UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| | | |
| þ | | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended December 31, 2007
OR
| | | |
| o | | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
Isolagen, Inc.
(Exact name of registrant as specified in its Charter.)
| | | | | |
Delaware
(State or other jurisdiction
of incorporation) | | 001-31564
(Commission File Number) | | 87-0458888
(I.R.S. Employer
Identification No.) |
405 Eagleview Boulevard
Exton, Pennsylvania 19341
(Address of principal executive offices, including zip code)
(484) 713-6000
(Issuer’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | | |
| Title of Each Class | | Name of Each Exchange on which Registered |
| Common Stock, $.001 par value | | American Stock Exchange |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.Yeso Noþ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.Yeso Noþ
Indicate by check mark whether the registrant: (1) filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for any shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.Yesþ Noo
Indicate by check mark if there is no disclosure of delinquent filers in response to Item 405 of Regulation S-K contained in this form, and no disclosure will be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to thisForm 10-K.o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
| Large accelerated filero | | Accelerated filerþ | | Non-accelerated filero | | Smaller reporting companyo |
| | | | | (Do not check if a smaller
reporting company) | | |
Indicate by check mark whether the registrant is shell company (as defined in the Exchange Act Rule 12b-2)Yeso Noþ
As of June 30, 2007, the aggregate market value of the issuer’s common stock held by non-affiliates of the issuer based upon the price at which such common stock was sold on the American Stock Exchange as of such date was $129,057,544.
As of March 3, 2008, issuer had 41,639,408 shares issued and 37,639,408 shares outstanding of common stock, par value $0.001.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Proxy Statement for the 2008 Annual Meeting of Stockholders (the “Proxy Statement”), to be filed within 120 days of the end of the fiscal year ended December 31, 2007, are incorporated by reference in Part III hereof. Except with respect to information specifically incorporated by reference in this Form 10-K, the Proxy Statement is not deemed to be filed as part hereof.
Part 1
This Annual Report on Form 10-K (including the section regarding Management’s Discussion and Analysis of Financial Condition and Results of Operations) contains certain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, as well as information relating to Isolagen, Inc. and its subsidiaries (referred to as “Isolagen,” “Company,” “we,” or “our”) that is based on management’s exercise of business judgment and assumptions made by and information currently available to management. Although forward-looking statements in this Annual Report on Form 10-K reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. When used in this document and other documents, releases and reports released by us, the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “the facts suggest” and words of similar import, are intended to identify any forward-looking statements. You should not place undue reliance on these forward-looking statements. These statements reflect our current view of future events and are subject to certain risks and uncertainties as noted below. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, our actual results could differ materially from those anticipated in these forward-looking statements. Actual events, transactions and results may materially differ from the anticipated events, transactions or results described in such statements. Although we believe that our expectations are based on reasonable assumptions, we can give no assurance that our expectations will materialize. Many factors could cause actual results to differ materially from our forward looking statements including those set forth in Item 1A of this report. Other unknown, unidentified or unpredictable factors could materially and adversely impact our future results. We undertake no obligation and do not intend to update, revise or otherwise publicly release any revisions to our forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of any unanticipated events.
We file reports with the Securities and Exchange Commission (“SEC” or “Commission”). We make available on our website (www.Isolagen.com) free of charge our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file such materials with or furnish them to the SEC. Information appearing at our website is not a part of this Annual Report on Form 10-K. You can also read and copy any materials we file with the Commission at its Public Reference Room at 100 F Street, NE, Washington, DC 20549. You can obtain additional information about the operation of the Public Reference Room by calling the Commission at 1-800-SEC-0330. In addition, the Commission maintains an Internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the Commission, including Isolagen.
Our corporate headquarters is located at 405 Eagleview Boulevard, Exton, Pennsylvania 19341. Our phone number is (484) 713-6000. Our fiscal year begins on January 1, and ends on December 31, and any references herein to “Fiscal 2007” mean the year ended December 31, 2007, and references to other “Fiscal” years mean the year ending December 31, of the year indicated.
We own or have rights to various copyrights, trademarks and trade names used in our business including but not limited to the following: Isolagen, Isolagen Therapy, Isolagen Process, Agera and Agera Rx. This report also includes other trademarks, service marks and trade names of other companies. Other trademarks and trade names appearing in this report are the property of the holder of such trademarks and trade names.
We obtained statistical data, market data and other industry data and forecasts used in this Form 10-K from publicly available information. While we believe that the statistical data, industry data, forecasts and market research are reliable, we have not independently verified the data, and we do not make any representation as to the accuracy of that information.
1
Overview
We are an aesthetic and therapeutic development stage company focused on developing novel skin and tissue rejuvenation products. Our clinical development product candidates are designed to improve the appearance of skin injured by the effects of aging, sun exposure, acne and burn scars with a patient’s own, or autologous, fibroblast cells produced by our proprietary Isolagen Process. Our clinical development programs encompass both aesthetic and therapeutic indications. Our most advanced indication utilizing the Isolagen Therapy is for the treatment of nasolabial folds/wrinkles and is currently in Phase III clinical development. We also have an ongoing Phase II/III study for the treatment of acne scars and ongoing Phase II studies related to full face rejuvenation, restrictive burns scars and periodontal disease.
We also develop and market an advanced skin care product line through our Agera Laboratories, Inc. subsidiary, in which we acquired a 57% interest in August 2006.
Going Concern
As of December 31, 2007, we had cash, cash equivalents and restricted cash of $17.0 million and working capital of $13.8 million (including our cash, cash equivalents and restricted cash). We believe our existing capital resources are adequate to finance our current operating plan through September 1, 2008; however, our long-term viability is dependent upon successful operation of our business, our ability to improve our manufacturing process, the approval of our products and the ability to raise additional funding to meet our business objectives. We estimate that we will require additional cash resources during the third quarter of 2008 based upon our current operating plan and condition. This estimate excludes any proceeds that would be realized from the sale of our Swiss campus, which we are attempting to sell (refer to Note 3 of the Consolidated Financial Statements). We will add any proceeds from the sale of the Swiss campus to our working capital, which would partially alleviate our need to obtain financing from other sources. There is no assurance that capital in any form would be available to us, and if available, on terms and conditions that are acceptable.
As a result of the above discussed conditions, and in accordance with generally accepted accounting principles in the United States, there exists substantial doubt about our ability to continue as a going concern, and our ability to continue as a going concern is contingent upon our ability to secure additional adequate financing or capital prior to or during the third quarter of 2008. If we are unable to obtain additional sufficient funds prior to this time, we will be required to terminate or delay regulatory approval of one, more than one, or all of our product candidates, curtail or delay the implementation of manufacturing process improvements or delay the expansion of our sales and marketing capabilities. Further, if we do not obtain additional funding prior to or during the third quarter of 2008, we may enter into bankruptcy during 2008 and possibly cease operations thereafter. Any of these actions would have an adverse affect on our operations, the realization of our assets and the timely satisfaction of our liabilities. Our financial statements are presented on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The financial statements do not include any adjustments relating to the recoverability of the recorded assets or the classification of liabilities that may be necessary should it be determined that we are unable to continue as a going concern.
Isolagen’s Technology Platform
We use our proprietary Isolagen Process to produce an autologous living cell therapy. We refer to this autologous living cell therapy as the Isolagen Therapy. We believe this therapy addresses the normal effects of aging or injury to the skin. Each of our product candidates is designed to use Isolagen Therapy to treat an indicated condition. We use our Isolagen Process to harvest autologous fibroblasts from a small skin punch biopsy from behind the ear with the use of a local anesthetic. We chose this location both because of limited exposure to the sun and to avoid creating a visible scar. In the case of our dental product candidate, the biopsy is taken from the patient’s palette. The biopsy is then packed in a vial in a special shipping container and shipped to our laboratory where the fibroblast cells are released from the biopsy and initiated into our cell culture process where the cells proliferate until they reach the required cell count. The fibroblasts are then harvested, tested by quality control and released by quality assurance prior to shipment. The number of cells and the frequency of injections may vary and will depend on the indication or application being studied.
2
If and when approved, we expect our product candidates will offer patients their own living fibroblast cells in a personalized therapy designed to improve the appearance of damaged skin and wrinkles; or in the case of restrictive burn scars, improve range of motion. Our product candidates are intended to be a minimally invasive alternative to surgical intervention and a viable natural alternative to other chemical, synthetic or toxic treatments. We also believe that because our product candidates are autologous, the risk of an immunological or allergic response is low. With regard to the therapeutic markets, we believe that our product candidates may address an insufficiently met medical need for the treatment of each of restrictive burn scars, acne scars and dental papillary insufficiency, or gum recession, and potentially help patients avoid surgical intervention. Our product candidates are still in clinical development and, as such, benefits we expect to see associated with our product candidates may not be validated in our clinical trials. In addition, disadvantages of our product candidates may become known in the future.
Our business strategy is primarily focused on our current Phase III nasolabial fold/wrinkle study. Specifically, during 2008 our efforts will be focused on our Phase III wrinkle study milestones, such as completing the six month efficacy follow-up, accumulating and analyzing the related study data, and continuing our Biologics License Application (BLA) efforts (assuming efficacious results from this Phase III study). Our additional objectives include achieving regulatory milestones related to our other Phase II/III and Phase II studies currently underway (refer to Clinical Development Programs below).
Recent Management Changes
In January 2008, Mr. Declan Daly assumed the role of our Chief Executive Officer. Our former Chief Executive Officer, Mr. Nicholas L. Teti, Jr., will continue to serve as Chairman of the Board of Directors and as a consultant to the Company.
Clinical Development Programs
Our product development programs are focused on the aesthetic and therapeutic markets. These programs are supported by a number of clinical trial programs at various stages of development.
Our aesthetics development programs include product candidates to treat targeted areas or wrinkles and to provide full-face rejuvenation that includes the improvement of fine lines, wrinkles, skin texture and appearance. Our therapeutic development programs are designed to treat acne scars, restrictive burn scars and dental papillary recession. All of our product candidates are non-surgical and minimally invasive. Although the discussions below include estimates of when we expect trials to be completed, the prediction of when a clinical trial will complete is subject to a number of factors and uncertainties.
Aesthetic Development Programs
Wrinkles/Nasolabial Folds —Phase III Trials: In October 2006, we reached an agreement with the U.S. Food and Drug Administration, or FDA, on the design of a Phase III pivotal study protocol for the treatment of nasolabial folds (lines which run from the sides of the nose to the corners of the mouth). The randomized, double-blind protocol was submitted to the FDA under the agency’s Special Protocol Assessment, or SPA. Pursuant to this assessment process, the FDA has agreed that our study design for two identical trials, including subject numbers, clinical endpoints, and statistical analyses, is adequate to provide the necessary data that, depending on the outcome, could form the basis of an efficacy claim for a marketing application. The pivotal Phase III trials will evaluate the efficacy and safety of Isolagen Therapy against placebo in approximately 400 subjects total with approximately 200 subjects enrolled in each trial. We completed enrollment of the study and commenced injection of subjects in early 2007. As we have previously disclosed, we encountered certain delays related to manufacturing and scheduling in connection with the study. We conferred with the FDA regarding these issues, and based on our findings and our dialogue with the FDA, we submitted an amendment to our study protocol and chemistry, manufacturing and control (CMC) submission. In June 2007, we received approval of the amendment and the related Informed Consent Forms from the Investigational Review Board. In July 2007, the FDA notified us that our proposed changes to the protocol were acceptable and within the scope of the original SPA review. We completed injections related to this study during January 2008 and are currently in the six-month efficacy follow-up period.
3
Refer to Part I, Item 1A of this Form 10-K for a discussion of our risk factors, including “Higher than anticipated dropout rates of subjects in our clinical trials could adversely affect trial results and make it more difficult to obtain regulatory approval.”
In March 2004, we announced positive results of a first Phase III exploratory clinical trial for our lead product candidate, and in July 2004 we commenced a 200 subject Phase III study of Isolagen Therapy for facial wrinkles consisting of two identical, simultaneous trials. The study was concluded during the second half of 2005. In August 2005 we announced that results of this study failed to meet co-primary endpoints. Based on the results of this study, we commenced preparations for our second Phase III pivotal study discussed in the preceding paragraph.
Full Face Rejuvenation —Phase II Trial: In March 2007 we commenced an open label (unblinded) trial of approximately 50 subjects. Injections of Isolagen Therapy began to be administered in July 2007. This trial is designed to further evaluate the safety and use of Isolagen Therapy to treat fine lines and wrinkles for the full face. Five investigators across the United States are participating in this trial. The subjects will receive two series of injections approximately one month apart. The subjects will be followed for six months following each subject’s last injection. In late December 2007, all 45 remaining subjects completed injections. This study is currently in the six-month efficacy follow-up period.
Therapeutic Development Programs
Acne Scars —Phase II/ III Trial: In November 2007, we commenced an acne scar Phase II/III study. This study is to include approximately 120 subjects. This placebo controlled trial is designed to evaluate the use of our Isolagen Therapy to correct or improve the appearance of acne scars. Each subject will serve as their own control, receiving Isolagen Therapy on one side of their face and placebo on the other. The subjects will receive three injections two weeks apart. The follow-up and evaluation period will be complete four months after each subject’s last injection.
We filed a pre-Investigational New Drug application, or pre-IND, for a Phase III clinical trial program in July 2007 together with our protocol. In September 2007, the IND was accepted by the FDA. We held an investigator meeting in early November 2007 and commenced biopsies shortly thereafter.
In connection with this acne scar program, we have developed a validated photo guide for use in the evaluators’ assessment of acne study subjects. We had originally designed the acne scar clinical program as two randomized, double-blind, Phase III, placebo-controlled trials of approximately 120 subjects each. However, our evaluator assessment scale and photo guide have never been utilized in a clinical trial. In November 2007, the FDA recommended that we consider conducting a Phase II study in order to address certain study issues, including issues related to our evaluator assessment scale. As such, we modified our clinical plans to initiate a single Phase II/III trial. This Phase II/III study, which is powered to demonstrate efficacy, will allow for a closer assessment of the evaluator assessment scale and photo guide. Upon successful completion of the Phase II/III study, we expect to initiate a subsequent, additional Phase III trial. We believe that the two trials may have the potential to form the basis of a licensure submission to the FDA.
Restrictive Burn Scars- Phase II Trial: In January 2007, we met with the FDA to discuss our clinical program for the use of Isolagen Therapy for restrictive burn scar patients. This Phase II trial will evaluate the use of Isolagen Therapy to improve range of motion, function and flexibility, among other parameters, in existing restrictive burn scars in approximately 20 patients. We filed IND in February 2007 and are currently in the process of obtaining Investigational Review Board approval related to the investigative sites.
Dental Study- Phase II Trial: In late 2003, we completed a Phase I clinical trial for the treatment of condition relating to periodontal disease, specifically to treat Interdental Papillary Insufficiency. In the second quarter of 2005, we concluded the Phase II dental clinical trial with the use of Isolagen Therapy and subsequently announced that investigator and subject visual analog scale assessments demonstrated that the Isolagen Therapy was statistically superior to placebo at four months after treatment. Although results of the investigator and subject assessment demonstrated that the Isolagen Therapy was statistically superior to placebo, an analysis of objective linear measurements did not yield statistically significant results.
4
In 2006, we commenced a Phase II open-label dental trial for the treatment of Interdental Papillary Insufficiency. This single site study includes 11 subjects and the trial is expected to be completed and the data evaluated during the first half of 2008. We are identifying a development strategy for this application as we do not expect to fund an additional trial related to this application.
Agera Skincare Systems
We market and sell a skin care product line through our majority-owned subsidiary, Agera Laboratories, Inc., which we acquired in August 2006. Agera offers a complete line of skincare systems based on a wide array of proprietary formulations, trademarks and nano-peptide technology. These skincare products can be packaged to offer anti-aging, anti-pigmentary and acne treatment systems. Agera markets its products in both the United States and Europe (primarily the United Kingdom).
| | | Our Target Market Opportunities |
Aesthetic Market Opportunity
Our Isolagen product candidate for wrinkles/nasolabial folds and full face rejuvenation are directed primarily at the aesthetic market. Aesthetic procedures have traditionally been performed by dermatologists, plastic surgeons and other cosmetic surgeons. According to the American Society for Aesthetic Plastic Surgery, or ASAPS, the total market for non-surgical cosmetic procedures was approximately $4.7 billion in 2007. We believe the aesthetic procedure market is driven by:
| | • | | aging of the “baby boomer” population, which currently includes ages approximately 44 to 62; |
| | • | | increasing desire of many individuals to improve their appearance; |
| | • | | impact of managed care and reimbursement policies on physician economics, which has motivated physicians to establish or expand the menu of elective, private-pay aesthetic procedures that they offer; and |
| | • | | broadening base of the practitioners performing cosmetic procedures beyond dermatologists and plastic surgeons to non-traditional providers. |
According to the ASAPS, 11.7 million surgical and non-surgical cosmetic procedures were performed in 2007, as compared to 11.4 million in 2006. Also according to the ASAPS, approximately 9.6 million non-surgical procedures were performed in 2007 and approximately 9.5 million non-surgical procedures were performed in 2006. We believe that the concept of non-surgical cosmetic procedures involving injectable materials has become more mainstream and accepted. According to the ASAPS, the following table shows the top five non-surgical cosmetic procedures performed in 2007:
| | | | | |
| Procedure | | Number | |
| Botox injection | | | 2,775,176 | |
| Hyaluronic acids | | | 1,448,716 | |
| Laser hair removal | | | 1,412,657 | |
| Microdermabrasion | | | 829,658 | |
| Laser skin resurfacing | | | 647,707 | |
Procedures among the 35 to 50 year old age group made up approximately 47% of all cosmetic procedures in 2007. The 51 to 64 year old age group made up 25% of all cosmetic procedures in 2007, while the 19 to 34 year old age group made up 21% of cosmetic procedures in 2007. Botox injection was the most popular treatment among the 35 to 50 year old age group.
Therapeutic Market Opportunities
In addition to the aesthetic market, we believe there are opportunities for our Isolagen Therapy to treat certain medical conditions such as acne scars, restrictive burn scars and tissue loss due to papillary recession. Presently, we are studying therapeutic applications of our technology for acne scars, restrictive burn scars and periodontal disease. We are not aware of other autologous cell-based treatments for any of these therapeutic applications.
5
Acne Scars.Acne is the most common skin disorder in the United States. The term acne includes conditions ranging from clogged pores to outbreaks of severe lesions. According to the American Academy of Dermatology and the National Institute of Health, nearly 80% of people aged 11 to 30 have acne outbreaks at some point, and approximately 95% of these patients will have some degree of scarring depending on the severity and duration of the condition. Over time, as facial tone declines and facial fat stores are depleted, the scars typically become more noticeable. Current treatments for acne scarring are dermabrasion, laser resurfacing, surgical excision, and certain temporary fillers. We believe this market represents a significant opportunity for our acne scar product candidate.
Burns and Burn Scars.According to a Kalorama Information study on burns (Wound Care Volume II: Burns, Kalorama Information, August 2005), an estimated 2.5 million Americans seek medical care each year for burns and approximately 100,000 are hospitalized. Approximately 50% of patients with deep second degree, third and fourth degree burns develop restrictive scarring which are often painful, and reduce flexibility and functionality of the area affected. We believe this market represents a significant opportunity for our non-surgical treatment of existing restrictive burn scars. We also believe additional market opportunity exists for the use of our product candidate prior to the formation of a restrictive scar to promote healing in the acute phase of burn wound healing.
Periodontal.In the dental field, a majority of the population will experience periodontal disease at some point in their lives; therefore a market opportunity exists for an effective therapy for treating papillary recession. Therapeutic options that decrease the depth of the periodontal pockets make the patient’s daily home care more effective and reduce the chance of further gum and bone loss.
Papillary recession, also known as “black triangles,” can be associated with the progression of periodontal disease, and involves the recession of the triangular section of gum tissue between two teeth. While the number of Americans with some form of gum disease is significant (up to 30% of the population may be genetically susceptible to gum disease—American Academy of Periodontology—perio.org), we are focused on a targeted subset of this patient population with papillary recession (black triangles). Currently, the loss of tissue associated with severe periodontal disease can only be treated through surgical procedures. These surgical procedures are expensive and painful, can potentially result in complications and have variable outcomes.
Agera Skincare Market Opportunities
Based on the Kline & Company, Inc. study, “The U.S. Professional Skin Care Market 2003,” the 2008 U.S. professional skin care market is estimated at $742 million. This report describes the market as comprised of the following sub-markets: Salons and spas (59%), Retail stores (22%) and Medical care (19%). The doctor dispensing market is primarily focused in the Dermatology and Plastic Surgeon segments but we believe is gaining interest with a broader audience of physician specialties, including the medical spa environment.
Sales and Marketing
While our Isolagen Therapy product candidates are still in the pre-approval phase in the United States, no marketing or sales can occur within the United States. Our Agera skincare products are primarily sold directly to our established distributors and salons, with historically very little focus on marketing efforts. We are currently attempting to identify additional third party distributors for our Agera product line. We believe that our Agera products have the strong potential to complement our Isolagen Therapy product candidates in the future.
Intellectual Property
We believe that patents, trademarks, copyrights, proprietary formulations (related to our Agera skincare products) and other proprietary rights are important to our business. We also rely on trade secrets, know-how and continuing technological innovations to develop and maintain our competitive position. We seek to protect our intellectual property rights by a variety of means, including obtaining patents, maintaining trade secrets and proprietary know-how, and technological innovation to operate without infringing on the proprietary rights of others and to prevent others from infringing on our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, actively seeking patent protection in the United States and certain foreign countries.
6
As of December 31, 2007, we had 8 issued U.S. patents, 6 pending U.S. patent applications, 32 granted foreign patents and 19 pending foreign patent applications. Our issued patents and patent applications primarily cover the method of using autologous cell fibroblasts for the repair of skin and soft tissue defects and the use of autologous fibroblast cells for tissue regeneration. We are in the process of pursuing several other patent applications.
In January 2003, we acquired two pending U.S. patent applications. As consideration, we issued 100,000 shares of our common stock and agreed to pay a royalty on revenue from commercial applications and licensing, up to a maximum of $2.0 million.
In August 2006, we acquired 57% of the common stock of Agera Laboratories. Agera has a number of trade names, trademarks, exclusive proprietary rights to product formulations and specified peptides that are used in the Agera skincare products.
Our success depends in part on our ability to maintain our proprietary position through effective patent claims and their enforcement against our competitors, and through the protection of our trade secrets. Although we believe our patents and patent applications provide a competitive advantage, the patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. We do not know whether any of our patent applications or those patent applications which we have acquired will result in the issuance of any patents. Our issued patents, those that may be issued in the future or those acquired by us, may be challenged, invalidated or circumvented, and the rights granted under any issued patent may not provide us with proprietary protection or competitive advantages against competitors with similar technology. In particular, we do not know if competitors will be able to design variations on our treatment methods to circumvent our current and anticipated patent claims. Furthermore, competitors may independently develop similar technologies or duplicate any technology developed by us. Because of the extensive time required for the development, testing and regulatory review of a potential product, it is possible that, before any of our products can be commercialized or marketed, any related patent claim may expire or remain in force for only a short period following commercialization, thereby reducing the advantage of the patent.
We also rely upon trade secrets, confidentiality agreements, proprietary know-how and continuing technological innovation to remain competitive, especially where we do not believe patent protection is appropriate or obtainable. We continue to seek ways to protect our proprietary technology and trade secrets, including entering into confidentiality or license agreements with our employees and consultants, and controlling access to and distribution of our technologies and other proprietary information. While we use these and other reasonable security measures to protect our trade secrets, our employees or consultants may unintentionally or willfully disclose our proprietary information to competitors.
Our commercial success will depend in part on our ability to operate without infringing upon the patents and proprietary rights of third parties. It is uncertain whether the issuance of any third party patents would require us to alter our products or technology, obtain licenses or cease certain activities. Our failure to obtain a license to technology that we may require to discover, develop or commercialize our future products may have a material adverse impact on us. One or more third-party patents or patent applications may conflict with patent applications to which we have rights. Any such conflict may substantially reduce the coverage of any rights that may issue from the patent applications to which we have rights. If third parties prepare and file patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference proceedings in the United States Patent and Trademark Office to determine priority of invention.
We have collaborated and may collaborate in the future with other entities on research, development and commercialization activities. Disputes may arise about inventorship and corresponding rights in know-how and inventions resulting from the joint creation or use of intellectual property by us and our subsidiaries, collaborators, partners, licensors and consultants. As a result, we may not be able to maintain our proprietary position.
Competition
The pharmaceutical and dermal aesthetics industries are characterized by intense competition, rapid product development and technological change. Competition is intense among manufacturers of prescription pharmaceuticals and dermal injection products, such as for our core products.
7
If certain of our product candidates are approved, we will compete with a variety of companies in the dermatology and plastic surgery markets, many of which offer substantially different treatments for similar problems. These include silicone injections, laser procedures, facial surgical procedures, such as facelifts and eyelid surgeries, fat injections, dermabrasion, collagen and hyaluronic acid injections and Botulinum toxin injections, and other dermal fillers. Indirect competition comes from facial care treatment products. Items catering to the growing demand for therapeutic skin care products include facial scrubs, anti-aging treatments, tonics, astringents and skin-restoration formulas.
Many of our competitors are large, well-established pharmaceutical, chemical, cosmetic or health care companies with considerably greater financial, marketing, sales and technical resources than those available to us. Additionally, many of our present and potential competitors have research and development capabilities that may allow them to develop new or improved products that may compete with our product lines. Our products could be rendered obsolete or made uneconomical by the development of new products to treat the conditions addressed by our products, technological advances affecting the cost of production, or marketing or pricing actions by one or more of our competitors. Our facial aesthetics product may compete for a share of the existing market with numerous products and/or technologies that have become relatively accepted treatments recommended or prescribed by dermatologists and administered by plastic surgeons and aesthetic dermatologists.
There are several dermal filler products under development and/or in the FDA pipeline for approval which claim to offer certain facial aesthetic benefits. Depending on the clinical outcomes of the Isolagen Therapy trials in aesthetics, the success or failure of gaining approval and the label granted by the FDA if and when the therapy is approved, the competition for the Isolagen Therapy may prove to be direct competition to certain dermal fillers, laser technologies or new technologies. However, if we gain approval, we believe our Isolagen Therapy would be a “first to market” autologous cellular technology that could complement other modalities of treatment and represent a significant additional market opportunity.
The field for therapeutic treatments or tissue regeneration for use in wound healing is rapidly evolving. A number of companies are either developing or selling therapies involving stem cells, human-based, animal-based or synthetic tissue products. If approved as a therapy for acne scars, restrictive burn scars or periodontal disease, our product candidates would compete with synthetic, human or animal derived cell or tissue products marketed by companies like Genzyme, Integra Life Sciences, Johnson & Johnson, C.R. Bard, LifeCell, Organogenesis, and others.
The market for skincare products is quite competitive with low barriers to entry. We believe Agera’s dominant competitors in this market include companies like Obagi Medical Products, Inc., Skin Medica, Murad, Inc., Dermalogica, Pevonia Botanica and others.
Government Regulation
Our Isolagen Therapy technologies are subject to extensive government regulation, principally by the FDA and state and local authorities in the United States and by comparable agencies in foreign countries. Governmental authorities in the United States extensively regulate the pre-clinical and clinical testing, safety, efficacy, research, development, manufacturing, labeling, storage, record-keeping, advertising, promotion, import, export, marketing and distribution, among other things, of pharmaceutical products under various federal laws including the Federal Food, Drug and Cosmetic Act, or FFDCA, and under comparable laws by the states and in most foreign countries.
Domestic Regulation
In the United States, the FDA, under the FFDCA, the Public Health Service Act and other federal statutes and regulations, subjects pharmaceutical and biologic products to rigorous review. If we do not comply with applicable requirements, we may be fined, the government may refuse to approve our marketing applications or allow us to manufacture or market our products or product candidates, and we may be criminally prosecuted. The FDA also has the authority to discontinue or suspend manufacture or distribution, require a product withdrawal or recall or revoke previously granted marketing authorizations if we fail to comply with regulatory standards or if we encounter problems following initial marketing.
8
FDA Approval Process
To obtain approval of a new product from the FDA, we must, among other requirements, submit data demonstrating the product’s safety and efficacy as well as detailed information on the manufacture and composition of the product candidate. In most cases, this entails extensive laboratory tests and pre-clinical and clinical trials. This testing and the preparation of necessary applications and processing of those applications by the FDA are expensive and typically take many years to complete. The FDA may deny our applications or may not act quickly or favorably in reviewing these applications, and we may encounter significant difficulties or costs in our efforts to obtain FDA approvals that could delay or preclude us from marketing any products we may develop. The FDA also may require post-marketing testing and surveillance to monitor the effects of approved products or place conditions on any approvals that could restrict the commercial applications of these products. Regulatory authorities may withdraw product approvals if we fail to comply with regulatory standards or if we encounter problems following initial marketing. With respect to patented products or technologies, delays imposed by the governmental approval process may materially reduce the period during which we will have the exclusive right to exploit the products or technologies.
The FDA does not apply a single regulatory scheme to human tissues and the products derived from human tissue. On a case-by-case basis, the FDA may choose to regulate such products as transplanted human tissue, medical devices or biologics. A fundamental difference in the treatment of products under these classifications is that the FDA generally permits human tissue for transplantation to be commercially distributed without marketing approval. In contrast, products that require manufacturing or processing are regulated as medical devices or biologics and require FDA approval.
The process required by the FDA before a new drug or biologic may be marketed in the United States generally involves the following:
| | • | | completion of pre-clinical laboratory tests or trials and formulation studies; |
| |
| | • | | submission to the FDA of an IND for a new drug or biologic, which must become effective before human clinical trials may begin; |
| |
| | • | | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug or biologic for its intended use; |
| |
| | • | | detailed information on product characterization and manufacturing process; and |
| |
| | • | | submission and approval of a New Drug Application, or NDA, for a drug, or a Biologics License Application, or BLA, for a biologic. |
Pre-clinical tests include laboratory evaluation of product chemistry formulation and stability, as well as animal and other studies to evaluate toxicity. In view of the autologous nature of our product candidates and our prior clinical experience with our product candidates, we concluded that it was reasonably safe to initiate clinical trials without pre-clinical studies and that the clinical trials would be adequate to further assess both the safety and efficacy of our product candidates. The results of pre-clinical testing, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND application. The FDA requires a 30-day waiting period after the filing of each IND application before clinical trials may begin, in order to ensure that human research subjects will not be exposed to unreasonable health risks. At any time during this 30-day period or at any time thereafter, the FDA may halt proposed or ongoing clinical trials, or may authorize trials only on specified terms. The IND application process may become extremely costly and substantially delay development of our products. Moreover, positive results of pre-clinical tests will not necessarily indicate positive results in clinical trials.
9
The sponsor typically conducts human clinical trials in three sequential phases, which may overlap. These phases generally include the following:
| | • | | Phase I: The product is usually first introduced into healthy humans or, on occasion, into patients, and is tested for safety, dosage tolerance, absorption, distribution, excretion and metabolism. |
| |
| | • | | Phase II: The product is introduced into a limited subject population to: |
| | • | | assess its efficacy in specific, targeted indications; |
| |
| | • | | assess dosage tolerance and optimal dosage; and |
| |
| | • | | identify possible adverse effects and safety risks. |
| | • | | Phase III: These are commonly referred to as pivotal studies. If a product is found to have an acceptable safety profile and to be potentially effective in Phase II clinical trials, new clinical trials will be initiated to further demonstrate clinical efficacy, optimal dosage and safety within an expanded and diverse subject population at geographically-dispersed clinical study sites. |
| |
| | • | | If the FDA does ultimately approve the product, it may require post-marketing testing, including potentially expensive Phase IV studies, to confirm or further evaluate its safety and effectiveness. |
Before proceeding with a study, sponsors may seek a written agreement from the FDA regarding the design, size, and conduct of a clinical trial. This is known as a Special Protocol Assessment, or SPA. Among other things, SPAs can cover clinical studies for pivotal trials whose data will form the primary basis to establish a product’s efficacy. Where the FDA agrees to an SPA, the agreement may not be changed by either the sponsor or the FDA except if the sponsor and the FDA agree to a change, or a senior FDA official determines that a substantial scientific issue essential to determining the safety or effectiveness of the product was identified after the testing began. SPAs thus help establish up-front agreement with the FDA about the adequacy of a clinical trial design to support a regulatory approval, but the agreement is not binding if new circumstances arise. There is no guarantee that a study will ultimately be adequate to support an approval even if the study is subject to an SPA.
Clinical trials must meet requirements for Institutional Review Board, or IRB, oversight, patient informed consent and the FDA’s Good Clinical Practices. Prior to commencement of each clinical trial, the sponsor must submit to the FDA a clinical plan, or protocol, accompanied by the approval of the committee responsible for overseeing clinical trials at the clinical trial sites. The FDA or the IRB at each institution at which a clinical trial is being performed may order the temporary or permanent discontinuation of a clinical trial at any time if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial subjects. Data safety monitoring committees, who monitor certain studies to protect the welfare of study subjects, may also require that a clinical study be discontinued or modified.
The sponsor must submit to the FDA the results of the pre-clinical and clinical trials, together with, among other things, detailed information on the manufacturing and composition of the product, and proposed labeling, in the form of an NDA, or, in the case of a biologic, a BLA. The applicant must also submit with the NDA or BLA a substantial user fee payment, unless a waiver or reduction applies. For fiscal year 2008 this fee is $1,178,000. The FDA has advised us it is regulating our Isolagen Therapy as a biologic. Therefore, we expect to submit BLAs to obtain approval of our product candidates. Each NDA or BLA submitted for FDA approval is usually reviewed for administrative completeness and reviewability within 45 to 60 days following submission of the application. If deemed complete, the FDA will “file” the NDA or BLA, thereby triggering substantive review of the application. The FDA can refuse to file any NDA or BLA that it deems incomplete or not properly reviewable. Once the submission has been accepted for filing, the FDA will review the application and respond to the applicant in accordance with performance goals the FDA has established for the review of NDAs and BLAs — six months for priority applications and 10 months for regular applications. The review process is often significantly extended by FDA requests for additional information or clarification, or changes to the application submitted by the applicant in the form of amendments. The FDA may refer the BLA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved, but the FDA is not bound by the recommendation of an advisory committee.
10
It is possible that our product candidates will not successfully proceed through this approval process or that the FDA will not approve them in any specific period of time, or at all. The FDA may deny or delay approval of applications that do not meet applicable regulatory criteria, or if the FDA determines that the clinical data do not adequately establish the safety and efficacy of the product. Satisfaction of FDA pre-market approval requirements for a new biologic is a process that may take several years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease. The FDA reviews these applications and, when and if it decides that adequate data are available to show that the product is both safe and effective and that other applicable requirements have been met, approves the drug or biologic for marketing. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures upon our activities. Success in early stage clinical trials does not assure success in later stage clinical trials. Data obtained from clinical activities is not always conclusive and may be susceptible to varying interpretations that could delay, limit or prevent regulatory approval. Upon approval, a product candidate may be marketed only for those indications approved in the BLA or NDA and may be subject to labeling and promotional requirements or limitations, including warnings, precautions, contraindications and use limitations, which could materially impact profitability. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-market regulatory standards is not maintained or if safety, efficacy or other problems occur after the product reaches the marketplace.
The FDA may, during its review of an NDA or BLA, ask for additional test data. If the FDA does ultimately approve the product, it may require post-marketing testing, including potentially expensive Phase IV studies, to confirm or otherwise further evaluate the safety and effectiveness of the product. In addition, the FDA may, in some circumstances, impose restrictions on the use of the product, which may be difficult and expensive to administer and may require prior approval of promotional materials. Following approval, FDA may require labeling changes or impose new post-approval study, risk management, or distribution restriction requirements.
Ongoing FDA Requirements
Before approving an NDA or BLA, the FDA often will inspect the facilities at which the product is manufactured and will not approve the product unless the manufacturing facilities are in compliance with the FDA’s current Good Manufacturing Practices, or cGMP, requirements which govern the manufacture, holding and distribution of a product. Manufacturers of biologics also must comply with the FDA’s general biological product standards. Following approval, the FDA periodically inspects drug and biologic manufacturing facilities to ensure continued compliance with the cGMP requirements. Manufacturers must continue to expend time, money and effort in the areas of production, quality control, record keeping and reporting to ensure compliance with those requirements. Failure to comply with these requirements subjects the manufacturer to possible legal or regulatory action, such as suspension of manufacturing, seizure of product, voluntary recall of product, withdrawal of marketing approval or civil or criminal penalties. Adverse experiences with the product must be reported to the FDA and could result in the imposition of marketing restrictions through labeling changes or market removal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following approval.
The labeling, advertising, promotion, marketing and distribution of a drug or biologic product also must be in compliance with FDA and FTC requirements which include, among others, standards and regulations for direct-to-consumer advertising, industry-sponsored scientific and educational activities, and promotional activities involving the internet. In general, all product promotion must be consistent with the FDA approval for such product, contain a balanced presentation of information on the products uses and benefits and important safety information and limitations on use, and otherwise not be false or misleading. The FDA and FTC have very broad enforcement authority, and failure to abide by these regulations can result in penalties, including the issuance of a Warning Letter directing a company to correct deviations from regulatory standards and enforcement actions that can include seizures, injunctions and criminal prosecution.
Manufacturers are also subject to various laws and regulations governing laboratory practices, the experimental use of animals and the use and disposal of hazardous or potentially hazardous substances in connection with their research. In each of the above areas, the FDA has broad regulatory and enforcement powers, including the ability to levy fines and civil penalties, suspend or delay issuance of approvals, seize or recall products and deny or withdraw approvals.
11
HIPAA Requirements
Other federal legislation may affect our ability to obtain certain health information in conjunction with our research activities. The Health Insurance Portability and Accountability Act of 1996, or HIPAA, mandates, among other things, the adoption of standards designed to safeguard the privacy and security of individually identifiable health information. In relevant part, the U.S. Department of Health and Human Services, or HHS, has released two rules to date mandating the use of new standards with respect to such health information. The first rule imposes new standards relating to the privacy of individually identifiable health information. These standards restrict the manner and circumstances under which covered entities may use and disclose protected health information so as to protect the privacy of that information. The second rule released by HHS establishes minimum standards for the security of electronic health information. While we do not believe we are directly regulated as a covered entity under HIPAA, the HIPAA standards impose requirements on covered entities conducting research activities regarding the use and disclosure of individually identifiable health information collected in the course of conducting the research. As a result, unless they meet these HIPAA requirements, covered entities conducting clinical trials for us may not be able to share with us any results from clinical trials that include such health information.
Other U.S. Regulatory Requirements
In the United States, the research, manufacturing, distribution, sale, and promotion of drug and biological products are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services (formerly the Health Care Financing Administration), other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and state and local governments. For example, sales, marketing and scientific/educational grant programs must comply with the anti-fraud and abuse provisions of the Social Security Act, the False Claims Act, and similar state laws, each as amended. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990 and the Veterans Health Care Act of 1992, each as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection, unfair competition, and other laws.
International Regulation
The regulation of our product candidates outside of the United States varies by country. Certain countries regulate human tissue products as a pharmaceutical product, which would require us to make extensive filings and obtain regulatory approvals before selling our product candidates. Certain other countries classify our product candidates as human tissue for transplantation but may restrict its import or sale. Other countries have no application regulations regarding the import or sale of products similar to our product candidates, creating uncertainty as to what standards we may be required to meet.
Manufacturing
We currently have one operational manufacturing facility located in Exton, Pennsylvania. As part of our continuing efforts to evaluate the best uses of our resources, in the fourth quarter of 2006, the Board of Directors approved the proposed closing of our UK operation. We completed the closure of our London manufacturing facility on March 31, 2007. We previously used our London facility for the commercialization of our process (for which we earned revenue from the sale of Isolagen Therapy in the United Kingdom and other non-US markets) and as a means to improve our manufacturing process.
The costs incurred in operating our Exton facility (except for costs related to general corporate administration) are currently classified as research and development expenses. All component parts used in our Exton, Pennsylvania manufacturing process are readily available with short lead times, and all machinery is maintained and calibrated. We believe we have made improvements in our manufacturing processes, and we expect to continue such efforts in the future.
Our Agera products are manufactured by a third-party contract manufacturer under a contract manufacturing agreement. The agreement is effective through July 2014.
12
Research and Development
In addition to our clinical development activities, our research and development activities include improving our manufacturing processes and reducing manufacturing costs. Fibroblasts are a general support cell for the tissue and, in addition to their direct production of collagen and elastin, produce endocrine factors, which we believe may assist in the growth or repair of surrounding tissues, such as the epidermis. We believe this effect is responsible for some of the positive results that physicians have observed when treating patients with severe scarring. We expense research and development costs as they are incurred. For the years ended December 31, 2007, 2006 and 2005, we incurred research and development expenses of $13.3 million, $8.8 million and $10.6 million, respectively.
Employees
As of March 1, 2008, we employed 42 people on a full-time basis, all located in the United States, and one employee, our Chief Executive Officer, who is based in Ireland and works in both Ireland and the United States. We anticipate hiring additional employees in the areas of executive management, sales and marketing, quality assurance, manufacturing and research and development as the need arises. We also employ 2 part-time Agera employees. None of our employees are covered by a collective bargaining agreement, and we consider our relationship with our employees to be good. We also employ consultants and temporary labor on an as needed basis to supplement existing staff.
Segment Information
Financial information concerning the Company’s business segments and geographic areas of operation is included in Note 15 in the Notes to Consolidated Financial Statements contained in Item 8 of this Form 10-K.
Discontinued Operations
As part of our continuing efforts to evaluate the best uses of our resources, in the fourth quarter of 2006 our Board of Directors approved the closing of our United Kingdom operation. On March 31, 2007, we completed the closure of the United Kingdom manufacturing facility. As a result of the completion of the closure of the United Kingdom manufacturing facility, as of March 31, 2007 our United Kingdom operation was classified as a discontinued operation. In addition, as a result of the closure of our United Kingdom operation, the operations that we previously conducted in Switzerland and Australia, which had been absorbed into the United Kingdom operation, were also classified as discontinued operations as of March 31, 2007. Accordingly, the historical results of the United Kingdom, Switzerland and Australia have been retrospectively adjusted herein, for all period presented, to reflect the treatment of these operations as discontinued operations.
Corporate History
On August 10, 2001, our company, then known as American Financial Holding, Inc., acquired Isolagen Technologies through the merger of our wholly-owned subsidiary, Isolagen Acquisition Corp., and an affiliated entity, Gemini IX, Inc., with and into Isolagen Technologies. As a result of the merger, Isolagen Technologies became our wholly owned subsidiary. On November 13, 2001, we changed our name to Isolagen, Inc.
Potential and current investors should carefully consider the following risk factors prior to making any investment decisions regarding our securities.
We could fail to remain a going concern. We will need to raise substantial additional capital to fund our operations through the near term and through commercialization of our product candidates, and we do not have any commitments for that capital.
There exists substantial doubt regarding our ability to continue as a going concern. As discussed in Note 2 to the Consolidated Financial Statements-Going Concern,as of December 31, 2007 we had cash, cash equivalents and restricted cash of $17.0 million and working capital of $13.8 million (including our cash, cash equivalents and restricted cash). We believe our existing capital resources are adequate to finance our operations through September 1, 2008. We are focused on the advancement of our clinical trials, research and development, are incurring losses from operations, have limited capital resources, and do not have access to a line of credit or other debt facility.
