EXHIBIT 99.2

Second Quarter 2020 Update August 5, 2020 Science - Based Innovation - Focused ADC Company

Q2 2020 Overview Behzad Aghazadeh, PhD Executive Chairman Today’s Speakers 2 Commercial Update Brendan Delaney Chief Commercial Officer Clinical Update Loretta M. Itri, MD Chief Medical Officer F inancial Update U sama Malik Chief Financial Officer and Chief Business Officer

Forward - Looking Statements 3 This presentation and various remarks we make during this presentation, may contain forward - looking statements made pursuant to the Private Securities Litigation Reform Act of 1995. Such statements, including statements regarding expectations for achieving full FDA approval b ase d on our confirmatory data for TRODELVY and the Company’s development of TRODELVY for additional indications, clinical trials (including the funding the ref or, anticipated patient enrollment, trial outcomes, timing or associated costs), regulatory applications and related timelines, including the filing and approval timelines for BLAs and BLA supplements , out - licensing arrangements, forecasts of future operating results, potential collaborations, capital raising activities, and the timing for bringing any product candidate to market, involve significant risks and uncertainties and actual results could differ materia lly from those expressed or implied herein. Factors that could cause such differences include, but are not limited to, the Company’s reliance on third - party relationships and outsourcing arrangements (for example in connection with manufacturing, logistics and distribution, and sales and marketing) over which i t m ay not always have full control, including the failure of third parties on which the Company is dependent to meet the Company’s business and operatio nal needs for investigational or commercial products and, or to comply with the Company’s agreements or laws and regulations that impact the Company’s busi nes s; the Company’s ability to meet post - approval compliance obligations; imposition of significant post - approval regulatory requirements on our pro ducts, including a requirement for a post - approval confirmatory clinical study, or failure to maintain or obtain full regulatory approval for the C ompany’s products, if received, due to a failure to satisfy post - approval regulatory requirements, such as the submission of sufficient data from a confirmatory clinical study; the uncertainties inherent in research and development; safety and efficacy concerns related to the Company’s products and produc t c andidates; uncertainties in the rate and degree of market acceptance of products and product candidates, if approved; inability to create an effective di rect sales and marketing infrastructure or to partner with third parties that offer such an infrastructure for distribution of the Company’s products and product candidates, if approved; inaccuracies in the Company’s estimates of the size of the potential markets for the Company’s products and product candidate s o r limitations by regulators on the proposed treatment population for the Company’s products and product candidates; decisions by regulatory authorities r ega rding labeling and other matters that could affect the availability or commercial potential of the Company’s products and product candidates; the Comp any ’s dependence on business collaborations or availability of required financing from capital markets, or other sources on acceptable terms, if at all, in order to further develop our products and finance our operations; new product development (including clinical trials outcome and regulatory requiremen ts/ actions); the risk that we or any of our collaborators may be unable to secure regulatory approval of and market our drug candidates; risks relating to the COVID - 19 pandemic in the U.S. and around the world; risks associated with litigation to which the Company is or may become a party, including the cost an d potential reputational damage resulting from such litigation; loss of key personnel; competitive risks to marketed products; and the Company’s abili ty to repay its outstanding indebtedness, if and when required, as well as the risks discussed in the Company’s filings with the Securities and Exchange Com mission. The Company is not under any obligation, and the Company expressly disclaims any obligation, to update or alter any forward - looking statements, whether as a result of new information, future events or otherwise.

