
Corporate Presentation November 5, 2015 1

2 Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. All statements that are not historical facts are hereby identified as forward-looking statements for this purpose and include, among others, statements relating to: the potential advantages of our product candidates; the initiation, design and timing of preclinical and clinical studies and the reporting of the results thereof; the timing of regulatory submissions and actions; anticipated milestones; and all other statements regarding our objectives, strategies, prospects, future operations, future financial performance, future financial position and other future events (including our assumptions relating thereto). Forward-looking statements are based upon our current expectations, intentions and beliefs and are subject to a number of risks, uncertainties, assumptions and other factors that could cause actual results to differ materially and adversely from those expressed or implied by such statements. Factors that could cause or contribute to such differences include, among others: our ability to successfully complete the additional toxicology study requested by the FDA and to adequately address the FDA’s other feedback relating to our IND for FCX-007; the outcome of the FDA’s review of the amended IND for FCX-007 and approval to commence the Phase I/II trial; varying interpretation of clinical and preclinical data; the risk that results seen in preclinical studies may not be replicated in humans; uncertainties relating to the initiation and completion of clinical trials and whether the results will validate and support the safety and efficacy of our product candidates; our ability to maintain our collaboration with Intrexon; and the other factors discussed under the caption “Item 1A. Risk Factors” in our most recent annual report on Form 10-K and our most recent quarterly report on Form 10-Q, which are available through the “Investors & Media—SEC filings” page of our website at www.fibrocell.com. As a result, you should not place undue reliance on forward-looking statements. The forward-looking statements made during this presentation represent our views only as of the date of this presentation (or any earlier date indicated in such statement). While we may update certain forward-looking statements from time to time, we specifically disclaim any obligation to do so, whether as a result of new information, future developments or otherwise.

• Fibrocell is the leader of autologous fibroblast therapy for skin and connective tissue diseases – Developing a pipeline of cell and gene therapies for orphan skin diseases using autologous fibroblasts to deliver a targeted gene • Phase II azficel-T sBLA program: autologous fibroblasts for Chronic Dysphonia – Significant voice impairment due to severe vocal cord scarring or atrophy – Phase II data expected in 2Q16 • FCX-007: Novel gene-therapy product candidate for Recessive Dystrophic Epidermolysis Bullosa (RDEB), a congenital orphan skin disease – Devastating, progressive, painful blistering disease that leads to death – Granted rare pediatric disease designation by FDA – Collaboration with Intrexon Corporation (NYSE: XON); expect to initiate Phase I/II trial in 2Q16 • FCX-013: Novel gene-therapy product candidate for Linear Scleroderma – Autoimmune disease causes debilitating, painful linear scars and skin thickening that can lead to pain, reduced joint mobility and deformity – Collaboration with Intrexon; proof-of-concept expected 1H16; IND planned 4Q16 Company Highlights 3

Autologous Fibroblasts as a Therapy • Fibroblasts repair tissue infrastructure by producing extracellular matrix proteins including collagen and growth factors • Most common cell in skin and connective tissue • Key advantages of our autologous fibroblast/gene-therapy approach: ‐ Monogenic (one gene) disorder with a known mechanism of action ‐ Fibroblasts are genetically-modified ex vivo, with safety testing prior to administration ‐ Protein target characterized by multiple assays ‐ Localized injection (avoids systemic delivery) ‐ Reduced rejection concern • We are using our proprietary technology to create personalized biologics 4

Gene Therapy Product Engine integrates Intrexon’s synthetic biology FCX-007, FCX-013 Autologous Fibroblast Product Engine azficel-T BLA* Two Product Engines – Multiple Therapeutics in Development Collection Culture Local Administration Vector Preparation Gene Packaging Gene Transduction Personalized Biologics Approach 5 * azficel-T BLA currently approved as LAVIV® for the treatment of nasolabial fold wrinkles in adults

