UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
x Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the fiscal year ended December 31, 2007
or
¨ Transition report pursuant to Section 13 or 15(d) of the Securities Act of 1934
For the transition period from to
Commission File Number 001-09781 (0-1052)
MILLIPORE CORPORATION
(Exact name of registrant as specified in its charter)
| | |
| Massachusetts | | 04-2170233 |
| (State or Other Jurisdiction of Incorporation or Organization) | | (I. R. S. Employer Identification No.) |
| |
| 290 Concord Road, Billerica, MA | | 01821 |
| (Address of principal executive offices) | | (Zip Code) |
(978) 715-4321
(Registrant’s telephone number, including area code)
SECURITIES REGISTERED PURSUANT TO SECTION 12(B) OF THE ACT:
| | |
| Title of Class | | Name of Exchange on Which Registered |
| Common Stock, $1.00 Par Value | | New York Stock Exchange, Inc. |
SECURITIES REGISTERED PURSUANT TO SECTION 12(G) OF THE ACT: NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
x Yes ¨ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
¨ Yes x No
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. x Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part Ill of Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act.
x Large accelerated filer ¨ Accelerated filer ¨ Non-accelerated filer ¨ Smaller reporting company
(Do not check if smaller reporting company)
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2). ¨ Yes x No
The aggregate market value of Common Stock held by non-affiliates of the registrant, based upon the closing sale price of the registrant’s Common Stock on June 30, 2007, the last business day of its most recently completed second fiscal quarter, as reported on the New York Stock Exchange, was approximately $3,123,753,685. Shares of Common Stock held by each executive officer and director and by each person known to beneficially own more than 5 percent of the outstanding Common Stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of February 20, 2008, 55,028,903 shares of the registrant’s Common Stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
| | |
Document Definitive Proxy Statement for the 2008 Annual Meeting | | Incorporated into Form 10-K Part Ill |
| | |
MILLIPORE FORM 10-K 2007 | | 1 |
Table of Contents
| | |
| 2 | | MILLIPORE FORM 10-K 2007 |
PART I
ITEM 1BUSINESS
| | |
Summary Millipore is a global leader in life science, providing
innovative products, services, and solutions so our customers can advance their research, development, and
production. Our academic, biotechnology, and pharmaceutical
customers use our consumable products and services to increase
their speed and to improve their consistency while saving costs in
laboratory applications and in biopharmaceutical manufacturing.
With our extensive technical expertise and applications knowledge,
we have the unique ability to engage in peer-to-peer discussions
with scientists to help them confront challenging scientific and
human health issues. | | 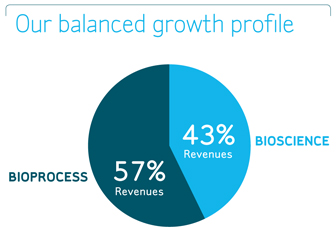 |

We help scientists conduct their research easily, efficiently and economically.
| | |
DEVELOPMENT | | |
BIOSCIENCE | | BIOPROCESS |
We help pharmaceutical and biotech companies discover new drugs. | | We help companies develop manufacturing processes to bring new drugs to market. |
PRODUCTION | | |
| | BIOPROCESS |
We help companies manufacture drugs efficiently and ensure drug purity and safety. |
| | |
MILLIPORE FORM 10-K 2007 | | 3 |
PART I
Millipore is organized around two operating divisions. Our Bioscience Division, which contributed approximately 43% of our 2007 revenues, improves laboratory productivity and workflows by providing innovative products and technologies for life science research. Our Bioprocess Division, which contributed approximately 57% of our 2007 revenues, helps pharmaceutical and biotechnology companies develop their manufacturing processes, optimize their manufacturing productivity, and ensure the quality of drugs.
RESEARCH
In the bioscience research market, we improve laboratory productivity by providing a range of products and solutions that help scientists conduct their research more easily, efficiently, and economically. We focus primarily on highly technical areas such as the cell biology and protein research markets.
DEVELOPMENT
Our Bioscience Division focuses on the drug discovery market and provides products and services that help pharmaceutical and biotechnology companies discover, evaluate, and prioritize potential drugs before moving them into clinical development. Our Bioprocess Division helps biopharmaceutical companies develop the processes used to manufacture drugs for clinical development and the processes to manufacture the drugs at commercial scale once the drugs receive regulatory approval.
PRODUCTION
Our products and expertise help biotechnology and pharmaceuticaI companies efficiently manufacture therapeutics, particularly biologic drugs and vaccines. Our products and expertise ensure the purity and safety of biologic drugs by removing contaminants such as viruses and bacteria and by providing tests so companies can monitor their manufacturing processes.
Our History
Millipore Corporation was formed as a Massachusetts corporation in 1954. During much of our history, we have

developed and sold products based on our proprietary filtration and other separations technologies to a variety of industries. In 2001, we made a strategic decision to focus primarily on the life science markets. Beginning in 2005, we began implementing a new strategy that sharpened our focus on the fast growing biopharmaceutical manufacturing and laboratory research markets. In 2005 and 2006, we made four acquisitions to transform Millipore into a larger and more innovative company. These acquisitions expanded and improved the products and services we offer and complemented our brand, sales force, and customer relationships.
Our Strategy
Our corporate strategy is to provide differentiated solutions to the life science research and biopharmaceutical manufacturing markets, which we believe have significant needs for new products that drive results, productivity improvements and new research goals. Since 2005, our strategy has been organized around five objectives:
| | |
1 | | Strengthen our leadership position with biotechnology manufacturing customers by expanding our bioprocess product offerings |
2 | | Establish Millipore as a strategic supplier in bioscience research markets by increasing our laboratory productivity platforms and market reach |
3 | | Lead our industry in product quality and manufacturing effectiveness |
4 | | Attract, retain and develop talented and motivated employees |
5 | | Double the value of the company between 2005 and 2009 |
| | |
| 4 | | MILLIPORE FORM 10-K 2007 |
PART I
Our Bioscience Division strategy is to capitalize on its global infrastructure and core capabilities in filtration, reagents and assay development to provide differentiated offerings in fast growing market segments. The division pursues targeted, market-specific strategies in laboratory water, drug discovery, and life science research. The division leverages three expert sales organizations to execute the multiple-segment strategy under one premium brand.
Our Bioprocess Division strategy is to leverage its leading position and broad portfolio of products to offer its biopharmaceutical customers integrated solutions that improve their productivity. By enabling companies to move from a product-centric approach to an integrated approach, the division can uniquely help customers to increase their speed, lower their costs, minimize their risk, and increase their quality. The division’s global sales organization is focused on selling products, services, and applications expertise that provide its customers with a comprehensive approach to optimize their biopharmaceutical manufacturing process.
RECENT DEVELOPMENTS
Some of our 2007 achievements that continued to advance our strategy and transform our company include:
| n | | Launching many new products, including |
| | — | successful new product launches for laboratory water in our Bioscience Division, and |
| | — | an upgrade to our core product portfolio in our Bioprocess Division along with 15 new product launches, compared to 8 in 2006. |
| n | | Establishing or broadening several key alliances to expand our product offerings and extend our development capabilities. |
| n | | Completing the integration of our Serologicals acquisition. The integration consisted of more than 800 milestones that significantly strengthened our capabilities, broadened our product portfolio, and advanced our position as a life science leader. |
| n | | Launching a new corporate brand to communicate our expanded capabilities to our customers and the market. |
| n | | Introducing a new corporate website making it easier for customers to do business with us. |

| n | | Achieving a record level of sales in Asia and continuing to expand in other emerging markets. |
| n | | Launching a formal sustainability initiative to reduce our overall impact on the environment. |
| n | | Continuing a number of initiatives to further improve our profitability, such as significant reductions in the number of our worldwide manufacturing facilities, improvements to our procurement and manufacturing processes, and enhancements of sales productivity. |
| | |
STRATEGIC ALLIANCES |

| | Recombinant Insulin |

| | Animal-free Cell Culture Supplements |

| | New Chromatography Media |

| | Real-time tests for microbial contamination |

| | Biomarkers for the drug discovery market |

| | Combining our Lab Water products in Siemens’ Vista® Intelligent Lab System for clinical laboratories |
| | |
MILLIPORE FORM 10-K 2007 | | 5 |
PART I
Our Customers
| | | | |
| | |
MILLIPORE | | | | |
| | |
DIVISIONS | | PRODUCT APPLICATIONS | | CUSTOMERS |
| | | |
BIOSCIENCE | | LAB WATER DRUG DISCOVERY LIFE SCIENCE | | n Research departments at biotechnology and pharmaceutical companies |
| | | n Life science research companies |
| | | n Private and public research laboratories, such as universities, medical research centers, and government institutions |
| | | n Hospitals and clinical laboratories |
| | | n Clinical research organizations |
| | | n Environmental, industrial, and other analytical laboratories |
| | | |
BIOPROCESS | | UPSTREAM BIOPROCESSING DOWNSTREAM BIOPROCESSING PROCESS MONITORING | | n Biotechnology companies |
| | | n Pharmaceutical companies |
| | | n Contract drug manufacturers |
| | | n Diagnostics and medical device companies |
| | | n Beverage companies |
| | | | | n Environmental testing companies |
Almost all of our revenue is related to customers engaged in life science research or biopharmaceutical production. A small portion of our business is related to customers in the food and beverage, environmental, diagnostics, and other industries.
BIOSCIENCE CUSTOMERS
Our Bioscience Division serves the life science research market, principally composed of companies and institutions conducting basic research, drug discovery, and other analytical laboratory work.
While the overall bioscience market is very broad, Millipore does not seek to serve all segments of the market. For example, in the life science research market we focus primarily on scientists conducting research in cell biology, protein research, and drug discovery.
These customers focus on applications such as stem cells, cell signaling and nuclear function, target identification, and compound screening. Our laboratory water and some lab filtration products are sold into many different types of research, analytical, and clinical laboratories worldwide and serve a more general, diverse customer base.
BIOPROCESS CUSTOMERS
Our Bioprocess Division serves the biopharmaceutical manufacturing market, principally composed of biotechnology and pharmaceutical companies that develop, manufacture, and sell products for the diagnosis, prevention, and treatment of diseases.
These Bioprocess customers are engaged in the development, scale-up, manufacturing, and testing of therapeutic, vaccine, and diagnostic products, as well as a variety of healthcare and other products.
Although no single customer accounts for 10 percent or more of our sales, some of our individual biotechnology customers do purchase significant quantities of our products. Our Bioprocess Division has significantly higher customer concentration than our Bioscience Division.
| | |
| 6 | | MILLIPORE FORM 10-K 2007 |
PART I
Our Business
OVERVIEW
We compete in two related markets, life science research and biopharmaceutical manufacturing.
In response to demand for healthcare improvement and disease prevention, new therapeutic products and vaccines, particularly biologics based on recombinant proteins, are being developed, approved, and produced in growing numbers.
We have worldwide operations with a strong brand, global infrastructure, proprietary technologies, highly qualified sales force, and manufacturing operations. We sell thousands of products, and we are continually developing and/or acquiring new proprietary products and technologies to advance our businesses. We believe we offer a balanced product mix that offers strong growth and profitability.
Most of our products are consumables that are used, disposed, and replaced, such as reagent kits or filtration cartridges. We derive a small portion of our revenue from standard hardware products ranging from small benchtop laboratory water systems to large filtration systems.
| | | | |
OUR BIOSCIENCE STRATEGY IMPROVES SCIENTIFIC WORK FLOWS |
RESEARCHERS WANT |
| | | |
n Reduced complexity in experiment work flow | | n Increased confidence in outcomes | | n Ongoing support during experiments |

|
MILLIPORE PRODUCTS AND SERVICES |
| | | |
n Simplify researchers’ work flow | | n Offer consolidated and validated solutions | | n Provide technical support |
In addition, we provide a variety of services, including drug target screening and selectivity testing, microbial contamination testing, consulting, manufacturing process validation, and product maintenance services. Although service revenues represent a small percentage of our revenues, they have grown significantly over the past two years due to strong market growth and the acquisition of service businesses.
Because of the differing applications required by each of our target markets, we believe our approach to these markets benefits from more specialized and focused attention. Accordingly, we have aligned our business to better address each of these markets. The following describes more specifically the principal markets in which we compete.
LABORATORY/LIFE SCIENCE
RESEARCH MARKETS
Industry Background
As researchers seek to understand complex biological systems and to identify and characterize new therapeutic targets, the market demand for tools that improve productivity and efficiency in the laboratory has grown. Intensive and expensive laboratory research is required to feed the pipeline of biologics, bioengineered vaccines, and other therapeutic and diagnostic products in development. Research organizations have come under increasing competitive and economic pressure to screen and identify possible new drugs with more speed and accuracy. In particular, the rapid growth in the development of new therapeutics has brought a heightened focus on protein research, including protein identification and characterization. Laboratory markets have also grown with the increase in concerns about bioterrorism and the emergence of new public health threats.
Our Bioscience Division serves major fields of life science research, including cellular biology, protein research, and drug discovery, which we believe are high growth market segments. Researchers want reduced complexity in their experimental work flows, increased confidence in the outcome, and ongoing support during their experiments. Our bioscience strategy is to create products and services which span the entire work flow, by simplifying the work flow for
| | |
MILLIPORE FORM 10-K 2007 | | 7 |
PART I
researchers, offering consolidated and validated solutions, and providing the necessary support along the way.
We offer products to advance life science research in a wide variety of areas from neuroscience, infectious disease, oncology, and metabolic disorders to stem cells, cell signaling, nuclear function, and chromatin biology.
Our Bioscience Division is organized around three specific market areas within the broad bioscience research market as described below.
Laboratory Water
All life science research starts with the use of pure water. Water purification systems are present in nearly every laboratory. Daily demand for purified water can range from a few liters to several thousand liters. We offer a wide selection of sophisticated bench-top and central laboratory water systems that ensure water purity for critical laboratory analysis and clinical testing. These systems provide the flexibility to produce the water quality needed for a variety of laboratory needs and applications.
| | |
MILLIPORE BIOSCIENCE VALUE PROPOSITION |
| | |
| FASTER | | INTEGRATED, PRE-VALIDATED KITS reduce the number of individual steps required CUSTOMIZED PRODUCTS (such as an antibody) fit the specific experiment |
| | |
| BETTER | | BEST-IN-CLASS QUALITY PRODUCTS yield reliable and consistent results TARGETED SOLUTIONSmeet researcher’s specific protocol |
| | |
| EASIER | | MILLIPORE APPLICATION PROTOCOLSguide scientist through the experiment EASY TO USE KITS ADDRESS SEVERAL STEPSin a combined format SERVICES AND STAND ALONE PRODUCTSfill any gaps in the experiment protocol WORLD-CLASS CUSTOMER SUPPORTto provide technical support on key experiments |
Drug Discovery
Millipore provides products and services that help pharmaceutical and biotech companies discover, evaluate, and prioritize potential drugs. A major challenge is to find promising drug candidates faster and then ensure that they will not generate unwanted or unexpected side effects in clinical trials or when they are commercially on the market. To improve the efficiency and economy of this research, we provide tools and services to identify disease targets and better understand how to improve the efficacy of drugs on targeted patient groups. In each case, the overriding goal is the transformation of medical practice from a “diagnose and treat” model to one of “predict and prevent”.
When researchers identify potential drugs, they must evaluate which drugs are most likely to function effectively and safely in the human body. This complex task of prioritizing possible drugs requires that the drugs be screened both for specificity and affinity for the specific target of interest, and for potential side effects.
The majority of new biotherapeutic targets are proteins, either newly discovered or those for which their function is better understood through recent research. Protein-based therapeutics are often more effective than chemically-based drugs in treating a number of diseases.
We provide bulk reagents required to perform the complex analyses involved in prioritizing drug candidates through screening for specificity and affinity for a target class of interest.
We also offer outsourced drug discovery screening services to ascertain activity and safety for drug candidates. As an outsourcing partner to the world’s leading biotechnology and pharmaceutical firms, we offer an efficient, comprehensive service to screen molecules before the expensive and time consuming development of drugs to treat cardiovascular, oncology, neurology, metabolic, and many other disorders.
| | |
| 8 | | MILLIPORE FORM 10-K 2007 |
PART I
Life Science
Our life science business is focused on serving the cell biology and protein research markets. All life science researchers conduct experiments on biological samples, such as cells, proteins, and nucleic acids. Most experiments follow a work flow protocol which requires the use of a broad range of research products, including consumable devices, reagents, kits, antibodies, and other molecular biology tools for purifying, preparing, or screening biological samples.
We offer product and service solutions designed around the entire protocol, so researchers can work faster, better, and easier than if they did not use our products and services.
The consistency and reproducibility of experimental results requires that the samples used by researchers are pure and properly isolated. The varying physical and biochemical characteristics of biological samples make the processes of isolation extremely complex. Research, clinical, and analytical laboratories use many sample preparation steps for a variety of laboratory procedures.
Millipore filtration devices and specialty membranes can be designed to accommodate the parameters of a wide variety of experiments. Millipore’s customized antibodies serve as biological markers that can be used to produce consistent and repeatable results, saving time and reducing costs.
Research scientists also outsource various services such as the development and production of custom antibodies, which we provide.
Life science researchers who study the structure and function of cells and proteins require innovative and high quality biological reagents to conduct their experiments consistently. A reagent is a substance used to detect, quantify, produce, modify or otherwise manipulate a biological target. Millipore offers a wide range of biological reagents.
Kits enhance research productivity by combining in one box all the disposables, reagents, and protocols needed to reliably and reproducibly conduct a particular experiment. Millipore offers a wide variety of kits that improve research productivity and efficiency.

BIOPHARMACEUTICAL MANUFACTURING MARKET
Industry Background
Our Bioprocess Division provides tools and services for the commercial production of bioengineered and pharmaceutical substances, including biologics, vaccines and other biotherapeutic products. Manufacturers of these products are under increasing competitive and economic pressure to:
| n | | maintain safety and quality |
| n | | minimize process deviations |
| n | | shorten production time |
| n | | improve manufacturing productivity and yield |
| n | | ensure security of supply |
Manufacturing of therapeutics generally encompasses production of two broad categories of molecules, small molecule drugs and large molecule drugs. Small molecule therapeutics are primarily chemical compounds that are made through an organic or inorganic chemistry process. These are sometimes referred to as synthetic pharmaceuticals. Chemical or pharmaceutical companies manufacture these therapeutics and their active ingredients in bulk. Large molecule therapeutics are primarily protein-based biologics. These represent the fastest growing segment of the biotech industry.
Biologics are products derived from living organisms, generated in a bioreactor or fermentor, and used in the prevention or treatment of disease. They include therapeutic products and vaccines based on recombinant proteins, such as monoclonal antibodies. About half of our Bioprocess business is related to biotechnology products.
| | |
MILLIPORE FORM 10-K 2007 | | 9 |
PART I
In many instances, recombinant proteins replace or mimic naturally occurring human proteins and are produced by cells containing modified DNA. One subset of recombinant protein-based drugs, monoclonal antibodies, has been shown to be extremely effective at treating otherwise intractable diseases such as cancer. This has led to a fast growing market for monoclonal antibodies, which are difficult to produce and require a variety of complex technologies and processes to enable their development and production.
Industry sources predict that volumes of monoclonal antibodies and bioengineered vaccines will continue to grow substantially over the next five years. There are approximately 2,300 biologics in various stages of pre-clinical and clinical development, of which approximately 660 are monoclonal antibodies and approximately 640 are bioengineered vaccines. In addition, applications and approvals for biologics drug supplements, which address incremental indications for a previously approved biologic, are also on the rise. In contrast, growth in sales of small molecule pharmaceuticals is expected to be lower because of intense generic competition as the patents expire on the chemical compounds comprising the drugs. Synthetic pharmaceuticals, however, continue to constitute a significant percentage of all marketed therapeutic products and are manufactured in large volumes.
As the demand for marketed biologics and vaccines grows and new products and applications are approved, the market for products that facilitate and accelerate the
identification, development and production of biologics and bioengineered vaccines is expanding.
Successfully bringing a biologic or a bioengineered vaccine to the market is a complex and lengthy process. It begins with extensive laboratory research and discovery, continues with years of development, clinical trials and scale-up of the manufacturing process, and culminates with establishing a manufacturing process that meets regulatory approvals and generates sufficient quantities of a safe and effective drug. Going from the initial research to full production scale cannot be achieved without increasingly effective cell culture and purification processes.
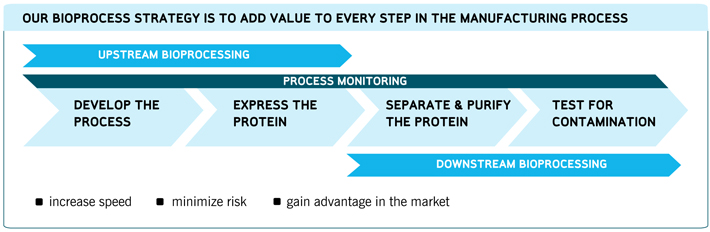
We view the value chain of our bioprocess customers to include:
| n | | process development and scale up; |
| n | | upstream bioprocessing (the growth and expression process in bioreactors); |
| n | | downstream purification and filtration (harvesting); and |
| n | | compliance monitoring and testing. |
We believe we offer one of the industry’s broadest offerings for biopharmaceutical manufacturing. Additionally, we believe we are the only company to offer consumable products in both upstream and downstream bioprocessing. Our Bioprocess Division serves the breadth of this development and manufacturing process by targeting the three principal applications described below. The development of a customer’s process involves aspects from each of these three product categories.
| | |
| 10 | | MILLIPORE FORM 10-K 2007 |
PART I
Upstream Bioprocessing
Biologic products must be grown in living cells since they cannot be synthesized chemically. We provide products and technologies that improve the ability of cells to efficiently produce proteins that ultimately become therapeutic drugs and vaccines.
The cells are grown in cell cultures held in large bioreactor or fermentation tanks of varying capacity. In order to achieve high protein concentrations, cells in the bioreactor require nutrients and supplements. As the cells grow and metabolize, they secrete into the cell culture medium the therapeutic protein that is then harvested, purified, and further processed.
To facilitate the manufacture of biologic drugs in mammalian cell cultures, we offer high quality nutrients and supplements for these cultures. Our products include the leading branded fatty acid supplements, recombinant insulin, bovine serum albumin, and other growth factors that improve the ability of cells to produce proteins efficiently. We also offer unique gene expression technology which permits screening and isolation of highly productive cell lines much faster than conventional technologies. The technology enables our customers to more efficiently manufacture recombinant proteins in mammalian cells by generating higher protein yields. Today, some of our upstream products are derived using materials from animals. Many customers are increasingly demanding products that do not use animal-derived products. We are actively expanding our portfolio of animal-free cell culture supplements. In December 2007, we entered into an exclusive long-term strategic alliance with Novozymes to bring recombinant albumin and transferrin to the market.
Downstream Bioprocessing
FILTRATION & CHROMATOGRAPHY
The production of biologics requires the extraction of proteins from the fluids in which these proteins are grown. The process also requires the removal of impurities such as bacteria, viruses, cellular debris, and other contaminants. Accordingly, manufacturing processes for biologics, particularly for monoclonal antibodies, are separation-intensive, often requiring numerous filtration and chromatography steps for clarification, concentration, and sterilization.

A complex biologic, such as a monoclonal antibody, can require as many as ten different separation processes. A typical synthetic drug may require between one and four filtration and sterilization steps.
We offer the broadest range of filtration, purification, and chromatography technologies to clarify, concentrate, purify, and remove viruses or other biological contaminants from biologics, synthetic pharmaceuticals, and beverages. Approximately half of our business is related to biologic drug production, with the remaining portion of our business primarily related to synthetic pharmaceutical manufacturing and food and beverage processing. We also sell membrane sheets and rolls and bulk chromatographic media to original equipment manufacturers of medical devices, environmental testing equipment, or other products for use as a material or component in these products. We also offer a line of monoclonal antibodies that serve as reagents for classifying antigens on red blood cells and detecting regular and irregular antibodies in blood specimens.
MIXING AND DISPOSABLE SOLUTIONS
Until recently, all biotherapeutic drugs were produced with stainless steel or glass equipment. Although these types of equipment are currently the prevalent processing tools, the industry has sought ways to reduce the costs and delays associated with cleaning fixed equipment in place between manufacturing runs. Contamination risks also arise if the equipment is not thoroughly purged of all residual materials from prior production runs. Companies have begun to migrate to single use, disposable technologies that eliminate the need for cleaning, thus shortening the time between processing runs. Biopharmaceutical manufacturers are also seeking flexible manufacturing components and solutions that can be configured and validated to meet customized biological manufacturing needs.
| | |
MILLIPORE FORM 10-K 2007 | | 11 |
PART I
We design and manufacture sophisticated systems for use in sterile biomanufacturing environments, such as chromatography columns, manufacturing skids, mixers, and valves. We are also a leading innovator in the transition from such systems to disposable manufacturing, offering a broad range of disposable manufacturing components. In the past several years, we have developed and/or acquired rights to products and technologies that simplify and reduce the time and expense of some steps in the downstream and final fill processes of biotechnology and pharmaceutical manufacturing primarily by replacing stainless steel hardware with disposable plastic products. Our hardware products range from large stainless steel process scale filtration and chromatography systems and columns that can sell for more than a million dollars to small filter housings or valves that cost much less.
Process Monitoring
Regulatory agencies such as the U.S. Federal Drug Administration (FDA) require drug manufacturers to ensure the purity and sterility of products before they are released to the public. This requires sampling and testing of therapeutics throughout the manufacturing process. During nearly all phases of drug development and production, companies take multiple steps to ensure their products
| | | | |
MILLIPORE COMPETITORS |
| | | |
| | | BIOSCIENCE | | BIOPROCESS |
Becton Dickinson | | n | | |
Bio-Rad Laboratories | | n | | |
Corning | | n | | |
GE Healthcare | | n | | n |
Invitrogen | | n | | n |
MDS Pharma | | n | | |
Pall Corporation | | n | | n |
Perkin Elmer | | n | | |
Sartorius Stedim | | n | | n |
Sigma-Aldrich | | n | | n |
TECHNE Corporation | | n | | |
Thermo Fisher | | n | | n |
are produced safely and without contamination. Millipore provides a broad range of products and services that enable sampling and testing of drugs throughout the manufacturing process to ensure the safety and purity of drugs.
Companies that produce beverages (including wine, beer, and bottled juices and water) also benefit from using our process monitoring products to monitor for microbiological contamination and remove bacteria and yeast.
Our process monitoring products are designed to test for microbiological, viral or other contamination in biologics and synthetic pharmaceuticals as a quality control or assurance step in their manufacture or processing. We are also developing next-generation technologies that are faster and more sensitive so bioprocess manufacturers can identify contamination earlier in their processes. Our alliance with Gen-Probe Incorporated (“Gen-Probe”) is designed to produce new process monitoring tools capable of significantly reducing the time-to-result from days or weeks to hours. In January 2008, we announced the launch of MiIIiPROBE™, the alliance’s first product. We also offer outsourced testing for biological and viral contamination of biologics.
Competition
The markets for our products and services are intensely competitive and we compete with a variety of public and private companies. Given the breadth of our product and service offerings, our competition comes from a wide array of competitors, ranging from specialized companies with strengths in niche segments of the life science markets to large manufacturers offering a broad portfolio of products, tools, and services. Many of these competitors have significant financial, operational, sales, and marketing resources, and experience in research and development. In some cases, these and other competitors are also our customers, distributors, and suppliers, and in some circumstances we serve these roles for such competitors as well.
We believe that a company’s competitive position in any of our markets is determined by a varying mix of product availability and performance, quality, responsiveness, technical support, price, and breadth of product line. Our customers are diverse
| | |
| 12 | | MILLIPORE FORM 10-K 2007 |
PART I
and we believe they place varying degrees of importance on these competitive attributes. In our judgment we are well positioned to compete in each of these categories.
Sales, Marketing, and
Customer Support
We sell our products to end users worldwide, primarily through our own direct global sales force. Augmenting our direct sales, we also sell our products through our website and, in selected locations and markets, through independent distributors.
We market to our customers through advertising, trade shows, conferences, and other techniques. Our marketing efforts focus on application development for existing products and on new and differentiated products for newly identified and proposed customer needs. We seek to educate customers about the variety of problems that may be addressed by our products as well as to adapt our products and technologies to the problems identified by our customers. Our technical support services are important to our marketing efforts. These services include assisting in defining a customer’s needs, evaluating alternative solutions, selecting or designing a specific system to perform the desired application, training users, and assisting the customer in compliance with relevant government regulations.
Our direct sales organization is a critical competitive differentiator for us. An important component of our strategy is to leverage our direct sales organization by expanding our product portfolio available for sale and increasing our penetration of our current customer base.
Research and Development
We believe that a strong research and product development effort is important to our future growth.
Our research and development activities are intended both to improve on our extensive core technologies and to create
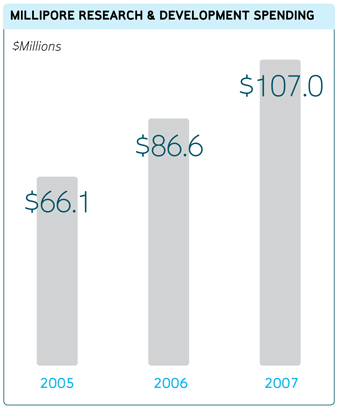
new applications and breakthroughs that complement our business. Our core technologies include:
| n | | Separation and purification membranes |
| n | | Customized monoclonal antibodies |
| n | | Cell culture supplements |
| n | | Disposable manufacturing |
Some of these technologies are incorporated into devices, cartridges, and modules of different configurations that span many of our markets while others are focused on a specific or customized application.
We do most of our own research and development and do not provide material amounts of research and development services for others. We have followed a practice of
| | |
MILLIPORE FORM 10-K 2007 | | 13 |
PART I
supplementing our internal research and development efforts by acquiring or licensing new technologies from unaffiliated third parties, acquiring distribution rights for new technologies, and undertaking collaborative or sponsored research and development activities with unaffiliated companies and academic or research institutions when we believe it is in our interests to do so.
For example, through our alliance with Gen-Probe, we are working to develop next-generation process monitoring tools for the biopharmaceutical manufacturing market by coupling Millipore membrane-based sample preparation technologies with Gen-Probe’s nucleic acid amplification and gene sequencing technologies. Additionally, through our acquisition of Serologicals, we have greatly expanded our product development capabilities to include antibodies, enzymes, labeling and detection reagents, molecular biology kits, multiplexed immunoassays, cell based assays, and drug screening services.
Quality Assurance
To compete effectively in our markets, we maintain a global quality assurance system and program designed to assure compliance with the stringent requirements of regulatory authorities, voluntary quality standards, industry trade associations, and our customers. Using our quality assurance program and an internally maintained regulatory compliance program, we conduct periodic audits of each of our facilities to ascertain the status and compliance of the quality system as implemented. The audits, in combination with performance metrics, are designed to ensure adherence to regulations and our procedures and to assess the effectiveness of our quality system as a whole. The audits are one component of the key performance indicators that we collect, review, and monitor in order to maintain our program of continuous improvement and compliance with our established systems and programs.
Most of our operating facilities are registered to ISO 9001:2000 quality standards. The ISO 9001:2000 series of standards is a voluntary quality standard recognized throughout the world.
Global Supply Chain—
Manufacturing and Sourcing
We manufacture the majority of our products in our own manufacturing facilities, primarily at those properties described and listed under Item 2 of this Form 10-K. Our global supply chain initiative, which began in 2004, is expected to result, over five years, in a new manufacturing landscape through the consolidation of current sites, the implementation of new raw material procurement practices by consolidating our current supplier base, and streamlined manufacturing processes through improvements using lean manufacturing and Six Sigma methodologies.
Our Employees
As of December 31, 2007, Millipore employed approximately 6,000 people worldwide, of whom approximately 2,300 were employed in the United States.
Patents, Trademarks and Licenses
We have been granted and have licensed rights under a number of patents and have other patent applications pending both in the United States and abroad. While these patents and licenses in the aggregate are viewed as valuable assets, we believe that no individual patent is material to our ongoing operations. We also own a number of trademarks, the most significant being “Millipore”.
Many of our research reagent products are sold under licenses that have varying terms and conditions. We expect to continue to in-license new technologies from academic and government institutions, as well as biotechnology and pharmaceutical companies. We use licensed technologies to create new products, including high value kits and services, many of which address bottlenecks in the research or drug discovery laboratories.
| | |
| 14 | | MILLIPORE FORM 10-K 2007 |
PART I
Our ability to obtain licenses to allow the introduction of new products is very important to allow us to offer new, innovative, and technologically superior research products. The licenses from others typically cover patents or biological materials, such as cell lines, that we use to develop new products. Most of them are for fixed terms with options for renewal and typically impose obligations on us to market the licensed technology. No single license is material to our business.
Government and Industry
Regulation
Many of our activities are subject to regulation by governmental authorities within the United States and similar bodies outside of the United States. The regulatory authorities may govern the collection, testing, manufacturing, safety, efficacy, labeling, storage, record keeping, transportation, approval, advertising, and promotion of our products, as well as the training of our employees. However, some of these products are subject to import and export regulations specific to the country of import. Certain of our products are considered “medical devices” under the Food, Drug and Cosmetic Act. Accordingly, these products are subject to the law’s general control provisions that include requirements for registration, listing of devices, quality regulations, labeling, and prohibitions against misbranding and adulteration. These products subject us to regulatory inspection and scrutiny. We believe that we are in substantial compliance with all relevant laws and regulations.
Environmental Matters
We are subject to numerous federal, state, and foreign laws and regulations that impose strict requirements for the control and abatement of air, water, and soil pollutants and the manufacturing, storage, handling, and disposal of hazardous substances and waste. We believe we are in substantial compliance with all applicable environmental requirements. We continue to invest in maintaining facilities that enable our compliance with these
environmental laws. These environmental related expenditures have not had a material effect on our financial results. Because regulatory standards under environmental laws and regulations have become increasingly stringent, there can be no assurance that future developments will not cause us to incur material environmental liabilities or costs. See the applicable risk factor under Item 1A of this Form 10-K.
Raw Materials
Our products are made from a wide variety of raw materials that are generally available from alternate sources of supply. For certain critical raw materials, we have qualified only a single source. We periodically purchase quantities of some of these critical raw materials in excess of current requirements in anticipation of future manufacturing needs. With sufficient lead times, we believe we would be able to validate alternate suppliers for each of these raw materials. As described in the applicable risk factor under Item 1A of this Form 10-K, several of these critical raw materials are used in a significant portion of our products, and if we were unable to obtain supply of any one of them, our loss of revenues would be material.
Seasonality
In general, we do not believe our business is inherently seasonal.
Backlog
We do not have a material amount of firm commitments that serve as backlog orders.
| | |
MILLIPORE FORM 10-K 2007 | | 15 |
PART I
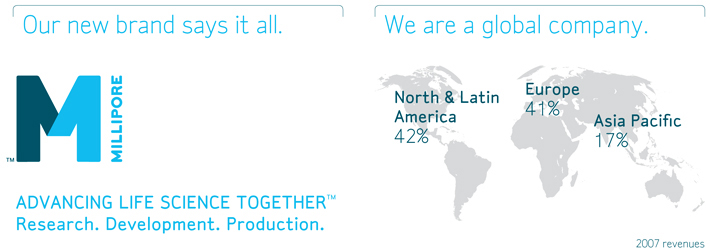
Geographic and Segment Information
We are a multinational company with approximately 63 percent of our 2007 sales outside the United States and approximately 50 percent of our long-lived assets outside the United States at December 31, 2007. Geographic and segment information, including the identification of operating segments and their aggregation, is discussed in Note 16 to our Consolidated Financial Statements.
Other Information
Millipore’s corporate headquarters are at 290 Concord Road, Billerica, Massachusetts, and our telephone number at that location is 1-978-715-4321.
The U.S. Securities and Exchange Commission (the “SEC”) maintains an internet website at http://www.sec.gov that contains our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and proxy statements, and all amendments thereto. All reports that we file with the SEC may be read and copied at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, DC 20549. Information about the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330.
Millipore’s internet website address is www.millipore.com. Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and proxy statements, and all amendments thereto, are available free of charge on our website as soon as reasonably practicable after such reports are electronically filed with, or furnished to, the SEC. In addition, our corporate governance guidelines, the charters of each of the committees of our Board of Directors, our code of ethics (consisting of our Corporate Compliance Policy, our Employee Code of Conduct and our Rules of Conduct) and our Director Code of Conduct are
available on our website and are available in print to any Millipore shareholder upon request in writing to “General Counsel, Millipore Corporation, 290 Concord Road, Billerica, MA 01821”.
The certifications of Millipore’s Chief Executive Officer and Chief Financial Officer, as required by the rules adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, are filed as exhibits to this Form 10-K. Millipore’s Chief Executive Officer, Martin D. Madaus, provided an annual certification to the New York Stock Exchange dated May 29, 2007, that he was not aware of any violations by the Company of the New York Stock Exchange corporate governance listing standards.
| | |
| 16 | | MILLIPORE FORM 10-K 2007 |
PART I
ITEM 1A. RISK FACTORS.
Lack of early success with our pharmaceutical and biotechnology customers can shut us out of future business with those customers.
Many of the products we sell to the pharmaceutical and biotechnology customers are incorporated into the customers’ drug manufacturing processes. In some cases, once a customer chooses a particular product for use in a drug manufacturing process, it is unlikely that the customer will later switch to a competing alternative. In many cases, the regulatory license for the product will specify the separation and cell culture supplement products qualified for use in the process. Obtaining the regulatory approvals needed for a change in the manufacturing process is time consuming, expensive and uncertain. Accordingly, if we fail to convince a pharmaceutical or biotechnology customer to choose our products early in its manufacturing design phase, we may lose permanently the opportunity to participate in the customer’s production of such product. Because we face vigorous competition in this market from companies with substantial financial and technical resources, we run the risk that our competitors will win significant early business with a customer making it difficult for us to recover that opportunity.