Q2 2020 Overview and Recent Highlights Behzad Aghazadeh, PhD Executive Chairman

Second Quarter 2020 was Transformative for IMMU 5 FDA approved TRODELVY™ in mTNBC – first drug in Company history 1 2 3 Phase 3 ASCENT topline confirmed compelling safety and efficacy profile of TRODELVY Strong commercial launch of TRODELVY in the United States 4 Balance sheet bolstered with $483 million equity offering + Everest milestone payment 5 Organization executing effectively amid COVID - 19 pandemic

6 TRODELVY – First ADC Approved Specifically for mTNBC • Approved on April 22, 2020 in U.S. • Strong initial launch execution – Product in channel within one week of approval – NCCN guidelines updated within two weeks of approval • Rapid adoption in advanced mTNBC – $20.1M net sales in first two months of commercial launch
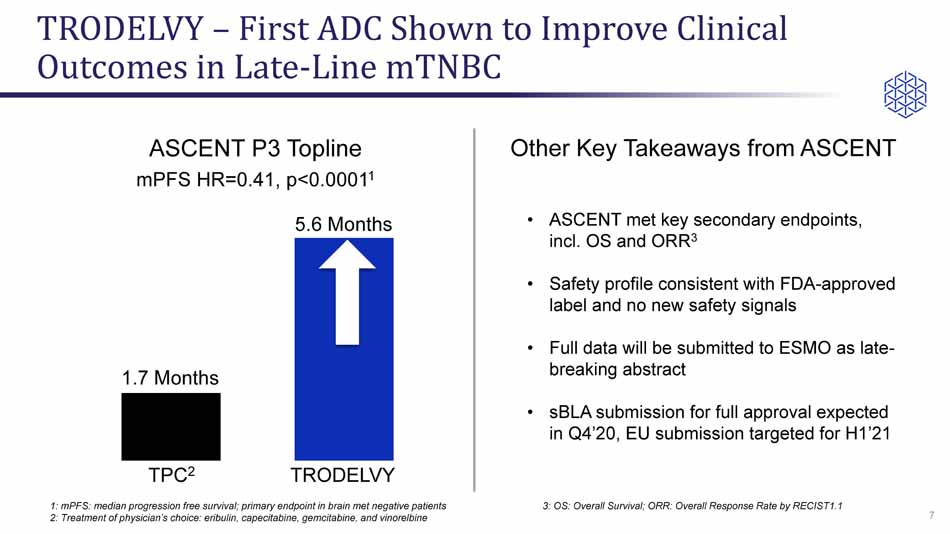
TRODELVY – First ADC Shown to Improve Clinical Outcomes in Late - Line mTNBC 7 TPC 2 TRODELVY 1.7 Months 5.6 Months ASCENT P3 Topline mPFS HR= 0.41, p<0.0001 1 1: mPFS : median progression free survival; primary endpoint in brain met negative patients 2: Treatment of physician’s choice: eribulin , capecitabine, gemcitabine, and vinorelbine • ASCENT met key secondary endpoints, incl. OS and ORR 3 • Safety profile consistent with FDA - approved label and no new safety signals • Full data will be submitted to ESMO as late - breaking abstract • sBLA submission for full approval expected in Q4’20, EU submission targeted for H1’21 Other Key Takeaways from ASCENT 3: OS: Overall Survival; ORR: Overall Response Rate by RECIST1.1

