UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
ANNUAL REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
| | | |
| þ | | ANNUAL REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | For the Fiscal Year Ended June 30, 2007 |
OR |
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 0-11250
DIONEX CORPORATION
(Exact name of registrant as specified in its charter)
| | | |
Delaware
(State or other jurisdiction of
Incorporation or organization) | | 94-2647429
(I.R.S. Employer
Identification No.) |
| | | |
1228 Titan Way,
Sunnyvale, California
(Address of principal executive offices) | | 94085
(Zip Code) |
Registrant’s telephone number, including area code
(408) 737-0700
Securities registered pursuant to Section 12(b) of the Act:
| | | |
Title of Each Class | | Name of Each Exchange on Which Registered |
| |
| Common Stock, par value $.001 per share | | The Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES þ NO o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES o NO þ
Indicate by check market whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES þ NO o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 ofRegulation S-K is not contained herein and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of thisForm 10-K or any amendment to thisForm 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” inRule 12b-2 of the Exchange Act.
Large accelerated filer þ Accelerated Filer o Non-Accelerated filer o
Indicate by check mark whether the registrant is a shell company (as defined inRule 12b-2 of the Exchange Act). Yes o No þ
The aggregate market value of the registrant’s Common Stock held by non-affiliates on December 31, 2006 (based upon the closing price of such stock as of such date) was $1,157,528,055.
As of August 27, 2007, 18,693,043 shares of the registrant’s Common Stock were outstanding.
Portions of the registrant’s definitive Proxy Statement for the Annual Meeting of Stockholders to be held on October 30, 2007 are incorporated by reference in Part III of this Annual Report.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
Certain statements set forth or incorporated by reference in thisForm 10-K, as well as in the Dionex Annual Report to Stockholders for the year ended June 30, 2007, constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, Section 21E of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), and the Private Securities Litigation Reform Act of 1995, and are made under the safe harbor provisions thereof. Such statements are subject to certain risks, uncertainties and other factors that may cause actual results, performance or achievements, or industry conditions, to be materially different from any future results, performance or achievements, or industry results, expressed or implied by such forward-looking statements. Such risks and uncertainties include: general economic conditions, foreign currency fluctuations, fluctuation in worldwide demand for analytical instrumentation, fluctuations in quarterly operating results, competition from other products, existing product obsolescence, new product development, including market receptiveness, the ability to manufacture products on an efficient and timely basis and at a reasonable cost and in sufficient volume, the ability to attract and retain talented employees and other risks as set forth under “Item 1A. “Risk Factors” and elsewhere in thisForm 10-K. Readers are cautioned not to place undue reliance on these forward-looking statements that reflect management’s analysis only as of the date hereof. Dionex undertakes no obligation to update these forward-looking statements.
OVERVIEW
Dionex Corporation designs, manufactures, markets and services analytical instrumentation and related accessories and chemicals. Our products are used to analyze chemical substances in the environment and in a broad range of industrial and scientific applications.
We have evaluated those business activities that are regularly reviewed by our senior management and have determined that we have two operating segments that are aggregated into one reportable segment.
Unless the context otherwise requires, the terms “Dionex,” “we,” “our” and “us” and words of similar import as used in this report include Dionex Corporation and its consolidated subsidiaries. Dionex Corporation was incorporated as a California corporation in 1980 and reincorporated in Delaware in 1986.
PRODUCTS AND SERVICES
We design, manufacture, market and service a range of liquid chromatography systems, sample preparation devices and related products that are used by chemists to separate and quantify the individual components of complex chemical mixtures in many major industrial, research and laboratory markets.
Our liquid chromatography systems are currently focused in two product areas: ion chromatography (IC) and high performance liquid chromatography (HPLC). We offer a mass spectrometer coupled with either an IC or HPLC system. For sample preparation, we provide automated solvent extraction systems. In addition, we develop and manufacture consumables, detectors, automation and analysis systems for use in or with liquid chromatographs. Each of these product areas is described below.
Ion Chromatography
Ion chromatography is a form of chromatography that separates ionic (charged) molecules, usually found in water-based solutions, and typically detects them based on their electrical conductivity. The sale of our IC systems and related columns, suppressors, detectors, automation and other products accounted for over 60% of our net sales in fiscal 2007, 2006 and 2005, respectively.
Our IC products are used in a wide range of analytical applications, including environmental monitoring, quality control of pharmaceuticals, corrosion monitoring, and evaluation of raw materials, quality control of industrial processes and products, research and development, and regulation of the chemical composition of food,
3
beverage and cosmetic products. Major customers include environmental testing laboratories, life science and food companies, chemical and petrochemical firms, power generation facilities, electronics manufacturers, government agencies and academic institutions.
In fiscal 2003, we introduced the industry’s first Reagent Free IC (RFIC) systems by combining eluent generation and advanced eluent suppression technologies, eliminating the need for manual preparation of eluents and reagents. Eluents are solvents used to separate materials in the chromatography process. Reagents are substances used in a chemical reaction to detect, measure, examine or produce other substances. RFIC systems simplify ion chromatography while increasing its sensitivity and reproducibility. RFIC systems also eliminate the errors associated with manual reagent preparation. In fiscal 2005, we further expanded our RFIC capabilities by offering dual RFIC capabilities. In fiscal 2006, we expanded this powerful technology further by introducing RFIC for carbonate/bi-carbonate eluents. In fiscal 2007, we further expanded this innovative technology by introducing RFIC-Eluent Regeneration. Using this technology, our customers are able to regenerate their eluent, thus saving time and money on eluent preparation while providing greater consistency of results.
We offer a broad range of systems for IC:
Our ICS-3000 RFIC System, introduced in fiscal 2005, is a modular system that provides a wide range of capabilities to our customers and expanding our RFIC systems to a broader range of eluents (liquids to carry a sample through a liquid chromatography system). This premier product in our IC family is expandable from a single to a dual system in one footprint. The ICS-3000 features the latest advancements in an RFIC system including dual eluent generation capabilities, continuously regenerated trap column capabilities, and the SRS Self-Regenerating Suppressor technology. The ICS-3000 comes as single or dual configurations with flow rates ranging from microbore to semi preparative. Flexible analytical capability is offered by interfacing with several detectors including conductivity, electrochemical, UV-Vis, photodiode array, and mass spectrometry. For sample introduction, the system is available with the full-featured autosampler (AS) capable of sample preparation functions, pre-concentration and matrix elimination for removal of interfering analytes and enhancing detection abilities. One autosampler (AS) can be shared between two systems, keeping costs to a minimum. The ICS-3000 RFIC system has the capabilities to allow customers to run parallel or 2D chromatography analyses. Chromeleon chromatography data management software, available with the ICS-3000, provides powerful data processing, control features, audit trail, intuitive database management, and full client-server capabilities using an easy to navigate graphical user interface. Together, the hardware and software offer “system wellness”, a feature that automatically schedules calibration, validation, and routine maintenance on each module. Built-in diagnostics can be prompted from the software to help troubleshoot and track useful parameters such as absorbance detector lamp life and column usage. For customers that do not require the full capabilities of our Chromeleon software, we offer Chromeleon Xpress for stand-alone control of the ICS-3000.
Our ICS-2000 RFIC System, introduced in fiscal 2003, is the industry’s first totally integrated and preconfigured RFIC system designed to perform with all types of electrolytically generated eluents for isocratic and gradient IC separations using suppressed conductivity detection. The system is controlled from an LCD touch pad front panel or using Chromeleon software. RFIC systems benefits include ease-of use, consistency and superior performance as compared to systems with manually prepared eluents.
Our ICS-1500 System, introduced in fiscal 2003, is a fully integrated and preconfigured system designed to perform IC separations using conductivity detection. The system is available with a dual-piston pump, thermally controlled conductivity cell, column heater, and optional in-line vacuum degassing. The system is controlled from an LCD touch pad front panel or using Chromeleon software.
Our ICS-1000, introduced in fiscal 2003, is an integrated and preconfigured system that performs IC separations using conductivity detection. The system features a dual-piston pump, LED status front panel and is controlled by our Chromeleon software (see “Automation Products — Chromeleon Software” for a description of this software’s capabilities). Options include column heating and in-line vacuum degassing. The ICS-1000 provides built-in control for electrolytic suppression technology to provide high performance and ease of use.
Our ICS-90 integrated IC system, introduced in fiscal 2002, is designed for routine ion analysis and provides rapid start up times, easy operation and stable performance.
4
High Performance Liquid Chromatography
HPLC is a form of chromatography that separates a wide range of molecules, such as proteins, carbohydrates, amino acids, and pharmaceuticals, and quantifies the components by measuring the amount of light that the molecules absorb or emit when exposed to a light source. Our HPLC customers include life science companies active in biological research, biotechnology, pharmaceutical drug discovery and development, and other industrial companies. The sales of our HPLC products and related columns, suppressors, detectors, automation and other products accounted for approximately 30% of our net sales in each of fiscal 2007, 2006 and 2005.
Analytical HPLC —We offer the following products for traditional and analytical HPLC:
Our UltiMate 3000 HPLC system for analytical, micro and semipreparative flowrates, introduced in fiscal 2006, consists of single or dual-high precision pumps providing accurate, highly reproducible isocratic or gradient separations from 2.5ml/min to 100ml/min depending on the configuration. The UltiMate 3000 systems allow advanced chromatography techniques such as tandem, two-dimensional, one-line SPE and parallel applications. Chromeleon chromatography data management software, available with the UltiMate 3000, provides full system control, multi-instrument control and other powerful features.
Our ICS-3000 RFIC System — The ICS-3000 features an inert, metal-free, polyetheretherketone (PEEK) flow path to preserve chemically unstable biomolecules. The ICS-3000 provides high performance dual piston pumps and detectors, including a choice of electrochemical, absorbance and photodiode array detectors. These options allow for a wide range of applications including quantification of carbohydrates, amino acids, proteins and peptides, nucleic acids and small biomolecules. The ICS-3000 system is available with the Chromeleon software.
Capillary-/nano-LC — Capillary-/nano-LC is a form of HPLC that uses low flow rates for analyzing sample volumes much smaller than those analyzed using traditional analytical HPLC. We offer the following products for capillary-/nano-HPLC:
Our UltiMate 3000 Capillary-/Nano-LC System, introduced in fiscal 2005, is a dedicated microseparation system consisting of single or dual high-precision pumps coupled with patented flow control capabilities utilizing a proprietary method of flow splitting to provide accurate, reproducible isocratic and gradient separations from 50nl/min to 2.5ml/min, depending on the configuration. The UltiMate also has a specially developed UV detector. This detector, coupled with our proprietary capillary flow cells, allows the most sensitive UV detection in microcolumn separations. Accessories for the UltiMate system include theWPS-3000 Wellplate autosampler and the FLM-3100 Flow Control module for flow control in a thermostated column compartment that also allows column switching.
Our APS-2000 System Series, introduced in fiscal 2004, provides the purification of synthesized biological and natural compounds. The APS-2000 brings the power of Ultimate HPLC system and Chromeleon software to purification. The APS-2000 is designed to increase throughput in the purification of compounds.
We offer the Probot microfraction collector for the micro-analysis market. The Probot allows collection or dispensation of micro-fractions and is also used for precision spotting of MALDI-TOF mass spectrometer target plates.
Sample Preparation
We offer a number of solutions for sample preparation:
Our ASE 300 and ASE 100 are automated sample extraction (ASE) instruments for large samples up to 100 mL and our ASE 200 is for samples less than 34 mL. The ASE 200 and ASE 300 can automatically extract 24 samples and 12 samples, respectively, while the ASE 100 is a single-sample automated extractor. Each ASE system extracts components of interest from solid and semi-solid samples using common solvents (the same used in traditional soxhlet techniques) at elevated temperatures and pressures. Competitive techniques include soxhlet, sonication, microwave extraction and supercritical fluid extraction. The ASE 200 and 300 systems offer several advantages over other solvent based extraction techniques including lower solvent consumption,
5
reduced extraction time, higher throughput automation and ease of use. We offer the Solvent Controller Module, which automates the delivery of multiple solvents to the ASE systems, and AutoASE, an add-on software feature which allows control of up to eight ASE systems from one location, as well as provides methods of data storage and documentation.
In fiscal 2005, we added the SE 400 and SE 500 Solvent Evaporation Systems to our sample preparation offerings. The systems are designed to rapidly evaporate organic solvents or water from ASE collection vials or bottles. ASE systems and related accessories are used worldwide for a number of environmental, pharmaceutical, industrial and food and beverage applications.
Detectors
Detectors are used to measure the quantity of various sample components after they have been separated in a chromatography column. We currently offer several detector products based on conductivity, electrochemistry, absorbance (including the PDA-3000 photodiode array detector), fluorescence and refractive index absorbance. This range of detectors is designed to meet customer requirements for analysis of organics, inorganics, metals, amino acids, biological compounds and pharmaceuticals.
Mass Spectrometry
Mass spectrometry (MS) is used to identify the molecular weight of compounds within a sample substance and renders structural molecular information. Through an agreement with Thermo Fisher Scientific, we offer the MSQ Plus mass spectrometer (MSQ Plus) together with the Ultimate 3000 Analytical HPLC and ICS-3000 systems.LC/MS and IC/MS systems using the MSQ Plus are used worldwide, particularly in the pharmaceutical market, but also for environmental testing, drug, food and beverage quality control and many other applications. The MSQ Plus mass spectrometer is a compact, benchtop, single quadrupole mass detector. The standard system is supplied with both Electrospray (ESI) and Atmospheric Pressure Chemical Ionization (APCI) for maximum analytical flexibility. The agreement with Thermo Fisher Scientific enables us to reach chemists desiring MS capabilities who previously were not among our potential customers.
In addition, in fiscal 2006, we introduced the Dionex Chromatography Mass Spectrometry Link (DCMSLink) software. This software package provides an interface for controlling a wide range of our chromatography instruments from third-party mass spectrometry software (MS software) such as Analyst from Applied Biosystems/MDS Sciex, Xcalibur from Thermo Fisher Scientific, and HyStar from Bruker Daltonics. When using DCMSLink, all instruments are controlled and all data is stored, reviewed, quantitated and reported by the MS software. DCMSLink enables us to expand to additional potential markets for our chromatography systems to customers who desire the quality and features of our chromatography with higher-end mass spectrometers.
Process Instrumentation
We offer the DX-800 Process Analyzer for continuous on-line monitoring necessary in a variety of industrial applications. Major applications for the DX-800 are in the power generation industry for the continuous monitoring of corrosive contaminants in boiler water, the semiconductor industry for continuous monitoring of contaminants in high purity water, and the pharmaceutical and chemical industries for continuous monitoring of biological and chemical synthesis processes. TheDX-800 uses our Chromeleon chromatography software for automation, data acquisition, reporting and security. The software allows the user to view analyzer status, handle alarms, and interface with the computing and control systems in place at the enterprise where the DX-800 is installed.
Automation Products
As part of our efforts to make chemical analyses simpler, faster and more reliable, we offer a family of products that automate sample handling, system operation and data analysis for chromatography systems. These products include Chromeleon software and several automated sample injection modules available for IC and HPLC applications.
Chromeleon Software — In fiscal 2006, we introduced Chromeleon 6.8, the latest version of our chromatography data management system. From a single user interface, Chromeleon provides full control of over 200 LC
6
and GC instruments from more than 25 vendors of liquid and gas chromatography systems. It is an easy-to-use, adaptable data management system with scalable client/server architecture for customers requiring a single workstation to lab- or campus-wide deployment. Data is organized by instrument, user, project or product. All data is stored locally on a personal computer, centrally on a network server, or both. Chromeleon features a flexible graphical user interface and report generator which can be adapted to dedicated applications. Chromeleon also offers a complete suite of features for regulatory compliance: security, validation, audit trails and electronic signatures. Chromeleon provides all the features that laboratories need to comply with GLP, GMP and 21 CFR Part 11 without losing productivity.
In fiscal 2007, we expanded Chromeleon by adding validation templates to assist customers who require validation of their HPLC systems.
Autosamplers — We offer a number of autosamplers that address a variety of customer needs.
The AS40 Automated Sample Injection module (AS40) is a low-cost, metal-free, rugged automated sample loading device designed especially for ion chromatography applications. The AS40 can be used with our IC systems.
For more complex needs, we offer the AS Autosampler (AS). The AS is a high-performance, random vial access autosampler with automated sample injection which is used primarily with the high-end RFIC systems. We also provide the simultaneous injection AS Autosampler for concurrent injection of a sample onto two analytical systems or an ICS-3000 Dual RFIC System for running unique or similar applications.
In fiscal 2007, we introduced the AS-HV Autosampler (AS-HV). The AS-HV allows customers to inject volumes up to 250 ml and is designed for customers performing trace analysis.
For HPLC applications, we offer the WPS 3000 series of Autosamplers with optional thermal control for temperature-sensitive samples. The WPS provides speed, simplicity, reliability and precision with either pull-loop or in-line split-loop injection technology. The removable carousels and programmable needle depth allows the WPS to accommodate a variety of sample vials and sizes including wellplates.
For capillary and nano-LC flow rates, we offer the WPS-3000 WellPlate micro Autosampler. The WPS-3000 micro is a fully automated micro autosampler for sample injections from up to three 96 or 386 well plates or conventional 1.5 milliliter vials. The unique design allows for automated injection of volumes down to 20 nanoliters. WPS-3000 has a proprietary injection technique that guarantees high performance and virtually no sample dispersion.
Consumables
We offer a number of consumables for IC and HPLC customers. The primary consumables provided by us are columns, suppressors and RFIC eluent cartridges. These consumables are replaced at regular intervals depending on the volume of use and sample composition.
Columns — A chromatography column consists of a hollow cylinder packed with a unique separation material. The column’s function is to separate various chemical components in a sample. We develop and manufacture separation materials such as ion-exchange resins, silica-based bonded phases and monolithic phases using proprietary processes. We currently manufacture and market a broad range of column types designed and tested for specific applications in the HPLC and IC markets. We offer a wide range of polymer-based ion-exchange and reversed-phase columns supporting capillary, analytical and semipreparative scale applications.
Recent column introductions augment a continuing program of product launches geared to address developing market requirements. For IC, we introduced the IonPac AS17-C anion exchange column for the determination of key anions in high-purity drinking water matrices and the IonPac AS24 anion exchange column for separation of haloacetic acids in drinking water prior to mass spectrometry analysis.
In fiscal 2006, we introduced the IonPac CS 18, AS22 and IonPac AS23 anion exchange column.
For HPLC customers, we introduced in fiscal 2007 a number of new columns designed to address the expanding needs of our customers. We introduced the Acclaim Mixed-Mode HILIC-1 silica-based column, the
7
Acclaim Mixed-Mode WAX-1 silica-based column and the Acclaim Fast LC silica-based family of columns. The Acclaim Fast LC column for Fast LC separations uses improved 3 micron particles coupled with our Ultimate 3000 system and provides 15 fold faster separations than conventional HPLC. The Acclaim Fast LC columns are designed for faster throughput while maintaining robustness and ease of use. The Acclaim Mixed-Mode HILIC-1 allows chromatographers to control the elution of both highly polar and nonpolar molecules to optimize resolution of polar compounds that are otherwise unretained by reversed-phase chromatography. The Acclaim Mixed-Mode WAX-1 allows the chromatographer to control the elution of acids, bases and neutral molecules with one column.
In fiscal 2006 and 2005, we introduced a number of silica-based columns to expand our Acclaim family of HPLC columns.
For the life sciences market, we introduced the ProSwift Ion-Exchange Monolith columns, the ProSwiftSAX-1S and the ProSwift WCX-1S in 4.6 mm format, for increase resolution, speed and capacity for protein separations. In addition, we introduced the ProSwift WAX-1S and ProSwift WCX-1S in 1 mm format for separation of proteins and large peptides. The introductions further expand our polymeric monolith family of columns.
