
White Paper On Cancer Theories and Cancer Detection
January 2007
1 THE ANEUPLOIDY THEORY OF CANCER AND BARRIERS TO ACCEPTANCE .................3
1.1 INTRODUCTION ........................................................................................................................................ 3
1.2 OVERCOMING THE BARRIERS: BOVERI'S ANEUPLOIDY THEORY OF CANCER....................................... 6
1.3 METABOLIC CONTROL ANALYSIS SUPPORTS THE ANEUPLOIDY THEORY OF CANCER ........................... 7
1.4 POLITICAL & SOCIOLOGICAL BARRIERS ................................................................................................ 8
2 CURRENT STATE OF CANCER DETECTION...................................................................................9
2.1 PROSTATE CANCER................................................................................................................................ 10
2.2 CERVICAL CANCER................................................................................................................................ 12
3 FAILURE OF CURRENT THEORIES AND THE RISE OF CHROMOSOMAL IMBALANCE
THEORY.............................................................................................................................................................14
3.1 FAILURE OF GENE-MUTATION THEORY................................................................................................ 14
3.2 THE RISE OF CHROMOSOMAL IMBALANCE........................................................................................... 15
3.3 100% CORRELATION BETWEEN CANCER AND CHROMOSOMAL IMBALANCE ...................................... 16
3.4 CANCER THEORY COMPARISONS.......................................................................................................... 16
3.5 CHROMOSOMAL IMBALANCE THE MOST DIRECT, SIMPLE, AND ACCURATE WAY TO DETECT CANCER
17
4 OVERVIEW OF ANUCYTE....................................................................................................................18
4.1 SYSTEM TESTING ................................................................................................................................... 18
5 ANUCYTE SYSTEM CLAIMS ...............................................................................................................19
5.1 MOST ACCURATE CANCER DETECTION ................................................................................................. 19
5.2 OBJECTIVE MEASUREMENT................................................................................................................... 19
5.3 FIRST AND ONLY IN THE WORLD ........................................................................................................... 19
5.4 PROPRIETARY CELL PREPARATION........................................................................................................ 19
6 BASIS FOR CLAIMS ................................................................................................................................20
6.1 SUMMARY .............................................................................................................................................. 20
6.2 BACKGROUND OF CHROMOSOMAL IMBALANCE NATURE OF CANCER ................................................. 20
6.2.1 Boveri's aneuploidy or chromosomal imbalance theory of cancer...........................................21
6.3 DUESBERG AND RASNICK REVIVE BOVERI'S ANEUPLOIDY THEORY OF CANCER ............................... 21
6.4 GENE MUTATION THEORY FAILS TO EXPLAIN CANCER......................................................................... 22
7 ANUCYTE CANCER DETECTION METHODOLOGY...................................................................23
7.1 INTRODUCTION AND BACKGROUND ..................................................................................................... 23
7.2 ANUCYTE TESTING PROCEDURE ........................................................................................................... 25
7.3 A SAMPLE OUTPUT REPORT FROM THE ANUCYTE SYSTEM: ................................................................ 26
7.4 ANUCYTE MOST ACCURATE METHOD OF TESTING FOR CANCER ......................................................... 26
7.5 ANUCYTE'S EFFECTIVENESS AND ACCURACY HAS BEEN DEMONSTRATED ........................................ 27
8 ANUCYTE CLAIMS FULLY SUPPORTED ........................................................................................28
8.1 FIRST IN THE WORLD OF ITS TYPE: ........................................................................................................ 28
8.2 OBJECTIVE MEASUREMENT: .................................................................................................................. 28
8.3 MOST ACCURATE IN THE WORLD: ......................................................................................................... 28
9 REFERENCES............................................................................................................................................28
1 The aneuploidy theory of cancer and barriers to acceptance
1.1 Introduction
Normal human cells have 23 different chromosomes that come in pairs. They yield a total of 46 chromosomes. Such cells are said to be "diploid." Cells found in solid tumors, on the other hand, typically have between 60 to 90 chromosomes (1). Their ploidy is "not good," in other words, and the Greek version of that is "aneuploid." It is a word that you will have a hard time finding in the cancer textbooks.
Recall that the genes (of which there may be 40,000 or so in humans) are strung along the chromosomes, so that each chromosome contains thousands of genes. Any cell with a chromosome number different from 46, or with an abnormal complement of chromosomes that add up to 46, is an aneuploid cell. Thus, aneuploid cells contain an imbalance in the complement of genes and chromosomes compared to the normal or "diploid" cell. This imbalance in the chromosomes leads to a wide variety of problems, one of which is cancer.
Another problem caused by aneuploidy is Down's Syndrome. This results when a baby is born with three copies of chromosome 21 instead of the normal two. Just one extra copy of the smallest chromosome, with its thousand or so normal genes, is sufficient to cause the syndrome (2). Most Down's fetuses are spontaneously aborted. Nonetheless, the imbalance is small enough (47 chromosomes) to permit occasional live births. The level of aneuploidy is therefore far below the threshold of 60-90 chromosomes found in invasive cancer, but it gives these patients a head start toward developing the same cancers that normal people get. Down's Syndrome patients have up to a 30-fold increased risk of leukemia, for example, compared to the general population (3, 4).
There is one important difference between the small aneuploidy found in Down's Syndrome, and the more pronounced aneuploidy of cancer cells. With Down's, the defect occurs in the germ line and so the chromosomal error is present in every cell in the body. But the defect that gives rise to the unbalanced complement of chromosomes in cancer cells is "somatic". That is, it occurs in a particular cell after the body is formed. In the course of life, cells constantly divide by a process called mitosis. When errors in mitosis occur, as they often do, the possibility exists that a daughter cell will be aneuploid.
Aneuploidy destabilizes a cell in much the same way that a dent disrupts the symmetry of a wheel. It leads to ever-greater distortions with each revolution. As aneuploid cells divide, their genomes become increasingly disorganized to the point where most of these cells stop dividing and die. But rarely, and disastrously, an aneuploid cell with the right number and combination of extra chromosomes wins the genetic lottery and keeps right on going. Then it has become a cancer cell.
Cells with a normal number of chromosomes are intrinsically stable and not prone to transformation into cancer. What, therefore, causes normal cells to become aneuploid? That is a hotly contested question. It is known, however, that if radioactive particles strike the nucleus of a cell, chromosomes can be shattered. When that damaged cell then divides by mitosis, an error may arise. Chromosomal imbalance may then result. In short, radiation can cause aneuploidy. And certain chemicals, such as tars, also give rise to aneuploid cells. Tars and radiation sources are known carcinogens. In fact, all carcinogens that have been examined so far do cause aneuploidy.
That is a very convincing argument for the aneuploidy theory of cancer, but in order to understand the controversy one must understand the alternative theory. Everyone has heard of it because it is in the newspapers all the time. It is the gene-mutation theory of cancer. According to this theory, certain genes, when they are mutated, turn a normal cell into a cancer cell. This theory has endured since the 1970s, and more than one Nobel Prize has been awarded to researchers who have made claims about it. One prize-winner was the former director of the National Institutes of Health, Harold Varmus. According to some researchers, the mutation of just one, or perhaps several genes, may be sufficient to transform a normal cell into a cancer cell.
In contrast, chromosomal imbalance disrupts the normal balance and interactions of many thousands of genes, because just one chromosome typically contains several thousand genes. And a cancer cell may have several copies of a given chromosome. For this reason alone, aneuploidy is likely to be far more devastating to the life of a cell than a small handful of gene mutations.
The fundamental difference between the aneuploidy theory and the reigning gene-mutation theory may be put this way. If the whole genome is a biological dictionary, divided into volumes called chromosomes, then the life of a cell is a Shakespearean drama. If one were to misspell a word here and there, in Hamlet for example, such "mutations" would be irrelevant to the vast majority of readers, or theater-goers. A multicellular organism is at least as resistant to "gene mutations" as a Shakespeare play.
On the other hand, without "mutating" a single word, one could transform the script of Hamlet into a legal document, a love letter, a declaration of independence, or more likely gibberish, by simply shifting and shuffling, copying and deleting numerous individual words, sentences and whole paragraphs. That is the literary equivalent of what aneuploidy does. The most efficient means of rewriting a cell's script is the wholesale shifting and shuffling of the genes, which aneuploidy or chromosomal imbalance accomplishes admirably.
Aneuploidy is known to be an efficient mechanism for altering the properties of cells, and it is also conceded that aneuploid cells are found in virtually all solid tumors. Bert Vogelstein of Johns Hopkins University has said that "at least 90 percent of human cancers are aneuploid." The true figure may be 100 percent. For references supporting the claim that cancers are invariably aneuploid see Li et al. 2000 (5).
Nonetheless, the presence of mutations in a handful of genes continues to be viewed as a significant, even a causal factor in carcinogenesis, even though any given mutated gene is found in only a minority of cancers. Cells with mutated genes can indeed be found in cancerous as well as normal cells, but the most likely reason is that they are innocuous. Hence they are readily accommodated during the expansion of barely viable aneuploid cells as they compete for survival with their more viable chromosomally balanced counterparts. The current emphasis in cancer research on the search for mutant genes in a perpetual background of aneuploidy is a classic example of not seeing the forest for the trees.
