Exhibit 10.7
CREDIT AGREEMENT AND GUARANTY
dated as of July 25, 2014
by and between
VARIATION BIOTECHNOLOGIES (US), INC.,
as the Borrower,
THE GUARANTORS PARTY HERETO,
and
PCOF 1, LLC,
as the Lender
TABLE OF CONTENTS
| | | Page |
| | | |
Article I | DEFINITIONS AND ACCOUNTING TERMS | 1 |
| Section 1.1 | Defined Terms | 1 |
| Section 1.2 | Use of Defined Terms | 18 |
| Section 1.3 | Cross-References | 18 |
| Section 1.4 | Accounting and Financial Determinations | 18 |
Article II | COMMITMENT and BORROWING procedures | 18 |
| Section 2.1 | Commitment | 18 |
| Section 2.2 | Borrowing Procedures | 18 |
| Section 2.3 | Funding | 19 |
| Section 2.4 | Reduction of the Commitment Amounts | 19 |
Article III | REPAYMENTS, PREPAYMENTS, INTEREST AND FEES | 19 |
| Section 3.1 | Repayments and Prepayments; Application | 19 |
| Section 3.2 | Repayments and Prepayments | 19 |
| Section 3.3 | Application | 20 |
| Section 3.4 | Interest Rate | 20 |
| Section 3.5 | Default Rate | 20 |
| Section 3.6 | Payment Dates | 21 |
| Section 3.7 | Exit Fee | 21 |
| Section 3.8 | Other Fees | 21 |
Article IV | LIBO RATE AND OTHER PROVISIONS | 21 |
| Section 4.1 | Increased Costs, Etc | 21 |
| Section 4.2 | Increased Capital Costs | 22 |
| Section 4.3 | Taxes | 22 |
| Section 4.4 | Payments, Computations; Proceeds of Collateral, Etc | 23 |
| Section 4.5 | Setoff | 24 |
| Section 4.6 | LIBOR Rate Not Determinable | 24 |
Article V | CONDITIONS TO LOAN | 24 |
| Section 5.1 | Initial Loan | 24 |
| | Section 5.2 | Delayed Draw Loan | 28 |
| Article VI | REPRESENTATIONS AND WARRANTIES | 29 |
TABLE OF CONTENTS
| | | | Page |
| | | | |
| | Section 6.1 | Organization, Etc | 29 |
| | Section 6.2 | Due Authorization, Non-Contravention, Etc | 30 |
| | Section 6.3 | Government Approval, Regulation, Etc | 30 |
| | Section 6.4 | Validity, Etc | 30 |
| | Section 6.5 | Financial Information | 30 |
| | Section 6.6 | No Material Adverse Change | 31 |
| | Section 6.7 | Litigation, Labor Matters and Environmental Matters 30Section 6.8 Subsidiaries | 31 |
| | Section 6.8 | Subsidiaries | 31 |
| | Section 6.9 | Ownership of Properties | 31 |
| | Section 6.10 | Taxes | 31 |
| | Section 6.11 | Pension Plans, Etc | 32 |
| | Section 6.12 | Accuracy of Information | 32 |
| | Section 6.13 | Regulations U and X | 32 |
| | Section 6.14 | Solvency | 32 |
| | Section 6.15 | Intellectual Property | 32 |
| | Section 6.16 | Material Agreements | 33 |
| | Section 6.17 | Permits | 34 |
| | Section 6.18 | Regulatory Matters | 34 |
| | Section 6.19 | Transactions with Affiliates | 36 |
| | Section 6.20 | Investment Company Act | 36 |
| | Section 6.21 | OFAC | 36 |
| | Section 6.22 | Anti-Corruption | 36 |
| | Section 6.23 | Deposit and Disbursement Accounts | 36 |
| | Section 6.24 | Registration Rights | 37 |
| | Section 6.25 | Royalty and Other Payments | 37 |
| Article VII | AFFIRMATIVE COVENANTS | 37 |
| | Section 7.1 | Financial Information, Reports, Notices, Etc | 37 |
| | Section 7.2 | Maintenance of Existence; Compliance with Contracts, Laws, Etc | 39 |
| | Section 7.3 | Maintenance of Properties | 39 |
| | Section 7.4 | Insurance | 39 |
TABLE OF CONTENTS
| | | | Page |
| | | | |
| | Section 7.5 | Books and Records | 40 |
| | Section 7.6 | Environmental Law Covenant | 40 |
| | Section 7.7 | Use of Proceeds | 40 |
| | Section 7.8 | Future Guarantors, Security, Etc | 41 |
| | Section 7.9 | Obtaining of Permits, Etc | 41 |
| | Section 7.10 | Product Licenses | 41 |
| | Section 7.11 | Maintenance of Regulatory Authorizations, Contracts, Intellectual Property, Etc | 41 |
| | Section 7.12 | Inbound Licenses | 42 |
| | Section 7.13 | Cash Management | 42 |
| | Section 7.14 | Modification of Organic Documents | 43 |
| | Section 7.15 | Inconsistent Agreements | 43 |
| | Section 7.16 | Restriction of Amendments to Certain Documents | 43 |
| | Section 7.17 | PIC | 43 |
| | Section 7.18 | Required Milestones | 43 |
| | Section 7.19 | Minimum Liquidity | 43 |
| Article VIII | NEGATIVE COVENANTS | 43 |
| | Section 8.1 | Business Activities | 44 |
| | Section 8.2 | Indebtedness | 44 |
| | Section 8.3 | Liens | 44 |
| | Section 8.4 | [INTENTIONALLY OMITTED] | 45 |
| | Section 8.5 | Investments | 45 |
| | Section 8.6 | Restricted Payments, Etc | 46 |
| | Section 8.7 | [INTENTIONALLY OMITTED] | 46 |
| | Section 8.8 | Consolidation, Merger; Permitted Acquisitions, Etc | 46 |
| | Section 8.9 | Permitted Dispositions | 46 |
| | Section 8.10 | Modification of Certain Agreements | 46 |
| | Section 8.11 | Transactions with Affiliates | 47 |
| | Section 8.12 | Restrictive Agreements, Etc | 47 |
| | Section 8.13 | Sale and Leaseback | 47 |
| | Section 8.14 | Product Sales | 47 |
TABLE OF CONTENTS
| | | | Page |
| | | | |
| | Section 8.15 | Outbound Licenses | 47 |
| | Section 8.16 | Change in Name, Location, Executive Office, or Executive Management; Change in Fiscal Year | 47 |
| Article IX | EVENTS OF DEFAULT | 48 |
| | Section 9.1 | Listing of Events of Default | 48 |
| | Section 9.2 | Action if Bankruptcy | 51 |
| | Section 9.3 | Action if Other Event of Default | 51 |
| Article X | GUARANTY | 51 |
| | Section 10.1 | Guaranty | 51 |
| | Section 10.2 | Waivers | 52 |
| | Section 10.3 | Benefit of Guaranty | 52 |
| | Section 10.4 | Subordination of Subrogation, Etc | 52 |
| | Section 10.5 | Election of Remedies | 52 |
| | Section 10.6 | Limitation | 53 |
| | Section 10.7 | Liability Cumulative | 53 |
| Article XI | MISCELLANEOUS PROVISIONS | 53 |
| | Section 11.1 | Waivers, Amendments, Etc | 53 |
| | Section 11.2 | Notices; Time | 53 |
| | Section 11.3 | Payment of Costs and Expenses | 54 |
| | Section 11.4 | Indemnification | 54 |
| | Section 11.5 | Survival | 55 |
| | Section 11.6 | Severability | 55 |
| | Section 11.7 | Headings | 55 |
| | Section 11.8 | Execution in Counterparts, Effectiveness, Etc | 55 |
| | Section 11.9 | Governing Law; Entire Agreement | 55 |
| | Section 11.10 | Successors and Assigns | 56 |
| | Section 11.11 | Other Transactions | 56 |
| | Section 11.12 | Forum Selection and Consent to Jurisdiction | 56 |
| | Section 11.13 | Waiver of Jury Trial | 57 |
TABLE OF CONTENTS
| SCHEDULES: | | |
| | | |
| Schedule 5.1.19 | VBI Convertible Notes | |
| Schedule 6.7(a) | Litigation | |
| Schedule 6.8 | Existing Subsidiaries | |
| Schedule 6.11 | Pension Plans | |
| Schedule 6.15(a) | Intellectual Property | |
| Schedule 6.16 | Material Agreements | |
| Schedule 6.19 | Transactions with Affiliates | |
| Schedule 6.23 | Deposit and Disbursement Accounts | |
| Schedule 6.24 | Registration Rights | |
| Schedule 6.25 | Royalty Payments | |
| Schedule 8.2(b) | Existing Indebtedness | |
| Schedule 8.3(b) | Existing Liens | |
| Schedule 8.5(a) | Investments | |
| Schedule 10.02 | Notice Information | |
EXHIBITS: | | | |
Exhibit A-1 | - | Form of Initial Term Note | |
Exhibit A-2 | - | Form of Delayed Draw Note | |
Exhibit B | - | Form of Loan Request | |
Exhibit C | - | Form of Compliance Certificate | |
Exhibit D | - | Form of Pledge and Security Agreement | |
Exhibit E | - | Form of Closing Date Warrant | |
Exhibit F | - | Form of Delayed Draw Warrant | |
Exhibit G | - | Intercompany Subordinated Note Provisions | |
CREDIT AGREEMENT AND GUARANTY
THIS CREDIT AGREEMENT AND GUARANTY dated as ofJuly 25, 2014 (as amended, supplemented or otherwise modified from time to time, this “Agreement”), is by and between VARIATION BIOTECHNOLOGIES (US), INC., a Delaware corporation (the “Borrower”), each Guarantor (as defined below) party hereto and PCOF 1, LLC (together with its Affiliates, successors, transferees and assignees, the “Lender”).
WITNESSETH:
WHEREAS, the Borrower has requested that the Lender provide a senior term loan facility to the Borrower in an aggregate principal amount of $6,000,000 (with up to $3,000,000 available on the Closing Date and up to $3,000,000 available on the Delayed Draw Date, in each case subject to the terms and conditions set forth herein); and
WHEREAS, the Lender is willing, on the terms and subject to the conditions hereinafter set forth, to extend the Commitment and make the Loans to the Borrower.
NOW, THEREFORE, the parties hereto agree as follows:
Article I
DEFINITIONS AND ACCOUNTING TERMS
Section 1.1Defined Terms. The following terms (whether or not underscored) when used in this Agreement, including its preamble and recitals, shall, except where the context otherwise requires, have the following meanings (such meanings to be equally applicable to the singular and plural forms thereof):
“Affiliate” of any Person means any other Person which, directly or indirectly, controls, is controlled by or is under common control with such Person. “Control” (and its correlatives) by any Person means the power of such Person, directly or indirectly, (i) to vote 10% or more of the Capital Securities (on a fully diluted basis) of another Person which Capital Securities have ordinary voting power for the election of directors, managing members or general partners (as applicable), or (ii) to direct or cause the direction of the management and policies of such other Person (whether by contract or otherwise).
“Agreement” is defined in the preamble.
“Applicable Margin” means 11.00%, as such percentage may be increased pursuant toSection 3.5.
“Authorized Officer” means, relative to each Loan Party, those of its officers, general partners or managing members (as applicable) whose signatures and incumbency shall have been certified to the Lender pursuant toSection 5.1.1.
“Benefit Plan” means any employee benefit plan, as defined in section 3(3) of ERISA,that either (i) is a Multiemployer Plan, (ii) is subject to section 412 of the Code, section 302 ofERISA or Title IV of ERISA or (iii) provides welfare benefits to terminated employees, otherthan to the extent required by section 4980B(f) of the Code and the corresponding provisions of ERISA or similar state law.
“BLA” means (i) (x) a biologics license application (as defined in the FD&C Act) to introduce, or deliver for introduction, a biologic product, including vaccines into commerce in the U.S., or any successor application or procedure and (y) any similar application or functional equivalent relating to biologics licensing applicable to or required by any country, jurisdiction or Governmental Authority other than the U.S. and (ii) all supplements and amendments that may be filed with respect to the foregoing.
“Borrower” is defined in thepreamble.
“Business Day” means any day which is neither a Saturday nor Sunday nor a legal holiday on which banks are authorized or required to be closed in New York, New York.
“Capital Securities” means, with respect to any Person, all shares of, interests or participations in, or other equivalents in respect of (in each case however designated, whether voting or non-voting), such Person’s capital stock, whether now outstanding or issued after the Closing Date.
“Capitalized Lease Liabilities” means, with respect to any Person, all monetary obligations of such Person and its Subsidiaries under any leasing or similar arrangement which have been (or, in accordance with GAAP, should be) classified as capitalized leases, and for purposes of each Loan Document the amount of such obligations shall be the capitalized amount thereof, determined in accordance with GAAP, and the stated maturity thereof shall be the date of the last payment of rent or any other amount due under such lease prior to the first date upon which such lease may be terminated by the lessee without payment of a premium or a penalty.
“Cash Equivalent Investment” means, at any time:
(a) any direct obligation of (or unconditionally guaranteed by) the United States or a state thereof (or any agency or political subdivision thereof, to the extent such obligations are supported by the full faith and credit of the United States or a state thereof) maturing not more than one year after such time;
(b) commercial paper maturing not more than 270 days from the date of issue, which is issued by a corporation (other than an Affiliate of the Borrower or any of its Subsidiaries) organized under the laws of any state of the United States or of the District of Columbia and rated A-1 or higher by S&P or P-1 or higher by Moody’s; or
(c) any certificate of deposit, time deposit or bankers acceptance, maturing not more than one year after its date of issuance, which is issued by any bank organized under the laws of the United States (or any state thereof) and which has (x) a credit rating of A2 or higher from Moody’s or A or higher from S&P and (y) a combined capital and surplus greater than $1,000,000,000.
“Casualty Event” means the damage, destruction or condemnation, as the case may be, of property of any Person or any of its Subsidiaries.
“Change in Control” means and shall be deemed to have occurred if (i) any “person” or “group” (within the meaning of Rule 13d-5 of the Securities Exchange Act of 1934 as in effect on the date hereof) (other than the Permitted Investors) shall own, directly or indirectly, beneficially or of record, determined on a fully diluted basis, more than 37.5% of the Voting Securities of Holdco, (ii) a majority of the seats (other than vacant seats) on the board of directors (or equivalent) of Holdco shall at any time be occupied by persons who were neither (x) nominated by the board of directors of Holdco nor (y) appointed by directors so nominated, (iii) Holdco shall cease to own directly, beneficially and of record, 100% of the issued and outstanding Capital Securities of the Borrower or (iv) Holdco shall cease to own directly or indirectly, beneficially and of record, 100% of the issued and outstanding Capital Securities of each of its other Subsidiaries.
“Change in Law” means the occurrence, after the date of this Agreement, of any of the following: (i) the adoption or taking effect of any law, rule, regulation or treaty, (ii) any change in any law, rule, regulation or treaty or in the administration, interpretation, implementation or application thereof by any Governmental Authority or (iii) the making or issuance of any request, rule, guideline or directive (whether or not having the force of law) by any Governmental Authority;provided that, notwithstanding anything herein to the contrary, (x) the Dodd-Frank Wall Street Reform and Consumer Protection Act and all requests, rules, guidelines or directives thereunder or issued in connection therewith and (y) all requests, rules, guidelines or directives promulgated by the Bank for International Settlements, the Basel Committee on Banking Supervision (or any successor or similar authority) or the United States or foreign regulatory authorities, in each case pursuant to Basel III, shall in each case be deemed to be a “Change in Law”, regardless of the date enacted, adopted or issued.
“Closing Date” means the date of the making of the Initial Loan hereunder.
“Closing Date Certificate” is defined inSection 5.1.2.
“Closing Date Warrant” means the warrant dated as of the date hereof, executed and delivered by the Lender and an Authorized Officer of Holdco, substantially in the form ofExhibit E hereto, as amended, supplemented, amended and restated or otherwise modified from time to time.
“Code” means the Internal Revenue Code of 1986, and the regulations thereunder, in each case as amended, reformed or otherwise modified from time to time.
“Commitment” means the Lender’s obligation (if any) to make Loans hereunder.
“Commitment Amount” means the Initial Commitment Amount plus the Delayed Draw Commitment Amount.
“Compliance Certificate” means a certificate duly completed and executed by an Authorized Officer of the Borrower and Holdco, substantially in the form ofExhibit C hereto, together with such changes thereto as the Lender may from time to time request for the purpose of monitoring the Borrower’s compliance with the financial covenants contained herein.
“Contingent Liability” means any agreement, undertaking or arrangement by which any Person guarantees, endorses or otherwise becomes or is contingently liable upon (by direct or indirect agreement, contingent or otherwise, to provide funds for payment, to supply funds to, or otherwise to invest in, a debtor, or otherwise to assure a creditor against loss) the Indebtedness of any other Person (other than by endorsements of instruments in the course of collection), or guarantees the payment of dividends or other distributions upon the Capital Securities of any other Person. The amount of any Person’s obligation under any Contingent Liability shall (subject to any limitation set forth therein) be deemed to be the outstanding principal amount of the debt, obligation or other liability guaranteed thereby;provided that, in the event there is not an outstanding principal amount or other similar readily discernable outstanding, actual or liquidated amount with respect to such debt, obligation or other liability guaranteed thereby, then, as of any time of determination, the amount of the Contingent Liability in respect thereof shall be the amount that, in light of then existing facts and circumstances, is reasonably expected to become an actual or matured liability.
“Control” is defined within the definition of “Affiliate”.
“Controlled Account” is defined inSection 7.13(a).
“Copyrights” means all copyrights, whether statutory or common law, and all exclusive and nonexclusive licenses from third parties or rights to use copyrights owned by such third parties, along with any and all (i) renewals, revisions, extensions, derivative works, enhancements, modifications, updates and new releases thereof, (ii) income, royalties, damages, claims and payments now and hereafter due and/or payable with respect thereto, including, without limitation, damages and payments for past, present or future infringements thereof, (iii) rights to sue for past, present and future infringements thereof, and (iv) foreign copyrights and any other rights corresponding thereto throughout the world.
“Copyright Security Agreement” means any Copyright Security Agreement executed and delivered by a Grantor substantially in the form of Exhibit C to the Pledge and Security Agreement, as amended, supplemented, amended and restated or otherwise modified from time to time.
“Default” means any Event of Default or any condition, occurrence or event which, after notice or lapse of time or both, would constitute an Event of Default.
“Delayed Draw Date” means the date of the making of the Delayed Draw Loan hereunder, which shall be no sooner than the date on which each of the conditions precedent set forth inSection 5.2 shall have been satisfied.
“Delayed Draw Certificate” is defined inSection 5.2.1.
“Delayed Draw Commitment Amount” means $3,000,000.
“Delayed Draw Loan” is defined inSection 2.1(b).
“Delayed Draw Note” means a promissory note of the Borrower payable to the Lender, in the form ofExhibit A-2 hereto (as such promissory note may be amended, endorsed or otherwise modified from time to time), evidencing the aggregate Indebtedness of the Borrower to the Lender resulting from the outstanding amount of the Delayed Draw Loans, and also means all other promissory notes accepted from time to time in substitution therefor or renewal thereof.
“Delayed Draw Warrant” means the warrant dated as of the Delayed Draw Date, executed and delivered by the Lender and an Authorized Officer of Holdco, substantially in the form ofExhibit F hereto, as amended, supplemented, amended and restated or otherwise modified from time to time.
“Designated Jurisdiction” means any country or territory to the extent that such country or territory is the subject of any Sanction.
“Disposition” (or similar words, such as “Dispose”) means any sale, transfer, lease, contribution or other conveyance (including by way of merger) of, or the granting of options, warrants or other rights to, any Loan Party’s assets (including accounts receivable and Capital Securities of Subsidiaries) to any other Person (other than to Holdco or one of its wholly-owned Subsidiaries) in a single transaction or series of transactions.
“DOH” means the Department of Health (Canada) and any successor entity.
“Dollars” and the sign “$” mean lawful money of the United States.
“Early Prepayment Fee” means (i) with respect to any prepayment of any Loan during the period from the Closing Date up to (and including) the first anniversary of the Closing Date, an amount equal to 5.00% of the principal amount of the Loans being prepaid and (ii) with respect to any prepayment of any Loan during the period from the day following the first anniversary of the Closing Date up to (and including) the second anniversary of the Closing Date, an amount equal to 2.00% of the principal amount of the Loans being prepaid.
“Environmental Laws” means all federal, state, local or international laws, statutes, rules, regulations, codes, directives, treaties, requirements, ordinances, orders, decrees, judgments, injunctions, notices or binding agreements issued, promulgated or entered into by any Governmental Authority, relating in any way to the environment, natural resources, Hazardous Material or health and safety matters.
“Environmental Liability” means any liability, loss, claim, suit, action, investigation, proceeding, damage, commitment or obligation, contingent or otherwise (including any liability for damages, costs of environmental remediation, fines, penalties or indemnities), of or affecting any Loan Party directly or indirectly arising from, in connection with or based upon (i) any Environmental Law or Environmental Permit, (ii) the generation, use, handling, transportation, storage, treatment, recycling, presence, disposal, Release or threatened Release of, or exposure to, any Hazardous Materials or (iii) any contract, agreement, penalty, order, decree, settlement, injunction or other arrangement (including operation of law) pursuant to which liability is assumed, entered into, inherited or imposed with respect to any of the foregoing.
“Environmental Permit” is defined inSection 6.7(c).
“ERISA” means the Employee Retirement Income Security Act of 1974, as amended, and any successor statute thereto of similar import, together with the regulations thereunder, in each case as in effect from time to time. References to Sections of ERISA also refer to any successor Sections thereto.
“ERISA Affiliate” means any person that for purposes of Title I and Title IV of ERISA and Section 412 of the Code would be deemed to be a single employer with the Borrower, pursuant to Section 414(b), (c), (m) or (o) of the Code or Section 4001 of ERISA.
“ERISA Event” means (a) any reportable event, as defined in Section 4043 of ERISA, with respect to a Pension Plan, as to which PBGC has not by regulation waived the requirement of Section 4043(a) of ERISA that it be notified of such event, (b) the filing of a notice of intent to terminate any Pension Plan, if such termination would require material additional contributions in order to be considered a standard termination within the meaning of Section 4041(b) of ERISA, the filing under Section 4041(c) of ERISA of a notice of intent to terminate any Pension Plan or the termination of any Pension Plan under Section 4041(c) of ERISA, (c) the institution of proceedings under Section 4042 of ERISA by the PBGC for the termination of, or the appointment of a trustee to administer, any Pension Plan, (d) any failure by any Pension Plan to satisfy the minimum funding requirements of Sections 412 and 430 of the Code or Section 302 of ERISA applicable to such Pension Plan, whether or not waived, (e) the failure to make a required contribution to any Pension Plan that would result in the imposition of an encumbrance on any Loan Party or any ERISA Affiliate under Section 412 or 430 of the Code or at any time prior to date hereof, a filing under Section 412 of the Code or Section 302 of ERISA of any request for a minimum funding variance with respect to any Pension Plan or Multiemployer Plan, (f) an engagement in a non-exempt prohibited transaction within the meaning of Section 4975 of the Code or Section 406 of ERISA with respect to which a Loan Party would incur liability which would reasonably be expected to have a Material Adverse Effect, (g) the complete or partial withdrawal of any Loan Party or any material ERISA Affiliate from a Multiemployer Plan, (h) any Loan Party or an ERISA Affiliate incurring any material liability under Title IV of ERISA with respect to any Pension Plan (other than premiums due and not delinquent under Section 4007 of ERISA) and (i) a determination that any Pension Plan is, or is expected to be, in “at risk” status (as defined in Section 303(i)(4) of ERISA or Section 430(i)(4) of the Code).
“Event of Default” is defined inSection 9.1.
“Event of Loss” means, with respect to any asset of any Loan Party, any of the following: (i) any loss, destruction or damage of such asset, (ii) any pending or threatened institution of any proceedings for the condemnation or seizure of such asset or of any right of eminent domain, or (iii) any actual condemnation, seizure or taking, by exercise of the power of eminent domain or otherwise, of such asset, or confiscation of such asset or requisition of the use of such asset.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Excluded Collateral” is defined in the Pledge and Security Agreement.
“Excluded Subsidiary” means any of PIC, VBI Acquisition, Paulson Investment I LLC, an Oregon limited liability company, Paulson Capital Properties LLC, an Oregon limited liability company, and PCP I LLC, an Oregon limited liability company.
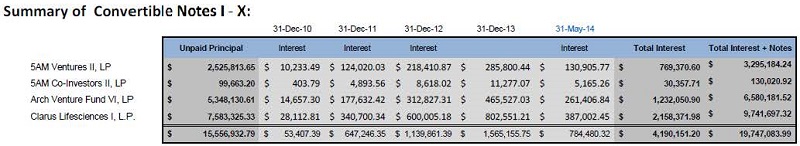
“Existing Investors” means Perceptive Life Sciences Master Fund Ltd.; Titan-Perc Ltd.; 5AM Ventures II, L.P.; 5AM Co-Investors II, L.P.; ARCH Venture Fund VI, L.P.; and Clarus Lifesciences I, L.P.
“Expense Deposit” means an amount equal to $30,000 deposited by the Borrower with the Lender to be applied to the expenses of the Lender pursuant toSection 11.3.
“FDA” means the U.S. Food and Drug Administration and any successor entity.
“FD&C Act” means the U.S. Food, Drug and Cosmetic Act of 1938 (or any successor thereto), as amended from time to time, and the rules and regulations promulgated thereunder.
“Fiscal Quarter” means a quarter ending on the last day of March, June, September or December.
“Fiscal Year” means any period of twelve consecutive calendar months ending on December 31; references to a Fiscal Year with a number corresponding to any calendar year (e.g., the “2013 Fiscal Year”) refer to the Fiscal Year ending on December 31 of such calendar year.
“F.R.S. Board” means the Board of Governors of the Federal Reserve System or any successor thereto.
“GAAP” is defined inSection 1.4.
“Governmental Authority” means any national, supranational, federal, state, county, provincial, local, municipal or other government or political subdivision thereof (including any Regulatory Authority), whether domestic or foreign, and any agency, authority, commission, ministry, instrumentality, regulatory body, court, tribunal, arbitrator, central bank or other Person exercising executive, legislative, judicial, taxing, regulatory or administrative powers or functions of or pertaining to any such government.
“Grantor” means, collectively, Holdco and each of its Subsidiaries.
“Guarantors” means, collectively, Holdco and each of its Subsidiaries (other than the Excluded Subsidiaries).
“Hazardous Material” means any material, substance, chemical, mixture or waste which is capable of damaging or causing harm to any living organism, the environment or natural resources, including all explosive, special, hazardous, polluting, toxic, industrial, dangerous, biohazardous, medical, infectious or radioactive substances, materials or wastes, noise, odor, electricity or heat, and including petroleum or petroleum products, byproducts or distillates, asbestos or asbestos-containing materials, urea formaldehyde, polychlorinated biphenyls, radon gas, ozone-depleting substances, greenhouse gases, and all other substances or wastes of any nature regulated pursuant to any Environmental Law or as to which any Governmental Authority requires investigation, reporting or remedial action.
“Hedging Obligations” means, with respect to any Person, all liabilities of such Person under currency exchange agreements, interest rate swap agreements, interest rate cap agreements and interest rate collar agreements, and all other agreements or arrangements designed to protect such Person against fluctuations in interest rates or currency exchange rates.
“herein”, “hereof”, “hereto”, “hereunder” and similar terms contained in any Loan Document refer to such Loan Document as a whole and not to any particular Section, paragraph or provision of such Loan Document.
“Holdco” means VBI Vaccines Inc., a Delaware corporation.
“Impermissible Qualification” means any qualification or exception to the opinion or certification of any independent public accountant as to any financial statement of Holdco and its Subsidiaries (i) which is of a “going concern” or similar nature, (ii) which relates to the limited scope of examination of matters relevant to such financial statement or (iii) which relates to the treatment or classification of any item in such financial statement and which, as a condition to its removal, would require an adjustment to such item the effect of which would be to cause the Borrower to be in Default.
“including” and “include” means including without limiting the generality of any description preceding such term, and, for purposes of each Loan Document, the parties hereto agree that the rule of ejusdem generis shall not be applicable to limit a general statement, which is followed by or referable to an enumeration of specific matters, to matters similar to the matters specifically mentioned.
“IND” means (i) (x) an investigational new drug application (as defined in the FD&C Act) that is required to be filed with the FDA before beginning clinical testing in human subjects, or any successor application or procedure and (y) any similar application or functional equivalent relating to any investigational new drug application applicable to or required by any country, jurisdiction or Governmental Authority other than the U.S. and (ii) all supplements and amendments that may be filed with respect to the foregoing.
“Indebtedness” of any Person means:
(a) all obligations of such Person for borrowed money or advances and all obligations of such Person evidenced by bonds, debentures, notes or similar instruments;
(b) all obligations, contingent or otherwise, relative to the face amount of all letters of credit, whether or not drawn, and banker’s acceptances issued for the account of such Person;
(c) all Capitalized Lease Liabilities of such Person;
(d) net Hedging Obligations of such Person and all obligations of such Person arising under Synthetic Leases, excluding amounts due under Synthetic Leases that may be terminated by the lessee on no more than 180 days prior notice and without penalty or further obligation in respect of the period following any notice of termination period required thereunder;
(e) all obligations issued, undertaken or assumed as the deferred purchase price of property or services, including earnouts, purchase price adjustments and seller notes in connection with acquisitions permitted hereunder (to the extent due and payable and included as a liability on the balance sheet in accordance with GAAP) (other than trade payables entered into in the ordinary course of business);
(f) whether or not so included as liabilities in accordance with GAAP, all obligations of such Person to pay the deferred purchase price of property or services (excluding trade accounts payable in the ordinary course of business which are not overdue for a period of more than 90 days or, if overdue for more than 90 days, as to which a dispute exists and adequate reserves in conformity with GAAP have been established on the books of such Person), and indebtedness secured by (or for which the holder of such indebtedness has an existing right, contingent or otherwise, to be secured by) a Lien on property owned or being acquired by such Person (including indebtedness arising under conditional sales or other title retention agreements), whether or not such indebtedness shall have been assumed by such Person or is limited in recourse; and
(g) all Contingent Liabilities of such Person in respect of any of the foregoing.
The Indebtedness of any Person shall include the Indebtedness of any other Person (including any partnership in which such Person is a general partner) to the extent such Person is liable therefor as a result of such Person’s ownership interest in or other relationship with such Person, except to the extent the terms of such Indebtedness provide that such Person is not liable therefor.
“Indemnified Liabilities” is defined inSection 11.4.
“Indemnified Parties” is defined inSection 11.4.
“Infringement” and “Infringes” mean the misappropriation of know-how, trade secrets and/or confidential information.
“Initial Commitment Amount” means $3,000,000.
“Initial Loan” is defined inSection 2.1(a).
“Initial Term Note” means a promissory note of the Borrower payable to the Lender, in the form ofExhibit A-1 hereto (as such promissory note may be amended, endorsed or otherwise modified from time to time), evidencing the aggregate Indebtedness of the Borrower to the Lender resulting from the outstanding amount of the Initial Loans, and also means all other promissory notes accepted from time to time in substitution therefor or renewal thereof.
“Intellectual Property” means all (i) Patents, (ii) Trademarks, (iii) Copyrights and other works of authorship (registered or unregistered), and all applications, registrations and renewals therefor, (iv) Product Authorizations, (v) Product Agreements, (vi) computer software, databases, data and documentation, (vii) trade secrets and confidential business information, whether patentable or unpatentable and whether or not reduced to practice, know-how, inventions, manufacturing processes and techniques, research and development information, data and other information included in or supporting Product Authorizations, (viii) financial, marketing and business data, pricing and cost information, business, finance and marketing plans, customer and prospective customer lists and information, and supplier and prospective supplier lists and information, (ix) other intellectual property or similar proprietary rights, (x) copies and tangible embodiments of any of the foregoing (in whatever form or medium) and (xi) any and all improvements to any of the foregoing.
“Intercompany Subordinated Note” means a promissory note executed and delivered by the Borrower or another Loan Party that includes subordination provisions substantially as set forth inExhibit G hereto and is otherwise satisfactory in form and substance to the Lender.
“Intercompany Subordinated Debt” means Indebtedness of any Loan Party owing to any other Loan Party;provided that (i) such Indebtedness is unsecured and evidenced by an Intercompany Subordinated Note, (ii) such Intercompany Subordinated Note is pledged to the Lender pursuant to the Pledge and Security Agreement on a first-priority basis and (iii) such Indebtedness will not mature or otherwise become due and payable earlier than 90 days following the Maturity Date.
“Interest Period” means, (i) initially, for any Loan made hereunder, the period beginning on (and including) the date on which such Loan is made hereunder pursuant toSection 2.2 and ending on (and including) the last day of the calendar month in which such Loan was made, and (ii) thereafter, the period beginning on (and including) the first day of each succeeding calendar month and ending on the earlier of (and including) (x) the last day of such calendar month and (y) the Maturity Date.
“Investment” means, relative to any Person, (i) any loan, advance or extension of credit made by such Person to any other Person, including the purchase by such Person of any bonds, notes, debentures or other debt securities of any other Person, (ii) Contingent Liabilities in favor of any other Person and (iii) any Capital Securities held by such Person in any other Person. The amount of any Investment shall be the original principal or capital amount thereof less all returns of principal or equity thereon and shall, if made by the transfer or exchange of property other than cash, be deemed to have been made in an original principal or capital amount equal to the fair market value of such property at the time of such Investment.
“Lender” is defined in thepreamble.
“Lender’s Designee” is defined inSection 5.1.20.
“LIBO Rate” means, with respect to any applicable Interest Period hereunder, the one-month London Interbank Offered Rate for deposits in Dollars at approximately 11:00 a.m. (London, England time), as determined by the Lender from the appropriate Bloomberg or Telerate page selected by the Lender (or any successor thereto or similar source reasonably determined by the Lender from time to time), which shall be that one-month London Interbank Offered Rate for deposits in Dollars in effect two Business Days prior to the first Business Day of such Interest Period rounded up to the nearest 1/16 of 1%, with such rate to be reset effective as of the first Business Day of each succeeding Interest Period. If the Initial Loan or the Delayed Draw Loan is advanced other than on the first Business Day of a Fiscal Quarter, the initial LIBO Rate for such Loan shall be that one-month London Interbank Offered Rate for deposits in Dollars in effect two Business Days prior to the date of the Initial Loan or the Delayed Draw Loan, as the case may be, which rate shall be in effect until (and including) the last Business Day of the first Interest Period relative to such Loan. The Lender’s internal records of applicable interest rates shall be determinative in the absence of manifest error. Notwithstanding the foregoing, in no event shall the LIBO Rate for any Loan at any time be greater than 5.00%.
“Lien” means any security interest, mortgage, pledge, hypothecation, assignment, deposit arrangement, encumbrance, lien (statutory or otherwise), charge against or interest in property, or other priority or preferential arrangement of any kind or nature whatsoever, to secure payment of a debt or performance of an obligation.
“Loan” means, as the context may require, the Initial Loan, the Delayed Draw Loan or both.
“Loan Documents” means, collectively, this Agreement, the Notes, the Pledge and Security Agreement, the Copyright Security Agreement, the Patent Security Agreement, the Trademark Security Agreement, each other agreement pursuant to which the Lender is granted a Lien to secure the Obligations, the Proposal Letter, the Closing Date Warrant, the Delayed Draw Warrant, and each other agreement, certificate, document or instrument delivered in connection with any Loan Document, whether or not specifically mentioned herein or therein.
“Loan Parties” means, collectively, the Borrower, Holdco and each other Guarantor.
“Loan Request” means a Loan request and certificate duly executed by an Authorized Officer of the Borrower substantially in the form ofExhibit B hereto.
“Material Adverse Effect” means a material adverse effect on (i) the business, condition (financial or otherwise), operations, performance, properties or prospects of Holdco and its Subsidiaries, taken as a whole, (ii) the rights and remedies of the Lender under any Loan Document or (iii) the ability of Holdco and its Subsidiaries to perform their respective Obligations under any Loan Document.
“Material Agreements” means (i) each contract or agreement to which any Loan Party is a party involving aggregate payments of more than $100,000, whether such payments are being made by such Loan Party to a non-Affiliated Person, or by a non-Affiliated Person to such Loan Party; and (ii) all other contracts or agreements, individually or in the aggregate, material to the business, operations, assets, prospects, conditions (financial or otherwise), performance or liabilities of the Loan Parties.
“Maturity Date” means July 25, 2017;provided that if the Delayed Draw Loan is made pursuant hereto, the Maturity Date shall be July 25, 2018.
“Merger Agreement” means the Agreement and Plan of Merger dated as of May 8, 2014, between the Borrower, Holdco and VBI Acquisition.
“Merger Transaction” means the acquisition of the Borrower by Holdco pursuant to the Merger Agreement.
“Milestone Clinical Trial” means a Phase I clinical trial for a CMV (VLP) vaccine candidate.
“MMA” has the meaning ascribed to such term in the definition of “FDA Requirements”.
“Moody’s” means Moody’s Investors Service, Inc.
“Multiemployer Plan” means a “multiemployer plan” (as defined in Section 4001(a)(3) of
ERISA) that is subject to Title IV of ERISA contributed to for any employees of a Loan Party or any ERISA Affiliate.
“NDA” means (i) (x) a new drug application (as defined in the FD&C Act) and (y) any similar application or functional equivalent relating to any new drug application applicable to or required by any country, jurisdiction or Governmental Authority other than the U.S. and (ii) all supplements and amendments that may be filed with respect to the foregoing.
“Net Cash Proceeds” means when used in respect of (i) any Disposition, (ii) any issuance of any debt or equity securities, or (iii) the receipt of any proceeds in connection with any Event of Loss suffered, in each case by any Loan Party, the gross proceeds in cash or cash equivalents received by such Person (including such proceeds subsequently received in respect of noncash consideration initially received and amounts initially placed in escrow that subsequently become available) from such Disposition, issuance or Event of Loss, less all direct costs and expenses incurred or to be incurred, and all federal, state, local and foreign Taxes assessed or to be assessed (if any), in connection therewith.
“Non-Excluded Taxes” means any Taxes other than net income and franchise Taxes imposed on the Lender or its properties by any Governmental Authority under the laws of which the Lender is organized or in which it maintains its applicable lending office.
“Note” means the Initial Term Note or the Delayed Draw Note, as the case may be.
“Obligations” means all obligations (monetary or otherwise, whether absolute or contingent, matured or unmatured) of each Loan Party arising under or in connection with a Loan Document and the principal of and premium, if any, and interest (including interest accruing during the pendency of any proceeding of the type described inSection 9.1.8, whether or not allowed in such proceeding) on the Loans.
“Organic Document” means, relative to each Loan Party, its certificate of incorporation, by-laws, certificate of partnership, partnership agreement, certificate of formation, limited liability agreement, operating agreement and all shareholder agreements, voting trusts and similar arrangements applicable to each Loan Party’s Capital Securities.
“Other Taxes” means any and all stamp, documentary or similar Taxes, or any other excise or property Taxes or similar levies that arise on account of any payment made or required to be made under any Loan Document or from the execution, delivery, registration, recording or enforcement of any Loan Document.
“Other Administrative Proceeding” means any administrative proceeding relating to a dispute involving a patent office or other relevant intellectual property registry which relates to validity, opposition, revocation, ownership or enforceability or the relevant Intellectual Property.
“Patent” means any patent, patent application and invention disclosure, including any divisions, continuations, continuations in-part, provisionals, continued prosecution applications, substitutions, reissues, reexaminations, renewals, extensions, restorations, supplemental protection certificates and other additions in connection therewith, whether in or related to the United States or any foreign country or other jurisdiction.
“Patent Security Agreement” means any Patent Security Agreement executed and delivered by a Grantor in substantially the form of Exhibit A to the Pledge and Security Agreement, as amended, supplemented, amended and restated or otherwise modified from time to time.
“PBGC” means the Pension Benefit Guaranty Corporation, or any entity succeeding to all or any of its functions under ERISA.
“PDMA” means the Prescription Drug Marketing Act.
“Pension Plan” means a “pension plan”, as such term is defined in Section 3(2) of ERISA, which is subject to Title IV of ERISA (other than a Multiemployer Plan), to which a Loan Party or any ERISA Affiliate sponsors, contributes to, or provides benefits under, or has any obligation to contribute or provide benefits under, and to which such Loan Party or ERISA Affiliate may have liability, including any liability by reason of having been a substantial employer under Section 4063 of ERISA at any time during the preceding five years, or by reason of being deemed to be a contributing sponsor under Section 4069 of ERISA.
“Permits” means all permits, licenses, registrations, certificates, orders, approvals, authorizations, consents, waivers, franchises, variances and similar rights issued by or obtained from any Governmental Authority or any other Person, including, without limitation, those relating to Environmental Laws.
“Permitted Investor” means any Existing Investor or any of its Affiliates as to which (i) such Existing Investor (or the Person that administers or manages such Existing Investor) acts as the sole managing member, general partner or equivalent of such Affiliate, and (ii) such Existing Investor (or other Person) has the power to direct or cause the direction of the management and policies of such Affiliate (whether by way of voting equity, contract or otherwise).
“Person” means any natural person, corporation, limited liability company, partnership, joint venture, association, trust or unincorporated organization, Governmental Authority or any other legal entity, whether acting in an individual, fiduciary or other capacity.
“PIC” means Paulson Investment Company, Inc.
“Pledge and Security Agreement” means the Pledge and Security Agreement executed and delivered by each Grantor, substantially in the form ofExhibit D hereto, as amended, supplemented, amended and restated or otherwise modified from time to time.
“PPSA” means the Personal Property Security Act as in effect from time to time in the Province of Ontario; provided that, if, with respect to any financing statement or by reason of any provisions of law, the perfection or the effect of perfection or non-perfection of the security interests granted to the Lender pursuant to the applicable Loan Document is governed by the Personal Property Security Act as in effect in a jurisdiction of Canada other than the Province of Ontario, then “PPSA” means the Personal Property Security Act as in effect from time to time in such other jurisdiction for purposes of the provisions of each Loan Document and any financing statement relating to such perfection or effect of perfection or non-perfection.
“Product” means any current or future product developed, manufactured, licensed, marketed, sold or otherwise commercialized by any Loan Party, including any such product in development or which may be developed.
“Product Agreement” means each agreement, license, document, instrument, interest (equity or otherwise) or the like under which one or more Persons grants or receives any right, title or interest with respect to any Product Development and Commercialization Activities in respect of one or more Products specified therein, or receives or is granted the right to exclude any third parties from engaging in any Product Development and Commercialization Activities with respect thereto, including each contract or agreement with suppliers, manufacturers, distributors, clinical research organizations, wholesalers, pharmacies or with any other Person related to any such entity.
“Product Authorizations” means any and all approvals (including applicable supplements, amendments, pre and post approvals, drug master files, governmental price and reimbursement approvals and approvals of applications for regulatory exclusivity), licenses, registrations or authorizations of any Governmental Authority necessary for the manufacture, development, distribution, use, storage, import, export, transport, promotion, marketing, sale or other commercialization of a Product in any country or jurisdiction, including without limitation INDs, NDAs and BLAs or similar applications.
“Product Development and Commercialization Activities” means, with respect to any Product, any combination of research, development, manufacture, importation, use, sale, storage, design, labeling, marketing, promotion, supply, distribution, testing, packaging, purchasing or other commercialization activities, receipt of payment in respect of any of the foregoing, or like activities the purpose of which is to commercially exploit such Product.
“Prohibited Payment” means any bribe, rebate, payoff, influence payment, kickback or other payment or gift of money or anything of value (including meals or entertainment) to any officer, employee or ceremonial office holder of any government or instrumentality thereof, political party or supra-national organization (such as the United Nations), any political candidate, any royal family member or any other person who is connected or associated personally with any of the foregoing that is prohibited under any applicable law or regulation or otherwise for the purpose of influencing any act or decision of such payee in his official capacity, inducing such payee to do or omit to do any act in violation of his lawful duty, securing any improper advantage or inducing such payee to use his influence with a government or instrumentality thereof to affect or influence any act or decision of such government or instrumentality.
“Proposal Letter” means, collectively, the proposal letter, dated as of March 18, 2014, between the Lender and the Borrower regarding the transactions contemplated hereby and the outline of proposed terms and conditions attached thereto.
“Regulatory Authority” means any Governmental Authority that is concerned with or has regulatory oversight with respect to the use, control, safety, efficacy, reliability, manufacturing, marketing, distribution, sale or other Product Development and Commercialization Activities relating to any Product of a Loan Party, including the FDA, the DOH and all equivalent of such agencies in other jurisdictions, and includes Standard Bodies.
“Regulatory Authorizations” means, with respect to the Products, all approvals, clearances, authorizations, orders, exemptions, registrations, certifications, licenses and Permits granted by any Regulatory Authorities, including all NDAs and Product Authorizations held by the Loan Parties or any of their respective licensors, as applicable, or that are pending before the FDA or equivalent non-United States Governmental Entity with respect to the Products.
“Release” means any releasing, disposing, discharging, injecting, spilling, leaking, leaching, pumping, pouring, dumping, depositing, emitting, escaping, emptying, seeping, dispersal, migrating or placing, including movement through, into or upon the environment or any natural or man-made structure.
“Required Milestone” means any event or occurrence described inSection 7.18.
“Restricted Payment” means (i) the declaration or payment of any dividend (other than dividends payable solely in Capital Securities of a Loan Party) on, or the making of any payment or distribution on account of, or setting apart assets for a sinking or other analogous fund for the purchase, redemption, defeasance, retirement or other acquisition of, any class of Capital Securities of a Loan Party or any warrants, options or other right or obligation to purchase or acquire any such Capital Securities, whether now or hereafter outstanding, (ii) the making of any other distribution in respect of such Capital Securities, in each case either directly or indirectly, whether in cash, property or obligations of a Loan Party or otherwise, or (iii) any payments to officers, directors or employees of a Loan Party, other than ordinary course wages or similar compensation or ordinary course reimbursements of customary business expenses incurred on behalf of such Loan Party or its operations.
“S&P” means Standard & Poor’s Rating Services, a division of The McGraw-Hill Companies, Inc.
“Sanction” means any international economic sanction administered or enforced by theUnited States Government (including, without limitation, OFAC), the United Nations SecurityCouncil, the European Union or its Member States, Her Majesty’s Treasury or other relevant sanctions authority.
“SEC” means the Securities and Exchange Commission.
“Solvent” means, with respect to the Borrower and its Subsidiaries on a particular date, that on such date (i) the fair value of the property of the Borrower and its Subsidiaries on a consolidated basis is greater than the total amount of liabilities, including Contingent Liabilities, of the Borrower and its Subsidiaries on a consolidated basis, (ii) the present fair saleable value of the assets of the Borrower and its Subsidiaries on a consolidated basis is not less than the amount that will be required to pay the probable liability of the Borrower and its Subsidiaries on a consolidated basis on its debts as they become absolute and matured, (iii) the Borrower does not intend to, and does not believe that it or its Subsidiaries will, incur debts or liabilities beyond the ability of the Borrower and its Subsidiaries to pay as such debts and liabilities mature, (iv) the Borrower and its Subsidiaries on a consolidated basis are not engaged in business or a transaction, and the Borrower and its Subsidiaries on a consolidated basis are not about to engage in a business or a transaction, for which the property of the Borrower and its Subsidiaries on a consolidated basis would constitute an unreasonably small capital and (v) the Borrower and its Subsidiaries have not executed this Agreement or any other Loan Document or made any transfer or incurred any obligations hereunder, with actual intent to hinder, delay or defraud either present or future creditors. The amount of Contingent Liabilities at any time shall be computed as the amount that, in light of all the facts and circumstances existing at such time, can reasonably be expected to become an actual or matured liability.
“Subordinated Debt” means any unsecured Indebtedness that (i) is of the type described inclause (a) of the definition of “Indebtedness”, and (ii) is permitted pursuant toSection 8.2(f).
“Subsidiary” means, with respect to any Person, any other Person of which more than 50% of the outstanding Voting Securities of such other Person (irrespective of whether at the time Capital Securities of any other class or classes of such other Person shall or might have voting power upon the occurrence of any contingency) is at the time directly or indirectly owned or controlled by such Person, by such Person and one or more other Subsidiaries of such Person, or by one or more other Subsidiaries of such Person. Unless the context otherwise specifically requires, the term “Subsidiary” shall be a reference to a Subsidiary of Holdco.
“Synthetic Lease” means, as applied to any Person, any lease (including leases that may be terminated by the lessee at any time) of any property (whether real, personal or mixed) (i) that is not a capital lease in accordance with GAAP and (ii) in respect of which the lessee retains or obtains ownership of the property so leased for federal income tax purposes, other than any such lease under which that Person is the lessor.
“Taxes” means all income, stamp or other taxes, duties, levies, imposts, charges, assessments, fees, deductions or withholdings, now or hereafter imposed, levied, collected, withheld or assessed by any Governmental Authority, and all interest, penalties or similar liabilities with respect thereto.
“Termination Date” means the date on which all Obligations (other than (i) any obligations contained in or arising out of the Closing Date Warrant or the Delayed Draw Warrant, and (ii) inchoate indemnification obligations) have been paid in full in cash and the Commitment shall have terminated.
“Trademark” means any trademark, service mark, trade name, logo, symbol, trade dress, domain name, corporate name and other indicator of source or origin, and all applications and registrations therefor, together with all of the goodwill associated with the therewith.
“Trademark Security Agreement” means any Trademark Security Agreement executed and delivered by a Grantor substantially in the form of Exhibit B to the Pledge and Security Agreement, as amended, supplemented, amended and restated or otherwise modified from time to time.
“UCC” means the Uniform Commercial Code as in effect from time to time in the State of New York;provided that, if, with respect to any financing statement or by reason of any provisions of law, the perfection or the effect of perfection or non-perfection of the security interests granted to the Lender pursuant to the applicable Loan Document is governed by the Uniform Commercial Code as in effect in a jurisdiction of the United States other than New York, then “UCC” means the Uniform Commercial Code as in effect from time to time in such other jurisdiction for purposes of the provisions of each Loan Document and any financing statement relating to such perfection or effect of perfection or non-perfection.
“United States” or “U.S.” means the United States of America, its fifty states and the District of Columbia.
“VBI Acquisition” means VBI Acquisition Corp., a Delaware corporation.
“VBI Convertible Notes” means, collectively, the convertible promissory notes listed onSchedule 5.1.19 hereto.
“Voting Securities” means, with respect to any Person, Capital Securities of any class or kind ordinarily having the power to vote for the election of directors, managers or other voting members of the governing body of such Person.
“Welfare Plan” means a “welfare plan”, as such term is defined in Section 3(1) of ERISA.
“wholly owned Subsidiary” means any direct or indirect Subsidiaries of Holdco, all of the outstanding Capital Securities of which (other than any director’s qualifying shares or investments by foreign nationals mandated by applicable laws) is owned directly or indirectly by Holdco.
Section 1.2Use of Defined Terms. Unless otherwise defined or the context otherwise requires, terms for which meanings are provided in this Agreement shall have such meanings when used in each other Loan Document and the schedules attached hereto.
Section 1.3Cross-References. Unless otherwise specified, references in a Loan Document to any Article or Section are references to such Article or Section of such Loan Document, and references in any Article, Section or definition to any clause are references to such clause of such Article, Section or definition.
Section 1.4Accounting and Financial Determinations. Unless otherwise specified, all accounting terms used in each Loan Document shall be interpreted, and all accounting determinations and computations thereunder (including underSection 7.19 and any definitions used in such calculations) shall be made, in accordance with those generally accepted accounting principles (“GAAP”) applied in the preparation of the financial statements referred to inSections 5.1.4(a). Unless otherwise expressly provided, all financial covenants and defined financial terms shall be computed on a consolidated basis for Holdco and its Subsidiaries, in each case without duplication.
Article II
COMMITMENT and BORROWING procedures
Section 2.1Commitment.
(a) On the terms and subject to the conditions of this Agreement, the Lender agrees to make a term loan (the “Initial Loan”) to the Borrower on the Closing Date in an amount not to exceed the Initial Commitment Amount.
(b) On the terms and subject to the conditions of this Agreement, the Lender agrees to make a term loan (the “Delayed Draw Loan”) to the Borrower on the Delayed Draw Date in an amount equal to or greater than $1,000,000, not to exceed the Delayed Draw Commitment Amount.
(c) No amounts paid or prepaid with respect to any Loan may be reborrowed.
Section 2.2Borrowing Procedures. In each case subject to the terms and conditions hereof:
(a) The Borrower may irrevocably request that the Initial Loan be made by delivering to the Lender a Loan Request on or before 10:00 a.m. on a Business Day at least three (3) (but not greater than five (5)) Business Days prior to the Closing Date;provided, however, that the Borrower and the Lender shall have mutually agreed upon the date of the Closing Date at least three (3) Business Days prior thereto, provided further that if no such agreement is reached then the Initial Loan will be funded within ten (10) days of delivery of the Loan Request.
(b) The Borrower may irrevocably request that the Delayed Draw Loan be made by delivering to the Lender a Loan Request on or before 10:00 a.m. on a Business Day at least 15 (but not greater than 20) Business Days prior to the proposed Delayed Draw Date;provided, however, that the Borrower and the Lender shall have mutually agreed upon the Delayed Draw Date at least three Business Days prior thereto,provided further that if no such agreement is reached then the Delayed Draw Loan will be funded within ten (10) days of delivery of the Loan Request.