13
We will need additional capital to achieve commercialization of our product candidates and to execute our business strategy, and if we are unsuccessful in raising additional capital we will be unable to achieve commercialization of our product candidates or unable to fully execute our business strategy on a timely basis, if at all. If we raise additional capital through the issuance of debt securities, the debt securities may be secured and any interest payments would reduce the amount of cash available to operate and grow our business. If we raise additional capital through the issuance of equity securities, such issuances will likely cause dilution to our stockholders, particularly if we are required to do so during periods when our common stock is trading at historically low price levels.
Additionally, we do not know whether any financing, if obtained, will be adequate to meet our capital needs and to support our growth. If adequate capital cannot be obtained on satisfactory terms, we may terminate or delay our efforts related to regulatory approval of one or more of our product candidates, curtail or delay the implementation of manufacturing process improvements or delay the expansion of our sales and marketing capabilities, any of which could cause our business may fail. Further, if we do not obtain additional funding prior to or during the third quarter of 2008, we may enter into bankruptcy during 2008 and possibly cease operations thereafter.
Our independent registered public accounting firm has modified their report for our fiscal year ended December 31, 2007 with respect to our ability to continue as a going concern. Our consolidated financial statements have been prepared on the basis of a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. If we became unable to continue as a going concern, we would have to liquidate our assets and we might receive significantly less than the values at which they are carried on our consolidated financial statements. The inclusion of a going concern modification in our independent registered public accounting firm’s audit opinion for the year ended December 31, 2007 may materially and adversely affect our stock price and our ability to raise new capital.
Clinical trials may fail to demonstrate the safety or efficacy of our product candidates, which could prevent or significantly delay regulatory approval and prevent us from raising additional financing.
Prior to receiving approval to commercialize any of our product candidates, we must demonstrate with substantial evidence from well-controlled clinical trials, and to the satisfaction of the FDA and other regulatory authorities in the United States and abroad, that our product candidates are both safe and effective. We will need to demonstrate our product candidates’ efficacy and monitor their safety throughout the process. We are conducting a pivotal Phase III clinical trial related to our lead facial aesthetic product candidate. The success of prior pre-clinical or clinical trials does not ensure the success of these trials, which are being conducted in populations with different racial and ethnic demographics than our previous trials. If our current trials or any future clinical trials are unsuccessful, our business and reputation would be harmed and the price at which our stock trades could be adversely affected. In addition, if our Phase III clinical trial related to our lead facial aesthetic product candidate is unsuccessful, we may be unable to raise additional equity or debt financing that we may require to continue to our operations.
All of our product candidates are subject to the risks of failure inherent in the development of biotherapeutic products. The results of early-stage clinical trials of our product candidates do not necessarily predict the results of later-stage clinical trials. Product candidates in later-stage clinical trials may fail to demonstrate desired safety and efficacy traits despite having successfully progressed through initial clinical testing. Even if we believe the data collected from clinical trials of our product candidates is promising, this data may not be sufficient to support approval by the FDA or any other U.S. or foreign regulatory approval. Pre-clinical and clinical data can be interpreted in different ways. Accordingly, FDA officials could reach different conclusions in assessing such data than we do, which could delay, limit or prevent regulatory approval. In addition, the FDA, other regulatory authorities, our Institutional Review Boards or we, may suspend or terminate clinical trials at any time.
14
Unlike our Phase III Nasolabial/Wrinkle trial, our Phase II/III Acne Scar trial is not subject to a Special Protocol Assessment (“SPA”) with the FDA. Isolagen has a photo guide for use in the evaluators’ assessment of acne study subjects. Our evaluator assessment scale and photo guide have not yet been used in a clinical trial.
Any failure or delay in completing clinical trials for our product candidates, or in receiving regulatory approval for the sale of any product candidates, has the potential to materially harm our business, and may prevent us from raising necessary, additional financing that we may need in the future.
Higher than anticipated dropout rates of subjects in our clinical trials could adversely affect trial results and make it more difficult to obtain regulatory approval.
Enrollment of 421 patients in our Phase III nasolabial/wrinkle trials was completed in February 2007. Our Phase III clinical trials include three separate treatment sessions for each subject followed by a 26 week efficacy observation period. Patient dropouts are expected and can occur for a variety of reasons. A subject who drops out of the trial prior to the 26 week post-treatment observation would, under the current protocol, be considered a “failure to respond” in the results of the clinical trial. As fewer patients complete a trial, a higher positive response rate to the therapy must be obtained for the group of remaining treated subjects in order to demonstrate statistically significant benefit compared to placebo. Our Phase III nasolabial/wrinkle trial consists of two studies, 006 and 005. Our 006 study enrolled 218 subjects and, as of March 3, 2008, has 191 remaining. Our 005 study enrolled 203 subjects and, as of March 3, 2008, has 168 remaining.
In October 2007, we submitted a protocol amendment to the FDA which, among other things, attempted to amend our Special Protocol Assessment to redefine the intent-to-treat population to exclude subjects for whom adequate cell yields could not be obtained for treatment. In December 2007, the FDA declined the Company’s protocol amendment, and as such the subject numbers remain as stated above. We continue to focus our efforts to prevent additional dropouts in both trials.
Higher than anticipated dropout rates may result in additional time, expense and uncertainty which may also affect our ability to obtain FDA clearance of our product and which could ultimately adversely affect our profitability and financial position.
Obtaining FDA and other regulatory approvals is complex, time consuming and expensive, and the outcomes are uncertain.
The process of obtaining FDA and other regulatory approvals is time consuming, expensive and difficult. Clinical trials are required and the marketing and manufacturing of our product candidates are subject to rigorous testing procedures. We have finished injections related to our pivotal Phase III clinical trial for our lead facial product candidate. Our other product candidates will require additional clinical trials. The commencement and completion of clinical trials for any of our product candidates could be delayed or prevented by a variety of factors, including:
| | • | | delays in obtaining regulatory approvals to commence a study; |
| |
| | • | | delays in identifying and reaching agreement on acceptable terms with prospective clinical trial sites; |
| |
| | • | | delays in the enrollment of subjects; |
| |
| | • | | manufacturing difficulties; |
| |
| | • | | lack of efficacy during clinical trials; or |
| |
| | • | | unforeseen safety issues. |
15
We do not know whether our clinical trials will need to be restructured or will be completed on schedule, if at all, or whether they will provide data necessary to support necessary regulatory approval. Significant delays in clinical trials will impede our ability to commercialize our product candidates and generate revenue, and could significantly increase our development costs.
We utilize bovine-sourced materials to manufacture our Isolagen Therapy. Future FDA regulations, as well as currently proposed regulations, may require us to change the source of the bovine-sourced materials we use in our products or to cease using bovine-sourced materials. If we are required to use alternative materials in our products, and in the event that such alternative materials are available to us, or if we choose to change the materials used in our products in the future, we would need to validate the new manufacturing process and run comparability trials with the reformulated product, which could delay our submission for regulatory approval.
Even if marketing approval from the FDA is received for one or more of our product candidates, the FDA may impose post-marketing requirements, such as:
| | • | | labeling and advertising requirements, restrictions or limitations, including the inclusion of warnings, precautions, contra-indications or use limitations that could have a material impact on the future profitability of our product candidates; |
| |
| | • | | testing and surveillance to further evaluate or monitor our future products and their continued compliance with regulatory standards and requirements; |
| |
| | • | | submitting products for inspection; |
| |
| | • | | suspending manufacturing; or |
| |
| | • | | withdrawing marketing clearance. |
We may issue additional equity securities and thereby materially and adversely affect the price of our common stock.
Sales of substantial amounts of shares of our common stock in the public market, or the perception that those sales may occur, could cause the market price of our common stock to decline. We have used and it is likely that we will continue to use our common stock or securities convertible into or exchangeable for our common stock to fund our working capital needs or to acquire technology, product rights or businesses, or for other purposes. If we issue additional equity securities, particularly during times when our common stock is trading at relatively low price levels, the price of our common stock may be materially and adversely affected.
We have yet to be profitable, losses may continue to increase from current levels and we will continue to experience significant negative cash flow as we expand our operations, which may limit or delay our ability to become profitable.
We have incurred losses since our inception, have never generated significant revenue from commercial sales of our products, and have never been profitable. We are focused on product development, and we have expended significant resources on our clinical trials, personnel and research and development. We expect these costs to continue to rise in the future. In addition, we have incurred, and will continue to incur, marketing and brand development costs for Agera, and will continue to incur such costs in the future. Our consolidated net losses for the years ended 2007, 2006 and 2005 were $35.6 million, $35.8 million and $35.8 million, respectively. As of December 31, 2007, we had an accumulated development stage net loss attributable to common shareholders of $162.6 million. We expect to continue to experience increasing operating losses and negative cash flow as we expand our operations.
We expect to continue to incur significant additional costs and expenses related to:
| | • | | FDA clinical trials and regulatory approvals; |
| |
| | • | | expansion of laboratory and manufacturing operations; |
16
| | • | | research and development; |
| |
| | • | | brand development; |
| |
| | • | | personnel costs; |
| |
| | • | | development of relationships with strategic business partners, including physicians who might use our future products; |
| |
| | • | | interest expense and amortization of issuance costs related to our outstanding note payables; and |
| |
| | • | | legal defense costs related to our class action and derivative action matters and any threatened actions. |
If our product candidates fail in clinical trials or do not gain regulatory approval, if our product candidates do not achieve market acceptance, or if we do not succeed in effectively and efficiently implementing manufacturing process and technology improvements to make our product commercially viable, we will not be profitable. If we fail to become and remain profitable, or if we are unable to fund our continuing losses, our business may fail.
We will continue to experience operating losses and significant negative cash flow until we begin to generate significant revenue from (a) the sale of our product candidates, which is dependent on the receipt of FDA approval for our product candidates and is dependent on our ability to successfully market and sell such product candidates, and (b) our Agera product line, which is dependent on achieving significant market penetration in its approved markets.
We may be unable to successfully commercialize any of our product candidates currently under development.
Before we can commercialize any of our product candidates in the United States, we will need to:
| | • | | conduct substantial additional research and development; |
| |
| | • | | successfully complete lengthy and expensive pre-clinical and clinical testing, including the Phase III clinical trial for our lead facial aesthetic product candidate; |
| |
| | • | | successfully improve our manufacturing process; and |
| |
| | • | | obtain FDA approvals. |
Even if our product development efforts are successful, we cannot assure you that we will be able to commercialize any of our product candidates currently under development. In that event, we will be unable to generate significant revenue, and our business will fail.
We have not generated significant revenue from commercial sales of our products to date, and we do not know whether we will ever generate significant revenue.
We are focused on product development and have not generated significant revenue from commercial sales of our products to date. Prior to the fourth quarter of 2006 we offered the Isolagen Therapy for sale in the United Kingdom. Our United Kingdom operation had been operating on a negative gross margin as we investigated means to improve manufacturing technologies for the Isolagen Process. During the fourth quarter of 2006 we determined to cease offering our Isolagen Therapy in the United Kingdom, as part of our continuing efforts to evaluate the best uses of our resources. Our revenue for the years ended December 31, 2007, 2006 and 2005 was $1.4 million and $0.4 million and $0, respectively.
17
We do not currently offer any products for sale that are based upon our Isolagen Therapy, and we cannot guarantee that we will ever market any such products. We must demonstrate that our product candidates satisfy rigorous standards of safety and efficacy before the FDA and other regulatory authorities in the United States and abroad will approve the product candidates for commercial marketing. We will need to conduct significant additional research, pre-clinical testing and clinical testing before we can file applications with the FDA for approval of our product candidates. We must also develop, validate and obtain FDA approval of any improved manufacturing process. In addition, to compete effectively our future products must be easy to use, cost-effective and economical to manufacture on a commercial scale. We may not achieve any of these objectives, and we may never generate revenue from our product candidates.
Our ability to effectively commercialize our product candidates depends on our ability to improve our manufacturing process and validate such future improvements.
As part of the approval process, we must pass a pre-approval inspection of our manufacturing facility before we can obtain marketing approval for our product candidates. We have never gone through a FDA pre-approval regulatory inspection of our manufacturing facility and we cannot guarantee that we will satisfy the requirements for approval. All of our manufacturing methods, equipment and processes must comply with the FDA’s current Good Manufacturing Practices, or cGMP, requirements. We will also need to perform extensive audits of our suppliers, vendors and contract laboratories. The cGMP requirements govern all areas of recordkeeping, production processes and controls, personnel and quality control. To ensure that we meet these requirements, we will expend significant time, money and effort. Due to the unique nature of our Isolagen Therapy, we cannot predict the likelihood that the FDA will approve our facility as compliant with cGMP requirements even if we believe that we have taken the steps necessary to achieve compliance.
The FDA, in its regulatory discretion, may require us to undergo additional clinical trials with respect to any new or improved manufacturing process we develop or utilize, in the future, if any. This could include a requirement to change the materials used in our manufacturing process. These improvements or modifications could delay or prevent approval of our product candidates. If we fail to comply with cGMP requirements, pass an FDA pre-approval inspection or obtain FDA approval of our manufacturing process, we would not receive FDA approval and would be subject to possible regulatory action. The failure to successfully implement our manufacturing process may delay or prevent our future profitability.
Even if we obtain FDA approval in the future and satisfy the FDA with regard to a validated manufacturing process, we still may be unable to commercially manufacture the Isolagen Therapy profitably. Our manufacturing cost has been subject to fluctuation, depending, in part, on the yields obtained from our manufacturing process. There is no guarantee that future manufacturing improvements will result in a manufacturing cost low enough to effectively compete in the market. Further, we currently manufacture the Isolagen Therapy on a limited basis (for research and development and for trial purposes only) and we have not manufactured commercial levels of the Isolagen Therapy in the United States. Such commercial manufacturing volumes, in the future, could lead to unexpected inefficiencies and result in unprofitable performance results.
We may not be successful in our efforts to develop commercial-scale manufacturing technology and methods.
In order to successfully commercialize any approved product candidates, we will be required to produce such products on a commercial scale and in a cost-effective manner. As stated in the preceding risk factor, we intend to seek FDA approval of our manufacturing process as a component of the BLA application and approval process. However, we can provide no assurance that we will be able to cost-effectively and commercially scale our operations using our current manufacturing process. If we are unable to develop suitable techniques to produce and manufacture our product candidates, our business prospects will suffer.
We depend on a third-party manufacturer for our Agera product line, the loss or unavailability of which would require us to find a substitute manufacturer, if available, resulting in delays in production and additional expenses.
Our Agera skin care product line is manufactured by a third party. We are dependent on this third party to manufacture Agera’s products, and the manufacturer is responsible for supplying the formula ingredients for the Agera product lines. If for any reason the manufacturer discontinues production of Agera’s products at a time when we have a low volume of inventory on hand or are experiencing a high demand for the products, significant delays in production of the products and interruption of product sales may result as we seek to establish a relationship and commence production with a new manufacturer, which would negatively impact our results of operation.
18
If our Isolagen Therapy is found to be unsafe or ineffective, or if our Isolagen Therapy is perceived to be unsafe or ineffective, our business would be materially harmed.
Our product candidates utilize our Isolagen Therapy. In addition, we expect to utilize our Isolagen Therapy in the development of any future product candidates. If our Isolagen Therapy is found to be, or perceived to be, unsafe or ineffective, we will not be successful in obtaining marketing approval for any product candidates then pending, and we may have to modify or cease production of any products that previously may have received regulatory approval. Negative media exposure, whether founded or unfounded, related to the safety and/or effectiveness of our Isolagen Therapy may harm our reputation and/or competitive position.
Subjects in our clinical development programs are required to sign Informed Consent Forms and amendments made to our Informed Consent Form could give rise to delays in our clinical development programs.
The subjects in our clinical trials are required to sign Informed Consent Forms. These forms are subject to amendment based on new knowledge obtained during the execution of our clinical trials or based on changes to the basic design or administration of our clinical trials. In the early stages of producing our Isolagen Therapy, we utilize certain raw materials, which include antibiotics, bovine-sourced materials and other animal-based materials. We have in the past amended our Informed Consent Form to address these items. Amendments made to our Informed Consent Form could give rise to delays in our clinical development programs.
If physicians do not follow our established protocols, the efficacy and safety of our product candidates may be adversely affected.
We are dependent on physicians to follow our established protocols both as to the administration and the handling of our product candidates in connection with our clinical trials, and we will continue to be dependent on physicians to follow such protocols if our product candidates are commercialized. The treatment protocol requires each physician to verify the patient’s name and date of birth with the patient and the patient records immediately prior to injection. In the event more than one patient’s cells are delivered to a physician or we deliver the wrong patient’s cells to the physician, which has occurred in the past, it is the physician’s obligation to follow the treatment protocol and assure that the patient is treated with the correct cells. If the physicians do not follow our protocol, the efficacy and safety of our product candidates may be adversely affected.
Our business, which depends on one facility, is vulnerable to natural disasters, telecommunication and information systems failures, terrorism and similar problems, and we are not fully insured for losses caused by all of these incidents.
We currently conduct all our research, development and manufacturing operations in one facility located in Exton, Pennsylvania. As a result, if we obtain FDA approval of any of our product candidates, all of the commercial manufacturing for the U.S. market will take place at a single U.S. facility. If regulatory, manufacturing or other problems require us to discontinue production at that facility, we will not be able to supply product, which would adversely impact our business.
Our Exton facility could be damaged by fire, floods, power loss, telecommunication and information systems failures or similar events. Our insurance policies have limited coverage levels for loss or damages in these events and may not adequately compensate us for any losses that may occur. In addition, terrorist acts or acts of war may cause harm to our employees or damage our Exton facility. The potential for future terrorist attacks, the national and international responses to terrorist attacks or perceived threats to national security, and other acts of war or hostility have created many economic and political uncertainties that could adversely affect our business and results of operations in ways that we cannot predict, and could cause our stock price to fluctuate or decline. We are uninsured for these types of losses.
19
As a result of our limited operating history, we may not be able to correctly estimate our future operating expenses, which could lead to cash shortfalls.
We have a limited operating history and our primary business activities consist of conducting clinical trials. As such, our historical financial data is of limited value in estimating future operating expenses. Our budgeted expense levels are based in part on our expectations concerning the costs of our clinical trials, which depend on the success of such trials and our ability to effectively and efficiently conduct such trials. In addition, our budgeted expense levels are based in part on our expectations of future revenue that we may receive from our Agera product line, and the size of future revenue depends on the choices and demand of individuals. Our limited operating history and clinical trial experience make these costs and revenues difficult to forecast accurately. We may be unable to adjust our operations in a timely manner to compensate for any unexpected increase in costs or shortfall in revenue. Further, our fixed manufacturing costs and business development and marketing expenses will increase significantly as we expand our operations. Accordingly, a significant increase in costs or shortfall in revenue could have an immediate and material adverse effect on our business, results of operations and financial condition.
Our operating results may fluctuate significantly in the future, which may cause our results to fall below the expectations of securities analysts, stockholders and investors.
Our operating results may fluctuate significantly in the future as a result of a variety of factors, many of which are outside of our control. These factors include, but are not limited to:
| | • | | the level of demand for the products that we may develop; |
| |
| | • | | the timely and successful implementation of improved manufacturing processes; |
| |
| | • | | our ability to attract and retain personnel with the necessary strategic, technical and creative skills required for effective operations; |
| |
| | • | | the amount and timing of expenditures by practitioners and their patients; |
| |
| | • | | introduction of new technologies; |
| |
| | • | | product liability litigation, class action and derivative action litigation, or other litigation; |
| |
| | • | | the amount and timing of capital expenditures and other costs relating to the expansion of our operations; |
| |
| | • | | the state of the debt and/or equity markets at the time of any proposed offering we choose to initiate; |
| |
| | • | | our ability to successfully integrate new acquisitions into our operations; |
| |
| | • | | government regulation and legal developments regarding our Isolagen Therapy in the United States and in the foreign countries in which we may operate in the future; and |
| |
| | • | | general economic conditions. |
As a strategic response to changes in the competitive environment, we may from time to time make pricing, service, technology or marketing decisions or business or technology acquisitions that could have a material adverse effect on our operating results. Due to any of these factors, our operating results may fall below the expectations of securities analysts, stockholders and investors in any future period, which may cause our stock price to decline.
20
We are party to securities and derivative litigation that distracts our management, is expensive to conduct and seeks a damage award against us.
We and certain of our current and former officers have been named as defendants in a consolidated putative shareholder class action lawsuit in the United States District Court for the Eastern District of Pennsylvania. The complaint purports to seek unspecified damages on behalf of an alleged class of persons who purchased our publicly traded securities between March 3, 2004 and August 9, 2005. The complaints allege that we and our officers violated Section 10(b) and Rule 10b-5 of the Exchange Act and Sections 11 and 12(a)(2) of the Securities Act by making certain false statements and omissions to the investing public regarding our business operations, management, and intrinsic value of our publicly traded securities. The complaints also allege liability against the individual defendants under Sections 20(a) and 20A of the Exchange Act and Section 15 of the Securities Act. In addition, stockholders have filed derivative actions seeking recovery on behalf of Isolagen against certain of our current and former officers and directors, alleging, among other things, breach of fiduciary duties and other wrongful conduct by those individual defendants. While we have directors and officers liability insurance, it is uncertain whether the insurance will be sufficient to cover all damages, if any, that we may be required to pay. In addition, the securities and derivative lawsuits may distract the attention of our management, and are expensive to conduct. We have and may continue to incur substantial legal and other professional service costs in connection with the stockholder lawsuits. The amount of any future costs in this respect cannot be determined at this time, and could have a material adverse effect on our business results.
We may be liable for product liability claims not covered by insurance, and we have been publicly threatened with claims related to our product in the United Kingdom.
Physicians who used our facial aesthetic product in the past, or who may use any of our future products, and patients who have been treated by our facial aesthetic product in the past, or who may use any of our future products, may bring product liability claims against us. In particular, we have received negative publicity and negative correspondence from patients in the United Kingdom that had previously received our treatment. More recently, we received a written demand by an attorney representing approximately 82 former patients each claiming, on average, £3,500 (or approximately $7,000), plus unquantified interest and incidental expenses. To date, no formal legal action has been brought by the attorney against us. While we have taken, and continue to take, what we believe are appropriate precautions, we may be unable to avoid significant liability exposure. We currently keep in force product liability insurance, although such insurance may not be adequate to fully cover any potential claims or may lapse in accordance with its terms prior to the assertion of claims. We may be unable to obtain product liability insurance in the future, or we may be unable to do so on acceptable terms. Any insurance we obtain or have obtained in the past may not provide adequate coverage against any asserted claims. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| | • | | diversion of management’s time and attention; |
| |
| | • | | expenditure of large amounts of cash on legal fees, expenses and payment of damages; |
| |
| | • | | decreased demand for our products or any of our future products and services; or |
| |
| | • | | injury to our reputation. |
If we are the subject of product liability claims our business could be adversely affected, and if these claims are in excess of insurance coverage, if any, that we may possess, our financial position will suffer.
21
Our failure to comply with extensive governmental regulation may significantly affect our operating results.
Even if we obtain regulatory approval for some or all our product candidates, we will continue to be subject to extensive requirements by a number of foreign, national, state and local agencies. These regulations will impact many aspects of our operations, including testing, research and development, manufacturing, safety, efficacy, labeling, storage, quality control, adverse event reporting, record keeping, approval, advertising and promotion of our future products. We must also submit new or supplemental applications and obtain FDA approval for certain changes to an approved product, product labeling or manufacturing process. Application holders must also submit advertising and other promotional material to the FDA and report on ongoing clinical trials. The FDA enforces post-marketing regulatory requirements, including the cGMP requirements, through periodic unannounced inspections. We do not know whether we will pass any future FDA inspections. Failure to pass an inspection could disrupt, delay or shut down our manufacturing operations. Failure to comply with applicable regulatory requirements could, among other things, result in:
| | • | | fines; |
| |
| | • | | changes to advertising; |
| |
| | • | | failure to obtain marketing approvals for our product candidates; |
| |
| | • | | revocation or suspension of regulatory approvals of products; |
| |
| | • | | product seizures or recalls; |
| |
| | • | | court-ordered injunctions; |
| |
| | • | | import detentions; |
| |
| | • | | delay, interruption or suspension of product manufacturing, distribution, marketing and sales; or |
| |
| | • | | civil or criminal sanctions. |
The discovery of previously unknown problems with our future products may result in restrictions of the products, including withdrawal from the market. In addition, the FDA may revisit and change its prior determinations with regard to the safety or efficacy of our future products. If the FDA’s position changes, we may be required to change our labeling or cease to manufacture and market our future products. Even prior to any formal regulatory action, we could voluntarily decide to cease the distribution and sale or recall any of our future products if concerns about their safety or efficacy develop.
In their regulation of advertising and other promotion, the FDA and the FTC may issue correspondence alleging that some advertising or promotional practices are false, misleading or deceptive. The FDA and FTC are authorized to impose a wide array of sanctions on companies for such advertising and promotion practices, which could result in any of the following:
| | • | | incurring substantial expenses, including fines, penalties, legal fees and costs to comply with the FDA’s requirements; |
| |
| | • | | changes in the methods of marketing and selling products; |
| |
| | • | | taking FDA mandated corrective action, which may include placing advertisements or sending letters to physicians rescinding previous advertisements or promotions; or |
| |
| | • | | disruption in the distribution of products and loss of sales until compliance with the FDA’s position is obtained. |
Improper promotional activities may also lead to investigations by federal or state prosecutors, and result in criminal and civil penalties. If we become subject to any of the above requirements, it could be damaging to our reputation and restrict our ability to sell or market our future products, and our business condition could be adversely affected. We may also incur significant expenses in defending ourselves.
22
Physicians may prescribe pharmaceutical or biologic products for uses that are not described in a product’s labeling or differ from those tested by us and approved by the FDA. While such “off-label” uses are common and the FDA does not regulate physicians’ choice of treatments, the FDA does restrict a manufacturer’s communications on the subject of off-label use. Companies cannot promote FDA-approved pharmaceutical or biologic products for off-label uses, but under certain limited circumstances they may disseminate to practitioners articles published in peer-reviewed journals. To the extent allowed by law, we intend to disseminate peer-reviewed articles on our future products to practitioners. If, however, our activities fail to comply with the FDA’s regulations or guidelines, we may be subject to warnings from, or enforcement action by, the FDA or other regulatory or law enforcement authorities.
Our sales, marketing, and scientific/educational grant programs, if any in the future, must also comply with applicable requirements of the anti-fraud and abuse provisions of the Social Security Act, the False Claims Act and similar state laws, each as amended. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990 and the Veteran’s Health Care Act of 1992, each as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws. The distribution of product samples to physicians must comply with the requirements of the Prescription Drug Marketing Act.
Depending on the circumstances, failure to meet post-approval requirements can result in criminal prosecution, fines or other penalties, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre-marketing product approvals, or refusal to allow us to enter into supply contracts, including government contracts. Any government investigation of alleged violations of law could require us to expend significant time and resources in response, and could generate negative publicity.
Legislative or regulatory reform of the healthcare system may affect our ability to sell our future products profitably.
In the United States and a number of foreign jurisdictions, there have been legislative and regulatory proposals to change the healthcare system in ways that could impact our ability to sell our future products profitably. The FDA’s policies may change and additional government regulations may be enacted, which could prevent or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are not able to maintain regulatory compliance, we might not be permitted to market our future products and our business could suffer.
We currently conduct business in foreign markets, and our business strategy involves selling our product candidates in foreign markets. These operations are and will be subject to a variety of regulations in those foreign markets that could have a material adverse effect on our business in a particular market or in general.
Our Agera product line is currently sold in the United Kingdom. In addition, our business strategy includes the sale of our product candidates in foreign markets. With respect to our product candidates, we will be required to comply with local laws regulating and approving the sale of biologics in each foreign market that we attempt to operate in. As such, we may become subject to a variety of foreign regulations. In addition, to the extent that our currently available product lines are regulated in any foreign markets, we will be required to comply with such regulations. Our failure to comply, or assertions that we fail to comply, with any foreign regulations could have a material adverse effect on our business in a particular market or in general. Government regulations in international markets could delay or prevent the introduction, or require the reformulation or withdrawal, of some of our future products.
23
Our foreign operations and any foreign operations we may commence in the future are exposed to risks associated with exchange rate fluctuations, trade restrictions and political, economic and social instability.
Our foreign operations and any foreign operations we commence in the future will subject us to the risks of doing business abroad, including:
| | • | | unexpected changes in regulatory requirements; |
| |
| | • | | export and import restrictions, tariffs and other trade barriers; |
| |
| | • | | difficulties in staffing and managing foreign operations; |
| |
| | • | | longer payment cycles and problems in collecting accounts receivable; |
| |
| | • | | potential adverse tax consequences; |
| |
| | • | | exchange rate fluctuations; |
| |
| | • | | increased risks of piracy and limits on our ability to enforce our intellectual property rights; |
| |
| | • | | limits on repatriation of funds; and |
| |
| | • | | political risks that may limit or disrupt international sales. |
A foreign government may impose trade or foreign exchange restrictions or increased tariffs, which could adversely affect our operations. Our operations in some markets also may be adversely affected by political, economic and social instability in foreign countries, including terrorism. Any limitations or interruptions in our foreign operations could have a material adverse effect on our business.
Any future products that we develop may not be commercially successful.
Even if we obtain regulatory approval for our product candidates in the United States and other countries, those products may not be accepted by the market. A number of factors may affect the rate and level of market acceptance of our products, including:
| | • | | labeling requirements or limitations; |
| |
| | • | | market acceptance by practitioners and their patients; |
| |
| | • | | our ability to successfully improve our manufacturing process; |
| |
| | • | | the effectiveness of our sales efforts and marketing activities; and |
| |
| | • | | the success of competitive products. |
If our current or future product candidates fail to achieve market acceptance, our profitability and financial condition will suffer.
Our competitors in the pharmaceutical, medical device and biotechnology industries may have superior products, manufacturing capabilities, financial resources or marketing position.
The human healthcare products and services industry is extremely competitive. Our competitors include major pharmaceutical, medical device and biotechnology companies. Most of these competitors have more extensive research and development, marketing and production capabilities and greater financial resources than we do. Our future success will depend on our ability to develop and market effectively our future products against those of our competitors. If our future products receive marketing approval but cannot compete effectively in the marketplace, our results of operations and financial position will suffer.
24
Difficulties managing growth could adversely affect our business, operating results and financial condition.
If we achieve growth in our operations in the next few years, which we must do to succeed, such growth could place a strain on our management, and our administrative, operational and financial infrastructure. We would need to hire additional management, financial, sales and marketing personnel to manage our operations. In addition, our ability to manage our future operations and growth would require the continued improvement of operational, financial and management controls, reporting systems and procedures. If we are unable to manage our growth effectively or if we are unable to attract additional highly qualified personnel, our business, operating results and financial condition may be materially adversely affected.
We are dependent on our key scientific and other management personnel, and the loss of any of these individuals could harm our business.
We are dependent on the efforts of our key management and scientific staff. The loss of any of these individuals, or our inability to recruit and train additional key personnel in a timely manner, could materially and adversely affect our business and our future prospects. A loss of one or more of our current officers or key personnel could severely and negatively impact our operations. We have employment agreements with most of our key management personnel, but some of these people are employed “at-will,” and any of them may elect to pursue other opportunities at any time. We have no present intention of obtaining key man life insurance on any of our executive officers or key management personnel.
We will need to attract, train and retain additional highly qualified senior executives and technical and managerial personnel in the future.
In the future, we may need to seek additional senior executives, as well as technical and managerial staff members. There is a high demand for highly trained executive, technical and managerial personnel in our industry. We do not know whether we will be able to attract, train and retain highly qualified technical and managerial personnel in the future, which could have a material adverse effect on our business, financial condition and results of operations.
If we are unable to effectively promote our brands and establish a competitive position in the marketplace, our business may fail.
Our Isolagen Therapy brand names are new and unproven. We believe that the importance of brand recognition will increase over time. In order to gain brand recognition, we may increase our marketing and advertising budgets to create and maintain brand loyalty. We do not know whether these efforts will lead to greater brand recognition. If we are unable effectively to promote our brands, including our Agera product line, and establish competitive positions in the marketplace, our business results will be materially adversely affected.
If we are unable to adequately protect our intellectual property and proprietary technology, the value of our technology and future products will be adversely affected, and if we are unable to enforce our intellectual property against unauthorized use by third parties our business may be materially harmed.
Our long-term success largely depends on our future ability to market technologically competitive products. Our ability to achieve commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection of our technology and future products, as well as successfully defending these patents against third party challenges. In order to do so we must:
| | • | | obtain and protect commercially valuable patents or the rights to patents both domestically and abroad; |
| |
| | • | | operate without infringing upon the proprietary rights of others; and |
| |
| | • | | prevent others from successfully challenging or infringing our proprietary rights. |
25
As of December 31, 2007, we had 8 issued U.S. patents, 6 pending U.S. patent applications, 32 granted foreign patents, and 19 pending foreign applications. However, we may not be able to obtain additional patents relating to our technology or otherwise protect our proprietary rights. If we fail to obtain or maintain patents from our pending and future applications, we may not be able to prevent third parties from using our proprietary technology. We will be able to protect our proprietary rights from unauthorized use only to the extent that these rights are covered by valid and enforceable patents that we control or are effectively maintained by us as trade secrets. Furthermore, the degree of future protection of our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep a competitive advantage.
The patent situation of companies in the markets in which we compete is highly uncertain and involves complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in the United States. The laws of other countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology and/or pharmaceuticals, which could make it difficult for us to stop the infringement of our patents in foreign countries in which we hold patents. Proceedings to enforce our patent rights in the United States or in foreign jurisdictions would likely result in substantial cost and divert our efforts and attention from other aspects of our business. Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents.
Other risks and uncertainties that we face with respect to our patents and other proprietary rights include the following:
| | • | | the inventors of the inventions covered by each of our pending patent applications might not have been the first to make such inventions; |
| |
| | • | | we might not have been the first to file patent applications for these inventions or similar technology; |
| |
| | • | | the future and pending applications we will file or have filed, or to which we will or do have exclusive rights, may not result in issued patents or may take longer than we expect to result in issued patents; |
| |
| | • | | the claims of any patents that are issued may not provide meaningful protection; |
| |
| | • | | our issued patents may not provide a basis for commercially viable products or may not be valid or enforceable; |
| |
| | • | | we might not be able to develop additional proprietary technologies that are patentable; |
| |
| | • | | the patents licensed or issued to us may not provide a competitive advantage; |
| |
| | • | | patents issued to other companies, universities or research institutions may harm our ability to do business; |
26
| | • | | other individual companies, universities or research institutions may independently develop or have developed similar or alternative technologies or duplicate our technologies and commercialize discoveries that we attempt to patent; |
| |
| | • | | other companies, universities or research institutions may design around technologies we have licensed, patented or developed; and |
| |
| | • | | many of our patent claims are method, rather than composition of matter, claims; generally composition of matter claims are easier to enforce and are more difficult to circumvent. |
Our business may be harmed and we may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights.
A third party may assert that we, one of our subsidiaries or one of our strategic collaborators has infringed his, her or its patents and proprietary rights or challenge the validity or enforceability of our patents and proprietary rights. Likewise, we may need to resort to litigation to enforce our patent rights or to determine the scope and validity of a third party’s proprietary rights.
We cannot be sure that other parties have not filed for or obtained relevant patents that could affect our ability to obtain patents or operate our business. Even if we have previously filed patent applications or obtain issued patents, others may file their own patent applications for our inventions and technology, or improvements to our inventions and technology. We have become aware of published patent applications filed after the issuance of our patents that, should the owners pursue and obtain patent claims to our inventions and technology, could require us to challenge such patent claims. Others may challenge our patent or other intellectual property rights or sue us for infringement. In all such cases, we may commence legal proceedings to resolve our patent or other intellectual property disputes or defend against charges of infringement or misappropriation. An adverse determination in any litigation or administrative proceeding to which we may become a party could subject us to significant liabilities, result in our patents being deemed invalid, unenforceable or revoked, or drawn into an interference, require us to license disputed rights from others, if available, or to cease using the disputed technology. In addition, our involvement in any of these proceedings may cause us to incur substantial costs and result in diversion of management and technical personnel. Furthermore, parties making claims against us may be able to obtain injunctive or other equitable relief that could effectively block our ability to develop, commercialize and sell products, and could result in the award of substantial damages against us.
The outcome of these proceedings is uncertain and could significantly harm our business. If we do not prevail in this type of litigation, we or our strategic collaborators may be required to:
| | • | | pay monetary damages; |
| |
| | • | | expend time and funding to redesign our Isolagen Therapy so that it does not infringe others’ patents while still allowing us to compete in the market with a substantially similar product; |
| |
| | • | | obtain a license, if possible, in order to continue manufacturing or marketing the affected products or services, and pay license fees and royalties, which may be non-exclusive. This license may be non-exclusive, giving our competitors access to the same intellectual property, or the patent owner may require that we grant a cross-license to our patented technology; or |
| |
| | • | | stop research and commercial activities relating to the affected products or services if a license is not available on acceptable terms, if at all. |
Any of these events could materially adversely affect our business strategy and the value of our business.
In addition, the defense and prosecution of intellectual property suits, interferences, oppositions and related legal and administrative proceedings in the United States and elsewhere, even if resolved in our favor, could be expensive and time consuming and could divert financial and managerial resources. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater financial resources.
27
If we are unable to keep up with rapid technological changes, our future products may become obsolete or unmarketable.
Our industry is characterized by significant and rapid technological change. Although we attempt to expand our technological capabilities in order to remain competitive, research and discoveries by others may make our future products obsolete. If we cannot compete effectively in the marketplace, our potential for profitability and financial position will suffer.
Our acquisitions of companies or technologies may result in disruptions in business and diversion of management attention.
We have made and may in the future make acquisitions of complementary companies, products or technologies. Any acquisitions will require the assimilation of the operations, products and personnel of the acquired businesses and the training and motivation of these individuals. Acquisitions may disrupt our operations and divert management’s attention from day-to-day operations, which could impair our relationships with current employees, customers and strategic partners. We may also have to, or we may choose to, incur debt or issue equity securities to pay for any future acquisitions. The issuance of equity securities for an acquisition could be substantially dilutive to our security holders. In addition, our results of operations may suffer because of acquisition-related costs or amortization or impairment costs for acquired goodwill and other intangible assets. If management is unable to fully integrate acquired businesses, products, technologies or personnel with existing operations, we may not receive the intended benefits of the acquisitions.
We have not declared any dividends on our common stock to date, and we have no intention of declaring dividends in the foreseeable future.
The decision to pay cash dividends on our common stock rests with our Board of Directors and will depend on our earnings, unencumbered cash, capital requirements and financial condition. We do not anticipate declaring any dividends in the foreseeable future, as we intend to use any excess cash to fund our operations. Investors in our common stock should not expect to receive dividend income on their investment, and investors will be dependent on the appreciation of our common stock to earn a return on their investment.
Provisions in our charter documents could prevent or delay stockholders’ attempts to replace or remove current management.
Our charter documents provide for staggered terms for the members of our Board of Directors. Our Board of Directors is divided into three staggered classes, and each director serves a term of three years. At stockholders’ meetings, only those directors comprising one of the three classes will have completed their term and be subject to re-election or replacement.
In addition, our Board of Directors is authorized to issue “blank check” preferred stock, with designations, rights and preferences as they may determine. Accordingly, our Board of Directors may, without stockholder approval, issue shares of preferred stock with dividend, liquidation, conversion, voting or other rights that could adversely affect the voting power or other rights of the holders of our common stock. This type of preferred stock could also be issued to discourage, delay or prevent a change in our control.
In May 2006, our Board of Directors declared a dividend of one right for each share of our common stock to purchase our newly created Series C participating preferred stock in connection with the adoption of a stockholder rights plan. These rights may have certain anti-takeover effects. For example, the rights may cause substantial dilution to a person or group that attempts to acquire us in a manner which causes the rights to become exercisable. As such, the rights may have the effect of rendering more difficult or discouraging an acquisition of our company which is deemed undesirable by our board of directors.
28
The use of a staggered Board of Directors, the ability to issue “blank check” preferred stock, and the adoption of stockholder rights plans are traditional anti-takeover measures. These provisions in our charter documents make it difficult for a majority stockholder to gain control of the Board of Directors and of our company. These provisions may be beneficial to our management and our Board of Directors in a hostile tender offer and may have an adverse impact on stockholders who may want to participate in such a tender offer, or who may want to replace some or all of the members of our Board of Directors.
Provisions in our bylaws provide for indemnification of officers and directors, which could require us to direct funds away from our business and future products.
Our bylaws provide for the indemnification of our officers and directors. We have in the past and may in the future be required to advance costs incurred by an officer or director and to pay judgments, fines and expenses incurred by an officer or director, including reasonable attorneys’ fees, as a result of actions or proceedings in which our officers and directors are involved by reason of being or having been an officer or director of our company. Funds paid in satisfaction of judgments, fines and expenses may be funds we need for the operation of our business and the development of our product candidates, thereby affecting our ability to attain profitability.
Future sales of our common stock may depress our stock price.
The market price of our common stock could decline as a result of sales of substantial amounts of our common stock in the public market, or as a result of the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of common stock. As of March 1, 2008, there were 41,639,408] shares of common stock issued and 37,639,408 outstanding. All of our outstanding shares are freely transferable without restriction or further registration under the Securities Act.
There is a limited public trading market for our common stock.
There is a limited public trading market for our common stock. Without an active trading market, there can be no assurance of any liquidity or resale value of our common stock, and stockholders may be required to hold shares of our common stock for an indefinite period of time.
Lack of effectiveness of internal controls over financial reporting could adversely affect the value of our securities.
As directed by Section 404 of the Sarbanes-Oxley Act, the SEC adopted rules requiring public companies to include a report of management on the company’s internal control over financial reporting in their annual reports on Form 10-K that contains an assessment by management of the effectiveness of the company’s internal control over financial reporting. In addition, the public accounting firm auditing the company’s financial statements must attest to and report on the company’s internal control over financial reporting. Ineffective internal controls over our financial reporting have occurred in the past and may arise in the future. As a consequence, our investors could lose confidence in the reliability of our financial statements, which could result in a decrease in the value of our securities.
Our debt obligations expose us to risks that could adversely affect our business, operating results and financial condition, and prevent us from fulfilling our obligations under the notes.
We have a substantial level of debt. As of December 31, 2007, we had approximately $90.5 million of indebtedness outstanding (including related accrued interest of $0.5 million). The level and nature of our indebtedness, among other things, could:
| | • | | make it difficult for us to make payments on our debt outstanding from time to time or to refinance it; |
| |
| | • | | make it difficult for us to obtain any necessary financing in the future for working capital, capital expenditures, debt service, acquisitions or general corporate purposes; |
29
| | • | | limit our flexibility in planning for or reacting to changes in our business; |
| |
| | • | | reduce funds available for use in our operations, as we will be required to use a portion of our cash for the payment of any principal or interest due on our outstanding indebtedness; |
| |
| | • | | make us more vulnerable in the event of a downturn in our business; |
| |
| | • | | place us at a possible competitive disadvantage relative to less leveraged competitors and competitors that have better access to capital resources; |
| |
| | • | | increase the impact to us of negative changes in general economic and industry conditions, as compared to less leveraged competitors; or |
| |
| | • | | impair our ability to merge or otherwise affect the sale of the company due to the right of the holders of certain of our indebtedness to accelerate the maturity date of the indebtedness in the event of a change of control of the company. |
If we do not grow our revenues, we could have difficulty making required payments on our indebtedness. If we are unable to generate sufficient cash flow or otherwise obtain funds necessary to make required payments, or if we fail to comply with the various requirements of our indebtedness, we would be in default, which would permit the holders of our indebtedness to accelerate the maturity of the indebtedness and could cause defaults under any indebtedness we may incur in the future. Any default under our indebtedness would have a material adverse effect on our business, operating results and financial condition.