Thomas Kuhn remarked that the great theoretical advances of Copernicus, Newton, Lavoisier, and Einstein had less to do with definitive experiments than with looking at old data from a new perspective. Sufficient (indeed overwhelming) evidence is already in hand to convict aneuploidy of the crime of cancer and release gene mutations from custody (5-16). Nevertheless, the gene-mutation theorists, when faced with the undeniable evidence that aneuploidy is necessary for cancer, have adopted a fall-back position. They argue that gene mutations must initiate the aneuploidy, (6) or as the Scientific American reported, referring to a researcher in Vogelstein's lab, "[Christoph] Lengauer insists aneuploidy must be a consequence of gene mutations" (7).
There would be no need for him to "insist" if there were proof that gene mutations really do cause cancer. What would gravely weaken the aneuploidy theory would be confirmed cases of diploid cancer (in which the tumor cells have balanced chromosomes), and with the culprit genes found lurking in every cell. That would go a long way toward proving the gene mutation theory. But where has that been demonstrated? It would be a front-page story. The truth is that researchers have not yet produced any convincing examples of diploid cancer.
In fact, the evidence is going the other way. There is a growing list of carcinogens that do not mutate genes at all. In addition, there are no cancer-specific gene mutations. Even tumors of a single organ rarely have uniform genetic alterations. And, in a rebuttal that should be decisive, no genes have yet been isolated from cancers that can transform normal human or animal cells into cancer cells. Furthermore, the latent periods between the application of a carcinogen and the appearance of cancer are exceedingly long, ranging from many months to decades. In contrast, the effects of mutation are instantaneous.
If the medical profession and biotechnology industries were to embrace the aneuploidy theory of cancer, cancer research and the flood of new technologies would at last become biologically and clinically relevant. There are, however, two formidable barriers to the ascendance of the aneuploidy theory of cancer: the first is conceptual; the second is political and sociological.
1.2 Overcoming the Barriers: Boveri's Aneuploidy theory of cancer
The aneuploidy theory was introduced by David von Hansemann in 1890 (8) and first formally stated by Theodor Boveri in 1914 (9). Almost the first thing that researchers noticed when they looked at cancer cells under the microscope was that they had excess chromosomes. Aneuploidy provides a simple and coherent explanation for all the properties of cancer (9-16). But precisely because the aneuploidy theory was proposed so long ago, scientists today are inclined to think (if they know about Boveri at all) that some fundamental flaw in the theory must have been discovered. They also assume that the gene mutation theory of cancer must be superior because it is newer and uses the latest sexy technologies.
Some American researchers, eager to dismiss the aneuploidy theory, ask, "What is the mechanism?" They remind Athel Cornish-Bowden (10) of the obstinate rejection of Alfred Wigener's theory of Continental drift by American geologists
(11) on the grounds that he could offer no mechanism for how the continents moved. In 1914, Boveri offered the first coherent explanation (including a mechanism) of how chromosomal imbalance leads to cancer (9). The developmental consequences of chromosomal imbalance in sea-urchin eggs suggested to him that malignant tumors could be due to an abnormal chromosome constitution originating during cell division.
The only other author with similar ideas was von Hansemann (8). He captured the essence of cancer as, "a process carrying the cell to some entirely new direction-- a direction, moreover, which is not the same in all tumors, nor even constant in the same tumor. . . The [cancer] cell then is one in which, through some unknown agency, a progressive disorganization . . . occurs, which in turn results in . . . a new biologic entity, differing from any cell present at any time in normal [development]." (Translated by Whitman (12)). Hansemann's "unknown agency" is the relentless randomization of the genome caused by aneuploidy.
Boveri extended Hansemann's insight. The essence of Boveri's hypothesis is that cancer results from "a certain abnormal [chromosome] constitution, the way in which it originates having no significance. Each process which brings about this constitution would result in the origin of a malignant tumor" (9). His theory predicts that cancer results from a single cell that has acquired an abnormal chromosome constitution. In other words, he predicted the clonal origin of cancer.
It is well known that a tumor cell has an abnormal metabolism. According to Boveri, "if the individual chromosomes have different qualities, chromosome aberrations will result in deviant metabolic functions. If, therefore, certain chromosomes are missing and others are present in abundance, certain substances will be produced also in abundance, and there will be a deficiency in others." (13)
In Boveri's time X-rays and certain chemicals were known to cause chromosomal imbalance. Boveri said the time interval between the time of the insult and the origin of a tumor may be explained by the assumption that the cancer-causing agent first interferes with the process of cell division, producing an aneuploid cell. In the second step, the aneuploid cell must be stimulated to divide further, producing daughter aneuploid cells. In heavily proliferating tissues, the risk of a tumor is increased.
Boveri points out that a natural consequence of his aneuploidy theory is that the risk of tumors would increase with age since in aging cells the process of cell division is more frequently disturbed (13). (In addition, enough time has elapsed in an older organism for many cell divisions to have occurred.) Boveri even predicted tumors that had the correct number of chromosomes but with an abnormal complement--the so-called pseudodiploid cancers. Boveri's aneuploidy theory of cancer is as valid today as it was in 1914.
1.3 Metabolic control analysis supports the aneuploidy theory of cancer
Dr. David Rasnick, PhD, remembers: "In November, 1996, Peter Duesberg left for the first of many trips to Mannheim to work on aneuploidy as a possible cause of cancer. I stayed at Berkeley and studied the literature on aneuploidy and the consequences of changes in gene dose. One day I came across Charles Epstein's book The consequences of chromosome imbalance: principles, mechanisms, and models (14). When I happened upon a figure extracted from a paper by Henrik Kacser and James Burns, it changed my life. I immediately realized that the reigning gene mutation hypothesis of cancer was almost certainly wrong and that the aneuploidy theory of cancer was almost certainly right."
In 1973 Kacser and Burns (15), and independently Heinrich and Rapoport (16), invented the field of metabolic control analysis. It is a quantifiable means of analyzing changes in a cell, tissue, or organ by taking into consideration the combined activities of all the metabolic elements (all the gene products) that contribute to the phenotype (stable characteristics) of the whole. For systems as complex as a cell, changes in the activities of a few or even scores of specific genes would be buffered by the many thousands of other genes contributing to the overall properties of a cell. There was simply no way for a handful of "oncogenes" or "tumor suppressor" genes to perturb a normal cell sufficiently to turn it into a massively abnormal cell.
At UC Berkeley Duesberg and Rasnick have shown that transforming the robust normal cell into a cancer cell requires massive changes in the number and composition of chromosomes (17). Aneuploidy provides the necessary boost in genetic material leading to cancer. It is entirely independent of gene mutation.
The effect of aneuploidy on cells can be visualized by analogy with an automobile factory, in which each assembly line corresponds to a chromosome. An "aneuploid" assembly line would randomize the output of an automobile factory and produce cars with five wheels, three brakes, two engines, no transmission, etc., and every car would be different from the one before. Most such cars wouldn't function, and would go directly to the junkyard. By chance, however, the aneuploid factory would also produce the rare, bizarre car that worked well enough to appear on the highways and keep right on running when you slammed on the brakes! It would be a menace to the society of normal cars.
In this analogy, the genes correspond to individual workers on the assembly lines. The effect of "mutating" individual workers is much more limited than randomly altering the number and composition of the assembly lines. Workers typically work at a fraction of their capacity. If the output of a few individual workers in an assembly line was "mutated" by sickness, death or vacation, the effects would be buffered by the remaining un-mutated workers upstream and downstream and by the redundant capacity built in to the workforce. The overall output and quality of cars would not noticeably change. By the same token, alterations in a handful of specific genes (18, 19) are insufficient and probably irrelevant to the generation of cancer because their numbers are too few to alter the normal cell.
The attraction of the gene mutation theory of cancer was its promise of simplicity: cancer resulted from a manageable number of specific mutations. A manageable number was the hoped-for key to unlocking the mysteries of cancer and to the taming of an ever growing modern scourge (20). Instead, we find that the seven mutations proposed to cause colon cancer (21) are drowned in an aneuploid sea of nearly 5,000 additional genes in the aneuploid cells of a cancerous colon (22).
Far from providing insights into the nature of cancer, and hence into prevention and more effective treatments, the gene mutation theory is now so burdened with the complexity of its details that it has lost all explanatory power. Analyzing close to 49,000 genes of normal and cancer cells (colon and pancreas), Zhang et al. acknowledged that, "most of the genes could not have been predicted to be differentially expressed in cancers" (22).
Results such as these will eventually kill what Stephen Friend, CEO of Rosetta Inpharmatics in Seattle, calls the "my-favorite-gene approach." He adds: "God, were we stupid!" (23).
1.4 Political & Sociological Barriers
The conceptual barriers to accepting aneuploidy as the cause of cancer are not trivial but they shrink in comparison with the political and sociological obstacles.
US taxpayers have forked over tens of billions of dollars in the war on cancer only to find that after 20 years of battling viruses, "oncogenes", and "tumor suppressor" genes we are losing the war (20). But it is a one-front war with almost no resources devoted to alternative approaches. In spite of a century of evidence implicating aneuploidy as the cause of cancer, a leading researcher guesses that "If you were to poll researchers ... 95 percent would say that the accumulation of mutations [to key genes] causes cancer" (7). If 50 percent of cancer-research funds went towards investigating the role aneuploidy plays in cancer, a poll of researchers would soon show that close to half would say that chromosomal imbalance causes cancer. Scientists, these days, tend to accept or reject a theory depending on whether or not there is funding for it.
With so many careers and reputations dependent on the failed gene mutation theory, researchers cannot afford to question something that has supported them for decades. The Federal Government decides scientific dogma and the research community falls in line. The highly publicized sequencing of the human genome, the commercialization of diagnostic tests for cancer genes (34-36), and the recent hype about Gleevec being "at the forefront of a new wave of cancer treatments [that] differs from other existing chemotherapies because it affects a protein that directly causes cancer" (24) make it even more difficult for researchers to consider the possibility that mutant genes may not cause cancer after all.