Section 2.3Funding. After receipt of the applicable Loan Request for the Initial Loan, the Lender shall, on the Closing Date and subject to the terms and conditions hereof, make the requested proceeds of the Initial Loan available to the Borrower by wire transfer to the account the Borrower shall have specified in its Loan Request. After receipt of the Loan Request for the Delayed Draw Loan, the Lender shall, on the Delayed Draw Date and subject to the terms and conditions hereof, make the requested proceeds of the Delayed Draw Loan available to the Borrower by wire transfer to the account the Borrower shall have specified in its Loan Request.
Section 2.4Reduction of the Commitment Amounts. The Initial Commitment Amount shall automatically and permanently be reduced to zero immediately after the making of the Initial Loan on the Closing Date. The Delayed Draw Commitment Amount shall automatically and permanently be reduced to zero immediately after the making of the Delayed Draw Loan on the Delayed Draw Date.
Article III
REPAYMENTS, PREPAYMENTS, INTEREST AND FEES
Section 3.1Repayments and Prepayments; Application. The Borrower agrees that the Loans, and any fees or interest accrued or accruing thereon, shall be repaid and prepaid solely in Dollars pursuant to the terms of thisArticle III.
Section 3.2Repayments and Prepayments. The Borrower shall repay in full the entire unpaid principal amount of the Loans on the Maturity Date. Prior thereto, payments and prepayments of the Loans shall be made as set forth below.
(a) From the Closing Date through the first anniversary thereof, no scheduled repayment of the aggregate outstanding principal amount of the Loans shall be required. Thereafter, on the last Business Day of each calendar month, the Borrower shall make a scheduled principal payment of $75,000 on the Initial Loan and, to the extent funded, $75,000 on the Delayed Draw Loan, with the remaining unpaid balance of the Loans payable in cash on the Maturity Date.
(b) The Borrower may, upon five (5) Business Days prior written notice to the Lender, prepay the outstanding amount of the Loans in whole or in part.
(c) Upon the issuance, sale or other incurrence of any debt securities or other Indebtedness (other than Subordinated Debt or Intercompany Subordinated Debt, in either case solely to the extent expressly permitted hereunder) by any Loan Party, the Borrower shall, within three Business Days of such Person’s receipt of the proceeds thereof, prepay the outstanding principal amount of the Loans in an amount equal to 100% of the Net Cash Proceeds therefrom. The provisions of this clause shall not be deemed to be implied consent to any such issuance, sale or incurrence otherwise prohibited by the terms and conditions of this Agreement.
(d) Upon a Disposition by any Loan Party (other than a Disposition permitted pursuant to Section 8.9), the Borrower shall within three Business Days of such Person’s receipt of the proceeds thereof, prepay the outstanding principal amount of the Loans in an amount equal to 100% of the Net Cash Proceeds therefrom. The provisions of this clause shall not be deemed to be implied consent to any Disposition otherwise prohibited by the terms and conditions of this Agreement.
(e) If any Event of Loss shall occur with respect to any Loan Party, the Borrower shall prepay the outstanding principal amount of the Loans in an amount equal to 100% the Net Cash Proceeds therefrom, if any.
(f) Immediately upon any acceleration of the Maturity Date of the Loans pursuant toSection 9.2 orSection 9.3, the Borrower shall repay in full the Loans, unless, pursuant toSection 9.3, only a portion of the Loans are so accelerated (in which case the portion so accelerated shall be so repaid).
(g) If any Loan hereunder is prepaid for any reason on or prior to the date that is two (2) years after the Closing Date, (excluding, however, payments made pursuant toclause (a) of thisSection 3.2), the Borrower shall pay the Early Prepayment Fee to the Lender at the time of such prepayment, together with all other fees payable hereunder (if any), including pursuant toSections 3.7 and3.8.
Section 3.3Application. Amounts repaid or prepaid in respect of the Loans shall be applied as set forth in thisSection 3.3.
(a) Subject toclause (b), each prepayment or repayment of the Loans shall be applied to the principal amount of the Loans then outstanding.
(b) Each prepayment of the Loans made pursuant toclauses (b),(c),(d) or(e) ofSection 3.2 shall be applied in reverse order of the scheduled repayments set forth inclause (a) ofSection 3.2.
Section 3.4Interest Rate. During any applicable Interest Period, the Loans shall accrue interest during such Interest Period at a rate per annum equal to the sum of (i) the Applicable Margin plus (ii) the higher of (x) the LIBO Rate for such Interest Period and (y) 1.00%. The interest rate shall be recalculated and, if necessary, adjusted for each Interest Period, in each case pursuant to the terms hereof.
Section 3.5Default Rate. At all times commencing upon the date any Event of Default occurs, and continuing until such Event of Default is no longer continuing, the Applicable Margin shall be increased by 4.00% per annum.
Section 3.6Payment Dates. Interest accrued on any Loan shall be payable in cash, without duplication:
(a) on the Maturity Date therefor;
(b) on the date of any payment or prepayment, in whole or in part, of principal outstanding on such Loan on the principal amount so paid or prepaid;
(c) the last day of each Interest Period for such Loan;provided that if such day is not a Business Day, then such payment shall be made on the next succeeding Business Day; and
(d) on that portion of such Loan that is accelerated pursuant toSection 9.2 orSection 9.3, immediately upon such acceleration.
Interest accrued on any Loan or other monetary Obligations after the date such amount is due and payable (whether on the Maturity Date, upon acceleration or otherwise) shall be payable upon demand.
Section 3.7Exit Fee. On the day when all Loans outstanding hereunder are paid in full, whether by voluntary or involuntary prepayment, scheduled amortization, acceleration, on the Maturity Date or otherwise, the Borrower will pay an exit fee equal to two percent (2%) multiplied by the sum of (i) the original principal amount of the Initial Loan and (ii) the original principal amount of the Delayed Draw Loan (if made);provided, however, that in the event that (x) the commitment for the Delayed Draw Loan has been permanently terminated and (y) all outstanding Loans have been repaid in full in cash prior to the first anniversary of the Closing Date, such exit fee shall not be payable.
Section 3.8Other Fees and Expenses. The Borrower shall pay to the Lender all fees and expenses of the Lender as required hereby and by each other Loan Document.
Article IV
LIBO RATE AND OTHER PROVISIONS
Section 4.1Increased Costs, Etc. If any Change in Law shall (i) impose, modify or deem applicable any reserve, special deposit, compulsory loan, insurance charge or similar requirement against assets of, deposits with or for the account of, or credit extended by, the Lender or any Person controlling the Lender (except any reserve requirement reflected in the LIBO Rate) or (ii) impose on the Lender or the London interbank market any other condition, cost or expense (other than Taxes) affecting this Agreement or Loans made by the Lender, and the result of any of the foregoing shall be to increase the cost to the Lender of making, converting to, continuing or maintaining any Loan or of maintaining its obligation to make any such Loan (whether of principal, interest or any other amount) then, upon written notice from the Lender, the Borrower shall within 30 days following receipt of such notice pay directly to the Lender such additional amount or amounts sufficient to compensate the Lender for such additional costs incurred or reduction suffered. A certificate of the Lender setting forth the amount or amounts necessary to compensate the Lender or a Person controlling the Lender, as the case may be, as specified in thisSection 4.1 and delivered to the Borrower, shall be conclusive absent manifest error. Failure or delay on the part of the Lender to demand compensation pursuant to thisSection 4.1 shall not constitute a waiver of the Lender’s right to demand such compensation; provided that the Borrower shall not be required to compensate the Lender pursuant to thisSection 4.1 for any increased costs incurred or reductions suffered more than nine months prior to the date that the Lender notifies the Borrower of the Change in Law giving rise to such increased costs or reductions, and of the Lender’s intention to claim compensation therefor (except that, if the Change in Law giving rise to such increased costs or reductions is retroactive, then the nine-month period referred to above shall be extended to include the period of retroactive effect thereof).
Section 4.2Increased Capital Costs. If any Change in Law affects or would affect the amount of capital required or expected to be maintained by the Lender or any Person controlling the Lender, and the Lender determines (in good faith but in its sole and absolute discretion) that the rate of return on its or such controlling Person’s capital as a consequence of the Commitment or any Loan made by it hereunder is reduced to a level below that which the Lender or such controlling Person could have achieved but for the occurrence of any such circumstance, then upon notice from time to time by the Lender to the Borrower, the Borrower shall within five days following receipt of such notice pay directly to the Lender additional amounts sufficient to compensate the Lender or such controlling Person for such reduction in rate of return. A statement of the Lender as to any such additional amount or amounts shall, in the absence of manifest error, be conclusive and binding on the Borrower. In determining such amount, the Lender may use any method of averaging and attribution that it (in its sole and absolute discretion) shall deem applicable.
Section 4.3Taxes. The Borrower covenants and agrees as follows with respect to Taxes.
(a) Any and all payments by the Borrower under each Loan Document shall be made without setoff, counterclaim or other defense, and free and clear of, and without deduction or withholding for or on account of, any Taxes. In the event that any Taxes are imposed and required to be deducted or withheld from any payment required to be made by any Loan Party to or on behalf of the Lender under any Loan Document, then:
(i) if such Taxes are Non-Excluded Taxes, the amount of such payment shall be increased as may be necessary so that such payment is made, after withholding or deduction for or on account of such Taxes, in an amount that is not less than the amount provided for in such Loan Document; and
(ii) such Loan Party shall withhold the full amount of such Taxes from such payment (as increased pursuant toclause (a)(i) and shall pay such amount to the Governmental Authority imposing such Taxes in accordance with applicable law.
(b) In addition, the Borrower shall pay all Other Taxes imposed to the relevant Governmental Authority imposing such Other Taxes in accordance with applicable law.
(c) As promptly as practicable after the payment of any Taxes or Other Taxes, and in any event within 45 days of any such payment being due, the Borrower shall furnish to the Lender a copy of an official receipt (or a certified copy thereof) evidencing the payment of such Taxes or Other Taxes.
(d) The Borrower shall indemnify the Lender for any Non-Excluded Taxes and Other Taxes levied, imposed or assessed on (and whether or not paid directly by) the Lender whether or not such Non-Excluded Taxes or Other Taxes are correctly or legally asserted by the relevant Governmental Authority. Promptly upon having knowledge that any such Non-Excluded Taxes or Other Taxes have been levied, imposed or assessed, and promptly upon notice thereof by the Lender, the Borrower shall pay such Non-Excluded Taxes or Other Taxes directly to the relevant Governmental Authority (provided that, the Lender shall not be under any obligation to provide any such notice to the Borrower). In addition, the Borrower shall indemnify the Lender for any incremental Taxes that may become payable by the Lender as a result of any failure of the Borrower to pay any Taxes when due to the appropriate Governmental Authority or to deliver to the Lender, pursuant toclause (c), documentation evidencing the payment of Taxes or Other Taxes. With respect to indemnification for Non-Excluded Taxes and Other Taxes actually paid by the Lender or the indemnification provided in the immediately preceding sentence, such indemnification shall be made within 30 days after the date the Lender makes written demand therefor. The Borrower acknowledges that any payment made to the Lender or to any Governmental Authority in respect of the indemnification obligations of the Borrower provided in this clause shall constitute a payment in respect of which the provisions ofclause (a) and this clause shall apply.
Section 4.4Payments, Computations; Proceeds of Collateral, Etc.
(a) Unless otherwise expressly provided in a Loan Document, all payments by the Borrower pursuant to each Loan Document shall be made without setoff, deduction or counterclaim not later than 11:00 a.m. on the date due in same day or immediately available funds to such account as the Lender shall specify from time to time by notice to the Borrower. Funds received after that time shall be deemed to have been received by the Lender on the next succeeding Business Day. All interest and fees shall be computed on the basis of the actual number of days (including the first day but excluding the last day) occurring during the period for which such interest or fee is payable over a year comprised of 360 days. Payments due on other than a Business Day shall be made on the next succeeding Business Day and such extension of time shall be included in computing interest and fees in connection with that payment.
(b) All amounts received as a result of the exercise of remedies under the Loan Documents (including from the proceeds of collateral securing the Obligations) or under applicable law shall be applied upon receipt to the Obligations as follows: (i) first, to the payment in full in cash of all interest (including interest accruing after the commencement of a proceeding in bankruptcy, insolvency or similar law, whether or not permitted as a claim under such law) and fees owing under the Loan Documents, and all costs and expenses owing to the Lender pursuant to the terms of the Loan Documents, until paid in full in cash, (ii) second, after payment in full in cash of the amounts specified inclause (b)(i), to the payment of the principal amount of the Loans then outstanding, (iii) third, after payment in full in cash of the amounts specified inclauses (b)(i) and(b)(ii), to the payment of all other Obligations owing to the Lender, and (iv) fourth, after payment in full in cash of the amounts specified inclauses (b)(i) through(b)(iii), and following the Termination Date, to the Borrower or any other Person lawfully entitled to receive such surplus.
Section 4.5Setoff. The Lender shall, upon the occurrence and during the continuance of any Event of Default described inclauses (a) through(d) ofSection 9.1.8 or, upon the occurrence and during the continuance of any other Event of Default declared by the Lender pursuant toSection 9.3, have the right to appropriate and apply to the payment of the Obligations owing to it (whether or not then due), and (as security for such Obligations) each Loan Party hereby grants to the Lender a continuing security interest in, any and all balances, credits, deposits, accounts or moneys of each Loan Party then or thereafter maintained with the Lender. The Lender agrees promptly to notify the Borrower after any such appropriation and application made by the Lender; provided that, the failure to give such notice shall not affect the validity of such setoff and application. The rights of the Lender under this Section are in addition to other rights and remedies (including other rights of setoff under applicable law or otherwise) which the Lender may have.
Section 4.6LIBOR Rate Not Determinable. If prior to the commencement of any Interest Period, adequate and reasonable means do not exist for ascertaining the LIBO Rate for such Interest Period, then the Lender shall give notice thereof to the Borrower as promptly as practicable. In the event of any such determination, the Loans shall, until the Lender has advised the Borrower that the circumstances giving rise to such notice no longer exist, bear interest at the interest rate in effect for the immediately preceding Interest Period.
Article V
CONDITIONS TO LOAN
Section 5.1Initial Loan. The obligation of the Lender to make the Initial Loan shall be subject to the execution and delivery of this Agreement by the parties hereto, the delivery of a Loan Request as requested pursuant toSection 2.2, and the prior or concurrent satisfaction of each of the conditions precedent set forth below in this Article.
Section 5.1.1Secretary’s Certificate, Etc. The Lender shall have received from each Loan Party and its respective Subsidiaries party to a Loan Document, (i) a copy of a good standing certificate, dated a date reasonably close to the Closing Date, for each such Person and (ii) a certificate, dated as of the Closing Date, duly executed and delivered by such Person’s Secretary or Assistant Secretary, managing member or general partner, as applicable, as to:
(a) resolutions of each such Person’s board of directors (or other managing body, in the case of other than a corporation) then in full force and effect authorizing the execution, delivery and performance of each Loan Document to be executed by such Person and the transactions contemplated hereby and thereby;
(b) the incumbency and signatures of those of its officers, managing member or general partner, as applicable, authorized to act with respect to each Loan Document to be executed by such Person; and
(c) the full force and validity of each Organic Document of such Person and copies thereof;
upon which certificates the Lender may conclusively rely until it shall have received a further certificate of the Secretary, Assistant Secretary, managing member or general partner, as applicable, of any such Person cancelling or amending the prior certificate of such Person.
Section 5.1.2Closing Date Certificate. The Lender shall have received a certificate, dated as of the Closing Date and in form and substance satisfactory to the Lender (the “Closing Date Certificate”), duly executed and delivered by an Authorized Officer of the Borrower and Holdco, in which certificate each of the Borrower and Holdco shall agree and acknowledge, among other things, that the statements made therein shall be deemed to be true and correct representations and warranties of each of the Borrower and Holdco as of such date, and, at the time such certificate is delivered, such statements shall in fact be true and correct, and such statements shall include that (i) both immediately before and after giving effect to the Initial Loan, (x) the representations and warranties set forth in each Loan Document shall, in each case, be true and correct and (y) no Default shall have then occurred and be continuing, or would result from the Initial Loan being advanced on the Closing Date and (ii) all of the conditions set forth inSection 5.1 have been satisfied. All documents and agreements required to be appended to the Closing Date Certificate, if any, shall be in form and substance satisfactory to the Lender, shall have been executed and delivered by the requisite parties, and shall be in full force and effect.
Section 5.1.3Delivery of Notes. The Lender shall have received a Note for the Initial Loan duly executed and delivered by an Authorized Officer of the Borrower.
Section 5.1.4Financial Information, Etc. The Lender shall have received:
(a) audited consolidated financial statements of Holdco and its Subsidiaries for each of the Fiscal Year ended December 31, 2013; and
(b) unaudited consolidated balance sheets of Holdco and its Subsidiaries for each Fiscal Quarter ended after December 31, 2013 and at least ten Business Days prior to the Closing Date, together with the related consolidated statement of operations, shareholder’s equity and cash flows for such Fiscal Quarter.
Section 5.1.5Compliance Certificate. The Lender shall have received an initial Compliance Certificate, prepared on a pro forma basis as of March 31, 2014, consistent in form with the pro forma financial statements included in the Definitive Proxy Statement on Schedule 14A of Holdco, and giving effect to the Initial Loan, the Merger Transaction and the Private Placement (as defined in the Merger Agreement), dated as of the Closing Date, duly executed (and with all schedules thereto duly completed) and delivered by the chief financial or accounting Authorized Officer of each of the Borrower and Holdco.
Section 5.1.6Solvency, Etc. The Lender shall have received, a solvency certificate duly executed and delivered by the chief financial or accounting Authorized Officer of the Borrower, dated as of the Closing Date, in form and substance satisfactory to the Lender.
Section 5.1.7Pledge and Security Agreement. The Lender shall have received executed counterparts of the Pledge and Security Agreement, dated as of the date hereof, duly executed and delivered by each Grantor, together with:
(a) certificates (in the case of Capital Securities that are securities (as defined in the UCC)) evidencing all of the issued and outstanding capital Securities owned by each Grantor, which certificates in each case shall be accompanied by undated instruments of transfer duly executed in blank, or, if any Capital Securities (in the case of Capital Securities that are uncertificated securities (as defined in the UCC)), confirmation and evidence satisfactory to the Lender that the security interest therein has been transferred to and perfected by the Lender in accordance with Articles 8 and 9 of the UCC and all laws otherwise applicable to the perfection of the pledge of such Capital Securities.
(b) financing statements suitable in form for naming each Grantor as a debtor and the Lender as the secured party, or other similar instruments or documents to be filed under the UCC and the PPSA of all jurisdictions as may be necessary or, in the opinion of the Lender, desirable to perfect the security interests of the Lender pursuant to the Pledge and Security Agreement;
(c) UCC Form UCC-3 termination statements and PPSA Form 2C discharge statements, if any, necessary to release all Liens and other rights of any Person (i) in any collateral described in the Pledge and Security Agreement previously granted by any Person, and (ii) securing any of the Indebtedness identified inSchedule 8.2(b), together with such other UCC Form UCC-3 termination statements and PPSA Form 2C discharge statements as the Lender may reasonably request from any Grantor;
(d) evidence that all deposit accounts, lockboxes, disbursement accounts, investment accounts or other similar accounts of each Grantor are Controlled Accounts; and
(e) evidence that all such Controlled Accounts are subject to one or more account control agreement, in favor of, and satisfactory in form and substance to, the Lender.
Section 5.1.8Intellectual Property Security Agreements. The Lender shall have received a Patent Security Agreement, a Copyright Security Agreement and a Trademark Security Agreement, as applicable, each dated as of the Closing Date, duly executed and delivered by each Grantor that, pursuant to the Pledge and Security Agreement, is required to provide such intellectual property security agreements to the Lender.
Section 5.1.9Closing Date Warrant. The Lender shall have received the Closing Date Warrant, dated as of the Closing Date, duly executed, delivered and validly issued by Holdco in favor of the Lender.
Section 5.1.10Insurance. The Lender shall have received, certified copies of the insurance policies (or binders in respect thereof), from one or more insurance companies satisfactory to the Lender, evidencing coverage required to be maintained pursuant to each Loan Document. All such insurance policies required pursuant to this Section shall (i) name the Lender as mortgagee (in the case of property insurance) or loss payee or additional insured (in the case of liability insurance), as applicable, and provide that no cancellation or modification of the policies will be made without the prior written consent of the Lender and (ii) be in addition to any requirements to maintain specific types of insurance contained in the other Loan Documents.
Section 5.1.11Material Agreements.The Lender shall be satisfied, in its sole discretion, with the terms, conditions and other provisions of each Material Agreement.
Section 5.1.12Opinions of Counsel. The Lender shall have received opinions, dated the Closing Date and addressed to the Lender, from
(a) Richardson & Patel, LLP, New York counsel to each Loan Party and its respective Subsidiaries, in form and substance satisfactory to the Lender; and
(b) Borden Ladner Gervais LLP, Canadian counsel to each Loan Party, in form and substance satisfactory to the Lender.
Section 5.1.13Closing Fees, Expenses, Etc. The Lender shall have received for its own account, all fees, costs and expenses due and payable pursuant toSection 11.3 and the Proposal Letter.
Section 5.1.14Equity Investment. The Lender shall have received evidence, satisfactory to it, that Holdco has received an equity investment of no less than $6,000,000 in cash from investors other than the Lender or any of its Affiliates, all on terms and conditions satisfactory to the Lender. The Lender shall have received documentation for such equity investment in form and substance satisfactory to the Lender.
Section 5.1.15Holdco Cash Contribution. Holdco shall have deposited at least $5,250,000 of cash in one or more Controlled Accounts that is free and clear of all Liens, other than Liens granted hereunder in favor of the Lender.
Section 5.1.16Anti-Terrorism Laws. The Lender shall have received, as applicable, all documentation and other information required by bank regulatory authorities under applicable “know your customer” and anti-money laundering rules and regulations, including the U.S.A. Patriot Act.
Section 5.1.17Due Diligence. The Lender shall have received and be satisfied with all due diligence (including without limitation legal, intellectual property, commercial market forecasts clinical and regulatory assessments, supply chain, securities, labor, tax, litigation, environmental, reimbursement and regulatory authority matters) in its sole discretion.
Section 5.1.18Material Adverse Change. No material adverse change shall have occurred in the business, financial performance or condition, operations (including the results thereof), assets, properties or prospects of Holdco and its Subsidiaries, taken as a whole, since December 31, 2013 (after giving pro forma effect to the Merger Transaction).
Section 5.1.19Merger Transaction, Etc. The Merger Transaction shall have been consummated in accordance with the terms of the Merger Agreement (or the Lender shall be satisfied with the arrangements in place for the consummation of the Merger Transaction). The Lender shall be satisfied with the ownership and capital structure of Holdco and its Subsidiaries (including in respect of debt and equity capital and all terms and legal and economic rights related thereto), after giving effect to the Merger Transaction. Without limiting the foregoing, each of the VBI Convertible Notes shall have been converted into shares of common stock of Holdco on terms satisfactory to the Lender. The Lender shall have received all documentation relating to the Merger Transaction and the debt and equity capital structure of Holdco and its Subsidiaries (including the Merger Agreement) and such documentation shall be reasonably satisfactory to the Lender.
Section 5.1.20Board Representatives. The Lender shall designate one individual who will be appointed to the board of directors (or equivalent) of each of Holdco and the Borrower (the “Lender’s Designee”), such Lender’s Designee to be reasonably acceptable to the Borrower. For so long as such Lender’s Designee is a member of the board of directors (or equivalent) of Holdco or the Borrower, the Lender shall not take any action (or advise such Lender’s Designee to take any action) that would cause the Lender’s Designee to be in violation of its fiduciary duties as a director (or equivalent) under applicable Delaware law.
Section 5.1.21Satisfactory Legal Form. All documents executed or submitted pursuant hereto by or on behalf of each Loan Party or any of its respective Subsidiaries shall be satisfactory in form and substance to the Lender and its counsel, and the Lender and its counsel shall have received all information, approvals, resolutions, opinions, documents or instruments as the Lender or its counsel may reasonably request.
Section 5.2Delayed Draw Loan. The obligation of the Lender to make the Delayed Draw Loan shall be subject to the prior making of the Initial Loan, the delivery of a Loan Request for such Delayed Draw Loan as required pursuant toSection 2.2, and the satisfaction of each of the conditions precedent set forth below in thisSection 5.2.
Section 5.2.1Delayed Draw Certificate. The Lender shall have received a certificate, dated as of the Delayed Draw Date and in form and substance satisfactory to the Lender (the “Delayed Draw Certificate”), duly executed and delivered by an Authorized Officer of each of the Borrower and Holdco, in which certificate each of the Borrower and Holdco shall agree and acknowledge, among other things, that the statements made therein shall be deemed to be true and correct representations and warranties of each of the Borrower and Holdco as of such date, and, at the time such certificate is delivered, such statements shall in fact be true and correct, and such statements shall include that (i) both immediately before and after giving effect to the Delayed Draw Loan (x) the representations and warranties set forth in this Agreement and each other Loan Document shall, in each case, be true and correct and (y) no Default shall have then occurred and be continuing, or would result from the Delayed Draw Loan to be advanced on the Delayed Draw Date, and (ii) all of the conditions set forth inSection 5.2 have been satisfied. All documents and agreements required to be appended to the Delayed Draw Certificate, if any, shall be in form and substance reasonably satisfactory to the Lender, shall have been executed and delivered by the requisite parties, and shall be in full force and effect.
Section 5.2.2Milestone Clinical Trial. The Borrower shall have commenced the Milestone Clinical Trial.
Section 5.2.3Delivery of Note. The Lender shall have received a Note for the Delayed Draw Loan duly executed and delivered by an Authorized Officer of the Borrower.
Section 5.2.4Compliance Certificate. The Lender shall have received a Compliance Certificate, prepared on a pro forma basis as if the Delayed Draw Loan had been made as of the first day of the most recently ended Fiscal Quarter for which a report pursuant toSection 7.1(b) has been delivered to the Lender, duly executed (and with all schedules thereto duly completed) and delivered by the chief financial or accounting Authorized Officer of each Loan Party.
Section 5.2.5Closing Fee, Expenses, Etc. The Lender shall have received for its own account, all fees, costs and expenses due and payable pursuant toSection 11.3 and the Proposal Letter.
Section 5.2.6Delayed Draw Warrant. The Lender shall have received the Delayed Draw Warrant, dated as of the Delayed Draw Date, duly executed, delivered and validly issued by the Borrower in favor of the Lender.
Section 5.2.7Disclosure Schedules. Immediately prior to the Delayed Draw Date, the Borrower shall deliver to the Lender updates toSchedules 6.15(a),6.16 and6.23, each such updated Schedule to be complete and accurate as of the Delayed Draw Date.
Section 5.2.8Delayed Draw Date. The Loan Request for the Delayed Draw Loan shall have been submitted to the Lender on or before the second anniversary of the Closing Date.
Section 5.2.9Satisfactory Legal Form. All documents executed or submitted pursuant hereto by or on behalf of the Borrower or any Subsidiary shall be reasonably satisfactory in form and substance to the Lender and its counsel, and the Lender and its counsel shall have received all information, approvals, resolutions, opinions, documents or instruments as the Lender or its counsel may reasonably request.
Article VI
REPRESENTATIONS AND WARRANTIES
In order to induce the Lender to enter into this Agreement and to make the Loans hereunder, each Loan Party jointly and severally represents and warrants to the Lender as set forth in this Article.
Section 6.1Organization, Etc. Each Loan Party is (i) validly organized and existing and in good standing under the laws of the jurisdiction of its incorporation or organization, (ii) to the extent that failure to do so could not reasonably be expected to have a Material Adverse Effect, is duly qualified to do business and is in good standing as a foreign entity in each jurisdiction where the nature of its business requires such qualification, and (iii) has full power and authority and holds all requisite governmental licenses, permits and other approvals to enter into and perform its Obligations under each Loan Document to which it is a party, to own and hold under lease its property and to conduct its business substantially as currently conducted by it.
Section 6.2Due Authorization, Non-Contravention, Etc. The execution, delivery and performance by each Loan Party of each Loan Document executed or to be executed by it are in each case within such Person’s powers, have been duly authorized by all necessary action, and do not:
(a) contravene (i) any Loan Party’s Organic Documents, (ii) any court decree or order binding on or affecting any Loan Party or (iii) any law or governmental regulation binding on or affecting any Loan Party; or
(b) result in (i) or require the creation or imposition of, any Lien on any Loan Party’s properties (except as permitted by this Agreement) or (ii) a default under any material contractual restriction binding on or affecting any Loan Party.
Section 6.3Government Approval, Regulation, Etc. No authorization or approval or other action by, and no notice to or filing with, any Governmental Authority or other Person (other than those that have been, or on the Closing Date will be, duly obtained or made and which are, or on the Closing Date will be, in full force and effect) is required for the due execution, delivery or performance by any Loan Party of any Loan Document to which it is a party. Each Loan Party and its respective properties and businesses are in compliance in all material respects with all laws, rules regulations, orders and court decrees applicable to such Persons, properties or businesses, as the case may be.
Section 6.4Validity, Etc. Each Loan Document to which any Loan Party is a party constitutes the legal, valid and binding obligations of such Person enforceable against such Person in accordance with its respective terms (except, in any case, as such enforceability may be limited by applicable bankruptcy, insolvency, reorganization or similar laws affecting creditors’ rights generally and by principles of equity).
Section 6.5Financial Information. The consolidated financial statements of Holdco and its Subsidiaries furnished to the Lender pursuant toSection 5.1.4 andSection 7.1(a) and(b) have been prepared in accordance with GAAP consistently applied, and present fairly the consolidated financial condition of the Persons covered thereby as at the dates thereof and the results of their operations for the periods then ended.
Section 6.6No Material Adverse Change. Other than the Merger Transaction, there has been no material adverse change in the business, financial performance or condition, operations (including the results thereof), assets, properties or prospects of Holdco and its Subsidiaries, taken as a whole, since December 31, 2013.
Section 6.7Litigation, Labor Matters and Environmental Matters.
(a) Except as described onSchedule 6.7(a), there are no actions, suits or proceedings by or before any arbitrator or Governmental Authority pending against or, to the knowledge of the Borrower and Holdco, threatened against or affecting, any Loan Party (i) as to which there is a reasonable likelihood of an adverse determination and that, if adversely determined, would reasonably be expected, individually or in the aggregate, to result in liabilities in excess of $250,000 or (ii) that would reasonably be likely to adversely affect this Agreement or the transaction contemplated hereby.
(b) There are no labor controversies pending against or, to the knowledge of the Borrower and Holdco, threatened against or affecting any Loan Party (i) that would reasonably be expected, individually or in the aggregate, to result in liabilities in excess of $250,000 or (ii) that would reasonably be likely to adversely affect this Agreement or the transaction contemplated hereby.
(c) No Loan Party (i) has failed to comply with any Environmental Law or to obtain, maintain or comply with any Permit under or in connection with any Environmental Law (“Environmental Permit”), (ii) is or has been subject to any Environmental Liability, (iii) has received notice of any Environmental Liability or (iv) knows of any basis for any Environmental Liability.
Section 6.8Subsidiaries. Holdco has no Subsidiaries, except those Subsidiaries which are identified onSchedule 6.8, or which are permitted to have been organized or acquired in accordance withSection 8.5 orSection 8.8.
Section 6.9Ownership of Properties. Each Loan Party owns (i) in the case of owned real property, good and marketable fee title to, and (ii) in the case of owned personal property, good and valid title to, or, in the case of leased real or personal property, valid and enforceable leasehold interests (as the case may be) in, all of its material properties and assets, tangible and intangible, of any nature whatsoever, free and clear in each case of all Liens or claims, except for Liens permitted pursuant toSection 8.3.
Section 6.10Taxes. Each Loan Party has filed all tax returns and reports required by law to have been filed by it and has paid all Taxes thereby shown to be due and owing, except any such Taxes which are being diligently contested in good faith by appropriate proceedings and for which adequate reserves in accordance with GAAP shall have been set aside on its books.
Section 6.11Pension Plans, Etc. During the twelve-consecutive-month period prior to the Closing Date and prior to the date of any Loans hereunder, no formal steps have been taken to terminate any Pension Plan, and no contribution failure has occurred with respect to any Pension Plan sufficient to give rise to a Lien on any Loan Party or any ERISA Affiliate under Section 303(k) of ERISA or under Section 430(k) of the Code. No condition exists or event or transaction has occurred with respect to any Pension Plan which would reasonably be expected to result in the incurrence by any Loan Party or any ERISA Affiliate of any material liability, fine or penalty. Except as disclosed inSchedule 6.11, neither any Loan Party nor any ERISA Affiliate has any Contingent Liability with respect to any post-retirement benefit under a Welfare Plan, other than liability for continuation coverage described in Part 6 of Title I of ERISA or similar state law. No Pension Plan is a Multiemployer Plan and no Loan Party has any actual or Contingent Liability in respect of such plan.
Section 6.12Accuracy of Information. None of the factual information heretofore or contemporaneously furnished in writing to the Lender by or on behalf of any Loan Party in connection with any Loan Document or any transaction contemplated hereby contains any untrue statement of a material fact, or omits to state any material fact necessary to make any information not misleading.
Section 6.13Regulations U and X. No Loan Party is engaged in the business of extending credit for the purpose of buying or carrying margin stock, and no proceeds of any Loan will be used to purchase or carry margin stock or otherwise for a purpose which violates, or would be inconsistent with, F.R.S. Board Regulation U or Regulation X. Terms for which meanings are provided in F.R.S. Board Regulation U or Regulation X or any regulations substituted therefor, as from time to time in effect, are used in this Section with such meanings.
Section 6.14Solvency. The Borrower, both before and after giving effect to each Loan, is Solvent.
Section 6.15Intellectual Property.
(a)Schedule 6.15(a) sets forth a complete and accurate list of all (i) Patents, (ii) registered and material unregistered Trademarks (including domain names) and any pending registrations for Trademarks and (iii) any other registered Intellectual Property and (iv) any commercially significant unregistered Intellectual Property, in each case owned or licensed by each Loan Party. For each item of Intellectual Property listed onSchedule 6.15(a), the applicable Loan Party has, where relevant, indicated (A) the countries in each case in which such item is patented, (B) the application numbers, (C) the registration or patent numbers, (D) with respect to the Patents, the earliest expected expiration date of the issued Patents as of the Closing Date, (E) the owner of such item of Intellectual Property and (F) with respect to Intellectual Property owned by any third party, the agreement pursuant to which that Intellectual Property is licensed to any Loan Party.
(b) With respect to all Intellectual Property of the Loan Parties listed onSchedule 6.15(a):
(i) such Loan Party owns or has a valid license to such Intellectual Property free and clear of any and all Liens other than Liens permitted pursuant toSection 8.3 and all such Intellectual Property are in full force and effect, and have not expired, lapsed or been forfeited, cancelled or abandoned;
(ii) such Loan Party has taken commercially reasonable actions to maintain and protect such Intellectual Property and there are no unpaid maintenance or renewal fees payable by such Loan Party that are currently overdue for any of such registered Intellectual Property;
(iii) there is no proceeding challenging the validity or enforceability of any such Intellectual Property, no Loan Party is involved in any such proceeding with any Person and none of the Intellectual Property is the subject of any Other Administrative Proceeding;
(iv) to the knowledge of the Borrower and Holdco, (A) such Intellectual Property is valid, enforceable and subsisting and (B) no event has occurred, and nothing has been done or omitted to have been done, that would effect the validity or enforceability of such Intellectual Property; and
(v) such Loan Party is the sole and exclusive owner of all right, title and interest in and to, all such Intellectual Property that is owned by such Loan Party.
(c) To the knowledge of the Borrower and Holdco, no third party is committing any act of Infringement of any Intellectual Property listed onSchedule 6.15(a).
(d) With respect to each license agreement listed onSchedule 6.15(a), such license agreement (i) is in full force and effect and is binding upon and enforceable against each Loan Party party thereto and, to the knowledge of the responsible officer of the Borrower and Holdco, all other parties party thereto in accordance with its terms, (ii) has not been amended or otherwise modified and (iii) has not suffered a default thereunder. No Loan Party has taken any action that would permit any other Person party to any Material Agreement to have, and to the knowledge of any responsible officer of the Borrower and Holdco, no such Person otherwise has, any defenses, counterclaims or rights of setoff thereunder.
(e) No Loan Party has received written notice from any third party alleging that the conduct of its business (including the development, manufacture, use, sale or other commercialization of any Product) Infringes any Intellectual Property of that third party and, to the knowledge of the Borrower and Holdco, the conduct of its business (including the development, manufacture, use, sale or other commercialization of any Product) does not Infringe any Intellectual Property of any third party.
Section 6.16Material Agreements. Set forth onSchedule 6.16 is a complete and accurate list as of the Closing Date or Delayed Draw Date, as applicable, of all Material Agreements of each Loan Party, with an adequate description of the parties, subject matter thereof and amendments and modifications thereto. Each such Material Agreement (i) is in full force and effect and is binding upon and enforceable against each Loan Party party thereto and, to the knowledge of any responsible officer of the Borrower and Holdco, all other parties thereto in accordance with its terms, (ii) has not been amended or otherwise modified and (iii) has not suffered a default thereunder. No Loan Party has taken any action that would permit any other Person party to any Material Agreement to have, and to the knowledge of any responsible officer of the Borrower and Holdco, no such Person otherwise has, any defenses, counterclaims or rights of setoff thereunder.
Section 6.17Permits. Each Loan Party has all material Permits, including Environmental Permits, necessary or required for the ownership, operation and conduct of its business and the distribution of the Products. All such Permits are validly held and there are no defaults thereunder.
Section 6.18Regulatory Matters. With respect to each Product:
(a) (i) All regulatory filings required by any Regulatory Authority or in respect of any Regulatory Authorization or Product Authorization with respect to any Product or any Product Development and Commercialization Activities have been made, and all such filings are complete and correct and have complied in all material respects with all applicable laws and regulations, (ii) all clinical and pre-clinical trials, if any, of investigational Products have been and are being conducted by each Loan Party according to all applicable laws and regulations along with appropriate monitoring of clinical investigator trial sites for their compliance, and (iii) each Loan Party has disclosed to the Lender all such regulatory filings and all material communications between representatives of each Loan Party and any Regulatory Authority.
(b) The Borrower and each other Loan Party and the Borrower’s agents and the agents of each other Loan Party are in compliance in all material respects with all applicable statutes, rules and regulations (including all Regulatory Authorizations and Product Authorizations) of all applicable Governmental Authorities, including the FDA, the DOH and all other Regulatory Authorities, with respect to each Product and all Product Development and Commercialization Activities related thereto. The Borrower and each other Loan Party has and maintains in full force and effect all the necessary and requisite Regulatory Authorizations and Product Authorizations. The Borrower and each other Loan Party is in compliance in all material respects with all applicable registration and listing requirements set forth in the FD&C Act, 21 U.S.C. § 360, and all similar applicable laws (whether U.S. or non-U.S.), including the Food and Drugs Act (R.S.C., 1985, c.F-27) in Canada. Each Loan Party adheres in all material respects to all applicable regulations of all Regulatory Authorities with respect to the Products and all Product Development and Commercialization Activities related thereto, including applicable provisions of the FDA’s Quality System regulation as set forth in Title 21 of the Code of Federal Regulations.
(c) Neither the Borrower nor any other Loan Party has received from any Regulatory Authority any notice of adverse findings with respect to any Product or any Product Development and Commercialization Activities related thereto, including any FDA Form 483 inspectional observations, notices of violations, Warning Letters, criminal proceeding notices under Section 305 of the FD&C Act, or any other similar communication from any Regulatory Authority. There have been no seizures conducted or, to the Borrower’s knowledge, threatened by any Regulatory Authority with respect to any Product, and no recalls, market withdrawals, field notifications, notifications of misbranding or adulteration or safety alerts conducted, requested or, to the Borrower’s knowledge, threatened by any Regulatory Authority with respect to any Product, and no recalls, market withdrawals, field notifications, notifications of misbranding or adulteration or safety alerts have been conducted, requested or, to the Borrower’s knowledge, threatened by any Regulatory Authority relating to any Products. Neither the Borrower nor any other Loan Party has received any written notification that remains unresolved from the FDA, the DOH or any other Regulatory Authority indicating any breach or violation of any applicable Product Authorization or Regulatory Authorization, including that any of the Products is misbranded or adulterated as defined in the FD&C Act or the rules and regulations promulgated thereunder.
(d) Neither the Borrower nor any other Loan Party nor any officer, employee or agent thereof, has made an untrue statement of a material fact or fraudulent statements to the FDA, the DOH or any other Regulatory Authority, failed to disclose a material fact required to be disclosed to the FDA, the DOH or any other Regulatory Authority, or committed an act, made a statement, or failed to make a statement that, at the time such disclosure was made (or was not made), could reasonably be expected to provide a basis for the FDA, the DOH or any other Regulatory Authority to invoke its policy respecting Fraud, Untrue Statements of Material Facts, Bribery and Illegal Gratuities, set forth in 56 Fed. Reg. 46191 (September 10, 1991) or any similar policy.
(e) Neither the Borrower nor any other Loan Party has received any written notice that the FDA, the DOH or any other applicable Regulatory Authority has commenced or initiated, or, to the knowledge of the Borrower or any such Loan Party, threatened to commence or initiate, any action to withdraw any Regulatory Authorization or Product Authorization or requested the recall of any Products or commenced or initiated or, to the knowledge of the Borrower or any such Loan Party, threatened to commence or initiate, any action to enjoin any Product Development and Commercialization Activities of the Borrower or any such Loan Party.
(f) The clinical, preclinical, safety and other studies and tests conducted by or on behalf of or sponsored by the Borrower and each other Loan Party, or in respect of which any Products or Product candidates under development have participated, were (and if still pending, are) being conducted in accordance with standard medical and scientific research procedures and all applicable Product Authorizations. The Borrower and each other Loan Party has operated within, and currently is in compliance in all material respects with, all applicable laws, Product Authorizations and Regulatory Authorizations, as well as the rules and regulations of the FDA, the DOH and each other Regulatory Authority, including but not limited to those rules and regulations governing studies for which an investigational new drug application has been filed in accordance with 21 C.F.R. Part 312 and all other rules and laws regarding clinical studies. Neither the Borrower nor any other Loan Party has received any notices or other correspondence from the FDA, the DOH or any other Regulatory Authority requiring the termination or suspension of any clinical, preclinical, safety or other studies or tests used to support regulatory clearance of, or any Product Authorization or Regulatory Authorization for, any Product.
Section 6.19Transactions with Affiliates. Except as set forth onSchedule 6.19, neither the Borrower nor any Subsidiary has entered into, renewed, extended or been a part to, any transaction (including the purchase, sale, lease, transfer or exchange of property or assets of any kind or the rendering of services of any kind) with any Affiliate during the three-year period prior to the Closing Date.
Section 6.20Investment Company Act. No Loan Party is an “investment company” or is “controlled” by an “investment company,” as such terms are defined in, or subject to regulation under, the Investment Company Act of 1940, as amended.
Section 6.21OFAC. No Loan Party or, to the knowledge of the Borrower and Holdco, any Related Party nor any of their respective directors, officers, or employees nor, to the knowledge of the Borrower and Holdco, any agents or other persons acting on behalf of any of the foregoing (a) is currently the target of any Sanctions, (b) is located, organized or residing in any Designated Jurisdiction, (c) is or has been (within the previous five (5) years) engaged in any transaction with, or for the benefit of, any Person who is now or was then the target of Sanctions or who is located, organized or residing in any Designated Jurisdiction or (d) is or has ever been in violation of or subject to an investigation relating to Sanctions. No Loan, nor the proceeds from any Loan, has been or will be used, directly or indirectly, to lend, contribute or provide to, or has been or will be otherwise made available to fund, any activity or business in any Designated Jurisdiction or to fund any activity or business of any Person located, organized or residing in any Designated Jurisdiction or who is the subject of any Sanctions, or in any other manner that will result in any violation by any Person (including the Lender and its Affiliates) of Sanctions.
Section 6.22Anti-Corruption. No Loan Party or, to the knowledge of the Borrower and Holdco, any Related Party, nor any of their respective directors, officers, or employees nor, to the knowledge of the Borrower and Holdco, any agents or other persons acting on behalf of any of the foregoing, directly or indirectly, has (a) violated or is in violation of any applicable anti-corruption law, (b) made, offered to make, promised to make or authorized the payment or giving of, directly or indirectly, any Prohibited Payment and (c) been subject to any investigation by any Governmental Authority with regard to any actual or alleged Prohibited Payment.
Section 6.23Deposit and Disbursement Accounts.Schedule 6.23 contains a list as of the Closing Date or the Delayed Draw Date, as applicable, of all banks and other financial institutions at which each Loan Party maintains deposit accounts, lockboxes, disbursement accounts, investment accounts or other similar accounts, and such Schedule correctly identifies the name, address and telephone number of each bank or financial institution, the name in which the account is held, the type of account, and the complete account number therefor.
Section 6.24Registration Rights. Except as set forth onSchedule 6.24, no Loan Party has granted or agreed to grant any registration rights, including piggyback rights (other than in respect of the Closing Date Warrant and the Delayed Draw Warrant), to any Person.
Section 6.25Royalty and Other Payments. Except as set forth onSchedule 6.25, no Loan Party is obligated to pay any royalty, milestone payment, deferred payment or any other contingent payment in respect of any Product.
Article VII
AFFIRMATIVE COVENANTS
Each Loan Party jointly and severally covenants and agrees with the Lender that until the Termination Date has occurred, each Loan Party will perform the obligations set forth below.
Section 7.1Financial Information, Reports, Notices, Etc. The Borrower will furnish the Lender copies of the following financial statements, reports, notices and information:
(a) as soon as available and in any event within 25 days after the end of each calendar month, an unaudited consolidated balance sheet of Holdco and its Subsidiaries as of the end of such month, and consolidated statements of income and cash flow of Holdco and its Subsidiaries for such applicable period, including (in each case), in comparative form, the figures for the corresponding month in, and year to date portion of, the immediately preceding Fiscal Year, certified as complete and correct by the chief financial or accounting Authorized Officer of Holdco (subject to normal year-end audit adjustments);
(b) as soon as available and in any event within 45 days after the end of each Fiscal Quarter, an unaudited consolidated balance sheet of Holdco and its Subsidiaries as of the end of such Fiscal Quarter, and consolidated statements of income and cash flow of Holdco and its Subsidiaries for such period, including (in each case), in comparative form, the figures for the corresponding Fiscal Quarter in, and year to date portion of, the immediately preceding Fiscal Year, certified as complete and correct by the chief financial or accounting Authorized Officer of Holdco (subject to normal year-end audit adjustments);
(c) commencing with the Fiscal Year ending December 31, 2014, as soon as available and in any event within 90 days after the end of each Fiscal Year, a copy of the consolidated balance sheet of Holdco and its Subsidiaries, and the related consolidated statements of income and cash flow of Holdco and its Subsidiaries for such Fiscal Year, setting forth in comparative form the figures for the immediately preceding Fiscal Year, audited (without any Impermissible Qualification) by independent public accountants acceptable to the Lender, which shall include a calculation of the financial covenants set forth inSection 7.19 and stating that, in performing the examination necessary to deliver the audited financial statements of Holdco, no knowledge was obtained of any Event of Default;
(d) concurrently with the delivery of the financial information pursuant toclauses (a) and(b), a Compliance Certificate, executed by the chief financial or accounting Authorized Officer of each of the Borrower and Holdco, (i) showing compliance with the financial covenants set forth inSection 7.19 and stating that no Default has occurred and is continuing (or, if a Default has occurred, specifying the details of such Default and the action that Holdco or any of its Subsidiaries has taken or proposes to take with respect thereto) and (ii) stating that no Subsidiary has been formed or acquired since the delivery of the last Compliance Certificate (or, if a Subsidiary has been formed or acquired since the delivery of the last Compliance Certificate, a statement that such Subsidiary has complied withSection 7.8);
(e) as soon as available upon approval of the board of directors of Holdco, but in any event within 30 days after the end of each Fiscal Year, an annual budget, a business plan and financial forecasts of Holdco and its Subsidiaries for the then current Fiscal Year of Holdco, in form and substance as approved by the board of directors of Holdco, which shall include at least a projection of income and a projected cash flow statement for each Fiscal Quarter in such Fiscal Year and a projected balance sheet as of the end of each Fiscal Quarter in such Fiscal Year, in each case prepared in reasonable detail, with appropriate presentation and discussion (in reasonable detail) of the principal assumptions upon which such budgets and projections are based, which shall be accompanied by the statement of an Authorized Officer of Holdco to the effect that such budget and projections are based on reasonable and good faith estimates and assumptions made by the management of Holdco for the respective periods covered thereby;
(f) as soon as possible, but in any event within (i) three Business Days after the Borrower or Holdco obtains knowledge of the occurrence of an Event of Default described inSection 9.1.1 or (ii) five Business Days after the Borrower or Holdco obtains knowledge of the occurrence of any other Event of Default, in each case a statement of an Authorized Officer of the Borrower setting forth details of such Event of Default and the action which the Borrower has taken and proposes to take with respect thereto;
(g) as soon as possible and in any event within five Business Days after the Borrower or Holdco obtains knowledge of (i) the occurrence of any material adverse development with respect to any litigation, action, proceeding or labor controversy described inSchedule 6.7(a) or (ii) the commencement of any litigation, action, proceeding or labor controversy of the type and materiality described inSection 6.7, notice thereof and, to the extent the Lender requests, copies of all documentation relating thereto;
(h) as soon as possible and in any event within five Business Days after the Borrower or Holdco obtains knowledge of any return, recovery, dispute or claim related to any Product or inventory that involves more than $250,000, written notice thereof from an Authorized Officer of the Borrower which notice shall include any statement setting forth details of such return, recovery, dispute or claim;
(i) promptly upon becoming aware of (i) the institution of any steps by any Person to terminate any Pension Plan, (ii) the failure of any Loan Party or any ERISA Affiliate to make a required contribution to any Pension Plan if such failure is sufficient to give rise to a Lien on any Loan Party or any ERISA Affiliate under Section 303(k) of ERISA or under Section 430(k) of the Code, (iii) the taking of any action with respect to a Pension Plan which would reasonably be expected to result in the requirement that any such Person furnish a bond or other security to the PBGC or such Pension Plan, or (iv) the occurrence of any event with respect to any Pension Plan which would reasonably be expected to result in the incurrence by any Loan Party or any ERISA Affiliate of any material liability, fine or penalty, notice thereof and copies of all documentation relating thereto, written notice thereof from an Authorized Officer of the Borrower, which notice shall include a statement setting forth details of such events;
(j) promptly after the filing thereof, written notice (which may be in the form of an email) of all reports, notices, prospectuses and registration statements which Holdco or any of its Subsidiaries files with the SEC;
(k) promptly upon receipt thereof, copies of all formal “management letters” (or equivalent) submitted to Holdco or any of its Subsidiaries by the independent public accountants referred to inclause (b) in connection with each audit made by such accountants; and
(l) such other financial and other information as the Lender may from time to time reasonably request (including information and reports in such detail as the Lender may request with respect to the terms of and information provided pursuant to the Compliance Certificate).
Section 7.2Maintenance of Existence; Compliance with Contracts, Laws, Etc. Each Loan Party will (i) preserve and maintain its legal existence (except as otherwise permitted bySection 8.8), (ii) perform in all material respects its obligations under each Material Agreement to which it is a party, and (iii) comply in all material respects with all applicable laws, rules, regulations and orders, including (x) the FD&C Act and the PDMA and in connection with the preparation and submission to the FDA of NDAs, and (y) the payment (before the same become delinquent) of all Taxes imposed upon such Loan Party or upon its property, except to the extent being diligently contested in good faith by appropriate proceedings and for which adequate reserves in accordance with GAAP have been set aside on the books of such Loan Party, as applicable.
Section 7.3Maintenance of Properties. Each Loan Party will maintain, preserve, protect and keep its and their respective properties in good repair, working order and condition (ordinary wear and tear excepted), and make necessary repairs, renewals and replacements so that the business carried on by such Loan Party may be properly conducted at all times, unless such Loan Party determines in good faith that the continued maintenance of such property is no longer economically desirable, necessary or useful to the business of such Loan Party or the Disposition of such property is otherwise permitted bySection 8.8 orSection 8.9.
Section 7.4Insurance. Each Loan Party will maintain:
(a) insurance on its property with financially sound and reputable insurance companies against loss and damage in at least the amounts (and with only those deductibles) customarily maintained, and against such risks as are typically insured against in the same general area, by Persons of comparable size engaged in the same or similar business as such Loan Party; and
(b) all worker’s compensation, employer’s liability insurance or similar insurance as may be required under the laws of any state or jurisdiction in which it may be engaged in business.
Without limiting the foregoing, all insurance policies required pursuant to this Section shall (i) name the Lender as mortgagee (in the case of property insurance) or loss payee or additional insured (in the case of liability insurance), as applicable, and provide that no cancellation or modification of the policies will be made without the prior written consent of the Lender and (ii) be in addition to any requirements to maintain specific types of insurance contained in the other Loan Documents.
Section 7.5Books and Records. Each Loan Party will keep books and records that accurately reflect all of its business affairs and transactions and permit the Lender or any of its respective representatives, at reasonable times and intervals upon reasonable notice to the Borrower, to visit such Loan Party’s offices, to discuss such Loan Party’s financial matters with its officers and employees, and its independent public accountants (and such Loan Party hereby authorizes such independent public accountant to discuss such Loan Party’s financial matters with the Lender or its representatives whether or not any representative of such Loan Party is present) and to examine (and photocopy extracts from) any of its books and records. Each Loan Party shall pay any fees of such independent public accountant incurred in connection with the Lender’s exercise of its rights pursuant to this Section.
