Insight Medical Group, Business Plan Overview
AnuCyte: Measuring Chromosomal Imbalance to Detect Cancer
TABLE OF CONTENTS
1 Overview of the AnuCyte System *
1.1 System Testing *
1.2 Sample Output of the AnuCyte System *
2 Business Summary *
2.1 MARKETS *
2.1.1 Prostate Cancer *
2.1.2 Cervical Cancer *
2.1.3 Bladder Cancer *
2.2 UNIQUE MARKETING OPPORTUNITY *
2.2.1 MARKET PROJECTIONS *
2.2.2 SUMMARY OF MARKETING OPPORTUNITY *
3 Failure of Current Cancer Theories and the Rise of Chromosomal Imbalance Theory *
3.1 Failure of Gene-Mutation theory *
3.2 The Rise of Chromosomal Imbalance *
3.3 100% Correlation between cancer and chromosomal imbalance *
3.4 Theory Comparisons *
3.5 Chromosomal Imbalance the most direct, simple, and accurate way to detect cancer *
4 Cancer Detection Markets *
4.1 Prostate & Cervical Cancers *
5 Key Science Team Members *
6 MARKET OPPORTUNITY *
6.1 Prostate Cancer *
6.2 Cervical Cancer *
7 Product Development Strategy and Market Entry *
7.1 Option A: prostate cancer diagnosis *
7.2 Option B: cervical cancer diagnosis *
8 COMPETITION *
8.1 Prostate Cancer *
8.2 Cervical Cancer *
8.3 Microscope/Imaging Systems *
8.3.1 Fluorescence Microscopy Industry *
8.3.2 Conventional Light Microscopy *
9 STATEMENT OF RISKS *
9.1 Limited History with No Profitable Operations *
9.2 Dependence on Key and Professional Personnel *
9.3 Dependence on Strategic Partners, Alliances and Sub-contractors *
9.4 FDA approval and Convincing Medical Professionals to Request AnuCyte Tests *
9.5 Competition *
9.6 Time to Market *
9.7 Revenue Projections *
9.8 Product Liability Potential *
10 SALES PROJECTIONS *
10.1 Prostate Cancer Diagnosis *
10.2 Entry into the competition-free 'indeterminate' Cervical cancer market *
10.3 Overall Cancer Diagnostic Marketplace *
10.4 Summary and Background for Revenue Modeling *
10.4.1 Spreadsheet: ASCUS Revenue Projections *
10.4.2 Spreadsheet: Prostate Cancer Diagnostics Revenue Projections *
11 REFERENCES *
1 Overview of the AnuCyte System
In the last several years, scientific breakthroughs in DNA probe technology, advancements in detection instrumentation, and inexpensive computing power have made it possible to construct an automated system for the routine detection of aneuploidy (chromosomal imbalance) in real-world human specimens. AnuCyte is the first commercial high-throughput image cytometer, to quantify chromosomal imbalance for the purpose of detecting all solid cancers. AnuCyte will enable clinical laboratories to test millions of patient specimens rapidly and accurately for all types of cancer.
AnuCyte is not an add-on feature of existing commercial microscopes. It is constructed for the specific purpose of diagnosing cancer in human specimens, thus providing unparalleled sensitivity and specificity. Because our system is assembled from standard components, it is simple to manufacture and maintain. AnuCyte features: 1) a proprietary method of sample preparation, 2) an automated, high-throughput image cytometer using proprietary control software, 3) proprietary software that analyzes aneuploid cells from the digital fluorescence signals attached to chromosomes, and 4) a printed report containing: (a) patient and physician information, (b) table of normal and aneuploid cells present, (c) histogram for quick inspection of results, (d) images of normal and aneuploid cells that are representative of the sample.
As discussed below, measuring chromosomal imbalance is the absolute method of distinguishing cancerous and precancerous cells from normal. Therefore, Management believes the automated, high-throughput analysis of chromosomal imbalance will necessarily increase accuracy, result in lower cost per sample, and increase throughput by at least ten-fold compared to manual inspection.
1.1 System Testing
In collaboration with the Cleveland Clinic and Texas Southwestern University, AnuCyte has successfully detected cancerous and precancerous aneuploid cells in hundreds of cervical samples and scores of fine needle aspirates from breast tumors.
The system worked as anticipated and as designed. It demonstrated its ability to accurately detect cancer using automated measurement of chromosomal imbalance within the nuclei of cells.
1.2 Sample Output of the AnuCyte System

2 Business Summary
We plan to generate revenue through marketing cancer detection and analysis services using our automated, state-of-the-art Fluorescence In situ Hybridization (FISH) microscope that measures chromosome imbalance (aneuploidy) to detect/diagnose cancer cells. Management is not aware of any such system currently in existence. We will market this service directly to clinical labs, pathologists, hospitals, and other organizations currently performing cancer diagnostic work.
2.1 MARKETS
2.1.1 Prostate Cancer
There are estimated to be more than 218,000 new cases of prostate cancer diagnosed in the USA in 2007 and over 27, 000 deaths . The prostate biopsy market was $240 million in 1997, which will continue to grow over the next 20 years as the "Baby Boomers" reach retirement age. As the population ages, prostate cancer can be expected to increase over the next ten to twenty years. Prostate cancer is one of the most difficult to definitively diagnose using conventional techniques.
However, current clinical laboratory methods cannot determine the cancer status of intermediate grade tumors, which account for the majority of prostate tumors . The measurement of chromosomal imbalance (aneuploidy) provides clinically useful prognostic information for patients with these intermediate grade tumors. However, pathologists do not perform chromosomal analysis in deciding if cancer is present in prostate biopsies because there is a lack of appropriate instrumentation. AnuCyte's automated microscope will fill the instrumentation void and allow pathologists to correctly characterize the intermediate grade prostate tumors (see Product Opportunity: Prostate Cancer). As a start up company with no operational history concerning the AnuCyte System, there are significant assumptions and risks associated with our ability to successfully introduce AnyCyte into the market as discussed in Section 9 below. However, based on the figures discussed above and the assumption that we can both fund this expansion and penetrate these markets, we believe that we can potentially capture up to 25% of the prostate cancer diagnostics revenue the first year on the market. If we can capture this percentage of the market as we hope, revenues of $12 million could be obtained the first year on the market and over $50 million by the end of the third year (equating to 70% of the prostate cancer diagnostics revenue) (see Fig. 1b and Projections and discussion of assumptions and risks).
2.1.2 Cervical Cancer
Based on Management's research () , Each year more than 100 million Pap smears are collected worldwide, with over 55 million being performed in the USA alone at a cost of $5-20 per test. This puts the Pap smear market in the USA at $1 billion annually. We will not attempt initially to compete in the Pap smear screening market, which is well established and limited to low costs per test (see Markets and Competition). Instead, we will pioneer the competition-free market of eliminating the 2-5 million annual ASCUS (indeterminate) cervical specimens . It is well-known in the industry that at present there is no other test or technique for eliminating indeterminate results.
Measuring aneuploidy in cervical smears is an independent predictor of biological outcome and should readily sort out the non-cancerous cells, precancerous cells, and cancerous cells in the ASCUS population (see Product Opportunity: Cervical Cancer). Since eliminating ASCUS is a completely untapped market at present, through discussions with leaders in the field, we estimate that we can charge $100 (Management has chosen the figure of $100 as market-competitive and comparably priced to other tests in use today as per our conversations and research with industry veterans and therefore believes the amount to be reasonable and justified.) to properly classify an indeterminate Pap smear, which represents a potential USA market of $220-440 million annually. As a start up company with no operational history concerning the AnuCyte System, there are significant assumptions and risks associated with our ability to successfully introduce AnyCyte into the market as discussed in Section 9 below. However, based on the figures discussed above and the assumption that we can both fund this expansion and penetrate these markets, we believe that we can potentially capture up to 5% of the ASCUS cervical cancer diagnostics revenue the first year on the market. If we can capture this percentage of the market as we hope, revenues of $16.5 million could be obtained the first year on the market and $50 million by the end of the third year (equating to 15% of the ASCUS cervical cancer diagnostics revenue) (see Fig. 1b and Projections).
2.1.3 Bladder Cancer
Bladder cancer is the fifth most common cancer in the USA . There are more than 50,000 new cases a year and more than 10,000 deaths. Of special interest is carcinoma in situ of the bladder, presenting problems of diagnosis and unpredictable behavior. Management believes the Bladder Cancer market to be a viable market for entry of our product. Since our AnuCyte system works for all cancer types, we feel the issues surrounding properly detecting bladder cancer can be addressed with our service. We have no immediate plans to enter this market proactively. We will however accept bladder cell samples for analysis should the test be requested by customers.
2.2 UNIQUE MARKETING OPPORTUNITY
Principals of the company have contributed to basic scientific cancer research by showing how and why cancer can best be diagnosed by measuring the level of the abnormal number of chromosomes (aneuploidy) in cells. Based on 11 years of research and development work on aneuploidy and cancer, we believe we are the first to have converted this aneuploidy research into a completely integrated and automated FISH system to diagnose (Option A) the majority of prostate tumors, which cannot be determined by current clinical laboratory methods, and (Option B) to pioneer the competition-free market of 2-5 million ASCUS (indeterminate) Pap smears generated annually. This system can work for any tissue sample to detect cancer and not just for the limited number of markets so far of interest to the company.
2.2.1 MARKET PROJECTIONS
There are significant assumptions and risks associated with our ability to successfully introduce AnyCyte into the market as discussed in Section 9 below and these projection results may not be realized at all or in fact differ materially from what we project. We state (Option A) prostate and (Option B) cervical cancer revenues 5 years after funding is secured with market entry in year 2007 (see Projections).
| | 2007 | 2008 | 2009 |
Prostate | 12,500 | 32,800 | 53,000 |
Cervical | 16,500 | 33,000 | 49,500 |
2.2.2 SUMMARY OF MARKETING OPPORTUNITY
According to a Frost & Sullivan report, applying DNA probe technology to clinical diagnoses represents a large untapped market worth hundreds of millions of dollars . In contrast to the current (and so far unproductive) approach of focusing on specific genes for each of the hundreds of different cancers, we will provide the first automated microscope system that uses Fluorescence In situ Hybridization (FISH) to clinically diagnose the broad spectrum of cancers by measuring aneuploidy (abnormal number of chromosomes).
Cancer detection in the clinical lab (where analysis is performed as a part of patient health care rather than a research lab) currently suffers from a general lack of automated diagnosis of solid cancers using "state-of-the-art" technologies . The research community, on the other hand, is addressed well through the offering of a large and growing number of different fluorescent DNA probes provided by Vysis, Oncor, and others (see Competition) for the study of cancer and carcinogensis. However, the needs of the clinical diagnostic laboratories are very different. The sheer volume of clinical tests requires an entirely different strategy than that of the research setting. For example, a test like the Pap smear for cervical cancer is performed over 55 million times each year in the USA alone . In addition, the lack of accuracy is distressingly high in evaluating Pap smears: False negative rates are typically 20-25% , but can run as high as 50% . The extent of false positive results is not generally reported but can run as high as 20%.
The question is, why haven't the DNA probe technologies of the 1990s moved dramatically into the clinical market? In our opinion, the chief roadblock is the prevailing, but terminally and demonstrably flawed, oncogene/tumor suppressor theory of cancer itself . Essentially all current thinking about the genetics of cancer is contained within the gene mutation hypothesis, which contends that mutation causes cancer by activating certain cellular genes, converting them to dominant cancer genes (oncogenes), and inactivating other, tumor suppressor, genes . The gene mutation hypothesis, which once promised a relatively simple entry into the massively disregulated genetic programs of the cancer cell, has become so burdened with the complexity of its details that it has become an empirical exercise devoid of theoretical and explanatory power (see Table I below).
