as defined below) by (y) the fully diluted common shares amount (as defined below).
The term “aggregate merger consideration” means 28,571,405 shares of Genome common stock less the sum of (x) the preference shares (as defined below) and (y) the accrued interest shares (as defined below).
The term “fully diluted common shares amount” means the sum of (x) the number of shares of Genesoft common stock issued and outstanding immediately prior to the effective time of the initial merger and (y) the number of shares of Genesoft common stock issuable upon exercise, conversion and/or exchange of all convertible securities, including options and warrants, of Genesoft issued and outstanding immediately prior to the effective time of the merger.
The term “preference shares” means the quotient obtained by dividing (x) the sum of (i) the interest accrued up to December 10, 2003 on the Genesoft promissory notes issued in December 2002 and January 2003, (ii) 150% of the principal amount of such promissory notes and (iii) the amount of interest payable on each such
81
Table of Contents
promissory note from and after December 10, 2003 until the closing of the initial merger by (y) the lower of (i) the closing financing price (as defined below) and (ii) the average closing sale price (as defined below).
The term “accrued interest shares” means the quotient obtained by dividing (x) the sum of (i) the interest accrued up to December 15, 2003 on the Genesoft promissory notes issued in April and May 2003 and (ii) the amount of interest payable on each such promissory from and after December 15, 2003 until the closing of the initial merger by (y) the lower of (i) the closing financing price and (ii) the average closing sale price.
The term “closing financing price” means the issuance price per share of Genome common stock issued in the closing financing (as defined below).
The term “average closing sale price” means the average closing price of Genome common stock on the Nasdaq for the five trading days immediately preceding the date of public announcement of the transactions contemplated by the merger agreement, which was $2.84.
The term “closing financing” means the sale of shares of Genome common stock, warrants exercisable for shares of Genome common stock, notes convertible into shares of Genome common stock or notes in an amount equal to at least $32 million.
As indicated above, the common exchange ratio and the amount of the merger consideration is subject to change depending on several variables, including (i) the date of the closing of the merger (which will affect the amount of interest accrued on the promissory notes and thus the number of preference shares and accrued interest shares) and (ii) the closing financing price (which, if lower than the average closing sale price, will be used to calculate the number of preference shares and accrued interest shares). Since these variables will not be determined until shortly prior to the effective time of the merger, the precise amount of the merger consideration to be issued to he holders of Genesoft stock in the merger will not be known until immediately prior to the effective time of the merger. Assuming the merger will close on February 28, 2004 and that the closing financing price equals or exceeds the average closing sale price, the common exchange ratio would be approximately 1.188. Thus, Genesoft stockholders would receive 1.188 shares of Genome common stock for each share of Genesoft common stock held by them.
The following chart shows the common exchange ratio that would determine the per share consideration to be received by Genesoft stockholders assuming different merger closing dates and closing financing prices:
| Merger Closing Date | ||||||||
| February 28, 2004 | March 31, 2004 | April 30, 2004 | ||||||
| Closing Financing Price | Prices of $2.84 or higher | 1.188 | 1.186 | 1.184 | ||||
$2.66 | 1.171 | 1.170 | 1.168 | |||||
$2.48 | 1.153 | 1.151 | 1.149 | |||||
$2.30 | 1.131 | 1.129 | 1.127 | |||||
$2.12 | 1.106 | 1.103 | 1.101 | |||||
Exchange of Genesoft Certificates
Within three business days after the effective time of the merger, Genome and the exchange agent will cause to be mailed to each record holder of certificates representing Genesoft stock a letter of transmittal and instructions for use in surrendering such certificates and receiving the merger consideration therefor. Upon the surrender of each such certificate for cancellation to the exchange agent, together with a properly completed and executed letter of transmittal and such other documents as may reasonably be required by Genome, Genome will cause to be issued to the holder of such Genesoft stock certificate in exchange therefore a separate stock certificate representing the number of Genome shares due to such holder and the certificate representing Genesoft stock will be cancelled. Each such Genesoft stock certificate will represent solely the right to receive the merger consideration therefor until so surrendered and exchanged.
82
Table of Contents
Representations and Warranties
The merger agreement contains representations and warranties by each of Genesoft, Genome, and Guardian as to, among other things:
| • | Corporate organization and similar corporate matters |
| • | Capitalization |
| • | Authorization of the merger agreement and required consents, approvals and permits |
| • | Financial statements |
| • | Absence of material changes or events |
| • | Absence of litigation |
| • | Employee benefit plans and employee matters |
| • | Contracts |
| • | Environmental matters |
| • | Intellectual property |
| • | Filing of tax returns and payment of taxes |
| • | Assets; absence of liens and encumbrances |
| • | Affiliate transactions |
| • | Insurance |
In the merger agreement, Genesoft also made representations and warranties as to:
| • | Its ownership of lots of FACTIVE drug product and the saleability of that product |
In the merger agreement, Genome and Guardian also made representations and warranties as to:
| • | Genome’s filings with the Securities and Exchange Commission |
| • | Interim operations of Guardian |
| • | Valid issuance of shares of Genome common stock |
Conduct of Business Pending the Merger
Pursuant to the merger agreement, Genesoft has agreed that prior to the effective time of the merger, Genesoft will conduct its business only in the ordinary and usual course consistent with past practice and will use all reasonable efforts to preserve the business, and maintain good relations with, its employees, customers, suppliers, and distributors. Except as expressly contemplated by the merger agreement (including the schedules thereto), or as approved by Genome (which consent will not be unreasonably withheld), Genesoft will not take certain actions, including the following:
| • | amend or otherwise change its certificate of incorporation or by-laws or equivalent organizational documents; |
| • | issue, sell, pledge, dispose of, grant, encumber, authorize or propose the issuance, sale, pledge, disposition, grant or encumbrance of any shares of its capital stock of any class, or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital stock or any other ownership interest (including, without limitation, any phantom interest), of Genesoft, except pursuant to the terms of options, warrants or preferred stock outstanding on the date of the merger agreement and except for grants of options to purchase up to 100,000 shares of Genesoft common stock pursuant to Genesoft’s stock plan; |
83
Table of Contents
| • | sell, lease, license, pledge, grant, encumber or otherwise dispose of any of its properties or assets unrelated to FACTIVE, except in the ordinary course of business, consistent with past practice; |
| • | sell, lease, license, pledge, grant, encumber or otherwise dispose of any of its properties or assets related to FACTIVE, including any co-promotion agreement relating to FACTIVE; |
| • | declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital stock; |
| • | cancel any indebtedness or waive any claims or rights of substantial value; |
| • | make any change in any method of accounting or accounting practice or policy other than those required by U.S. GAAP; |
| • | enter into, amend, modify or terminate any contract, commitment or agreement related to Genesoft’s ability to sell, have sold, market, develop, distribute, import or manufacture FACTIVE (including, without limitation, any co-promotion agreement related to FACTIVE), or waive any material rights thereunder; |
| • | enter into, amend, modify or terminate any contract, commitment or agreement that obligates Genesoft to make payments other than any payments contemplated and permitted by the pre-closing budget and spending plan agreed to by Genesoft and Genome; |
| • | split, combine, subdivide, redeem or reclassify any of its capital stock or issue or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of its capital stock, or purchase or otherwise acquire, directly or indirectly, any shares of its capital stock except from former employees, directors and consultants in accordance with agreements providing for the repurchase of shares in connection with any termination of service by such party; |
| • | acquire (including, without limitation, by merger, consolidation, or acquisition of stock or assets) any interest or any assets in any corporation, partnership, other business organization or any division thereof; |
| • | incur, other than to Genome, any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise as an accommodation become responsible for, the obligations of any person, or make any loans or advances; |
| • | authorize any capital expenditure in excess of $50,000, in the aggregate; |
| • | enter into any lease or contract for the purchase or sale of any property, real or personal, except in the ordinary course of business, consistent with past practice; |
| • | increase, or agree to increase, the compensation payable, or to become payable, to its officers or employees, except for increases in accordance with past practice in salaries or wages of its employees who are not its officers, or grant any severance or termination pay to, or enter into any employment or severance agreement with, any of its directors, officers or other employees, or establish, adopt, enter into or amend any collective bargaining, bonus, profit sharing, compensation, stock option, restricted stock, retirement, employment, severance or other plan, agreement, or arrangement for the benefit of any director, officer or employee; except for amendments that may be required by law; |
| • | accelerate, amend or change the period of exercisability or the vesting schedule of restricted stock or options granted under any option plan, employee stock plan or other agreement or authorize cash payments in exchange for any options granted under any of such plans except as specifically required by the terms of such plans or any such agreement; |
| • | extend any offers of employment to potential employees who would receive cash compensation at a rate of $100,000 per year or more or extend any consulting or independent contracting offers that are not cancelable on prior notice of 30 days or less; |
84
Table of Contents
| • | other than as contemplated by the merger agreement, enter into, or perform, any transaction with, or for the benefit of any affiliate of Genesoft (other than payments made to officers, directors and employees in the ordinary course of business); |
| • | initiate any clinical trial; |
| • | schedule or conduct any meeting with FDA or other regulatory authority; |
| • | settle any litigation; |
| • | amend, modify or terminate in any material respect any material contract; |
| • | waive any rights related to confidentiality under any contract or agreement; |
| • | make, change or revoke any material tax election, elect or change any method of accounting for tax purposes, settle any action in respect of taxes or enter into any contractual obligation in respect of taxes with any tax authority; or |
| • | authorize any of, or commit or agree to take, whether in writing or otherwise, to do any of the foregoing actions. |
Pursuant to the merger agreement, Genome has agreed that prior to the effective time of the merger, Genome will conduct its business only in the ordinary and usual course consistent with past practice and will use all reasonable efforts to preserve the business, and maintain good relations with, its employees, customers, suppliers, and distributors. Except as expressly contemplated by the merger agreement (including the schedules thereto), or as approved by Genesoft (which consent may not be unreasonably withheld), Genome will not take certain actions, including the following:
| • | amend or otherwise change its certificate of incorporation or by-laws or equivalent organizational documents; |
| • | issue, sell, pledge, dispose of, grant, encumber, authorize or propose the issuance, sale, pledge, disposition, grant or encumbrance of any shares of its capital stock of any class, or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital stock or any other ownership interest (including, without limitation, any phantom interest), of Genome, except pursuant to the terms of options, warrants or preferred stock outstanding on the date of the merger agreement and except for grants of options to purchase up to 644,000 shares of Genome common stock pursuant to Genome’s stock plan; |
| • | sell, lease, license, pledge, grant, encumber or otherwise dispose of any of its properties or assets, except in the ordinary course of business, consistent with past practice; |
| • | declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital stock; |
| • | cancel any indebtedness or waive any claims or rights of substantial value; |
| • | make any change in any method of accounting or accounting practice or policy other than those required by U.S. GAAP; |
| • | split, combine, subdivide, redeem or reclassify any of its capital stock or issue or authorize the issuance of any other securities in respect of shares of its capital stock, or purchase or otherwise acquire, directly or indirectly, any shares of its capital stock except from former employees, directors and consultants in accordance with agreements providing for the repurchase of shares in connection with any termination of service by such party; |
| • | acquire (including, without limitation, by merger, consolidation, or acquisition of stock or assets) any interest or any assets in any corporation, partnership, other business organization; |
85
Table of Contents
| • | incur, other than to Genesoft, any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise as an accommodation become responsible for, the obligations of any person, or make any loans or advances; |
| • | authorize any capital expenditure in excess of $100,000, in the aggregate; |
| • | enter into any lease or contract for the purchase or sale of any property, real or personal except in the ordinary course of business, consistent with past practice; |
| • | increase, or agree to increase, the compensation payable, or to become payable, to its officers or employees, except for increases in accordance with past practice in salaries or wages of its employees who are not its officers, or grant any severance or termination pay to, or enter into any employment or severance agreement with, any of its directors, officers or other employees, or establish, adopt, enter into or amend any collective bargaining, bonus, profit sharing, compensation, stock option, restricted stock, retirement, employment, severance or other plan, agreement, or arrangement for the benefit of any director, officer or employee; except for amendments that may be required by law; |
| • | accelerate, amend or change the period of exercisability or the vesting schedule of restricted stock or options granted under any option plan, employee stock plan or other agreement or authorize cash payments in exchange for any options granted under any of such plans except as specifically required by the terms of such plans or any such agreement; |
| • | extend any offers of employment to potential employees who would receive cash compensation at a rate of $100,000 per year or more or extend any consulting or independent contracting offers that are not cancelable on prior notice of 30 days or less; |
| • | other than as contemplated by the merger agreement, enter into, or perform, any transaction with, or for the benefit of any affiliate of Genome (other than payments made to officers, directors and employees in the ordinary course of business); |
| • | initiate any clinical trial; |
| • | schedule or conduct any meeting with FDA or other regulatory authority; |
| • | settle any litigation; |
| • | amend, modify or terminate in any material respect any material contract or waive any material rights thereunder; |
| • | waive any rights related to confidentiality under any contract or agreement; |
| • | make, change or revoke any material tax election, elect or change any method of accounting for tax purposes, settle any action in respect of taxes or enter into any contractual obligation in respect of taxes with any tax authority; or |
| • | authorize any of, or commit or agree to take, whether in writing or otherwise, to do any of the foregoing actions. |
Covenants
Both Genome and Genesoft have agreed not to solicit, initiate or knowingly encourage (including by way of furnishing nonpublic information), any inquiries or the making of any proposal by any person concerning a competing merger or other acquisition transaction, or enter into or maintain or continue discussions or negotiate with any person in furtherance of any inquiries or agree to or endorse any other merger or acquisition transaction; except that either company’s board of directors may furnish information to, or entering into discussions or negotiations with, any person or entity that makes a “superior proposal.” A “superior proposal” means an unsolicited proposal or offer regarding a merger or acquisition transaction that the company’s board of directors determines in good faith provides greater value to the company’s stockholders than the merger between Genome
86
Table of Contents
and Genesoft, is not subject to regulatory approvals that give rise to a significant risk that the proposal will not be consummated and for which financing is committed or, in the good faith judgment of the company’s board of directors, is reasonably capable of being obtained.
Genome and Genesoft have agreed to notify the other promptly after receipt of any proposal for, or inquiry respecting, any competing merger or other acquisition transaction, or any request for nonpublic information in connection with such proposal. Genome and Genesoft have agreed to cease and terminate all discussions or negotiations concerning competing merger or acquisition transactions that existed prior to the signing of the merger agreement.
Each of Genome and Genesoft has agreed that it will afford to the other party access, at all reasonable times, from the date of the merger agreement to the effective time of the merger, to its properties, records, contracts and personnel.
Genesoft has agreed to use its reasonable best efforts to obtain stockholder approval of the merger agreement.
Genome has agreed to use its reasonable best efforts to obtain stockholder approval of the increase in the number of authorized shares of Genome common stock and the issuance, pursuant to the merger agreement, of shares of Genome common stock to Genesoft stockholders.
Each of Genome and Genesoft has agreed to notify the other in writing promptly after learning of any claim, action, suit, arbitration, mediation, proceeding or investigation by or before any court, arbitrator or arbitration panel, board or other governmental entity initiated by it or against it, or known by it to be threatened against it or any of its officers, directors, employees or stockholders in their official capacity.
Pursuant to the merger agreement, Genome and Genesoft have agreed to give each other prompt notice of (i) the occurrence, or non-occurrence, of any event the occurrence, or non-occurrence, of which would be likely to cause any representation or warranty contained in the merger agreement to be untrue or inaccurate, in any material respect, or any covenant, condition or agreement contained in the merger agreement not to be complied with or satisfied, in any material respect; and (ii) any failure or inability of Genome or Genesoft, as the case may be, to comply, in any material respect, with or satisfy any covenant, condition or agreement to be complied with or satisfied by it under the merger agreement.
Genome and Genesoft have agreed to comply with, and will cause their respective representatives to comply with, all of their respective obligations under the confidentiality agreement dated as of July 29, 2003, by and between Genome and Genesoft.
Genome has agreed to indemnify and hold harmless Genesoft’s past and present officers and directors for a period of six years following the closing of the merger to the same extent they are indemnified on the date of the merger agreement for acts or omissions prior to the closing of the merger. Genome will provide such insurance for the same period on terms no less favorable than in effect on the date of the merger agreement, provided that Genome will not be required to pay an annual premium for the insurance in excess of approximately $54,000.
Genome has agreed to use its reasonable best efforts to enter into and complete agreements to sell shares of its common stock, warrants, convertible notes or notes in an amount equal to at least $32 million.
Genome and Genesoft have agreed to take all appropriate action and do all things necessary to consummate the merger and the other transactions contemplated by the merger agreement.
87
Table of Contents
Conditions to Completion of the Merger
The obligations of Genesoft and Genome to consummate the merger are subject to the satisfaction or waiver, where permissible, of certain conditions, including the following:
| • | The merger agreement must be approved and adopted by the requisite affirmative vote of the stockholders of Genesoft in accordance with applicable law and Genesoft’s certificate of incorporation and by-laws; |
| • | The increase in Genome authorized shares and the issuance of shares to Genesoft stockholders must be approved and adopted by the requisite affirmative vote of the stockholders of Genome in accordance with the Nasdaq rules and Genome’s by-laws; |
| • | No governmental entity or court of competent jurisdiction located or having jurisdiction in the United States will have enacted, issued, promulgated, enforced or entered any statute, rule, regulation, decree, judgment, injunction or other order, whether temporary, preliminary or permanent which is then in effect and has the effect of making the merger illegal or otherwise prohibiting consummation of the merger; |
| • | Any waiting period (and any extension thereof) applicable to the consummation of the merger under the HSR Act will have expired or been terminated; |
| • | Genome will have obtained the proceeds from the sale of at least $32 million of Genome securities; |
| • | The appropriate registration statement relating to the issuance of the shares of Genome common stock pursuant to the merger agreement must have become effective under the Securities Act of 1933, as amended, and will not be the subject of any stop order or proceeding seeking a stop order; and |
| • | The exchange by Genesoft note holders of Genesoft promissory notes for Genome promissory notes must have occurred. |
The obligation of Genesoft to consummate the merger is subject to the satisfaction or waiver, where permissible, of certain additional conditions, including:
| • | The representations and warranties made by Genome in the merger agreement must be true and correct as of the effective time of the merger except (i) that those representations and warranties that address matters only as of a particular date must remain true and correct as of such date, (ii) for changes contemplated by the merger agreement, and (iii) where the failure of the representations and warranties to be so true and correct would not be reasonably likely to have a material adverse effect, and Genesoft must have received a certificate of the Chief Executive Officer of Genome to that effect; |
| • | Each of Genome and Guardian must have performed or complied in all material respects with all agreements and covenants required by the merger agreement to be performed or complied with by it on or prior to the effective time of the merger, and Genesoft must have received a certificate of a duly authorized officer of Genome to that effect; |
| • | Genesoft must have received the opinion of Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP (counsel to Genesoft) to the effect that the merger will be treated for Federal income tax purposes as a reorganization qualifying under the provisions of Section 368(a) of the United States Internal Revenue Code of 1986, as amended, which opinion must not have been withdrawn or modified in any material respect; |
| • | Genesoft must have received the opinion of Ropes & Gray LLP (counsel to Genome) substantially in the form attached to the merger agreement; |
| • | If necessary, Genome must have filed with the Nasdaq National Market a Notification Form for Listing Additional Shares with respect to the shares of Genome common stock to be issued pursuant to the merger agreement and pursuant to Genesoft options; |
88
Table of Contents
| • | Since September 27, 2003, there will have occurred no events nor will there exist any circumstances which singly or in the aggregate have resulted in, or are reasonably likely to result in, a material adverse effect on Genome; |
| • | Genome and an escrow agent must have entered into an escrow agreement as provided by the merger agreement; |
| • | Genome must have taken all necessary action to constitute the Genome board of directors, effective upon the effective time of the merger, as provided in the merger agreement; and |
| • | Genome must have taken all necessary action to appoint, effective upon the effective time of the merger, the executive officers listed in the merger agreement. |
The obligation of Genome to consummate the merger is subject to the satisfaction or waiver, where permissible, of certain additional conditions, including:
| • | The representations and warranties made by Genesoft in the merger agreement must be true and correct as of the effective time of the merger except (i) that those representations and warranties that address matters only as of a particular date will remain true and correct as of such date, (ii) for changes contemplated by the merger agreement, and (iii) where the failure of such representations and warranties to be so true and correct would not be reasonably likely to have a material adverse effect, and Genome must have received a certificate of the Chief Executive Officer of Genesoft to that effect; |
| • | Genesoft must have performed or complied in all material respects with all agreements and covenants required by the merger agreement to be performed or complied with by it on or prior to the effective time of the merger and Genome must have received a certificate of the Chief Executive Officer of Genesoft to that effect; |
| • | Genesoft must have received the consents and approvals set forth in the Genesoft disclosure schedule to the merger agreement and all other authorizations, consents, orders and approvals the failure of which to obtain would be reasonably likely to have a material adverse effect on Genesoft; |
| • | Genome must have received the opinion of Ropes & Gray LLP (counsel to Genome) to the effect that the merger will be treated for Federal income tax purposes as a reorganization qualifying under the provisions of Section 368(a) of the United States Internal Revenue Code of 1986, as amended, which opinion must not have been withdrawn or modified in any material respect; |
| • | Since September 30, 2003, there will have occurred no events nor will there exist any circumstances which singly or in the aggregate have resulted in, or are reasonably likely to result in, a material adverse effect on Genesoft; |
| • | Genome must have received written confirmation of matters relating to Genome’s license to FACTIVE from LG Life Sciences, Ltd., in a form and substance agreed to by Genome and Genesoft prior to the date of the merger agreement; |
| • | Genome must be reasonably satisfied that LG Life Sciences has the capability to and will, upon the terms set forth in the License and Option Agreement by and between Genesoft and LG Life Sciences dated as of October 22, 2002, as amended, including, without limitation, the product specifications and manufacturing practices referred to in the agreement, supply the active pharmaceutical ingredient in FACTIVE and FACTIVE final product (as provided in the License Agreement) to Genesoft in a timeframe and in sufficient amounts to meet the need for FACTIVE final product anticipated by Genesoft and Genome; |
| • | Each of the Third Amended and Restated Investors Rights Agreement by and between Genesoft, John D. Baldeschwieler, Peter B. Dervan, Ph.D., and the other stockholders party to that agreement, dated as of August 8, 2002, as amended, the Fifth Amended and Restated Voting Agreement by and between Genesoft and the other stockholders party to that agreement, dated as of August 8, 2001, and all rights to observe or obtain notice of meetings of the Board of Directors of Genesoft must have been terminated effective as of the effective time of the merger; |
89
Table of Contents
| • | There must not be pending any suit, action, investigation or proceeding to which a governmental entity is a party prohibiting the consummation of the merger or any of the other transactions contemplated by the merger agreement; |
| • | The escrow agent and the stockholders’ representative must have entered into an escrow agreement; |
| • | Genome must have received the opinion of Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP (counsel to Genesoft), reasonably satisfactory to Genome, substantially in the form attached to the merger agreement; |
| • | Genome must have received a certificate executed by the Secretary of Genesoft attaching and certifying as to matters customary for a transaction of this sort; |
| • | Genesoft must have taken appropriate steps, which steps are reasonably satisfactory to Genome, to ensure that the consummation of the merger or any of the other transactions contemplated by the merger agreement will not, by themselves or after taking into account the satisfaction of any other condition or conditions or the occurrence of any other event or events, result in the payment of any “excess parachute payment” within the meaning of Section 280G of the United States Internal Revenue Code of 1986, as amended; |
| • | Genesoft will have delivered to Genome a certification (in such form as may be reasonably requested by counsel to Genome) conforming to the requirements of Treasury Regulations 1.1445-2(c)(3) and 1.897-2(h); and |
| • | Genesoft will have amended its certificate of incorporation so that no Genesoft preferred stock will be authorized. |
Voting Agreement
Each of the directors and executive officers of Genome, who collectively hold an aggregate of approximately 0.5% of the outstanding shares of Genome’s stock, have agreed to vote their shares of Genome stock in favor of the increase in Genome’s authorized shares and the issuance of Genome common stock to the Genesoft stockholders pursuant to the merger agreement, in favor of any other matter relating to consummation of the transactions contemplated by the merger agreement and against any other merger or similar transaction involving Genome.
Stockholders of Genesoft, who collectively hold an aggregate of approximately 63% of the shares of Genesoft’s stock outstanding as of November 30, 2003, have agreed to vote their shares in favor of the merger agreement, in favor of any other matter relating to consummation of the transactions contemplated by the merger agreement and against any other merger or similar transaction involving Genesoft. The percentage of shares subject to these agreements will be reduced if Genesoft’s board of directors changes its recommendations in favor of the merger agreement due to a superior proposal.
Indemnification
If the merger agreement is approved and the merger occurs, all holders of Genesoft capital stock who have not perfected appraisal or dissenters rights under Delaware law or California law, as applicable, will be deemed to have agreed, subject to the limitations described below, to indemnify Genome and its affiliates, officers, directors and employees against losses due to:
| • | any inaccuracy or breach of any representation or warranty of Genesoft contained in the merger agreement or any certificate required to be delivered in connection with the merger agreement; |
| • | any breach of, non-compliance with or non-fulfillment of any covenant or agreement made by Genesoft in the merger agreement or any certificate required to be delivered in connection with the merger agreement; |
90
Table of Contents
| • | any fraudulent action, and any violation of any criminal law by Genesoft; or |
| • | any claim by a holder or former holder of Genesoft’s equity interests or any other person seeking to assert, or based upon: (i) ownership or rights of ownership to any shares of capital stock of Genesoft; (ii) any rights of a stockholder of Genesoft, including any option, preemptive rights, rights to notice or to vote or any appraisal rights under the applicable provisions of the DGCL; (iii) any rights under the organizational documents of Genesoft; (iv) any claim that his, her or its equity interests were wrongfully repurchased, canceled, terminated or otherwise limited by Genesoft; or (v) any claim in connection with the issuance of any equity interests or otherwise, regardless of whether an action, suit or proceeding can or has been made against Genesoft. |
Any party seeking indemnification under the merger agreement must deliver notice to the indemnifying party within 30 days of the incurrence of any losses. Genome’s right to indemnification is limited to the merger consideration placed in escrow pursuant to the escrow agreement attached to this joint proxy statement/prospectus as Annex B (and described on page97), representing 20% of the Genome common stock that would otherwise be due to Genesoft stockholders in the merger. If a holder of a Genesoft option assumed by Genome pursuant to the merger agreement exercises any portion of such holder’s option prior to the termination of the escrow fund, such holder will contribute a portion of the shares of Genome common stock issued upon exercise to the escrow fund in accordance with the provisions of the escrow agreement. Genome is entitled to indemnification after all losses exceed $676,458.64 in the aggregate, and then only for losses in excess of such amount. Luke Evnin will serve as stockholders’ representative under the escrow agreement. Subject to any claims made by Genome or its affiliates, officers, directors or employees and any payments related to those claims, up to one-half of the indemnity escrow amount will be released from escrow one year after closing of the merger and the remainder will be released 18 months after closing. Luke Evnin is a general partner of MPM Capital. MPM Capital is a direct or indirect parent and/or control person of MPM Asset Management LLC, funds managed or advised by it (including BB BioVentures LP, MPM Bio Ventures Parallel Fund, LP and MPM Asset Management Investors 1998 LLC), and is the general partner of such funds. BB BioVentures LP directly owns 1,779,496 shares of Genesoft common stock, MPM BioVentures Parallel Fund, LP directly owns 254,372 shares of Genesoft common stock and MPM Asset Management Investors 1998 LLC directly owns 23,659 shares of Genesoft common stock, in each case, as of November 30, 2003.
ADOPTION AND APPROVAL OF THE MERGER AGREEMENT BY GENESOFT’S STOCKHOLDERS SHALL CONSTITUTE APPROVAL BY SUCH STOCKHOLDERS OF THE INDEMNIFICATION OBLIGATIONS SET FORTH IN THE MERGER AGREEMENT, THE TERMS OF THE ESCROW AGREEMENT AND THE AUTHORITY OF THE STOCKHOLDER’S REPRESENTATIVE.
Genome has also agreed to indemnify Genesoft’s stockholders against losses due to:
| • | any inaccuracy or breach of any representation or warranty of Genome contained in the merger agreement or any certificate required to be delivered in connection with the merger agreement; |
| • | any breach of, non-compliance with or non-fulfillment of any covenant or agreement made by Genome in the merger agreement or any certificate required to be delivered in connection with the merger agreement; or |
| • | any fraudulent action, and any violation of any criminal law by Genome. |
The aggregate indemnification obligations of Genome will not exceed $13,529,172.87 and are available once Genesoft losses exceed $676,458.64 in the aggregate, and then for losses in excess of such amount.
91
Table of Contents
Termination of the Merger Agreement
Genome and Genesoft may terminate the merger agreement by mutual written consent duly authorized by the boards of directors of each of Genome and Genesoft. In addition, either Genome or Genesoft may terminate the merger agreement if:
| • | The merger has not been consummated by April 30, 2004; provided that the party seeking to terminate has not materially breached its obligations in a manner that contributed to the failure to complete the merger; and provided further, that this period shall be extended to May 30, 2004 if the merger has not closed by April 30, 2004 as a result of the related registration statement filed with the Securities and Exchange Commission not having become effective; |
| • | Genome or Genesoft’s respective stockholders have not approved the merger and related transactions; or |
| • | There is any final and non-appealable order preventing consummation of the merger. |
The merger agreement may also be terminated by Genome if:
| • | Genesoft has breached any representation, warranty, covenant or agreement, or any representation or warranty has become untrue, except for such exceptions as are provided in the merger agreement; provided, that if the breach is curable, and for so long as Genesoft continues to exercise best efforts to cure the breach, Genome may not terminate; |
| • | Genesoft’s board shall have withdrawn or adversely modified its approval or recommendation of the merger; |
| • | Genesoft’s board shall have recommended to the stockholders a competing merger or acquisition transaction or shall have entered into any letter of intent accepting any competing merger or acquisition transaction; |
| • | Genesoft’s board fails to reject a competing merger or acquisition transaction within 10 days following public announcement or receipt of the proposal for such competing merger or acquisition transaction; |
| • | Genesoft shall have failed to include in the joint proxy statement its board recommendation of the merger or shall have failed to hold its stockholder meeting as promptly as practicable; |
| • | Genesoft’s board fails to reaffirm its board recommendation of the merger within five business days after Genome requests in writing that such recommendation be reaffirmed; or |
| • | Genesoft shall have willfully breached its non-solicitation obligations. |
The merger agreement may also be terminated by Genesoft if:
| • | Genome and Guardian have breached any representation, warranty, covenant or agreement, or any representation or warranty has become untrue, except for such exceptions as are provided in the merger agreement; provided, that if the breach is curable, and for so long as Genome and Guardian continue to exercise best efforts to cure the breach, Genesoft may not terminate; |
| • | Genome’s board shall have withdrawn or adversely modified its approval or recommendation of the merger; |
| • | Genome’s board shall have recommended to the stockholders a competing merger or acquisition transaction or shall have entered into any letter of intent accepting any competing merger or acquisition transaction; |
| • | Genome’s board fails to reject a competing merger or acquisition transaction within 10 days following public announcement or receipt of the proposal for such competing merger or acquisition transaction; |
| • | Genome shall have failed to include in the joint proxy statement its board recommendation or shall have failed to hold its stockholder meeting as promptly as practicable; |
92
Table of Contents
| • | Genome’s board fails to reaffirm its board recommendation of the merger within five business days after Genesoft requests in writing that such recommendation be reaffirmed; or |
| • | Genome shall have willfully breached its non-solicitation obligations. |
Termination Fees
Genesoft has agreed to pay Genome’s transaction expenses up to $1,000,000 if the merger agreement is terminated:
| • | By Genome or Genesoft due to the failure of Genesoft’s stockholders to approve the merger; |
| • | By Genome due to Genesoft’s board withdrawing or adversely modifying its approval or recommendation of the merger; |
| • | By Genome due to Genesoft’s board recommending to the stockholders a competing merger or acquisition transaction or entering into any letter of intent accepting any competing merger or acquisition transaction; |
| • | By Genome due to Genesoft’s board’s failure to reject a competing merger or acquisition transaction within 10 days following public announcement or receipt of the proposal for such competing merger or acquisition transaction; |
| • | By Genome due to Genesoft’s failure to include in the joint proxy statement its board recommendation of the merger or failure to hold its stockholder meeting as promptly as practicable; |
| • | By Genome due to Genesoft’s board’s failure to reaffirm its board recommendation of the merger within five business days after Genome requests in writing that such recommendation be reaffirmed; or |
| • | By Genome due to Genesoft willfully breaching its non-solicitation obligations. |
Genesoft has agreed to pay Genome an additional $3,044,063.90 if the merger is terminated for any of the above reasons and within 12 months of such termination Genesoft enters into (or announces its intention to enter into) an agreement to consummate a competing merger or acquisition transaction.
Genome has agreed to pay Genesoft’s transaction expenses up to $1,000,000 if the merger agreement is terminated:
| • | By Genesoft or Genome due to the failure of Genome’s stockholders to approve the merger; |
| • | By Genesoft due to Genome’s board withdrawing or adversely modifying its approval or recommendation of the merger; |
| • | By Genesoft due to Genome’s board recommending to the stockholders a competing merger or acquisition transaction or entering into any letter of intent accepting any competing merger or acquisition transaction; |
| • | By Genesoft due to Genome’s board’s failure to reject a competing merger or acquisition transaction within 10 days following public announcement or receipt of the proposal for such competing merger or acquisition transaction; |
| • | By Genesoft due to Genome’s failure to include in the joint proxy statement its board recommendation or failure to hold its stockholder meeting as promptly as practicable; |
| • | By Genesoft due to Genome’s board’s failure to reaffirm its board recommendation of the merger within five business days after Genesoft requests in writing that such recommendation be reaffirmed; or |
| • | By Genesoft due to Genome willfully breaching its non-solicitation obligations. |
Genome has agreed to pay Genesoft an additional $3,044,063.90 if the merger is terminated for any of the above reasons and within 12 months of such termination Genome enters into (or announces its intention to enter into) an agreement to consummate a competing merger or acquisition transaction.
93
Table of Contents
Amendment of the Merger Agreement
Genesoft and Genome may mutually amend any provision of the merger agreement at any time prior to the closing by action taken by or on behalf of their respective boards of directors. Any amendment of the merger agreement must be in writing and signed by all of the parties. Any waiver of any right or remedy under the merger agreement must be in writing and signed by the party giving such waiver.
Material United States Federal Income Tax Consequences of the Merger
The discussion below summarizes the material United States federal income tax considerations of the merger generally applicable to common stockholders of Genesoft who are “United States persons,” as defined for United States federal income tax purposes, and who hold their Genesoft common stock as a capital asset. For United States federal income tax purposes, a “United States person” is:
| • | a United States citizen or resident alien as determined under the Internal Revenue Code; |
| • | a corporation or partnership (as defined by the Internal Revenue Code) that is organized under the laws of the United States, any state or the District of Columbia; |
| • | an estate, the income of which is subject to United States federal income taxation regardless of its source; or |
| • | a trust, if a court within the United States is able to exercise primary supervision over its administration and at least one United States person is authorized to control all of its substantial decisions. |
The discussion below is based on current provisions of the Internal Revenue Code, currently applicable United States Treasury regulations promulgated thereunder, and judicial and administrative decisions and rulings.
No ruling from the Internal Revenue Service has been or will be sought. Future legislative, judicial or administrative changes or interpretations could alter or modify the statements and conclusions set forth herein, and such changes or interpretations could be retroactive and could affect the tax consequences of the merger to Genome, Genesoft and the stockholders of Genesoft.
The discussion below does not purport to deal with all aspects of federal income taxation that may affect particular stockholders or note holders in light of their individual circumstances or that may affect stockholders subject to special treatment under federal income tax law. Stockholders subject to special treatment include insurance companies, tax-exempt organizations, financial institutions, mutual funds, broker-dealers, foreign individuals and entities, stockholders subject to the alternative minimum tax provisions of the Internal Revenue Code, stockholders who hold Genesoft capital stock as qualified small business stock within in the meaning of Section 1202 of the Internal Revenue Code, stockholders who hold their stock as part of a hedge, wash sale, appreciated financial position, straddle, conversion or other risk reduction transaction, and stockholders who have acquired their stock upon exercise of employee options or otherwise as compensation. In addition, the discussion below does not consider the effect of any applicable state, local or foreign tax laws, nor does it consider the tax consequences of other transactions effectuated before, after or concurrently with the merger (whether or not any such transaction are undertaken in connection with the merger, including, but not limited to, transactions effected pursuant to the note amendment and exchange agreement entered into by and among Genome, Genesoft and holders of certain Genesoft promissory notes). Finally, the discussion below does not consider the tax consequences of the merger to holders of notes or of options, warrants or other similar rights to acquire Genesoft stock, including the assumption by Genome of outstanding options and subscriptions to acquire Genesoft stock.
You are urged to consult with your tax advisor as to the tax consequences of the merger to you in light of your particular circumstances, including the applicability and effect of any state, local or foreign tax laws.
94
Table of Contents
It is the opinion of Genome’s counsel, Ropes & Gray LLP, and Genesoft’s counsel, Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP, that, for United States federal income tax purposes, the merger of a wholly-owned subsidiary of Genome with and into Genesoft, and the immediate subsequent merger of the surviving entity with and into a second wholly-owned subsidiary of Genome, will be treated as a reorganization within the meaning of section 368(a) of the Internal Revenue Code. It is a condition to completion of the merger that Ropes & Gray LLP and Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP issue such opinions to Genome and to Genesoft, respectively. Such opinions will be based on certificates executed by officers of Genome and Genesoft containing representations regarding past, current and future matters that are customary for transactions of this nature. If any of the representations is inaccurate or incorrect, the conclusions stated in the opinions could be affected. The opinions will not be binding on the Internal Revenue Service or the courts, and there can be no assurance that the Internal Revenue Service or the courts will not take a contrary view.
The following are the material United States federal income tax consequences that will generally result from treatment of the merger as a reorganization described in Section 368(a) of the Internal Revenue Code:
| • | A holder of Genesoft common stock will not recognize any gain or loss upon the receipt of Genome common stock (including escrowed shares) in exchange for Genesoft common stock in the merger. |
| • | A Genesoft stockholder’s aggregate tax basis in the Genome common stock received in the merger (including escrowed shares) in exchange for such stockholder’s Genesoft common stock will be the same as the aggregate basis of the Genesoft common stock surrendered in the exchange. |
| • | A Genesoft stockholder’s holding period for the Genome common stock received in the merger (including escrowed shares) in exchange for such stockholder’s Genesoft common stock will include the holding period for the Genesoft common stock surrendered in the exchange. |
| • | Holders of Genesoft stock who exercise dissenters’ rights with respect to their shares of Genesoft stock and who receive payment for the shares in cash will generally recognize gain or loss measured by the difference between the amount of cash received and the stockholders’ basis in the shares surrendered, provided that the payment is neither essentially equivalent to a dividend within the meaning of Section 302 of the Internal Revenue Code nor has the effect of a distribution of a dividend within the meaning of Section 356(a)(2) of the Internal Revenue Code. These payments are hereinafter collectively referred to as a “dividend equivalent transaction.” A sale of Genesoft stock pursuant to an exercise of dissenters’ rights will generally not be a dividend equivalent transaction if, as a result of such exercise, such stockholder owns no shares of Genesoft stock (either actually or constructively within the meaning of Section 318 of the Internal Revenue Code) immediately after the merger. If the payment of cash received by a Genesoft stockholder exercising his, her or its dissenters’ rights is considered a dividend equivalent transaction, then such stockholder may recognize ordinary income for federal income tax purposes in an amount equal to the entire amount of cash so received. |
| • | No gain or loss will be recognized by a Genesoft stockholder upon the distribution of escrowed shares to the Genesoft stockholder upon termination of the escrow. |
| • | Upon the distribution of escrowed shares to Genome in satisfaction of an indemnification claim, a Genesoft stockholder will likely recognize gain or loss in an amount equal to the difference between the value of the stockholder’s escrowed shares distributed to Genome (as determined under the escrow agreement) and the stockholder’s adjusted basis in such shares. |
| • | None of Genome, Genesoft, the combined company, or any stockholder of Genome will recognize any gain or loss as a result of the merger. |
As noted above, no ruling from the Internal Revenue Service has been or will be sought in connection with the merger, and the opinions issued by Genome and Genesoft’s respective counsel will not be binding upon the Internal Revenue Service. The Internal Revenue Service is therefore not precluded from asserting a contrary
95
Table of Contents
opinion. If the Internal Revenue Service were to challenge successfully the “reorganization” status of the merger, a holder of Genesoft common stock would recognize gain or loss with respect to such stockholder’s shares of Genesoft common stock surrendered in the merger. The gain or loss would be equal to the difference between (i) the fair market value of the Genome common stock received in the merger, and (ii) the Genesoft stockholder’s adjusted tax basis in the Genesoft common stock surrendered in the merger. Such stockholder’s total tax basis in the Genome common stock received would equal its fair market value and such stockholder’s holding period for the stock would begin the day after the merger.
Each Genesoft stockholder who receives shares of Genome common stock in the merger is required to file a statement with his, her or its federal income tax return setting forth the stockholder’s basis in the shares of Genesoft common stock surrendered and the fair market value of Genome common shares received in the merger, and is required to retain permanent records of these facts.
Accounting Treatment of the Merger
The merger will be accounted for as a purchase by Genome under accounting principles generally accepted in the United States. Under the purchase method of accounting, Genome will be considered the acquiror and the assets and liabilities of Genesoft will be recorded, as of the completion of the merger, at their respective fair values and added to those of Genome. Reported financial condition and results of operations of Genome issued after completion of the merger will reflect Genesoft’s balances and results after completion of the merger, but will not be restated retroactively to reflect the historical financial position or results of operations of Genesoft. Following the completion of the merger, the earnings of the combined company will reflect purchase accounting adjustments, including in-process research and development charges and amortization and depreciation expense for acquired tangible and intangible assets. The most significant of the intangible assets identified will have finite lives and relate to FACTIVE. These amounts will be amortized over their expected useful lives. Goodwill will also be recorded, however, pursuant to SFAS No. 141, “Business Combinations” and SFAS No. 142, “Goodwill and Other Intangible Assets,” goodwill will no longer be subject to amortization. Rather, goodwill be subject to at least an annual assessment for impairment based on a fair value test. For purposes of disclosing pro forma information in this joint proxy statement/prospectus, the combined company has made a preliminary determination of the purchase price allocation, based upon current estimates and assumptions, which is subject to revision upon consummation of the merger.
Regulatory Filings and Approvals Required to Complete the Merger
Neither Genome nor Genesoft is aware of any material governmental or regulatory approval required for completion of the merger, other than compliance with applicable corporate laws of the Commonwealth of Massachusetts and the State of Delaware and federal and state securities laws.
Restrictions on Sales of Genome Common Stock by Affiliates of Genesoft
The shares of Genome common stock to be issued in connection with the merger have been registered under the Securities Act and will be freely transferable under the Securities Act, except for shares of Genome common stock issued to any person who is deemed to be an “affiliate” of Genesoft at the time of the special meetings and shares subject to other contractual restrictions. Persons who may be deemed to be affiliates of Genesoft include individuals or entities that control, are controlled by, or are under common control of Genesoft and may include Genesoft’s officers and directors, as well as its principal stockholders. Affiliates of Genesoft may resell their shares of Genome common stock acquired in connection with the merger only (1) in transactions permitted by Rule 145 under the Securities Act, (2) under an effective registration statement under the Securities Act or (3) in compliance with an exemption from the registration requirements of the Securities Act. Generally, Rule 145 permits resales of stock received in a registered offering by an affiliate of Genesoft as long as Genome has complied with certain reporting requirements and the selling stockholder complies with volume and manner of sale restrictions set forth in Rules 144 and 145.
96
Table of Contents
This joint proxy statement/prospectus does not cover resales of Genome common stock received by any person upon completion of the merger, and no person is authorized to make any use of this joint proxy statement/prospectus in connection with any resale.
Listing on the Nasdaq National Market of Genome Common Stock to be Issued in the Merger
Genome common stock is listed in the Nasdaq National Market under the symbol “GENE.”
Other Material Agreements Relating to the Merger
Escrow Agreement
Upon the closing of the merger, Genome will enter into an escrow agreement with Luke Evnin, as stockholders’ representative of the Genesoft stockholders, and a commercial bank, or its designee, as escrow agent. Under the terms of the merger agreement and the escrow agreement, twenty percent (20%) of the shares of the Genome common stock issuable to the Genesoft stockholders in the merger will be placed in escrow to cover potential indemnity claims by Genome under the merger agreement and an additional 400,000 shares of Genome common stock issuable to the Genesoft stockholders will be placed in escrow to fund potential issuances of equity to a senior clinical development officer that may be hired by our combined company. These amounts will be deducted from the shares of Genome common stock to be received by the Genesoft stockholders on a pro rata basis. Additionally, if a holder of a Genesoft option assumed by Genome pursuant to the merger agreement exercises any portion of such holder’s option prior to the termination of the escrow fund, a portion of the shares of Genome common stock issued upon such exercise will be withheld and contributed to the escrow fund. Subject to any claims made by Genome or its affiliates, officers, directors or employees and any payments related to those claims, up to one half of the indemnity escrow amount will be released from escrow one year after closing of the merger and the remainder, if any, will be released 18 months after closing. Subject to any claims made by Genome and payments related to those claims, the employment escrow amount will be released from escrow 12 months after closing. The escrow agent will disburse the indemnity escrow amounts only pursuant to an instrument signed by Genome and not objected to by the stockholders representative or an order of a court of competent jurisdiction or an arbitrator as provided under the escrow agreement or under the provisions of the escrow agreement governing distribution of the escrow fund following termination of the escrow agreement. The escrow agent will disburse the employment escrow amounts pursuant to an instrument signed by Genome and notice given to the stockholders’ representative. Any amounts withdrawn from the escrow will be withdrawn from each stockholder and optionholder that has contributed to the escrow on a pro rata basis, according to each such stockholder’s or optionholder’s respective share of the escrowed amount. The escrowed shares will be valued for purposes of indemnity claims based on the sales prices per share of Genome common stock on the Nasdaq National Market over the 5 trading days immediately preceding the date distribution of such shares is required under the escrow agreement. A stockholder with shares in escrow will have all rights with respect to the shares in escrow except for the right to possess and sell, assign or pledge such shares.
Bridge Loan
Genome has loaned $6.2 million to Genesoft for operations prior to close. The loan is subject to spending in accordance with a pre-closing budget and spending plan agreed to by Genesoft and Genome. The note issued by Genesoft to Genome is due and payable 60 days following the termination of the merger agreement, except if the merger agreement terminates due to a failure of Genome’s stockholders to approve the merger, in which case the note will be payable 180 days after termination of the merger agreement. The note will become immediately due and payable upon any of the following events of default:
| • | Genesoft fails to pay when due any principal or interest due under the note; |
| • | Genesoft breaches any covenant under the note or the merger agreement; |
| • | Genesoft defaults on any of its other outstanding debt obligations; |
97
Table of Contents
| • | Genesoft enters into bankruptcy, whether voluntarily or involuntarily; |
| • | The merger agreement, the note, or any warrants issued to Genome by Genesoft shall cease to be enforceable; or |
| • | Genesoft spends cash or incurs obligations to expend cash in a manner that does not conform with the pre-closing budget and spending plan. |
Genome has agreed to subordinate its right to payment under the note to the rights of payment of equipment lenders of Genesoft and has agreed with these lenders, under particular circumstances, to forebear from taking action to collect upon the note for up to 180 days from the time it is due. Interest accrues on the note at a rate of 5% per annum, except that the interest will be recalculated at a rate of 4% per month from the date of issuance upon the occurrence of any of the above listed events of default or if the merger terminates as a result of any of the following:
| • | the merger has not been consummated by April 30, 2004 as a result of Genesoft’s failure to satisfy its obligations under the merger agreement; |
| • | Genesoft has breached any representation, warranty, covenant or agreement, or any representation or warranty has become untrue, except for such exceptions as are provided in the merger agreement; |
| • | Genesoft’s board shall have withdrawn or adversely modified its approval or recommendation of the merger; |
| • | Genesoft’s board shall have recommended to the stockholders a competing merger or acquisition transaction or shall have entered into any letter of intent accepting any competing merger or acquisition transaction; |
| • | Genesoft’s board fails to reject a competing merger or acquisition transaction within 10 days following public announcement or receipt of the proposal for such competing merger or acquisition transaction; |
| • | Genesoft shall have failed to include in the joint proxy statement its board recommendation of the merger or shall have failed to hold its stockholder meeting as promptly as practicable; |
| • | Genesoft’s board fails to reaffirm its board recommendation of the merger within five business days after Genome requests in writing that such recommendation be reaffirmed; |
| • | Genesoft shall have willfully breached its non-solicitation obligations; or |
| • | Genesoft’s stockholders fail to approve the merger. |
If the merger terminates for any of the reasons listed above, Genome is entitled to convert all or any portion of the outstanding principal and accrued interest under the promissory note into, at Genome’s option, (i) Genesoft common stock at the per share price of $3.39 or (ii) any consideration that holders of Genesoft notes issued pursuant to the note and warrant purchase agreement dated as of April 15, 2003, as amended, receive or become entitled to receive prior to the repayment date of the promissory note.
Pursuant to the loan, Genome was granted a security interest in all of Genesoft’s assets. This security interest is junior to the security interests of Genesoft’s equipment lessors. In connection with the loan, Genome was issued a warrant to purchase 457,838 shares of Genesoft common stock at $3.39 per share. The warrant is only exercisable if the merger agreement is terminated for one of the reasons listed above and will become null and void upon the completion of the merger with Genesoft.
Note Amendment and Exchange Agreement
Genome, Genesoft, and holders of Genesoft promissory notes issued during financing rounds in December 2002-January 2003 and April-May 2003, referred to as the December and April notes, respectively, have entered into a note amendment and exchange agreement that restructures $22,309,647 of Genesoft notes. As a result of this agreement, the maturity date of the December and April notes was extended to the later of the current
98
Table of Contents
maturity dates of the notes and the date 60 days following the termination of merger agreement. The interest rate of the notes was amended to 5% per annum commencing on December 10, 2003 for the December notes and December 15, 2003 for the April notes. Upon the closing of the merger, the December and April notes will be converted into Genome notes. Outstanding principal under the December and April notes will be converted into the initial principal amount under new promissory notes issued by Genome. These new notes will have a maturity date five years from the date of the closing of the merger and will bear interest at 5% per annum and will be convertible at any time at the option of the holder into shares of Genome common stock at a 10% premium to the average trading price of Genome common stock for the five trading days immediately preceding the date of the closing of the merger. Accrued interest through the closing of the merger under the December and April notes, and the amount that would be payable upon a change in control under the December notes, will be converted into shares of Genome common stock as described in “The Merger—The Merger Agreement—Conversion of Genesoft Stock.” The shares issued at the closing of the merger and the shares issuable upon conversion of the new promissory notes are subject to a registration rights agreement between Genome and the Genesoft note holders. The note amendment and exchange agreement places limitations on Genome’s ability to incur additional debt that has a maturity prior to six months after the maturity of the Genome promissory notes to be issued to the Genesoft note holders.
Registration Rights Agreement
Genome and the holders of the Genesoft promissory notes to be converted pursuant to the note amendment and exchange agreement have entered into a registration rights agreement. Under this agreement, Genome must file a shelf registration statement covering the resale of shares of Genome common stock (i) issued by it as payment in respect of the interest and related amounts and (ii) issuable by it upon conversion of the notes within 30 days of the closing of the merger. Genome must use its best efforts to have this registration statement declared effective within 120 days of the closing of the merger. If Genome fails to meet these deadlines, the former Genesoft debtholders will be entitled to customary damages payments.
99
Table of Contents
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The following unaudited pro forma condensed combined financial statements combine the historical consolidated balance sheets and statements of operations of Genome and Genesoft, giving effect to the merger using the purchase method of accounting under accounting principles generally accepted in the United States and the assumptions and adjustments described below. The unaudited pro forma condensed combined financial statements are presented for illustrative purposes only to aid you in your analysis of the financial aspects of the merger, and do not purport to be indicative of the consolidated financial position and results of operations for future periods or the results that actually would have been realized had Genome and Genesoft been a consolidated company during the specified periods.
The unaudited pro forma condensed combined financial statements are based on the respective audited and unaudited historical consolidated financial statements and the notes thereto of Genome and Genesoft.
The pro forma adjustments were based upon available information and certain assumptions described in the notes to the unaudited pro forma condensed combined financial statements that Genome’s management believes are reasonable under the circumstances. The pro forma adjustments are based on the information available at the date of this joint proxy statement/prospectus and a preliminary determination of the purchase price allocation and are subject to change based on completion of the transaction, and such changes may be material. The closing of the merger is contingent on Genome raising at least $32 million to finance the combined companies (unless waived by both parties). These unaudited pro forma condensed combined financial statements do not include any adjustment to record the expected proceeds from this offering or the dilutive effect of the issuance of shares related to this offering.
The unaudited pro forma condensed combined financial statements and accompanying notes should be read in conjunction with the historical consolidated financial statements and notes thereto of Genome included in its Annual Report on Form 10-K for the year ended December 31, 2002, and its quarterly report on Form 10-Q for the nine months ended September 27, 2003, incorporated by reference in this joint proxy statement/prospectus, and the separate historical financial statements and notes thereto of Genesoft for the year ended December 31, 2002 and the nine months ended September 30, 2003 included in this joint proxy statement/prospectus.
The unaudited pro forma condensed consolidated balance sheet is as of September 27, 2003 as it relates to Genome and is as of September 30, 2003 as it relates to Genesoft. The unaudited pro forma condensed consolidated statements of operations for the year ended December 31, 2002 and for the nine months ended September 27, 2003 assume that the merger occurred as of January 1, 2002. For the interim period, Genome’s nine months ended September 27, 2003 was combined with Genesoft’s nine months ended September 30, 2003.
Under the purchase method of accounting, the total estimated purchase price, calculated as described in Note 1 to these unaudited pro forma condensed combined financial statements, is allocated to the net tangible and intangible assets to be acquired in connection with the merger, based on their estimated fair values. A preliminary valuation and purchase price allocation was conducted to determine the fair value of these assets at the transaction date. This preliminary valuation and purchase price allocation is the basis for the estimates of fair value reflected in these unaudited pro forma condensed combined financial statements.
The unaudited pro forma condensed combined financial information has been prepared based upon available information and certain assumptions described in the accompanying notes and the estimated fair value of assets to be acquired and liabilities to be assumed from Genesoft. The unaudited pro forma condensed combined financial statements do not include any adjustments for liabilities resulting from integration plans.
100
Table of Contents
Unaudited Pro Forma Condensed Consolidated
Statements of Operations
Nine Months Ended September 27, 2003
(in thousands, except per share amounts)
| Genome | Genesoft | Pro Forma Adjustments | Pro Forma Combined | |||||||||||||
Total Revenues | $ | 7,318 | $ | 3,072 | $ | — | $ | 10,390 | ||||||||
Costs and Expenses: | ||||||||||||||||
Cost of revenues | 1,902 | — | — | 1,902 | ||||||||||||
Research and development | 17,541 | 8,896 | 4,514 | (2b) | 30,951 | |||||||||||
Restructuring charge | 4,733 | — | — | 4,733 | ||||||||||||
Convertible debt retirement expense | 5,540 | — | — | 5,540 | ||||||||||||
Selling, general and administrative | 5,463 | 7,306 | 1,311 | (2a) | 14,080 | |||||||||||
Total costs and expenses | 35,179 | 16,202 | 5,825 | 57,206 | ||||||||||||
Loss from operations | (27,861 | ) | (13,130 | ) | (5,825 | ) | (46,816 | ) | ||||||||
Other Income (Expense): | ||||||||||||||||
Other income | 460 | 59 | — | 519 | ||||||||||||
Other expense | (1,054 | ) | (6,725 | ) | — | (7,779 | ) | |||||||||
Net other income (expense) | (594 | ) | (6,666 | ) | — | (7,260 | ) | |||||||||
Net loss | $ | (28,455 | ) | $ | (19,796 | ) | $ | (5,825 | ) | $ | (54,076 | ) | ||||
Net Loss per Common Share: | ||||||||||||||||
Basic and diluted | $ | (1.16 | ) | $ | (1.69 | ) | $ | — | $ | (1.08 | ) | |||||
Weighted Average Shares Used in Computing Net Loss per Share: | ||||||||||||||||
Basic and diluted | 24,581 | 11,729 | — | 50,061 | ||||||||||||
See accompanying notes to pro forma condensed combined financial statements.
101
Table of Contents
Unaudited Pro Forma Condensed Consolidated
Statements of Operations
Year Ended December 31, 2002
(in thousands, except per share amounts)
| Genome | Genesoft | Pro Forma Adjustments | Pro Forma Combined | |||||||||||||
Total Revenues: | $ | 22,987 | $ | 5,402 | $ | — | $ | 28,389 | ||||||||
Costs and Expenses: | ||||||||||||||||
Cost of services | 15,020 | — | — | 15,020 | ||||||||||||
Research and development | 32,435 | 26,283 | 6,018 | (2b) | 64,736 | |||||||||||
Selling, general and administrative | 9,382 | 4,542 | 1,748 | (2a) | 15,672 | |||||||||||
Total costs and expenses | 56,837 | 30,825 | 7,766 | 95,428 | ||||||||||||
Loss from operations | (33,850 | ) | (25,423 | ) | (7,766 | ) | (67,039 | ) | ||||||||
Other Income (Expense): | ||||||||||||||||
Other income | 1,769 | 564 | — | 2,333 | ||||||||||||
Other expense | (1,936 | ) | (710 | ) | — | (2,646 | ) | |||||||||
Net other income (expense) | (167 | ) | (146 | ) | — | (313 | ) | |||||||||
Net loss | $ | (34,017 | ) | $ | (25,569 | ) | $ | (7,766 | ) | $ | (67,352 | ) | ||||
Net Loss per Common Share: | ||||||||||||||||
Basic and diluted | $ | (1.48 | ) | $ | (12.81 | ) | $ | — | $ | (1.39 | ) | |||||
Weighted Average Shares Used in Computing Net Loss per Share: | ||||||||||||||||
Basic and diluted | 22,921 | 1,996 | — | 48,401 | ||||||||||||
See accompanying notes to pro forma condensed combined financial statements.
102
Table of Contents
Unaudited Pro Forma Condensed Consolidated
Balance Statement
September 27, 2003
(in thousands)
| Genome | Genesoft | Pro Forma Adjustments | Pro Forma Combined | |||||||||||||
ASSETS | ||||||||||||||||
Current Assets: | ||||||||||||||||
Cash and cash equivalents | $ | 14,270 | $ | 4,129 | $ | (9,697 | )(2c),(2e) | $ | 8,702 | |||||||
Marketable securities (held-to-maturity) | 9,832 | — | — | 9,832 | ||||||||||||
Marketable securities (available-for-sale) | 983 | — | — | 983 | ||||||||||||
Interest receivable | 240 | — | — | 240 | ||||||||||||
Accounts receivable | 179 | 1,131 | — | 1,310 | ||||||||||||
Unbilled costs and fees | 129 | — | — | 129 | ||||||||||||
Prepaid expenses and other current assets | 350 | 51 | — | 401 | ||||||||||||
Total current assets | 25,983 | 5,311 | (9,697 | ) | 21,597 | |||||||||||
Property and equipment, net | 3,907 | 10,170 | — | 14,077 | ||||||||||||
Long-term marketable securities (held-to-maturity) | 701 | — | — | 701 | ||||||||||||
Restricted cash | — | 3,697 | — | 3,697 | ||||||||||||
Intangible assets | — | 6,575 | 73,797 | (2i) | 80,372 | |||||||||||
Goodwill | — | — | 19,306 | (2i) | 19,306 | |||||||||||
Other assets | 148 | 46 | — | 194 | ||||||||||||
Total Assets | $ | 30,739 | $ | 25,799 | $ | 83,406 | $ | 139,944 | ||||||||
LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||||||||
Current Liabilities: | ||||||||||||||||
Current maturities of long-term obligations | $ | 1,167 | $ | 19,689 | $ | (1,697 | )(2c) | $ | 19,159 | |||||||
Accounts payable | 247 | 1,168 | — | 1,415 | ||||||||||||
Clinical trial expense accrual and other accrued liabilities | 8,544 | 5,447 | 4,000 | (2d) | 17,991 | |||||||||||
Deferred revenue | 852 | — | — | 852 | ||||||||||||
Total Current Liabilities | 10,810 | 26,304 | 2,303 | 39,417 | ||||||||||||
Long-term obligations, net of current maturities | 583 | 6,620 | — | 7,203 | ||||||||||||
Shareholders’ Equity: | ||||||||||||||||
Common stock, par value | 2,617 | 1 | 2,547 | (2f) | 5,165 | |||||||||||
Additional paid-in capital | 170,797 | 68,238 | 19,628 | (2g) | 258,663 | |||||||||||
Accumulated deficit | (154,231 | ) | (75,364 | ) | 63,587 | (2h) | (166,008 | ) | ||||||||
Other shareholders’ equity | 163 | — | (4,659 | ) | (4,496 | ) | ||||||||||
Total Shareholders’ Equity | 19,346 | (7,125 | ) | 81,103 | 93,324 | |||||||||||
Total Liabilities and Shareholders’ Equity | $ | 30,739 | $ | 25,799 | $ | 83,406 | $ | 139,944 | ||||||||
See accompanying notes to pro forma condensed combined financial statements.
103
Table of Contents
Notes to Unaudited Pro Forma Condensed Combined Financial Statements
Note 1—Description of Merger and Purchase Price
On November 17, 2003, Genome entered into a definitive agreement to acquire Genesoft in a transaction to be accounted for as a purchase under accounting principles generally accepted in the United States. Under the terms of the merger agreement, Genome will issue an aggregate of 28,571,405 shares of its common stock, options and warrants to purchase Genome common shares to existing shareholders, promissory note holders and holders of stock options and warrants of Genesoft. The exact amount of common stock, stock options, and warrants to be issued by Genome will be determined at the closing date of the merger based on a common exchange ratio as determined by:
| • | deducting the shares of Genome common stock to be issued to the holders of Genesoft’s promissory notes as payment of accrued interest and related amounts from the total of 28,571,405 shares of Genome common stock issuable in the merger and |
| • | dividing that remaining amount of Genome shares by the fully-diluted number of shares of Genesoft common stock outstanding on the closing date (assuming conversion or exercise of all Genesoft options and warrants). |
The exact exchange ratio between Genesoft and Genome common stock will depend on the closing date of the merger, which will determine how much interest has accrued on the Genesoft promissory notes, as well as the price at which the accrued interest and other related amounts of the Genesoft promissory note holders are converted into Genome common stock. The interest and other related amounts will be converted into Genome common stock at a price of $2.84 per share, unless the issuance price per share of Genome common stock expected to be issued in the capital raising transaction to raise a minimum of $32 million to finance the combined company, which is a condition to the merger agreement (unless waived by both parties), is less than $2.84, in which case that lesser per share price will become the conversion price. As noted above, these unaudited pro forma condensed combined financial statements do not include the proceeds from this offering or the dilutive effect of the shares that would be issued. Had these shares been included in unaudited condensed combined pro forma financials, pro forma earnings per share would have been approximately $1.13 and $0.89 for the year ended December 31, 2002 and nine months ended September 30, 2003, respectively, assuming 11 million shares were sold at $3.05 per share less closing costs.