Personalized Biologics Indication Pre-Clinical Development Phase 1 Phase 2 Phase 3 azficel-T sBLA* Chronic Dysphonia FCX-007 Gene-Therapy Orphan Product Candidate Recessive Dystrophic Epidermolysis Bullosa (RDEB) FCX-013 Gene-Therapy Potential Orphan Product Candidate Linear Scleroderma Development Pipeline 6 * azficel-T BLA currently approved as LAVIV® for the treatment of nasolabial fold wrinkles in adults
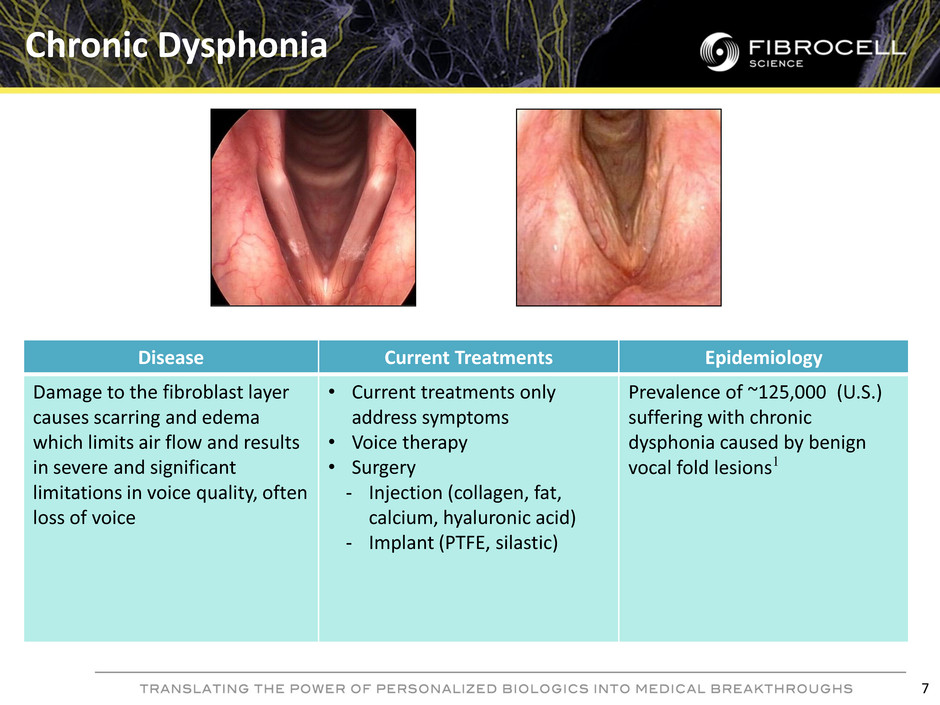
Disease Current Treatments Epidemiology Damage to the fibroblast layer causes scarring and edema which limits air flow and results in severe and significant limitations in voice quality, often loss of voice • Current treatments only address symptoms • Voice therapy • Surgery - Injection (collagen, fat, calcium, hyaluronic acid) - Implant (PTFE, silastic) Prevalence of ~125,000 (U.S.) suffering with chronic dysphonia caused by benign vocal fold lesions1 Chronic Dysphonia 7

Positive Phase I clinical trial results published in peer-reviewed journal2 • Injection of azficel-T into scarred fold lamina propria layer was found to be safe and well-tolerated in trial (n=5) ₋ All patients completed the trial ₋ No serious adverse events reported • Positive trend of sustained improvement – Sustained improvement from Month 3 through Month 12 was noted in a majority of patients in the mucosal wave grade assessment, voice handicap index and patient-assessed voice quality 8 Clinical Data Support Our Approach in Chronic Dysphonia
• Fully enrolled 20+ subjects • Double-blind, randomized, placebo-controlled • Validated scales with a 4-month efficacy endpoint • Dosing expected to be complete by year-end 2015 • Expect to report primary endpoint results in 2Q16 Chronic Dysphonia - Phase II Trial 9

Providing Hope for RDEB Patients 10 RDEB patients do not produce type VII collagen (COL7) due to mutation in COL7A1 gene ‐ Main component of anchoring fibrils that connect skin layers FCX-007 is an autologous human dermal fibroblast transduced with a lentiviral (LV) vector containing the gene for COL7A1 ‐ Simple, local injection to the papillary dermis

