- CVM Dashboard
- Financials
- Filings
-
Holdings
-
Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
S-8 Filing
CEL-SCI (CVM) S-8Registration of securities for employees
Filed: 30 Aug 24, 4:32pm
EXHIBIT 99
CEL-SCI CORPORATION
Common Stock
This Prospectus relates to shares (the "Shares") of common stock (the "Common Stock") of CEL-SCI Corporation which may be issued pursuant to certain employee compensation plans adopted by CEL-SCI. The employee compensation plans provide for the grant, to selected employees of CEL-SCI and other persons, of either shares of CEL-SCI’s common stock or options to purchase shares of CEL-SCI’s common stock. Persons who received Shares pursuant to the Plans and who are offering such shares to the public by means of this Prospectus are referred to as the "Selling Shareholders".
CEL-SCI has Incentive Stock Option Plans, Non-Qualified Stock Option Plans, Stock Bonus Plans, Stock Compensation Plans and a 2014 Incentive Stock Bonus Plan. In some cases these plans are collectively referred to as the "Plans". The terms and conditions of any stock grants and the terms and conditions of any options, including the price of the shares of Common Stock issuable on the exercise of options, are governed by the provisions of the respective Plans and any particular agreements between CEL-SCI and the Plan participants.
The Selling Shareholders may offer the shares from time to time in negotiated transactions through the NYSE American Exchange, at fixed prices which may be changed from time to time, at market prices prevailing at the time of sale, at prices related to such prevailing market prices or at negotiated prices. The Selling Shareholders may effect such transactions by selling the Shares to or through securities broker/dealers, and such broker/dealers may receive compensation in the form of discounts, concessions, or commissions from the Selling Shareholders and/or the purchasers of the Shares for whom such broker/dealers may act as agent or to whom they sell as principal, or both (which compensation as to a particular broker/dealer might be in excess of customary commissions). See "Selling Shareholders" and "Plan of Distribution".
None of the proceeds from the sale of the Shares by the Selling Shareholders will be received by CEL-SCI. CEL-SCI has agreed to bear all expenses of registering the Shares with the Securities and Exchange Commission (other than underwriting discounts, selling commissions and fees and expenses of counsel and other advisers to the Selling Shareholders).
The purchase of the securities offered by this prospectus involves a high degree of risk. Risk factors include the lack of revenues and history of loss, need for additional capital and need for FDA approval. See the “Risk Factors” section of this prospectus beginning on page 16.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or has passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this Prospectus is August __, 2024.
| 1 |
AVAILABLE INFORMATION
CEL-SCI is subject to the information requirements of the Securities Exchange Act of 1934 (the "Exchange Act") and in accordance therewith, files reports and other information with the Securities and Exchange Commission (the "Commission"). Proxy statements, reports and other information concerning CEL-SCI is available at the Internet Web Site maintained by the Securities and Exchange Commission at www.sec.gov. This Prospectus does not contain all information set forth in the Registration Statement of which this Prospectus forms a part and exhibits thereto which CEL-SCI has filed with the Commission under the Securities Act and to which reference is hereby made.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents filed with the Commission by CEL-SCI (Commission File No. 001-11889) are incorporated by reference into this prospectus:
| · | our Annual Report on Form 10-K for the fiscal year ended September 30, 2023; |
|
|
|
| · | our quarterly reports on Form 10-Q for the periods ended December 31, 2023, March 31, 2024 and June 30, 2024; |
|
|
|
| · | our current reports on Form 8-K filed with the SEC on October 24, 2023, October 30, 2023, November 20, 2023, February 13, 2024, April 19, 2024, April 23, 2024, June 20, 2024, July 26, 2024 and July 29, 2024; |
|
|
|
| · | our Proxy Statement relating to our April 19, 2024 Annual Meeting of Shareholders; and |
|
|
|
| · | the description of our common stock contained in our Registration Statement on Form 8-A filed with the SEC on July 2, 1996 and all amendments and reports updating that description. |
CEL-SCI will provide, without charge, to each person to whom a copy of this Prospectus is delivered, including any beneficial owner, upon the written or oral request of such person, a copy of any or all of the documents incorporated by reference herein (other than exhibits to such documents, unless such exhibits are specifically incorporated by reference into this Prospectus). Requests should be directed to:
CEL-SCI Corporation
8229 Boone Blvd., Suite 802
Vienna, Virginia 223l4
(703) 506-9460
Attention: Secretary
| 2 |
All documents filed with the Commission by CEL-SCI pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act subsequent to the date of this prospectus and prior to the termination of this offering shall be deemed to be incorporated by reference into this prospectus and to be a part of this prospectus from the date of the filing of such documents. Any statement contained in a document incorporated or deemed to be incorporated by reference shall be deemed to be modified or superseded for the purposes of this prospectus to the extent that a statement contained in this prospectus or in any subsequently filed document which also is or is deemed to be incorporated by reference in this prospectus modifies or supersedes such statement. Such statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
Investors are entitled to rely upon information in this prospectus or incorporated by reference at the time it is used by CEL-SCI even though that information may be superseded or modified by information subsequently incorporated by reference into this prospectus.