It would not help the images of the cancer research establishment and the multibillion dollar biotech industry if it became widely known that an unfunded lab has a preferable explanation of the cause and progress of cancer. If a small group with small funding has rediscovered the cause, why should taxpayers continue to dole out billions of dollars for work on mutant genes that has never panned out? And what would happen to the biotech industry that has bet so heavily on cancer diagnostics and therapeutics based entirely on the gene mutation theory?
Max Planck said that, "A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die, and a new generation grows up that is familiar with it" (25).
The failures of the cancer research establishment of the past 30 years steadily accumulate. The scientific dogma decreed by the Federal Government shows itself demonstrably incorrect with each passing year and each wasted dollar. We are on the forefront of a new vanguard of cancer theory and approaches that gains more acknowledgement and acceptance with each passing moment.
2 Current State of Cancer Detection
Cancer detection in the clinical lab (where analysis is performed as a part of patient health care rather than a research lab) currently suffers from a general lack of automated diagnosis of solid cancers using "state-of-the-art" technologies. The sheer volume of clinical tests requires an entirely different strategy than that of the research setting. For example, a test like the Pap smear for cervical cancer is performed over 55 million times each year in the USA alone. In addition, the lack of accuracy is distressingly high in evaluating Pap smears. Management's research shows false negative rates are typically 20-25% ,(1,2) but can run as high as 50% (3) The extent of false positive results is not generally reported but can run as high as 20%.
The question is, why haven't new ideas in cancer detection moved into the clinical market? In our opinion the chief roadblock is the prevailing, but terminally and demonstrably flawed, oncogene/tumor suppressor theory of cancer itself.(4-8). Essentially all current thinking about the genetics of cancer is contained within the gene mutation hypothesis, which contends that mutation causes cancer by activating certain cellular genes, converting them to dominant cancer genes (oncogenes), and inactivating other, tumor suppressor, genes (5). The gene mutation hypothesis, which once promised a relatively simple entry into the massively disregulated genetic programs of the cancer cell, has become so burdened with the complexity of its details that it has become an empirical exercise devoid of theoretical and explanatory power (see Table I below).
Key Cancer diagnostics
We design our cancer detection products to take advantage of the aneuploidy theory of cancer (13, 43, 55) instead of the unproductive oncogene/tumor suppressor-gene theory of cancer (4-8, 49-51).
We can offer clinical laboratories a fully automated Fluorescence In situ Hybridization (FISH) microscopic analysis for the quantitative measurement of aneuploidy (chromosomal imbalance) in pap smears, prostate cancer biopsies, and ultimately for all solid cancers. The quantitative measurement of aneuploidy should substantially reduce the false-negative and false-positive results of conventional methods and still allow the pathologist the flexibility to review individual slides as needed.
While the FDA has approved some karyotyping (chromosome counting) software packages and several fluorescent DNA probe kits for clinical use, the authors are not aware of any provider of a completely integrated and automated system employing the FISH technology to detect cancer based on aneuploidy. The quantitative output of our automated FISH analysis provides the pathologist and physician with information upon which to more reliably classify the various types and severity of cervical and prostate cancers. This is important since the choice of therapy is determined by the classification and stage of malignancy (62, 65).
2.1 Prostate Cancer
In spite of the 1993 consensus review (37) advocating the clinical utility of DNA analysis in prostate cancer, pathologists continue to rely on cell morphology and do not perform chromosomal analysis in deciding if cancer is present in prostate biopsies primarily because there is a lack of appropriate instrumentation. Solid cancers produce inadequate numbers of mitoses for
conventional chromosomal characterization, and needle biopsies of prostate tissues do not provide a large enough number of cells for flow cytometric analysis. Automated flow cytometry is a well established and broadly available technology that can be used to characterize the gross genetic and chromosomal differences between populations of normal and cancer cells. However, flow cytometry lacks the sensitivity of FISH to be able to characterize the abnormalities of individual chromosomes in specific cells (72).
The AnuCyte Cancer Detection System uses FISH analysis technology to eliminate these problems because it does not require the presence of mitoses for chromosomal analysis, and can analyze all cells in a biopsy. FISH analysis also requires much smaller sample size than flow cytometry and allows a pathologist to conveniently go back and inspect individual prostate specimens and specific cells, which is not possible with flow cytometry. In short, the prostate market suffers from an absence of an automated FISH system and the AnuCyte System is the first for this marketplace.
Although histopathologic criteria are important in detecting the presence of prostatic carcinomas and in determining probable disease course, significant limitations still exist in the ability of current histologic markers to predict the course for individual patients. (From the Consensus Review (37) of the clinical utility of measuring chromosome imbalance in prostate cancer.)
The majority of prostate tumors (intermediate grade), cannot be determined by current clinical laboratory methods. The measurement of aneuploidy provides the only clinically useful prognostic information for patients with these intermediate grade tumors (37, 73) (Table I). In prostate cancer, which is heterogeneous, multifocal, and intermixed with benign cells, FISH can accurately detect small changes in the number of chromosomes. In addition, because FISH can be performed on tissue sections, it has greater clinical utility as a prognostic marker tool than flow cytometry. Using FISH, Henke et al. (66) report that numerical chromosome alterations in prostate cancer coincide with aggressive tumor behavior. Two recent reports further illustrate the value of FISH in diagnosing prostate cancer. FISH analysis of chromosomes 7, 8, 11, and 12 was performed by Takahashi et al. on 50 radical prostectomy specimens obtained by 18-gauge needle biopsy (67). Their analysis using FISH was more sensitive than traditional flow cytometry analysis in detecting ploidy anomalies, and gains of chromosomes 7 and 8 were associated with higher-grade tumors.
TABLE I
Prostate Cancer Diagnosis
Existing Methods Our Chromosomal Imbalance
Approach
- * histologic markers prone to subjective * chromosome number is quantitative grading and difficult to automate and our system is automated
- * intermediate grade tumors are
- indeterminate * intermediate grade tumors readily characterized
- * cannot predict clinical course of
- disease * diploid (normal number of chromosomes) tumors have favorable outcome; aneuploid tumors show poor outcome irrespective of stage or therapy
- * flow cytometry requires large samples
- * small biopsy samples more than
- * flow cytometry has low sensitivity adequate
- * image analysis sensitive to fractions of
- * cells are lost at end of flow cytometric a chromosome analysis
* cells and images are permanent records
A study by Macoska et al. compared benign and matched malignant specimens for chromosome 8 abnormalities in 10 prostate cancer patients (68). The authors concluded that FISH was more sensitive than more traditional techniques in detecting chromosome 8 abnormalities. "[T]hese studies suggest great promise for the use of FISH in elucidating genetic alterations in prostate cancer and in developing FISH-based, clinically, useful prognostic markers." (69) Indeed, a consensus report (37) concluded that, "DNA content measurement by image cytometry [FISH] provides a significant predictor of disease course or survival. ...[I]mage cytometry may identify populations of cells or nuclei that flow cytometry studies do not report. Additionally, image cytometry may provide the only means to measure DNA ploidy of small samples, particularly needle biopsies containing small areas of tumor."
2.2 Cervical Cancer
Four to nine percent of Pap smears (2-5 million) are classified as ASCUS, which stands for Atypical Squamous Cells of Undetermined Significance. At present, clinical laboratories are not able to properly characterize ASCUS slides. To be on the "safe side," many ASCUS patients are hospitalized and treated as if they were cancer patients. However, the vast majority of ASCUS cases are cancer-free since there are only 16,000 confirmed new cervical cancers annually but millions of ASCUS cases. The elimination of ASCUS (indeterminate) cases represents potential annual savings of billions of dollars in unnecessary hospitalizations and treatments, not to mention saving millions of women the trauma of being treated as cancer patients. Table II compares our approach to existing methods of cervical cancer diagnosis.
TABLE II
Cervical Cancer Diagnosis
Existing Methods
Our chromosomal Imbalance Approach
- * 50 year-old Pap smear technique * 100 year-old correlation between chromosomal imbalance and cancer
- * 20-25% false negative rate * chromosomal imbalance is a better predictive value than histolopathologic characteristics
- * histologic markers prone to subjective
- grading and difficult to automate * chromosome number is quantitative and our system is automated
- * 2-5 million ASCUS (indeterminate) Pap smears
- * chromosomal imbalance is a
- * flow cytometry has low sensitivity quantitative predictor of clinical outcome
- * cells are lost at end of flow cytometric * image analysis sensitive to fractions of analysis a chromosome
* cells and images are permanent records
During the last 50 years in the USA, the use of screening programs based on the Papanicolaou (Pap) smear and pelvic examination has led to a steep decline in incidence and deaths from cervical cancer (64). However, the methods of specimen acquisition, preparation, and evaluation of the Pap smear have changed little since its introduction in the 1940s. Although it is highly effective in screening for pre-invasive lesions of the cervix, a single Pap smear has a false-negative rate estimated to be 20-25% (1, 2), but can run as high as 50% (3).
One-half of the false negatives are due to inadequate sampling, and the other half are attributed to a failure to identify the abnormal cells or to interpret them accurately (62, 64, 70).