3 Failure of Current Cancer Theories and the Rise of Chromosomal Imbalance Theory
3.1 Failure of Gene-Mutation theory
The gene mutation hypothesis is problematic in a number of ways:
1) It cannot account for the fact that aneuploidy (abnormal number of chromosomes) is "the rule rather than the exception" in cancer, since it predicts the occurrence of diploid (normal number of chromosomes) tumors. In fact, except for the rare cancers produced in the laboratory , diploidy does not seem to occur in human solid tumors . After a decade of searching we are not aware of any example of any type of cancer with balanced chromosomes.
2) The gene mutation hypothesis has yet to offer experimental proof of malignant transformation of a normal diploid cell by one or a combination of cellular oncogenes, or
3) to explain the acquisition of the many new functional and structural hallmarks of the cancer cell, such as invasiveness, dedifferentiation, altered morphology and genetic instability (despite essentially normal mutation rates), since most mutations are either silent or lead to loss of function.
4) The oncogene hypothesis cannot explain the growing list of non-mutating carcinogens like asbestos, aromatic hydrocarbons, mineral oil, or mitotic spindle blockers such as colcemid.
5) Nor can gene mutation explain the almost 1000-fold increase in cancer risk with age. As most (if not all) suspected oncogenes are perfectly heritable, cancer should be a disease of youth if tumor progression required the gradual accumulation of mutations.
The mounting evidence against the mutation theory of cancer explains why specific cancer genes have not resulted in clinical diagnostic markers of cancer and targets of therapeutic intervention. We, on the other hand, have chosen a different strategy of cancer diagnosis. We have considered a hundred years of scientific and medical observation, looking for the most characteristic differences between cancer cells and normal tissues.
3.2 The Rise of Chromosomal Imbalance
In 1890 David von Hansemann first described abnormal chromatin content and asymmetric mitoses in cancer cells . In 1914, Theodor Boveri proposed that cancer was caused by chromosomal imbalance (aneuploidy ) . There have been literally thousands of publications consistent with Boveri's historic proposal that aneuploidy causes/is cancer . However, over the last 25 years the gene mutation hypothesis has become so dominant that aneuploidy, still considered a viable candidate for cancer etiology by the editors of the Journal of the National Cancer Institute in 1966 , is today not even listed in the indexes of the most important molecular biology texts , and this is in spite of the fact that numeric chromosome imbalance is the most prominent genetic change in essentially all of over 20,000 solid tumors that have been examined to date .
While none of the "oncogenes" is a general diagnostic for cancer (not even a specific type of cancer), aneuploidy is always present in solid cancers . The mass of data accumulating on cancer "demonstrates the more general principle that some increase in chromosome number (most often to a mode centered in the triploid or hypotetraploid range) is associated with poorer prognosis, atypia, and other measures of tumor progression" .
3.3 100% Correlation between cancer and chromosomal imbalance
The scientific team behind AnuCyte have recently provided extremely strong experimental support and a firm theoretical foundation for the chromosomal imbalance theory of cancer. Peter Duesberg et al. have shown that aneuploidy is correlated 100% with chemical transformation of Chinese hamster cells using carcinogens that do not cause mutations . The fact that these non-mutagenic carcinogens always lead to aneuploid cells at the earliest stages of transformation is strong evidence that aneuploidy causes/is cancer.
3.4 Theory Comparisons
David Rasnick and Peter Duesberg have adapted the method of metabolic control analysis to investigate the aneuploidy hypothesis of cancer . The results show that transforming normal cells into robust cancer requires a 2-fold increase in the expression of thousands of normal genes. The massive change in gene dose produces abnormal changes in the physiology and metabolism of cells and tissues. Aneuploidy explains virtually all of the gross biochemical abnormalities of cancer cells (including increased cellular size, the appearance of numerous membrane-bound tumor-associated antigens and the high levels of secreted proteins that are responsible for invasiveness) as the natural consequence of aneuploidy. The well known genetic instability of cancer cells is due to the perpetual regrouping of the genome following the disruption of nuclear symmetry by aneuploidy. Aneuploid cells are less robust than normal cells. This result may be the basis for the age dependence of most cancers and the spontaneous remission of some. Finally, the results show that mutated genes are neither necessary nor sufficient to produce the cancer phenotype in wild-type organisms. Table I compares the explanatory powers of the gene mutation and aneuploidy theories of cancer.
TABLE I
Competing Hypotheses of Cancer
Properties of Cancer Explained | Gene Mutation 1 | Aneuploidy 2 |
* large size of cancer cells * numerous new membrane cancer antigens * high levels of cancer secreted proteins * invasiveness * genetic instability of cancer cells * non-mutagenic carcinogens * 1000-fold increased cancer risk with age * aneuploidy in all of more than 20,000 human cancers catalogued * 60-90 chromosomes in cancer cells (normal cells have 46 chromosomes) | no no no no no no no no no | yes yes yes yes yes yes yes yes yes |
1 Cooper, G. M. Oncogenes. Boston: Jones and Bartlett Publishers, 1990; Bishop, J. M. The molecular genetics of cancer, Science. 235: 305-311, 1987; Weiss, R., Teich, N., Varmus, H., and Coffin, J. Molecular Biology of RNA Tumor Viruses. Plainview, NY: Cold Spring Harbor Lab. Press, 1985; Varmus, H. E. The Molecular Genetics of Cellular Oncogenes, Annu. Rev. Genet. 18: 533-612, 1984; Huebner, R. J. and Todaro, G. Oncogenes of RNA tumor viruses as determinants of cancer, Proceedings of the National Academy of Sciences (USA). 64: 1087-1094, 1969.
2 Li, R., Yerganian, G., Duesberg, P., Kraemer, A., Willer, A., Rausch, C., and Hehlmann, R. Aneuploidy 100% correlated with chemical transformation of Chinese hamster cells, Proceedings of the National Academy of Sciences (USA). 94: 14506-14511, 1997; Rasnick, D. and Duesberg, P. H. The aneuploidy theory of cancer and the analysis of phenotypes, submitted to Proceedings of the National Academy of Sciences (USA), 1998.
3.5 Chromosomal Imbalance the most direct, simple, and accurate way to detect cancer
The measurement of aneuploidy is a simple and robust way to diagnose/detect cancer, and provides an excellent market entry opportunity for an affordable state-of-the-art diagnostic test. Using chromosomal imbalance to diagnose cancer is so generally applicable and reliable that we expect it will become the gold standard of the industry worldwide.
In summary, the hundred-year-long correlation between chromosomal imbalance (aneuploidy) and all types of cancer provides a sound foundation upon which to detect cancer and offers an important opportunity for the company to pioneer and dominate an un-served niche of cancer diagnosis by:
1) analyzing whole chromosomes for chromosomal imbalance rather than the individual genes thought to cause cancer,
2) using a highly automated instrument operated through our proprietary software rather than human operators and manual testing,
3) successfully detecting the broad spectrum of solid cancers (the most lethal of cancers) since chromosomal imbalance is associated with them all,
4) diagnosing the cancer status of tumors that are currently impossible to characterize,
5) focusing on the market of standardized high volume tests, such as the Pap smear, carried out by clinical diagnostics laboratories rather than specializing as the current closest competitors do (see Competition) in the very different low volume FISH market for research laboratories.
Based on 11 years of research and development, we understand that no current company offers an automated system for the analysis of aneuploidy (chromosomal imbalance) in solid cancers. Accordingly, we believe that our entry into the market should be of significant use to clinical laboratories in the diagnosis of cancers of all types.
4 Cancer Detection Markets
4.1 Prostate & Cervical Cancers
According to the National Cancer Institute there are 207 different types of cancer . These cancers are among the leading causes of morbidity and mortality in the industrialized world. Since cancer is a disease of aging, the incidence of these diseases will increase dramatically as the "Baby Boomers" enter retirement age. Prostate cancer, for example, is a major health problem in the USA (Figs. 1a & 1b, 1995 and 2002 respectively).
In 1995, there were 244,000 new cases (up 44,000 from 1994) of prostate cancer and 40,400 deaths (up 2,400 from 1994) due to this disease . Carcinoma of the cervix is one of the most common malignancies in women, accounting for 15,700 new cases (6% of all cancers) and 4,900 deaths in the USA each year (Fig. 1a). Worldwide, cervical cancer is second only to breast cancer as the most common malignancy in both incidence and mortality .
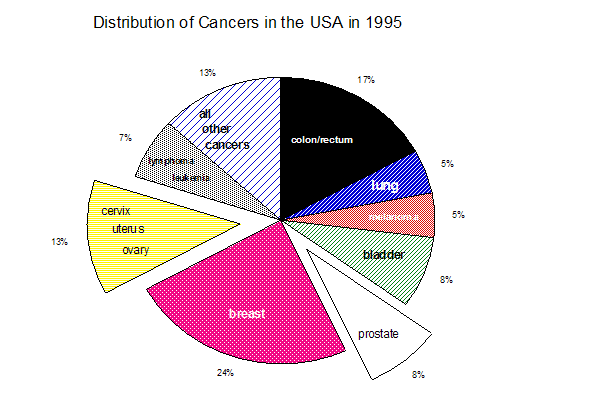

Fig. 1b
Cervical and prostate cancers provide an ideal point of market entry for an aneuploidy-based diagnostic methodology since chromosomal imbalance has been established as a prognostic indicator for these cancers .
There are 350,000 operations done in the USA each year for benign hyperplasias of the prostate (BPH), and the tissue removed is routinely checked for hidden cancer cells. About 69% of the suspicious results following routine digital rectal examination (DRE) or the prostate specific antigen (PSA) blood test ($50 per test) require prostate biopsies . Each year more than 100 million Pap smears are collected worldwide, with 55 million being performed in the USA alone at a cost of $5-20 per test. This puts the Pap smear market in the USA at $1 billion annually. This large market is currently serviced by obsolete and error-prone technologies . Furthermore, 4-8% of Pap smears (2-5 million) are classified as ASCUS, which stands for Atypical Squamous Cells of Undetermined Significance. At present, clinical laboratories are not able to properly characterize ASCUS slides (see Product Development Strategy and Market Entry). Measuring aneuploidy in cervical smears is an independent predictor of biological outcome and should readily sort out the non-cancerous cells, precancerous cells, and cancerous cells in the ASCUS population (see Product Opportunity: Cervical Cancer). Since ASCUS represents a completely untapped market at present, Boveran will charge $100 to properly classify an indeterminate Pap smear, which represents a potential USA market of $220-440 million annually.
5 Key Science Team Members
David Rasnick, PhD, brings 25-years experience in the pharmaceutical/biotech industry. As co-founder of Prototek, Inc., Dublin CA, Dr. Rasnick was instrumental in securing a $5 million deal with Marion Laboratories in 1987 to jointly develop his invention the peptidylfluoro ketones as potential therapeutic drugs for the treatment of arthritis. At Prototek he put together a team consisting of: a production manager who established a GMP facility to synthesize kilogram quantities of the fluoro ketone compounds that were required for the toxicological and pharmacological evaluations necessary for FDA approval; a manager of quality assurance and control responsible for ensuring that all specifications for FDA compliance were established and met; a specific R/D facility to explore the scope of the fluoro ketone technologies. The corporate alliance between Prototek and Marion Laboratories demonstrated that cysteine protease are responsible for the destruction of cartilage and bone in arthritis, identifying these enzymes are important targets for drug development. In 1990, Marion Laboratories was acquired by Merrill Dow, which resulted in the termination of the collaboration between Prototek and Marion. As a visiting scientist in Professor Peter Duesberg's lab at UC Berkeley, Dr. Rasnick developed the theoretical underpinnings of the aneuploidy theory of cancer. The results show that aneuploidy--not gene mutations--is the cause of cancer.