Each holder of a stock option or warrant to purchase shares of Genesoft common stock that does not terminate by its terms prior to the merger will receive an option or warrant to purchase a number of shares of Genome common stock equal to the product of the number of Genesoft shares for which such option or warrant was exercisable multiplied by the common exchange ratio.
Coincident with the signing of the merger agreement, Genome made a bridge loan of $6.2 million to Genesoft pursuant to a promissory note issued by Genesoft, which is repayable within 60 days of an event of default (as defined in the note) or termination of the merger agreement, unless the merger agreement is terminated by Genesoft due to a failure of Genome to obtain the stockholder vote necessary to approve the merger, in which case it is repayable within 180 days of termination.
104
Table of Contents
The estimated total purchase price of the merger is calculated as follows (in thousands):
Issuance of 25,479,517 shares of Genome common stock to existing Genesoft common shareholders, promissory note holders and warrant holders | $ | 75,674 | |
Fair value of 3,043,547 options issued in exchange for Genesoft stock options | 8,372 | ||
Payment to LG Life Sciences related to FACTIVE license | 8,000 | ||
Bridge loan and related accrued interest to be forgiven at closing | 6,287 | ||
Fair value of 48,341 warrants issued in exchange for Genesoft warrants | 81 | ||
Estimated direct transaction costs incurred by Genome | 4,000 | ||
| 102,414 | |||
| Less: Amount related to unvested stock options allocated to deferred compensation | (4,659) | ||
Total Estimated Purchase Price | $ | 97,755 | |
The fair value of the Genome shares used in determining the purchase price was $2.97 per share based on the average closing price of Genome’s stock from the two days before through two days after November 18, 2003, the date of the public announcement of the merger. The fair value of the options and warrants to be assumed by Genome in connection with the merger is determined based on a stock price of $2.97 per share using the Black-Scholes method with the following assumptions: risk free interest rate of 3.8%, volatility of 84% and no expected dividend. The options have an expected life of four years, which is based on historical Genome experience. The warrants expire in October 2007 and June 2011.
Deferred compensation reflects the estimated intrinsic value of approximately 1.7 million shares of unvested stock options that will be outstanding as of February 28, 2004.
The preliminary allocation of the purchase price is as follows (in thousands):
Current assets | $ | 5,311 | ||
Property, plant and equipment, net | 10,170 | |||
In-process research and development | 11,777 | |||
Intangible assets | 80,372 | |||
Goodwill | 19,306 | |||
Other assets | 46 | |||
Restricted cash | 3,697 | |||
Current liabilities | (26,304 | ) | ||
Long-term liabilities | (6,620 | ) | ||
Total | $ | 97,755 | ||
The final determination of the purchase price allocation will be based on the fair values of the assets, including the fair value of in-process research and development and other intangibles, and the fair value of liabilities assumed at the date of the closing of the merger. The purchase price will remain preliminary until Genome is able to finalize its valuation of significant intangible assets acquired, including in-process research and development, and adjust the fair value of other assets and liabilities acquired. The final determination of the purchase price allocation is expected to be completed as soon as practicable after the date of the closing of the merger. Once the merger is complete, the final amounts allocated to assets and liabilities acquired could differ significantly from the amounts presented in the unaudited pro forma condensed consolidated financial information above.
The valuation of the purchased intangible assets of $80.4 million was based on the result of a valuation using the income approach and applying a risk–adjusted discount rate of between 15% to 22%. The valuation of purchased intangible assets include Genesoft’s lead product and developed technology, FACTIVE, valued at
105
Table of Contents
$72.7 million, an orally administered, broad-spectrum flouroquinolone antibiotic which was approved by the FDA for the treatment of acute bacterial exacerbation of chronic bronchitis (ABECB) and community-acquired pneumonia (CAP) of mild to moderate severity. The valuation of purchased intangible assets also includes the value of a manufacturing and supply agreement for FACTIVE with a third party of $5.2 million. The valuation of purchased intangible assets also includes a Biowarfare Countermeasures / DNA-Nanobinder research program, valued at $2.5 million, supported by the U.S. Department of Defense to develop oral, small molecule treatments for bio-warfare threats, including smallpox, anthrax and malaria. FACTIVE is expected to be launched by September 2004 with cash flows from product sales to begin in the fourth quarter of 2004. The valuation of the Biowarfare Countermeasures / DNA-Nanobinder research program assumes that funding from the U.S government or other sources would be available to support this research program through 2006 . However, there is no guarantee that funding to support this program would be available beyond early 2004.
The valuation of the in-process research and development of $11.8 million represents a peptide deformylase inhibitor research program (PDF) for the development of GSQ-83698 and oral PDF inhibitors, licensed from British Biotech (now Vernalis) for the treatment of community-acquired infections. In-process research and development also includes three novel metalloenzyme bacterial targets from Vernalis that the combined company may elect to initiate a drug discovery program to develop therapeutics directed against these targets.
Goodwill of $19.3 million represents the excess of the purchase price over the fair market value of the tangible and identifiable intangible assets. The unaudited pro forma condensed combined consolidated statements of operations do not reflect the amortization of goodwill acquired in the proposed merger consistent with the guidance in Financial Accounting Standards Board (FASB) Statement No, 142,Goodwill and Other Intangible Assets.
Note 2—Pro Forma Adjustments
The pro forma adjustments included in the unaudited pro forma condensed combined financial statements are as follows:
| (a) | An adjustment has been made to reflect the amortization of deferred compensation related to the intrinsic value of the unvested portion of stock options issued by Genome to holders of Genesoft stock options at the close of the merger. Deferred compensation will be amortized over the remaining vesting period of these options. Amounts adjusted for the year ended December 31, 2002 and nine months ended September 27, 2003 were $1,748,000 and $1,311,000, respectively. |
| (b) | An adjustment to reflect amortization expense on estimated intangible assets based on an estimated useful life of 15 years for FACTIVE and the related manufacturing and supply agreement, and an estimated useful life of 3 years for the Biowarfare Countermeasures / DNA-Nanobinder research program. Amounts adjusted for the year ended December 31, 2002 and nine months ended September 27, 2003 were $6,018,000 and $4,514,000, respectively. |
| (c) | An adjustment has been made for payment of $1,697,000 by Genome to certain promissory note holders of Genesoft at the closing date of the merger. |
| (d) | An adjustment has been made to accrue estimated merger costs of $4,000,000 expected to be incurred by Genome in connection with the merger, consisting primarily of financial advisory and legal and accounting fees. |
| (e) | An adjustment has been made to reflect a payment of $8,000,000 by Genome to LG Life Sciences at the closing of the merger under Genesoft’s License Agreement with LG Life Sciences for FACTIVE. |
| (f) | An adjustment to eliminate the par value of Genesoft historical common stock of $1,000 has been made in consideration of the merger offset by the par value of $2,548,000 of new Genome securities issued in consideration of the merger. |
106
Table of Contents
| (g) | The reduction in pro forma combined additional paid-in-capital is as follows (in thousands): |
Elimination of Genesoft additional paid-in capital | $ | (68,238 | ) | |
Value of new Genome securities issued in consideration of the merger (including options and warrants of $8,453 and a bridge loan of $6,287) | 90,414 | |||
Less par value assigned to common stock | (2,548 | ) | ||
| $ | 19,628 | |||
| (h) | The reduction in pro forma combined accumulated deficit is as follows (in thousands): |
Elimination of Genesoft’s historical accumulated deficit | $ | 75,364 | ||
Charge for in-process research and development | (11,777 | ) | ||
| $ | 63,587 | |||
| (i) | An adjustment has been made to reflect the estimated valuation of the purchased intangible assets of $80.4 million less the historical value of Genesoft’s intangible assets of $6.6 million and goodwill of $19.3 million, as further explained above. |
107
Table of Contents
Genesoft is a specialty pharmaceutical company based in South San Francisco focused on the discovery and development of novel anti-infective agents. FACTIVE (gemifloxacin mesylate) is the company’s lead product, an orally administered, broad-spectrum fluoroquinolone antibiotic recently approved by the FDA for the treatment of acute bacterial exacerbations of chronic bronchitis, or ABECB, and community-acquired pneumonia, or CAP, of mild to moderate severity. Under an agreement with LG Life Sciences, Genesoft exclusively licensed the rights to develop and commercialize FACTIVE in North America, France, Germany, the United Kingdom, Luxembourg, Ireland, Italy, Spain, Portugal, Belgium, the Netherlands, Austria, Greece, Sweden, Denmark, Finland, Norway, Iceland, Switzerland, Andorra, Monaco, San Marino and Vatican City. By virtue of itsin vitro potency, favorable pharmacokinetic profile, and clinical efficacy as demonstrated in clinical trials, Genesoft believes that FACTIVE is well positioned to become an important antibiotic for the treatment of respiratory tract infections. See “FACTIVE—Competitive Advantages” below.
Genesoft is also developing two classes of novel mode of action antibiotics. The first, peptide deformylase, or PDF, inhibitors, represent a new class of molecules that target an essential bacterial enzyme and have antibacterial activities suitable for the potential treatment of respiratory tract infections. The second, DNA-Nanobinder compounds, target certain DNA sequences and have the potential to serve as biological warfare countermeasures.
Infectious Diseases Market
Bacterial infections comprise the sixth leading cause of death in the U.S. and anti-infectives, consisting of antibacterials, antivirals, and antifungals, are the third largest product segment in the pharmaceutical industry, accounting for more than $30 billion in annual sales worldwide in 2002. Antibacterials represent the largest segment of the anti-infective market, accounting for $20 billion of total worldwide anti-infective sales in 2002. The principal structural classes of antibiotics include beta-lactams, quinolones, macrolides, tetracyclines, aminoglycosides, glycopeptides and trimethoprim combinations. Penicillin, a member of the beta-lactam class, which also includes extended-spectrum penicillins, cephalosporins and carbapenems, was first developed in the 1940s. Nalidixic acid, the earliest member of the quinolone class, was discovered in the 1960s. Major advances were made in the 1970s with the development of new beta-lactams and in the 1980s with the development of new quinolones and macrolides.
Bacterial resistance to existing antibiotics has been increasing in recent years, leading to bacterial infection recurrences, treatment failures and higher costs. These factors have fueled a growing need for more effective products in existing antibiotic classes, as well as for products with new mechanisms of action.
Community Respiratory Diseases
Acute Bacterial Exacerbation of Chronic Bronchitis (ABECB).Chronic bronchitis is a health problem associated with significant morbidity and mortality. It is estimated that chronic bronchitis affects up to 13 million individuals or approximately 4% to 6% of adults in the United States. Patients with chronic bronchitis are prone to frequent exacerbations, characterized by increased cough and other symptoms of respiratory distress. Longitudinal studies have estimated that 1 to 4 exacerbations occur each year in patients with chronic bronchitis, and such exacerbations are estimated to account for approximately 12 million physician visits per year in the U.S. Antibiotic therapy, the standard treatment for ABECB, is typically effective in reducing the course of illness for patients.
Community-Acquired Pneumonia (CAP).CAP is a common and serious illness in the United States. The 3 to 4 million reported cases per year of CAP result in approximately 10 million physician visits, 1 million hospitalizations, 64 million days of restricted activity, and 64,000 deaths annually, making CAP the seventh leading cause of death in the United States, and the most common cause of death due to infectious diseases.
108
Table of Contents
Antibiotics are the mainstay of treatment for most patients with pneumonia, and where possible, antibiotic treatment should be specific and individualized. However, since the responsible pathogen is not identified in a high proportion of patients with CAP, an empiric approach to treatment is usually necessary. Over the last decade, resistance to penicillin and macrolides has increased significantly, and in many cases, quinolones are now recommended as a first line of therapy due to their efficacy against a wide range of respiratory pathogens, including many resistant strains. The recent treatment guidelines from the Infectious Diseases Society of America recommend quinolones as a first line treatment for certain higher-risk patients with CAP.
FACTIVE
In April 2003, FACTIVE (gemifloxacin mesylate) was approved by the FDA for the treatment of ABECB and CAP of mild to moderate severity. In July 2003, FACTIVE was approved to treat CAP caused by susceptible strains of multi-drug resistantStreptococcus pneumoniae, orS. pneumoniae, a growing clinical concern. Multi-drug resistantS. pneumoniae, or MDRSP, is defined asS. pneumoniae resistant to two or more of the following antibiotics: penicillin, second-generation cephalosporins (such as cefuroxime), macrolides, tetracyclines, and trimethoprim/sulfamethoxazole. FACTIVE is the only antimicrobial currently approved for this indication.
FACTIVE has potentin vitro activity against a wide range of Gram-positive, Gram-negative and atypical pathogens, including key respiratory pathogens, such asS. pneumoniae, Haemophilus influenzae,andMoraxella catarrhalis, and is bactericidal at clinically achievable concentrations. FACTIVE targets two enzymes in bacteria and has minimum inhibitory concentrations, or MICs, as low as 0.03 µg/ml for S. pneumoniae. FACTIVE has been studied in nearly 7,000 patients and has a good overall safety and tolerability profile comparable to other currently marketed antibiotics.
FACTIVE has been the subject of over 200 publications. Among the research published are data indicating FACTIVE’s ability to reduce the number of ABECB recurrences over a six-month period following treatment.
Within the antibiotic market, quinolones, a product class with close to $3 billion in annual sales in the U.S., have been gaining market share at the expense of older antibiotics, according to IMS Health. Genesoft expects this trend to continue as resistance to older antibiotic classes increases. Due to its microbiological activity and clinical efficacy, FACTIVE, a new branded quinolone, represents an alternative choice for the treatment of certain respiratory tract infections.
Mechanism of Action
FACTIVE acts by inhibiting bacterial DNA synthesis through the inhibition of both DNA gyrase and topoisomerase IV, two enzymes that are essential for bacterial growth and survival.S. pneumoniae showing mutations in both DNA gyrase and topoisomerase IV (double mutants) are resistant to most fluoroquinolones. Since FACTIVE has the ability to inhibit both target enzymes at therapeutically relevant drug levels, some of theseS. pneumoniae double mutants remain susceptible to FACTIVE.
FACTIVE is also active against many strains ofS. pneumoniae that are resistant to other classes of antibiotics. There is no known bacterial cross-resistance between FACTIVE and any other class of antimicrobials.
Clinical Efficacy
FACTIVE was studied for the treatment of acute bacterial exacerbation of chronic bronchitis in three pivotal, double-blind, randomized, active-controlled clinical trials using 320 mg once daily for 5 days. In these non-inferiority studies, a total of 826 patients received treatment with FACTIVE and 822 patients received treatment with active comparator, namely levofloxacin, clarithromycin, or amoxicillin/clavulanate. The primary
109
Table of Contents
efficacy parameter was clinical response at follow-up. The results for the principal ABECB studies demonstrate that FACTIVE given once daily for 5 days was at least as effective as the comparators given for 7 days. The clinical success rates for each of these three trials were as follows:
• | FACTIVE | 5 days (320 mg): | 88.2 | % | |||
Levofloxacin | 7 days (500 mg): | 85.1 | % | ||||
• | FACTIVE | 5 days (320 mg): | 86.0 | % | |||
Clarithromycin | 7 days (500 mg bid): | 84.8 | % | ||||
• | FACTIVE | 5 days (320 mg): | 93.6 | % | |||
| Amoxicillin/clavulanate | 7 days (500 mg/125 mg, 3 times/day, or tid): | 93.2 | % | ||||
FACTIVE was also studied for the treatment of community-acquired pneumonia in three double-blind, randomized, active-controlled clinical studies, one open, active-controlled study, and two uncontrolled studies. In total, 1,349 patients with CAP were treated with FACTIVE, including 1,037 patients treated for 7 days; 927 patients with CAP were treated with an active comparator. The primary efficacy parameter for each of these three trials was clinical response at follow-up. The results of these studies showed that FACTIVE was effective in the treatment of mild to moderate CAP. The clinical success rates for FACTIVE in studies with a fixed 7-day duration ranged from 89% to 92%.
In the pivotal CAP comparator study, a 7-day treatment regimen of FACTIVE 320 mg once daily was shown to be as effective as a 10-day treatment course of amoxicillin/clavulanate (500 mg/125 mg tid). The clinical success rates for the two treatment arms were:
• | FACTIVE | 7 days (320 mg): | 88.7 | % | |||
| Amoxicillin/clavulanate | 10 days (500 mg/125 mg tid): | 87.6 | % |
Clinical studies showed that FACTIVE was effective in the treatment of CAP due to penicillin-resistantS. pneumoniae, or PRSP. Of 11 patients with PRSP treated with FACTIVE for 7 days, 100% achieved both clinical and bacteriological success at follow-up.
FACTIVE is also effective in the treatment of CAP due to MDRSP. In clinical trials, of 22 patients with MDRSP treated with FACTIVE for 7 days, 19 (87%) achieved both clinical and bacteriological success at follow-up. FACTIVE is the first antibiotic approved to treat mild to moderate CAP caused by these multi-drug resistant organisms.
Competitive Advantages
The potential competitive advantages of FACTIVE include the following:
| • | FACTIVE is active against many bacterial isolates resistant to other classes of antibiotics, and is the only antibiotic approved to treat community-acquired pneumonia of mild to moderate severity due to multi-drug resistantS. pneumoniae. |
| • | FACTIVE has a dual targeting mechanism of action inS. pneumoniae, which targets two enzymes essential for bacterial growth and survival at therapeutically relevant drug levels, and has low in vitro potential for resistance generation. |
| • | FACTIVE can be dosed once daily, with short courses of therapy for both ABECB (5 days) and CAP (7 days). |
| • | FACTIVE has patent protection into 2015 (with possible regulatory extension), longer than any currently marketed fluoroquinolones or other antibiotics widely used to treat respiratory tract infections. |
110
Table of Contents
Safety and Tolerability
FACTIVE has been studied extensively in nearly 7,000 patients and has a favorable safety profile. The incidence of adverse events reported for FACTIVE was low and comparable to comparator drugs, namely beta-lactam antibiotics, macrolides and other fluoroquinolones. Most adverse events were described as mild to moderate.
Although rash was more frequent among FACTIVE-treated patients in the total patient population than among those who received comparator drugs, in the adult population most at risk for CAP of mild to moderate severity and ABECB (patients over 40 years of age) and at the approved dosage (320 mg for 7 days or less), the rate of rash with FACTIVE was low and comparable to that seen with other antibiotics.
As a post-marketing study commitment, the FDA required that Genesoft conduct a prospective, randomized study comparing FACTIVE (5,000 patients) to an active comparator (2,500 patients) in patients with CAP or ABECB. This study will include patients of different ethnicities, to gain safety information in populations not substantially represented in the existing clinical trial program, specifically as it relates to rash. Patients will be evaluated for clinical and laboratory safety. This Phase IV trial is in the design stage and the FDA required, as a condition to its approval, that the trial be initiated by March 2004.
Additional Development Plans
FACTIVE has also been the subject of additional clinical trials for other indications. Two double-blind, randomized, active-controlled clinical studies were conducted to examine the efficacy of FACTIVE 320 mg once daily for 7 days in the treatment of patients with acute bacterial sinusitis, or ABS. In these studies, 540 patients received FACTIVE and 536 patients received active comparator, namely trovafloxacin or cefuroxime. The primary efficacy parameter was clinical success at follow-up. The result of these clinical trials showed comparable clinical success for patients treated with FACTIVE and those treated with comparator drugs. In addition, a double-blind, randomized, active-controlled clinical study comparing a FACTIVE 7-day treatment regimen for ABS with a FACTIVE 5-day treatment regimen showed similar efficacy between the two treatment arms. Two open-label studies also support the efficacy of FACTIVE given for 5 days for the treatment of ABS. Genesoft anticipates pursuing this indication in the future.
An intravenous formulation of FACTIVE is also in development. Genesoft is currently evaluating plans for the completion of this intravenous formulation program.
Product Pipeline
Genesoft’s current pharmaceutical programs reflect its commitment to the research and development of novel anti-infective therapeutics. The pipeline spans discovery research and preclinical development to early clinical trials and pre-launch activities.
Peptide Deformylase Inhibitors. In August 2002, Genesoft entered into a research and license agreement with British Biotech Pharmaceuticals Ltd., now Vernalis, to co-develop inhibitors of peptide deformylase, or PDF, a novel iron-binding enzyme essential for bacterial growth but not involved in human cytoplasmic protein synthesis. Genesoft believes that PDF inhibitors represent an excellent opportunity for the development of novel mode of action antibiotics. In September 2003, Genesoft assumed full responsibility for the development and commercialization of these compounds.
Preclinical studies of GSQ-83698, Genesoft’s most advanced PDF inhibitor, indicated that the compound may have potential for the treatment of hospitalized patients suffering from CAP. An intravenous formulation of GSQ-83698 entered Phase I clinical trials in October 2002, and the drug was well tolerated and demonstrated good pharmacokinetic properties. GSQ-83698 has exhibited goodin vitro activity against many of the important
111
Table of Contents
respiratory tract pathogens, but has limited activity againstH. influenzae. Rather than devote additional resources to the clinical development of GSQ-83698, Genesoft has chosen to focus on the optimization of second-generation PDF inhibitors.
This second-generation research program has focused on developing orally available PDF compounds with the potential to target the broader community-based antibiotic market. Several compounds have been identified with improved properties, including good activity againstH. influenzae. With continued success, Genesoft anticipates selecting a development candidate and initiating IND-enabling studies.
Biowarfare Countermeasures/DNA-Nanobinder Program.In an ongoing research effort supported by the Defense Advanced Research Projects Agency, or DARPA, Genesoft is developing DNA-Nanobinder compounds to target biological warfare agents, Gram-positive pathogens, and some parasitic organisms. DNA-Nanobinder compounds selectively target pathogen DNA and bind with high affinity to functionally important adenine/thymine, or A/T, rich DNA sequences, thereby inhibiting DNA and RNA synthesis. These compounds derive their spectrum of activity from the fact that most biowarfare threat agents contain A/T rich DNA sequences in essential elements of their genome. DNA-Nanobinder compounds are being investigated as a medical defense against anthrax, smallpox, and malaria. GSQ-7302, Genesoft’s most advanced DNA-Nanobinder compound, has demonstratedin vitro activity against these pathogens, and efficacy in a small animal model for anthrax infection.
Intellectual Property
In October 2002, Genesoft exclusively licensed from LG Life Sciences the rights to develop and commercialize FACTIVE in North America, France, Germany, the United Kingdom, Luxembourg, Ireland, Italy, Spain, Portugal, Belgium, the Netherlands, Austria, Greece, Sweden, Denmark, Finland, Norway, Iceland, Switzerland, Andorra, Monaco, San Marino and Vatican City. This license covers 11 issued U.S. patents and a broad portfolio of corresponding foreign patents and patent applications. The U.S. patents are currently set to expire at various dates, ranging from June 2015, in the case of the principal patents relating to FACTIVE, to September 2019. Genesoft has filed patent term extension applications, covering the regulatory review process, for the principal patents related to FACTIVE. If granted, these extensions would extend the exclusivity period through April 2017.
The patents that Genesoft licenses to FACTIVE under the agreement with LG Life Sciences include claims related to the chemical composition of FACTIVE, its use for the prophylaxis and treatment of bacterial infections, and methods of manufacturing FACTIVE. Genesoft also has the exclusive right to use FACTIVE trademarks, trade names, domain names and logos in conjunction with the use or sale of the product in the territories covered by the license.
Genesoft has exclusively licensed rights from Vernalis for the research, development, and commercialization of certain anti-infectives under Vernalis’ patent portfolio of 5 issued U.S. patents, 1 pending U.S. patent, 24 issued foreign patents, and 36 pending foreign patent applications. The patents that Genesoft licenses from Vernalis relate to metalloenzyme inhibitors (including peptide deformylase inhibitors), their uses, and their targets.
Genesoft’s patent portfolio related to DNA-Nanobinder compounds and their applications as anti-infective therapeutics consists of one issued U.S. patent, 10 pending U.S. patent applications and 8 pending foreign patent applications. In addition, Genesoft licenses 14 issued U.S. patents, 10 pending U.S. patents, 10 issued foreign patents, and 36 pending foreign patent applications from the California Institute of Technology. Some of Genesoft’s patents and patent applications related to DNA-Nanobinder compounds resulted from research funded by the U.S. government, and the government has a standard statutory nonexclusive government purpose license and march-in rights if, for example, Genesoft fails to actively develop the technology or public health concerns are implicated.
112
Table of Contents
Partnerships and Collaborations
LG Life Sciences.In October of 2002, Genesoft entered into a partnership with LG Life Sciences to license exclusive commercialization rights to FACTIVE in the territories specified above under “Intellectual Property.” The term of the agreement coincides with FACTIVE’s patent life which currently expires in 2015, but the patent could be extended for an additional two years. The arrangement included the payment to LG Life Sciences of an up-front fee of $5.5 million and the issuance to LG Life Sciences of approximately 14% of Genesoft’s fully-diluted shares outstanding as of April 2003. The arrangement also provides for Genesoft’s payment of royalties on future product sales. Genesoft is required to buy bulk drug requirements from LG Life Sciences (see below), and will pay LG Life Sciences a royalty on sales in the U.S. and the territories covered by the license in Europe. The gross margin on product sales, including royalty obligations, is projected to be approximately 75% during the first two years, and in the 65 to 70% range after those periods. Genesoft is responsible, at its expense and through consultation with LG Life Sciences, for the clinical and commercial development of FACTIVE in the territories covered by the license. This arrangement requires a minimum sales commitment over a period of time, which if not met, could result in the technology being returned to LG Life Sciences. Genesoft is obligated to purchase from LG Life Sciences, and LG is obligated to supply to Genesoft, all of Genesoft’s anticipated commercial requirements for FACTIVE bulk drug substance as further described in the “Manufacturing” section below. Upon delivery of the first shipment of FACTIVE, which is anticipated to occur within the next two months, Genesoft will be obligated to make a $2.5 million milestone payment to LG Life Sciences as well as a payment of $4.8 million for the purchase of the drug inventory. Upon the closing of the merger, the combined company will be obligated to make an $8 million milestone payment to LG Life Sciences. The arrangement also provides for potential additional milestone payments to LG Life Sciences of up to $22 million, primarily upon achieving sales targets.
Vernalis.In August of 2002, Genesoft entered into a strategic partnership with British Biotech Pharmaceuticals Ltd., now Vernalis, to co-develop GSQ-83698 and oral PDF inhibitors for the treatment of community-acquired infections. In 2002, Genesoft paid fees to Vernalis totaling $5 million in connection with the original agreement and issued 356,252 shares of Genesoft common stock upon the achievement of a milestone under the agreement. In September 2003, the companies entered into an agreement whereby Genesoft would assume sole responsibility for the development and commercialization of these compounds. Genesoft also obtained an exclusive worldwide license or sub-license, as applicable, to develop and commercialize three novel bacterial targets for purposes of the treatment of infections from Vernalis as part of this agreement.Genesoft is obligated to pursue the development of these targets and, if appropriate, to pursue the regulatory approval and commercialization of them. Under the agreement, Genesoft has obligations to make royalty payments to Vernalis on future product sales. Additionally, Genesoft may be required to make future milestone payments to Vernalis of up to $18.8 million.
Defense Advanced Research Projects Agency. In December 1998, Genesoft received a three-year, $12.3 million grant in the aggregate from DARPA to conduct research on the regulation of pathogen gene expression and to endeavor to develop oral therapeutics against bio-warfare threat agents, including anthrax, smallpox and malaria. This grant ended in June 2002. In November 2002, Genesoft entered into a $3.0 million contract with DARPA to continue the same research. This contract was amended in April 2003 to include the U.S. Army as a party and to provide for an additional $5.5 million to fund the research through early 2004.
California Institute of Technology.In September of 1998, Genesoft entered into a license agreement with CIT for the development of DNA-Nanobinders for human gene regulation, under which Genesoft obtained an exclusive worldwide license to a number of patents described above under “Intellectual Property.” As an up-front fee, Genesoft paid CIT $5,000 and issued CIT 42,750 shares of its common stock. Professor Peter Dervan, one of Genesoft’s founders and a director of the company, leads the research effort related to this collaboration at CIT. Genesoft is obligated to pursue the development and commercialization of products based on the technology licensed from CIT. Genesoft is also obligated to pay royalties on possible future product sales and any costs relating to the preparation, filing, prosecution and maintenance of existing and new patents covered by the license agreement.
113
Table of Contents
Manufacturing
Under the terms of Genesoft’s licensing agreement with LG Life Sciences, LG Life Sciences agreed to supply all of Genesoft’s anticipated commercial requirements for FACTIVE bulk drug substance and Genesoft agreed to purchase all of its requirements for the bulk drug substance from LG Life Sciences. LG Life Sciences is expected to supply the FACTIVE bulk drug substance from its manufacturing facility in South Korea. The LG Life Sciences facility is subject to on-going government regulation, including FDA regulations requiring compliance with current Good Manufacturing Practices, or cGMP. For 2004, the final drug product will be tableted and packaged for LG Life Sciences by SB Pharmco at its manufacturing facility in Puerto Rico. This arrangement with SB Pharmco is expected to conclude by the end of 2004. Genesoft is in discussions with a new secondary manufacturer to assume these responsibilities for subsequent periods.
Genesoft subleases approximately 68,000 square feet of laboratory and administrative space at 7000 Shoreline Court, South San Francisco, California 94080. The yearly base rent for this facility is approximately $3,697,000. Genesoft’s sublease for this facility expires on March 31, 2011. Genesoft has sub-subleased approximately 30,200 square feet of the facility through December 31, 2004. Genesoft receives approximately $1,700,000 in
yearly base rent from the sub-sublease. Genesoft is considering additional subleases and other options for portions of this space.
Genesoft is not aware of any actual, threatened or pending legal proceeding to which it is a party or to which any of its property is subject that could result in material adverse change in the business or financial condition of Genesoft.
114
Table of Contents
GENESOFT MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with Genesoft’s financial statements and notes thereto appearing elsewhere in this joint proxy statement/prospectus. This discussion and analysis contains forward-looking statements about Genesoft within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements represent the judgment of the management of Genesoft regarding future events. Forward-looking statements typically are identified by use of terms such as “may,” “will,” “should,” “plan,” “expect,” “intend,” “anticipate,” “estimate,” and similar words, although some forward-looking statements are expressed differently. Genesoft does not plan to update these forward-looking statements. You should be aware that actual results could differ materially from those contained in the forward-looking statements due to a number of risks affecting the business of Genesoft.
Although Genesoft believes that its plans, intentions and expectations as reflected in or suggested by these forward-looking statements are reasonable, it can give no assurance that these plans, intentions or expectations will be achieved. Genesoft stockholders are cautioned that all forward-looking statements involve risks and uncertainties and actual results may differ materially from those discussed as a result of various risk factors described in the section entitled “Risk Factors” and elsewhere in this joint proxy statement/prospectus.
Some of the important risk factors that could cause Genesoft’s actual results to differ materially from those expressed in Genesoft’s forward-looking statements include, but are not limited to:
| • | risks related to Genesoft’s approved product, FACTIVE, such as (i) Genesoft’s inability to obtain the financial resources and personnel to commercialize FACTIVE, (ii) competitors in the antibiotic market introducing superior products that are more effective, more cost-effective and marketed more effectively and (iii) Genesoft’s business in the future could expose it to potential product liability risks; |
| • | Genesoft’s inability to successfully develop and obtain regulatory approval of products based on metalloenzyme inhibitors, including peptide deformylase (PDF) inhibitors, and DNA-Nanobinder technology; |
| • | Genesoft’s history of operating losses, and negative working capital which resulted in a going concern qualification to its December 31, 2002 financial statements, and Genesoft’s need to raise future capital to support Genesoft’s product development and research initiatives; |
| • | intensified competition from pharmaceutical or biotechnology companies that may have greater resources and more experience than Genesoft; |
| • | Genesoft’s inability to obtain or enforce Genesoft’s intellectual property rights; |
| • | Genesoft’s dependence on key personnel; and |
| • | Genesoft’s issued debt burden which totaled approximately $22.0 million at September 30, 2003. |
Since its inception in 1997, Genesoft has devoted its efforts to the research and development of its licensed technology. To date, Genesoft has generated no revenues from product sales and has depended upon equity financings, interest on invested funds, research funding from the government and financing through debt to provide the capital required to pursue its intended business activities. Genesoft has a net accumulated deficit of $75.4 million through September 30, 2003. The accumulated deficit has resulted principally from Genesoft’s efforts to develop drug candidates and the associated administrative costs required to support these efforts.
115
Table of Contents
Genesoft expects to incur significant additional operating losses over the next several years due to the costs associated with launching FACTIVE and its ongoing development and clinical efforts. Genesoft’s potential for future profitability is dependent on its ability to successfully launch FACTIVE, its ability to effectively develop its metalloenzyme inhibitor compounds and its ability to license and develop new compounds.
Major Research and Development Projects
FACTIVE
Genesoft’s ongoing regulatory activities related to FACTIVE (gemifloxacin mesylate), its lead product, comprised 28% of its total research and development expenditures for the fiscal year ended December 31, 2002 (including $5.5 million in licensing fees paid to LG Life Sciences), 3% of total research and development expenditures for the nine month period ended September 30, 2002, and 13% of total research and development expenditures for the nine month period ended September 30, 2003.
In October 2002, the company entered into a partnership with LG Life Sciences to develop and commercialize FACTIVE, a novel quinolone antibiotic, in North America, France, Germany, the United Kingdom, Luxembourg, Ireland, Italy, Spain, Portugal, Belgium, the Netherlands, Austria, Greece, Sweden, Denmark, Finland, Norway, Iceland, Switzerland, Andorra, Monaco, San Marino and Vatican City. The term of the agreement coincides with the compound’s patent life which currently expires in 2015. The patent could be extended for an additional two years pursuant to Genesoft’s request for an extension related to the regulatory process. The product was approved for sale in the United States in April 2003 for the treatment of acute bacterial exacerbation of chronic bronchitis and community-acquired pneumonia of mild to moderate severity. The arrangement with LG Life Sciences included up-front fees, milestone payments and royalties on sales. In addition, Genesoft issued LG Life Sciences common stock equivalent to 14% of the equity in Genesoft on a fully diluted basis as of the time of FDA approval. The bulk product will be manufactured by LG Life Sciences. Genesoft will purchase its requirements for the final drug product from LG Life Sciences for 2004, which final drug product will be tableted and packaged for LG Life Sciences by SB Pharmco at its manufacturing facility in Puerto Rico. This arrangement with SB Pharmco is expected to conclude by the end of 2004. Genesoft is in discussions with a new secondary manufacturer to assume these responsibilities for subsequent periods.
The successful commercialization of FACTIVE is subject to many risks and uncertainties, including an inability to successfully market the product due to competition from other competing drugs, inability to recruit and retain a successful sales management team and sales force, and the inability to raise the financial resources required to launch the drug. A failure to successfully commercialize FACTIVE would have a significant negative impact on Genesoft’s operations, financial position and liquidity.
Metalloenzyme Inhibitors (MEI), including PDF Inhibitors
Genesoft’s ongoing clinical trials and other research activities related to Genesoft’s MEI program comprised 23% of Genesoft’s total research and development expenditures for fiscal 2002 (including $5 million in in-license and milestone fees paid in August and October 2002 to British Biotech Pharmaceuticals Ltd. (now Vernalis)), 29% of total research and development expenditures for the nine month period ended September 30, 2002 and 25% of total research and development expenditures for the nine month period ended September 30, 2003.
In August 2002, Genesoft entered into a three-year joint collaboration with Vernalis to co-develop GSQ-83698, a novel PDF inhibitor which, based on human pharmacokinetic and tolerability information, may have potential to treat patients hospitalized with community-acquired pneumonia, or CAP. In addition, Genesoft commenced an optimization research project to deliver second-generation oral peptide deformylase development candidates for the treatment of respiratory tract infections, or RTI. In September 2003, Genesoft assumed full responsibility for the MEI program, including some additional limited research assets such as three novel
116
Table of Contents
metalloenzyme bacterial targets. The transfer of remaining Vernalis assets to Genesoft related to this program is nearly complete. Genesoft’s license agreement with Vernalis provides Genesoft with exclusive rights to develop and market GSQ-83698 and any molecules that are developed from the oral PDF inhibitor program. Genesoft is obligated to pay a royalty on product sales and to make other milestone payments.
Rather than devote additional resources to the clinical development of GSQ-83698, Genesoft has chosen to focus on the optimization of second-generation orally available PDF compounds. Although GSQ-83698 has exhibited goodin vitro activity against many of the important respiratory tract pathogens, it has limited activity againstH. influenzae. The second-generation PDF compounds have demonstrated improved properties, including good activity againstH. influenzae. With continued success, Genesoft anticipates selecting a development candidate and initiating IND-enabling studies.
The successful commercialization of the PDF inhibitor molecules is subject to many risks and uncertainties, including Genesoft’s inability to realize the potential of Genesoft’s initial discoveries due to scientific failures or lack of skilled personnel. In addition, Genesoft’s success in achieving its goals depends, for example, upon whether Genesoft’s compounds warrant clinical development, whether any such compounds demonstrate the required safety and efficacy in clinical trials in order to support a regulatory approval and whether Genesoft is able to successfully manufacture and commercialize the product. As a result of these many risks and uncertainties, Genesoft cannot predict when material cash inflows from Genesoft’s MEI inhibitor project will commence, if ever. A failure to successfully commercialize Genesoft’s PDF inhibitor compounds would have a significant negative impact on Genesoft’s operations, financial position and liquidity.
Department of Defense Collaboration
A second major research and development project of Genesoft’s is the fulfillment of Genesoft’s research obligations related to Genesoft’s contract with the U.S. Department of Defense and related agencies.
The research and development expense to support this program was 34% of total research and development expenses in fiscal 2001, 18% of total research and development expenses in fiscal 2002, 27% of total research and development expenses for the nine months ended September 30, 2002 and 26% of total research and development expenses for the nine months ended September 30, 2003. Research and development expense to support this alliance was 29% of the total research and development expense from January 1, 1999 through September 30, 2003.
Genesoft has had substantial funding since 1999 from agencies within the U.S. Department of Defense to develop oral, small molecule treatments for bio-warfare threats, including smallpox, anthrax and malaria. In research, Genesoft has shown its compounds to be efficaciousin vitroagainst smallpox, anthrax and malaria andin vivo against cowpox, anthrax and malaria.
In December 1998, Genesoft received a three-year, $12.3 million grant in the aggregate from DARPA to conduct research on the regulation of pathogen gene expression and to endeavor to develop oral therapeutics against bio-warfare threat agents, including anthrax, smallpox and malaria. This grant ended in June 2002. In November 2002, Genesoft entered into a $3.0 million contract with DARPA to continue the same research. This contract was amended in April 2003 to include the U.S. Army as a party and to provide for an additional $5.5 million to fund the research through early 2004. Genesoft is subject to the risk that this contract may be terminated prior to its specified expiration date or that the contract may not be renewed further.
Genesoft’s ability to obtain the goals for this collaboration is subject to numerous risks, including Genesoft’s inability to realize the potential of Genesoft’s initial discoveries due to scientific failures or lack of skilled personnel. In addition, Genesoft’s success in achieving its goals depends, for example, upon whether Genesoft’s compounds warrant clinical development, whether any such compounds demonstrate the required safety and efficacy in clinical trials in order to support a regulatory approval and whether Genesoft is able to
117
Table of Contents
successfully manufacture and commercialize the product. Due to these uncertainties, Genesoft cannot be certain if Genesoft will obtain additional funding under this program. A failure to obtain additional funding and to advance Genesoft’s program towards product approvals would have a significant negative impact on Genesoft’s operations, financial position and liquidity.
Internally Funded Research Program
Genesoft conducts its own internally funded program which stems from technology that was licensed from California Institute of Technology, or CIT, called DNA-Nanobinder technology. The use of compounds generated from this technology has been explored in various therapeutic areas. However, a number of technical hurdles associated with the early development of this technology, including limited cellular uptake, binding specificity, and building block stability has slowed progress in some of the therapy areas. Genesoft’s current focus has been to use the DNA-Nanobinder compounds in the discovery and research of potential drug candidates in the anti-infective area. In June 2002, Genesoft entered into a contract with Dow Pharmaceuticals for the development of a topical antibacterial to treat skin infections such as infected diabetic foot ulcers and secondarily infected traumatic lesions. Under this collaboration, a topical DNA-Nanobinder preparation was investigated. This program is currently on hold for financial reasons.
These research efforts represented 66% of total research and development expenditures in fiscal 2001, 31% of expenditures in fiscal 2002, 41% of expenditures during the nine month period ended September 30, 2002 and 36% of expenditures during the nine month period ended September 30, 2003. These efforts comprised 47% of the total research and development expense from inception in August 1997 through September 30, 2003.
Genesoft’s ability to obtain its goals for its internally funded research program is subject to numerous risks, including Genesoft’s inability to make new discoveries due to scientific failures or lack of skilled personnel. Even if Genesoft succeeds in identifying novel lead series, Genesoft may not be successful in developing these discoveries further due to lack of resources and skilled personnel and the inability to find a strategic partner in an increasingly competitive environment for strategic alliances. Due to all of these uncertainties, Genesoft can provide no assurance that Genesoft will ever receive any material cash inflows from this program.
Going Concern
Genesoft has generated negative cash flows from operations since inception and has minimal capital resources at December 31, 2002. Genesoft has been able to fund its cash needs to date through the sale of its preferred and common stock and debt financings. The ability of Genesoft to manage its operating expenses to a level that can be financed by existing cash flows and its ability to obtain additional funding is therefore critical to Genesoft’s ability to continue operating as a going concern. These conditions raise substantial doubt about Genesoft’s ability to continue as a going concern. Genesoft’s management intends to merge Genesoft with Genome (See Note 12 to its financial statements included elsewhere in this joint proxy statement/prospectus) and obtain additional financing or enter into collaborative arrangements. The outcome of management’s intentions is not presently determinable. As such, no adjustments have been made that might result from this situation.
Genesoft’s continuation as a going concern is primarily dependent upon its ability to merge or obtain alternative sources of capital. In the event Genesoft is unable to secure alternative financing sources, it is likely that any of the following alternatives will be pursued: (1) pursue a co-promotion collaboration; or (2) pursue other available protective remedies.
Critical Accounting Policies & Estimates
Genesoft’s management discussion and analysis of its financial condition and results of operations are based on its financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires Genesoft to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of
118
Table of Contents
contingent assets and liabilities at the date of the financial statements as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, Genesoft evaluates its estimates and judgments. Genesoft bases its estimates on historical experience and on various other factors that it believes are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While Genesoft’s significant accounting policies are more fully described in Note 1 to its financial statements included elsewhere in this joint proxy/prospectus, Genesoft believes that the following accounting
policies relating to the fair value of common stock, the impairment of assets, revenue recognition and stock compensation are most critical to a full understanding and evaluation of its reported financial results.
Fair Value of Common Stock
Genesoft has issued various equity instruments including common stock, warrants and options as part of the various transactions it has entered into including those related to the FACTIVE license agreement with LG Life Sciences, the license agreement with Vernalis and option grants to consultants and employees. Genesoft must make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported revenues and expenses during the reporting periods related to the valuation of equity instruments issued in these transactions. Genesoft utilizes third party valuation experts and industry accepted valuation models to estimate the fair market value of these equity instruments; however, the methods utilized by these various valuation methodologies require the use of estimates and assumptions. On an ongoing basis, Genesoft evaluates its estimates and judgments.
Impairment of Assets
Genesoft is required to make judgments about the recoverability of its long-lived assets whenever events or changes in circumstances indicate that the carrying value of these assets may be impaired or not recoverable. In order to make such judgments, Genesoft is required to make assumptions about the value of these assets in the future including future prospects for earnings and cash flows. If impairment is indicated, Genesoft writes those assets down to their fair value that is generally determined based on discounted cash flows. Judgments and assumptions about the future are complex, subjective and can be affected by a variety of factors including industry and economic trends, Genesoft’s market position and the competitive environment of the businesses in which Genesoft operates.
Revenue Recognition
Grant revenue is recognized as the costs stipulated under the grant contracts are incurred.
Stock Compensation
Genesoft accounts for employee stock options using the intrinsic-value method in accordance with Accounting Principles Board, or APB, Opinion No. 25, Accounting for Stock Issued to Employees, Financial Accounting Standards Board, or FASB, Interpretation No. 44, Accounting for Certain Transactions involving Stock Compensation, an interpretation of APB No. 25, and related interpretations and have adopted the disclosure-only provisions of Statement of Financial Accounting Standards, or SFAS, No. 123, Accounting for Stock-Based Compensation, or SFAS No. 123.
In December 2002, the FASB issued SFAS No. 148, Accounting for Stock-Based Compensation—Transition and Disclosure. SFAS No. 148 amends SFAS No. 123 to provide alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation. Genesoft has elected to continue to follow the intrinsic value method of accounting as prescribed by APB No. 25. The information regarding net loss as required by SFAS No. 123, presented in Note 1 to its financial statements,
119
Table of Contents
has been determined as if Genesoft had accounted for its employee stock options under the fair value method of that statement. The resulting effect on net loss pursuant to SFAS No. 123 is not likely to be representative of the effects on net loss pursuant to SFAS No. 123 in future years, since future years are likely to include additional grants and the irregular impact of future years’ vesting.
Nine Months Ended September 30, 2002 and September 30, 2003
Genesoft’s total revenues increased to $3.1 million for the nine months ended September 30, 2003 compared with no revenue for the period ended September 30, 2002. In November 2002, DARPA awarded Genesoft a new four month contract for $3 million. This was increased by $5.5 million for 12 months in April 2003. Genesoft believes that revenues from DARPA will remain relatively stable, if the contract is renewed in April 2004. Genesoft also believes that it will recognize product revenues as a result of its first product launch (FACTIVE) projected for late summer of 2004.
Research and development expenses decreased $5.6 million (39%) to $8.9 million for the nine month period ended September 30, 2003 compared to $14.5 million for the nine month period ended September 30, 2002. The decrease was primarily due to a decrease of $1.3 million (51%) in salary expense to scientific management and staff due to reduction in staffing levels in order to control expenditures (which primarily impacted the MEI inhibitor program), a decrease of $2.0 million (45%) in facility related allocation due to the reduction in scientific headcount and a decrease of approximately $470,000 (24%) in consultants used in the internal programs. Additionally, in August 2002, Genesoft paid Vernalis $4 million in technology license fees. There was no comparable payment to Vernalis in 2003. The decrease was partially offset by an increase in research and development expenses due to increased regulatory related fees for FACTIVE of $1.0 million and a payable of $775,000 in research costs to Vernalis to reconcile program costs and FTE requirements per the contract. Genesoft believes that R&D expenses will remain relatively stable or be reduced as Genesoft tries to partner its MEI inhibitor program.
Marketing expenses increased to $2.1 million for the nine month period ended September 30, 2003 compared to no expenses in the period ended September 30, 2002. Genesoft licensed FACTIVE in October 2002, and started its marketing efforts when the product was approved in April 2003. Marketing expenses will continue to increase as Genesoft incurs expenses for the launch of FACTIVE in late summer 2004.
General and administrative expenses increased by $1.6 million (44%) to $5.2 million for the nine months ended September 30, 2003 compared to $3.6 million for the nine months ended September 30, 2002. The increase in general and administrative expense was primarily due to an increase of $1.5 million (136%) in facility related allocation due to the change in ratio of scientific to general and administrative staff and an increase of approximately $224,000 (26%) in travel and legal services due to increased activity in seeking alliances and potential strategic transactions. These increases were somewhat offset by a decrease in consulting expenses of approximately $319,000 (45%) as a result of reduced expenditures in the areas of business development and human resources. General and administrative expenses should remain relatively stable or be reduced as Genesoft continues to control its expenditures in this area.
Other income decreased by approximately $262,000 (82%) to $59,000 for the nine months ended September 30, 2003 compared to $321,000 for the nine months ended September 30, 2002. The decrease was as a result of lower interest income as a result of lower average cash balances for the period ended December 31, 2002 and lower interest rates earned on invested cash balances in that period. Other income should increase as Genesoft raises more funds through financings resulting in higher cash balances earning interest.
Other expense increased by $6.2 million (1170%) to $6.7 million for the nine months ended September 30, 2003 compared to approximately $530,000 for the nine months ended September 30, 2002. The increase was a
120
Table of Contents
result of the interest on the bridge loans which were entered into in December 2002 and April 2003. Interest expense accrued on the bridge loans was $5.5 million through September 30, 2003. Other expense should decrease as Genesoft either pays off or converts its loans in the upcoming months. This decrease is subject to the completion of the merger as planned.
Twelve Months Ended December 31, 2002 compared with Twelve Months Ended December 31, 2001
Genesoft’s total revenues increased by $3.3 million (157%) to $5.4 million for the twelve months ended December 31, 2002 compared to $2.1 million for the comparable period ended December 31, 2001. This increase was due to additional funding from DARPA. In September 2002, DARPA awarded Genesoft additional grant funds of $3.5 million. Additionally, a new four month contract for $3 million was awarded in November 2002.
Research and development expenses increased by $10.1 million (62%) to $26.3 million for the twelve months ended December 31, 2002 compared to $16.2 million for twelve months ended December 31, 2001. The increase in research and development expenses was primarily due to $5 million in technology licensing and milestone fees paid to Vernalis for access to the Metalloenzyme Inhibitor technology platform; a $5.5 million license fee paid to LG Life Sciences for rights to FACTIVE, a quinolone antibiotic; increased lab supply and contract service expenses of approximately $741,000 (26%) due to toxicology and other analytical expenses related to the DARPA contract. These expenses were somewhat offset by a decrease of approximately $369,000 (10%) in salary expense to scientific management and staff due to reduction in staffing levels in order to control expenditures, which primarily impacted the internal DNA-Nanobinder related programs, as well as a reduction in rent and related facility expense of $1.5million (23%) as a result of subleasing additional space in its facility to a subtenant.
General and administrative expenses decreased by approximately $286,000 (6%) to $4.5 million for the twelve months ended December 31, 2002 compared to $4.8 million for the twelve months ended December 31, 2001. The decrease in general and administrative expenses was primarily due to approximately $233,000 (14%) decrease in administrative salaries and relocation expenses due to reduction in staffing levels and one time relocation charges in 2001 and a decrease of approximately $432,000 (28%) due to subleasing additional space in its facility. This was somewhat offset by an increase of approximately $333,000 (29%) in professional service fees such as legal and consulting fees due to increased activities in business development and human resources related to the licensing of FACTIVE from LG Life Sciences and analyzing other licensing and collaborative opportunities.
Other income decreased by approximately $688,000 (55%) to $564,000 in the twelve months ended December 31, 2002 compared to $1.3 million for the twelve months ended December 31, 2001. The decrease was due to lower interest income resulting from lower cash balances for the period ended December 31, 2002 and lower interest rates earned on invested cash balances.
Other expense increased by approximately $152,000 (27%) to $710,000 in the twelve months ended December 31, 2002 compared to approximately $558,000 for the twelve months ended December 31, 2001. The increase was primarily due to a full year of interest expense on Genesoft’s equipment financing . In 2001, the first installment of $3.7 million was drawn in June 2001, followed by the second draw in August 2001 of approximately $512,000 and the last draw of approximately $ 464,000 in October 2001.
Twelve Months Ended December 31, 2001 compared with Twelve Months Ended December 31, 2000
Genesoft’s total revenues decreased by $2.1 million (51%) to $2.1 million for the twelve months ended December 31, 2001 compared to $4.2 million for the comparable period ended December 31, 2000. This decrease was due to decreased funding from DARPA. Funds from the DARPA grant were depleted by May 2001 whereas in the comparable period ended December 31, 2000 Genesoft was fully funded for twelve months.
121
Table of Contents
Research and development expenses increased by $4.8 million (42%) to $16.2 million in the twelve months ended December 31, 2001, from $11.4 million for the twelve months ended December 31, 2000. The increase in research and development expenses was primarily due to an increase of $1.3 million (54%) in salaries for scientific management and staff due to increased headcount primarily to support the DNA-Nanobinder antibacterial and mammalian programs, an increase of approximately $618,000 (42%) in professional service fees related to consultants used in the DNA-Nanobinder antibacterial and mammalian programs, an increase of $4.4 million (197%) in facility related expenses comprised primarily of an increase in rent expense due to the move to a new, larger facility and associated expenses, including depreciation expense due to the depreciation commencing on leasehold improvements related to the new facility, and other facility-related expenses such as utilities, repairs and maintenance, and office-related expenses. These expenses were somewhat offset by decreased lab supply and outside contract services expenses of $1.0 million (25%) as toxicology and scale up synthesis related to the DNA-Nanobinder antibacterial program were completed in the prior year.
General and administrative expenses increased $3.0 million (164%) to $4.8 million in the twelve months ended December 31, 2001, from $1.8 million for the twelve months ended December 31, 2000. The increase in general and administrative expenses was primarily due to approximately $857,000 (138%) increase in administrative salaries and relocation expenses due to increased headcount to build Genesoft’s infrastructure; an
increase of approximately $446,000 (162%) in professional service fees for consulting in the areas of business development and human resources; an increase of $1.2 million (367%) comprised primarily of an increase in rent expense due to the move to a new larger facility and associated expenses, including depreciation expense due to the depreciation on leasehold improvements for the new facility, and other facility-related expenses such as utilities, repairs and maintenance, and office-related expenses.
Other income increased approximately $20,000 (2%) to $1.25 million in the twelve months ended December 31, 2001 compared to $1.23 million for the twelve months ended December 31, 2000. This was primarily a result of interest income being stable between years as the cash balances and interest rates were relatively unchanged during the two years.
Other expense increased approximately $504,000 (927%) to $558,000 in the twelve months ended December 31, 2001 from approximately $54,000 for the twelve months ended December 31, 2000. The increase was primarily due to an increase in interest expense as a result of equipment and leasehold related financing transactions in 2001.
Income Taxes
At December 31, 2002, Genesoft had net operating loss carry-forwards for federal income taxes of $10.0 million. If not utilized, federal net operating loss carry-forwards will begin to expire in 2007. Genesoft’s utilization of the net operating loss and tax credit carry-forwards may be subject to annual limitations pursuant to Section 382 of the Internal Revenue Code, and similar state provisions, as a result of changes in its ownership structure. The annual limitations may result in the expiration of net operating losses and credits prior to utilization.
At December 31, 2001 and 2002, Genesoft had deferred tax assets representing the benefit of net operating loss carryforwards and certain start-up costs capitalized for tax purposes. Genesoft did not record a benefit for the deferred tax assets because realization of the benefit was uncertain and, accordingly, a valuation allowance is provided to offset the deferred tax assets.
Liquidity and Capital Resources
Genesoft’s primary sources of cash have been through government grants and contracts, borrowings under equipment lending facilities and proceeds from the sale of equity and debt securities.
As of September 30, 2003, Genesoft had cash, cash equivalents and short-term and long-term marketable securities of approximately $7,826,000, of which $3,697,000 was restricted.
122
Table of Contents
In June of 2000 and August of 2001, Genesoft completed private placements of its series C and series D convertible preferred stock, respectively. The series C involved the issuance of 4,890,000 shares at $5.00 per share raising $24,405,000 in net proceeds. The series D involved the issuance of 5,450,000 shares at $4.00 per share raising $20,650,000 in net proceeds. Each share of preferred stock was convertible, at the option of the holder, into one share of common stock. In December 2002, in connection with Genesoft’s raising of funds from a bridge loan (see further discussion below), all convertible preferred stock was converted to common stock.
Genesoft’s operating activities used cash of approximately $6,992,000, $16,647,000 , $22,006,000, $16,153,000 and $13,342,000 for the years ended December 31, 2000, 2001 and 2002 and the nine months ended September 30, 2002 and 2003, respectively. Cash used in Genesoft’s operating activities for the fiscal year ended 2000 was primarily due to its net loss and increases in accounts receivable and prepaid expenses. These uses of cash were partially offset by increases in accounts payable, other assets, accrued bonus and other accrued liabilities as well as non-cash expenses, such as, depreciation and amortization, interest expense and accounting charges for stock issuances to consultants. Cash used in Genesoft’s operating activities for the fiscal year ended 2001 was primarily due to its net loss and decreases in accounts payable, accrued patent expenses and accrued leasehold improvements. These uses of cash were partially offset by decreases in accounts receivable, other assets, accrued bonus and other accrued liabilities, increases in deferred rent payable as well as non-cash expenses, such as, depreciation and amortization, interest expense and accounting charges for stock issuances to consultants. Cash used in Genesoft’s operating activities for the fiscal year ended 2002 was primarily due to its net loss and increases in accounts receivable and other assets. These uses of cash were partially offset by increases in accounts payable, other accrued liabilities, deferred rent payable and decreases in prepaid expenses as well as non-cash expenses, such as, depreciation and amortization, interest expense and accounting charges for stock issuances to consultants and collaborators. Additionally, Genesoft realized gains on its short-term investments as well as disposal of equipment. Cash used in its operating activities for the nine months ended September 30, 2002 was primarily due to its net loss and increases in accounts receivable, other assets and decreases in its accounts payable. These uses of cash were partially offset by increases in other accrued liabilities, deferred rent payable and decreases in prepaid expenses as well as non-cash expenses, such as, depreciation and amortization, interest expense. Additionally, Genesoft realized gains on its short-term investments as well as disposal of equipment. Cash used in Genesoft’s operating activities for the nine months ended September 30, 2003 was primarily due to its net loss and increases in accounts receivable and decreases in its accounts payable. These uses of cash were partially offset by increases in bridge loans, other accrued liabilities, deferred rent payable and decreases in prepaid expenses and other assets as well as non-cash expenses, such as, depreciation and amortization, interest expense and stock issued to consultants and collaborators. Additionally, Genesoft realized gains on its short-term investments.
Genesoft’s investing activities (used)/provided cash of approximately ($12,946,000), ($15,905,000), $16,320,000, $14,614,000 and $369,000 for the years ended December 31, 2000, 2001, 2002 and the nine months ended September 30, 2002 and 2003, respectively. Cash used by Genesoft’s investing activities for fiscal year 2000 was primarily due to purchases of marketable securities and property and equipment and the issuance of a standby letter of credit to its landlord for the building deposit, which is secured by a restricted cash account. Cash used by Genesoft’s investing activities for fiscal year 2001 was primarily due to purchases of marketable securities and property and equipment. The uses were partially offset by the conversion of marketable securities to cash and cash equivalents. Cash provided by Genesoft’s investing activities for the fiscal year 2002, was primarily through the conversion of marketable securities to cash and cash equivalents, proceeds received from the sale of property and equipment. These uses were partially offset by the purchases of marketable securities and property and equipment. Cash provided by Genesoft’s investing activities for the nine months ended September 30 and September 30, 2002, respectively, was primarily through the conversion of marketable securities to cash and cash equivalents, proceeds received from the sale of property and equipment. These uses were partially offset by the purchases of marketable securities and property and equipment. Cash provided by Genesoft’s investing activities for the nine months ended September 30, 2003, was primarily through the conversion of marketable securities to cash and cash equivalents. These uses were partially offset by the purchases of marketable securities and property and equipment.
123
Table of Contents
Capital expenditures totaled $2,386,000, $12,174,000, $209,000, $151,000 and $7,000 for the years ended December 31, 2000, 2001, 2002 and the nine months ended September 30, 2002 and 2003, respectively, consisting primarily of the investment in leasehold improvements and purchases of laboratory and computer equipment. Genesoft currently estimates that it will not acquire any new equipment or make additions to leasehold improvements prior to the consummation of the proposed merger with Genome. Genesoft’s capital expenditures will mainly result from the replacement of any defective equipment.
Genesoft’s financing activities provided cash of approximately $26,206,000, $24,016,000, $3,435,000 and $15,222,000 for the years ended December 31, 2000, 2001, 2002 and the nine months ended September 30, 2003, respectively. For the nine months ended September 30, 2002, Genesoft’s financing activities used cash of $1,427,000. For the fiscal year ended 2000, Genesoft’s cash was provided primarily from proceeds received from the sale of convertible preferred stock totaling $24.4 million in net proceeds, proceeds received from entering into an additional loan agreement for $1.9 million, as well as proceeds received from issuances of stock from employee early exercise of options through its employee option plan. These proceeds from financing activities were partially offset by payments of obligations of $323,000 and the repurchase of unvested stock from terminated employees. For the fiscal year ended 2001, Genesoft’s cash was provided primarily from proceeds received from the sale of convertible preferred stock totaling $20.6 million in net proceeds, proceeds received from entering into an additional loan agreement for $4.7 million, as well as proceeds received from issuances of stock from employee early exercise of options through the employee option plan. These proceeds from financing activities were partially offset by payments of obligations of $1,279,000 and the repurchase of unvested stock from terminated employees. For the fiscal year ended 2002, Genesoft’s cash was provided primarily from proceeds received from entering into a bridge loan and additional loan agreements for $6.5 million, as well as proceeds received from issuances of stock from employee early exercise of options through the employee option plan. These proceeds from financing activities were partially offset by payments of obligations of $3,032,000 and the repurchase of unvested stock from terminated employees. For the nine months ended September 30, 2002, Genesoft’s cash was used by payments of obligations of $1,409,000 and the repurchase of unvested stock from terminated employees. The use was partially offset by issuances of stock from the employee early exercise of options through the employee option plan. For the nine months ended September 30, 2003, Genesoft’s cash was provided primarily from proceeds received from entering into a bridge loan for $18.8 million as well as proceeds received from the issuances of stock from employee early exercise of options through the employee option plan. These proceeds from financing activities were partially offset by payments of obligations of $3,625,000 and the repurchase of unvested stock from terminated employees.
Contractual obligations
In December of 2002 and April of 2003, Genesoft entered into convertible bridge loan agreements with various existing and new investors in the aggregate principal amount of $22,300,000. The December bridge loan was in the original principal amount of approximately $5 million, had an interest rate of 6% per annum, carries a liquidation preference of $7.5 million and required the conversion of all existing Genesoft preferred stock to common stock. The April bridge loan was in the original principal amount of approximately $17.3 million and had an initial interest rate of 17% per annum which increased to 4% per month on August 15, 2003 since the loan was not repaid by that date. The December bridge loan is convertible, at the option of the holders, into common stock of Genesoft upon the closing of a financing transaction at the price per share paid in that financing transaction. The April bridge loan is convertible, at the option of the holders, into common stock of Genesoft at any time after December 15, 2003 at a price of $5.00 per share. In connection with the signing of the merger agreement with Genome, the December and April bridge loans were amended to provide that interest would accrue on the loans at a rate of 5% per annum from and after December 10, 2003, in the case of the December bridge loans, and from and after December 15, 2003, in the case of the April bridge loans. The maturity date of the December bridge loans was amended to be the later of December 10, 2005 and 60 days following the termination or expiration of the merger agreement. The maturity date of the April bridge loans was amended to be the later of December 15, 2005 and 60 days following the termination or expiration of the merger agreement. Upon the closing of the merger, the December and April bridge loans will be exchanged for convertible
124
Table of Contents
promissory notes of Genome. See “The Merger and Related Transactions—Other Material Agreements Relating to the Merger—Note Amendment and Exchange Agreement” for more detail.