Section 7.6Environmental Law Covenant. Each Loan Party will (i) use and operate all of its and their businesses, facilities and properties in material compliance with all Environmental Laws, and keep and maintain all Environmental Permits and remain in compliance therewith, and (ii) promptly notify the Lender of, and provide the Lender with copies of all material claims, complaints, notices or inquiries relating to, any actual or alleged non-compliance with any Environmental Laws or Environmental Permits or any actual or alleged Environmental Liabilities. Each Loan Party will promptly resolve, remedy and mitigate any such non-compliance or Environmental Liabilities, and shall keep the Lender informed as to the progress of same.
Section 7.7Use of Proceeds. Proceeds of the Loans shall be used for general corporate purposes of Holdco and its Subsidiaries, including the payment of fees, costs and expenses related to the transactions contemplated hereby.
Section 7.8Future Guarantors, Security, Etc. Each Loan Party will execute any documents, UCC-1 financing statements, UCC-3 termination statements, PPSA-1C financing statements, PPSA-2C discharge statements, agreements and instruments, and take all further action that may be required under applicable law, or that the Lender may reasonably request, in order to effectuate the transactions contemplated by the Loan Documents and in order to grant, preserve, protect and perfect the validity and first priority (subject to Liens permitted bySection 8.3) of the Liens created or intended to be created by the Loan Documents. Holdco will promptly cause any Subsidiary acquired or organized after the date hereof to execute a supplement (in form and substance reasonably satisfactory to the Lender) to this Agreement and each other applicable Loan Document in favor of the Lender. The Borrower will promptly notify the Lender of any subsequently acquired real property of any Loan Party and will provide the Lender with a description of such real property, the acquisition date thereof and the purchase price therefor. In addition, from time to time, each Loan Party will, at its cost and expense, promptly secure the Obligations by pledging or creating, or causing to be pledged or created, perfected Liens with respect to such of its assets and properties as the Lender shall designate, it being agreed that it is the intent of the parties that the Obligations shall at all times be secured by, among other things, substantially all the assets of Holdco and its Subsidiaries (including personal property acquired subsequent to the Closing Date), except for the Excluded Collateral. Such Liens will be created under the Loan Documents in form and substance reasonably satisfactory to the Lender, and each Loan Party shall deliver or cause to be delivered to the Lender all such instruments and documents (including legal opinions and lien searches) as the Lender shall reasonably request to evidence compliance with thisSection 7.8.
Section 7.9Obtaining of Permits, Etc. With respect to Products, each Loan Party shall obtain, maintain and preserve, and take all necessary action to timely renew all Permits and accreditations which are necessary in the proper conduct of its business.
Section 7.10Product Licenses. Each Loan Party shall (i) maintain each Permit, including each Regulatory Authorization, from, or file any notice or registration in, each jurisdiction in which such Loan Party is required to obtain any Permit or Regulatory Authorization or to file any notice or registration, in order to sell or distribute the Products and (ii) promptly provide evidence of same to the Lender.
Section 7.11Maintenance of Regulatory Authorizations, Contracts, Intellectual Property, Etc. With respect to the Products, each Loan Party will (i) maintain in full force and effect all Regulatory Authorizations (including the Product Authorizations), contract rights, or other rights necessary for the operations of its business, (ii) notify the Lender, promptly after learning thereof, of any product recalls, safety alerts, corrections, withdrawals, marketing suspensions, removals or the like conducted, to be undertaken or issued by any Loan Party or its respective suppliers whether or not at the request, demand or order of any Governmental Authority or otherwise with respect to any Product, or any basis for undertaking or issuing any such action or item, (iii) maintain in full force and effect, and pay all costs and expenses relating to, all Intellectual Property owned or controlled by any Loan Party that is used in the operations of the business of such Loan Party, or in connection with any Product Development and Commercialization Activities, and all Material Agreements, (iv) notify the Lender, promptly after learning thereof, of any Infringement or other violation by any Person of its Intellectual Property that is used in the operations of the business of such Loan Party, or in connection with any Product Development and Commercialization Activities, and aggressively pursue any such Infringement or other violation except in any specific circumstances where both (x) such Loan Party is able to demonstrate that it is not commercially reasonable to do so and (y) where not doing so does not materially adversely affect any Product, (v) use commercially reasonable efforts to pursue and maintain in full force and effect legal protection for all new Intellectual Property developed or controlled by such Loan Party that is used in the operations of the business of such Loan Party, or in connection with any Product Development and Commercialization Activities, and (vi) notify the Lender, promptly after learning thereof, of (x) any claim by any Person that the conduct of such Loan Party’s business (including the development, manufacture, use, sale or other commercialization of any Product) Infringes any Intellectual Property of such Loan Party and, if requested by the Lender, use commercially reasonable efforts to resolve such claim, or (y) any event, circumstance, act or omission that would cause any representation or warranty contained inSection 6.18 to be incorrect in any material respect if such representation or warranty was to be made at the time such Loan Party learned of such event, circumstance, act or omission.
Section 7.12Inbound Licenses. Prior to any Loan Party entering into or becoming bound by any inbound license or agreement requiring the Borrower to make payments in excess of $1,000,000 in any twelve-month period during the term of such license or agreement (other than over-the-counter software that is commercially available to the public), the Borrower shall: (i) provide written notice to the Lender of the material terms of such license or agreement with a description of its anticipated and projected impact on such Loan Party’s business or financial condition, (ii) obtain written consent of the Lender to such inbound license or agreement, such consent not to be unreasonably withheld and (iii) take such commercially reasonable actions as the Lender may reasonably request to obtain the consent of, or waiver by, any Person whose consent or waiver is necessary for the Lender to be granted and perfect a valid security interest in such license or agreement and to fully exercise its rights under any of the Loan Documents in the event of a disposition or liquidation of the rights, assets or property that is the subject of such license or agreement.
Section 7.13Cash Management. Each Loan Party will:
(a) maintain all deposit accounts, disbursement accounts, investment accounts (and other similar accounts) and lockboxes with a bank or financial institution that has executed and delivered to the Lender an account control agreement, in form and substance reasonably acceptable to the Lender; each such deposit account, disbursement account, investment account (or similar account) and lockbox (each, a “Controlled Account”) shall be a cash collateral account, with all cash, checks and other similar items of payment in such account securing payment of the Obligations, and each Loan Party shall have granted a Lien to the Lender over such Controlled Accounts;
(b) deposit promptly, and in any event no later than five Business Days after the date of receipt thereof, all cash, checks, drafts or other similar items of payment relating to or constituting payments made in respect of any and all accounts and other rights and interests into Controlled Accounts; and
(c) at any time after the occurrence and during the continuance of an Event of Default, at the request of the Lender, each Loan Party will cause all payments constituting proceeds of accounts to be directed into lockbox accounts under agreements in form and substance satisfactory to the Lender.
Section 7.14Modification of Organic Documents. No Loan Party will amend, modify or otherwise change its Organic Documents without the Lender’s prior written consent, which shall not be unreasonably withheld.
Section 7.15Inconsistent Agreements. No Loan Party will enter into any agreement containing any provision which would (i) be violated or breached by such Person hereunder or by the performance by such Person of any of its obligations hereunder or under any other Loan Document, (ii) prohibit any such Person from granting to the Lender a Lien on any of its assets or (iii) create or permit to exist or become effective any encumbrance or restriction on the ability of any Loan Party to (x) pay dividends or make other distributions to the Borrower, or pay any Indebtedness owed to the Borrower, (y) make loans or advances to the Borrower or (z) transfer any of its assets or properties to the Borrower.
Section 7.16Restriction of Amendments to Certain Documents. No Loan Party will amend or otherwise modify, or waive any rights under, any other document, instrument or agreement if, in any case, such amendment, modification or waiver could be materially adverse to the Lender’s Lien in any Collateral (as defined in the Pledge and Security Agreement).
Section 7.17PIC. Holdco’s percentage ownership of PIC’s Capital Securities shall be diluted to approximately 0.01% as provided in the Merger Agreement.
Section 7.18Required Milestones. Holdco and the Borrower covenant and agree that (i) on or before September 30, 2014 the Borrower will have entered into a licensing agreement with a global pharmaceuticals company with respect to the Thermostable LPV technology, on terms satisfactory to the Lender, (ii) on or before September 25, 2015 the Borrower will have commenced toxicology work with respect to the CMV (VLP) Product, in the form of a first immunization of an animal for GLP toxicology, and (iii) on or before April 25 2016 the Borrower will have commenced Phase I clinical trials with respect to the CMV (VLP) Product, in the form of a patient vaccination.
Section 7.19Minimum Liquidity. The Loan Parties shall at all times maintain a minimum aggregate balance of $1,000,000 of cash in one or more Controlled Accounts that is free and clear of all Liens, other than Liens granted hereunder in favor of the Lender.
Article VIII
NEGATIVE COVENANTS
Each Loan Party jointly and severally covenants and agrees with the Lender that until the Termination Date has occurred, each Loan Party will perform or cause to be performed the obligations set forth below.
Section 8.1Business Activities. No Loan Party will engage in any business activity except those business activities engaged in on the date of this Agreement and activities reasonably incidental thereto.
Section 8.2Indebtedness. No Loan Party will create, incur, assume or permit to exist any Indebtedness without the Lender’s prior written consent (which may be withheld in the Lender’s sole discretion), other than:
(a) Indebtedness in respect of the Obligations;
(b) Indebtedness existing as of the Closing Date which is identified inSchedule
8.2(b), and refinancing of such Indebtedness in a principal amount not in excess of that
which is outstanding on the Closing Date (as such amount has been reduced following the Closing Date);
(c) unsecured Indebtedness in respect of performance, surety or appeal bonds provided in the ordinary course of business in an aggregate amount at any time outstanding not to exceed $250,000, but excluding (in each case) Indebtedness incurred through the borrowing of money or Contingent Liabilities in respect thereof;
(d) purchase money Indebtedness and Capitalized Lease Liabilities in an aggregate amount at any time outstanding not to exceed $250,000;
(e) Intercompany Subordinated Debt that does not exceed an aggregate principal amount of $1,500,000 at any time outstanding; and
(f) Subordinated Debt of any Loan Party owing to a non-Affiliate that (i) is unsecured and subject to a written subordination agreement that is satisfactory to the Lender (in form and substance) and (ii) does not exceed an aggregate principal amount of $2,000,000 at any time outstanding;
provided that, no Indebtedness otherwise permitted byclauses (b),(d),(e) or(f) shall be assumed, created or otherwise incurred if a Default has occurred and is then continuing or would result therefrom.
Section 8.3Liens. No Loan Party will create, incur, assume or permit to exist any Lien upon any of its property (including Capital Securities of any Person), revenues or assets, whether now owned or hereafter acquired, except:
(a) Liens securing payment of the Obligations;
(b) Liens existing as of the Closing Date and disclosed inSchedule 8.3(b) securing Indebtedness described inclause (b) ofSection 8.2, and refinancings of such Indebtedness;provided that, no such Lien shall encumber any additional property and the amount of Indebtedness secured by such Lien is not increased from that existing on the Closing Date (as such Indebtedness may have been permanently reduced subsequent to the Closing Date);
(c) Liens in favor of carriers, warehousemen, mechanics, materialmen and landlords granted in the ordinary course of business for amounts not overdue or being diligently contested in good faith by appropriate proceedings and for which adequate reserves in accordance with GAAP shall have been set aside on its books;
(d) Liens incurred or deposits made in the ordinary course of business in connection with worker’s compensation, unemployment insurance or other forms of governmental insurance or benefits, or to secure performance of tenders, statutory obligations, bids, leases or other similar obligations (other than for borrowed money) entered into in the ordinary course of business or to secure obligations on surety and appeal bonds or performance bonds;
(e) judgment Liens in existence for less than 45 days after the entry thereof or with respect to which execution has been stayed or the payment of which is covered in full (subject to a customary deductible) by insurance maintained with responsible insurance companies and which do not otherwise result in an Event of Default underSection 9.1.6;
(f) easements, rights-of-way, zoning restrictions, minor defects or irregularities in title and other similar encumbrances not interfering in any material respect with the value or use of the property to which such Lien is attached;
(g) Liens for Taxes not at the time delinquent or thereafter payable without penalty or being diligently contested in good faith by appropriate proceedings and for which adequate reserves in accordance with GAAP shall have been set aside on its books; and
(h) Liens securing purchase money Indebtedness and Capitalized Lease Liabilities permitted underSection 8.2(d).
Section 8.4[INTENTIONALLY OMITTED].
Section 8.5Investments. No Loan Party will purchase, make, incur, assume or permit to exist any Investment in any other Person, except:
(a) Investments existing on the Closing Date and identified inSchedule 8.5(a);
(b) Cash Equivalent Investments;
(c) Investments received in connection with the bankruptcy or reorganization of, or settlement of delinquent accounts and disputes with, customers and suppliers, in each case in the ordinary course of business;
(d) Investments consisting of any deferred portion of the sales price received by any Loan Party in connection with any Disposition permitted underSection 8.9;
(e) Investments constituting (i) accounts receivable arising, (ii) trade debt granted, or (iii) deposits made in connection with the purchase price of goods or services, in each case in the ordinary course of business; and
(f) Intercompany Subordinated Debt permitted underSection 8.2(e).
Section 8.6Restricted Payments, Etc. No Loan Party will declare or make a Restricted Payment, or make any deposit for any Restricted Payment, other than (i) Restricted Payments made by Loan Parties to the Borrower or to other wholly owned Subsidiaries of Holdco and (ii) distributions of assets of the liquidating trust of Holdco described in Holdco’s Annual Report on Form 10-K for the year ended December 31, 2013 filed with the SEC, as amended and as may be updated in subsequent periodic reports of Holdco filed with the SEC.
Section 8.7[INTENTIONALLY OMITTED]
Section 8.8Consolidation, Merger; Permitted Acquisitions, Etc. No Loan Party will liquidate or dissolve, consolidate with, or merge into or with, any other Person, or purchase or otherwise acquire any other Person or all or substantially all of the assets of any other Person (or any division thereof), except that, so long as no Event of Default has occurred and is continuing (or would occur), (i) any Subsidiary of Holdco (other than the Borrower) may liquidate or dissolve voluntarily into, and may merge with and into, Holdco or any wholly owned Subsidiary of Holdco, (ii) any Loan Party may from time to time acquire another Person or all or substantially all of the assets of another Person (or any division thereof); provided that (v) such acquisition is approved by the boards of director of each of the Borrower and Holdco, (w) the newly acquired (or continuing or surviving) Person shall comply with Section 7.8 hereof and shall be engaged in a line of business similar to the Borrower, (x) the aggregate consideration paid for all acquisitions pursuant to this clause (ii) shall not exceed $5,000,000, (y) the aggregate consideration paid in cash for all such acquisitions pursuant to this clause (ii) shall not exceed $1,500,000, and (z) Holdco and the Borrower shall, prior to consummating any such acquisition, certify in writing to the Lender that, after giving effect to such acquisition, it reasonably expects to comply withSections 7.18 and7.19 hereof.
Section 8.9Permitted Dispositions. No Loan Party will dispose of any of its assets (including accounts receivable and Capital Securities) to any Person in one transaction or series of transactions unless such Disposition (i) is inventory or obsolete, damaged, worn out or surplus property Disposed of in the ordinary course of its business, (ii) has an aggregate fair market value that, when taken together with all other Dispositions made pursuant to thisclause (ii), does not exceed $250,000, (iii) is required pursuant toSection 7.17 or (iv) is an outbound license of Intellectual Property permitted bySection 8.15.
Section 8.10Modification of Certain Agreements. No Loan Party will consent to any amendment, supplement, waiver or other modification of, or enter into any forbearance from exercising any rights with respect to the terms or provisions contained in any Organic Documents of any Loan Party, if the result would have a material adverse effect on the rights or remedies of the Lender.
Section 8.11Transactions with Affiliates. No Loan Party will enter into or cause or permit to exist any arrangement, transaction or contract (including for the purchase, lease or exchange of property or the rendering of services) with any of its other Affiliates, other than Intercompany Subordinated Debt, unless such arrangement, transaction or contract (i) is on fair and reasonable terms no less favorable to such Loan Party than it could obtain in an arm’s-length transaction with a Person that is not an Affiliate and (ii) is of the kind which would be entered into by a prudent Person in the position of such Loan Party with a Person that is not one of its Affiliates.
Section 8.12Restrictive Agreements, Etc. No Loan Party will enter into any agreement prohibiting (i) the creation or assumption of any Lien upon its properties, revenues or assets, whether now owned or hereafter acquired, (ii) the ability of such Loan Party to amend or otherwise modify any Loan Document or (iii) the ability of any Subsidiary to make any payments, directly or indirectly, to the Borrower or Holdco, including by way of dividends, advances, repayments of loans, reimbursements of management and other intercompany charges, expenses and accruals or other returns on investments. The foregoing prohibitions shall not apply to restrictions contained (x) in any Loan Document, or (y) in the case ofclause (i), any agreement governing any Indebtedness permitted byclause (e) ofSection 8.2 as to the assets financed with the proceeds of such Indebtedness.
Section 8.13Sale and Leaseback. No Loan Party will directly or indirectly enter into any agreement or arrangement providing for the sale or transfer by it of any property (now owned or hereafter acquired) to a Person and the subsequent lease or rental of such property or other similar property from such Person.
Section 8.14Product Sales. No Loan Party will sell or distribute Products or cause any sale or distribution where such Loan Party is required to obtain any Permit, or to file any notice or registration in any jurisdiction prior to any such sale or distribution, in each case, until such Loan Party has obtained such required Permit or filed such notice or registration.
Section 8.15Outbound Licenses. So long as no Default has occurred and is continuing, no Loan Party will enter into or become bound by any outbound license of Intellectual Property unless such outbound license (i) is approved by the board of directors of each of the Borrower and Holdco, (ii) is entered into on an arm’s-length basis, on commercially reasonable terms and in the ordinary course of business, (iii) does not otherwise constitute a Disposition prohibited pursuant toSection 8.9, and (iv) does not impair the Lender from fully exercising its rights under any of the Loan Documents in the event of a disposition or liquidation of the rights, assets or property that is the subject of such license or agreement.
Section 8.16Change in Name, Location, Executive Office, or Executive Management; Change in Fiscal Year. No Loan Party will (i) change its legal name or any trade name used to identify it in the conduct of its business or ownership of its properties (other than Holdco in connection with the Merger Transaction), (ii) change its jurisdiction of organization or legal structure, (iii) relocate its chief executive office, principal place of business or any office in which it maintains books or records relating to its business (including the establishment of any new office or facility), (iv) change its federal taxpayer identification number or organizational number (or equivalent) without 30 days prior written notice to the Lender, (v) replace its chief financial officer without written notification to the Lender within 30 days thereafter or (vi) change its Fiscal Year or any of its Fiscal Quarters.
Article IX
EVENTS OF DEFAULT
Section 9.1Listing of Events of Default. Each of the following events or occurrences described in this Article shall constitute an “Event of Default”.
Section 9.1.1Non-Payment of Obligations. The Borrower shall default in the payment or prepayment when due of (i) any principal of or interest on any Loan, or (ii) any fee described inArticle III or any other monetary Obligation, and in the case ofclause (ii) such default shall continue unremedied for a period of two (2) Business Days after such amount was due.
Section 9.1.2Breach of Warranty. Any representation or warranty made or deemed to be made by any Loan Party in any Loan Document (including any certificates delivered pursuant toArticle V) is or shall be incorrect when made or deemed to have been made in any material respect.
Section 9.1.3Non-Performance of Certain Covenants and Obligations. Any Loan Party shall default in the due performance or observance of any of its obligations underSections��7.1(f),7.13,7.17,7.18,7.19 orArticle VIII.
Section 9.1.4Non-Performance of Other Covenants and Obligations. Any Loan Party shall default in the due performance and observance of any other covenant, obligation or agreement contained in any Loan Document executed by it, and such default shall continue unremedied for a period of fifteen (15) days after the earlier to occur of (a) notice thereof given to the Borrower by the Lender or (b) the date on which the Borrower or Holdco has knowledge of such default;provided that, if the Borrower shall default in the due performance and observance of any covenant, obligation or agreement underSection 7.1(c) solely resulting from the inclusion of an Impermissible Qualification, then such fifteen (15) day cure period shall be extended to ninety (90) days;provided further that, if the Borrower delivers to the Lender a detailed plan in form and substance reasonably satisfactory to the Lender and approved by the board of directors of the Borrower addressing such Impermissible Qualification in a commercially reasonable manner, then such ninety (90) day cure period shall be extended for up to an additional ninety (90) days (but in no event shall such cure period exceed one hundred eighty (180) days in the aggregate).
Section 9.1.5Default on Other Indebtedness. A default shall occur in the payment of any amount when due (subject to any applicable grace period), whether by acceleration or otherwise, of any principal or stated amount of, or interest or fees on, any Indebtedness (other than Indebtedness permitted underSection 8.2) of any Loan Party having a principal or stated amount, individually or in the aggregate, in excess of $250,000, or a default shall occur in the performance or observance of any obligation or condition with respect to such Indebtedness if the effect of such default is to accelerate the maturity of any such Indebtedness or such default shall continue unremedied for any applicable period of time sufficient to permit the holder or holders of such Indebtedness, or any trustee or agent for such holders, to cause or declare such Indebtedness to become due and payable or to require such Indebtedness to be prepaid, redeemed, purchased or defeased, or require an offer to purchase or defease such Indebtedness to be made, prior to its expressed maturity.
Section 9.1.6Judgments. Any judgment or order for the payment of money individually or in the aggregate in excess of $250,000 (exclusive of (i) any amounts fully covered by insurance (less any applicable deductible) and as to which the insurer has acknowledged its responsibility to cover such judgment or order and (ii) any amounts indemnified and covered pursuant to the Indemnification Escrow/Reserve and Liquidating Trust Indemnification Agreement (each as provided by and defined in the Merger Agreement)) shall be rendered against any Loan Party and such judgment shall not have been vacated or discharged or stayed or bonded pending appeal within 30 days after the entry thereof or enforcement proceedings shall have been commenced by any creditor upon such judgment or order.
Section 9.1.7Change in Control. Any Change in Control shall occur.
Section 9.1.8Bankruptcy, Insolvency, Etc. Any Loan Party shall:
(a) become insolvent or generally fail to pay, or admit in writing its inability or unwillingness generally to pay, debts as they become due;
(b) apply for, consent to, or acquiesce in the appointment of a trustee, receiver, sequestrator or other custodian for any substantial part of the property of any thereof, or make a general assignment for the benefit of creditors;
(c) in the absence of such application, consent or acquiescence in or permit or suffer to exist the appointment of a trustee, receiver, sequestrator or other custodian for a substantial part of the property of any thereof, and such trustee, receiver, sequestrator or other custodian shall not be discharged within 60 days;provided that, each Loan Party hereby expressly authorizes the Lender to appear in any court conducting any relevant proceeding during such 60-day period to preserve, protect and defend its rights under the Loan Documents;
(d) permit or suffer to exist the commencement of any bankruptcy, reorganization, debt arrangement or other case or proceeding under any bankruptcy or insolvency law or any dissolution, winding up or liquidation proceeding, in respect thereof, and, if any such case or proceeding is not commenced by a Loan Party, such case or proceeding shall be consented to or acquiesced in by a Loan Party, as the case may be, or shall result in the entry of an order for relief or shall remain for 60 days undismissed;provided that, each Loan Party hereby expressly authorizes the Lender to appear in any court conducting any such case or proceeding during such 60-day period to preserve, protect and defend its rights under the Loan Documents; or
(e) take any action authorizing, or in furtherance of, any of the foregoing.
Section 9.1.9Impairment of Security, Etc. Any Loan Document or any Lien granted thereunder shall (except in accordance with its terms), in whole or in part, terminate, cease to be effective or cease to be the legally valid, binding and enforceable obligation of any Loan Party party thereto; any Loan Party shall, directly or indirectly, contest in any manner such effectiveness, validity, binding nature or enforceability; or, except as permitted under any Loan Document, any Lien securing any Obligation shall, in whole or in part, cease to be a perfected first priority Lien.
Section 9.1.10Material Adverse Change. Any circumstance occurs that could reasonably be expected to have a Material Adverse Effect.
Section 9.1.11Key Person Event. If Jeff Baxter ceases to be employed full time by both the Borrower and Holdco and actively working as Chief Executive Officer of each such Person, unless within 30 days after such individual ceases to be employed full time and actively working as President and Chief Executive Officer of each such Person the Borrower or Holdco, as the case may be, hires a replacement for such individual approved by the Lender in its sole discretion.
Section 9.1.12Regulatory Matters. If any of the following occurs: (i) the FDA or any other Governmental Authority initiates enforcement action against, or issues a warning letter with respect to, any Loan Party, or any of their Products or the manufacturing facilities therefor, that causes any Loan Party to discontinue marketing or withdraw any of its material Products, or causes a delay in the manufacture of any of its material Products, which discontinuance, withdrawal or delay could reasonably be expected to last for more than 90 days, (ii) a recall of any Product that has generated or is expected to generate at least $1,000,000 in revenue for Holdco and its Subsidiaries over any consecutive twelve (12) month period or (iii) any Loan Party enters into a settlement agreement with the FDA or any other Governmental Authority that results in aggregate liability as to any single or related series of transactions, incidents or conditions, in excess of $250,000.
Section 9.1.13Pension Plans. Any of the following events shall occur with respect to any Pension Plan:
(a) the institution of any steps by any Loan Party, any ERISA Affiliate or any other Person to terminate a Pension Plan if, as a result of such termination, any Loan Party or any such ERISA Affiliate would be required to make a contribution to such Pension Plan, or would reasonably expect to incur a liability or obligation to such Pension Plan, in excess of $250,000;
(b) a contribution failure occurs with respect to any Pension Plan sufficient to give rise to a Lien on the Borrower or any ERISA Affiliate under section 303(k) of ERISA or under Section 430(k) of the Code; or
(c) any ERISA Event shall occur.
Section 9.2Action if Bankruptcy. If any Event of Default described inclauses (a) through(d) ofSection 9.1.8 with respect to any Loan Party shall occur, the Commitments (if not theretofore terminated) shall automatically terminate and the outstanding principal amount of the Loans and all other Obligations shall automatically be and become immediately due and payable, without notice or demand to any Person.
Section 9.3Action if Other Event of Default. If any Event of Default (other than any Event of Default described inclauses (a) through(d) ofSection 9.1.8) shall occur for any reason, whether voluntary or involuntary, and be continuing, the Lender may, by notice to the Borrower declare all or any portion of the outstanding principal amount of the Loans and other Obligations to be due and payable and/or the Commitments (if not theretofore terminated) to be terminated, whereupon the full unpaid amount of the Loans and other Obligations which shall be so declared due and payable shall be and become immediately due and payable, without further notice, demand or presentment, and the Commitments shall terminate.
Article X
GUARANTY
Section 10.1Guaranty. Each Guarantor hereby agrees that such Guarantor is jointly and severally liable for, and hereby absolutely and unconditionally guarantees to the Lender and its successors and assigns, the full and prompt payment (whether at stated maturity, by acceleration or otherwise) and performance of, all Obligations owed or hereafter owing to Lender by each Loan Party. Each Guarantor agrees that its guaranty obligation hereunder is a continuing guaranty of payment and performance and not of collection, and that its obligations under thisArticle X shall be absolute and unconditional, irrespective of, and unaffected by,
(a) the genuineness, validity, regularity, enforceability or any future amendment of, or change in, this Agreement, any other Loan Document or any other agreement, document or instrument to which any Loan Party is or may become a party;
(b) the absence of any action to enforce this Agreement (including thisArticle X) or any other Loan Document or the waiver or consent by the Lender with respect to any of the provisions thereof;
(c) the existence, value or condition of, or failure to perfect its Lien against, any security for the Obligations or any action, or the absence of any action, by the Lender in respect thereof (including the release of any such security);
(d) the insolvency of any Loan Party; or
(e) any other action or circumstances which might otherwise constitute a legal or equitable discharge or defense of a surety or guarantor,
it being agreed by each Guarantor that its obligations under thisArticle X shall not be discharged until the Termination Date. Each Guarantor shall be regarded, and shall be in the same position, as principal debtor with respect to the Obligations guaranteed hereunder.
Section 10.2Waivers. Each Guarantor expressly waives all rights it may have now or in the future under any statute, or at common law, or at law or in equity, or otherwise, to compel the Lender to marshal assets or to proceed in respect of the Obligations guaranteed hereunder against the Borrower or any other Guarantor, any other party or against any security for the payment and performance of the Obligations before proceeding against, or as a condition to proceeding against, such Guarantor. It is agreed among each Guarantor and the Lender that the foregoing waivers are of the essence of the transaction contemplated by this Agreement and the other Loan Documents and that, but for the provisions of thisArticle X and such waivers, the Lender would decline to enter into this Agreement.
Section 10.3Benefit of Guaranty. Each Guarantor agrees that the provisions of thisArticle X are for the benefit of the Lender and its successors, transferees, endorsees and assigns, and nothing herein contained shall impair, as between the Borrower, on the one hand, and the Lender, on the other hand, the obligations of the Borrower and each Guarantor under the Loan Documents.
Section 10.4Subordination of Subrogation, Etc. Notwithstanding anything to the contrary in this Agreement or in any other Loan Document, each Guarantor hereby expressly and irrevocably subordinates to the prior payment in full, in cash, of the Obligations any and all rights at law or in equity to subrogation, reimbursement, exoneration, contribution, indemnification or set off and any and all defenses available to a surety, guarantor or accommodation co-obligor until all Commitments have expired or been terminated and the Obligations are indefeasibly paid in full in cash. Each Guarantor acknowledges and agrees that this subordination is intended to benefit the Lender and shall not limit or otherwise affect such Guarantor’s liability hereunder or the enforceability of thisArticle X, and that the Lender and its successors and assigns are intended third party beneficiaries of the waivers and agreements set forth in thisSection 10.4.
Section 10.5Election of Remedies. If the Lender may, under applicable law, proceed to realize its benefits under any of the Loan Documents giving the Lender a Lien upon any collateral, whether owned by any Grantor or by any other Person, either by judicial foreclosure or by non-judicial sale or enforcement, the Lender may, at its sole option, determine which of its remedies or rights it may pursue without affecting any of its rights and remedies under thisArticle X. If, in the exercise of any of its rights and remedies, the Lender shall forfeit any of its rights or remedies, including its right to enter a deficiency judgment against any Loan Party or any other Person, whether because of any applicable laws pertaining to “election of remedies” or the like, each Guarantor hereby consents to such action by the Lender and waives any claim based upon such action, even if such action by the Lender shall result in a full or partial loss of any rights of subrogation which each Guarantor might otherwise have had but for such action by the Lender. Any election of remedies which results in the denial or impairment of the right of the Lender to seek a deficiency judgment against the any Loan Party shall not impair any Guarantor’s obligation to pay the full amount of the Obligations. In the event the Lender shall bid at any foreclosure or trustee’s sale or at any private sale permitted by law or the Loan Documents, the Lender may bid all or less than the amount of the Obligations and the amount of such bid need not be paid by the Lender but shall be credited against the Obligations. The amount of the successful bid at any such sale, whether the Lender or any other party is the successful bidder, shall be conclusively deemed to be the fair market value of the collateral and the difference between such bid amount and the remaining balance of the Obligations shall be conclusively deemed to be the amount of the Obligations guaranteed under thisArticle X, notwithstanding that any present or future law or court decision or ruling may have the effect of reducing the amount of any deficiency claim to which the Lender might otherwise be entitled but for such bidding at any such sale.
Section 10.6Limitation. Notwithstanding any provision herein contained to the contrary, each Guarantor’s liability under thisArticle X shall be limited to an amount not to exceed the amount which could be claimed by the Lender from such Guarantor under thisArticle X without rendering such claim voidable or avoidable under Section 548 of Chapter 11 of the United States Bankruptcy Code or under any applicable state Uniform Fraudulent Transfer Act, Uniform Fraudulent Conveyance Act or similar statute or common law after taking into account, among other things, such Guarantor’s right of contribution and indemnification from each other Guarantor.
Section 10.7Liability Cumulative. The liability of each Guarantor under thisArticle X is in addition to and shall be cumulative with all liabilities of each Loan Party to the Lender under this Agreement and the other Loan Documents to which each Loan Party is a party or in respect of any Obligations or obligation of such Loan Party, without any limitation as to amount, unless the instrument or agreement evidencing or creating such other liability specifically provides to the contrary.
Article XI
MISCELLANEOUS PROVISIONS
Section 11.1Waivers, Amendments, Etc. The provisions of each Loan Document may from time to time be amended, modified or waived, if such amendment, modification or waiver is in writing and consented to by the Lender, Holdco and the Borrower.
No failure or delay on the part of the Lender in exercising any power or right under any Loan Document shall operate as a waiver thereof, nor shall any single or partial exercise of any such power or right preclude any other or further exercise thereof or the exercise of any other power or right. No notice to or demand on any Loan Party in any case shall entitle it to any notice or demand in similar or other circumstances. No waiver or approval by the Lender under any Loan Document shall, except as may be otherwise stated in such waiver or approval, be applicable to subsequent transactions. No waiver or approval hereunder shall require any similar or dissimilar waiver or approval thereafter to be granted hereunder.
Section 11.2Notices; Time. All notices and other communications provided under any Loan Document shall be in writing or by facsimile and addressed, delivered or transmitted, if to the Borrower or the Lender, to the applicable Person at its address or facsimile number set forth onSchedule 11.02 hereto, or at such other address or facsimile number as may be designated by such party in a notice to the other parties. Any notice, if mailed and properly addressed with postage prepaid or if properly addressed and sent by pre-paid courier service, shall be deemed given when received; any notice, if transmitted by facsimile, shall be deemed given when the confirmation of transmission thereof is received by the transmitter. Unless otherwise indicated, all references to the time of a day in a Loan Document shall refer to New York City time.
Section 11.3Payment of Costs and Expenses. The Borrower agrees to pay on demand all reasonable expenses of the Lender (including the reasonable fees and out-of-pocket expenses of Morrison & Foerster LLP, counsel to the Lender and of local counsel, if any, who may be retained by or on behalf of the Lender) in connection with:
(a) the negotiation, preparation, execution and delivery of each Loan Document, including schedules and exhibits, and any amendments, waivers, consents, supplements or other modifications to any Loan Document as may from time to time hereafter be required, whether or not the transactions contemplated hereby are consummated; and
(b) the filing or recording of any Loan Document (including any financing statements) and all amendments, supplements, amendment and restatements and other modifications to any thereof, searches made following the Closing Date in jurisdictions where financing statements (or other documents evidencing Liens in favor of the Lender) have been recorded and any and all other documents or instruments of further assurance required to be filed or recorded by the terms of any Loan Document; and
(c) the preparation and review of the form of any document or instrument relevant to any Loan Document;
provided that the Expense Deposit (if any) shall be applied by the Lender from time to time for purposes of satisfying the foregoing expenses of the Borrower.
The Borrower further agrees to pay, and to save the Lender harmless from all liability for, any stamp or other taxes which may be payable in connection with the execution or delivery of each Loan Document, the Loans or the issuance of the Note. The Borrower also agrees to reimburse the Lender upon demand for all reasonable out-of-pocket expenses (including reasonable attorneys’ fees and legal expenses of counsel to the Lender) incurred by the Lender in connection with (i) the negotiation of any restructuring or “work-out” with the Borrower, whether or not consummated, of any Obligations and (ii) the enforcement of any Obligations;provided that the Borrower shall not be liable for indemnification of any expenses under thisclause (ii) to the extent such expenses arise as a result of the bad faith, gross negligence or willful misconduct of the Lender, as finally determined by a court of competent jurisdiction in a non-appealable decision.
Section 11.4Indemnification. In consideration of the execution and delivery of this Agreement by the Lender, the Borrower hereby indemnifies, agrees to defend, exonerates and holds the Lender and each of its officers, directors, employees and agents (collectively, the “Indemnified Parties”) free and harmless from and against any and all actions, causes of action, suits, losses, costs, liabilities, obligations and damages, and expenses incurred in connection therewith (irrespective of whether any such Indemnified Party is a party to the action for which indemnification hereunder is sought), including reasonable attorneys’ and professionals’ fees and disbursements, whether incurred in connection with actions between the parties hereto or the parties hereto and third parties (collectively, the “Indemnified Liabilities”), incurred by the Indemnified Parties or any of them as a result of, or arising out of, or relating to (i) the entering into and performance of any Loan Document by any of the Indemnified Parties (including any action brought by or on behalf of the Borrower as the result of any determination by the Lender pursuant toArticle V not to fund any Loan; provided that, any such action is resolved in favor of such Indemnified Party) or (ii) any Environmental Liability, any actual or alleged breach of or non-compliance with Environmental Laws or Environmental Permits, any Hazardous Materials, or any other decision, act, omission or matter relating to the environment, natural resources, health, safety or welfare. If and to the extent that the foregoing indemnification may be unenforceable for any reason, the Borrower agrees to make the maximum contribution to the payment and satisfaction of each of the Indemnified Liabilities which is permissible under applicable law.
Section 11.5Survival. The obligations of the Borrower underSection 4.1,Section 4.2,Section 4.3,Section 11.3 andSection 11.4, shall in each case survive any assignment by the Lender and the occurrence of the Termination Date. The representations and warranties made by each Loan Party in each Loan Document shall survive the execution and delivery of such Loan Document until the Termination Date.
Section 11.6Severability. Any provision of any Loan Document which is prohibited or unenforceable in any jurisdiction shall, as to such provision and such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions of such Loan Document or affecting the validity or enforceability of such provision in any other jurisdiction.
Section 11.7Headings. The various headings of each Loan Document are inserted for convenience only and shall not affect the meaning or interpretation of such Loan Document or any provisions thereof.
Section 11.8Execution in Counterparts, Effectiveness, Etc. This Agreement may be executed by the parties hereto in several counterparts, each of which shall be an original and all of which shall constitute together but one and the same agreement. This Agreement shall become effective when counterparts hereof executed on behalf of the Borrower, each other Loan Party and the Lender, shall have been received by the Lender. Delivery of an executed counterpart of a signature page to this Agreement by email (e.g. “pdf” or “tiff”) or telecopy shall be effective as delivery of a manually executed counterpart of this Agreement.
Section 11.9Governing Law; Entire Agreement. EACH LOAN DOCUMENT AND ANY CLAIMS, CONTROVERSY, DISPUTE OR CAUSE OF ACTION (WHETHER IN CONTRACT OR TORT OR OTHERWISE) BASED UPON, ARISING OUT OF OR RELATING TO THIS AGREEMENT OR ANY OTHER LOAN DOCUMENT CONTEMPLATED HEREBY AND THEREBY SHALL BE GOVERNED BY, AND CONSTRUED IN ACCORDANCE WITH, THE INTERNAL LAWS OF THE STATE OF NEW YORK (INCLUDING FOR SUCH PURPOSE SECTIONS 5-1401 AND 5-1402 OF THE GENERAL OBLIGATIONS LAW OF THE STATE OF NEW YORK). The Loan Documents constitute the entire understanding among the parties hereto with respect to the subject matter thereof and supersede any prior agreements, written or oral, with respect thereto.
Section 11.10Successors and Assigns. This Agreement shall be binding upon and shall inure to the benefit of the parties hereto and their respective successors and assigns;provided that, no Loan Party may assign or transfer its rights or obligations hereunder without the prior written consent of the Lender.
Section 11.11Other Transactions. Nothing contained herein shall preclude the Lender, from engaging in any transaction, in addition to those contemplated by the Loan Documents, with any Loan Party or any of their respective Affiliates in which such Loan Party or such Affiliate is not restricted hereby from engaging with any other Person.
Section 11.12Forum Selection and Consent to Jurisdiction. ANY LITIGATION BASED HEREON, OR ARISING OUT OF, UNDER, OR IN CONNECTION WITH, ANY LOAN DOCUMENT, OR ANY COURSE OF CONDUCT, COURSE OF DEALING, STATEMENTS (WHETHER ORAL OR WRITTEN) OR ACTIONS OF THE LENDER OR THE LOAN PARTIES IN CONNECTION HEREWITH OR THEREWITH MAY BE BROUGHT AND MAINTAINED IN THE COURTS OF THE BOROUGH OF MANHATTAN IN THE CITY OF NEW YORK IN THE STATE OF NEW YORK OR IN THE UNITED STATES DISTRICT COURT FOR THE SOUTHERN DISTRICT OF NEW YORK; PROVIDED THAT, ANY SUIT SEEKING ENFORCEMENT AGAINST ANY COLLATERAL OR OTHER PROPERTY MAY BE BROUGHT, AT THE LENDER’S OPTION, IN THE COURTS OF ANY JURISDICTION WHERE SUCH COLLATERAL OR OTHER PROPERTY MAY BE FOUND. EACH LOAN PARTY IRREVOCABLY CONSENTS TO THE SERVICE OF PROCESS BY REGISTERED MAIL, POSTAGE PREPAID, OR BY PERSONAL SERVICE WITHIN OR WITHOUT THE STATE OF NEW YORK AT THE ADDRESS FOR NOTICES SPECIFIED INSection 11.2. EACH LOAN PARTY HEREBY EXPRESSLY AND IRREVOCABLY WAIVES, TO THE FULLEST EXTENT PERMITTED BY LAW, ANY OBJECTION WHICH IT MAY HAVE OR HEREAFTER MAY HAVE TO THE LAYING OF VENUE OF ANY SUCH LITIGATION BROUGHT IN ANY SUCH COURT REFERRED TO ABOVE AND ANY CLAIM THAT ANY SUCH LITIGATION HAS BEEN BROUGHT IN AN INCONVENIENT FORUM. TO THE EXTENT THAT EACH LOAN PARTY HAS OR HEREAFTER MAY ACQUIRE ANY IMMUNITY FROM JURISDICTION OF ANY COURT OR FROM ANY LEGAL PROCESS (WHETHER THROUGH SERVICE OR NOTICE, ATTACHMENT PRIOR TO JUDGMENT, ATTACHMENT IN AID OF EXECUTION OR OTHERWISE) WITH RESPECT TO ITSELF OR ITS PROPERTY, EACH LOAN PARTY HEREBY IRREVOCABLY WAIVES TO THE FULLEST EXTENT PERMITTED BY LAW SUCH IMMUNITY IN RESPECT OF ITS OBLIGATIONS UNDER THE LOAN DOCUMENTS.
Section 11.13Waiver of Jury Trial. THE LENDER AND EACH LOAN PARTY HEREBY KNOWINGLY, VOLUNTARILY AND INTENTIONALLY WAIVE TO THE FULLEST EXTENT PERMITTED BY LAW ANY RIGHTS THEY MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION BASED HEREON, OR ARISING OUT OF, UNDER, OR IN CONNECTION WITH, EACH LOAN DOCUMENT, OR ANY COURSE OF CONDUCT, COURSE OF DEALING, STATEMENTS (WHETHER ORAL OR WRITTEN) OR ACTIONS OF THE LENDER OR EACH LOAN PARTY IN CONNECTION THEREWITH. EACH LOAN PARTY ACKNOWLEDGES AND AGREES THAT IT HAS RECEIVED FULL AND SUFFICIENT CONSIDERATION FOR THIS PROVISION (AND EACH OTHER PROVISION OF EACH OTHER LOAN DOCUMENT TO WHICH IT IS A PARTY) AND THAT THIS PROVISION IS A MATERIAL INDUCEMENT FOR THE LENDER ENTERING INTO THE LOAN DOCUMENTS.
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be executed by their respective officers thereunto duly authorized as of the day and year first above written.
| VARIATION BIOTECHNOLOGIES (US), INC., | |
| | as the Borrower | |
| | | |
| | | | |
| | | | |
| By: | /s/ Jeff Baxter | |
| | Name: Jeff Baxter | |
| | Title: Chief Executive Officer | |
| | | | |
| | | | |
| | | | |
| | VBI VACCINES INC., | |
| | as Guarantor | |
| | | | |
| | | | |
| | | | |
| | By: | /s/ Jeff Baxter | |
| | | Name: | |
| | | Title: | |
| | | | |
| | | | |
| | | | |
| | VARIATION BIOTECHNOLOGIES, INC., | |
| | as Guarantor | |
| | | | |
| | | | |
| | | | |
| | By: | /s/ Jeff Baxter | |
| | | Name: Jeff Baxter | |
| | | Title: Chief Executive Officer | |
SIGNATURE PAGE TO CREDIT AGREEMENT AND GUARANTY
| PCOF 1, LLC, | |
| | as the Lender | |
| | | |
| | | |
| | | |
| By: | /s/ Sandeep Dixit | |
| | Name: Sandeep Dixit | |
| | Title: Chief Credit Officer | |
| | | | |
| | | | |
| | | | |
| | By: | /s/ Sam Chawla | |
| | | Name: Sam Chawla | |
| | | Title: Portfolio Manager | |
SIGNATURE PAGE TO CREDIT AGREEMENT AND GUARANTY
Disclosure Schedule to Credit Agreement and Guaranty
Variation Biotechnologies (US), Inc., a Delaware corporation (“Borrower” or “Company”) and VBI Vaccines Inc., a Delaware corporation (“Holdco” or “Parent”) do hereby disclose certain information as called for under, and as exceptions to the representations and warranties contained in the Credit Agreement and Guaranty, dated July 25, 2014, by and among the Borrower and Lender and the Guarantors (as such terms are defined therein) (the “Agreement”). Schedule numbers and references to sections herein correspond to relevant sections of the Agreement. All capitalized terms not otherwise defined herein shall have the meanings set forth in the Agreement or the Merger Agreement.