Commercial Update Brendan Delaney Chief Commercial Officer
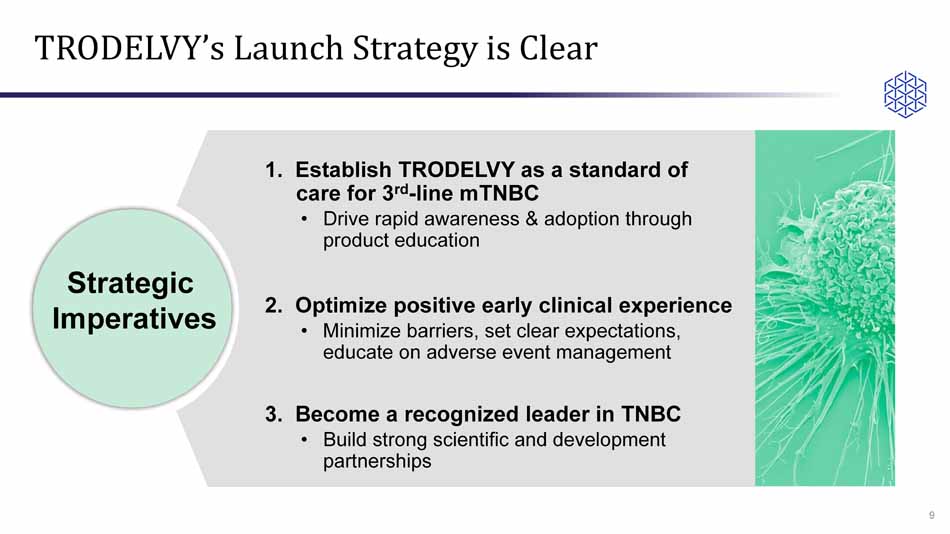
TRODELVY’s Launch Strategy is Clear 1. Establish TRODELVY as a standard of care for 3 rd - line mTNBC • Drive rapid awareness & adoption through product education 2. Optimize positive early clinical experience • Minimize barriers, set clear expectations, educate on adverse event management 3. Become a recognized leader in TNBC • Build strong scientific and development partnerships Goals 9 Strategic Imperatives
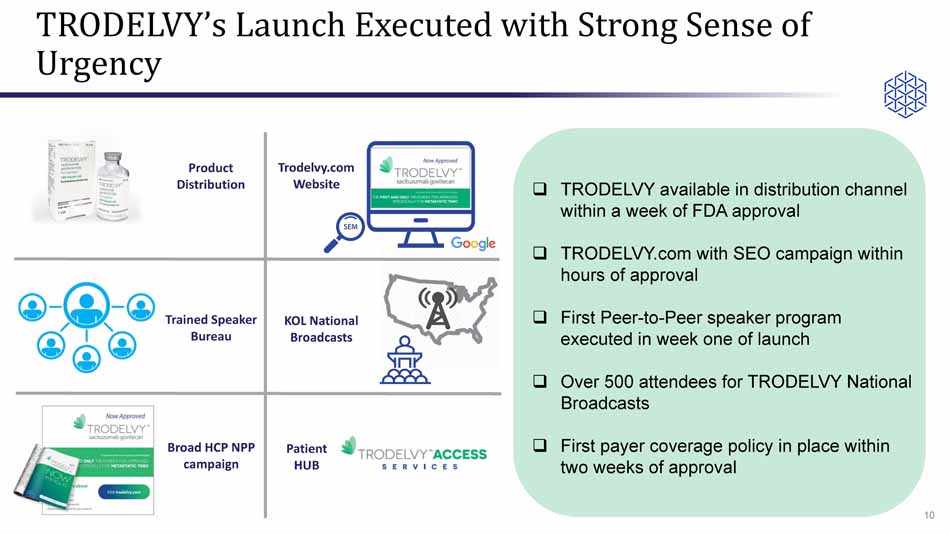
TRODELVY’s Launch Executed with Strong Sense of Urgency □ TRODELVY available in distribution channel within a week of FDA approval □ TRODELVY.com with SEO campaign within hours of approval □ First Peer - to - Peer speaker program executed in week one of launch □ Over 500 attendees for TRODELVY National Broadcasts □ First payer coverage policy in place within two weeks of approval KOL National Broadcasts Trained Speaker Bureau Broad HCP NPP campaign Product Distribution Trodelvy.com Website Patient HUB 10
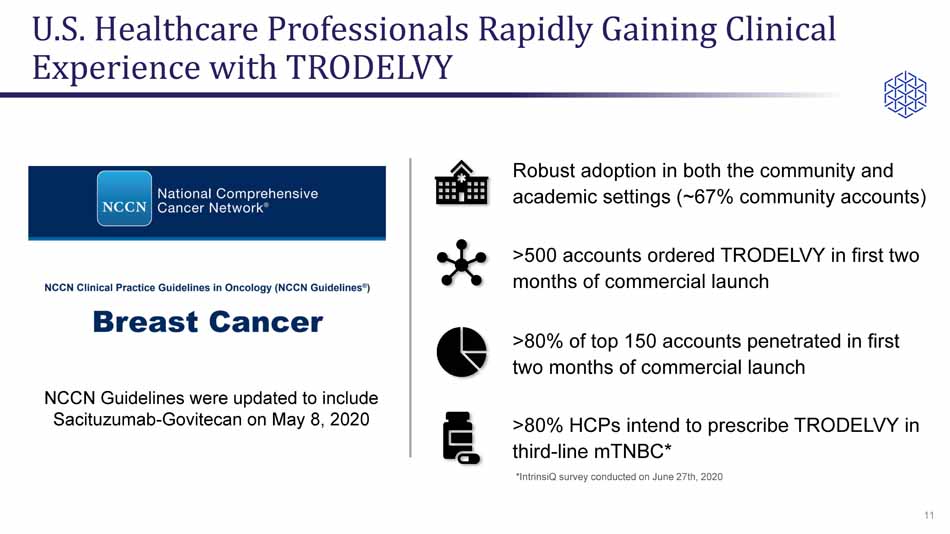