The suspension or termination of production of a customer’s therapeutic product may result in the abrupt suspension or termination of their purchases of our products, resulting in an unexpected reduction in our revenue.
Success in our Bioprocess division substantially depends on the incorporation of our products into a customer’s manufacturing process. If this “design in” is achieved, we will likely have the opportunity to sell consumable products to the customer during the life cycle of the customer’s product, which could continue for many years. Our planning and growth projections are built in part on the volume assumptions deriving from these customer successes. If a customer stops production of its product, either temporarily or permanently, our sales to the customer for the applicable product will drop or stop. A customer may suspend or terminate production of a product, either voluntarily or involuntarily, and related sales and distribution for many reasons. These may include adverse regulatory, competitive, legal or economic circumstances. We have had in the past, and expect to have in the future, situations in which a customer suspends its purchases of our products. A suspension or permanent cessation of a process in which we would otherwise anticipate selling a significant volume of consumables will reduce our revenues and negatively impact our earnings.
Disruptions in the supply of raw materials and distributed products from our single source suppliers could result in a significant disruption in sales and profitability.
Our products are made from a wide variety of raw materials that are generally available from alternate sources of supply. However, certain critical raw materials and supplies required for the production of some of our principal products are available only from a single supplier, as are some products that we distribute. Such raw materials and distributed products cannot be obtained from other sources without significant delay or at all. If such suppliers were to limit or terminate production or otherwise fail to supply these materials for any reason, such failures could have a material adverse impact on our product sales and our business.
If our efforts to integrate acquired or licensed businesses or technologies into our business are not successful, our business could be harmed.
As part of our business strategy, we have grown our business through acquisitions of technologies or of companies that offer products, services and technologies that we believe complement our products, technologies and services. In 2005, we acquired NovAseptic and MicroSafe B.V. In April 2006, we acquired Newport and in July 2006, we acquired Serologicals. We expect to continue to grow our business through additional acquisitions if appropriate opportunities arise.
| | |
MILLIPORE FORM 10-K 2007 | | 17 |
PART I
Managing these recent acquisitions and any future acquisitions will entail numerous operational, legal and financial risks, including:
| | n | | difficulties in assimilating new technologies, operations, sites and personnel; |
| | n | | diversion of resources and management attention from our existing businesses and technologies; |
| | n | | inability to maintain uniform quality standards, controls, and procedures; |
| | n | | inability to retain key employees of any acquired businesses or hire enough qualified personnel to staff any new or expanded operations; |
| | n | | impairment or loss of relationships with key customers and suppliers of acquired businesses; |
| | n | | issuance of dilutive equity securities; |
| | n | | incurrence or assumption of debt; |
| | n | | exposure to unknown or unanticipated liabilities; |
| | n | | additional expenses associated with future amortization or impairment of acquired intangible assets or potential businesses; and |
| | n | | exposure to federal, state, local and foreign tax liabilities in connection with any acquisition or the integration of any acquired businesses. |
Our failure to address these risks successfully in the future could harm our business and prevent our achievement of anticipated growth.
Increased leverage as a result of our debt offerings and other debt we have incurred to finance our acquisition of Serologicals may harm our financial condition and results of operations.
As of December 31, 2007, our total long term debt was $1,260.0 million and approximately $331.6 million represents indebtedness of our subsidiaries under our revolving credit facilities that is guaranteed by us. Our revolving credit facilities permit us to borrow in either the United States or Europe, with a combined maximum borrowing not to exceed€465.0 million, or $678.3 million.
Our level of indebtedness could have important consequences because:
| | n | | a substantial portion of our cash flows from operations will be dedicated to interest and principal payments and may not be available for operations, working capital, capital expenditures, expansion, acquisitions or general corporate or other purposes; |
| | n | | it may impair our ability to obtain additional or replacement financing in the future; |
| | n | | it may limit our flexibility in planning for, or reacting to, changes in our business and industry; and |
| | n | | it may make us more vulnerable to downturns in our business, our industry or the economy in general. |
Our operations may not generate sufficient cash to enable us to service our debt. If we fail to make a payment on any of our debt obligations or comply with financial covenants in our debt agreements, we could be in default on such debts, and this default could cause us to be in default on our other outstanding indebtedness. In each case of default, we may be required to repay all of our outstanding indebtedness or renegotiate the terms of our indebtedness on unfavorable terms.
If we fail to maintain adequate quality standards for our products and services, our business may be adversely affected and our reputation harmed.
Our customers are subject to rigorous quality standards in order to maintain their products and the manufacturing processes and testing methods that generate them. A failure to sustain the specified quality requirements, including the processing and testing functions performed by our products, could result in the loss of the applicable regulatory license. Delays or quality lapses in our customer’s production line could result in substantial economic losses to them and to us. For example, large production lots of biotherapeutics are very delicate and expensive and a failure of a separation mem-
| | |
| 18 | | MILLIPORE FORM 10-K 2007 |
PART I
brane could result in the contamination of the entire lot, requiring its destruction. We also perform services that may be considered an extension of our customers’ manufacturing and quality assurance processes, which also require the maintenance of prescribed levels of quality. Although we believe that our continued focus on quality throughout the company adequately addresses these risks, there can be no assurance that we will not experience occasional or systemic quality lapses in our manufacturing and service operations. If we experience significant or prolonged quality problems, our business and reputation may be harmed, which may result in the loss of customers, our inability to participate in future customer product opportunities, and reduced revenues and earnings.
We may be unable to establish and to maintain collaborative development and marketing relationships with business partners, which could result in a decline in revenues or slower than anticipated growth rates.
As a part of our business strategy, we have formed, and intend to continue to form, strategic alliances, license agreements and marketing and distribution arrangements with corporate partners relating to the development, commercialization, marketing and distribution of certain of our existing and potential products to increase our revenues and to leverage our product and service offerings. Our success will depend, in part, on our ability to maintain these relationships and to cultivate additional corporate alliances with such companies. In 2005, we entered into a joint development agreement with Gen-Probe. In 2007, we entered into agreements with Novozymes A/S, Rohm and Haas, and Stem Cell Sciences, among others.
We cannot ensure that our historical collaborative relationships will be commercially successful or yield the desired results, that we will be able to negotiate additional collaborative relationships, that such additional collaborative relationships will be available to us on acceptable terms, or that any such relationships, if established, will be commercially successful. In addition, we cannot ensure that parties with which we have established, or will establish, collaborative relationships will not, either directly or in collaboration with others, pursue alternative technologies or develop alternative products in addition to, or instead of, our products. Such parties may also be acquired by our competitors to terminate our relationship. They may also experience financial or other difficulties that lessen their value to us and to our customers. Our results of operations and opportunities for growth may be adversely affected by our failure to establish and maintain successful collaborative relationships.
Demand for our bioprocess products and services are subject to the commercial success of our customers’ products which may vary for reasons outside our control.
Even if we are successful in securing participation for our products in a customer’s manufacturing process, sales of many of our bioprocess products and services remain dependent on the timing and volume of the customer’s production, over which we have no control. The customer’s demand for our products will depend on the regulatory approval and commercial success of the supported product. The regulatory process is complex, lengthy and expensive and can often take years to complete, if at all. Commercial success of a customer’s product, which would drive demand in production and commensurate demand for our products and services, is dependent on many factors, some of which can change rapidly, despite early positive indications. Any delay or cancellation by a customer of volume manufacturing may harm our revenues and earnings.
Technology innovations in the markets that we serve may create alternatives to our products and result in reduced sales.
Our customers constantly attempt to reduce their manufacturing costs and to improve product quality. Technology innovations to which our current and potential customers would have access could reduce or eliminate their need for our membrane or chromatography products. For example, if a new membrane or chromatography technology of one of our competitors is accepted by the pharmaceutical or biotechnology industry as a market standard, sales of our membrane or
| | |
MILLIPORE FORM 10-K 2007 | | 19 |
PART I
chromatography products would be negatively impacted. In addition, a disruptive technology that reduces or eliminates the use of our core technologies would negatively impact the sale of our products. As an example, animal–free serum products are generally favored over bovine serum. We may be unable to respond on a timely basis to the changing needs of our customer base and the new technologies we design for our customers may prove to be ineffective. Our failure to develop and to introduce or to enhance products able to compete with such new technologies in a timely manner could have a material adverse effect on our business, results of operations, and financial condition. We may be unable to respond on a timely basis to the changing needs of our customer base and the new technologies we design for our customers may prove to be ineffective.
We may be unable to realize our growth strategy if we cannot identify suitable acquisition opportunities in the future.
As part of our business strategy, we expect to continue to grow our business through acquisitions of technologies or companies. We may not identify or complete complementary acquisitions in a timely manner, on a cost-effective basis, or at all. In addition, we compete with other companies, including large, well funded competitors, to acquire suitable targets, and may not be able to acquire certain targets that we seek. There can be no assurance that we will be able to execute this component of our growth strategy which may harm our business and hinder our future growth.
To achieve desired growth rates as we become larger, we may seek larger or public companies as potential acquisition candidates. The acquisition of a public company may involve additional risks, including the potential for lack of recourse against public shareholders for undisclosed material liabilities of the acquired business. In addition, if we were to proceed with one or more significant future acquisitions in which the consideration consisted of cash, a substantial portion of our available cash resources could be used.
Our continued growth is dependent on our development and successful commercialization of new products.
Our future success will depend in part on timely development and introduction of new products that address changing market requirements. We believe that successful new product introductions provide a significant competitive advantage because customers make an investment of time in selecting and learning to use a new product. Customers are reluctant to switch to a competing product after making their initial selection. To the extent that we fail to introduce new and innovative products, we may lose market share to our competitors, which will be difficult or impossible to regain. An inability, for technological or other reasons, to successfully develop and introduce new products could reduce our growth rate or otherwise damage our business. In the past, we have experienced, and are likely to experience in the future, delays in the development and introduction of products. We cannot assure that we will keep pace with the rapid rate of change in life sciences research, or that our new products will adequately meet the requirements of the marketplace or achieve market acceptance.
If we fail to attract, hire, develop and retain qualified personnel, we may not be able to design, manufacture, market or sell our products or successfully grow our business.
Competition for individuals with skills including sales, marketing, research, product development, engineering and others is strong and we may not be able to secure the personnel we need. The loss of the services of any key personnel, or our inability to hire new personnel with the requisite skills, could restrict our ability to develop new products and services or enhance existing products and services in a timely manner, sell products to our customers, or manage our business effectively. As part of our global supply chain initiative to improve customer service and to amplify our product expertise, we have begun to concentrate our facilities in fewer geographical areas in which there is high demand for qualified staff.
| | |
| 20 | | MILLIPORE FORM 10-K 2007 |
PART I
If our consolidated manufacturing operations were disrupted, we may be unable to supply products to our customers and achieve expected revenues.
We are in the process of executing a coordinated reorganization of our supply chain and manufacturing operations. In an effort to better serve our customers and to attain efficiencies of scale and expertise, we are consolidating the majority of our production facilities into fewer sites. Each of these remaining facilities serves as our primary production facility for specific product lines. This concentration of production, however, exposes us to a greater risk of disruption to our ability to manufacture and supply our products. If operation at any of these facilities were disrupted, we may not be able to deliver products to our customers and achieve expected revenues or earnings. If we were unable to reestablish production in a timely manner, we may lose customers and have difficulty regaining them. It is uncertain whether the safety measures and contingency plans that we have implemented or may implement will successfully address the risks that may arise if production is disrupted. Also, there can be no assurance that the insurance that we maintain to protect against business interruption loss will be adequate or that such insurance will continue to remain available on acceptable terms, if at all. The extent of the coverage of our insurance could limit our ability to mitigate for lost sales and could result in such losses materially and adversely affecting our operating results.
Sales of several of our products are dependent on a small number of customers, the loss of which may harm our business and result in a reduction in revenues and earnings.
No single customer represents more than 10 percent of our annual sales. However, sales of some of our products are dependent on a limited number of customers, who account for a significant portion of such sales. Some of these products are in areas in which we plan to grow substantially. The loss of such key customers for such products, or a significant reduction in sales to those customers, could significantly reduce our revenues in these products and adversely affect our future growth in such markets.
We may become involved in disputes regarding our patents and other intellectual property rights, which could result in prohibition on the use of certain technology in current or planned products, exposure of the business to significant liability and diversion of management’s focus.
We and our major competitors spend substantial time and resources developing and patenting new and improved products and technologies. Many of our products are based on complex, rapidly developing technologies. Although we try to identify all relevant third party patents and intellectual property rights, these products could be developed by the business without knowledge of published or unpublished patent applications that cover or use some aspect of these technologies. We also license products and technologies developed by other biotechnology companies or academic research laboratories for further resale. We have been and may in the future be sued by third parties alleging that we are infringing their intellectual property rights. These lawsuits are expensive, take significant time and divert management’s focus from other business concerns. If we are found to be infringing the intellectual property of others, we could be required to stop the infringing activity, or we may be required to design around or license the intellectual property in question. If we are unable to obtain a required license on acceptable terms, or are unable to design around any third party patent, we may be unable to sell some of our products and services, which could result in reduced revenue. In addition, if we do not prevail, a court may find damages or award other remedies in favor of the opposing party in any of these suits, which may adversely affect our earnings.
Concern about the transmission of “mad-cow disease” could reduce the demand for our cell culture products that are derived from bovine serum.
The demand for several of our cell culture products could be adversely affected by concerns about the use of bovine material in the process by which they are manufactured. The concern arises from the risk that the agent causing bovine
| | |
MILLIPORE FORM 10-K 2007 | | 21 |
PART I
spongiform encephalopathy, or “mad-cow disease,” might be present in the raw materials used in the production process and that the agent might be introduced into a therapeutic substance manufactured by one of our customers. The regulatory authorities of certain countries, including Japan, have refused to approve pharmaceuticals that are manufactured using a product that was derived from bovine serum or that was manufactured by a process that uses bovine material. The regulatory authorities of other countries could adopt similar restrictions.
Our operations must comply with environmental statutes and regulations, and any failure to comply could result in extensive costs which would harm our business.
The manufacture of some of our products involves the use, transportation, storage and disposal of hazardous, radioactive or toxic materials and is subject to various environmental protection and occupational health and safety laws and regulations in the countries in which we operate. This has exposed us in the past, and could expose us in the future, to risks of accidental contamination and events of non-compliance with environmental laws. Any such occurrences could result in regulatory enforcement or personal injury and property damage claims or could lead to a shutdown of some of our operations, which could have an adverse effect on our business and results of operations. We currently incur costs to comply with environmental laws and regulations and these costs may become more significant.
The environmental laws of many jurisdictions impose actual and potential obligations on us to remediate contaminated sites. These obligations may relate to sites:
| | n | | that we currently own or operate; |
| | n | | that we formerly owned or operated; or |
| | n | | where waste from our operations was disposed. |
These environmental remediation obligations could reduce our operating results. In particular, our accruals for these obligations may be insufficient if the assumptions underlying the accruals prove incorrect or if we are held responsible for additional, currently undiscovered contamination.
A substantial fine or penalty, the payment of significant environmental remediation costs or the loss of a permit or other authorization to operate or engage in our ordinary course of business could result in material, unanticipated expenses and the possible inability to satisfy customer demand.
Our sales may be negatively affected by the implementation of second source programs by our customers.
For many customers, we are the single source supplier for one or more critical components used in their production lines. We are aware of customers that have begun to implement second sourcing programs to reduce the potential risk of disruptions to their production due to a supply bottleneck. These can include diversifying purchases of one component among vendors or spreading the sources of components of a process, such as purification, among different suppliers. If, as a result of these second sourcing programs, existing customers were to choose another company to supply components that we currently supply, or if we lose future business opportunities for which we would otherwise be qualified, our future revenues may be harmed.
Our use of third party manufacturers exposes us to increased risks that may affect our ability to supply our customers.
As part of our efforts to consolidate our manufacturing operations, we have increased the outsourcing of certain manufacturing operations. For example, in 2006 we migrated most of our standard bioprocess systems production to a company in India in which we have a minority equity interest. In addition, we often source products resulting from collaborative development relationships from such development partners. Our increased dependence on third party contract manufacturers exposes us to increased risks associated with delivery schedules, manufacturing capability, quality control, quality
| | |
| 22 | | MILLIPORE FORM 10-K 2007 |
PART I
assurance and costs. If any of our third party manufacturers experiences delays, disruptions, capacity constraints or quality control problems in its manufacturing operations or becomes insolvent, then product shipments to our customers could be delayed, which would decrease our revenues and harm our competitive position and reputation.
Because we compete directly with one of our key suppliers and one of our significant distributors, our results of operations could be adversely affected if either of these parties discontinues or materially changes the terms of the agreement.
We currently source a key raw material from a significant competitor in the market into which we sell the resulting products. Although we purchase these materials under a supply agreement which provides for some supply protections, our business could be adversely affected if this supplier discontinues selling the raw materials to us and if we have not established an alternate source of supply. In addition, one of our competitors also serves as a significant distributor. If this distributor discontinued selling our products or materially changed the terms, our sales and earnings could be adversely affected in the short term.
Violation of government regulations or voluntary quality programs could result in loss of sales and customers and additional expense to attain compliance.
Several of our facilities are subject to extensive regulation by the FDA and similar governmental bodies in other countries. These facilities are subject to periodic inspection by the FDA and other similar governmental bodies to ensure their compliance with applicable laws and regulations. New facilities, products and operating procedures also may require approval by the FDA and/or similar governmental bodies in other countries. Failure to comply with these laws and regulations could lead to sanctions by the governmental bodies, such as written observations of deficiencies made following inspections, warning letters, product recalls, fines, product seizures and consent decrees, which would be made available to the public. Such actions and publicity could affect our ability to sell products and to provide our services.
Several of our operations are also subject to U.S. Department of Agriculture regulations and various foreign regulations for the sourcing, manufacturing and distribution of animal based proteins, all of which now apply to us as a result of the acquisition. Our failure to comply with these requirements could negatively impact our business and potentially cause the loss of customers and sales. ISO 9001:2000 quality standards are an internationally recognized set of voluntary quality standards that require compliance with a variety of quality requirements somewhat similar to the requirements of the FDA’s Quality System Regulations, which were formerly known as Good Manufacturing Practices or GMP. Some of our facilities are registered under the ISO standards. Failure to comply with this voluntary standard can lead to observations of non-compliance or even suspension of ISO certification by the certifying unit. Loss of ISO certification could cause some customers to purchase products from other suppliers.
If we experience a significant disruption in our information technology systems or if we fail to implement new systems and software successfully, our business could be adversely affected.
We rely on one centralized information system throughout our company to keep financial records, process orders, manage inventory, process shipments to customers and operate other critical functions. If we were to experience a prolonged system disruption in the information technology systems that involve our interactions with customers and suppliers, it could result in the loss of sales and customers, which could adversely affect our business.
We are subject to economic, governmental, political, legal and other risks associated with our significant international sales and operations, which could adversely affect our business.
We conduct operations throughout the world through a variety of subsidiaries and distributors. Sales outside the United States were approximately 63 percent and 61 percent of total sales in 2007 and 2006, respectively. A significant portion
| | |
MILLIPORE FORM 10-K 2007 | | 23 |
PART I
of our revenues, approximately 41 percent and 17 percent in 2007, is generated in Europe and Asia, respectively. We anticipate that revenue from international operations will continue to represent a significant portion of our revenues. In addition, two of our primary manufacturing facilities, Molsheim, France and Cork, Ireland, and many of our employees and suppliers, are located outside the United States. Our sales and earnings could be adversely affected by a variety of factors resulting from our international operations, including:
| | n | | changes in the political or economic conditions in a country or region, particularly in developing or emerging markets; |
| | n | | trade protection measures and import or export licensing requirements; |
| | n | | our failure or the failure of our commercial partners to comply with U.S. laws applicable to foreign operations or with applicable local laws; |
| | n | | differing tax laws and changes in those laws; |
| | n | | difficulty in staffing and managing widespread operations; and |
| | n | | differing regulatory requirements and changes in those requirements. |
Foreign exchange fluctuations may adversely affect our reported earnings, the value of our assets and the cash outflow for our debt repayment.
We prepare our consolidated financial statements in U.S. dollars, but a significant portion of our earnings and expenditures are in other currencies. In 2007, we derived about 63 percent of our revenues from customers outside the United States. Our sales made in countries other than the United States are typically made in the local currencies of those countries. As a result, fluctuations in exchange rates have caused and will continue to cause foreign currency gains and losses. Fluctuations in exchange rates between the U.S. dollar and other currencies may also affect the book value of our assets outside the United States. We intend to repay our Euro-denominated debt from our European profits. There can be no assurance that such cash flow from our European operations will be sufficient to repay such debt, in which case we may need to repay from profits denominated in U.S. dollars. In such an event, a significant appreciation of the Euro with respect to the U.S. dollar could expose us to additional foreign currency risk. Due to the number of currencies involved, the variability of currency exposures and the potential volatility of currency exchange rates, we cannot predict the effects of exchange rate fluctuations on future operating results. We seek to minimize our currency exposure by coordinating our worldwide supply sourcing, actively managing cross-border currency flows, and engaging in foreign exchange hedging transactions. Despite these steps, there can be no assurance that our foreign currency management strategy will adequately protect our operating results from the effects of future exchange rate fluctuations.
Reduction in our customers’ research and development budgets and government funding may result in reduced sales.
Our customers include researchers at pharmaceutical and biotechnology companies, academic institutions and government and private laboratories throughout the world. Their research and development budgets and activities have a large effect on the demand for our products and services. Fluctuations in our customers’ research and development budgets occur due to changes in available resources, mergers of pharmaceutical and biotechnology companies, spending priorities and institutional budgetary policies. Our bioscience business could be adversely impacted by any significant decrease in life sciences research and development expenditures by pharmaceutical and biotechnology companies, academic institutions or government and private laboratories. In addition, short term changes in administrative, regulatory or purchasing-related procedures can create uncertainties or other impediments which can contribute to lower sales.
| | |
| 24 | | MILLIPORE FORM 10-K 2007 |
PART I
A portion of our bioscience sales have been to researchers, universities, government laboratories and private foundations whose funding may be dependent in part upon grants from government agencies such as the U.S. National Institutes of Health (“NIH”) and similar domestic and international agencies. NIH funds are subject to reallocation, reduction or discontinuation, which could impact research projects using our products. Government funding of research and development is subject to the political process, which is inherently fluid and unpredictable. Our revenues may be adversely affected if our customers delay purchases as a result of uncertainties surrounding the approval of government or industrial budget proposals. If researchers were not able to obtain, for any extended period, government funding necessary to purchase our products or if there is a decrease in overall research funding, it could reduce our bioscience sales and damage our business.
Our revenues may fluctuate, and this fluctuation could cause financial results to be below expectations.
Fluctuations in our operating results from period to period may occur for a number of reasons. In planning our operating expenses for the foreseeable future, we assume that revenues will continue to grow. Generally operating expenses cannot be adjusted quickly in the short term because we have significant fixed costs. If our revenues decline or do not grow as anticipated, we may not be able to reduce our operating expenses accordingly. Failure to achieve anticipated levels of revenue could therefore significantly harm our operating results for a particular period.
A revenue shortfall could arise from any number of factors, some of which we cannot control. For example, factors that may cause our results to vary by period include:
| | n | | the volume and timing of orders from customers for our products and services; |
| | n | | the level and timing of our customers’ research and commercialization efforts; |
| | n | | changes in the mix of our products and services; |
| | n | | the number, timing and significance of new products and services introduced by our customers; |
| | n | | our ability to develop, market and introduce new and enhanced products and services on a timely basis; |
| | n | | changes in the cost, quality and availability of materials and components required to manufacture or use our products; |
| | n | | the timing and costs of any acquisitions of businesses or technologies; |
| | n | | the introduction of new products by us or our competitors; |
| | n | | exchange rate fluctuations; and |
| | n | | general economic conditions. |
Increased exposure to product liability claims could adversely affect our earnings.
Product liability is a major risk in testing and marketing biotechnology and pharmaceutical products offered by our customers. Currently these risks are primarily borne by our customers. As our products and services are further integrated into our customers’ production processes, we may become increasingly exposed to product liability and other claims in the event that the use of our products or services is alleged to have resulted in adverse effects. There can be no assurance that a future product liability claim or series of claims brought against us would not have an adverse effect on our business or the results of operations. Our business may be materially and adversely affected by a successful product liability claim or claims in excess of any insurance coverage that we may have. In addition, product liability claims, regardless of their merits, could be costly and divert management’s attention, and adversely affect our reputation and the demand for our products.
| | |
MILLIPORE FORM 10-K 2007 | | 25 |
PART I
The stated value of long-lived and intangible assets may become impaired and result in an impairment charge.
As of December 31, 2007, we had approximately $2,040.9 million of long-lived and intangible assets. We continue to invest in the construction and upgrading of our manufacturing and research facilities which may have the effect of increasing the recorded value of our long-lived assets. If we are successful in acquiring additional complementary businesses and technologies, a substantial portion of the value of these may be recorded as goodwill, an intangible asset. The carrying amounts of long-lived and intangible assets are affected whenever events or changes in circumstances indicate that the carrying amount of any asset may not be recoverable. Such events or changes might include a significant decline in market share, a significant decline in profits, rapid changes in technology, failure to achieve the benefits of capacity increases and utilization, significant litigation arising out of an acquisition or other matters. Adverse events or changes in circumstances may affect the estimated undiscounted future operating cash flows expected to be derived from long-lived and intangible assets. If at any time we determine that an impairment has occurred, we will be required to reflect the impaired value as a charge, resulting in a reduction in earnings in the quarter such impairment is identified and a corresponding reduction in our net asset value. The potential recognition of impairment in the carrying value, if any, could have a material and adverse affect on our results of operations.
We may require substantial additional capital to pursue strategic acquisitions or alliances, which capital we may not be able to obtain on commercially reasonable terms, if at all.
We anticipate that our currently planned capital requirements will be satisfied by the future operating cash flow, current cash balances, borrowings under our revolver, or other existing financing sources. To the extent that we desire to pursue a strategic acquisition or alliance requiring substantial cash expenditures for which our existing resources and credit facilities are insufficient, we may need to raise funds through public or private debt or equity financings. There is no assurance that such additional funds will be available or, if available, that we can obtain such funds on terms acceptable to us.
If adequate funds are not available, we may have to forgo desired acquisitions or alliances, or reduce expenditures for research and development, production or marketing, which could have an adverse effect on our business. To the extent that additional capital is raised through the sale of equity or convertible securities, the issuance of such securities could result in dilution to our shareholders.
Future issuances of common stock may depress the trading price of our common stock and our convertible notes.
Any issuance of equity securities, including the issuance of shares upon conversion of our convertible notes, could dilute the interests of our existing stockholders, including holders who have received shares upon conversion of their notes, and could substantially decrease the trading price of our common stock and our convertible notes. We may issue equity securities in the future for a number of reasons, including to finance our operations and business strategy (including in connection with acquisitions, strategic collaborations or other transactions), to adjust our ratio of debt to equity, to satisfy our obligations upon the exercise of outstanding warrants or options or for other reasons.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
Not applicable
| | |
| 26 | | MILLIPORE FORM 10-K 2007 |
PART I
ITEM 2. PROPERTIES.
Our headquarters are located in leased facilities in Billerica, Massachusetts. We own or lease various other facilities worldwide for manufacturing, distribution, warehousing, research and development, sales and demonstration, service, and administration. The following is a list of our principal and other materially important facilities. We use substantially all of the space in these facilities and we believe these facilities are maintained in good working order and suitable for their present uses.
| | | | | | |
| Location | | Facility Use | | Owned
or Leased | | Approximate Floor Space Sq. Ft. (000s) |
Bedford, MA | | Manufacturing, research, warehouse and office | | Owned | | 341 |
Molsheim, France | | Manufacturing, research, warehouse and office | | Owned | | 321 |
Jaffrey, NH | | Manufacturing, warehouse and office | | Owned | | 255 |
Cork, Ireland | | Manufacturing, warehouse and office | | Owned | | 178 |
Burlington, MA | | Warehouse and distribution | | Leased | | 130 |
Billerica, MA | | Research and office | | Both | | 127 |
Temecula, CA | | Manufacturing, research, warehouse and office | | Owned | | 111 |
Danvers, MA | | Manufacturing, research and office | | Owned | | 108 |
Billerica, MA | | Office (headquarters) | | Leased | | 104 |
Kankakee, IL | | Manufacturing, research, warehouse and office | | Both | | 83 |
St. Charles, MO | | Manufacturing, research, warehouse and office | | Owned | | 81 |
Livingston, Scotland | | Manufacturing, research, warehouse and office | | Both | | 60 |
Consett, England | | Manufacturing, research, warehouse and office | | Leased | | 36 |
None of our owned facilities are subject to any material encumbrances, except for a finance lease on a portion of the Molsheim, France property.
As part of a coordinated program to optimize our global manufacturing operations, we sold our Cidra, Puerto Rico facility in 2006. We are currently leasing this facility from the new owner until we complete the transition of manufacturing operations in accordance with our manufacturing consolidation strategy in 2008.
ITEM 3. LEGAL PROCEEDINGS.
We are not currently a party to any material legal proceeding.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS.
This item is not applicable.
| | |
MILLIPORE FORM 10-K 2007 | | 27 |
PART I
SUPPLEMENTARY ITEM. EXECUTIVE OFFICERS OF THE REGISTRANT (PURSUANT TO INSTRUCTION 3 TO ITEM 401(b) OF REGULATION S-K).
The following is a list, as of February 19, 2008, of the executive officers of Millipore Corporation. Except as noted, all of such executive officers were elected to serve until the first Directors Meeting following our 2008 Annual Shareholders Meeting.
| | | | | | | | |
| | | | | | | First Elected or
Appointed |
| Name | | Age | | Office | | An
Executive
Officer | | To
Present
Office |
Martin D. Madaus | | 48 | | Chairman of the Board, President and Chief Executive Officer | | 2005 | | 2005 |
Dominique F. Baly | | 59 | | Vice President, President of Bioscience Division | | 2000 | | 2005 |
Bruce J. Bonnevier | | 49 | | Vice President, Global Human Resources | | 2006 | | 2006 |
Dennis W. Harris | | 51 | | Vice President and Chief Scientific Officer | | 2006 | | 2006 |
Geoffrey F. Ide | | 54 | | Vice President, Millipore International | | 2006 | | 2006 |
Peter C. Kershaw | | 54 | | Vice President, Global Supply Chain | | 2004 | | 2005 |
Jean-Paul Mangeolle | | 46 | | Vice President, President of Bioprocess Division | | 2005 | | 2005 |
Jeffrey Rudin | | 56 | | Vice President, General Counsel and Secretary | | 1996 | | 1996 |
Gregory J. Sam | | 49 | | Vice President, Quality | | 2003 | | 2003 |
Charles F. Wagner, Jr. | | 39 | | Vice President and Chief Financial Officer | | 2003 | | 2007 |
Wei Zhang * | | 40 | | Vice President, Strategy and Corporate Development
| | 2008 | | 2008 |
| * | | Dr. Zhang was not elected by the Board of Directors, but we have determined that she is an executive officer as such term is defined in Rule 3b-7 under the Exchange Act of 1934, as amended. |
Dr. Madaus joined Millipore Corporation as our President and Chief Executive Officer, and as a Director, on January 1, 2005, and was appointed Chairman of the Board effective March 1, 2005. From 2000 until December 2004, Dr. Madaus served as President and Chief Executive Officer of Roche Diagnostics Corporation, heading the North American diagnostics business of Hoffmann-La Roche, a leading pharmaceutical and diagnostics company. Prior to that, Dr. Madaus held various management positions from 1989 to 1999 with Hoffmann-La Roche and with Boehringer Mannheim (prior to its 1998 acquisition by Hoffmann-La Roche). Dr. Madaus also serves as a board member of each of the New England Healthcare Initiative, the Analytical & Life Science Systems Association, the Massachusetts High Technology Council, Predictive Biosciences, Inc., a privately held company in Lexington, MA and the YMCA of Greater Boston.
Mr. Baly was elected Vice President of Millipore Corporation in December 2000 and serves as President of our Bioscience Division, which was formed in February 2005 as a combination of our Laboratory Water and Life Science Divisions. Mr. Baly also served as President of Millipore International to which he was appointed in February 2001. From February 2001 through February 2005, Mr. Baly was President of the Laboratory Water Division. Prior to that, Mr. Baly held a wide variety of positions since joining us in 1972, most recently as Vice President of the Analytical Divisions of Millipore from 1994 until 2001.
| | |
| 28 | | MILLIPORE FORM 10-K 2007 |
PART I
Mr. Bonnevier joined Millipore Corporation as Vice President of Global Human Resources in January 2006. From 2004 to 2005, Mr. Bonnevier served as Vice President of Human Resources for Hillenbrand Industries, Inc., a company that owns and operates businesses that provide products and services for the health care and funeral services industries. From 2000 to 2004, he was Vice President of Human Resources for Shipley Company, now the Electronic Materials Division of the Rohm and Haas Company, a leading producer of specialty materials used in a wide variety of applications, including electronic materials, paints and personal care products. From 1989 through 2000, Mr. Bonnevier held various senior management roles at Rohm and Haas, including Director of International Human Resources and Business Human Resources Manager.
Dr. Harris joined Millipore Corporation as our Chief Scientific Officer following our acquisition of Serologicals in July 2006. From 2004 to 2006, Dr. Harris served as Vice President, Global Research & Development and business development and Chief Scientific Officer at Serologicals. From 2002 to 2003, Dr. Harris served as Executive Vice President, Research & Development for Vitra Biosciences, Inc., a developer of cell-based drug screening array systems for drug discovery. From 2001 to 2003, Dr. Harris held senior Research & Development and business positions at ACLARA Biosciences, Inc., a developer of novel technologies in the areas of microfluidics and gene and protein analysis. For approximately twenty years prior to joining ACLARA, Dr. Harris held positions of increasing responsibility at Amersham Pharmacia Biotech, Inc. and its affiliates, most recently as Vice President of Research and Development for North America and global genomics Research and Development from 1997 to 2001. Amersham (acquired by General Electric Corporation in 2004) is a manufacturer of pharmaceutical products for the diagnosis and treatment of disease and of technologies for biotechnology research and drug discovery.
Mr. Ide joined Millipore Corporation in 2005 as Vice President, Millipore International, with responsibility for market development opportunities in Japan, Asia, India, South America, Eastern Europe, the Middle East and Africa. In August 2006 Mr. Ide became a member of the Corporate Executive Committee. Prior to joining Millipore, Mr. Ide was employed by Bausch & Lomb Incorporated, a world leader in the development, manufacture and marketing of eye health products, from 1988 to 2005. He served Bausch & Lomb in positions of increasing responsibility, most recently as corporate Vice President and President of Japan Operations from 1999 to 2005.
Mr. Kershaw was elected Vice President, Worldwide Manufacturing Operations, of Millipore Corporation effective February 2004 and, in August 2005, was appointed head of our newly created Global Supply Chain organization, a combination of the Company’s worldwide manufacturing and customer service functions. Prior to joining Millipore, Mr. Kershaw served Hologic, Inc., a manufacturer of medical imaging systems, as Corporate Vice President, Manufacturing Operations (2003-2004) and Vice President and General Manager, LORAD Division (2001-2003). Prior to that, Mr. Kershaw served as President (1998-2001) and Vice President and General Manager (1996-1998) of the Medical Device Division of Bespak plc, a manufacturer of plastic injection molded components and finished medical devices.
Mr. Mangeolle was elected Vice President of Millipore Corporation in October 2005 and is President of the Bioprocess Division. From 2002 to 2005, he served as Vice President of the Division’s Worldwide Field Operations. From 2001 to 2002, Mr. Mangeolle was Vice President of Operations of Mykrolis Corporation, a spin-off of Millipore’s former Microelectronics Division. Prior to 2001, Mr. Mangeolle held a number of senior management positions in Millipore’s Microelectronics and Laboratory Water Divisions, as well as Millipore’s Asian Operations. Mr. Mangeolle joined Millipore SA, our wholly-owned subsidiary in France, as a sales applications specialist in 1984.
Mr. Rudin was elected Vice President and General Counsel of Millipore Corporation in December 1996 and as Clerk (that office is now known as Secretary) of Millipore in 1999. Prior to joining Millipore, Mr. Rudin served Ciba Corning Diagnostics Corp. as Senior Vice President and General Counsel (1993-1996) and as Vice President and General Counsel (1988-1993).
| | |
MILLIPORE FORM 10-K 2007 | | 29 |
PART I
Mr. Sam was elected Vice President, Quality, of Millipore Corporation in March 2003. Prior to joining Millipore, Mr. Sam served from 2001-2002 as Vice President, Quality, for the Drug Delivery Business Unit of Elan Corporation, a pharmaceutical company focused on the development, manufacturing and marketing of novel therapeutic products, and from 2000- 2001 as Vice President, Quality, of Dura Pharmaceuticals (acquired by Elan Corporation in 2000), a manufacturer of pre- scription pharmaceutical products. From 1999 to 2000, Mr. Sam was Senior Director, Corporate QA – Quality Management, at Watson Pharmaceuticals, Inc., a specialty pharmaceutical company.
Mr. Wagner was elected Vice President and Chief Financial Officer of Millipore Corporation effective in August 2007. Mr. Wagner joined Millipore Corporation in December 2002 as Director of Strategic Planning and Business Development and was elected Vice President, Strategic Planning and Business Development (now Strategy and Corporate Development), in March 2003, serving in this role until his election as Chief Financial Officer. Prior to joining Millipore, Mr. Wagner served as a Manager (2001-2002) and Consultant (1998-2001) at Bain & Company.
