UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year ended December 31, 2008
OR
¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Transition Period From to
Commission file number 0-3338
MILLENNIUM BIOTECHNOLOGIES GROUP, INC.
(Exact Name of Registrant as Specified in its Charter)
| | Delaware | 22-1558317 | |
| | (State or other Jurisdiction of | (IR..S Employer | |
| | Incorporation or Organization) | Identification No.) | |
| | | | |
| | 665 Martinsville Road, Suite 219 Basking Ridge, NJ | 07920 | |
| | (Address of Principal Executive Offices) | (Zip Code) | |
(908) 604-2500
(Registrant’s telephone number including area code)
Securities registered pursuant to Section 12(b) of the Act: None
| Title of each class | | Name of each exchange on which registered |
| | | |
| None | | |
| | | |
| | | |
Securities registered pursuant to Section 12(g) of the Act:
(Title of class)
Common Stock, $.001 par value
| Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. | o Yes o No |
| Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. | o Yes o No |
Note - Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Exchange Act from their obligations under those Sections.
| Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. | x Yes o No |
| Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T(§229.405 of this chapter) during the preceeding 12 months (or for such shorter period that the registrant was required to submit and post such files). | o Yes o No |
| Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. | o |
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer o | Accelerated filer o |
| | |
Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). o Yes x No
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.
The Registrant’s revenues for the fiscal year ended December 31, 2008, were $1,492,262.
Common stock, par value $.001 per share (“Common Stock”), was the only class of voting stock of the Registrant outstanding on March 31, 2008. Based on the closing price of the Common Stock on the OTC Electronic Bulletin Board as reported on March 31, 2009, ($0.01), the aggregate market value of the 16,546,901 shares of the Common Stock held by persons other than officers, directors and persons known to the Registrant to be the beneficial owners (as the term is defined under the rules of the Securities and Exchange Commission) of more than five percent of the Common Stock on March 31, 2009, was approximately $165,469. By the foregoing statements, the Registrant does not intend to imply that any of the officers, directors, or beneficial owners are affiliates of the registrant or that the aggregate market value, as computed pursuant to rules of the Securities and Exchange Commission, is in any way indicative of the amount which could be obtained for such shares of Common Stock.
As of March 31, 2009, 257,062,363 shares of Common Stock, $0.001 par value, 65,141 shares of Series B Convertible Preferred Stock, $1.00 par value, and 64,763 shares of Series C Cumulative Preferred Stock, $1.00 par value, were outstanding.
Note - If determination as to weather a particular person or entity is an affiliate cannot be made without involving unreasonable effort and expense, the aggregated market value of the common stock held by non-affiliates may be calculated on the basis of assumptions reasonable under the circumstance, provided that the assumptions are set forth in this Form.
DOCUMENTS INCORPORATED BY REFERENCE: SEE EXHIBIT INDEX
Transitional Small Business Disclosure Format: Yes ¨ No x
MILLENNIUM BIOTECHNOLOGIES GROUP, INC.
CONTENTS
| | | Page |
| | | |
| PART I. | | |
| | | |
| Item 1. | Business | 2 |
| | | |
| Item 1.A. | Risk Factors | 20 |
| | | |
| Item 2. | Properties | 24 |
| | | |
| Item 3. | Legal Proceedings | 24 |
| | | |
| Item 4. | Submission of Matters to a Vote of Security Holders | 24 |
| | | |
| PART II. | | |
| | | |
| Item 5. | Market for Registrant's Common Equity and Related Stockholder Matters | 25 |
| | | |
| Item 6. | Selected Financial Data | 27 |
| | | |
| Item 7. | Management’s' Discussion and Analysis of Financial Condition | |
| | and Results of Operations | 28 |
| | | |
| Item 7.A. | Quantitative and Qualitative Disclosures about Market Risks | 29 |
| | | |
| Item 8. | Financial Statements | 29 |
| | | |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and | |
| | Financial Disclosure | 29 |
| | | |
| Item 9.A. | Controls and Procedures | 29 |
| | | |
| Item 9.B. | Other Information | 30 |
| | | |
| PART III. | | |
| | | |
| Item 10. | Directors, Executive Officers, Promoters and Control Persons; | |
| | Compliance with Section 16(a) of the Exchange Act | 31 |
| | | |
| Item 11. | Executive Compensation | 34 |
| | | |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management | |
| | and Related Stockholder Matters | 35 |
| | | |
| Item 13. | Certain Relationships and Related Transactions | 36 |
| | | |
| Item 14. | Principal Accountant Fees and Services | 37 |
| | | |
| PART IV. | | |
| | | |
| Item 15. | Exhibits | 38 |
| | | |
| | Signatures | 39 |
| | | |
| | Exhibit Index | 38 |
CAUTIONARY STATEMENT PURSUANT TO "SAFE HARBOR" PROVISIONS OF SECTION 21E OF THE SECURITIES EXCHANGE ACT OF 1934
Except for historical information, the Company's reports to the Securities and Exchange Commission on Form 10-KSB and Form 10-Q and 10-QSB and periodic press releases, as well as other public documents and statements, contain "forward-looking statements" within the meaning of Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the statements. These risks and uncertainties include general economic and business conditions, development and market acceptance of the Company’s products, current dependence on the willingness of investors to continue to fund operations of the Company and other risks and uncertainties identified in the Company's reports to the Securities and Exchange Commission, periodic press releases, or other public documents or statements.
Readers are cautioned not to place undue reliance on forward-looking statements. The Company undertakes no obligation to republish or revise forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrences of unanticipated events.
PART I
The Company
Millennium Biotechnologies Group, Inc. (the Company or “Millennium Group”), formerly Regent Group, Inc., is a holding company for its subsidiary Millennium Biotechnologies, Inc. (“Millennium”).
Millennium was incorporated in the State of Delaware on November 9, 2000, and is located in New Jersey. Millennium is a research based bio-nutraceutical corporation involved in the field of nutritional science. Millennium's principal source of revenue is from sales of its nutraceutical supplements.
The Company acquired Millennium on July 27, 2001, when it completed a merger with Millennium. During the year prior to the merger, the Company had no material operations. For more information on the Company's operations prior to the merger and the general terms of the merger we refer to the Company’s report on Form 10-KSB for the period ended July 31, 2001, and related filings with the Securities and Exchange Commission. In the merger, new Convertible Preferred Series D stock was issued in exchange for all the outstanding stock of Millennium. Such preferred shares were subsequently converted into approximately 96% of the outstanding common stock of the Company. For accounting purposes, the merger has been treated as an acquisition of Millennium Group by Millennium, and a re-capitalization of Millennium. The historical financial statements prior to July 27, 2001, are those of Millennium. Subsequent to July 27, 2001, the financial statements are those of the Company and its wholly-owned subsidiary Millennium, on a consolidated basis.
Narrative Description of Business
Introduction
Millennium Biotechnologies Group, Inc. (“Millennium” or “the Company”) engages in the research, development, and marketing of specialized nutritional supplements as an adjunct to medical treatments for select medical conditions, as well as for athletes seeking improved recovery and advanced performance. The Company’s currently marketed products are targeted toward immuno-compromised individuals undergoing medical treatment for diseases, such as cancer, as well as individuals living with Human Immunodeficiency Virus (HIV)/Acquired Immune Deficiency Syndrome (AIDS) and wound healing and post-surgical healing and geriatric among other conditions. Millennium also is currently marketing the Surgex™ product line to elite athletes. Millennium currently manufactures and markets five proprietary product lines and 21 SKU’s to 7 separate target markets. Millennium is headquartered in Basking Ridge, New Jersey, and trades on the Over-the-Counter (OTC) market under the symbol “MBTG.OB.”
Three product lines form the Company’s Resurgex® Continuum of Care, include Resurgex Select®, Resurgex®, andResurgex Plus®. Resurgex Select® is a whole foods-based, calorically dense, high-protein nutritional formula developed for cancer patients undergoing chemotherapy or radiation treatments. Resurgex® and Resurgex Plus® are specialized, anabolic nutritional supplements rich in antioxidants that provide nutritional support post-treatment. Resurgex Essential™ and Resurgex Essential Plus™ represent Millennium’s Ready-To-Drink product line are currently being sold into the Long-Term Care-Geriatric markets. In November of 2007 Millennium obtained a 5 Year $20,000,000 exclusive purchase agreement from Provider Services, Inc., Ohio’s second largest nursing home network.
The Company’s products are unique in that they deliver healthy, whole food calories and do not contain high-fructose corn syrup or corn oil, which are not healthy or suitable forms of calories for Millennium’s targeted markets. These ingredients have also been shown to correlate with an increase in obesity, a promotion of insulin resistance, and are implicated in inflammation and cancer. Additionally, the use of high levels of Omega-6 fats (corn oil) has been shown to promote tumor growth in animal models. In contrast, Millennium’s nutritional products deliver 15 nutraceutical ingredients that specifically address the needs of chronically ill patients.
Millennium additionally developed Surgex™ (www.surgexsports.com) sports nutritional formula in late 2007. Surgex™ is clinically proven in two double-blind placebo controlled clinical trials conducted on the Division 1A Football and Soccer players at Rutgers University. Surgex™ was proven to address the nutritional concerns of both professional and amateur elite athletes. These athletes often experience similar symptoms post-workout to those battling immuno-compromised conditions, such as fatigue, loss of lean muscle, oxidative stress, and reduced immune function.
The Products
Since the initial launch of Resurgex® in September 2001, the Product lines have expanded to include 5 separate product lines and 21 SKU’s. Each of the Company’s specialized nutritional products—Resurgex Select®, Resurgex®, and Resurgex Plus®, the newly developed Ready-To-Drink Resurgex Essentiial™ and Essential Plus® form the Company’s Continuum of Care. The Surgex™ sports nutritional formula was created to open up new business outside of the medical market in which the Company’s Continuum of Care products are currently sold and marketed.
Resurgex Select®
Resurgex Select® is a whole foods-based nutritional product that is designed to be used throughout the course of cancer treatment (chemotherapy, radiation, etc.), as many times patients lose weight and cannot consume adequate nutrition. This product combines dietary fiber (3 g), low sugar (5 g), and high protein (15 g) with no added antioxidants to be a high-calorie (350 calorie) supplement. The omission of antioxidants is important because many oncologists prefer to avoid the risk that antioxidants may pose during their patient’s treatments. With this nutritional formula (which may be administered orally or fed through a gastrointestinal [GI] tube), the right balance of nutritional support can be provided in one drink. It is available in three flavors (Vanilla Bean, Chocolate Fudge, and Fruit Smoothie) and each can be mixed with water, milk, juices, or in soft cold foods such as yogurt, apple sauce, or pudding.
Resurgex Plus®
Resurgex Plus® is a high-protein (21 g), high-calorie (400 calorie), antioxidant-rich, enteral nutritional formula providing multiple comprehensive nutritional regimens into one drink. This product (which is available in Chocolate Cream and Vanilla Cream) may serve as a meal replacement or as a sole source of nutrition when directed by a physician following a patient’s treatment. Concentrated calories are derived from high-quality food sources rather than corn oil, sucrose, and corn syrup, which are commonly found in more well-known nutritional supplements.
Resurgex®
Resurgex® is a high-protein (12 g), low-sugar (5 g), low-calorie (90 calorie), antioxidant-rich, nutritional formula, which may be administered orally or fed through a GI tube. This drink, which is available in two flavors (Vanilla Supreme and Fruit Blast), is commonly used as a nutritional supplement to meals and is intended to promote wellness. It can also be mixed with water, milk, juices, or in soft cold foods such as yogurt, apple sauce, or pudding.
Surgex™
While Resurgex® andResurgex Plus® are the first multi-component nutritional supplements to address the various dietary needs of immuno-compromised individuals, Surgex™ (www.surgexsports.com), a nutritional support formula that aims to address the concerns of many elite athletes who suffer from like symptoms (fatigue, lean muscle loss, lactic acid buildup, oxidative stress, and stressed immune systems). This formula includes many of the benefits of the Resurgex® products and is designed to improve recovery parameters in efforts to enhance the performance of professional and collegiate athletes.
While Resurgex® or Resurgex Plus® can be effective when taken by athletes, the National Collegiate Athletic Association (NCAA) has specific rules about the use of certain amino acids, or anabolic agents. In fact, colleges and universities are prohibited from purchasing any product that contains amino acids or anabolic agents for athletes. This led to the development of the Surgex™ nutritional support formula.
Millennium has successfully tested its sports nutrition formula products at Rutgers University in New Jersey, among both the Men’s Division I Soccer Team and the Men’s Division I Football Team in two separate double-blind placebo-controlled studies. Both studies illustrated the product’s beneficial effects as a post-workout recovery aid, assisting the athlete in maximizing training responses and optimal recovery and improving performance. In addition, each batch of Surgex™ is certified by the Banned Substance Control Group (BSCG) to not contain any banned substances.
Resurgex Essential™
Millennium exited Research and Development on the Company’s first Ready-to-Drink Product line Resurgex Essential™ in July 2008. The Essential™ line is a ready to drink alternative to Ensure® and Boost® designed to be marketed into the long-term care channel. Millennium will fill approximately 50% of the existing $20 million dollar 5-year exclusive purchase commitment from Provider Services, Inc. (“PSI”) with this new product line. The remaining 50% of the purchase order from PSI requires Millennium to develop a tube feeding version and diabetic version of the Essential™ product line. Millennium plans to raise the capital to fund this research and development project in 2009. The Resurgex Essential™ has 250 whole food calories containing no corn syrup or corn oil. The product also contains fruit and vegetable extracts, and FOS Fiber to provide superior quality calories and taste when compared to the competition. Resurgex Essential Plus™ contains the same high quality ingredients with a higher caloric value of 450 calories per 8 oz serving.
Product Functionality Overview
Several health concerns must be addressed when it comes to effectively providing nutritional support for any major disease or immuno-compromised condition. While other “single magic bullet” products on the market largely only focus on one area while potentially neglecting others, Millennium’s products have been developed to address the major dietary concerns that may be influenced by nutritional support, when incorporated as an adjunct to a patient’s medical care. Thus, rather than providing significant amounts of calories from corn oil, low-quality proteins, sucrose, and corn syrup combined with an inexpensive multivitamin blend, Millennium’s Resurgex Select®, Resurgex®, Resurgex Plus® and Resurgex Essential™ have been developed to provide a comprehensive and complex array of nutrients, which fulfill necessary requirements in the health and well being of patients.
Mitochondrial (Energy) Support
Found within all human cells, mitochondria are tiny organelles that act as the cell’s “power plants,” producing energy (Adenosine Tri-Phosphate [ATP]) and involved in protein and fat processing. Damage to the mitochondria is typically attributed to the buildup of free radicals and other noxious byproducts, such as lactic acid, that accumulate during and after strenuous activity. Free radicals are highly reactive compounds created in the body during normal metabolic functions or introduced from the environment. Free radicals are inherently unstable, since they contain “extra” energy. To reduce their energy load, free radicals react with certain chemicals in the body, and in the process, interfere with the cells’ ability to function normally.
Factors that may influence the manner in which mitochondria function effectively include age, the presence of infections or certain diseases, strenuous activity, and select medications. Such changes or mutations may damage the mitochondria (known as mitochondrial toxicity) and either disrupt the normal function or cause function to stop completely. Millennium’s patented blend of ingredients, described below, is intended to support the function of the mitochondria and assist in producing energy within a cell.
| § | Co-Enzyme Q10 (CoQ10). CoQ10 is a vital component of cellular energy production and respiration. Participating in the mitochondrial electron transport system, which supplies ATP for a variety of physiological functions, muscle mitochondria lack adequate CoQ10 due to several chronic conditions. |
| § | L-Carnitine. L-Carnitine functions primarily to regulate fat metabolism. It is also a carrier of fatty acids into the mitochondria, where they are oxidized and then converted into ATP. It is believed that serum carnitine deficiency is common in select conditions. Furthermore, it is thought that several medications can be linked to mitochondrial destruction. |
| § | Ribose. Ribose is a key carbohydrate used by cells to form the body’s primary source of all energy, ATP. It further plays a central part in generating and recovering ATP. Ribose can offer powerful complimentary support to other nutrients addressing energy depletion by assisting in the production of normal ATP. |
| § | Nucleotides. Nucleotides are any group of molecules that, when linked together, form the building blocks of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleotides are a dietary source required to promote optimal tissue growth. |
Reduce Oxidative Stress
Oxygen consumption greatly increases during exercise, which leads to increased free radical production. Free radical formation within the muscle during exercise can easily damage muscle tissue, thereby inhibiting performance by inducing fatigue. Oxidative stress in the body is caused by an imbalance or overload of oxidants (such as free radicals from air, food, metabolism, medications, stress and disease). Prolonged oxidative stress causes disruption of the cell structure and defenses, which can lead to damage or death to the cell and play a part in the pathophysiology of a host of diseases.
Proper antioxidant defense is essential for recovery from disease. While there are many important antioxidants, vitamin C, vitamin E, zinc, selenium, polyphenols, and carotenoids, the most important is the cell’s master antioxidant defense enzyme, called superoxide dismutase (SOD). Millennium has developed a blend of ingredients, described below, to help support the cell’s ability to defend against oxidative stress.
| § | SOD/Gliadin. SOD/Gliadin has been shown to reduce oxidative stress in humans by reducing a cell’s genetic damage and reducing isoprostanes. In particular, SOD has been shown to lower oxidative stress, and is the master cellular defense enzyme of the cell. It further serves as significant support for the immune system, countering the harmful effects of free radicals and reducing their negative effects within the body. SOD may consequently support immune function, and reduce oxidative stress. The clinical relevancy of SOD has been demonstrated in numerous scientific studies in cardiology, immunology, oncology, inflammatory conditions, asthma, vision, and liver support. |
| § | Undenatured Whey Protein. A well-known source of protein for building and retaining lean muscle mass, undenatured whey has become a nutritional staple for immuno-compromised patients. Undenatured whey has also been shown to assist in cellular defense by increasing available glutathione, another important cellular antioxidant required by the body to ward off the effects of oxidative stress. |
| § | Beta Glucans. Beta glucans are sugar molecules bound together as a sugar/protein complex. Beta glucans spur macrophages (a type of white blood cell that surrounds and kills microorganisms, removes dead cells, and stimulates the action of other immune system cells) into action. Increased macrophage activity triggers a cascade of immune events that boost immune response and stimulate the production of immune cells. |
| § | Multi-Vitamin/Mineral Mix, Polyphenols. Polyphenols are a class of phytochemicals that have been associated with preventing heart disease and cancer. This mix also provides essential vitamins required by the body to provide a nutritional balance. |
Maintain Lean Muscle
Lean muscle loss or wasting is a common problem as people age and among many chronic degenerative or immuno-compromised conditions. Also known as cachexia, this problem can diminish a person’s quality of life and aggravate an existing illness. Some of the common issues that are associated with muscle loss from illness and aging consist of inadequate caloric intake, problems with metabolism, elevations of inflammatory compounds that break down muscle (some cytokines), or malabsorption.
Nutritional supplementation is important for boosting caloric intake. However, there are a variety of conditions where increasing calories may not be an effective solution. Millennium’s products contain ingredients that are designed to help maintain lean tissue by providing high-quality protein and compounds that assist in building muscle and preventing its breakdown. Some of the ingredients incorporated into Millennium’s products that help maintain lean muscle mass are bulleted below.
| § | Undenatured Whey A well-known source of protein for building and retaining lean muscle mass, undenatured whey has become a nutritional staple for immuno-compromised patients. Undenatured whey has also been shown to assist in cellular defense by increasing available glutathione, another important cellular antioxidant required by the body to ward off the effects of oxidative stress. |
| § | Ornithine Alpha-ketoglutarate (OKG). OKG affects wasting through three primary mechanisms: (1) as an anabolic agent (buildup of muscle tissue); (2) as an anti-catabolic agent (prevents breakdown of muscle tissue); and (3) as an inducer of protein synthesis. Each of these mechanisms contributes to muscular development and enhanced recovery. OKG also spares the loss of glutamine in muscle. This is vital for recovery and repair. |
| § | Branched Chain Amino Acids (BCAAs). BCAAs play an important role in muscle recovery, muscle growth, and energy maintenance. Each must be present in the muscle cells to promote protein synthesis. There is scientific evidence that BCAAs may help build and retain lean muscle mass. |
| § | Nucleotides. Nucleotides are any group of molecules that, when linked together, form the building blocks of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleotides are a dietary source required to promote optimal tissue growth. |
Support Immune Function
Good dietary support supplying essential macronutrients and micronutrients is critically important for maintaining a proper immune system. Nevertheless, if the cells of the immune system cannot produce energy efficiently (mitochondrial dysfunction), have poor antioxidant defenses (oxidative stress), and the body is losing important lean muscle, immune support cannot effectively be achieved. Millennium’s products contain a blend of ingredients, listed below, that can support the immune system while protecting against mitochondrial dysfunction, oxidative stress, and lean muscle loss.
| § | SOD/Gliadin, Beta Glucans, Nucleotides, OKG, CoQ10 and L-Carnitine. |
| § | Undenatured Whey High in Immunoglobulins. |
| § | Fruit and Vegetable Extract Blend. This blend has the phytonutrients equivalent to one to two servings of fruits and vegetables per day. The phytonutrients found in fruits and vegetables directly contribute to a healthy immune system. |
Principal Market
Size of the Market
The nutritional supplement market is expected to increase to over $6 billion by 2011 from approximately $4.7 billion at year-end 2006, according to Nutritional Supplements in the U.S., a report from Packaged Facts published in November 2006. This growth is likely to be attributed to several factors, including increased awareness of the value of adequate nutrition in helping to recover from illness or injury, the advancement and further understanding of nutritional science, the availability of newer and more sophisticated nutritional formulas, and pressure from many managed care organizations to reduce overall healthcare costs.
Target Markets
Millennium’s products are intended to benefit patients with chronic debilitating diseases, such as cancer, as well as patients needing homecare for HIV/AIDS and those undergoing wound healing or post-surgical healing, among other conditions. Surgex™ has been clinically proven to address sports recovery needs for amateur and professional elite athletes. Greater details on these markets are provided below.
Cancer
Cancer is a class of diseases or disorders characterized by uncontrolled division of cells and the ability of these cells to spread, either by direct growth into adjacent tissue through invasion, or by implantation into distant sites by metastasis (where cancer cells are transported through the bloodstream or lymphatic system). Cancer may affect people at all ages, but risk tends to increase with age and is one of the principal causes of death in developed countries. There are many types of cancer, all of which require specific treatments such as surgery, chemotherapy, and radiation. These treatments, as well as the disease itself, often have a direct impact on a person’s nutritional health.
Millennium has recognized the need for a product that is specific to people receiving cancer treatment. For that reason, the Company has formulated its Resurgex Select®, designed to be utilized during cancer treatment.