Robust adoption in both the community and academic settings (~67% community accounts) U.S. Healthcare Professionals Rapidly Gaining Clinical Experience with TRODELVY 11 * IntrinsiQ survey conducted on June 27th, 2020 NCCN Guidelines were updated to include Sacituzumab - Govitecan on May 8, 2020 >80% of top 150 accounts penetrated in first two months of commercial launch >80% HCPs intend to prescribe TRODELVY in third - line mTNBC* >500 accounts ordered TRODELVY in first two months of commercial launch
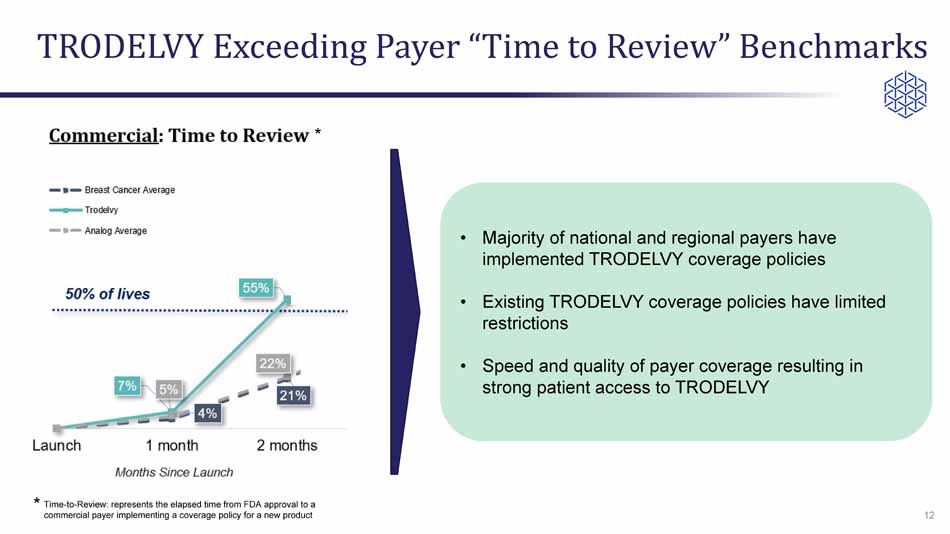
TRODELVY Exceeding Payer “Time to Review” Benchmarks • Majority of national and regional payers have implemented TRODELVY coverage policies • Existing TRODELVY coverage policies have limited restrictions • Speed and quality of payer coverage resulting in strong patient access to TRODELVY 12 * * Time - to - Review: represents the elapsed time from FDA approval to a commercial payer implementing a coverage policy for a new product
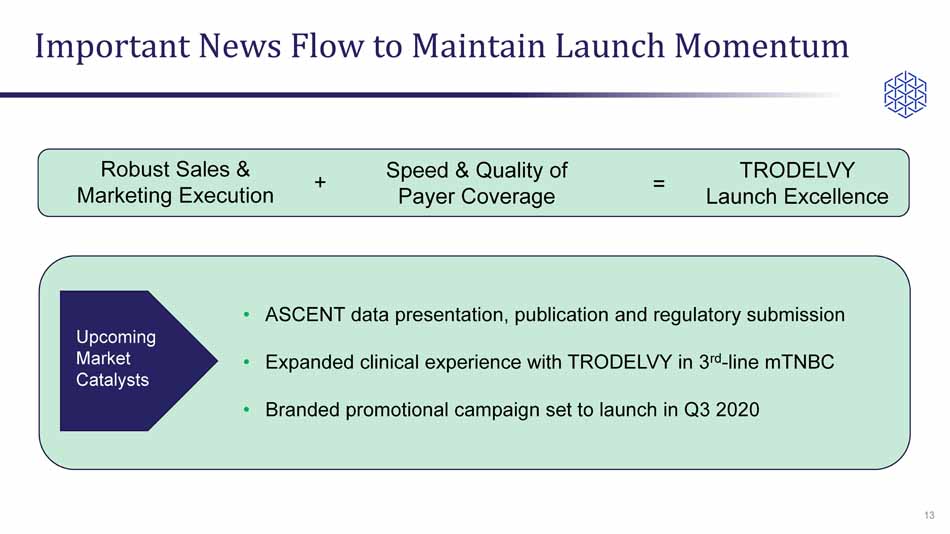
Important News Flow to Maintain Launch Momentum 13 • ASCENT data presentation, publication and regulatory submission • Expanded clinical experience with TRODELVY in 3 rd - line mTNBC • Branded promotional campaign set to launch in Q3 2020 Robust Sales & Marketing Execution Speed & Quality of Payer Coverage TRODELVY Launch Excellence + = Upcoming Market Catalysts