Dr. Zhang joined Millipore Corporation in February 2008 as Vice President of Strategy and Corporate Development. Prior to joining Millipore, since 2004, Dr. Zhang served as President of Harvard Square Consulting, Inc., a boutique consulting firm focusing on helping multinational companies enter, invest, and compete in China. From 1997 to 2003, Dr. Zhang worked at McKinsey & Company, a leading management consulting firm. Her responsibilities increased from Associate to Senior Engagement Manager while serving clients in healthcare, high tech, and financial services on strategy, operations, and financial management.
| | |
| 30 | | MILLIPORE FORM 10-K 2007 |
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON STOCK, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Millipore’s Common Stock, $1.00 par value, is listed on the New York Stock Exchange and is traded under the symbol “MIL”. The following table sets forth, for the indicated fiscal periods, (i) the high and low sales prices of Millipore’s Common Stock (as reported on the New York Stock Exchange Composite Tape). On February 11, 2008, there were approximately 37,137 registered and beneficial shareholders of record.
| | | | | | | | | | | | |
| | | Range of Stock Prices |
| | | 2007 | | 2006 |
| | | High | | Low | | High | | Low |
First Quarter | | $ | 75.27 | | $ | 65.81 | | $ | 74.52 | | $ | 63.84 |
Second Quarter | | $ | 77.47 | | $ | 71.96 | | $ | 76.95 | | $ | 60.53 |
Third Quarter | | $ | 80.39 | | $ | 69.07 | | $ | 67.36 | | $ | 59.58 |
Fourth Quarter | | $ | 82.43 | | $ | 72.49 | | $ | 70.16 | | $ | 60.51 |
We did not declare any cash dividends during 2007 or 2006. We do not currently have plans to make future cash dividend declarations or payments.
| | |
MILLIPORE FORM 10-K 2007 | | 31 |
PART II
ITEM 6. SELECTED FINANCIAL DATA.
The following selected consolidated financial data are derived from our Consolidated Financial Statements and notes thereto and should be read in connection with and are qualified in their entirety by our Consolidated Financial Statements and notes thereto and other financial information included elsewhere in this Form 10-K report.
FIVE YEAR SUMMARY OF OPERATIONS
| | | | | | | | | | | | | | | | | | | | |
| (In thousands, except per share data) | | 2007 | | | 2006(2) | | | 2005 | | | 2004 | | | 2003 | |
| Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Net sales | | $ | 1,531,555 | | | $ | 1,255,371 | | | $ | 991,031 | | | $ | 883,263 | | | $ | 799,622 | |
Cost of sales | | | 721,092 | | | | 625,608 | | | | 472,023 | | | | 412,129 | | | | 369,174 | |
Gross profit | | | 810,463 | | | | 629,763 | | | | 519,008 | | | | 471,134 | | | | 430,448 | |
Selling, general and administrative expenses | | | 486,737 | | | | 398,842 | | | | 309,029 | | | | 270,796 | | | | 246,819 | |
Research and development expenses | | | 106,999 | | | | 86,617 | | | | 66,052 | | | | 62,485 | | | | 58,385 | |
Restructuring and other | | | – | | | | – | | | | 3,149 | (4) | | | – | | | | (1,400 | )(6) |
Operating profit | | | 216,727 | | | | 144,304 | | | | 140,778 | | | | 137,853 | | | | 126,644 | |
Interest income | | | 1,453 | | | | 21,415 | | | | 3,466 | | | | 2,073 | | | | 2,035 | |
Interest expense | | | (65,757 | ) | | | (45,336 | ) | | | (6,711 | ) | | | (9,447 | ) | | | (16,505 | ) |
Income before income taxes and minority interest | | | 152,423 | | | | 120,383 | | | | 137,533 | | | | 130,479 | | | | 112,174 | |
Provision for income taxes | | | 12,424 | (1) | | | 21,462 | | | | 57,365 | (5) | | | 24,923 | | | | 11,378 | (7) |
Minority interest | | | 3,527 | | | | 1,937 | | | | – | | | | – | | | | – | |
Net income | | $ | 136,472 | | | $ | 96,984 | | | $ | 80,168 | | | $ | 105,556 | | | $ | 100,796 | |
| | | | | |
Earnings per share: | | | | | | | | | | | | | | | | | | | | |
Basic earnings per share | | $ | 2.52 | | | $ | 1.82 | | | $ | 1.57 | | | $ | 2.13 | | | $ | 2.08 | |
Diluted earnings per share | | $ | 2.48 | | | $ | 1.79 | | | $ | 1.55 | | | $ | 2.10 | | | $ | 2.06 | |
| | | | | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | | |
Basic | | | 54,263 | | | | 53,160 | | | | 50,953 | | | | 49,469 | | | | 48,574 | |
Diluted | | | 55,028 | | | | 54,245 | | | | 51,659 | | | | 50,201 | | | | 49,046 | |
| | | | | |
| Balance Sheet Data (at end of year): | | | | | | | | | | | | | | | | | | | | |
Working capital | | $ | 407,848 | | | $ | 307,525 | | | $ | 824,502 | | | $ | 377,846 | | | $ | 316,070 | |
Total assets | | | 2,777,257 | | | | 2,771,491 | | | | 1,646,665 | | | | 1,013,819 | | | | 960,298 | |
Long-term debt | | | 1,260,043 | | | | 1,316,256 | (3) | | | 552,285 | | | | 147,000 | | | | 216,000 | |
Total shareholders’ equity | | | 1,136,568 | | | | 948,411 | | | | 791,563 | | | | 638,850 | | | | 464,681 | |
| (1) | | In 2007, we recorded $11,900 of previously unrecognized tax benefits in our statement of operations as a result of the completion of tax examinations and statute of limitations closures. |
| (2) | | Our 2006 statement of operations and balance sheet data included the effect of our acquisition of Serologicals. The operating results of Serologicals’ operations have been included in our consolidated statement of operations since July 14, 2006, the date of the acquisition. |
| (3) | | In 2006, we issued $565,000 of 3.75 percent convertible notes and€250,000, or $330,033, of 5.875 percent senior notes to fund the acquisition of Serologicals. |
| (4) | | In the 2005 third quarter, we expensed purchased in-process research and development related to the acquisition of NovAseptic A.B. because these costs had no alternative future uses and had not reached technological feasibility. |
| (5) | | Provision for income taxes for 2005 included $30,634 of tax obligations related to the repatriation of foreign earnings under the provisions of the American Jobs Creation Act of 2004 and $3,177 related to the release of tax valuation allowance. |
| (6) | | Amount represents the reversal of accruals initially related to restructuring charges taken in connection with our 2001 restructuring program which included reducing, consolidating and outsourcing certain manufacturing operations, centralizing European shared services (including order processing, cash collections and cash application processes) and streamlining certain corporate shared services and divisional overhead functions. |
| (7) | | Provision for income taxes for 2003 included a tax valuation allowance release of $21,971 related to certain foreign tax credits and a $10,000 additional tax provision related to exposures previously mitigated by the reserved foreign tax credits. |
| | |
| 32 | | MILLIPORE FORM 10-K 2007 |
PART II
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following Management’s Discussion and Analysis (“MD&A”) is intended to help the reader understand the results of operations and financial condition of Millipore Corporation. MD&A is provided as a supplement to, and should be read in conjunction with our financial statements and the accompanying notes to the financial statements.
Business Overview
We are a global leader in life science providing innovative products, services, and solutions so our customers can advance their research, development, and production. Our academic, biotechnology, and pharmaceutical customers use our consumable products and services to increase their speed and to improve their consistency while saving costs in laboratory applications and in biopharmaceutical manufacturing. With our extensive technical expertise and applications knowledge, we have the unique ability to engage in peer-to-peer discussions with scientists to help them confront challenging scientific and human health issues.
We are organized around two operating divisions. Our Bioscience Division, which contributed approximately 43% of our 2007 revenues, improves laboratory productivity and workflows by providing innovative products and technologies for life science research. Our Bioprocess Division, which contributed approximately 57% of our 2007 revenues, helps pharmaceutical and biotechnology companies develop their manufacturing processes, to optimize their manufacturing productivity, and ensure the quality of drugs.
BUSINESS DRIVERS
Our Bioscience market is primarily driven by the amount of research activity conducted by pharmaceutical and biotechnology companies, academic institutions, governments and other organizations. The more research that is conducted worldwide, the higher the demand is for our consumable products used in these research activities. Some of the key market trends affecting our Bioscience Division include the global expansion of laboratories, particularly in Asia, the move from genomic-based research toward protein research and cell biology, and higher demand for workflow-based solutions that improve laboratory productivity. For example, pressure on global pharmaceutical and biotechnology companies to identify new drug candidates has led to increasing demand for our products that increase laboratory productivity. Products that are pre-validated and optimized with each other save time and increase efficiency for the researcher, particularly when combined in kits. We have expanded our number of new products and incorporated our lab filtration, reagents and other products into critical laboratory protocols. We believe customers are willing to pay a premium for innovation, expertise and streamlined purchase and service benefits.
The market drivers of our Bioprocess Division include increasing demand and production volumes of marketed therapeutics and the number of approvals for new biologics and new indications for existing biologics. In particular, a higher number of approvals for monoclonal antibodies, recombinant vaccines and other recombinant protein-based therapeutics are driving the market. Pharmaceutical companies are shifting more of their drug pipeline from chemically-based drugs toward biologic drugs.
Monoclonal antibodies are one of the fastest growing biologic drugs. They are being produced in larger volumes because of increasing demand and their ability to treat diseases that previously had a limited number of therapies. They are separation-intensive, complex to produce, and require significant use of our products. The growth in biologics is creating an increase in demand for our consumable products that enable the production of therapeutic drugs. We provide a number of technologies that can be used in small-scale production of a drug and be reliably scaled up to commercial size manufacturing volumes. We are strategically positioned to gain customer access, to increase our applications knowledge, and to
| | |
MILLIPORE FORM 10-K 2007 | | 33 |
PART II
identify new technologies and customer needs. This enables us to help optimize our customers’ productivity. As a result, we expect our revenues related to a specific drug will increase over the various stages of the drug approval process, particularly, as the drug moves into later stage clinical trials and ultimately into commercial production.
OUR STRATEGY
Our corporate strategy is to provide differentiated solutions to the life science research and biopharmaceutical manufacturing markets, which we believe have significant needs for new products that drive results, productivity improvements and new research goals. Since 2005, our strategy has been organized around five objectives:
| | n | | Strengthen our leadership position with biotechnology manufacturing customers by expanding our bioprocess product offerings; |
| | n | | Establish Millipore as a strategic supplier in bioscience research markets by increasing our laboratory productivity platforms and market reach; |
| | n | | Lead our industry in product quality and manufacturing effectiveness; |
| | n | | Attract, retain and develop talented and motivated employees; and |
| | n | | Double the value of the company between 2005 and 2009. |
Our Bioscience Division’s strategy is to capitalize on its global infrastructure and core capabilities in filtration, reagents, and assay development to provide differentiated offerings in fast growing market segments. The division pursues targeted, market-specific strategies in laboratory water, drug discovery, and life science research. The division leverages three expert sales organizations to execute the multiple-segment strategy under one premium brand.
The Bioprocess Division’s strategy is to leverage its leading position and broad portfolio of products to offer its biopharmaceutical customers integrated solutions that improve their productivity. By enabling companies to move from a product-centric approach to an integrated approach, the division can uniquely help customers increase their speed, lower their costs, minimize their risk, and increase their quality. The division’s global sales organization is focused on selling products, services, and applications expertise that provide its customers with a comprehensive approach to optimize their biopharmaceutical manufacturing process.
2007 HIGHLIGHTS
We provide a wide range of products and services to a range of customers across a range of geographies. The breadth of our business portfolio allows us to target growth on a number of dimensions, rather than relying on any single business, market, or economy.
In 2007, we completed the integration of Serologicals Corporation (“Serologicals”) into our business. The proportion of our Bioscience revenue to total revenue increased in 2007 as a result of including a full year of Serologicals revenue, which had a greater percentage of its revenue derived from the life science research market. Since our Bioprocess revenues tend to fluctuate with the timing of our customers’ major drug campaigns and finished goods inventory levels, a higher mix of Bioscience revenues helps offset the effect of these fluctuations.
We also re-branded the company and launched a new website and e-business platform in 2007. Our Bioprocess Division strengthened its core product portfolio and increased the number of new product launches from 8 products in 2006 to 15 products in 2007. These new products included new chromatography media with the highest capacity and flow rate now on the market and a new range of disposable mixers that combine technologies from companies we previously acquired. Our Bioscience Division also launched a new laboratory water product (Milli-Q Advantage™), which was one of our most successful product launches in terms of first-year sales and customer orders.
| | |
| 34 | | MILLIPORE FORM 10-K 2007 |
PART II
The following table sets forth revenues derived from the Bioprocess and Bioscience divisions as a percentage of our total revenue.
| | | | | | | | | |
| | | Year ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
Bioprocess | | 57 | % | | 60 | % | | 61 | % |
Bioscience | | 43 | % | | 40 | % | | 39 | % |
Total | | 100 | % | | 100 | % | | 100 | % |
The composition of our geographic revenues is as follows:
| | | | | | | | | |
| | | Year ended
December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
Americas | | 42 | % | | 45 | % | | 43 | % |
Europe | | 41 | % | | 39 | % | | 40 | % |
Asia/Pacific | | 17 | % | | 16 | % | | 17 | % |
Total | | 100 | % | | 100 | % | | 100 | % |
The performance of our broader business portfolio, the underlying growth of our business in Europe and Asia, and the execution of our strategy resulted in 2007 revenue growth.
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Bioprocess | | | Bioscience | | | Consolidated | |
| | | 2007 | | | 2006 | | | 2005 | | | 2007 | | | 2006 | | | 2005 | | | 2007 | | | 2006 | | | 2005 | |
Reported growth | | 17 | % | | 25 | % | | 16 | % | | 29 | % | | 30 | % | | 7 | % | | 22 | % | | 27 | % | | 12 | % |
Less: Foreign currency translation | | 5 | % | | 0 | % | | 0 | % | | 5 | % | | 0 | % | | 0 | % | | 5 | % | | 0 | % | | 0 | % |
Acquisitions | | 7 | % | | 17 | % | | 4 | % | | 16 | % | | 20 | % | | 0 | % | | 11 | % | | 18 | % | | 2 | % |
Organic growth | | 5 | % | | 8 | % | | 12 | % | | 8 | % | | 10 | % | | 7 | % | | 6 | % | | 9 | % | | 10 | % |
Consolidated revenue of $1,531.6 million for 2007 increased $276.2 million, or 22 percent, compared to 2006. The 2007 revenue increase included a 5 percent favorable effect of changes in foreign currency translation rates and an 11 percent favorable effect of business acquisitions. Adjusting for these items, our consolidated revenues for 2007 grew 6 percent. Changes in product pricing had an insignificant effect on the year-over-year comparison. The revenue growth came primarily from the strength in the life sciences research market and our downstream bioprocessing products.
After adjusting for the effect of acquisitions and foreign currency translation, Bioscience Division revenue grew 8 percent in 2007 compared to 2006. The primary drivers of this growth were higher sales of laboratory water products attributable to new product launches and our expansion into rapidly growing markets, such as China and India. Additionally, our drug discovery products that we acquired from Serologicals were among the Bioscience Division’s fastest growing products in 2007. The successful execution of our sales and marketing initiatives and the increased productivity in 2007 of our combined sales organization following the Serologicals acquisition were also key factors causing this revenue growth. The growth in the Asian markets reflected the amount of research and development activities that are occurring in the region. We expect Bioscience Division revenue growth to continue through our focus on marketing and re-branding programs, a new e-commerce sales channel, broadening distributor relationships, and new products.
After adjusting for the effect of acquisitions and foreign currency translation, Bioprocess Division revenue grew 5 percent in 2007 compared to 2006. The lower year-over-year growth rate was the result of lower purchases of our products from a limited number of our largest biotechnology customers in the U.S. We believe these customers have re-evaluated market demand for their products and are reducing inventory levels to lower costs and improve working capital. These customers have also been restructuring operations, including closing manufacturing facilities, reducing manufacturing campaigns, and
| | |
MILLIPORE FORM 10-K 2007 | | 35 |
PART II
delaying expansion plans, which also contributed to lower sales of our products. We expect this trend to continue into 2008 and to improve later in the year as these customers’ drug inventory levels and spending patterns become more normal. Despite the lower demand from these customers, we believe that the overall biotechnology industry remains healthy. Increasing levels of investment by private equity and large pharmaceutical companies, expected growth in commercially available antibodies, anticipated 2008 approvals of new biologic drugs and new indications of existing biologic drugs, new biologic manufacturing facilities, and expected overall biotechnology market growth are all trends that we anticipate will result in higher sales of our products in the long run.
Our 2007 operating income increased compared to 2006 from $144.3 million to $216.7 million. This was the result of higher sales volume, a more profitable business mix, productivity improvements associated with our supply chain initiatives, achievement of Serologicals related integration cost synergies, and the favorable effect of foreign currency translation. These factors resulted in an increase of our 2007 operating profit margin to 14 percent from 11 percent in 2006.
Diluted earnings per share (“EPS”) of $2.48 in 2007 increased $0.69 compared to 2006. In addition to the higher operating income, the reversal of reserves related to uncertain tax positions upon the completion of tax examinations and statute of limitations closures contributed to the higher EPS. Higher interest expense associated with a full year inclusion of Serologicals financing costs somewhat offset these increases.
We generated $222.2 million of operating cash flows in 2007, which was a 51 percent increase over 2006. During 2007, we repaid $205.6 million of our debt. Our focus on operating cash flow generation will continue in 2008, which we expect to use primarily for debt reduction.
Results of Operations
REVENUES
Net sales and percent sales growth by division, as compared with the prior years, is summarized in the table below:
| | | | | | | | | | | | | | | |
| | | Year ended December 31, | | Percent sales growth | |
| Net sales by division ($ in millions): | | 2007 | | 2006 | | 2005 | | 2007 | | | 2006 | |
Bioprocess | | $ | 878.5 | | $ | 749.8 | | $ | 601.4 | | 17 | % | | 25 | % |
Bioscience | | | 653.1 | | | 505.6 | | | 389.6 | | 29 | % | | 30 | % |
Total | | $ | 1,531.6 | | $ | 1,255.4 | | $ | 991.0 | | 22 | % | | 27 | % |
Net sales and percent sales growth by geography, as compared with the prior years, is summarized in the table below:
| | | | | | | | | | | | | | | |
| | | Year ended December 31, | | Percent sales growth | |
| Net sales by geography ($ in millions): | | 2007 | | 2006 | | 2005 | | 2007 | | | 2006 | |
Americas | | $ | 647.7 | | $ | 564.8 | | $ | 419.6 | | 15 | % | | 35 | % |
Europe | | | 623.0 | | | 491.0 | | | 399.6 | | 27 | % | | 23 | % |
Asia/Pacific | | | 260.9 | | | 199.6 | | | 171.8 | | 31 | % | | 16 | % |
Total | | $ | 1,531.6 | | $ | 1,255.4 | | $ | 991.0 | | 22 | % | | 27 | % |
Bioprocess Division
2007 versus 2006
Bioprocess revenue of $878.5 million for 2007 increased $128.7 million, or 17 percent, compared to 2006. The 2007 revenue increase included a 5 percent favorable effect of foreign currency translation and a 7 percent favorable effect of business acquisitions. Adjusting for these items, Bioprocess revenues for 2007 grew 5 percent. Our year-over-year revenue
| | |
| 36 | | MILLIPORE FORM 10-K 2007 |
PART II
growth rate was adversely affected by reduction in purchases of our chromatography media and cell culture supplements products from a limited number of our key U.S. customers in the 2007 second half. Revenue growth in 2007 was primarily attributable to higher sales of our products used in downstream bioprocessing, particularly our filtration and systems hardware and components products. Sales of our systems hardware and components products grew in 2007 because of our customers’ drug manufacturing campaigns occurring in Europe and Asia. Sales of our disposable systems and components products grew because more customers migrated to single-use, disposable technologies that eliminate the need for cleaning stainless steel and glass equipment. Biopharmaceutical manufacturers also seek flexible manufacturing components and solutions because they enable reduced time between manufacturing runs and they can be configured and validated to meet customized biological manufacturing needs. Sales of our process monitoring tools, which are used to test for biopharmaceutical contaminants, increased because of the overall health of the biopharmaceutical markets in Europe and Asia.
From a geographic perspective and excluding the favorable effects of foreign currency translation and acquired businesses, revenues in the Americas, Europe and Asia/Pacific decreased $20.2 million, increased $33.2 million, and increased $23.8 million, respectively, in 2007 compared to 2006. The Americas decrease was primarily attributable to the sales decline of our chromatography media and cell culture supplements products. The European and Asia/Pacific increases were primarily attributable to higher sales of our downstream processing systems hardware products. Our core process filtration products also had strong growth in Europe and Asia/Pacific regions, particularly in China and India, which was the result of our direct investment in sales and marketing and infrastructure for these markets.
2006 versus 2005
Bioprocess revenue of $749.8 million for 2006 increased $148.4 million, or 25 percent, compared to 2005. Revenue growth was primarily attributable to business acquisitions and higher sales volume as a result of the strong demand for our differentiated products. Changes in foreign currency rates and product pricing had insignificant effects on the year- over-year comparisons. Revenue contributed by acquired businesses in 2006 represented approximately $100.1 million, or 67 percent, of the year-over-year revenue increase. Excluding business acquisitions, Bioprocess Division revenue increased $48.2 million, or 8 percent. Revenue growth was primarily attributable to strong demand for our core filtration and chromatography media products in the biotechnology market as a result of continued increase in biopharmaceutical production, particularly for monoclonal antibodies. Our customers are making investments to increase manufacturing capacity for biopharmaceutical drugs and expanding biotechnology product offerings through acquisitions and internal development. Revenue growth was also positively affected by increased sales of our NovaSeptum products, which are used by biotechnology customers for disposable sampling. We acquired this product line early in the third quarter of 2005. This increased revenue level was an example of the effectiveness of our sales force at integrating acquired products into our existing distribution network.
From a geographic perspective and excluding the effects of acquired businesses, revenues in the Americas, Europe and Asia/Pacific increased $22.1 million, $19.2 million, and $7.0 million, respectively, in 2006 as compared to 2005. The Americas and European increases were primarily driven by sales of our downstream bioprocessing products, such as our core process filtration and chromatography media products. The majority of the remaining sales increase occurred in international growth markets, particularly in China and India. These increases were the result of our direct investment in sales and marketing and infrastructure for these markets. Weaker market conditions for our products in Japan somewhat offset the Asia/Pacific market revenue growth.
| | |
MILLIPORE FORM 10-K 2007 | | 37 |
PART II
Bioscience Division
2007 versus 2006
Bioscience revenue of $653.1 million for 2007 increased $147.5 million, or 29 percent, compared to 2006. The 2007 revenue increase included a 5 percent favorable effect of foreign currency translation and a 16 percent favorable effect of business acquisitions. Adjusting for these items, Bioscience revenue for 2007 grew 8 percent. This year-over-year increase was primarily driven by the overall strength of the life sciences research market and increased levels of life sciences research and development in both universities and pharmaceutical and biotechnology companies, particularly in international markets. Revenue growth in 2007 was primarily attributable to strong demand for our laboratory water products and higher sales of our life sciences products, such as analytical sample preparation and molecular biology products. Our successful implementation of initiatives designed to align sales and product management goals, to prioritize key customer relationships, and to execute targeted sales and marketing campaigns has positioned us well with our research customers. Our drug discovery business also grew in the 2007 second half, particularly in sales of multiplex immunoassays, because of strong market demand for such products.
From a geographic perspective and excluding the favorable effects of foreign currency translation and the acquired businesses, revenues in the Americas, Europe, and Asia/Pacific increased $11.9 million, $9.3 million, and $19.3 million, respectively, in 2007 compared to 2006. The increases in the Americas and Europe were primarily attributable to higher sales of laboratory water and drug discovery products. The majority of the remaining sales increase occurred in international growth markets, particularly India and China. This reflected the increased levels of life sciences research and the return on our continued investment in sales and marketing infrastructure in growing Asia/Pacific markets.
2006 versus 2005
Bioscience revenue of $505.6 million for 2006 increased $116.0 million, or 30 percent, compared to 2005. Changes in foreign currency rates and product pricing had insignificant effects on the year-over-year comparisons. The Serologicals acquisition in 2006 represented approximately $78.1 million, or 67 percent, of the year-over-year revenue increase. Excluding the Serologicals acquisition, Bioscience Division revenue increased $37.9 million, or 10 percent. Revenue growth was primarily attributable to higher demand for our laboratory water and life science filtration products, higher sales in growing international markets, and the impact of new products launched late in 2005. This growth was driven by increased levels of life science research and development occurring in both universities and pharmaceutical companies, particularly in North America. The international market growth was also the result of higher sales of laboratory water products. Our customers are building and expanding their research laboratories in these markets and one of the first investments they make are in systems to produce purified water. Our successful implementation of initiatives designed to align sales and product management goals, to prioritize key customer relationships, and to launch targeted sales and marketing campaigns has positioned us well with these research customers, allowing us to serve them early in the drug development process.
From a geographic perspective and excluding the effects of Serologicals, revenues in the Americas, Europe and Asia/Pacific increased $13.3 million, $14.0 million, and $10.6 million, respectively, in 2006 as compared to 2005. These increases were primarily driven by sales of our laboratory water and life science products, and in the case of China and India, our sales and marketing and infrastructure investments in these growth markets.
| | |
| 38 | | MILLIPORE FORM 10-K 2007 |
PART II
GROSS PROFIT MARGIN
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| ($ in millions): | | 2007 | | | 2006 | | | 2005 | |
Gross profit | | $ | 810.5 | | | $ | 629.8 | | | $ | 519.0 | |
Percentage of sales | | | 52.9 | % | | | 50.2 | % | | | 52.4 | % |
2007 versus 2006
Gross profit increased $180.7 million, or 29 percent, in 2007 versus 2006. This was attributable to the increased sales volume, an improved business mix caused by a higher proportion of Bioscience revenues in 2007, productivity improvements and lower spending as a result of our supply chain initiatives, and lower amortization of business acquisition inventory fair value adjustments and lower acquisition integration costs related to the Serologicals acquisition. The higher sales volume was primarily caused by the full year inclusion of Serologicals in our 2007 operating results compared to 24 weeks in 2006. Significant factors affecting the increase in our gross profit margin were the favorable business mix, lower costs associated with our manufacturing consolidation strategy (primarily employee separation costs, facility closure costs and accelerated depreciation) amounting to $11.3 million in 2007 compared to $23.2 million in 2006, lower amortization of business acquisition inventory fair value adjustments of $11.1 million in 2007 compared to $24.9 million in 2006, and lower acquisition integration costs amounting to $2.7 million in 2007 compared to $4.5 million in 2006. Somewhat offsetting the gross profit margin increase was the effect that the stronger Euro had on translating the results of our significant manufacturing operations in Ireland and France into U.S. dollars. This caused the cost of those operations to represent a higher proportion of our total manufacturing costs in 2007. Amortization of acquired intangibles also lowered the gross profit margin, which amounted to $9.5 million in 2007 compared to $4.6 million in 2006. We expect 2008 full year amortization of acquired intangibles affecting gross profit to be approximately $9.8 million and we plan to continue with our supply chain initiatives in 2008, which will include the relocation of manufacturing operations and product lines.
2006 versus 2005
Gross profit increases in 2006 resulted from lower costs realized in connection with our ongoing manufacturing consolidation strategy and a business mix favoring our high margin Bioscience Division laboratory water products. However, the gross profit margin declined 2.2 percentage points as these increases were more than offset primarily by the amortization of business acquisition inventory fair value adjustments of $24.9 million, or 2.0 percentage points; amortization of intangible assets acquired of $4.6 million, or 0.4 percentage points; and acquisition integration costs of $4.5 million, or 0.4 percentage points. Costs associated with our manufacturing consolidation strategy (primarily employee separation costs, facility closure costs and accelerated depreciation) also lowered our gross margins in both 2006 and 2005. These costs amounted to $23.2 million in 2006 compared with $12.5 million in 2005. Stock-based compensation charges associated with the implementation of Statement of Financial Accounting Standards (“SFAS”) No. 123 (Revised 2004), “Share-Based Payment” (“SFAS No. 123(R)”) also had an unfavorable effect on the year-over-year comparisons. SFAS No. 123(R) costs lowered gross profit by $1.8 million in 2006.
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| ($ in millions): | | 2007 | | | 2006 | | | 2005 | |
Selling, general and administrative expenses | | $ | 486.7 | | | $ | 398.8 | | | $ | 309.0 | |
Percentage of sales | | | 31.8 | % | | | 31.8 | % | | | 31.2 | % |
| | |
MILLIPORE FORM 10-K 2007 | | 39 |
PART II
2007 versus 2006
Selling, general and administrative (“SG&A”) expenses increased $87.9 million, or 22 percent, in 2007 compared to 2006. The SG&A expense increase was primarily attributable to the inclusion of Serologicals’ SG&A expenses in our operating results for the full year in 2007 compared to 24 weeks in 2006, significantly higher amortization of intangible assets, the unfavorable translation effect of the weaker U.S. dollar, increased labor related costs attributable to our continued investment in our sales and marketing infrastructure, and increased stock-based compensation expense. The increase in our average headcount was a significant driver of our SG&A expense growth because employee-related expenses represents over 65 percent of our total SG&A costs. In 2007, our average employee headcount increased approximately 15 percent compared to 2006. Amortization expense related to acquired intangible assets increased $37.6 million to $48.9 million in 2007 compared to $11.3 million in 2006. We anticipate 2008 amortization expense affecting our SG&A expenses to increase $5.2 million. Serologicals integration costs were $10.2 million in 2007 compared to $9.7 million in 2006. The Serologicals integration was completed in 2007 and therefore, we do not expect to incur integration costs in 2008. Stock based compensation expense of $11.8 million increased $2.9 million, or 33 percent, compared to 2006 because of changes we made to our equity compensation plans in anticipation of the adoption of SFAS No. 123(R). We anticipate 2008 stock-based compensation expense to increase approximately $7.0 million to $8.0 million, of which $5.0 million to $6.0 million is expected to increase SG&A expense. In 2006, we incurred $2.1 million of expense related to an environmental liability and $8.7 million of expense relating to the curtailment of our retirement plan. These charges did not recur in 2007.
2006 versus 2005
SG&A expenses increased $89.8 million, or 29 percent, in 2006 compared to 2005. The primary drivers of the higher SG&A expenses were the inclusion of Serologicals’ expenses amounting to $30.3 million, Serologicals integration costs, significantly higher amortization of intangible assets, and SFAS No. 123(R) costs. Serologicals integration costs were $9.7 million in 2006 and were primarily attributable to professional advisor fees, employee separations, and incremental travel. Amortization expense related to acquisitions increased $7.0 million in 2006 compared to 2005. SG&A also increased $2.1 million in 2006 for estimated costs related to an existing environmental liability and $8.7 million attributable to the curtailment of our retirement plan. Employee stock-based compensation expenses contributed $8.8 million to the year-over-year increase, as a result of adopting SFAS No. 123(R). Additional drivers for higher SG&A expenses were increased investments in international growth markets and increased headcount in both Bioprocess and Bioscience divisions to support our sales and marketing initiatives in 2006.
RESEARCH AND DEVELOPMENT EXPENSES
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| ($ in millions): | | 2007 | | | 2006 | | | 2005 | |
Research and development expenses | | $ | 107.0 | | | $ | 86.6 | | | $ | 66.1 | |
Percentage of sales | | | 7.0 | % | | | 6.9 | % | | | 6.7 | % |
2007 versus 2006
Research and development (“R&D”) expenses increased $20.4 million, or 24 percent, in 2007 compared to 2006. Higher R&D expenses in 2007 were primarily attributable to the full year inclusion of Serologicals’ R&D expenses in our operating results in 2007 compared to 24 weeks in 2006, increased labor related costs attributable to increased headcount, and increased spending on new product development.
| | |
| 40 | | MILLIPORE FORM 10-K 2007 |
PART II
2006 versus 2005
R&D expenses increased $20.6 million, or 31 percent, in 2006 compared to 2005. Higher R&D expenses in 2006 were primarily attributable to the inclusion of a half year of Serologicals expenses amounting to $9.8 million and related integration costs of $1.8 million. Employee stock-based compensation expense accounted for $1.6 million of the year-over-year increase, which was the result of adopting SFAS No. 123(R) as of January 1, 2006.
PURCHASED IN-PROCESS RESEARCH AND DEVELOPMENT
In 2005, we wrote off $3.1 million of purchased in-process R&D costs in connection with our NovAseptic acquisition. This represented the fair value of two R&D projects that were still in development stage prior to reaching technological feasibility and were deemed to have no alternative future use. The estimated fair value of these projects was determined based on the use of a discounted cash flow model. For each project, the estimated after-tax cash flows were discounted to the present value using a discount rate of 18.0 percent.
INTEREST INCOME/EXPENSE
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| ($ in millions): | | 2007 | | | 2006 | | | 2005 | |
Interest income | | $ | 1.5 | | | $ | 21.4 | | | $ | 3.5 | |
Interest expense | | $ | 65.8 | | | $ | 45.3 | | | $ | 6.7 | |
Average interest rate during the year | | | 4.7 | % | | | 4.3 | % | | | 5.8 | % |
2007 versus 2006
Interest income decreased $20.0 million, or 93 percent, in 2007 compared to 2006. This was the result of lower investment balances attributable to prior year sales of marketable securities. The proceeds of those sales were used, in part, to fund our Serologicals acquisition on July 14, 2006.
Interest expense increased $20.4 million, or 45 percent, in 2007 compared to 2006. This increase was attributable to a full year of interest in 2007 related to our $565.0 million 3.75 percent convertible senior notes and our€250.0 million 5.875 percent senior notes issued in June 2006 to fund the acquisition of Serologicals. The effect of higher average interest rates in 2007 were partially offset by a lower overall debt balance as we continued to repay our debt. Our revolving credit facility is comprised of floating rate borrowings based on LIBOR. Increases or decreases in these rates cause increases or decreases to our interest expense, respectively.
In August 2007, the Financial Accounting Standards Board (the “FASB”) proposed FASB Staff Position (“FSP”) APB 14-a, “Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement)” (the “Proposed FSP”). The public comment period for the Proposed FSP ended in October 2007. The FASB has not issued any final standards to date. The Proposed FSP would require the proceeds from the issuance of such convertible debt instruments to be allocated between a liability component (issued at a discount) and an equity component. The resulting debt discount would be amortized over the period the convertible debt is expected to be outstanding as additional non-cash interest expense. If adopted, the Proposed FSP would change the accounting treatment for our $565.0 million of 3.75 percent convertible senior notes that were issued in June 2006. Such a change would impact the presentation of our consolidated financial statements and could result in an increase to our non-cash interest expense beginning in 2008 and for financial statements covering the 2006 and 2007 fiscal years. We cannot determine whether or not such accounting treatment will eventually be adopted by the FASB.
| | |
MILLIPORE FORM 10-K 2007 | | 41 |
PART II
2006 versus 2005
Interest income increased $17.9 million in 2006 compared to 2005. We earned interest income as a result of investing the proceeds of borrowings under our revolver in December 2005 and under our 3.75 percent convertible senior notes and the 5.875 percent senior notes issued in June 2006 in connection with the acquisition of Serologicals until the consummation of the acquisition.
Interest expense increased $38.6 million in 2006 compared to 2005. The increases were primarily attributable to a full year of borrowings under our revolving credit facility as well as borrowings under the 3.75 percent convertible senior notes and the 5.875 percent senior notes issued in June 2006 in connection with the Serologicals acquisition. The Serologicals acquisition in July 2006 was financed with the borrowings under these debt instruments. Initial revolver borrowings occurred in December 2005 in connection with the repatriation of earnings under the American Jobs Creation Act. Commitment fees of $1.3 million associated with a bridge loan commitment we secured in connection with the Serologicals acquisition also contributed to the interest expense increase in 2006 compared to 2005.
PROVISION FOR INCOME TAXES
| | | | | | | | | |
| | | Year ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
Effective income tax rate | | 8.2 | % | | 17.8 | % | | 41.7 | % |
2007 versus 2006
The effective income tax rates for 2007, 2006 and 2005 reflected the tax benefit associated with lower tax rates on international earnings, which we intend to indefinitely reinvest outside of the United States.
The decrease in the 2007 effective tax rate compared to 2006 was primarily attributable to the release of tax reserves amounting to $11.9 million and a pretax income mix favoring lower tax rate jurisdictions in 2007. Lower U.S. pretax profits as a result of reduced purchases from our large U.S. biotechnology customers and significantly higher expenses for interest and amortization were the primary causes of the mix shift. In addition, the shift in the mix of our pretax income was also the result of the continued shift of our production activities to Ireland in accordance with the manufacturing consolidation strategy. In 2008, we anticipate a higher effective income tax rate because of forecasted levels of taxable income in higher tax rate jurisdictions compared to our 2007 profit mix.
In the normal course of business, we are examined by various tax authorities, including the Internal Revenue Service (“IRS”). In 2006, the IRS completed the examination phase of years 2002 and 2003 and commenced an examination of years 2004 and 2005. In 2007, the IRS continued the examination phase of years 2004 and 2005. Although the 2004 and 2005 examinations were not settled at December 31, 2007, we believe appropriate provision was made for any potential unfavorable financial statement impact upon settlement of each of these years. Any reduction of these contingent liabilities or additional assessment would increase or decrease net income, respectively, in the period such determination is made.
