UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
| þ | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended September 30, 2017
OR
| ¨ | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from ____ to ____
Commission File No. 1-6651

HILL-ROM HOLDINGS, INC.
(Exact name of registrant as specified in its charter)
| Indiana | 35-1160484 |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
130 East Randolph Street, Suite 1000 Chicago, IL | 60601 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (312) 819-7200
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on Which Registered |
| Common Stock, without par value | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes þ No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company (as defined in Rule 12b-2 of the Exchange Act).
Large accelerated filer þ Accelerated filer o Non-accelerated filer o Smaller reporting company o Emerging growth company o
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ¨ No þ
The aggregate market value of the registrant’s voting common equity, held by non-affiliates of the registrant, was approximately $4.6 billion, based on the closing sales price of $70.60 per share as of March 31, 2017 (the last business day of the registrant’s most recently completed second fiscal quarter). There is no non-voting common equity held by non-affiliates.
The registrant had 65,820,999 shares of its common stock, without par value, outstanding as of November 14, 2017.
Documents incorporated by reference.
Certain portions of the registrant’s definitive Proxy Statement to be delivered to shareholders in connection with the Annual Meeting of Shareholders to be held on March 6, 2018 are incorporated by reference into Part III of this Annual Report on Form 10-K.
HILL-ROM HOLDINGS, INC.
Annual Report on Form 10-K
For the Fiscal Year Ended September 30, 2017
TABLE OF CONTENTS
| Page | ||
| PART I | ||
| PART II | ||
| PART III | ||
| PART IV | ||
2
PART I
DISCLOSURE REGARDING FORWARD LOOKING STATEMENTS
Certain statements in this Annual Report on Form 10-K ("Form 10-K") contain forward-looking statements within the meanings of the Private Securities Litigation Reform Act of 1995 regarding our future plans, objectives, beliefs, expectations, representations and projections.
Forward-looking statements are not guarantees of future performance, and our actual results could differ materially from those set forth in any forward-looking statements. Factors that could cause actual results to differ from forward-looking statements include, but are not limited to, the factors discussed in Part I, Item 1A "Risk Factors" in this Form 10-K and in Part II, Item 7 "Management's Discussion and Analysis of Financial Condition and Results of Operations" in this Form 10-K. We assume no obligation to update or revise any forward-looking statements, unless required by law.
| Item 1. | BUSINESS |
General
Hill-Rom Holdings, Inc. (the "Company," "Hill-Rom," "we," "us," or "our") was incorporated on August 7, 1969 in the State of Indiana and is headquartered in Chicago, Illinois. We are a leading global medical technology company with more than 10,000 employees worldwide. We partner with health care providers in more than 100 countries by focusing on patient care solutions that improve clinical and economic outcomes. Hill-Rom's people, products and programs work towards one mission: Every day, around the world, we enhance outcomes for patients and their caregivers.
Segment Information
During our first quarter of fiscal 2017, we changed our segment reporting to reflect changes in our organizational structure and management’s operation and view of the business. We combined the prior year North America Patient Support Systems segment and International Patient Support Systems segment into a new segment called Patient Support Systems. Our revised operating structure is generally aligned by product type and contains the following reporting segments:
| • | Patient Support Systems – globally provides our specialty bed frames and surfaces and mobility solutions, as well as our clinical workflow solutions which specializes in software and information technologies to improve care and deliver actionable insight to caregivers and patients. |
| • | Front Line Care – globally provides respiratory care products, and sells medical diagnostic monitoring equipment and a diversified portfolio of physical assessment tools that assess, diagnose, treat, and manage a wide variety of illnesses and diseases. |
| • | Surgical Solutions – globally provides products that improve surgical safety and efficiency in the operating room including tables, lights, pendants, positioning devices and various other surgical products and accessories. |
Net revenue, segment profitability and other measures of segment reporting for each reporting segment are set forth in Note 11 of our Consolidated Financial Statements.
Products and Services
Patient Support Systems. Our innovative patient support systems include a variety of specialty frames and surfaces, such as Medical Surgical ("Med-Surg") beds, Intensive Care Unit ("ICU") beds, and Bariatric patient beds, patient mobility solutions (such as lifts and other devices used to safely move patients), non-invasive therapeutic products and surfaces, and our communications technologies and software solutions. These patient support systems are sold globally and can be designed for use in high, mid, and low acuity settings, depending on the specific design options, and are built to advance mobility, reduce patient falls and caregiver injuries, improve caregiver efficiency and prevent and care for pressure injuries. In addition, we also sell equipment service contracts for our capital equipment, primarily in the U.S. Approximately 52%, 55% and 72% of our revenue during fiscal 2017, 2016 and 2015, respectively, was derived from this segment.
Front Line Care. Our Front Line Care products include our patient monitoring and diagnostics products from Welch Allyn, Inc. ("Welch Allyn") and Mortara Instruments, Inc. ("Mortara") and our respiratory health products. Our patient monitoring and diagnostics products include blood pressure, physical assessment, vital signs monitoring, diagnostic cardiopulmonary, diabetic
3
retinopathy screening, and thermometry products. We also see exciting opportunities to integrate Welch Allyn and Mortara technologies and patient data in the care environment to further enhance our product offerings. Our respiratory health products include the Vest® System, VitalCough® System, MetaNeb® System and new MonarchTM System. These products are designed to assist patients in the mobilization of retained blockages that, if not removed, may lead to increased rates of respiratory infection, hospitalization, and reduced lung function. Front Line Care products are sold globally within multiple care settings including primary care (Welch Allyn and Mortara products), acute care, extended care and home care (primarily respiratory health products). Approximately 32%, 30% and 7% of our revenue during fiscal 2017, 2016 and 2015, respectively, were derived from products within this segment.
Surgical Solutions. Our Surgical Solutions products include surgical tables, lights, and pendants utilized within the operating room setting. We also offer a range of positioning devices for use in shoulder, hip, spinal and lithotomy surgeries as well as platform-neutral positioning accessories for nearly every model of operating room table. In addition, we offer operating room surgical safety and accessory products such as scalpels and blades, light handle systems, skin markers and other disposable products. The products offered within this category are both capital sales and recurring consumable revenue streams that are sold globally. Approximately 16%, 15% and 21% of our revenue during fiscal 2017, 2016 and 2015, respectively, were derived from products within this segment.
We have extensive distribution capabilities and broad reach across all health care settings. We primarily operate in the following channels: (1) sales and rentals of products to acute and extended care facilities worldwide through both a direct sales force and distributors; (2) sales and rentals of products directly to patients in the home; and (3) sales into primary care facilities (primarily Welch Allyn products) through distributors. Through our network of approximately 140 North American and 42 international service centers, and approximately 1,900 service professionals, we provide technical support and services and rapidly deliver our products to customers on an as-needed basis, providing our customers flexibility to purchase or rent select products. No single customer accounts for more than 10% of our revenue.
Raw Materials
Principal materials used in our products for each business segment include carbon steel, aluminum, stainless steel, wood and laminates, petroleum based products, such as foams and plastics, and other materials, substantially all of which are available from multiple sources. Motors and electronic controls for electrically operated beds and certain other components are purchased from one or more manufacturers.
Prices fluctuate for raw materials and sub-assemblies used in our products based on a number of factors beyond our control. Specifically, over the past several years, the fluctuating prices of certain raw materials, including metals, fuel, plastics and other petroleum-based products in particular, and fuel related delivery costs, had a direct effect on our profitability. Although we generally have not engaged in hedging transactions with respect to raw material purchases, we have entered into fixed price supply contracts at times.
Most of our extended contracts with hospital Group Purchasing Organizations ("GPOs") and other customers for the sale of products in North America permit us to institute annual list price increases, although we may not be able to raise prices sufficiently to offset all raw material cost inflation.
Competition
Across our business, we compete on the basis of clinical expertise and resulting product clinical utility and ability to produce favorable outcomes, as well as value, quality, customer service, innovation and breadth of product offerings. We evaluate our competition based on our product categories.
4
The following table displays our significant competitors with respect to each product category:
| Product Categories | Competitors | ||
| Patient Support Systems | ArjoHuntleigh (Division of Getinge AB) Ascom Holding Joerns Healthcare Linet Rauland, a Division of AMETEK, Inc. | SIZEWise Rentals, LLC Stiegelmeyer Stryker Corporation Universal Hospital Services, Inc. | |
| Front Line Care | Covidien, Ltd. Electromed, Inc. Exergen Corporation GE Healthcare Heine Optotechnik International Biophysics, Inc. Keeler Littman (3M) | Mindray Midmark Omron Healthcare Philips Resmed Respirtech (Part of Philips) Riester Schiller Thayer Medical | |
| Surgical Solutions | Action Medical DeRoyal Draeger Maquet (Division of Getinge AB) MizuhoOSI | Skytron Steris Stryker Corporation Swann-Morton | |
Additionally, we compete with a large number of smaller and regional manufacturers.
Regulatory Matters
FDA Regulation. We design, manufacture, install and distribute medical devices that are regulated by the Food and Drug Administration ("FDA") in the U.S. and similar agencies in other countries. The regulations and standards of these agencies evolve over time and require us to make changes in our manufacturing processes and quality systems to remain in compliance. The FDA’s Quality System regulations and the regulatory equivalents internationally set forth standards for our product design and manufacturing processes, require the maintenance of certain records and provide for inspections of our facilities. From time to time, the FDA performs routine inspections of our facilities and may inform us of certain deficiencies in our processes or facilities. In addition, there are certain state and local government requirements that must be complied with in the manufacturing and marketing of our products. See Item 1A. Risk Factors for additional information.
Environmental. We are subject to a variety of federal, state, local and foreign environmental laws and regulations relating to environmental and health and safety concerns, including the handling, storage, discharge and disposal of hazardous materials used in, or derived from, our manufacturing processes. When necessary, we provide for reserves in our financial statements for environmental matters. We do not expect the remediation costs for any environmental issues in which we are currently involved to exceed $1.0 million.
Health Care Regulations. In March 2010, comprehensive health care reform legislation was signed into law through the passage of the Patient Protection and Affordable Health Care Act and the Health Care and Education Reconciliation Act. The health care industry continues to undergo significant change as this law is executed. Currently, the Trump Administration and the U.S. Congress are seeking to modify, repeal or otherwise invalidate all or part of this health care reform legislation and it remains unclear what new framework may emerge as a result of such efforts. In addition to health care reform, Medicare, Medicaid and managed care organizations, such as health maintenance organizations and preferred provider organizations, traditional indemnity insurers and third-party administrators are under increasing pressure to control costs and limit utilization, while improving quality and health care outcomes. These objectives are being advanced through a variety of reform initiatives including: accountable care organizations, value based purchasing, bundling initiatives, competitive bidding programs, etc. We are also subject to a number of other regulations around the world related to the sale and distribution of health care products. The potential impact of these regulations to our business is discussed further in Item 1A. Risk Factors and Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations, included in this Form 10-K.
5
Product Development
Most of our products and product improvements are developed internally. We maintain close working relationships with various medical professionals who assist in product research and development. New and improved products play a critical role in our sales growth. We continue to place emphasis on the development of proprietary products and product improvements to complement and expand our existing product lines. Our significant research and development activities are located in Acton, Massachusetts; Batesville, Indiana; Beaverton, Oregon; Bologna, Italy; Cary, North Carolina; Milwaukee, Wisconsin; Skaneateles Falls, New York; Pluvigner, France; Singapore; and Saalfeld and Pucheim, Germany.
Research and development is expensed as incurred. Research and development expense for the fiscal years ended September 30, 2017, 2016 and 2015, was $133.7 million, $133.5 million and $91.8 million, respectively.
In addition, certain software development technology costs for software to be sold or licensed to customers are capitalized as intangibles and are amortized over a period of three to five years once the software is ready for its intended use. The amounts capitalized during fiscal years 2017, 2016 and 2015 were approximately $2.3 million, $2.4 million and $2.6 million, respectively.
Patents and Trademarks
We own, and from time-to-time license, a number of patents on our products and manufacturing processes, but we do not believe any single patent or related group of patents is of material significance to any business segment or our business as a whole. We also own a number of trademarks and service marks relating to our products and services. Except for the marks "Hill-Rom®", "Bard-Parker®", and "Welch Allyn®", we do not believe any single trademark or service mark is of material significance to any business segment or our business as a whole.
Foreign Operations
Information about our foreign operations is set forth in tables relating to geographic information in Note 11 of our Consolidated Financial Statements, included herein under Part II, Item 8 of this Form 10-K.
Employees
At September 30, 2017, we had more than 10,000 employees worldwide. Approximately 3% of our employees in the U.S. work under collective bargaining agreements. We are also subject to various collective bargaining arrangements or national agreements outside the U.S. covering approximately 16% of our employees. The collective bargaining agreement at our primary U.S. manufacturing facility expires in January 2019. We have not experienced a work stoppage in the U.S. in over 40 years, and we believe that our employee relations are satisfactory. Refer to Item 1A. Risk Factors in this Form 10-K for additional information about our employees.
Executive Officers
The following sets forth certain information regarding our executive officers. The term of office for each executive officer expires on the date his or her successor is chosen and qualified. No director or executive officer has a "family relationship" with any other director or executive officer of the Company, as that term is defined for purposes of this disclosure requirement. There is no understanding between any executive officer and any other person pursuant to which the executive officer was selected.
John J. Greisch, 62, was elected President and Chief Executive Officer of Hill-Rom, effective January 2010. Mr. Greisch was most recently President, International Operations for Baxter International, Inc., a position he held since 2006. Prior to this, he held several other positions with Baxter, serving as Baxter's Chief Financial Officer and as President of Baxter's BioScience division.
Carlos Alonso, 58, was elected Senior Vice President and President, Hill-Rom International, effective April 2015. Before joining Hill-Rom, Mr. Alonso served as the President and CEO of the Esaote Group, a medical imaging leader based in Genova, Italy. Prior to the Esaote Group, Mr. Alonso served as the CEO of Esteve Pharmaceuticals based in Barcelona, Spain, and held various leadership roles of increasing responsibility with Baxter International, Inc. over the course of fifteen years, including serving as Global President of the Renal Division.
Andreas Frank, 41, was elected as Senior Vice President Corporate Development and Strategy, effective October 2011. Before joining Hill-Rom, Mr. Frank was Director, Corporate Development at Danaher Corporation. Previously, he worked in the Corporate Finance and Strategy practice at the consulting firm McKinsey & Company.
6
Paul Johnson, 52, was elected as Senior Vice President and President of Patient Support Systems, effective November 2016. He had previously served as president, PSS North America. Before joining Hill-Rom in 2013, Mr. Johnson held various commercial leadership positions at Life Technologies and GE Healthcare.
Kenneth Meyers, 55, was elected Senior Vice President and Chief Human Resources Officer, effective September 2015. Before joining Hill-Rom, Mr. Meyers was Senior Vice President and Chief Human Resources Officer at Hospira, Inc. Previously, he was a partner at Mercer / Oliver Wyman Consulting. Prior to Mercer / Oliver Wyman, he served as Senior Vice President, Human Resources, for Starbucks International.
Deborah Rasin, 51, was elected Senior Vice President, Chief Legal Officer and Secretary for Hill-Rom, effective January 2016. Previously she was General Counsel for Dentsply Sirona, Inc. Prior to Dentsply, Ms. Rasin served as General Counsel at Samsonite Corporation (for which she worked in Denver and London) and as a senior attorney at GM (in Detroit and Zurich).
Jason A. Richardson, 40, was elected Vice President, Controller and Chief Accounting Officer of the Company, effective March 2016. Mr. Richardson previously served in a variety of finance and accounting positions with Hill-Rom, including Assistant Controller and head of finance for Hill-Rom’s Surgical and Respiratory Care division.
Alton Shader, 44, was elected Senior Vice President and President, Front Line Care, effective September 2015. He had served as Senior Vice President and President, North America since July 2012 and previously as Senior Vice President and President, Post-Acute Care with Hill-Rom since July 2011. Before joining Hill-Rom, Mr. Shader was General Manager of Renal at Baxter International, Inc. Previously, he served as General Manager for Baxter Ireland and held senior marketing positions in Baxter's operations in Zurich and in California.
Steven J. Strobel, 59, was elected Senior Vice President, effective November 2014 and Chief Financial Officer, effective December 2014. Before joining Hill-Rom, Mr. Strobel was President of McGough Road Advisors, a corporate finance consulting firm, from 2012 to 2014 and previously Chief Financial Officer of BlueStar Energy, an independent retail energy services company, from 2009 to 2012. Prior to BlueStar, he served as Treasurer and Corporate Controller at Motorola, and in the same positions at Owens Corning. Mr. Strobel serves on the Board of Directors of Newell Brands Inc., where he chairs the Audit Committee.
Francisco Canal Vega, 56, was elected Senior Vice President and President, Surgical Solutions, effective June 2017. He had served as President of our Europe region. Before joining Hill-Rom, Mr. Canal held several senior executive roles at Baxter, Gambro, and Smith & Nephew.
Availability of Reports and Other Information
Our website is www.hill-rom.com. We make available on this website, free of charge, access to our annual, quarterly and current reports and other documents we file with, or furnish to, the Securities and Exchange Commission ("SEC") as soon as practicable after such reports or documents are filed or furnished. We also make available on our website position specifications for the Chairman, members of the Board of Directors and the Chief Executive Officer, our Global Code of Conduct (and any amendments or waivers), the Corporate Governance Standards of our Board of Directors and the charters of each of the standing committees of the Board of Directors. All of these documents are also available to shareholders in print upon request.
All reports filed with the SEC are also available via the SEC website, www.sec.gov, or may be read and copied at the SEC Public Reference Room at 100 F Street, NE, Washington, DC 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330.
| Item 1A. | RISK FACTORS |
Our business involves risks. The following information about these risks should be considered carefully together with the other information contained herein. The risks described below are not the only risks we face. Additional risks not currently known or deemed immaterial also might result in adverse effects on our business. Any of these risks could have a material adverse impact on our business, financial condition, or future results. The order in which these factors appear should not be construed to indicate their relative importance or priority.
7
We face significant uncertainty in the industry due to government health care reform, changes in Medicare, Medicaid and other governmental medical program reimbursements, and we cannot predict how these reforms will impact our operating results.
In March 2010, the U.S. Congress adopted and President Obama signed into law comprehensive health care reform legislation through the passage of the Patient Protection and Affordable Health Care Act (H.R. 3590) and the Health Care and Education Reconciliation Act (H.R. 4872). We cannot predict with certainty what additional healthcare initiatives, if any, will be implemented at the federal or state level, or what the ultimate effect of federal health care reform or any future legislation or regulation will have on us. Currently, the Trump Administration and the U.S. Congress are seeking to modify, repeal or otherwise invalidate all or part of this health care reform legislation and it remains unclear what new framework may emerge as a result of such efforts. Further, regardless of the prevailing political environment in the United States, Medicare, Medicaid, managed care organizations and foreign governments are increasing pressure to both control health care utilization and to limit reimbursement. Changes in reimbursement programs or their regulations, including retroactive and prospective rate and coverage criteria changes, competitive bidding for certain products and services, and other changes intended to reduce expenditures (domestically or internationally), could adversely affect the portions of our businesses that are dependent on third-party reimbursement or direct governmental payments. Moreover, to the extent that our customers experience reimbursement pressure resulting in lower revenue for them, their demand for our products and services might decrease. The impact of the above mentioned items could have a material adverse impact on our business, results of operations and cash flows.
Failure by us or our suppliers to comply with the FDA regulations and similar foreign regulations applicable to the products we design, manufacture, install or distribute could expose us to enforcement actions or other adverse consequences.
We design, manufacture, install and distribute medical devices that are regulated by the FDA in the U.S. and similar agencies in other countries. Failure to comply with applicable regulations could result in future product recalls, injunctions preventing the shipment of products or other enforcement actions that could have a material adverse effect on our revenue and profitability. Additionally, certain of our suppliers are subject to FDA regulations, and the failure of these suppliers to comply with regulations could adversely affect us as regulatory actions taken by the FDA against those manufacturers can result in product shortages, recalls or modifications.
We could be subject to substantial fines or damages and possible exclusion from participation in federal or state health care programs if we fail to comply with the laws and regulations applicable to our business.
We are subject to stringent laws and regulations at both the federal and state levels governing the participation of durable medical equipment suppliers in federal and state health care programs. From time to time, the government seeks additional information related to our claims submissions, and in some instances government contractors perform audits of payments made to us under Medicare, Medicaid, and other federal health care programs. On occasion, these reviews identify overpayments for which we submit refunds. At other times, our own internal audits identify the need to refund payments. We believe the frequency and intensity of government audits and review processes has intensified and we expect this will continue in the future, due to increased resources allocated to these activities at both the federal and state Medicaid level, and greater sophistication in data review techniques.
If we are deemed to have violated these laws and regulations, we could be subject to substantial fines, damages, possible exclusion from participation in federal health care programs such as Medicare and Medicaid and possible recoupment of any overpayments related to such violations. While we believe that our practices materially comply with applicable state and federal requirements, the requirements might be interpreted in a manner inconsistent with our interpretation. Failure to comply with applicable laws and regulations, even if inadvertent, could have a material adverse impact on our business.
We operate in a highly competitive industry that is subject to the risk of declining demand and pricing pressures, which could adversely affect our operating results.
Demand for our products and services depends in large part on overall demand in the health care market. Additionally, with the health care market’s increased focus on hospital asset and resource efficiency as well as reimbursement constraints, spending for some of our products is on a long-term declining trend. Further, the competitive pressures in our industry could cause us to lose market share unless we increase our expenditures or reduce our prices, which could adversely impact our operating results. The nature of this highly competitive marketplace demands that we successfully introduce new products into the market in a cost effective manner (more fully detailed below). These factors, along with possible legislative developments and others, might result in significant shifts in market share among the industry's major participants, including us. Accordingly, if we are unable to effectively differentiate ourselves from our competitors in terms of both new products and diversification of our product portfolio through business acquisitions, then our market share, sales and profitability could be adversely impacted through lower volume or decreased prices.
8
We have a substantial amount of indebtedness. This level of indebtedness could adversely affect our ability to raise additional capital to fund operations, our flexibility in operating our business and our ability to react to changes in the economy or our industry.
At September 30, 2017, we had $2,309.3 million of indebtedness outstanding net of certain issuance costs. As a result of this debt, we have significant demands on our cash resources. The level of debt could, among other things:
| • | require us to dedicate a large portion of our cash flow from operations to the servicing and repayment of our debt, thereby reducing funds available for working capital, capital expenditures, research and development expenditures and other general corporate requirements; |
| • | limit our ability to obtain additional financing to fund future working capital, capital expenditures, research and development expenditures and other general corporate requirements; |
| • | limit our flexibility in planning for, or reacting to, changes in its business and the industry in which we operate; |
| • | restrict our ability to make strategic acquisitions or dispositions or to exploit business opportunities; |
| • | place us at a competitive disadvantage compared to competitors that have less debt; |
| • | adversely affect our credit rating, with the result that the cost of servicing our indebtedness might increase; |
| • | adversely affect the market price of Hill-Rom common stock; |
| • | limit our ability to apply proceeds from an offering or asset sale to purposes other than the servicing and repayment of debt; and |
| • | cause us to fail to meet payment obligations or otherwise default under our debt, which will give our lenders the right to accelerate the indebtedness and exercise other rights and remedies against us. |
In addition, we might incur substantial additional indebtedness in the future, which could cause the related risks to intensify. We might need to refinance all or a portion of our indebtedness on or before their respective maturities. We cannot assure you that we will be able to refinance any of our indebtedness on commercially reasonable terms or at all. The terms of any additional debt might give the holders rights, preferences, and privileges senior to those of holders of our common stock, particularly in the event of liquidation. The terms of any new debt might also impose additional and more stringent restrictions on our operations than are currently in place. If we are unable to refinance our debt, we might default under the terms of our indebtedness, which could lead to an acceleration of the debt. We do not expect that we could repay all of our outstanding indebtedness if the repayment of such indebtedness was accelerated.
Our future financial performance will depend in part on the successful introduction of new products into the marketplace on a cost-effective basis.
Our future financial performance will depend in part on our ability to influence, anticipate, identify and respond to changing consumer preferences and needs. We can provide no assurances that our new products will achieve the same degree of success as in the past. We might not correctly anticipate or identify trends in consumer preferences or needs, or might identify them later than competitors do. In addition, difficulties in manufacturing or in obtaining regulatory approvals might delay or prohibit introduction of new products into the marketplace. Further, we might not be able to develop and produce new products at a cost that allows us to meet our goals for profitability. Warranty claims and service costs relating to our new products might be greater than anticipated, and we might be required to devote significant resources to address any quality issues associated with our new products, which could reduce the resources available for further new product development and other matters. In addition, the introduction of new products might also cause customers to defer purchases of existing products.
Failure to successfully introduce new products on a cost-effective basis, or delays in customer purchasing decisions related to the evaluation of new products, could cause us to lose market share and could materially adversely affect our business, financial condition, results of operations and cash flow.
Adverse developments in general domestic and worldwide economic conditions and instability and disruption of credit markets could have an adverse effect on our operating results, financial condition, or liquidity.
We are subject to risks arising from adverse changes in general domestic and global economic conditions, including recession or economic slowdown and disruption of domestic and international credit markets. The credit and capital markets could experience extreme volatility and disruption which could lead to periods of recessionary conditions and depressed levels of consumer and commercial spending. These recessionary conditions could cause customers to reduce, modify, delay or cancel plans to purchase our products and services. If our customers reduce investments in capital expenditures or utilize their limited capital funds to invest in products that we do not offer or that do not comprise a large percentage of our product portfolio, it could negatively impact our operating results. Moreover, even if our revenue remains constant, our profitability could decline if there is a shift to sales of
9
product mix or geographic locations with less favorable margins. If worldwide economic conditions worsen, we would expect our customers to scrutinize costs resulting from pressures on operating margin due to rising supply costs, reduced investment income and philanthropic giving, increased interest expense, reimbursement pressure, reduced elective healthcare spending and uncompensated care.
We might not be able to grow or achieve expected cost savings or profitability if we are unable to successfully acquire and integrate, or form business relationships with, other companies.
We have in the past, and expect in the future, to grow our business through mergers, acquisitions and other similar business arrangements. We might not be able to identify suitable acquisition candidates or business relationships, negotiate acceptable terms for such acquisitions or relationships or receive necessary financing on acceptable terms for such acquisitions or relationships. Additionally, we might become responsible for liabilities associated with businesses that we acquire to the extent they are not covered by indemnification from the sellers or by insurance. Even if we are able to consummate acquisitions, such acquisitions could be dilutive to earnings and we might not be fully successful in our integration efforts or fully realize expected benefits from the integration. Our integration efforts might also divert management and other resources from other important matters, and we could experience delays or unusual expenses in the integration process, including intangible asset impairments which could result in significant charges in our Statements of Consolidated Income. Moreover, the margins for these companies might differ from our historical gross and operating margins resulting in a material adverse effect on our results of operations.
Failure to comply with regulations due to our contracts with U.S. government entities could adversely affect our business and results of operations.
Our U.S. business contracts with U.S. government entities and is subject to specific rules, regulations and approvals applicable to government contractors. U.S. government agencies often reserve the right to conduct audits and investigations of our business practices to assure our compliance with these requirements. Our failure to comply with these or other laws and regulations could result in contract terminations, suspension or debarment from contracting with the U.S. federal government, civil fines and damages and criminal prosecution. In addition, changes in procurement policies, budget considerations, unexpected U.S. developments, such as changes in the funding or structure of Department of Veterans Affairs or other government agencies to which we sell, might adversely affect sales to government entities.
The assets in our pension plans are subject to market disruptions. In addition, our pension plans are underfunded.
Our primary pension plan invests in a variety of equity and debt securities subject to market risks. In addition, our pension plans are underfunded by $61.4 million based on our projected benefit obligation and fair value of plan assets at September 30, 2017. Market volatility and disruption could cause declines in asset values or fluctuations in assumptions used to value our liability and expenses. If this occurs, we might need to make additional pension plan contributions and our pension expense in future years might increase.
Our business is significantly dependent on major contracts with GPOs, IDNs, and certain other distributors and purchasers.
A majority of our U.S. hospital sales and rentals are made pursuant to contracts with hospital GPOs. At any given time, we are typically at various stages of responding to bids and negotiating and renewing expiring GPO agreements. Failure to be included in certain of these agreements could have a material adverse effect on our business, including product sales and service and rental revenue.
Participation by us in such programs often requires increased discounting or restrictions on our ability to raise prices, and failure to participate or to be selected for participation in such programs might result in a reduction of sales to the member hospitals. In addition, the industry is showing an increased focus on contracting directly with health systems or IDNs (which typically represent influential members and owners of GPOs). IDNs and health systems often make key purchasing decisions and have influence over the GPO’s contract decisions, and often request additional discounts or other enhancements. Further, certain other distributors and purchasers have similar processes to the GPOs and IDNs and failure to be included in agreements with these other purchasers could have a material adverse effect on our business.
10
Increased prices for, or unavailability of, raw materials or sub-assemblies used in our products could adversely affect profitability or revenue. In particular, our results of operations could be adversely affected by high prices for metals, fuel, plastics and other petroleum-based products. We also procure several raw materials and sub-assemblies from single suppliers.
Our profitability is affected by the prices and availability of the raw materials and sub-assemblies used in the manufacture of our products. These prices might fluctuate based on a number of factors beyond our control, including changes in supply and demand, general economic conditions, labor costs, fuel related delivery costs, competition, import duties, tariffs, currency exchange rates, and government regulation. Significant increases in the prices of raw materials or sub-assemblies that cannot be recovered through increases in the prices of our products could adversely affect our results of operations. There can be no assurance that the marketplace will support higher prices or that such prices and productivity gains will fully offset any commodity price increases in the future. We generally have not engaged in hedging transactions with respect to raw material purchases, but do enter into fixed price supply contracts at times. Future decisions not to engage in hedging transactions or ineffective hedging transactions might result in increased price volatility, potentially adversely impacting our profitability.
Our dependency upon regular deliveries of supplies from particular suppliers means that interruptions or stoppages in such deliveries could adversely affect our operations until arrangements with alternate suppliers could be made. Several of the raw materials and sub-assemblies used in the manufacture of our products currently are procured only from a single source. If any of these sole-source suppliers were unable or unwilling to deliver these materials for an extended period of time we might not be able to manufacture one or more products for a period of time, and our business could suffer. We might not be able to find acceptable alternatives, and any such alternatives could result in increased costs. Difficulties in the credit markets could adversely affect our suppliers’ access to capital and therefore their ability to continue to provide an adequate supply of the materials we use in our products.
