Searchable text section of graphics shown above
[LOGO]
Novartis Merger:
Presentation to Stockholders
March 2006
Agenda
1. Chiron’s Perspective on the Transaction
2. Questions & Answers
3. Session with Chiron Directors*
* “Directors” throughout this presentation refers to the Non-Novartis Directors. See Annex A for biographical material on the Directors.
[LOGO]
2
Fair Price and Better Than Status Quo
• Novartis has had the right to acquire Chiron since 1994
• Appropriate time
• 11 month process to allow time to address business challenges
• No significant near-term milestones to drive value
• Fair price: Determined by Directors following careful consideration of opportunities and risks
• BioPharma - tifacogin significant opportunity but high risk; molecular oncology promising but very early
• Vaccines - substantial opportunity but ongoing challenges and intensified competition
• Blood Testing - strong commercial capabilities but key patents expiring and no proven development or manufacturing capabilities
• Royalties - significant to earnings but expected to decline as key patents expire
• Better than status quo: significant downside risk in absence of Novartis deal
• Valuation of management’s long range plan shows significant risk of lower value
• Continued operational challenges and execution risks
• Potentially protracted uncertainty may impact operations (including ability to hire and retain key personnel) and share price
3
Novartis Has the Right to Acquire Chiron
• Under the 1994 Agreements, Novartis has the right to acquire Chiron in accordance with a specified process
• Directors leveraged Chiron’s rights under Governance Agreement to achieve optimal outcome for Chiron stockholders
Governance Agreement enabled Directors to optimize outcome
4
Appropriate time | |
| |
Better than status quo | |
| |
Fair price | |
5
11-Month Process Allowed Chiron to Address Business Challenges
• The Directors conducted discussions with Novartis at a deliberate pace
• 11 months elapsed from first discussions to definitive merger agreement
• During this period, Chiron achieved several significant milestones:
• Re-entry to U.S. flu market
• Completion of Phase 3 trial in EU for flu cell culture
• Initiation of Phase 1 / Phase 2 trial in U.S. for flu cell culture
• Positive data on MF59 adjuvant with potential pandemic strain
• Steady progress on patient enrollment in tifacogin trial
• Initiation of Phase 3 trial for TIP
• Initiation of Phase 1 trials for CHIR-258 and CHIR-12.12
• Geographic expansion and ex-U.S. Procleix Ultrio Assay penetration
• These achievements were expected and well communicated to investors and marketplace
Directors managed process to increase value
6