We also offer a variety of micro, capillary and nano-LC columns in varying formats packed with a variety of “stationary phase” materials.
Eluent Suppressors —We manufacture eluent suppressors that are used to enhance detection and sensitivity in ion chromatography. Our suppressors’ lower background conductivity and support a wide range of ion exchange columns separations including separations using high capacity columns and more concentrated eluents liquids used to carry a sample through a liquid chromatography system. We offer an array of suppressors that include the Self Regenerating Suppressor (SRS Ultra II), the Atlas Suppressor and the MicroMembrane Suppressor (MMS III).
The Self Regenerating Suppressor enhances IC performance during long-term operation, without user intervention. The SRS Ultra II provides superior performance for all IC applications, particularly traces level ion chromatography using hydroxide eluents, and is a key component of the ICS-3000 Reagent Free IC (RFIC) systems.
The Atlas Suppressor (AES) provides improved sensitivity, lower noise and fasterstart-up over our previous, industry leading AutoSuppression technology, raising performance goals even higher for IC electrolytic suppression. The AES suppressor offers optimal performance for most anion applications using carbonate eluents and cation applications using methanesulfonic acid eluents.
The MMS III MicroMembrane Suppressor, with an innovative displacement chemical regeneration mode of operation, can be used with our ion-exchange columns, providing ultra-low noise operation, and is recommended for anion and cation separations when using eluents containing organic solvents.
RFIC Eluent Cartridges — We manufacture eluent generation cartridges used with RFIC systems for the automatic production of high-purity eluents. We offer cartridges for generation of hydroxide Aethane Sulforic Acid and carbonate/bicarbonate eluents.
Service and Other — We also generate sales from our customer service organization through maintenance contracts, spare part sales, customer training and sales of other products and valued-added services. (See “Technical Support, Installation and Service” below.)
CUSTOMERS, MARKETING, AND SALES
Our products are used extensively in the environmental, life science and industrial markets using chromatography and extraction technologies. The environmental market is characterized by water analysis, safety and security applications and pollution testing, with chemists from private and governmental laboratories being our primary customers in this field. The life science markets we serve include the pharmaceutical segment, biosciences and medical sciences, with customers from industrial, academic and governmental accounts. The industrial markets we serve include the electronics and power industries with a demand for analyzing the higher-purity water quality in their production facilities. Furthermore, we serve a number of the largest industrial companies worldwide within the chemical industry market, which produce specialty chemicals, petrochemicals, consumer products and more, and the food and beverage market, which test for product quality assurance.
8
One of our marketing strategies is to target all market segments mentioned above where our technology is already established. Here, we seek to increase demand for our chromatography solutions by approaching all existing and potential customers through direct marketing activities including direct sales calls, mailings, advertising, electronic marketing, seminars, and workshops. In addition, we build visibility and branding for our global presence through scientific conferences and exhibitions. Continuous growth in all these markets results from identifying new customers in existing sales regions, extending geographic penetration and increasing demand for our products and technical support capabilities.
The second component of our marketing strategy is to explore and develop new application fields in close collaboration with existing and potential customers, and to leverage this competence into other market areas. A prerequisite to establish this process is the availability of highly skilled technical developing and support staff working to also assist customers in solution definition and development. To meet and exceed customer expectations in our developing commercial markets, our effort is to optimize and diversify our technology interests in the chromatography market, including sample preparation, purification, analysis, testing, and data management.
Geographically, we currently market and distribute our products and services through our own sales force in Austria, Australia, Brazil, Canada, China, Denmark, France, Germany, India, Ireland, Italy, Japan, Korea, Netherlands, Switzerland, Taiwan, the United Kingdom and the United States. In each of these countries, we maintain one or more local sales offices in order to support and service our customers in the regions. In other international locations where we do not have a direct sales force, we have developed a network of distributors and sales agents. Sales to customers by geographic region were approximately 29% in North America, 44% in Europe and 27% in Asia/Pacific and other of net sales in fiscal 2007. In fiscal 2006, sales to customers by geographic region were approximately 31% in North America, 42% in Europe and 27% in Asia/Pacific and other.
We manufacture our products based upon our forecast of customer demand and maintain adequate inventories of completed modules or finished goods in advance of receipt of firm orders. System or instrument orders are generally placed by the customer on an as-needed basis, and instruments are usually shipped within four to six weeks after receipt of an order. We do not maintain a substantial backlog, and backlog as of any particular date may not be indicative of our actual sales in any succeeding period. The level of backlog at June 30, 2007 was $49.3 million and at June 30, 2006 was $46.9 million.
FINANCIAL INFORMATION ABOUT GEOGRAPHIC AREAS
For financial information by geographic area, refer to footnote 14 of the Notes to Consolidated Financial Statements in Item 8 of thisForm 10-K.
COMPETITION
Competition in our industry is based upon the performance capabilities of the analytical instruments, technical support and after-market services, the manufacturer’s reputation as a technological leader and selling prices. Management believes that performance capabilities are the most important of these criteria. Customers measure system performance based on sensitivity (the ability to discern minute quantities of a particular sample component), selectivity (the ability to distinguish between similar components), speed and throughput of analysis, and the range of chemical and biological samples the system can effectively analyze. Management believes that we enjoy a favorable reputation in terms of performance capabilities, technical support and service.
Companies competing with us in the analytical instruments market include Agilent Technologies, Inc., Waters Corporation, Shimadzu Corporation, Thermo Fisher Scientific Inc., Varian, Inc., Perkin-Elmer, Inc. and Metrohm Ltd.
We believe we have a substantial market share in the IC market, which is a segment of the LC market. Our IC systems generally compete with a number of analytical techniques used in identifying and quantifying ionic and polar compounds. The primary source of competition are conventional manual and automated wet chemistry procedures and certain possibly modified liquid chromatography systems using a single column method without or including an ion suppression device. Companies competing with us in IC include such vendors as Metrohm AG, Shimadzu Corporation, Waters Corporation and other smaller companies.
9
We believe we have a smaller but growing market share in the analytical and capillary/nano-LC HPLC portions of the LC market. Our UltiMate 3000 systems compete directly with other manufacturers’ HPLC systems in traditional and capillary-/nano- HPLC applications. We believe that the UltiMate HPLC system has certain benefits over competing systems, including advanced pump and dual pump technology, thermostatted temperature control, high performance auto sampling capabilities, all technologies designed for intelligent LC (LCi). In addition, UltiMate 3000 offers many benefits over competing systems including the ability to analyze minute contents of sample at very low flow rates. We also believe that our Chromeleon software package not only provides competitive advantages over our competitors’ software offerings but is respected as the most advanced chromatography data management system in the market. Our competitors in the HPLC market include such vendors as Agilent Technologies, Inc., Shimadzu Corporation, Thermo Fisher Scientific, Varian, Inc., Waters Corporation and various smaller companies.
Our Accelerated Solvent Extraction (ASE) systems compete directly with standard Soxhlet, sonication, supercritical fluid extraction and microwave extraction techniques provided by other companies. Management believes that our ASE systems have certain benefits compared to competing techniques, including faster extraction time, reduced solvent usage, built-in automation and ease of use.
PATENTS AND LICENSES
We have a patent portfolio covering certain technologies of our products. Our patents are presently issued to the United States and number of foreign countries. As a matter of company policy, we vigorously protect our intellectual property rights and seek patent coverage on developments that we regard as strategic, material and patentable. Our patents, including those licensed from others, expire between 2008 through 2024. We believe that, while our patent portfolio has value, no single patent or patent application is in itself essential and that the invalidity or expiration of any single patent would not have a material adverse effect on our business.
We regard our Chromeleon software as proprietary and we rely on a combination of copyrights, trademarks, trade secret laws and other proprietary rights, laws, license agreements and other restrictions on disclosure, copying and transferring title to protect our rights to our software products. We have no patents covering our software, and existing copyright laws afford only limited protection. In addition, the laws of some foreign countries do not protect our proprietary rights to the same extent as do the laws of the United States.
INTERNATIONAL OPERATIONS
Financial information about foreign and domestic operations and export sales is provided in Note 14 of the Notes to Consolidated Financial Statements found elsewhere in this report.
We have subsidiaries in Austria, Australia, Brazil, Canada, China, Denmark, France, Germany, India, Ireland, Italy, Japan, Korea, Netherlands, Switzerland, Taiwan, the United Kingdom and the United States. Our foreign sales are affected by fluctuations in currency exchange rates and by regulation adopted by foreign governments. Such fluctuations have materially affected, both positively and negatively, our results of operation in past periods and will likely materially affect our results of operations in the future. Export sales are subject to certain controls and restrictions, but we have not experienced any material difficulties related to these limitations.
MANUFACTURING AND SUPPLIERS
We produce chemicals and resins and assemble IC systems and modules in our California manufacturing facilities. We assemble the systems and modules for our UltiMate 3000 systems in our manufacturing facility in Germany. We have developed proprietary processes for the manufacture of polystyrene-based resins and for packing columns with these resins. We believe that our resins, columns and suppressor manufacturing know-how are critical to the performance and reliability of our chromatography systems. We require each employee and consultant to sign a nondisclosure agreement to protect our proprietary processes. However, there can be no assurances that these agreements will provide meaningful protection or adequate remedies for our proprietary processes in the event of unauthorized use or disclosure.
We have emphasized a modular design for the principal subsystems of our pumping and flow systems, sample injection systems, chromatography modules, detectors, and control and data analysis systems. We believe that this
10
modular approach has enabled us to meet the wide range of system configurations required by our customers. Manufacturing has transitioned into flow-line production for our major systems while maintaining subassembly cell production for our integrated modules. These practices have enhanced our ability to effectively manage our inventory levels.
Many subassemblies used in our products are manufactured by us. Components, including formed-plastic and sheet-metal packaging materials, machine-metal parts, integrated circuits, microprocessors, microcomputers and certain detector and data analysis modules, are purchased from other manufacturers. Most of the raw materials, components and supplies purchased by us are available from a number of different suppliers, although a number of items are purchased from limited or single source of supply.
TECHNICAL SUPPORT, INSTALLATION AND SERVICE
Users of our chromatography systems may require technical support before and after a system sale. Services provided before the sale are recorded in operating expenses as incurred. Chromatography systems sold by us generally include a one-year warranty. These costs are accrued for at the time of the system sale. Installation and certain basic user training are provided to the customer, with revenues for these services recognized at the time the services are provided. Maintenance contracts may be purchased by customers to cover equipment no longer under warranty. Maintenance work not performed under warranty or maintenance contracts is performed on a time and materials basis. We offer training courses and periodically send our customers information on applications development. We install and service our products through our own field service organizations in Austria, Australia, Brazil, Canada, China, Denmark, France, Germany, India, Italy, Ireland, Japan, Korea, Netherlands, Taiwan, Switzerland, the United Kingdom and the United States. Installation and service in other foreign countries are typically provided by our distributors or agents.
RESEARCH AND PRODUCT DEVELOPMENT
Our research and product development efforts are focused on increasing the performance of our chromatography and other products and expanding the number of chemical and biological compounds that can be analyzed efficiently with our products. Research and product development expenditures were $24.7 million, $22.4 million and $20.4 million in fiscal 2007, 2006 and 2005, respectively. We pursue active development programs in the areas of system hardware, applications, computer software, suppressors, and resin and column technologies. There can be no assurances that our product development efforts will be successful or that the products developed will be accepted by the marketplace.
EMPLOYEES
We had 1,193 employees at June 30, 2007, compared with 1,135 employees at June 30, 2006.
AVAILABLE INFORMATION
We maintain a website at www.dionex.com; however, information found on our website is not incorporated by reference into this report. We make available free of charge on or through our website our annual report onForm 10-K, quarterly reports onForm 10-Q, current reports onForm 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission (the “SEC”). Our Board of Directors has adopted charters for the Audit, Compensation and Nominating and Governance Committees of our Board of Directors and a Code of Business Conduct and Ethics applicable to all of our officers and employees. These charters and our Code of Business Ethics and Values are available on our website athttp://investor.dionex.com/governance-PDFs.cfm and a printed copy of this information is available without charge by sending a written request to: Investor Relations, Dionex Corporation, 1228 Titan Way, Sunnyvale, California 94085. The public may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Room 1580, Washington, DC 20549, and may obtain information on the operation of the Public Reference Room by calling the SEC at1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC athttp://www.sec.gov.
11
You should consider carefully the following risk factors as well as other information in this report before investing in any of our securities. If any of the following risks actually occur, our business operating results and financial condition could be adversely affected. This could cause the market price for our common stock to decline, and you may lose all or part of your investment. These risk factors include any material changes to, and supersede, the risk factors previously disclosed in our most recent annual report onForm 10-K and subsequently filed quarterly reports onForm 10-Q.
Economic, political and other risks associated with international sales and operations could adversely affect our results of operations.
Because we sell our products worldwide and have significant operations outside of the United States, our business is subject to risks associated with doing business internationally. We anticipate that revenue from international operations will continue to represent a majority of our total sales. In addition, we expect that the proportion of our employees, contract manufacturers, suppliers, job functions and manufacturing facilities located outside the United States may increase. Accordingly, our future results could be harmed by a variety of factors, including:
| | |
| | • | interruption to transportation flows for delivery of parts to us and finished goods to our customers; |
| |
| | • | changes in a specific country’s or region’s political, economic or other conditions; |
| |
| | • | trade protection measures and import or export licensing requirements; |
| |
| | • | negative consequences from changes in tax laws; |
| |
| | • | difficulty in staffing and managing widespread operations; |
| |
| | • | differing labor regulations; |
| |
| | • | differing protection of intellectual property; |
| |
| | • | unexpected changes in regulatory requirements; and |
| |
| | • | geopolitical turmoil, including terrorism and war. |
Foreign currency fluctuations related to international operations may adversely affect our operating results.
We derived approximately 74% of our net sales from outside the United States in fiscal 2007 and expect to continue to derive the majority of net sales from outside the United States for the foreseeable future. Most of our sales outside the United States are denominated in the local currency of our customers. As a result, the U.S. dollar value of our net sales varies with currency rate fluctuations. Significant changes in the value of the U.S. dollar relative to certain foreign currencies could have a material adverse effect on our results of operations. In recent periods, our results of operations have been positively affected from the depreciation of the U.S. dollar against the Euro, the Japanese yen and other foreign currencies, but there can be no assurance that this positive impact will continue. In the past, our results of operations have been negatively impacted by the appreciation of the U.S. dollar against other currencies.
Fluctuations in worldwide demand for analytical instrumentation could affect our operating results.
The demand for analytical instrumentation products can fluctuate depending upon capital expenditure cycles. Most companies consider our instrumentation products capital equipment and some customers may be unable to secure the necessary capital expenditure approvals due to general economic or customer specific conditions. Significant fluctuations in demand could harm our results of operations.
12
Fluctuations in our quarterly operating results may cause our stock price to decline.
A high proportion of our costs are fixed due in part to our significant sales, research and product development and manufacturing costs. Declines in revenue caused by fluctuations in currency rates, worldwide demand for analytical instrumentation or other factors could disproportionately affect our quarterly operating results, which may in turn cause our stock price to decline.
Our results of operations and financial condition will suffer if we do not introduce new products that are attractive to our customers on a timely basis.
Our products are highly technical in nature. As a result, many of our products must be developed months or even years in advance of the potential need by a customer. If we fail to introduce new products and enhancements as demand arises or in advance of the competition, our products are likely to become obsolete over time, which would harm operating results. Also, if the market is not receptive to our newly developed products, we may be unable to recover costs of research and product development and marketing, and may fail to achieve material components of our business plan.
The analytical instrumentation market is highly competitive, and our inability to compete effectively in this market would adversely affect our results of operations and financial condition.
The analytical instrumentation market is highly competitive and we compete with many companies on a local and international level that are significantly larger than us and have greater resources, including larger sales forces and technical staff. Competitors may introduce more effective and less costly products and, in doing so, may make it difficult for us to acquire and retain customers. If this occurs, our market share may decline and operating results could suffer.
We may experience difficulties with obtaining components from sole- or limited-source suppliers, or manufacturing delays, either of which could adversely affect our results of operations.
Most raw materials, components and supplies that we purchase are available from many suppliers. However, certain items are purchased from sole or limited-source suppliers and a disruption of these sources could adversely affect our ability to ship products as needed. A prolonged inability to obtain certain materials or components would likely reduce product inventory, hinder sales and harm our reputation with customers. Worldwide demand for certain components may cause the cost of such components to rise or limit the availability of these components, which could have an adverse affect our results of operations.
We manufacture products in our facilities in Germany and the United States. Any prolonged disruption to the operations at these facilities, whether due to labor unrest, supplier issues, damage to the physical plants or equipment or other reasons, could also adversely affect our results of operations.
Our executive officers and other key employees are critical to our business, they may not remain with us in the future and finding talented replacements may be difficult.
Our operations require managerial and technical expertise. Each of the executive officers and key employees located in the United States is employed “at will” and may leave our employment at any time. In addition, we operate in a variety of locations around the world where the demand for qualified personnel may be extremely high and is likely to remain so for the foreseeable future. As a result, competition for personnel can be intense and the turnover rate for qualified personnel may be high. The loss of any of our executive officers or key employees could cause us to incur increased operating expenses and divert senior management resources in searching for replacements. An inability to hire, train and retain sufficient numbers of qualified employees would seriously affect our ability to conduct our business.
13
We may be unable to protect our intellectual property rights and may face intellectual property infringement claims.
Our success will depend, in part, on our ability to obtain patents, maintain trade secret protection and operate without infringing the proprietary rights of third parties. We cannot be certain that:
| | |
| | • | any of our pending patent applications or any future patent applications will result in issued patents; |
| |
| | • | the scope of our patent protection will exclude competitors or provide competitive advantages to us; |
| |
| | • | any of our patents will be held valid if subsequently challenged; or |
| |
| | • | others will not claim rights in or ownership of the patents and other proprietary rights held by us. |
Furthermore, we cannot be certain that others have not or will not develop similar products, duplicate any of our products or design around any patents issued, or that may be issued, in the future to us or to our licensors. Whether or not patents are issued to us or to our licensors, others may hold or receive patents which contain claims having a scope that covers products developed by us. We could incur substantial costs in defending any patent infringement suits or in asserting any patent rights, including those granted by third parties. In addition, we may be required to obtain licenses to patents or proprietary rights from third parties. There can be no assurance that such licenses will be available on acceptance terms, if at all.
Our issued U.S. patents, and corresponding foreign patents, expire at various dates ranging from 2008 to 2024. When each of our patents expires, competitors may develop and sell products based on the same or similar technologies as those covered by the expired patent. We have invested in significant new patent applications, and we cannot be certain that any of these applications will result in an issued patent to enhance our intellectual property rights.
EXECUTIVE OFFICERS OF DIONEX CORPORATION
The following table lists the names and positions of all our current executive officers, and their ages as of August 29, 2007. There are no family relationships between any director and executive officer of Dionex Corporation. Executive officers serve at the discretion of the Board of Directors.
| | | | | | | |
Name | | Age | | Position(s) |
| |
| Lukas Braunschweiler | | | 51 | | | President, Chief Executive Officer and Director |
| Bruce Barton | | | 48 | | | Vice President, Sales and Service International |
| David Bow | | | 43 | | | Vice President, North American Sales/Service |
| Kevin Chance | | | 40 | | | Executive Vice President |
| David Fairbanks | | | 47 | | | Vice President, Information Technology |
| Dietrich Hauffe | | | 48 | | | Vice President, Marketing and Business Development |
| Peter Jochum | | | 56 | | | Vice President, Life Sciences Business Unit |
| Craig McCollam | | | 47 | | | Vice President, and Chief Financial Officer |
| John Plohetski | | | 48 | | | Vice President, Chemical Analysis Business Unit |
| Christopher Pohl | | | 56 | | | Vice President, Research and Development and Chief Technology Officer |
Dr. Braunschweiler has served as President and Chief Executive Officer and a member of our Board of Directors since joining us in August 2002. Prior to that time, Dr. Braunschweiler was employed by Mettler-Toledo, a supplier of precision instruments, where he served most recently as Group Vice-President and Head of the Laboratory and Packaging Division. Prior to that, he served in a variety of management positions at Mettler-Toledo. Dr. Braunschweiler has been residing in Switzerland since 2006. Dr. Braunschweiler primarily operates from Switzerland and our European facilities, but also spends a substantial portion of his time in the United States.
