
STAAR Surgical NASDAQ: STAA www.staar.com / www.discoverevo.com Investor Presentation | August 7, 2019 Exhibit 99.1 Exhibit 99.1

Forward Looking Statements All statements in this presentation that are not statements of historical fact are forward-looking statements, including statements about any of the following: any financial projections, including those relating to the plans, strategies, and objectives of management for 2019 or prospects for achieving such plans, expectations for sales, revenue, or earnings, product safety or effectiveness, the status of our pipeline of ICL products with regulators, including our EDOF lens for Presbyopia and our EVO family of lenses in the U.S., and any statements of assumptions underlying any of the foregoing, including those relating to our product pipeline and market expansion activities. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements are set forth in the Company’s Annual Report on Form 10-K for the year ended December 28, 2018 under the caption “Risk Factors,” which is on file with the Securities and Exchange Commission and available in the “Investor Information” section of the company’s website under the heading “SEC Filings.” We disclaim any intention or obligation to update or revise any financial projections or forward-looking statement due to new information or events. These statements are based on expectations and assumptions as of the date of this press release and are subject to numerous risks and uncertainties, which could cause actual results to differ materially from those described in the forward-looking statements. The risks and uncertainties include the following: global economic conditions; the discretion of regulatory agencies to approve or reject existing, new or improved products, or to require additional actions before approval, or to take enforcement action; potential international trade disputes; and the willingness of surgeons and patients to adopt a new or improved product and procedure. The The Visian ICL with CentraFLOW, now known as EVO Visian ICL, is not yet approved for sale in the United States.