Nutrition in Cancer
Cancer patients have specific nutritional needs in order to fight the disease and maintain their energy and quality of life. According to the Canadian Cancer Journal Clin. 2005;55:319-321, some of the requirements of cancer patients include protein from high biological value sources, specific amino acids (BCAAs), “good fats” (Medium Chain Triglycerides [MCTs], Omega-3 fatty acids, low in Omega-6 fats), healthy fruits and vegetables, and highly soluble fiber (oat fiber, fructooligosaccharide [FOS], etc.). Enteral nutritional support during cancer treatment or recovery can help to restore normal body protein levels, restore immune function, and promote weight gain.
Diet is an important part of cancer treatment since malnutrition is a common problem in cancer patients due to side effects, such as anorexia, nausea, vomiting, diarrhea, constipation, mouth sores, trouble with swallowing, and pain, which make it difficult for cancer patients to eat well (where up to 85% of cancer patients experience malnutrition during their treatments). Malnutrition may also cause the patient to be weak, tired, and unable to resist infections or withstand some cancer therapies. Inadequate nutrition may further play an important role in adverse outcomes for patients, such as increased morbidity and mortality, as well as a decreased quality of life.
According to the NIH, the cancer and cancer treatments described below may cause nutrition-related side effects.
| § | Surgery. Depending on the procedure, surgery can cause mechanical or physiologic barriers to adequate nutrition, such as a short gut, which results in malabsorption after bowel resection. In addition, surgery frequently imposes an immediate metabolic response that increases the energy needs and changes the nutritional requirements necessary for wound healing and recovery at a time when baseline needs and requirements are often not being met. A well-balanced diet that contains the recommended amounts of essential nutrients and calories may help promote wound healing. |
| § | Chemotherapy. Chemotherapy is a systemic treatment that affects the whole body and causes potentially more side effects than surgery or radiation therapy. The most commonly experienced nutrition-related side effects are anorexia, taste changes, early satiety, nausea, vomiting, mucositis/esophagitis, diarrhea, and constipation. Such side effects in combination with the cancer can greatly affect a patient’s nutritional status. Nutritional support or high-calorie/high-protein liquid supplements may be used in an effort to maintain adequate calorie and nutrient intake. |
| § | Radiation. Radiation therapy can produce changes in normal physiologic function of healthy tissue that may ultimately diminish a patient’s nutritional status by interfering with ingestion, digestion, or absorption of nutrients. Side effects of radiation therapy depend on the area irradiated, total dose, fractionation, duration, and volume irradiated. Adequate calories and protein can help maintain patient strength and prevent body tissues from further catabolism. Individuals who do not consume adequate calories and protein make use of stored nutrients as an energy source, which leads to protein wasting and further weight loss. |
| | o | Many patients who are undergoing radiation therapy can benefit from nutritional supplements between meals. Aggressive nutritional support is indicated when oral intake alone fails to maintain an individual’s weight. Tube feedings, which are used more frequently than parenteral nutrition (primarily to preserve GI function), are usually well tolerated, pose less risk to the patient than parenteral feedings, and are more cost effective. Although Millennium is not yet addressing the tube-feeding market, the Company is in the process of developing a formula and could enter this market by third quarter of 2009. |
It is noteworthy that the use of antioxidants to promote healing during cancer treatments has been highly debated. According to the American Cancer Society, some recently published studies state that antioxidant supplementation may cause new disease or interference with treatment in some cancer patients. Thus, based on conflicting information as to the benefits and harms of antioxidants on patients undergoing treatment, Millennium has developed Resurgex Select® to support a cancer patient’s nutritional needs without containing antioxidants.
Protein-Calorie Malnutrition (PCM)
Protein-calorie malnutrition (PCM) is one of the most common secondary diagnoses in individuals with cancer. This condition stems from the inadequate intake of carbohydrate, protein, and fat to meet metabolic requirements or the reduced absorption of macronutrients. PCM in cancer may result from multiple factors, most often associated with anorexia, cachexia, and the early satiety sensation frequently experienced by individuals with cancer. These factors range from loss of taste or change in taste to a physical inability to digest food—both leading to inadequate nutrient intake. Furthermore, cancer-induced abnormalities may further increase the incidence of PCM. Such abnormalities could lead to glucose intolerance and insulin resistance, increased lipolysis, and increased whole-body protein turnover.
Good nutritional practices can help cancer patients maintain weight and the body’s nutrition stores, as well as aid the body in keeping its immune system strong so that it is able to fight off infection or disease following cancer treatment. Major concerns in the oncology patient that can be influenced by proper nutritional support include the following:
| § | Mitochondrial Dysfunction. Mitochondrial function is depressed in liver, kidney, colon, breast, gastric, esophageal, and lung carcinomas; |
| § | Oxidative Stress. Oxidative stress is involved in the pathophysiology of most cancers. Additionally, low levels of SOD, CAT, etc. and elevated free radicals have been found in the plasma of certain cancer patients; and |
| § | Wasting. Elevated inflammatory cytokines are strongly associated with muscle wasting and cancer cachexia. |
Each of these conditions, described in greater detail on pages 6-7, can be addressed with Millennium’s Resurgex Select®.
Product Distribution in Oncology Markets
Many hospitals and pharmacies already prescribe Millennium’s products for support during oncology treatments or for recovery.
Treatment of HIV/AIDS and Other Immuno-Compromised Conditions
According to Kalorama Information (www.kaloramainformation.com), the U.S. market for homecare products and equipment continues to increase, primarily as a result of the aging population and increasing shifts of chronic care patients from hospitals to home. In 2004, an estimated 16 million people received homecare in the U.S. Through 2009, the numbers of homecare patients are expected to continue to grow as patient life spans are extended with more sophisticated medical care and new technologies, and homecare becomes an increasingly important and accepted means to contain healthcare costs for managed care providers.
Homecare represents a wide range of community-based services to support someone that is recuperating from an acute situation, such as a hip fracture, or services needed by people with ongoing chronic conditions, such as cancer or HIV/AIDS. Homecare is often preferred to hospital stays by patients as it makes it possible for care recipients to remain in a safe, comfortable, familiar environment, and possibly have more independence than they would in a hospital or clinical setting. Furthermore, hospitals are discharging patients earlier to long-term care or homecare situations in order to cut costs.
HIV/AIDS
People living with HIV/AIDS often experience a loss of lean muscle (wasting), nutrient depletion, immune deficiencies, mitochondrial dysfunction (energy loss), and oxidative stress (free radical damage). The Company’s two patented nutritional formulas, Resurgex® and Resurgex Plus®, address the nutritional needs of patients with HIV/AIDS, whose immune systems have been compromised as a result of chronic and acute viral-based infections and who are receiving medical care. These products and their use in HIV/AIDS patients was the impetus behind the Company’s expansion into oncology.
Nutrition in HIV/AIDS
Antiretroviral medications (ARVs) are used to treat HIV/AIDS. Although ARVs do not completely destroy HIV, they significantly reduce the replication of the virus in the blood, which slows down the progression of the disease to AIDS. Many of the ARVs on the market today, however, can impair the body’s ability to use sugar. Additionally, side effects of medication, such as taste changes, loss of appetite (i.e., anorexia), nausea, bloating, heartburn, constipation, vomiting, and diarrhea, indirectly affect nutritional status by causing a reduction in food intake or nutrient absorption. Reduced food intake and poor nutrient absorption can lead to weight loss and continued impairment of the immune system, which, in turn, allows HIV to more quickly progress to AIDS.
Some side effects of medications can be similar to select AIDS-related symptoms and call for similar dietary management. Dietary management of these side effects can help maintain food intake, compensate for nutrient losses, and prevent weight loss.
Major concerns in the HIV/AIDS patient that can be influenced by nutritional support include the following:
| § | Energy Loss (Mitochondrial Dysfunction). Highly Active Antiretroviral Therapy (HAART) causes damage to the cell’s mitochondria; |
| § | Oxidative Stress. People with AIDS are under chronic stress as demonstrated by low levels of superoxide dismutase (SOD); and |
| § | Wasting (Muscle Loss). Wasting is not necessarily due to lack of fat and sugar. Patients infected with HIV lose lean body mass, even on HAART and with adequate dietary intake, due to excessive production of inflammatory compounds. |
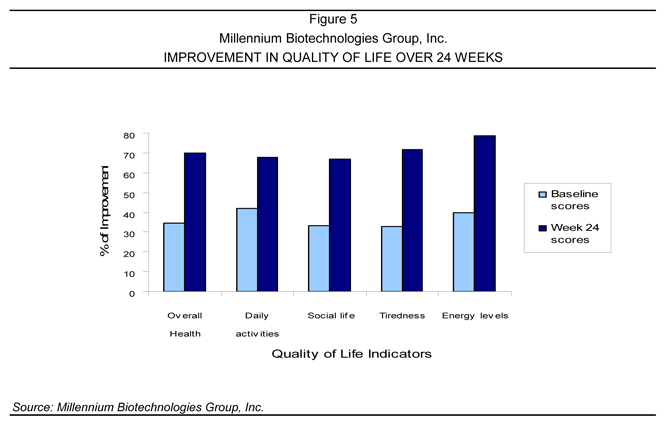
Resurgex® Metabolic/Virological: Quality of Life Study
A six-month study of 26 HIV-1-infected patients on HAART was conducted at the Center for Family Health at the University of Medicine and Dentistry of New Jersey (UMDNJ)/St. Mary’s Hospital (Hoboken, New Jersey). In the study, patients were given Resurgex® twice daily. A standardized quality of life questionnaire, approved by the Investigational Review Board (IRB), was completed at baseline and follow-up visits. The results of this questionnaire are summarized in figure 5. This Study was displayed as a poster presentation at the Annual International Conference on HIV/AIDS in Brazil.
Sports Recovery
Competitive athletes often suffer from similar symptoms to those battling cancer or immuno-compromised diseases, such as fatigue, oxidative stress, muscle wasting, and possibly lack of immune support due to the demands they put on their bodies daily. In order to compete effectively, these athletes need a solution to address some of the key concerns they face, described below.
| § | Fatigue and Mitochondrial Dysfunction. Damage done to the mitochondria is typically attributed to the buildup of free radicals and other noxious byproducts, such as lactic acid that accumulates during and after strenuous activity. |
| § | Oxidative Stress. Oxygen consumption greatly increases during exercise, which leads to increased free radical production. Also, free radical formation within the muscle during exercise can easily damage muscle tissue and inhibit performance by the induction of fatigue. |
| § | Muscle Wasting. A common problem during strenuous activity is lean muscle loss or wasting as muscle tends to breakdown after vigorous activity. This problem can have a serious impact on performance and recovery. |
| § | Immune Support. Exercise has also been shown to have a positive effect on the immune system if one does not overtrain. Signs and signals of overtraining are a constant feeling of fatigue, loss of strength or endurance, and although still exercising, a feeling of burnout. Additionally, excessive exercise or periods of very heavy conditioning could lead to suppression of immunity for several hours to a week or longer, creating a brief period of vulnerability when the risk of upper respiratory tract infections is increased. |
Effect of Banned Nutritionals
In recent years, professional athletes have come under considerable scrutiny for taking different substances or performance enhancers. Whether it is the Olympics, Tour de France, baseball, or other professional sports, organizations constantly test athletes for chemicals within their body that would indicate they have taken an illegal substance to boost their performance. Millennium’s Surgex™ sports nutritional formula product is devoid of any banned substances.
Each batch of the Company’s product is tested for a list of substances by the Banned Substance Control Group (BSCG) that are outlawed by organizations such as the International Olympic Committee (IOC), the World Anti-Doping Agency (WADA), the U.S. Anti-Doping Agency (USADA), the NCAA, and the National Football League (NFL). All of the Surgex™ products receive a seal from the BSCG, a WADA-approved laboratory. Therefore, athletes need not worry about consuming any substance that could disqualify them from competition by using the Company’s products. The product also meets all NCAA guidelines for Nutritional Supplements.
Rutgers Studies: Demonstrating the Effect of Surgex™ Sports Nutrition Formula vs. Placebo
Millennium has conducted two clinical trials at Rutgers University among the Men’s Division I Soccer and Football teams, demonstrating the effect of the Surgex™ sports nutrition formula versus a placebo. Both studies showed the product’s beneficial effects as post-workout recovery aid, assisting the athlete in maximizing training responses and aiding in optimal recovery.
Division I Men’s Soccer Team-Results of the Trial-Accepted for Publication in The Journal for Strength and Conditioning Research
Millennium conducted its first clinical trial among the Rutgers University Men’s Division I Soccer team during their preseason training regimen. The clinical trial was accepted for publication in The Journal for Strength and Conditioning Research (“JSCR”). The JCSR is the official research journal of the National Strength and Conditioning Association, (``NSCA''). This journal is recognized as the preeminent publication for strength and conditioning coaches worldwide. These thought leaders rely on this journal for the future of leading edge science as applied to their profession.
Preseason training often places a high demand on athletes, and requires them to engage in frequent, high-intensity workouts with limited time devoted to recovery. Soccer players spend a considerable portion of a match at an intensity close to 75% of VO2 max (the maximum amount of oxygen in milliliters that one can use in one minute per kilogram of body weight) and rely on anaerobic metabolism and power during brief bursts of sprinting, kicking, and jumping. These players must be able to perform near maximal capacity for extended periods, which may result in increased oxidative stress. With outside supplementation of protective nutraceuticals, it may be possible to reduce acute and chronic oxidative stress, as well as capitalize on gains from intense preseason training.
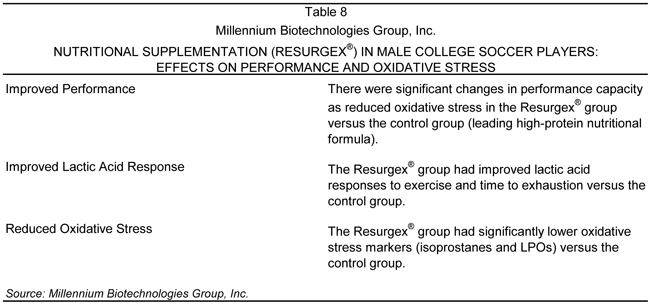
The purpose of this study was to examine changes in performance and oxidative stress in collegiate soccer players over the course of preseason preparation, and to determine the impact of a supplemental proprietary nutraceutical blend proposed to reduce oxidative stress and enhance recovery.
Results
A summary of the findings of the soccer trial is provided in Table 8.
This double-blind, placebo-controlled trial found that the Company’s Surgex™ sports nutrition formula enhanced performance parameters and reduced oxidative stress levels (free radical damage caused by exercise) in players. Oxidative stress in the body is caused by imbalance or overload of oxidants (free radicals from air, food, metabolism, medications, stress, disease, etc.). Sustained oxidative stress disrupts the cells’ defenses, resulting in damage that contributes to the development of many diseases.
Results indicated a beneficial effect of the Surgex™ sports nutrition formula, including improvements in performance capacity, time to exhaustion, lactic acid response, and reduced oxidative stress. This data suggests an important role for Resurgex® in the sports fitness field in addition to its proven benefits for cancer and immuno-compromised medical patients. The fact that this trial is approved for publication in the JSCR confirms the results of this clinical trial have been reviewed and approved by an independent and accredited third party.
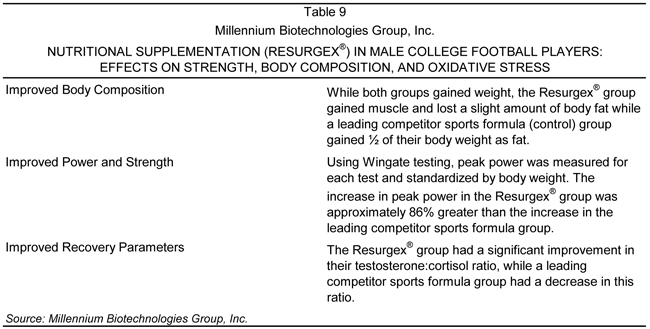
Rutgers’ Division I Football Team
The Company also conducted a second clinical trial on the Rutgers University Division I Football team versus a placebo group. The study tested the recovery time, strength, and body composition of the players versus a placebo (a leading competitor sports formula). The results concluded that the Surgex™ sports nutrition formula significantly enhanced recovery parameters, strength, and body composition in the football players.
Results
Those receiving the Surgex™ sports nutrition formula showed significant increases in their peak power, as measured by Wingate testing (assessment for peak anaerobic power, anaerobic fatigue, and total anaerobic capacity through a combination of running, vertical jumping, and resistance training), better muscle to fat weight gains, and an improved testosterone:cortisol ratio, as well as reduction of interleukin 6 (IL6), creatine kinase, and isoprostanes versus the control group.
Testosterone and cortisol are the two hormones affected by training. The cortisol levels typically elevate and break down lean muscle. Testosterone, in contrast, which is the supporter of lean muscle, decreases, therefore causing lean muscle breakdown in the body after strenuous exercise. In this study, the Resurgex® group had significantly improved testosterone to cortisol ratios versus the control group. A summary of the findings of this study is provided in Table 9.
The practical application emerging from the study demonstrates the beneficial application of the Surgex™ sports nutrition formula as a post-workout recovery aid, assisting the athlete in maximizing training responses by helping to buffer the acute and chronic biochemical challenges to optimal recovery. The product also has the ability to reduce inflammatory and oxidative stress markers, as well as compounds that can break down muscle. Millennium believes that these findings can help the Company position the Surgex™ sports nutrition formula as an aid to help athletes increase their performance by improving recovery, strength, and energy parameters.
Intellectual Property
Millennium owns all rights to the formulations of Resurgex Select®, Resurgex®, Resurgex Plus®, Resurgex Essential™ and Surgex™, and has filed compositional patent applications with respect to these formulations. Resurgex®, Resurgex Plus®, Resurgex Select®, and Surgex™ are registered trademarks filed with the U.S. Patent and Trademark Office (USPTO). Additionally, the Company has patents pending for all product lines in 57 countries worldwide.
On January 7, 2003, Resurgex® was issued a use and composition patent (U.S. Patent 6,503,506, Nutrient therapy for immuno-compromised patients). Millennium was granted a composition patent for Resurgex Select® in December of 2006. In addition, the Surgex™ line of products is patent pending in the United States and 57 countries worldwide. The Company relies on trade secrets and unpatented proprietary technology in addition to their patented technologies.
On March 20, 2006, Millennium received the Healthcare Common Procedure Coding System (HCPCS) code for Resurgex Select®. HCPCS is one of the formats in which nutritional formulas may be coded for Medicare reimbursement and is specifically required for Medicaid reimbursement in many states. The Resurgex® and Resurgex Plus® product lines have also received Federal HCPCS Codes.
Regulatory Environment
The manufacturing, processing, formulation, packaging, labeling and advertising of all of Millennium’s product lines are subject to regulation by federal agencies, including the Food and Drug Administration (the "FDA"), the Federal Trade Commission, the Consumer Product Safety Commission, the United States Department of Agriculture, the United States Postal Service and the United States Environmental Protection Agency. These activities are also subject to regulation by various agencies of the states and localities in which the Company sells and plans to sell its products.
The Dietary Supplement Health and Education Act of 1994 (the "Dietary Supplement Law") broadly regulates nutritional labeling, claims and manufacturing requirements for dietary supplements. The Dietary Supplement Law provides for regulation of Statements of Nutritional Support ("Statements"). These Statements may be made if they are truthful and not misleading and if "adequate" substantiation for the claims is available. Statements can describe claims of enhanced well-being from use of the dietary supplement or product statements that relate to affecting a structure or function of the body. However, statements cannot claim to diagnose, treat, cure, or prevent any disease, regardless of the possible existence of scientific reports substantiating such claims.
Statements appearing in dietary supplement labeling must be accompanied by disclaimer stating that the FDA has not evaluated the Statements. Notification to the FDA of these Statements is not considered approval of the Statements. If the FDA determines in possible future proceedings that dietary supplement Statements fail to meet the requirements of the Dietary Supplement Law, a product may be subject to regulation as a drug. The FDA retains all enforcement means available to it (i.e. seizure, civil or criminal penalties, etc.), when investigating or enforcing labeling claims.
The Federal Trade Commission ("FTC") regulates advertising of dietary supplements which includes all of Millennium’s products. The Federal Trade Commission Act prohibits unfair or deceptive trade practices and false or misleading advertising. The FTC has recently been very active in its enforcement of advertising against manufacturers and distributors of nutritional dietary supplements having instituted several enforcement actions resulting in signed agreements and payment of large fines. Although the Company has not been the target of a FTC investigation, there can be no assurance that the FTC will not investigate the Company's advertising in the future.
The Company is unable to predict the nature of any future laws, regulations, interpretations, or applications, nor can it predict what effect additional governmental regulations or administrative orders, when and if promulgated, would have on its business in the future. They could, however, require the reformulation of certain products not possible to be reformulated, imposition of additional record keeping requirements, and expanded documentation of the properties of certain products, expanded or different labeling and scientific substantiation regarding product ingredients, safety or usefulness. Any or all such requirements could have a material adverse effect on the Company's results of operations and financial condition.
Medicaid Reimbursement
Medicare/Medicaid
Millennium has received federal government approval to have its nutritional product lines (Resurgex®, Resurgex Plus® and Resurgex Select®) covered by Medicare. After receiving Medicare approval by the federal government, Millennium started lobbying state governments for Medicaid approval, which would allow the Company to have its products distributed or retailed in HIV clinics, oncology centers, long term care facilities and pharmacies throughout each state. Currently, Millennium has received Medicaid and Medicare approval for the Resurgex® line products in several states including New York, New Jersey, Connecticut and Nevada.
Growth Strategy and Distribution
Millennium has implemented a variety of strategies and formed partnerships that are intended to penetrate the various specialized markets for nutritional products, which are targeted by the Company (oncology patients, immuno-compromised individuals, and athletes seeking effective sports recovery). Currently, products can be purchased through the Company’s websites, www.surgexsports.com www.milbiotech.com and www.resurgex.com, also through the website www.caring4cancer.com, or ordered by phone (877) RESURGX. Additionally, in select areas, Medicaid-associated pharmacies distribute the Company’s products. Furthermore, select international distribution agreements are in place (as described below), which are intended to expand the Company beyond U.S. markets. Each of the Company’s growth strategies in terms of product distribution are detailed below.
Oncology Market Distribution
Canadian Distribution
In March 2007, Millennium signed a Letter of Intent with Ferring Pharmaceutical the Canadian subsidiary of an international pharmaceutical company currently producing gross revenue in excess of $1,000,000,000 USD. Subsequently in March 2008 Millennium entered into a definitive distribution agreement where Millennium granted exclusive marketing rights to this pharmaceutical company to distribute and market its line of Resurgex® products for cancer patients in Canada.
Under the proposed terms of the agreement Millennium would profit from the manufacturing and royalty of all product sold in Canada. This agreement was finalized in March 2008.
Greek Distribution
In April 2007, the Company entered into an international distribution partnership with Nutrimedica, a Greek distributor of nutritional products with knowledge of the local market and regulations in Greece. Under this agreement, Nutrimedica is to import and distribute the Resurgex® products to hospitals, pharmacies, and directly to patients. Nutrimedica has initiated introduction of the Resurgex® products to the market, and sales have significantly increased over the past year. The distribution agreement with Nutrimedica produced annual average revenue of $22,000 for the years 2006 and 2007. Millennium recorded revenue totaling $313,000 in 2008 from Nutrimedica in Greece.