General economic conditions, industry cycles and financial, business and other factors affecting our operations, many of which are beyond our control, may affect our future performance, which may affect our ability to make principal and interest payments on our indebtedness. If we cannot generate sufficient cash flow from operations in the future to service our debt, we may, among other things:
| | • | | seek additional financing in the debt or equity markets, and the documentation governing any future financing may contain covenants that limit or restrict our strategic, operating or financing activities; |
| |
| | • | | refinance or restructure all or a portion of our indebtedness; |
| |
| | • | | sell selected assets; |
| |
| | • | | reduce or delay planned capital expenditures; |
| |
| | • | | reduce or delay planned research and development expenditures; and/or |
| |
| | • | | enter into bankruptcy protection. |
These measures might not be sufficient to enable us to service our indebtedness. In addition, any financing, refinancing or sale of assets might not be available, or available on economically favorable terms.
30
The price of our common stock has in the past and may in the future fluctuate significantly, which may make it difficult for holders of our common stock to sell our common stock when desired or at attractive prices.
On March 3, 2008, the per share closing price of our common stock was $0.61. From January 1, 2006 until December 31, 2007, the per share closing price of our common stock ranged from $1.76 to $5.00 per share. The value of our common stock may decline regardless of our operating performance or prospects. The market price of our common stock is subject to significant fluctuations in response to the factors in this section and other factors, including:
| | • | | market reaction to our capitalization, cash reserves and utilization of cash; |
| |
| | • | | market reaction to announcements regarding our management; |
| |
| | • | | the success or failure of our product development efforts, especially those related to obtaining regulatory approvals domestically and internationally; |
| |
| | • | | the implementation of improved manufacturing processes; |
| |
| | • | | technological innovations developed by us or our competitors; |
| |
| | • | | variations in our operating results and the extent to which we achieve our key business targets; |
| |
| | • | | differences between our reported results and those expected by investors and securities analysts; |
| |
| | • | | market reaction to any acquisitions or joint ventures announced by us or our competitors; and |
| |
| | • | | developments with respect to the class and derivative action litigation of which we are currently defendants, or development with respect to threatened litigation. |
In addition, in recent years, the stock market in general, and the market for life sciences companies in particular, have experienced significant price and volume fluctuations. This volatility has affected the market prices of securities issued by many companies, often for reasons unrelated to their operating performance, and it may adversely affect the price of our common stock. In the past, securities class action litigation has often been instituted following periods of volatility in the market price of a company’s securities. The current class and derivative action suits or a future securities class action suit against us could result in potential liabilities, substantial costs and the diversion of management’s attention and resources, regardless of whether we win or lose. These broad market fluctuations may adversely affect the price of our securities, regardless of our operating performance.
| | |
| Item 1B. | | Unresolved Staff Comments |
None.
Our corporate headquarters and manufacturing operations are located in Exton, Pennsylvania. We currently lease approximately 86,500 square feet. This lease is noncancelable through March 31, 2013.
During 2006, we entered into a lease for approximately 2,200 square feet of office space in Santa Barbara, California to support the Office of the Chief Executive and his staff, Investor Relations and our Business Development management team. This lease is noncancelable through August 31, 2008. We will not renew this lease as we are in the process of closing this Santa Barbara, California office.
We currently lease approximately 14,800 square feet of office and laboratory space in Houston, Texas. Our lease expires April 30, 2008 and we do not expect to renew this lease. During 2006, we relocated our research and development activities to our Exton, Pennsylvania facility and we also subleased approximately 50% of the Houston, Texas facility to a third-party tenant.
31
During 2006, the Board of Directors approved the proposed closing of our UK operation, including our commercial manufacturing facility and cellular laboratory located in London, England. This London, England facility consists of approximately 11,800 square feet under a lease that expires in March 2010. The facility was closed during the first half of 2007. We are attempting to sublease the facility to a third-party.
In April 2005, we acquired a two-building, 100,000 square foot corporate campus in Bevaix, Canton of Neuchâtel, Switzerland. During 2006, management placed the corporate campus on the real estate market for sale. See “Assets Held for Sale” in Note 3 of Notes to Consolidated Financial Statements.
| | |
| Item 3. | | Legal Proceedings |
Federal Securities Litigation
The Company and certain of its current and former officers and directors are defendants in class action cases pending in the United States District Court for the Eastern District of Pennsylvania.
In August 2005 and September 2005, various lawsuits were filed alleging securities fraud and asserting claims on behalf of a putative class of purchasers of publicly traded Isolagen securities between March 3, 2004 and August 1, 2005. These lawsuits wereElliot Liff v. Isolagen, Inc. et al., C.A. No. H-05-2887, filed in the United States District Court for the Southern District of Texas;Michael Cummiskey v. Isolagen, Inc. et al., C.A. No. 05-cv-03105, filed in the United States District Court for the Southern District of Texas;Ronald A. Gargiulo v. Isolagen, Inc. et al., C.A. No. 05-cv-4983, filed in the United States District Court for the Eastern District of Pennsylvania, andGregory J. Newman v. Frank M. DeLape, et al., C.A. No. 05-cv-5090, filed in the United States District Court for the Eastern District of Pennsylvania.
TheLiffandCummiskeyactions were consolidated on October 7, 2005. TheGargioloandNewmanactions were consolidated on November 29, 2005. On November 18, 2005, the Company filed a motion with the Judicial Panel on Multidistrict Litigation (the “MDL Motion”) to transfer the Federal Securities Actions and theKeenederivative case (described below) to the United States District Court for the Eastern District of Pennsylvania. TheLiffandCummiskey actions were stayed on November 23, 2005 pending resolution of the MDL Motion. TheGargiuloandNewmanactions were stayed on December 7, 2005 pending resolution of the MDL Motion. On February 23, 2006, the MDL Motion was granted and the actions pending in the Southern District of Texas were transferred to the Eastern District of Pennsylvania, where they have been captionedIn re Isolagen, Inc. Securities & Derivative Litigation, MDL No. 1741 (the “Federal Securities Litigation”).
On April 4, 2006, the United States District Court for the Eastern District of Pennsylvania appointed Silverback Asset Management, LLC, Silverback Master, Ltd., Silverback Life Sciences Master Fund, Ltd., Context Capital Management, LLC and Michael F. McNulty as Lead Plaintiffs, and the law firms of Bernstein Litowitz Berger & Grossman LLP and Kirby McInerney & Squire LLP as Lead Counsel in the Federal Securities Litigation.
On July 14, 2006, Lead Plaintiffs filed a Consolidated Class Action Complaint in the Federal Securities Litigation on behalf of a putative class of persons or entities who purchased or otherwise acquired Isolagen common stock or convertible debt securities between March 3, 2004 and August 9, 2005. The complaint purports to assert claims for securities fraud in violation of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 against Isolagen and certain of its former officers and directors. The complaint also purports to assert claims for violations of Section 11 and 12 of the Securities Act of 1933 against the Company and certain of its current and former directors and officers in connection with the registration and sale of certain shares of Isolagen common stock and certain convertible debt securities. The complaint also purports to assert claims against CIBC World Markets Corp., Legg Mason Wood Walker, Inc., Canaccord Adams, Inc. and UBS Securities LLC as underwriters in connection with an April 2004 public offering of Isolagen common stock and a 2005 sale of convertible notes. On November 1, 2006, the defendants moved to dismiss the complaint. On September 26, 2007, the court denied the Company’s motions to dismiss the complaint. On November 6, 2007, the court entered a scheduling order that provides for discovery to be complete by June 8, 2009.
32
The Company intends to defend these lawsuits vigorously. However, the Company cannot currently estimate the amount of loss, if any, that may result from the resolution of these actions, and no provision has been recorded in the consolidated financial statements. The Company will expense its legal costs as they are incurred and will record any insurance recoveries on such legal costs in the period the recoveries are received.
Derivative Actions
The Company is the nominal defendant in derivative actions (the “Derivative Actions”) pending in State District Court in Harris County, Texas, the United States District Court for the Eastern District of Pennsylvania, and the Court of Common Pleas of Chester County, Pennsylvania.
On September 28, 2005, Carmine Vitale filed an action styled, Case No. 2005-61840,Carmine Vitale v. Frank DeLape, et al.in the 55th Judicial District Court of Harris County, Texas and in February 2006 Mr. Vitale filed an amended petition. In this action, the plaintiff purports to bring a shareholder derivative action on behalf of the Company against certain of the Company’s current and former officers and directors. The Plaintiff alleges that the individual defendants breached their fiduciary duties to the Company and engaged in other wrongful conduct. Jeffrey Tomz, who formerly served as Isolagen’s Chief Financial Officer, was accused of engaging in insider trading of Isolagen stock through a proxy. The plaintiff did not make a demand on the Board of Isolagen prior to bringing the action and plaintiff alleges that a demand was excused under the law as futile.
On December 2, 2005, the Company filed its answer and special exceptions pursuant to Rule 91 of the Texas Rules of Civil Procedure based on pleading defects inherent in the Vitale petition. The plaintiff filed an amended petition on February 15, 2006, to which the defendants renewed their special exceptions. On September 6, 2006, the Court granted the special exceptions and permitted the plaintiff thirty days to attempt to replead. Thereafter the plaintiff moved the Court for an order compelling discovery, which the Court denied on October 2, 2006. On October 18, 2006, the Court entered an order explaining its grounds for granting the special exceptions. On November 3, 2006, the plaintiff filed a second amended petition. On February 8, 2007, the Company filed its answer and special exceptions to the second amended petition. On August 9, 2007, the Court granted the special exceptions and dismissed the second amended petition with prejudice. On September 4, 2007, the plaintiff moved for reconsideration of the dismissal with prejudice of the second amended petition, for a new trial, and for leave to further amend the petition, and the defendants opposed that motion on September 20, 2007. On October 23, 2007, that motion was deemed denied by operation of law because the court had not acted on it by that date.
On October 8, 2005, Richard Keene filed an action styled, C.A. No. H-05-3441, Richard Keene v. Frank M. DeLape et al., in the United States District Court for the Southern District of Texas. This action makes substantially similar allegations as the original complaint in the Vitale action. The plaintiff also alleges that his failure to make a demand on the Board prior to filing the action is excused as futile.
The Company sought to transfer the Keene action to the United States District Court for the Eastern District of Pennsylvania as part of the MDL Motion. On January 21, 2006, the court stayed the Keene action pending resolution of the MDL Motion. On February 23, 2006, the Keene action was transferred with the Federal Securities Actions from the Southern District of Texas to the Eastern District of Pennsylvania. Thereafter, on May 15, 2006, the plaintiff filed an amended complaint, and on June 5, 2006, the defendants moved to dismiss the amended complaint. On August 21, 2006, the plaintiff moved for leave to file a second amended complaint, and on September 15, 2006, defendants filed an opposition to that motion. On January 24, 2007, the court denied the plaintiff’s motion to file a second amended complaint, and on April 10, 2007 the court granted the defendants’ motion to dismiss and dismissed the amended complaint without prejudice. On May 9, 2007, plaintiff filed a notice of appeal from the January 24, 2007 order denying plaintiff’s motion to file a second amended complaint, and from the April 10, 2007 order dismissing plaintiff’s amended complaint without prejudice. The appeal is fully briefed.
On October 31, 2005, William Thomas Fordyce filed an action styled, C.A. No. GD-05-08432, William Thomas Fordyce v. Frank M. DeLape, et al., in the Court of Common Pleas of Chester County, Pennsylvania. This action makes substantially similar allegations as the original complaint in the Vitale action. The plaintiff also alleges that his failure to make a demand on the Board prior to filing the action is excused as futile.
33
On January 20, 2006, the Company filed its preliminary objections to the complaint. On August 31, 2006, the Court of Common Pleas entered an opinion and order sustaining the preliminary objections and dismissing the complaint with prejudice. On September 19, 2006, Fordyce filed a motion for reconsideration, which the Court of Common Pleas denied. On September 28, 2006, Fordyce filed a notice of appeal to the Superior Court of Pennsylvania. On July 27, 2007, the Superior Court affirmed the decision of the Court of Common Pleas.
On February 14, 2008, Ronald Beattie filed an action styled C.A. No. 08-724, Ronald Beattie v. Michael Macaluso, et al., in the United States District Court for the Eastern District of Pennsylvania. This action makes substantially similar allegations as the original complaint in the Vitale action. The complaint has not yet been served on the Company.
The Derivative Actions are purportedly being prosecuted on behalf of the Company and any recovery obtained, less any attorneys’ fees awarded, will go to the Company. The Company is advancing legal expenses to certain current and former directors and officers of the Company who are named as defendants in the Derivative Actions and expects to receive reimbursement for those advances from its insurance carriers. The Company will expense its legal costs as they are incurred and will record any insurance recoveries on such legal costs in the period the recoveries are received. The Company cannot currently estimate the amount of loss, if any, that may result from the resolution of these actions, and no provision has been recorded in the consolidated financial statements.
Indemnity Demands
Mr. Jeffrey Tomz
After the above referenced litigations were commenced, Mr. Jeffrey Tomz, who formerly served as Isolagen’s Chief Financial Officer, demanded reimbursement of his costs of defense, and reimbursement for the costs of responding to a Securities and Exchange Commission investigation of his alleged insider trading in Isolagen stock. It is understood that Tomz’s defense costs to date amount to approximately $0.3 million.
As the Vitale matter has now been resolved in favor of all defendants, including Mr. Tomz, the Company is presently obligated to reimburse him for the reasonable and necessary costs of defending all claims asserted therein other than the insider trading allegations. Although decided on jurisdictional grounds, it is likely the Company is also obligated to reimburse Mr. Tomz for the reasonable and necessary costs incurred in defending the Fordyce matter given that it has also been resolved in favor of all defendants. The Company would be liable to reimburse Mr. Tomz for the reasonable and necessary costs of defense in the Keene case if it is affirmed on appeal and in the putative securities cases should he prevail in that action. The Company has refused to pay the amount of fees and expenses for which Mr. Tomz has sought reimbursement because it believes they are excessive, duplicative and have not been properly segregated between reimbursable and non-reimbursable claims. The Company has negotiated an acceptable compromise for the amounts billed by Mr. Tomz’s local Pennsylvania counsel for an amount less than $0.1 million.
Prior to the resolution of the various derivative actions, Mr. Tomz filed a demand for arbitration seeking advancement of his defense costs. He subsequently agreed to stay those proceedings. At present, Mr. Tomz has not sought to lift this stay and it is uncertain whether he will attempt to do so in the future.
The Company has accrued less than $0.1 million in the accompanying Consolidated Financial Statements as of December 31, 2007 with respect to Mr. Tomz’s existing defense costs in dispute. The Company intends to seek reimbursement under its directors and officers liability policy for any amounts paid to reimburse Mr. Tomz for his defense costs, which includes less than $0.1 million paid to date.
Underwriters
The Underwriters have each demanded that the Company indemnify, hold harmless and defend them with respect to the claims asserted in the putative securities actions. The total amount demanded to date is less than $0.3 million, however, we believe that cumulative future amounts of these defense costs are likely to be significant, although these cumulative future amounts cannot be estimated at this time.
34
Isolagen has denied this demand on numerous grounds, including that: (i) as to the November 2004 convertible notes offering, it only agreed to indemnify the Underwriters for any losses resulting from any untrue statement, or alleged untrue statement, of material fact in the offering documents provided by the Company to a holder or prospective purchaser of securities, and plaintiffs’ claims in the securities action are not based upon alleged untrue statements in any such documents; (ii) as to the June 2004 secondary offering, it only agreed to indemnify the Underwriters for untrue statements, or alleged untrue statements, of material fact, in the preliminary prospectus, registration statement, prospectus or any amendment thereof, and there were no such untrue statements in any such document; (iii) Isolagen assumed no duty to defend or advance defense costs; and (iv) Isolagen satisfied whatever duty to defend it may have to the Underwriters by offering to assume the Underwriters’ defense. Accordingly, we have not accrued any amounts related to the Underwriters’ defense costs in our Consolidated Financial Statements.
United Kingdom
Subsequent to the Company’s public announcement regarding the closure of the United Kingdom operation, the Company received negative publicity and negative correspondence from former patients in the United Kingdom that previously received our treatment. More recently, the Company received a written demand by an attorney representing approximately 82 former patients each claiming negligent misstatements were made and each claiming, on average, £3,500 (or approximately $7,000), plus unquantified interest and incidental expenses. The Company is in the process of evaluating the merits of the claims made in the demand. To date, no formal legal action has been brought by the attorney against the Company, and no provision has been recorded in the consolidated financial statements related to this matter.
Other
We are involved in various other legal matters that are being defended and handled in the ordinary course of business. Although it is not possible to predict the outcome of these matters, management believes that the results will not have a material impact on the Company’s financial statements.
| | |
| Item 4. | | Submission of Matters to a Vote of Security Holders |
On November 5, 2007, we held our 2007 Annual Meeting of Stockholders at our office in Exton, Pennsylvania. Dr. Kenneth A. Selzer, Mr. Steven Morrell, and Mr. Marshall G. Webb were elected as directors by our stockholders at the meeting to serve until the 2010 annual meeting of stockholders or until their respective successors have been duly elected and qualified. The Company’s stockholders ratified the appointment of BDO Seidman, LLP as the Company’s auditors for the year ending December 31, 2007.
The results of the vote were as follows:
| 1. | | To ratify the appointment of BDO Seidman, LLP as the Company’s auditors for the year ending December 31, 2007. |
| | |
| | | |
| Shares voted FOR / AGAINST / ABSTAINING: | | 33,120,713 / 136,638 / 54,576 |
| 2. | | To elect three directors to hold office until the Company’s 2010 annual meeting of stockholders, or until his successor is duly elected and qualified. |
| | |
| | | |
| Shares voted FOR / WITHHELD Mr. Steven Morrell: | | 31,935,719 / 1,376,208 |
| | | |
| Shares voted FOR / WITHHELD Mr. Marshall G. Webb: | | 32,069,017 / 1,242,910 |
| | | |
| Shares voted FOR / WITHHELD Dr. Kenneth A. Selzer: | | 33,009,911 / 302,016 |
35
Part II
| | |
| Item 5. | | Market For Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Since December 11, 2002, our common stock has been traded on the American Stock Exchange under the symbol “ILE.” Prior to December 11, 2002, our common stock was quoted on the OTC Bulletin Board under the symbol “ISLG.” The market for our common stock is limited and volatile. The following table sets forth the high and low sales prices, as applicable, for our common stock for each of the periods indicated as reported by the AMEX.
| | | | | | | | | | | | | | | | | |
| | | December 31, | | | December 31, | |
| | | 2007 | | | 2006 | |
| | | High | | | Low | | | High | | | Low | |
| First Quarter | | $ | 3.93 | | | $ | 1.98 | | | $ | 2.72 | | | $ | 1.84 | |
| Second Quarter | | | 5.00 | | | | 3.54 | | | | 4.20 | | | | 1.76 | |
| Third Quarter | | | 4.19 | | | | 2.04 | | | | 3.99 | | | | 3.20 | |
| Fourth Quarter | | | 3.40 | | | | 2.24 | | | | 3.67 | | | | 2.84 | |
The closing price of our common stock on March 3, 2008 was $0.61.
Holders
As of March 1, 2008, we had 383 stockholders of record of our common stock.
Dividends
We have never paid any cash dividends on our common stock. We anticipate that we will retain future earnings, if any, to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends in the foreseeable future.
Recent Sales of Unregistered Securities
We did not sell unregistered securities during the fourth quarter of 2007.
Purchases of Equity Securities.
We did not repurchase any of our equity securities during the fourth quarter of 2007.
36
Stock Performance Graph
The following graphs our performance in the form of cumulative total return to holders of our common stock since December 31, 2002 in comparison to the AMEX Composite Index, and the AMEX Biotech Index for that same period. The graph assumes that $100 was invested on December 31, 2002 in each of our common stock, the AMEX Composite Index, and the AMEX Biotech Index, and that all dividends were reinvested. The comparisons shown in the graph below are based upon historical data. The stock price performance shown in the graph below is not necessarily indicative of, or intended to forecast, the potential future performance of our common stock. The stock performance graph shall not be deemed to be “soliciting material” or to be “filed” with the SEC under the Securities Act or the Exchange Act, or incorporated by reference in any document so filed.
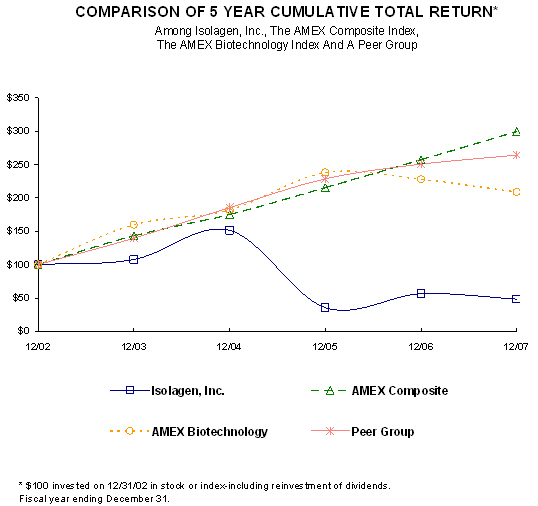
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 12/02 | | | 12/03 | | | 12/04 | | | 12/05 | | | 12/06 | | | 12/07 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Isolagen, Inc. | | | 100.00 | | | | 107.69 | | | | 151.35 | | | | 35.58 | | | | 56.35 | | | | 48.27 | |
AMEX Composite | | | 100.00 | | | | 143.18 | | | | 175.20 | | | | 215.26 | | | | 257.04 | | | | 299.37 | |
AMEX Biotechnology | | | 100.00 | | | | 159.74 | | | | 181.93 | | | | 237.92 | | | | 227.81 | | | | 208.94 | |
Peer Group | | | 100.00 | | | | 139.54 | | | | 185.38 | | | | 228.39 | | | | 250.49 | | | | 264.13 | |
37
| | |
| Item 6. | | Selected Financial Data |
Our selected consolidated financial information presented as of December 31, 2007, 2006, 2005, 2004 and 2003 and for each of the five years ended December 31, 2007 was derived from our audited consolidated financial statements.
This information should be read in conjunction with the historical consolidated financial statements and related notes included herein, and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” We have classified our discontinued operations as discontinued operations for all periods presented herein (see Note 5 of Notes to Consolidated Financial Statements).
| | | | | | | | | | | | | | | | | | | | | |
| | | For the Year Ended December 31, | |
| | | 2007 (1)(3) | | | 2006 (1)(3) | | | 2005 | | | 2004 | | | 2003 | |
| Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
| Revenue | | $ | 1,400,986 | | | $ | 384,389 | | | $ | — | | | $ | — | | | $ | — | |
| License fees | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
| Total revenue | | | 1,400,986 | | | | 384,389 | | | | — | | | | — | | | | — | |
| Cost of sales | | | 656,029 | | | | 194,197 | | | | — | | | | — | | | | — | |
| Selling, general and administrative expenses | | | 18,730,863 | | | | 13,174,960 | | | | 14,000,977 | | | | 9,717,713 | | | | 4,491,259 | |
| Research and development | | | 13,298,338 | | | | 8,796,219 | | | | 10,592,170 | | | | 4,647,553 | | | | 3,100,059 | |
| | | | | | | | | | | | | | | | |
| Operating loss | | | (31,284,244 | ) | | | (21,780,987 | ) | | | (24,593,147 | ) | | | (14,365,266 | ) | | | (7,591,318 | ) |
| Other income (expense) | | | | | | | | | | | | | | | | | | | | |
| Interest income | | | 901,262 | | | | 2,261,899 | | | | 2,808,328 | | | | 558,536 | | | | 40,663 | |
| Other income | | | 150,138 | | | | — | | | | — | | | | 84,359 | | | | 55,663 | |
| Interest expense | | | (3,899,239 | ) | | | (3,899,239 | ) | | | (3,912,059 | ) | | | (636,676 | ) | | | — | |
| Minority interest | | | 246,347 | | | | 78,132 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
| Loss before income taxes from continuing operations | | | (33,885,736 | ) | | | (23,340,195 | ) | | | (25,696,878 | ) | | | (14,359,047 | ) | | | (7,494,993 | ) |
| Income tax benefit | | | — | | | | 190,754 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
| Loss from continuing operations | | | (33,885,736 | ) | | | (23,149,441 | ) | | | (25,696,878 | ) | | | (14,359,047 | ) | | | (7,494,993 | ) |
| Loss from discontinued operations, net of tax | | | (1,687,378 | ) | | | (12,671,965 | ) | | | (10,080,706 | ) | | | (7,115,422 | ) | | | (3,773,301 | ) |
| | | | | | | | | | | | | | | | |
| Net loss | | | (35,573,114 | ) | | | (35,821,406 | ) | | | (35,777,584 | ) | | | (21,474,469 | ) | | | (11,268,294 | ) |
| | | | | | | | | | | | | | | | |
| Deemed dividend associated with beneficial conversion of preferred stock | | | — | | | | — | | | | — | | | | — | | | | (1,244,880 | ) |
| Preferred stock dividends | | | — | | | | — | | | | — | | | | — | | | | (1,087,200 | ) |
| | | | | | | | | | | | | | | | |
| Net loss attributable to common shareholders | | $ | (35,573,114 | ) | | $ | (35,821,406 | ) | | $ | (35,777,584 | ) | | $ | (21,474,469 | ) | | $ | (13,600,374 | ) |
| | | | | | | | | | | | | | | | |
| Per share information | | | | | | | | | | | | | | | | | | | | |
| Net loss from continuing operations—basic and diluted | | $ | (1.02 | ) | | $ | (0.76 | ) | | $ | (0.85 | ) | | $ | (0.47 | ) | | $ | (0.39 | ) |
| Net loss from discontinued operations— basic and diluted | | | (0.05 | ) | | | (0.42 | ) | | | (0.33 | ) | | | (0.24 | ) | | | (0.19 | ) |
| Deemed dividend associated with beneficial conversion of preferred stock | | | — | | | | — | | | | — | | | | — | | | | (0.06 | ) |
| Preferred stock dividends | | | — | | | | — | | | | — | | | | — | | | | (0.06 | ) |
| | | | | | | | | | | | | | | | |
| Net loss attributable to common shareholders | | $ | (1.07 | ) | | $ | (1.18 | ) | | $ | (1.18 | ) | | $ | (0.71 | ) | | $ | (0.70 | ) |
| | | | | | | | | | | | | | | | |
| Weighted average shares outstanding | | | 33,093,370 | | | | 30,309,439 | | | | 30,245,283 | | | | 30,116,827 | | | | 19,297,865 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | |
| | | 2007 (2) | | | 2006 (2) | | | 2005 | | | 2004 | | | 2003 | |
| Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents, restricted cash and available-for-sale investments | | $ | 17,042,102 | | | $ | 33,266,742 | | | $ | 67,013,659 | | | $ | 116,139,016 | | | $ | 15,935,558 | |
| Working capital | | | 13,805,981 | | | | 29,487,802 | | | | 61,130,870 | | | | 111,061,724 | | | | 14,367,768 | |
| Total assets | | | 39,491,190 | | | | 57,286,875 | | | | 90,179,922 | | | | 128,121,138 | | | | 19,644,465 | |
| Total liabilities | | | 96,284,125 | | | | 96,806,084 | | | | 98,276,819 | | | | 99,135,713 | | | | 2,380,740 | |
| Total shareholders equity (deficit) | | | (58,650,961 | ) | | | (41,623,582 | ) | | | (8,096,897 | ) | | | 28,985,425 | | | | 17,263,725 | |
| | | |
| (1) | | Includes the results of operations of Agera, which was acquired August 10, 2006 from the date of acquisition to December 31, 2006. See Note 4 of Notes to Consolidated Financial Statements. |
| |
| (2) | | Includes the assets and liabilities of Agera which was acquired August 10, 2006. |
| |
| (3) | | Effective January 1, 2006 the Company adopted the provisions of Statement of Financial Accounting Standards (“SFAS”) No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123(R)”). SFAS No. 123(R) requires entities to recognize compensation expense for all share-based payments to employees and directors, including grants of employee stock options, based on the grant-date fair value of those share-based payments, adjusted for expected forfeitures. As a result of adopting Statement 123(R) on January 1, 2006, the Company’s loss before income taxes and net loss for the years ended December 31, 2007 and 2006 was $1.8 million and $1.1 million higher, respectively, than if it had continued to account for share-based compensation under APB No. 25 (refer to Note 13 in Notes to Consolidated Financial Statements for further stock-based compensation discussion). |
38
| | |
| Item 7. | | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
General
We are an aesthetic and therapeutic company focused on developing novel skin and tissue rejuvenation products. Our clinical development product candidates are designed to improve the appearance of skin injured by the effects of aging, sun exposure, acne and burns with a patient’s own, or autologous, fibroblast cells produced in our proprietary Isolagen Process. Our clinical development programs encompass both aesthetic and therapeutic indications. Our most advanced indication utilizing the Isolagen Process is for the treatment of wrinkles and is currently in Phase III clinical development. We have also initiated a Phase II/III study in acne scars, a Phase II study for restrictive burn scars and have ongoing Phase II trials in full face rejuvenation and periodontal disease. We also develop and market an advanced skin care product line through our Agera Laboratories, Inc. subsidiary, in which we acquired a 57% interest in August 2006.
We sometimes refer to our product candidates in the aggregate as Isolagen Therapy. From 2002 through 2006, we made Isolagen Therapy available to physicians primarily in the United Kingdom. In the fourth quarter of 2006, our Board of Directors approved closing our United Kingdom operation. Our United Kingdom operation was shutdown on March 31, 2007 (as more fully discussed in Note 5 in Notes to the Consolidated Financial Statements and below). We have refocused our management and capital resources on the management of our clinical trials, and funding thereof.
We market and sell an advanced skin care line through our majority-owned subsidiary, Agera, which we acquired in August 2006. Agera offers a complete line of skincare systems based on a wide array of proprietary formulations, trademarks and nano-peptide technology. Agera markets its product in both the United States and Europe (primarily the United Kingdom).
We are considered to be a “development stage” enterprise.
Going Concern
At December 31, 2007, we have cash, cash equivalents and restricted cash of $17.0 million and working capital of $13.8 million (including our cash, cash equivalents and restricted cash). We believe that its existing capital resources are adequate to finance our operations through at least September 1, 2008. We estimate that we will require additional cash resources during the third quarter of 2008 based upon our current operating plan and condition. This estimate excludes any proceeds that would be realized upon the sale of the Swiss campus (discussed further below underFactors Affecting Our Capital Resources).
Through December 31, 2007, we have been primarily engaged in developing our initial product technology and recruiting personnel. In the course of our development activities, we have sustained losses and expect such losses to continue through at least 2008. We expect to finance our operations primarily through our existing cash and any future financing, including the sale of assets. However, there exists substantial doubt about our ability to continue as a going concern. We will be required to obtain additional capital in the future to continue our operations. There is no assurance that we will be able to obtain any such additional capital as we need to finance these efforts, through asset sales, equity or debt financing, or any combination thereof, or on satisfactory terms or at all. Additionally, no assurance can be given that any such financing, if obtained, will be adequate to meet our ultimate capital needs and to support our growth. If adequate capital cannot be obtained on a timely basis and on satisfactory terms, our operations would be materially negatively impacted. Further, if we do not obtain additional funding prior to or during the third quarter of 2008, we may enter into bankruptcy during 2008 and possibly cease operations thereafter.
We filed a shelf registration statement on Form S-3 during June 2007, which was subsequently declared effective by the SEC. The shelf registration allows us the flexibility to offer and sell, from time to time, up to an original amount of $50 million of common stock, preferred stock, debt securities, warrants or any combination of the foregoing in one or more future public offerings. In August 2007, we sold under this shelf registration statement 6,746,647 shares of common stock to institutional investors, raising proceeds of $13.8 million, net of offering costs. We may offer and sell up to an additional $36.2 of common stock pursuant to this shelf registration.
39
Our ability to complete additional offerings, including any additional offerings under our shelf registration statement, is dependent on the state of the debt and/or equity markets at the time of any proposed offering, and such market’s reception of our company and the offering terms. In addition, our ability to complete an offering may be dependent on the status of our clinical trials, and in particular, the status of our Phase III clinical trial for the treatment of wrinkles, which cannot be predicted. We are also continuing its efforts to sell our Swiss campus (see Note 3). We will add any proceeds from the sale of the Swiss campus to our working capital, which would partially alleviate our need to obtain financing from other sources. There is no assurance that capital in any form would be available to us, and if available, on terms and conditions that are acceptable.
As a result of the above discussed conditions, and in accordance with generally accepted accounting principles in the United States, there exists substantial doubt about our ability to continue as a going concern, and our ability to continue as a going concern is contingent upon our ability to secure additional adequate financing or capital prior to or during the third quarter of 2008. If we are unable to obtain additional sufficient funds during this time, we will be required to terminate or delay our efforts to obtain regulatory approval of one, more than one, or all of our product candidates, curtail or delay the implementation of manufacturing process improvements and/or delay the expansion of our sales and marketing capabilities. Any of these actions would have an adverse effect on our operations, the realization of our assets and the timely satisfaction of our liabilities. Our financial statements are presented on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The financial statements do not include any adjustments relating to the recoverability of the recorded assets or the classification of liabilities that may be necessary should it be determined that we are unable to continue as a going concern.
The report of our independent registered public accounting firm, BDO Seidman, LLP, contains a modification concerning our ability to continue as a going concern.
Closure of the United Kingdom Operation
As part of our continuing efforts to evaluate the best uses of our resources, in the fourth quarter of 2006 our Board of Directors approved the proposed closing of the United Kingdom operation. On March 31, 2007, we completed the closure of the United Kingdom manufacturing facility. The United Kingdom operation was located in London, England with two locations; a manufacturing site and an administrative site. Both sites are under operating leases. The manufacturing site lease expires February 2010 and, as of December 31, 2007, the remaining lease obligation approximated $0.5 million. The administrative site lease expired in April 2007.
Since our public announcement regarding the closure of the United Kingdom operation, we have received negative publicity and negative correspondence from former patients in the United Kingdom that previously received our treatment. More recently, we received a written demand by an attorney representing approximately 82 former patients each claiming negligent misstatements were made and each claiming, on average, £3,500 (or approximately $7,000), plus unquantified interest and incidental expenses. We are in the process of evaluating the merits of the claims made in the demand. To date, no formal legal action has been brought by the attorney against the Company, and no provision has been recorded in the consolidated financial statements related to this matter.
With the closure of the United Kingdom operation on March 31, 2007, our European operations (both the United Kingdom and Switzerland) and Australian operations have been presented in the financial statements as discontinued operations for all periods presented. See Note 5 of Notes to Consolidated Financial Statements.
Critical Accounting Policies
The following discussion and analysis of financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in conformity with accounting principles generally accepted in the United States of America. Our significant accounting policies are more fully described in Note 3 of the Notes to the Consolidated Financial Statements. However, certain accounting policies and estimates are particularly important to the understanding of our financial position and results of operations and require the application of significant judgment by our management or can be materially affected by changes from period to period in economic factors or conditions that are outside of the control of management. As a result they are subject to an inherent degree of uncertainty. In applying these policies, our management uses their judgment to determine the appropriate assumptions to be used in the determination of certain estimates. Those estimates are based on our historical operations, our future business plans and projected financial results, the terms of existing contracts, our observance of trends in the industry, information provided by our customers and information available from other outside sources, as appropriate. The following discusses our critical accounting policies and estimates.
40
Going Concern:As disclosed in Note 2 to the Consolidated Financial Statements, management has concluded that substantial doubt exists about the Company’s ability to continue as a going concern. This conclusion is based on estimates of our future spending and future funding required during 2008. We will be required to obtain additional capital in 2008 to continue and expand our operations. There is no assurance that we will be able to obtain any such additional capital as it needs to finance these efforts, through asset sales, equity or debt financing, or any combination thereof, or on satisfactory terms or at all.
At December 31, 2007, our cash, cash equivalents and restricted cash balance was $17.0 million. For the year ended December 31, 2007, our cash used in operations and investing activities was nearly $30 million. These factors, as well as our future spending estimates, were important factors in concluding that substantial doubt exists about our ability to continue as a going concern. We believe these estimates are particularly important to the understanding of our financial position.
Our financial statements are presented on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The financial statements do not include any adjustments relating to the recoverability of the recorded assets or the classification of liabilities that may be necessary should it be determined that we are unable to continue as a going concern.
Stock-Based Compensation: In December 2004, the Financial Accounting Standards Board (“FASB”) issued Statement of Financial Accounting Standards (“SFAS”) No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123 (R)”). SFAS No. 123 (R) replaces SFAS No. 123, “Accounting for Stock-Based Compensation,” supersedes APB Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB No. 25”), and amends SFAS No. 95, “Statement of Cash Flows.” SFAS No. 123 (R) requires entities to recognize compensation expense for all share-based payments to employees and directors, including grants of employee stock options, based on the grant-date fair value of those share-based payments, adjusted for expected forfeitures.
We adopted SFAS No. 123(R) as of January 1, 2006 using the modified prospective application method. Under the modified prospective application method, the fair value measurement requirements of SFAS No. 123(R) is applied to new awards and to awards modified, repurchased, or cancelled after January 1, 2006. Additionally, compensation cost for the portion of awards for which the requisite service has not been rendered that were outstanding as of January 1, 2006 is recognized as the requisite service is rendered on or after January 1, 2006. The compensation cost for that portion of awards is based on the grant-date fair value of those awards as calculated for pro forma disclosures under SFAS No. 123. Changes to the grant-date fair value of equity awards granted before January 1, 2006 are precluded.
The fair value of stock options is determined using the Black-Scholes valuation model, which is consistent with our valuation techniques previously utilized for awards in footnote disclosures required under SFAS No. 123. Prior to the adoption of SFAS No. 123(R), we followed the intrinsic value method in accordance with APB No. 25 to account for our employee and director stock options. Historically, substantially all stock options have been granted with an exercise price equal to the fair market value of the common stock on the date of grant. Accordingly, no compensation expense was recognized from substantially all option grants to employees and directors prior to the adoption of SFAS No. 123(R). However, compensation expense was recognized in connection with the issuance of stock options to non-employee consultants in accordance with EITF 96-18, “Accounting for Equity Instruments That are Issued to Other than Employees for Acquiring, or in Conjunction with Selling Goods and Services.” SFAS No. 123(R) did not change the accounting for stock-based compensation related to non-employees in connection with equity based incentive arrangements.
41
The adoption of SFAS No. 123(R) requires additional accounting related to the income tax effects and additional disclosure regarding the cash flow effects resulting from share-based payment arrangement. This change in accounting resulted in the recognition of compensation expense of $1.8 million and $1.1 million related to our employee and director stock options for the years ended December 31, 2007 and 2006, respectively. During the year ended December 31, 2007, we granted stock options to purchase 0.7 million shares of our common stock. As of December 31, 2007, there was $1.8 million of total unrecognized compensation cost related to non-vested director and employee stock options which vest over time. That cost is expected to be recognized over a weighted-average period of 1.6 years. As of December 31, 2007, there was $0.8 million of total unrecognized compensation cost related to performance-based, non-vested employee stock options. That cost will begin to be recognized when the performance criteria within the respective performance-base option grants become probable of achievement.
During December 2005, the board of directors approved the full vesting of all unvested, outstanding stock options issued to current employees and directors. The board decided to take this action (the “acceleration event”) in anticipation of the adoption of SFAS No. 123 (R). As a result of this acceleration event, stock options to purchase approximately 1.4 million shares of our common stock were vested that would have otherwise vested during 2006 and later periods. At the time of the acceleration event, the unamortized grant date fair value of the affected options was approximately $3.6 million (for SFAS No. 123 and SFAS No. 148 pro forma disclosure purposes), which was charged to pro forma expense in the fourth quarter of 2005. Substantially all of the unvested employee stock options that were subject to the acceleration event had exercise prices above market price of our common stock at the time the board approved the acceleration event. However, in accordance with SFAS 123 (R) if we had not completed this acceleration event in December 2005, the majority of the $3.6 million amount discussed above would have been charged against the future results of operations, beginning in the first quarter of fiscal 2006 and continuing through later periods as the options vested. As discussed above, substantially all of the unvested employee stock options which were accelerated had exercise prices above market price at the time of acceleration. For the purposes of applying APB No. 25 to such stock options in the statement of operations for the year ended December 31, 2005, the acceleration event was treated as the acceleration of the vesting of employee and director options that otherwise would have vested as originally scheduled, and accordingly was not a modification requiring the remeasurement of the intrinsic value of the options, or the application of variable option accounting, under APB No. 25. For stock options that had exercise prices below market price at the time of acceleration and that would not have vested originally, a charge of approximately $15,000 was recorded in the statement of operations for the year ended December 31, 2005.
In March 2007, and in connection with the separation of the Company’s President, the Company agreed to modify certain of the President’s stock options such that (1) 120,000 unvested, time-based stock options would vest immediately and (2) of 400,000 performance based stock options, 100,000 would be cancelled and the remaining 300,000 would be extended such that the 300,000 options would expire 10 years from the original grant date, as opposed to expiring upon termination of employment. The 300,000 performance based stock options will continue to be subject to the same performance based vesting requirements. The 120,000 modified stock options were valued using the Black-Scholes valuation model, and resulted in $0.3 million charge to selling, general and administrative expense during the year ended December 31, 2007. The 300,000 modified performance stock options were valued using the Black-Scholes valuation model, and resulted in $0.8 million charge to selling, general and administrative expense during the year ended December 31, 2007. Two other employee stock option modifications resulted in less than $0.1 million charge to selling, general and administrative expense during the year ended December 31, 2007.
Accounting for Legal Matters:As discussed in Note 11 of Notes to Consolidated Financial Statements, set forth elsewhere in this Report, we are currently defending ourselves against various class and derivative actions. We have also received threats of litigation and demands from former patients associated with our United Kingdom operation. We intend to defend ourselves vigorously against these actions. We cannot currently estimate the amount of loss, if any, that may result from the resolution of these actions, and no provision has been recorded in our consolidated financial statements. Generally, a loss must be both reasonably estimable and probable in order to record a provision for loss. We will expense our legal costs as they are incurred and will record any insurance recoveries on such legal costs in the period the recoveries are received. Although we have not recorded a provision for loss regarding these matters, a loss could occur in a future period.
We are involved in various other legal matters that are being defended and handled in the ordinary course of business. Although it is not possible to predict the outcome of these matters, management currently believes that the results will not have a material impact on our financial statements.