Mr. Barton will serve as Vice President of Sales and Service International effective September 1, 2007. Prior to this new role, he served as Vice President, Asia/Pacific and Rest of World since October 2003. Upon joining us in
14
1987, he served in numerous positions in the Sales, Accounting and Finance departments. Previously he held various positions including Head of Distributor Operations as well as Vice President-International Operations.
Mr. Bow has served as Vice President of North American Sales/Service since joining us in September 2003. Prior to that, he founded and served as President of Genesis Chemicals, Buffers & Biochemicals Corp, GW Incorporated and GenChem GmbH.
Mr. Chance has served as Executive Vice President since January 2007. He was the Vice President of our Chemical Analysis Business Unit (CABU) from April 2003 to January 2007. From 2000 to 2003, Mr. Chance served as Chief Executive Officer of Aptus Pharmaceuticals, Inc., a biotech company. From 1989 through 2000, he served as a business unit general manager and in various other positions at Siemens Industrial Automation (Moore Products Company), an automation technology provider.
Mr. Fairbanks has served as Vice President, Information Technology since May 1, 2007. Prior to joining Dionex and since June 2005, he served as Chief Information Officer of Silicon Graphics, Inc., a leading provider of products, services, and solutions for use in high-performance computing and data management. Prior to that, from 1993 to 2005, Mr. Fairbanks served in various senior director roles in Information technology at Silicon Graphics, Inc.
Dr. Hauffe has served as Vice President, Marketing and Business Development since December 2005. Between 2000 and 2005, Mr. Hauffe served as General Manager of our subsidiary, Dionex Idstein, in Germany. From 1993 to 1997, Dr. Hauffe served as our Product Manager Life Sciences.
Dr. Jochum has served as our Vice President, Life Sciences Business Unit since October 2000. Prior to that, he served as Managing Director of our subsidiary, Dionex Softron, since joining us in October 1998. Prior to joining us, he served as Managing Director of Softron GmbH, which we acquired in 1998
Mr. McCollam has served as Vice President and Chief Financial Officer since October 1999. Prior to that, he served as Director of Finance and Corporate Controller since joining us in 1993.
Mr. Plohetski has served as our Vice President, Chemical Analysis Business Unit since December 2006. From March 2003 to November 2006, he served as our Director of Manufacturing. Prior to joining us in 2003, Mr. Plohetski served as Director of Engineering at Carl Zeiss, Inc. from 1982 to 2002.
Mr. Pohl has served as our Vice President, Research and Development and Chief Technology Officer since May 2004. Prior to that, he served as Vice President, Research and Development since June 2001. From March 2000 to June 2001, Mr. Pohl served as Vice President, Research and Development of Ciphergen Biosystems, Inc., a provider of enabling tools for proteomics. From 1981 to 2000, he served as our Vice President, Consumables and in various other capacities.
| |
| Item 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
We own nine buildings in Sunnyvale, California, providing 252,000 square feet of space utilized for administration, marketing, sales, service, research and product development and manufacturing. We also own a 20,000 square feet building utilized for sales, service and administration in Idstein, Germany, a 77,000 square foot building for manufacturing and administration in Germering, Germany and a 32,000 square foot building in Osaka, Japan for sales, service and administration.
We lease sales and service offices in Austria, Australia, Brazil, Canada, China, Denmark, France, Germany, India, Ireland, Italy, Japan, Korea, Netherlands, Switzerland, Taiwan, the United Kingdom and the United States. In addition, we lease marketing and research and product development offices in Salt Lake City, Utah. We also lease marketing and research and product development offices in Amsterdam, the Netherlands. Our facilities are well maintained, adequate to conduct our current business.
15
| |
| Item 3. | LEGAL PROCEEDINGS |
We are a party to various legal proceedings arising in the ordinary course of our business, but are not currently a party to any legal proceeding that management believes will have a material adverse effect on our financial position or results of operations.
| |
| Item 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
Not applicable.
| |
| Item 5. | MARKET FOR OUR COMMON STOCK, RELATED STOCKHOLDER MATTERS AND ISSUER REPURCHASES OF EQUITY SECURITIES |
MARKET PRICE OF COMMON STOCK
Our common stock is traded in the over-the-counter market through the Nasdaq Global Market under the symbol DNEX. The following table sets forth, for the periods indicated, the high and low sales price as reported by the Nasdaq Global Market.
| | | | | | | | | | | | | | | | | |
| | | Fiscal 2007 | | | Fiscal 2006 | |
Quarter | | High | | | Low | | | High | | | Low | |
| |
| First | | $ | 56.58 | | | $ | 45.76 | | | $ | 54.50 | | | $ | 42.90 | |
| Second | | $ | 58.99 | | | $ | 48.67 | | | $ | 54.00 | | | $ | 47.15 | |
| Third | | $ | 68.11 | | | $ | 54.62 | | | $ | 61.48 | | | $ | 48.19 | |
| Fourth | | $ | 74.85 | | | $ | 66.04 | | | $ | 61.25 | | | $ | 50.06 | |
As of August 28, 2007, there were 784 holders of record of our common stock as shown on the records of our transfer agent.
DIVIDENDS
As of August 28, 2007, we have paid no cash dividends on our common stock and we do not anticipate doing so in the foreseeable future.
16
ISSUER PURCHASES OF EQUITY SECURITIES
During the fourth quarter of fiscal 2007, we repurchased shares of our common stock under a systematic program to manage the dilution created by shares issued under employee stock plans and for other purposes. This program authorizes repurchases in the open market or in private transactions. We started a series of repurchase programs in fiscal 1989, with the Board of Directors most recently authorizing in August 2006 future repurchases of an aggregate of 1.5 million shares of common stock as well as authorizing the repurchase of additional shares of common stock equal to the number of common shares issued pursuant to our employee stock plans.
The following table indicates common shares repurchased and additional shares added to the program during the three months ended June 30, 2007:
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Total
| | | | | | | |
| | | | | | | | | Number of
| | | | | | Maximum
| |
| | | | | | | | | Shares
| | | | | | Number of
| |
| | | | | | | | | Purchased
| | | | | | Shares that
| |
| | | Total
| | | Avg.
| | | as Part of
| | | Additional
| | | May Yet be
| |
| | | Number of
| | | Price
| | | Publicly
| | | Shares
| | | Purchased
| |
| | | Shares
| | | Paid per
| | | Announced
| | | Authorized for
| | | Under the
| |
Period | | Purchased | | | Share | | | Program(1) | | | Purchase(1) | | | Program(2) | |
| |
| April 1 - 30, 2007 | | | — | | | | — | | | | 5,812,153 | | | | 156 | | | | 1,323,281 | |
| May 1 - 31, 2007 | | | 224,215 | | | $ | 71.85 | | | | 6,036,368 | | | | 174,551 | | | | 1,273,617 | |
| June 1 - 30, 2007 | | | 58,009 | | | $ | 68.95 | | | | 6,094,377 | | | | 11,562 | | | | 1,227,170 | |
| | |
| (1) | | The number of shares represents the number of shares issued pursuant to employee stock plans that are authorized for purchase. |
| |
| (2) | | The number of shares includes 1.5 million shares of common stock approved for repurchase in August 2006 plus that number of shares of common stock equal to the number of shares issued pursuant to employee stock plans subsequent to August 2006 minus the number of shares purchased since August 2006. |
17
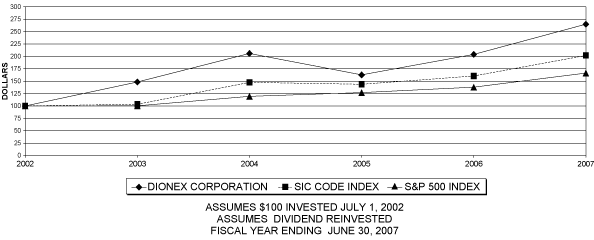
PERFORMANCE GRAPH
The following graph demonstrates a comparison of cumulative total returns for our Common Stock, the SIC Code and the Standard & Poor’s 500 Stock Index, assuming $100 invested as of July 1, 2002:
COMPARE 5-YEAR CUMULATIVE TOTAL RETURN
AMONG DIONEX CORPORATION,
S&P 500 INDEX AND SIC CODE INDEX
COMPARISON OF CUMULATIVE TOTAL RETURN OF ONE OR MORE
COMPANIES, PEER GROUPS, INDUSTRY INDEXES AND/OR BROAD MARKETS
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | FISCAL YEAR ENDING |
| COMPANY/INDEX/MARKET | | | 6/30/2002 | | | 6/30/2003 | | | 6/30/2004 | | | 6/30/2005 | | | 6/30/2006 | | | 6/30/2007 |
| Dionex Corporation | | | | 100.00 | | | | | 148.34 | | | | | 205.94 | | | | | 162.75 | | | | | 204.03 | | | | | 264.99 | |
| Analytical Instruments (SIC) | | | | 100.00 | | | | | 103.43 | | | | | 147.58 | | | | | 143.77 | | | | | 160.46 | | | | | 201.88 | |
| S&P Composite | | | | 100.00 | | | | | 100.25 | | | | | 119.41 | | | | | 126.96 | | | | | 137.92 | | | | | 166.32 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
18
| |
| Item 6. | SELECTED CONSOLIDATED FINANCIAL DATA |
Statement of Operations Information:
| | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended June 30 | |
| | | 2007 | | | 2006 | | | 2005 | | | 2004 | | | 2003 | |
| | | (In thousands, except per share amounts) | |
| |
| Net sales | | $ | 327,284 | | | $ | 291,300 | | | $ | 279,317 | | | $ | 258,834 | | | $ | 214,909 | |
| Cost of sales | | | 109,015 | | | | 99,857 | | | | 91,754 | | | | 88,944 | | | | 73,273 | |
| | | | | | | | | | | | | | | | | | | | | |
| Gross profit | | | 218,269 | | | | 191,443 | | | | 187,563 | | | | 169,890 | | | | 141,636 | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | | |
| Selling, general and administrative | | | 123,525 | | | | 113,241 | | | | 102,539 | | | | 89,100 | | | | 76,565 | |
| Research and product development | | | 24,737 | | | | 22,392 | | | | 20,354 | | | | 19,155 | | | | 16,888 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 148,262 | | | | 135,633 | | | | 122,893 | | | | 108,255 | | | | 93,453 | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating income | | | 70,007 | | | | 55,810 | | | | 64,670 | | | | 61,635 | | | | 48,183 | |
| Interest income | | | 1,435 | | | | 1,874 | | | | 1,276 | | | | 801 | | | | 504 | |
| Interest expense | | | (335 | ) | | | (184 | ) | | | (176 | ) | | | (240 | ) | | | (192 | ) |
| Other income/(expense) | | | 183 | | | | 1,013 | | | | 801 | | | | (340 | ) | | | 133 | |
| Write-off of a non-affiliated investment | | | — | | | | — | | | | — | | | | — | | | | (2,067 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Income before taxes on income | | | 71,290 | | | | 58,513 | | | | 66,571 | | | | 61,856 | | | | 46,561 | |
| Taxes on income | | | 25,968 | | | | 22,820 | | | | 21,081 | | | | 20,481 | | | | 15,133 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income | | $ | 45,322 | | | $ | 35,693 | | | $ | 45,490 | | | $ | 41,375 | | | $ | 31,428 | |
| | | | | | | | | | | | | | | | | | | | | |
| Basic earnings per share | | $ | 2.37 | | | $ | 1.78 | | | $ | 2.20 | | | $ | 1.96 | | | $ | 1.49 | |
| Diluted earnings per share | | $ | 2.31 | | | $ | 1.74 | | | $ | 2.13 | | | $ | 1.89 | | | $ | 1.45 | |
| Shares used in computing earnings per share amounts: | | | | | | | | | | | | | | | | | | | | |
| Basic | | | 19,136 | | | | 20,013 | | | | 20,655 | | | | 21,056 | | | | 21,057 | |
| Diluted | | | 19,615 | | | | 20,527 | | | | 21,388 | | | | 21,943 | | | | 21,632 | |
We have paid no cash dividends.
Balance sheet information:
| | | | | | | | | | | | | | | | | | | | | |
| | | At June 30 | |
| | | 2007 | | | 2006 | | | 2005 | | | 2004 | | | 2003 | |
| | | (In thousands) | |
| |
| Working capital | | $ | 93,361 | | | $ | 97,769 | | | $ | 102,006 | | | $ | 103,719 | | | $ | 88,014 | |
| Total assets | | | 271,769 | | | | 250,402 | | | | 238,153 | | | | 235,465 | | | | 213,100 | |
| Long-term debt | | | — | | | | — | | | | — | | | | — | | | | 500 | |
| Stockholders’ equity | | | 185,708 | | | | 185,382 | | | | 183,049 | | | | 183,454 | | | | 159,280 | |
19
| |
| Item 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Cautionary Statement Regarding Forward-Looking Statements
Except for historical information contained herein, the discussion below and in the footnotes to our financial statements contained in this Report onForm 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, Section 21E of the Exchange Act and the Private Securities Litigation Reform Act of 1995, and are made under the safe harbor provisions thereof. Such statements are subject to certain risks, uncertainties and other factors that may cause actual results, performance or achievements, or industry results, to be materially different from any future results, performance or achievements, or industry results, expressed or implied by such forward-looking statements. Such risks and uncertainties include, among other things: general economic conditions, foreign currency fluctuations, fluctuations in worldwide demand for analytical instrumentation, fluctuations in quarterly operating results, competition from other products, existing product obsolescence, new product development, including market receptiveness, the ability to manufacture products on an efficient and timely basis and at a reasonable cost and in sufficient volume, the ability to attract and retain talented employees and other risks as described in more detail below under the heading “Risk Factors.” Readers are cautioned not to place undue reliance on these forward-looking statements that reflect management’s analysis only as of the date hereof. We undertake no obligation to update these forward-looking statements.
THE COMPANY
Dionex Corporation designs, manufactures, markets and services analytical instrumentation and related accessories and chemicals. Our products are used to analyze chemical substances in the environment and in a broad range of industrial and scientific applications. Our systems are used in environmental analysis and by the pharmaceutical, life sciences, chemical, petrochemical, power generation, and food and electronics industries in a variety of applications. Unless the context otherwise requires, the terms “Dionex,” “we,” “our” and “us” and words of similar import as used in these financial statements include Dionex Corporation and its consolidated subsidiaries.
Our liquid chromatography systems are currently focused in two product areas: ion chromatography (IC) and high performance liquid chromatography (HPLC). We offer a mass spectrometer coupled with either an IC or HPLC system. For sample preparation, we provide automated solvent extraction systems. In addition, we develop and manufacture consumables, detectors, automation and analysis systems for use in or with liquid chromatographs.
We market and distribute our products and services through our own sales force in Austria, Australia, Brazil, Canada, China, Singapore, Denmark, France, Germany, India, Ireland, Italy, Japan, Korea, Netherlands, Switzerland, Taiwan, the United Kingdom and the United States. In each of these countries, we maintain one or more local sales offices in order to support and service our customers in the regions. We manufacture our products based upon a forecast of customer demand and we generally try to maintain adequate inventories of completed modules or finished goods in advance of receipt of firm orders. System or instrument orders are generally placed by the customer on an as-needed basis, and instruments are usually shipped within two to six weeks after receipt of an order.
OVERVIEW
Net sales for fiscal 2007 were $327.3 million, an increase of 12% compared with the $291.3 million reported in fiscal 2006. Operating income for fiscal 2007 was $70.0 million, growing 25% from fiscal 2006 and representing 21% of sales. Fiscal 2006 operating income of $55.8 million declined 14% compared to fiscal 2005 and represented 19% of sales. Diluted earnings per share for fiscal 2007 were $2.31, an increase of 33% compared to $1.74 in fiscal 2006. Net income for fiscal 2007 included costs of approximately $700,000, net of tax, or $0.04 per diluted share, related to our initiative to centralize some of our field-related technical, administrative and support functions in North America and Europe, and a discrete tax benefit from the extension of the federal research credit of approximately $550,000, or $0.03 per diluted share, reported in the second quarter of fiscal 2007. Net income in fiscal 2006 included costs of approximately $1.8 million, net of tax, or $0.09 per diluted share, related to our initiative to centralize some of our field-related technical, administrative and support functions in North America
20
and Europe, higher corporate taxes due to a one-time tax charge of $2.2 million, or $0.11 per diluted share, resulting from the resolution of two tax audits and other income of $1.0 million, net of tax, or $0.05 per diluted share, related to a one-time gain from the favorable settlement of a patent litigation.
Cash flow from operations during fiscal 2007 was strong at $68.5 million. For fiscal 2007, we repurchased 1,185,100 shares of our common stock for $69.6 million.
RESULTS OF OPERATIONS
The following table summarizes our consolidated statement of income items for the last three fiscal years as a percentage of net sales.
| | | | | | | | | | | | | |
| | | Years Ended June 30 | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Net sales | | | 100.0 | % | | | 100.0 | % | | | 100.0 | % |
| Cost of sales | | | 33.3 | | | | 34.3 | | | | 32.9 | |
| | | | | | | | | | | | | |
| Gross profit | | | 66.7 | | | | 65.7 | | | | 67.1 | |
| Operating expenses: | | | | | | | | | | | | |
| Selling, general and administrative | | | 37.7 | | | | 38.9 | | | | 36.7 | |
| Research and product development | | | 7.6 | | | | 7.7 | | | | 7.2 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 45.3 | | | | 46.6 | | | | 43.9 | |
| | | | | | | | | | | | | |
| Operating income | | | 21.4 | | | | 19.1 | | | | 23.2 | |
| Interest income, net | | | 0.3 | | | | 0.6 | | | | 0.4 | |
| Other income, net | | | 0.1 | | | | 0.3 | | | | 0.2 | |
| | | | | | | | | | | | | |
| Income before taxes | | | 21.8 | | | | 20.0 | | | | 23.8 | |
| Taxes on income | | | 7.9 | | | | 7.8 | | | | 7.5 | |
| | | | | | | | | | | | | |
| Net income | | | 13.8 | % | | | 12.2 | % | | | 16.3 | % |
| | | | | | | | | | | | | |
Net Sales. The increase in net sales from fiscal 2005 to fiscal 2006 and from fiscal 2006 to fiscal 2007 was the result of increased sales in all of the major geographic regions in which we do business, as described in greater detail below. We are subject to the effects of foreign currency fluctuations that have an impact on net sales and gross profits. Currency fluctuations increased net sales by 3% in fiscal 2007 and decreased net sales 3% in fiscal 2006. Growth rates for the last two fiscal years are indicated in the tables below:
Percentage increase in net sales:
| | | | | | | | | |
| | | From Fiscal 2006
| | | From Fiscal 2005
| |
| | | to
| | | to
| |
| | | Fiscal 2007 | | | Fiscal 2006 | |
| |
| Total: | | | 12 | % | | | 4 | % |
| By geographic region: | | | | | | | | |
| North America | | | 5 | % | | | 4 | % |
| Europe | | | 17 | % | | | 3 | % |
| Asia/Pacific | | | 14 | % | | | 6 | % |
21
Percentage change in net sales excluding currency fluctuations:
| | | | | | | | | |
| | | From Fiscal 2006
| | | From Fiscal 2005
| |
| | | to
| | | to
| |
| | | Fiscal 2007 | | | Fiscal 2006 | |
| |
| Total: | | | 9 | % | | | 7 | % |
| By geographic region: | | | | | | | | |
| North America | | | 5 | % | | | 4 | % |
| Europe | | | 9 | % | | | 8 | % |
| Asia/Pacific | | | 15 | % | | | 9 | % |
Net sales in North America grew 5% from fiscal 2006 to fiscal 2007 driven by growth in most markets, including an improvement in the second half of the fiscal year in the life sciences segment and very strong growth in sales of both RFIC instrumentation and consumables. Net sales in Europe grew 17% in reported dollars (9% net of currency fluctuations) from fiscal 2006 to fiscal 2007 led by growth in the life sciences and environmental markets and strong HPLC product sales. Net sales in the Asia/Pacific region grew 14% in reported dollars (15% net of currency fluctuation) driven by continued expansion in China, Korea, India and Australia and growth in the chemical, environmental and food safety markets.