On a percentage basis the revenue received in 2008 as compared to average revenue received from Nutrimedica in Greece in the years 2006 and 2007 increased 1,323%. Furthermore Millennium received a purchase order in the first quarter of 2009 totaling $478,000 form Nutrimedica in Greece. Management anticipates the exponential revenue growth in Greece to continue based on the positive reports and exponentially large increases in orders from Greece which are continuing into 2009.
Sports Nutrition Market Product Distribution
In order to penetrate the sports nutrition market, the Company directly targeted the strength/conditioning coaches of professional sports teams. During the National Basketball Association (NBA) 2006-2007 and 2007-2008 seasons, several NBA organizations that purchased and used Surgex™, and had players using the products before and after practices and games. Importantly, since many of the NBA coaches have come from the NCAA, many coaches at the collegiate level are aware of the Surgex® line of products. However, due to NCAA rules of fairness, no university may offer or distribute supplements containing amino acids or anabolic agents to its athletes. For that reason, Millennium has developed an NCAA-compliant Surgex™ sports nutrition formula, devoid of amino acids or anabolic agents, which is fully compliant with the NCAA rules and could therefore be used by universities. In addition, each batch of Surgex™ is certified by the BSCG to not contain any banned substances.
Research and Development
During 2008 and 2007 the Company spent $476,806 and $97,171, respectively, on research and development of its products. The increased research and development expenses incurred in 2008 are directly related to the development of the Resurgex Essential™ ready-to-drink product line. Millennium received other income totaling $400,000 during the fourth quarter of 2007 pursuant to the terms of the research and development contract between Millennium and Provider Services, Inc. The funds received from PSI helped subsidize the cash requirement related to the development of the Resurgex Essential™ Ready-to-Drink product lines.
Competition
Millennium’s products target the nutritional supplement market, specifically the ready-to-drink beverage market, as an adjunct or meal replacement. The products that most directly compete with Millennium’s Resurgex® Continuum of Care in the adult nutrition market are produced by mainstream manufacturers—Boost® by Novartis AG, Ensure® by the Ross Product Division of Abbott Laboratories Inc., and Carnation® Instant Breakfast® by Nestlé—as well as generic (store branded) products that are marketed head-to-head against these products.
Millennium has chosen to target the medical community, vis-à-vis a pharmaceutical sales approach and distribution model for its currently marketed products, which is unique from that of its competitors, as the Company believes that this approach provides credibility and legitimacy that competitive brands on the market may lack. Additional factors that Millennium believes distinguish it from other competitively marketed nutritional products are listed below.
| § | Resurgex®, Resurgex Plus®, Resurgex Select® and Resurgex Essential™ address multiple issues that cause diminished fatigue and quality of life in immuno-compromised individuals. To the Company’s knowledge, no other product on the market can make this claim. These include the following: |
| | o | Mitochondrial support (energy); |
| | o | Reducing oxidative stress; |
| | o | Providing healthy, whole food calories. |
| § | Many of the mass-market competitors manufacture their products using the least expensive ingredients, which ensures low retail price and high profitability. This means diminished bioavailability and low biological value, as well as less benefit to the end user. |
| § | Resurgex®, Resurgex Plus®, Resurgex Select® and Resurgex Essential™ were developed with the ingredients necessary in order to deliver optimal performance. |
| § | Resurgex® and Resurgex Plus® deliver over 15 nutraceutical ingredients that specifically address the needs of the chronically ill. Other products generally contain only one highlighted ingredient. |
| § | Resurgex®, Resurgex Plus®, Resurgex Select® and Resurgex Essential™ deliver therapeutic levels of active ingredients based on the scientific research. |
| § | Millennium has a use and composition patent for the existing formulas. Ensure® and Boost® are composed of mostly corn syrup and provide “empty calories,” which could do more harm than good, especially in glucose-sensitive individuals. |
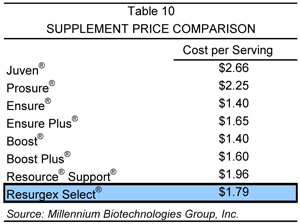
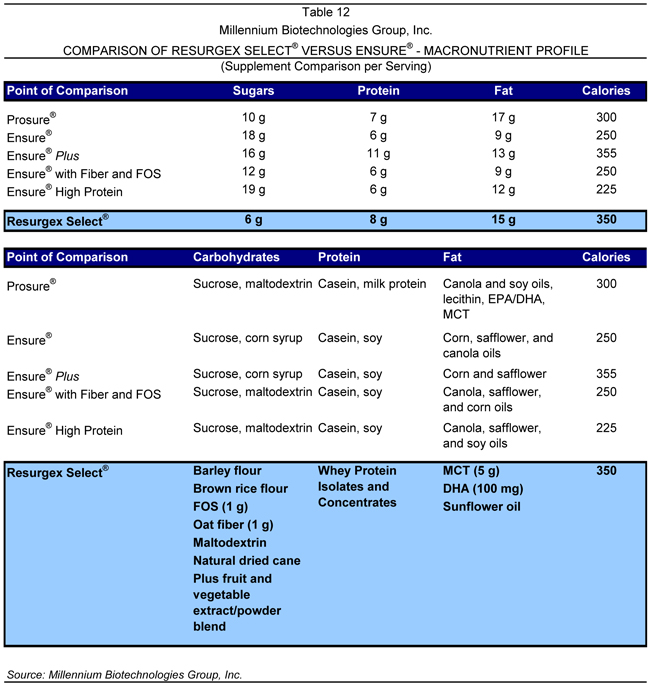
While the majority of other companies in this market sell the bulk of their product through the mass market, these formulations may not transfer well into the highly critical medical market, which Millennium’s products target. Additionally, many of these products have been slow to update their formulas and incorporate the latest nutritional ingredients. Descriptions of the products which Millennium believes could be considered competitors to its own product line are provided in the accompanying section, along with a price summary of each of these products, relative to Resurgex Select®, which is provided in Table 10.
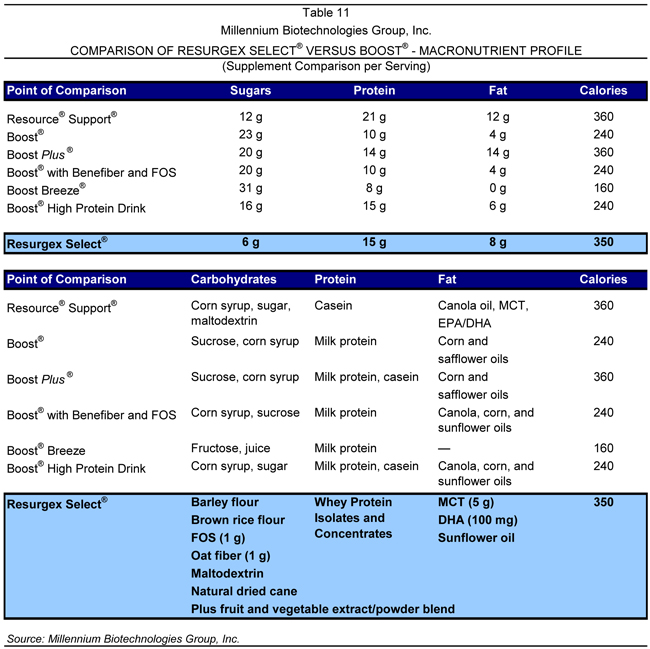
Boost® and Boost Plus®
Boost® is marketed as an energy beverage for healthy adults, providing a balance of essential vitamins and minerals to promote physical and mental health. As a meal replacement or between-meal snack alternative, Boost® is a nutritional drink with 25 vitamins and minerals, carbohydrates, and protein. The product additionally contains antioxidants, such as beta-carotene, selenium, and vitamins C and E, as well as natural milk calcium. Boost Plus® is marketed as a high-protein, high-calorie, nutritionally complete drink for people on fluid- or volume-restricted diets, people with HIV suffering from weight
loss, or for general weight gain. A comparison between Resurgex Select® and Boost® from a macronutrient perspective is provided in Table 11.
Ensure® and Ensure Plus®
Ensure® was developed to provide a source of complete, balanced nutrition for supplemental use between or with meals and for interim sole-source feeding. Ensure® is marketed as a benefit to people who are at nutrition risk, experiencing involuntary weight loss, recovering from illness or surgery, or on modified or low-residue diets. Ensure Plus® is marketed as a source of complete, balanced nutrition that provides concentrated calories and protein to help patients gain or maintain healthy weight. It can be used with or between meals or as a meal replacement. A comparison between Resurgex Select® and Ensure® from a macronutrient perspective is provided in Table 12.
Sports Nutrition Market
Millennium is currently seeking sales, marketing and distribution partner which will assist in distribution Surgex™ to the sports nutrition market. Millennium’s management feels a partner is necessary for the mass market distribution launch of Surgex into the retail sports nutrition marketplace. Management is pursuing this alternative because Millennium’s shareholders would benefit from the anti-dilutive capital which would be allocated to the marketing expense of a mass market product launch. Millennium would also benefit from the marketing expertise and capabilities of their distribution partner. Based on historical acceptance by 10 NBA teams, PGA golfers, and NFL players with little or no marketing budget, management expects to exponentially improve results with proper marketing and distribution strategies which cater to the mass market of sports nutrition consumers.
Products such as those listed below, as well as many other “high protein” drinks, are marketed as sports nutrition supplements and may be considered competitive products to the Company’s Surgex™ sports nutrition formula. It is important to note that as the high-protein drink market is expansive, these competitive products are not an exhaustive list of competitors. Rather, these products are representative of some of the high-protein drink products on the market today that may compete with Surgex™ sports nutrition formula. Surgex™ sports nutrition formula will likely provide athletes with the important calories they need while being competitively priced with other products.
| § | BSN Syntha-6™ Extended Release Protein Blend by BSN Inc. |
| § | Muscle Milk® by CytoSport Inc. |
| § | FRS, an antioxidant health drink, by New Sun Nutrition, Inc. |
| § | Gatorade® Performance Series |
Employees
As of December 31, 2008, the Company employed 6 persons, of whom one is primarily engaged in research and development and product support activities, two are primarily engaged in overall managerial functions associated with operations, capital raising, distribution partnerships and sales and marketing, one is primarily engaged in day to day managerial operations and two are engaged in general administrative and wholesale/direct to consumer sales functions. The Company has no collective bargaining agreements with its employees.
RISK FACTORS
The following cautionary statements identify important factors that could cause our actual result to differ materially from those projected in the forward-looking statements made in this report.
We have operated at a loss and cannot assure that we will be able to attain profitable operations.
Although we are generating revenues, we continue to operate at a loss. During the year ended December 31, 2008, we generated revenues of $1,492,262 from sales of our six products. However, during this period we realized net losses of $11,361,216, of which $6,517,142 were non-cash items primarily related to issuance of shares and warrants for compensation, services, and associated with financing transactions during the period. We expect to continue incurring operating losses until we are able to derive meaningful revenues from marketing our three products and other products we intend to bring to market. We cannot assure that we will be able to attain profitable operations.
We require additional funding to maintain our operations and to further develop our business. Our inability to obtain additional financing would have an adverse effect on our business.
Our success depends on our ability to develop a market for our three products and other nutraceutical supplements we intend to bring to market. This means having an adequate advertising and marketing budget and adequate funds to continue to promote our products. Although our revenues have increased, our operating expenses are significantly greater than our revenues. During 2008, the Company obtained new capital in the form of equity that supplied a major portion of the funds that were needed to finance operations during the reporting period. Such new investments resulted in the receipt by the Company of $575,000. In addition, the Company obtained $2,302,925 from borrowings, net of debt repayments, through issuance of promissory notes (see Note 7 to our audited Financial Statements below). These funds in conjunction with on going operating revenues provided adequate capital for our operating needs for 2009. We need to continue to raise funds to cover working capital requirements until we are able to raise revenues to a point of positive cash flow. We plan to do this, as before, through additional equity or debt financings. We may not be able to raise such funds on terms acceptable to us or at all. Financings may be on terms that are dilutive or potentially dilutive to our stockholders. If sources of financing are insufficient or unavailable, we will be required to modify our operating plans to the extent of available funding or curtail or suspend operations.
Our year end audited financial statements contain a “going concern” explanatory paragraph. Our inability to continue as a going concern would require a restatement of assets and liabilities on a liquidation basis, which would differ materially and adversely from the going concern basis on which our financial statements included in this report have been prepared.
Our consolidated financial statements for the year ended December 31, 2008 included herein have been prepared on the basis of accounting principles applicable to a going concern. Our auditors’ report on the consolidated financial statements contained herein includes an additional explanatory paragraph following the opinion paragraph on our ability to continue as a going concern. A note to these consolidated financial statements describes the reasons why there is substantial doubt about our ability to continue as a going concern and our plans to address this issue. Our December 31, 2008 and 2007 consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our inability to continue as a going concern would require a restatement of assets and liabilities on a liquidation basis, which would differ materially and adversely from the going concern basis on which our consolidated financial statements have been prepared. See, “Part II. Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations; Liquidity and Capital Resources.”
We are subject to significant government regulation.
The packaging, labeling, advertising, promotion, distribution and sale of Resurgex®, Resurgex Plus® Resurgex Select®, Surgex™, Resurgex Essential™ and Resurgex Essential Plus™ and other products we plan to produce and market are subject to regulation by numerous governmental agencies, the most active of which is the U.S. Food and Drug Administration (the "FDA"), which regulates our products under the Federal Food, Drug and Cosmetic Act (the "FDCA") and regulations promulgated there under. Our products are also subject to regulation by, among other regulatory entities, the Consumer Product Safety Commission (the "CPSC"), the U.S. Department of Agriculture (the "USDA") and the Environmental Protection Agency (the "EPA"). Advertising and other forms of promotion and methods of marketing of our products are subject to regulation by the U.S. Federal Trade Commission (the "FTC"), which regulates these activities under the Federal Trade Commission Act (the "FTCA"). The manufacture, labeling and advertising of our products are also regulated by various state and local agencies. Failure to comply with applicable regulatory requirements may result in, among other things, injunctions, product withdrawals, recalls, product seizures, and fines.
Our involvement in defending product liability claims could have a detrimental effect on our operations.
Like other retailers and distributors of products designed for human consumption, we face an inherent risk of exposure to product liability claims in the event that the use of our products results in injury. We may be subjected to various product liability claims, including, among others, that our products include inadequate instructions for use or inadequate warnings concerning possible side effects and interactions with other substances. We carry $10,000,000 of product liability insurance. Thus, any product liabilities exceeding our coverage relating to our products could have a material adverse effect on our business, financial condition and results of operations.
We face significant competition.
The biotechnology and nutraceutical supplement industries are highly competitive and subject to significant and rapid technological change. Developments by our competitors may render our products obsolete or noncompetitive. Numerous companies compete in our market, many of which have greater size and financial, personnel, distribution and other resources greater than ours. Our principal competition in the distribution channels where we are marketing our current products and where we intend to market other products comes from a limited number of large nationally known manufacturers and many smaller manufacturers of nutraceutical supplements. In addition, large pharmaceutical companies compete with us on a limited basis in the nutraceutical supplement market. Increased competition from such companies could have a material adverse effect on us because such companies have greater financial and other resources available to them and possess distribution and marketing capabilities far greater than ours. We also face competition in mass market distribution channels from private label nutraceutical supplements offered by health and natural food store chains and drugstore chains. We cannot assure that we will be able to compete.
If we are unable to protect our intellectual property or we infringe on intellectual property of others, our business and financial condition may be materially and adversely affected.
We own all rights to the formulation of Resurgex®, Resurgex Plus®, Resurgex Select®, Surgex™ Resurgex Essential™ and Resurgex Essential Plus™ and have a use and compositional patent with respect to Resurgex® (which covers Resurgex Plus®), and Resurgex Select®. Surgex™ is patent pending. We also have registered trademarks for the names "Resurgex", “Resurgex Plus” and “Resurgex Select”. “Surgex” has preliminary Trade mark reservation status. We have filed patent applications internationally with regards to all patents and patents pending. No assurance can be given that patents will be issued from pending applications or that there right, if issued and the rights from our existing patents and registered name will afford us adequate protections. In addition, we rely on trade secrets and unpatented proprietary technology. There is no assurance that others may not independently develop the same or similar technology or produce products which provide the same benefits as the current product lines.
Although we will seek to ensure that our products do not infringe the intellectual property rights of others, there can be no assurance that third parties will not assert intellectual property infringement claims against us. Any infringement claims by third parties against us may have a material adverse effect on our business, financial condition and results of operations.
Because our Board can issue common stock without stockholder approval, you could experience substantial dilution.
Our Board of Directors has the authority to issue up to 400,000,000 shares of common stock and to issue options and warrants to purchase shares of our common stock without stockholder approval. As of March 31, 2009, 314,465,510 shares are issued and outstanding or reserved for issuance on a fully-diluted basis. Future issuance of our additional shares of common stock could be at values substantially below the current market price of our common stock and, therefore, could represent substantial dilution to investors in this offering. In addition, our Board could issue large blocks of our common stock to fend off unwanted tender offers or hostile takeovers without further stockholder approval.
Anti-takeover provisions of the Delaware General Corporation Law could discourage a merger or other type of corporate reorganization or a change in control even if they could be favorable to the interests of our stockholders.
The Delaware General Corporation Law contains provisions which may enable our management to retain control and resist a takeover of us. These provisions generally prevent us from engaging in a broad range of business combinations with an owner of 15% or more of our outstanding voting stock for a period of three years from the date that this person acquires his stock. Accordingly, these provisions could discourage or make more difficult a change in control or a merger or other type of corporate reorganization even if they could be favorable to the interests of our stockholders.
We do not intend to pay cash dividends in the foreseeable future.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our earnings, if any, for use in its business and do not anticipate paying any cash dividends in the foreseeable future. The payment of any future dividends will be at the discretion of our Board of Directors and will depend upon a number of factors, including future earnings, the success of our business activities, our general financial condition and future prospects, general business conditions and such other factors as the Board of Directors may deem relevant. In addition, no cash dividends may be declared or paid on our Common Stock if, and as long as, the Series B Preferred Stock is outstanding or there are unpaid dividends on outstanding shares of Series C Preferred Stock. No dividends may be declared on the Series C Preferred Stock if, and as long as, the Series B Preferred Stock is outstanding. Accordingly, it is unlikely that we will declare any cash dividends in the foreseeable future.
We cannot assure that there will be a sustained public market for our common stock.
At present, our common stock is quoted on the OTC Bulletin Board and tradable in the over-the-counter market. Our common stock is not traded on a sustained basis or with significant volume. In addition, we currently do not meet the requirements for listing our common stock on NASDAQ or a national securities exchange and we cannot assure if or when our common stock will be listed on such an exchange. For the foregoing reasons, we cannot assure that there will be a significant and sustained public market for the sale of our common stock. Accordingly, if you purchase our common stock, you may be unable to resell it. In the absence of any readily available secondary market for our common stock, you may experience great difficulty in selling your shares at or near the price that you originally paid.
The market price of our common stock may be volatile.
The market price of our common stock may fluctuate significantly in response to the following factors:
| | · | variations in quarterly operating results; |
| | · | our announcements of significant contracts, milestones, acquisitions; |
| | · | our relationships with other companies or capital commitments; |
| | · | additions or departures of key personnel; |
| | · | sales of common stock or termination of stock transfer restrictions; |
| | · | changes in financial estimates by securities analysts; and |
| | · | fluctuations in stock market price and volume. |
Our stock price may be adversely affected if a significant amount of shares are sold in the public market.
As of March 31, 2009, approximately 63,719,238 shares of our common stock constituted "restricted securities" as defined in Rule 144 under the Securities Act of 1933. In addition, as of March 31, 2009, we had warrants outstanding for the purchase of an aggregate of 51,014,147 shares of our common stock, and stock options for 6,469,000 shares. None of the shares issuable upon exercise of the warrants are currently registered pursuant to agreements between us and the selling stockholders, requiring us to register their shares for resale under the Securities Act. Registration of the shares permits the sale of the shares of common stock in the open market or in privately negotiated transactions without compliance with the requirements of Rule 144. To the extent the exercise price of the warrants is less than the market price of the common stock, the holders of the warrants are likely to exercise them and sell the underlying shares of common stock and to the extent that the exercise price of the warrants are adjusted pursuant to anti-dilution protection, the warrants could be exercisable or convertible for even more shares of common stock. Moreover, some of the remaining restricted shares as well as shares resulting from the exercise of the other warrants and options may, after certain holding periods, enter the market, and we may issue additional shares to raise funding or compensate employees, consultants and/or directors. We are unable to estimate the amount, timing or nature of future sales of outstanding common stock. Sales of substantial amounts of our common stock in the public market could cause the market price for our common stock to decrease. Furthermore, a decline in the price of our common stock would likely impede our ability to raise capital through the issuance of additional shares of common stock or other equity securities.
Our shares are subject to the Penny Stock Reform Act.
Our shares are subject to the Penny Stock Reform Act of 1990 which may potentially decrease your ability to easily transfer our shares. Broker-dealer practices in connection with transactions in "penny stocks" are regulated. Penny stocks generally are equity securities with a price of less than $5.00. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer's account. In addition, the penny stock rules generally require that prior to a transaction in a penny stock, the broker-dealer make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for a stock that becomes subject to the penny stock rules. As our shares immediately following this offering will likely be subject to such penny stock rules, investors in this offering will in all likelihood find it more difficult to sell their securities.
Because the risk factors referred to above could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us, you should not place undue reliance on any such forward-looking statements. Further, any forward-looking statement speaks only as of the date on which it is made and we undertake no obligation to update any forward-looking statement or statements to reflect events or circumstances after the date on which such statement is made or reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
The Company leases certain office space and equipment under operating leases.
In October 2007, the Company extended its lease commitment an additional 5 years with an annual right to renew or cancel commencing in December 2007, for approximately 4,500 square feet of office space. The terms of the lease provide for a monthly rental rate of $10,635 per month, plus an allocated portion of certain operating expenses. The lease expense in 2008 was subsidized by $16,000 of sublease income. The Company presently occupies these facilities along with a new sub lessee paying approximately 30% of the rental expense projected for 2009. The lease is personally guaranteed by the Company’s former Chairman of the Board of Directors and Chief Executive Officer Jerry E. Swon.
Creative Healthcare Solutions, LLC vs. Millennium Biotechnologies Inc, Ct. of Common Pleas of Delaware County Ohio, Case No. 07 CV H 11 1420) Millennium was not satisfied with the service rendered by Creative Healthcare Solutions, LLC in 2005 which were associated with the development of Resurgex Select collateral materials developed in December of 2005. Millennium subsequently was forced to destroy and dispose of over 80% of the materials provided by Creative Healthcare Solutions due to the poor quality of the materials. Millennium has been unsuccessful in resolving the dispute and subsequently Creative Healthcare Solutions, LLC has filed legal action for demand of payment in the amount of $63,718 for services rendered. Millennium continues to negotiate a settlement through counsel with regards to this legal proceeding. Neither the Company nor Millennium has received any additional legal correspondence with regards to this matter as of May 4, 2009.