Clinical Update Loretta M. Itri, M.D. Chief Medical Officer
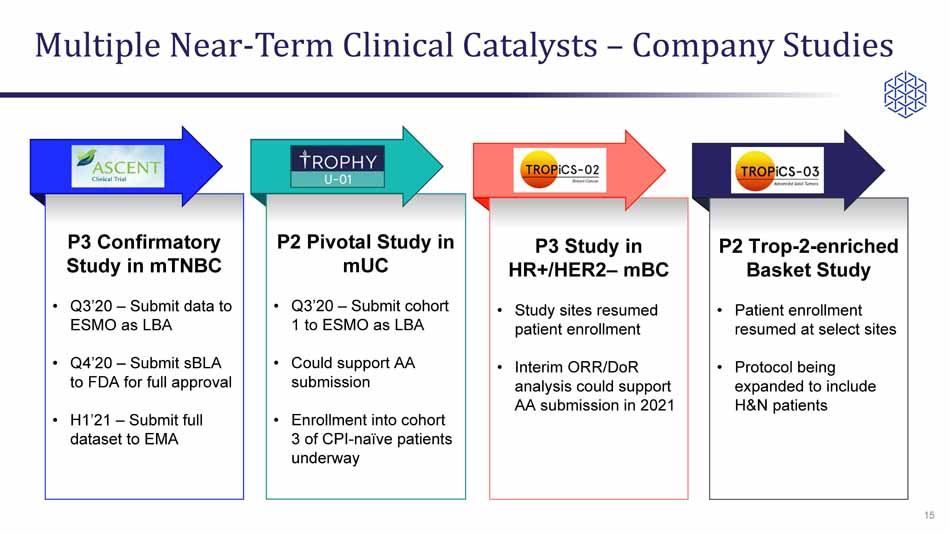
Multiple Near - Term Clinical Catalysts – Company Studies 15 P2 Pivotal Study in mUC • Q3’20 – Submit cohort 1 to ESMO as LBA • Could support AA submission • Enrollment into cohort 3 of CPI - naïve patients underway P2 Trop - 2 - enriched Basket Study • Patient enrollment resumed at select sites • Protocol being expanded to include H&N patients P3 Confirmatory Study in mTNBC • Q3’20 – Submit data to ESMO as LBA • Q4’20 – Submit sBLA to FDA for full approval • H1’21 – Submit full dataset to EMA P3 Study in HR+/HER2 – mBC • Study sites resumed patient enrollment • Interim ORR/ DoR analysis could support AA submission in 2021