Disease Current Treatments Epidemiology • Devastating, progressive, painful blistering disease that leads to death • Diagnosed at infancy • High mortality rate • Cause: A mutation in the COL7A1 gene which encodes for COL7 • Current treatments only address symptoms • Bandaging & antibiotics – bandaging alone can exceed $10,000 per month3 • Feeding tubes • Surgery, including hand and esophageal Dystrophic EB (DEB) ~5,500 – 12,500 US4 • RDEB ~1,100 – 2,500 US5 Recessive Dystrophic Epidermolysis Bullosa 11

COL7 Expression Confirmation COL7 Trimer COL7 FORMATION 290kDa ~900kDa Culture supernatant evaluated for COL7 expression: • ELISA assay indicates virus dose-dependent protein expression • Trimeric form of COL7 produced by RDEB patient fibroblasts transduced with LV-COL7 ~ 900kDa 12

FCX-007 In Vitro Results Summary 13 Study Results: Results Suggest: Production of LV-COL7 was demonstrated Ability to successfully make the vector FCX-007 was produced by expanding LV-COL7-transduced RDEB patient fibroblasts from a biopsy sample Ability for full-scale FCX-007 production The integrated transgene copy number per cell was dependent on the virus dose Full gene integration into cell genome The COL7 expression from the FCX-007 was confirmed by qRT- PCR, immunofluorescence staining, and ELISA COL7 is being produced from FCX-007 The structure of the COL7 expressed by FCX-007 was confirmed to be predominantly trimeric by immunoprecipitation/SDS- PAGE/Western blot analysis COL7 produced from FCX-007 is the correct size and structure The COL7 produced from FCX-007 was demonstrated to be functional by binding to Laminin 332 in an in vitro binding assay and by correction of the hypermotility phenotype of RDEB cells in an in vitro migration assay COL7 produced from FCX-007 is functional Results replicated over two additional FCX-007 cultures Study results are reproducible

Positive Pre-Clinical Results & Next Steps Toxicity • Hybrid pharmacology/toxicology study with SCID mice grafted with normal human skin graft & injected with FCX-007 to determine toxicity and systemic distribution of vector • Results: – No adverse observations at 2 and 6 weeks post-injection – No apparent vector distribution based on qPCR of organ samples Proof-of-Concept • SCID mice grafted with human RDEB skin substitutes & injected with FCX-007 • Results: – Production of COL7 observed in vivo – COL7 detected in the dermal-epidermal junction Next Steps • Perform toxicology-specific mouse study in non-grafted SCID mice • Amended IND with new toxicology data expected in 1Q 2016 • Expect to initiate Phase I/II trial in 2Q2016 14

FCX-007 Proposed Clinical Trial Design Title A Phase I/II Trial of FCX-007 (Genetically-Modified Autologous Human Dermal Fibroblasts) for Recessive Dystrophic Epidermolysis Bullosa (RDEB) Statement of Purpose The purpose of this study is threefold: 1) To evaluate the safety of FCX-007 2) To evaluate COL7 expression and the presence of anchoring fibrils resulting from FCX-007 3) To analyze wound healing as a result of FCX-007 administration Objectives Primary 1) The primary objective of this protocol is to evaluate the safety of FCX-007 Secondary 1) To evaluate mechanism of action of FCX-007 at weeks 4,12, 25, 52, and unscheduled visits through the evaluation of skin biopsies for COL7 expression and the presence of anchoring fibrils 2) To evaluate the efficacy of FCX-007 through an intra-subject paired analysis of target wound area at weeks 4, 8, 12, 25, 52, and unscheduled visits, comparing FCX-007 treated wounds to untreated wounds in Phase I and to wounds administered sterile saline in Phase II through the evaluation of digital imaging of wounds # of Subjects Nine (three adults in Phase I followed by six pediatrics in Phase II) 15