Positioning the ICL™ as a premium and primary procedure for visual freedom Expanding market opportunity and winning share with a pipeline of products to address near, intermediate and distance vision We Transform Lives by Providing Visual Freedom through Premium Lens-Based Surgical Correction of Refractive Error Please Note: The ICL is not visible to other people after implantation.
The ICL™ A Remarkable Technology 99.4% of Patients Would Elect STAAR’s Implantable Collamer® Lens Again* Upgradeable Removeable Eco-Friendly An Evolution in Visual Freedom™ * Patient registry data on file.
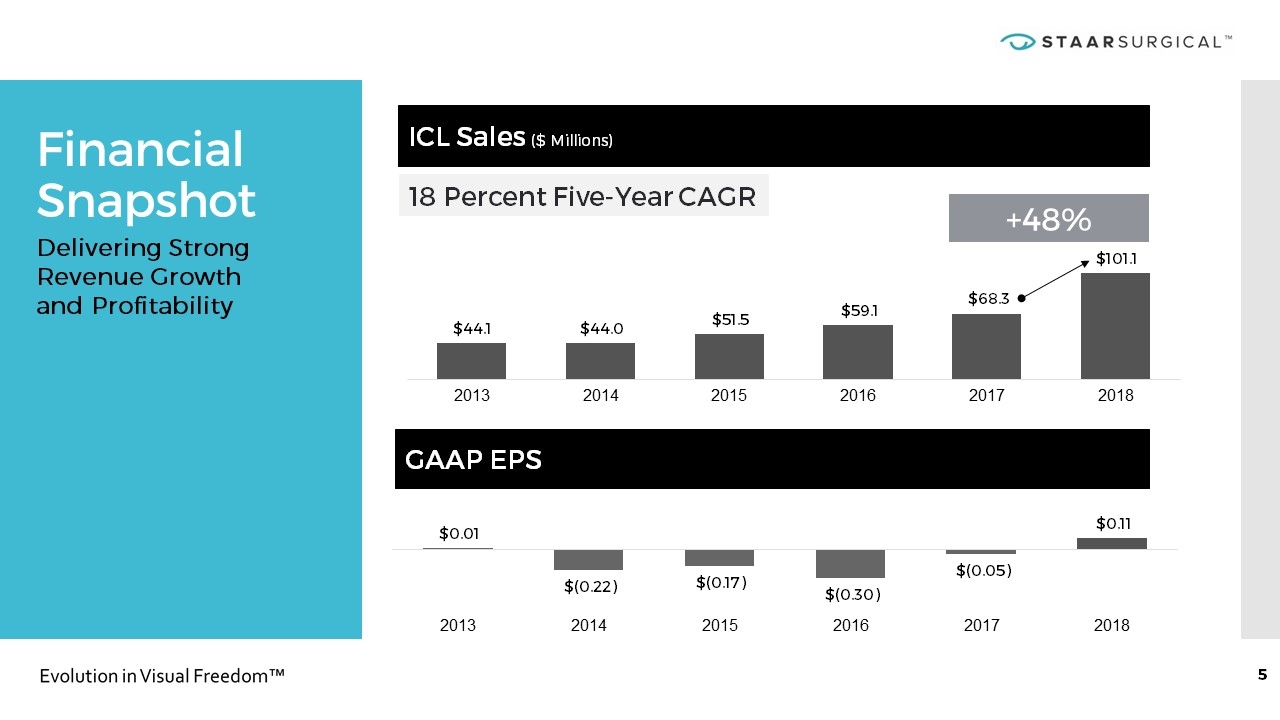
Financial Snapshot ICL Sales ($ Millions) GAAP EPS 18 Percent Five-Year CAGR +48% Delivering Strong Revenue Growth and Profitability
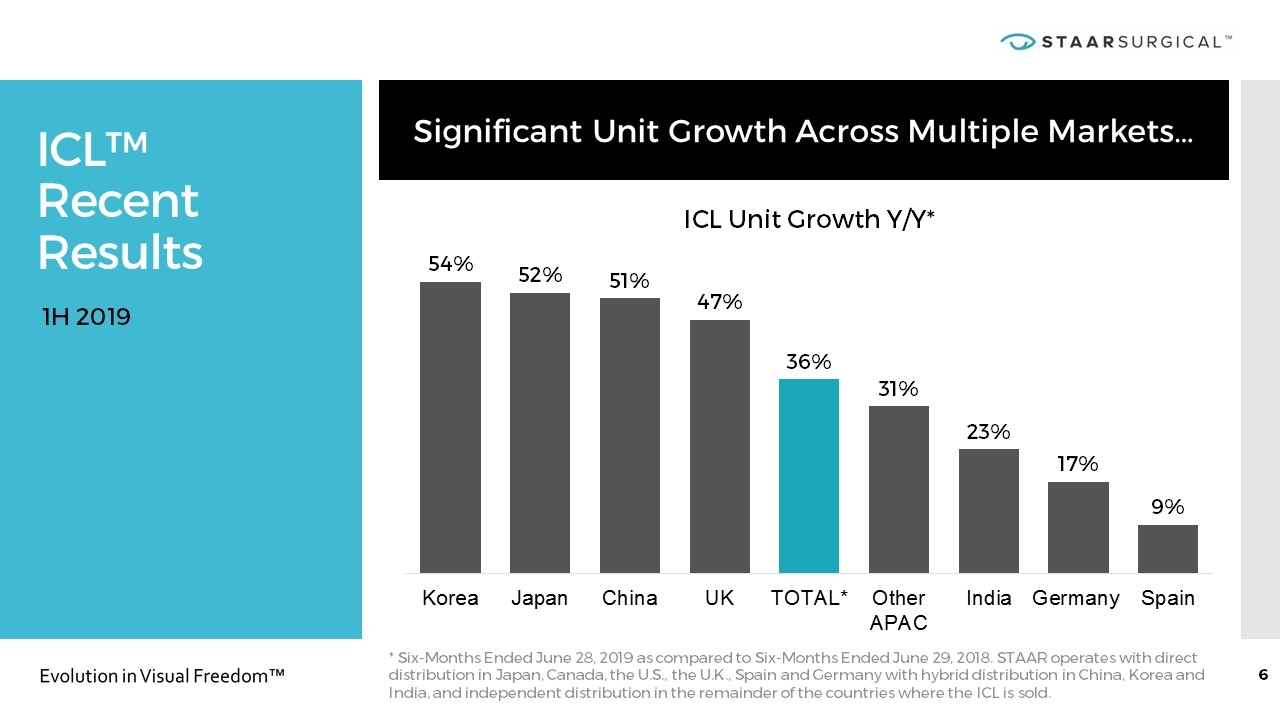
ICL™ Recent Results Significant Unit Growth Across Multiple Markets… 1H 2019 * Six-Months Ended June 28, 2019 as compared to Six-Months Ended June 29, 2018. STAAR operates with direct distribution in Japan, Canada, the U.S., the U.K., Spain and Germany with hybrid distribution in China, Korea and India, and independent distribution in the remainder of the countries where the ICL is sold.

ICL™ Milestone 1 Million+ ICLs 75+ Countries 86% of Sales Mix* 1 Million+ ICLs™ Implanted Globally * Six-Months ended June 28, 2019.
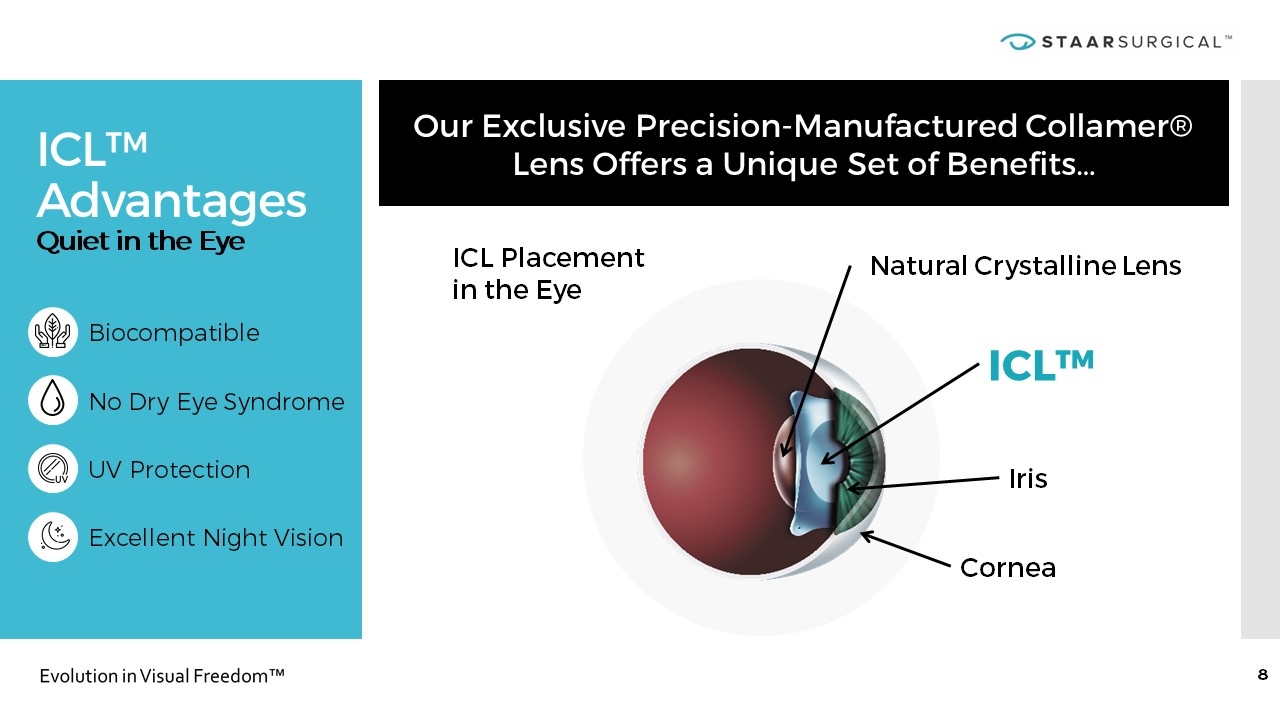
ICL™ Advantages Quiet in the Eye Our Exclusive Precision-Manufactured Collamer® Lens Offers a Unique Set of Benefits… Biocompatible No Dry Eye Syndrome UV Protection Excellent Night Vision ICL Placement in the Eye Iris Cornea ICL™ Natural Crystalline Lens

EVO ICL™ *Published in Clinical Ophthalmology, Packer, December 2018. Safety and Effectiveness Outcomes Reported in Literature Stellar Safety and Effectiveness of STAAR’s EVO Lens… “Improved safety and effectiveness across a broad range of refractive errors make EVO an attractive option for surgeons and patients.” 2018 Literature Review authored by Dr. Mark Packer is a review of 67 papers from 10 countries* Review covers > 6,000 Eye data points with up to 5 years of Follow-Up Outstanding outcomes for Safety and Effectiveness
Investment Highlights Surgeon training and consumer education and marketing regarding latest ICL technology is driving faster growth STAAR Surgical… …is pursuing a large vision correction market opportunity. …is gaining market share from laser-based refractive procedures. …market share gains have accelerated in recent quarters.