Manual screening of Pap smears is very labor intensive and demands that the cytotechnologist be capable of high levels of concentration for extended periods. In addition, cytotechnologists may feel pressure to examine as many slides as they can legally despite recent regulatory changes attributable to the Clinical Laboratory Improvement Act of 1988 (CLIA '88). A slide may have only a few abnormal cells, which could be easily overlooked by a fatigued technologist at the end of a busy workday. Recent reports suggest that some of the false negative results are caused by these screening errors. An automated screening machine, such as our AnuCyte system, would not be subject to fatigue and, therefore, could reduce this type of error (64, 70).
Failure to recognize abnormal cells is another possible source of error. A few states have instituted proficiency testing programs in an effort to minimize this type of error. A hypothetical ideal automated screening machine could be programmed so that it would recognize all types of potentially abnormal cells. Of course, no machine performs ideally in practice. Several companies are developing automated screening machines in an attempt to reduce the false negative rate of Pap smears without having an unreasonably high rate of false positives. The systems currently under development generally automate the 50year old Pap smear technology without incorporating the advanced fluorescent probe technologies of the 1990s. A couple of firms include the additional feature of automating the preparation of conventional Pap smear slides.
In the fall of 1995, the Food and Drug Administration (FDA) approved two automated instruments for re-screening smears evaluated as negative on the initial screen. Data from the trials suggest that the new instruments could reduce the rate of false-negative smears. However, neither the efficacy in routine practice nor the cost-benefit of these devices has been determined.
None of the supposed 'advances' in screening technology use chromosomal imbalance as the sole or primary means of detecting cancer. The failure to scan the nucleus for chromosomal imbalance (anueploidy) compromises and reduces any cancer detection system's ideal accuracy rate (100%).
Aneuploidy is the most common chromosomal abnormality observed in cervical and ovarian cancers (46, 71). According to a number of authorities on cancer of the cervix, "[Aneu]ploidy appears to be a good predictor of biologic behavior and may have better predictive value than histopathologic characteristics judged by the eye." (72) The same authorities are critical of flow cytometry since it is not as sensitive as microscopic image analysis for the detection of cervical cancer.
3 Failure of Current Theories and the Rise of Chromosomal Imbalance Theory
The current popular theories of cancer fail completely in their ability to explain and predict cancer. After 30 years of research and billions upon billions of dollars spent, these cancer theories have yielded precisely zero results.
3.1 Failure of Gene-Mutation theory
The gene mutation hypothesis is problematic in a number of ways:
1) It cannot account for the fact that aneuploidy (abnormal number of chromosomes) is "the rule rather than the exception" (9) in cancer, since it predicts the occurrence of diploid (normal number of chromosomes) tumors. In fact, except for the rare cancers produced in the laboratory (10), diploidy does not seem to occur in human solid tumors (11). After a decade of searching we are not aware of any example of any type of cancer with balanced chromosomes.
2) The gene mutation hypothesis has yet to offer experimental proof of malignant transformation of a normal diploid cell by one or a combination of cellular oncogenes, or 3) to explain the acquisition of the many new functional and structural hallmarks of the cancer cell, such as invasiveness, dedifferentiation, altered morphology and genetic instability (despite essentially normal mutation rates), since most mutations are either silent or lead to loss of function.
4) The oncogene hypothesis cannot explain the growing list of non-mutating carcinogens like asbestos, aromatic hydrocarbons, mineral oil, or mitotic spindle blockers such as colcemid. 5) Nor can gene mutation explain the almost 1000-fold increase in cancer risk with age. As most (if not all) suspected oncogenes are perfectly heritable, cancer should be a disease of youth if tumor progression required the gradual accumulation of mutations.
The mounting evidence against the mutation theory of cancer explains why specific cancer genes have not resulted in clinical diagnostic markers of cancer and targets of therapeutic intervention. We, on the other hand, have chosen a different strategy of cancer diagnosis. We have considered a hundred years of scientific and medical observation, looking for the most characteristic differences between cancer cells and normal tissues.
3.2 The Rise of Chromosomal Imbalance
In 1890 David von Hansemann first described abnormal chromatin content and asymmetric mitoses in cancer cells (12). In 1914, Theodor Boveri proposed that cancer was caused by chromosomal imbalance (aneuploidy ) (13). There have been literally thousands of publications consistent with Boveri's historic proposal that aneuploidy causes/is cancer (11, 14-48). However, over the last 25 years the gene mutation hypothesis has become so dominant that aneuploidy, still considered a viable candidate for cancer etiology by the editors of the Journal of the National Cancer Institute in 1966 (16), is today not even listed in the indexes of the most important molecular biology texts (49-51), and this is in spite of the fact that numeric chromosome imbalance is the most prominent genetic change in essentially all of over 20,000 solid tumors that have been examined to date (11, 52).
While none of the "oncogenes" is a general diagnostic for cancer (not even a specific type of cancer), aneuploidy is always present in solid cancers (12-17, 28, 30, 31, 34-37, 52-54). The mass of data accumulating on cancer "demonstrates the more general principle that some increase in chromosome number (most often to a mode centered in the triploid or hypotetraploid range) is associated with poorer prognosis, atypia, and other measures of tumor progression." (31)
3.3 100% Correlation between cancer and chromosomal imbalance
The scientific team behind AnuCyte have recently provided extremely strong experimental support and a firm theoretical foundation for the chromosomal imbalance theory of cancer. Peter Duesberg et al. have shown that aneuploidy is correlated 100% with chemical transformation of Chinese hamster cells using carcinogens that do not cause mutations (43). The fact that these non-mutagenic carcinogens always lead to aneuploid cells at the earliest stages of transformation is strong evidence that aneuploidy causes/is cancer.
3.4 Cancer Theory Comparisons
David Rasnick and Peter Duesberg have adapted the method of metabolic control analysis to investigate the aneuploidy hypothesis of cancer (55). The results show that transforming normal cells into robust cancer requires a 2-fold increase in the expression of thousands of normal genes. The massive change in gene dose produces abnormal changes in the physiology and metabolism of cells and tissues. Aneuploidy explains virtually all of the gross biochemical abnormalities of cancer cells (including increased cellular size, the appearance of numerous membrane-bound tumor-associated antigens and the high levels of secreted proteins that are responsible for invasiveness) as the natural consequence of aneuploidy. The well known genetic instability of cancer cells is due to the perpetual regrouping of the genome following the disruption of nuclear symmetry by aneuploidy. Aneuploid cells are less robust than normal cells. This result may be the basis for the age dependence of most cancers and the spontaneous remission of some. Finally, the results show that mutated genes are neither necessary nor sufficient to produce the cancer phenotype in wild-type organisms. Table III compares the explanatory powers of the gene mutation and aneuploidy theories of cancer.
TABLE III
Competing Hypotheses of Cancer
Properties of Cancer Explained Gene Mutation 1 Aneuploidy 2
- * large size of cancer cells no yes
- * numerous new membrane cancer antigens no yes
- * high levels of cancer secreted proteins no yes
- * invasiveness no yes
- * genetic instability of cancer cells no yes
- * non-mutagenic carcinogens no yes
- * 1000-fold increased cancer risk with age no yes
- * aneuploidy in all of more than 20,000 human cancers catalogued no yes
- * 60-90 chromosomes in cancer cells (normal cells have 46 chromosomes)
no yes
1 Cooper, G. M. Oncogenes. Boston: Jones and Bartlett Publishers, 1990; Bishop,
J. M. The molecular genetics of cancer, Science. 235: 305-311, 1987; Weiss, R.,
Teich, N., Varmus, H., and Coffin, J. Molecular Biology of RNA Tumor Viruses.
Plainview, NY: Cold Spring Harbor Lab. Press, 1985; Varmus, H. E. The
Molecular Genetics of Cellular Oncogenes, Annu. Rev. Genet. 18: 533-612,
1984; Huebner, R. J. and Todaro, G. Oncogenes of RNA tumor viruses as
determinants of cancer, Proceedings of the National Academy of Sciences (USA).
64: 1087-1094, 1969.
2 Li, R., Yerganian, G., Duesberg, P., Kraemer, A., Willer, A., Rausch, C., and
Hehlmann, R. Aneuploidy 100% correlated with chemical transformation of
Chinese hamster cells, Proceedings of the National Academy of Sciences (USA).
94: 14506-14511, 1997; Rasnick, D. and Duesberg, P. H. The aneuploidy theory
of cancer and the analysis of phenotypes, submitted to Proceedings of the
National Academy of Sciences (USA), 1998.
3.5 Chromosomal Imbalance the most direct, simple, and accurate way to detect cancer
The measurement of aneuploidy is a simple (56-60) and robust (43, 55) way to diagnose/detect cancer, and provides an excellent market entry opportunity for an affordable state-of-the-art diagnostic test. Using chromosomal imbalance to diagnose cancer is so generally applicable and reliable that we expect it will become the gold standard of the industry worldwide.
In summary, the hundred-year-long correlation between chromosomal imbalance (aneuploidy) and all types of cancer (12-17, 28, 30, 31, 34-37, 52-54) provides a sound foundation upon which to detect cancer and offers an important opportunity for the company to pioneer and dominate an unserved niche of cancer diagnosis by:
1) analyzing whole chromosomes for chromosomal imbalance rather than the individual genes thought to cause cancer, 2) using a highly automated instrument operated through our proprietary software rather than human operators and manual testing,
3) successfully detecting the broad spectrum of solid cancers (the most lethal of cancers) since chromosomal imbalance is associated with them all,
4) diagnosing the cancer status of tumors that are currently impossible to characterize,
5) focusing on the market of standardized high volume tests, such as the Pap smear, carried out by clinical diagnostics laboratories rather than specializing as the current closest competitors do (see Competition) in the very different low volume FISH market for research laboratories.