42
Research and Development Expenses:Research and development costs are expensed as incurred and include salaries and benefits, costs paid to third-party contractors to perform research, conduct clinical trials, develop and manufacture drug materials and delivery devices, and a portion of facilities cost. Clinical trial costs are a significant component of research and development expenses and include costs associated with third-party contractors. Invoicing from third-party contractors for services performed can lag several months. We accrue the costs of services rendered in connection with third-party contractor activities based on our estimate of management fees, site management and monitoring costs and data management costs. Actual clinical trial costs may differ from estimated clinical trial costs and are adjusted for in the period in which they become known.
Results of Operations—Comparison of Years Ending December 31, 2007 and 2006
REVENUES. Revenue increased $1.0 million to $1.4 million for the year ended December 31, 2007, as compared to $0.4 million for the year ended December 31, 2006. Our revenue from continuing operations is from the operations of Agera which we acquired on August 10, 2006. Agera markets and sells a complete line of advanced skin care systems based on a wide array of proprietary formulations, trademarks and non-peptide technology. On a pro forma basis, assuming that Agera had been acquired on January 1, 2006, our revenue would have been $1.2 million for the year ended December 31, 2006. Due to our financial statement presentation of our United Kingdom operation as a discontinued operation, our revenue for all periods presented is representative of only Agera, as all historical United Kingdom revenue is reflected in loss from discontinued operations. We currently expect first quarter 2008 revenue to be approximately $0.2 million.
COST OF SALES. Costs of sales increased to $0.7 million for the year ended December 31, 2007, as compared to $0.2 million for the year ended December 31, 2006. Our cost of sales relates to the operation of Agera.
As a percentage of revenue, Agera cost of sales were approximately 47% for the year ended December 31, 2007 and approximately 50% for the year ended December 31, 2006. On a pro forma basis, assuming Agera had been acquired on January 1, 2006, our cost of sales as a percentage of revenue would have been 50% for the full year ended December 31, 2006. The decrease in 2007 cost of sales as a percentage of revenue, as compared to 2006, is primarily due to selling price increases implemented during 2007.
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES. Selling, general and administrative expenses increased approximately $5.6 million, or 42%, to $18.7 million for the year ended December 31, 2007, 2007, as compared to $13.2 million for the year ended December 31, 2006. The increase in selling, general and administrative expense is primarily due to the following:
a) Additional severance expense and related costs associated with the termination of our President, pursuant to a settlement agreement executed in June 2007, resulted in an additional $4.6 million of selling, general and administrative expense for the year ended December 31, 2007 (see Notes 11 and 13 of Notes to Consolidated Financial Statements).
b) Salaries, bonuses and payroll taxes increased by approximately $0.1 million to $5.3 million for the year ended December 31, 2007, as compared to $5.2 million for the year ended December 31, 2006, due to an increase in the average number of our employees, primarily at the executive management level, which resulted in higher salary expense. The higher salary expense was partially offset by lower bonuses earned for the year ended December 31, 2007 as compared to December 31, 2006.
c) Marketing expense increased by approximately $0.2 million to $0.8 million for the year ended December 31, 2007, as compared to $0.6 million during the year ended December 31, 2006 due primarily to marketing and promotional efforts related to marketing and selling our Agera line of advanced skin care systems.
d) Travel expense increased by approximately $0.1 million to $0.7 million for the year ended December 31, 2007, as compared to $0.6 million for the year ended December 31, 2006 due to the increase in the number of our employees, primarily at the executive management level, and increase in business development activities during 2007.
43
e) Other general and administrative operating costs increased by approximately $1.6 million to $6.8 million for the year ended December 31, 2007, as compared to $5.2 million for the year ended December 31, 2006 due primarily to costs of approximately $0.8 million related to our withdrawn and terminated debt offering, increased business development activities and increased investor relations costs of $0.6 million for the year ended December 31, 2007, as well as the addition of general expenses related to our August 2006 acquisition of Agera. We incurred non-cash amortization expense related to our Agera intangible assets of approximately $0.3 million for the year ended December 31, 2007, as compared to $0.1 million for the year ended December 31, 2006.
f) Legal expenses decreased by approximately $1.0 million to $0.6 million for the year ended December 31, 2007, as compared to $1.6 million for the year ended December 31, 2006. For the years ended December 31, 2007 and December 30, 2006, we received $1.7 million and $0.9 million, respectively, of reimbursements from our insurance carrier as reimbursement for defense costs related to our class action and derivative matters. If we had not received these reimbursements, our legal expenses would have been $2.3 million for the year ended December 31, 2007 and $2.5 million for the year ended December 31, 2006. Excluding the insurance carrier reimbursement, legal expenses remained relatively consistent. In general, our legal expense, net, fluctuates primarily as a result of the level of class action and derivative action defense costs incurred during a particular period and as a result of the timing of related insurance carrier reimbursements for defense costs. Such reimbursements are recorded when received. As discussed in Note 11 of Notes to Consolidated Financial Statements, on September 26, 2007 the Court denied our motion to dismiss the complaint in the federal securities litigation against us, and on November 6, 2007 the court entered a scheduling order that provides that discovery be completed by June 8, 2009. The commencement of the discovery process in this matter will likely cause legal expenses to increase in 2008.
RESEARCH AND DEVELOPMENT. Research and development expenses increased by approximately $4.5 million for the year ended December 31, 2007 to $13.3 million, as compared to $8.8 million for the year ended December 31, 2006. Research and development costs are composed primarily of costs related to our efforts to gain FDA approval for our Isolagen Therapy for specific dermal applications in the United States, as well as costs related to other potential indications for our Isolagen Therapy, such as acne scars and burn scars. Also, research and development expense includes costs to develop manufacturing, cell collection and logistical process improvements. Research and development costs primarily include personnel and laboratory costs related to these FDA trials and certain consulting costs. The total inception to date cost of research and development as of December 31, 2007 was $44.0 million.
The FDA approval process is extremely complicated and is dependent upon our study protocols and the results of our studies. In the event that the FDA requires additional studies for our dermal product candidate or requires changes in our study protocols or in the event that the results of the studies are not consistent with our expectations, as occurred during 2005 with respect to our previously conducted Phase III wrinkle/nasolabial fold trial, the process will be more expensive and time consuming. Due to the complexities of the FDA approval process, we are unable to predict what the cost of obtaining approval for our dermal product candidate will be at this time.
The major changes in research and development expenses are due primarily to the following:
a) Consulting expense increased by approximately $3.0 million to $6.6 million for the year ended December 31, 2007, as compared to $3.6 million for the year ended December 31, 2006, as a result of increased expenditures related to our current wrinkle/nasolabial fold study and acne scar study, and preparations related to other clinical trials such as restrictive burn scars.
b) Salaries, bonuses and payroll taxes increased by approximately $0.6 million to $3.0 million for the year ended December 31, 2007, as compared to $2.4 million for the year ended December 31, 2006, as a result of increased employees engaged in research and development activities.
c) Laboratory costs increased by approximately $0.6 million to $1.5 million for the year ended December 31, 2007, as compared to $0.9 million for the year ended December 31, 2006, as a result of increased clinical and manufacturing activities in our Exton, Pennsylvania location.
44
d) Contract labor support related to our clinical manufacturing operation increased $0.5 million to $0.5 million for the year ended December 31, 2007, as compared to less than $0.1 million for the year ended December 31, 2006, as a result of increased clinical activities in our Exton, Pennsylvania location.
e) Facilities, depreciation and travel costs decreased $0.2 million to $1.7 million for the year ended December 31, 2007, as compared to $1.9 million for the year ended December 31, 2006.
LOSS FROM DISCONTINUED OPERATIONS. As discussed above under “—Closure of the United Kingdom Operation,” during the three months ended December 31, 2006, the Board of Directors approved the closure of our United Kingdom operation. The United Kingdom operation was closed in March 2007, as compared to normal operations during the year ended December 31, 2006.
The loss from discontinued operations decreased by approximately $11.0 million for the year ended December 31, 2007 to $1.7 million, as compared to $12.7 million for the year ended December 31, 2006. The $1.7 million loss from discontinued operations during the year ended December 31, 2007 consisted of $1.1 million of losses incurred during the first quarter 2007, which was the United Kingdom’s last quarter of full operations, and $0.6 million of second, third and fourth quarter 2007 losses (which, after closure of the operation, primarily consisted of facility expense, legal expense and public relations costs, as well as costs to maintain our Switzerland corporate campus held for sale). The $1.1 million loss from discontinued operations during the three months ended March 31, 2007 primarily consisted of the following:
a) Salaries, severance expense and payroll taxes were approximately $0.3 million for the three months ended March 31, 2007.
b) Other general and administrative operating costs were approximately $0.5 million primarily related to lease expense and operating costs incurred during the three months ended March 31, 2007.
c) Gross loss was $0.3 million during the three months ended March 31, 2007, primarily due to low production volumes during the shutdown period and due to the write-off of unrealizable inventory used in the manufacturing process.
INTEREST INCOME. Interest income decreased approximately $1.4 million to $0.9 million for the year ended December 31, 2007, as compared to $2.3 million for the year ended December 31, 2006. The decrease in interest income of $1.4 million resulted from a decrease in the amount of cash, cash equivalents and restricted cash balances, as a result of our use of these balances primarily to fund our normal operating activities related to our efforts to gain FDA approval for our Isolagen Therapy.
INTEREST EXPENSE. Interest expense remained constant at $3.9 million for the year ended December 31, 2007, as compared to the year ended December 31, 2006. Our interest expense is related to our $90.0 million, 3.5% convertible subordinated notes, as well as the related amortization of deferred debt issuance costs of $0.8 million for each of the years ended December 31, 2007 and 2006, respectively.
NET LOSS. Net loss decreased $0.2 million to $35.6 million for the year ended December 31, 2007, as compared to a net loss of $35.8 million for the year ended December 31, 2006. This decrease in net loss primarily represents the effects of our decrease in loss from discontinued operation and additional gross profit of $0.6 million, offset by the increases in our selling, general and administrative expenses and research and development expenses and reductions in interest income, as discussed above.
Results of Operations—Comparison of Years Ending December 31, 2006 and 2005
REVENUE. Revenue increased $0.4 million for the year ended December 31, 2006, as compared to zero for the year ended December 31, 2005. The increase in revenue is due to the acquisition of Agera on August 10, 2006, which markets and sells a complete line of advanced skin care systems based on a wide array of proprietary formulations, trademarks and nano-peptide technology. On a pro forma basis, assuming that Agera had been acquired on January 1, 2006 and 2005, respectively, our revenue would have been $1.2 million and $1.0 million for the years ended December 31, 2006 and 2005, respectively.
45
COST OF SALES. Costs of sales increased to $0.2 million for the year ended December 31, 2006, as compared to zero for the year ended December 31, 2005.
As a percentage of revenue, Agera cost of sales were approximately 50% for the year ended December 31, 2006. Agera’s cost of sales, as a percentage of revenue, for all of 2006 and 2005 (including the periods prior to our August 10, 2006 acquisition of a 57% interest in Agera) were 46% and 37%, respectively.
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES. Selling, general and administrative expenses decreased approximately $0.8 million, or 6%, to $13.2 million for the year ended December 31, 2006, as compared to $14.0 million for the year ended December 31, 2005. This decrease was net of the effect of a $0.6 million increase in selling, general and administrative expenses for expenses of Agera for the period August 10, 2006 to December 31, 2006. The decrease in selling, general and administrative expense is primarily due to the following:
a) Salaries, bonuses and payroll taxes increased by approximately $2.1 million to $5.2 million for the year ended December 31, 2006, as compared to $3.1 million for year ended December 31, 2005, due to an increase in the number of our employees, primarily at the executive management level, which resulted in higher salary and bonus expense of $1.3 million during the year ended December 31, 2006. Additionally, as the result of the adoption of SFAS 123(R) on January 1, 2006, equity-based compensation was approximately $1.1 million higher in 2006 as compared to the prior year. These increases were offset by a decrease in severance expense during 2006 of $0.3 million. We incurred $0.2 million of severance expense in the year ended December 31, 2006, as compared to $0.5 million in the year ended December 31, 2005.
b) Marketing expense increased by approximately $0.2 million to $0.6 million for the year ended December 31, 2006, as compared to $0.4 million for year ended December 31, 2005 due primarily to increased marketing and promotional efforts related to marketing and selling our complete line of advanced skin care systems under the trade name Agera, since our acquisition of Agera on August 10, 2006.
c) Travel expense decreased by approximately $0.2 million to $0.6 million for the year ended December 31, 2006, as compared to $0.8 million for year ended December 31, 2005, due to less travel between our Houston, Texas and Exton, Pennsylvania facilities as a result of our closing of the Houston office during 2006.
d) Consulting expense decreased by approximately $0.1 million to $0.7 million for the year ended December 31, 2006, as compared to $0.8 million for the year ended December 31, 2005 primarily due to decreased recruiting costs during 2006.
e) Legal expenses, net, increased approximately $0.3 million to $1.6 million for the year ended December 31, 2006, as compared to $1.3 million for the year ended December 31, 2005, due primarily to costs related to the securities and derivative lawsuits, for which we are defendants, and employment termination matters. While the change in legal expense, net, is not significant, included in the net legal expenses are insurance refunds of $0.9 million and $0 in the years ended December 31, 2006 and 2005, respectively. Insurance refunds received related to the reimbursement of legal defense costs are recorded in the period that they are received.
f) Other general and administrative operating costs decreased approximately $3.1 million to $4.5 million for the year ended December 31, 2006, as compared to $7.6 million for the year ended December 31, 2005, due primarily to the write-off of third party developed software costs of approximately $1.4 million in the year ended December 31, 2005. In addition, with the completion of the Exton laboratory in the latter half of 2005, there were costs of approximately $1.4 million that were allocated from selling, general and administrative cost to research and development costs. Additionally, there was a decrease of approximately $0.3 million related to other corporate expenses, including public relations, investor relations and accounting fees.
46
RESEARCH AND DEVELOPMENT. Research and development expenses decreased by approximately $1.8 million during the year ended December 31, 2006 to $8.8 million, as compared to $10.6 million for the year ended December 31, 2005. Research and development costs are composed primarily of costs related to our efforts to gain FDA approval for the Isolagen Therapy for specific dermal applications in the United States and also include costs to develop manufacturing, cell collection and logistical process improvements. Research and development costs include personnel and laboratory costs related to these FDA trials and certain consulting costs. The total inception to date cost of research and development as of December 31, 2006 was $30.7 million. The FDA approval process is extremely complicated and is dependent upon our study protocols and the results of our studies. In the event that the FDA requires additional studies for dermal applications or requires changes in our study protocols or in the event that the results of the studies are not consistent with our expectations, as occurred during 2005 with respect to our first pivotal Phase III dermal trial, the process will be more expensive and time consuming. Due to the complexities of the FDA approval process, we are unable to predict what the costs of obtaining approval for the dermal applications will be at this time. We have other research projects currently underway. However, research and development costs related to these projects were not material during 2006 and 2005.
The major changes in research and development expense are due primarily to the following: a) consulting expense decreased by approximately $2.4 million to $4.5 million for the year ended December 31, 2006, as compared to $6.9 million for the year ended December 31, 2005, as a result of decreased expenditures related to our clinical trials and manufacturing process research and development, b) salaries and payroll taxes decreased by approximately $0.9 million to $2.4 million for the year ended December 31, 2006, as compared to $3.3 million for the year ended December 31, 2005, as a result of the closure of our research and development facility in Houston, Texas, during 2006, and termination of related personnel, as well as the termination of certain Exton, Pennsylvania personnel during 2006 and c) facility costs, including rent, utilities, depreciation and other related costs, increased approximately $1.5 million, due primarily to the new Exton, Pennsylvania lease which commenced during 2005.
LOSS FROM DISCONTINUED OPERATIONS. As discussed above under “Closure of the United Kingdom Operation,” during the three months ended December 31, 2006, the Board of Directors approved the closure of our United Kingdom operation, and on March 31, 2007, the United Kingdom manufacturing facility was closed.
The loss from discontinued operations increased by approximately $2.6 million for the year ended December 31, 2006 to $12.7 million, as compared to $10.1 million for the year ended December 31, 2005, and primarily consisted of the following:
a) Gross loss increased by approximately $0.7 million to $1.2 million for the year ended December 31, 2006, as compared to $0.5 million for the year ended December 31, 2005, due to a decrease in the number of biopsies, thus resulting in a decrease in revenue. With a decrease in revenue as a result of less marketing and promotional activities, and a large portion of relatively fixed manufacturing costs, our gross loss increased.
b) Salaries, bonuses and payroll taxes increased by approximately $1.0 million to $2.9 million for the year ended December 31, 2006, as compared to $1.9 million for year ended December 31, 2005, due primarily to an increase in the number of our employees dedicated to sales and marketing and customer service of our Isolagen Therapy in the United Kingdom. Further, severance expenses related to the decision to shutdown the United Kingdom operation were approximately $0.3 million for the year ended December 31, 2006.
c) Marketing expense decreased by approximately $1.1 million to $1.7 million for the year ended December 31, 2006, as compared to $2.8 million for year ended December 31, 2005 due primarily to decreased marketing and promotional efforts related to our Isolagen Therapy in the United Kingdom as a result of continued losses from the sale of the Isolagen Therapy and our related decision to shutdown the United Kingdom operations.
d) Other general and administrative operating costs increased by approximately $1.6 million to $5.7 million for the year ended December 31, 2006, as compared to $4.1 million for the year ended December 31, 2005, due primarily to impairment charges of approximately $2.6 million related to the United Kingdom and Switzerland fixed assets; offset by approximately $1.0 million in decreased United Kingdom and Swiss general office costs due to the anticipated United Kingdom shutdown and due to placing the Swiss campus for sale during the year ended December 31, 2006.
47
e) The United Kingdom customer settlement charge was $0.8 million for the year ended December 31, 2006, as compared to zero for the year ended December 31, 2005. As discussed further under Note 11, the United Kingdom customer settlement occurred during the first quarter of 2006.
f) Research and development expenses decreased by approximately $0.4 million to $0.4 million for the year ended December 31, 2006, as compared to $0.8 million for the year ended December 31, 2005, due to a decrease in consulting related to process improvements related to our Isolagen manufacturing process as a result of the decision to shutdown the United Kingdom operation.
INTEREST INCOME. Interest income decreased approximately $0.5 million to $2.3 million for the year ended December 31, 2006, as compared to $2.8 million for the year ended December 31, 2005. The decrease in interest income resulted principally from a decrease in the amount of cash, restricted cash and short-term investment balances, as a result of our normal operating activities related to our efforts to gain FDA approval for the Isolagen Therapy for specific dermal applications in the United States.
INTEREST EXPENSE. Interest expense remained constant at $3.9 million for the year ended December 31, 2006, as compared to the year ended December 31, 2005. Our interest expense is related to the issuance in November 2004 of $90 million in principal amount of 3.5% convertible subordinated debt, as well as the related amortization of deferred debt issuance costs of $0.8 million for each of the years ended December 31, 2006 and 2005.
NET LOSS. Net loss for the year ended December 31, 2006 was $35.8 million as compared to a net loss of $35.8 million for the year ended December 31, 2005. Our net loss, in total, was unchanged from 2005. However, the individual components of net loss have fluctuated, as discussed above.
Liquidity and Capital Resources
Net cash provided by (used in) operating, investing and financing activities for the three years ended December 31, 2007 were as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | (in millions) | |
| Cash flows from operating activities | | $ | (29.7 | ) | | $ | (29.6 | ) | | $ | (34.0 | ) |
| Cash flows from investing activities | | | (0.1 | ) | | | 19.8 | | | | 11.6 | |
| Cash flows from financing activities | | $ | 14.7 | | | $ | 0.2 | | | $ | 0.1 | |
OPERATING ACTIVITIES. Cash used in operating activities during the year ended December 31, 2007 amounted to $29.7 million, an increase of $0.1 million over the year ended December 31, 2006. The increase in our cash used in operating activities over the prior year is primarily due to an increase in net losses (adjusted for non-cash items) of $1.7 million, offset by changes in operating assets and liabilities of $1.6 million. While our 2007 net loss was $0.2 million lower than the prior year, our net loss (adjusted for non-cash items) increased by $1.7 million; specifically our depreciation and amortization, impairment losses and bad debt provision were $3.4 million lower in 2007 as compared to 2006 (primarily due to the March 31, 2007 closing of our United Kingdom operation), offset by $1.6 million of additional stock option expense (primarily due to stock option modification expense incurred during 2007; see Note 13 to the Consolidated Financial Statements). In part as the result of the closure of our United Kingdom operation during 2007, the majority of our operating assets and liabilities have fluctuated as compared to the prior year. For example, the closure of our United Kingdom operations eliminated the accounts receivable, prepaid expenses and deferred revenue related to those operations. In addition, cash outflows related to inventory purchases have increased due to large inventory purchases by Agera during the first half of 2007.
Our negative operating cash flows in 2007 were funded from cash on hand at December 31, 2006 and the proceeds from the sale of our common stock in August 2007 (discussed further below under Financing Activities).
48
INVESTING ACTIVITIES. Cash used in investing activities during the year ended December 31, 2007 amounted to $0.1 million, an decrease of $19.9 million over the year ended December 31, 2006 cash inflow of $19.8 million. The cash used in investing activities of $0.1 million during the year ended December 31, 2007 related to capital expenditures of $0.2 million offset by $0.1 million of proceeds from property and equipment sold.
The cash provided by investing activities of $19.8 million during the year ended December 31, 2006 was generated from the liquidation of $23.0 million of short-term investments (net of purchases) offset by $2.0 million of cash used (net of cash acquired) for the acquisition of a 57% interest in Agera, and further offset by $1.2 million of capital expenditures during 2006. The reduction of short-term investments was used to fund our 2006 purchases of property and equipment, our acquisition of Agera and to partially fund our negative operating cash flows.
FINANCING ACTIVITIES. Cash provided by financing activities was $14.7 million during the year ended December 31, 2007, as compared to cash provided of $0.2 million during the year ended December 31, 2006. In June 2007, we filed a shelf registration statement on Form S-3, which was subsequently declared effective by the SEC. The shelf registration allowed us the flexibility to offer and sell, from time to time, up to an original amount of $50 million of common stock, preferred stock, debt securities, warrants or any combination of the foregoing in one or more future public offerings. In August 2007, the Company sold under this shelf registration statement 6,767,647 shares of common stock to institutional investors, raising proceeds of $13.8 million, net of offering costs. In addition, during the year ended December 31, 2007 we generated $0.9 million of cash proceeds related to the exercise of stock options and warrants. The 2006 cash proceeds of $0.2 million related solely to stock option exercises.
Cash Flows Related to Discontinued Operations
Cash flows related to discontinued operations, which are included in the table of cash flows above, were as follows:
| | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | (in millions) | |
| Cash flows used in operating activities | | $ | (2.6 | ) | | $ | (10.8 | ) | | $ | (9.0 | ) |
| Cash flows used in investing activities | | | 0.1 | | | | (0.3 | ) | | | (13.0 | ) |
Total cash flows used in discontinued operations during the year ended December 31, 2007 was $2.5 million, as compared to $11.1 million during the year ended December 31, 2006. The large majority of cash outflow associated with discontinued operations related to the first quarter of 2007. Cash flows used in discontinued operations during the three months ended March 31, 2007 was $2.1 million. Our United Kingdom was in operation during the three months ended March 31, 2007, and was shutdown on March 31, 2007. The net loss from our United Kingdom operation during the three months ended March 31, 2007 was $1.1 million. In addition, accrued expenses and deferred revenue decreased by $1.2 million during this shutdown period (cash outflows), primarily related to the payment of severance and refunds to customers. The United Kingdom net loss and the large decrease in United Kingdom accrued expenses and United Kingdom deferred revenue during the three months ended March 31, 2007 is what primarily generated the cash outflow from discontinued operations for the year ended December 31, 2007. The remaining cash outflows associated with discontinued operations during the second, third and fourth quarters of 2007 primarily related to the payment of our lease obligation, public relations costs and legal costs in the United Kingdom, as well as operating costs to maintain our Switzerland campus.
Cash outflows from operating activities related to the years ended December 31, 2006 and 2005 related primarily to the normal, historical operation of the United Kingdom and Swiss operations, prior to the decision to shutdown the United Kingdom operation. The cash outflow from investing activities of $13.0 million during the year ended December 31, 2005 is primarily due to the purchase of the Swiss campus, and related capital improvements.
49
WORKING CAPITAL: At December 31, 2007, we had cash, cash equivalents and restricted cash of $17.0 million and working capital of $13.8 million (including our cash, cash equivalents and restricted cash). The substantial majority of our working capital change, as compared to December 31, 2006, relates to the use of our cash, cash equivalents, restricted cash and available-for-sale investments for the purpose of funding and operating our business, offset by the cash proceeds from our $13.8 million common stock sale in August 2007. Our ability to operate profitably is contingent upon our success in obtaining additional capital or financing, obtaining regulatory approval of our product candidates, development of markets for our products, and development of profitable scalable manufacturing processes. We believe our existing capital resources are adequate to finance our operations through at least September 1, 2008, but we will need to engage in a capital-raising transaction during 2008 or we will need to significantly modify our business plan in order to sustain our operations. We can not assure you that we will be able to obtain regulatory approvals of our product candidates, successfully develop the markets for our product candidates or develop profitable scalable manufacturing processes or obtain the capital we require, on a timely basis or on terms that we would find acceptable, or at all. Further, if we do not obtain additional funding prior to or during the third quarter of 2008, we may enter into bankruptcy during 2008 and possibly cease operations thereafter. Our ability to raise equity or debt financing may be dependent on the status of our Phase III clinical trial related to our lead facial aesthetic product candidate, which cannot be predicted. If we are able to raise additional capital, it will likely result in dilution to our current stockholders, which may be substantial.
FACTORS AFFECTING OUR CAPITAL RESOURCES: In April 2005, we acquired a two-building, 100,000 square foot corporate campus in Bevaix, Canton of Neuchâtel, Switzerland for $10 million. The $10 million purchase price was paid using cash on hand from the proceeds of our 2004 issuances of common stock and 3.5% convertible subordinated notes. Our initial estimate of the total cost of acquisition and renovation of the facility, including the purchase of required equipment, was $25 million, which includes approximately $1.8 million we had spent in renovations. The corporate campus is classified as “assets of discontinued operations held for sale” at December 31, 2007 with a value of $11.2 million, reflecting the fair value of the corporate campus. Fair value is defined as the price at which an asset would change hands in a transaction between a willing buyer and willing seller in an unforced transaction. However, if we were to sell the Swiss campus in a forced transaction or a distress sale, it is likely that the price we would receive would be less, and we would recognize a loss, which may be significant, upon such sale. As the result of our cash position at December 31, 2007 and our need for funding, it is possible that we will choose or be required to sell the Swiss campus under such conditions.
Contractual Obligations
The following table summarizes the amounts of payments due under specified contractual obligations as of December 31, 2007:
| | | | | | | | | | | | | | | | | |
| (in millions) | | Payments Due by Period | |
| | | Less than | | | 1 - 3 | | | 4 - 5 | | | More than | |
| Contractual Obligations | | 1 Year | | | Years | | | Years | | | 5 Years | |
| Long-Term Debt Obligations, excluding interest* | | $ | — | | | $ | 90.0 | | | $ | — | | | $ | — | |
| Interest* | | | 3.2 | | | | 3.2 | | | | — | | | | — | |
| Lease Obligations | | | 1.4 | | | | 3.6 | | | | 1.4 | | | | — | |
| Purchase Obligations** | | | 1.3 | | | | 0.1 | | | | — | | | | — | |
| Obligations in Connection with Acquisition*** | | | 0.2 | | | | 1.1 | | | | 0.8 | | | | 5.8 | |
| | | | | | | | | | | | | |
| Total | | $ | 6.1 | | | $ | 98.0 | | | $ | 2.2 | | | $ | 5.8 | |
| | | | | | | | | | | | | |
| | | |
| * | | The table above assumes that our 3.5% convertible subordinated notes will be called due on November 1, 2009. Refer to the below for a description of our 3.5% convertible subordinated notes. |
| |
| ** | | In addition to the above, we have, in the ordinary course of business, various contractual agreements with various consultants and service providers whereby a fee or rate per hour has been agreed to, but no guaranteed minimums have been established. Generally, such agreements are related to our research and development efforts or general operating matters. The above table should be read in conjunction with our consolidated financial statements, which illustrate a 2007 net loss of $35.6 million and net cash used in operations of $29.7 million during 2007. |
| |
| *** | | In August 2006, we paid $2.7 million in cash to acquire a 57% interest in Agera and also we agreed to contribute $0.3 million to the working capital of Agera to support marketing and inventory acquisitions. In addition, the acquisition agreement includes future contingent payments up to a maximum of $8.0 million. Such additional purchase price is based upon certain percentages of Agera’s cost of sales incurred after June 30, 2007. Accordingly, based upon the financial performance of Agera, up to an additional $8.0 million of purchase price may be due to the selling shareholder in future periods. We believe any such payments will not be significant in fiscal year 2007. The timing of future payments may differ from the estimates above, and will depend on the future operating performance of Agera. |
50
In November 2004, we issued $90.0 million in principal amount of 3.5% convertible subordinated notes due November 1, 2024, although these notes may be due sooner as discussed below. The notes are our general, unsecured obligations. The notes are subordinated in right of payment, which means that they rank in right of payment behind other indebtedness of ours. In addition, the notes are effectively subordinated to all existing and future liabilities of our subsidiaries. We will be required to repay the full principal amount of the notes on November 1, 2024 unless they are previously converted, redeemed or repurchased.
The notes bear interest at an annual rate of 3.5% from the date of issuance of the notes. We will pay interest twice a year, on each May 1 and November 1, until the principal is paid or made available for payment or the notes have been converted. Interest will be calculated on the basis of a 360-day year consisting of twelve 30-day months.
The note holders may convert the notes into shares of our common stock at any time before the close of business on November 1, 2024, unless the notes have been previously redeemed or repurchased. The initial conversion rate (which is subject to adjustment) for the notes is 109.2001 shares of common stock per $1,000 principal amount of notes, which is equivalent to an initial conversion price of approximately $9.16 per share. Holders of notes called for redemption or submitted for repurchase will be entitled to convert the notes up to and including the business day immediately preceding the date fixed for redemption or repurchase.
At any time on or after November 1, 2009, we may redeem some or all of the notes at a redemption price equal to 100% of the principal amount of such notes plus accrued and unpaid interest (including additional interest, if any) to, but excluding, the redemption date.
The note holders will have the right to require us to repurchase their notes on November 1 of 2009, 2014 and 2019. In addition, if we experience a fundamental change (which generally will be deemed to occur upon the occurrence of a change in control or a termination of trading of our common stock), note holders will have the right to require us to repurchase their notes. In the event of certain fundamental changes that occur on or prior to November 1, 2009, we will also pay a make-whole premium to holders that require us to purchase their notes in connection with such fundamental change.
Off-Balance Sheet Transactions
We do not engage in material off-balance sheet transactions.
Other
INFLATION. Inflation did not have a significant impact on our results for year ended December 31, 2007.
51
| | |
| Item 7A. | | Quantitative and Qualitative Disclosures About Market Risk |
Market risk is the potential loss arising from adverse changes in market rates and prices, such as foreign currency exchange rates or interest rates. We are exposed to market risk in the form of foreign exchange rate risk and interest rate risk.
Foreign Exchange Rate Risk
The effect of U.S. dollar currency fluctuations against the foreign currency in these countries is somewhat mitigated by the fact that expenses are generally incurred in the same currencies in which the revenue is generated. Our income will be higher or lower depending on the weakening or strengthening of the U.S. dollar against the respective foreign currency. Additionally, approximately 29% of our assets at December 31, 2007 (see Note 5 to our Consolidated Financial Statements, “Discontinued Operations”) were based in our foreign operations and translated into U.S. dollars at the foreign currency exchange rate in effect as of the end of each accounting period, with the effect of such translation reflected as a separate component of consolidated shareholders’ deficit. Substantially all of the 28% in foreign assets relates to our Swiss campus held for sale, with a value of $11.2 million at December 31, 2007. Accordingly, our consolidated shareholders’ deficit will fluctuate depending on the weakening or strengthening of the U.S. dollar against the respective foreign currency.
As a result of changing foreign currency exchange rates since December 31, 2006, specifically the exchange rate between the US dollar and the Swiss franc, our accumulated other comprehensive loss of $0.1 million at December 31, 2006 has swung to an accumulated other comprehensive gain of $0.7 million at December 31, 2007; or a change of approximately $0.8 million. However, this $0.8 million gain is considered unrealized and is reflected on the Consolidated Balance Sheet. Accordingly, this unrealized gain may increase or decrease in the future, based on the movement of foreign currency exchange rates, but will not have an impact on net income (loss) until the related foreign capital investments are sold or otherwise realized.
Interest Rate Risk
Our 3.5%, $90.0 million convertible subordinated notes, pay interest at a fixed rate and, accordingly, we are not exposed to interest rate risk as a result of this debt. However, the fair value of our $90.0 million convertible subordinated notes does vary based upon, among other factors, the price of our common stock and current interest rates on similar instruments.
We do not enter into derivatives or other financial instruments for trading or speculative purposes.
| | |
| Item 8. | | Financial Statements and Supplementary Data |
The financial statements, including the notes thereto and report of the independent auditors thereon, are included in this report as set forth in the “Index to Financial Statements.” See F-1 for Index to Consolidated Financial Statements.
52
| | |
| Item 9. | | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
None.
| | |
| Item 9A. | | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
During the fourth quarter of 2007, management, including our principal executive officer and principal financial officer, evaluated the disclosure controls and procedures related to the recording, processing, summarization and reporting of information in the periodic reports that the Company files with the SEC. These disclosure controls and procedures have been designed to ensure that (a) material information relating to the Company, including its consolidated subsidiaries, is made known to management, including these officers, by other employees of the Company, and (b) this information is recorded, processed, summarized, evaluated and reported, as applicable, within the time periods specified in the SEC’s rules and forms.
Accordingly, as of December 31, 2007, these officers (the principal executive officer and principal financial officer) concluded that the Company’s disclosure controls and procedures were effective.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission.
Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Our internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Based on our evaluation under the framework inInternal Control — Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2007.
BDO Seidman, LLP, the independent registered public accounting firm who also audited our consolidated financial statements, has issued its own attestation report on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2007, which is filed herewith.
53
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Isolagen, Inc. (a development stage company)
Exton, Pennsylvania
We have audited Isolagen, Inc.’s (in the development stage) internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Isolagen, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Management’s Report on Internal Control Over Financial Reporting”. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Isolagen, Inc., maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Isolagen, Inc. as of December 31, 2007 and 2006, and the related consolidated statements of operations and comprehensive loss, shareholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2007 and the statements of shareholders’ equity (deficit) for the period from December 28, 1995 (inception) to December 31, 2004, and our report dated March 5, 2008 contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
/s/ BDO Seidman, LLP
Houston, Texas
March 5, 2008
Changes in Internal Controls
There was no change in our internal control over financial reporting that occurred during the fourth fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| | |
| Item 9B. | | Other Information |
None.
54
Part III
| | |
| Item 10. | | Directors, Executive Officers and Corporate Governance |
The information required by this Item 10 will be included in the Company’s Proxy Statement for the 2008 Annual Meeting of Stockholders which will be filed with the Securities and Exchange Commission no later than April 29, 2008 and is incorporated into this Item 10 by reference.
Code of Ethics.We have adopted a written code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller and any persons performing similar functions. The code of ethics is incorporated into this Item 10 by reference.
| | |
| Item 11. | | Executive Compensation |
The information required by this Item 11 will be included in the Company’s Proxy Statement for the 2008 Annual Meeting of Stockholders which will be filed with the Securities and Exchange Commission no later than April 29, 2008 and is incorporated into this Item 11 by reference.
| | |
| Item 12. | | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Except as provided below, the information required by this Item 12 will be included in the Company’s Proxy Statement for the 2008 Annual Meeting of Stockholders which will be filed with the Securities and Exchange Commission no later than April 29, 2008 and is incorporated into this Item 12 by reference.
Securities Authorized for Issuance Under Equity Compensation Plans
As of December 31, 2007, our equity compensation plan information was as follows:
| | | | | | | | | | | | | |
| | | Number of Securities | | | | |
| | | to be issued upon | | Weighted-average | | Number of |
| | | exercise of | | exercise price of | | securities remaining |
| | | outstanding options | | outstanding options | | for future issuance |
| Equity compensation plans approved by security holders | | | 4,155,666 | | | $ | 4.34 | | | | 4,486,320 | |
| Equity compensation plans not approved by security holders (1) | | | 3,568,333 | | | $ | 2.42 | | | — |
| | | | | | | | | | | | | |
| Total | | | 7,723,999 | | | $ | 3.45 | | | | 4,486,320 | |
| | | | | | | | | | | | | |
| | | |
| (1) | | Represents options issued to employees, in connection with initial employment, outside of our approved plans. |
| | |
| Item 13. | | Certain Relationships and Related Transactions, and Director Independence |
The information required by this Item 13 will be included in the Company’s Proxy Statement for the 2008 Annual Meeting of Stockholders which will be filed with the Securities and Exchange Commission no later than April 29, 2008 and is incorporated into this Item 13 by reference.
| | |
| Item 14. | | Principal Accountant Fees and Services |
The information required by this Item 14 will be included in the Company’s Proxy Statement for the 2008 Annual Meeting of Stockholders which will be filed with the Securities and Exchange Commission no later than April 29, 2008 and is incorporated into this Item 14 by reference.
55
Part IV
| | |
| Item 15. | | Exhibits and Financial Statement Schedule |
(a)(1) Financial Statements.
| | • | | Report of Independent Registered Public Accounting Firm |
| |
| | • | | Consolidated Balance Sheets as of December 31, 2007 and 2006 |
| |
| | • | | Consolidated Statements of Operations for the years ended December 31, 2007, 2006, and 2005 and inception to December 31, 2007 |
| |
| | • | | Consolidated Statements of Shareholders’ Equity and Comprehensive Loss from inception to December 31, 2007 |
| |
| | • | | Consolidated Statements of Cash Flows for the years ended December 31, 2007, 2006 and 2005 and inception to December 31, 2007 |
| |
| | • | | Notes to Consolidated Financial Statements |
(a)(2) Financial Statement Schedule.
All schedules are omitted because of the absence of conditions under which they are required or because the required information is presented in the Financial Statements or Notes thereto.
(a)(3) The exhibits listed under Item 15(b) are filed or incorporated by reference herein.
56
(b) Exhibits.
The following exhibits are filed as part of this annual report:
| | | |
| EXHIBIT | | |
| NO. | | IDENTIFICATION OF EXHIBIT |
| 2 | | Agreement and Plan of Merger by and among American Financial Holding, Inc., ISO Acquisition Corp., Isolagen Technologies, Inc., Gemini IX, Inc., and William K. Boss, Jr., Olga Marko and Dennis McGill dated August 1, 2001(1) |
| 3(i) | | Amended Certificate of Incorporation(17) |
| 3(ii) | | Third Amended and Restated Bylaws(25) |
| 4.1 | | Specimen of Common Stock certificate(2) |
| 4.2 | | Certificate of Designations of Series A Convertible Preferred Stock(7) |
| 4.3 | | Certificate of Designations of Series B Convertible Preferred Stock(5) |
| 4.4 | | Indenture, dated November 3, 2004, between the Company and The Bank of New York Trust Company, N.A., as trustee(11) |
| 4.5 | | Rights Agreement, dated as of May 12, 2006, by and between the registrant and American Stock Transfer & Trust Company, including the Form of Certificate of Designation, Preferences and Rights of Series C Junior Participating Preferred Stock attached as Exhibit A thereto, the Form of Rights Certificate attached as Exhibit B thereto and the Summary of Rights to Purchase Preferred Stock attached as Exhibit C thereto. (21) |
| 10.1 | | 2003 Stock Option and Stock Appreciation Rights Plan(3)* |
| 10.2 | | 2001 Stock Option and Appreciation Rights Plan(4)* |
| 10.3 | | Lease Agreement dated March 24, 2002 by and between the Registrant as Lessee and Claire O Aceti Gbmh asLessor(7) |
| 10.4 | | Intellectual Property Purchase Agreement between Isolagen Technologies, Inc., Gregory M. Keller, and PacGen Partners(8) |
| 10.5 | | Purchase Agreement among CIBC World Market Corp., UBS Securities LLC, and Adams, Harkness & Hill, Inc. dated October 28, 2004(11) |
| 10.6 | | Registration Rights Agreement among CIBC World Market Corp., UBS Securities LLC, and Adams, Harkness & Hill, Inc. dated November 3, 2004(11) |
| 10.7 | | Lease Agreement between Isolagen Technologies, Inc. and Beltway 8 Service Center Investors Ltd. dated February 16, 2005(13) |
| 10.8 | | Lease Agreement between Isolagen, Inc and The Hankin Group dates April 7, 2005(15) |
| 10.9 | | Purchase Option Agreement between Isolagen, Inc and 405 Eagleview Associates dated April 7, 2005(15) |
| 10.10 | | 2005 Equity Incentive Plan, as amended(18) |
| 10.11 | | Separation and Release Agreement, dated October 27, 2005, among Isolagen, Inc., Isolagen Technologies, Inc. and Frank DeLape(19) |
| 10.12 | | Amended Employment Agreement between Isolagen, Inc. and Susan Ciallella(20)* |
| 10.13 | | Employment Agreement between Isolagen, Inc. and Todd Greenspan(20)* |
| 10.14 | | Employment Agreement dated June 5, 2006 between Isolagen, Inc. and Nicholas L. Teti(22)* |
| 10.15 | | Employment Agreement dated March 12, 2007 between Isolagen, Inc. and Declan Daly(23)* |
| 10.16 | | Employment Agreement dated March 12, 2007 between Isolagen, Inc. and Steven Trider(23)* |
| 10.17 | | Settlement Agreement and Release between Susan Stranahan Ciallella and Isolagen, Inc. dated June 8, 2007 (24) |
| 10.18 | | Consulting and Non-Competition Agreement dated January 7, 2008 between Isolagen, Inc. and Nicholas L.Teti * (26) |
| 10.19 | | Employment Agreement dated January 7, 2008 between Isolagen, Inc. and Declan Daly * (26) |
| 14 | | Code of Ethics(9) |
| 21 | | List of Subsidiaries(23) |
| 23 | | BDO Seidman, LLP Consent(26) |
| 31 | | Certification pursuant to Rule 13a-14(a) and 15d-14(a), required under Section 302 of the Sarbanes-Oxley Act of2002(26) |
| 32 | | Certification pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002(26) |
57
| | | |
| * | | Indicates a management contract or a compensatory plan or arrangement. |
| |
| (1) | | Previously filed as an exhibit to the company’s Form 8-K, filed on August 22, 2001, and is incorporated by reference hereto. |
| |
| (2) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-KSB for the fiscal year ended December 31, 2001, and is incorporated by reference hereto. |
| |
| (3) | | Previously filed as an appendix to the company’s Definitive Proxy Statement, as filed on May 6, 2003, in connection with the 2003 Annual Stockholder Meeting, and is incorporated by reference hereto. |
| |
| (4) | | Previously filed as an appendix to the company’s Definitive Proxy Statement, as filed on October 23, 2001, in connection with the 2001 Annual Stockholder Meeting, and is incorporated by reference hereto. |
| |
| (5) | | Previously filed as an exhibit to the company’s Form 10-Q for the fiscal quarter ended March 31, 2003, as filed on May 15, 2003, and is incorporated by reference hereto. |
| |
| (6) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-KSB for the fiscal year ended December 31, 2002, and is incorporated by reference hereto. |
| |
| (7) | | Previously filed as an exhibit to the company’s Form S-1, as filed on September 12, 2003, and is incorporated by reference hereto. |
| |
| (8) | | Previously filed as an exhibit to the company’s amended Form S-1, as filed on October 24, 2003, and is incorporated by reference hereto. |
| |
| (9) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2003, and is incorporated by reference hereto. |
| |
| (10) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-K/A for the fiscal year ended December 31, 2003, and is incorporated by reference hereto. |
| |
| (11) | | Previously filed as an exhibit to the company’s Current Report on Form 8-K dated November 4, 2004, and is incorporated by reference hereto. |
| |
| (12) | | Reserved. |
| |
| (13) | | Previously filed as an exhibit to the company’s Form 8-K, filed on February 23, 2005, and is incorporated by reference hereto. |
| |
| (14) | | Reserved. |
| |
| (15) | | Previously filed as an exhibit to the company’s Form 8-K, filed on April 12, 2005, and is incorporated by reference hereto. |
| |
| (16) | | Reserved. |
| |
| (17) | | Previously filed as an exhibit to the company’s Form 10-Q for the fiscal quarter ended June 30, 2005, as filed on August 9, 2005, and is incorporated by reference hereto. |
| |
| (18) | | Previously filed as an exhibit to the company’s Form S-8, filed on February 13, 2006, and is incorporated by reference hereto. |
| |
| (19) | | Previously filed as an exhibit to the company’s Form 8-K, filed on November 2, 2005, and is incorporated by reference hereto. |
| |
| (20) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2005, and is incorporated by reference hereto. |
| |
| (21) | | Previously filed as an exhibit to the company’s Form 8-K, filed on May 15, 2006, and is incorporated by reference hereto. |
| |
| (22) | | Previously filed as an exhibit to the company’s Form 8-K, filed on June 9, 2006, and is incorporated by reference hereto. |
| |
| (23) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2007, and is incorporated by reference hereto. |
| |
| (24) | | Previously filed as an exhibit to the company’s Form 8-K, filed on June 13, 2007, and is incorporated by reference hereto. |
| |
| (25) | | Previously filed as an exhibit to the company’s Form 8-K, filed on January 8, 2007, and is incorporated by reference hereto. |
| |
| (26) | | Filed herewith. |
58
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
Isolagen, Inc.
| | |
| By: | /s/ Declan Daly | | |
| | Declan Daly, Chief Executive Officer &
Chief Financial Officer | | |
| | | | |
| |
Date: March 6, 2008
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the date indicated.
| | | | | |
| Signature | | Title | | Date |
| | | | | |
/s/ Nicholas L. Teti Nicholas L. Teti | | Chairman of the Board of Directors | | March 6, 2008 |
| /s/ Declan Daly Declan Daly | | Chief Executive Officer and Chief Financial Officer | | March 6, 2008 |
| /s/ Todd J. Greenspan Todd J. Greenspan | | Vice President of Finance and Administration, Corporate Controller | | March 6, 2008 |
| /s/ Steven Morrell Steven Morrell | | Director | | March 6, 2008 |
| /s/ Henry Toh Henry Toh | | Director | | March 6, 2008 |
| /s/ Ralph De Martino Ralph De Martino | | Director | | March 6, 2008 |
| /s/ Marshall G. Webb Marshall G. Webb | | Director | | March 6, 2008 |
| /s/ Terry E. Vandewarker Terry E. Vandewarker | | Director | | March 6, 2008 |
| /s/ Kenneth A. Selzer Kenneth A. Selzer | | Director | | March 6, 2008 |
59
Isolagen, Inc.