No Compelling Reason to Further Extend Discussions
• No significant value-enhancing milestones in the near term beyond those considered
• While Chiron successfully addressed many critical challenges, significant issues still lie ahead, including:
• Flu vaccine manufacturing challenges, including regulatory agencies interactions relating to Fluvirin and Begrivac, and increased competition
• Heavy dependence of BioPharma business on tifacogin
• Risks intrinsic to drug development and regulatory approval (e.g. Pulminiq “approvable” letter)
• Slower growth and regulatory delay in Blood Testing business (Procleix Ultrio and Procleix Tigris)
• Ongoing litigation relating to Fluvirin
• Managerial and operational challenges of running complex, global business
• Risk of Novartis invoking arbitration process, with unpredictable results
Discussions with Novartis concluded at appropriate time for Chiron
7
Appropriate time | |
| |
Better than status quo | |
| |
Fair price | |
8
Better Than Status Quo: Significant Downside Risk If No Novartis Deal
• Valuation of long range plan shows significant risk of lower value
• Prepared by management and thoroughly reviewed by Directors
• No milestones have been achieved since the date of the transaction that are not captured in the long range plan and reflected in the valuation
• Continued operational challenges and execution risks
• Earnings misses
• MMR recall and withdrawal
• Potentially protracted uncertainty may impact operations (including ability to hire and retain key personnel) and share price
• Novartis veto power over certain strategic transactions, publicly stated intention not to sell its 44% stake, and right to initiate new buy-out proposal at any time
9
Appropriate time | |
| |
Better than status quo | |
| |
Fair price | |
• Determined by Directors with significant industry and financial expertise following careful consideration of opportunities and risks
10
BioPharma Business is Challenging for Chiron
• Scale of business: high levels of R&D spend relative to current sales
• And current R&D spend is only a fraction of what will be required to advance promising early stage programs
• Need to make significant investments in manufacturing and commercial capabilities pre-launch
• Mixed record of internal product development – most existing products have been obtained via acquisition or licensing, including Betaseron, Proleukin, TOBI, and Cubicin
• Certain current products are under competitive pressure or subject to near-term patent expiration – Proleukin, Betaseron
• The molecular oncology program, while showing initial promise, is very early in development
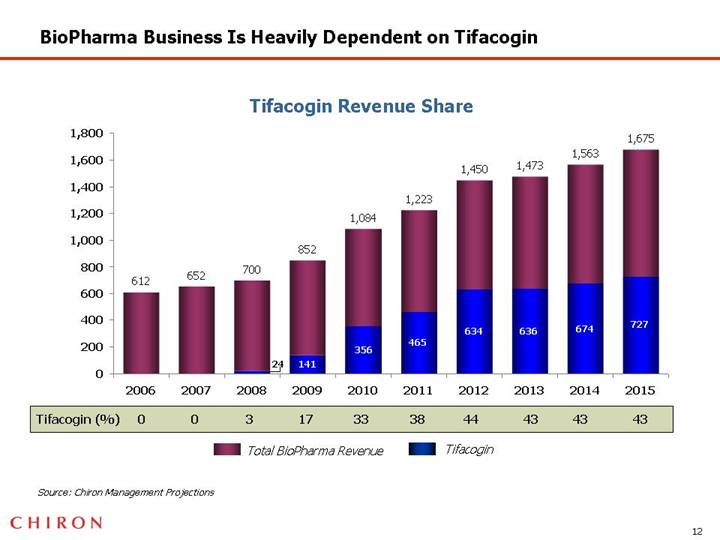
• Tifacogin is potentially a substantial opportunity but entails significant risk