4 Overview of AnuCyte
In the last several years, scientific breakthroughs in DNA probe technology, advancements in detection instrumentation, and inexpensive computing power have made it possible to construct an automated system for the routine detection of aneuploidy (chromosomal imbalance) in real-world human specimens. AnuCyte is the first commercial high-throughput image cytometer, to quantify chromosomal imbalance for the purpose of detecting all solid cancers. AnuCyte will enable clinical laboratories to test millions of patient specimens rapidly and accurately for all types of cancer.
AnuCyte is not an add-on feature of existing commercial microscopes. It is constructed for the specific purpose of diagnosing cancer in human specimens, thus providing unparalleled sensitivity and specificity. Because our system is assembled from standard components, it is simple to manufacture and maintain. AnuCyte features: 1) a proprietary method of sample preparation, 2) an automated, high-throughput image cytometer using proprietary control software, 3) proprietary software that analyzes aneuploid cells from the digital fluorescence signals attached to chromosomes, and 4) a printed report containing: (a) patient and physician information, (b) table of normal and aneuploid cells present, (c) histogram for quick inspection of results, (d) images of normal and aneuploid cells that are representative of the sample.
As discussed below, measuring chromosomal imbalance is the absolute method of distinguishing cancerous and precancerous cells from normal. Therefore, Management believes the automated, high-throughput analysis of chromosomal imbalance will necessarily increase accuracy, result in lower cost per sample, and increase throughput by at least ten-fold compared to manual inspection.
4.1 System Testing
In collaboration with the Cleveland Clinic and Texas Southwestern University, AnuCyte has successfully detected cancerous and precancerous aneuploid cells in hundreds of cervical samples and scores of fine needle aspirates from breast tumors.
The system worked as anticipated and as designed. It demonstrated its ability to accurately detect cancer using automated measurement of chromosomal imbalance within the nuclei of cells.
5 AnuCyte system claims
Based upon a decade of scientific research and confirmation of the science with device testing we make the following claims regarding the AnuCyte Cancer Detection System
5.1 Most accurate cancer detection
The AnuCyte system is the single most accurate method in the world for
detecting cancer in any tissue samples because it provides an automated
method for detecting the presence or absence of advanced chromosomal
imbalance called aneuploidy. Research (67, 70, 26) has shown that
aneuploidy is a more accurate predictor of cancer than
cytological/histological analysis or genetic marker-based diagnostics, the
only other methods in existence today.
5.2 Objective Measurement
The AnuCyte system delivers an objective measurement of the presence or
absence of cancer in any tissue sample because it employs an objective
criterion for detecting cancer--the presence of aneuploidy.
5.3 First and only in the world
The AnuCyte system is the first and only system in the world that
determines the presence or absence of cancer by solely measuring the
presence or absence of aneuploidy in an automated manner. No other
system uses the automated detection of advanced aneuploidy (or its
absence) as the sole determining factor for labeling any cell sample
'cancerous' (or non-cancerous) for all types of cancer.
5.4 Proprietary cell preparation
The AnuCyte system utilizes a proprietary preparation method for samples that allows a clear view of the cell nucleus. Difficulty in seeing the nucleus introduces errors in conventional cancer diagnosis. The AnuCyte system eliminates this source of error. This technique allows for the clearest view of the nucleus and the ability to obtain the most accurate assessment of the cell's interior and the presence or absence of chromosomal imbalance. AnuCyte, therefore, eliminates the sources of error inherent in many tissue types, in particular cervical samples.
6 Basis for claims
6.1 Summary
The central and most important justification is the following:
The progression of a cell to an advanced state of chromosomal imbalance (known as aneuploidy) is in fact cancer.
Thus, the detection of advanced chromosomal imbalance in a cell's nucleus is identical with the detection of cancer since they are one and the same.
Cancer and advanced aneuploidy are synonymous and are different ways of describing the exact same cell state. Therefore, cancer (all types of cancer) is an advanced state of chromosomal imbalance (aneuploidy). Testing for this advanced state of chromosomal imbalance is synonymous with testing for cancer.
This statement succinctly encapsulates and explains everything that is known about all types of cancer. Aneuploidy is responsible for all the characteristics of cancer (17, 27-33), including the abnormal cellular size and appearance of cancer cells, the production of tumor-associated antigens, as well as the high levels of cell-bound and secreted proteins responsible for invasiveness and metastasis. Aneuploidy is the self-perpetuating source of chromosomal instability, which is the hallmark of cancer cells. Aneuploidy also explains the finite lifetime of normal cells in culture, the time course of the appearance of papillomas and carcinomas in carcinogen-treated animals, and the age-dependence of human cancers. Finally, aneuploidy theory explains the absence of immune surveillance protecting against cancer and the failure of chemotherapy. This correct explanation of cancer was first formally published in 1914 by Theodor Boveri.
Since 1996, professor Peter Duesberg and David Rasnick investigated Boveri's aneuploidy theory of cancer and have published their extensive theoretical and experimental evidence establishing the fact that Boveri was right: the initiation and progression of all types of cancer is simply the initiation and progression of aneuploidy.
Based on this correct explanation and understanding of cancer, Duesberg and Rasnick created the AnuCyte system, to detect and quantify aneuploidy in order to detect cancerous and pre-cancerous samples from any tissue specimen. An equally important benefit of this approach is that the absence of aneuploid cells guarantees the absence of cancer in that sample.
6.2 Background of chromosomal imbalance nature of cancer
6.2.1 Boveri's aneuploidy or chromosomal imbalance theory of cancer
The aneuploidy theory was introduced by David von Hansemann in 1890 (8) and first formally stated by Theodor Boveri in 1914 (9). Almost the first thing that researchers noticed when they looked at cancer cells under the microscope was that they had excess chromosomes. The extra chromosomes are responsible for the dramatically larger nuclei that pathologists use to diagnosis cancer cells. In other words, pathologists have always been using the presence of aneuploid nuclei to diagnose cancer but were not aware of this connection.
As stated above, aneuploidy provides a simple and coherent explanation for all the properties of cancer (17, 27-33). The essence of Boveri's theory is that cancer results from a certain abnormal chromosome constitution, the way in which it originates having no significance. Each process which brings about this constitution would result in the origin of a malignant tumor (9). His theory predicts that cancer results from a single cell that has acquired an abnormal chromosome constitution. In other words, he predicted the clonal origin of cancer.
It is well known that a tumor cell has an abnormal metabolism. According to Boveri, "if the individual chromosomes have different qualities, chromosome aberrations will result in deviant metabolic functions. If, therefore, certain chromosomes are missing and others are present in abundance, certain substances will be produced also in abundance, and there will be a deficiency in others" (13).
In Boveri's time, X-rays and certain chemicals were known to cause chromosomal imbalance. Boveri said the interval between the time of the initiation of aneuploidy and the origin of a tumor may be explained by the assumption that the cancer-causing agent first interferes with the process of cell division, producing an aneuploid cell. In the second step, the aneuploid cell must be stimulated to divide further, producing daughter aneuploid cells. In heavily proliferating tissues, the risk of a tumor is increased.
Boveri points out that a natural consequence of his aneuploidy theory is that the risk of tumors would increase with age since in aging cells the process of cell division is more frequently disturbed (13). (In addition, enough time has elapsed in an older organism for many cell divisions to have occurred.) Boveri even predicted tumors that had the correct number of chromosomes but with an abnormal complement--the so-called pseudodiploid cancers.
Boveri's aneuploidy theory of cancer is as valid today as it was in 1914.
6.3 Duesberg and Rasnick revive Boveri's aneuploidy theory of cancer
At UC Berkeley, Duesberg and Rasnick showed that transforming the robust normal cell into a cancer cell requires massive changes in the number and composition of chromosomes (17). Aneuploidy provides the necessary boost in genetic material leading to cancer. It is entirely independent of gene mutation.
The effect of aneuploidy on cells can be visualized by analogy with an automobile factory, in which each assembly line corresponds to a chromosome. An "aneuploid" assembly line would randomize the output of an automobile factory and produce cars with five wheels, three brakes, two engines, no transmission, etc., and every car would be different from the one before. Most such cars wouldn't function, and would go directly to the junkyard. By chance, however, the aneuploid factory would also produce the rare, bizarre car that worked well enough to appear on the highways and keep right on running when you slammed on the brakes! It would be a menace to the society of normal cars.
In this analogy, the genes correspond to individual workers on the assembly lines. The effect of "mutating" individual workers is much more limited than randomly altering the number and composition of the assembly lines. Workers typically work at a fraction of their capacity. If the output of a few individual workers in an assembly line was "mutated" by sickness, death or vacation, the effects would be buffered by the remaining un-mutated workers upstream and downstream and by the redundant capacity built in to the workforce. The overall output and quality of cars would not noticeably change. By the same token, alterations in a handful of specific genes (18, 19) are insufficient and probably irrelevant to the generation of cancer because their numbers are too few to alter the normal cell.
6.4 Gene mutation theory fails to explain cancer
The fundamental difference between the aneuploidy theory and the reigning gene-mutation theory may be put this way. Gene mutations are the smallest possible modification to the information stored in genetic material. Anueploidy is the shifting of very large components of this information. The make the changes in a cell to create the behavior found in cancer, one needs to change massive components of the machine, not change a few screws here and there.
The most efficient means of rewriting a cell's script is the wholesale shifting and shuffling of the genes, which aneuploidy or chromosomal imbalance accomplishes admirably.