The majority of our products are manufactured at a single facility or location, and the material damage or loss of, or partial or complete labor-related work stoppage at, one or more of these facilities or locations could prevent us from manufacturing some of the various products we sell.
We manufacture the majority of our products in only a single facility or location. If an event (including any weather or natural disaster-related event) occurred that resulted in material damage or loss of, or partial or complete labor-related work stoppage at, one or more of these manufacturing facilities or we lacked sufficient labor to fully operate the facility, we might be unable to transfer the manufacture of the relevant products to another facility or location in a cost-effective or timely manner, if at all. This potential inability to transfer production could occur for a number of reasons, including but not limited to a lack of necessary relevant manufacturing capability at another facility, or the regulatory requirements of the FDA or other governmental regulatory bodies. Such an event could materially negatively impact our financial condition, results of operations and cash flows.
Our international sales and operations are subject to risks and uncertainties that vary by country and which could have a material adverse effect on our business and/or results of operations.
International sales account for a significant percent of our total sales in fiscal 2017. We anticipate that international sales will continue to represent a significant portion of our total sales in the future. In addition, we have multiple manufacturing facilities and third-party suppliers that are located outside of the U.S. As a result, our international sales, as well as our sales in the U.S. of products produced or sourced internationally, are subject to risks and uncertainties that can vary by country, such as political instability, economic conditions, foreign currency exchange rate fluctuations, changes in tax laws, regulatory and reimbursement programs and policies, and the protection of intellectual property rights. In addition, our collections of international receivables are subject to economic pressures and the actions of some governmental authorities who have initiated various austerity measures to control healthcare and other governmental spending.
Unfavorable outcomes related to uncertain tax positions could result in significant tax liabilities.
We have recorded tax benefits related to various uncertain tax positions taken or expected to be taken in a tax return. While we believe our positions are appropriate, the Internal Revenue Service ("IRS"), state or foreign tax authorities could disagree with our positions, which could result in a significant tax payment.
11
We are involved on an ongoing basis in claims, lawsuits and governmental proceedings relating to our operations, as well as product liability or other liability claims that could expose us to adverse judgments or could adversely affect the sales of our products.
We are involved in the design, manufacture and sale of health care products, which face an inherent risk of exposure to product liability claims or if our products are alleged to have caused injury or are found to be unsuitable for their intended use. Amongst other claims, we are, from time to time, a party to claims and lawsuits alleging that our products have caused injury or death or are otherwise unsuitable. It is possible that we will receive adverse judgments in such lawsuits, and any such adverse judgments could be material. Although we carry insurance with respect to such matters, this insurance is subject to varying deductibles and self-insured retentions and might not be adequate to cover the full amount of any particular claim. In addition, any such claims could negatively impact the sales of products that are the subject of such claims or other products.
We might not be able to attract, retain and develop key personnel.
Our future performance depends in significant part upon the continued service of our executive officers and other key personnel. The loss of the services of one or more of our executive officers or other key employees could have a material adverse effect on our business, prospects, financial condition and results of operations. Our success also depends on our continuing ability to attract, retain and develop highly qualified personnel, and as competition for such personnel is intense, there can be no assurance that we can do so in the future.
A portion of our workforce is unionized, and we could face labor disruptions that would interfere with our operations.
Approximately 3% of our employees in the U.S. work under collective bargaining agreements. We are also subject to various collective bargaining arrangements or national agreements outside the U.S. covering approximately 16% of our employees. Although we have not recently experienced any significant work stoppages as a result of labor disagreements, we cannot ensure that such a stoppage will not occur in the future. Our labor contract at our primary U.S. manufacturing facility expires in January 2019. Our ability to negotiate satisfactory new agreements or a labor disturbance at one of our principal facilities could have a material adverse effect on our operations.
We might not be successful in achieving expected operating efficiencies and sustaining or improving operating expense reductions, and might experience business disruptions and adverse tax consequences associated with restructuring, realignment and cost reduction activities.
Over the past few years we have initiated several restructuring, realignment and cost reduction initiatives. While we expect to realize efficiencies from these actions, these activities might not produce the full efficiency and cost reduction benefits we expect. Further, such benefits might be realized later than expected, and the ongoing costs of implementing these measures might be greater than anticipated. If these measures are not successful or sustainable, we might undertake additional realignment and cost reduction efforts, which could result in future charges. Moreover, our ability to achieve our other strategic goals and business plans might be adversely affected and we could experience business disruptions with customers and elsewhere if our restructuring and realignment efforts and our cost reduction activities prove ineffective.
These actions, the resulting costs, and potential delays or potential lower than anticipated benefits might also impact our foreign tax positions and might require us to record tax reserves against certain deferred tax assets in our international business.
We are increasingly dependent on consistent functioning of our information technology and cybersecurity systems and if we are exposed to any intrusions or if we fail to maintain the integrity of our data, our business and our reputation could be materially adversely affected.
We are increasingly dependent on consistent functioning of our information technology and cybersecurity systems for our infrastructure and products. Our information systems require an ongoing commitment of significant resources to maintain, protect, and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards, integration of acquisitions, and the increasing need to protect patient, customer and supplier information. In addition, third parties might attempt to hack into our products or systems and might obtain proprietary information. If we fail to maintain or protect our information and cybersecurity systems and data integrity effectively, we could lose existing customers or suppliers, have difficulty attracting new customers or suppliers, have problems that adversely impact internal controls, have difficulty preventing, detecting, and controlling fraud, have disputes with customers and suppliers, have regulatory sanctions or penalties imposed, have increases in operating expenses, incur expenses or lose revenues as a result of a data privacy breach, or suffer other adverse consequences. Any significant breakdown, intrusion, interruption, corruption, or destruction of these systems, as well as any data breaches, could have a material adverse effect on our business.
12
We might be adversely affected by new regulations relating to conflict minerals.
The SEC has adopted rules regarding disclosure for public companies whose products contain conflict minerals (commonly referred to as tin, tantalum, tungsten and gold) which originate from the Democratic Republic of the Congo (DRC) and/or adjoining countries. The implementation of these requirements could adversely affect the sourcing, availability and pricing of materials used in the manufacturing of our products. In addition, we will incur additional costs to comply with the disclosure requirements, including costs related to determining the source of any of the relevant minerals used in our products. Since our supply chain is complex and multilayered, we might be unable to ascertain with sufficient certainty the origins for these minerals despite our due diligence procedures, which in turn might harm our reputation. We might also face difficulties in satisfying customers who might require that our products be certified as DRC conflict free, which could harm our relationships with these customers and/or lead to a loss of revenue. These requirements also could have the effect of limiting the pool of suppliers from which we source these minerals, and we might be unable to obtain conflict-free minerals at prices similar to the past, which could increase our costs and adversely affect our manufacturing operations and our profitability.
| Item 1B. | UNRESOLVED STAFF COMMENTS |
We have not received any comments from the staff of the SEC regarding our periodic or current reports that remain unresolved.
13
| Item 2. | PROPERTIES |
The principal properties used in our operations are listed below. All facilities are suitable for their intended purpose, are being efficiently utilized and are believed to provide adequate capacity to meet demand for the next several years.
| Location | Description and Primary Use | Owned/Leased |
| Acton, MA | Light manufacturing, development and distribution of health care equipment; Office administration | Leased |
| Batesville, IN | Manufacturing, development and distribution of health care equipment; Office administration | Owned |
| Beaverton, OR | Development of health care equipment; Office administration | Leased |
| Caledonia, MI | Manufacturing, development and distribution of surgical products; Office administration | Leased |
| Cary, NC | Development of health care equipment; Office administration | Leased |
| Charleston, SC | Light manufacturing and distribution of health care equipment; Office administration | Leased |
| Chicago, IL | Office administration | Leased |
| Coral Springs, FL | Manufacturing and distribution of health care equipment; Office administration | Leased |
| Corona, CA | Manufacturing, engineering and distribution of health care equipment | Leased |
| Fishers, IN | Manufacturing of health care equipment | Leased |
| Milwaukee, WI | Manufacturing, development and distribution of health care equipment; Office administration | Owned |
| St. Paul, MN | Office administration and distribution of health care equipment | Leased |
| Skaneateles Falls, NY | Manufacturing, development and distribution of health care equipment; Office administration | Owned |
| Sydney, Australia | Distribution of healthcare equipment; Office administration | Leased |
| Shanghai, China | Manufacturing and development of health care equipment; Office administration | Leased |
| Taicang, China | Light manufacturing and distribution of health care equipment | Leased |
| Pluvigner, France | Manufacturing, development and distribution of health care equipment; Office administration | Owned |
| Puchheim, Germany | Manufacturing, development and distribution of health care equipment; Office administration | Owned/Leased |
| Saalfeld, Germany | Manufacturing, development and distribution of health care equipment; Office administration | Owned |
| Navan, County Meath, Ireland | Office administration | Owned |
| Bologna, Italy | Research and development | Leased |
| Tijuana, Mexico | Manufacturing and distribution of health care equipment; Office administration | Leased |
| Monterrey, Mexico | Manufacturing of health care equipment | Owned |
| Amsterdam, Netherlands | Office administration | Leased |
| Las Piedras, Puerto Rico | Manufacturing of surgical products | Owned |
| Singapore | Research and development of health care equipment; Office administration | Leased |
| Lulea, Sweden | Manufacturing, development and distribution of health care equipment; Office administration | Owned |
In addition to the foregoing, we lease or own a number of other facilities, warehouse distribution centers, service centers and sales offices throughout the U.S., Canada, Western Europe, Mexico, Australia, Middle East, the Far East, and Latin America.
14
| Item 3. | LEGAL PROCEEDINGS |
See Note 13 of our Consolidated Financial Statements included under Part II, Item 8 of this Form 10-K for information regarding legal proceedings in which we are involved.
| Item 4. | MINE SAFETY DISCLOSURES |
Not applicable.
15
PART II
| Item 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is traded on the New York Stock Exchange under the ticker symbol "HRC". The closing price of our common stock on the New York Stock Exchange on November 14, 2017 was $76.93 per share. The following table reflects the range of high and low selling prices of our common stock and cash dividends declared by quarter for each of the last two fiscal years.
| Year Ended September 30 | ||||||||||||||||||||||||
| 2017 | 2016 | |||||||||||||||||||||||
| Quarter Ended: | High | Low | Cash Dividends Declared | High | Low | Cash Dividends Declared | ||||||||||||||||||
| December 31 | $ | 63.12 | $ | 50.50 | $ | 0.17 | $ | 55.26 | $ | 46.31 | $ | 0.16 | ||||||||||||
| March 31 | $ | 71.22 | $ | 55.04 | $ | 0.18 | $ | 51.11 | $ | 42.99 | $ | 0.17 | ||||||||||||
| June 30 | $ | 81.33 | $ | 69.47 | $ | 0.18 | $ | 54.57 | $ | 46.79 | $ | 0.17 | ||||||||||||
| September 30 | $ | 84.65 | $ | 71.91 | $ | 0.18 | $ | 62.17 | $ | 49.42 | $ | 0.17 | ||||||||||||
Holders
As of November 14, 2017, there were approximately 42,500 shareholders of record.
Dividends
The declaration and payment of cash dividends is at the sole discretion of our Board of Directors ("Board") and depends upon many factors, including our financial condition, earnings potential, capital requirements, alternative uses of cash, covenants associated with debt obligations, legal requirements, and other factors deemed relevant by our Board. We have paid cash dividends on our common stock every quarter since our initial public offering in 1971. We intend to continue to pay quarterly cash dividends comparable to those paid in the periods covered by these financial statements.
Issuer Purchases of Equity Securities
| Period | Total Number of Shares Purchased (1) | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs (2) | Approximate Dollar Value of Shares That May Yet Be Purchased Under the Programs (2) | |||||||||
| July 1, 2017 - July 31, 2017 | 544 | $ | 82.80 | — | $ | 34.7 | |||||||
| August 1, 2017 - August 31, 2017 | 271,834 | $ | 75.41 | 265,160 | $ | 14.7 | |||||||
| September 1, 2017 - September 30, 2017 | 78,012 | $ | 73.74 | — | $ | 14.7 | |||||||
| Total | 350,390 | 265,160 | |||||||||||
| (1) | Shares purchased during the quarter ended September 30, 2017 were in connection with the share repurchase program discussed below as well as employee payroll tax withholding for restricted and deferred stock distributions. |
| (2) | In September 2013, the Board approved an expansion of its previously announced share repurchase authorization to a total of $190.0 million. As of September 30, 2017, a cumulative total of $175.3 million had been used under the then existing authorization. On November 6, 2017, the Board of Directors approved an increase to the share repurchase program in an amount of $150.0 million, leaving the company approximately $165.0 million of availability under the share repurchase program as of such date. The plan does not have an expiration date and currently there are no plans to terminate this program in the future. |
16
Stock Performance Graph
The following graph compares the return on our common stock with that of Standard & Poor’s 500 Stock Index ("S&P 500") and our peer groups* for the five years ended September 30, 2017. Because the composition of our current peer group (the "2017 Peer Group") has changed since the date of our Annual Report on Form 10-K for the fiscal year ended September 30, 2016, we have included the data for the 2017 Peer Group as well as for our prior year’s peer group (the "2016 Peer Group") in the graph below. The changes reflected in the 2017 Peer Group were made in order to more closely align with the peer group used in our most recent compensation study done for executive compensation purposes. The graph assumes that the value of the investment in our common stock, the S&P 500, our 2017 Peer Group and our 2016 Peer Group was $100 on October 1, 2012 and that all dividends were reinvested.
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |||||||||||||||||||
| HRC | $ | 100 | $ | 123 | $ | 143 | $ | 179 | $ | 213 | $ | 255 | ||||||||||||
| S & P 500 | $ | 100 | $ | 117 | $ | 137 | $ | 133 | $ | 151 | $ | 175 | ||||||||||||
| 2016 Peer Group | $ | 100 | $ | 107 | $ | 121 | $ | 129 | $ | 170 | $ | 200 | ||||||||||||
| 2017 Peer Group | $ | 100 | $ | 106 | $ | 120 | $ | 135 | $ | 176 | $ | 205 | ||||||||||||
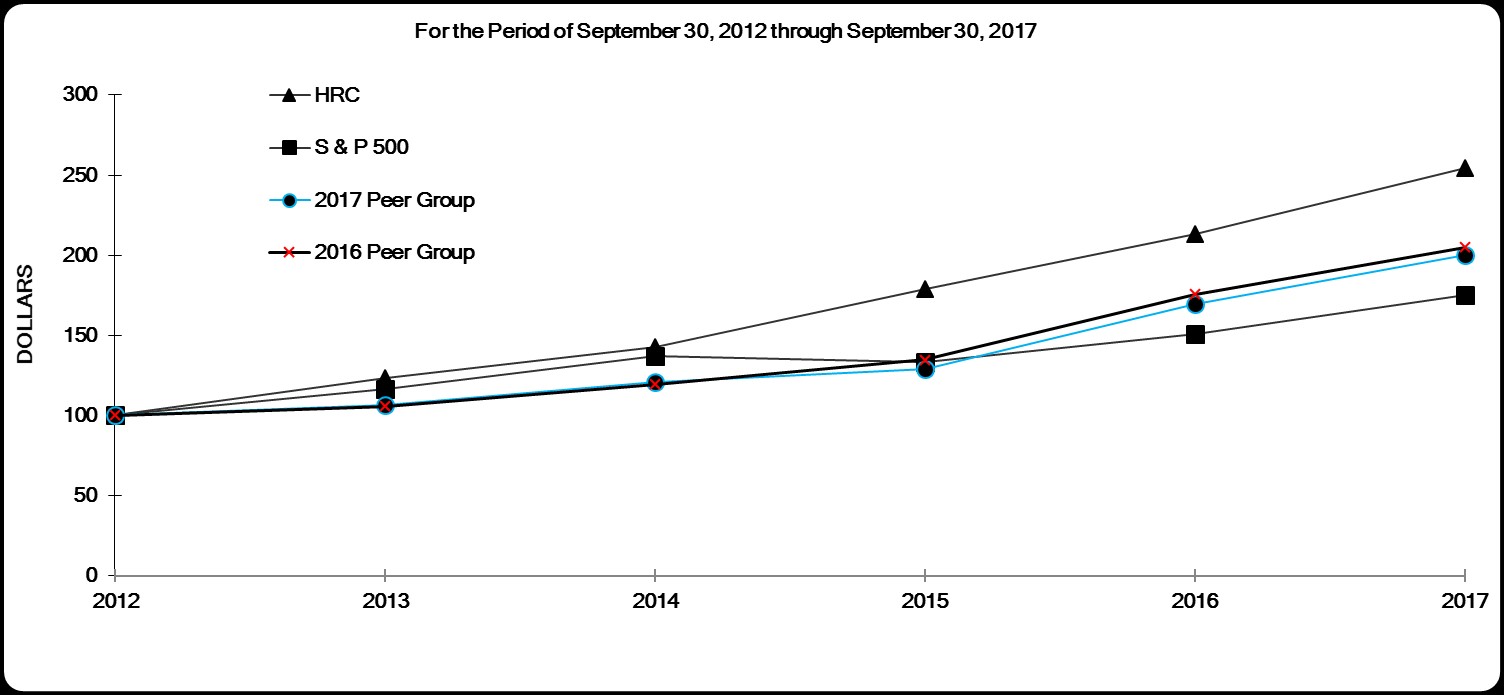
| * | For purposes of the Stock Performance Graph above, our 2017 Peer Group is comprised of: Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., Bruker Corporation, C.R. Bard, Inc., The Cooper Companies, Inc., Dentsply Sirona Inc., Edwards Lifesciences Corporation, Halyard Health, Inc., Hologic, Inc., Intuitive Surgical, Inc., Mednax, Inc., Patterson Companies, Inc., PerkinElmer, Inc., Quest Diagnostics Incorporated, St. Jude Medical, Inc., Steris plc, Teleflex, Incorporated, Varian Medical Systems, Inc. and Waters Corporation. |
Our 2016 Peer Group was comprised of: Bruker Corporation, C.R. Bard, Inc., The Cooper Companies, Inc., Dentsply Sirona Inc., Edwards Lifesciences Corporation, Halyard Health, Inc., Hologic, Inc., Intuitive Surgical, Inc., Laboratory Corporation of America Holdings, Mednax, Inc., Patterson Companies, Inc., PerkinElmer, Inc., Quest Diagnostics Incorporated, St. Jude Medical, Inc., Steris plc, Teleflex, Incorporated, Varian Medical Systems, Inc. and Waters Corporation.
Certain other information required by this item will be contained under the caption "Equity Compensation Plan Information" in our definitive Proxy Statement to be delivered to shareholders in connection with the Annual Meeting of Shareholders to be held on March 6, 2018, and such information is incorporated herein by reference.
17
| Item 6. | SELECTED FINANCIAL DATA |
The following table presents our selected consolidated financial data for each of the last five fiscal years ended September 30. Refer to Note 2 of our Consolidated Financial Statements included under Part II, Item 8 of this Form 10-K for disclosure of business combinations for each of the last three fiscal years. Also see Note 12 of our Consolidated Financial Statements included under Part II, Item 8 of this Form 10-K for selected unaudited quarterly financial information for each of the last two fiscal years.
| 2017 | 2016 | 2015 | 2014 | 2013 | |||||||||||||||
| Net revenue | $ | 2,743.7 | $ | 2,655.2 | $ | 1,988.2 | $ | 1,686.1 | $ | 1,716.2 | |||||||||
| Net income | $ | 132.3 | $ | 122.8 | $ | 46.8 | $ | 60.6 | $ | 105.0 | |||||||||
| Net income attributable to common shareholders | $ | 133.6 | $ | 124.1 | $ | 47.7 | $ | 60.6 | $ | 105.0 | |||||||||
| Net income attributable to common shareholders per share - Basic | $ | 2.04 | $ | 1.90 | $ | 0.83 | $ | 1.05 | $ | 1.75 | |||||||||
| Net income attributable to common shareholders per share - Diluted | $ | 1.99 | $ | 1.86 | $ | 0.82 | $ | 1.04 | $ | 1.74 | |||||||||
| Total assets | $ | 4,528.7 | $ | 4,262.4 | $ | 4,457.6 | $ | 1,751.3 | $ | 1,586.8 | |||||||||
| Long-term obligations | $ | 2,120.4 | $ | 1,938.4 | $ | 2,175.2 | $ | 364.1 | $ | 225.8 | |||||||||
| Cash flows from operating activities | $ | 311.1 | $ | 281.2 | $ | 213.8 | $ | 210.3 | $ | 263.2 | |||||||||
| Capital expenditures | $ | 97.5 | $ | 83.3 | $ | 121.3 | $ | 62.7 | $ | 65.3 | |||||||||
| Cash flows from investing activities | $ | (389.4 | ) | $ | (97.7 | ) | $ | (1,756.4 | ) | $ | (294.5 | ) | $ | (58.6 | ) | ||||
| Cash flows from financing activities | $ | 70.6 | $ | (141.9 | ) | $ | 1,642.7 | $ | 63.8 | $ | (161.5 | ) | |||||||
| Cash dividends per share | $ | 0.7100 | $ | 0.6700 | $ | 0.6325 | $ | 0.5950 | $ | 0.5250 | |||||||||
18
| Item 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
This Form 10-K contains "forward-looking statements" within the meaning of the federal securities laws with respect to general economic conditions, our financial condition, results of operations, cash flows and business and our expectations or beliefs concerning future events, including the demand for our products, the ability to operate our manufacturing sites at full capacity, future supplies of raw materials for our operations, share repurchases, international market conditions, expectations regarding our liquidity, our capital spending, plans for future acquisitions and divestitures, and our operating plans. These forward-looking statements can generally be identified by phrases such as we or our management "expects," "anticipates," "believes," "estimates," "intends," "plans to," "ought," "could," "will," "should," "likely," "appears," "projects," "forecasts," "outlook" or other similar words or phrases. There are inherent risks and uncertainties in any forward-looking statements. We caution readers not to place undue reliance on any forward-looking statements.
Our forward-looking statements are based on management's expectations and beliefs as of the time this Form 10-K is filed with the SEC or, with respect to any document incorporated by reference, as of the time such document was prepared. Although we believe that our expectations are reasonable, we can give no assurance that these expectations will prove to have been correct, and actual results may vary materially. These factors include those described in Part I, Item 1A "Risk Factors" of this Form 10-K. Except as required by law, we undertake no obligation to update, amend or clarify any forward-looking statements to reflect changed assumptions, the occurrence of anticipated or unanticipated events, new information or circumstances or any other changes.
Overview
Hill-Rom Holdings, Inc. ("we," "us," or "our"), is a leading global medical technology company with more than 10,000 employees worldwide. We partner with health care providers in more than 100 countries, across all care settings, by focusing on patient care solutions that improve clinical and economic outcomes in five core areas: Advancing Mobility, Wound Care and Prevention, Patient Monitoring and Diagnostics, Surgical Safety and Efficiency and Respiratory Health. Hill-Rom's people, products, and programs work towards one mission: Every day, around the world, we enhance outcomes for patients and their caregivers.
Industry Trends
Provider Consolidation. Economic considerations, competition and other factors have led to ongoing consolidation of customers and the centralization of purchasing decision-making. We believe this has influenced the criteria customers use to evaluate the value proposition offered by Hill-Rom for various product and service offerings.
Emerging Markets Healthcare Access. While industry growth rates in more mature geographic regions such as western and northern Europe and Japan have moderated, in many other geographic markets, the relative spending on health care is expanding. We expect long-term increasing demand for medical technologies as a result. New hospital construction and hospital refurbishments are expected in regions such as Latin America, the Middle East and many parts of Asia.
Information and Connectivity. Patient and provider demand for health care products and services will continue to grow over the long-term as a result of a number of factors, including an aging population, longer life expectancies, and an increasing number of sicker patients across all care settings, including hospitals, extended care facilities and in the home. In contrast, however, health care providers are under continued pressure to improve efficiency and control costs. As a result, utilizing connected devices to generate meaningful and real time information about patients and products has become critical to providing quality healthcare, enhancing patient experience, lowering length of stay and driving efficiencies across the healthcare continuum.
Economic and Clinical Value. We believe an increasing emphasis is being placed within hospitals to assure quality of care through increased accountability and public disclosure. As an example, several pieces of legislation have been enacted over the past few years to address these areas including the "pay for performance" initiative by the Centers for Medicare and Medicaid Services ("CMS") which aims to better align reimbursement with improved patient outcomes and the reduction of adverse events including bedsores (or pressure ulcers), ventilator associated pneumonia, patient falls, deep vein thrombosis and patient entrapment. Hospitals may experience reduced reimbursement for hospital-acquired adverse events, creating a stronger connection between these adverse events and hospital revenue levels. Therefore, we believe that health care providers will seek to do business with partners that can demonstrate improved clinical, and consequently economic, outcomes.
Lower Cost Care Settings. Growing pressures on healthcare costs are resulting in a migration of care from the acute care hospital into lower cost care setting. We believe that this trend increases the demand for more solutions to care for these patients in lower acuity settings, including improved medical technologies, communication tools and information technologies.
19
Strategic Priorities
We believe we have aligned our strategic priorities to accommodate the evolving global healthcare landscape.
Accelerating Growth Across Care Settings and Markets. As we continue to diversify our portfolio, we are extending our presence into new care settings and markets. We are expanding internationally, entering new product areas and accelerating revenue contributions from new products, and expect our U.S. and international businesses to be key contributors to solid growth. Among other measures, Hill-Rom established Enterprise Accounts teams in the U.S. and an integrated international commercial organization to serve as an adjunct to our traditional sales representatives, and better address customer needs for products and services that deliver solutions for quality patient care. With the acquisition of Welch Allyn, we also added a significant distributor component serving the U.S. primary care setting.
Innovation. Due to the growing population of sick patients, health care systems are challenged to treat the rising incidence of complex diseases and conditions while also reducing costs and improving efficiency. This trend increases the demand for our innovative medical technologies, communication tools and information technologies, and we are constantly innovating to ensure doctors, nurses and caregivers have the products they need to protect patients, speed recovery, and manage their conditions.
Transforming Our Portfolio. We have made several acquisitions and this continues to be a key component of our strategy going forward. We have also recently divested non-strategic assets from our portfolio. We believe these actions are helping optimize our portfolio and enhance our focus on long-term higher margin growth.
Driving Operational Execution. We will continue to undertake initiatives to improve our operating efficiency, including consolidation of our manufacturing footprint, realignment and optimization of business processes, and lowering sourcing costs. We believe our operating expenses and margins will be positively impacted by these actions. We may utilize savings generated from these actions to reinvest in our growth priorities and improve our profitability.
Risk Factors
Our ability to sustain long-term growth and successfully execute the strategies discussed above depends in part on our ability to manage within an increasingly competitive and regulated environment and to address the other risk factors described in Item 1A on this Form 10-K.
Use of Non-GAAP Financial Measures
The accompanying Consolidated Financial Statements, including the related notes, are presented in accordance with accounting principles generally accepted in the U.S. ("GAAP"). We routinely provide gross margin, operating margin and earnings per share results on an adjusted basis because the Company’s management believes these measures contribute to an understanding of our financial performance, provide additional analytical tools to understand our results from core operations and reveal underlying trends. These measures exclude strategic developments, acquisition and integration costs, special charges or other unusual events. The Company also excludes expenses associated with the amortization of intangible assets associated with prior business acquisitions. These adjustments are made to allow investors to evaluate and understand operating trends excluding the non-cash impact of acquired intangible amortization on operating income and earnings per share.
Management uses these measures internally for planning, forecasting and evaluating the performance of the business. Investors should consider non-GAAP measures in addition to, not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP.
In addition, we present certain results on a constant currency basis. Constant currency information compares results between periods as if foreign currency exchange rates had remained consistent period-over-period. We monitor sales performance on a constant currency basis that eliminates the positive or negative effects that result from translating international sales into U.S. dollars. We calculate constant currency by applying the foreign currency exchange rate for the prior period to the local currency results for the current period. We believe that evaluating growth in net revenue on a constant currency basis provides an additional and meaningful assessment to both management and investors.
20
Results of Operations
Fiscal Year Ended September 30, 2017 Compared to Fiscal Year Ended September 30, 2016
In this section, we provide an overview of our results of operations. We disclose segment information that is consistent with the way in which management operates and views the business. During our first quarter of fiscal 2017, we changed our segment reporting to reflect changes in our organizational structure and management’s operation and view of the business. We combined the prior year North America Patient Support Systems segment and International Patient Support Systems segment into a new segment called Patient Support Systems. Our Patient Support Systems segment now also includes an additional component of global marketing spend that was previously unallocated. The prior year segment information included in this Form 10-K has been updated to reflect these changes. Our revised operating structure contains the following reporting segments:
| • | Patient Support Systems – globally provides our specialty bed frames and surfaces and mobility solutions, as well as our clinical workflow solutions which specializes in software and information technologies to improve care and deliver actionable insight to caregivers and patients. |
| • | Front Line Care – globally provides respiratory care products, and sells medical diagnostic monitoring equipment and a diversified portfolio of physical assessment tools that assess, diagnose, treat, and manage a wide variety of illnesses and diseases. |
| • | Surgical Solutions – globally provides products that improve surgical safety and efficiency in the operating room including tables, lights, pendants, positioning devices and various other surgical products and accessories. |
Net Revenue
| U.S. | OUS | |||||||||||||||||||||
| Year Ended September 30 | Change As Reported | Constant Currency | Change As Reported | Change As Reported | Constant Currency | |||||||||||||||||
| 2017 | 2016 | |||||||||||||||||||||
| Revenue: | ||||||||||||||||||||||
| Product sales and service | $ | 2,358.1 | $ | 2,263.4 | 4.2 | % | 4.6 | % | 4.7 | % | 3.2 | % | 4.4 | % | ||||||||
| Rental revenue | 385.6 | 391.8 | (1.6 | )% | (1.3 | )% | (1.1 | )% | (5.1 | )% | (2.6 | )% | ||||||||||
| Total revenue | $ | 2,743.7 | $ | 2,655.2 | 3.3 | % | 3.7 | % | 3.6 | % | 2.7 | % | 4.0 | % | ||||||||
| Revenue: | ||||||||||||||||||||||
| Patient Support Systems | $ | 1,423.9 | $ | 1,437.2 | (0.9 | )% | (0.6 | )% | 0.2 | % | (3.9 | )% | (2.8 | )% | ||||||||
| Front Line Care | 885.3 | 809.7 | 9.3 | % | 9.7 | % | 8.0 | % | 12.8 | % | 14.0 | % | ||||||||||
| Surgical Solutions | 434.5 | 408.3 | 6.4 | % | 7.2 | % | 8.1 | % | 4.7 | % | 6.4 | % | ||||||||||
| Total revenue | $ | 2,743.7 | $ | 2,655.2 | 3.3 | % | 3.7 | % | 3.6 | % | 2.7 | % | 4.0 | % | ||||||||
| OUS - Outside of the U.S. | ||||||||||||||||||||||
Consolidated Revenue
Consolidated revenue increased 3.3% on a reported basis and 3.7% on a constant currency basis for the year ended September 30, 2017 with growth in both the U.S. and OUS. This growth was impacted by the acquisition of Mortara in February 2017, partially offset by the disposition of our Architectural Products and Völker businesses in fiscal 2017 and the disposition of our products related to our perinatal data management system in fiscal 2016. All three dispositions were within our Patient Support Systems segment. Excluding the impact of businesses we recently divested and the impact of the Mortara acquisition, our consolidated revenue grew approximately 3% on a constant currency basis.
