
Development of an Autologous Gene-Modified Cell Therapy for the Treatment of Linear Scleroderma Tathagata Chaudhuri,1 Scott Moncrief,1 Anna Malyala,2 John Maslowski,2 Darby L. Thomas1 1Intrexon Corporation, Germantown MD; 2Fibrocell, Exton PA Presented at the 20th Annual American Society of Gene and Cell Therapy meeting, Washington, D.C., May 13, 2017

© 2017 Intrexon Corp. All rights reserved. 2 © 2017 Intrexon Corp. All rights reserved. Intrexon Corporation is sharing the following materials for informational purposes only. Such materials do not constitute an offer to sell or the solicitation of an offer to buy any securities of Intrexon. Any offer and sale of Intrexon’s securities will be made, if at all, only upon the registration and qualification of such securities under all applicable federal and state securities laws or pursuant to an exemption from such requirements. The attached information has been prepared in good faith by Intrexon. However, Intrexon makes no representations or warranties as to the completeness or accuracy of any such information. Any representations or warranties as to Intrexon shall be limited exclusively to any agreements that may be entered into by Intrexon and to such representations and warranties as may arise under law upon distribution of any prospectus or similar offering document by Intrexon. Forward-Looking Statements Some of the statements made in this presentation are forward-looking statements. These forward-looking statements are based upon our current expectations and projections about future events and generally relate to our plans, objectives and expectations for the development of our business. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. All information in this presentation is as of the date marked on the cover page, and Intrexon undertakes no duty to update this information unless required by law.