2006 versus 2005
The significant decrease in the 2006 effective tax rate compared to 2005 was attributable to our 2005 repatriation of foreign earnings in accordance with the American Jobs Creation Act of 2004. The 2006 income tax provision was $30.6 lower, and the effective tax rate was 22.2 percentage points lower, than 2005 as a result of the repatriation. The 2006 effective tax rate was also lowered by significant Serologicals integration costs incurred in the United States in the second half of the year and costs surrounding the transfer of production activities from Puerto Rico, both of which caused a shift of pre-tax income to lower tax rate jurisdictions compared to 2005.
| | |
| 42 | | MILLIPORE FORM 10-K 2007 |
PART II
NET INCOME AND DILUTED EARNINGS PER SHARE
| | | | | | | | | |
| | | Year ended December 31, |
| ($ in millions, except share data): | | 2007 | | 2006 | | 2005 |
Net income | | $ | 136.5 | | $ | 97.0 | | $ | 80.2 |
Diluted earnings per share | | $ | 2.48 | | $ | 1.79 | | $ | 1.55 |
2007 versus 2006
Net income increased $39.5 million, or 41 percent, in 2007 compared to 2006. The increase was primarily the result of higher 2007 operating income and the lower effective tax rate, somewhat offset by higher interest expense associated with the financing of the Serologicals acquisition.
Diluted earnings per share increased $0.69, or 39 percent, in 2007 compared to 2006. The increase was the result of the reasons discussed above.
2006 versus 2005
Net income increased $16.8 million, or 21 percent, in 2006 compared to 2005. The increase was primarily the result of lower effective tax rate, higher interest income, and higher 2006 operating income. Net income in 2006 was also adversely affected by higher interest expense associated with the financing of the Serologicals acquisition.
Diluted earnings per share increased $0.24, or 15 percent, in 2006 as compared to 2005. The increase was the result of the reasons discussed above.
Capital Resources and Liquidity
The following table shows information about our capitalization as of the dates indicated:
| | | | | | | | |
| Total capitalization ($ in millions, except ratio amounts) | | December 31, 2007 | | | December 31, 2006 | |
Cash and cash equivalents | | $ | 36 | | | $ | 77 | |
Total debt | | $ | 1,265 | | | $ | 1,416 | |
Total capitalization (debt plus equity) | | $ | 2,402 | | | $ | 2,365 | |
Debt to total capitalization | | | 52.7 | % | | | 59.9 | % |
We assess our liquidity in terms of our ability to generate cash to fund our operating, investing, and financing activities. Our primary ongoing cash requirements will be to fund operations, capital expenditures, investments in businesses, product development, employee benefit plans, and debt service. Our primary sources of liquidity are internally generated cash flows and borrowings under our revolving credit facility. Significant factors affecting the management of our ongoing cash requirements are the adequacy of available bank lines of credit and our ability to attract long term capital with satisfactory terms. The sources of our liquidity are subject to all of the risks of our business and could be adversely affected by, among other factors, a decrease in demand for our products, our ability to integrate acquisitions, deterioration in certain financial ratios, and market changes in general.
Our ability to obtain debt financing at comparable risk-based interest rates is partly a function of our existing debt to capitalization levels as well as our current credit standing. Our credit ratings are reviewed regularly by major debt rating agencies such as Standard & Poor’s and Moody’s Investors Service. Our senior unsecured notes are rated BBB by Standard & Poor’s and Ba2 by Moody’s Investors Service and our revolving credit facility is rated BBB and Baa2 by Standard and Poor’s and Moody’s Investors Service, respectively. Our senior convertible notes are rated BB- by Standard & Poor’s and have not been rated by Moody’s Investors Service.
| | |
MILLIPORE FORM 10-K 2007 | | 43 |
PART II
We believe our future operating cash flows will be sufficient to meet our future operating and investing cash needs. Furthermore, our ability to obtain equity financing, as well as availability of additional borrowings under our revolving credit facility, provide additional potential sources of liquidity should they be required.
In 2007, we reduced our cash balances reflecting our focus on debt repayment. We intend to continue to maintain our cash balances at a level that reflects the minimum operating needs of our subsidiaries in which we conduct our business. The repatriation of cash balances from certain of our subsidiaries could have adverse tax consequences. However, these cash balances are generally available without legal restrictions to fund ordinary business operations. We have transferred, and will continue to transfer, cash from our subsidiaries to us and to other international subsidiaries when it is cost effective to do so.
CASH FLOWS
The following table summarizes our sources and uses of cash over the periods indicated:
| | | | | | | | | | | | |
| ($ in millions) | | 2007 | | | 2006 | | | 2005 | |
Net cash provided by operating activities | | $ | 222.2 | | | $ | 147.3 | | | $ | 185.1 | |
Net cash (used for) investing activities | | | (113.5 | ) | | | (1,168.8 | ) | | | (301.6 | ) |
Net cash (used for) provided by financing activities | | | (153.4 | ) | | | 557.1 | | | | 515.5 | |
(Decrease) increase in cash and cash equivalents | | | (41.3 | ) | | | (459.6 | ) | | | 384.9 | |
OPERATING CASH FLOWS
Cash provided by operating activities was $222.2 million for the year ended December 31, 2007 and was primarily attributable to our net income of $136.5 million and non-cash adjustments for depreciation and amortization expenses of $123.7 million, stock-based compensation expense of $16.0 million, and business acquisition inventory fair value adjustments of $11.1 million. Offsetting this were uses of operating cash flows attributable to deferred income tax benefits of $20.6 million and working capital of $46.5 million. Our deferred income tax benefits were the result of a decrease in our deferred tax liabilities associated with the amortization of acquired intangible assets from business acquisitions, which are not tax deductible, and higher net operating loss and tax credit carryforwards. The increase in our net working capital was primarily attributable to decreases in accrued expenses of $37.1 million resulting from the timing of accrued interest payments; employee separation payments and facility payments associated with the Serologicals acquisition; reductions of retirement plan contributions as a result of the change in our plans; and overall decreases in accrued general expenses. We also used operating cash flow to fund increased inventory levels amounting to $15.6 million attributable to increased safety stock levels required in connection with our manufacturing consolidation strategy and inventories associated with new products. In the 2007 second half, we managed cash collections and disbursements to maximize cash available for debt reduction.
The increased inventory balances discussed above caused the number of days supply in ending inventory to increase 20 days to 138 days at December 31, 2007 compared to 118 days at December 31, 2006. The number of days sales outstanding in ending accounts receivable remained the same at 67 days at December 31, 2007 compared with December 31, 2006, showing our continued focus on cash collections.
INVESTING CASH FLOWS
Cash used for investing activities was $113.5 million during 2007 compared with $1,168.8 million during 2006. The decrease was primarily attributable to our acquisition of Serologicals in 2006. During 2007, we paid $101.7 million for capital expenditures and $17.9 million for the settlement of a forward exchange contract used to hedge our exposure to
| | |
| 44 | | MILLIPORE FORM 10-K 2007 |
PART II
foreign exchange risks associated with certain European borrowings. We also received $6.0 million for the sale of property, plant and equipment, primarily facilities we acquired from Serologicals. We expect our capital expenditures to be approximately $102.0 million for 2008.
FINANCING CASH FLOWS
Cash used in financing activities was $153.4 million during 2007 compared with cash provided by financing activities of $557.1 million during 2006. The decrease was primarily attributable to the debt we raised in 2006 for our July 14, 2006 acquisition of Serologicals. Repayments of debt in 2007 included repayment of our $100.0 million 7.5 percent ten-year unsecured notes and net revolver repayments of $105.6 million. Cash used in financing activities was partially offset by cash received from employees upon the exercise of stock options amounting to $49.9 million.
FINANCING COMMITMENTS
Short-term debt
Short-term debt at December 31, 2007 consisted of borrowings under our operating bank facilities. Short-term debt at December 31, 2006 consisted of our 7.5 percent ten-year unsecured notes in the aggregate amount of $100.0 million. These notes were due on April 1, 2007 and were paid off, including accrued interest of $3.8 million, with cash on hand and borrowings under our revolving credit facility.
Revolving credit facility
We entered into an agreement for a five-year unsecured revolving credit facility (the “Revolver”) in December 2005. The acquisition of Serologicals on July 14, 2006 and the related financing required us to change certain terms of the Revolver agreement. Accordingly, we amended the agreement in June 2006 (some of which became effective on July 14, 2006) to:
| | n | | permit the consummation of the Serologicals acquisition and issuance and incurrence of certain additional indebtedness in connection with the acquisition; |
| | n | | extend the maturity date to June 6, 2011; |
| | n | | require interest rate and commitment fee adjustments based on specified credit ratings; |
| | n | | require the pledge of substantially all our assets to secure our obligations under the Revolver if specified credit rating levels are reached; and |
| | n | | adjust certain restrictions and financial covenants. |
We further amended the Revolver agreement in July 2006 to increase the borrowing availability under the domestic facility from€430.0 million, or $627.3 million, to€465.0 million, or $678.3 million. In the second quarter of 2006, we recorded $2.8 million of deferred financing costs associated with amending the Revolver agreement.
We are required to pay an unused commitment fee ranging between 0.0675 percent and 0.60 percent annually based on the Revolver’s debt rating.
We are required to maintain certain leverage and interest coverage ratios as set forth in the Revolver agreement. The agreement also includes limitations on our ability to incur additional indebtedness, to merge, consolidate, or sell assets, to create liens, to make payments in respect of capital stock or subordinated debt, as well as other customary covenants and representations. In general, the leverage ratio is calculated by dividing our total outstanding indebtedness at December 31, 2007 by our cumulative adjusted cash earnings for the twelve months ended at December 31, 2007. The interest coverage ratio is calculated by dividing our cumulative adjusted cash earnings for the twelve months ended at December 31, 2007 by our cumulative gross interest expense for the twelve months ended at December 31, 2007. The definitions of the factors used to calculate these leverage and interest coverage ratios are included in the Revolver agreement.
| | |
MILLIPORE FORM 10-K 2007 | | 45 |
PART II
The following table summarizes the financial covenant requirements as of December 31, 2007 and thereafter and our compliance with these covenants as of December 31, 2007:
| | | | |
| Covenant | | Requirement | | Actual at
December 31, 2007 |
Maximum leverage ratio | | 3.50:1.0 | | 3.37:1.0 |
Minimum interest coverage ratio | | 3.50:1.0 | | 5.65:1.0 |
Our ability to continue to comply with these covenants will depend primarily on the success in growing our business and generating substantial operating cash flow. Future compliance with the covenants may be adversely affected by various economic, financial, and industry factors. Noncompliance with the covenants would constitute an event of default under the Revolver, allowing the lenders to accelerate repayment of any outstanding borrowings. In the event of any potential failure by us to continue to be in compliance with any covenants, we would seek to negotiate amendments to the applicable covenants or to obtain compliance waivers from our lenders.
As of December 31, 2007, we had borrowings outstanding under the Revolver of $331.6 million, which were classified as long-term debt because of our intent and ability to continuously refinance them. As of December 31, 2007, we had€237.7 million, or $346.8 million, available for borrowing under the Revolver.
3.75% convertible senior notes due 2026
In June 2006, we issued $565.0 million in aggregate principal amount of convertible senior notes (the “Convertible Notes”) in a private placement offering. The Convertible Notes bear interest at 3.75 percent per annum, payable semi-annually in arrears on June 1 and December 1 of each year. Commencing with the six-month period beginning on December 1, 2011, if the average trading price of the Convertible Notes for the five consecutive trading days preceding such six-month periods equals 120 percent or more of the principal amount, contingent interest will accrue at the rate of 0.175 percent of the average trading price of the Convertible Notes. The Convertible Notes are our senior unsecured obligations and rank equally with all of our existing and future senior unsecured indebtedness. The Convertible Notes are effectively subordinated to all of our existing and future secured indebtedness and all existing and future liabilities of our subsidiaries, including trade payables. The Convertible Notes will mature on June 1, 2026. We used the net proceeds from this offering to complete the acquisition of Serologicals on July 14, 2006. We recorded $13.4 million of deferred financing costs associated with this offering.
Holders of the Convertible Notes may convert their notes into cash and, if applicable, shares of our common stock prior to June 1, 2026 under certain conditions. The Convertible Notes may be converted if the closing sale price of our common stock for each of the 20 or more trading days in a period of 30 consecutive trading days ending on the last trading day of the immediately preceding calendar quarter exceeds 120 percent of the conversion price in effect on the last trading day of the immediately preceding calendar quarter. The Convertible Notes may also be converted during the five consecutive business days immediately after any five consecutive trading day period in which the average trading price per $1,000 principal amount of the Convertible Notes was equal to or less than 97 percent of the average conversion value of the notes during this period. The Convertible Notes will also be convertible if we make certain distributions on our common stock or engage in certain transactions, if we call the Convertible Notes for redemption, and at any time from November 1, 2011 through December 1, 2011 and any time on or after June 1, 2024. Upon conversion, the Convertible Notes will be converted into cash for the principal amount and shares of our common stock for the conversion premium, if any, based on an initial conversion rate of 11.0485 shares per $1,000 principal amount (which represents an initial conversion price of approximately $90.51 per share), subject to adjustments.
| | |
| 46 | | MILLIPORE FORM 10-K 2007 |
PART II
On or after December 1, 2011, we have the option to redeem the Convertible Notes at a redemption price equal to 100 percent of the principal amount of the notes, plus accrued but unpaid interest. On each of December 1, 2011, June 1, 2016 and June 1, 2021, holders of the Convertible Notes have the option to require us to purchase all or a portion of their notes at a purchase price in cash equal to 100 percent of the principal amount of the notes, plus accrued but unpaid interest. Holders may also require us to repurchase all or a portion of their notes upon a fundamental change at a repurchase price in cash equal to 100 percent of the principal amount of the notes to be repurchased, plus accrued but unpaid interest.
Although we are not required to maintain any specified financial ratios under the Convertible Notes agreement, we will be considered in default if we fail to fulfill our conversion or redemption obligations, make required interest payments, provide notice to holders of the Convertible Notes in certain specified circumstances, or cure our default on any of our indebtedness or that of our subsidiaries in the aggregate principal amount of $50 million or more. If an event of default has occurred and is continuing, the principal amount of the Convertible Notes plus interest thereon may become immediately due and payable. We are currently in compliance with the covenant restrictions.
5.875% senior notes due 2016
In June 2006, we issued€250.0 million, or $364.7 million, in aggregate principal amount of 5.875 percent senior notes (the “Euro Notes”) due 2016. Interest is payable semi-annually in arrears on June 30 and December 30 of each year. The Euro Notes were issued at 99.611 percent of the principal amount, which resulted in an original issue discount of€1.0 million, or $1.4 million. We recorded $3.3 million of deferred financing costs associated with the issuance of the Euro Notes. The Euro Notes are our senior unsecured obligations and rank equally with all of our existing and future senior unsecured indebtedness.
Upon the occurrence of any change in control, holders of the Euro Notes may require us to repurchase all of their Euro Notes for a cash price equal to 101 percent of the principal amount, plus accrued and unpaid interest thereon. Before June 30, 2016, we may, at our option, redeem the Euro Notes, in whole or in part, for cash, at a redemption price equal to 100 percent of the principal amount of the Euro Notes we redeem, plus applicable “make-whole” premium. In addition, we may redeem, at our option, in whole but not in part, at a redemption price equal to 100 percent of the principal amount, plus accrued and unpaid interest, upon the occurrence of certain tax events in the United States.
The indenture for the Euro Notes places certain restrictions on our ability to create, incur, assume or suffer liens on our manufacturing plants and other principal facilities in the United States and ability to enter into certain sale lease-back transactions. We would also be considered in default if we fail to fulfill our redemption obligations, make required interest payments, provide notice to holders of the Euro Notes in certain specified circumstances, or cure our default on any of our indebtedness or that of our subsidiaries in the aggregate principal amount of $50 million or more. If an event of default has occurred and is continuing, the principal amounts of the Euro Notes plus any accrued interest thereon may become immediately due and payable. We are currently in compliance with the covenant restrictions.
| | |
MILLIPORE FORM 10-K 2007 | | 47 |
PART II
CONTRACTUAL OBLIGATIONS AND COMMERCIAL COMMITMENTS
The following table summarizes our minimum future payments under our contractual obligations at December 31, 2007:
| | | | | | | | | | | | | | | |
| | | Payment due |
| (in millions) | | Total | | Less than
1 year | | 1-3 years | | 3-5 years | | More than
5 years |
Long-term debt obligations | | $ | 1,838.8 | | $ | 42.6 | | $ | 85.2 | | $ | 420.3 | | $ | 1,290.7 |
Non-cancellable operating leases | | | 104.5 | | | 21.0 | | | 38.8 | | | 24.0 | | | 20.7 |
Employee pension and postretirement medical plans | | | 61.2 | | | 3.9 | | | 8.9 | | | 11.0 | | | 37.4 |
Non-cancellable purchase obligations | | | 119.2 | | | 74.3 | | | 42.6 | | | 0.7 | | | 1.6 |
Total | | $ | 2,123.7 | | $ | 141.8 | | $ | 175.5 | | $ | 456.0 | | $ | 1,350.4 |
Long-term debt obligations include estimated interest payments on our 3.75 percent Convertible Notes and our 5.875 percent Euro Notes for the respective periods presented above. Outstanding borrowings of $335.0 million under our Revolver are included in the table above as payments due in 3-5 years because we intend to refinance the Revolver borrowings on a long-term basis and the maturity date of the Revolver will be June 6, 2011.
We maintain various defined benefit pension and postretirement plans for the benefit of our employees. At December 31, 2007, our U.S. pension plan and postretirement benefit plans were under-funded by $19.4 million and $8.7 million, respectively. At December 31, 2007, our foreign retirement plans were under-funded by $16.1 million. We anticipate funding for these plans will be approximately $11.9 million in 2008. Amounts included in the table above for employee pension and postretirement medical plans reflect projected benefit payments. Our future pension expense and pension liabilities will be affected by fluctuations in future discount rates as well as the fair market value of assets used to fund these plans.
Our purchase obligations include obligations related to the future purchase of goods and services, capital lease obligations, and other long term liabilities reflected on our balance sheet.
The above table does not reflect unrecognized tax benefits of $20.2 million, the timing of which is uncertain. We cannot make reasonably reliable estimates of the period of cash settlement with tax authorities.
Critical Accounting Estimates
Preparation of our financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses. Note 2 to the consolidated financial statements describes the significant accounting policies used in the preparation of our consolidated financial statements. Management believes the most complex and sensitive judgments, because of their significance to the consolidated financial statements, result primarily from the need to make estimates about the effects of matters that are inherently uncertain. The most significant areas involving management judgments and estimates are described below. Actual results in these areas could differ from management’s estimates.
Revenue Recognition. Revenue from the sale of products is recognized when we meet all of the criteria specified in Securities Exchange Commission Staff Accounting Bulletin No. 104 (“SAB 104”), “Revenue Recognition in Financial Statements.” These criteria include:
| | n | | evidence of an arrangement is in place; |
| | n | | related prices are fixed or determinable; |
| | n | | delivery or performance has occurred; and |
| | n | | collection of the resulting receivable is reasonably assured. |
| | |
| 48 | | MILLIPORE FORM 10-K 2007 |
PART II
Customer purchase orders or sales agreements evidence our sales arrangements. These purchase orders and sales agreements specify both selling prices and quantities, which are the basis for recording sales revenue. Trade terms for the majority of our sales contracts indicate that title and risk of loss pass from us to our customer when we ship products from our facilities, which is when revenue is recognized. Revenue is deferred until our products arrive at customers’ facilities in situations where trade terms indicate that title and risk of loss pass from us to the customers upon their receipt of our products. We perform ongoing credit evaluations of our customers and ship products only to customers that satisfy our credit evaluation. We also maintain allowances for doubtful accounts for estimated losses resulting from our customers’ inability to make required payments.
Standard consumable and hardware products account for over 90 percent of our total consolidated revenues and are typically sold with standard terms and conditions. Revenues for these products are generally recognized upon shipment or delivery to the customers. In instances where we sell filtration systems products with a related installation obligation, we generally recognize revenue related to the filtration systems when title passes and recognize revenue related to the installation when installation is complete. The allocation of revenue between the filtration system and the installation is based on relative fair value at the time of sale.
In limited cases, our customers may require site acceptance testing for certain customized products built to customers’ specifications. Revenues on these products are deferred upon shipment and are recognized when site acceptance testing is completed.
Revenue from service arrangements is recognized when the services are provided. For laboratory water systems, installation and maintenance service revenues are recognized when the site service visit is completed. For validation testing services provided to customers, revenue is recognized when the contracted study is completed and accepted by the customer. For sample analysis services provided to customers, revenue is recognized as each sample analysis is completed. For assay development and assay validation services provided to customers, revenue is recognized on a proportional performance model as contractually defined deliverables are provided to the customer.
Revenue for fixed price contracts associated with our large, custom process equipment business is recognized under the percentage of completion method (“POC”). Approximately 1 percent of our revenue was derived from POC sales in 2007. Revenue is recognized based on the ratio of hours expended compared with the total estimated hours to complete the construction of the process equipment. The cumulative impact of any revisions in estimates of the percent completed is reflected in the period in which the changes become known. In the event that assumptions used in calculating POC during the construction of the process equipment are later revised, total revenue and expenses estimated for contracts upon completion could differ from the latter estimate. If it is estimated that the project will result in a loss when completed, the entire loss is recognized at that point. Actual results related to POC estimates have been materially the same as the assumptions used at the beginning of each contract. In addition, should a POC contract be cancelled while in progress, we would generally be able to recover expenses incurred with progress payments previously received during the design and construction period. Typically, such progress payments can range between 20 percent and 60 percent of the total contract sales value. Historically, we have experienced few cancellations.
We recognize license and royalty revenue when the amounts are determinable and we have fulfilled our obligations under the applicable agreement. This generally occurs when cash payments are received or licensed sales are reported to us.
Inventory Valuation. Our product life cycle is generally a minimum of 5 years and may be in excess of 20 years. Therefore, we generally rely upon recent historic usage, expiration dates, and estimated future demand in estimating the realizable value of our inventory. Finished goods and components that are determined to be obsolete are written-off when such determination is made. In certain cases, such as for newly introduced products and overstocked products, estimated future demand is considered in establishing inventory write-downs. Raw material and work-in-process inventories are also
| | |
MILLIPORE FORM 10-K 2007 | | 49 |
PART II
reviewed for obsolescence and alternative or future use based on reviewing manufacturing plans, estimated future demand and market conditions. In situations where it is determined that work-in-process inventories cannot be converted into finished goods, the inventories are written down to net realizable value. Inventory at December 31, 2007 reflected cumulative net realizable value write-downs of $37.0 million. Should it be determined that write-downs are insufficient, we would be required to record additional inventory write-downs, which would have a negative impact on gross profit margin. Once recorded, inventory valuation provisions are not subsequently reversed unless the related inventory items are subsequently sold.
Valuation of Long-lived Assets. Valuation of certain long-lived assets including property, plant and equipment, intangible assets, and goodwill requires significant judgment. Assumptions and estimates are used in determining the fair value of assets acquired and liabilities assumed in a business combination. A significant portion of the purchase price in our acquisitions is assigned to intangible assets and goodwill. Assigning value to intangible assets requires that we use significant judgment in determining (i) the fair value; and (ii) whether such intangibles are amortizable or non-amortizable and, if the former, the period and the method by which the intangible assets will be amortized. We utilize commonly accepted valuation techniques, such as the income approach and the cost approach, as appropriate, in establishing the fair value of long-lived assets. Typically, key assumptions include projected revenue and expense levels used in establishing the fair value of business acquisitions as well as discount rates based on an analysis of our weighted average cost of capital, adjusted for specific risks associated with the assets. Changes in the initial assumptions could lead to changes in amortization expense recorded in our future financial statements.
For intangible assets and property, plant and equipment, we assess the carrying value of these assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors we consider important which could trigger an impairment review include but are not limited to the following:
| | n | | significant underperformance relative to expected historical or projected future operating results; |
| | n | | significant negative industry or economic trends; or |
| | n | | significant changes or developments in strategy or operations which affect our intellectual or tangible properties. |
Should we determine that the carrying value of long-lived assets and intangible assets may not be recoverable, we will measure any impairment based on a projected discounted cash flow method using a discount rate determined by management to be commensurate with the risk inherent in our current business model. Significant judgments are required to estimate future cash flows, including the selection of appropriate discount rates and other assumptions. Changes in these estimates and assumptions could materially affect the determination of fair value for these assets.
We perform annual reviews in our second quarter for impairment of goodwill or whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Goodwill may be considered to be impaired if we determine that the carrying value of the reporting unit, including goodwill, exceeds the reporting unit’s fair value. Assessing the impairment of goodwill requires us to make assumptions and judgments regarding the fair value of the net assets of our reporting units. We estimate the fair value of our reporting units using a combination of valuation techniques, including discounted cash flows and cash earnings multiples, and compare the values to our estimated overall market capitalization.
Stock-based Compensation. On January 1, 2006, we adopted SFAS No. 123(R), which required us to recognize share-based payments to employees and directors as compensation expense using a fair value-based method in the results of operations. Prior to the adoption of SFAS No. 123(R) and as permitted by SFAS No. 123, “Accounting for Stock-Based Compensation,” we accounted for share-based payments to employees using the intrinsic value method pursuant to Accounting Principles Board (“APB”) Opinion No. 25, “Accounting for Stock Issued to Employees,” and related interpretations. Therefore, no stock-based employee compensation expense had been recorded in connection with the issuance of employee and director stock options as all options granted under these plans were fixed awards and had an exercise
| | |
| 50 | | MILLIPORE FORM 10-K 2007 |
PART II
price equal to the market value of our common stock at the time of the grant. Stock-based employee compensation expense relating to separation agreements for certain executive officers and the vesting of restricted stock awards and restricted stock units granted at no cost to the employees was reflected in net income. We used the modified prospective method when we adopted SFAS No.123(R) and, accordingly, did not restate the results of operations for the prior periods. In the year ended December 31, 2007, compensation expense of $16.0 million was recognized for all awards granted on or after January 1, 2006 as well as for the unvested portion of awards granted before January 1, 2006.
Stock-based compensation expense is estimated as of the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which generally represents the vesting period. We estimate the fair value of our stock options using the Black-Scholes option-pricing model and the fair value of our restricted stock awards and restricted stock units based on the quoted market price of our common stock at the time of grant. We recognize the associated compensation expense on a straight-line basis over the vesting periods of the awards, net of estimated forfeitures. Forfeiture rates are estimated based on historical pre-vesting forfeiture history and are updated on a quarterly basis to reflect actual forfeitures of unvested awards and other known events.
Estimating the fair value for stock options requires judgment, including estimating stock-price volatility, expected term, expected dividends and risk-free interest rates. The expected volatility rates are estimated based on historical volatilities of our common stock over a period of time that approximates the expected term of the options. The expected term represents the average time that options are expected to be outstanding and is estimated based on the historical exercise, post-vesting cancellation and expiration patterns of our stock options. Expected dividends are estimated based on our dividend history as well as our current projections. The risk-free interest rate for periods approximating the expected terms of the options is based on the U.S. Treasury yield curve in effect at the time of grant. These assumptions are updated at least on an annual basis or when there is a significant change in circumstances that could affect these assumptions.
Income Taxes. We recognize income taxes when transactions are recorded in our consolidated statement of operations, with deferred taxes provided for items that are recognized in different periods for financial statement and tax reporting purposes. We record a valuation allowance to reduce the deferred tax assets to the amount that is more likely than not to be realized. At December 31, 2007, we had valuation allowances of $1,530 related to federal research credits, $22,223 related to state tax credits and net operating loss carryforwards, $2,587 related to capital loss carryforward, and $4,413 related to foreign net operating loss carryforwards.
We are a worldwide business. We are subject to tax audits on a regular basis. Because significant judgment is required in determining our worldwide provision for income taxes, we periodically assess our income tax positions and record tax benefits for all years subject to examination based upon our evaluation of the facts, circumstances and information available at the reporting date. For those tax positions where it is more likely than not that a tax benefit will be sustained, we record the largest amount of tax benefit with a greater than 50 percent likelihood of being realized upon ultimate settlement with a taxing authority that has full knowledge of all relevant information. For those income tax positions where it is not more likely than not that a tax benefit will be sustained, no tax benefit is recognized in the financial statements. We believe our tax reserves are necessary to appropriately reflect tax obligations that may arise out of current and future audits. Any reduction of these contingent liabilities or additional assessment would increase or decrease income, respectively, in the period such determination is made.
| | |
MILLIPORE FORM 10-K 2007 | | 51 |
PART II
In the normal course of business, we are examined by various tax authorities, including the IRS. In 2007, the IRS continued the examination phase of years 2004 and 2005. Although the examinations were not settled at December 31, 2007, we believe an appropriate provision was made for any potential unfavorable financial statement impact.
We provide for U.S. income taxes on the earnings of foreign subsidiaries unless they are considered indefinitely invested outside the U.S. The earnings of our Ireland, United Kingdom and Sweden subsidiaries were considered indefinitely invested outside the U.S. These elections were made based on our operating plans and foreign debt service requirements.
Employee Retirement Plans. In the U.S., we sponsor a pension plan and a postretirement medical plan covering substantially all employees who meet certain eligibility requirements. For both plans, we determine several key assumptions that are used in calculating the expense and liability of the plans, typically on an annual basis.
For the pension plan, these key assumptions include the discount rate and expected return on plan assets. In selecting the expected long-term rate of return on assets, we considered the average rate of earnings expected on the funds invested or to be invested to provide for the benefits under the pension plan. This included considering the trusts’ asset allocations and the expected returns likely to be earned over the life of this plan. The assumed discount rate is intended to approximate the actual rate at which benefits could effectively be settled. We used the Citigroup Pension Discount Curve as the benchmark rate for estimating our discount rate for 2007 pension expense. In addition, we update, as needed, other assumptions used in determining the expense and liabilities of the plan, such as withdrawal and mortality assumptions based on the average age grouping of our plan participants and updated mortality tables published by the Society of Actuaries. The actuarial assumptions used by us may differ materially from actual results due to changing market and economic conditions, higher or lower withdrawal rates or longer or shorter life spans of the participants. These differences may have a significant effect on the amount of pension expense recorded by us in future years. During 2007, we recognized our pension expense using a discount rate of 5.75 percent and an expected return on plan assets of 8.0 percent related to our U.S. pension plan. The most sensitive assumptions used in calculating the expense and liability of our U.S. pension plan were the discount rates and the expected rate of return on plan assets. Although they were the most sensitive assumptions, a 0.5 percentage point change in either assumption would be immaterial to our results of operations and financial position.
For the postretirement medical plan, significant assumptions included the discount rate, the future medical cost escalation rate, withdrawal rates and mortality rates. The actuarial assumptions used by us may differ materially from future actual results because of changing conditions in the growth of medical expenses or longer or shorter life spans of the participants. These differences may have a significant effect on the amount of postretirement medical expense recorded by us. During 2007, we recognized our expense using a discount rate of 5.75 percent and an expected medical cost escalation rate that declines gradually from 9.0 percent in 2007 to 5.0 percent in 2013. Although these are the most sensitive assumptions, a 0.5 percentage point change in either assumption would be immaterial to our results of operations and financial position.
In certain foreign subsidiaries, we also sponsor pension plans for our employees. Accounting and reporting for these plans requires the use of country specific assumptions for discount rates, expected returns on assets, and rates of compensation increases. We apply a consistent methodology, year over year, in determining the key assumptions. Our discount rates are based on high quality bond indices for durations that approximate the average remaining service periods of our plan participants in each country. We select expected long-term rates of return on assets based on the average rate of earnings expected on funds invested or to be invested to provide for the benefits under these plans. Although the most sensitive assumptions used in calculating the expense and liability of our foreign pension plans are the discount rate and the expected rate of return on plan assets, a 0.5 percentage point change in either assumption would be immaterial to our results of operations and financial position.
| | |
| 52 | | MILLIPORE FORM 10-K 2007 |
PART II
In the 2006 fourth quarter, our Board of Directors approved an amendment to the Retirement Plan for Employees of Millipore Corporation (the “Retirement Plan”) and the Employees’ Participation and Savings Plan (the “Participation Plan”). The effect of the amendment was to freeze the Retirement Plan effective December 31, 2006, after which no benefits will accrue. We provided eligible participants a one-time final opportunity in early 2007 to transfer balances in their Participation Plan accounts to the Retirement Plan for the purpose of purchasing an annuity under the existing terms of the Retirement Plan. We recognized a curtailment loss of $8.7 million in the 2006 fourth quarter as a result of this amendment.
We used an assumption that 17.1 percent of available balances in the Participation Plan as of December 31, 2006 would be transferred into the Retirement Plan for purposes of determining the curtailment loss associated with the amendment. The 17.1 percent assumption was selected based on a review of our actual transfer experience for the 2002-2005 period. Actual transfer experience was analyzed to determine the percentage by age grouping of available Participation Plan balances that were transferred to the Retirement plan. These percentages were then applied to projected balances by age grouping as of December 31, 2006 to determine the estimated balances that would be transferred by age grouping. Upon completion of the transfer of Participation Plan balances as of July 1, 2007, the actual transfers represented 35.1 percent of the final Participation Plan asset balance as of that time. The 35.1 percent actual transfer experience had no impact on 2007 expense because the actuarial loss attributable to the actual transfer percent exceeding the 17.1 percent transfer rate assumption will be amortized over the average remaining service period of plan participants beginning in 2008. The balances disclosed at December 31, 2007 for benefit obligations and plan assets reflect the impact of the actual transfers from the Participation Plan.
Market Risk
We are exposed to market risks, which include changes in foreign currency exchange rates, interest rate risk, and credit risk. We manage these market risks through our normal financing and operating activities and, when appropriate, through the use of derivative financial instruments.
Foreign Currency Exchange Rate Risk
We are exposed to foreign currency exchange rate risk inherent in sales, net income, and assets and liabilities denominated in currencies other than the U.S. dollar. The potential change in foreign currency exchange rates represents a substantial risk to us because approximately 63 percent of our business was conducted outside of the United States for the year ended December 31, 2007, generally in foreign currencies. Our primary risk management strategy is to use forward exchange contracts to hedge certain foreign currency transaction exposures. The intent of this strategy is to offset gains and losses that occur on the underlying booked exposures with gains and losses resulting from the forward exchange contracts that hedge these exposures. Principal hedged currencies include the Euro, Japanese Yen, and Great Britain Pound. Gains and losses resulting from changes in the value of the derivative are recognized currently in earnings or reported in accumulated other comprehensive income, a separate component of shareholders’ equity. This depends on the use of the derivative and whether it has been designated and qualifies as an effective hedge.
As of December 31, 2007, we had open forward exchange contracts designated as cash flow hedges of forecasted intercompany sales with total U.S. dollar equivalent notional amounts of approximately $55.2 million. These forward exchange
| | |
MILLIPORE FORM 10-K 2007 | | 53 |
PART II
contracts are generally short-term in nature and mature through February 2009. Based on our analysis, a hypothetical adverse foreign exchange rate movement of 10 percent against our forward exchange contracts would have resulted in a net loss in fair value of these contracts of approximately $6.0 million at December 31, 2007. All such losses on these forward exchange contracts would be substantially offset by gains on the underlying transactions that were hedged.
In addition, we also hold forward exchange contracts to mitigate the impact of foreign exchange risk related to certain foreign currency denominated receivable and payable balances. Changes in fair value of these forward exchange contracts are recorded through current earnings because these instruments do not qualify for hedge accounting. As of December 31, 2007, the U.S. dollar equivalent notional amounts of the forward exchange contracts related to foreign currency denominated receivable and payable balances totaled $243.1 million. The periods of these forward exchange contracts typically span less than three months. The fair value of these forward exchange contracts was a net loss of $0.4 million at December 31, 2007.
During 2007, we entered into forward exchange contracts to hedge the foreign exchange risk related to foreign currency denominated debt. The initial forward exchange contracts matured in October 2007, resulting in a realized loss of $17.9 million. At the same time, we entered into additional forward exchange contracts, which were outstanding at December 31, 2007 and mature in April 2008. At December 31, 2007, these forward exchange contracts had an aggregate U.S. dollar equivalent notional amount of $291.9 million and an aggregate U.S. dollar equivalent fair value of a net loss of $5.7 million. The net realized and unrealized losses on these forward exchange contracts were substantially offset by gains on the underlying transactions, which resulted in a net gain of $0.9 million in 2007.
Our risk management policy allows for hedging our net investments in foreign subsidiaries, using both derivative and non-derivative instruments. In June 2006, we issued€250.0 million of Euro-denominated senior notes which gives rise to foreign exchange risk when the debt is remeasured into U.S. dollars at the end of each period. The remeasurement gains and losses are recorded in other comprehensive income because we designated this debt as an economic hedge of our net investments in European subsidiaries. Upon maturity, however, we could be exposed to significant exchange rate risk because we will be required to repay the debt at the then current market exchange rates, which could be higher than the rates at which we borrowed the debt in June 2006.As of December 31, 2007, we have recorded a cumulative loss of $50.9 million in accumulated other comprehensive income attributable to the change in value of the U.S. dollar versus the Euro since the issuance of the notes. A further 10 percent strengthening or weakening of the Euro against the U.S. dollar will cause this cumulative loss to increase or decrease by $36.5 million.
We do not enter into derivatives for trading or other speculative purposes, nor do we use leveraged financial instruments.