In connection with the December and April bridge loans, Genesoft issued warrants to purchase 5,000,678 of its shares of common stock at an exercise price of $0.01 per share and 360,593 of its common stock at an exercise price of $12 per share. These warrants and the conversion feature on the bridge loans were valued, using the Black-Scholes option pricing model, at $1.2 million which is being amortized to interest expense over the term of the notes.
Genesoft has two loan agreements under which it has financed the purchase of office and laboratory equipment and leasehold improvements. Genesoft has borrowed approximately $6,600,000 in the aggregate from financial institutions, of which approximately $1,889,000 remains outstanding at September 30, 2003. This amount is repayable over the next 15 months, with $1,518,000 repayable over the next 12 months. At the closing of the merger with Genome, the combined company will be required to pay off one of these loans under which $1,019,306 is currently outstanding. In connection with these financing arrangements, Genesoft issued warrants to purchase 40,702 shares of common stock at an exercise price of $13.47 per share. These warrants were valued, using the Black-Scholes option pricing model, at $408,000 which is being amortized to interest expense over the term of the agreement.
In December 2002, Genesoft issued a promissory note to LG Life Sciences for $3,000,000 which represented the balance due on the up-front in-license fee of $5,500,000. The note, which had a maturity date of April 29, 2003, was unsecured and had an interest rate of 10% per annum compounded quarterly. In December 2002, Genesoft prepaid $125,000 of the outstanding balance and in April 2003 Genesoft paid back the entire remaining amount due under the note.
The future minimum payments under the operating leases (gross) and financing arrangements, by year, are as follows:
| Operating Leases | Notes Payable and Bridge Loan | ||||||
| Three months ending December 31, 2003 | $ | 533,925 | $ | 24,714,412 | |||
Year ending December 31, | |||||||
2004 | 2,198,940 | 1,714,354 | |||||
2005 | 4,075,654 | 5,918,301 | |||||
2006 | 4,218,296 | — | |||||
2007 | 4,365,944 | — | |||||
Thereafter | 13,384,023 | — | |||||
| $ | 28,776,782 | 32,347,067 | |||||
Less interest | (6,897,842 | ) | |||||
Less discount | (731,631 | ) | |||||
| 24,717,595 | |||||||
Less current portion | (19,689,234 | ) | |||||
Long-term portion | $ | 5,028,361 | |||||
Genesoft plans to continue to invest in the launch of FACTIVE as well as its internal research programs, primarily the MEI inhibitors. Pursuant to its partnership with LG Life Sciences, upon delivery of the first shipment of FACTIVE, which is anticipated to occur in the next two months, Genesoft will be obligated to make a $2.5 million milestone payment to LG Life Sciences as well as a payment of $4.8 million for the purchase of the drug inventory. Upon the closing of the merger, the combined company will be obligated to make an $8 million milestone payment to LG Life Sciences.
125
Table of Contents
On November 17, 2003, Genome loaned to Genesoft $6.2 million in connection with the signing of the merger. This loan, along with Genesoft’s existing capital resources are expected to fund Genesoft’s operations through the closing of the merger. If, however, the closing of the merger is delayed or if Genesoft’s liabilities increase, there can be no assurance that these funds will be sufficient. For further detail on the terms of this loan, see “The Merger and Related Transactions—Other Material Agreements Relating to the Merger—Bridge Loan.”
In the future, Genesoft, as part of the combined company following the merger, will need to raise additional capital in order to continue to fund its programs. Additional financing may not be available when needed or, if available, it may not be on terms acceptable to the combined company. Any additional capital that the combined company raises by issuing equity or convertible debt securities will dilute the ownership of existing stockholders of Genesoft in the combined company.
Quantitative and Qualitative Disclosures about Market Risk
The primary objective of Genesosft’s investment activities is to preserve its capital for the purpose of funding operations while at the same time maximizing the income Genesoft receives from its investments without significantly increasing risk. To achieve these objectives, Genesoft’s investment policy allows it to maintain a portfolio of cash equivalents and short-term investments in a variety of securities, including commercial paper, money market funds and corporate debt securities. Genesoft’s cash and cash equivalents through September 30, 2003 included liquid money market accounts. Genesoft’s short-term investments included readily marketable debt securities. Due to the short-term nature of these instruments, a 1% movement in market interest rates would not have a significant impact on the total value of Genesoft’s portfolio as of September 30, 2003.
126
Table of Contents
GENESOFT PRINCIPAL AND MANAGEMENT STOCKHOLDERS
The following table sets forth information regarding the beneficial ownership of Genesoft common stock as of November 30, 2003 by:
| • | each person known by Genesoft to own beneficially 5% or more of the Genesoft stock; |
| • | each director of Genesoft; |
| • | each executive officer of Genesoft; and |
| • | all of the directors and executive officers of Genesoft as a group. |
The percentages shown are based on 12,378,931 shares of Genesoft common stock outstanding as of November 30, 2003. Unless otherwise indicated, the address for each stockholder is c/o GeneSoft Pharmaceuticals, Inc., 7300 Shoreline Court, South San Francisco, California 94080. Unless otherwise indicated, each person or entity named in the table has sole voting power and investment power (or shares such power with his or her spouse) with respect to all shares of capital stock listed as owned by such person or entity.
Name and Address of Beneficial Owner | Amount and Nature of Beneficial Ownership | Percent of Class | ||||
David B. Singer | 1,229,778 | (1) | 9.3 | % | ||
Gary Patou | 811,790 | (2) | 6.2 | % | ||
Peter B. Dervan | 618,096 | (3) | 5.0 | % | ||
Vernon R. Loucks | 195,938 | (4) | 1.6 | % | ||
Luke B. Evnin | 5,594,802 | (5) | 35.2 | % | ||
William J. Rutter | 818,095 | (6) | 6.4 | % | ||
Edward M. Scolnick | 17,812 | (7) | 0.1 | % | ||
LG Life Sciences | 2,856,368 | (8) | 23.1 | % | ||
Entities affiliated with MPM Capital | 5,594,802 | (9) | 35.2 | % | ||
Novartis Forschungsttiftung | 1,647,344 | (10) | 12.1 | % | ||
SunAmerica Investments, Inc. | 1,440,330 | (11) | 10.4 | % | ||
Entities affiliated with Maverick Capital, Ltd. | 1,728,393 | (12) | 12.3 | % | ||
All directors and executive officers as a group (7 persons) | 9,286,311 | (13) | 51.5 | % |
| (1) | Includes 14,250 shares of common stock held by the Singer-Kapp Family 2000 Trust and 200,000 shares of common stock held by the Singer-Kapp Long-Term Trust. Includes 826,965 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of options. |
| (2) | Includes 811,790 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of options. |
| (3) | The address of this stockholder is 1200 E. California Boulevard, MS 164-30, Pasadena, CA 91125. |
| (4) | The address of this stockholder is 1101 Skokie Boulevard, Suite 240, Northbrook, Illinois 60062. |
| (5) | Includes 1,779,496 shares of common stock held by BB BioVentures L.P.; 23,659 shares of common stock held by MPM Asset Management Investors 1998 LLC; and 254,372 shares of common stock held by MPM BioVentures Parallel Fund, L.P. Includes 2,477,964 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants held by BB BioVentures, L.P.; 32,343 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants held by MPM Asset Management Investors 1998 LLC; and 302,190 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants held by MPM BioVentures Parallel Fund, L.P. Includes 706,340 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes held by BB BioVentures L.P.; 9,219 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes held by MPM Asset Management Investors 1998 LLC; and 86,139 shares of common stock that may be acquired within 60 days |
127
Table of Contents
of November 30, 2003 upon conversion of promissory notes held by MPM BioVentures Parallel Fund, L.P. Dr. Evnin has shared voting and dispositive power over shares held by BB BioVentures L.P., MGM Asset Management Investors 1998 LLC and MPM BioVentures Parallel Fund, L.P. The address of this stockholder is 601 Gateway Boulevard, Suite 360, South San Francisco, California 94080. |
| (6) | Includes 356,251 shares of common stock held by the William J. Rutter Revocable Trust U/A/D 4/11/02. Includes 310,416 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants held by the William J. Rutter Revocable Trust U/A/D 4/11/02. Includes 133,616 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes held by the William J. Rutter Revocable Trust U/A/D 4/11/02. Includes 17,812 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of options. The address of this stockholder is One Market Street, Suite 1475, San Francisco, CA 94105. |
| (7) | Includes 17,812 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of options. The address of this stockholder is 770 Sunneytown Pike, WP26-25, West Point, Pennsylvania 19486. |
| (8) | The address of this stockholder is LG Twin Tower, 20, Yoido-dong, Youngdungpo-gu, Seoul, Korea. |
| (9) | Includes 1,779,496 shares of common stock held by BB BioVentures L.P.; 23,659 shares of common stock held by MPM Asset Management Investors 1998 LLC; and 254,372 shares of common stock held by MPM BioVentures Parallel Fund, L.P. Includes 2,477,964 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants held by BB BioVentures, L.P.; 32,343 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants held by MPM Asset Management Investors 1998 LLC; and 302,190 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants held by MPM BioVentures Parallel Fund, L.P. Includes 706,340 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes held by BB BioVentures L.P.; 9,219 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes held by MPM Asset Management Investors 1998 LLC; and 86,139 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes held by MPM BioVentures Parallel Fund, L.P. The address of this stockholder is 601 Gateway Boulevard, Suite 360, South San Francisco, California 94080. |
| (10) | Includes 1,020,833 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants. Includes 267,232 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes. The address of this stockholder is WSJ-200.220, Lichstrasse 354056, Basel, Switzerland. |
| (11) | Includes 104,166 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants. Includes 1,336,164 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes. The address of this stockholder is 1 SunAmerica Center, Los Angeles, California 90067. |
| (12) | Includes 76,437 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants held by Maverick Fund LDC; 34,520 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants held by Maverick Fund USA, Ltd; 14,041 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of warrants held by Maverick Fund II, Ltd. Includes 980,477 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes held by Maverick Fund LDC; 442,804 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes held by Maverick Fund, Ltd.; and 180,114 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes held by Maverick Fund II, Ltd. The address of this stockholder is 300 Crescent Court, Suite 1850, Dallas, TX 75201. |
| (13) | Includes 1,674,379 shares of common stock that may be acquired within 60 days of November 30, 2003 upon exercise of options. Includes 3,122,913 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of warrants. Includes 935,314 shares of common stock that may be acquired within 60 days of November 30, 2003 upon conversion of promissory notes. |
128
Table of Contents
The following directors of Genesoft will become directors of Genome following the closing of the merger:
Name | Age | Position | ||
David B. Singer | 41 | Chairman | ||
Luke Evnin, Ph.D. | 40 | Director | ||
Vernon R. Loucks, Jr. | 69 | Director | ||
William Rutter, Ph.D. | 76 | Director |
David B. Singer joined Genesoft as founding President and Chief Executive Officer in September of 1998. Mr. Singer previously served as founding President and Chief Executive Officer of both Affymetrix, Inc., a company focused on developing state-of-the-art technology for acquiring, analyzing and managing complex genetic information for use in biomedical research, and Corcept Therapeutics, Inc. Prior to Genesoft, Mr. Singer was Senior Vice President and Chief Financial Officer of Heartport, Inc. He is a member of the board of directors at Affymetrix (NASDAQ: AFFX), Corcept and Physician Dynamics, Inc. Mr. Singer received his B.A. in History from Yale College and his M.B.A. from The Graduate School of Business at Stanford University. He is a Henry Crown Fellow of the Aspen Institute and Sterling Fellow of Yale University.
Luke Evnin, Ph.D., is a Managing Director of MPM Asset Management LLC, a venture capital firm. Prior to joining MPM in 1998, Dr. Evnin was a general partner at Accel Partners, focusing on investing in a broad range of life sciences companies. From October 1998 to July 2002, Dr. Evnin served as a director of Sonic Innovations. Dr. Evnin received his A.B. degree from Princeton University and his Ph.D. in Molecular Biology from the University of California, San Francisco. Dr. Evnin also serves on the boards of several private companies.
Vernon R. Loucks, Jr. is the Chief Executive Officer of Segway LLC, a company providing solutions to short distance travel, since January 2003. Mr. Loucks served as Chairman of Baxter International Inc., and held the position of Chief Executive Officer from May 1980 to January 2000. He is a director of Affymetrix, Inc., Anheuser-Busch Companies, Inc., Capital and Limited (Singapore) and Emerson Electric Co. He is a member of The Business Council and is the former chairman and co-founder of the Healthcare Leadership Council. Mr. Loucks is a trustee of Rush-Presbyterian/St. Luke’s Medical Center in Chicago, and has served as a director of the Harvard Business School Board of Directors and as Senior Fellow of the Yale Corporation. Mr. Loucks holds a B.A. degree in History from Yale College and a M.B.A. from the Harvard Graduate School of Business Administration. He is a veteran of the U.S. Marine Corps. Mr. Loucks also serves on the board of a private equity firm.
William Rutter, Ph.D., is Professor Emeritus of Biochemistry at the University of California, San Francisco. Dr. Rutter is Chairman, Chief Executive Officer and principal shareholder of Synergenics LLC, a company that provides financial resources, facilities, financial, legal support and strategic advice to start-up biotech companies, since July 2002. Dr. Rutter was a founder of Chiron and served as the company’s Chief Executive Officer and Chairman of the Board. Dr. Rutter also was a consultant to Chiron from February 2000 until May 2002. He continues to serve as a Director of Chiron. Dr. Rutter services as a director of Ciba-Geigy, Ltd. and subsequently Novartis from 1995 until April 1999. From January 2000 to present, Dr. Rutter has served as a director of Sangamo Biosciences, Inc. From 1969 to 1982, Dr. Rutter was Chairman of the Department of Biochemistry and Biophysics at the University of California, San Francisco. Dr. Rutter received his B.A. from Harvard University and his Ph.D. from the University of Illinois. Dr. Rutter has received numerous awards for his scientific work and is a member of the National Academy of Sciences and the American Academy of Arts and Sciences. Dr. Rutter also serves on the boards of other privately-held biotechnology companies.
129
Table of Contents
MANAGEMENT OF THE COMBINED COMPANY AFTER THE MERGER
The board of the combined company will consist of Luke Evnin, Robert J. Hennessey, Vernon R. Loucks, Jr., Steven Rauscher, William S. Reardon, Norbert G. Riedel, William Rutter, David B. Singer and David K. Stone. David B. Singer will serve as Chairman of the board of directors.
Committees of the Board of Directors
The board of directors will have an audit committee and a stock option and compensation committee, each consisting of at least three independent directors, and a nominating committee, consisting of two independent directors. Each committee will perform the functions traditionally performed by such committee.
Directors of the combined company will be subject to the existing compensation structure for Genome’s current directors. Each non-employee director of the combined company will receive his annual retainer, currently set at $10,000, for the fiscal year, and a non-employee chairman of each sub-committee will also receive an additional retainer, currently set at $4,000, for the fiscal year, each in the form of a stock option grant that provides the right to purchase share of Genome common stock at a 70% discount to the fair market value. These grants will vest quarterly over a year from the date of grant. The grant size (number of options) will be determined by dividing the annual retainer fee by 70% of the fair market value of the Genome common stock on the date of grant. In addition, upon their initial election to the board, non-employee directors will also be granted options to receive an aggregate 17,000 shares of Genome common stock that will vest equally over three years with an exercise price equal to the fair market value at date of grant. As a long term incentive in connection with their re-election to the board, directors will, upon their re-election to the board, also be granted options to receive an aggregate of 8,500 shares of Genome common stock that will vest equally over three years with an exercise price equal to the fair market value at date of grant. Upon a change of control if, within two years following the change of control, a director is either not nominated to serve as a director or is not elected by the shareholders to serve as a director, all of such director’s unvested options will become exercisable upon such director ceasing to be a director of Genome and all of the director’s options will remain exercisable until the earlier of two years from the date such director ceases to be a director of Genome and the final exercise date of the option. In addition, each director will have the option to receive all of his board meeting fees and sub-committee fees, currently set at $2,000 and $1,250, respectively, per meeting, in the form of cash or a stock option grant on the same terms described above for the annual retainer. Meeting fees will be reduced by fifty percent if the director attends a meeting via teleconference.
The management of the combined company will consist of the following: Steven Rauscher as Chief Executive Officer and President, Stephen Cohen as Senior Vice President and Chief Financial Officer and Martin Williams as Senior Vice President of Corporate Development and Marketing.
130
Table of Contents
GENOME PRINCIPAL AND MANAGEMENT STOCKHOLDERS
The following table sets forth certain information regarding the beneficial ownership of Genome common stock as of November 30, 2003 by:
| • | each person who has reported to the Securities and Exchange Commission beneficial ownership of more than 5% of the outstanding shares of Genome common stock; |
| • | each director of Genome; |
| • | the chief executive officer of Genome and the three other most highly compensated executive officers of Genome who were serving as executive officers on December 31, 2002; and |
| • | all executive officers and directors of Genome as a group. |
The percentages shown are based on 31,451,099 shares of Genome common stock outstanding as of November 30, 2003.
Unless otherwise indicated, each person or entity named in the table has sole voting power and investment power (or shares such power with his or her spouse) with respect to all shares of capital stock listed as owned by such person or entity.
Name and Address of Beneficial Owner(1) | Amount and Nature of Beneficial Ownership | Percent of Class | ||||
Marc B. Garnick | 51,590 | (2) | * | |||
Robert J. Hennessey | 782,777 | (2) | 2.4 | % | ||
Philip Leder | 135,412 | (2) | * | |||
Lawrence Levy(3) | 18,131 | (2) | * | |||
Steven M. Rauscher | 542,877 | (2) | 1.7 | % | ||
Norbert G. Riedel | 55,527 | (2) | * | |||
David K. Stone | 40,599 | (2) | * | |||
William S. Reardon | 8,964 | (2) | ||||
Stephen Cohen | 126,525 | (2) | * | |||
Richard F. Labaudiniere | 118,538 | (2) | * | |||
Martin D. Williams | 96,463 | (2) | * | |||
All directors and executive officers as a group (11 persons) | 1,977,403 | (4) | 5.9 | % |
| * | Less than 1%. |
| (1) | The address of all such persons is c/o the Company, 100 Beaver Street, Waltham, Massachusetts, 02453. |
| (2) | Includes 49,330 shares for Dr. Garnick, 734,317 shares for Mr. Hennessey, 128,154 shares for Dr. Leder, 15,051 shares for Mr. Levy, 496,952 shares for Mr. Rauscher, 8,964 shares for Mr. Reardon, 52,008 shares for Dr. Riedel, 40,599 shares for Mr. Stone, 107,476 shares for Mr. Cohen, 116,496 shares for Dr. Labaudiniere and 96,463 shares for Mr. Williams, which shares are issuable upon the exercise of vested options or options that are to become vested within 60 days following November 30, 2003. Includes 24,000 restricted shares for Mr. Rauscher, which are subject to repurchase by the company based on a vesting schedule. Excludes options which have been granted to directors and executive officers which will not become vested within 60 days following November 30, 2003. |
| (3) | Represents shares held by Northern Ventures Corporation, over which Mr. Levy has shared voting and |
dispositive power.
| (4) | Includes a total of 1,845,810 shares that may be issuable upon the exercise of vested options or options that are to become vested within 60 days following November 30, 2003. Includes 24,000 restricted shares, which are subject to repurchase by the company based on a vesting schedule. Excludes options which have been granted to directors and executive officers which will not become vested within 60 days following November 30, 2003. |
131
Table of Contents
COMPARISON OF RIGHTS OF HOLDERS
OF GENOME COMMON STOCK AND HOLDERS
OF GENESOFT CAPITAL STOCK
Genome is a Massachusetts corporation subject to the provisions of the Massachusetts Business Corporation Law or MBCL. Genesoft is a Delaware corporation subject to the provisions of the Delaware General Corporation Law or DGCL. Upon completion of the merger, Genesoft stockholders, whose rights are currently governed by the Genesoft charter, by-laws and the DGCL, will become stockholders of Genome and their rights will be governed by the Genome charter, by-laws and the MBCL.
The following description summarizes material differences which may affect the rights of holders of Genome common stock and Genesoft capital stock. This is not a complete statement of all those differences, or a complete description of the specific provisions referred to in this summary. The identification of specific differences is not intended to indicate that other equally or more significant differences do not exist. For additional information regarding the specific rights of holders of Genome common stock, you should read the section of this proxy statement/prospectus entitled “Description of Genome Common Stock” beginning on page 143.You should also read carefully the relevant provisions of the MBCL and the DGCL and the respective charters and by-laws of Genome (which are incorporated by reference in this proxy statement/prospectus) and Genesoft for a more complete understanding of these differences.
RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
The rights of Genome stockholders are governed by Massachusetts law and Genome’s charter and by-laws. Upon completion of the merger, the rights of Genome stockholders will continue to be governed by Massachusetts law and Genome’s charter and by-laws. | The rights of Genesoft stockholders are currently governed by Delaware law and Genesoft’s charter and by-laws. Upon completion of the merger, the rights of Genesoft stockholders will be governed by Massachusetts law and Genome’s charter and by-laws. | |||
Genome has authority to issue 50,000,000 shares of common stock (i) 49,375,000 shares are designated as common stock, par value $.10 per share, and (ii) 625,000 shares are designated series B restricted stock, par value $.10 per share.
Genome is seeking approval from its stockholders through this proxy statement/prospectus to amend its Articles of Organization to increase the number of shares of common stock the company is authorized to issue from 50,000,000 to 175,000,000. | The authorized capital stock of Genesoft consists of 43,450,000 shares of common stock and 24,975,000 shares of preferred stock. Of the authorized preferred stock, 5,425,000 shares are designated as series A preferred stock, 6,000,000 shares are designated as series B preferred stock, 6,600,000 shares are designated as series C preferred stock, 5,950,000 shares are designated as series D preferred stock and 1,000,000 shares are designated as series 1 preferred stock.
Genesoft is seeking approval from its stockholders through this proxy statement/prospectus to amend and restated its certificate of incorporation to eliminate any authorized shares of preferred stock if the merger is completed. | |||
132
Table of Contents
| RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
The Genome by-laws provide that the directors may, at any time and from time to time, issue additional shares of capital stock up to the amount authorized in the charter. | Section 161 of the DGCL provides that the directors may, at any time and from time to time, issue additional shares of capital stock up to the amount authorized in the charter. | |||
Under the MBCL, a corporation may pay dividends or repurchase its own stock so long as: — the corporation is solvent; — the dividend or repurchase does not render the corporation insolvent; and — the dividend or repurchase does not violate the corporation’s charter.
Genome’s board of directors may declare and pay dividends on common stock only from legally available funds for the payment of such dividends. Genome has never paid cash dividends on any of its series of common stock.
The holders of Genome’s series B restricted stock, none of which is outstanding, are not entitled to receive dividends. | Under the DGCL, a corporation may pay dividends out of surplus or net profits for the current or preceding fiscal year, provided that the capital of the corporation is not less than the aggregate liquidation preference of the corporation’s outstanding stock having a preference upon distribution of assets.
Genesoft’s board of directors may declare and pay dividends on capital stock only from legally available funds for the payment of such dividends.
Under Genesoft’s charter, the holders of the series A preferred stock, series B preferred stock, series C preferred stock and series D preferred stock, none of which are outstanding, are entitled to receive dividends at the rate of 8% of the original purchase price of such shares, per share, per annum, and the holders of the series 1 preferred stock shall be entitled to receive dividends at the rate of $0.24 per share, per annum (in each case adjusted for any combinations, consolidations, stock splits, or stock distributions or dividends or the like with respect to such shares). Such dividends are payable only when, as, and if declared by the board of directors and are non-cumulative.
No dividends (other than those payable solely in Genesoft common stock) may be paid on any common stock during any fiscal year until dividends required to be paid on the preferred stock have been paid or declared and set apart for that fiscal year and any prior year in which dividends accumulated but remain unpaid.
No right accrues to holders of shares of preferred stock by reason of the fact that dividends on said shares were not declared in any prior year, nor will any unpaid dividend bear or accrue any interest.
Genesoft has never paid cash dividends on its capital stock. | |||
133
Table of Contents
| RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
Upon a liquidation, dissolution or winding up of Genome’s affairs, the holders of Genome common stock are entitled to receive, prior to the holders of series B restricted stock, the greater of $5.00 per share of common stock or an amount for each share of common stock equal to 10 times the amount available to holders of series B restricted stock. No series B restricted stock is currently outstanding. | In the event of any liquidation, dissolution or winding up of the Corporation, the holders of the series D preferred stock shall be entitled to receive, prior and in preference to any distribution to any other stockholders, the amount of $4.00 for each share of series D preferred stock then held by them (adjusted for any combinations, consolidations, stock splits, or stock distributions or dividends or the like with respect to such shares), plus any declared but unpaid dividends on such shares. No series D preferred stock is currently outstanding.
Upon completion of the above distribution, the holders of the series A preferred stock, series B preferred stock and series C preferred stock shall be entitled to receive, prior and in preference to any distribution to any other stockholders, (i) the amount of $1.00 per share for each share of series A preferred stock then held by them, (ii) the amount of $2.50 per share for each share of series B preferred stock then held by them and (iii) the amount of $5.00 per share for each share of series C preferred stock then held by them (in each case adjusted for any combinations, consolidations, stock splits, or stock distributions or dividends or the like with respect to such shares); plus any declared but unpaid dividends onsuch shares. No series A preferred stock, series B preferred stock or series C preferred stock is currently outstanding.
Upon completion of the above distributions, the holders of the series 1 preferred stock shall be entitled to receive, prior and in preference to any distribution to any other stockholders, the amount of $1.50 per share for each share of series 1 preferred stock then held by them (adjusted for any combinations, consolidations, stock splits, or stock distributions or dividends or the like with respect to such shares), plus any declared but unpaid dividends on such shares. No series 1 preferred stock is currently outstanding.
Upon the completion of the above distributions, the remaining assets of Genesoft available for distribution to stockholders shall be distributed among the holders of series A preferred stock, series 1 preferred stock and common stock pro rata based on the number of shares of common stock held by each (assuming full conversion of all such series A preferred stock and series 1 preferred stock), until with respect to the holders of series A preferred stock and the holders of series 1 preferred stock, such holders shall have received an aggregate of $2.50 per share and $3.00 per share, respectively (adjusted for any combinations, consolidations, stock splits, or stock distributions or dividends or the like with respect to such shares); thereafter, if assets remain, the holders of the common stock of Genesoft shall receive all of the remaining assets of the corporation pro rata based on the number of shares of common stock held by each. | |||
134
Table of Contents
| RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
Each holder of Genome common stock is entitled to one vote per share in all matters.
The holders of Genome’s series B restricted stock are not entitled to vote. | Holders of Genesoft common stock have one vote per share held by them. The holders of preferred stock are entitled to the number of votes equal to the number of shares of common stock into which their preferred shares could be converted. Holders of preferred stock have voting rights and powers equal to the voting rights and powers of the common stock. | |||
Under Genome’s charter, holders of common stock have no redemption rights and Genome has no option to exchange or redeem any shares of common stock. | Under Genesoft’s charter, holders of capital stock have no redemption rights and Genesoft has no option to exchange or redeem any shares of capital stock.
Each share of series A preferred stock, series B preferred stock, series D preferred stock and series 1 preferred stock is convertible at any time, at the option of the holder thereof, into such number of shares of common stock as is determined by dividing the sum of the original purchase price of such share (in each case as adjusted for any combinations, consolidations, stock splits, or stock distributions or dividends or the like) plus any declared but unpaid dividends by the original purchase price of such share (in each case adjusted for any dilutive events).
Each share of series C preferred stock is convertible at any time, at the option of the holder thereof, into such number of shares of common stock as is determined by dividing the sum of the original purchase price of such share (in each case as adjusted for any combinations, consolidations, stock splits, or stock distributions or dividends or the like) plus any declared but unpaid dividends by $4.79617 (in each case adjusted for any dilutive events). | |||
135
Table of Contents
| RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
A special meeting of stockholders may be called at any time by the president or by the board of directors.
A written notice stating the time, place and purpose of the meeting shall be given at least 7 days before the meeting to each stockholder entitled to notice. |
Special meetings of the stockholders may be called at any time by the President. A special meeting will also be called by the President or Secretary at the written request of either (i) a majority of the board of directors or (ii) stockholders owning a majority in amount of the entire issued and outstanding capital stock of Genesoft. Such request shall state the purpose or purposes of the proposed meeting.
A written notice stating the place, date and hour of the meeting and the purpose or purposes for which the meeting is called, shall be given not fewer than 10 nor more than 60 days before the date of the meeting to each stockholder entitled to vote. Business transacted at any special meeting shall be limited to the purposes stated in the notice. | |||
Under the MBCL and Genome’s charter, stockholders may take any action without a meeting so long as they act by unanimous written consent. |
Under the DGCL, stockholders may take any action without a meeting.
Genesoft’s by-laws provide that any action allowed or required to be taken at any annual or special meeting, may be taken without a meeting, without prior notice and without a vote, if a consent in writing, setting forth the action so taken, shall be signed by the holders of outstanding stock having at least the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted. Prompt notice of an action by less than unanimous written consent shall be given to those stockholders who have not consented in writing. | |||
136
Table of Contents
RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
For a stockholder proposal to be considered at Genome’s annual meeting, the proposal must be received by the company not less than 120 days before the date of the company’s proxy statement released to stockholders in connection with the previous year’s annual meeting. However, if the date of the annual meeting changes by more than 30 days from the date of the previous year’s annual meeting, the deadline is a reasonable time before the company begins to print and mail its proxy materials. Stockholders have a reasonable time before the company begins to print and mail its proxy materials to submit proposals for a special meeting of stockholders. | For a stockholder proposal to be considered at Genesoft’s annual meeting, the proposal must be properly brought before the meeting. | |||
The holders of a majority in interest of all outstanding stock entitled to vote at a Genome stockholder meeting, present in person or represented by proxy, constitutes a quorum for transacting business at a meeting. | The holders of fifty percent (50%) of the issued and outstanding shares of Genesoft stock entitled to vote at a meeting, present in person or represented by proxy, constitutes a quorum for transacting business at any meeting of the stockholders. | |||
By law, stockholders have the right for a proper purpose to inspect the company’s charter, by-laws, records of all meetings of incorporators and stockholders, and stock and transfer records, including the stockholder list. Additionally, stockholders have a qualified right to inspect other books and records of the corporation. | Under the DGCL any stockholder has the right to inspect the company’s stock ledger, stockholder list, and other books and records for a purpose reasonably related to the person’s interest as a stockholder. | |||
Genome currently has eight directors. Genome’s by-laws provide that the board of directors shall be at least three and not more than nine. The number of directors is fixed by the board and may be enlarged at any time by a vote of the stockholders or by a vote of the majority of directors. | Genesoft currently has seven directors. The number of directors may be determined by resolution of the board of directors or by the stockholders at the annual meeting of stockholders. | |||
137
Table of Contents
RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
Genome’s board of directors consists of a single class. Each director is elected for a one year term at the annual meeting of the stockholders. | Genesoft’s board of directors consists of a single class. Each director holds office until his successor is elected and qualified. | |||
A director may be removed (i) with or without cause by a majority of stockholders or (ii) with cause by the majority of the directors. Vacancies, including vacancies resulting from the enlargement of the board, may be filled by the stockholders or, in the absence of stockholder action, by the majority of the directors. | Any director may be removed, with or without cause, by the holders of a majority of shares entitled to vote at an election of directors. Under Genesoft’s by-laws vacancies on the board of directors may be filled by a majority of the directors then in office. | |||
Genome’s charter provides that directors shall not be personally liable to Genome or its stockholders for monetary damages for breaching their fiduciary duties except to the extent eliminating or limiting their liability is not permitted under the MBCL as in effect at the time such liability is determined. |
Genesoft’s charter provides that directors shall not be personally liable to Genesoft or its stockholders for monetary damages for breaching their fiduciary duties except to the extent eliminating or limiting their liability is not permitted under the DGCL. | |||
Massachusetts law permits, and Genome’s by-laws provide for, indemnification of directors and officers for all expenses and liabilities imposed upon them due to any proceeding in which they may become involved by serving or having served as directors or officers. Indemnification is denied, however, if the person is found not to have acted in good faith with the reasonable belief that his or her action was in Genome’s best interest.
The MBCL does not explicitly address indemnifying persons against judgments in actions brought by or in the right of the corporation. The previously discussed standard applies to these cases. |
Genesoft’s by-laws provide that Genesoft shall, to the fullest extent permitted by the DGCL as amended from time to time, indemnify any director or officer who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding whether civil, criminal, administrative or investigative, by reason of being or having been a director or officer of Genesoft.
The DGCL does not permit a corporation to indemnify persons against judgments in actions brought by or in the right of the corporation. | |||
138
Table of Contents
RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
Under the MBCL, a majority vote of stockholders is required to amend some charter provisions primarily involving changes in the amount and par value of the company’s authorized stock. For most other amendments, the MBCL requires a two-thirds vote, such as to authorize the sale, mortgage, pledge, lease or exchange of all the company’s property or assets. The MBCL does, however, permit a corporate charter to specify a threshold vote of less than two-thirds, but of at least a majority. | Under DGCL, a majority vote of stockholders is required to amend a company’s charter. However, under Genesoft’s charter, any amendments to the preferred stock conversion provisions must be approved by two-thirds of each series of preferred stock (voting as separate classes) and any amendment to the charter which would adversely change any of the rights, preferences or privileges of any shares of preferred stock must be approved by a vote of two-thirds of the outstanding preferred stock that would be adversely effected. | |||
Genome’s by-laws may be amended, altered or repealed, and new by-laws may be adopted, at any annual or special meeting of the stockholders called for the purpose, by a majority vote of the stockholders. The directors may also make, amend or repeal the by-laws, except that the directors shall not take any action with respect to the indemnification and amendment provisions or take any action unless permitted by law. | Genesoft’s by-laws may be adopted, amended or repealed by the vote or the written consent of stockholders entitled to exercise a majority of the voting power of the corporation. Genesoft’s by-laws may also be adopted, amended or repealed by the board of directors. | |||
139
Table of Contents
RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
The Massachusetts “Business Combination” statute prohibits a Massachusetts corporation from engaging in a “business combination” with a person owning 5% or more of the corporation’s voting stock without the approval of its board of directors to acquire that stock (referred to as an interested stockholder) for three years from the time the person became an interested stockholder, unless the:
— board of directors approves the stock acquisition or the combination transaction before the person becomes an interested stockholder; — the interested stockholder acquires 90% of the outstanding voting stock of the company (excluding stock owned by directors-officers or some employee stock plans) in one transaction; or — the combination transaction is approved by the board of directors and by two-thirds of the outstanding voting stock not owned by the interested stockholder.
Genome is subject to the Massachusetts Business Combination statute unless it elects, with stockholder approval, not to be. Under its by-laws, Genome has elected not to be governed by this statute. | Section 203 of the DGCL prohibits a Delaware corporation from engaging in a “business combination” with a person owning 15% or more of the corporation’s voting stock, or an interested stockholder, for three years following the time that person became an interested stockholder, unless (i) the board of directors approves the stock acquisition or the business combination before the person becomes an interested stockholder; (ii) the person became an interested stockholder and 85% owner of the voting stock in the same transaction, excluding shares owned by directors and officers and shares owned by some employee stock plans; or (iii) the combination transaction is approved by the board of directors and by holders of at least two-thirds of the outstanding voting stock not owned by the interested stockholder.
A Delaware corporation can elect in its charter or by-laws not to be governed by Section 203. Genesoft has not made that election. | |||
The Massachusetts Control Share Acquisition statute provides that a person that acquires 20%, 33 1/3% or a majority of the corporation’s voting stock, in each case, cannot vote the shares exceeding that threshold unless a majority of the outstanding shares not owned by the acquiror and the corporation’s officers and employee-directors vote to permit it to do so with respect to such acquisition. Under its by-laws, Genome has elected not to be governed by this statute. |
Delaware does not have a Control Share Acquisition statute. | |||
140
Table of Contents
RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
Genome does not have a stockholders rights plan. | Genesoft does not have a stockholders rights plan. | |||
Under the MBCL, the affirmative vote of two-thirds of the outstanding shares of each class of stock (or such lower proportion permitted by the charter, but not less than a majority) is required to authorize a merger or consolidation of Genome into any other corporation, or the sale, lease, or exchange of all or substantially all of Genome’s property and assets.
Under the MBCL, unless the corporation’s charter otherwise provides for a stockholder vote, a surviving corporation need not obtain stockholder approval for a merger if: — any shares of the surviving corporation to be issued or delivered in the merger will not increase the number of shares of common stock outstanding before the merger by more than 15%; and — the merger agreement does not amend the charter of the surviving corporation. |
Under the DGCL, the affirmative vote of a majority of the outstanding stock entitled to vote is required to authorize a merger or consolidation.
Under Genesoft’s charter, the affirmative vote of two-thirds of the then outstanding shares of preferred stock (voting together as a single class and on an as converted basis) is necessary to effect any sale or other conveyance of all or substantially all of the assets of Genesoft or any of its subsidiaries, or any consolidation or merger involving Genesoft or any of its subsidiaries, in which in excess of 50% of Genesoft’s voting power is transferred, or any reorganization or recapitalization of Genesoft. | |||
141
Table of Contents
RIGHTS OF GENOME STOCKHOLDERS | RIGHTS OF GENESOFT STOCKHOLDERS | |||
Under the MBCL, a properly dissenting stockholder is entitled to receive the appraised value of his shares when the corporation votes to:
— sell, lease, or exchange all or substantially all of its property and assets; — adopt an amendment to its charter that adversely affects the rights of the stockholder; or — merge or consolidate with another corporation.
No appraisal rights are available, however, to stockholders of a corporation surviving the merger, if the merger does not require the approval of these stockholders.
In order to exercise their appraisal rights, stockholders must not vote in favor of the corporate action triggering the appraisal right. Also, they must send the corporation a written objection to the corporate action stating their intention to demand payment for their shares. If stockholders follow the appraisal procedures set out under Massachusetts law, the “fair value” of their stock will be determined as of the day before effectiveness of the corporate action. The appraisal rights provisions are the only remedy for stockholders who object to the corporate action, unless the corporate action is determined to have been illegal, fraudulent or in breach of the board’s fiduciary duties. |
Under the DGCL, the right of dissenting stockholders to obtain the fair value for their shares is available in connection with some mergers or consolidations. Unless otherwise provided in the corporate charter, appraisal rights are not available to stockholders when the corporation will be the surviving corporation in a merger and no vote of its stockholders is required to approve the merger. In addition, no appraisal rights are available to holders of shares of any class of stock which is either:
(i) listed on a national securities exchange or designated as a national market system security on an interdealer quotation system by the NASD;
(ii) held of record by more than 2,000 stockholders; and, in the case of both (i) and (ii), those stockholders are not required by the terms of the merger to accept anything other than (1) shares of stock of the surviving corporation, (2) shares of stock of another corporation which, on the effective date of the merger or consolidation, are nationally listed or held of record by more than 2,000 holders, (3) cash instead of fractional shares of stock, or (4) any combination of the consideration set forth in (1) through (3).
Under the California Corporations Code, a properly dissenting stockholder is entitled to receive the fair market value of his shares in connection with a reorganization or merger requiring stockholder approval. In order to exercise its appraisal rights, a stockholder must not vote in favor of the reorganization or merger. Also, it must make written demand upon the corporation no later than the date of the special meeting. The fair market value is determined as of the day before the first announcement of the terms of the proposed reorganization or merger. If the corporation and a stockholder cannot agree as to the fair market value, the stockholder may file within six months a complaint with the superior court demanding judicial determination of such value. If the complaint is not filed within the specified six-month period, the stockholder’s rights as a dissenter are terminated. | |||
142
Table of Contents
DESCRIPTION OF GENOME COMMON STOCK
The following summary of the terms of Genome common stock does not purport to be complete and is subject to and qualified in its entirety by reference to Genome’s charter and by-laws, copies of which are on file with the Securities and Exchange Commission as exhibits to previous Securities and Exchange Commission filings by Genome. Please refer to “Where You Can Find Additional Information” below for directions on obtaining these documents.
Genome has authority to issue 50,000,000 shares of common stock (i) 49,375,000 shares are designated as common stock, par value $.10 per share, and (ii) 625,000 shares are designated series B restricted stock, par value $.10 per share. As of November 30, 2003, Genome had 31,451,099 shares of its common stock outstanding. There are no shares of series B restricted stock issued or outstanding.
Each holder of Genome common stock is entitled to one vote per share in all matters. The holders of Genome common stock are entitled to share ratably in any dividends that may be declared by the Genome board of directors. Upon a liquidation or dissolution, the holders of Genome common stock are entitled to receive, prior to the holders of series B restricted stock, the greater of $5.00 per share of Genome common stock or an amount for each share of Genome common stock equal to 10 times the amount available to holders of series B restricted stock. There are no preemptive or other subscription rights, conversion rights, or redemption or sinking fund provisions with respect to shares of Genome common stock. All of the outstanding shares of Genome common stock are, and the shares of Genome common stock offered by this joint proxy statement/prospectus will be, duly authorized, validly issued, fully paid and nonassessable.
Genome Series B Restricted Stock
Genome’s Restated Articles of Incorporation, as amended, provide that the holders of Genome series B restricted stock are not entitled to vote or receive dividends. No shares of Genome series B restricted stock are outstanding and Genome has no current intention to issue any shares of series B restricted stock.
The transfer agent and registrar for Genome common stock is EquiServe Trust Company N.A. Its telephone number is (781) 575-3400 and its address is: PO Box 43010, Providence, Rhode Island 02940-3010.
143
Table of Contents
The validity of the shares of Genome common stock offered by this joint proxy statement/prospectus will be passed upon for Genome by Ropes & Gray LLP.
The financial statements of Genesoft (a development stage company) at December 31, 2001 and 2002, and for each of the three years in the period ended December 31, 2002, included in this joint proxy statement/prospectus have been audited by Ernst & Young LLP, independent auditors, as set forth in their reports thereon (which contain an explanatory paragraph describing conditions that raise substantial doubt about the ability of Genesoft to continue as a going concern as described in Note 1 to the financial statements) appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The consolidated financial statements of Genome at December 31, 2002, and for the year ended December 31, 2002, incorporated by reference in this joint proxy statement/prospectus have been audited by Ernst & Young LLP, independent auditors, as set forth in their report thereon incorporated by reference herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The consolidated financial statements for the years ended December 31, 2001 and 2000 and as of December 31, 2001, and included in Genome’s 2002 Annual Report had been audited by Arthur Andersen LLP, independent accountants, as indicated in their report thereon included therein and incorporated herein by reference. Such consolidated financial statements are incorporated herein by reference in reliance upon such report given the authority of such firm as experts in auditing and accounting. Arthur Andersen has not consented to the inclusion of their report in this prospectus, and in reliance on Rule 437a under the Securities Act, Genome has not obtained their consent to do so. Genome refers you to “Risk Factors—Risks Related to the Securities Market—Certain of our financial statements have been audited by Arthur Andersen LLP, and the ability to recover damages from Arthur Andersen may be limited.” contained in the “Risk Factors” of this joint proxy statement/prospectus.
As of the date of this joint proxy statement/prospectus, neither the Genome board of directors nor Genesoft’s board of directors knows of any matter that will be presented for consideration at the Genome special meeting or the Genesoft special meeting, respectively, other than as described in this joint proxy statement/prospectus.
144
Table of Contents
WHERE YOU CAN FIND ADDITIONAL INFORMATION
This joint proxy statement/prospectus incorporates other reports by reference that are not presented in or delivered with this document.
All reports, proxy and information statements and other information filed by Genome pursuant to Section 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, after the date of this joint proxy statement/prospectus and before the date of the Genome special meeting described herein are incorporated by reference into this joint proxy statement/prospectus from the date of filing of those reports, proxy and information statements and other information. This means that Genome can disclose important information to you by referring you to other information Genome has filed with the Securities and Exchange Commission.
You should rely only on the information contained in this joint proxy statement/prospectus or which is referred to herein. Neither Genome nor Genesoft has authorized anyone to provide you with different information.
The following documents, which have been filed by Genome with the Securities and Exchange Commission, are incorporated by reference into this joint proxy statement/prospectus:
| (a) | Annual Report on Form 10-K for the fiscal year ended December 31, 2002. |
| (b) | Annual Report on Form 10-K/A for the fiscal year ended December 31, 2002. |
| (c) | Quarterly Report on Form 10-Q for the fiscal quarter ended March 29, 2003. |
| (d) | Quarterly Report on Form 10-Q for the fiscal quarter ended June 28, 2003. |
| (e) | Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2003. |
| (f) | Current Report on Form 8-K as filed on January 2, 2003. |
| (g) | Current Report on Form 8-K as filed June 5, 2003. |
| (h) | Current Report on Form 8-K as filed June 13, 2003. |
| (i) | Current Report on Form 8-K as filed on October 1, 2003. |
| (j) | Current Report on Form 8-K as filed on October 16, 2003. |
| (k) | Current Report on Form 8-K as filed on November 18, 2003. |
| (l) | The description of Genome common stock contained in its registration statement on Form 10/A filed with the Securities and Exchange Commission on January 9, 1996 under the Exchange Act, including any amendment or reports filed for the purpose of updating such description. |
| (m) | Proxy Statement filed on April 2, 2003 for the stockholders meeting held on May 8, 2003. |
Genome will provide to you, without charge, upon your written or oral request, a copy of any or all of the documents that it incorporates by reference, (other than exhibits to such documents unless such exhibits are specifically incorporated by reference into the documents that this prospectus incorporates). Written or oral requests for copies should be directed to Christopher Taylor, Investor Relations, 100 Beaver Street, Waltham, Massachusetts 02453, telephone number (781) 398-2300.
Any request for documents should be made by , 2004 to ensure timely delivery prior to the special meeting of Genome stockholders at which the adoption and approval of the merger and the related transactions will be considered and voted upon and the special meeting of Genesoft stockholders at which the adoption and approval of the merger and the related transactions will be considered and voted upon.
Any statement contained herein or in a document incorporated or deemed to be incorporated by reference into this joint proxy statement/prospectus will be deemed to be modified or superseded for purposes of this joint proxy statement/prospectus to the extent that a statement contained in this joint proxy statement/prospectus or
145
Table of Contents
any other subsequently filed document that is deemed to be incorporated by reference into this joint proxy statement/prospectus modifies or supersedes the statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this joint proxy statement/prospectus.
Genome files reports, proxy statements and other information with the Securities and Exchange Commission. Copies of these reports, proxy statements and other information may be inspected and copied at the public reference facility maintained by the Securities and Exchange Commission at the following location:
Public Reference Room
Judiciary Plaza
Room 1024
450 Fifth Street, N.W.
Washington, D.C. 20549
Copies of these materials also can be obtained by mail at prescribed rates from the Public Reference Section of the Securities and Exchange Commission, 450 Fifth Street, N.W., Washington, D.C. 20549 or by calling the Securities and Exchange Commission at 1-800-SEC-0330. These materials are also available to the public on the Securities and Exchange Commission’s website at http://www.sec.gov and the Investors section of Genome’s website at http://www.genomecorp.com.
Genome has filed a registration statement on Form S-4 under the Securities Act with the Securities and Exchange Commission with respect to Genome common stock to be issued to Genesoft stockholders in the merger. This joint proxy statement/prospectus constitutes the prospectus of Genome as part of the registration statement. This joint proxy statement/prospectus does not contain all of the information set forth in the registration statement because certain parts of the registration statement are omitted in accordance with the rules and regulations of the Securities and Exchange Commission. Statements made in this joint proxy statement/prospectus as to the content of any contract, agreement or other document referred to are not necessarily complete. With respect to each contract, agreement or other document to be filed or incorporated by reference as an exhibit to the registration statement, you should refer to the corresponding exhibit, when it is filed, for a more complete description of the matter involved and read all statements in this joint proxy statement/prospectus in light of that exhibit. The registration statement and its exhibits are available for inspection and copying as set forth above.
Genesoft stockholders should call Asha Rajagopal at 7300 Shoreline Court, South San Francisco, California 94080, telephone number (650) 837-1800 with any request for any documentation referred to in this joint proxy statement/prospectus.
This joint proxy statement/prospectus does not constitute an offer to sell, or a solicitation of an offer to purchase, the securities offered by this joint proxy statement/prospectus, or the solicitation of a proxy, in any jurisdiction to or from any person to whom or from whom it is unlawful to make such offer, solicitation of an offer or proxy solicitation in such jurisdiction. Neither the delivery of this joint proxy statement/prospectus nor any distribution of securities pursuant to this joint proxy statement/prospectus shall, under any circumstances, create any implication that there has been no change in the information set forth or incorporated into this joint proxy statement/prospectus by reference or in the affairs of Genome, or Genesoft since the date of this joint proxy statement/prospectus. The information contained in this joint proxy statement/prospectus with respect to Genome was provided by Genome and the information contained in this joint proxy statement/prospectus with respect to Genesoft was provided by Genesoft.
146
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Index to Financial Statements
| F-1 | ||
| F-2 | ||
| F-3 | ||
| F-4 | ||
| F-7 | ||
| F-9 | ||
147
Table of Contents
Report of Ernst & Young LLP, Independent Auditors
The Board of Directors and Stockholders
GeneSoft Pharmaceuticals, Inc.
We have audited the accompanying balance sheets of GeneSoft Pharmaceuticals, Inc. (a development stage company) as of December 31, 2002 and 2001, and the related statements of operations, stockholders’ equity (net capital deficiency), and cash flows for the years then ended and for the period from August 12, 1997 (inception) through December 31, 2002. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of GeneSoft Pharmaceuticals, Inc. (a development stage company) at December 31, 2002 and 2001, and the results of its operations and its cash flows for the years then ended and for the period from August 12, 1997 (inception) through December 31, 2002, in conformity with accounting principles generally accepted in the United States.
The accompanying financial statements have been prepared assuming that GeneSoft Pharmaceuticals, Inc. (a development stage company) will continue as a going concern. As more fully described in Note 1, the Company has incurred recurring operating losses and has a working capital deficiency. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Palo Alto, California
April 28, 2003, except for Note 12
as to which the date is
November 17, 2003
F-1
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Balance Sheets
| December 31, | September 30, | |||||||||||
| 2002 | 2001 | 2003 | ||||||||||
| Assets | (Unaudited) | |||||||||||
Current assets: | ||||||||||||
Cash and cash equivalents | $ | 1,880,794 | $ | 4,132,162 | $ | 4,129,274 | ||||||
Short-term investments | 373,437 | 16,885,099 | — | |||||||||
Grants receivable | 713,437 | 109,950 | 1,130,850 | |||||||||
Tenant allowance receivable | — | — | — | |||||||||
Prepaid expenses and other current assets | 141,334 | 368,676 | 50,851 | |||||||||
Total current assets | 3,109,002 | 21,495,887 | 5,310,975 | |||||||||
Restricted cash | 3,696,840 | 3,696,840 | 3,696,840 | |||||||||
Property and equipment, net | 12,290,802 | 14,969,544 | 10,170,004 | |||||||||
Intangible and other assets | 335,000 | — | 6,621,236 | |||||||||
Total assets | $ | 19,431,644 | $ | 40,162,271 | $ | 25,799,055 | ||||||
Liabilities and stockholders’ equity | ||||||||||||
Current liabilities: | ||||||||||||
Accounts payable | $ | 1,554,167 | $ | 1,134,372 | $ | 1,167,845 | ||||||
Other accrued liabilities | 770,314 | 362,292 | 5,447,333 | |||||||||
Accrued leasehold improvements | — | — | — | |||||||||
Accrued bonus | — | — | — | |||||||||
Accrued patent expenses | — | — | — | |||||||||
Current portion of lease commitments, promissory notes, and bridge loan | 3,860,021 | 1,790,931 | 19,689,234 | |||||||||
Total current liabilities | 6,184,502 | 3,287,595 | 26,304,412 | |||||||||
Long-term liabilities: | ||||||||||||
Long-term portion of commitments, promissory notes, and bridge loan | 4,511,510 | 3,428,970 | 5,028,361 | |||||||||
Deferred rent payable | 927,498 | 421,590 | 1,231,455 | |||||||||
Security deposit | 359,775 | 359,775 | 359,775 | |||||||||
Total long-term liabilities | 5,798,783 | 4,210,335 | 6,619,591 | |||||||||
Commitments | ||||||||||||
Stockholders’ equity: | ||||||||||||
Preferred stock, $0.0001 par value: 24,975,000 shares are authorized at September 30, 2003 (unaudited) and December 31, 2002, 31,025,000 shares are authorized at December 31, 2001: | ||||||||||||
Series A convertible preferred stock: 5,425,000 shares designated at September 30, 2003 (unaudited), December 31, 2002 and December 31, 2001. None issued and outstanding at September 30, 2003 (unaudited) and December 31, 2002, and December 31, 2001 | — | 5,350,417 | — | |||||||||
Series B convertible preferred stock: 6,000,000 shares designated at September 30, 2003 (unaudited), December 31, 2002, and December 31, 2001. None issued and outstanding at September 30, 2003 (unaudited) and 5,420,000 outstanding at December 31, 2002, and 4,527,400 shares issued and outstanding at December 31, 2001 | — | 11,190,814 | — | |||||||||
Series C convertible preferred stock: 6,600,000 shares designated at September 30, 2003 (unaudited) and December 31, 2002, 4,890,000 shares designated at December 31, 2001. None issued and outstanding at September 30, 2003 (unaudited) and December 31, 2002, and 4,890,000 shares issued and outstanding at December 31, 2001 | — | 24,814,092 | — | |||||||||
Series D convertible preferred stock: 5,950,000 shares designated at September 30, 2003 (unaudited) and December 31, 2002, 13,000,000 shares designated at December 31, 2001. None issued and outstanding at September 30, 2003 (unaudited) and December 31, 2002, and 5,450,000 shares issued and outstanding at December 31, 2001 | — | 20,649,701 | — | |||||||||
Series 1 convertible preferred stock: 1,000,000 shares designated in 2002, none outstanding at December 31, 2002 and September 30, 2003 (unaudited) | — | — | — | |||||||||
Common stock, $0.0001 par value: 43,450,000 shares are authorized at September 30, 2003 (unaudited) and December 31, 2002, 45,000,000 shares are authorized at December 31, 2001. 12,378,931, 10,808,540, and 1,504,047 shares issued and outstanding at September 30, 2003 (unaudited), December 31, 2002, and December 31, 2001, respectively | 63,016,056 | 420,789 | 68,238,679 | |||||||||
Other accumulated comprehensive income | — | 237,193 | 194 | |||||||||
Deficit accumulated during the development stage | (55,567,697 | ) | (29,998,665 | ) | (75,363,821 | ) | ||||||
Total stockholders’ equity (net capital deficiency) | 7,448,359 | 32,664,341 | (7,124,948 | ) | ||||||||
Total liabilities and stockholders’ equity (net capital deficiency) | $ | 19,431,644 | $ | 40,162,271 | $ | 25,799,055 | ||||||
See accompanying notes.
F-2
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Statements of Operations
| Year ended December 31, | Period from August 12, 2002 | Nine months ended September 30, | Period from August 12, 1997 2003 | |||||||||||||||||||||||||
| 2002 | 2001 | 2000 | 2003 | 2002 | ||||||||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||||||
Grant revenue | $ | 5,401,895 | $ | 2,059,176 | $ | 4,186,751 | $ | 14,167,895 | $ | 3,072,350 | $ | — | $ | 17,240,245 | ||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||
Research and development | 26,283,501 | 16,245,449 | 11,454,934 | 59,536,123 | 8,895,882 | 14,534,786 | 68,432,005 | |||||||||||||||||||||
Marketing | — | — | — | — | 2,059,396 | — | 2,059,396 | |||||||||||||||||||||
General and administrative | 4,541,718 | 4,828,042 | 1,825,410 | 12,276,734 | 5,246,839 | 3,623,601 | 17,523,573 | |||||||||||||||||||||
Total operating expenses | 30,825,219 | 21,073,491 | 13,280,344 | 71,812,857 | 16,202,117 | 18,158,387 | 88,014,974 | |||||||||||||||||||||
Operating loss | (25,423,324 | ) | (19,014,315 | ) | (9,093,593 | ) | (57,644,962 | ) | (13,129,767 | ) | (18,158,387 | ) | (70,744,729 | ) | ||||||||||||||
Other income | 564,099 | 1,251,633 | 1,226,872 | 3,436,073 | 59,097 | 321,135 | 3,495,170 | |||||||||||||||||||||
Other expense | (709,807 | ) | (557,970 | ) | (54,309 | ) | (1,358,808 | ) | (6,725,454 | ) | (530,291 | ) | (8,084,262 | ) | ||||||||||||||
Net loss | $ | (25,569,032 | ) | $ | (18,320,652 | ) | $ | (7,921,030 | ) | $ | (55,567,697 | ) | $ | (19,796,124 | ) | $ | (18,367,543 | ) | $ | (75,363,821 | ) | |||||||
Basic and diluted net loss per share | $ | (12.81 | ) | $ | (15.69 | ) | $ | (8.27 | ) | $ | (1.69 | ) | $ | (14.04 | ) | |||||||||||||
Weighted-average shares used in calculating basic and diluted net loss per share | 1,996,472 | 1,167,611 | 957,311 | 11,728,821 | 1,307,881 | |||||||||||||||||||||||
F-3
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Statements of Stockholders’ Equity (Net Capital Deficiency)
Period from August 12, 1997 (inception) through September 30, 2003
Series A Convertible Preferred Stock | Series B Convertible Preferred Stock | Series C Convertible Preferred Stock | Series D Preferred Stock | Series 1 Preferred Stock | Common Stock | Other (Loss) | Deficit Stage | Total Deficiency) | ||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||
Issuance of common stock to founders in October 1997 at $0.0048 per share for cash | — | $ | — | — | $ | — | — | $ | — | — | $ | — | — | $ | — | 730,317 | $ | 3,500 | $ | — | $ | — | $ | 3,500 | ||||||||||||||||||||
Issuance of common stock in September 1998 at $0.14 per share for license to technology | — | — | — | — | — | — | — | — | — | — | 42,750 | 6,000 | — | — | 6,000 | |||||||||||||||||||||||||||||
Issuance of common stock in November 1998 at $0.0048 per share for cash | — | — | — | — | — | — | — | — | — | — | 42,750 | 204 | — | — | 204 | |||||||||||||||||||||||||||||
Issuance of Series A convertible preferred stock at $1.00 per share to investors in October 1998 through December 1998 for cash, net of issuance costs of $69,583 | 3,537,500 | 3,467,917 | — | — | — | — | — | — | — | — | — | — | — | — | 3,467,917 | |||||||||||||||||||||||||||||
Issuance of Series A convertible preferred stock at $1.00 per share to investors in October 1998 upon conversion of notes payable to stockholders | 150,000 | 150,000 | — | — | — | — | — | — | — | — | — | — | — | — | 150,000 | |||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (769,840 | ) | (769,840 | ) | |||||||||||||||||||||||||||
Balance at December 31, 1998 | 3,687,500 | 3,617,917 | — | — | — | — | — | — | — | — | 815,817 | 9,704 | — | (769,840 | ) | 2,857,781 | ||||||||||||||||||||||||||||
Issuance of Series A convertible preferred stock in February 1999 at $1.00 per share | 1,732,500 | 1,732,500 | — | — | — | — | — | — | — | — | — | — | — | — | 1,732,500 | |||||||||||||||||||||||||||||
Issuance of Series B convertible preferred stock in September 1999 at $2.50 per share, net of issuance costs of $127,686 | — | — | 4,527,400 | 11,190,814 | — | — | — | — | — | — | — | — | — | — | 11,190,814 | |||||||||||||||||||||||||||||
Options exercised for cash during 1999 | — | — | — | — | — | — | — | — | — | — | 420,912 | 75,900 | — | — | 75,900 | |||||||||||||||||||||||||||||
Options exercised in October in connection with Series B convertible preferred stock issuance | — | — | — | — | — | — | — | — | — | — | 8,100 | 5,684 | — | — | 5,684 | |||||||||||||||||||||||||||||
Compensation expense related to issuance of stock awards to consultants | — | — | — | — | — | — | — | — | — | — | — | 10,246 | — | — | 10,246 | |||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (2,987,143 | ) | (2,987,143 | ) | |||||||||||||||||||||||||||
Unrealized loss on available-for-sale securities | — | — | — | — | — | — | — | — | — | — | — | — | (48,907 | ) | — | (48,907 | ) | |||||||||||||||||||||||||||
Comprehensive loss | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (3,036,050 | ) | ||||||||||||||||||||||||||||
Balance at December 31, 1999 | 5,420,000 | 5,350,417 | 4,527,400 | 11,190,814 | — | — | — | — | — | — | 1,244,829 | 101,534 | (48,907 | ) | (3,756,983 | ) | 12,836,875 | |||||||||||||||||||||||||||
Issuance of Series C convertible preferred stock at $5.00 per share in June 2000, net of issuance costs of $44,277 | — | — | — | — | 4,890,000 | 24,405,724 | — | — | — | — | — | — | — | — | 24,405,724 | |||||||||||||||||||||||||||||
Issuance of a warrant to purchase 13,600 shares of Series C convertible preferred stock at $5.00 per share | — | — | — | — | — | 37,808 | — | — | — | — | — | — | — | — | 37,808 | |||||||||||||||||||||||||||||
Options exercised for cash | — | — | — | — | — | — | — | — | — | — | 282,180 | 187,075 | — | — | 187,075 | |||||||||||||||||||||||||||||
Shares repurchased at $0.70 per share | — | — | — | — | — | — | — | — | — | — | (3,562 | ) | (2,500 | ) | — | — | (2,500 | ) | ||||||||||||||||||||||||||
Compensation expense related to issuance of stock awards to consultants | — | — | — | — | — | — | — | — | — | — | — | 26,714 | — | — | 26,714 | |||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (7,921,030 | ) | (7,921,030 | ) | |||||||||||||||||||||||||||
Unrealized gain on available-for-sale securities | — | — | — | — | — | — | — | — | — | — | — | — | 145,545 | — | 145,545 | |||||||||||||||||||||||||||||
Comprehensive loss | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (7,775,485 | ) | ||||||||||||||||||||||||||||
Balance at December 31, 2000 (carried forward) | 5,420,000 | 5,350,417 | 4,527,400 | 11,190,814 | 4,890,000 | 24,443,532 | — | — | — | — | 1,523,447 | 312,823 | 96,638 | (11,678,013 | ) | 29,716,211 | ||||||||||||||||||||||||||||
F-4
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Statements of Stockholders’ Equity (Net Capital Deficiency) (continued)
Period from August 12, 1997 (inception) through September 30, 2003
Series A Convertible Preferred Stock | Series B Convertible Preferred Stock | Series C Convertible Preferred Stock | Series D Convertible Preferred Stock | Series 1 Preferred Stock | Common Stock | Other (Loss) | Deficit Stage | Total Deficiency) | ||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2000 (brought forward) | 5,420,000 | $ | 5,350,417 | 4,527,400 | $ | 11,190,814 | 4,890,000 | $ | 24,443,532 | — | $ | — | — | $ | — | 1,523,447 | $ | 312,823 | $ | 96,638 | $ | (11,678,013 | ) | $ | 29,716,211 | |||||||||||||||||||||||||||||
Issuance of Series D convertible preferred stock at $4.00 per share in August 2001, net of issuance costs of $1,150,299 | — | — | — | — | — | — | 5,450,000 | 20,649,701 | — | — | — | — | — | — | 20,649,701 | |||||||||||||||||||||||||||||||||||||||
Issuance of warrants to purchase 96,000 shares of Series C convertible preferred stock at $5.00 per share in June 2001 | — | — | — | — | — | 370,560 | — | — | — | — | — | — | — | — | 370,560 | |||||||||||||||||||||||||||||||||||||||
Options exercised for cash | — | — | — | — | — | — | — | — | — | — | 57,231 | 53,222 | — | — | 53,222 | |||||||||||||||||||||||||||||||||||||||
Shares repurchased at $0.001 to $0.50 per share | — | — | — | — | — | — | — | — | — | — | (129,712 | ) | (77,462 | ) | — | — | (77,462 | ) | ||||||||||||||||||||||||||||||||||||
Compensation expense related to issuance of stock awards to consultants | — | — | — | — | — | — | — | — | — | — | — | 57,706 | — | — | 57,706 | |||||||||||||||||||||||||||||||||||||||
Issuance of common stock at $0.50 per share for services to collaborators in 2001 | — | — | — | — | — | — | — | — | — | — | 4,631 | 6,500 | — | — | 6,500 | |||||||||||||||||||||||||||||||||||||||
Issuance of common stock at $0.50 per share for services to consultants in December 2001 | — | — | — | — | — | — | — | — | — | — | 48,450 | 68,000 | — | — | 68,000 | |||||||||||||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (18,320,652 | ) | (18,320,652 | ) | |||||||||||||||||||||||||||||||||||||
Unrealized gain on available-for-sale securities | — | — | — | — | — | — | — | — | — | — | — | — | 140,555 | — | 140,555 | |||||||||||||||||||||||||||||||||||||||
Comprehensive loss | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (18,180,097 | ) | ||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2001 | 5,420,000 | 5,350,417 | 4,527,400 | 11,190,814 | 4,890,000 | 24,814,092 | 5,450,000 | 20,649,701 | — | — | 1,504,047 | 420,789 | 237,193 | (29,998,665 | ) | 32,664,341 | ||||||||||||||||||||||||||||||||||||||
Issuance of common stock at $0.50 per share for services to consultants in 2002 | — | — | — | — | — | — | — | — | — | — | — | 1,829 | — | — | 1,829 | |||||||||||||||||||||||||||||||||||||||
Issuance of shares to British Biotech at $0.08 per share in October 2002 | — | — | — | — | — | — | — | — | 356,252 | 28,500 | — | — | — | — | 28,500 | |||||||||||||||||||||||||||||||||||||||
Issuance of shares to LG Life Sciences at $0.08 per share in October 2002 | — | — | — | — | — | — | — | — | — | — | 1,692,076 | 135,366 | — | — | 135,366 | |||||||||||||||||||||||||||||||||||||||
Options exercised for cash at $0.70 to $1.40 per share | — | — | — | — | — | — | — | — | — | — | 4,688 | 4,996 | — | — | 4,996 | |||||||||||||||||||||||||||||||||||||||
Shares repurchased at $0.28 to $1.40 per share | — | — | — | — | — | — | — | — | — | — | (49,906 | ) | (38,392 | ) | — | — | (38,392 | ) | ||||||||||||||||||||||||||||||||||||
Issuance of warrants to purchase 3.5 million shares of common stock at .01 per share in December 2002 | — | — | — | — | — | — | — | — | — | — | — | 457,944 | — | — | 457,944 | |||||||||||||||||||||||||||||||||||||||
Conversion of preferred stock to common stock in December 2002 (Note 9) | (5,420,000 | ) | (5,350,417 | ) | (4,527,400 | ) | (11,190,814 | ) | (4,890,000 | ) | (24,814,092 | ) | (5,450,000 | ) | (20,649,701 | ) | (356,252 | ) | (28,500 | ) | 7,657,635 | 62,033,524 | — | — | — | |||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (25,569,032 | ) | (25,569,032 | ) | |||||||||||||||||||||||||||||||||||||
Unrealized gain (loss) on available-for-sale securities | — | — | — | — | — | — | — | — | — | — | — | — | (237,193 | ) | — | (237,193 | ) | |||||||||||||||||||||||||||||||||||||
Comprehensive loss | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (25,806,225 | ) | ||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2002 | — | $ | — | — | $ | — | — | $ | — | — | $ | — | — | $ | — | 10,808,540 | $ | 63,016,056 | $ | — | $ | (55,567,697 | ) | $ | 7,448,359 | |||||||||||||||||||||||||||||
F-5
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Statements of Stockholders’ Equity (Net Capital Deficiency) (continued)
Period from August 12, 1997 (inception) through September 30, 2003
Series A Convertible Preferred Stock | Series B Convertible Preferred Stock | Series C Convertible Preferred Stock | Series D Convertible Preferred Stock | Series 1 Convertible Preferred Stock | Common Stock | Other (Loss) | Deficit Stage | Total Deficiency) | |||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||||||||
Balance at December 31, 2002 (brought forward) | – | $ | — | — | $ | — | — | $ | — | — | $ | — | — | $ | — | 10,808,540 | $ | 63,016,056 | $ | — | $ | (55,567,697 | ) | $ | 7,448,359 | ||||||||||||||||||
Warrants exercised for cash to $0.01 per share (unaudited) | — | — | — | — | — | — | — | — | — | — | 376,000 | 3,760 | — | — | 3,760 | ||||||||||||||||||||||||||||
Options exercised for cash at $0.70 to $1.40 per share through 2003 (unaudited) | — | — | — | — | — | — | — | — | — | — | 37,552 | 36,441 | — | — | 36,441 | ||||||||||||||||||||||||||||
Shares repurchased at $0.28 to $1.40 per share through 2003 (unaudited) | — | — | — | — | — | — | — | — | — | — | (7,453 | ) | (2,958 | ) | — | — | (2,958 | ) | |||||||||||||||||||||||||
Issuance of warrants to purchase 360,593 shares of common stock at $12 per share in April 2003 (unaudited) | — | — | — | — | — | — | — | — | — | — | — | 766,634 | — | — | 766,634 | ||||||||||||||||||||||||||||
Common stock issued to LG at $3.50 per share in April 2003 (unaudited) | — | — | — | — | — | — | — | — | — | — | 1,164,292 | 4,075,022 | — | — | 4,075,022 | ||||||||||||||||||||||||||||
Common stock issued to consultants at $0.08 to $3.50 per share in 2003 | — | — | — | — | — | — | — | — | — | — | — | 343,724 | — | — | 343,724 | ||||||||||||||||||||||||||||
Net loss (unaudited) | — | — | — | — | — | — | — | — | — | — | — | — | — | (19,796,124 | ) | (19,796,124 | ) | ||||||||||||||||||||||||||
Unrealized gain (loss) on available-for-sale securities (unaudited) | — | — | — | — | — | — | — | — | — | — | — | — | 194 | — | 194 | ||||||||||||||||||||||||||||
Comprehensive loss (unaudited) | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (19,795,930 | ) | |||||||||||||||||||||||||||
Balance at September 30, 2003 (unaudited) | — | $ | — | — | $ | — | — | $ | — | — | $ | — | — | $ | — | 12,378,931 | $ | 68,238,679 | $ | 194 | $ | (75,363,821 | ) | $ | (7,124,948 | ) | |||||||||||||||||
See accompanying notes.