Product sales and service revenue increased 4.2% on a reported basis and 4.6% on a constant currency basis for the year ended September 30, 2017, primarily due to growth in our Surgical Solutions segment as well as our acquisition of Mortara. This growth was partially offset by declines from businesses we recently divested within our Patient Support Systems segment.
21
Rental revenue decreased 1.6% on a reported basis or 1.3% on a constant currency basis for the year ended September 30, 2017, primarily due to volume declines in our third party rental business.
Business Segment Revenue
Patient Support Systems revenue decreased 0.9% on a reported basis and 0.6% on a constant currency basis for the year ended September 30, 2017 compared to the prior year. Fiscal 2017 was impacted by lower revenue from businesses we recently divested. Excluding the impact of these completed divestitures from all periods, revenue grew by approximately 3% on a constant currency basis for the year ended September 30, 2017 led by growth in the U.S. and Middle East.
Front Line Care revenue increased 9.3% on a reported basis and 9.7% on a constant currency basis for the year ended September 30, 2017 compared to the prior year, primarily due to growth in Europe and Asia Pacific from our Welch Allyn business, as well as additional revenue from our Mortara acquisition in February 2017.
Surgical Solutions revenue increased 6.4% on a reported basis and 7.2% on a constant currency basis for the year ended September 30, 2017 compared to the prior year, mainly due to double digit growth in surgical equipment and patient positioning businesses, which included strong OUS growth across most regions and new product growth in the U.S.
Gross Profit
| Year Ended September 30 | |||||||
| 2017 | 2016 | ||||||
| Gross Profit | |||||||
| Product sales and service | $ | 1,122.3 | $ | 1,054.0 | |||
| Percent of Related Revenue | 47.6 | % | 46.6 | % | |||
| Rental | 198.3 | 203.0 | |||||
| Percent of Related Revenue | 51.4 | % | 51.8 | % | |||
| Total Gross Profit | $ | 1,320.6 | $ | 1,257.0 | |||
| Percent of Total Revenue | 48.1 | % | 47.3 | % | |||
Product sales and service gross margin increased 100 basis points for the year ended September 30, 2017. The prior year included an impact of $19.9 million for the inventory step-up associated with the Welch Allyn acquisition compared to the current year impact of $4.8 million for inventory step-up associated with the Mortara acquisition. Excluding these items, product sales and service gross margin increased 30 basis points for the year ended September 30, 2017, primarily due to product mix and supply chain improvements.
Rental gross margin decreased 40 basis points for the year ended September 30, 2017 compared to the prior year due to reduced leverage of our fleet and field service infrastructure driven by lower revenue.
Operating Expenses
| Year Ended September 30 | |||||||
| 2017 | 2016 | ||||||
| Research and development expenses | $ | 133.7 | $ | 133.5 | |||
| Percent of Total Revenue | 4.9 | % | 5.0 | % | |||
| Selling and administrative expenses | $ | 876.1 | $ | 853.3 | |||
| Percent of Total Revenue | 31.9 | % | 32.1 | % | |||
Research and development expenses remained relatively flat for the year ended September 30, 2017 compared to the prior year. As a percentage of revenue, research and development expenses have been consistent year over year.
22
As a percentage of total revenue, selling and administrative expenses decreased during the year to date period compared to the prior year. Selling and administrative expenses include $132.7 million of acquisition-related intangible asset amortization, acquisition and integration costs, and certain litigation charges for the year ended September 30, 2017 and $114.8 million for the year ended September 30, 2016. Excluding these items, selling and administrative expenses decreased 70 basis points as a percentage of revenue as a result of disciplined cost management.
Business Segment Divisional Income
| Year Ended September 30 | Change As Reported | |||||||||
| 2017 | 2016 | |||||||||
| Divisional income: | ||||||||||
| Patient Support Systems | $ | 249.6 | $ | 245.2 | 1.8 | % | ||||
| Front Line Care | 231.8 | 202.1 | 14.7 | % | ||||||
| Surgical Solutions | 42.5 | 46.2 | (8.0 | )% | ||||||
Refer to Note 11 of our Consolidated Financial Statements for a description of how divisional income is determined.
Patient Support Systems divisional income increased 1.8% for the year ended September 30, 2017 primarily due to lower operating expenses and an increase in margins from product mix and supply chain improvements.
Front Line Care divisional income increased 14.7% for the year ended September 30, 2017 compared to the prior year as a result of our Mortara acquisition and higher margins from supply chain improvements.
Surgical Solutions divisional income decreased 8.0% for the year ended September 30, 2017 compared to the prior year, primarily due to increased operating expenses and lower margins due to increases in supply chain costs.
Special Charges and Other
| Year Ended September 30 | |||||||
| 2017 | 2016 | ||||||
| Special charges | $ | 37.4 | $ | 39.9 | |||
| Interest expense | $ | (88.9 | ) | $ | (90.4 | ) | |
| Loss on extinguishment of debt | $ | — | $ | (10.8 | ) | ||
| Investment income and other, net | $ | (1.5 | ) | $ | 9.2 | ||
In connection with various organizational changes to improve our business alignment and cost structure, we recognized special charges of $37.4 million and $39.9 million for the year to date periods ended September 30, 2017 and 2016. These charges relate to the initiatives described in Note 8 of our Consolidated Financial Statements.
Interest expense was lower in the year to date period ended September 30, 2017 mainly due to the improved terms under our prior year amendment to our Senior Credit Agreement. These favorable terms were partially offset by higher outstanding debt as a result of the Mortara acquisition. See Note 4 of our Consolidated Financial Statements for additional information.
Loss on extinguishment of debt in the prior year relates to the amendment of our Senior Credit Agreement. Refer to Note 4 of our Consolidated Financial Statements for additional information.
Investment income and other, net decreased due to the fiscal 2016 gain from the disposition of our products related to our perinatal data management system.
23
GAAP and Adjusted Earnings
Operating margin, income before income taxes, income tax expense, and earnings attributable to common shareholders per diluted share are summarized in the table below. GAAP amounts are adjusted for certain items to aid management in evaluating the performance of the business. Income tax expense is computed by applying a blended statutory tax rate based on the jurisdictional mix of the respective before tax adjustment.
| Year Ended September 30, 2017 | Year Ended September 30, 2016 | ||||||||||||||||||||||||||||
Operating Margin1 | Income Before Income Taxes | Income Tax Expense | Diluted EPS | Operating Margin | Income Before Income Taxes | Income Tax Expense | Diluted EPS1 | ||||||||||||||||||||||
| GAAP Basis | 10.0 | % | $ | 183.0 | $ | 50.7 | $ | 1.99 | 8.7 | % | $ | 138.3 | $ | 15.5 | $ | 1.86 | |||||||||||||
| Adjustments: | |||||||||||||||||||||||||||||
| Acquisition and integration costs | 0.9 | % | 23.5 | 9.7 | 0.21 | 1.5 | % | 38.9 | 11.3 | 0.41 | |||||||||||||||||||
| Acquisition-related intangible asset amortization | 4.0 | % | 108.4 | 34.2 | 1.10 | 3.6 | % | 95.9 | 31.7 | 0.96 | |||||||||||||||||||
| Field corrective actions | — | % | — | (0.2 | ) | — | — | % | 0.2 | (0.1 | ) | — | |||||||||||||||||
Litigation settlements and expenses2 | (0.3 | )% | (9.4 | ) | (3.4 | ) | (0.09 | ) | — | % | — | — | — | ||||||||||||||||
| Special charges | 1.9 | % | 52.5 | 10.3 | 0.63 | 1.5 | % | 39.9 | 13.4 | 0.40 | |||||||||||||||||||
| Foreign tax law change | — | % | — | (2.2 | ) | 0.03 | — | % | — | — | — | ||||||||||||||||||
| Foreign valuation allowance | — | % | — | — | — | — | % | — | 19.5 | (0.29 | ) | ||||||||||||||||||
| Debt refinancing | — | % | — | — | — | — | % | 12.9 | 4.7 | 0.12 | |||||||||||||||||||
| Gain on disposition | — | % | (1.0 | ) | (0.4 | ) | (0.01 | ) | — | % | (10.1 | ) | (3.7 | ) | (0.10 | ) | |||||||||||||
| Adjusted Basis | 16.3 | % | $ | 357.0 | $ | 98.7 | $ | 3.86 | 15.3 | % | $ | 316.0 | $ | 92.3 | $ | 3.38 | |||||||||||||
1 Total may not add due to rounding | |||||||||||||||||||||||||||||
2 Fiscal 2017 includes favorable litigation settlement of $15.1 million which was recognized as Special charges in our Statements of Consolidated Income. Refer to Note 8 of our Consolidated Financial Statements for additional information. | |||||||||||||||||||||||||||||
The effective tax rate for the year to date period ended September 30, 2017 was 27.7% compared to 11.2% for the prior year. The effective tax rate for the current year is higher than the comparable period in fiscal 2016 due primarily to the difference in the amount of discrete tax benefits recognized in each period. The tax rate for fiscal 2017 was unfavorably impacted by the non-deductible impairment loss related to the sale of our Völker business compared to the large favorable period tax benefits of $20.0 million in the prior year primarily related to the release of the valuation allowance on our deferred tax assets in France. Fiscal 2017 also includes period tax benefits of $8.9 million related to the adoption of the ASU 2016-09, as discussed in Note 7 of our Consolidated Financial Statements.
The adjusted effective tax rate for the year ended September 30, 2017 was 27.6% compared to 29.2% for the comparable period in the prior year. The lower adjusted tax rate is due primarily to period tax items related to the adoption of ASU 2016-09.
Diluted earnings per share increased 7.0% on a reported basis and 14.2% on an adjusted basis for the year ended September 30, 2017.
24
Fiscal Year Ended September 30, 2016 Compared to Fiscal Year Ended September 30, 2015
Net Revenue
| U.S. | OUS | |||||||||||||||||||||
Year Ended September 30 | Change As Reported | Constant Currency | Change As Reported | Change As Reported | Constant Currency | |||||||||||||||||
| 2016 | 2015 | |||||||||||||||||||||
| Revenue: | ||||||||||||||||||||||
| Product sales and service | $ | 2,263.4 | $ | 1,604.5 | 41.1 | % | 43.1 | % | 57.7 | % | 17.5 | % | 22.4 | % | ||||||||
| Rental revenue | 391.8 | 383.7 | 2.1 | % | 2.7 | % | 4.2 | % | (11.3 | )% | (6.8 | )% | ||||||||||
| Total revenue | $ | 2,655.2 | $ | 1,988.2 | 33.5 | % | 35.3 | % | 43.7 | % | 15.5 | % | 20.3 | % | ||||||||
| Revenue: | ||||||||||||||||||||||
| Patient Support Systems | $ | 1,437.2 | $ | 1,426.6 | 0.7 | % | 1.8 | % | 8.2 | % | (14.5 | )% | (11.1 | )% | ||||||||
| Front Line Care | 809.7 | 139.0 | N/M | N/M | N/M | N/M | N/M | |||||||||||||||
| Surgical Solutions | 408.3 | 422.6 | (3.4 | )% | (1.4 | )% | 5.2 | % | (10.9 | )% | (7.2 | )% | ||||||||||
| Total revenue | $ | 2,655.2 | $ | 1,988.2 | 33.5 | % | 35.3 | % | 43.7 | % | 15.5 | % | 20.3 | % | ||||||||
| OUS - Outside of the U.S. | ||||||||||||||||||||||
| N/M - Not meaningful | ||||||||||||||||||||||
Consolidated Revenue
Product sales and service revenue increased 41.1% on a reported basis and 43.1% on a constant currency basis in fiscal 2016 mainly due to the Welch Allyn acquisition. On a proforma basis, reflecting the inclusion of Welch Allyn in both the fiscal 2016 and 2015 periods, product sales and service revenue increased 1.1% on a reported basis and increased 2.5% on a constant currency basis. These movements were driven by proforma growth of the Welch Allyn business and higher sales of specialty frames and surfaces and clinical workflow solutions in our Patient Support Systems segment. These increases were partially offset by lower international sales of specialty frames and surfaces and surgical products primarily in the Middle East and Latin America as a result of macro-economic conditions in these regions. Revenue for the period in Europe was also lower in fiscal 2016 compared to fiscal 2015.
Rental revenue increased 2.1% on a reported basis and 2.7% on a constant currency basis. This increase was mainly driven by higher volumes in our Patient Support Systems segment, partially offset by decreased international rental revenue resulting from volume declines and pricing pressures in Europe.
Business Segment Revenue
Patient Support Systems revenue increased 0.7% in fiscal 2016 compared to fiscal 2015. Product sales and service revenue increased primarily due to higher sales of specialty frames and surfaces and clinical workflow solutions products, partially offset by declines in the Middle East, Europe, and Latin America. Rental revenue increased by 2.1% primarily due to increased volumes in the U.S., partially offset by lower volume and pricing pressures in Europe.
Front Line Care revenue increased in 2016 primarily as a result of the Welch Allyn acquisition. On a proforma constant currency basis, reflecting the inclusion of Welch Allyn in fiscal 2016 and 2015, Front Line Care revenue grew 6.0% due mainly to growth in Welch Allyn. Fiscal year 2015 results for this segment primarily reflects our respiratory care business, which achieved low single digit revenue growth in fiscal 2016.
Surgical Solutions revenue decreased 3.4% on a reported basis and 1.4% on a constant currency basis. On a constant currency basis, sales declines were mainly in the Middle East and Latin America as a result of macro-economic difficulties in these regions. These declines were partially offset by increases in our Allen Medical and Trumpf businesses in the U.S.
25
Gross Profit
| Year Ended September 30 | |||||||
| 2016 | 2015 | ||||||
| Gross Profit | |||||||
| Product sales and service | $ | 1,054.0 | $ | 683.3 | |||
| Percent of Related Revenue | 46.6 | % | 42.6 | % | |||
| Rental | $ | 203.0 | $ | 197.0 | |||
| Percent of Related Revenue | 51.8 | % | 51.3 | % | |||
| Total Gross Profit | $ | 1,257.0 | $ | 880.3 | |||
| Percent of Related Revenue | 47.3 | % | 44.3 | % | |||
Product sales and service gross margin increased 400 basis points in fiscal 2016. The increase in gross margin was driven primarily by the addition of Welch Allyn’s higher gross margins. Excluding the impact of the Welch Allyn acquisition, organic gross margin improved 180 basis points in fiscal 2016 driven by favorable product mix in our Patient Support Systems segment, as well as manufacturing efficiencies and favorable geographic mix. These increases were partially offset by gross margin declines in our international regions.
Rental gross margin increased 50 basis points in fiscal 2016. Gross margin was favorably impacted by product mix and increased leverage of fleet and field service infrastructure in our Patient Support Systems segment, partially offset by lower volumes and pricing pressures in our international regions.
Operating Expenses
| Year Ended September 30 | |||||||
| 2016 | 2015 | ||||||
| Research and development expenses | $ | 133.5 | $ | 91.8 | |||
| Percent of Total Revenue | 5.0 | % | 4.6 | % | |||
| Selling and administrative expenses | $ | 853.3 | $ | 664.2 | |||
| Percent of Total Revenue | 32.1 | % | 33.4 | % | |||
Research and development expenses increased 45.4% primarily due to the addition of Welch Allyn and additional investment in new product development initiatives in Surgical Solutions and in our respiratory care business.
Selling and administrative expenses as a percent of total revenue decreased 130 basis points. Selling and administrative expenses include acquisition and integration costs, acquisition-related intangible asset amortization, FDA remediation expenses, a supplemental stock compensation charge, and litigation settlements and expenses that totaled $114.8 million in 2016, compared with $90.0 million in 2015. Excluding these items, selling and administrative expenses decreased 110 basis points as a percentage of revenue.
26
Business Segment Divisional Income
| Year Ended September 30 | Change As Reported | |||||||||
| 2016 | 2015 | |||||||||
| Divisional income: | ||||||||||
| Patient Support Systems | $ | 245.2 | $ | 206.5 | 18.7 | % | ||||
| Front Line Care | 202.1 | 41.5 | N/M | |||||||
| Surgical Solutions | 46.2 | 56.0 | (17.5 | )% | ||||||
| N/M - Not meaningful | ||||||||||
Refer to Note 11 of our Consolidated Financial Statements for a description of how divisional income is determined.
Patient Support Systems divisional income increased 18.7% due primarily to improved gross margins, along with improved operating leverage as operating expenses were lower. Product sales and service margins increased compared with 2015, primarily due to favorable product mix and manufacturing efficiencies, partially offset by reduced leverage of manufacturing costs in our international regions. Rental margins also increased during the year as a result of product mix and increased leverage of our fleet and field service infrastructure due to higher rental revenue, partially offset by pricing pressures in our international regions.
Front Line Care divisional income increased in 2016 primarily as a result of the Welch Allyn acquisition.
Surgical Solutions divisional income decreased 17.5%. Divisional income was impacted by higher investments in research and development and sales and marketing in support of long-term growth initiatives.
Special Charges and Other
| Year Ended September 30 | ||||||||
| 2016 | 2015 | |||||||
| Special charges | $ | 39.9 | $ | 41.2 | ||||
| Interest expense | $ | (90.4 | ) | $ | (18.4 | ) | ||
| Loss on extinguishment of debt | $ | (10.8 | ) | $ | — | |||
| Investment income and other, net | $ | 9.2 | $ | 0.4 | ||||
We recognized special charges of $39.9 million in fiscal 2016 and $41.2 million in fiscal 2015, related to various organizational changes that we implemented to improve our business alignment and cost structure. These charges relate to the initiatives described in Note 8 of our Consolidated Financial Statements.
Interest expense was higher compared with 2015 due to additional borrowings made in connection with the Welch Allyn acquisition.
Loss on extinguishment of debt represents the write-off of deferred financing fees in connection with the refinancing of our outstanding debt in the fourth quarter of fiscal 2016. Refer to Note 4 of our Consolidated Financial Statements for additional information regarding our debt refinancing.
Investment income and other, net increased due to the fiscal 2016 gain from the disposition of our perinatal data management system in the fourth quarter of 2016.
27
GAAP and Adjusted Earnings
Operating margin, income before income taxes, income tax expense, and earnings attributable to common shareholders per diluted share are summarized in the table below. GAAP amounts are adjusted for certain items to aid management in evaluating the performance of the business. Income tax expense is computed by applying a blended statutory tax rate based on the jurisdictional mix of the respective before tax adjustment.
| Year Ended September 30 | |||||||||||||||||||||||||||||
| 2016 | 2015 | ||||||||||||||||||||||||||||
Operating Margin | Income Before Income Taxes | Income Tax Expense | Diluted EPS 1 | Operating Margin 1 | Income Before Income Taxes | Income Tax Expense | Diluted EPS | ||||||||||||||||||||||
| GAAP Basis | 8.7 | % | $ | 138.3 | $ | 15.5 | $ | 1.86 | 4.2 | % | $ | 65.1 | $ | 18.3 | $ | 0.82 | |||||||||||||
| Adjustments: | |||||||||||||||||||||||||||||
| Acquisition and integration costs | 1.5 | % | 38.9 | 11.3 | 0.41 | 3.2 | % | 62.8 | 18.0 | 0.76 | |||||||||||||||||||
| Acquisition-related intangible asset amortization | 3.6 | % | 95.9 | 31.7 | 0.96 | 1.7 | % | 34.1 | 9.8 | 0.42 | |||||||||||||||||||
| FDA remediation expenses | — | — | — | — | 0.2 | % | 3.8 | 1.2 | 0.04 | ||||||||||||||||||||
| Field corrective actions | — | 0.2 | (0.1 | ) | — | 0.2 | % | 4.5 | 1.4 | 0.05 | |||||||||||||||||||
| Litigation settlements and expenses | — | — | — | — | — | (0.6 | ) | (0.2 | ) | (0.01 | ) | ||||||||||||||||||
| Special charges | 1.5 | % | 39.9 | 13.4 | 0.40 | 2.1 | % | 41.2 | 10.7 | 0.52 | |||||||||||||||||||
| Supplemental stock compensation charge | — | — | — | — | 0.3 | % | 6.1 | 2.2 | 0.07 | ||||||||||||||||||||
| Foreign valuation allowance | — | — | 19.5 | (0.29 | ) | — | — | 1.9 | (0.03 | ) | |||||||||||||||||||
| Debt refinancing | — | 12.9 | 4.7 | 0.12 | — | — | — | — | |||||||||||||||||||||
| Gain on disposition | — | (10.1 | ) | (3.7 | ) | (0.10 | ) | — | — | — | — | ||||||||||||||||||
| Adjusted Basis | 15.3 | % | $ | 316.0 | $ | 92.3 | $ | 3.38 | 11.8 | % | $ | 217.0 | $ | 63.3 | $ | 2.64 | |||||||||||||
1 Total does not add due to rounding | |||||||||||||||||||||||||||||
The effective tax rate for fiscal 2016 was 11.2% compared to 28.1% in 2015. The effective tax rate for fiscal 2016 is lower than fiscal 2015 due primarily to the release of the valuation allowance on our deferred tax assets discussed in Note 1 of our Consolidated Financial Statements in Part II, Item 8 of this Form 10-K.
The adjusted effective tax rate was 29.2% for both fiscal years 2016 and 2015.
Liquidity and Capital Resources
| Year Ended September 30 | |||||||||||
| (Dollars in millions) | 2017 | 2016 | 2015 | ||||||||
| Cash Flows Provided By (Used In): | |||||||||||
| Operating activities | $ | 311.1 | $ | 281.2 | $ | 213.8 | |||||
| Investing activities | (389.4 | ) | (97.7 | ) | (1,756.4 | ) | |||||
| Financing activities | 70.6 | (141.9 | ) | 1,642.7 | |||||||
| Effect of exchange rate changes on cash | 7.3 | (2.2 | ) | (6.6 | ) | ||||||
| Increase (Decrease) in Cash and Cash Equivalents | $ | (0.4 | ) | $ | 39.4 | $ | 93.5 | ||||
28
Net cash flows from operating activities and selected borrowings represented our primary sources of funds for growth of the business, including capital expenditures and acquisitions. Our financing agreements contain certain restrictions relating to dividend payments, the making of restricted payments, and the incurrence of additional secured and unsecured indebtedness. None of our financing agreements contain any credit rating triggers which would increase or decrease our cost of borrowings. Credit rating changes can, however, impact the cost of borrowings and any potential future borrowings under any new financing agreements.
Operating Activities
Cash provided by operating activities increased $29.9 million in fiscal 2017 compared to the prior year due primarily to higher net income and a prior year pension contribution partially offset by working capital activities. Cash provided by operating activities was driven primarily by net income, adjusted for the non-cash effects of depreciation, amortization, the impairment of our Völker business and stock compensation expense, along with working capital activities.
Cash provided by operating activities during fiscal 2016 was driven primarily by net income, adjusted for the non-cash effects of depreciation, amortization, loss on extinguishment of debt, stock compensation expense and the rollout of inventory step-up from the Welch Allyn acquisition. These sources of cash were offset by the payout of performance-based compensation related to our 2015 fiscal year, a pension contribution of $30 million, acquisition and restructuring costs related mainly to Welch Allyn and other working capital activities. Cash provided by operating activities increased compared to the prior year due mainly to higher net income adjusted for the non-cash effects of the items previously listed.
Cash provided by operating activities during fiscal 2015 was driven by net income, adjusted up for non-cash expenses including depreciation, amortization, stock compensation, and a pension settlement charge, offset by the provision for deferred income taxes and changes in working capital.
Investing Activities
Cash used in investing activities increased $291.7 million in fiscal 2017 compared to the prior year, primarily due to our acquisition of Mortara in the second quarter of fiscal 2017, partially offset by proceeds on the sale of property, plant and equipment and our recently divested Architectural Products and Völker businesses. See Note 2 of our Consolidated Financial Statements for additional information on our acquisition of Mortara.
Cash used for investing activities during fiscal 2016 consisted mainly of capital expenditures and payment for the acquisition of Anodyne Medical Device, Inc., known as Tridien Medical ("Tridien"). The fiscal 2015 cash used for investing activities was higher due to the acquisition of Welch Allyn and higher than normal capital expenditures due to investments in our rental fleet to support volume increases.
Financing Activities
Cash provided by financing activities increased $212.5 million in fiscal 2017 primarily due to increases in our net borrowings in connection with the Mortara acquisition, offset by $50.0 million of share repurchases. See Note 4 of our Consolidated Financial Statements for additional information on our financing agreements. During the year ended September 30, 2017, we increased our dividends paid by $0.04 per share compared to the prior year.
Cash used in financing activities during fiscal 2016 consisted mainly of the pay down of long-term debt and payments of cash dividends. During the year ended September 30, 2016, we increased our dividends paid by $0.0375 per share compared to the prior year. The net cash used in financing activities for fiscal 2016 compares to net cash provided by financing activities in fiscal 2015, as borrowings for the acquisition of Welch Allyn exceeded stock repurchases and the payment of dividends in the prior year period.
Cash provided by financing activities during fiscal 2015 consisted mainly of new borrowings which were used to fund the Welch Allyn acquisition. Borrowings under our prior credit facility were also used to fund the higher rental fleet investment previously discussed. This was offset by treasury stock acquired, dividend payments, and payments to retire previously outstanding debt as this was replaced with the financing obtained in conjunction with the Welch Allyn acquisition. During the year ended September 30, 2015, we increased our dividends paid by $0.0375 per share compared to the prior year.
The treasury stock acquired refers to purchases in the open market and the repurchases of shares associated with employee payroll tax withholdings for restricted and deferred stock distributions.
Our debt-to-capital ratio was 62.8%, 63.5% and 65.9% at September 30, 2017, 2016 and 2015, respectively.
29
Other Liquidity Matters
In addition to the discussion of our financing agreements detailed in Note 4 of our Consolidated Financial Statements and our retirement and postretirement benefit plans detailed in Note 6 of our Consolidated Financial Statements, we intend to continue to pay quarterly cash dividends comparable to those paid in the periods covered by these financial statements. However, the declaration and payment of dividends by us will be subject to the sole discretion of our Board and will depend upon many factors, including our financial condition, earnings, capital requirements, covenants associated with debt obligations, legal requirements and other factors deemed relevant by our Board.
During fiscal 2017 and fiscal 2015, we purchased 0.8 million shares and 1.2 million shares of our common stock valued at $50.0 million and $54.8 million in the open market, leaving $14.7 million available for purchase as of September 30, 2017 under a $190.0 million share repurchase program approved by the Board of Directors in September 2013. Repurchases may be made on the open market or via private transactions and are used for general business purposes. On November 6, 2017, the Board of Directors approved an increase to the share repurchase program in an amount of $150.0 million, leaving the company approximately $165.0 million of availability under the share repurchase program as of such date. This program does not have an expiration date and there are no plans to terminate this program in the future.
Over the long term, we intend to continue to pursue inorganic growth in certain areas of our business, but the timing, size or success of any acquisition effort and the related potential capital commitments cannot be predicted.
We believe that cash on hand and generated from operations, along with amounts available under our financing agreements, will be sufficient to fund operations, working capital needs, capital expenditure requirements, and financing obligations for at least the next twelve months from the date of this filing. However, disruption and volatility in the credit markets could impede our access to capital. Our $700.0 million revolving credit facility is with a syndicate of banks, which we believe reduces our exposure to any one institution and would still leave us with significant borrowing capacity in the event that any one of the institutions within the group is unable to comply with the terms of our agreement.
As of September 30, 2017, approximately 80.3% of our cash and cash equivalents were held by our foreign subsidiaries. Portions of this may be subject to U.S. income taxation if repatriated to the U.S., however, because cash and cash equivalents held by our foreign subsidiaries are largely used for operating needs outside the U.S. we have no need to repatriate this cash for other uses. We believe that cash on hand and cash generated from operations, along with amounts available under our Revolving Credit Facility and Securitization Program, will be sufficient to fund operations, working capital needs, capital expenditure requirements and financing obligations.
Credit Ratings
In fiscal 2017, Standard and Poor’s Rating Services and Moody’s Investor Service issued credit ratings for Hill-Rom of BB+ and Ba2, respectively, with stable outlooks.
Other Uses of Cash
We expect capital spending in 2018 to be approximately $110.0 million. Capital spending will be monitored and controlled as the year progresses.
Off-Balance Sheet Arrangements
We have no material off-balance sheet arrangements.