Interest Rate Risk
We are exposed to changes in interest rates in the normal course of our business operations as a result of our ongoing investing and financing activities, which affect our debt as well as cash and cash equivalents. As of December 31, 2007, our debt portfolio was comprised of a combination of fixed and floating rate borrowings. Our exposure to interest rate risk primarily relates to our revolving credit facilities, under which the interest rates on our borrowings float with LIBOR rates. The fair market value of our long-term fixed interest rate debt is subject to interest rate risk. Generally, the fair market value of fixed interest rate debt will increase as interest rates fall and decrease as interest rates rise. In addition, the fair value of our convertible notes is affected by our stock price. The total estimated fair value of our fixed rate debt at December 31, 2007 was $951.0 million. Fair values were determined from available market prices using current interest rates and terms to maturity. If interest rates were to increase or decrease by 1 percent, the fair value of our long-term debt would decrease or increase by approximately $33.8 million.
| | |
| 54 | | MILLIPORE FORM 10-K 2007 |
PART II
We assess our interest rate risks on a regular basis and do not currently use financial instruments to mitigate these risks.
Credit Risk
We are exposed to concentrations of credit risk in cash and cash equivalents, trade receivables, and forward exchange contracts. Cash and cash equivalents are placed with major financial institutions with high quality credit ratings. The amount placed with any one institution is limited by policy. Trade receivables credit risk exposure is limited because of our large number of established customers and their dispersion across different geographies. No single customer accounted for 10 percent or more of our consolidated trade receivables as of December 31, 2007.
We are exposed to credit risk on our hedging instruments in the event of nonperformance by counterparties. However, we do not anticipate nonperformance by any of these counterparties because our hedging activities are transacted only with financial institutions with high credit ratings.
Related Party Agreements
Rolf A. Classon, a Director of Millipore since December 2005, retired as Chairman and President of Bayer Healthcare LLC in July 2004. He is currently a member of the Supervisory Board of Bayer Healthcare AG. During 2007, Bayer AG (including Bayer Healthcare LLC), purchased a total of $17.7 million of products from Millipore. The relationship between Millipore and Bayer predates Mr. Classon’s election as a Director.
Dividends
We did not declare any cash dividends in 2007 or 2006. We do not currently have plans to make future cash dividend declarations or payments.
Legal Proceedings
We currently are not a party to any material legal proceeding.
Following our decision to consolidate the results of our 40 percent owned Indian Joint-Venture (the “India JV”) in January 2006, we learned as a result of our internal controls procedures that certain payment and commission practices at the India JV raise issues of compliance with the U.S. Foreign Corrupt Practices Act. Promptly upon learning of this, our Audit and Finance Committee engaged outside counsel and commenced an investigation. We have implemented certain corrective actions. We have notified the Securities and Exchange Commission and the Department of Justice of this matter. The operations and financial results of the India JV are not currently, and have not to date been, material to us.
New Accounting Pronouncements
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurement” (“SFAS No. 157”). SFAS No. 157 clarifies the principle that fair value should be based on the assumptions market participants would use when pricing an asset or liability and establishes a fair value hierarchy that prioritizes the information used to develop those assumptions. Under
| | |
MILLIPORE FORM 10-K 2007 | | 55 |
PART II
the standard, fair value measurements would be separately disclosed by level within the fair value hierarchy. In November 2007, the FASB deferred the effective date of SFAS No. 157 for certain nonfinancial and nonrecurring assets and liabilities. Other than the partial deferral, SFAS No. 157 is effective for us as of the beginning of fiscal 2008. SFAS No. 157 does not have a material impact on our financial assets and liabilities. We are currently evaluating the impact of SFAS No. 157 on our nonfinancial assets and liabilities within the scope of SFAS No. 157.
In September 2006, the FASB issued SFAS No. 158, “Employers Accounting for Defined Benefit Pension and Other Retirement Plans—an amendment of FASB Statements No. 87, 88, 106, and 132(R)” (“SFAS No. 158”). Under this standard, we are required to recognize the overfunded or underfunded status of our defined benefit postretirement plan as an asset or liability in our statement of financial position and to recognize changes in that funded status in the year in which the changes occur through comprehensive income. We were required to initially recognize the funded status of our defined benefit postretirement plans and to provide the required disclosures as of the end of 2006. The requirement to measure plan assets and benefit obligations as of year end in the statement of financial position will be effective for us in 2008. We adopted the funded status provisions of SFAS No. 158 effective December 31, 2006, but have not adopted the measurement date provision. Our adoption of the measurement date provisions of SFAS No. 158 in 2008 is not expected to have a material impact on our financial position or results of operations.
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Liabilities, Including an amendment of FASB Statement No. 115” (“SFAS No. 159”). SFAS No. 159 permits entities to measure many financial instruments and certain other items at fair value that are not currently required to be measured at fair value. SFAS No. 159 is effective for us as of the beginning of fiscal year 2008. We do not anticipate the adoption of SFAS No. 159 to have a material impact on our financial position or results of operations.
In June 2007, the Emerging Issues Task Force (“EITF”) reached a consensus on EITF Issue No. 07-3, “Accounting for Nonrefundable Advance Payments for Goods or Services Received to Be Used in Future Research and Development Activities” (“EITF 07-3”). EITF 07-3 requires companies that are involved in research and development activities to defer nonrefundable advance payments for future research and development activities and to recognize those payments as goods and services are delivered. We will be required to assess on an ongoing basis whether or not the goods or services will be delivered and to expense the nonrefundable advance payments immediately if we determine that the delivery of such goods or services is unlikely. EITF 07-3 is effective for new arrangements entered into subsequent to the beginning of our fiscal year 2008. Adoption of EITF 07-3 will not have a material impact on our financial position or results of operations.
In December 2007, the FASB issued SFAS No. 141 (Revised 2007), “Business Combinations” (“SFAS No. 141(R)”), which replaces SFAS No. 141, “Business Combinations.” SFAS No. 141(R) retains the underlying concepts of SFAS No. 141 in that all business combinations are still required to be accounted for at fair value under the acquisition method. However, SFAS No. 141(R) changes the method of applying the acquisition method in a number of significant aspects: acquisition costs will generally be expensed as incurred; noncontrolling interests will be valued at fair value at the acquisition date; in-process research and development will be recorded at fair value as an indefinite-lived intangible asset at the acquisition date; restructuring costs associated with a business combination will generally be expensed subsequent to the acquisition date; and changes in deferred tax asset valuation allowances and income tax uncertainties after the acquisition date generally will affect income tax expense. SFAS No. 141(R) is effective on a prospective basis for all business combinations for which the acquisition date is on or after the beginning of the first annual period subsequent to December 15, 2008, with the exception of the accounting for valuation allowances on deferred taxes and acquired tax contingencies. SFAS No. 141(R) amends SFAS No. 109 such that adjustments made to valuation allowances on deferred taxes and acquired tax contingencies associated with acquisitions that closed prior to the effective date of SFAS No. 141(R) would
| | |
| 56 | | MILLIPORE FORM 10-K 2007 |
PART II
also follow the provisions of SFAS No. 141(R). Early adoption of the provisions of No. SFAS 141(R) is not permitted. We are currently evaluating the effects that SFAS No. 141(R) may have on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 160, “Noncontrolling Interests in Consolidated Financial Statements—an amendment of ARB No. 51” (“SFAS No. 160”). This statement is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008, with earlier adoption prohibited. This statement requires the recognition of a noncontrolling interest (minority interest) as equity in the consolidated financial statements and separate from the parent’s equity. The amount of net income attributable to the noncontrolling interest will be included in consolidated net income on the face of the income statement. It also amends certain consolidation procedures for consistency with the requirements of SFAS No. 141(R).This statement also includes expanded disclosure requirements regarding the interests of the parent and its noncontrolling interest. We are currently evaluating this new statement and anticipate that the statement will not have a significant impact on our results of operations.
In December 2007, the EITF reached consensus on Issue No. 07-1, “Accounting for Collaborative Arrangements” (“EITF 07-1”). EITF 07-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years, and shall be applied retrospectively to all prior periods presented for all collaborative arrangements existing as of the effective date. EITF 07-1 requires that transactions with third parties (i.e., revenue generated and costs incurred by the partners) should be reported in the appropriate line item in each company’s financial statement pursuant to the guidance in EITF Issue No. 99-19, “Reporting Revenue Gross as a Principal versus Net as an Agent.” EITF 07-1 also includes enhanced disclosure requirements regarding the nature and purpose of the arrangement, rights and obligations under the arrangement, accounting policy, amount and income statement classification of collaboration transactions between the parties, and amounts due from or owed to other participants under the collaborative arrangements. We are currently evaluating the effects that EITF 07-1 may have on our consolidated financial statements.
Forward-Looking Statements
The matters discussed in this Form 10-K Annual Report, as well as in future oral and written statements by our management, that are forward-looking statements are based on our current management expectations. These expectations involve substantial risks and uncertainties which could cause actual results to differ materially from the results expressed in, or implied by, these forward-looking statements. Potential risks and uncertainties that could affect our future operating results include, without limitation, the risk factors and uncertainties set forth in Item 1A and elsewhere in this Form 10-K Annual Report.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
The information called for by this item is set forth under the heading “Market Risk” in Management’s Discussion and Analysis of Financial Condition and Results of Operations contained in Item 7 above which information is hereby incorporated by reference.
| | |
MILLIPORE FORM 10-K 2007 | | 57 |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
Index to Consolidated Financial Statements
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our CEO and CFO, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment our management concluded that, as of December 31, 2007, our internal control over financial reporting was effective based on those criteria.
The effectiveness of our internal control over financial reporting as of December 31, 2007 has been audited by PricewaterhouseCoopers LLP, an Independent Registered Public Accounting Firm, as stated in their report which is included herein.
| | |
| 58 | | MILLIPORE FORM 10-K 2007 |
Report of Independent Registered Public Accounting Firm
To the Shareholders and Directors of Millipore Corporation:
In our opinion, the consolidated financial statements listed in the accompanying index, present fairly, in all material respects, the financial position of Millipore Corporation and its subsidiaries at December 31, 2007 and 2006, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2007 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in “Management’s Annual Report on Internal Control over Financial Reporting” appearing under Item 8. Our responsibility is to express opinions on these financial statements and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in Notes 2, 11, 12 and 13 to the consolidated financial statements, the Company changed the manner in which it accounts for share-based compensation and the manner in which it accounts for defined benefit pension and other post retirement plans in 2006, and the manner in which it accounts for income tax contingencies in 2007.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
February 28, 2008
| | |
MILLIPORE FORM 10-K 2007 | | 59 |
Consolidated Statements of Operations
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| (In thousands, except per share data) | | 2007 | | | 2006 | | | 2005 | |
Net sales | | $ | 1,531,555 | | | $ | 1,255,371 | | | $ | 991,031 | |
Cost of sales | | | 721,092 | | | | 625,608 | | | | 472,023 | |
Gross profit | | | 810,463 | | | | 629,763 | | | | 519,008 | |
Selling, general and administrative expenses | | | 486,737 | | | | 398,842 | | | | 309,029 | |
Research and development expenses | | | 106,999 | | | | 86,617 | | | | 66,052 | |
Purchased in-process research and development | | | – | | | | – | | | | 3,149 | |
Operating profit | | | 216,727 | | | | 144,304 | | | | 140,778 | |
Interest income | | | 1,453 | | | | 21,415 | | | | 3,466 | |
Interest expense | | | (65,757 | ) | | | (45,336 | ) | | | (6,711 | ) |
Income before income taxes and minority interest | | | 152,423 | | | | 120,383 | | | | 137,533 | |
Provision for income taxes | | | 12,424 | | | | 21,462 | | | | 57,365 | |
Minority interest | | | 3,527 | | | | 1,937 | | | | – | |
Net income | | $ | 136,472 | | | $ | 96,984 | | | $ | 80,168 | |
Earnings per share: | | | | | | | | | | | | |
Basic | | $ | 2.52 | | | $ | 1.82 | | | $ | 1.57 | |
Diluted | | $ | 2.48 | | | $ | 1.79 | | | $ | 1.55 | |
Weighted average shares outstanding: | | | | | | | | | | | | |
Basic | | | 54,263 | | | | 53,160 | | | | 50,953 | |
Diluted | | | 55,028 | | | | 54,245 | | | | 51,659 | |
The accompanying notes are an integral part of the consolidated financial statements.
| | |
| 60 | | MILLIPORE FORM 10-K 2007 |
Consolidated Balance Sheets
| | | | | | | | |
| | | December 31, | |
| (In thousands, except per share data) | | 2007 | | | 2006 | |
| Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 36,177 | | | $ | 77,481 | |
Accounts receivable (less allowance for doubtful accounts of $3,613 and $3,700 at December 31, 2007 and 2006, respectively) | | | 292,143 | | | | 277,410 | |
Inventories | | | 277,355 | | | | 256,666 | |
Deferred income taxes | | | 66,451 | | | | 62,978 | |
Assets held for sale | | | – | | | | 17,150 | |
Other current assets | | | 17,963 | | | | 17,670 | |
Total current assets | | | 690,089 | | | | 709,355 | |
Property, plant and equipment, net | | | 589,161 | | | | 525,903 | |
Deferred income taxes | | | 21,973 | | | | 8,366 | |
Intangible assets, net | | | 432,108 | | | | 488,303 | |
Goodwill | | | 1,019,581 | | | | 1,014,194 | |
Other assets | | | 24,345 | | | | 25,370 | |
Total assets | | $ | 2,777,257 | | | $ | 2,771,491 | |
| Liabilities and Shareholders’ Equity | | | | | | | | |
Current liabilities: | | | | | | | | |
Short-term debt and notes payable | | $ | 5,240 | | | $ | 100,000 | |
Accounts payable | | | 96,915 | | | | 90,843 | |
Income taxes payable | | | 11,248 | | | | 15,539 | |
Accrued expenses | | | 164,996 | | | | 191,265 | |
Deferred income taxes | | | 3,842 | | | | 4,183 | |
Total current liabilities | | | 282,241 | | | | 401,830 | |
Deferred income taxes | | | 9,384 | | | | 16,121 | |
Long-term debt | | | 1,260,043 | | | | 1,316,256 | |
Other liabilities | | | 82,778 | | | | 83,793 | |
Total liabilities | | | 1,634,446 | | | | 1,818,000 | |
Minority interest | | | 6,243 | | | | 5,080 | |
Commitments and contingencies (Note 15) | | | | | | | | |
Shareholders’ equity: | | | | | | | | |
Common stock, par value $1.00 per share, 120,000 shares authorized; 54,772 shares issued and outstanding as of December 31, 2007; 53,524 shares issued and outstanding as of December 31, 2006 | | | 54,772 | | | | 53,524 | |
Additional paid-in capital | | | 260,334 | | | | 196,774 | |
Retained earnings | | | 842,558 | | | | 706,686 | |
Accumulated other comprehensive loss | | | (21,096 | ) | | | (8,573 | ) |
Total shareholders’ equity | | | 1,136,568 | | | | 948,411 | |
Total liabilities, minority interest and shareholders’ equity | | $ | 2,777,257 | | | $ | 2,771,491 | |
The accompanying notes are an integral part of the consolidated financial statements.
| | |
MILLIPORE FORM 10-K 2007 | | 61 |
Consolidated Statements of Shareholders’ Equity
Years Ended December 31, 2007, 2006 and 2005
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional Paid-In Capital | | | Retained Earnings | | | Unearned Compensation | | | Accumulated Other Comprehensive Income (Loss) | | | Total Shareholders’
Equity | |
| (In thousands) | | Shares | | Par Value | | | | | Unrealized Gain (Loss) on Securities | | | Gain (Loss) on
Cash Flow
Hedges | | | Translation Adjustments | | | Unfunded Pension Liabilities | | | Total | | |
Balance at December 31, 2004 | | 49,816 | | $ | 49,816 | | $ | 10,654 | | | $ | 529,534 | | | $ | (4 | ) | | $ | 203 | | | $ | – | | | $ | 55,309 | | | $ | (6,662 | ) | | $ | 48,850 | | | $ | 638,850 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | | | | | | | | | | 80,168 | | | | | | | | | | | | | | | | | | | | | | | | | | | | 80,168 | |
Net unrealized losses on securities available for sale,
net of tax of $111 | | | | | | | | | | | | | | | | | | | | (203 | ) | | | | | | | | | | | | | | | (203 | ) | | | (203 | ) |
Minimum pension liability adjustments,
net of tax of $745 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (1,304 | ) | | | (1,304 | ) | | | (1,304 | ) |
Translation adjustments, net of tax of $6,850 | | | | | | | | | | | | | | | | | | | | | | | | | | | | (47,267 | ) | | | | | | | (47,267 | ) | | | (47,267 | ) |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 31,394 | |
Stock issued under stock plans | | 2,411 | | | 2,411 | | | 104,492 | | | | | | | | (386 | ) | | | | | | | | | | | | | | | | | | | | | | | 106,517 | |
Amortization of unearned compensation | | | | | | | | | | | | | | | | 100 | | | | | | | | | | | | | | | | | | | | | | | | 100 | |
Stock-based compensation expense related to officer severance | | | | | | | | 5,505 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 5,505 | |
Tax benefit from stock plan activities | | | | | | | | 9,197 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 9,197 | |
Balance at December 31, 2005 | | 52,227 | | | 52,227 | | | 129,848 | | | | 609,702 | | | | (290 | ) | | | – | | | | – | | | | 8,042 | | | | (7,966 | ) | | | 76 | | | | 791,563 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | | | | | | | | | | 96,984 | | | | | | | | | | | | | | | | | | | | | | | | | | | | 96,984 | |
Minimum pension liability adjustments,
net of tax of $1,189 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (1,238 | ) | | | (1,238 | ) | | | (1,238 | ) |
Translation adjustments, net of tax of $5,087 | | | | | | | | | | | | | | | | | | | | | | | | | | | | (4,774 | ) | | | | | | | (4,774 | ) | | | (4,774 | ) |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 90,972 | |
SFAS No. 158 adoption adjustment, net of tax of $707 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (2,637 | ) | | | (2,637 | ) | | | (2,637 | ) |
Stock issued under stock plans | | 1,297 | | | 1,297 | | | 54,921 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 56,218 | |
Reclassification upon adoption of SFAS No. 123R | | | | | | | | (290 | ) | | | | | | | 290 | | | | | | | | | | | | | | | | | | | | | | | | – | |
Stock-based compensation expense | | | | | | | | 12,295 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 12,295 | |
Balance at December 31, 2006 | | 53,524 | | | 53,524 | | | 196,774 | | | | 706,686 | | | | – | | | | – | | | | – | | | | 3,268 | | | | (11,841 | ) | | | (8,573 | ) | | | 948,411 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | | | | | | | | | | 136,472 | | | | | | | | | | | | | | | | | | | | | | | | | | | | 136,472 | |
Net realized loss on cash flow hedges, net of tax of $49 | | | | | | | | | | | | | | | | | | | | | | | | (73 | ) | | | | | | | | | | | (73 | ) | | | (73 | ) |
Net unrealized loss on cash flow hedges,
net of tax of $254 | | | | | | | | | | | | | | | | | | | | | | | | (340 | ) | | | | | | | | | | | (340 | ) | | | (340 | ) |
Change in additional pension liability,
net of tax of $1,065 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 3,753 | | | | 3,753 | | | | 3,753 | |
Translation adjustments, net of tax of $0 | | | | | | | | | | | | | | | | | | | | | | | | | | | | (15,863 | ) | | | | | | | (15,863 | ) | | | (15,863 | ) |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 123,949 | |
Stock issued under stock plans | | 1,248 | | | 1,248 | | | 47,600 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 48,848 | |
FIN 48 adoption adjustment | | | | | | | | | | | | (600 | ) | | | | | | | | | | | | | | | | | | | | | | | | | | | (600 | ) |
Stock-based compensation expense | | | | | | | | 15,960 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 15,960 | |
Balance at December 31, 2007 | | 54,772 | | $ | 54,772 | | $ | 260,334 | | | $ | 842,558 | | | $ | – | | | $ | – | | | $ | (413 | ) | | $ | (12,595 | ) | | $ | (8,088 | ) | | $ | (21,096 | ) | | $ | 1,136,568 | |
The accompanying notes are an integral part of the consolidated financial statements.
| | |
| 62 | | MILLIPORE FORM 10-K 2007 |
Consolidated Statements of Cash Flows
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| (In thousands) | | 2007 | | | 2006 | | | 2005 | |
Cash flows from operating activities: | | | | | | | | | | | | |
Net income | | $ | 136,472 | | | $ | 96,984 | | | $ | 80,168 | |
Minority interest | | | 3,527 | | | | 1,937 | | | | – | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 123,747 | | | | 72,277 | | | | 50,657 | |
Business acquisition inventory fair value adjustments | | | 11,121 | | | | 24,871 | | | | 2,172 | |
Amortization of deferred debt issuance costs | | | 3,791 | | | | 2,097 | | | | 699 | |
Deferred income tax (benefit) provision | | | (20,555 | ) | | | (14,238 | ) | | | 11,231 | |
Excess tax benefit from stock plan activities | | | – | | | | – | | | | 9,197 | |
Stock-based compensation | | | 15,960 | | | | 12,295 | | | | 5,605 | |
Curtailment loss on pension | | | – | | | | 8,664 | | | | – | |
Other | | | (7,504 | ) | | | 2,025 | | | | 3,149 | |
Changes in operating assets and liabilities, net of effects of business acquisitions: | | | | | | | | | | | | |
Decrease (increase) in accounts receivable | | | 3,196 | | | | (31,653 | ) | | | (18,534 | ) |
(Increase) in inventories | | | (15,553 | ) | | | (4,984 | ) | | | (17,933 | ) |
Decrease (increase) in other current assets | | | 8,577 | | | | 1,633 | | | | (5,964 | ) |
(Increase) in other assets | | | (1,786 | ) | | | (309 | ) | | | (2,167 | ) |
Increase in accounts payable | | | 2,176 | | | | 6,950 | | | | 8,640 | |
(Decrease) increase in accrued expenses | | | (37,120 | ) | | | (9,534 | ) | | | 33,407 | |
(Decrease) increase in income taxes payable | | | (7,779 | ) | | | (26,431 | ) | | | 30,320 | |
Increase (decrease) in other liabilities | | | 3,900 | | | | 4,752 | | | | (5,574 | ) |
Net cash provided by operating activities | | | 222,170 | | | | 147,336 | | | | 185,073 | |
Cash flows from investing activities: | | | | | | | | | | | | |
Additions to property, plant and equipment | | | (101,662 | ) | | | (110,346 | ) | | | (86,429 | ) |
Proceeds from sale of property, plant and equipment | | | 6,049 | | | | 3,939 | | | | – | |
Acquisition of businesses, net of cash acquired | | | – | | | | (1,176,368 | ) | | | (101,298 | ) |
Purchases of marketable securities | | | – | | | | (1,481,205 | ) | | | (130,703 | ) |
Proceeds from sale of marketable securities | | | – | | | | 1,595,152 | | | | 16,864 | |
Settlement of derivative transactions | | | (17,926 | ) | | | – | | | | – | |
Net cash (used in) investing activities | | | (113,539 | ) | | | (1,168,828 | ) | | | (301,566 | ) |
Cash flows from financing activities: | | | | | | | | | | | | |
Proceeds from issuance of common stock under stock plans | | | 49,897 | | | | 56,218 | | | | 106,517 | |
Issuance of 3.75% convertible senior notes due 2026, net of debt issuance costs | | | – | | | | 551,639 | | | | – | |
Issuance of 5.875% senior notes due 2016, net of debt issuance costs | | | – | | | | 309,238 | | | | – | |
Repayment of Serologicals 4.75% convertible debentures | | | – | | | | (277,313 | ) | | | – | |
Repayments of 7.5% ten-year unsecured notes | | | (100,000 | ) | | | – | | | | – | |
(Repayments of) net proceeds from revolver borrowings | | | (105,610 | ) | | | (79,285 | ) | | | 405,976 | |
Other | | | 2,272 | | | | (3,394 | ) | | | 2,973 | |
Net cash (used in) provided by financing activities | | | (153,441 | ) | | | 557,103 | | | | 515,466 | |
Effect of foreign exchange rates on cash and cash equivalents | | | 3,506 | | | | 4,818 | | | | (14,065 | ) |
Net (decrease) increase in cash and cash equivalents | | | (41,304 | ) | | | (459,571 | ) | | | 384,908 | |
Cash and cash equivalents at beginning of year | | | 77,481 | | | | 537,052 | | | | 152,144 | |
Cash and cash equivalents at end of year | | $ | 36,177 | | | $ | 77,481 | | | $ | 537,052 | |
| Supplemental Disclosure of Cash Flow Information: | | | | | | | | | | | | |
Interest paid, net of amounts capitalized | | $ | 72,989 | | | $ | 30,412 | | | $ | 6,690 | |
Income taxes paid, net of refunds | | $ | 37,361 | | | $ | 58,397 | | | $ | 12,759 | |
The accompanying notes are an integral part of the consolidated financial statements.
| | |
MILLIPORE FORM 10-K 2007 | | 63 |
Notes to Consolidated Financial Statements
(In thousands, except per share data)
1. DESCRIPTION OF OPERATIONS
Millipore is a global leader in life science providing innovative products, services, and solutions so our customers can advance their research, development, and production. Our academic, biotechnology, and pharmaceutical customers use our products and services to increase their speed and to improve their consistency in laboratory applications and in biopharmaceutical manufacturing while saving costs.
Millipore is organized around two divisions. Our Bioscience division improves laboratory productivity and workflows by providing innovative products and technologies for life science research. Our Bioprocess division helps pharmaceutical and biotechnology companies develop their manufacturing processes, optimize their manufacturing productivity, and ensure the quality of drugs.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The consolidated financial statements include the accounts of Millipore Corporation and our subsidiaries. We consolidate entities that we control or own more than fifty percent of the voting shares and variable interest entities for which we are considered the primary beneficiary. All intercompany accounts and transactions have been eliminated in consolidation.
Translation of Foreign Currencies
Local currencies are the functional currencies of our subsidiaries outside of the United States. The financial statements of these subsidiaries are translated into U.S. dollars in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 52, “Foreign Currency Translation.” Assets and liabilities are translated at prevailing exchange rates on the balance sheet date, revenues and expenses are translated at average exchange rates during the period, and elements of shareholders’ equity are translated at historical rates. The resulting translation adjustments are reported as a separate component of other comprehensive income in shareholders’ equity. Exchange gains and losses on foreign currency transactions are included in selling, general and administrative expenses in the consolidated statements of operations.
Use of Estimates in the Preparation of Financial Statements
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We base our estimates on historical experience, current conditions and various other assumptions that we believe are reasonable under the circumstances. Estimates and assumptions are reviewed on an on-going basis and the effects of revisions are reflected in the consolidated financial statements in the period in which they are determined to be necessary. Actual results could differ from those estimates.
Reclassifications
Certain reclassifications have been made to prior years’ financial statements to conform to the 2007 presentation.
| | |
| 64 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
Cash Equivalents
Cash equivalents, consisting primarily of investments in money market mutual funds and commercial paper, are carried at cost plus accrued interest, which approximates fair market value. All cash equivalents are highly liquid investments with original maturities of three months or less.
Concentration of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist principally of cash and cash equivalents and accounts receivable. We place our cash and cash equivalents in various financial institutions with high credit ratings and, by policy, limit the amount of credit exposure to any one financial institution.
Concentrations of credit risk with respect to accounts receivable is limited because of the large number of customers comprising our customer base and the dispersion of those customers across different geographies. No single customer accounted for 10 percent or more of the consolidated accounts receivable as of December 31, 2007 and 2006, respectively. We perform ongoing credit evaluations of our customers and generally do not require collateral. We maintain allowances for doubtful accounts for specifically identified estimated losses resulting from the inability of our customers to make required payments. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required.
The following table presents changes in our allowance for doubtful accounts:
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
Balance at beginning of the year | | $ | 3,700 | | | $ | 2,851 | | | $ | 4,500 | |
Provisions | | | 1,361 | | | | 820 | | | | (98 | ) |
Write-offs | | | (1,438 | ) | | | (1,761 | ) | | | (1,120 | ) |
Recoveries | | | (262 | ) | | | (77 | ) | | | (67 | ) |
Acquisitions | | | – | | | | 1,648 | | | | – | |
Foreign exchange | | | 252 | | | | 219 | | | | (364 | ) |
Balance at end of year | | $ | 3,613 | | | $ | 3,700 | | | $ | 2,851 | |
Inventories
We value our inventories at the lower of market value or actual cost, determined on a first-in, first-out (“FIFO”) basis. We generally rely upon recent usage history, expected future demand, and product expiration dates in estimating the realizable value of our inventories. Finished goods and components that are determined to be obsolete are written off when such determination is made. In certain cases, such as newly introduced products and overstocked products, expected future demand is considered in establishing inventory write-downs. Raw material and work-in-process inventories are also reviewed for obsolescence based on evaluating manufacturing plans, expected future demand, alternative use, and market conditions. In situations where we determine that work-in-process inventories cannot be converted into finished goods, the inventories are written down to net realizable value. Should we determine that current levels of write-downs are insufficient, we may record additional inventory write-downs, which would have a negative impact on gross profit. Inventory valuation provisions are not subsequently reversed after they are recorded unless the inventory items are sold.
Our products are made from a wide variety of raw materials that are generally available from alternate sources of supply. However, certain critical raw materials and supplies required for the production of certain principal products are available only from a single supplier as are some products that we distribute. Such raw materials and distributed products cannot be obtained from other sources without significant delay or at all. If such suppliers were to limit or terminate production or
| | |
MILLIPORE FORM 10-K 2007 | | 65 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
otherwise fail to supply these materials for any reason, such failure could have a significant adverse impact on our results of operations. To mitigate such risks, we periodically purchase quantities of some of these critical raw materials in excess of current requirements in anticipation of future manufacturing needs. With sufficient lead time, we will also be able validate alternate suppliers for each of these critical raw materials.
Assets Held for Sale
In connection with the acquisition of Serologicals Corporation (“Serologicals”) on July 14, 2006, we acquired certain idle facilities located in Lawrence, Kansas and Lake Placid, New York. The estimated net realizable value of these assets was $17,150, which we reported as assets held for sale in the current assets section of the consolidated balance sheets as of December 31, 2006 in accordance with SFAS No. 144, “Accounting for Impairment or Disposal of Long-Lived Assets (“SFAS No. 144”). These assets were not used in our operations and were not being depreciated. We also ceased to use, and reported as an asset held for sale, an additional former Serologicals facility located in Toronto, Ontario in the 2007 first quarter.
During the 2007 third quarter, we sold the Lake Placid and Toronto facilities for $5,584 and recognized gains amounting to $400 in our consolidated statements of operations. Because we were not successful selling the Lawrence facility during the one year period following the Serologicals acquisition, we reclassified this facility from assets held for sale to property, plant and equipment at the lower of the facility’s fair value or depreciated carrying value. The reclassification amounted to $13,180 and we simultaneously recorded depreciation expense in the amount of $870 in 2007, which represented the cumulative adjustment for depreciation expense from the date of acquisition to September 29, 2007. The reclassification and depreciation adjustments were made in accordance with SFAS No. 144 and represented a non-cash investing activity.
Property, Plant and Equipment
Property, plant and equipment are recorded at cost. Expenditures for maintenance and repairs are charged to expense and the costs of significant improvements that extend the life of underlying assets are capitalized. Assets are generally depreciated using the straight-line method. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation are eliminated and the related gains or losses are recorded in net income.
We capitalize internal use software development costs. These costs are included in property, plant and equipment and other equipment and are amortized on a straight-line basis over the estimated useful lives of the related software, which is generally three years. We also capitalize interest costs associated with the construction of certain capital assets. Amounts capitalized in 2007, 2006 and 2005 were $3,232, $3,686, and $3,861, respectively.
The estimated useful lives of our depreciable assets are as follows:
| | |
Leasehold improvements | | Shorter of the life of the improvement or the initial term of the lease |
Buildings and improvements | | 4 to 40 years |
Production and other equipment | | 2 to 15 years |
Goodwill and Other Intangible Assets
Goodwill is the excess of the purchase price paid for business acquisitions over the fair value of net assets acquired. Intangible assets were primarily acquired through business acquisitions and consist almost entirely of patented and unpatented technology, trade names and trademarks, customer related intangibles, and licenses. Goodwill and intangible assets deemed to have indefinite lives are not amortized. All other intangible assets are amortized over periods ranging from 1.5 to 20 years either on a straight-line basis or in proportion to the projected economic consumption of the intangible assets. Goodwill and indefinite life intangible assets are subject to annual impairment testing using the guidance and cri-
| | |
| 66 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
teria described in SFAS No. 142,“Goodwill and Other Intangible Assets.” This testing compares carrying values to fair values and, when appropriate, the carrying value of these assets is reduced to fair value. We completed the annual impairment tests in 2007, 2006 and 2005 and concluded that our goodwill or indefinite life intangible assets were not impaired.
Other Long-Lived Assets
We evaluate the potential impairment of other long-lived assets whenever events or changes in circumstances indicate that their carrying value may not be recoverable. If the carrying value exceeds the sum of undiscounted expected future cash flows of the grouping of assets to which the long-lived assets relate, the carrying value of the asset is written down to fair value.
Derivatives and Financial Instruments
Derivative financial instruments are used to mitigate foreign currency risks and are not used for trading or speculative purposes. We account for derivative financial instruments and hedging activities in accordance with SFAS No. 133“Accounting for Derivative Instruments and Hedging Activities” (“SFAS No. 133”). All derivatives are recognized on the balance sheet at fair value. Changes in the fair value of derivatives are recognized periodically either in earnings or in shareholders’ equity as a component of other comprehensive income depending on whether the derivative financial instrument qualifies for hedge accounting. Gains and losses on derivatives designated as cash flow hedges, to the extent they are effective, are recorded in other comprehensive income and subsequently reclassified to earnings to offset the impact of the hedged items when they occur. In the event it becomes probable that the forecasted transaction to which a cash flow hedge relates will not occur, the amount in other comprehensive income would be recognized in earnings immediately and generally the derivative would be terminated. Changes in the fair value of derivatives used as hedges of our net investments in foreign operations are reported in other comprehensive income. Changes in the fair value of derivatives not designated as hedges are recorded in selling, general and administrative expenses in the consolidated statements of operations. Cash flows from derivatives that are accounted for as hedges under SFAS No. 133 or as economic hedges where cash flows are similar to the hedged transactions are classified in the same line as the cash flows from the underlying hedged transactions. Cash flows associated with derivatives that are either not designated as hedges or the timing of the cash flows are not similar to the hedged transactions are classified as investing cash flows.
Stock-based Compensation
On January 1, 2006, we adopted SFAS No. 123 (Revised 2004), “Share-Based Payment” (“SFAS No. 123(R)”), which required us to recognize share-based payments to employees and directors as compensation expense using a fair value-based method in the consolidated statements of operations. Prior to the adoption of SFAS No. 123(R) and as permitted by SFAS No. 123, “Accounting for Stock-Based Compensation” (“SFAS No. 123”), we accounted for share-based payments to employees using the intrinsic value method pursuant to Accounting Principles Board (“APB”) Opinion No. 25, “Accounting for Stock Issued to Employees,” and related interpretations. Under APB Opinion No. 25, no stock-based employee compensation expense had been recorded in connection with the issuance of employee and director stock options as all options granted under these plans were fixed awards and had an exercise price equal to the market value of our common stock at the time of the grant. Stock-based employee compensation expense relating to separation agreements for certain executive officers and the vesting of restricted stock awards and restricted stock units granted at no cost to the employees was recorded in net income.
| | |
MILLIPORE FORM 10-K 2007 | | 67 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
We elected to use the modified prospective method upon adoption of SFAS No. 123(R) and, accordingly, did not restate the results of operations for the prior periods. Under the modified prospective method, compensation expense is recognized for all awards granted on or after January 1, 2006 as well as for the unvested portion of awards granted before January 1, 2006.
Stock-based compensation expense is estimated as of the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which generally represents the vesting period. We estimate the fair value of our stock options using the Black-Scholes option-pricing model and the fair value of our restricted stock awards and restricted stock units based on the quoted market price of our common stock. We recognize the associated compensation expense on a straight-line basis over the vesting periods of the awards, net of estimated forfeitures. Forfeiture rates are estimated based on historical pre-vesting forfeiture history and are updated on a quarterly basis to reflect actual forfeitures of unvested awards and other known events.
Estimating the fair value for stock options requires judgment, including estimating stock-price volatility, expected term, expected dividends and risk-free interest rates. The expected volatility rates are estimated based on historical volatilities of our common stock over a period of time that approximates the expected term of the options. The expected term represents the average time that options are expected to be outstanding and is estimated based on the historical exercise, post-vesting cancellation, and expiration patterns of our stock options. Expected dividends are estimated based on our dividend history as well as our current projections. The risk-free interest rate for periods approximating the expected terms of the options is based on the U.S. Treasury yield curve in effect at the time of grant. These assumptions are updated at least on an annual basis or when there is a significant change in circumstances that could affect these assumptions.
Employee Retirement Plans
We sponsor domestic and foreign defined benefit pension and postretirement benefit plans covering employees who meet certain eligibility requirements. Effective December 31, 2006, we adopted the recognition and disclosure provisions of SFAS No. 158, “Employers’ Accounting for Defined Benefit Pension and Other Retirement Plans—an amendment of FASB Statements No. 87, 88, 106, and 132(R)” (“SFAS No. 158”). Accordingly, we decreased our other assets by $145, increased our liability for pension benefits by $2,560, and increased our accumulated other comprehensive income by $2,705, based on the funded status of our plans at December 31, 2006. Under SFAS No. 158, we are required to recognize the overfunded or underfunded status of our defined benefit pension and postretirement benefit plans as an asset or liability in our statement of financial position and to recognize changes in that funded status in the year in which the changes occur through other comprehensive income. We use a December 31 measurement date for all our defined benefit pension and postretirement benefit plans, except for one foreign plan that uses September 30. We will adopt the measurement date provision of SFAS No. 158 in 2008.