Peter H. Duesberg, PhD, is a professor of molecular and cell biology at the University of California, Berkeley. In 1968-1970 he demonstrated that influenza virus has a segmented genome. He isolated the first cancer gene through his work on retroviruses in 1970, and mapped the genetic structure of these viruses. This, and his subsequent work in the same field, resulted in his election to the National Academy of Sciences in 1986. He is also the recipient of a seven-year Outstanding Investigator Grant from the National Institutes of Health. Professor Duesberg brings over 30-years of cancer research experience to the team. His most recent findings show a 100% correlation between the level of aneuploidy and the extent of transformation of cells grown in culture after treatment with non-mutagenic carcinogens.
6 MARKET OPPORTUNITY
We design our cancer detection products to take advantage of our unique marketing approach (see Unique Marketing Opportunity) based on the aneuploidy theory of cancer instead of the unproductive oncogene/tumor suppressor-gene theory of cancer .
We will offer clinical laboratories a fully automated Fluorescence In situ Hybridization (FISH) microscopic analysis for the quantitative measurement of aneuploidy (chromosomal imbalance) in pap smears, prostate cancer biopsies, and ultimately for all solid cancers. Since chromosomal imbalance causes all cancer quantitative measurement of aneuploidy should substantially reduce the false-negative and false-positive results of conventional methods and still allow the pathologist the flexibility to review individual slides as needed.
While the FDA has approved some karyotyping (chromosome counting) software packages and several fluorescent DNA probe kits for clinical use (see Competition), Management is not aware of any provider of a completely integrated and automated system employing the FISH technology to detect cancer based on aneuploidy. The quantitative output of our automated FISH analysis will provide the pathologist and physician with information upon which to more reliably classify the various types and severity of cervical and prostate cancers. This is important since the choice of therapy is determined by the classification and stage of malignancy .
6.1 Prostate Cancer
Although histopathologic criteria are important in detecting the presence of prostatic carcinomas and in determining probable disease course, significant limitations still exist in the ability of current histologic markers to predict the course for individual patients. (From the Consensus Review of the clinical utility of measuring chromosome imbalance in prostate cancer.)
The majority of prostate tumors (intermediate grade), cannot be determined by current clinical laboratory methods. The measurement of aneuploidy provides the only clinically useful prognostic information for patients with these intermediate grade tumors (Table II).
In prostate cancer, which is heterogeneous, multifocal, and intermixed with benign cells, FISH can accurately detect small changes in the number of chromosomes. In addition, because FISH can be performed on tissue sections, it has greater clinical utility as a prognostic marker tool than flow cytometry.
Using FISH, Henke et al. report that numerical chromosome alterations in prostate cancer coincide with aggressive tumor behavior. Two recent reports further illustrate the value of FISH in diagnosing prostate cancer. FISH analysis of chromosomes 7, 8, 11, and 12 was performed by Takahashi et al. on 50 radical prostectomy specimens obtained by 18-gauge needle biopsy . Their analysis using FISH was more sensitive than traditional flow cytometry analysis in detecting ploidy anomalies, and gains of chromosomes 7 and 8 were associated with higher-grade tumors.
TABLE II
Prostate Cancer Diagnosis
Existing Methods | Our Chromosomal Imbalance Approach |
* histologic markers prone to subjective grading and difficult to automate * intermediate grade tumors are indeterminate * cannot predict clinical course of disease * flow cytometry requires large samples * flow cytometry has low sensitivity * cells are lost at end of flow cytometric analysis | * chromosome number is quantitative and our system is automated * intermediate grade tumors readily characterized * diploid (normal number of chromosomes) tumors have favorable outcome; aneuploid tumors show poor outcome irrespective of stage or therapy * small biopsy samples more than adequate * image analysis sensitive to fractions of a chromosome * cells and images are permanent records |
A study by Macoska et al. compared benign and matched malignant specimens for chromosome 8 abnormalities in 10 prostate cancer patients . The authors concluded that FISH was more sensitive than more traditional techniques in detecting chromosome 8 abnormalities. "[T]hese studies suggest great promise for the use of FISH in elucidating genetic alterations in prostate cancer and in developing FISH-based, clinically, useful prognostic markers" . Indeed, a consensus report concluded that, "DNA content measurement by image cytometry [FISH] provides a significant predictor of disease course or survival. . . . [I]mage cytometry may identify populations of cells or nuclei that flow cytometry studies do not report. Additionally, image cytometry may provide the only means to measure DNA ploidy of small samples, particularly needle biopsies containing small areas of tumor."
6.2 Cervical Cancer
Four to nine percent of Pap smears (2-5 million) are classified as ASCUS, which stands for Atypical Squamous Cells of Undetermined Significance. At present, clinical laboratories are not able to properly characterize ASCUS slides. To be on the "safe side," many ASCUS patients are hospitalized and treated as if they were cancer patients. However, the vast majority of ASCUS cases are cancer-free since there are only 16,000 confirmed new cervical cancers annually but millions of ASCUS cases. We believe (based upon guidance from industry veterans and experts that an estimated it costs approximately $1000 to keep patients in ASCUS status until they show 3 normals or show cancer.) Given this dollar figure and the number of ASCUS patients (millions per year) The elimination of ASCUS (indeterminate) cases represents potential annual savings of billions of dollars in unnecessary hospitalizations and treatments, not to mention saving millions of women the trauma of being treated as cancer patients. Table III compares our approach to existing methods of cervical cancer diagnosis.
TABLE III
Cervical Cancer Diagnosis
Existing Methods | Our chromosomal Imbalance Approach |
* 50 year-old Pap smear technique * 20-25% false negative rate * histologic markers prone to subjective grading and difficult to automate * 2-5 million ASCUS (indeterminate) Pap smears * flow cytometry has low sensitivity * cells are lost at end of flow cytometric analysis | * 100 year-old correlation between chromosomal imbalance and cancer * chromosomal imbalance is a better predictive value than histolopathologic characteristics * chromosome number is quantitative and our system is automated * chromosomal imbalance is a quantitative predictor of clinical outcome * image analysis sensitive to fractions of a chromosome * cells and images are permanent records |
During the last 50 years in the USA, the use of screening programs based on the Papanicolaou (Pap) smear and pelvic examination has led to a steep decline in incidence and deaths from cervical cancer . However, the methods of specimen acquisition, preparation, and evaluation of the Pap smear have changed little since its introduction in the 1940s. Although it is highly effective in screening for pre-invasive lesions of the cervix, a single Pap smear has a false-negative rate estimated to be 20-25% , but can run as high as 50% . One-half of the false negatives are due to inadequate sampling, and the other half are attributed to a failure to identify the abnormal cells or to interpret them accurately .
Manual screening of Pap smears is very labor intensive and demands that the cytotechnologist be capable of high levels of concentration for extended periods. In addition, cytotechnologists may feel pressure to examine as many slides as they can legally despite recent regulatory changes attributable to the Clinical Laboratory Improvement Act of 1988 (CLIA '88). A slide may have only a few abnormal cells, which could be easily overlooked by a fatigued technologist at the end of a busy workday. Recent reports suggest that some of the false negative results are caused by these screening errors. An automated screening machine, such as our AnuCyte system, would not be subject to fatigue and, therefore, could reduce this type of error .
Failure to recognize abnormal cells is another possible source of error. A few states have instituted proficiency testing programs in an effort to minimize this type of error. A hypothetical ideal automated screening machine could be programmed so that it would recognize all types of potentially abnormal cells. Of course, no machine performs ideally in practice. Several companies are developing automated screening machines in an attempt to reduce the false negative rate of Pap smears without having an unreasonably high rate of false positives (see Competition). The systems currently under development generally automate the 50-year old Pap smear technology without incorporating the advanced fluorescent probe technologies of the 1990s. A couple of firms include the additional feature of automating the preparation of conventional Pap smear slides.
In the fall of 1995, the Food and Drug Administration (FDA) approved two automated instruments for rescreening smears evaluated as negative on the initial screen. Data from the trials suggest that the new instruments could reduce the rate of false-negative smears. However, neither the efficacy in routine practice nor the cost-benefit of these devices has been determined.
Aneuploidy is the most common chromosomal abnormality observed in cervical and ovarian cancers . According to a number of authorities on cancer of the cervix, "[Aneu]ploidy appears to be a good predictor of biologic behavior and may have better predictive value than histopathologic characteristics judged by the eye" . The same authorities are critical of flow cytometry since it is not as sensitive as microscopic image analysis for the detection of cervical cancer.
7 Product Development Strategy and Market Entry
7.1 Option A: prostate cancer diagnosis
We selected prostate cancer as a candidate for market entry because the prostate tumors with an intermediate histological grade, which represent the majority of prostate tumors, cannot be determined by current clinical laboratory methods. The measurement of chromosomal imbalance provides the only clinically useful prognostic information for patients with these intermediate grade tumors . Management's research indicates there are approximately 2 million men living with prostate cancer in the USA , with a growth rate of about 200,000 new cases per year. As a start up company with no operational history concerning the AnuCyte System, there are significant assumptions and risks associated with our ability to successfully introduce AnuCyte into the market as discussed in Section 9 below. However, based on the figures discussed above and the assumption that we can both fund this expansion and penetrate these markets, we believe that we can potentially capture up to 25% of the prostate cancer diagnostics revenue the first year on the market. If we can capture this percentage of the market as we hope, revenues of $12 million could be obtained the first year on the market and over $50 million by the end of the third year (equating to 70% of the prostate cancer diagnostics revenue) (see Fig. 1b and Projections and discussion of assumptions and risks.
7.2 Option B: cervical cancer diagnosis
Currently, cervical cancer represents a larger market than prostate cancer (see Markets), with 55 million Pap smears collected in the USA annually . We will not attempt initially to compete in the Pap smear screening market, which is well established and limited to low costs per test. Our strategy will be to pioneer the competition-free market of 2-5 million ASCUS (indeterminate) Pap smears generated annually. Measuring chromosomal imbalance in cervical smears is an independent predictor of biological outcome and should readily sort out the non-cancerous cells, precancerous cells, and cancerous cells in the ASCUS population . Management believes there is no method at present in clinical laboratories for deciding the status of ASCUS slides, a cost of $100 to classify a previously indeterminate ASCUS slide is justified. Management estimates that this puts the ASCUS market in the USA at $200-500 million annually. As a start up company with no operational history concerning the AnuCyte System, there are significant assumptions and risks associated with our ability to successfully introduce AnuCyte into the market as discussed in Section 9 below. However, based on the figures discussed above and the assumption that we can both fund this expansion and penetrate these markets, we project cervical cancer diagnostics revenues of $16 million the first year on the market and $50 million by the end of the third year (see Projections).
8 COMPETITION
The pathology and cytology departments are separated in the big clinical laboratories, producing two distinct markets. Tissue biopsies come under the domain of pathology, whereas Pap smears are analyzed in the cytology laboratory. To the best of our knowledge there are no integrated, fully automated systems for chromosomal imbalance and FISH diagnostic methodologies currently being used in clinical laboratories or in development.
8.1 Prostate Cancer
In spite of the 1993 consensus review advocating the clinical utility of DNA analysis in prostate cancer, pathologists continue to rely on cell morphology and do not perform chromosomal analysis in deciding if cancer is present in prostate biopsies primarily because there is a lack of appropriate instrumentation. Solid cancers produce inadequate numbers of mitoses for conventional chromosomal characterization, and needle biopsies of prostate tissues do not provide a large enough number of cells for flow cytometric analysis. Automated flow cytometry is a well established and broadly available technology that can be used to characterize the gross genetic and chromosomal differences between populations of normal and cancer cells. However, flow cytometry lacks the sensitivity of FISH to be able to characterize the abnormalities of individual chromosomes in specific cells .
Our automated system uses FISH analysis technology to eliminate these problems because it does not require the presence of mitoses for chromosomal analysis, and can analyze all cells in a biopsy. FISH analysis also requires much smaller sample size than flow cytometry and allows a pathologist to conveniently go back and inspect individual prostate specimens and specific cells, which is not possible with flow cytometry. In short, there is no direct competition to an automated FISH analysis in the prostate market.