Aneuploidy is known to be an efficient mechanism for altering the properties of cells, and it is also conceded that aneuploid cells are found in virtually all solid tumors. Bert Vogelstein of Johns Hopkins University has said that "at least 90 percent of human cancers are aneuploid." The true figure is 100 percent. For references supporting the claim that cancers are invariably aneuploid see Li et al. 2000 (5).
Nonetheless, the presence of mutations in a handful of genes continues to be viewed as a significant, even a causal factor in carcinogenesis, even though any given mutated gene is found in only a minority of cancers. Cells with mutated genes can indeed be found in cancerous as well as normal cells, but the most likely reason is that they are innocuous. Hence they are readily accommodated during the expansion of barely viable aneuploid cells as they compete for survival with their more viable chromosomally balanced counterparts. The current emphasis in cancer research on the search for mutant genes in a perpetual background of aneuploidy is a classic example of not seeing the forest for the trees.
The evidence against the gene mutation theory of cancer continues to mount. There is a growing list of carcinogens that do not mutate genes at all. In addition, there are no cancer-specific gene mutations. Even tumors of a single organ rarely have uniform genetic alterations. And, in a rebuttal that should be decisive, no genes have yet been isolated from cancers that can transform normal human or animal cells into cancer cells. Furthermore, the latent periods between the application of a carcinogen and the appearance of cancer are exceedingly long, ranging from many months to decades. In contrast, the effects of mutation are instantaneous.
The attraction of the gene mutation theory of cancer was its promise of simplicity: cancer resulted from a manageable number of specific mutations. A manageable number was the hoped-for key to unlocking the mysteries of cancer and to the taming of an ever growing modern scourge (20). Instead, we find that the seven mutations proposed to cause colon cancer (21) are drowned in an aneuploid sea of nearly 5,000 additional genes in the aneuploid cells of a cancerous colon (22).
Far from providing insights into the nature of cancer leading to better prevention and more effective treatments, the gene mutation theory is now so burdened with the complexity of its details that it has lost all explanatory power. Analyzing close to 49,000 genes of normal and cancer cells (colon and pancreas), Zhang et al. acknowledged that, "most of the genes could not have been predicted to be differentially expressed in cancers" (22).
7 AnuCyte cancer detection methodology
The Automated AnuCyte system provides a fast, accurate and unique detection and quantification of aneuploid cells present in any human tissue sample.
7.1 Introduction and Background
DNA probe technology applied to the clinical diagnosis of solid cancers represents a large untapped market worth hundreds of millions of dollars. In contrast to the current (and so far unproductive) approach of focusing on specific genes for each of the hundreds of different cancers, AnuCyte provides the first automated microscope system that uses Fluorescence In Situ Hybridization (FISH) to clinically diagnose the broad spectrum of solid cancers by measuring aneuploidy (abnormal number of chromosomes).
Cancer detection in clinical labs (where analysis is performed as a part of patient health care rather than a research lab) currently suffers from a general lack of automated diagnosis of solid cancers using "state-of-the-art" technologies. The research community, on the other hand, is addressed well through the offering of a large and growing number of different fluorescent DNA probes provided by Vysis, Oncor, and others for the study of cancer and carcinogensis. However, the needs of the clinical diagnostic laboratories are very different. The sheer volume of clinical tests requires an entirely different strategy than that of the research setting. For example, a test like the Pap smear for cervical cancer is performed over 55 million times each year in the USA alone. In addition, the lack of accuracy is distressingly high in evaluating Pap smears. False negative rates are typically 20-25% (34, 35), but can run as high as 50% (36). The extent of false positive results is not generally reported but can run as high as 20%.
The question is, why haven't the DNA probe technologies of the 1990s moved dramatically into the clinical market? In our opinion the chief problem with current strategies is that they are trying to be too specific in seeking unique oncogene/tumor suppressor gene markers for specific cancers (37-41). Essentially all current thinking about the genetics of cancer is contained within the gene mutation hypothesis, which contends that mutation causes cancer by activating certain cellular genes, converting them to dominant cancer genes (oncogenes), and inactivating other, tumor suppressor, genes (38). The gene mutation hypothesis, which once promised a relatively simple entry into the massively disregulated genetic programs of the cancer cell, has become so burdened with the complexity of its details that it has become very difficult to link specific genetic markers with specific cancers.
Insight Medical Group, on the other hand, has chosen a different strategy of cancer detection. We have considered a hundred years of scientific and medical observation, looking for the most characteristic differences between cancer cells and normal tissues.
In 1890 David Hansemann first described abnormal chromatin content and asymmetric mitoses in cancer cells (8). In 1914, Theodor Boveri proposed that cancer was caused by chromosomal imbalance (aneuploidy) (9). There have been literally thousands of publications consistent with Boveri's historic proposal that aneuploidy causes/is cancer (1, 14, 22, 31, 42-74).
A wealth of scientific research demonstrates that while none of the "oncogenes" is a general diagnostic for cancer, aneuploidy is always present in solid cancers ((1, 8, 9, 42-45, 55, 57, 58, 60-62, 75-77). The mass of data accumulating on cancer "demonstrates the more general principle that some increase in chromosome number (most often to a mode centered in the triploid or hypotetraploid range) is associated with poorer prognosis, atypia, and other measures of tumor progression." (58)
The measurement of aneuploidy is a simple (78-82) and robust (17, 31, 74) way to diagnose cancer, and provides an excellent market entry opportunity for an affordable state-of-the-art diagnostic test that is doable now. Using aneuploidy to diagnose cancer is so general and reliable that we expect it will become the gold standard of the industry.
In summary, the hundred-year-long correlation between chromosomal imbalance (aneuploidy) and all types of cancer (1, 8, 9, 42-45, 55, 57, 58, 60-62, 75-77) provides a sound foundation upon which to develop a cancer detection/diagnostic test and offers an important opportunity for the anucyte System to pioneer and dominate an unserved niche of cancer diagnosis by:
1) analyzing whole chromosomes for aneuploidy rather than the individual genes thought to cause cancer,
2) using a highly automated instrument operated through proprietary software rather than manual testing,
3) successfully detecting/diagnosing the broad spectrum of solid cancers (the most lethal of cancers) since aneuploidy is associated with them all,
4) detecting/diagnosing the cancer status of tumors that are currently impossible to characterize in the clinical lab,
5) focusing on the market of standardized high volume tests, such as the Pap smear, carried out by clinical diagnostics laboratories rather than specializing as the current closest competitors do in the very different low volume FISH market for research laboratories.
Since no current company offers an automated system for the analysis of aneuploidy (chromosomal imbalance) in solid cancers, we believe that our entry should be of significant use to clinical laboratories in the diagnosis of cancers of all types.
7.2 AnuCyte testing procedure
The process is as follows:
The cells of a patient's sample are disaggregated, suspended in alcohol/water and applied to microscope slides using standard methods. Individual chromosomes are labeled with commercially available DNA probes using standard methods of fluorescence in situ hybridization (FISH). The automated microscope, using a specially adapted digital imager and related imaging software, locates the nuclei of the cells that have been stained with a blue fluorescence dye. The automated microscope then counts the number of chromosomes labeled with the red and green DNA probes. Normal, non-cancerous cells have exactly 2 copies of each chromosome for a total of exactly 46 chromosomes. Cancer and precancerous cells will have abnormal numbers of chromosomes that do not have the normal balance of exactly 46 chromosomes. The number of aneuploid cells and the extent of aneuploidy in each cell are indicative of the severity of cancer or precancer. The current version of the AnuCyte automated system analyzes a patient's slide in under 10 minutes. The planned next generation system employing a new high-throughput slide preparation system will allow analysis in under 1 minute.
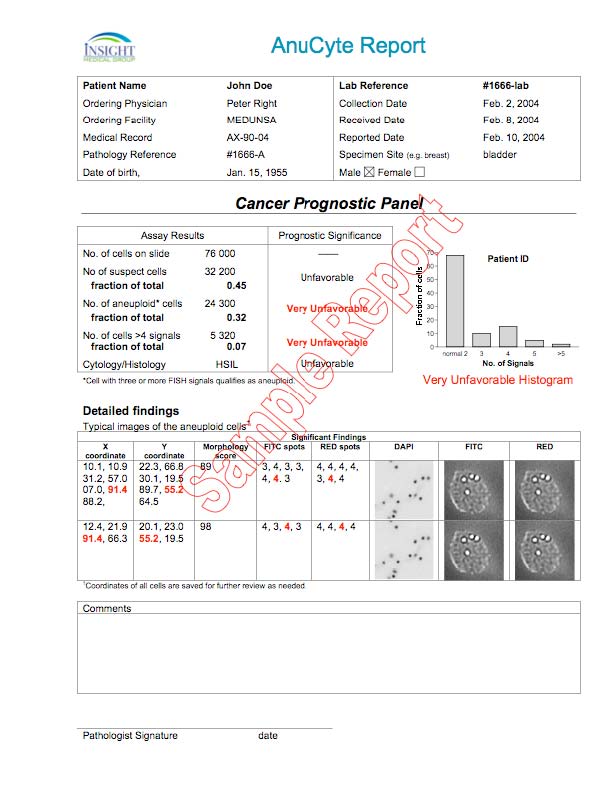
Two things make AnuCyte unique and the most accurate method of detecting cancer and precancer in any tissue:
As outlined above and detailed in the supporting documents, cancer is caused by the initiation and progression of aneuploidy. Cancer and advanced aneuploidy are synonymous and are different ways of describing the exact same cell state. Since cancer (all types of cancer) is an advanced state of chromosomal imbalance (aneuploidy) there is no superior method for detecting cancer than the detection of aneuploidy, even in principle. AnuCyte is the only system available that exclusively employs the detection of aneuploidy to detect any type of cancer and precancer and thus eliminates indeterminates and yield the highest possible accuracy.