F-6
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Statements of Cash Flows
| Year ended December 31, | Period from 2002 | Nine month period ended September 30, | Period from 2003 | |||||||||||||||||||||||||
| 2002 | 2001 | 2000 | 2003 | 2002 | ||||||||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||||||
Operating activities | ||||||||||||||||||||||||||||
Net loss | $ | (25,569,032 | ) | $ | (18,320,652 | ) | $ | (7,921,030 | ) | $ | (55,567,697 | ) | $ | (19,796,124 | ) | $ | (18,367,543 | ) | $ | (75,363,821 | ) | |||||||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||||||||||||||||||
Depreciation and amortization | 2,867,731 | 2,073,610 | 692,695 | 5,919,627 | 2,396,246 | 2,176,583 | 8,315,875 | |||||||||||||||||||||
Stock awards to consultants for services | 1,829 | 125,706 | 26,714 | 164,495 | 343,724 | — | 508,219 | |||||||||||||||||||||
Stock issued to licensor | 163,866 | — | — | 163,866 | — | 163,866 | ||||||||||||||||||||||
Amortization of note payable discount | 141,375 | 81,125 | 1,512 | 224,012 | 677,300 | 99,445 | 901,312 | |||||||||||||||||||||
(Gain)/loss on property and equipment disposal | (10,000 | ) | — | 8,378 | (1,622 | ) | — | (10,000 | ) | (1,622 | ) | |||||||||||||||||
Realized gain on sale of short-term investments | (224,586 | ) | — | — | (224,586 | ) | (1,590 | ) | (242,493 | ) | (226,176 | ) | ||||||||||||||||
Changes in assets and liabilities: | ||||||||||||||||||||||||||||
Accounts receivable | (603,487 | ) | 1,229,902 | (622,016 | ) | (351,130 | ) | (417,413 | ) | (3,567 | ) | (768,543 | ) | |||||||||||||||
Prepaid expenses and other current assets | 227,342 | (38,657 | ) | (169,819 | ) | (90,535 | ) | 90,483 | 132,712 | (52 | ) | |||||||||||||||||
Other assets | (335,000 | ) | — | 41,500 | (335,000 | ) | (2,480,000 | ) | (20,000 | ) | (2,815,000 | ) | ||||||||||||||||
Accounts payable | 419,795 | (432,951 | ) | 556,534 | 815,473 | (386,321 | ) | (455,461 | ) | 429,152 | ||||||||||||||||||
Accrued patent expenses | — | (240,000 | ) | — | (240,000 | ) | — | — | (240,000 | ) | ||||||||||||||||||
Accrued leasehold improvements | — | (1,681,619 | ) | — | (1,681,619 | ) | — | — | (1,681,619 | ) | ||||||||||||||||||
Accrued lease deposit | — | 359,775 | — | 359,775 | — | — | 359,775 | |||||||||||||||||||||
Accrued interest on bridge loan | — | — | — | — | 5,452,607 | — | 5,452,607 | |||||||||||||||||||||
Deferred rent payable | 505,908 | 421,590 | — | 927,498 | 303,957 | 379,431 | 1,231,455 | |||||||||||||||||||||
Accrued bonus | — | (238,274 | ) | 148,808 | — | — | — | — | ||||||||||||||||||||
Other accrued liabilities | 408,022 | 13,051 | 244,930 | 1,010,314 | 474,665 | 157,928 | 1,484,979 | |||||||||||||||||||||
Net cash used in operating activities | (22,006,237 | ) | (16,647,394 | ) | (6,991,794 | ) | (48,907,129 | ) | (13,342,466 | ) | (16,152,965 | ) | (62,249,593 | ) | ||||||||||||||
Investing activities | ||||||||||||||||||||||||||||
Purchase of short-term investments | (887,947 | ) | (22,806,019 | ) | (6,863,453 | ) | (42,610,853 | ) | 193 | (360,316 | ) | (42,610,660 | ) | |||||||||||||||
Maturities of short-term investments | — | — | — | 5,000,000 | — | — | 5,000,000 | |||||||||||||||||||||
Sales of short-term investments | 17,407,000 | 19,075,000 | — | 37,482,000 | 375,027 | 15,115,000 | 37,857,027 | |||||||||||||||||||||
Purchase of property and equipment | (209,216 | ) | (12,174,339 | ) | (2,385,967 | ) | (16,181,028 | ) | (6,660 | ) | (151,096 | ) | (16,187,688 | ) | ||||||||||||||
Sale of property and equipment | 10,227 | — | — | 10,227 | — | 10,227 | 10,227 | |||||||||||||||||||||
Restricted cash | — | — | (3,696,840 | ) | (3,696,840 | ) | — | — | (3,696,840 | ) | ||||||||||||||||||
Net cash provided (used) in investing activities | 16,320,064 | (15,905,358 | ) | (12,946,260 | ) | (19,996,494 | ) | 368,560 | 14,613,815 | (19,627,934 | ) | |||||||||||||||||
Financing activities | ||||||||||||||||||||||||||||
Proceeds from issuance of lease commitments, promissory notes, and bridge loan | 6,500,000 | 4,663,737 | 1,938,925 | 13,823,124 | 18,809,666 | — | 32,632,790 | |||||||||||||||||||||
Payment of lease commitments and notes payable | (3,031,801 | ) | (1,279,455 | ) | (322,776 | ) | (4,710,092 | ) | (3,624,523 | ) | (1,409,204 | ) | (8,334,615 | ) | ||||||||||||||
Proceeds from issuance of convertible preferred stock, net of issuance costs | — | 20,649,701 | 24,405,724 | 61,452,340 | — | — | 61,452,340 | |||||||||||||||||||||
Proceeds from issuance of common stock | 4,996 | 59,722 | 187,075 | 337,397 | 40,201 | 5,038 | 377,598 | |||||||||||||||||||||
Repurchase of common stock | (38,392 | ) | (77,462 | ) | (2,500 | ) | (118,354 | ) | (2,958 | ) | (22,817 | ) | (121,312 | ) | ||||||||||||||
Net cash provided by (used in) financing activities | 3,434,803 | 24,016,243 | 26,206,448 | 70,784,415 | 15,222,386 | (1,426,983 | ) | 86,006,801 | ||||||||||||||||||||
Net increase (decrease) in cash | (2,251,370 | ) | (8,536,509 | ) | 6,268,394 | 1,880,794 | 2,248,480 | (2,966,133 | ) | 4,129,274 | ||||||||||||||||||
Cash at beginning of period | 4,132,164 | 12,668,671 | 6,400,277 | — | 1,880,794 | 4,132,162 | — | |||||||||||||||||||||
Cash at end of period | $ | 1,880,794 | $ | 4,132,162 | $ | 12,668,671 | $ | 1,880,794 | $ | 4,129,274 | $ | 1,166,029 | $ | 4,129,274 | ||||||||||||||
See accompanying notes.
F-7
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Statements of Cash Flows (continued)
| Year ended December 31, | Period from 2002 | Nine month period ended September 30, | Period from 2003 | ||||||||||||||||||
| 2002 | 2001 | 2000 | 2003 | 2002 | |||||||||||||||||
| (Unaudited) | (Unaudited) | ||||||||||||||||||||
Supplemental disclosure of cash flow information | |||||||||||||||||||||
Cash paid for interest | $ | 546,599 | $ | 450,484 | $ | 52,777 | $ | 1,086,602 | $ | 257,816 | $ | 430,784 | $ | 1,344,418 | |||||||
Schedule of noncash investing and financing activities | |||||||||||||||||||||
Conversion of placement fees to common stock | $ | — | $ | 68,000 | $ | — | $ | 68,000 | $ | — | $ | — | $ | 68,000 | |||||||
Conversion of notes payable to preferred stock | $ | — | $ | — | $ | — | $ | 150,000 | $ | — | $ | — | $ | 150,000 | |||||||
Issuance of stock to collaborators | $ | 163,866 | $ | — | $ | — | $ | 163,866 | $ | — | $ | — | $ | 163,866 | |||||||
Conversion of preferred to common stock | $ | 62,033,524 | $ | — | $ | — | $ | 62,085,024 | $ | — | $ | — | $ | 62,085,024 | |||||||
Issuance of warrants in connection with financing agreement | $ | 457,944 | $ | 370,560 | $ | 37,808 | $ | 866,312 | $ | 766,632 | $ | — | $ | 1,632,944 | |||||||
See accompanying notes.
F-8
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements
1. �� Summary of Significant Accounting Policies
Organization, Business, and Basis of Presentation
GeneSoft Pharmaceuticals, Inc. (a development stage company) (the “Company”) was incorporated in the state of Delaware on August 12, 1997. The Company was organized to develop a family of small molecule drugs to treat gene-mediated diseases. The Company’s activities to date have consisted principally of raising capital, acquiring intellectual property, recruiting staff, and conducting research and development. Accordingly, the Company is considered to be in the development stage, and expects to incur continuing losses and require additional financial resources to achieve commercialization of its products. The Company operates in only one segment, the development of biopharmaceutical products.
The Company anticipates working on a number of long-term development projects which will involve experimental and unproven technology. The projects may require many years and substantial expenditures to complete, and may ultimately be unsuccessful. Additionally, the Company’s approved product, FACTIVE, will require substantial funds to market and launch. Therefore, the Company will need to obtain additional funds from outside sources to continue its research and development activities, fund operating expenses, pursue regulatory approvals, and build production, sales, and marketing capabilities, as necessary.
Going Concern
The Company has generated negative cash flows from operations since inception and has a working capital deficiency and has minimal capital resources at September 30, 2003. The company has been able to fund its cash needs to date through the sale of its preferred and common stock and debt financings. The ability of the Company to manage its operating expenses to a level that can be financed by existing cash flows and its ability to obtain additional funding is therefore critical to the Company’s ability to continue operating as a going concern.These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The Company’s management intends to merge the Company with another corporation and obtain additional financing or enter into collaborative arrangements. The outcome of management’s intentions is not presently determinable. As such, no adjustments have been made that might result from this situation.
The Company’s continuation as a going concern is primarily dependent upon its ability to merge and obtain alternative sources of capital.
In the event the Company is unable to secure alternative financing sources, it is likely that any of the following alternatives will be pursued: (1) pursue a co-promotion collaboration; or (2) pursue other available protective remedies.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the financial statements. Actual results could differ from these estimates.
Unaudited Interim Consolidated Results
The accompanying balance sheet as of September 30, 2003, the statements of operations and cash flows for the nine months ended September 30, 2002 and 2003 and the statements of stockholders’ equity (net capital deficiency) for the nine months ended September 30, 2003 are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of
F-9
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
management, reflect all adjustments, which include only normal recurring adjustments, necessary to present fairly the Company’s financial position as of September 30, 2003 and the results of operations and cash flows for the nine months ended September 30, 2003 and 2002. The financial data and other information disclosed in these notes to financial statements as of September 30, 2003 and related to the nine-month periods ended September 30, 2003 and 2002 are unaudited. The results for the nine months ended September 30, 2003 are not necessarily indicative of the results to be expected for the year ending December 31, 2003 or for any other interim period or for any other future year.
Cash Equivalents and Short-Term Investments
The Company considers all highly liquid investments in debt securities with a remaining maturity from the date of purchase of 90 days or less to be cash equivalents. Cash equivalents consist of money market funds. The Company’s short-term investments consist entirely of mutual funds with investments in debt securities.
All cash equivalents and short-term investments are classified as available-for-sale as the Company may sell the investment prior to the maturity date in order to take advantage of market conditions. Available-for-sale securities are carried at estimated market values at December 31, 2002 and 2001. Unrealized gains and losses on available-for-sale securities are excluded from earnings and recorded as a separate component of stockholders’ equity (net capital deficiency). The cost of securities sold is based on the specific identification method.
Other Intangible Assets
Intangible assets with definite useful lives are amortized on a straight-line basis over a period of fifteen years, the life of the agreement. Intangible assets are tested for impairment whenever events or changes in circumstances indicate the carrying amount of the assets may not be recoverable from future undiscounted cash flows. If impaired, the assets are recorded at fair value. Intangible assets consist of capitalized license costs incurred through the Company’s licensing arrangement with LG Life Sciences subsequent to approval of FACTIVE in April 2003 and are being amortized over the term of the license. License costs prior to approval were charged to research and development expense.
The Company will periodically evaluate whether changes have occurred that would require revision of the remaining estimated useful lives of these assets or otherwise render the assets unrecoverable. If such an event occurred, the Company would determine whether the other intangibles were impaired. To date, no such impairment losses have been recorded.
Property and Equipment
Property and equipment are stated at cost. Depreciation is recorded using the straight-line method over the estimated useful lives of the respective assets, generally three years. Leasehold improvements are amortized on a straight-line basis over the shorter of their useful life or the remaining life of the lease.
Research and Development
Research and development expenses consist of costs incurred for internally sponsored research and development as well as costs for in-licensed technology. These costs include direct labor and supplies, in-license fees and indirect research-related overhead expenses consisting primarily of facility costs.
Stock-Based Compensation
In accordance with the provisions of Statement of Financial Accounting Standards (“SFAS”) No. 123,Accounting for Stock-Based Compensation (“SFAS 123”), as amended by SFAS No. 148,Accounting for Stock-Based Compensation—Transition and Disclosure (“SFAS 148”), the Company has elected to follow Accounting
F-10
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
Principles Board Opinion No. 25,Accounting for Stock Issued to Employees (“APB 25”), and related interpretations, and to adopt the pro forma disclosure alternative described in SFAS 123 in accounting for stock awards to employees.
No compensation expense is recognized in the Company’s financial statements in connection with the stock options granted to employees. The weighted-average fair value of these options at December 31, 2002 and 2001 of $0.60 and $0.57, and at September 30, 2003 and 2002 of $0.22 and $0.50, was estimated at the date of grant using a minimum value option pricing model with the following assumptions: a risk-free interest rate of 3.0% and 6.0%, respectively, a weighted-average expected life of the option of eight years and nine years, respectively, and a dividend yield of zero.
The following table illustrates the effect on net loss if the Company had applied the fair value recognition provisions of SFAS 123, as amended by SFAS 148, to stock-based employee compensation.
| Year ended December 31, | Nine-month period ended September 30, | |||||||||||||||||||
| 2002 | 2001 | 2000 | 2003 | 2002 | ||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
Net loss as reported | $ | (25,569,032 | ) | $ | (18,320,652 | ) | $ | (7,921,030 | ) | $ | (19,796,124 | ) | $ | (18,367,543 | ) | |||||
Less: Total stock-based employee compensation expense determined under fair-value-based method for all awards | (240,720 | ) | (183,609 | ) | (85,523 | ) | (284,160 | ) | (237,314 | ) | ||||||||||
Pro forma net loss | $ | (25,809,752 | ) | $ | (18,504,261 | ) | $ | (8,006,553 | ) | $ | (20,080,284 | ) | $ | (18,604,857 | ) | |||||
Net loss per share as reported | $ | (12.81 | ) | $ | (15.69 | ) | $ | (8.27 | ) | $ | (1.69 | ) | $ | (14.04 | ) | |||||
Pro forma net loss per share | $ | (12.93 | ) | $ | (15.85 | ) | $ | (8.36 | ) | $ | (1.71 | ) | $ | (14.22 | ) | |||||
Total shares used in calculation | 1,996,472 | 1,167,611 | 957,311 | 11,728,821 | 1,307,881 | |||||||||||||||
Option grants to non-employees are accounted for in accordance with SFAS 123 and Emerging Issues Task Force Consensus No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services,” which requires the value of such options to be periodically remeasured as they vest over a performance period.
Net Loss per Share
Basic loss per share is calculated by dividing net loss by the weighted-average number of shares of common stock outstanding. Basic earnings per share does not include shares subject to the Company’s right of repurchase, which lapse ratably over the related vesting term. Diluted loss per share is calculated by dividing net loss available to common stockholders by the weighted-average number of shares of common stock outstanding plus shares of potential common stock. Shares of potential common stock are composed of shares of common stock subject to the Company’s right of repurchase and shares of common stock issuable upon the exercise of stock options (using the treasury stock method). The calculation of diluted net loss per share excludes shares of potential common stock if the effect is anti-dilutive.
Revenue
Grant revenue is recorded as grant costs are incurred as stipulated by the underlying contract.
Comprehensive Income (Loss)
The only item of other comprehensive income (loss) that the Company currently reports is unrealized gains (losses) on short-term investments, which are included in comprehensive loss in the statements of stockholders’ equity (net capital deficiency).
F-11
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
Recently Issued Accounting Standards
In November 2002, the Financial Accounting Standards Board (the FASB) issued the FASB Interpretation No. 45,Guarantor’s Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others(“FIN 45”), which clarifies the requirements for a guarantor’s accounting and disclosures of certain guarantees issued and outstanding. This interpretation elaborates on the disclosures to be made by a guarantor in its interim and annual financial statements about its obligations under certain guarantees that it has issued. It also clarifies that a guarantor is required to recognize, at its inception of guarantee, a liability for the fair value of the obligation undertaken in issuing the guarantee. The initial recognition and initial measurement provisions of this interpretation are applicable on a prospective basis to guarantees issued or modified after December 31, 2002, irrespective of the guarantor’s fiscal year-end. The disclosure requirements on this interpretation are effective for financial statements of interim or annual periods ending after December 15, 2002. The adoption of FIN 45 did not have a material impact on the Company’s results of operations or financial position.
In November 2002, the EITF issued EITF Issue No. 00-21,Accounting for Revenue Arrangements with Multiple Deliverables (“EITF 00-21). EITF 00-21 addresses how to account for arrangements that may involve delivery or performance of multiple products, services, and/or rights to use assets, and if so, how an arrangement involving multiple deliverables should be divided into separate units of accounting. It does not change otherwise applicable revenue recognition criteria. It applies to arrangements entered into in fiscal periods beginning after June 15, 2003, with early adoption permitted. The adoption of EITF 00-21 did not have a material impact on the Company’s results of operations or financial position.
In May 2003, the FASB issued SFAS No. 150,Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity(“SFAS 150”). SFAS 150 establishes standards for the classification and measurement of financial instruments with characteristics of both liabilities and equity. FAS 150 is effective for financial instruments entered into or modified after May 31, 2003, except for certain mandatorily redeemable financial instruments for which the FASB announced on November 5, 2003 deferred effective dates for certain provisions of FAS 150. The adoption of FAS 150 and the subsequent deferred effective dates did not and will not have a material effect on the Company’s financial position or results of operations.
2. Cash and Cash Equivalents
At September 30, 2003, the Company reported $4,129,274 as cash and cash equivalents. Additionally, as a requirement of a lease agreement, the Company obtained a letter of credit with a bank for $3,696,840. The Company is obligated to maintain a minimum balance of $3,696,840 in the bank’s security accounts, which has been recorded as restricted cash. At December 31, 2002 and September 30, 2003, the Company was in compliance with this requirement.
F-12
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
3. Investments
Gross unrealized gains on available-for-sale securities were $0 and $237,193 as of December 31, 2002 and 2001, respectively, and $194 as of September 30, 2003. The following is a summary of available-for-sale securities:
September 30, 2003 | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | ||||||||
| (Unaudited) | ||||||||||||
Cash equivalents: | ||||||||||||
Money market funds | $ | 4,129,080 | $ | 194 | $ | — | $ | 4,129,274 | ||||
December 31, 2002 | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | ||||||||
Cash equivalents: | ||||||||||||
Money market funds | $ | 28,532 | $ | — | $ | — | $ | 28,532 | ||||
Short-term investments: | ||||||||||||
Mutual fund securities | $ | 373,437 | $ | — | $ | — | $ | 373,437 | ||||
December 31, 2001 | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | ||||||||
Cash equivalents: | ||||||||||||
Money market funds | $ | 2,850,761 | $ | — | $ | — | $ | 2,850,761 | ||||
Short-term investments: | ||||||||||||
Mutual fund securities | $ | 16,647,906 | $ | 237,193 | $ | — | $ | 16,885,099 | ||||
4. Property and Equipment
Property and equipment consisted of the following:
| December 31, | September 30, 2003 | |||||||||||
| 2002 | 2001 | |||||||||||
| (Unaudited) | ||||||||||||
Laboratory equipment | $ | 4,479,650 | $ | 4,363,210 | $ | 4,479,650 | ||||||
Computer and office equipment | 1,077,478 | 994,702 | 1,081,138 | |||||||||
Leasehold improvements | 12,643,956 | 12,654,183 | 12,643,956 | |||||||||
| 18,201,084 | 18,012,095 | 18,207,744 | ||||||||||
Less: accumulated depreciation and amortization | (5,910,282 | ) | (3,042,551 | ) | (8,037,740 | ) | ||||||
| $ | 12,290,802 | $ | 14,969,544 | $ | 10,170,004 | |||||||
At September 30, 2003, all of the Company’s property and equipment was pledged as security to repay the outstanding notes payable to a financing company.
F-13
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
5. Leases, Commitments, Promissory Notes, and Bridge Loan
The Company leases its office facilities under an operating lease arrangement that expires on March 1, 2011. Rent expense under the operating lease amounted to $4,202,748, $3,568,253, $541,995 and $9,175,463 for the years ended December 31, 2002, 2001, 2000 and for the period from August 12, 1997 (inception) through December 31, 2002, respectively. Rent expense under the operating leases amounted to $3,152,064, $3,153,061, and $12,327,527 for the period ended September 30, 2003 and 2002 and for the period from August 12, 1997 (inception) through September 30, 2003, respectively.
A portion of the leased facilities is subleased to an external party. Rental income under this sublease which is offset against lease expense was $1,584,190, $351,000, and $0 during the years ended December 31, 2002, 2001, and 2000, respectively. Rental income under this sublease was $1,258,219, $1,176,070 and $3,193,409 during the period ended September 30, 2003, 2002, and the period from August 12, 1997 (inception) through September 30, 2003, respectively. The aggregate future minimum rental to be received under the noncancelable sublease amounts to $2,161,515 at September 30, 2003 and is due through December 2004.
To fund purchases of equipment required for research, the Company and a financial institution entered into a Master Security Agreement effective September 15, 2000. Under the terms of this agreement, the Company granted the financial institution a security interest in and against all property acquired under all existing and future debts, obligations, and liabilities between the parties.
On October 26, 2000 and December 28, 2000, the Company issued promissory notes to the financial institution in the amount of $744,177 and $1,194,748, respectively, to finance equipment under the Master Security Agreement. The promissory notes are payable in 48 equal monthly installments of $19,497 and $31,302, and bear interest at 12.27% per annum. At December 31, 2002 and September 30, 2003, $1,029,951 and $652,374 remained outstanding, respectively.
In connection with the Master Security Agreement, the Company issued warrants to the financial institution to purchase 13,600 shares of Series C convertible preferred stock at $5.00 per share (converted in 2002 to 5,050 warrants to purchase common stock at $14 per share as a result of the conversion and reverse split discussed in Note 8), the fair value on the date of issuance. The warrants vested immediately and are exercisable until October 3, 2007. The warrants are outstanding as of September 30, 2003.
The fair value of the warrants issued to the financial institution of $37,808 was recorded as a discount against the promissory notes. The discount is being amortized to interest expense over the term of the promissory notes. The Company calculated the fair value of the warrants issued to the financial institution using the Black-Scholes option pricing model with the following assumptions: a risk-free interest rate of 6%, a contractual life of seven years, a dividend yield of 0%, and a volatility of 65%.
In April 2001, the Company entered into a new Master Security Agreement jointly with two financial institutions. Under the terms of this agreement, the Company granted the financial institutions a security interest in and against all property acquired under all existing and future debts, obligations, and liabilities between parties.
In June, July, and September 2001, the Company issued promissory notes to the financial institutions under a new Master Security Agreement for $3,668,585, $511,613, and $463,538, respectively. In December 2002, the Company renegotiated its promissory notes and prepaid $1,000,000 of the outstanding liability and re-amortized the balance payable under the promissory notes to $37,302 per month for January to May 2003, increasing to $96,938 per month thereafter through the end of the term on December 2004. The interest rate on this loan is 11.44%. At September 30, 2003, $1,236,935 remained outstanding under these notes.
F-14
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
In connection with the new Master Security Agreement, the Company issued warrants to the financial institutions to purchase 96,000 shares of Series C convertible preferred stock at $5.00 per share (converted to 35,652 shares at $13.47 per share in December 2002). The warrants vested immediately and are exercisable until June 13, 2011. The warrants are outstanding as of September 30, 2003.
The fair value of the warrants issued to the financial institutions of $370,560 was recorded as a discount against the promissory notes. The discount is being amortized to interest expense over the term of the promissory notes. The Company calculated the fair value of the warrants issued to the financial institution using the Black-Scholes option pricing model with the following assumptions: a risk-free interest rate of 5.5%, a contractual life of 10 years, a dividend yield of 0%, and a volatility factor of 65%.
In December 2002 and January 2003, the Company entered into a bridge loan agreement for $5,000,678. The bridge loan is unsecured and bears interest at a fixed rate of 6% per annum. The bridge loan may be converted into the Company’s common stock upon the close of an equity financing round of at least $2,500,000. The bridge loan has a liquidation preference of up to $10 million payable in cash upon sale or in the event of an initial public offering. The bridge loan becomes due on December 6, 2005 if not converted to common stock by that date.
In connection with the issuance of the bridge loan agreement, the Company issued warrants to purchase 5,000,678 shares of the Company’s common stock at an exercise price of $0.01 per share. The fair value of the warrants issued to the financial institution of $245,000 was recorded as a discount against the promissory notes, of which $61,250 was amortized to interest expense in the nine months ended September 30, 2003. The discount is being amortized to interest expense over the term of the promissory notes. The Company calculated the fair value of the warrants issued to the financial institution using the Black-Scholes option pricing model with the following assumptions: a risk-free interest rate of 5.5%, a contractual life of 10 years, a dividend yield of 0%, and a volatility factor of 65%.
After deducting the fair value of the warrants from the proceeds of the bridge loan issuance, the convertible bridge loan proceeds were subject to a beneficial conversion feature valued at $228,972 of which $53,235 was recorded as interest expense in 2003 in accordance with Emerging Issues Task Force (“EITF”) 98-5,Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios, as amended by EITF No. 00-27 Application of Issue 98-5 to Certain Convertible Instruments. The remaining $175,737 will be amortized to interest expense over the remaining term of the bridge loan.
In April of 2003, the Company entered into a second bridge loan agreement (the “loan”) for approximately $17.3 million. The loan matures on December 15, 2003. The interest rate on the loan is 17% through August 15, 2003, and 4% per month from August 16, 2003 through December 15, 2003. In the event that repayment does not occur as of the extended maturity date, the note may be converted into common stock at a value of $5.00 per share. In conjunction with the loan, the Company issued 360,593 warrants to purchase common stock at $12 per share. The fair value of the warrants issued to the lender of $385,837 was recorded as a discount against the bridge loan. The discount is being amortized to interest expense over the term of the bridge loan. The Company calculated the fair value of the warrants using the Black-Scholes option pricing model. With the following assumptions: a risk-free interest rate of 5%, a contractual Life of 5 years, dividend yield of 0% and a volatility factor of 65%.
The conditions of the second loan amended the terms of the December 2002 bridge loan. The liquidation preference was revised from the $10 million liquidation preference to $7.5 million. The amendment provided for
F-15
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
the liquidation preference to be paid either upon the sale, an initial public offering or maturity of the note. Beginning in April 2003, the Company began accreting up to the liquidation preference to the maturity date of the note through charges to interest expense. As of September 30, 2003, $1,251,795 was recorded as interest expense related to this liquidation preference.
Interest expense of $709,807, $531,610, $54,309, and $1,342,448 was incurred during the years ended December 31, 2002, 2001, 2000, and the period from inception to December 31, 2002, respectively in relation to notes payable. Interest expense of $6,456,666, $530,291 and $7,799,114 was incurred during the period ended September 30, 2003, 2002, and the period from inception to September 30, 2003, respectively, in relation to notes payable.
After deducting the fair value of the warrants from the proceeds of the bridge loan issuance, the convertible bridge loan proceeds were subject to a beneficial conversion feature valued at $380,797 of which $237,998 was recorded as interest expense in 2003 in accordance with Emerging Issues Task Force (“EITF”) 98-5,Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios, as amended by EITF No. 00-27,Application of Issue 98-5 to Certain Convertible Instruments. The remaining $142,799 will be amortized to interest expense over the term of the bridge loan.
The future minimum payments under the operating leases (gross of sublease income) and financing arrangements, by year, are as follows:
| Operating Leases | Notes Payable and Bridge Loan | ||||||
Year ending December 31, | |||||||
2003 (three months) | $ | 533,925 | $ | 24,714,412 | |||
2004 | 2,198,940 | 1,714,354 | |||||
2005 | 4,075,654 | 5,918,301 | |||||
2006 | 4,218,296 | — | |||||
2007 | 4,365,944 | — | |||||
Thereafter | 13,384,023 | — | |||||
| $ | 28,776,782 | 32,347,067 | |||||
Less interest | (6,897,841 | ) | |||||
Less discount | (731,631 | ) | |||||
| 24,717,595 | |||||||
Less current portion | (19,689,234 | ) | |||||
Long-term portion | $ | 5,028,361 | |||||
F-16
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
6. License and Collaboration Agreements
California Institute of Technology
In September 1998, the Company entered into a license agreement (“the Agreement”) with the California Institute of Technology (“CalTech”), which was amended twice, in February 1999 and in January 2000. Under the Agreement, CalTech granted an exclusive license to the Company, with the right to grant and authorize sublicenses. As an up-front fee, the Company paid CalTech $5,000 and issued 42,750 shares of its common stock. The Company has to pay an annual minimum fee of $10,000 as well as any cost related to preparation, filing, prosecution, and maintenance of existing and new patents covered under the Agreement. The Company is also obligated to pay future royalties on product sales. A portion (16.7%) of these costs are reimbursable by CalTech. Prosecution costs incurred in the amount of $240,000, were paid in full during 2001. No such cost were incurred in 2002 or for the nine months ended September 30, 2003.
Dow Pharmaceuticals
In June 2002, the Company entered into a contract with Dow Pharmaceuticals for the development of a topical antibacterial to treat skin infections such as infected diabetic foot ulcers and secondarily infected traumatic lesions. Under this collaboration, a topical DNA-Nanobinder preparation was investigated. This program is currently on hold for financial reasons.
British Biotech Pharmaceuticals Limited
In August 2002, the Company entered into a three-year strategic partnership with British Biotech Pharmaceutical Ltd. (now “Vernalis”) to co-develop GSQ-83698, a novel antibiotic to treat intractable Gram-positive respiratory infections in hospital-based patients. GSQ-83698 entered Phase I clinical trials in the United Kingdom on October 1, 2002. The Company will be responsible for commercializing the product in the United States and the rest of the world, excluding Europe and Japan. The parties will split development funding and worldwide profits equally.
Additionally, the Company agreed to co-develop oral PDF inhibitors for the treatment of community-acquired Gram-positive and Gram-negative infections. The Company will be responsible for commercializing the product in the United States and all countries other than those in Europe and Japan. The parties will split development funding and worldwide profits such that the Company is responsible for approximately 63% of the costs that included at least 12 full-time equivalent (“FTE”) personnel. Any shortfall in the required FTEs will be reimbursable to the other party at a rate of $250,000 and $150,000 per FTE per year for employees working in the United States and the United Kingdom, respectively.
The Company also licensed three novel metalloenzyme bacterial targets from Vernalis. The Company intends to initiate a drug discovery program to develop small molecule therapeutics against three of these targets. The targets provide an important set of early stage discovery programs for the Company.
During 2002, the Company made an up-front payment of $4,000,000, a $1,000,000 clinical milestone payment and issued equity in order to access this technology. This technology is at an early stage of development and the risks inherent in drug development in order to take compounds such as these to commercial viability are very high. This risk assessment resulted in recording the up-front payment and milestone as research and development expenses during 2002.
In September 2003, the Company agreed to assume full responsibility for the program. The respective parties performed a reconciliation of FTEs and costs to date and the Company agreed to pay Vernalis $775,000 by December 20, 2003. This amount is recorded in other accrued liabilities at September 30, 2003.
As a result of this amendment, the Company is obligated to make royalty payments on future product sales. Additionally, milestone payments of up to $18.8 million could also be payable over the term of the license.
F-17
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
LG Life Sciences, LTD
In October 2002, the Company entered into a partnership with LG Life Sciences (“LG”) to license rights in North America and the territories covered by the license in Europe to FACTIVE® (gemifloxacin) (the “product”), a novel quinolone antibiotic. The term of the agreement coincides with the compound’s patent life which currently expires in 2015. The patent could be extended for an additional two years. The product was approved for sale in the United States in April 2003. The arrangement with LG includes up-front fees, milestone payments, and royalties on sales. In addition, the Company issued LG 1,164,292 shares of common stock in April 2003.
To secure the license to this product, the Company made an up-front payment of $5,500,000, $3,000,000 of which was settled by way of a promissory note. The Company paid all costs required to obtain regulatory approval of the product. Although the product is approved, the agreement with LG requires a minimum sales commitment over a period of time, which if not met, could result in the technology being returned to LG. Because of the risks inherent with successfully obtaining Federal Drug Administration (“FDA”) approval of a product in 2002, the Company included the up-front payments made to LG in research and development costs.
The Company is subject to future milestone payments of up to $35.0 million over the term of the license. In April 2003, the Company obtained FDA approval for the sale of FACTIVE in the United States. The approval triggered the first milestone payment to LG. The amount of the milestone payment ($5 million) is payable as follows: $2.5 million payable 30 days after approval and $2.5 million payable contingent on the receipt and acceptance of the first order of drug product scheduled to occur by the end of the year. The first installment was paid as scheduled, and has been capitalized and being amortized over the term of the license.
The Company is obligated to pay LG Life Sciences a royalty on sales in the U.S. and the territories covered by the license in Europe.
The Company is obligated to purchase its requirements for the final drug product from LG Life Sciences for 2004. In 2004, the final drug product will be tableted and packaged for LG Life Sciences by SB Pharmco at its manufacturing facility in Puerto Rico. This arrangement with SB Pharmco is expected to conclude by the end of 2004. Genesoft is in discussions with a new secondary manufacturer to assume these responsibilities for subsequent periods.
Pursuant to its partnership with LG Life Sciences, upon delivery of the first shipment of FACTIVE, which is anticipated to occur in the next two months, Genesoft will be obligated to make a $2.5 million milestone payment to LG Life Sciences as well as a payment of $4.8 million for the purchase of the drug inventory. Upon the closing of the merger, the combined company will be obligated to make an $8 million milestone payment to LG Life Sciences.
7. Accounts Receivable and Grant Revenue
In December 1998, the Company was awarded a government grant to research the regulation of pathogen gene expression by DNA-binding polyamides. The original term of the grant commenced on the effective date of the grant, December 1998, and continues for a period of three years thereafter. Defense Advance Research Project Agency (“DARPA”) was assigned as project officer. The amount of this grant available to the Company was $2,263,000. The Company was entitled to receive payments made on a cost reimbursement basis. Title to all property and equipment purchased by the Company with grant proceeds will vest to the Company upon acquisition of the property and equipment. Either party may terminate this grant, in whole or in part, upon notice to and consultation with the other party, and upon agreement of the parties that continuation of the project would not produce beneficial results commensurate with the further expenditures of funds. In addition, the grant may be revoked upon a finding that the Company had failed materially to meet the provisions of the grant. The Company recognizes grant revenue as costs reimbursable under the grant are incurred and the terms of the government agreement are met.
F-18
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
Effective November 2, 1999, an amendment to the grant was approved by DARPA. The amendment stipulated that the amount available under the grant increased by $3,008,200 for a total of $5,271,200. An additional amendment was approved on April 4, 2000, which increased the funds available for payment to a total amount of $5,786,600. On November 21, 2000, DARPA approved an amendment to the grant and made a total budget of $8,766,000 fully available to the Company. In September 2002, DARPA further amended the grant and made funds of $12,282,194 available to the Company. During 2002, all amounts available under the grant were billed. As of December 31, 2002, the Company had received $12,107,194 in cash and the remaining $175,000 was included in accounts receivable. As of September 30, 2003, the $175,000 remains in accounts receivable.
In November 2002, the Company entered into a new contract with DARPA for $3,000,000 for further research. In April 2003, the Company entered into an extension of this contract for an additional $5,500,000 for further research. At September 30, 2003, the Company had billed $4,916,675 under this contract. At September 30, 2003, the Company had received $4,003,069 in cash and the remaining $913,606 was included in accounts receivable.
8. Stockholders’ Equity (Net Capital Deficiency)
Stock Split
In December 2002, the Board of Directors approved a 1 for 2.807 reverse stock split of the Company’s common stock in conjunction with the bridge financing. All stock information in these financial statements has been retroactively adjusted to reflect the reverse split.
Common Stock
In October 1997, the Company issued 730,317 shares of common stock to the founders at $0.0048 per share, subject to repurchase by the Company with repurchase rights lapsing over a 60-month period from the date of issuance. At September 30, 2003, the repurchase rights have lapsed.
In November 1998, the Company issued 21,375 shares of common stock to a consultant at $0.0048 per share. The shares are subject to repurchase by the Company, with repurchase rights lapsing ratably over a 48-month period commencing November 2, 1999. The Company recorded compensation expense relating to the stock issuance as services are provided by the consultant, which approximates the vesting schedule. Compensation charges of $1,760 and $7,500 were recorded in 2002 and 2001, respectively, related to these consultant grants. The Company calculated these charge using the Black-Scholes option pricing model with the following assumptions: a risk-free interest rate of 6%, a contractual life of seven years, a dividend yield of 0%, and a volatility of 65%. At December 31, 2002, the repurchase rights had lapsed.
In October 2002, in conjunction with the licensing of FACTIVE (Note 6), the Company issued 1,692,076 shares of common stock to LG Life Sciences. This stock was issued at fair value of $0.08 and recorded in the Company’s financial statements as a charge to research and development. The agreement with LG provided for anti-dilution protection from the date of the licensing agreement (October 2002) until the approval of FACTIVE, with the issuance of antidilution shares being contingent upon product approval by the FDA. In April 2003, in conjunction with the approval of FACTIVE, the Company issued an additional 1,164,292 shares of common stock to LG. The stock was issued at fair value of $3.50 and was capitalized in the Company’s financial statements as an intangible asset being amortized over the term of the license.
F-19
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
Convertible Preferred Stock
At September 30, 2003, the Company was authorized to issue up to 24,975,000 shares of preferred stock, issuable in series, with the rights and preferences of each designated series to be determined by the Company’s board of directors. To date, 5,425,000, 6,000,000, 6,600,000, 13,000,000 and 1,000,000 shares have been designated as Series A, B, C, D and Series 1 convertible non-redeemable preferred stock, respectively.
In August 2002, the Company issued 1,000,000 shares of Series 1 preferred stock (converted to 356,252 shares of common stock, as described below) to British Biotech Pharmaceuticals Ltd. in accordance with the technology agreement which provided for issuance of Series 1 Preferred Stock on the meeting of a clinical milestone. This stock was issued at fair value of $0.08 per share and recorded as research and development expense in August 2002.
In December 2002, in conjunction with the bridge financing, the Company converted all shares of Series A, Series B, Series D and Series 1 convertible non-redeemable preferred stock to common stock at a ratio of one share of common stock for each 2.807 shares of nonredeemable convertible preferred stock held and all shares of Series C convertible nonredeemable preferred stock at a rate of one share of common stock for each 2.693 shares of nonredeemable convertible preferred stock held, after taking into account antidilution protection which resulted from the issuance of Series D preferred stock.
9. Accounting for Stock-Based Compensation Stock Options
1998 Stock Plan
The 1998 Stock Plan (the “Plan”) was adopted in June 1998 and provides for the issuance of stock options. As of December 31, 2002, the Company had reserved 4,011,814 shares of common stock for issuance under the Plan.
Stock options granted under the Plan may be either incentive stock options or nonstatutory stock options. Incentive stock options may be granted to employees with exercise prices of no less than the fair value and nonstatutory options may be granted to employees, directors, or consultants at exercise prices of no less than 85% of the fair value of the common stock on the grant date, as determined by the board of directors. If, at the time the Company grants an option, the optionee directly or by attribution owns stock possessing more than 10% of the total combined voting power of all classes of stock of the Company, the option price shall be at least 110% of the fair value and shall not be exercisable more than five years after the date of grant. For all grants prior to December 31, 2001, options become exercisable as determined by the board of directors, at the rate of 20% at the end of the first year with the remaining balance vesting ratably over the next four years. For all grants beginning in 2002, options become exercisable as determined by the board of directors at a rate of 25% at the end of the first year with the remaining balance vesting ratably over the next three years. Except as noted above, options expire no more than 10 years after the date of grant or earlier if employment is terminated.
The Plan allows for the early exercise of options before they have vested. Any unvested shares so purchased are subject to repurchase by the Company upon termination of the purchaser’s employment or services. The repurchase right lapses over the normal vesting schedule. At December 31, 2002, and September 30, 2003, there were 88,703 and 22,208 shares subject to repurchase relating to the early exercise of options at a weighted-average exercise price of $0.70 and $0.41 per share, respectively.
F-20
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
Stock Options
Option activity under the Plan is as follows:
| Outstanding Options | |||||||||
Shares Available for Grant | Number of Shares | Weighted- Exercise Price | |||||||
Balance at December 31, 1999 | 212,777 | 323,654 | $ | 0.508 | |||||
Shares reserved | 712,504 | — | — | ||||||
Shares repurchased | 3,562 | — | — | ||||||
Options granted | (726,042 | ) | 726,042 | $ | 1.178 | ||||
Options exercised | — | (282,180 | ) | $ | 0.654 | ||||
Options canceled | 91,528 | (91,528 | ) | $ | 0.691 | ||||
Balance at December 31, 2000 | 294,329 | 675,988 | $ | 1.142 | |||||
Shares reserved | 178,126 | — | — | ||||||
Shares repurchased | 100,499 | — | — | ||||||
Options granted | (282,864 | ) | 282,864 | $ | 1.40 | ||||
Options exercised | — | (57,231 | ) | $ | 1.024 | ||||
Options canceled | 115,017 | (115,017 | ) | $ | 1.142 | ||||
Balance at December 31, 2001 | 405,107 | 786,604 | $ | 1.229 | |||||
Shares reserved | 2,155,766 | — | — | ||||||
Shares repurchased | 49,906 | — | — | ||||||
Options granted | (140,274 | ) | 140,274 | $ | 1.40 | ||||
Options exercised | — | (4,688 | ) | $ | 1.06 | ||||
Options canceled | 211,230 | (211,230 | ) | $ | 1.32 | ||||
Balance at December 31, 2002 | 2,681,735 | 710,960 | $ | 1.23 | |||||
Shares reserved(Unaudited) | — | — | — | ||||||
Shares repurchased(Unaudited) | 3,178 | — | — | ||||||
Options granted(Unaudited) | (2,312,544 | ) | 2,312,544 | $ | 0.17 | ||||
Options exercised(Unaudited) | (37,552 | ) | $ | 1.06 | |||||
Options canceled(Unaudited) | 116,205 | (116,205 | ) | $ | 0.83 | ||||
Balance at September 30, 2003(unaudited) | 488,574 | 2,869,747 | $ | 0.39 | |||||
F-21
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
Details of the Company’s stock options at December 31, 2002 are as follows:
Options Outstanding and Exercisable | ||||
Exercise Price | Number of Shares Under Option | Weighted- Average Remaining Contractual Life | ||
| (In years) | ||||
$0.28 | 91,616 | 6.12 | ||
$0.70 | 28,500 | 7.21 | ||
$1.40 | 590,844 | 8.26 | ||
| 710,960 | ||||
In the nine months ended September 30, 2003 and 2002, the Company granted 56,800 and 26,718 options, respectively, to consultants under the Plan with exercise prices equal to the market price of the options determined by the Company’s board of directors with vesting periods from one to five years. Additionally, on August 2003, the Company issued 70,000 options outside of the Plan. The Company recognized compensation charges of $1,829 in 2002, $50,205 in 2001, and $26,714 in 2000 and $343,724 for the nine months ended September 30, 2003 relating to these options. To determine the fair value of options earned by consultants in 2003 and 2002, the Black-Scholes valuation model was applied, using the following assumptions: a risk-free interest rate of 5.0%, respectively; a weighted-average contractual life of approximately 10 years; a volatility of 65%; common stock prices of $0.08 and $3.50 per share in 2002 and 2003, respectively; and no dividends
Stock Issuance Program
A stock issuance program was adopted in June 1998 (the “Stock Issuance Program”) and provides for the direct and immediate issuance of shares of common stock without any intervening option grants at purchase prices of not less than 85% of the fair market value of the common stock on the issue date, as determined by the board of directors. If, at the time the Company issues shares, the purchaser directly or by attribution owns stock possessing more than 10% of the total combined voting power of all classes of stock of the Company, the purchase price shall be at least 100% of the fair value on the issue date. Shares of common stock may be issued under the Stock Issuance Program for cash or check, payable to the Company, or for past services rendered to the Company.
Shares of common stock issued under the Stock Issuance Program may, at the discretion of the board of directors, be fully and immediately vested upon issuance or may vest in one or more installments over the participant’s period of service or upon attainment of specified performance objectives. However, the board of directors may not impose a vesting schedule upon any stock issuance affected under the Stock Issuance Program which is more restrictive than 20% per year vesting, with initial vesting to occur not later than one year after the issuance date. Such limitation shall not apply to any common stock issuances made to the officers of the Company, nonemployee board members, or independent consultants.
F-22
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
Common Shares Reserved
The Company had reserved shares of common stock for future issuances as follows:
| December 31, 2002 | September 30, 2003 (Unaudited) | |||
1998 Stock Plan | 3,392,695 | 3,428,321 | ||
Warrants to purchase common stock | 3,540,702 | 5,025,970 | ||
| 6,933,397 | 8,454,291 | |||
10. Income Taxes
As of December 31, 2002, 2001 and 2000, the Company had deferred tax assets of approximately $22.9 million, $10.4 million and $5.0 million, respectively. Realization of the deferred tax assets is dependent upon future taxable income, if any, the amount and timing of which are uncertain. Accordingly, the deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by approximately $12.5 million, $5.4 million and $3.3 million during the years ended December 31, 2002, 2001 and 2000, respectively. Deferred tax assets primarily relate to net operating losses and capitalized research and development costs.
As of December 31, 2002, the Company had federal and state net operating loss carryforwards of approximately $10.0 million and $7.0 million respectively, which begin to expire in 2005 if not utilized. The Company has also capitalized research and development costs for federal and state purposes of approximately $42.0 million, which are amortized over a 10-year period.
Utilization of the net operating loss carryforwards and credits may be subject to a substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986, as amended, and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization.
11. Related Party
In December 2002, the Company loaned $315,000 to an officer. The funds are secured by a loan agreement that matures on December 20, 2005. The note carries interest of 1.84% per annum.
12. Subsequent Events
In November 2003, the Company entered into a definitive agreement with Genome Therapeutics Corp., a publicly traded company to merge in an all stock transaction (the merger agreement). Consummation of this transaction is subject to the receipt of certain third-party approvals and consents, as well as approval by both parties’ shareholders and raising additional capital, approximately $32 million, to fund the merged company. Selected stockholders of Genome Therapeutics Corp. and the Company have agreed to vote in favor of the merger.
On November 17, 2003, Genome loaned to the Company $6.2 million in connection with the signing of the merger. This loan, along with the Company’s existing capital resources are expected to fund the Company’s operations through the closing of the merger. If, however, the closing of the merger is delayed or if the Company’s liabilities increase, there can be no assurance that these funds will be sufficient.
F-23
Table of Contents
GeneSoft Pharmaceuticals, Inc.
(a development stage company)
Notes to Financial Statements—(Continued)
In November 2003, the Company and the holders of the Company’s promissory notes entered into a note amendment and exchange agreement that restructures $22,309,647 of the Company’s notes. As a result of this agreement, the maturity date of the December and April notes was extended to the later of the current maturity dates of the notes or the date 60 days following the termination of the merger agreement. The interest rate of the notes was amended to 5% per annum commencing on December 10, 2003 for the December notes and December 15, 2003 for the April notes. Upon the closing of the merger, the December and April notes will be converted into Genome Therapeutics Corp. notes. Accrued interest through the closing of the merger under the December and April notes, and the amount that would be payable upon a change in control under the December notes, will be converted into shares of Genome Therapeutics Corp. common stock.
F-24
Table of Contents
Annex A
AGREEMENT AND PLAN OF MERGER AND REORGANIZATION
among
GENOME THERAPEUTICS CORP.,
GUARDIAN ACQUISITION, INC.,
GENESOFT PHARMACEUTICALS, INC.
and
LUKE EVNIN, as
STOCKHOLDERS’ REPRESENTATIVE
(solely for purposes of Section 9.05)
Dated as of November 17, 2003
Table of Contents
TABLE OF CONTENTS
| Page | ||||
ARTICLE I THE MERGER | 2 | |||
SECTION 1.01 | The Merger | 2 | ||
SECTION 1.02 | Effective Time; Closing | 2 | ||
SECTION 1.03 | Effect of the Merger | 2 | ||
SECTION 1.04 | Certificate of Incorporation and Bylaws of the Surviving Corporation | 2 | ||
SECTION 1.05 | Directors and Officers of Merger Sub | 3 | ||
SECTION 1.06 | Directors and Officers of Parent | 3 | ||
ARTICLE II MERGER CONSIDERATION; EXCHANGE OF CERTIFICATES | 3 | |||
SECTION 2.01 | Merger Consideration | 3 | ||
SECTION 2.02 | Exchange of Certificates | 4 | ||
SECTION 2.03 | Stock Transfer Books | 6 | ||
SECTION 2.04 | Company Stock Options; Company Warrants | 6 | ||
SECTION 2.05 | Dissenting Shares | 7 | ||
ARTICLE III REPRESENTATIONS AND WARRANTIES OF THE COMPANY | 7 | |||
SECTION 3.01 | Organization and Qualification | 8 | ||
SECTION 3.02 | Certificate of Incorporation and Bylaws | 8 | ||
SECTION 3.03 | No Subsidiaries | 8 | ||
SECTION 3.04 | Capitalization | 8 | ||
SECTION 3.05 | Authority Relative to This Agreement | 9 | ||
SECTION 3.06 | No Conflict; Required Filings and Consents | 9 | ||
SECTION 3.07 | Permits; Compliance | 10 | ||
SECTION 3.08 | Financial Statements | 10 | ||
SECTION 3.09 | Absence of Certain Changes or Events | 10 | ||
SECTION 3.10 | Absence of Litigation | 10 | ||
SECTION 3.11 | Employee Benefit Plans; Labor Matters | 10 | ||
SECTION 3.12 | Contracts | 13 | ||
SECTION 3.13 | Environmental Matters | 14 | ||
SECTION 3.14 | Intellectual Property | 15 | ||
SECTION 3.15 | Taxes | 16 | ||
SECTION 3.16 | Vote Required | 16 | ||
SECTION 3.17 | Assets; Absence of Liens and Encumbrances | 17 | ||
SECTION 3.18 | Owned Real Property | 17 | ||
SECTION 3.19 | Brokers | 17 | ||
SECTION 3.20 | Tax Matters | 17 | ||
SECTION 3.21 | State Takeover Statutes | 17 | ||
SECTION 3.22 | Accounts Receivables | 17 | ||
SECTION 3.23 | Illegal Payments, etc | 17 | ||
SECTION 3.24 | Affiliate Transactions | 17 | ||
SECTION 3.25 | Employees | 17 | ||
SECTION 3.26 | Insurance | 18 | ||
SECTION 3.27 | Factive Supply | 18 | ||
i
Table of Contents
| Page | ||||
SECTION 3.28 | Disclosure | 18 | ||
ARTICLE IV REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB | 18 | |||
SECTION 4.01 | Organization and Qualification | 18 | ||
SECTION 4.02 | Certificate of Incorporation and Bylaws | 19 | ||
SECTION 4.03 | No Subsidiaries | 19 | ||
SECTION 4.04 | Capitalization | 19 | ||
SECTION 4.05 | Authority Relative to This Agreement | 19 | ||
SECTION 4.06 | No Conflict; Required Filings and Consents | 20 | ||
SECTION 4.07 | Permits; Compliance | 20 | ||
SECTION 4.08 | SEC Filings; Financial Statements | 21 | ||
SECTION 4.09 | Absence of Certain Changes | 21 | ||
SECTION 4.10 | Absence of Litigation | 21 | ||
SECTION 4.11 | Employee Benefit Plans; Labor Matters | 21 | ||
SECTION 4.12 | Contracts | 23 | ||
SECTION 4.13 | Environmental Matters | 24 | ||
SECTION 4.14 | Intellectual Property | 24 | ||
SECTION 4.15 | Taxes | 25 | ||
SECTION 4.16 | Vote Required | 25 | ||
SECTION 4.17 | Assets; Absence of Liens and Encumbrances | 26 | ||
SECTION 4.18 | Owned Real Property | 26 | ||
SECTION 4.19 | Brokers | 26 | ||
SECTION 4.20 | Tax Matters | 26 | ||
SECTION 4.21 | State Takeover Statutes | 26 | ||
SECTION 4.22 | Interim Operations of Merger Sub | 26 | ||
SECTION 4.23 | Valid Issuance of Parent Shares | 26 | ||
SECTION 4.24 | Accounts Receivables | 26 | ||
SECTION 4.25 | Illegal Payments, etc | 26 | ||
SECTION 4.26 | Affiliate Transactions | 26 | ||
SECTION 4.27 | Employees | 27 | ||
SECTION 4.28 | Insurance | 27 | ||
SECTION 4.29 | Disclosure | 27 | ||
ARTICLE V CONDUCT OF BUSINESSES PENDING THE MERGER | 27 | |||
SECTION 5.01 | Conduct of Business by the Company Pending the Merger | 27 | ||
SECTION 5.02 | Conduct of Business by Parent Pending the Merger | 29 | ||
SECTION 5.03 | Litigation | 30 | ||
SECTION 5.04 | Notification of Certain Matters | 30 | ||
ARTICLE VI ADDITIONAL AGREEMENTS | 30 | |||
SECTION 6.01 | Registration Statement; Joint Proxy Statement | 30 | ||
SECTION 6.02 | Company Stockholders’ Meeting | 32 | ||
SECTION 6.03 | Parent Stockholders’ Meeting | 33 | ||
SECTION 6.04 | Access to Information; Confidentiality | 33 | ||
SECTION 6.05 | No Company Solicitation of Transactions | 33 | ||
SECTION 6.06 | No Parent Solicitation of Transactions | 34 | ||
ii
Table of Contents
| Page | ||||
SECTION 6.07 | Employee Benefits Matters | 34 | ||
SECTION 6.08 | Further Action; Consents; Filings | 35 | ||
SECTION 6.09 | Plan of Reorganization | 35 | ||
SECTION 6.10 | No Public Announcement | 36 | ||
SECTION 6.11 | Affiliate Agreements | 36 | ||
SECTION 6.12 | Indemnification of Officers and Directors | 36 | ||
SECTION 6.13 | Nasdaq National Market Listing | 36 | ||
SECTION 6.14 | Section 16 Relief | 36 | ||
SECTION 6.15 | Financing | 37 | ||
SECTION 6.16 | Promissory Note | 37 | ||
ARTICLE VII CONDITIONS TO THE MERGER | 37 | |||
SECTION 7.01 | Conditions to the Obligations of Each Party | 37 | ||
SECTION 7.02 | Conditions to the Obligations of Parent and Merger Sub | 38 | ||
SECTION 7.03 | Conditions to the Obligations of the Company | 39 | ||
ARTICLE VIII TERMINATION, AMENDMENT AND WAIVER | 40 | |||
SECTION 8.01 | Termination | 40 | ||
SECTION 8.02 | Effect of Termination | 41 | ||
SECTION 8.03 | Amendment | 41 | ||
SECTION 8.04 | Waiver | 41 | ||
SECTION 8.05 | Expenses | 41 | ||
SECTION 8.06 | Payment | 42 | ||
ARTICLE IX HOLD HARMLESS; INDEMNIFICATION | 42 | |||
SECTION 9.01 | Survival of Representations and Warranties | 42 | ||
SECTION 9.02 | Escrow; Hold Harmless | 42 | ||
SECTION 9.03 | Indemnification by Parent | 44 | ||
SECTION 9.04 | Indemnification Procedures | 44 | ||
SECTION 9.05 | Stockholders’ Representative | 45 | ||
ARTICLE X GENERAL PROVISIONS | 46 | |||
SECTION 10.01 | Notices | 46 | ||
SECTION 10.02 | Certain Definitions | 47 | ||
SECTION 10.03 | Severability | 52 | ||
SECTION 10.04 | Assignment; Binding Effect; Benefit | 52 | ||
SECTION 10.05 | Incorporation of Exhibits | 53 | ||
SECTION 10.06 | Specific Performance | 53 | ||
SECTION 10.07 | Governing Law; Forum | 53 | ||
SECTION 10.08 | Time of the Essence | 53 | ||
SECTION 10.09 | Construction and Interpretation | 53 | ||
SECTION 10.10 | Further Assurances | 53 | ||
SECTION 10.11 | Headings | 53 | ||
SECTION 10.12 | Counterparts | 53 | ||
SECTION 10.13 | Entire Agreement | 53 | ||
iii
Table of Contents
| Exhibit A-1 | Form of Company Voting Agreement | |
| Exhibit A-2 | Form of Parent Voting Agreement | |
| Exhibit B | Form of Escrow Agreement | |
| Exhibit C | Form of Company Affiliate Agreement | |
| Exhibit D | Form of Company Counsel Legal Opinion | |
| Exhibit E | Form of Parent Counsel Legal Opinion | |
| Schedule 1.06(a) | Members of the Board of Directors of Parent at the Effective Time | |
| Schedule 1.06(b) | Executive Management of Parent at the Effective Time | |
iv
Table of Contents
AGREEMENT AND PLAN OF MERGER AND REORGANIZATION
AGREEMENT AND PLAN OF MERGER AND REORGANIZATION,dated as of November 17, 2003 (this “Agreement”), among Genome Therapeutics Corp., a Massachusetts corporation (“Parent”), Guardian Acquisition, Inc., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”), Genesoft Pharmaceuticals, Inc., a Delaware corporation (the “Company”), and Luke Evnin, as Stockholders’ Representative (as defined in Section 9.05 hereof and solely for purposes thereof).