8.2 Cervical Cancer
In contrast to prostate cancer, there is a 50 year-long established market in cervical cancer diagnosis. The Papanicolaou stain (and variations of it) has the advantage of wide familiarity and low costs, which present serious obstacles to market penetration by superior technology. Nevertheless, the persistent high levels of false positive (5-20%) and false negative (20-25%) results that plague conventional Pap smear analysis presents an opening to market entry which few have pursued successfully. For the reasons stated above (see Product Development Strategy and Market Entry: Option B), We will not attempt initially to compete in the Pap smear screening market. Instead, we will start by analyzing the competition-free ASCUS (indeterminate) Pap smear market. The number of ASCUS customers are 2+ million per year in the USA .
8.3 Microscope/Imaging Systems
8.3.1 Fluorescence Microscopy Industry
To the best of our knowledge, no company, listed below or otherwise, possesses either a automated device to measure chromosomal imbalance as the sole or primary means of detecting cancer, nor do they possess our proprietary method of preparing tissue samples to enable accurate inspection of the cell nucleus.
Our closest potential competitor used to be Vysis before the company was acquired by Abbott Laboratories. Vysis provides Abbott with DNA probe reagents for its UroVysion(tm) test. However, to the best of our knowledge, Vysis has not gone the next step, as we have, of providing an integrated, fully automated system for the general diagnosis of solid cancers.
Abbott is the first to introduce chromosomal analysis as part of its bladder cancer diagnostic UroVysion. Unlike AnuCyte, Abbott's test is not automated and relies on cytologists to examine the slides. UroVysion(tm) quantifies three specific chromosomes because "Individual genes are often too small to be effective probes, so a long stretch of DNA that contains the gene of interest is used as the probe, e.g. 17p13 DNA for TP53 , and 9p21 DNA for cell-dependent kinase inhibitor 2A ( CDKN2A, also known as p16 , p14, ARF, INK4a and others) a major bladder cancer tumor suppressor gene" .. Based on our research, we understand that the fact UroVysion(tm) is detecting aneuploidy, and that its detection is incidental to the their belief that specific genes on those chromosomes are responsible for bladder cancer. By describing UroVysion(tm) as gene-based, Abbott indicates it does not understand that testing for aneuploidy is synonymous with testing for cancer. Abbot's system does not therefore purport to use chromosomal imbalance as the sole or primary means of detecting cancer. Furthermore, their system is not automated and requires a person to perform the analysis. Our system is both fully automated, objective, and uses chromosomal imbalance as the sole and primary means of detecting cancer and functions for all cancer types. We conclude Abbot is not a likely competitor.
BioView LTD is an Israeli company. The company says it develops, manufactures and assembles, markets and services an automated cell diagnostic system for use in cytology, cytogenetics, hematology and pathology laboratories. The company's major system is comprised of an automated scanning imaging workstation called Duet(tm), an offline-workstation with proprietary software called Solo(tm), and preparation kits called BioWhite(tm), BioRed(tm) and BioBlue(tm). In 2005 the Company introduced two new scanning workstations: the ALLEGRO for automated scanning of FISH probes without Brightfield scanning and the ACCORD for the semi-automated imaging of FISH for Breast Cancer (Her-2/Neu) and for FISH on Tissue. The Company has received FDA clearance to use the Duet for cells in urine specimens, stained by FISH using the Vysis UroVysiontm(tm) Bladder Cancer Recurrence Kit for chromosomes 3, 7, 17 and 9p21 locus, from subjects with transitional cell carcinoma of the bladder. However, to the best of our knowledge, the BioView system is not being used with the UroVysion test. It is difficult to find citations in the scientific/medical literature where the BioView systems have been used to analyze samples for cancer. Attempts to contact BioView have failed. We continue our efforts to contact BioView to explore the possibility of synergy between our approaches. The threat BioView poses is unknown at present because there is so little information about the company's activities that impinge upon our plans.
PSI (Perceptive Scientific Instruments, Inc.) provides an interactive Apple Macintosh controlled FISH workstation for the research laboratory only and does not have FDA approval for clinical use. The PowerGene probe software package incorporates a useful feature that automatically counts probe signals (dots) in the interphase nuclei. A statistical summary automatically presents the resultant data. To the best of our knowledge, they do not have a system for the automated detection of cancer using chromosomal imbalance as the primary or sole indicator and as such does not appear to be poised to enter in a meaningful way into direct competition with us in the automated diagnosis of cancer.
Leica Imaging Systems Ltd provides the QPloidy application software for the LEICA Q600 image analysis system for pathology and oncology research laboratory environments but does not have FDA approval for clinical use. The Leica system is similar to the PSI product but uses the Microsoft Windows environment instead of the Macintosh. To the best of our knowledge, they do not have a system for the automated detection of cancer using chromosomal imbalance as the primary or sole indicator and as such does not appear to be poised to enter in a meaningful way into direct competition with us in the automated diagnosis of cancer.
The BioDx, Inc., MultiFluor Interactive Analysis system is designed for interphase spot counting, fluorescence intensity screenings, and general cell morphometry measurements. With the Optional SlideScan Scanning Module, Automated Analysis becomes a fully automated system for rapidly scanning and analyzing microscope slide-based samples. With microscope automation present, the SlideScan Module adds field-to-field stage control, automated focus, multiple wavelength and multiple focal plane image acquisition, and object relocation capabilities. The MultiFluor imaging software runs on a standard Pentium-based PC operating under Microsoft Windows. The MultiFluor system is not approved for clinical use, but represents a good starting point for the development of a clinical system. To the best of our knowledge, they do not have a system for the automated detection of cancer using chromosomal imbalance as the primary or sole indicator and as such does not appear to be poised to enter in a meaningful way into direct competition with us in the automated diagnosis of cancer.
CompuCyte Corp., uses a laser scanning cytometer adaptation of flow cytometry to perform FISH analysis of microscopic slides. The system is quite advanced, but lacks full automation and is for research use only and is not approved by the FDA for clinical use. To the best of our knowledge, they do not have a system for the automated detection of cancer using chromosomal imbalance as the primary or sole indicator and as such does not appear to be poised to enter in a meaningful way into direct competition with us in the automated diagnosis of cancer.
8.3.2 Conventional Light Microscopy
The NeoPath AutoPap 300 is designed to work with the conventional Papanicolaou stain and has a stated goal of removing 50% of the negative slides from the workload in the prescreening mode. The company has proposed a rescreening protocol using the AutoPap 300 QC machine in which 100% of the negative slides would be examined. This protocol was recommended for approval by the FDA advisory panel. To the best of our knowledge, they do not have a system for the automated detection of cancer using chromosomal imbalance as the primary or sole indicator and as such does not appear to be poised to enter in a meaningful way into direct competition with us in the automated diagnosis of cancer.
The PAPNET system from Neuromedical Systems, Inc., uses conventionally prepared smears. Slides are sent to Neuromedical Systems (the manufacturer), where they are scanned, and 128 digital images of potentially abnormal cells or clusters of cells are stored on a digital tape. The tape and the slides are returned to the laboratory, where the 128 potentially abnormal fields are reviewed manually by a cytolotechnologist on a PAPNET review station. The system is totally interactive and does not attempt to remove any cases from the workload. In the USA, PAPNET is only approved for quality control of primary screening, but in the UK, Dulcie Coleman (Imperial College School of Medicine, London) is coordinating a multi-center trial to evaluate PAPNET for use in primary screening. The study, which started in September 1997 and is due for completion in May, 1998, will assess accuracy and cost-effectiveness (70). Charges of $10-20 per slide have been quoted for utilizing the PAPNET service. In addition, the PAPNET review station must be leased or purchased from Neuromedical Systems, Inc. To the best of our knowledge, they do not have a system for the automated detection of cancer using chromosomal imbalance as the primary or sole indicator and as such does not appear to be poised to enter in a meaningful way into direct competition with us in the automated diagnosis of cancer.
Morphometrix Technologies Inc. ("Morphometrix") was founded in early 1993 to develop, manufacture and market an automated instrument to visually analyze and diagnose Pap specimens.
The aim of Morphometrix is to be the leader in producing automated systems for the early detection of cervical cancer and other cytopathologies. The Company has developed advanced proprietary image recognition and interpretation technologies which automate the screening process, increasing the throughput speed and substantially reducing its screening costs.
Morphometrix's first product, the CYMET A40 Automated Pap Screening System, automatically analyzes monolayer Pap specimens for cervical cancer and its precursors.
To the best of our knowledge, they do not have a system for the automated detection of cancer using chromosomal imbalance as the primary or sole indicator and as such does not appear to be poised to enter in a meaningful way into direct competition with us in the automated diagnosis of cancer.
The Xillix Cyto-Savant has been designed to work with conventional preparations that have been air dried, as is standard practice in British Colombia, Canada, where it is being developed. A difference with the Xillix system is that slides are stained initially with a Feulgen-Thionin stain, which is stoichiometric for DNA. The Papanicolaou stain may be applied later for human review. A goal of the system is to remove 60-80% of the negative slides from the workload in a prescreening mode. It also may be used in a rescreening mode or in an interactive mode in which the cytolotechnologist reviews cells from each slide on the monitor. The machine is currently undergoing clinical trial evaluation. Oncometrics is a spin-off of Xillix, which uses imagining of conventionally (non-fluorescent) stained slides to diagnose lung cancers for research purposes only. To the best of our knowledge, they do not have a system for the automated detection of cancer using chromosomal imbalance as the primary or sole indicator and as such does not appear to be poised to enter in a meaningful way into direct competition with us in the automated diagnosis of cancer.
The Roche AutoCyte system is designed to be fully interactive. Specimens are evaluated with statistical algorithms. The system presents the 120 most significant cellular events on its video monitor for evaluation by the cytolotechnologist. A noteworthy feature is the automated preparation of monolayer slides by the AutoRich process to facilitate image analysis. When compared with conventional smears, the monolayers have been shown to allow equal or slightly higher rates of detection of squamous intraepithelial lesions than manual examination. To the best of our knowledge, they do not have a system for the automated detection of cancer using chromosomal imbalance as the primary or sole indicator and as such does not appear to be poised to enter in a meaningful way into direct competition with us in the automated diagnosis of cancer.
The CYTYC CDS-1000 cytology workstation uses monolayer preparations as well, produced on the CYTYC ThinPrep Processor. For use with the CDS-1000 workstation, the slides are stained with the CYTYC EnhanCyt Nuclear Papanicolaou stain, which is formulated to look similar to the standard Papanicolaou stain to the human eye, but is said to be more reproducible and therefore superior for computer analysis. A specially modified Apple Macintosh computer is used in the workstation. The system is designed to be used in the interactive mode. To the best of our knowledge, they do not have a system for the automated detection of cancer using chromosomal imbalance as the primary or sole indicator and as such does not appear to be poised to enter in a meaningful way into direct competition with us in the automated diagnosis of cancer.
9 STATEMENT OF RISKS
9.1 Limited History with No Profitable Operations
The AnuCyte system has no history of profitable operations. There are no assurances that Insight Medical Group will be able to develop its markets successfully or that the concept will be received as intended, and that the business will be operated profitably. Accordingly, the projections outlined herein may not materialize at all or may be materially less than we have stated.
9.2 Dependence on Key and Professional Personnel
The Company's success depends to a significant extent on the efforts, knowledge, and skills of certain key management personnel. If any of their services were to become unavailable, it may have a material adverse affect on the Company and its ability to meet the projections outlined herein. Also, the Company relies on its ability to recruit and retain highly qualified management personnel. The extent to which the Company fails to attract and retain such individuals could have a material adverse effect on the Company (see Section 5).
9.3 Dependence on Strategic Partners, Alliances and Sub-contractors
The AnuCyte diagnostics concept may depend on successful partnering with various parties. If a partner fails to deliver on its agreements or the Company fails to resolve any disputes or reach agreement, there will be a material adverse effect on the Company's business. At present, Insight Medical Group has formed no partnerships.