For decades, aneuploidy has been proposed as a criterion for diagnosing or detecting cancer. However, the unreliable and inadequate techniques used in the 1970s and 1980s gave aneuploidy a bad name. See Failure of Flow Cytometry in supporting documents.
To this day, total DNA content is frequently used by oncologists as adjunctive information for establishing the severety cancer. Total DNA content is a crude measure of aneuploidy that confirms the presence of well-established, advanced cancer. However, it is too insensitive and unreliable for detecting precancer and early stage cancer and is rarely used for those purposes. Total DNA content is also not reliable for eliminating indeterminate samples.
Cells suspended in blood or urine are the easiest to analyze for the presence of cancer since they are free and unobscured by the presence of protein.
Extensively keratinized (protein obscured) cervical samples, for example, are difficult for cytologists to grade because the keratin obscures the nucleus and cytoplasm. As a result, keratinized cervical samples have been associated with false-negative cytology, some even masking an invasive cancer of the cervix (83).
Keratin-coated cells will also obscure the fluorescently labeled chromosomes used to detect aneuploidy. Approximately 40 percent of cervical samples have varying degrees of keratinization that interferes with the fluorescence measurements. Therefore, removal of the protein obscuring cells is essential for accurate detection and measuring of aneuploidy.
The completely automated AnuCyte system incorporates a proprietary sample preparation method which facilitates chromosomal analysis in any tissue sample.
In the AnuCyte system, the hybridized slides are loaded into an automated slide handler for placement on the microscope stage for automated analysis. AnuCyte uses the blue fluorescence to automatically catalog the coordinates, area and fluorescence intensity of every nucleus on the slide. All cells are then automatically surveyed to objectively count the green and red signals for the chromosomes analyzed.
Finally, AnuCyte generates a report that lists the total number of cells, the number that have the normal number of chromosomes, the number that are aneuploid, and their percentages. A histogram of the results is provided for rapid visual inspection.
We are not aware of any automated or manual cancer detection system currently available that does this anywhere in the world.
7.5 AnuCyte's effectiveness and accuracy has been demonstrated
The AnuCyte system has been demonstrated to function to expectations and the results of the pre-clinical testing with Cleveland Clinic support the claims of accuracy.
8 AnuCyte Claims Fully Supported
8.1 First in the world of its type:
No other cancer diagnostic system targets advanced anueploidy as the sole means of detecting cancer. The AnuCyte system is the first system and at present only system to ever specifically and solely target the presence of anueploidy as the means to detect cancer. We remind the reader cancer is the precise same thing as advanced anueploidy.
8.2 Objective measurement:
AnuCyte employs the ultimate objective criterion provided by Nature for detecting cancer. Normal cells have exactly two copies each of the 23 unique human chromosomes for a total of 46. Cancer and precancer cells have an abnormal number and composition of chromosomes. The AnuCyte system uses an automated digital imaging system to objectively discriminate between normal cells, which have an identical composition of chromosomes, and cancer or precancer cells, which are always aneuploid. No other system uses this strategy to detect all types of cancer.
8.3 Most accurate in the world:
The AnuCyte system measures and quantifies the presence of advanced anueploidy. Advanced anueploidy is the exact same thing as cancer. Any other type of methodology for detecting cancer that does not use anueploidy as its primary means of detecting cancer can not, by definition, be as accurate. Standard cancer diagnoses involve an assessment of the cell sample by human beings using microscopes. The presence of manual examination of the cell samples combined with the fact anueploidy is not used as the defining criteria introduces the errors in cancer diagnostics prevalent in the industry. The AnuCyte system eliminates the human factor and eliminates all other criteria for detecting cancer not related to anueploidy. Therefore, for these two reasons, AnuCyte is, and must be, the single most accurate system and method in the world for detecting cancer in a cell sample.
9 References
- 1. Shackney SE, Berg G, Simon SR, et al. Origins and clinical implications of aneuploidy in early bladder cancer. Cytometry 1995;22:307-16.
- 2. Shapiro BL. Down syndrome--a disruption of homeostasis. American Journal of Medical Genetics 1983;14:241-69.
- 3. Zipursky A, Thorner P, De Harven E, Christensen H, Doyle J. Myelodysplasia and acute megakaryoblastic leukemia in Down's syndrome. Leuk Res 1994;18(3):163-71.
- 4. Shen JJ, Williams BJ, Zipursky A, et al. Cytogenetic and molecular studies of Down syndrome individuals with leukemia. Am J Hum Genet 1995;56(4):915-25.
- 5. Li R, Sonik A, Stindl R, Rasnick D, Duesberg P. Aneuploidy versus gene mutation hypothesis: recent study claims mutation, but is found to support aneuploidy. Proceedings of the National Academy of Sciences (USA) 2000;97(7):3236-41.
- 6. Sen S. Aneuploidy and cancer. Curr Opin Oncol 2000;12(1):82-8.
- 7. Gibbs WW. Dissident or Don Quixote? Scientific American 2001;265(2):30-2.
- 8. Hansemann D. Ueber asymmetrische Zelltheilung in epithel Krebsen und deren biologische Bedeutung. Virschows Arch Pathol Anat 1890;119:299-326.
- 9. Boveri T. Zur Frage der Entstehung maligner Tumoren. Jena: Fischer; 1914.
- 10. Cornish-Bowden A. Metabolic control analysis in biotechnology and medicine. Nature Biotechnology 1999;17:641-3.
- 11. Oreskes N. The Rejection of Continental Drift. New York: Oxford University Press; 1999.
- 12. Whitman RC. Somatic mutation as a factor in the production of cancer; a critical review of v. Hansemann's theory of anaplasia in the light of modern knowledge of genetics. Journal of Cancer Research 1919;4:181-202.
- 13. Wolf U. Theodor Boveri and his book "On the problem of the origin of malignant tumors". In: German J, editor. Chromosomes and Cancer. New York: John Wiley & Sons; 1974. p. 3-20.
- 14. Epstein CJ. The consequences of chromosome imbalance: principles, mechanisms, and models. New York: Cambridge University Press; 1986.
- 15. Kacser H, Burns JA. The control of flux. Symposia of the Society for Experimental Biology 1973;27:65-104.
- 16. Heinrich R, Rapoport TA. Linear theory of enzymatic chains; its application for the analysis of the crossover theorem and of the glycolysis of human erythrocytes. Acta Biologica et Medica Germanica 1973;31(4):479-94.
- 17. Rasnick D, Duesberg PH. How aneuploidy affects metabolic control and causes cancer. Biochemical Journal 1999;340(3):621-30.
- 18. Kinzler KW, Vogelstein B. Cancer-susceptibility genes: gatekeepers and caretakers. Nature 1997;386:761,3.
- 19. Tomlinson I, Bodmer W. Selection, the mutation rate and cancer: ensuring that the tail does not wag the dog. Nature Medicine 1999;5(1):11-2.
- 20. Epstein SS. The Politics of Cancer Revisited. New York: East Ridge Press; 1998.
- 21. Kinzler K, Vogelstein B. Lessons from hereditary colorectal cancer. Cell 1996;87:159-70.
- 22. Zhang L, Zhou W, Velculescu VE, et al. Gene expression profiles in normal and cancer cells. Science 1997;276:1268-72.
- 23. Wortman M. DNA chips target Cancer. Technology Review 2001:1-5.
- 24. McCormick F. New-age drug meets resistance. Nature 2001;412(6844):281-2.
- 25. Planck M. Scientific Autobiography and Other Papers pp. 33-34. New York: translated by F. Gaynor; 1949.
- 26. Mitchel MF, Hittelman WK, Lotan R, et al. Chemoprevention trials and surrogate end point biomarkers in the cervix. Cancer Supplement 1995;76(10):1956-77.
- 27. Duesberg P, Li R, Rasnick D, et al. Aneuploidy precedes and segregates with chemical carcinogenesis. Cancer Genetics and Cytogenetics 2000;119(2):83-93.
- 28. Duesberg P, Rasnick D. Aneuploidy, the somatic mutation that makes cancer a species of its own. Cell Motility and Cytoskeleton 2000;47(2):81-107.
- 29. Duesberg P, Stindl R, Hehlmann R. Explaining the high mutation rates of cancer cells to drug and multidrug resistance by chromosome reassortments that are catalyzed by aneuploidy. Proc Natl Acad Sci U S A 2000;97(26):14295-300.
- 30. Duesberg P, Stindl R, Hehlmann R. Origin of multidrug resistance in cells with and without multidrug resistance genes: Chromosome reassortments catalyzed by aneuploidy. Proc Natl Acad Sci U S A 2001;98(20):11283-8.
- 31. Li R, Yerganian G, Duesberg P, et al. Aneuploidy 100% correlated with chemical transformation of Chinese hamster cells. Proceedings of the National Academy of Sciences (USA) 1997;94:14506-11.
- 32. Rasnick D. Auto-catalyzed progression of aneuploidy explains the Hayflick limit of cultured cells, carcinogen-induced tumours in mice, and the age distribution of human cancer. Biochemical Journal 2000;348(3):497-506.
- 33. Rasnick D. Aneuploidy theory explains tumor formation, the absence of immune surveillance and the failure of chemotherapy. Cancer Genetics Cytogenetics 2001;Submitted.