Investment Highlights Surgeon training and consumer education and marketing regarding latest ICL technology is driving faster growth STAAR Surgical… ….gross margins have been expanding for multiple quarters enabling near-term investments for additional growth. …sales are globally-diversified and not subject to government payer or insurance reimbursement risk. …is targeting at least 20% to 30% market share for the ICL™.
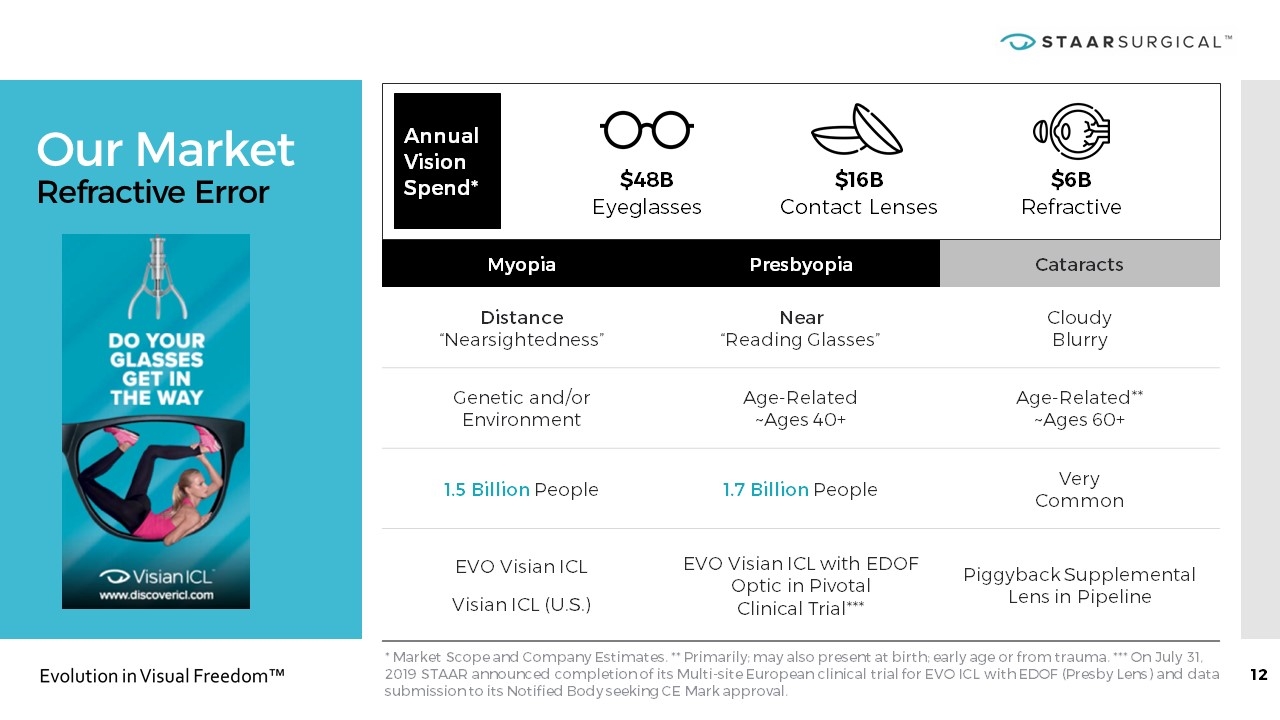
Our Market Refractive Error * Market Scope and Company Estimates. ** Primarily; may also present at birth; early age or from trauma. *** On July 31, 2019 STAAR announced completion of its Multi-site European clinical trial for EVO ICL with EDOF (Presby Lens) and data submission to its Notified Body seeking CE Mark approval. Myopia Presbyopia Cataracts Distance “Nearsightedness” Near “Reading Glasses” Cloudy Blurry Genetic and/or Environment Age-Related ~Ages 40+ Age-Related** ~Ages 60+ 1.5 Billion People 1.7 Billion People Very Common EVO Visian ICL Visian ICL (U.S.) EVO Visian ICL with EDOF Optic in Pivotal Clinical Trial*** Piggyback Supplemental Lens in Pipeline Annual Vision Spend* $48B Eyeglasses $16B Contact Lenses $6B Refractive
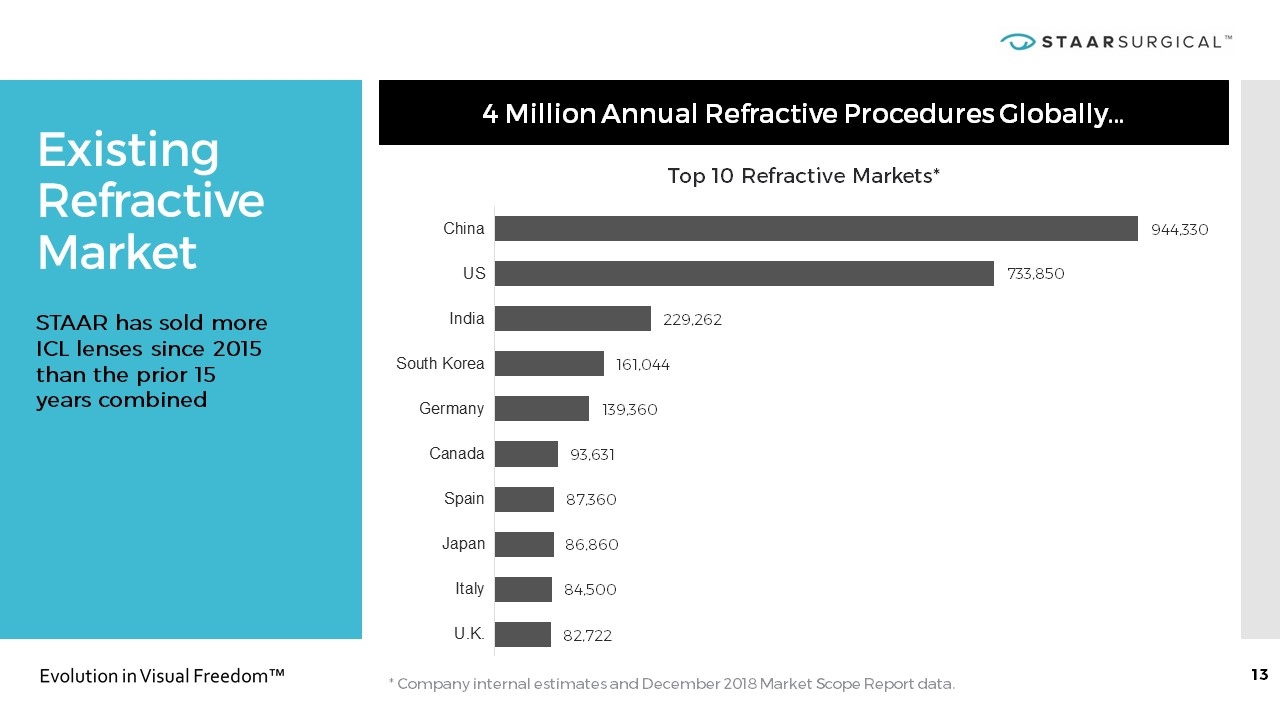