Income Taxes
We account for income taxes in accordance with SFAS No. 109, “Accounting for Income Taxes” (“SFAS No. 109”). The asset and liability approach under SFAS No. 109 requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of our assets and liabilities. Deferred tax assets and liabilities are measured using enacted tax rates for the years in which those temporary differences are expected to be recovered or settled. With respect to the unremitted earnings of our foreign subsidiaries, deferred taxes are provided on amounts expected to be repatriated. We record a valuation allowance to reduce the deferred tax assets to the amount that is more likely than not to be realized.
| | |
| 68 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
We periodically assess our exposures related to our provisions for income taxes and accrue for contingencies that may result in potential tax obligations under the provisions of Financial Accounting Standards Board (“FASB”) Interpretation No. 48 (“FIN 48”),“Accounting for Uncertainty in Income Taxes, an interpretation of FASB Statement No. 109,” which we adopted as of January 1, 2007. As a result of the implementation of FIN 48, we recognized a $600 adjustment to retained earnings which increased the liability for unrecognized income tax benefits. At the adoption date of January 1, 2007 and including the opening adjustment, we had $29,200 of unrecognized tax benefits, of which $21,300 would affect our effective tax rate if recognized. The remaining unrecognized benefits that would not affect our effective tax rate if recognized relate to the pre-acquisition periods of Serologicals. We recognize interest and penalties related to uncertain tax positions in income tax expense. At December 31, 2007, we had approximately $400 of accrued interest related to uncertain tax positions.
Earnings per Share
Basic earnings per share is calculated by dividing the net income for the period by the weighted average number of shares outstanding for the period. Diluted earnings per share is calculated by considering the dilutive impact of common stock equivalents (e.g., outstanding stock options, unvested restricted stock, unvested restricted stock units and convertible debt) under the treasury stock method as if they were converted into common stock as of the beginning of the period or as of the date of grant, if later.
Contingently issuable shares under convertible debt agreements will be included in the diluted earnings per share calculation when our stock price exceeds the conversion price.
Revenue Recognition
Revenue from the sale of products is recognized when we meet all of the criteria specified in U.S. Securities Exchange Commission (the “SEC”) Staff Accounting Bulletin No. 104 (“SAB 104”), “Revenue Recognition in Financial Statements.” These criteria include:
| | n | | evidence of an arrangement is in place; |
| | n | | related prices are fixed or determinable; |
| | n | | delivery or performance has occurred; and |
| | n | | collection of the resulting receivable is reasonably assured. |
Customer purchase orders or sales agreements evidence our sales arrangements. These purchase orders and sales agreements specify both selling prices and quantities, which are the basis for recording sales revenue. Any deviation from this policy requires management review and approval. Trade terms for the majority of our sales contracts indicate that title and risk of loss pass from us to the customer when we ship products from our facilities, which is when revenue is recognized. Revenue is deferred until our products arrive at customers’ facilities in situations where trade terms indicate that title and risk of loss pass from us to the customers upon their receipt of the products. We perform ongoing credit evaluations of our customers and ship products only to customers that satisfy our credit evaluation. We also maintain allowances for doubtful accounts for estimated losses resulting from our customers’ inability to make required payments.
Standard consumable and hardware products account for over 90 percent of our total consolidated revenues and are typically sold with standard terms and conditions. Revenues for these products are generally recognized upon shipment or delivery to the customers. In instances where installation is required for the sale of laboratory water filtration systems products, we generally recognize revenue related to the filtration systems when title passes and recognize revenue related to the installation when installation is complete. The allocation of revenue between the filtration system and the installation is
| | |
MILLIPORE FORM 10-K 2007 | | 69 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
based on relative fair value at the time of sale. In limited cases, our customers may require site acceptance testing for certain customized products built to the customers’ specifications. Revenues on these products are deferred upon shipment and are recognized when site acceptance testing is completed.
Revenue for certain fixed price contracts associated with our Bioprocess division equipment business is recognized under the percentage of completion method. Revenue is recognized based on the ratio of hours expended to the total estimated hours to complete the construction of the equipment. The cumulative impact of any revisions in estimates of the percentage of completion is reflected in the period in which the changes become known. Losses are accrued when known.
Revenue from service arrangements is recognized when the services are provided.
We recognize license and royalty revenue when the amounts are determinable and we have fulfilled our obligations under the applicable agreement. This generally occurs when cash payments are received or licensed sales are reported to us.
Warranty Costs
We accrue for estimated warranty costs for products at the time of sale. Warranty liabilities are based on estimated future repair costs using historical statistical models and were not material as of December 31, 2007 and 2006.
Research and Development
Research and development costs are expensed as incurred. The fair value of acquired in-process research and development costs is expensed as of the acquisition date if the related projects have not reached technological feasibility and were determined to have no alternative future use.
Recent Accounting Pronouncements
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurement” (“SFAS No. 157”). SFAS No. 157 clarifies the principle that fair value should be based on the assumptions market participants would use when pricing an asset or liability and establishes a fair value hierarchy that prioritizes the information used to develop those assumptions. Under the standard, fair value measurements would be separately disclosed by level within the fair value hierarchy. In November 2007, the FASB deferred the effective date of SFAS No. 157 for certain nonfinancial and nonrecurring assets and liabilities. Other than the partial deferral, SFAS No. 157 is effective for us as of the beginning of 2008. SFAS No. 157 does not have a material impact on our financial assets and liabilities. We are currently evaluating the impact of SFAS No. 157 on our nonfinancial assets and liabilities within the scope of SFAS No. 157.
In September 2006, the FASB issued SFAS No. 158, “Employers Accounting for Defined Benefit Pension and Other Retirement Plans—an amendment of FASB Statements No. 87, 88, 106, and 132(R)” (“SFAS No. 158”). Under this standard, we are required to recognize the overfunded or underfunded status of our defined benefit postretirement plan as an asset or liability in our statement of financial position and to recognize changes in that funded status in the year in which the changes occur through comprehensive income. We were required to initially recognize the funded status of our defined benefit postretirement plans and to provide the required disclosures as of the end of 2006. The requirement to measure plan assets and benefit obligations as of year end in the statement of financial position will be effective for us in 2008. We adopted the funded status provisions of SFAS No. 158 effective December 31, 2006, but have not adopted the measurement date provision. Our adoption of the measurement date provisions of SFAS No. 158 in 2008 is not expected to have a material impact on our financial position or results of operations.
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Liabilities, Including an amendment of FASB Statement No. 115” (“SFAS No. 159”). SFAS No. 159 permits entities to measure many finan-
| | |
| 70 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
cial instruments and certain other items at fair value that are not currently required to be measured at fair value. SFAS No. 159 is effective for us as of the beginning of 2008. We do not anticipate the adoption of SFAS No. 159 to have a material impact on our financial position or results of operations.
In June 2007, the Emerging Issues Task Force (“EITF”) reached a consensus on EITF Issue No. 07-3, “Accounting for Nonrefundable Advance Payments for Goods or Services Received to Be Used in Future Research and Development Activities” (“EITF 07-3”). EITF 07-3 requires companies that are involved in research and development activities to defer nonrefundable advance payments for future research and development activities and to recognize those payments as goods and services are delivered. We will be required to assess on an ongoing basis whether or not the goods or services will be delivered and to expense the nonrefundable advance payments immediately if we determine that the delivery of such goods or services is unlikely. EITF 07-3 is effective for new arrangements entered into subsequent to the beginning of 2008. Adoption of EITF 07-3 will not have a material impact on our financial condition or results of operations.
In December 2007, the FASB issued SFAS No. 141 (Revised 2007), “Business Combinations” (“SFAS No. 141(R)”), which replaces SFAS No. 141, “Business Combinations.” SFAS No. 141(R) retains the underlying concepts of SFAS No. 141 in that all business combinations are still required to be accounted for at fair value under the acquisition method. However, SFAS No. 141(R) changes the method of applying the acquisition method in a number of significant aspects: acquisition costs will generally be expensed as incurred; noncontrolling interests will be valued at fair value at the acquisition date; in-process research and development will be recorded at fair value as an indefinite-lived intangible asset at the acquisition date; restructuring costs associated with a business combination will generally be expensed subsequent to the acquisition date; and changes in deferred tax asset valuation allowances and income tax uncertainties after the acquisition date generally will affect income tax expense. SFAS No. 141(R) is effective on a prospective basis for all business combinations for which the acquisition date is on or after the beginning of the first annual period subsequent to December 15, 2008, with the exception of the accounting for valuation allowances on deferred taxes and acquired tax contingencies. SFAS No. 141(R) amends SFAS No. 109 such that adjustments made to valuation allowances on deferred taxes and acquired tax contingencies associated with acquisitions that closed prior to the effective date of SFAS No. 141(R) would also follow the provisions of SFAS No. 141(R). Early adoption of the provisions of SFAS No. 141(R) is not permitted. We are currently evaluating the effects that SFAS No. 141(R) may have on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 160, “Noncontrolling Interests in Consolidated Financial Statements—an amendment of ARB No. 51” (“SFAS No. 160”). This statement is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008, with earlier adoption prohibited. This statement requires the recognition of a noncontrolling interest (minority interest) as equity in the consolidated financial statements and separate from the parent’s equity. The amount of net income attributable to the noncontrolling interest will be included in consolidated net income on the face of the income statement. It also amends certain consolidation procedures for consistency with the requirements of SFAS No. 141(R).This statement also includes expanded disclosure requirements regarding the interests of the parent and its noncontrolling interest. We are currently evaluating this new statement and anticipate that the statement will not have a significant impact on the reporting of our results of operations.
In December 2007, the EITF reached a consensus on Issue No. 07-1, “Accounting for Collaborative Arrangements” (“EITF 07-1”). EITF 07-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years, and shall be applied retrospectively to all prior periods presented for all collaborative arrangements existing as of the effective date. EITF 07-1 requires that transactions with third parties (i.e., revenue generated and costs incurred by the partners) should be reported in the appropriate line item in each company’s financial statement pursuant to the guidance in EITF Issue No. 99-19, “Reporting Revenue Gross as a Principal versus Net as an Agent.”
| | |
MILLIPORE FORM 10-K 2007 | | 71 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
EITF 07-1 also includes enhanced disclosure requirements regarding the nature and purpose of the arrangement, rights and obligations under the arrangement, accounting policy, amount and income statement classification of collaboration transactions between the parties, and amounts due from or owed to other participants under the collaborative arrangements. We are currently evaluating the effects that EITF 07-1 may have on our consolidated financial statements.
3. BUSINESS ACQUISITIONS
2006 Acquisitions
Serologicals Corporation
On July 14, 2006, we acquired Serologicals. This acquisition strengthened the market position of our Bioscience division by increasing its product portfolio into markets such as drug discovery products and services, nuclear function and stem cell research products. The acquisition also facilitated our entrance into the upstream bioprocessing market by gaining a cell culture supplements offering for our Bioprocess division. The total purchase price was $1,474,928 including debt assumed. The acquisition was financed with cash on hand and net proceeds from the issuance of the 3.75 percent senior convertible notes and the 5.875 percent senior notes.
The total Serologicals purchase price is shown below:
| | | |
Cash paid for common stock | | $ | 1,079,280 |
Cash paid for stock options, restricted stock, and performance shares | | | 32,191 |
Cash paid for Serologicals debt at closing | | | 75,954 |
Direct acquisition costs | | | 10,190 |
Total cash consideration | | | 1,197,615 |
Conversion value of 4.75% Serologicals convertible debentures assumed | | | 277,313 |
Total purchase price, including debt assumed | | $ | 1,474,928 |
The acquisition purchase price was allocated to net assets acquired and identifiable intangible assets based on their estimated fair values. These fair values were based on management’s estimates and assumptions. The excess purchase price over those assigned values was recorded as goodwill. Goodwill and intangible assets recorded as a result of this acquisition are not deductible for tax purposes. In 2007, we finalized the purchase price allocation for this acquisition and recorded adjustments to increase goodwill by $188, decrease deferred tax liabilities by $811 and increase other liabilities by $999.
The final Serologicals purchase price was allocated as follows:
| | | | |
| | | Amount | |
Cash | | $ | 29,713 | |
Accounts receivable | | | 37,599 | |
Inventories | | | 107,613 | |
Assets held for sale | | | 17,150 | |
Property, plant and equipment | | | 73,683 | |
Other assets | | | 22,886 | |
Identifiable intangible assets: | | | | |
Customer related intangibles (weighted average useful life of 18 years) | | | 385,100 | |
Patented and unpatented technology (weighted average useful life of 12 years) | | | 49,680 | |
Trademarks and trade names (weighted average useful life of 15 years) | | | 18,600 | |
Total identifiable intangible assets (weighted average useful life of 17 years) | | | 453,380 | |
Goodwill | | | 915,691 | |
4.75% convertible debentures assumed | | | (277,313 | ) |
Deferred tax liabilities | | | (113,369 | ) |
Other liabilities | | | (69,418 | ) |
Total cash consideration | | $ | 1,197,615 | |
| | |
| 72 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
We paid $277,313 to the holders of the 4.75 percent Serologicals convertible debentures when these holders converted their notes in August 2006.
At the time of acquisition, we committed to a preliminary plan of integration of certain Serologicals activities, which included closure of facilities, the abandonment or redeployment of equipment, and employee terminations. As of July 14, 2006, we recorded severance and relocation cost liabilities amounting to $6,675 and facility closure cost liabilities amounting to $5,877 with corresponding adjustments to goodwill in accordance with EITF Issue No. 95-3,“Recognition of Liabilities in Connection with a Purchase Business Combination” (“EITF 95-3”). The following table is a summary of these liabilities:
| | | | | | | | | | | | |
| | | Severance
and
Relocation
Costs | | | Other
Facility
Exit
Costs | | | Total | |
Balance at July 14, 2006 | | $ | 6,675 | | | $ | 5,877 | | | $ | 12,552 | |
Payments | | | (238 | ) | | | – | | | | (238 | ) |
Revision of previously recorded costs | | | (250 | ) | | | (651 | ) | | | (901 | ) |
Balance at December 31, 2006 | | | 6,187 | | | | 5,226 | | | | 11,413 | |
Provisions | | | 905 | | | | 69 | | | | 974 | |
Payments | | | (5,401 | ) | | | (1,840 | ) | | | (7,241 | ) |
Other | | | 66 | | | | 181 | | | | 247 | |
Balance at December 31, 2007 | | $ | 1,757 | | | $ | 3,636 | | | $ | 5,393 | |
Amounts accrued for severance and relocation cost will be paid in 2008. Accruals for facility exit costs are expected to be paid over the remaining lease term for certain idle facilities.
The results of Serologicals’ operations have been included in the consolidated statements of operations since the acquisition date. The following unaudited pro forma financial information presents the combined results of operations of Millipore and Serologicals as if the acquisition had occurred as of the beginning of the periods presented below. The combined results of operations have been adjusted to reflect the amortization of purchased intangible assets and inventory fair value adjustments, additional financing expenses, and other direct costs incurred by Serologicals in connection with the acquisition. The unaudited pro forma financial information is not intended to represent, or be indicative of, our consolidated results of operations that would have been reported had the acquisition been completed as of the dates presented and should not be taken as representative of our future consolidated results of operations.
| | | | | | |
| | | December 31, |
| | | 2006 | | 2005 |
Net sales | | $ | 1,385,735 | | $ | 1,264,888 |
Net income | | $ | 56,199 | | $ | 11,867 |
Basic earnings per share | | $ | 1.06 | | $ | 0.23 |
Diluted earnings per share | | $ | 1.04 | | $ | 0.23 |
Newport Bio Systems, Inc.
On April 27, 2006, we acquired Newport Bio Systems, Inc. (“Newport”), a provider of disposable process containers used in biopharmaceutical production. The acquisition broadened the scope of the process equipment product offerings of our Bioprocess division. The total purchase price was $8,602.
| | |
MILLIPORE FORM 10-K 2007 | | 73 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
The purchase price was allocated to the net assets acquired, identifiable intangible assets, and goodwill based on their estimated fair values at the time of acquisition, as follows:
| | | | |
| | | Amount | |
Current assets | | $ | 1,746 | |
Property, plant and equipment | | | 218 | |
Identifiable intangible assets: | | | | |
Customer related intangibles (weighted average useful life of 19 years) | | | 2,500 | |
Patented and unpatented technology (weighted average useful life of 4 years) | | | 300 | |
Trademarks and trade names (weighted average useful life of 6 years) | | | 200 | |
Total identifiable intangible assets (weighted average useful life of 13 years) | | | 3,000 | |
Goodwill | | | 6,212 | |
Current liabilities | | | (1,431 | ) |
Deferred tax liability | | | (1,143 | ) |
Total purchase price | | $ | 8,602 | |
The results of the acquired operations have been included in the consolidated statements of operations since the acquisition date. The excess purchase price allocated to intangible assets and goodwill is not deductible for income tax purposes. Pro forma results of operations have not been presented because such information is not material to our consolidated financial statements.
2005 Acquisitions
During the third quarter of 2005, we acquired NovAseptic A.B. (“NovAseptic”) and MicroSafe B.V. (“MicroSafe”) for $96,296 and $9,088, respectively. NovAseptic provided innovative solutions for aseptic processing applications in biotechnology and pharmaceutical manufacturing operations while MicroSafe developed assays and provided testing services that help biotechnology and pharmaceutical customers monitor quality and compliance in the drug manufacturing process. The purchase prices for the NovAseptic and MicroSafe acquisitions were allocated to the net assets acquired, identifiable intangible assets, and goodwill based on their estimated fair values at the time of acquisition. In the second quarter of 2006, we finalized the purchase price allocation for these acquisitions and recorded adjustments to reduce goodwill and deferred tax liabilities by $1,634. The excess purchase price allocated to intangible assets and goodwill is not deductible for income tax purposes. The amount allocated to the in-process research and development costs of $3,149 was written off at the date of acquisition because these costs had no alternative future uses and the underlying projects had not reached technological feasibility. Pro forma results of operations have not been presented because such information is not material to our consolidated financial statements.
4. GOODWILL
The following table presents changes in the carrying amounts of goodwill:
| | | | | | | |
| | | 2007 | | 2006 | |
Balance at beginning of year | | $ | 1,014,194 | | $ | 82,718 | |
Additions for current year acquisitions | | | – | | | 921,715 | |
Adjustments for prior year acquisitions | | | 188 | | | (1,634 | ) |
Effect of foreign exchange rate changes | | | 5,199 | | | 11,395 | |
Balance at end of year | | $ | 1,019,581 | | $ | 1,014,194 | |
| | |
| 74 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
5. INTANGIBLE ASSETS
Identifiable intangible assets consisted of the following:
| | | | | | | | | | | | |
| December 31, 2007 | | Gross Intangible
Assets | | Accumulated
Amortization | | | Net Intangible
Assets | | Estimated
Useful Life |
Patented and unpatented technologies | | $ | 81,134 | | $ | (30,407 | ) | | $ | 50,727 | | 5 – 20 years |
Trademarks and trade names | | | 42,534 | | | (15,810 | ) | | | 26,724 | | 5 – 20 years |
Customer relationships | | | 405,235 | | | (52,886 | ) | | | 352,349 | | 15 – 18 years |
Licenses and other | | | 8,159 | | | (5,851 | ) | | | 2,308 | | 5 – 10 years |
Total | | $ | 537,062 | | $ | (104,954 | ) | | $ | 432,108 | | |
| | | | | | | | | | | | |
| December 31, 2006 | | Gross Intangible
Assets | | Accumulated
Amortization | | | Net Intangible
Assets | | Estimated
Useful Life |
Patented and unpatented technologies. | | $ | 80,461 | | $ | (20,719 | ) | | $ | 59,742 | | 5 – 20 years |
Trademarks and trade names | | | 42,292 | | | (11,914 | ) | | | 30,378 | | 5 – 20 years |
Customer relationships | | | 404,138 | | | (7,798 | ) | | | 396,340 | | 15 – 18 years |
Licenses and other | | | 6,363 | | | (4,520 | ) | | | 1,843 | | 5 – 10 years |
Total | | $ | 533,254 | | $ | (44,951 | ) | | $ | 488,303 | | |
Amortization expense for the years ended December 31, 2007, 2006 and 2005 was $59,448, $16,453 and $4,333, respectively.
The estimated aggregate amortization expense for intangible assets owned as of December 31, 2007 for each of the five succeeding years and thereafter is as follows:
| | | |
2008 | | $ | 63,879 |
2009 | | | 56,657 |
2010 | | | 50,462 |
2011 | | | 45,297 |
2012 | | | 39,668 |
Thereafter | | | 176,145 |
Total | | $ | 432,108 |
6. BASIC AND DILUTED EARNINGS PER SHARE
The following table sets forth the computation of basic and diluted earnings per share:
| | | | | | | | | |
| | | Year ended December 31, |
| | | 2007 | | 2006 | | 2005 |
Numerator: | | | | | | | | | |
Net income | | $ | 136,472 | | $ | 96,984 | | $ | 80,168 |
Denominator: | | | | | | | | | |
Weighted average common shares outstanding for basic EPS | | | 54,263 | | | 53,160 | | | 50,953 |
Dilutive effect of stock-based compensation awards | | | 765 | | | 1,085 | | | 706 |
Weighted average common shares outstanding for diluted EPS | | | 55,028 | | | 54,245 | | | 51,659 |
Earnings per share: | | | | | | | | | |
Basic | | $ | 2.52 | | $ | 1.82 | | $ | 1.57 |
Diluted | | $ | 2.48 | | $ | 1.79 | | $ | 1.55 |
| | |
MILLIPORE FORM 10-K 2007 | | 75 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
For the years ended December 31, 2007, 2006 and 2005, outstanding stock options and restricted stock units amounting to 497 shares, 290 shares and 41 shares, respectively, had exercise prices in excess of the average fair market value of our common stock for the related years and were excluded from the calculation of diluted earnings per share because of their antidilutive effect. Antidilutive options could become dilutive in the future. In addition, shares issuable for the conversion premium upon conversion of the 3.75 percent convertible senior notes were excluded from the calculation of diluted earnings per share as of December 31, 2007 and 2006 because our stock price had not exceeded the conversion price.
7. INVENTORIES
Inventories, stated at the lower of FIFO cost or market, consisted of the following:
| | | | | | |
| | | December 31, |
| | | 2007 | | 2006 |
Raw materials | | $ | 52,011 | | $ | 50,085 |
Work in process | | | 77,642 | | | 79,577 |
Finished goods | | | 147,702 | | | 127,004 |
Total inventories | | $ | 277,355 | | $ | 256,666 |
8. PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consisted of the following:
| | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
Land | | $ | 21,497 | | | $ | 20,673 | |
Leasehold improvements | | | 24,970 | | | | 17,593 | |
Buildings and improvements | | | 339,793 | | | | 310,654 | |
Production and other equipment | | | 407,530 | | | | 356,688 | |
Construction in progress | | | 114,134 | | | | 91,665 | |
| | | 907,924 | | | | 797,273 | |
Less: accumulated depreciation | | | (318,763 | ) | | | (271,370 | ) |
Property, plant and equipment, net | | $ | 589,161 | | | $ | 525,903 | |
Depreciation expense for the years ended December 31, 2007, 2006 and 2005 was $64,299, $55,824 and $46,324, respectively.
We excluded accrued liabilities of $1,182, $9,139 and $0 as non-cash investing activity from the consolidated statements of cash flows in 2007, 2006, and 2005, respectively, related to property, plant and equipment that had not yet been paid as of those respective year ends.
9. ACCRUED EXPENSES
Accrued expenses consisted of the following:
| | | | | | |
| | | December 31, |
| | | 2007 | | 2006 |
Deferred revenue | | $ | 15,656 | | $ | 11,586 |
Accrued compensation | | | 85,247 | | | 88,238 |
Other | | | 64,093 | | | 91,441 |
Total | | $ | 164,996 | | $ | 191,265 |
| | |
| 76 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
10. DEBT
Short-term debt and notes payable
Short-term debt at December 31, 2007 consisted of borrowings under our operating bank facilities. Short-term debt at December 31, 2006 consisted of our 7.5 percent ten-year unsecured notes in the aggregate amount of $100,000. These notes were due on April 1, 2007 and were paid off, including accrued interest of $3,750, with cash on hand and borrowings under our revolving credit facility.
Long-term debt
Our long-term debt consisted of the following:
| | | | | | |
| | | December 31, |
| | | 2007 | | 2006 |
Revolving credit facility | | $ | 331,552 | | $ | 422,442 |
3.75% convertible senior notes due 2026 | | | 565,000 | | | 565,000 |
5.875% senior notes due 2016, net of discount | | | 363,491 | | | 328,814 |
Total long-term debt | | $ | 1,260,043 | | $ | 1,316,256 |
In June 2006, we issued $895,033 of long-term debt. These proceeds and borrowings under our revolving credit facility were used primarily to fund the Serologicals acquisition.
REVOLVING CREDIT FACILITY
We entered into an agreement for a five-year unsecured revolving credit facility (the “Revolver”) in December 2005. The Revolver agreement originally provided for a domestic revolving credit facility and a foreign credit facility each with a maximum borrowing of€430,000. The combined borrowings at any one time under both revolving credit facilities may not exceed€430,000 in the aggregate. The domestic revolving credit facility includes a€65,000 letter of credit subfacility and a€17,500 swing line subfacility. We may elect to increase the credit facilities by an amount not in excess of€130,000. We may prepay any outstanding borrowings in whole or in part without premium or penalty. As of December 31, 2007 and 2006, outstanding letters of credit were $1,881 and $1,808, respectively.
The acquisition of Serologicals on July 14, 2006 and the related financing required us to change certain terms of the Revolver agreement. Accordingly, we amended the agreement in June 2006 (some of which became effective on July 14, 2006) to:
| | n | | permit the consummation of the Serologicals acquisition and issuance and incurrence of certain additional indebtedness in connection with the acquisition; |
| | n | | extend the maturity date to June 6, 2011; |
| | n | | modify interest rate and commitment fee adjustments based on specified credit ratings; |
| | n | | require the pledge of substantially all of our assets to secure our obligations under the Revolver if specified credit rating levels are reached; and |
| | n | | adjust certain restrictions and financial covenants. |
We again amended the Revolver agreement in July 2006 to increase the borrowing availability under the domestic facility from€430,000, or $627,279, to€465,000, or $678,337. We recorded $2,806 of deferred financing costs associated with amending the Revolver agreement and will amortize the costs over the term of the agreement, or five years.
| | |
MILLIPORE FORM 10-K 2007 | | 77 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
We may choose an interest rate equal to either LIBOR plus an applicable margin as provided for in the new credit agreement or a base rate defined as the higher of the annual rate of the lead bank’s prime rate or the federal funds rate plus 0.50 percentage points for borrowings under the new credit agreement. Interest is payable quarterly or, if earlier, at the end of an interest period. We are required to pay a commitment fee on unused commitments ranging between 0.0675 percent and 0.60 percent annually, based on the Revolver’s debt rating. As of December 31, 2007, we had€237,721, or $346,785, available for borrowing on the Revolver.
We are required to maintain certain leverage and interest coverage ratios set forth in the Revolver agreement. As of December 31, 2007, we were compliant with all financial covenants specified in the amended Revolver agreement. The agreement also includes limitations on our ability to incur additional indebtedness; to merge, consolidate, or sell assets; to create liens; and to make payments in respect of capital stock or subordinated debt, as well as other customary covenants and representations.
The following table summarizes the financial covenant requirements as of December 31, 2007 and thereafter, and our compliance with these covenants as of December 31, 2007:
| | | | |
| Covenant | | Requirement | | Actual at
December 31, 2007 |
Maximum leverage ratio | | 3.50:1.0 | | 3.37:1.0 |
Minimum interest coverage ratio | | 3.50:1.0 | | 5.65:1.0 |
As of December 31, 2007, we borrowed€227,279, or $331,552, under the Revolver. The borrowings were classified as long-term debt because of our ability and intent to continuously refinance such borrowings. For the years ended December 31, 2007 and 2006, the weighted average interest rate for the Revolver was 4.8 percent and 3.3 percent, respectively.
3.75% CONVERTIBLE SENIOR NOTES DUE 2026
In June 2006, we issued $565,000 in aggregate principal amount of convertible senior notes (the “Convertible Notes”) in a private placement offering. The Convertible Notes bear interest at 3.75 percent per annum, payable semi-annually in arrears on June 1 and December 1 of each year. Commencing with the six-month period beginning on December 1, 2011, we will accrue contingent interest on the Convertible Notes at the rate of 0.175 percent of the average trading price of the Convertible Notes (“the Contingent Interest feature”), if the average trading price of the Convertible Notes for the five consecutive trading days preceding such six-month periods equals 120 percent or more of the principal amount. The Convertible Notes are senior unsecured obligations and rank equally with all of our existing and future senior unsecured indebtedness. The Convertible Notes are effectively subordinated to all of our existing and future secured indebtedness and all existing and future liabilities of our subsidiaries, including trade payables. The Convertible Notes will mature on June 1, 2026. We recorded $13,361 of deferred financing costs associated with the issuance of the Convertible Notes and will amortize the amount over 5.5 years.
Holders of the Convertible Notes may convert their notes into cash and, if applicable, shares of our common stock prior to June 1, 2026 under certain conditions. The Convertible Notes may be converted if the closing sale price of our common stock for each of the 20 or more trading days in a period of 30 consecutive trading days ending on the last trading day of the immediately preceding calendar quarter exceeds 120 percent of the conversion price in effect on the last trading day of the immediately preceding calendar quarter. The Convertible Notes may also be converted during the five consecutive business days immediately after any five consecutive trading day period in which the average trading price per $1,000 principal amount of the Convertible Notes was equal to or less than 97 percent of the average conversion value of the notes during this period. The Convertible Notes will also be convertible if we make certain distributions on our common stock or
| | |
| 78 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
engage in certain transactions; if we call the Convertible Notes for redemption; and at any time from November 1, 2011 through December 1, 2011; and on or after June 1, 2024. Upon conversion, the Convertible Notes will be convertible into cash for the principal amount and shares of our common stock for the conversion premium, if any, based on an initial conversion rate of 11.0485 shares per $1,000 principal amount (which represents an initial conversion price of approximately $90.51 per share), subject to adjustments.
On or after December 1, 2011, we have the option to redeem the Convertible Notes at a redemption price equal to 100 percent of the principal amount of the notes, plus accrued but unpaid interest (the “Call Option”). On each of December 1, 2011, June 1, 2016 and June 1, 2021, holders of the Convertible Notes have the option to require us to purchase all or a portion of their notes at a purchase price in cash equal to 100 percent of the principal amount of the notes, plus accrued but unpaid interest (the “Put Option”). Holders may also require us to repurchase all or a portion of their notes upon a fundamental change at a repurchase price in cash equal to 100 percent of the principal amount of the notes to be repurchased, plus accrued but unpaid interest.
A holder that surrenders the Convertible Notes for conversion in connection with a “make-whole fundamental change” that occurs before December 1, 2011 may in certain circumstances be entitled to an increased conversion rate (the “Make-whole Payment”). However, in lieu of increasing the conversion rate applicable to those Convertible Notes, we may in certain circumstances elect to adjust the conversion rate and the related conversion obligation so that the Convertible Notes will be convertible into shares of the acquiring company’s common stock, except that the principal return due upon conversion will continue to be payable in cash.
Although we are not required to maintain any specified financial ratios under the Convertible Notes agreement, we will be considered in default if we fail to fulfill our conversion or redemption obligations, make required interest payments, provide notice to holders of the Convertible Notes in certain specified circumstances, or cure our default on any indebtedness of ours or our subsidiaries in the aggregate principal amount of $50,000 or more. If an event of default has occurred and is continuing, the principal amount of the Convertible Notes plus interest thereon may become immediately due and payable. We are currently in compliance with the covenant restrictions.
As of December 31, 2007, the Convertible Notes had a fair market value of $608,166.
We evaluated the Convertible Notes agreement for potential embedded derivatives under SFAS No. 133, and related applicable accounting literature, including EITF Issue No. 00-19, “Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock”, and EITF Issue No. 05-4, “The Effect of a Liquidated Damages Clause on a Freestanding Financial Instrument Subject to Issue No. 00-19 (“EITF Issue No. 05-4”). The conversion feature, the Make-whole Payment, the Put Option of the holder, and our Call Option were determined to not meet the embedded derivative criteria as set forth by SFAS No. 133. Therefore, no fair value has been recorded for these items. The Contingent Interest feature and the conversion feature related to the trading price of the Convertible Notes represent embedded derivatives that require separate recognition of fair value apart from the Convertible Notes under SFAS No. 133. As a result, we are required to separate the value of these items from the Convertible Notes and record a liability on the consolidated balance sheet. As of December 31, 2007, both the Contingent Interest feature and the conversion feature had a nominal fair value. We evaluated the “Additional Interest” provision of the registration rights clause in accordance with EITF Issue No. 05-4 and concluded that the item should be evaluated separately as a liability. As we fulfilled our initial registration obligation on August 9, 2006 and have continued to maintain an effective registration statement, we have no liability in connection with this provision.
| | |
MILLIPORE FORM 10-K 2007 | | 79 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
5.875% SENIOR NOTES DUE 2016
In June 2006, we issued€250,000, or $364,697, in aggregate principal amount of 5.875 percent senior notes (the “Euro Notes”) due in 2016. Interest is payable semi-annually in arrears on June 30 and December 30 of each year. The Euro Notes were issued at 99.611 percent of the principal amount, which resulted in an original issue discount of€973, or $1,419. The Euro Notes are senior unsecured obligations and rank equally with all of our existing and future senior unsecured indebtedness. We recorded $3,321 of deferred financing costs associated with the issuance of the Euro Notes and will amortize the amount over 10 years.
If the acquisition of Serologicals did not occur, we would have been required to repurchase the Euro Notes on or before October 31, 2006 at a redemption price, payable in cash, equal to 101 percent of the principal amount, plus accrued and unpaid interest (the “Repurchase Obligation”). Upon the occurrence of any change in control, holders of the Euro Notes may require us to repurchase all of their Euro Notes for a cash price equal to 101 percent of the principal amount, plus accrued and unpaid interest thereon (the holder’s “Put Option”). Before June 30, 2016, we may, at our option, redeem the Euro Notes, in whole or in part, for cash, at a redemption price equal to 100 percent of the principal amount of the Euro Notes we redeem, plus applicable “make-whole” premium (“call options”). In addition, we may redeem our option in whole, but not in part, at a redemption price equal to 100 percent of the principal amount, plus accrued and unpaid interest, upon the occurrence of certain tax events in the United States (“call options”). We evaluated the Euro Notes agreement for potential embedded derivatives under SFAS No. 133 and determined that our call options, the holders’ Put Option, and our Repurchase Obligation do not meet the embedded derivative criteria as set forth by SFAS No. 133.
The indenture for the Euro Notes places certain restrictions on our ability to create, incur, assume or suffer liens on our manufacturing plants and other principal facilities in the United States and to enter into certain sale-leaseback transactions. We would also be considered in default if we fail to fulfill our redemption obligations, make required interest payments, provide notice to holders of the Euro Notes in certain specified circumstances, or cure our default on any of our indebtedness or that of our subsidiaries in the aggregate principal amount of $50,000 or more. If an event of default has occurred and is continuing, the principal amounts of the Euro Notes plus any accrued interest thereon may become immediately due and payable. We are currently in compliance with the covenant restrictions.
As of December 31, 2007, the Euro Notes had a fair market value of€235,000, or $342,815.
11. INCOME TAXES
Our provisions for income taxes are summarized as follows:
| | | | | | | | | | | |
| | | Year ended December 31, |
| | | 2007 | | | 2006 | | | 2005 |
U.S. and foreign (loss) income before income taxes: | | | | | | | | | | | |
U.S. | | $ | (30,621 | ) | | $ | (12,564 | ) | | $ | 38,650 |
Foreign | | | 183,044 | | | | 132,947 | | | | 98,883 |
Income before income taxes | | $ | 152,423 | | | $ | 120,383 | | | $ | 137,533 |
Domestic and foreign (benefit from) provision for income taxes: | | | | | | | | | | | |
U.S. Federal | | $ | (22,735 | ) | | $ | (8,405 | ) | | $ | 33,591 |
Foreign | | | 35,536 | | | | 29,796 | | | | 21,560 |
U.S. State | | | (377 | ) | | | 71 | | | | 2,214 |
Total | | $ | 12,424 | | | $ | 21,462 | | | $ | 57,365 |
Current and deferred provision for (benefit from) income taxes: | | | | | | | | | | | |
Current | | $ | 32,979 | | | $ | 35,700 | | | $ | 46,134 |
Deferred | | | (20,555 | ) | | | (14,238 | ) | | | 11,231 |
Total | | $ | 12,424 | | | $ | 21,462 | | | $ | 57,365 |
| | |
| 80 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
Deferred income taxes represent the tax effects of transactions that are reported in different periods for tax and financial reporting purposes. These amounts consist of the tax effects of temporary differences between the tax and financial reporting balances and tax carryforwards. Pursuant to SFAS No. 109, current and non-current deferred income tax assets and liabilities within the same tax jurisdiction are generally offset for presentation in the consolidated balance sheets.