SCHEDULE 5.1.19
VBI Convertible Notes
● As at May 31, 2014, Borrower has issued USD $15.6 million of convertible notes I - X to existing venture capital investors. The accrued interest as of May 31, 2014 is estimated to be $4.2M as detailed in the following table:
● See AttachedSchedule5.1.19 – Company Convertible Notes I – Xfor a complete list of outstanding convertibles notes and accrued interest to be converted in Company Series A Preferred and then converted into Company Common Shares prior to Closing; all earn 10% interest per annum and mature September 8, 2014; and,
● See Attached Schedule 5.1.19– Company Convertible Notes XIfor a list of convertibles notes issued on March 10, 2014; all earn 5% interest per annum and mature September 8, 2014. The accrued interest as at July 15, 2014 is estimated to be $52,602.74, as detailed in the following table:
Convertible Notes XI Issued Date | | 10-Mar-14 | | | | | | | | | |
Annual Percentage Rate | | 10% | | | | | | | | | |
| | | Amount | | | Interest (to15-Jul-14) | | | Total Interest + Notes | |
5AM Ventures II, LP | | $ | 95,805.89 | | | $ | 3,359.77 | | | $ | 99,165.66 | |
5AM Co-Investors II, LP | | | 3,780.29 | | | | 132.57 | | | | 3,912.86 | |
Arch Venture Fund VI, LP | | | 137,221.65 | | | | 4,812.16 | | | | 142,033.81 | |
Clarus Lifesciences I, L.P. | | | 263,192.17 | | | | 9,229.75 | | | | 272,421.92 | |
Perceptive Advisors LLC | | | 437,500.00 | | | | 15,342.47 | | | | 452,842.47 | |
Titan-Perc Ltd. | | | 62,500.00 | | | | 2,191.78 | | | | 64,691.78 | |
Hudson Bay Master Fund Ltd | | | 250,000.00 | | | | 8,767.12 | | | | 258,767.12 | |
DKR Ventures | | | 250,000.00 | | | | 8,767.12 | | | | 258,767.12 | |
| | | $ | 1,500,000.00 | | | $ | 52,602.74 | | | $ | 1,552,602.74 | |
● | On March 10, Borrower issued convertible notes to new and existing investors in the aggregate principal amount and for total gross proceeds of $1,500,000 (the “Bridge Notes”) which bear interest at 5% per annum, except upon and during the continuation of an event of default the interest rate shall be 15% per annum. The maturity date of the Bridge Notes is September 8, 2014, which may be extended by three months if Borrower fails to close the Merger, or a similar transaction with an Applicable Entity (as defined below) prior to the maturity date despite Borrower reasonable and good faith best efforts to do so and through no fault of Borrower, in which case the maturity date shall be extended by three months for a maturity date (as extended) of December 8, 2014. The maturity date for all other issued and outstanding Notes was amended to be September 8, 2014. |
| | |
| | If prior to the maturity date of the Bridge Notes (i) Borrower completes a financing resulting in aggregate gross proceeds to Borrower of at least $9,000,000.00 (excluding conversion of the Bridge Notes) (a “Qualified Financing”), (ii) the Merger is consummated or (iii) Borrower enters into a similar merger with an alternative NASDAQ listed public company (a “NASDAQ Pubco”) or a company whose common stock is listed in an over-the-counter market maintained by the OTC Markets Group, Inc. (an “OTC Pubco” and together with the PubCo and the NASDAQ Pubco, each an “Applicable Entity,” as applicable), then the Bridge Notes shall be automatically converted into shares of Borrower or the Applicable Entity at a 15% discount to the price per share issued as part of such Qualified Financing or the PIPE. |
| | |
| | If the Merger is not consummated prior to the maturity date of the Bridge Notes or Borrower does not consummate a merger with a NASDAQ Pubco or an OTC Pubco prior to the maturity date of the Bridge Notes, in addition to full repayment of the Bridge Notes and any accrued interest thereon, purchasers of the Bridge Notes shall be entitled to on a pro rata basis based on the principal amount of their respective Bridge Notes, a total amount of common stock of Borrower determined by dividing 15% of the total outstanding principal and interest of the Bridge Notes as of the maturity date by $1.455 per share (as adjusted for any forward or reverse stock splits), which share price is equivalent to 75% of the price per share of Borrower’s Series A Preferred Stock last issued by Borrower. The Bridge Notes are not subject to optional conversion of the holders thereof. |
Variation Biotechnologies (US), Inc. | Schedule5.1.19- VBI Convertible Notes I-X |
Summary of Outstanding Convertible Notes I to X | Page 1 / 2 |
As at Date | |
31-May-14 | |
| | | | | | | | 31-Dec-10 | | | 31-Dec-11 | | | 31-Dec-12 | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Unpaid Principal | | | Interest | | | Interest | | | Interest | | | | | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | 2,525,813.65 | | | $ | 10,233.49 | | | $ | 124,020.03 | | | $ | 218,410.87 | | | $ | 285,800.44 | | | $ | 130,905.77 | | | $ | 769,370.60 | | | $ | 3,295,184.24 | |
| | 5AM Co-Investors II, LP | | $ | 99,663.20 | | | $ | 403.79 | | | $ | 4,893.56 | | | $ | 8,618.02 | | | $ | 11,277.07 | | | $ | 5,165.26 | | | $ | 30,357.71 | | | $ | 130,020.92 | |
| | Arch Venture Fund VI, LP | | $ | 5,348,130.61 | | | $ | 14,657.30 | | | $ | 177,632.42 | | | $ | 312,827.31 | | | $ | 465,527.03 | | | $ | 261,406.84 | | | $ | 1,232,050.90 | | | $ | 6,580,181.52 | |
| | Clarus Lifesciences I, L.P. | | $ | 7,583,325.33 | | | $ | 28,112.81 | | | $ | 340,700.34 | | | $ | 600,005.18 | | | $ | 802,551.21 | | | $ | 387,002.45 | | | $ | 2,158,371.98 | | | $ | 9,741,697.32 | |
| | | | $ | 15,556,932.79 | | | $ | 53,407.39 | | | $ | 647,246.35 | | | $ | 1,139,861.39 | | | $ | 1,565,155.75 | | | $ | 784,480.32 | | | $ | 4,190,151.20 | | | $ | 19,747,083.99 | |
| | | | | | | | | | | | | | | | Cumulative balance | | | $ | 18,962,603.68 | | | $ | 19,747,083.99 | | | | | | | | | |
Detail by Convertible Debt Financing: | | | | | | | | | | | | | | | | - | | | | | | | | | | | | | |
I | Issued Date | | 17-Nov-10 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | 31-Dec-10 | | | 31-Dec-11 | | | 31-Dec-12 | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | Interest | | | Interest | | | Interest | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | 830,049.36 | | | $ | 10,233.49 | | | $ | 84,028.28 | | | $ | 92,431.11 | | | $ | 101,674.22 | | | $ | 46,268.74 | | | $ | 334,635.84 | | | $ | 1,164,685.20 | |
| | 5AM Co-Investors II, LP | | $ | 32,751.96 | | | $ | 403.79 | | | $ | 3,315.58 | | | $ | 3,647.13 | | | $ | 4,011.85 | | | $ | 1,825.66 | | | $ | 13,204.01 | | | $ | 45,955.97 | |
| | Arch Venture Fund VI, LP | | $ | 1,188,869.91 | | | $ | 14,657.30 | | | $ | 120,352.72 | | | $ | 132,387.99 | | | $ | 145,626.79 | | | $ | 66,270.16 | | | $ | 479,294.97 | | | $ | 1,668,164.88 | |
| | Clarus Lifesciences I, L.P. | | $ | 2,280,261.56 | | | $ | 28,112.81 | | | $ | 230,837.44 | | | $ | 253,921.18 | | | $ | 279,313.30 | | | $ | 127,106.68 | | | $ | 919,291.41 | | | $ | 3,199,552.97 | |
| | | | $ | 4,331,932.79 | | | $ | 53,407.39 | | | $ | 438,534.02 | | | $ | 482,387.42 | | | $ | 530,626.16 | | | $ | 241,471.25 | | | $ | 1,746,426.24 | | | $ | 6,078,359.03 | |
II | Issued Date | | 3-Jun-11 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | 31-Dec-11 | | | 31-Dec-12 | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | | | | | Interest | | | Interest | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | 670,641.24 | | | | | | | $ | 38,952.31 | | | $ | 70,959.36 | | | $ | 78,055.29 | | | $ | 35,520.50 | | | $ | 223,487.46 | | | $ | 894,128.70 | |
| | 5AM Co-Investors II, LP | | $ | 26,462.06 | | | | | | | $ | 1,536.97 | | | $ | 2,799.90 | | | $ | 3,079.89 | | | $ | 1,401.56 | | | $ | 8,818.33 | | | $ | 35,280.39 | |
| | Arch Venture Fund VI, LP | | $ | 960,551.53 | | | | | | | $ | 55,790.94 | | | $ | 101,634.25 | | | $ | 111,797.67 | | | $ | 50,875.60 | | | $ | 320,098.46 | | | $ | 1,280,649.99 | |
| | Clarus Lifesciences I, L.P. | | $ | 1,842,345.17 | | | | | | | $ | 107,007.45 | | | $ | 194,935.26 | | | $ | 214,428.79 | | | $ | 97,579.79 | | | $ | 613,951.28 | | | $ | 2,456,296.45 | |
| | | | $ | 3,500,000.01 | | | | | | | $ | 203,287.67 | | | $ | 370,328.77 | | | $ | 407,361.64 | | | $ | 185,377.45 | | | $ | 1,166,355.53 | | | $ | 4,666,355.54 | |
IIIa | Issued Date | | 14-Dec-11 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | 31-Dec-11 | | | 31-Dec-12 | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | | | | | Interest | | | Interest | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | 210,772.97 | | | | | | | $ | 1,039.43 | | | $ | 21,181.24 | | | $ | 23,299.36 | | | $ | 10,602.81 | | | $ | 56,122.84 | | | $ | 266,895.80 | |
| | 5AM Co-Investors II, LP | | $ | 8,316.65 | | | | | | | $ | 41.01 | | | $ | 835.77 | | | $ | 919.34 | | | $ | 418.36 | | | $ | 2,214.49 | | | $ | 10,531.14 | |
| | Arch Venture Fund VI, LP | | $ | 301,887.61 | �� | | | | | | $ | 1,488.76 | | | $ | 30,337.64 | | | $ | 33,371.40 | | | $ | 15,186.27 | | | $ | 80,384.07 | | | $ | 382,271.68 | |
| | Clarus Lifesciences I, L.P. | | $ | 579,022.77 | | | | | | | $ | 2,855.45 | | | $ | 58,187.82 | | | $ | 64,006.60 | | | $ | 29,127.39 | | | $ | 154,177.27 | | | $ | 733,200.04 | |
| | | | $ | 1,100,000.00 | | | $ | - | | | $ | 5,424.66 | | | $ | 110,542.47 | | | $ | 121,596.71 | | | $ | 55,334.83 | | | $ | 292,898.67 | | | $ | 1,392,898.67 | |
IIIb | Issued Date | | 9-Mar-12 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | 31-Dec-12 | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | | | | | | | | | Interest | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | 210,772.97 | | | | | | | | | | | $ | 17,208.31 | | | $ | 22,798.13 | | | $ | 10,374.71 | | | $ | 50,381.15 | | | $ | 261,154.12 | |
| | 5AM Co-Investors II, LP | | $ | 8,316.65 | | | | | | | | | | | $ | 679.00 | | | $ | 899.57 | | | $ | 409.36 | | | $ | 1,987.93 | | | $ | 10,304.59 | |
| | Arch Venture Fund VI, LP | | $ | 301,887.61 | | | | | | | | | | | $ | 24,647.26 | | | $ | 32,653.49 | | | $ | 14,859.57 | | | $ | 72,160.32 | | | $ | 374,047.93 | |
| | Clarus Lifesciences I, L.P. | | $ | 579,022.77 | | | | | | | | | | | $ | 47,273.64 | | | $ | 62,629.64 | | | $ | 28,500.78 | | | $ | 138,404.06 | | | $ | 717,426.83 | |
| | | | $ | 1,100,000.00 | | | $ | - | | | $ | - | | | $ | 89,808.22 | | | $ | 118,980.82 | | | $ | 54,144.42 | | | $ | 262,933.46 | | | $ | 1,362,933.46 | |
IV | Issued Date | | 20-Jun-12 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | 31-Dec-12 | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | | | | | | | | | Interest | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | 229,934.14 | | | | | | | | | | | $ | 12,284.15 | | | $ | 24,221.83 | | | $ | 11,022.59 | | | $ | 47,528.57 | | | $ | 277,462.71 | |
| | 5AM Co-Investors II, LP | | $ | 9,072.71 | | | | | | | | | | | $ | 484.71 | | | $ | 955.74 | | | $ | 434.93 | | | $ | 1,875.38 | | | $ | 10,948.09 | |
| | Arch Venture Fund VI, LP | | $ | 329,331.95 | | | | | | | | | | | $ | 17,594.45 | | | $ | 34,692.64 | | | $ | 15,787.53 | | | $ | 68,074.61 | | | $ | 397,406.56 | |
| | Clarus Lifesciences I, L.P. | | $ | 631,661.20 | | | | | | | | | | | $ | 33,746.28 | | | $ | 66,540.75 | | | $ | 30,280.60 | | | $ | 130,567.63 | | | $ | 762,228.83 | |
| | | | $ | 1,200,000.00 | | | | | | | | | | | $ | 64,109.59 | | | $ | 126,410.96 | | | $ | 57,525.64 | | | $ | 248,046.19 | | | $ | 1,448,046.19 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | Schedule 5.1.19 – VBI Convertible Notes I - X | |
| | | | | | | | | | | | | | | | | | | | | | | | Page 2 / 2 | |
V | Issued Date | | 24-Oct-12 | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | 31-Dec-12 | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | | | | | | | | | Interest | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | 229,934.14 | | | | | | | | | | | $ | 4,346.70 | | | $ | 23,428.08 | | | $ | 10,661.38 | | | $ | 38,436.17 | | | $ | 268,370.30 | |
| | 5AM Co-Investors II, LP | | $ | 9,072.71 | | | | | | | | | | | $ | 171.51 | | | $ | 924.42 | | | $ | 420.68 | | | $ | 1,516.61 | | | $ | 10,589.32 | |
| | Arch Venture Fund VI, LP | | $ | 329,331.95 | | | | | | | | | | | $ | 6,225.73 | | | $ | 33,555.77 | | | $ | 15,270.17 | | | $ | 55,051.67 | | | $ | 384,383.62 | |
| | Clarus Lifesciences I, L.P. | | $ | 631,661.20 | | | | | | | | | | | $ | 11,940.99 | | | $ | 64,360.22 | | | $ | 29,288.31 | | | $ | 105,589.52 | | | $ | 737,250.72 | |
| | | | $ | 1,200,000.00 | | | | | | | | | | | $ | 22,684.93 | | | $ | 122,268.49 | | | $ | 55,640.54 | | | $ | 200,593.96 | | | $ | 1,400,593.96 | |
VI | Issued Date | | 22-Feb-13 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | | | | | | | | | | | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | 143,708.84 | | | | | | | | | | | | | | | $ | 12,323.53 | | | $ | 6,455.04 | | | $ | 18,778.56 | | | $ | 162,487.40 | |
| | 5AM Co-Investors II, LP | | $ | 5,670.45 | | | | | | | | | | | | | | | $ | 486.26 | | | $ | 254.70 | | | $ | 740.96 | | | $ | 6,411.41 | |
| | Arch Venture Fund VI, LP | | $ | 337,428.54 | | | | | | | | | | | | | | | $ | 28,935.65 | | | $ | 15,156.44 | | | $ | 44,092.09 | | | $ | 381,520.63 | |
| | Clarus Lifesciences I, L.P. | | $ | 263,192.17 | | | | | | | | | | | | | | | $ | 22,569.63 | | | $ | 11,821.93 | | | $ | 34,391.56 | | | $ | 297,583.73 | |
| | | | $ | 750,000.00 | | | | | | | | | | | | | | | $ | 64,315.07 | | | $ | 33,688.10 | | | $ | 98,003.17 | | | $ | 848,003.17 | |
VII | Issued Date | | 10-Jun-13 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | | | | | | | | | | | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | - | | | | | | | | | | | | | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | 5AM Co-Investors II, LP | | $ | - | | | | | | | | | | | | | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | Arch Venture Fund VI, LP | | $ | 500,225.84 | | | | | | | | | | | | | | | $ | 28,094.88 | | | $ | 21,856.56 | | | $ | 49,951.43 | | | $ | 550,177.27 | |
| | Clarus Lifesciences I, L.P. | | $ | 249,774.16 | | | | | | | | | | | | | | | $ | 14,028.41 | | | $ | 10,913.48 | | | $ | 24,941.89 | | | $ | 274,716.05 | |
| | | | $ | 750,000.00 | | | | | | | | | | | | | | | $ | 42,123.29 | | | $ | 32,770.03 | | | $ | 74,893.32 | | | $ | 824,893.32 | |
VIII | Issued Date | | 26-Aug-13 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | | | | | | | | | | | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | - | | | | | | | | | | | | | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | 5AM Co-Investors II, LP | | $ | - | | | | | | | | | | | | | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | Arch Venture Fund VI, LP | | $ | 118,403.92 | | | | | | | | | | | | | | | $ | 4,152.25 | | | $ | 5,070.13 | | | $ | 9,222.38 | | | $ | 127,626.30 | |
| | Clarus Lifesciences I, L.P. | | $ | 131,596.08 | | | | | | | | | | | | | | | $ | 4,614.88 | | | $ | 5,635.03 | | | $ | 10,249.90 | | | $ | 141,845.98 | |
| | | | $ | 250,000.00 | | | | | | | | | | | | | | | $ | 8,767.12 | | | $ | 10,705.16 | | | $ | 19,472.28 | | | $ | 269,472.28 | |
IX | Issued Date | | 30-Sep-13 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | | | | | | | | | | | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | - | | | | | | | | | | | | | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | 5AM Co-Investors II, LP | | $ | - | | | | | | | | | | | | | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | Arch Venture Fund VI, LP | | $ | 355,211.75 | | | | | | | | | | | | | | | $ | 9,050.60 | | | $ | 15,069.48 | | | $ | 24,120.08 | | | $ | 379,331.83 | |
| | Clarus Lifesciences I, L.P. | | $ | 394,788.25 | | | | | | | | | | | | | | | $ | 10,058.99 | | | $ | 16,748.47 | | | $ | 26,807.46 | | | $ | 421,595.71 | |
| | | | $ | 750,000.00 | | | | | | | | | | | | | | | $ | 19,109.59 | | | $ | 31,817.96 | | | $ | 50,927.55 | | | $ | 800,927.55 | |
X | Issued Date | | 11-Dec-13 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | 31-Dec-13 | | | 31-May-14 | | | | | | | | | |
| | | | Note | | | | | | | | | | | | | | | Interest | | | Interest | | | Total Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | - | | | | | | | | | | | | | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | 5AM Co-Investors II, LP | | $ | - | | | | | | | | | | | | | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | Arch Venture Fund VI, LP | | $ | 625,000.00 | | | | | | | | | | | | | | | $ | 3,595.89 | | | $ | 26,004.93 | | | $ | 29,600.82 | | | $ | 654,600.82 | |
| | Clarus Lifesciences I, L.P. | | $ | - | | | | | | | | | | | | | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | | | $ | 625,000.00 | | | | | | | | | | | | | | | $ | 3,595.89 | | | $ | 26,004.93 | | | $ | 29,600.82 | | | $ | 654,600.82 | |
Variation Biotechnologies (US), Inc. | Schedule5.1.19- VBI Convertible Notes XI |
Summary of Outstanding Convertible Notes XI | |
As at Date | |
15-Jul-14 | |
XI | Issued Date | | 10-Mar-14 | | | | | | | | | |
| | Day Count Convention | | ACTUAL/ACTUAL | | | | | | | | | |
| | Annual Percentage Rate | | 10% | | | | | |
| | | | | | | | 15-Jul-14 | | | | | |
| | | | Note | | | Interest | | | Total Interest + Notes | |
| | 5AM Ventures II, LP | | $ | 95,805.89 | | | $ | 3,359.77 | | | $ | 99,165.66 | |
| | 5AM Co-Investors II, LP | | $ | 3,780.29 | | | $ | 132.57 | | | $ | 3,912.86 | |
| | Arch Venture Fund VI, LP | | $ | 137,221.65 | | | $ | 4,812.16 | | | $ | 142,033.81 | |
| | Clarus Lifesciences I, L.P. | | $ | 263,192.17 | | | $ | 9,229.75 | | | $ | 272,421.92 | |
| | Perceptive Advisors LLC | | $ | 436,250.00 | | | $ | 15,298.63 | | | $ | 451,548.63 | |
| | Titan-Perc Ltd. | | $ | 63,750.00 | | | $ | 2,235.62 | | | $ | 65,985.62 | |
| | Hudson Bay Master Fund Ltd | | $ | 250,000.00 | | | $ | 8,767.12 | | | $ | 258,767.12 | |
| | DKR Ventures, LLC | | $ | 250,000.00 | | | $ | 8,767.12 | | | $ | 258,767.12 | |
| | | | $ | 1,500,000.00 | | | $ | 52,602.74 | | | $ | 1,552,602.74 | |
SCHEDULE 6.7(a)
Litigation, Labor Matters and Environmental Matters
None.
SCHEDULE 6.8
Subsidiaries
● Variation Biotechnologies (US), Inc., a Delaware corporation
● Variation Biotechnologies Inc., a Canadian company (“Canadian Subsidiary”) incorporated on August 24, 2001 under the Canada Business Corporations Act, is a wholly-owned subsidiary of Borrower.
● ePixis SA (“ePixis”), a French company, was a wholly-owned subsidiary of Canadian Subsidiary. Effective March 1, 2014, ePixis was dissolved and all remaining assets and liabilities were transferred to Borrower Cda.
● Paulson Investment Company, Inc., an Oregon corporation (“PIC”), which upon consummation of the Merger and post-recapitalization of PIC, Holdco will own .1% of PIC.
● Paulson Investment I LLC, an Oregon limited liability company wholly-owned by Holdco.
SCHEDULE 6.11
Pension Plans
None.
SCHEDULE 6.15(a)
Intellectual Property
(a) | All intellectual property listed on Schedules 6.15(a) and (b) is owned by Canadian Subsidiary. |
| | |
| i. | Please see attachment Schedule 6.15(a) for summary of Borrower Patents, updated as of July 11, 2014. |
| | |
| ii. | Please see attachment Schedule 6.15(b) for summary of Borrower Trademarks, updated as of May 27, 2014. |
| | |
| iii. | Domain Names: |
Domain Name | Name of
Registrant | Registration | Renewal Date | Registrar Name and Contact Information |
www.variationbiotech.com | Egidio Nascimento | July 27, 2001 | July 27, 2014 | NAMESCOUT CORP / http://www.namescout.com |
www.variationbiotechnologies.com | Egidio Nascimento | July 27, 2001 | July 27, 2014 | NAMESCOUT CORP / http://www.namescout.com |
www.variationbiotech.ca | Egidio Nascimento | January 3, 2008 | January 3, 2015 | DomainsAtCost Corp. http://www.domainsatcost.ca |
www.Borrowervaccines.com | Egidio Nascimento | November 11, 2010 | November 11, 2014 | DomainsAtCost Corp. http://www.domainsatcost.ca |
www.Borrowervaccines.ca | Egidio Nascimento | November 11, 2010 | November 11, 2014 | DomainsAtCost Corp. http://www.domainsatcost.ca |
www.vbiv.com | Egidio Nascimento | June 5, 2004 | June 5, 2015 | Godaddy.com LLC / Godaddy.com |
www.vbiv.ca | Egidio Nascimento | July 2, 2014 | July 2, 2015 | Go Daddy Domains Canada, Inc / Godaddy.com |
Borrower maintains certain trade secrets, information and proprietary rights as are necessary to the conduct of Borrower business.
(F) Intellectual Property License Agreements
● AMRIC Exclusive License Agreement for Variosite patent families (see Schedule 6.15(a) – patent families VBI-001, VBI-002, VBI-003, VBI-004, VBI-008 and associated VariositeTMTrademarks)
● NRC License Agreement for HEK 293 proprietary manufacturing cell line for eVLPs
● Epixis S.A. UPMC/INSERM License Agreements for eVLP base patents (see Schedule 6.15(a) – patent families P1, P2 and P3)
Schedule 6.15(a) VBI Patent Summary
(July 11th, 2014)
Article I.VARIATION OWNED PEPTIDES PATENTS AND PATENT APPLICATIONS (Published) |
Inventors: José Vidal Torres | Assignee:Variation Biotechnologies Inc. Licensee:AMRIC/Lilly Creek Vaccines |
Patent Family: Immunogenic Formulations of Variable Peptidic Epitopes and Process for Preparation Thereof (Variosite Process Patent – Abstract - A process is disclosed for preparation of a immunogenic peptide mixture in a single synthesis. The peptide mixture collectively represents thein vivo variability seen in immunogenic epitopes from a pathogen. The mixture is termed a hypervariable epitope construct (HEC). Immunization with a HEC evokes broadly reactive immunity against divergent strains of a pathogen upon which the HEC is based.) | Priority Date: October 9, 1998. VBI-001-1 (US 60/103,642) |
Country | Number | Agent | Filing Date Dd/mm/yr | Reference No. | Status or Patent No. |
United States | 12/636,204 Continuation of 11/425,610 Continuation of 10/072,084 CIP of 09/414,484 | Choate (0028) BLG (568B) (568A) | 11/12/09 21/06/06 08/02/02 08/10/99 | VBI-001USCN2 VBI-001USCN VBI-001USCIP VBI-001US | ●abandoned ●abandoned ●Issued; 7,118,874 Issue date 10/10/06; Earliest expected expiration date 08/02/22 ●11.5 year Renewal due 10/04/18 ●abandoned |
PCT | PCT/CA02/00137 WO 03/066090 (publish 14/08/03) | BLG | 08/02/02 | VBI-001PC | ●National Phase ●No priority claim to VBI-001-1 |
Canada | 2,472,265 | BLG | 08/02/02 | VBI-001CA | ●Issued; 2,472,265 Issue date 05/02/13; Earliest expected expiration date 08/02/22 |
Europe | 02711695.3 | BLG | 08/02/02 | VBI-001EP | ●Issued; 1476182 Issue date 09/11/11; Earliest expected expiration date 08/02/22 ●Validated in UK, France & Germany; |
Japan | 2003-565513 | BLG | 08/02/02 | VBI-001JP | ●Issued; 4391827 Issue date 16/10/09; Earliest expected expiration date 08/02/22 |
Australia | 2002231510 | BLG | 08/02/02 | VBI-001AU | ●Issued;2002231510 Issue date 17/07/08; Earliest expected expiration date 08/02/22 |
China | 02828706.1 | BLG | 08/02/02 | VBI-001CN | ●Issued; ZL02828706.1 Issue date 28/07/10; Earliest expected expiration date 08/02/22 |
India | 1995/CHENP/2004 | BLG | 08/02/02 | VBI-001IN | ●Issued; 243388 Issue date 12/10/10; Earliest expected expiration date 08/02/22 |
Mexico | PA/A/2004/007646 | BLG | 08/02/02 | VBI-001MX | ●Issued; 263534 Issue date 07/01/09; Earliest expected expiration date 08/02/22 |
Eurasia | 2004 00897 | BLG | 08/02/02 | VBI-001EA | ●Issued; 008143 Issue date 27/04/07; Earliest expected expiration date 08/02/22 |
Russia | 2004 00897 | BLG | 08/02/02 | VBI-001RU | ●Issued; 008143 RU Issue date 27/04/07; Earliest expected expiration date 08/02/22 |
ARIPO | AP/P/2004/003081 | BLG | 08/02/02 | VBI-001AR | ●Issued; AP 2393 Issue date 30/04/12; Earliest expected expiration date 08/02/22 |
South Africa | 2004/5492 | BLG | 08/02/02 | VBI-001ZA | ●Issued; 2004/5492 Issue date 28/09/05; Earliest expected expiration date 08/02/22 |
Korea | | BLG | 08/02/02 | VBI-001KP | ●Abandoned |
Inventors: José Vidal Torres, David Evander Anderson and Franciso J. Diaz-Mitoma | Assignee:Variation Biotechnologies Inc. Licensee:AMRIC/Lilly Creek Vaccines |
Patent Family: HIV Vaccine Composition (Abstract - An anti-HIV vaccine composition is disclosed. The vaccine comprises an combination of immunogenic peptide mixtures, which mixtures may be prepared in a single synthesis. The composition collectively represents thein vivo variability seen in immunogenic epitopes from highly variable regions of HIV. Immunization with the vaccine elicits broadly reactive immunity (CTL and T helper cell responses) against the divergent strains of HIV upon which it is based. The vaccine may be formulated to target regionally distinct variability based on an HIV clade predominant in a geographical region.) | Priority Date: March 1, 2005 VBI-002-1 (US 60/656,908) |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/CA06/000295 WO 06/092046 (publish 08/09/06) | BLG (2767) | 28/02/06 | VBI-002PC | ●National Phase |
Canada | 2,540,279 | BLG | 28/02/06 | VBI-002CA | ●Issued; 2,540,279 Issue date 20/10/09; Earliest expected expiration date 28/02/26 |
United States | 11/817,640 | BLG | 28/02/06 | VBI-002US | ●Issued; 8,029,797 Issue date 04/10/11; Earliest expected expiration date 28/02/26 |
Europe | 06705249.8 | BLG | 28/02/06 | VBI-002EP | ●Issued; 1861121 Issue date 11/04/12; Earliest expected expiration date 28/02/26 ●Validated in UK, France & Germany |
Inventors: José Vidal Torres | Assignee:Variation Biotechnologies Inc. Licensee:AMRIC/Lilly Creek Vaccines |
Patent Family: Peptide-Based Influenza Vaccine Formulation (Abstract - Peptide-based anti-influenza formulations against influenza A and B are disclosed. The peptides are derived from influenza-based epitopes. The formulations are based on peptide mixtures which may be formulated so that variability is present at particular residues. The formulations can be used to prepare vaccines for preventing influenza in human, avian, murine or equine animals.) | Priority Date: June 1, 2005 VBI-003-1 (US 60/686,041) |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/CA06/000891 WO 06/128294 (publish 07/12/06) | BLG (2849) | 01/06/06 | VBI-003PC | ●National Phase |
Canada | 2,610,667 | BLG | 01/06/06 | VBI-003CA | ●Abandoned |
United States | 11/921,436 | BLG | 01/06/06 | VBI-003US | ●Abandoned |
Europe | 06741591.9 | BLG | 01/06/06 | VBI-003EP | ●Abandoned |
Japan | 2008-513881 | BLG | 01/06/06 | VBI-003JP | ●Issued; 4939531 Issue date 02/03/12; Earliest expected expiration date 01/06/26 |
Mexico | MX/A/2007/015105 | BLG | 01/06/06 | VBI-003MX | ●Abandoned |
India | 9663/DELNP/2007 | BLG | 01/06/06 | VBI-003IN | ●Abandoned |
Singapore | 200718225-6 | BLG | 01/06/06 | VBI-003SG | ●Issued; 137978 Issue date 15/06/10; Earliest expected expiration date 01/06/26 |
China | 200680023950.X | BLG | 01/06/06 | VBI-003CN | ●Issued; ZL20068023950.X Issue date 05/10/11; Earliest expected expiration date 01/06/26 |
Hong Kong | 08105712.8 | BLG | 01/06/06 | VBI-003HK | ●Abandoned |
Korea | 2007-7029582 | BLG | 01/06/06 | VBI-003KR | ●Abandoned |
Inventors: Andrei Ogrel | Assignee:Variation Biotechnologies Inc. Licensee:AMRIC/Lilly Creek Vaccines |
Patent Family: Influenza Vaccine Formulation (Abstract– Peptide-based anti-influenza formulations against influenza are disclosed. The peptides derived from influenza-based epitopes. The formulations are based on peptide mixtures which may be formulated so that variability is present at particular residues. The formulations can be used to prepare vaccines for preventing influenza, particularly avian influenza.) | Priority Date: November 30, 2006 VBI-004-1 (US 60/868,008) |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/CA07/002164 WO 08/064488 (publish 05/06/08) | BLG (3556) | 30/11/07 | VBI-004PC | ●National Phase |
United States | 12/875,638 Divisional of 11/948,505 | BLG/ Choate | 03-09-10 30/11/07 | VBI-004USDV VBI-004US | ●Abandoned ●Issued; 7,807,173 Issue date 05/10/10; Earliest expected expiration date 30/11/27 |
Canada | 2, 670, 965 | BLG | 30/11/07 | VBI-004CA | ●Pending – 1st Office Action Response due 11/09/14 |
Europe | 07855446.6 | BLG | 30/11/07 | VBI-004EP | ●Issued; 2097103 Issue date 03/10/12; Earliest expected expiration date 30/11/27 ● Validated in UK, France, Germany & Switzerland |
India | 3777/CHENP/2009 | BLG | 30/11/07 | VBI-004IN | ●Pending –1st Office Action Response due 23/08/14 |
China | 200780048923.2 | BLG | 30/11/07 | VBI-004CN | ●Issued; ZL200780048923.2 Issue date 22/08/12; Earliest expected expiration date 30/11/27 |
Japan | 2009-538564 | BLG | 30/11/07 | VBI-004JP | ●Issued; 5177451 Issue date 18/01/13; Earliest expected expiration date 30/11/27 |
Inventors: Franciso J. Diaz-Mitoma, Andrei Ogrel, José Vidal Torres and David Evander Anderson | Assignee:Variation Biotechnologies Inc. Licensee:AMRIC/Lilly Creek Vaccines |
Patent Family: Compositions and Methods for Treating Influenza (Abstract – The present application provides compositions and methods useful for treating influenza. As described herein, the compositions and methods are based on the development of peptides and peptide combinations which exhibit immunogenic properties against influenza. In some embodiments, the peptide combinations induce a protective response against multiple strains of influenza, e.g., seasonal strains of influenza or even the new pandemic influenza A (H1N1) virus of swine origin.) | Priority Date: June 19, 2008 VBI-005PC (PCT/US08/067471) Priority Date: May 29, 2009 VBI-008-1 (US 61/182,614) |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US09/047911 WO 09/155489 (publish 23/12/09) | Choate (0019) | 19/06/09 | VBI-008PC | ●National Phase |
Canada | 2,735,724 | Choate (0055) to BLG | 28/02/11 | VBI-008CA | ●Pending – Examination requested 19/06/14 |
United States | 14/161,842 Continuation of 12/999,725 | Choate (0098) to BLG Choate (0052) | 23/01/14 17/12/10 | VBI-008USCN VBI-008US | ●Pending ●Abandoned |
Japan | 2011-514829 | Choate (0060) to BLG | 17/02/11 | VBI-008JP | ●Pending – 1st Office Action Response filed 19/05/14 |
Australia | 2009259964 | Choate (0053) to BLG | 17/01/11 | VBI-008AU | ●Pending – 1st Office Action Response due 15/01/15 |
China | 80132130.8 | Choate (0056) to BLG | 17/02/11 | VBI-008CN | ●Pending – 1st & 2nd Office Action Responses filed |
India | 348/CHENP/2011 | Choate (0058) to BLG | 17/01/11 | VBI-008IN | ●Pending |
Israel | 210097 | Choate (0059) to BLG | 19/01/11 | VBI-008IL | ●Pending – 1st Office Action Response due 16/12/14 |
Korea | 2011-7001217 | Choate (0062) to BLG | 17/01/11 | VBI-008KR | ●Pending – Examination requested 19/06/14 |
VARIATION OWNED HEPATITIS A PATENT APPLICATIONS (Published) |
Inventors: Franciso J. Diaz-Mitoma and Thanh Le | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Hepatitis A (Abstract – The present application provides compositions and methods useful for treating hepatitis A. In particular, while hepatitis A vaccines are currently limited to parenteral administration routes (i.e., intramuscular injection), we have identified compositions that induce a protective response when administered orally.) | Priority Date: September 18, 2008 VBI-006-1 (US 60/098,177) |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US09/057492 WO 10/033812 (publish 25/03/10) | Choate (0020) | 18/09/09 | VBI-006PC | ●National Phase |
United States | 13/119,583 | Choate (0072) | 18/09/09 | VBI-006US | ●Abandoned |
Inventors: Ali Aziz | Owner:Variation Biotechnologies Inc. |
Patent Family: Method and Kit for Detection of Hepatitis A Virus Neutralizing Antibodies | Priority Date: April 15, 2009 VBI-007-1 (US 61/169,344) |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
Canada | 2,700,078 | BLG (6043) | 15/04/10 | VBI-007CA | ●Abandoned |
United States | 12/760,858 | BLG (6043) | 15/04/10 | VBI-007US | ●Abandoned |
VARIATION OWNED FORMULATION PROCESS PATENT APPLICATIONS (Published) |
Inventors: David Evander Anderson and Andrei Ogrel | Owner:Variation Biotechnologies Inc. |
Patent Family: Methods for Preparing Liposomes and Formulation Produced Therefrom | Priority Date(s): July 6, Oct. 30th, 2009 VBI-009-1/VBI-009-2 US 61/223,196 and US 61/256,912 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US10/041078 WO 11/005769 (publish 13/01/11) | Choate (0041) | 06/07/10 | VBI-009PC | ●National Phase |
Canada | 2,767,392 | BLG | 06/07/10 | VBI-009CA | ●Pending – Examination to be requested 06/07/15 |
United States | 13/377,365 | Choate | 06/07/10 | VBI-009US | ●Pending – 1st Office Action Response due 06/09/14 |
Europe | 10797727.4 | BLG | 06/07/10 | VBI-009EP | ●Pending – Response filed to Extended European Search Report |
Japan | 2012-519672 | BLG | 06/07/10 | VBI-009JP | ●Pending |
China | 201080039405.6 | BLG | 06/07/10 | VBI-009CN | ●Pending – 1st & 2nd Office Action Response filed |
Australia | 2010270722 | BLG | 06/07/10 | VBI-009AU | ●Pending |
Brazil | 112012 0008269 | BLG | 06/07/10 | VBI-009BR | ●Pending |
Israel | 217375 | BLG | 06/07/10 | VBI-009IL | ●Pending – Response filed to Examination Notice |
India | 1069/DELNP/2012 | BLG | 06/07/10 | VBI-009IN | ●Pending |
Mexico | MX/A/2012/000372 | BLG | 06/07/10 | VBI-009MX | ●Pending – 1st Office Action Response filed 24/06/14 |
Inventors: David Evander Anderson, Francisco Diaz-Mitoma and Thanh Le | Owner:Variation Biotechnologies Inc. |
Patent Family: Methods for Preparing Liposomes and Formulation Produced Therefrom | Priority Date(s): July 6, Oct. 30th, 2009 VBI-010-1/VBI-010-2 US 61/223,192 and US 61/256, 909 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US10/041081 WO 11/005772 (publish 13/01/11) | Choate (0043) | 06/07/10 | VBI-010PC | ●National Phase |
Canada | 2,803,282 | BLG | 06/07/10 | VBI-010CA | ●Pending – Examination to be Requested 06/07/15 |
United States | 13/377,371 | Choate | 06/07/10 | VBI-010US | ●Pending – 1st Office Action Response filed 30/06/14 |
VARIATION OWNED ORAL FORMULATION PATENT APPLICATIONS (Unpublished) |
Inventors: David Evander Anderson | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Meningococcal and Pneumococcal Infections | Priority Date: December 23, 2009 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
US Provisional | 61/289,603 | Choate (0024) | 23/12/09 | VBI-011-1 | ●Not converted to PCT |
Inventors: David Evander Anderson, Marc J. Kirchmeier, Tanvir Ahmed and Catalina P. Soare | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Enteric Diseases *Priority claim withdrawn prior to publication | Priority Date: December 23, 2009 VBI-012-1US 61/289,615 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US10/061673 | Choate (0045) | 21/12/10 | VBI-012PC | ●PCT withdrawn prior to publication |
Inventors: David Evander Anderson, Francisco Diaz-Mitoma, Thanh Le and Andrei Ogrel | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Influenza *Priority claim withdrawn prior to publication | Priority Date: December 23, 2009 VBI-013-1 US 61/289,580 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US10/61685 | Choate (0047) | 22/12/10 | VBI-013PC | ●PCT withdrawn prior to publication |
Inventors: David Evander Anderson | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Measles, Mumps, and Rubella | Priority Date: December 23, 2009 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
US Provisional | 61/289,586 | Choate (0027) | 23/12/09 | VBI-014-1 | ●Not converted to PCT |
VARIATION OWNED THERMOSTABLE FORMULATION PATENT APPLICATIONS (published) |
Inventors: David Evander Anderson | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Influenza | Priority Date: December 23, 2009 VBI-015-1 61/289,556 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US10/62079 | Choate (0049) | 23/12/10 | VBI-015PC | ●PCT withdrawn prior to publication |
Inventors: David Evander Anderson, Jeff Baxter, Andrei Ogrel and Ron Boch | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Influenza | Priority Date(s): July 6, 2010 & Jan. 10, 2011 VBI-015-2/ VBI-015-4 US 61/361,898 and US 61/431,218 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US11/43094 WO 12/006367 (publish 12/01/12) | Choate (0073) | 06/07/11 | VBI-015PC1 | ●National Phase |
Canada | 2,840,079 | BLG | 06/07/11 | VBI-015CA | ●Pending – Examination to be Requested 06/07/16 |
United States | 13/808,155 | Choate | 06/07/11 | VBI-015US | ●Pending – 1st Office Action response filed |
Europe | 11804305.8 | BLG | 06/07/11 | VBI-015EP | ●Pending |
Japan | 2013-518810 | BLG | 06/07/11 | VBI-015JP | ●Pending – Examination Requested 05/07/14 |
China | 201180042971.7 | BLG | 06/07/11 | VBI-015CN | ●Pending |
Australia | 2011276223 | BLG | 06/07/11 | VBI-015AU | ●Pending – 1st Office Action Response due 14/01/15 |
Brazil | 1120130003944 | BLG | 06/07/11 | VBI-015BR | ●Pending – Examination Requested 05/07/14 |
Israel | 224022 | BLG | 06/07/11 | VBI-015IL | ●Pending |
India | 1077/DELNP/2013 | BLG | 06/07/11 | VBI-015IN | ●Pending – Examination Requested 05/07/14 |
Mexico | MX/A/2012/015232 | BLG | 06/07/11 | VBI-015MX | ●Pending |
Inventors: David Evander Anderson, Jeff Baxter, Andrei Ogrel and Ron Boch | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Influenza | Priority Date(s): July 6, 2010 & Jan. 10, 2011 VBI-015-3/VBI-015-5 US 61/361,899 and US 61/431,278 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US11/43095 WO 12/006368 (publish 12/01/12) | Choate (0074) | 06/07/11 | VBI-015PC2 | ●Not converted at National Phase |
Inventors: David Evander Anderson | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Melanoma | Priority Date: December 23, 2009 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
US Provisional | 61/289,560 | Choate (0030) | 23/12/09 | VBI-016-1 | ●Not converted to PCT |
Inventors: David Evander Anderson | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Hepatitis B | Priority Date: December 23, 2009 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
US Provisional | 61/289,576 | Choate (0031) | 23/12/09 | VBI-017-1 | ●Not converted to PCT |
Inventors: Francisco Diaz-Mitoma, David Evander Anderson and Ali Aziz | Owner: Variation Biotechnologies Inc. |
Patent Family: Compositions and Method for Immunogenic Peptide Vaccines *Priority claim(s) withdrawn prior to publication | Priority Date(s): Dec. 23, 2009 & Feb 5, 2010 VBI-018-1/VBI-018-2 US 61/289,588 and US 61/301,784 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US11/061683 | Choate (0051) | 23/12/10 | VBI-018PC | ●PCT withdrawn prior to publication |
Inventors: David Evander Anderson | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Hepatitis A | Priority Date: March 25, 2009 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
US Provisional | 61/317,421 | Choate (0035) | 25/03/10 | VBI-019-1 | ●Not converted to PCT |
US Provisional | 61/317,418 | Choate (0034) | 25/03/10 | VBI-020-1 | ●Not converted to PCT |
Inventors: Maura Ellen Campbell | Owner:Variation Biotechnologies Inc. |
Patent Family: Synthetic Derivatives of MPL and Uses Thereof | Priority Date(s): November 18, 2011 VBI-021-1/VBI-022-1 61/561,795 and 61/561,797 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US12/65466 PCT/IB12/002855 WO 13/072768 (publish 23/05/13) | Choate (0087) BLG | 18/11/12 | VBI-022PC | ●Pending ●EP Application filed ●US Application filed ●Assignment filed |
Inventors: David Evander Anderson, Tanvir Ahmed, Jasminka Bozic and Marc Kirchmeier | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Viral Infections | Priority Date: January 13, 2011 VBI-023-1 61/432,567 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US12/21388 WO 12/097346 (Publish 19/07/12) | Choate (0080) | 13/01/12 | VBI-023PC | ●National Phase |
Canada | NA | BLG | 13/01/12 | VBI-023CA | ●Reinstate NPE late entry 13/07/14 |
United States | 13/979,322 | BLG | 13/01/12 | VBI-023US | ●Pending – Preliminary Amendment filed |
Europe | 12734104.8 | BLG | 13/01/12 | VBI-023EP | ●Pending – Claim Amendments filed |
China | 201280008709.5 | BLG | 13/01/12 | VBI-023CN | ●Pending |
Brazil | 1120130179392 | BLG | 13/01/12 | VBI-023BR | ●Pending – Examination to be Requested 12/01/15 |
India | 7052/DELNP/2013 | BLG | 13/01/12 | VBI-023IN | ●Pending – Examination to be Requested 12/01/15 |
Mexico | MX/A/2013/008106 | BLG | 13/01/12 | VBI-023MX | ●Pending |
Inventors: David Evander Anderson, Yvonne Perrie, Jit Wilkhu and Marc Kirchmeier | Owner:Variation Biotechnologies Inc. |
Patent Family: Methods for Preparing Vesicles and Formulations Produced Therefrom | Priority Date: January 13, 2011 VBI-024-1 61/432,569 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US12/21389 WO 12/097347 (Publish 19/07/12) | Choate (0081) | 13/01/12 | VBI-024PC | ●National Phase |
Canada | NA | BLG | 13/01/12 | VBI-024CA | ●Reinstate NPE late entry 13/07/14 |
United States | 13/979,317 | BLG | 13/01/12 | VBI-024US | ●Pending – Preliminary Amendment filed |
Europe | 12733900.0 | BLG | 13/01/12 | VBI-024EP | ●Pending – Claim Amendments filed |
China | 201280008692.3 | BLG | 13/01/12 | VBI-024CN | ●Pending |
Australia | 2012205315 | BLG | 13/01/12 | VBI-024AU | ●Pending – Examination to be Requested 12/01/17 |
Brazil | 1120130180749 | BLG | 13/01/12 | VBI-024BR | ●Pending – Examination to be Requested 12/01/15 |
India | 7053/DELNP/2013 | BLG | 13/01/12 | VBI-024IN | ●Pending – Examination to be Requested 12/01/15 |
Mexico | MX/A/2013/008104 | BLG | 13/01/12 | VBI-024MX | ●Pending |
Inventors: David Evander Anderson and Anne-Catherine Fluckiger | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treatment of Cytomegalovirus | Priority Date(s): Nov 11, 2011 and July 1, 2012 VBI-025-1/ VBI-025-2 61/558,800 and 61/654,157 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US12/64556 PCT/IB12/002854 WO 13/068847 (publish 16/05/13) | Choate (0086) BLG | 09/11/12 | VBI-025PC | ●Pending – NPE (31 months) & late entries ●30 month NPE Applications filed 10/05/14 ●Assignment filed |
Inventors: David Evander Anderson | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Treating Viral Infections | Priority Date: January 12, 2012 VBI-026-1 61/585,971 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US13/21277 PCT/IB13/000453 WO 13/104995 (publish 18/07/13) | Choate (0091) BLG | 12/01/13 | VBI-026PC | ●Pending – NPE (30 months) due 11/07/14 ●Assignment filed |
Inventors: Marc Kirchmeier | Owner:Variation Biotechnologies Inc. |
Patent Family: Methods and Compositions for Therapeutic Agents | Priority Date: January 27, 2012 VBI-027-1 61/591,837 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US13/23079 PCT/IB13/000454 WO 13/111012 (publish 01/08/13) | Choate (0092) BLG | 25/01/13 | VBI-027PC | ●Pending – NPE (30 months) due 26/07/14 ●Assignment filed |
Inventors: David Evander Anderson, Jasminka Bozic and Barthelemy Ontsouka | Owner:Variation Biotechnologies Inc. |
Patent Family: Methods for Detection of Anti-Cytomegalovirus Neutralizing Antibodies | Priority Date: January 27, 2012 VBI-028-1 61/616,204 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US13/34157 PCT/IB13/001021 WO 13/144722 (publish 03/10/13) | Choate (0093) BLG | 27/03/13 | VBI-028PC | ●Pending –NPE (30 months) due 26/09/14 ●Assignment filed |
Inventors: David Evander Anderson, and David Klatzmann | Owner:Variation Biotechnologies Inc. |
Patent Family: Compositions and Methods for Inducing a CTL Response | Priority Date(s): February 25, 2013 and March 21, 2013 VBI-029-1/ VBI-029-2 61/769,140 and 61/803,940 |
Country | Number | Agent | Filing Date | Reference No. | Status or Patent No. |
PCT | PCT/US14/18277 PCT/IB14/000815 | Choate(0099) BLG | 25/02/14 | VBI-029PC | ●Pending ●Assignment to be filed |
Inventors: David Klatzmann and Jean-Loup Salzmann | Assignee: Universite Pierre et Marie Curies (Paris VI) Licensee: Variation Biotechnologies Inc. |
Patent Family: Defective Viral Vaccine Particles Obtained in Vivo or Ex Vivo (Particules Virales Defectives Vaccinales Obtenues in Vivo ou ex Vivo) Filed in French (Abstract– The present invention relates to a vaccine consisting of defective viral particles as are obtained in vivo or ex vivo, in individuals infected or capable of being infected with a virus, after expression of the genes carried by a vector or a combination of vectors and comprising at least the structural genes necessary for the constitution of the viral particle.) | Priority Date: April 5, 1996 (FR 96/04370) |
Country | Number | Agent | Filing Date Dd/mm/yr | Reference No. | Status or Patent No. |
PCT | PCT/FR97/00619 WO 97/38118 (published 16/10/97) | Becker B0014 | 07/04/97 | P1-PC | ●National Phase |
United States | 09/166,147 | Becker | 07/04/97 | P1-US | ●Issued; 6,140,114 Issue date 31/10/00; Earliest expected expiration date 07/04/17 |
Europe | EP 97 400796.5 | Becker | 07/04/97 | P1-EP | ●Issued; EP0799893B2 Issue date 12/09/01; Earliest expected expiration date 07/04/17 ●Validated in France, Germany, Great Britain,Spain & Italy |
Canada | 2,251,027 | Becker | 07/04/97 | P1-CA | ●Issued; 2,251,027 Issue date 10/11/09; Earliest expected expiration date 07/04/17 |
Japan | JP 1997-535922 | Becker | 07/04/97 | P1-JP | ●Abandoned |
Inventors: David Klatzmann, Jean-Loup Salzmann, Bertrand Bellier, Charlotte Frisen and Francoise-Loic Cosset | Assignee: Universite Pierre et Marie Curies (Paris VI) Licensee: Variation Biotechnologies Inc. |
Patent Family: Synthetic Viruses and Uses Thereof (Abstract– The present invention relates to compositions and methods for producing an immune response or reaction, as well as to vaccines, kits, processes, cells and uses thereof. This invention more particularly relates to compositions and methods of using a synthetic viral particle to produce, modify or regulate an immune response in a subject. In a more preferred embodiment, the invention is based, generally, on compositions using synthetic viral particles as an adjuvant and/or vehicle to raise immune response against selected antigen(s) or epitopes, in particular a cellular and/or a humoral immune response. | Priority Date: October 26, 2000 (EP 00 402978.1) |
Country | Number | Agent | Filing Date Dd/mm/yr | Reference No. | Status or Patent No. |
PCT | PCT/EP01/12356 WO 02/34893 (published 02/05/02) | Becker B0047 | 25/10/01 | P2-PC | ●National Phase |
United States | 14/163,315 Continuation of 10/415,242 | Choate Becker | 24/01/14 25/10/01 | P2-US2 P2-US1 | ●Pending ●Issued; 8,673,612 Issue date 18/03/14; Earliest expected expiration date 25/10/21 |
Europe | 10 183343.2 Divisional of 01 988766.0 | Becker | 30/09/10 25/10/01 | P2-EP2 P2-EP1 | ●Pending – 1st & 2nd Office Action responses filed ●Notice to Grant; 15/10/13; Earliest expected expiration date 25/10/21 |
Inventors: Birke Bartosch and Francois-Loic Cosset | Assignee: Institut National de la Sante et de la Recherche Medicale (INSERM) Licensee: Variation Biotechnologies Inc. |
Patent Family: Infectious Hepacivirus Pseudo-Particles Containing Functional E1, E2 Envelope Proteins | Priority Date(s): Sept 13, 2002 & March 3, 2003 (EP 02292254.6) & (EP 03290505.1) |
Country | Number | Agent | Filing Date Dd/mm/yr | Reference No. | Status or Patent No. |
PCT | PCT/IB03/003882 WO 04/024904 (published 25/03/04) | Lavoix | 12/09/03 | P3-PC | ●National Phase |
United States | 10/527,422 | Lavoix | 12/09/03 | P3-US | ●Pending – Response filed to RCE |
Europe | 03 795150.6 | Lavoix | 12/09/03 | P3-EP | ●Issued; EP 1 537 206 B1 Issue date 06/05/09; Earliest expected expiration date 12/09/23 ●Validated in France, Germany, Spain, Greece, Italy, Great Britain and Turkey |
Canada | 2,498,770 | Lavoix | 12/09/03 | P3-CA | ●Pending – 3rd Office Action response filed |
Japan | JP 2004-571924 | Lavoix | 12/09/03 | P3-JP | ●Issued; 4634152 Issue date 26/11/10; Earliest expected expiration date 12/09/23 |
Schedule 6.15(b) VBITrademark Summary
(May 27th, 2014)
Variation Owned Trademark Applications |
Trademark Family: Variosite™ | Priority Date: August 11, 2010 |
Country | Number | Agent | Filing Date/ Registration Date (Dd/mm/yr) | Reference No. | Status |
Canada | S.N. 1,491,977 | BLG | 11/08/2010 (Filing date) | TM 102449-1 CA | Pending |
US | S.N. 76/440,352 | BLG | 11/02/2002 (Priority filing date) 09/08/2002 (Filing date) | TM 54051-2 US | Pending |
Mexico | S.N. 1,027,921 (Class 5) R.N. 1120583 | BLG | 19/08/2009 (Filing date) 11/09/2009 (Registration Date) | TM 100380C5-21 | Registered |
Mexico | S.N. 1,027,927 (Class 42) R.N. 1120585 | BLG | 19/08/2009 (Filing date) 11/09/2009 (Registration Date) | TM 100380C42-21 | Registered |
EU | S.N. 2,823,367 R.N. 2,823,367 | BLG | 09/08/2002 (Filing date) 12/03/2004 (Registration Date) | TM 54051-81 | Registered |
Trademark Family: Lipid Particle Vaccine™ | Priority Date: May 20, 2011 |
Country | Number | Agent | Filing Date Dd/mm/yr | Reference No. | Status |
Canada | S.N. 1,528,640 | BLG | 20/05/2011 | TM 103841-1 CA | ●Pending |
Trademark Family: LPV™ | Priority Date: May 20, 2011 |
Country | Number | Agent | Filing Date Dd/mm/yr | Reference No. | Status |
Canada | S.N. 1,528,635 | BLG | 20/05/2011 | TM 103842-1 CA | ●Pending |
SCHEDULE 6.16
Agreements, Actions
| | Material Agreements of Borrower |
| | |
| | See Attachment Schedule 6.16 for list of agreements and contracts of the Borrower. |
| | |
● | Signed a contribution agreement for CAD $499,000 on July 18, 2013 with the National Research Council under their Industrial Research Assistance program. |
● | Amendments to the letter agreements with Middlebury Securities, LLC (“Middlebury”) and Evolution Venture Partners (“Evolution”) : |
o | Initial Term extended from September 30, 2013 to December 31, 2013. |
o | For the balance of the Initial Term, being June 1, 2013 to December 31, 2013, an additional US $50,000 payable upon the successful completion of a transaction (instead of $15,000 per month). |
o | Both agreements were terminated on May 30, 2014 |
o | Success fees payable and shares to be issued under these agreements are summarized below: |
Broker / Agent | Fees | Shares / Warrants |
Middlebury | Success fees based on 6% of funds sourced which are expected to be: ●$300,000 being 6% of the $5,000,000 equity investment by Perceptive Advisors, LLC or 6% of any lesser total amount invested by Perceptive in the Private Placement; and ●Upon the initial drawdown of $3,000,000 in Venture Debt from Perceptive, 6% of such initial drawdown ($180,000) | ●600,000 common shares of Parent shall be issuable to Middlebury and 2,400,000 common shares of Parent to Evolution post-Merger and Private Placement (see below) |
Palladium | Success fees based on 7% of funds sourced which are expected to be: ●$35,000 being 7% of the $500,000 they sourced for the March 10, 2014 convertible notes financing (already paid); and, ●$367,500 being 7% of the $5,250,000 unencumbered cash and cash equivalents owned by Parent at the time of the Merger (net of any unpaid payables or debt of Parent at the time of the Merger) | ●1,708,657 common shares of Parent, representing 1.27% of the total common shares of Parent post-Merger and Private Placement |
Evolution | ●A financial advisory and consulting fees and deferred retainers of $570,000 | ●2,400,000 shares shall be issuable to Evolution, upon the concurrent closings of the Paulson Merger and the Paulson Private Placement, in each case subject to the lock-up agreements described in Section 4(g) of the Middlebury Letter Agreement Amendment #2 |
● | On December 12, 2013 Borrower a signed term sheet with Bezalel Partners, LLC (“Bezalel”) for the “Proposed Acquisition of and Equity Investment in Variation Biotechnologies (US), Inc.” Related to this term sheet, Borrower signed a Nondisclosure and Noncircumvent Agreement with Bezalel on December 18, 2013. The term sheet has been superseded but the Letter of Intent signed with Paulson Capital Corp. as described below. |
| | |
● | On March 7, 2014, Borrower signed an agreement with PALLADIUM CAPITAL ADVISORS, LLC (“Palladium”) whereby Palladium agreed to act its non-exclusive agent to arrange bridge financing prior to the Merger. |
| | |
| | Borrower agreed to pay Palladium, upon the closing of the Bridge with Investors introduced by Palladium 7% of the aggregate consideration raised in each Closing from such Investors introduced by Palladium. In respect of the Bridge consisting of the sale of senior secured convertible promissory notes of Borrower, Palladium acknowledges that the only Investors introduced by Palladium are Hudson Bay Capital and DKR Ventures, which combined are purchased $500,000 in aggregate principle amount of such Bridge notes, therefore requiring Borrower to pay Palladium a total of $35,000 at the Closing of the sale thereof. |
| | |
| | In addition, if a Merger with a Pubco introduced by Palladium to Borrower during the Term is consummated during the one year term of the agreement, or if Borrower enters into a definitive agreement with a Pubco introduced by Palladium that subsequently results in a Merger, Borrower agreed to pay Palladium the following compensation: (i) 7% of the unencumbered cash and cash equivalents owned by Pubco at the time of the Merger (net of any unpaid payables or debt of the Pubco at the time of the Merger), and (ii) the number of shares of common stock of Pubco immediately after the Merger that represents 1.27% of the surviving post-Merger entity on a fully diluted basis. |
| | |
● | On March 10, 2014, Borrower signed an agreement with Bezalel Partners, LLC (“Bezalel”) whereby Bezalel agreed provide consulting services to the Surviving Company after the Merger. Upon the consummation of the Merger, Bezalel shall receive an award of 5,342,510 shares of Pubco’s common stock (subject to proportionate adjustment for forward or reverse stock splits) (the “Awarded Shares”). The Awarded Shares shall be one hundred percent (100%) vested on the date of issuance and deemed to have been fully earned on such date. The Awarded Shares, together with any related expense reimbursement are the sole compensation payable to Bezalel with respect to its performance of the consulting services. |
● | On May 8, 2014, Borrower signed an Agreement and Plan of Merger with Paulson Capital (Delaware) Corp. a NASDAQ listed public company (“Holdco”) whereby the Company merged with a subsidiary of Holdco (the “Merger”). On closing, the shareholders of the Borrower will be issued 42,772,713 of Holdco common shares, representing 71% of the PubCo voting shares immediately post-merger. The Merger also contemplates closing an additional $11 million of private equity financing (the “PIPE”) contemporaneously with the merger and a $6 million of venture debt financing. |
Material Agreements of Holdco
| | ● | Subscription Agreement, dated as of January 29, 2014, by and among Parent and the subscribers party thereto, relating to the sale of $250,000 in aggregate amount of Parent Common Stock, at a price of $0.50 per share. |
| | ● | Agreements with Broadridge Corporate Issuer Solutions with respect to transfer agent and warrant agent services. |
| | ● | Insurance arrangements as described on Schedule 4.18, which is incorporated by reference herein. |
| | ● | Agreement with Donohoe Advisory Associates LLC, related to advice in connection with NASDAQ matters. |
| | ● | Engagement letter with Grant Thornton, related to tax services. |
| | ● | Agreement with Holland & Knight LLP, related to legal representation. |
| | ● | Agreement with Nasdaq related to the listing of Parent shares. |
| | ● | Engagement letter with Peterson Sullivan, related to accounting services. |
| | ● | Agreement with Sichenzia Ross Friedman Ference LLP, relate to escrow services and the payment of certain legal fees. |
Variation Biotechnologies (US), Inc.