Existing Refractive Market * Company internal estimates and December 2018 Market Scope Report data. 4 Million Annual Refractive Procedures Globally… STAAR has sold more ICL lenses since 2015 than the prior 15 years combined Top 10 Refractive Markets*
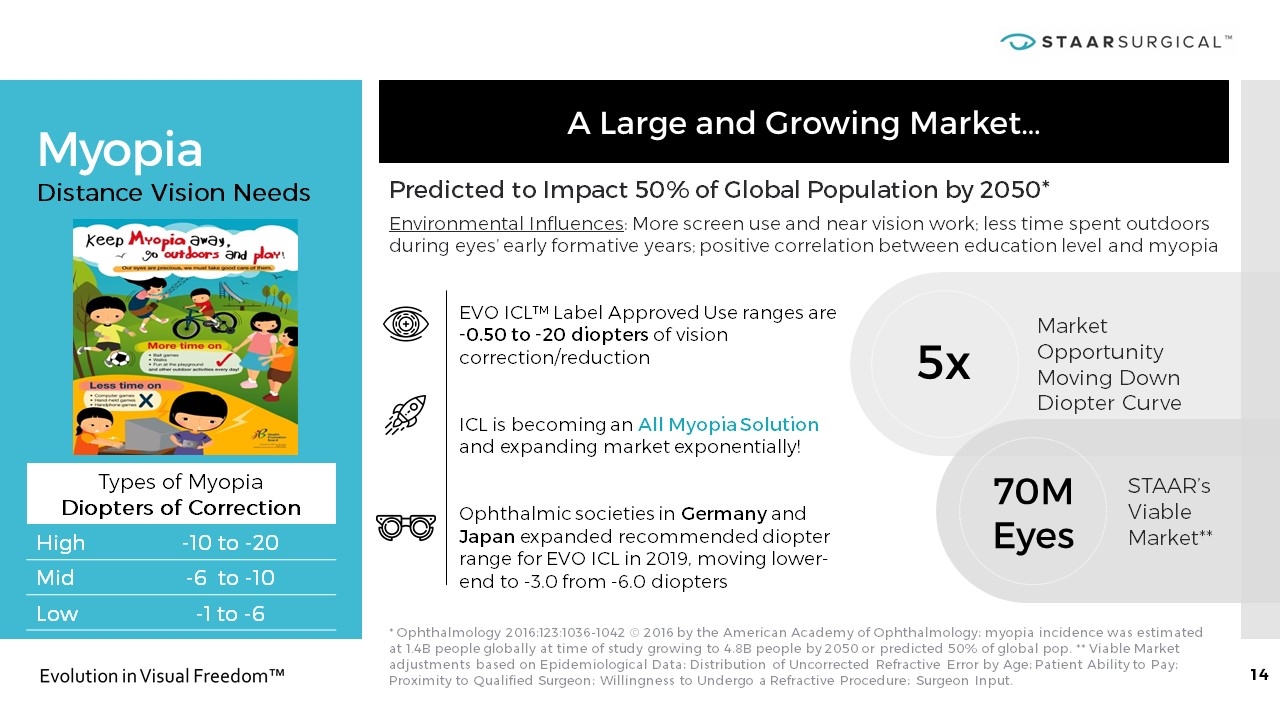
Myopia * Ophthalmology 2016;123:1036-1042 © 2016 by the American Academy of Ophthalmology; myopia incidence was estimated at 1.4B people globally at time of study growing to 4.8B people by 2050 or predicted 50% of global pop. ** Viable Market adjustments based on Epidemiological Data; Distribution of Uncorrected Refractive Error by Age; Patient Ability to Pay; Proximity to Qualified Surgeon; Willingness to Undergo a Refractive Procedure; Surgeon Input. A Large and Growing Market… Distance Vision Needs Types of Myopia Diopters of Correction High -10 to -20 Mid -6 to -10 Low -1 to -6 Predicted to Impact 50% of Global Population by 2050* Environmental Influences: More screen use and near vision work; less time spent outdoors during eyes’ early formative years; positive correlation between education level and myopia EVO ICL™ Label Approved Use ranges are -0.50 to -20 diopters of vision correction/reduction ICL is becoming an All Myopia Solution and expanding market