Net sales in North America grew 4% in reported dollars from fiscal 2005 to fiscal 2006 reflecting an improvement in economic conditions throughout the fiscal year. Net sales in Europe grew 3% in reported dollars (8% net of currency fluctuations) from fiscal 2005 to fiscal 2006 as a result of a rebound in sales to the life sciences and environmental markets, partially offset by a weaker demand from our larger pharmaceutical and industrial customers in the first half of fiscal 2006. Net sales in the Asia/Pacific region grew 6% in reported dollars (9% net of currency fluctuation) in spite of the continued challenging environment in Japan in the first three quarters of fiscal 2006. The increases are primarily fueled by strong sales growth in China, Korea, Australia and India. For fiscal 2006, sales in Japan were down over 10% compared to fiscal 2005.
Sales outside North America accounted for 71% of net sales in fiscal 2007, 69% in fiscal 2006 and 69% in fiscal 2005. We sell directly through our sales forces in Austria, Australia, Brazil, Canada, China, Denmark, France, Germany, India, Ireland, Italy, Japan, Korea, Netherlands, Switzerland, Taiwan, the United Kingdom and the United States. Direct sales accounted for 94% of net sales in fiscal 2007, compared with 90% in fiscal 2006 and 91% in fiscal 2005. International distributors and representatives in Europe, Asia and other international markets accounted for the balance of net sales. There was no significant price changes during the three-year period ended June 30, 2007.
Net sales of IC products grew 14% from fiscal 2006 to fiscal 2007 driven mostly by increases in sales of RFIC instrumentation, consumables and services across all major geographies. Net sales of IC products grew 3% from fiscal 2005 to fiscal 2006 due partially to weaker demand in Japan offset by increased sales surrounding the introduction of our ICS-3000 Premiere IC system.
Net sales of HPLC and Nano/capillary-flow products grew by 9% from fiscal 2006 to fiscal 2007 driven primarily by increased sales in Europe, partially offset by weaker demand in North America in the first half of fiscal 2007. Sales of HPLC and Nano/capillary-flow products grew 17% from fiscal 2005 to fiscal 2006 primarily as a result of our continued expansion of our global HPLC business, offset by the negative impact of a stronger U.S. dollar, some weakness in certain larger pharmaceutical accounts and the product transition from our older Summit HPLC product line to the new Ultimate 3000 analytical/micro/prep flow system.
Gross Profit. Gross profit for fiscal 2007 was $218.3 million compared to $191.4 million in fiscal 2006, an increase of $26.9 million or 14%. Gross profit as a percentage of sales was 66.7%, 65.7% and 67.2% in fiscal 2007, 2006 and 2005, respectively. Gross profit as a percentage of sales for fiscal 2007 increased primarily due to product mix, currency fluctuations and product cost cutting initiatives related to our newer ICS-3000 and Ultimate-3000 product lines. The decrease in gross margin as a percentage of sales from fiscal 2005 to fiscal 2006 was mainly due to the effect of a stronger U.S. dollar, product mix, higher manufacturing andramp-up costs related to new product introductions, lower margin as a result of a one-time sales promotion for Summit systems and compensation cost related to the impact of SFAS 123R. We anticipate gross margin will be approximately 66% for fiscal year 2008.
22
Operating Expenses — Fiscal 2006 to fiscal 2007. From fiscal 2006 to fiscal 2007, overall operating expenses increased by 9% to $148.2 million. Increased expenses in fiscal 2007 were primarily attributable to the following factors:
| | | | | |
| • currency fluctuations | | | 3 | % |
| • continued expansion efforts in the Asia/Pacific region | | | 2 | % |
| • increased depreciation and infrastructure expense | | | 2 | % |
| • increased sales and marketing efforts in Europe | | | 1 | % |
Selling, general and administrative (SG&A) expenses as a percentage of net sales were 38% in fiscal 2007 compared with 39% in fiscal 2006. SG&A expenses were $123.5 million in fiscal 2007, an increase of 9% from $113.2 million in fiscal 2006. Increases in fiscal 2007 over 2006 include $3.3 million due to currency fluctuations, $3.0 million due to our continued expansion into the Asia/Pacific geography, $2.5 million due to increased expense for depreciation and distributed costs attributable to capital investments and facilities and IT infrastructure costs, $2.0 million due to increased selling and marketing efforts in Europe. The increase was partially offset by lower expenses related to our centralization initiative in North America and Europe of approximately $785,000.
Research and product development expenses were 7.6% of net sales in fiscal 2007 compared with 7.7% in fiscal 2006. Research and product development expenses in fiscal 2007 were $24.7 million, an increase of $2.3 million compared with $22.4 million in fiscal 2006. Research and product development expenses increased by approximately $1.1 million due to higher product development costs, $510,000 for increased salary expense and $447,000 increase due to currency fluctuations.
Operating Expenses — Fiscal 2005 to fiscal 2006. From fiscal 2005 to fiscal 2006, overall operating expenses grew by 10%. Increased expenses in fiscal 2006 was primarily attributable to the following factors:
| | | | | |
| • stock-based compensation resulting from adoption of SFAS 123(R) | | | 4 | % |
| • expansion efforts in the Asia/Pacific region | | | 3 | % |
| • centralization initiative in North America and Europe | | | 2 | % |
| • increased sales and marketing efforts in Europe | | | 2 | % |
| • increased marketing costs related to new product introductions | | | 1 | % |
| • currency fluctuations | | | (2 | )% |
Selling, general and administrative (SG&A) expenses as a percentage of net sales were 39% in fiscal 2006 compared with 37% in fiscal 2005. SG&A expenses were $113.2 million in fiscal 2006, an increase of 10% from $102.5 million in fiscal 2005. Increases over 2005 include $3.8 million stock-based compensation charges, $2.2 million for our centralization initiative in North America and Europe, $3.5 million due to our expansion efforts in Asia/Pacific region in connection with the greater sales and marketing effort in Europe and $1.0 million in marketing costs associated with new product introductions. The increase was offset in part by $2.0 million in currency fluctuations.
Research and product development expenses were 7.7% of net sales in fiscal 2006 compared with 7.3% in fiscal 2005. Research and product development expenses in fiscal 2006 of $22.4 million increased by $2.0 million compared with $20.4 million in fiscal 2005. Research and product development expenses have increased due to $1.4 million of stock-based compensation expense, and approximately $0.5 million due to higher spending to develop our IC and HPLC products introduced in fiscal 2006.
Interest Income. Interest income in fiscal 2007 of $1.4 million was lower than the $1.8 million reported for fiscal 2006 and higher than the $1.3 million reported in fiscal 2005. The decrease in fiscal 2007 from fiscal 2006 was due to lower yields on cash reserves held outside of the U.S., which reserves grew from fiscal 2006 to fiscal 2007. The increase from fiscal 2005 to fiscal 2006 was due to higher average cash balances and increasing interest rates in the United States and Europe.
Interest Expense. Interest expense of $0.3 million remained constant in fiscal 2007, 2006 and 2005. The interest expense was primarily due to short-term borrowings.
23
Other Income, Net. Other income in fiscal 2007 was $0.2 million, which was primarily due to the gains on foreign currency exchange. We had other income in fiscal 2006 of $1.0 million, which was attributable primarily to settlement of a patent litigation partially offset by losses on certain foreign currency contracts.
Taxes on Income. Our effective tax rate was 36.4% for fiscal 2007, 39.0% for fiscal 2006 and 31.7% for fiscal 2005. Our effective tax rate is affected by the mix of taxable income among the various tax jurisdictions in which we do business. The decrease in the effective tax rate from fiscal 2006 to fiscal 2007 was primarily due to special tax charges incurred in fiscal 2006 resulting from the resolution of two tax audits.
Earnings per Share. Diluted earnings per share were $2.31, an increase of 33% compared with the $1.74 reported for fiscal 2006. Net income for fiscal 2007 included costs of approximately $700,000, net of tax, or $0.04 per share, related to the Company’s initiative to centralize some of its field related technical, administrative and support functions within North America and Europe, and a discrete tax benefit from the extension of the federal research credit of approximately $550,000 or $0.03 per share. Net income for fiscal 2006 included costs of approximately $1.8 million, net of tax, or $0.09 per share, related to the company’s initiative to centralize some of its field related technical, administrative and support functions in North America and Europe, higher corporate taxes due to a one-time tax charge of $2.2 million or $0.11 per share resulting from a tax audit, and other income of $1.0 million, net of tax, or $0.05 per share, related primarily to a one-time gain from the favorable settlement of patent litigation. Earnings per share decreased between fiscal 2005 and fiscal 2006 due to higher costs in connection with our centralization in North America (4%), special tax charges related to two unfavorable tax audits in Europe (5%), and due to stock-based compensation expense (9%) in fiscal 2006.
Liquidity and Capital Resources. At June 30, 2007, we had cash and equivalents and short-term investments of $55.0 million. Our working capital was $93.4 million at June 30, 2007, compared with $97.8 million at June 30, 2006.
Cash generated by operating activities was $68.5 million in fiscal 2007, compared with $51.0 million in fiscal 2006 and $57.2 million in fiscal 2005. The increase in operating cash flows was due to better operating results, increases in deferred revenue, increase in accrued liabilities due to higher accrued payroll costs and higher income taxes payable resulting from lower tax payments, partially offset by higher prepaid expenses. The decrease in operating cash flows in fiscal 2006 as compared to fiscal 2005 was primarily due to decreases in net income, an increase in accounts receivable due to slower cash collections from customers and exclusion of tax benefits related to stock option exercises that are now reported under cash flows from financing activities as required by SFAS 123R. The decrease was partially offset by increases in accrued liabilities due to higher deferred revenue and income tax payable due to lower tax payments.
Cash used for investing activities was $4.0 million in fiscal 2007 compared with $8.7 million in fiscal 2006 and $10.9 million in fiscal 2005. The decreases from fiscal 2005 to fiscal 2006 and from fiscal 2006 to fiscal 2007 were due to lower net proceeds from sale of marketable securities in fiscal 2006 and fiscal 2007, partially offset by higher capital expenditures due to expansion of our HPLC manufacturing and development facility in Germany and the acquisition of the polymer based monolithic technology from Teledyne-Isco, a subsidiary of Teledyne Technologies, Inc. Our estimate is that capital expenditures will be approximately $10.0 million in fiscal 2008.
Cash used for financing activities was $52.4 million in fiscal 2007, compared with $42.3 million in fiscal 2006 and $50.0 million in fiscal 2005. Financing activities for all three years consisted primarily of common stock repurchases, partially offset by proceeds from issuances of shares pursuant to and the tax benefits related to stock option plans in fiscal 2007. We repurchased 1,185,100 shares of our common stock for $69.6 million in fiscal 2007 under our repurchase program. We repurchased 1,409,577 shares for $73.9 million in fiscal 2006 and 1,355,900 shares for $66.7 million in fiscal 2005. We have 1,227,170 remaining shares authorized for repurchase under our repurchase programs at June 30, 2007.
At June 30, 2007, our available bank lines of credit totaled $28.5 million, compared with $31.7 million at June 30, 2006. We believe our cash flow from operations, our existing cash and cash equivalents and our bank lines of credit will be adequate to meet our cash requirements for at least the next 12 months. The impact of inflation on our financial position and results of operations was not significant during any of the periods presented.
24
CONTRACTUAL OBLIGATIONS
The following summarizes our contractual obligations at June 30, 2007, and the effect such obligations are expected to have on our liquidity and cash flows in future periods (in thousands):
| | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period |
| | | | | Less Than
| | 1-3
| | 4-5
| | After 5
|
| | | Total | | 1 Year | | Year | | Years | | Years |
| |
| Operating Lease Obligations | | $ | 17,750 | | | $ | 4,864 | | | $ | 6,546 | | | $ | 3,465 | | | $ | 2,875 | |
| | | | | | | | | | | | | | | | | | | | | |
EFFECT OF NEW ACCOUNTING STANDARDS
In February 2007, the Financial Accounting Standards Board (“FASB”) issued Statement of Financial Accounting Standards No. 159,The Fair Value Option for Financial Assets and Financial Liabilities(“SFAS 159”). SFAS 159 permits entities to choose, at specified election dates, to measure eligible items at fair value (or “fair value option”) and to report in earnings unrealized gains and losses on those items for which the fair value option has been elected. SFAS 159 also requires entities to display the fair value of those assets and liabilities on the face of the balance sheet. SFAS 159 establishes presentation and disclosure requirements designed to facilitate comparisons between entities that choose different measurement attributes for similar types of assets and liabilities. SFAS 159 is effective for us as of the first quarter of fiscal 2009. We are currently evaluating the impact of this pronouncement on our financial statements.
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements(“SFAS No. 157”). SFAS No. 157 establishes a framework for measuring fair value and expands disclosures about fair value measurements. The changes to current practice resulting from the application of SFAS No. 157 relate to the definition of fair value, the methods used to measure fair value, and the expanded disclosures about fair value measurements. SFAS No. 157 is effective for fiscal years beginning after November 15, 2007 and interim periods within those fiscal years. We do not believe that the adoption of the provisions of SFAS No. 157 will materially impact our financial position and results of operations.
In June 2006, the FASB issued Interpretation No. (“FIN”) 48,Accounting for Uncertainty in Income Taxes, which is effective for fiscal years beginning after December 15, 2006. FIN 48 clarifies the accounting for uncertainty in income taxes by prescribing rules for recognition, measurement, classification and disclosure in our financial statements of tax positions taken or expected to be taken in a tax return. We are currently evaluating the provisions in FIN 48 and have not yet determined its expected impact. We plan to adopt this new standard effective July 1, 2007.
In February 2006, the FASB issued Statement of Financial Accounting Standards (“SFAS”) No. 155,Accounting for Certain Hybrid Financial Instruments — an amendment of FASB Statements No. 133 and 140. This standard permits fair value re-measurement for any hybrid financial instrument that contains an embedded derivative that otherwise would require bifurcation; clarifies which interest-only strips and principal-only strips are not subject to the requirements of SFAS No. 133; requires evaluation of interests in securitized financial assets; clarifies that concentrations of credit risk in the form of subordination are not embedded derivatives; and eliminates the prohibition of a qualifying special-purpose entity from holding a derivative financial instrument that pertains to a beneficial interest of another derivative financial instrument. This standard is effective for all financial instruments acquired or issued after fiscal years beginning after September 15, 2006. The adoption of SFAS No. 155 did not have a material effect on our financial position, results of operations or cash flows.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Summary. The preparation of consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of net sales and expenses during the reporting period. We evaluate our estimates, including those related to product returns and allowances, bad debts, inventory valuation, goodwill and other intangible assets, income taxes, warranty and installation provisions, and contingencies, on an on-going basis.
25
We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates. We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition Policy. We derive revenues from the sale of products and the delivery of services to our customers, including installation, training, time and material repairs, and maintenance, which consists of product repair obligations, telephone support, and/or unspecified software upgrades.
We recognize revenue in accordance with Staff Accounting Bulletin No. 104,Revenue Recognition, and Statement of Position97-2,Software Revenue Recognition, as amended, when persuasive evidence of an arrangement exists, the product has been delivered, or service performed, the price is fixed or determinable, collection is probable and vendor specific objective evidence or objective reliable evidence of fair value, as applicable, exists to allocate revenue to the various elements of the arrangement. In all cases, the portion of revenue allocated to the undelivered elements is deferred until those items are delivered to the customer or the services are performed. Delivery of the product is generally considered to have occurred when shipped. Undelivered elements in our sales arrangements, which are not considered to be essential to the functionality of a product, generally include maintenance, installation servicesand/or training that are delivered after the related products have been delivered. Installation consists of systemset-up, calibration and basic functionality training and generally requires one to three days depending on the product. Fair value for training services is based on the price charged when the element is sold separately or, if not sold separately, when the price is established by authorized management. The fair value of installation services is calculated by applying standard service billing rates to the estimate of the number of hours to install a specific product based on historical experience. These estimated hours for installation have historically been accurate and consistent from product to product. However, to the extent these estimates were to reflect unfavorable variability, our ability to maintain objective reliable evidence of fair value for such element could be impacted which in turn could delay the recognition of the revenue currently allocated to the delivered elements.
Fair value of the maintenance contracts is based on the price charged when an element is sold separately or, if not yet sold separately, when the price is established by authorized management. Maintenance fees are recognized ratably over the period of the related maintenance contracts, which range from one to two years. Maintenance consists of product repair obligations, telephone support, and/or unspecified software upgrades.
Our sales are typically not subject to rights of return and, historically, actual sales returns have not been significant. The ability to maintain fair value for undelivered elements and other judgments may affect our reported revenues.
Product Warranty. Our equipment typically includes a one-year warranty. The estimated cost of product warranty claims is accrued at the time the sale is recognized, based on historical experience. While we believe our historical experience provides a reliable basis for estimating such warranty cost, unforeseen quality issues or component failure rates could result in future costs in excess of such estimates, or alternatively, improved quality and reliability in our products could result in actual expenses that are below those currently estimated.
Loss Provisions on Accounts Receivable and Inventory. We maintain allowances for doubtful accounts for estimated losses resulting from the inability of customers to make required payments. If the financial condition of any of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required. We assess collectibility based on a number of factors including, but not limited to, past transaction history with the customer, the credit-worthiness of the customer, independent credit reports, industry trends and the macro-economic environment. Sales returns and allowances are estimates of future product returns related to current period revenue. Material differences may result in the amount and timing of our revenue for any period. Historically, we have not experienced significant sales returns or bad debt losses.
We value our inventory at the lower of standard cost (which approximates cost on afirst-in, first-out basis) or market. Our estimated valuation provisions on inventory are based on technical obsolescence, historical demand,
26
projections of future demand and industry and market conditions. If actual future demand or market conditions are less favorable than those projected by management, additional valuation provisions may be required. If demand or market conditions are more favorable than projected, higher margins could be realized to the extent inventory is sold which had previously been written down.
Long-Lived Assets, Intangible Assets with Finite Lives and Goodwill. We assess for the impairment of long-lived assets, intangible assets with finite lives and goodwill whenever events or changes in circumstances indicate that the carrying value may not be recoverable. In addition, we assess goodwill for impairment at least annually. Factors we consider important which could trigger an impairment review include but are not limited to the following:
• significant underperformance relative to historical or projected future operating results;
• significant negative industry or economic trends; and
• significant changes or developments in strategic technology.
When we determine that the carrying value of long-lived assets and intangible assets with finite lives may not be recoverable based upon the existence of one or more of the above or other indicators, we measure any impairment based on a projected discounted cash flow method using a discount rate determined by our management to be commensurate with the risk inherent in our current business model. Goodwill is tested for impairment by comparing the fair values of related reporting units to their carrying values. We are required to perform an impairment review for goodwill at least annually.