CEL-SCI has filed with the Securities and Exchange Commission a Registration Statement under the Securities Act of l933, as amended, with respect to the securities offered by this prospectus. This prospectus does not contain all of the information set forth in the Registration Statement. For further information with respect to CEL-SCI and such securities, reference is made to the Registration Statement and to the exhibits filed with the Registration Statement. Statements contained in this prospectus as to the contents of any contract or other documents are summaries which are not necessarily complete, and in each instance reference is made to the copy of such contract or other document filed as an exhibit to the Registration Statement, each such statement being qualified in all respects by such reference. The Registration Statement and related exhibits may be examined at the Commission’s internet site (www.sec.gov).
| 3 |
TABLE OF CONTENTS
|
| PAGE |
|
|
|
|
|
THE COMPANY |
| 5 |
|
|
|
|
|
FORWARD LOOKING STATEMENTS |
| 15 |
|
|
|
|
|
RISK FACTORS |
| 16 |
|
|
|
|
|
DILUTION |
| 16 |
|
|
|
|
|
USE OF PROCEEDS |
| 16 |
|
|
|
|
|
MARKET FOR CEL-SCI'S COMMON STOCK |
| 17 |
|
|
|
|
|
SELLING SHAREHOLDERS |
| 18 |
|
|
|
|
|
PLAN OF DISTRIBUTION |
| 20 |
|
|
|
|
|
DESCRIPTION OF SECURITIES |
| 20 |
|
| 4 |
THE COMPANY
OVERVIEW AND PRODUCT CANDIDATES
CEL-SCI Corporation is a late clinical-stage biotechnology company dedicated to research and development directed at improving the treatment of cancer and other diseases by using the immune system, the body’s natural defense system. CEL-SCI is currently focused on the development of the following product candidates and technologies:
1) Multikine, an investigational immunotherapy under development for the potential treatment of certain head and neck cancers; and
2) L.E.A.P.S. (Ligand Epitope Antigen Presentation System) technology, or LEAPS, with several product candidates under development for the potential treatment of rheumatoid arthritis.
Multikine (Leukocyte Interleukin, Injection) is the full name of this investigational therapy, which, for simplicity, is referred to in this prospectus as Multikine. Multikine is the trademark that the Company has registered for this investigational therapy, and this proprietary name is subject to FDA review under the Company’s future anticipated regulatory submission for approval. None of the Company’s product candidates have been approved for sale, barter or exchange by the Food and Drug Administration (FDA) or any other regulatory agency for any use to treat disease in humans nor has the safety or efficacy of these products been established for any use. There can be no assurance that obtaining marketing approval from the FDA in the United States and by comparable agencies in most foreign countries will be granted.
MULTIKINE, THE PHASE III CLINICAL TRIAL RESULTS AND PATH FORWARD
Immunotherapy is a large, high growth market. Immunotherapies use the patient’s own immune system to fight disease. These “targeted therapies” are at the forefront of modern cancer research. A recent Bloomberg report from January 2023 asserted that:
The global cancer immunotherapy market is expected to reach USD $196.45 billion by 2030, registering CAGR of 7.2% during the forecast period, according to a new report by Grand View Research, Inc. The rising adoption of immunotherapy over other therapy options for cancer owing to its targeted action is anticipated to increase the adoption during the forecast period. Moreover, increasing regulatory approvals from authoritarian establishments for novel immunotherapy used for oncology is also expected to further fuel the market growth.