Ronald Burgert vs. Millennium Biotechnologies, Inc., et al. filed on the 9th day of October 2008 in District Court of Dallas County, Dallas, Texas. Mr. Burgert has filed a claim in the amount of $25,000 based on a note dated May 18, 2006. As of March 26, 2008 the balance due on the note, including unpaid principal and interest, was $31,635.12. On December 1, 2008, the 14th Judicial District, Dallas County, Dallas, Texas issued a default judgment against Millennium Biotechnologies, Inc. in the amount of $31,635.62 plus interest and unpaid attorney’s fees.
ESI Global Logistics, Inc. vs. Millennium Biotechnologies, Inc. filed on March 31, 2009 in the Superior Court of New Jersey, Law Division, Somerset County, Case #SOM-L-581-09. The ESI Global Logistics, Inc. claims a total of $54,111.95 plus costs and reasonable attorney fees based upon the Millennium Biotechnologies, Inc.’s failure to pay the plaintiff as an air freight carrier. The charges incurred by the plaintiff for the transfer of defendant’s product from the United States to Greece. Millennium Biotechnologies, Inc. has not responded to this claim as of May 4, 2009.
Riverwalk Village Center, LLC vs. Millennium Biotechnologies, Inc. filed on March 3, 2009 in the Superior Court of New Jersey, Law Division-Special Civil Part, Somerset County, Case #SOM-LT-572-09. Riverwalk Village Center, LLC brought a claim for unpaid rent and other charges against Millennium Biotechnologies, Inc. in the total amount of $68,076.02. The plaintiff and defendant have entered into a settlement order dated April 3, 2009 where in the plaintiff has paid a stipulated sum in the amount of $5,000 and has agreed to make subsequent payments to landlord in conjunction with certain subtenants of Millennium Biotechnologies, Inc. as the defendants. Three payments in the amount of approximately $20,000 each are to commence on 4/30/09 and to be completed by 6/18/09. In addition the rent payments for May and June 2009 must be made prior to the tenth day of each month in order to maintain the terms agreed to in the settlement agreement and prevent further action by the Riverwalk Village Center, LLC. Against Millennium Biotechnologies, Inc.
| ITEM 4: | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
No matters were submitted to a vote of the security holders during the fourth quarter of this fiscal period.
PART II
| ITEM 5: | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND SMALL BUSINESS ISSUER PURCHASES OF EQUITY SECURITIES |
(a) Market Information
The Company’s common stock currently trades in the OTC market and is quoted on the Electronic Bulletin Board of the OTC market, under the symbol MBTG. The following table sets forth, for the calendar quarters indicated during the last two fiscal years, the high and low quotations of the Company’s common stock. The quotations reflect inter-dealer prices, without retail mark-up, mark-down, or commission and may not represent actual transactions. The market for the common stock has been sporadic and there have been long periods during which there were few, if any, transactions in the common stock and no reported quotations. Accordingly, reliance should not be placed on the quotes listed below, as the trades and depth of the market may be limited, and therefore, such quotes may not be a true indication of the current market value of the Company's common stock.
| | | OTC-BB | |
| | | High/Bid | | | Low/Bid | |
| 2007 | | | | | | |
| First Quarter | | $ | 0.22 | | | $ | 0.14 | |
| Second Quarter | | | 0.24 | | | | 0.12 | |
| Third Quarter | | | 0.21 | | | | 0.16 | |
| Fourth Quarter | | | 0.24 | | | | 0.12 | |
| | | | | | | | | |
| 2008 | | | | | | | | |
| First Quarter | | $ | 0.17 | | | $ | 0.08 | |
| Second Quarter | | | 0.12 | | | | 0.07 | |
| Third Quarter | | | 0.10 | | | | 0.01 | |
| Fourth Quarter | | | 0.07 | | | | 0.01 | |
(b) Stockholders
As of March 31, 2009, there were approximately 1,511 stockholders of record for the Company’s Common Stock. The number of record holders does not include stockholders whose securities are held in street names the Company estimates over 1,000 holders in street names. In addition, there were approximately 10 holders of record of the Company's Series B Convertible Preferred Stock and 67 holders of record of the Company's Series C Preferred Stock.
(c) Dividends
The Company has not declared or paid, nor has it any present intention to pay, cash dividends on its common stock. No cash dividends may be declared or paid on the Company's Common Stock if, and as long as, the Series B Preferred Stock is outstanding or there are unpaid dividends on outstanding shares of Series C Preferred Stock. No dividends may be declared on the Series C Preferred Stock if, and as long as, the Series B Preferred Stock is outstanding. Accordingly, it is unlikely the Company will declare any cash dividends in the foreseeable future.
Recent Issues of Unregistered Securities
During the fourth quarter of 2008 the Company issued the following unregistered securities
| (i) | 5,400,000 shares of common stock to certain officers, directors and employees of the Company as compensation (see “Related Party Transactions”). |
| (ii) | 2,310,000 shares of common stock to six accredited investors pursuant to subscriptions for private placements, for aggregate cash receipts by the Company of $151,915. |
| (iii) | 27,121,000 shares of common stock to 28 creditors as loan origination and loan due date extension fees. |
| (iv) | 4,327,979 shares of common stock against conversion of promissory notes and accrued interest totaling $432,798. |
| (v) | 17,604,588 shares of common stock and warrants for the purchase of 5,000,000 shares, exercisable at $0.10 during three years, to twelve consultants and service providers, as remuneration. |
| (vi) | 750,000 shares of common stock to an investor in lieu of interest. |
| (vii) | 8,250,000 shares of common stock to four employees pursuant to agreements whereby such persons surrendered their respective right to royalties on the sale of some of the Company’s products. |
The foregoing issuances of securities were private transactions and exempt from registration under section 4(2) of the Securities Act and/or regulation D rule 506 promulgated under the Securities Act.
At December 31, 2008, stock certificates for an aggregate 16,193,905 shares issuable for investments and financing costs, and as compensation for services rendered had not yet been issued and the Company classified the valuation of such shares as current liabilities at year-end, totaling $1,263,239.
Information about common stock that may be issued upon the exercise of options and warrants is contained in the Notes to Consolidated Financial Statements attached hereto.
Securities authorized for issuance under equity compensation plans
Plan category | | Number of securities to be issued upon exercise of outstanding options, warrants and rights | | | Weighted-average exercise price of outstanding options, warrants and rights | | | Number of securities remaining available for future issuance under equity compensation plans | |
Equity compensation plans approved by security holders | | | 0 | | | | - | | | | 0 | |
| | | | | | | | | | | | | |
Equity compensation plans not approved by security holders | | | 0 | | | | - | | | | 500,000 | |
| | | | | | | | | | | | | |
| Total | | | 0 | | | | - | | | | 500,000 | |
Information about common stock that may be issued upon the exercise of options and warrants is contained in the Notes to Consolidated Financial Statements attached hereto.
Purchases of Equity Securities
None.
| ITEM 6: | SELECTED FINANCIAL DATA |
Except for historical information, the Company's reports to the Securities and Exchange Commission on Form 10-K, and Forms 10-QSB and 10-Q and periodic press releases, as well as other public documents and statements, contain "forward-looking statements" within the meaning of Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the statements. These risks and uncertainties include general economic and business conditions, development and market acceptance of the Company’s products, current dependence on the willingness of investors to continue to fund operations of the Company and other risks and uncertainties identified in the Company's reports to the Securities and Exchange Commission, periodic press releases, or other public documents or statements.
Readers are cautioned not to place undue reliance on forward-looking statements. The Company undertakes no obligation to republish or revise forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrences of unanticipated events.
The selected financial information presented below under the captions "Statement of Operations" and "Balance Sheet" for the years ended December 31, 2007 through 2008 is derived from the financial statements of the Company and should be read in conjunction with the financial statements and notes thereto.
The financial data are those of the Company including the operations of Millennium Biotechnologies Inc. All inter-company accounts and transactions have been eliminated in consolidation.
Balance Sheet
| | | December 31, | |
| | | 2008 | | | 2007 | |
| Total assets | | $ | 719,214 | | | $ | 832,680 | |
| Current liabilities | | | 15,549,779 | | | | 13,098,828 | |
| Long-term debt | | | 5,000 | | | | 10,000 | |
| Working capital | | | (14,862,083 | ) | | | (12,293,000 | ) |
| | | | | | | | | |
| Shareholders’ equity (deficit) | | $ | (14,835,565 | ) | | $ | (12,276,148 | ) |
Statement of Operations
| | | For the Year Ended December 31, | |
| | | 2008 | | | 2007 | |
| Total revenues | | $ | 1,492,262 | | | $ | 882,395 | |
| Operating income (loss) | | | (6,068,433 | ) | | | (7,852,169 | ) |
| Net (loss) | | | (11,361,216 | ) | | | (13,132,488 | ) |
| Net loss per common share | | $ | (0.06 | ) | | $ | (0.14 | ) |
| Number of shares used in | | | | | | | | |
| computing per share data | | | 175,885,553 | | | | 91,322,305 | |
| ITEM 7: | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Results of Operations for the year ended December 31, 2008 compared to the year ended December 31, 2007:
Total revenues generated from the sales of Resurgex®, Resurgex Plus®, Resurgex Select®, Surgex™, Resurgex Essential™ and Resurgex Essential Plus™ for the year ended December 31, 2008 totaled $1,492,262 an increase of 69% from the year ended December 31, 2007 which totaled $882,395.
At this stage in the Company’s development, revenues are not yet sufficient to cover ongoing operating expenses.
Gross profits for the year ended December 31, 2008 amounted to $756,896 for a 51% gross margin. Gross profits increased $330,531 or 78% for the year ended December 31, 2008 compared to $426,365 for the year ended December 31, 2007. The increase in gross profits is a direct result of increased revenues.
After deducting research and development costs of $476,806 and selling, general and administrative expenses of $6,348,523, which included $2,964,927 in non-cash outlays in the form of restricted stock and warrants issued for professional fees, and compensation, the Company realized an operating loss of $6,068,433 for the period ending December 31,2008. Operating losses for 2008 of $6,068,433 were down $1,783,736 or 23% as compared to the 2007 operating loss of $7,852,169. This is the second consecutive year he Company has realized a reduction of operating loss greater than 23%. Non-operating expenses totaled $5,292,783, which included non-cash outlays in the form of restricted stock and warrants issued for interest and financing costs totaling $4,117,871 for the year ended December 31, 2008. Non-operating expenses totaling $5,292,783 increased 2% as compared to $5,280,319 for the year ended December 31, 2007. The non-operating expenses continued to as a burden to the Company’s income statement due to due to in interest and financing expenses related to the requirements of short-term and long-term debt holders. Management was able to moderately subsidize non-operating expense by receiving $377,978 in miscellaneous income by receiving contributions made by long-term care facilities which were pledged to develop the Resurgex Essential™ ready to drink product line.
The net result for the year ended December 31, 2008 was a loss of $11,361,216 or $0.06 per share, compared to a loss of $13,132,488 or $0.14 per share for the prior year, net losses decreased by $1,771,272 or $0.08 per share and 13% or 57% respectively as compared to the prior year. The 2008 net results were significantly affected by the sustained high costs of interest and financing expenses which eliminated the positive effect of management’s ability to increase revenue 69%, gross profit by 78% while reducing operating expenses 18% during 2008. The Company will continue to invest in further expanding its operations and a comprehensive marketing campaign with the goal of accelerating the education of potential clients and promoting the name and products of the Company. Given the fact that most of the operating expenses are fixed or have quasi-fixed character management expects them to significantly decrease as a percentage of revenues as revenues increase as financial results during 2007 and 2008 have proven.
Liquidity and Capital Resources
The Company’s business operations generally have been financed by new equity and debt investments through private placements and promissory notes with accredited investors. During 2008, the Company obtained new equity capital totaling $575,000 and new debt financings totaling $2,641,911 which supplied the majority of the funds that were needed to finance operations during the reporting period. Such new investments resulted in the receipt by the Company of $3,216,911. While these funds sufficed to compensate for a majority of the negative cash flow from operations they were not sufficient to build up a liquidity reserve or sustain all of the operating costs. As a result, the Company’s financial position at the end of the year showed a working capital showing a deficit of $14,862,083. During the fourth quarter of 2008 and in the first quarter of 2009 the Company obtained new financing sufficient to fund a portion of the ongoing working capital requirements. We need to continue to raise funds to cover working capital requirements until we are able to raise revenues to a point of positive cash flow. See “Risk Factors: We require additional funding to maintain our operations and to further develop our business.
| ITEM 7 A: | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
The Company is subject to certain market risks, for changes in financial market conditions. The Company does not undertake any special actions to limit those exposures. We do not have a significant interest rate risk because the interest on all our debt obligations is based on fixed rates in accordance with the terms of such indebtedness.
| ITEM 8: | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The Company's Financial Statements and Notes to Financial Statements are attached hereto as Exhibit A and incorporated herein by reference.
| ITEM 9: | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
There have been no changes in or disagreements with the Registrant’s independent auditors during the last two years.
| Item 9A (T). | Controls and Procedures |
Evaluation of disclosure controls and procedures
Management of the Company has evaluated, with the participation of the Chief Executive Officer and Chief Financial Officer of the Company, the effectiveness of the Company's disclosure controls and procedures (as defined in Rules 13a-15(e) or 15d-15(e) promulgated by the Securities and Exchange Commission pursuant to the Securities Exchange Act of 1934, as amended (the "Exchange Act")) as of the end of the period covered by this Annual Report on Form 10-K. Based on that evaluation, the Chief Executive Officer and Chief Financial Officer of the Company had concluded that the Company's disclosure controls and procedures as of the period covered by this Annual Report on Form 10-K were not effective for the following reasons:
a) The deficiency was identified as the Company's limited segregation of duties amongst the Company's employees with respect to the Company's control activities. This deficiency is the result of the Company's limited number of employees. This deficiency may affect management's ability to determine if errors or inappropriate actions have taken place. Management is required to apply its judgment in evaluating the cost-benefit relationship of possible changes in our disclosure controls and procedures.
b) The deficiency was identified in respect to the Company's Board of Directors. This deficiency is the result of the Company's limited number of external board members. This deficiency may give the impression to the investors that the board is not independent from management. Management and the Board of Directors are required to apply their judgment in evaluating the cost-benefit relationship of possible changes in the organization of the Board of Directors.
Management's Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Management conducted an evaluation of the effectiveness of the Company's internal control over financial reporting as of December 31, 2008. In making this assessment, management used the framework set forth in the report entitled "Internal Control-Integrated Framework" issued by the Committee of Sponsoring Organizations of the Treadway Commission, or COSO. The COSO framework summarizes each of the components of a Company's internal control system, including (i) the control environment, (ii) risk assessment, (iii) control activities, (iv) information and communication, and (v) monitoring. Based on that evaluation, the Chief Executive Officer and Chief Financial Officer of the Company have concluded, as of the end of the fiscal year covered by this Annual Report on Form 10-K, due to a lack of segregation of duties that our internal control over financial reporting has not been effective. However, at this time, our resources and size prevent us from being able to employ sufficient resources to enable us to have adequate segregation of duties within our internal control system. The Company intends to remedy the material weakness by hiring additional employees and reallocating duties, including responsibilities for financial reporting, among the Company's employees as soon as the Company has the financial resources to do so. Management is required to apply judgment in evaluating the cost-benefit relationship of possible changes in our disclosure controls and procedures.
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by our registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit us to provide only management's report in this annual report. Our registered public accounting firm will be required to attest to our management's assessment of internal control over financial reporting beginning with our annual report for the year ended December 31, 2009.
Changes in internal controls
Management of the Company has evaluated, with the participation of the Chief Executive Officer of the Company, any change in the Company's internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the fiscal year covered by this Annual Report on Form 10-K. There was no change in the Company's internal control over financial reporting identified in that evaluation that occurred during the fiscal year covered by this Annual Report on Form 10-K that has materially affected, or is reasonably likely to materially affect, the Company's internal control over financial reporting, other than what has been reported above.
ITEM 9B: OTHER INFORMATION
None.
PART III
| ITEM 10: | DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS; COMPLIANCE WITH SECTION 16(a) OF THE EXCHANGE ACT |
Information about all directors and executive officers of the Company are as follows:
| Name | | Position | | Term(s) of Office |
| | | | | |
| Jerry E. Swon, 59 | | Chief Executive Officer, | | Jul.27, 2001 until Aug 4, 2008 |
| | | Chairman of the Board | | Jul.27, 2001 until Aug 4, 2008 |
| | | Company Director | | Aug 4, 2008 until present |
| | | | | |
| Mark Mirken, 64 | | President, Chief Operating Officer | | September 2007 until present |
| | | Chief Executive Officer | | Aug 4, 2008 until present |
| | | Chairman of the Board | | Aug 4, 2008 until present |
| | | | | |
| Frank Guarino, 34 | | Chief Financial Officer | | Oct.15, 2001 until present |
| | | | | |
| Michael G. Martin, 57 | | Company Director | | Oct.15, 2001 until present |
| | | | | |
| David Sargoy, 49 | | Company Director | | Oct.15, 2001 until present |
| | | | | |
| Benjamin Custodio, 66 | | Company Director | | Oct 28, 2008 until present |
| | | | | |
| Kenneth Sadowsky, 46 | | Company Director | | Sep 17, 2008 until present |
| | | | | |
| Carl Germano, 54 | | Executive Vice President, Research | | May 15, 2001 until present |
| | | and Product Development | | |
There are no other family relationships among the Company's officers and directors. All directors hold office until the next annual meeting of stockholders and the election and qualification of their successors. Vacancies on the Board of Directors may be filled by the remaining directors until the next annual stockholders' meeting. Officers serve at the discretion of the Board.
A summary of the business experience for each of our officers and directors is as follows:
Jerry E. Swon
Mr. Swon, a founder of Millennium, has been Millennium's President and Chief Executive Officer since its formation, and the Company's President, Chief Executive Officer and Chairman of the Board since July 27, 2001. Prior to joining Millennium, and since 1992, Mr. Swon was the Chief Executive Officer of Royal Capital Inc., a New Jersey based company, which provided financial consulting and corporate structuring services to private and public companies. In 1998, Mr. Swon served as Chief Executive Officer and as a Director of Magnitude Information Systems Inc., a company engaged in the design and development of ergonomic software for office uses. Mr. Swon was also co-founder of the Tax Transfer Corporation of New Jersey in 1999. Mr. Swon received a B.A. degree from Hamline University in 1972. Mr. Swon resigned the position of chief executive officer on August 4, 2008.
Mark C. Mirken
Mr. Mark C. Mirken has been Millennium’s President and Chief Operating Office since August 2007 and its chief executive officer since August 2008. He was previously employed by Turbo Chef Technologies, Inc. (NASDAQ:OVEN). Mr. Mirken reported to the Chairman & Board of Directors and had global P&L accountability for the entire company. He conceived and executed business strategies, steered direction of development and growth, and managed all aspects of operations including R&D, engineering, product development, new business development, sales (domestic/international and direct/indirect), marketing (strategies, campaigns, collaterals), branding (differentiating features and benefits in six categories), manufacturing (in-house/contract and onshore/offshore), investor relations, PR, media affairs, and major/global channels and accounts management (Subway, Starbucks, BP, HMS Host). He also mentored and led a core management team of 10 executives (including CFO, CTO and Director of Manufacturing in China), directed 24-person global sales force, and provided indirect oversight to a worldwide workforce of 100 plus. Mr. Mirken earned a Bachelor of Science from the University of North Carolina, in addition he eared a J.D. from the University of North Carolina School of Law.
Frank Guarino
Mr. Frank Guarino has been the Chief Financial Officer (CFO) of Millennium Biotechnologies Group, Inc. and Millennium Biotechnologies, Inc. since 2001. Mr. Guarino actively participates in all fundraising activities with other members of senior management which have yielded Millennium over $20,000,000 in new capital throughout his tenure. His role also includes steady contributions as an active member of the senior management team in all operations, systems management, technology implementation and internal controls. Additionally Mr. Guarino along with the CEO of Millennium currently manages investor relations activities on a daily basis. Mr. Guarino was previously employed from 1997 through February 2001 as the Controller of First National Funding Corporation of America, a mortgage banking firm which grew from a small family business to a medium sized corporation with 55 branches nationwide producing over $350 million in annual volume at the time of his departure. Eight accounting and human resource employees reported directly to Mr. Guarino. From 1995 to 1997 Mr. Guarino was employed by Panasonic Broadcast and Television Systems Co., where his responsibilities evolved to an independent supervisory position in the accounts receivable department which collected over $400,000,000 annually in accounts receivable from clients which included national television networks. Mr. Guarino earned a Bachelor of Science in Accounting from St. Peter’s College in 1997.
Michael G. Martin
Mr. Martin was appointed a Director of the Company on October 15, 2001. In 1991, Mr. Martin founded Magnitude, Inc. (then known as Proformix, Inc.), a company engaged in the design and development of ergonomic software for office uses. Mr. Martin served as the Chairman and President of Magnitude, Inc. and its parent, Magnitude Information Systems Inc. from 1991 until 1999. Since June 2000, Mr. Martin has served as the director of business development for the Behrle Group, a regional interior office design company. Mr. Martin received a B.A. degree from Bloomsburg State College in 1972.
David Sargoy
Mr. Sargoy was appointed a Director of the Company on October 15, 2001. From January 1997 to the present, Mr. Sargoy has been a Director of the commercial real estate division of Brown Harris Stevens. Prior to 1997, Mr. Sargoy was a partner in Willrock L.I. Inc., a commercial real estate company. Mr. Sargoy received a Bachelor of Science degree from Ithaca College in 1979 and an M.B.A. from Washington University in 1981.
Carl Germano
Mr. Carl Germano serves as Millennium’s executive vice president of research and product development. He is a registered, certified, and licensed nutritionist. Mr. Germano holds a Master’s degree in clinical nutrition from New York University and has over 27 years of experience using innovative, complementary nutritional therapies in private practice. For the past 20 years, he has dedicated his efforts to research and product development for the dietary supplement and medical foods industries, where he has been instrumental in bringing unique nutritional substances and formulations to the health/dietary supplement industry. From April 1999 to July 2001, Mr. Germano was senior vice president of research and product development with Nutratech, Inc., a nutraceutical raw materials supplier. From 1992 to 1999, he was vice president of product development and research with Solgar Vitamin and Herb (noting that in 2005 NBTY Inc. [NTY-NYSE] purchased the Solgar Vitamin and Herb Co. from Wyeth Consumer Healthcare [WYE-NYSE]).
Benjamin Custodio
Mr. Custodio brings over 30 years experience in Pharmaceuticals, Nutrition and business development with expertise in the commercialization of consumer products to the Millennium Board. Currently, Mr. Custodio is the President and CEO for LCI Group, Ltd; a privately owned business development group which works with various companies commercializing products in the international arena. Prior to his time with LCI, Mr. Custodio worked with Johnson and Johnson, Ciba-Geigy and Roberts Pharmaceuticals, Del-Monte Beverage Corp, Wampole Inc., and Webber in the vitamin and nutrition field. Mr. Custodio has previously sat on the Board of Directors for Nu-Life Nutrition Ltd.