Diverse Collaborative Studies to Drive Further Growth 16 • Collaboration expanded into urothelial cancer and non - small cell lung cancer P2 studies of TRODELVY ± Keytruda in ( i ) 1L PD - L1 – mTNBC (ii) 1L and 2L HR+/HER2 – PD - L1+ mBC P1b/2 MORPHEUS study of Tecentriq + TRODELVY in first - line TNBC P2 NeoSTAR study of response - guided neoadjuvant TRODELVY in patients with localized TNBC • Registration - oriented study launched • HR+/ HER2 - CTA being finalized IIT of TRODELVY bioavailability in patients with glioblastoma and metastatic brain tumors from breast • Both studies launched • Abstract on early results accepted at ESMO • First patients dosed P2b mTNBC study in Chinese patients with at least 2 prior treatments for metastatic disease

Financial Update Usama Malik Chief Financial Officer and Chief Business Officer
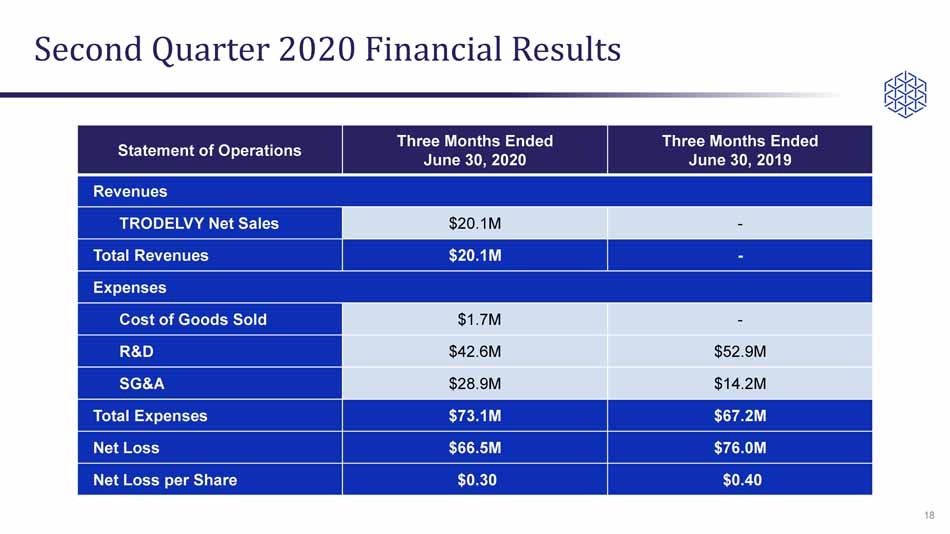
Second Quarter 2020 Financial Results Statement of Operations Three Months Ended June 30, 2020 Three Months Ended June 30, 2019 Revenues TRODELVY Net Sales $20.1M - Total Revenues $20.1M - Expenses Cost of Goods Sold $1.7M - R&D $42.6M $52.9M SG&A $28.9M $14.2M Total Expenses $73.1M $67.2M Net Loss $66.5M $76.0M Net Loss per Share $0.30 $0.40 18
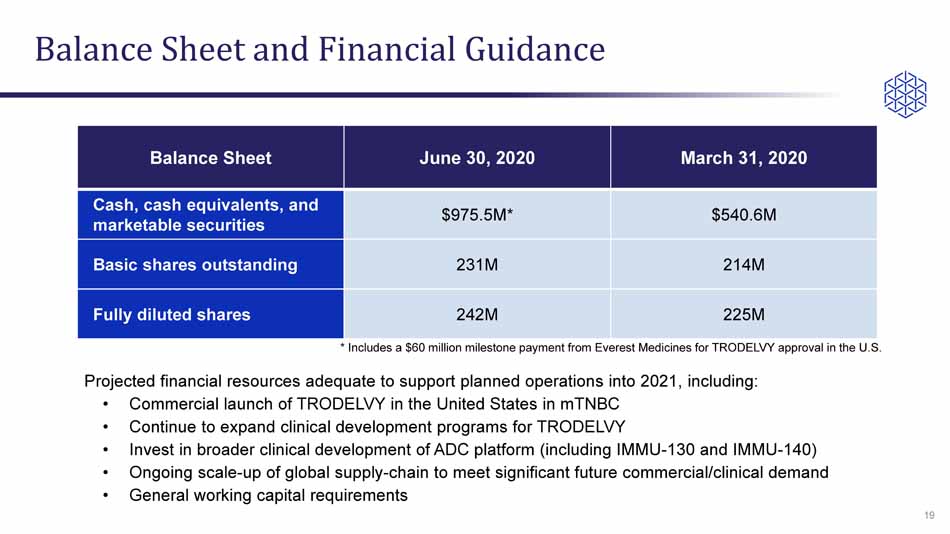
Balance Sheet and Financial Guidance Balance Sheet June 30, 2020 March 31, 2020 Cash, cash equivalents, and marketable securities $975.5M* $540.6M Basic shares outstanding 231M 214M Fully diluted shares 242M 225M 19 Projected financial resources adequate to support planned operations into 2021, including: • Commercial launch of TRODELVY in the United States in mTNBC • Continue to expand clinical development programs for TRODELVY • Invest in broader clinical development of ADC platform (including IMMU - 130 and IMMU - 140) • Ongoing scale - up of global supply - chain to meet significant future commercial/clinical demand • General working capital requirements * Includes a $60 million milestone payment from Everest Medicines for TRODELVY approval in the U.S.