- 34. Mayeaux J, E. J. The Papanicolaou smear. Shreveport: Louisiana State University Medical Center; 1994.
- 35. Koss LG. Cervical (Pap) smear: new directions. Cancer Supplement 1993;71(4):1406-12.
- 36. van der Graaf Y, Voois GP, Gaillard HJL, Go DMDS. Screening errors in cervical cytology screening (sic). Acta Cytology 1987;31:434-8.
- 37. Cooper GM. Oncogenes. Boston: Jones and Bartlett Publishers; 1990.
- 38. Bishop JM. The molecular genetics of cancer. Science 1987;235:305-11.
- 39. Weiss R, Teich N, Varmus H, Coffin J. Molecular Biology of RNA Tumor Viruses. Plainview, NY: Cold Spring Harbor Lab. Press; 1985.
- 40. Varmus HE. The Molecular Genetics of Cellular Oncogenes. Annu Rev Genet 1984;18:533-612.
- 41. Huebner RJ, Todaro G. Oncogenes of RNA tumor viruses as determinants of cancer. Proceedings of the National Academy of Sciences (USA) 1969;64:1087-94.
- 42. Hauschka TS. The chromosomes in ontogeny and oncogeny. Cancer Res 1961;21(8):957-81.
- 43. Winge O. Zytologische Untersuchungen ueber die Natur maligner Tumoren. II. Teerkarzinome bei Maeusen. Zeitschrift fuer Zellforschung und Mikroskopische Anatomie 1930;10:683-735.
- 44. Atkin NB, Baker MC. Chromosome abnormalities as primary events in human malignant disease: evidence from marker chromosomes. Journal of the National Cancer Institute 1966;36:539-57.
- 45. Levan A, Biesele JJ. Role of chromosomes in cancerogenesis, as studied in serial tissue culture of mammalian cells. Annals of New York Academy of Sciences 1958;71:1022-53.
- 46. Sennerstam R, Kato H, Auer GU. Dissociation of cellular protein and DNA content in mild and moderate dysplasia as a reflection of the degree of aneuploidy in cancer. Analytical and Quantitative Cytology and Histology 1989;11(4):255-60.
- 47. Foley GE, Handler AH, Lynch PM, Wolman SR, Stulberg CS, Eagle H. Loss of neoplasatic properties in vitro. II. Observations on KB sublines. Cancer Research 1965;25:1254-61.
- 48. Caspersson TO. Disturbed systems for protein formation in the metazoan cell. In: Cell growth and cell function: A cytochemical study. New York: W. W. Norton & Company; 1950. p. 141-51.
- 49. Caspersson T, Foley GE, Killander D, Lomakka G. Cytochemical differences between mammalian cell lines of normal and neoplastic origins: correlation with heterotransplantability in Syrian hamsters. Experimental Cell Research 1963;32:553-65.
- 50. Frati L, Cortesi E, Ficorella C, Manzari V, Verna R. Phenotypic and genetic markers of cancer: turning point in research. In: Aaronson SA, Frati L, Verna R, editors. Genetic and phenotypic markers of tumors. New York: Plenum Press; 1984. p. 1-20.
- 51. Alderman EM, Lobb RR, Fett JW. Isolation of tumor-secreted products from human carcinoma cells maintained in a defined protein-free medium. Proceedings of the National Academy of Sciences (USA) 1985;82:5771-5.
- 52. Atkin NB, Mattinson G, Baker MC. A comparison of the DNA content and chromosome number of fifty human tumors. British Journal of Cancer 1996;20:87-101.
- 53. Atkin NB, Baker MC, Fox MF. Chromosome changes in 43 carcinomas of the cervix uteri. Cancer Genetics and Cytogenetics 1990;44:229-41.
- 54. Atkin NB. Solid tumor cytogenetics: progress since 1979. Cancer Genetics and Cytogenetics 1989;40:3-12.
- 55. Atkin NB. Modal DNA value and chromosome number in ovarian neoplasia: a clinical and histopathologic assessment. Cancer 1971;27(5):1064-73.
- 56. Atkin NB, Baker MC. Possible differences between the karyotypes of preinvasive lesions and malignant tumours. British Journal of Cancer 1969;23:329-36.
- 57. Giaretti W, Santi L. Tumor progression by DNA flow cytometry in human colorectal cancer. International Journal of Cancer 1990;45:597-603.
- 58. Wolman SR. Karyotypic progression in human tumors. Cancer Metastasis Reviews 1983;2:257-93.
- 59. Chaganti RSK. The question of normal karotypes in tumor cells. In: German J, editor. Chromosome mutation and neoplasia. New York: Alan R. Liss, Inc.; 1983. p. 370
- 2.
- 60. Shackney SE, Smith CA, Miller BW, et al. Model for the genetic evolution of human solid tumors. Cancer Research 1989;49:3344-54.
- 61. Shackney SE, Singh SG, Yakulis R, et al. Aneuploidy in breast cancer: a fluorescence in situ hybridization study. Cytometry 1995;22:282-91.
- 62. Shankey TV, Kallioniemi O-P, Koslowski JM, et al. Consensus review of the clinical utility of DNA content cytometry in prostate cancer. Cytometry 1993;14:497-500.
- 63. Sreekantaiah C, De Braekeleer M, Hass O. Cytogenetic findings in cervical carcinoma: a statistical approach. Cancer Genetics and Cytogenetics 1991;53:75-81.
- 64. Segers P, Haesen S, Castelain P, et al. Study of numerical aberrations of chromosome 1 by fluorescent in situ hybridization and DNA content by densitometric analysis on (pre)-malignant cervical lesions. Histochemical Journal 1995;27(1):24-34.
- 65. Segers P, Haesen S, Amy J-J, de Sutter P, van Dam P, Kirsch-Volders M. Detection of premalignant stages in cervical smears with a biotinylated probe for chromosome 1. Cancer Genetics and Cytogenetics 1994;75:120-9.
- 66. Sandberg AA, Chen Z. Cancer cytogenetics: nomenclature and clinical applications. In: Razelle K, Talpaz M, editors. Molecular biology in cancer medicine. London: Martin Dunitz; 1996.
- 67. Johansson B, Mertens F, Mitelman F. Primary vs. secondary neoplasia-associated chromosomal abnormalities- balanced rearrangements vs. genomic imbalances? Genes Chromosomes and Cancer 1996;16:155-63.
- 68. Sandberg AA. The chromosomes in human cancer and leukemia. 2 ed. New York: Elsevier Science Publishing; 1990.
- 69. Sandberg AA, Kakati S. Chromosome alterations and oncogenes in human neoplasia. In: Sharma T, editor. Trends in chromosome research: Springer-Verlag; 1990.
p. 190-203.
- 70. Sandberg AA. Tumors of the female. In: The chromosomes in human cancer and leukemia. New York: Elsevier; 1990. p. 827-51.
- 71. Sandberg AA, Turc-Carel C, Gemmill RM. Chromosomes in solid tumors and beyond. Cancer Research 1988;48:1049-59.
- 72. Sandberg AA, Turc-Carel C. The cytogenetics of solid tumors: relation to diagnosis, classification and pathology. Cancer 1987;59:387-95.
- 73. Sandberg AA. The chromosomes in human cancer and leukemia. Amsterdam: Elsevier/North-Holland; 1980.
- 74. Duesberg P, Rausch C, Rasnick D, Hehlmann R. Genetic instability of cancer cells is proportional to their degree of aneuploidy. Proceedings of the National Academy of Sciences (USA) 1998;95:13692-7.
- 75. Mitelman F. Catalogue of chromosome aberrations in cancer. 4 ed. New York: Wiley-Liss; 1994.
- 76. Mitelman F, Mark J, Levan G, Levan A. Tumor etiology and chromosome pattern. Science 1972;176:1340-1.
- 77. Mitelman F, Mertens F, Johansson B. A breakpoint map of recurrent chromosomal rearrangements in human neoplasia. Nature Genetics 1997;Special issue(April 15):417-74.
- 78. Eastmond DA, Tucker JD. Identification of aneuploidy-inducing agents using cytokinesis-blocked human lymphocytes and an antikinetochore antibody. Environmental and Molecular Mutagenesis 1989;13:34-43.
- 79. Eastmond DA, Pinkel D. Detection of aneuploidy and aneuploidy-inducing agents in human lymphocytes using fluorescence in situ hybridization with chromosome-specific DNA probes. Mutation Research 1990;234:303-18.
- 80. Eastmond DA, Rupa DS, Hasegawa LS. Detection of hyperdiploidy and chromosomal breakage in interphase human lymphocytes following exposure to the benzene metabolite hydroquinone using multicolor fluorescence in situ hybridization with DNA probes. Mutation Research 1994;322:9-20.
- 81. Eastmond DA, Schuler M, Rupa DS. Advantages and limitations of using fluorescence in situ hybridization for the detection of aneuploidy in interphase human cells. Mutation Research 1995;348:153-62.
- 82. Rupa DS, Schuler M, Eastmond DA. Detection of hyperdiploidy and breakage affecting the 1cen-1q12 region of cultured interphase human lymphocytes treated with various genotoxic agents. Environmental and Molecular Mutagenesis 1997;29:161-7.
- 83. Faquin WC, Brown FM, Krane JF, Renshaw AA, Cibas ES. Extensively keratinized squamous intraepithelial lesions of the cervix are difficult to grade. Am J Clin Pathol 2001;115(1):80-4.