Source: https://www.bloomberg.com/press-releases/2023-01-18/cancer-immunotherapy-market-worth-196-45-billion-by-2030-grand-view-research-inc
CEL-SCI hopes to participate in this growing market with its lead investigational therapy Multikine® (Leukocyte Interleukin, Injection). Multikine is unique among approved cancer immunotherapies because it is given first, right after diagnosis, before any other treatment including surgery.
Multikine has been tested in approximately 740 patients in Phase 1, 2 and 3 clinical studies conducted in the U.S., Canada, Europe, Israel and Asia. In these studies, it has been administered in multiple doses by various routes and various frequencies to determine its safety and efficacy. The data from these studies allowed CEL-SCI to determine the patient population most responsive to Multikine and most likely to benefit from it. The target population is newly diagnosed advanced primary head and neck cancer patients with no lymph node involvement (determined via PET imaging) and with low PD-L1 tumor expression (determined via biopsy), two features that physicians routinely assess at baseline as part of standard practice.
| 5 |
In the target patient population CEL-SCI believes Multikine significantly extended life. In the Phase III study, CEL-SCI observed a 73% survival rate with Multikine vs. only 45% without Multikine at 5 years after treatment, and a Hazard ratio of 0.35 (95% CIs [0.19, 0.66]).
CEL-SCI completed a bias analysis for the target population in the Phase III study in preparation for submission of data to regulatory agencies including the FDA for confirmatory registration study. The detailed data on parameters including patient age, sex, race, tumor locations, and staging demonstrate balance between the treatment and control arms. Therefore, no bias was found, which supports confidence in Multikine’s efficacy results.
CEL-SCI applied to the FDA for a 212-patient randomized controlled confirmatory registration study focusing only on those patients in the target population, this accounts for approximately 100,000 patients worldwide per year. In May 2024, CEL-SCI announced that the FDA indicated CEL-SCI may move forward with a confirmatory registration study of Multikine in the target population.
What is Multikine and who is it for? Multikine is a biological medicinal immunotherapy comprised of a mixture of natural cytokines and small biological molecules. Multikine is injected around the tumor and adjacent lymph nodes for three weeks as a first-line treatment before the standard of care (SOC), which is surgery followed by either radiotherapy or chemoradiotherapy. Multikine’s rationale for use is to incite a locoregional immune response against the tumor before the local immune system has been compromised by the standard of care and/or disease progression.
The Multikine target population is not yet treated adult patients with resectable locally advanced primary squamous cell carcinoma of the head and neck (SCCHN) in the oral cavity and who have:
| · | No lymph node involvement (via PET imaging) |
| · | Low PD-L1 tumor expression (TPS<10) (via biopsy) |
PD-L1 is a protein receptor on the tumor surface that helps the tumor repel cells of the immune system. CEL-SCI believes that patients with tumors having low PD-L1 would be more likely to respond to Multikine because their tumors have lower defenses against the patient’s immune system. CEL-SCI estimates that patients with tumors having low PD-L1 represent about 70% of locally advanced primary SCCHN patients.
Targeting low PD-L1 also differentiates Multikine from other immunotherapies. For example, checkpoint inhibitors like Keytruda and Opdivo appear to best serve patients having high PD-L1, because these drugs work by blocking PD1/PD-L1 receptors interaction; when this interaction (PD1/PD-L1) happens it leads to inactivation/death of the immune cells attacking the tumor. These checkpoint inhibitors appear to act best when tumors express high levels of PD-L1 receptors (usually TPS >20 to TPS >50). While none of these drugs are currently approved as a first-line treatment before surgery, even if such approvals were to come in the future, we believe the large majority of patients in this group having low PD-L1 would still be expected to need Multikine.