9.4 FDA approval and Convincing Medical Professionals to Request AnuCyte Tests
At present, the Company does not nor does the Company have immediate plans to operate a laboratory in the United States. However, Should the Company choose to operate a laboratory in the United States, the FDA would have jurisdiction but the Company does not require FDA approval because AnuCyte is an in-house-developed assay. In-house-developed assays are commonly know as home brews. Despite the informal name, home-brew assays are widely accepted as scientifically valid and are relied upon routinely throughout the healthcare system. They are regulated by the Centers for Medicare & Medicaid Services (CMS) under the Clinical Laboratory Improvement of 1988 (CLIA).
In August 1992, FDA issued a draft compliance policy guideline proposing to apply general medical device regulation to home-brew assays. The laboratory community objected, arguing that they were adequately regulated under CLIA, that FDA regulation would be duplicative, and that FDA lacked legal authority to regulate laboratory testing services. FDA withdrew its proposal, but insisted that it had authority to regulate home brews should it wish to do so.
In November 1997, however, CDRH published a final rule governing the use of ASRs in certain in vitro diagnostic products (IVDs) and in-house laboratory assays. The final rule was the culmination of a lengthy process in which FDA sought to determine how, if at all, it would regulate clinical laboratories that prepare in-house assays using ingredients purchased from third-party biological and chemical suppliers . In the ASR regulation, FDA invoked the restricted-device authority in section 520(e) of the Federal Food, Drug, and Cosmetic Act (FD&C Act). It did so to impose certain restrictions on the sale, distribution, and use of ASRs when used as ingredients of home-brew assays and in certain IVDs.
As FDA made clear, the agency was not actively regulating the in-house tests and had determined that strong public health reasons existed for continuing this approach. In the final rule, FDA recognized that "the use of in-house-developed tests has contributed to enhanced standards of medical care in many circumstances, and that significant regulatory changes in this area could have negative effects on the public health." The agency said that the laboratories would be responsible for both the quality and interpretation of results generated from those tests . Thus, the final rule focused not on the in-house tests, but on the ASRs that are used in preparing such tests.
Furthermore, the company does not treat, cure, or diagnose any disease. We offer laboratory services to detect the presence or absence of advanced chromosomal imbalances within the nucleus of cells. The pathologist, doctor, or other licensed health-care practitioner makes a diagnosis to the patient. However in order to expand its marketing capabilities (for example: direct marketing of our services to consumers) within the USA the Company may wish to obtain FDA approval and undertake clinical trials to provide proof of the cancer diagnostic benefits associated with assessing chromosomal imbalance. There is the risk that the clinical trials may not show sufficient utility to obtain FDA approval. Furthermore, if in the future the Company is unable to convince sufficient medical professionals of the merits of the AnuCyte chromosomal imbalance test, it may materially impair the Company's ability to succeed, which could result in the projections outlined herein not materializing at all or be negatively affected in a material way.
9.5 Competition
The Company's competitors are only partially defined at the present, however, several large diagnostics companies could become interested in the market in which the Company plans to compete. Potential competitors could possess greater financial, technical, and marketing resources than the Company. The extent to which the competition offers better products or services than the Company, could have a material adverse effect on the business (see Competition)
9.6 Time to Market
The Aneuploidy Theory of Cancer has been contemplated for nearly a century. There is the risk that a competitor with greater resources could develop a similar diagnostic device and achieve FDA approval in advance of the Company, which could have a material adverse effect on the business. We seek to protect the Company with international patents for our test and techniques. (see Markets and Competition)
9.7 Revenue Projections
The sales/revenue projections are provided by Management. Although Management has no reason to question the validity of the assumptions used in the projections, there is no guarantee that the projected results will be achieved.
The revenue models and associated spreadsheets were acquired as part of the Asset Acquisition of the Boveran Assets. We believe these models and spreadsheets are of significant value and were an important part of the assets acquired. These spreadsheets and model were crafted at great expense of both time and money by industry professionals. Management believes the validity of the methodology and the models themselves are as valid today as they were in 1999. Management reviewed these models carefully in 2006 and determined that no changes were required or desired. The population, rates of incidence, disease prevalence, etc data used in the models are from 1997. The current data have increased from the 1997 data. Therefore the original data was purposely used in our current revenue projections in order to bias the revenue projections to a lower sales number in order to bias the overall revenue modeling and sales projections as conservatively as reasonably possible. Should the Company choose to replace the data used in the model with the most currently available data on population, rates of incidence, etc, the revenue projections would increase accordingly. The more current data is provided herein as demonstration of this fact.
The revenue models only cover the USA market. As part of our initial marketing strategy, we will market our services to the Carribean and Central and South America concurrently with our entry into the US Market. Our revenue projections do not include the sales figures from the international marketplace. We have no reasonably accurate data or management knowledge of these markets sufficient to create a good-faith model. However, we have selected these markets to offer our services into as part of our overall marketing strategy of entry into the USA marketplace as a means to establish and pre-existing customers and enhance marketplace credibility. The revenue projections, therefore, could easily be increased by a substantial amount should we meet with nominal success in the early international marketplace. However, if we fail in the international marketplace, the projections outlined herein will likely not materialize at all or be affected negatively in a material way. We are not willing to make good-faith projections on these international markets at this time due to the reasons stated. If we included our revenue expectations for our international markets, the revenue projections would be adjusted upward accordingly. However, Management is not prepared at this time to make good-faith estimates on these markets. We have therefore not included our international revenue expectations.
Assumptions used in the revenue models and projections are based on a gradual assumed increase of the Company's share of the target market and a gradual assumed increase of the general acceptance of chromosomal imbalance testing within that market. For example, for our first target market for 'indeterminates', we assume, based on a good-faith estimate by Management, an initial Market Penetration of testing chromosomal imbalance at 5% increasing by 5% each year. Of this calculated amount, we assumed, based on Management's good faith estimate, that the Company will represent 100% of this portion of the overall market. We assume the company will have 100% of the market because no competition exists within that market space. Therefore, the main limiting factor of our ability to penetrate the target market, in the example of 'indeterminates' , is the estimated percent of that market that will decide to use chromosomal imbalance testing.
The size of the markets, for the purposes of revenue modeling, were based on data and population figure from 1997. For example, the number of Pap smear test performed annually has been fairly constant at 55 million. The revenue model for 'indeterminates' uses the number of 6% as taken directly from the US Federal Government's NCI data as the number used in our model. The revenue analysis purposely assumes the number of tests will not increase over time in order to bias the revenue projection to a lower sales number in order to bias the overall revenue modeling and sales projections as conservatively as reasonably possible. As another example, the prostate revenue model uses prostate cancer prevalence figure of 10 million men how have prostate cancer at some stage, including those unaware of the disease. This figure was originally obtained from CapCure.Org as well as recently reconfirmed from the US Federal Government's National Cancer Institute . Per the NCI, one our of three men over 50 have some form of prostate cancer. According to the 2000 Census, there were 38 million men over 50 in the USA. One-third of those would be just under 13 million. Therefore, according the National Cancer Institute, there were just under 13 million men over 50 who had some form of prostate cancer. We have purposely chosen the lower number 10 million and purposely used census data from 2000 in order to bias the overall revenue modeling and sales projections as conservatively as reasonably possible. If we used the actual reported number of 13 million and the most recent census data, the revenue projections would rise accordingly.
Furthermore, the revenue models uses population data from 1997 that has purposely not been update to the most recent 2005 population data in order to bias the projections to a more conservative output. Should the Company choose to update the revenue models with the most recent, larger, population numbers, the revenue projections would increase proportionately. We have instead purposely chosen to retain the original 1997 population numbers in order to bias the overall revenue modeling and sales projections as conservatively as reasonably possible.
Additionally, the rates of disease incidence were taken from the original data set of the 1997 data used in the construction of the revenue analysis models. The most recent rates of disease incidence (included herein: Prostate Revenue Spreadsheet, Demographic Detail) are higher than 1997. Should the Company choose to update the revenue models with the most recent, higher, rates of incidence data, the revenue projections would increase proportionately.
The population data and the rates of disease incidence data have been purposely taken from the model's original 1997 dataset in order to bias the overall revenue modeling and sales projections as conservatively as reasonably possible.
The assumed and expected charge we have used in our revenue models for our ASCUS test to be $100 to properly classify an indeterminate Pap smear. We estimate $100 to be our price for prostate tests also. Management has chosen the figure of $100 as market-competitive and comparably priced to other tests in use today as per our conversations and research with industry veterans and therefore believes the amount to be reasonable and justified.
9.8 Product Liability Potential
Although Management believes that the AnuCyte test methodology is a great improvement over existing test procedures, there is no "fool-proof" method of cancer detection. While we believe the situation will not occur, there is the potential that our product could provide false negative and false positive test results. In the event of a false positive result, the individual is incorrectly diagnosed to have cancer, while a false negative incorrectly diagnoses the individual to be without cancer. There is the risk that an erroneous test result may expose the Company to litigation. Although the Company will use its best efforts to obtain product liability insurance, there is the risk that the cost of such insurance may be prohibitively expensive or that the coverage will not be sufficient to cover a claim against the Company impairing continued operations.
10 SALES PROJECTIONS
The following revenue projections are based on our best, good-faith estimate and rely heavy on a number of assumptions (See "Risk Factors and Revenue Projection spreadsheets). Actual results may vary substantially from these projections, either higher or lower than expected. These projections are based on estimates of market share capture and our ability enter these markets successfully. If we are unable to achieve our marketing goals or fail to raise sufficient funding to expand the business we may not meet these projections.
10.1 Prostate Cancer Diagnosis
Prostate tumors with an intermediate histological grade represent the majority of prostate tumors that cannot be determined by current clinical laboratory methods. We believe the measurement of chromosomal imbalance (aneuploidy) provides the only clinically useful prognostic information for patients with these intermediate grade tumors.
Our market research indicates that there are approximately 2 million men living with prostate cancer in the USA , with a growth rate of about 200,000 new cases per year. As a start up company with no operational history concerning the AnuCyte System, there are significant assumptions and risks associated with our ability to successfully introduce AnuCyte into the market as discussed in Section 9 above. However, based on these figures and assuming we can penetrate these markets and properly fund this expansion, the company estimates prostate cancer diagnostic revenues of potentially $12 million the first year for the market and potentially over $50 million by the end of the third year.
10.2 Entry into the competition-free 'indeterminate' Cervical cancer market
Currently, cervical cancer represents a larger market than prostate cancer with 55 million Pap smears collected in the USA annually . The company's first entry into this market space will be the competition free market of 2-5 million (and rising) "ASCUS" (indeterminate) Pap smears generated annually.
Based on scientific research and system testing, we believe measuring chromosomal imbalance in cervical smears is an independent predictor of biological outcome and will sort out the non-cancerous cells, precancerous cells, and cancerous cells in the ASCUS slide's cell population. We are not aware of any method at present in clinical laboratories for deciding the status of an ASCUS slide. Based on the science and the system test results we believe that the AnuCyte system can quickly deliver comprehensive and accurate results for any ASCUS slide. Our estimates put the ASCUS market in the USA at $200-$500 million annually. As a start up company with no operational history concerning the AnuCyte System, there are significant assumptions and risks associated with our ability to successfully introduce AnuCyte into the market as discussed in Section 9 above However, based on these figures and the assumption that we can both fund this expansion and penetrate these markets, the company projects cervical cancer detection revenues of potentially $16 million the first year for the market and potentially $50 million by the end of the third year.
