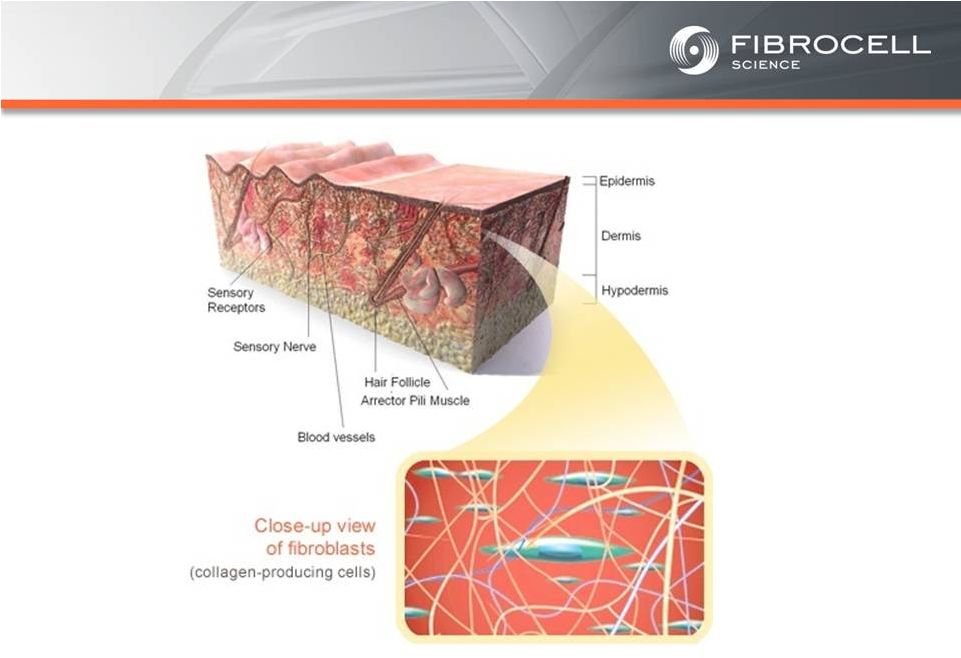
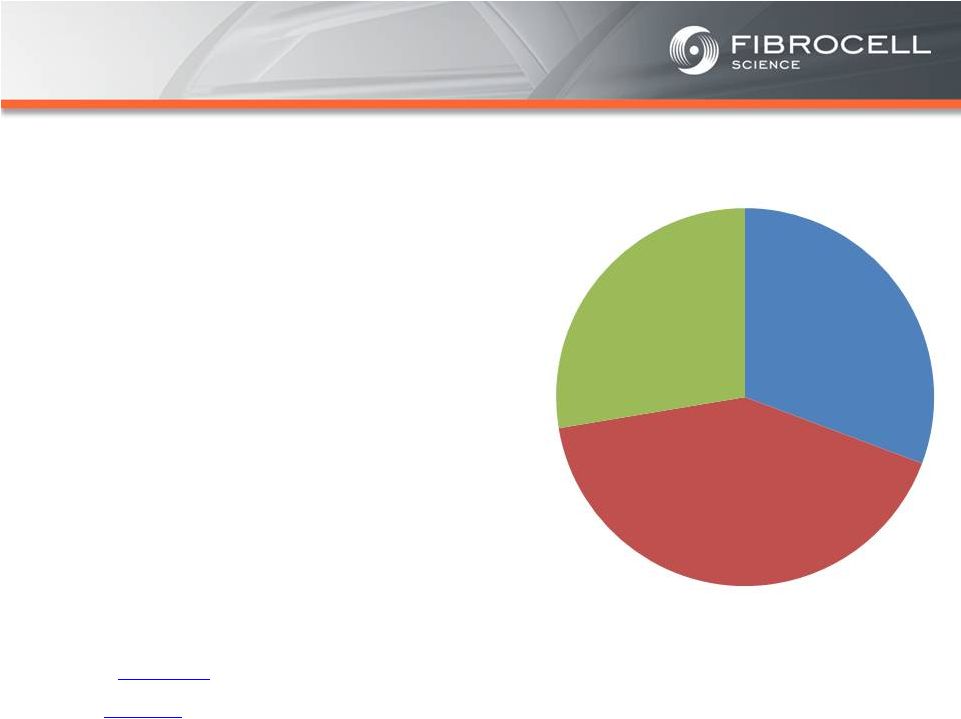
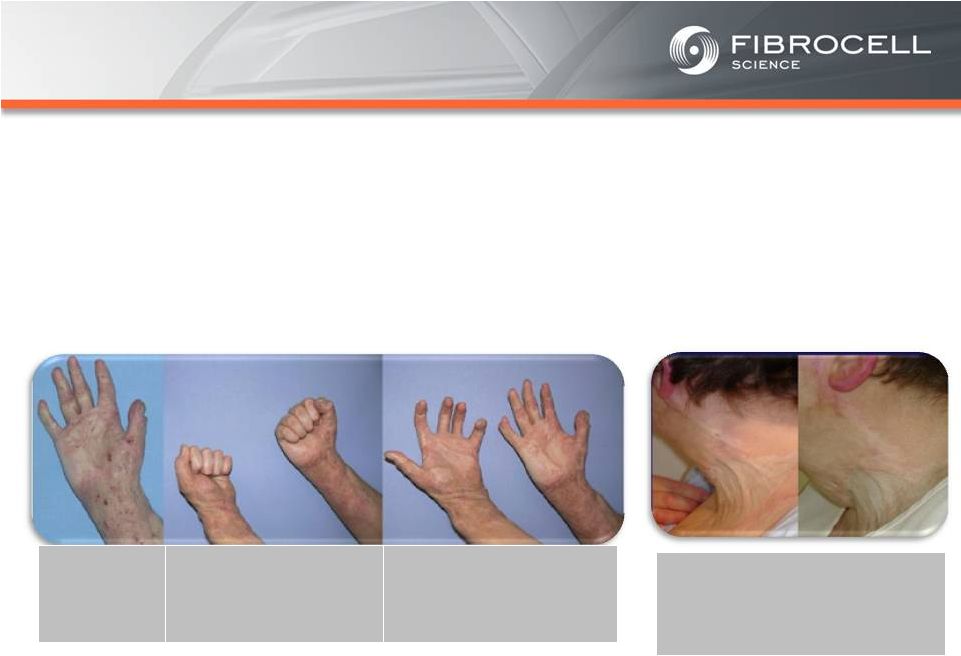
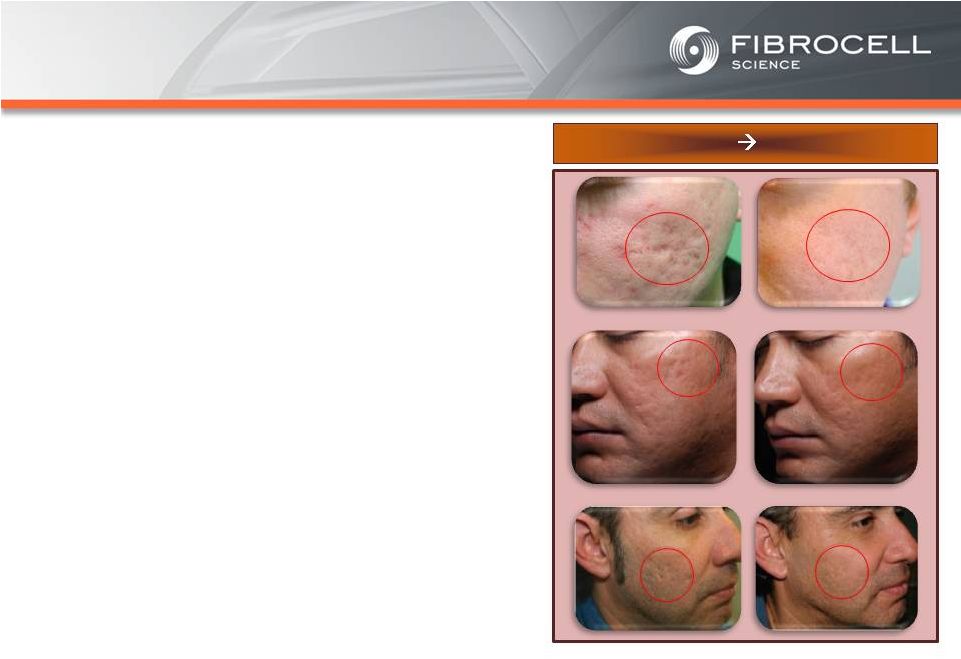
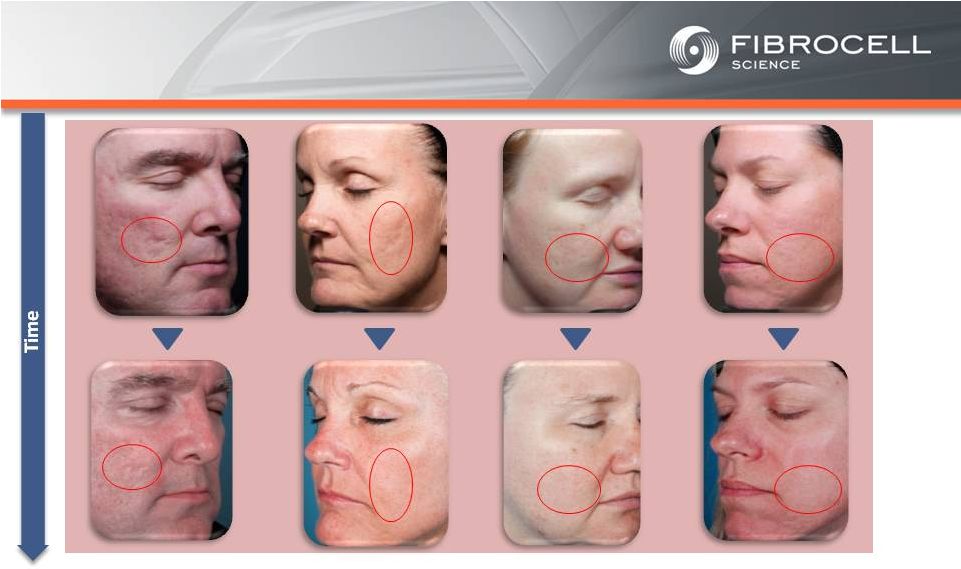
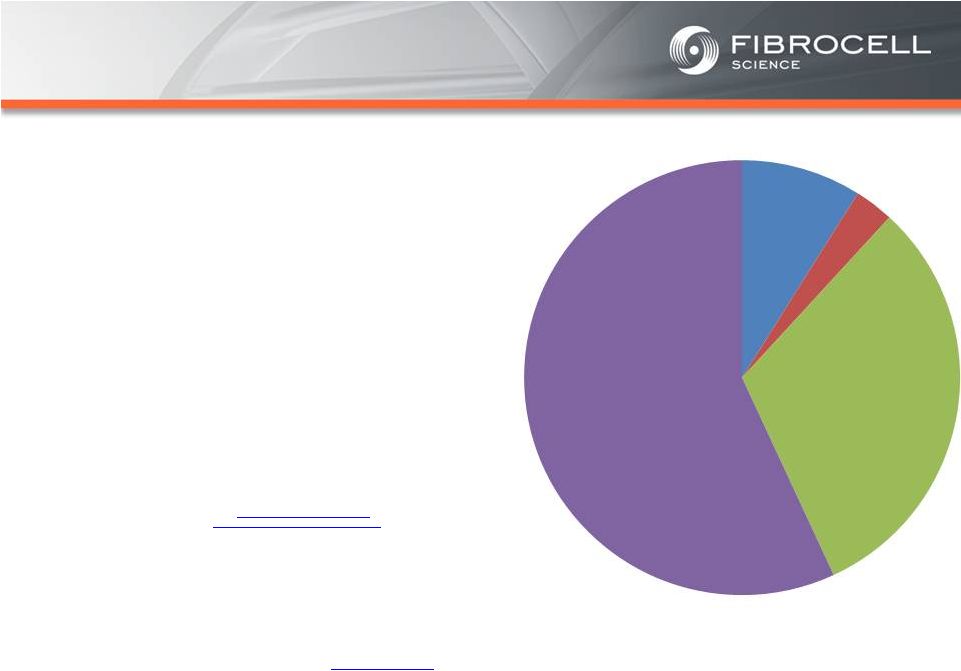
Intrexon Collaboration: Executive Summary Based on market analyses, the annual market potential for each indication is as follows: • $1.7-$3.4 billion for a treatment for moderate to severe psoriasis (1,2,3,4) • $120-$140 million for a treatment for cutaneous eosinophilias (5,6) • $300-$350 million for a treatment for morphea (localized scleroderma) (7,8) • $560 million-$2.2 billion for a treatment for Recessive Dystrophic Epidermolysis Bullosa (RDEB) (9,10,11, 12) Cutaneous Eosinophilias $120-$140 million Moderate to Severe Psoriasis $1.7-$3.4 Billion RDEB $560million- $2.2 billion Morphea $300-$500 million (1) National Psoriasis