Significant components of our net deferred tax assets and liabilities are as follows:
| | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| Deferred tax assets: | | | | | | | | |
Inventory related transactions | | $ | 49,666 | | | $ | 41,516 | |
Retirement plans and postretirement benefits | | | 16,505 | | | | 17,359 | |
Tax credits | | | 55,578 | | | | 46,927 | |
Net operating loss carryforwards | | | 46,767 | | | | 32,458 | |
Capitalized research and development costs | | | 14,076 | | | | 17,708 | |
Intangible assets and goodwill | | | 46,735 | | | | 47,630 | |
Deferred state tax assets | | | 30,097 | | | | 26,781 | |
Accrued expenses | | | 16,502 | | | | 27,585 | |
Other | | | 22,155 | | | | 25,719 | |
Total deferred tax assets | | | 298,081 | | | | 283,683 | |
Valuation allowance | | | (30,753 | ) | | | (29,146 | ) |
Total deferred tax assets, net of valuation allowance | | | 267,328 | | | | 254,537 | |
| Deferred tax liabilities: | | | | | | | | |
Purchased intangible assets | | | 164,647 | | | | 182,967 | |
Other | | | 27,483 | | | | 20,530 | |
Total deferred tax liabilities | | | 192,130 | | | | 203,497 | |
Net deferred tax assets | | $ | 75,198 | | | $ | 51,040 | |
At December 31, 2007, we had gross federal net operating loss carryforwards of approximately $172,333 that will begin to expire in 2025 through 2027. We also have foreign net operating loss carryforwards of approximately $46,578 that will begin to expire in 2009 through 2027 or can be carried forward indefinitely. When net operating losses are realized, tax benefits associated with tax deductions of $21,794 attributable to our stock plan activities will be recorded in additional paid in capital. We have general business credit carryforwards of approximately $12,097 that expire in the years 2008 through 2027. In addition, we have alternative minimum tax credit carryforwards of approximately $10,878, which can be carried forward indefinitely.
Valuation allowances were established for the expiration of federal and state research credits, state investment credit carryforwards, some foreign and state net operating loss carryforwards, and a capital loss carryforward. Although realization is not assured, we believe it is more likely than not that the remainder of deferred tax assets, net of valuation allowances, will be realized. The amount of deferred tax assets considered realizable, however, could be reduced in the near term if estimates of future taxable income are reduced.
At December 31, 2007, we had valuation allowances of $1,530 related to federal research credits, $22,223 related to state tax credits and net operating loss carryforwards, $2,587 related to capital loss carryforward, and $4,413 related to foreign net operating loss carryforwards. During 2007, $596 of federal research credits expired and were written off against the valuation allowance. We also recorded additional valuation allowances of $1,005 and $1,198, respectively, for federal research credits and state tax credits projected to expire unused.
| | |
MILLIPORE FORM 10-K 2007 | | 81 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
We provide for U.S. income taxes on the earnings of foreign subsidiaries unless they are considered indefinitely invested outside the U.S. The pre-tax income from Ireland, Sweden and United Kingdom are considered indefinitely reinvested overseas and we have not recorded deferred income taxes applicable to undistributed earnings of our subsidiaries in these countries.
The American Jobs Creation Act of 2004 (the “AJCA”) provided a temporary incentive for U.S. companies to repatriate funds deemed to be permanently reinvested outside the U.S. at a reduced effective federal tax rate on qualified amounts. Under this provision of the AJCA, we repatriated approximately $500,000 and provided associated taxes of $30,634 in December 2005. As a result of this repatriation transaction, there were no cumulative earnings outside the United States upon which U.S. income taxes were not provided at December 31, 2005. These earnings amounted to $215,776 at December 31, 2007. If earnings of such foreign subsidiaries were not indefinitely reinvested, a deferred tax liability of $56,601 would have been required at December 31, 2007.
A summary of the differences between the worldwide effective tax rate and the United States statutory federal income tax rate is as follows:
| | | | | | | | | |
| | | Year ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
U.S. statutory federal income tax rate | | 35.0 | % | | 35.0 | % | | 35.0 | % |
Puerto Rico tax rate benefit | | – | | | – | | | (3.2 | ) |
Ireland, Sweden and UK tax rate benefit | | (20.8 | ) | | (16.1 | ) | | (10.8 | ) |
State income tax, net of federal income tax benefit | | (0.2 | ) | | 0.1 | | | 0.2 | |
Export sales benefit | | – | | | (0.5 | ) | | (0.7 | ) |
Change in valuation allowance | | 0.7 | | | – | | | (2.5 | ) |
U.S. tax on repatriation of foreign earnings | | – | | | – | | | 22.2 | |
Net decrease in tax reserves | | (7.3 | ) | | – | | | – | |
Write-off of purchased in-process research and development | | – | | | – | | | 0.8 | |
Other | | 0.8 | | | (0.7 | ) | | 0.7 | |
Effective tax rate | | 8.2 | % | | 17.8 | % | | 41.7 | % |
Tax exemptions relating to Ireland operations are effective through 2010. The special U.S. federal tax regime applicable to our Puerto Rico operations expired on December 31, 2005.
We are a worldwide business and are subject to tax audits on a regular basis. Significant judgment is required in determining our worldwide provision for income taxes and we periodically assess our income tax positions and record tax benefits for all years subject to examination based upon our evaluation of the facts, circumstances and information available at the reporting date. For those tax positions where it is more likely than not that a tax benefit will be sustained, we record the largest amount of tax benefit with a greater than 50 percent likelihood of being realized upon ultimate settlement with a taxing authority that has full knowledge of all relevant information. For those income tax positions where it is not more likely than not that a tax benefit will be sustained, no tax benefit is recognized in the financial statements.
We adopted the provisions of FIN 48 as of January 1, 2007. As a result of the implementation of FIN 48, we recognized a $600 adjustment to retained earnings, which increased the liability for unrecognized income tax benefits. At the adoption date of January 1, 2007 and including the opening adjustment, we had $29,200 of unrecognized tax benefits, of which $21,300 would affect our effective tax rate if recognized. The remaining unrecognized benefits relate to the pre-acquisition
| | |
| 82 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
periods of Serologicals. We recognize interest and penalties related to uncertain tax positions in income tax expense. At December 31, 2007, we had approximately $400 of accrued interest related to uncertain tax positions. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| | | | |
Balance at January 1, 2007 | | $ | 29,200 | |
Additions based on tax positions related to current year | | | 5,200 | |
Settlements | | | (600 | ) |
Lapse of statute of limitations | | | (13,600 | ) |
Balance at December 31, 2007 | | $ | 20,200 | |
We file income tax returns in the United States and multiple foreign jurisdictions. In the normal course of business, we are subject to examination by taxing authorities throughout the world, including such major jurisdictions as France, Ireland, Japan, Sweden, the United Kingdom, and the United States. In 2007, the IRS continued the examination phase of years 2004 and 2005. Although the examinations were not settled at December 31, 2007, we believe an appropriate provision was made for any potential unfavorable financial statement impact. The major taxing jurisdictions in which we operate and the tax years that remain subject to examination are as follows:
| | |
France | | 2005 – 2007 |
Ireland | | 2003 – 2007 |
Japan | | 2007 |
Sweden | | 1998 – 2007 |
United Kingdom | | 2003 – 2007 |
United States | | 2004 – 2007 |
During the year, we recorded $13,600 of previously unrecognized tax benefits as a result of the completion of tax examinations and statute of limitations closures. Of this amount, $11,900 was recorded as a reduction to our 2007 provision for income taxes and the remaining $1,700 was recorded as a reduction to goodwill related to our recent business acquisitions. At December 31, 2007, we had $20,200 of unrecognized tax benefits, of which $14,600 would affect our effective tax rate when they are recognized. Over the next twelve months, we do not reasonably foresee any material changes in our unrecognized tax benefits.
12. STOCK PLANS AND STOCK-BASED COMPENSATION
Stock Incentive Plan
As of January 1, 2006, we had two share-based compensation plans, the “1999 Stock Incentive Plan” (the “1999 Plan”) and the “1999 Stock Option Plan for Non-Employee Directors” (the “Directors’ Plan”). On April 26, 2006, our shareholders approved amendments to the 1999 Plan to permit awards of equity incentive compensation to our non-employee directors under the 1999 Plan and to add to the 1999 Plan the 119 shares of our common stock then remaining available for grant under the Directors’ Plan. Also as of April 26, 2006, the Directors’ Plan was terminated, except that any option grant previously made under the Directors’ Plan remained in effect pursuant to its terms.
The 1999 Plan, as in effect at December 31, 2007, allowed for the issuance of a total of 11,321 shares of common stock, of which 119 represent new shares from the Directors’ Plan. The types of awards permitted under the 1999 Plan include stock options, restricted stock, stock appreciation rights and stock units (including restricted stock units). In the future, we may condition the grant or vesting of awards on the satisfaction of performance conditions. The exercise price of the stock options may not be less than the fair market value of our common stock at the time of grant. Stock options generally vest over a four-year period and expire no later than ten years from the date of grant. Restricted stock awards represent shares of common stock issued to employees subject to forfeiture if the vesting conditions are not satisfied. Restricted stock units
| | |
MILLIPORE FORM 10-K 2007 | | 83 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
represent the right to receive shares of common stock upon meeting specified vesting requirements. The vesting conditions for our restricted stock awards and restricted stock units are determined by the Board of Directors at the time of grant. Restricted stock and restricted stock units, which are awarded at no cost to employees, cannot be sold, assigned, transferred or pledged during the restriction period. The restriction or vesting period ranges from two to four years. In most instances, shares are subject to forfeiture should employment terminate during the restriction period.
A summary of stock option activities with respect to the 1999 Plan and the Directors Plan is as follows:
| | | | | | | | |
| | | Stock Options |
| | | Shares
(in thousands) | | | Weighted Average
Exercise Price | | Weighted
Average
Remaining
Contractual
Life (in
years) |
Outstanding at December 31, 2004 | | 7,055 | | | $ | 44.08 | | |
Granted | | 305 | | | $ | 53.45 | | |
Exercised | | (2,397 | ) | | $ | 44.41 | | |
Canceled or expired | | (303 | ) | | $ | 43.04 | | |
Outstanding at December 31, 2005 | | 4,660 | | | $ | 44.60 | | |
Granted | | 258 | | | $ | 67.20 | | |
Exercised | | (1,296 | ) | | $ | 43.42 | | |
Canceled or expired | | (123 | ) | | $ | 45.90 | | |
Outstanding at December 31, 2006. | | 3,499 | | | $ | 46.66 | | |
Granted | | 299 | | | $ | 74.71 | | |
Exercised | | (1,213 | ) | | $ | 41.12 | | |
Canceled or expired | | (26 | ) | | $ | 48.84 | | |
Outstanding at December 31, 2007 | | 2,559 | | | $ | 52.55 | | 6.18 |
Exercisable at December 31, 2005 | | 3,166 | | | $ | 45.96 | | |
Exercisable at December 31, 2006 | | 2,280 | | | $ | 45.59 | | |
Exercisable at December 31, 2007 | | 1,678 | | | $ | 47.38 | | 5.25 |
The following table summarizes information about stock options at December 31, 2007:
| | | | | | | | | | | | |
| | | Options Outstanding | | Options Exercisable |
| Range of Exercise Price | | Outstanding | | Weighted Average
Remaining
Contractual
Life (in
years) | | Weighted
Average
Exercise
Price | | Exercisable | | Weighted
Average Exercise
Price |
$25.59–$48.72 | | 993 | | 5.5 | | $ | 41.71 | | 776 | | $ | 39.77 |
$49.78–$51.99 | | 561 | | 6.3 | | $ | 51.34 | | 427 | | $ | 51.53 |
$52.22–$66.79 | | 681 | | 5.7 | | $ | 58.84 | | 469 | | $ | 55.82 |
$68.48–$76.76 | | 324 | | 9.1 | | $ | 74.71 | | 6 | | $ | 74.63 |
$25.59–$76.76 | | 2,559 | | 6.2 | | $ | 52.55 | | 1,678 | | $ | 47.38 |
At December 31, 2007, the total aggregate intrinsic value for options currently exercisable and options outstanding was $43,304 and $53,314, respectively. These values represent the total pre-tax intrinsic value based on our closing common stock price of $73.18 as of December 31, 2007. This intrinsic value represents the value that would have been received by the option holders had option holders exercised all of their options as of that date. Intrinsic value for stock options is defined as the difference between the current market value and the grant price. The total intrinsic value of options exercised
| | |
| 84 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
during the years ended December 31, 2007, 2006, and 2005 was $42,290, $32,118, and $32,297, respectively. The total fair value of shares of restricted stock and restricted stock units vested during the years ended December 31, 2007, 2006, and 2005 was $3,865, $166, and $1,450, respectively. The following table summarizes the status of unvested restricted stock awards and restricted stock units.
| | | | | | |
| | | Shares | | | Weighted Average
Grant-date Fair
Value |
Unvested January 1, 2006 | | 15 | | | $ | 55.09 |
Granted | | 243 | | | $ | 66.74 |
Vested | | (3 | ) | | $ | 63.79 |
Forfeited | | (10 | ) | | $ | 66.79 |
Unvested at December 31, 2006 | | 245 | | | $ | 66.08 |
Granted | | 339 | | | $ | 74.17 |
Vested | | (51 | ) | | $ | 66.62 |
Forfeited | | (27 | ) | | $ | 71.14 |
Unvested at December 31, 2007 | | 506 | | | $ | 71.17 |
Non-Employee Director Deferred Compensation Agreements
Through 2001, deferred compensation agreements for non-employee directors allowed for deferral of directors’ fees by converting them to deferred compensation phantom stock units based on 100 percent of the fair market value of our common stock on periodic conversion dates. Upon retirement or earlier termination of service from the Board of Directors, the cash equivalent of the phantom stock units will be distributed in annual installments over ten years. We record a compensation adjustment related to the change in the fair market value of stock at the grant date as compared to the current fair market value of the stock. In June 2002, such conversion to phantom stock units was discontinued, and deferred compensation agreements between us and certain non-employee directors thereafter allowed for a cash deferral of directors’ fees. In connection with these deferred compensation arrangements, we recorded compensation expense of $331, $191 and $889 in 2007, 2006 and 2005, respectively.
Stock-based Compensation Expense
The following table presents stock-based compensation expense included in our consolidated statements of operations for the years ended December 31, 2007 and December 31, 2006:
| | | | | | | | |
| | | Year ended December 31, | |
| | | 2007 | | | 2006 | |
Increase (decrease) in: | | | | | | | | |
Cost of sales | | $ | 2,432 | | | $ | 1,803 | |
Selling, general and administrative expenses | | | 11,798 | | | | 8,860 | |
Research and development expenses | | | 1,730 | | | | 1,632 | |
Income before income taxes and minority interest | | | 15,960 | | | | 12,295 | |
Provision for income taxes | | | (5,025 | ) | | | (3,881 | ) |
Net income | | | 10,935 | | | | 8,414 | |
Earnings per share: | | | | | | | | |
Basic | | $ | 0.20 | | | $ | 0.16 | |
Diluted | | $ | 0.20 | | | $ | 0.16 | |
| | |
MILLIPORE FORM 10-K 2007 | | 85 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
The weighted average grant-date fair value of options granted during the years ended December 31, 2007, 2006 and 2005 was $25.93, $25.00, and $19.90 per option, respectively. The weighted average grant-date fair value of restricted stock and restricted stock units awarded during the years ended December 31, 2007, 2006, and 2005 was $74.17, $66.75, and $61.56 per unit, respectively. The fair value of the fixed option grants was estimated using the Black-Scholes option-pricing model with the following weighted average assumptions for option grants:
| | | | | | | | | |
| | | Year ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
Risk-free interest rate | | 4.7 | % | | 4.7 | % | | 3.8 | % |
Volatility factor | | 29.5 | % | | 33.0 | % | | 35.0 | % |
Weighted average expected life (in years) | | 5 | | | 5 | | | 5 | |
Dividend rate | | 0.0 | % | | 0.0 | % | | 0.0 | % |
We did not capitalize any stock-based compensation related costs as such costs were not material for the year ended December 31, 2007 and 2006. Unrecognized stock-based compensation expense was $33,335 at December 31, 2007 and is expected to be recognized over an estimated weighted average amortization period of 2.0 years. The forfeiture rate used in the share-based compensation expense calculation for the years ended December 31, 2007 and 2006 was 5.8 percent and 4.0 percent, respectively.
Pursuant to requirements in SFAS No. 123(R), we reclassified unearned compensation balance of $290 related to restricted stock awards to additional paid-in capital as of January 1, 2006.
Prior to adoption of SFAS No. 123(R) on January 1, 2006, we applied the recognition and measurement provisions of APB Opinion No. 25 in accounting for stock-based compensation plans and complied with the disclosure requirements under SFAS No. 123. The following table illustrates the effect on net income and earnings per share for the year ended December 31, 2005 as if we had accounted for our stock-based compensation under the fair value method:
| | | | |
| | | December 31,
2005 | |
Net income, as reported | | $ | 80,168 | |
Add: | | | | |
Stock-based employee compensation expense included in reported net income, net of related tax effects | | | 3,634 | |
Deduct: | | | | |
Pro forma stock-based employee compensation expense determined under fair value based method, net of related tax effects | | | (9,937 | ) |
Pro forma net income | | $ | 73,865 | |
Earnings per share: | | | | |
Basic, as reported | | $ | 1.57 | |
Basic, pro forma | | $ | 1.45 | |
Diluted, as reported | | $ | 1.55 | |
Diluted, pro forma | | $ | 1.41 | |
13. EMPLOYEE BENEFIT PLANS
We sponsor numerous domestic and foreign employee benefit plans. The following is a discussion of our significant plans.
U.S. Employee Savings Plans. The Millipore Corporation Employees’ Participation and Savings Plan (the “Participation and Savings Plan”) was maintained for the benefit of all U.S. employees and comprises both a defined contribution plan (the “Participation Plan”) and an employee §401(K) savings plan (the “Savings Plan”). Our contributions to the Participation Plan were allocated among eligible U.S. employees on the basis of the compensation they received during the year
| | |
| 86 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
for which the contribution was made. Prior to January 1, 2007, the Savings Plan allowed employees to make certain tax-deferred voluntary contributions upon hire date, which we would make a 25 percent matching contribution after one year of service or a 50 percent matching contribution after ten years of service for up to 6 percent of the employees’ eligible compensation. In October 2006, our Board of Directors approved amendments to the Participation and Savings Plan, effective January 1, 2007, to discontinue annual employer contributions to eligible employees’ Participation Plan accounts, to allow eligible employees to begin to participate in the Savings Plan without any waiting period, and to increase our 401(k) matching contribution rates, dollar for dollar, up to the first 6 percent of compensation deferred by the employee. Total expense under the Participation and Savings Plan was $9,460, $8,322, and $7,455 in 2007, 2006 and 2005, respectively.
We offer a Supplemental Savings and Retirement Plan for Key Salaried Employees (the “Supplemental Plan”) to certain senior executives. This unfunded plan allows certain salary deferral benefits that would otherwise be lost by reason of restrictions imposed by the Internal Revenue Code limiting the amount of compensation that may be deferred under tax-qualified plans. Amounts deferred are converted into shares of mutual funds selected by the employees and are valued at the closing market prices of those mutual funds. During periods when the market values of the investments increase, our obligations increase and we recognize additional compensation expense. Total expense recorded under the Supplemental Plan was $223, $796 and $405 in 2007, 2006 and 2005, respectively.
The Millipore Corporation 2000 Deferred Compensation Plan for Senior Management (the “Deferred Compensation Plan”) provides that certain members of senior management may elect to defer a portion of their salary and bonus payments until retirement, termination of employment or the passage of a period of time (not less than three years). The amounts deferred are invested in certain publicly traded mutual funds. Plan participants are fully vested in their respective account balances at all times. We recognize compensation expense related to our obligations to pay the employee’s deferred compensation in the year such compensation is earned. In subsequent periods, we recognize increases or decreases to compensation expense based on the performance of the underlying investments in the Deferred Compensation Plan. Total increase in the market value of the underlying investments recognized as expense under the Deferred Compensation Plan was $79, $44 and $42 in 2007, 2006 and 2005, respectively.
U.S. Pension Plans. Our Retirement Plan for Employees of Millipore Corporation (the “Retirement Plan”) is a defined benefit offset pension plan for all eligible U.S. employees. The Retirement Plan provides benefits to the extent that assets of the Participation Plan, described above, do not provide guaranteed retirement income levels set forth under the terms of the Retirement Plan. Guaranteed retirement income levels are determined based on years of service and salary level as integrated with Social Security benefits. Prior to January 1, 2007, employees became eligible under the Retirement Plan after one year of continuous service and vested after five years of service.
In October 2006, our Board of Directors approved certain amendments to the Retirement Plan to freeze the Retirement Plan effective December 31, 2006, after which no benefits will accrue. All participants’ accrued benefits under the Retirement Plan became fully vested as of December 31, 2006. Eligible participants were also provided a one-time final opportunity in early 2007 to transfer balances from their Participation Plan accounts to the Retirement Plan for the purpose of purchasing an annuity under the existing terms of the Retirement Plan. We recorded a curtailment loss of $8,664 in the statement of operations in the 2006 fourth quarter as a result of this amendment.
For purposes of determining the curtailment loss associated with the freeze of the Retirement Plan and the related one-time opportunity to transfer Participation Plan balances that were offered to employees in 2007, we used an assumption that 17.1 percent of available balances in the Participation Plan would be transferred into the Retirement Plan. The 17.1 per-
| | |
MILLIPORE FORM 10-K 2007 | | 87 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
cent assumption was selected based on a review of our actual transfer experience between the Plans from 2002 to 2005. Actual transfer experience was analyzed to determine the percentage by age grouping of available Participation Plan balances that were transferred to the Retirement Plan. These percentages were then applied to projected balances by age grouping as of December 31, 2006 to determine the estimated balances that would be transferred by age grouping. Upon completion of the transfer of Participation Plan balances as of July, 1, 2007, the actual transfers represented 35.1 percent of the final Participation Plan assets balances at that time. The 35.1 percent actual transfer experience had no effect on 2007 expense because the actuarial loss attributable to the actual transfer experience exceeding the 17.1 percent transfer rate assumption will be amortized over the average remaining service period of plan participants beginning in 2008. The balances disclosed at December 31, 2007 for benefit obligations and plan assets reflected the impact of the actual transfers from the Participation Plan.
For accounting purposes, we use the projected unit credit cost method of actuarial valuation to determine the service cost and the projected benefit obligations. The actuarial method for funding purposes was the entry age normal cost method. Our funding policy is to contribute amounts annually to the Retirement Plan to satisfy the minimum funding requirements set forth in the Employee Retirement Income Security Act of 1974 (“ERISA”) plus additional tax deductible amounts as may be advisable under the circumstances. Plan assets are invested primarily in mutual funds that maintain a portfolio of U.S. equity and fixed income securities.
U.S. Postretirement Benefit Plans. We sponsor unfunded postretirement benefit plans covering all U.S. employees. The plans provide medical and life insurance benefits and are, depending on the plan, either contributory or non-contributory. The accounting for the postretirement benefit plans anticipates future cost-sharing changes that are at our discretion. The postretirement benefit plans include a limitation on our share of costs for recent and future retirees.
Foreign Pension Plans.We sponsor defined benefit retirement plans at various foreign subsidiaries. We recognize the periodic pension expense in the statements of operations and the associated liabilities in the balance sheets of these foreign subsidiaries.
The following tables summarize the funded status of the Retirement Plan, postretirement benefit plans, and significant foreign employee retirement plans and amounts reflected in our consolidated balance sheets. We use a December 31 measurement date for all of our retirement and postretirement benefit plans, except for one foreign plan that uses September 30.
| | |
| 88 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
Obligations and Funded Status
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | U.S. Pension Benefits | | | U. S.
Postretirement Benefits | | | Foreign Pension Benefits | |
| | | December 31, | | | December 31, | | | December 31, | |
| | | 2007 | | | 2006 | | | 2007 | | | 2006 | | | 2007 | | | 2006 | |
| Change in benefit obligations: | | | | | | | | | | | | | | | | | | | | | | | | |
Benefit obligations at beginning of year | | $ | 37,326 | | | $ | 22,676 | | | $ | 10,305 | | | $ | 10,432 | | | $ | 39,502 | | | $ | 33,444 | |
Service cost/(benefit) | | | 50 | | | | (348 | ) | | | 605 | | | | 425 | | | | 2,804 | | | | 2,480 | |
Interest cost | | | 2,336 | | | | 1,420 | | | | 602 | | | | 548 | | | | 1,699 | | | | 1,361 | |
Actuarial value of transfers from Participation Plan / Participants’ contributions | | | 28,055 | | | | 6,340 | | | | 277 | | | | 225 | | | | – | | | | – | |
Foreign exchange effect | | | – | | | | – | | | | – | | | | – | | | | 2,865 | | | | 3,285 | |
Actuarial loss /(gain) | | | 54 | | | | 378 | | | | 523 | | | | (554 | ) | | | (4,500 | ) | | | (439 | ) |
Benefits paid | | | (2,155 | ) | | | (1,804 | ) | | | (811 | ) | | | (771 | ) | | | (1,121 | ) | | | (629 | ) |
Plan changes | | | – | | | | – | | | | (2,848 | ) | | | – | | | | – | | | | – | |
Settlements | | | – | | | | – | | | | – | | | | – | | | | (478 | ) | | | – | |
Curtailments | | | – | | | | 8,664 | | | | – | | | | – | | | | (44 | ) | | | – | |
Benefit obligations at end of year | | | 65,666 | | | | 37,326 | | | | 8,653 | | | | 10,305 | | | | 40,727 | | | | 39,502 | |
| Change in plan assets: | | | | | | | | | | | | | | | | | | | | | | | | |
Fair value of plan assets at beginning of year | | | 19,373 | | | | 15,189 | | | | – | | | | – | | | | 21,209 | | | | 16,622 | |
Actual return on plan assets | | | 1,193 | | | | 1,252 | | | | – | | | | – | | | | 1,411 | | | | 1,531 | |
Company contributions | | | 1,442 | | | | 1,037 | | | | 534 | | | | 546 | | | | 2,472 | | | | 1,650 | |
Transfers from Participation Plan and Participants’ contributions | | | 26,436 | | | | 3,699 | | | | 277 | | | | 225 | | | | – | | | | – | |
Foreign exchange effect | | | – | | | | – | | | | – | | | | – | | | | 1,164 | | | | 2,035 | |
Benefits paid | | | (2,155 | ) | | | (1,804 | ) | | | (811 | ) | | | (771 | ) | | | (1,121 | ) | | | (629 | ) |
Settlements | | | – | | | | – | | | | – | | | | – | | | | (478 | ) | | | – | |
Fair value of plan assets at end of year | | | 46,289 | | | | 19,373 | | | | – | | | | – | | | | 24,657 | | | | 21,209 | |
Funded status at end of year | | $ | (19,377 | ) | | $ | (17,953 | ) | | $ | (8,653 | ) | | $ | (10,305 | ) | | $ | (16,070 | ) | | $ | (18,293 | ) |
Amounts recognized in the statement of financial position consist of:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | U.S. Pension Benefits | | | U.S.
Postretirement Benefits | | | Foreign Pension Benefits | |
| | | December 31, | | | December 31, | | | December 31, | |
| | | 2007 | | | 2006 | | | 2007 | | | 2006 | | | 2007 | | | 2006 | |
Non-current asset | | $ | – | | | $ | – | | | $ | – | | | $ | – | | | $ | 2,618 | | | $ | 703 | |
Current liabilities | | | – | | | | – | | | | (548 | ) | | | – | | | | (177 | ) | | | – | |
Non-current liability | | | (19,377 | ) | | | (17,953 | ) | | | (8,105 | ) | | | (10,305 | ) | | | (18,511 | ) | | | (18,996 | ) |
Net | | $ | (19,377 | ) | | $ | (17,953 | ) | | $ | (8,653 | ) | | $ | (10,305 | ) | | $ | (16,070 | ) | | $ | (18,293 | ) |
Amounts recognized in accumulated other comprehensive income consist of:
| | | | | | | | | | | | | | | | | | | | |
| | | U.S. Pension Benefits | | U.S.
Postretirement Benefits | | | Foreign Pension Benefits |
| | | December 31, | | December 31, | | | December 31, |
| | | 2007 | | 2006 | | 2007 | | | 2006 | | | 2007 | | 2006 |
Net loss/(gain) | | $ | 16,348 | | $ | 14,539 | | $ | (2,223 | ) | | $ | (2,823 | ) | | $ | 1,315 | | $ | 5,666 |
Prior service benefit | | | – | | | – | | | (2,848 | ) | | | – | | | | – | | | – |
Total | | $ | 16,348 | | $ | 14,539 | | $ | (5,071 | ) | | $ | (2,823 | ) | | $ | 1,315 | | $ | 5,666 |
| | |
MILLIPORE FORM 10-K 2007 | | 89 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
The Medicare Prescription Drug, Improvement and Modernization Act of 2003 (the “Act”) introduced a prescription drug benefit under Medicare as well as a federal subsidy to sponsors of retiree healthcare benefit plans that provide a benefit that is at least actuarially equivalent to Medicare Part D. In May 2004, the FASB issued FASB Staff Position No. 106-2, “Accounting and Disclosure Requirements Related to the Medicare Prescription Drug, Improvement and Modernization Act of 2003” (“FSP No. 106-2”). As permitted under FSP No. 106-2, we elected to defer the accounting for the Act until the issuance of authoritative guidance on the determination of actuarial equivalence for purposes of receiving the federal subsidy. On January 21, 2005, the Center for Medicare and Medicaid Services released the final regulations implementing the Act. Based on these final regulations, we determined that most benefits provided by the postretirement benefit plan were at least actuarially equivalent to Medicare Part D. In 2007, changes to the postretirement benefit plan resulted in it no longer providing prescription drug coverage that is at least actuarially equivalent to the Medicare Part D drug benefit. In accordance with FSP No. 106-2, the combined net effect on the accumulated benefit obligation (“ABO”) of the postretirement benefit plan change was accounted for as a prior service benefit.
The projected benefit obligations are equal to the accumulated benefit obligations under the Retirement Plan because the plan was frozen effective December 31, 2006 and no additional benefits will accrue. As of December 31, 2007 and 2006, the projected and accumulated obligations of the Retirement Plan were $65,666 and $37,326, respectively, and the fair value of plan assets were $46,289 and $19,373, respectively.
The accumulated benefit obligations for foreign retirement plans were $31,416 and $30,858 at December 31, 2007 and 2006, respectively. Information for certain foreign retirement plans with an accumulated benefit obligation in excess of plan assets is as follows:
| | | | | | |
| | | Foreign Pension Benefits |
| | | December 31, |
| | | 2007 | | 2006 |
Projected benefit obligations | | $ | 22,168 | | $ | 20,339 |
Accumulated benefit obligations | | $ | 16,677 | | $ | 15,654 |
Fair value of plan assets | | $ | 3,776 | | $ | 3,339 |
| | |
| 90 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
Components of Net Periodic Benefit Cost and Other Amounts Recognized in Other Comprehensive Income:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | U.S. Pension Benefits | | | U.S. Postretirement Benefits | | | Foreign Pension Benefits | |
| | | Year ended December 31, | | | Year ended December 31, | | | Year ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | | | 2007 | | | 2006 | | | 2005 | | | 2007 | | | 2006 | | | 2005 | |
| Net Periodic Benefit Cost: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Service cost/(benefit) | | $ | 50 | | | $ | (348 | ) | | $ | (272 | ) | | $ | 604 | | | $ | 425 | | | $ | 410 | | | $ | 2,805 | | | $ | 2,480 | | | $ | 2,156 | |
Interest cost | | | 2,336 | | | | 1,420 | | | | 1,121 | | | | 602 | | | | 548 | | | | 541 | | | | 1,699 | | | | 1,361 | | | | 1,241 | |
Expected return on plan assets | | | (2,017 | ) | | | (1,258 | ) | | | (1,078 | ) | | | – | | | | – | | | | – | | | | (1,435 | ) | | | (1,145 | ) | | | (918 | ) |
Amortization of net loss/(gain) | | | 689 | | | | 902 | | | | 677 | | | | (77 | ) | | | (92 | ) | | | (107 | ) | | | 153 | | | | 169 | | | | 129 | |
Other | | | – | | | | 7 | | | | 8 | | | | – | | | | – | | | | – | | | | 36 | | | | – | | | | 47 | |
Net periodic benefit cost | | | 1,058 | | | | 723 | | | | 456 | | | | 1,129 | | | | 881 | | | | 844 | | | | 3,258 | | | | 2,865 | | | | 2,655 | |
| Other Changes in Plan Assets and Benefit Obligations Recognized in Other Comprehensive Income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss/(gain) | | | 2,498 | | | | 3,027 | | | | N/A | | | | 523 | | | | (554 | ) | | | N/A | | | | (4,521 | ) | | | 5,666 | | | | N/A | |
Prior service cost | | | – | | | | (7 | ) | | | N/A | | | | (2,848 | ) | | | – | | | | N/A | | | | – | | | | – | | | | N/A | |
Amortization of net (loss)/gain | | | (689 | ) | | | (902 | ) | | | N/A | | | | 77 | | | | 92 | | | | N/A | | | | (153 | ) | | | – | | | | N/A | |
Other | | | – | | | | – | | | | N/A | | | | – | | | | – | | | | N/A | | | | 323 | | | | – | | | | N/A | |
Total recognized in other comprehen- sive income | | | 1,809 | | | | 2,118 | | | | N/A | | | | (2,248 | ) | | | (462 | ) | | | N/A | | | | (4,351 | ) | | | 5,666 | | | | N/A | |
Total recognized in net periodic benefit cost and other comprehensive income | | $ | 2,867 | | | $ | 2,841 | | | | N/A | | | $ | (1,119 | ) | | $ | 419 | | | | N/A | | | $ | (1,093 | ) | | $ | 8,531 | | | | N/A | |
The estimated net loss for the Retirement Plan that will be amortized from accumulated other comprehensive income into net periodic benefit cost over 2008 is $783. The estimated net gain for the other defined benefit postretirement plans and prior service benefit that will be amortized from accumulated other comprehensive income into net periodic benefit cost over 2008 is $82 and $182, respectively. The estimated net loss for the foreign defined benefit pension plans that will be amortized from accumulated other comprehensive income into net periodic benefit cost over 2008 is $10.
| | |
MILLIPORE FORM 10-K 2007 | | 91 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
Assumptions
Weighted-average assumptions used to determine benefit obligations are as follows:
| | | | | | | | | | | | | | | | |
| | | U.S.
Pension Benefits | | | U.S.
Postretirement Benefits | | Foreign Pension
Benefits | |
| | | December 31, | | | December 31, | | December 31, | |
| | | 2007 | | | 2006 | | | 2007 | | 2006 | | 2007 | | | 2006 | |
Discount rate | | 6.50 | % | | 5.75 | % | | 6.25% | | 5.75% | | 4.74 | % | | 4.16 | % |
Rate of compensation increase | | N/A | | | 4.00 | % | | N/A | | N/A | | 3.00 | % | | 3.01 | % |
Weighted-average assumptions used to determine net periodic benefit cost are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | U.S. Pension Benefits | | | U.S. Postretirement Benefits | | | Foreign Pension Benefits | |
| | | Year ended December 31, | | | Year ended December 31, | | | Year ended December 31, | |
| | | 2007 | | | 2006 | | 2005 | | | 2007 | | | 2006 | | | 2005 | | | 2007 | | | 2006 | | | 2005 | |
Discount rate | | 5.75 | % | | 5.50% / 5.75% | | 5.75 | % | | 5.75 | % | | 5.50 | % | | 5.75 | % | | 4.16 | % | | 3.86 | % | | 4.15 | % |
Expected return on plan assets | | 8.00 | % | | 8.00% | | 8.00 | % | | N/A | | | N/A | | | N/A | | | 6.41 | % | | 6.29 | % | | 6.17 | % |
Rate of compensation increase | | N/A | | | 4.00% | | 4.00 | % | | N/A | | | N/A | | | N/A | | | 3.01 | % | | 2.91 | % | | 2.92 | % |
Net periodic benefit cost for U.S. pension benefits for 2006 was calculated utilizing a discount rate of 5.50 percent for 10 months and 5.75 percent for 2 months because the amendments to the Retirement Plan in October 2006 triggered a new measurement date under SFAS No. 87.
In selecting the expected return on plan assets, we considered the average rate of earnings expected on the funds invested or to be invested to provide for the benefits when they become due. This included considering the asset allocations and the expected returns likely to be earned on these assets over the life of these plans. Our method is consistent with last year.
The discount rates reflect the rates at which amounts that are invested in a portfolio of high-quality debt instruments would provide the future cash flows necessary to pay benefits when they come due.
The rate of compensation increase reflected the expected annual salary increase for the plan participants. Since the Retirement Plan was frozen at December 31, 2006, there will be no further compensation increases. The rate for our foreign retirement plans was estimated based on historical experience and our current employee compensation strategy.
Plan assets
The weighted average asset allocations by asset category of our Retirement Plan and foreign retirement plans were as follows:
| | | | | | | | | | | | |
| | | U.S.
Pension Benefits | | | Foreign
Pension Benefits | |
| | | December 31, | | | December 31, | |
| | | 2007 | | | 2006 | | | 2007 | | | 2006 | |
Equity securities | | 60 | % | | 59 | % | | 71 | % | | 71 | % |
Debt securities | | 40 | % | | 40 | % | | 9 | % | | 7 | % |
Other | | – | | | 1 | % | | 20 | % | | 22 | % |
Total | | 100 | % | | 100 | % | | 100 | % | | 100 | % |
| | |
| 92 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
Our investment policy includes a periodic review of the Retirement Plan’s and foreign retirement plans’ investments in the various asset classes. The current asset allocation target for our Retirement Plan is 60 percent equities and 40 percent fixed income. The current weighted average asset allocation target for our foreign retirement plans’ is 71 percent equities, 12 percent fixed income securities, and 17 percent other investments. Other investments include investments in money market mutual funds and general funds at certain insurance companies.