W I T N E S S E T H
WHEREAS,upon the terms and subject to the conditions of this Agreement and in accordance with the Delaware General Corporation Law (the “DGCL”), Parent and the Company will enter into a two-step business combination transaction pursuant to which (i) Merger Sub will merge with and into the Company (the “Reverse Merger”) and (ii) immediately thereafter the Company will merge with and into a limited liability company wholly owned by Parent (“Second Acquisition Subsidiary”) (the “Second-Step Merger”), both steps of which will occur as part of a single integrated plan. As used in this Agreement, “Merger” shall mean the Reverse Merger and the Second-Step Merger, collectively or seriatim, as appropriate;
WHEREAS,the Board of Directors of the Company has (i) determined that the Merger (as defined below) is fair to, and in the best interests of, the Company and its stockholders, (ii) approved and adopted this Agreement, the Merger, and the other transactions contemplated by this Agreement, and (iii) determined to recommend that the stockholders of the Company approve and adopt this Agreement and the Merger;
WHEREAS,the Boards of Directors of each of Parent and Merger Sub have (i) determined that the Merger is consistent with and in furtherance of the long-term business strategy of Parent and fair to, and in the best interests of, Parent, Merger Sub and their respective stockholders, (ii) approved and adopted this Agreement, the Merger, and the other transactions contemplated by this Agreement and (iii) determined to unanimously recommend that the stockholders of Parent approve the issuance of Parent Common Stock (as defined below) pursuant to the Merger (the “Share Issuance”);
WHEREAS,for Federal income tax purposes, the Merger is intended to qualify as a reorganization under the provisions of Section 368(a) of the United States Internal Revenue Code of 1986, as amended (the “Code”);
WHEREAS, certain stockholders of the Company own such number of shares of common stock, $0.0001 par value, of the Company (the “Company Stock”), as is set forth opposite such stockholder’s name in Section 1.01 of the Company Disclosure Schedule (as defined in Article III) (such stockholders being referred to herein as the “Principal Stockholders”);
WHEREAS,pursuant to the Merger, each outstanding share of Company Stock shall be converted into the right to receive shares of Parent’s authorized common stock, par value $0.10 per share (“Parent Common Stock”), at the rate determined in this Agreement;
WHEREAS,as a condition and inducement to Parent’s and Merger Sub’s entering into this Agreement and incurring the obligations set forth herein, concurrently with the execution and delivery of this Agreement, each of the Principal Stockholders is entering into a voting agreement with Parent (a “Company Voting Agreement”), dated the date hereof and substantially in the form attached hereto asExhibit A-1;
WHEREAS,as a condition and inducement to the Company’s entering into this Agreement and incurring the obligations set forth herein, concurrently with the execution and delivery of this Agreement, certain of the Parent stockholders are entering into a voting agreement with the Company (a “Parent Voting Agreement”), dated the date hereof and substantially in the form attached hereto asExhibit A-2;
WHEREAS,a portion of the Parent Common Stock otherwise issuable by Parent to the equity holders of the Company in connection with the Merger shall be placed in escrow by Parent, the release of which amount shall be contingent upon certain events and conditions, all as set forth in this Agreement and the Escrow Agreement (as defined in Section 2.02(b));
A-1
Table of Contents
WHEREAS,concurrently with the execution of this Agreement, the Company and Parent are entering into a Note Amendment and Exchange Agreement (the “Note Amendment and Exchange Agreement”) with holders of the Company’s outstanding convertible promissory notes, pursuant to which such holders are exchanging such notes for new notes and shares of Common Stock of Parent; and
WHEREAS,certain capitalized terms used in this Agreement are defined in Section 10.02 of this Agreement.
NOW, THEREFORE,in consideration of the foregoing and the mutual covenants and agreements herein contained, and intending to be legally bound hereby, Parent, Merger Sub, the Company and the Stockholders’ Representative hereby agree as follows:
ARTICLE I
THE MERGER
SECTION 1.01 The Merger. Upon the terms of this Agreement and subject to the conditions set forth in this Agreement, and in accordance with the DGCL, at the Effective Time (as defined in Section 1.02), as part of a single integrated plan, Merger Sub shall be merged with and into the Company, and immediately thereafter the Company shall be merged with and into the Second Acquisition Subsidiary. As a result of the Reverse Merger, the separate corporate existence of Merger Sub shall cease, and the Company shall continue as the surviving corporation of the Reverse Merger (the “Surviving Corporation”) and as a wholly-owned subsidiary of Parent. As a result of the Second-Step Merger, the separate corporate existence of the Company shall cease, and Second Acquisition Subsidiary shall continue as the surviving limited liability company of the Second-Step Merger, wholly owned by Parent.
SECTION 1.02 Effective Time; Closing. As promptly as practicable following the satisfaction or, if permissible by the express terms of this Agreement, waiver of the conditions set forth in Article VII (or such other date as may be agreed by each of the parties hereto), the parties hereto shall cause (a) the Reverse Merger to be consummated by (i) filing a certificate of merger (the “Certificate of Merger”) with the Secretary of State of the State of Delaware in such form as is required by, and executed in accordance with, the relevant provisions of the DGCL and (ii) making all other filings and recordings required under the DGCL and (b) the Second-Step Merger to be consummated by making all filings and recordings required under the Delaware Limited Liability Company Act. The term “Effective Time” means the date and time of the filing of the Certificate of Merger (or such later time as may be agreed by each of the parties hereto and specified in the Certificate of Merger). Immediately prior to the filing of the Certificate of Merger, a closing (the “Closing”) will be held at the offices of Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP (“Gunderson Dettmer”), 155 Constitution Drive, Menlo Park, California (or such other place as the parties may agree). The date on which the Closing shall occur is referred to herein as the “Closing Date.”
SECTION 1.03 Effect of the Merger. At and after the Effective Time, the Reverse Merger shall have the effects as set forth in the applicable provisions of the DGCL. Without limiting the generality of the foregoing, and subject thereto, at the Effective Time, all the property, rights, privileges, powers and franchises of each of the Company and Merger Sub shall vest in the Surviving Corporation, and all debts, liabilities, obligations, restrictions, disabilities and duties of each of the Company and Merger Sub shall become the debts, liabilities, obligations, restrictions, disabilities and duties of the Surviving Corporation, and, following the Second-Step Merger, all the property, rights, privileges, powers and franchises of the Surviving Corporation shall vest in Second Acquisition Subsidiary, and all debts, liabilities, obligations, restrictions, disabilities and duties of the Surviving Corporation shall become the debts, liabilities, obligations, restrictions, disabilities and duties of Second Acquisition Subsidiary.
SECTION 1.04 Certificate of Incorporation and Bylaws of the Surviving Corporation.
(a) Subject to Section 6.12, at the Effective Time, the Certificate of Incorporation of the Company as the Surviving Corporation shall be amended and restated to read the same as the Certificate of Incorporation of Merger Sub as in effect immediately prior to the Effective Time, except that Section 1 of the amended and restated Certificate of Incorporation of the Surviving Corporation, instead of reading the same as Section 1 of the Certificate of Incorporation of Merger Sub, shall read as follows: “The name of this corporation is GeneSoft Pharmaceuticals, Inc.,” or such other name as determined by Parent, and following the Second-Step Merger, the
A-2
Table of Contents
Certificate of Formation of Second Acquisition Subsidiary shall be amended to provide that the name of Second Acquisition Subsidiary shall be “GeneSoft Pharmaceuticals, LLC,” or such other name as determined by Parent.
(b) Subject to Section 6.12, at the Effective Time, the Bylaws of the Company as the Surviving Corporation shall be amended to read the same as the Bylaws of Merger Sub as in effect immediately prior to the Effective Time, except that all references to Merger Sub in the Bylaws of the Surviving Corporation shall be changed to refer to GeneSoft Pharmaceuticals, Inc., and following the Second Step Merger, the limited liability company agreement of Second Acquisition Subsidiary (the “LLC Agreement”) shall be modified to refer to GeneSoft Pharmaceuticals, LLC.
SECTION 1.05 Directors and Officers of Merger Sub. The directors of Merger Sub immediately prior to the Effective Time shall be the initial directors of the Surviving Corporation, each to hold office in accordance with the Certificate of Incorporation and Bylaws of the Surviving Corporation, and the officers of the Company immediately prior to the Effective Time shall be the initial officers of the Surviving Corporation, in each case until their respective successors are duly elected or appointed and qualified, and the Managers of the Second Acquisition Subsidiary immediately prior to the Second Step Merger shall be the initial managers of the surviving limited liability company, each to hold office in accordance with the LLC Agreement, and the officers of the Second Acquisition Subsidiary immediately prior to the Second Step Merger shall be the initial officers of the surviving limited liability company, in each case until their respective successors are duly elected or appointed and qualified.
SECTION 1.06 Directors and Officers of Parent.
(a) Prior to the Effective Time, Parent shall take such action so that, from and after the Effective Time, until duly changed in compliance with applicable Law and the Certificate of Incorporation and Bylaws of Parent, the Board of Directors of Parent shall consist of the nine (9) members listed on Schedule 1.06(a) attached hereto.
(b) Parent shall take such action so that, upon the Effective Time, the persons set forth on Schedule 1.06(b) attached hereto shall hold the executive positions with Parent set forth opposite their name.
ARTICLE II
MERGER CONSIDERATION; EXCHANGE OF CERTIFICATES
SECTION 2.01 Merger Consideration.
(a) At the Effective Time, by virtue of the Merger and without any action on the part of Parent, Merger Sub, the Company or the holders of any of the following securities:
(i) each share of Company Stock issued and outstanding as of the Effective Time (other than Dissenting Shares (as defined in Section 2.05)) shall, by virtue of the Merger and without any action on the part of the holders of Company Stock, be cancelled and terminated as set forth below and converted into the right to receive the applicable portion of the Aggregate Merger Consideration (as defined in Section 2.01(b)) allocated to each share of Company Stock issued and outstanding immediately prior to the Effective Time (other than any Dissenting Shares (as defined in Section 2.05)) as follows: each outstanding share of Company Stock shall be converted automatically into the right to receive such number of shares of Parent Common Stock equal to the Common Exchange Ratio (as defined in Section 2.01(b));
(ii) each share of Company Stock held in the treasury of the Company immediately prior to the Effective Time shall be cancelled and extinguished without any conversion thereof and no payment or distribution shall be made with respect thereto; and
(iii) each share of common stock, par value $0.01 per share, of Merger Sub issued and outstanding immediately prior to the Effective Time shall be converted into and exchanged for one validly issued, fully paid and nonassessable share of common stock, par value $0.01 per share, of the Surviving Corporation. The stock certificate evidencing shares of common stock of Merger Sub shall then evidence ownership of the outstanding share of common stock of the Surviving Corporation. As a result of the Second Step Merger, each share of common stock of the Surviving Corporation shall be converted into and exchanged for a limited liability company interest in Second Acquisition Subsidiary.
A-3
Table of Contents
(b) As used in this Agreement, the following terms have the following meanings:
(i) “Aggregate Merger Consideration” means (x) 28,571,405 shares of Parent Common Stock (the “Parent Shares”) less (y) the sum of the Preference Shares (as defined below) and the Accrued Interest Shares (as defined below).
(ii) “Escrow Shares” means the number of Parent Shares (rounded up to the next whole number) obtained from the sum of (i) the number of Parent Shares determined by multiplying the Aggregate Merger Consideration by 0.20 (the “Indemnity Escrow Shares”) and (ii) 400,000 Parent Shares (the “Other Escrow Shares”).
(iii) “Common Exchange Ratio” means the number of Parent Shares determined by dividing (x) the Aggregate Merger Consideration by (y) the Fully Diluted Common Shares Amount (as defined below).
(iv) “Fully Diluted Common Shares Amount” means a number of shares of Company Stock equal to the sum of (x) the number of shares of Company Stock issued and outstanding immediately prior to the Effective Time and (y) the number of shares of Company Stock issuable upon exercise, conversion and/or exchange of all securities issued and outstanding immediately prior to the Effective Time that are exercisable, convertible and/or exchangeable for shares of Company Stock, including, without limitation, the Company Options (as defined in Section 2.04 below), whether or not exercisable and whether or not vested or contingent, and the Company Warrants (as defined in Section 2.04 below), whether or not exercisable and whether or not vested or contingent.
(v) “Preference Shares” shall have the meaning given it in the Note Amendment and Exchange Agreement.
(vi) “Accrued Interest Shares” shall have the meaning given it in the Note Amendment and Exchange Agreement.
(c) If, during the period between the date hereof and the Effective Time, any change in the capital stock of Parent shall occur by reason of reclassification, recapitalization, stock split or combination, exchange or readjustment of shares, or any stock dividend thereon with a record date during such period or any similar event, the Aggregate Merger Consideration and Other Escrow Shares shall be correspondingly adjusted to the extent appropriate to reflect such stock dividend, subdivision, reclassification, recapitalization, split, combination, exchange or readjustment of shares.
(d) If any shares of Company Stock outstanding immediately prior to the Effective Time are unvested or are subject to a repurchase option, risk of forfeiture or other condition under any applicable restricted stock purchase agreement, stock option exercise agreement or other agreement with the Company, then the Parent Shares issued in exchange for such shares of Company Stock will also be unvested and/or subject to the same repurchase option, risk of forfeiture or other condition, and the certificates representing such Parent Shares may accordingly be marked with appropriate legends.
SECTION 2.02 Exchange of Certificates.
(a)Exchange Procedures. From and after the Effective Time, a bank or trust company to be designated by Parent shall act as exchange agent (the “Exchange Agent”) in effecting the exchange of the applicable Parent Shares for certificates which immediately prior to the Effective Time represented outstanding shares of Company Stock (“Company Share Certificates”) and which were converted into the right to receive the applicable Parent Shares pursuant to Section 2.01. As promptly as practicable after the Effective Time but in no event later than three business days following the Effective Time, Parent and the Exchange Agent shall mail to each record holder of Company Share Certificates a letter of transmittal (the “Letter of Transmittal”) in a form approved by Parent and the Company and instructions for use in surrendering such Company Share Certificates and receiving the applicable Parent Shares pursuant to Section 2.01. Promptly after the Effective Time, but in no event later than ten business days following the Effective Time, Parent shall cause to be deposited in trust with the Exchange Agent the Aggregate Merger Consideration less the Escrow Shares.
Upon the surrender of each Company Share Certificate for cancellation to the Exchange Agent, together with a properly completed Letter of Transmittal and such other documents as may reasonably be required by Parent:
(i) Parent shall cause to be issued to the holder of such Company Share Certificate in exchange therefor a separate stock certificate representing the Parent Shares to which such holder is entitled pursuant to Section 2.01 (less the Escrow Shares attributable to the pro rata interest of such holder in the Escrow Funds pursuant to Section 2.02(b)); and
(ii) the Company Share Certificates so surrendered shall forthwith be cancelled.
A-4
Table of Contents
In the event of a transfer of ownership of shares of Company Stock that is not registered in the transfer records of the Company, the applicable Parent Shares may be issued to a person other than the person in whose name the Company Share Certificate so surrendered is registered if the Company Share Certificate representing such shares of Company Stock is presented to Parent, accompanied by all documents required by Parent to evidence and effect such transfer and evidence that (i) the shares are transferable and (ii) any applicable stock transfer taxes have been paid.
Until surrendered as contemplated by this Article II, each Company Share Certificate shall, subject to dissenters rights under the DGCL (and if the Company is subject to Section 2115 of the California Corporations Code, Chapter 13 of the California Corporations Code) and Section 2.05, be deemed at any time after the Effective Time to represent only the right to receive upon surrender the applicable Parent Shares with respect to the shares of Company Stock formerly represented thereby to which such holder is entitled pursuant to Section 2.01.
(b) Escrow Fund. Prior to or simultaneously with the Closing, the Stockholders’ Representative and Parent shall enter into escrow agreement with an escrow agent selected by Parent and reasonably acceptable to the Stockholders’ Representative (the “Escrow Agent”) substantially in the form ofExhibit B hereto (the “Escrow Agreement”). Pursuant to the terms of the Escrow Agreement, Parent shall deposit (i) one or more certificates in the name of the Escrow Agent representing the shares of Parent Common Stock that are the Company Stockholders’ pro-rata portion of the Indemnity Escrow Shares into an escrow account, which account is to be managed by the Escrow Agent (the “Indemnity Escrow Account”) and (ii) one or more certificates in the name of the Escrow Agent representing the 400,000 shares of Parent Common Stock that are the Other Escrow Shares into an escrow account, which account is to be managed by the Escrow Agent (the “Other Escrow Account”, and together with the Indemnity Escrow Account, the “Escrow Accounts”). Any Escrow Shares in the Indemnity Escrow Account are referred to herein as the “Indemnity Escrow Fund”, any Escrow Shares in the Other Escrow Account are referred to herein as the “Other Escrow Fund”, and the Indemnity Escrow Fund and Other Escrow Fund together are the “Escrow Funds”. In connection with such deposit of the Escrow Shares with the Escrow Agent and as of the Effective Time, each holder of Company Stock will be deemed to have received and deposited with the Escrow Agent each stockholder’s pro rata interest in the Escrow Funds (plus any additional shares as may be issued upon any stock split, stock dividend or recapitalization effected by Parent after the Effective Time with respect to shares constituting the Escrow Funds) as determined as of Closing by reference to such stockholder’s ownership of shares of Company Stock, without any act of the stockholders of the Company (the “Company Stockholders”). If a holder of assumed Company Options exercises any portion of such holder’s option prior to the termination of the Escrow Funds, such holder shall contribute a portion of the shares of Parent Common Stock issued upon exercise to the Escrow Funds in accordance with the provisions of the Escrow Agreement, and such shares shall thereafter be “Escrow Shares” for purposes hereunder. Distributions of any Escrow Shares from the Escrow Accounts shall be governed by the terms and conditions of the Escrow Agreement. The adoption of this Agreement and the approval of the Merger by the Company Stockholders shall constitute approval of the Escrow Agreement and of all the arrangements relating thereto, including, without limitation, the placement of the Escrow Shares in escrow and the appointment of the Stockholders’ Representative.
(c) Distributions with Respect to Unexchanged Parent Shares. No dividends or other distributions declared or made after the Effective Time with respect to Parent Shares comprising part of the Aggregate Merger Consideration with a record date after the Effective Time shall be paid to the holder of any unsurrendered Company Share Certificate with respect to the Parent Shares represented thereby until the holder of such Company Share Certificate shall surrender such Company Share Certificate in accordance with this Section 2.02.
(d) No Further Rights in Company Stock. The Parent Shares issued upon the conversion of shares of Company Stock in accordance with the terms hereof shall be deemed to have been issued in full satisfaction of all rights pertaining to such shares of Company Stock.
(e) No Fractional Shares. Notwithstanding any other provision of this Agreement, no fractional shares of Parent Common Stock shall be issued upon the conversion and exchange of Company Share Certificates, and no holder of Company Share Certificates shall be entitled to receive a fractional share of Parent Common Stock. In the event that any holder of Company Stock would otherwise be entitled to receive a fractional share of Parent Common Stock (after aggregating all shares and fractional shares of Parent Common Stock issuable to such holder), then such holder will receive an aggregate number of shares of Parent Common Stock rounded up or down to the nearest whole share (with 0.5 being rounded up).
A-5
Table of Contents
(f) No Liability. Neither Parent nor the Surviving Corporation shall be liable to any holder of shares of Company Stock for any such shares of Parent Common Stock (or dividends or distributions with respect thereto) or cash properly and legally delivered to a public official pursuant to any abandoned property, escheat or similar Law (as defined in Section 3.06(a)).
(g) Withholding Rights. Each of the Exchange Agent, the Surviving Corporation and Parent shall be entitled to deduct and withhold from the consideration otherwise payable pursuant to this Agreement to any holder of shares of Company Stock such amounts as it is required to deduct and withhold with respect to the making of such payment under the Code, or any provision of state, local or foreign Tax (as defined in Section 3.15(c)) Law. To the extent that amounts are so withheld by the Exchange Agent, the Surviving Corporation or Parent, as the case may be, such withheld amounts shall be treated for all purposes of this Agreement as having been paid to the holder of the shares of Company Stock in respect of which such deduction and withholding were made by the Exchange Agent, the Surviving Corporation or Parent, as the case may be.
(h) Lost Certificates. If any Company Share Certificate shall have been lost, stolen or destroyed, upon the making of an affidavit of that fact by the person claiming such Company Share Certificate to be lost, stolen or destroyed and, if required by the Surviving Corporation, the posting by such person of a bond, in such reasonable amount as the Surviving Corporation may direct, as indemnity against any claim that may be made against it with respect to such Company Share Certificate, Parent shall issue in exchange for such lost, stolen or destroyed Company Share Certificate, the applicable Parent Shares (and dividends or other distributions pursuant to Section 2.02(c)) to which such person is entitled pursuant to the provisions of this Article II.
(i) Return of Parent Shares. Promptly following the end of the sixth full calendar month after the Effective Time, the Exchange Agent shall return to Parent all of the remaining Parent Shares in the Exchange Agent’s possession and the Exchange Agent’s duties shall terminate. Thereafter, upon the surrender of a Company Share Certificate to Parent, together with a properly executed Letter of Transmittal and forms of stock power and such other documents as may reasonably be required by Parent, and subject to applicable abandoned property, escheat and similar Laws, the holder of such Company Share Certificate shall be entitled to receive in exchange therefor the applicable Parent Shares (and dividends or other distributions pursuant to Section 2.02(c)) without any interest thereon.
SECTION 2.03 Stock Transfer Books. At the Effective Time, the stock transfer books of the Company shall be closed and there shall be no further registration of transfers of shares of Company Stock thereafter on the records of the Company. From and after the Effective Time, the holders of certificates representing shares of Company Stock outstanding immediately prior to the Effective Time shall cease to have any rights with respect to such shares of Company Stock, except as otherwise provided in this Agreement or by Law.
SECTION 2.04 Company Stock Options; Company Warrants.
(a) At the Effective Time, Parent shall assume all options to purchase Common Stock issued by the Company pursuant to the Company Stock Plan (as defined in Section 3.04(b)) whether vested or unvested and whether exercisable or unexercisable (each a “Company Option”). The Company’s repurchase right with respect to any unvested shares acquired by the exercise of Company Options shall be assigned to Parent by virtue of the Merger and without any further action on the part of the Company or the holder of such unvested shares. Immediately after the Effective Time, each Company Option outstanding immediately prior to the Effective Time shall be deemed to constitute an option to acquire, on the same terms and conditions as were applicable under such Company Option at the Effective Time (subject to Parent’s right to withhold a percentage of Parent Common Stock upon exercise of such assumed Company Option as contemplated by the terms of the Escrow Agreement), such number of shares of Parent Common Stock that is equal to the number of shares of Company Stock subject to the unexercised portion of such Company Option multiplied by the Common Exchange Ratio (rounded down to the nearest whole number). The per share exercise price for the shares of Parent Common Stock issuable upon exercise of such assumed Company Option shall be equal to the exercise price per share of such Company Option in effect immediately prior to the Effective Time divided by the Common Exchange Ratio (rounded up to the nearest whole cent). The term, vesting schedule, status as an “incentive stock option” under Section 422 of the Code, if applicable, and all of the other terms of the Company Options shall otherwise remain unchanged. It is the intention of the parties that each Company Option so assumed by Parent shall qualify following the Effective Time as an incentive stock option as defined in Section 422 of the Code to the extent permitted under Section 422 of the Code and to the extent such Company Option qualified as an incentive stock option prior to the Effective Time. Shares of Parent Common Stock issuable upon exercise of any assumed Company Options shall be subject to the escrow provisions of
A-6
Table of Contents
Section 2.02(b) and Article IX hereof. Within 45 business days after the Effective Time, Parent will issue to each person who, immediately prior to the Effective Time, was a holder of a Company Option a document evidencing the foregoing assumption of such option by Parent. Within 5 business days after the Effective Time, Parent shall file a registration statement on Form S-8 (or any successor or other appropriate forms) that will register the shares of Parent Common Stock subject to assumed Company Options to the extent permitted by Federal securities laws and shall use its commercially reasonable efforts to maintain the effectiveness of such registration statement or registration statements (and maintain the current status of the prospectus or prospectuses contained therein) for so long as such options remain outstanding.
(b) At the Effective Time, each warrant to acquire shares of Company Stock granted and outstanding immediately prior to the Effective Time (each a “Company Warrant”), whether or not contingent or earned and whether exercisable or unexercisable, that does not terminate by its terms at or prior to the Effective Time, shall be converted into a warrant to acquire shares of Parent Common Stock in accordance with its terms. Each Company Warrant so converted shall continue to have, and be subject to, the same terms and conditions set forth in such Company Warrant immediately prior to the Effective Time, except that (i) such Company Warrant shall be exercisable (or become exercisable in accordance with its terms) for that number of whole shares of Parent Common Stock (rounded down to the nearest whole number) equal to the product of the number of shares of Company Stock that were issuable upon exercise of such Company Warrant immediately prior to the Effective Time multiplied by the Common Exchange Ratio, and (ii) the per share exercise price for the shares of Parent Common Stock issuable upon exercise of such assumed Company Warrant shall be equal to the exercise price per share of Company Stock at which such Company Warrant was exercisable immediately prior to the Effective Time divided by the Common Exchange Ratio (rounded up to the nearest whole cent).
SECTION 2.05 Dissenting Shares.
(a) Notwithstanding any provision of this Agreement to the contrary, shares of Company Stock that are outstanding immediately prior to the Effective Time and which are held by stockholders who have exercised and perfected appraisal rights for such shares of Company Stock in accordance with the DGCL (and, if the Company is subject to Section 2115 of the California Corporations Code, such rights as may be granted to such persons in Chapter 13 of the California Corporations Code) (collectively, the “Dissenting Shares”) shall not be converted into or represent the right to receive the applicable Parent Shares. Such stockholders shall be entitled to receive payment of the appraised value of such shares of Company Stock held by them in accordance with the DGCL (and, if the Company is subject to Section 2115 of the California Corporations Code, such rights as may be granted to such persons in Chapter 13 of the California Corporations Code), unless and until such stockholders fail to perfect or effectively withdraw or otherwise lose their appraisal rights under the DGCL (or, if applicable, Chapter 13 of the California Corporations Code). All Dissenting Shares held by stockholders who shall have failed to perfect or who effectively shall have withdrawn or lost their right to appraisal of such shares of Company Stock under the DGCL (or, if applicable, Chapter 13 of the California Corporations Code) shall thereupon be deemed to have been converted into and to have become exchangeable for, as of the Effective Time, the right to receive the applicable Parent Shares, without any interest thereon, upon the surrender, in the manner provided in Section 2.02 (including the provision for the Escrow Shares pursuant to Section 2.02(b)), of the corresponding Company Share Certificate.
(b) The Company shall give Parent (i) reasonably prompt notice of any demands for appraisal received by the Company, withdrawals of such demands, and any other related instruments served pursuant to the DGCL (or, if applicable, Chapter 13 of the California Corporations Code) and received by the Company and (ii) the opportunity to direct all negotiations and proceedings with respect to demands for appraisal under the DGCL (or, if applicable, Chapter 13 of the California Corporations Code). The Company shall not, except with the prior written consent of Parent (which shall not be unreasonably withheld), make any payment with respect to any demands for appraisal or offer to settle or settle any such demands.
ARTICLE III
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
The Company hereby represents and warrants to Parent and Merger Sub that the statements contained in this Article III are true and correct as of the date of this Agreement and as of the Closing Date (except that those representations and warranties that address matters only as of a particular date shall remain true and correct as of such date) in each case, subject to the matters set forth in the
A-7
Table of Contents
disclosure schedule delivered by the Company to Parent and Merger Sub concurrently with the execution of this Agreement (the “Company Disclosure Schedule”).
SECTION 3.01 Organization and Qualification. The Company is a corporation duly organized, validly existing and in good standing under the laws of the State of Delaware and has all requisite corporate power and authority to own, lease and otherwise hold and operate its properties and other assets and to carry on its business as it is now being conducted and as currently proposed to be conducted, except where the failure to be so organized, existing or in good standing or to have such corporate power and authority would not be reasonably likely to have, individually or in the aggregate, a Company Material Adverse Effect (as defined below). The Company is duly qualified or licensed as a foreign corporation to do business, and is in good standing, in each jurisdiction where the character of the properties owned, leased or operated by it or the nature of its business makes such qualification or licensing necessary, except where the failure to be so qualified or licensed and in good standing has not had, and could not reasonably be expected to have, individually or in the aggregate, a Company Material Adverse Effect. Section 3.01 of the Company Disclosure Schedule sets forth each jurisdiction where the Company is qualified or licensed as a foreign corporation and each other jurisdiction in which the Company owns, uses, licenses or leases real property or has employees. The term “Company Material Adverse Effect” means any event, change, violation, inaccuracy, circumstance or effect that is individually or in the aggregate, materially adverse to the condition (financial or otherwise), assets (tangible or intangible), liabilities, or results of operations of the Company, except for any such events, changes, violations, inaccuracies, circumstances or effects resulting from or arising in connection with (i) any changes in general economic, political or business conditions and not specifically related to the Company, (ii) any changes or events affecting the industry in which the Company operates and not specifically related to the Company, (iii) any matter otherwise known to Parent prior to the date of this Agreement, (iv) any matters resulting from or arising in connection with this Agreement or the transactions contemplated by this Agreement or the announcement hereof (v) any fees or expenses payable as a result of the performance of the transactions contemplated by this Agreement, (vi) any pre-clinical or clinical results related to any of the Company’s programs or potential products other than Factive or (vii) any violations or other matters arising from changes in law or generally accepted accounting principles. Notwithstanding the foregoing, the Company acknowledges that any event, change, violation, inaccuracy, circumstance or effect, including without limitation, the revocation, withdrawal, suspension, cancellation, termination or material adverse modification (or notice of any threatened revocation, withdrawal, suspension, cancellation, termination or modification) of any FDA Company Permit (as defined in Section 3.07(b)) or Non-FDA Company Permit (as defined in Section 3.07(a)) related to Factive, that is individually or in the aggregate, materially adverse to the Company’s ability to sell, have sold, market, develop, distribute, import or manufacture Factive shall constitute a Company Material Adverse Effect.
SECTION 3.02 Certificate of Incorporation and Bylaws. The Company has made available to Parent a complete and correct copy of (a) the Certificate of Incorporation and the Bylaws of the Company including all amendments thereto, (b) the minute books containing all consents, actions and meeting of the stockholders of the Company and the Company’s Board of Directors and any committees thereof, and (c) the stock transfer books of the Company setting forth all issuances or transfers of any capital stock of the Company. Such Certificate of Incorporation and Bylaws are in full force and effect.
SECTION 3.03 No Subsidiaries. The Company does not own, of record or beneficially, or control any direct or indirect equity or other interest, or any right (contingent or otherwise) to acquire the same, in any corporation, partnership, limited liability company, joint venture, association or other entity.
SECTION 3.04 Capitalization.
(a) The authorized capital stock of the Company consists of 43,450,000 shares of Company Stock, 5,425,000 shares of Series A Preferred Stock (“Company Series A Preferred Stock”), 6,000,000 shares of Series B Preferred Stock (“Company Series B PreferredStock”), 6,600,000 shares of Series C Preferred Stock (“Company Series C Preferred Stock”), 5,950,000 shares of Series D Preferred Stock (“Company Series D Preferred Stock”) and 1,000,000 shares of Series 1 Preferred Stock (“Company Series 1 Preferred Stock”, and collectively with each foregoing series of preferred stock, the “Company Preferred Stock”). As of the date hereof, (i) 12,378,931 shares of Company Stock are issued and outstanding, all of which are duly authorized, validly issued, fully paid and nonassessable, (ii) no shares of Company Stock are held in the treasury of the Company and (iii) 2,939,747 shares of Company Stock are reserved for future issuance pursuant to outstanding Company Options. As of the date of this Agreement, (A) no shares of Company Series A Preferred Stock are issued and outstanding, (B) no shares of Company Series B Preferred Stock are issued and outstanding, (C) no shares of Company Series C Preferred Stock are issued and outstanding, (D) no shares of Company Series D Preferred Stock are issued and outstanding, and (E) no shares of Company Series 1 Preferred Stock are issued and outstanding. There are no other shares
A-8
Table of Contents
of Company Preferred Stock outstanding. As of the date hereof, the outstanding shares of Company Stock are owned as set forth in Section 3.04(a) of the Company Disclosure Schedule. Section 3.04(a) of the Company Disclosure Schedule also provides an accurate and complete description of the terms of each repurchase option or right of first refusal which is held by the Company and to which any of such shares is subject.
(b) The Company has reserved 4,011,814 shares of Company Stock for issuance under the Company’s 1998 Stock Option Plan/Stock Issuance (the “Company Stock Plan”) of which options to purchase 2,939,747 shares of Company Stock are outstanding as of the date of this Agreement. Section 3.04(b) of the Company Disclosure Schedule accurately sets forth with respect to each Company Option that is outstanding as of the date of this Agreement: (i) the name of the holder of such Company Option; (ii) the total number of shares of Company Stock that was originally subject to such Company Option; (iii) the number of shares of Company Stock that remain subject to such Company Option, (iv) the date on which such Company Option was granted and the term of such Company Option; (v) the vesting schedule and vesting commencement date for such Company Option; (vi) the exercise price per share of Company Stock purchasable under such Company Option; (vii) whether such Company Option has been designated an “incentive stock option” as defined in Section 422 of the Code; and (viii) the current employee or independent contractor or Board member status of the holder of such Company Option.
(c) The Company has reserved 40,702 shares of Company Stock for future issuance pursuant to the exercise of Company Warrants. Section 3.04(c) of the Company Disclosure Schedule sets forth, with respect to each Company Warrant issued to any person: (i) the name of the holder of such Company Warrant; (ii) the total number of shares of Company Stock that are subject to such Company Warrant; (iii) the exercise price per share of Company Stock purchasable under such Company Warrant; (iv) the total number of shares of Company Stock with respect to which such warrant is immediately exercisable; (v) the vesting schedule for such Company Warrant; and (vi) the term of such Company Warrant.
(d) Except as described in Section 3.04(b) above or as set forth in Section 3.04(b) or Section 3.04(c) of the Company Disclosure Schedule, there are no options, warrants or other rights, agreements, arrangements or commitments of any character, whether or not contingent, relating to the issued or unissued capital stock of the Company or obligating the Company to issue or sell any share of capital stock of, or other equity interest in, the Company. All shares of Company Stock so subject to issuance, upon issuance in accordance with the terms and conditions specified in the instruments pursuant to which they are issuable, will be duly authorized, validly issued, fully paid and nonassessable.
(e) Except as set forth on Schedule 3.04(d) of the Company Disclosure Schedule, the Company does not have outstanding any bonds, debentures, notes or other obligations the holders of which have the right to vote (or which are convertible into or exercisable for securities having the right to vote) with the stockholders of the Company on any matter.
SECTION 3.05 Authority Relative to This Agreement. The Company has all necessary corporate power and authority to execute this Agreement and, subject to obtaining the necessary approvals of the Company Stockholders, to perform its obligations hereunder and to consummate the Merger and the other transactions contemplated by this Agreement. The execution and delivery of this Agreement by the Company and the consummation by the Company of the Merger and the other transactions contemplated by this Agreement have been duly and validly authorized by all necessary corporate action and no other corporate proceedings on the part of the Company are necessary to authorize this Agreement or to consummate the Merger and the other transactions contemplated by this Agreement (other than the approval and adoption of this Agreement and the Merger by the Company Stockholders as described in Section 3.16 hereof and the filing and recordation of appropriate merger documents as required by the DGCL). This Agreement has been duly and validly executed and delivered by the Company and, assuming the due authorization, execution and delivery by Parent and Merger Sub, constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, subject to the effect of any applicable bankruptcy, reorganization, insolvency, moratorium or similar Laws affecting creditors’ rights generally and subject, as to enforceability, to the effect of general principles of equity.
SECTION 3.06 No Conflict; Required Filings and Consents.
(a) The execution and delivery of this Agreement by the Company do not, the performance of this Agreement by the Company will not, and the consummation of the Merger and the other transactions contemplated hereby will not, (i) conflict with or violate the Certificate of Incorporation or Bylaws of the Company, (ii) assuming that all consents, approvals, authorizations and other actions
A-9
Table of Contents
described in Section 3.06(b)(i) and Section 3.06(b)(ii) have been obtained and all filings and obligations described in Section 3.06(b)(i) and Section 3.06(b)(ii) have been made or complied with, conflict with or violate in any material respect any foreign or domestic (Federal, state or local) law, statute, ordinance, franchise, permit, concession, license, writ, rule, regulation, order, injunction, judgment or decree (“Law”) applicable to the Company or by which any property or asset of the Company is bound or affected, or (iii) conflict with, result in any breach of or constitute a default (or an event which with notice or lapse of time or both would become a default) under, result in the loss of any benefit under, require consent, approval or notice under, give to others any right of termination, amendment, acceleration or cancellation of, require any payment under, or result in the creation of a lien or other encumbrance on, or the forfeiture of, any material property or asset of the Company pursuant to, any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which the Company is a party.
(b) The execution and delivery of this Agreement by the Company do not, and the performance of this Agreement by the Company will not, require any consent, approval, order, permit, or authorization from, or registration, notification or filing with, any domestic or foreign governmental, regulatory or administrative authority, agency or commission, any court, tribunal or arbitral body, or any quasi-governmental or private body exercising any regulatory, taxing, importing or other governmental authority (a “Governmental Entity”), except (i) for the pre-merger notification requirements of the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and the rules and regulations promulgated thereunder (the “HSR Act”) and (ii) for the filing and recordation of appropriate merger documents as required by the DGCL.
SECTION 3.07 Permits; Compliance.
(a) The Company is in possession of all material franchises, grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental Entity (other than the FDA (as defined below)) necessary for the Company to own, lease and otherwise hold and operate its properties and other assets and to carry on its business as it is now being conducted, and to sell, market, develop, distribute, import and manufacture Factive (the “Non-FDA Company Permits”). All Non-FDA Company Permits are in full force and effect and will remain so, in all material respects, after the Closing and no suspension or cancellation of any Non-FDA Company Permit is pending or, to the knowledge of the Company, threatened. The Company is in compliance in all material respects with the Non-FDA Company Permits and has not received any written notice or other written communication from any Governmental Entity regarding (i) any actual or possible violation of or failure to comply with any term or requirement of any Non-FDA Company Permit, or (ii) any actual or possible revocation, withdrawal, suspension, cancellation, termination or modification of any Non-FDA Company Permit.
(b) The Company holds all new drug applications, abbreviated new drug applications, product license applications, and investigational new drug applications, product export applications and other approvals issuable by the U.S. Food and Drug Administration (the “FDA”) (the “FDA Company Permits”), necessary for the conduct of the business as currently conducted and for the sale, marketing, development, distribution, importation and manufacturing of Factive in the United States and no such FDA Company Permits have been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified in any adverse manner, other than immaterial adverse modifications. Licensor (as defined below in Section 3.12(xvii)) holds all FDA Company Permits necessary for the manufacture and exportation to the United States of Factive and no such FDA Company Permit has, to the knowledge of the Company, been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified in any adverse manner, other than immaterial adverse modifications. The Company is not aware of the existence of, or any basis for, any limitation on the ability of Licensor to manufacture and export Factive in accordance with the Company’s specifications. The Company is in compliance in all material respects with the FDA Company Permits and has not received any written notice or other written communication from the FDA regarding (i) any actual or possible violation of or failure to comply with any term or requirement of any FDA Company Permits or (ii) any actual or possible revocation, withdrawal, suspension, cancellation, termination or modification of any FDA Company Permit. Except for the information and files identified on Section 3.07(b)(X) of the Company Disclosure Schedule, the Company has made available to Parent all information in its possession or control relating to Factive and the development, manufacture and sale of Factive, including without limitation, complete and correct copies of the following (to the extent there are any): (i) adverse event reports; clinical study reports and material study data; and FDA inspection reports, notices of adverse findings, warning letters, FDA filings and letters and other correspondence with the FDA and (ii) similar reports, study data, notices, letters, filings and correspondence with any other Governmental Entity. The information and files identified on Section 3.07(b)(X) of the Company Disclosure Schedule do not reflect circumstances regarding the matters referred to therein that are more unfavorable to the Company than the information regarding such matters contained in the materials made available to Parent pursuant to the immediately preceding sentence.
A-10
Table of Contents
SECTION 3.08 Financial Statements.
(a) True and complete copies of (i) the audited consolidated balance sheet of the Company as of December 31, 2001, and the related audited statements of operations, changes in stockholders’ equity and changes in cash flows for the year then ended, together with all related notes and schedules thereto and (ii) the unaudited consolidated balance sheet of the Company as of December 31, 2002, and the related audited statements of operations, changes in stockholders’ equity and changes in cash flows for the years then ended, together with all related notes and schedules thereto ((i) and (ii) collectively referred to herein as the “Company AuditedFinancial Statements”), and (ii) the unaudited consolidated balance sheet of the Company as of September 30, 2003 (the “CompanyReference Balance Sheet”), and the related statements of operations and changes in cash flows for the nine months ended September 30, 2003 (collectively referred to herein as the “Company Interim Financial Statements”), are attached as Section 3.08(a) of the Company Disclosure Schedule. The Audited Financial Statements and the Company Interim Financial Statements (including, in each case, any notes thereto) were prepared in accordance with United States generally accepted accounting principles (“U.S. GAAP”) applied on a consistent basis throughout the periods indicated (except as may be indicated in the notes thereto or, in the case of unaudited statements, as permitted by U.S. GAAP) and each present fairly, in all material respects, the consolidated financial position of the Company as at the respective dates thereof and for the respective periods indicated therein, except as otherwise noted therein (subject, in the case of unaudited statements, to normal and recurring year-end adjustments which were not and are not expected, individually or in the aggregate, to be material).
(b) Except as set forth in Section 3.08(b) of the Company Disclosure Schedule, the Company does not have any debts, liabilities or obligations (“Liabilities”), other than Liabilities (i) recorded or reserved against on the Company Reference Balance Sheet and set forth in the footnotes thereto and (ii) incurred since September 30, 2003 in the ordinary course of the Company’s business, consistent with past practice.
SECTION 3.09 Absence of Certain Changes or Events. Since January 1, 2003, except as contemplated by or as disclosed in this Agreement (including the Company Disclosure Schedule), the Company has conducted its business only in the ordinary course and in a manner consistent with past practice and, since such date, there has not been any Company Material Adverse Effect nor any action reasonably likely to result in a Company Material Adverse Effect, nor any action that, if taken after the date hereof, would violate Section 5.01.
SECTION 3.10 Absence of Litigation. Except as set forth on Schedule 3.10 of the Company Disclosure Schedule, there is no litigation, suit, claim, action, proceeding or investigation pending involving the Company (whether or not before any Governmental Entity (a “Legal Proceeding”)) or, to the knowledge of the Company, any threat or any basis for any of the foregoing.
SECTION 3.11 Employee Benefit Plans; Labor Matters.
(a) Schedule 3.11(a) of the Company Disclosure Schedule lists, in all cases whether or not reduced to writing and whether covering a single individual or group, (i) all employee benefit plans (as defined in Section 3(3) of the Employee Retirement Income Security Act of 1974, as amended (“ERISA”)) and all bonus, stock option, stock purchase, stock appreciation right, restricted stock, phantom stock, incentive, deferred compensation, retiree medical, disability or life insurance, cafeteria benefit, dependent care, disability, director or employee loan, fringe benefit, sabbatical, supplemental retirement, severance or other benefit plans, programs or arrangements, and all employment, termination, severance or other contracts or agreements to which the Company is a party, with respect to which the Company has any obligation or which are maintained, contributed to or sponsored by the Company for the benefit of any current or former employee, officer or director of the Company, or any consultant or other independent contractor who provides services to or for the benefit of the Company, (ii) each employee benefit plan for which the Company could incur liability under Section 4069 of ERISA in the event such plan has been or were to be terminated, (iii) any plan in respect of which the Company could incur liability under Section 4212(c) of ERISA, and (iv) any employment agreements or other contracts between the Company and any employee of the Company (each, a “Company Plan,” and collectively, the “Company Plans”).
(b) The Company has made available to Parent a true and complete copy of each Company Plan and, to the extent applicable, a true and complete copy of (i) each trust or other funding arrangement including any insurance policies, (ii) each summary plan description and summary of material modifications, (iii) the three (3) most recent annual reports (Form 5500 series and all schedules and financial statements attached thereto), if any, required under ERISA or the Code in connection with each Company Plan, (iv) the most recently received Internal Revenue Service determination letter for each Company Plan intended to qualify under the Code,
A-11
Table of Contents
(v) the most recently prepared actuarial report or financial statement in connection with each such Company Plan, (vi) each form of notice of grant and stock option agreement used to document Company Options, (vii) custodial agreements, administrative services agreements, investment management or investment advisory agreements, and (viii) employee handbooks.
(c) None of the Company Plans is subject to Title IV of ERISA and no corporation, trust, partnership or other entity that would be considered a single employer with the Company under Section 4001(b)(1) of ERISA or Section 414 of the Code (a “CompanyERISA Affiliate”) has ever maintained a plan subject to Title IV of ERISA.
(d) Except as set forth in Company Disclosure Schedule 3.11(d), none of the Company Plans provides for the payment of separation, severance, termination or similar benefits to any person. Neither the execution and delivery of this Agreement nor the consummation of the transactions contemplated hereby, either alone or together with a termination of service, will (i) result in any payment (including, without limitation, severance, unemployment compensation, golden parachute, forgiveness of indebtedness or otherwise) becoming due under any Company Plan, whether or not such payment is contingent, (ii) increase any benefits otherwise payable under any Company Plan or other arrangement, (iii) result in the acceleration of the time of payment, vesting or funding of any benefits including, but not limited to, the acceleration of the vesting and exercisability of any Company Option, whether or not contingent, or (iv) affect in any material respects any Company Plan’s current treatment under any Laws, including any Tax or social contribution Law. No Company Plan provides, or reflects or represents any liability to provide, retiree health, retiree disability, or retiree life insurance benefits to any person for any reason, except as may be required by the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended (“COBRA”), or other applicable statute, and the Company has never represented, promised or contracted (whether in oral or written form) to any employee (either individually or to employees as a group) or any other person that such employee or other person would be provided with retiree health, retiree disability, or retiree life insurance benefits, except to the extent required by statute. Nothing has occurred with respect to any Company Plan described in Section 4980B of the Code that could subject the Company to a tax under Section 4980B of the Code.
(e) Each Company Plan has been operated in accordance with its terms and the requirements of all applicable Laws, regulations and rules promulgated thereunder including, without limitation, ERISA and the Code. No action, claim or proceeding is pending or, to the knowledge of the Company, threatened with respect to any Company Plan (other than claims for benefits in the ordinary course), and, to the knowledge of the Company, no fact or event exists that could give rise to any such action, claim or proceeding. Neither the Company nor any Company ERISA Affiliate is subject to any penalty or Tax with respect to any Company Plan under Section 502(i) of ERISA, Section 4972 of the Code or Sections 4975 through 4980 of the Code. No Company Plan is under examination by the IRS, the DOL or any other Government Authority. Neither the Company nor any Company Plan has any pending application or filing before the IRS, the DOL or any other Government Authority pursuant to any voluntary compliance, amnesty or similar program sponsored by the IRS, the DOL or any other Government Authority.
(f) Each Company Plan intended to qualify under Section 401(a) or Section 401(k) of the Code and each trust intended to qualify under Section 501(a) of the Code (i) has received a favorable determination, opinion, notification or advisory letter from the Internal Revenue Service with respect to each such Company Plan as to its qualified status under the Code, including all amendments to the Code effected by the Tax Reform Act of 1986 and subsequent legislation, and no fact or event has occurred since the date of such determination letter or letters from the Internal Revenue Service that is reasonably likely to adversely affect the qualified status of any such Company Plan or the exempt status of any such trust, or (ii) has remaining a period of time under applicable Treasury regulations or Internal Revenue Service pronouncements in which to apply for such a letter and make any amendments necessary to obtain a favorable determination as to the qualified status of each such Company Plan.
(g) All contributions, premiums or payments required to be made or accrued with respect to any Company Plan have been made on or before their due dates.
(h) Except as set forth in Section 3.11(h) of the Company Disclosure Schedule, (i) the Company is not a party to any collective bargaining agreement or other labor union contract applicable to persons employed by the Company or in the Company’s business, and as of the date of this Agreement, to the knowledge of the Company, there are no organizational campaigns, petitions or other unionization activities seeking recognition of a collective bargaining unit that could affect the Company; (ii) there are no controversies, strikes, slowdowns or work stoppages pending or, to the knowledge of the Company, threatened between the Company and any of its employees, and the Company has not experienced any such controversy, strike, slowdown or work stoppage within the
A-12
Table of Contents
past three years; (iii) the Company has not breached or otherwise failed to comply with the provisions of any collective bargaining or union contract and there are no grievances outstanding against the Company under any such agreement or contract; (iv) the Company is not a party to, or otherwise bound by, any consent decree with, or citation by, any Governmental Entity relating to employees or employment practices; (v) there is no charge or proceeding with respect to a violation of any occupational safety or health standards that has been asserted or is now pending with respect to the Company; and (vi) there is no charge of discrimination in employment or employment practices for any reason, including, without limitation, age, gender, race, religion or other legally protected category, which has been asserted or, to the knowledge of the Company, threatened against the Company or that is now pending before the United States Equal Employment Opportunity Commission or any other Governmental Entity. Each individual who currently provides, or formerly provided, services to the Company or any Company ERISA Affiliate and who is or was categorized by the Company as an independent contractor is and was at all times properly categorized as such and does not or did not, as the case may be, constitute a common law employee of the Company or any Company ERISA Affiliate during any time that such individual is or was categorized as an independent contractor.
SECTION 3.12 Contracts.
(a) Section 3.12(a) of the Company Disclosure Schedule lists each of the following written or oral contracts and agreements to which the Company is a party or otherwise subject or bound or to which or by which any property, business, operation or right of the Company is subject or bound as of the date hereof (such contracts and agreements being the “Material Company Contracts”):
(i) each contract and agreement for the purchase or lease of personal property with any supplier or for the furnishing of services to the Company with payments greater than $25,000 per year;
(ii) all broker, exclusive dealing or exclusivity, distributor, dealer, manufacturer’s representative, franchise, agency, sales promotion, market research, marketing, consulting and advertising contracts to which the Company is a party or any other contract that compensates any person based on any sales by the Company;
(iii) all leases and subleases of real property, including without limitation, any capital lease;
(iv) all leases, licenses or sublicenses of any asset, including any intellectual property not listed on Schedule 3.14 of the Company Disclosure Schedule, other than nonexclusive leases, licenses or sublicenses of commercially available software that the Company has procured through shrink wrap, click wrap or purchase orders;
(v) all employment agreements and employment contracts that have an aggregate future liability in excess of $25,000, other than offer letters in a standard form that do not provide for any severance or bonus payments or provide for the grant of equity incentives not pursuant to the existing Company Plans;
(vi) all contracts and agreements for any joint venture, partnership or similar arrangement;
(vii) all contracts and agreements under which the Company has advanced or loaned an amount to any of its affiliates or employees other than in the ordinary course of business;
(viii) any contract or agreement relating to the acquisition or disposition of (a) any business of the Company (whether by merger, consolidation or other business combination, sale of securities, sale of assets or otherwise) or (b) any asset (tangible or intangible) other than sales of supplies pursuant to purchase orders in the ordinary course of business;
(ix) any contract or agreement under which the Company is, or may become, obligated to pay an amount in respect of indemnification obligations, purchase price adjustment or otherwise in connection with any (a) acquisition or disposition of assets or securities (other than the sale of inventory in the ordinary course of business), (b) merger, consolidation or other business combination or (c) series or group of related transactions or events of the type specified in clauses (a) and (b) above;
(x) all contracts and agreements relating to indebtedness other than trade indebtedness of the Company in an aggregate amount not to exceed $100,000, including any contracts and agreements in which the Company is a guarantor of indebtedness;
(xi) all contracts and agreements under which any person has guaranteed any indebtedness of the Company;
(xii) all contracts and agreements that limit or purport to limit the ability of the Company to compete in any line of business or with any person or in any geographic area or during any period of time;
(xiii) all contracts and agreements relating to the voting and any rights or obligations of a stockholder of the Company;
A-13
Table of Contents
(xiv) all contracts or agreements under which the Company is, or may become, obligated to incur any severance pay or other compensation obligations and all profit sharing, stock option, stock purchase, stock appreciation, deferred compensation, or other plan or arrangement for the benefit of the Company’s current or former directors, officers and employees;
(xv) all contracts regarding the acquisition, issuance or transfer of any securities of the Company and each contract affecting or dealing with any securities of the Company, including, without limitation, any restricted stock agreements or escrow agreements;
(xvi) any agreement of guarantee, assumption or endorsement of, or any similar commitment with respect to, the obligations, liabilities (whether accrued, absolute, contingent or otherwise) or indebtedness of any person other than software licenses or professional services contracts entered into in the ordinary course of business; and
(xvii) all contracts and agreements with Licensor (as defined hereinafter), including, without limitation, the License and Option Agreement by and between the Company and LG Life Sciences, Ltd. (“Licensor”) dated as of October 22, 2002, as amended by Amendment No. 1 to the License and Option Agreement dated November 21, 2002, Amendment No. 2 to the License and Option Agreement dated December 6, 2002, and Amendment No. 3 to the License and Option Agreement dated October 16, 2003 (the “License Agreement”).
(b) Each Material Company Contract (i) is legal, valid and binding on the Company, and, to the knowledge of the Company, on the other parties thereto, is in full force and effect, and is enforceable against the Company and, to the knowledge of the Company, is enforceable against each other party thereto and (ii) upon consummation of the transactions contemplated by this Agreement, shall continue in full force and effect without penalty or other adverse consequence. The Company is not in breach or violation of, or default under, any Material Company Contract and, to the knowledge of the Company, no other party to any Material Company Contract is in breach or violation thereof or default thereunder. The Company has made available to Parent true, accurate and complete copies of each written agreement set forth on Schedule 3.12 of the Company Disclosure Schedule, in each case, as amended or otherwise modified and in effect. There are no payments due and unpaid by the Company under any Material Company Contract, whether or not any other party thereto has agreed to forbear or waive any rights in connection with such non-payment (other than payments set forth on Section 3.12(b) of the Company Disclosure Schedule and identified as not being made due to the amount in dispute).
SECTION 3.13 Environmental Matters.
(a) The Company (i) is in compliance with all applicable Environmental Laws (as defined below), (ii) holds all Environmental Permits (as defined below) necessary to conduct the Company’s business, all of which are set forth on Schedule 3.13 of the Company Disclosure Schedule and (iii) is in compliance with its Environmental Permits.
(b) The Company has not released, and no other person has released, Hazardous Materials (as defined below) on, upon, into or from any real property owned or leased by the Company or, during their ownership or occupancy of such property, on any property formerly owned, operated or leased by the Company.
For purposes of this Agreement:
“Environmental Laws” means any Federal, state or local statute, law, ordinance, regulation, rule, code or order of the United States, or any other jurisdiction and any enforceable judicial or administrative interpretation thereof, including any judicial or administrative order, consent decree or judgment, relating to pollution or protection of the environment, human health or natural resources, including, without limitation, those relating to the use, handling, transportation, treatment, storage, disposal, assessment, cleanup, release or discharge of Hazardous Materials, as in effect as of the date of this Agreement.
“Environmental Permits” means any permit, approval, identification number, license and other authorization required under any applicable Environmental Law.
“Hazardous Materials” means (i) any petroleum, petroleum products, by-products or breakdown products, radioactive materials, asbestos-containing materials or polychlorinated biphenyls or (ii) any chemical, material or substance defined or regulated or identified as toxic or hazardous or as a pollutant or contaminant or waste under any applicable Environmental Law.
A-14
Table of Contents
SECTION 3.14 Intellectual Property.
(a) The Company owns, is licensed for, or possesses sufficient rights with respect to, all Company Intellectual Property (as defined below) (i) that is material to the business of the Company, (ii) that is necessary to conduct any such business, as such business is presently conducted and (iii) as required for the sale of Factive in the United States or in any other country in which the License Agreement purports to grant to the Company the right to sell Factive. The Company has not received any written communication alleging that the Company or Company Intellectual Property infringes or misappropriates any right of a third party (an “Infringement”), nor is the Company aware of any Infringement. The Company has not infringed upon or misappropriated, and the sale by the Company, Parent or a sub-licensee of Factive will not infringe or misappropriate, any Intellectual Property right of any third party. The Company has not brought or threatened any action, suit or proceeding against any third party for any Infringement of any Company Intellectual Property or any breach of any license, sublicense or agreement involving Company Intellectual Property and is not aware of a bona fide basis for such a proceeding. “Intellectual Property” means (i) inventions (whether or not patentable); trade names, trade and service marks, logos, domains, URLs, websites, addresses and other designations (“Marks”); works of authorship; mask works; data; technology, know-how, trade secrets, ideas and information; designs; formulas; algorithms; processes; methods; schematics; computer software (in source code and/or object code form); and all other intellectual property of any sort (“Inventions”) and (ii) patent rights; Mark rights; copyrights; mask work rights;sui generis database rights; trade secret rights; and all other intellectual and industrial property rights of any sort throughout the world, and all applications, registrations, issuances and the like with respect thereto (“IP Rights”). “Company Intellectual Property” means all Intellectual Property that was or is used, exercised, or exploited (“Used”) in any business of the Company, or that is necessary to conduct any such business, as such business is presently conducted or as required for the sale of Factive in the United States or in any other country in which the License Agreement purports to grant to the Company the right to sell Factive. With respect to patent rights and mark rights, and notwithstanding anything to the contrary, the representations and warranties of this Section 3.14(a) are made only to the Company’s knowledge and without having conducted any special investigation or patent or trademark search.
(b) To the extent included in Company Intellectual Property (but excluding Intellectual Property licensed to the Company only on a nonexclusive basis), Section 3.14(b) of the Company Disclosure Schedule lists (by name, number, jurisdiction and owner) all issued patents and patent applications; all registered Marks; and all registered copyrights and mask works. No cancellation, termination, expiration or abandonment of any of the foregoing (except natural expiration or termination at the end of the full possible term, including extensions and renewals) has occurred or is anticipated by the Company. Except as referenced in written documentation previously provided to Parent (including without limitation file wrappers), the Company is not aware of any material questions or challenges with respect to the validity of any claims of any of the foregoing patents or the validity (or any other aspect or status) of any such IP Rights.
(c) Section 3.14(c) of the Company Disclosure Schedule lists: (i) all licenses, sublicenses and other agreements to which the Company is a party that assign, authorize to Use, encumber, or give access to any Company Intellectual Property to a third party other than access provided under a standard form nondisclosure/nonuse agreement; (ii) all licenses, sublicenses and other agreements pursuant to which the Company has received rights to Use any third party Intellectual Property other than commercially available shrink wrap software; and (iii) all Marks. The Company has not entered into any agreement to indemnify, hold harmless or defend any other person with respect to any assertion of Infringement, other than indemnification provisions contained in agreements used in transactions arising in the ordinary course of business (including, but not limited to, purchase and supply agreements, distribution agreements, and service agreements). No event or circumstance has occurred or exists (including, without limitation, the authorization, execution or delivery of this Agreement or the consummation of any of the transactions contemplated hereby) that would result in a material breach or material violation by the Company of any license, sublicense or other agreement required to be listed in Section 3.14(c) of the Company Disclosure Schedule or, to the Company’s knowledge, in such a breach or violation of any such license, sublicense or other agreement by any other party thereto.
(d) The Company has taken reasonable steps to protect and preserve the confidentiality of all Company Intellectual Property with respect to which the Company has exclusivity and is not otherwise disclosed in published patents or patent applications or registered copyrights (“Company Confidential Information”). Each current and former employee and contractor of the Company who contributed to the creation or development of any Company Intellectual Property executed an agreement in substantially the form of the Company’s standard Proprietary Information and Inventions Agreement (in the case of an employee) or Consulting Agreement (in the case of a contractor), except where failure to do so would not be reasonably likely to have a Company Material Adverse Effect.
A-15
Table of Contents
SECTION 3.15 Taxes.