Taxes on Income. As part of the process of preparing our consolidated financial statements, we are required to estimate our income taxes in each of the jurisdictions in which we operate. This process involves estimating our actual current tax exposure together with assessing temporary differences resulting from differing treatment of items, such as depreciation, amortization and inventory reserves, for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheets. We must then assess the likelihood that our deferred tax assets will be recovered from future taxable income and, to the extent management believes that recovery is more likely than not, a valuation allowance must be established. In the event that actual results differ from these estimates, we may need to revise the valuation allowance, which could materially impact our financial position and results of operations.
Stock-Based Compensation. SFAS No. 123 (Revised 2004),Share-Based Payment(SFAS No. 123R) requires that all share-based payments to employees be recognized in the statements of operations based on their fair value. We have used the Black-Scholes model to determine the fair value of our stock option awards. Under the fair value recognition provisions of this statement, share-based compensation cost is measured at the grant date based on the value of the award and is recognized as expense over the vesting periods. Determining the fair value of share-based awards at the grant date required judgment, including estimating stock price volatility and employee stock options exercise behaviors. If actual results differ significantly from these estimates, stock-based compensation expense recognized in our results of operations could be materially affected. As stock-based compensation expense recognized in the consolidated statements of operations is based on awards that ultimately are expected to vest, the amount of the expense has been reduced for estimated forfeitures. SFAS No. 123R requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures were estimated based on historical experience. If factors change and we employ different assumptions in the application of SFAS No. 123R, the compensation expense that we record in the future periods may differ significantly from what we have recorded in the current period.
| |
| Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are exposed to financial market risks from fluctuations in foreign currency exchange rates, interest rates and stock prices of marketable securities. With the exception of the stock price volatility of our marketable equity securities, we manage our exposure to these and other risks through our regular operating and financing activities and, when appropriate, through our hedging activities. Our policy is not to use hedges or other derivative financial instruments for speculative purposes. We deal with a diversified group of major financial institutions to limit the risk of nonperformance by any one institution on any financial instrument. Separate from our financial hedging
27
activities, material changes in foreign exchange rates, interest rates and, to a lesser extent, commodity prices could cause significant changes in the costs to manufacture and deliver our products and in customers’ buying practices. We have not substantially changed our risk management practices during fiscal 2007 and we do not currently anticipate significant changes in financial market risk exposures in the near future that would require us to change our current risk management practices.
Foreign Currency Exchange. Revenues generated from international operations are generally denominated in foreign currencies. We entered into forward foreign exchange contracts to hedge against fluctuations of intercompany account balances. Market value gains and losses on these hedge contracts are substantially offset by fluctuations in the underlying balances being hedged, and the net financial impact is not expected to be material in future periods. At June 30, 2007, we had forward exchange contracts to sell foreign currencies totaling $10.5 million, including approximately $4.5 million in Euros, $3.5 million in Japanese yen, $1.2 million in Australian dollars and $1.3 million in Canadian dollars. At June 30, 2006, we had forward exchange contracts to sell foreign currencies totaling $14.8 million, including approximately $9.0 million in Euros, $4.9 million in Japanese yen, $592,000 in Australian dollars and $305,000 in Canadian dollars. The foreign exchange contracts at the end of each fiscal year mature within the first quarter of the following fiscal year. Additionally, contract values and fair market values are the same.
In March 2007, we entered into a cross-currency swap of Japanese Yen for $10.0 million which matures in March 2010. This derivate instrument did not qualify for net investment hedge accounting and was deemed to be an ineffective hedge instrument, given that, at the inception of the hedge transaction, there was no formal documentation of the hedging relationship and our risk management objective and strategy for undertaking the hedge. Therefore, we marked to market the increase in value of approximately $462,000 and this amount was recorded in Other income, net. A sensitivity analysis assuming a hypothetical 10% movement in foreign currency exchange rates applied to our hedging contracts and underlying balances being hedged at June 30, 2007 and 2006 indicated that these market movements would not have a material effect on the our business, operating results or financial condition.
Foreign currency rate fluctuations can impact the U.S. dollar translation of our foreign operations in our consolidated financial statements. Currency fluctuations increased sales by 3% in fiscal 2007 and decreased sales by 3% in fiscal 2006. Currency fluctuations increased sales by 4% in fiscal 2005.
Interest and Investment Income. Our interest and investment income is subject to changes in the general level of U.S. interest rates. Changes in U.S. interest rates affect the interest earned on our cash equivalents and short-term investments. A sensitivity analysis assuming a hypothetical 10% movement in interest rates applied to our investment balances at June 30, 2007 and 2006 indicated that such market movement would not have a material effect on our business, operating results or financial condition. Actual gains or losses in the future may differ materially from this analysis, depending on our actual balances and changes in the timing and amount of interest rate movements.
Debt and Interest Expense. At June 30, 2007, we had notes payable of $231,000. A sensitivity analysis assuming a hypothetical 10% movement in interest rates applied to our outstanding debt balance at June 30, 2007, indicated that such market movement would not have a material effect on our business, operating results or financial condition. Actual gains or losses in the future may differ materially from this analysis, depending on changes in the timing and amount of interest rate movements and the level of borrowings maintained by us.
28
| |
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
| | | | | |
| | | Page |
| |
| | | | | |
FINANCIAL STATEMENTS | | | | |
| | | 30 | |
| | | 31 | |
| | | 32 | |
| | | 33 | |
| | | 34 | |
| | | 35 | |
29
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Dionex Corporation:
We have audited the consolidated balance sheets of Dionex Corporation and its subsidiaries (collectively, the “Company”) as of June 30, 2007 and 2006, and the related consolidated statements of income, stockholders’ equity and comprehensive income, and cash flows for each of the three years in the period ended June 30, 2007. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of the Company at June 30, 2007 and 2006, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2007, in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 1 to the consolidated financial statements, in fiscal year 2006, the Company changed its method of accounting for stock-based compensation in accordance with guidance provided in Statement of Financial Accounting Standards No. 123 (revised 2004),Share-Based Payment.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of the Company’s internal control over financial reporting as of June 30, 2007, based on the criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated August 29, 2007, expressed an unqualified opinion on management’s assessment of the effectiveness of the Company’s internal control over financial reporting and an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
/s/ Deloitte & Touche LLP
San Jose, California
August 29, 2007
30
CONSOLIDATED BALANCE SHEETS
AT JUNE 30
| | | | | | | | | |
| | | 2007 | | | 2006 | |
| | | (In thousands, except per share amounts) | |
| |
| ASSETS |
| Current assets: | | | | | | | | |
| Cash and equivalents | | $ | 54,938 | | | $ | 43,524 | |
| Short-term investments | | | 124 | | | | 7,490 | |
| Accounts receivable (net of allowance for doubtful accounts of $610 in 2007 and $674 in 2006) | | | 65,990 | | | | 63,008 | |
| Inventories | | | 28,626 | | | | 27,702 | |
| Deferred income taxes | | | 8,983 | | | | 9,915 | |
| Prepaid expenses and other | | | 12,113 | | | | 5,791 | |
| | | | | | | | | |
| Total current assets | | | 170,774 | | | | 157,430 | |
| Property, plant and equipment, net | | | 62,366 | | | | 58,700 | |
| Goodwill | | | 25,443 | | | | 24,982 | |
| Intangible assets, net | | | 6,955 | | | | 4,522 | |
| Other assets | | | 6,231 | | | | 4,768 | |
| | | | | | | | | |
| | | $ | 271,769 | | | $ | 250,402 | |
| | | | | | | | | |
| |
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
| Current liabilities: | | | | | | | | |
| Notes payable | | $ | 231 | | | $ | — | |
| Accounts payable | | | 12,293 | | | | 9,395 | |
| Accrued liabilities | | | 30,329 | | | | 24,377 | |
| Deferred revenues | | | 18,617 | | | | 15,296 | |
| Income taxes payable | | | 13,068 | | | | 7,100 | |
| Accrued product warranty | | | 2,875 | | | | 3,493 | |
| | | | | | | | | |
| Total current liabilities | | | 77,413 | | | | 59,661 | |
| Deferred income taxes | | | 5,060 | | | | 3,952 | |
| Other long-term liabilities and deferred revenues | | | 3,588 | | | | 1,407 | |
| Commitments and other contingencies (Note 13) | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Preferred stock (par value $.001 per share; 1,000,000 shares authorized; none outstanding) | | | — | | | | — | |
| Common stock (par value $.001 per share; 80,000,000 shares authorized; shares outstanding: 18,845,802 in 2007 and 19,624,839 in 2006) | | | 161,409 | | | | 148,214 | |
| Retained earnings | | | 13,223 | | | | 28,589 | |
| Accumulated other comprehensive income | | | 11,076 | | | | 8,579 | |
| | | | | | | | | |
| Total stockholders’ equity | | | 185,708 | | | | 185,382 | |
| | | | | | | | | |
| | | $ | 271,769 | | | $ | 250,402 | |
| | | | | | | | | |
See notes to consolidated financial statements.
31
CONSOLIDATED STATEMENTS OF INCOME
YEARS ENDED JUNE 30
| | | | | | | | | | | | | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | (In thousands, except per share amounts) | |
| |
| Net sales | | $ | 327,284 | | | $ | 291,300 | | | $ | 279,317 | |
| Cost of sales | | | 109,015 | | | | 99,857 | | | | 91,754 | |
| | | | | | | | | | | | | |
| Gross profit | | | 218,269 | | | | 191,443 | | | | 187,563 | |
| Operating expenses: | | | | | | | | | | | | |
| Selling, general and administrative | | | 123,525 | | | | 113,241 | | | | 102,539 | |
| Research and product development | | | 24,737 | | | | 22,392 | | | | 20,354 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 148,262 | | | | 135,633 | | | | 122,893 | |
| | | | | | | | | | | | | |
| Operating income | | | 70,007 | | | | 55,810 | | | | 64,670 | |
| Interest income | | | 1,435 | | | | 1,874 | | | | 1,276 | |
| Interest expense | | | (335 | ) | | | (184 | ) | | | (176 | ) |
| Other income, net | | | 183 | | | | 1,013 | | | | 801 | |
| | | | | | | | | | | | | |
| Income before taxes on income | | | 71,290 | | | | 58,513 | | | | 66,571 | |
| Taxes on income | | | 25,968 | | | | 22,820 | | | | 21,081 | |
| | | | | | | | | | | | | |
| Net income | | $ | 45,322 | | | $ | 35,693 | | | $ | 45,490 | |
| | | | | | | | | | | | | |
| Basic earnings per share: | | $ | 2.37 | | | $ | 1.78 | | | $ | 2.20 | |
| | | | | | | | | | | | | |
| Diluted earnings per share: | | $ | 2.31 | | | $ | 1.74 | | | $ | 2.13 | |
| | | | | | | | | | | | | |
| Shares used in computing earnings per share: | | | | | | | | | | | | |
| Basic | | | 19,136 | | | | 20,013 | | | | 20,655 | |
| Diluted | | | 19,615 | | | | 20,527 | | | | 21,388 | |
See notes to consolidated financial statements.
32
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY AND COMPREHENSIVE INCOME
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Accumulated
| | | | | | | |
| | | | | | | | | | | | Other
| | | | | | | |
| | | Common Stock | | | Retained
| | | Comprehensive
| | | | | | Comprehensive
| |
| | | Shares | | | Amount | | | Earnings | | | Income(Loss) | | | Total | | | Income | |
| | | (Dollars in thousands) | |
| |
| Balance, June 30, 2004 | | | 20,840,881 | | | $ | 103,943 | | | $ | 71,217 | | | $ | 8,294 | | | $ | 183,454 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income, net of tax: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | | | | | | | | | 45,490 | | | | | | | | 45,490 | | | $ | 45,490 | |
| Foreign currency translation adjustments (net of tax of $1,392) | | | | | | | | | | | | | | | (2,434 | ) | | | (2,434 | ) | | | (2,434 | ) |
| Unrealized loss on securities (net of tax of $365) | | | | | | | | | | | | | | | (621 | ) | | | (621 | ) | | | (621 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | $ | 42,435 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Common stock issued under employee stock-based compensation plans | | | 676,111 | | | | 18,168 | | | | | | | | | | | | 18,168 | | | | | |
| Repurchase of common stock | | | (1,355,900 | ) | | | (7,407 | ) | | | (59,256 | ) | | | | | | | (66,663 | ) | | | | |
| Tax benefit from employee stock transactions | | | | | | | 5,655 | | | | | | | | | | | | 5,655 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, June 30, 2005 | | | 20,161,092 | | | | 120,359 | | | | 57,451 | | | | 5,239 | | | | 183,049 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income, net of tax: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | | | | | | | | | 35,693 | | | | | | | | 35,693 | | | $ | 35,693 | |
| Foreign currency translation adjustments (net of tax of $233) | | | | | | | | | | | | | | | 3,319 | | | | 3,319 | | | | 3,319 | |
| Unrealized gain on securities (net of tax of $0) | | | | | | | | | | | | | | | 21 | | | | 21 | | | | 21 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | $ | 39,033 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Common stock issued under employee stock-based compensation plans | | | 873,324 | | | | 25,438 | | | | | | | | | | | | 25,438 | | | | | |
| Repurchase of common stock | | | (1,409,577 | ) | | | (9,328 | ) | | | (64,555 | ) | | | | | | | (73,883 | ) | | | | |
| Stock-based compensation expense | | | | | | | 5,610 | | | | | | | | | | | | 5,610 | | | | | |
| Tax benefit from employee stock transactions | | | | | | | 6,135 | | | | | | | | | | | | 6,135 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, June 30, 2006 | | | 19,624,839 | | | | 148,214 | | | | 28,589 | | | | 8,579 | | | | 185,382 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income, net of tax: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | | | | | | | | | 45,322 | | | | | | | | 45,322 | | | $ | 45,322 | |
| Foreign currency translation adjustments (net of tax of $1,582) | | | | | | | | | | | | | | | 2,470 | | | | 2,470 | | | | 2,470 | |
| Unrealized gain on securities (net of tax of $0) | | | | | | | | | | | | | | | 27 | | | | 27 | | | | 27 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | $ | 47,819 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Common stock issued under employee stock-based compensation plans | | | 406,063 | | | | 13,517 | | | | | | | | | | | | 13,517 | | | | | |
| Repurchase of common stock | | | (1,185,100 | ) | | | (8,904 | ) | | | (60,688 | ) | | | | | | | (69,592 | ) | | | | |
| Stock-based compensation expense | | | | | | | 5,125 | | | | | | | | | | | | 5,125 | | | | | |
| Tax benefit from employee stock transactions | | | | | | | 3,457 | | | | | | | | | | | | 3,457 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, June 30, 2007 | | | 18,845,802 | | | $ | 161,409 | | | $ | 13,223 | | | $ | 11,076 | | | $ | 185,708 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
See notes to consolidated financial statements.
33
CONSOLIDATED STATEMENTS OF CASH FLOWS
YEARS ENDED JUNE 30
| | | | | | | | | | | | | | | | | |
| | | 2007 | | | 2006 | | | 2005 | | | | |
| | | (In thousands) | | | | |
| |
| Cash flows from operating activities: | | | | | | | | | | | | | | | | |
| Net income | | $ | 45,322 | | | $ | 35,693 | | | $ | 45,490 | | | | | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | | | | | |
| Depreciation and amortization | | | 8,544 | | | | 6,514 | | | | 6,007 | | | | | |
| Stock-based compensation | | | 5,125 | | | | 5,572 | | | | — | | | | | |
| Gain on sale of marketable securities | | | — | | | | — | | | | (1,188 | ) | | | | |
| Loss on disposal of fixed assets | | | 422 | | | | 538 | | | | — | | | | | |
| Tax benefit related to stock transactions | | | (3,457 | ) | | | (6,135 | ) | | | 5,655 | | | | | |
| Deferred income taxes | | | 443 | | | | 78 | | | | (106 | ) | | | | |
| Changes in assets and liabilities: | | | | | | | | | | | | | | | | |
| Accounts receivable | | | (1,174 | ) | | | (6,351 | ) | | | (2,515 | ) | | | | |
| Inventories | | | 407 | | | | (276 | ) | | | (1,676 | ) | | | | |
| Prepaid expenses and other assets | | | (6,332 | ) | | | 214 | | | | (1,668 | ) | | | | |
| Accounts payable | | | 2,694 | | | | (797 | ) | | | 1,882 | | | | | |
| Deferred revenues | | | 3,594 | | | | 2,518 | | | | 2,716 | | | | | |
| Accrued liabilities | | | 4,431 | | | | 2,097 | | | | 3,562 | | | | | |
| Income taxes payable | | | 9,206 | | | | 11,439 | | | | (930 | ) | | | | |
| Accrued product warranty | | | (714 | ) | | | (104 | ) | | | (57 | ) | | | | |
| | | | | | | | | | | | | | | | | |
| Net cash provided by operating activities | | | 68,511 | | | | 51,000 | | | | 57,172 | | | | | |
| | | | | | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | | | | | |
| Proceeds from sale of marketable securities | | | 9,700 | | | | 39,096 | | | | 69,428 | | | | | |
| Purchase of marketable securities | | | (2,600 | ) | | | (35,050 | ) | | | (64,652 | ) | | | | |
| Purchase of property, plant and equipment | | | (9,388 | ) | | | (9,742 | ) | | | (12,261 | ) | | | | |
| Purchase of intangible assets | | | (1,723 | ) | | | (3,005 | ) | | | — | | | | | |
| Acquisition, net of cash acquired | | | — | | | | — | | | | (3,500 | ) | | | | |
| Other | | | (26 | ) | | | (38 | ) | | | 76 | | | | | |
| | | | | | | | | | | | | | | | | |
| Net cash used for investing activities | | | (4,037 | ) | | | (8,739 | ) | | | (10,909 | ) | | | | |
| | | | | | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| Net change in notes payable | | | 231 | | | | — | | | | (940 | ) | | | | |
| Principal payments on debt | | | — | | | | — | | | | (564 | ) | | | | |
| Proceeds from issuance of stock pursuant to stock-based compensation plans | | | 13,517 | | | | 25,438 | | | | 18,168 | | | | | |
| Tax benefit related to stock transactions | | | 3,457 | | | | 6,135 | | | | — | | | | | |
| Repurchase of common stock | | | (69,592 | ) | | | (73,883 | ) | | | (66,663 | ) | | | | |
| | | | | | | | | | | | | | | | | |
| Net cash used for financing activities | | | (52,387 | ) | | | (42,310 | ) | | | (49,999 | ) | | | | |
| | | | | | | | | | | | | | | | | |
| Effect of exchange rate changes on cash | | | (673 | ) | | | 894 | | | | (258 | ) | | | | |
| | | | | | | | | | | | | | | | | |
| Net increase (decrease) in cash and equivalents | | | 11,414 | | | | 845 | | | | (3,994 | ) | | | | |
| Cash and equivalents, beginning of year | | | 43,524 | | | | 42,679 | | | | 46,673 | | | | | |
| | | | | | | | | | | | | | | | | |
| Cash and equivalents, end of year | | $ | 54,938 | | | $ | 43,524 | | | $ | 42,679 | | | | | |
| | | | | | | | | | | | | | | | | |
| Supplemental disclosures of cash flow information: | | | | | | | | | | | | | | | | |
| Income taxes paid | | $ | 21,349 | | | $ | 9,289 | | | $ | 17,308 | | | | | |
| Interest expense paid | | $ | 162 | | | $ | 71 | | | $ | 94 | | | | | |
| Supplemental schedule of non-cash investing and financing activities: | | | | | | | | | | | | | | | | |
| Accrued purchases of property, plant and equipment | | $ | 219 | | | $ | 162 | | | $ | 419 | | | | | |
| Accrued purchases of intangible assets | | $ | 2,000 | | | $ | — | | | $ | — | | | | | |
See notes to consolidated financial statements.
34
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 — SIGNIFICANT ACCOUNTING POLICIES
Organization. Dionex Corporation is a leading manufacturer and marketer of chromatography systems for chemical analysis. Our systems are used in environmental analysis and by the pharmaceutical, life sciences, chemical, petrochemical, power generation, and food and electronics industries in a variety of applications. Unless the context otherwise requires, the terms “Dionex,” “we,” “our” and “us” and words of similar import as used in these notes to consolidated financial statements include Dionex Corporation and its consolidated subsidiaries.