10.3 Overall Cancer Diagnostic Marketplace
The worldwide market potential is unquantifiable. The 'AnuCyte Cancer Detection System' is not limited to the cancers mentioned above. A wealth of scientific research and system testing lead us to believe that the system functions for any slide preparation and delivers rapid and accurate results in all suspected cancerous samples. It must be noted that our predictions only cover the USA market. As part of our initial marketing strategy, we will market our service to the Carribean and Central and South America concurrently with our entry into the US Market. Our revenue projections do not include the sales figures from the international marketplace. The initial and concurrent marketing of our services to areas outside the USA are intended to establish early sales and credibility for our product. We believe the international market is larger and will be part of the company's initial marketing efforts alongside the USA.
10.4 Summary and Background for Revenue Modeling
Our revenue projection models originate from 1999-2000 and were originally based on population and cancer incidence rate from the latest data of 1997. Based on the fact both population and rates of incidence data have increased since 1997 and a reasonably small number of market penetration percentages were estimated by Management, we believe that the models are conservative. The revenue calculations would be higher if the most recent higher population data were used alongside the more recent higher rates of incidence . The models and the data were reviewed in 2006 and determined by Management to be valid for the purposes of revenue modeling and projections. The rates of incidence data and the population data were not updated to the most recent data in order to purposely bias the revenue projections to the most reasonably conservative number output. The market percentage penetration numbers were arrived at based on careful consideration by management and advisors to Management at the time of the model's creation as well as reconsideration of the entire model and data in 2006. Based on the input from the original models' authors, Management's expertise and industry knowledge, Management concluded the percentages chosen were both reasonable and in Management's opinion, conservative. The actual results and percentage of market penetration may be higher or lower than Management's estimates. We believe the revenue projection models consistent with industry projection modeling techniques and have found to reason to change the calculations or methodology used in the original 1999-2000 models. Based on our belief that the 1997 data creates conservative modeling numbers, we chose to retain the 1997 dataset in order to bias the revenue projections to a more conservative output for our planning purposes. If we updated the models to the 2005 dataset, and based on the fact the revenue projection are based on percentages of these base numbers, therefore the revenue projections would rise accordingly as the rates of incidence as well as population numbers have risen.
10.4.1 Spreadsheet: ASCUS Revenue Projections
| AnuCyte Diagnostics | | | | Scenario: | | Base Case | | | | | | | |
| Cancer Test Revenue Analysis | | | | Product: | | ASCUS - Cervical Cancer Test | | | | | |
| ASCUS - Cervical Cancer Test | | | | Geographic Market: | USA/World | | | | | | |
| | | | | | | | | | | | | | |
| A. Total Patient Population | | | | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
| A1. Total Pap Smear Tests Annualy (000s) | | | (1) | 55,000 | 55,000 | 55,000 | 55,000 | 55,000 | 55,000 | 55,000 | 55,000 | 55,000 | |
| A2. Avg. Rate of ASCUS smear results (%) | | | (2) | 6% | 6% | 6% | 6% | 6% | 6% | 6% | 6% | 6% | |
| A3. Annual ASCUS Results (Total Patient Pop. in 000s) | | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 | |
Growth Rate (%) | | | | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| B. Prime Diagnostic Patient Population | | | | | | | | | | | | | |
| B1. % of ASCUS slides capable of bDNA FISH Analysis | | | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
| B2. Prime Diagnostic Patient Population (000s) | | | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 | |
Growth Rate (%) | | | | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| C. Market Penetration of Ploidy Tests (%) | | | 0% | 5% | 10% | 15% | 20% | 25% | 35% | 45% | 60% | |
Growth Rate (%) | | | | N/A | N/A | 100% | 50% | 33% | 25% | 40% | 29% | 33% | |
| D. Market Share of AnuCyte Test (%) | | | | 0% | 100% | 100% | 100% | 90% | 80% | 70% | 60% | 50% | |
Growth Rate (%) | | | | N/A | N/A | 0% | 0% | -10% | -11% | -13% | -14% | -17% | |
| E. Tests Per Patient Per Year | | | | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
Growth Rate (%) | | | | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| F. Total Tests (000s) (B*C*D*E) | | | | 0 | 165 | 330 | 495 | 594 | 660 | 809 | 891 | 990 | |
Growth Rate (%) | | | | N/A | N/A | 100% | 50% | 20% | 11% | 23% | 10% | 11% | |
| G. Average Selling Price Per Test ($) | | | | $0.00 | $100.00 | $100.00 | $100.00 | $95.00 | $90.00 | $85.00 | $80.00 | $75.00 | |
Growth Rate (%) | | | | N/A | N/A | 0% | 0% | -5% | -5% | -6% | -6% | -6% | |
| H. Product Revenue ($000s) (F*H) | | | | $0 | $16,500 | $33,000 | $49,500 | $56,430 | $59,400 | $68,723 | $71,280 | $74,250 | |
Growth Rate (%) | | | | N/A | N/A | 100% | 50% | 14% | 5% | 16% | 4% | 4% | |
| I. Reagent Costs ($000s) (Expense Item) | | | $0 | $0 | $0 | $0 | $0 | $0 | $0 | $0 | $0 | |
| Either | | | | | | | | | | | | | |
| 1. % of Sales (Input %) | | | | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Or | | | | | | | | | | | | | |
| 2. Reagent Costs as ($ per Test) | | | | $- | $- | $- | $- | $- | $- | $- | $- | $- | |
| | | | | | | | | | | | | | |
| (1) The number of Pap smear tests performed annually has been fairly constant at 55 million. This analysis conservatively assumes that the number of tests does not grow over time. |
| (2) The annual rate of ASCUS (abnormal Pap smear with indeterminant results) has ranged consistently between 4% and 8%. This analysis assumes a constant average rate of 6%. |
| | | | | | | | | | | | | | | | |
10.4.2 Spreadsheet: Prostate Cancer Diagnostics Revenue Projections
| AnuCyte Diagnostics | | | | Scenario: | | Base Case | | | | | | | |
| Cancer Test Revenue Analysis | | | Product: | | Prostate Cancer Ploidy Diagnostics Test | | | | |
| | | | | Geographic Market: | USA | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
| Prostate Hyperplasia (PHP) New Cases (000s) | (1) | 2,314 | 2,430 | 2,551 | 2,679 | 2,813 | 2,954 | 3,101 | 3,256 | 3,419 | |
| Growth Rate (%) in New Cases | | | | 5% | 5% | 5% | 5% | 5% | 5% | 5% | 5% | 5% | |
| Prostate Cancer Population | | | | | | | | | | | | | |
| Growth Rate (%) in New Cases | | | | 5% | 5% | 5% | 5% | 5% | 5% | 5% | 5% | 5% | |
| A1. Beg. of Year Pros. Can. Patient Pop. (000s) | | (2) | 2,442 | 2,662 | 2,891 | 3,132 | 3,384 | 3,649 | 3,926 | 4,216 | 4,521 | |
| A2. Total New Pros. Cancer Cases (000s) | | | (1) | 231 | 243 | 255 | 268 | 281 | 295 | 310 | 326 | 342 | |
| A3. Reductions in Pop. (deaths/other) (000s) | | (3) | (12) | (13) | (14) | (16) | (17) | (18) | (20) | (21) | (23) | |
| A. Total Patient Population (000s) | | | | 2,662 | 2,891 | 3,132 | 3,384 | 3,649 | 3,926 | 4,216 | 4,521 | 4,840 | |
| Population Growth Rate (%) | | | | 9% | 9% | 8% | 8% | 8% | 8% | 7% | 7% | 7% | |
| Annual Biopsies = Prime Patient Population | | | | | | | | | | | | | |
| B1. Existing Cancer Patient Pop. (% Biopsies) | | | 0.1% | 0.5% | 5.0% | 7.0% | 10.0% | 15.0% | 18.0% | 20.0% | 20.0% | |
| B1. Prime Patient Pop. Existing Cancer (000s) | | | 2 | 13 | 145 | 219 | 338 | 547 | 707 | 843 | 904 | |
| B2. Prime Patient Pop. New PHP Cases (000s) | | | 463 | 486 | 511 | 536 | 563 | 591 | 621 | 652 | 684 | |
| B2. New PHP Cases Patient Pop. (% Biopsies) | | (4) | 20% | 20% | 20% | 20% | 20% | 20% | 20% | 20% | 20% | |
| B. Prime Diagnostic Patient Population (000s) | | 466 | 500 | 655 | 756 | 902 | 1,139 | 1,328 | 1,495 | 1,589 | |
Growth Rate (%) | | | | 5% | 7% | 31% | 15% | 19% | 26% | 17% | 13% | 6% | |
| C. Market Penetration of Ploidy Tests (%) | | | 0% | 25% | 50% | 70% | 75% | 80% | 85% | 90% | 99% | |
Growth Rate (%) | | | | N/A | N/A | 100% | 40% | 7% | 7% | 6% | 6% | 10% | |
| D. Market Share of AnuCyte Test (%) | | | 0% | 100% | 100% | 100% | 90% | 80% | 70% | 60% | 50% | |
Growth Rate (%) | | | | N/A | N/A | 0% | 0% | -10% | -11% | -13% | -14% | -17% | |
| E. Tests Per Patient Per Year | | | | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
Growth Rate (%) | | | | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| F. Total Tests (000s) (B*C*D*E) | | | | 0 | 125 | 328 | 529 | 609 | 729 | 790 | 807 | 786 | |
Growth Rate (%) | | | | N/A | N/A | 162% | 61% | 15% | 20% | 8% | 2% | -3% | |
| G. Average Selling Price Per Test ($) | | | | $0.00 | $100.00 | $100.00 | $100.00 | $95.00 | $90.00 | $85.00 | $85.00 | $85.00 | |
Growth Rate (%) | | | | N/A | N/A | 0% | 0% | -5% | -5% | -6% | 0% | 0% | |
| H. Product Revenue ($000s) (F*H) | | | | $0 | $12,493 | $32,767 | $52,889 | $57,813 | $65,585 | $67,140 | $68,630 | $66,844 | |
Growth Rate (%) | | | | N/A | N/A | 162% | 61% | 9% | 13% | 2% | 2% | -3% | |
| I. Reagent Costs ($000s) (Expense Item) | | | $0 | $0 | $0 | $0 | $0 | $0 | $0 | $0 | $0 | |
| Either | | | | | | | | | | | | | |
| 1. % of Sales (Input %) | | | | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Or | | | | | | | | | | | | | |
| 2. Reagent Costs as ($ per Test) | | | | $- | $- | $- | $- | $- | $- | $- | $- | $- | |
| | | | | | | | | | | | | | |
| (1) One out of every ten cases of Prostatic Hyperplasia (PHP) is found to be cancerous. It is then assumed that the number of New PHP cases is 10x the annual prostate cancer rate. | |
| (2) CapCure estimates Prostate cancer prevalence at upwards of 10 million (including individuals that are not currently aware of the disease). | | | |
| Prostate cancer prevalence for those that have already been diagnosed with the disease is estimated at ten times the annual incidence rate. | | | |
| (3) Annual deaths/reductions in number of existing Prostate Cancer is estimated based on historical rate. | | | | | | |