(a) All material Tax (as defined below) returns, statements, reports, declarations and other forms and documents (including without limitation estimated Tax returns and reports and material information returns and reports) required to be filed with any Tax Authority (as defined below) before the Closing (after giving effect to any extensions of such due date) (collectively, “Tax Returns” and individually, a “Tax Return”), by or on behalf of the Company, have been or will be completed and filed when due (after giving effect to any extensions of such due date) and all such Tax Returns are true, correct, and complete in all material respects. All amounts shown due on such Tax Returns on or before the Effective Time, and all other material Taxes (whether or not shown on any Tax Return) that are due on or before the Effective Time, have been or will be paid on or before such date. The Company Interim Financial Statements (i) accrue on the face of such Company Interim Financial Statements (rather than in any footnote thereto) all material actual and contingent liabilities for Taxes (as defined below) with respect to all periods through September 30, 2003 and the Company has not and will not incur any Tax liability in excess of the amount reflected (excluding any amount thereof that reflects timing differences between the recognition of income for purposes of U.S. GAAP and for Tax purposes) on the face of the Company Reference Balance Sheet included in the Company Interim Financial Statements (rather than in any footnote thereto) with respect to such periods, and (ii) properly accrue in accordance with U.S. GAAP all material liabilities for Taxes payable after September 30, 2003, with respect to all transactions and events occurring on or prior to such date. The Company has not incurred any material Tax liability since September 30, 2003 other than (i) in the ordinary course of business or (ii) that may result from potential discharge of indebtedness income in connection with the transactions contemplated by the Note Amendment and Exchange Agreement and the Company has made adequate provisions for all Taxes since that date in accordance with U.S. GAAP on at least a quarterly basis.
(b) The Company has withheld and paid to the applicable financial institution or Tax Authority all amounts required to be withheld by the Company, and the Company has complied with all material Tax reporting and recordkeeping requirements. To the best knowledge of the Company, no Tax Returns filed with respect to Taxable years through the Taxable year ended December 31, 2002 have been examined by any Tax Authority. The Company has not granted any extension or waiver of the limitation period applicable to any Tax Returns that is still in effect and there is no material claim, audit, action, suit, proceeding, or (to the knowledge of the Company) investigation now pending or threatened in writing against or with respect to the Company in respect of any Tax or assessment. No notice of deficiency or similar document of any Tax Authority has been received by the Company, and there are no liabilities for Taxes with respect to the issues that have been raised (and are currently pending) by any Tax Authority that could, if determined adversely to the Company, materially and adversely affect the liability of the Company for Taxes. There are no liens for Taxes (other than for current Taxes not yet due and payable) upon the assets of the Company. The Company has never been a party (either as a distributing corporation, a distributed corporation or otherwise) to any transaction intended to qualify under Section 355 of the Code or any corresponding provision of state Law. The Company has previously provided or made available to Parent true and complete copies of all income, franchise, and sales Tax Returns. The Company has not made any payments, or has been or is a party to any agreement, contract, arrangement or plan that could result in it making payments, that have resulted or would result, separately or in the aggregate, in the payment of any “excess parachute payment” within the meaning of Section 280G of the Code or in the imposition of an excise Tax under Section 4999 of the Code (or any corresponding provisions of state, local, or foreign Tax law) or that were not or would not be deductible under Section 162 (other than 162(a)) or Section 404 of the Code. The Company does not have any liability for the Taxes of any person under Treas. Reg. §1.1502-6 (or any similar provision of state, local, or foreign law), as a transferee or successor, by contract or otherwise.
(c) For purposes of this Agreement, the following terms have the following meanings: “Tax” (and, with correlative meaning, “Taxes” and “Taxable”) means any and all taxes including, without limitation, any net income, alternative or add-on minimum tax, gross income, gross receipts, sales, use, ad valorem, transfer, franchise, profits, value added, net worth, license, withholding, payroll, employment, excise, severance, stamp, occupation, premium, property, environmental or windfall profit tax, custom, duty or other tax, governmental fee or other like assessment or charge of any kind whatsoever in the nature of a tax, together with any interest or any penalty, addition to tax or additional amount imposed by any Governmental Entity responsible for the imposition of any such tax (domestic or foreign) (a “Tax Authority”).
SECTION 3.16 Vote Required. The only vote of the holders of any classes or series of capital stock of the Company necessary to approve and adopt this Agreement, the Merger and the other transactions contemplated by this Agreement is the affirmative vote of the holders of at least a majority of the outstanding shares of the Company Stock in favor of the approval and adoption of this Agreement and the Merger.
A-16
Table of Contents
SECTION 3.17 Assets; Absence of Liens and Encumbrances. Except as set forth in Section 3.17 of the Company Disclosure Schedule, the Company owns, leases or has the legal right to use all of the material assets, properties and rights of every kind, nature, character and description, including, without limitation, real property and personal property (other than Intellectual Property, which is covered by Section 3.14 hereof), used in the conduct of the business of the Company or otherwise owned or leased by the Company and, with respect to contract rights, is a party to and enjoys the right to the benefits of all material contracts, agreements and other arrangements used by the Company in or relating to the conduct of the business of the Company (all such properties, assets and contract rights being the “Company Assets”). The equipment of the Company used in the operations of their business is, taken as a whole, in good operating condition and repair, ordinary wear and tear excepted.
SECTION 3.18 Owned Real Property. The Company does not own any real property.
SECTION 3.19 Brokers. Except for Merrill Lynch & Co., Inc., no broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the origination, negotiation or execution of this Agreement, the Merger or the other transactions contemplated by this Agreement based upon arrangements made by or on behalf of the Company.
SECTION 3.20 Tax Matters. Neither the Company nor any of its affiliates has taken or agreed to take any action that would prevent the Merger from constituting a reorganization qualifying under Section 368(a) of the Code. The Company is not aware of any agreement, plan or other circumstance that would prevent the Merger from qualifying as a reorganization under Section 368(a) of the Code.
SECTION 3.21 State Takeover Statutes. The Board of Directors of the Company has taken all action necessary to ensure that any restrictions on business combinations contained in the DGCL will not apply to the Merger and the other transactions contemplated by this Agreement. No other “fair price,” “moratorium,” “control share acquisition” or other similar anti-takeover statute or regulation or any anti-takeover provision in the Company’s Certificate of Incorporation or Bylaws is, or at the Effective Time will be, applicable to the Company, the shares of Company Stock, the Merger or the other transactions contemplated by this Agreement.
SECTION 3.22 Accounts Receivable. All accounts and notes receivable reflected on the Company Reference Balance Sheet and all accounts and notes receivable arising subsequent to the date of the Company Reference Balance Sheet and on or prior to the Closing Date, have arisen or will arise in the ordinary course of business, represent or will represent legal, valid, binding and enforceable obligations to the Company and, subject only to consistently recorded reserves for bad debts established as of a date prior to the Closing Date.
SECTION 3.23 Illegal Payments, etc. In the conduct of the business of the Company, the Company has not, and none of its directors, officers, employees or agents, has (a) directly or indirectly, given, or agreed to give, any illegal gift, contribution, payment or similar benefit to any supplier, customer, governmental official or employee or other person who was, is or may be in a position to help or hinder the Company (or assist in connection with any actual or proposed transaction) or made, or agreed to make, any illegal contribution, or reimbursed any illegal political gift or contribution made by any other person, to any candidate for federal, state, local or foreign public office or (b) established or maintained any unrecorded fund or asset or made any false entries on any books or records for any purpose.
SECTION 3.24 Affiliate Transactions. Except for the matters disclosed on Section 3.24 of the Company Disclosure Schedule, no Company Stockholder or any affiliate of any Company Stockholder is an officer, director, employee, consultant, competitor, creditor, debtor, customer, distributor, supplier or vendor of, or is a party to any Material Company Contract with, the Company. Except as disclosed on Section 3.24 of the Company Disclosure Schedule, no Company Stockholder or any affiliate of any Company Stockholder owns any asset used in, or necessary to, the business of the Company.
SECTION 3.25 Employees. Except as disclosed on Section 3.25 of the Company Disclosure Schedule, no employee of the Company is represented by a labor union. Except as set forth on Section 3.25 of the Company Disclosure Schedule, no executive officer or other key employee has been terminated for any reason nor has any such officer or key employee notified the Company of his or her intention to resign or retire since January 1, 2002.
A-17
Table of Contents
SECTION 3.26 Insurance. The insurance policies owned and maintained by the Company are set forth on Section 3.26 of the Company Disclosure Schedule with their respective expiration dates. All such policies, taken as a whole, are in full force and effect, all premiums due and payable thereon have been paid (other than retroactive or retrospective premium adjustments that are not yet, but may be, required to be paid with respect to any period ending prior to the Closing Date), and no notice of cancellation or termination has been received with respect to any such policy which has not been replaced on substantially similar terms prior to the date of such cancellation. Except as set forth on Section 3.26 of the Company Disclosure Schedule, no insurer (a) has questioned, denied or disputed (or otherwise reserved its rights with respect to) the coverage of any claim pending under any such policy or (b) has threatened to cancel any such policy.
SECTION 3.27 Factive Supply. Section 3.27 of the Company Disclosure Schedule sets forth the quantity, location and owner of all (a) Final Product and (b) Bulk Product (each as defined below) in existence on the date hereof. All Final Product and Bulk Product referenced on Section 3.27 of the Company Disclosure Schedule is owned free and clear of all liens and Liabilities by the owner identified therein. All of such Final Product and Bulk Product has been manufactured in accordance with all applicable laws and regulations including, without limitation, good manufacturing practice compliance standards and necessary FDA Company Permits and conforms to the specification for Final Product and Bulk Product set forth in the License Agreement. All such Final Product is saleable in the ordinary course of business and all such Bulk Product is fit for processing into Final Product. For purposes of this Section 3.27, “Final Product” shall mean finished products including Gemifloxacin as an active ingredient in final form for commercialization, distribution and sale, labeled in accordance with applicable regulatory requirements and custom and meeting the specifications set forth in the License Agreement, and “Bulk Product” shall mean Gemifloxacin in active bulk form meeting the specifications set forth in the License Agreement.
SECTION 3.28 Disclosure. The representations and warranties contained in this Article III (including, without limitation, the Company Disclosure Schedule) do not contain and will not contain any untrue statement of fact or omit to state any material fact necessary in order to make the statements and information contained therein not misleading.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB
Parent and Merger Sub, jointly and severally, hereby represent and warrant to the Company that the statements contained in this Article IV are true and correct as of the date of this Agreement and as of the Closing Date (except that those representations and warranties that address matters only as of a particular date shall remain true and correct as of such date) in each case, subject to the matters set forth in the Parent SEC Reports where it is clear, upon a reading of such disclosure in the Parent SEC Reports without any independent knowledge on the part of the reader regarding the matter disclosed, that the disclosure in the Parent SEC Reports applies, or the disclosure schedule delivered by Parent to the Company concurrently with the execution of this Agreement (the “Parent Disclosure Schedule”).
SECTION 4.01 Organization and Qualification.
(a) Parent is a corporation duly incorporated, validly existing and in good standing under the laws of the Commonwealth of Massachusetts and has all requisite corporate power and authority to own, lease and otherwise hold and operate its properties and other assets and to carry on its business as it is now being conducted, except where the failure to be so organized, existing or in good standing or to have such corporate power and authority would not be reasonably likely to have, individually or in the aggregate, a Parent Material Adverse Effect (as defined below). Parent is duly qualified or licensed as a foreign corporation to do business, and is in good standing, in each jurisdiction where the character of the properties owned, leased or operated by it or the nature of its business makes such qualification or licensing necessary, except where the failure to be so qualified or licensed and in good standing has not had, and could not reasonably be expected to have, individually or in the aggregate, a Parent Material Adverse Effect. The term “Parent Material Adverse Effect” means any event, change, violation, inaccuracy, circumstance or effect that is, individually or in the aggregate, materially adverse to the condition (financial or otherwise), assets (tangible or intangible), liabilities, or results of operations of Parent and its subsidiaries taken as a whole, except for any such events, changes, violations, inaccuracies, circumstances or effects resulting from or arising in connection with (i) any changes in general economic, political or business conditions and not specifically related to Parent, (ii) any changes or events affecting the industry in which Parent and its subsidiaries operate and not
A-18
Table of Contents
specifically related to Parent, (iii) any matter otherwise known to the Company prior to the date of this Agreement, (iv) any matters resulting from or arising in connection with this Agreement or the transactions contemplated by this Agreement or the announcement hereof, (v) any fees or expenses payable as a result of the performance of the transactions contemplated by this Agreement, (vi) any pre-clinical or clinical results related to any of Parent’s programs or potential products other than Ramoplanin, (vii) any violations or other matter arising from changes in law or generally accepted accounting principles or (viii) sales or other dispositions of Parent properties or assets other than Ramoplanin. Notwithstanding the foregoing, Parent acknowledges that any event, change, violation, inaccuracy, circumstance or effect, including without limitation, the revocation, withdrawal, suspension, cancellation, termination or material adverse modification (or notice of any threatened revocation, withdrawal, suspension, cancellation, termination or modification)of any FDA Parent Permit (as defined in Section 4.07(b)) or Non-FDA Parent Permit (as defined in Section 4.07(a)) related to Ramoplanin, that is individually or in the aggregate, materially adverse to Parent’s ability to sell, have sold, market, develop, distribute, import or manufacture Ramoplanin shall constitute a Parent Material Adverse Effect, provided that the Company acknowledges and agrees that in no event shall the circumstances and events, or any results or changes arising from such circumstances and events, described specifically on Section 4.01 of the Parent Disclosure Schedule be deemed to constitute a Parent Material Adverse Effect.
(b) Merger Sub is a corporation duly incorporated, validly existing and in good standing under the laws of the State of Delaware.
SECTION 4.02 Certificate of Incorporation and Bylaws. Parent has made available to the Company a complete and correct copy of (a) the Certificate of Incorporation and the Bylaws of Parent (or equivalent organizational documents) including all amendments thereto and (b) the minute books containing all consents, actions and meeting of the stockholders of Parent and Parent’s Board of Directors and any committees thereof.
SECTION 4.03 No Subsidiaries. Except as set forth on Section 4.03 of the Parent Disclosure Schedule, Parent does not own, of record or beneficially, or control any direct or indirect equity or other interest, or any right (contingent or otherwise) to acquire the same, in any corporation, partnership, limited liability company, joint venture, association or other entity. References to “Parent” in this Agreement shall be deemed to include all subsidiaries of Parent, if any, unless the context clearly requires otherwise.
SECTION 4.04 Capitalization.
(a) As of the date hereof, the authorized capital stock of Parent consists of 50,000,000 shares of Parent Common Stock, consisting of 49,375,000 shares of common stock, par value $0.10 per share and 625,000 shares of Series B restricted stock, par value $0.10 per share. No shares of Series B restricted stock are outstanding. As of September 27, 2003, (i) 26,172,776 shares of Parent Common Stock were issued and outstanding, all of which are duly authorized, validly issued, fully paid and nonassessable, (ii) 4,213,052 shares of Parent Common Stock were reserved for future issuance pursuant to outstanding, unexercised options to purchase Parent Common Stock (“Parent Options”), and 1,328,179 shares of Parent Common Stock were reserved for future issuance pursuant to Parent Options available for future grant, (iii) 586,646 shares of Parent Common Stock were reserved for issuance pursuant to outstanding, unexercised warrants to purchase Parent Common Stock, (iv) 183,784 shares of Parent Common Stock were reserved for issuance under the Parent’s Employee Stock Purchase Plan (the “Parent Stock Plan”) and (v) 217 shares of Parent Common Stock were reserved for issuance under the Company’s 1997 Directors Deferred Plan.
(b) Except as described in Section 4.04(a) above or as set forth in Section 4.04(b) of the Parent Disclosure Schedule, there are no options, warrants or other rights, agreements, arrangements or commitments of any character, whether or not contingent, relating to the issued or unissued capital stock of Parent or obligating Parent to issue or sell any share of capital stock of, or other equity interest in, Parent. All shares of Parent Common Stock so subject to issuance, upon issuance in accordance with the terms and conditions specified in the instruments pursuant to which they are issuable, will be duly authorized, validly issued, fully paid and nonassessable.
SECTION 4.05 Authority Relative to This Agreement. Each of Parent and Merger Sub has all necessary corporate power and authority to execute and deliver this Agreement, and, subject to obtaining the necessary approvals of the stockholders of Parent, to perform its obligations hereunder and to consummate the Merger and the other transactions contemplated by this Agreement. The execution and delivery of this Agreement by each of Parent and Merger Sub and the consummation by each of Parent and Merger Sub of the Merger and the other transactions contemplated by this Agreement have been duly and validly authorized by all necessary corporate action, and no other corporate proceedings on the part of Parent or Merger Sub are necessary to authorize this Agreement or to consummate the Merger and the other transactions contemplated by this Agreement (other than with respect to the Merger, the
A-19
Table of Contents
filing and recordation of appropriate merger documents as required by the DGCL). This Agreement has been duly and validly executed and delivered by each of Parent and Merger Sub and, assuming the due authorization, execution and delivery by the Company, constitutes a legal, valid and binding obligation of each of Parent and Merger Sub, enforceable against each of Parent and Merger Sub in accordance with its terms, subject to the effect of any applicable bankruptcy, reorganization, insolvency, moratorium or similar Laws affecting creditors’ rights generally and subject, as to enforceability, to the effect of general principles of equity.
SECTION 4.06 No Conflict; Required Filings and Consents.
(a) The execution and delivery of this Agreement by each of Parent and Merger Sub do not, the performance of this Agreement by each of Parent and Merger Sub will not, and the consummation of the Merger and the other transactions contemplated hereby will not, (i) conflict with or violate their respective organizational documents, (ii) assuming that all consents, approvals, authorizations and other actions described in Section 4.06(b) have been obtained and all filings and obligations described in Section 4.06(b) have been made or complied with, conflict with or violate in any material respect any Law applicable to Parent or Merger Sub or by which any property or asset of Parent or Merger Sub is bound or affected, or (iii) conflict with, result in any breach of or constitute a default (or an event which with notice or lapse of time or both would become a default) under, result in the loss of any benefit under, or give to others any right of termination, amendment, acceleration or cancellation of, or result in the creation of a lien or other encumbrance on or forfeiture of, any material property or asset of Parent or Merger Sub pursuant to, any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which Parent or Merger Sub is a party or by which any property or asset of Parent or Merger Sub is bound or affected.
(b) The execution and delivery of this Agreement by each of Parent and Merger Sub do not, and the performance of this Agreement by each of Parent and Merger Sub will not, require any consent, approval, order, authorization, registration or permit of, or filing with or notification to, any Governmental Entity, except (i) for the pre-merger notification requirements of the HSR Act, (ii) for the filing and recordation of appropriate merger documents as required by the DGCL, and (iii) for applicable requirements, if any, of the Exchange Act of 1934, as amended (the “Exchange Act”), Federal and state securities laws (including, without limitation, Section 25121 of the California General Corporation Law) and The Nasdaq National Market.
SECTION 4.07 Permits; Compliance.
(a) Parent is in possession of all material franchises, grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental Entity (other than the FDA) necessary for Parent to own, lease and otherwise hold and operate its properties and other assets and to carry on its business as it is now being conducted, including, without limitation, to conduct the clinical trials of Ramoplanin now being conducted (the “Non-FDA Parent Permits”). All Non-FDA Parent Permits are in full force and effect and will remain so, in all material respects, after the Closing and no suspension or cancellation of any Non-FDA Parent Permit is pending or, to the knowledge of Parent, threatened. Parent is in compliance in all material respects with the Non-FDA Parent Permits and has not received any written notice or other written communication from any Governmental Entity regarding (i) any actual or possible violation of or failure to comply with any term or requirement of any Non-FDA Parent Permit, or (ii) any actual or possible revocation, withdrawal, suspension, cancellation, termination or modification of any Non-FDA Parent Permit.
(b) Parent holds all investigational new drug applications and other approvals issuable by the FDA (the “FDA Parent Permits”), necessary for the conduct of the business as currently conducted, including, without limitation, to conduct the clinical trials of Ramoplanin now being conducted in the United States and no such FDA Parent Permits have been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified in any adverse manner, other than immaterial adverse modifications. Parent is in compliance in all material respects with the FDA Parent Permits and has not received any written notice or other written communication from the FDA regarding (i) any actual or possible violation of or failure to comply with any term or requirement of any FDA Parent Permits or (ii) any actual or possible revocation, withdrawal, suspension, cancellation, termination or modification of any FDA Parent Permit. Parent has made available to the Company all information in its possession or control relating to Ramoplanin and the development and manufacture of Ramoplanin, including without limitation, complete and correct copies of the following (to the extent there are any): adverse event reports; clinical study reports and material study data; and FDA inspection reports, notices of adverse findings, warning letters, FDA filings and letters and other correspondence with the FDA.
A-20
Table of Contents
SECTION 4.08 SEC Filings; Financial Statements.
(a) Parent has filed all forms, reports and documents required to be filed by it with the Securities and Exchange Commission (the “SEC”) since January 1, 2000 through the date of this Agreement (collectively, the “Parent SEC Reports”). As of the respective dates they were filed (and if amended or superceded by a filing prior to the date of this Agreement, then on the date of such filing), (i) the Parent SEC Reports complied in all material respects with the requirements of the Securities Act of 1933, as amended (the “Securities Act”) or the Exchange Act, as the case may be, and (ii) none of the Parent SEC Reports contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading.
(b) Each of the consolidated financial statements (including, in each case, any notes thereto) contained in the Parent SEC Reports was prepared in accordance with U.S. GAAP applied on a consistent basis throughout the periods indicated (except as may be indicated in the notes thereto or, in the case of unaudited statements, as permitted by Form 10-Q or 8-K promulgated by the SEC) and each presented fairly, in all material respects, the consolidated financial position of Parent and its consolidated subsidiaries as at the respective dates thereof and for the respective periods indicated therein, except as otherwise noted therein (subject, in the case of unaudited statements, to normal and recurring year-end adjustments which would not, individually or in the aggregate, have a Parent Material Adverse Effect).
SECTION 4.09 Absence of Certain Changes. Since January 1, 2003, except as contemplated by or as disclosed in this Agreement (including the Parent Disclosure Schedule), Parent has conducted its business only in the ordinary course and in a manner consistent with past practice and, since such date, there has not been a Parent Material Adverse Effect nor any action reasonably likely to result in a Parent Material Adverse Effect, nor any action that, if taken after the date hereof, would violate Section 5.02.
SECTION 4.10 Absence of Litigation .. Other than as set forth on Section 4.10 of the Parent Disclosure Schedule, there is no litigation, suit, claim, action, hearing, investigation or proceeding pending involving Parent or Merger Sub (whether or not before any Governmental Entity) or, to the knowledge of Parent, any threat or basis for any of the foregoing.
SECTION 4.11 Employee Benefit Plans; Labor Matters.
(a) Schedule 4.11(a) of the Parent Disclosure Schedule lists, in all cases whether or not reduced to writing and whether covering a single individual or group, (i) all employee benefit plans (as defined in Section 3(3) of ERISA) and all bonus, stock option, stock purchase, stock appreciation right, restricted stock, phantom stock, incentive, deferred compensation, retiree medical, disability or life insurance, cafeteria benefit, dependent care, disability, director or employee loan, fringe benefit, sabbatical, supplemental retirement, severance or other benefit plans, programs or arrangements, and all employment, termination, severance or other contracts or agreements to which Parent is a party, with respect to which Parent has any obligation or which are maintained, contributed to or sponsored by Parent for the benefit of any current or former employee, officer or director of Parent, or any consultant or other independent contractor who provides services to or for the benefit of Parent, (ii) each employee benefit plan for which Parent could incur liability under Section 4069 of ERISA in the event such plan has been or were to be terminated, (iii) any plan in respect of which Parent could incur liability under Section 4212(c) of ERISA, and (iv) any employment agreements or other contracts between Parent and any employee of Parent (each, a “Parent Plan,” and collectively, the “Parent Plans”).
(b) Parent has made available to the Company a true and complete copy of each Parent Plan and, to the extent applicable, a true and complete copy of (i) each trust or other funding arrangement including any insurance policies, (ii) each summary plan description and summary of material modifications, (iii) the three (3) most recent annual reports (Form 5500 series and all schedules and financial statements attached thereto), if any, required under ERISA or the Code in connection with each Parent Plan, (iv) the most recently received Internal Revenue Service determination letter for each Parent Plan intended to qualify under the Code, (v) the most recently prepared actuarial report and financial statement in connection with each such Parent Plan, (vi) each form of notice of grant and stock option agreement used to document Parent Options, and (vii) custodial agreements, administrative services agreements, investment management or investment advisory agreements, and (viii) employee handbooks.
(c) None of the Parent Plans is subject to Title IV of ERISA and no corporation, trust, partnership or other entity that would be considered a single employer with Parent under Section 4001(b)(1) of ERISA or Section 414 of the Code (a “Parent ERISA Affiliate”) has ever maintained a plan subject to Title IV of ERISA.
A-21
Table of Contents
(d) Except as set forth in Section 4.11(d) of the Parent Disclosure Schedule, none of the Parent Plans provides for the payment of separation, severance, termination or similar benefits to any person. Neither the execution and delivery of this Agreement nor the consummation of the transactions contemplated hereby, either alone or together with a termination of service, will (i) result in any payment (including, without limitation, severance, unemployment compensation, golden parachute, forgiveness of indebtedness or otherwise) becoming due under any Parent Plan, whether or not such payment is contingent, (ii) increase any benefits otherwise payable under any Parent Plan or other arrangement, (iii) result in the acceleration of the time of payment, vesting or funding of any benefits including, but not limited to, the acceleration of the vesting and exercisability of any Parent Option, whether or not contingent, or (iv) affect in any material respects any Parent Plan’s current treatment under any Laws, including any Tax or social contribution Law. No Parent Plan provides, or reflects or represents any liability to provide, retiree health, retiree disability, or retiree life insurance benefits to any person for any reason, except as may be required by COBRA, or other applicable statute, and Parent has never represented, promised or contracted (whether in oral or written form) to any employee (either individually or to employees as a group) or any other person that such employee or other person would be provided with retiree health, retiree disability, or retiree life insurance benefits, except to the extent required by statute. Nothing has occurred with respect to any Parent Plan described in Section 4980B of the Code that could subject Parent to a tax under Section 4980B of the Code. Except as set forth on Section 4.11(d) of the Parent Disclosure Schedule, the consummation of the transactions contemplated by Article I of this Agreement will not be deemed an involuntary termination for any officer of director of Parent such that such officer or director is entitled to severance benefits or acceleration or other changes of the vesting provisions for any securities held by such officer or director, in each case, solely as a result of the consummation of the transactions contemplated by Article I of this Agreement.
(e) Each Parent Plan has been operated in accordance with its terms and the requirements of all applicable Laws, regulations and rules promulgated thereunder including, without limitation, ERISA and the Code. No action, claim or proceeding is pending or, to the knowledge of Parent, threatened with respect to any Parent Plan (other than claims for benefits in the ordinary course), and, to the knowledge of Parent, no fact or event exists that could give rise to any such action, claim or proceeding. Neither Parent nor any Parent ERISA Affiliate is subject to any penalty or Tax with respect to any Parent Plan under Section 502(i) of ERISA, Section 4972 of the Code or Sections 4975 through 4980 of the Code. No Parent Plan is under examination by the IRS, the DOL or any other Government Authority. Neither Parent nor any Parent Plan has any pending application or filing before the IRS, the DOL or any other Government Authority pursuant to any voluntary compliance, amnesty or similar program sponsored by the IRS, the DOL or any other Government Authority.
(f) Each Parent Plan intended to qualify under Section 401(a) or Section 401(k) of the Code and each trust intended to qualify under Section 501(a) of the Code (i) has received a favorable determination, opinion, notification or advisory letter from the Internal Revenue Service with respect to each such Parent Plan as to its qualified status under the Code, including all amendments to the Code effected by the Tax Reform Act of 1986 and subsequent legislation, and no fact or event has occurred since the date of such determination letter or letters from the Internal Revenue Service that is reasonably likely to adversely affect the qualified status of any such Parent Plan or the exempt status of any such trust, or (ii) has remaining a period of time under applicable Treasury regulations or Internal Revenue Service pronouncements in which to apply for such a letter and make any amendments necessary to obtain a favorable determination as to the qualified status of each such Parent Plan.
(g) All contributions, premiums or payments required to be made or accrued with respect to any Parent Plan have been made on or before their due dates.
(h) Except as set forth in Section 4.11(h) of the Parent Disclosure Schedule, (i) Parent is not a party to any collective bargaining agreement or other labor union contract applicable to persons employed by Parent or in Parent’s business, and as of the date of this Agreement, to the knowledge of Parent, there are no organizational campaigns, petitions or other unionization activities seeking recognition of a collective bargaining unit that could affect Parent; (ii) there are no controversies, strikes, slowdowns or work stoppages pending or, to the knowledge of Parent, threatened between Parent and any of its employees, and Parent has not experienced any such controversy, strike, slowdown or work stoppage within the past three years; (iii) Parent has not breached or otherwise failed to comply with the provisions of any collective bargaining or union contract and there are no grievances outstanding against Parent under any such agreement or contract; (iv) Parent is not a party to, or otherwise bound by, any consent decree with, or citation by, any Governmental Entity relating to employees or employment practices; (v) there is no charge or proceeding with respect to a violation of any occupational safety or health standards that has been asserted or is now pending with respect to Parent; and (vi) there is no charge of discrimination in employment or employment practices for any reason, including, without limitation, age,
A-22
Table of Contents
gender, race, religion or other legally protected category, which has been asserted or, to the knowledge of Parent, threatened against Parent or that is now pending before the United States Equal Employment Opportunity Commission or any other Governmental Entity. Each individual who currently provides, or formerly provided, 3services to Parent or any Parent ERISA Affiliate and who is or was categorized by Parent as an independent contractor is and was at all times properly categorized as such and does not or did not, as the case may be, constitute a common law employee of Parent or any Parent ERISA Affiliate during any time that such individual is or was categorized as an independent contractor.
SECTION 4.12 Contracts.
(a) Section 4.12(a) of the Parent Disclosure Schedule lists each of the following written or oral contracts and agreements to which Parent is a party or otherwise subject or bound or to which or by which any property, business, operation or right of the Parent is subject or bound as of the date hereof (such contracts and agreements being the “Material Parent Contracts”):
(i) each contract and agreement for the purchase or lease of personal property with any supplier or for the furnishing of services to Parent with payments greater than $100,000 per year;
(ii) all broker, exclusive dealing or exclusivity, distributor, dealer, manufacturer’s representative, franchise, agency, sales promotion, market research, marketing, consulting and advertising contracts to which Parent is a party or any other contract that compensates any person based on any sales by Parent;
(iii) all leases and subleases of real property including, without limitation, any capital lease;
(iv) all leases, licenses or sublicenses of any asset, including any intellectual property not listed on Schedule 4.12 of the Parent Disclosure Schedule, other than nonexclusive leases, licenses or sublicenses of commercially available software that Parent has procured through shrink wrap, click wrap or purchase orders;
(v) all employment agreements and employment contracts that have an aggregate future liability in excess of $25,000, other than offer letters in a standard form that do not provide for any severance or bonus payments or provide for the grant of equity incentives not consistent with the existing Company Plans;
(vi) all contracts and agreements for any joint venture, partnership or similar arrangement;
(vii) all contracts and agreements under which Parent has advanced or loaned an amount to any of its affiliates or employees other than in the ordinary course of business;
(viii) any contract or agreement relating to the acquisition or disposition of (a) any business of Parent (whether by merger, consolidation or other business combination, sale of securities, sale of assets or otherwise) or (b) any asset (tangible or intangible) other than sales of supplies pursuant to purchase orders in the ordinary course of business;
(ix) any contract or agreement under which Parent is, or may become, obligated to pay an amount in respect of indemnification obligations, purchase price adjustment or otherwise in connection with any (a) acquisition or disposition of assets or securities (other than the sale of inventory in the ordinary course of business), (b) merger, consolidation or other business combination or (c) series or group of related transactions or events of the type specified in clauses (a) and (b) above;
(x) all contracts and agreements relating to indebtedness other than trade indebtedness of Parent in an aggregate amount not to exceed $100,000, including any contracts and agreements in which Parent is a guarantor of indebtedness;
(xi) all contracts and agreements under which any person has guaranteed any indebtedness of Parent;
(xii) all contracts and agreements that limit or purport to limit the ability of Parent to compete in any line of business or with any person or in any geographic area or during any period of time;
(xiii) all contracts and agreements relating to the voting and any rights or obligations of a stockholder of Parent;
(xiv) all contracts or agreements under which Parent is, or may become, obligated to incur any severance pay or other compensation obligations and all profit sharing, stock option, stock purchase, stock appreciation, deferred compensation, or other plan or arrangement for the benefit of Parent’s current or former directors, officers and employees;
(xv) all contracts regarding the acquisition, issuance or transfer of any securities of Parent and each contract affecting or dealing with any securities of Parent, including, without limitation, any restricted stock agreements or escrow agreements; and
A-23
Table of Contents
(xvi) any agreement of guarantee, assumption or endorsement of, or any similar commitment with respect to, the obligations, liabilities (whether accrued, absolute, contingent or otherwise) or indebtedness of any person other than software licenses or professional services contracts entered into in the ordinary course of business.
(b) Each Material Parent Contract (i) is legal, valid and binding on Parent, and, to the knowledge of Parent, on the other parties thereto, is in full force and effect and is enforceable against Parent and, to the knowledge of Parent, is enforceable against each other party thereto, and (ii) upon consummation of the transactions contemplated by this Agreement, shall continue in full force and effect without penalty or other adverse consequence. Parent is not in breach or violation of, or default under, any Material Parent Contract and, to the knowledge of Parent, no other party to any Material Parent Contract is in breach or violation thereof or default thereunder. Parent has made available to the Company true, accurate and complete copies of each written agreement set forth on Schedule 4.12 of the Parent Disclosure Schedule, in each case, as amended or otherwise modified and in effect. There are no payments due and unpaid by Parent under any Material Parent Contract, whether or not any other party thereto has agreed to forbear or waive any rights in connection with such non-payment (other than payments set forth on Section 4.12(b) of the Parent Disclosure Schedule and identified as not being made due to the amount in dispute).
SECTION 4.13 Environmental Matters.
(a) Parent (i) is in compliance with all applicable Environmental Laws (as defined below), (ii) holds all Environmental Permits (as defined below) necessary to conduct Parent’s business, all of which are set forth on Schedule 4.13 of the Parent Disclosure Schedule and (iii) is in compliance with its Environmental Permits.
(b) Parent has not released, and no other person has released, Hazardous Materials (as defined below) on, upon, into or from any real property owned or leased by Parent or, during their ownership or occupancy of such property, on any property formerly owned, operated or leased by Parent.
SECTION 4.14 Intellectual Property.
(a) Parent owns, is licensed for, or possesses sufficient rights with respect to, all Parent Intellectual Property (as defined below), that is material to the business of Parent, and that is necessary to conduct any such business, as such business is presently conducted. Parent has not received any written communication alleging or suggesting that Parent or Parent Intellectual Property has caused an Infringement, nor is Parent aware of any Infringement. Parent has not infringed upon or misappropriated, and the sale by Parent of Ramoplanin will not infringe or misappropriate, any Intellectual Property right of any third party. Parent has not brought or threatened any action, suit or proceeding against any third party for any Infringement of any Parent Intellectual Property or any breach of any license, sublicense or agreement involving Parent Intellectual Property and is not aware of a bona fide basis for such a proceeding. “Parent Intellectual Property” means all Intellectual Property that was or is Used in any business of Parent, or that is necessary to conduct any such business, as such business is presently conducted.With respect to patent rights and mark rights, and notwithstanding anything to the contrary, the representations and warranties of this Section 4.14(a) are made only to Parent’s knowledge and without having conducted any special investigation or patent or trademark search.
(b) To the extent included in Parent Intellectual Property (but excluding Intellectual Property licensed to Parent only on a nonexclusive basis), Section 4.14(b) of the Parent Disclosure Schedule lists (by name, number, jurisdiction and owner) all issued patents and patent applications; all registered Marks; and all registered copyrights and mask works. No cancellation, termination, expiration or abandonment of any of the foregoing (except natural expiration or termination at the end of the full possible term, including extensions and renewals) has occurred or is anticipated by Parent. Except as referenced in written documentation previously provided to the Company (including without limitation file wrappers), Parent is not aware of any material questions or challenges with respect to the validity of any claims of any of the foregoing patents or the validity (or any other aspect or status) of any such IP Rights.
(c) Section 4.14(c) of the Parent Disclosure Schedule lists: (i) all licenses, sublicenses and other agreements to which Parent is a party that assign, authorize to Use, encumber, or give access to any Parent Intellectual Property to a third party other than access provided under a standard form nondisclosure/nonuse agreement; and (ii) all licenses, sublicenses and other agreements pursuant to which Parent has received rights to Use any third party Intellectual Property other than commercially available shrink wrap software, and (iii) all Marks. Parent has not entered into any agreement to indemnify, hold harmless or defend any other person with respect to
A-24
Table of Contents
any assertion of Infringement, other than indemnification provisions contained in agreements used in transactions arising in the ordinary course of business (including, but not limited to, purchase and supply agreements, distribution agreements, and service agreements). To Parent’s knowledge, no event or circumstance has occurred or exists (including, without limitation, the authorization, execution or delivery of this Agreement or the consummation of any of the transactions contemplated hereby) that would result in a material breach or material violation of any license, sublicense or other agreement required to be listed in Section 4.14(c) of the Parent Disclosure Schedule.
(d) Parent has taken reasonable steps to protect and preserve the confidentiality of all Parent Intellectual Property with respect to which Parent has exclusivity and is not otherwise disclosed in published patents or patent applications or registered copyrights (“Parent Confidential Information”). Each current and former employee and contractor of Parent who contributed to the creation or development of any Parent Intellectual Property executed an agreement in substantially the form of the Parent’s standard Proprietary Information and Inventions Agreement (in the case of an employee) or Consulting Agreement (in the case of a contractor), except where failure to do so would not have a Parent Material Adverse Effect.
SECTION 4.15 Taxes.
(a) All material Tax Returns, by or on behalf of Parent, have been or will be completed and filed when due (after giving effect to any extensions of such due date) and all such Tax Returns are true, correct, and complete in all material respects. All amounts shown due on such Tax Returns on or before the Effective Time, and all other material Taxes (whether or not shown on any Tax Return) that are due on or before the Effective Time, have been or will be paid on or before such date. Parent’s unaudited consolidated balance sheet as of September 27, 2003 (the “Parent Reference Balance Sheet”), and related statements of operation, changes in stockholders’ equity and changes in cash flows for the 39 week period ended September 27, 2003 previously provided to the Company (collectively referred to herein as the “Parent Interim Financial Statements”) (i) accrue on the face of such Parent Interim Financial Statements (rather than in any footnote thereto) all material actual and contingent liabilities for Taxes with respect to all periods through September 27, 2003 and Parent has not and will not incur any Tax liability in excess of the amount reflected (excluding any amount thereof that reflects timing differences between the recognition of income for purposes of U.S. GAAP and for Tax purposes) on the face of the Parent Reference Balance Sheet included in the Parent Interim Financial Statements (rather than in any footnote thereto) with respect to such periods, and (ii) properly accrue in accordance with U.S. GAAP all material liabilities for Taxes payable after September 27, 2003, with respect to all transactions and events occurring on or prior to such date. Parent has not incurred any material Tax liability since September 27, 2003 other than in the ordinary course of business and Parent has made adequate provisions for all Taxes since that date in accordance with U.S. GAAP on at least a quarterly basis.
(b) Parent has withheld and paid to the applicable financial institution or Tax Authority all amounts required to be withheld by Parent, and Parent has complied with all material Tax reporting and recordkeeping requirements. To the best knowledge of Parent, no Tax Returns filed with respect to Taxable years through the Taxable year ended December 31, 2002 have been examined by any Tax Authority. Parent has not granted any extension or waiver of the limitation period applicable to any Tax Returns that is still in effect and there is no material claim, audit, action, suit, proceeding, or (to the knowledge of Parent) investigation now pending or threatened in writing against or with respect to Parent in respect of any Tax or assessment. No notice of deficiency or similar document of any Tax Authority has been received by Parent, and there are no liabilities for Taxes with respect to the issues that have been raised (and are currently pending) by any Tax Authority that could, if determined adversely to Parent, materially and adversely affect the liability of Parent for Taxes. There are no liens for Taxes (other than for current Taxes not yet due and payable) upon the assets of Parent. Parent has never been a party (either as a distributing corporation, a distributed corporation or otherwise) to any transaction intended to qualify under Section 355 of the Code or any corresponding provision of state Law. Parent has previously provided or made available to the Company true and complete copies of all income, franchise, and sales Tax Returns.
SECTION 4.16 Vote Required. The only votes of the holders of any classes or series of capital stock of Parent necessary to approve the Share Issuance and the issuance of the Parent Securities in connection with the Combined Company Financing are (i) the votes prescribed by Marketplace Rule 4350 of the National Association of Securities Dealers, Inc. (as such rule may be amended after the date hereof, the “NASD Rule”) and (ii) the vote of the holders of Parent Common Stock to increase the number of authorized shares of Parent Common Stock to 200,000,000 (the “Parent Charter Vote,” and (i) and (ii) the “Parent Stockholder Approvals”). The Parent Stockholder Approvals are the only votes of holders of any class or series of the capital stock of Parent necessary for Parent to effect the Merger and the transactions contemplated in this Agreement.
A-25
Table of Contents
SECTION 4.17 Assets; Absence of Liens and Encumbrances. Except as set forth in Section 4.17 of the Parent Disclosure Schedule, Parent owns, leases or has the legal right to use all of the material assets, properties and rights of every kind, nature, character and description, including, without limitation, real property and personal property (other than Intellectual Property, which is covered by Section 4.14 hereof), used in the conduct of the business of Parent or otherwise owned or leased by Parent and, with respect to contract rights, is a party to and enjoys the right to the benefits of all material contracts, agreements and other arrangements used by Parent in or relating to the conduct of the business of Parent (all such properties, assets and contract rights being the “Parent Assets”). The equipment of Parent used in the operations of their business is, taken as a whole, in good operating condition and repair, ordinary wear and tear excepted.
SECTION 4.18 Owned Real Property. Parent does not own any real property.
SECTION 4.19 Brokers. Except as set forth on Section 4.19 of the Parent Disclosure Schedule, no broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the Merger or the other transactions contemplated by this Agreement based upon arrangements made by or on behalf of Parent or Merger Sub.
SECTION 4.20 Tax Matters. Neither Parent nor Merger Sub nor any of their affiliates has taken or agreed to take any action that would prevent the Merger from constituting a reorganization qualifying under Section 368(a) of the Code. Parent is not aware of any agreement, plan or other circumstance that would prevent the Merger from qualifying as a reorganization under Section 368(a) of the Code.
SECTION 4.21 State Takeover Statutes. The Board of Directors of Parent has taken all action necessary to ensure that any restrictions on business combinations contained in the Massachusetts Business Combination Law will not apply to the Merger and the other transactions contemplated by this Agreement. No other “fair price,” “moratorium,” “control share acquisition” or other similar anti-takeover statute or regulation or any anti-takeover provision in Parent’s Certificate of Incorporation or Bylaws is, or at the Effective Time will be, applicable to Parent, the shares of Parent Common Stock, the Merger or the other transactions contemplated by this Agreement.
SECTION 4.22 Interim Operations of Merger Sub. Merger Sub was formed by Parent solely for the purpose of engaging in the transactions contemplated by this Agreement, has engaged in no other business activities and has conducted its operations only as contemplated by this Agreement. Merger Sub has no liabilities and, except for a subscription agreement pursuant to which all of its authorized capital stock was issued to Parent, is not a party to any agreement other than this Agreement and agreements with respect to the appointment of registered agents and similar matters.
SECTION 4.23 Valid Issuance of Parent Shares. The shares of Parent Common Stock to be issued pursuant to this Agreement will, when issued, be duly authorized, validly issued, fully paid and non-assessable, and issued in compliance with all applicable federal and state securities laws.
SECTION 4.24 Accounts Receivable. All accounts and notes receivable reflected on the Parent Reference Balance Sheet and all accounts and notes receivable arising subsequent to the date of the Parent Reference Balance Sheet and on or prior to the Closing Date, have arisen or will arise in the ordinary course of business, represent or will represent legal, valid, binding and enforceable obligations to Parent and, subject only to consistently recorded reserves for bad debts established as of a date prior to the Closing Date.
SECTION 4.25 Illegal Payments, etc. In the conduct of the business of Parent, Parent has not, and none of its directors, officers, employees or agents, has (a) directly or indirectly, given, or agreed to give, any illegal gift, contribution, payment or similar benefit to any supplier, customer, governmental official or employee or other person who was, is or may be in a position to help or hinder Parent (or assist in connection with any actual or proposed transaction) or made, or agreed to make, any illegal contribution, or reimbursed any illegal political gift or contribution made by any other person, to any candidate for federal, state, local or foreign public office or (b) established or maintained any unrecorded fund or asset or made any false entries on any books or records for any purpose.
SECTION 4.26 Affiliate Transactions. Except for the matters disclosed on Section 4.26 of the Parent Disclosure Schedule, no stockholder of Parent or any affiliate of any stockholder of Parent is an officer, director, employee, consultant, competitor, creditor,
A-26
Table of Contents
debtor, customer, distributor, supplier or vendor of, or is a party to any Material Parent Contract with, the Company. Except as disclosed on Section 4.26 of the Parent Disclosure Schedule, no stockholder of Parent or any affiliate of any stockholder of Parent owns any asset used in, or necessary to, the business of Parent.
SECTION 4.27 Employees. Except as disclosed on Section 4.27 of the Parent Disclosure Schedule, no employee of Parent is represented by a labor union.
SECTION 4.28 Insurance. The insurance policies owned and maintained by Parent are set forth on Section 4.28 of the Parent Disclosure Schedule with their respective expiration dates. All such policies, taken as a whole, are in full force and effect, all premiums due and payable thereon have been paid (other than retroactive or retrospective premium adjustments that are not yet, but may be, required to be paid with respect to any period ending prior to the Closing Date), and no notice of cancellation or termination has been received with respect to any such policy which has not been replaced on substantially similar terms prior to the date of such cancellation. Except as set forth on Section 4.28 of the Parent Disclosure Schedule, no insurer (a) has questioned, denied or disputed (or otherwise reserved its rights with respect to) the coverage of any claim pending under any such policy or (b) has threatened to cancel any such policy.
SECTION 4.29 Disclosure. The representations and warranties contained in this Article IV (including, without limitation, the Parent Disclosure Schedule) do not contain and will not contain any untrue statement of fact or omit to state any material fact necessary in order to make the statements and information contained therein not misleading.
ARTICLE V
CONDUCT OF BUSINESSES PENDING THE MERGER
SECTION 5.01 Conduct of Business by the Company Pending the Merger. During the period from the date of this Agreement and continuing until the earlier of the termination of this Agreement or the Effective Time, the Company agrees (except to the extent that Parent shall otherwise consent in writing (such consent not to be unreasonably withheld, delayed or modified)), to carry on its business in the usual, regular and ordinary course and in substantially the same manner as previously conducted, to use all reasonable efforts consistent with past practices and policies to keep available the services of its present officers and key employees and consultants and preserve its relationships with customers, suppliers, distributors, licensors, licensees, and others having business dealings with it, to the end that its goodwill and ongoing businesses would be unimpaired, in any material respect, at the Effective Time. The Company shall promptly notify Parent of any event or occurrence not in the ordinary course of business of the Company.
By way of amplification and not limitation, except as contemplated by this Agreement or as set forth in Section 5.01 of the Company Disclosure Schedule, the Company shall not, between the date of this Agreement and the Effective Time, do, any of the following without the prior written consent of Parent (such consent not to be unreasonably withheld, delayed or modified):
(a) amend or otherwise change its Certificate of Incorporation or Bylaws or equivalent organizational documents;
(b) issue, sell, pledge, dispose of, grant, encumber, authorize or propose the issuance, sale, pledge, disposition, grant or encumbrance of any shares of its capital stock of any class, or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital stock or any other ownership interest (including, without limitation, any phantom interest), of the Company, except pursuant to the terms of options, warrants or preferred stock outstanding on the date of this Agreement and except for grants of options to purchase up to 100,000 shares of Company Stock pursuant to the Company Stock Plan;
(c) sell, lease, license, pledge, grant, encumber or otherwise dispose of any of its properties or assets unrelated to Factive, except in the ordinary course of business, consistent with past practice;
(d) sell, lease, license, pledge, grant, encumber or otherwise dispose of any of its properties or assets related to Factive, including any co-promotion agreement relating to Factive;
(e) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital stock;
A-27
Table of Contents
(f) cancel any indebtedness or in the aggregate or waive any claims or rights of substantial value;
(g) make any change in any method if accounting or accounting practice or policy other than those required by U.S. GAAP;
(h) enter into, amend, modify or terminate any contract, commitment or agreement related to the Company’s ability to sell, have sold, market, develop, distribute, import or manufacture Factive (including, without limitation, any co-promotion agreement related to Factive), or waive any material rights thereunder;
(i) enter into, amend, modify or terminate any contract, commitment or agreement that obligates the Company to make payments other than any payments contemplated and permitted by the budget and spending plan attached as an exhibit to the Promissory Note dated the date hereof, issued by the Company to Parent (the “Promissory Note”);
(j) split, combine, subdivide, redeem or reclassify any of its capital stock or issue or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of its capital stock, or purchase or otherwise acquire, directly or indirectly, any shares of its capital stock except from former employees, directors and consultants in accordance with agreements providing for the repurchase of shares in connection with any termination of service by such party;
(k) acquire (including, without limitation, by merger, consolidation, or acquisition of stock or assets) any interest or any assets in any corporation, partnership, other business organization or any division thereof;
(l) incur, other than to Parent, any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise as an accommodation become responsible for, the obligations of any person, or make any loans or advances;
(m) authorize any capital expenditure in excess of $50,000, in the aggregate;
(n) enter into any lease or contract for the purchase or sale of any property, real or personal except in the ordinary course of business, consistent with past practice;
(o) increase, or agree to increase, the compensation payable, or to become payable, to its officers or employees, except for increases in accordance with past practice in salaries or wages of its employees who are not its officers, or grant any severance or termination pay to, or enter into any employment or severance agreement with, any of its directors, officers or other employees, or establish, adopt, enter into or amend any collective bargaining, bonus, profit sharing, thrift, compensation, stock option, restricted stock, pension, retirement, deferred compensation, employment, termination, severance or other Plan, agreement, trust, fund, policy or arrangement for the benefit of any director, officer or employee;provided,however, that the foregoing provisions of this subsection shall not apply to any amendments to employee benefit plans described in Section 3(3) of ERISA that may be required by Law;
(p) accelerate, amend or change the period of exercisability or the vesting schedule of restricted stock or Company Options granted under any option plan, employee stock plan or other agreement or authorize cash payments in exchange for any Company Options granted under any of such plans except as specifically required by the terms of such plans or any such agreement or any related agreement in effect as of the date of this Agreement and disclosed in the Company Disclosure Schedule;
(q) extend any offers of employment to potential employees who would receive cash compensation at a rate of $100,000 per year or more or extend any consulting or independent contracting offers that are not cancelable on prior notice of 30 days or less;
(r) other than as contemplated by this Agreement, enter into, or perform, any transaction with, or for the benefit of any affiliate of the Company (other than payments made to officers, directors and employees in the ordinary course of business);
(s) initiate any clinical trial;
(t) schedule or conduct any meeting with FDA or other regulatory authority;
(u) settle any litigation;
(v) amend, modify or terminate in any material respect any Material Company Contract or waive any material rights thereunder;
(w) waive any rights related to confidentiality under any contract or agreement;
A-28
Table of Contents
(x) make, change or revoke any material Tax election, elect or change any method of accounting for Tax purposes, settle any action in respect of Taxes or enter into any contractual obligation in respect of Taxes with any Tax Authority; or
(y) authorize any of, or commit or agree to take, whether in writing or otherwise, to do any of the foregoing actions.
SECTION 5.02 Conduct of Business by Parent Pending the Merger. During the period from the date of this Agreement and continuing until the earlier of the termination of this Agreement or the Effective Time, Parent agrees (except to the extent that the Company shall otherwise consent in writing (such consent not to be unreasonably withheld, delayed or modified)), to carry on its business in the usual, regular and ordinary course and in substantially the same manner as previously conducted, to use all reasonable efforts consistent with past practices and policies to keep available the services of its present officers and key employees and consultants and preserve its relationships with customers, suppliers, distributors, licensors, licensees, and others having business dealings with it, to the end that its goodwill and ongoing businesses would be unimpaired, in any material respect, at the Effective Time. Parent shall promptly notify the Company of any event or occurrence not in the ordinary course of business of Parent.
By way of amplification and not limitation, except as contemplated by this Agreement or as set forth in Section 5.02 of the Parent Disclosure Schedule, Parent shall not, between the date of this Agreement and the Effective Time, do, any of the following without the prior written consent of the Company (such consent not to be unreasonably withheld, delayed or modified):
(a) amend or otherwise change its Certificate of Incorporation or Bylaws or equivalent organizational documents;
(b) issue, sell, pledge, dispose of, grant, encumber, authorize or propose the issuance, sale, pledge, disposition, grant or encumbrance of any shares of its capital stock of any class, or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital stock or any other ownership interest (including, without limitation, any phantom interest), of Parent, except pursuant to the terms of options, warrants or preferred stock outstanding on the date of this Agreement and except for grants of options to purchase up to 644,000 shares of Parent Common Stock pursuant to the Parent Stock Plan;
(c) sell, lease, license, pledge, grant, encumber or otherwise dispose of any of its properties or assets, except in the ordinary course of business, consistent with past practice;
(d) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital stock;
(e) cancel any indebtedness or waive any claims or rights of substantial value;
(f) make any change in any method if accounting or accounting practice or policy other than those required by U.S. GAAP;
(g) split, combine, subdivide, redeem or reclassify any of its capital stock or issue or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of its capital stock, or purchase or otherwise acquire, directly or indirectly, any shares of its capital stock except from former employees, directors and consultants in accordance with agreements providing for the repurchase of shares in connection with any termination of service by such party;
(h) acquire (including, without limitation, by merger, consolidation, or acquisition of stock or assets) any interest or any assets in any corporation, partnership, other business organization or any division thereof;
(i) incur, other than to the Company, any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise as an accommodation become responsible for, the obligations of any person, or make any loans or advances;
(j) authorize any capital expenditure in excess of $100,000, in the aggregate;
(k) enter into any lease or contract for the purchase or sale of any property, real or personal except in the ordinary course of business, consistent with past practice;
(l) increase, or agree to increase, the compensation payable, or to become payable, to its officers or employees, except for increases in accordance with past practice in salaries or wages of its employees who are not its officers, or grant any severance or termination pay to, or enter into any employment or severance agreement with, any of its directors, officers or other employees, or establish, adopt, enter into or amend any collective bargaining, bonus, profit sharing, thrift, compensation, stock option, restricted stock, pension, retirement, deferred compensation, employment, termination, severance or other Plan, agreement, trust, fund, policy or arrangement for the benefit of any director, officer or employee;provided,however, that the foregoing provisions
A-29
Table of Contents
of this subsection shall not apply to any amendments to employee benefit plans described in Section 3(3) of ERISA that may be required by Law;
(m) accelerate, amend or change the period of exercisability or the vesting schedule of restricted stock or Parent Options granted under any option plan, employee stock plan or other agreement or authorize cash payments in exchange for any Parent Options granted under any of such plans except as specifically required by the terms of such plans or any such agreement or any related agreement in effect as of the date of this Agreement and disclosed in the Parent Disclosure Schedule;
(n) extend any offers of employment to potential employees who would receive cash compensation at a rate of $100,000 per year or more or extend any consulting or independent contracting offers that are not cancelable on prior notice of 30 days or less
(o) other than as contemplated by this Agreement, enter into, or perform, any transaction with, or for the benefit of any affiliate of Parent (other than payments made to officers, directors and employees in the ordinary course of business);
(p) initiate any clinical trial;
(q) schedule or conduct any meeting with FDA or other regulatory authority;
(r) settle any litigation;
(s) amend, modify or terminate in any material respect any Material Parent Contract or waive any material rights thereunder;
(t) waive any rights related to confidentiality under any contract or agreement;
(u) make, change or revoke any material Tax election, elect or change any method of accounting for Tax purposes, settle any action in respect of Taxes or enter into any contractual obligation in respect of Taxes with any Tax Authority; or
(v) authorize any of, or commit or agree to take, whether in writing or otherwise, to do any of the foregoing actions.
SECTION 5.03 Litigation. Each of the Company and Parent shall notify the other in writing promptly after learning of any claim, action, suit, arbitration, mediation, proceeding or investigation by or before any court, arbitrator or arbitration panel, board or other Governmental Entity initiated by it or against it, or known by it to be threatened against it or any of its officers, directors, employees or stockholders in their capacity as such.
SECTION 5.04 Notification of Certain Matters. Parent shall give reasonably prompt notice to the Company, and the Company shall give reasonably prompt notice to Parent, of (i) the occurrence, or non-occurrence, of any event the occurrence, or non-occurrence, of which would be likely to cause (x) any representation or warranty contained in this Agreement to be untrue or inaccurate, in any material respect, or (y) any covenant, condition or agreement contained in this Agreement not to be complied with or satisfied, in any material respect; and (ii) any failure or inability of Parent or the Company, as the case may be, to comply, in any material respect, with or satisfy any covenant, condition or agreement to be complied with or satisfied by it hereunder.
ARTICLE VI
ADDITIONAL AGREEMENTS
SECTION 6.01 Registration Statement; Joint Proxy Statement.
(a) As promptly as practicable after the execution of this Agreement, (i) Parent and the Company shall prepare and Parent shall file with the SEC a joint proxy statement (together with any amendments thereof or supplements thereto, the “Joint Proxy Statement”) relating to the respective meetings of the Company stockholders (the “Company Stockholders’ Meeting”) to be held to consider approval of this Agreement (including the principal terms hereof) and the Merger and of the Parent stockholders (the “Parent Stockholders’ Meeting”) to be held to consider approval of the Share Issuance and (ii) Parent shall prepare and file with the SEC a registration statement on Form S-4 (together with all amendments and supplements thereto, the ”Registration Statement”) in which the Joint Proxy Statement shall be included as part of the prospectus, in connection with the registration under the Securities Act of the Parent Shares to be issued to the stockholders of the Company pursuant to the Merger. Each of Parent and the Company shall use its reasonable best efforts to cause the Registration Statement to become effective as promptly as practicable. Prior to the Effective Time,
A-30
Table of Contents
Parent shall take all or any action reasonably required under applicable state securities Laws in connection with the issuance of shares of Parent Common Stock to be issued in the Merger. Each of Parent and the Company shall furnish all information concerning itself as the other may reasonably request in connection with such actions and the preparation of the Registration Statement and Joint Proxy Statement. As promptly as practicable, but in no event later than the third business day, after the Registration Statement shall have become effective, each of Parent and the Company shall mail or cause to be mailed the Joint Proxy Statement to their respective stockholders.
(b) Subject to paragraph (c) of this Section 6.01, the Joint Proxy Statement shall include the unanimous recommendation of the Board of Directors of the Company to the stockholders of the Company to vote in favor of approving this Agreement (including the principal terms hereof) and the Merger (the “Company Board Recommendation”) and neither the Board of Directors of the Company nor any committee thereof shall withhold, withdraw, amend, modify or change, or propose or resolve to withhold, withdraw, amend, modify or change, in each case in a manner adverse to Parent, the Company Board Recommendation. For purposes of this Agreement, the Company Board Recommendation shall be deemed to have been modified in a manner adverse to Parent if such recommendation shall no longer be unanimous.
(c) Prior to the approval of this Agreement and the approval of the Merger by the requisite vote of the stockholders of the Company, nothing in this Agreement shall prevent the Company’s Board of Directors from withholding, withdrawing, amending, modifying or changing the Company Board Recommendation if (i) a Company Superior Proposal (as defined in Section 6.05(a) below) is made to the Company and is not withdrawn, (ii) the Company shall have promptly, but in no event more than 24 hours after receiving such Company Superior Proposal, provided written notice to Parent advising Parent that the Company has received a Company Superior Proposal, specifying the terms and conditions of such Company Superior Proposal in reasonable detail and identifying the person or entity making such Company Superior Proposal (a “Notice of Company Superior Proposal”), (iii) Parent shall not have, within five business days of Parent’s receipt of the Notice of Company Superior Proposal, made an offer that the Company’s Board of Directors by a majority vote determines in its good faith judgment (based on the written advice of a reputable financial advisor) to be at least as favorable to the Company and its stockholders as such Company Superior Proposal (it being agreed that the Company’s Board of Directors shall convene a meeting to consider any such offer by Parent promptly following the receipt thereof), (iv) the Board of Directors of the Company concludes in good faith, after consultation with its outside legal counsel, that, in light of such Company Superior Proposal, the failure to withhold, withdraw, amend, modify or change such recommendation would create a substantial risk of liability for breach of its fiduciary obligations to the Company and its stockholders under applicable Law and (v) the Company shall not have violated any of the restrictions set forth in Section 6.05 and shall have complied with this Section 6.01(c). The Company shall provide Parent with reasonable advance notice of any meeting of the Company’s Board of Directors (provided that in no event shall such notice be given less than 36 hours prior to any such meeting) at which the Company’s Board of Directors is reasonably expected to consider any Company Competing Transaction (as defined in Section 6.05(b) below). Subject to applicable Law, nothing contained in this Section 6.01(c) shall limit the Company’s obligation to convene and hold the Company Stockholders’ Meeting (regardless of whether the Company Board Recommendation shall have been withheld, withdrawn, amended, modified or changed).
(d) Subject to paragraph (e) of this Section 6.01, the Joint Proxy Statement shall include the unanimous recommendation of the Board of Directors of Parent to the stockholders of Parent to vote in favor of the Share Issuance (the“Parent Board Recommendation”) and neither the Board of Directors of Parent nor any committee thereof shall withhold, withdraw, amend, modify or change, or propose or resolve to withhold, withdraw, amend, modify or change, in each case in a manner adverse to the Company, the Parent Board Recommendation. For purposes of this Agreement, such recommendation of the Board of Directors shall be deemed to have been modified in a manner adverse to the Company if such recommendation shall no longer be unanimous.
(e) Prior to the adoption and approval of this Agreement and the approval of the Merger by the requisite vote of the stockholders of Parent, nothing in this Agreement shall prevent the Parent’s Board of Directors from withholding, withdrawing, amending, modifying or changing the Parent Board Recommendation if (i) a Parent Superior Proposal (as defined in Section 6.06(a) below) is made to Parent and is not withdrawn, (ii) Parent shall have promptly, but in no event more than 24 hours after receiving such Parent Superior Proposal, provided written notice to the Company advising the Company that Parent has received a Parent Superior Proposal, specifying the terms and conditions of such Parent Superior Proposal in reasonable detail and identifying the person or entity making such Parent Superior Proposal (a “Notice of Parent Superior Proposal”), (iii) the Company shall not have, within five business days of the Company’s receipt of the Notice of Parent Superior Proposal, made an offer that the Parent’s Board of Directors by a majority vote determines in its good faith judgment (based on
A-31
Table of Contents
the written advice of a reputable financial advisor) to be at least as favorable to Parent and its stockholders as such Parent Superior Proposal (it being agreed that the Parent’s Board of Directors shall convene a meeting to consider any such offer by the Company promptly following the receipt thereof), (iv) the Board of Directors of Parent concludes in good faith, after consultation with its outside legal counsel, that, in light of such Parent Superior Proposal, the failure to withhold, withdraw, amend, modify or change such recommendation would create a substantial risk of liability for breach of its fiduciary obligations to Parent and its stockholders under applicable Law and (v) Parent shall not have violated any of the restrictions set forth in Section 6.06 and shall have complied with this Section 6.01(e). Parent shall provide the Company with reasonable advance notice of any meeting of the Parent’s Board of Directors at which the Parent’s Board of Directors (provided that in no event shall such notice be given less than 36 hours prior to any such meeting) is reasonably expected to consider any Parent Competing Transaction (as defined in Section 6.06(b) below). Subject to applicable Law, nothing contained in this Section 6.01(e) shall limit the Parent’s obligation to convene and hold the Parent Stockholders’ Meeting (regardless of whether the Parent Board Recommendation shall have been withheld, withdrawn, amended, modified or changed).