Kenneth Sadowsky
Mr. Sadowsky brings to Millennium extensive experience in the beverage industry. Mr. Sadowsky was a principal of Atlas Distributing, Inc. overseeing the non-alcoholic beverage division which he created. The division was founded in 1988 and sales were $50,000.00 that year. In 2007 sales were over $16,000,000 and the total company sales were in excess of $75 million. He was director of Energy Brands, Inc (glaceau) makers of Glaceau Vitaminwater, Smartwater and Fruitwater from 2000-2006 when the company sold a minority interest for over 4.1 Billion. In addition, Mr. Sadowsky is currently on the board of directors of All Market Inc., makers of Vita Coco Coconut water and Hint, Inc. makers of Hint Water. Mr. Sadowsky graduated with a BA from Tulane in 1984.
Compliance with Section 16(a) of the Securities Exchange Act of 1934
To our knowledge, based solely on a review of such materials as are required by the Securities and Exchange Commission, no officer, director or beneficial holder of more than ten percent of our issued and outstanding shares of common stock failed to file in a timely manner with the Securities and Exchange Commission any form or report required to be so filed pursuant to Section 16(a) of the Securities Exchange Act of 1934 during the fiscal year ended December 31, 2008 except for Messrs. Jerry E. Swon, David Sargoy, Michael Martin, Mark C. Mirken, Frank Guarino, Kenneth Sadowsky, who are currently late in filing forms 4 pertaining to certain security acquisitions from the Company in 2008 and 2007.
Audit Committee and Audit Committee Expert
Audit Committee. We have do not have an audit committee at this time. Our securities are not listed on a national securities exchange. Accordingly, all members of the audit committee are not required to be independent. We do not have a financial expert as defined in Securities and Exchange Commission rules on the committee in the true sense of the description.
Corporate Governance And Code Of Ethics
The Company has always been committed to good corporate governance. In furtherance of this commitment, in February 2003 the Board of Directors appointed an Audit Committee whose duties specifically include responsibility and oversight of corporate governance matters and adherence to the Company’s Code of Ethics.
A copy of the Corporate Code of Ethics and Conduct was set forth as an exhibit to Form 10-KSB for the fiscal year ended December 31, 2002, and is included herein by reference. A copy may be obtained free of charge by submitting a request in writing to the Company at the address shown on the first page of this report.
| ITEM 11: | EXECUTIVE COMPENSATION |
The following table sets forth certain compensation information for: (i) the person who served as the Chief Executive Officer of Magnitude during the year ended December 31, 2008, regardless of the compensation level, and (ii) each of our other executive officers, serving as an executive officer at any time during 2008, as well as the most highly compensated employees who did not serve as executive officers during 2008. Compensation information is shown for the fiscal years ended December 31, 2007 and 2006:
Name and Principal Position | | Year | | Salary ($) | | | Directors Fee ($) | | | Other Annual Compensation($) | | | Restricted Stock Awards ($) | | | Securities Underlying Options ($) | | | All Other Compens.($) | |
| | | | | (1) | | | | | | (2) | | | | | | (3) | | | | |
Jerry E. Swon (4) | | 2008 | | | 175,000 | | | | - | | | | 10,500 | | | | - | | | | - | | | | |
| Former CEO, | | 2007 | | | 300,000 | | | | 65,719 | | | | 18,000 | | | | - | | | | - | | | - | |
| Director | | 2006 | | | 300,000 | | | | 25,000 | | | | 18,000 | | | | - | | | | - | | | | |
Mark C. Mirken (6) | | 2008 | | | 350,000 | | | | - | | | | - | | | | - | | | | - | | | | - | |
| President and CEO, | | 2007 | | | 87,166 | | | | - | | | | - | | | | 1,080,000 | | | | - | | | | - | |
| Director | | 2006 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
Carl Germano (5) | | 2008 | | | 200,000 | | | | - | | | | - | | | | 47,286 | | | | - | | | | - | |
| Exec. Vice President | | 2007 | | | 200,000 | | | | - | | | | - | | | | 51,300 | | | | 214,701 | | | | - | �� |
| | | 2006 | | | 200,000 | | | | - | | | | - | | | | - | | | | - | | | | - | |
Frank Guarino (7) | | 2008 | | | 200,000 | | | | - | | | | 12,000 | | | | 30,000 | | | | - | | | | | |
| Chief Financial Officer | | 2007 | | | 150,000 | | | | - | | | | 12,000 | | | | 34,000 | | | | - | | | | - | |
| | | 2006 | | | 147,017 | | | | - | | | | 12,000 | | | | - | | | | - | | | | - | |
| (1) | The value of other non-cash compensation, except for the items listed under (2), (3), (4) and (5), that was extended to or paid for individuals named above did not exceed 10% of the aggregate cash compensation paid to such individual, or to all executive officers as a group. |
| (2) | Consists of automobile expenses allowances. |
| (3) | The Company did not expense options and warrants granted to the named executives in the years 2002 to 2004. Beginning with the year 2005 the Company recognized expenses for options and warrants granted to employees on the basis of fair value calculated using the Black-Scholes formula (see below). |
| (5) | In 2008, Mr. Germano received 750,000 restricted shares valued at $47,286 pursuant to an agreement whereby he surrendered his rights to royalties on the sales of certain Company products. In 2007 Mr. Germano received 285,000 restricted shares in connection with an agreement which terminated his royalty rights on the sales of the Company’s products; the market price of such shares is shown in column “Restricted Stock Awards”. He also conducted the cashless exercise of stock options which resulted in the issuance of 1,341,884 common shares to him, the value of which, at the market price for the Company’s stock, is shown in column “Other Compensation”. |
| (6) | In 2007, Mr. Mirken received 6,000,000 shares as a signing bonus in accordance with the terms of his employment agreement. These shares, priced at the market price of the Company’s stock, are shown in column “Restricted Stock Awards”. |
| (7) | In 2008, Mr. Guarino received 1,000,000 restricted shares, valued at $30,000, as bonus payment. In 2007, Mr. Guarino received 100,000 shares. These shares, priced at the market price of the Company’s stock, are shown in the “Restricted Stock Awards” column. |
Stock Options /Stock Purchase Warrants:
There were no options and stock purchase warrants granted during 2008, to executive officers, certain other employees with highest remuneration, directors, and beneficial owners of more than 10 percent of any class of equity securities of the Company.
There were no exercises of stock options or warrants during 2008 by executive officers, other employees with highest remuneration, directors or beneficial owners of more than 10 percent of any class of equity securities of the Company.
Compensation of our Directors
During 2008 none of our Directors received cash compensation. Michael Martin and David Sargoy received 1,250,000 restricted shares each, in lieu of $125,000 accrued director’s fees.
Employment Agreements
Certain employees have received employment agreements the details of which are outlined in the section “Employment Agreements” in the Notes to the Financial Statements attached as an exhibit to this report.
| ITEM 12: | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT |
The following table sets forth, as of March 31, 2009, the record and beneficial ownership of common stock of the Company by each executive officer and director, all executive officers and directors as a group, and each person known to the Company to own beneficially, or of record, five percent or more of the outstanding shares of the Company:
| Title | | Name and Address of | | Amount and Nature of | | | Percent | |
| of Class | | Beneficial Owner | | Beneficial Ownership (1) | | | of Class | |
| Common | | Jerry E. Swon | | | 1,870,390 | (2) | | | 0.8 | % |
| Stock | | Frank Guarino | | | 1,914,000 | (3) | | | 0.8 | % |
| | | Carl Germano | | | 2,842,313 | (4) | | | 1.2 | % |
| | Michael G. Martin | | | 1,829,099 | (5) | | | 0.8 | % |
| | David Sargoy | | | 1,841,099 | (6) | | | 0.8 | % |
| | Mark C. Mirken | | | 6,000,000 | | | | 2.5 | % |
| | | Kenneth Sadowsky | | | 250,000 | | | | - | % |
| | | All Directors and Executive Officers | | | 16,546,901 | | | | 6.9 | % |
| | | as a Group (7 persons) | | | | | | | | |
Address of all persons above: c/o the Company.
| (1) | For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares of common stock which such person has the right to acquire within 60 days of March 31, 2007. For purposes of computing the percentage of outstanding shares of common stock held by each person or group of persons named above, any security which such person or persons has or have the right to acquire within such date is deemed to be outstanding but is not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. Except as indicated in the footnote to this table and pursuant to applicable community property laws, the Company believes based on information supplied by such persons, that the persons named in this table have sole voting and investment power with respect to all shares of common stock which they beneficially own. |
| (2) | Includes 183,333 shares issuable upon exercise of options and warrants. These options and warrants have a cashless exercise provision and include certain piggyback registration rights. Does not include any securities owned by Jane Swon, Mr. Swon's spouse, as to which securities Mr. Swon disclaims beneficial ownership. |
| (3) | Includes 764,000 shares issuable upon exercise of warrants. |
| (4) | Mr. Germano holds options to purchase 1,708,979 shares of the Company’s common stock. Also included are shares issuable upon exercise of warrants to purchase 241,667 shares of Company common stock. All of these options and warrants have a cash-less exercise provision. |
| (5) | Includes 183,333 shares issuable upon exercise of warrants. These warrants have a cash-less exercise provision and include certain piggyback registration rights. |
| (6) | Includes 183,333 shares issuable upon exercise of warrants. These warrants have a cash-less exercise provision and include certain piggyback registration rights. |
** Less than 1%
| ITEM 13: | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
On September 11, 2007, the boards of directors of Millennium Biotechnologies, Inc. (the “Company”) and its parent Millennium Biotechnologies Group, Inc. (“Group”, and together with the Company, the “Companies”) appointed Mark C. Mirken as President and Chief Operating Officer of each of the Companies, effective immediately, in accordance with an employment agreement, dated as of September 11, 2007. On August 4, 2008, Mr. Mirken assumed the position of chief executive officer of the Company.
The term of Mr. Mirken’s employment under the employment agreement (the “Term”) commenced as of September 11, 2007 and will continue for a period of three years; provided, Mr. Mirken has the right to extend the term of employment for two additional years. Pursuant to the employment agreement, Mr. Mirken will receive an annual salary of $350,000 per year. In addition, during the Term, Mr. Mirken is entitled to receive an annual bonus of up to $2,725,000, depending on the gross sales of the Company during such year. For gross sales in excess of $100,000,000 during any year, the amount of additional bonuses, if any, will be at the discretion of the Board of Directors. Mr. Mirken also is entitled to receive temporary housing expense for one year, reimbursement of his moving expenses and a vehicle allowance during the Term. In addition, Mr. Mirken received a cash signing bonus of $100,000, and Group agreed to issue 6,000,000 shares of its Common Stock to Mr. Mirken. The employment agreement also provides that during each year of the Term, Mr. Mirken will receive three year options to purchase such number of Group’s common stock, at an exercise price of $.01 per share, as is equal to the greater of (i) one percent of all issued and outstanding shares of common stock of Group at the end of such year, for each $20,000,000 incremental increase in gross sales for such fiscal year which is in excess of the prior high point of gross sales reported in any prior fiscal year by the Company; or (ii) one percent of all issued and outstanding shares of common stock of Group at the end of such year, for each $30,000,000 increase in market capitalization of Group over and above the high point of market capitalization of Group for any fifteen consecutive trading days preceding such year. Pursuant to the employment agreement Mr. Mirken is entitled to a gross-up of his base salary to create a neutral tax impact for the issuance of any shares or options to him under the employment agreement.
The employment agreement terminates upon Mr. Mirken’s death and may be terminated at the option of the Company as a result of Mr. Mirken’s disability or for “cause” as defined in the employment agreement. Mr. Mirken has the right to terminate the employment agreement for “good reason” as defined in the employment agreement. In the event that the employment agreement is terminated due to Mr. Mirken’s death or disability, he is entitled to receive his annual salary for a period equal to the lessor of (i) three months from the date of death or disability or (ii) the balance of the Term; and all other accrued but unpaid compensation and benefits. If the employment agreement is terminated by the Company for “cause”, Mr. Mirken is not entitled to receive any compensation other than accrued but unpaid compensation and benefits. In the event Mr. Mirken terminates the employment agreement for “good reason”, the Company shall pay to Mr. Mirken his annual salary through the date of the end of the contract term; bonuses that have accrued and are unpaid as of the date of termination; and any Options which have been granted to Mr. Mirken as of the date of the termination. The employment agreement also provides for Mr. Mirken is subject to confidentiality, non-solicitation and non-compete covenants for a period of one year following his termination, provided such termination is not by the Company without “cause” or by Mr. Mirken for “good reason”.
During 2008, the Company issued restricted stock awards to certain officers and directors, as follows:
750,000 restricted shares to Carl Germano valued at $47,286 pursuant to an agreement whereby he surrendered his rights to royalties on the sales of certain Company products;
1,000,000 restricted shares to Frank Guarino, valued at $30,000, as bonus payment;
1,250,000 shares each to Michael Martin and David Sargoy, in lieu of $125,000 /each accrued director’s fees;
250,000 shares to Kenneth Sadowsky in connection with a $50,000 loan extended by Mr. Sadowsky to the Company,. Mr. Sadowsky extended another loan, for $156,000 to the Company for which he will receive 1,000,000 shares, to be issued in 2009.
| ITEM 14: | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
AUDIT FEES
Bagell, Josephs, Levine & Company LLC. billed us $56,227 for professional services rendered from their audit of our annual financial statements, during 2008, and $40,000 for audit services during 2007.
AUDIT-RELATED FEES
Bagell, Josephs, Levine & Company LLC did not bill us for, nor perform professional services rendered for assurance and related services that were reasonably related to the performance of audit or review of the Company's financial statements during the fiscal years ended December 31, 2008 and December 31, 2007.
TAX FEES
Bagell, Josephs, Levine & Company, LLC billed us in the aggregate amount of $1,563 and $0 for professional services rendered for tax related services during the fiscal years ended December 31, 2008 and December 31, 2007, respectively.
ALL OTHER FEES
The aggregate fees billed by Bagell, Josephs, Levine & Company, LLC for services rendered to the Company during the last two fiscal years, other than as reported above, were $0 and $0, respectively.
PART IV
| Exhibit | | Description |
| 3.1 | | Certificate of Incorporation and Bylaws of the Company.(1) |
| | | |
| 3.2 | | Certificate of Incorporation and Bylaws of Millennium.* |
| | | |
| 4.1 | | Certificate of Designations filed July 26, 2001* |
| | | |
| 10.1 | | Agreement and Plan of Reorganization between the Company, Millennium and the Stockholders of Millennium dated July 26, 2001.(2) |
| | | |
| 10.2 | | License Agreement with Isocell SA.(3) |
| | | |
| 10.3 | | Royalty and Investment Agreement between Millennium and P. Elayne Wishart dated January 11, 2001.* |
| | | |
| 10.4 | | Royalty and Investment Agreement between Millennium and Jane Swon dated January 11, 2001.* |
| | | |
| 10.5 | | Royalty and Investment Agreement between Millennium and David Miller dated January 11, 2001.* |
| | | |
| 10.6 | | Employment Agreement between Millennium and Jerry E. Swon dated April 1, 2001.* |
| | | |
| 10.7 | | Letter of Intent, among Millennium Biotechnologies Group, Inc., Millennium Biotechnologies Inc., Aisling Capital II, LP, dated April 5, 2006 (5) |
| | | |
| 21 | | Subsidiaries of the Company: |
| | | (i) Millennium Biotechnologies, Inc. is a corporation formed under the laws of the State of Delaware and is the name under which it conducts business. |
| | | |
| 14 | | Corporate Code of Ethics and Business Conduct (4) |
| | | |
| 23.1 | | Consent of Bagell, Josephs & Company, L.L.C., Independent Registered Public Accounting Firm. |
| | | |
| 31.1 | | Certification of Jerry E. Swon, Chief Executive Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 31.2 | | Certification of Frank Guarino, Chief Financial Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.1 | | Certification of Jerry E. Swon, Chief Executive Officer, pursuant to Sections 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350. |
| | | |
| 32.2 | | Certification of Frank Guarino, Chief Financial Officer pursuant to Sections 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350. |
| * | Previously filed as an exhibit to the Company's Annual Report on Form 10-KSB for the fiscal year ended July 31, 2001. |
| (1) | Previously filed as an exhibit to the Company's Annual Report on Form 10-K for the fiscal year ended July 31, 1981, and incorporated herein by reference. |
| (2) | Previously filed as an exhibit to the Company's report on Form 8-K filed on August 10, 2001, and incorporated herein by reference. |
| (3) | Portions of this Exhibit were omitted and have been filed separately with the Secretary of the Securities and Commission pursuant to the Company's Application requesting Confidential Treatment under Rule 406 of the Securities Act of 1933. |
| (4) | Previously filed as an exhibit to the Company’s Annual report on Form 10-KSB for the fiscal year ended December 31, 2002. |
| (5) | Previously filed as an exhibit to the Company's report on Form 8-K filed on April 5, 2006, and incorporated herein by reference. |
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act, the Registrant has caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | MILLENNIUM BIOTECHNOLOGIES GROUP, INC. |
| | | | |
| | | | |
| | By: | /s/ Mark C. Mirken | | Date: May 4, 2009 |
| | | Mark C. Mirken | | |
| | | Chief Executive Officer | | |
| | | (Principal Executive Officer), | | |
| | | | | |
| | | | | |
| | By: | /s/ Frank Guarino | | Date: May 4, 2009 |
| | | Frank Guarino | | |
| | | Chief Financial Officer | | |
| | | (Principal Financial Officer) | | |
In accordance with the requirements of the Securities Exchange Act, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | Name | | | Date |
| | | | | |
| | /s/ Jerry E. Swon | | | May 4, 2009 |
| | Jerry E. Swon, Director | | | |
| | Chairman of the Board | | | |
| | | | | |
| | /s/ Benjamin Custodio | | | May 4, 2009 |
| | Benjamin Custodio, Director | | | |
| | /s/ Michael G. Martin | | | May 4, 2009 |
| | Michael G. Martin, Director | | | |
| | | | | |
| | | | | |
| | /s/ Kenneth Sadowsky | | | May 4, 2009 |
| | Kenneth Sadowsky, Director | | | |
| | | | | |
| | | | | |
| | /s/ David Sargoy | | | May 4, 2009 |
| | David Sargoy, Director | | | |
Millennium Biotechnologies Group, Inc.
and Subsidiary
Consolidated Financial Statements
December 31, 2008
Millennium Biotechnologies Group, Inc. and Subsidiary
Index to the Consolidated Financial Statements
December 31, 2008
| | Page |
| | |
| Report of Independent Registered Public Accounting Firm | 1 |
| | |
| Financial Statements | |
| | |
| Consolidated Balance Sheets | 2 |
| | |
| Consolidated Statements of Operations | 3 |
| | |
| Consolidated Statement of Stockholders’ (Deficit) | 4-5 |
| | |
| Consolidated Statements of Cash Flows | 6-7 |
| | |
| Notes to the Consolidated Financial Statements | 8-28 |
Bagell, Josephs, Levine & Company, LLC
406 Lippincott Drive Suite J, Marlton, NJ 08053
Tel: 856.355.5900 Fax: 856.396.0022
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders’
Millennium Biotechnologies Group, Inc.
Basking Ridge, NJ 07920
We have audited the accompanying consolidated balance sheets of Millennium Biotechnologies Group, Inc. (the “Company”) as of December 31, 2008 and 2007, and the related consolidated statements of operations, changes in stockholders’ deficit and cash flows for each of the years in the two-year period ended December 31, 2008. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Millennium Biotechnologies Group, Inc., as of December 31, 2008 and 2007, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2008 in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company did not generate sufficient cash flows from revenues during the year ended December 31, 2008, to fund its operations. Also at December 31, 2008, the Company had negative net working capital of $14,862,083. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plan in regard to these matters is also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
BAGELL, JOSEPHS, LEVINE & COMPANY, LLC
BAGELL, JOSEPHS LEVINE & COMPANY, LLC
May 4, 2009
Millennium Biotechnologies Group, Inc. and Subsidiary
Consolidated Balance Sheets
For the Years Ended December 31, 2008 and 2007
| | | December 31, | |
| | | 2008 | | | 2007 | |
| Assets | | | | | (Restated) | |
Current Assets: | | | | | | |
Cash | | $ | 121,009 | | | $ | 107,042 | |
Accounts receivable, net of allowance for doubtful accounts of $0 and $50,000 | | | 39,966 | | | | 346,580 | |
Inventories | | | 54,671 | | | | 271,398 | |
Prepaid expenses | | | 472,050 | | | | 80,808 | |
Total Current Assets | | | 687,696 | | | | 805,828 | |
| | | | | | | | | |
| Property and equipment, net of accumulated depreciation of $131,809 and $129,651 | | | 5,920 | | | | 678 | |
| Patents, net of accumulated amortization of $4,271 and $3,695 | | | 7,246 | | | | 7,822 | |
| Deposits | | | 18,352 | | | | 18,352 | |
Total Assets | | $ | 719,214 | | | $ | 832,680 | |
| Liabilities and Stockholders’ (Deficit) | | | | | | | | |
Current Liabilities: | | | | | | | | |
Accounts payable and accrued expenses | | $ | 3,258,764 | | | $ | 2,390,197 | |
Obligations to be settled in stock | | | 1,263,239 | | | | 1,215,602 | |
Prepayments | | | 74,965 | | | | - | |
Short-term debt | | | 10,952,811 | | | | 9,493,029 | |
Total Current Liabilities | | | 15,549,779 | | | | 13,098,828 | |
| Accrued royalties, long-term portion | | | 5,000 | | | | 10,000 | |
Total Liabilities | | | 15,554,779 | | | | 13,108,828 | |
| | | | | | | | | |
| Stockholders’ (Deficit) | | | | | | | | |
Preferred stock, par value $1; 1,810,360 shares authorized, none issued and outstanding: | | | | | | | | |
Convertible Series B, 65,141 shares issued and outstanding; at redemption value | | | 130,282 | | | | 130,282 | |
Cumulative Series C, non-voting 64,763 shares issued and outstanding | | | 64,763 | | | | 64,763 | |
Convertible Series D, voting, 0 shares issued and outstanding | | | - | | | | - | |
| Common stock, par value $0.001; authorized 400,000,000 shares; issued and outstanding 240,904,713 shares as of 12/31/08; 133,238,812 as of 12/31/07 | | | 240,905 | | | | 133,239 | |
Additional paid-in capital | | | 46,448,394 | | | | 38,147,595 | |
Deferred compensation | | | (704,722 | ) | | | (1,098,055 | ) |
Accumulated Deficit | | | (61,015,187 | ) | | | (49,653,972 | ) |
Total Stockholders’ (Deficit) | | | (14,835,565 | ) | | | (12,276,148 | ) |
| | | | | | | | | |
Total Liabilities and Stockholders’ (Deficit) | | $ | 719,214 | | | $ | 832,680 | |
The accompanying notes are an integral part of the consolidated financial statements.