CEL-SCI believes Multikine leads to longer survival with no safety issues. Clinical investigations of Multikine, presented at ESMO (Europe Society for Medical Oncology) in October 2023, have demonstrated in the randomized controlled Phase III trial (RCT) the following in the target population:
| · | risk of death cut in half at five years versus the control; |
| · | 28.6% absolute 5-year overall survival benefit versus control (p=0.0015); |
| · | 0.349 hazard ratio vs control (95% CIs [0.18, 0.66], Wald p=0.0012); |
| · | >35% rate of pre-surgery reductions and/or downstages (p<0.01); and |
| · | low PD-L1 tumor expression (vs high PD-L1 where Keytruda and Opdivo work best). |
| 6 |
There were no demonstrable safety signals or toxicities observed in approximately 740 Multikine-treated subjects across multiple clinical trials. Adverse event (AE) and serious adverse event (SAE) incidences were not significantly different among treatment and control groups. There were no Multikine-related deaths, no Multikine-related delays of surgery, no Multikine-related interference with post-surgical treatment, and only two discontinuations. Multikine-related AEs before surgery were local and resolved after surgery. Although the literature reports that some of Multikine’s components may be toxic when administered systemically (e.g., TNFα, IFN γ, IL-1β), these toxicities did not emerge with Multikine, even at doses many times higher than those administered in the Phase III trial, primarily due to Multikine’s delivery by local injection and dosage.
CEL-SCI published its data as abstracts and posters at the annual conferences for the 2022 American Society of Clinical Oncology (ASCO), 2022 and 2023 European Society for Medical Oncology (ESMO), and the 2023 European Head and Neck Society’s (EHNS’s) annual European Conference on Head And Neck Oncology (ECHNO) and the 2023 European Society for Therapeutic Radiology and Oncology (ESTRO) and 2023 American Head and Neck Society (AHNS). These publications can be accessed at http://www.cel-sci.com.
Multikine works by inducing pre-surgical responses. CEL-SCI observed statistically significant pre-surgical responses after Multikine treatment, and therefore CEL-SCI believes in the following:
| ➢ | Multikine causes pre-surgical responses; |
| ➢ | Pre-surgical responses lead to longer life; |
| ➢ | Therefore, selecting more patients predicted to have a pre-surgical response should lead to much better survival in the target population. |
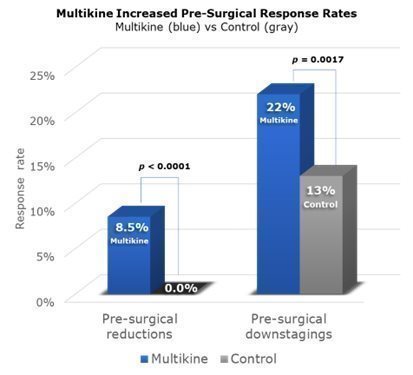
A “pre-surgical response” is a significant change in disease before surgery. CEL-SCI saw two kinds of responses in the Phase III trial. First, there were “reductions” in the size of the tumor—a reduction of 30% or more qualified as a “pre-surgical reduction,” or “PSR” for short. Second, there were disease “downstages” (e.g., the disease improved from Stage IV to Stage III) pre-surgery. CEL-SCI calls this a “pre-surgical downstaging” or “PSD” for short. CEL-SCI’s 2022 ESMO cancer conference presentation reported on PSR, and CEL-SCI’s new 2023 ESMO presentation reported on PSD.
Across the whole Phase III trial, PSRs were seen in 8.5% of Multikine patients compared to none in the control group. PSDs were seen in 22% of Multikine patients as compared to 13% in the control group. Because Multikine was the only therapy given to these patients before surgery, it is CEL-SCI’s strong belief that Multikine had to be the cause of the higher rates of PSR and PSD.
These data are presented visually below. The taller blue columns show PSR and PSD rates in all 529 Multikine-treated patients in the Phase III trial, and the gray columns show PSR and PSD rates for all 394 control patients.
| 7 |
It was not enough for us to show that Multikine likely leads to PSRs and PSDs as compared to a control group, CEL-SCI also had to test if PSRs and PSDs lead to improved survival. CEL-SCI’s Phase III trial demonstrated that PSR patients were 72% likely to be alive after five years, whereas control patients were only about 49% likely to be alive after five years. Patients with PSD saw similar improvement in CEL-SCI’s Phase III trial. Their five-year chance of survival was approximately 68%. Therefore, CEL-SCI believes that the Phase III trial demonstrated that those patients who had PSR or PSD resulting from Multikine lived longer than those who were not treated with Multikine. It is important to note that these results are from the entire Phase III study population, not from a subgroup. The likelihood of living at least five years is shown in the graphic below for patients with PSR (blue), patients with PSD (orange) and control patients who did not receive Multikine (gray).
| 8 |

Multikine cut the 5-year risk of death in half in the target population. CEL-SCI’s results show that Multikine can cut the risk of death in half at five years versus the control group in the target population. Survival increased from 45% in the control group to 73% in the Multikine group at five years. This means the risk of death fell to 27% in the Multikine group from 55% in the control, shown below.