30
Contractual Obligations, Contingent Liabilities and Commitments
To give a clear picture of matters potentially impacting our liquidity position, the following table outlines our contractual obligations as of September 30, 2017:
| Payments Due by Period | |||||||||||||||||||
| (Dollars in millions) | Total | Less Than 1 Year | 1 - 3 Years | 3 - 5 Years | After 5 Years | ||||||||||||||
| Contractual Obligations | |||||||||||||||||||
| Long-term debt obligations | $ | 2,331.7 | $ | 188.9 | $ | 294.6 | $ | 1,504.9 | $ | 343.3 | |||||||||
| Interest payments relating to long-term debt (1) | 403.3 | 77.0 | 137.7 | 105.8 | 82.8 | ||||||||||||||
| Operating lease obligations | 105.1 | 31.7 | 39.0 | 20.8 | 13.6 | ||||||||||||||
Pension and postretirement health care benefit funding (2) | 26.3 | 2.8 | 4.5 | 4.7 | 14.3 | ||||||||||||||
| Purchase obligations (3) | 182.3 | 166.8 | 15.5 | — | — | ||||||||||||||
| Other long-term liabilities (4) | 32.4 | 0.1 | 12.8 | 12.8 | 6.7 | ||||||||||||||
| Total contractual cash obligations | $ | 3,081.1 | $ | 467.3 | $ | 504.1 | $ | 1,649.0 | $ | 460.7 | |||||||||
| (1) | Interest payments on our long-term debt are projected based on the contractual rates of outstanding debt securities. |
| (2) | Based on our funded status as of September 30, 2017, we are not required to make any further contributions to our master pension plan in fiscal 2018. |
| (3) | Purchase obligations represent contractual obligations under various take-or-pay arrangements executed in the normal course of business. These commitments represent future purchases in line with expected usage to obtain favorable pricing. Also included are obligations arising from purchase orders for which we have made firm commitments. As a result, we believe that the purchase obligations portion of our contractual obligations is substantially those obligations for which we are certain to pay, regardless of future facts and circumstances. We expect to fund purchase obligations with operating cash flows and current cash balances. |
| (4) | Other long-term liabilities include deferred compensation arrangements, self-insurance reserves, and other various liabilities. |
We also had commercial commitments related to standby letters of credit at September 30, 2017 of $8.4 million.
In addition to the contractual obligations and commercial commitments disclosed above, we also have a variety of other agreements related to the procurement of materials and services and other commitments. While many of these agreements are long-term supply agreements, some of which are exclusive supply or complete requirements-based contracts, we are not committed under these agreements to accept or pay for requirements which are not needed to meet production needs. Also, we have an additional $4.5 million of other liabilities as of September 30, 2017, which represent uncertain tax positions for which it is not possible to determine in which future period the tax liability might be settled.
In conjunction with our acquisition and divestiture activities, we have entered into certain guarantees and indemnifications of performance, as well as, non-competition agreements for varying periods of time. Potential losses under the indemnifications are generally limited to a portion of the original transaction price, or to other lesser specific dollar amounts for certain provisions. Guarantees and indemnifications with respect to acquisition and divestiture activities, if triggered, could have a materially adverse impact on our financial condition and results of operations.
We are also subject to potential losses from adverse litigation results that are not accounted for by a self-insurance or other reserves; however, such potential losses are not quantifiable at this time, and may never occur.
Critical Accounting Policies and Estimates
Our accounting policies, including those described below, require management to make significant estimates and assumptions using information available at the time the estimates are made. Such estimates and assumptions significantly affect various reported amounts of assets, liabilities, revenue and expenses. If future experience differs materially from these estimates and assumptions, results of operations and financial condition could be affected. Our most critical accounting policies are described below.
31
Revenue Recognition
Net revenue reflects gross revenue less sales discounts and allowances and customer returns for product sales and rental revenue reserves. Revenue is evaluated under the following criteria and recognized when each is met:
| • | Evidence of an arrangement: An agreement with the customer reflecting the terms and conditions to deliver products or services serves as evidence of an arrangement. |
| • | Delivery: For products, delivery is generally considered to occur upon transfer of title and risk of loss per the respective sales terms. For rental services, delivery is considered to occur when the services are rendered. |
| • | Fixed or determinable price: The sales price is considered fixed or determinable if it is not subject to refund or adjustment. |
| • | Collection is deemed probable: At or prior to the time of a transaction, credit reviews of each customer are performed to determine the creditworthiness of the customer. Collection is deemed probable if the customer is expected to be able to pay amounts under the arrangement as those amounts become due. If collection is not probable, revenue is recognized when collection becomes probable, generally upon cash collection. |
As a general interpretation of the above guidelines, revenue for health care and surgical products are generally recognized upon delivery of the products to the customer and their assumption of risk of loss and other risks and rewards of ownership. Local business customs and sales terms specific to certain customers or products can sometimes result in deviations to this normal practice; however, in no case is revenue recognized prior to the transfer of risk of loss and rewards of ownership.
For non-invasive therapy products and medical equipment management services, the majority of product offerings are rental products for which revenue is recognized consistent with the rendering of the service and use of products. For The Vest ® product, revenue is generally recognized at the time of receipt of authorization for billing from the applicable paying entity as this serves as evidence of the arrangement and sets a fixed or determinable price.
For health care products and services aimed at improving operational efficiency and asset utilization, various revenue recognition techniques are used, depending on the offering. Arrangements to provide services, routinely under separately sold service and maintenance contracts, result in the deferral of revenue until specified services are performed. Service contract revenue is generally recognized ratably over the contract period, if applicable, or as services are rendered. Product-related goods are generally recognized upon delivery to the customer.
Revenue and Accounts Receivable Reserves
Revenue is presented in the Statements of Consolidated Income net of certain discounts, GPO fees, and sales adjustments. For product sales, we record reserves resulting in a reduction of revenue for contractual discounts, as well as price concessions and product returns. Likewise, rental revenue reserves, reflecting contractual and other routine billing adjustments, are recorded as a reduction of revenue. Reserves for revenue are estimated based upon historical rates for revenue adjustments.
Provisions for doubtful accounts are recorded as a component of operating expenses and represent our best estimate of the amount of probable credit losses and collection risk in our existing accounts receivable. We determine such reserves based on historical write-off experience by industry. Receivables are generally reviewed on a pooled basis based on historical collection experience for each receivable type and are also reviewed individually for collectability. Account balances are charged against the allowance when we believe it is probable the receivable will not be recovered. We do not have any off-balance sheet credit exposure related to our customers.
If circumstances change, such as higher than expected claims denials, payment defaults, changes in our business composition or processes, adverse changes in general economic conditions, instability or disruption of credit markets, or an unexpected material adverse change in a major customer’s or payer’s ability to meet its obligations, our estimates of the realizability of trade receivables could be reduced by a material amount.
32
Liabilities for Loss Contingencies Related to Lawsuits
We are involved on an ongoing basis in claims, investigations and lawsuits relating to our operations, including patent infringement, business practices, commercial transactions and other matters. The ultimate outcome of these actions cannot be predicted with certainty. An estimated loss from these contingencies is recognized when we believe it is probable that a loss has been incurred and the amount of the loss can be reasonably estimated. However, it is difficult to measure the actual loss that might be incurred related to claims, investigations and lawsuits. The ultimate outcome of these actions could have a material adverse effect on our financial condition, results of operations and cash flow.
We are also involved in other possible claims, including product and general liability, workers’ compensation, auto liability and employment related matters. Refer to Note 13 of our Consolidated Financial Statements for additional information.
The recorded amounts represent our best estimate of the costs we will incur in relation to such exposures, but it is possible that actual costs could differ from those estimates.
Goodwill and Intangible Assets
We account for acquired businesses using the acquisition method of accounting. This method requires that the identifiable assets acquired and liabilities assumed be measured at their fair value, with goodwill being the excess value of consideration paid less the fair value of the net identifiable assets acquired. Judgments and estimates are required in the determination of fair values, including the setting of discount rates, growth rates and forecasted business results for the acquired business and portions of the acquired business, along with estimated useful lives. Changes in these judgments or estimates can have a material impact on the valuation of the respective assets and liabilities acquired and our results of operations.
We perform an impairment assessment on goodwill and other indefinite-lived intangibles annually during the third fiscal quarter, or whenever events or changes in circumstances indicate that the carrying value of a reporting unit may not be recoverable. These events or conditions include, but are not limited to, a significant adverse change in the business environment; regulatory environment or legal factors; a current period operating or cash flow loss combined with a history of such losses or a projection of continuing losses; a substantial decline in market capitalization of our stock; or a sale or disposition of a significant portion of a reporting unit.
The goodwill impairment assessment requires either evaluating qualitative factors or performing a quantitative assessment to determine if a reporting unit’s carrying value is likely to exceed its fair value. The qualitative goodwill impairment assessment requires evaluating factors to determine that a reporting unit’s carrying value would not more likely than not exceed its fair value. As part of our goodwill qualitative testing process for each reporting unit, when utilized, we evaluate various factors that are specific to the reporting unit as well as industry and macroeconomic factors in order to determine whether it is reasonably likely to have a material impact on the fair value of our reporting units. Examples of the factors that are considered include the results of the most recent impairment test, current and long-range forecasted financial results, and changes in the strategic outlook or organizational structure of the reporting units. The long-range financial forecasts of the reporting units, which are based upon management’s long-term view of our markets and are used by senior management and the Board of Directors to evaluate operating performance, are compared to the forecasts used in the prior year analysis to determine if management expectations for the business have changed. Management changes in strategic outlook or organizational structure represent internally driven strategic or organizational changes that could have a material impact on our results of operations or product offerings. Industry, market changes and macroeconomic indicators represent our view on changes outside of the Company that could have a material impact on our results of operations, product offerings or future cash flow forecasts. In the event we were to determine that a reporting unit’s carrying value would more likely than not exceed its fair value, quantitative testing would be performed comparing carrying values to estimated fair values. Changes in management intentions, market conditions, operating performance and other similar circumstances could affect the assumptions used in this qualitative impairment test. Changes in the assumptions could result in impairment charges that could be material to our Consolidated Financial Statements in any given period.
Quantitative testing involves a two-step process. The first step, used to identify potential impairment, is a comparison of each reporting unit’s estimated fair value to its carrying value, including goodwill. If the fair value of a reporting unit exceeds its carrying value, applicable goodwill is considered not to be impaired. If the carrying value exceeds fair value, there is an indication of impairment and the second step is performed to measure the amount of the impairment. The second step requires us to calculate an implied fair value of goodwill. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination, which is the excess of the fair value of the reporting unit, as determined in the first step, over the aggregate fair values of the individual assets, liabilities and identifiable intangibles as if the reporting unit was being acquired in a business combination. If the goodwill assigned to a reporting unit exceeds the implied fair value of the goodwill, an impairment charge is recorded for the excess.
33
Measurement of the fair value of reporting units in the first step of a quantitative impairment process requires significant management judgment with respect to forecasted sales, gross margin and selling, general and administrative expenses, capital expenditures, the selection and use of an appropriate discount rate, the selection of comparable public companies and the determination of an appropriate control premium. In addition, the use of third-party appraisals of significant tangible and intangible assets as part of the second step of the impairment test also requires management judgment related to certain inputs and assumptions. There are inherent uncertainties related to each of the above listed assumptions and inputs, and our judgment in applying them. The use of different assumptions, estimates or judgments in either step of the process could trigger the need for an impairment charge, or materially increase or decrease the amount of any such impairment charge.
Retirement Benefit Plans
We sponsor retirement and postretirement benefit plans covering select employees. Expense recognized in relation to these defined benefit retirement and postretirement health care plans is based upon actuarial valuations and inherent in those valuations are key assumptions including discount and mortality rates, and where applicable, expected returns on assets, projected future salary rates and projected health care cost trends. The discount rates used in the valuation of our defined benefit pension and postretirement plans are evaluated annually based on current market conditions. In setting these rates we utilize long-term bond indices and yield curves as a preliminary indication of interest rate movements, and then make adjustments to the respective indices to reflect differences in the terms of the bonds covered under the indices in comparison to the projected outflow of our obligations. Our overall expected long-term rate of return on pension assets is based on historical and expected future returns, which are inflation adjusted and weighted for the expected return for each component of the investment portfolio. Our rate of assumed compensation increase is also based on our specific historical trends of past wage adjustments.
Changes in retirement and postretirement benefit expense and the recognized obligations may occur in the future as a result of a number of factors, including changes to any of these assumptions. Our expected rate of return on pension plan assets was 5.8% for fiscal 2017, 5.8% for fiscal 2016 and 6.8% for 2015. At September 30, 2017, we had pension plan assets of $284.4 million. A 25 basis point increase in the expected rate of return on pension plan assets reduces annual pension expense by approximately $0.6 million. Differences between actual and projected investment returns, especially in periods of significant market volatility, can also impact estimates of required pension contributions. The discount rate for our defined benefit pension plans obligation was 3.9% in 2017, 3.7% in 2016 and 4.4% in 2015. The discount rate for our postretirement obligations may vary up to 100 basis points from that of our retirement obligations. For each 50 basis point change in the discount rate, the impact to annual pension expense ranges from an increase of $1.9 million to a decrease of $1.7 million, while the impact to our postretirement health care expense would be insignificant. Impacts from assumption changes could be positive or negative depending on the direction of the change in rates.
Income Taxes
We compute our income taxes using an asset and liability approach to reflect the net tax effects of temporary differences between the financial reporting carrying amounts of assets and liabilities and the corresponding income tax amounts. We have a variety of deferred tax assets in numerous tax jurisdictions. These deferred tax assets are subject to periodic assessment as to recoverability and if it is determined that it is more likely than not that the benefits will not be realized, valuation allowances are recognized. In evaluating whether it is more likely than not that we would recover these deferred tax assets, future taxable income, the reversal of existing temporary differences and tax planning strategies are considered.
We believe that our estimates for the valuation allowances recorded against deferred tax assets are appropriate based on current facts and circumstances. As of September 30, 2017, we had $58.2 million of valuation allowances on deferred tax assets, on a tax-effected basis, primarily related to certain foreign deferred tax attributes that are not expected to be utilized.
We account for uncertain income tax positions using a threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The difference between the tax benefit recognized in the financial statements for an uncertain income tax position and the tax benefit claimed in the tax return is referred to as an unrecognized tax benefit.
We also have on-going audits in various stages of completion with the IRS and several state and foreign jurisdictions, one or more of which may conclude within the next 12 months. Such settlements could involve some or all of the following: the payment of additional taxes, the adjustment of certain deferred taxes and/or the recognition of previously unrecognized tax benefits. The resolution of these matters, in combination with the expiration of certain statutes of limitations in various jurisdictions, make it reasonably possible that our unrecognized tax benefits may decrease as a result of either payment or recognition by approximately $0.5 million to $1.0 million in the next twelve months, excluding interest.
34
Guarantees
We routinely grant limited warranties on our products with respect to defects in material and workmanship. The terms of these warranties are generally one year, however, certain components and products have substantially longer warranty periods. We recognize a reserve with respect to these obligations at the time of product sale, with subsequent warranty claims recorded directly against the reserve. The amount of the warranty reserve is determined based on historical trend experience for the covered products. For more significant warranty-related matters which might require a broad-based correction, separate reserves are established when such events are identified and the cost of correction can be reasonably estimated.
Inventory
We review the net realizable value of inventory on an ongoing basis, considering factors such as excess, obsolescence, and other items. We record an allowance for estimated losses when the facts and circumstances indicate that particular inventories will not be sold at prices in excess of current carrying costs. These estimates are based on historical experience and expected future trends. If future market conditions vary from those projected, and our estimates prove to be inaccurate, we may be required to write down inventory values and record an adjustment to cost of revenue.
Recently Issued Accounting Guidance
For a summary of recently issued accounting guidance applicable to us, see Note 1 of our Consolidated Financial Statements included under Part II, Item 8 of this Form 10-K.
| Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are exposed to various market risks, including fluctuations in interest rates, collection risk associated with our accounts and notes receivable portfolio and variability in currency exchange rates. We have established policies, procedures, and internal processes governing our management of market risks and the use of financial instruments to manage our exposure to such risks.
We are subject to variability in foreign currency exchange rates in our international operations. Exposure to this variability is periodically managed primarily through the use of natural hedges, whereby funding obligations and assets are both managed in the local currency. We, from time-to-time, enter into currency exchange agreements to manage our exposure arising from fluctuating exchange rates related to specific and forecasted transactions. We operate this program pursuant to documented corporate risk management policies and do not enter into derivative transactions for speculative purposes. The sensitivity of earnings and cash flows to variability in exchange rates is assessed by applying an appropriate range of potential rate fluctuations to our assets, obligations and projected results of operations denominated in foreign currencies.
Our currency risk consists primarily of foreign currency denominated firm commitments and forecasted foreign currency denominated intercompany and third-party transactions. At September 30, 2017, the notional amount of open foreign exchange contracts was $6.7 million. These contracts were in a net liability position with an aggregate fair value of $0.5 million. The maximum length of time over which we hedge transaction exposures is generally 15 months. Derivative gains/(losses), initially reported as a component of Accumulated Other Comprehensive Loss, are reclassified to earnings in the period when the transaction affects earnings.
Refer to Note 4 and Note 6 of our Consolidated Financial Statements for additional discussions about our interest rate swap agreements and our pension plan assets. We may need to make additional pension plan contributions and our pension expense in future years may increase if market volatility and disruption causes declines in asset values and low interest rates result in a high pension obligation. Investment strategies and policies are set by the plan’s fiduciaries. Long-term strategic investment objectives utilize a diversified mix of equity and fixed income securities to preserve the funded status of the trusts and balance risk and return. The plan fiduciaries oversee the investment allocation process, which includes selecting investment managers, setting long-term strategic targets and monitoring asset allocations.
35
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | |
| Financial Statements: | |
| Financial Statement Schedule: | |
| All other schedules are omitted because they are not applicable or the required information is shown in the financial statements or the notes thereto. | |
36
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management is responsible for establishing and maintaining adequate internal control over financial reporting for Hill-Rom Holdings, Inc. ("we" or "our"). Our internal control over financial reporting is a process designed, under the supervision of our principal executive, principal financial and principal accounting officers, and effected by our Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our Consolidated Financial Statements for external purposes in accordance with accounting principles generally accepted in the United States. Our internal control over financial reporting includes policies and procedures that:
| 1) | Pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; |
| 2) | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of our Consolidated Financial Statements in accordance with accounting principles generally accepted in the United States and that our receipts and expenditures are being made only in accordance with authorizations of our management and our Board of Directors; and |
| 3) | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our Consolidated Financial Statements. |
Because of its inherent limitations, our internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures may deteriorate.
Management performed an assessment of the effectiveness of our internal control over financial reporting as of September 30, 2017 using criteria established in the Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on these criteria, management concluded that we maintained effective internal control over financial reporting as of September 30, 2017.
The effectiveness of our internal control over financial reporting as of September 30, 2017 has been audited by PricewaterhouseCoopers LLP, our independent registered public accounting firm, who also audited our Consolidated Financial Statements, as stated in their report included herein.
We have excluded Mortara Instrument, Inc. ("Mortara") from our assessment of internal control over financial reporting as of September 30, 2017, because Mortara was acquired by us in a purchase business combination in the second quarter of 2017. Mortara is a wholly-owned subsidiary whose total assets and total revenues represent approximately 2.0% and 2.5%, respectively, of the related consolidated financial statement amounts as of and for the year ended September 30, 2017.
/s/ John J. Greisch
John J. Greisch
President and Chief Executive Officer
/s/ Steven J. Strobel
Steven J. Strobel
Senior Vice President and Chief Financial Officer
/s/ Jason A. Richardson
Jason A. Richardson
Vice President, Controller and Chief Accounting Officer
37
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Hill-Rom Holdings, Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of income, comprehensive income (loss), shareholders’ equity and cash flows present fairly, in all material respects, the financial position of Hill-Rom Holdings, Inc. and its subsidiaries ("the Company") as of September 30, 2017 and 2016, and the results of their operations and their cash flows for each of the three years in the period ended September 30, 2017 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of September 30, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in Note 7 to the consolidated financial statements, the Company changed the manner in which it accounts for share-based compensation in 2017.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As described in Management’s Report on Internal Control over Financial Reporting, management has excluded Mortara Instrument, Inc. ("Mortara") from its assessment of internal control over financial reporting as of September 30, 2017, because it was acquired by the Company in a purchase business combination during 2017. We have also excluded Mortara from our audit of internal control over financial reporting. Mortara is a wholly-owned subsidiary whose total assets and total revenues excluded from management’s assessment and our audit of internal control over financial reporting represent approximately 2.0% and 2.5%, respectively, of the related consolidated financial statement amounts as of and for the year ended September 30, 2017.
/s/ PricewaterhouseCoopers LLP
Indianapolis, Indiana
November 17, 2017
38
Hill-Rom Holdings, Inc. and Subsidiaries
STATEMENTS OF CONSOLIDATED INCOME
(In millions, except per share data)
| Year Ended September 30 | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Net Revenue | ||||||||||||
| Product sales and service | $ | 2,358.1 | $ | 2,263.4 | $ | 1,604.5 | ||||||
| Rental revenue | 385.6 | 391.8 | 383.7 | |||||||||
| Total revenue | 2,743.7 | 2,655.2 | 1,988.2 | |||||||||
| Cost of Revenue | ||||||||||||
| Cost of goods sold | 1,235.8 | 1,209.4 | 921.2 | |||||||||
| Rental expenses | 187.3 | 188.8 | 186.7 | |||||||||
| Total cost of revenue | 1,423.1 | 1,398.2 | 1,107.9 | |||||||||
| Gross Profit | 1,320.6 | 1,257.0 | 880.3 | |||||||||
| Research and development expenses | 133.7 | 133.5 | 91.8 | |||||||||
| Selling and administrative expenses | 876.1 | 853.3 | 664.2 | |||||||||
| Special charges (Note 8) | 37.4 | 39.9 | 41.2 | |||||||||
| Operating Profit | 273.4 | 230.3 | 83.1 | |||||||||
| Interest expense | (88.9 | ) | (90.4 | ) | (18.4 | ) | ||||||
| Loss on extinguishment of debt | — | (10.8 | ) | — | ||||||||
| Investment income and other, net | (1.5 | ) | 9.2 | 0.4 | ||||||||
| Income Before Income Taxes | 183.0 | 138.3 | 65.1 | |||||||||
| Income tax expense (Note 9) | 50.7 | 15.5 | 18.3 | |||||||||
| Net Income | 132.3 | 122.8 | 46.8 | |||||||||
| Less: Net loss attributable to noncontrolling interests | (1.3 | ) | (1.3 | ) | (0.9 | ) | ||||||
| Net Income Attributable to Common Shareholders | $ | 133.6 | $ | 124.1 | $ | 47.7 | ||||||
| Net Income Attributable to Common Shareholders per Common Share - Basic | $ | 2.04 | $ | 1.90 | $ | 0.83 | ||||||
| Net Income Attributable to Common Shareholders per Common Share - Diluted | $ | 1.99 | $ | 1.86 | $ | 0.82 | ||||||
| Dividends per Common Share | $ | 0.7100 | $ | 0.6700 | $ | 0.6325 | ||||||
| Average Common Shares Outstanding - Basic (thousands) (Note 10) | 65,599 | 65,333 | 57,249 | |||||||||
| Average Common Shares Outstanding - Diluted (thousands) (Note 10) | 67,225 | 66,596 | 58,536 | |||||||||
See Notes to Consolidated Financial Statements.
39
Hill-Rom Holdings, Inc. and Subsidiaries
STATEMENTS OF CONSOLIDATED COMPREHENSIVE INCOME (LOSS)
(In millions)
| Year Ended September 30 | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Net Income | $ | 132.3 | $ | 122.8 | $ | 46.8 | ||||||
| Other Comprehensive Income (Loss), Net of Tax: | ||||||||||||
| Available-for-sale securities and hedges | 7.4 | (3.1 | ) | — | ||||||||
| Foreign currency translation adjustment | 33.9 | (22.4 | ) | (58.6 | ) | |||||||
| Change in pension and postretirement defined benefit plans | 17.8 | (2.8 | ) | (8.1 | ) | |||||||
| Total Other Comprehensive Income (Loss), Net of Tax | 59.1 | (28.3 | ) | (66.7 | ) | |||||||
| Total Comprehensive Income (Loss) | 191.4 | 94.5 | (19.9 | ) | ||||||||
| Less: Comprehensive loss attributable to noncontrolling interests | (1.3 | ) | (1.3 | ) | (0.9 | ) | ||||||
| Total Comprehensive Income (Loss) Attributable to Common Shareholders | $ | 192.7 | $ | 95.8 | $ | (19.0 | ) | |||||
See Notes to Consolidated Financial Statements.
40
Hill-Rom Holdings, Inc. and Subsidiaries
CONSOLIDATED BALANCE SHEETS
| (In millions, except share amounts) | September 30 | |||||||
| 2017 | 2016 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 231.8 | $ | 232.2 | ||||
| Trade accounts receivable, less allowances of $25.1 in 2017 and $26.8 in 2016 (Note 1) | 579.3 | 515.1 | ||||||
| Inventories (Note 1) | 284.5 | 252.0 | ||||||
| Other current assets | 70.6 | 82.8 | ||||||
| Total current assets | 1,166.2 | 1,082.1 | ||||||
| Property, plant, and equipment (Note 1) | 979.6 | 961.8 | ||||||
| Less accumulated depreciation | (624.2 | ) | (611.8 | ) | ||||
| Property, plant, and equipment, net | 355.4 | 350.0 | ||||||
| Intangible assets: | ||||||||
| Goodwill (Notes 1, 2 and 3) | 1,759.6 | 1,584.4 | ||||||
| Other intangible assets and software, net (Notes 1, 2 and 3) | 1,144.0 | 1,143.3 | ||||||
| Deferred income taxes (Notes 1 and 9) | 40.9 | 43.1 | ||||||
| Other assets | 62.6 | 59.5 | ||||||
| Total Assets | $ | 4,528.7 | $ | 4,262.4 | ||||
| LIABILITIES | ||||||||
| Current Liabilities | ||||||||
| Trade accounts payable | $ | 167.9 | $ | 136.0 | ||||
| Short-term borrowings (Note 4) | 188.9 | 210.1 | ||||||
| Accrued compensation | 126.9 | 127.0 | ||||||
| Accrued product warranties (Note 1) | 25.5 | 27.5 | ||||||
| Accrued rebates | 39.7 | 40.8 | ||||||
| Other current liabilities | 109.8 | 120.9 | ||||||
| Total current liabilities | 658.7 | 662.3 | ||||||
| Long-term debt (Note 4) | 2,120.4 | 1,938.4 | ||||||
| Accrued pension and postretirement benefits (Note 6) | 78.1 | 99.0 | ||||||
| Deferred income taxes (Notes 1 and 9) | 266.2 | 287.8 | ||||||
| Other long-term liabilities | 39.7 | 39.0 | ||||||
| Total Liabilities | 3,163.1 | 3,026.5 | ||||||
| Commitments and Contingencies (Note 13) | ||||||||
| SHAREHOLDERS' EQUITY (Note 7) | ||||||||
| Capital Stock: | ||||||||
| Preferred stock - without par value: | ||||||||
| Authorized - 1,000,000 shares; none issued or outstanding | ||||||||
| Common stock - without par value: | ||||||||
| Authorized - 199,000,000 | ||||||||
| Issued - 88,457,634 shares in 2017 and 2016 | 4.4 | 4.4 | ||||||
| Additional paid-in capital | 584.4 | 575.9 | ||||||
| Retained earnings | 1,676.2 | 1,589.7 | ||||||
| Accumulated other comprehensive loss (Note 1) | (110.0 | ) | (169.1 | ) | ||||
| Treasury stock, common shares at cost: 22,643,840 and 22,752,381 | (796.8 | ) | (773.7 | ) | ||||
| Total Shareholders' Equity Attributable to Common Shareholders | 1,358.2 | 1,227.2 | ||||||
| Noncontrolling interests | 7.4 | 8.7 | ||||||
| Total Shareholders' Equity | 1,365.6 | 1,235.9 | ||||||
| Total Liabilities and Shareholders' Equity | $ | 4,528.7 | $ | 4,262.4 | ||||
See Notes to Consolidated Financial Statements.
41
Hill-Rom Holdings, Inc. and Subsidiaries
STATEMENTS OF CONSOLIDATED CASH FLOWS
| (In millions) | Year Ended September 30 | |||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Operating Activities | ||||||||||||
| Net income | $ | 132.3 | $ | 122.8 | $ | 46.8 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
| Depreciation | 82.0 | 86.2 | 73.6 | |||||||||
| Amortization | 20.4 | 26.9 | 10.5 | |||||||||
| Acquisition-related intangible asset amortization | 108.4 | 95.9 | 34.1 | |||||||||
| Loss on extinguishment of debt | — | 10.8 | — | |||||||||
| Provision for deferred income taxes | (32.8 | ) | (0.5 | ) | (22.3 | ) | ||||||
| Loss on disposal of property, equipment leased to others, intangible assets and impairments | 24.7 | 1.9 | 0.5 | |||||||||
| Pension settlement charge | — | — | 9.6 | |||||||||
| Pension contribution to master pension plan | — | (30.0 | ) | — | ||||||||
| Gain on sale of businesses | (1.0 | ) | (10.1 | ) | — | |||||||
| Stock compensation | 23.0 | 23.1 | 25.0 | |||||||||
| Excess tax benefits from employee stock plans | — | (3.6 | ) | (3.6 | ) | |||||||
| Change in working capital excluding cash, current debt, acquisitions and dispositions: | ||||||||||||
| Trade accounts receivable | (42.5 | ) | (15.8 | ) | (39.7 | ) | ||||||
| Inventories | (14.9 | ) | 21.3 | 11.0 | ||||||||
| Other current assets | 15.0 | 27.7 | (7.7 | ) | ||||||||
| Trade accounts payable | 21.6 | (0.5 | ) | 0.7 | ||||||||
| Accrued expenses and other liabilities | (32.3 | ) | (73.0 | ) | 53.8 | |||||||
| Other, net | 7.2 | (1.9 | ) | 21.5 | ||||||||
| Net cash provided by operating activities | 311.1 | 281.2 | 213.8 | |||||||||
| Investing Activities | ||||||||||||
| Capital expenditures and purchases of intangible assets | $ | (97.5 | ) | $ | (83.3 | ) | $ | (121.3 | ) | |||
| Proceeds on sale of property and equipment leased to others | 15.1 | 2.2 | 1.5 | |||||||||
| Payment for acquisition of businesses, net of cash acquired | (311.4 | ) | (25.3 | ) | (1,638.7 | ) | ||||||
| Proceeds on sale of businesses | 5.8 | 10.3 | — | |||||||||
| Other | (1.4 | ) | (1.6 | ) | 2.1 | |||||||
| Net cash used in investing activities | (389.4 | ) | (97.7 | ) | (1,756.4 | ) | ||||||
| Financing Activities | ||||||||||||
| Proceeds from borrowings on long-term debt | $ | 300.0 | $ | 530.4 | $ | 2,225.0 | ||||||
| Payment of long-term debt | (73.2 | ) | (767.9 | ) | (401.6 | ) | ||||||
| Net change in short-term debt | — | — | (0.7 | ) | ||||||||
| Borrowings on Revolving Credit Facility | 180.0 | 156.9 | 95.0 | |||||||||
| Payments on Revolving Credit Facility | (325.8 | ) | (20.0 | ) | (135.0 | ) | ||||||
| Borrowings on Securitization Program | 124.5 | — | — | |||||||||
| Payments on Securitization Program | (45.4 | ) | — | — | ||||||||
| Repurchase of registered debentures | — | — | (5.9 | ) | ||||||||
| Debt issuance costs | (5.1 | ) | (2.3 | ) | (50.3 | ) | ||||||
| Purchase of noncontrolling interest of former joint venture | — | (0.4 | ) | (1.9 | ) | |||||||
| Payment of cash dividends | (46.6 | ) | (43.8 | ) | (37.1 | ) | ||||||
| Proceeds from exercise of stock options | 17.8 | 6.2 | 12.1 | |||||||||
| Proceeds from stock issuance | 5.0 | 3.8 | 2.8 | |||||||||
| Excess tax benefits from employee stock plans | — | 3.6 | 3.6 | |||||||||
| Treasury stock acquired | (60.6 | ) | (8.4 | ) | (63.3 | ) | ||||||
| Net cash provided by (used in) financing activities | 70.6 | (141.9 | ) | 1,642.7 | ||||||||
| Effect of exchange rate changes on cash | 7.3 | (2.2 | ) | (6.6 | ) | |||||||
| Net Cash Flows | (0.4 | ) | 39.4 | 93.5 | ||||||||
| Cash and Cash Equivalents | ||||||||||||
| At beginning of period | 232.2 | 192.8 | 99.3 | |||||||||
| At end of period | $ | 231.8 | $ | 232.2 | $ | 192.8 | ||||||
| Supplemental cash flow information: | ||||||||||||
| Cash paid for income taxes | $ | 70.4 | $ | 10.9 | $ | 49.1 | ||||||
| Cash paid for interest | $ | 81.3 | $ | 80.9 | $ | 6.3 | ||||||
| Non-cash investing and financing activities: | ||||||||||||
| Treasury stock issued under stock compensation plans | $ | 37.5 | $ | 23.3 | $ | 32.4 | ||||||
| Common stock issued for acquisition of businesses | $ | — | $ | — | $ | 416.3 | ||||||
See Notes to Consolidated Financial Statements.