Disclosure Schedule Section 6.16 – Agreements Summary
**1
1 Information omitted pursuant to a Confidential Treatment Request filed with the SEC on February 25, 2016.
SCHEDULE 6.19
Transactions with Affiliates
| | | VBI (US) (Borrower) | | VBI Cda (Canadian Subsidiary) | | ePixis SA |
| | | 2013 | 2012 | 2011 | | 2013 | 2012 | 2011 | | 2013 | 2012 | 2011 |
| | | | | | | | | | | | | |
1) Intercompany transactions between VBI Subsidiary & Borrower | | | | | | | | | | | |
| | Relationship:Intercompany management agreement between companies which covers transfer pricing charges for services rendered (PDF document 4.25 available for review) | | | | | | | | | | | |
| | Fees charged – based on intercompany entries for services rendered (eliminated on consolidation) | Yes | Yes | Yes | | Yes | Yes | Yes | | N/A | N/A | N/A |
| | | | | | | | | | | | | |
2) Intercompany transactions between VBI Subsidiary & ePixis SA | | | | | | | | | | | |
| | Relationship:Canadian Subsidiary is the parent company of ePixis SA (a French company); prior to acquisition ePixis SA had a research collaboration MOU agreement with Canadian Subsidiary | | | | | | | | | | | |
| | a) R&D fees paid to ePixis SA (as per related MOU and amendments listed in Schedule 16.6) | N/A | N/A | N/A | | No | No | Yes | | N/A | N/A | N/A |
| | | | | | | | | | | | | |
| | b) Short-term loan(s) | N/A | N/A | N/A | | Yes | Yes | No | | N/A | N/A | N/A |
| | - 1,500 Euro loan in 2013 | | | | | | | | | | | |
| | - 20,000 Euro loan in 2013 | | | | | | | | | | | |
| | Principal & interest now fully repaid | | | | | | | | | | | |
| | c) Incurred ePixis SA related professional fees | N/A | N/A | N/A | | Yes | Yes | Yes | | N/A | N/A | N/A |
| | | | | | | | | | | | | |
SCHEDULE 6.23
Deposit and Disbursement Accounts
Name of bank or other financial institution | Name of Account Holder | Account number | Type of Account |
Silicon Valley Bank 3003 Tasman Drive, Santa Clara, CA 95054 888-782-4140 | Variation Biotechnologies (US), Inc. (“Borrower’) | ### | Main operating account for Borrower |
Caisse Desjardins 655, boulevard Saint-René Ouest Gatineau, Québec J8T 8M4 819-568-5368 | Variation Biotechnologies (US), Inc. (“Borrower’) | ### | USD account for Borrower in Canada |
Caisse Desjardins 655, boulevard Saint-René Ouest Gatineau, Québec J8T 8M4 819-568-5368 | Variation Biotechnologies Inc. (“VBI Subsidiary”) | ### | CAD and USD account for R&D operations in Canada |
Capital Advisors Group 29 Crafts Street Newton, MA 02458 617-630-8100 | Variation Biotechnologies (US), Inc. (“Borrower’) | ### | Investment account |
State Street PO Box 710 South Windsor, CT 06074-0710 800-392-9244 | Variation Biotechnologies (US), Inc. (“Borrower’) | ### | Safekeeping investment account (custodian) |
Silicon Valley Bank 3003 Tasman Drive, Santa Clara, CA 95054 888-782-4140 | VBIV Vaccines Inc. | NEW bank account to be opened concurrent with Merger | USD Operating account |
SCHEDULE 6.24
Registration Rights
Capitalized terms not defined herein shall be as defined in the Merger Agreement.
(a) The Merger Agreement provides demand registration rights to the Pre-Merger Company Sharesholders holding at least twenty-five percent (25%) of shares of Parent Common Stock held by all Pre-Merger Company Shareholders immediately after the Merger, including any shares of Parent Common Stock issued in the Merger, issued in the Private Placement and/or issuable upon exercise or conversion of any options, warrants or convertible securities held by such Pre-Merger Company Shareholders immediately after the Merger or issued in the Private Placement (the “Required Registration Holders”), Parent shall, as soon as practicable and in any event within thirty (30) days after Parent’s receipt of the Registration Request, file with the SEC, and thereafter use its commercially reasonable efforts to have declared effective as soon as practicable and in any event within ninety (90) days after the initial filing thereof with the SEC, a registration statement on Form S-3 (or if Parent is not eligible to use Form S-3, any other form that Parent is eligible to use) (a “S-3 Registration Statement”) under the Securities Act covering the resale by all Pre-Merger Company Shareholders (collectively, the “Registration Holders”) of such shares of Parent Common Stock (the “Registrable Shares”). In its discretion, Parent will be permitted to register any other shares for resale by other eligible selling stockholders using the S-3 Registration Statement and other shares on its own behalf on the S-3 Registration Statement. Parent shall use commercially reasonable efforts to keep the S-3 Registration Statement continuously effective and usable for the resale of the Registrable Shares covered thereby for a period commencing on the date on which the SEC declares the S-3 Registration Statement effective or it otherwise becomes automatically effective and ending on the earlier of (i) the date upon which all of the Registrable Shares first become eligible for resale under the Securities Act without restriction or (ii) the first date upon which all of the Registrable Shares covered by the S-3 Registration Statement have been sold pursuant to such S-3 Registration Statement or otherwise. The Registration Holders agree to cooperate with and provide such assistance to Parent, as Parent may reasonably request, in connection with any registration and sale of the Registrable Shares, including without limitation, accurately completing and executing selling shareholder questionnaires prior to the filing of any S-3 Registration Statement.
(b) The agreements entered into by Parent in connection with the transactions contemplated by the October 2013 Proxy Statement contain certain provisions with respect to the voting rights of the shares of Parent Capital Stock, the registration of shares of Parent Capital Stock, participation rights with respect to Parent Capital Stock, and anti-dilution rights with respect to Parent Capital Stock. Those agreements, which are attached as exhibits to Parent's Form 8/K filed with the SEC on August 30, 2013, include (A) the Subscription Agreement, (B) the Class A Warrant, (C) the Class B Warrants, and (D) the Interest Preservation Letter Agreement.
(c) DKR and Hudson Bay were granted certain registration rights pursuant to the Hudson Bay Financing Documents (as defined in the Merger Agreement).
SCHEDULE 6.25
Royalty and Other Payments
| 1. | | Inbound Intellectual Property Contracts that include royalties or other payments: |
| | | |
| | ● | NRC License Agreement for HEK 293 proprietary manufacturing cell line for eVLPs |
| | | |
| | | Borrower acquired a non-exclusive license to the National Research Council’s (“NRC”) 293SF-3F6 cell line for use in vaccine manufacturing. Under the terms of the agreement, NRC is entitled to, and Borrower is obligated to pay milestone payments on successful advancement of a product using the cell line in clinical development. The milestone payments total up to $155,000, excluding a commercial annual fee of $10,000 once a product is approved. |
| | ● | Epixis S.A. UPMC/INSERM License Agreements for eVLP base patents (see Schedule 6.15(a) – patent families P1, P2 and P3) |
| | On July 18, 2011, VBI entered into a Sale and Purchase Agreement where it is obligated to make the following milestone payments: |
| | |
● | EUR 101,720 upon successful technology transfer to a contract manufacturing organization; |
● | EUR 500,000 to EUR 1,000,000 upon first approval by the United States Food and Drug Administration; |
● | EUR 750,000 to EUR 1,500,000 upon reaching Cumulative Net Sales of EUR 25,000,000, in the case of a sublicense the payments, are reduced by 50%; |
● | EUR 1,000,000 to EUR 2,000,000 upon reaching Cumulative Net Sales of EUR 50,000,000 , in the case of a sublicense, the payments are reduced by 50%; and |
● | in the case of a sublicense only, EUR 500,000 to EUR 1,000,000 upon reaching Cumulative Net Sales of EUR 75,000,000 and EUR 100,000,000 |
The events obliging VBI to make these payments have not yet occurred and the probability of them occurring is not determinable; consequently, no amounts are accrued in respect of these contingencies.
2. The Consulting Agreement with Dr. David Klatzman dated January 17, 2013 includes the following provisions in Appendix II to the agreement:
“EQUITY
Upon execution of this Agreement, subject to VBI Board Approval, the Consultant shall be entitled to a grant of $100K in equity of VBI as determined by the price of the Series B financing. For greater clarity, the shares will be issued coincident with the Series B financing.
RISK ADJUSTED PERFORMANCE BONUSES
To provide incentive to the Consultant for the successful development of the HCV vaccine, the Company will provide incentive based pay for each of the following events (the “Milestones”):
| | ● | For each new eVLP vaccine patent filed (in which Consultant is an inventor AND which does not include other inventors/rights other than those already secured owned by VBI) |
| | ● | FDA or EMEA approval to begin clinical testing of an eVLP based HCV vaccine |
| | ● | Start of Ph II of an eVLP based HCV vaccine |
| | ● | Start of Ph III of an eVLP based HCV vaccine |
| | ● | Approval of an eVLP based HCV vaccine product |
| | ● | Cumulative sales of $100M for an eVLP based HCV vaccine product |
Event/Compensation | Each New Patent | FDA/EMEA IND/CTA Approval | Start of Ph II | Start ofPh III | Approval of HCV Vaccine | Cumulative Sales of $100M |
Compensation Due on Milestone | $10K | $40K | $50K | $75K | $125K | $125K |
Implied Present Value | $8.9K | $35.7K | $12.6K | $3.7K | $5.7K | $5.7K |
The above chart describes the Compensation Due on Milestone to the Consultant on successful achievement of each event. Note that Implied Present Values are also included as they provide an estimate of the current value of the compensation once risk and time-value of money are considered. These values were calculated using industry standard risk-adjustment of potential future revenues in relation to the time and development risk involved in developing a potential HCV vaccine.”
With respect to the equity commitment noted above, management is exploring possible alternative equity grants that are mutually acceptable and will be submitted to the post-merger board of directors for approval.
3. The Consulting Agreement Dr. Charlotte Fribert dated January 28, 2013 includes the following provisions:
“EQUITY
Upon execution of this Agreement, subject to VBI Board Approval, the Consultant shall be entitled to a grant of $100K in equity of VBI as determined by the price of the Series B financing.
PERFORMANCE BONUSES
To provide incentive to the Consultant for the successful development of the HCV vaccine, the Company will provide incentive based pay for each of the following:
● | $10K for each new eVLP vaccine patent filed (in which Consultant is an inventor AND which does not include other inventors/rights other than those already secured owned by VBI) |
● | $40K on FDA or EMEA approval to begin clinical testing of an eVLP based HCV vaccine |
● | $50K on start of Ph II of an eVLP based HCV vaccine |
● | $75K on start of Ph III of an eVLP based HCV vaccine |
● | $125K on approval of an eVLP based HCV vaccine product |
● | $125K on cumulative sales of $100M for an eVLP based HCV vaccine product” |
With respect to the equity commitment noted above, management is exploring possible alternative equity grants that are mutually acceptable and will be submitted to the post-merger board of directors for approval.
SCHEDULE 8.2(b)
Existing Indebtedness
None, other than convertible notes and trade payables.
See Schedule 5.1.19 –VBI Convertible Notes.
SCHEDULE 8.3(b)
Existing Liens
● | As part of the Credit Facility and Guaranty Agreement, the lender will be placing a lien on VBI Subsidiary’s intellectual property. |
SCHEDULE 8.5(a)
Investments
None, other than investment in respective subsidiaries of Holdco and Borrower.
SCHEDULE 11.02
Notice Information
If to VBI Vaccines Inc. or Variation Biotechnologies (US), Inc.:
222 Third Street, Suite 2241
Cambridge, MA 02142
Attn: Jeff Baxter
eFacsimile: 888-391-2579
Email: jbaxter@vbivaccines.com
With a copy to:
Richardson & Patel LLP
1100 Glendon Avenue, Suite 850
Los Angeles, California 90024
Attn: Kevin Friedmann, Esq.
Facsimile: 310-208-1154
Email: kfriedmann@richardsonpatel.com
If to Perceptive Advisors LLC:
499 Park Avenue, 25th Floor
New York, New York 10022
Attention: Sandeep Dixit
E-mail: sandeep@perceptivelife.com
Fax: 646-205-5301
EXHIBIT A-1
FORM OF INITIAL TERM NOTE
| $3,000,000 | July 25, 2014, |
FOR VALUE RECEIVED, VARIATION BIOTECHNOLOGIES (US), INC., a Delaware corporation (the “Borrower”), promises to pay to PCOF 1, LLC (together with any of its successors, transferees and assignees, the “Lender”) on the Maturity Date (as such date may be accelerated pursuant to the Credit Agreement, defined below) the principal sum of $THREE MILLION DOLLARS ($3,000,000) or, if less, the aggregate unpaid principal amount of the Initial Loan shown on the schedule attached hereto (and any continuation thereof) made by the Lender pursuant to the Credit Agreement and Guaranty, dated as of July 25, 2014] (as amended or otherwise modified from time to time, the “Credit Agreement”), by and among the Borrower, each Guarantor party thereto and the Lender. Unless otherwise defined, capitalized terms used herein have the meanings provided in the Credit Agreement.
The Borrower also promises to pay interest on the unpaid principal amount hereof from time to time outstanding from the date hereof until maturity (whether by acceleration or otherwise) and, after maturity upon demand, until paid in full, at the rates per annum and on the dates specified in the Credit Agreement, as well as any other amounts that may be due to the Lender upon maturity (whether by acceleration or otherwise) under or in respect of this Initial Term Note.
Payments of both principal and interest are to be made in Dollars in same day or immediately available funds to the account designated by the Lender pursuant to the Credit Agreement.
The Borrower hereby irrevocably authorizes the Lender to make (or cause to be made) appropriate notations on the grid attached to this Initial Term Note (or on any continuation of such grid), which notations, if made, shall evidence,interalia the date of, the outstanding principal amount of, and the interest rate and Interest Period applicable to the Initial Loan evidenced hereby. Such notations shall, to the extent not inconsistent with notations made by the Lender in the Register, be conclusive and binding on the Borrower absent manifest error;provided, that the failure of the Lender to make any such notations shall not limit or otherwise affect any Obligations of the Borrower or any Guarantors.
This Initial Term Note is one of the Notes referred to in, and evidences Indebtedness incurred under, the Credit Agreement, to which reference is made for a description of the security for this Initial Term Note and for a statement of the terms and conditions on which the Borrower is permitted and required to make prepayments and repayments of the unpaid principal amount of the Indebtedness evidenced by this Initial Term Note and on which such Indebtedness may be declared to be immediately due and payable. Any prepaid principal of this Initial Term Note may not be reborrowed.
All parties hereto, whether as makers, endorsers or otherwise, severally waive presentment for payment, demand, protest and notice of dishonor.
THIS INITIAL TERM NOTE HAS BEEN DELIVERED IN NEW YORK, NEW YORK AND SHALL BE DEEMED TO BE A CONTRACT MADE UNDER AND GOVERNED BY THE INTERNAL LAWS OF THE STATE OF NEW YORK (INCLUDING FOR SUCH PURPOSE SECTIONS 5-1401 AND 5-1402 OF THE GENERAL OBLIGATIONS LAW OF THE STATE OF NEW YORK).
VARIATION BIOTECHNOLOGIES (US), INC. |
By: | |
| | Name: Title: |
INITIAL LOAN AND PRINCIPAL PAYMENTS
Date | Amount of Initial Loan Made | Interest Period | Amount of Principal Repaid | Unpaid Principal Balance | Total | Notation Made By |
LIBO Rate | LIBO Rate | LIBO Rate |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
EXHIBIT A-2
FORM OF DELAYED DRAW NOTE
FOR VALUE RECEIVED, VARIATION BIOTECHNOLOGIES (US), INC., a Delaware corporation (the “Borrower”), promises to pay to PCOF 1, LLC (together with any of its successors, transferees and assignees, the “Lender”) on the Maturity Date (as such date may be accelerated pursuant to the Credit Agreement, defined below) the principal sum of [_______] [($_______)] or, if less, the aggregate unpaid principal amount of the Delayed Draw Loan shown on the schedule attached hereto (and any continuation thereof) made by the Lender pursuant to the Credit Agreement and Guaranty, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Credit Agreement”), by and among the Borrower, each Guarantor party thereto and the Lender. Unless otherwise defined, capitalized terms used herein have the meanings provided in the Credit Agreement.
The Borrower also promises to pay interest on the unpaid principal amount hereof from time to time outstanding from the date hereof until maturity (whether by acceleration or otherwise) and, after maturity upon demand, until paid in full, at the rates per annum and on the dates specified in the Credit Agreement, as well as any other amounts that may be due to the Lender upon maturity (whether by acceleration or otherwise) under or in respect of this Delayed Draw Note.
Payments of both principal and interest are to be made in Dollars in same day or immediately available funds to the account designated by the Lender pursuant to the Credit Agreement.
The Borrower hereby irrevocably authorizes the Lender to make (or cause to be made) appropriate notations on the grid attached to this Delayed Draw Note (or on any continuation of such grid), which notations, if made, shall evidence,interalia the date of, the outstanding principal amount of, and the interest rate and Interest Period applicable to the Delayed Draw Loan evidenced hereby. Such notations shall, to the extent not inconsistent with notations made by the Lender in the Register, be conclusive and binding on the Borrower absent manifest error;provided, that the failure of the Lender to make any such notations shall not limit or otherwise affect any Obligations of the Borrower or any Guarantors.
This Delayed Draw Note is one of the Notes referred to in, and evidences Indebtedness incurred under, the Credit Agreement, to which reference is made for a description of the security for this Delayed Draw Note and for a statement of the terms and conditions on which the Borrower is permitted and required to make prepayments and repayments of the unpaid principal amount of the Indebtedness evidenced by this Delayed Draw Note and on which such Indebtedness may be declared to be immediately due and payable. Any prepaid principal of this Delayed Draw Note may not be reborrowed.
All parties hereto, whether as makers, endorsers or otherwise, severally waive presentment for payment, demand, protest and notice of dishonor.
THIS DELAYED DRAW NOTE HAS BEEN DELIVERED IN NEW YORK, NEW YORK AND SHALL BE DEEMED TO BE A CONTRACT MADE UNDER AND GOVERNED BY THE INTERNAL LAWS OF THE STATE OF NEW YORK (INCLUDING FOR SUCH PURPOSE SECTIONS 5-1401 AND 5-1402 OF THE GENERAL OBLIGATIONS LAW OF THE STATE OF NEW YORK).
VARIATION BIOTECHNOLOGIES (US), INC. |
By: | |
| | Name: Title: |
DELAYED DRAW LOAN AND PRINCIPAL PAYMENTS
Date | Amount of Delayed Draw Loan Made | Interest Period | Amount of Principal Repaid | Unpaid Principal Balance | Total | Notation Made By |
LIBO Rate | LIBO Rate | LIBO Rate |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
EXHIBIT B
FORM OF LOAN REQUEST
| | Date: [•] |
| | |
To: | PCOF 1, LLC, as Lender under the Credit Agreement (as defined below). |
| | |
Re: | Credit Agreement and Guaranty, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Credit Agreement”) by and among VARIATION BIOTECHNOLOGIES (US), INC., a Delaware corporation, each Guarantor party thereto and PCOF 1, LLC. Capitalized terms used herein and not otherwise defined shall have the meanings provided in the Credit Agreement. |
Ladies and Gentlemen:
The undersigned hereby requests a borrowing of (select one):
☐ the Initial Loan | ☐ the Delayed Draw Loan |
| | |
| | 1. | Date of borrowing: [●] (a Business Day) |
With respect to any borrowing requested hereby, the undersigned Borrower hereby represents and warrants that (i) such request complies with the requirements ofSections 2.1 and 2.2 of the Credit Agreement, as applicable, (ii) all representations and warranties set forth in each Loan Document are true and correct and (iii) no Default or Event of Default shall exist, or would result from such proposed Loan.
VARIATION BIOTECHNOLOGIES (US), INC., a Delaware corporation |
By: | |
Name: |
Title: |
| |
[Signature Page to Loan Request]
EXHIBIT C
FORM OF COMPLIANCE CERTIFICATE
VARIATION BIOTECHNOLOGIES (US), INC.
COMPUTATION DATE: _______ __, 201_
This Compliance Certificate (this “Compliance Certificate”) is delivered pursuant to [Section 5.1.5] [Section 5.2.5] [clause (c) ofSection 7.1] of the Credit Agreement and Guaranty, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Credit Agreement”), by and among VARIATION BIOTECHNOLOGIES (US), INC., a Delaware corporation (the “Borrower”), each Guarantor party thereto and PCOF 1, LLC (together with its Affiliates, successors, transferees and assignees, the “Lender”). Unless otherwise defined herein or the context otherwise requires, terms used herein have the meanings provided in the Credit Agreement.
The undersigned are duly authorized to execute and deliver this Compliance Certificate on behalf of each of the Borrower and Holdco. By executing this Compliance Certificate the Borrower and Holdco hereby certify to the Lender on behalf of the Loan Parties as follows:
(a) The financial statements delivered pursuant toSection 5.1.4 or, if later, pursuant toclause (a) or(b) ofSection 7.1 of the Credit Agreement have been prepared in accordance with GAAP consistently applied, and fairly present the financial condition of the Loan Parties as of the dates thereof and the related results of their operations for the periods then ended (subject to the absence of footnotes and to normal year-end adjustments in the case of unaudited financial statements).
(b) All other information presented in connection with this Compliance Certificate (including the Attachments hereto) is correct and complete in all material respects.
(c) Borrower has maintained at all times a minimum balance of $1,000,000 of cash in one or more Controlled Accounts that is free and clear of all Liens, other than Liens granted hereunder in favor of the Lender. As a result, the minimum liquidity requirement pursuant toSection 7.19 of the Credit Agreement [has been][has not been] satisfied.
(d) No Default or Event of Default has occurred and is continuing[, except as set forth onAttachment 4 hereto, which includes a description of the nature and period of existence of such Default or Event of Default and what action the Borrower has taken, is taking and proposes to take with respect thereto.]
(e) Except as set forth onAttachment 5 hereto, subsequent to the date of the most recent Compliance Certificate submitted by the undersigned pursuant toclause (c) ofSection 7.1 of the Credit Agreement no Subsidiary has been formed or acquired or, if a Subsidiary has been formed or acquired since the delivery of the last Compliance Certificate, such Subsidiary has, to the extent required, complied withSection 7.8 of the Credit Agreement).
IN WITNESS WHEREOF, each undersigned has caused this Compliance Certificate to be executed and delivered, and the certification and warranties contained herein to be made, by its chief financial or accounting Authorized Officer as of the date first above written.
VARIATION BIOTECHNOLOGIES (US), INC. |
| |
By: | |
Name: |
Title: |
EXHIBIT D
FORM OF PLEDGE AND SECURITY AGREEMENT
This PLEDGE AND SECURITY AGREEMENT, dated as of July 25, 2014 (as amended or otherwise modified from time to time, this “Security Agreement”), is made by and among Variation Biotechnologies (US), Inc., a Delaware corporation (the “Borrower”) and the Guarantors party to the Credit Agreement (defined below); (the Borrower, the Guarantors and any Subsidiary that becomes a party to this Security Agreement are herein referred to as the “Grantors”) (terms used in the preamble and the recitals have the definitions set forth in or incorporated by reference inArticle I), in favor of PCOF 1, LLC (together with its successors, transferees or assignees, the “Secured Party”).
WITNESSETH:
WHEREAS, pursuant to a Credit Agreement and Guaranty, dated as of July 25, 2014, (as amended or otherwise modified from time to time, the “Credit Agreement”), by and among the Borrower, each Guarantor party thereto and the Secured Party, the Secured Party has extended Commitments to make Loans to the Borrower; and
WHEREAS, as a condition precedent to the making of Loans under the Credit Agreement, the Grantors are required to execute and deliver this Security Agreement;
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, each Grantor agrees, for the benefit of the Secured Party, as follows:
ARTICLE I
DEFINITIONS
Certain Terms. The following terms (whether or not underscored) when used in this Security Agreement, including its preamble and recitals, shall have the following meanings (such definitions to be equally applicable to the singular and plural forms thereof):
“Borrower” is defined in thepreamble.
“Collateral” is defined inSection 2.1.
“Collateral Account” is defined inclause (b) ofSection 4.3.
“Computer Hardware and Software Collateral” means:
all computer and other electronic data processing hardware, integrated computer systems, central processing units, memory units, display terminals, printers, features, computer elements, card readers, tape drives, hard and soft disk drives, cables, electrical supply hardware, generators, power equalizers, accessories and all peripheral devices and other related computer hardware, including all operating system software, utilities and application programs in whatsoever form;
all software programs (including both source code, object code and all related applications and data files), designed for use on the computers and electronic data processing hardware described inclause (a) above;
all firmware associated therewith;
all documentation (including flow charts, logic diagrams, manuals, guides, specifications, training materials, charts and pseudo codes) with respect to such hardware, software and firmware described in the precedingclauses (a) through(c);and
all rights with respect to all of the foregoing, including copyrights, licenses, options, warranties, service contracts, program services, test rights, maintenance rights, support rights, improvement rights, renewal rights and indemnifications and any substitutions, replacements, improvements, error corrections, updates, additions or model conversions of any of the foregoing.
“Control Agreement” means an authenticated record in form and substance reasonably satisfactory to the Secured Party, that provides for the Secured Party to have “control” (as defined in the UCC) over the Controlled Accounts.
“Copyright Collateral” means all copyrights of any Grantor, whether statutory or common law, registered or unregistered and whether published or unpublished, now or hereafter in force throughout the world including all of such Grantor’s right, title and interest in and to all copyrights registered in the United States Copyright Office or anywhere else in the world and also including the copyrights referred to inItem A ofSchedule V, and registrations and recordings thereof and all applications for registration thereof, all copyright licenses, including each copyright license referred to inItem B ofSchedule V, the right to sue for past, present and future infringements of any of the foregoing, all rights corresponding thereto, all extensions and renewals of any thereof and all Proceeds of the foregoing, including licenses, royalties, income, payments, claims, damages and Proceeds of suit.
“Credit Agreement” is defined in thefirst recital.
“Distributions” means all dividends or other distributions paid on Capital Securities, including in connection with (or in connection with the exercise of) stock splits, reclassifications, warrants, options, non-cash dividends, mergers, consolidations, and all other distributions (whether similar or dissimilar to the foregoing) on or with respect to any Capital Securities.
“Excluded Collateral” is defined inSection 2.1.
“Filing Statements” is defined inclause (b) ofSection 3.7.
“General Intangibles” means all “general intangibles” and all “payment intangibles”, each as defined in the UCC.
“Grantor” is defined in thepreamble.
“Guarantor” means, collectively, Holdco and each of its Subsidiaries (other than the Borrower).
“Intellectual Property Collateral” means, collectively, the Computer Hardware and Software Collateral, the Copyright Collateral, the Patent Collateral, the Trademark Collateral and the Trade Secrets Collateral.
“Patent Collateral” means:
(a) Inventions, invention disclosures and discoveries, whether patentable or not, all letters patent and applications for letters patent throughout the world, including provisional, design and utility patents each patent and patent application referred to inItem A ofSchedule III;
(b) all reissues, divisions, continuations, continuations-in-part, extensions, renewals and reexaminations of any of the items described inclause (a);
all patent licenses, and other agreements providing any Grantor with the right to use any items of the type referred to inclauses (a) and(b) above, including each patent license referred to inItem B ofSchedule III; and
all Proceeds of, and rights associated with, the foregoing (including licenses, royalties and Proceeds of infringement suits), the right to sue third parties for past, present or future infringements of any patent or patent application, and for breach or enforcement of any patent license.
“Permitted Liens” means all Liens permitted by Section 8.3 of the Credit Agreement.
“PIC” has the meaning defined in the Credit Agreement.
“Secured Party” is defined in thepreamble.
“Securities Act” is defined inclause (a) ofSection 6.2.
“Security Agreement” is defined in thepreamble.
“Termination Date” has the meaning defined in the Credit Agreement.
“Trademark Collateral” means:
(a) (i) all trademarks, trade names, corporate names, company names, business names, fictitious business names, trade styles, service marks, certification marks, collective marks, logos and other source or business identifiers, and all goodwill of the business associated therewith, now existing or hereafter adopted or acquired including those referred to inItem A ofSchedule IV, whether currently in use or not, all registrations and recordings thereof and all applications in connection therewith, including registrations, recordings and applications in the United States Patent and Trademark Office or in any office or agency of the United States of America, or any State thereof or any other country or political subdivision thereof or otherwise, and all common-law rights relating to the foregoing, and (ii) the right to obtain all reissues, extensions or renewals of the foregoing (collectively referred to as the “Trademark”);
(c) all Trademark licenses for the grant by or to any Grantor of any right to use any trademark, including each trademark license referred to inItem B ofSchedule IV; and
all of the goodwill of the business connected with the use of, and symbolized by the items described in,clause (a) above, and to the extent applicableclause (b) above;
the right to sue third parties for past, present and future infringements of any Trademark Collateral described inclause (a) above and, to the extent applicable,clause (b) above; and
all Proceeds of, and rights associated with, the foregoing, including any claim by any Grantor against third parties for past, present or future infringement or dilution of any Trademark, Trademark registration or Trademark license, or for any injury to the goodwill associated with the use of any such Trademark or for breach or enforcement of any Trademark license and all rights corresponding thereto throughout the world.
“Trade Secrets Collateral” means all common law and statutory trade secrets and all other confidential, proprietary or useful information and all know-how (all of the foregoing being collectively called a “Trade Secret”), whether or not such Trade Secret has been reduced to a writing or other tangible form, including all Documents and things embodying, incorporating or referring in any way to such Trade Secret, all Trade Secret licenses, including each Trade Secret license referred to inSchedule VI, and including the right to sue for and to enjoin and to collect damages for the actual or threatened misappropriation of any Trade Secret and for the breach or enforcement of any such Trade Secret license.
Credit Agreement Definitions. Unless otherwise defined herein or the context otherwise requires, terms used in this Security Agreement, including its preamble and recitals, have the meanings provided in the Credit Agreement, including, without limitation, Section 1.4 thereof.
UCC and PPSA Definitions. When used herein the terms “Account”, “Certificate of Title”, “Certificated Securities”, “Chattel Paper”, “Commercial Tort Claim”, “Commodity Account”, “Commodity Contract”, “Deposit Account”, “Document”, “Electronic Chattel Paper”, “Equipment”, “Goods”, “Instrument”, “Inventory”, “Investment Property”, “Letter-of-Credit Rights”, “Payment Intangibles”, “Proceeds”, “Promissory Notes”, “Securities Account”, “Security Entitlement”, “Supporting Obligations” and “Uncertificated Securities” have the meaning provided in Article 8 or Article 9, as applicable, of the UCC or their corresponding meaning or definition in Section 1(1) of the PPSA. “Letters of Credit” has the meaning provided in Section 5-102 of the UCC.
SECURITY INTEREST
Grant of Security Interest. Each Grantor hereby grants to the Secured Party, a continuing security interest in all of such Grantor’s right, title, and interest in and to the following property, whether now or hereafter existing, owned or acquired by such Grantor, and wherever located, (collectively, the “Collateral”):
Accounts;
Chattel Paper;
Commercial Tort Claims listed onItem I ofSchedule II (as such schedule may be amended or supplemented from time to time);
Deposit Accounts;
Documents;
General Intangibles;
Goods;
Instruments;
Intellectual Property Collateral;
Investment Property;
Letter-of-Credit Rights and Letters of Credit;
Supporting Obligations;
all books, records, writings, databases, information and other property relating to, used or useful in connection with, evidencing, embodying, incorporating or referring to, any of the foregoing in this Section;
all Proceeds of the foregoing and, to the extent not otherwise included, (A) all payments under insurance (whether or not the Secured Party is the loss payee thereof) and (B) all tort claims; and
all other property and rights of every kind and description and interests therein.
Notwithstanding the foregoing, the term “Collateral” shall not include the following (collectively the “Excluded Collateral”):
any Grantor’s real property interests (including fee real estate, leasehold interests and fixtures);
any General Intangibles or other rights arising under any contracts, instruments, licenses or other documents as to which the grant of a security interest would (A) constitute a violation of a valid and enforceable restriction in favor of a third party on such grant, unless and until any required consents shall have been obtained, (B) give any other party to such contract, instrument, license or other document the right to terminate its obligations thereunder or accelerate any of relevant Grantor’s obligations thereunder, or (C) result in a breach or violation of, or constitute a default under, the agreement or instrument governing such Collateral;
any asset, the granting of a security interest in which would be void or illegal under any applicable governmental law, rule or regulation, or pursuant thereto would result in, or permit the termination of, such asset;
any asset subject to a Permitted Lien (other than Liens in favor of the Secured Party) to the extent that the grant of other Liens on such asset (A) would result in a breach or violation of, or constitute a default under, the agreement or instrument governing such Permitted Lien, (B) would result in the loss of use of such asset or (C) would permit the holder of such Permitted Lien to terminate the relevant Grantor’s use of such asset or accelerate any of such Grantor’s obligations thereunder;
vehicles and other goods subject to a Certificate of Title; and
all of PIC’s assets and any Capital Securities of PIC held by Holdco.
Security for Obligations. This Security Agreement and the Collateral in which the Secured Party is granted a security interest hereunder by the Grantors secures the payment and performance by the Grantors of all of the Obligations under the Credit Agreement and each other Loan Document including, without limitation, the payment of all principal of and premium, if any, and interest (including interest accruing during the pendency of any proceeding of the type described inSection 9.1.8 of the Credit Agreement) on the Loans.
Grantors Remain Liable. Anything herein to the contrary notwithstanding:
the Grantors will remain liable under their respective contracts and agreements included in the Collateral to the extent set forth therein, and will perform all of their respective duties and obligations under such contracts and agreements to the same extent as if this Security Agreement had not been executed;
the exercise by the Secured Party of any of its rights hereunder will not release any Grantor from any of its duties or obligations under any such contracts or agreements included in the Collateral; and
the Secured Party will have no obligation or liability under any contracts or agreements included in the Collateral by reason of this Security Agreement, nor will it be obligated to perform any of the obligations or duties of any Grantor thereunder or to take any action to collect or enforce any claim for payment assigned hereunder.
Distributions on Pledged Shares. In the event that any Distribution with respect to any Capital Securities pledged hereunder is permitted to be paid (in accordance with Section 8.6 of the Credit Agreement), such Distribution or payment may be paid directly to the relevant Grantor. If any Distribution is made in contravention ofSection 8.6 of the Credit Agreement, the relevant Grantor shall hold the same segregated and in trust for the Secured Party until paid to the Secured Party in accordance withSection 4.1.5.
Security Interest Absolute, etc. This Security Agreement shall in all respects be a continuing, absolute, unconditional and irrevocable grant of security interest, and shall remain in full force and effect until the Termination Date. All rights of the Secured Party and the security interests granted to the Secured Party hereunder, and all obligations of the each Grantor hereunder, shall, in each case, be absolute, unconditional and irrevocable irrespective of:
any lack of validity, legality or enforceability of any Loan Document;
the failure of the Secured Party (i) to assert any claim or demand or to enforce any fight or remedy against any Obligor or any other Person (including any Grantor) under the provisions of any Loan Document or otherwise, or (ii) to exercise any right or remedy against any other guarantor (including any Grantor) of, or collateral securing, any Obligations;
any change in the time, manner or place of payment of, or in any other term of, all or any part of the Obligations, or any other extension, compromise or renewal of any Obligations;
any reduction, limitation, impairment or termination of any Obligations for any reason, including any claim of waiver, release, surrender, alteration or compromise, and shall not be subject to (and each Grantor hereby waives any right to or claim of) any defense or setoff, counterclaim, recoupment or termination whatsoever by reason of the invalidity, illegality, non-genuineness, irregularity, compromise, unenforceability of, or any other event or occurrence affecting, any Obligations or otherwise;
any amendment to, rescission, waiver, or other modification of, or any consent to or departure from, any of the terms of any Loan Document;
any addition, exchange or release of any Collateral or of any Person that is (or will become) a Grantor (including each Grantor hereunder) of the Obligations, or any surrender or non-perfection of any collateral, or any amendment to or waiver or release or addition to, or consent to or departure from, any other guarantee held by the Secured Party securing any of the Obligations; or
any other circumstance which might otherwise constitute a defense available to, or a legal or equitable discharge of, any Obligor, any surety or any guarantor.
REPRESENTATIONS AND WARRANTIES
In order to induce the Secured Party to enter into the Credit Agreement and make Loans thereunder, the Grantors represent and warrant to the Secured Party as set forth below.
As to Capital Securities of the Subsidiaries, Investment Property.
With respect to any direct Subsidiary of each Grantor that is
a corporation, business trust, joint stock company or similar Person, all Capital Securities issued by such Subsidiary are duly authorized and validly issued, fully paid and non-assessable, and represented by a certificate; and
a partnership or limited liability company, no Capital Securities issued by such Subsidiary (A) are dealt in or traded on securities exchanges or in securities markets, (B) expressly provide that such Capital Securities is a security governed by Article 8 of the UCC or (C) are held in a Securities Account, except, with respect to thisclause (a)(ii), Capital Securities (x) for which the Secured Party is the registered owner or (y) with respect to which the issuer has agreed in an authenticated record with such Grantor and the Secured Party to comply with any instructions of the Secured Party without the consent of such Grantor.
Each Grantor has delivered all Certificated Securities constituting Collateral held by such Grantor on the Closing Date to the Secured Party, together with duly executed undated blank stock powers, or other equivalent instruments of transfer acceptable to the Secured Party.
With respect to Uncertificated Securities constituting Collateral owned by any Grantor, such Grantor has caused the issuer thereof either to (i) register the Secured Party as the registered owner of such security or (ii) agree in an authenticated record with such Grantor and the Secured Party that such issuer will comply with instructions with respect to such security originated by the Secured Party without further consent of such Grantor.
The percentage of the issued and outstanding Capital Securities of each Subsidiary pledged by each Grantor hereunder is as set forth onSchedule I.
All deposit accounts, lockboxes, disbursement accounts, investment accounts or other similar accounts of each Grantor are Controlled Accounts.
Grantor Name, Location, etc.
The jurisdictions in which the Grantors are located for purposes of Sections 9-301 and 9-307 of the UCC or its PPSA equivalent, as applicable, are set forth inItem A ofSchedule II.
Each location in which a secured party would have filed a UCC or PPSA financing statement in the five years prior to the date hereof to perfect a security interest in Equipment, Inventory and General Intangibles owned by a Grantor is set forth inItem B ofSchedule II.
No Grantor has any trade names other than those set forth inItem C ofSchedule II hereto.
During the four months preceding the date hereof, no Grantor has been known by any legal name different from the one set forth on the signature page hereto and no Grantor has been the subject of any merger or other corporate reorganization, except as set forth inItem D ofSchedule II hereto.
Each Grantor’s federal taxpayer identification number is (and, during the four months preceding the date hereof, such Grantor has not had a federal taxpayer identification number different from that) set forth inItem E ofSchedule II hereto.
No Grantor is a party to any federal, state or local government contract except as set forth inItem F ofSchedule II hereto.
No Grantor maintains any Deposit Accounts, Securities Accounts or Commodity Accounts with any Person, in each case, except as set forth onItem G ofSchedule II.
No Grantor is the beneficiary of any Letters of Credit, except as set forth onItem H ofSchedule II.
No Grantor has Commercial Tort Claims except as set forth onItem I ofSchedule II.
The name set forth on the signature page attached hereto is the true and correct legal name (as defined in the UCC or PPSA, as applicable) of each Grantor.
Each Grantor has obtained a legal, valid and enforceable consent of each issuer of any Letter of Credit pledged by such Grantor to the assignment of the Proceeds of such Letter of Credit to the Secured Party and such Grantor has not consented to, and is not otherwise aware of, any Person (other than the Secured Party pursuant hereto) having control (within the meaning of Section 9-104 of the UCC) over, or any other interest in any of such Grantor’s rights in respect thereof.
Ownership, No Liens, etc. Except as disclosed onSchedules III throughV, each Grantor owns its Collateral free and clear of any Lien, except for any security interest (i) created by the Loan Documents, (ii) in the case of Collateral other than the Capital Securities of each Subsidiary pledged hereunder, that is a Permitted Lien, or (iii) which will be released simultaneously with the making of the Initial Loan under the Credit Agreement. No effective UCC or PPSA financing statement or other filing similar in effect covering all or any part of the Collateral is on file in any recording office, except those filed in favor of the Secured Party relating to this Security Agreement, Permitted Liens or as to which a duly authorized termination statement relating to such UCC or PPSA financing statement or other instrument has been delivered to the Secured Party on the Closing Date.
Possession of Inventory, Control; etc.
Except as otherwise permitted in the Credit Agreement, each Grantor has, and agrees that it will maintain, exclusive possession of its Documents, Instruments, Promissory Notes, Goods, Equipment and Inventory, other than (i) Equipment and Inventory in transit in the ordinary course of business, (ii) Equipment and Inventory that is in the possession or control of a warehouseman, bailee agent or other Person (other than a Person controlled by or under common control with such Grantor) that has been notified of the security interest created in favor of the Secured Party pursuant to this Security Agreement, and has authenticated a record acknowledging that it holds possession of such Collateral for the Secured Party’s benefit and waives any Lien held by it against such Collateral, and (iii) Instruments or Promissory Notes that have been delivered to the Secured Party pursuant toSection 3.5. In the case of Equipment or Inventory described inclause (ii) above, no lessor or warehouseman of any premises or warehouse upon or in which such Equipment or Inventory is located has (i) issued any warehouse receipt or other receipt in the nature of a warehouse receipt in respect of any such Equipment or Inventory, (ii) issued any Document for any such Equipment or Inventory, (iii) received notification of the Secured Party’s interest (other than the security interest granted hereunder) in any such Equipment or Inventory or (iv) any Lien on any such Equipment or Inventory.
Each Grantor is the sole entitlement holder of its Accounts and no other Person (other than the Secured Party pursuant to this Security Agreement or any other Person with respect to Permitted Liens) has control or possession of, or any other interest in, any of its Accounts or any other securities or property credited thereto.
Negotiable Documents, Instruments and Chattel Paper. Each Grantor has delivered to the Secured Party possession of all originals of all Documents, Instruments, Promissory Notes, and tangible Chattel Paper owned or held by such Grantor on the Closing Date.
Intellectual Property Collateral. Except as disclosed onSchedules III throughV and except for standard off-the-shelf software used by any Grantor, with respect to any Intellectual Property Collateral:
each Grantor has made all necessary filings and recordations to protect its interest in such Intellectual Property Collateral, including recordations of all of its interests in the Patent Collateral and Trademark Collateral in the United States Patent and Trademark Office and in corresponding offices throughout the world, and its claims to the Copyright Collateral in the United States Copyright Office and in corresponding offices throughout the world;
no Grantor has made a previous assignment, sale, transferor agreement constituting a present or future assignment, sale or transfer of any Intellectual Property for purposes of granting a security interest or as Collateral that has not been terminated or released;
each Grantor has executed and delivered to the Secured Party, Intellectual Property Collateral security agreements for all Copyrights, Patents and Trademarks owned by such Grantor, including all Copyrights, Patents and Trademarks onSchedule III throughV (as such schedules may be amended or supplemented from time to time); and
the consummation of the transactions contemplated by the Credit Agreement and this Security Agreement will not result in the termination or material impairment of any of the Intellectual Property Collateral.
Validity, etc.
This Security Agreement creates a valid security interest in the Collateral securing the payment of the Obligations.
Each Grantor has filed or caused to be filed all UCC-1 or PPSA-1C financing statements, as applicable, in the filing office for such Grantor’s jurisdiction of organization listed inItem A of Schedule II (collectively, the “Filing Statements”) (or has authenticated and delivered to the Secured Party the Filing Statements suitable for filing in such offices) and has taken all other actions requested by the Secured Party necessary for the Secured Party to obtain control of the Collateral as provided in Sections 9-104, 9-105, 9-106 and 9-107 of the UCC or its PPSA equivalent, as applicable.
Upon the filing of the Filing Statements with the appropriate agencies therefor, the security interests created under this Security Agreement shall constitute a perfected security interest in the Collateral described on such Filing Statements in favor of the Secured Party to the extent that a security interest therein may be perfected by filing pursuant to the relevant UCC or PPSA, as applicable, prior to all other Liens, except for Permitted Liens (in which case such security interest shall be second in priority of right only to the Permitted Liens until the obligations secured by such Permitted Liens have been satisfied).
Authorization, Approval, etc. Except as have been obtained or made and are in full force and effect, no authorization, approval or other action by, and no notice to or filing with, any Governmental Authority or any other third party is required either:
for the grant by any Grantor of the security interest granted hereby or for the execution, delivery and performance of this Security Agreement by any Grantor;
for the perfection or maintenance of the security interests hereunder including the first priority (subject to Permitted Liens) nature of such security interest (except with respect to the Filing Statements or, with respect to Intellectual Property Collateral, the recordation of any agreements with the U.S. Patent and Trademark Office or the U.S. Copyright Office or the Canadian Intellectual Property Office) or the exercise by the Secured Party of its rights and remedies hereunder; or
for the exercise by the Secured Party of the voting or other rights provided for in this Security Agreement, or, except (i) with respect to any securities issued by a Subsidiary of a Grantor, as may be required in connection with a disposition of such securities by laws affecting the offering and sale of securities generally, the remedies in respect of the Collateral pursuant to this Security Agreement and (ii) any “change of control” or similar filings required by state licensing agencies.
COVENANTS
Each Grantor covenants and agrees that, until the Termination Date, such Grantor will perform, comply with and be bound by the obligations set forth below.
As to Investment Property, etc. Capital Securities of Subsidiaries. No Grantor will allow any of its Subsidiaries:
that is a corporation, business trust, joint stock company or similar Person, to issue Uncertificated Securities;
that is a partnership or limited liability company, to (i) issue Capital Securities that are to be dealt in or traded on securities exchanges or in securities markets, expressly provide in its Organic Documents that its Capital Securities are securities governed by Article 8 of the UCC or its PPSA equivalent, or (ii) place such Subsidiary’s Capital Securities in a Securities Account; and
to issue Capital Securities in addition to or in substitution for the Capital Securities pledged hereunder, except to such Grantor (and such Capital Securities are immediately pledged and delivered to the Secured Party pursuant to the terms of this Security Agreement).
SECTION 1.1.2 Investment Property (other than Certificated Securities).
With respect to any Deposit Accounts, Securities Accounts, Commodity Accounts, Commodity Contracts or Security Entitlements constituting Investment Property owned or held by any Grantor, such Grantor will, upon the Secured Party’s request, cause the intermediary maintaining such Investment Property to execute a Control Agreement relating to such Investment Property pursuant to which such intermediary agrees to comply with the Secured Party’s instructions with respect to such Investment Property without further consent by such Grantor.
With respect to any Uncertificated Securities (other than Uncertificated Securities credited to a Securities Account) constituting Investment Property owned or held by any Grantor, such Grantor will cause the issuer of such securities to either (i) register the Secured Party as the registered owner thereof on the books and records of the issuer or (ii) execute a Control Agreement relating to such Investment Property pursuant to which the issuer agrees to comply with the Secured Party’s instructions with respect to such Uncertificated Securities without further consent by such Grantor.
Certificated Securities (Stock Powers). Each Grantor agrees that all Certificated Securities delivered to the Secured Party by such Grantor pursuant to this Security Agreement, will be accompanied by duly executed undated blank stock powers, or other equivalent instruments of transfer reasonably acceptable to the Secured Party.
Continuous Pledge. Each Grantor will (except as otherwise permitted under the Credit Agreement or this Security Agreement) deliver to the Secured Party and at all times keep pledged to the Secured Party pursuant hereto, on a first-priority, perfected basis all of its Investment Property, all Dividends and Distributions with respect thereto, all of its Payment Intangibles to the extent they are evidenced by a Document, Instrument, Promissory Note or Chattel Paper, and all interest and principal with respect to such Payment Intangibles, and all Proceeds and rights from time to time received by or distributable to such Grantor in respect of any of the foregoing Collateral. Each Grantor agrees that it will, promptly following receipt thereof, deliver to the Secured Party possession of all originals of negotiable Documents, Instruments, Promissory Notes and Chattel Paper that it acquires following the Closing Date.
Voting Rights; Dividends, etc. Each Grantor agrees:
promptly upon receipt of notice of the occurrence and continuance of an Event of Default from the Secured Party and without any request therefor by the Secured Party, so long as such Event of Default shall continue, to deliver (properly endorsed where required hereby or requested by the Secured Party) to the Secured Party all Dividends and Distributions with respect to Investment Property, all interest, principal, other cash payments on Payment Intangibles, and all Proceeds of the Collateral, in each case thereafter received by such Grantor, all of which shall be held by the Secured Party as additional Collateral; and
with respect to Collateral consisting of general partner interests or limited liability company interests, to promptly modify its Organic Documents to admit the Secured Party as a general partner or member, as applicable, immediately upon the occurrence and continuance of an Event of Default and so long as the Secured Party has notified such Grantor of the Secured Party’s intention to exercise its voting power under this clause,
that the Secured Party may exercise (to the exclusion of such Grantor) the voting power and all other incidental rights of ownership with respect to any Investment Property constituting Collateral and such Grantor hereby grants the Secured Party an irrevocable proxy, exercisable under such circumstances, to vote such Investment Property; and
to promptly deliver to the Secured Party such additional proxies and other documents as may be necessary to allow the Secured Party to exercise such voting power.
All dividends, Distributions, interest, principal, cash payments, Payment Intangibles and Proceeds that may at any time and from time to time be held by any Grantor, but which such Grantor is then obligated to deliver to the Secured Party, shall, until delivery to the Secured Party, be held by such Grantor separate and apart from its other property in trust for the Secured Party. The Secured Party agrees that unless an Event of Default shall have occurred and be continuing and the Secured Party shall have given the notice referred to in clause (b), the Grantors will have the exclusive voting power with respect to any of their Investment Property constituting Collateral and the Secured Party will, upon the written request of a Grantor, promptly deliver such proxies and other documents, if any, as shall be reasonably requested by such Grantor which are necessary to allow such Grantor to exercise that voting power;provided that no vote shall be cast, or consent, waiver, or ratification given, or action taken by any Grantor that would impair any such Collateral or be inconsistent with or violate any provision of any Loan Document.
Change of Name, etc. No Grantor will change its name or place of incorporation or organization or federal taxpayer identification number except upon 30 days’ prior written notice to the Secured Party.
As to Accounts.
The Grantors shall have the right to collect all Accounts so long as no Event of Default shall have occurred and be continuing.
Upon (i) the occurrence and continuance of an Event of Default and (ii) the delivery of notice by the Secured Party to a Grantor, all Proceeds of Collateral received by such Grantor shall be delivered in kind to the Secured Party for deposit in a Deposit Account of such Grantor maintained with the Secured Party or with any depositary institution that has entered into a Control Agreement in favor of the Secured Party (together with any other Accounts pursuant to which any portion of the Collateral is deposited with the Secured Party, the “Collateral Accounts”), and such Grantor shall not commingle any such Proceeds, and shall hold separate and apart from all other property, all such Proceeds in express trust for the benefit of the Secured Party until delivery thereof is made to the Secured Party.
Following the delivery of notice pursuant toclause (b)(ii) and during the continuance of an Event of Default, the Secured Party shall have the right to apply any amount in the Collateral Account to the payment of any Obligations which are due and payable.
With respect to each of the Collateral Accounts, it is hereby confirmed and agreed that (i) deposits in such Collateral Account are subject to a security interest as contemplated hereby, (ii) such Collateral Account shall be under the control of the Secured Party and (iii) the Secured Party shall have the sole right of withdrawal over such Collateral Account.
As to Grantors’ Use of Collateral.
Subject toclause (b), each Grantor (i) may in the ordinary course of its business, at its own expense, sell, lease or furnish under the contracts of service any of the Inventory normally held by such Grantor for such purpose, and use and consume, in the ordinary course of its business, any raw materials, work in process or materials normally held by such Grantor for such purpose, (ii) will, at its own expense, endeavor to collect, as and when due and in accordance with its customary practices, all amounts due with respect to any of the Collateral, including the taking of such action with respect to such collection as the Secured Party may request following the occurrence of an Event of Default or, in the absence of such request, as such Grantor may deem advisable, and (iii) may grant, in the ordinary course of business, to any party obligated on any of the Collateral, any rebate, refund or allowance to which such party may be lawfully entitled, and may accept, in connection therewith, the return of Goods, the sale or lease of which shall have given rise to such Collateral.
At any time following the occurrence and during the continuance of an Event of Default, whether before or after the maturity of any of the Obligations, the Secured Party may (i) revoke any or all of the rights of any Grantor set forth inclause (a), (ii) notify any parties obligated on any of the Collateral to make payment to the Secured Party of any amounts due or to become due thereunder and (iii) enforce collection of any of the Collateral by suit or otherwise and surrender, release, or exchange all or any part thereof, or compromise or extend or renew for any period (whether or not longer than the original period) any indebtedness thereunder or evidenced thereby.
Upon request of the Secured Party following the occurrence and during the continuance of an Event of Default, each Grantor will, at its own expense, notify any parties obligated on any of its Collateral to make payment to the Secured Party of any amounts due or to become due thereunder.
