U.S. SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
T ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2009
Commission file number 0-12183
BOVIE MEDICAL CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware No. | | 11-2644611 |
| (State or other jurisdiction of incorporation or organization) | | (IRS Employer Identification No.) |
734 Walt Whitman Rd., Melville, New York 11747
(Address of principal executive offices)
(631) 421-5452
(Issuer's telephone number)
| | Title of each Class | Name of each Exchange on which registered |
| | Common Stock, $.001 Par Value | NYSE Amex Market |
Securities registered under Section 12(g) of the Exchange Act
None
Indicate by check mark if the Company is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes: £ No T
Indicate by check mark if the Company is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes: £ No T
Indicate by check mark whether the registrant (I) filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act of 1934 during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes T No £
Indicate by checkmark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. £
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a small reporting company. See definition of “large accelerated filer”, “accelerated filer” and “small reporting company” in Rule 12b-2 of the Exchange Act (Check one):
Large accelerated filer £ | Accelerated filer T | Non-accelerated filer £ | Small reporting company £ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes £ No T
The aggregate market value of the voting stock held by non-affiliates computed by reference to the price at which the stock was sold, or the average bid and asked prices of such stock, as of March 1, 2010 was approximately $123,711,995
The number of shares of the registrant's $.001 par value common stock outstanding on the NYSE Amex exchange as of March 1, 2010 was 17,110,926
Company Symbol-BVX
Company SIC (Standard Industrial Code)-3841
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive Proxy Statement relating to the Annual Meeting of Shareholders which was held on December 29, 2009 are incorporated by reference into Part I.
Bovie Medical Corporation 2009 Form 10-K Annual Report
Table of Contents
| Part I | | | Page |
| Item 1 | | | 1 |
| Item 1A | | | 7 |
| Item 1B | | | 13 |
| Item 2 | | | 13 |
| Item 3 | | | 13 |
| Item 4. | | | 13 |
| | | | |
| Part II | | | |
| Item 5. | | | 14 |
| Item 6. | | | 16 |
| Item 7 | | | 18 |
| Item 7A | | | 27 |
| Item 8 | | | 27 |
| Item 9 | | | 27 |
| Item 9A | | | 27 |
| Item 9B | | | 28 |
| | | | |
| Part III | | | |
| Item 10 | | | 28 |
| Item 11 | | | 33 |
| Item 12 | | | 40 |
| Item 13. | | | 42 |
| Item 14 | | | 43 |
| | | | 44 |
| | | | |
| Part IV | | | |
| Item 15 | | | |
BOVIE MEDICAL CORPORATION
Cautionary Notes Regarding “Forward-Looking” Statements
This report contains statements that we believe to be “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements give our current expectations or forecasts of future events. Forward-looking statements generally can be identified by the use of forward-looking terminology such as “may,” “will,” “expect,” “intend,” “estimate,” “anticipate,” “believe,” “project,” or “continue,” or similar words or the negative thereof. From time to time, we also may provide oral or written forward-looking statements in other materials we release to the public. Any or all of our forward-looking statements in this report and in any public statements we make could be materially different from actual results. They can be affected by assumptions we might make or by known or unknown risks or uncertainties. Consequently, we cannot guarantee any forward-looking statements. Investors are cautioned not to place undue reliance on any forward-looking statements. Investors should also understand that it is not possible to predict or identify all such factors and should not consider the risk factors discussed in Item 1A below to be a complete statement of all potential risks and uncertainties.
Part I
General
Bovie Medical Corporation (“Company” or “Bovie”) was incorporated in 1982, under the laws of the State of Delaware and has its principal executive office at 734 Walt Whitman Road, Melville, New York 11747.
We are actively engaged in the business of developing, manufacturing, and marketing medical products and devices with a strong emphasis in electrosurgical generators and electrosurgical disposables. We sell a broad range of products designed for doctor’s offices, surgi-centers and hospitals.
Significant Subsidiaries
Aaron Medical Industries, Inc. is a wholly owned Florida Corporation based in Clearwater, Florida. It is principally engaged in the business of marketing our medical products.
Bovie Canada ULC (a wholly owned subsidiary of BVX Holdings, LLC, which is wholly owned by Bovie), is an Alberta, Canada Corporation with its facility located in Windsor, Ontario. The principal function of this facility is product development and manufacturing focused mainly on endoscopic devices.
Industry
We believe the medical device industry will continue to have a long term growth outlook with surgical procedures performed increasing annually as a result of the aging “baby boomer” population. We also anticipate a continued increase in minimally invasive surgical procedures due to ongoing advancements in technology coupled with continued overall pressure to reduce healthcare costs via a reduction in patient trauma and recovery time. Expanding global markets will also continue to provide growth opportunities for the medical device industry.
Business Strategy
We manufacture and market products, both under private label and the Bovie brands (Aaron, IDS, ICON, and SEER), to distributors worldwide. Additionally, Bovie has original equipment manufacturing (OEM) agreements with other medical device manufacturers. These OEM and private label arrangements and our use of the Bovie brands allow us to gain greater market share for the distribution of our products.
During 2009 we continued to progress on the development and marketing of our new products and technologies. Management is encouraged by the continued positive acceptance of our new SEER tissue resection device (orders have already been received) and we have already established a direct and specialty sales team. Although entry for this product into the hospital market (liver resection) has been slower and more challenging than initially anticipated due to changes in hospital purchasing procedures and environments which now consist of several levels of approval boards, we remain optimistic on the sales potential for this and other related products.
In addition, although the challenging economic conditions and global recession have adversely impacted our capital equipment sales in 2009 (as evidenced by the downward trend in the sale of our generators), we are cautiously optimistic that this trend will show modest improvement starting in the second half of 2010 due to improved economic conditions and the anticipated introduction of new products.
We have continued the development of new products in 2009 and submitted 510K’s to the Food and Drug Administration (“FDA”) for the BOSS, laproscopy SEER, Seal-N-Cut, Icon VS, Icon GS (J-Plasma) and Resistick II (coated blades). The 510K’s for the laproscopy SEER and ICON GS/J-Plasma, and coated blades were received in 2009. Recently, we received FDA clearance to market Resistick II and the ICON VS generator.
In August 2009, we received clearance to market our J-Plasma technology (ICON GS). J-Plasma includes an improved, redesigned system with added features to increase efficiency for the surgeon, while reducing manufacturing costs. We are developing marketing strategies for J-Plasma, and believe the product will be versatile, with possible uses in a range of surgical specialties.
Cautery sales have trended up from 2007 to 2008 but remained flat in 2009.
Company Products
We group our products into three main categories: electrosurgery, cauteries, and other products. Information regarding sales by product categories and related percentages is included in the Management’s Discussion and Analysis of Financial Condition and Results of Operations section of this report (page 18) and is incorporated by reference herein.
Electrosurgery Products
Electrosurgery is our largest product line and includes desiccators, generators, electrodes, electrosurgical pencils and various ancillary disposable products. In addition, in 2009 we expanded into the saline enhanced electrosurgical market, which has a market estimated in excess of 1.3 million procedures and $500,000,000 in the United States annually and has only one other competitor. These electrosurgical products are used during surgical procedures in gynecology, urology, plastic surgery, dermatology, veterinary, and other surgical markets for the cutting and coagulation of tissue. It is estimated that 80% of all surgical procedures performed worldwide are accomplished by electrosurgery. Our electrosurgery products fall under two categories, monopolar or bipolar. Monopolar products require the use of a grounding pad attached to the patient for the return of the electrical current, while bipolar products consist of two electrodes; one for the inbound current and one for the return current and therefore do not require the use of a grounding pad.
Aaron 900 and Aaron 940
These products are low powered (30 and 40 watt) and high frequency desiccators. These units were designed primarily for dermatology and family practice physicians. The units are used mainly for removing small skin lesions and growths.
Aaron 950
Bovie has developed a high frequency desiccator with cut capacity for outpatient surgical procedures. These generators allow physicians to change the power settings with one action. They were designed mainly for use in doctors’ offices and are utilized in a variety of specialties including dermatology, gynecology, family and general practice, urology, plastic surgery and ophthalmology.
Aaron 1250U
We have also developed a 120-watt multipurpose electrosurgery generator. The unit features monopolar and bipolar functions with pad sensing. This generator was recently redesigned to allow one unit to work with a line voltage ranging from 100 – 240 VAC whereas previously there was a need for three different versions.
Aaron 2250 / 3250 and IDS 200 / 300 / 400
Given the market interest in more powerful electrosurgical generators, we have developed 200-watt, 300-watt, and 400-watt multipurpose digital electrosurgery generators designed for the rapidly expanding surgi-center market and the hospital market. The digital hardware allows very high parallel data processing throughout the operation. All data is sampled and processed digitally. For the first time in electrosurgery, through digital technology, we are able to measure tissue impedance in real time (5,000 times a second). These units have been designed based on a digital feedback system. Bovie is using dedicated digital hardware instead of a general purpose controller for processing data. As the impedance varies, the power is adjusted to deliver a consistent clinical effect. The IDS 200 / Aaron 2250 have the capability to do m ost procedures performed today in the surgi-center or outpatient settings and was introduced in 2003. Although 200 watts is more than enough power to do most procedures in the operating room, 300 watts is considered the standard and believed to be what most hospitals and surgi-centers will require. Therefore, we developed the IDS 300/ Aaron 3250. The Bovie IDS 400 is a 400 watt generator designed primarily for sale in the overseas markets. These units feature both monopolar and bipolar functions, have pad and tissue sensing, plus nine blended cutting settings.
ICON GI and ICON GP
The ICON product lines are innovative, custom designed specialty electrosurgical generators that incorporate an easy to use touch-screen interface which provides the user flexibility in achieving a desired effect through different digitally built-in modes. In addition, the ICON product line was designed to improve safety and convenience by requiring the use of only split pads with digital technology to protect against pad burns. It features specialized error messaging to prevent misinterpretation and allows for quicker troubleshooting, and has specialized audible alerts to indicate improper cable connections. The ICON line represents a new foundation platform that can be readily expanded thereby reducing the development time and cost for future new specialized generators and also allowing the user to easily upgrade existing units. The ICON GI is designed for the gastrointestinal (“GI”) niche market, while the ICON GP is designed for a more general purpose market like hospital operating rooms, surgery centers, etc.
SEER line
The SEER line consists of conductive sintered steel as an electrode for radio frequency (RF) coupled with sterile saline used in cutting and coagulation, intended to lower blood loss, quicken procedure times and provide cost savings for hospitals. Initial fields of therapy for the technology include liver, pancreatic and kidney tumor therapies.
Cauteries
Battery Operated Cauteries
Battery operated cauteries constitute our second largest product line. Cauteries were originally designed for precise hemostasis (to stop bleeding) in ophthalmology. The current use of cauteries has been substantially expanded to include sculpting woven grafts in bypass surgery, vasectomies, evacuation of subungual hematoma (smashed fingernail) and for arresting bleeding in many types of surgery. Battery operated cauteries are primarily sterile one-time use products. Bovie manufactures one of the broadest lines of cauteries in the world, including but not limited to, a line of replaceable battery and tip cauteries, which are popular in overseas markets.
Other Products
Battery Operated Medical Lights
We manufacture a variety of specialty lighting instruments for use in ophthalmology as well as specialty lighting instruments for general surgery, hip replacement surgery and for the placement of endotracheal tubes in emergency and surgical procedures. We also manufacture and market physicians’ office use penlights.
Nerve Locator Stimulator
Bovie manufactures a nerve locator stimulator primarily used for identifying motor nerves in hand and facial reconstructive surgery. This instrument is a self-contained, battery-operated unit, used for single surgical procedures.
Research and Development and New Products
Our research and development activities are an essential component of our efforts to develop new innovative products for introduction in the marketplace. New and improved products play a critical role in the Company’s sales growth. The Company continues to place emphasis on the development of proprietary products and product improvements to complement and expand its existing product lines. We maintain close working relationships with physicians and medical personnel in hospitals and universities who assist in product research and areas of development. Our research and development activities are primarily developed internally. Information regarding the Company’s research and development costs for each of the past three years is included on page 22 in the Management’s Discussion and Analysis of Financial Condition and R esults of Operations section of this report and Footnote 2 of the Notes to Consolidated Financial Statements and is incorporated by reference herein.
BOSS product lines
The BOSS product line expands on the premise of the SEER patent in using conductive sintered steel as an electrode for radio frequency (RF) coupled with sterile saline but uses dual electrodes. This product is used in cutting and coagulation and is intended to lower blood loss, quicken procedure times and provide cost savings for hospitals. The BOSS is designed mainly for the orthopedic market which has a worldwide market size expected to total approximately $500 million in 2010. We recently filed for 510K FDA approval to market the BOSS which is anticipated to go into production in 2010.
Aaron 1450
The Aaron 1450-RF is a high frequency generator designed for surgery in the doctor office setting. This unit operates at 4 MHz, eight times higher frequency than a standard electrosurgical generator which operates at approximately 500 KHz. This unit is intended to be the first in a family of 4MHz generators initially designed for several office based specialties. The 1450-RF has been designed to include universal power control which allows the unit to be used in any power setting world wide.
Seal-N-Cut Handle and Accessories (formerly Polarian)
The Seal-N-Cut is a new disposable endoscopic surgical handle that supports a plurality of electrical and mechanical modes. This technologically advanced endoscopic device will target the growing vessel and tissue sealing and cutting market. We have recently filed for 510K FDA approval to market the Seal-N-Cut.
We acquired assets related to this product line in October 2006 from Lican Developments LTD (Lican), an Ontario, Canada Corporation. As a result of the asset acquisition, Steve Livneh became President of Bovie Canada. The assets acquired included proprietary patent pending technologies, working prototypes in various stages of development and production equipment.
ICON VS
This generator expands further on our ICON platform which incorporates a flexible and simple user interface and allows for customization of the output modes for a variety of electrosurgical applications. This product, like the ICON GI and GP, its predecessor generators, is designed and being developed to add safety features and improve convenience in performing general purpose procedures and includes a vessel sealing component. This generator will also be used with our Seal-N-Cut handle and accessories. We have recently received 510K FDA clearance to market the ICON VS.
ICON GS (J-Plasma)
Our J-Plasma technology is the foundation for the ICON GS plasma system, which utilizes a gas ionization process producing a stable thin focused beam of ionized gas that can be controlled in a wide range of temperatures and intensities, providing the surgeon greater precision, minimal invasiveness and an absence of conductive currents during surgery. The development of this new gas system generator also includes the design of a new proprietary handpiece. We have received 510K FDA approval to market the ICON GS.
Prior to our acquiring the J-Plasma technology, Soring, a German company, had licensed the same technology. The license agreement was terminated but Soring has filed its own patent possibly using the J-Plasma technology as its basis. Management of Bovie believes Soring may be infringing on our patent and as a result, there is no assurance that there will not be future litigation involving Soring or the possible loss of our competitive advantage.
MEG Handle and Accessories
These innovative modular forceps are ergonomically designed to provide surgeons added comfort and improved safety while reducing per-procedure costs. The modular forceps offer a unique and simpler assembly process for laparoscopic procedures and are the first modular design for the arthroscopy market.
We launched our first generation of the MEG line in 2009. However, after receiving various sources of feedback requesting further abilities to be incorporated in the design, we have temporarily suspended the manufacture of the first generation MEG line in an effort to provide our customers with the features they requested in our second generation MEG design.
We acquired the MEG patent-pending technology in January 2006 from Henvil Corp. Ltd. (“Henvil”) and Steve Livneh, its principal owner. Pursuant to this agreement, commencing with the year following the first sale or commercial delivery of the Product, Bovie will pay to Henvil’s principal, Steve Livneh, an initial minimum royalty of the greater of $35,000 per year or 3% of adjusted gross sales received from the sale of the instruments for a period of four years. Thereafter, Mr. Livneh will be paid a royalty equal to 2.5% of adjusted gross sales for the life of the patents established for the technology.
Suture Cutter
The Suture Cutter was not well accepted by the doctors who tried the device on patients for a variety of reasons. Obstacles consisted of resistance to the higher price verses current method of suture removal, the requirement to change standard procedures related to suture removal procedures, and partial concerns related to the heating element. Based on this feedback, we are evaluating the potential of this product but feel with a modification in design and an introduction to classes at both the teaching university and at medical trade shows that this still could become an accepted method of technique in a niche market. The investment in this product line has been minimal to date.
Resistick II
Resistick II is coating applied to stainless steel which resists eschar (scab or scar tissue caused by burning) during surgery. The coated electrodes continue the expansion of the Bovie line of electrosurgical disposables. the Company has received 510(k) clearance from the Food and Drug Administration (FDA) to market its line of coated blades, needles and ball electrodes used for cutting and coagulating soft tissues during surgical procedures
Sales & Marketing
The majority of the Company’s products are marketed through medical distributors, which distribute to more than 6,000 hospitals and to doctors and other health-care facilities. New distributors are contacted through responses to our advertising in international and domestic medical journals and domestic or international trade shows. International sales represented 16% of total revenues in 2009 as compared with 17% in 2008 and 15% in 2007. The Company’s products are sold in more than 150 countries through local dealers which are coordinated by sales and marketing personnel at the Clearwater, Florida facility.
Our business is generally not seasonal in nature.
Competition
The Company competes with numerous manufacturers and distributors of medical supplies and devices, many of which are large and well established.
We sell our products and compete in various ways including by private labeling some of our products for major distributors under their label, and selling the majority of our other products through distributors. Private labeling allows us to increase our position in the marketplace and thereby compete from two different approaches, our Aaron or Bovie label, and our customers’ private label. Our private label customers distribute our products under their name through their internal sales force. Selling the majority of our other products through distribution increases our sales potential and helps level the playing field in regards to our larger competitors as most of the companies we compete with sell direct. Domestically, we believe, we have a substantial market share in the field of electrosurgical generator manufacturing which c onsists of our Company label and OEM units. We sell our products and compete with other manufacturers in various ways.
Our main competitors are Conmed, Valleylab (a division of Covidien) and Erbe Electromedizine, in the electrosurgery market, Xomed (a division of Medtronics), in the battery operated cautery market, Salient Surgical Technologies (formerly Tissuelink) in the saline enhanced sintered steel market and Ethicon and U.S. Surgical in the endoscopic instrumentation market. We believe our competitive position did not change in 2009.
Intellectual Property
We rely on our intellectual property that we have acquired over recent years including patents, trade secrets, technical innovations, and various licensing agreements to provide our future growth and build our competitive position. We own 20 outstanding patents and trademarks with some of our earlier patents currently having diminished useful lives. We also have filed several U.S. and international patent applications pending for various new products. As we continue to expand our intellectual property portfolio we believe it is critical for our Company to continue to invest in filing patent applications to protect our technology, inventions, and improvements, however we can give no assurance that competitors will not infringe on our patent rights or otherwise create similar or non-infringi ng competing products that are technically patentable in their own right.
Manufacturing and Suppliers
We are committed to producing products of the highest quality and technical advancements. Bovie manufactures the majority of its products on its premises in Clearwater, Florida which is certified under the ISO international quality standards and may be subject to continuing regulation and routine inspections by the FDA to determine compliance with regulations in our quality system, medical device reporting, and FDA restrictions on promoting products for unapproved or off-label uses. In addition, we are also subject to regulations under the Occupational Safety and Health Act, the Environmental Protection Act and other federal, state and local regulations.
Customers
We sell the majority of our current products through major distributors which include Allegiance (a Cardinal Company), IMCO, McKesson Medical Surgical, Inc., Medline, NDC (Abco, Cida and Starline), Owens & Minor, and Physician Sales & Service. In addition we have a major OEM customer, Arthrex, Inc. for which we manufacture products on a private label basis, pursuant to an agreement. On August 31, 2007 we amended and extended this manufacturing agreement for an additional three year period. The main change to the amended manufacturing agreement is the elimination of the provision that required Arthrex to exclusively purchase the products from us as well as the elimination of the provision that required us not to compete in the same Arthrex markets with said products. This amended Arthrex Agreement has termination date s of December 6, 2010 and March 2011 for the generators. In fiscal 2009, Arthrex orders represented approximately 22% of our total revenues. As such, should Arthrex determine to reduce or cease placement of orders for the products, our business will likely be adversely affected.
Backlog
The dollar value of unshipped factory orders is not material.
Employees
Bovie has 163 full time employees consisting of 4 executive officers, 21 supervisory personnel, 11 sales personnel, and 127 technical support, administrative, and production employees. None of our current employees are covered by any collective bargaining agreement and we have never experienced a work stoppage. We consider our employee relations to be good.
In addition to risks and uncertainties in the ordinary course of business, important risk factors that may affect us are discussed below.
Risks Relating to Our Business
Current challenges in the credit and capital markets may adversely affect our business and financial condition.
The current global economic crisis described below should also be considered when reviewing each of the subsequent paragraphs setting forth the various aspects of our business, operations, and products.
The recent global economic and financial market crisis has caused, among other things, a general tightening in the credit and capital markets, lower levels of liquidity, increases in the rates of default and bankruptcy, and lower consumer and business spending. Although the ultimate outcome of these events cannot be predicted, they may have a material adverse effect on the Company and our ability to raise capital or borrow money in the credit markets and potentially to draw on our revolving credit facility or otherwise obtain financing. Similarly, current or potential customers and suppliers may no longer be in business, may be unable to fund purchases or may decide to reduce purchases, all of which could lead to reduced demand for our products, reduced gross margins, and increased customer payment delays or defaults. Furthe r, suppliers may not be able to supply us with needed raw materials on a timely basis, may increase prices or go out of business, which could result in our inability to meet customer demand in a timely manner or affect our gross margins. We are also limited in our ability to reduce costs to offset the results of a prolonged or severe economic downturn given certain fixed costs associated with our operations.
We do a substantial amount of business with certain original equipment manufacturers (“OEM”) which as a group have produced substantial revenues for our Company. Loss of business from a major OEM customer will likely adversely affect our business.
Bovie manufactures the majority of its products on its premises in Clearwater, Florida. Labor-intensive sub-assemblies and labor-intensive products may be out-sourced to our specification. Although we sell through distributors, we market our products through national trade journal advertising, direct mail, distributor sales representatives and trade shows, under the Bovie name, the Bovie/Aaron name and private label. Major distributors include Allegiance (a Cardinal Company), IMCO, McKesson Medical Surgical, Inc., Medline, NDC (Abco, Cida and Starline), Owens & Minor, and Physician Sales & Service. If any of these distributor relationships are terminated or not replaced, our revenue from the territories served by these distributors could be adversely affected.
We have a major OEM customer, Arthrex, Inc. for which we manufacture products on a private label basis, pursuant to an agreement. On August 31, 2007, we amended and extended this manufacturing agreement for an additional three year period. The amended terms continue to provide that we will be reimbursed for our expenses in developing any changes or modifications to products according to Arthrex’s specifications, and that Arthrex continues to own the intellectual property. In addition, general provisions for product warranties, insurance, termination, and confidentiality remain the same. The main change to the amended manufacturing agreement is the elimination of the provision that required Arthrex to exclusively purchase the products from us as well as the elimination of the provision that required us to forego competi ng in the same Arthrex markets with said products. This amended Arthrex Agreement has termination dates of December 6, 2010 and March 2011 for the generators. In fiscal 2009, Arthrex orders represented approximately 22% of our total revenues. As such, should Arthrex determine to reduce or cease placement of orders for the products, or decline to extend or review its agreements with us, our business will likely be materially and adversely affected.
We are also dependent on other OEM customers who have no legal obligation to purchase products. Should the collaborative customer fail to give us purchase orders for the product after development, our future business and value of related assets could be negatively affected. Furthermore, no assurance can be given that a collaborative customer will give sufficient high priority to our products. Finally, disagreements or disputes may arise between Bovie and its contractual customers, which could adversely affect production of our products.
We rely on certain suppliers and manufacturers for raw materials and other products and are vulnerable to fluctuations in the availability and price of such products and services.
Fluctuations in the price, availability, and quality of the raw materials we use in our manufacturing could have a negative effect on our cost of sales and our ability to meet the demands of our customers. Inability to meet the demands of our customers could result in the loss of future sales. In addition, the costs to manufacture our products depends in part on the market prices of the raw materials used to produce the them. We may not be able to pass along to our customers all or a portion of our higher costs of raw materials due to competitive and marketing pressures, which could decrease our earnings.
We also have informal collaborative arrangements with three foreign suppliers under which we request the development of certain items and components and we purchase them pursuant to purchase orders. Our purchase order commitments are never more than one year in duration and are supported by orders from our customers.
If we are unable to protect our patents or other proprietary rights, or if we infringe on the patents or other proprietary rights of others, our competitiveness and business prospects may be materially damaged.
We own 20 outstanding patents and trademarks with some of our earlier patents currently having diminished useful lives. We also have several U.S. and international patent applications pending for various new products. We can give no assurance that competitors will not infringe on our patent rights or otherwise create similar or non-infringing competing products that are technically patentable in their own right.
We have recently filed new patent applications for various new products including a scanning cannula, modular laparoscopic and endoscopic instruments, the output stage to our generator platform, our ICON product line and a Plasma Stream patent application relating to the plasma technology.
Divestitures of some of our operations or product lines may materially and adversely affect our business and results of operations.