Assumed healthcare cost trend rates
The following assumptions were used to determine the accumulated postretirement benefit obligations under our postretirement benefit plans at December 31, 2007 and 2006, respectively.
| | | | | | |
| | | U.S. Postretirement Benefits | |
| | | 2007 | | | 2006 | |
Healthcare cost trend rate assumed for next year | | 8.00 | % | | 9.00 | % |
Rate to which the cost trend rate is assumed to decline (the ultimate trend rate) | | 5.00 | % | | 5.00 | % |
Year that the rate reaches the ultimate trend rate | | 2013 | | | 2012 | |
Assumed healthcare cost trend rates could have a significant effect on the amounts reported for the postretirement plans. A one-percentage point change in assumed healthcare cost trend rates would have the following effects:
| | | | | | | |
| | | 1% Point
Increase | | 1% Point
Decrease | |
Increase/(decrease) to total of service and interest cost components | | $ | 48 | | $ | (40 | ) |
Increase/(decrease) to postretirement benefit obligations | | $ | 397 | | $ | (342 | ) |
Cash flows
In 2008, we expect to contribute $9,834 to our Retirement Plan, $548 to our postretirement benefit plans, and $1,495 to our foreign retirement plans.
Estimated future benefit payments
The following benefit payments, which reflect expected future service, as appropriate, are expected to be paid:
| | | | | | | | | |
| | | U.S. Pension
Benefits | | U.S.
Postretirement
Benefits | | Foreign Pension
Benefits |
2008 | | $ | 2,432 | | $ | 548 | | $ | 960 |
2009 | | $ | 2,733 | | $ | 571 | | $ | 1,137 |
2010 | | $ | 3,015 | | $ | 624 | | $ | 800 |
2011 | | $ | 3,288 | | $ | 641 | | $ | 958 |
2012 | | $ | 3,743 | | $ | 663 | | $ | 1,717 |
2013 – 2017 | | $ | 22,287 | | $ | 3,393 | | $ | 11,651 |
| | |
MILLIPORE FORM 10-K 2007 | | 93 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
14. DERIVATIVE INSTRUMENTS AND HEDGING
The primary purpose of our foreign currency hedging activities is to mitigate the impact of volatility associated with foreign currency transactions. We do not hold derivative instruments for trading or speculative purposes.
Cash Flow Hedges
We began entering into foreign currency forward exchange contracts designated as cash flow hedges in the 2007 second quarter to mitigate the currency risk associated with forecasted intercompany sales attributable to changes in the related foreign exchange rates. We entered into forward exchange contracts that match the currency, timing, and notional amount of the underlying forecasted transactions. Therefore, no ineffectiveness resulted or was recorded through the consolidated statement of operations. In the event it becomes probable that the forecasted transaction to which a cash flow hedge relates will not occur, the amount in other comprehensive income would be recognized in earnings immediately and generally the derivative would be terminated. At December 31, 2007, these forward exchange contracts had an aggregate U.S. dollar equivalent notional amount of $55,152 and an aggregate U.S. dollar equivalent fair value of a net loss of $594, which was recorded as part of other comprehensive income. Our forward exchange contracts are primarily short term in nature with the maximum hedge period not exceeding fifteen months. The net gain or loss from these cash flow hedging contracts reported in accumulated other comprehensive income will be reclassified to earnings when the underlying transactions affect earnings. The ultimate amount recognized will vary based on fluctuations of the hedged currencies through the contract maturity dates. Gains and losses on forward exchange contracts intended as hedges of intercompany sales are recorded in net sales in our consolidated statement of operations when the related inventory is sold to third-party customers. For the year ended December 31, 2007, we recorded net realized losses of $599 from these hedging contracts, of which $477 has been recognized in our consolidated statement of operations as a reduction to net sales.
Net Investment Hedge
We designated our 5.875 percent senior notes, which are denominated in Euro, as an economic hedge of our net investments in our European subsidiaries. Accordingly, we reported cumulative unrealized losses of $50,918 and $16,253 in accumulated other comprehensive income in our consolidated balance sheets as of December 31, 2007 and December 31, 2006, respectively, which represented the amounts to remeasure the Euro notes into U.S. dollars at those dates.
Other Derivatives
In addition to cash flow hedges and the net investment hedge, we also enter into forward exchange contracts to mitigate the impact of foreign exchange risk related to certain foreign currency denominated intercompany debt, external debt and non-functional currency denominated receivable and payable balances. We do not designate these forward exchange contracts as hedges under SFAS No. 133. The aggregate U.S. dollar equivalent notional amount of the forward exchange contracts related to foreign currency receivable and payable balances was $243,126 and $286,626 at December 31, 2007 and December 31, 2006, respectively. The fair values of these forward exchange contracts were losses of $372 and gains of $1,125 at December 31, 2007 and December 31, 2006, respectively. The forward exchange contracts related to the intercompany and external debt outstanding at December 31, 2007 mature in April 2008. At December 31, 2007, these contracts had U.S. dollar aggregate notional amount of $291,943 and an aggregate U.S. dollar equivalent fair value of a net unrealized loss of $5,709. During 2007, we recorded net realized and unrealized derivative losses of $17,926 and $5,489, respectively. These losses were substantially offset by gains on the underlying transactions, which resulted in a net gain of $944 for the year ended December 31, 2007. Both realized and unrealized gains and losses on these forward exchange contracts are recorded in selling, general, and administrative expenses.
| | |
| 94 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
15. COMMITMENTS AND CONTINGENCIES
Leases. We occupy space and use certain equipment under lease arrangements. At December 31, 2007, future minimum rental payments under non-cancelable operating leases with initial terms exceeding one year and the amounts due from tenants on related subleases were as follows:
| | | |
2008 | | $ | 21,611 |
2009 | | | 20,359 |
2010 | | | 19,280 |
2011 | | | 15,532 |
2012 | | | 9,227 |
Thereafter | | | 24,858 |
Total minimum future rental payments | | | 110,867 |
Less: amounts due from subleases | | | 6,397 |
Total minimum future rental payments less sublease income | | $ | 104,470 |
Rental expense under these lease arrangements in 2007, 2006 and 2005 was $ 28,283, $23,794 and $18,356 respectively.
Environmental. Our operations are subject to environmental regulation by federal, state, and local authorities in the United States and regulatory authorities with jurisdiction over our foreign operations. We have accrued for the costs of environmental remediation activities and periodically reassess these amounts. We believe that the likelihood of incurring losses materially in excess of amounts accrued is remote.
Other. We have purchase commitments totaling $119,213 at December 31, 2007.
We currently are not a party to any material legal proceeding and have no knowledge of any material legal proceeding contemplated by any governmental authority or third party. We are subject to a number of claims and legal proceedings which, in the opinion of our management, are incidental to our normal business operations. In our opinion, although final settlement of these suits and claims may impact our financial statements in a particular period, they will not, in the aggregate, have a material adverse effect on our financial position, cash flows or results of operations.
Following our decision to consolidate the results of our 40 percent owned Indian Joint-Venture (the “India JV”) in January 2006, we learned as a result of our internal controls procedures that certain payment and commission practices at the India JV raise issues of compliance with the U.S. Foreign Corrupt Practices Act. Promptly upon learning of this, our Audit and Finance Committee engaged outside counsel and commenced an investigation. We have implemented certain corrective actions. We have notified the Securities and Exchange Commission and the Department of Justice of this matter. The operations and financial results of the India JV are not currently, and have not to date been, material to us.
As permitted under Massachusetts law and required by our corporate by-laws, we indemnify our officers and directors for certain events or occurrences while the director or officer is or was serving in such capacity. The maximum potential amount of future payments that could be required under these indemnification obligations is unlimited; however, we have a Directors and Officers liability insurance policy that enables us to recover a portion of any future amounts paid. As there were no known or pending claims, we have not accrued a liability for these agreements as of December 31, 2007.
In the ordinary course of business, we warrant to customers that our products will conform to published or agreed specifications. Generally, the applicable product warranty period is one year from the date of delivery of the product to the customer or of site acceptance, if required. Additionally, we typically provide limited warranties with respect to our services. From time to time, we also make other warranties to customers, including warranties that our products are manufactured in accordance with applicable laws and not in violation of third party rights. We provide for estimated warranty costs at the
| | |
MILLIPORE FORM 10-K 2007 | | 95 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
time of the product sale. We believe our warranty accrual as of December 31, 2007 appropriately reflected the estimated cost of such warranty obligations.
In the ordinary course of business, we agree from time to time to indemnify certain customers against certain third party claims for property damage, bodily injury, personal injury or intellectual property infringement arising from the operation or use of our products. Also, from time to time in agreements with suppliers, licensors and other business partners, we agree to indemnify these partners against certain liabilities arising out of the sale or use of our products. The maximum potential amount of future payments we could be required to make under these indemnification obligations is unlimited; however, we have general and umbrella insurance policies that enable us to recover a portion of any amounts paid. Based on our experience with such indemnification claims, we believe the estimated fair value of these obligations is minimal. Accordingly, we have no liabilities recorded for these agreements as of December 31, 2007.
As part of past acquisitions and divestitures of businesses or assets, we have provided a variety of warranties and indemnifications to the sellers and purchasers that are typical for such transactions. Typically certain of the warranties and the indemnifications expire after a defined period of time following the transaction, but certain warranties and indemnifications may survive indefinitely. As of December 31, 2007, no material claims under these warranties or indemnifications are outstanding, and we do not know of any such claims being contemplated.
16. BUSINESS SEGMENT AND GEOGRAPHIC INFORMATION
SFAS No. 131,“Disclosures about Segments of an Enterprise and Related Information,” establishes standards for reporting information about operating segments in annual financial statements and requires selected information for those segments to be presented in interim financial reports. It also establishes standards for related disclosures about products and services, geographic areas and major customers. We have evaluated our business activities that are regularly reviewed by the chief operating decision-maker for which separate discrete financial information is available. As a result of this evaluation, we have determined that we have two operating segments as of December 31, 2007, Bioprocess and Bioscience, which are aggregated into one reporting segment.
The Bioprocess operating segment develops, manufactures and sells consumable products and hardware and provides related services used principally in the development and manufacturing of therapeutic products. The Bioscience operating segment manufactures and sells instrumentation, consumable products and services used in drug discovery and other laboratory applications. For both operating segments, economic characteristics, production processes, products and services, types and classes of customers, methods of distribution and regulatory environments are similar.
| | |
| 96 | | MILLIPORE FORM 10-K 2007 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(In thousands, except per share data)
We attribute net sales to different geographic areas on the basis of the location of the customer. Net sales and long-lived assets (property, plant and equipment and other non-current assets) information by geographic area is as follows:
| | | | | | | | | |
| | | Year ended December 31, |
| | | 2007 | | 2006 | | 2005 |
| Net Sales | | | | | | | | | |
United States | | $ | 560,257 | | $ | 488,240 | | $ | 353,136 |
Other Americas | | | 87,372 | | | 76,523 | | | 66,530 |
Americas | | | 647,629 | | | 564,763 | | | 419,666 |
Europe | | | 623,032 | | | 491,006 | | | 399,592 |
Japan | | | 133,017 | | | 117,623 | | | 119,990 |
Other Asia/Pacific | | | 127,877 | | | 81,979 | | | 51,783 |
Asia/Pacific | | | 260,894 | | | 199,602 | | | 171,773 |
Total | | $ | 1,531,555 | | $ | 1,255,371 | | $ | 991,031 |
| | | | | | |
| | | December 31, |
| | | 2007 | | 2006 |
| Long-Lived Assets | | | | | | |
United States | | $ | 298,816 | | $ | 279,871 |
Other Americas | | | 7,990 | | | 14,088 |
Americas | | | 306,806 | | | 293,959 |
France | | | 85,076 | | | 83,170 |
Ireland | | | 161,029 | | | 115,779 |
Other Europe | | | 28,444 | | | 26,056 |
Europe | | | 274,549 | | | 225,005 |
Asia/Pacific | | | 7,806 | | | 6,939 |
Total | | $ | 589,161 | | $ | 525,903 |
Long-lived assets are net fixed assets attributed to the specific geographic regions.
17. INVESTMENTS IN AFFILIATED COMPANIES
We have an equity investment in a South African company that is accounted for using the equity method. During 2007, 2006, and 2005, we recorded $724, $548, and $659 of income, respectively. During 2007, 2006, and 2005 we received dividends totaling $448, $523, and $0, respectively.
In addition, we have an equity investment in an Indian company that is engaged in the manufacture and sale of certain types of filtration systems and laboratory water purification systems. This investment was previously accounted for using the equity method. In 2006, we identified this entity as a variable interest entity under the FASB Interpretation No. 46(R), “Consolidation of Variable Interest Entities.” Since we are deemed the primary beneficiary of this joint venture, the financial results of this Indian entity have been consolidated in our consolidated financial statements beginning January 1, 2006. The entity had total net assets of $10,405 at December 31, 2007.
| | |
MILLIPORE FORM 10-K 2007 | | 97 |
Quarterly Results (Unaudited)
Our quarterly unaudited results are summarized below:
| | | | | | | | | | | | | | | | | | | | |
| (In thousands, except per share data) | | First
Quarter | | | Second
Quarter | | | Third
Quarter | | | Fourth
Quarter | | | Full Year | |
| 2007 | | | | | | | | | | | | | | | | | | | | |
Net sales | | $ | 371,992 | | | $ | 383,175 | | | $ | 371,174 | | | $ | 405,214 | | | $ | 1,531,555 | |
Cost of sales | | | 182,129 | | | | 182,782 | | | | 169,128 | | | | 187,053 | | | | 721,092 | |
Gross profit | | | 189,863 | | | | 200,393 | | | | 202,046 | | | | 218,161 | | | | 810,463 | |
Selling, general and administrative expenses | | | 122,844 | | | | 123,060 | | | | 118,143 | | | | 122,690 | | | | 486,737 | |
Research and development expenses | | | 27,464 | | | | 25,993 | | | | 26,492 | | | | 27,050 | | | | 106,999 | |
Operating income | | | 39,555 | | | | 51,340 | | | | 57,411 | | | | 68,421 | | | | 216,727 | |
Interest income | | | 471 | | | | 299 | | | | 382 | | | | 301 | | | | 1,453 | |
Interest expense | | | (16,748 | ) | | | (16,325 | ) | | | (16,542 | ) | | | (16,142 | ) | | | (65,757 | ) |
Income before income taxes | | | 23,278 | | | | 35,314 | | | | 41,251 | | | | 52,580 | | | | 152,423 | |
(Benefit from) provision for income taxes | | | (4,350 | ) | | | 5,869 | | | | 4,130 | | | | 6,775 | | | | 12,424 | |
Minority interest | | | 969 | | | | 1,032 | | | | 859 | | | | 667 | | | | 3,527 | |
Net income | | $ | 26,659 | | | $ | 28,413 | | | $ | 36,262 | | | $ | 45,138 | | | $ | 136,472 | |
Earnings per share: | | | | | | | | | | | | | | | | | | | | |
Basic | | $ | 0.50 | | | $ | 0.52 | | | $ | 0.67 | | | $ | 0.83 | | | $ | 2.52 | |
Diluted | | $ | 0.49 | | | $ | 0.52 | | | $ | 0.66 | | | $ | 0.81 | | | $ | 2.48 | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | | |
Basic | | | 53,751 | | | | 54,147 | | | | 54,472 | | | | 54,669 | | | | 54,263 | |
Diluted | | | 54,734 | | | | 54,910 | | | | 55,184 | | | | 55,399 | | | | 55,028 | |
| | | | | |
| 2006 | | | | | | | | | | | | | | | | | | | | |
Net sales | | $ | 268,415 | | | $ | 273,775 | | | $ | 330,117 | | | $ | 383,064 | | | $ | 1,255,371 | |
Cost of sales | | | 125,772 | | | | 130,249 | | | | 169,261 | | | | 200,326 | | | | 625,608 | |
Gross profit | | | 142,643 | | | | 143,526 | | | | 160,856 | | | | 182,738 | | | | 629,763 | |
Selling, general and administrative expenses | | | 82,286 | | | | 87,538 | | | | 106,785 | | | | 122,233 | | | | 398,842 | |
Research and development expenses | | | 18,413 | | | | 19,717 | | | | 24,637 | | | | 23,850 | | | | 86,617 | |
Operating income | | | 41,944 | | | | 36,271 | | | | 29,434 | | | | 36,655 | | | | 144,304 | |
Interest income | | | 6,892 | | | | 9,268 | | | | 4,713 | | | | 542 | | | | 21,415 | |
Interest expense | | | (4,193 | ) | | | (7,992 | ) | | | (16,548 | ) | | | (16,603 | ) | | | (45,336 | ) |
Income before income taxes | | | 44,643 | | | | 37,547 | | | | 17,599 | | | | 20,594 | | | | 120,383 | |
Provision for income taxes | | | 10,015 | | | | 7,986 | | | | 2,347 | | | | 1,114 | | | | 21,462 | |
Minority interest | | | 97 | | | | 424 | | | | 439 | | | | 977 | | | | 1,937 | |
Net income | | $ | 34,531 | | | $ | 29,137 | | | $ | 14,813 | | | $ | 18,503 | | | $ | 96,984 | |
Earnings per share: | | | | | | | | | | | | | | | | | | | | |
Basic | | $ | 0.66 | | | $ | 0.55 | | | $ | 0.28 | | | $ | 0.35 | | | $ | 1.82 | |
Diluted | | $ | 0.64 | | | $ | 0.54 | | | $ | 0.27 | | | $ | 0.34 | | | $ | 1.79 | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | | | | | |
Basic | | | 52,713 | | | | 53,183 | | | | 53,286 | | | | 53,452 | | | | 53,160 | |
Diluted | | | 53,883 | | | | 54,207 | | | | 54,172 | | | | 54,468 | | | | 54,245 | |
For the year ended December 31, 2006, each of the quarters’ basic earnings per share do not sum to the full year basic earnings per share because of rounding.
| | |
| 98 | | MILLIPORE FORM 10-K 2007 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
This item is not applicable.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
An evaluation was carried out under the supervision and with the participation of our management, including our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (the “Exchange Act”)) as of the end of the fiscal year covered by this report. Based upon that evaluation, our CEO and CFO have concluded that our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported in accordance with and within the time periods specified in Securities and Exchange Commission rules and forms.
Management’s Annual Report on Internal Control over Financial Reporting
Management’s annual report on internal control over financial reporting can be found on page 58 of this Form 10-K. The Independent Registered Public Accounting Firm’s report on the effectiveness of our internal control over financial reporting can be found on page 59 of this Form 10-K.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting identified during the three months ended December 31, 2007 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION.
This item is not applicable.
| | |
MILLIPORE FORM 10-K 2007 | | 99 |
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
The information required by this item with respect to our directors is incorporated by reference to our definitive Proxy Statement for Millipore’s Annual Meeting of Stockholders scheduled to be held on May 8, 2008 (the “Proxy Statement”) under the caption “MANAGEMENT AND ELECTION OF DIRECTORS”. The Proxy Statement will be filed with the Securities and Exchange Commission not later than April 29, 2008.
The information required by this item with respect to compliance with Section 16(a) of the Securities Exchange Act of 1934, and our Audit and Finance Committee and our Audit Committee Financial Expert(s) is incorporated by reference to the Proxy Statement under the captions “OWNERSHIP OF MILLIPORE COMMON STOCK – Section 16(a) Beneficial Ownership Reporting Compliance”, and “Committees, Meetings and Compensation of Directors; Shareholder Communications with Directors – Audit and Finance Committee”, respectively.
Information required by this item with respect to our executive officers is set forth in Part I of this Form 10-K report under the heading “Supplementary Item. Executive Officers of the Registrant (pursuant to Instruction 3 to Item 401(b) of Regulation S-K)”.
We have adopted a code of ethics that applies to our principal executive officer, our principal financial officer, and our principal accounting officer, as well as to our other employees. This code of ethics consists of our Corporate Compliance Policy, our Employee Code of Conduct and our Rules of Conduct. We have made this code of ethics available on our website, as described under “Other Information” in Item 1 of this Form 10-K report. We also intend to provide disclosure on our website regarding any amendments to our code of ethics, or waivers from our code of ethics as relate to our principal executive officer, principal financial officer or principal accounting officer, or persons performing similar functions, within four days following any such amendments or waivers.
ITEM 11. EXECUTIVE COMPENSATION.
The information required by this item with respect to executive compensation, compensation committee interlocks and compensation committee report (as furnished information and not filed information) is incorporated by reference to the Proxy Statement under the caption “Executive Compensation” and “Compensation Discussion and Analysis of Executive Compensation”, “Committees, Meetings and Compensation of Directors; Shareholder Communications with Directors – Management Development and Compensation Committee – Compensation Committee Interlocks and Insider Participation” and “Report of the Management Development and Compensation Committee”, respectively.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERSANDMANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The information required by this item with respect to Securities Authorized for Issuance under Equity Compensation Plans is incorporated by reference to the Proxy Statement under the caption “Equity Compensation Plan Benefit Information”.
The information required by this item with respect to security ownership of certain beneficial owners and management of the Company is incorporated by reference to the Proxy Statement under the captions “Ownership of Millipore Common Stock – Other Principal Holders of Millipore Common Stock” and “Ownership of Millipore Common Stock – Management Ownership of Millipore Common Stock”.
| | |
| 100 | | MILLIPORE FORM 10-K 2007 |
PART III
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
The information required by this item with respect to certain relationships and related transactions is incorporated by reference to the Proxy Statement under the caption “Certain Relationships and Related Transactions”. The information required by this item with respect to director independence is incorporated by reference to the Proxy Statement under the caption “Corporate Governance – Committees, Meetings and Compensation of Directors; Shareholder Communications with Directors”.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
The information required by this item is incorporated by reference to the Proxy Statement under the caption “Report of the Audit and Finance Committee”.
| | |
MILLIPORE FORM 10-K 2007 | | 101 |
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
The following documents are filed or furnished, or incorporated by reference, as a part of this Report:
1. FINANCIAL STATEMENTS.
The following Financial Statements are filed as part of this report
2. FINANCIAL STATEMENT SCHEDULES.
No financial statement schedules have been included because they are not applicable or not required under Regulation S-X, or the required information is included in the Company’s Financial Statements.
3. LIST OF EXHIBITS.
A. The following exhibits are incorporated herein by reference. All referenced Forms 10-K, 10-Q and 8-K are those of Millipore Corporation [Commission File No. 0-1052]:
| | | | |
Reg. S-K Item 601(b) Reference | | Document Incorporated | | Referenced Document on file with the Commission |
| | |
| 1.1 | | Purchase Agreement dated as of June 7, 2006 among Millipore, UBS Limited and Goldman, Sachs & Co., as representatives for the initial purchasers named therein | | Form 8-K filed June 9, 2006 |
| | |
| 1.2 | | Purchase Agreement dated as of June 23, 2006, among Millipore, UBS Limited and Banc of America Securities Limited, as representatives for the initial purchasers named therein | | Form 8-K filed June 28, 2006 |
| | |
| 2.1 | | Form of Master Separation and Distribution Agreement between Millipore and Mykrolis Corporation+ | | Form 10-Q for the quarter ended June 30, 2001 |
| | |
| 2.2 | | Form of General Assignment and Assumption Agreement between Millipore and Mykrolis Corporation+ | | Form 10-Q for the quarter ended June 30, 2001 |
| | |
| 3(i) | | Restated Articles of Organization, as amended May 6, 1996 | | Form 10-K for year ended December 31, 1996 |
| | |
| 3(ii) | | By Laws, as amended | | Form 8-K filed February 14, 2005 |
| | |
| 4.1 | | Specimen Stock Certificate | | Registration Statement on Form S-3 ASR (No. 333-136451) |
| | |
| 4.2.1 | | Common Stock Rights Agreement dated as of April 15, 1988, as amended and restated April 16, 1998 between Millipore and The First National Bank of Boston | | Form 8-K filed April 30, 1998 |
| | |
| 4.2.2 | | Agreement of Substitution and Amendment of Common Stock Rights Agreement dated as of February 14, 2003 between Millipore and American Stock Transfer and Trust Company | | Form 10-Q for the quarter ended March 31, 2003 |
| | |
| 102 | | MILLIPORE FORM 10-K 2007 |
| | | | |
Reg. S-K Item 601(b) Reference | | Document Incorporated | | Referenced Document on file with the Commission |
| | |
| 4.2.3 | | Amendment of Common Stock Rights Agreement dated May 16, 2003 between Millipore and American Stock Transfer and Trust Company | | Form 10-Q for the quarter ended June 30, 2003 |
| | |
| 4.3 | | Indenture dated as of June 13, 2006 among Millipore, Wilmington Trust Company and Citibank, N.A. | | Form 8-K filed June 16, 2006 |
| | |
| 4.4 | | Registration Rights Agreement dated as of June 13, 2006 among Millipore and the initial purchasers named therein | | Form 8-K filed June 16, 2006 |
| | |
| 4.5 | | Indenture dated as of June 30, 2006 among Millipore, Citibank, N.A., and Citibank International plc | | Form 8-K filed July 6, 2006 |
| | |
| 10.1.1 | | Form of letter agreement with directors relating to the deferral of directors fees and conversion into phantom stock units* | | Form 10-K for the year ended December 31, 1998 |
| | |
| 10.1.2 | | Form of letter agreement with directors relating to the deferral of directors’ cash compensation* | | Form 10-K for the year ended December 31, 2002 |
| | |
| 10.1.3 | | Form of Amendment dated August 12, 2004 to Deferral Letter Agreement with Directors of Millipore Corporation* | | Form 10-K for the year ended December 31, 2004 |
| | |
| 10.1.4 | | Director Compensation* | | Form 8-K filed February 21, 2006 |
| | |
| 10.2.1 | | 1989 Stock Option Plan for Non-Employee Directors* | | Form 10-K for the year ended December 31, 1998 |
| | |
| 10.2.2 | | Amendment dated November 18, 2003 to 1989 Stock Option Plan for Non-Employee Directors* | | Form 10-K for the year ended December 31, 2003 |
| | |
| 10.2.3 | | Form of Stock Option Grant to Directors under 1989 Stock Option Plan for Non-Employee Directors* | | Form 10-K for the year ended December 31, 2004 |
| | |
| 10.3.1 | | Amended and Restated 1999 Stock Option Plan for Non-Employee Directors* | | Form 10-Q for the quarter ended June 30, 2003 |
| | |
| 10.3.2 | | Amendment dated November 18, 2003 to 1999 Stock Option Plan for Non-Employee Directors* | | Form 10-K for the year ended December 31, 2003 |
| | |
| 10.3.3 | | Form of Stock Option Grant to Directors under 1999 Stock Option Plan for Non-Employee Directors* | | Form 10-K for the year ended December 31, 2004 |
| | |
| 10.4.1 | | Amended and Restated 1999 Stock Incentive Plan dated April 26, 2006* | | Form 10-Q for the quarter ended April 1, 2006 |
| | |
| 10.4.2 | | Form of Stock Option Grant to Executive Officers and other employees under 1999 Stock Incentive Plan* | | Form 10-K for the year ended December 31, 2004 |
| | |
| 10.4.3 | | Form of Restricted Stock Unit Grant to Executive Officers and other employees under 1999 Stock Incentive Plan* | | Form 8-K filed February 21, 2006 |
| | |
| 10.4.4 | | Form of Nonqualified Stock Option Grant for Nonemployee Directors under 1999 Stock Incentive Plan* | | Form 10-Q for the quarter ended September 30, 2006 |
| | |
| 10.4.5 | | Form of Restricted Stock Unit Award Document for Nonemployee Directors under 1999 Stock Incentive Plan* | | Form 10-Q for the quarter ended September 30, 2006 |
| | |
| 10.5.1 | | 2000 Deferred Compensation Plan for Senior Management* | | Form 10-K for the year ended December 31, 2000 |
| | |
| 10.5.2 | | Amendment No. 1 dated March 31, 2001 to 2000 Deferred Compensation Plan for Senior Management * | | Form 10-K for the year ended December 31, 2001 |
| | |
| 10.5.3 | | Standard Deferred Compensation Agreement* | | Form 10-K for the year ended December 31, 2000 |
| | |
MILLIPORE FORM 10-K 2007 | | 103 |
| | | | |
Reg. S-K Item 601(b) Reference | | Document Incorporated | | Referenced Document on file with the Commission |
| | |
| 10.6.1 | | Supplemental Savings and Retirement Plan for Key Salaried Employees of Millipore Corporation, as amended through 2000* | | Form 10-K for the year ended December 31, 2000 |
| | |
| 10.6.2 | | Amendment dated March 31, 2001 to Supplemental Savings and Retirement Plan for Key Salaried Employees of Millipore Corporation* | | Form 10-K for the year ended December 31, 2001 |
| | |
| 10.6.3 | | Amendment dated November 18, 2003 to Supplemental Savings and Retirement Plan for Key Salaried Employees of Millipore Corporation* | | Form 10-K for the year ended December 31, 2003 |
| | |
| 10.7 | | Millipore Incentive Plan (f/k/a 2000 Management Incentive Plan)* | | Form 10-K for the year ended December 31, 2000 |
| | |
| 10.8.1 | | Offer Letter to Martin D. Madaus dated October 11, 2004* | | Form 10-K for the year ended December 31, 2004 |
| | |
| 10.8.2 | | Executive Termination Agreement dated August 8, 2007 between Millipore and Martin D. Madaus* | | Form 8-K filed August 18, 2007 |
| | |
| 10.8.3 | | Officer Severance Agreement dated August 8, 2007 between Millipore and Martin D. Madaus* | | Form 8-K filed August 18, 2007 |
| | |
| 10.8.4 | | Restricted Stock Agreement dated January 1, 2005 between Millipore and Martin D. Madaus* | | Form 10-Q for the quarter ended October 1, 2005 |
| | |
| 10.9.1 | | Executive Termination Agreement dated September 10, 2007 between Millipore and Jean-Paul Mangeolle* | | Form 8-K filed September 13, 2007 |
| | |
| 10.9.2 | | Officer Severance Agreement dated September 10, 2007 between Millipore and Jean-Paul Mangeolle* | | Form 8-K filed September 13, 2007 |
| | |
| 10.10.1 | | Executive Termination Agreement dated September 10, 2007 between Millipore and Jeffrey Rudin* | | Form 8-K filed September 13, 2007 |
| | |
| 10.10.2 | | Officer Severance Agreement dated September 10, 2007 between Millipore and Jeffrey Rudin* | | Form 8-K filed September 13, 2007 |
| | |
| 10.11.1 | | Executive Termination Agreement dated September 10, 2007 between Millipore and Charles F. Wagner, Jr.* | | Form 8-K filed September 13, 2007 |
| | |
| 10.11.2 | | Officer Severance Agreement dated September 10, 2007 between Millipore and Charles F. Wagner, Jr.* | | Form 8-K filed September 13, 2007 |
| | |
| 10.11.3 | | Letter Agreement dated May 1, 2007 between Millipore and Charles F. Wagner, Jr.* | | Form 8-K filed May 2, 2007 |
| | |
| 10.12.1 | | Executive Termination Agreement dated September 13, 2007 between Millipore and Dominique Baly* | | Form 8-K filed September 13, 2007 |
| | |
| 10.12.2 | | Officer Severance Agreement dated September 13, 2007 between Millipore and Dominique Baly* | | Form 8-K filed September 13, 2007 |
| | |
| 10.13 | | Letter Agreement dated May 1, 2007 between Millipore and Kathleen B. Allen* | | Form 8-K filed May 2, 2007 |
| | |
| 10.14.1 | | Management and Director Compensation Changes, Equity Grants and Approval of Payments under the Millipore Incentive Plan* | | Form 8-K filed February 14, 2007 |
| | |
| 10.14.2 | | Management Equity Grants* | | Form 8-K filed February 25, 2007 |
| | |
| 10.14.3 | | Description of 2007 Metrics under the Millipore Incentive Plan* | | Form 8-K filed February 14, 2007 |
| | |
| 10.15.1 | | Master Patent License Agreement dated as of March 31, 2001 between Millipore and Mykrolis Corporation | | Form 10-Q for the quarter ended June 30, 2001 |
| | |
| 104 | | MILLIPORE FORM 10-K 2007 |
| | | | |
Reg. S-K Item 601(b) Reference | | Document Incorporated | | Referenced Document on file with the Commission |
| | |
| 10.15.2 | | Master Patent Grantback License Agreement dated as of March 31, 2001 between Millipore and Mykrolis Corporation | | Form 10-Q for the quarter ended June 30, 2001 |
| | |
| 10.15.3 | | Master Trade Secret and Know-How Agreement dated as of March 31, 2001 between Millipore and Mykrolis Corporation | | Form 10-Q for the quarter ended June 30, 2001 |
| | |
| 10.15.4 | | Tax Sharing Agreement dated as of March 31, 2001 between Millipore and Mykrolis Corporation | | Form 10-Q for the quarter ended June 30, 2001 |
| | |
| 10.16 | | Net Lease dated August 12, 2002 between Millipore and Getronics Wang Co., LLC, with respect to the Company’s headquarters in Billerica, Massachusetts | | Form 10-K for the year ended December 31, 2002 |
| | |
| 10.17 | | Officer Severance Agreement dated November 18, 2003 between Millipore and Francis J. Lunger | | Form 10-K for the year ended December 31, 2003 |
| | |
| 10.18.1 | | Credit Agreement dated as of December 15, 2005 among Millipore and certain of its subsidiaries, Bank of America, N.A., and certain other lenders and arrangers | | Form 8-K filed December 20, 2005 |
| | |
| 10.18.2 | | Amendment No. 1 and Consent dated as of June 6, 2006 among Millipore and certain of its subsidiaries, Bank of America, N.A., and certain other lenders and arrangers | | Form 8-K filed June 9, 2006 |
| | |
| 10.18.3 | | Amendment No. 2 dated as of July 13, 2006 among Millipore and certain of its subsidiaries, Bank of America, N.A., and certain other lenders and arrangers | | Form 8-K filed July 18, 2006 |
| + | | Millipore Corporation agrees to furnish supplementally to the Commission a copy of any omitted schedule or exhibit to such agreement upon request by the Commission. |
| * | | A “management contract or compensatory plan” |
B. The following exhibits are filed or furnished herewith:
| | |
Reg. S-K
Item 601(b)
Reference | | Documents Filed Herewith |
| |
| (21) | | Subsidiaries of Millipore |
| |
| (23) | | Consent of Independent Registered Public Accounting Firm |
| |
| (24) | | Power of Attorney |
| |
| (31) | | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) (17 CFR 240.13a-14(a)) or Rule 15d-14(a) (17 CFR 240.15d-14(a)), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| | Certification of Chief Financial Officer Pursuant to Rule 13a-14(a) (17 CFR 240.13a-14(a)) or Rule 15d-14(a) (17 CFR 240.15d-14(a)), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| | | Documents Furnished Herewith |
| |
| (32) | | Certification of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | |
MILLIPORE FORM 10-K 2007 | | 105 |
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | | | | |
| | | | MILLIPORE CORPORATION |
| | | |
| Dated: February 28, 2008 | | | | By: | | /S/ CHARLES F. WAGNER, JR. |
| | | | | | | | Charles F. Wagner, Jr., Vice President and Chief Financial Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacity and on the dates indicated.
| | | | |
| Signature | | Title | | Date |
| | |
/S/ MARTIN D. MADAUS Martin D. Madaus | | Chairman of the Board of Directors, President and Chief Executive Officer | | February 28, 2008 |
| | |
/S/ CHARLES F. WAGNER, JR. Charles F. Wagner, Jr. | | Vice President, Chief Financial Officer (Principal Financial Officer) | | February 28, 2008 |
| | |
/S/ ANTHONY L. MATTACCHIONE Anthony L. Mattacchione | | Vice President, Corporate Controller and Chief Accounting Officer (Principal Accounting Officer) | | February 28, 2008 |
| | |
/S/ DANIEL BELLUS* Daniel Bellus | | Director | | February 28, 2008 |
| | |
/S/ ROBERT C. BISHOP* Robert C. Bishop | | Director | | February 28, 2008 |
| | |
/S/ MELVIN D. BOOTH* Melvin D. Booth | | Director | | February 28, 2008 |
| | |
/S/ ROLF CLASSON* Rolf Classon | | Director | | February 28, 2008 |
| | |
/S/ MAUREEN A. HENDRICKS* Maureen A. Hendricks | | Director | | February 28, 2008 |
| | |
/S/ MARK HOFFMAN* Mark Hoffman | | Director | | February 28, 2008 |
| | |
/S/ JOHN F. RENO* John F. Reno | | Director | | February 28, 2008 |
| | |
/S/ EDWARD M. SCOLNICK* Edward M. Scolnick | | Director | | February 28, 2008 |
| | |
/S/ KAREN E. WELKE* Karen E. Welke | | Director | | February 28, 2008 |
| | |
| |
| *By: | | /S/ JEFFREY RUDIN |
| | Jeffrey Rudin, Attorney-in-Fact |
| | |
| 106 | | MILLIPORE FORM 10-K 2007 |
Index to Exhibits
| | |
| Exhibit No. | | Description |
| |
| 21.1 | | Subsidiaries of Millipore |
| |
| 23.1 | | Consent of Independent Registered Public Accounting Firm |
| |
| 24.1 | | Power of Attorney |
| |
| 31.1 | | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) (17 CFR 240.13a-14(a)) or Rule 15d-14(a) (17 CFR 240.15d-14(a)), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 31.2 | | Certification of Chief Financial Officer Pursuant to Rule 13a-14(a) (17 CFR 240.13a-14(a)) or Rule 15d-14(a) (17 CFR 240.15d-14(a)), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 32.1 | | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | |
MILLIPORE FORM 10-K 2007 | | 107 |