At any time following the occurrence and during the continuation of an Event of Default, the Secured Party may endorse, in the name of any Grantor, any item, howsoever received by the Secured Party, representing any payment on or other Proceeds of any of the Collateral.
As to Intellectual Property Collateral. Each Grantor covenants and agrees to comply with the following provisions as such provisions relate to any Intellectual Property Collateral material to the operations or business of such Grantor:
the Grantor shall use commercially reasonable efforts to pursue and maintain, at its own expense, legal protection for all Intellectual Property owned or controlled by the Borrower or any of the Subsidiaries, including (i) initiating proceedings before the United States Patent and Trademark Office, the United States Copyright Office or similar offices or agencies in other countries or political subdivisions thereof, and filing applications for renewal, affidavits of use, affidavits of in contestability and opposition, interference and cancellation proceedings and the paying fees and taxes and (ii) not doing or failing to perform acts whereby such Intellectual Property may lapse or become abandoned or dedicated to the public, invalid or unenforceable;
the Grantor shall promptly notify the Secured Party if it knows, or has reason to know, that any application or registration relating to any material item of the Intellectual Property Collateral may become abandoned or dedicated to the public or placed in the public domain or invalid or unenforceable, or of any adverse determination or development (including the institution of, or any such determination or development in, any proceeding in the United States Patent and Trademark Office, the United States Copyright Office or any foreign counterpart thereof or any court) regarding such Grantor’s ownership of any of the Intellectual Property Collateral, its right to register the same or to keep and maintain and enforce the same;
in no event will the Grantor or any of its agents, employees, designees or licensees file an application for the registration of any Intellectual Property Collateral with the United States Patent and Trademark Office, the United States Copyright Office or any similar office or agency in any other country or any political subdivision thereof, unless it promptly informs the Secured Party, and upon request of the Secured Party (subject to the terms of the Credit Agreement), executes and delivers all agreements, instruments and documents as the Secured Party may request to evidence the Secured Party’s security interest in such Intellectual Property Collateral; and
Within 30 days from the end of each Fiscal Quarter the Grantor will execute and deliver to the Secured Party (as applicable) a Patent Security Agreement, Trademark Security Agreement and/or Copyright Security Agreement, as the case may be, in the forms ofExhibit A,Exhibit B andExhibit C hereto in connection with its obtaining an interest in any such Intellectual Property, and shall execute and deliver to the Secured Party any other document reasonably required to acknowledge or register or perfect the Secured Party’s interest in any part of such item of Intellectual Property Collateral unless such Grantor shall determine in good faith (with the consent of the Secured Party) that any Intellectual Property Collateral is of negligible economic value to such Grantor.
As to Letter-of-Credit Rights.
Each Grantor, by granting a security interest in its Letter-of-Credit Rights to the Secured Party, intends to (and hereby does) collaterally assign to the Secured Party its rights (including its contingent rights) to the Proceeds of all Letter-of-Credit Rights of which it is or hereafter becomes a beneficiary or assignee. Each Grantor will promptly use commercially reasonable efforts to cause the issuer of each Letter of Credit and each nominated person (if any) with respect thereto to consent to such assignment of the Proceeds thereof in a consent agreement in form and substance reasonably satisfactory to the Secured Party and deliver written evidence of such consent to the Secured Party.
Upon the occurrence of an Event of Default, each Grantor will, promptly upon request by the Secured Party, (i) notify (and such Grantor hereby authorizes the Secured Party to notify) the issuer and each nominated person with respect to each of the Letters of Credit that the Proceeds thereof have been assigned to the Secured Party hereunder and any payments due or to become due in respect thereof are to be made directly to the Secured Party and (ii) arrange for the Secured Party to become the transferee beneficiary Letter of Credit.
As to Commercial Tort Claims. Each Grantor covenants and agrees that, until the payment in full of the Obligations and termination of all Commitments, with respect to any Commercial Tort Claim hereafter arising asserting damages in excess of $100,000 (individually or in the aggregate for all such claims), it shall deliver to the Secured Party a supplement in form and substance satisfactory to the Secured Party, together with all supplements to schedules thereto identifying such new Commercial Tort Claims.
Electronic Chattel Paper and Transferable Records. If any Grantor at any time holds or acquires an interest in any electronic chattel paper or any “transferable record,” as that term is defined in Section 201 of the U.S. Federal Electronic Signatures in Global and National Commerce Act, or in Section 16 of the U.S. Uniform Electronic Transactions Act as in effect in any relevant jurisdiction, with a value in excess of $1,000,000, such Grantor shall promptly notify the Secured Party thereof and, at the request of the Secured Party, shall take such action as the Secured Party may request to vest in the Secured Party control under Section 9-105 of the U.C.C. of such electronic chattel paper or control under Section 201 of the Federal Electronic Signatures in Global and National Commerce Act or, as the case may be, Section 16 of the Uniform Electronic Transactions Act, as so in effect in such jurisdiction, of such transferable record. The Secured Party agrees with each Grantor that the Secured Party will arrange, pursuant to procedures satisfactory to the Secured Party and so long as such procedures will not result in the Secured Party’s loss of control, for such Grantor to make alterations to the electronic chattel paper or transferable record permitted under Section 9-105 of the U.C.C. or, as the case may be, Section 201 of the U.S. Federal Electronic Signatures in Global and National Commerce Act or Section 16 of the U.S. Uniform Electronic Transactions Act for a party in control to allow without loss of control, unless an Event of Default has occurred and is continuing or would occur after taking into account any action by such Grantor with respect to such electronic chattel paper or transferable record.
Further Assurances, etc. Each Grantor agrees that, from time to time at its own expense, it will promptly execute and deliver all further instruments and documents, and take all further action, that may be necessary or that the Secured Party may request, in order to perfect, preserve and protect any security interest granted or purported to be granted hereby or to enable the Secured Party to exercise and enforce its rights and remedies hereunder with respect to any Collateral. Without limiting the generality of the foregoing, each Grantor will:
from time to time upon the request of the Secured Party, promptly deliver to the Secured Party such stock powers, instruments and similar documents, reasonably satisfactory in form and substance to the Secured Party, with respect to such Collateral as the Secured Party may request and will, from time to time upon the request of the Secured Party, after the occurrence and during the continuance of any Event of Default, promptly transfer any securities constituting Collateral into the name of any nominee designated by the Secured Party; if any Collateral shall be evidenced by an Instrument, negotiable Document, Promissory Note or tangible Chattel Paper, deliver and pledge to the Secured Party hereunder such Instrument, negotiable Document, Promissory Note or tangible Chattel Paper duly endorsed and accompanied by duly executed instruments of transfer or assignment, all in form and substance satisfactory to the Secured Party;
file (and hereby authorize the Secured Party to file) such Filing Statements or continuation statements, or amendments thereto, and such other instruments or notices (including any assignment of claim form under or pursuant to the federal assignment of claims statute, 31 U.S.C. § 3726, any successor or amended version thereof or any regulation promulgated under or pursuant to any version thereof), as may be necessary or that the Secured Party may request in order to perfect and preserve the security interests and other rights granted or purported to be granted to the Secured Party hereby;
deliver to the Secured Party and at all times keep pledged to the Secured Party pursuant hereto, on a first-priority, perfected basis, at the request of the Secured Party, all Investment Property constituting Collateral, all Dividends and Distributions with respect thereto, and all interest and principal with respect to Promissory Notes, and all Proceeds and rights from time to time received by or distributable to such Grantor in respect of any of the foregoing Collateral;
not take or omit to take any action the taking or the omission of which would result in any material impairment or alteration of any obligation of the maker of any Payment Intangible or other Instrument constituting Collateral, except as provided inSection 4.4;
not create any tangible Chattel Paper without placing a legend on such tangible Chattel Paper reasonably acceptable to the Secured Party indicating that the Secured Party has a security interest in such Chattel Paper;
furnish to the Secured Party, from time to time at the Secured Party’s request, statements and schedules further identifying and describing the Collateral and such other reports in connection with the Collateral as the Secured Party may request, all in reasonable detail; and
do all things reasonably requested by the Secured Party in accordance with this Security Agreement in order to enable the Secured Party to have and maintain control over the Collateral consisting of Investment Property, Deposit Accounts, Letter-of-Credit-Rights and Electronic Chattel Paper.
With respect to the foregoing and the grant of the security interest hereunder, each Grantor hereby authorizes the Secured Party to file one or more financing or continuation or financing change statements, and amendments thereto, relative to all or any part of the Collateral. Each Grantor agrees that a carbon, photographic or other reproduction of this Security Agreement or any UCC or PPSA financing statement covering the Collateral or any part thereof shall be sufficient as a UCC or PPSA financing statement where permitted by law. Each Grantor hereby authorizes the Secured Party to file financing statements describing as the collateral covered thereby “all of the debtor’s personal property or assets” or words to that effect, notwithstanding that such wording may be broader in scope than the Collateral described in this Security Agreement.
Deposit Accounts. Following the occurrence and during the continuance of an Event of Default, at the request of the Secured Party, each Grantor will maintain all of its Deposit Accounts only with the Secured Party or with any depositary institution that has entered into a Control Agreement in favor of the Secured Party.
Inbound Licenses. Each Grantor will, promptly after entering into or becoming bound by any inbound license or similar agreement relating to the sale, distribution, licensing or other commercialization of any Product or any Intellectual Property covering any Product (other than over-the-counter software that is commercially available to the public), take such commercially reasonable actions as the Secured Party may reasonably request to obtain the consent of, or waiver by, any Person whose consent or waiver is necessary for the Secured Party to be granted and perfect a valid security interest in such license or similar agreement and to fully exercise its rights under any of the Loan Documents in the event of a disposition or liquidation of the rights, assets or property that is the subject of such license or similar agreement.
THE SECURED PARTY
Secured Party Appointed Attorney-in-Fact. Each Grantor hereby irrevocably appoints the Secured Party its attorney-in-fact, with full authority in the place and stead of such Grantor and in the name of such Grantor or otherwise, from time to time in the Secured Party’s discretion, following the occurrence and during the continuance of an Event of Default, to take any action and to execute any instrument which the Secured Party may deem necessary or advisable to accomplish the purposes of this Security Agreement, including:
to ask, demand, collect, sue for, recover, compromise, receive and give acquittance and receipts for moneys due and to become due under or in respect of any of the Collateral;
to receive, endorse, and collect any drafts or other Instruments, Documents and Chattel Paper, in connection withclause (a) above;
to file any claims or take any action or institute any proceedings which the Secured Party may deem necessary or desirable for the collection of any of the Collateral or otherwise to enforce the rights of the Secured Party with respect to any of the Collateral;
dispose of all or any part of any Collateral as provided inSection 6.1(a)(iv) below; and
to perform the affirmative obligations of such Grantor hereunder.
Each Grantor hereby acknowledges, consents and agrees that the power of attorney granted pursuant to this Section is irrevocable and coupled with an interest.
Secured Party May Perform. If any Grantor fails to perform any agreement contained herein, the Secured Party may itself perform, or cause performance of, such agreement, and the expenses of the Secured Party incurred in connection therewith shall be payable by the Borrower pursuant to Section 10.3 of the Credit Agreement.
Secured Party Has No Duty. The powers conferred on the Secured Party hereunder are solely to protect its interest in the Collateral and shall not impose any duty on it to exercise any such powers. Except for reasonable care of any Collateral in its possession and the accounting for moneys actually received by it hereunder, the Secured Party shall have no duty as to any Collateral or responsibility for
ascertaining or taking action with respect to calls, conversions, exchanges, maturities, tenders or other matters relative to any Investment Property, whether or not the Secured Party has or is deemed to have knowledge of such matters, or
taking any necessary steps to preserve rights against prior parties or any other rights pertaining to any Collateral.
Reasonable Care. The Secured Party is required to exercise reasonable care in the custody and preservation of any of the Collateral in its possession;provided that the Secured Party shall be deemed to have exercised reasonable care in the custody and preservation of any of the Collateral, if it takes such action for that purpose as any Grantor reasonably requests in writing at times other than upon the occurrence and during the continuance of any Event of Default, but failure of the Secured Party to comply with any such request at any time shall not in itself be deemed a failure to exercise reasonable care.
REMEDIES
Certain Remedies. If any Event of Default shall have occurred and be continuing:
The Secured Party may exercise in respect of the Collateral, in addition to other rights and remedies provided for herein or otherwise available to it, all the rights and remedies of a Secured Party on default under the UCC or PPSA, as applicable (whether or not the UCC or the PPSA applies to the affected Collateral) and also may
take possession of any Collateral not already in its possession without demand and without legal process;
require any Grantor to, and each Grantor hereby agrees that it will, at its expense and upon request of the Secured Party forthwith, assemble all or part of the Collateral as directed by the Secured Party and make it available to the Secured Party at a place to be designated by the Secured Party that is reasonably convenient to both parties,
enter onto the property where any Collateral is located and take possession thereof without demand and without legal process;
without notice except as specified below, lease, license, sell or otherwise dispose of the Collateral or any part thereof in one or more parcels at public or private sale, at any of the Secured Party’s offices or elsewhere, for cash, on credit or for future delivery, and upon such other terms as the Secured Party may deem commercially reasonable. Each Grantor agrees that, to the extent notice of sale shall be required by law, at least ten days’ prior notice to such Grantor of the time and place of any public sale or the time after which any private sale is to be made shall constitute reasonable notification. The Secured Party shall not be obligated to make any sale of Collateral regardless of notice of sale having been given. The Secured Party may adjourn any public or private sale from time to time by announcement at the time and place fixed therefor, and such sale may, without further notice, be made at the time and place to which it was so adjourned.
All cash Proceeds received by the Secured Party in respect of any sale of, collection from, or other realization upon, all or any part of the Collateral shall be applied by the Secured Party against, all or any part of the Obligations as set forth in Section 4.4 of the Credit Agreement.
The Secured Party may:
transfer all or any part of the Collateral into the name of the Secured Party or its nominee, with or without disclosing that such Collateral is subject to the Lien hereunder,
notify the parties obligated on any of the Collateral to make payment to the Secured Party of any amount due or to become due thereunder,
withdraw, or cause or direct the withdrawal, of all funds with respect to the Collateral Account;
enforce collection of any of the Collateral by suit or otherwise, and surrender, release or exchange all or any part thereof, or compromise or extend or renew for any period (whether or not longer than the original period) any obligations of any nature of any party with respect thereto,
endorse any checks, drafts, or other writings in any Grantor’s name to allow collection of the Collateral,
take control of any Proceeds of the Collateral, and
execute (in the name, place and stead of any Grantor) endorsements, assignments, stock powers and other instruments of conveyance or transfer with respect to all or any of the Collateral.
Securities Laws. If the Secured Party shall determine to exercise its right to sell all or any of the Collateral that are Capital Securities pursuant toSection 6.1, each Grantor agrees that, upon request of the Secured Party, such Grantor will, at its own expense:
execute and deliver, and cause (or, with respect to any issuer which is not a Subsidiary of such Grantor, use commercially reasonable efforts to cause) each issuer of the Collateral contemplated to be sold and the directors and officers thereof to execute and deliver, all such instruments and documents, and do or cause to be done all such other acts and things, as may be necessary or, in the opinion of the Secured Party, advisable to register such Collateral under the provisions of the Securities Act of 1933, as from time to time amended (the “Securities Act”), and cause the registration statement relating thereto to become effective and to remain effective for such period as prospectuses are required by law to be furnished, and to make all amendments and supplements thereto and to the related prospectus which, in the opinion of the Secured Party, are necessary or advisable, all in conformity with the requirements of the Securities Act and the rules and regulations of the SEC applicable thereto;
use commercially reasonable efforts to exempt the Collateral under the state securities or “Blue Sky” laws and to obtain all necessary governmental approvals for the sale of the Collateral, as requested by the Secured Party;
cause (or, with respect to any issuer that is not a Subsidiary of such Grantor, use commercially reasonable efforts to cause) each such issuer to make available to its security holders, as soon as practicable, an earnings statement that will satisfy the provisions of Section 11(a) of the Securities Act; and
do or cause to be done all such other acts and things as may be necessary to make such sale of the Collateral or any part thereof valid and binding and in compliance with applicable law.
Compliance with Restrictions. Each Grantor agrees that in any sale of any of the Collateral whenever an Event of Default shall have occurred and be continuing, the Secured Party is hereby authorized to comply with any limitation or restriction in connection with such sale as it may be advised by counsel is necessary in order to avoid any violation of applicable law (including compliance with such procedures as may restrict the number of prospective bidders and purchasers, require that such prospective bidders and purchasers have certain qualifications, and restrict such prospective bidders and purchasers to Persons who will represent and agree that they are purchasing for their own account for investment and not with a view to the distribution or resale of such Collateral), or in order to obtain any required approval of the sale or of the purchaser by any Governmental Authority or official, and each Grantor further agrees that such compliance shall not result in such sale being considered or deemed not to have been made in a commercially reasonable manner, nor shall the Secured Party be liable nor accountable to any Grantor for any discount allowed by the reason of the fact that such Collateral is sold in compliance with any such limitation or restriction.
Protection of Collateral. The Secured Party may from time to time, at its option, perform any act which any Grantor fails to perform after being requested in writing so to perform (it being understood that no such request need be given after the occurrence and during the continuance of an Event of Default) and the Secured Party may from time to time take any other action which the Secured Party deems necessary for the maintenance, preservation or protection of any of the Collateral or of its security interest therein.
MISCELLANEOUS PROVISIONS
Loan Document. This Security Agreement is a Loan Document executed pursuant to the Credit Agreement and shall (unless otherwise expressly indicated herein) be construed, administered and applied in accordance with the terms and provisions thereof, including Article X thereof.
Binding on Successors, Transferees and Assigns; Assignment. This Security Agreement shall remain in full force and effect until the Termination Date has occurred, shall be binding upon each Grantor and its successors, transferees and assigns and shall inure to the benefit of and be enforceable by the Secured Party and its successors, transferees and assigns;provided that no Grantor may (unless otherwise permitted under the terms of the Credit Agreement or this Security Agreement) assign any of its obligations hereunder without the prior written consent of the Secured Party.
Amendments, etc. No amendment to or waiver of any provision of this Security Agreement, nor consent to any departure by any Grantor from its obligations under this Security Agreement, shall in any event be effective unless the same shall be in writing and signed by the Secured Party and the Grantors and then such waiver or consent shall be effective only in the specific instance and for the specific purpose for which given.
Notices. All notices and other communications provided for hereunder shall be in writing or by facsimile and addressed, delivered or transmitted to the appropriate party at the address or facsimile number of such party specified in the Credit Agreement or at such other address or facsimile number as may be designated by such party in a notice to the other party. Any notice or other communication, if mailed and properly addressed with postage prepaid or if properly addressed and sent by pre-paid courier service, shall be deemed given when received; any such notice or other communication, if transmitted by facsimile, shall be deemed given when transmitted and electronically confirmed.
Release of Liens. Upon (a) the Disposition of Collateral in accordance with the Credit Agreement or (b) the occurrence of the Termination Date, the security interests granted herein shall automatically terminate with respect to (i) such Collateral (in the case ofclause (a)) or (ii) all Collateral (in the case of clause (b)). Upon any such Disposition or termination, the Secured Party will, at the Grantors’ sole expense, deliver to the relevant Grantor, without any representations, warranties or recourse of any kind whatsoever, all Collateral held by the Secured Party hereunder, and execute and deliver to such Grantor such documents as such Grantor shall reasonably request to evidence such termination.
Additional Grantor. Upon the execution and delivery by any other Person of a supplement in the form ofAnnex I hereto, such Person shall become a “Grantor” hereunder with the same force and effect as if it were originally a party to this Security Agreement and named as a “Grantor” hereunder. The execution and delivery of such supplement shall not require the consent of any Grantor hereunder, and the rights and obligations of each Grantor hereunder shall remain in full force and effect notwithstanding the addition of any new Grantor as a party to this Security Agreement.
No Waiver; Remedies. In addition to, and not in limitation ofSection 2.5, no failure on the part of the Secured Party to exercise, and no delay in exercising, any right hereunder shall operate as a waiver thereof, nor shall any single or partial exercise of any right hereunder preclude any other or further exercise thereof or the exercise of any other right. The remedies herein provided are cumulative and not exclusive of any remedies provided by law.
Headings. The various headings of this Security Agreement are inserted for convenience only and shall not affect the meaning or interpretation of this Security Agreement or any provisions thereof.
Severability. Any provision of this Security Agreement which is prohibited or unenforceable in any jurisdiction shall, as to such provision and such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions of this Security Agreement or affecting the validity or enforceability of such provision in any other jurisdiction.
Governing Law, Entire Agreement, etc. THIS SECURITY AGREEMENT SHALL BE DEEMED TO BE A CONTRACT MADE UNDER AND GOVERNED BY THE INTERNAL LAWS OF THE STATE OF NEW YORK (INCLUDING FOR SUCH PURPOSE SECTIONS 5-1401 AND 5-1402 OF THE GENERAL OBLIGATIONS LAW OF THE STATE OF NEW YORK), EXCEPT TO THE EXTENT THAT THE PERFECTION, EFFECT OF PERFECTION OR NON-PERFECTION, AND PRIORITY OF THE SECURITY INTEREST HEREUNDER, OR REMEDIES HEREUNDER, IN RESPECT OF ANY PARTICULAR COLLATERAL ARE GOVERNED BY THE LAWS OF A JURISDICTION OTHER THAN THE STATE OF NEW YORK. This Security Agreement and the other Loan Documents constitute the entire understanding among the parties hereto with respect to the subject matter hereof and thereof and supersede any prior agreements, written or oral, with respect thereto.
Counterparts. This Security Agreement may be executed by the parties hereto in several counterparts, each of which shall be deemed to be an original and all of which shall constitute together but one and the same agreement. Delivery of an executed counterpart of a signature page to this Security Agreement by facsimile shall be effective as delivery of a manually executed counterpart of this Security Agreement.
IN WITNESS WHEREOF, each of the parties hereto has caused this Security Agreement to be duly executed and delivered by its Authorized Officer as of the date first above written.
VARIATION BIOTECHNOLOGIES (US), INC. |
| |
By: | |
| | |
| | Name: |
| | Title: |
VBI VACCINES INC. |
| |
By: | |
| |
Name:
|
| | Title: |
VARIATION BIOTECHNOLOGIES INC. |
| |
By: | |
| |
Name:
|
| | Title: |
| |
PCOF 1, LLC |
By: | |
| |
Name:
|
| | Title: |
Signature Page to Pledge and Security Agreement
SCHEDULE I
to Security Agreement
Name of Grantor: Variation Biotechnologies (US), Inc. | | | Common Stock | |
| | | | | | |
Issuer (corporate) | Cert. # | # of Shares | Authorized Shares | Outstanding Shares | % of Shares Pledged |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | Limited Liability Company Interests |
Issuer (limited liability company | % of Limited Liability Company Interests Pledged | Type of Limited Liability Company Interests Pledged |
| | | |
| | | |
| | | |
| | | |
| | Partnership Interests |
Issuer (partnership) | % of Partnership Interests Owned | % of Partnership Interests Pledged |
N/A | | |
SCHEDULE II
to Security Agreement
Item ALocation of Grantor. |
| |
Name of Grantor: | Location for purposes of UCC or PPSA: |
| | |
| | |
| |
Item BFiling locations last five years. |
| |
Name of Grantor: | Filing locations last five years |
| | |
| | |
| |
Item CTrade names. |
| |
Name of Grantor: | Trade Names: |
| | |
| | |
| |
Item D Merger or other corporate reorganization. |
| |
Name of Grantor: | Merger or other corporate reorganization: |
| | |
| | |
| |
Item ETaxpayer ID numbers. |
| |
Name of Grantor: | Taxpayer ID numbers: |
| | |
| | |
| |
Item FGovernment Contracts. |
| |
Name of Grantor: | Description of Contract: |
| | |
| | |
Item GDeposit Accounts and Securities Accounts. |
| |
Name of Grantor: | Description of Deposit Accounts and Securities Accounts: |
| | |
| | |
| | |
| | |
| | |
| | |
| |
Item HLetter of Credit Rights. |
| |
Name of Grantor: | Description of Deposit Accounts and Securities Accounts: |
| | |
| | |
| |
Item ICommercial Tort Claims. |
| |
Name of Grantor: | Description of Commercial Tort Claims: |
| | |
| | |
SCHEDULE III
to Security Agreement
Item APatents
Issued Patents
Pending Patent Applications
Item B Patent Licenses
SCHEDULE IV
to Security Agreement
Item ATrademarks
Registered Trademarks
Country | Trademark | Registration No. | Registration Date |
|
| | | | |
| | | | |
Pending Trademark Applications
Country | Trademark | Application No. | Filing Date |
| | | | |
| | | | |
Domain Names
Item BTrademark Licenses
SCHEDULE V
to Security Agreement
Item ACopyrights/Mask Works
Issued Copyrights/Mask Works
Pending Copyrights/Mask Works Registration Applications
Item BCopyrights/Mask Work Licenses
SCHEDULE VI
to Security Agreement
Trade Secret or Know-How Licenses
EXHIBIT A
to Security Agreement
PATENT SECURITY AGREEMENT
This PATENT SECURITY AGREEMENT, dated as of_______, 201_ (thisAgreement”), is made by [NAME OF GRANTOR], a _______ corporation (the “Grantor”), in favor of PCOF 1, LLC (together with its successors, transferees or assignees, the “Secured Party”).
WITNESSETH:
WHEREAS, pursuant to a Credit Agreement and Guaranty, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Credit Agreement”), by and among the Borrower, each Guarantor party thereto and the Secured Party, the Secured Party has extended Commitments to make Loans to the Borrower;
WHEREAS, in connection with the Credit Agreement, the Grantor has executed and delivered a Pledge and Security Agreement, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Security Agreement”);
WHEREAS, pursuant to the Credit Agreement and pursuant toclause (d) ofSection 4.5of the Security Agreement, the Grantor is required to execute and deliver this Agreement and to grant to the Secured Party a continuing security interest in all of the Patent Collateral (as defined below) to secure all Obligations; and
WHEREAS, the Grantor has duly authorized the execution, delivery and performance of this Agreement; and
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Grantor agrees, for the benefit of the Secured Party, as follows:
SECTION 1.Definitions. Unless otherwise defined herein or the context otherwise requires, terms used in this Agreement, including its preamble and recitals, have the meanings provided in the Security Agreement.
SECTION 2.Grant of Security Interest. The Grantor hereby assigns, pledges, hypothecates, charges, mortgages, delivers, and transfers to the Secured Party, and hereby grants to the Secured Party, a continuing security interest in all of the following property, whether now or hereafter existing or acquired by the Grantor (the “Patent Collateral”):
(a) all of its letters patent and applications for letters patent throughout the world, including each patent and patent application referred to inItem A ofSchedule I;
(b) all reissues, divisions, continuations, continuations-in-part, extensions, renewals and reexaminations of any of the items described inclause (a);
(c) all of its patent licenses, and other agreements providing the Grantor with the right to use any items of the type referred to inclauses (a) and(b) above, including each patent license referred to inItem B ofSchedule I; and
(d) all Proceeds of, and rights associated with, the foregoing (including license royalties and Proceeds of infringement suits), the right to sue third parties for past, present or future infringements of any patent or patent application, and for breach or enforcement of any patent license.
SECTION 3.Security Agreement. This Agreement has been executed and delivered by the Grantor for the purpose of registering the security interest of the Secured Party in the Patent Collateral with the United States Patent and Trademark Office and corresponding offices in other countries of the world. The security interest granted hereby has been granted as a supplement to, and not in limitation of, the security interest granted to the Secured Party under the Security Agreement. The Security Agreement (and all rights and remedies of the Secured Party thereunder) shall remain in full force and effect in accordance with its terms.
SECTION 4.Release of Liens. Upon (i) the Disposition of Patent Collateral in accordance with the Credit Agreement or (ii) the occurrence of the Termination Date, the security interests granted herein shall automatically terminate with respect to (A) such Patent Collateral (in the case ofclause (i)) or (B) all Patent Collateral (in the case ofclause (ii)). Upon any such Disposition or termination, the Secured Party will, at the Grantor’s sole expense, deliver to the Grantor, without any representations, warranties or recourse of any kind whatsoever, all Patent Collateral held by the Secured Party hereunder, and execute and deliver to the Grantor such Documents as the Grantor shall reasonably request to evidence such termination.
SECTION 5.Acknowledgment. The Grantor does hereby further acknowledge and affirm that the rights and remedies of the Secured Party with respect to the security interest in the Patent Collateral granted hereby are more fully set forth in the Security Agreement, the terms and provisions of which (including the remedies provided for therein) are incorporated by reference herein as if fully set forth herein.
SECTION 6.Loan Document. This Agreement is a Loan Document executed pursuant to the Credit Agreement and shall (unless otherwise expressly indicated herein) be construed, administered and applied in accordance with the terms and provisions thereof, including Article X thereof.
SECTION 7.Counterparts. This Agreement may be executed by the parties hereto in several counterparts, each of which shall be deemed to be an original and all of which shall constitute together but one and the same agreement.
* * * * *
IN WITNESS WHEREOF, each of the parties here to has caused this Agreement to be duly executed and delivered by its Authorized Officer as of the date first above written.
[NAME OF GRANTOR] |
| |
| |
By: | |
| | Name: |
| | Title: |
| |
| |
PCOF 1, LLC |
| |
By: | |
| | Name: |
| | Title: |
SCHEDULE I
to Patent Security Agreement
Item APatents
Issued Patents | | | |
Country | Patent No. | Issue Date | Inventor(s) | Title |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
Pending Patent Applications
Country | Serial No. | Filing Date | Inventor(s) | Title |
| | | | | |
| | | | | |
| | | | | |
Item BPatent Licenses
Country or Territory | Licensor | Licensee | EffectiveDate | Expiration Date | SubjectMatter |
| | | | | | |
| | | | | | |
EXHIBIT B
to Security Agreement
TRADEMARK SECURITY AGREEMENT
This TRADEMARK SECURITY AGREEMENT, dated as of _______, 201_ (this “Agreement”), is made by [GRANTOR], a _______ corporation (the “Grantor”), in favor of PCOF 1, LLC (together with its successors, transferees or assignees, the “Secured Party”).
WITNESSETH:
WHEREAS, pursuant to a Credit Agreement and Guaranty, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Credit Agreement”), by and among the Borrower, each Guarantor party thereto and the Secured Party, the Secured Party has extended Commitments to make Loans to the Borrower;
WHEREAS, in connection with the Credit Agreement, the Grantor has executed and delivered a Pledge and Security Agreement, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Security Agreement”);
WHEREAS, pursuant to the Credit Agreement and pursuant toclause (d) ofSection 4.5 of the Security Agreement, the Grantor is required to execute and deliver this Agreement and to grant to the Secured Party a continuing security interest in all of the Trademark Collateral (as defined below) to secure all Obligations; and
WHEREAS, the Grantor has duly authorized the execution, delivery and performance of this Agreement; and
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Grantor agrees, for the benefit of the Secured Party, as follows:
SECTION 1.Definitions. Unless otherwise defined herein or the context otherwise requires, terms used in this Agreement, including its preamble and recitals, have the meanings provided in the Security Agreement.
SECTION 2.Grant of Security Interest. The Grantor hereby assigns, pledges, hypothecates, charges, mortgages, delivers, and transfers to the Secured Party, and hereby grants to the Secured Party, a continuing security interest in all of the following property, whether now or hereafter existing or acquired by the Grantor (the “Trademark Collateral”):
(a) (i) all of its trademarks, trade names, corporate names, company names, business names, fictitious business names, trade styles, service marks, certification marks, collective marks, logos and other source or business identifiers of the Grantor, and all goodwill of the business associated therewith, now existing or hereafter adopted or acquired including those referred to inItem A ofSchedule I, whether currently in use or not, all registrations and recordings thereof and all applications in connection therewith, including registrations, recordings and applications in the United States Patent and Trademark Office or in any office or agency of the United States of America or any State thereof or any other country or political subdivision thereof or otherwise, and all common-law rights relating to the foregoing, and (ii) the right to obtain all reissues, extensions or renewals of the foregoing (collectively referred to as the “Trademark”);
(b) all Trademark licenses for the grant by or to the Grantor of any right to use any Trademark, including each Trademark license referred to inItem B ofSchedule I;
(c) all of the goodwill of the business connected with the use of, and symbolized by the items described in,clause (a), and to the extent applicableclause (b);
(d) the right to sue third parties for past, present and future infringements of any Trademark Collateral described inclause (a) and, to the extent applicable,clause (b); and
(e) all Proceeds of, and rights associated with, the foregoing, including any claim by the Grantor against third parties for past, present or future infringement or dilution of any Trademark, Trademark registration or Trademark license, or for any injury to the goodwill associated with the use of any such Trademark or for breach or enforcement of any Trademark license and all rights corresponding thereto throughout the world.
SECTION 3.Security Agreement. This Agreement has been executed and delivered by the Grantor for the purpose of registering the security interest of the Secured Party in the Trademark Collateral with the United States Patent and Trademark Office and corresponding offices in other countries of the world. The security interest granted hereby has been granted as a supplement to, and not in limitation of, the security interest granted to the Secured Party under the Security Agreement. The Security Agreement (and all rights and remedies of the Secured Party thereunder) shall remain in full force and effect in accordance with its terms.
SECTION 4.Release of Liens. Upon (i) the Disposition of Trademark Collateral in accordance with the Credit Agreement or (ii) the occurrence of the Termination Date, the security interests granted herein shall automatically terminate with respect to (A) such Trademark Collateral (in the case ofclause (i)) or (B) all Trademark Collateral (in the case ofclause (ii)). Upon any such Disposition or termination, the Secured Party will, at the Grantor’s sole expense, deliver to the Grantor, without any representations, warranties or recourse of any kind whatsoever, all Trademark Collateral held by the Secured Party hereunder, and execute and deliver to the Grantor such Documents as the Grantor shall reasonably request to evidence such termination.
SECTION 5.Acknowledgment. The Grantor does hereby further acknowledge and affirm that the rights and remedies of the Secured Party with respect to the security interest in the Trademark Collateral granted hereby are more fully set forth in the Security Agreement, the terms and provisions of which (including the remedies provided for therein) are incorporated by reference herein as if fully set forth herein.
SECTION 6.Loan Document. This Agreement is a Loan Document executed pursuant to the Credit Agreement and shall (unless otherwise expressly indicated herein) be construed, administered and applied in accordance with the terms and provisions thereof, including Article X thereof.
SECTION 7.Counterparts. This Agreement may be executed by the parties hereto in several counterparts, each of which shall be deemed to be an original and all of which shall constitute together but one and the same agreement.
* * * * *
IN WITNESS WHEREOF, each of the parties hereto has caused this Agreement to be duly executed and delivered by Authorized Officer as of the date first above written.
[NAME OF GRANTOR] |
| |
| |
By: | |
| | Name: |
| | Title: |
| |
| |
PCOF 1, LLC |
| |
| |
By: | |
| | Name: |
| | Title: |
SCHEDULE I
to Trademark Security Agreement
Item ATrademarks
Registered Trademarks
Country | Trademark | Registration No. | Registration Date |
| | | | |
| | | | |
| | | | |
Pending Trademark Applications
Country | Trademark | Serial No. | Filing Date |
| | | | |
| | | | |
| | | | |
Item BTrademark Licenses
Country or Territory | Trademark | Licensor | Licensee | EffectiveDate | Expiration Date |
| | | | | | |
| | | | | | |
| | | | | | |
EXHIBIT C
to Security Agreement
COPYRIGHT SECURITY AGREEMENT
This COPYRIGHT SECURITY AGREEMENT, dated as of _______, 201_ (this “Agreement”), is made by [GRANTOR], a _______ corporation (the “Grantor”), in favor of PCOF 1, LLC (together with its successors, transferees or assignees, the “Secured Party”).
WITNESSETH:
WHEREAS, pursuant to a Credit Agreement and Guaranty, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Credit Agreement”), by and among the Borrower, each Guarantor party thereto and the Secured Party, the Secured Party has extended Commitments to make Loans to the Borrower;
WHEREAS, in connection with the Credit Agreement, the Grantor has executed and delivered a Pledge and Security Agreement, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Security Agreement”);
WHEREAS, pursuant to the Credit Agreement and pursuant toclause (d) ofSection 4.5 of the Security Agreement, the Grantor is required to execute and deliver this Agreement and to grant to the Secured Party a continuing security interest in all of the Copyright Collateral (as defined below) to secure all Obligations; and
WHEREAS, the Grantor has duly authorized the execution, delivery and performance of this Agreement; and
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Grantor agrees, for the benefit of the Secured Party, as follows:
SECTION 1.Definitions. Unless otherwise defined herein or the context otherwise requires, terms used in this Agreement, including its preamble and recitals, have the meanings provided in the Security Agreement.
SECTION 2.Grant of Security Interest. The Grantor hereby assigns, pledges, hypothecates, charges, mortgages, delivers, and transfers to the Secured Party, and hereby grants to the Secured Party, a continuing security interest in all of the following (the “Copyright Collateral”), whether now or hereafter existing or acquired by the Grantor: all copyrights owned by the Grantor, whether statutory or common law, registered or unregistered and whether published or unpublished, now or hereafter in force throughout the world including all of the Grantor’s right, title and interest in and to all copyrights registered in the United States Copyright Office or anywhere else in the world and also including the copyrights referred to inItem A ofSchedule I, and registrations and recordings thereof and all applications for registration thereof, all copyright licenses, including each copyright license referred to inItem B ofSchedule I, the right to sue for past, present and future infringements of any of the foregoing, all rights corresponding thereto, all extensions and renewals of any thereof and all Proceeds of the foregoing, including licenses, royalties, income, payments, claims, damages and Proceeds of suit.
SECTION 3.Security Agreement. This Agreement has been executed and delivered by the Grantor for the purpose of registering the security interest of the Secured Party in the Copyright Collateral with the United States Copyright Office and corresponding offices in other countries of the world. The security interest granted hereby has been granted as a supplement to, and not in limitation of, the security interest granted to the Secured Party under the Security Agreement. The Security Agreement (and all rights and remedies of the Secured Party thereunder) shall remain in full force and effect in accordance with its terms.
SECTION 4.Release of Liens. Upon (i) the Disposition of Copyright Collateral in accordance with the Credit Agreement or (ii) the occurrence of the Termination Date, the security interests granted herein shall automatically terminate with respect to (A) such Copyright Collateral (in the case ofclause (i)) or (B) all Copyright Collateral (in the case of clause (ii)). Upon any such Disposition or termination, the Secured Party will, at the Grantor’s sole expense, deliver to the Grantor, without any representations, warranties or recourse of any kind whatsoever, all Copyright Collateral held by the Secured Party hereunder, and execute and deliver to the Grantor such Documents as the Grantor shall reasonably request to evidence such termination.
SECTION 5.Acknowledgment. The Grantor does hereby further acknowledge and affirm that the rights and remedies of the Secured Party with respect to the security interest in the Copyright Collateral granted hereby are more fully set forth in the Security Agreement, the terms and provisions of which (including the remedies provided for therein) are incorporated by reference herein as if fully set forth herein.
SECTION 6.Loan Document. This Agreement is a Loan Document executed pursuant to the Credit Agreement and shall (unless otherwise expressly indicated herein) be construed, administered and applied in accordance with the terms and provisions thereof, including Article X thereof.
SECTION 7.Counterparts. This Agreement may be executed by the parties hereto in several counterparts, each of which shall be deemed to be an original and all of which shall constitute together but one and the same agreement.
* * * * *
IN WITNESS WHEREOF, each of the parties here to has caused this Agreement to be duly executed and delivered by its Authorized Officer as of the date first above written.
[NAME OF GRANTOR] |
| |
| |
By: | |
| | Name: |
| | Title: |
| |
| |
PCOF 1, LLC |
| |
| |
By: | |
| | Name: |
| | Title: |
SCHEDULE I
to Copyright Security Agreement
Item ACopyrights/Mask Works
Registered Copyrights/Mask Works
Country | Registration No. | Registration Date | Author(s) | Title |
| | | | | |
| | | | | |
| | | | | |
Copyright/Mask Work Pending Registration Applications
Country | Serial No. | Filing Date | Author(s) | Title |
| | | | | |
| | | | | |
| | | | | |
Item BCopyright/Mask Work Licenses
Country or Territory | Licensor | Licensee | Effective Date | Expiration Date |
| | | | | |
| | | | | |
| | | | | |
ANNEX I
to Security Agreement
SUPPLEMENT TO
PLEDGE AND SECURITY AGREEMENT
This SUPPLEMENT, dated as of _______, 201_ (this “Supplement”), is to the Pledge and Security Agreement, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Security Agreement”), among the Grantors (such term, and other terms used in this Supplement, to have the meanings set forth in Article I of the Security Agreement) from time to time party thereto, in favor of the Secured Party (together with its successors, transferees or assignees, the “Secured Party”).
WITNESSETH:
WHEREAS, pursuant to a Credit Agreement and Guaranty, dated as of July 25, 2014 (as amended or otherwise modified from time to time, the “Credit Agreement”), by and among the Borrower, each Guarantor party thereto and the Secured Party, the Secured Party has extended Commitments to make Loans to the Borrower; and
WHEREAS, pursuant to the provisions ofSection 7.6 of the Security Agreement, each of the undersigned is becoming a Grantor under the Security Agreement; and
WHEREAS, each of the undersigned desires to become a “Grantor” under the Security Agreement in order to induce the Secured Party to continue to extend Loans under the Credit Agreement;
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, each of the undersigned agrees, for the benefit of the Secured Party, as follows.
SECTION 1.Party to Security Agreement, etc. In accordance with the terms of the Security Agreement, by its signature below each of the undersigned hereby irrevocably agrees to become a Grantor under the Security Agreement with the same force and effect as if it were an original signatory thereto and each of the undersigned hereby (i) agrees to be bound by and comply with all of the terms and provisions of the Security Agreement applicable to it as a Grantor and (ii) represents and warrants that the representations and warranties made by it as a Grantor thereunder are true and correct as of the date hereof, unless stated to relate solely to an earlier date, in which case such representations and warranties shall be true and correct as of such earlier date. In furtherance of the foregoing, each reference to a “Grantor” and/or “Grantors” in the Security Agreement shall be deemed to include each of the undersigned.
SECTION 2.Representations. Each of the undersigned Grantor hereby represents and warrants that this Supplement has been duly authorized, executed and delivered by it and that this Supplement and the Security Agreement constitute the legal, valid and binding obligation of each of the undersigned, enforceable against it in accordance with its terms.
SECTION 3.Full Force of Security Agreement. Except as expressly supplemented hereby, the Security Agreement shall remain in full force and effect in accordance with its terms.
SECTION 4.Severability. Wherever possible each provision of this Supplement shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Supplement shall be prohibited by or invalid under such law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provision or the remaining provisions of this Supplement or the Security Agreement.
SECTION 5.Governing Law, Entire Agreement, etc. THIS SUPPLEMENT SHALL BE DEEMED TO BE A CONTRACT MADE UNDER AND GOVERNED BY THE INTERNAL LAWS OF THE STATE OF NEW YORK (INCLUDING FOR SUCH PURPOSE SECTIONS 5-1401 AND 5-1402 OF THE GENERAL OBLIGATIONS LAW OF THE STATE OF NEW YORK). This Supplement and the other Loan Documents constitute the entire understanding among the parties hereto with respect to the subject matter thereof and supersede any prior agreements, written or oral, with respect thereto.
SECTION 6.Counterparts. This Supplement may be executed by the parties hereto in several counterparts, each of which shall be deemed to be an original and all of which shall constitute together but one and the same agreement.
* * * * *
IN WITNESS WHEREOF, each of the parties here to has caused this Agreement to be duly executed and delivered by its Authorized Officer as of the date first above written.
| | [NAME OF ADDITIONAL SUBSIDIARY] |
| | |
| | |
| | By: | |
| | | Name: |
| | | Title: |
| | |
| | |
| | [NAME OF ADDITIONAL SUBSIDIARY] |
| | |
| | |
| | By: | |
| | | Name: |
| | | Title: |
| |
ACCEPTED AND AGREED |
| |
| |
PCOF 1, LLC |
| |
| |
By: | | |
| | Name: | |
| | Title: | |
EXHIBIT E
FORM OF CLOSING DATE WARRANT AGREEMENT
THIS WARRANT AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS WARRANT HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE "ACT"), OR QUALIFIED UNDER ANY STATE OR FOREIGN SECURITIES LAWS AND MAY NOT BE OFFERED FOR SALE, SOLD, PLEDGED, HYPOTHECATED OR OTHERWISE TRANSFERRED OR ASSIGNED UNLESS (I) A REGISTRATION STATEMENT COVERING SUCH SHARES IS EFFECTIVE UNDER THE ACT AND IS QUALIFIED UNDER APPLICABLE STATE AND FOREIGN LAW OR (II) THE TRANSACTION IS EXEMPT FROM THE REGISTRATION AND PROSPECTUS DELIVERY REQUIREMENTS UNDER THE ACT AND THE QUALIFICATION REQUIREMENTS UNDER APPLICABLE STATE AND FOREIGN LAW.
Warrant Certificate No.: 1
Original Issue Date: July 25, 2014
FOR VALUE RECEIVED, VBI VACCINES INC., a Delaware corporation (the "Company"), hereby certifies that PCOF 1, LLC or its permitted transferees and assigns (the "Holder") is entitled to purchase from the Company up to 699,281 duly authorized, validly issued, fully paid and nonassessable shares of Common Stock (defined below) at a per share purchase price of $2.145 (subject to adjustment as provided herein, the "Exercise Price"), all subject to the terms, conditions and adjustments set forth below in this Warrant (defined below). Certain capitalized terms used herein are defined inSection0 hereof.
Definitions. As used in this Warrant, the following terms have the following meanings:
“Acknowledgement” has the meaning set forth inSection 3(e).
"Aggregate Exercise Price" means, with respect to any exercise of this Warrant, an amount equal to the product of (i) the number of Warrant Shares in respect of which this Warrant is then being exercised pursuant toSection0 hereof, multiplied by (ii) the Exercise Price in effect as of the Exercise Date.
“Assignment” has the meaning set forth inSection 7.
"Board" means the board of directors of the Company.
"Business Day" means any day which is neither a Saturday or Sunday nor a legal holiday on which banks are authorized or required to be closed in New York, New York.
“Cashless Method” means, as the context may require, one or both of the Exercise Price payment methods described inclauses (ii) and(iii) ofSection 3(b).
"Commission" means the Securities and Exchange Commission or any other federal agency administering the Securities Act and the Exchange Act at the time.
"Common Stock" means the common stock, par value $0.0001 per share, of the Company, and any capital stock into which such Common Stock shall have been converted, exchanged or reclassified following the date hereof.
"Company" has the meaning set forth in the preamble.
"Convertible Securities" means any securities, whether debt, equity or other securities (directly or indirectly) convertible into or exchangeable for Common Stock, but excluding Options.
"Covered Persons" has the meaning set forth inSection 3(i).
“Credit Agreement” means the Credit Agreement and Guaranty dated as of the date hereof among Variation Biotechnologies (US), Inc., as borrower, PCOF 1, LLC, as lender, the Company, as guarantor and the other guarantors party thereto.
"Demand Registration" has the meaning set forth inSection 6(a)(ii).
"Disqualification Events" has the meaning set forth inSection 3(i).
"Exercise Date" means, for any given exercise of this Warrant, the date on which the conditions to such exercise as set forth inSection0 shall have been satisfied at or prior to 5:00 p.m., New York time, on a Business Day, including, without limitation, the receipt by the Company of the Subscription Agreement, the Warrant and the Aggregate Exercise Price.
"Exercise Period" has the meaning set forth inSection0.
"Exercise Price" has the meaning set forth in the preamble.
"Fair Market Value" means, as of any particular date: (i) the volume weighted average of the closing sales prices of the Common Stock for such day on all domestic securities exchanges on which the Common Stock may at the time be listed; (ii) if there have been no sales of the Common Stock on any such exchange on any such day, the average of the highest bid and lowest asked prices for the Common Stock on all such exchanges at the end of such day; (iii) if on any such day the Common Stock is not listed on a domestic securities exchange, the closing sales price of the Common Stock as quoted on Nasdaq, the OTC Bulletin Board, the “pink sheets” or similar quotation system or association for such day; or (iv) if there have been no sales of the Common Stock on Nasdaq, the OTC Bulletin Board, the “pink sheets” or similar quotation system or association on such day, the average of the highest bid and lowest asked prices for the Common Stock quoted on Nasdaq, the OTC Bulletin Board, the “pink sheets” or similar quotation system or association at the end of such day; in each case, averaged over twenty (20) consecutive Business Days ending on the Business Day immediately prior to the day as of which "Fair Market Value" is being determined;provided, that if the Common Stock is listed on any domestic securities exchange, the term "Business Day" as used in this sentence means Business Days on which such exchange is open for trading. If at any time the Common Stock is not listed on any domestic securities exchange or quoted on Nasdaq, the OTC Bulletin Board, the “pink sheets” or similar quotation system or association, the "Fair Market Value" of the Common Stock shall be the fair market value per share as determined jointly by the Board and the Holder.
"Holder" has the meaning set forth in the preamble.
"Indemnified Liabilities" has the meaning set forth inSection 18(b).
"Indemnitees" has the meaning set forth inSection 18(b).
“Inspectors” has the meaning set forth inSection 6(c)(viii).
“Long-Form Registration” has the meaning set forth inSection 6(a)(i).
“Merger Agreement Demand” means any demand for registration under the Securities Act of Registrable Securities pursuant to Section 6.14 of the Merger Agreement (as defined in the Credit Agreement).
"Nasdaq" means The Nasdaq Stock Market, Inc.
"Options" means any warrants or other rights or options to subscribe for or purchase Common Stock or Convertible Securities.
"Original Issue Date" means the date hereof.
"OTC Bulletin Board" means the National Association of Securities Dealers, Inc. OTC Bulletin Board.
"Person" means any individual, sole proprietorship, partnership, limited liability company, corporation, joint venture, trust, incorporated organization or government or department or agency thereof.
“Piggyback Registration” has the meaning set forth inSection 6(b)(i).
"Prospectus" means the prospectus or prospectuses included in any Registration Statement, as amended or supplemented by any prospectus supplement with respect to the terms of the offering of any portion of the Registrable Securities covered by such Registration Statement and by all other amendments and supplements to the prospectus, including post-effective amendments and all material incorporated by reference in such prospectus or prospectuses.
“Records” has the meaning set forth inSection 6(c)(viii).
"Registrable Securities" means (x) any shares of Common Stock held by any Person or issuable upon conversion, exercise or exchange of any securities owned by any Person at any time (including Warrant Shares exercisable upon exercise of this Warrant), and (y) any shares of Common Stock issued or issuable with respect to any shares described in subsection (x) above by way of a stock dividend or stock split or in connection with a combination of shares, recapitalization, merger, consolidation or other reorganization (it being understood that for purposes of this Warrant, a Person shall be deemed to be a holder of Registrable Securities whenever such Person has the right to then acquire or obtain from the Company any Registrable Securities, whether or not such acquisition has actually been effected). As to any particular Registrable Securities, such securities shall cease to be Registrable Securities when (i) a Registration Statement covering such securities has been declared effective by the Commission and such securities have been disposed of pursuant to such effective Registration Statement, (ii) such securities are sold under circumstances in which all of the applicable conditions of Rule 144 (or any similar provisions then in force) under the Securities Act are met, (iii) such securities are otherwise transferred and such securities may be resold without subsequent registration under the Securities Act, or (iv) such securities shall have ceased to be outstanding.
"Registration Statement" means any registration statement of the Company which covers any of the Registrable Securities pursuant to the provisions of this Agreement, including the Prospectus, amendments and supplements to such Registration Statement, including post-effective amendments, all exhibits and all materials incorporated by reference in such Registration Statement.
“Securities Act” means the Securities Act of 1933, as amended.
“Short-Form Registration” has the meaning set forth inSection 6(a)(ii).
"Solicitor" has the meaning set forth inSection 3(i).
"Subscription Agreement" has the meaning set forth inSection 3(a)(i).
"Warrant" means this Warrant and all warrants issued upon division or combination of, or in substitution for, this Warrant.
"Warrant Shares" means the shares of Common Stock or other capital stock of the Company purchasable upon exercise of this Warrant in accordance with its terms.
2. Term of Warrant. Subject to the terms and conditions hereof, at any time or from time to time after the date hereof up to and including 5:00 p.m., New York time, on the fifth (5th) anniversary of the Original Issue Date or, if such day is not a Business Day, on the next preceding Business Day (the "Exercise Period"), the Holder of this Warrant may exercise this Warrant for all or any part of the Warrant Shares purchasable hereunder (subject to adjustment as provided herein).
| 3. | | Exercise of Warrant. |
| | | |
| | (a) | Exercise Procedure. This Warrant may be exercised from time to time on any Business Day during the Exercise Period, for all or any part of the unexercised Warrant Shares, upon: |
| | | |
| | (i) | surrender of this Warrant to the Company at its then principal executive offices (or an indemnification undertaking with respect to this Warrant in the case of its loss, theft or destruction), together with a Subscription Agreement in the form attached hereto asExhibit A (each, a "Subscription Agreement"), duly completed (including specifying the number of Warrant Shares to be purchased) and executed; and |
| | | |
| | (ii) | payment to the Company of the Aggregate Exercise Price in accordance withSectionSection 1.01(a)(ii). |
| | | |
| | (b) | Payment of the Aggregate Exercise Price. Payment of the Aggregate Exercise Price shall be made, at the option of the Holder as expressed in the Subscription Agreement, by the following methods: |
| | | |
| | (i) | by delivery to the Company of a certified or official bank check payable to the order of the Company or by wire transfer of immediately available funds to an account designated in writing by the Company, in the amount of such Aggregate Exercise Price; |
| | | |
| | (ii) | by instructing the Company to withhold a number of Warrant Shares then issuable upon exercise of this Warrant with an aggregate Fair Market Value as of the Exercise Date equal to such Aggregate Exercise Price; |
| | | |
| | (iii) | by surrendering to the Company (x) Warrant Shares previously acquired by the Holder with an aggregate Fair Market Value as of the Exercise Date equal to such Aggregate Exercise Price and/or (y) other securities or debt obligations of the Company (including loans outstanding under the Credit Agreement) having a value as of the Exercise Date equal to the Aggregate Exercise Price (which value in the case of debt securities or obligations shall be the principal amount thereof plus accrued and unpaid interest, in the case of preferred stock shall be the liquidation value thereof plus accumulated and unpaid dividends and in the case of shares of Common Stock shall be the Fair Market Value thereof); or |
| | | |
| | (iv) | any combination of the foregoing; |
provided;however; that the Holder may only use the Cashless Method to pay the Exercise Price for up to (but not in excess of) 279,706 Warrant Shares (subject to proportionate adjustment for any stock split, dividend, distribution, subdivision, recapitalization or combination).