42
Hill-Rom Holdings, Inc. and Subsidiaries
STATEMENTS OF CONSOLIDATED SHAREHOLDERS’ EQUITY
(In millions, except share amounts)
| Common Stock | Additional Paid-in Capital | Retained Earnings | Accumulated Other Comprehensive Income (Loss) | Common Stock in Treasury | Total Equity Attributable to Common Shareholders | Noncontrolling Interests | |||||||||||||||||||||||||||||||
| Shares Outstanding | Amount | ||||||||||||||||||||||||||||||||||||
| Shares | Amount | Total | |||||||||||||||||||||||||||||||||||
| Balance at September 30, 2014 | 57,439,911 | $ | 4.4 | $ | 134.1 | $ | 1,499.8 | $ | (74.1 | ) | 22,884,001 | $ | (757.7 | ) | $ | 806.5 | $ | — | $ | 806.5 | |||||||||||||||||
| Net Income Attributable to Common Shareholders | — | — | — | 47.7 | — | — | — | 47.7 | (0.9 | ) | 46.8 | ||||||||||||||||||||||||||
| Consolidation of noncontrolling interest | — | — | — | — | — | — | — | — | 10.9 | 10.9 | |||||||||||||||||||||||||||
| Other comprehensive loss, net of tax of $5.1 | — | — | — | — | (66.7 | ) | — | — | (66.7 | ) | — | (66.7 | ) | ||||||||||||||||||||||||
| Dividends | — | — | 0.5 | (37.6 | ) | — | — | — | (37.1 | ) | — | (37.1 | ) | ||||||||||||||||||||||||
| Issuance of common stock | 8,133,722 | — | 416.3 | — | — | — | — | 416.3 | — | 416.3 | |||||||||||||||||||||||||||
| Treasury shares acquired | (1,373,321 | ) | — | — | — | — | 1,373,321 | (63.3 | ) | (63.3 | ) | — | (63.3 | ) | |||||||||||||||||||||||
| Stock awards and option exercises | 965,584 | — | 11.1 | — | — | (965,584 | ) | 32.4 | 43.5 | — | 43.5 | ||||||||||||||||||||||||||
| Balance at September 30, 2015 | 65,165,896 | 4.4 | 562.0 | 1,509.9 | (140.8 | ) | 23,291,738 | (788.6 | ) | 1,146.9 | 10.0 | 1,156.9 | |||||||||||||||||||||||||
| Net Income Attributable to Common Shareholders | — | — | — | 124.1 | — | — | — | 124.1 | (1.3 | ) | 122.8 | ||||||||||||||||||||||||||
| Other comprehensive loss, net of tax of $3.2 | — | — | — | — | (28.3 | ) | — | — | (28.3 | ) | — | (28.3 | ) | ||||||||||||||||||||||||
| Dividends | — | — | 0.5 | (44.3 | ) | — | — | — | (43.8 | ) | — | (43.8 | ) | ||||||||||||||||||||||||
| Treasury shares acquired | (148,203 | ) | — | — | — | — | 148,203 | (8.4 | ) | (8.4 | ) | — | (8.4 | ) | |||||||||||||||||||||||
| Stock awards and option exercises | 687,560 | — | 13.4 | — | — | (687,560 | ) | 23.3 | 36.7 | — | 36.7 | ||||||||||||||||||||||||||
| Balance at September 30, 2016 | 65,705,253 | 4.4 | 575.9 | 1,589.7 | (169.1 | ) | 22,752,381 | (773.7 | ) | 1,227.2 | 8.7 | 1,235.9 | |||||||||||||||||||||||||
| Net Income Attributable to Common Shareholders | — | — | — | 133.6 | — | — | — | 133.6 | (1.3 | ) | 132.3 | ||||||||||||||||||||||||||
| Other comprehensive loss, net of tax of $(14.6) | — | — | — | 59.1 | — | — | 59.1 | — | 59.1 | ||||||||||||||||||||||||||||
| Dividends | — | — | 0.5 | (47.1 | ) | — | — | — | (46.6 | ) | — | (46.6 | ) | ||||||||||||||||||||||||
| Treasury shares acquired | (976,473 | ) | — | — | — | — | 976,473 | (60.6 | ) | (60.6 | ) | — | (60.6 | ) | |||||||||||||||||||||||
| Stock awards and option exercises | 1,085,014 | — | 8.0 | — | (1,085,014 | ) | 37.5 | 45.5 | — | 45.5 | |||||||||||||||||||||||||||
| Balance at September 30, 2017 | 65,813,794 | $ | 4.4 | $ | 584.4 | $ | 1,676.2 | $ | (110.0 | ) | 22,643,840 | $ | (796.8 | ) | $ | 1,358.2 | $ | 7.4 | $ | 1,365.6 | |||||||||||||||||
See Notes to Consolidated Financial Statements.
43
Hill-Rom Holdings, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars in millions except per share data)
Note 1. Summary of Significant Accounting Policies
Nature of Operations
Hill-Rom Holdings, Inc. (the "Company," "Hill-Rom," "we," "us," or "our") was incorporated on August 7, 1969 in the State of Indiana and is headquartered in Chicago, Illinois. We are a leading global medical technology company with more than 10,000 employees worldwide. We partner with health care providers in more than 100 countries, across all care settings, by focusing on patient care solutions that improve clinical and economic outcomes in five core areas: Advancing Mobility, Wound Care and Prevention, Patient Monitoring and Diagnostics, Surgical Safety and Efficiency and Respiratory Health. We have three reportable segments, each of which is generally aligned by region and/or product type. Around the world, Hill-Rom's people, products, and programs work towards one mission: Enhancing outcomes for patients and their caregivers.
Basis of Presentation and Principles of Consolidation
The Consolidated Financial Statements include the accounts of Hill-Rom and its wholly-owned subsidiaries. In addition, we also consolidate variable interest entities ("VIEs") where Hill-Rom is deemed to have a controlling financial interest. Intercompany accounts and transactions have been eliminated in consolidation, including the intercompany transactions with consolidated VIEs. Where our ownership interest is less than 100%, the noncontrolling interests are reported in our Consolidated Financial Statements. Certain prior year amounts have been reclassified to conform to current year presentation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires our management to make estimates and assumptions that affect the reported amounts of certain assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expense during the reporting period. Actual results could differ from those estimates. Examples of such estimates include our accounts receivable reserves (Note 1), accrued warranties (Note 1), the impairment of intangibles and goodwill (Note 3), use of the spot yield curve approach for pension expense (Note 6), income taxes (Notes 1 and 9) and commitments and contingencies (Note 13), among others.
Cash and Cash Equivalents
We consider investments in marketable securities and other highly liquid instruments with a maturity of three months or less at date of purchase to be cash equivalents. All of our marketable securities may be freely traded.
Trade Accounts Receivable
Trade accounts receivable are recorded at the invoiced amount and do not bear interest, unless the transaction is an installment sale with extended payment terms. Reserves for uncollectible accounts represent our best estimate of the amount of probable credit losses and collection risk in our existing accounts receivable. We determine such reserves based on historical write-off experience by industry and reimbursement platform. Receivables are generally reviewed on a pooled basis based on historical collection experience for each reimbursement and receivable type. Receivables for sales transactions are also reviewed individually for collectability. Account balances are charged against the allowance when we believe it is probable the receivable will not be recovered. We do not have any off-balance sheet credit exposure related to our customers. If circumstances change, such as higher than expected claims denials, payment defaults, changes in our business composition or processes, adverse changes in general economic conditions, unfavorable impacts of austerity measures initiated by some governmental authorities, instability or disruption of credit markets, or an unexpected material adverse change in a major customer’s or payer’s ability to meet its obligations, our estimates of the realizability of trade receivables could be reduced by a material amount.
Within rental revenue, the domestic third-party payers’ reimbursement process requires extensive documentation, which has had the effect of slowing both the billing and cash collection cycles relative to the rest of the business, and therefore, increasing total accounts receivable. Because of the extensive documentation required and the requirement to settle a claim with the primary payer prior to billing the secondary and/or patient portion of the claim, the collection period for a claim in a portion of our business may, in some cases, be extended.
44
We generally hold our trade accounts receivable until they are paid. Certain long-term receivables are occasionally sold to third parties; however, any recognized gain or loss on such sales has historically not been material.
Inventories
Inventories are valued at the lower of cost or market. Inventory costs are determined by the last-in, first-out ("LIFO") method for approximately 21% and 26% of our inventories at September 30, 2017 and 2016. Costs for other inventories have been determined principally by the first-in, first-out ("FIFO") method. Inventories consist of the following:
| September 30 | ||||||||
| 2017 | 2016 | |||||||
| Finished products | $ | 147.5 | $ | 124.2 | ||||
| Work in process | 38.8 | 35.7 | ||||||
| Raw materials | 98.2 | 92.1 | ||||||
| Total | $ | 284.5 | $ | 252.0 | ||||
If the FIFO method of inventory accounting, which approximates current cost, had been used for all inventories, they would have been approximately $2.0 million and $2.1 million higher than reported at September 30, 2017 and 2016.
Property, Plant and Equipment
Property, plant and equipment is recorded at cost and depreciated over the estimated useful life of the assets using principally the straight-line method. Ranges of estimated useful lives are as follows:
| Useful Life | |
| Land improvements | 6 - 15 years |
| Buildings and building equipment | 10 - 40 years |
| Machinery and equipment | 3 - 10 years |
| Equipment leased to others | 2 -10 years |
When property, plant and equipment is retired from service or otherwise disposed of, the cost and related amount of depreciation or amortization are eliminated from the asset and accumulated depreciation accounts. The difference, if any, between the net asset value and the proceeds on sale are charged or credited to income. Total depreciation expense for fiscal years 2017, 2016 and 2015 was $82.0 million, $86.2 million and $73.6 million, respectively. The major components of property and the related accumulated depreciation were as follows:
| September 30 | ||||||||||||||||
| 2017 | 2016 | |||||||||||||||
| Cost | Accumulated Depreciation | Cost | Accumulated Depreciation | |||||||||||||
| Land and land improvements | $ | 18.4 | $ | 3.3 | $ | 21.5 | $ | 3.1 | ||||||||
| Buildings and building equipment | 196.1 | 84.7 | 186.9 | 91.9 | ||||||||||||
| Machinery and equipment | 402.6 | 265.1 | 380.6 | 239.2 | ||||||||||||
| Equipment leased to others | 362.5 | 271.1 | 372.8 | 277.6 | ||||||||||||
| Total | $ | 979.6 | $ | 624.2 | $ | 961.8 | $ | 611.8 | ||||||||
Intangible Assets
Intangible assets are stated at cost and consist predominantly of goodwill, software, patents, acquired technology, trademarks, and acquired customer relationship assets. With the exception of goodwill and certain trademarks, our intangible assets are amortized on a straight-line basis over periods generally ranging from 1 to 20 years.
We assess the carrying value of goodwill and non-amortizable intangibles annually, during the third quarter of each fiscal year, or more often if events or changes in circumstances indicate there may be impairment.
45
The majority of our goodwill and many of our intangible assets are not deductible for income tax purposes. A summary of intangible assets and the related accumulated amortization and impairment losses follows:
| September 30 | ||||||||||||||||
| 2017 | 2016 | |||||||||||||||
| Cost | Amortization and Impairment | Cost | Amortization and Impairment | |||||||||||||
| Goodwill | $ | 2,232.4 | $ | 472.8 | $ | 2,057.2 | $ | 472.8 | ||||||||
| Software | 166.1 | 133.0 | 174.1 | 140.0 | ||||||||||||
| Patents and Trademarks | 504.2 | 16.4 | 497.1 | 19.2 | ||||||||||||
| Other | 966.0 | 342.9 | 870.4 | 239.1 | ||||||||||||
| Total | $ | 3,868.7 | $ | 965.1 | $ | 3,598.8 | $ | 871.1 | ||||||||
Amortization expense for fiscal years 2017, 2016 and 2015 was $128.8 million, $122.8 million and $44.6 million, respectively. As further discussed in Note 3 of our Consolidated Financial Statements, we have various indefinite-lived intangible assets representing trade names with a carrying value of $466.9 million at September 30, 2017 and September 30, 2016. Amortization expense for all other intangibles is expected to approximate the following for each of the next five fiscal years and thereafter:
| Amount | |||
| 2018 | $ | 119.5 | |
| 2019 | $ | 109.7 | |
| 2020 | $ | 98.0 | |
| 2021 | $ | 89.0 | |
| 2022 | $ | 76.1 | |
| 2023 and beyond | $ | 184.8 | |
Software consists mainly of capitalized costs associated with internal use software, including applicable costs associated with the implementation/upgrade of our Enterprise Resource Planning systems. In addition, software includes capitalized development costs for software products to be sold. Capitalized software costs are amortized on a straight-line basis over periods ranging from three to ten years. Software amortization expense approximated $12.8 million, $17.0 million and $9.8 million for fiscal years 2017, 2016 and 2015 and is included in the total intangibles amortization presented earlier.
Other includes mainly customer relationships and developed technology at Welch Allyn and Mortara. The cost and amortization amounts of customer relationships at Welch Allyn were $517.4 million and $125.5 million as of September 30, 2017 and $514.1 million and $62.1 million as of September 30, 2016. The cost and amortization amounts of developed technology at Welch Allyn were $54.0 million and $16.6 million as of September 30, 2017 and $54.0 million and $8.6 million as of September 30, 2016. The cost and amortization amounts of customer relationships at Mortara were $52.3 million and $4.6 million as of September 30, 2017. The cost and amortization amounts of developed technology at Mortara were $37.9 million and $2.9 million as of September 30, 2017.
Fair Value Measurements
Fair value measurements are classified and disclosed in one of the following three categories:
| • | Level 1: Financial instruments with unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets and liabilities. |
| • | Level 2: Financial instruments with observable inputs other than those included in Level 1 such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| • | Level 3: Financial instruments with unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Unobservable inputs reflect our own assumptions that market participants would use in pricing the asset or liability (including assumptions about risk). Unobservable inputs shall be developed based on the best information available in the circumstances, which might include our own data. |
46
We record cash and cash equivalents, as disclosed on our Consolidated Balance Sheets, as Level 1 instruments and certain other investments and derivatives as either Level 2 or 3 instruments. Except for the adoption of revised disclosure guidance related to investments held by our pension plan as discussed in Note 6, there have been no significant changes in our classification among assets and liabilities. Refer to Note 4 of our Consolidated Financial Statements for disclosure of our debt instrument fair values.
Guarantees
We routinely grant limited warranties on our products with respect to defects in material and workmanship. The terms of these warranties are generally one year; however, certain components and products have substantially longer warranty periods. We recognize a reserve with respect to these obligations at the time of product sale, with subsequent warranty claims recorded directly against the reserve. The amount of the warranty reserve is determined based on historical trend experience for the covered products. For more significant warranty-related matters which might require a broad-based correction, separate reserves are established when such events are identified and the cost of correction can be reasonably estimated.
A reconciliation of changes in our warranty reserve is as follows:
| 2017 | 2016 | 2015 | ||||||||||
| Balance at October 1 | $ | 27.5 | $ | 32.1 | $ | 28.4 | ||||||
| Provision for warranties during the period | 13.9 | 13.9 | 14.7 | |||||||||
| Warranty reserves acquired | 1.5 | 2.6 | 7.1 | |||||||||
| Warranty claims incurred during the period | (17.4 | ) | (21.1 | ) | (18.1 | ) | ||||||
| Balance at September 30 | $ | 25.5 | $ | 27.5 | $ | 32.1 | ||||||
In the normal course of business, we enter into various other guarantees and indemnities in our relationships with suppliers, service providers, customers, business partners and others. Examples of these arrangements would include guarantees of product performance, indemnifications to service providers and indemnifications of our actions to business partners. These guarantees and indemnifications have not historically nor do we expect them to have a material impact on our financial condition or results of operations, although indemnifications associated with our actions generally have no dollar limitations.
In conjunction with our acquisition and divestiture activities, we have entered into select guarantees and indemnifications of performance with respect to the fulfillment of our commitments under applicable purchase and sale agreements. The arrangements generally indemnify the buyer or seller for damages associated with breach of contract, inaccuracies in representations and warranties surviving the closing date and satisfaction of liabilities and commitments retained under the applicable contract. With respect to sale transactions, we also routinely enter into non-competition agreements for varying periods of time. Guarantees and indemnifications with respect to acquisition and divestiture activities, if triggered, could have a materially adverse impact on our financial condition and results of operations.
Accrued Rebates
We provide rebates and sales incentives to certain customer groups and distributors. Provisions for rebates are recorded as a reduction in net revenue when revenue is recognized. In some cases, rebates may be payable directly to the customer or distributor. We also have arrangements where we provide rebates to certain distributors that sell to end-user customers at prices determined under a contract between us and the end-user customer.
Retirement Plans
We sponsor retirement and postretirement plans covering select employees. Expense recognized in relation to these defined benefit retirement plans and postretirement health care plans in the U.S. is based upon actuarial valuations and inherent in those valuations are key assumptions including discount rates, and where applicable, expected returns on assets, projected future salary rates and projected health care cost trends. The discount rates used in the valuation of our defined benefit pension and postretirement plans are evaluated annually based on current market conditions. In setting these rates we utilize long-term bond indices and yield curves as a preliminary indication of interest rate movements, and then make adjustments to the respective indices to reflect differences in the terms of the bonds covered under the indices in comparison to the projected outflow of our obligations. Our overall expected long-term rate of return on pension assets is based on historical and expected future returns, which are inflation adjusted and weighted for the expected return for each component of the investment portfolio. Our rate of assumed compensation increase is also based on our specific historical trends of wage adjustments.
47
We account for our defined benefit pension and other postretirement plans by recognizing the funded status of a benefit plan in the statement of financial position. We also recognize in accumulated other comprehensive income (loss) certain gains and losses that arose during the period. See Note 6 of our Consolidated Financial Statements for key assumptions and further discussion related to our pension and postretirement plans.
Environmental Liabilities
Expenditures that relate to an existing condition caused by past operations, and which do not contribute to future revenue generation, are expensed. A reserve is established when it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. These reserves are determined without consideration of possible loss recoveries from third parties.
Specific costs included in environmental expense and reserves include site assessment, development of a remediation plan, clean-up costs, post-remediation expenditures, monitoring, fines, penalties and legal fees. Reserve amounts represent the expected undiscounted future cash outflows associated with such plans and actions.
Self Insurance
We are involved in various claims, including product and general liability, workers’ compensation, auto liability and employment related matters. Refer to Note 13 of our Consolidated Financial Statements for additional information.
Treasury Stock
Treasury stock consists of our common shares that have been issued, but subsequently reacquired. We account for treasury stock purchases under the cost method. When these shares are reissued, we use an average-cost method to determine cost. Proceeds in excess of cost are credited to additional paid-in capital.
Revenue Recognition — Sales and Rentals
Revenue is presented in the Statements of Consolidated Income net of sales discounts and allowances, rebates and customer returns for product sales and rental revenue reserves. Revenue is evaluated under the following criteria and recognized when each is met:
| • | Evidence of an arrangement: An agreement with the customer reflecting the terms and conditions to deliver products or services serves as evidence of an arrangement. |
| • | Delivery: For products, delivery is generally considered to occur upon transfer of title and risk of loss per the respective sales terms. For rental services, delivery is considered to occur when the services are rendered. |
| • | Fixed or determinable price: The sales price is considered fixed or determinable if it is not subject to refund or measurable adjustment. |
| • | Collection is deemed probable: At or prior to the time of a transaction, credit reviews of each customer are performed to determine the creditworthiness of the customer. Collection is deemed probable if the customer is expected to be able to pay amounts under the arrangement as those amounts become due. If collection is not probable, revenue is recognized when collection becomes probable, generally upon cash collection. |
As a general interpretation of the above guidelines, revenue for health care and surgical products are generally recognized upon delivery of the products to the customer and their assumption of risk of loss and other risks and rewards of ownership. Local business customs and sales terms specific to certain customers or products can sometimes result in deviations to this normal practice; however, in no case is revenue recognized prior to the transfer of risk of loss and rewards of ownership.
For non-invasive therapy products and medical equipment management services, the majority of product offerings are rental products for which revenue is recognized consistent with the rendering of the service and use of products. For The Vest ® product, revenue is generally recognized at the time of receipt of authorization for billing from the applicable paying entity as this serves as evidence of the arrangement and sets a fixed or determinable price.
For health care products and services aimed at improving operational efficiency and asset utilization, various revenue recognition techniques are used, depending on the offering. Arrangements to provide services, routinely under separately sold service and maintenance contracts, result in the deferral of revenue until specified services are performed. Service contract revenue is generally
48
recognized ratably over the contract period, if applicable, or as services are rendered. Product-related goods are generally recognized upon delivery to the customer.
For product sales, we record reserves resulting in a reduction of revenue for contractual discounts, as well as price concessions and product returns. Likewise, rental revenue reserves, reflecting contractual and other routine billing adjustments, are recorded as a reduction of revenue. Reserves for revenue are estimated based upon historical rates for revenue adjustments.
Taxes Collected from Customers and Remitted to Governmental Units
Taxes assessed by a governmental authority that are directly imposed on a revenue producing transaction between us and our customers, including but not limited to sales taxes, use taxes, and value added taxes, are accounted for on a net (excluded from revenue and cost) basis.
Cost of Revenue
Cost of goods sold for product sales consists primarily of purchased material costs, fixed manufacturing expense, variable direct labor, overhead costs and costs associated with the distribution and delivery of products to our customers. Rental expenses consist of costs associated directly with rental revenue, including depreciation, maintenance, logistics and service center facility and personnel costs.
Research and Development Costs
Research and development costs are expensed as incurred. Costs were $133.7 million, $133.5 million and $91.8 million for fiscal years 2017, 2016 and 2015.
In addition, certain costs for software development technology held for sale are capitalized as intangibles and are amortized over a period of three to five years once the software is ready for its intended use. The amount capitalized during fiscal years 2017, 2016 and 2015 was approximately $2.3 million, $2.4 million and $2.6 million.
Advertising Costs
Advertising costs are expensed as incurred. Costs were $13.8 million, $10.4 million and $6.8 million for fiscal years 2017, 2016 and 2015.
Comprehensive Income
We include the net-of-tax effect of unrealized gains or losses on our available-for-sale securities, interest and foreign currency hedges, foreign currency translation adjustments and pension or other defined benefit postretirement plans’ actuarial gains or losses and prior service costs or credits in comprehensive income. See Note 5 of our Consolidated Financial Statements for further details.
Foreign Currency Translation
The functional currency of foreign operations is generally the local currency in the country of domicile. Assets and liabilities of foreign operations are primarily translated into U.S. dollars at year-end rates of exchange and the income statements are translated at the average rates of exchange prevailing during the year. Adjustments resulting from translation of the financial statements of foreign operations into U.S. dollars are excluded from the determination of net income, but included as a component of accumulated other comprehensive income (loss). Foreign currency gains and losses resulting from foreign currency transactions are included in our results of operations and are not material.
49
Stock-Based Compensation
We account for stock-based compensation under fair value provisions. Stock-based compensation cost is measured at the grant date based on the value of the award and is recognized as expense over the vesting period. In order to determine the fair value of stock options and other performance-based stock awards on the date of grant, we utilize a Binomial model. Inherent in this model are assumptions related to a volatility factor, expected life, risk-free interest rate, dividend yield and expected forfeitures. The risk-free interest rate is based on factual data derived from public sources. The volatility factor, expected life, dividend yield and expected forfeiture assumptions require judgment utilizing historical information, peer data and future expectations. Deferred stock (also known as restricted stock units ("RSUs")) is measured based on the fair market price of our common stock on the date of grant, as reported by the New York Stock Exchange, multiplied by the number of units granted. See Note 7 of our Consolidated Financial Statements for further details.
Income Taxes
Hill-Rom and its eligible domestic subsidiaries file a consolidated U.S. income tax return. Foreign operations file income tax returns in a number of jurisdictions. Deferred income taxes are computed using an asset and liability approach to reflect the net tax effects of temporary differences between the financial reporting carrying amounts of assets and liabilities and the corresponding income tax amounts. We have a variety of deferred tax assets in numerous tax jurisdictions. These deferred tax assets are subject to periodic assessment as to recoverability. If it is determined that it is more likely than not that the benefits will not be realized, valuation allowances are recognized. In evaluating whether it is more likely than not that we would recover these deferred tax assets, future taxable income, the reversal of existing temporary differences and tax planning strategies are considered.
As of September 30, 2017, we had $58.2 million of valuation allowances on deferred tax assets, on a tax-effected basis, primarily related to certain foreign deferred tax attributes that are not expected to be utilized. We believe that our estimates for the valuation allowances recorded against deferred tax assets are appropriate based on current facts and circumstances.
We account for uncertain income tax positions using a threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The difference between the tax benefit recognized in the financial statements for an uncertain income tax position and the tax benefit claimed in the tax return is referred to as an unrecognized tax benefit. See Note 9 of our Consolidated Financial Statements for further details.
Derivative Instruments and Hedging Activity
We use derivative financial instruments to manage the economic impact of fluctuations in currency exchange and interest rates. Derivative financial instruments related to currency exchange rates include forward purchase and sale agreements which generally have terms no greater than 15 months. Additionally, interest rate swaps are sometimes used to convert some or all of our long-term debt to either a fixed or variable rate.
Derivative financial instruments are recognized on the Consolidated Balance Sheets as either assets or liabilities and are measured at fair value. Changes in the fair value of derivatives are recorded each period in the Statement of Consolidated Income or the Statement of Consolidated Comprehensive Income, depending on whether a derivative is designated and considered effective as part of a hedge transaction, and if it is, the type of hedge transaction. Gains and losses on derivative instruments reported in accumulated other comprehensive income (loss) are subsequently included in the Statement of Consolidated Income in the periods in which earnings are affected by the hedged item. These activities have not had a material effect on our financial position or results of operations for the periods presented herein.
Dispositions
During the fourth quarter of fiscal 2017, we sold our Völker business. We recorded impairment charges of $25.4 million during the third quarter, relating mainly to non-cash write-downs of long-lived assets and working capital associated with the Völker brand portfolio, and fiscal 2017 transaction related costs of approximately $3.0 million in Special charges. We do not expect a majority of the impairment related to the disposition to be tax deductible.
During the first quarter of fiscal 2017, we sold our Architectural Products business for $4.5 million in cash proceeds and recorded an immaterial gain in Investment income and other, net.
During the fourth quarter of 2016, we sold our perinatal data management system for $10.5 million and recorded a gain of $10.1 million in Investment income and other, net.
50
All businesses recently disposed were part of our Patient Support Systems segment.
Recently Issued Accounting Guidance
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). From the lessee’s perspective, the new standard establishes a right-of-use ("ROU") model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement for a lessee. From the lessor’s perspective, the new standard requires a lessor to classify leases as either sales-type, finance or operating. A lease will be treated as a sale if it transfers all of the risks and rewards, as well as control of the underlying asset, to the lessee. If risks and rewards are conveyed without the transfer of control, the lease is treated as a financing lease. If the lessor does not convey risks and rewards or control, an operating lease results. ASU 2016-02 is effective for our first quarter of fiscal 2020. A modified retrospective transition approach is required for leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. We are currently in the process of evaluating the impact of the amended guidance on our Consolidated Financial Statements.
In May 2014, the FASB issued ASU 2014-09, "Revenue from Contracts with Customers," which provides guidance for revenue recognition. The standard’s core principle is that a company will recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. In August 2015, the FASB issued ASU 2015-14 which delayed the effective date of the new revenue guidance by one year, while permitting companies to early adopt the new standard as of the original effective date. As a result, the provisions of ASU 2014-09 and subsequent amendments, are effective for us in the first quarter of fiscal 2019 using either of the following transition methods: (i) a full retrospective approach reflecting the application of the standard in each prior reporting period with the option to elect certain practical expedients, or (ii) a modified retrospective approach with the cumulative effect of initially adopting ASU 2014-09 recognized at the date of adoption. We plan to adopt the new standard effective October 1, 2018 and are continuing to evaluate the impact of adoption on our Consolidated Financial Statements and the implementation approach to be used.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s consolidated financial statements upon adoption.