Principles of Consolidation. The consolidated financial statements include Dionex Corporation and its consolidated subsidiaries. All significant wholly-owned intercompany transactions and accounts are eliminated in consolidation.
Certain Risks and Uncertainties. The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of net sales and expenses during the reporting period. Actual results could differ from those estimates.
Financial instruments which potentially subject us to concentrations of credit risk consist principally of investments and trade receivables. We invest in high-grade instruments which we place for safekeeping with high quality financial institutions. We sell our products primarily to large organizations in diversified industries worldwide. Credit risk is further mitigated by our credit evaluation process and the reasonably short collection terms. We do not require collateral or other security to support accounts receivable and we maintain allowances for potential credit losses.
We are subject to certain risks and uncertainties and believe that changes in any of the following areas could have a material adverse effect on our future financial position or results of operations. Such factors include, among others: the continuation or spread of economic uncertainties; risks related to international operations, including foreign currency fluctuations; the importance of meeting customer demand for new products; competition in the analytical instrumentation market; our ability to maintain inventories; the importance of attracting and retaining key personnel; our ability to protect our proprietary information and acceptance of new products.
Cash Equivalents. We consider all highly liquid investments with maturities of three months or less at the date of purchase to be cash equivalents. We place our cash, cash equivalents and marketable debt securities with high-credit quality financial institutions and to date, we have not experienced credit losses on investments in these instruments.
Short-Term Investments. We classify our debt and equity securities as “held to maturity” or “available for sale.” Securities classified as “held to maturity” are reported at amortized cost and “available for sale” securities are reported at fair market value, with a corresponding recognition of the unrealized gains and losses (net of tax effect) as a separate component of stockholders’ equity. Our investments in marketable debt securities have been classified as “available for sale.”
Inventories. Inventories are stated at the lower of standard cost or market (cost approximatesfirst-in, first-out method). We write down product inventory based on forecasted demand and technological obsolescence and parts inventory based on past usage. These factors are impacted by market and economic conditions, technology changes, new product introductions and changes in strategic direction and require estimates that may include uncertain elements. Actual demand may differ from forecasted demand and such differences may have a material effect on recorded inventory values. In addition, when the inventory carrying value exceeds the market estimated value, we write-down the value of the inventory for the difference between the cost and the estimated market value. These write-downs are determined based on management’s estimates. Inventories consist of finished goods,work-in-process and raw materials.
35
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Property, Plant and Equipment. Property, plant and equipment are stated at cost. Depreciation is computed using the straight-line method based on estimated useful lives of 3 to 30 years. Leasehold improvements are amortized over the lesser of the useful life or the remaining term of the lease.
Purchased Technology and Goodwill. Purchased technology amounts are recorded at their fair market values as of the date of acquisition and amortized over their estimated useful lives of up to ten years. Identifiable intangible assets are recognized separately from goodwill if certain criteria are met and those assets are amortized over their estimated useful economic life. Goodwill is not amortized but is tested for impairment as required. We test goodwill for impairment in April each year and more often if circumstances indicate that goodwill may be impaired. If impaired, a charge is recorded in income from operations. We found no impairment as a result of our fiscal 2007 and 2006 annual impairment tests performed in April of each year.
Valuation of Long Lived Assets. The carrying value of our long-lived assets is reviewed for impairment whenever events or changes in circumstances indicated that an asset may not be recoverable. We look to current and future profitability, as well as current and future undiscounted cash flows, as primary indicators of recoverability. If impairment is determined to exist, any related impairment loss is calculated based on the amount by which the carrying value of the asset exceeds the fair value of the asset with fair value determined on a discounted cash flow basis.
Revenue Recognition. We derive revenues from the sale of products and the delivery of services to our customers, including installation, training, time and material repairs, and maintenance, which consists of product repair obligations, telephone support, and/or unspecified software upgrades.
We recognize revenue in accordance with Staff Accounting Bulletin No. 104,Revenue Recognition, and Statement of Position97-2,Software Revenue Recognition, as amended, when persuasive evidence of an arrangement exists, the product has been delivered, or service performed, the price is fixed or determinable, collection is probable and vendor specific objective evidence or objective reliable evidence of fair value, as applicable, exists to allocate revenue to the various elements of the arrangement. In all cases, the portion of revenue allocated to the undelivered elements is deferred until those items are delivered to the customer or the services are performed. Delivery of the product is generally considered to have occurred when shipped. Undelivered elements in our sales arrangements, which are not considered to be essential to the functionality of a product, generally include maintenance, installation servicesand/or training that are delivered after the related products have been delivered. Installation consists of systemset-up, calibration and basic functionality training and generally requires one to three days depending on the product. Fair value for training services is based on the price charged when the element is sold separately or, if not sold separately, when the price is established by authorized management. The fair value of installation services is calculated by applying standard service billing rates to the estimate of the number of hours to install a specific product based on historical experience. These estimated hours for installation have historically been accurate and consistent from product to product. However, to the extent these estimates were to reflect unfavorable variability, our ability to maintain objective reliable evidence of fair value for such element could be impacted which in turn could delay the recognition of the revenue currently allocated to the delivered elements.
Fair value of the maintenance contracts is based on the price charged when an element is sold separately or, if not yet sold separately, when the price is established by authorized management. Maintenance fees are recognized ratably over the period of the related maintenance contracts, which range from one to two years. Maintenance consists of product repair obligations, telephone support, and/or unspecified software upgrades.
Our sales are typically not subject to rights of return and, historically, actual sales returns have not been significant. We sell our products through our direct sales force and through distributors and resellers. Sales through distributors and resellers are recognized as revenue upon sale to the distributor or reseller as these sales are considered to be final and no right of return or price protection exists. Customer acceptance is generally limited to performance under our published product specifications. When additional customer acceptance conditions apply, all revenue related to the sale is deferred until acceptance is obtained.
36
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Product Warranty. Our equipment typically includes a one-year warranty. The estimated cost of product warranty claims is accrued at the time the sale is recognized, based on historical experience. While we believe our historical experience provides a reliable basis for estimating such warranty cost, unforeseen quality issues or component failure rates could result in future costs in excess of such estimates, or alternatively, improved quality and reliability in our products could result in actual expenses that are below those currently estimated.
Reclassifications. Certain reclassifications have been made of amounts previously reported to conform to the current year presentation. Such reclassifications consist of separate line item disclosures for the current portion of deferred revenues as of June 30, 2006 on the consolidated balance sheet, the change in deferred revenues in the consolidated statements of cash flows for the years ended June 30, 2006 and 2005, and tax benefits from employee stock transactions in the consolidated statements of stockholders’ equity and comprehensive income for the years ended June 30, 2006 and 2005.
Stock-based Compensation Plans. On July 1, 2005, we adopted Statement of Financial Accounting Standards (“SFAS”) No. 123 (revised 2004) Share-Based Payment(SFAS No. 123R), which requires companies to measure the cost of employee services received in exchange for an award of equity instruments based on the grant date fair value of the award. We have elected to use the modified prospective transition method such that SFAS No. 123R applies to new awards and to awards modified, repurchased or canceled after the effective date. We have a stock-based employee compensation plan and an employee stock purchase plan. Generally, stock options granted to employees fully vest four years from the grant date and have a term of ten years. For options granted beginning on July 1, 2006, we recognize stock-based compensation expense over the requisite service period of the individual grants, generally, equal to the vesting period.
Also, commencing in fiscal 2006, SFAS No. 123R required the benefits of tax deductions in excess of the tax-effected compensation that would have been recognized as if we had always accounted for our stock-based award activity under SFAS No. 123R to be reported as a cash flow from financing activities, rather than as a cash flow from operating activities.
Prior to July 1, 2005, we accounted for these plans under the intrinsic value method described in APB Opinion No. 25,Accounting for Stock Issued to Employees, and related interpretations. We applied the intrinsic value method, did not record stock-based compensation cost in net income because the exercise price of our stock options equaled the market price of the underlying stock at the date of grant. Compensation costs for the portion of awards for which the required service period has not been rendered (such as unvested options) that were outstanding as of July 1, 2005 shall be recognized using the multiple-option method as the remaining required services are rendered. The compensation costs relating to unvested awards is based on the grant date fair value of those awards as calculated under SFAS No. 123Accounting for Stock-Based Compensation(SFAS No. 123), adjusted for forfeitures as required by SFAS No. 123R.
Prior to the adoption of SFAS No. 123R, we presented all tax benefits of deductions resulting from the exercise of stock options as operating cash flows in our statement of cash flows. In accordance with SFAS No. 123R, the cash flows resulting from excess tax benefits (from equity-based compensation plans) are classified as financing cash flows. For the years ended June 30, 2007 and 2006, we recorded $3.5 million and $6.1 million of excess tax benefits from equity-based compensation plans as financing cash inflows.
We elected to adopt the alternative transition method provided in the FSP 123R-3 for calculating the tax effects of stock-based compensation pursuant to SFAS No. 123R. The alternative transition method includes simplified
37
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
methods to establish the beginning balance of the additionalpaid-in-capital (“APIC pool”) related to the tax effects of employee stock-based compensation, and to determine the subsequent impact on the APIC pool and Consolidated Statements of Cash Flows of the tax effects of employee stock-based compensation awards that are outstanding upon adoption of SFAS No. 123R.
The following table shows total stock-based compensation expense included in the Consolidated Statements of Income for the years ended June 30, 2007 and 2006 (in thousands):
| | | | | | | | | |
| | | Year Ended
| | | Year Ended
| |
| | | June 30,
| | | June 30,
| |
| | | 2007 | | | 2006 | |
| |
| Cost of sales | | $ | 393 | | | $ | 372 | |
| Selling, general and administrative expenses | | | 3,494 | | | | 3,832 | |
| Research and product development expenses | | | 1,238 | | | | 1,368 | |
| Tax benefit | | | (1,542 | ) | | | (1,751 | ) |
| | | | | | | | | |
| Total | | $ | 3,583 | | | $ | 3,821 | |
| | | | | | | | | |
For pro forma disclosures, the estimated fair value of the options at the date of grant is amortized to expense on a multiple-option method over the service period, which generally equals the vesting period. Had the compensation costs under our stock-based compensation plans been recorded in fiscal 2005, the effect on our net income and earnings per share would have been as follows (in thousands, except per share amounts):
| | | | | |
| | | Year Ended
| |
| | | June 30,
| |
| | | 2005 | |
| |
| Net income, as reported | | $ | 45,490 | |
| Less: Total stock-based employee compensation expense determined under fair value method for all awards, net of taxes | | | 8,707 | |
| Tax benefit | | | (2,790 | ) |
| | | | | |
| Pro forma net income | | $ | 39,573 | |
| | | | | |
| Earnings per share: | | | | |
| Basic — as reported (pro forma) | | $ | 2.20 | |
| | | | | |
| Basic — pro forma (pro forma) | | $ | 1.92 | |
| | | | | |
| Diluted — as reported | | $ | 2.13 | |
| | | | | |
| Diluted — pro forma | | $ | 1.85 | |
| | | | | |
Common Stock Repurchases. We repurchase shares in the open market under our ongoing stock repurchase program. For each share repurchased, we reduce the common stock account by the average value per share reflected in the account prior to the repurchase with the excess allocated to retained earnings. We currently retire all shares upon repurchase.
During fiscal 2007, we repurchased 1,185,100 shares of our common stock on the open market for $69.6 million (an average of $58.72 per share), compared with 1,409,577 shares repurchased for $73.9 million (an average of $52.41 per share) for fiscal 2006 and 1,355,900 shares repurchased for $66.7 million (an average of $49.17 per share) for fiscal 2005.
Translation of Foreign Currency. Our foreign operations are measured using local currencies as the functional currency. Assets and liabilities are translated into U.S. dollars at year-end rates of exchange, and
38
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
results of operations are translated at average rates for the year. Translation adjustments are included in stockholders’ equity as accumulated other comprehensive income/(loss).
Derivative Securities. Derivative instruments, including certain derivative instruments embedded in other contracts, are recorded on the consolidated balance sheet at their fair value as required by SFAS No. 133Accounting for Derivative Instruments and Hedging Activities. Changes in the fair value of derivatives are recorded each period in current earnings or other comprehensive income, depending on whether a derivative is designated as part of a hedge transaction and, if it is, the type of hedge transaction. We formally document, designate and assess the effectiveness of transactions that receive hedge accounting. The changes in fair value of an ineffective portion of a hedge instrument are included in earnings through a credit or charge to other income/expense.
We enter into foreign exchange forward contracts with high quality financial institutions to manage our exposure to the impact of fluctuations in foreign currency exchange rates on our intercompany receivables balances. These contracts generally have maturities of approximately 30 days and require us to exchange foreign currencies for U.S. dollars at maturity. We have not designated these contracts as hedging instruments. The contracts are recorded at fair value on the consolidated balance sheet. Changes in the fair values of these derivative instruments are recognized in earnings in the period they occur.
At June 30, 2007, we had forward exchange contracts to sell foreign currencies totaling $10.5 million, including approximately $4.5 million in Euros, $3.5 million in Japanese yen, $1.2 million in Australian dollars and $1.3 million in Canadian dollars. At June 30, 2006, we had forward exchange contracts to sell foreign currencies totaling $14.8 million, including approximately $9.0 million in Euros, $4.9 million in Japanese yen, $592,000 in Australian dollars and $305,000 in Canadian dollars. At June 30, 2007 and 2006, the aggregate unrealized gains or losses on the forward exchange contracts were not material.
In March 2007, we entered into a cross-currency swap of Japanese Yen for $10.0 million which matures in March 2010. This derivate instrument did not qualify for net investment hedge accounting and was deemed to be an ineffective hedge instrument, given that, at the inception of the hedge transaction, there was no formal documentation of the hedging relationship and our risk management objective and strategy for undertaking the hedge. Therefore, we marked to market the increase in value of approximately $462,000 and this amount was recorded in Other income, net.
Comprehensive Income. We are required to report comprehensive income in the financial statements, in addition to net income. For us, the primary differences between net income and comprehensive income are foreign currency translation adjustments and net unrealized gains or losses on available for sale securities. At June 30, 2007, 2006 and 2005, the components of accumulated other comprehensive income was as follows:
| | | | | | | | | | | | | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | (In thousands) | |
| |
| Foreign currency translation adjustments | | $ | 11,075 | | | $ | 8,605 | | | $ | 5,286 | |
| Unrealized gain/(loss) on securities available for sale, net | | | 1 | | | | (26 | ) | | | (47 | ) |
| | | | | | | | | | | | | |
| | | $ | 11,076 | | | $ | 8,579 | | | $ | 5,239 | |
| | | | | | | | | | | | | |
New Accounting Pronouncements. In February 2007, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities(“SFAS No. 159”). SFAS No. 159 permits entities to choose, at specified election dates, to measure eligible items at fair value (or “fair value option”) and to report in earnings unrealized gains and losses on those items for which the fair value option has been elected. SFAS No. 159 also requires entities to display the fair value of those assets and liabilities on the face of the balance sheet. SFAS No. 159 establishes presentation and disclosure requirements designed to facilitate comparisons between entities that choose different measurement attributes for similar types of assets and liabilities. SFAS No. 159 is effective for us as of the first quarter of fiscal 2009. We are currently evaluating the impact of this pronouncement on our financial statements.
39
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements(“SFAS No. 157”). SFAS No. 157 establishes a framework for measuring fair value and expands disclosures about fair value measurements. The changes to current practice resulting from the application of SFAS No. 157 relate to the definition of fair value, the methods used to measure fair value, and the expanded disclosures about fair value measurements. SFAS No. 157 is effective for fiscal years beginning after November 15, 2007 and interim periods within those fiscal years. We do not believe that the adoption of the provisions of SFAS No. 157 will materially impact our financial position and results of operations.
In June 2006, the FASB issued Interpretation No. (“FIN”) 48,Accounting for Uncertainty in Income Taxes, which is effective for fiscal years beginning after December 15, 2006. FIN 48 clarifies the accounting for uncertainty in income taxes by prescribing rules for recognition, measurement, classification and disclosure in our financial statements of tax positions taken or expected to be taken in a tax return. We are currently evaluating the provisions in FIN 48 and have not yet determined its expected impact. We plan to adopt this new standard effective July 1, 2007.
In February 2006, the FASB issued SFAS No. 155,Accounting for Certain Hybrid Financial Instruments— an amendment of FASB Statements No. 133 and 140. This standard permits fair value re-measurement for any hybrid financial instrument that contains an embedded derivative that otherwise would require bifurcation; clarifies which interest-only strips and principal-only strips are not subject to the requirements of SFAS No. 133; requires evaluation of interests in securitized financial assets; clarifies that concentrations of credit risk in the form of subordination are not embedded derivatives; and eliminates the prohibition of a qualifying special-purpose entity from holding a derivative financial instrument that pertains to a beneficial interest of another derivative financial instrument. This standard is effective for all financial instruments acquired or issued after fiscal years beginning after September 15, 2006. The adoption of SFAS No. 155 did not have a material effect on our financial position, results of operations or cash flows.
Note 2 — EARNINGS PER SHARE
Basic earnings per share are determined by dividing net income by the weighted average number of common shares outstanding during the period. Diluted earnings per share is determined by dividing net income by the weighted average number of common shares used in the basic earnings per share calculation, plus the number of common shares that would be issued assuming conversion of all potentially dilutive securities outstanding under the treasury stock method.
The following table is a reconciliation of the numerators and denominators used in computing basic and diluted earnings per share (in thousands, except per share amounts):
| | | | | | | | | | | | | |
| | | Years Ended June 30 | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Numerator: | | | | | | | | | | | | |
| Net income | | $ | 45,322 | | | $ | 35,693 | | | $ | 45,490 | |
| | | | | | | | | | | | | |
| Denominator: | | | | | | | | | | | | |
| Shares used to compute net income per common share — basic | | | 19,136 | | | | 20,013 | | | | 20,655 | |
| Effect of dilutive stock options | | | 479 | | | | 514 | | | | 733 | |
| | | | | | | | | | | | | |
| Shares used to compute net income per common share — diluted | | | 19,615 | | | | 20,527 | | | | 21,388 | |
| | | | | | | | | | | | | |
| Basic earnings per share | | $ | 2.37 | | | $ | 1.78 | | | $ | 2.20 | |
| | | | | | | | | | | | | |
| Diluted earnings per share | | $ | 2.31 | | | $ | 1.74 | | | $ | 2.13 | |
| | | | | | | | | | | | | |
40
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Antidilutive common equivalent shares related to stock options excluded from the calculation of diluted shares were approximately 327,894, 448,228 and 300,000 for fiscal 2007, 2006 and 2005, respectively.
Note 3 — SHORT-TERM INVESTMENTS
| | | | | | | | | | | | | |
| | | | | | Gross
| | | | |
| | | Cost | | | Unrealized Losses | | | Fair Value | |
| | | (In thousands) | |
| |
| June 30, 2007 | | | | | | | | | | | | |
| Corporate debt securities(1) | | $ | 127 | | | $ | (3 | ) | | $ | 124 | |
| | | | | | | | | | | | | |
| | | $ | 127 | | | $ | (3 | ) | | $ | 124 | |
| | | | | | | | | | | | | |
| June 30, 2006 | | | | | | | | | | | | |
| Auction rate securities | | $ | 7,100 | | | $ | — | | | $ | 7,100 | |
| Corporate debt securities(1) | | | 415 | | | | (25 | ) | | | 390 | |
| | | | | | | | | | | | | |
| | | $ | 7,515 | | | $ | (25 | ) | | $ | 7,490 | |
| | | | | | | | | | | | | |
| | |
| (1) | | These investments have been in a loss position for greater than 12 months. |
Investments with maturities greater than three months, but less than one year are classified as short-term investments. Investments with maturities greater than one year are classified as long-term investments and are recorded in other assets. Auction rate debt securities with interest rates that reset in less than three months but with maturity dates longer than three months, are classified as short-term investments. At June 30, 2006, all such investments have been classified as “held-to-maturity” and recorded as short-term investments. The corporate debt securities are classified as “available-for-sale” securities and are carried at fair value. At June 30, 2007, the fair value of the securities was $124,000 reported in short-term investments.