We periodically evaluate the performance of all of operations and although we have not to date, we may sell, consolidate, or close a portion of our business or product lines. Any divestitures may result in significant write-offs, including those related to goodwill and other intangible assets, which could have a material adverse effect on our business and results of operations. Divestitures could involve additional risks, including difficulties in the separation of operations, services, products and personnel, the diversion of management’s attention from other business concerns, the disruption of our business and the potential loss of key employees. We may not be successful in managing these or any other significant risks that we encounter in divesting a business or product line.
Although we carry liability insurance, due to the nature of our products and their use by professionals, we may, from time to time, be subject to litigation from persons who sustain injury during medical procedures in hospitals, physician’s offices or in clinics and defending such litigations is expensive, disruptive, time consuming and could adversely affect our business.
The manufacture and sale of medical products entail significant risks of product liability claims. Bovie currently maintains product liability insurance with combined coverage limits of $10 million on a claims made basis. There is no assurance that this coverage will be adequate to protect us from any possible liabilities we might incur in connection with the sale or testing of our products. In addition, we may need increased product liability coverage as products are commercialized. This insurance is expensive and in the future may not be available on acceptable terms, if at all.
Our business is subject to the potential for defects or failures associated with our products which could lead to recalls or safety alerts and negative publicity.
Manufacturing flaws, component failures, design defects, off-label uses or inadequate disclosure of product-related information could result in an unsafe condition or the injury or death of a patient. These problems could lead to a recall of, or issuance of a safety alert relating to, our products and result in significant costs and negative publicity. Due to the strong name recognition of our brands, an adverse event involving one of our products could result in reduced market acceptance and demand for all products within that brand, and could harm our reputation and our ability to market our products in the future. In some circumstances, adverse events arising from or associated with the design, manufacture or marketing of our products could result in the suspension or delay of our current regulatory reviews of our applications for n ew product approvals. We also may undertake voluntarily to recall products or temporarily shut down production lines based on internal safety and quality monitoring and testing data. Any of the foregoing problems could disrupt our business and have a material adverse effect on our business, results of operations, financial condition and cash flows.
Our manufacturing facilities are located in Clearwater, Florida and could be affected due to multiple risks from fire, hurricanes and the like.
Our manufacturing facilities are located in Clearwater, Florida and could be affected by multiple weather risks, most notably hurricanes (one of which previously caused damage to the roof of one of our buildings as well as some of our furniture and equipment). The damage was mildly disruptive to operations. Although we carry casualty insurance and business interruption insurance, future possible disruptions of operations due to hurricanes or other weather risks could affect our ability to meet our commitments to our customers and impair important business relationships, the loss of which could adversely affect our operations and profitability.
Risks Related to Our Industry
The medical device industry is highly competitive and we may be unable to compete effectively.
The medical device industry is highly competitive. Many competitors in this industry are well established, do a substantially greater amount of business, and have greater financial resources and facilities than we do.
Domestically, we believe we rank third in the number of units sold in the field of electrosurgical generator manufacturing and we sell our products and compete with other manufacturers in various ways. In addition to advertising, attending trade shows and supporting our distribution channels, we strive to enhance product quality, improve user friendliness and expand product exposure.
We also compete by private labeling our products for major distributors under their label. This allows us to increase our position in the marketplace and thereby compete from two different approaches, our Aaron or Bovie label, and our customers’ private label. Our private label customers distribute our products under their name through their internal sales force. We believe our main competitors do not private label their products.
Lastly, at this time we sell the majority of our products through distributors. Many of the companies we compete with sell direct, thus competing directly with distributors they sometimes use.
Our main competitors are Conmed, Valleylab (a division of Covidien) and Erbe Electromedizine, in the electrosurgery market, Xomed (a division of Medtronics), in the battery operated cautery market, Salient Surgical Technologies (formerly Tissuelink) in the saline enhanced sintered steel market and Ethicon and U.S. Surgical in the endoscopic instrumentation market. We believe our competitive position did not materially change in 2009.
Our industry is highly regulated by the U.S. Food and Drug Administration and internationally including other governmental, state and federal agencies which have substantial authority to establish criteria which must be complied with in order for us to continue in operation.
United States
The Company’s products and research and development activities are subject to regulation by the FDA and other regulatory bodies. FDA regulations govern, among other things, the following activities:
| · | Pre-market clearance or approval. |
| · | Advertising and promotion. |
| · | Product traceability, and |
In the United States, medical devices are classified on the basis of control deemed necessary to reasonably ensure the safety and effectiveness of the device. Class I devices are subject to general controls. These controls include registration and listing, labeling, pre-market notification and adherence to the FDA Quality System Regulation. Class II devices are subject to general and special controls. Special controls include performance standards, post market surveillance, patient registries and FDA guidelines. Class III devices are those which must receive pre-market approval by the FDA to ensure their safety and effectiveness. Currently, we only manufacture Class I and Class II devices. Pre-market notification clearance must be obtained for some Class I and most Class II devices when the FDA does not require pre-market ap proval. All Bovie products have been cleared by the Pre-market notification process. To date, the FDA has not failed to clear any devices we have submitted.
A pre-market approval application is required for most Class III devices. A pre-market approval application must be supported by valid scientific evidence to demonstrate the safety and effectiveness of the device. The pre-market approval application typically includes:
| · | Results of bench and laboratory tests, animal studies, and clinical studies |
| · | A complete description of the device and its components |
| · | A detailed description of the methods, facilities and controls used to manufacture the device, and proposed labeling. |
The pre-market approval process can be expensive, uncertain and lengthy. A number of devices for which pre-market approval has been sought by other companies have never been approved for marketing.
International Regulation
To market products in the European Union, our products must bear the “CE” mark. Manufacturers of medical devices bearing the CE mark have gone through a conformity assessment process that assures that products are manufactured in compliance with a recognized quality system and to comply with the European Medical Devices Directive.
Each device that bears a CE mark has an associated technical file that includes a description of the following:
| · | Description of the device and its components, |
| . | A summary of how the device complies with the essential requirements of the medical devices directive, |
| · | Safety (risk assessment) and performance of the device, |
| · | Clinical evaluations with respect to the device, |
| · | Methods, facilities and quality controls used to manufacture the device, and |
| · | Proposed labeling for the device. |
Manufacturing and distribution of a device is subject to ongoing surveillance by the appropriate regulatory body to ensure continued compliance with quality system and reporting requirements.
We began CE marking of devices for sale in the European Union in 1999. In addition to the requirement to CE mark, each member country of the European Union maintains the right to impose additional regulatory requirements.
Outside of the European Union, regulations vary significantly from country to country. The time required to obtain approval to market products may be longer or shorter than that required in the United States or the European Union. Certain European countries outside of the European Union do recognize and give effect to the CE mark certification. We are permitted to market and sell our products in those countries.
If we are unable to successfully introduce new products or fail to keep pace with advances in technology, our business, financial condition and results of operations could be adversely affected. In addition, our research and development efforts rely upon investments and alliances, and we cannot guarantee that any previous or future investments or alliances will be successful.
Our research and development activities are an essential component of our efforts to develop new innovative products for introduction in the marketplace. New and improved products play a critical role in our sales growth. We continue to place emphasis on the development of proprietary products and product improvements to complement and expand its existing product lines. We maintain close working relationships with physicians and medical personnel in hospitals and universities who assist in product research and areas of development. Our research and development activities are primarily conducted internally and are expensed as incurred. These expenses include direct expenses for wages, materials and services associated with the development of our products net of any reimbursements from customers. Research and development expenses do not include any portion of general and administrative expenses. The Company has two complementary facilities that both contribute to a centralized research and development focus. Our Clearwater, FL facility has been our flagship research and design location, followed later by our addition of the Canadian facility in October 2006. Currently both facilities are working synergistically developing our new products the ICON GP/VS and ICON GS, as well as the accompanying Endoscopic Modular Instruments, the Polarian handle and accessories. We expect to make future investments to enable us to develop new technologies and products to further our strategic objectives and strengthen our existing business. However, we cannot guarantee that any of our previous or future investments will be successful.
The amount expended by us on research and development of our products during the years 2009, 2008 and 2007, totaled approximately $2.1, $2.1, and $1.6 million respectively. During the past three years, we invested in the J-Plasma technology, currently used in one of our new products under development, the ICON GS plasma system. In addition, we invested in the BOSS and other saline enhanced electrosurgical devices, Endoscopic Modular Instruments, and accompanying new generators. We have not incurred any direct costs relating to environmental regulations or requirements. For 2010, we expect our expenditures for research and development activities to remain around the same level as 2009.
Our international operations subject us to foreign currency fluctuations and other risks associated with operating in foreign countries.
We operate internationally and enter into transactions denominated in foreign currencies (most notably the Canadian dollar and the Euro). To date, we have not hedged our exposure to changes in foreign currency exchange rates, and as a result, we are subject to foreign currency transaction and translation gains and losses. We purchase goods and services in U.S. and Canadian dollars and have recently begun to invoice certain product sales in Euros. Foreign exchange risk is managed primarily by satisfying foreign denominated expenditures with cash flows or assets denominated in the same currency. We charged currency value fluctuations to our accumulated other comprehensive account which amounts were not material for 2009.
Our operations are subject to certain proposed healthcare reform legislative proposals that could be enacted into law, our business, financial condition, results of operations and cash flows could be significantly and adversely affected.
In 2009, both the U.S. Senate and House of Representatives released various draft proposals related to healthcare reform legislation which could include provisions that would impose a fee or excise tax on certain medical devices. These proposals may apply to some or all of our medical device and supply products. Many details of the proposals remain uncertain, and any healthcare reform legislation must still be enacted by both Houses of Congress and signed by the President. If any of these medical device proposals is enacted into law, our results of operations could be materially and adversely affected.
Our operations may experience higher costs to produce our products as a result of changes in prices for oil, gas and other commodities.
We use some plastics and other petroleum-based materials along with precious metals contained in electronic components as raw materials in many of our products. Prices of oil and gas also significantly affect our costs for freight and utilities. Oil, gas and precious metal prices are volatile and may increase, resulting in higher costs to produce and distribute our products. Due to the highly competitive nature of the healthcare industry and the cost-containment efforts of our customers we may be unable to pass along cost increases through higher prices. If we are unable to fully recover these costs through price increases or offset through other cost reductions, our results of operations could be materially and adversely affected.
Risks related to climate change
Our manufacturing facilities (Clearwater and Canada) do not produce hazardous materials or emissions that would adversely impact the environment. We do however, have air conditioning units and consume electricity which could be impacted by climate change in the form of increased rates. We do not however believe the increase in expense from the rate increases, as a percentage of sales, would be material in the near term.
The effect on the Company’s business and operations related to physical changes in the planet caused by climate change such as increased storms (hurricanes) could result in impaired production of products or damages to property, plant and equipment. We do however maintain a backup generator at our Clearwater facility and a disaster recovery plan in place to help mitigate this risk.
ITEM 1B. Unresolved Staff Comments
There are no outstanding unresolved comments from the staff of the Securities and Exchange Commission.
Bovie currently maintains the following locations:
| | · | Our executive office at 734 Walt Whitman Road, Melville, New York which is leased for approximately $1,500 per month. |
| | · | A 60,000 square foot facility which consists of office, warehousing, manufacturing and research space located at 5115 Ulmerton Rd., Clearwater, FL which was acquired on September 11, 2008, renovated, and occupied during June 2009. Monthly principal and interest payments are approximately $24,000 per month. |
| | · | A 28,000 square foot manufacturing facility at 7100 30th Ave N., St Petersburg, Florida which we own. |
| | · | A research and manufacturing facility at 4056 North Services Rd. E., Windsor, Canada which is leased for approximately $4,200 per month through December 2010. |
| | · | A research and manufacturing facility at 3200 Tyrone Blvd., St. Petersburg, Florida which is leased for approximately $13,000 per month under a lease that expires in September 2013. |
The Company currently has the facility at 7100 30th Ave. N., St. Petersburg listed with a broker for sale along with a sublease listed with the same broker for its facility at 3200 Tyrone Blvd., St. Petersburg.
ITEM 3. Legal Proceedings
In 2008, Erbe USA, Inc. (“Erbe”) filed a civil action in the U.S. District Court for the Northern District of Georgia, Atlanta Division, against Bovie and a former employee, seeking equitable relief and unspecified damages. The complaint essentially alleges that the employee, among other things, breached his employment agreement with Erbe by wrongfully taking Erbe’s confidential information and trade secrets for use in his new employment position, with the assistance of Bovie. In a mutual effort to resolve the dispute, on November 4, 2009, Bovie and Erbe signed a full and final settlement agreement and mutual general release of all claims. We continue to deny Erbe’s claims and allegations. Given that both parties desire to end the litigation and mit igate ongoing legal costs, however, we agreed to pay Erbe $160,000 as part of the terms of the settlement. We also agreed not to use or disclose, and to destroy, any information that Erbe alleged constituted trade secrets and confidential business information related to Erbe. Additional terms of the settlement include a two-year period in which we agreed not to solicit (a) Erbe’s current employees and (b) a limited number of dealers and independent representatives who currently market Erbe products (see Note 12. Commitments and Contingency).
ITEM 4. Submission of Matters to a Vote of Security Holders
Reserved
PART II
ITEM 5. Market for Registrant’s Common Equity and Related Stockholder Matters
Bovie’s common stock currently is traded on the NYSE Amex exchange and previously was traded on the American Stock Exchange from November 5, 2003. The table shows the reported high and low bid prices for the common stock during each quarter of the last eight respective quarters. These prices do not represent actual transactions and do not include retail markups, markdowns or commissions.
| 2009 | | High | | | Low | |
| | | | | | | |
4th Quarter | | $ | 9.11 | | | $ | 7.17 | |
3rd Quarter | | | 10.00 | | | | 7.04 | |
2nd Quarter | | | 9.69 | | | | 6.24 | |
1st Quarter | | | 7.60 | | | | 5.75 | |
| | | | | | | | | |
| 2008 | | High | | | Low | |
| | | | | | | |
4th Quarter | | $ | 7.57 | | | $ | 3.90 | |
3rd Quarter | | | 8.05 | | | | 6.51 | |
2nd Quarter | | | 9.27 | | | | 6.27 | |
1st Quarter | | | 6.69 | | | | 5.50 | |
On March 1, 2010, the closing bid for Bovie’s Common Stock as reported by the NYSE Amex exchange was $7.23 per share. As of March 1, 2010, the total number of shareholders of Bovie’s Common Stock was approximately 3,500, of which approximately 2,800 are estimated to be shareholders whose shares are held in the name of their broker, stock depository or the escrow agent holding shares for the benefit of Bovie Medical Corporation shareholders and the balance are shareholders who keep their shares registered in their own name.
Recent Sales of Unregistered Equity Securities
None
Issuer Purchases of Equity Securities
| | | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | | | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights | | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) | |
| | | (a) | | | (b) | | | (c) | |
| Equity compensation plans approved by security holders | | | 1,621,525 | | | $ | 3.69 | | | | 148,875 | |
| Equity compensation plans not approved by security holders | | | 120,000 | (1) | | | 3.87 | | | | — | |
| | | | | | | | | | | | | |
| TOTAL | | | 1,741,525 | | | $ | 3.61 | | | | 148,875 | |
(1) Includes an issuance on January 11, 2006 for 100,000 restricted stock options to Henvil Corporation related to the acquisition of the MEG technology and 20,000 restricted stock options granted to Howard Stallard pursuant to an employment agreement dated October 1, 2006 related to the Lican Development asset purchase agreement.
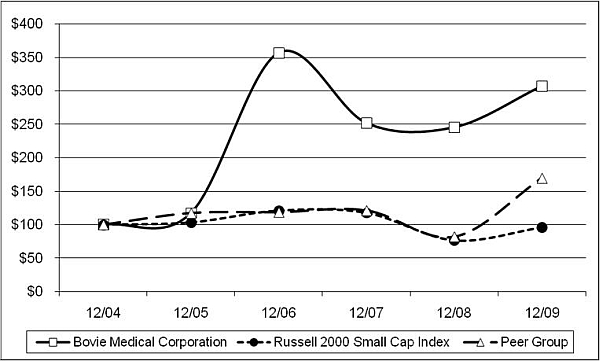
Performance Graph
The following graph shows a comparison of the cumulative total stockholder return for our common stock, the Russell 2000 Small Cap Index, and a peer group that we believe in good faith is an appropriate basis for comparison. The comparison for each of the periods assumes that $100 was invested on December 31, 2004 in our common stock, the Russell 2000 Small Cap Index, and the stocks in the peer group, and that all dividends were reinvested. The results shown in the graph below are not necessarily indicative of future performance.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Bovie Medical Corporation, Russell 2000 Small Cap Index,
And a Peer Group
* $100 invested on 12/31/2004 in stock or index, including reinvesting of any dividends. Fiscal year ended December 31.
| | | Cumulative Total Return | | | | |
| | | | 12/04 | | | | 12/05 | | | | 12/06 | | | | 12/07 | | | | 12/08 | | | | 12/09 | |
| Bovie Medical Corporation | | | 100.00 | | | | 117.32 | | | | 357.09 | | | | 251.97 | | | | 245.67 | | | | 307.48 | |
| Russell 2000 Small Cap Index | | | 100.00 | | | | 103.32 | | | | 120.89 | | | | 117.57 | | | | 76.65 | | | | 95.98 | |
| Peer Group | | | 100.00 | | | | 117.44 | | | | 118.70 | | | | 121.32 | | | | 82.00 | | | | 169.90 | |
This peer group consists of five companies, Atrion Corp. (ATRI), Alpha Pro Tech Ltd. (APT), Endologix (ELGX), Utah Medical Products (UTMD), and Trinity Biotech plc. (TRIB). These companies were chosen using the following criteria: a listing on either the NYSE or Nasdaq Exchange, they were in the medical supply industry, they had similar market capitalization, and similar sales volume and number of employees.
This information shall not be deemed to be ''soliciting material’’ or to be ''filed’’ with the Commission or subject to Regulation 14A (17 CFR 240.14a-1-240.14a-104), other than as provided in Item 201(e) of Regulation S-K, or subject to the liabilities of section 18 of the Exchange Act (15 U.S.C. 78r).
We have never declared or paid any cash dividends on our common stock and we do not intend to pay cash dividends in the foreseeable future. We currently expect to retain any future earnings to fund the operation and expansion of our business.
ITEM 6. Selected Financial Data
The following selected consolidated financial data (presented in thousands, except per share amounts and employee data) are derived from our consolidated financial statements. This data should be read in conjunction with the consolidated financial statements and notes thereto, and with Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Year Ended December 31,
(in thousands, except per share amounts)
| | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | |
| | | | | | | | | (As restated) | | | | | | | |
| Sales, net | | $ | 26,953 | | | $ | 28,097 | | | $ | 28,779 | | | $ | 26,676 | | | $ | 20,211 | |
| Cost of sales | | | 15,098 | | | | 16,248 | | | | 17,464 | | | | 16,075 | | | | 12,649 | |
| | | | | | | | | | | | | | | | | | | | | |
| Gross Profit | | | 11,855 | | | | 11,849 | | | | 11,315 | | | | 10,601 | | | | 7,562 | |
| | | | | | | | | | | | | | | | | | | | | |
| Gain on cancellation of agreement | | | -- | | | | 1,496 | | | | -- | | | | -- | | | | -- | |
| | | | | | | | | | | | | | | | | | | | | |
| Other costs: | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | 2,083 | | | | 2,061 | | | | 1,643 | | | | 1,048 | | | | 986 | |
| Professional services | | | 1,398 | | | | 991 | | | | 738 | | | | 520 | | | | 447 | |
| Salaries and related costs | | | 3,003 | | | | 3,017 | | | | 2,805 | | | | 2,558 | | | | 2,011 | |
| Selling, general and administration | | | 4,656 | | | | 4,489 | | | | 4,023 | | | | 3,712 | | | | 3,553 | |
| Development cost - joint venture | | | -- | | | | -- | | | | -- | | | | 139 | | | | 161 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total other costs | | | 11,140 | | | | 10,558 | | | | 9,209 | | | | 7,977 | | | | 7,158 | |
| | | | | | | | | | | | | | | | | | | | | |
| Income from operations | | | 715 | | | | 2,787 | | | | 2,106 | | | | 2,624 | | | | 404 | |
| | | | | | | | | | | | | | | | | | | | | |
| Other income and (expense): | | | | | | | | | | | | | | | | | | | | |
| Interest income | | | 24 | | | | 49 | | | | 143 | | | | 103 | | | | 47 | |
| Minority interest | | | -- | | | | -- | | | | 5 | | | | 20 | | | | 10 | |
| Interest expense | | | (76 | ) | | | (59 | ) | | | (3 | ) | | | (16 | ) | | | (23 | ) |
| Total other income (expense) - net | | | (52 | ) | | | (10 | ) | | | 145 | | | | 107 | | | | 34 | |
| | | | | | | | | | | | | | | | | | | | | |
| Income before income taxes | | | 663 | | | | 2,777 | | | | 2,251 | | | | 2,731 | | | | 438 | |
| | | | | | | | | | | | | | | | | | | | | |
| Benefit (provision) for income taxes | | | (67 | ) | | | (945 | ) | | | 1,550 | | | | (48 | ) | | | (32 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net income | | $ | 596 | | | $ | 1,832 | | | $ | 3,801 | | | $ | 2,683 | | | $ | 406 | |
| | | | | | | | | | | | | | | | | | | | | |
| Earnings per common share: | | | | | | | | | | | | | | | | | | | | |
| Basic | | $ | 0.04 | | | $ | 0.11 | | | $ | 0.25 | | | $ | 0.19 | | | $ | 0.03 | |
| Diluted | | $ | 0.03 | | | $ | 0.11 | | | $ | 0.22 | | | $ | 0.16 | | | $ | 0.03 | |
| | | | | | | | | | | | | | | | | | | | | |
| Balance Sheet Information: | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 2,155 | | | $ | 2,565 | | | $ | 3,535 | | | $ | 2,953 | | | $ | 1,295 | |
| Working capital | | $ | 10,741 | | | $ | 9,943 | | | $ | 10,071 | | | $ | 7,955 | | | $ | 5,501 | |
| Total assets | | $ | 27,584 | | | $ | 26,725 | | | $ | 20,213 | | | $ | 16,686 | | | $ | 11,771 | |
| Long-term debt | | $ | 3,958 | | | $ | 4,143 | | | $ | 318 | | | $ | 368 | | | $ | 0 | |
| Stockholders’ equity | | $ | 21,153 | | | $ | 20,128 | | | $ | 18,192 | | | $ | 14,060 | | | $ | 9,802 | |
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Our future results of operations and the other forward-looking statements contained herein, particularly the statements regarding growth in the medical products industry, capital spending, research and development, and marketing and general and administrative expenses, involve a number of risks and uncertainties. In addition to the factors discussed above, there are other factors that could cause actual results to differ materially, such as business conditions and the general state of the economy; competitive factors including rival manufacturers’ availability of components at reasonable prices; risk of nonpayment of accounts receivable; risks associated with foreign operations; and litigation involving intellectual property and consumer issues.
We believe that we have the product mix, facilities, personnel, competitive edge, operating cash flows and financial resources for business success in the immediate future (1 year) and distant future (after 1 year), but future revenues, costs, margins, product mix and profits are all subject to the influence of a number of factors, as discussed above.
The following discussion should be read in conjunction with the Selected Financial Data and the Consolidated Financial Statements and Notes.
Executive Level Overview
We are a medical device company engaged in the manufacturing and marketing of electrosurgical devices. Our medical products include a wide range of devices including electrosurgical generators and accessories, cauteries, medical lighting, nerve locators and other products.
We internally divide our operations into three product lines. Electrosurgical products, battery operated cauteries and other products. The electrosurgical line sells electrosurgical products which include desiccators, generators, electrodes, electrosurgical pencils and various ancillary disposable products. These products are used in surgery for the cutting and coagulation of tissue. Battery operated cauteries are used for precise hemostasis (to stop bleeding) in ophthalmology and in other fields. Our other revenues are derived from nerve locators, disposable and reusable penlights, medical lighting, license fees, development fees and other miscellaneous income.
Most of the Company’s products are marketed through medical distributors, which distribute to more than 6,000 hospitals, and to doctors and other health-care facilities. New distributors are contacted through responses to our advertising in international and domestic medical journals and domestic or international trade shows. International sales represented 16% of total revenues in 2009 as compared with 17% in 2008 and 15% in 2007. The Company’s products are sold in more than 150 countries through local dealers which are coordinated by sales and marketing personnel at the Clearwater, Florida facility. Our business is generally not seasonal in nature.
Outlook for 2010
During 2009 we continued to make progress on the development and marketing of our new products and technologies. We are encouraged by the continued positive acceptance of our new SEER tissue resection device (orders have already been received) and we have already established a direct and specialty sales team for this product. Although entry for this product into the hospital market (liver resection) has been slower and more challenging than initially anticipated due to changes in hospital purchasing procedures and environments which now consist of several levels of approval boards, we remain optimistic on the sales potential for this and other related products.
In addition, although the challenging economic conditions and global recession have adversely impacted our capital equipment sales in 2009 (as evidenced by the downward trend in the sale of our generators), we are cautiously optimistic that this trend will show modest improvement starting in the second half of 2010 due to improved economic conditions and the anticipated introduction of new products.