exponentially! Ophthalmic societies in Germany and Japan expanded recommended diopter range for EVO ICL in 2019, moving lower-end to -3.0 from -6.0 diopters 5x Market Opportunity Moving Down Diopter Curve 70M Eyes STAAR’s Viable Market**
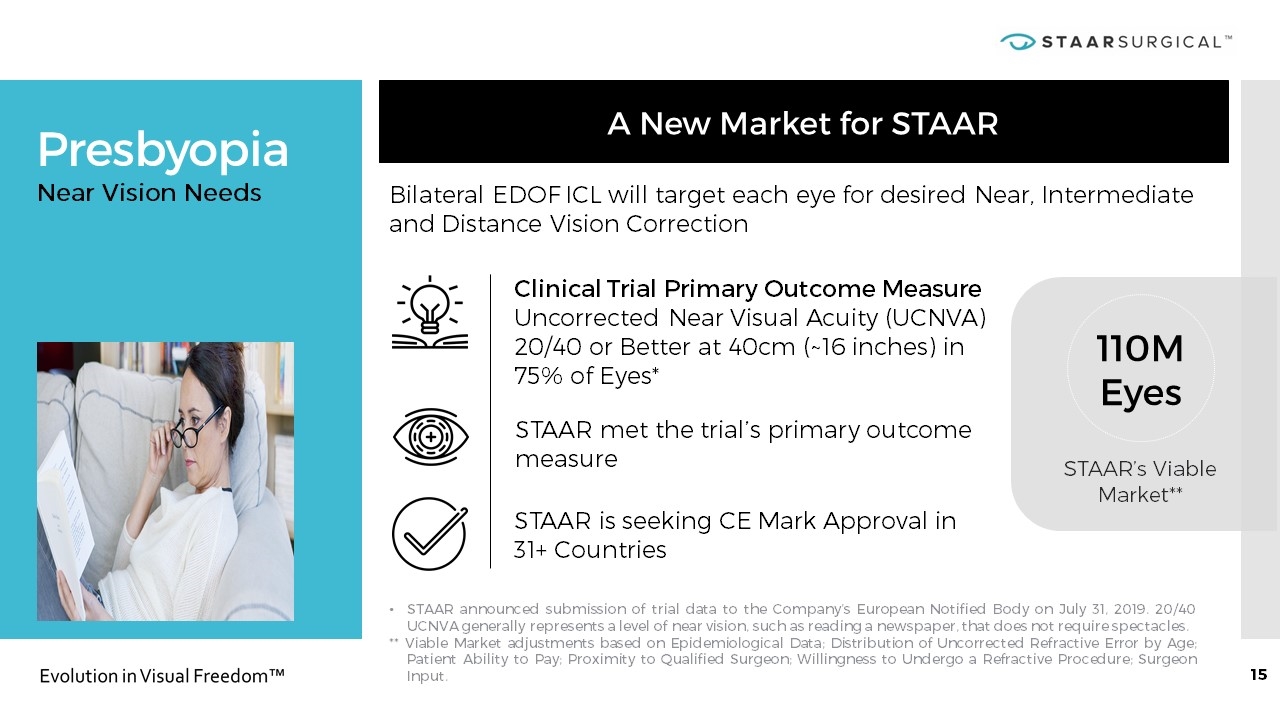
Presbyopia STAAR announced submission of trial data to the Company’s European Notified Body on July 31, 2019. 20/40 UCNVA generally represents a level of near vision, such as reading a newspaper, that does not require spectacles. ** Viable Market adjustments based on Epidemiological Data; Distribution of Uncorrected Refractive Error by Age; Patient Ability to Pay; Proximity to Qualified Surgeon; Willingness to Undergo a Refractive Procedure; Surgeon Input. Near Vision Needs 110M Eyes STAAR’s Viable Market** A New Market for STAAR STAAR is seeking CE Mark Approval in 31+ Countries Bilateral EDOF ICL will target each eye for desired Near, Intermediate and Distance Vision Correction Clinical Trial Primary Outcome Measure Uncorrected Near Visual Acuity (UCNVA) 20/40 or Better at 40cm (~16 inches) in 75% of Eyes* STAAR met the trial’s primary outcome measure