(f) Subject to Sections 6.01(c) and (e) with respect to the Company Board Recommendation or the Parent Board Recommendation, no amendment or supplement to the Joint Proxy Statement or the Registration Statement will be made by Parent or the Company without the approval of the other party (such approval not to be unreasonably withheld or delayed). Each of Parent and the Company will advise the other, promptly after it receives notice thereof, of the time the SEC has issued formal comments to the Registration Statement, of the time at which the Registration Statement has become effective or any supplement or amendment has been filed, of the issuance of any stop order, of the suspension of the qualification of the Parent Shares issuable in connection with the Merger for offering or sale in any jurisdiction, or of any request by the SEC for amendment of the Joint Proxy Statement or the Registration Statement or comments thereon and responses thereto or requests by the SEC for additional information.
(g) The information supplied by Parent for inclusion in the Registration Statement and the Joint Proxy Statement shall not, at (i) the time the Registration Statement is declared effective, (ii) any time the Joint Proxy Statement is mailed to the stockholders of the Company and/or Parent, (iii) the time of the Company Stockholders’ Meeting, (iv) the time of the Parent Stockholders’ Meeting, and (v) the Effective Time, contain any untrue statement of a material fact or fail to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. If, at any time prior to the Effective Time, any event or circumstance relating to Parent or any Parent Subsidiary, or their respective officers or directors, that should be set forth in an amendment or a supplement to the Registration Statement or Joint Proxy Statement is discovered by Parent, Parent shall promptly inform the Company. All documents that Parent is responsible for filing with the SEC in connection with the Merger or the other transactions contemplated by this Agreement will comply as to form and substance in all material respects with the applicable requirements of the Securities Act and the Exchange Act.
(h) The information supplied by the Company for inclusion in the Registration Statement and the Joint Proxy Statement shall not, at (i) the time the Registration Statement is declared effective, (ii) any time the Joint Proxy Statement is mailed to the stockholders of the Company and/or Parent, (iii) the time of the Company Stockholders’ Meeting, (iv) the time of the Parent Stockholders’ Meeting and (v) the Effective Time, contain any untrue statement of a material fact or fail to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. If, at any time prior to the Effective Time, any event or circumstance relating to the Company or any Company Subsidiary, or their respective officers or directors, that should be set forth in an amendment or a supplement to the Registration Statement or Joint Proxy Statement is discovered by the Company, the Company shall promptly inform Parent. All documents that the Company is responsible for filing with the SEC in connection with the Merger or the other transactions contemplated by this Agreement will comply as to form and substance in all material respects with the applicable requirements of the Securities Act and the Exchange Act.
SECTION 6.02 Company Stockholders’ Meeting. The Company shall (i) call and hold the Company Stockholders’ Meeting as promptly as practicable for the purpose of voting upon the approval of this Agreement (including the principal terms hereof) and the Merger, (ii) use its reasonable best efforts to hold the Company Stockholders’ Meeting as soon as practicable after the date on which the Registration Statement becomes effective and (iii) shall in any event hold such Company Stockholders’ Meeting within 45 days after the date on which the Registration Statement becomes effective. The Company shall use its reasonable best efforts to solicit from its stockholders proxies in favor of the approval of the Merger and this Agreement (including the principal terms hereof), and shall take all other commercially reasonable action necessary or advisable to secure the vote or consent of stockholders required by the DGCL, in each case in compliance with applicable Laws.
A-32
Table of Contents
SECTION 6.03 Parent Stockholders’ Meeting. Parent shall (i) call and hold the Parent Stockholders’ Meeting as promptly as practicable for the purpose of obtaining the approval of the Share Issuance and the Parent Charter Vote by the Parent stockholders, (ii) use its reasonable best efforts to hold the Parent Stockholders’ Meeting as soon as practicable after the date on which the Registration Statement becomes effective and (iii) shall in any event hold such Parent Stockholders’ Meeting within 45 days after the date on which the Registration Statement becomes effective. Parent shall use its reasonable best efforts to solicit from its stockholders proxies in favor of the Share Issuance and the Parent Charter Vote, and shall take all other commercially reasonable action necessary or advisable to secure the vote or consent of stockholders required by the NASD Rule and Massachusetts Business Corporation Law, in compliance with applicable Laws.
SECTION 6.04 Access to Information; Confidentiality.
(a) From the date of this Agreement to the Effective Time, each of the Company and Parent shall: (i) provide the other (and its officers, directors, employees, accountants, consultants, legal counsel, advisors, agents and other representatives (collectively, “Representatives”)) access at reasonable times upon prior notice to the directors, officers, employees, agents, properties, offices, books, records and other facilities of the Company and Parent (as the case may be) and their respective subsidiaries and (ii) furnish promptly such information concerning the business, properties, contracts, assets, liabilities, personnel and other aspects of the Company or Parent (as the case may be) and their respective subsidiaries as reasonably requested by the other party or its Representatives.
(b) The parties shall comply with, and shall cause their respective Representatives to comply with, all of their respective obligations under the Confidentiality Agreement dated as of July 29, 2003 (the “Non-Disclosure Agreement”), by and between the Company and Parent. Notwithstanding the foregoing confidentiality provisions or anything in this Agreement to the contrary, each of the Company and Parent and its respective Representatives may consult any tax advisor regarding the tax treatment and tax structure of the transactions contemplated hereby (the “Transactions”) and may disclose to any and all persons, without limitation of any kind, the tax treatment and tax structure of the Transactions and all materials of any kind (including opinions or other tax analyses) that are provided to such person relating to such tax treatment or tax structure.
SECTION 6.05 No Company Solicitation of Transactions.
(a) The Company will not solicit, initiate or knowingly encourage (including by way of furnishing nonpublic information), or take any other action to facilitate, any inquiries or the making of any proposal or offer (including, without limitation, any proposal or offer to its stockholders) that constitutes, or may reasonably be expected to lead to, any Company Competing Transaction (as defined below), or enter into or maintain or continue discussions or negotiate with any person in furtherance of such inquiries or to obtain a Company Competing Transaction, or agree to or endorse any Company Competing Transaction, or authorize or permit any of the officers, directors or employees of the Company, or any investment banker, financial advisor, attorney, accountant or other representative retained by the Company, to take any such action;provided,however, that nothing contained in this Section 6.05 shall prohibit the Board of Directors of the Company from furnishing information to, or entering into discussions or negotiations with, any person or entity that makes an unsolicited proposal or offer regarding a Company Competing Transaction or other similar transaction, if, the Board of Directors of the Company determines in good faith, after consultation with the Company’s financial advisor, that such proposal provides greater value to the Company Stockholders than the Merger, is not subject to regulatory approvals that give rise to a significant risk that the Company Competing Transaction will not be consummated and for which financing is committed or, in the good faith judgment of the Board of Directors of the Company, is reasonably capable of being obtained (a “Company Superior Proposal”); provided that the Company shall not furnish any information to any person making such proposal unless the Company and such other party shall have entered into a confidentiality agreement as least as favorable to the Company as the Non-Disclosure Agreement. The Company will notify Parent promptly (and in any event within 48 hours) after receipt by the Company (or any of its officers, directors, employees, agents, advisors or other representatives) of any proposal for, or inquiry respecting, any Company Competing Transaction, or any request for nonpublic information in connection with such proposal or inquiry or for access to the properties, books or records of the Company by any person that informs or has informed the Company that it is considering making or has made such a proposal or inquiry. The Company immediately shall cease and cause to be terminated all existing discussions or negotiations with any parties conducted heretofore with respect to a Company Competing Transaction.
(b) A “Company Competing Transaction” means any of the following involving the Company (other than the Merger and the other transactions contemplated by this Agreement): (i) a merger, consolidation, share exchange, business combination or other
A-33
Table of Contents
similar transaction; (ii) any sale, lease, exchange, transfer or other disposition of a material portion of the assets or debt or equity securities of such party; and (iii) a tender offer or exchange offer for 15% or more of the outstanding voting securities of such party.
SECTION 6.06 No Parent Solicitation of Transactions.
(a) Parent will not solicit, initiate or knowingly encourage (including by way of furnishing nonpublic information), or take any other action to facilitate, any inquiries or the making of any proposal or offer (including, without limitation, any proposal or offer to its stockholders) that constitutes, or may reasonably be expected to lead to, any Parent Competing Transaction (as defined below), or enter into or maintain or continue discussions or negotiate with any person in furtherance of such inquiries or to obtain a Parent Competing Transaction, or agree to or endorse any Parent Competing Transaction, or authorize or permit any of the officers, directors or employees of Parent, or any investment banker, financial advisor, attorney, accountant or other representative retained by Parent, to take any such action;provided, however, that nothing contained in this Section 6.06 shall prohibit the Board of Directors of Parent from furnishing information to, or entering into discussions or negotiations with, any person or entity that makes an unsolicited proposal or offer regarding a Parent Competing Transaction or other similar transaction, if, the Board of Directors of Parent determines in good faith, after consultation with Parent’s financial advisor, that such proposal provides greater value to Parent Stockholders than the Merger, is not subject to regulatory approvals that give rise to a significant risk that the Parent Competing Transaction will not be consummated and for which financing is committed or, in the good faith judgment of the Board of Directors of Parent, is reasonably capable of being obtained (a “Parent SuperiorProposal”); provided that Parent shall not furnish any information to any person making such proposal unless Parent and such other party shall have entered into a confidentiality agreement as least as favorable to Parent as the Non-Disclosure Agreement. Parent will notify the Company promptly (and in any event within 48 hours) after receipt by Parent (or any of its officers, directors, employees, agents, advisors or other representatives) of any proposal for, or inquiry respecting, any Parent Competing Transaction, or any request for nonpublic information in connection with such proposal or inquiry or for access to the properties, books or records of Parent by any person that informs or has informed Parent that it is considering making or has made such a proposal or inquiry. Parent immediately shall cease and cause to be terminated all existing discussions or negotiations with any parties conducted heretofore with respect to a Parent Competing Transaction.
(b) A “Parent Competing Transaction” means any of the following involving Parent (other than the Merger and the other transactions contemplated by this Agreement): (i) a merger, consolidation, share exchange, business combination or other similar transaction; (ii) any sale, lease, exchange, transfer or other disposition of a material portion of the assets or debt or equity securities of such party; and (iii) a tender offer or exchange offer for 15% or more of the outstanding voting securities of such party.
SECTION 6.07 Employee Benefits Matters. All employees of the Company shall continue in their existing benefit plans until such time as, in Parent’s reasonable discretion, an orderly transition can be accomplished to employee benefit plans and programs maintained by Parent for its and its affiliates’ employees in the United States. Parent shall take such reasonable actions, to the extent permitted by Parent’s benefits programs, as are necessary to allow eligible employees of the Company to participate in the health, welfare and other benefit programs of Parent or alternative benefits programs in the aggregate that are substantially equivalent to those applicable to employees of Parent in similar functions and positions on similar terms (it being understood that equity incentive plans are not considered employee benefits). Pending such action, Parent shall maintain the effectiveness of the Company’s benefit plans. To the extent that Parent transitions employees of the Company from the Company’s benefit plans to employee benefit plans and programs maintained by Parent, from and after the Effective Time, Parent shall, to the extent permitted by Parent’s plans, grant all employees of the Company credit for all service (to the same extent as service with Parent is taken into account with respect to similarly situated employees of Parent) with the Company prior to the Effective Time for (i) eligibility and vesting purposes and (ii) for purposes of vacation accrual after the Effective Time as if such service with the Company was service with Parent; provided, that such service shall not be recognized to the extent that such recognition would result in a duplication of benefits or to the extent that such service was not recognized under the applicable Company benefit plan. Parent and the Company agree that where applicable with respect to any welfare benefit plan, including without limitation medical or dental benefit plan, of Parent, Parent shall, to the extent permitted by Parent’s plans, waive any pre-existing condition exclusion and actively-at-work requirements (provided, however, that no such waiver shall apply to a pre-existing condition of any employee of the Company who was, as of the Effective Time, excluded from participation in a plan maintained by the Company by virtue of such pre-existing condition) and similar limitations, eligibility waiting periods and evidence of insurability requirements under any of Parent’s group health plans to the extent permitted by such plans. Parent shall provide that any covered expenses incurred on or before the Effective Time by the Company’s employees or such employees’ covered dependents shall be taken into account for purposes of satisfying applicable deductible, coinsurance and maximum out-of-pocket provisions after the Effective Time to the same extent as such expenses are taken into account for the benefit
A-34
Table of Contents
of similarly situated employees of Parent. Notwithstanding the preceding, at the request of Parent, the Company shall terminate any and all 401(k) plans of the Company, effective not later than the day immediately preceding the Closing Date, and shall submit an application for a favorable determination letter on the termination of any such plans to the IRS.
SECTION 6.08 Further Action; Consents; Filings.
(a) Upon the terms and subject to the conditions hereof, each of the parties hereto shall use its reasonable best efforts to (i) take, or cause to be taken, all appropriate action and do, or cause to be done, all things necessary, proper or advisable under applicable Law or otherwise to consummate and make effective the Merger and the other transactions contemplated by this Agreement, including, without limitation, seeking in good faith the opinions described in Sections 7.02(d) and 7.03(c), (ii) obtain from any Governmental Entity or any other person all consents, licenses, permits, waivers, approvals, authorizations or orders required to be obtained or made by Parent or the Company or any of their subsidiaries in connection with the authorization, execution and delivery of this Agreement and the consummation of the Merger and the other transactions contemplated by this Agreement, including those required under the HSR Act, and (iii) make all necessary filings, and thereafter make any other required submission, with respect to this Agreement, the Merger and the other transactions contemplated by this Agreement required under applicable Law. The parties hereto shall cooperate with each other in connection with the making of all such filings, including by providing copies of all such documents to the nonfiling party and its advisors prior to filing and, if requested, by accepting all reasonable additions, deletions or changes suggested in connection therewith.
(b) Parent and the Company shall file as soon as practicable after the date hereof notifications under the HSR Act and each of Parent and the Company shall use commercially reasonable efforts to respond as promptly as practicable to all reasonable inquiries or requests and to resolve such objections, if any, as may be asserted by any Governmental Entity with respect to the transactions contemplated by this Agreement under the HSR Act, the Sherman Act, as amended, the Clayton Act, as amended, the Federal Trade Commission Act, as amended, and any other Federal, state or foreign statutes, rules, regulations, orders or decrees that are designed to prohibit, restrict or regulate actions having the purpose or effect of monopolization or restraint of trade. Parent shall be responsible for all filing fees arising from the filings under the HSR Act contemplated by this Section 6.08(b).
(c) From the date of this Agreement until the earlier of the Effective Time or the termination of this Agreement, the Company and Parent shall promptly notify the other in writing of any pending or, to the knowledge of such party, threatened action, proceeding or investigation by any Governmental Entity or any other person.
SECTION 6.09 Plan of Reorganization.
(a) This Agreement is intended to constitute a “plan of reorganization” within the meaning of Section 1.368-2(g) of the income tax regulations promulgated under the Code. From and after the date of this Agreement and until the Effective Time, each party hereto will not knowingly take any action, cause any action to be taken, fail to take any action or cause any action to fail to be taken which action or failure to act could prevent the Merger from qualifying as a reorganization under the provisions of Section 368(a) of the Code. Following the Effective Time, neither the Surviving Corporation, Parent nor any of their affiliates shall knowingly take any action, cause any action to be taken, fail to take any action or cause any action to fail to be taken, which action or failure to act could cause the Merger to fail to qualify as a reorganization under Section 368(a) of the Code.
(b) As of the date hereof, the Company does not know of any reason (i) why it would not be able to deliver to Gunderson Dettmer (counsel to the Company) and Ropes & Gray LLP (counsel to Parent), at the date of the legal opinions referred to below, representation letters substantially in compliance with Internal Revenue Service published advance ruling guidelines, with customary exceptions and modifications thereto, to enable such firms to deliver the legal opinions contemplated by Sections 7.02(d) and 7.03(c), and the Company hereby agrees to deliver such representation letters effective as of the date of such opinions, or (ii) why Gunderson Dettmer (counsel to the Company) and Ropes & Gray LLP (counsel to Parent) would not be able to deliver the opinions required by Sections 7.02(d) and 7.03(c). As of the date hereof, Parent and Merger Sub do not know of any reason why they would not be able to deliver to Gunderson Dettmer (counsel to the Company) or Ropes & Gray LLP (counsel to Parent), at the date of the legal opinions referred to below, representation letters substantially in compliance with the Internal Revenue Service published advance ruling guidelines, with customary exceptions and modifications thereto, to enable such firms to deliver the legal opinions contemplated by Sections 7.02(d) and 7.03(c), and Parent hereby agrees to deliver such representation letters effective as of the date of such opinions, or (ii) Gunderson Dettmer (counsel to the Company) or Ropes & Gray LLP (counsel to Parent) would not be able to deliver the opinions required by Sections 7.02(d) and 7.03(c).
A-35
Table of Contents
SECTION 6.10 No Public Announcement. The initial press release relating to this Agreement shall be a joint press release the text of which has been agreed to by each of Parent and the Company, except as may be required by applicable law, court process or by the rules and regulations of any national securities exchange.
SECTION 6.11 Affiliate Agreements. The Company shall use its reasonable efforts to cause each person that could reasonably be deemed to be an “affiliate” of the Company for purposes of the Securities Act to execute and deliver to Parent, as promptly as practicable after the execution of this Agreement, an Affiliate Agreement in the form attached hereto asExhibit C.
SECTION 6.12 Indemnification of Officers and Directors.
(a) For a period of six years from and after the Closing Date, Parent and the Surviving Corporation agree to indemnify (including advancement of expenses) and hold harmless all past and present officers and directors of the Company to the same extent such persons are indemnified by the Company as of the date of this Agreement pursuant to the Company’s Certificate of Incorporation or Bylaws, employment agreements, indemnification agreements identified on the Company Disclosure Schedule or under applicable Law for acts or omissions which occurred at or prior to the Effective Time. The Certificate of Incorporation and Bylaws of the Surviving Corporation shall contain provisions with respect to indemnification and exculpation that are at least as favorable to the past and present officers and directors of the Company as those provisions contained in the Company’s Certificate of Incorporation and Bylaws in effect on the date hereof, and such provisions shall not be amended, repealed or otherwise modified for a period of six years in any manner that would adversely affect the rights of the past and present officers and directors of the Company (unless such modification is required by applicable Law).
(b) For a period of six years for and after the Effective Time, each of the Parent and the Surviving Corporation agrees to provide officers’ and directors’ liability insurance with respect to acts or omissions occurring at or prior to the Effective Time covering each past and present officer and director of the Company who are currently covered by the Company’s officers’ and directors’ liability insurance policy (a true and complete copy of which has been delivered to Parent). The terms and coverage amounts of the liability insurance policy shall be at least as favorable as the terms and coverage amounts of the liability insurance policy in effect on the date hereof. Notwithstanding the foregoing, in no event shall Parent be required to expend per annum pursuant to this Section 6.05 in excess of 150% of the amount per annum the Company paid in its last full fiscal year for such insurance, which the Company represents was $35,775. Alternatively and in satisfaction of its obligations under this Section 6.12(b), prior to the Closing, Parent may purchase an insurance policy to extend the coverage of the Company’s current officers’ and directors’ liability insurance policy for a period of six years for and after the Effective Time.
(c) If Parent, the Surviving Corporation or any of its successors or assigns (i) consolidates with or merges into any other person and shall not be the continuing or surviving corporation or entity of such consolidation or merger or (ii) transfers or conveys all or substantially all of its properties and assets to any person, then, and in each such case, to the extent necessary, proper provision shall be made so that the successors and assigns of Parent or the Surviving Corporation, as the case may be, shall assume the obligations set forth in this Section 6.12.
(d) The provisions of this Section 6.12 are intended for the benefit of, and shall be enforceable by, all past and present officers and directors of the Company and his or her heirs and representatives. The rights of all past and present officers and directors of the Company under this Section 6.12 are in addition to, and not in substitution for, any other rights to indemnification or contribution that any such person may have by contract, applicable Law or otherwise.
SECTION 6.13 Nasdaq National Market Listing. If necessary, Parent shall promptly prepare and file with The Nasdaq National Market a Notification Form for Listing Additional Shares with respect to the shares of Parent Common Stock to be issued pursuant to this Agreement and pursuant to the Company Options, and shall use its reasonable efforts to obtain, prior to the Effective Time, approval for the listing of such shares of Parent Common Stock, subject to official notice to The Nasdaq National Market of issuance, and the Company shall cooperate with Parent with respect to such filing.
SECTION 6.14 Section 16 Relief. Provided that the Company delivers to Parent the Section 16 Information (as defined below) in a timely fashion, the Board of Directors of Parent, or a committee of two or more Non-Employee Directors thereof (as such term is defined for purposes of Rule 16b-3 under the Exchange Act), shall adopt resolutions prior to the consummation of the Merger providing that the receipt by the Company Insiders (as defined below) of the Parent Common Stock upon conversion of the Company
A-36
Table of Contents
Stock, and of options for Parent Common Stock upon conversion of the Company Options, in each case pursuant to the transactions contemplated hereby and to the extent such securities are listed in the Section 16 Information, are intended to be exempt from liability pursuant to Section 16(b) under the Exchange Act. Such resolutions shall comply with the approval conditions of Rule 16b-3 under the Exchange Act for purposes of such Section 16(b) exemption, including, but not limited to, specifying the name of the Company Insiders, the number of securities to be acquired or disposed of for each such person, the material terms of any derivative securities, and that the approval is intended to make the receipt of such securities exempt pursuant to Rule 13b-3(d). “Section 16 Information”shall mean information regarding the Company Insiders, the number of shares of the Company Stock held by each such Company Insider and expected to be exchanged for Parent Common Stock in connection with the Merger, and the number and description of the Company Options held by each such Company Insider and expected to be converted into options for Parent Common Stock in connection with the Merger. “Company Insiders”shall mean those officers and directors of the Company who will be subject to the reporting requirement of Section 16(b) of the Exchange Act with respect to Parent and who are listed in the Section 16 Information.
SECTION 6.15 Financing. Parent shall use its reasonable best efforts to enter into and consummate agreements to sell shares of Parent Common Stock, warrants exercisable for shares of Parent Common Stock, notes convertible into shares of Common Stock or notes (collectively, the “Parent Securities”) in an amount equal to at least $32 million (the “Combined Company Financing”).Promptly following the execution of this Agreement, Parent and the Company shall form a committee that shall be charged with creating and implementing a plan to obtain the Combined Company Financing (the “Financing Committee”). The Financing Committee shall (i) be co-chaired by the Chief Executive Officers of each of Parent and the Company, (ii) include such other members designated by the co-chairs and (iii) meet regularly (in any case, no less than weekly). The Company shall use its reasonable best efforts to cooperate with Parent in attempting to obtain the Combined Company Financing.
SECTION 6.16 Promissory Note. Parent and the Company shall perform their respective obligations under the Promissory Note, dated the date of this Agreement, between Parent and the Company.
ARTICLE VII
CONDITIONS TO THE MERGER
SECTION 7.01 Conditions to the Obligations of Each Party. The respective obligations of the Company, Parent and Merger Sub to consummate the Merger are subject to the satisfaction or waiver (where permissible) of the following conditions:
(a)Company Stockholder Approval. This Agreement shall have been approved and adopted by the requisite affirmative vote of the stockholders of the Company in accordance with applicable Law and the Company’s Certificate of Incorporation and Bylaws;
(b)Parent Stockholder Approval. The Share Issuance and the Parent Charter Vote shall have been approved and adopted by the requisite affirmative vote of the stockholders of Parent in accordance with the NASD Rule and Parent’s Bylaws;
(c)No Order. No Governmental Entity or court of competent jurisdiction located or having jurisdiction in the United States shall have enacted, issued, promulgated, enforced or entered any statute, rule, regulation, decree, judgment, injunction or other order, whether temporary, preliminary or permanent (each an “Order”) which is then in effect and has the effect of making the Merger illegal or otherwise prohibiting consummation of the Merger;
(d)HSR Act. Any waiting period (and any extension thereof) applicable to the consummation of the Merger under the HSR Act shall have expired or been terminated;
(e)Financing. Parent shall have obtained the proceeds from the sale of Parent Securities the Combined Company Financing;
(f)Registration Statement. The appropriate registration statement relating to the issuance of the shares of Parent Common Stock hereunder shall have become effective under the Securities Act and shall not be the subject of any stop order or proceeding seeking a stop order; or
(g)Note Amendment and Exchange Agreement. The Closing (as that term is defined in the Note Amendment and Exchange Agreement) shall have occurred.
A-37
Table of Contents
SECTION 7.02 Conditions to the Obligations of Parent and Merger Sub. The obligations of Parent and Merger Sub to consummate the Merger are subject to the satisfaction or waiver (where permissible) of the following additional conditions:
(a)Representations and Warranties. The representations and warranties made by the Company in this Agreement shall be true and correct as of the Effective Time except (i) that those representations and warranties that address matters only as of a particular date shall remain true and correct as of such date (subject to the following clauses (ii) and (iii)), (ii) for changes contemplated by this Agreement, and (iii) where the failure of such representations and warranties to be so true and correct (without giving effect to any materiality qualifications contained in such representations and warranties) would not be reasonably likely to have a Company Material Adverse Effect, and Parent shall have received a certificate of the Chief Executive Officer of the Company to that effect;
(b)Agreements and Covenants. The Company shall have performed or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied with by it on or prior to the Effective Time and Parent shall have received a certificate of the Chief Executive Officer of the Company to that effect;
(c)Approvals. The Company shall have received the consents and approvals set forth on Section 7.02(c) of the Company Disclosure Schedule and all other authorizations, consents, orders and approvals the failure of which to obtain would be reasonably likely to have a Company Material Adverse Effect;
(d)Tax Opinion of Parent’s Counsel. Parent shall have received the opinion of Ropes & Gray LLP (counsel to Parent), based upon representations of Parent, Merger Sub and the Company and normal assumptions, to the effect that the Merger will be treated for Federal income tax purposes as a reorganization qualifying under the provisions of Section 368(a) of the Code, which opinion shall not have been withdrawn or modified in any material respect. The issuance of such opinion shall be conditioned on receipt by Ropes & Gray LLP (counsel to Parent) of representation letters from each of Parent, Merger Sub, Second Acquisition Subsidiary and the Company as contemplated in Section 6.09 of this Agreement. Each such representation letter shall be dated on or before the date of such opinion and shall not have been withdrawn or modified in any material respect as of the Effective Time;
(e) [Intentionally omitted];
(f)No Material Adverse Change. Since the date of the Company Reference Balance Sheet, there will have occurred no events nor will there exist any circumstances which singly or in the aggregate have resulted in, or are reasonably likely to result in, a Company Material Adverse Effect;
(g)Licensor Letter. Parent shall have received written confirmation from Licensor, in a form and substance agreed to by Parent and the Company prior to the date of this Agreement;
(h)Manufacturing. Parent shall be reasonably satisfied that Licensor has the capability to and will, upon the terms set forth in the License Agreement, including, without limitation, the product specifications and manufacturing practices referred to therein, supply the active pharmaceutical ingredient in Factive and Factive final product (as provided in the License Agreement) to the Company in a timeframe and in sufficient amounts to meet the need for Factive final product anticipated by the Company and Parent;
(i)Termination of Agreements. Each of the Third Amended and Restated Investors Rights Agreement by and between the Company, John D. Baldeschwieler, Peter B. Dervan, Ph.D., and the stockholders listed onSchedule A thereto, dated as of August 8, 2002, as amended by the Admission and Amendment Agreements, dated as of October 1, 2002 and December 4, 2002, the Fifth Amended and Restated Voting Agreement by and between the Company and the stockholders listed onSchedule Athereto, dated as of August 8, 2001, and all rights to observe or obtain notice of meetings of the Board of Directors of the Company shall have been terminated effective as of the Effective Time;
(j)No Restraints. There shall not be pending any suit, action, investigation or proceeding to which a Governmental Entity is a party prohibiting the consummation of the Merger or any of the other transactions contemplated by this Agreement;
(k)Escrow Agreement. The Escrow Agent and the Stockholders’ Representative shall have entered into the Escrow Agreement;
(l)Opinion of the Company’s Counsel. Parent shall have received the opinion of Gunderson Dettmer (counsel to the Company), or another counsel reasonably satisfactory to Parent, substantially in the form attached hereto as Exhibit D;
A-38
Table of Contents
(m)Secretary’s Certificate. Parent shall have received a certificate executed by the Secretary of the Company attaching and certifying as to matters customary for a transaction of this sort, including, without limitation, the true and correct copies of the Company’s current Certificate of Incorporation and Bylaws and copies of the resolutions of the Company’s Board of Directors and the Company Stockholders approving and adopting this Agreement and the transactions relating hereto;
(n)Excess Parachute Payment. The Company shall have taken appropriate steps, which steps are reasonably satisfactory to Parent, to ensure that the consummation of the Merger or any of the other transactions contemplated by this Agreement will not, by themselves or after taking into account the satisfaction of any other condition or conditions or the occurrence of any other event or events, result in the payment of any “excess parachute payment” within the meaning of Section 280G of the Code;
(o)FIRPTA Certificate. The Company will have delivered to Parent a certification (in such form as may be reasonably requested by counsel to Parent) conforming to the requirements of Treasury Regulations 1.1445-2(c)(3) and 1.897-2(h); or
(p)Amendment. The Company shall have amended its Certificate of Incorporation so that no Company Preferred Stock shall be authorized.
SECTION 7.03 Conditions to the Obligations of the Company. The obligations of the Company to consummate the Merger are subject to the satisfaction or waiver (where permissible) of the following additional conditions:
(a)Representations and Warranties. The representations and warranties made by Parent in this Agreement shall be true and correct as of the Effective Time except (i) that those representations and warranties that address matters only as of a particular date shall remain true and correct as of such date (subject to the following clauses (ii) and (iii)), (ii) for changes contemplated by this Agreement, and (iii) where the failure of such representations and warranties to be so true and correct (without giving effect to any materiality qualifications contained in such representations and warranties) would not be reasonably likely to have a Parent Material Adverse Effect, and the Company shall have received a certificate of the Chief Executive Officer of Parent to that effect;
(b)Agreements and Covenants. Each of Parent and Merger Sub shall have performed or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied with by it on or prior to the Effective Time, and the Company shall have received a certificate of a duly authorized officer of Parent to that effect;
(c)Tax Opinion of Company’s Counsel. The Company shall have received the opinion of Gunderson Dettmer (counsel to the Company), based upon representations of Parent, Merger Sub, Second Acquisition Subsidiary and the Company and normal assumptions, to the effect that the Merger will be treated for Federal income tax purposes as a reorganization qualifying under the provisions of Section 368(a) of the Code, which opinion shall not have been withdrawn or modified in any material respect. The issuance of such opinion shall be conditioned on receipt by Gunderson Dettmer (counsel to the Company) of representation letters from each of Parent, Merger Sub and the Company as contemplated in Section 6.09 of this Agreement. Each such representation letter shall be dated on or before the date of such opinion and shall not have been withdrawn or modified in any material respect as of the Effective Time;
(d)Opinion of Parent’s Counsel. The Company shall have received the opinion of Ropes & Gray LLP (counsel to Parent), or another counsel reasonably satisfactory to the Company, substantially in the form attached hereto asExhibit E;
(e)Listing. If necessary, Parent shall have filed with The Nasdaq National Market a Notification Form for Listing Additional Shares with respect to the shares of Parent Common Stock to be issued pursuant to this Agreement and pursuant to the Company Options;
(f)No Material Adverse Change. Since September 27, 2003, there will have occurred no events nor will there exist any circumstances which singly or in the aggregate have resulted in, or are reasonably likely to result in, a Parent Material Adverse Effect;
(g)Escrow Agreement. Parent and the Escrow Agent shall have entered into the Escrow Agreement;
(h)Board Composition. Parent shall have taken all necessary action to constitute the Board of Directors of Parent, effective upon the Effective Time, consistent with Schedule 1.06(a); or
(i)Executive Group. Parent shall have taken all necessary action to appoint, effective upon the Effective Time, the individuals listed on Schedule 1.06(b) to the executive positions set forth opposite their name.
A-39
Table of Contents
ARTICLE VIII
TERMINATION, AMENDMENT AND WAIVER
SECTION 8.01 Termination. This Agreement may be terminated and the Merger and the other transactions contemplated by this Agreement may be abandoned at any time prior to the Effective Time, notwithstanding any requisite approval and adoption of this Agreement and the transactions contemplated by this Agreement, as follows:
(a) by mutual written consent duly authorized by the Boards of Directors of each of Parent and the Company;
(b) by either Parent or the Company if the Effective Time shall not have occurred on or before April 30, 2004;provided,however, that the right to terminate this Agreement under this Section 8.01(b) shall not be available to any party whose failure to fulfill any obligation under this Agreement has been the cause of, or resulted in, the failure of the Effective Time to occur on or before April 30, 2004;provided further, that if the Effective Time has not occurred as a result of the Registration Statement not having become effective, or the Registration Statement not having become effective in a timely manner so as to hold the Company Stockholders’ Meeting and Parent Stockholders’ Meeting by April 30, 2004, then the right of either Party to terminate this Agreement under this Section 8.01(b) shall not be available unless the Effective Time shall not have occurred on or before May 30, 2004;
(c) by either Parent or the Company upon the issuance of any Order which is final and nonappealable which would prevent the consummation of the Merger;provided, however,that the right to terminate this Agreement under this Section 8.01(c) shall not be available to any party whose failure to fulfill any obligation under this Agreement has been the cause of, or resulted in, such Order;
(d) by Parent upon a breach of any representation, warranty, covenant or agreement on the part of the Company set forth in this Agreement, or if any representation or warranty of the Company shall have become untrue, in either case such that the conditions set forth in Sections 7.02(a) and 7.02(b) would not be satisfied (“Terminating Company Breach”);
provided,however, that, if such Terminating Company Breach is curable by the Company through the exercise of its best efforts and for so long as the Company continues to exercise such best efforts, Parent may not terminate this Agreement under this Section 8.01(d);
(e) by the Company upon a breach of any representation, warranty, covenant or agreement on the part of Parent and Merger Sub set forth in this Agreement, or if any representation or warranty of Parent and Merger Sub shall have become untrue, in either case such that the conditions set forth in Sections 7.03(a) and 7.03(b) would not be satisfied (“Terminating Parent Breach”);provided,however, that, if such Terminating Parent Breach is curable by Parent and Merger Sub through the exercise of their respective best efforts and for so long as Parent and Merger Sub continue to exercise such best efforts, the Company may not terminate this Agreement under this Section 8.01(e);
(f) by Parent if (i) the Board of Directors of the Company withholds, withdraws, amends, modifies or changes the Company Board Recommendation in a manner adverse to Parent or shall have resolved to do so, (ii) the Board of Directors of the Company shall have recommended to the stockholders of the Company a Company Competing Transaction or shall have resolved to do so or shall have entered into any letter of intent or similar document or any agreement, contract or commitment accepting any Company Competing Transaction, (iii) the Board of Directors of the Company fails to reject a Company Competing Transaction within 10 days following public announcement or receipt by the Company of the proposal for such Company Competing Transaction; it being understood that taking no position or indicating its inability to take a position does not constitute a rejection of such Company Competing Transaction, (iv) the Company shall have failed to include in the Joint Proxy Statement the Company Board Recommendation or shall have failed to hold the Company Stockholder Meeting as promptly as practicable and in any event within 45 days after the Registration Statement is declared effective, (v) the Company’s Board of Directors fails to reaffirm the Company Board Recommendation within five business days after Parent requests in writing that such recommendation be reaffirmed, or (vi) the Company shall have willfully breached its obligations under Section 6.05;
(g) by the Company if (i) the Board of Directors of Parent withholds, withdraws, amends, modifies or changes the Parent Board Recommendation in a manner adverse to the Company or shall have resolved to do so, (ii) the Board of Directors of Parent shall have recommended to the stockholders of Parent a Parent Competing Transaction or shall have resolved to do so or shall have entered into any letter of intent or similar document or any agreement, contract or commitment accepting any Parent Competing Transaction, (iii) the Board of Directors of Parent fails to reject a Parent Competing Transaction within 10 days
A-40
Table of Contents
following public announcement or receipt by Parent of the proposal for such Parent Competing Transaction; it being understood that taking no position or indicating its inability to take a position does not constitute a rejection of such Parent Competing Transaction, (iv) Parent shall have failed to include in the Joint Proxy Statement the Parent Board Recommendation or shall have failed to hold the Parent Stockholder Meeting as promptly as practicable and in any event within 45 days after the Registration Statement is declared effective, (v) the Parent’s Board of Directors fails to reaffirm the Parent Board Recommendation within five business days after Parent requests in writing that such recommendation be reaffirmed or (vi) Parent shall have willfully breached its obligations under Section 6.06;
(h) by either Parent or the Company if (i) the Company Stockholders’ Meeting (including any adjournments or postponements thereof) shall have been held and completed and the Company stockholders shall have taken a vote on a proposal to approve this Agreement (including the principal terms hereof) and the Merger, and (ii) this Agreement (including the principal terms hereof) or the Merger shall not have been approved at such meeting by the Company Stockholder Approval (and shall not have been approved at any adjournment or postponement thereof);provided,however, that a party shall not be permitted to terminate this Agreement pursuant to this Section 8.01(h) if the failure to obtain the Company Stockholder Approval is attributable to a failure on the part of such party to perform any material obligation required to be performed by such party at or prior to the Effective Time; or
(i) by either Parent or the Company if (i) the Parent Stockholders’ Meeting (including any adjournments or postponements thereof) shall have been held and completed and the Parent stockholders shall have taken a vote on the Share Issuance and the Parent Charter Vote, and (ii) the Share Issuance and the Parent Charter Vote shall not have been approved at such meeting (and shall not have been approved at any adjournment or postponement thereof) by the Parent Stockholder Approval;provided,however, that a party shall not be permitted to terminate this Agreement pursuant to this Section 8.01(i) if the failure to obtain the Parent Stockholder Approval is attributable to a failure on the part of such party to perform any material obligation required to be performed by such party at or prior to the Effective Time.
SECTION 8.02 Effect of Termination. In the event of termination of this Agreement pursuant to Section 8.01, this Agreement shall forthwith become void, there shall be no liability under this Agreement on the part of Parent, Merger Sub or the Company or any of their respective officers or directors, and all rights and obligations of each party hereto shall cease;provided,however, that (i) Section 6.04(b), and this Section 8.02 shall remain in full force and effect and survive any termination of this Agreement and (ii) nothing herein shall relieve any party from liability for the willful breach of any of its representations or warranties or the breach of any of its covenants or agreements set forth in this Agreement.
SECTION 8.03 Amendment. This Agreement may be amended by the parties hereto by action taken by or on behalf of their respective Boards of Directors at any time prior to the Effective Time. This Agreement may not be amended except by an instrument in writing signed by the parties hereto.
SECTION 8.04 Waiver. At any time prior to the Effective Time, any party hereto may (a) extend the time for the performance of any obligation or other act of any other party hereto, (b) waive any inaccuracy in the representations and warranties contained herein or in any document delivered pursuant hereto, and (c) waive compliance with any agreement or condition contained herein. Any such extension or waiver shall be valid if set forth in an instrument in writing signed by the party or parties to be bound thereby.
SECTION 8.05 Expenses.
(a) Except as set forth in this Section 8.05, all Expenses (as defined below) incurred in connection with this Agreement, the Merger and the other transactions contemplated by this Agreement shall be paid by the party incurring such expenses, whether or not the Merger or any other transaction is consummated. “Expenses” as used in this Agreement shall include all reasonable out-of-pocket expenses (including, without limitation, all fees and expenses of counsel, accountants, investment bankers, experts and consultants to a party hereto and its affiliates) incurred by a party or on its behalf in connection with or related to the authorization, preparation, negotiation, execution and performance of this Agreement, the preparation, printing, filing and mailing of the Registration Statement and the Joint Proxy Statement, the solicitation of stockholder approval, the filing of any required notices and all other matters related to the closing of the Merger and the other transactions contemplated by this Agreement.
(b) The Company agrees that the Company shall pay to Parent an amount equal to all of Parent’s Expenses up to $1,000,000 if Parent shall terminate this Agreement pursuant to Section 8.01(f) or either Parent or the Company shall terminate the Agreement pursuant to Section 8.01(h).
A-41
Table of Contents
(c) The Company agrees that the Company shall pay to Parent an additional $3,044,063.90 if (i) this Agreement is terminated by Parent pursuant to Section 8.01(f) or by either Parent or the Company pursuant Section 8.01(h) and (ii) the Company shall have, within 12 months of the date of such termination, entered into (or announced its intention to enter into) an agreement to consummate a Company Competing Transaction.
(d) Parent agrees that Parent shall pay to the Company an amount equal to all of the Company’s Expenses up to $1,000,000 if the Company shall terminate this Agreement pursuant to Section 8.01(g) or either Parent or the Company shall terminate the Agreement pursuant to Section 8.01(i).
(e) Parent agrees that Parent shall pay to the Company an additional $3,044,063.90 if (i) this Agreement is terminated by the Company pursuant to Section 8.01(g) or by either Parent or the Company pursuant Section 8.01(i) and (ii) Parent shall have, within 12 months of the date of such termination, entered into (or announced its intention to enter into) an agreement to consummate a Parent Competing Transaction.
(f) Any payment required to be made pursuant to Section 8.05(b), (c), (d) or (e) shall be made not later than five business days after delivery to Parent or the Company, as the case may be (the “Requesting Party”), of notice of demand for payment and an itemization setting forth in reasonable detail all Expenses being demanded by the Requesting Party (which itemization may be supplemented and updated from time to time by the Requesting Party until the 60th day after the Requesting Party delivers such notice of demand for payment), and shall be made by wire transfer of immediately available funds to an account designated by the Requesting Party. The payments described in Section 8.05(b), (c), (d) and (e) shall not be in lieu of damages incurred in the event of willful breach of the representations and warranties set forth in this Agreement or the material breach of any of the covenants or agreements set forth in this Agreement.
SECTION 8.06 Payment. In the event that Parent or the Company shall fail to pay the Expenses of the Requesting Party as required by Section 8.05(b), (c), (d) or (e) as applicable, when due, then such Expenses shall be increased to include the costs and expenses actually incurred or accrued by the Requesting Party (including, without limitation, fees and expenses of counsel) in connection with the collection under and enforcement of this Section 8.06, together with interest on such unpaid Expenses, commencing on the date that such Expenses became due, at a rate equal to the rate of interest publicly announced by Citibank, N.A., from time to time, in The City of New York, as such bank’s Prime Rate (the “Prime Rate”) plus 2.00%.
ARTICLE IX
HOLD HARMLESS; INDEMNIFICATION
SECTION 9.01 Survival of Representations and Warranties. The representations and warranties of the Company, Merger Sub and Parent contained in this Agreement shall survive the Effective Time for a period of 18 months following the Effective Time (the “Survival Period”). If written notice of a claim has been given prior to the expiration of the applicable representations and warranties by a party hereto to another party hereto in accordance with Section 9.04(a), then the relevant representations and warranties shall survive as to such claim until such claim has been finally resolved;provided,however, that such claim may only be asserted during the Survival Period.
SECTION 9.02 Escrow; Hold Harmless. (a) After the Effective Time, Parent and its affiliates (including, after the Effective Time, the Surviving Corporation), officers, directors and employees (collectively, the “Parent Indemnified Parties”) shall be held harmless for any actual liabilities, losses, damages, costs, expenses, and penalties (including, without limitation, reasonable attorneys’ fees (other than costs relating to in-house legal counsel) and expenses suffered or paid by them (net of (i) any recoveries under insurance policies or indemnities from third parties if and when actually received by a Parent Indemnified Party, reduced by the present value of any increases in insurance premiums which the Parent Indemnified Party reasonably estimates to result from such recoveries and (ii) any tax benefit actually recognized and realized by the Parent Indemnified Party as a reduction in the amount of its United States federal and state income tax liability in the year in which such Loss is suffered or incurred and which is determined by the applicable Parent Indemnified Party’s tax return preparers to be without material risk of being disallowed on audit) in connection with the payment of such liabilities, losses, damages, costs, expenses, and penalties (collectively, “Losses”), arising out of or resulting from:
A-42
Table of Contents
(i) any inaccuracy or breach of any representation or warranty made by the Company in this Agreement or in any certificate required to be delivered in connection herewith (as each such representation or warranty would read if all materiality or Company Material Adverse Effect provisions were not contained therein);
(ii) any breach of, non-compliance with or non-fulfillment of any covenant or agreement made by the Company in this Agreement or in any certificate delivered in connection herewith;
(iii) any fraudulent action, and any violation of any criminal law by the Company; and
(iv) any claim by a holder or former holder of the Company’s Equity Interests or any other person seeking to assert, or based upon: (i) ownership or rights of ownership to any shares of capital stock of the Company; (ii) any rights of a stockholder of the Company, including any option, preemptive rights, rights to notice or to vote or any appraisal rights under the applicable provisions of the DGCL; (iii) any rights under the Organizational Documents of the Company; (iv) any claim that his, her or its Equity Interests were wrongfully repurchased, canceled, terminated or otherwise limited by the Company; or (v) any claim in connection with the issuance of any Equity Interests or otherwise, regardless of whether an action, suit or proceeding can or has been made against the Company.
(b) Notwithstanding anything to the contrary contained in this Agreement:
(i) The Indemnity Escrow Fund shall be the sole and exclusive right and remedy for any Losses arising out of any and all claims relating to the subject matter of this Agreement (other than claims pursuant to Section 9.02(a)(iii)), and the maximum amount that may be recovered from any single Company Stockholder or holder of assumed Company Options shall be limited to such holder’s pro-rata share of the Indemnity Escrow Fund; and
(ii) no payment from the Indemnity Escrow Fund with respect to any Losses otherwise payable under Section 9.02(a) and arising out of or resulting from the causes enumerated in Section 9.02(a) shall be payable until such time as all such Losses shall aggregate to more than an amount equal to $676,458.64, after which time only such Losses in excess of such amount may be reimbursable from the Indemnity Escrow Fund. An Indemnified Party (as defined in Section 9.04) shall take all reasonable steps to mitigate all Losses upon and after becoming aware of any event that could reasonably be expected to give rise to any Losses.
(c) Parent acknowledges and agrees that it (i) has made its own inquiry and investigation into, and, based thereon, has formed an independent judgment concerning the Company, (ii) has been furnished with or given adequate access to such information about the Company as it has requested and (iii) will not assert any claim against the Company or any of its directors, officers, employees, agents, stockholders, affiliates, consultants, counsel, accountants, investment bankers or representatives, or hold the Company or any such persons liable, for any inaccuracies, misstatements or omissions with respect to information (other than, with respect to the Company, the representations and warranties contained in this Agreement) furnished by the Company or any such persons concerning the Company or any of its affiliates.
(d) Company acknowledges and agrees that it (i) has made its own inquiry and investigation into, and, based thereon, has formed an independent judgment concerning Parent, (ii) has been furnished with or given adequate access to such information about Parent as it has requested and (iii) will not assert any claim against Parent or any of its directors, officers, employees, agents, stockholders, affiliates, consultants, counsel, accountants, investment bankers or representatives, or hold Parent or any such persons liable, for any inaccuracies, misstatements or omissions with respect to information (other than, with respect to Parent, the representations and warranties contained in this Agreement) furnished by Parent or any such persons concerning Parent or any of its affiliates.
(e) In connection with Parent’s investigation of the Company, Parent has received from the Company certain estimates, projections and other forecasts for the Company, and certain plan and budget information. Parent acknowledges that there are uncertainties inherent in attempting to make such projections, forecasts, plans and budgets, that Parent is familiar with such uncertainties, that Parent is taking full responsibility for making its own evaluation of the adequacy and accuracy of all estimates, projections, forecasts, plans and budgets so furnished to it, and that Parent will not assert any claim against the Company or any of its directors, officers, employees, agents, stockholders, affiliates, consultants, counsel, accountants, investment bankers or representatives, or hold any such persons liable, with respect thereto. Except for the representations and warranties of the Company set forth in this Agreement, the Company makes no representation or warranty with respect to any estimates, projections, forecasts, plans or budgets referred to in this Section 9.02(e).
A-43
Table of Contents
(f) In connection with the Company’s investigation of Parent, Company has received from Parent certain estimates, projections and other forecasts for Parent, and certain plan and budget information. Company acknowledges that there are uncertainties inherent in attempting to make such projections, forecasts, plans and budgets, that Company is familiar with such uncertainties, that Company is taking full responsibility for making its own evaluation of the adequacy and accuracy of all estimates, projections, forecasts, plans and budgets so furnished to it, and that Company will not assert any claim against Parent or any of its directors, officers, employees, agents, stockholders, affiliates, consultants, counsel, accountants, investment bankers or representatives, or hold any such persons liable, with respect thereto. Except for the representations and warranties of Parent set forth in this Agreement, Parent makes no representation or warranty with respect to any estimates, projections, forecasts, plans or budgets referred to in this Section 9.02(f).
SECTION 9.03 Indemnification by Parent. (a)After the Effective Time, the Company Stockholders and their respective affiliates, officers, directors, employees, agents, successors and assigns (collectively, the “Stockholder Indemnified Parties”) shall be indemnified and held harmless by Parent for any and all Losses, arising out of or resulting from:
(i) the breach of any representation or warranty made by Parent in this Agreement or in any certificate required to be delivered in connection herewith (as each such representation or warranty would read if all materiality or Parent Material Adverse Effect provisions were not contained therein);
(ii) any breach of, non-compliance with or non-fulfillment of any covenant or agreement made by Parent in this Agreement or in any certificate delivered in connection herewith; and
(iii) any fraudulent action, and any violation of any criminal law by Parent.
(b) Notwithstanding anything to the contrary contained in this Agreement:
(i) no payment with respect to any Losses otherwise payable under Section 9.03(a) and arising out of or resulting from the causes enumerated in Section 9.03(a) shall be payable until such time as all such Losses shall aggregate to more than an amount equal to $676,458.64, after which time the Stockholder Indemnified Parties shall be entitled to recover only such Losses in excess of such amount. An Indemnified Party (as defined in Section 9.04) shall take all reasonable steps to mitigate all Losses upon and after becoming aware of any event that could reasonably be expected to give rise to any Losses; and
(ii) Parent’s indemnification obligations under this Section 9.03 shall be the sole and exclusive right and remedy for any Losses arising out of any and all claims relating to the subject matter of this Agreement (other than claims pursuant to Section 9.03(a)(iii)), and Parent shall have no liability under this Section 9.03 to Stockholder Indemnified Parties for Losses in excess of an amount equal to $13,529,172.87.
SECTION 9.04 Indemnification Procedures.
(a) For purposes of this Section 9.04, a party against which indemnification may be sought is referred to as the “Indemnifying Party” and the party which may be entitled to indemnification is referred to as the “Indemnified Party.” Within 30 days following the incurrence of any Losses by an Indemnified Party who believes that such party is entitled to indemnification pursuant to this Article IX, the Indemnified Party shall deliver to the Indemnifying Party a certificate which shall:
(i) state that the Indemnified Party has paid Losses for which such Indemnified Party is entitled to indemnification pursuant to Article IX;
(ii) specify in reasonable detail each individual item of Loss included in the amount so stated, the date such item was paid and the nature of the misrepresentation, breach of warranty, breach of covenant or claim to which each such item is related and the computation of the amount to which such Indemnified Party claims to be entitled hereunder; and
(iii) attached thereto all supporting documents, calculations, correspondence and all other documents related to each item of Loss set forth on the certificate.
No delay on the part of the Indemnified Party in notifying any Indemnifying Party shall relieve the Indemnifying Party from any obligation hereunder unless (and then solely to the extent) the Indemnifying Party thereby is prejudiced.
(b) The obligations and liabilities of Indemnifying Parties under this Article IX with respect to Losses arising from actual or threatened claims or demands by any third party which are subject to the indemnification provided for in this Article IX (“Third Party
A-44
Table of Contents
Claims”) shall be governed by and contingent upon the following additional terms and conditions: if an Indemnified Party shall receive notice of any Third Party Claim, the Indemnified Party shall give the Indemnifying Party notice of such Third Party Claim within 30 days of the receipt by the Indemnified Party of such notice; provided, that no delay on the part of the Indemnified Party in notifying any Indemnifying Party shall relieve the Indemnifying Party from any obligation hereunder unless (and then solely to the extent) the Indemnifying Party thereby is prejudiced. The notice of claim shall describe in reasonable detail the facts known to the Indemnified Party giving rise to such indemnification claim, and the amount or good faith estimate of the amount arising therefrom.
(c) The Indemnifying Party shall be entitled to assume and control the defense of any Third Party Claim through counsel of its choice (such counsel to be reasonably acceptable to the Indemnified Party) so long as (i) the Indemnifying Party notifies the Indemnified Party (based on the facts and circumstances then known by the Indemnifying Party) in writing within 30 calendar days after the Indemnified Party has given notice of the Third Party Claim that the Indemnifying Party will indemnify the Indemnified Party from and against the entirety of any Losses the Indemnified Party may suffer resulting from, arising out of, relating to, in the nature of, or caused by the Third Party Claim, (ii) the Indemnifying Party provides the Indemnified Party with evidence acceptable to the Indemnified Party that the Indemnifying Party will have the financial resources to defend against the Third Party Claim and fulfill its indemnification obligations hereunder, (iii) the Third Party Claim involves only money damages and does not seek an injunction or other equitable relief, (iv) settlement of, or an adverse judgment with respect to, the Third Party Claim is not, in the good faith judgment of the Indemnified Party, likely to establish a precedential custom or practice adverse to the continuing business interests of the Indemnified Party and (v) the Indemnifying Party conducts the defense of the Third Party Claim actively and diligently. The Indemnified Party shall cooperate with the Indemnifying Party in such defense and make available to the Indemnifying Party, at the Indemnifying Party’s expense, all witnesses, pertinent records, materials and information in the Indemnified Party’s possession or under the Indemnified Party’s control relating thereto as is reasonably requested by the Indemnifying Party.
(d) So long as the Indemnifying Party is conducting the defense of the Third Party Claim in accordance with Section 9.04(c) above, (i) the Indemnified Party may retain separate co-counsel at its sole cost and expense and participate in the defense of the Third Party Claim, (ii) the Indemnified Party will not consent to the entry of any judgment or enter into any settlement with respect to the Third Party Claim without the prior written consent of the Indemnifying Party (which consent shall not unreasonably be withheld) and (iii) except with the written consent of the Indemnified Party (not to be unreasonably withheld), the Indemnifying Party will not consent to the entry of any judgment or enter into any settlement with respect to the Third Party Claim unless written agreement is obtained releasing the Indemnified Party from all liability thereunder.
(e) In the event any of the conditions in Section 9.04(c) which has been satisfied thereafter becomes unsatisfied, however, the Indemnified Party may defend against, and consent to the entry of any judgment or enter into any settlement with respect to, the Third Party Claim in any manner it may deem appropriate (and the Indemnified Party need not consult with, or obtain any consent from, any Indemnifying Party in connection therewith).
SECTION 9.05 Stockholders’ Representative.
(a) Effective only upon the Effective Time, Luke Evnin (such person and any successor or successors being the “Stockholders’ Representative”) shall act as the representative of the Company Stockholders, and shall be authorized to act on behalf of the Company Stockholders and to take any and all actions required or permitted to be taken by the Stockholders’ Representative under this Agreement with respect to any claims (including the settlement thereof) made by a Parent Indemnified Party for indemnification pursuant to this Article IX and with respect to any actions to be taken by the Stockholders’ Representative pursuant to the terms of the Escrow Agreement (including, without limitation, the exercise of the power to (i) authorize the delivery of Escrow Shares to a Parent Indemnified Party in satisfaction of claims by a Parent Indemnified Party, (ii) agree to, negotiate, enter into settlements and compromises of, and comply with orders of courts with respect to any claims for indemnification and (iii) take all actions necessary in the judgment of the Stockholders’ Representative for the accomplishment of the foregoing). In all matters relating to this Article IX as described in the preceding sentence, the Stockholders’ Representative shall be the only party entitled to assert the rights of the Company Stockholders, and the Stockholders’ Representative shall perform all of the obligations of the Company Stockholders hereunder. The Parent Indemnified Parties shall be entitled to rely on all statements, representations and decisions of the Stockholders’ Representative. The Stockholders’ Representative is not entitled to amend this Agreement or take any actions relating to this Agreement prior to the Effective Time. Following the Effective Time, the Stockholders’ Representative may amend this Agreement with the prior written consent of the holders of a majority-in-interest in the Escrow Funds. The Stockholders’ Representative may resign upon not less than 20
A-45
Table of Contents
business days’ prior written notice to Parent and the Company Stockholders. The Company Stockholders by the vote of a majority-in-interest of the Escrow Funds may remove the Stockholders’ Representative from time to time upon not less than 20 business days’ prior written notice to Parent. Any vacancy in the position of the Stockholders’ Representative may be filled by the approval of the holders of a majority-in-interest in the Escrow Funds. Any successor Stockholders’ Representative shall acknowledge in writing to Parent her acceptance of her appointment as Stockholders’ Representative.
(b) The Company Stockholders shall be bound by all actions taken by the Stockholders’ Representative in his, her or its capacity thereof, except for any action that conflicts with the limitations set forth in subsection (d) below. The Stockholders’ Representative shall promptly, and in any event within ten business days, provide written notice to the Company Stockholders of any action taken on behalf of them by the Stockholders’ Representative pursuant to the authority delegated to the Stockholders’ Representative under this Section 9.05. The Stockholders’ Representative shall at all times act in his or her capacity as Stockholders’ Representative in a manner that the Stockholders’ Representative believes to be in the best interest of the Company Stockholders. Neither the Stockholders’ Representative nor any of its directors, officers, agents or employees, if any, shall be liable to any person for any error of judgment, or any action taken, suffered or omitted to be taken under this Agreement or the Escrow Agreement, except in the case of its gross negligence, bad faith or willful misconduct. The Stockholders’ Representative may consult with legal counsel, independent public accountants and other experts selected by it. The Stockholders’ Representative shall not have any duty to ascertain or to inquire as to the performance or observance of any of the terms, covenants or conditions of this Agreement or the Escrow Agreement. As to any matters not expressly provided for in this Agreement or the Escrow Agreement, the Stockholders’ Representative shall not exercise any discretion or take any action.
(c) Each Company Stockholder on whose behalf the Escrow Shares were contributed to the Escrow Account shall, severally and not jointly, hold harmless and reimburse the Stockholders’ Representative from and against such Company Stockholder’s ratable share of any and all liabilities, losses, damages, claims, costs or expenses suffered or incurred by the Stockholders’ Representative arising out of or resulting from any action taken or omitted to be taken by the Stockholders’ Representative under this Agreement or the Escrow Agreement, other than such liabilities, losses, damages, claims, costs or expenses (including the reasonable fees and expenses of any legal counsel retained by the Stockholders’ Representative) arising out of or resulting from the Stockholders’ Representative’s gross negligence, bad faith or willful misconduct;provided,however, that no such Company Stockholder shall be liable in excess of such Company Stockholder’s pro rata portion of the Aggregate Merger Consideration. In the event there are any remaining funds in the Escrow Accounts to be distributed to stockholders of Company immediately prior to any interim distribution or the final distribution from the Escrow Accounts pursuant to the Escrow Agreement, the Stockholders’ Representative shall be entitled to recover any such expenses from the Escrow Accounts prior to the distribution of funds to the Company Stockholders. The Stockholders’ Representative shall not be entitled to any compensation for his or her services in such capacity.
(d) Notwithstanding anything to the contrary herein or in the Escrow Agreement, the Stockholders’ Representative is not authorized to, and shall not, accept on behalf of any Company Stockholder any merger consideration to which such Company Stockholder is entitled under this Agreement and the Stockholders’ Representative shall not in any manner exercise, or seek to exercise, any voting power whatsoever with respect to shares of capital stock of the Company or Parent now or hereafter owned of record or beneficially by any Company Stockholder unless the Stockholders’ Representative is expressly authorized to do so in a writing signed by such Company Stockholder.
ARTICLE X
GENERAL PROVISIONS
SECTION 10.01 Notices. All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be deemed to have been duly given upon receipt) by delivery in person, by cable, telecopy, facsimile, telegram or telex or by registered or certified mail (postage prepaid, return receipt requested) to the respective parties at the following addresses (or at such other address for a party as shall be specified in a notice given in accordance with this Section 10.01):
(a) if to Parent or Merger Sub:
Genome Therapeutics Corp.
100 Beaver Street
A-46
Table of Contents
Waltham, MA 02453
Facsimile No.: 781-893-8277
Attention: Chief Financial Officer
with a copy to:
Ropes & Gray LLP
One International Place
Boston, MA 02110
Facsimile No.: (617) 951-7050
Attention: Patrick O’Brien
(b) if to the Company:
Genesoft Pharmaceuticals, Inc.
7300 Shoreline Court
South San Francisco, CA 94080
Facsimile No.: 650-827-0477
Attention: Chief Executive Officer
with a copy to:
Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP
155 Constitution Drive
Menlo Park, California 94025
Facsimile No.: (650) 321-2800
| Attention: | Christopher D. Dillon | |
| David T. Young |
(c) if to the Stockholders’ Representative:
Luke Evnin, Ph.D.
601 Gateway Blvd.
Suite 350
South San Francisco, CA 94080
Facsimile No.: (650) 553-3301
SECTION 10.02 Certain Definitions.
(a) As used in this Agreement, the following terms shall have the following meanings:
(i) “affiliate” of a specified person means a person who directly or indirectly through one or more intermediaries controls, is controlled by, or is under common control with such specified person.
(ii) “beneficial owner” with respect to any shares means a person who shall be deemed to be the beneficial owner of such shares (i) which such person or any of its affiliates or associates (as such term is defined in Rule 12b-2 promulgated under the Exchange Act) beneficially owns, directly or indirectly, (ii) which such person or any of its affiliates or associates has, directly or indirectly, (A) the right to acquire (whether such right is exercisable immediately or subject only to the passage of time), pursuant to any agreement, arrangement or understanding or upon the exercise of consideration rights, exchange rights, warrants or options, or otherwise, or (B) the right to vote pursuant to any agreement, arrangement or understanding, or (iii) which are beneficially owned, directly or indirectly, by any other persons with whom such person or any of its affiliates or associates or person with whom such person or any of its affiliates or associates has any agreement, arrangement or understanding for the purpose of acquiring, holding, voting or disposing of any shares.
(iii) “business day” means any day on which banks are not required or authorized to close in San Francisco, California.