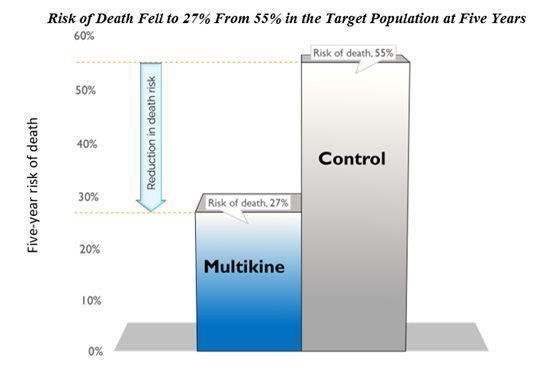
| 9 |
Another way to see the survival benefit of Multikine in the target population is the Kaplan-Meier curve from our ESMO ’23 poster, shown below. On the vertical axis is the probability of survival and the horizontal axis is time in months. The blue Multikine line is far above the green control line, meaning the chance of survival is much higher in the Multikine group at every point in time compared to the control. These results had a low (log rank) p-value of 0.0015, which is very significant as a statistical matter.
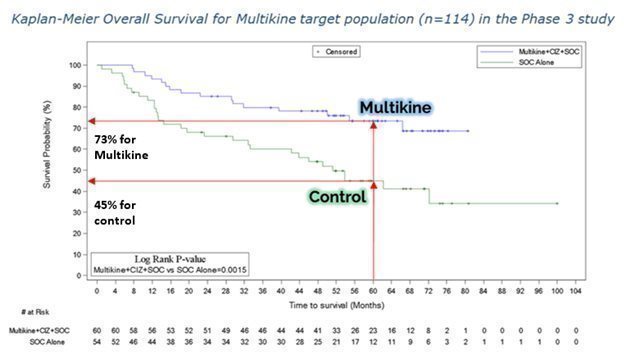
CEL-SCI’s physician consultants tell CEL-SCI that the early separation of these two survival curves (e.g., at 12 months) adds validation to the potential positive effects of Multikine.
Another measure of survival benefit is called the “hazard ratio,” which compares the rate of an event (chances) of dying between two different groups. Here, in the Multikine target population, the hazard ratio was 0.35, which means that deaths occurred in the Multikine group about one-third as frequently as in the control group. It is also important to note that the hazard ratio’s 95% confidence interval remained far below 1.0 (which would mean parity between the compared groups). In the case of Multikine, statistically speaking, there is a 95% chance that the hazard ratio would fall between 0.18 and 0.66 if Multikine were tested in the target population in another study. A hazard ratio of 0.66 as the “so called worst case scenario” (the upper limit of the 95% confidence interval - for the hazard ration - in this case) is still below (better) than the hazard ratio required for most drug approvals.
CEL-SCI completed a bias analysis for the target population in the Phase III study in preparation for submission of data to regulatory agencies including the FDA for confirmatory registration study. The detailed data on parameters including patient age, sex, race, tumor locations, and staging demonstrate balance between the treatment and control arms. Therefore, no bias was found, which supports confidence in Multikine’s efficacy results.
| 10 |
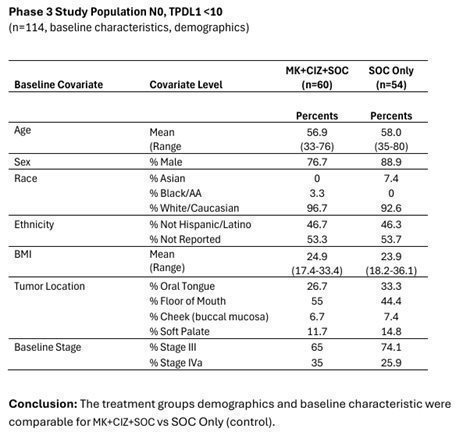
These positive survival outcomes—increased overall survival, reduced risk of death, widely separated Kaplan-Meier curves with early separation, low hazard ratio, low p-values, low confidence intervals—CEL-SCI believes were driven by high PSR/PSD rates in the target population, as shown in the graphic below:
| 11 |

CEL-SCI relies on all of these data together to support its plan to request accelerated/conditional approval in the new target population without waiting until the completion of another clinical trial. CEL-SCI’s regulatory strategy going forward is to seek approval of Multikine following full enrollment of our confirmatory study wherever possible.
