Exhibit 99.1

Building a Leading Oncology and Acute Care Focused Biopharmaceutical Company
UBS Global Life Sciences Conference September 27, 2005

Forward-Looking Statements
This presentation contains forward-looking statements that involve risks and uncertainties, including those described in the Company’s Securities and Exchange Commission filings.
MGI PHARMA
Building A Leading Biopharmaceutical Company
Revenue potential >$1B within 5 years
Aloxi® injection
Best-in-class 5-HT3 RA for CINV
#1 share position in IV 5-HT 3 CINV market
Product portfolio
Pending acquisition of Guilford Pharmaceuticals
Aquavan® injection pivotal program to begin (procedural sedation) Gliadel® wafer marketed for high grade malignant glioma
Progress made with late-stage product candidates
Dacogen™ injection approvable letter received (MDS)
Saforis™ oral suspension NDA to be submitted (oral mucositis) Other pivotal programs ongoing
Aloxi injection for PONV & oral Aloxi formulation ZYC101a

MGI PHARMA
A Leading Biopharmaceutical Company
MARKETED PRODUCTS
DEVELOPMENT PIPELINE
Oncology
Dacogen™ injection Saforis™ oral suspension Irofulven ZYC300
Acute Care
Aloxi® injection for PONV Aquavan® injection ZYC101a

The Best in Class 5-HT 3 Antagonist for CINV
Approved for both acute and delayed chemotherapy-induced nausea & vomiting (CINV) Favorable head-to-head trials vs. competitive agents #1 share position in IV CINV market $1 billion market opportunity $500 M peak sales potential

2005 CINV Market Dynamics
Approximately 6 M Day 1 IV doses used annually
Aloxi injection share gains on target Pricing has remained stable
CINV market growth has moderated in 2005
Reimbursement methodology change: AWP to ASP system Forecasted Day 1 IV volume growth: ~3% in 2005 Actual 1H05 IV volume 3% lower than 2H04
2005 Aloxi sales anticipated to be $250 to $260 M
Q3 sales estimated to be $63 to $65 M
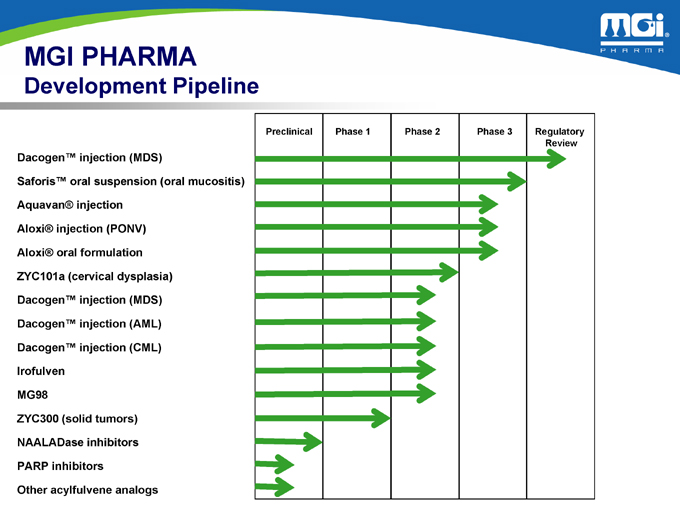
MGI PHARMA
Development Pipeline
Preclinical Phase 1 Phase 2 Phase 3 Regulatory Review
Dacogen™ injection (MDS)
Saforis™ oral suspension (oral mucositis) Aquavan® injection Aloxi® injection (PONV) Aloxi® oral formulation ZYC101a (cervical dysplasia) Dacogen™ injection (MDS) Dacogen™ injection (AML) Dacogen™ injection (CML) Irofulven MG98 ZYC300 (solid tumors) NAALADase inhibitors PARP inhibitors Other acylfulvene analogs

Anti-Cancer Agent with Broad Clinical Activity
Myelodysplastic syndromes (MDS)
MAA under review by EMEA Approvable Letter received from FDA
Over 40 trials ongoing
Alternative dosing regimen studies ongoing Phase 2 acute myeloid leukemia (AML) trial in elderly patients ongoing Expanded AML clinical program planned for 2005 $>250 M peak sales potential