In December 1989, we invested $3.0 million in the stock of Molecular Devices Corporation (MDC). Two of our directors served on the Board of Directors of MDC until its purchase by another company in fiscal 2007. We sold our remaining ownership interest in MDC in fiscal 2005 resulting in a gain of approximately $1.2 million.
Note 4 — INVENTORIES
Inventories at June 30 consisted of the following:
| | | | | | | | | |
| | | 2007 | | | 2006 | |
| | | (In thousands) | |
| |
| Finished goods | | $ | 16,535 | | | $ | 13,962 | |
| Work in process | | | 1,329 | | | | 1,840 | |
| Raw materials and subassemblies | | | 10,762 | | | | 11,900 | |
| | | | | | | | | |
| | | $ | 28,626 | | | $ | 27,702 | |
| | | | | | | | | |
Note 5 — PROPERTY, PLANT AND EQUIPMENT, NET
Property, plant and equipment, net at June 30 consisted of:
| | | | | | | | | |
| | | 2007 | | | 2006 | |
| | | (In thousands) | |
| |
| Land | | $ | 23,246 | | | $ | 23,141 | |
| Buildings and improvements | | | 40,409 | | | | 37,364 | |
| Machinery, equipment and tooling | | | 29,281 | | | | 25,579 | |
| Furniture and fixtures | | | 9,980 | | | | 9,147 | |
| | | | | | | | | |
| | | | 102,916 | | | | 95,231 | |
| Accumulated depreciation and amortization | | | (40,550 | ) | | | (36,531 | ) |
| | | | | | | | | |
| Property, plant and equipment, net | | $ | 62,366 | | | $ | 58,700 | |
| | | | | | | | | |
41
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Note 6 — GOODWILL AND INTANGIBLE ASSETS
Information regarding our goodwill and other intangible assets reflect current foreign exchange rates.
Changes in the carrying amount of goodwill for the years ended June 30, 2007 and 2006 are as follows (in thousands):
| | | | | |
| Balance as of June 30, 2005 | | $ | 24,638 | |
| Translation adjustments | | | 344 | |
| | | | | |
| Balance as of June 30, 2006 | | $ | 24,982 | |
| Translation adjustments | | | 461 | |
| | | | | |
| Balance as of June 30, 2007 | | $ | 25,443 | |
| | | | | |
Our reporting units represent our operating segments, see Note 14. All goodwill has been assigned to one reporting unit. The evaluation of goodwill is based upon the fair value of this reporting unit. Pursuant to the provisions of SFAS No. 142,Goodwill and Other Intangible Assets, we performed an annual impairment test on goodwill in April 2007 and 2006 and determined that goodwill was not impaired.
Information regarding our other intangible assets having a finite life is as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | As of June 30, 2007 | | | As of June 30, 2006 | |
| | | Carrying
| | | Accumulated
| | | | | | Carrying
| | | Accumulated
| | | | |
| | | Amount | | | Amortization | | | Net | | | Amount | | | Amortization | | | Net | |
| |
| Patents and trademarks | | $ | 5,958 | | | $ | (779 | ) | | $ | 5,179 | | | $ | 3,337 | | | $ | (735 | ) | | $ | 2,602 | |
| Developed technology | | | 10,013 | | | | (9,805 | ) | | | 208 | | | | 9,695 | | | | (8,773 | ) | | | 922 | |
| Customer relationships | | | 2,205 | | | | (637 | ) | | | 1,568 | | | | 1,411 | | | | (413 | ) | | | 998 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 18,176 | | | $ | (11,221 | ) | | $ | 6,955 | | | $ | 14,443 | | | $ | (9,921 | ) | | $ | 4,522 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
During fiscal 2007, we pursued our strategic initiative of strengthening our base in the eluent generation and membrane suppression for ion chromatography applications and to support new liquid chromatography applications with the acquisition of the IC patent portfolio from Alltech Associates, Inc., for approximately $3.0 million. At June 30, 2007, we had paid $1.0 million of the purchase price and the remaining $2.0 million is recorded in accrued liabilities.
During fiscal 2006, we also pursued our strategic initiative of strengthening our base in the separations chemistry with the acquisition of the patent for polymer based monolith technology from Teledyne-Isco, a subsidiary of Teledyne Technologies Incorporated, for a cash payment of approximately $3.0 million.
We amortize patents and trademarks over a period of ten years and the remaining weighted average amortization period for this category is approximately seven years.
We amortize developed technology over a period of three to seven years and the remaining weighted average amortization period for this category is less than one year. We amortize other intangibles over a period of five to ten years and the remaining weighted average amortization period for this category is approximately four years.
42
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Amortization expense related to intangible assets was $1.3 million, $1.4 million and $1.5 million for the years ended June 30, 2007, 2006 and 2005, respectively. The estimated amortization for each of the five fiscal years subsequent to June 30, 2007 is as follows (in thousands):
| | | | | |
| | | Remaining
| |
| | | Amortization
| |
Year Ending June 30, | | Expense | |
| |
| 2008 | | $ | 1,046 | |
| 2009 | | | 842 | |
| 2010 | | | 792 | |
| 2011 | | | 792 | |
| 2012 | | | 792 | |
| Thereafter | | | 2,691 | |
| | | | | |
| Total | | $ | 6,955 | |
| | | | | |
Note 7 — FINANCING ARRANGEMENTS
We have unsecured lines of credit with various domestic and foreign banks which have been used primarily to minimize our exposure to foreign currency fluctuations and to fund acquisitions. The available lines of credit totaled $28.4 million and $31.7 million at June 30, 2007 and 2006, respectively. The decrease in existing lines of credit was due to the contractual expiration of certain lines with foreign banks. Borrowings in each country bear interest at local reference rates which ranged from 5.5% to 9.3% at June 30, 2007. There was $231,000 outstanding under these lines at June 30, 2007. Such line of credit agreements impose certain financial restrictions relating to cash dividends, working capital and tangible net worth.
One of our foreign subsidiaries discounts trade notes receivable with banks. Total notes receivable discounted were approximately $7.3 million in fiscal 2007 and $7.9 million in fiscal 2006. The uncollected balances of notes receivable due to the discounting banks at June 30, 2007 and 2006 were approximately $2.4 million and $1.3 million, respectively. We have a contingent liability to repurchase these notes under certain conditions. We have determined that the carrying amount of our contingent liability under this guarantee was insignificant at June 30, 2007 and 2006 based on its past experience of discounting trade notes receivable.
Total interest paid was $162,000 in 2007, $71,000 in 2006 and $94,000 in 2005.
Note 8 — WARRANTY
Product warranties are recorded at the time revenue is recognized for certain product shipments. While we engage in extensive product quality programs and processes, our warranty obligation is affected by product failure rates, material usage and service costs incurred in correcting a product failure. Should actual product failure rates, material usage or service costs differ from our previous estimates, revisions to the estimated warranty liability would be required.
Details of the change in accrued product warranty for fiscal 2007, 2006 and 2005 are as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Charged
| | | | | | | |
| | | Balance
| | | | | | (Credited)
| | | | | | Balance
| |
| | | Beginning of
| | | | | | to Other
| | | | | | End of
| |
| | | Year | | | Additions | | | Accounts(1) | | | Deductions(2) | | | Year | |
| | | (In thousands) | |
| |
| Accrued product warranty: | | | | | | | | | | | | | | | | | | | | |
| June 30, 2007 | | $ | 3,493 | | | $ | 2,669 | | | $ | 100 | | | $ | (3,386 | ) | | $ | 2,876 | |
| June 30, 2006 | | | 3,514 | | | | 3,810 | | | | 134 | | | | (3,965 | ) | | | 3,493 | |
| June 30, 2005 | | | 3,584 | | | | 3,400 | | | | (3 | ) | | | (3,467 | ) | | | 3,514 | |
| | |
| (1) | | Effects of exchange rate changes. |
| |
| (2) | | Product warranty costs. |
43
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Note 9 — ACCRUED LIABILITIES
Accrued liabilities at June 30 consist of:
| | | | | | | | | |
| | | 2007 | | | 2006 | |
| | | (In thousands) | |
| |
| Accrued payroll and related expenses | | $ | 18,469 | | | $ | 15,919 | |
| Other accrued liabilities | | | 11,860 | | | | 8,458 | |
| | | | | | | | | |
| | | $ | 30,329 | | | $ | 24,377 | |
| | | | | | | | | |
Note 10 — STOCK OPTION AND PURCHASE PLANS
Stock Option Plans. We have one stock option plan (the “Option Plan”) under which incentive and nonqualified options may be granted. Options are granted at the stock’s fair market value at the grant date. Options generally become exercisable in increments over a period of four years from the date of grant and expire generally ten years from the grant date.
In August 2006, the Board of Directors approved an amendment to our Option Plan to increase the number of shares authorized for issuance by 1,500,000 shares. The amendment was approved by our stockholders at the Annual Meeting of Stockholders on October 27, 2006.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | As of June 30, | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | | | | Wtd. Avg.
| | | | | | Wtd. Avg.
| | | | | | Wtd. Avg.
| |
| | | | | | Exercise
| | | | | | Exercise
| | | | | | Exercise
| |
| | | Shares | | | Price | | | Shares | | | Price | | | Shares | | | Price | |
| |
| Options outstanding, beginning of year | | | 1,736,593 | | | $ | 37.43 | | | | 2,261,642 | | | $ | 32.65 | | | | 2,642,714 | | | $ | 29.33 | |
| Granted | | | 346,250 | | | | 53.58 | | | | 356,650 | | | | 48.23 | | | | 300,050 | | | | 47.70 | |
| Exercised | | | (367,479 | ) | | | 32.29 | | | | (831,347 | ) | | | 28.63 | | | | (635,998 | ) | | | 26.04 | |
| Canceled | | | (20,893 | ) | | | 48.61 | | | | (50,352 | ) | | | 44.21 | | | | (45,124 | ) | | | 31.73 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Options outstanding, end of year | | | 1,694,471 | | | $ | 41.91 | | | | 1,736,593 | | | $ | 37.43 | | | | 2,261,642 | | | $ | 32.65 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Options vested and expected to vest | | | 1,664,063 | | | $ | 41.74 | | | | 1,703,277 | | | $ | 37.24 | | | | 2,249,001 | | | $ | 32.58 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Options exercisable at year end | | | 1,036,706 | | | $ | 36.13 | | | | 1,052,726 | | | $ | 32.17 | | | | 1,491,853 | | | $ | 28.93 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Weighted average fair value of options granted during the year | | | | | | $ | 18.38 | | | | | | | $ | 19.38 | | | | | | | $ | 22.54 | |
The total intrinsic value of options exercised were $12.3 million, $18.6 million and $18.1 million in fiscal 2007, 2006 and 2005 respectively. As of June 30, 2007, there was $12.5 million of total unrecognized compensation cost related to nonvested stock options. That cost is expected to be recognized over a weighted average period of 2.3 years. The total intrinsic value of options exercisable at June 30, 2007 was $36.1 million based upon a market value of $70.99 per share. The total intrinsic value of options outstanding is $49.3 million based upon a market value of $70.99 per share. The total intrinsic value of the options excercisable and expected to vest, based on a market value of $70.99 per share was $48.7 million with a weighted average remaining contractual life of 6.48 years.
44
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Additional information regarding options outstanding and exercisable as of June 30, 2007 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Options Outstanding | | | Options Exercisable | |
| | | | | | Weighted Avg.
| | | Weighted
| | | | | | Weighted Avg.
| | | Weighted
| |
| | | | | | Remaining
| | | Avg.
| | | | | | Remaining
| | | Avg.
| |
Range of
| | Number
| | | Contractual
| | | Exercise
| | | Number
| | | Contractual
| | | Exercise
| |
Exercise Prices | | Outstanding | | | Life (Yrs) | | | Price | | | Exercisable | | | Life (Yrs) | | | Price | |
| |
| $23-98 - 29.80 | | | 301,965 | | | | 4.50 | | | $ | 25.28 | | | | 301,965 | | | | | | | $ | 25.28 | |
| 29.80 - 34.88 | | | 195,275 | | | | 2.69 | | | | 31.89 | | | | 195,275 | | | | | | | | 31.89 | |
| 39.47 - 41.03 | | | 304,986 | | | | 5.96 | | | | 39.66 | | | | 267,943 | | | | | | | | 39.61 | |
| 41.03 - 47.19 | | | 223,597 | | | | 7.08 | | | | 47.19 | | | | 143,983 | | | | | | | | 47.19 | |
| 47.19 - 49.93 | | | 313,648 | | | | 8.12 | | | | 48.22 | | | | 119,540 | | | | | | | | 48.11 | |
| 49.93 - 70.32 | | | 355,000 | | | | 9.06 | | | | 54.59 | | | | 8,000 | | | | | | | | 54.86 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| $23.98 - 70.32 | | | 1,694,471 | | | | 6.52 | | | $ | 41.91 | | | | 1,036,706 | | | | 5.33 | | | $ | 36.13 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
At June 30, 2007, 1,497,570 shares were available for future grants under the Option Plan.
The fair value of each option on the date of grant is estimated using the Black-Scholes option-pricing model using the multiple option approach for options granted, prior to June 30, 2005 and using the single option approach for options granted after June 30, 2005. These are the following weighted-average assumptions:
| | | | | | | | | | | | | |
| | | Years Ended June 30, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Volatility for options | | | 29 | % | | | 40 | % | | | 47 | % |
| Volatility for employee stock purchase plan | | | 23 - 26 | % | | | 30 | % | | | 27 - 29 | % |
| Risk-free interest rate for options | | | 4.50 - 4.88 | % | | | 4 - 4.5 | % | | | 3.56 | % |
| Risk-free interest rate for employee stock purchase plan | | | 4.98 | % | | | 3.6 - 4.4 | % | | | 1.74 - 2.71 | % |
| Expected life of options | | | 4.7 years | | | | 4.75 years | | | | 5.6 years | |
| Expected life of employee stock purchase plan | | | 6 months | | | | 6 months | | | | 6 months | |
| Expected dividend | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | |
Exercise and post-vesting forfeiture assumptions based on analysis of historical data.
Determining Fair Value
Valuation and amortization method — We estimate the fair value of stock options granted using the Black-Scholes-Merton option – pricing formula and a single option award approach. This fair value is then amortized on a straight-line basis over the requisite service periods of the awards, which is generally the vesting period.
Expected Term – The expected term represents the period that our stock-based awards are expected to be outstanding and was determined based on historical experience of similar awards, giving consideration to the contractual terms of the stock-based awards, vesting schedules and expectations of future employee behavior as influenced by changes to the terms of our stock-based awards.
Expected Volatility – Our computation of expected volatility for the years ended June 30, 2007 and 2006 is based on a combination of historical and market-based implied volatility. Expected volatilities for the year ended June 30, 2005 were based mainly on the historical volatility of our stock price.
Risk-Free Interest Rate – The risk-free interest rate used in the Black-Scholes-Merton valuation method is based on the implied yield currently available on U.S. Treasury zero-coupon issues with an equivalent remaining term.
45
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Expected Dividend — The expected dividend assumption is based on our current expectations about our anticipated dividend policy.
Employee Stock Purchase Plan. Under our Employee Stock Purchase Plan (the “Purchase Plan”), eligible employees are permitted to have salary withholdings to purchase shares of common stock at a price equal to 85% of the lower of the market value of the stock at the beginning or end of each six-month offer period, subject to certain annual limitations. The number of shares of stock issued under the Purchase Plan was 38,584, 41,977 and 40,113 shares in fiscal 2007, 2006 and 2005, respectively, at weighted average prices of $44.36, $39.39 and $40.09, respectively. The weighted average fair value of the fiscal 2007, 2006 and 2005 awards was $14.10, $11.72 and $12.34, respectively. At June 30, 2007, 668,219 shares were reserved for future issuances under the Purchase Plan.
Note 11 — EMPLOYEE 401(K) PLAN
We have a 401(k) tax deferred savings plan covering most U.S. employees. Participants may contribute up to 10% of their compensation and we make matching contributions ($1.4 million in fiscal 2007, 2006 and 2005, respectively) limited to 5% of each participant’s compensation. In fiscal 2007, matching contributions vest in 25% increments each year. In prior years, matching contributions vested in 25% increments each year beginning two years after the participant’s date of employment.
Note 12 — TAXES ON INCOME
The provision for taxes on income consists of:
| | | | | | | | | | | | | |
| | | Years Ended June 30 | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | (In thousands) | |
| |
| Current: | | | | | | | | | | | | |
| Federal | | $ | 10,488 | | | $ | 10,599 | | | $ | 10,584 | |
| State | | | 1,770 | | | | 1,221 | | | | 664 | |
| Foreign | | | 13,166 | | | | 10,082 | | | | 9,415 | |
| | | | | | | | | | | | | |
| Total current | | | 25,424 | | | | 21,902 | | | | 20,663 | |
| | | | | | | | | | | | | |
| Deferred: | | | | | | | | | | | | |
| Federal | | | 1,474 | | | | (32 | ) | | | (84 | ) |
| State | | | (78 | ) | | | 81 | | | | 82 | |
| Foreign | | | (852 | ) | | | 869 | | | | 420 | |
| | | | | | | | | | | | | |
| Total deferred | | | 544 | | | | 918 | | | | 418 | |
| | | | | | | | | | | | | |
| | | $ | 25,968 | | | $ | 22,820 | | | $ | 21,081 | |
| | | | | | | | | | | | | |
Domestic and foreign income before taxes on income is as follows:
| | | | | | | | | | | | | |
| | | Years Ended June 30 | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | (In thousands) | |
| |
| Domestic | | $ | 55,126 | | | $ | 47,012 | | | $ | 53,918 | |
| Foreign | | | 16,164 | | | | 11,501 | | | | 12,653 | |
| | | | | | | | | | | | | |
| | | $ | 71,290 | | | $ | 58,513 | | | $ | 66,571 | |
| | | | | | | | | | | | | |
46
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The components of the current and non-current deferred tax assets and liabilities are as follows:
| | | | | | | | | |
| | | Years Ended June 30 | |
| | | 2007 | | | 2006 | |
| | | (In thousands) | |
| |
| Current deferred tax assets: | | | | | | | | |
| Accounting accruals deductible in different periods for tax purposes | | $ | 8,271 | | | $ | 9,342 | |
| State income tax | | | 448 | | | | 316 | |
| Other | | | 264 | | | | 257 | |
| | | | | | | | | |
| Total current deferred tax assets | | | 8,983 | | | | 9,915 | |
| | | | | | | | | |
| Non-current deferred tax asset — Difference in tax basis from acquisition | | | 919 | | | | 1,208 | |
| Non-current deferred tax liabilities: | | | | | | | | |
| Accelerated depreciation | | | 1,084 | | | | 993 | |
| Excess tax basis from acquisition | | | 919 | | | | 1,208 | |
| Accumulated translation adjustment | | | 3,094 | | | | 1,567 | |
| Other | | | (38 | ) | | | 184 | |
| | | | | | | | | |
| Total deferred tax liabilities | | | 5,059 | | | | 3,952 | |
| | | | | | | | | |
| Net deferred tax assets | | $ | 4,843 | | | $ | 7,171 | |
| | | | | | | | | |
Total income tax expense differs from the amount computed by applying the statutory Federal income tax rate to income before taxes on income as follows:
| | | | | | | | | | | | | |
| | | Years Ended June 30 | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Statutory Federal income tax rate | | | 35.0 | % | | | 35.0 | % | | | 35.0 | % |
| State income taxes, net of Federal income tax effect | | | 1.6 | | | | 1.5 | | | | 0.7 | |
| Extraterritorial income exclusion/Manufacturing deduction | | | (1.1 | ) | | | (2.2 | ) | | | (2.3 | ) |
| Taxes on foreign income | | | 1.8 | | | | 4.3 | | | | 0.5 | |
| Other | | | (0.9 | ) | | | 0.4 | | | | (2.2 | ) |
| | | | | | | | | | | | | |
| | | | 36.4 | % | | | 39.0 | % | | | 31.7 | % |
| | | | | | | | | | | | | |
Income taxes paid were $21.3 million in fiscal 2007, $9.3 million in fiscal 2006 and $17.3 million in fiscal 2005.