CEL-SCI applied to the FDA for a 212-patient randomized controlled confirmatory registration study focusing only on those patients in the target population, this accounts for approximately 100,000 patients worldwide per year. In May 2024, CEL-SCI announced that the FDA indicated CEL-SCI may move forward with a confirmatory registration study of Multikine in the target population.
| 12 |
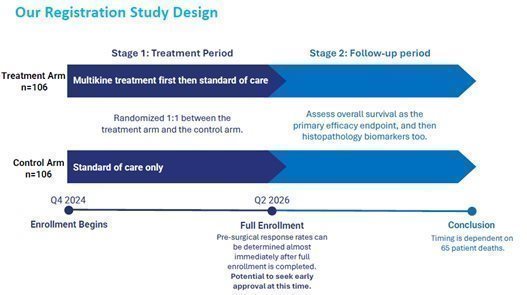
| · | The confirmatory study will be a randomized controlled trial with two arms: Multikine treatment plus standard of care versus standard of care alone. CEL-SCI expects to commence enrollment in Q4 2024/Q1 2025 and reach full enrollment in Q2 2026. |
|
|
|
| · | If approved as a pre-surgical treatment, CEL-SCI believes Multikine should be added to the standard of care for the target population in this unmet medical need. |
|
|
|
| · | CEL-SCI believes that the confirmatory study has a high likelihood of success based on the large survival benefit that has already been observed in the target population from the completed Phase III study. The planned confirmatory study will be much smaller—less than a quarter the size of the prior study— and will focus on the patients who saw the greatest survival benefit when treated with Multikine. |
Why Do We Believe Our Confirmatory Study Will Be Successful?

| 13 |
An “unmet need” is a factor for approval considered by all major regulatory bodies worldwide. In the Multikine target population, there is also a great unmet need for improved survival. The current standard of care provides only about a 50/50 chance of surviving five years, whereas Multikine could increase that survival rate to over 70% in the target population based on the Phase III data. Chemotherapy (in addition to radiotherapy following surgery) has improved survival outcome for some head and neck patients, but chemotherapy is only indicated for high-risk patients, who are not likely to fall within the Multikine target population. Currently available immunotherapies are given after surgery or where surgery is not indicated, in contrast to Multikine, which is given before surgery to patients with resectable tumors. Available checkpoint inhibitors work best on tumors with high PD-L1 expression, whereas Multikine works best in tumors with low PD-L1 expression. Therefore, Multikine’s target population is underserved, and will continue to be underserved, by current therapies, but Multikine can meet the need for improved survival.
The major regulatory bodies with whom we are working, U.S. FDA, Health Canada, European Medicines Agency (EMA) and the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom (UK) all have conditional approval pathways designed for situations where the target population has not been fully tested prospectively and there is strong data supporting clinical benefit for patients. The reason is that regulators understand that in many cases patients should not have to wait for additional data before being offered the chance to benefit from a new drug, especially if the drug has shown to be safe. Every situation is different and depends on the specific facts.