Future growth and profitability of BioPharma is heavily dependent on tifacogin, which remains a high-risk program
11
BioPharma Business Is Heavily Dependent on Tifacogin
Tifacogin Revenue Share
[CHART]
Source: Chiron Management Projections
12
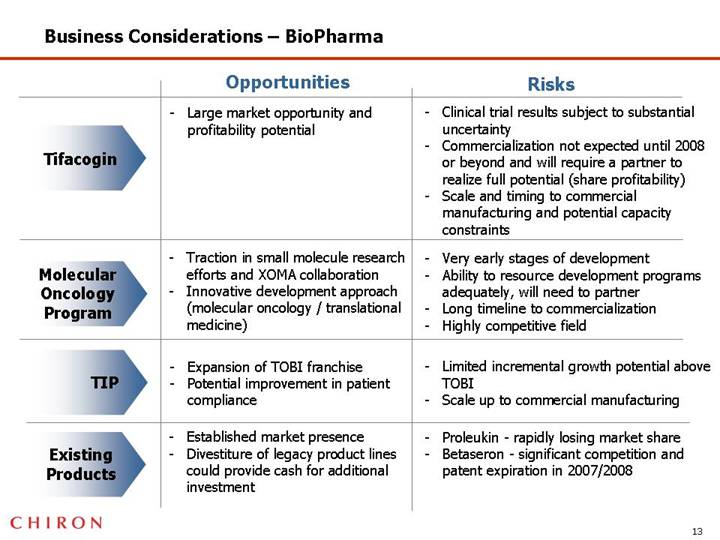
Business Considerations – BioPharma
| | Opportunities | | Risks |
| | | | |
Tifacogin | | • Large market opportunity and profitability potential | | • Clinical trial results subject to substantial uncertainty • Commercialization not expected until 2008 or beyond and will require a partner to realize full potential (share profitability) • Scale and timing to commercial manufacturing and potential capacity constraints |
| | | | |
Molecular Oncology Program | | • Traction in small molecule research efforts and XOMA collaboration • Innovative development approach (molecular oncology / translational medicine) | | • Very early stages of development • Ability to resource development programs adequately, will need to partner • Long timeline to commercialization • Highly competitive field |
| | | | |
TIP | | • Expansion of TOBI franchise • Potential improvement in patient compliance | | • Limited incremental growth potential above TOBI • Scale up to commercial manufacturing |
| | | | |
Existing Products | | • Established market presence • Divestiture of legacy product lines could provide cash for additional investment | | • Proleukin - rapidly losing market share • Betaseron - significant competition and patent expiration in 2007/2008 |
13
Vaccines Represents Substantial Opportunity But Has Risks
• Traditional egg-based influenza vaccines, including Fluvirin vaccine and Begrivac vaccine, have fueled Chiron’s vaccines growth
• While remediation efforts to date have been successful, financial and reputational costs to Chiron are substantial
• GMP compliance will require continuous improvement to meet ever higher regulatory standards over time
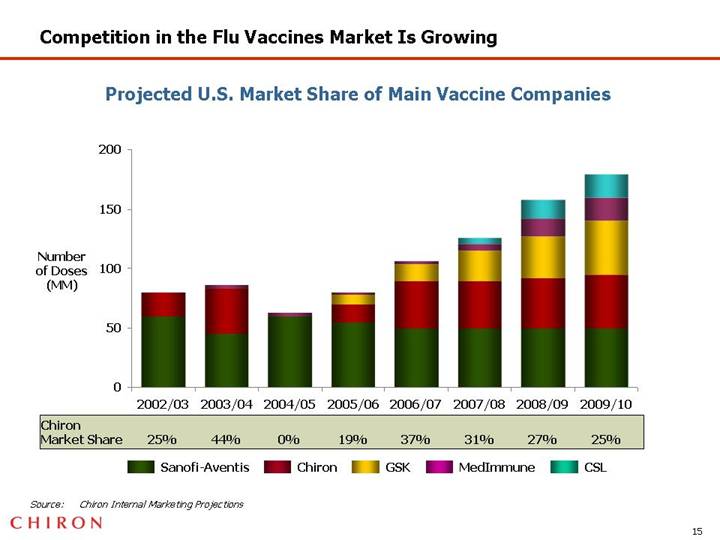
• Chiron’s competitive position in influenza market has declined with new market entrants, including GSK and CSL
• Flu cell culture conversion, which is a significant opportunity for Vaccines segment, faces developmental, regulatory, and manufacturing hurdles
• Pandemic flu is an important strategic opportunity, although incremental commercial value is uncertain and technical challenges must be overcome
• Meningitis B program is promising and proprietary, but development, manufacturing, and commercial risks remain; MenACWY will be second to market
Vaccines business remains an attractive opportunity but key products and programs face intensifying competition and on-going challenges
14
Competition in the Flu Vaccines Market Is Growing