Ex-U.S. Partnering Strategy
Active negotiations ongoing with potential partners Criteria for Dacogen injection European partner:
Strong commercial presence in EU Development expertise in oncology Provide R&D support
Process to identify ROW partners ongoing
Supportive Care Agent for Oral Mucositis
Proprietary oral formulation (Up-Tec™) of L-glutamine
Pivotal phase 3 trial complete in breast cancer patients
Primary endpoint met (p=0.026): overall 22% risk reduction (WHO=2) vs. placebo Significant activity in prevention of WHO grade 3 mucositis
NDA submission targeted for early Q405 Fast track designation Additional clinical trials planned

Oral Mucositis Market Opportunity
Significant unmet medical need
1.3 M patients receive cytotoxic chemotherapy annually Incidence: 10% to >50% depending on regimen Opportunity: >200,000 patients in U.S.
Grade 3 or 4 mucositis associated with:
3-fold increase in risk of chemotherapy dose reduction 2-fold increase in ER visits Additional 7 days of hospitalization per episode Incremental cost of over $3500 per episode
No FDA approved product for patients with solid tumors Peak sales potential ~$100 M

Post Operative Nausea & Vomiting (PONV)
PONV is common with anesthesia and surgical procedures
Prevalent in gynecological, abdominal, and ear, nose and throat surgeries
~ 30 million doses of 5-HT 3 RAs used annually in U.S. for
PONV
Can delay hospital discharge, cause hospital admissions and increase healthcare costs
Phase 3 program ongoing in >1,000 patients
2 | | trials evaluating 3 Aloxi injection doses |
Peak sales potential >$250 M
ZYC101a
Immunotherapeutic for Cervical Dysplasia
Immune response therapeutic for treatment of cervical dysplasia, a precancerous condition Phase 2 study completed in 161 patients:
In a prospectively defined population of patients under age 25, resolution of high grade dysplasia observed in 70% vs. 23% of placebo patients
Pivotal program ongoing
Component of acute care franchise Peak sales potential >$250 M

MGI PHARMA
Guilford Transaction Overview
Transaction timeline
Announced: July 21 HSR expiration: August 31
Shareholder vote scheduled: September 29 Projected closing: October 3
Aquavan® Injection
Rapid progress in ongoing dosing study Plan to finalize phase 3 protocol during Q405
>$250 million U.S. revenue potential
Gliadel® Wafer
Fits current oncology focus Augments hospital presence
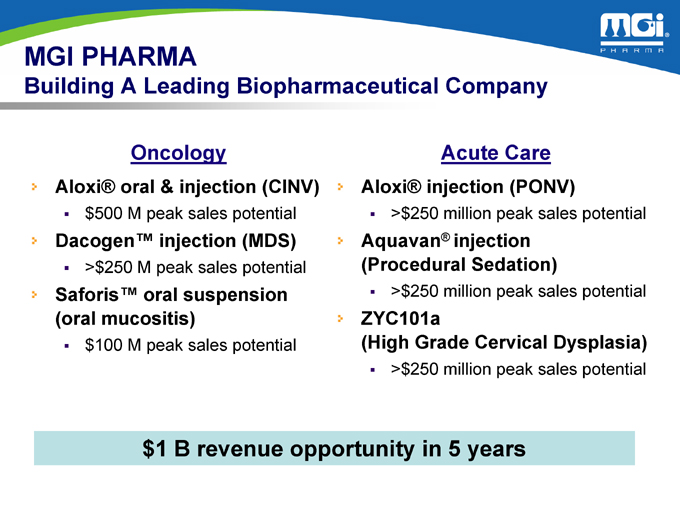
MGI PHARMA
Building A Leading Biopharmaceutical Company
Oncology
Aloxi® oral & injection (CINV) $500 M peak sales potential
Dacogen™ injection (MDS)
>$250 M peak sales potential
Saforis™ oral suspension (oral mucositis) $100 M peak sales potential
Acute Care
Aloxi® injection (PONV)
>$250 million peak sales potential
Aquavan® injection (Procedural Sedation)
>$250 million peak sales potential
ZYC101a
(High Grade Cervical Dysplasia)
>$250 million peak sales potential $1 B revenue opportunity in 5 years

Building a Leading Oncology and Acute Care Focused Biopharmaceutical Company
UBS Global Life Sciences Conference September 27, 2005