IN CONCLUSION
| · | Strong survival data: Multikine-treated patients in the target population had a 73% 5-year survival vs a 45% 5-year survival in the control group who did not receive Multikine in the Phase III study. In addition, no safety signals or toxicities versus standard of care. The Hazard ratio is 0.35 with an upper limit of 0.66. |
|
|
|
| · | Addressing an unmet medical need: Multikine focuses on the 70% of patients not well served by the two leading approved drugs for head and neck cancer, Keytruda and Opdivo. Multikine’s proposed indication is a pre-surgical treatment in head & neck cancer, where no drug is currently approved. |
|
|
|
| · | Multikine’s Target Population: The confirmatory registration study will focus on newly diagnosed locally advanced primary head and neck cancer patients with no lymph node involvement (determined via PET scan) and with low PD-L1 tumor expression (determined via biopsy). |
|
|
|
| · | FDA pathway: CEL-SCI is aiming to begin enrollment for a 212-patient confirmatory registration study in Q4 2024/Q1 2025, with full enrollment expected about Q2 2026. Potential to seek early approval after full enrollment. |
| 14 |
FORWARD LOOKING STATEMENTS
This prospectus and the documents that are incorporated or deemed to be incorporated by reference into this prospectus, contain or incorporate by reference “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. You can generally identify these forward-looking statements by forward-looking words such as “anticipates,” “believes,” “expects,” “intends,” “future,” “could,” “estimates,” “plans,” “would,” “should,” “potential,” “continues” and similar words or expressions (as well as other words or expressions referencing future events, conditions or circumstances). These forward-looking statements involve risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements, including, but not limited to:
| · | the progress and timing of, and the amount of expenses associated with, our research, development and commercialization activities for our product candidates, including Multikine; |
| · | our expectations regarding the timing, costs and outcome of any pending or future litigation matters, lawsuits or arbitration proceeding; |
| · | the success of our clinical studies for our product candidates; |
| · | our ability to obtain U.S. and foreign regulatory approval for our product candidates and the ability of our product candidates to meet existing or future regulatory standards; |
| · | our expectations regarding federal, state and foreign regulatory requirements; |
| · | the therapeutic benefits and effectiveness of our product candidates; |
| · | the safety profile and related adverse events of our product candidates; |
| · | our ability to manufacture sufficient amounts of Multikine or our other product candidates for use in our clinical studies or, if approved, for commercialization activities following such regulatory approvals; |
| · | our plans with respect to collaborations and licenses related to the development, manufacture or sale of our product candidates; |
| · | our expectations as to future financial performance, expense levels and liquidity sources; |
| · | our ability to compete with other companies that are or may be developing or selling products that are competitive with our product candidates; |
| · | anticipated trends and challenges in our potential markets; |
| · | our ability to attract, retain and motivate key personnel; |
| · | our ability to continue as a going concern; and |
| · | our liquidity. |
All forward-looking statements contained herein are expressly qualified in their entirety by this cautionary statement, the risk factors set forth under the heading “Risk Factors” and information elsewhere in this prospectus and in the documents incorporated or deemed to be incorporated by reference into this prospectus. The forward-looking statements contained in this prospectus and any document incorporated or deemed to be incorporated by reference in this prospectus, speak only as of their respective dates. Except to the extent required by applicable laws and regulations, we undertake no obligation to update these forward-looking statements to reflect new information, events or circumstances after the date of this prospectus or to reflect the occurrence of unanticipated events. In light of these risks and uncertainties, the forward-looking events and circumstances described in this prospectus and the documents that are incorporated by reference into this prospectus may not occur and actual results could differ materially from those anticipated or implied in such forward-looking statements. Accordingly, you are cautioned not to place undue reliance on these forward-looking statements.
| 15 |
RISK FACTORS
Investing in our securities involves a high degree of risk. In addition to the other information contained in this prospectus and in the documents we incorporate by reference, you should carefully consider the risks discussed below and under the heading “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended September 30, 2023 before making a decision about investing in our securities. The risks and uncertainties discussed below and in our Annual Report on Form 10-K for the fiscal year ended September 30, 2023 are not the only ones facing us. Additional risks and uncertainties not presently known to us may also harm our business. If any of these risks occur, our business, financial condition and operating results could be harmed, the trading price of our common stock could decline and you could lose part or all of your investment.
The exercise of outstanding warrants and options will cause dilution.
As of August 20, 2024, there were outstanding warrants which allow the holders to purchase 1,092,470 shares of common stock with a weighted average exercise price of $3.16 per share, pre-funded warrants to purchase 2,380,000 shares of common stock with an exercise price of $0.01 per share, and outstanding options which allow the holders to purchase up to 16,313,110 shares of common stock, with a weighted average exercise price of $6.92 per share. The exercise of these outstanding warrants and options will cause dilution to holders of our common stock.
A provision in our Bylaws regarding shareholder claims may not be enforceable.
Article X of our bylaws provides that stockholder claims brought against us, or our officers or directors, including any derivative claim or claim purportedly filed on our behalf, must be brought in the U.S. District Court for the district of Delaware.