Concluding Remarks Behzad Aghazadeh, PhD Executive Chairman
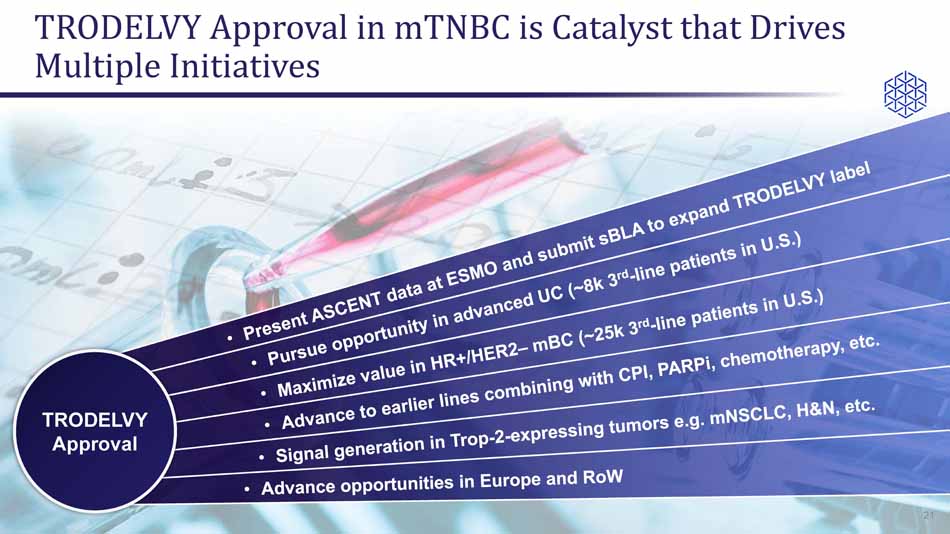
TRODELVY Approval in mTNBC is Catalyst that Drives Multiple Initiatives 21 TRODELVY Approval

Question & Answer

Second Quarter 2020 Update August 5, 2020 Science - Based Innovation - Focused ADC Company






