We have continued the development of new products in 2009 and submitted 510K’s to the Food and Drug Administration (“FDA”) for the BOSS, laproscopy SEER, Seal-N-Cut, Icon VS, Icon GS (J-Plasma) and Resistick II (coated blades). The 510K’s for the laproscopy SEER and ICON GS/J-Plasma, and coated blades were received in 2009. Recently, we received FDA clearance to market Resistick II and the ICON VS generator.
In August 2009, we received clearance to market our J-Plasma technology (ICON GS). J-Plasma includes an improved, redesigned system with added features to increase efficiency for the surgeon, while reducing manufacturing costs. We are developing marketing strategies for J-Plasma, and believe the product will be versatile, with possible uses in a range of surgical specialties.
In today’s economic environment, marked by historic uncertainty, forecasting has become increasingly more difficult. We have and will always, take a conservative approach. Every effort has been made to provide an outlook based on our experience and knowledge; however, variations beyond our ability to predict or control often impact forecasting which may result in a change in this outlook. We strongly encourage investors to visit our website: www.boviemedical.com to view the most current news and to review our filings with the Securities and Exchange Commission.
Results of Operations –
Sales
| | | 2009 vs. 2008 | | | 2008 vs. 2007 | |
| Sales by Product Line (in thousands) | | 2009 | | | 2008 | | | Percent change | | | 2008 | | | 2007 | | | Percent change | |
| Electrosurgical | | $ | 18,576 | | | | 19,535 | | | | (4.9 | )% | | $ | 19,535 | | | | 20,284 | | | | (3.7 | )% |
| Cauteries | | | 6,252 | | | | 6,265 | | | | (0.2 | )% | | | 6,265 | | | | 6,131 | | | | 2.2 | % |
| Other | | | 2,125 | | | | 2,296 | | | | (7.5 | )% | | | 2,296 | | | | 2,364 | | | | (2.9 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 26,953 | | | | 28,096 | | | | (4.1 | )% | | $ | 28,096 | | | | 28,779 | | | | (2.4 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Sales by Domestic and International (in thousands) | | | | | | | | | | | | | | | | | | | | | | | | |
| Domestic | | $ | 22,506 | | | | 23,176 | | | | (2.9 | )% | | $ | 23,176 | | | | 24,474 | | | | (5.3 | )% |
| International | | | 4,447 | | | | 4,920 | | | | (9.6 | )% | | | 4,920 | | | | 4,305 | | | | 14.3 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 26,953 | | | | 28,096 | | | | (4.1 | )% | | $ | 28,096 | | | | 28,779 | | | | (2.4 | )% |
Sales for the year ended 2009 decreased approximately $1.1 million or 4.1% compared to the same period in 2008. This decrease was due to the following reasons:
| | · | sales of generators were down approximately $930,000 or 7.2% due to lower capital expenditures by hospitals and doctor offices in the current economy; |
| | · | sales of other products were down approximately $170,000 or 7.4%. This consisted of a $41,000 reduction in royalty income due to royalty contracts ending in 2008; a $50,000 reduction in the sales of penlights; $74,000 reduction in medical lighting sales; and a $5,000 reduction in miscellaneous other products. These decreases are mainly the result of distributors electing to reduce their inventories in the current economy. |
Sales for the year ended 2008 decreased approximately $683,000 or 2.4% compared to the same period in 2007. This decrease was due to the following reasons:
| | · | sales of generators were down approximately $940,000 or 26% due to a reduction in the sales to OEM customers. |
The decrease in generator sales was offset by:
| | · | an increase in sales of cauteries of approximately $130,000 due to increased demand; |
| | · | new products sales of SEER, MEG, and plasma probes of approximately $62,000; and |
| | · | an increase in sales of disposable electrosurgical accessories of approximately $65,000. |
Our ten largest customers accounted for approximately 71.5%, 70%, and 71% of net revenues for 2009, 2008, and 2007 respectively. In 2009, 2008 and 2007, Arthrex was our only customer that accounted for over 10% of total revenues (22%, 20%, and 21% of our revenues for each such year). Arthrex sales of generators and accessories increased by approximately $245,000, or 4.3% to approximately $6.0 million for the year ended December 31, 2009 from approximately $5.7 million for the year ended December 31, 2008.
Gross Profit
| | | Years ended December 31, | |
| (in thousands) | | 2009 | | | 2008 | | | Percent change 08’vs 09’ | | | 2007 | | | Percent change 07’vs.08 | |
| Cost of sales | | $ | 15,099 | | | $ | 16,248 | | | | (7.1 | )% | | $ | 17,464 | | | | (7.0 | )% |
| Cost of sales as a percentage of revenue | | | 56.0 | % | | | 57.8 | % | | | | | | | 60.7 | % | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Gross profit | | $ | 11,855 | | | $ | 11,849 | | | | 0.1 | % | | $ | 11, 316 | | | | 4.7 | % |
| Gross profit as a percentage of revenue | | | 44.0 | % | | | 42.2 | % | | | | | | | 39.3 | % | | | | |
In relation to the $1.1 million reduction in sales, the 1.8% increase in gross profit as a percentage of sales in the year ended 2009 compared to the same period for 2008 was primarily a result of:
| | · | approximately $308,000 increase in capitalized manufacturing overhead; |
| | · | approximately $105,000 reduction in annual bonuses; |
| | · | approximately $108,000 reduction of our company match to our employees’ 401(k) contributions; |
| | · | an additional decrease in direct and indirect labor costs amounting to approximately $101,000; and |
| | · | approximately a $476,000 reduction in direct material cost as a result of the $1.1 million reduction in sales. |
In relation to the $683,000 reduction in sales, the 2.9% increase in gross profit as a percentage of sales in the year ended 2008 compared to the same period for 2007 was primarily a result of:
| | · | material cost reductions of approximately $170,000 from a decrease in shipping costs and a $460,000 decrease in component costs related to an OEM customer; |
| | · | approximately $116,000 reduction in manufacturing overhead costs; |
| | · | an additional decrease in direct and indirect labor costs amounting to approximately $63,000; |
| | · | certain portions of the workforce in our Canadian facility were reallocated from manufacturing to research and development activities amounting to approximately $175,000 to expand the development of our new products; and |
| | · | approximately a $216,000 reduction in direct material cost as a result of the $683,000 reduction in sales. |
Gain on Cancellation of Agreement
| | | Year ended December 31, | |
| (in thousands) | | 2009 | | | 2008 | | | Percent change 08’vs 09’ | | | 2007 | | | Percent change 07’vs.08 | |
| Gain on cancellation of agreement | | | -- | | | $ | 1,496 | | | | -- | | | | -- | | | | -- | |
| Gain as a percentage of revenue | | | -- | | | | 5.3 | % | | | -- | | | | -- | | | | -- | |
During the year ended 2008, we recognized a one time gain from a cancellation of a contract with Boston Scientific Corporation of approximately $1.5 million. We had no such activity in 2009 or 2007. For an explanation of this gain, please see Note 15 in our consolidated financial statements..
Research and development
| | | Year ended December 31, | |
| (in thousands) | | 2009 | | | 2008 | | | Percent change 08’vs 09’ | | | 2007 | | | Percent change 07’vs.08 | |
| Research and Development expense | | $ | 2,083 | | | $ | 2,061 | | | | 1.1 | % | | $ | 1,643 | | | | 25.4 | % |
| R&D expense as a percentage of revenue | | | 7.7 | % | | | 7.3 | % | | | | | | | 5.7 | % | | | | |
The 1.1% increase in R&D expenses in the year ended 2009 compared to the same period for 2008 was primarily a result of:
| | · | increased costs of approximately $120,000 related to continued development of the Boss and other related sintered steel product line; |
| | · | increase in costs of approximately $22,000 related to the end stage development of the ICON GS (J-Plasma). |
These increases were offset by:
| | · | a decrease in the R&D costs related to our Canadian facility in the amount of approximately $120,000 which was the result of a reduction of the labor force and other related overhead costs. |
The 25.4% increase in R&D expenses in the year ended 2008 compared to the same period for 2007 was primarily a result of:
| | · | certain portions of the workforce in our Canadian facility were reallocated from manufacturing to research and development activities amounting to approximately $175,000 to expand the development of our new products; |
| | · | increases in development costs of the Seal-N-Cut product line, including additional R&D labor, amounting to approximately $243,000; |
Professional services
| | | Year ended December 31, | |
| (in thousands) | | 2009 | | | 2008 | | | Percent change 08’vs 09’ | | | 2007 | | | Percent change 07’vs.08 | |
| Professional services expense | | $ | 1,398 | | | $ | 991 | | | | 41.1 | % | | $ | 738 | | | | 34.3 | % |
| Professional services as a percentage of revenue | | | 5.2 | % | | | 3.5 | % | | | | | | | 2.6 | % | | | | |
The 41.1% increase in professional services costs in the year ended 2009 compared to the same period for 2008 was primarily a result of:
| | · | legal fees related to the Erbe lawsuit increased approximately $339,000; |
| | · | legal fees related to Security and Exchange Commission filings and correspondence increased by approximately $57,000; and |
| | · | audit and accounting costs increased by $51,000 which were related to additional tax related issues and Sarbanes-Oxley compliance and related testing. |
These increases were offset by:
| | · | A decrease of approximately $30,000 in legal fees attributable to patent costs incurred by our Canadian facility for the Seal-N-Cut and other developing products. |
The 34.3% increase in professional services costs in the year ended 2008 compared to the same period for 2007 was primarily a result of:
| | · | legal fees related to the Erbe lawsuit increased approximately $212,000; |
| | · | audit and accounting costs increased by $41,000 which were primarily related to Sarbanes-Oxley compliance and related testing. |
Salaries and related costs
| | | Year ended December 31, | |
| (in thousands) | | 2009 | | | 2008 | | | Percent change 08’vs 09’ | | | 2007 | | | Percent change 07’vs.08 | |
| Salaries and related expenses | | $ | 3,003 | | | $ | 3,016 | | | | (0.5 | )% | | $ | 2,805 | | | | 7.5 | % |
| Salaries & related expenses as a percentage of revenue | | | 11.1 | % | | | 10.7 | % | | | | | | | 9.7 | % | | | | |
The 0.5% decrease in salaries and related costs in the year ended 2009 compared to the same period for 2008 was primarily a result of:
| | · | management’s cost cutting plan implemented in 2009 which resulted in a decrease of approximately $94,000 related to the suspension of employee bonuses, salary increases, and vacation cash out ability. In addition, we had a decrease of approximately $52,000 related to the suspension of the company’s 401(k) match; |
| | · | a decrease in the administrative labor force located at our Canadian facility amounting to approximately $18,000; |
| | · | a reduction in the amount of employee stock option expense booked in the amount of approximately $29,000; and |
| | · | a reduction in other employee benefits by approximately $31,000. |
These decreases were offset by:
| | · | adding additional sales force employees related to our SEER and MEG product lines of approximately $179,000; and |
| | · | an increase in employee health insurance costs of approximately $32,000. |
The 7.5% increase in salaries and related costs in the year ended 2008 compared to the same period for 2007 was primarily a result of:
| | · | annual salary increases which amounted to approximately $168,000; |
| | · | addition of administrative personnel in our Canadian facility of approximately $22,000; and |
| | · | an increase in employee health insurance costs of approximately $21,000. |
Selling, general and administration
| | | Year ended December 31, | |
| (in thousands) | | 2009 | | | 2008 | | | Percent change 08’vs 09’ | | | 2007 | | | Percent change 07’vs.08 | |
| SG&A expense | | $ | 4,656 | | | $ | 4,489 | | | | 3.7 | % | | $ | 4,018 | | | | 11.7 | % |
| SG&A expense as a percentage of revenue | | | 17.3 | % | | | 16.0 | % | | | | | | | 14.0 | % | | | | |
The 3.7% increase in selling, general and administration costs in the year ended 2009 compared to the same period for 2008 was primarily a result of:
| | · | a one time cost for the legal settlement paid to Erbe in the amount of approximately $160,000; |
| | · | increases in real estate and franchise taxes which amounted to approximately $73,000; |
| | · | a one time cost incurred for moving expenses related to the consolidation of our manufacturing to our new facility in the amount of approximately $58,000; |
| | · | an increase in utilities expense of approximately $89,000 mainly due to the addition of our new facility coupled with power and water company rate increases; |
| | · | one time cost related to the installation, transferring, and expansion of a new leased phone and communication system in our new facility which amounted to approximately $47,000; |
| | · | an increase of approximately $84,000 in amortization expense for new products that went into production in 2009; |
| | · | an increase in depreciation expense of approximately $36,000, most of which was attributable to our Canadian facility; |
| | · | during 2009 we accrued for the initial minimum royalty expense to be paid on the MEG product line which amounted to approximately $19,000; |
| | · | increase in general insurance premiums of approximately $40,000; |
| | · | an increase in our bad debt reserve of approximately $15,000; and |
| | · | an increase in our stock exchange fees and expenses of approximately $18,000; |
These increases were offset by:
| | · | a decrease in advertising costs in the amount of approximately $97,000; |
| | · | management’s consolidating of trade show and travel expenses which resulted in a decrease of approximately $275,000; |
| | · | sales and marketing consulting costs decreased by approximately $77,000 from management re-negotiating the terms of the agreement at the renewal; and |
| | · | a decrease in regulatory expenses of approximately $23,000. |
The 11.7% increase in selling, general and administration costs in the year ended 2008 compared to the same period for 2007 was primarily a result of:
| | · | increased costs related to our Canadian facility of approximately $56,000, $38,000, and $21,000 for depreciation, travel, and taxes expenses respectively; |
| | · | an increase of approximately $88,000 in amortization expense for new products that went into production in 2008; |
| | · | an increase in general insurance premiums of approximately $29,000; |
| | · | an increase in show and travel costs of approximately $47,000; |
| | · | an increase due to consulting costs of establishing a distribution channel in Europe for the MEG and SEER product lines of approximately $160,000; and |
| | · | an increase in commission expense of approximately $95,000 due to increased sales upon which we pay commissions. |
These increases were offset by:
| | · | a decrease in advertising costs in the amount of approximately $63,000; |
Other Income
| | | Year ended December 31, | |
| (in thousands) | | 2009 | | | 2008 | | | Percent change 08’vs 09’ | | | 2007 | | | Percent change 07’vs.08 | |
| Interest income | | $ | 24 | | | $ | 49 | | | | (51.0 | )% | | $ | 143 | | | | (65.7 | )% |
| Interest expense | | $ | (76 | ) | | $ | (59 | ) | | | 28.8 | % | | $ | (3 | ) | | | 1,867 | % |
| Total other income (expense) | | $ | (52 | ) | | $ | (10 | ) | | | | | | $ | 140 | | | | | |
| Other income (expense) as a percentage of revenue | | | (0.2 | )% | | | (0.0 | )% | | | | | | | 0.5 | % | | | | |
Interest income decreased by approximately $25,000 during the year ended December 31, 2009 when compared with 2008, and decreased by approximately $94,000 during the year ended December 31, 2008 when compared with 2007. This was due to lower interest rates on our sweep account coupled with lower cash balances year over year.
Interest expense increased progressively from 2007 through 2009 mainly as a direct result of interest incurred on debt originated in 2008 (we had no debt in 2007). This debt was used to primarily acquire and renovate our new facility and for working capital purposes.
Income Taxes
Our income tax provision for the year ended December 31, 2009 was approximately $67,000 compared with approximately $945,000 for the year ended December 31, 2008. Our effective tax rate was approximately 10% for the year ended December 31, 2009, which is less than our statutory rates because of the deductibility of stock based compensation and certain research and development tax credits we used during the year. Our effective tax rate was approximately 34% for the year ended December 31, 2008, which percentage is somewhat less than statutory rates because of certain research and development tax credits we used during the year.
| | | 2009 | | | 2008 | | | 2007 | |
| | | | | | | | | | |
| Federal tax provision | | % | 34.0 | | | | 34.0 | | | | 34.0 | |
| State taxes (net of federal benefit) | | | 2.6 | | | | 5.8 | | | | 5.8 | |
| Stock based compensation | | | (15.9 | ) | | | - | | | | (24.1 | ) |
| Research and development credits | | | (12.8 | ) | | | (2.6 | ) | | | (7.5 | ) |
| Other | | | 2.3 | | | | (3.2 | ) | | | (1.0 | ) |
| Valuation allowance | | | - | | | | - | | | | (76.0 | ) |
| | | % | 10.2 | | | | 34.0 | | | | (68.8 | ) |
At December 31, 2006, management believed there was a risk that substantially all of our net operating losses might not be realizable and, accordingly, a valuation allowance was recorded against them. During the year ended December 31, 2007, management determined that such valuation allowances were no longer necessary, and accordingly, the valuation allowances were reversed, resulting in a benefit for income taxes being recorded for the anticipated utilization.
Liquidity and Capital Resources
Our working capital at December 31, 2009 was $10.7 million compared with $9.9 million at December 31, 2008. Accounts receivable days sales outstanding were 39 days and 43 days at December 31, 2009 and 2008 respectively. The number of days worth of sales in inventory, which is the total inventory available for production divided by the 12 month average cost of materials, increased 28 days to 172 days equating to an inventory turn ratio of 1.7 at December 31, 2009 from 144 days and an inventory turn ratio of 2.2 at December 31, 2008. The higher number of days worth of sales in inventory which translated into a lower inventory turnover rate is mainly due to the decrease in sales related to our generator product lines which contain a greater number of parts compared to all our other products.
In fiscal 2009, net cash used by operating activities amounted to approximately $98,000 compared with net cash provided of approximately $740,000 from operations in 2008. The decrease in cash generated by operations in 2009 compared with the prior year is primarily due to a large decrease in the balance of accounts payables offset by changes in the balances of various assets.
Net cash used in investing activities was $2.5 million and $4.5 million during 2009 and 2008, respectively, which amounts were used primarily for the purchase of property and equipment.
Net cash provided by financing activities was approximately $2.2 million for fiscal 2009, a decrease of approximately $735,000 compared with fiscal 2008. During fiscal 2008, we borrowed $4.0 million under industrial revenue bonds for the purchase and renovation of our new facility through RBC Bank, of which we received $2.7 million in 2008 and the remaining escrow portion of approximately $1.3 million in 2009. The bonds, which are being amortized over a 20 year term, balloon in 10 years and bear interest at a fixed interest rate of 4.6%. Scheduled maturities of this indebtedness are $135,000, $140,000, $145,000, $155,000 and $165,000 for 2010, 2011, 2012, 2013 and 2014 respectively. During 2009 we borrowed $1.0 million on our line of credit with RBC Bank which was used for working capital.
We had approximately $2.2 million in cash and cash equivalents at December 31, 2009. We believe our cash on hand, as well as anticipated cash flows from operations, will be sufficient to meet our operating cash commitments for the next year. Should additional funds be required, we have secured additional borrowing capacity with RBC Bank (USA). (see below)
On December 2, 2009 RBC Bank (USA) increased our secured revolving line of credit facility to $8 million from the previous $5 million and on the same date provided us with a separate additional credit facility for up to $1 million specific to financing new equipment purchases.
Advances under the $8 million line of credit are due on demand and bear interest at a rate of LIBOR plus 2% with a minimum floor rate of 4.0% and are secured by a perfected first security interest in our inventory, accounts receivable, and equipment.
The $1 million facility related to equipment purchases provides for a 2 year draw up period followed by a 5 year term period and bears interest also at LIBOR plus 2% with a minimum floor of 4% and will be secured by a perfected first security interest in the new equipment purchased. This equipment credit facility also allows the Company the option of financing purchased equipment at 75% of the cost through either a traditional loan or through RBC leasing at the time of purchase.
Subsequent available borrowings for both these credit facilities is subject to a borrowing base utilizing a percentage of eligible receivables, inventories, and any assigned cash along with certain financial ratios, specifically maintaining: a ratio of debt to tangible net worth of less than 2.0 to 1.0, a ratio of total funded debt to EBITDA of less than 3.25 to 1.0 excluding the industrial revenue bond note balance which had an original principal amount of $4.0 million, and a ratio of minimum debt service coverage of 1.5 to 1.0 measured on a rolling four quarter basis.
At December 31, 2009 the Company was in full compliance with the loan covenants and ratios of both the credit facilities. According to our most recent borrowing base calculation we had approximately $4.0 million total availability under the $8 million credit line, of which we have a current balance of $1.0 million, and we have available all of the $1 million under the equipment line of credit.
The Company’s future contractual obligations for agreements with initial terms greater than one year and agreements to purchase materials in the normal course of business are summarized as follows (in thousands):
| Description | | Years Ending December 31, | |
| | | 2010 | | | 2011 | | | 2012 | | | 2013 | | | 2014 | | | 2015 | |
| Operating leases | | | 278 | | | | 252 | | | | 247 | | | | 223 | | | | 11 | | | | - | |
| Employment agreements | | | 811 | | | | 865 | | | | 871 | | | | 881 | | | | 73 | | | | - | |
| Purchase Commitments | | | 3,756 | | | | - | | | | - | | | | - | | | | - | | | | - | |
Critical Accounting Estimates
In preparing the consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP), we have adopted various accounting policies. Our most significant accounting policies are disclosed in Note 2 to the consolidated financial statements.
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires us to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Our estimates and assumptions, including those related to inventories, intangible assets, property, plant and equipment, legal proceedings, research and development, warranty obligations, product liability, sales returns and discounts, and income taxes are updated as appropriate, which in most cases is at least quarterly. We base our estimates on historical experience, or various assumptions that are believed to be reasonable under the circumstances and the results form the basis for making judgments about the reported values of assets, liabilities, revenues and expenses. Actual results may materially differ f rom these estimates.
Estimates are considered to be critical if they meet both of the following criteria: (1) the estimate requires assumptions about material matters that are uncertain at the time the accounting estimates are made, and (2) other materially different estimates could have been reasonably made or material changes in the estimates are reasonably likely to occur from period to period. Our critical accounting estimates include the following:
Inventory reserves
When necessary we maintain reserves for excess and obsolete inventory resulting from the potential inability to sell our products at prices in excess of current carrying costs. The markets in which we operate are highly competitive, with new products and surgical procedures introduced on an ongoing basis. Such marketplace changes may cause our products to become obsolete. We make estimates regarding the future recoverability of the costs of these products and record a provision for excess and obsolete inventories based on historical experience, and expected future trends. If actual product life cycles, product demand or acceptance of new product introductions are less favorable than projected by management, additional inventory write-downs may be required, which would unfavorably affect future operating results.
Long-lived assets
We review long-lived assets which are held and used, including property and equipment and intangible assets, for impairment whenever changes in circumstances indicate that the carrying amount of the assets may not be recoverable. Such evaluations compare the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset over its expected useful life and are significantly impacted by estimates of future prices and volumes for our products, capital needs, economic trends and other factors that are inherently difficult to forecast. If the asset is considered to be impaired, we record an impairment charge equal to the amount by which the carrying value of the asset exceeds its fair value determined by either a quoted market price, if any, or a value determined by utilizing a discounted cash flow tec hnique.
Share-based Compensation
Under the Company’s stock option plan, options to purchase Common Shares of the Company may be granted to key employees, officers and directors of the Company by the Board of Directors. The Company accounts for stock options in accordance with FASB ASC Topic 718, compensation-stockcompensation (SFAS Statement 123 (R)) with option expense amortized over the vesting period based on the binomial lattice option-pricing model fair value on the grant date, which includes a number of estimates that affect the amount of our expense.
Income Taxes
The provision for income taxes includes federal, foreign, state and local income taxes currently payable and those deferred because of temporary differences between the financial statement and tax bases of assets and liabilities. Deferred tax assets or liabilities are computed based on the difference between the financial statement and income tax bases of assets and liabilities using enacted marginal tax rates. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. Deferred income tax expenses or credits are based on the changes in the asset or liability from period to period.
We periodically have net operating loss and tax credit carry forwards available to reduce future taxable income. Future tax benefits for net operating loss and tax credit carry forwards are recognized to the extent that realization of these benefits is considered more likely than not. This determination is based on the expectation that related operations will be sufficiently profitable or various tax, business and other planning strategies will enable us to utilize the operating loss and tax credit carry forwards. We cannot be assured that we will be able to realize these future tax benefits or that future valuation allowances will not be required. To the extent that available evidence raises doubt about the realization of a deferred income tax asset, a valuation allowance is established.
It is our policy to provide for uncertain tax positions and the related interest and penalties based upon management’s assessment of whether a tax benefit is more likely than not to be sustained upon examination by tax authorities. To the extent that the probable tax outcome of these uncertain tax positions changes, such changes in estimate will impact the income tax provision in the period in which such determination is made. At December 31, 2009, we believe we have appropriately accounted for any unrecognized tax benefits. To the extent we prevail in matters for which a liability for an unrecognized tax benefit is established or we are required to pay amounts in excess of the liability, our effective tax rate in a given financial statement period may be affected.
.
Recent Accounting Pronouncements
See Note 7 of the Notes to Consolidated Financial Statements, which is incorporated by reference herein.