Foundation, “About Psoriasis” Available at: http://www.psoriasis.org/about-psoriasis. Accessed June 27, 2013. (2) PhotoMedex, Inc., “Severity of Psoriasis” Available at: http://xtracnow.com/physicians/psoriasis_stats.htm/ Accessed June 27, 2013. (3) Beyer et al. “Recent Trends in Systemic Psoriasis Treatment Costs” Arch Dermatol2010;146(1):46-54. (4) Stern et al. “Psoriasis Is Common, Carries a Substantial Burden Even When Not Extensive, and Is Associated with Widespread Treatment Satisfaction” J InvestigDermatolzzz Symp2004;9:136-139. (5) Cutaneous eosinophilias represent a family of more than 30 different conditions ranging from eosinophilic cellulitis (Wells’ syndrome) to eosinophilicdermatosis and eosinophilic fasciitis. The prevalence varies based on which conditions are targeted for treatment. It could be as low as a few hundred patients (Wells’ syndrome and eosinophilic fasciitis) to thousands of patients (Duhring disease, which affects 15% to 25% of celiac patients). We chose 4,000 patients based on the Fibrocell press release dated July 1, 2013. (6) Beyer et al. “Recent Trends in Systemic Psoriasis Treatment Costs” Arch Dermatol 2010;146(1):46-54. We assume that the cutaneous eosinophiliasindication will command a higher price than the psoriasis indication due to the need for new treatment options, the frequency of treatment, and the severity of the condition. (7) Kaplan et al. “Localized Fibrosing Disorders – Linear Scleroderma, Morphea, and Regional Fibrosis” eMedicine March 6, 2013. (8) Beyer et al. “Recent Trends in Systemic Psoriasis Treatment Costs” Arch Dermatol2010;146(1):46-54. We assume that the morpheaindication will command a higher price than the psoriasis indication due to the need for new treatment options, the frequency of treatment, and the severity of the condition. (9) Stanford School of Medicine, “EpidermolysisBullosa Clinic Frequently Asked Questions” Available at: http://dermatology.stanford.edu/gsdc/eb_clinic/eb-faqs.html. Accessed July 11, 2013. (10) The Dystrophic EpidermolysisBullosa Research Association of America (DEBRA), “About EB” http://www.debra.org/abouteb. Accessed July 11, 2013. (11) Herper, Matthew. “How A $440,000 Drug Is Turning Alexion Into Biotech‘s New Innovation Powerhouse.” Forbes. 5 September 2012 (12) The price range represents the price potential for a new therapy for a severe ultra rare disease based on currently marketed rare disease therapies such as Soliris® (eculizumab - ~$400,000/year), Elaprase® (idursulfase - ~$375,000/year), Naglazyme® (galsulfase - ~$365,000/year), Myozyme® (alglucosidasealfa - ~$300,000/year), and Fabrazyme® (agalsidase beta - ~$200,000/year). |