Global Consumer Marketing Display, SEO/SEM in High Potential & Focus Markets Consumer Advertising & Social Media Campaigns Generate “Peer to Peer” Brand Desirability Increasing EVO Visian ICL Brand Awareness, Desirability, Conversion and Activating Influencer Communications for Key Markets…
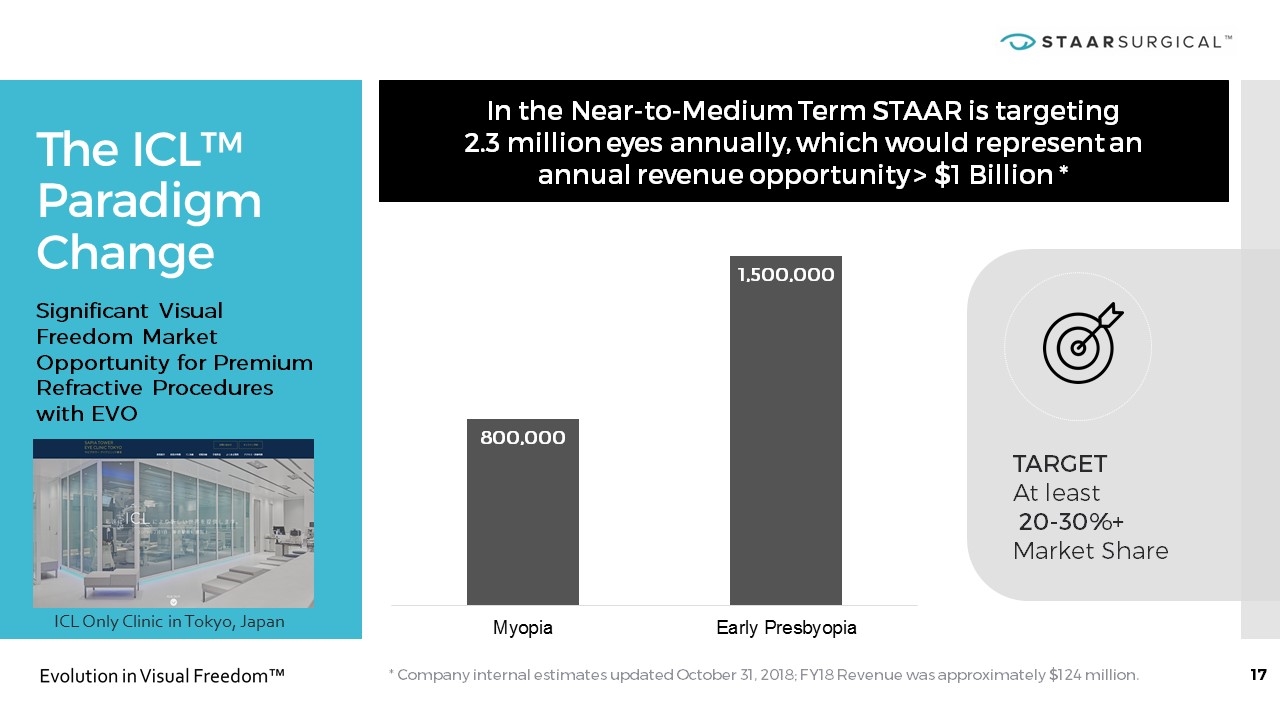
The ICL™ Paradigm Change * Company internal estimates updated October 31, 2018; FY18 Revenue was approximately $124 million. In the Near-to-Medium Term STAAR is targeting 2.3 million eyes annually, which would represent an annual revenue opportunity > $1 Billion * Significant Visual Freedom Market Opportunity for Premium Refractive Procedures with EVO TARGET At least 20-30%+ Market Share ICL Only Clinic in Tokyo, Japan

Financial Overview Business Results, Outlook and Execution
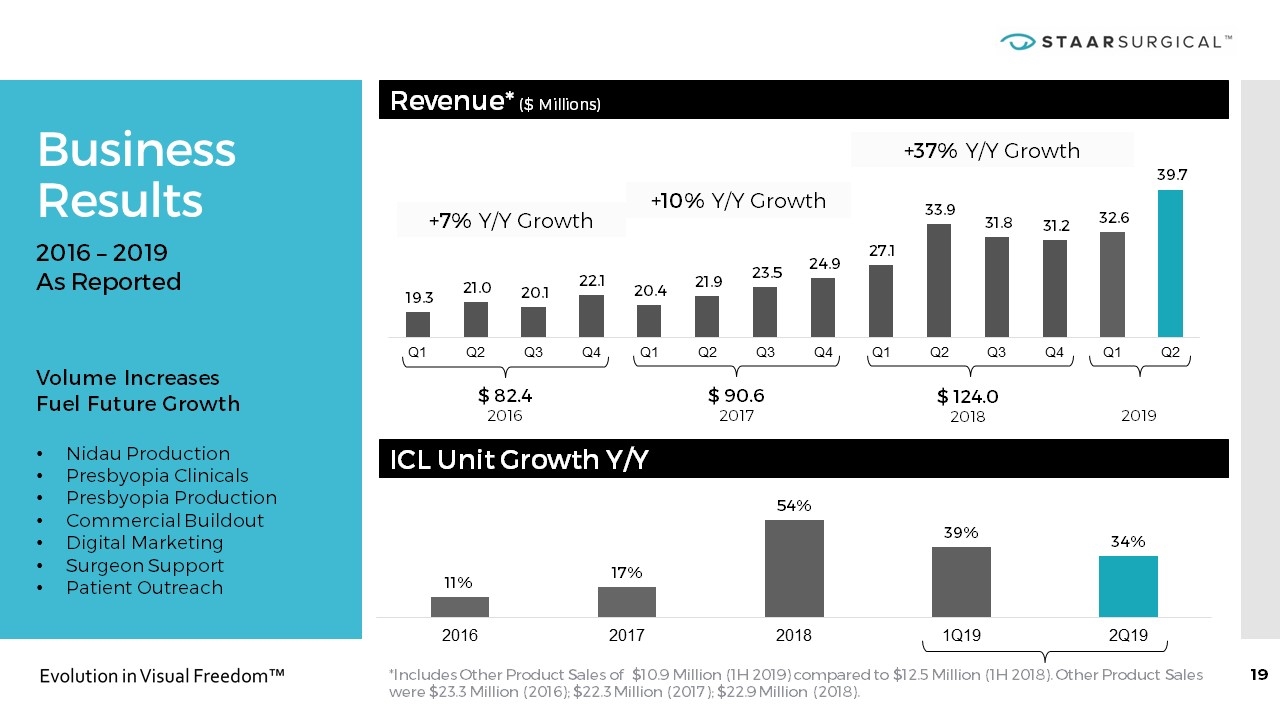
Business Results 2016 – 2019 As Reported Volume Increases Fuel Future Growth Nidau Production Presbyopia Clinicals Presbyopia Production Commercial Buildout Digital Marketing Surgeon Support Patient Outreach Revenue* ($ Millions) +7% Y/Y Growth +10% Y/Y Growth +37% Y/Y Growth $ 82.4 2016 $ 90.6 2017 $ 124.0 2018 2019 ICL Unit Growth Y/Y *Includes Other Product Sales of $10.9 Million (1H 2019) compared to $12.5 Million (1H 2018). Other Product Sales were $23.3 Million (2016); $22.3 Million (2017); $22.9 Million (2018).