ITEM 7A. Quantitative and Qualitative Disclosures about Market Risk
Our short term investments consist of cash, cash equivalents and overnight investments. As such we do not believe we are exposed to significant interest rate risk. The primary objective of our investment activities is to preserve principal while at the same time maximizing yields without significantly increasing risk. To achieve this objective, we invest in highly liquid overnight money market investments. If a 10% change in interest rates were to have occurred on December 31, 2009, this change would not have had a material effect on the fair value of our investment portfolio as of that date.
ITEM 8. Financial Statements and Supplementary Data
The information required by this item may be found beginning on page F-1 of this Annual Report.
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
There were no disagreements with our current accountants on accounting and financial disclosures.
ITEM 9A. Disclosure Controls and Procedures
We have carried out an evaluation, under the supervision of and with the participation of our management, including our Chief Executive Officer (CEO) and Chief Financial Officer (CFO), of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended), as of December 31, 2009. Based upon that evaluation, our CEO and CFO concluded that, as of the end of that period, our disclosure controls and procedures are effective in providing reasonable assurance that (a) the information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and (b) such informati on is accumulated and communicated to our management, including our CEO and CFO, as appropriate to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Changes in Internal Control over Financial Reporting
Section 404 of the Sarbanes-Oxley Act of 2002 requires us to evaluate annually the effectiveness of our internal controls over financial reporting as of the end of each fiscal year, and to include a management report assessing the effectiveness of our internal control over financial reporting in all annual reports. There were no changes in our internal control over financial reporting during the year ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management's Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act as a process designed by, or under the supervision of, a company’s principal executive and principal financial officers and effected by a company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that:
| | • | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; |
| | • | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| | • | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2009. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control — Integrated Framework. Based on our assessment, our management has concluded that, as of December 31, 2009, our internal control over financial reporting is effective based on those criteria.
The effectiveness of our internal control over financial reporting as of December 31, 2009 has been audited by Kingery & Crouse, P.A., our independent registered public accounting firm, as stated in their report, which is attached to our audited financial statements.
ITEM 9B. Other Information
None.
Part III
ITEM 10. Directors, Executive Officers, and Corporate Governance
BACKGROUND AND EXPERIENCE OF DIRECTORS
When considering whether directors and nominees have the experience, qualifications, attributes or skills, taken as a whole, to enable the Board of Directors to satisfy its oversight responsibilities effectively in light of the Company’s business and structure, Governance and Nominating Committee focused primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth immediately above. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of Bovie’s business. As more specifically described in such person’s individual biographies set forth above, our directors possess relevant and industry-specific experience and knowledge in the medical, engineering and business fields, as the case may be, which we believe enhances the Board’s ability to oversee, evaluate and direct our overall corporate strategy. The Governance and Nominating Committee annually reviews and makes recommendations to the Board regarding the composition and size of the Board so that the Board consists of members with the proper expertise, skills, attributes, and personal and professional backgrounds needed by the Board, consistent with applicable regulatory requirements.
The Governance and Nominating Committee believes that all directors, including nominees, should possess the highest personal and professional ethics, integrity, and values, and be committed to representing the long-term interests of our stockholders. The Governance and Nominating Committee will consider criteria including the nominee’s current or recent experience as a senior executive officer, whether the nominee is independent, as that term is defined in existing independence requirements of the NYSE Amex Market and the Securities and Exchange Commission, the business, scientific or engineering experience currently desired on the Board, geography, the nominee’s industry experience, and the nominee’s general ability to enhance the overall composition of the Board.
The Governance and Nominating Committee does not have a formal policy on diversity; however, in recommending directors, the Board and the Committee consider the specific background and experience of the Board members and other personal attributes in an effort to provide a diverse mix of capabilities, contributions and viewpoints which the Board believes enables it to function effectively as the Board of Directors of a company with our size and nature of business.
Set forth below is information regarding the executive officers and directors of Bovie Medical as of March 1, 2010:
Name | | Position | | Director Since |
| Andrew Makrides | | Chairman of the Board, President, and CEO | | December 1982 |
| | | | | |
| J. Robert Saron | | Chief Sales and Marketing Officer and Director | | August 1994 |
| | | | | |
| George Kromer | | Research Analyst and Director | | October 1995 |
| | | | | |
| Michael Norman | | Director | | September 2004 |
| | | | | |
| August Lentricchia | | Director | | October 2007 |
| | | | | |
| Moshe Citronowicz | | Executive Vice President and Chief Operating Officer | | |
| | | | | |
| Gary D. Pickett | | Chief Financial Officer, Treasurer, and Secretary | | |
| | | | | |
| Steve Livneh | | President of Bovie Canada and Director | | April 2008 |
| | | | | |
| Steven MacLaren | | Director | | April 2008 |
| | | | | |
| Dr. Peter Pardoll | | Director | | April 2009 |
| | | | | |
| Gregory Konesky | | Director | | December 2009 |
Directors serve for one-year terms and are elected at the annual shareholders’ meeting.
Andrew Makrides, Esq. Age 68, Chairman of the Board and President, member of the Board of Directors, received a Bachelor of Arts degree in Psychology from Hofstra University and a Juris Doctor Degree from Brooklyn Law School. He is a member of the Bar of the State of New York and practiced law from 1968 until joining Bovie Medical Corporation as a co-founder and Executive Vice President and director, in 1982. Mr. Makrides became President of the Company in 1985 and the CEO in December 1998 and has served as such to date. Mr. Makrides employment contract extends to December 31, 2014. Mr. Makrides has over 28 years of executive experience in the medical industry.
J. Robert Saron, age 57, Director, holds a Bachelor degree in Social and Behavioral Science from the University of South Florida. From 1988 to present Mr. Saron has served as a director of the Company. Mr. Saron previously served as CEO and chairman of the Board of the Company from 1994 to December 1998 and is currently the Chief Sales and Marketing Officer. Mr. Saron also serves on two industry boards, as a director on the Health Industry Distributors Association Education Foundation and as President of the Health Care Manufacturing Marketing Council. Mr. Saron’s employment contract extends to December 31, 2014. Mr. Saron brings over 22 years of executive marketing and distribution experience in the medical industry.
George Kromer, Jr., age 69, became a director on October 1, 1995. On January 1, 2006 Mr. Kromer accepted an employment position with Bovie Medical Corporation as research analyst for the company in which he still maintains his capacity as a director. Mr. Kromer had been writing for business publications since 1980. In 1976, he received a Master’s Degree in health administration from Long Island University. He was engaged as a Senior Hospital Care Investigator for the City of New York Health & Hospital Corporation from 1966 to 1986. He also holds a Bachelor of Science Degree from Long Island University’s Brooklyn Campus and an Associate in Applied Science Degree from New York City Community College, Brooklyn, New York. Mr. Kromer has over 30 years of business analyst experience with a specialty in the medical industry.
Moshe Citronowicz, age 57, is a graduate of the University of Be’er Sheva, Be’er Sheva, Israel, with a Bachelor of Science Degree in electrical engineering. Since coming to the United States in 1978, Mr. Citronowicz has worked in a variety of manufacturing and high technology industries. In October 1993, Mr. Citronowicz joined the Company as Vice President of Operations. He is responsible for all areas of manufacturing, purchasing, product redesign, as well as new product design. In September 1997, Mr. Citronowicz was appointed by the Board of Directors to the position of Executive Vice President and Chief Operating Officer. Mr. Citronowicz’s employment contract extends to December 31, 2014.
Gary D. Pickett, CPA, age 58, holds an MBA from the University of Tampa, a BS degree in Accounting from Florida State University, and served five years as a field artillery officer in the United States Army. Mr. Pickett joined as controller of Bovie in March 2006 and became Chief Financial Officer in October 2006. During the past five years, Mr. Pickett held positions of Director of Financial Systems with Progress Energy Services of Raleigh, NC, Vice President and Controller of Progress Rail Services, a subsidiary of Progress Energy Services in Albertville, AL, each of which were non-affiliated with Bovie. He has had extensive experience in Sarbanes-Oxley implementation as well as GAAP accounting and SEC Reporting. Mr. Pickett’s employment contract extends to June 2012.
Michael Norman, CPA age 52, joined Bovie in 2004. He manages the CPA firm, Michael Norman, CPA, PC since 1994 specializing in business financial planning as well as governmental and financial auditing. Mr. Norman is a member of the Nassau County Board of Assessors, Treasurer of the Don Monti Memorial Research Foundation and a Glen Cove City Councilman, all located on Long Island, New York. Mr. Norman provides the board with over 20 years of experience as a CPA and also serves as the expert member of our audit committee.
August Lentricchia, age 55, is presently employed by Freedom Tax and Financial Services Bohemia as a Registered Representative since 2001. He is also licensed as a Registered Representative and investment consultant of HD Vest Investment Services, a non-bank subsidiary of Wells Fargo and Company. He has also served as an investment consultant for Citibank. He received a BA degree from the University of Arizona in 1977 and has received a Masters degree in Education from Dowling College in 2004. Mr. Lentricchia has over 25 years of financial and investment experience and also serves on our Audit Committee.
Steve Livneh, age 61, became President of Bovie Canada in October 2006 following the asset purchase of certain intellectual properties by Bovie from Lican Development of Ontario, Canada, and then a director in April 2008. Mr. Livneh, is a mechanical engineer and inventor, and has developed and manufactured varied products, including aerial munitions, consumer goods, irrigation and hydraulic devices and guidance systems. During the past several years he has been engaged in developing endoscopic electrosurgery instruments, targeting the general surgery, gynecology, urology and thoracic surgery markets.
Steven MacLaren, age 39, joined Bovie as a director in April 2008. Mr. MacLaren is a 1991 graduate of The Ohio State University in Columbus, Ohio with a BSBA degree in accounting. He is currently the president and a shareholder of Ronin Consulting Group, Inc. of Belleair Bluffs, Florida, which he started in February 2004 and which has provided consulting services for Bovie Medical since August 2005. Previous to this he served as the CFO and a technical currency trader of Capital Management Group, LLC, an investment company located in Naples, FL from November 2001 through February 2004. Mr. MacLaren has a history with the Company as he also served as Bovie Medical’s Controller from November 1996 through October 2001. Mr. MacLaren serves as the Chairman of our Compensation Committee and the Nominating Committee and bring s approximately 20 years of business and financial experience to the board.
Dr. Peter Pardoll, age 63, joined Bovie as a director in April 2009 and is board certified in Internal Medicine and Gastroenterology. He attended Emory University for undergraduate studies and graduated with honors from the Medical College of Virginia in 1971. Dr. Pardoll then completed three years internal medicine training at the University of Miami Affiliated Hospitals, and subsequently completed his GI fellowship at University of South Florida. He was the founding partner of the Center for Digestive Diseases (“CDD”) in St. Petersburg, Florida. During his 28 years of practice, he established a successful clinical research division of CDD performing studies and research for major pharmaceutical companies. He developed t echniques to educate physician colleagues on laser therapeutics in GI endoscopy, bronchoscopy and urology. Dr. Pardoll is the past president of the Florida GI Society. He has been elected a Master of the American College of Gastroenterology (“ACG”) and was elected as a Trustee for a 6-year term to the ACG Board. He remains active with his national organizations and as a healthcare consultant. Dr. Pardoll brings 28 years of physician experience to the board and serves on our Compensation Committee and Nominating Committee.*
Gregory A. Konesky, age 56, joined Bovie as a director in December 2009 and is a 1977 graduate of Polytechnic University Brooklyn, NY with a BSEE degree in computer science. He has been a scientific consultant to Bovie Medical Corporation for over 12 years. He is a member of the Bovie’s Scientific Advisory Board and is Lead Scientist for the Company’s J-Plasma technology. He has two patents granted and three pending, and has authored over 51 articles on a wide range of subjects including medical plasma technology, optical communications, astrobiology, and others. He has also served as a technical advisor to the investment community and is a member of numerous professional and scientific associations. **
*Dr. Pardoll replaced Randy Rossi who resigned as a director in March, 2009 due to a change in employment.
** Gregory A. Konesky replaced Brian H. Madden who recently resigned in November, 2009 for personal reasons.
Independent Board Members
The board has five members, Michael Norman, August Lentricchia, Steven MacLaren, Dr. Peter Pardoll, and Greg Konesky that meet the existing independence requirements of the NYSE Amex Market and the Securities and Exchange Commission.
Board Leadership
The Board has no formal policy with respect to separation of the positions of Chairman and CEO or with respect to whether the Chairman should be a member of management or an independent director, and believes that these are matters that should be discussed and determined by the Board from time to time. Currently, Andrew Makrides serves as our Chairman and CEO. Given the fact that Mr. Makrides, in his capacity as our CEO is tasked with the responsibility of implementing our corporate strategy, we believe he is best suited for leading discussions, at the Board level, regarding performance relative to our corporate strategy, and this discussion accounts for a significant portion of the time devoted at our Board meetings.
Risk Management
The Board believes that risk management is an important component of the Company’s corporate strategy. While we assess specific risks at our committee levels, the Board, as a whole, oversees our risk management process, and discusses and reviews with management major policies with respect to risk assessment and risk management. The Board is regularly informed through its interactions with management and committee reports about risks we face in the course of our business. Finally, the Board believes the combined Chairman and CEO role assists us in our implementation of major policies addressing our risks.
Audit Committee
The Audit Committee assists the full Board of Directors in its general oversight of our financial reporting, internal controls, and audit functions, and is directly responsible for the appointment, compensation and oversight of the work of our independent registered public accounting firm. The Audit Committee reviews and discusses with management and our independent accountants the annual audited and quarterly financial statements (including the disclosures under “Management’s Discussion and Analysis of Financial Condition and Results of Operations”), reviews the integrity of the financial reporting processes, both internal and external, reviews the qualifications, performance and independence of our independent accountants, and prepares the Audit Committee Report included in this Annual Report on Form 10-K in accorda nce with rules and regulations of the Securities and Exchange Commission. The Audit Committee has the power to investigate any matter brought to its attention within the scope of its duties. It also has the authority to retain counsel and advisors to fulfill its responsibilities and duties. The Audit Committee also acts as a qualified legal compliance committee.
Due to the recent resignation of Brian Madden from our board, our Audit Committee currently consists of only two independent members of the Board of Directors, Michael Norman CPA, and August Lentricchia. We are required to have at least three independent members comprising our Audit Committee in accordance with Rule 10A-3 of the Securities Exchange Act of 1934 and as such are temporarily not in compliance. Our Nominating Committee is actively searching out potential candidates that fulfill the requirements of an audit committee member. Michael Norman serves as our Chairman and financial expert for the Committee. The Audit Committee meets as often as it determines necessary but not less frequently than once every fiscal quarter.
AUDIT COMMITTEE REPORT
Our Audit Committee is composed of “independent” directors, as determined in accordance with Rule 10A-3 of the Securities Exchange Act of 1934. The Audit Committee operates pursuant to a written charter adopted by the Board of Directors.
As described more fully in its charter, the purpose of the Audit Committee is to assist the Board of Directors with its oversight responsibilities regarding the integrity of our financial statements, our compliance with legal and regulatory requirements, assessing the independent registered public accounting firm’s qualifications, independence and performance for us. Management is responsible for preparation, presentation and integrity of our financial statements as well as our financial reporting process, accounting policies, internal audit function, internal accounting controls and disclosure controls and procedures. The independent registered public accounting firm is responsible for performing an independent audit of our consolidated financial statements in accordance with generally accepted auditing standards and to issue a report thereon. The Audit Committee’s responsibility is to monitor and oversee these processes. The following is the Audit Committee’s report submitted to the Board of Directors for 2009.
The Audit Committee has:
| | • | reviewed and discussed our audited financial statements with management and Kingery & Crouse, P. A., the independent public accountants |
| | • | discussed with Kingery & Crouse, P.A. matters required to be discussed by Auditing Standard No. 5 of the PCAOB, as may be modified or supplemented; and |
| | • | received from Kingery & Crouse, P. A. the written disclosures and the letter regarding their independence as required by PCAOB Rule 3526, Communication with Audit Committees Concerning Independence, as may be modified or supplemented, and discussed the auditors’ independence with them. |
In addition, the Audit Committee has met separately with management and with Kingery & Crouse, P. A.
Based on the review and discussions referred to above, the Audit Committee recommended to the Board of Directors that the audited financial statements be included in our Annual Report on Form 10-K for the year ended December 31, 2009 for filing with the Securities and Exchange Commission.
The foregoing Audit Committee Report shall not be deemed incorporated by reference into any filing under the Securities Act of 1933 or the Securities Exchange Act of 1934, and shall not otherwise be deemed filed under these acts, except to the extent we specifically incorporate by reference into such filings.
Governance and Nominating Committee
The Governance and Nominating Committee is responsible for matters relating to the corporate governance of our company and the nomination of members of the board and committees thereof. Our Governance and Nominating Committee consists of four independent members of the Board of Directors, Michael Norman CPA, August Lentricchia, Steven MacLaren who serves as Chairman, and Dr. Peter Pardoll. The Governance and Nominating Committee meets as often as it determines necessary, but not less than once a year.
Compensation Committee
The Compensation Committee is responsible for overseeing our compensation and employee benefit plans (including those involving the issuance of our equity securities) and practices, including formulating, evaluating, and approving the compensation of our executive officers and reviewing and recommending to the full Board of Directors the compensation of our Chief Executive Officer. The Committee is also responsible for recommending the level of Board of Directors' compensation to the full Board of Directors. Our Compensation Committee consists of four independent members of the Board of Directors, Michael Norman CPA, August Lentricchia, Steven MacLaren who serves as Chairman, and Dr. Peter Pardoll. The Compensation Committee meets as often as it determines necessary, but not les s than once a year.
In 2009, the Compensation Committee did not engage any independent consultants.
Code of Ethics
On March 30, 2004 Bovie adopted an executive employee ethics code.
A copy of the code of ethics which expressly relates to the CEO and CFO will be provided without charge to any person upon request to Bovie Medical Corporation, 734 Walt Whitman Road, Melville, NY 11747, Attn: Andrew Makrides.
ITEM 11. Executive Compensation Discussion and Analysis
General Compensation Philosophy
The primary objective of our compensation program for employees, including our compensation program for executive officers, is to attract, retain, and motivate qualified individuals and reward them in a manner that is fair to all stockholders. We strive to provide incentives for every employee that rewards them for their contribution to the Company.
Our compensation program is designed to be competitive with other employment opportunities and to align the interests of all employees, including executive officers, with the long-term interests of our stockholders. Historically, for our executive officers, we link a much higher percentage of total compensation to incentive compensation such as stock based compensation than we do for other employees.
With these objectives in mind, our Board has built executive and non-executive compensation programs that consists of two principal elements - Base Salary and grants of stock options and/or shares of restricted stock.
Compensation Program
Base Salary
Bovie pays base salaries to its Named Executive Officers (as defined below) in order to provide a consistent, minimum level of pay that sustained individual performance warrants. The Company also believes that a competitive annual base salary is important to attract and retain an appropriate caliber of talent for each position over time.
The annual base salaries of Bovie's Named Executive Officers are determined by its Compensation Committee and approved by the Board of Directors. All salary decisions are based on each Named Executive Officer's level of responsibility, experience and recent and past performance, as determined by the Compensation Committee. The Compensation Committee does not benchmark its base salaries in any way, nor do they presently employ the services of a compensation consultant.
Stock Options
The second component of executive compensation is equity grants which have mainly come in the form of stock options. Bovie believes that equity ownership in the Company is important to provide its Named Executive Officers with long-term incentives to better align interests of executives with the interests of stockholders and build value for Bovie stockholders. In addition, the equity compensation is designed to attract and retain the executive management team. Stock options have value only if the stock price increases over time and, therefore, provide executives with an incentive to build Bovie's value. This characteristic ensures that the Named Executive Officers have a meaningful portion of their compe nsation tied to future stock price increases and rewards management for long-term strategic planning through the resulting enhancement of the stock price.
Stock option awards to Named Executive Officers are entirely discretionary. The CEO and COO recommend to the Compensation Committee which individuals should be awarded stock options. The Compensation Committee considers the prior contribution of these individuals and their expected future contributions to the growth of Bovie then formulates and presents the recommended allocation of stock option awards to the Board of Directors for approval. The Board of Directors approves or, if necessary, modifies the Committee’s recommendations.
Perquisites and Other Benefits
Bovie's Named Executive Officers are eligible for the same health and welfare programs and benefits as the rest of its employees in their respective locations. In addition, Bovie's CEO, COO, and Chief Sales and Marketing Officer each receive an automobile allowance of approximately $6,300 per year.
Bovie's Named Executive Officers are entitled to participate in and receive employer contributions to Bovie's 401(k) Savings Plan. However, during January of 2009 management made the decision to suspend the employer 401(k) match for 2009. For more information on employer contributions to the 401(k) Savings Plan see the Summary Compensation Table and its footnotes.
Tax and Accounting Considerations.
Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”), places a limit of $1,000,000 on the amount of compensation that we may deduct as a business expense in any year with respect to each of our most highly paid executives unless, among other things, such compensation is performance-based and has been approved by stockholders. The non-performance-based compensation paid to our executive officers for the 2009 fiscal year did not exceed the $1 million limit per executive officer. Accounting considerations also play an important role in the design of our executive compensation program. Accounting rules such as FAS 123R require us to expense the cost of our stock option grants which reduces the amount of our reported profits. Because of option expensing and the impact of dilution on our stockholders, we p ay close attention to the number and value of the shares underlying stock options we grant.
Compensation of Named Executive Officers
The following table sets forth the compensation paid to each of Bovie’s Named Executive Officers for the three years ended December 31, 2009 for services to our company in all capacities:
Summary Compensation Table
| Name And Principal Position | Year | | Salary ($) | | | Bonus ($) ** | | | Stock Awards ($) | | | Option Awards ($) * | | | Non- Equity Incentive Plan Compensa- tion Earnings ($) | | | Change in Pension Value and Nonquali-fied Deferred compen- sation Earnings ($) | | | All Other Compen-Sation ($) | | | Total ($) | |
| (a) | (b) | | (c) | | | (d) | | | (e) | | | (f) | | | (g) | | | (h) | | | (i) | | | (j) | |
| Andrew Makrides President, CEO, Chairman of the Board | 2009 | | $ | 205,252 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 18,463 | (1) | | $ | 223,715 | |
| 2008 | | $ | 208,598 | | | $ | 3,870 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 20,553 | (9) | | $ | 233,021 | |
| 2007 | | $ | 195,452 | | | $ | 3,685 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 21,770 | (6) | | $ | 220,907 | |
| Gary D. Pickett CFO, Treasurer, Secretary | 2009 | | $ | 101,186 | | | | 0 | | | | 0 | | | $ | 43,750 | (8) | | | 0 | | | | 0 | | | $ | 483 | (2) | | $ | 145,419 | |
| 2008 | | $ | 104,083 | | | $ | 1,961 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 3,316 | (16) | | $ | 109,360 | |
| 2007 | | $ | 94,457 | | | $ | 1,904 | | | | 0 | | | $ | 88,200 | (7) | | | 0 | | | | 0 | | | $ | 3,097 | (10) | | $ | 187,658 | |
| J. Robert Saron Chief Sales and Marketing Officer and Director | 2009 | | $ | 290,651 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 10,972 | (3) | | $ | 301,623 | |
| 2008 | | $ | 295,650 | | | $ | 5,480 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 21,312 | (12) | | $ | 322,442 | |
| 2007 | | $ | 276,680 | | | $ | 5,218 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 20,413 | (11) | | $ | 302,311 | |
| Moshe Citronowicz Vice President Chief Operating Officer | 2009 | | $ | 213,549 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 14,069 | (4) | | $ | 227,618 | |
| 2008 | | $ | 213,197 | | | $ | 4,026 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 21,055 | (14) | | $ | 238,278 | |
| 2007 | | $ | 203,349 | | | $ | 3,834 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 20,109 | (13) | | $ | 227,292 | |
| Steve Livneh President Bovie Canada | 2009 | | $ | 190,225 | (18) | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 1,849 | (5) | | $ | 192,074 | |
| 2008 | | $ | 164,959 | | | $ | 2,747 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 6,575 | (17) | | $ | 174,281 | |
| 2007 | | $ | 174,155 | | | $ | 3,523 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | $ | 12,664 | (15) | | $ | 190,342 | |
* These columns represent the grant date fair value of the awards as calculated in accordance with FASB ASC 718 (Stock Compensation). Pursuant to SEC rule changes effective February 28, 2010, we are required to reflect the total grant date fair values of the option grants in the year of grant, rather than the portion of this amount that was recognized for financial statement reporting purposes in a given fiscal year which was required under the prior SEC rules, resulting in a change to the amounts reported in prior Annual Reports.
** Column (d) consists of amounts for annual bonuses given to all employees equal to one week of base compensation.
(1) This amount includes: $155 of employer contributions under the Bovie Employee 401(k) savings plan; car allowance of $6,310; life insurance premiums of $432; and health insurance premiums of $11,567.
(2) This amount includes: $118 of employer contributions under the Bovie Employee 401(k) savings plan; and life insurance premiums of $365.
(3) This amount includes: $336 of employer contributions under the Bovie Employee 401(k) savings plan; car allowance of $6,310; life insurance premiums of $470; and health insurance premiums of $3,856.
(4) This amount includes: $242 of employer contributions under the Bovie Employee 401(k) savings plan; car allowance of $6,310; life insurance premiums of $470; and health insurance premiums of $7,047.