| (4) Prostatic Hyperplasia cases are typically inspected via a Digital Rectal Exam. 69% of the cases found to be suspicious (assumed at approximately 30%) are subjected to a biopsy. | |
| | | | | | | | |
AnuCyte Diagnostics | 2005 SEER Incidence Rates | |
Population Demographics Detail | Race/Ethnicity | Men |
| | | | | | | | All Races | 170.3 per 100,000 men |
| Scenario: | | Base Case | | | | | White | 163.4 per 100,000 men |
| Product: | | Prostate Cancer Ploidy Diagnostics Test | | | Black | 258.3 per 100,000 men |
| Geographic Market: | | USA | | | | | Asian/Pacific Islander | 96.8 per 100,000 men |
| | | | | | | | American Indian/Alaska Native+ | 70.7 per 100,000 men |
(amounts in 000's) | | | | Hispanic~ | 141.1 per 100,000 men |
| Population Estimates (1) | | | 1997 | | | | |
| Aged from 0 to 39 | | | | 79,543 | | | | |
| Aged from 40 to 44 | | | | 10,712 | | | | |
| Aged from 45 to 49 | | | | 9,166 | | | | |
| Aged from 50 to 54 | | | | 7,524 | | | | |
| Aged from 55 to 59 | | | | 5,795 | | | | |
| Aged from 60 to 64 | | | | 4,781 | | | | |
| Aged from 65 to 69 | | | | 4,432 | | | | |
| Aged from 70 to 74 | | | | 3,830 | | | | |
| Aged from 75 to 79 | | | | 2,949 | | | | |
| Aged from 80 to 84 | | | | 1,733 | | | | |
| Aged from 85 to 89 | | | | 784 | | | | |
| Aged from 90+ | | | | 354 | | | | |
| Total Males (as of 12/1/97) | | | 131,603 | | | | |
| (1) Per U.S. Census Bureau Estimates as of December 1997. These numbers are now significantly higher. | | |
| | | | | | | | | |
| Benign Prostate Hyperplasia (BHP) | Rate of BHP | | | | | |
| Total Prevalence by Age Category | by Age Cat. | | | | | |
| Aged from 0 to 39 | | 0% | | - | | | | |
| Aged from 40 to 44 | | 0% | | - | | | | |
| Aged from 45 to 49 | | 0% | | - | | | | |
| Aged from 50 to 54 | | 15% | est. | 1,129 | | | | |
| Aged from 55 to 59 | | 30% | est. | 1,739 | | | | |
| Aged from 60 to 64 | | 45% | est. | 2,151 | | | | |
| Aged from 65 to 69 | | 60% | est. | 2,659 | | | | |
| Aged from 70 to 74 | | 70% | (2) | 2,681 | | | | |
| Aged from 75 to 79 | | 75% | est. | 2,212 | | | | |
| Aged from 80 to 84 | | 80% | est. | 1,386 | | | | |
| Aged from 85 to 89 | | 85% | est. | 666 | | | | |
| Aged from 90+ | | 90% | (2) | 319 | | | | |
| Total BHP Est. (as of 12/1/97) | | | 14,942 | | | | |
| (2) Per Epidemiology of Prostate Cancer 1997, G.Haas & W.Sakr | | | | | |
| | | | | | | | | |
Prostate Cancer: | Incidence Rate | | | | | |
Est. of Annual Incidence | by Age Cat. | | | | | |
| Aged from 0 to 39 | | 0% | | - | | | | |
| Aged from 40 to 44 | | 0% | | - | | | | |
| Aged from 45 to 49 | | 0% | | - | | | | |
| Aged from 50 to 54 | | 0.05% | (2) | 3 | | | | |
| Aged from 55 to 59 | | 0.2% | est. | 11 | | | | |
| Aged from 60 to 64 | | 0.3% | (2) | 16 | | | | |
| Aged from 65 to 69 | | 1.0% | (2) | 44 | | | | |
| Aged from 70 to 74 | | 1.0% | (2) | 38 | | | | |
| Aged from 75 to 79 | | 1.0% | (2) | 29 | | | | |
| Aged from 80 to 84 | | 1.0% | (2) | 17 | | | | |
| Aged from 85 to 89 | | 1.0% | (2) | 8 | | | | |
| Aged from 90+ | | 1.0% | (2) | 4 | | | | |
| Est. Total New Cancer Cases per yr. (in 000's) | | 171 | | | | |
| (2) Per Epidemiology of Prostate Cancer 1997, G.Haas & W.Sakr | | | | | |
| | | | | | | | | |
Estimate of Prost. Cancer Incidence | Incidence (1,3) | Current (2) | Est. Cancer | | | | |
Est. of Incidence Rate by Race | Rate by Race | Pop. (in 000) | Incidence | | | | |
| Total White Males in U.S. | 0.15% | 109,363 | 167.9 | | | | |
| Total Black Males in U.S. | 0.20% | 16,232 | 32.5 | | | | |
| Total Asian & other Males in U.S. | 0.12% | 6,091 | 7.5 | | | | |
| Total Males in U.S. | | | 131,686 | 207.9 | | | | |
| (1) Per American Cancer Society Surveilance, Epidemeology, & End Results (SEER). | | | | |
| (2) Per U.S. Census Bureau Estimates as of December 1997. | | | | | |
| (3) The incidence rate is from SEER data from 1997. | | | | | | |
| The most recent 2005 data shows that incidence rates to be HIGHER than 1997 rates. | | | | | |
11 REFERENCES
1. National Cancer Institute. (2007) Prostate cancer in National Cancer Institute Online Bethesday.
2. Lee, S.E., Currin, S. M., Paulson, D.F. & Walther, P. J. (1988) Flow cytometric determination of ploidy in prostatic adenocarcinoma: a comparison with seminal vesicle involvment and histopathological grading as a predictor of clinical recurrence, Journal of Urology. 140, 769-774.
3. Shankey, T. V., Kallioniemi, O.-P., Koslowski, J. M., Lieber, M. L., Mayall, B. H., Miller, G. & Smith, G. J. (1993) Consensus review of the clinical utility of DNA content cytometry in prostate cancer, Cytometry, 14, 497-500.
4. National Cancer Institute, (2006) The Pap Test: Questions and Answer in Online National Cancer Institute
5. National Cervical Cander Coalition. (2007) Research and Treatment in
6. Jemal, A., Murray, T., Samuels, A., Ghafoor, A., Ward, E. & Thun, M. J. (2003) Cancer statistics, 2003, CA Cancer J Clin. 53, 5-26.
7. Crum, C. P., Cibas, E. S. & Lee, K. R. (1997) Pathology of early cervical neoplasia, Churchill Livingstone, New York.
8. Koss, L. G. (1933) Cervical (Pap) smear: new directions, Cancer Supplement. 71, 1406-1412.
9. Mayeaux, J. E. (1994) The Papanicolaou smear in AAFP Scientific Assembly, Louisiana State University Medical Center, Shreveport.
10. van der Graaf, Y., Voois, G. P., Gaillard H. J. L. & Go, D. M.D.S. (1987) Screening errors in cervical cytology screening (sic), Acta Cytology, 31, 434-438.
11. Bishop, J. M. (1987) The molecular genetics of cancer, Science. 235, 305-311.
12. Cooper, G. M. (1990) Oncogenes, Jones and Bartlett Publishers, Boston.
13. Huebner, R. J. & Todaro, G. (1969) Oncogenes of RNA tumor viruses as determinants of cancer, Proceedings of the National Acadamy of Sciences (USA). 64, 1087-1094.
14. Varmus, H. E. (1984) The Molecular Genetics of Cellular Oncogenes, Annu. Rev. Genet. 18, 533-612.
15. Weiss, R., Teich, N., Varmus, H. & Coffin, J. (1985) Molecular Biology of RNA Tumor Viruses, Cold Spring Harbor Lab. Press, Plainview, NY.
16. Pitot, H. C. (1986) Fundamentals of Oncology, 3rd. edn, Marcel Dekker, Inc., New York.
17. Mitelman, F. (1974) The Rous sarcoma virus story: cytogenetics of tumors induced by RSV in Chromosomes and cancer (German, J., ed) pp. 675-693, John Wiley & Sons, Inc.
18. Sandbrg, A. A. (1980) The chromosomes in human cancer and leukemia, Elsevier/North-Holland, Amsterdam.
19. Hansemann, D. (1890) Ueber asymmetrische Zelltheilung in epithel Krebsen und deren biologische Bedeutung, Virschows Arch. Pathol. anat. 119, 299-326.
20. Boveri, T. (1914) Zur Frage der Entstehung maligner Tumoren, Fischer, Jena.
21. Atkin, N. B. & Baker, M. C. (1966) Chromosome abnormalities as primary events in human malignant disease: evidence from marker chromosomes, Journal of the National Cancer Institute. 36, 539-557.
22. Hauschka, T. S. (1961) The chromosomes in ontegeny and oncogeny, Cancer Res. 21, 957-981.
23. Levan, A. & Biesele, J. J. (1958) Role of chromosomes in cancerogenesis, as studied in serial tissue culture of mammalian cells, Annals of New York Academy of Sciences, 71, 1022-1053.
24. Winge, O. (1930) zytologische Untersuchungen ueger die Natur maligner Tumoren. II. Teerkarzinome bei Maeusen, Zeitschrift fuer Zellforschung und Mikroskopische Anatomie. 10, 683-735.
25. Caspersson, T., Foley, G.E., Killander, D. & Lomakka, G. (1963) Cytochemical differences between mammalian cell lines of normal and neoplastic origins: correlation with heterotransplantability in Syrian hamsters, Experimental Cell Research. 32, 553-565.
26. Caspersson, T. O. (1950) Disturbed systesm for protein formation in the metazoan cell in Cell growth and cell function: A cytochemical study pp. 141-151, W. W. Norton & Company, New York.
27. Foley, G. E., Handler, A. H., Lynch, P. M., Woman, S. R., Stulberg, C. S. & Eagle, H. (1965) Loss of neoplasatic properties in vitro. II. Observations of KB sublines, Cancer Research. 25, 1254-1261.
28. Sennerstam, R., Kato, H. & Auer, G. U. (1989) Dissociation of cellular protein and DNA content in mild and moderate dysplasia as a reflection of the degree of aneuploidy in cancer, Analytical and Quantitative Cytology and Histology. 11, 255-260.
29. Alderman, E. M., Lobb, R. R. & Fett, J. W. (1985) Isolation of tumor-secreted products from human carcinoma cells maintained in a defined protein-ree medium, Proceedings of the National Academy of Sciences (U.S.A.). 82, 5771-5775.
30. Atkin, N. B. (1971) Modal DNA value and chromosome number in ovarian neoplasia: a clinical and histopathologic assessment, Cancer. 27, 1064-1073.
31. Atkin, N. B. (1989) Solid tumor cytogenetics: progress since 1979, Cancer Genetics and Cytogenetics. 40, 3-12.
32. Atkin, N. B. & Baker, M. C. (1969) Possible differences between the karyotypes of preinvasive lesions and malignant tumours, British Journal of Cancer. 23, 329-336.
33. Atkin, N. B. & Baker, M. C. (1990) Are human cancers are diploid-or often trisomic? Conflicting evidence from direct preparations and culture, Cytogenetics and Cell Genetics. 53, 58-60.
34. Atkin, N. B. Mattinson, G. & Baker, M. C. (1996) A comparison of the DNA content and chromosome number of fifty human tumors, British Journal of Cancer. 20,87-101.
35. Frati, L., Cortei, E., Ficorella, C., Manzari, V. & Verna, R. (1984) Phenotypic and genetic markers of cancer: turning point in research in Genetic and phenotypic markers of tumors (Aaronson, S. A., Frati, L. & Verna, R., eds) pp. 1-20, Plenum Press, New York.
36. Zhang, L., Zhou, W. Velculescu, V. E. Kern, S. E. Hruban, R. H. Hamilton, S. R., Vogelstein, B. & Kinzler, K. W. (1997) Gene expressions profiles in normal and cancer cells, Science. 276, 1268-1272.
37. Chaganti, R. S. K. (1983) The question of normal karotypes in tumor cells in chromosome mutation and neoplasia (German., J. ed) pp. 370-372, Alan R. Liss, Inc., New York.
38. Giaratti, W. & Santi, L. (1990) Tumor progression by DNA flow cytometry in human colorectal cancerl, International Journal of Cancer. 45, 597-603.
39. Wolman, S. R. (1983) Karyotypic progression in human tumors, Cancer Metastasis Reviews. 2. 257-293.
40. Epstein, C. J. (1986) The consequences of chromosom imbalance: principles,, mechanisms, and models, Cambridge University Press, New York.
41. Sandberg, A. A. & Chen, Z. (1996) Cancer cytogenetics: nomenclature and clinical applications in Molecular biology in cancer medicine (Razelle, K. & Talpaz, M., eds), Martin Dunitz, London.