Note 2. Acquisitions
Mortara Instrument
On February 14, 2017, we completed the acquisition of Mortara Instrument, Inc. ("Mortara") for consideration of $330.0 million in cash ($311.2 million, net of cash acquired), primarily financed through a private offering of $300.0 million of senior unsecured notes (see Note 4 of our Consolidated Financial Statements). Mortara provides a portfolio of diagnostic cardiology devices designed to serve the full continuum of clinical care, from acute care to primary care and clinical research organizations.
The results of Mortara are included in the Consolidated Financial Statements since the date of acquisition. The impact to reported revenue and net income was not significant. The impact to our year to date revenue and net income on an unaudited proforma basis, as if the Mortara acquisition had been consummated at the beginning of our fiscal 2016 year, would not have been significant.
51
The following summarizes the preliminary estimate of the fair value of assets acquired and liabilities assumed at the date of the Mortara acquisition. During fiscal 2017, we made certain adjustments to the opening balance sheet as of the acquisition date which were insignificant. The fair value of assets acquired and liabilities assumed are still considered to be preliminary, however we do not expect further adjustments to be significant.
| Amount | |||
| Trade receivables | $ | 16.4 | |
| Inventory | 22.1 | ||
| Other current assets | 2.8 | ||
| Property, plant and equipment | 18.2 | ||
| Goodwill | 164.8 | ||
| Trade names (7-year weighted average useful life) | 15.8 | ||
| Customer relationships (8-year useful life) | 37.9 | ||
| Developed technology (7-year useful life) | 52.3 | ||
| Other noncurrent assets | 4.7 | ||
| Current liabilities | (22.5 | ) | |
| Noncurrent liabilities | (1.3 | ) | |
| Total purchase price, net of cash acquired | $ | 311.2 | |
Goodwill in connection with the Mortara acquisition was allocated entirely to our Front Line Care segment. The preliminary fair value attributes a majority of the goodwill to the acquired U.S. operations which is deductible for tax purposes.
Tridien Medical
On September 21, 2016, we acquired all of the outstanding shares of Tridien for a purchase price of $26.0 million, net of cash acquired. Tridien develops, manufactures and markets support surfaces and patient positioning devices. We funded the transaction primarily with borrowings under our Senior Secured Revolving Credit Facility ("Revolving Credit Facility"). The fair value of assets acquired included $10.4 million of working capital consisting primarily of inventories and accounts receivable, $7.4 million of goodwill and $6.3 million of acquisition-related intangible assets. The results of Tridien are included in the Consolidated Financial Statements since the date of acquisition. Goodwill in connection with the Tridien acquisition was allocated entirely to our Patient Support Systems segment and is not deductible for tax purposes.
During fiscal 2017, we made certain adjustments to the opening balance sheet as of the acquisition date which were insignificant.
Welch Allyn
On September 8, 2015, we completed the acquisition of Welch Allyn Holdings, Inc. and its subsidiaries (collectively, "Welch Allyn") for consideration of $1,686.8 million in cash ($1,633.1 million, net of cash acquired) and 8,133,722 shares of Hill-Rom common stock for a total combined purchase price of approximately $2.1 billion. Welch Allyn is a leading manufacturer of medical diagnostic equipment and offers a diversified portfolio of devices that assess, diagnose, treat, and manage a wide variety of illnesses and diseases.
The transaction was funded with new borrowings, including $1.8 billion in term loans and $425.0 million of senior notes issued in a private placement debt offering. Refer to Note 4 of our Consolidated Financial Statements for additional information regarding our debt obligations.
52
The following summarizes the fair value of assets acquired and liabilities assumed at the date of the acquisition. These results are considered final.
| Amount | |||
| Trade receivables | $ | 62.9 | |
| Inventory | 110.5 | ||
| Other current assets | 53.8 | ||
| Property, plant, and equipment | 91.5 | ||
| Goodwill | 1,179.8 | ||
| Trade name (indefinite life) | 434.0 | ||
| Customer relationships (12-year useful life) | 516.8 | ||
| Developed technology (7-year weighted average useful life) | 54.0 | ||
| Other intangibles | 19.5 | ||
| Other noncurrent assets | 26.5 | ||
| Current liabilities | (166.1 | ) | |
| Noncurrent deferred income taxes | (309.0 | ) | |
| Other noncurrent liabilities | (24.8 | ) | |
| Total purchase price, net of cash acquired | $ | 2,049.4 | |
| Fair value of common stock issued | $ | 416.3 | |
| Cash payment, net of cash acquired | 1,633.1 | ||
| Total consideration | $ | 2,049.4 | |
Final purchase accounting adjustments were made in fiscal 2016 reducing goodwill by $23.7 million primarily due to the finalization of deferred income taxes. These adjustments are reflected in the table above.
Goodwill from the Welch Allyn acquisition, which is not deductible for tax purposes, is primarily due to enhanced customer relevance and a stronger competitive position resulting from the business combination, including a complementary commercial position, product portfolio, and enhanced synergies. The goodwill from the Welch Allyn acquisition has been allocated entirely to our Front Line Care segment.
Our total revenue on an unaudited proforma basis, as if the Welch Allyn acquisition had been consummated at the beginning of our 2014 fiscal year, would have been higher by approximately $638 million for the year ended September 30, 2015. On the same unaudited proforma basis, our net income would have been lower by approximately $59 million for the year ended September 30, 2015. The proforma net income for fiscal 2015 has been adversely impacted by significant costs related to the transaction including deal costs, financing costs, restructuring costs incurred in relation to our synergy initiatives, costs associated with triggering the change-in-control provisions of certain equity-based compensation programs at Welch Allyn, and purchase price accounting, including the nonrecurring effects of the inventory step-up. These results are not indicative of expected future performance.
The unaudited proforma results are based on the Company’s historical financial statements and those of the Welch Allyn business and do not necessarily indicate the results of operations that would have resulted had the acquisition been completed at the beginning of the comparable period presented and are not indicative of the results of operations in future periods.
53
Note 3. Goodwill and Indefinite-Lived Intangible Assets
The following summarizes goodwill activity by reportable segment:
| Patient Support Systems | Front Line Care | Surgical Solutions | Total | ||||||||||||
| Balances at September 30, 2015 | |||||||||||||||
| Goodwill | $ | 536.0 | $ | 1,232.2 | $ | 315.1 | $ | 2,083.3 | |||||||
| Accumulated impairment losses | (472.8 | ) | — | — | (472.8 | ) | |||||||||
| Goodwill, net at September 30, 2015 | 63.2 | 1,232.2 | 315.1 | 1,610.5 | |||||||||||
| Changes in Goodwill during the period: | |||||||||||||||
| Goodwill related to acquisitions | 7.9 | (23.7 | ) | 1.1 | (14.7 | ) | |||||||||
| Currency translation effect | 0.2 | (3.0 | ) | (8.6 | ) | (11.4 | ) | ||||||||
| Balances at September 30, 2016 | |||||||||||||||
| Goodwill | 544.1 | 1,205.5 | 307.6 | 2,057.2 | |||||||||||
| Accumulated impairment losses | (472.8 | ) | — | — | (472.8 | ) | |||||||||
| Goodwill, net at September 30, 2016 | 71.3 | 1,205.5 | 307.6 | 1,584.4 | |||||||||||
| Changes in Goodwill during the period: | |||||||||||||||
| Goodwill related to acquisitions | (0.5 | ) | 164.8 | — | 164.3 | ||||||||||
| Currency translation effect | 1.4 | 5.3 | 4.2 | 10.9 | |||||||||||
| Balances at September 30, 2017 | |||||||||||||||
| Goodwill | 545.0 | 1,375.6 | 311.8 | 2,232.4 | |||||||||||
| Accumulated impairment losses | (472.8 | ) | — | — | (472.8 | ) | |||||||||
| Goodwill, net at September 30, 2017 | $ | 72.2 | $ | 1,375.6 | $ | 311.8 | $ | 1,759.6 | |||||||
We acquired Mortara, Tridien and Welch Allyn during 2017, 2016 and 2015, respectively. All goodwill associated with Mortara and Welch Allyn was assigned to the Front Line Care segment and all goodwill associated Tridien was assigned to the Patient Support Systems segment. During fiscal 2017 and 2016, we recorded adjustments to goodwill related to the Mortara, Tridien, and
Welch Allyn acquisitions. Refer to Note 2 of our Consolidated Financial Statements for additional information regarding these acquisitions.
As discussed in Note 11 of our Consolidated Financial Statements, we operate in three reportable business segments. Goodwill impairment testing is performed at the reporting unit level. Goodwill is assigned to reporting units at the date the goodwill is initially recorded and is reallocated as necessary based on the restructuring of reporting units over time. Once goodwill is assigned to reporting units, it no longer retains its association with a particular acquisition, and all of the activities within a reporting unit, whether acquired or organically grown, are available to support the value of the goodwill.
Testing for impairment must be performed annually, or on an interim basis upon the occurrence of a triggering event or change in circumstances that would more likely than not reduce the fair value of a reporting unit below its carrying amount. The annual evaluation of goodwill performed during the third quarter of fiscal 2017 and 2016 did not result in any impairments.
Indefinite-lived intangible assets
We have various indefinite-lived intangible assets representing trade names with a carrying value of $466.9 million as of September 30, 2017 and September 30, 2016. Testing for impairment must be performed annually, or on an interim basis upon the occurrence of a triggering event or change in circumstances that would more likely than not reduce the fair value of an indefinite-lived intangible asset below its carrying amount. The annual evaluation of indefinite-lived intangible assets performed during the third quarter of fiscal 2017 and 2016 did not result in impairment.
54
Note 4. Financing Agreements
Total debt consists of the following:
| September 30, 2017 | September 30, 2016 | ||||||
| Revolving credit facilities | $ | 90.0 | $ | 235.8 | |||
| Current portion of long-term debt | 109.8 | 73.2 | |||||
| Senior secured Term Loan A, long-term portion | 1,266.7 | 1,372.3 | |||||
| Senior unsecured 5.75% notes due on September 1, 2023 | 419.9 | 419.1 | |||||
| Senior unsecured 5.00% notes due on February 14, 2025 | 295.8 | — | |||||
| Unsecured 7.00% debentures due on February 15, 2024 | 13.6 | 13.7 | |||||
| Unsecured 6.75% debentures due on December 15, 2027 | 29.6 | 29.6 | |||||
| Securitization Program | 79.1 | — | |||||
| Other | 4.8 | 4.8 | |||||
| Total debt | 2,309.3 | 2,148.5 | |||||
| Less Short-term borrowings | 188.9 | 210.1 | |||||
| Total Long-term debt | $ | 2,120.4 | $ | 1,938.4 | |||
In May 2017, we entered into a 364-day $110.0 million accounts receivable securitization program (the "Securitization Program") with certain financial institutions. Under the terms of the Securitization Program, certain of our accounts receivable secure the amounts borrowed and cannot be used to pay our other debts or liabilities. The amount that we may borrow at a given point in time is determined based on the amount of qualifying accounts receivable that are present at such point in time. As of September 30, 2017, $79.1 million was outstanding under the Securitization Program. Such outstanding borrowings under the Securitization Program bear interest at London Interbank Offered Rate ("LIBOR") plus the applicable margin of 0.675% and are included as a component of Short-term borrowings, while the accounts receivable securing these obligations remain as a component of Trade accounts receivable, net of allowances in our Consolidated Balance Sheets. In addition, the agreement governing the Securitization Program contains various customary affirmative and negative covenants, and customary default and termination provisions. As of September 30, 2017, we were in compliance with these covenants and provisions.
In February 2017, we entered into $300.0 million of senior unsecured notes maturing February 2025 for purposes of financing the Mortara acquisition. These notes bear interest at a fixed rate of 5.00% annually. We also have outstanding senior unsecured notes of $425.0 million maturing in September 2023 that bear interest at a fixed rate of 5.75% annually (collectively, the "Senior Notes"). These Senior Notes were issued at par in a private placement offering and are not registered securities on any public market. All of the notes were outstanding as of September 30, 2017. We are not required to make any mandatory redemption or sinking fund payments with respect to the Senior Notes, other than in certain circumstances such as a change in control or material sale of assets. We may redeem the 5.75% and 5.00% notes prior to maturity, but doing so prior to September 1, 2021 and February 15, 2020 would require payment of a premium on any amounts redeemed, the amount of which varies based on the timing of the redemption. The indentures governing the Senior Notes contain certain covenants which impose limitations on the amount of dividends we may pay and the amount of common shares we may repurchase in the open market, but we do not expect these covenants to affect our current dividend policy or open share repurchase program. The terms of these indentures also impose certain restrictions on the amount and type of additional indebtedness we may obtain in the future, as well as the types of liens and guarantees we may provide.
In September 2016, we entered into an amended and restated senior credit agreement ("Senior Credit Agreement") for purposes of refinancing our credit facilities (originally entered into as part of the Welch Allyn acquisition) and funding the payoff of our then outstanding senior secured Term Loan B facility. The Senior Credit Agreement consists of two facilities as follows:
| • | $1,462.5 million senior secured Term Loan A facility ("TLA Facility"), maturing in September 2021 |
| • | Revolving Credit Facility, providing borrowing capacity of up to $700.0 million, maturing in September 2021 |
The TLA Facility and Revolving Credit Facility (collectively, the "Senior Secured Credit Facilities") bear interest at variable rates which are currently less than 3.0%. These interest rates are based primarily on the LIBOR, but under certain conditions could also be based on the U.S. Federal Funds Rate or the U.S. Prime Rate, at our option. We are able to voluntarily prepay outstanding loans under the TLA Facility at any time.
55
The following table summarizes the scheduled maturities of the TLA Facility for fiscal years 2018 through 2022:
| Amount | |||
| 2018 | $ | 109.7 | |
| 2019 | $ | 146.3 | |
| 2020 | $ | 146.3 | |
| 2021 | $ | 987.2 | |
| 2022 | $ | — | |
On September 30, 2017, there were $90.0 million of outstanding borrowings on the Revolving Credit Facility, and available borrowing capacity was $601.6 million after giving effect to $8.4 million of outstanding standby letters of credit. The availability of borrowings under our Revolving Credit Facility is subject to our ability at the time of borrowing to meet certain specified conditions, including compliance with covenants contained in the Senior Credit Agreement.
The Senior Secured Credit Facilities are held with a syndicate of banks, which includes over 30 institutions. Our general corporate assets, including those of certain of our subsidiaries, collateralize these obligations. The Senior Credit Agreement contains financial covenants which specify a maximum secured net leverage ratio and a minimum interest coverage ratio, as such terms are defined in the Senior Credit Agreement. These financial covenants are measured at the end of each fiscal quarter. The required ratios vary providing a gradually decreasing maximum secured net leverage ratio and a gradually increasing minimum interest coverage ratio, as set forth in the following table:
| Fiscal Quarter Ended | Maximum Secured Net Leverage Ratio | Minimum Interest Coverage Ratio |
| December 31, 2017 | 4.00x | 3.50x |
| December 31, 2018 | 3.50x | 3.75x |
| December 31, 2019 and thereafter | 3.00x | 4.00x |
We are in compliance with all applicable financial covenants under the Senior Credit Agreement as of September 30, 2017.
In conjunction with the amendment of the Senior Secured Credit Facilities in September 2016, we recorded a $10.8 million loss on extinguishment of debt related to a majority of the debt issuance costs previously capitalized for the Term Loan B facility. We also incurred $6.5 million of costs related to the amendment, $4.5 million of which were capitalized as part of the new Senior Secured Credit Facilities.
We are exposed to market risk from fluctuations in interest rates. We sometimes manage our exposure to interest rate fluctuations through the use of interest rate swaps. As of September 30, 2017, we had nine interest rate swap agreements, with notional amounts of $750.0 million, in aggregate, to hedge the variability of cash flows associated with a portion of the variable interest rate payments through September 2021 on the Senior Secured Credit Facilities. The interest rate swaps have effective start dates ranging between December 31, 2016 and September 8, 2020 and are designated as cash flow hedges. At September 30, 2017, these swaps were in a net asset position with an aggregate fair value of $7.3 million, of which $8.5 million were classified as Other assets and $1.2 million were classified as Other current liabilities. We classify fair value measurements on our interest rate swaps as Level 2, as described in Note 1.
The fair value of our debt is estimated based on the quoted market prices for the same or similar issues or on the current rates offered to us for debt of the same remaining maturities. The book values of our short-term debt instruments approximate fair value. The estimated fair values of our long-term debt instruments are described in the table below:
| September 30, 2017 | September 30, 2016 | ||||||
| Senior secured Term Loan A | $ | 1,364.8 | $ | 1,441.0 | |||
| Senior unsecured 5.75% notes due on September 1, 2023 | 449.3 | 454.0 | |||||
| Senior unsecured 5.00% notes due on February 14, 2025 | 311.9 | — | |||||
| Unsecured debentures | 46.8 | 45.8 | |||||
| Total debt | $ | 2,172.8 | $ | 1,940.8 | |||
56
The estimated fair values of our long-term unsecured debentures were based on observable inputs such as quoted prices in markets that are not active. The estimated fair values of our term loans and the Senior Notes were based on quoted prices for similar liabilities. These fair value measurements were classified as Level 2, as described in Note 1 of our Consolidated Financial Statements.
Note 5. Other Comprehensive Income
The following tables represent the changes in accumulated other comprehensive loss by component for the fiscal years ended September 30, 2017 and 2016:
| Year Ended September 30, 2017 | |||||||||||||||||||||||||||||||
| Other comprehensive income (loss) | Accumulated other comprehensive income (loss) | ||||||||||||||||||||||||||||||
| Prior to reclassification | Reclassification from | Pre-tax | Tax effect | Net of tax | Beginning balance | Net activity | Ending balance | ||||||||||||||||||||||||
| Derivative instruments and hedges | $ | 12.7 | $ | (0.9 | ) | $ | 11.8 | $ | (4.4 | ) | $ | 7.4 | $ | (3.1 | ) | $ | 7.4 | $ | 4.3 | ||||||||||||
| Foreign currency translation adjustment | 32.9 | 1.0 | 33.9 | — | 33.9 | (115.2 | ) | 33.9 | (81.3 | ) | |||||||||||||||||||||
| Change in pension and postretirement defined benefit plans | 22.1 | 5.9 | 28.0 | (10.2 | ) | 17.8 | (50.8 | ) | 17.8 | (33.0 | ) | ||||||||||||||||||||
| Total | $ | 67.7 | $ | 6.0 | $ | 73.7 | $ | (14.6 | ) | $ | 59.1 | $ | (169.1 | ) | $ | 59.1 | $ | (110.0 | ) | ||||||||||||
| Year Ended September 30, 2016 | |||||||||||||||||||||||||||||||
| Other comprehensive income (loss) | Accumulated other comprehensive income (loss) | ||||||||||||||||||||||||||||||
Prior to reclassification | Reclassification from | Pre-tax | Tax effect | Net of tax | Beginning balance | Net activity | Ending balance | ||||||||||||||||||||||||
| Derivative instruments and hedges | $ | (4.9 | ) | $ | (0.1 | ) | $ | (5.0 | ) | $ | 1.9 | $ | (3.1 | ) | $ | — | $ | (3.1 | ) | $ | (3.1 | ) | |||||||||
| Foreign currency translation adjustment | (22.4 | ) | — | (22.4 | ) | — | (22.4 | ) | (92.8 | ) | (22.4 | ) | (115.2 | ) | |||||||||||||||||
| Change in pension and postretirement defined benefit plans | (8.5 | ) | 4.4 | (4.1 | ) | 1.3 | (2.8 | ) | (48.0 | ) | (2.8 | ) | (50.8 | ) | |||||||||||||||||
| Total | $ | (35.8 | ) | $ | 4.3 | $ | (31.5 | ) | $ | 3.2 | $ | (28.3 | ) | $ | (140.8 | ) | $ | (28.3 | ) | $ | (169.1 | ) | |||||||||
The following table represents the items reclassified out of accumulated other comprehensive loss and the related tax effects during fiscal 2017 and 2016:
| Year Ended September 30 | |||||||||||||||||||||||
| 2017 | 2016 | ||||||||||||||||||||||
Amount reclassified | Tax effect | Net of tax | Amount reclassified | Tax effect | Net of tax | ||||||||||||||||||
| Derivative instruments and hedges (a) | $ | (0.9 | ) | $ | 0.3 | $ | (0.6 | ) | $ | (0.1 | ) | $ | — | $ | (0.1 | ) | |||||||
| Foreign currency translation adjustment (b) | $ | 1.0 | $ | — | $ | 1.0 | $ | — | $ | — | $ | — | |||||||||||
| Change in pension and postretirement defined benefit plans (c) | $ | 5.9 | $ | (2.2 | ) | $ | 3.7 | $ | 4.4 | $ | (1.3 | ) | $ | 3.1 | |||||||||
(a) Reclassified from accumulated other comprehensive income (loss) into Interest expense.
(b) Reclassified from accumulated other comprehensive income (loss) into Special charges.
(c) Reclassified from accumulated other comprehensive income (loss) into Cost of goods sold and Selling and administrative expenses. These components are included in the computation of net periodic pension expense.
57
Note 6. Retirement and Postretirement Benefit Plans
Our retirement plans consist of defined benefit plans, postretirement healthcare plans and defined contribution savings plans. Plans cover certain employees both in and outside of the U.S.
Retirement Plans
We sponsor five defined benefit plans. Those plans include a master defined benefit retirement plan, a nonqualified supplemental executive defined benefit retirement plan and three defined benefit retirement plans covering employees in Germany and France. Benefits for such plans are based primarily on years of service and the employee’s level of compensation during specific periods of employment. We contribute funds to trusts as necessary to provide for current service and for any unfunded projected future benefit obligation over a reasonable period of time. All of our plans have a September 30 measurement date.
Effect on Operations
The components of net periodic benefit cost for our defined benefit retirement plans were as follows:
| Year Ended September 30 | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Service cost | $ | 5.8 | $ | 5.0 | $ | 5.4 | ||||||
| Interest cost | 9.9 | 10.9 | 14.6 | |||||||||
| Expected return on plan assets | (14.6 | ) | (13.0 | ) | (16.7 | ) | ||||||
| Amortization of unrecognized prior service cost, net | 0.2 | 0.3 | 0.6 | |||||||||
| Amortization of net loss | 6.1 | 4.5 | 5.2 | |||||||||
| Net periodic benefit cost | 7.4 | 7.7 | 9.1 | |||||||||
| Settlement charge | — | — | 9.6 | |||||||||
| Special termination benefits | 0.1 | — | — | |||||||||
| Net pension expense | $ | 7.5 | $ | 7.7 | $ | 18.7 | ||||||
Beginning in the first quarter of fiscal 2016, we elected to change the method we use to estimate the service and interest cost components of net periodic benefit cost for our defined benefit pension plans to a spot yield curve approach. Previously, we estimated the service and interest cost components of pension expense using a single weighted-average discount rate derived from the yield curve used to measure the benefit obligation at the beginning of the period. Under the new approach, we apply discounting using individual spot rates from a yield curve composed of the rates of return on several hundred high-quality, fixed income corporate bonds available at the measurement date. These spot rates align to each of the projected benefit obligations and service cost cash flows. The service cost component relates to the active participants in the plan, so the relevant cash flows on which to apply the yield curve are considerably longer in duration on average than the total projected benefit obligation cash flows, which also include benefit payments to retirees. Interest cost is computed by multiplying each spot rate by the corresponding discounted projected benefit obligation cash flows. The spot yield curve approach reduces any actuarial gains and losses based upon interest rate expectations (e.g., built-in gains in interest cost in an upward sloping yield curve scenario), or gains and losses merely resulting from the timing and magnitude of cash outflows associated with our benefit obligations. The change does not affect the measurement of the total benefit obligations as the change in service and interest costs offsets the actuarial gains and losses recorded in other comprehensive income.
We made this change to improve the correlation between projected benefit cash flows and the corresponding yield curve spot rates and to provide a better measurement of service and interest costs. This change is considered a change in estimate and is accounted for on a prospective basis starting in fiscal year 2016.
In April 2015, we offered all terminated vested participants of our domestic master defined benefit retirement plan an option to receive a lump sum cash payout in lieu of their right to future periodic benefit payments under the plan upon their retirement. Lump sums of $42.3 million were paid to participants in September 2015, triggering a plan settlement charge of $9.6 million, which is recorded as a component of Special charges on the Statements of Consolidated Income.
58
Obligations and Funded Status
The change in benefit obligations, plan assets and funded status, along with amounts recognized in the Consolidated Balance Sheets for our defined benefit retirement plans were as follows:
| Year Ended September 30 | ||||||||
| 2017 | 2016 | |||||||
| Change in benefit obligation: | ||||||||
| Benefit obligation at beginning of year | $ | 347.1 | $ | 315.5 | ||||
| Service cost | 5.8 | 5.0 | ||||||
| Interest cost | 9.9 | 10.9 | ||||||
| Actuarial loss (gain) | (5.7 | ) | 27.4 | |||||
| Benefits paid | (12.5 | ) | (11.9 | ) | ||||
| Special termination benefits | 0.1 | — | ||||||
| Exchange rate loss | 1.1 | 0.2 | ||||||
| Benefit obligation at end of year | 345.8 | 347.1 | ||||||
| Change in plan assets: | ||||||||
| Fair value of plan assets at beginning of year | 267.0 | 219.1 | ||||||
| Actual return on plan assets | 28.8 | 28.8 | ||||||
| Employer contributions | 1.1 | 31.0 | ||||||
| Benefits paid | (12.5 | ) | (11.9 | ) | ||||
| Fair value of plan assets at end of year | 284.4 | 267.0 | ||||||
| Funded status and net amounts recognized | $ | (61.4 | ) | $ | (80.1 | ) | ||
| Amounts recorded in the Consolidated Balance Sheets: | ||||||||
| Accrued pension benefits, current portion | $ | (1.2 | ) | $ | (1.1 | ) | ||
| Accrued pension benefits, long-term | (60.2 | ) | (79.0 | ) | ||||
| Net amount recognized | $ | (61.4 | ) | $ | (80.1 | ) | ||
In addition to the amounts above, net actuarial losses of $59.9 million and prior service costs of $0.6 million, less an applicable aggregate tax effect of $22.8 million are included as components of accumulated other comprehensive loss at September 30, 2017. In addition to the amounts above, net actuarial losses of $85.7 million and prior service costs of $0.8 million, less an applicable aggregate tax effect of $32.2 million are included as components of accumulated other comprehensive loss at September 30, 2016. The estimated net actuarial loss and prior service cost for our defined benefit retirement plans that will be amortized from accumulated other comprehensive loss into net periodic benefit cost over the next fiscal year are $4.5 million and $0.1 million, respectively.
Accumulated Benefit Obligation
The accumulated benefit obligation for all defined benefit pension plans was $325.7 million and $326.3 million at September 30, 2017 and 2016. Selected information for our plans, including plans with accumulated benefit obligations exceeding plan assets, was as follows:
| September 30 | ||||||||||||||||||||||||
| 2017 | 2016 | |||||||||||||||||||||||
| PBO | ABO | Plan Assets | PBO | ABO | Plan Assets | |||||||||||||||||||
| Master plan | $ | 319.8 | $ | 301.5 | $ | 284.1 | $ | 319.6 | $ | 300.7 | $ | 266.8 | ||||||||||||
| International plans | 20.8 | 19.0 | 0.3 | 22.2 | 20.3 | 0.2 | ||||||||||||||||||
| Supplemental executive plan | 5.2 | 5.2 | — | 5.3 | 5.3 | — | ||||||||||||||||||
| $ | 345.8 | $ | 325.7 | $ | 284.4 | $ | 347.1 | $ | 326.3 | $ | 267.0 | |||||||||||||
59
Actuarial Assumptions
The weighted average assumptions used in accounting for our domestic pension plans were as follows:
| 2017 | 2016 | 2015 | ||||
Weighted average assumptions to determine benefit obligations at the measurement date: | ||||||
| Discount rate for obligation | 3.9% | 3.7% | 4.4% | |||
| Rate of compensation increase | 3.0% | 3.0% | 3.0% | |||
Weighted average assumptions to determine benefit cost for the year: | ||||||
| Discount rate for expense | 3.7% | 4.4% | 4.5% | |||
| Expected rate of return on plan assets | 5.8% | 5.8% | 6.8% | |||
| Rate of compensation increase | 3.0% | 3.0% | 3.0% | |||
The discount rates used in the valuation of our defined benefit pension plans are evaluated annually based on current market conditions. In setting these rates we utilize long-term bond indices and yield curves as a preliminary indication of interest rate movements, and then make adjustments to the respective indices to reflect differences in the terms of the bonds covered under the indices in comparison to the projected outflow of our pension obligations. The overall expected long-term rate of return is based on historical and expected future returns, which are inflation adjusted and weighted for the expected return for each component of the investment portfolio, as well as taking into consideration economic and capital market conditions. The rate of assumed compensation increase is also based on our specific historical trends of past wage adjustments. The weighted average discount rate assumptions used for our international plans are lower than our domestic plan assumptions and do not significantly affect the consolidated net benefit obligation or net periodic benefit cost balances.
Plan Assets
The weighted average asset allocations of our master defined benefit retirement plan at September 30, 2017 and 2016, by asset category, along with target allocations, are as follows:
| 2017 Target Allocation | 2016 Target Allocation | 2017 Actual Allocation | 2016 Actual Allocation | |||||
| Equity securities | 37% - 45% | 39 - 49% | 42% | 43% | ||||
| Fixed income securities | 55% - 63% | 51 - 61% | 58% | 57% | ||||
| Total | 100% | 100% | ||||||
We have a Plan Committee that sets investment guidelines with the assistance of an external consultant. These guidelines are established based on market conditions, risk tolerance, funding requirements and expected benefit payments. The Plan Committee also oversees the investment allocation process and monitors asset performance. As pension liabilities are long-term in nature, we employ a long-term total return approach to maximize the long-term rate of return on plan assets for a prudent level of risk. Target allocations are guidelines, not limitations, and plan fiduciaries may occasionally approve allocations above or below a target range or elect to rebalance the portfolio within the targeted range.
The investment portfolio contains a diversified portfolio of primarily equities and fixed income securities. Securities are also diversified in terms of domestic and international securities, short- and long-term securities, growth and value styles, large cap and small cap stocks. The primary investment strategy is a dynamic target allocation method that periodically rebalances among various investment categories depending on the current funded positions. This program is designed to actively move from return-seeking investments (such as equities) toward liability-hedging investments (such as long-duration fixed income) as funding levels improve.