(5) This amount includes: $207 of employer contributions under the Bovie Employee 401(k) savings plan; life insurance premiums of $192; and health insurance premiums of $1,450.
(6) This amount includes: $3,759 of employer contributions under the Bovie Employee 401(k) savings plan; car allowance of $6,310; life insurance premiums of $396; and health insurance premiums of $11,305.
(7) In 2007 a total of 25,000 options were granted to Mr. Pickett as follows: 20,000 stock options granted on January 12, 2007 with a fair value of $3.66 per option; 5,000 stock options granted on March 29, 2007 with a fair value of $3.00 per option.
(8) On October 26, 2009 a total of 12,500 options were granted to Mr. Pickett with a fair value of $3.50 per option.
(9) This amount includes: $4,151 of employer contributions under the Bovie Employee 401(k) savings plan; car allowance of $6,431; life insurance premiums of $396; and health insurance premiums of $9,576.
(10) This amount includes: $2,834 of employer contributions under the Bovie Employee 401(k) savings plan; and life insurance premiums of $263.
(11) This amount includes: $8,140 of employer contributions under the Bovie Employee 401(k) savings plan; car allowance of $6,310; life insurance premiums of $434; and health insurance premiums of $5,529.
(12) This amount includes: $8,738 of employer contributions under the Bovie Employee 401(k) savings plan; car allowance of $6,431; life insurance premiums of $434; and health insurance premiums of $5,709.
(13) This amount includes: $5,982 of employer contributions under the Bovie Employee 401(k) savings plan; car allowance of $6,310; life insurance premiums of $434; and health insurance premiums of $7,383.
(14) This amount includes: $6,470 of employer contributions under the Bovie Employee 401(k) savings plan; car allowance of $6,431; life insurance premiums of $434; and health insurance premiums of $7,720.
(15) This amount includes: $4,591 of employer contributions under the Bovie Employee 401(k) savings plan; car allowance of $6,310; life insurance premiums of $192; and health insurance premiums of $1,571.
(16) This amount includes: $2,970 of employer contributions under the Bovie Employee 401(k) savings plan; and life insurance premiums of $346.
(17) This amount includes: $4,976 of employer contributions under the Bovie Employee 401(k) savings plan; life insurance premiums of $192; and health insurance premiums of $1,407.
(18) This amount includes unused vacation pay for the 3 year period of employment in the amount of $31,704
Employment Agreements and Potential Payments Upon Termination or Change in Control
At December 31, 2009, we were obligated under employment contracts with Mr. Makrides, Mr. Saron, and Mr. Citronowicz that are set to expire in January 2014 and contain an automatic extension for a period of one year unless we provide the executives with appropriate written notice pursuant to the contracts. The employment agreements provide, among other things, that the Executive may be terminated as follows:
| | (a) | Upon the death of the Executive, in which case the Executive’s estate shall be paid the basic annual compensation due the Employee pro-rated through the date of death. |
| | (b) | By the resignation of the Executive at any time upon at least thirty (30) days prior written notice to Bovie in which case Bovie shall be obligated to pay the Employee the basic annual compensation due him pro-rated to the effective date of termination, |
| | (c) | By Bovie, “for cause” if during the term of the Employment Agreement the Employee violates the non-competition provisions of his employment agreement, or is found guilty in a court of law of any crime of moral turpitude in which case the contract would be terminated and provisions for future compensation forfeited. |
| | (d) | By Bovie, without cause, with the majority approval of the Board of Directors, at any time upon at least thirty (30) days prior written notice to the Executive. In this case Bovie shall be obligated to pay the Executive compensation in effect at such time, including all bonuses, accrued or prorated, and expenses up to the date of termination. Thereafter, for the period remaining under the contract, Bovie shall pay the Executive the salary in effect at the time of termination payable weekly until the end of their contract. |
| | (e) | If Bovie fails to meet its obligations to the Executive on a timely basis, or if there is a change in the control of Bovie, the Executive may elect to terminate his employment agreement. Upon any such termination or breach of any of its obligations under the Employment Agreement, Bovie shall pay the Executive a lump sum severance equal to three times the annual salary and bonus in effect the month preceding such termination or breach as well as any other sums which may be due under the terms of the Employment Agreement up to the date of termination. |
We have an employment contract with Mr. Pickett to serve as Chief Financial Officer which has a current expiration date of June 2012. In the event of a change of control, the contract provides that Mr. Pickett will receive salary and bonus in effect up to the date of the remaining portion of the contract.
On October 10, 2006, the Company entered into a three year contract with Mr. Livneh to serve as President of Bovie Canada which contract allows for a two year extension, unless the Company provides written notice of its intention not to renew prior to the expiration date. During August of 2009, in line with the provisions of the contract, the Company provided written notice to Mr. Livneh of its intent not to renew his employment contract. Mr. Livneh remains as a director of Bovie Medical Corporation and as of December 31, 2009 continues to provide consulting support towards the development of the Seal-N-Cut product line via Lican Developments, Ltd. (see ITEM 13. Certain Relationships and Related Transactions)
There are no other employment contracts that have non-cancelable terms in excess of one year.
Grants of Plan-Based Awards
The only incentive award granted to a Named Executive Officer in fiscal 2009 was to Gary Pickett in the amount of 12,500 stock options.
Options Exercises During Fiscal 2009
The following table summarizes the options exercised during the year ended December 31, 2009 and the value realized upon exercise:
| | | Option Awards | |
| | Number of Shares Acquired on Exercise | | | Value Realized Upon Exercise ($) (1) | |
| Andrew Makrides | | | -- | | | | -- | |
| J. Robert Saron | | | -- | | | | -- | |
| Moshe Citronowicz | | | -- | | | | -- | |
| Steve Livneh | | | -- | | | | -- | |
| Gary Pickett | | | -- | | | | -- | |
| (1) | The value realized equals the excess of the fair market value of our common stock on the exercise date over the option exercise price, multiplied by the number of options exercised. |
Outstanding Equity Awards
The following table presents information with respect to each unexercised stock option held by Bovie's Named Executive Officers as of December 31, 2009.
| | | Outstanding Equity Awards at 12/31/09 | |
| Name | | # of Securities Underlying Unexercised Options (# Exercisable) | | | # of Securities Underlying Unexercised Options (# Unexercisable) (*) | | | Option Exercise Price ($/sh) | | Option Expiration Date 10 Years After Grant Date | |
| Andrew Makrides | | | 25,000 | | | | -- | | | | 3.25 | | 9/29/2013 | |
| | | | 25,000 | | | | -- | | | | 2.13 | | 9/23/2014 | |
| | | | 25,000 | | | | -- | | | | 2.25 | | 5/5/2015 | |
| J. Robert Saron | | | 12,500 | | | | -- | | | | 3.25 | | 9/29/2013 | |
| | | | 12,500 | | | | -- | | | | 2.13 | | 9/23/2014 | |
| | | | 12,500 | | | | -- | | | | 2.25 | | 5/5/2015 | |
| Moshe Citronowicz | | | 25,000 | | | | -- | | | | 3.25 | | 9/29/2013 | |
| | | | 25,000 | | | | -- | | | | 2.13 | | 9/23/2014 | |
| | | | 25,000 | | | | -- | | | | 2.25 | | 5/5/2015 | |
Gary Pickett | | | 20,000 | | | | 14,286 | | | | 8.66 | | 1/12/2017 | |
| | | | 5,000 | | | | 3,572 | | | | 7.10 | | 3/29/2017 | |
| | | | 12,500 | | | | 12,500 | | | | 8.32 | | 10/26/2019 | |
| Steve Livneh | (1) | | 100,000 | | | | -- | | | | 3.26 | | 1/1/2016 | |
(1) Issued in the name of Henvil Corporation as part of the Purchase Agreement to acquire the MEG technology. Steve Livneh is the principal owner of Henvil Corporation.
Compensation of Non-Employee Directors
The following is a table showing the director compensation for the year ended December 31, 2009:
| Name | | Fees Earned Or Paid In Cash ($) | | | Stock Awards ($) | | | Option Awards ($) *** | | | Non-Equity Incentive Plan Compensa- tion ($) | | | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | | | All Other Compensa- tion ($) | | | Total ($) | |
| (a) | | (b) | | | (c) | | | (d) | | | (e) | | | (f) | | | (g) | | | (h) | |
| Brian Madden ** | | | 0 | | | | 0 | | | $ | 35,000 | * (1) | | | 0 | | | | 0 | | | | 0 | | | $ | 35,000 | |
| Michael Norman | | | 0 | | | | 0 | | | $ | 35,000 | * (2) | | | 0 | | | | 0 | | | | 0 | | | $ | 35,000 | |
| August Lentricchia | | | 0 | | | | 0 | | | $ | 35,000 | * (3) | | | 0 | | | | 0 | | | | 0 | | | $ | 35,000 | |
| Steven MacLaren | | | 0 | | | | 0 | | | $ | 35,000 | * (4) | | | 0 | | | | 0 | | | | 0 | | | $ | 35,000 | |
| Dr. Peter Pardoll | | | 0 | | | | 0 | | | $ | 26,250 | * (5) | | | 0 | | | | 0 | | | | 0 | | | $ | 26,250 | |
| Greg Konesky | | | 0 | | | | 0 | | | $ | 26,250 | * (6) | | | 0 | | | | 0 | | | | 0 | | | $ | 26,250 | |
*** These columns represent the grant date fair value of the awards as calculated in accordance with FASB ASC 718 (Stock Compensation). Pursuant to SEC rule changes effective February 28, 2010, we are required to reflect the total grant date fair values of the option grants in the year of grant, rather than the portion of this amount that was recognized for financial statement reporting purposes in a given fiscal year which was required under the prior SEC rules, resulting in a change to the amounts reported in prior Annual Reports.
* (1) Mr. Madden was granted 10,000 stock options on October 26, 2009 which had a fair value of $3.50 per option.
* (2) Mr. Norman was granted 10,000 stock options on October 26, 2009 which had a fair value of $3.50 per option.
* (3) Mr. Lentricchia was granted 10,000 stock options on October 26, 2009 which had a fair value of $3.50 per option.
* (4) Mr. MacLaren was granted 10,000 stock options on October 26, 2009 which had a fair value of $3.50 per option.
* (5) Dr. Pardoll was granted 7,500 stock options on October 26, 2009 which had a fair value of $3.50 per option.
* (6) Mr. Konesky was granted 7,500 stock options on December 29, 2009 which had a fair value of $3.50 per option.
** Mr. Madden resigned from the board in November 2009 for personal reasons and was replaced by Gregory Konesky.
Directors' compensation is determined by the Board of Directors based upon recommendations from the Compensation Committee. The Board periodically grants directors stock options in order to assure that they have proper incentives and an opportunity for an ownership interest in common with other stockholders.
Our Board of Directors presently consists of J. Robert Saron, Andrew Makrides, Chairman, CEO, and President, George Kromer, Jr., Michael Norman, August Lentricchia, Steve Livneh, Steven MacLaren, Dr. Peter Pardoll, and Gregory Konesky.
In 2003, the Board of Directors adopted and shareholders approved Bovie’s 2003 Executive and Employee Stock Option Plan covering a total of one million two hundred thousand (1,200,000) shares of common stock issuable upon exercise of options to be granted under the Plan. In 2001, the Board of Directors adopted the 2001 Executive and Employee Stock Option Plan which reserved for issuance 1,200,000 stock options.
On October 30, 2007, shareholders approved and the Board of Directors adopted an amendment to the 2003 Executive and Employee Stock Option Plan to increase the maximum aggregate number of shares of common stock reserved for issuance under the 2003 Plan from 1.2 million shares (already reserved against outstanding options) to 1.7 million shares, or an increase of 500,000 shares of common stock for future issuance pursuant to the terms of the Plan. Except for the increase in the number of shares covered by the Plan, the Plan remains otherwise unchanged from its present status. In 2009, the Board of Directors granted 85,500 options to purchase a like number of shares of common stock.
There have been no changes in the pricing of any options previously or currently awarded.
COMPENSATION COMMITTEE REPORT
Our Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis contained in this Annual Report on Form 10-K with management. Based on our Compensation Committee’s review of and the discussions with management with respect to the Compensation Discussion and Analysis, our Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in our Proxy Statement and in this Annual Report on Form 10-K for the fiscal year ended December 31, 2009 for filing with the SEC. Our compensation committees’ members are Steven MacLaren, Michael Norman (CPA), Dr. Peter Pardoll and August Lentricchia.
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Equity Compensation Plan Information
See “ITEM 5. Market for Registrant’s Common Equity and Related Stockholder Matters”.
Security Ownership of Certain Beneficial Owners
The following table sets forth certain information as of December 31, 2009, with respect to the beneficial ownership of the Company’s common stock by its executive officers, directors, all persons known by the Company to be the beneficial owners of more than 5% of its outstanding shares and by all officers and directors as a group.
| | Number of Shares | | |
| Name and Address | Title | Owned (i) | Nature of Ownership | Percentage of Ownership (i) |
| | | | | |
| Andrew Makrides | Common | 674,213(ii) | Beneficial | 4.0% |
| 734 Walt Whitman Road | | | | |
| Melville, NY 11746 | | | | |
| | | | | |
| George Kromer | Common | 341,508(iii) | Beneficial | 2.0% |
| P.O. Box 188 | | | | |
| Farmingville, NY 11738 | | | | |
| | | | | |
| J. Robert Saron | Common | 424,819(iv) | Beneficial | 2.5% |
| 5115 Ulmerton Rd. | | | | |
| Clearwater, FL 33760 | | | | |
| | | | | |
Gregory Konesky | Common | 32,550 (vi) | Beneficial | 0.2% |
| Rolling Hill Rd. | | | | |
| Hampton Bays, NY 11946 | | | | |
| | | | | |
| Mike Norman | Common | 95,000(vii) | Beneficial | 0.6% |
| 410 Jericho Tpke. | | | | |
| Jericho, NY | | | | |
| | | | | |
| Dr. Peter Pardoll | Common | 29,873 (viii) | Beneficial | 0.2% |
| 34 Paradise Lane | | | | |
| Treasure Island, FL 33706 | | | | |
| | | | | |
| Moshe Citronowicz | Common | 481,504 (v) | Beneficial | 2.8% |
| 5115 Ulmerton Rd. | | | | |
| Clearwater, FL 33760 | | | | |
| | | | | |
| Gary Pickett | Common | 37,500 (ix) | Beneficial | 0.2% |
| 5115 Ulmerton Rd. | | | | |
| Clearwater, FL 33760 | | | | |
| | | | | |
| Steve Livneh | Common | 300,000 (x) | Beneficial | 1.8% |
| 4056 North Services Rd. E. | | | | |
| Windsor, Canada | | | | |
| | | | | |
| August Lentricchia | Common | 19,100 (xi) | Beneficial | 0.1% |
| 734 Walt Whitman Road | | | | |
| Melville, NY 11746 | | | | |
| | | | | |
| Steven MacLaren | Common | 22,500 (xii) | Beneficial | 0.1% |
| 5115 Ulmerton Rd. | | | | |
| Clearwater, FL 33760 | | | | |
| | | | | |
| Officers and Directors as a group (11 Persons) | 2,458,567(xiii) | | 14.5% |
(i) Based on 17,094,773 outstanding shares of Common Stock and 1,741,525 outstanding options to acquire a like number of shares of Common Stock as of December 31, 2009, of which officers and directors owned a total of 575,000 options and 1,883,567 shares at December 31, 2009. We have calculated the percentages on the basis of the amount of outstanding securities plus, for each person or group, any securities that person or group has current or future right to acquire pursuant to options, warrants, conversion privileges or other rights.
(ii) Includes 599,213 shares and 75,000 ten year options owned by Mr. Makrides to purchase shares of Common Stock of the Company. Exercise prices for his options range from $2.13 for 25,000 shares to $3.25 for 25,000 shares.
(iii) Includes 266,508 shares and 75,000 ten year options owned by Mr. Kromer to purchase shares of the Company. Exercise prices for his options range from $2.13 for 25,000 shares to $3.25 for 25,000 shares.
(iv) Includes 387,319 shares and 37,500 ten year options owned by Mr. Saron, exercisable at prices ranging from $2.13 per share for 12,500 shares, and $3.25 per share for 12,500 shares.
(v) Includes 406,504 shares and 75,000 ten year options owned by Mr. Citronowicz exercisable at prices ranging from $2.13 for 25,000 shares to $3.25 for 25,000 shares.
(vi) Includes 50 shares and 32,500 ten year options owned by Mr. Konesky exercisable at prices ranging from $1.30 for 10,000 shares, $2.93 for 15,000 shares, to $7.85 for 7,500 shares.
(vii) Includes 95,000 ten year options owned by Mr. Norman exercisable at prices ranging from $2.13 for 25,000 shares to $8.66 for 12,500 shares.
(viii) Includes 22,373 shares and 7,500 ten year options owned by Mr. Pardoll exercisable at a price of $8.32.
(ix) Includes 37,500 ten year options owned to Mr. Pickett exercisable at prices ranging from $7.10 for 5,000 shares to $8.66 for 20,000 shares. These options vest over a 7 year period.
(x) Includes 100,000 ten year options owned by Mr. Livneh. These options were part of the Henvil Purchase Agreement and were issued under the name Henvil Corporation. Mr. Livneh is the principal owner of Henvil Corporation. (see Item 1 Business - New Products) Also includes 200,000 restricted shares issued to Lican Developments, Inc. of which Mr. Livneh is also the principal owner.
(xi) Includes 1,600 Shares owned by Mr. Lentricchia and 17,500 ten year options issued to Mr. Lentricchia exercisable at prices ranging from $7.68 for 7,500 shares to $8.32 for 10,000 shares. These options vest over a period of 7 years.
(xii) Includes 22,500 ten year options owned to Mr. MacLaren exercisable at prices ranging from $7.33 for 7,500 shares to $8.66 for 5,000 shares. These options vest over a 7 year period.
(xiii) Includes 575,000 shares reserved for outstanding options owned by all Executive Officers and directors as a group. The last date options can be exercised is December 29, 2019.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires our officers and directors, and persons who own more than ten percent of a registered class of our equity securities, to file reports of ownership and changes in ownership with the Securities and Exchange Commission. Officers, directors and greater than ten-percent shareholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file.
Based solely on a review of the copies of such forms furnished to us, we believe that during the year ended December 31, 2009 all officers, directors and ten percent beneficial owners who were subject to the provisions of Section 16(a) complied with all of the filing requirements during the year.
ITEM 13. Certain Relationships and Related Transactions
Our policy is that employees, non-employees, and third parties must obtain authorization from the appropriate department executive manager, for any business relationship or proposed business transaction in which they or an immediate family member has a direct or indirect interest, or from which they or an immediate family member may derive a personal benefit (a “related party transaction”). The maximum dollar amount of related party transactions that may be approved as described above in this paragraph in any calendar year is $120,000. Any related party transactions that would bring the total value of such transactions to greater than $120,000 must be referred to the Audit Committee to determine the procedure for approval, and then have the recommendations presented to the Board of Directors for approval.
Steve Livneh, a director and former officer of Bovie, is the founder and principal of Lican Developments LTD (“Lican”), an Ontario, Canada Corporation.. During 2009 Lican was paid $50,000 related to an installment payment from an October 2006 purchase agreement. The remaining amounts owed under this arrangement are reflected as a Due to Lican in our consolidated financial statements. Lican may in the future also receive ongoing royalties ranging from 2.5% to 3% of sales of certain products.
Steven MacLaren, a director of Bovie, is president and a shareholder of Ronin Consulting Group, Inc., a company which provided various financial and analytical project consulting services to Bovie. Ronin Consulting Group, Inc. was paid fees approximating $99,800 and $72,400 during 2009, and for the portion of 2008 he was a director, respectively.
Dr. Peter Pardoll, a director of Bovie, is the principal owner of Medical Education Associates, LLC, which provided medical field consulting services to Bovie. Medical Education Associates, LLC was paid fees approximating $40,000 since he became a director in April 2009.
A relative of Bovie’s chief operating officer is considered a related party. Arik Zoran, is an employee of the Company in charge of the engineering department and was paid inclusive of benefits $188,363, $197,272, and $166,487 for 2009, 2008 and 2007 respectively.
Starting November 1, 2009, Lican provided research and development consulting in the continued development of our Seal-N-Cut product line and was paid consulting fees approximating $21,000.
ITEM 14. Principal Accountant Fees and Services
The following table sets forth the aggregate fees billed to us for fiscal years ended December 31, 2009 and 2008 by our current and previous accountants (Kingery & Crouse P.A. and Bloom & Co. LLP, respectively):
| | | 2009 | | | 2008 | |
| Audit Fees (1) | | $ | 143,021 | | | $ | 162,651 | |
| | | | | | | | | |
| Non-Audit Fees: | | | | | | | | |
| Related Fees(2) | | | 51,659 | | | | 52,935 | |
| Tax Fees(3) | | | 6,648 | | | | 5,689 | |
| All other Fees(4) | | | 12,565 | | | | 12,882 | |
| Total Fees billed | | $ | 213,803 | | | $ | 234,157 | |
(1) Audit fees consist of fees billed for professional services rendered for the audit of Bovie’s annual financial statements and reviews of its interim consolidated financial statements included in quarterly reports and other services related to statutory and regulatory filings or engagements.
(2) Related fees consist of fees billed for assurance and related services that are reasonably related to the performance of the audit or reviews of Bovie’s consolidated financial statements and are not reported under “Audit Fees”.
(3) Tax fees consist of fees billed for professional services rendered for tax compliance and tax advice (domestic and international). These services include assistance regarding federal, state and international tax compliance, acquisitions and international tax planning.
(4) All other fees consist of fees for products and services other than the services reported above.
Effective for the returns for the year ended December 31, 2008, we engaged Hobson, Bishoff & Dowdy, PLLC to handle our tax services. Previous to this engagement, the Board of Directors had considered the role of our independent auditors in providing certain tax services to Bovie and had concluded that such services were compatible with their independence as our auditors. In addition, since the effective date of the SEC rules stating that an auditor is not independent of an audit client if the services it provides to the client are not appropriately approved, the Audit Committee pre-approves all audit and permissible non-audit services provided by our independent auditors.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in Melville, New York on March 16, 2010.
| | Bovie Medical Corporation |
| | |
| | |
| | By: /s/ ANDREW MAKRIDES |
| | Andrew Makrides |
| | President |
| | Chairman of the Board |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | | Title | | Date |
| | | | | |
| Principal Executive Officer: | | | | |
| | | | | |
/s/ ANDREW MAKRIDES Andrew Makrides | | Chief Executive Officer and Chairman of the Board | | March 16, 2010 |
| | | | | |
| Principal Financial Officer: | | | | |
| | | | | |
/s/ GARY D. PICKETT Gary D. Pickett | | Chief Financial Officer, Treasurer, and Secretary | | March 16, 2010 |
| | | | | |
| Directors: | | | | |
| | | | | |
/s/ J. ROBERT SARON J. Robert Saron | | Chief Sales and Marketing Officer and Director | | March 16, 2010 |
| | | | | |
/s/ GEORGE KROMER George Kromer | | Director | | March 16, 2010 |
| | | | | |
/s/ MICHAEL NORMAN Michael Norman | | Director | | March 16, 2010 |
| | | | | |
/s/ AUGUST LENTRICCHIA August Lentricchia | | Director | | March 16, 2010 |
| | | | | |
/s/ STEVE LIVNEH Steve Livneh | | President of Bovie Canada and Director | | March 16, 2010 |
| | | | | |
/s/ STEVEN MACLAREN Steven MacLaren | | Director | | March 16, 2010 |
| | | | | |
/s/ DR. PETER PARDOLL Dr. Peter Pardoll | | Director | | March 16, 2010 |
| | | | | |
/s/ GREG KONESKY Greg Konesky | | Director | | March 16, 2010 |
PART II
ITEM 15. Exhibits and Financial Statement Schedules
The financial statements and exhibits filed as part of this annual report on Form 10-K are provided below:
ITEM 15A. Financial Statements
| BOVIE MEDICAL CORPORATION INDEX TO FINANCIAL STATEMENTS | Page |
| | |
| Report of Independent Registered Public Accounting Firm | F-1 |
| | |
| Consolidated Balance Sheets at December 31, 2009 and 2008 | F-2 |
| | |
| Consolidated Statements of Operations for the years ended December 31, 2009, 2008 and 2007 | F-4 |
| | |
| Consolidated Statements of Stockholders' Equity and Comprehensive Income for the years ended December 31, 2009, 2008 and 2007 | F-5 |
| | |
| Consolidated Statements of Cash Flows for the years ended December 31, 2009, 2008 and 2007 | F-6 |
| | |
| Notes to Consolidated Financial Statements | F-7 |
[LETTERHEAD OF KINGERY & CROUSE, P.A.]
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Bovie Medical Corporation:
We have audited the accompanying consolidated balance sheets of Bovie Medical Corporation (the “Company”), as of December 31, 2009 and 2008, and the related consolidated statements of operations, stockholders’ equity and comprehensive income, and of cash flows for the years ended December 31, 2009, 2008 and 2007. We also have audited the Company’s internal control over financial reporting as of December 31, 2009, based on criteria established in ”Internal Control – Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). The Company’s management is responsible for these financial statements, for maintaining effective control over financial reporting and for its asses sment of the effectiveness of internal control over financial reporting included in Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on these financial statements and an opinion on the Company’s internal control over financial reporting based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States of America). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of in ternal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of a company’s assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of a company are being made only in accordance with authorizations of it’s management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of a company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2009 and 2008, and the results of its operations and its cash flows for the years ended December 31, 2009, 2008 and 2007 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on criteria established in “Internal Control – Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”).