(A Development Stage Company)
Index to Consolidated Financial Statements
| | | |
| | | PAGE |
| | | |
| | F-2 |
| | | |
| | F-3 |
| | | |
| | F-4 |
| | | |
| | F-5-13 |
| | | |
| | F-14 |
| | | |
| | F-15-46 |
| | | |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Isolagen, Inc. (a development stage company)
Exton, Pennsylvania
We have audited the accompanying consolidated balance sheets of Isolagen, Inc. (in the development stage) as of December 31, 2007 and 2006 and the related consolidated statements of operations and comprehensive loss, shareholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2007. We have also audited the statements of shareholders’ equity (deficit) for the period from December 28, 1995 (inception) to December 31, 2004. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Isolagen, Inc. at December 31, 2007 and 2006, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2007 and the statements of shareholders’ equity (deficit) for the period from December 28, 1995 (inception) to December 31, 2007 in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Isolagen, Inc.’s internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 5, 2008 expressed an unqualified opinion thereon.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficit that raise substantial doubt about its ability to continue as a going concern. Management’s plan in regard to these matters is also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ BDO Seidman, LLP
Houston, Texas
March 5, 2008
F-2
Isolagen, Inc.
(A Development Stage Company)
Consolidated Balance Sheets
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
Assets | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 16,590,720 | | | $ | 31,783,545 | |
| Restricted cash | | | 451,382 | | | | 1,483,197 | |
| Accounts receivable, net | | | 319,674 | | | | 81,502 | |
| Inventory | | | 669,119 | | | | 181,865 | |
| Other receivables | | | 29,250 | | | | 357 | |
| Prepaid expenses | | | 701,214 | | | | 860,244 | |
| Current assets of discontinued operations, net | | | 14,515 | | | | 739,009 | |
| | | | | | | |
| Total current assets | | | 18,775,874 | | | | 35,129,719 | |
| | | | | | | |
| Property and equipment, net of accumulated depreciation and amortization of $2,921,651 and $1,816,125, respectively | | | 3,395,723 | | | | 4,331,605 | |
| Intangibles, net of amortization of $718,762 and $381,795, respectively | | | 4,599,538 | | | | 4,936,505 | |
| Other assets, net of amortization of $2,372,589 and $1,623,352, respectively | | | 1,424,456 | | | | 2,204,685 | |
| Assets of discontinued operations held for sale | | | 11,202,725 | | | | 10,503,234 | |
| Other long-term assets of discontinued operations | | | 92,874 | | | | 181,127 | |
| | | | | | | |
| Total assets | | $ | 39,491,190 | | | $ | 57,286,875 | |
| | | | | | | |
| | | | | | | | | |
Liabilities, Minority Interests and Shareholders’ Deficit | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 403,815 | | | $ | 886,598 | |
| Accrued expenses | | | 4,348,256 | | | | 2,886,041 | |
| Deferred revenue | | | — | | | | 5,421 | |
| Current liabilities of discontinued operations | | | 217,822 | | | | 1,863,857 | |
| | | | | | | |
| Total current liabilities | | | 4,969,893 | | | | 5,641,917 | |
| Long term debt | | | 90,000,000 | | | | 90,000,000 | |
| Other long term liabilities of continuing operations | | | 1,206,721 | | | | 999,940 | |
| Long term liabilities of discontinued operations | | | 107,511 | | | | 164,227 | |
| | | | | | | |
| Total liabilities | | | 96,284,125 | | | | 96,806,084 | |
| | | | | | | |
| Commitments and contingencies (see Note 10) | | | | | | | | |
| Minority interests | | | 1,858,026 | | | | 2,104,373 | |
| | | | | | | |
| | | | | | | | | |
| Shareholders’ deficit: | | | | | | | | |
| Preferred stock, $.001 par value; 5,000,000 shares authorized | | | — | | | | — | |
| Series C junior participating preferred stock, $.001 par value; 10,000 shares authorized | | | — | | | | — | |
| Common stock, $.001 par value; 100,000,000 shares authorized | | | 41,640 | | | | 34,363 | |
| Additional paid-in capital | | | 129,208,631 | | | | 111,516,561 | |
| Treasury stock, at cost, 4,000,000 shares | | | (25,974,000 | ) | | | (25,974,000 | ) |
| Accumulated other comprehensive income (loss) | | | 718,926 | | | | (127,462 | ) |
| Accumulated deficit during development stage | | | (162,646,158 | ) | | | (127,073,044 | ) |
| | | | | | | |
| Total shareholders’ deficit | | | (58,650,961 | ) | | | (41,623,582 | ) |
| | | | | | | |
| Total liabilities, minority interests and shareholders’ deficit | | $ | 39,491,190 | | | $ | 57,286,875 | |
| | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Isolagen, Inc.
(A Development Stage Company)
Consolidated Statements of Operations
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Cumulative Period | |
| | | | | | | | | | | | | | | from December 28, | |
| | | | | | | | | | | | | | | 1995 (date of | |
| | | | | | | | | | | | | | | inception) to | |
| | | For the Year Ended December 31, | | | December 31, 2007 | |
| | | 2007 | | | 2006 | | | 2005 | | | (Unaudited) | |
| Revenue | | | | | | | | | | | | | | | | |
| Product sales | | $ | 1,400,986 | | | $ | 384,389 | | | $ | — | | | $ | 3,175,489 | |
| License fees | | | — | | | | — | | | | — | | | | 260,000 | |
| | | | | | | | | | | | | |
| Total revenue | | | 1,400,986 | | | | 384,389 | | | | — | | | | 3,435,489 | |
| Cost of sales | | | 656,029 | | | | 194,197 | | | | — | | | | 1,252,685 | |
| | | | | | | | | | | | | |
| Gross profit | | | 744,957 | | | | 190,192 | | | | — | | | | 2,182,804 | |
| Selling, general and administrative expenses | | | 18,730,863 | | | | 13,174,960 | | | | 14,000,977 | | | | 66,146,085 | |
| Research and development | | | 13,298,338 | | | | 8,796,219 | | | | 10,592,170 | | | | 43,989,034 | |
| | | | | | | | | | | | | |
| Operating loss | | | (31,284,244 | ) | | | (21,780,987 | ) | | | (24,593,147 | ) | | | (107,952,315 | ) |
| Other income (expense) | | | | | | | | | | | | | | | | |
| Interest income | | | 901,262 | | | | 2,261,899 | | | | 2,808,328 | | | | 6,807,777 | |
| Other income | | | 150,138 | | | | — | | | | — | | | | 322,581 | |
| Interest expense | | | (3,899,239 | ) | | | (3,899,239 | ) | | | (3,912,059 | ) | | | (12,658,841 | ) |
| Minority interest | | | 246,347 | | | | 78,132 | | | | — | | | | 324,479 | |
| | | | | | | | | | | | | |
| Loss before income taxes from continuing operations | | | (33,885,736 | ) | | | (23,340,195 | ) | | | (25,696,878 | ) | | | (113,156,319 | ) |
| Income tax benefit | | | — | | | | 190,754 | | | | — | | | | 190,754 | |
| | | | | | | | | | | | | |
| Loss from continuing operations | | | (33,885,736 | ) | | | (23,149,441 | ) | | | (25,696,878 | ) | | | (112,965,565 | ) |
| Loss from discontinued operations, net of tax | | | (1,687,378 | ) | | | (12,671,965 | ) | | | (10,080,706 | ) | | | (36,666,908 | ) |
| | | | | | | | | | | | | |
| Net loss | | | (35,573,114 | ) | | | (35,821,406 | ) | | | (35,777,584 | ) | | | (149,632,473 | ) |
| Deemed dividend associated with beneficial conversion of preferred stock | | | — | | | | — | | | | — | | | | (11,423,824 | ) |
| Preferred stock dividends | | | — | | | | — | | | | — | | | | (1,589,861 | ) |
| | | | | | | | | | | | | |
| Net loss attributable to common shareholders | | $ | (35,573,114 | ) | | $ | (35,821,406 | ) | | $ | (35,777,584 | ) | | $ | (162,646,158 | ) |
| | | | | | | | | | | | | |
| Per share information: | | | | | | | | | | | | | | | | |
| Loss from continuing operations — basic and diluted | | $ | (1.02 | ) | | $ | (0.76 | ) | | $ | (0.85 | ) | | $ | (7.59 | ) |
| Loss from discontinued operations — basic and diluted | | | (0.05 | ) | | | (0.42 | ) | | | (0.33 | ) | | | (2.47 | ) |
| Deemed dividend associated with beneficial conversion of preferred stock | | | — | | | | — | | | | — | | | | (0.77 | ) |
| Preferred stock dividends | | | — | | | | — | | | | — | | | | (0.11 | ) |
| | | | | | | | | | | | | |
| Net loss per common share—basic and diluted | | $ | (1.07 | ) | | $ | (1.18 | ) | | $ | (1.18 | ) | | $ | (10.94 | ) |
| | | | | | | | | | | | | |
| Weighted average number of basic and diluted common shares outstanding | | | 33,093,370 | | | | 30,309,439 | | | | 30,245,283 | | | | 14,873,948 | |
| | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Isolagen, Inc.
(A Development Stage Company)
Consolidated Statements of Shareholders’ Equity (Deficit) and Comprehensive Loss
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | Series A | | | Series B | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | Deficit | | | Total | |
| | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Additional | | | Treasury Stock | | | Other | | | During | | | Shareholders’ | |
| | | Number of | | | | | | | Number of | | | | | | | Number of | | | | | | | Paid-In | | | Number of | | | | | | | Comprehensive | | | Development | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Shares | | | Amount | | | Income | | | Stage | | | (Deficit) | |
| Issuance of common stock for cash on 12/28/95 | | | — | | | $ | — | | | | — | | | $ | — | | | | 2,285,291 | | | $ | 2,285 | | | $ | (1,465 | ) | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 820 | |
| Issuance of common stock for cash on 11/7/96 | | | — | | | | — | | | | — | | | | — | | | | 11,149 | | | | 11 | | | | 49,989 | | | | — | | | | — | | | | — | | | | — | | | | 50,000 | |
| Issuance of common stock for cash on 11/29/96 | | | — | | | | — | | | | — | | | | — | | | | 2,230 | | | | 2 | | | | 9,998 | | | | — | | | | — | | | | — | | | | — | | | | 10,000 | |
| Issuance of common stock for cash on 12/19/96 | | | — | | | | — | | | | — | | | | — | | | | 6,690 | | | | 7 | | | | 29,993 | | | | — | | | | — | | | | — | | | | — | | | | 30,000 | |
| Issuance of common stock for cash on 12/26/96 | | | — | | | | — | | | | — | | | | — | | | | 11,148 | | | | 11 | | | | 49,989 | | | | — | | | | — | | | | — | | | | — | | | | 50,000 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (270,468 | ) | | | (270,468 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, 12/31/96 | | | — | | | $ | — | | | | — | | | $ | — | | | | 2,316,508 | | | $ | 2,316 | | | $ | 138,504 | | | | — | | | $ | — | | | $ | — | | | $ | (270,468 | ) | | $ | (129,648 | ) |
| Issuance of common stock for cash on 12/27/97 | | | — | | | | — | | | | — | | | | — | | | | 21,182 | | | | 21 | | | | 94,979 | | | | — | | | | — | | | | — | | | | — | | | | 95,000 | |
| Issuance of common stock for services on 9/1/97 | | | — | | | | — | | | | — | | | | — | | | | 11,148 | | | | 11 | | | | 36,249 | | | | — | | | | — | | | | — | | | | — | | | | 36,260 | |
| Issuance of common stock for services on 12/28/97 | | | — | | | | — | | | | — | | | | — | | | | 287,193 | | | | 287 | | | | 9,968 | | | | — | | | | — | | | | — | | | | — | | | | 10,255 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (52,550 | ) | | | (52,550 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, 12/31/97 | | | — | | | $ | — | | | | — | | | $ | — | | | | 2,636,031 | | | $ | 2,635 | | | $ | 279,700 | | | | — | | | $ | — | | | $ | — | | | $ | (323,018 | ) | | $ | (40,683 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | Series A | | | Series B | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | Deficit | | | Total | |
| | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Additional | | | Treasury Stock | | | Other | | | During | | | Shareholders’ | |
| | | Number of | | | | | | | Number of | | | | | | | Number of | | | | | | | Paid-In | | | Number of | | | | | | | Comprehensive | | | Development | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Shares | | | Amount | | | Income | | | Stage | | | (Deficit) | |
| Issuance of common stock for cash on 8/23/98 | | | — | | | $ | — | | | | — | | | $ | — | | | | 4,459 | | | $ | 4 | | | $ | 20,063 | | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 20,067 | |
| Repurchase of common stock on 9/29/98 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,400 | | | | (50,280 | ) | | | — | | | | — | | | | (50,280 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (195,675 | ) | | | (195,675 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, 12/31/98 | | | — | | | $ | — | | | | — | | | $ | — | | | | 2,640,490 | | | $ | 2,639 | | | $ | 299,763 | | | | 2,400 | | | $ | (50,280 | ) | | $ | — | | | $ | (518,693 | ) | | $ | (266,571 | ) |
| Issuance of common stock for cash on 9/10/99 | | | — | | | | — | | | | — | | | | — | | | | 52,506 | | | | 53 | | | | 149,947 | | | | — | | | | — | | | | — | | | | — | | | | 150,000 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1,306,778 | ) | | | (1,306,778 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, 12/31/99 | | | — | | | $ | — | | | | — | | | $ | — | | | | 2,692,996 | | | $ | 2,692 | | | $ | 449,710 | | | | 2,400 | | | $ | (50,280 | ) | | $ | — | | | $ | (1,825,471 | ) | | $ | (1,423,349 | ) |
| Issuance of common stock for cash on 1/18/00 | | | — | | | | — | | | | — | | | | — | | | | 53,583 | | | | 54 | | | | 1,869 | | | | — | | | | — | | | | — | | | | — | | | | 1,923 | |
| Issuance of common stock for services on 3/1/00 | | | — | | | | — | | | | — | | | | — | | | | 68,698 | | | | 69 | | | | (44 | ) | | | — | | | | — | | | | — | | | | — | | | | 25 | |
| Issuance of common stock for services on 4/4/00 | | | — | | | | — | | | | — | | | | — | | | | 27,768 | | | | 28 | | | | (18 | ) | | | — | | | | — | | | | — | | | | — | | | | 10 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (807,076 | ) | | | (807,076 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, 12/31/00 | | | — | | | $ | — | | | | — | | | $ | — | | | | 2,843,045 | | | $ | 2,843 | | | $ | 451,517 | | | | 2,400 | | | $ | (50,280 | ) | | $ | — | | | $ | (2,632,547 | ) | | $ | (2,228,467 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | Series A | | | Series B | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | Deficit | | | Total | |
| | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Additional | | | Treasury Stock | | | Other | | | During | | | Shareholders’ | |
| | | Number of | | | | | | | Number of | | | | | | | Number of | | | | | | | Paid-In | | | Number of | | | | | | | Comprehensive | | | Development | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Shares | | | Amount | | | Income | | | Stage | | | (Deficit) | |
| Issuance of common stock for services on 7/1/01 | | | — | | | $ | — | | | | — | | | $ | — | | | | 156,960 | | | $ | 157 | | | $ | (101 | ) | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 56 | |
| Issuance of common stock for services on 7/1/01 | | | — | | | | — | | | | — | | | | — | | | | 125,000 | | | | 125 | | | | (80 | ) | | | — | | | | — | | | | — | | | | — | | | | 45 | |
| Issuance of common stock for capitalization of accrued salaries on 8/10/01 | | | — | | | | — | | | | — | | | | — | | | | 70,000 | | | | 70 | | | | 328,055 | | | | — | | | | — | | | | — | | | | — | | | | 328,125 | |
| Issuance of common stock for conversion of convertible debt on 8/10/01 | | | — | | | | — | | | | — | | | | — | | | | 1,750,000 | | | | 1,750 | | | | 1,609,596 | | | | — | | | | — | | | | — | | | | — | | | | 1,611,346 | |
| Issuance of common stock for conversion of convertible shareholder notes payable on 8/10/01 | | | — | | | | — | | | | — | | | | — | | | | 208,972 | | | | 209 | | | | 135,458 | | | | — | | | | — | | | | — | | | | — | | | | 135,667 | |
| Issuance of common stock for bridge financing on 8/10/01 | | | — | | | | — | | | | — | | | | — | | | | 300,000 | | | | 300 | | | | (192 | ) | | | — | | | | — | | | | — | | | | — | | | | 108 | |
| Retirement of treasury stock on 8/10/01 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (50,280 | ) | | | (2,400 | ) | | | 50,280 | | | | — | | | | — | | | | — | |
| Issuance of common stock for net assets of Gemini on 8/10/01 | | | — | | | | — | | | | — | | | | — | | | | 3,942,400 | | | | 3,942 | | | | (3,942 | ) | | | — | | | | — | | | | — | | | | — | | | | — | |
| Issuance of common stock for net assets of AFH on 8/10/01 | | | — | | | | — | | | | — | | | | — | | | | 3,899,547 | | | | 3,900 | | | | (3,900 | ) | | | — | | | | — | | | | — | | | | — | | | | — | |
| Issuance of common stock for cash on 8/10/01 | | | — | | | | — | | | | — | | | | — | | | | 1,346,669 | | | | 1,347 | | | | 2,018,653 | | | | — | | | | — | | | | — | | | | — | | | | 2,020,000 | |
| Transaction and fund raising expenses on 8/10/01 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (48,547 | ) | | | — | | | | — | | | | — | | | | — | | | | (48,547 | ) |
| Issuance of common stock for services on 8/10/01 | | | — | | | | — | | | | — | | | | — | | | | 60,000 | | | | 60 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 60 | |
| Issuance of common stock for cash on 8/28/01 | | | — | | | | — | | | | — | | | | — | | | | 26,667 | | | | 27 | | | | 39,973 | | | | — | | | | — | | | | — | | | | — | | | | 40,000 | |
| Issuance of common stock for services on 9/30/01 | | | — | | | | — | | | | — | | | | — | | | | 314,370 | | | | 314 | | | | 471,241 | | | | — | | | | — | | | | — | | | | — | | | | 471,555 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | Series A | | | Series B | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | Deficit | | | Total | |
| | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Additional | | | Treasury Stock | | | Other | | | During | | | Shareholders’ | |
| | | Number of | | | | | | | Number of | | | | | | | Number of | | | | | | | Paid-In | | | Number of | | | | | | | Comprehensive | | | Development | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Shares | | | Amount | | | Income | | | Stage | | | (Deficit) | |
| Uncompensated contribution of services—3rd quarter | | | — | | | $ | — | | | | — | | | $ | — | | | | — | | | $ | — | | | $ | 55,556 | | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 55,556 | |
| Issuance of common stock for services on 11/1/01 | | | — | | | | — | | | | — | | | | — | | | | 145,933 | | | | 146 | | | | 218,754 | | | | — | | | | — | | | | — | | | | — | | | | 218,900 | |
| Uncompensated contribution of services—4th quarter | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1,652,004 | ) | | | (1,652,004 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, 12/31/01 | | | — | | | $ | — | | | | — | | | $ | — | | | | 15,189,563 | | | $ | 15,190 | | | $ | 5,321,761 | | | | — | | | $ | — | | | $ | — | | | $ | (4,284,551 | ) | | $ | 1,052,400 | |
| Uncompensated contribution of services—1st quarter | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | |
| Issuance of preferred stock for cash on 4/26/02 | | | 905,000 | | | | 905 | | | | — | | | | — | | | | — | | | | — | | | | 2,817,331 | | | | — | | | | — | | | | — | | | | — | | | | 2,818,236 | |
| Issuance of preferred stock for cash on 5/16/02 | | | 890,250 | | | | 890 | | | | — | | | | — | | | | — | | | | — | | | | 2,772,239 | | | | — | | | | — | | | | — | | | | — | | | | 2,773,129 | |
| Issuance of preferred stock for cash on 5/31/02 | | | 795,000 | | | | 795 | | | | — | | | | — | | | | — | | | | — | | | | 2,473,380 | | | | — | | | | — | | | | — | | | | — | | | | 2,474,175 | |
| Issuance of preferred stock for cash on 6/28/02 | | | 229,642 | | | | 230 | | | | — | | | | — | | | | — | | | | — | | | | 712,991 | | | | — | | | | — | | | | — | | | | — | | | | 713,221 | |
| Uncompensated contribution of services—2nd quarter | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | |
| Issuance of preferred stock for cash on 7/15/02 | | | 75,108 | | | | 75 | | | | — | | | | — | | | | — | | | | — | | | | 233,886 | | | | — | | | | — | | | | — | | | | — | | | | 233,961 | |
| Issuance of common stock for cash on 8/1/02 | | | — | | | | — | | | | — | | | | — | | | | 38,400 | | | | 38 | | | | 57,562 | | | | — | | | | — | | | | — | | | | — | | | | 57,600 | |
| Issuance of warrants for services on 9/06/02 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 103,388 | | | | — | | | | — | | | | — | | | | — | | | | 103,388 | |
| Uncompensated contribution of services—3rd quarter | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | |
| Uncompensated contribution of services—4th quarter | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | |
| Issuance of preferred stock for dividends | | | 143,507 | | | | 144 | | | | — | | | | — | | | | — | | | | — | | | | 502,517 | | | | — | | | | — | | | | — | | | | (502,661 | ) | | | — | |
| Deemed dividend associated with beneficial conversion of preferred stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 10,178,944 | | | | — | | | | — | | | | — | | | | (10,178,944 | ) | | | — | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (5,433,055 | ) | | | (5,433,055 | ) |
| Other comprehensive income, foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 13,875 | | | | — | | | | 13,875 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (5,419,180 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, 12/31/02 | | | 3,038,507 | | | $ | 3,039 | | | | — | | | $ | — | | | | 15,227,963 | | | $ | 15,228 | | | $ | 25,573,999 | | | | — | | | $ | — | | | $ | 13,875 | | | $ | (20,399,211 | ) | | $ | 5,206,930 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-8
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | Series A | | | Series B | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | Deficit | | | Total | |
| | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Additional | | | Treasury Stock | | | Other | | | During | | | Shareholders’ | |
| | | Number of | | | | | | | Number of | | | | | | | Number of | | | | | | | Paid-In | | | Number of | | | | | | | Comprehensive | | | Development | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Shares | | | Amount | | | Income | | | Stage | | | (Deficit) | |
| Issuance of common stock for cash on 1/7/03 | | | — | | | $ | — | | | | — | | | $ | — | | | | 61,600 | | | $ | 62 | | | $ | 92,338 | | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 92,400 | |
| Issuance of common stock for patent pending acquisition on 3/31/03 | | | — | | | | — | | | | — | | | | — | | | | 100,000 | | | | 100 | | | | 539,900 | | | | — | | | | — | | | | — | | | | — | | | | 540,000 | |
| Cancellation of common stock on 3/31/03 | | | — | | | | — | | | | — | | | | — | | | | (79,382 | ) | | | (79 | ) | | | (119,380 | ) | | | — | | | | — | | | | — | | | | — | | | | (119,459 | ) |
| Uncompensated contribution of services—1st quarter | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | |
| Issuance of preferred stock for cash on 5/9/03 | | | — | | | | — | | | | 110,250 | | | | 110 | | | | — | | | | — | | | | 2,773,218 | | | | — | | | | — | | | | — | | | | — | | | | 2,773,328 | |
| Issuance of preferred stock for cash on 5/16/03 | | | — | | | | — | | | | 45,500 | | | | 46 | | | | — | | | | — | | | | 1,145,704 | | | | — | | | | — | | | | — | | | | — | | | | 1,145,750 | |
| Conversion of preferred stock into common stock—2nd qtr | | | (70,954 | ) | | | (72 | ) | | | — | | | | — | | | | 147,062 | | | | 147 | | | | 40,626 | | | | — | | | | — | | | | — | | | | — | | | | 40,701 | |
| Conversion of warrants into common stock—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | 114,598 | | | | 114 | | | | (114 | ) | | | — | | | | — | | | | — | | | | — | | | | — | |
| Uncompensated contribution of services—2nd quarter | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | |
| Issuance of preferred stock dividends | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1,087,200 | ) | | | (1,087,200 | ) |
| Deemed dividend associated with beneficial conversion of preferred stock | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,244,880 | | | | — | | | | — | | | | — | | | | (1,244,880 | ) | | | — | |
Issuance of common stock for cash—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | 202,500 | | | | 202 | | | | 309,798 | | | | — | | | | — | | | | — | | | | — | | | | 310,000 | |
| Issuance of common stock for cash on 8/27/03 | | | — | | | | — | | | | — | | | | — | | | | 3,359,331 | | | | 3,359 | | | | 18,452,202 | | | | — | | | | — | | | | — | | | | — | | | | 18,455,561 | |
Conversion of preferred stock into common stock—3rd qtr | | | (2,967,553 | ) | | | (2,967 | ) | | | (155,750 | ) | | | (156 | ) | | | 7,188,793 | | | | 7,189 | | | | (82,875 | ) | | | — | | | | — | | | | — | | | | — | | | | (78,809 | ) |
Conversion of warrants into common stock—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | 212,834 | | | | 213 | | | | (213 | ) | | | — | | | | — | | | | — | | | | — | | | | — | |
| Compensation expense on warrants issued to non-employees | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 412,812 | | | | — | | | | — | | | | — | | | | — | | | | 412,812 | |
Issuance of common stock for cash—4th qtr | | | — | | | | — | | | | — | | | | — | | | | 136,500 | | | | 137 | | | | 279,363 | | | | — | | | | — | | | | — | | | | — | | | | 279,500 | |
Conversion of warrants into common stock—4th qtr | | | — | | | | — | | | | — | | | | — | | | | 393 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (11,268,294 | ) | | | (11,268,294 | ) |
| Other comprehensive income, foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 360,505 | | | | — | | | | 360,505 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (10,907,789 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, 12/31/03 | | | — | | | $ | — | | | | — | | | $ | — | | | | 26,672,192 | | | $ | 26,672 | | | $ | 50,862,258 | | | | — | | | $ | — | | | $ | 374,380 | | | $ | (33,999,585 | ) | | $ | 17,263,725 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-9
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | Series A | | | Series B | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | Deficit | | | Total | |
| | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Additional | | | Treasury Stock | | | Other | | | During | | | Shareholders’ | |
| | | Number of | | | | | | | Number of | | | | | | | Number of | | | | | | | Paid-In | | | Number of | | | | | | | Comprehensive | | | Development | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Shares | | | Amount | | | Income | | | Stage | | | (Deficit) | |
Conversion of warrants into common stock—1st qtr | | | — | | | | — | | | | — | | | | — | | | | 78,526 | | | $ | 79 | | | $ | (79 | ) | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
Issuance of common stock for cash in connection with exercise of stock options—1st qtr | | | — | | | | — | | | | — | | | | — | | | | 15,000 | | | | 15 | | | | 94,985 | | | | — | | | | — | | | | — | | | | — | | | | 95,000 | |
Issuance of common stock for cash in connection with exercise of warrants—1st qtr | | | — | | | | — | | | | — | | | | — | | | | 4,000 | | | | 4 | | | | 7,716 | | | | — | | | | — | | | | — | | | | — | | | | 7,720 | |
Compensation expense on options and warrants issued to non-employees and directors—1st qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,410,498 | | | | — | | | | — | | | | — | | | | — | | | | 1,410,498 | |
Issuance of common stock in connection with exercise of warrants—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | 51,828 | | | | 52 | | | | (52 | ) | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of common stock for cash—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | 7,200,000 | | | | 7,200 | | | | 56,810,234 | | | | — | | | | — | | | | — | | | | — | | | | 56,817,434 | |
Compensation expense on options and warrants issued to non-employees and directors—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 143,462 | | | | — | | | | — | | | | — | | | | — | | | | 143,462 | |
Issuance of common stock in connection with exercise of warrants—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | 7,431 | | | | 7 | | | | (7 | ) | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of common stock for cash in connection with exercise of stock options—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | 110,000 | | | | 110 | | | | 189,890 | | | | — | | | | — | | | | — | | | | — | | | | 190,000 | |
Issuance of common stock for cash in connection with exercise of warrants—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | 28,270 | | | | 28 | | | | 59,667 | | | | — | | | | — | | | | — | | | | — | | | | 59,695 | |
Compensation expense on options and warrants issued to non-employees and directors—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 229,133 | | | | — | | | | — | | | | — | | | | — | | | | 229,133 | |
Issuance of common stock in connection with exercise of warrants—4th qtr | | | — | | | | — | | | | — | | | | — | | | | 27,652 | | | | 28 | | | | (28 | ) | | | — | | | | — | | | | — | | | | — | | | | — | |
Compensation expense on options and warrants issued to non-employees, employees, and directors—4th qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 127,497 | | | | — | | | | — | | | | — | | | | — | | | | 127,497 | |
Purchase of treasury stock—4th qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4,000,000 | | | | (25,974,000 | ) | | | — | | | | — | | | | (25,974,000 | ) |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (21,474,469 | ) | | | (21,474,469 | ) |
| Other comprehensive income, foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 79,725 | | | | — | | | | 79,725 | |
| Other comprehensive income, net unrealized gain on available-for-sale investments | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 10,005 | | | | — | | | | 10,005 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (21,384,739 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, 12/31/04 | | | — | | | $ | — | | | | — | | | $ | — | | | | 34,194,899 | | | $ | 34,195 | | | $ | 109,935,174 | | | | 4,000,000 | | | $ | (25,974,000 | ) | | $ | 464,110 | | | $ | (55,474,054 | ) | | $ | 28,985,425 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-10
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | Series A | | | Series B | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | Deficit | | | Total | |
| | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Additional | | | Treasury Stock | | | Other | | | During | | | Shareholders’ | |
| | | Number of | | | | | | | Number of | | | | | | | Number of | | | | | | | Paid-In | | | Number of | | | | | | | Comprehensive | | | Development | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Shares | | | Amount | | | Income (Loss) | | | Stage | | | (Deficit) | |
Issuance of common stock for cash in connection with exercise of stock options—1st qtr | | | — | | | $ | — | | | | — | | | $ | — | | | | 25,000 | | | $ | 25 | | | $ | 74,975 | | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 75,000 | |
Compensation expense on options and warrants issued to non-employees—1st qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 33,565 | | | | — | | | | — | | | | — | | | | — | | | | 33,565 | |
Conversion of warrants into common stock—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | 27,785 | | | | 28 | | | | (28 | ) | | | — | | | | — | | | | — | | | | — | | | | — | |
Compensation expense on options and warrants issued to non-employees—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (61,762 | ) | | | — | | | | — | | | | — | | | | — | | | | (61,762 | ) |
Compensation expense on options and warrants issued to non-employees—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (137,187 | ) | | | — | | | | — | | | | — | | | | — | | | | (137,187 | ) |
Conversion of warrants into common stock—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | 12,605 | | | | 12 | | | | (12 | ) | | | — | | | | — | | | | — | | | | — | | | | — | |
Compensation expense on options and warrants issued to non-employees—4th qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 18,844 | | | | — | | | | — | | | | — | | | | — | | | | 18,844 | |
Compensation expense on acceleration of options—4th qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 14,950 | | | | — | | | | — | | | | — | | | | — | | | | 14,950 | |
Compensation expense on restricted stock award issued to employee—4th qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 606 | | | | — | | | | — | | | | — | | | | — | | | | 606 | |
| Conversion of predecessor company shares | | | — | | | | — | | | | — | | | | — | | | | 94 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (35,777,584 | ) | | | (35,777,584 | ) |
| Other comprehensive loss, foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1,372,600 | ) | | | — | | | | (1,372,600 | ) |
| Foreign exchange gain on substantial liquidation of foreign entity | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 133,851 | | | | | | | | 133,851 | |
| Other comprehensive loss, net unrealized gain on available-for-sale investments | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (10,005 | ) | | | — | | | | (10,005 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (37,026,338 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, 12/31/05 | | | — | | | $ | — | | | | — | | | $ | — | | | | 34,260,383 | | | $ | 34,260 | | | $ | 109,879,125 | | | | 4,000,000 | | | $ | (25,974,000 | ) | | $ | (784,644 | ) | | $ | (91,251,638 | ) | | $ | (8,096,897 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
F-11
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | Series A | | | Series B | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | Deficit | | | Total | |
| | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Additional | | | Treasury Stock | | | Other | | | During | | | Shareholders’ | |
| | | Number of | | | | | | | Number of | | | | | | | Number of | | | | | | | Paid-In | | | Number of | | | | | | | Comprehensive | | | Development | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Shares | | | Amount | | | Income | | | Stage | | | (Deficit) | |
Compensation expense on options and warrants issued to non-employees—1st qtr | | | — | | | $ | — | | | | — | | | $ | — | | | | — | | | $ | — | | | $ | 42,810 | | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 42,810 | |
Compensation expense on option awards issued to employee and directors—1st qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 46,336 | | | | — | | | | — | | | | — | | | | — | | | | 46,336 | |
Compensation expense on restricted stock issued to employees—1st qtr | | | — | | | | — | | | | — | | | | — | | | | 128,750 | | | | 129 | | | | 23,368 | | | | — | | | | — | | | | — | | | | — | | | | 23,497 | |
Compensation expense on options and warrants issued to non-employees—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 96,177 | | | | — | | | | — | | | | — | | | | — | | | | 96,177 | |
Compensation expense on option awards issued to employee and directors—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 407,012 | | | | — | | | | — | | | | — | | | | — | | | | 407,012 | |
Compensation expense on restricted stock to employees—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4,210 | | | | — | | | | — | | | | — | | | | — | | | | 4,210 | |
Cancellation of unvested restricted stock — 2nd qtr | | | — | | | | — | | | | — | | | | — | | | | (97,400 | ) | | | (97 | ) | | | 97 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of common stock for cash in connection with exercise of stock options—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | 10,000 | | | | 10 | | | | 16,490 | | | | — | | | | — | | | | — | | | | — | | | | 16,500 | |
Compensation expense on options and warrants issued to non-employees—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 25,627 | | | | — | | | | — | | | | — | | | | — | | | | 25,627 | |
Compensation expense on option awards issued to employee and directors—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 389,458 | | | | — | | | | — | | | | — | | | | — | | | | 389,458 | |
Compensation expense on restricted stock to employees—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3,605 | | | | — | | | | — | | | | — | | | | — | | | | 3,605 | |
Issuance of common stock for cash in connection with exercise of stock options—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | 76,000 | | | | 76 | | | | 156,824 | | | | — | | | | — | | | | — | | | | — | | | | 156,900 | |
Compensation expense on options and warrants issued to non-employees—4th qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 34,772 | | | | — | | | | — | | | | — | | | | — | | | | 34,772 | |
Compensation expense on option awards issued to employee and directors—4th qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 390,547 | | | | — | | | | — | | | | — | | | | — | | | | 390,547 | |
Compensation expense on restricted stock to employees—4th qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 88 | | | | — | | | | — | | | | — | | | | — | | | | 88 | |
Cancellation of unvested restricted stock award— 4th qtr | | | — | | | | — | | | | — | | | | — | | | | (15,002 | ) | | | (15 | ) | | | 15 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (35,821,406 | ) | | | (35,821,406 | ) |
| Other comprehensive gain, foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 657,182 | | | | — | | | | 657,182 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (35,164,224 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance 12/31/06 | | | — | | | $ | — | | | | — | | | $ | — | | | | 34,362,731 | | | $ | 34,363 | | | $ | 111,516,561 | | | | 4,000,000 | | | $ | (25,974,000 | ) | | $ | (127,462 | ) | | $ | (127,073,044 | ) | | $ | (41,623,582 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-12
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | Series A | | | Series B | | | | | | | | | | | | | | | | | | | | | | | Accumulated | | | Deficit | | | Total | |
| | | Preferred Stock | | | Preferred Stock | | | Common Stock | | | Additional | | | Treasury Stock | | | Other | | | During | | | Shareholders’ | |
| | | Number of | | | | | | | Number of | | | | | | | Number of | | | | | | | Paid-In | | | Number of | | | | | | | Comprehensive | | | Development | | | Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Shares | | | Amount | | | Income (Loss) | | | Stage | | | (Deficit) | |
Compensation expense on options and warrants issued to non-employees—1st qtr | | | — | | | $ | — | | | | — | | | $ | — | | | | — | | | $ | — | | | $ | 39,742 | | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 39,742 | |
Compensation expense on option awards issued to employee and directors—1st qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 448,067 | | | | — | | | | — | | | | — | | | | — | | | | 448,067 | |
Compensation expense on restricted stock issued to employees—1st qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 88 | | | | — | | | | — | | | | — | | | | — | | | | 88 | |
Issuance of common stock for cash in connection with exercise of stock options—1st qtr | | | — | | | | — | | | | — | | | | — | | | | 15,000 | | | | 15 | | | | 23,085 | | | | — | | | | — | | | | — | | | | — | | | | 23,100 | |
Expense in connection with modification of employee stock options —1st qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,178,483 | | | | — | | | | — | | | | — | | | | — | | | | 1,178,483 | |
Compensation expense on options and warrants issued to non-employees—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 39,981 | | | | — | | | | — | | | | — | | | | — | | | | 39,981 | |
Compensation expense on option awards issued to employee and directors—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 462,363 | | | | — | | | | — | | | | — | | | | — | | | | 462,363 | |
Compensation expense on restricted stock issued to employees—2nd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 88 | | | | — | | | | — | | | | — | | | | — | | | | 88 | |
Compensation expense on option awards issued to employee and directors—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 478,795 | | | | — | | | | — | | | | — | | | | — | | | | 478,795 | |
Compensation expense on restricted stock issued to employees—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 88 | | | | — | | | | — | | | | — | | | | — | | | | 88 | |
Issuance of common stock upon exercise of warrants—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | 492,613 | | | | 493 | | | | 893,811 | | | | — | | | | — | | | | — | | | | — | | | | 894,304 | |
Issuance of common stock for cash, net of offering costs—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | 6,767,647 | | | | 6,767 | | | | 13,745,400 | | | | — | | | | — | | | | — | | | | — | | | | 13,752,167 | |
Issuance of common stock for cash in connection with exercise of stock options—3rd qtr | | | — | | | | — | | | | — | | | | — | | | | 1,666 | | | | 2 | | | | 3,164 | | | | — | | | | — | | | | — | | | | — | | | | 3,166 | |
Compensation expense on option awards issued to employee and directors—4th qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 378,827 | | | | — | | | | — | | | | — | | | | — | | | | 378,827 | |
Compensation expense on restricted stock issued to employees—4th qtr | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 88 | | | | — | | | | — | | | | — | | | | — | | | | 88 | |
| Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (35,573,114 | ) | | | (35,573,114 | ) |
| Other comprehensive gain, foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 846,388 | | | | — | | | | 846,388 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (34,726,726 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance 12/31/07 | | | — | | | $ | — | | | | — | | | $ | — | | | | 41,639,657 | | | $ | 41,640 | | | $ | 129,208,631 | | | | 4,000,000 | | | $ | (25,974,000 | ) | | $ | 718,926 | | | $ | (162,646,158 | ) | | $ | (58,650,961 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-13
Isolagen, Inc.