© 2017 Intrexon Corp. All rights reserved. 3 Collaboration

© 2017 Intrexon Corp. All rights reserved. 4 FCX-013 for Linear Scleroderma • Localized scleroderma is characterized by excess collagen production resulting in skin fibrosis • Underlying tissues and muscle may also be affected which can impair growth in affected legs, arms or forehead in linear scleroderma • Prevalence ~160,000 in the U.S.; initial target linear scleroderma, prevalence ~40,000 • FCX-013 – Genetically modified autologous fibroblasts expressing MMP1 to address excess collagen deposits and improve mobility • Orphan Drug Designation in April 2016 from Christen-Zaech et al. 2008
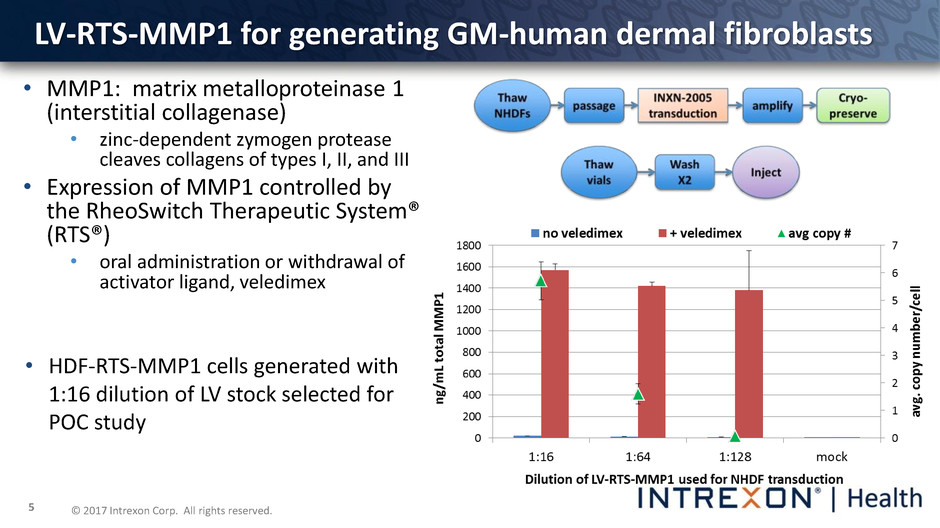
© 2017 Intrexon Corp. All rights reserved. 5 • MMP1: matrix metalloproteinase 1 (interstitial collagenase) • zinc-dependent zymogen protease cleaves collagens of types I, II, and III • Expression of MMP1 controlled by the RheoSwitch Therapeutic System® (RTS®) • oral administration or withdrawal of activator ligand, veledimex LV-RTS-MMP1 for generating GM-human dermal fibroblasts • HDF-RTS-MMP1 cells generated with 1:16 dilution of LV stock selected for POC study
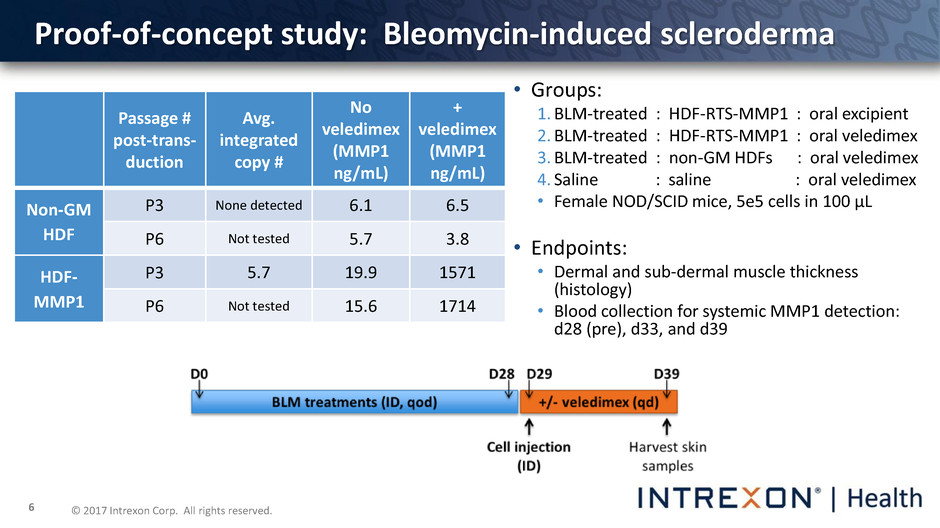
© 2017 Intrexon Corp. All rights reserved. 6 • Groups: 1. BLM-treated : HDF-RTS-MMP1 : oral excipient 2. BLM-treated : HDF-RTS-MMP1 : oral veledimex 3. BLM-treated : non-GM HDFs : oral veledimex 4. Saline : saline : oral veledimex • Female NOD/SCID mice, 5e5 cells in 100 µL • Endpoints: • Dermal and sub-dermal muscle thickness (histology) • Blood collection for systemic MMP1 detection: d28 (pre), d33, and d39 Proof-of-concept study: Bleomycin-induced scleroderma Passage # post-trans- duction Avg. integrated copy # No veledimex (MMP1 ng/mL) + veledimex (MMP1 ng/mL) Non-GM HDF P3 None detected 6.1 6.5 P6 Not tested 5.7 3.8 HDF- MMP1 P3 5.7 19.9 1571 P6 Not tested 15.6 1714

© 2017 Intrexon Corp. All rights reserved. 7 Representative histological images 4) Saline (no BLM) No cells Oral veledimex (normal skin) 1) Bleomycin HDF-RTS-MMP1 Oral excipient 2) Bleomycin HDF-RTS-MMP1 Oral veledimex (experimental) 3) Bleomycin Non-GM HDFs Oral veledimex (disease control)

© 2017 Intrexon Corp. All rights reserved. 8 HDF-RTS-MMP1 Reduction of Dermal Thickness 0 50 100 150 200 250 1: Bleomycin HDF-RTS-MMP1 oral excipient 2: Bleomycin HDF-RTS-MMP1 oral veledimex 3: Bleomycin non-GM HDFs oral veledimex 4: saline (no BLM) no cells oral veledimex Mean D e rmal Th ickne ss, µ m • Image analyses: 5 slides per mouse, 5 images per slide, means +/- SD presented • 10X magnification, 1 pixel = 0.8686 µm conversion n=8 n=9 n=6 n=7