Projected U.S. Market Share of Main Vaccine Companies
[CHART]
Source: Chiron Internal Marketing Projections
15
Pandemic Influenza Vaccine Strategy Presents Challenges
PRIOR TO A PANDEMIC
• Until there is an actual pandemic, opportunity may be limited to government stockpiling (manufacturing between seasonal campaigns) and government funding of R&D
• Commercial potential for stockpiling is unclear
• So far, limited demand…government tenders have been small
• Larger demand would require additional capacity
• Narrow window between seasonal campaigns heightens capacity constraints
• Each country has different specifications
• Government pressure on pricing
• Competition is intensifying
• GSK, MedImmune and Sanofi-Pasteur have initiated or plan to initiate development
16
Pandemic Influenza Vaccine Strategy Presents Challenges
DURING A PANDEMIC
• An actual pandemic may lead to year-round production of pandemic strain in lieu of seasonal campaigns for a brief period
• Proprietary adjuvant MF59 may reduce antigen requirements and increase supplies
• Government pressure on pricing may lead to lower margins
• But in order to produce a commercial pandemic vaccine, technical obstacles must first be addressed…
• Pandemic vaccine may require higher number of doses than seasonal vaccine
• Adjuvants may be required to improve immunogenicity
• Manufacturing yields expected to be relatively low
• Unclear that these technical obstacles will be resolved before pandemic arrives
• …and the regulatory pathway is still not clear
• No currently approved pandemic vaccines
• FDA and EMEA may have different requirements
• FDA has not approved any adjuvant other than alum
• Flu cell culture production, which is not reliant on egg supply, may afford an advantage
• Significant capital investment is required (U.S.: $350-$400MM, EU: $80-$100MM)
17
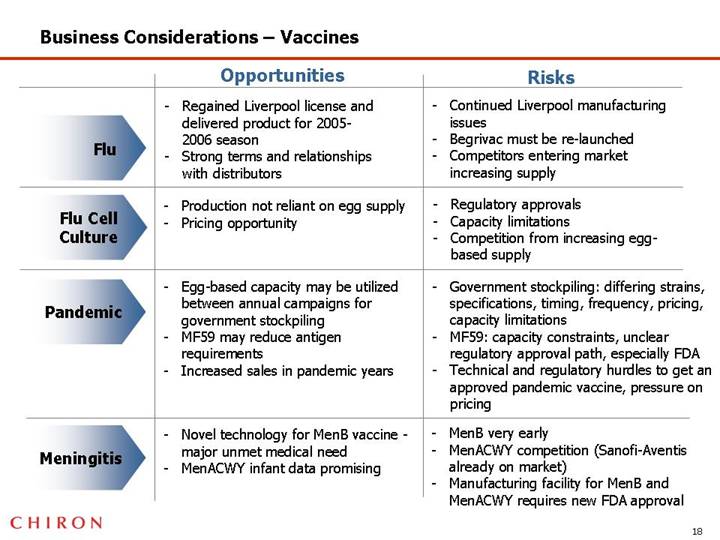
Business Considerations – Vaccines
| | Opportunities | | Risks |
| | | | |
Flu | | • Regained Liverpool license and delivered product for 2005-2006 season • Strong terms and relationships with distributors | | • Continued Liverpool manufacturing issues • Begrivac must be re-launched • Competitors entering market increasing supply |
| | | | |
Flu Cell Culture | | • Production not reliant on egg supply • Pricing opportunity | | • Regulatory approvals • Capacity limitations • Competition from increasing egg-based supply |
| | | | |
Pandemic | | • Egg-based capacity may be utilized between annual campaigns for government stockpiling • MF59 may reduce antigen requirements • Increased sales in pandemic years | | • Government stockpiling: differing strains, specifications, timing, frequency, pricing, capacity limitations • MF59: capacity constraints, unclear regulatory approval path, especially FDA • Technical and regulatory hurdles to get an approved pandemic vaccine, pressure on pricing |