We have not provided for Federal income taxes on approximately $46.9 million of undistributed earnings of certain foreign subsidiaries, which we intend to permanently reinvest in subsidiary operations. If these earnings were distributed to us as the parent, foreign tax credits available under current law would substantially eliminate the resulting Federal income tax liability.
Note 13 — COMMITMENTS AND OTHER CONTINGENCIES
Certain facilities and equipment are leased under non-cancelable operating leases. We generally pay taxes, insurance and maintenance costs on leased facilities and equipment. Minimum annual rental commitments under these non-cancelable operating leases are $4.9 million for fiscal 2008, $3.7 million for fiscal 2009, $2.9 million for fiscal 2010, $1.9 million for fiscal 2011, $1.6 million for fiscal 2012 and $2.9 million thereafter.
47
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Total rental expense for all operating leases was $6.7 million in fiscal 2007, $5.6 million in fiscal 2006 and $5.7 million in fiscal 2005.
We enter into standard indemnification agreements with many of our customers and certain other business partners in the ordinary course of business. These agreements include provisions for indemnifying the customer against any claim brought by a third party to the extent any such claim alleges that our product infringes a patent, copyright or trademark, or violates any other proprietary rights of that third party. We also indemnify against general negligence claims. While the maximum potential amount of future payments we could be required to make under these indemnification agreements is not estimable, we have not incurred any costs to defend lawsuits or settle claims related to these indemnification agreements. To date, no material claims for such indemnifications are outstanding as of June 30, 2007. We have not recorded any liabilities for these indemnification agreements at June 30, 2007 or June 30, 2006.
Note 14 — BUSINESS SEGMENT INFORMATION
SFAS No. 131 establishes standards for reporting information about operating segments in annual financial statements of public business enterprises. It also establishes standards for related disclosures about products and service, geographic areas and major customers.
We have two operating segments, the Chemical Analysis Business Unit (“CABU”) and the Life Sciences Business Unit (“LSBU”). CABU sells ion chromatography and accelerated solvent extraction products, services and related consumables. LSBU sells high performance liquid chromatography products, services and related consumables. These two operating segments are aggregated into one reportable segment for financial statement purposes.
We sell products, installation and training services and maintenance within this reportable segment, detailed as follows:
| | | | | | | | | | | | | |
| | | Years Ended June 30 | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | (In thousands) | |
| |
| Products | | $ | 286,767 | | | $ | 254,054 | | | $ | 242,581 | |
| Installation and training services | | | 9,365 | | | | 8,945 | | | | 9,218 | |
| Maintenance | | | 31,152 | | | | 28,301 | | | | 27,518 | |
| | | | | | | | | | | | | |
| | | $ | 327,284 | | | $ | 291,300 | | | $ | 279,317 | |
| | | | | | | | | | | | | |
48
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Geographic information is presented below:
| | | | | | | | | | | | | |
| | | Years Ended June 30 | |
| | | 2007 | | | 2006 | | | 2005 | |
| | | (In thousands) | |
| |
| Net sales to unaffiliated customers: | | | | | | | | | | | | |
| United States | | $ | 85,419 | | | $ | 80,860 | | | $ | 79,553 | |
| Europe, excluding Germany | | | 106,781 | | | | 91,795 | | | | 88,075 | |
| Japan | | | 32,642 | | | | 30,428 | | | | 34,391 | |
| Germany | | | 37,683 | | | | 32,145 | | | | 32,107 | |
| Other International | | | 64,759 | | | | 56,072 | | | | 45,191 | |
| | | | | | | | | | | | | |
| Consolidated net sales | | $ | 327,284 | | | $ | 291,300 | | | $ | 279,317 | |
| | | | | | | | | | | | | |
| At June 30 | | | | | | | | | | | | |
| Long-lived assets: | | | | | | | | | | | | |
| United States | | $ | 51,728 | | | $ | 45,170 | | | $ | 41,495 | |
| Europe, excluding Germany | | | 9,022 | | | | 9,170 | | | | 10,010 | |
| Japan | | | 8,738 | | | | 9,420 | | | | 10,304 | |
| Germany | | | 30,251 | | | | 27,420 | | | | 22,678 | |
| Other International | | | 1,256 | | | | 1,792 | | | | 1,583 | |
| | | | | | | | | | | | | |
| Consolidated long-lived assets | | $ | 100,995 | | | $ | 92,972 | | | $ | 86,070 | |
| | | | | | | | | | | | | |
No individual customer accounted for greater than 5% of net sales in fiscal 2007, 2006 and 2005 or greater than 10% of consolidated accounts receivable at June 30, 2007 and 2006.
Note 15 — RELATED PARTY TRANSACTION
In fiscal 2005, we purchased land for 2.3 million Euros (approximately $3.1 million) for expansion of our manufacturing facility in Germany. The property was owned 25% by one of our vice presidents. We believe that the price was negotiated at an arms-length fair value.
49
DIONEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Note 16 — QUARTERLY RESULTS OF OPERATIONS (unaudited)
The following is a summary of the unaudited quarterly results of operations for the years ended June 30, 2007 and 2006.
| | | | | | | | | | | | | | | | | |
| | | Quarter | |
| | | First | | | Second | | | Third | | | Fourth | |
| | | (In thousands, except per share amounts) | |
| |
| Fiscal 2007: | | | | | | | | | | | | | | | | |
| Net sales | | $ | 72,857 | | | $ | 83,519 | | | $ | 84,954 | | | $ | 85,954 | |
| Gross profit | | | 47,898 | | | | 55,864 | | | | 55,782 | | | | 58,725 | |
| Net income | | | 8,671 | | | | 13,267 | | | | 11,497 | | | | 11,887 | |
| Basic earnings per share | | | 0.45 | | | | 0.69 | | | | 0.60 | | | | 0.63 | |
| Diluted earnings per share | | | 0.44 | | | | 0.68 | | | | 0.59 | | | | 0.61 | |
| Fiscal 2006: | | | | | | | | | | | | | | | | |
| Net sales | | $ | 68,100 | | | $ | 74,341 | | | $ | 73,674 | | | $ | 75,185 | |
| Gross profit | | | 44,330 | | | | 49,812 | | | | 48,548 | | | | 48,753 | |
| Net income | | | 9,014 | | | | 10,900 | | | | 10,365 | | | | 5,414 | |
| Basic earnings per share | | | 0.45 | | | | 0.54 | | | | 0.52 | | | | 0.27 | |
| Diluted earnings per share | | | 0.44 | | | | 0.53 | | | | 0.50 | | | | 0.27 | |
50
| |
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
| |
| Item 9A. | CONTROLS AND PROCEDURES |
Management’s Evaluation of Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our “disclosure controls and procedures” (as defined in rules promulgated under the Exchange ActRules 13a-15(e) and15d-15(e)). Based on that evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures as of June 30, 2007 were effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is (i) recorded processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to our management, including our principal executive and principal financial officers, to allow timely decisions regarding required disclosures.
Management’s Annual Report on Internal Control over Financial Reporting
Our management, including our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining internal control over financial reporting (as defined in Exchange ActRules 13a-15(f) and15d-15(f)) for us. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). Our management conducted an assessment of the effectiveness of our internal control over financial reporting as of June 30, 2007 based on criteria established in the “Internal Control — Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on our assessment, our management concluded that, as of June 30, 2007, our internal control over financial reporting was effective.
Our independent registered public accounting firm, Deloitte & Touche LLP, has audited the effectiveness of our internal control over financial reporting and management’s assessment of the effectiveness of our internal control over financial reporting as of June 30, 2007, as stated in their report that appears below.
Changes in Internal Control over Financial Reporting
No changes in our internal control over financial reporting occurred during the quarter ended June 30, 2007 that have materially affected or are reasonably likely to materially affect our internal control over financial reporting.
| |
| Item 9B. | OTHER INFORMATION |
Not applicable.
51
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Dionex Corporation:
We have audited management’s assessment, included in the accompanying Management’s Report on Internal Control Over Financial Reporting, that Dionex Corporation and its subsidiaries (collectively, the “Company”) maintained effective internal control over financial reporting as of June 30, 2007, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on management’s assessment and an opinion on the effectiveness of the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, management’s assessment that the Company maintained effective internal control over financial reporting as of June 30, 2007, is fairly stated, in all material respects, based on the criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of June 30, 2007, based on the criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements and financial statement schedule as of and for the year ended June 30, 2007 of the Company and our reports dated August 29, 2007 expressed unqualified opinions on those consolidated financial statements and financial statement schedule (which report on the consolidated financial statements includes an explanatory paragraph relating to the adoption of Statement of Financial Accounting Standards No. 123 (revised 2004),Share-Based Payment).
/s/ Deloitte & Touche LLP
San Jose, California
August 29, 2007
52
| |
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
IDENTIFICATION OF DIRECTORS
The information required by Item 10 ofForm 10-K with respect to identification of directors is incorporated by reference to the information contained in the sections captioned “Election of Directors” and “Section 16(a) Beneficial Ownership Reporting and Compliance” in our definitive Proxy Statement for the Annual Meeting of Stockholders to be held October 30, 2007, the “2007 Proxy Statement”, which will be filed in accordance with Regulation 14A under the Exchange Act.
IDENTIFICATION OF OFFICERS
See Page 14 of this Report captioned “Executive Officers of Dionex Corporation,” which is incorporated herein by reference.
CORPORATE GOVERNANCE
The information required by Item 10 ofForm 10-K with respect to the Audit Committee is incorporated by reference to the information contained in “Election of Directors” in the 2007 Proxy Statement.
CODE OF ETHICS
See Page 11 of this Report captioned “Available Information,” which is incorporated herein by reference.
| |
| Item 11. | EXECUTIVE COMPENSATION |
The information required by Item 11 ofForm 10-K regarding executive compensation is incorporated by reference to the information contained in the sections captioned “Executive Compensation,” “Compensation Discussion and Analysis,” “Compensation Committee Interlocks and Insider Participation” and “Compensation Committee Report” in the 2007 Proxy Statement.
| |
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by Item 12 ofForm 10-K is incorporated by reference to the information contained in the section captioned “Security Ownership of Certain Beneficial Owners and Management” in the 2007 Proxy Statement.
| |
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by Item 13 ofForm 10-K is incorporated by reference to the information contained in the section captioned “Election of Directors” in the 2007 Proxy Statement.
| |
| Item 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by Item 14 ofForm 10-K is incorporated by reference to the information contained in the sections captioned “Independent Registered Public Accounting Firm’s Fees,” “Policy on Audit Committee Pre-Approval” and “Audit Committee Disclosure” in the 2007 Proxy Statement.
| |
| Item 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| | |
| | (a) | (1) Financial Statements — See Index to Financial Statements at page 29 of this Report. |
(2) Financial Statement Schedule — See Index to Financial Statement Schedules at page 55 of this Report.
(3) Exhibits — See Exhibit Index at page 58 through 59 of this Report.
53
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, we have duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
DIONEX CORPORATION
| | |
| | By | /s/ Lukas Braunschweiler |
Lukas Braunschweiler
President and Chief Executive Officer
Date: August 29, 2007
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Lukas Braunschweiler and Craig A. McCollam, and each or either of them, each with the power of substitution, his attorney-in-fact, to sign any amendments to this report, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of Dionex Corporation and in the capacities and on the dates indicated.
| | | | | | | |
Signature | | Title | | Date |
| |
| | | | | |
/s/ Lukas Braunschweiler
Lukas Braunschweiler | | President, Chief Executive Officer, and Director (Principal Executive Officer) | | August 29, 2007 |
| | | | | |
/s/ Craig A. McCollam
Craig A. McCollam | | Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) | | August 29, 2007 |
| | | | | |
/s/ A. Blaine Bowman
A. Blaine Bowman | | Director | | August 29, 2007 |
| | | | | |
/s/ David L. Anderson
David L. Anderson | | Director | | August 29, 2007 |
| | | | | |
/s/ Roderick McGeary
Roderick McGeary | | Director | | August 29, 2007 |
| | | | | |
/s/ Riccardo Pigliucci
Riccardo Pigliucci | | Lead Director | | August 29, 2007 |
| | | | | |
/s/ Michael W. Pope
Michael W. Pope | | Director | | August 29, 2007 |
54
INDEX TO FINANCIAL STATEMENT SCHEDULES
| | | | | |
| | | Page |
| |
| FINANCIAL STATEMENT SCHEDULE | | | | |
| | | 56 | |
| | | 57 | |
All other schedules are omitted because they are not required, are not applicable or the information is included in the consolidated financial statements or notes thereto.
55
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Dionex Corporation:
We have audited the consolidated financial statements of Dionex Corporation and its subsidiaries (collectively, the “Company”) as of June 30, 2007 and 2006, and for each of the three years in the period ended June 30, 2007, management’s assessment of the effectiveness of the Company’s internal control over financial reporting as of June 30, 2007, and the effectiveness of the Company’s internal control over financial reporting as of June 30, 2007, and have issued our reports thereon dated August 29, 2007 (which report on the consolidated financial statements includes an explanatory paragraph relating to the adoption of Statement of Financial Accounting Standards No. 123 (revised 2004),Share-Based Payment). Such reports are included elsewhere in this Annual Report onForm 10-K for the year ended June 30, 2007. Our audits also included the consolidated financial statement schedule of the Company listed in Item 15(a)(2). The consolidated financial statement schedule is the responsibility of the Company’s management. Our responsibility is to express an opinion based on our audits. In our opinion, such consolidated financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
/s/ Deloitte & Touche LLP
San Jose, California
August 29, 2007
56
DIONEX CORPORATION
VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
YEARS ENDED JUNE 30, 2007, 2006 AND 2005
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Charged
| | | | | | | |
| | | Balance
| | | | | | (Credited)
| | | | | | Balance
| |
| | | Beginning of
| | | | | | to Other
| | | | | | End of
| |
| | | Year | | | Additions | | | Accounts(1) | | | Deductions(2) | | | Year | |
| | | (In thousands) | |
| |
| YEAR ENDED JUNE 30, 2007: | | $ | 674 | | | $ | — | | | $ | 41 | | | $ | (105 | ) | | $ | 610 | |
| Allowance for doubtful accounts | | | | | | | | | | | | | | | | | | | | |
| YEAR ENDED JUNE 30, 2006: | | $ | 953 | | | $ | — | | | $ | 37 | | | $ | (316 | ) | | $ | 674 | |
| Allowance for doubtful accounts | | | | | | | | | | | | | | | | | | | | |
| YEAR ENDED JUNE 30, 2005: | | $ | 760 | | | $ | 350 | | | $ | (15 | ) | | $ | (142 | ) | | $ | 953 | |
| Allowance for doubtful accounts | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Effects of exchange rate changes |
| |
| (2) | | Accounts written off, net of recoveries |
57
| | | | | | | | | |
Exhibit
| | | | |
Number | | Description | | Reference |
| |
| | 3 | .1 | | Restated Certificate of Incorporation, filed December 12, 1988 | | | (2) | |
| | 3 | .2 | | Certificate of Amendment of Restated Certificate of Incorporation, filed December 1, 1999 | | | | |
| | 3 | .3 | | Bylaws, as amended on July 29, 2002 | | | (5) | |
| | 3 | .4 | | Amendment to Bylaws, adopted on January 11, 2007 | | | (11) | |
| | 4 | .1 | | Stockholder Rights Agreement dated January 21, 1999, between Dionex Corporation and Bank Boston N.A. | | | (3) | |
| | 10 | .1 | | Medical Care Reimbursement Plan (Exhibit 10.17) | | | (1) | |
| | 10 | .2 | | Credit Agreement dated November 13, 2000 between Wells Fargo Bank and Dionex Corporation (Exhibit 10.15) | | | (4) | |
| | 10 | .3 | | First amendment to Credit Agreement dated November 13, 2000 between Wells Fargo Bank and Dionex Corporation | | | (6) | |
| | 10 | .4 | | Dionex Corporation 2004 Equity Incentive Plan, as amended October 2006 | | | | |
| | 10 | .5 | | Form of Stock Option Agreement for non-employee directors (Exhibit 99.2) | | | (8) | |
| | 10 | .6 | | Form of Stock Option Agreement for other than non-employee directors (Exhibit 99.3) | | | (8) | |
| | 10 | .7 | | Employee Stock Participation Plan (Exhibit 10.13) | | | (7) | |
| | 10 | .8 | | Second amendment to Credit Agreement dated November 13, 2000 between Wells Fargo Bank and Dionex Corporation (Exhibit 10.1) | | | (9) | |
| | 10 | .9 | | Change in Control Severance Benefit Plan (Exhibit 10.15) | | | (10) | |
| | 10 | .10 | | Third amendment to Credit Agreement dated December 1, 2006 between Wells Fargo Bank and Dionex Corporation | | | (12) | |
| | 10 | .11 | | Executive Employment Agreement for Lukas Braunschweiler dated November 20, 2006 | | | (13) | |
| | 21 | .1 | | Subsidiaries of Dionex Corporation | | | | |
| | 23 | .1 | | Consent of Independent Registered Public Accounting Firm | | | | |
| | 24 | .1 | | Power of Attorney (reference is made to the signature page of this report onForm 10-K) | | | | |
| | 31 | .1 | | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | | | |
| | 31 | .2 | | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | | | |
| | 32 | .1 | | Certification of the Chief Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | | | |
| | 32 | .2 | | Certification of the Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | | | |
| | |
| (1) | | Incorporated by reference to the indicated exhibit in Amendment No. 1 of our Registration Statement onForm S-1 filed December 7, 1982. |
| |
| (2) | | Incorporated by reference to the corresponding exhibit in our Annual Report onForm 10-Q filed September 20, 1989. |
| |
| (3) | | Incorporated by reference to the corresponding exhibit in our Quarterly Report onForm 10-Q filed February 16, 1999. |
| |
| (4) | | Incorporated by reference to the indicated exhibit in our Quarterly Report onForm 10-Q filed February 14, 2001. |
| |
| (5) | | Incorporated by reference to the indicated Exhibit 10.17 in our Annual Report onForm 10-K filed August 28, 2002. |
58
| | |
| (6) | | Incorporated by reference to the indicated Exhibit in our Annual Report onForm 10-K filed September 24, 2003. |
| |
| (7) | | Incorporated by reference to the indicated exhibit in our Annual Report onForm 10-K filed September 10, 2004. |
| |
| (8) | | Incorporated by reference to our Registration Statement onForm S-8 filed December 8, 2004. |
| |
| (9) | | Incorporated by reference to the indicated exhibit in our current Report onForm 8-K filed December 22, 2004. |
| |
| (10) | | Incorporated by reference to the indicated exhibit in our Quarterly Report onForm 10-Q filed May 10, 2005. |
| |
| (11) | | Incorporated by reference to the indicated exhibit in ourForm 8-K filed January 17, 2007. |
| |
| (12) | | Incorporated by reference to the indicated exhibit in our Quarterly Report onForm 10-Q filed May 10, 2007. |
| |
| (13) | | Incorporated by reference to the indicated exhibit in ourForm 8-K filed November 21, 2006. |
59