Trust assets are invested subject to the following policy restrictions: short-term securities must be rated A2/P2 or higher; all fixed-income securities shall have a credit quality rating "BBB" or higher; investments in equities in any one company may not exceed 10% of the equity portfolio.
60
Fair Value Measurements of Plan Assets
The following table summarizes the valuation of our pension plan assets by pricing categories:
| Balance at September 30, 2017 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
| Cash | $ | 7.2 | $ | 7.2 | $ | — | $ | — | ||||||||
| Equities (a) | ||||||||||||||||
| U.S. companies | 58.6 | — | — | — | ||||||||||||
| International companies | 59.4 | — | — | — | ||||||||||||
| Fixed income securities (a) | 159.2 | — | — | — | ||||||||||||
| Total plan assets at fair value | $ | 284.4 | $ | 7.2 | $ | — | $ | — | ||||||||
| Balance at September 30, 2016 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
| Cash | $ | 3.7 | $ | 3.7 | $ | — | $ | — | ||||||||
| Equities (a) | ||||||||||||||||
| U.S. companies | 59.3 | — | — | — | ||||||||||||
| International companies | 56.4 | — | — | — | ||||||||||||
| Fixed income securities (a) | 147.6 | — | — | — | ||||||||||||
| Total plan assets at fair value | $ | 267.0 | $ | 3.7 | $ | — | $ | — | ||||||||
(a) These investments are commingled funds and/or collective trusts valued using the net asset value ("NAV") unit price provided by the fund administrator. The NAV is based on the value of the underlying assets owned by the fund. During fiscal 2017, we adopted revised disclosure guidance related to investments measured at NAV as a practical expedient, under which they are no longer categorized in the fair value hierarchy. For further descriptions of the asset Levels used in the above chart, refer to Note 1 of our Consolidated Financial Statements.
Cash Flows
Our U.S. qualified defined benefit plan is funded in excess of 89%, as measured under the requirements of the Pension Protection Act of 2006, and therefore we expect that the plan will not be subject to the "at risk" funding requirements of this legislation.
During 2017 and 2016, we contributed cash of $1.1 million and $31.0 million to our defined benefit retirement plans. We will not be required to contribute to our master defined benefit retirement plan for fiscal year 2018 due to the current funding level; however, minimal contributions will be required for our unfunded plans.
Estimated Future Benefit Payments
The benefit payments, which are expected to be funded through plan assets and company contributions and reflect expected future service, are expected to be paid as follows:
| Pension Benefits | |||
| 2018 | $ | 13.7 | |
| 2019 | $ | 13.8 | |
| 2020 | $ | 14.6 | |
| 2021 | $ | 15.4 | |
| 2022 | $ | 16.0 | |
| 2023-2027 | $ | 91.4 | |
61
Defined Contribution Savings Plans
We have defined contribution savings plans that cover substantially all U.S. employees and certain non-U.S. employees. The general purpose of these plans is to provide additional financial security during retirement by providing employees with an incentive to make regular savings. Company contributions to the plans are based on eligibility and employee contributions. Expense under these plans was $26.8 million in fiscal years 2017 and 2016. During fiscal 2015, the expense was $17.4 million.
Postretirement Health Care Plans
In addition to defined benefit retirement plans, we also offer two domestic postretirement health care plans, one of which was assumed in the acquisition of Welch Allyn, that provide health care benefits to qualified retirees and their dependents. The plans are closed to new participants and include retiree cost sharing provisions and generally extends retiree coverage for medical and prescription benefits beyond the COBRA continuation period to the date of Medicare eligibility. We use a measurement date of September 30, for these plans.
The expense related to postretirement health care plans, including the Welch Allyn plan on a post-acquisition basis, has not been significant during fiscal years 2017, 2016 or 2015. The change in the accumulated postretirement benefit obligation was as follows:
| Year Ended September 30 | ||||||||
| 2017 | 2016 | |||||||
| Change in benefit obligation: | ||||||||
| Benefit obligation at beginning of year | $ | 21.6 | $ | 25.1 | ||||
| Service cost | 0.4 | 0.3 | ||||||
| Interest cost | 0.5 | 0.8 | ||||||
| Plan amendments | (0.7 | ) | — | |||||
| Actuarial gain | (1.8 | ) | (3.7 | ) | ||||
| Benefits paid | (0.9 | ) | (1.5 | ) | ||||
| Retiree contributions | 0.3 | 0.6 | ||||||
| Benefit obligation at end of year | $ | 19.4 | $ | 21.6 | ||||
| Amounts recorded in the Consolidated Balance Sheets: | ||||||||
| Accrued benefits obligation, current portion | $ | 1.5 | $ | 1.6 | ||||
| Accrued benefits obligation, long-term | 17.9 | 20.0 | ||||||
| Net amount recognized | $ | 19.4 | $ | 21.6 | ||||
We contributed approximately $0.9 million to the plans in fiscal 2017, compared with $1.5 million in fiscal 2016.
In addition to the amounts above, net actuarial gains of $7.3 million and prior service credits of $1.0 million, less an applicable aggregate tax effect of $3.1 million are included as components of accumulated other comprehensive loss at September 30, 2017. Net actuarial gains of $5.9 million and prior service credits of $0.5 million, less an applicable aggregate tax effect of $2.4 million are included as components of accumulated other comprehensive loss at September 30, 2016.
The estimated net actuarial gain and prior service benefit for our postretirement health care plans that will be amortized from accumulated other comprehensive loss into net periodic benefit cost over the next fiscal year are $(0.6) million and $(0.2) million.
The discount rate used to determine the net periodic benefit cost for the postretirement health care plans during the fiscal years ended September 30, 2017, 2016 and 2015 was 3.0%, 3.5% and 3.7%, respectively. The discount rate used to determine the benefit obligation as of September 30, 2017 was 3.3%. For fiscal year ended 2016 the discount rate ranged from 2.9% to 3.0%. For the fiscal year ended 2015 the discount rate was 3.5%. As of September 30, 2017, the health care cost trend rates for the plans were generally assumed to be in the ranges of 6.9% to 8.7%, trending down to a rate of 4.5% over the long-term.
A one-percentage-point increase/decrease in the assumed health care cost trend rates as of September 30, 2017 would cause an increase/decrease in service and interest costs of less than $0.1 million, along with an increase in the benefit obligation of $1.3 million and a decrease of $1.2 million.
62
We fund the postretirement health care plans as benefits are paid, and current plan benefits are expected to require net company contributions of approximately $1.6 million in fiscal 2017 and approximately $2.0 million per fiscal year thereafter.
Note 7. Common Stock
Share Repurchases
As part of the $190.0 million share repurchase program approved by the Board of Directors in September 2013, we repurchased 0.8 million shares of our common stock in the open market during fiscal year 2017 valued at $50.0 million. We did not repurchase shares in fiscal year 2016 in the open market. We repurchased 1.2 million shares of our common stock in the open market during fiscal year 2015 valued at $54.8 million. As of September 30, 2017, a cumulative total of $175.3 million had been used under the then existing authorization. Repurchases may be made on the open market or via private transactions, and are used for general business purposes. On November 6, 2017, the Board of Directors approved an increase to the share repurchase program in an amount of $150.0 million, leaving the company approximately $165.0 million of availability under the share repurchase program as of such date. This program does not have an expiration date and there are no plans to terminate this program in the future.
Stock-Based Compensation
We have stock-based compensation plans under which employees and non-employee directors may be granted options to purchase shares of Company common stock at the fair market value at the time of grant. In addition to stock options, we grant performance share units ("PSUs") and RSUs to certain management level employees and vested deferred stock to non-employee directors. We also offer eligible employees the opportunity to buy shares of our common stock at a discount via an Employee Stock Purchase Plan ("ESPP").
Our primary stock-based compensation program is the Stock Incentive Plan, which has been approved by our shareholders. Under the Stock Incentive Plan, we have a total of 15.3 million authorized shares. As of September 30, 2017, approximately 3.0 million shares were available for future grants under our stock-based compensation plans. We generally settle our stock-based awards with treasury shares. As of September 30, 2017, we had 22.6 million treasury shares available for use to settle stock-based awards.
The following table sets forth a summary of the annual stock-based compensation cost that was charged against income for all types of awards:
| Year Ended September 30 | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Total stock-based compensation cost (pre-tax) | $ | 23.0 | $ | 23.1 | $ | 25.0 | ||||||
| Total income tax benefit | (16.5 | ) | (7.9 | ) | (7.5 | ) | ||||||
| Total stock-based compensation cost, net of tax | $ | 6.5 | $ | 15.2 | $ | 17.5 | ||||||
In March 2016, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2016-09, Compensation – Stock Compensation (Topic 718), "Improvements to Employee Share-Based Payment Accounting." During the first quarter of fiscal 2017, we elected to early adopt ASU 2016-09, as permitted. Under ASU 2016-09, the tax effects of stock compensation will be recognized as income tax expense or benefit in the income statement and the tax effects of exercised or vested awards will be treated as discrete items in the reporting period in which they occur. Amendments related to accounting for excess tax benefits have been adopted prospectively, resulting in recognition of excess tax benefits against income tax expense rather than additional paid-in capital of $8.9 million in the year ended September 30, 2017. As a result of the adoption, we did not record an adjustment to retained earnings as we did not have net operating loss carryforwards attributable to excess tax benefits on stock compensation that had not been previously recognized to additional paid-in capital. Excess tax benefits for share-based payments are now included as net operating activities rather than net financing activities in the Statements of Consolidated Cash Flows. The changes have been applied prospectively in accordance with the ASU and prior periods have not been adjusted. Cash paid by an employer when directly withholding shares for tax withholding purposes will continue to be classified as financing activities. We elected not to change our accounting policy for forfeitures. The threshold to qualify for equity classification permits withholding up to the maximum statutory tax rates in the applicable jurisdictions.
Stock Options
Stock options granted by our Compensation Committee of our Board under the Stock Incentive Plan are non-qualified stock options. These awards are generally granted with exercise prices equal to the average of the high and low prices of our common stock on the date of grant. They vest in equal annual installments over a three or four-year period and the maximum contractual term is ten years. We use a Binomial option-pricing model to estimate the fair value of stock options, and compensation cost is recognized on a straight-line basis over the requisite service period.
63
The following table sets forth the weighted average fair value per share of stock options and the related valuation assumptions used in the determination of those fair values:
| Year Ended September 30 | ||||||
| 2017 | 2016 | 2015 | ||||
| Weighted average fair value per share | $15.05 | $14.07 | $12.83 | |||
| Valuation assumptions: | ||||||
| Risk-free interest rate | 1.7% | 1.6% | 1.6% | |||
| Expected dividend yield | 1.3% | 1.2% | 1.4% | |||
| Expected volatility | 33.2% | 33.1% | 35.0% | |||
| Weighted average expected life (years) | 4.9 | 4.9 | 4.9 | |||
The risk-free interest rate is based upon observed U.S. Treasury interest rates appropriate for the term of our employee stock options. Expected dividend yield is based on the history and our expectation of dividend payouts. Expected volatility was based on our historical stock price volatility. Expected life represents the weighted average period the stock options are expected to remain outstanding and is a derived output of the Binomial model. The expected life of employee stock options is impacted by the above assumptions as well as the post-vesting forfeiture rate and the exercise factor used in the Binomial model. These two variables are based on the history of exercises and forfeitures for previous stock options granted by us.
The following table summarizes transactions under our stock option plans for fiscal year 2017:
| Weighted Average Number of Shares (in thousands) | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value (1) (in millions) | ||||||||||
| Balance Outstanding at October 1, 2016 | 2,006 | $ | 37.31 | ||||||||||
| Granted | 353 | 53.81 | |||||||||||
| Exercised | (556 | ) | 31.94 | ||||||||||
| Cancelled/Forfeited | (98 | ) | 49.28 | ||||||||||
| Balance Outstanding at September 30, 2017 | 1,705 | $ | 41.79 | 6.2 | $ | 54.9 | |||||||
| Exercisable at September 30, 2017 | 981 | $ | 35.29 | 4.6 | $ | 38.0 | |||||||
| Options Expected to Vest | 623 | $ | 50.36 | 8.2 | $ | 14.7 | |||||||
| (1) | The aggregate intrinsic value represents the total pre-tax intrinsic value, based on our closing stock price of $74.00, as reported by the New York Stock Exchange on September 30, 2017. This amount, which changes continuously based on the fair value of our common stock, would have been received by the option holders had all option holders exercised their options as of the balance sheet date. |
The total intrinsic value of options exercised during fiscal years 2017, 2016 and 2015 was $19.4 million, $4.0 million and $6.3 million, respectively.
As of September 30, 2017, there was $5.2 million of unrecognized compensation expense related to stock options granted under the Stock Incentive Plan. This unrecognized compensation expense does not reflect a reduction for our estimate of potential forfeitures, and is expected to be recognized over a weighted average period of 2.1 years.
Restricted Stock Units
RSUs are granted to certain employees with fair values equal to the average of the high and low prices of our common stock on the date of grant, multiplied by the number of units granted. RSU grants are contingent upon continued employment and vest over periods ranging from one to four years. Dividends, payable in common stock equivalents, accrue on the grants and are subject to the same specified terms as the original grants, including the risk of forfeiture.
64
The following table summarizes transactions for our nonvested RSUs for fiscal year 2017:
Number of Share Units (in thousands) | Weighted Average Grant Date Fair Value | |||||
| Nonvested RSUs at October 1, 2016 | 556 | $ | 46.32 | |||
| Granted | 255 | 57.47 | ||||
| Vested | (241) | 45.86 | ||||
| Forfeited | (49) | 49.14 | ||||
| Nonvested RSUs at September 30, 2017 | 521 | $ | 51.39 | |||
As of September 30, 2017, there was $12.3 million of total unrecognized compensation expense related to nonvested RSUs granted under the Stock Incentive Plan. This unrecognized compensation expense does not reflect a reduction for our estimate of potential forfeitures, and is expected to be recognized over a weighted average period of 2 years. The total vest date fair value of shares that vested during fiscal years 2017, 2016 and 2015 was $14.5 million, $14.4 million and $4.3 million, respectively.
Performance Share Units
Our Compensation Committee grants PSUs to certain employees and these awards are subject to any stock dividends, stock splits, and other similar rights inuring to common stock, but unlike our RSUs are not entitled to dividend reinvestment. Vesting of the grants is contingent upon achievement of performance targets and corresponding service requirements.
The fair value of the PSUs is equal to the average of the high and low prices of our common stock on the date of grant, multiplied by the number of units granted. For PSUs with a market condition such as total shareholder return, the Monte-Carlo simulation method is used to determine fair value. The Monte-Carlo simulation is a generally accepted statistical technique used to generate a defined number of stock price paths in order to develop a reasonable estimate of the range of our and the Peer Group’s future expected stock prices.
The following table sets forth the weighted average fair value per share for PSUs and the related valuation assumptions used in the determination of those fair values. PSUs granted in fiscal 2017, 2016 and 2015 are based on company-specific performance targets, with a total shareholder return collar.
| Year Ended September 30 | ||||||
| 2017 | 2016 | 2015 | ||||
| Weighted average fair value per share | $55.95 | $50.51 | $47.82 | |||
| Valuation assumptions: | ||||||
| Risk-free interest rate | 1.2% | 1.1% | 0.9% | |||
| Expected dividend yield | 0.0% | 0.0% | 0.0% | |||
| Expected volatility | 22.6% | 22.3% | 23.5% | |||
The basis for the assumptions listed above is similar to the valuation assumptions used for stock options, as discussed previously.
The following table summarizes transactions for our nonvested PSUs for fiscal 2017:
Number of Share Units (in thousands) | Weighted Average Grant Date Fair Value | |||||
| Nonvested PSUs as of October 1, 2016 | 455 | $ | 49.50 | |||
| Granted | 143 | 55.95 | ||||
| Vested | (196) | 48.46 | ||||
| Forfeited | (57) | 49.92 | ||||
| Nonvested PSUs at September 30, 2017 | 345 | $ | 53.40 | |||
65
As of September 30, 2017, there was $14.0 million of unrecognized compensation expense related to PSUs granted under the Stock Incentive Plan based on the expected achievement of certain performance targets or market conditions. This unrecognized compensation expense as of September 30, 2017 does not reflect a reduction for our estimate of potential forfeitures, and is expected to be recognized by the end of fiscal 2019. The total vest date fair value of shares that vested during fiscal 2017, 2016 and 2015 was $14.2 million, $10.2 million and $20.5 million.
Note 8. Special Charges
In connection with various organizational changes to improve our business alignment and cost structure, as well as legal settlements, we recognized special charges of $37.4 million, $39.9 million and $41.2 million for the year to date periods ended September 30, 2017, 2016 and 2015. These charges are summarized as follows:
Dispositions
During the fourth quarter of fiscal 2017, we sold our Völker business. We recorded impairment charges of $25.4 million during the third quarter, relating mainly to non-cash write-downs of long-lived assets and working capital associated with the Völker brand portfolio, and fiscal 2017 transaction related costs of approximately $3.0 million in Special charges.
During the first quarter of fiscal 2017, we sold our Architectural Products business and recorded special charges of $1.1 million, primarily related to severance.
Legal Settlements
During the fourth quarter of fiscal 2017, we entered into a confidential agreement with Stryker Corporation, resolving alleged infringement of certain Hill-Rom patents covering proprietary communications networks whereby Stryker Corporation paid the Company $15.1 million.
Integration and Business Realignment
We recently acquired Mortara and Tridien and initiated integration activities to optimize the available synergies of our combined company. Additionally, with the acquisition of Welch Allyn in September 2015, we initiated plans to realign our business structure to facilitate the integration, take full advantage of available synergies, and position our existing businesses to capitalize on opportunities for growth. We also incurred costs, including severance and benefit costs, associated with other business realignment and integration activities. During the year ended September 30, 2017, we incurred total integration and business realignment charges of approximately $7.6 million, of which $3.5 million were severance and benefit costs. These amounts compare to charges of $19.0 million, of which $14.0 million were severance and benefit costs during the year ended September 30, 2016. Additionally, we recorded special charges of $14.4 million in the fourth quarter of fiscal 2015 related to the acquisition of Welch Allyn. We continue to evaluate additional actions related to integration and business realignment and expect additional special charges to be incurred. However, it is not practicable to estimate the amount of these future expected costs until such time as the evaluations are complete.
Site Consolidation
In the third quarter of fiscal 2015, we initiated a plan to streamline our operations and simplify our supply chain by consolidating certain manufacturing and distribution operations ("Site Consolidation"). As part of this action, we have announced the closure of five sites. During the year ended September 30, 2017, we recorded total charges of $19.7 million, related to these efforts, of which $5.1 million were severance and benefit costs. During fiscal 2016, we recorded total charges related to the combined activities of $15.9 million related to these actions, including $7.2 million of severance and benefit costs. During fiscal 2015, we recorded severance and benefit charges of $2.7 million, as well as $1.8 million of other related costs.
During the second quarter of fiscal 2017, we sold our Charleston property for $6.1 million in cash proceeds and recorded a gain of $5.2 million.
Since the inception of the Site Consolidation program through September 30, 2017, we have recognized aggregate special charges of $34.9 million. We continue to evaluate our facilities footprint and expect to incur additional costs with respect to other actions in the future, however, it is not practicable to estimate the amount of these future expected costs until such time as the evaluations are complete.
2014 Global Transformation
During the second quarter of fiscal 2014, we announced a global transformation program focused on improving our cost structure. The domestic portion of this action was completed in fiscal 2015. Part of this program included reducing our European manufacturing capacity and streamlining our global operations by, among other things, executing a back office process transformation program in Europe. The restructuring in Europe is complete and, for the year to date period ended September 30,
66
2017, resulted in charges of $0.9 million for legal and professional fees, temporary labor, project management, severance and benefit costs, and other administrative functions. These amounts compare to charges of $5.1 million and $12.7 million (net of reversals) in the prior year to date periods ended 2016 and 2015. Since the inception of the 2014 global transformation program through September 30, 2017, we have recognized aggregate special charges of $43.6 million. We do not expect to incur further costs related to this action.
Pension Settlement Charge
As disclosed in Note 6 of our Consolidated Financial Statements, we offered lump sum settlements to all terminated vested participants in our domestic master defined benefit retirement plan, which resulted in a settlement charge of $9.6 million. This charge was recorded as a component of special charges in fiscal 2015.
For all accrued severance and other benefit charges described above, we record restructuring reserves within other current liabilities. The reserve activity for severance and other benefits during the year to date period ended September 30, 2017 was as follows:
| Balance at September 30, 2016 | $ | 14.7 | |
| Expenses | 9.7 | ||
| Cash Payments | (14.3 | ) | |
| Reversals | (1.1 | ) | |
| Balance at September 30, 2017 | $ | 9.0 | |
Note 9. Income Taxes
The significant components of income before income taxes and the consolidated income tax provision were as follows:
| Year Ended September 30 | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Income before income taxes: | ||||||||||||
| Domestic | $ | 129.0 | $ | 92.2 | $ | 49.2 | ||||||
| Foreign | 54.0 | 46.1 | 15.9 | |||||||||
| Total | $ | 183.0 | $ | 138.3 | $ | 65.1 | ||||||
| Income tax expense: | ||||||||||||
| Current provision | ||||||||||||
| Federal | $ | 61.6 | $ | 4.7 | $ | 35.3 | ||||||
| State | 8.6 | 2.2 | 3.6 | |||||||||
| Foreign | 13.3 | 9.1 | 1.7 | |||||||||
| Total current provision | 83.5 | 16.0 | 40.6 | |||||||||
| Deferred provision: | ||||||||||||
| Federal | (34.9 | ) | 21.8 | (18.1 | ) | |||||||
| State | 1.3 | 1.2 | (1.3 | ) | ||||||||
| Foreign | 0.8 | (23.5 | ) | (2.9 | ) | |||||||
| Total deferred provision | (32.8 | ) | (0.5 | ) | (22.3 | ) | ||||||
| Income tax expense | $ | 50.7 | $ | 15.5 | $ | 18.3 | ||||||
67
Differences between income tax expense reported for financial reporting purposes and that computed based upon the application of the statutory U.S. Federal tax rate to the reported income before income taxes were as follows:
| Year Ended September 30 | |||||||||||||||||||||
| 2017 | 2016 | 2015 | |||||||||||||||||||
| Amount | % of Pretax Income | Amount | % of Pretax Income | Amount | % of Pretax Income | ||||||||||||||||
Federal income tax (a) | $ | 64.1 | 35.0 | % | $ | 48.4 | 35.0 | % | $ | 22.8 | 35.0 | % | |||||||||
State income tax (b) | 4.1 | 2.2 | % | 2.9 | 2.1 | % | 1.6 | 2.4 | % | ||||||||||||
Foreign income tax (c) | (35.6 | ) | (19.4 | )% | (14.0 | ) | (10.1 | )% | (10.2 | ) | (15.7 | )% | |||||||||
| Application of federal research tax credits | (3.6 | ) | (2.0 | )% | (5.6 | ) | (4.0 | )% | (2.2 | ) | (3.4 | )% | |||||||||
| Application of foreign tax credits | (15.0 | ) | (8.2 | )% | (0.5 | ) | (0.4 | )% | (0.2 | ) | (0.3 | )% | |||||||||
| Adjustment of estimated income tax accruals | (0.8 | ) | (0.4 | )% | 0.3 | 0.2 | % | (1.6 | ) | (2.4 | )% | ||||||||||
| Valuation of tax attributes | 36.3 | 19.8 | % | (14.4 | ) | (10.4 | )% | 4.0 | 6.2 | % | |||||||||||
| Foreign Inclusions | 11.5 | 6.3 | % | 0.9 | 0.6 | % | 0.3 | 0.5 | % | ||||||||||||
| Domestic manufacturer's deduction | (4.4 | ) | (2.4 | )% | (1.8 | ) | (1.3 | )% | (1.5 | ) | (2.3 | )% | |||||||||
| Excess tax benefits from share based awards | (8.9 | ) | (4.9 | )% | — | — | % | — | — | % | |||||||||||
| Capitalized transaction costs | — | — | % | — | — | % | 2.5 | 3.8 | % | ||||||||||||
| Other, net | 3.0 | 1.7 | % | (0.7 | ) | (0.5 | )% | 2.8 | 4.3 | % | |||||||||||
| Income tax expense | $ | 50.7 | 27.7 | % | $ | 15.5 | 11.2 | % | $ | 18.3 | 28.1 | % | |||||||||
| (a) | At statutory rate. |
| (b) | Net of Federal benefit. |
| (c) | Federal tax rate differential. |
The tax effect of temporary differences that gave rise to the deferred tax balance sheet accounts were as follows:
| Year Ended September 30 | ||||||||
| 2017 | 2016 | |||||||
| Deferred tax assets: | ||||||||
| Employee benefit accruals | $ | 64.5 | $ | 76.5 | ||||
| Inventory | 16.6 | 13.9 | ||||||
| Net operating loss carryforwards | 70.7 | 47.1 | ||||||
| Tax credit carryforwards | 23.3 | 14.2 | ||||||
| Other, net | 41.4 | 46.4 | ||||||
| 216.5 | 198.1 | |||||||
| Less: Valuation allowance | (58.2 | ) | (26.9 | ) | ||||
| Total deferred tax assets | 158.3 | 171.2 | ||||||
| Deferred tax liabilities: | ||||||||
| Depreciation | (28.6 | ) | (41.6 | ) | ||||
| Amortization | (349.7 | ) | (371.2 | ) | ||||
| Other, net | (5.3 | ) | (3.1 | ) | ||||
| Total deferred tax liabilities | (383.6 | ) | (415.9 | ) | ||||
| Deferred tax asset (liability) - net | $ | (225.3 | ) | $ | (244.7 | ) | ||
At September 30, 2017, we had $68.6 million of deferred tax assets related to operating loss carryforwards in foreign jurisdictions that are subject to various carryforward periods with the majority eligible to be carried forward for an unlimited period. Additionally, we had $1.8 million of deferred tax assets related to federal net operating loss carryforwards which will expire between 2019 and 2033 and $0.3 million of deferred tax assets related to state net operating loss carryforwards, which expire between 2018 and 2035. We had $15.0 million of deferred tax assets related to state tax credits, some of which will be carried forward for an unlimited
68
period and some of which will expire between 2018 and 2031. Additionally, we had $8.3 million of deferred tax assets related to foreign tax credits, which will expire between 2026 and 2027.We had $3.8 million of deferred tax assets related to capital loss carryforwards, which will expire in 2021.
The gross deferred tax assets as of September 30, 2017 were reduced by valuation allowances of $58.2 million primarily related to certain foreign deferred tax attributes and state tax credit carryforwards as it is more likely than not that some portion or all of these tax attributes will not be realized. In evaluating whether it is more likely than not that we would recover our deferred tax assets, future taxable income, the reversal of existing temporary differences and tax planning strategies were considered. We believe that our estimates for the valuation allowances recorded against deferred tax assets are appropriate based on current facts and circumstances. In connection with our integration of recent acquisitions, in 2017 the Company completed a restructuring to further align its capital and debt structure. As a result, non-US NOLs were generated for which a full valuation allowance was recorded. This was the primary driver of the increase in the valuation allowance from the prior year end.
We operate under tax holidays in both Singapore and Puerto Rico. The Singapore tax holiday is effective through 2018 while the Puerto Rico tax holiday is effective through 2025. Both incentives are conditional on meeting certain employment and/or investment thresholds. The impact of these tax holidays decreased foreign taxes by $3.6 million in fiscal 2017, $4.1 million for fiscal 2016 and $4.3 million for fiscal 2015. The benefit of the tax holidays on net income per share (diluted) was $0.05, $0.06 and $0.07 for fiscal 2017, 2016 and 2015, respectively.
With regard to our non-U.S. subsidiaries, it is our practice and intention to reinvest the earnings in those businesses, to fund capital expenditures and other operating cash needs. Because the undistributed earnings of non-U.S. subsidiaries are considered to be permanently reinvested, no U.S. deferred income taxes or foreign withholding taxes have been provided. As of September 30, 2017, we have approximately $335.9 million of undistributed earnings in our non-U.S. subsidiaries that are considered to be permanently reinvested. If such earnings were repatriated, additional tax expense may result. It is not practicable to estimate the amount of deferred tax liability related to these undistributed earnings due to the assumptions necessary to compute the tax.
We file a consolidated federal income tax return as well as multiple state, local and foreign jurisdiction tax returns. In the normal course of business, we are subject to examination by the taxing authorities in each of the jurisdictions where we file tax returns. During fiscal 2017, the Internal Revenue Service ("IRS") concluded its audit for fiscal year 2015 and initiated its post-filing examination of the fiscal 2016 consolidated federal return. We continue to participate in the IRS Compliance Assurance Program ("CAP") for fiscal year 2017 and 2018 and have submitted the application to remain in the CAP for fiscal year 2019. The CAP provides the opportunity for the IRS to review certain tax matters prior to us filing our tax return for the year, thereby reducing the time it takes to complete the post-filing examination. We are also subject to state and local or foreign income tax examinations by taxing authorities for years back to fiscal 2013.
We also have on-going audits in various stages of completion in several state and foreign jurisdictions, one or more of which may conclude within the next 12 months. Such settlements could involve some or all of the following: the payment of additional taxes, the adjustment of certain deferred taxes and/or the recognition of unrecognized tax benefits. The resolution of these matters, in combination with the expiration of certain statutes of limitations in various jurisdictions, make it reasonably possible that our unrecognized tax benefits may decrease as a result of either payment or recognition by approximately $0.5 million to $1.0 million in the next twelve months, excluding interest.
The total amount of gross unrecognized tax benefits as of September 30, 2017, 2016 and 2015 was $4.5 million, $5.1 million and $5.8 million, which includes $3.3 million, $3.6 million and $3.3 million that, if recognized, would impact the effective tax rate in future periods. The remaining amount relates to items which, if recognized, would not impact our effective tax rate.