| | | | |
Meningitis | | • Novel technology for MenB vaccine - major unmet medical need • MenACWY infant data promising | | • MenB very early • MenACWY competition (Sanofi-Aventis already on market) • Manufacturing facility for MenB and MenACWY requires new FDA approval |
18
Blood Testing Business is Strong But Has Limited Growth Potential
• Strong commercial capabilities and solid intellectual property
• Growth is slowing
• Key patents are facing expiration
• Chiron has no proven internal development or manufacturing capabilities for “next generation” platform
19
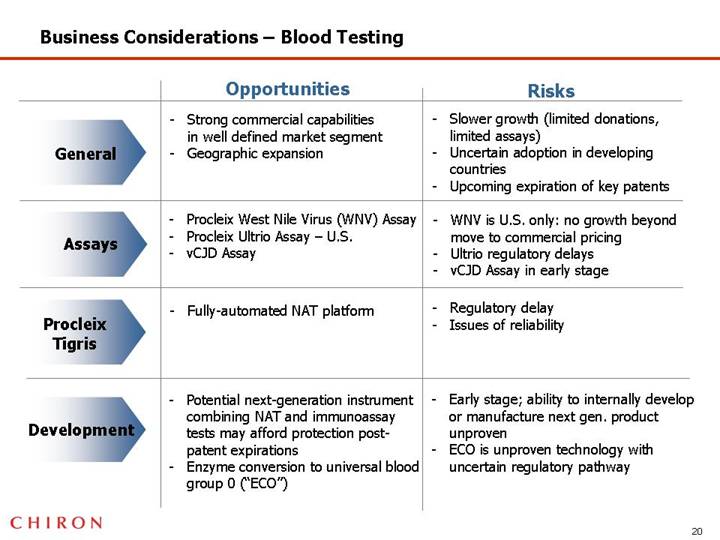
Business Considerations – Blood Testing
| | Opportunities | | Risks |
| | | | |
General | | • Strong commercial capabilities in well defined market segment • Geographic expansion | | • Slower growth (limited donations, limited assays) • Uncertain adoption in developing countries • Upcoming expiration of key patents |
| | | | |
Assays | | • Procleix West Nile Virus (WNV) Assay • Procleix Ultrio Assay – U.S. • vCJD Assay | | • WNV is U.S. only: no growth beyond move to commercial pricing • Ultrio regulatory delays • vCJD Assay in early stage |
| | | | |
Procleix Tigris | | • Fully-automated NAT platform | | • Regulatory delay • Issues of reliability |
| | | | |
Development | | • Potential next-generation instrument combining NAT and immunoassay tests may afford protection post-patent expirations • Enzyme conversion to universal blood group 0 (“ECO”) | | • Early stage; ability to internally develop or manufacture next gen. product unproven • ECO is unproven technology with uncertain regulatory pathway |
20
Directors Managed Process to Achieve Fair Price
• Conducted by Directors with significant industry and financial expertise *
• Independent, top-tier financial and legal advisors
• Transaction timeline managed by Directors to achieve an optimal outcome
• Threat of invoking additional procedural rights provided for in Governance Agreement, such as arbitration and delay, resulted in fair price for stockholders
• Unanimous approval by Directors
* See Annex A
21
$45 Offer Represents Fair Value for Chiron Stockholders
• In assessing the transaction, Directors carefully considered the business risks and opportunities described above
• The Directors required and relied on certain analyses, and also obtained fairness opinions from two independent financial advisors, Credit Suisse and Morgan Stanley
• Industry-accepted methodologies and sensitivities to projected business performance
• Historical trading ranges