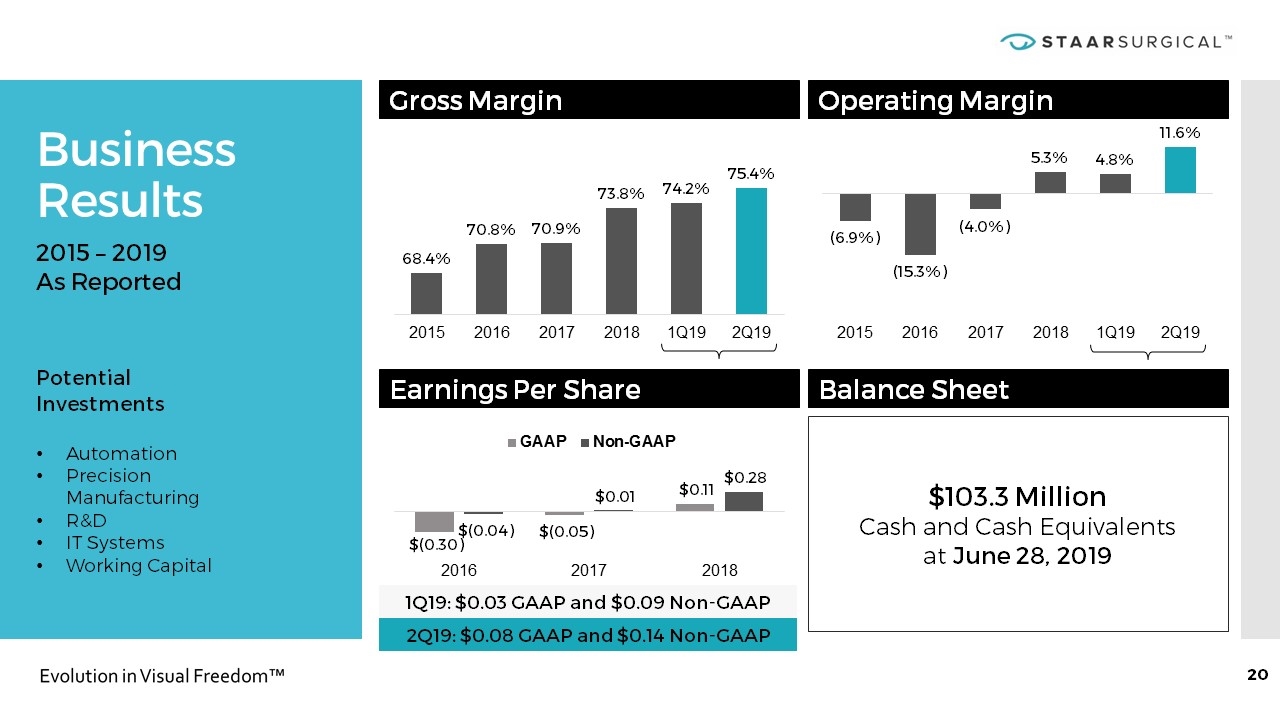
Business Results 2015 – 2019 As Reported Potential Investments Automation Precision Manufacturing R&D IT Systems Working Capital Gross Margin Operating Margin Earnings Per Share Balance Sheet 1Q19: $0.03 GAAP and $0.09 Non-GAAP $103.3 Million Cash and Cash Equivalents at June 28, 2019 2Q19: $0.08 GAAP and $0.14 Non-GAAP
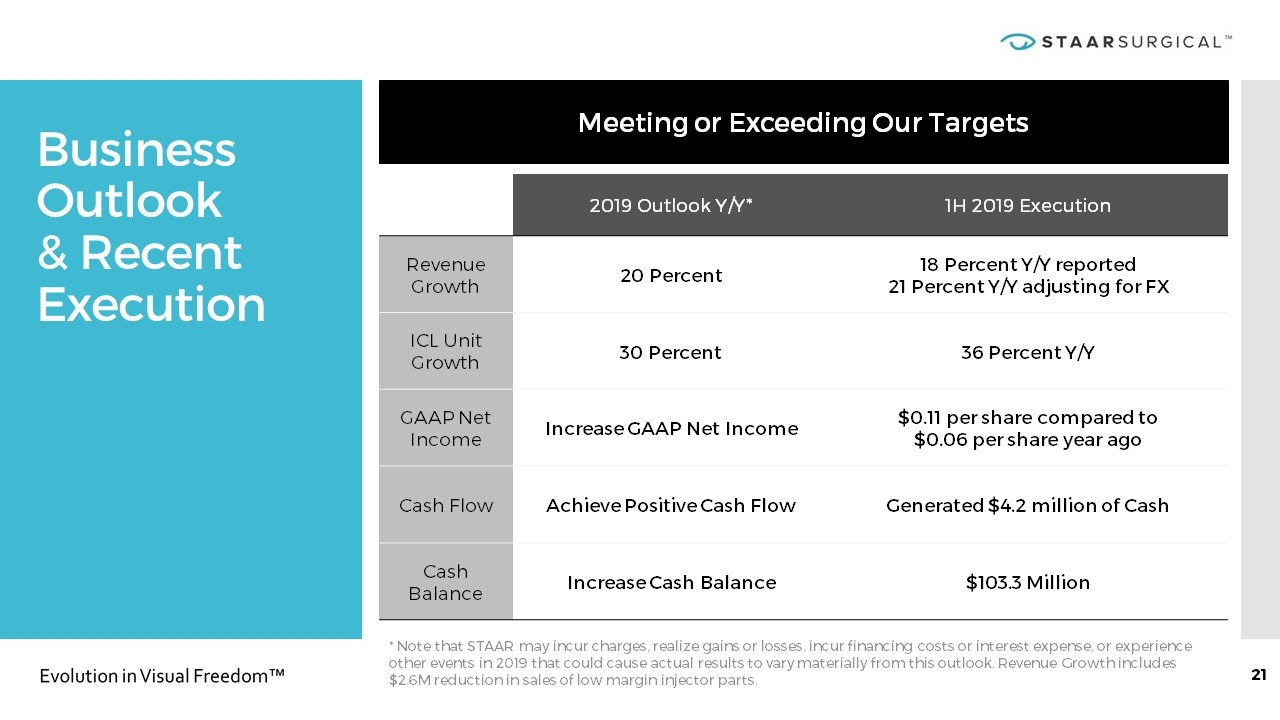
Business Outlook & Recent Execution * Note that STAAR may incur charges, realize gains or losses, incur financing costs or interest expense, or experience other events in 2019 that could cause actual results to vary materially from this outlook. Revenue Growth includes $2.6M reduction in sales of low margin injector parts. Meeting or Exceeding Our Targets 2019 Outlook Y/Y* 1H 2019 Execution Revenue Growth 20 Percent 18 Percent Y/Y reported 21 Percent Y/Y adjusting for FX ICL Unit Growth 30 Percent 36 Percent Y/Y GAAP Net Income Increase GAAP Net Income $0.11 per share compared to $0.06 per share year ago Cash Flow Achieve Positive Cash Flow Generated $4.2 million of Cash Cash Balance Increase Cash Balance $103.3 Million
Growth Drivers Continued ICL market penetration globally; market share gains in mid-to-low diopter (-1 to -10) lenses (moving down the diopter curve) expands STAAR’s opportunity. Increasing investment in surgeon training, DTC marketing and patient education. EVO with EDOF (Presby Lens) in CE Mark Countries; clinical trial data for this bilateral intraocular custom solution for Presby treatment submitted to DEKRA in July 2019.