Disease Epidemiology • Excess production of extracellular matrix characterized by skin fibrosis and linear scars • The linear areas of skin thickening may extend to underlying tissue and muscle in children which may impair growth in affected legs and arms or forehead • Lesions appearing across joints impair motion and may be permanent • Localized Scleroderma ~200,000 sufferers US6 comprised of many different sub-types - Linear Scleroderma Initial target for FCX-013 is a group of ~40,000 patients who have scleroderma over a major joint and exhibit severe joint pain7 Current Treatments • Current treatments only address symptoms • Systemic or topical corticosteroids • UVA light therapy • Physical therapy Linear Scleroderma Photo: © 2015 American College of Rheumatology. Used with permission. 16
• Demonstrated protein expression • Product Profile – Genetically-modified autologous dermal fibroblasts – Gene construct identified, target gene selected – Preparing scale-up manufacturing using lentivirus • Animal model in development • Anticipated milestones – Proof-of-concept animal study data 1H16 – IND submission in 4Q16 FCX-013 Development Progressing 17

Anticipated Milestones & Corporate Info 18 • Cash = $36.9 million at 30 Sept 2015 • 43.9 million common shares of FCSC outstanding at 30 Sept 2015 – 5.9 million warrants; 3.2 million options • Analyst research – Wedbush Securities – David Nierengarten, PhD – Griffin Securities – Keith Markey, PhD Anticipated Milestones Timing FCX-007 Phase I/II Trial Initiation 2Q16 azficel-T sBLA Phase II Data 2Q16 FCX-013 Proof-of-Concept Data 1H16 FCX-013 IND Submission 4Q16

• Fibrocell is the leader of autologous fibroblast therapy for skin and connective tissue diseases – Developing a pipeline of cell and gene therapies for orphan skin diseases using autologous fibroblasts to deliver a targeted gene • Phase II azficel-T sBLA program: autologous fibroblasts for Chronic Dysphonia – Significant voice impairment due to severe vocal cord scarring or atrophy – Phase II data expected in 2Q16 • FCX-007: Novel gene-therapy product candidate for Recessive Dystrophic Epidermolysis Bullosa (RDEB), a congenital orphan skin disease – Devastating, progressive, painful blistering disease that leads to death – Granted rare pediatric disease designation by FDA – Collaboration with Intrexon Corporation (NYSE: XON); expect to initiate Phase I/II trial in 2Q16 • FCX-013: Novel gene-therapy product candidate for Linear Scleroderma – Autoimmune disease causes debilitating, painful linear scars and skin thickening that can lead to pain, reduced joint mobility and deformity – Collaboration with Intrexon; proof-of-concept expected 1H16; IND planned 4Q16 Company Highlights 19

References 1Data on file. Fibrocell Science, Inc. 2Chhetri, Dinesh, Injection of Cultured Autologous Fibroblasts for Human Vocal Fold Scars. The Laryngoscope 121(4):785-792, 2011. 3 The Dystrophic Epidermolysis Research Association of America (DebRA). DEB brochure, page 6: http://www.debra.org/pdfs/Debra-of-America-Brochure.pdf; accessed 07/20/15. 4 DEBRA International. What is EB Infographic: http://www.debra-international.org/epidermolysis-bullosa.html.; accessed 10/06/2014. 5Petrof G., et. al. Fibroblast cell therapy enhances initial healing in recessive dystrophic epidermolysis bullosa wounds: results of a randomised, vehicle-controlled trial. Brit J Dermatol. 2013 Nov;169(5):1025-33. 6The Scleroderma Foundation. What is Scleroderma? http://www.scleroderma.org/site/PageServer?pagename=patients_whatis#.VaUwk7BFBMw, paragraph 6; accessed 10/09/2014—states, “It’s estimated that about 300,000 Americans have scleroderma. About one third of those people have the systemic form of scleroderma (i.e., 200,000 have a form of localized scleroderma).“ 7The Scleroderma Foundation. “Localized Scleroderma” brochure, pages 4, 6-7: http://www.scleroderma.org/site/DocServer/Localized.pdf?docID=317; accessed 07/20/15—states, “Some patients with localized scleroderma, an estimated 10 to 20 percent (20% of 200,000 = 40,000 patients), develop joint pain (arthralgia) during the course of their disease.” 20