Millennium Biotechnologies Group, Inc. and Subsidiary
Consolidated Statements of Operations
For the Years Ended December 31, 2008 and 2007
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | |
| | | | | | (Restated) | |
| Net Sales | | $ | 1,492,262 | | | $ | 882,395 | |
| Cost of Sales | | | 735,366 | | | | 456,030 | |
| Gross Profit | | | 756,896 | | | | 426,365 | |
| | | | | | | | | |
| Research and development costs | | | 476,806 | | | | 97,171 | |
| Selling, general and administrative expenses | | | 6,348,523 | | | | 8,181,363 | |
| | | | | | | | | |
| Loss from operations | | | (6,068,433 | ) | | | (7,852,169 | ) |
| Other income (expense) | | | | | | | | |
| Loss on disposal of assets | | | - | | | | (282,901 | ) |
| Miscellaneous income | | | 377,978 | | | | 402,393 | |
| Miscellaneous expenses | | | (87,055 | ) | | | (25,608 | ) |
Interest and financing expense | | | (5,583,706 | ) | | | (5,374,203 | ) |
| Total other income (expense) | | | (5,292,783 | ) | | | (5,280,319 | ) |
| | | | | | | | | |
| Net loss before taxes | | | (11,361,216 | ) | | | (13,132,488 | ) |
| Provision for income taxes | | | - | | | | - | |
| | | | | | | | | |
| Net Loss | | $ | (11,361,216 | ) | | $ | (13,132,488 | ) |
| | | | | | | | | |
| Net Loss Per Common Share | | $ | (0.06 | ) | | $ | (0.14 | ) |
| | | | | | | | | |
| Weighted average number of basic and fully diluted shares outstanding | | | 175,885,553 | | | | 91,322,305 | |
The accompanying notes are an integral part of the consolidated financial statements.
Millennium Biotechnologies Group, Inc. and Subsidiary
Consolidated Statement of Stockholders’ (Deficit)
For the Years Ended December 31, 2008 and 2007
| | | Preferred Stock | | | Common Stock | | | | | | | | | | | | | |
| | | Convertible Series B Shares | | | Convertible Series B Amount | | | Cumulative Series C Shares | | | Cumulative Series C Amount | | | Convertible Series D Shares | | | Convertible Series D Amount | | | Shares | | | Amount | | | Deferred Compensation | | | Additional Paid in Capital | | | Accumulated Deficit | | | Total | |
| Balance, January 1, 2007 | | | 65,141 | | | $ | 130,282 | | | | 64,763 | | | $ | 64,763 | | | | - | | | $ | - | | | | 69,128,623 | | | $ | 69,129 | | | $ | - | | | $ | 27,118,745 | | | $ | (36,521,483 | ) | | $ | (9,138,564 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock and warrants - private placements | | | | | | | | | | | | | | | | | | | | | | | | | | | 24,569,474 | | | | 24,569 | | | | | | | | 2,950,351 | | | | | | | | 2,974,920 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock pursuant to exercise of warrants | | | | | | | | | | | | | | | | | | | | | | | | | | | - | | | | - | | | | | | | | 15,649 | | | | | | | | 15,649 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for compensation | | | | | | | | | | | | | | | | | | | | | | | | | | | 6,020,000 | | | | 6,020 | | | | | | | | 1,078,780 | | | | | | | | 1,084,800 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock pursuant to note conversion | | | | | | | | | | | | | | | | | | | | | | | | | | | 5,427,299 | | | | 5,427 | | | | | | | | 596,999 | | | | | | | | 602,426 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock and warrants for loan origination fees | | | | | | | | | | | | | | | | | | | | | | | | | | | 8,106,000 | | | | 8,106 | | | | | | | | 1,625,433 | | | | | | | | 1,633,539 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock and warrants for services | | | | | | | | | | | | | | | | | | | | | | | | | | | 14,436,563 | | | | 14,437 | | | | | | | | 3,201,823 | | | | | | | | 3,216,260 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Employee stock options | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 189,644 | | | | | | | | 189,644 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock issued for interest | | | | | | | | | | | | | | | | | | | | | | | | | | | 20,869 | | | | 21 | | | | | | | | 3,944 | | | | | | | | 3,965 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Change in deferred compensation | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (1,098,055 | ) | | | | | | | | | | | (1,098,055 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock and stock warrants for interest and note due date extensions | | | | | | | | | | | | | | | | | | | | | | | | | | | 3,034,000 | | | | 3,034 | | | | | | | | 581,018 | | | | | | | | 584,052 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exercise of stock options | | | | | | | | | | | | | | | | | | | | | | | | | | | 1,341,884 | | | | 1,342 | | | | | | | | 213,360 | | | | | | | | 214,702 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Beneficial conversion rights of convertible notes | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 570,396 | | | | | | | | 570,396 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Buy-out of royalty rights with newly issued common stock | | | | | | | | | | | | | | | | | | | | | | | | | | | 1,154,100 | | | | 1,154 | | | | | | | | (3,547 | ) | | | | | | | (2,393 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of equity investment versus deferred royalties | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 5,000 | | | | | | | | 5,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net (loss) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (13,132,488 | ) | | | (13,132,488 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2007 | | | 65,141 | | | $ | 130,282 | | | | 64,763 | | | $ | 64,763 | | | | - | | | $ | - | | | | 133,238,812 | | | $ | 133,239 | | | $ | (1,098,055 | ) | | $ | 38,147,595 | | | $ | (49,653,972 | ) | | $ | (12,276,148 | ) |
The accompanying notes are an integral part of the consolidated financial statements.
Millennium Biotechnologies Group, Inc. and Subsidiary
Consolidated Statement of Stockholders’ (Deficit)
For the Years Ended December 31, 2008 and 2007
| | | Preferred Stock | | | Common Stock | | | | | | | | | | | | | |
| | | Convertible Series B Shares | | | Convertible Series B Amount | | | Cumulative Series C Shares | | | Cumulative Series C Amount | | | Convertible Series D Shares | | | Convertible Series D Amount | | | Shares | | | Amount | | | Deferred Compensation | | | Additional Paid in Capital | | | Accumulated Deficit | | | Total | |
| Balance, January 1, 2008 | | | 65,141 | | | $ | 130,282 | | | | 64,763 | | | $ | 64,763 | | | | - | | | $ | - | | | | 133,238,812 | | | $ | 133,239 | | | $ | (1,098,055 | ) | | $ | 38,147,595 | | | $ | (49,653,972 | ) | | $ | (12,276,148 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock - private placements | | | | | | | | | | | | | | | | | | | | | | | | | | | 5,650,000 | | | | 5,650 | | | | | | | | 569,350 | | | | | | | | 575,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for license fees | | | | | | | | | | | | | | | | | | | | | | | | | | | 1,500,000 | | | | 1,500 | | | | | | | | 148,500 | | | | | | | | 150,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for compensation | | | | | | | | | | | | | | | | | | | | | | | | | | | 2,800,000 | | | | 2,800 | | | | | | | | 174,200 | | | | | | | | 177,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock pursuant to note conversion | | | | | | | | | | | | | | | | | | | | | | | | | | | 11,120,000 | | | | 11,120 | | | | | | | | 1,127,880 | | | | | | | | 1,139,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock and warrants for loan origination fees | | | | | | | | | | | | | | | | | | | | | | | | | | | 12,055,667 | | | | 12,056 | | | | | | | | 1,230,770 | | | | | | | | 1,242,826 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for services | | | | | | | | | | | | | | | | | | | | | | | | | | | 27,872,588 | | | | 27,873 | | | | | | | | 1,877,556 | | | | | | | | 1,905,429 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock and warrants for directors’ fees | | | | | | | | | | | | | | | | | | | | | | | | | | | 2,600,000 | | | | 2,600 | | | | | | | | 281,400 | | | | | | | | 284,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock issued for interest | | | | | | | | | | | | | | | | | | | | | | | | | | | 5,137,146 | | | | 5,137 | | | | | | | | 428,583 | | | | | | | | 433,720 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Change in deferred compensation | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 393,333 | | | | | | | | | | | | 393,333 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock and stock warrants for note due date extensions | | | | | | | | | | | | | | | | | | | | | | | | | | | 21,245,000 | | | | 21,245 | | | | | | | | 1,787,365 | | | | | | | | 1,808,610 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for late payment penalties | | | | | | | | | | | | | | | | | | | | | | | | | | | 9,435,500 | | | | 9,435 | | | | | | | | 623,280 | | | | | | | | 632,715 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Buy-out of royalty rights with newly issued common stock | | | | | | | | | | | | | | | | | | | | | | | | | | | 8,250,000 | | | | 8,250 | | | | | | | | 46,915 | | | | | | | | 55,165 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of equity investment versus deferred royalties | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 5,000 | | | | | | | | 5,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net (loss) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (11,361,216 | ) | | | (11,361,216 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2008 | | | 65,141 | | | $ | 130,282 | | | | 64,763 | | | $ | 64,763 | | | | - | | | $ | - | | | | 240,904,713 | | | $ | 240,905 | | | $ | (704,722 | ) | | $ | 46,448,394 | | | $ | (61,015,187 | ) | | $ | (14,835,565 | ) |
The accompanying notes are an integral part of the consolidated financial statements.
Millennium Biotechnologies Group, Inc. and Subsidiary
Consolidated Statements of Cash Flows
For the Years Ended December 31, 2008 and 2007
| | | 2008 | | | 2007 | |
| | | | | | (Restated) | |
| Cash Flows from Operating Activities: | | | | | | |
Net loss | | $ | (11,361,216 | ) | | $ | (13,132,488 | ) |
Adjustments to reconcile net (loss) to net cash (used) by Operating Activities: | | | | | | | | |
Depreciation and amortization | | | 2,734 | | | | 9,788 | |
Stock issued for services | | | 1,905,429 | | | | 2,284,982 | |
Stock issued for compensation | | | 461,000 | | | | 391,090 | |
Stock issued for royalty rights and license fees | | | 205,165 | | | | - | |
Change in inventory and receivables reserve | | | (50,000 | ) | | | (120,000 | ) |
Convertible feature of notes | | | - | | | | 704,818 | |
Amortization of deferred compensation | | | 393,333 | | | | - | |
Stock issued for interest and financing expenses | | | 4,117,871 | | | | 1,954,772 | |
Changes in assets and liabilities | | | | | | | | |
Decrease in inventory | | | 216,727 | | | | 127,527 | |
Decrease (Increase) in accounts receivable | | | 356,614 | | | | (220,044 | ) |
| (Increase) in prepaid expenses | | | (391,242 | ) | | | (53,022 | ) |
Increase in customer prepayments | | | 74,965 | | | | - | |
Liability for stock to be issued | | | 47,637 | | | | 747,251 | |
Increase (Decrease) in accounts payable and accrued expenses | | | 1,164,424 | | | | 1,948,548 | |
Net Cash (Used) by Operating Activities | | | (2,856,559 | ) | | | (5,356,778 | ) |
Cash Flows from Investing Activities: | | | | | | | | |
Purchases of property and equipment | | | (7,400 | ) | | | - | |
Net Cash (Used) by Investing Activities | | | (7,400 | ) | | | - | |
Cash Flows from Financing Activities: | | | | | | | | |
Proceeds from borrowings | | | 2,641,911 | | | | 2,324,685 | |
Repayment of loans and notes | | | (338,986 | ) | | | (818,408 | ) |
Proceeds from issuance of common and preferred stock | | | 575,000 | | | | 3,945,571 | |
Net Cash Provided by Financing Activities | | | 2,877,925 | | | | 5,451,848 | |
Net Increase (Decrease) in Cash | | | 13,967 | | | | 95,070 | |
Cash - beginning of year | | | 107,042 | | | | 11,972 | |
Cash - end of year | | $ | 121,009 | | | $ | 107,042 | |
| | | | | | | | | |
| Supplemental information: | | | | | | | | |
Cash paid during the year for: | | | | | | | | |
Interest | | $ | 391,340 | | | $ | 296,575 | |
Income taxes | | $ | - | | | $ | - | |
The accompanying notes are an integral part of the consolidated financial statements.
Millennium Biotechnologies Group, Inc. and Subsidiary
Consolidated Statements of Cash Flows
For the Years Ended December 31, 2008 and 2007
| | | 2008 | | | 2007 | |
| Schedule of non-cash investing and financing activities: | | | | | | | |
| | | | | | | | |
| In consideration for services and compensation, 33,272,588 common shares and 3,200,000 warrants were issued | | $ | 2,366,429 | | | | |
| | | | | | | | |
| In consideration of the extension of due dates, interest, and late payment penalties on promissory notes, 35,817,646 common shares and 4,462,176 warrants were issued | | $ | 2,924,841 | | | | |
| | | | | | | | |
| In consideration for loan origination fees, 12,055,667 common shares and 7,000,000 warrants were issued | | $ | 1,193,030 | | | | |
| | | | | | | | |
| In consideration for services and compensation, 20,456,563 common shares and 4,122,500 warrants were issued | | | | | | $ | 4,301,060 | |
| | | | | | | | | |
| In consideration of the extension of due dates and interest on promissory notes, 3,034,000 common shares and 251,060 warrants were issued | | | | | | $ | 584,052 | |
| | | | | | | | | |
| In consideration for loan origination fees, 8,106,600 common shares and 10,686,667 warrants were issued | | | | | | $ | 1,633,539 | |
| | | | | | | | | |
| In connection with conversion of convertible notes, 5,427,299 common shares were issued | | | | | | $ | 602,426 | |
The accompanying notes are an integral part of the consolidated financial statements.
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization
| | Millennium Biotechnologies Group, Inc. (the Company or "Millennium Group"), formerly Regent Group, Inc., is a holding company for its subsidiary Millennium Biotechnologies, Inc. ("Millennium"). |
| | Millennium was incorporated in the State of Delaware on November 9, 2000 and is located in New Jersey. Millennium is a research based bio-nutraceutical corporation involved in the field of nutritional science. Millennium’s principal source of revenue is from sales of its nutraceutical supplements, Resurgex®, Resurgex Plus®, Resurgex Select® and Surgex™ which serve as a nutritional support for immuno-compromised individuals undergoing medical treatment for chronic debilitating diseases. |
| | The Company acquired Millennium on July 27, 2001, when it completed a merger with Millennium. In the merger, new Convertible Preferred Series D stock was issued in exchange for all the outstanding stock of Millennium. Such preferred shares were convertible into approximately 96% of the outstanding common stock of the Company at the time of issuance. Under the terms of the Agreement and Plan of Reorganization, a new wholly-owned Millennium Group subsidiary merged into Millennium. For accounting purposes, the merger has been treated as an acquisition of Millennium Group by Millennium, and a re-capitalization of Millennium. The financial statements are those of the Company and its wholly-owned subsidiary Millennium on a consolidated basis. |
Principles of Consolidation
| | The Company’s operations presently consist almost exclusively of the operations of Millennium. The consolidated financial statements include the accounts of the Company and its subsidiary. All significant inter-company transactions and balances have been eliminated. |
In the opinion of the Company’s management, all adjustments (consisting of normal recurring accruals) necessary to present fairly the Company’s financial position as of December 31, 2008 and December 31, 2007, the results of operations for the years ended December 31, 2008 and 2007, and the cash flows for the years ended December 31, 2008 and 2007, have been included.
Use of Estimates
| | The preparation of the financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. |
Property and Equipment
| | Property and equipment are stated at cost less accumulated depreciation. Depreciation, which includes amortization of assets under capital leases, is calculated using the straight-line method over the estimated useful lives of the assets: 3-8 years for machinery and equipment, leasehold improvements are amortized over the shorter of the estimated useful lives of the underlying lease term. Repairs and maintenance expenditures which do not extend the useful lives of related assets are expensed as incurred. For Federal income tax purposes, depreciation is computed under accelerated methods over the assets class life. |
Patents
| | Patents are capitalized and amortized over 240 months. Amortization expense was $576 and $576 for 2008 and 2007, respectively. |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES, Continued
Evaluation of Long-Lived Assets
Long-lived assets are assessed for recoverability on an ongoing basis. In evaluating the fair value and future benefits of long-lived assets, their carrying value would be reduced by the excess, if any, of the long-lived asset over management’s estimate of the anticipated undiscounted future net cash flows of the related long-lived asset.
Revenue Recognition
| | Revenue is recognized at the date of shipment to customers provided that the resulting receivable is deemed probable of collection. |
Advertising costs
| | Advertising costs are charged to operations when incurred. Advertising expense was $3,121 and $76,044 for the years ended December 31, 2008 and 2007, respectively. |
Shipping and Handling Costs
| | Shipping costs are included in cost of sales. Handling costs are included in general and administrative expenses and were accounted for with $67,326 and $92,164 for the years ended December 31, 2008 and 2007, respectively. |
Stock-Based Compensation
| | Effective January 1, 2006, the Company adopted the provisions of Financial Accounting Standards Board Statement of Financial Accounting Standard ("SFAS") No. 123(R), "Share-Based Payments," which establishes the accounting for employee stock-based awards. Under the provisions of SFAS No. 123(R), stock-based compensation is measured at the grant date, based on the calculated fair value of the award, and is recognized as an expense over the requisite employee service period (generally the vesting period of the grant). The Company adopted SFAS No. 123(R) using the modified prospective method and, as a result, periods prior to December 31, 2005 have not been restated. The Company recognized stock-based compensation for awards issued under the Company's stock option plans in other income/expenses included in the Consolidated Statement of Operations. Additionally, no modifications were made to outstanding stock options prior to the adoption of SFAS No. 123(R), and no cumulative adjustments were recorded in the Company's financial statements. |
Income Taxes
| | The Company provides for income taxes based on enacted tax law and statutory tax rates at which items of income and expenses are expected to be settled in the Company’s income tax return. Certain items of revenue and expense are reported for Federal income tax purposes in different periods than for financial reporting purposes, thereby resulting in deferred income taxes. Deferred taxes are also recognized for operating losses that are available to offset future taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. The Company has incurred net operating losses for financial-reporting and tax-reporting purposes. Accordingly, for Federal and state income tax purposes, the benefit for income taxes has been offset entirely by a valuation allowance against the related federal and state deferred tax asset for the years ended December 31, 2008 and 2007. |
Loss Per Common Share
| | Basic and diluted loss per common share are computed by dividing net loss by the weighted average number of common shares outstanding during the periods. Potential common shares used in computing diluted earnings per share related to stock options, warrants, convertible preferred stock and convertible debt which, if exercised, would have an anti- dilutive effect on earnings per share, have not been included. |
Fair Value of Financial Instruments
| | For financial instruments including cash, prepaid expenses and other current assets, short-term debt, accounts payable and accrued expenses, it was assumed that the carrying values approximated fair value because of their short-term maturities. |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial StatementsDecember 31, 2008 and 2007
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES, Continued
Limitations
| | Fair value estimates are made at a specific point in time, based on relevant market information and information about the financial statement. These estimates are subjective in nature and involve uncertainties and matters of significant judgment and therefore cannot be determined with precision. Changes in assumptions could significantly affect the estimates. |
Reclassification
| | Certain reclassifications have been made to prior year balances to conform to the current year’s presentation. These reclassifications has no effect on financial position or operations. |
2. GOING CONCERN
As shown in the accompanying consolidated financial statements, the Company incurred substantial net losses for the years ended December 31, 2008 and 2007. There is no guarantee that the Company will be able to generate enough revenue and/or raise capital to support those operations. This raises substantial doubt about the Company’s ability to continue as a going concern.
The Company’s future success is dependent upon its ability to achieve profitable operations and generate cash from operating activities, and upon additional financing. There is no guarantee that the Company will be able to raise enough capital or generate revenues to sustain its operations. Management believes they can raise the appropriate funds needed to support their business plan and acquire an operating, cash flow positive company.
The consolidated financial statements do not include any adjustments relating to the recoverability or classification of recorded assets and liabilities that might result should the Company be unable to continue as a going concern.
3. CONCENTRATIONS OF BUSINESS AND CREDIT RISK
The Company maintains cash balances in several financial institutions which are insured by the Federal Deposit Insurance Corporation up to $250,000. Balances in these accounts may, at times, exceed the federally insured limits.
The Company provides credit in the normal course of business to customers located throughout the U. S. The Company performs ongoing credit evaluations of its customers and maintains allowances for doubtful accounts based on factors surrounding the credit risk of specific customers, historical trends, and other information.
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
4. INVENTORIES
Inventories consist of finished goods, samples and packaging for the Company’s Resurgex®, Resurgex Plus®, Resurgex Select®, and Surgex™ product lines. Cost-of-goods sold are calculated using the average costing method. Inventories at December 31, 2008 and 2007, consisted of the following:
| | | 2008 | | | 2007 | |
| Finished Goods | | $ | 115,179 | | | $ | 323,416 | |
| Samples | | | - | | | | 8,490 | |
| Packaging | | | 3,746 | | | | 3,746 | |
| | | | 118,925 | | | | 335,652 | |
| Less: Reserve for losses | | | (64,254 | ) | | | (64,254 | ) |
| Total | | $ | 54,671 | | | $ | 271,398 | |
5. PROPERTY AND EQUIPMENT
Property and equipment at cost, less accumulated depreciation, at December 31, 2008 and 2007, consisted of the following:
| | | 2008 | | | 2007 | |
| Furniture | | $ | 46,127 | | | $ | 46,127 | |
| Equipment | | | 22,445 | | | | 22,445 | |
| Leasehold improvements | | | 69,157 | | | | 61,757 | |
| Subtotal | | | 137,729 | | | | 130,329 | |
| Less accumulated depreciation | | | (131,809 | ) | | | (129,651 | ) |
| Total | | $ | 5,920 | | | $ | 678 | |
Depreciation expense charged to operations was $2,158 and $9,712 for the years ended December 31, 2008 and 2007, respectively.