© 2017 Intrexon Corp. All rights reserved. 9 HDF-RTS-MMP1 Reduction of Sub-Dermal Muscle Thickness • Image analyses: 5 slides per mouse, 5 images per slide, means +/- SD presented • 10X magnification, 1 pixel = 0.8686 µm conversion 0 50 100 150 200 250 300 350 1: Bleomycin HDF-RTS-MMP1 oral excipient 2: Bleomycin HDF-RTS-MMP1 oral veledimex 3: Bleomycin non-GM HDFs oral veledimex 4: saline (no BLM) no cells oral veledimex Mean mu scl e t h ickne ss, µ m n=8 n=9 n=6 n=7
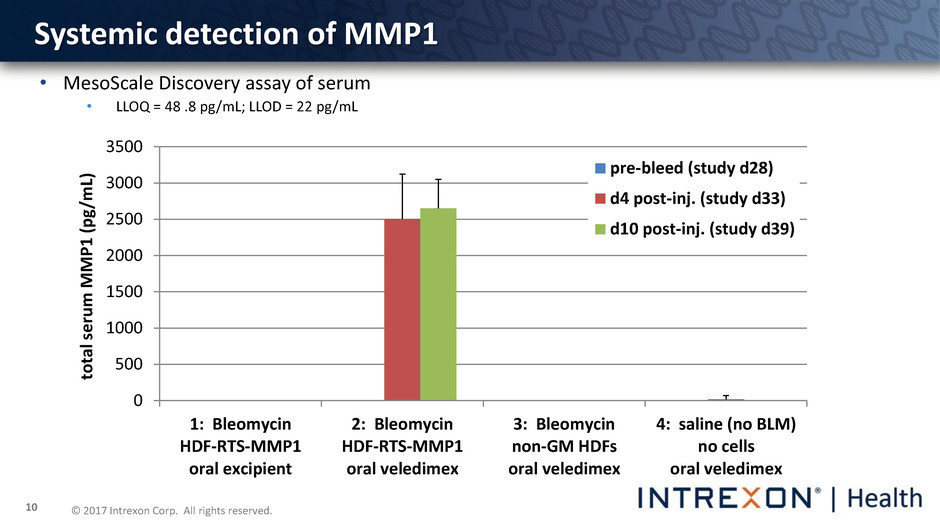
© 2017 Intrexon Corp. All rights reserved. 10 Systemic detection of MMP1 • MesoScale Discovery assay of serum • LLOQ = 48 .8 pg/mL; LLOD = 22 pg/mL 0 500 1000 1500 2000 2500 3000 3500 1: Bleomycin HDF-RTS-MMP1 oral excipient 2: Bleomycin HDF-RTS-MMP1 oral veledimex 3: Bleomycin non-GM HDFs oral veledimex 4: saline (no BLM) no cells oral veledimex to ta l s e rum M M P 1 ( p g/ m L) pre-bleed (study d28) d4 post-inj. (study d33) d10 post-inj. (study d39)

© 2017 Intrexon Corp. All rights reserved. 11 • Non-GLP toxicology / biodistribution / dose ranging study -- in data collection and analysis phase • Next step -- toxicology/pharmacology study • Engineering runs for manufacturing ongoing Pre-Clinical/IND-enabling development
© 2017 Intrexon Corp. All rights reserved. 12 • FCX-013: autologous dermal fibroblasts genetically-modified to express MMP1 under RTS® gene switch control • Initial proof-of-concept study demonstrated significant reduction in dermal and sub-dermal muscle thickness after intradermal injection of GM-HDFs expressing MMP1 in a bleomycin model of scleroderma • High levels of MMP1 expression are not needed to see efficacious effect • IND-enabling activities ongoing Summary

© 2017 Intrexon Corp. All rights reserved. 13 Acknowledgements • Tathagata Chaudhuri, PhD • Scott Moncrief, PhD • Jacques Plummer • Rahim Johnson • Ernie Boyd • Anna Malyala, PhD • John Maslowski