69
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Year Ended September 30 | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Balance at October 1 | 5.1 | 5.8 | 4.1 | |||||||||
| Increases in tax position of prior years | 0.1 | 0.8 | 0.4 | |||||||||
| Decreases in tax position of prior years | — | (0.1 | ) | (1.3 | ) | |||||||
| Settlements with taxing authorities | — | (0.3 | ) | (1.2 | ) | |||||||
| Lapse of applicable statute of limitations | (0.8 | ) | (0.5 | ) | (1.3 | ) | ||||||
| Change in positions due to acquisitions | — | (0.6 | ) | 5.5 | ||||||||
| Foreign currency adjustments | 0.1 | — | (0.4 | ) | ||||||||
| Total change | (0.6 | ) | (0.7 | ) | 1.7 | |||||||
| Balance at September 30 | $ | 4.5 | $ | 5.1 | $ | 5.8 | ||||||
We recognize accrued interest and penalties related to unrecognized tax benefits as a component of income tax expense. Accrued interest and penalties, which are not presented in the reconciliation table above, were $2.6 million, $3.0 million and $3.0 million at September 30, 2017, 2016 and 2015, respectively. Related to interest and penalties, we recognized an income tax benefit of $0.4 million in 2017 and $0.2 million in 2015. There was no income tax impact of interest or penalties in 2016.
Note 10. Earnings per Common Share
Basic earnings per share is calculated based upon the weighted average number of outstanding common shares for the period, plus the effect of deferred vested shares. Diluted earnings per share is calculated consistent with the basic earnings per share calculation plus the effect of dilutive unissued common shares related to stock-based employee compensation programs. For all periods presented, anti-dilutive stock options were excluded from the calculation of diluted earnings per share. Cumulative treasury stock acquired, less cumulative shares reissued, have been excluded in determining the average number of shares outstanding.
Earnings per share are calculated as follows (share information in thousands):
| Year Ended September 30 | |||||||||||
| 2017 | 2016 | 2015 | |||||||||
| Net income attributable to common shareholders | $ | 133.6 | $ | 124.1 | $ | 47.7 | |||||
| Average shares outstanding - Basic | 65,599 | 65,333 | 57,249 | ||||||||
| Add potential effect of exercise of stock options and other unvested equity awards | 1,626 | 1,263 | 1,287 | ||||||||
| Average shares outstanding - Diluted | 67,225 | 66,596 | 58,536 | ||||||||
| Net income attributable to common shareholders per common share - Basic | $ | 2.04 | $ | 1.90 | $ | 0.83 | |||||
| Net income attributable to common shareholders per common share - Diluted | $ | 1.99 | $ | 1.86 | $ | 0.82 | |||||
Note 11. Segment Reporting
We disclose segment information that is consistent with the way in which management operates and views the business. During our first quarter of fiscal 2017, we changed our segment reporting to reflect changes in our organizational structure and management’s operation and view of the business. We combined the prior year North America Patient Support Systems segment and International Patient Support Systems segment into a new segment called Patient Support Systems. Our Patient Support Systems segment now also includes an additional component of global marketing spend that was previously unallocated. The prior year segment information included in this Form 10-K has been updated to reflect these changes. Our revised operating structure contains the following reporting segments:
70
| • | Patient Support Systems – globally provides our specialty bed frames and surfaces and mobility solutions, as well as our clinical workflow solutions which specializes in software and information technologies to improve care and deliver actionable insight to caregivers and patients. |
| • | Front Line Care – globally provides respiratory care products, and sells medical diagnostic monitoring equipment and a diversified portfolio of physical assessment tools that assess, diagnose, treat, and manage a wide variety of illnesses and diseases. |
| • | Surgical Solutions – globally provides products that improve surgical safety and efficiency in the operating room including tables, lights, pendants, positioning devices and various other surgical products and accessories. |
Under our revised segments, our performance within each reportable segment continues to be measured on a divisional income basis before non-allocated operating and administrative costs, litigation, special charges, acquisition and integration costs, acquisition-related intangible asset amortization, and other unusual events. Divisional income generally represents the division’s gross profit less its direct operating costs along with an allocation of manufacturing and distribution costs, research and development and certain corporate functional expenses.
Non-allocated operating costs, administrative costs, and other includes functional expenses that support the entire organization such as administration, finance, legal and human resources, expenses associated with strategic developments, acquisition-related intangible asset amortization, and other events that are not indicative of operating trends. We exclude such amounts from divisional income to allow management to evaluate and understand divisional operating trends. The chief operating decision maker does not receive any asset information by operating segment and, accordingly, we do not report asset information by operating segment.
| Year Ended September 30 | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Revenue: | ||||||||||||
| Patient Support Systems | $ | 1,423.9 | $ | 1,437.2 | $ | 1,426.6 | ||||||
| Front Line Care | 885.3 | 809.7 | 139.0 | |||||||||
| Surgical Solutions | 434.5 | 408.3 | 422.6 | |||||||||
| Total revenue | $ | 2,743.7 | $ | 2,655.2 | $ | 1,988.2 | ||||||
| Divisional income: | ||||||||||||
| Patient Support Systems | $ | 249.6 | $ | 245.2 | $ | 206.5 | ||||||
| Front Line Care | 231.8 | 202.1 | 41.5 | |||||||||
| Surgical Solutions | 42.5 | 46.2 | 56.0 | |||||||||
| Other operating costs: | ||||||||||||
| Non-allocated operating costs, administrative costs, and other | 213.1 | 223.3 | 179.7 | |||||||||
| Special charges | 37.4 | 39.9 | 41.2 | |||||||||
| Operating profit | 273.4 | 230.3 | 83.1 | |||||||||
| Interest expense | (88.9 | ) | (90.4 | ) | (18.4 | ) | ||||||
| Loss on extinguishment of debt | — | (10.8 | ) | — | ||||||||
| Investment income and other, net | (1.5 | ) | 9.2 | 0.4 | ||||||||
| Income before income taxes | $ | 183.0 | $ | 138.3 | $ | 65.1 | ||||||
71
Geographic Information
Geographic data for net revenue and long-lived assets (which consist mainly of property and equipment leased to others) were as follows:
| Year Ended September 30 | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
Net revenue to unaffiliated customers: (a) | ||||||||||||
| United States | $ | 1,895.2 | $ | 1,829.4 | $ | 1,273.0 | ||||||
| Foreign | 848.5 | 825.8 | 715.2 | |||||||||
| Total revenue | $ | 2,743.7 | $ | 2,655.2 | $ | 1,988.2 | ||||||
Long-lived assets: (b) | ||||||||||||
| United States | $ | 243.9 | $ | 234.2 | $ | 263.9 | ||||||
| Foreign | 111.5 | 115.8 | 114.5 | |||||||||
| Total long-lived assets | $ | 355.4 | $ | 350.0 | $ | 378.4 | ||||||
| (a) | Net revenue is attributed to geographic areas based on the location of the customer. |
| (b) | Includes property and equipment leased to others. |
72
Note 12. Quarterly Financial Information (Unaudited)
The following table presents selected consolidated financial data by quarter for each of the last two fiscal years.
| 2017 Quarter Ended | December 31, 2016 | March 31, 2017 | June 30, 2017 | September 30, 2017 | ||||||||||||
| Net Revenue | $ | 637.4 | $ | 678.9 | $ | 689.1 | $ | 738.3 | ||||||||
| Gross Profit | $ | 302.6 | $ | 324.4 | $ | 331.1 | $ | 362.5 | ||||||||
| Net Income Attributable to Common Shareholders | $ | 23.8 | $ | 34.4 | $ | 6.0 | $ | 69.4 | ||||||||
Net Income Attributable to Common Shareholders per Common Share - Basic | $ | 0.36 | $ | 0.52 | $ | 0.09 | $ | 1.06 | ||||||||
Net Income Attributable to Common Shareholders per Common Share - Diluted | $ | 0.36 | $ | 0.51 | $ | 0.09 | $ | 1.03 | ||||||||
| 2016 Quarter Ended | December 31, 2015 | March 31, 2016 | June 30, 2016 | September 30, 2016 | ||||||||||||
| Net Revenue | $ | 661.2 | $ | 632.6 | $ | 655.4 | $ | 706.0 | ||||||||
| Gross Profit | $ | 290.7 | $ | 304.2 | $ | 315.4 | $ | 346.7 | ||||||||
| Net Income Attributable to Common Shareholders | $ | 4.8 | $ | 22.3 | $ | 45.3 | $ | 51.7 | ||||||||
| Net Income Attributable to Common Shareholders per Common Share - Basic | $ | 0.07 | $ | 0.34 | $ | 0.69 | $ | 0.79 | ||||||||
| Net Income Attributable to Common Shareholders per Common Share - Diluted | $ | 0.07 | $ | 0.33 | $ | 0.68 | $ | 0.77 | ||||||||
Note 13. Commitments and Contingencies
Lease Commitments
Rental expense for fiscal years 2017, 2016 and 2015 was $30.5 million, $31.7 million and $25.2 million, respectively. The table below indicates the minimum annual rental commitments (excluding renewable periods) aggregating $105.1 million, for manufacturing facilities, warehouse distribution centers, service centers and sales offices, under non-cancelable operating leases.
| Amount | |||
| 2018 | $ | 31.7 | |
| 2019 | $ | 23.4 | |
| 2020 | $ | 15.7 | |
| 2021 | $ | 11.9 | |
| 2022 | $ | 8.8 | |
| 2023 and beyond | $ | 13.6 | |
Self Insurance
We are involved in various claims, including product and general liability, workers’ compensation, auto liability and employment related matters. Such claims in the United States have deductibles and self-insured retentions ranging from $25.0 thousand to $1.0 million per occurrence or per claim, depending upon the type of coverage and policy period. International deductibles and self-insured retentions are lower. We are also generally self-insured up to certain stop-loss limits for certain employee health benefits, including medical, drug and dental. Our policy is to estimate reserves based upon a number of factors including known claims, estimated incurred but not reported claims and outside actuarial analysis, which are based on historical information along with certain assumptions about future events. Such estimated reserves are classified as Other Current Liabilities and Other Long-Term Liabilities within the Consolidated Balance Sheets.
73
Legal Proceedings
General
We are subject to various other claims and contingencies arising out of the normal course of business, including those relating to governmental investigations and proceedings, commercial transactions, product liability, employee related matters, antitrust, safety, health, taxes, environmental and other matters. Litigation is subject to many uncertainties and the outcome of individual litigated matters is not predictable with assurance. It is possible that some litigation matters for which reserves have not been established could be decided unfavorably to us, and that any such unfavorable decisions could have a material adverse effect on our financial condition, results of operations and cash flows.
Universal Hospital Services, Inc. Litigation
On January 13, 2015, Universal Hospital Services, Inc. filed a complaint against us in the United States District Court for the Western District of Texas. The plaintiff alleges, among other things, that we engaged in certain customer contracting practices in violation of state and federal antitrust laws. The plaintiff also has asserted claims for tortious interference with business relationships. The plaintiff seeks injunctive relief and money damages in an unspecified amount. No trial date has been set. We believe that the allegations are without merit and intend to defend this matter vigorously.
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| Item 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Our management, with the supervision and participation of our President and Chief Executive Officer and our Senior Vice President and Chief Financial Officer (the "Certifying Officers"), has evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of September 30, 2017. Our disclosure controls and procedures are designed to ensure that information required to be disclosed in the reports we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and such information is accumulated and communicated to management, including our Certifying Officers and our Board, as appropriate to allow timely decisions regarding required disclosure.
Based upon that evaluation, the Certifying Officers concluded that our disclosure controls and procedures were effective as of September 30, 2017.
Management’s Report on Internal Control Over Financial Reporting
The report of management’s assessment of the effectiveness of our internal control over financial reporting as of September 30, 2017 and the related report of our independent registered public accounting firm, are included under Part II, Item 8 of this Form 10-K. We have excluded Mortara from our assessment of internal control over financial reporting as of September 30, 2017, because Mortara was acquired by us in a purchase business combination in the second quarter of 2017. Mortara is a wholly-owned subsidiary whose total assets and total revenues represent approximately 2.0% and 2.5%, respectively, of the related consolidated financial statement amounts as of and for the year ended September 30, 2017. We are currently in the process of evaluating and integrating Mortara’s historical internal control over financial reporting structure with ours. We expect to complete this integration in fiscal 2018.
Changes in Internal Control Over Financial Reporting
There have been no changes to our internal controls over financial reporting. Management’s report on our internal control over financial reporting is included under Part II, Item 8 of this Form 10-K.
| Item 9B. | OTHER INFORMATION |
None.
74
PART III
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this Item is incorporated herein by reference to our Proxy Statement to be filed with the SEC in January 2018 relating to our 2018 Annual Meeting of Shareholders (the "2018 Proxy Statement"), under the headings "Election of Directors", "Section 16(a) Beneficial Ownership Reporting Compliance", and "Corporate Governance." Information relating to our executive officers is included in this Form 10-K in Part I, Item 1 under the caption "Executive Officers."
| Item 11. | EXECUTIVE COMPENSATION |
The information required by this Item is incorporated herein by reference to the 2018 Proxy Statement, under the heading "Executive Compensation."
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this Item is incorporated herein by reference to the 2018 Proxy Statement, under the headings "Security Ownership of Certain Beneficial Owners and Management" and "Equity Compensation Plan Information."
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE |
The information required by this Item is incorporated herein by reference to the 2018 Proxy Statement, where such information is included under the heading "Corporate Governance."
| Item 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by this Item is incorporated herein by reference to the 2018 Proxy Statement, where such information is included under the heading "Proposals Requiring Your Vote - Ratification of Appointment of Independent Registered Public Accounting Firm."
75
PART IV
| Item 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| (a) | The following documents have been filed as a part of this Form 10-K or, where noted, incorporated by reference: |
| (1) | Financial Statements |
The financial statements of the Company and its consolidated subsidiaries are listed under Part II, Item 8 on the Index to Consolidated Financial Statements.
| (2) | Financial Statement Schedules |
The financial statement schedule filed in response to Part II, Item 8 and Part IV, Item 15(c) of Form 10-K is listed under Part II, Item 8 on the Index to Consolidated Financial Statements.
| (3) | Exhibits (See changes to Exhibit Index below): |
"The Exhibit Index, which follows the signature page to this Form 10-K and is hereby incorporated herein by reference, sets forth a list of those exhibits filed herewith, and includes and identifies management contracts or compensatory plans or arrangements required to be filed as exhibits to this Form 10-K by Item 601 (b)(10)(iii) of Regulation S-K."
The agreements included as exhibits to this Form 10-K are intended to provide information regarding their terms and not to provide any other factual or disclosure information about us or the other parties to the agreements. The agreements may contain representations and warranties by the parties to the agreements, including us, solely for the benefit of the other parties to the applicable agreement. Such representation and warranties:
| • | should not in all instances be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate; |
| • | may have been qualified by disclosures that were made to the other party in connection with the negotiation of the applicable agreement, which disclosures are not necessarily reflected in the agreement; |
| • | may apply standards of materiality in a way that is different from what may be viewed as material to certain investors; and |
| • | were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement and are subject to more recent developments. |
Accordingly, these representations and warranties may not describe the actual state of affairs as of the date they were made or at any other time.
76
SCHEDULE II
HILL-ROM HOLDINGS, INC. AND SUBSIDIARIES
Valuation and Qualifying Accounts
For The Fiscal Years Ended September 30, 2017, 2016 and 2015
(Dollars in millions)
| ADDITIONS | ||||||||||||||||||||||||
| DESCRIPTION | BALANCE AT BEGINNING OF PERIOD | CHARGED TO COSTS AND EXPENSES | CHARGED TO OTHER ACCOUNTS | DEDUCTIONS NET OF RECOVERIES | BALANCE AT END OF PERIOD | |||||||||||||||||||
| Reserves deducted from assets to which they apply: | ||||||||||||||||||||||||
| Allowance for possible losses and sales returns - accounts receivable: | ||||||||||||||||||||||||
| Period Ended: | ||||||||||||||||||||||||
| September 30, 2017 | $ | 26.8 | $ | 4.3 | $ | 2.0 | (a) | $ | (8.0 | ) | (b) | $ | 25.1 | |||||||||||
| September 30, 2016 | $ | 26.0 | $ | 2.1 | $ | 2.2 | (a) | $ | (3.5 | ) | (b) | $ | 26.8 | |||||||||||
| September 30, 2015 | $ | 31.4 | $ | 1.8 | $ | 0.1 | (a) | $ | (7.3 | ) | (b) | $ | 26.0 | |||||||||||
| Valuation allowance against deferred tax assets: | ||||||||||||||||||||||||
| Period Ended: | ||||||||||||||||||||||||
| September 30, 2017 | $ | 26.9 | $ | 30.8 | $ | — | (c) | $ | 0.5 | (d) | $ | 58.2 | ||||||||||||
| September 30, 2016 | $ | 40.7 | $ | (14.9 | ) | $ | — | (c) | $ | 1.1 | (d) | $ | 26.9 | |||||||||||
| September 30, 2015 | $ | 28.3 | $ | 4.0 | $ | 11.1 | $ | (2.7 | ) | (d) | $ | 40.7 | ||||||||||||
| (a) | Reduction of gross revenue for uncollectible health care rental reimbursements, cash discounts and other adjustments in determining net revenue. Also includes the effect of acquired businesses, if any. |
| (b) | Generally reflects the write-off of specific receivables against recorded reserves. |
| (c) | Generally reflects the effect of acquired businesses, if any. |
| (d) | Primarily reflects write-offs of deferred tax assets against the valuation allowance. |
77
HILL-ROM HOLDINGS, INC.
INDEX TO EXHIBITS
Management contracts and compensatory plans or arrangements are designated with "*".
| Agreement and Plan of Merger dated June 16, 2015 by and among Hill-Rom Holdings, Inc., Empire Merger Sub Corp., and Welch Allyn Holdings, Inc. (Incorporated herein by reference to Exhibit 2.1 filed with the Company’s Form 8-K dated June 17, 2015) | |
| Share Purchase and Transfer Agreement dated as of June 13, 2014 by and among TRUMPF International Beteiligungs-GmbH, Hill-Rom Holdings Netherlands B.V., HR Europe B.V. and Hill-Rom Holdings, Inc. (Incorporated herein by reference to Exhibit 1.1 filed with the Company’s Form 8-K dated June 16, 2014) | |
| Restated and Amended Articles of Incorporation of Hill-Rom Holdings, Inc., as currently in effect (Incorporated herein by reference to Exhibit 3.1 filed with the Company’s Form 8-K dated March 10, 2010) | |
| Amended and Restated Code of By-Laws of Hill-Rom Holdings, Inc., as currently in effect (Incorporated herein by reference to Exhibit 3.2 filed with the Company’s Form 8-K dated March 10, 2010) | |
| 4.1 | Indenture dated as of December 1, 1991, between Hill-Rom Holdings, Inc. and Union Bank, N.A. (as successor to LaSalle Bank National Association and Harris Trust and Savings Bank) as Trustee (Incorporated herein by reference to Exhibit (4) (a) to Registration Statement on Form S-3, Registration No. 33-44086) |
| Indenture dated as of September 1, 2015, between Hill-Rom Holdings, Inc. and MUFG Union Bank, N.A., as Trustee (Incorporated herein by reference to Exhibit 4.1 to the Company’s Form 8-K dated September 1, 2015) | |
| First Supplemental Indenture dated September 8, 2015, among Hill-Rom Holdings, Inc., the guarantors party thereto, and MUFG Union Bank, N.A., as Trustee (Incorporated herein by reference to Exhibit 4.3 to the Company's Form 10-K dated November 17, 2016) | |
| Second Supplemental Indenture dated as of September 29, 2016, among Hill-Rom Holdings, Inc., the guarantors party thereto, and MUFG Union Bank, N.A., as Trustee (Incorporated herein by reference to Exhibit 4.4 to the Company's Form 10-K dated November 17, 2016) | |
| Third Supplemental Indenture dated May 12, 2017, among Hill-Rom Holdings, Inc., the guarantors party thereto, and MUFG Union Bank, N.A., as Trustee | |
| Indenture dated as of February 14, 2017, between Hill-Rom Holdings, Inc., the guarantors party thereto, and MUFG Union Bank, N.A., as Trustee (Incorporated herein by reference to Exhibit 4.1 to the Company's Form 8-K dated February 14, 2017) | |
| First Supplemental Indenture dated May 12, 2017, among Hill-Rom Holdings, Inc., the guarantors party thereto, and MUFG Union Bank, N.A., as Trustee | |
| Hill-Rom Holdings, Inc. Amended and Restated Short Term Incentive Compensation Program (Incorporated herein by reference to Exhibit 10.1 filed with the Company’s Form 10-K dated November 24, 2009) | |
| Form of Director Indemnity Agreement (Incorporated herein by reference to Exhibit 10.6 filed with the Company’s Form 10-K dated December 23, 2003) | |
| Form of Indemnity Agreement between Hill-Rom Holdings, Inc. and certain executive officers (Incorporated herein by reference to Exhibit 10.6 filed with the Company’s Form 10-K dated November 16, 2011) | |
78
| Hill-Rom Holdings, Inc. Board of Directors’ Deferred Compensation Plan (Incorporated herein by reference to Exhibit 10.10 filed with the Company’s Form 10-Q dated July 13, 2001) | |
| Hill-Rom Holdings, Inc. Director Phantom Stock Plan and form of award (Incorporated herein by reference to Exhibit 10.11 filed with the Company’s Form 10-Q dated July 13, 2001) | |
| Amended and Restated Hill-Rom Holdings, Inc. Stock Incentive Plan, as currently in effect (Incorporated herein by reference to Exhibit 10.30 filed with the Company’s Form 10-K dated November 24, 2009) | |
| Hill-Rom Holdings, Inc. Employee Stock Purchase Plan (Incorporated herein by reference to Appendix I to the Company’s definitive Proxy Statement on Schedule 14A dated January 7, 2009) | |
| Employment Agreement dated January 6, 2010 between Hill-Rom Holdings, Inc. and John J. Greisch (Incorporated herein by reference to Exhibit 10.1 filed with the Company’s Form 8-K dated January 7, 2010) | |
| Form of Change in Control Agreement between Hill-Rom Holdings, Inc. and certain of its officers, including Named Executive Officers (other than the CEO) (Incorporated herein by reference to Exhibit 10.58 filed with the Company’s Form 10-K dated November 17, 2010) | |
| Amended Change in Control Agreement between Hill-Rom Holdings, Inc. and John J. Greisch dated September 30, 2010 (Incorporated herein by reference to Exhibit 10.59 filed with the Company’s Form 10-K dated November 17, 2010) | |
| Hill-Rom Holdings, Inc. Short-Term Incentive Plan (Incorporated herein by reference to Appendix 1 to the Hill-Rom Holdings, Inc. Definitive Proxy Statement on Schedule 14A dated January 18, 2011) | |
| Hill-Rom Holdings, Inc. Amended and Restated Supplemental Executive Retirement Plan (Incorporated herein by reference to Exhibit 10.69 filed with the Company’s Form 10-K dated November 16, 2011) | |
| Employment Agreement between Hill-Rom Holdings, Inc. and Alton Shader, dated July 11, 2011 (Incorporated herein by reference to Exhibit 10.2 filed with the Company’s Form 10-Q dated July 28, 2011) | |
| Employment Agreement between Hill-Rom Holdings, Inc. and Andreas Frank, dated October 3, 2011 (Incorporated herein by reference to Exhibit 10.72 filed with the Company’s Form 10-K dated November 16, 2011) | |
| Employment Agreement between Hill-Rom Holdings, Inc. and Steven Strobel, dated October 23, 2014 (Incorporated herein by reference to Exhibit 10.1 filed with the Company’s Form 8-K dated October 27, 2014) | |
| Form of Limited Recapture Agreement between Hill-Rom Holdings, Inc. and certain of its officers, including Named Executive Officers (Incorporated herein by reference to Exhibit 10.34 filed with the Company’s Form 10-K dated November 20, 2013) | |
| Employment Agreement between Hill-Rom Holdings, Inc. and Carlos Alonso-Marum dated March 19, 2015 (Incorporated herein by reference to Exhibit 10.2 to the Company’s Form 10-Q dated August 7, 2015) | |
| Employment Agreement between Hill-Rom Holdings, Inc. and Kenneth Meyers dated September 23, 2015 (Incorporated herein by reference to Exhibit 10.29 filed with the Company’s Form 10-K dated November 19, 2015) | |
| Employment Agreement between Hill-Rom Holdings, Inc. and Taylor Smith dated November 11, 2013 (Incorporated herein by reference to Exhibit 10.30 filed with the Company’s Form 10-K dated November 19, 2015) | |
| FY 2016 Non-Employee Director Compensation Policy (Incorporated herein by reference to Exhibit 10.31 filed with the Company’s Form 10-K dated November 19, 2015) | |
79
| Employment Agreement between Hill-Rom Holdings, Inc. and Deborah Rasin dated November 6, 2015 (Incorporated herein by reference to Exhibit 10.1 to the Company’s Form 10-Q dated February 1, 2016) | |
| Letter Agreement between Hill-Rom Holdings, Inc. and Jason Richardson (Incorporated herein by reference to Exhibit 10.1 filed with the Company’s Form 8-K dated March 16, 2016) | |
| Form of Indemnity Agreement between Hill-Rom Holdings, Inc. and certain executive officers (Incorporated herein by reference to Exhibit 10.9 filed with the Company’s Form 10-K dated December 23, 2003) | |
| Employment Agreement between Hill-Rom Holdings, Inc. and Dirk Ehlers (Incorporated herein by reference to Exhibit 10.3 to the Company’s Form 10-Q dated April 29, 2016) | |
| Amended and Restated Credit Agreement, dated as of September 21, 2016, among Hill-Rom Holdings, Inc., JP Morgan Chase Bank, N.A., as Administrative Agent and Collateral Agent, and the lenders party thereto (Incorporated herein by reference to Exhibit 10.1 to the Company’s Form 8-K dated September 22, 2016) | |
| Loan and Security Agreement dated May 5, 2017, among Hill-Rom Finance Company LLC, as Borrower, the persons from time to time party hereto, as lenders and as Group Agents, The Bank of Tokyo-Mitsubishi UFJ, Ltd., as Administrative Agent, and Hill-Rom Company, Inc., as initial Servicer (Incorporated herein by reference to Exhibit 10.1 to the Company's Form 8-K dated May 5, 2017) | |
| Purchase and Sale Agreement dated May 5, 2017, among Hill-Rom Company, Inc., as an originator and as servicer, other originators from time to time party hereto, as originators, and Hill-Rom Finance Company LLC, as Buyer (Incorporated herein by reference to Exhibit 10.2 to the Company's Form 8-K dated May 5, 2017) | |
| Performance Guaranty dated May 5, 2017, between Hill-Rom Holdings, Inc., the Bank of Tokyo-Mitsubishi UFJ, Ltd., as administrative agent, for and on behalf of the Credit Parties and other Secured Parties from time to time under the Loan and Security Agreement, dated as of the date hereof, among Hill-Rom Finance Company LLC, Hill-Rom Company, Inc., as initial servicer, the Administrative Agent and BTMU (Incorporated herein by reference to Exhibit 10.3 to the Company's Form 8-K dated May 5, 2017) | |
| Employment Agreement between HR Europe B.V. and Francisco Canal Vega (Incorporated herein by reference to Exhibit 10.1 to the Company's Form 10-Q dated July 28, 2017) | |
| Form of Non-Qualified Stock Option Agreement for employees hired prior to August 1, 2016, under the Amended and Restated Hill-Rom Holdings, Inc.'s Stock Incentive Plan | |
| Form of Non-Qualified Stock Option Agreement for employees hired on and after August 1, 2016, under the Amended and Restated Hill-Rom Holdings, Inc.'s Stock Incentive Plan | |
| Form of Non-Qualified Stock Option Agreement (CEO version), under the Amended and Restated Hill-Rom Holdings, Inc.'s Stock Incentive Plan | |
| Form of Restricted Stock Unit Award Agreement for employees hired prior to August 1, 2016, under the Amended and Restated Hill-Rom Holdings, Inc.'s Stock Incentive Plan | |
| Form of Restricted Stock Unit Award Agreement for employees hired on and after August 1, 2016, under the Amended and Restated Hill-Rom Holdings, Inc.'s Stock Incentive Plan | |
| Form of Restricted Stock Unit Award Agreement (CEO version), under the Amended and Restated Hill-Rom Holdings, Inc.'s Stock Incentive Plan | |
80
| Form of Performance-Based Restricted Stock Unit Award Agreement for employees hired prior to August 1, 2016, under the Amended and Restated Hill-Rom Holdings, Inc.'s Stock Incentive Plan | |
| Form of Performance-Based Restricted Stock Unit Award Agreement for employees hired on and after August 1, 2016, under the Amended and Restated Hill-Rom Holdings, Inc.'s Stock Incentive Plan | |
| Form of Performance-Based Restricted Stock Unit Award Agreement (CEO version), under the Amended and Restated Hill-Rom Holdings, Inc.'s Stock Incentive Plan | |
| Subsidiaries of the Registrant | |
| Consent of Independent Registered Public Accounting Firm | |
| Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| Certification of Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| Certification of Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101.INS | XBRL Instance Document |
| 101.SCH | XBRL Taxonomy Extension Schema Document |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document |
| 101.LAB | XBRL Extension Labels Linkbase Document |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
81
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| HILL-ROM HOLDINGS, INC. | ||
| By: | /s/ John J. Greisch | |
| John J. Greisch | ||
| President and Chief Executive Officer | ||
Date: November 17, 2017
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the date indicated.
| /s/ | Rolf A. Classon | /s/ | James R. Giertz | |
| Rolf A. Classon | James R. Giertz | |||
| Chairman of the Board | Director | |||
| /s/ | John J. Greisch | /s/ | Charles E. Golden | |
| John J. Greisch | Charles E. Golden | |||
| President and Chief Executive Officer and Director | Director | |||
| (Principal Executive Officer) | ||||
| /s/ | Steven J. Strobel | /s/ | William H. Kucheman | |
| Steven J. Strobel | William H. Kucheman | |||
| Senior Vice President and Chief Financial Officer | Director | |||
| (Principal Financial Officer) | ||||
| /s/ | Jason A. Richardson | /s/ | Ronald A. Malone | |
| Jason A. Richardson | Ronald A. Malone | |||
| Vice President — Controller and | Director | |||
| Chief Accounting Officer | ||||
| (Principal Accounting Officer) | ||||
| /s/ | William G. Dempsey | /s/ | Nancy M. Schlichting | |
| William G. Dempsey | Nancy M. Schlichting | |||
| Director | Director | |||
| /s/ | Gary L. Ellis | /s/ | Stacy Enxing Seng | |
| Gary L. Ellis | Stacy Enxing Seng | |||
| Director | Director | |||
| /s/ | Mary Garrett | |||
| Mary Garrett | ||||
| Director | ||||
Date: November 17, 2017
82