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
6. ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses consisted of the following at December 31, 2008 and 2007:
| | | 2008 | | | 2007 | |
| Accounts payable | | $ | 1,548,061 | | | $ | 769,105 | |
| Accrued interest | | | 1,037,214 | | | | 591,795 | |
| Accrued salaries, bonuses and payroll taxes | | | 505,280 | | | | 332,831 | |
| Accrued royalties | | | 5,000 | | | | 73,976 | |
| Accrued professional fees | | | 48,000 | | | | 48,000 | |
| Accrued minimum purchase obligations | | | 115,209 | | | | 265,209 | |
| Accrued directors’ fees | | | - | | | | 309,281 | |
| | | $ | 3,258,764 | | | $ | 2,390,197 | |
7. DEBT
Short-term debt at December 31, 2008 and 2007, is as follows:
| | | 2008 | | 2007 |
| Cash advances by three accredited investors, due on demand, non-interest bearing. | $ | 4,440 | $ | 11,440 |
| | | | | |
| Promissory note dated December 17, 2002, originally for $50,000 issued to an accredited investor, maturing September 28, 2003, bearing interest at the rate of 10% per annum. The note has been changed to be due on demand and remains outstanding at December 31, 2008. The holder of the note is entitled to convert all or a portion of the principal and interest at any time after the maturity date into shares of common stock of the Company at a price equal to $.10/share of the principal if the principal and interest is not fully repaid on or before the maturity date. Management has repaid $25,000 in December 2003. The Company issued 125,000 5-year common stock purchase warrants in conjunction with the note which were exercised at a rate of $0.01 per share. The computed discount (computed with Black-Scholes) related to the detachable stock purchase warrants has been fully amortized. | | 25,000 | | 25,000 |
| Convertible Promissory Note to an accredited investor dated May 20, 2003, maturing May 20, 2004, bearing interest at a rate 8% per annum payable in restrictedshares of common stock. | | 30,000 | | 30,000 |
| Convertible promissory note dated July 3, 2003 originally due December 31, 2003, bearing interest at 12% per year payable in restricted common stock, extended through December 31, 2004. The note has subsequently been changed to be due on demand and remains outstanding at December 31, 2008. The note is convertible at the option of the holder into restricted common stock at the rate of $0.20 per share. | | 50,000 | | 50,000 |
| | | | | |
| Promissory note issued to an accredited investor on February 18, 2005 and originally maturing June 18, 2005, carrying interest at the rate of 12% per year. The note remains open at December 31, 2008 and is now subject to a late payment penalty of $5,000 per month. | | 100,000 | | 100,000 |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
DEBT (Continued)
| | | 2008 | | 2007 |
| Two demand loans extended by two investors in March 2004 and January 2005, bearing no interest. | $ | 25,000 | $ | 25,000 |
| Promissory note for $200,000 issued to an accredited investor on July 12, 2005 and maturing December 31, 2005, carrying interest at the rate of 10% per year. The note is presently due on demand. The note is convertible into common shares of the Company at the option of the holder, at $0.25 per share. | | 200,000 | | 200,000 |
| Promissory grid note issued to an accredited investor on December 22, 2005, due on demand. The note carries interest at the rate of 12% per year and has an open balance of $552,237 on December 31, 2008. | | 552,237 | | 632,637 |
| Promissory note issued to an accredited investor on January 12, 2006, maturing on 12/31/06, presently due on demand. The note carries interest at the rate of 10% per year and is convertible into common shares at the rate of $0.25 /share. | | 113,432 | | 113,432 |
| 12 promissory notes issued to 12 accredited investors in May 2006, originally maturing in June 2006. All notes have since changed to be due on demand. The notes carried interest at the rate of 10% per year and are convertible into common shares at the rate of $0.25 /share | | 299,640 | | 298,664 |
| | | | | |
| Two promissory notes issued to two accredited investors on June 29, 2006 and July 5, 2006, originally maturing 8/1/06 and 9/15/06, since changed to be due on demand. The Company had issued warrants for an aggregate 3,000,000 shares, exercisable at $0.25/share as loan origination fee. The notes carried interest at the rate of 10% per year until maturity and are now subject to a rate of 14% per year, and are convertible into common shares at the rate of $0.25 /share. | | 750,000 | | 750,000 |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
DEBT (Continued)
| | | 2008 | | 2007 |
| 6 promissory notes issued to 6 accredited investors between July and September 2006, originally maturing at various dates between 9/15/06 and 1/31/07, all of which since having been changed to due on demand. The notes carried interest at the rate of 10% per year until maturity and thereafter are subject to a rate of 14% per year, and are convertible into common shares at the rate of $0.25 /share. | $ | 340,000 | $ | 515,000 |
| | | | | |
| 3 promissory notes issued to 3 accredited investors in September 2006, maturing at various dates between 11/30/06 and 1/31/07, since been changed to be due on demand. The Company had issued warrants for an aggregate 392,000 shares, exercisable at $0.25/share as loan origination fee. The notes carried interest at the rate of 10% per year until maturity and thereafter are subject to a rate of 14% per year, and are convertible into common shares at the rate of $0.25 /share. | | 63,000 | | 88,000 |
| | | | | |
| 5 promissory notes issued to 5 accredited investors in October 2006, maturing on 1/31/07, subsequently changed to be due on demand. The Company had issued warrants for an aggregate 1,060,000 shares, exercisable at $0.25/share as loan origination fee. The notes carried interest at the rate of 10% per year until maturity and thereafter are subject to a rate of 14% per year, and are convertible into common shares at the rate of $0.25 /share. | | 325,462 | | 325,462 |
| | | | | |
| Three promissory notes issued to accredited investors in November and December 2006, maturing on 1/31/07, now due on demand. The Company had issued warrants for an aggregate 1,120,000 shares, exercisable at $0.25/share as loan origination fee. The notes carried interest at the rate of 10% per year until maturity and thereafter are subject to a rate of 14% per year, and are convertible into common shares at the rate of $0.25 /share. | | 95,000 | | 95,000 |
| | | | | |
| Two promissory notes issued to two accredited investors in January 2007, maturing on 3/31/07, since changed to be due on demand. The Company had issued warrants for an aggregate 900,000 shares, exercisable at between $0.15 and $0.25/share as loan origination fees. The notes carried interest at the rate of 10% per year until maturity and thereafter are subject to a rate of 14% per year, and are convertible into common shares at rates between $0.15 and $0.25 /share. | | 325,000 | | 325,000 |
| | | | | |
| Non interest bearing advance from an outside director, repayable on demand, originally for $20,000, increased to $50,000 in August 2008 and changed into a promissory note, carrying interest of 12% until 11/30/08, since increased to 18% p.a. and due on demand. The note is convertible at $0.10 /share. | | 50,000 | | 20,000 |
| | | | | |
Two promissory notes issued to accredited investors in March 2007, originally maturing in May and June 2007, now due on demand. The notes initially carried interest at rates between 10% and 24% per year until maturity and thereafter are subject to rates of between 14% and 28% per year. One note for $250,000 is convertible into common shares at the rate of $0.15 /share, and one note at a rate equal to market price for the stock at the time of conversion less 30%. One note of $30,000 has been repaid in August 2008. One note for $30,000 was repaid by another creditor in August 2008. | | 250,000 | | 380,000 |
| | | | | |
| Six promissory notes issued to five accredited investors in May and June 2007, maturing between September 30, 2007 and October 31, 2007, since changed to be due on demand. The notes carried interest at the rate of 10% per year until maturity and thereafter are subject to a rate of 12% per year. One note calls for the interest payable in form of common stock, calculated at $0.10 per share. All notes are convertible into common shares at the rate of $0.10 /share. | | 187,000 | | 312,000 |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
DEBT (Continued)
| | | 2008 | | 2007 |
| Promissory note issued to an accredited investor on July 11, 2007, maturing October 11, 2007, now due on demand. The note carried interest at the rate of 12% per year until maturity and thereafter is subject to a penalty rate of 5% per month. | $ | 27,000 | $ | 27,000 |
| | | | | |
| Revolving non-interest bearing loan by an accredited investor. The loan presently has an outstanding balance of $38,000 | | 38,000 | | 21,900 |
| | | | | |
| Promissory note issued to an accredited investor in July, 2007, originally maturing December 31, 2007 and now due on demand. The note carried interest at the rate of 10% per year until maturity and thereafter is subject to a rate of 18% per year. | | 25,000 | | 25,000 |
| | | | | |
| Five promissory notes issued to an accredited investor in July 2007, due on demand, The notes carry interest at the rate of 10% per year. | | 100,000 | | 100,000 |
| | | | | |
| Promissory note originally for $25,000 issued to an accredited investor in August 2007, originally due on August 15, 2008, since changed to be due on demand. The note carries interest at the rate of 10% per year and is convertible, at the option of the holder, into common shares at the rate of $0.15 per share. In October 2007 $15,000 was converted. | | 10,000 | | 10,000 |
| | | | | |
| In August 2007 the Company and a creditor agreed to convert $605,577.75 in outstanding payables into a note, repayable six months after demand for repayment has been issued, demand for repayment has not been issued. The note currently carries an interest rate of 10% per year. | | 605,578 | | 605,578 |
| | | | | |
| Three promissory notes issued to an accredited investor in September 2007, originally due on September 12, 2008, since extended to March 31, 2009. Note is now due on demand. The notes carry interest at the rate of 6% per year and are convertible, at the option of the holder, into common shares at the rate of $0.25 per share. The notes had been issued pursuant to an agreement whereby the investor repaid a third-party creditor of the Company for outstanding promissory notes and accrued interest totaling the same face amount. Those notes have been cancelled. During the third quarter in 2008, $151,320 have been repaid. The balance is payable on demand. | | 63,710 | | 215,030 |
| | | | | |
| Promissory note for $550,000 issued to an accredited investor in November 2007, due on November 30, 2008. The note carries interest at the rate of 6% per year and is convertible, at the option of the holder, into common shares at the rate of $0.14 per share. In December 2008 $150,000 was repaid and the note extended to March 31, 2009. Note is now due on demand. | | 400,000 | | 550,000 |
| | | | | |
| Promissory note issued to an accredited investor in November 2007, originally for $200,000 due on March 31, 2008, presently due on March 31, 2009. Note is now due on demand. The note carries interest at the rate of 15% per year. In June, $12,500 accrued interest had been capitalized. | | 212,500 | | 200,000 |
| | | | | |
| Promissory note issued to an accredited investor in November 2007, due on demand. The note carries interest at the rate of 10% per year. | | 5,000 | | 5,000 |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
DEBT (Continued)
| | | 2008 | | 2007 |
| Promissory note issued to an accredited investor, originally due on January 26, 2008, now due on demand. The note carried interest at the rate of 18% per year which rate, at maturity increased to 24% per year. | $ | 25,000 | $ | 25,000 |
| | | | | |
| Promissory note issued to an accredited investor in September 2007, originally due on September 18, 2008, since changed to be due on demand. The note carries interest at the rate of 18% per year which rate, upon default would increase to 24% per year. | | 50,000 | | 50,000 |
| | | | | |
| Promissory note, originally in the amount of $2,710,563 issued to a service provider, due on July 31, 2008. The note carries interest at the rate of 10% per year compounded monthly. The compounding has increased the face amount of the note to $3,121,258 at December 31, 2008. (please see footnote 19: Subsequent Events.) | | 3,121,258 | | 2,825,401 |
| | | | | |
| Promissory note issued to an accredited investor in March 2008, due on June 18, 2008, presently due on March 31, 2009. The note carries interest at the rate of 6% per year which rate, upon default, has increased to 18% per year. A portion of $200,000 had been repaid since issuance. Note is now payable upon demand. | | 300,000 | | |
| | | | | |
| Promissory notes for $375,000 and $300,000 issued to two accredited investors in April 2008, the first currently due on demand, and the second due on March 31, 2009. The Company had issued 3,000,000 shares as interest and loan origination fees for one of the notes. The second note carried interest at the rate of 15% per year which rate has increased to 18% per year, and is convertible, at the holder’s option into common shares at $0.10 per share. Note is now payable upon demand. | | 675,000 | | |
| | | | | |
| Promissory note issued to two accredited investors in April 2008 for $40,000, due on demand. The note carries interest at 12% per year and is convertible at $0.10 /share. A partial repayment of $20,000 had been made I September 2008. Note is now payable upon demand. | | 20,000 | | |
| | | | | |
| Promissory note issued to two accredited investors in June 2008, due on demand. The note carries interest at 12% per year. | | 32,000 | | |
| | | | | |
| Two promissory notes issued to two accredited investors in July 2008, one in the amount of $200,000 due on demand and the other, in the amount of $350,000 originally due on November 7, 2008 and since also changed to be due on demand. The Company had issued restricted 1,200,000 shares as loan origination fees for the notes. They carry interest at the rate of 12% per year which rate and are convertible, at the holder’s option into common shares at $0.10 per share. | | 550,000 | | |
| | | | | |
| Six promissory notes issued to six accredited investors in September 2008, due at various dates in December 2008. The Company had issued 2,175,000 restricted shares as loan origination fees. The notes carry interest at 15% per year and are convertible, at the option of the holders, into common shares at market rates. | | 485,000 | | |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
DEBT (Continued)
| | | 2008 | | 2007 |
| Promissory note issued to an accredited investor in October 2008, due on December 31, 2008. The note carries interest at the rate of 15% per year. Note is payable upon demand. | $ | 50,000 | $ | |
| | | | | |
| Promissory note issued to an accredited investor in October 2008, due on December 31, 2008. The note carries interest at the rate of 15% per year. Note is payable upon demand. | | 25,000 | | |
| | | | | |
| Promissory note issued to an accredited investor in October 2008, due on December 31, 2008. The note carries interest at the rate of 15% per year. Note is payable upon demand. | | 105,000 | | |
| | | | | |
| Promissory note issued to an accredited investor in October 2008, due on December 31, 2008. The note carries interest at the rate of 15% per year. Note is payable upon demand. | | 156,000 | | |
| | | | | |
| Loan extended by a payroll financing company, presently showing a principal balance of $86,554. There presently is a dispute about the interest rate to be applied on this open balance. The company has accrued interest at the rate of 30% p.a. Note is payable upon demand. | | 86,554 | | |
| | | | | |
| Promissory note issued to an accredited investor on March 17, 2005 and maturing June 15, 2005 carrying interest at the rate of 10% per year. In the event of default by debtor, the interest rate increases to 20% per year. $147,515 had been repaid during 2006, with the balance remaining open at December 31, 2007, payable on demand. The note was subject to a $20,000 origination fee and assumption of $3,000 related legal expenses. | | | | 72,485 |
| | | | | |
| Promissory note issued to an accredited investor on March 31, 2006, originally maturing on 5/31/06. The note had been changed to be due on demand, against issuance of 300,000 shares, against issuance of warrants for the purchase of 100,000 shares, exercisable at $0.25 share. | | | | 214,000 |
| | | | | |
| Four promissory notes assigned by the holders of such previously issued notes, to an accredited investor in December 2007, due on demand. The notes carry interest at the rate of 10% per year and are convertible, at the option of the holder, into common shares at the rate of $0.25 per share. | | | | 250,000 |
| Total Short Term Debt | $ | 10,952,811 | $ | 9,493,029 |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
8. INCOME TAX
The income tax provision (benefit) is comprised of the following:
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | |
| State current provision (benefit) | | $ | - | | | $ | - | |
| State deferred provision (benefit) | | | - | | | | - | |
| | | $ | - | | | $ | - | |
There was no provision for income tax, on the federal or state level, for the fiscal years ended December 31, 2008 and 2007.
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
The Company’s total deferred tax asset and valuation allowance are as follows:
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | |
Total deferred tax asset, non-current | | $ | 15,663,000 | | | $ | 14,862,000 | |
Less valuation allowance | | | (15,663,000 | ) | | | (14,862,000 | ) |
| Net deferred tax asset, non-current | | $ | - | | | $ | - | |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
9. EMPLOYMENT AGREEMENTS
On September 11, 2007, the boards of directors of Millennium Biotechnologies, Inc. (the “Company”) and its parent Millennium Biotechnologies Group, Inc. (“Group”, and together with the Company, the “Companies”) appointed Mark C. Mirken as President and Chief Operating Officer of each of the Companies, effective immediately, in accordance with an employment agreement, dated as of September 11, 2007. On August 4, 2008, Mr. Mirken assumed the position of chief executive officer of the Company.
The term of Mr. Mirken’s employment under the employment agreement (the “Term”) commenced as of September 11, 2007 and will continue for a period of three years; provided, Mr. Mirken has the right to extend the term of employment for two additional years. Pursuant to the employment agreement, Mr. Mirken will receive an annual salary of $350,000 per year. In addition, during the Term, Mr. Mirken is entitled to receive an annual bonus of up to $2,725,000, depending on the gross sales of the Company during such year. For gross sales in excess of $100,000,000 during any year, the amount of additional bonuses, if any, will be at the discretion of the Board of Directors. Mr. Mirken also is entitled to receive temporary housing expense for one year, reimbursement of his moving expenses and a vehicle allowance during the Term. In addition, Mr. Mirken received a cash signing bonus of $100,000, and Group agreed to issue 6,000,000 shares of its Common Stock to Mr. Mirken. The employment agreement also provides that during each year of the Term, Mr. Mirken will receive three year options to purchase such number of Group’s common stock, at an exercise price of $.01 per share, as is equal to the greater of (i) one percent of all issued and outstanding shares of common stock of Group at the end of such year, for each $20,000,000 incremental increase in gross sales for such fiscal year which is in excess of the prior high point of gross sales reported in any prior fiscal year by the Company; or (ii) one percent of all issued and outstanding shares of common stock of Group at the end of such year, for each $30,000,000 increase in market capitalization of Group over and above the high point of market capitalization of Group for any fifteen consecutive trading days preceding such year. Pursuant to the employment agreement Mr. Mirken is entitled to a gross-up of his base salary to create a neutral tax impact for the issuance of any shares or options to him under the employment agreement.
The employment agreement terminates upon Mr. Mirken’s death and may be terminated at the option of the Company as a result of Mr. Mirken’s disability or for “cause” as defined in the employment agreement. Mr. Mirken has the right to terminate the employment agreement for “good reason” as defined in the employment agreement. In the event that the employment agreement is terminated due to Mr. Mirken’s death or disability, he is entitled to receive his annual salary for a period equal to the lessor of (i) three months from the date of death or disability or (ii) the balance of the Term; and all other accrued but unpaid compensation and benefits. If the employment agreement is terminated by the Company for “cause”, Mr. Mirken is not entitled to receive any compensation other than accrued but unpaid compensation and benefits. In the event Mr. Mirken terminates the employment agreement for “good reason”, the Company shall pay to Mr. Mirken his annual salary through the date of the end of the contract term; bonuses that have accrued and are unpaid as of the date of termination; and any Options which have been granted to Mr. Mirken as of the date of the termination. The employment agreement also provides for Mr. Mirken is subject to confidentiality, non-solicitation and non-compete covenants for a period of one year following his termination, provided such termination is not by the Company without “cause” or by Mr. Mirken for “good reason”.
10. CAPITAL STOCK
| | Convertible Series B preferred shares ("Series B") are non-dividend bearing, and are convertible into shares of the Company’s common stock at any time at the option of the holder and are subject to adjustment in accordance with certain anti-dilution clauses. Cumulative Series C preferred shares ("Series C") are not convertible but are entitled to cumulative cash dividends at the rate of $.65 per share per annum, payable in each year commencing the year after all the shares of Series B are retired. Convertible Series D preferred shares ("Series D") are non-dividend bearing and are convertible into shares of the Company’s common stock at the option of the Company and are subject to adjustment in accordance with certain anti-dilution clauses. Pursuant to the Agreement and Plan of Reorganization with Millennium, 237,049.7 Series D shares were issued in exchange for all outstanding common stock of Millennium. An additional 4,148.8 shares were issued in July 2001 at prices between $24.00 and $36.00 per share to four individual accredited investors. All Series D Preferred Shares were converted into common stock in April 2002. |
| | The holders of Series B and Series C preferred stock have no voting rights. Each share of common stock is entitled to one vote. |
| | No cash dividends may be declared or paid on the Company’s common stock if, and as long as, Series B preferred stock is still outstanding or there are dividends in arrears on outstanding shares of Series C preferred stock. No dividends may be declared on Series C shares if, and as long as, any Series B shares are outstanding. |
d) Other information is summarized as follows:
| | | Convertible Series B | | | Cumulative Series C | | | Convertible Series D | |
| Number of common shares to be issued upon conversion of each preferred share | | | 10 | | | None | | | | 641.215 | |
| Redemption price and involuntary liquidation value per preferred shares (if redeemed, ranking would be Convertible Series D then , Convertible Series B then Cumulative Series C) | | $ | 2.00 | | | $ | 10.00 | (1) | | $ | 1.00 | |
| (1) Plus any dividend in arrears. | | | | | | | | | | | | |
Because the Series B preferred stock had mandatory redemption requirements at the time of its issuance (which are no longer applicable), these shares are stated at redemption value. Series C shares are stated at par value.
The Company issued shares of common stock pursuant to the schedule below for the fiscal years 2008 and 2007:
| | | Shares of Common | | | | | | Shares of Common | | | | |
| | | Stock Issued | | | | | | Stock Issued | | | | |
Issuance of Common Stock: | | FY 2008 | | | Value | | | FY 2007 | | | Value | |
| | | | | | | | | | | | | |
| for Private Placements | | | 5,650,000 | | | $ | 575,000 | | | | 24,569,474 | | | $ | 2,974,920 | |
| | | | | | | | | | | | | | | | | |
| for License Fees | | | 1,500,000 | | | $ | 150,000 | | | | | | | $ | - | |
| | | | | | | | | | | | | | | | | |
| for Compensation | | | 2,800,000 | | | $ | 177,000 | | | | 6,020,000 | | | $ | 1,084,800 | |
| | | | | | | | | | | | | | | | | |
| pursuant to Note Conversion | | | 11,120,000 | | | $ | 1,139,000 | | | | 5,427,299 | | | $ | 602,426 | |
| | | | | | | | | | | | | | | | | |
| for Loan Origination Fees | | | 12,055,667 | | | $ | 1,242,826 | | | | 8,106,000 | | | $ | 1,633,539 | |
| | | | | | | | | | | | | | | | | |
| for Services | | | 27,872,588 | | | $ | 1,905,429 | | | | 14,436,563 | | | $ | 3,216,260 | |
| | | | | | | | | | | | | | | | | |
| for Directors Fees | | | 2,600,000 | | | $ | 284,000 | | | | | | | $ | - | |
| | | | | | | | | | | | | | | | | |
| for Interest | | | 5,137,146 | | | $ | 433,720 | | | | 20,869 | | | $ | 3,965 | |
| | | | | | | | | | | | | | | | | |
| for Note Due Date Extensions | | | 21,245,000 | | | $ | 1,808,610 | | | | 3,034,000 | | | $ | 584,052 | |
| | | | | | | | | | | | | | | | | |
| for Exercise of Stock Options | | | | | | $ | - | | | | 1,341,884 | | | $ | 214,702 | |
| | | | | | | | | | | | | | | | | |
| for Late Payment Penalties | | | 9,435,500 | | | $ | 632,715 | | | | | | | $ | - | |
| | | | | | | | | | | | | | | | | |
| for Buy-Out of Royalty Rights | | | 8,250,000 | | | $ | 55,165 | | | | 1,154,100 | | | $ | (2,393 | ) |
| | | | | | | | | | | | | | | | | |
| Total Common Stock Issued for Period: | | | 107,665,901 | | | $ | 8,403,465 | | | | 64,110,189 | | | $ | 10,312,271 | |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
Effective January 1, 2006, the Company adopted the provisions of Financial Accounting Standards Board Statement of Financial Accounting Standard (“SFAS”) No. 123(R), “Share-Based Payments,” which establishes the accounting for employee stock-based awards. Under the provisions of SFAS No. 123(R), stock-based compensation is measured at the grant date, based on the calculated fair value of the award, and is recognized as an expense over the requisite employee service period (generally the vesting period of the grant). The Company adopted SFAS No. 123(R) using the modified prospective method and, as a result, periods prior to December 31, 2005 have not been restated. The Company recognized stock-based compensation for awards issued under the Company’s stock option plans in other income/expenses included in the Consolidated Statement of Operations. Additionally, no modifications were made to outstanding stock options prior to the adoption of SFAS No. 123(R), and no cumulative adjustments were recorded in the Company’s financial statements.