42. Segers, P., Haesen, S., Amy, J.-J., de Sutter, P., van Dam, P. & Kirsch-Volders, M. (1994) Detection of premalignant stages in cervical smears with a biotinylated probe for chromosome 1, Cancer Genetics and Cytogenetics. 75, 120-120.
43. Segers, P., Haesen, S., Castelain, P., Amy, J.-J., de Sutter, P., van Dam, P. & Kirch-Volders, M. (1995) Study of numerical aberrations of chromosome 1 by flurescent in situ hybridization and DNA content by densitometric analysis on (pre)-malignant cervical lesions, Histochemical Journal, 27, 24-34.
44. Shackney, S. E., Berg, G., Simon, S. R., Cohen, J., Amina, S., Pommersheim, W., Yakulis, R., Wang, S., Uhl, M., Smith, C. A., Pollice, A. A. & Hartsock, R. J. (1995) Origins and clinical implications of aneuploidy in early bladder cancer, Cytometry. 22, 307-316.
45. Shackney, S. E., Singh, S. G. Yakulis, R., Smith, C. A. Pollice, A. A. Petruolo, S., Waggoner, A. & Hartsock, R. J. (1995) Aneuploidy in breast cancer: a flourescence in situ hybridization study, Cytometry. 22, 282-291.
46. Shackney, S. E., Smith,, C. A., Miller, B. W., Burholt, D. R., Murtha, K., Giles, H. R., Ketterer, D. M. & Pollice, A. A. (1989) Model for the genetic evolution of human solid tumors, Cancer Research. 49, 3344-3354.
47. Sreekantaiah, C., De Braekeleer, M. & Hass, O. (1991) Cytogenetic findings in cervical carcinoma: a statistical approach, Cancer Genetics and Cytogenetics. 53, 75-81.
48. Johansson, B., Mertens, F. & Mitelman, F. (1996) Primary vs. secondary neoplasia-associated chromosomal abnormalities- balanced rearrangements vs. genomic imbalances? GenesChromosomes and Cancer. 16, 155-163.
49. Li, R., Yerganian, G., Duesberg, P., Kraemer, A., Willer, A., Rausch, C. & Hehlmann, R. (1997) Aneuploidy 100% correlated with chemical transformation of Chinese hamster cells, Proceedings of the National Academy of Sciences (U.S.A.). 94, 14506-14511.
50. Sandberge, A. A. (1990) The chromosomes in human cancer and leukemia, 2 edn, Elsevier Science Publishing, New York.
51. Sandberg, A. A. (1990) Tumors of the female in The chromosomes in human cancer and leukemia pp. 827-851, Elsevier, New York.
52. Sandberg, A. A. & Kakati, S. (1990) Chromosome alterationas and oncogenes in human neoplasia in Trends in chromosome research (Sharma, T., ed) pp. 190-203, Springer-Verlag.
53. Sandberg, A. A. & Turc-Carel, C. (1987) The cytogenetics of solid tumors: relation to diagnosis, classification and pathalogy, Cancer. 59, 387-395.
54. Alberts, B., Bray, D., Lewis, J., Raff, M., Roberts, K. & Watson, J. D. (1994) Molecular Biology of the Cell, Garland Publishing, Inc., New York.
55. Alberts, B., Bray, D., lewis, J., Raff, M., Roberts, K. & Watson, J. D. (1994) Molecular Biology of the Cell, Garland Publishing, Inc., New York.
56. Lewin, B. (1994) Genes V, fifth edn, Oxford University Press, New York.
57. Lodish, H., Baltimore, D., Berk, A., Zipursky, S. L., Matsudaira, P. & Darnell, J. (1995) Molecular Cell Biology, Scientific American Books, Inc. by W. H. Freeman & Co., New York and Oxford UK.
58. Mitelman, F. (1994) Catalogue of chromosome aberrations in cancer, 4. edn, Wiley-Liss, New York.
59. Mitelman, F., Mark, J., Levan, G. & Levan, A. (1972) Tumor etiology and chromosome pattern, Science 176, 1340-1341.
60. Mitelman, F., mertens, F. & Johansson, B. (1997) A breakpoint map of recurrent chromosomal rearrangemetns in human neoplasia, Nature Genetics. Special issue, 417-474.
61. Rasnick, D. & Duesberg, P. H. (1999) How aneuploidy affects metabolic control and causes cancer, Biochemical Journal. 340 621-630.
62. Eastmond, D. A. & Pinkel, D. (1990) Detection of aneuploidy and aneuloidy-inducing agents in human lymphocytes using fluerescence in situ hybridization with chromosome-specific DNA probes., Mutation Research. 234, 303-318.
63. Eastmond, D. A., Rupa, D. S. & Hasegawa, L. S. (1994) Detection of hyperdiploidy and chromosomal breakage in interphase human lymphocytes following exposure to the benzene metabolite hydroquinone using mutlicolor fluorescence in situ hybridization with DNA probes, Mutation Research. 322, 9-20.
64. Eastmond, D. A., Schuler, M. & Rupa, D. S. (1995) Advantages and limitations of using fluorescence in situ hybridization for the detection of aneuploidy in interphase human cells, Mutation Research. 348, 153-162.
65. Eastmond, D. A. & Tucker, J. D. (1989) Identification of aneuploidy-inducing agents using cytokineses-blocked human lymphocytes and an antikinetochore antibody, Environmental and Molecular Mutagenesis. 13, 34-43.
66. Rupa, D. S., Schuler, M. & Eastmond, D. A. (1997) Detection of hyperdiploidy and breakage affecting the 1cen-1q12 region of cultured interphase human lymphocytes treated with various genotoxic agents, Environmental and Molecular Mutagenesis. 29, 161-167.
67. National Cancer Institute. (2007) A to Z list of cancers in
68. Office of Technology Assessment. (1995) "Costs and effectiveness of prostate cancer screening in elderly men" in OTA-BP-H-145 Washington, DC.
69. NIH. (1996) Cervical cancer, National Institutes of Health consensus development statement. 14, 1-38.
70. Cohen, C. (1996) Image cytometric analysis of pathology, Human Pathology. 27, 482-493.
71. Birdson, G. G. (1996) Automated screening of cervical cytology specimens, Human Pathology, 27, 468-481.
72. Rasnick, D. (2000) Auto-catalyzed progression of aneuploidy explains the Hayflick limit of cultered cells, carcinogen-induced tumours in mice, and the age distribution of human cancer, Biochemical Journal. 348, 497-506.
73. Rasnick, D. (2002) Aneuploidy theory explains tumor formation, the absence of immune surveillance and the failure of chemotherapy, Cancer Genetics Cytogenetics. 136, 66.72.
74. Duesberg, P. (1999) Are centrosomes or aneuploidy the key to cancer? Science, 284, 2091-2.
75. Duesberg, P., Fabarius, A. & Hehlmann, R. (2004) Aneuploidy, the primary cause of the miltilateral genomic instability of neoplastic and preneoplastic cells, IUBMB Life. 56, 65-81.
76. Duesberg, P. & Li, R. (2003) Multistep carcinogenesis: a chain reaction of aneuploidizations, Cell Cycle. 2, 202-10.
77. Duesberg, P., Li, R., Fabarius, A. & Hehlmann, R. (2006) Aneuploidy and cancer: from correlation to causation, Contrib Microbiol. 13, 16-44.
78. Duesberg, P., Li, R. & Rasnick, D. (2004) Aneuploidy Approaching a Perfect Score in Predicting and Preventing Cancer: Highlights from a Conference Held in Oakland in January 2004, Cell Cycle. 3.
79. Duesberg, P., Li, R., Rasnick, D., Rausch, C., Willer, A., Kraemer, A., Yerganian, G. & Hehlmann, R. (2000) Aneuploidy precedes and segragates with chemical carcinogenesis, Cancer Genetics and Cytogenetics. 119, 83-93.
80. Duesberg, P., Li, R., Rausch, C., Willer, A., Kraemer, A., Yerganian, G., Hehlmann, R. & Rasnick, D. (2000) Mechanism of carcinegenesis by polycyclic aromatic hydrocarbons: aneoploidy precedes malagnant transformation and occurs in all cancers in Technology and Medical Implications of Metabolic Control Analysis (Cornish-Bowden, A. & Cardenas, M. L., eds) pp. 83-98, Kluwer Academic Publishers, Dordrecht.
81. Duesberg, P. & Rasnick, D. (2000) Aneuploidy, the somatic mutation that makes cancer a species of its own, Cell Motility and Cytoskeleton, 47, 81-107.
82. Duesberg, P., Rausch, C., Rasnick, D. & Hehlmann, R. (1998) Genetic instability of cancer cells is proportional to their degree of aneuploidy, Proceedings of the National Academy of Sciences (U.S.A.). 95, 13692-13697.
83. Duesberg, P., Stindl, R. & Hehlmann, R. (2000) Explaining the high mutation rates of cancer cells to drug and multidrug resistance by chromosome reassortments that are catalyzed by aneuploidy, Proc Natl Acad Sci U S A. 97, 14295-300.
84. Duesberg, P., Stindl, R. & Hehlmann, R. (2001) Origin of multidrug resistance in cells with and without multidrug reistance genes: Chromosome reassortments catalyzed by aneuploidy, Proc Natl Acad Sci U S A. 98, 11283-8.
85. Duesberg, P., Stindl, R., Li, R., Hehlmann, R. & Rasnick, D. (2001) Aneuploidy versus gene mutation as cause of cancer, Current Science (India). 81, 490-500.
86. National Cancer Institute (1997) Prostate cancer staging and treatment.
87. Henke, R. P., Kruger, E., Ayhan, N., Hubner, D. & Hammerer, P. (1993) Numerical chromosomal aberrations in prostate cancer: Correlation with morphology and cell kinetics, Virchows Archievs [A]. 422, 61-62.
88. Takahashi, S., Qian, J., Brown, J. A., Alcaraz, A., Bostwick, D. G., Lieber, M. M. & Jenkins, R. B. (1994) Potential markers of prostate cancer aggressiveness detected by flourescence in situ hybridizations in needle biopsies, Cancer Research. 54, 3574-3579.
89. Macoska, J. A., Trybus, T. M., Sakr, W. A., Wolf, M. C., Benson, P. D., Powell, I. J. & Pontes, J. E. (1994) Flourescence in situ hybridization analysis of 8p allelic loss and chromosome 8 instability in human prostate cancer, Cancer Research. 54, 3824-3830.
90. Moul, J. W. (1997) The Clinical Relevance of Oncogenes and Tumor Suppresor Genes in Prostate Cancer in, Center for Prostate Disease Research, a program of the Henry M. Jackson Foundation for the Advancement of Military Medicine, Rockville, MD.
91. Fricker, J. (1997) Cervical-cancer screening comes of age - or does it? Lancet. 350, Oct. 4.
92. Heim, S. & Mitelman, F. (1995) Tumors of the femail genital organs in Cancer cytogenetics pp. 389-407, Wiley-Liss, New York.
93. Mitchel, M. F., Hittelman, W. K., Lotan,, R., Nishioka, K., Tortolero-Luna, G., Richards-Kortum, R., Whart, J. T. & Hong, W., K (1995) Chemoprevention trials and surrogate end point biomarkers in the cervix, Cancer Supplement. 76, 1956-1977.
94. National Cancer Institute. (2003) Tumors included in prevalence estimates: SEER cancer statistics review 1975-2003 in Surveillance Epidemiology and End Results (SEER),
95. Phillips, J. L. & Richardson, I. C. (2006) Aneuploidy in bladder cancers: the utility of fluorescent in situ hybridization in clinical practice, BJU Int. 98, 33-7
96. US Government. (1996) Medical Devices: Analyte specific regents; classification/reclassification as restricted devices in pp. 10480-10483, Federal REgister,
97. US Government. (1997) Amendments forTesting and Monitoring Provisions in pp. 62243-62273, Federal Register,