As discussed in Note 2 to the consolidated financial statements, the Company has restated certain amounts in the consolidated financial statements from those previously reported as of and for the years ended December 31, 2008 and 2007.
Kingery & Crouse, P.A s/s
Tampa, FL
March 16, 2010
BOVIE MEDICAL CORPORATION
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2009 AND 2008
| ASSETS | | 2009 | | | 2008 | |
| Current assets: | | | | | | |
| | | | | | | |
| Cash and cash equivalents | | $ | 2,154,825 | | | $ | 2,564,443 | |
| Trade accounts receivable, net | | | 2,565,734 | | | | 2,991,473 | |
| Inventories | | | 6,774,166 | | | | 5,339,983 | |
| Prepaid expenses and other current assets | | | 919,222 | | | | 1,069,438 | |
| Deferred income tax asset, net | | | 800,000 | | | | 216,885 | |
| | | | | | | | | |
| Total current assets | | | 13,213,947 | | | | 12,182,222 | |
| | | | | | | | | |
| Property and equipment, net | | | 8,813,882 | | | | 7,125,943 | |
| | | | | | | | | |
| Other assets: | | | | | | | | |
| | | | | | | | | |
| Brand name and trademark | | | 1,509,662 | | | | 1,509,662 | |
| Purchased technology, net | | | 3,270,067 | | | | 3,479,752 | |
| License rights, net | | | 152,549 | | | | 215,673 | |
| Restricted cash held in escrow | | | 35,635 | | | | 1,285,117 | |
| Deferred income tax asset, net | | | 158,641 | | | | 802,134 | |
| Deposits | | | 430,076 | | | | 124,707 | |
| | | | | | | | | |
| Total other assets | | | 5,556,630 | | | | 7,417,045 | |
| Total Assets | | $ | 27,584,459 | | | $ | 26,725,210 | |
The accompanying notes are an integral part of the consolidated financial statements.
BOVIE MEDICAL CORPORATION
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2009 AND 2008
(Continued)
LIABILITIES AND STOCKHOLDERS' EQUITY
| LIABILITIES | | 2009 | | | 2008 | |
| Current liabilities: | | | | | | |
| | | | | | | |
| Accounts payable | | $ | 589,407 | | | $ | 1,317,336 | |
| Deferred revenue | | | 3,994 | | | | 24,538 | |
| Accrued payroll | | | 77,779 | | | | 61,168 | |
| Accrued vacation | | | 170,514 | | | | 237,633 | |
| Customer deposits | | | 5,930 | | | | 168 | |
| Current portion of amounts due to Lican | | | 50,000 | | | | 50,000 | |
| Current portion of mortgage note payable to bank | | | 135,000 | | | | 125,000 | |
| Line of credit | | | 1,000,000 | | | | - | |
| Accrued and other liabilities | | | 440,253 | | | | 422,941 | |
| | | | | | | | | |
| Total current liabilities | | | 2,472,877 | | | | 2,238,784 | |
| | | | | | | | | |
| | | | | | | | | |
| Mortgage note payable to bank, net of current portion | | | 3,740,000 | | | | 3,875,000 | |
| | | | | | | | | |
| Due to Lican, net of current portion | | | 218,150 | | | | 268,150 | |
| | | | | | | | | |
| Total liabilities | | | 6,431,027 | | | | 6,381,934 | |
| | | | | | | | | |
| Commitments and Contingencies (see Note 12) | | | | | | | | |
| | | | | | | | | |
| Stockholders' equity: | | | | | | | | |
| | | | | | | | | |
| Preferred stock, par value $.001; 10,000,000 shares authorized; none issued or outstanding | | | -- | | | | -- | |
| Common stock, par value $.001 par value; 40,000,000 shares authorized; 17,094,773 and 16,982,707 issued and 16,951,695 and 16,795,269 outstanding on December 31, 2009 and December 31, 2008, respectively | | | 16,952 | | | | 16,796 | |
| Additional paid-in capital | | | 23,056,526 | | | | 22,841,545 | |
| Accumulated other comprehensive (loss) | | | (88,967 | ) | | | (88,464 | ) |
| Deficit | | | (1,831,079 | ) | | | (2,426,601 | ) |
| | | | | | | | | |
| Total stockholders' equity | | | 21,153,432 | | | | 20,343,276 | |
| | | | | | | | | |
| Total Liabilities and Stockholders' Equity | | $ | 27,584,459 | | | $ | 26,725,210 | |
The accompanying notes are an integral part of the consolidated financial statements.
BOVIE MEDICAL CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2008 AND 2007
| | | 2009 | | | 2008 | | | 2007 (as restated) | |
| | | | | | | | | | |
| Sales, net | | $ | 26,953,447 | | | $ | 28,096,510 | | | $ | 28,779,157 | |
| Cost of sales | | | 15,098,696 | | | | 16,247,702 | | | | 17,463,644 | |
| Gross Profit | | | 11,854,751 | | | | 11,848,808 | | | | 11,315,513 | |
| | | | | | | | | | | | | |
| Gain on cancellation of agreement | | | -- | | | | 1,495,634 | | | | -- | |
| | | | | | | | | | | | | |
| Other costs: | | | | | | | | | | | | |
| Research and development | | | 2,082,960 | | | | 2,060,854 | | | | 1,643,092 | |
| Professional services | | | 1,398,029 | | | | 990,814 | | | | 737,800 | |
| Salaries and related costs | | | 3,002,862 | | | | 3,016,447 | | | | 2,805,082 | |
| Selling, general and administration | | | 4,656,051 | | | | 4,489,415 | | | | 4,023,033 | |
| Total other costs | | | 11,139,902 | | | | 10,557,530 | | | | 9,209,007 | |
| | | | | | | | | | | | | |
| Income from operations | | | 714,849 | | | | 2,786,912 | | | | 2,106,506 | |
| | | | | | | | | | | | | |
| Other income (expense): | | | | | | | | | | | | |
| Interest income | | | 24,362 | | | | 48,762 | | | | 142,721 | |
| Minority interest | | | -- | | | | -- | | | | 5,000 | |
| Interest expense | | | (76,370 | ) | | | (58,463 | ) | | | (2,471 | ) |
| Total other income (expense), net | | | (52,008 | ) | | | (9,701 | ) | | | 145,250 | |
| | | | | | | | | | | | | |
| Income before income taxes | | | 662,841 | | | | 2,777,211 | | | | 2,251,756 | |
| | | | | | | | | | | | | |
| Provision for current income taxes | | | (6,941 | ) | | | (34,423 | ) | | | (20,802 | ) |
| Benefit (provision) for deferred income taxes | | | (60,378 | ) | | | (911,000 | ) | | | 1,570,000 | |
| Total benefit (provision) for income taxes - net | | | (67,319 | ) | | | (945,423 | ) | | | 1,549,198 | |
| | | | | | | | | | | | | |
| Net income | | $ | 595,522 | | | $ | 1,831,788 | | | $ | 3,800,954 | |
| | | | | | | | | | | | | |
| Earnings per common share: | | | | | | | | | | | | |
| Basic | | $ | 0.04 | | | $ | 0.11 | | | $ | 0.25 | |
| Diluted | | $ | 0.03 | | | $ | 0.11 | | | $ | 0.22 | |
| | | | | | | | | | | | | |
| Weighted average number of common shares | | | | | | | | | | | | |
| outstanding | | | 16,899,297 | | | | 16,071,229 | | | | 15,324,508 | |
| | | | | | | | | | | | | |
| Weighted average number of common shares outstanding adjusted for dilutive securities | | | 17,836,212 | | | | 17,086,798 | | | | 17,684,705 | |
The accompanying notes are an integral part of the consolidated financial statements.
BOVIE MEDICAL CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY AND COMPREHENSIVE INCOME
FOR THE YEARS ENDED DECEMBER 31, 2009, 2008 AND 2007
| | | Common | | | Additional Paid-in | | | Accumulated Other Comprehensive Gain | | | | | | | |
| | | Shares | | | Par Value | | | Capital | | | (Loss) | | | Deficit | | | Total | |
| | | | | | | | | | | | | | | | | | | |
| December 31, 2006 | | | 15,223,538 | | | $ | 15,241 | | | $ | 22,104,399 | | | $ | - | | | $ | (8,059,343 | ) | | $ | 14,060,297 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Options exercised | | | 225,300 | | | | 225 | | | | 309,925 | | | | - | | | | - | | | | 310,150 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock based compensation | | | - | | | | - | | | | 72,089 | | | | - | | | | - | | | | 72,089 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock swap to acquire options | | | (9,179 | ) | | | (9 | ) | | | (56,241 | ) | | | - | | | | - | | | | (56,250 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Other | | | 17,429 | | | | - | | | | 4,989 | | | | - | | | | - | | | | 4,989 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | - | | | | - | | | | - | | | | - | | | | 3,800,954 | | | | 3,800,954 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2007 (as restated) | | | 15,457,088 | | | | 15,457 | | | | 22,435,161 | | | | - | | | | (4,258,389 | ) | | | 18,192,229 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Options exercised | | | 1,488,750 | | | | 1,489 | | | | 1,195,606 | | | | - | | | | - | | | | 1,197,095 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock based compensation | | | - | | | | - | | | | 184,697 | | | | - | | | | - | | | | 184,697 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock swap to acquire options | | | (150,569 | ) | | | (150 | ) | | | (973,919 | ) | | | - | | | | - | | | | (974,069 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | - | | | | - | | | | - | | | | - | | | | 1,831,788 | | | | 1,831,788 | |
| Foreign currency remeasurement | | | | | | | | | | | | | | | (88,464 | ) | | | - | | | | (88,464 | ) |
| Comprehensive income | | | - | | | | - | | | | - | | | | - | | | | - | | | | 1,743,324 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2008 | | | 16,795,269 | | | | 16,796 | | | | 22,841,545 | | | | (88,464 | ) | | | (2,426,601 | ) | | | 20,343,276 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Options exercised | | | 183,250 | | | | 183 | | | | 286,233 | | | | - | | | | - | | | | 286,416 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock based compensation | | | | | | | | | | | 136,383 | | | | - | | | | - | | | | 136,383 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock swap to acquire options | | | (26,824 | ) | | | (27 | ) | | | (207,635 | ) | | | - | | | | - | | | | (207,662 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | - | | | | - | | | | - | | | | - | | | | 595,522 | | | | 595,522 | |
| Foreign currency remeasurement | | | - | | | | - | | | | - | | | | (503 | ) | | | - | | | | (503 | ) |
| Comprehensive income | | | - | | | | - | | | | - | | | | - | | | | - | | | | 595,019 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2009 | | | 16,951,695 | | | $ | 16,952 | | | $ | 23,056,526 | | | $ | (88,967 | ) | | $ | (1,831,079 | ) | | $ | 21,153,432 | |
The accompanying notes are an integral part of the consolidated financial statements.
BOVIE MEDICAL CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2008 AND 2007
| | | 2009 | | | 2008 | | | 2007 (as restated) | |
Cash flows from operating activities: | | | | | | | | | |
| Net income | | $ | 595,522 | | | $ | 1,831,788 | | | $ | 3,800,954 | |
| Adjustments to reconcile net income to net cash provided by (used in) operating activities: | | | | | | | | | | | | |
| Depreciation and amortization of property and equipment | | | 776,825 | | | | 784,411 | | | | 666,162 | |
| Amortization of intangible assets | | | 272,808 | | | | 188,658 | | | | 104,664 | |
| Provision for (recovery of) inventory obsolescence | | | 27,459 | | | | (29,118 | ) | | | (100,565 | ) |
| Loss (gain) on disposal of fixed assets | | | (750 | ) | | | 6,557 | | | | 10,806 | |
| Stock-based compensation | | | 136,385 | | | | 184,698 | | | | 72,089 | |
| Noncash reclassification adjustment | | | -- | | | | 10,325 | | | | 4,989 | |
| Provision (benefit) for deferred income taxes | | | 60,378 | | | | 911,000 | | | | (1,570,000 | ) |
| Provision for (recovery of) bad debts | | | (14,681 | ) | | | (89 | ) | | | 3,375 | |
| Minority interest in net loss of joint venture | | | -- | | | | -- | | | | (5,000 | ) |
| Gain on cancellation of agreement | | | -- | | | | (1,495,634 | ) | | | -- | |
| Change in assets and liabilities: | | | | | | | | | | | | |
| Trade receivables | | | 440,422 | | | | (466,175 | ) | | | 288,731 | |
| Prepaid expenses and other current assets | | | 150,216 | | | | (726,176 | ) | | | 124,161 | |
| Inventories | | | (1,461,642 | ) | | | (788,873 | ) | | | (812,127 | ) |
| Deposits | | | (305,368 | ) | | | (80,269 | ) | | | (23,223 | ) |
| Accounts payable | | | (727,929 | ) | | | 510,141 | | | | (148,014 | ) |
| Accrued and other liabilities | | | 17,312 | | | | 13,440 | | | | (215,737 | ) |
| Accrued payroll | | | 16,610 | | | | (52,140 | ) | | | 23,401 | |
| Accrued vacation | | | (67,118 | ) | | | 8,042 | | | | 39,399 | |
| Customer deposits | | | 5,763 | | | | (35,909 | ) | | | (55,121 | ) |
| Deferred revenue | | | (20,544 | ) | | | (31,848 | ) | | | (117,600 | ) |
| Net cash provided by (used in) operating activities | | | (98,332 | ) | | | 742,829 | | | | 2,091,344 | |
| | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | |
| Purchases of property and equipment | | | (2,465,247 | ) | | | (4,465,879 | ) | | | (881,401 | ) |
| Proceeds from sale of property and equipment | | | 1,233 | | | | 10,573 | | | | -- | |
| Increase in purchased technology | | | -- | | | | (57,283 | ) | | | (516,356 | ) |
| Increase in license rights | | | -- | | | | -- | | | | (315,620 | ) |
| Net cash used in investing activities | | | (2,464,014 | ) | | | (4,512,589 | ) | | | (1,713,377 | ) |
| | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | |
Proceeds from mortgage note payable to bank (net of amounts in escrow) | | | 1,249,481 | | | | 2,714,883 | | | | -- | |
| Proceeds from line of credit | | | 1,000,000 | | | | -- | | | | -- | |
| Proceeds from sales of common stock | | | 78,750 | | | | 223,025 | | | | 253,900 | |
| Repayments of long-term debt and due to Lican | | | (175,000 | ) | | | (50,000 | ) | | | (50,000 | ) |
| Net cash provided by financing activities | | | 2,153,231 | | | | 2,887,908 | | | | 203,900 | |
| | | | | | | | | | | | | |
| Effect of exchange rate changes on cash and cash equivalents | | | (503 | ) | | | (88,464 | ) | | | - | |
| | | | | | | | | | | | | |
| Net change in cash and cash equivalents | | | (409,618 | ) | | | (970,316 | ) | | | 581,867 | |
| | | | | | | | | | | | | |
| Cash and cash equivalents at beginning of year | | | 2,564,443 | | | | 3,534,759 | | | | 2,952,892 | |
| | | | | | | | | | | | | |
| Cash and cash equivalents at end of year | | $ | 2,154,825 | | | $ | 2,564,443 | | | $ | 3,534,759 | |
| Cash paid for: | | | | | | | | | | | | |
| Interest paid, net of amounts capitalized of $119,000 in 2009 | | $ | 127,532 | | | $ | 58,463 | | | $ | 2,471 | |
| Income taxes | | $ | 244,753 | | | $ | 135,583 | | | $ | 73,504 | |
The accompanying notes are an integral part of the consolidated financial statements.
BOVIE MEDICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1. DESCRIPTION OF BUSINESS
Bovie Medical Corporation (“Bovie”) was incorporated in 1982, under the laws of the State of Delaware and is a medical device company engaged in the manufacturing and marketing of electrosurgical devices. Our medical products include a wide range of devices including electrosurgical generators and accessories, cauteries, medical lighting, nerve locators and other products.
NOTE 2. SIGNIFICANT ACCOUNTING POLICIES
Consolidated Financial Statements
The accompanying consolidated financial statements include the accounts of Bovie and its wholly owned subsidiaries, Aaron Medical Industries, Inc., BVX Holdings, LLC (which in turn owns 100% of Bovie Canada ULC) and Jump Agentur Fur Electrotechnik GMBH (“JAG”) (collectively, the “Company” or “we”, “our” or “us”). The latter entity was a 50% owned joint venture until May 2007 (see Note 14). All intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates in the Preparation of Financial Statements
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements. The reported amounts of revenues and expenses during the reporting period may be affected by the estimates and assumptions we are required to make. Estimates that are critical to the accompanying consolidated financial statements relate principally to the adequacy of our inventory allowances, and the the recoverability of certain intangibles and our deferred income tax assets. In addition, stock-based compensation expense represents a significant estimate as such expe nse is derived from a formula that uses various assumptions to estimate the future but unknown value of our common stock. The markets for the Company’s products are characterized by intense price competition, rapid technological development, evolving standards and short product life cycles, all of which could impact the future realization of its assets. Estimates and assumptions are reviewed periodically and the effects of revisions are reflected in the period that they are determined to be necessary. It is at least reasonably possible that the Company’s estimates could change in the near term with respect to these matters.
Restatement
Prior to filing our Annual Report on Form 10-K for the year ended December 31, 2009, we determined there were errors in the computation of deferred tax assets and the related income tax benefit. In preparing our 2009 income tax returns, we initiated a review of our net operating loss carryforwards which were initially fully used in 2008. During this review we became aware that since as early as 2003 we had not taken a tax deduction for the taxable compensation issued to holders of certain Non-Qualified Stock Options as a result of their exercise of such options (the difference between the exercise value and the amounts booked as a deduction). As a result of this review we determined that our net operating losses were understated. Prior to 2007, management determined that the deferred tax assets related to net operating losses and credits might not be realizable and, accordingly, maintained a valuation allowance against them. During the year ended December 31, 2007, management determined that such valuation allowances were no longer necessary, and accordingly, the valuation allowances were reversed, resulting in a benefit for income taxes being recorded for the anticipated utilization. Because management would have also concluded that the additional deferred tax assets resulting from the increase in net operating losses were fully realizable, we have included the entire amount as income in 2007 as an income tax benefit. There was no effect on the 2008 consolidated statement of income; however this adjustment did result in an increase in amounts refundable, an increase in deferred tax assets and the allocation of such assets between current and non-current in 2007. The Company’s current liquidity increased by approximately $65,000 and $79,000 for 2007 a nd 2008, respectively, as a result of these changes.
As such the December 31, 2007 consolidated financial statements have been restated and various amounts in the 2008 consolidated balance sheet have been reclassified . The effect of correcting these errors in the consolidated financial statements was as follows (in thousands, except per share amounts):
| Balance Sheet | | December 31, 2008 | |
| | | As Reported | | | Adjustment | | | Revised | |
| Prepaid and other current assets | | $ | 501 | | | $ | 568 | | | $ | 1,069 | |
| Prior year reclassifications from inventories | | | 499 | | | | (499 | ) | | | - | |
| Prepaid and other current assets (as reclassified) | | $ | 1,000 | | | $ | 69 | | | $ | 1,069 | |
| | | | | | | | | | | | | |
| Current assets | | $ | 12,113 | | | $ | 69 | | | $ | 12,182 | |
| | | | | | | | | | | | | |
| Deferred income tax assets, non current | | $ | - | | | $ | 802 | | | $ | 802 | |
| | | | | | | | | | | | | |
| Total Assets | | $ | 25,779 | | | $ | 946 | | | $ | 26,725 | |
| | | | | | | | | | | | | |
| Current income taxes payable | | $ | 78 | | | $ | (78 | ) | | $ | - | |
| | | | | | | | | | | | | |
| Current liabilities | | $ | 2,317 | | | $ | (78 | ) | | $ | 2,239 | |
| | | | | | | | | | | | | |
| Deferred tax liabilities, non current | | $ | 531 | | | $ | (531 | ) | | $ | - | |
| | | | | | | | | | | | | |
| Total liabilities | | $ | 6,991 | | | $ | (609 | ) | | $ | 6,382 | |
| | | | | | | | | | | | | |
| Retained earnings | | $ | (3,982 | ) | | $ | 1,555 | | | $ | (2,427 | ) |
| | | | | | | | | | | | | |
| Total stockholders’ equity | | $ | 18,788 | | | $ | 1,555 | | | $ | 20,343 | |
| | | | | | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 25,779 | | | $ | 1,555 | | | $ | 26,725 | |
| Balance Sheet | | December 31, 2007 | |
| | | As Reported | | | Adjustment | | | Revised | |
| | | | | | | | | | |
| Prepaid expenses | | $ | 278 | | | $ | 65 | | | $ | 343 | |
| | | | | | | | | | | | | |
| Current assets | | $ | 11,709 | | | $ | 65 | | | $ | 11,774 | |
| | | | | | | | | | | | | |
| Deferred tax assets, net | | $ | 849 | | | $ | 1,082 | | | $ | 1,931 | |
| | | | | | | | | | | | | |
| Total Assets | | $ | 19,066 | | | $ | 1,147 | | | $ | 20,213 | |
| | | | | | | | | | | | | |
| Current liabilities | | $ | 1,703 | | | $ | - | | | $ | 1,703 | |
| | | | | | | | | | | | | |
| Deferred tax liabilities | | $ | 408 | | | $ | (408 | ) | | $ | - | |
| | | | | | | | | | | | | |
| Total liabilities | | $ | 2,429 | | | $ | (408 | ) | | $ | 2,021 | |
| | | | | | | | | | | | | |
| Retained earnings | | $ | (5,814 | ) | | $ | 1,555 | | | $ | (4,258 | ) |
| | | | | | | | | | | | | |
| Total stockholders’ equity | | $ | 16,637 | | | $ | 1,555 | | | $ | 18,192 | |
| | | | | | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 19,066 | | | $ | 1,147 | | | $ | 20,213 | |
| Statement of operations | | Year Ended December 31, 2007 | |
| | | As Reported | | | Adjustment | | | Revised | |
| | | | | | | | | | |
| Benefit (provision) for income taxes | | $ | (6 | ) | | $ | 1,555 | | | $ | 1,549 | |
| | | | | | | | | | | | | |
| Net income | | $ | 2,246 | | | $ | 1,555 | | | $ | 3,801 | |
| | | | | | | | | | | | | |
| Earnings per share - basic | | $ | 0.15 | | | $ | 0.10 | | | $ | 0.25 | |
| | | | | | | | | | | | | |
| Earnings per share - diluted | | $ | 0.13 | | | $ | 0.09 | | | $ | 0.22 | |
Cash and Cash Equivalents
Holdings of highly liquid investments with initial maturities of three months or less are considered to be cash equivalents.
Fair Values of Financial Instruments and Concentration of Credit Risk
The carrying amount of our financial instruments included in current assets and liabilities approximates fair value due to their short term nature. In addition, management believes the carrying balance of the “Due to Lican” approximates its fair value. Finally, we believe the book value of our note payable obligation approximates its fair values as the terms of such obligation approximates the terms at which similar types of borrowing arrangements could be currently obtained.
Financial instruments, which potentially subject us to significant concentrations of credit risk, consist primarily of cash and cash equivalents, and trade accounts receivable. With respect to cash, we frequently maintain cash and cash equivalent balances in excess of federally insured limits. We have not experienced any losses in such accounts.
With respect to receivables, our ten largest customers accounted for approximately 65%, 76% and 73% of trade receivables as of December 31, 2009, 2008 and 2007, respectively, and 71.5%, 70% and 71% of net revenues for the respective years then ended. In 2009, 2008 and 2007, Arthrex was our only customer that accounted for over 10% of total revenues, accounting for 22%, 20% and 21%, respectively of such revenues. All of these entities are customers of our U.S. Operations. We perform ongoing credit evaluations of our customers and generally do not require collateral because we believe we have procedures in place to limit potential for significant losses, and because of the nature of our customer base.
Accounts Receivable and Allowance for Doubtful Accounts
Our credit terms for our billings range from net 10 days to net 30 days, depending on the customer agreement. Accounts receivable are determined to be past due if payments are not made in accordance with such agreements and a reserve is created for them when they become three months past due or sooner if there are other indicators that the receivables may not be recovered. Customary collection efforts are initiated and receivables are written off when we determine they are not collectible and abandon these collection efforts. We gave negotiated sales volume discounts, which amounted to $533,541, $500,225 and $580,605 for 2009, 2008 and 2007, respectively. Sales are reported net of all discounts.
We evaluate the allowance for doubtful accounts on a regular basis for adequacy based upon our periodic review of the collectability of the receivables in light of historical experience, adverse situations that may affect our customers’ ability to pay, estimated value of any underlying collateral and prevailing economic conditions. This evaluation is inherently subjective, as it requires estimates that are susceptible to significant revision as more information becomes available. Substantially all of the receivables included in the accompanying balance sheets were recovered subsequent to the respective year ends. Because of this, and because historical losses on accounts receivable have not been material, management believes that the allowances for doubtful accounts of $20,000 and $8,645 at Decemb er 31, 2009 and 2008, respectively, are, or were, adequate to provide for possible bad debts.