In February 2000, Millennium adopted its 2001 Stock Option Plan ("The 2001 Plan"). The 2001 Plan provides that certain options granted thereunder are intended to qualify as "Incentive Stock Options" (ISO) within the meaning of Section 422A of the United States Internal Revenue Code of 1986, while non-qualified options may also be granted under the Plan. The Plan provided for the grant of options for up to 500,000 shares. The purchase price per common stock deliverable upon exercise of each ISO shall not be less than 100% of the fair market value of the common stock on the date such option is granted. If an ISO is issued to an individual who owns, at the time of grant, more than 10% of the total enhanced voting power of all classes of Millennium’s common stock, the exercise price of such option shall be at least 110% of the fair market value of the common stock on the date of grant and the term of the option shall not exceed five years from the date of grant. The purchase price of shares subject to non-qualified stock options shall be determined by a committee established by the Board of Directors with the condition that such prices shall not be less than 85% of the fair market value of the common stock at the time of grant. Millennium had no options issued pursuant to this Plan as of December 31, 2008.
The granting of the following Company stock options was not under a formal stock option plan.
Information regarding the Company’s stock options and warrants for fiscal years ended December 31, 2008 and 2007 is as follows:
| | | December 31, 2008 | | | December 31, 2007 | |
| | | Shares | | | Weighted Average Exercise Price | | | Shares | | | Weighted Average Exercise Price | |
| Options outstanding - beginning of year | | | 8,569,000 | | | $ | 0.35 | | | | 10,207,979 | | | $ | 0.31 | |
| Options expired | | | 2,100,000 | | | | 0.01 | | | | 230,000 | | | | 0.37 | |
| Options granted | | | | | | | | | | | | | | | | |
| Options cancelled | | | | | | | | | | | 1,408,979 | | | | | |
| Options outstanding - end of year | | | 6,469,000 | | | $ | 0.46 | | | | 8,569,000 | | | $ | 0.35 | |
| | | | | | | | | | | | | | | | | |
| Stock price at end of year | | $ | 0.02 | | | | | | | $ | 0.17 | | | | | |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
OPTIONS AND WARRANTS, Continued
| | | December 31, 2008 | | | December 31, 2007 | |
| | | Shares | | | Weighted Average Exercise Price | | | Shares | | | Weighted Average Exercise Price | |
| Option price range for exercised shares | | | N/A | | | | N/A | | | | N/A | | | | N/A | |
| Options available for grant at end of year | | | N/A | | | | N/A | | | | N/A | | | | N/A | |
| | | | | | | | | | | | | | | | | |
| Warrants outstanding - beginning of year | | | 38,446,356 | | | $ | 0.28 | | | | 21,257,711 | | | $ | 0.45 | |
| Warrants exercised | | | - | | | | | | | | - | | | | - | |
| Warrants granted | | | 13,631,326 | | | | 0.14 | | | | 19,033,651 | | | | 0.15 | |
| Warrants expired | | | 1,063,535 | | | | 0.20 | | | | 1,845,006 | | | | 2.33 | |
| | | | | | | | | | | | | | | | | |
| Warrants outstanding - end of year | | | 51,014,147 | | | $ | 0.22 | | | | 38,446,356 | | | $ | 0.28 | |
| | | | | | | | | | | | | | | | | |
| Warrants price range at end of year | | $0.10 - $0.75 | | | $0.01 - $0.75 | |
| | | | | | | | | | | | | | | | | |
| Warrants price for exercised shares | | $ | | | $ | |
| Warrants available for grant at end of year | | | N/A | | | | N/A | | | | N/A | | | | N/A | |
The weighted exercise price and weighted fair value of options and warrants granted by the Company for years ended 2008 and 2007, are as follows:
| | | December 31, 2008 | | | December 31, 2007 | |
| | | Weighted Average Exercise Price | | | Weighted Average Fair Value | | | Weighted Average Exercise Price | | | Weighted Average Fair Value | |
| | | | | | | | | | | | | |
| Weighted average of options and warrants granted during the year whose exercise price exceeded fair market value at the date of grant | | $ | 0.14 | | | $ | 0.07 | | | $ | 0.20 | | | $ | 0.18 | |
| | | | | | | | | | | | | | | | | |
| Weighted average of options and warrants granted during the year whose exercise price was equal or lower than fair market value at the date of grant | | $ | 0.10 | | | $ | 0.14 | | | $ | 0.12 | | | $ | 0.20 | |
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
OPTIONS AND WARRANTS, Continued
The following table summarizes information about fixed-price stock options and warrants outstanding at December 31, 2008.
Range of Exercise Prices | | Number Outstanding at December 31, 2008 | | Average Remaining Contractual Life | | Weighted Average Exercise Price | | | Number Exercisable at December 31, 2008 | | | Weighted Average Exercise Price | |
| $ 0.10 – 0.17 | | | 30,701,741 | | 25 Mo’s | | $ | 0.11 | | | | 30,701,741 | | | $ | 0.11 | |
| $ 0.25 – 0.37 | | | 15,577,609 | | 10 Mo’s | | $ | 0.26 | | | | 15,577,609 | | | $ | 0.26 | |
| $ 0.50 – 0.75 | | | 11,203,797 | | 13.Mo’s | | $ | 0.63 | | | | 11,203,797 | | | $ | 0.63 | |
| | | | 57,483,147 | | | | | | | | | 57,483,147 | | | | | |
The Company has used the fair value based method of accounting for its employee stock options beginning with the year ended December 31, 2006, as prescribed by Statement of Financial Accounting Standards No. 123(R). The value of each option grant is estimated on the date of grant using the Black-Scholes option pricing model with the following weighted average assumptions: expected dividend, 0%; risk-free interest rate, 5%; and expected volatility 80%.
Total compensation cost recognized in the income statement for stock-based employee and directors’ compensation awards was $461,000 and $269,444 in 2008 and 2007, respectively.
12. OPERATING LEASE COMMITMENTS
The Company leases certain office space and equipment under operating leases.
In October 2007, the Company extended its lease commitment an additional 5 years with an annual right to renew or cancel commencing in December 2007, for approximately 4,500 square feet of office space. The terms of the lease provide for a monthly rental rate of $10,635 per month, plus an allocated portion of certain operating expenses. The lease expense in 2008 was subsidized by $16,000 of sublease income. The Company presently occupies these facilities along with a new sub lessee paying approximately 30% of the rental expense projected for 2009. The lease is personally guaranteed by the Company’s former Chief Executive Officer and Chairman of the Board of Directors, Jerry E. Swon.
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
OPERATING LEASE COMMITMENTS, Continued
The following is a schedule of future minimum rental payments (exclusive of allocated expenses) required under operating leases that have initial or non-cancelable lease terms in excess of one year as of December 31, 2008:
| Year Ending December 31, | | | |
| 2009 | | 168,000 | |
| 2010 | | | 168,000 | |
| Total minimum payments required | | $ | 336,000 | |
Net rent expense for the Company under operating leases for the years ended December 31, 2008 and 2007 was $90,353 and $107,271, respectively. The Company expects to receive $100,000 per year in sublease income for the years 2009 and 2010.
13. NEW ACCOUNTING PRONOUNCEMENTS
In September 2006, the FASB released SFAS No. 157, Fair Value Measurements ("SFAS 157"), which defines fair value and establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. Although SFAS 157 applies to (and amends) the provisions of existing authoritative literature, it does not, of itself, require any new fair value measurements or establish valuation standards. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. Adoption of SFAS 157 did have a material impact on our Consolidated Financial Statements.
In February, 2007, the FASB issued FASB Statement No. 159, The Fair Value Option for Financial Assets and Financial Liabilities ("SFAS 159"), which creates an alternative measurement treatment for certain financial assets and financial liabilities. SFAS 159 permits fair value to be used for both the initial and subsequent measurements on an instrument by instrument basis, with changes in the fair value to be recognized in earnings as those changes occur. This election is referred to as the fair value option. SFAS 159 also requires additional disclosures to compensate for the lack of comparability that will arise from the use of the fair value option. SFAS 159 is effective for fiscal years beginning after November 15, 2007. The fair value option has not been elected for any financial assets or liabilities at December 31, 2008.
In December 2007, the FASB issued SFAS No. 141R, Business Combinations ("SFAS 141R"), which replaced FASB Statement 141, Business Combination, which changes the accounting for business combinations and non-controlling interests. Among other things, when compared to the predecessor guidance SFAS 141R will require (i) more assets acquired and liabilities assumed to be measured at fair value as of the acquisition date, (ii) liabilities related to contingent consideration to be remeasured to fair value each subsequent reporting period, and (iii) acquirer in preacquisition periods to expense all acquisition-related costs. SFAS 141R must be applied prospectively for fiscal years beginning after December 15, 2008. To date, the adoption of SFAS 141R has not had a material impact on our Consolidated Financial Statements.
In December 2007, the FASB issued SFAS No. 160, Non-controlling Interests in Consolidated Financial Statements - an Amendment of ARB No. 51 ("SFAS 160"), which changes the accounting and reporting for minority interests, which will be re-characterized as non-controlling interests and classified as a component of equity. SFAS 160 must be adopted no later than January 1, 2009. To date, the adoption of SFAS 160 has not had a material impact on our Consolidated Financial Statements.
In March 2008, the FASB issued SFAS No. 161, Disclosures about Derivative Instruments and Hedging Activities - an Amendment of FASB Statement No. 133 ("SFAS 161"), which requires enhanced disclosures about (i) how and why an entity uses derivative instruments, (ii) how derivative instruments and related hedged items are accounted for under SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities, as amended ("SFAS 133") and its related interpretations and (iii) how derivative instruments and related hedged items affect an entity's financial position, financial performance and cash flows. SFAS 161 will be effective for financial statements issued for fiscal years and interim periods beginning after November 15, 2008. We do not expect adoption of SFAS 161 to have a material impact on our Consolidated Financial Statements.
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
NEW ACCOUNTING PRONOUNCEMENTS (continued)
In June 2008, the Emerging Issues Task Force ("EITF") issued No. 03-6-1, Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities ("EITF 03-6-1"), which addresses whether instruments granted in share-based payment transactions are participating securities prior to vesting and, therefore, need to be included in the earnings allocation in computing earnings per share ("EPS") under the two-class method. EITF 03-6-1 will be effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those years. All prior-period EPS data presented will be adjusted retrospectively to conform to the provisions of EITF 03-6-1. We are evaluating the expected impact of adoption of EITF 03-6-1.
In May 2008, FASB issued FASB Staff Position ("FSP") APB 14-1, "Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement)" ("FSP APB 14-1"). FSP APB 14-1 clarifies that convertible debt instruments that may be settled in cash upon either mandatory or optional conversion (including partial cash settlement) are not addressed by paragraph 12 of APB Opinion No. 14, "Accounting for Convertible Debt and Debt issued with Stock Purchase Warrants."
Additionally, FSP APB 14-1 specifies that issuers of such instruments should separately account for the liability and equity components in a manner that will reflect the entity's nonconvertible debt borrowing rate when interest cost is recognized in subsequent periods. FSP APB 14-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. We will adopt FSP APB 14-1 beginning in the first quarter of 2009, and this standard must be applied on a retrospective basis. We are evaluating the impact the adoption of FSP APB 14-1 will have on our financial position and results of operations.
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
14. RELATED PARTY TRANSACTIONS
On September 11, 2007, the boards of directors of Millennium Biotechnologies, Inc. (the “Company”) and its parent Millennium Biotechnologies Group, Inc. (“Group”, and together with the Company, the “Companies”) appointed Mark C. Mirken as President and Chief Operating Officer of each of the Companies, effective immediately, in accordance with an employment agreement, dated as of September 11, 2007. On August 4, 2008, Mr. Mirken assumed the position of Chief Executive Officer of the Company.
The term of Mr. Mirken’s employment under the employment agreement (the “Term”) commenced as of September 11, 2007 and will continue for a period of three years; provided, Mr. Mirken has the right to extend the term of employment for two additional years. Pursuant to the employment agreement, Mr. Mirken will receive an annual salary of $350,000 per year. In addition, during the Term, Mr. Mirken is entitled to receive an annual bonus of up to $2,725,000, depending on the gross sales of the Company during such year. For gross sales in excess of $100,000,000 during any year, the amount of additional bonuses, if any, will be at the discretion of the Board of Directors. Mr. Mirken also is entitled to receive temporary housing expense for one year, reimbursement of his moving expenses and a vehicle allowance during the Term. In addition, Mr. Mirken received a cash signing bonus of $100,000, and Group agreed to issue 6,000,000 shares of its Common Stock to Mr. Mirken. The employment agreement also provides that during each year of the Term, Mr. Mirken will receive three year options to purchase such number of Group’s common stock, at an exercise price of $.01 per share, as is equal to the greater of (i) one percent of all issued and outstanding shares of common stock of Group at the end of such year, for each $20,000,000 incremental increase in gross sales for such fiscal year which is in excess of the prior high point of gross sales reported in any prior fiscal year by the Company; or (ii) one percent of all issued and outstanding shares of common stock of Group at the end of such year, for each $30,000,000 increase in market capitalization of Group over and above the high point of market capitalization of Group for any fifteen consecutive trading days preceding such year. Pursuant to the employment agreement Mr. Mirken is entitled to a gross-up of his base salary to create a neutral tax impact for the issuance of any shares or options to him under the employment agreement.
The employment agreement terminates upon Mr. Mirken’s death and may be terminated at the option of the Company as a result of Mr. Mirken’s disability or for “cause” as defined in the employment agreement. Mr. Mirken has the right to terminate the employment agreement for “good reason” as defined in the employment agreement. In the event that the employment agreement is terminated due to Mr. Mirken’s death or disability, he is entitled to receive his annual salary for a period equal to the lessor of (i) three months from the date of death or disability or (ii) the balance of the Term; and all other accrued but unpaid compensation and benefits. If the employment agreement is terminated by the Company for “cause”, Mr. Mirken is not entitled to receive any compensation other than accrued but unpaid compensation and benefits. In the event Mr. Mirken terminates the employment agreement for “good reason”, the Company shall pay to Mr. Mirken his annual salary through the date of the end of the contract term; bonuses that have accrued and are unpaid as of the date of termination; and any Options which have been granted to Mr. Mirken as of the date of the termination. The employment agreement also provides for Mr. Mirken is subject to confidentiality, non-solicitation and non-compete covenants for a period of one year following his termination, provided such termination is not by the Company without “cause” or by Mr. Mirken for “good reason”.
During 2008, the Company issued restricted stock awards to certain officers and directors, as follows:
750,000 restricted shares to Carl Germano valued at $47,286 pursuant to an agreement whereby he surrendered his rights to royalties on the sales of certain Company products;
1,000,000 restricted shares to Frank Guarino, valued at $30,000, as bonus payment;
1,250,000 shares each to Michael Martin and David Sargoy, in lieu of $125,000 /each accrued director’s fees;
250,000 shares to Kenneth Sadowsky in connection with a $50,000 loan extended by Mr. Sadowsky to the Company. Mr. Sadowsky extended another loan, for $156,000 to the Company for which he will receive 1,000,000 shares, to be issued in 2009.
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
15. CONVERTIBLE NOTES
At December 31, 2008 the Company had an aggregate of $5,307,244 payable in twenty-two (22) convertible notes of which $463,710 bear interest at 6% per annum, $30,000 at 8% per annum, $648,072 at 10% per annum, $807,000 bear interest at 12% per annum, $1,898,462 bear interest at 14% per annum, $485,000 bear interest at 15% per annum, $725,000 bear interest at 18% per annum and, $250,000 bear interest at 24% per annum. The terms of the Company’s convertible notes generally provide that the holder of the note is entitled, at its option at any time on or before the maturity date, to convert all or a portion of the principal amount into shares of common stock of the Company at a fixed price. The holding period for the shares would be one year from the funding of the convertible note. The convertible notes are included in Note 7: Debt. In comparison, at December 31, 2007 the Company had an aggregate of $6,667,628 payable in eighty-three convertible notes of which $66,900 are non-interest bearing, $4,047,621 bear interest at 10% per annum, $1,071,637 at 12% per annum, $325,000 at 14% per annum, $200,000 bear interest at 15% per annum, $75,000 bear interest at 18% per annum and $765,030 bear interest at 6% per annum. The terms of the Company’s convertible notes generally provide that the holder of the note is entitled, at its option at any time on or before the maturity date, to convert all or a portion of the principal amount into shares of common stock of the Company at a fixed price. The holding period for the shares would be one year from the funding of the convertible note.
The Company follows EITF 98-5 in accounting for convertible notes with "beneficial conversion features" (i.e., the notes may be converted into common stock at the lower of a fixed rate at the commitment date or a fixed discount to the market price of the underlying common stock at the conversion date). Because the Company’s convertible notes contained a beneficial conversion feature on the date of issuance, the Company measured and recognized the intrinsic value of the beneficial conversion feature of the convertible notes when the convertible notes were issued. During the years ended December 31, 2008 and 2007, interest expense of $720,904 and $704,818 respectively, was recognized as the intrinsic value of the beneficial conversion feature of the convertible notes that were issued during such periods.
Millennium Biotechnologies Group, Inc. and Subsidiary
Notes to Consolidated Financial Statements
December 31, 2008 and 2007
16. MAJOR SUPPLIERS
For the sourcing of raw materials, procurement of inherent specialty ingredients, manufacture of bulk product; quality control and testing; and contact research assistance, the Company has retained the services of numerous suppliers. The Company had a major supplier in the year presented. A major supplier is defined as one that provides ten-percent or more of total cost-of-sales in a particular year. In 2008, all of the Company's purchases were provided by one supplier. The Company, in an effort to minimize their risk, is negotiating with other potential suppliers and manufactures.
17. COMMITMENTS
None.
18. LITIGATION
Creative Healthcare Solutions, LLC vs. Millennium Biotechnologies Inc, Ct. of Common Pleas of Delaware County Ohio, Case No. 07 CV H 11 1420) Millennium was not satisfied with the service rendered by Creative Healthcare Solutions, LLC in 2005 which were associated with the development of Resurgex Select collateral materials developed in December of 2005. Millennium subsequently was forced to destroy and dispose of over 80% of the materials provided by Creative Healthcare Solutions due to the poor quality of the materials. Millennium has been unsuccessful in resolving the dispute and subsequently Creative Healthcare Solutions, LLC has filed legal action for demand of payment in the amount of $63,718 for services rendered. Millennium continues to negotiate a settlement through counsel with regards to this legal proceeding. Neither the Company nor Millennium has received any additional legal correspondence with regards to this matter as of May 4, 2009.
Ronald Burgert vs. Millennium Biotechnologies, Inc., et al. filed on the 9th day of October 2008 in District Court of Dallas County, Dallas, Texas. Mr. Burgert has filed a claim in the amount of $25,000 based on a note dated May 18, 2006. As of March 26, 2008 the balance due on the note, including unpaid principal and interest, was $31,635.12. On December 1, 2008, the 14th Judicial District, Dallas County, Dallas, Texas issues a default judgment against Millennium Biotechnologies, Inc. in the amount of $31,635.62 plus interest and unpaid attorney’s fees.
ESI Global Logistics, Inc. vs. Millennium Biotechnologies, Inc. filed on March 31, 2009 in the Superior Court of New Jersey, Law Division, Somerset County, Case #SOM-L-581-09. The ESI Global Logistics, Inc. claims a total of $54,111.95 plus costs and reasonable attorney fees based upon the Millennium Biotechnologies, Inc.’s failure to pay the plaintiff as an air freight carrier. The charges incurred by the plaintiff for the transfer of defendant’s product from the United States to Greece.
Riverwalk Village Center, LLC vs. Millennium Biotechnologies, Inc. filed on March 3, 2009 in the Superior Court of New Jersey, Law Division-Special Civil Part, Somerset County, Case #SOM-LT-572-09. Riverwalk Village Center, LLC brought a claim for unpaid rent and other charges against Millennium Biotechnologies, Inc. in the total amount of $68,076.02. The plaintiff and defendant have entered into a settlement order dated April 3, 2009 wherein the plaintiff has paid a stipulated sum in the amount of $5,000 and has agreed to make subsequent payments to landlord in conjunction with certain subtenants of Millennium Biotechnologies, Inc. as the defendants. Payments are to commence on 4/30/09 and to be completed by 6/15/09.
19. SUBSEQUENT EVENTS
On January 8, 2009 the Company received a $487,690 Purchase Order from Greek Distributor, Nutrimedica S.A. This order is the Company’s largest international order and encompasses the complete medical line. The Purchase Order is 104% greater than the total revenue the Company received from Greece in 2008. Nutrimedica S.A. has been distributing Resurgex products in Greece since March of 2006. Over a three year time period, the Company and Nurtrimedica, S.A. have been able to establish a strong international relationship in manufacturing and distributing elite, targeted nutritional supplements to the medical markets in Greece.
On January 20, 2009 the Company announced that its clinical recovery and performance trial in Division 1 Athletes will be published in The Journal of Strength and Conditioning Research (``JSCR''). JSCR is the official research journal of the National Strength and Conditioning Association, (``NSCA''). This journal is recognized as the preeminent publication for strength and conditioning coaches worldwide. These thought leaders rely on this journal for the future of leading edge science as applied to their profession.
On March 4, 2009 the Company’s largest Senior Secured Creditor agreed to subordinate and restructure $3,400,000 of Senior Secured Debt. Additionally in a separate agreement, the largest senior secured creditor has entered into a Contract Services Agreement pursuant to which they will represent the Company in certain identified institutional channels, such as Long-Term Care, U.S Veterans Administration and U.S. Department of Defense. Pursuant to the terms of this Services Agreement Millennium will repay the creditor 20% of the net profits directly related to revenue generated from their clients under the terms of the Agreement. The payments will be applied to the existing debenture due to the creditor which now extends through January 31, 2012.
On March 24, 2009 the Company received a $705,000 Purchase Order from Provider Services, Inc. for Resurgex Essential and Resurgex Essential Plus Ready-to-Drink products. The Purchase Order exclusively requests the delivery of Millennium's two new Ready-to-Drink product lines Resurgex Essential(tm) and Resurgex Essential Plus(tm). Millennium will add a new flavor of Resurgex Essential Plus ``Chocolate Truffle,'' as requested by the customer, in the upcoming production run at Farmland Dairies.
20. RESTATEMENT OF PREVIOUSLY ISSUED FINANCIAL STATEMENTS AS OF FOR THE YEAR ENDED DECEMBER 31, 2007
The Company has restated its previously issued financial statements for the year ended December 31, 2007 on its report dated April 15, 2008. The Company has restated its financial statements to properly reflect the effect of a conversion of an accounts payable balance to a note payable and the resulting accrued interest thereon. This transaction resulted in an increase in the net loss applicable to common shares of $114,837 for the year ended December 31, 2007, to a net loss of $13,132,488, as restated, and an increase in the accumulated deficit to $49,653,972 at December 31, 2007.