In the event of any withholding of Warrant Shares or surrender of other equity securities pursuant toclause (ii),(iii) or(iv) above where the number of shares whose value is equal to the Aggregate Exercise Price is not a whole number, the number of shares withheld by or surrendered to the Company shall be rounded up to the nearest whole share and the Company shall make a cash payment to the Holder (by delivery of a certified or official bank check or by wire transfer of immediately available funds) based on the incremental fraction of a share being so withheld by or surrendered to the Company in an amount equal to the product of (x) such incremental fraction of a share being so withheld or surrendered multiplied by (y) in the case of Common Stock, the Fair Market Value per Warrant Share as of the Exercise Date, and, in all other cases, the value thereof as of the Exercise Date determined in accordance withclause (iii)(y) above.
(c) Delivery of Stock Certificates. Upon receipt by the Company of the Subscription Agreement, surrender of this Warrant and payment of the Aggregate Exercise Price (in accordance withSectionSection 1.01(a) hereof), the Company shall, as promptly as practicable, and in any event within three (3) Business Days thereafter, execute (or cause to be executed) and deliver (or cause to be delivered) to the Holder a certificate or certificates representing the Warrant Shares issuable upon such exercise, together with cash in lieu of any fraction of a share, as provided inSectionSection 1.01(c) hereof. The stock certificate or certificates so delivered shall be, to the extent possible, in such denomination or denominations as the exercising Holder shall reasonably request in the Subscription Agreement and shall be registered in the name of the Holder or, subject to compliance withSection0 below, such other Person's name as shall be designated in the Subscription Agreement. This Warrant shall be deemed to have been exercised and such certificate or certificates of Warrant Shares shall be deemed to have been issued, and the Holder or any other Person so designated to be named therein shall be deemed to have become a holder of record of such Warrant Shares for all purposes, as of the Exercise Date.
(d) Fractional Shares. The Company shall not be required to issue a fractional Warrant Share upon exercise of any Warrant. As to any fraction of a Warrant Share that the Holder would otherwise be entitled to purchase upon such exercise, the Company shall pay to such Holder an amount in cash (by delivery of a certified or official bank check or by wire transfer of immediately available funds) equal to the product of (i) such fraction multiplied by (ii) the Fair Market Value of one Warrant Share on the Exercise Date.
(e) Acknowledgement; Partial Exercise. Unless the purchase rights represented by this Warrant shall have expired or shall have been fully exercised, the Company shall, at the time of delivery of the certificate or certificates representing the Warrant Shares being issued in accordance withSectionSection 1.01(c) hereof, deliver to the Holder within a reasonable time an acknowledgement in substantially the form attached hereto asExhibit B (each, an “Acknowledgement”) indicating the number of Warrant Shares which remain issuable upon exercise of this Warrant, if any.
(f) Valid Issuance of Warrant and Warrant Shares; Payment of Taxes. With respect to the exercise of this Warrant, the Company hereby represents, covenants and agrees:
(i) This Warrant is, and any Warrant issued in substitution for or replacement of this Warrant shall be, upon issuance, duly authorized and validly issued.
(ii) All Warrant Shares issuable upon the exercise of this Warrant pursuant to the terms hereof shall be, upon issuance, and the Company shall take all such actions as may be necessary or appropriate in order that such Warrant Shares are, validly issued, fully paid and non-assessable, issued without violation of any preemptive or similar rights of any stockholder of the Company and free and clear of all taxes, liens and charges.
(iii) The Company shall take all such actions as may be necessary to ensure that all such Warrant Shares are issued without violation by the Company of any applicable law or governmental regulation or any requirements of any domestic securities exchange upon which shares of Common Stock or other securities constituting Warrant Shares may be listed at the time of such exercise (except for official notice of issuance which shall be immediately delivered by the Company upon each such issuance).
(iv) Without in any way limitingSection 6 hereof, the Company shall use its best efforts to cause the Warrant Shares, immediately upon such exercise, to be listed on any domestic securities exchange upon which shares of Common Stock or other securities constituting Warrant Shares are listed at the time of such exercise.
(v) The Company shall pay all expenses in connection with, and all taxes and other governmental charges that may be imposed with respect to, the issuance or delivery of Warrant Shares upon exercise of this Warrant;provided, that the Company shall not be required to pay any tax or governmental charge that may be imposed with respect to any applicable withholding or the issuance or delivery of the Warrant Shares to any Person other than the Holder, and no such issuance or delivery shall be made unless and until the Person requesting such issuance has paid to the Company the amount of any such tax, or has established to the satisfaction of the Company that such tax has been paid.
(g) Conditional Exercise. Notwithstanding any other provision hereof, if an exercise of any portion of this Warrant is to be made in connection with a public offering or a sale of the Company (pursuant to a merger, sale of stock, or otherwise), such exercise may at the election of the Holder be conditioned upon the consummation of such transaction, in which case such exercise shall not be deemed to be effective until immediately prior to the consummation of such transaction.
(h) Reservation of Shares. During the Exercise Period, the Company shall at all times reserve and keep available out of its authorized but unissued Common Stock or other securities constituting Warrant Shares, solely for the purpose of issuance upon the exercise of this Warrant, the maximum number of Warrant Shares issuable upon the exercise of this Warrant, and the par value per Warrant Share shall at all times be less than or equal to the applicable Exercise Price. The Company shall not increase the par value of any Warrant Shares receivable upon the exercise of this Warrant above the Exercise Price then in effect, and shall take all such actions as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable shares of Common Stock upon the exercise of this Warrant.
(i) No “Bad Actor” Disqualification. The Company has exercised reasonable care, in accordance with Commission rules and guidance, to determine whether any Covered Person (as defined below) is subject to any of the “bad actor” disqualifications described in Rule 506(d)(1)(i) through (viii) under the Securities Act (“Disqualification Events”). To the Company’s knowledge, no Covered Person is subject to a Disqualification Event. The Company has complied, to the extent applicable, with all disclosure obligations under Rule 506(e) under the Securities Act. “Covered Persons” are those persons specified in Rule 506(d)(1) under the Securities Act, including the Company, any predecessor or affiliate of the Company, any director, executive officer, other officer participating in the offering, general partner or managing member of the Company, any beneficial owner of 20% or more of the Company’s outstanding voting equity securities, calculated on the basis of voting power, any promoter (as defined in Rule 405 under the Securities Act) connected with the Company in any capacity at the time of the exercise of this Warrant, and any person that has been or will be paid (directly or indirectly) remuneration for solicitation of purchasers in connection with the sale of any securities of the Company (a “Solicitor”), any general partner or managing member of any Solicitor, and any director, executive officer or other officer participating in the offering of any Solicitor or general partner or managing member of any such Solicitor.
4. Adjustment to Exercise Price and Number of Warrant Shares. The Exercise Price and the number of Warrant Shares issuable upon exercise of this Warrant shall be subject to adjustment from time to time as provided in thisSection0.
(a) Dividends and Distributions of Non-Company Securities. If, at any time or from time to time after the Original Issue Date, the Company makes or declares, or fixes a record date for the determination of holders of Common Stock entitled to receive, a dividend or any other distribution payable in securities of the Company (other than a dividend or distribution of shares of Common Stock or Options or Convertible Securities in each case in respect of Common Stock), cash or other property, then, and in each such event, provision shall be made so that the Holder shall receive upon exercise of the Warrant, in addition to the number of Warrant Shares receivable thereupon, the kind and amount of securities of the Company, cash or other property which the Holder would have been entitled to receive had the Warrant been exercised in full into Warrant Shares on the date of such event and had the Holder thereafter, during the period from the date of such event to and including the Exercise Date, retained such securities, cash or other property receivable by them as aforesaid during such period, giving application to all adjustments called for during such period under thisSection0 with respect to the rights of the Holder;provided, that no such provision shall be made if the Holder receives, simultaneously with the distribution to the holders of Common Stock, a dividend or other distribution of such securities, cash or other property in an amount equal to the amount of such securities, cash or other property as the Holder would have received if the Warrant had been exercised in full into Warrant Shares on the date of such event.
(b) Adjustment to Exercise Price and Warrant Shares Upon Dividend, Subdivision or Combination of Common Stock. If the Company shall, at any time or from time to time after the Original Issue Date, (i) pay a dividend or make any other distribution upon the Common Stock or any other capital stock of the Company payable in shares of Common Stock or in Options or Convertible Securities (in each case in respect of Common Stock), or (ii) subdivide (by any stock split, recapitalization or otherwise) its outstanding shares of Common Stock into a greater number of shares, the Exercise Price in effect immediately prior to any such dividend, distribution or subdivision shall be proportionately reduced and the number of Warrant Shares issuable upon exercise of this Warrant shall be proportionately increased. If the Company at any time combines (by combination, reverse stock split or otherwise) its outstanding shares of Common Stock into a smaller number of shares, the Exercise Price in effect immediately prior to such combination shall be proportionately increased and the number of Warrant Shares issuable upon exercise of this Warrant shall be proportionately decreased. Any adjustment under thisSection 4(b) shall become effective at the close of business on the date the dividend, subdivision or combination becomes effective.
(c) Adjustment to Exercise Price and Warrant Shares Upon Reorganization, Reclassification, Consolidation or Merger. In the event of any (i) capital reorganization of the Company, (ii) reclassification of the stock of the Company (other than a change in par value or from par value to no par value or from no par value to par value or as a result of a stock dividend or subdivision, split-up or combination of shares), (iii) consolidation or merger of the Company with or into another Person, (iv) sale of all or substantially all of the Company's assets to another Person, (v) Change of Control (as defined in the Credit Agreement) or (vi) other similar transaction, in each case which entitles the holders of Common Stock to receive (either directly or upon subsequent liquidation) cash, stock, securities or assets with respect to or in exchange for Common Stock, each Warrant shall, immediately after such reorganization, reclassification, consolidation, merger, sale, Change of Control or similar transaction, remain outstanding and shall thereafter, in lieu of or in addition to (as the case may be) the number of Warrant Shares then exercisable under this Warrant, be exercisable for the amount of cash or the kind and number of shares of stock or other securities or assets of the Company or of the successor Person resulting from such transaction to which the Holder would have been entitled upon such reorganization, reclassification, consolidation, merger, sale, Change of Control or similar transaction if the Holder had exercised this Warrant in full immediately prior to the time of such reorganization, reclassification, consolidation, merger, sale, Change of Control or similar transaction and acquired the applicable number of Warrant Shares then issuable hereunder as a result of such exercise (without taking into account any limitations or restrictions on the exercisability of this Warrant); and, in such case, appropriate adjustment (in form and substance satisfactory to the Holder) shall be made with respect to the Holder's rights under this Warrant to insure that the provisions of thisSection0 hereof shall thereafter be applicable, as nearly as possible, to this Warrant in relation to any cash, shares of stock, securities or assets thereafter acquirable upon exercise of this Warrant (including, in the case of any consolidation, merger, sale, Change of Control or similar transaction in which the successor or purchasing Person is other than the Company, an immediate adjustment in the Exercise Price to the value per share for the Common Stock reflected by the terms of such consolidation, merger, sale, Change of Control or similar transaction, and a corresponding immediate adjustment to the number of Warrant Shares acquirable upon exercise of this Warrant without regard to any limitations or restrictions on exercise, if the value so reflected is less than the Exercise Price in effect immediately prior to such consolidation, merger, sale, Change of Control or similar transaction). The provisions of thisSection 4(c) shall similarly apply to successive reorganizations, reclassifications, consolidations, mergers, sales, Changes of Control or similar transactions. The Company shall not effect any such reorganization, reclassification, consolidation, merger, sale, Change of Control or similar transaction unless, prior to the consummation thereof, the successor Person (if other than the Company) resulting from such reorganization, reclassification, consolidation, merger, sale, Change of Control or similar transaction, shall assume, by written instrument substantially similar in form and substance to this Warrant and satisfactory to the Holder, the obligation to deliver to the Holder such shares of stock, securities or assets which, in accordance with the foregoing provisions, such Holder shall be entitled to receive upon exercise of this Warrant. Notwithstanding anything to the contrary contained herein, with respect to any corporate event or other transaction contemplated by the provisions of thisSection 4(c), the Holder shall have the right to elect prior to the consummation of such event or transaction, to give effect to the exercise rights contained inSection0 instead of giving effect to the provisions contained in thisSection 4(c) with respect to this Warrant.
(d) Certain Events. If any event of the type contemplated by the provisions of thisSection0 but not expressly provided for by such provisions occurs, then the Board shall make an appropriate adjustment in the Exercise Price and the number of Warrant Shares issuable upon exercise of this Warrant so as to protect the rights of the Holder in a manner consistent with the provisions of thisSection0;provided, that no such adjustment pursuant to thisSection 4(d) shall increase the Exercise Price or decrease the number of Warrant Shares issuable as otherwise determined pursuant to thisSection0.
(e) Certificate as to Adjustment.
(i) As promptly as reasonably practicable following any adjustment of the Exercise Price, but in any event not later than three (3) Business Days thereafter, the Company shall furnish to the Holder a certificate of an executive officer setting forth in reasonable detail such adjustment and the facts upon which it is based and certifying the calculation thereof.
(ii) As promptly as reasonably practicable following the receipt by the Company of a written request by the Holder, but in any event not later than three (3) Business Days thereafter, the Company shall furnish to the Holder a certificate of an executive officer certifying the Exercise Price then in effect and the number of Warrant Shares or the amount, if any, of other shares of stock, securities or assets then issuable upon exercise of the Warrant.
| | (f) | Notices. In the event: |
| | | |
| | (i) | that the Company shall take a record of the holders of its Common Stock (or other capital stock or securities at the time issuable upon exercise of the Warrant) for the purpose of entitling or enabling them to receive any dividend or other distribution, to vote at a meeting (or by written consent), to receive any right to subscribe for or purchase any shares of capital stock of any class or any other securities, or to receive any other security; or |
| | | |
| | (ii) | of any capital reorganization of the Company, any reclassification of the Common Stock of the Company, any consolidation or merger of the Company with or into another Person, or sale of all or substantially all of the Company's assets to another Person; or |
| | | |
| | (iii) | of the voluntary or involuntary dissolution, liquidation or winding-up of the Company; |
then, and in each such case, the Company shall send or cause to be sent to the Holder at least ten (10) Business days prior to the applicable record date or the applicable expected effective date, as the case may be, for the event, a written notice specifying, as the case may be, (A) the record date for such dividend, distribution, meeting or consent or other right or action, and a description of such dividend, distribution or other right or action to be taken at such meeting or by written consent, or (B) the effective date on which such reorganization, reclassification, consolidation, merger, sale, dissolution, liquidation or winding-up is proposed to take place, and the date, if any is to be fixed, as of which the books of the Company shall close or a record shall be taken with respect to which the holders of record of Common Stock (or such other capital stock or securities at the time issuable upon exercise of the Warrant) shall be entitled to exchange their shares of Common Stock (or such other capital stock or securities) for securities or other property deliverable upon such reorganization, reclassification, consolidation, merger, sale, dissolution, liquidation or winding-up, and the amount per share and character of such exchange applicable to the Warrant and the Warrant Shares.
5. Purchase Rights. In addition to (and not in limitation or in lieu of) any adjustments pursuant toSection0 above, if at any time the Company grants, issues or sells any shares of Common Stock, Options, Convertible Securities or rights to purchase stock, warrants, securities or other property pro rata to the record holders of Common Stock (the "Purchase Rights"), then the Holder shall be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder would have acquired if the Holder had held the number of Warrant Shares acquirable upon complete exercise of this Warrant immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights.
| 6. | | Registration Rights. |
| | | |
| | (a) | Demand Registration. |
(i) At any time after the one hundred eightieth (180) day following the Original Issue Date, the Holder may request registration under the Securities Act of all or any portion of the Warrant Shares on Form S-1 or any successor form thereto (each a "Long-Form Registration"). Each request for a Long-Form Registration shall specify the approximate number of Warrant Shares required to be registered. Upon receipt of such request, the Company shall promptly (but in no event later than five days following receipt thereof) deliver notice of such request to all other holders of Registrable Securities having “piggy back” rights or equivalent, if any, who shall then have ten days from the date such notice is given to notify the Company in writing of their desire to be included in such registration. The Company shall cause a Registration Statement on Form S-1 (or any successor form) to be filed within 30 days after the date on which the initial request is given and shall use its best efforts to cause such Registration Statement to be declared effective by the Commission as soon as practicable thereafter. The Company shall not be required to effect a Long-Form Registration more than five times by the Holder;provided, that a Registration Statement shall not count as a Long-Form Registration requested under thisSectionSection 1.01(a)(i) unless and until it has become effective and the Holder is able to register and sell at least 35% of the Warrant Shares requested to be included in such registration.
(ii) The Company shall use its best efforts to qualify and remain qualified to register securities under the Securities Act pursuant to a Registration Statement on Form S-3 or any successor form thereto. At such time as the Company shall have qualified for the use of a Registration Statement on Form S-3, the Holder shall have the right to request an unlimited number of registrations of Warrant Shares on Form S-3 or any similar short-form registration (each a "Short-Form Registration" and, together with each Long-Form Registration, a "Demand Registration"). Each request for a Short-Form Registration shall specify the approximate number of Warrant Shares requested to be registered. Upon receipt of any such request, the Company shall promptly (but in no event later than five days following receipt thereof) deliver notice of such request to all other holders of Registrable Securities having “piggy back” rights or equivalent, if any, who shall then have ten days from the date such notice is given to notify the Company in writing of their desire to be included in such registration. The Company shall cause a Registration Statement on Form S-3 (or any successor form) to be filed within 30 days after the date on which the initial request is given and shall use its best efforts to cause such Registration Statement to be declared effective by the Commission as soon as practicable thereafter.
(iii) The Company shall not be obligated to effect any Long-Form Registration within 90 days after the effective date of a previous Long-Form Registration or a previous Piggyback Registration in which the Holder was permitted to register, and actually sold, at least 35% of the shares of Registrable Securities requested to be included therein. The Company may postpone for up to 60 days the filing or effectiveness of a Registration Statement for a Demand Registration if the Company's Board determines in its reasonable good faith judgment that such Demand Registration would (i) materially interfere with a significant acquisition, corporate organization or other similar transaction involving the Company; (ii) require premature disclosure of material information that the Company has a bona fide business purpose for preserving as confidential; or (iii) render the Company unable to comply with requirements under the Securities Act or Exchange Act. The Company may delay a Demand Registration hereunder only two times in any period of twelve consecutive months.
(iv) If the Holder elects to distribute Warrant Shares covered by its request in an underwritten offering, it shall so advise the Company as a part of its request made pursuant toSectionSection 1.01(a)(i)(i) orSection 6(a)(ii). The Holder shall select the investment banking firm or firms to act as the managing underwriter or underwriters in connection with such offering.
(v) The Company shall not include in any Demand Registration any securities which are not Registrable Securities without the prior written consent of the Holder, which consent shall not be unreasonably withheld or delayed. If a Demand Registration involves an underwritten offering and the managing underwriter of the requested Demand Registration advises the Company in writing that in its opinion the number of shares of Common Stock proposed to be included in the Demand Registration, including all Registrable Securities and all other shares of Common Stock proposed to be included in such underwritten offering, exceeds the number of shares of Common Stock which can be sold in such underwritten offering and/or the number of shares of Common Stock proposed to be included in such registration would adversely affect the price per share of the Registrable Securities proposed to be sold in such underwritten offering, the Company shall include in such Demand Registration (A) first, all of the Warrant Shares requested to be included in such registration by the Holder, and (B) second, any other Registrable Securities the Company may permit to be included in such registration, allocated pro rata among the respective holders thereof on the basis of the number of Registrable Securities owned by each such holder.
| | (b) | Piggyback Registration. |
(i) Whenever the Company proposes to register any shares of its Common Stock under the Securities Act (other than a registration effected solely to implement an employee benefit plan or a transaction to which Rule 145 of the Securities Act is applicable, or a Registration Statement on Form S-4, S-8 or any successor form thereto or another form not available for registering the Registrable Securities for sale to the public), whether for its own account or for the account of one or more stockholders of the Company and the form of Registration Statement to be used may be used for any registration of Warrant Shares (a"Piggyback Registration"), the Company shall give prompt written notice (in any event no later than twenty (20) days prior to the filing of such Registration Statement) to the Holder of its intention to effect such a registration and, subject toSection 6(b)(ii) andSection 6(b)(iii), shall include in such registration all Warrant Shares with respect to which the Company has received written requests for inclusion from the Holder within ten days after the Company's notice has been given to the Holder.
(ii) If a Piggyback Registration is initiated as a primary underwritten offering on behalf of the Company and the managing underwriter advises the Company and the Holder (if the Holder has elected to include Warrant Shares in such Piggyback Registration) in writing that in its opinion the number of shares of Common Stock proposed to be included in such registration, including all Registrable Securities and all other shares of Common Stock proposed to be included in such underwritten offering, exceeds the number of shares of Common Stock which can be sold in such offering and/or that the number of shares of Common Stock proposed to be included in any such registration would adversely affect the price per share of the Common Stock to be sold in such offering, the Company shall include in such registration (A) first, the number of shares of Common Stock that the Company proposes to sell; (B) second, the number of shares of Common Stock requested to be included therein by the Holder; and (C) third, the number of shares of Common Stock requested to be included therein by holders of Common Stock (other than Warrant Shares held by the Holder);provided, that in any event the Holder shall be entitled to register at least 35% of the securities to be included in any such registration.
(iii) If a Piggyback Registration is initiated as an underwritten offering on behalf of one or more holders of Common Stock other than Warrant Shares, and the managing underwriter advises the Company in writing that in its opinion the number of shares of Common Stock proposed to be included in such registration, including all Warrant Shares and all other shares of Common Stock proposed to be included in such underwritten offering, exceeds the number of shares of Common Stock which can be sold in such offering and/or that the number of shares of Common Stock proposed to be included in any such registration would adversely affect the price per share of the Common Stock to be sold in such offering, the Company shall, subject to the proviso below, include in such registration (i) first, the number of shares of Common Stock requested to be included therein by the Holder (on a fully diluted, as converted basis); and (ii) second, the number of shares of Common Stock requested to be included therein by other holders of Common Stock, allocated among such holders in such manner as they may agree;provided that, in the event of a registration resulting from a Merger Agreement Demand, the Company shall include in such registration, on a pro rata basis, (x) those shares of Common Stock (other than Warrant Shares) of the holders thereof requesting such Merger Agreement Demand, and (y) those Warrant Shares requested to be included in such registration by the Holder.
(iv) If any Piggyback Registration is initiated as a primary underwritten offering on behalf of the Company, the Company shall select the investment banking firm or firms to act as the managing underwriter or underwriters in connection with such offering.
(c) Registration Procedures. If and whenever the Holder requests that any Warrant Shares be registered pursuant to the provisions of this Warrant, the Company shall use its best efforts to effect the registration and the sale of such Warrant Shares in accordance with the intended method of disposition thereof, and pursuant thereto the Company shall as soon as practicable:
(i) subject toSection 6(a)(i) andSection 6(a)(ii), prepare and file with the Commission a Registration Statement with respect to such Warrant Shares and use its best efforts to cause such Registration Statement to become effective;
(ii) prepare and file with the Commission such amendments, post-effective amendments and supplements to such Registration Statement and the Prospectus used in connection therewith as may be necessary to keep such Registration Statement effective for a period of not less than thirty (30) days, or if earlier, until all of such Warrant Shares have been disposed of and to comply with the provisions of the Securities Act with respect to the disposition of such Warrant Shares in accordance with the intended methods of disposition set forth in such Registration Statement;
(iii) at least fifteen (15) Business Days before filing such Registration Statement, Prospectus or amendments or supplements thereto, furnish to counsel of the Holder copies of such documents proposed to be filed, which documents shall be subject to the review, comment and approval of such counsel;
(iv) notify the Holder, promptly after the Company receives notice thereof, of the time when such Registration Statement has been declared effective or a supplement to any Prospectus forming a part of such Registration Statement has been filed;
(v) furnish to the Holder such number of copies of the Prospectus included in such Registration Statement (including each preliminary Prospectus) and any supplement thereto (in each case including all exhibits and documents incorporated by reference therein) and such other documents as the Holder may request in order to facilitate the disposition of the Warrant Shares;
(vi) use its best efforts to register or qualify such Warrant Shares under such other securities or "blue sky" laws of such jurisdictions as any selling holder requests and do any and all other acts and things which may be necessary or advisable to enable the Holder to consummate the disposition;provided, that the Company shall not be required to qualify generally to do business, subject itself to general taxation or consent to general service of process in any jurisdiction where it would not otherwise be required to do so but for thisSection 6(c)(vi);
(vii) notify the Holder, at any time when a Prospectus relating thereto is required to be delivered under the Securities Act, of the happening of any event as a result of which the Prospectus included in such Registration Statement contains an untrue statement of a material fact or omits any fact necessary to make the statements therein not misleading, and, at the request of any such holder, the Company shall prepare a supplement or amendment to such Prospectus so that, as thereafter delivered to the purchasers of such Warrant Shares, such Prospectus shall not contain an untrue statement of a material fact or omit to state any fact necessary to make the statements therein not misleading;
(viii) make available for inspection by the Holder, any underwriter participating in any disposition pursuant to such Registration Statement and any attorney, accountant or other agent retained by the Holder or any such underwriter (collectively, the "Inspectors"), all financial and other records, pertinent corporate documents and properties of the Company (collectively, the "Records"), and cause the Company's officers, directors and employees to supply all information requested by any such Inspector in connection with such Registration Statement;
(ix) use its best efforts to cause such Warrant Shares to be listed on each securities exchange on which the Common Stock is then listed or, if the Common Stock is not then listed, on a national securities exchange selected by the Holder;
(x) in connection with an underwritten offering, enter into such customary agreements (including underwriting and lock-up agreements in customary form) and take all such other customary actions as the Holder or the managing underwriter of such offering request in order to expedite or facilitate the disposition of such Warrant Shares (including, without limitation, making appropriate officers of the Company available to participate in "road show" and other customary marketing activities (including one-on-one meetings with prospective purchasers of the Warrant Shares);
(xi) otherwise use its best efforts to comply with all applicable rules and regulations of the Commission and make available to its stockholders an earnings statement (in a form that satisfies the provisions of Section 11(a) of the Securities Act and Rule 158 thereunder) no later than thirty (30) days after the end of the 12-month period beginning with the first day of the Company's first full fiscal quarter after the effective date of such Registration Statement, which earnings statement shall cover said 12-month period, and which requirement will be deemed to be satisfied if the Company timely files complete and accurate information on Forms 10-Q, 10-K and 8-K under the Exchange Act and otherwise complies with Rule 158 under the Securities Act;
(xii) furnish to the Holder and each underwriter, if any, with (i) a legal opinion of the Company's outside counsel, dated the effective date of such Registration Statement (and, if such registration includes an underwritten public offering, dated the date of the closing under the underwriting agreement), in form and substance as is customarily given in opinions of the Company's counsel to underwriters in underwritten public offerings; and (ii) a "comfort" letter signed by the Company's independent certified public accountants in form and substance as is customarily given in accountants' letters to underwriters in underwritten public offerings;
(xiii) without limitingSection 6(c)(vi) above, use its best efforts to cause such Warrant Shares to be registered with or approved by such other governmental agencies or authorities as may be necessary by virtue of the business and operations of the Company to enable the Holder to consummate the disposition of such Warrant Shares in accordance with their intended method of distribution thereof;
(xiv) notify the Holder promptly of any request by the Commission for the amending or supplementing of such Registration Statement or Prospectus or for additional information;
(xv) advise the Holder, promptly after it shall receive notice or obtain knowledge thereof, of the issuance of any stop order by the Commission suspending the effectiveness of such Registration Statement or the initiation or threatening of any proceeding for such purpose and promptly use its best efforts to prevent the issuance of any stop order or to obtain its withdrawal at the earliest possible moment if such stop order should be issued; and
(xvi) otherwise use its best efforts to take all other steps necessary to effect the registration of such Warrant Shares contemplated hereby.
7. Transfer of Warrant. Subject to the transfer conditions referred to in the legend endorsed hereon, this Warrant and all rights hereunder are freely transferable, in whole or in part, by the Holder without charge to the Holder, upon delivery to the Company of a written request for assignment in the form attached hereto asExhibit C (each, an “Assignment”) by the Holder and surrender of this Warrant to the Company at its then principal executive offices, together with funds sufficient to pay any transfer taxes described inSection 3(f)(v) in connection with the making of such transfer. If requested by the Company, the Holder will also provide an opinion of counsel satisfactory to the Company to the effect that the transfer or assignment is in compliance with (or is exempt from) applicable federal and state securities laws. Upon such compliance, surrender and delivery and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees and in the denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant, if any, not so assigned and this Warrant shall promptly be cancelled.
8. Holder Not Deemed a Stockholder; Limitations on Liability. Except as otherwise specifically provided herein (includingSection 4(a)), prior to the issuance to the Holder of the Warrant Shares to which the Holder is then entitled to receive upon the due exercise of this Warrant, the Holder shall not be entitled to vote or receive dividends or be deemed the holder of shares of capital stock of the Company for any purpose, nor shall anything contained in this Warrant be construed to confer upon the Holder, as such, any of the rights of a stockholder of the Company or any right to vote, give or withhold consent to any corporate action (whether any reorganization, issue of stock, reclassification of stock, consolidation, merger, conveyance or otherwise), receive notice of meetings, receive dividends or subscription rights, or otherwise. In addition, nothing contained in this Warrant shall be construed as imposing any liabilities on the Holder to purchase any securities (upon exercise of this Warrant or otherwise) or as a stockholder of the Company, whether such liabilities are asserted by the Company or by creditors of the Company. Notwithstanding thisSection0, the Company shall provide the Holder with copies of the same notices and other information given to the stockholders of the Company generally, contemporaneously with the giving thereof to the stockholders.
9. Replacement on Loss; Division and Combination.
(a) Replacement of Warrant on Loss. Upon receipt of evidence reasonably satisfactory to the Company of the loss, theft, destruction or mutilation of this Warrant and upon delivery of an indemnity reasonably satisfactory to it (it being understood that a written indemnification agreement or affidavit of loss of the Holder shall be a sufficient indemnity) and, in case of mutilation, upon surrender of such Warrant for cancellation to the Company, the Company at its own expense shall execute and deliver to the Holder, in lieu hereof, a new Warrant of like tenor and exercisable for an equivalent number of Warrant Shares as the Warrant so lost, stolen, mutilated or destroyed;provided, that, in the case of mutilation, no indemnity shall be required if this Warrant in identifiable form is surrendered to the Company for cancellation.
(b) Division and Combination of Warrant. Subject to compliance with the applicable provisions of this Warrant as to any transfer or other assignment which may be involved in such division or combination, this Warrant may be divided or, following any such division of this Warrant, subsequently combined with other Warrants, upon the surrender of this Warrant or Warrants to the Company at its then principal executive offices, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the respective Holders or their agents or attorneys. Subject to compliance with the applicable provisions of this Warrant as to any transfer or assignment which may be involved in such division or combination, the Company shall at its own expense execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants so surrendered in accordance with such notice. Such new Warrant or Warrants shall be of like tenor to the surrendered Warrant or Warrants and shall be exercisable in the aggregate for an equivalent number of Warrant Shares as the Warrant or Warrants so surrendered in accordance with such notice.
10. No Impairment. The Company shall not, by amendment of its Certificate of Incorporation or Bylaws, or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities, or any other voluntary action, avoid or circumvent or seek to avoid or circumvent the observance or performance of any of the terms to be observed or performed by it hereunder, but shall at all times in good faith assist in the carrying out of all the provisions of this Warrant and in the taking of all such action as may reasonably be requested by the Holder in order to protect the exercise rights of the Holder against dilution or other impairment, consistent with the tenor and purpose of this Warrant.
11. Compliance with the Securities Act. The Holder, by acceptance of this Warrant, agrees to comply in all respects with the provisions of thisSection0 and the restrictive legend requirements set forth on the face of this Warrant and further agrees that such Holder shall not offer, sell or otherwise dispose of this Warrant or any Warrant Shares to be issued upon exercise hereof except under circumstances that will not result in a violation of the Securities Act. This Warrant and all Warrant Shares issued upon exercise of this Warrant (unless registered under the Securities Act) shall be stamped or imprinted with a legend in substantially the following form:
"THIS WARRANT AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS WARRANT HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE "ACT"), OR QUALIFIED UNDER ANY STATE OR FOREIGN SECURITIES LAWS AND MAY NOT BE OFFERED FOR SALE, SOLD, PLEDGED, HYPOTHECATED OR OTHERWISE TRANSFERRED OR ASSIGNED UNLESS (I) A REGISTRATION STATEMENT COVERING SUCH SHARES IS EFFECTIVE UNDER THE ACT AND IS QUALIFIED UNDER APPLICABLE STATE AND FOREIGN LAW OR (II) THE TRANSACTION IS EXEMPT FROM THE REGISTRATION AND PROSPECTUS DELIVERY REQUIREMENTS UNDER THE ACT AND THE QUALIFICATION REQUIREMENTS UNDER APPLICABLE STATE AND FOREIGN LAW."
12. Representations of the Holder. In connection with the issuance of this Warrant, the Holder specifically represents, as of the date hereof, to the Company by acceptance of this Warrant as follows:
(a) The Holder is an "accredited investor" as defined in Rule 501 of Regulation D promulgated under the Securities Act. The Holder is acquiring this Warrant and the Warrant Shares to be issued upon exercise hereof for investment for its own account and not with a current view towards, or for resale in connection with, the public sale or distribution of this Warrant or the Warrant Shares, except pursuant to sales registered or exempted under the Securities Act.
(b) The Holder understands and acknowledges that this Warrant and the Warrant Shares to be issued upon exercise hereof are "restricted securities" under the federal securities laws inasmuch as they are being acquired from the Company in a transaction not involving a public offering and that, under such laws and applicable regulations, such securities may be resold without registration under the Securities Act only in certain limited circumstances. In addition, the Holder represents that it is familiar with Rule 144 under the Securities Act, as presently in effect, and understands the resale limitations imposed thereby and by the Securities Act.
(c) The Holder acknowledges that it can bear the economic and financial risk of its investment for an indefinite period, and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment in the Warrant and the Warrant Shares. The Holder has had an opportunity to ask questions and receive answers from the Company regarding the terms and conditions of the offering of the Warrant and the business, properties, prospects and financial condition of the Company.
13. Warrant Register. The Company shall keep and properly maintain at its principal executive offices books for the registration of the Warrant and any transfers thereof. The Company may deem and treat the Person in whose name the Warrant is registered on such register as the Holder thereof for all purposes, and the Company shall not be affected by any notice to the contrary, except any assignment, division, combination or other transfer of the Warrant effected in accordance with the provisions of this Warrant.
14. Notices. All notices and other communications provided hereunder shall be in writing or by facsimile and addressed, delivered or transmitted, if to the Company or the Holder, to the applicable party at its address or facsimile number set forth on the signature pages hereto, or at such other address or facsimile number as may be designated by such party in a notice to the other party. Any notice, if mailed and properly addressed with postage prepaid or if properly addressed and sent by pre-paid courier service, shall be deemed given when received; any notice, if transmitted by facsimile, shall be deemed given when the confirmation of transmission thereof is received by the transmitter. Unless otherwise indicated, all references to the time of a day shall refer to New York City time.
15. Cumulative Remedies. Except to the extent expressly provided inSection0 to the contrary, the rights and remedies provided in this Warrant are cumulative and are not exclusive of, and are in addition to and not in substitution for, any other rights or remedies available at law, in equity or otherwise.
16. Equitable Relief. Each of the Company and the Holder acknowledges that a breach or threatened breach by such party of any of its obligations under this Warrant would give rise to irreparable harm to the other party hereto for which monetary damages would not be an adequate remedy and hereby agrees that in the event of a breach or a threatened breach by such party of any such obligations, the other party hereto shall, in addition to any and all other rights and remedies that may be available to it in respect of such breach, be entitled to equitable relief, including a restraining order, an injunction, specific performance and any other relief that may be available from a court of competent jurisdiction.
17. Finder’s Fee. Each party represents to the other party that it is not and will not be obligated for any finder’s fee or commission in connection with the transactions contemplated by this Warrant. The Holder agrees to indemnify and to hold harmless the Company from any liability for any commission or compensation in the nature of a finder’s fee (and the costs and expenses of defending against such liability or asserted liability) for which the Holder or any of its officers, employees or representatives is responsible. The Company agrees to indemnify and hold harmless the Holder from any liability for any commission or compensation in the nature of a finder’s fee (and the costs and expenses of defending against such liability or asserted liability) for which the Company or any of its officers, employees or representatives is responsible.
18. Expenses; Indemnification.
The Company will reimburse the reasonable fees and expenses of the Holder, including reasonable legal fees and expenses, with respect to the negotiation, execution and delivery of this Warrant as provided inSection 11.3 of the Credit Agreement.
In further consideration of the Holder’s acquiring the Warrant hereunder and in addition to all of the Company’s other obligations hereunder, the Company will defend, protect, indemnify and hold harmless the Holder and each other holder of the Warrant and all of their shareholders, partners, members, officers, directors, employees and direct or indirect investors and any of the foregoing Persons’ agents or other representatives (including, without limitation, those retained in connection with the transactions contemplated hereby) (collectively, the “Indemnitees”) from and against any and all actions, causes of action, suits, claims, losses, costs, penalties, fees, liabilities and damages, and expenses in connection therewith (irrespective of whether any such Indemnitee is a party to the action for which indemnification hereunder is sought), and including attorneys’ fees and disbursements (the “IndemnifiedLiabilities”), incurred by any Indemnitee as a result of, or arising out of, or relating to (i) any misrepresentation or breach of any representation or warranty made by the Company in this Warrant or any other certificate, instrument or document contemplated hereby or thereby, (ii) any breach of any covenant, agreement or obligation of the Company contained in this Warrant or any other certificate, instrument or document contemplated hereby or thereby, or (iii) any cause of action, suit or claim brought or made against such Indemnitee by a third party (including for these purposes a derivative action brought on behalf of the Company) and arising out of or resulting from (A) the execution, delivery, performance or enforcement of this Warrant or any other certificate, instrument or document contemplated hereby or thereby, or (B) the status of the Holder or holder of the Warrant as an investor in the Company pursuant to the transactions contemplated hereby. To the extent that the foregoing undertaking by the Company may be unenforceable for any reason, the Company will make the maximum contribution to the payment and satisfaction of each of the Indemnified Liabilities which is permissible under applicable law.
19. Entire Agreement. This Warrant constitutes the sole and entire agreement of the parties to this Warrant with respect to the subject matter contained herein, and supersedes all prior and contemporaneous understandings and agreements, both written and oral, with respect to such subject matter.
20. Successor and Assigns. This Warrant and the rights evidenced hereby shall be binding upon and shall inure to the benefit of the parties hereto and the successors of the Company and the successors and permitted assigns of the Holder. Such successors and/or permitted assigns of the Holder shall be deemed to be a Holder for all purposes hereunder.
21. No Third-Party Beneficiaries. This Warrant is for the sole benefit of the Company and the Holder and their respective successors and, in the case of the Holder, permitted assigns and nothing herein, express or implied, is intended to or shall confer upon any other Person any legal or equitable right, benefit or remedy of any nature whatsoever, under or by reason of this Warrant.
22. Headings. The headings in this Warrant are for reference only and shall not affect the interpretation of this Warrant.
23. Amendment and Modification; Waiver. Except as otherwise provided herein, this Warrant may only be amended, modified or supplemented by an agreement in writing signed by each party hereto. No waiver by the Company or the Holder of any of the provisions hereof shall be effective unless explicitly set forth in writing and signed by the party so waiving. No waiver by any party shall operate or be construed as a waiver in respect of any failure, breach or default not expressly identified by such written waiver, whether of a similar or different character, and whether occurring before or after that waiver. No failure to exercise, or delay in exercising, any rights, remedy, power or privilege arising from this Warrant shall operate or be construed as a waiver thereof; nor shall any single or partial exercise of any right, remedy, power or privilege hereunder preclude any other or further exercise thereof or the exercise of any other right, remedy, power or privilege.
24 Severability. If any term or provision of this Warrant is invalid, illegal or unenforceable in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other term or provision of this Warrant or invalidate or render unenforceable such term or provision in any other jurisdiction.
25. Governing Law. This Warrant shall be governed by and construed in accordance with the internal laws of the State of New York without giving effect to any choice or conflict of law provision or rule (whether of the State of New York or any other jurisdiction) that would cause the application of laws of any jurisdiction other than those of the State of New York.
26. Submission to Jurisdiction. Any legal suit, action or proceeding arising out of or based upon this Warrant or the transactions contemplated hereby may be instituted in the federal courts of the United States of America or the courts of the State of New York, in either case sitting in the Borough of Manhattan, and each party irrevocably submits to the exclusive jurisdiction of such courts in any such suit, action or proceeding. Service of process, summons, notice or other document by certified or registered mail to such party's address set forth herein shall be effective service of process for any suit, action or other proceeding brought in any such court. The parties irrevocably and unconditionally waive any objection to the laying of venue of any suit, action or any proceeding in such courts and irrevocably waive and agree not to plead or claim in any such court that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.
27. Waiver of Jury Trial. Each party acknowledges and agrees that any controversy which may arise under this Warrant is likely to involve complicated and difficult issues and, therefore, each such party irrevocably and unconditionally waives any right it may have to a trial by jury in respect of any legal action arising out of or relating to this Warrant or the transactions contemplated hereby.
28. Counterparts. This Warrant may be executed in counterparts, each of which shall be deemed an original, but all of which together shall be deemed to be one and the same agreement. A signed copy of this Warrant delivered by facsimile, e-mail or other means of electronic transmission shall be deemed to have the same legal effect as delivery of an original signed copy of this Warrant.
29. No Strict Construction. This Warrant shall be construed without regard to any presumption or rule requiring construction or interpretation against the party drafting an instrument or causing any instrument to be drafted.
[SIGNATURE PAGE FOLLOWS]
IN WITNESS WHEREOF, the Company has duly executed this Warrant on the Original Issue Date.
| VBI VACCINES INC. | |
| | | |
| By: | | |
| Name: | | |
| Title: | | |
Accepted and agreed, |
PCOF 1, LLC By: _____________________ Name: Title: | |
EXHIBIT A
FORM OF SUBSCRIPTION AGREEMENT
(To be signed only upon exercise of Warrant)
To:__________________________
The undersigned, as holder of a right to purchase shares of Common Stock of VBI VACCINES INC., a Delaware corporation (the “Company”), pursuant to that certain Warrant of VBI VACCINES INC.(the “Warrant”), dated as of July 25, 2014, hereby irrevocably elects to exercise the purchase right represented by such Warrant for, and to purchase thereunder, __________________________ (_________) shares of Common Stock of the Company and herewith makes payment of _________________________________ Dollars ($__________) therefor by the following method:
(Check all that apply):
_______ (check if applicable) | The undersigned hereby elects to make payment of the Aggregate Exercise Price of ______________ Dollars ($___________) in cash for _____________ (_________) shares of Common Stock using the method described inSection 3(b)(i). |
_______ (check if applicable) | The undersigned hereby elects to make payment of the Aggregate Exercise Price of ______________ Dollars ($___________) for _____________ (_________) shares of Common Stock using the method described inSection 3(b)(ii) of the Warrant. |
_______ (check if applicable) | The undersigned hereby elects to make payment of the Aggregate Exercise Price of ______________ Dollars ($___________) for _____________ (_________) shares of Common Stock using the method described inSection 3(b)(iii) of the Warrant. |
Requested Denomination of
Common Stock: | __________________ shares |
Registered Holder: | __________________ |
In order to induce the issuance of such securities the undersigned makes to the Company, as of the date hereof, the representations and warranties set forth inSection 12 of the Warrant. Unless otherwise defined herein, capitalized terms have the meanings provided in the Warrant.
DATED: ________________ | | |
| | PCOF 1, LLC | |
| | | |
| | | | |
| | | |
| By: | | |
| | | |
| | Name: | | |
| | | | |
| Title: | | |
EXHIBIT B
FORM OF ACKNOWLEDGMENT
To: PCOF 1, LLC
The undersigned hereby acknowledges that as of the date hereof, __________________ (___________) shares of Common Stock remain subject to the right of purchase in favor of PCOF 1, LLC pursuant to that certain Warrant of VBI VACCINES INC. in favor of PCOF 1, LLC, dated as of July 25, 2014.
DATED: ________________ | | |
| | VBI VACCINES INC. | |
| | | |
| | | | |
| | | |
| By: | | |
| | | |
| Name: | | |
| | | | |
| | Title: | | |
EXHIBIT C
FORM OF ASSIGNMENT
FOR VALUE RECEIVED, the undersigned Holder of record of this Warrant of VBI VACCINES INC. (the “Company”), which is dated ___________, hereby sells, assigns and transfers unto the Assignee named below all of the rights, including, without limitation, the Purchase Rights (as such term is defined in this Warrant) of the undersigned under the within Warrant, with respect to the number of shares of Common Stock set forth below:
Name of Transferee/Assignee | Address | No. of Shares |
and does hereby irrevocably constitute and appoint the Secretary of VBI VACCINES INC. to make such transfer on the books of VBI VACCINES INC., maintained for the purpose, with full power of substitution in the premises.
Attached hereto, if and to the extent requested by the Company, is an opinion of counsel that the assignment is in compliance with or is exempt from, applicable federal and state securities laws. As provided in the Warrant, including but not limited toSection 7 of the Warrant, the Company may, in its sole discretion, decide whether such opinion is satisfactory, and Assignee and Holder agree to any reasonable delay in transfer caused by such evaluation.
The Assignee acknowledges and agrees that this Warrant and the shares of Common Stock to be issued upon exercise hereof or conversion thereof are being acquired for investment and that the Assignee will not offer, sell or otherwise dispose of this Warrant or any shares of stock to be issued upon exercise hereof or conversion thereof except under circumstances which will not result in a violation of the Securities Act of 1933, as amended (the “Act”), or any applicable state securities laws.
Accordingly, the following restrictive legend is made applicable to this assignment (and to this Warrant and securities covered by this Warrant as assigned hereby to Assignee):
This Assignment and this Warrant and the securities underlying this Warrant as assigned hereby, have not been registered under the Act, and may not be offered, sold or otherwise transferred, assigned, pledged or hypothecated in the absence of such registration or an exemption therefrom under such Act, any applicable state securities laws and the rules and regulations thereunder.
| Dated: __________________________ | HOLDER: | | |
| | | |
| By: | | |
| Name: | | |
| Title: | | |
| | | | |
| Dated: __________________________ | ASSIGNEE: | | |
| | | | |
| | By: | | |
| | Name: | | |
| | Title: | | |
EXHIBIT F
FORM OF DELAYED DRAW WARRANT
THIS WARRANT AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS WARRANT HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE "ACT"), OR QUALIFIED UNDER ANY STATE OR FOREIGN SECURITIES LAWS AND MAY NOT BE OFFERED FOR SALE, SOLD, PLEDGED, HYPOTHECATED OR OTHERWISE TRANSFERRED OR ASSIGNED UNLESS (I) A REGISTRATION STATEMENT COVERING SUCH SHARES IS EFFECTIVE UNDER THE ACT AND IS QUALIFIED UNDER APPLICABLE STATE AND FOREIGN LAW OR (II) THE TRANSACTION IS EXEMPT FROM THE REGISTRATION AND PROSPECTUS DELIVERY REQUIREMENTS UNDER THE ACT AND THE QUALIFICATION REQUIREMENTS UNDER APPLICABLE STATE AND FOREIGN LAW.
Warrant Certificate No.: [___]
Original Issue Date: [__________ __], 201[_]
FOR VALUE RECEIVED, VBI VACCINES INC., a Delaware corporation (the "Company"), hereby certifies that PCOF 1, LLC or its permitted transferees and assigns (the "Holder") is entitled to purchase from the Company up to [_________]2 duly authorized, validly issued, fully paid and nonassessable shares of Common Stock (defined below) at a per share purchase price of $[______]3 (subject to adjustment as provided herein, the "Exercise Price"), all subject to the terms, conditions and adjustments set forth below in this Warrant (defined below). Certain capitalized terms used herein are defined inSection0 hereof.
1. Definitions. As used in this Warrant, the following terms have the following meanings:
“Acknowledgement” has the meaning set forth inSection 3(e).
"Aggregate Exercise Price" means, with respect to any exercise of this Warrant, an amount equal to the product of (i) the number of Warrant Shares in respect of which this Warrant is then being exercised pursuant toSection0 hereof, multiplied by (ii) the Exercise Price in effect as of the Exercise Date.
“Assignment” has the meaning set forth inSection 7.
"Board" means the board of directors of the Company.
2 NTD: To be determined based on the amount of the Delayed Draw Loan. If the Delayed Draw Loan is $3 million, then insert 699,281 shares.
3 NTD: Insert the 10-day volume weighted average stock price prior to the date of the Milestone Draw.
"Business Day" means any day which is neither a Saturday or Sunday nor a legal holiday on which banks are authorized or required to be closed in New York, New York.
“Cashless Method” means, as the context may require, one or both of the Exercise Price payment methods described inclauses (ii) and(iii) ofSection 3(b).
"Commission" means the Securities and Exchange Commission or any other federal agency administering the Securities Act and the Exchange Act at the time.
"Common Stock" means the common stock, par value $0.0001 per share, of the Company, and any capital stock into which such Common Stock shall have been converted, exchanged or reclassified following the date hereof.
"Company" has the meaning set forth in the preamble.
"Convertible Securities" means any securities, whether debt, equity or other securities (directly or indirectly) convertible into or exchangeable for Common Stock, but excluding Options.
"Covered Persons" has the meaning set forth inSection 3(i).
“Credit Agreement” means the Credit Agreement and Guaranty dated as of July 25, 2014 among Variation Biotechnologies (US), Inc., as borrower, PCOF 1, LLC, as lender, the Company, as guarantor and the other guarantors party thereto.
"Demand Registration" has the meaning set forth inSection 6(a)(ii).
"Disqualification Events" has the meaning set forth inSection 3(i).
"Exercise Date" means, for any given exercise of this Warrant, the date on which the conditions to such exercise as set forth inSection0 shall have been satisfied at or prior to 5:00 p.m., New York time, on a Business Day, including, without limitation, the receipt by the Company of the Subscription Agreement, the Warrant and the Aggregate Exercise Price.
"Exercise Period" has the meaning set forth inSection0.
"Exercise Price" has the meaning set forth in the preamble.
"Fair Market Value" means, as of any particular date: (i) the volume weighted average of the closing sales prices of the Common Stock for such day on all domestic securities exchanges on which the Common Stock may at the time be listed; (ii) if there have been no sales of the Common Stock on any such exchange on any such day, the average of the highest bid and lowest asked prices for the Common Stock on all such exchanges at the end of such day; (iii) if on any such day the Common Stock is not listed on a domestic securities exchange, the closing sales price of the Common Stock as quoted on Nasdaq, the OTC Bulletin Board, the “pink sheets” or similar quotation system or association for such day; or (iv) if there have been no sales of the Common Stock on Nasdaq, the OTC Bulletin Board, the “pink sheets” or similar quotation system or association on such day, the average of the highest bid and lowest asked prices for the Common Stock quoted on Nasdaq, the OTC Bulletin Board, the “pink sheets” or similar quotation system or association at the end of such day; in each case, averaged over twenty (20) consecutive Business Days ending on the Business Day immediately prior to the day as of which "Fair Market Value" is being determined;provided, that if the Common Stock is listed on any domestic securities exchange, the term "Business Day" as used in this sentence means Business Days on which such exchange is open for trading. If at any time the Common Stock is not listed on any domestic securities exchange or quoted on Nasdaq, the OTC Bulletin Board, the “pink sheets” or similar quotation system or association, the "Fair Market Value" of the Common Stock shall be the fair market value per share as determined jointly by the Board and the Holder.
"Holder" has the meaning set forth in the preamble.
"Indemnified Liabilities" has the meaning set forth inSection 18(b).
"Indemnitees" has the meaning set forth inSection 18(b).
“Inspectors” has the meaning set forth inSection 6(c)(viii).
“Long-Form Registration” has the meaning set forth inSection 6(a)(i).
“Merger Agreement Demand” means any demand for registration under the Securities Act of Registrable Securities pursuant to Section 6.14 of the Merger Agreement (as defined in the Credit Agreement).
"Nasdaq" means The Nasdaq Stock Market, Inc.
"Options" means any warrants or other rights or options to subscribe for or purchase Common Stock or Convertible Securities.
"Original Issue Date" means the date hereof.