Inventories and Repair Parts
Inventories are stated at the lower of average cost or market. Finished goods and work-in-process inventories include material, labor, and overhead costs. Factory overhead costs are allocated to inventory manufactured in-house based upon cost of materials.
We monitor usage reports to determine if the carrying value of any items should be adjusted due to lack of demand and adjust the inventory for estimated obsolescence (inventory judged to be unused in the manufacturing process for 2 years and eventually discarded) or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by management, additional inventory write-downs may be required.
Inventories at December 31, 2009 and 2008 were as follows:
| | | | | | | |
| | | 2009 | | | 2008 | |
| | | | | | | |
| Raw materials | | $ | 4,254,044 | | | $ | 3,368,939 | |
| Work in process | | | 1,944,266 | | | | 1,621,032 | |
| Finished goods | | | 1,116,893 | | | | 890,915 | |
| Gross inventories | | | 7,315,203 | | | | 5,880,886 | |
| Less: reserve for obsolescence | | | (541,037 | ) | | | (540,903 | ) |
| | | | | | | | | |
| Net inventories | | $ | 6,774,166 | | | $ | 5,339,983 | |
During 2009 the reserves and related costs of sales were increased by $15,174. In 2008 and 2007 the reserves and related cost of sales were reduced by $29,118 and $100,565 respectively as a result of changes in estimates regarding the recoverability of certain types of our inventory. There were no reserves for finished goods or work in progress.
Property and Equipment
These assets are recorded at cost. Depreciation and amortization are provided for using the straight-line method over the estimated useful lives of the assets. The amortization of leasehold improvements is based on the shorter of the lease term or the life of the improvement. Betterments and large improvements, which extend the life of the asset, are capitalized, whereas maintenance and repairs and small improvements are expensed as incurred. The estimated useful lives are: machinery and equipment, 3-10 years; buildings, 39 years; molds, 7-15 years and furniture and fixtures, 5-10 years.
Intangible Assets
These assets consist of licenses, purchased technology and brand name and trademarks. The licenses and purchased technology (other intangibles) are being amortized by the straight-line method over a 5-17 year period commencing with the date they were placed in service. Estimated aggregate amortization expense for the five years ending December 31, 2014 is expected to approximate $1,485,000. Brand name and trademark qualifies as an indefinite-lived intangible asset and is not subject to amortization; rather it is reviewed for impairment on an annual basis (see Long-Lived Assets)
Long-Lived Assets
We review our long-lived assets for recoverability if events or changes in circumstances indicate that the assets may have been impaired. In the event of impairment of any long-lived asset, the excess of the carrying amount over the fair value is recognized as an impairment loss. Any impairment losses are not restored in the future if the fair value increases. At December 31, 2009, we believe all of our long-lived assets are recoverable.
Restricted Cash
At December 31, 2009 and 2008, restricted cash of $35,635 and $1.3 million respectively, represents the amount of cash held in escrow related to the issuance of industrial revenue bonds. The remaining balance of these funds will be disbursed to us in 2010 with the final payment for the renovations to our new facility.
Revenue Recognition
Revenue is recognized when title has been transferred to the customer, which is generally at the time of shipment. The following policies apply to our major categories of revenue transactions:
| | · | Sales to customers are evidenced by firm purchase orders. Title and the risks and rewards of ownership are transferred to the customer when the product is shipped. Payment by the customer is due under fixed payment terms. |
| | · | Product returns are only accepted at our discretion and in accordance with our “Returned Goods Policy”. Historically, the level of product returns has not been significant. We accrue for sales returns, rebates and allowances based upon an analysis of historical customer returns and credits, rebates, discounts and current market conditions. |
| | · | Our terms of sale to customers generally do not include any obligations to perform future services. Limited warranties are generally provided for sales and provisions for warranty are provided at the time of product sale based upon an analysis of historical data. |
| | · | Amounts billed to customers related to shipping and handling are included in net sales. Shipping and handling costs included in cost of sales were $105,539, $118,891 and $124,424 in 2009, 2008 and 2007, respectively. |
We have no consignment inventory with customers but we do have inventory located at contract manufacturers that produce components for us. At December 31, 2009 and 2008, we had consigned work in progress of $936,085 and $527,906, respectively.
Advertising Costs
All advertising costs are expensed as incurred. The amounts of advertising costs were $215,588, $397,068, and $470,890 for 2009, 2008 and 2007, respectively.
Net Earnings Per Common Share
We compute basic earnings per share by dividing net income by the weighted average number of common shares outstanding for the reporting period. Diluted earnings per share gives effect to all potential dilutive shares outstanding (in our case, stock options) during the period.
Research and Development Costs
With the exception of development costs that are purchased from another enterprise and have alternative future use, research and development expenses are charged to operations as incurred.
Research and Development Costs for Others
For research and development activities that are partially or completely funded by other parties, and when the obligation is incurred solely to perform contractual services, expenses are charged to cost of sales and all revenues are shown as sales (see Note 13).
Income Taxes
The provision for income taxes includes federal, foreign, state and local income taxes currently payable and those deferred because of temporary differences between the financial statement and tax bases of assets and liabilities. Deferred tax assets or liabilities are computed based on the difference between the financial statement and income tax bases of assets and liabilities using enacted marginal tax rates. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. Deferred income tax expenses or credits are based on the changes in the asset or liability from period to period.
We have net operating loss and tax credit carry forwards available in certain jurisdictions to reduce future taxable income. Future tax benefits for net operating loss and tax credit carry forwards are recognized to the extent that realization of these benefits is considered more likely than not. This determination is based on the expectation that related operations will be sufficiently profitable or various tax, business and other planning strategies will enable us to utilize the operating loss and tax credit carry forwards. We cannot be assured that we will be able to realize these future tax benefits or that future valuation allowances will not be required. To the extent that available evidence raises doubt about the realization of a deferred income tax asset, a valuation allowance is established.
It is our policy to provide for uncertain tax positions and the related interest and penalties based upon management’s assessment of whether a tax benefit is more likely than not to be sustained upon examination by tax authorities. To the extent that the probable tax outcome of these uncertain tax positions changes, such changes in estimate will impact the income tax provision in the period in which such determination is made. At December 31, 2009, we believe we have appropriately accounted for any unrecognized tax benefits. To the extent we prevail in matters for which a liability for an unrecognized tax benefit is established or we are required to pay amounts in excess of the liability, our effective tax rate in a given financial statement period may be affected.
Since inception, we have been subject to tax by both federal and state taxing authorities. Until the respective statutes of limitations expire, we are subject to income tax audits in the jurisdictions in which we operate. We are no longer subject to U.S. federal tax examinations for fiscal years prior to 2005, and we are not subject to audits prior to the 2005 fiscal year for the majority of the state jurisdictions.”
Foreign Currency Transactions
The United States dollar is the functional currency of the Company’s operations in the United States and in line with determining guidance outlined in FASB ASC Topic 830, has also been determined to be the functional currency for the Company’s Canadian subsidiary. FASB ASC Topic 830 provides for using the remeasurement method in converting the foreign subsidiary’s financial statements into U.S. dollars. Monetary assets and liabilities denominated in foreign currency are converted at the current rate, while nonmonetary assets, liabilities, and shareholder equity accounts are converted at the appropriate historical rate. Revenue and expenses are converted at the weighted-average exchange rate for the period. FASB ASC Topic 830 requires any gain or loss as a result of remeasurement to be included in current pe riod income unless the investment in the subsidiary is not expected to be recovered in the foreseeable future. As our investment in the Canadian subsidiary is not expected to be recovered in the near future, we have reflected the net gains and losses from the remeasurement as other accumulated comprehensive loss in the accompanying 2009 and 2008 statements of stockholders’ equity and comprehensive income. The impact of remeasuring these accounts and balances were insignificant as of December 31, 2007.
Reclassifications
Certain amounts in the 2008 financial statements have been reclassified to conform to the current year presentation.
NOTE 3. TRADE ACCOUNTS RECEIVABLE
As of December 31, 2009 and 2008, trade accounts receivable were as follows:
| | | 2009 | | | 2008 | |
| Trade accounts receivable | | $ | 2,585,734 | | | $ | 3,000,118 | |
| Less: allowance for doubtful accounts | | | (20,000 | ) | | | (8,645 | ) |
| | | | | | | | | |
| Trade accounts receivable, net | | $ | 2,565,734 | | | $ | 2,991,473 | |
NOTE 4. PROPERTY, PLANT AND EQUIPMENT
As of December 31, 2009 and 2008, property, plant and equipment consisted of the following:
| | | 2009 | | | 2008 | |
| | | | | | | |
| Land | | $ | 1,600,000 | | | $ | 1,600,000 | |
| Equipment | | | 3,382,713 | | | | 2,992,694 | |
| Building and improvements | | | 5,017,586 | | | | 3,239,756 | |
| Furniture and fixtures | | | 1,700,568 | | | | 1,601,671 | |
| Leasehold improvements | | | 448,134 | | | | 443,853 | |
| Molds | | | 1,083,663 | | | | 964,813 | |
| | | | 13,232,664 | | | | 10,842,787 | |
| Less accumulated depreciation and amortization | | | (4,418,782 | ) | | | (3,716,844 | ) |
| | | | | | | | | |
| Net property, plant, and equipment | | $ | 8,813,882 | | | $ | 7,125,943 | |
NOTE 5. INTANGIBLE ASSETS
At December 31, 2009 and 2008, intangible assets consisted of the following:
| | | 2009 | | | 2008 | |
| | | | | | | |
| Trade name (life indefinite) | | $ | 1,509,662 | | | $ | 1,509,662 | |
| | | | | | | | | |
| Purchased technology (9-17 year lives) | | $ | 3,940,618 | | | $ | 3,940,618 | |
| Less accumulated amortization | | | (670,551 | ) | | | (460,866 | ) |
| | | | | | | | | |
| Net carrying amount | | $ | 3,270,067 | | | $ | 3,479,752 | |
| | | | | | | | | |
| | | | | | | | | |
| License rights (5 year life) | | $ | 315,619 | | | $ | 315,619 | |
| Less accumulated amortization | | | (163,070 | ) | | | (99,946 | ) |
| | | | | | | | | |
| Net carrying amount | | $ | 152,549 | | | $ | 215,673 | |
With respect to our trademark and brand name, we continue to market products, release new products and product extensions and maintain and promote these trademarks and brand names in the marketplace through legal registration and such methods as advertising, medical education and trade shows. It is our belief that these trademarks and brand names will generate cash flow for an indefinite period of time. Therefore, our trademarks and trade names intangible assets are not being amortized.
NOTE 6. FAIR VALUE MEASUREMENTS
Our assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2009 are measured in accordance with FASB ASC Topic 820-10-05, Fair Value Measurements (FASB 157). FASB ASC Topic 820-10-05 defines fair value, establishes a framework for measuring fair value and expands the disclosure requirements regarding fair value measurements for financial assets and liabilities as well as for non-financial assets and liabilities that are recognized or disclosed at fair value on a recurring basis in the financial statements.
The statement requires fair value measurement be classified and disclosed in one of the following three categories:
Level 1: Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
Level 2: Quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability; and
Level 3: Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e., supported by little or no market activity).
The following tables summarize our financial instruments as of December 31, 2009 and 2008 (in thousands):
| | | December 31, 2009 | |
| | | Fair Value Measurements | |
| | | Total | | | Level 1 | | | Level 2 | | | Level 3 | |
| Assets: | | | | | | | | | | | | |
| Cash and equivalents – United States | | $ | 2,237 | | | $ | 2,237 | | | $ | – | | | $ | – | |
| Cash and equivalents - Foreign currency | | | (82 | ) | | | (82 | ) | | | – | | | | – | |
| | | | | | | | | | | | | | | | | |
| Total | | $ | 2,155 | | | $ | 2,155 | | | $ | – | | | $ | – | |
| | | December 31, 2008 | |
| | | Fair Value Measurements | |
| | | Total | | | Level 1 | | | Level 2 | | | Level 3 | |
| Assets: | | | | | | | | | | | | |
| Cash and equivalents – United States | | $ | 2,497 | | | $ | 2,497 | | | $ | – | | | $ | – | |
| Cash and equivalents – Foreign currency | | | 67 | | | | 67 | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | |
| Total | | $ | 2,564 | | | $ | 2,564 | | | $ | – | | | $ | – | |
NOTE 7. RECENT ACCOUNTING PRONOUNCEMENTS
In January 2010, the FASB issued ASU 2010-06, Fair Value Measurements and Disclosures (Topic 820) – Improving Disclosures about Fair Value Measurements (“ASU 2010-06”), which amends Topic 820 to add new requirements for disclosures about transfers into and out of Levels 1 and 2 and separate disclosures about purchases, sales, issuances, and settlements related to Level 3 measurements. ASU 2010-06 also clarifies existing fair value disclosures about the level of disaggregation and about inputs and valuation techniques used to measure fair value. The ASU is effective for the first reporting period beginning after December 15, 2009, except for the requirements to provide the Level 3 activity of purchases, sales, issuances, and settlements on a gross basis, which will be effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. Early adoption is permitted
In September 2009, ASU 2009-13, Revenue Recognition (Topic 605) — Multiple-Deliverable Revenue Arrangements (“ASU 2009-13”) was issued and will change the accounting for multiple-deliverable arrangements to enable vendors to account for products or services (deliverables) separately rather than as a combined unit. Specifically, this guidance amends the criteria in Subtopic 605-25, Revenue Recognition-Multiple-Element Arrangements, for separating consideration in multiple-deliverable arrangements. This guidance establishes a selling price hierarchy for determining the selling price of a deliverable, which is based on: (a) vendor-specific objective evidence; (b) third-party evidence; or (c) estimates. This guidance also eliminates the residual method of alloc ation and requires that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method. In addition, this guidance significantly expands required disclosures related to a vendor’s multiple-deliverable revenue arrangements. ASU 2009-13 is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010 with early adoption permitted. We are currently evaluating the potential impact of this standard on our business, financial condition or results of operations.
In June 2009, the Financial Accounting Standards Board, or FASB, issued FASB Statement No. 168, The FASB Accounting Standards CodificationTM and the Hierarchy of Generally Accepted Accounting Principles – a replacement of FASB Statement No. 162, which was titled The Hierarchy of Generally Accepted Accounting Principles (the “Codification”). The Codification is effective for financial statements issued for interim and annual periods ending after September 15, 2009. The Codification is the single source of authoritative accounting principles recogn ized by the FASB to be applied by non-governmental entities in the preparation of financial statements in conformity with GAAP. Although the adoption of this statement did not materially affect our financial statements, the references to accounting literature within the notes to the condensed consolidated financial statements and elsewhere in this report conform to the Codification. For convenience, we have also included a corresponding parenthetical reference to the pre-Codification literature.
In June 2009, the FASB issued FASB ASC Topic 810-10-05, Amendments to FASB Interpretation No. 46R (SFAS 167). FASB ASC Topic 810-10-05 amends certain requirements of FASB Interpretation No. 46 (revised December 2003), Consolidation of Variable Interest Entities, to improve financial reporting by enterprises involved with variable interest entities and to provide more relevant and reliable information to users of financial statements. FASB ASC Topic 810-10-05 is effective for fiscal years beginning after November 15, 2009. Because we do not currently have any significant variable interests in unconsolidated entities, we do not anticipate that the adoption of this guidance will affect our consolidated financial statements.
In June 2009, the FASB issued FASB ASC Topic 860-10-05, Accounting for Transfers of Financial Assets, an amendment to SFAS No. 140 (SFAS 166). The new standard eliminates the concept of a “qualifying special-purpose entity,” changes the requirements for derecognizing financial assets and requires additional disclosures to enhance information reported to users of financial statements by providing greater transparency about transfers of financial assets, including securitization transactions, and an entity’s continuing involvement in and exposure to the risks related to transferred financial assets. FASB ASC Topic 860-10-05 i s effective for fiscal years beginning after November 15, 2009. We are evaluating the impact it will have on our consolidated financial statements.
In May 2009, the FASB issued FASB ASC Topic 855-10-05, Subsequent Events (SFAS 165). This standard is intended to establish general standards of accounting for and disclosures of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. Specifically, this standard sets forth the period after the balance sheet date during which management of a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements, the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements, and the disclosures that an entity should make about events or tran sactions that occurred after the balance sheet date. FASB ASC Topic 855-10-05 is effective for fiscal years and interim periods ending after June 15, 2009. We adopted this standard effective June 15, 2009 and have evaluated any subsequent events through the date of this filing. We do not believe there are any material subsequent events that would require further disclosure.
In December 2007, the FASB issued FASB ASC Topic 810-10-65, Non-controlling Interests in Consolidated Financial Statements, an amendment of ARB No. 51 (SFAS 160). FASB ASC Topic 810-10-65 will change the accounting and reporting for minority interests, which will be recharacterized as non-controlling interests and classified as a component of equity. This new consolidation method will significantly change the accounting for partial and/or step acquisitions. FASB ASC Topic 810-10-65 will be effective for us in the first quarter of fiscal year 2010. We do not believe adoption of this standard will have a material impact on our consolidated financial statements.
In April 2009, the FASB issued FASB ASC Topic 158-320-05 and 320-10-05, Recognition and Presentation of Other-Than-Temporary Impairments (FASB Staff Position, or FSP, No. FAS 115-2 and FAS 124-2), to amend the other-than-temporary impairment guidance in debt securities to be based on intent to sell instead of ability to hold the security and to improve the presentation and disclosure of other-than-temporary impairments on debt and equity securities in the financial statements. This pronouncement is effective for periods ending after June 15, 2009. We adopted this standard effective June 15, 2009, and it did not have a material impact on our consolidated financial position and results of operations .
In April 2009, the FASB issued FASB ASC Topic 820-10-05, Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly (FSP 157-4). FASB ASC Topic 820-10-05 provides additional authoritative guidance to assist both issuers and users of financial statements in determining whether a market is active or inactive, and whether a transaction is distressed. This pronouncement is effective for periods ending after June 15, 2009. We adopted this standard effective June 15, 2009, and it did not have a material impact on our consolidated financial position and results of operations.
In April 2009, the FASB issued FASB ASC Topic 270-10-05, Interim Disclosures about Fair Value of Financial Instruments (FSP FAS 107-1 and APB 28-1). FASB ASC Topic 270-10-05 enhances consistency in financial reporting by increasing the frequency of fair value disclosures. This guidance relates to fair value disclosures for any financial instruments that are not currently reflected on the balance sheet of companies at fair value. Before this guidance was adopted, fair values for these assets and liabilities were disclosed only once a year. The guidance now requires these disclosures to be made on a quarterly basis, providing qualitative and quantitative information about fair value estimates for all those financial instruments not m easured on the balance sheet at fair value. This pronouncement is effective for periods ending after June 15, 2009. We adopted this standard effective June 15, 2009, and it did not have a material impact on our consolidated financial position and results of operations.
In April 2009, the FASB issued FASB ASC Topic 805-10-10, Accounting for Assets Acquired and Liabilities Assumed in a Business Combination That Arise from Contingencies (SFAS 141(R)-1), to amend the provisions related to the initial recognition and measurement, subsequent measurement and disclosure of assets and liabilities arising from contingencies in a business combination under FASB ASC Topic 805-10-10, Business Combinations (SFAS 141(R)). Under this guidance, assets acquired and liabilities assumed in a business combination that arise from contingencies should be recognized at fair value on the acquisition date if fair value can be determined during the measurement period. If fair value cannot be determined, companies should typically account for the acquired contingencies using existing guidance. We do not believe adoption of FASB ASC Topic 805-10-10 will have a material impact on our consolidated financial statements.
NOTE 8. LINE OF CREDIT AND NOTE PAYABLE TO BANK
Line of Credit
On December 2, 2009 RBC Bank (USA) increased our secured revolving line of credit facility to $8 million from the previous $5 million and on the same date provided us with a separate additional credit facility for up to $1 million specific to financing new equipment purchases (although no amounts were drawn on this facility as of December 31, 2009). Advances under the $8 million line of credit are due on demand and bear interest at a rate of LIBOR plus 2% with a minimum floor rate of 4.0% and are secured by a perfected first security interest in our inventories, accounts receivable, and equipment.
The $1 million facility related to equipment purchases provides for a 2 year draw up period followed by a 5 year term period and bears interest also at LIBOR plus 2% with a minimum floor of 4% and will be secured by a perfected first security interest in the new equipment purchased. This equipment credit facility also allows the Company the option of financing purchased equipment at 75% of the cost through either a traditional loan or through RBC leasing at the time of purchase.
Subsequent available borrowings for both these credit facilities is subject to a borrowing base utilizing a percentage of eligible receivables, inventories, and any assigned cash along with certain financial ratios, specifically maintaining: a ratio of debt to tangible net worth of less than 2.0 to 1.0, a ratio of total funded debt to EBITDA of less than 3.25 to 1.0 excluding the industrial revenue bond note balance which had an original principal amount of $4.0 million, and a ratio of minimum debt service coverage of 1.5 to 1.0 measured on a rolling four quarter basis. At December 31, 2009 the Company was in full compliance with the loan covenants and ratios of both the credit facilities. According to our most recent borrowing base calculation we had approximately $4.0 million total available under the $8 million credit line, of which we have a current balance of $1.0 million, and we have available all of the $1 million under the equipment line of credit.
Mortgage Note Payable
In November 2008, we received $4.0 million from industrial revenue bonds issued through RBC Bank. These bonds, which have a current fixed interest rate of 4.6%, are repayable over a 20 year amortization period but mature after 10 years, with a balloon payment due at that time. The $4.0 million that was financed covered approximately $2.7 million of the $3 million purchase price for the facility plus approximately $1.3 million worth of the renovation costs to prepare the facility for our manufacturing needs and requirements. At December 31, 2009 approximately $35,000 of the $4.0 million was being held in escrow pending completion and final billing of the renovations. Scheduled maturities of this indebtedness are $135,000, $140,000, $145,000, $155,000 and $160,000 for 2010, 2011, 2012, 2013 and 2014 respectively. Th e balance on the mortgage as of December 31, 2009 is $3,875,000.
NOTE 9. TAXES AND NET OPERATING LOSS CARRYFORWARDS
Deferred income taxes reflect the impact of temporary differences between the amount of assets and liabilities recognized for financial reporting purposes and such amounts recognized for income tax purposes. The tax effects of these temporary differences representing the components of deferred tax assets (liabilities) at December 31 were approximatly as follows:
| | | 2009 | | | 2008 | |
| Deferred tax assets, current: | | | | | | |
| U.S. net operating loss carryforwards | | $ | 882,000 | | | $ | 937,000 | |
| Canadian net operating loss carryforwards | | | 570,000 | | | | 304,000 | |
| State net operating loss carryforwards | | | 102,000 | | | | 112,000 | |
| Research and development credits | | | 494,000 | | | | 409,000 | |
| AMT credits | | | 59,000 | | | | 32,000 | |
| Accounts receivable | | | 8,000 | | | | 3,000 | |
| Inventory Reserves | | | 215,000 | | | | 215,000 | |
| Accrued expenses | | | 74,000 | | | | 61,000 | |
| Unrecognized tax benefit liability for current temporary differences | | | (249,000 | ) | | | (257,000 | ) |
| Valuation allowance for Canadian loss carryforward | | | (570,000 | ) | | | (304,000 | ) |
| Non-current estimate of loss and credit carryforwards | | | (785,000 | ) | | | (1,295,000 | ) |
| Total deferred tax assets, current | | $ | 800,000 | | | $ | 217,000 | |
| | | | | | | | | |
| Deferred tax assets, non-current: | | | | | | | | |
| Loss and credit carryforwards | | $ | 785,000 | | | $ | 1,295,000 | |
| Stock based compensation | | | 31,000 | | | | 13,000 | |
| Total deferred tax assets, non- current | | | 816,000 | | | | 1,308,000 | |
| | | | | | | | | |
| Deferred tax liabilities, non-current: | | | | | | | | |
| Property and equipment | | | (383,000 | ) | | | (258,000 | ) |
| Intangibles | | | (236,000 | ) | | | (87,000 | ) |
| Unrecognized tax benefit liability for non-current temporary differences | | | (38,000 | ) | | | (161,000 | ) |
| Total deferred tax liabilities, non- current | | | (657,000 | ) | | | (506,000 | ) |
| | | | | | | | | |
| Net deferred tax asset, non-current | | $ | 159,000 | | | $ | 802,000 | |
Pursuant to ASC 740, we must consider all positive and negative evidence regarding the realization of deferred tax assets, including past operating results and future sources of taxable income. Under the provisions of ASC 740, we determined that the entire net deferred tax asset related to the Canadian net operating loss needed to be reserved given a lack of historical earnings at that location. Our remaining U.S. net operating losses expire in years beginning in 2018.
We have adopted the provisions of FIN 48, now under ASC 740. Under ASC 740, the impact of an uncertain tax position taken or expected to be taken on an income tax return must be recognized in the financial statements at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized in the financial statements unless it is more likely than not of being sustained. All of our positions arise from taxable temporary differences and as such the liability has been recognized in the net deferred tax asset, current and non-current items to which the relate. The calculated amount of penalties and interest related to these timing differences were immaterial at December 31, 2009 and 2008. In addition, because the amounts are related to temporary timing differences there would be no material impact on our effective tax rate if recognized.