(iv) “control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly or as trustee or executor, of the power to direct or cause the direction of the management and policies of a person, whether through the ownership of voting securities, as trustee or executor, by contract or credit arrangement or otherwise.
A-47
Table of Contents
(v) “Equity Interests” means (a) any capital stock, share, partnership or membership interest, unit of participation or other similar interest (however designated) in any person and (b) any option, warrant, purchase right, conversion right, exchange rights or other contract or agreement, whether written or oral, which would entitle any person to acquire any such interest in such person or otherwise entitle any person to share in the equity, profit, earnings, losses or gains of such person (including stock appreciation, phantom stock, profit participation or other similar rights).
(vi) “knowledge” means, with respect to the Company, actual knowledge of David B. Singer, the Company’s Chairman and Chief Executive Officer, Gary Patou, the Company’s President, Asha Rajagopal, the Company’s Director of Finance, and Sofia Touami, the Company’s Business Development Manager.
(vii) “organizational documents” means, with respect to any person (other than an individual), (a) the certificate or articles of incorporation or organization and any joint venture, limited liability company, operating or partnership agreement and other similar documents adopted or filed in connection with the creation, formation or organization of such person and (b) all by-laws, voting agreements and similar documents, instruments or agreements relating to the organization or governance of such person, in each case, as amended or supplemented.
(viii) “person” means an individual, corporation, partnership, limited partnership, syndicate, person (including, without limitation, a “person” as defined in Section 13(d)(3) of the Exchange Act), trust, association or entity or government, political subdivision, agency or instrumentality of a government.
(ix) “subsidiary” or “subsidiaries” of any person means any corporation, partnership, joint venture or other legal entity of which such person (either alone or through or together with any other subsidiary) owns, directly or indirectly, more than 50% of the stock or other equity interests, the holders of which are generally entitled to vote for the election of the board of directors or other governing body of such corporation or other legal entity.
A-48
Table of Contents
(b) The following terms shall have the meanings defined for such terms in the Sections of this Agreement set forth:
Term | Section | |
Accrued Interest Shares | 2.01(b)(vi) | |
affiliate | 10.02(a) | |
Aggregate Merger Consideration | 2.01(b) | |
Agreement | Preamble | |
beneficial owner | 10.02(a) | |
Bulk Product | 3.27 | |
business day | 10.02(a) | |
Certificate of Merger | 1.02 | |
Closing | 1.02 | |
Closing Date | 1.02 | |
COBRA | 3.11(d) | |
Code | Recitals | |
Combined Company Financing | 6.15 | |
Common Exchange Ratio | 2.01(b) | |
Company | Preamble | |
Company Assets | 3.17 | |
Company Audited Financial Statements | 3.08(a) | |
Company Board Recommendation | 6.01(b) | |
Company Competing Transaction | 6.05(b) | |
Company Confidential Information | 3.14(d) | |
Company Disclosure Schedule | Article III | |
Company ERISA Affiliate | 3.11(c) | |
Company Insiders | 6.14 | |
Company Intellectual Property | 3.14(a) | |
Company Interim Financial Statements | 3.08(a) | |
Company Material Adverse Effect | 3.01 | |
Company Options | 2.04(a) | |
Company Plan(s) | 3.11(a) | |
Company Reference Balance Sheet | 3.08(a) | |
Company Series A Preferred Stock | 3.04(a) | |
Company Series B Preferred Stock | 3.04(a) | |
Company Series C Preferred Stock | 3.04(a) |
A-49
Table of Contents
Term | Section | |
Company Series D Preferred Stock | 3.04(a) | |
Company Series 1 Preferred Stock | 3.04(a) | |
Company Share Certificates | 2.02(a) | |
Company Stock | Recitals | |
Company Stockholders | 2.02(b) | |
Company Stockholders’ Meeting | 6.01(a) | |
Company Stock Plan | 3.04(b) | |
Company Superior Proposal | 6.05(a) | |
Company Voting Agreement | Recitals | |
Company Warrant | 2.04(b) | |
control | 10.02(a) | |
DGCL | Recitals | |
Dissenting Shares | 2.05(a) | |
Effective Time | 1.02 | |
Equity Interests | 10.02(a) | |
Environmental Laws | 3.13 | |
Environmental Permits | 3.13 | |
ERISA | 3.11(a) | |
Escrow Accounts | 2.02(b) | |
Escrow Agent | 2.02(b) | |
Escrow Agreement | 2.02(b) | |
Escrow Funds | 2.02(b) | |
Escrow Shares | 2.01(b) | |
Exchange Act | 4.06(a) | |
Exchange Agent | 2.02(a) | |
Expenses | 8.05(a) | |
FDA | 3.07(b) | |
FDA Company Permits | 3.07(b) | |
FDA Parent Permits | 4.07(b) | |
Final Product | 3.27 | |
Financing Committee | 6.15 | |
Fully Diluted Common Shares Amount | 2.01(b) | |
Governmental Entity | 3.06(b) | |
Gunderson Dettmer | 1.02 | |
Hazardous Materials | 3.13 | |
HSR Act | 3.06(b) | |
Indemnified Party | 9.04(a) | |
Indemnifying Party | 9.04(a) | |
Indemnity Escrow Account | 2.02(b) | |
Indemnity Escrow Fund | 2.02(b) | |
Indemnity Escrow Shares | 2.01(b) | |
Infringement | 3.14(a) | |
Intellectual Property | 3.14(a) | |
IP Rights | 3.14(a) | |
Inventions | 3.14(a) |
A-50
Table of Contents
Term | Section | |
Joint Proxy Statement | 6.01(a) | |
knowledge | 10.02(a) | |
Law | 3.06(a) | |
Legal Proceeding | 3.10 | |
Letter of Transmittal | 2.02(a) | |
Liabilities | 3.08(b) | |
License Agreement | 3.12(a) | |
Licensor | 3.12(a) | |
LLC Agreement | 1.04(b) | |
Losses | 9.02(a) | |
Marks | 3.14(a) | |
Material Company Contracts | 3.12(a) | |
Material Parent Contracts | 4.12(a) | |
Merger | Recitals | |
Merger Sub | Preamble | |
NASD Rule | 4.16 | |
Non-Disclosure Agreement | 6.04(b) | |
Non-FDA Company Permits | 3.07(a) | |
Non-FDA Parent Permits | 4.07(a) | |
Note Amendment and Exchange Agreement | Recitals | |
Notice of Company Superior Proposal | 6.01(c) | |
Notice of Parent Superior Proposal | 6.01(e) | |
Order | 7.01(c) | |
organizational documents | 10.02(a) | |
Other Escrow Account | 2.02(b) | |
Other Escrow Fund | 2.02(b) | |
Other Escrow Shares | 2.01(b) | |
Parent | Preamble | |
Parent Assets | 4.17 | |
Parent Board Recommendation | 6.01(d) | |
Parent Charter Vote | 4.16 | |
Parent Common Stock | Recitals | |
Parent Competing Transaction | 6.06(b) | |
Parent Confidential Information | 4.14(d) | |
Parent Disclosure Schedule | Article IV | |
Parent ERISA Affiliate | 4.11(c) | |
Parent Indemnified Parties | 9.02(a) | |
Parent Intellectual Property | 4.14(a) | |
Parent Interim Financial Statements | 4.15(a) | |
Parent Material Adverse Effect | 4.01(a) | |
Parent Options | 4.04(a) | |
Parent Plan(s) | 4.11(a) | |
Parent Reference Balance Sheet | 4.15(a) | |
Parent SEC Reports | 4.08(a) | |
Parent Securities | 6.15 |
A-51
Table of Contents
Term | Section | |
Parent Shares | 2.01(b) | |
Parent Stockholder Approvals | 4.16 | |
Parent Stockholders’ Meeting | 6.01(a) | |
Parent Stock Plan | 4.04(a) | |
Parent Superior Proposal | 6.06(a) | |
Parent Voting Agreement | Recitals | |
person | 10.02(a) | |
Preference Shares | 2.01(b)(v) | |
Prime Rate | 8.06 | |
Principal Stockholders | Recitals | |
Promissory Notes | 5.01(i) | |
Registration Statement | 6.01(a) | |
Representatives | 6.04(a) | |
Requesting Party | 8.05(f) | |
Reverse Merger | Recitals | |
SEC | 4.08(a) | |
Second Acquisition Subsidiary | Recitals | |
Second-Step Merger | Recitals | |
Section 16 Information | 6.14 | |
Securities Act | 4.08(a) | |
Share Issuance | Recitals | |
Stockholder Indemnified Parties | 9.03(a) | |
Stockholders’ Representative | 9.05(a) | |
subsidiaries | 10.02(a) | |
subsidiary | 10.02(a) | |
Survival Period | 9.01 | |
Surviving Corporation | 1.01 | |
Tax | 3.15(c) | |
Taxable | 3.15(c) | |
Tax Authority | 3.15(c) | |
Taxes | 3.15(c) | |
Tax Return(s) | 3.15(a) | |
Terminating Company Breach | 8.01(d) | |
Terminating Parent Breach | 8.01(e) | |
Third Party Claims | 9.04(b) | |
Transactions | 6.04(b) | |
U.S. GAAP | 3.08(a) | |
Used | 3.14(a) |
SECTION 10.03 Severability. If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any rule of Law or public policy, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the transactions contemplated by this Agreement is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the transactions contemplated by this Agreement be consummated as originally contemplated to the fullest extent possible.
SECTION 10.04 Assignment; Binding Effect; Benefit. Neither this Agreement nor any of the rights, interests or obligations hereunder shall be assigned by any of the parties hereto (whether by operation of Law or otherwise) without the prior written consent of the other parties. Subject to the preceding sentence, this Agreement shall be binding upon and shall inure to the benefit of the parties hereto and their respective successors and assigns. Other than under Sections 6.04, 6.09 and 6.12, nothing in this Agreement,
A-52
Table of Contents
expressed or implied, is intended to confer on any person other than the parties hereto or their respective successors and assigns any rights, remedies, obligations or liabilities under or by reason of this Agreement.
SECTION 10.05 Incorporation of Exhibits. The Company Disclosure Schedule, the Parent Disclosure Schedule, the Schedules and all Exhibits attached hereto and referred to herein are hereby incorporated herein and made a part hereof for all purposes as if fully set forth herein.
SECTION 10.06 Specific Performance. The parties hereto agree that irreparable damage would occur in the event any provision of this Agreement was not performed in accordance with the terms hereof and that the parties shall be entitled to specific performance of the terms hereof in addition to any other remedy at law or in equity.
SECTION 10.07 Governing Law. This Agreement shall be governed by, and construed in accordance with, the laws of the State of New York and without regard to any applicable conflicts of law.
SECTION 10.08 Time of the Essence. For purposes of this Agreement and the transactions contemplated by this Agreement, time is of the essence.
SECTION 10.09 Construction and Interpretation.
(a) For purposes of this Agreement, whenever the context requires, the singular number shall include the plural, and vice versa; the masculine gender shall include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter gender shall include the masculine and feminine genders.
(b) Neither this Agreement nor any uncertainty or ambiguity herein shall be construed or resolved against any party, whether under any rule of construction or otherwise. No party to this Agreement shall be considered the draftsman. The parties acknowledge and agree that this Agreement has been reviewed, negotiated, and accepted by all parties and their attorneys and shall be construed and interpreted according to the ordinary meaning of the words used so as fairly to accomplish the purposes and intentions of all parties hereto.
(c) As used in this Agreement, the words “include” and “including,” and variations thereof, shall not be deemed to be terms of limitation, but rather shall be deemed to be followed by the words “without limitation.”
(d) Except as otherwise indicated, all references in this Agreement to “Articles,” “Sections,” “Schedules” and “Exhibits” are intended to refer to an Article or Section of, or Schedule or Exhibit to, this Agreement.
(e) Except as otherwise indicated, all references (i) to any agreement (including this Agreement), contract or Law are to such agreement, contract or Law as amended, modified, supplemented or replaced from time to time, and (ii) to any Governmental Entity include any successor to that Governmental Entity.
SECTION 10.10 Further Assurances. Each party hereto shall execute and cause to be delivered to each other party hereto such instruments and other documents, and shall take such other actions, as such other party may reasonably request (prior to, at or after the Closing) for the purpose of carrying out or evidencing any of the transactions contemplated by this Agreement.
SECTION 10.11 Headings. The descriptive headings contained in this Agreement are included for convenience of reference only and shall not affect in any way the meaning or interpretation of this Agreement.
SECTION 10.12 Counterparts. This Agreement may be executed and delivered (including by facsimile transmission) in two or more counterparts, each of which when executed and delivered shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
SECTION 10.13 Entire Agreement. This Agreement (including the Exhibits, the Schedules, the Company Disclosure Schedule and the Parent Disclosure Schedule) and the Non-Disclosure Agreement constitute the entire agreement among the parties with respect to the subject matter hereof and supersede all prior agreements and understandings among the parties with respect thereto. No
A-53
Table of Contents
addition to or modification of any provision of this Agreement shall be binding upon any party hereto unless made in writing and signed by all parties hereto.
[Remainder of page intentionally left blank]
A-54
Table of Contents
IN WITNESS WHEREOF,each of Parent, Merger Sub, the Company and the Stockholders’ Representative has executed or has caused this Agreement to be executed by its duly authorized officer as of the date first written above.
| GENOME THERAPEUTICS CORP. | ||
By: | /s/ STEVEN RAUSCHER | |
Steven Rauscher President and Chief Executive Officer | ||
| GUARDIAN ACQUISITION, INC. | ||
By: | /s/ STEVEN RAUSCHER | |
Steven Rauscher President | ||
| GENESOFT PHARMACEUTICALS, INC. | ||
| By: | /s/ DAVID SINGER | |
David Singer Chief Executive Officer | ||
| LUKE EVNIN | ||
/s/ LUKE EVNIN | ||
| Luke Evnin, solely for purposes of Section 9.05, as Stockholders’ Representative | ||
A-55
Table of Contents
EXHIBIT A-1
Form of Company Voting Agreement
E-1
Table of Contents
EXHIBIT A-2
Form of Parent Voting Agreement
E-2
Table of Contents
EXHIBIT B
Form of Escrow Agreement
E-3
Table of Contents
EXHIBIT C
Form of Company Affiliate Agreement
E-4
Table of Contents
EXHIBIT D
Form of Company Counsel Legal Opinion
E-5
Table of Contents
EXHIBIT E
Form of Parent Counsel Legal Opinion
E-6
Table of Contents
Schedule 1.06(a)
Members of the Board of Directors of Parent following the Effective Time
Members of the Board of Directors of Parent
Luke B. Evnin, Ph.D.
Robert J. Hennessey
Vernon R. Loucks, Jr.
William J. Rutter
Steven M. Rauscher
William S. Reardon
Norbert G. Riedel, Ph.D.
David B. Singer (Chairman)
David K. Stone
S-1
Table of Contents
Schedule 1.06(b)
Executive Management of Parent following the Effective Time
Name of Officer | Title | |
| Steven M. Rauscher | Chief Executive Officer | |
| Stephen Cohen | Senior Vice President and Chief Financial Officer | |
| Martin Williams | Senior Vice President, Corporate Development and Marketing |
S-2
Table of Contents
Annex B
Escrow Agreement
(to be filed by amendment)
B-1
Table of Contents
Annex C
Opinion of Harris Nesbitt Corp.
November 10, 2003
Board of Directors
Genome Therapeutics Corp.
100 Beaver Street
Waltham, Massachusetts 02453
Gentlemen:
GeneSoft Pharmaceuticals, Inc. (“GeneSoft” or the “Company”), Genome Therapeutics Corp. (“Genome” or the “Acquiror”), a wholly owned subsidiary of Genome (“Merger Sub”) and a representative of the stockholders of GeneSoft (the “Stockholders’ Representative”), propose to enter into an Agreement and Plan of Merger and Reorganization (the “Agreement”) pursuant to which GeneSoft will merge with and into Merger Sub, with Merger Sub surviving as a wholly owned subsidiary of Genome (the “Merger”).
Genome will issue an aggregate of 28.571 million shares (the “Aggregate Stock Consideration”) of Genome common stock, par value $0.10 per share, in connection with the Merger and related transactions (collectively with the Merger, the “Transaction”) under the Note Amendment and Exchange Agreement (as defined below). You have asked us whether or not, in our opinion, the Aggregate Stock Consideration to be issued by the Acquiror in the Transaction is fair to the Acquiror from a financial point of view.
In arriving at the opinion set forth below, we have, among other things:
| 1. | Reviewed the Acquiror’s Annual Reports on Form 10-K and related publicly-available financial information for the three fiscal years ended December 31, 2000, 2001, 2002 and the Acquiror’s Form 10-Q and the related unaudited financial information for the nine months ended September 30, 2003; |
| 2. | Reviewed certain financial and operating information relating to the business, earnings, cash flow, assets and prospects of the Company and the Acquiror, furnished to us by the Company and the Acquiror; |
| 3. | Reviewed certain financial projections prepared by the management of the Company and the Acquiror; |
| 4. | Conducted discussions with members of senior management of the Company and the Acquiror concerning their respective operations, financial condition and prospects; |
| 5. | Reviewed the historical market prices and trading activity for shares of the common stock of the Company and the Acquiror and compared them with those of certain publicly-traded companies which we deemed to be reasonably similar to the Company and the Acquiror; |
| 6. | Compared the financial performance of the Company and the Acquiror with that of certain companies which we deemed to be reasonably similar to the Company and the Acquiror, respectively; |
| 7. | Compared the proposed financial terms to the Acquiror of the Transaction with the financial terms of certain other mergers and acquisitions which we deemed to be relevant; |
| 8. | Reviewed a draft of the Agreement dated November 10, 2003; |
| 9. | Reviewed a draft dated November 10, 2003 of the Note Amendment and Exchange Agreement among Genome, the Company and the holders of certain notes and warrants issued by the Company (the “Note Amendment and Exchange Agreement”); |
| 10. | Reviewed drafts each dated November 10, 2003 of the forms of Voting Agreements (the “Voting Agreements”) to be entered into by certain principal stockholders of the Company for the benefit of Genome and by certain principal stockholders of Genome for the benefit of the Company; |
C-1
Table of Contents
| 11. | Reviewed a draft dated November 10, 2003 of the form of Registration Rights Agreement (the “Registration Rights Agreement”) to be entered into by Genome and certain holders of Genome Common Stock following the Merger; |
| 12. | Reviewed a draft dated November 10, 2003 of the form of escrow agreement (the “Escrow Agreement,” and, together with the Note Amendment and Exchange Agreement, the Voting Agreements, and the Registration Rights Agreement, the “Ancillary Agreements”) to be entered into by Genome, the Stockholders’ Representative and an escrow agent to be selected by Genome and the Stockholders’ Representative; and |
| 13. | Reviewed such other financial studies, performed such other analyses and investigations and took into account such other matters as we deemed appropriate. |
In preparing our opinion, we have relied on the accuracy and completeness of all information reviewed by us, and we have not independently verified such information or undertaken an independent valuation or appraisal of the assets of the Company or the Acquiror, nor have we been furnished with any such valuation or appraisal. We understand that in connection with the Merger, the Acquiror will lend $6,200,000 to the Company at the time of execution of the Agreement. We have not reviewed the documents providing for this loan and express no opinion on its terms. We understand that the parties intend that the Merger be treated for tax purposes as a tax free reorganization.
With respect to the financial projections supplied to us, we have assumed that they have been reasonably prepared on bases reflecting the best currently available estimates and good faith judgments of senior management of the Company and the Acquiror of their respective future competitive, operating and regulatory environments and related financial performance of the Company and the Acquiror. We express no opinion with respect to such projections or the assumptions on which they are based. Furthermore, we have neither reviewed the books and records of the Company or the Acquiror nor conducted a physical inspection of the properties or facilities of the Company or the Acquiror. We have assumed that the executed version of the Agreement and the Ancillary Agreements will not differ in any material respect from the last draft we reviewed, and that the Transaction will be consummated on the terms set forth therein, without waiver or modification of any material terms. Our opinion is necessarily based on economic, market and other conditions and circumstances as they exist and can be evaluated on, and the information made available to us as of, the date hereof.
This opinion does not constitute a recommendation to any stockholder of the Acquiror or the Company as to how any such stockholder should vote on the Transaction. This opinion does not address the relative merits of the Transaction and any other transactions or business strategies discussed by the Board of Directors of the Acquiror as alternatives to the Transaction or the decision of the Board of Directors of the Acquiror to proceed with the Transaction. We express no opinion as to the prices at which the Genome Common Stock or the Company Common Stock may trade after announcement of the Transaction or, with respect to the Genome Common Stock, following completion of the Transaction.
Our opinion addresses only the fairness from a financial point of view to the Acquiror of the Aggregate Stock Consideration to be issued by the Acquiror in the Transaction, and we do not express any views on any other terms of the Transaction.
It is understood that this letter is for the information of the Board of Directors and, except for inclusion of this letter in its entirety in a proxy statement of the Acquiror relating to the Transaction, may not be used or quoted for any other purpose without our prior written consent.
In rendering this opinion, we have not been engaged to act as an agent or fiduciary of the holders of common stock of the Acquiror, the Company or any other third party. We have acted as financial advisor to the Board of Directors in connection with the transaction described in this letter and we will receive a fee for our services.
C-2
Table of Contents
On the basis of, and subject to the foregoing, we are of the opinion that, as of the date hereof, the Aggregate Stock Consideration to be issued by the Acquiror in the Transaction is fair to the Acquiror from a financial point of view.
Very truly yours, | ||
HARRIS NESBITT CORP. | ||
By: | /s/ Kenneth Lerner | |
Kenneth Lerner | ||
Managing Director | ||
C-3
Table of Contents
Annex D
Opinion of Merrill, Lynch, Pierce, Fenner & Smith Incorporated
[Letterhead of Merrill Lynch]
November 12, 2003
Board of Directors
Genesoft Pharmaceuticals, Inc.
7300 Shoreline Court
South San Francisco, CA 94080
Members of the Board of Directors:
Genesoft Pharmaceuticals, Inc. (the “Company”), Genome Therapeutics Corporation (the “Acquiror”), Guardian Acquisition, Inc., a newly formed, wholly owned subsidiary of the Acquiror (the “Merger Sub”), and Luke Evnin, as Stockholders’ Representative, propose to enter into an Agreement and Plan of Merger and Reorganization, substantially in the form of the draft dated November 12, 2003 (the “Agreement”), pursuant to which the Merger Sub will be merged with the Company in a transaction (the “Transaction”) in which each outstanding share of the Company’s common stock, par value $0.0001 per share (the “Company Shares”), will be converted into the right to receive, subject to certain escrow provisions as to which we express no opinion, the number of shares of the common stock of the Acquiror, par value $0.10 per share (the “Acquiror Shares”) equal to the Common Exchange Ratio (as defined in the Agreement). Generally, the Common Exchange Ratio is defined as the quotient of (i) 28,571,405 Acquiror Shares less the number of Acquiror Shares issuable to the December Holders (as defined in the Note Amendment and Exchange Agreement, substantially in the form of the draft dated November 12, 2003 (the “Note Agreement”), among the Company, the Acquiror and the holders listed on the signature pages thereto) and the April Holders (as defined in the Note Agreement) pursuant to the terms of the Note Agreement, divided by (ii) the fully diluted number of Company Shares outstanding immediately prior to the effective time of the Transaction.
You have asked us whether, in our opinion, the Common Exchange Ratio is fair from a financial point of view to the holders of the Company Shares, other than the Acquiror and its affiliates.
In arriving at the opinion set forth below, we have, among other things:
| (1) | Reviewed certain publicly available business and financial information relating to the Company and Acquiror that we deemed to be relevant; |
| (2) | Reviewed certain information, including financial forecasts, relating to the business, earnings, cash flow, cash and other resource requirements, assets, liabilities and financing and other prospects of the Company and the Acquiror furnished to us by the Company and Acquiror, respectively; |
| (3) | Conducted discussions with certain members of senior management and representatives of the Company made available to us concerning the matters described in clauses 1 and 2 above; |
| (4) | Reviewed the market price of the Acquiror Shares and compared them with those of certain publicly traded companies that we deemed to be relevant; |
| (5) | Reviewed the valuation multiples for the Company Shares and the Acquiror Shares and compared them with those of certain publicly traded companies that we deemed to be relevant; |
| (6) | Compared the proposed financial terms of the Transaction with the financial terms of certain other transactions that we deemed to be relevant; |
| (7) | Participated in certain discussions and negotiations among representatives of the Company and the Acquiror and their financial and legal advisors; |
| (8) | Reviewed the draft dated November 12, 2003 of the Agreement; and |
| (9) | Reviewed such other financial studies and analyses and took into account such other matters as we deemed necessary, including our assessment of general economic, market and monetary conditions. |
D-1
Table of Contents
In preparing our opinion, we have assumed and relied on the accuracy and completeness of all information supplied or otherwise made available to us, discussed with or reviewed by or for us, or publicly available, and we have not assumed any responsibility for independently verifying such information or undertaken an independent evaluation or appraisal of any of the assets or liabilities of the Company or the Acquiror or been furnished with any such evaluation or appraisal, nor have we evaluated the solvency or fair value of the Company or the Acquiror under any state or federal laws relating to bankruptcy, insolvency or similar matters. In addition, we have not assumed any obligation to conduct any physical inspection of the properties or facilities of the Company or the Acquiror. With respect to the financial forecast information furnished to or discussed with us by the Company or the Acquiror, we have assumed that they have been reasonably prepared and reflect the best currently available estimates and judgment of the Company’s or Acquiror’s management as to the expected future financial performance of the Company or the Acquiror, as the case may be. We have also assumed that the final form of the Agreement will be substantially similar to the last draft reviewed by us and that the Transaction will be consummated in accordance with the terms of the Agreement without waiver of any of the conditions precedent to the Transaction contained in the Agreement. We have further assumed that the Transaction will qualify as a tax-free reorganization for U.S. federal income tax purposes.
Our opinion is necessarily based upon market, economic and other conditions as they exist and can be evaluated on, and on the information made available to us as of, the date hereof. We have assumed that in the course of obtaining the necessary regulatory or other consents or approvals (contractual or otherwise) for the Transaction, no restrictions, including any divestiture requirements or amendments or modifications, will be imposed that will have a material adverse effect on the contemplated benefits of the Transaction. We have also assumed that the consideration to be received by the holders of Company Shares pursuant to the Transaction will not be reduced as a result of hold harmless or indemnification provisions of the Agreement. In connection with our engagement, we were requested to solicit indications of interest from, and held discussions with, a substantial number of third parties regarding the possible acquisition of all or a part of the Company.
We are acting as financial advisor to the Company in connection with the Transaction and will receive a fee from the Company for our services, substantially all of which is contingent upon the consummation of the Transaction. In addition, the Company has agreed to indemnify us for certain liabilities arising out of our engagement. We have, in the past, provided financial advisory and financing services to the Company and the Acquiror and/or its affiliates and may continue to do so and have received, and may receive, fees for the rendering of such services. In addition, in the ordinary course of our business, we may actively trade the Acquiror Shares and other securities of the Acquiror for our own account and for the accounts of customers and, accordingly, may at any time hold a long or short position in such securities.
This opinion is for the use and benefit of the Board of Directors of the Company. Our opinion does not address the merits of the underlying decision by the Company to engage in the Transaction and does not constitute a recommendation to any shareholder as to how such shareholder should vote on the proposed Transaction or any matter related thereto. In addition, you have not asked us to address, and this opinion does not address, the fairness to, or any other consideration of, the holders of any class of securities, creditors or other constituencies of the Company, other than holders of the Company Shares, and our opinion does not address the fairness to any person of the terms of the Note Agreement or any other financing arrangement to be entered into by the Company or the Acquiror in connection with the Transaction.
We are not expressing any opinion herein as to the prices at which the Acquiror Shares will trade following the announcement or consummation of the Transaction.
On the basis of and subject to the foregoing, we are of the opinion that, as of the date hereof, the Common Exchange Ratio is fair from a financial point of view to the holders of the Company Shares, other than the Acquiror and its affiliates.
Very truly yours,
/s/ MERRILL LYNCH, PIERCE, FENNER & SMITH
INCORPORATED
D-2
Table of Contents
Annex E
THE GENERAL CORPORATION LAW
OF
THE STATE OF DELAWARE
SECTION 262 APPRAISAL RIGHTS.
(a) Any stockholder of a corporation of this State who holds shares of stock on the date of the making of a demand pursuant to subsection (d) of this section with respect to such shares, who continuously holds such shares through the effective date of the merger or consolidation, who has otherwise complied with subsection (d) of this section and who has neither voted in favor of the merger or consolidation nor consented thereto in writing pursuant to §228 of this title shall be entitled to an appraisal by the Court of Chancery of the fair value of the stockholder’s shares of stock under the circumstances described in subsections (b) and (c) of this section. As used in this section, the word “stockholder” means a holder of record of stock in a stock corporation and also a member of record of a nonstock corporation; the words “stock” and “share” mean and include what is ordinarily meant by those words and also membership or membership interest of a member of a nonstock corporation; and the words “depository receipt” mean a receipt or other instrument issued by a depository representing an interest in one or more shares, or fractions thereof, solely of stock of a corporation, which stock is deposited with the depository.
(b) Appraisal rights shall be available for the shares of any class or series of stock of a constituent corporation in a merger or consolidation to be effected pursuant to §251 (other than a merger effected pursuant to §251(g) of this title), §252, §254, §257, §258, §263 or §264 of this title:
(1) Provided, however, that no appraisal rights under this section shall be available for the shares of any class or series of stock, which stock, or depository receipts in respect thereof, at the record date fixed to determine the stockholders entitled to receive notice of and to vote at the meeting of stockholders to act upon the agreement of merger or consolidation, were either (i) listed on a national securities exchange or designated as a national market system security on an interdealer quotation system by the National Association of Securities Dealers, Inc. or (ii) held of record by more than 2,000 holders; and further provided that no appraisal rights shall be available for any shares of stock of the constituent corporation surviving a merger if the merger did not require for its approval the vote of the stockholders of the surviving corporation as provided in subsection (f) of §251 of this title.
(2) Notwithstanding paragraph (1) of this subsection, appraisal rights under this section shall be available for the shares of any class or series of stock of a constituent corporation if the holders thereof are required by the terms of an agreement of merger or consolidation pursuant to §§251, 252, 254, 257, 258, 263 and 264 of this title to accept for such stock anything except:
a. Shares of stock of the corporation surviving or resulting from such merger or consolidation, or depository receipts in respect thereof;
b. Shares of stock of any other corporation, or depository receipts in respect thereof, which shares of stock (or depository receipts in respect thereof) or depository receipts at the date of the merger or consolidation will be either listed on a national securities exchange or designated as a national market system security on an interdealer quotation system by the National Association of Securities Dealers, Inc. or held of record by more than 2,000 holders;
c. Cash in lieu of fractional shares or fractional depository receipts described in the foregoing subparagraphs a. and b. of this paragraph; or
d. Any combination of the shares of stock, depository receipts and cash in lieu of fractional shares or fractional depository receipts described in the foregoing subparagraphs a., b. and c. of this paragraph.
E-1
Table of Contents
(3) In the event all of the stock of a subsidiary Delaware corporation party to a merger effected under §253 of this title is not owned by the parent corporation immediately prior to the merger, appraisal rights shall be available for the shares of the subsidiary Delaware corporation.
(c) Any corporation may provide in its certificate of incorporation that appraisal rights under this section shall be available for the shares of any class or series of its stock as a result of an amendment to its certificate of incorporation, any merger or consolidation in which the corporation is a constituent corporation or the sale of all or substantially all of the assets of the corporation. If the certificate of incorporation contains such a provision, the procedures of this section, including those set forth in subsections (d) and (e) of this section, shall apply as nearly as is practicable.
(d) Appraisal rights shall be perfected as follows:
(1) If a proposed merger or consolidation for which appraisal rights are provided under this section is to be submitted for approval at a meeting of stockholders, the corporation, not less than 20 days prior to the meeting, shall notify each of its stockholders who was such on the record date for such meeting with respect to shares for which appraisal rights are available pursuant to subsection (b) or (c) hereof that appraisal rights are available for any or all of the shares of the constituent corporations, and shall include in such notice a copy of this section. Each stockholder electing to demand the appraisal of such stockholder’s shares shall deliver to the corporation, before the taking of the vote on the merger or consolidation, a written demand for appraisal of such stockholder’s shares. Such demand will be sufficient if it reasonably informs the corporation of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such stockholder’s shares. A proxy or vote against the merger or consolidation shall not constitute such a demand. A stockholder electing to take such action must do so by a separate written demand as herein provided. Within 10 days after the effective date of such merger or consolidation, the surviving or resulting corporation shall notify each stockholder of each constituent corporation who has complied with this subsection and has not voted in favor of or consented to the merger or consolidation of the date that the merger or consolidation has become effective; or
(2) If the merger or consolidation was approved pursuant to §228 or §253 of this title, then, either a constituent corporation before the effective date of the merger or consolidation, or the surviving or resulting corporation within ten days thereafter, shall notify each of the holders of any class or series of stock of such constituent corporation who are entitled to appraisal rights of the approval of the merger or consolidation and that appraisal rights are available for any or all shares of such class or series of stock of such constituent corporation, and shall include in such notice a copy of this section. Such notice may, and, if given on or after the effective date of the merger or consolidation, shall, also notify such stockholders of the effective date of the merger or consolidation. Any stockholder entitled to appraisal rights may, within 20 days after the date of mailing of such notice, demand in writing from the surviving or resulting corporation the appraisal of such holder’s shares. Such demand will be sufficient if it reasonably informs the corporation of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such holder’s shares. If such notice did not notify stockholders of the effective date of the merger or consolidation, either (i) each such constituent corporation shall send a second notice before the effective date of the merger or consolidation notifying each of the holders of any class or series of stock of such constituent corporation that are entitled to appraisal rights of the effective date of the merger or consolidation or (ii) the surviving or resulting corporation shall send such a second notice to all such holders on or within 10 days after such effective date; provided, however, that if such second notice is sent more than 20 days following the sending of the first notice, such second notice need only be sent to each stockholder who is entitled to appraisal rights and who has demanded appraisal of such holder’s shares in accordance with this subsection. An affidavit of the secretary or assistant secretary or of the transfer agent of the corporation that is required to give either notice that such notice has been given shall, in the absence of fraud, be prima facie evidence of the facts stated therein. For purposes of determining the stockholders entitled to receive either notice, each constituent corporation may fix, in advance, a record date that shall be not more than 10 days prior to the date the notice is given, provided, that if the notice is given on or after the
E-2
Table of Contents
effective date of the merger or consolidation, the record date shall be such effective date. If no record date is fixed and the notice is given prior to the effective date, the record date shall be the close of business on the day next preceding the day on which the notice is given.
(e) Within 120 days after the effective date of the merger or consolidation, the surviving or resulting corporation or any stockholder who has complied with subsections (a) and (d) hereof and who is otherwise entitled to appraisal rights, may file a petition in the Court of Chancery demanding a determination of the value of the stock of all such stockholders. Notwithstanding the foregoing, at any time within 60 days after the effective date of the merger or consolidation, any stockholder shall have the right to withdraw such stockholder’s demand for appraisal and to accept the terms offered upon the merger or consolidation. Within 120 days after the effective date of the merger or consolidation, any stockholder who has complied with the requirements of subsections (a) and (d) hereof, upon written request, shall be entitled to receive from the corporation surviving the merger or resulting from the consolidation a statement setting forth the aggregate number of shares not voted in favor of the merger or consolidation and with respect to which demands for appraisal have been received and the aggregate number of holders of such shares. Such written statement shall be mailed to the stockholder within 10 days after such stockholder’s written request for such a statement is received by the surviving or resulting corporation or within 10 days after expiration of the period for delivery of demands for appraisal under subsection (d) hereof, whichever is later.
(f) Upon the filing of any such petition by a stockholder, service of a copy thereof shall be made upon the surviving or resulting corporation, which shall within 20 days after such service file in the office of the Register in Chancery in which the petition was filed a duly verified list containing the names and addresses of all stockholders who have demanded payment for their shares and with whom agreements as to the value of their shares have not been reached by the surviving or resulting corporation. If the petition shall be filed by the surviving or resulting corporation, the petition shall be accompanied by such a duly verified list. The Register in Chancery, if so ordered by the Court, shall give notice of the time and place fixed for the hearing of such petition by registered or certified mail to the surviving or resulting corporation and to the stockholders shown on the list at the addresses therein stated. Such notice shall also be given by 1 or more publications at least 1 week before the day of the hearing, in a newspaper of general circulation published in the City of Wilmington, Delaware or such publication as the Court deems advisable. The forms of the notices by mail and by publication shall be approved by the Court, and the costs thereof shall be borne by the surviving or resulting corporation.
(g) At the hearing on such petition, the Court shall determine the stockholders who have complied with this section and who have become entitled to appraisal rights. The Court may require the stockholders who have demanded an appraisal for their shares and who hold stock represented by certificates to submit their certificates of stock to the Register in Chancery for notation thereon of the pendency of the appraisal proceedings; and if any stockholder fails to comply with such direction, the Court may dismiss the proceedings as to such stockholder.
(h) After determining the stockholders entitled to an appraisal, the Court shall appraise the shares, determining their fair value exclusive of any element of value arising from the accomplishment or expectation of the merger or consolidation, together with a fair rate of interest, if any, to be paid upon the amount determined to be the fair value. In determining such fair value, the Court shall take into account all relevant factors. In determining the fair rate of interest, the Court may consider all relevant factors, including the rate of interest which the surviving or resulting corporation would have had to pay to borrow money during the pendency of the proceeding. Upon application by the surviving or resulting corporation or by any stockholder entitled to participate in the appraisal proceeding, the Court may, in its discretion, permit discovery or other pretrial proceedings and may proceed to trial upon the appraisal prior to the final determination of the stockholder entitled to an appraisal. Any stockholder whose name appears on the list filed by the surviving or resulting corporation pursuant to subsection (f) of this section and who has submitted such stockholder’s certificates of stock to the Register in Chancery, if such is required, may participate fully in all proceedings until it is finally determined that such stockholder is not entitled to appraisal rights under this section.
E-3
Table of Contents
(i) The Court shall direct the payment of the fair value of the shares, together with interest, if any, by the surviving or resulting corporation to the stockholders entitled thereto. Interest may be simple or compound, as the Court may direct. Payment shall be so made to each such stockholder, in the case of holders of uncertificated stock forthwith, and the case of holders of shares represented by certificates upon the surrender to the corporation of the certificates representing such stock. The Court’s decree may be enforced as other decrees in the Court of Chancery may be enforced, whether such surviving or resulting corporation be a corporation of this State or of any state.
(j) The costs of the proceeding may be determined by the Court and taxed upon the parties as the Court deems equitable in the circumstances. Upon application of a stockholder, the Court may order all or a portion of the expenses incurred by any stockholder in connection with the appraisal proceeding, including, without limitation, reasonable attorney’s fees and the fees and expenses of experts, to be charged pro rata against the value of all the shares entitled to an appraisal.
(k) From and after the effective date of the merger or consolidation, no stockholder who has demanded appraisal rights as provided in subsection (d) of this section shall be entitled to vote such stock for any purpose or to receive payment of dividends or other distributions on the stock (except dividends or other distributions payable to stockholders of record at a date which is prior to the effective date of the merger or consolidation); provided, however, that if no petition for an appraisal shall be filed within the time provided in subsection (e) of this section, or if such stockholder shall deliver to the surviving or resulting corporation a written withdrawal of such stockholder’s demand for an appraisal and an acceptance of the merger or consolidation, either within 60 days after the effective date of the merger or consolidation as provided in subsection (e) of this section or thereafter with the written approval of the corporation, then the right of such stockholder to an appraisal shall cease. Notwithstanding the foregoing, no appraisal proceeding in the Court of Chancery shall be dismissed as to any stockholder without the approval of the Court, and such approval may be conditioned upon such terms as the Court deems just.
(l) The shares of the surviving or resulting corporation to which the shares of such objecting stockholders would have been converted had they assented to the merger or consolidation shall have the status of authorized and unissued shares of the surviving or resulting corporation.
E-4
Table of Contents
Annex F
CALIFORNIA CORPORATIONS CODE
TITLE 1. CORPORATIONS
DIVISION 1. GENERAL CORPORATION LAW
CHAPTER 13. Dissenters’ Rights
| Section 1300. | Reorganization or short form merger; dissenting shares; corporate purchase at the fair market value; definitions |
(a) If the approval of the outstanding shares (Section 152) of a corporation is required for a reorganization under subdivisions (a) and (b) or subdivision (e) or (f) of Section 1201, each shareholder of the corporation entitled to vote on the transaction and each shareholder of a subsidiary corporation in a short-form merger may, by complying with this chapter, require the corporation in which the shareholder holds shares to purchase for cash at their fair market value the shares owned by the shareholder which are dissenting shares as defined in subdivision (b). The fair market value shall be determined as of the day before the first announcement of the terms of the proposed reorganization or short-form merger, excluding any appreciation or depreciation in consequence of the proposed action, but adjusted for any stock split, reverse stock split, or share dividend which becomes effective thereafter.
(b) As used in this chapter, “dissenting shares” means shares which come within all of the following descriptions:
(1) Which were not immediately prior to the reorganization or short-form merger either (A) listed on any national securities exchange certified by the Commissioner of Corporations under subdivision (o) of Section 25100 or (B) listed on National Market System of the NASDAQ Stock Market, and the notice of meeting of shareholders to act upon the reorganization summarizes this section and Sections 1301, 1302, 1303 and 1304; provided, however, that this provision does not apply to any shares with respect to which there exists any restriction on transfer imposed by the corporation or by any law or regulation; and provided, further, that this provision does not apply to any class of shares described in subparagraph (A) or (B) if demands for payment are filed with respect to 5 percent or more of the outstanding shares of that class.
(2) Which were outstanding on the date for the determination of shareholders entitled to vote on the reorganization and (A) were not voted in favor of the reorganization or, (B) if described in subparagraph (A) or (B) of paragraph (1) (without regard to the provisos in that paragraph), were voted against the reorganization, or which were held of record on the effective date of a short-form merger; provided, however, that subparagraph (A) rather than subparagraph (B) of this paragraph applies in any case where the approval required by Section 1201 is sought by written consent rather than at a meeting.
(3) Which the dissenting shareholder has demanded that the corporation purchase at their fair market value, in accordance with Section 1301.
(4) Which the dissenting shareholder has submitted for endorsement, in accordance with Section 1302.
(c) As used in this chapter, “dissenting shareholder” means the recordholder of dissenting shares and includes a transferee of record.
| Section 1301. | Notice to holders of dissenting shares in reorganizations; demand for purchase; time; contents |
(a) If, in the case of a reorganization, any shareholders of a corporation have a right under Section 1300, subject to compliance with paragraphs (3) and (4) of subdivision (b) thereof, to require the corporation to purchase their shares for cash, such corporation shall mail to each such shareholder a notice of the approval of the reorganization by its outstanding shares (Section 152) within 10 days after the date of such approval, accompanied by a copy of Sections 1300, 1302, 1303, 1304 and this section, a statement of
F-1
Table of Contents
the price determined by the corporation to represent the fair market value of the dissenting shares, and a brief description of the procedure to be followed if the shareholder desires to exercise the shareholder’s right under such sections. The statement of price constitutes an offer by the corporation to purchase at the price stated any dissenting shares as defined in subdivision (b) of Section 1300, unless they lose their status as dissenting shares under Section 1309.
(b) Any shareholder who has a right to require the corporation to purchase the shareholder’s shares for cash under Section 1300, subject to compliance with paragraphs (3) and (4) of subdivision (b) thereof, and who desires the corporation to purchase such shares shall make written demand upon the corporation for the purchase of such shares and payment to the shareholder in cash of their fair market value. The demand is not effective for any purpose unless it is received by the corporation or any transfer agent thereof (1) in the case of shares described in clause (i) or (ii) of paragraph (1) of subdivision (b) of Section 1300 (without regard to the provisos in that paragraph), not later than the date of the shareholders’ meeting to vote upon the reorganization, or (2) in any other case within 30 days after the date on which the notice of the approval by the outstanding shares pursuant to subdivision (a) or the notice pursuant to subdivision (i) of Section 1110 was mailed to the shareholder.
(c) The demand shall state the number and class of the shares held of record by the shareholder which the shareholder demands that the corporation purchase and shall contain a statement of what such shareholder claims to be the fair market value of those shares as of the day before the announcement of the proposed reorganization or short-form merger. The statement of fair market value constitutes an offer by the shareholder to sell the shares at such price.
| Section 1302. | Submission of share certificates for endorsement; uncertificated securities |
Within 30 days after the date on which notice of the approval by the outstanding shares or the notice pursuant to subdivision (i) of Section 1110 was mailed to the shareholder, the shareholder shall submit to the corporation at its principal office or at the office of any transfer agent thereof, (a) if the shares are certificated securities, the shareholder’s certificates representing any shares which the shareholder demands that the corporation purchase, to be stamped or endorsed with a statement that the shares are dissenting shares or to be exchanged for certificates of appropriate denomination so stamped or endorsed or (b) if the shares are uncertificated securities, written notice of the number of shares which the shareholder demands that the corporation purchase. Upon subsequent transfers of the dissenting shares on the books of the corporation, the new certificates, initial transaction statement, and other written statements issued therefor shall bear a like statement, together with the name of the original dissenting holder of the shares.
| Section 1303. | Payment of agreed price with interest; agreement fixing fair market value; filing; time of payment |
(a) If the corporation and the shareholder agree that the shares are dissenting shares and agree upon the price of the shares, the dissenting shareholder is entitled to the agreed price with interest thereon at the legal rate on judgments from the date of the agreement. Any agreements fixing the fair market value of any dissenting shares as between the corporation and the holders thereof shall be filed with the secretary of the corporation.
(b) Subject to the provisions of Section 1306, payment of the fair market value of dissenting shares shall be made within 30 days after the amount thereof has been agreed or within 30 days after any statutory or contractual conditions to the reorganization are satisfied, whichever is later, and in the case of certificated securities, subject to surrender of the certificates therefor, unless provided otherwise by agreement.
| Section 1304. | Action to determine whether shares are dissenting shares or fair market value; limitation; joinder; consolidation; determination of issues; appointment of appraisers |
(a) If the corporation denies that the shares are dissenting shares, or the corporation and the shareholder fail to agree upon the fair market value of the shares, then the shareholder demanding purchase of such shares as
F-2
Table of Contents
dissenting shares or any interested corporation, within six months after the date on which notice of the approval by the outstanding shares (Section 152) or notice pursuant to subdivision (i) of Section 1110 was mailed to the shareholder, but not thereafter, may file a complaint in the superior court of the proper county praying the court to determine whether the shares are dissenting shares or the fair market value of the dissenting shares or both or may intervene in any action pending on such a complaint.
(b) Two or more dissenting shareholders may join as plaintiffs or be joined as defendants in any such action and two or more such actions may be consolidated.
(c) On the trial of the action, the court shall determine the issues. If the status of the shares as dissenting shares is in issue, the court shall first determine that issue. If the fair market value of the dissenting shares is in issue, the court shall determine, or shall appoint one or more impartial appraisers to determine, the fair market value of the shares.
| Section 1305. | Report of appraisers; confirmation; determination by court; judgment; payment; appeal; costs |
(a) If the court appoints an appraiser or appraisers, they shall proceed forthwith to determine the fair market value per share. Within the time fixed by the court, the appraisers, or a majority of them, shall make and file a report in the office of the clerk of the court. Thereupon, on the motion of any party, the report shall be submitted to the court and considered on such evidence as the court considers relevant. If the court finds the report reasonable, the court may confirm it.
(b) If a majority of the appraisers appointed fail to make and file a report within 10 days from the date of their appointment or within such further time as may be allowed by the court or the report is not confirmed by the court, the court shall determine the fair market value of the dissenting shares.
(c) Subject to the provisions of Section 1306, judgment shall be rendered against the corporation for payment of an amount equal to the fair market value of each dissenting share multiplied by the number of dissenting shares which any dissenting shareholder who is a party, or who has intervened, is entitled to require the corporation to purchase, with interest thereon at the legal rate from the date on which judgment was entered.
(d) Any such judgment shall be payable forthwith with respect to uncertificated securities and, with respect to certificated securities, only upon the endorsement and delivery to the corporation of the certificates for the shares described in the judgment. Any party may appeal from the judgment.
(e) The costs of the action, including reasonable compensation to the appraisers to be fixed by the court, shall be assessed or apportioned as the court considers equitable, but, if the appraisal exceeds the price offered by the corporation, the corporation shall pay the costs (including in the discretion of the court attorneys’ fees, fees of expert witnesses and interest at the legal rate on judgments from the date of compliance with Sections 1300, 1301 and 1302 if the value awarded by the court for the shares is more than 125 percent of the price offered by the corporation under subdivision (a) of Section 1301).
| Section 1306. | Prevention of immediate payment; status as creditors; interest |
To the extent that the provisions of Chapter 5 prevent the payment to any holders of dissenting shares of their fair market value, they shall become creditors of the corporation for the amount thereof together with interest at the legal rate on judgments until the date of payment, but subordinate to all other creditors in any liquidation proceeding, such debt to be payable when permissible under the provisions of Chapter 5.
| Section 1307. | Dividends on dissenting shares |
Cash dividends declared and paid by the corporation upon the dissenting shares after the date of approval of the reorganization by the outstanding shares (Section 152) and prior to payment for the shares by the corporation shall be credited against the total amount to be paid by the corporation therefor.
F-3
Table of Contents
| Section 1308. | Rights of dissenting shareholders pending valuation; withdrawal and demand for payment |
Except as expressly limited in this chapter, holders of dissenting shares continue to have all the rights and privileges incident to their shares, until the fair market value of their shares is agreed upon or determined. A dissenting shareholder may not withdraw a demand for payment unless the corporation consents thereto.
| Section 1309. | Termination of dissenting shareholder status |
Dissenting shares lose their status as dissenting shares and the holders thereof cease to be dissenting shareholders and cease to be entitled to require the corporation to purchase their shares upon the happening of any of the following:
(a) The corporation abandons the reorganization. Upon abandonment of the reorganization, the corporation shall pay on demand to any dissenting shareholder who has initiated proceedings in good faith under this chapter all necessary expenses incurred in such proceedings and reasonable attorneys’ fees.
(b) The shares are transferred prior to their submission for endorsement in accordance with Section 1302 or are surrendered for conversion into shares of another class in accordance with the articles.
(c) The dissenting shareholder and the corporation do not agree upon the status of the shares as dissenting shares or upon the purchase price of the shares, and neither files a complaint or intervenes in a pending action as provided in Section 1304, within six months after the date on which notice of the approval by the outstanding shares or notice pursuant to subdivision (i) of Section 1110 was mailed to the shareholder.
(d) The dissenting shareholder, with the consent of the corporation, withdraws the shareholder’s demand for purchase of the dissenting shares.
| Section 1310. | Suspension of right to compensation or valuation proceedings; litigation of shareholders’ approval |
If litigation is instituted to test the sufficiency or regularity of the votes of the shareholders in authorizing a reorganization, any proceedings under Sections 1304 and 1305 shall be suspended until final determination of such litigation.
| Section 1311. | Exempt shares |
This chapter, except Section 1312, does not apply to classes of shares whose terms and provisions specifically set forth the amount to be paid in respect to such shares in the event of a reorganization or merger.
| Section 1312. | Right of dissenting shareholder to attack, set aside or rescind merger or reorganization; restraining order or injunction; conditions |
(a) No shareholder of a corporation who has a right under this chapter to demand payment of cash for the shares held by the shareholder shall have any right at law or in equity to attack the validity of the reorganization or short-form merger, or to have the reorganization or short-form merger set aside or rescinded, except in an action to test whether the number of shares required to authorize or approve the reorganization have been legally voted in favor thereof; but any holder of shares of a class whose terms and provisions specifically set forth the amount to be paid in respect to them in the event of a reorganization or short-form merger is entitled to payment in accordance with those terms and provisions or, if the principal terms of the reorganization are approved pursuant to subdivision (b) of Section 1202, is entitled to payment in accordance with the terms and provisions of the approved reorganization.
F-4
Table of Contents
(b) If one of the parties to a reorganization or short-form merger is directly or indirectly controlled by, or under common control with, another party to the reorganization or short-form merger, subdivision (a) shall not apply to any shareholder of such party who has not demanded payment of cash for such shareholder’s shares pursuant to this chapter; but if the shareholder institutes any action to attack the validity of the reorganization or short-form merger or to have the reorganization or short-form merger set aside or rescinded, the shareholder shall not thereafter have any right to demand payment of cash for the shareholder’s shares pursuant to this chapter. The court in any action attacking the validity of the reorganization or short-form merger or to have the reorganization or short-form merger set aside or rescinded shall not restrain or enjoin the consummation of the transaction except upon 10 days’ prior notice to the corporation and upon a determination by the court that clearly no other remedy will adequately protect the complaining shareholder or the class of shareholders of which such shareholder is a member.
(c) If one of the parties to a reorganization or short-form merger is directly or indirectly controlled by, or under common control with, another party to the reorganization or short-form merger, in any action to attack the validity of the reorganization or short-form merger or to have the reorganization or short-form merger set aside or rescinded, (1) a party to a reorganization or short-form merger which controls another party to the reorganization or short-form merger shall have the burden of proving that the transaction is just and reasonable as to the shareholders of the controlled party, and (2) a person who controls two or more parties to a reorganization shall have the burden of proving that the transaction is just and reasonable as to the shareholders of any party so controlled.
F-5
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 20. Indemnification of Directors and Officers
The registrant is organized under the laws of The Commonwealth of Massachusetts. The Massachusetts Business Corporation Law provides that indemnification of directors, officers, employees, and other agents of another organization, or who serve at its request in any capacity with respect to any employee benefit plan, may be provided by the corporation to whatever extent specified in its charter documents or votes adopted by its stockholders, except that no indemnification may be provided for any person with respect to any matter as to which the person shall have been adjudicated in any proceeding not to have acted in good faith in the reasonable belief that his action was in the best interest of the corporation. Under Massachusetts law, a corporation can purchase and maintain insurance on behalf of any person against any liability incurred as a director, officer, employee, agent, or person serving at the request of the corporation as a director, officer, employee, or other agent of another organization or with respect to any employee benefit plan, in his capacity as such, whether or not the corporation would have power to itself indemnify him against such liability.
The registrant’s Restated Articles of Organization, as amended to date, provide that its directors shall not be liable to the registrant or its stockholders for monetary damages for breach of fiduciary duty as a director, except to the extent that the exculpation from liabilities is not permitted under the Massachusetts Business Corporation Law as in effect at the time such liability is determined. The By-Laws provide that the registrant shall indemnify its directors and officers to the full extent permitted by the laws of The Commonwealth of Massachusetts. In addition, the registrant holds a Directors and Officer Liability and Corporate Indemnification Policy.
Item 21. Exhibits and Financial Statement Schedules
(a) See Exhibit Index immediately following the signature page.
(b) The financial statement schedules for Genesoft are included on page F-1 of the joint proxy statement/prospectus included within this registration statement and the financial statement schedules for Genome are incorporated herein by reference to Genome’s Annual Report on Form 10-K for the fiscal year ended December 31, 2002.
Item 22. Undertakings
(a) The undersigned registrant hereby undertakes:
(1) That, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(2) That prior to any public reoffering of the securities registered hereunder through use of a prospectus which is a part of this registration statement, by any person or party who is deemed to be an underwriter within the meaning of Rule 145(c), the issuer undertakes that such reoffering prospectus will contain the information called for by the applicable registration form with respect to reofferings by persons who may be deemed underwriters, in addition to the information called for by the other Items of the applicable form.
(3) That every prospectus (i) that is filed pursuant to paragraph (2) immediately preceding, or (ii) that purports to meet the requirements of Section 10(a)(3) of the Securities Act of 1933 and is used in connection with an offering of securities subject to Rule 415, will be filed as a part of an amendment to the registration
II-1
Table of Contents
statement and will not be used until such amendment is effective, and that, for purposes of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(4) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the questions whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(b) The undersigned registrant hereby undertakes to respond to requests for information that is incorporated by reference into the prospectus pursuant to Item 4, 10(b), 11 or 13 of this form, within one business day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This includes information contained in documents filed subsequent to the effective date of the registration statement through the date of responding to the request.
(c) The undersigned registrant hereby undertakes to supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in the registration statement when it became effective.
II-2
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Waltham, Massachusetts, on December 15, 2003.
| GENOME THERAPEUTICS CORP. | ||
By: | /s/ STEVEN M. RAUSCHER | |
Steven M. Rauscher President, Chief Executive Officer and Chairman of the Board | ||
Each person whose signature appears below hereby constitutes and appoints Steven M. Rauscher and Stephen Cohen, and each of them singly, his true and lawful attorneys-in-fact and agents with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign this registration statement on Form S-4 and any and all amendments (including post-effective amendments) to said registration statement on Form S-4 and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ STEVEN M. RAUSCHER Steven M. Rauscher | Director, Chairman of the Board, President and Chief Executive Officer (Principal Executive Officer) | December 15, 2003 | ||
/s/ STEPHEN COHEN Stephen Cohen | Senior Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) | December 15, 2003 | ||
/s/ ROBERT J. HENNESSEY Robert J. Hennessey | Director | December 15, 2003 | ||
/s/ MARC B. GARNICK, M.D. Marc B. Garnick, M.D. | Director | December 15, 2003 | ||
/s/ PHILIP LEDER, M.D. Philip Leder, M.D. | Director | December 15, 2003 | ||
/s/ LAWRENCE LEVY Lawrence Levy | Director | December 15, 2003 | ||
/s/ NORBERT G. RIEDEL, PH.D. Norbert G. Riedel, Ph.D. | Director | December 15, 2003 | ||
/s/ DAVID K. STONE David K. Stone | Director | December 15, 2003 | ||
/s/ WILLIAM S. REARDON William S. Reardon | Director | December 15, 2003 | ||
II-3
Table of Contents
EXHIBIT INDEX
| Exhibit No. | Exhibit Description | |
| 2.1 | Agreement and Plan of Merger and Reorganization, dated as of November 17, 2003, among Genome Therapeutics Corp., Guardian Acquisition, Inc., GeneSoft Pharmaceuticals, Inc., and Luke Evnin, as Stockholders’ Representative.(41) | |
| 3.1 | Restated Articles of Organization and By-laws(1) | |
| 3.2 | Amendment dated January 5, 1982 to Restated Articles of Organization(2) | |
| 3.3 | Amendment dated January 24, 1983 to Restated Articles of Organization(3) | |
| 3.4 | Amendment dated January 17, 1984 to Restated Articles of Organization(4) | |
| 3.5 | Amendment dated October 20, 1987 to the By-laws(8) | |
| 3.6 | Amendment dated December 9, 1987 to Restated Articles of Organization(9) | |
| 3.7 | Amendment dated October 16, 1989 to the By-laws(11) | |
| 3.8 | Amendment dated January 24, 1994 to Restated Articles of Organization(14) | |
| 3.9 | Amendment dated August 31, 1994 to Restated Articles of Organization(14) | |
| 3.10 | Amendment dated March 15, 2001 to Restated Articles of Organization(33) | |
| 3.11 | By-Laws of Genome Therapeutics Corp. (as amended through July 24, 2001)(34) | |
| 4.1 | Specimen common stock certificate(5) | |
| 4.2 | See Exhibits 3.1 and 3.11 for provisions of the Articles of Organization and By-Laws of the Registrant defining the rights of holders of common stock of the Genome Therapeutics Corp. | |
| 5.1 | Opinion of Ropes & Gray LLP.(43) | |
| 9.1 | Form of Genome Voting Agreement(42) | |
| 10.1 | Security Agreement, dated as of November 17, 2003, between GeneSoft Pharmaceuticals, Inc. and Genome Therapeutics Corps.(42) | |
| 10.2 | Warrant for the purchase of common stock of GeneSoft Pharmaceuticals, Inc.(42) | |
| 10.3 | Note Amendment and Exchange Agreement, dated as of November 17, 2003, by and among GeneSoft Pharmaceuticals, Inc., Genome Therapeutics Corp. and certain creditors of GeneSoft Pharmaceuticals, Inc.(42) | |
| 10.4 | Registration Rights Agreement, dated as of November 17, 2003, by and between Genome Therapeutics Corp. and certain creditors of GeneSoft Pharmaceuticals, Inc.(42) | |
| 10.5 | Promissory Note of GeneSoft Pharmaceuticals, Inc., dated as of November 17, 2003.(42) | |
| 23.1 | Consent of Ernst & Young LLP(42) | |
| 23.2 | Consent of Ernst & Young LLP(42) | |
| 23.3 | Consent of Ropes & Gray LLP(42) | |
| 23.4 | Consent of Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP(42) | |
| 23.5 | Consent of Harris Nesbitt Corp.(42) | |
| 24.1 | Power of Attorney (part of signature page of this Registration Statement) | |
| 99.1 | Form of Escrow Agreement (attached as Annex B to the joint proxy statement/prospectus and incorporated herein by reference)(43) | |
| 99.2 | Opinion of Harris Nesbitt Corp. (attached as Annex C to the joint proxy statement/prospectus and incorporated herein by reference)(42) | |
II-4
Table of Contents
| Exhibit No. | Exhibit Description | |
| 99.3 | Opinion of Merrill Lynch, Pierce, Fenner & Smith Incorporated. (attached as Annex D to the joint proxy statement/prospectus and incorporated herein by reference)(42) | |
| 99.4 | Consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated.(42) | |
| 99.5 | Form of Genome Therapeutics Corp. proxy card(43) | |
| 99.6 | Form of GeneSoft Pharmaceuticals, Inc. proxy card(43) | |
| 99.7 | Form of Genesoft Voting Agreement(41) | |
| 99.8 | Form of Genesoft Voting Agreement(41) | |
| (1) | Filed as exhibits to the Company’s Registration Statement on Form S-1 (No. 2-75230) and incorporated herein by reference. |
| (2) | Filed as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended February 27, 1982 and incorporated herein by reference. |
| (3) | Filed as exhibits to the Company’s Quarterly Report on Form 10-Q for the quarter ended February 26, 1983 and incorporated herein by reference. |
| (4) | Filed as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended February 25, 1984 and incorporated herein by reference. |
| (5) | Filed as an exhibit to the Company’s Registration Statement on Form S-3 (File No. 333-00127) and incorporated herein by reference. |
| (8) | Filed as an exhibit to the Company’s Annual Report on Form 10-K for the year ended August 31, 1987 and incorporated herein by reference. |
| (9) | Filed as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended November 28, 1987 and incorporated herein by reference. |
| (11) | Filed as an exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended August 31, 1989 and incorporated herein by reference. |
| (14) | Filed as exhibits of the Company’s Annual Report on Form 10-K for the fiscal year ended August 31, 1994 and incorporated herein by reference. |
| (33) | Filed as an exhibit to the Company’s 10-Q for the quarter ended February 24, 2001 and incorporated herein by reference. |
| (34) | Filed as an exhibit to the Company’s 10-Q for the quarter ended September 29, 2001 and incorporated herein by reference. |
| (41) | Filed as an exhibit to the Company’s 8-K filed November 18, 2003 and incorporated herein by reference. |
| (42) | Filed herewith. |
| (43) | To be filed by amendment. |
II-5