• Prior to initial offer, research analysts forward price targets of $32 -$42 per share
• Selected companies analysis
• Discounted cash flow analysis, consolidated and sum of the parts
• Various sensitivity analyses and additional data
• $45 value is supported by and attractive relative to ranges implied by analysis and Chiron’s intrinsic value and reflects a significant portion of synergy value available to Novartis
• Value represents outcome of extensive negotiation and careful timing by Directors
$45 offer deemed by Directors to be fair and most attractive alternative available to Chiron
22
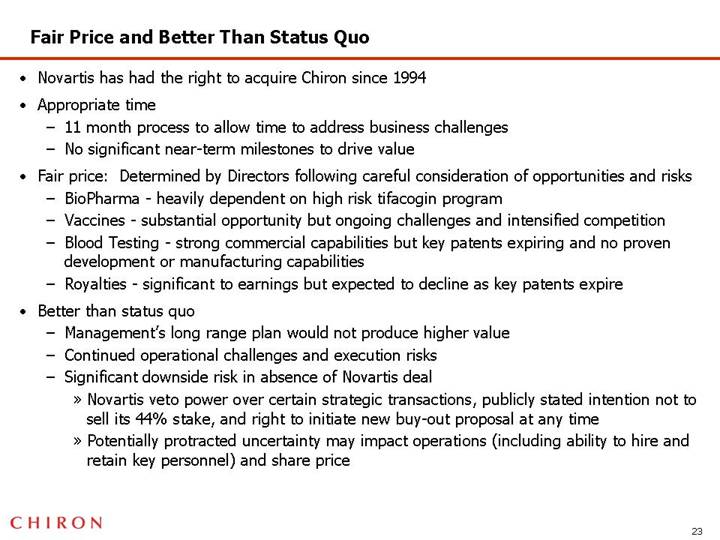
Fair Price and Better Than Status Quo
• Novartis has had the right to acquire Chiron since 1994
• Appropriate time
• 11 month process to allow time to address business challenges
• No significant near-term milestones to drive value
• Fair price: Determined by Directors following careful consideration of opportunities and risks
• BioPharma - heavily dependent on high risk tifacogin program
• Vaccines - substantial opportunity but ongoing challenges and intensified competition
• Blood Testing - strong commercial capabilities but key patents expiring and no proven development or manufacturing capabilities
• Royalties - significant to earnings but expected to decline as key patents expire
• Better than status quo
• Management’s long range plan would not produce higher value
• Continued operational challenges and execution risks
• Significant downside risk in absence of Novartis deal
• Novartis veto power over certain strategic transactions, publicly stated intention not to sell its 44% stake, and right to initiate new buy-out proposal at any time
• Potentially protracted uncertainty may impact operations (including ability to hire and retain key personnel) and share price
23
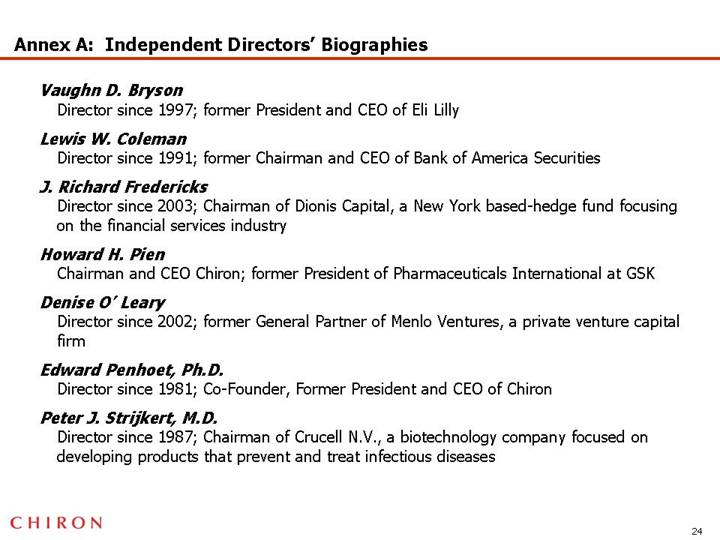
Annex A: Independent Directors’ Biographies
Vaughn D. Bryson
Director since 1997; former President and CEO of Eli Lilly
Lewis W. Coleman
Director since 1991; former Chairman and CEO of Bank of America Securities
J. Richard Fredericks
Director since 2003; Chairman of Dionis Capital, a New York based-hedge fund focusing on the financial services industry
Howard H. Pien
Chairman and CEO Chiron; former President of Pharmaceuticals International at GSK
Denise O’ Leary
Director since 2002; former General Partner of Menlo Ventures, a private venture capital firm
Edward Penhoet, Ph.D.
Director since 1981; Co-Founder, Former President and CEO of Chiron
Peter J. Strijkert, M.D.
Director since 1987; Chairman of Crucell N.V., a biotechnology company focused on developing products that prevent and treat infectious diseases
24
[LOGO]
Novartis Merger:
Presentation to Stockholders
March 2006