Growth Drivers EVO in the U.S. represents significant opportunity for the ICL in the 2nd largest global market. Discussions ongoing.* Strategic cooperation agreements and alliances with global partners drives base business growth. Increasing number of clinical studies, already 100+, support ICL safety, efficacy and advantages. * As previously disclosed, STAAR has been in interactive review with the FDA since Q4’ 2018 re: STAAR’s supplemental PMA for the EVO Visian ICL in the U.S. STAAR continues discussions with the FDA to bring EVO to the U.S. market and the Company announced on July 31, 2019 that it recently submitted an application to the FDA encompassing a prospective clinical trial design that reflects a least burdensome pathway, which of course must be finalized with FDA reviewers. STAAR will communicate any agreed-upon next steps for approval after determined, appropriate and permitted.
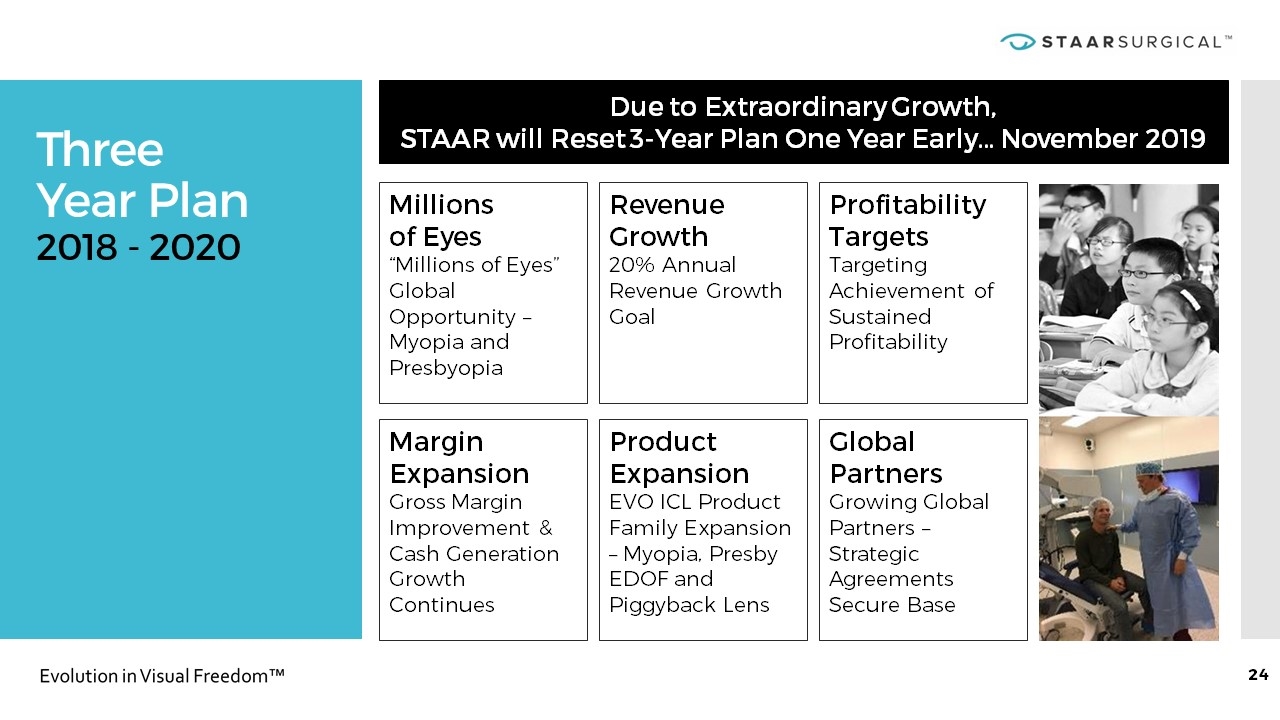
Three Year Plan 2018 - 2020 Due to Extraordinary Growth, STAAR will Reset 3-Year Plan One Year Early… November 2019 Millions of Eyes “Millions of Eyes” Global Opportunity – Myopia and Presbyopia Margin Expansion Gross Margin Improvement & Cash Generation Growth Continues Revenue Growth 20% Annual Revenue Growth Goal Product Expansion EVO ICL Product Family Expansion – Myopia, Presby EDOF and Piggyback Lens Profitability Targets Targeting Achievement of Sustained Profitability Global Partners Growing Global Partners – Strategic Agreements Secure Base

Thank You! STAAR Surgical | NASDAQ: STAA www.staar.com / www.discoverevo.com Investor Presentation | August 7, 2019
