(A Development Stage Company)
Consolidated Statements of Cash Flows
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Cumulative Period | |
| | | | | | | | | | | | | | | from December 28, | |
| | | | | | | | | | | | | | | 1995 (date of | |
| | | | | | | | | | | | | | | inception) to | |
| | | For the Year Ended December 31, | | | December 31, 2007 | |
| | | 2007 | | | 2006 | | | 2005 | | | (Unaudited) | |
| Cash flows from operating activities: | | | | | | | | | | | | | | | | |
| Net loss | | $ | (35,573,114 | ) | | $ | (35,821,406 | ) | | $ | (35,777,584 | ) | | $ | (149,632,473 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | | | | | |
| Equity awards issued for services | | | 3,026,610 | | | | 1,464,139 | | | | (130,984 | ) | | | 7,892,949 | |
| Uncompensated contribution of services | | | — | | | | — | | | | — | | | | 755,556 | |
| Depreciation and amortization | | | 1,505,218 | | | | 2,202,229 | | | | 1,701,423 | | | | 7,715,127 | |
| Provision for doubtful accounts | | | 11,803 | | | | 134,370 | | | | 133,412 | | | | 330,118 | |
| Amortization of debt issue costs | | | 749,239 | | | | 749,240 | | | | 749,239 | | | | 2,372,591 | |
| Amortization of debt discounts on investments | | | — | | | | — | | | | (508,983 | ) | | | (508,983 | ) |
| Loss on disposal or impairment of property and equipment | | | 59,871 | | | | 2,603,843 | | | | 1,369,527 | | | | 4,609,102 | |
| Foreign exchange gain on substantial liquidation of foreign entity | | | — | | | | — | | | | (133,851 | ) | | | (133,851 | ) |
| Minority interest | | | (246,347 | ) | | | (78,132 | ) | | | — | | | | (324,479 | ) |
| Change in operating assets and liabilities, excluding effects of acquisition: | | | | | | | | | | | | | | | | |
| Decrease (increase) in restricted cash | | | 1,031,815 | | | | 976,259 | | | | (2,459,456 | ) | | | (451,383 | ) |
| Decrease (increase) in accounts receivable | | | (187,603 | ) | | | 1,033,975 | | | | 489,961 | | | | (156,795 | ) |
| Decrease (increase) in other receivables | | | 268,912 | | | | (43,862 | ) | | | 252,854 | | | | 148,476 | |
| Decrease (increase) in inventory | | | (393,778 | ) | | | 228,345 | | | | 556,184 | | | | (596,355 | ) |
| Decrease (increase) in prepaid expenses | | | 431,390 | | | | (185,739 | ) | | | (159,046 | ) | | | (658,218 | ) |
| Decrease (increase) in other assets | | | 122,149 | | | | (13,248 | ) | | | 320,404 | | | | 140,504 | |
| Increase (decrease) in accounts payable | | | (898,831 | ) | | | (771,715 | ) | | | (336,103 | ) | | | 294,261 | |
| Increase in accrued expenses and other liabilities | | | 704,436 | | | | 5,136 | | | | 340,577 | | | | 4,317,908 | |
| Decrease in deferred revenue | | | (348,642 | ) | | | (2,095,914 | ) | | | (398,008 | ) | | | (50,096 | ) |
| | | | | | | | | | | | | |
| Net cash used in operating activities | | | (29,736,872 | ) | | | (29,612,480 | ) | | | (33,990,434 | ) | | | (123,936,041 | ) |
| | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | | | | | |
| Acquisition of Agera, net of cash acquired | | | — | | | | (2,009,841 | ) | | | — | | | | (2,009,841 | ) |
| Purchase of property and equipment | | | (184,538 | ) | | | (1,243,036 | ) | | | (17,712,723 | ) | | | (25,481,833 | ) |
| Proceeds from the sale of property and equipment | | | 57,153 | | | | 6,595 | | | | — | | | | 98,048 | |
| Purchase of investments | | | — | | | | (2,700,000 | ) | | | (77,498,313 | ) | | | (152,998,313 | ) |
| Proceeds from sales and maturities of investments | | | — | | | | 25,700,000 | | | | 106,807,000 | | | | 153,507,000 | |
| | | | | | | | | | | | | |
| Net cash provided by (used in) investing activities | | | (127,385 | ) | | | 19,753,718 | | | | 11,595,964 | | | | (26,884,939 | ) |
| | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| Proceeds from convertible debt | | | — | | | | — | | | | — | | | | 91,450,000 | |
| Offering costs associated with the issuance of convertible debt | | | — | | | | — | | | | — | | | | (3,746,193 | ) |
| Proceeds from notes payable to shareholders, net | | | — | | | | — | | | | — | | | | 135667 | |
| Proceeds from the issuance of preferred stock, net | | | — | | | | — | | | | — | | | | 12,931,800 | |
| Proceeds from the issuance of common stock, net | | | 14,672,737 | | | | 173,400 | | | | 75,000 | | | | 93,753,857 | |
| Cash dividends paid on preferred stock | | | — | | | | — | | | | — | | | | (1,087,200 | ) |
| Cash paid for fractional shares of preferred stock | | | — | | | | — | | | | — | | | | (38,108 | ) |
| Merger and acquisition expenses | | | — | | | | — | | | | — | | | | (48,547 | ) |
| Repurchase of common stock | | | — | | | | — | | | | — | | | | (26,024,280 | ) |
| | | | | | | | | | | | | |
| Net cash provided by financing activities | | | 14,672,737 | | | | 173,400 | | | | 75,000 | | | | 167,326,996 | |
| | | | | | | | | | | | | |
| Effect of exchange rate changes on cash balances | | | (1,305 | ) | | | (85,296 | ) | | | (455,683 | ) | | | 84,704 | |
| Net increase (decrease) in cash and cash equivalents | | | (15,192,825 | ) | | | (9,770,658 | ) | | | (22,775,153 | ) | | | 16,590,720 | |
| Cash and cash equivalents, beginning of period | | | 31,783,545 | | | | 41,554,203 | | | | 64,329,356 | | | | — | |
| | | | | | | | | | | | | |
| Cash and cash equivalents, end of period | | $ | 16,590,720 | | | $ | 31,783,545 | | | $ | 41,554,203 | | | $ | 16,590,720 | |
| | | | | | | | | | | | | |
| Supplemental disclosures of cash flow information: | | | | | | | | | | | | | | | | |
| Cash paid for interest | | $ | 3,150,000 | | | $ | 3,150,000 | | | $ | 3,115,000 | | | $ | 9,565,283 | |
| | | | | | | | | | | | | |
| Non-cash investing and financing activities: | | | | | | | | | | | | | | | | |
| Deemed dividend associated with beneficial conversion of preferred stock | | | — | | | | — | | | | — | | | $ | 11,423,824 | |
| | | | | | | | | | | | | |
| Preferred stock dividend | | | — | | | | — | | | | — | | | | 1,589,861 | |
| | | | | | | | | | | | | |
| Uncompensated contribution of services | | | — | | | | — | | | | — | | | | 755,556 | |
| | | | | | | | | | | | | |
| Common stock issued for intangible assets | | | — | | | | — | | | | — | | | | 540,000 | |
| | | | | | | | | | | | | |
| Equipment acquired through capital lease | | | — | | | | — | | | | — | | | $ | 167,154 | |
| | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-14
Isolagen, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
Note 1—Basis of Presentation, Business and Organization
Isolagen, Inc. f/k/a American Financial Holding, Inc., a Delaware corporation (“Isolagen”) is the parent company of Isolagen Technologies, Inc., a Delaware corporation (“Isolagen Technologies”) and Agera Laboratories, Inc., a Delaware corporation (“Agera”). Isolagen Technologies is the parent company of Isolagen Europe Limited, a company organized under the laws of the United Kingdom (“Isolagen Europe”), Isolagen Australia Pty Limited, a company organized under the laws of Australia (“Isolagen Australia”), and Isolagen International, S.A., a company organized under the laws of Switzerland (“Isolagen Switzerland”). The common stock of the Company, par value $0.001 per share, (“Common Stock”) is traded on the American Stock Exchange (“AMEX”) under the symbol “ILE.”
The Company is an aesthetic and therapeutic company focused on developing novel skin and tissue rejuvenation products. The Company’s clinical development product candidates are designed to improve the appearance of skin injured by the effects of aging, sun exposure, acne and burns with a patient’s own, or autologous, fibroblast cells produced in the Company’s proprietary Isolagen Process. The Company also develops and markets an advanced skin care line with broad application in core target markets through its Agera subsidiary.
The Company acquired 57% of the outstanding common shares of Agera on August 10, 2006. Agera offers a complete line of skincare systems based on a wide array of proprietary formulations, trademarks and nano-peptide technology. These technologically advanced skincare products can be packaged to offer anti-aging, anti-pigmentary and acne treatment systems. Agera markets its product in both the United States and Europe (primarily the United Kingdom). The results of Agera’s operations and cash flows have been included in the consolidated financial statements from the date of the acquisition. The assets and liabilities of Agera have been included in the consolidated balance sheet since the date of the acquisition.
In October 2006, the Company reached an agreement with the FDA on the design of a Phase III pivotal study protocol for the treatment of nasolabial folds. The randomized, double-blind protocol was submitted to the FDA under the agency’s Special Protocol Assessment (“SPA”) regulations. Pursuant to this assessment process, the FDA has agreed that the Company’s study design for two identical trials, including patient numbers, clinical endpoints, and statistical analyses, is acceptable to the FDA to form the basis of an efficacy claim for a marketing application. The randomized, double-blind, pivotal Phase III trials will evaluate the efficacy and safety of Isolagen Therapy against placebo in approximately 400 patients with approximately 200 patients enrolled in each trial. The Company completed enrollment of the study and commenced injection of subjects in early 2007.
In March 2004, the Company announced positive results of a first Phase III exploratory clinical trial for the Company’s lead facial aesthetics product candidate, and in July 2004 we commenced a 200 patient Phase III study of Isolagen Therapy for facial wrinkles consisting of two identical, simultaneous trials. The study was concluded during the second half of 2005. In August 2005 we announced that results of this study failed to meet co-primary endpoints. Based on the results of this study, the Company commenced preparations for our second Phase III pivotal study discussed in the preceding paragraph.
During 2006, 2005 and 2004, the Company sold its aesthetic product primarily in the United Kingdom. However, during the fourth quarter of fiscal 2006, the Company decided to close the United Kingdom operation. The Company completed the closure of the United Kingdom operation on March 31, 2007, and as of March 31, 2007, the United Kingdom, Swiss and Australian operations were presented as discontinued operations for all periods presented, as more fully discussed in Note 5.
Through December 31, 2007, the Company has been primarily engaged in developing its initial product technology and recruiting personnel. In the course of its development activities, the Company has sustained losses and expects such losses to continue through at least 2008. The Company expects to finance its operations primarily through its existing cash and any future financing. However, as described in Note 2, there exists substantial doubt about the Company’s ability to continue as a going concern. The Company’s ability to operate profitably is largely contingent upon its success in obtaining financing, obtaining regulatory approval to sell one or a variety of applications of the Isolagen Therapy, upon its successful development of markets for its products and upon the development of profitable scaleable manufacturing processes. The Company will be required to obtain additional capital in the future to continue and expand its operations. No assurance can be given that the Company will be able to obtain such regulatory approvals, successfully develop the markets for its products or develop profitable manufacturing methods. There is no assurance that the Company will be able to obtain any such additional capital as it needs to finance these efforts, through asset sales, equity or debt financing, or any combination thereof, or on satisfactory terms or at all. Additionally, no assurance can be given that any such financing, if obtained, will be adequate to meet the Company’s ultimate capital needs and to support the Company’s growth. If adequate capital cannot be obtained on a timely basis and on satisfactory terms, the Company’s operations would be materially negatively impacted.
F-15
If the Company achieves growth in its operations in the next few years, such growth could place a strain on its management, administrative, operational and financial infrastructure. The Company may find it necessary to hire additional management, financial and sales and marketing personnel to manage the Company’s expanding operations. In addition, the Company’s ability to manage its current operations and future growth requires the continued improvement of operational, financial and management controls, reporting systems and procedures. If the Company is unable to manage this growth effectively and successfully, the Company’s business, operating results and financial condition may be materially adversely affected.
Acquisition and merger and basis of presentation
On August 10, 2001, Isolagen Technologies consummated a merger with American Financial Holdings, Inc. (“AFH”) and Gemini IX, Inc. (“Gemini”). Pursuant to an Agreement and Plan of Merger, dated August 1, 2001, by and among AFH, ISO Acquisition Corp, a Delaware corporation and wholly-owned subsidiary of AFH (“Merger Sub”), Isolagen Technologies, Gemini, a Delaware corporation, and William J. Boss, Jr., Olga Marko and Dennis McGill, stockholders of Isolagen Technologies (the “Merger Agreement”), AFH (i) issued 5,453,977 shares of its common stock, par value $0.001 to acquire, in a privately negotiated transaction, 100% of the issued and outstanding common stock (195,707 shares, par value $0.01, including the shares issued immediately prior to the Merger for the conversion of certain liabilities, as discussed below) of Isolagen Technologies, and (ii) issued 3,942,400 shares of its common stock to acquire 100% of the issued and outstanding common stock of Gemini. Pursuant to the terms of the Merger Agreement, Merger Sub, together with Gemini, merged with and into Isolagen Technologies (the “Merger”), and AFH was the surviving corporation. AFH subsequently changed its name to Isolagen, Inc. on November 13, 2001. Prior to the Merger, Isolagen Technologies had no active business and was seeking funding to begin FDA trials of the Isolagen Therapy. AFH was a non-operating, public shell company with limited assets. Gemini was a non-operating private company with limited assets and was unaffiliated with AFH.
The consolidated financial statements presented include Isolagen, Inc., its wholly-owned subsidiaries and its majority-owned subsidiary. All significant intercompany transactions and balances have been eliminated. Isolagen Technologies was, for accounting purposes, the surviving entity of the Merger, and accordingly for the periods prior to the Merger, the financial statements reflect the financial position, results of operations and cash flows of Isolagen Technologies. The assets, liabilities, operations and cash flows of AFH and Gemini are included in the consolidated financial statements from August 10, 2001 onward.
Unless the context requires otherwise, the “Company” refers to Isolagen, Inc. and all of its consolidated subsidiaries, “Isolagen” refers to Isolagen, Isolagen Technologies, Isolagen Europe, Isolagen Australia and Isolagen Switzerland, and “Agera” refers to Agera Laboratories, Inc.
Note 2—Going Concern
At December 31, 2007, the Company had cash, cash equivalents and restricted cash of $17.0 million and working capital of $13.8 million (including our cash, cash equivalents and restricted cash). The Company believes that its existing capital resources are adequate to finance its operations through at least September 1, 2008. The Company estimates that it will require additional cash resources during the third quarter of 2008 based upon its current operating plan and condition. This estimate excludes any proceeds that would be realized upon the sale of the Swiss campus (see further discussion below and at Note 3).
Through December 31, 2007, the Company has been primarily engaged in developing its initial product technology and recruiting personnel. In the course of its development activities, the Company has sustained losses and expects such losses to continue through at least 2008. The Company expects to finance its operations primarily through its existing cash and any future financing, including the sale of assets. However, there exists substantial doubt about the Company’s ability to continue as a going concern. The Company will be required to obtain additional capital in the future to continue its operations. There is no assurance that the Company will be able to obtain any such additional capital as it needs to finance these efforts, through asset sales, equity or debt financing, or any combination thereof, or on satisfactory terms or at all. Additionally, no assurance can be given that any such financing, if obtained, will be adequate to meet the Company’s ultimate capital needs and to support the Company’s growth. If adequate capital cannot be obtained on a timely basis and on satisfactory terms, the Company’s operations would be materially negatively impacted. Further, if the Company does not obtain additional funding prior to or during the third quarter of 2008, it may enter into bankruptcy during 2008 and possibly cease operations thereafter.
F-16
The Company filed a shelf registration statement on Form S-3 during June 2007, which was subsequently declared effective by the SEC. The shelf registration allows the Company the flexibility to offer and sell, from time to time, up to an original amount of $50 million of common stock, preferred stock, debt securities, warrants or any combination of the foregoing in one or more future public offerings. In August 2007, the Company sold under this shelf registration statement 6,746,647 shares of common stock to institutional investors, raising proceeds of $13.8 million, net of offering costs. The Company may offer and sell up to an additional $36.2 million of common stock pursuant to this shelf registration.
The Company’s ability to complete additional offerings, including any additional offerings under its shelf registration statement, is dependent on the state of the debt and/or equity markets at the time of any proposed offering, and such market’s reception of the Company and the offering terms. In addition, the Company’s ability to complete an offering may be dependent on the status of its clinical trials, and in particular, the status of its Phase III clinical trial for the treatment of wrinkles, which cannot be predicted. The Company is also continuing its efforts to sell its Swiss campus (see Note 3). The Company will add any proceeds from the sale of the Swiss campus to its working capital, which would partially alleviate the Company’s need to obtain financing from other sources. There is no assurance that capital in any form would be available to the Company, and if available, on terms and conditions that are acceptable.
As a result of the above discussed conditions, and in accordance with generally accepted accounting principles in the United States, there exists substantial doubt about the Company’s ability to continue as a going concern, and the Company’s ability to continue as a going concern is contingent upon its ability to secure additional adequate financing or capital prior to or during the third quarter of 2008. If the Company is unable to obtain additional sufficient funds during this time, the Company will be required to terminate or delay its efforts to obtain regulatory approval of one, more than one, or all of its product candidates, curtail or delay the implementation of manufacturing process improvements and/or delay the expansion of its sales and marketing capabilities. Any of these actions would have an adverse effect on the Company’s operations, the realization of its assets and the timely satisfaction of its liabilities. The Company’s financial statements are presented on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The financial statements do not include any adjustments relating to the recoverability of the recorded assets or the classification of liabilities that may be necessary should it be determined that the Company is unable to continue as a going concern.
Note 3—Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Examples include provisions for bad debts and inventory obsolescence, useful lives of property and equipment and intangible assets, impairment of property and equipment and intangible assets, deferred taxes, the provision for and disclosure of litigation and loss contingencies (see Note 11) and estimates and assumptions related to equity-based compensation expense (see Note 13). Actual results may differ materially from those estimates.
Foreign Currency Translation
The financial position and results of operations of the Company’s foreign subsidiaries are determined using the local currency as the functional currency. Assets and liabilities of these subsidiaries are translated at the exchange rate in effect at each period-end. Income statement accounts are translated at the average rate of exchange prevailing during the period. Adjustments arising from the use of differing exchange rates from period to period are included in accumulated other comprehensive income in shareholders’ equity. Gains and losses resulting from foreign currency transactions are included in earnings and have not been material in any one period.
F-17
Balances of related after-tax components comprising accumulated other comprehensive income (loss) included in shareholders’ equity at December 31, 2007 and December 31, 2006 are as follows:
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| Foreign currency translation adjustment | | $ | 718,926 | | | $ | (127,462 | ) |
| | | | | | | |
| Accumulated other comprehensive income (loss) | | $ | 718,926 | | | $ | (127,462 | ) |
| | | | | | | |
Upon sale or upon complete or substantially complete liquidation of an investment in a foreign entity, the amount attributable to that entity and accumulated in the translation adjustment component of equity is removed from the separate component of equity and is reported as gain or loss for the period during which the sale or liquidation occurs. During December 2005, the Company substantially completed the liquidation of the Company’s Australian entity. As such, the accumulated translation adjustment component was removed from equity by recording $0.1 million as other income in the 2005 consolidated statement of operations.
Statement of cash flows
For purposes of the statements of cash flows, the Company considers all highly liquid investments (i.e., investments which, when purchased, have original maturities of three months or less) to be cash equivalents. At December 31, 2007 and December 31, 2006, the Company had $0.5 million and $1.5 million of cash restricted for the payment of the non-cancelable portion of the Exton, Pennsylvania facility lease, due monthly through March 2008.
Concentration of credit risk
The Company maintains its cash primarily with major U.S. domestic banks. The amounts held in these banks generally exceed the insured limit of $100,000. The terms of these deposits are on demand to minimize risk. The Company has not incurred losses related to these deposits. Cash equivalents are maintained in two financial institutions. The Company invests these funds primarily in government securities.
Allowance for Doubtful Accounts
The Company maintains an allowance for doubtful accounts related to its accounts receivable that have been deemed to have a high risk of collectibility. Management reviews its accounts receivable on a monthly basis to determine if any receivables will potentially be uncollectible. Management analyzes historical collection trends and changes in its customer payment patterns, customer concentration, and creditworthiness when evaluating the adequacy of its allowance for doubtful accounts. In its overall allowance for doubtful accounts, the Company includes any receivable balances that are determined to be uncollectible. Based on the information available, management believes the allowance for doubtful accounts is adequate; however, actual write-offs might exceed the recorded allowance.
The following is a rollforward of the allowance for doubtful accounts, which includes that related to both continuing operations and discontinued operations, for the years ended December 31, 2007 and 2006.
| | | | | |
| Balance, as of December 31, 2005 | | $ | 100,639 | |
| Provision during 2006 | | | 134,370 | |
| Charges to the allowance account | | | (159,200 | ) |
| | | | |
| Balance, as of December 31, 2006 | | $ | 75,809 | |
| Provision during 2007 | | | 13,143 | |
| Charges to the allowance account | | | — | |
| | | | |
| Balance, as of December 31, 2007 | | $ | 88,952 | |
| | | | |
The allowance for doubtful accounts related to continuing operations was $18,112 and $0 at December 31, 2007 and 2006, respectively. The allowance for doubtful accounts related to discontinued operations was $70,840 and $75,809 at December 31, 2007 and 2006, respectively.
F-18
Inventory
Agera purchases the large majority of its inventory from one contract manufacturer. Agera accounts for its inventory on the first-in-first-out method. At December 31, 2007, Agera’s inventory of $0.7 million consisted of $0.1 million of raw materials and $0.6 million of finished goods. At December 31, 2006, Agera’s inventory included $0.2 million of finished goods.
Asset of discontinued operations held for sale
In April 2005, the Company acquired land and a two-building, 100,000 square foot campus in Bevaix, Canton of Neuchâtel, Switzerland for $10 million. The Company subsequently spent approximately $1.8 million on the first phase of a renovation. In April 2006, management decided to place the Swiss campus on the market for sale in order to conserve capital. The Company commenced its selling efforts during June of 2006. As of December 31, 2007, the net book value of the Swiss campus was $11.2 million, reflecting the fair value of the corporate campus. Fair value is defined as the price at which an asset would change hands in a transaction between a willing buyer and willing seller in an unforced transaction. However, if the Company were to sell the Swiss campus in a forced transaction or a distress sale, it is likely that the price it would receive would be less, and the Company would recognize a loss, which may be significant, upon such sale. As the result of the cash position of the Company at December 31, 2007 and its need for funding, it is possible that the Company will choose or be required to sell the Swiss campus under such conditions. The corporate campus is included in assets of discontinued operations held for sale on the consolidated balance sheets for all periods presented.
Although the corporate campus was not being actively marketed for sale as of March 31, 2006, at that date management assessed whether the book value of the corporate campus was impaired based on its estimate of the realizable value of the corporate campus, and made a determination to write down the corporate campus by $0.7 million. The Company subsequently recorded a further impairment charge of $0.4 million during the fourth quarter of 2006 to reflect management’s estimate of the realizable value of the corporate campus at December 31, 2006. Accordingly, total impairment charges of $1.1 million have been recorded related to the Switzerland corporate campus, which charges are reflected in the loss from discontinuing operations in the consolidated statement of operations.
In March 2007, the Company completed the closing of its United Kingdom manufacturing facility. As described in Note 5, the Company recorded a fixed asset impairment charge related to its United Kingdom operation of $1.4 million during the fourth quarter of 2006, which is included in loss from discontinued operations in the consolidated statement of operations. The carrying value of the impaired United Kingdom fixed assets was $0.2 million at December 31, 2006, reflecting management’s estimate of realizable value. The United Kingdom fixed assets are included in assets of discontinued operations held for sale on the accompanying consolidated balance sheets for all periods presented (see Note 5 for further discussion of the Company’s discontinued operations).
Property and equipment
Property and equipment is carried at cost less accumulated depreciation and amortization. Generally, depreciation and amortization for financial reporting purposes is provided by the straight-line method over the estimated useful life of three years, except for leasehold improvements which are amortized using the straight-line method over the remaining lease term or the life of the asset, whichever is shorter. The cost of repairs and maintenance is charged as an expense as incurred.
Intangible assets
Intangible assets primarily include proprietary formulations and trademarks, which were acquired in connection with the acquisition of Agera (see Note 4), as well as certain in-process patents. Proprietary formulations and trademarks are amortized on a straight-line basis over their estimated useful lives, generally for periods ranging from 13 to 18 years. Amortization of intangible assets is expected to be approximately $0.3 million each year over the next five fiscal years. The Company continually evaluates the reasonableness of the useful lives of these assets. Intangibles are tested for recoverability whenever events or changes in circumstances indicate the carrying amount may not be recoverable. An impairment loss, if any, would be measured as the excess of the carrying value over the fair value determined by discounted cash flows.
F-19
Intangible assets are comprised as follows:
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| Proprietary formulations | | $ | 3,101,100 | | | $ | 3,101,100 | |
| Trademarks | | | 1,511,400 | | | | 1,511,400 | |
| Other intangibles | | | 705,800 | | | | 705,800 | |
| | | | | | | |
| | | | 5,318,300 | | | | 5,318,300 | |
| Less: Accumulated amortization | | | (718,762 | ) | | | (381,795 | ) |
| | | | | | | |
| Intangible assets, net | | $ | 4,599,538 | | | $ | 4,936,505 | |
| | | | | | | |
Debt Issue Costs
The costs incurred in issuing the Company’s 3.5% Convertible Subordinated Notes, including placement agent fees, legal and accounting costs and other direct costs are included in other assets and are being amortized to expense using the effective interest method over five years, through November 2009. Debt issuance costs, net of amortization, were approximately $1.4 million at December 31, 2007 and approximately $2.1 million at December 31, 2006 and were included in other assets, net, in the accompanying consolidated balance sheets.
The Company filed registration statements on Form S-3 and Form S-4 (the “Combined Registration Statements”) during July 2007. The Combined Registration Statements related to (1) a proposed exchange offer of new 3.5% convertible senior notes due 2024 to the holders of the currently outstanding $90 million, in principal amount, 3.5% convertible subordinated notes due 2024 and (2) a proposed offer to the public of an additional $30 million, in principal amount, of new 3.5% convertible senior notes due 2024. In August, 2007 the Company decided not to proceed with the offerings covered by the Combined Registration Statements, and the Combined Registration Statements were subsequently withdrawn by the Company in August 2007 prior to being declared effective by the SEC. During the year ended December 31, 2007, the Company incurred $0.8 million of costs related to the Combined Registration Statements. As a result of the Company’s withdrawal of the Combined Registration Statements in August 2007, such $0.8 million of costs were expensed and are included in selling, general and administrative expenses in the consolidated statement of operations for the year ended December 31, 2007.
Treasury Stock
The Company utilizes the cost method for accounting for its treasury stock acquisitions and dispositions.
Revenue recognition
The Company recognizes revenue over the period the service is performed in accordance with SEC Staff Accounting Bulletin No. 104, “Revenue Recognition in Financial Statements” (“SAB 104”). In general, SAB 104 requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists, (2) delivery has occurred or services rendered, (3) the fee is fixed and determinable and (4) collectibility is reasonably assured.
Continuing operations: Revenue from the sale of Agera’s products is recognized upon transfer of title, which is upon shipment of the product to the customer. The Company believes that the requirements of SAB 104 are met when the ordered product is shipped, as the risk of loss transfers to our customer at that time, the fee is fixed and determinable and collection is reasonably assured. Any advanced payments are deferred until shipment.
Discontinued operations:The Isolagen Therapy was administered, in the United Kingdom, to each patient using a recommended regimen of injections. Due to the short shelf life, each injection is cultured on an as needed basis and shipped prior to the individual injection being administered by the physician. The Company believes each injection had stand alone value to the patient. The Company invoiced the attending physician when the physician sent his or her patient’s tissue sample to the Company which created a contractual arrangement between the Company and the medical professional. The amount invoiced varied directly with the dose and number of injections requested. Generally, orders were paid in advance by the physician prior to the first injection. There was no performance provision under any arrangement with any physician, and there is no right to refund or returns for unused injections.
F-20
As a result, the Company believes that the requirements of SAB 104 were met as each injection was shipped, as the risk of loss transferred to our physician customer at that time, the fee was fixed and determinable and collection was reasonably assured. Advance payments were deferred until shipment of the injection(s). The amount of the revenue deferred represented the fair value of the remaining undelivered injections measured in accordance with Emerging Issues Task Force Issue (“EITF”) 00-21,“Accounting for Revenue Arrangements with Multiple Deliverables,”which addresses the issue of accounting for arrangements that involve the delivery of multiple products or services. Should the physician discontinue the regimen prematurely all remaining deferred revenue is recognized.
Shipping and handling costs
Agera charges its customers for shipping and handling costs. Such charges to customers are presented net of the costs of shipping and handling, as selling, general and administrative expense, and are not significant to the consolidated statements of operations.
Advertising cost
Agera advertising costs are expensed as incurred and include the costs of public relations and certain marketing related activities. These costs are included in selling, general and administrative expenses in the accompanying consolidated statements of operations.
Research and development expenses
Research and development costs are expensed as incurred and include salaries and benefits, costs paid to third-party contractors to perform research, conduct clinical trials, develop and manufacture drug materials and delivery devices, and a portion of facilities cost. Research and development costs also include costs to develop manufacturing, cell collection and logistical process improvements.
Clinical trial costs are a significant component of research and development expenses and include costs associated with third-party contractors. Invoicing from third-party contractors for services performed can lag several months. The Company accrues the costs of services rendered in connection with third-party contractor activities based on its estimate of management fees, site management and monitoring costs and data management costs. Actual clinical trial costs may differ from estimated clinical trial costs and are adjusted for in the period in which they become known.
Stock-based compensation
Effective January 1, 2006 the Company adopted the provisions of Statement of Financial Accounting Standards (“SFAS”) No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123(R)”). SFAS No. 123(R) replaces SFAS No. 123, “Accounting for Stock-Based Compensation”, supersedes APB Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB No. 25”), and amends SFAS No. 95, “Statement of Cash Flows.” SFAS No. 123(R) requires entities to recognize compensation expense for all share-based payments to employees and directors, including grants of employee stock options, based on the grant-date fair value of those share-based payments, adjusted for expected forfeitures. The Company adopted SFAS No. 123(R) using the modified prospective application method. Under the modified prospective application method, the fair value measurement requirements of SFAS No. 123(R) is applied to new awards and to awards modified, repurchased, or cancelled after January 1, 2006. Additionally, compensation cost for the portion of awards for which the requisite service has not been rendered that were outstanding as of January 1, 2006 is recognized as the requisite service is rendered on or after January 1, 2006. The compensation cost for that portion of awards is based on the grant-date fair value of those awards as calculated for pro forma disclosures under SFAS No. 123. Changes to the grant-date fair value of equity awards granted before January 1, 2006 are precluded.
Prior to the adoption of SFAS No. 123(R), the Company followed the intrinsic value method in accordance with APB No. 25 to account for its employee stock options. Historically, substantially all stock options have been granted with an exercise price equal to the fair market value of the common stock on the date of grant. Accordingly, no compensation expense was recognized from substantially all option grants to employees and directors. Compensation expense was recognized in connection with the issuance of stock options to non-employee consultants in accordance with EITF 96-18, “Accounting for Equity Instruments That are Issued to Other than Employees for Acquiring, or in Conjunction with Selling Goods and Services.” SFAS No. 123(R) did not change the accounting for stock-based compensation related to non-employees in connection with equity based incentive arrangements.
F-21
Income taxes
An asset and liability approach is used for financial accounting and reporting for income taxes. Deferred income taxes arise from temporary differences between income tax and financial reporting and principally relate to recognition of revenue and expenses in different periods for financial and tax accounting purposes and are measured using currently enacted tax rates and laws. In addition, a deferred tax asset can be generated by net operating loss carryforwards (“NOLs”). If it is more likely than not that some portion or all of a deferred tax asset will not be realized, a valuation allowance is recognized.
In the event the Company is charged interest or penalties related to income tax matters, the Company would record such interest as interest expense and would record such penalties as other expense in the consolidated statement of operations. No such charges have been incurred by the Company. As of December 31, 2007, the Company has no accrued interest related to uncertain tax positions.
The Company adopted the provisions of Financial Standards Accounting Board Interpretation No. 48 Accounting for Uncertainty in Income Taxes (“FIN 48”) an interpretation of FASB Statement No. 109 (“SFAS 109”) on January 1, 2007. No material adjustment in the liability for unrecognized income tax benefits was recognized as a result of the adoption of FIN 48. At the adoption date of January 1, 2007, we had $40.4 million of unrecognized tax benefits, all of which would affect the Company’s effective tax rate if recognized. At December 31, 2007, the Company has $54.8 million of unrecognized tax benefits, the large majority of which relates to loss carryforwards for which we have provided a full valuation allowance. The tax years 2004 through 2007 remain open to examination by the major taxing jurisdictions to which we are subject. Refer to Note 10 for further discussion of income tax related matters.
Loss per share data
Basic loss per share is calculated based on the weighted average common shares outstanding during the period, after giving effect to the manner in which the merger was accounted for as described in Note 1. Diluted earnings per share also gives effect to the dilutive effect of stock options, warrants (calculated based on the treasury stock method) and convertible notes and convertible preferred stock. The Company does not present diluted earnings per share for years in which it incurred net losses as the effect is antidilutive.
At December 31, 2007, options and warrants to purchase 7.9 million shares of common stock at exercise prices ranging from $1.50 to $9.81 per share were outstanding, but were not included in the computation of diluted earnings per share as their effect would be antidilutive. Also, 9.8 million shares issuable upon the conversion of the Company’s convertible notes, at a conversion price of approximately $9.16, were not included as their effect would be antidilutive.
At December 31, 2006, options and warrants to purchase 11.2 million shares of common stock at exercise prices ranging from $1.50 to $11.38 per share were outstanding, but were not included in the computation of diluted earnings per share as their effect would be antidilutive. Also, 9.8 million shares issuable upon the conversion of the Company’s convertible notes, at a conversion price of approximately $9.16, were not included as their effect would be antidilutive.
Fair Value of Financial Instruments
The Company’s financial instruments consist of accounts receivable, marketable debt securities, accounts payable and convertible subordinated debentures. The fair values of the Company’s accounts receivable and accounts payable approximate, in the Company’s opinion, their respective carrying amounts. The Company’s marketable debt security investments are carried at fair value. The Company’s convertible subordinated debentures were quoted at approximately 74% and 73% of par value at December 31, 2007 and 2006, respectively. Accordingly, the fair value of our convertible subordinated debentures was approximately $66.6 million and $65.7 million at December 31, 2007 and 2006, respectively.
Recently Issued Accounting Standards Not Yet Effective
In September 2006, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 157, “Fair Value Measurement,” effective for the Company’s fiscal year beginning January 1, 2008. SFAS No. 157 defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements. This Statement does not require any new fair value measurements, but simplifies and codifies related guidance within GAAP. This Statement applies under other accounting pronouncements that require or permit fair value measurements. In November 2007, FASB agreed to a one-year deferral of the effective date for nonfinancial assets and liabilities that are recognized or disclosed at fair value on a nonrecurring basis. The Company is currently assessing the impact the adoption of this pronouncement will have on the financial statements.
F-22
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities”. SFAS No. 159 allows entities the option to measure eligible financial instruments at fair value as of specified dates. Such election, which may be applied on an instrument by instrument basis, is typically irrevocable once elected. SFAS No. 159 is effective for fiscal years beginning after November 15, 2007, and early application is allowed under certain circumstances. The Company is currently assessing the impact the adoption of this pronouncement will have on the financial statements.
In December 2007, the Financial Accounting Standards Board released SFAS No. 141-R, “Business Combinations.” This Statement applies prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008, which is business combinations in the year ending December 31, 2009 for the Company. SFAS No. 141-R makes changes to the manner in which purchase business combinations, and in particular partial and step acquisitions, and minority interests, are measured and recorded. The objective of this Statement is to improve the relevance, representational faithfulness, and comparability of the information that a reporting entity provides in its financial reports about a business combination and its effects. The Company is currently assessing the impact the adoption of this pronouncement will have on the financial statements.
In December 2007, the Financial Accounting Standards Board released SFAS No. 160, “Noncontrolling Interests in Consolidated Financial Statements — an amendment of ARB No. 51”. This Statement is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008, which for the Company is the year ending December 31, 2009 and the interim periods within that fiscal year. SFAS No. 160 changes the manner in which minority interests are classified in consolidated financial statements, and the accounting for changes in minority interests. The objective of this pronouncement is to improve the relevance, comparability and transparency of the financial information that a reporting entity provides in its consolidated financial statements. This pronouncement currently does not impact the Company as it has full controlling interest of all of its subsidiaries.
Note 4—Acquisition of Agera Laboratories, Inc.
On August 10, 2006, the Company acquired 57% of the outstanding common shares of Agera Laboratories, Inc. (“Agera”). Agera is a skincare company that has proprietary rights to a scientifically-based advanced line of skincare products. Agera markets its product in both the United States and Europe. The Company believes that the acquisition of Agera will complement the Company’s Isolagen Therapy and will broaden the Company’s position in the skincare market as Agera has a comprehensive range of technologically advanced skincare products that can be packaged to offer anti-aging, anti-pigmentary and acne treatment systems.
The acquisition has been accounted for as a purchase. Accordingly, the bases in Agera’s assets and liabilities have been adjusted to reflect the allocation of the purchase price to the 57% interest the Company acquired (with the remaining 43% interest, and the minority interest in Agera’ net assets, recorded at Agera’s historical book values), and the results of Agera’s operations and cash flows have been included in the consolidated financial statements from the date of the acquisition.
The Company paid $2.7 million in cash to acquire the 57% interest in Agera and in connection with the acquisition contributed $0.3 million to the working capital of Agera. Included in the purchase price was an option to acquire an additional 8% of Agera’s outstanding common shares for an exercise price of $0.5 million in cash. This option expired unexercised in February 2007. In addition, the acquisition agreement includes future contingent payments up to a maximum of $8.0 million. Such additional purchase price is based upon certain percentages of Agera’s cost of sales incurred after June 30, 2007. Accordingly, based upon the financial performance of Agera, up to an additional $8.0 million of purchase price may be due the selling shareholder in future periods. No amounts have yet been paid with respect to the additional purchase price.
F-23
The following table summarizes the allocation of the purchase price to the estimated fair values of the assets acquired and liabilities assumed at the date of acquisition. These assets and liabilities were included in the consolidated balance sheet as of the acquisition date.
| | | | | |
| Current assets | | $ | 1,531,926 | |
| Intangible assets | | | 4,522,120 | |
| | | | |
| Total assets acquired | | | 6,054,046 | |
| | | | |
| | | | | |
| Current liabilities | | | 30,284 | |
| Deferred tax liability, net | | | 190,754 | |
| Other long—term liabilities | | | 695,503 | |
| Minority interest | | | 2,182,505 | |
| | | | |
| Total liabilities assumed and minority interest | | | 3,099,046 | |
| | | | |
| Net assets acquired | | $ | 2,955,000 | |
| | | | |
Of the $4.5 million, net, of acquired intangible assets, $4.4 million, net, was assigned to product formulations and trademarks, which have a weighted average useful life of approximately 16 years. No amount of the purchase price was assigned to goodwill.
The unaudited pro forma financial information below assumes that the Agera acquisition occurred on January 1 of the periods presented and includes the effect of amortization of intangibles from that date. The unaudited pro forma results of operations are being furnished solely for informational purposes and are not intended to represent or be indicative of the consolidated results of operations that would have been reported had these transactions been completed as of the dates and for the periods presented, nor are they necessarily indicative of future results.
| | | | | | | | | |
| (in millions, except per share data) | | 2006 | | | 2005 | |
| Pro forma revenue | | $ | 1.2 | | | $ | 1.0 | |
| Pro forma net loss | | | (35.8 | ) | | | (36.0 | ) |
| Pro forma basic and diluted loss per share | | $ | (1.18 | ) | | $ | (1.19 | ) |
Note 5—Discontinued Operations and Exit Costs
As part of the Company’s continuing efforts to evaluate the best uses of its resources, in the fourth quarter of 2006 the Company’s Board of Directors approved the proposed closing of the Company’s United Kingdom operation. On March 31, 2007, the Company completed the closure of its United Kingdom manufacturing facility. The United Kingdom operation was located in London, England with two locations; a manufacturing site and an administrative site. Both sites are under operating leases. The manufacturing site lease expires February 2010 and, as of December 31, 2007, the remaining lease obligation approximated $0.5 million. The administrative site lease expired in April 2007. The Company believes that substantially all costs related to the closure of the United Kingdom operation have been incurred by December 31, 2007, excluding the remaining lease obligation discussed above and any potential claims or contingencies unknown or which cannot be estimated at this time (see Note 11).
As a result of the closure of the Company’s United Kingdom operation, the operations that the Company previously conducted in Switzerland and Australia, which when closed had been absorbed into the United Kingdom operation, were also classified as discontinued operations as of March 31, 2007.
The Company recorded a fixed asset impairment charge related to its United Kingdom operation of $1.4 million during 2006, which is included in loss from discontinued operations in the consolidated statement of operations. All assets, liabilities and results of operations of the United Kingdom, Switzerland and Australian operations are reflected as discontinued operations in the accompanying consolidated financial statements. All prior period information has been restated to reflect the presentation of discontinued operations.