Below is a reconciliation of the statutory federal income tax rate to our effective tax rate for the fiscal years ended December 31,
| | | 2009 | | | 2008 | | | 2007 | |
| | | | | | | | | | |
| Federal tax provision | % | | 34.0 | | | | 34.0 | | | | 34.0 | |
| State taxes (net of federal benefit) | | | 2.6 | | | | 5.8 | | | | 5.8 | |
| Stock based compensation | | | (15.9 | ) | | | - | | | | (24.1 | ) |
| Research and development credits | | | (12.8 | ) | | | (2.6 | ) | | | (7.5 | ) |
| Other | | | 2.3 | | | | (3.2 | ) | | | (1.0 | ) |
| Valuation allowance | | | - | | | | - | | | | (76.0 | ) |
| | % | | 10.2 | | | | 34.0 | | | | (68.8 | ) |
At December 31, 2006, management believed there was a risk that substantially all of our net operating losses might not be realizable and, accordingly, a valuation allowance was recorded against them. During the year ended December 31, 2007, management determined that such valuation allowances were no longer necessary, and accordingly, the valuation allowances were reversed, resulting in a benefit for income taxes being recorded for the anticipated utilization.
NOTE 10. RETIREMENT PLAN
The Company provides a tax-qualified profit-sharing retirement plan under section 401k of the Internal Revenue Code for the benefit of eligible employees with an accumulation of funds for retirement on a tax-deferred basis and provides for annual discretionary contribution to individual trust funds.
All employees are eligible to participate. The employees may make voluntary contributions to the plan up to the maximum percentage allowed by the Internal Revenue Code. Vesting in employee matching contributions is graded and depends on the years of service. After three years from their date of hire, the employees are 100% vested. The Company makes matching contributions of 50% of the employee contributions up to a total of 3% of participant payroll. During 2009 the Company’s management suspended the matching contribution as a cost cutting measure.
The Company’s contributions and expense during 2009, 2008 and 2007 approximated $6,960, $167,000 and $149,000, respectively.
NOTE 11. OTHER RELATED PARTY TRANSACTIONS
A director and former officer of Bovie, is the founder and principal of Lican Developments LTD (“Lican”), an Ontario, Canada Corporation.. During 2009 Lican was paid $50,000 related to an installment payment from an October 2006 purchase agreement. The remaining amounts owed under this arrangement are reflected as a Due to Lican in our consolidated financial statements. Lican may in the future also receive ongoing royalties ranging from 2.5% to 3% of sales of certain products.
Compensation of Non-Employee Directors
During the year ended December 31, 2009, we granted 55,000 stock options having a fair value of approximately $192,500 to our independent directors as consideration for their service on our Board of Directors.
Professional Services
A director of Bovie, is president and a shareholder of Ronin Consulting Group, Inc., a company which provided various financial and analytical project consulting services to Bovie. Ronin Consulting Group, Inc. was paid fees approximating $99,800 and $72,400 during 2009, and for the portion of 2008 he was a director, respectively.
A director of Bovie, is the principal owner of Medical Education Associates, LLC, which provided medical field consulting services to Bovie. Medical Education Associates, LLC was paid fees approximating $40,000 since he became a director in April 2009.
Starting November 1, 2009, Lican provided research and development consulting in the continued development of our Seal-N-Cut product line and was paid consulting approximating $21,000..
NOTE 12. COMMITMENTS AND CONTINGENCIES
Property and Rental Agreements
The Company owns its main facility in St. Petersburg, but is also obligated under various operating leases for a manufacturing and warehouse facility in St. Petersburg, Florida (which lease requires monthly payments of approximately $12,400, and expires on October 31, 2013), a separate warehouse facility in St Petersburg (under a month to month arrangement requiring monthly payments of approximately $2,400), its Windsor, Canada facility (which lease requires monthly payments of approximately $2,400 through December 31, 2010) and its executive offices in New York (under a month to month arrangement requiring monthly payments of approximately $1,500). The following is a schedule of approximate future minimum lease payments under operating leases as of December 31, 2009 and assuming the renewal of all month to month leases:
| 2010 | | $ | 278,100 | |
| 2011 | | | 251,900 | |
| 2012 | | | 246,800 | |
| 2013 | | | 223,100 | |
| 2014 | | | 11,000 | |
| | | | | |
| Total | | $ | 1,010,900 | |
Rent expense for the years ended December 31, 2009, 2008 and 2007 approximated $283,300, $280,300 and $283,100, respectively.
Purchase Commitments
At December 31, 2009, we had non-cancelable purchase commitments for inventories totaling approximately $3.8 million, substantially all of which is expected to be paid by mid 2010.
Employment Agreements
At December 31, 2009, the Company is obligated under employment agreements with four employees which have expiration dates between June 2012 and January 2014. Approximate future minimum payments under these agreements are as follows as of December 31, 2009:
| 2010 | | $ | 811,000 | |
| 2011 | | | 865,000 | |
| 2012 | | | 871,000 | |
| 2013 | | | 881,000 | |
| 2014 | | | 73,000 | |
| | | | | |
| Total | | $ | 3,501,000 | |
During 2009, three of these agreements requiring annual base compensation of approximately $725,000 were automatically extended through January 2014. The employees also are eligible to receive bonuses and certain medical and other benefits. In addition, the agreements with our Chief Operating Officer, President, and Chief Sales and Marketing Officer contain the following:
| | · | Clauses that allow for continuous automatic extensions of one year unless timely written notice terminating the contract is provided to such officers (as defined in the agreements). |
| | · | Clauses which require the Company to make lump sum payments to such officers equal to three times their salary and bonus in effect at the time of any change in control and/or breach of the agreements by the Company. The 2010 base salaries for these officers are expected to approximate $700,000, and such amounts increase by 7.5% per year. |
Litigation
In 2008, Erbe USA, Inc. (“Erbe”) filed a civil action in the U.S. District Court for the Northern District of Georgia, Atlanta Division, against Bovie and a former employee, seeking equitable relief and unspecified damages. The complaint essentially alleges that the employee, among other things, breached his employment agreement with Erbe by wrongfully taking Erbe’s confidential information and trade secrets for use in his new employment position, with the assistance of Bovie. In a mutual effort to resolve the dispute, on November 4, 2009, Bovie and Erbe signed a full and final settlement agreement and mutual general release of all claims. We continue to deny Erbe’s claims and allegations. Given that both parties desire to end the litigation and mitigate ongoing l egal costs, however, we agreed to pay Erbe $160,000 as part of the terms of the settlement. We also agreed not to use or disclose, and to destroy, any information that Erbe alleged constituted trade secrets and confidential business information related to Erbe. Additional terms of the settlement include a two-year period in which we agreed not to solicit (a) Erbe’s current employees and (b) a limited number of dealers and independent representatives who currently market Erbe products.
NOTE 13. RESEARCH AND DEVELOPMENT PERFORMED FOR OTHERS
Bovie has entered into several manufacturing and development agreements to produce electrosurgical products for medical equipment companies. The agreements are considered Original Equipment Manufacturing (OEM) contracts that call for: (1) Bovie to develop specific use devices and components (2) the customer is not committed to a certain dollar amount of purchases and (3) Bovie charges what it believes will be its costs for the development of the product. If the customer rejects or terminates the contract, it forfeits the development payments incurred prior to termination. The customer must fulfill its agreement if Bovie delivers its working prototypes on a timely basis.
The following is research and development revenue and costs related to OEM contracts for 2009, 2008 and 2007:
Contracted Development Payments Received:
| | | 2009 | | | 2008 | | | 2007 | |
| | | | | | | | | | |
| Revenues included in sales revenue | | $ | - | | | $ | - | | | $ | 126,098 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Cost of OEM research and development contracts included in costs of sales | | $ | - | | | $ | - | | | $ | 45,860 | |
NOTE 14. PURCHASE OF MINORITY INTEREST IN JOINT VENTURE
In May 2007, we acquired the remaining 50% interest in JAG (previously our J-Plasma joint-venture) for total consideration of $500,000, resulting in us having 100% ownership of the medical device technology. We recorded the $500,000 investment, as well as certain direct costs incident to the acquisition and the reversal of the remaining balance of our minority interest ($115,000) as an increase in “Purchased technology”.
NOTE 15. GAIN FROM CONTRACT SETTLEMENT
On April 29, 2008 we signed an agreement with Boston Scientific Corporation to acquire technology, patents, and assets related to the use of conductive sintered steel as an electrode for radio frequency cutting and coagulation, intended to lower blood loss, quicken procedure times and provide cost savings for hospitals. The original development and manufacturing agreement signed in 2007 required us to develop and manufacture certain products using Boston Scientifics’ intellectual property, with which we complied. Boston Scientific terminated the original agreement and through the contract settlement negotiations we acquired the ownership rights to the intellectual property and equipment in consideration for releasing Boston Scientific from any further obligations as outlined in the original development and manufacturing agreement . A new agreement was signed in place of the previous distribution and marketing agreement between the companies for the technology’s use in Boston Scientifics’ oncology business. As part of this new agreement, we granted a limited license to Boston Scientific until 2016 for uses outside of our intended fields listed above, which management feels has no impact on the fair value of the asset received.
Management believed at the time of the transaction that the nonmonetary exchange had ‘commercial substance’, meaning there was an expectation that the future cash flows of the company would change significantly as a result of the exchange. The presence of ‘commercial substance’ determines that the measure of value to be utilized for transactions is fair value. Due to the fact that there was a degree of urgency in the transaction, namely the immediate need for Boston Scientific to terminate the contract due to some internal restructuring, one could not rely on it being an ‘arms length’ transaction. Management first conducted a thorough valuation of the non-monetary exchange and then engaged two independent third party appraisers to ensure that management’s valuation was within reasonable limits. These third party appraisers utilized the three general approaches to appraising assets: the market approach, the cost approach, and the income approach.
Management determined the fair value of the patent a few different ways, first by conducting an analysis of the costs to reproduce, second by reviewing similar market transactions, and lastly modeling expected future cash flows of the patent. Each approach to fair value measurement was analyzed based on the level of inputs and compared against the fair value hierarchy. The income approach utilized numerous Level 3 inputs in its determination of value, including rates of return and projected cash flow. The market approach utilized Level 2 inputs for market rates of royalties on similar technologies. The cost approach was considered, but excluded from the fair value determination as the cost to reproduce the patent does not adequately represent the value of the patent.
Management believes that the resulting valuations were within reasonable limits because the multiple probability weightings reduced the likelihood of uncertainties to the asset valuation and soon after the transaction, we generated revenues and began discussions for possible distribution agreements which allowed us to determine that our estimate of fair value was within reasonable limits when reviewing our projected sales. Management used revenue projections from Boston Scientific, discussions with the inventor (a Bovie employee) as to the market and any competitors, and research and projections from Bovie’s sales and marketing department. Management then reviewed the work of the third party appraisers to validate that the amount calculated was within reasonable limits. Management was responsible for calculating and re cording a gain of approximately $1,496,000 based on the fair values of the assets we received (i.e. intellectual property and molds of approximately $1,456,000 and $40,000, respectively).
Management utilized in its multiple probability weightings:
| | · | Number of procedures ranging from 30 to 70% of the market (assumed growth rate ranging from 2% to 7%) |
| | · | Adoption Rate ranging from 1% to 5% |
| | · | Average sales price of $800 based upon the current market price, which we were generating revenue at, with an assumed 5% growth rate |
| | · | Capital investment ranges from $500,000 to $1,100,000 |
| | · | Discount rate ranges from 10% to 30% (includes Risk Free rate, adjusted equity risk premium, risk premium for size and risk premium for Company specific risk factors) |
| | · | SEER device market opportunity projected revenues provided by Boston Scientific. |
Third Party appraisers utilized professional data from various sources as Capital IQ, Hoovers Online, OneSource, and Compustat Research Insight database from Standard & Poor’s, along with the SEER device market opportunity projected revenues provided by Boston Scientific.
NOTE 16. STOCK OPTIONS
On October 30, 2007, shareholders approved and the Board of Directors adopted an amendment to the 2003 Executive and Employee Stock Option Plan (the “Plan”) to increase the maximum aggregate number of shares of common stock reserved for issuance under the Plan from 1.2 Million shares (already reserved against outstanding options) to 1.7 Million shares. Except for the increase in the number of shares covered by the Plan, the Plan remained otherwise unchanged. In 2001, the Board of Directors adopted the 2001 Executive and Employee Stock Option Plan which reserved for issuance 1,200,000 stock options. Stock options typically have a ten year life and currently vest over a seven year period.
As of December 31, 2009, there was approximately $616,000 of total unrecognized compensation costs related to outstanding stock options, which is expected to be recognized over a period of 5 years.
The status of our stock options and stock awards are summarized as follows:
| | | | | | Weighted Average Exercise Price | |
| | | | | | | |
| Outstanding at December 31, 2007 | | | 3,148,400 | | | $ | 1.83 | |
| | | | | | | | | |
| Granted | | | 207,500 | | | $ | 7.29 | |
| Exercised | | | (1,488,750 | ) | | $ | 0.81 | |
| Canceled | | | - | | | | - | |
| Outstanding at December 31, 2008 | | | 1,867,150 | | | $ | 3.25 | |
| | | | | | | | | |
| Granted | | | 85,500 | | | $ | 8.17 | |
| Exercised | | | (183,250 | ) | | $ | 1.86 | |
| Cancelled | | | (27,875 | ) | | $ | 7.18 | |
| Outstanding at December 31, 2009 | | | 1,741,525 | | | $ | 3.61 | |
| | | | | | | | | |
| Exercisable at December 31, 2009 | | | 1,452,257 | | | $ | 2.87 | |
The following table summarizes information about our options outstanding at December 31, 2009:
| Exercise Prices | | | Number Outstanding | | Weighted Average Remaining Contractual Life | | Exercise Price | | | Options Exercisable | | | Exercise Price | |
| 0.50 | | | | 170,700 | | 2 years | | | .50 | | | | 170,700 | | | | .50 | |
| 0.70 | | | | 60,000 | | 4 years | | | .70 | | | | 60,000 | | | | .70 | |
| 0.75 | | | | 21,500 | | 2 – 4 years | | | .75 | | | | 21,500 | | | | .75 | |
| 1.30 | | | | 30,000 | | 4 years | | | 1.30 | | | | 30,000 | | | | 1.30 | |
| 2.13 | | | | 150,000 | | 5 years | | | 2.13 | | | | 150,000 | | | | 2.13 | |
| 2.25 | | | | 347,500 | | 6 years | | | 2.25 | | | | 345,000 | | | | 2.25 | |
| 2.41 | | | | 40,000 | | 5 years | | | 2.41 | | | | 40,000 | | | | 2.41 | |
| 2.93 | | | | 35,000 | | 6 years | | | 2.93 | | | | 35,000 | | | | 2.93 | |
| 2.95 | | | | 2,500 | | 5 years | | | 2.95 | | | | 2,500 | | | | 2.95 | |
| 3.25 | | | | 361,700 | | 4 years | | | 3.25 | | | | 361,700 | | | | 3.25 | |
| 3.26 | | | | 100,000 | | 6 years | | | 3.26 | | | | 100,000 | | | | 3.26 | |
| 6.93 | | | | 20,000 | | 7 years | | | 6.93 | | | | 20,000 | | | | 6.93 | |
| 7.10 | | | | 12,125 | | 9 years | | | 7.10 | | | | 8,572 | | | | 7.10 | |
| 7.18 | | | | 50,000 | | 10 years | | | 7.18 | | | | 50,000 | | | | 7.18 | |
| 7.33 | | | | 147,500 | | 10 years | | | 7.33 | | | | 21,071 | | | | 7.33 | |
| 7.68 | | | | 7,500 | | 9 years | | | 7.68 | | | | 2,142 | | | | 7.68 | |
| 8.66 | | | | 100,000 | | 9 years | | | 8.66 | | | | 28,572 | | | | 8.66 | |
| 6.60 | | | | 5,500 | | 10 years | | | 6.60 | | | | 5,500 | | | | 6.60 | |
| 8.32 | | | | 72,500 | | 10 years | | | 8.32 | | | | - | | | | 8.32 | |
| 7.85 | | | | 7,500 | | 10 years | | | 7.85 | | | | - | | | | 7.85 | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| | | | | 1,741,525 | | | | | | | | | 1,452,257 | | | | | |
The number and weighted average grant-date fair values of options non-vested at the beginning and end of 2009, as well as options granted, vested and forfeited during the year was as follows:
| | | Number Of Options | | | Weighted Average Exercise Prices | |
| Nonvested at January 1, 2009 | | | 294,357 | | | | 7.57 | |
| Granted in 2009 | | | 85,500 | | | | 8.17 | |
| Vested in 2009 | | | (62,714 | ) | | | 7.12 | |
| Forfeited in 2009 | | | (27,875 | ) | | | 7.18 | |
| | | | | | | | | |
| Nonvested at December 31, 2009 | | | 289,268 | | | | 7.88 | |
Common shares required to be issued upon the exercise of stock options and warrants would be issued from our authorized and unissued shares.
The grant date fair value of options granted in 2009 were estimated on the grant date using a binomial lattice option-pricing model and the following assumptions: expected volatility of 40% - 45%, expected term of 7 years, risk-free interest rate of 0% - 2.74%, and expected dividend yield of 0%.
The grant date fair value of options granted in 2008 were estimated on the grant date using a binomial lattice option-pricing model and the following assumptions: expected volatility of 30%, expected term of 7 years, risk-free interest rate of 2.7%, and expected dividend yield of 0%.
The grant date fair value of options granted in 2007 were estimated on the grant date using a binomial lattice option-pricing model and the following assumptions: expected volatility of 25%, expected term of 5 years, risk-free interest rate of 5.0%, and expected dividend yield of 0%.
Expected volatility is based on a weighted average of the historical volatility of the Company's stock and peer company volatility. The average expected life was calculated using the simplified method under SAB 107. The risk-free rate is based on the rate of U.S. Treasury zero-coupon issues with a remaining term equal to the expected life of the options. The Company uses historical data to estimate pre-vesting forfeiture rates.
Allocation of stock based compensation expense for the fiscal years ended December 31, 2009, 2008 and 2007 was as follows:
| | | 2009 | | | 2008 | | | 2007 | |
| | | | | | | | | | |
| Cost of sales | | $ | 29,931 | | | $ | 16,294 | | | $ | 36,185 | |
| Research and development | | | 32,240 | | | | 115,344 | | | | 10,072 | |
| Salaries and related costs | | | 74,214 | | | | 53,060 | | | | 25,832 | |
| | | | | | | | | | | | | |
| Total | | $ | 136,385 | | | $ | 184,698 | | | $ | 72,089 | |
NOTE 17. GEOGRAPHIC AND SEGMENT INFORMATION
The Company has two reportable business segments, our main operations, Bovie Medical Corporation located in the United States and Bovie Canada, our Canada operations located in Windsor, Canada. Because Bovie Canada operations represented a loss greater than 10% of our consolidated net income (on an absolute value basis) we are required to report certain information broken out by segment in the table listed below for the years ended December 31, 2009, 2008, and 2007 (in thousands).
| | | Bovie Medical Corp | | | Bovie Canada | | | Bovie Medical Corp | | | Bovie Canada | | | Bovie Medical Corp | | | Bovie Canada | |
| | | 2009 | | | 2009 | | | 2008 | | | 2008 | | | 2007 | | | 2007 | |
| | | | | | | | | | | | | | | | | | | |
| Sales, net | | $ | 26,768 | | | $ | 185 | | | $ | 27,441 | | | $ | 656 | | | $ | 28,432 | | | $ | 347 | |
| Gross profit | | $ | 11,713 | | | $ | 142 | | | $ | 11,781 | | | $ | 68 | | | $ | 11,569 | | | $ | (253 | ) |
| Operating expenses | | $ | 10,298 | | | $ | 842 | | | $ | 9,555 | | | $ | 1,003 | | | $ | 8,716 | | | $ | 488 | |
| Net income (loss) | | $ | 1,295 | | | $ | (700 | ) | | $ | 2,767 | | | $ | (935 | ) | | $ | 4,542 | | | $ | (741 | ) |
NOTE 18. SELECTED QUARTERLY INFORMATION (UNAUDITED)
The following table sets forth certain unaudited quarterly data for each of the four quarters in the years ended December 31, 2009, and 2008. The data has been derived from the Company's unaudited consolidated financial statements that, in management's opinion, include all adjustments (consisting of normal recurring adjustments) necessary for a fair presentation of such information when read in conjunction with the Consolidated Financial Statements and Notes thereto. The results of operations for any quarter are not necessarily indicative of the results of operations for any future period.
| | | First | | | Second | | | Third | | | Fourth | |
| Year ended December 31, 2009 | | Quarter | | | Quarter | | | Quarter | | | Quarter | |
| Total revenue | | $ | 7,217 | | | $ | 6,832 | | | $ | 6,371 | | | $ | 6,533 | |
| Gross profit | | $ | 3,320 | | | $ | 2,981 | | | $ | 2,760 | | | $ | 2,794 | |
| Net income | | $ | 403 | | | $ | 207 | | | $ | (38 | ) | | $ | 23 | |
| Diluted earnings per share (1) | | $ | 0.02 | | | $ | 0.01 | | | $ | 0.00 | | | $ | 0.00 | |
| Year ended December 31, 2008 | | | | | | | | | | | | | | | | |
| Total revenue | | $ | 6,678 | | | $ | 6,985 | | | $ | 7,296 | | | $ | 7,138 | |
| Gross profit | | $ | 2,586 | | | $ | 2,900 | | | $ | 3,233 | | | $ | 3,130 | |
| Net income | | $ | 190 | | | $ | 1,205 | | | $ | 366 | | | $ | 71 | |
| Diluted earnings per share (1) | | $ | .01 | | | $ | .08 | | | $ | .02 | | | $ | .00 | |
| (1) | Quarterly income (loss) per share may not equal the annual reported amounts. |
| Exhibit 10.1* | | Original Equipment Manufacturer Agreement between Arthrex, Inc. and Bovie Medical Corp. dated as of June, 2002. *** |
| Exhibit 10.2* | | Consulting and Intellectual Property Assignment Agreement dated January 12, 2006 among Bovie, Henvil Corp. Ltd and Steve Livneh. |
| Exhibit 10.3* | | Distribution Agreement between Bovie Medical Corporation and Boston Scientific dated October 6, 2006 amended and as re-filed, inclusive of Exhibit A..** |
| Exhibit 10.4* | | First Amendment to Distribution Agreement between Boston Scientific Corporation and Bovie Medical Corporation August 23, 2007, as re-filed. ** |
| Exhibit 10.5* | | Termination Purchase and License Agreement between Boston Scientific Corporation and Bovie Medical Corporation dated April 29, 2008 as amended and re-filed, inclusive of Exhibit A.** |
| Exhibit 10.6* | | Asset Purchase Agreement dated as of October 2, 2006 between Bovie Medical Corporation and Lican Developments, Ltd as re-filed, inclusive of Exhibit A, B, C and D. |
| Exhibit 10.7* | | First Amendment to Manufacturing and Development Agreement dated August 24, 2007 between Bovie Medical Corporation and Arthrex, Inc. ** |
| Exhibit 10.8* | | First Amendment to OEM Agreement between Arthrex, Inc. and Bovie Medical Corp. dated as of July, 2007. |
| Exhibit 10.9* | | Amended Employment Agreement dated January 15, 2006 between Bovie Medical Corporation and Andrew Makrides. |
| Exhibit 10.10* | | Amended Employment Agreement dated January 15, 2006 between J. Robert Saron and Bovie Medical Corporation. |
| Exhibit 10.11* | | Amended Employment Agreement dated January 15, 2006 between Moshe Citronowicz and Bovie Medical Corporation.. |
| Exhibit 10.12* | | Employment Agreement dated June 18, 2007 between Bovie Medical Corporation and Gary Pickett. |
| Exhibit 10.13* | | Employment Agreement dated October 2, 2006 between Steve Livneh and Bovie Medical Corporation. |
| Exhibit 10.14* | | Amendment to Consulting and Intellectual Property Assignment Agreement dated June 22, 2006 among Bovie, Henvil Corp. Ltd and Steve Livneh. |
Exhibit 10.15**** Exhibit 10.16***** | | Employment Agreement dated as of March 2, 2010 Modification Agreement dated December 2, 2009 |
| | Certification pursuant to Section 302 of Sarbanes-Oxley Act of 2002. |
| | Certification pursuant to Section 302 of Sarbanes-Oxley Act of 2002. |
| | Certification pursuant to Section 906 of Sarbanes-Oxley Act of 2002. |
| | Certification pursuant to Section 906 of Sarbanes-Oxley Act of 2002. |
* Previously filed report form 10-K/A filed with commission November 30, 2009.
**Subject to a confidential treatment application made by the Company.
*** Subject to a previous Confidential Treatment application which has been withdrawn; and agreement is re-filed in its entirety without redactions.
**** Previously filed report form 8K with commission on March 8, 2010.
***** Previously filed report form 8K with commission on March 15, 2010
F-25