"OTC Bulletin Board" means the National Association of Securities Dealers, Inc. OTC Bulletin Board.
"Person" means any individual, sole proprietorship, partnership, limited liability company, corporation, joint venture, trust, incorporated organization or government or department or agency thereof.
“Piggyback Registration” has the meaning set forth inSection 6(b)(i).
"Prospectus" means the prospectus or prospectuses included in any Registration Statement, as amended or supplemented by any prospectus supplement with respect to the terms of the offering of any portion of the Registrable Securities covered by such Registration Statement and by all other amendments and supplements to the prospectus, including post-effective amendments and all material incorporated by reference in such prospectus or prospectuses.
“Records” has the meaning set forth inSection 6(c)(viii).
"Registrable Securities" means (x) any shares of Common Stock held by any Person or issuable upon conversion, exercise or exchange of any securities owned by any Person at any time (including Warrant Shares exercisable upon exercise of this Warrant), and (y) any shares of Common Stock issued or issuable with respect to any shares described in subsection (x) above by way of a stock dividend or stock split or in connection with a combination of shares, recapitalization, merger, consolidation or other reorganization (it being understood that for purposes of this Warrant, a Person shall be deemed to be a holder of Registrable Securities whenever such Person has the right to then acquire or obtain from the Company any Registrable Securities, whether or not such acquisition has actually been effected). As to any particular Registrable Securities, such securities shall cease to be Registrable Securities when (i) a Registration Statement covering such securities has been declared effective by the Commission and such securities have been disposed of pursuant to such effective Registration Statement, (ii) such securities are sold under circumstances in which all of the applicable conditions of Rule 144 (or any similar provisions then in force) under the Securities Act are met, (iii) such securities are otherwise transferred and such securities may be resold without subsequent registration under the Securities Act, or (iv) such securities shall have ceased to be outstanding.
"Registration Statement" means any registration statement of the Company which covers any of the Registrable Securities pursuant to the provisions of this Agreement, including the Prospectus, amendments and supplements to such Registration Statement, including post-effective amendments, all exhibits and all materials incorporated by reference in such Registration Statement.
“Securities Act” means the Securities Act of 1933, as amended.
“Short-Form Registration” has the meaning set forth inSection 6(a)(ii).
"Solicitor" has the meaning set forth inSection 3(i).
"Subscription Agreement" has the meaning set forth inSection 3(a)(i).
"Warrant" means this Warrant and all warrants issued upon division or combination of, or in substitution for, this Warrant.
"Warrant Shares" means the shares of Common Stock or other capital stock of the Company purchasable upon exercise of this Warrant in accordance with its terms.
2. Term of Warrant. Subject to the terms and conditions hereof, at any time or from time to time after the date hereof up to and including 5:00 p.m., New York time, on the fifth (5th) anniversary of the Original Issue Date or, if such day is not a Business Day, on the next preceding Business Day (the "Exercise Period"), the Holder of this Warrant may exercise this Warrant for all or any part of the Warrant Shares purchasable hereunder (subject to adjustment as provided herein).
3. Exercise of Warrant.
(a) Exercise Procedure. This Warrant may be exercised from time to time on any Business Day during the Exercise Period, for all or any part of the unexercised Warrant Shares, upon:
(i) surrender of this Warrant to the Company at its then principal executive offices (or an indemnification undertaking with respect to this Warrant in the case of its loss, theft or destruction), together with a Subscription Agreement in the form attached hereto asExhibit A (each, a "Subscription Agreement"), duly completed (including specifying the number of Warrant Shares to be purchased) and executed; and
(ii) payment to the Company of the Aggregate Exercise Price in accordance withSectionSection 1.01(a)(ii).
(b) Payment of the Aggregate Exercise Price. Payment of the Aggregate Exercise Price shall be made, at the option of the Holder as expressed in the Subscription Agreement, by the following methods:
(i) by delivery to the Company of a certified or official bank check payable to the order of the Company or by wire transfer of immediately available funds to an account designated in writing by the Company, in the amount of such Aggregate Exercise Price;
(ii) by instructing the Company to withhold a number of Warrant Shares then issuable upon exercise of this Warrant with an aggregate Fair Market Value as of the Exercise Date equal to such Aggregate Exercise Price;
(iii) by surrendering to the Company (x) Warrant Shares previously acquired by the Holder with an aggregate Fair Market Value as of the Exercise Date equal to such Aggregate Exercise Price and/or (y) other securities or debt obligations of the Company (including loans outstanding under the Credit Agreement) having a value as of the Exercise Date equal to the Aggregate Exercise Price (which value in the case of debt securities or obligations shall be the principal amount thereof plus accrued and unpaid interest, in the case of preferred stock shall be the liquidation value thereof plus accumulated and unpaid dividends and in the case of shares of Common Stock shall be the Fair Market Value thereof); or
(iv) any combination of the foregoing;
provided;however; that the Holder may only use the Cashless Method to pay the Exercise Price for up to (but not in excess of) [_________] Warrant Shares (subject to proportionate adjustment for any stock split, dividend, distribution, subdivision, recapitalization or combination).
In the event of any withholding of Warrant Shares or surrender of other equity securities pursuant toclause (ii),(iii) or(iv) above where the number of shares whose value is equal to the Aggregate Exercise Price is not a whole number, the number of shares withheld by or surrendered to the Company shall be rounded up to the nearest whole share and the Company shall make a cash payment to the Holder (by delivery of a certified or official bank check or by wire transfer of immediately available funds) based on the incremental fraction of a share being so withheld by or surrendered to the Company in an amount equal to the product of (x) such incremental fraction of a share being so withheld or surrendered multiplied by (y) in the case of Common Stock, the Fair Market Value per Warrant Share as of the Exercise Date, and, in all other cases, the value thereof as of the Exercise Date determined in accordance withclause (iii)(y) above.
(c) �� Delivery of Stock Certificates. Upon receipt by the Company of the Subscription Agreement, surrender of this Warrant and payment of the Aggregate Exercise Price (in accordance withSectionSection 1.01(a) hereof), the Company shall, as promptly as practicable, and in any event within three (3) Business Days thereafter, execute (or cause to be executed) and deliver (or cause to be delivered) to the Holder a certificate or certificates representing the Warrant Shares issuable upon such exercise, together with cash in lieu of any fraction of a share, as provided inSectionSection 1.01(c) hereof. The stock certificate or certificates so delivered shall be, to the extent possible, in such denomination or denominations as the exercising Holder shall reasonably request in the Subscription Agreement and shall be registered in the name of the Holder or, subject to compliance withSection0 below, such other Person's name as shall be designated in the Subscription Agreement. This Warrant shall be deemed to have been exercised and such certificate or certificates of Warrant Shares shall be deemed to have been issued, and the Holder or any other Person so designated to be named therein shall be deemed to have become a holder of record of such Warrant Shares for all purposes, as of the Exercise Date.
(d) Fractional Shares. The Company shall not be required to issue a fractional Warrant Share upon exercise of any Warrant. As to any fraction of a Warrant Share that the Holder would otherwise be entitled to purchase upon such exercise, the Company shall pay to such Holder an amount in cash (by delivery of a certified or official bank check or by wire transfer of immediately available funds) equal to the product of (i) such fraction multiplied by (ii) the Fair Market Value of one Warrant Share on the Exercise Date.
(e) Acknowledgement; Partial Exercise. Unless the purchase rights represented by this Warrant shall have expired or shall have been fully exercised, the Company shall, at the time of delivery of the certificate or certificates representing the Warrant Shares being issued in accordance withSectionSection 1.01(c) hereof, deliver to the Holder within a reasonable time an acknowledgement in substantially the form attached hereto asExhibit B (each, an “Acknowledgement”) indicating the number of Warrant Shares which remain issuable upon exercise of this Warrant, if any.
(f) Valid Issuance of Warrant and Warrant Shares; Payment of Taxes. With respect to the exercise of this Warrant, the Company hereby represents, covenants and agrees:
(i) This Warrant is, and any Warrant issued in substitution for or replacement of this Warrant shall be, upon issuance, duly authorized and validly issued.
(ii) All Warrant Shares issuable upon the exercise of this Warrant pursuant to the terms hereof shall be, upon issuance, and the Company shall take all such actions as may be necessary or appropriate in order that such Warrant Shares are, validly issued, fully paid and non-assessable, issued without violation of any preemptive or similar rights of any stockholder of the Company and free and clear of all taxes, liens and charges.
(iii) The Company shall take all such actions as may be necessary to ensure that all such Warrant Shares are issued without violation by the Company of any applicable law or governmental regulation or any requirements of any domestic securities exchange upon which shares of Common Stock or other securities constituting Warrant Shares may be listed at the time of such exercise (except for official notice of issuance which shall be immediately delivered by the Company upon each such issuance).
(iv) Without in any way limitingSection 6 hereof, the Company shall use its best efforts to cause the Warrant Shares, immediately upon such exercise, to be listed on any domestic securities exchange upon which shares of Common Stock or other securities constituting Warrant Shares are listed at the time of such exercise.
(v) The Company shall pay all expenses in connection with, and all taxes and other governmental charges that may be imposed with respect to, the issuance or delivery of Warrant Shares upon exercise of this Warrant;provided, that the Company shall not be required to pay any tax or governmental charge that may be imposed with respect to any applicable withholding or the issuance or delivery of the Warrant Shares to any Person other than the Holder, and no such issuance or delivery shall be made unless and until the Person requesting such issuance has paid to the Company the amount of any such tax, or has established to the satisfaction of the Company that such tax has been paid.
(g) Conditional Exercise. Notwithstanding any other provision hereof, if an exercise of any portion of this Warrant is to be made in connection with a public offering or a sale of the Company (pursuant to a merger, sale of stock, or otherwise), such exercise may at the election of the Holder be conditioned upon the consummation of such transaction, in which case such exercise shall not be deemed to be effective until immediately prior to the consummation of such transaction.
(h) Reservation of Shares. During the Exercise Period, the Company shall at all times reserve and keep available out of its authorized but unissued Common Stock or other securities constituting Warrant Shares, solely for the purpose of issuance upon the exercise of this Warrant, the maximum number of Warrant Shares issuable upon the exercise of this Warrant, and the par value per Warrant Share shall at all times be less than or equal to the applicable Exercise Price. The Company shall not increase the par value of any Warrant Shares receivable upon the exercise of this Warrant above the Exercise Price then in effect, and shall take all such actions as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable shares of Common Stock upon the exercise of this Warrant.
(i) No “Bad Actor” Disqualification. The Company has exercised reasonable care, in accordance with Commission rules and guidance, to determine whether any Covered Person (as defined below) is subject to any of the “bad actor” disqualifications described in Rule 506(d)(1)(i) through (viii) under the Securities Act (“Disqualification Events”). To the Company’s knowledge, no Covered Person is subject to a Disqualification Event. The Company has complied, to the extent applicable, with all disclosure obligations under Rule 506(e) under the Securities Act. “Covered Persons” are those persons specified in Rule 506(d)(1) under the Securities Act, including the Company, any predecessor or affiliate of the Company, any director, executive officer, other officer participating in the offering, general partner or managing member of the Company, any beneficial owner of 20% or more of the Company’s outstanding voting equity securities, calculated on the basis of voting power, any promoter (as defined in Rule 405 under the Securities Act) connected with the Company in any capacity at the time of the exercise of this Warrant, and any person that has been or will be paid (directly or indirectly) remuneration for solicitation of purchasers in connection with the sale of any securities of the Company (a “Solicitor”), any general partner or managing member of any Solicitor, and any director, executive officer or other officer participating in the offering of any Solicitor or general partner or managing member of any such Solicitor.
4. Adjustment to Exercise Price and Number of Warrant Shares. The Exercise Price and the number of Warrant Shares issuable upon exercise of this Warrant shall be subject to adjustment from time to time as provided in thisSection0.
(a) Dividends and Distributions of Non-Company Securities. If, at any time or from time to time after the Original Issue Date, the Company makes or declares, or fixes a record date for the determination of holders of Common Stock entitled to receive, a dividend or any other distribution payable in securities of the Company (other than a dividend or distribution of shares of Common Stock or Options or Convertible Securities in each case in respect of Common Stock), cash or other property, then, and in each such event, provision shall be made so that the Holder shall receive upon exercise of the Warrant, in addition to the number of Warrant Shares receivable thereupon, the kind and amount of securities of the Company, cash or other property which the Holder would have been entitled to receive had the Warrant been exercised in full into Warrant Shares on the date of such event and had the Holder thereafter, during the period from the date of such event to and including the Exercise Date, retained such securities, cash or other property receivable by them as aforesaid during such period, giving application to all adjustments called for during such period under thisSection0 with respect to the rights of the Holder;provided, that no such provision shall be made if the Holder receives, simultaneously with the distribution to the holders of Common Stock, a dividend or other distribution of such securities, cash or other property in an amount equal to the amount of such securities, cash or other property as the Holder would have received if the Warrant had been exercised in full into Warrant Shares on the date of such event.
(b) Adjustment to Exercise Price and Warrant Shares Upon Dividend, Subdivision or Combination of Common Stock. If the Company shall, at any time or from time to time after the Original Issue Date, (i) pay a dividend or make any other distribution upon the Common Stock or any other capital stock of the Company payable in shares of Common Stock or in Options or Convertible Securities (in each case in respect of Common Stock), or (ii) subdivide (by any stock split, recapitalization or otherwise) its outstanding shares of Common Stock into a greater number of shares, the Exercise Price in effect immediately prior to any such dividend, distribution or subdivision shall be proportionately reduced and the number of Warrant Shares issuable upon exercise of this Warrant shall be proportionately increased. If the Company at any time combines (by combination, reverse stock split or otherwise) its outstanding shares of Common Stock into a smaller number of shares, the Exercise Price in effect immediately prior to such combination shall be proportionately increased and the number of Warrant Shares issuable upon exercise of this Warrant shall be proportionately decreased. Any adjustment under thisSection 4(b) shall become effective at the close of business on the date the dividend, subdivision or combination becomes effective.
(c) Adjustment to Exercise Price and Warrant Shares Upon Reorganization, Reclassification, Consolidation or Merger. In the event of any (i) capital reorganization of the Company, (ii) reclassification of the stock of the Company (other than a change in par value or from par value to no par value or from no par value to par value or as a result of a stock dividend or subdivision, split-up or combination of shares), (iii) consolidation or merger of the Company with or into another Person, (iv) sale of all or substantially all of the Company's assets to another Person, (v) Change of Control (as defined in the Credit Agreement) or (vi) other similar transaction, in each case which entitles the holders of Common Stock to receive (either directly or upon subsequent liquidation) cash, stock, securities or assets with respect to or in exchange for Common Stock, each Warrant shall, immediately after such reorganization, reclassification, consolidation, merger, sale, Change of Control or similar transaction, remain outstanding and shall thereafter, in lieu of or in addition to (as the case may be) the number of Warrant Shares then exercisable under this Warrant, be exercisable for the amount of cash or the kind and number of shares of stock or other securities or assets of the Company or of the successor Person resulting from such transaction to which the Holder would have been entitled upon such reorganization, reclassification, consolidation, merger, sale, Change of Control or similar transaction if the Holder had exercised this Warrant in full immediately prior to the time of such reorganization, reclassification, consolidation, merger, sale, Change of Control or similar transaction and acquired the applicable number of Warrant Shares then issuable hereunder as a result of such exercise (without taking into account any limitations or restrictions on the exercisability of this Warrant); and, in such case, appropriate adjustment (in form and substance satisfactory to the Holder) shall be made with respect to the Holder's rights under this Warrant to insure that the provisions of thisSection0 hereof shall thereafter be applicable, as nearly as possible, to this Warrant in relation to any cash, shares of stock, securities or assets thereafter acquirable upon exercise of this Warrant (including, in the case of any consolidation, merger, sale, Change of Control or similar transaction in which the successor or purchasing Person is other than the Company, an immediate adjustment in the Exercise Price to the value per share for the Common Stock reflected by the terms of such consolidation, merger, sale, Change of Control or similar transaction, and a corresponding immediate adjustment to the number of Warrant Shares acquirable upon exercise of this Warrant without regard to any limitations or restrictions on exercise, if the value so reflected is less than the Exercise Price in effect immediately prior to such consolidation, merger, sale, Change of Control or similar transaction). The provisions of thisSection 4(c) shall similarly apply to successive reorganizations, reclassifications, consolidations, mergers, sales, Changes of Control or similar transactions. The Company shall not effect any such reorganization, reclassification, consolidation, merger, sale, Change of Control or similar transaction unless, prior to the consummation thereof, the successor Person (if other than the Company) resulting from such reorganization, reclassification, consolidation, merger, sale, Change of Control or similar transaction, shall assume, by written instrument substantially similar in form and substance to this Warrant and satisfactory to the Holder, the obligation to deliver to the Holder such shares of stock, securities or assets which, in accordance with the foregoing provisions, such Holder shall be entitled to receive upon exercise of this Warrant. Notwithstanding anything to the contrary contained herein, with respect to any corporate event or other transaction contemplated by the provisions of thisSection 4(c), the Holder shall have the right to elect prior to the consummation of such event or transaction, to give effect to the exercise rights contained inSection0 instead of giving effect to the provisions contained in thisSection 4(c) with respect to this Warrant.
(d) Certain Events. If any event of the type contemplated by the provisions of thisSection0 but not expressly provided for by such provisions occurs, then the Board shall make an appropriate adjustment in the Exercise Price and the number of Warrant Shares issuable upon exercise of this Warrant so as to protect the rights of the Holder in a manner consistent with the provisions of thisSection0;provided, that no such adjustment pursuant to thisSection 4(d) shall increase the Exercise Price or decrease the number of Warrant Shares issuable as otherwise determined pursuant to thisSection0.
(e) Certificate as to Adjustment.
(i) As promptly as reasonably practicable following any adjustment of the Exercise Price, but in any event not later than three (3) Business Days thereafter, the Company shall furnish to the Holder a certificate of an executive officer setting forth in reasonable detail such adjustment and the facts upon which it is based and certifying the calculation thereof.
(ii) As promptly as reasonably practicable following the receipt by the Company of a written request by the Holder, but in any event not later than three (3) Business Days thereafter, the Company shall furnish to the Holder a certificate of an executive officer certifying the Exercise Price then in effect and the number of Warrant Shares or the amount, if any, of other shares of stock, securities or assets then issuable upon exercise of the Warrant.
(f) Notices. In the event:
(i) that the Company shall take a record of the holders of its Common Stock (or other capital stock or securities at the time issuable upon exercise of the Warrant) for the purpose of entitling or enabling them to receive any dividend or other distribution, to vote at a meeting (or by written consent), to receive any right to subscribe for or purchase any shares of capital stock of any class or any other securities, or to receive any other security; or
(ii) of any capital reorganization of the Company, any reclassification of the Common Stock of the Company, any consolidation or merger of the Company with or into another Person, or sale of all or substantially all of the Company's assets to another Person; or
(iii) of the voluntary or involuntary dissolution, liquidation or winding-up of the Company;
then, and in each such case, the Company shall send or cause to be sent to the Holder at least ten (10) Business days prior to the applicable record date or the applicable expected effective date, as the case may be, for the event, a written notice specifying, as the case may be, (A) the record date for such dividend, distribution, meeting or consent or other right or action, and a description of such dividend, distribution or other right or action to be taken at such meeting or by written consent, or (B) the effective date on which such reorganization, reclassification, consolidation, merger, sale, dissolution, liquidation or winding-up is proposed to take place, and the date, if any is to be fixed, as of which the books of the Company shall close or a record shall be taken with respect to which the holders of record of Common Stock (or such other capital stock or securities at the time issuable upon exercise of the Warrant) shall be entitled to exchange their shares of Common Stock (or such other capital stock or securities) for securities or other property deliverable upon such reorganization, reclassification, consolidation, merger, sale, dissolution, liquidation or winding-up, and the amount per share and character of such exchange applicable to the Warrant and the Warrant Shares.
5. Purchase Rights. In addition to (and not in limitation or in lieu of) any adjustments pursuant toSection0 above, if at any time the Company grants, issues or sells any shares of Common Stock, Options, Convertible Securities or rights to purchase stock, warrants, securities or other property pro rata to the record holders of Common Stock (the "Purchase Rights"), then the Holder shall be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder would have acquired if the Holder had held the number of Warrant Shares acquirable upon complete exercise of this Warrant immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights.
6. Registration Rights.
(a) Demand Registration.
(i) At any time after the one hundred eightieth (180) day following the Original Issue Date, the Holder may request registration under the Securities Act of all or any portion of the Warrant Shares on Form S-1 or any successor form thereto (each a "Long-Form Registration"). Each request for a Long-Form Registration shall specify the approximate number of Warrant Shares required to be registered. Upon receipt of such request, the Company shall promptly (but in no event later than five days following receipt thereof) deliver notice of such request to all other holders of Registrable Securities having “piggy back” rights or equivalent, if any, who shall then have ten days from the date such notice is given to notify the Company in writing of their desire to be included in such registration. The Company shall cause a Registration Statement on Form S-1 (or any successor form) to be filed within 30 days after the date on which the initial request is given and shall use its best efforts to cause such Registration Statement to be declared effective by the Commission as soon as practicable thereafter. The Company shall not be required to effect a Long-Form Registration more than five times by the Holder;provided, that a Registration Statement shall not count as a Long-Form Registration requested under thisSectionSection 1.01(a)(i) unless and until it has become effective and the Holder is able to register and sell at least 35% of the Warrant Shares requested to be included in such registration.
(ii) The Company shall use its best efforts to qualify and remain qualified to register securities under the Securities Act pursuant to a Registration Statement on Form S-3 or any successor form thereto. At such time as the Company shall have qualified for the use of a Registration Statement on Form S-3, the Holder shall have the right to request an unlimited number of registrations of Warrant Shares on Form S-3 or any similar short-form registration (each a "Short-Form Registration" and, together with each Long-Form Registration, a "Demand Registration"). Each request for a Short-Form Registration shall specify the approximate number of Warrant Shares requested to be registered. Upon receipt of any such request, the Company shall promptly (but in no event later than five days following receipt thereof) deliver notice of such request to all other holders of Registrable Securities having “piggy back” rights or equivalent, if any, who shall then have ten days from the date such notice is given to notify the Company in writing of their desire to be included in such registration. The Company shall cause a Registration Statement on Form S-3 (or any successor form) to be filed within 30 days after the date on which the initial request is given and shall use its best efforts to cause such Registration Statement to be declared effective by the Commission as soon as practicable thereafter.
(iii) The Company shall not be obligated to effect any Long-Form Registration within 90 days after the effective date of a previous Long-Form Registration or a previous Piggyback Registration in which the Holder was permitted to register, and actually sold, at least 35% of the shares of Registrable Securities requested to be included therein. The Company may postpone for up to 60 days the filing or effectiveness of a Registration Statement for a Demand Registration if the Company's Board determines in its reasonable good faith judgment that such Demand Registration would (i) materially interfere with a significant acquisition, corporate organization or other similar transaction involving the Company; (ii) require premature disclosure of material information that the Company has a bona fide business purpose for preserving as confidential; or (iii) render the Company unable to comply with requirements under the Securities Act or Exchange Act. The Company may delay a Demand Registration hereunder only two times in any period of twelve consecutive months.
(iv) If the Holder elects to distribute Warrant Shares covered by its request in an underwritten offering, it shall so advise the Company as a part of its request made pursuant toSectionSection 1.01(a)(i) orSection 6(a)(ii). The Holder shall select the investment banking firm or firms to act as the managing underwriter or underwriters in connection with such offering.
(v) The Company shall not include in any Demand Registration any securities which are not Registrable Securities without the prior written consent of the Holder, which consent shall not be unreasonably withheld or delayed. If a Demand Registration involves an underwritten offering and the managing underwriter of the requested Demand Registration advises the Company in writing that in its opinion the number of shares of Common Stock proposed to be included in the Demand Registration, including all Registrable Securities and all other shares of Common Stock proposed to be included in such underwritten offering, exceeds the number of shares of Common Stock which can be sold in such underwritten offering and/or the number of shares of Common Stock proposed to be included in such registration would adversely affect the price per share of the Registrable Securities proposed to be sold in such underwritten offering, the Company shall include in such Demand Registration (A) first, all of the Warrant Shares requested to be included in such registration by the Holder, and (B) second, any other Registrable Securities the Company may permit to be included in such registration, allocated pro rata among the respective holders thereof on the basis of the number of Registrable Securities owned by each such holder.
(b) Piggyback Registration.
(i) Whenever the Company proposes to register any shares of its Common Stock under the Securities Act (other than a registration effected solely to implement an employee benefit plan or a transaction to which Rule 145 of the Securities Act is applicable, or a Registration Statement on Form S-4, S-8 or any successor form thereto or another form not available for registering the Registrable Securities for sale to the public), whether for its own account or for the account of one or more stockholders of the Company and the form of Registration Statement to be used may be used for any registration of Warrant Shares (a "Piggyback Registration"), the Company shall give prompt written notice (in any event no later than twenty (20) days prior to the filing of such Registration Statement) to the Holder of its intention to effect such a registration and, subject toSection 6(b)(ii) andSection 6(b)(iii), shall include in such registration all Warrant Shares with respect to which the Company has received written requests for inclusion from the Holder within ten days after the Company's notice has been given to the Holder.
(ii) If a Piggyback Registration is initiated as a primary underwritten offering on behalf of the Company and the managing underwriter advises the Company and the Holder (if the Holder has elected to include Warrant Shares in such Piggyback Registration) in writing that in its opinion the number of shares of Common Stock proposed to be included in such registration, including all Registrable Securities and all other shares of Common Stock proposed to be included in such underwritten offering, exceeds the number of shares of Common Stock which can be sold in such offering and/or that the number of shares of Common Stock proposed to be included in any such registration would adversely affect the price per share of the Common Stock to be sold in such offering, the Company shall include in such registration (A) first, the number of shares of Common Stock that the Company proposes to sell; (B) second, the number of shares of Common Stock requested to be included therein by the Holder; and (C) third, the number of shares of Common Stock requested to be included therein by holders of Common Stock (other than Warrant Shares held by the Holder);provided, that in any event the Holder shall be entitled to register at least 35% of the securities to be included in any such registration.
(iii) If a Piggyback Registration is initiated as an underwritten offering on behalf of one or more holders of Common Stock other than Warrant Shares, and the managing underwriter advises the Company in writing that in its opinion the number of shares of Common Stock proposed to be included in such registration, including all Warrant Shares and all other shares of Common Stock proposed to be included in such underwritten offering, exceeds the number of shares of Common Stock which can be sold in such offering and/or that the number of shares of Common Stock proposed to be included in any such registration would adversely affect the price per share of the Common Stock to be sold in such offering, the Company shall, subject to the proviso below, include in such registration (i) first, the number of shares of Common Stock requested to be included therein by the Holder (on a fully diluted, as converted basis); and (ii) second, the number of shares of Common Stock requested to be included therein by other holders of Common Stock, allocated among such holders in such manner as they may agree;provided that, in the event of a registration resulting from a Merger Agreement Demand, the Company shall include in such registration, on a pro rata basis, (x) those shares of Common Stock (other than Warrant Shares) of the holders thereof requesting such Merger Agreement Demand, and (y) those Warrant Shares requested to be included in such registration by the Holder.
(iv) If any Piggyback Registration is initiated as a primary underwritten offering on behalf of the Company, the Company shall select the investment banking firm or firms to act as the managing underwriter or underwriters in connection with such offering.
(c) Registration Procedures. If and whenever the Holder requests that any Warrant Shares be registered pursuant to the provisions of this Warrant, the Company shall use its best efforts to effect the registration and the sale of such Warrant Shares in accordance with the intended method of disposition thereof, and pursuant thereto the Company shall as soon as practicable:
(i) subject toSection 6(a)(i) andSection 6(a)(ii), prepare and file with the Commission a Registration Statement with respect to such Warrant Shares and use its best efforts to cause such Registration Statement to become effective;
(ii) prepare and file with the Commission such amendments, post-effective amendments and supplements to such Registration Statement and the Prospectus used in connection therewith as may be necessary to keep such Registration Statement effective for a period of not less than thirty (30) days, or if earlier, until all of such Warrant Shares have been disposed of and to comply with the provisions of the Securities Act with respect to the disposition of such Warrant Shares in accordance with the intended methods of disposition set forth in such Registration Statement;
(iii) at least fifteen (15) Business Days before filing such Registration Statement, Prospectus or amendments or supplements thereto, furnish to counsel of the Holder copies of such documents proposed to be filed, which documents shall be subject to the review, comment and approval of such counsel;
(iv) notify the Holder, promptly after the Company receives notice thereof, of the time when such Registration Statement has been declared effective or a supplement to any Prospectus forming a part of such Registration Statement has been filed;
(v) furnish to the Holder such number of copies of the Prospectus included in such Registration Statement (including each preliminary Prospectus) and any supplement thereto (in each case including all exhibits and documents incorporated by reference therein) and such other documents as the Holder may request in order to facilitate the disposition of the Warrant Shares;
(vi) use its best efforts to register or qualify such Warrant Shares under such other securities or "blue sky" laws of such jurisdictions as any selling holder requests and do any and all other acts and things which may be necessary or advisable to enable the Holder to consummate the disposition;provided, that the Company shall not be required to qualify generally to do business, subject itself to general taxation or consent to general service of process in any jurisdiction where it would not otherwise be required to do so but for thisSection 6(c)(vi);
(vii) notify the Holder, at any time when a Prospectus relating thereto is required to be delivered under the Securities Act, of the happening of any event as a result of which the Prospectus included in such Registration Statement contains an untrue statement of a material fact or omits any fact necessary to make the statements therein not misleading, and, at the request of any such holder, the Company shall prepare a supplement or amendment to such Prospectus so that, as thereafter delivered to the purchasers of such Warrant Shares, such Prospectus shall not contain an untrue statement of a material fact or omit to state any fact necessary to make the statements therein not misleading;
(viii) make available for inspection by the Holder, any underwriter participating in any disposition pursuant to such Registration Statement and any attorney, accountant or other agent retained by the Holder or any such underwriter (collectively, the "Inspectors"), all financial and other records, pertinent corporate documents and properties of the Company (collectively, the "Records"), and cause the Company's officers, directors and employees to supply all information requested by any such Inspector in connection with such Registration Statement;
(ix) use its best efforts to cause such Warrant Shares to be listed on each securities exchange on which the Common Stock is then listed or, if the Common Stock is not then listed, on a national securities exchange selected by the Holder;
(x) in connection with an underwritten offering, enter into such customary agreements (including underwriting and lock-up agreements in customary form) and take all such other customary actions as the Holder or the managing underwriter of such offering request in order to expedite or facilitate the disposition of such Warrant Shares (including, without limitation, making appropriate officers of the Company available to participate in "road show" and other customary marketing activities (including one-on-one meetings with prospective purchasers of the Warrant Shares);
(xi) otherwise use its best efforts to comply with all applicable rules and regulations of the Commission and make available to its stockholders an earnings statement (in a form that satisfies the provisions of Section 11(a) of the Securities Act and Rule 158 thereunder) no later than thirty (30) days after the end of the 12-month period beginning with the first day of the Company's first full fiscal quarter after the effective date of such Registration Statement, which earnings statement shall cover said 12-month period, and which requirement will be deemed to be satisfied if the Company timely files complete and accurate information on Forms 10-Q, 10-K and 8-K under the Exchange Act and otherwise complies with Rule 158 under the Securities Act;
(xii) furnish to the Holder and each underwriter, if any, with (i) a legal opinion of the Company's outside counsel, dated the effective date of such Registration Statement (and, if such registration includes an underwritten public offering, dated the date of the closing under the underwriting agreement), in form and substance as is customarily given in opinions of the Company's counsel to underwriters in underwritten public offerings; and (ii) a "comfort" letter signed by the Company's independent certified public accountants in form and substance as is customarily given in accountants' letters to underwriters in underwritten public offerings;
(xiii) without limitingSection 6(c)(vi) above, use its best efforts to cause such Warrant Shares to be registered with or approved by such other governmental agencies or authorities as may be necessary by virtue of the business and operations of the Company to enable the Holder to consummate the disposition of such Warrant Shares in accordance with their intended method of distribution thereof;
(xiv) notify the Holder promptly of any request by the Commission for the amending or supplementing of such Registration Statement or Prospectus or for additional information;
(xv) advise the Holder, promptly after it shall receive notice or obtain knowledge thereof, of the issuance of any stop order by the Commission suspending the effectiveness of such Registration Statement or the initiation or threatening of any proceeding for such purpose and promptly use its best efforts to prevent the issuance of any stop order or to obtain its withdrawal at the earliest possible moment if such stop order should be issued; and
(xvi) otherwise use its best efforts to take all other steps necessary to effect the registration of such Warrant Shares contemplated hereby.
7. Transfer of Warrant. Subject to the transfer conditions referred to in the legend endorsed hereon, this Warrant and all rights hereunder are freely transferable, in whole or in part, by the Holder without charge to the Holder, upon delivery to the Company of a written request for assignment in the form attached hereto asExhibit C (each, an “Assignment”) by the Holder and surrender of this Warrant to the Company at its then principal executive offices, together with funds sufficient to pay any transfer taxes described inSection 3(f)(v) in connection with the making of such transfer. If requested by the Company, the Holder will also provide an opinion of counsel satisfactory to the Company to the effect that the transfer or assignment is in compliance with (or is exempt from) applicable federal and state securities laws. Upon such compliance, surrender and delivery and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees and in the denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant, if any, not so assigned and this Warrant shall promptly be cancelled.
8. Holder Not Deemed a Stockholder; Limitations on Liability. Except as otherwise specifically provided herein (includingSection 4(a)), prior to the issuance to the Holder of the Warrant Shares to which the Holder is then entitled to receive upon the due exercise of this Warrant, the Holder shall not be entitled to vote or receive dividends or be deemed the holder of shares of capital stock of the Company for any purpose, nor shall anything contained in this Warrant be construed to confer upon the Holder, as such, any of the rights of a stockholder of the Company or any right to vote, give or withhold consent to any corporate action (whether any reorganization, issue of stock, reclassification of stock, consolidation, merger, conveyance or otherwise), receive notice of meetings, receive dividends or subscription rights, or otherwise. In addition, nothing contained in this Warrant shall be construed as imposing any liabilities on the Holder to purchase any securities (upon exercise of this Warrant or otherwise) or as a stockholder of the Company, whether such liabilities are asserted by the Company or by creditors of the Company. Notwithstanding thisSection0, the Company shall provide the Holder with copies of the same notices and other information given to the stockholders of the Company generally, contemporaneously with the giving thereof to the stockholders.
9. Replacement on Loss; Division and Combination.
(a) Replacement of Warrant on Loss. Upon receipt of evidence reasonably satisfactory to the Company of the loss, theft, destruction or mutilation of this Warrant and upon delivery of an indemnity reasonably satisfactory to it (it being understood that a written indemnification agreement or affidavit of loss of the Holder shall be a sufficient indemnity) and, in case of mutilation, upon surrender of such Warrant for cancellation to the Company, the Company at its own expense shall execute and deliver to the Holder, in lieu hereof, a new Warrant of like tenor and exercisable for an equivalent number of Warrant Shares as the Warrant so lost, stolen, mutilated or destroyed;provided, that, in the case of mutilation, no indemnity shall be required if this Warrant in identifiable form is surrendered to the Company for cancellation.
(b) Division and Combination of Warrant. Subject to compliance with the applicable provisions of this Warrant as to any transfer or other assignment which may be involved in such division or combination, this Warrant may be divided or, following any such division of this Warrant, subsequently combined with other Warrants, upon the surrender of this Warrant or Warrants to the Company at its then principal executive offices, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the respective Holders or their agents or attorneys. Subject to compliance with the applicable provisions of this Warrant as to any transfer or assignment which may be involved in such division or combination, the Company shall at its own expense execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants so surrendered in accordance with such notice. Such new Warrant or Warrants shall be of like tenor to the surrendered Warrant or Warrants and shall be exercisable in the aggregate for an equivalent number of Warrant Shares as the Warrant or Warrants so surrendered in accordance with such notice.
10. No Impairment. The Company shall not, by amendment of its Certificate of Incorporation or Bylaws, or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities, or any other voluntary action, avoid or circumvent or seek to avoid or circumvent the observance or performance of any of the terms to be observed or performed by it hereunder, but shall at all times in good faith assist in the carrying out of all the provisions of this Warrant and in the taking of all such action as may reasonably be requested by the Holder in order to protect the exercise rights of the Holder against dilution or other impairment, consistent with the tenor and purpose of this Warrant.
11. Compliance with the Securities Act. The Holder, by acceptance of this Warrant, agrees to comply in all respects with the provisions of thisSection0 and the restrictive legend requirements set forth on the face of this Warrant and further agrees that such Holder shall not offer, sell or otherwise dispose of this Warrant or any Warrant Shares to be issued upon exercise hereof except under circumstances that will not result in a violation of the Securities Act. This Warrant and all Warrant Shares issued upon exercise of this Warrant (unless registered under the Securities Act) shall be stamped or imprinted with a legend in substantially the following form:
"THIS WARRANT AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS WARRANT HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE "ACT"), OR QUALIFIED UNDER ANY STATE OR FOREIGN SECURITIES LAWS AND MAY NOT BE OFFERED FOR SALE, SOLD, PLEDGED, HYPOTHECATED OR OTHERWISE TRANSFERRED OR ASSIGNED UNLESS (I) A REGISTRATION STATEMENT COVERING SUCH SHARES IS EFFECTIVE UNDER THE ACT AND IS QUALIFIED UNDER APPLICABLE STATE AND FOREIGN LAW OR (II) THE TRANSACTION IS EXEMPT FROM THE REGISTRATION AND PROSPECTUS DELIVERY REQUIREMENTS UNDER THE ACT AND THE QUALIFICATION REQUIREMENTS UNDER APPLICABLE STATE AND FOREIGN LAW."
12. Representations of the Holder. In connection with the issuance of this Warrant, the Holder specifically represents, as of the date hereof, to the Company by acceptance of this Warrant as follows:
(a) The Holder is an "accredited investor" as defined in Rule 501 of Regulation D promulgated under the Securities Act. The Holder is acquiring this Warrant and the Warrant Shares to be issued upon exercise hereof for investment for its own account and not with a current view towards, or for resale in connection with, the public sale or distribution of this Warrant or the Warrant Shares, except pursuant to sales registered or exempted under the Securities Act.
(b) The Holder understands and acknowledges that this Warrant and the Warrant Shares to be issued upon exercise hereof are "restricted securities" under the federal securities laws inasmuch as they are being acquired from the Company in a transaction not involving a public offering and that, under such laws and applicable regulations, such securities may be resold without registration under the Securities Act only in certain limited circumstances. In addition, the Holder represents that it is familiar with Rule 144 under the Securities Act, as presently in effect, and understands the resale limitations imposed thereby and by the Securities Act.
(c) The Holder acknowledges that it can bear the economic and financial risk of its investment for an indefinite period, and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment in the Warrant and the Warrant Shares. The Holder has had an opportunity to ask questions and receive answers from the Company regarding the terms and conditions of the offering of the Warrant and the business, properties, prospects and financial condition of the Company.
13. Warrant Register. The Company shall keep and properly maintain at its principal executive offices books for the registration of the Warrant and any transfers thereof. The Company may deem and treat the Person in whose name the Warrant is registered on such register as the Holder thereof for all purposes, and the Company shall not be affected by any notice to the contrary, except any assignment, division, combination or other transfer of the Warrant effected in accordance with the provisions of this Warrant.
14. Notices. All notices and other communications provided hereunder shall be in writing or by facsimile and addressed, delivered or transmitted, if to the Company or the Holder, to the applicable party at its address or facsimile number set forth on the signature pages hereto, or at such other address or facsimile number as may be designated by such party in a notice to the other party. Any notice, if mailed and properly addressed with postage prepaid or if properly addressed and sent by pre-paid courier service, shall be deemed given when received; any notice, if transmitted by facsimile, shall be deemed given when the confirmation of transmission thereof is received by the transmitter. Unless otherwise indicated, all references to the time of a day shall refer to New York City time.
15. Cumulative Remedies. Except to the extent expressly provided inSection0 to the contrary, the rights and remedies provided in this Warrant are cumulative and are not exclusive of, and are in addition to and not in substitution for, any other rights or remedies available at law, in equity or otherwise.
16. Equitable Relief. Each of the Company and the Holder acknowledges that a breach or threatened breach by such party of any of its obligations under this Warrant would give rise to irreparable harm to the other party hereto for which monetary damages would not be an adequate remedy and hereby agrees that in the event of a breach or a threatened breach by such party of any such obligations, the other party hereto shall, in addition to any and all other rights and remedies that may be available to it in respect of such breach, be entitled to equitable relief, including a restraining order, an injunction, specific performance and any other relief that may be available from a court of competent jurisdiction.
17. Finder’s Fee. Each party represents to the other party that it is not and will not be obligated for any finder’s fee or commission in connection with the transactions contemplated by this Warrant. The Holder agrees to indemnify and to hold harmless the Company from any liability for any commission or compensation in the nature of a finder’s fee (and the costs and expenses of defending against such liability or asserted liability) for which the Holder or any of its officers, employees or representatives is responsible. The Company agrees to indemnify and hold harmless the Holder from any liability for any commission or compensation in the nature of a finder’s fee (and the costs and expenses of defending against such liability or asserted liability) for which the Company or any of its officers, employees or representatives is responsible.
18. Expenses; Indemnification.
(a) The Company will reimburse the reasonable fees and expenses of the Holder, including reasonable legal fees and expenses, with respect to the negotiation, execution and delivery of this Warrant as provided inSection 11.3 of the Credit Agreement.
(b) In further consideration of the Holder’s acquiring the Warrant hereunder and in addition to all of the Company’s other obligations hereunder, the Company will defend, protect, indemnify and hold harmless the Holder and each other holder of the Warrant and all of their shareholders, partners, members, officers, directors, employees and direct or indirect investors and any of the foregoing Persons’ agents or other representatives (including, without limitation, those retained in connection with the transactions contemplated hereby) (collectively, the “Indemnitees”) from and against any and all actions, causes of action, suits, claims, losses, costs, penalties, fees, liabilities and damages, and expenses in connection therewith (irrespective of whether any such Indemnitee is a party to the action for which indemnification hereunder is sought), and including attorneys’ fees and disbursements (the “IndemnifiedLiabilities”), incurred by any Indemnitee as a result of, or arising out of, or relating to (i) any misrepresentation or breach of any representation or warranty made by the Company in this Warrant or any other certificate, instrument or document contemplated hereby or thereby, (ii) any breach of any covenant, agreement or obligation of the Company contained in this Warrant or any other certificate, instrument or document contemplated hereby or thereby, or (iii) any cause of action, suit or claim brought or made against such Indemnitee by a third party (including for these purposes a derivative action brought on behalf of the Company) and arising out of or resulting from (A) the execution, delivery, performance or enforcement of this Warrant or any other certificate, instrument or document contemplated hereby or thereby, or (B) the status of the Holder or holder of the Warrant as an investor in the Company pursuant to the transactions contemplated hereby. To the extent that the foregoing undertaking by the Company may be unenforceable for any reason, the Company will make the maximum contribution to the payment and satisfaction of each of the Indemnified Liabilities which is permissible under applicable law.
19. Entire Agreement. This Warrant constitutes the sole and entire agreement of the parties to this Warrant with respect to the subject matter contained herein, and supersedes all prior and contemporaneous understandings and agreements, both written and oral, with respect to such subject matter.
20. Successor and Assigns. This Warrant and the rights evidenced hereby shall be binding upon and shall inure to the benefit of the parties hereto and the successors of the Company and the successors and permitted assigns of the Holder. Such successors and/or permitted assigns of the Holder shall be deemed to be a Holder for all purposes hereunder.
21. No Third-Party Beneficiaries. This Warrant is for the sole benefit of the Company and the Holder and their respective successors and, in the case of the Holder, permitted assigns and nothing herein, express or implied, is intended to or shall confer upon any other Person any legal or equitable right, benefit or remedy of any nature whatsoever, under or by reason of this Warrant.
22. Headings. The headings in this Warrant are for reference only and shall not affect the interpretation of this Warrant.
23. Amendment and Modification; Waiver. Except as otherwise provided herein, this Warrant may only be amended, modified or supplemented by an agreement in writing signed by each party hereto. No waiver by the Company or the Holder of any of the provisions hereof shall be effective unless explicitly set forth in writing and signed by the party so waiving. No waiver by any party shall operate or be construed as a waiver in respect of any failure, breach or default not expressly identified by such written waiver, whether of a similar or different character, and whether occurring before or after that waiver. No failure to exercise, or delay in exercising, any rights, remedy, power or privilege arising from this Warrant shall operate or be construed as a waiver thereof; nor shall any single or partial exercise of any right, remedy, power or privilege hereunder preclude any other or further exercise thereof or the exercise of any other right, remedy, power or privilege.
24. Severability. If any term or provision of this Warrant is invalid, illegal or unenforceable in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other term or provision of this Warrant or invalidate or render unenforceable such term or provision in any other jurisdiction.
25. Governing Law. This Warrant shall be governed by and construed in accordance with the internal laws of the State of New York without giving effect to any choice or conflict of law provision or rule (whether of the State of New York or any other jurisdiction) that would cause the application of laws of any jurisdiction other than those of the State of New York.
26. Submission to Jurisdiction. Any legal suit, action or proceeding arising out of or based upon this Warrant or the transactions contemplated hereby may be instituted in the federal courts of the United States of America or the courts of the State of New York, in either case sitting in the Borough of Manhattan, and each party irrevocably submits to the exclusive jurisdiction of such courts in any such suit, action or proceeding. Service of process, summons, notice or other document by certified or registered mail to such party's address set forth herein shall be effective service of process for any suit, action or other proceeding brought in any such court. The parties irrevocably and unconditionally waive any objection to the laying of venue of any suit, action or any proceeding in such courts and irrevocably waive and agree not to plead or claim in any such court that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.
27. Waiver of Jury Trial. Each party acknowledges and agrees that any controversy which may arise under this Warrant is likely to involve complicated and difficult issues and, therefore, each such party irrevocably and unconditionally waives any right it may have to a trial by jury in respect of any legal action arising out of or relating to this Warrant or the transactions contemplated hereby.
28. Counterparts. This Warrant may be executed in counterparts, each of which shall be deemed an original, but all of which together shall be deemed to be one and the same agreement. A signed copy of this Warrant delivered by facsimile, e-mail or other means of electronic transmission shall be deemed to have the same legal effect as delivery of an original signed copy of this Warrant.
29. No Strict Construction. This Warrant shall be construed without regard to any presumption or rule requiring construction or interpretation against the party drafting an instrument or causing any instrument to be drafted.
[SIGNATURE PAGE FOLLOWS]
IN WITNESS WHEREOF, the Company has duly executed this Warrant on the Original Issue Date.
| VBI VACCINES INC. | |
| | | |
| By: | | |
| Name: | | |
| Title: | | |
Accepted and agreed, |
PCOF 1, LLC By: _____________________ Name: Title: | |
EXHIBIT A
FORM OF SUBSCRIPTION AGREEMENT
(To be signed only upon exercise of Warrant)
To:__________________________
The undersigned, as holder of a right to purchase shares of Common Stock of VBI VACCINES INC., a Delaware corporation (the “Company”), pursuant to that certain Warrant of VBI VACCINES INC.(the “Warrant”), dated as of [__________ __], 201[_], hereby irrevocably elects to exercise the purchase right represented by such Warrant for, and to purchase thereunder, __________________________ (_________) shares of Common Stock of the Company and herewith makes payment of _________________________________ Dollars ($__________) therefor by the following method:
(Check all that apply):
_______ (check if applicable) | The undersigned hereby elects to make payment of the Aggregate Exercise Price of ______________ Dollars ($___________) in cash for _____________ (_________) shares of Common Stock using the method described inSection 3(b)(i). |
_______ (check if applicable) | The undersigned hereby elects to make payment of the Aggregate Exercise Price of ______________ Dollars ($___________) for _____________ (_________) shares of Common Stock using the method described inSection 3(b)(ii) of the Warrant. |
_______ (check if applicable) | The undersigned hereby elects to make payment of the Aggregate Exercise Price of ______________ Dollars ($___________) for _____________ (_________) shares of Common Stock using the method described inSection 3(b)(iii) of the Warrant. |
Requested Denomination of
Common Stock: | __________________ shares |
Registered Holder: | __________________ |
In order to induce the issuance of such securities the undersigned makes to the Company, as of the date hereof, the representations and warranties set forth inSection 12 of the Warrant. Unless otherwise defined herein, capitalized terms have the meanings provided in the Warrant.
DATED: ________________ | | |
| | PCOF 1, LLC | |
| | | |
| | | |
| By: | | |
| | | |
| | Name: | | |
| | | | |
| Title: | | |
EXHIBIT B
FORM OF ACKNOWLEDGMENT
To: PCOF 1, LLC
The undersigned hereby acknowledges that as of the date hereof, __________________ (___________) shares of Common Stock remain subject to the right of purchase in favor of PCOF 1, LLC pursuant to that certain Warrant of VBI VACCINES INC. in favor of PCOF 1, LLC, dated as of [__________ __], 201[_].
DATED: ________________ | | |
| | VBI VACCINES INC. | |
| | | |
| | | |
| | | | |
| By: | | |
| | | |
| Name: | | |
| | | | |
| | Title: | | |
EXHIBIT C
FORM OF ASSIGNMENT
FOR VALUE RECEIVED, the undersigned Holder of record of this Warrant of VBI VACCINES INC. (the “Company”), which is dated ___________, hereby sells, assigns and transfers unto the Assignee named below all of the rights, including, without limitation, the Purchase Rights (as such term is defined in this Warrant) of the undersigned under the within Warrant, with respect to the number of shares of Common Stock set forth below:
Name of Transferee/Assignee | Address | No. of Shares |
and does hereby irrevocably constitute and appoint the Secretary of VBI VACCINES INC. to make such transfer on the books of VBI VACCINES INC., maintained for the purpose, with full power of substitution in the premises.
Attached hereto, if and to the extent requested by the Company, is an opinion of counsel that the assignment is in compliance with or is exempt from, applicable federal and state securities laws. As provided in the Warrant, including but not limited toSection 7 of the Warrant, the Company may, in its sole discretion, decide whether such opinion is satisfactory, and Assignee and Holder agree to any reasonable delay in transfer caused by such evaluation.
The Assignee acknowledges and agrees that this Warrant and the shares of Common Stock to be issued upon exercise hereof or conversion thereof are being acquired for investment and that the Assignee will not offer, sell or otherwise dispose of this Warrant or any shares of stock to be issued upon exercise hereof or conversion thereof except under circumstances which will not result in a violation of the Securities Act of 1933, as amended (the “Act”), or any applicable state securities laws.
Accordingly, the following restrictive legend is made applicable to this assignment (and to this Warrant and securities covered by this Warrant as assigned hereby to Assignee):
This Assignment and this Warrant and the securities underlying this Warrant as assigned hereby, have not been registered under the Act, and may not be offered, sold or otherwise transferred, assigned, pledged or hypothecated in the absence of such registration or an exemption therefrom under such Act, any applicable state securities laws and the rules and regulations thereunder.
Dated: ____________________ | HOLDER: | | |
| | | |
| By: | | |
| Name: | | |
| Title: | | |
| | | | |
| Dated: ____________________ | ASSIGNEE: | | |
| | | | |
| | By: | | |
| | Name: | | |
| | Title: | | |
EXHIBIT G
Intercompany Subordinated Note Provisions
Reference is made to that certain Credit Agreement and Guaranty, dated as of July 25, 2014 (as amended, extended, increased or otherwise modified from time to time, the “Credit Agreement”), by and among Variation Biotechnologies (US), Inc., as Borrower (the “Borrower”), each Guarantor party thereto and PCOF 1, LLC, as Lender (the “Lender”). Unless otherwise defined, capitalized terms used herein have the meanings provided in the Credit Agreement.
The [INSERT NAME OF (OR DEFINED FOR) THE ISSUER OF THE NOTE] and, by its acceptance hereof, any holder of this promissory note (together with any successor, transferee or assign, a “Holder”), each hereby unconditionally agrees that all obligations hereunder (collectively, the “Junior Obligations”) shall be subordinated in full to the prior payment in full in cash of all Obligations (as defined in the Credit Agreement), notwithstanding the maturity date hereof, any default by or insolvency of [INSERT NAME OF (OR DEFINED FOR) THE ISSUER OF THE NOTE] or otherwise. This agreement to subordinate is for the benefit of and shall be enforceable by the Lender and each of its successors and permitted transferees and assigns (collectively, the “Senior Creditor”).
Until all Obligations have been paid in full in cash, neither the [INSERT NAME OF (OR DEFINED FOR) THE ISSUER OF THE NOTE] may make, and no Holder shall accept, receive or collect, any direct or indirect payment or distribution of any kind or character whatsoever (in cash, securities, other property, by setoff, or otherwise) of any properties or assets of [INSERT NAME OF (OR DEFINED FOR) THE ISSUER OF THE NOTE], or otherwise from the [INSERT NAME OF (OR DEFINED FOR) THE ISSUER OF THE NOTE], on account of the Junior Obligations. Under no circumstance may any payment of the Junior Obligations be accelerated.
In the event that, notwithstanding the foregoing, the [INSERT NAME OF (OR DEFINED FOR) THE ISSUER OF THE NOTE] shall make any payment or distribution to any Holder prohibited by the foregoing provisions, then, until the Obligations have been repaid in full in cash, such payment or distribution shall be held in trust by the Holder for the benefit of and promptly shall be paid over to the Senior Creditor for application against the Obligations until paid in full in cash.