F-24
The following sets forth the components of assets and liabilities of discontinued operations as of December 31, 2007 and December 31, 2006:
| | | | | | | | | |
| | | December 31, | | | December 31, | |
| (in millions) | | 2007 | | | 2006 | |
| Accounts receivable, net | | $ | — | | | $ | — | |
| Inventory | | | — | | | | 0.1 | |
| Value-added tax refund due | | | — | | | | 0.3 | |
| Other current assets | | | — | | | | 0.3 | |
| | | | | | | |
| Total current assets | | | — | | | | 0.7 | |
| Assets held for sale | | | 11.2 | | | | 10.5 | |
| Long term assets | | | 0.1 | | | | 0.2 | |
| | | | | | | |
| Total assets | | $ | 11.3 | | | $ | 11.4 | |
| | | | | | | |
| | | | | | | | | |
| Accounts payable | | $ | — | | | $ | 0.5 | |
| Accrued expenses and other current liabilities | | | 0.2 | | | | 1.4 | |
| | | | | | | |
| Total current liabilities | | | 0.2 | | | | 1.9 | |
| Long term liabilities | | | 0.1 | | | | 0.2 | |
| | | | | | | |
| Total liabilities | | $ | 0.3 | | | $ | 2.1 | |
| | | | | | | |
The following sets forth the results of operations of discontinued operations for the years ended December 31, 2007, December 31, 2006 and December 31, 2005:
| | | | | | | | | | | | | |
| | | December 31, | | | December 31, | | | December 31, | |
| (in millions) | | 2007 | | | 2006 | | | 2005 | |
| Net revenue | | $ | 0.2 | | | $ | 5.7 | | | $ | 8.8 | |
| Gross loss | | | (0.3 | ) | | | (1.2 | ) | | | (0.5 | ) |
| Operating loss | | | (2.0 | ) | | | (13.0 | ) | | | (10.4 | ) |
| Other income | | | 0.3 | | | | 0.3 | | | | 0.3 | |
| | | | | | | | | | |
| Loss from discontinued operations | | $ | (1.7 | ) | | $ | (12.7 | ) | | $ | (10.1 | ) |
| | | | | | | | | | |
The following sets forth information about the major components of the United Kingdom operation exit costs incurred during 2007 and 2006:
| | | | | | | | | | | | | |
| | | Costs Incurred for | | | Costs Incurred for | | | | |
| | | the Year Ended | | | the Year Ended | | | Cumulative Costs | |
| | | December 31, 2006 | | | December 31, 2007 | | | Incurred to Date | |
| Employee severance | | $ | 284,096 | | | $ | 183,222 | | | $ | 467,318 | |
| Fixed asset impairment | | | 1,445,647 | | | | — | | | | 1,445,647 | |
| | | | | | | | | | |
| Total | | $ | 1,729,743 | | | $ | 183,222 | | | $ | 1,912,965 | |
| | | | | | | | | | |
The following sets forth information about the changes in the United Kingdom accrued exit costs for the year ended December 31, 2006:
| | | | | | | | | | | | | | | | | |
| | | Accrued Liability | | | Costs Charged | | | Costs Paid | | | Accrued Liability | |
| | | at January 1, 2006 | | | to Expense | | | or Settled | | | December 31, 2006 | |
| Employee severance | | $ | — | | | $ | 284,096 | | | $ | — | | | $ | 284,096 | |
| Fixed asset impairment | | | — | | | | 1,445,647 | | | | 1,445,647 | | | | — | |
| | | | | | | | | | | | | |
| Total | | $ | — | | | $ | 1,729,743 | | | $ | 1,445,647 | | | $ | 284,096 | |
| | | | | | | | | | | | | |
The following sets forth information about the changes in the United Kingdom accrued exit costs for the year ended December 31, 2007:
| | | | | | | | | | | | | | | | | |
| | | Accrued Liability | | | Costs Charged | | | Costs Paid | | | Accrued Liability | |
| | | at January 1, 2007 | | | to Expense | | | or Settled | | | December 31, 2007 | |
| Employee severance | | $ | 284,096 | | | $ | 183,222 | | | $ | 467,318 | | | $ | — | |
| | | | | | | | | | | | | |
| Total | | $ | 284,906 | | | $ | 183,222 | | | $ | 467,318 | | | $ | — | |
| | | | | | | | | | | | | |
F-25
Houston, Texas. On March 28, 2006, the Company’s board of directors approved the shut-down of the Houston, Texas facility. The Houston, Texas facility was used primarily for research and development purposes and is maintained under an operating lease which ends on April 30, 2008. The research and development activities conducted in Houston were transferred to the Company’s facilities located in Exton, Pennsylvania. An exit plan was communicated to the affected employees during the three months ended June 30, 2006. There were approximately 15 employees at the Houston, Texas facility at March 31, 2006 and the large majority of these employees had been terminated by June 30, 2006 at a severance cost of less than $0.1 million. The Company currently subleases approximately 50% of the facility to an unrelated third party. As of December 31, 2007, the remaining lease payments and common operating expenses due under the Houston, Texas lease agreement were less than $0.1 million.
Note 6—Available-for-Sale Investments
The Company had no available-for-sale investments at December 31, 2007 or 2006. Proceeds from the sale of available-for-sale marketable debt securities were $0.0 million, $25.7 million and $106.8 million for the years ended December 31, 2007, 2006 and 2005, respectively, and no realized gains and losses based on specific identification, were included in the results of operations upon those sales.
Note 7—Property and Equipment
Property and equipment is comprised of:
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| Leasehold improvements | | $ | 3,751,030 | | | $ | 3,747,561 | |
| Lab equipment | | | 1,423,840 | | | | 1,304,813 | |
| Computer equipment and software | | | 1,101,097 | | | | 1,059,205 | |
| Office furniture and fixtures | | | 41,407 | | | | 36,151 | |
| | | | | | | |
| | | | 6,317,374 | | | | 6,147,730 | |
| Less: Accumulated depreciation and amortization | | | (2,921,651 | ) | | | (1,816,125 | ) |
| | | | | | | |
| Property and equipment, net | | $ | 3,395,723 | | | $ | 4,331,605 | |
| | | | | | | |
The amounts of depreciation and amortization expense for the above property and equipment included in the statement of operations are as follows:
| | | | | | | | | | | | | |
| | | Year ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| Depreciation expense related to continuing operations: Selling, general, administrative, research and development expenses | | $ | 1,168,251 | | | $ | 1,174,404 | | | $ | 649,505 | |
During the third quarter of 2005, the Company determined that a certain third-party developed software system (the “MES” system) was impaired and, accordingly, recorded a charge of $1.3 million to selling, general and administrative expense in order to remove the related cost of the asset and associated depreciation. Previous to this charge, certain components of the MES system had been placed in use and certain components were still in development. The gross balance and accumulated depreciation related to the MES system components placed in use were $0.6 million and $0.1 million, respectively, at the time of the impairment charge and was being depreciated over five years. The balance related to the MES system components still in development, for which amortization had not commenced at the time of the impairment charge, was $0.9 million.
F-26
Note 8—Accrued Expenses
Accrued expenses are comprised of the following:
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| Accrued professional fees | | $ | 2,243,319 | | | $ | 1,057,110 | |
| Accrued compensation | | | 840,641 | | | | 916,172 | |
| Accrued severance | | | 379,402 | | | | 146,827 | |
| Accrued interest | | | 525,000 | | | | 525,000 | |
| Accrued other | | | 359,894 | | | | 240,932 | |
| | | | | | | |
| Accrued expenses | | $ | 4,348,256 | | | $ | 2,886,041 | |
| | | | | | | |
Note 9—Convertible Subordinated Notes
On November 3, 2004, the Company completed the private placement of $75.0 million aggregate principal amount of 3.5% Convertible Subordinated Notes Due 2024 (the “3.5% Subordinated Notes”). The 3.5% Subordinated Notes could be due sooner than 2024, as discussed below. The Company received net proceeds of approximately $71.7 million after the deduction of commissions and offering expenses. The Company also granted the purchasers of the 3.5% Subordinated Notes the option to purchase up to $15.0 million of additional 3.5% Subordinated Notes through December 2, 2004. On November 5, 2004, the Company completed the private placement of the additional $15.0 million aggregate principal amount of 3.5% Subordinated Notes. The Company received net proceeds of approximately $14.5 million after the deduction of discounts, commissions and offering expenses. The total net proceeds to the Company were approximately $86.2 million after the deduction of commissions and offering expenses.
The Company used approximately $26 million of the net proceeds to repurchase 4,000,000 shares of its common stock, of which 2,000,000 shares were repurchased from Frank DeLape, who was then the Chairman of the Board of Directors, Michael Macaluso, a former director and the former President and Chief Executive Officer, Olga Marko, the former Senior Vice President and Director of Research, Michael Avignon, the former Manager of International Operations, and Timothy J. Till, a shareholder. The purchase price from the insiders, affiliates and founders of the Company listed above was $6.33 per share which represented a 5% discount from the closing price of the Company’s common stock on the American Stock Exchange on October 28, 2004, the date the offering of the 3.5% Subordinated Notes was priced. The purchase of the shares from the insiders was approved by a special committee of independent directors in partial reliance on a fairness opinion issued by an investment bank. The remaining 2,000,000 shares were repurchased in private transactions at a price of $6.66 per share. The remaining net proceeds of approximately $60.2 million were added to the Company’s general working capital.
The 3.5% Subordinated Notes are unsecured obligations and are subordinated in right of payment to all of the Company’s existing and future senior indebtedness. The 3.5% Subordinated Notes are also effectively subordinated to all indebtedness and other liabilities of the Company’s subsidiaries.
The 3.5% Subordinated Notes require the semi-annual payment of interest, on May 1 and November 1 of each year beginning May 1, 2005, at 3.5% interest per annum on the principal amount outstanding. The 3.5% Subordinated Notes will mature on November 1, 2024. Prior to maturity the holders may convert their 3.5% Subordinated Notes into shares of the Company’s common stock. The initial conversion rate is 109.2001 shares per $1,000 principal amount of 3.5% Subordinated Notes, which is equivalent to an initial conversion price of approximately $9.16 per share.
On or after November 1, 2009, the Company may at its option redeem the 3.5% Subordinated Notes, in whole or in part, for cash, at a redemption price equal to 100% of the principal amount of the 3.5% Subordinated Notes to be redeemed plus accrued and unpaid interest.
On each of November 1, 2009, November 1, 2014 and November 1, 2019, the holders may require the Company to purchase all or a portion of their 3.5% Subordinated Notes at a purchase price in cash equal to 100% of the principal amount of 3.5% Subordinated Notes to be purchased plus accrued and unpaid interest. The holders of the 3.5% Subordinated Notes may also require the Company to repurchase their 3.5% Subordinated Notes in the event its common stock (or other common stock into which the 3.5% Convertible Subordinated Notes are then convertible) ceases to be listed for trading on a U.S. national securities exchange or approved for trading on an established automated over-the-counter market in the United States.
F-27
In the event a change in control occurs on or before November 9, 2009, the holders of the 3.5% Subordinated Notes may require the Company to purchase all or a portion of their notes at a purchase price equal to 100% of the principal amount of the 3.5% Subordinated Notes to be purchased plus accrued and unpaid interest and the payment of a “make-whole” payment which is based on the date on which the change in control occurs and the price per share paid for the Company’s common stock in such change in control transaction. The Company will be allowed to pay for the repurchase of the 3.5% Subordinated Notes and accrued and unpaid interest in cash or, at its option, shares of its common stock, and the Company will be allowed to make the make-whole payment in cash or, at its option, such other form of consideration as is paid to its common stockholders in the change in control transaction. In addition, in the event a change in control occurs on or before November 9, 2009, the holders of the 3.5% Subordinated Notes that convert their 3.5% Subordinated Notes into shares of the Company’s common stock in connection with such change in control transaction will also be entitled to receive the make-whole payment.
The 3.5% Subordinated Notes were issued in an offering not registered under the Securities Act of 1933, as amended (“the Securities Act”). However, the Company was obligated to file with the SEC a shelf registration statement covering resales of the 3.5% Subordinated Notes and the shares of the Company’s common stock issuable upon the conversion of the 3.5% Subordinated Notes. The shelf registration statement was subsequently declared effective on May 2, 2005. As of December 31, 2007, the Company has recorded $90.0 million of long-term debt as a long-term liability on the accompanying consolidated balance sheet.
Note 10—Income Taxes
Isolagen, Inc. and Isolagen Technologies, Inc. file a consolidated U.S. Federal income tax return. During the third quarter of 2006, the Company acquired a 57% interest in Agera (see Note 4). Agera files a separate U.S. Federal income tax return. The Company’s foreign subsidiaries, which comprise loss from discontinued operations, file income tax returns in their respective jurisdictions. The geographic source of loss from continuing operations is the United States.
The components of the income tax benefit below, which relate solely to continuing operations, are as follows:
| | | | | | | | | | | | | |
| | | Year ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| United States | | | | | | | | | | | | |
| Current | | $ | — | | | $ | — | | | $ | — | |
| Deferred | | | — | | | | 190,754 | | | | — | |
| Foreign | | | | | | | | | | | | |
| Current | | | — | | | | — | | | | — | |
| Deferred | | | — | | | | — | | | | — | |
| | | | | | | | | | |
| Total income tax benefit | | $ | — | | | $ | 190,754 | | | $ | — | |
| | | | | | | | | | |
The reconciliation between income tax expense (benefit) at the U.S. federal statutory rate and the amount recorded in the accompanying consolidated financial statements is as follows:
| | | | | | | | | | | | | |
| | | Year ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| Tax at U.S. federal statutory rate | | $ | (11,860,007 | ) | | $ | (8,169,068 | ) | | $ | (8,993,907 | ) |
| Increase in domestic valuation allowance | | | 13,833,925 | | | | 10,112,079 | | | | 10,124,461 | |
| State income taxes before valuation allowance, net of federal benefit | | | (1,694,287 | ) | | | (1,336,884 | ) | | | (1,284,798 | ) |
| Other | | | (279,631 | ) | | | (796,881 | ) | | | 154,244 | |
| | | | | | | | | | |
| | | $ | — | | | $ | (190,754 | ) | | $ | — | |
| | | | | | | | | | |
As discussed in Note 4, the purchase accounting for the acquisition of Agera’s resulted in the recording of a deferred tax liability of $0.2 million related to the difference between the tax and financial reporting bases (after the allocation of the purchase price) of Agera’s assets and liabilities. Agera recorded a deferred tax benefit during the three months ended December 31, 2006 related to its net operating losses during that period. Such deferred tax benefit was limited to the initial deferred tax liability recorded upon acquisition.
F-28
The components of the Company’s deferred tax assets (liabilities) at December 31, 2007 and 2006 are as follows:
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| Deferred tax assets and liabilities: | | | | | | | | |
| Loss carryforwards | | $ | 50,360,697 | | | $ | 37,251,873 | |
| Accrued expenses and other | | | 1,568,776 | | | | 1,160,496 | |
| Stock option compensation | | | 2,039,475 | | | | 1,316,388 | |
| Property and equipment | | | 814,503 | | | | 646,808 | |
| | | | | | | |
| | | | 54,783,451 | | | | 40,375,565 | |
| Less: Valuation allowance | | | (54,783,451 | ) | | | (40,375,565 | ) |
| | | | | | | |
| | | $ | — | | | $ | — | |
| | | | | | | |
As of December 31, 2007, the Company had generated U.S. net operating loss carryforwards of approximately $108.4 million which expire from 2011 to 2026 and net loss carryforwards in certain non-US jurisdictions of approximately $29.9 million. These net operating loss carryforwards are available to reduce future taxable income. However, a, change in ownership, as defined by federal income tax regulations, could significantly limit the Company’s ability to utilize its U.S. net operating loss carryforwards. Additionally, because federal tax laws limit the time during which the net operating loss carryforwards may be applied against future taxes, if the Company fails to generate taxable income prior to the expiration dates it may not be able to fully utilize the net operating loss carryforwards to reduce future income taxes. As the Company has had cumulative losses and there is no assurance of future taxable income, valuation allowances have been recorded to fully offset the deferred tax asset at December 31, 2007 and 2006. The valuation allowance increased $14.4 million, $13.4 million and $12.7 million during 2007, 2006 and 2005, respectively, due primarily to the Company’s 2007, 2006 and 2005 net losses, respectively.
Note 11—Commitments and Contingencies
Federal Securities Litigation
The Company and certain of its current and former officers and directors are defendants in class action cases pending in the United States District Court for the Eastern District of Pennsylvania.
In August 2005 and September 2005, various lawsuits were filed alleging securities fraud and asserting claims on behalf of a putative class of purchasers of publicly traded Isolagen securities between March 3, 2004 and August 1, 2005. These lawsuits wereElliot Liff v. Isolagen, Inc. et al., C.A. No. H-05-2887, filed in the United States District Court for the Southern District of Texas;Michael Cummiskey v. Isolagen, Inc. et al., C.A. No. 05-cv-03105, filed in the United States District Court for the Southern District of Texas;Ronald A. Gargiulo v. Isolagen, Inc. et al., C.A. No. 05-cv-4983, filed in the United States District Court for the Eastern District of Pennsylvania, andGregory J. Newman v. Frank M. DeLape, et al., C.A. No. 05-cv-5090, filed in the United States District Court for the Eastern District of Pennsylvania.
TheLiffandCummiskeyactions were consolidated on October 7, 2005. TheGargioloandNewmanactions were consolidated on November 29, 2005. On November 18, 2005, the Company filed a motion with the Judicial Panel on Multidistrict Litigation (the “MDL Motion”) to transfer the Federal Securities Actions and theKeenederivative case (described below) to the United States District Court for the Eastern District of Pennsylvania. TheLiffandCummiskey actions were stayed on November 23, 2005 pending resolution of the MDL Motion. TheGargiuloandNewmanactions were stayed on December 7, 2005 pending resolution of the MDL Motion. On February 23, 2006, the MDL Motion was granted and the actions pending in the Southern District of Texas were transferred to the Eastern District of Pennsylvania, where they have been captionedIn re Isolagen, Inc. Securities & Derivative Litigation, MDL No. 1741 (the “Federal Securities Litigation”).
On April 4, 2006, the United States District Court for the Eastern District of Pennsylvania appointed Silverback Asset Management, LLC, Silverback Master, Ltd., Silverback Life Sciences Master Fund, Ltd., Context Capital Management, LLC and Michael F. McNulty as Lead Plaintiffs, and the law firms of Bernstein Litowitz Berger & Grossman LLP and Kirby McInerney & Squire LLP as Lead Counsel in the Federal Securities Litigation.
F-29
On July 14, 2006, Lead Plaintiffs filed a Consolidated Class Action Complaint in the Federal Securities Litigation on behalf of a putative class of persons or entities who purchased or otherwise acquired Isolagen common stock or convertible debt securities between March 3, 2004 and August 9, 2005. The complaint purports to assert claims for securities fraud in violation of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 against Isolagen and certain of its former officers and directors. The complaint also purports to assert claims for violations of Section 11 and 12 of the Securities Act of 1933 against the Company and certain of its current and former directors and officers in connection with the registration and sale of certain shares of Isolagen common stock and certain convertible debt securities. The complaint also purports to assert claims against CIBC World Markets Corp., Legg Mason Wood Walker, Inc., Canaccord Adams, Inc. and UBS Securities LLC as underwriters in connection with an April 2004 public offering of Isolagen common stock and a 2005 sale of convertible notes. On November 1, 2006, the defendants moved to dismiss the complaint. On September 26, 2007, the court denied the Company’s motions to dismiss the complaint. On November 6, 2007, the court entered a scheduling order that provides for discovery to be complete by June 8, 2009.
The Company intends to defend these lawsuits vigorously. However, the Company cannot currently estimate the amount of loss, if any, that may result from the resolution of these actions, and no provision has been recorded in the consolidated financial statements. The Company will expense its legal costs as they are incurred and will record any insurance recoveries on such legal costs in the period the recoveries are received.
Derivative Actions
The Company is the nominal defendant in derivative actions (the “Derivative Actions”) pending in State District Court in Harris County, Texas, the United States District Court for the Eastern District of Pennsylvania, and the Court of Common Pleas of Chester County, Pennsylvania.
On September 28, 2005, Carmine Vitale filed an action styled, Case No. 2005-61840,Carmine Vitale v. Frank DeLape, et al.in the 55th Judicial District Court of Harris County, Texas and in February 2006 Mr. Vitale filed an amended petition. In this action, the plaintiff purports to bring a shareholder derivative action on behalf of the Company against certain of the Company’s current and former officers and directors. The Plaintiff alleges that the individual defendants breached their fiduciary duties to the Company and engaged in other wrongful conduct. Jeffrey Tomz, who formerly served as Isolagen’s Chief Financial Officer, was accused of engaging in insider trading of Isolagen stock through a proxy. The plaintiff did not make a demand on the Board of Isolagen prior to bringing the action and plaintiff alleges that a demand was excused under the law as futile.
On December 2, 2005, the Company filed its answer and special exceptions pursuant to Rule 91 of the Texas Rules of Civil Procedure based on pleading defects inherent in the Vitale petition. The plaintiff filed an amended petition on February 15, 2006, to which the defendants renewed their special exceptions. On September 6, 2006, the Court granted the special exceptions and permitted the plaintiff thirty days to attempt to replead. Thereafter the plaintiff moved the Court for an order compelling discovery, which the Court denied on October 2, 2006. On October 18, 2006, the Court entered an order explaining its grounds for granting the special exceptions. On November 3, 2006, the plaintiff filed a second amended petition. On February 8, 2007, the Company filed its answer and special exceptions to the second amended petition. On August 9, 2007, the Court granted the special exceptions and dismissed the second amended petition with prejudice. On September 4, 2007, the plaintiff moved for reconsideration of the dismissal with prejudice of the second amended petition, for a new trial, and for leave to further amend the petition, and the defendants opposed that motion on September 20, 2007. On October 23, 2007, that motion was deemed denied by operation of law because the court had not acted on it by that date.
On October 8, 2005, Richard Keene filed an action styled, C.A. No. H-05-3441, Richard Keene v. Frank M. DeLape et al., in the United States District Court for the Southern District of Texas. This action makes substantially similar allegations as the original complaint in the Vitale action. The plaintiff also alleges that his failure to make a demand on the Board prior to filing the action is excused as futile.
The Company sought to transfer the Keene action to the United States District Court for the Eastern District of Pennsylvania as part of the MDL Motion. On January 21, 2006, the court stayed the Keene action pending resolution of the MDL Motion. On February 23, 2006, the Keene action was transferred with the Federal Securities Actions from the Southern District of Texas to the Eastern District of Pennsylvania. Thereafter, on May 15, 2006, the plaintiff filed an amended complaint, and on June 5, 2006, the defendants moved to dismiss the amended complaint. On August 21, 2006, the plaintiff moved for leave to file a second amended complaint, and on September 15, 2006, defendants filed an opposition to that motion. On January 24, 2007, the court denied the plaintiff’s motion to file a second amended complaint, and on April 10, 2007 the court granted the defendants’ motion to dismiss and dismissed the amended complaint without prejudice. On May 9, 2007, plaintiff filed a notice of appeal from the January 24, 2007 order denying plaintiff’s motion to file a second amended complaint, and from the April 10, 2007 order dismissing plaintiff’s amended complaint without prejudice. The appeal is fully briefed.
F-30
On October 31, 2005, William Thomas Fordyce filed an action styled, C.A. No. GD-05-08432, William Thomas Fordyce v. Frank M. DeLape, et al., in the Court of Common Pleas of Chester County, Pennsylvania. This action makes substantially similar allegations as the original complaint in the Vitale action. The plaintiff also alleges that his failure to make a demand on the Board prior to filing the action is excused as futile.
On January 20, 2006, the Company filed its preliminary objections to the complaint. On August 31, 2006, the Court of Common Pleas entered an opinion and order sustaining the preliminary objections and dismissing the complaint with prejudice. On September 19, 2006, Fordyce filed a motion for reconsideration, which the Court of Common Pleas denied. On September 28, 2006, Fordyce filed a notice of appeal to the Superior Court of Pennsylvania. On July 27, 2007, the Superior Court affirmed the decision of the Court of Common Pleas.
On February 14, 2008, Ronald Beattie filed an action styled C.A. No. 08-724, Ronald Beattie v. Michael Macaluso, et al., in the United States District Court for the Eastern District of Pennsylvania. This action makes substantially similar allegations as the original complaint in the Vitale action. The complaint has not yet been served on the Company.
The Derivative Actions are purportedly being prosecuted on behalf of the Company and any recovery obtained, less any attorneys’ fees awarded, will go to the Company. The Company is advancing legal expenses to certain current and former directors and officers of the Company who are named as defendants in the Derivative Actions and expects to receive reimbursement for those advances from its insurance carriers. The Company will expense its legal costs as they are incurred and will record any insurance recoveries on such legal costs in the period the recoveries are received. The Company cannot currently estimate the amount of loss, if any, that may result from the resolution of these actions, and no provision has been recorded in the consolidated financial statements.
Indemnity Demands
Mr. Jeffrey Tomz
After the above referenced litigations were commenced, Mr. Jeffrey Tomz, who formerly served as Isolagen’s Chief Financial Officer, demanded reimbursement of his costs of defense, and reimbursement for the costs of responding to a Securities and Exchange Commission investigation of his alleged insider trading in Isolagen stock. It is understood that Tomz’s defense costs to date amount to approximately $0.3 million.
As the Vitale matter has now been resolved in favor of all defendants, including Mr. Tomz, the Company is presently obligated to reimburse him for the reasonable and necessary costs of defending all claims asserted therein other than the insider trading allegations. Although decided on jurisdictional grounds, it is likely the Company is also obligated to reimburse Mr. Tomz for the reasonable and necessary costs incurred in defending the Fordyce matter given that it has also been resolved in favor of all defendants. The Company would be liable to reimburse Mr. Tomz for the reasonable and necessary costs of defense in the Keene case if it is affirmed on appeal and in the putative securities cases should he prevail in that action. The Company has refused to pay the amount of fees and expenses for which Mr. Tomz has sought reimbursement because it believes they are excessive, duplicative and have not been properly segregated between reimbursable and non-reimbursable claims. The Company has negotiated an acceptable compromise for the amounts billed by Mr. Tomz’s local Pennsylvania counsel for an amount less than $0.1 million.
Prior to the resolution of the various derivative actions, Mr. Tomz filed a demand for arbitration seeking advancement of his defense costs. He subsequently agreed to stay those proceedings. At present, Mr. Tomz has not sought to lift this stay and it is uncertain whether he will attempt to do so in the future.
The Company has accrued less than $0.1 million in the accompanying Consolidated Financial Statements as of December 31, 2007 with respect to Mr. Tomz’s existing defense costs in dispute. The Company intends to seek reimbursement under its directors and officers liability policy for any amounts paid to reimburse Mr. Tomz for his defense costs, which includes less than $0.1 million paid to date.
Underwriters
The Underwriters have each demanded that the Company indemnify, hold harmless and defend them with respect to the claims asserted in the putative securities actions. The total amount demanded to date is less than $0.3 million, however, we believe that cumulative future amounts of these defense costs are likely to be significant, although these cumulative future amounts cannot be estimated at this time.
F-31
Isolagen has denied this demand on numerous grounds, including that: (i) as to the November 2004 convertible notes offering, it only agreed to indemnify the Underwriters for any losses resulting from any untrue statement, or alleged untrue statement, of material fact in the offering documents provided by the Company to a holder or prospective purchaser of securities, and plaintiffs’ claims in the securities action are not based upon alleged untrue statements in any such documents; (ii) as to the June 2004 secondary offering, it only agreed to indemnify the Underwriters for untrue statements, or alleged untrue statements, of material fact, in the preliminary prospectus, registration statement, prospectus or any amendment thereof, and there were no such untrue statements in any such document; (iii) Isolagen assumed no duty to defend or advance defense costs; and (iv) Isolagen satisfied whatever duty to defend it may have to the Underwriters by offering to assume the Underwriters’ defense. Accordingly, the Company has have not accrued any amounts related to the Underwriters’ defense costs in our Consolidated Financial Statements.
Dispute with Former President and Member of the Board of Directors
On March 16, 2007, the Company disclosed in its Form 10-K for the year ended December 31, 2006 (the “Form 10-K”), that the Company and Susan Ciallella had reached an understanding pursuant to which Ms. Ciallella would resign from the Company in all capacities. The understanding, which was described in the Form 10-K, was subject to the negotiation and execution of a definitive agreement. On May 10, 2007, the Company disclosed in its Form 10-Q for the quarter ended March 31, 2007, that no such definitive agreement had been concluded with Ms. Ciallella, and that Ms. Ciallella was asserting claims against the Company in connection with her separation from the Company. On June 8, 2007, the Company and Ms. Ciallella participated in a voluntary mediation before a former federal judge. Upon conclusion of the mediation, the Company and Ms. Ciallella entered into a Settlement Agreement and Release (the “Settlement Agreement”) pursuant to which the parties agreed to settle and resolve all claims that Ms. Ciallella may have against the Company as well as all aspects of Ms. Ciallella’s separation from the Company. The Settlement Agreement provided Ms. Ciallella the following:
(i) severance payments as follows: (a) $450,000, which was paid by June 2007; (b) $240,000 paid on September 17, 2007; and (c) $40,000 per month to be paid each month beginning October 17, 2007 through July 15, 2008 with a $20,000 payment to be made on July 30, 2008;
(ii) $1,745,000, which was paid during June 2007 in satisfaction and settlement of Ms. Ciallella’s legal claims relating to her termination;
(iii) $5,000 paid during June 2007 in connection with Ms. Ciallella’s release of claims under the Age Discrimination in Employment Act;
(iv) $159,245 paid during June 2007 for the reimbursement of Ms. Ciallella’s legal fees in connection with the negotiation and execution of the Settlement Agreement;
(v) $198,950 related to Ms. Ciallella’s legal support services to be provided to the Company, of which approximately $158,000 had been paid as of December 31, 2007;
(vi) Ms. Ciallella retained 300,000 of the 400,000 performance options issued to her in June 2006, which expire in June 2016; Ms. Ciallella retained 160,000 of the options issued to her in April 2006, which expire in April 2016 and which were fully vested; and Ms. Ciallella retained her vested 300,000 options, which were issued to her in April 2005 and expire in April 2015 (see Note 13).
Each of the Company and Ms. Ciallella released the other party from any and all claims that it/she may have; and Ms. Ciallella agreed to resign from all officer and director positions she held with the Company or any of its subsidiaries.
During the three months ended March 31, 2007, the Company recorded termination costs aggregating $2.6 million to reflect the March 16, 2007 understanding between the parties, which were included in selling, general, and administrative expenses. As a result of the June 2007 Settlement Agreement, during the three months ended June 30, 2007 the Company recorded an additional $2.0 million of termination costs, which are also included in selling, general, and administrative expenses. No such expenses were incurred during the three months ended September 30, 2007. Accordingly, during the year ended December 31, 2007, the Company recorded total termination costs of approximately $4.6 million, as follows:
F-32
| | | | | |
| | | Year ended | |
| (in millions) | | December 31, 2007 | |
| Salary and severance | | $ | 2.8 | |
| Consulting fee | | | 0.2 | |
| Legal fee reimbursement to Ms. Ciallella | | | 0.2 | |
| Company legal fees | | | 0.3 | |
| Stock option modifications (see Note 13) | | | 1.1 | |
| | | | |
| | | $ | 4.6 | |
| | | | |
Of the $0.3 million of Company legal fees discussed above, approximately $133,000 related to the Board of Directors’ Special Committee legal counsel retained in connection with the Ciallella matter and was paid directly by the Company. Less than $10,000 related to the legal fees of one the Company’s Board members in connection with the Ciallella matter was paid directly by the Company. Also, less than $10,000 related to the legal fees of our Chief Executive Officer in connection with the Ciallella matter was paid directly by the Company. The Company is pursuing reimbursement of the amounts paid in excess of Ms. Ciallella’s contractual severance, plus defense costs, from its insurance carriers. There can be, however, no assurance that there will be a recovery or if there is, of the amount thereof. As of December 31, 2007, $0.3 million remains due to Ms. Ciallella and is recorded in accrued expenses on the consolidated balance sheet.
United Kingdom Customer Settlement
During 2005, the Company began an informal study and surveyed a number of patients who had previously received the Isolagen treatment to assess patient satisfaction. Some patients surveyed reported sub-optimal results from treatment. One hundred forty-nine patients who claimed to have received sub-optimal results were retreated for the purpose of determining the reasons for sub-optimal results. Only those patients who completed the survey, provided adequate medical records including before and after photographs and who were deemed both to have received a sub-optimal result from a first treatment administered according to the Isolagen protocol and who were considered to be appropriate patients for treatment with the Isolagen Therapy received re-treatment. No one completing the survey was offered re-treatment unless they agreed to these conditions. Following re-treatment, a number of patients reported better results than first obtained through the initial treatment by their initial treating physician.
During the first quarter of 2006, the Company received a number of complaints from certain patients who had learned of the limited re-treatment program and also learned that a number of physicians with dissatisfied patients were generating public ill-will as a result of the Company’s decision to limit the number of patients offered re-treatment and were encouraging dissatisfied patients to seek recourse against the Company. In response, in March 2006 the Company decided that it was in its best interest to address these complaints to foster goodwill in the marketplace and avoid the cost of any potential patient claims. Accordingly, the Company agreed to resolve any properly documented and substantiated patient complaints by offering to retreat the patient pursuant to the same criteria stated above or pay £1,000 (approximately US$1,750) to the patients identified to the Company as having received a sub-optimal result. In order to qualify for re-treatment and in addition to the criteria set forth above, the patient will be treated by a physician identified by the Company who will treat these patients pursuant to a protocol. In addition, these patients must have agreed to follow-up visits and assessments of their response to treatment. No patient unlikely to benefit from Isolagen Therapy has been or will be retreated.
The Company made this offer to approximately 290 patients during late March 2006. Accordingly, the Company believed its range of liability was between £290,000 (or approximately $0.5 million), assuming all 290 patients were to choose the £1,000 payment, and approximately £580,000 (or approximately $1.0 million), assuming all 290 patients elected to be retreated. The estimated costs for retreatment include the cost of treatment, physician fees and other ancillary costs. The Company estimated that 60% of the patients would elect the £1,000 offer and 40% would elect to be retreated. Accordingly, the Company recorded a charge reflected under loss from discontinued operations for the three months ended March 31, 2006 of $0.7 million. During the three months ended June 30, 2006, an additional 31 patients were entered into the settlement program, resulting in an additional charge reflected under loss from discontinued operations of $0.1 million.
During the year ended December 31, 2006, payments to patients and retreatments reduced the accrual by $0.6 million. During the three months ended March 31, 2007 and June 30, 2007, payments and retreatments to patients reduced the accrual by approximately $0.1 million and $0.0 million, respectively. As of December 31, 2007, the accrual, which is included in current liabilities of discontinued operations in the consolidated balance sheet, was $0.1 million. The estimates related to this liability may change in future periods and the effects of any changes will be accounted for in the period in which the estimate changes.
F-33
Subsequent to the Company’s public announcement regarding the closure of the United Kingdom operation, the Company received negative publicity and negative correspondence from former patients in the United Kingdom that previously received our treatment. More recently, the Company received a written demand by an attorney representing approximately 82 former patients each claiming negligent misstatements were made and each claiming, on average, £3,500 (or approximately $7,000), plus unquantified interest and incidental expenses. The Company is in the process of evaluating the merits of the claims made in the demand. To date, no formal legal action has been brought by the attorney against the Company, and no provision has been recorded in the consolidated financial statements related to this matter.
Other Litigation
The Company is involved in various other legal matters that are being defended and handled in the ordinary course of business. Although it is not possible to predict the outcome of these other matters, management currently believes that the results will not have a material impact on the Company’s financial statements.
Leases
The Company has entered into leases for office, warehouse and laboratory facilities in Exton, Pennsylvania, Houston, Texas, Santa Barbara, California and London, England under third party non-cancelable operating leases through 2010. Future minimum lease commitments at December 31, 2007 are as follows:
| | | | | |
| Year Ending December 31, | | | | |
| 2008 | | $ | 1,350,058 | |
| 2009 | | | 1,329,907 | |
| 2010 | | | 1,154,411 | |
| 2011 | | | 1,119,312 | |
| 2012 | | | 1,119,312 | |
| Thereafter | | | 279,828 | |
| | | | |
| Total | | $ | 6,352,828 | |
| | | | |
For the years ended December 31, 2007, 2006 and 2005, rental expense totaled $1.7 million, $1.8 million and $1.8 million, respectively (which includes rent expense related to discontinued operations of $0.4 million, $0.7 million and $0.7 million for the years ended December 31, 2007, 2006 and 2005, respectively).
In August 2006, the Company entered into a non-cancelable two year operating lease for approximately 2,200 square feet in Santa Barbara, California. This office space houses certain members of our senior management team.
In April 2005, the Company entered into a non-cancelable three year operating lease for approximately 86,500 square feet in Exton, Pennsylvania. This facility houses members of our senior management team, quality and manufacturing personnel, and the corporate finance department. The Company began constructing a production line in a portion of this facility in anticipation of eventual FDA approval. The facility was completed during September 2005. This production line is expected to be utilized for the production of clinical supplies. The non-cancelable portion of the lease expires on March 31, 2008, provided however that if the lease is not cancelled by the Company prior to March 31, 2007, at least one year prior to the end of the non-cancelable portion of the lease, then the lease shall end on March 31, 2013. The Company would then have the option to extend the term of the lease for five years, beginning on April 1, 2013. During 2007, the Company extended the lease through March 31, 2013, and accordingly, the Company amortizes its leasehold improvements related to this facility through March 31, 2013. Lease expense is recognized on a straight-line basis through March 31, 2013. The Exton, Pennsylvania minimum lease payments have been included in the future minimum lease commitments table above through March 31, 2013.
Certain former officers of the Company had previously provided office space and laboratory facilities in Houston, Texas at no charge until August 2003. Beginning September 2003, the lease rate was approximately $1.80 per month per square foot. During the first quarter of 2005, this lease with certain former officers of the Company was terminated. Commencing March 2005, the Company entered into a new lease with a third-party for approximately 14,850 square feet lease of office and laboratory space in Houston, Texas. The lease term is through April 2008. The Company no longer leases or occupies office space or laboratory facilities from related parties.
As discussed in Note 5, in September 2004 the Company adopted a plan to close its Australia facility. The lease related to the Company’s Australia facility was originally due to expire on December, 31, 2004. The Company terminated this lease in October 2005.
F-34
As discussed in Note 3, in April 2005 the Company acquired a two-building corporate campus in Bevaix, Canton of Neuchâtel, Switzerland, which is reflected as assets of discontinued operations held for sale on the accompanying consolidated balance sheets. The Company leases one of these buildings to a third party for approximately $0.3 million per year. This lease began April 15, 2005 and concludes on December 31, 2010.
Distribution agreement
In April 2003, the Company entered into a distribution agreement with Equipmed Pty. Ltd (“Equipmed”). Equipmed had the exclusive right as the Company’s distributor in Australia and New Zealand of services utilizing the Company’s technology for its autologous cellular system for soft tissue regeneration and other therapies in the cosmetic dermatological surgery markets (i.e., exclusively for wrinkle and acne reduction) within Australia and New Zealand. The Company terminated this agreement in exchange for a payment to Equipmed of approximately $0.4 million during 2005. Approximately $0.1 million of this payment was charged to loss from discontinued operations in the consolidated statement of operations during the year ended December 31, 2005.
Departure of Former Chief Executive Officers
Effective October 27, 2005 Mr. Frank DeLape also resigned as Chairman of the Board and member of the Board of Directors. In connection with Mr. DeLape’s resignation, Mr. DeLape and the Company entered into a Separation and Release Agreement (the “Agreement”). Pursuant to the Agreement, Mr. DeLape agreed, among other things, to (a) resign all positions with the Company and all of its subsidiaries and to terminate his employment with the Company, (b) certain lock-up and standstill restrictions in respect of shares of the Company’s common stock he and his affiliates own through July 2006, and (c) execute a release for the benefit of the Company and its subsidiaries. The Company agreed, among other things, to pay a separation payment in the amount of $210,000, beginning on Mr. DeLape’s resignation date through March 15, 2006. Mr. DeLape also retained options to purchase (a) 650,000 shares of the Company’s common stock granted on September 1, 2001 at an exercise price of $6.00, which were fully vested as of his resignation date and will be exercisable for a period of two years following his resignation date, (b) 400,000 shares of the Company’s common stock granted on February 25, 2003 at an exercise price of $4.50, which were fully vested as of his resignation date and will be exercisable for a period of five years following his resignation date and (c) 150,000 shares of the Company’s common stock granted on September 5, 2003 at an exercise price of $9.81, which were fully vested as of his resignation date and will be exercisable for a period of three years following his resignation date. All other unexercised options granted to Mr. DeLape to purchase shares of the Company’s common stock were cancelled as of Mr. DeLape’s resignation date. The Amended and Restated Employment Agreement of June 2005 between Mr. DeLape and the Company was terminated. Any options to purchase stock under the 2005 Agreement were cancelled. The separation payment pursuant to the Separation and Release Agreement reflects the amount that would otherwise be owed under Mr. DeLape’s 2003 Employment Agreement reduced by the amount of certain office expense reimbursements paid to him pursuant to the 2005 Agreement.
Mr. Michael Macaluso, former Chief Executive Officer and former Director, entered into an employment agreement dated September 5, 2003, with an initial term ending July 31, 2006 and providing for a base salary of $300,000, subject to the right of the Board of Directors to increase his salary from time to time. Mr. Macaluso resigned from Chief Executive Officer and President effective September 1, 2004. Mr. Macaluso was paid his base salary until July 2006, which was fully accrued at the time of his resignation.
Note 12—Equity
Significant Common Stock Transactions
In August 2003, the Company sold in a private offering 3,359,331 shares of Common Stock, par value $0.001 per share, at an offering price of $6 per share. After deducting the costs and expenses associated with the sale, the Company received net cash totaling $18.5 million.
In June 2004, the Company issued a) 7,200,000 shares of common stock, at $8.50 per share, for cash totaling net $56.8 million in connection with the secondary offering completed in June 2004; and b) 51,828 shares of common stock in exchange for cashless exercise of warrants.
In June 2007, the Company filed a shelf registration statement on Form S-3, which was subsequently declared effective by the SEC. The shelf registration allowed the Company the flexibility to offer and sell, from time to time, up to an original amount of $50 million of common stock, preferred stock, debt securities, warrants or any combination of the foregoing in one or more future public offerings. In August 2007, the Company sold under this shelf registration statement 6,767,647 shares of common stock to institutional investors, raising proceeds of $13.8 million, net of offering costs. The Company may offer and sell up to an additional $36.2 million of common stock pursuant to this shelf registration.
F-35
Refer to the consolidated statement of shareholders’ equity (deficit) and comprehensive loss for common stock transactions from the period December 28, 1995 through December 31, 2007.
Treasury Stock
In November 2004, the Company repurchased 4,000,000 shares of its common stock for an aggregate of approximately $26.0 million, of which 2,000,000 shares were repurchased from Frank DeLape, who was then the Chairman of the Board of Directors, Michael Macaluso, a former director and the former President and Chief Executive Officer, Olga Marko, the former Senior Vice President and Director of Research, Michael Avignon, the former Manager of International Operations, and Timothy J. Till, a shareholder. The purchase price from the insiders, affiliates and founders of the Company listed above was $6.33 per share which represented a 5% discount from the closing price of the Company’s common stock on the American Stock Exchange on October 28, 2004, the date the offering of the 3.5% Subordinated Notes was priced. The purchase of the shares from the insiders was approved by a special committee of independent directors in partial reliance on a fairness opinion issued by an investment bank.
2003 Conversion of Series A Convertible Preferred Stock and Series B Convertible Preferred Stock
In July 2002, the Company completed a private offering of 2,895,000 shares of Series A Convertible Preferred Stock, par value $0.001 per share, at an offering price of $3.50 per share. Each share of Series A Preferred Stock was convertible into two shares of common stock at any time after issuance and accrued dividends at 8% per annum payable in cash or additional shares of Series A Preferred Stock. In conjunction with this private offering, the Company issued to the placement agent warrants to purchase 1,158,000 shares of common stock with an exercise price of $1.93 per share. The warrants were exercisable immediately after grant and expire five years thereafter. The fair market of the warrants granted to the placement agent, based on the Black-Scholes valuation model, is estimated to be $1.57 per warrant. The value of the warrants granted were offset against the proceeds received from the sale of the Series A Preferred Stock. During the year ended December 31, 2002, the Company issued an additional 143,507 shares of Series A Preferred Stock in lieu of cash for payment of dividends on the Series A Preferred Stock totaling approximately $0.5 million.
The price of the preferred stock sold was $3.50 per share. The market value of the Company’s common stock sold on the dates that the preferred stock sold or was issued as a dividend had a range of $2.30 — $5.40 per common share. In accordance with EITF 00-27 this created a beneficial conversion to the holders of the preferred stock and a deemed dividend to the preferred stockholders totaling $10.2 million was recorded by the Company with a corresponding amount recorded as additional paid-in capital. The deemed dividend associated with the beneficial conversion is calculated as the difference between the fair value of the underlying common stock less the proceeds that have been received for the Series A Preferred Stock limited to the value of the proceeds received.
In May 2003, the Company sold in a private offering 155,750 shares of Series B Convertible Preferred Stock, par value $0.001 per share, at an offering price of $28 per share. Each share of Series B preferred stock is convertible into 8 shares of common stock at any time after issuance and accrues dividends at 6% per annum payable in cash or additional shares of Series B Preferred Stock. After deducting the costs and expenses associated with the sale, the Company received cash totaling $3.9 million. In conjunction with the private offering, the Company issued to the placement agent warrants to purchase 124,600 shares of common stock with an exercise price of $3.50 per share. The warrants are exercisable immediately after grant and expire five years thereafter. The fair value of the warrants granted to the placement agent, based on the Black-Scholes valuation model is estimated to be $2.77 per warrant. The value of the warrants granted has been offset from the proceeds received from the sale of the Series B Preferred Stock and recorded as additional paid in capital.
The price of the preferred stock sold was $28 per share. The market value of the Company’s common stock sold on the dates that the preferred stock was sold had a range of $4.40 — $4.54 per common share. In accordance with EITF 00-27 this created a beneficial conversion to the holders of the preferred stock and a deemed dividend to the preferred stockholders totaling approximately $1.2 million was recorded by the Company with a corresponding amount recorded as additional paid-in capital. The deemed dividend associated with the beneficial conversion is calculated as the difference between the fair value of the underlying common stock less the proceeds that have been received for the Series B Preferred Stock limited to the value of the proceeds received.
In 2003, all outstanding shares of Series A and Series B Convertible Preferred Stock were converted into 7.3 million shares of common stock.
F-36
Stockholder Rights Plan
In May 2006, the Board of Directors of the Company adopted a Stockholder Rights Plan, as set forth in the Rights Agreement, dated as of May 12, 2006, by and between the Company and American Stock Transfer & Trust Company, a trust company organized under the laws of the State of New York (the “Rights Agent”). Pursuant to the Rights Agreement, stockholders of record at the close of business on May 22, 2006 received one right (“Right”) for each share of Isolagen common stock held on that date. The Rights, which will initially trade with the common stock and represent the right to purchase one ten-thousandth of a share of the Company’s newly created Series C Preferred Stock at $35 per Right, become exercisable when a person or group acquires 15% or more of the Company’s common stock (20% in the case of certain institutional stockholders) or announces a tender offer for 15% or more of the common stock. In that event, in lieu of purchasing the Series C Preferred Stock, the Rights permit the Company’s stockholders, other than the acquiror, to purchase Isolagen common stock having a market value of twice the exercise price of the Rights. In addition, in the event of certain business combinations, the Rights permit holders to purchase the common stock of the acquiror at a 50% discount. Rights held by the acquiror will become null and void in each case.
The Rights have certain anti-takeover effects, in that they would cause substantial dilution to a person or group that attempts to acquire a significant interest in the Company on terms not approved by the Board of Directors. In the event that the Board of Directors determines a transaction to be in the best interests of the Company and its stockholders, the Board of Directors will be entitled to redeem the Rights for $.001 per Right at any time before the tenth business day after the Company’s announcement that a person or group has acquired ownership of 15% or the tenth business day after commencement of a tender or exchange offer for more than 15% of the outstanding common stock. The Rights expire on May 12, 2016.
Note 13—Equity-based Compensation
In December 2004, the Financial Accounting Standards Board (“FASB”) issued Statement of Financial Accounting Standards (“SFAS”) No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123(R)”). SFAS No. 123(R) replaces SFAS No. 123, “Accounting for Stock-Based Compensation”, supersedes APB Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB No. 25”), and amends SFAS No. 95, “Statement of Cash Flows.” SFAS No. 123(R) requires entities to recognize compensation expense for all share-based payments to employees and directors, including grants of employee stock options, based on the grant-date fair value of those share-based payments, adjusted for expected forfeitures. The Company adopted SFAS No. 123(R) as of January 1, 2006 using the modified prospective application method. Under the modified prospective application method, the fair value measurement requirements of SFAS No. 123(R) is applied to new awards and to awards modified, repurchased, or cancelled after January 1, 2006. Additionally, compensation cost for the portion of awards for which the requisite service has not been rendered that were outstanding as of January 1, 2006 is recognized as the requisite service is rendered on or after January 1, 2006. The compensation cost for that portion of awards is based on the grant-date fair value of those awards as calculated for pro forma disclosures under SFAS No. 123. Changes to the grant-date fair value of equity awards granted before January 1, 2006 are precluded.
Prior to the adoption of SFAS No. 123(R), the Company followed the intrinsic value method in accordance with APB No. 25 to account for its employee stock options. Historically, substantially all stock options have been granted with an exercise price equal to the fair market value of the common stock on the date of grant. Accordingly, no compensation expense was recognized from substantially all option grants to employees and directors. Compensation expense was recognized in connection with the issuance of stock options to non-employee consultants in accordance with EITF 96-18, “Accounting for Equity Instruments That are Issued to Other than Employees for Acquiring, or in Conjunction with Selling Goods and Services.” SFAS No. 123(R) did not change the accounting for stock-based compensation related to non-employees in connection with equity based incentive arrangements.
The Company utilizes the straight-line attribution method for recognizing stock-based compensation expense under SFAS No. 123(R). The Company recorded $1.8 million and $1.1 million of compensation expense, net of tax, during the years ended December 31, 2007 and 2006, respectively, for stock option awards to employees and directors based on the estimated fair values, at the grant dates, of the awards. Further, in connection with the separation agreement with the Company’s former President and related modification of the former President’s stock options (see Note 11), the Company recorded $1.1 million in stock option modification expense during the three months ended March 31, 2007, as discussed further below.
F-37
Results for the years ended December 31, 2005 have not been restated. Had compensation expense for employee and director stock options been determined based on fair value at the grant date consistent with SFAS No. 123(R), with stock options expensed using the straight-line attribution method, the Company’s net loss and loss per share would have been increased to the pro forma amounts indicated below:
| | | | | |
| | | Year Ended | |
| | | December 31, | |
| | | 2005 | |
| Net loss—as reported | | $ | (35,777,584 | ) |
| Plus: stock-based employee compensation expense included in reported net loss, net of related tax effects of $0 | | | 44,329 | |
| Less: total stock based employee compensation determined under fair value based method for all awards granted to employees, net of related tax effect of $0 | | | (5,967,467 | ) |
| | | | |
| Net loss—pro forma | | $ | (41,700,722 | ) |
| | | | |
| Net loss per share—as reported | | | | |
| Basic and diluted | | $ | (1.18 | ) |
| | | | |
| Net loss per share—pro forma | | | | |
| Basic and diluted | | $ | (1.38 | ) |
| | | | |
The weighted average fair market value using the Black-Scholes option-pricing model of the options granted was $1.91, $1.42 and $2.90 for the years ended December 31, 2007, 2006 and 2005, respectively. The fair market value of the stock options at the date of grant was estimated using the Black-Scholes option-pricing model with the following weighted average assumptions:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| Expected life (years) | | | 4.1 | | | | 5.3 | | | | 5.0 | |
| Interest rate | | | 4.8 | % | | | 4.9 | % | | | 4.0 | % |
| Dividend yield | | | — | | | | — | | | | — | |
| Volatility | | | 76 | % | | | 79 | % | | | 78 | % |
The risk-free interest rate is based on U.S. Treasury interest rates at the time of the grant whose term is consistent with the expected life of the stock options. Expected volatility is based on the Company’s historical experience. Expected life represents the period of time that options are expected to be outstanding and is based on the Company’s historical experience or the simplified method, as permitted by SEC Staff Accounting Bulletin No. 107 where appropriate. Expected dividend yield was not considered in the option pricing formula since the Company does not pay dividends and has no current plans to do so in the future. The forfeiture rate used was based upon historical experience. As required by SFAS No. 123(R), the Company will adjust the estimated forfeiture rate based upon actual experience.
During December 2005, the Company’s Board of Directors approved the full vesting of all unvested, outstanding stock options issued to current employees and directors. The Board decided to take this action (“the acceleration event”) in anticipation of the adoption of SFAS No. 123(R). As a result of this acceleration event, approximately 1.4 million stock options were vested that would have otherwise vested during 2006 and later periods. At the time of the acceleration event, the unamortized grant date fair value of the affected options was approximately $3.6 million (for SFAS No. 123 and SFAS No. 148 pro forma disclosure purposes), which was charged to pro forma expense in the fourth quarter of 2005. As the Company accelerated the vesting of outstanding employee and director stock options during December 2005, there was no remaining expense related to such options to be recognized in the Company’s statements of operations in future periods.
There were 16,666 stock options exercised during the year ended December 31, 2007, resulting in cash proceeds to the Company of less than $0.1 million. There were 86,000 stock options exercised during the year ended December 31, 2006, resulting in cash proceeds to the Company of $0.2 million. There were 25,000 stock options exercised during the year ended December 31, 2005, resulting in cash proceeds to the Company of $0.1 million. These exercised options in 2007, 2006 and 2005, respectively, had an intrinsic value of less than $0.1 million, $0.2 million and less than $0.1 million. A summary of option activity for the year ended December 31, 2007 is as follows:
F-38
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Weighted | | | | |
| | | | | | | Weighted | | | Average | | | | |
| | | | | | | Average | | | Remaining | | | Aggregate | |
| | | | | | | Exercise | | | Contractual | | | Intrinsic | |
| | | Shares | | | Price | | | Term | | | Value | |
| Outstanding at January 1, 2007 | | | 10,474,833 | | | $ | 4.17 | | | | | | | | | |
| Granted | | | 717,500 | | | | 3.26 | | | | | | | | | |
| Exercised | | | (16,666 | ) | | | 1.58 | | | | | | | | | |
| Forfeited | | | (3,451,668 | ) | | | 5.61 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Outstanding at December 31, 2007 | | | 7,723,999 | | | | 3.45 | | | | 5.67 | | | $ | 2,365,230 | |
| | | | | | | | | | | | | |
| Options exercisable at December 31, 2007 | | | 5,177,161 | | | $ | 4.06 | | | | 5.04 | | | $ | 1,093,007 | |
| | | | | | | | | | | | | |
The following table summarizes the status of the Company’s non-vested stock options since January 1, 2007:
| | | | | | | | | |
| | | Non-vested Options | |
| | | | | | | Weighted- | |
| | | Number of | | | Average Fair | |
| | | Shares | | | Value | |
| Non-vested at January 1, 2007 | | | 3,571,089 | | | $ | 1.41 | |
| Granted | | | 717,500 | | | | 1.91 | |
| Vested | | | (1,345,083 | ) | | | 1.55 | |
| Forfeited | | | (396,668 | ) | | | 1.77 | |
| | | | | | | |
| Non-vested at December 31, 2007 | | | 2,546,838 | | | $ | 1.43 | |
| | | | | | | |
The total fair value of shares vested during the years ended December 31, 2007, 2006 and 2005 was $2.1 million, $1.2 million and $10.6 million, respectively. As discussed above, in December 2005 the Company’s Board of Directors approved the full vesting of all unvested, outstanding stock options issued to current employees and directors. As of December 31, 2007, there was $1.8 million of total unrecognized compensation cost related to non-vested director and employee stock options which vest over time. That cost is expected to be recognized over a weighted-average period of 1.6 years. As of December 31, 2007, there was $0.8 million of total unrecognized compensation cost related to performance-based, non-vested employee stock options. That cost will begin to be recognized when the performance criteria within the respective performance-base option grants become probable of achievement.
2001 Stock Option and Stock Appreciation Rights Plan
Effective August 10, 2001, the Company adopted the Isolagen, Inc. 2001 Stock Option and Stock Appreciation Rights Plan (the “2001 Stock Plan”). The 2001 Stock Plan is discretionary and allows for an aggregate of up to 5,000,000 shares of the Company’s common stock to be awarded through incentive and non-qualified stock options and stock appreciation rights. The 2001 Stock Plan is administered by the Company’s Board of Directors, who has exclusive discretion to select participants who will receive the awards and to determine the type, size and terms of each award granted. As of December 31, 2007, there were 1,984,000 options outstanding under the Stock Plan and 2,390,334 options are available to be issued under the Stock Plan.
During the three months ended March 31, 2007, the Company issued, under the 2001 Stock Plan, 395,000 options to employees and Board of Director members, with exercise prices ranging from $2.37 to $3.10 and with contractual lives of 5 years for employees and 10 years for Board members.
During the three months ended June 30, 2007, the Company issued, under the 2001 Stock Plan, 140,000 options to three employees, with exercise prices ranging from $4.20 to $4.55 and with contractual lives of 5 years.
During the three months ended September 30, 2007, the Company issued, under the 2001 Stock Plan, (1) a total of 102,500 options to four employees with an exercise price of $3.38 per share, which have a five year maximum contractual life and which vest annually over a three year period from the date of grant and (2) a total of 50,000 performance-based options to one employee with an exercise price of $3.38 per share and which have a maximum contractual life of five years. With respect to these 50,000 performance-based stock options, no compensation expense will be recorded until the performance-based vesting event is probable of occurrence. The grant date fair value of this award was less than $0.1 million.
F-39
During the three months ended December 31, 2007, the Company issued, under the 2001 Stock Plan, 30,000 options to one newly appointed board member, with exercise price of $3.14, with a contractual life of 10 years and a cliff-vesting period of one year.
2003 Stock Option and Stock Appreciation Rights Plan
On January 29, 2003, the Company’s Board of Directors approved the 2003 Stock Option and Appreciation Rights Plan (the “2003 Stock Plan”). The 2003 Stock Plan is discretionary and allows for an aggregate of up to 2,250,000 shares of the Company’s common stock to be awarded through incentive and non-qualified stock options and stock appreciation rights. The 2003 Stock Plan is administered by the Company’s Board of Directors, who has exclusive discretion to select participants who will receive the awards and to determine the type, size and terms of each award granted. As of December 31, 2007, there were 1,866,666 options outstanding under the 2003 Stock Plan and 383,334 shares were available for issuance under the 2003 Stock Plan. No options were granted under the 2003 Stock Plan during the year ended December 31, 2007.
2005 Equity Incentive Plan
On April 26, 2005, the Company’s Board of Directors approved the 2005 Equity Incentive Plan (the “2005 Stock Plan”). The 2005 Stock Plan is discretionary and allows for an aggregate of up to 2,100,000 shares of the Company’s common stock to be awarded through incentive and non-qualified stock options, stock units, stock awards, stock appreciation rights and other stock-based awards. The 2005 Stock Plan is administered by the Compensation Committee of the Company’s Board of Directors, who has exclusive discretion to select participants who will receive the awards and to determine the type, size and terms of each award granted. As of December 31, 2007, there were 305,000 options outstanding and 1,712,652 shares were available for issuance under the 2005 Stock Plan.
During the three months ended March 31, 2007, the Company modified a 400,000 share performance option grant, as discussed below underModification of Stock Options.This 400,000 share performance option grant was issued during the three months ended June 30, 2006 to purchase common stock with an exercise price of $1.88 per share to the Company’s President. These options had a ten year maximum contractual life and the options were to vest, and no longer be subject to forfeiture, upon the occurrence of any of the following events: (i) upon the closing of the sale of substantially all of the assets of the Company or the reorganization, consolidation or the merger of the Company; provided that the event results in the payment or distribution of consideration valued in good faith by the Board of Directors at $25 per share or more; or (ii) upon the closing of a tender offer or exchange offer to purchase 50% or more of the issued and outstanding shares of common stock of the Company at a price per share valued in good faith by the Board of Directors at $25 or more; or (iii) immediately following a “Stock Acquisition Date,” as that term is defined in the Rights Plan adopted by the Company on May 12, 2006 (provided that said rights are not subsequently redeemed by the Company or that the Rights Plan is not subsequently amended to preclude exercise of the rights issued thereunder, prior to the Distribution Date, as that term is defined in the Rights Pan), or (iv) at such other time as the Board of Directors, in its sole discretion, deems appropriate; provided in each case that the President is employed by the Company at the time of said event. The 400,000 share option grant was considered a grant of a performance stock option. No compensation cost was recorded during 2006 for this grant as the Company did not believe that the vesting events were probable of occurrence. The 2006 grant date fair value of the award was approximately $0.6 million. Subsequently, this 400,000 share option grant was modified during the three months ended March 31, 2007, as discussed below underModification of Stock Options.
Modification of Stock Options
In connection with the separation of the Company’s President (see Note 11), the Company agreed on March 16, 2007 to modify certain of the President’s stock options such that (1) 120,000 unvested, time-based stock options would vest immediately and (2) of 400,000 performance based stock options, 100,000 would be cancelled and the remaining 300,000 would be extended such that the 300,000 options would expire 10 years from the original grant date, as opposed to expiring upon termination of employment. The 300,000 performance based stock options would continue to be subject to the same performance based vesting requirements. The 120,000 modified stock options were valued using the Black-Scholes valuation model, and resulted in $0.3 million charge to selling, general and administrative expense during the months ended March 31, 2007. The 300,000 modified performance stock options were valued using the Black-Scholes valuation model, and resulted in $0.8 million charge to selling, general and administrative expense in the year ended December 31, 2007. Two other employee stock option modifications resulted in less than $0.1 million charge to selling, general and administrative expense in the year ended 2007.
During the year ended December 31, 2006, the Company modified 100,000 stock options previously granted to the former CFO of the Company during 2005, such that the options will expire five years from the date of grant, as opposed to 90 days after termination. In connection with this modification, the Company recorded a charge of $0.1 million to selling, general and administrative expenses in the consolidated statement of operations in the year ended December 31, 2006.
F-40
Also, refer to Note 17,Subsequent Event,for a discussion of the stock options modifications, which occurred in January 2008, related to our former Chief Executive Officer.
Restricted Stock
During the three months ended March 31, 2006, the Company issued 126,750 shares of restricted stock awards to various employees. The restricted common stock vested quarterly over three years, beginning on March 31, 2006 and ending on December 31, 2008. On March 31, 2006, 10,557 shares of the 126,750 shares of restricted stock vested.
During the three months ended June 30, 2006, 2,752 shares of the restricted stock were cancelled due to terminations, 94,648 shares of restricted stock were cancelled in a Board approved exchange for 206,500 stock options (which were issued under the 2001 Plan) and 3,707 shares of restricted stock vested.
During the three months ended September 30, 2006, 1,708 shares of restricted stock vested. During the three months ended December 31, 2006, 15,002 shares were cancelled due to terminations and 42 shares of restricted stock vested. Compensation expense related to the restricted stock was less than $0.1 million for the year ended December 31, 2007 and less than $0.1 million for the year ended December 31, 2006. As of December 31, 2007, 166 shares of unvested restricted stock were outstanding, which vest quarterly through March 31, 2009.
Other Stock Options
During the year ended December 31, 2007, the Company did not issue any options outside the 2001 Stock Plan, the 2003 Stock Plan or the 2005 Stock Plan. As of December 31, 2007, there were 3,568,333 nonqualified stock options outstanding outside of the shareholder approved plans discussed above.
During the year ended December 31, 2006, the Company issued (1) 2,000,000 options to purchase its common stock with an exercise price of $1.88 per share to the Company’s CEO, which have a ten year maximum contractual life and which vest each fiscal quarter end over a three year period from the date of grant, (2) 325,000 options to purchase its common stock with an exercise price of $1.87 per share to the Company’s CFO, with a five year maximum contractual life and which vest annually over a three year period from the date of grant and (3) 200,000 options to purchase its common stock with an exercise price of $1.87 per share to a separate employee, with a five year maximum contractual life and which vest annually over a three year period from the date of grant.
In addition, during the year ended December 31, 2006 the Company issued 500,000 options to purchase its common stock with an exercise price of $1.88 per share to the Company’s CEO. These options have a ten year maximum contractual life and the options have identical vesting terms to the Performance Stock Option Grant issued to the Company’s President under the 2005 Stock Plan as described above. No compensation cost has been recorded for this grant as the Company does not currently believe that the vesting events are probable of occurrence. The grant date fair value of the award was approximately $0.7 million. This fair value of $0.7 million, or any portion thereof, will not be recognized as compensation expense until the vesting of the award becomes probable.
Equity Instruments Issued for Services
As of December 31, 2007, the Company had outstanding 603,600 warrants and options issued to non-employees under consulting agreements. The following sets forth certain information concerning these warrants and options:
| | | | | |
| | | Vested |
| Warrants and options outstanding | | 603,600 |
| Range of exercise prices | | $1.50-6.00 |
| Weighted average exercise price | | $4.17 |
| Expiration dates | | 2009-2013 |
F-41
Expense related to these contracts was less than $0.1 million, $0.2 million and $(0.2) million for the years ended December 31, 2007, 2006 and 2005, respectively. The expense was calculated using the Black Scholes option-pricing model based on the following weighted average assumptions:
| | | |
| Expected life (years) | | 4-5 Years |
| Interest rate | | 4.0-4.97% |
| Dividend yield | | — |
| Volatility | | 71-83% |
Further, there were 168,246 and 688,256 warrants outstanding as of December 31, 2007 and as of December 31, 2006, which were primarily issued in connection with past equity offerings. During the years ended December 31, 2007, 2006 and 2005, there were 520,010, 0 and 60,000 warrants exercised, respectively. Of the warrants exercised during the year ended December 31, 2007, 463,370 were exercised via cash payment of the $1.93 exercise price, and the remaining 56,640 warrants were exercised via net share exercise. In total, 492,613 shares of common stock were issued during 2007 as a result of these warrants exercised and $0.9 million of cash proceeds were received by the Company in connection with the payment of the related warrant exercise price. The intrinsic value of the warrants exercised during the years ended December 31, 2007 and 2005 were $1.1 million and $0.4 million, respectively. There were no warrants converted or forfeited during the year ended December 31, 2006.
Note 14—Certain Relationships and Related Transactions
As discussed in Notes 9 and 12, in November 2004 the Company repurchased 2,000,000 shares of its common stock from Frank DeLape, who was then the Chairman of the Board of Directors, Michael Macaluso, a former director and the former President and Chief Executive Officer, Olga Marko, the former Senior Vice President and Director of Research, Michael Avignon, the former Manager of International Operations, and Timothy J. Till, a shareholder. The purchase price from the insiders, affiliates and founders of the Company listed above was $6.33 per share which represented a 5% discount from the closing price of the Company’s common stock on the American Stock Exchange on October 28, 2004, the date the offering of the 3.5% Subordinated Notes was priced. The purchase of the shares from the insiders was approved by a special committee of independent directors in partial reliance on a fairness opinion issued by an investment bank.
Five of the Company’s current Board members and seven of the Company’s former officers and directors are named defendants in certain pending class action and derivative legal proceedings discussed in Note 11 above. During 2007, the Company advanced an aggregate of $0.8 million, or approximately $0.1 million per person, for legal expenses incurred on behalf of those five Board members and seven former officers and directors in connection with their defense in those proceedings. During 2006, the Company advanced an aggregate of $0.9 million, or approximately $0.1 million per person, for legal expenses incurred on behalf of those five Board members and seven former officers and directors in connection with their defense in those proceedings. As of December 31, 2007, $1.5 million of the advanced amounts (approximately $0.1 per person) have been reimbursed to the Company by the Company’s insurance carriers.
Since June 2005, Mr. Ralph De Martino, a member of the Company’s Board of Directors, has been a member of the law firm Cozen O’Connor in the firm’s Washington, DC office. From January 2003 until June 2005, Mr. De Martino was the managing partner of the Washington, DC office of the law firm Dilworth Paxson LLP. Fees paid by the Company to Cozen O’Connor during 2007, 2006 and 2005 were $0.7 million, $0.4 million and $0.2 million, respectively. Fees paid by the Company to Dilworth Paxson LLP during 2007, 2006 and 2005 were $0.0 million, $0.0 million and $0.4 million, respectively.
See Note 11,Dispute with Former President and Member of the Board of DirectorsandDeparture of Former Chief Executive Officers,for discussion of severance arrangements with former senior employees. See Note 17, Subsequent Event, for discussion of the January 2008 departure of our former Chief Executive Officer.
F-42
Note 15—Segment Information and Geographical information
With the acquisition of Agera on August 10, 2006 (see Note 4), the Company now has two reportable segments: Isolagen Therapy and Agera. Prior to the acquisition of Agera, the Company reported one reportable segment. The Isolagen Therapy segment specializes in the development and commercialization of autologous cellular therapies for soft tissue regeneration. The Agera segment maintains proprietary rights to a scientifically-based advanced line of skincare products. The following table provides operating financial information for the continuing operations of the Company’s two reportable segments:
| | | | | | | | | | | | | |
| | | Segment | | | | |
| Year Ended December 31, 2007 | | Isolagen Therapy | | | Agera | | | Consolidated | |
| External revenue | | $ | — | | | $ | 1,400,986 | | | $ | 1,400,986 | |
| Intersegment revenue | | | — | | | | — | | | | — | |
| | | | | | | | | | |
| Total operating revenue | | | — | | | | 1,400,986 | | | | 1,400,986 | |
| Cost of revenue | | | — | | | | 656,029 | | | | 656,029 | |
| Selling, general and administrative expense | | | 17,429,016 | | | | 1,301,847 | | | | 18,730,863 | |
| Research and development expense | | | 13,278,796 | | | | 19,542 | | | | 13,298,338 | |
| Management fee | | | (600,000 | ) | | | 600,000 | | | | — | |
| | | | | | | | | | |
| Total operating expenses | | | 30,107,812 | | | | 1,921,389 | | | | 32,029,201 | |
| Operating loss | | | (30,107,812 | ) | | | (1,176,432 | ) | | | (31,284,244 | ) |
| Interest income | | | 897,731 | | | | 3,531 | | | | 901,262 | |
| Other income | | | 150,138 | | | | — | | | | 150,138 | |
| Interest expense | | | (3,899,239 | ) | | | — | | | | (3,899,239 | ) |
| Minority interest | | | — | | | | 246,347 | | | | 246,347 | |
| | | | | | | | | | |
| Segment loss | | $ | (32,959,182 | ) | | $ | (926,554 | ) | | $ | (33,885,736 | ) |
| | | | | | | | | | |
| | | | | | | | | | | | | |
Supplemental information related to continuing operations | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Depreciation and amortization expense | | $ | 1,178,376 | | | $ | 326,842 | | | $ | 1,505,218 | |
| Capital expenditures | | | 184,538 | | | | — | | | | 184,538 | |
| Equity awards issued for services | | | 3,026,609 | | | | — | | | | 3,026,609 | |
| Amortization of debt issuance costs | | | 749,239 | | | | — | | | | 749,239 | |
| Total assets, including assets from discontinued operations | | | 34,162,693 | | | | 5,328,497 | | | | 39,491,190 | |
| Property and equipment, net | | | 3,395,723 | | | | — | | | | 3,395,723 | |
| Intangible assets, net | | | 529,875 | | | | 4,069,663 | | | | 4,599,538 | |
An intercompany receivable of $1.1 million, due from the Agera segment to the Isolagen Therapy segment, is eliminated in consolidation. This intercompany receivable is primarily due to the intercompany management fee charge to Agera by Isolagen, as well as Agera working capital needs provided by Isolagen, and has been excluded from total assets of the Isolagen Therapy segment in the above table. Total assets on the consolidated balance sheet at December 31, 2007 are approximately $39.5 million, which includes assets of continuing operations of $28.2 million and assets of discontinued operations of $11.3 million.
F-43
| | | | | | | | | | | | | |
| | | Segment | | | | |
| Year Ended December 31, 2006 | | Isolagen Therapy | | | Agera | | | Consolidated | |
| External revenue | | $ | — | | | $ | 384,389 | | | $ | 384,389 | |
| Intersegment revenue | | | — | | | | — | | | | — | |
| | | | | | | | | | |
| Total operating revenue | | | — | | | | 384,389 | | | | 384,389 | |
| Cost of revenue | | | — | | | | 194,197 | | | | 194,197 | |
| Selling, general and administrative expense | | | 12,600,305 | | | | 574,655 | | | | 13,174,960 | |
| Research and development expense | | | 8,796,219 | | | | — | | | | 8,796,219 | |
| Management fee | | | (278,136 | ) | | | 278,136 | | | | — | |
| | | | | | | | | | |
| Total operating expenses | | | 21,118,388 | | | | 852,791 | | | | 21,971,179 | |
| Operating loss | | | (21,118,388 | ) | | | (662,599 | ) | | | (21,780,987 | ) |
| Interest income | | | 2,249,892 | | | | 12,007 | | | | 2,261,899 | |
| Interest expense | | | (3,899,239 | ) | | | — | | | | (3,899,239 | ) |
| Minority interest | | | — | | | | 78,132 | | | | 78,132 | |
| Income tax benefit | | | — | | | | 190,754 | | | | 190,754 | |
| | | | | | | | | | |
| Segment loss | | $ | (22,767,735 | ) | | $ | (381,706 | ) | | $ | (23,149,441 | ) |
| | | | | | | | | | |
| | | | | | | | | | | | | |
Supplemental information related to continuing operations | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Depreciation and amortization expense | | $ | 1,174,404 | | | $ | 125,616 | | | $ | 1,300,020 | |
| Capital expenditures | | | 917,811 | | | | — | | | | 917,811 | |
| Equity awards issued for services | | | 1,464,139 | | | | — | | | | 1,464,139 | |
| Amortization of debt issuance costs | | | 749,240 | | | | — | | | | 749,240 | |
| Total assets, including assets from discontinued operations | | | 51,269,373 | | | | 6,017,502 | | | | 57,286,875 | |
| Property and equipment, net | | | 4,331,605 | | | | — | | | | 4,331,605 | |
| Intangible assets, net | | | 540,000 | | | | 4,396,505 | | | | 4,936,505 | |
An intercompany receivable of $0.3 million, due from the Agera segment to the Isolagen Therapy segment, is eliminated during consolidation. This intercompany receivable is primarily due to the intercompany management fee charge and has been excluded from total assets of the Isolagen Therapy segment in the above table. Total assets on the consolidated balance sheet at December 31, 2006 are approximately $57.3 million, which includes assets of continuing operations of $45.9 million and assets of discontinued operations of $11.4 million.
Geographical information concerning the Company’s continuing operations and assets is as follows:
| | | | | | | | | | | | | |
| | | Revenue | |
| | | Year ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| United States | | $ | 368,784 | | | $ | 150,945 | | | $ | — | |
| United Kingdom | | | 967,313 | | | | 217,340 | | | | — | |
| Other | | | 64,889 | | | | 16,104 | | | | — | |
| | | | | | | | | | |
| | | $ | 1,400,986 | | | $ | 384,389 | | | $ | — | |
| | | | | | | | | | |
F-44
During 2007, revenue from one foreign customer and one domestic customer represented 69% and 16% of consolidated revenue, respectively.
| | | | | | | | | |
| | | Property and Equipment, net | |
| | | As of December 31, | |
| | | 2007 | | | 2006 | |
| United States | | $ | 3,395,723 | | | $ | 4,331,605 | |
| | | | | | | |
| | | | | | | | | |
| | | Intangible Assets, net | |
| | | As of December 31, | |
| | | 2007 | | | 2006 | |
| United States | | $ | 4,599,538 | | | $ | 4,936,505 | |
| | | | | | | |
Note 16—Summarized Quarterly Financial Data (unaudited)
| | | | | | | | | | | | | | | | | |
| (in millions, except net loss per share amounts) | | | | | | | | | | | | |
| For the following three-month periods ended | | March 31 | | | June 30 | | | September 30 | | | December 31 | |
2007 | | | | | | | | | | | | | | | | |
| Revenue | | $ | 313,622 | | | $ | 346,716 | | | $ | 331,115 | | | $ | 409,533 | |
| Cost of sales | | | 159,087 | | | | 157,373 | | | | 158,096 | | | | 181,473 | |
| Operating loss | | | (9,271,841 | ) | | | (8,889,214 | ) | | | (6,930,287 | ) | | | (6,192,902 | ) |
| Net loss from discontinued operations | | | (1,092,926 | ) | | | (318,952 | ) | | | (128,111 | ) | | | (147,389 | ) |
| Net loss | | | (10,941,393 | ) | | | (9,740,860 | ) | | | (7,798,473 | ) | | | (7,092,388 | ) |
| Net loss per share | | $ | (0.36 | ) | | $ | (0.32 | ) | | $ | (0.23 | ) | | $ | (0.19 | ) |
| | | | | | | | | | | | | | | | | |
| For the following three-month periods ended | | March 31 | | | June 30 | | | September 30 | | | December 31 | |
2006 | | | | | | | | | | | | | | | | |
| Revenue | | $ | — | | | $ | — | | | $ | 141,428 | | | $ | 242,961 | |
| Cost of sales | | | — | | | | — | | | | 72,444 | | | | 121,753 | |
| Operating loss | | | (5,696,305 | ) | | | (4,958,515 | ) | | | (4,478,833 | ) | | | (6,647,334 | ) |
| Net loss from discontinued operations | | | (3,888,686 | ) | | | (2,817,271 | ) | | | (1,797,416 | ) | | | (4,168,592 | ) |
| Net loss | | | (9,917,451 | ) | | | (8,131,714 | ) | | | (6,671,880 | ) | | | (11,100,361 | ) |
| Net loss per share | | $ | (0.33 | ) | | $ | (0.27 | ) | | $ | (0.22 | ) | | $ | (0.37 | ) |
Quarterly and year to date computations of per share amounts are made independently; therefore, the sum of per share amounts for the quarters may not equal per share amounts for the year.
Note 17—Subsequent Event
On January 7, 2008, the Company and Mr. Nicholas L. Teti, Jr. entered into a consulting and non-competition agreement (the “Consulting Agreement”), pursuant to which Mr. Teti agreed to continue as the Company’s non-executive Chairman of the Board and to become a consultant to the Company, and Mr. Teti resigned his position as Chief Executive Officer and President of the Company. Mr. Teti agreed to provide consulting services to the Company until June 30, 2009, subject to the prior termination of the Consulting Agreement, which may occur upon 30 days notice by either party. Mr. Teti will receive an annual consulting fee of $100,000 for his services. Pursuant to the Consulting Agreement, Mr. Teti’s original employment agreement, dated June 5, 2006, was terminated and the parties agreed that he was owed no severance payments under the original employment agreement. Mr. Teti will retain his previously issued stock options, which will continue to vest in accordance with their original terms. In connection with Mr. Teti’s service as non-executive Chairman of the Board, Mr. Teti will receive an annual retainer of $60,000.
As a result of the modifications to Mr. Teti’s stock options set forth in the Consulting Agreement, the Company will be required to record a non-cash compensation charge during the three months ended March 31, 2008 of approximately $1.3 million related to Mr. Teti’s 1,166,665 vested stock options, even though the number of shares, the vesting schedules and the exercise price of the stock options previously granted to Mr. Teti have not changed. Further, related to Mr. Teti’s 833,335 unvested stock options, the Company estimates that a non-cash charge of approximately $1.4 million will be recorded ratably over five fiscal quarters, beginning with the three months ended March 31, 2008. This estimate is based on certain stock option valuation assumptions as of January 2008. However, the ultimate charge will be based on the future valuation assumptions in effect upon the vesting dates of these currently unvested stock options.
F-45
On January 7, 2008, the Company and Mr. Declan Daly entered into an employment agreement (the “Agreement”) pursuant to which Mr. Daly agreed to serve as Chief Executive Officer of the Company until December 31, 2010, subject to the automatic renewal of the Agreement for an additional one-year term unless the Company notifies Mr. Daly 180 days prior to the expiration of the Agreement of its intention not to renew the Agreement. The Agreement supersedes the current employment agreement between the Company and Mr. Daly, effective June 5, 2006, which was terminated. In addition, Mr. Daly agreed to continue in his current role as Chief Financial Officer of the Company, but has resigned as Chief Operating Officer of the Company. The Agreement provides Mr. Daly with an annual base salary of $430,000, which will be periodically reviewed and may be increased at the Board’s discretion. Under the Agreement, Mr. Daly was granted the following ten-year option grants: (a) an option to purchase 350,000 shares of common stock at an exercise price equal to the closing of the common stock on the last trading day preceding execution of the Agreement, which vests in twelve equal quarterly installments commencing March 31, 2008; and (b) a performance stock option to purchase 100,000 shares of common stock at an exercise price equal to the closing of the common stock on the last trading day preceding execution of the Agreement that shall vest as follows: (i) 50% of performance stock option shall vest upon the Company’s accepted filing of a Biologics License Application by the FDA and (ii) the remaining 50% of the performance stock option shall vest upon the FDA’s approval of the Company’s Biologics License Application filing; provided in each case that Mr. Daly is the Company’s Chief Executive Officer at the time of said event.
F-46
EXHIBIT INDEX
| | | |
| EXHIBIT | | |
| NO. | | IDENTIFICATION OF EXHIBIT |
| 2 | | Agreement and Plan of Merger by and among American Financial Holding, Inc., ISO Acquisition Corp., Isolagen Technologies, Inc., Gemini IX, Inc., and William K. Boss, Jr., Olga Marko and Dennis McGill dated August 1, 2001(1) |
| 3(i) | | Amended Certificate of Incorporation(17) |
| 3(ii) | | Third Amended and Restated Bylaws(25) |
| 4.1 | | Specimen of Common Stock certificate(2) |
| 4.2 | | Certificate of Designations of Series A Convertible Preferred Stock(7) |
| 4.3 | | Certificate of Designations of Series B Convertible Preferred Stock(5) |
| 4.4 | | Indenture, dated November 3, 2004, between the Company and The Bank of New York Trust Company, N.A., as trustee(11) |
| 4.5 | | Rights Agreement, dated as of May 12, 2006, by and between the registrant and American Stock Transfer & Trust Company, including the Form of Certificate of Designation, Preferences and Rights of Series C Junior Participating Preferred Stock attached as Exhibit A thereto, the Form of Rights Certificate attached as Exhibit B thereto and the Summary of Rights to Purchase Preferred Stock attached as Exhibit C thereto. (21) |
| 10.1 | | 2003 Stock Option and Stock Appreciation Rights Plan(3)* |
| 10.2 | | 2001 Stock Option and Appreciation Rights Plan(4)* |
| 10.3 | | Lease Agreement dated March 24, 2002 by and between the Registrant as Lessee and Claire O Aceti Gbmh asLessor(7) |
| 10.4 | | Intellectual Property Purchase Agreement between Isolagen Technologies, Inc., Gregory M. Keller, and PacGen Partners(8) |
| 10.5 | | Purchase Agreement among CIBC World Market Corp., UBS Securities LLC, and Adams, Harkness & Hill, Inc. dated October 28, 2004(11) |
| 10.6 | | Registration Rights Agreement among CIBC World Market Corp., UBS Securities LLC, and Adams, Harkness & Hill, Inc. dated November 3, 2004(11) |
| 10.7 | | Lease Agreement between Isolagen Technologies, Inc. and Beltway 8 Service Center Investors Ltd. dated February 16, 2005(13) |
| 10.8 | | Lease Agreement between Isolagen, Inc and The Hankin Group dates April 7, 2005(15) |
| 10.9 | | Purchase Option Agreement between Isolagen, Inc and 405 Eagleview Associates dated April 7, 2005(15) |
| 10.10 | | 2005 Equity Incentive Plan, as amended(18) |
| 10.11 | | Separation and Release Agreement, dated October 27, 2005, among Isolagen, Inc., Isolagen Technologies, Inc. and Frank DeLape(19) |
| 10.12 | | Amended Employment Agreement between Isolagen, Inc. and Susan Ciallella(20)* |
| 10.13 | | Employment Agreement between Isolagen, Inc. and Todd Greenspan(20)* |
| 10.14 | | Employment Agreement dated June 5, 2006 between Isolagen, Inc. and Nicholas L. Teti(22)* |
| 10.15 | | Employment Agreement dated March 12, 2007 between Isolagen, Inc. and Declan Daly(23)* |
| 10.16 | | Employment Agreement dated March 12, 2007 between Isolagen, Inc. and Steven Trider(23)* |
| 10.17 | | Settlement Agreement and Release between Susan Stranahan Ciallella and Isolagen, Inc. dated June 8, 2007 (24) |
| 10.18 | | Consulting and Non-Competition Agreement dated January 7, 2008 between Isolagen, Inc. and Nicholas L.Teti * (26) |
| 10.19 | | Employment Agreement dated January 7, 2008 between Isolagen, Inc. and Declan Daly * (26) |
| 14 | | Code of Ethics(9) |
| 21 | | List of Subsidiaries(23) |
| 23 | | BDO Seidman, LLP Consent(26) |
| 31 | | Certification pursuant to Rule 13a-14(a) and 15d-14(a), required under Section 302 of the Sarbanes-Oxley Act of2002(26) |
| 32 | | Certification pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002(26) |
| | | |
| * | | Indicates a management contract or a compensatory plan or arrangement. |
| |
| (1) | | Previously filed as an exhibit to the company’s Form 8-K, filed on August 22, 2001, and is incorporated by reference hereto. |
| |
| (2) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-KSB for the fiscal year ended December 31, 2001, and is incorporated by reference hereto. |
| |
| (3) | | Previously filed as an appendix to the company’s Definitive Proxy Statement, as filed on May 6, 2003, in connection with the 2003 Annual Stockholder Meeting, and is incorporated by reference hereto. |
| |
| (4) | | Previously filed as an appendix to the company’s Definitive Proxy Statement, as filed on October 23, 2001, in connection with the 2001 Annual Stockholder Meeting, and is incorporated by reference hereto. |
| |
| (5) | | Previously filed as an exhibit to the company’s Form 10-Q for the fiscal quarter ended March 31, 2003, as filed on May 15, 2003, and is incorporated by reference hereto. |
| |
| (6) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-KSB for the fiscal year ended December 31, 2002, and is incorporated by reference hereto. |
| |
| (7) | | Previously filed as an exhibit to the company’s Form S-1, as filed on September 12, 2003, and is incorporated by reference hereto. |
| |
| (8) | | Previously filed as an exhibit to the company’s amended Form S-1, as filed on October 24, 2003, and is incorporated by reference hereto. |
| |
| (9) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2003, and is incorporated by reference hereto. |
| |
| (10) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-K/A for the fiscal year ended December 31, 2003, and is incorporated by reference hereto. |
| |
| (11) | | Previously filed as an exhibit to the company’s Current Report on Form 8-K dated November 4, 2004, and is incorporated by reference hereto. |
| |
| (12) | | Reserved. |
| |
| (13) | | Previously filed as an exhibit to the company’s Form 8-K, filed on February 23, 2005, and is incorporated by reference hereto. |
| |
| (14) | | Reserved. |
| |
| (15) | | Previously filed as an exhibit to the company’s Form 8-K, filed on April 12, 2005, and is incorporated by reference hereto. |
| |
| (16) | | Reserved. |
| |
| (17) | | Previously filed as an exhibit to the company’s Form 10-Q for the fiscal quarter ended June 30, 2005, as filed on August 9, 2005, and is incorporated by reference hereto. |
| |
| (18) | | Previously filed as an exhibit to the company’s Form S-8, filed on February 13, 2006, and is incorporated by reference hereto. |
| |
| (19) | | Previously filed as an exhibit to the company’s Form 8-K, filed on November 2, 2005, and is incorporated by reference hereto. |
| |
| (20) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2005, and is incorporated by reference hereto. |
| |
| (21) | | Previously filed as an exhibit to the company’s Form 8-K, filed on May 15, 2006, and is incorporated by reference hereto. |
| |
| (22) | | Previously filed as an exhibit to the company’s Form 8-K, filed on June 9, 2006, and is incorporated by reference hereto. |
| |
| (23) | | Previously filed as an exhibit to the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2007, and is incorporated by reference hereto. |
| |
| (24) | | Previously filed as an exhibit to the company’s Form 8-K, filed on June 13, 2007, and is incorporated by reference hereto. |
| |
| (25) | | Previously filed as an exhibit to the company’s Form 8-K, filed on January 8, 2007, and is incorporated by reference hereto. |
| |
| (26) | | Filed herewith. |