The exclusive forum provision may limit a stockholder’s ability to bring a claim in a judicial forum the stockholder finds favorable for disputes with CEL-SCI or its directors or officers, and may have the effect of discouraging lawsuits with respect to claims that may benefit CEL-SCI or its stockholders.
Although it is our intent that this provision applies to actions arising under the Securities Act of 1933 and the Securities Exchange Act of 1934 there is uncertainty as to whether a court would enforce this provision since Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations under the Securities Act.
In addition, since this provision in our bylaws applies to state law claims there is uncertainty as to whether any court would enforce this provision.
DILUTION
As of June 30, 2024, we had a net book value was approximately $0.15 per share. An investor will suffer dilution equal in amount to the difference between the price paid for the shares and our net tangible book value at the time of purchase.
USE OF PROCEEDS
All of the shares offered by this prospectus are being offered by certain owners of CEL-SCI’s common stock (the Selling Shareholders) and were issued by CEL-SCI in connection with CEL-SCI's employee stock option, bonus and compensation plans. None of the proceeds from this offering will be received by CEL-SCI. Expenses expected to be incurred by CEL-SCI in connection with this offering are estimated to be approximately $10,000. The Selling Shareholders have agreed to pay all commissions and other compensation to any securities broker/dealers through which they sell any of the Shares.
| 16 |
MARKET FOR CEL-SCI’S COMMON STOCK
Our common stock is publicly traded on the NYSE American under the symbol “CVM”. The following table sets forth, for the periods indicated, the high and low intraday sale prices of our common stock as reported by the NYSE American.
Quarter Ending |
| High |
|
| Low |
| ||
|
|
|
|
|
|
| ||
FY 2022 |
|
|
|
|
|
| ||
12/31/2021 |
| $ | 12.82 |
|
| $ | 7.06 |
|
3/31/2022 |
| $ | 7.73 |
|
| $ | 3.80 |
|
6/30/2022 |
| $ | 6.14 |
|
| $ | 2.49 |
|
9/30/2022 |
| $ | 5.42 |
|
| $ | 3.09 |
|
|
|
|
|
|
|
|
|
|
FY 2023 |
|
|
|
|
|
|
|
|
12/31/2022 |
| $ | 3.68 |
|
| $ | 1.88 |
|
3/31/2023 |
| $ | 3.33 |
|
| $ | 2.15 |
|
6/30/2023 |
| $ | 2.94 |
|
| $ | 1.86 |
|
9/30/2023 |
| $ | 2.99 |
|
| $ | 1.08 |
|
|
|
|
|
|
|
|
|
|
FY 2024 |
|
|
|
|
|
|
|
|
12/31/2023 |
| $ | 3.23 |
|
| $ | 1.04 |
|
3/31/2024 |
| $ | 3.08 |
|
| $ | 1.63 |
|
6/30/2024 |
| $ | 2.39 |
|
| $ | 1.10 |
|
As of August 20, 2024, there were 62,743,229 outstanding shares of our common stock outstanding held by approximately 480 holders of record.
Holders of common stock are entitled to receive dividends as may be declared by the Board of Directors out of legally available funds and, in the event of liquidation, to share pro rata in any distribution of CEL-SCI’s assets after payment of liabilities. The Board of Directors is not obligated to declare a dividend. CEL-SCI has not paid any dividends on its common stock and CEL-SCI does not have any current plans to pay any common stock dividends.
The provisions in CEL-SCI’s Articles of Incorporation relating to CEL-SCI’s preferred stock would allow CEL-SCI’s directors to issue preferred stock with rights to multiple votes per share and dividend rights which would have priority over any dividends paid with respect to CEL-SCI’s common stock. The issuance of preferred stock with such rights may make more difficult the removal of management, even if such removal would be considered beneficial to shareholders generally, and will have the effect of limiting shareholder participation in certain transactions such as mergers or tender offers if such transactions are not favored by incumbent management.
The market price of CEL-SCI’s common stock, as well as the securities of other biopharmaceutical and biotechnology companies, have historically been highly volatile, and the market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. Factors such as fluctuations in CEL-SCI’s operating results, announcements of technological innovations or new therapeutic products by CEL-SCI or its competitors, governmental regulation, developments in patent or other proprietary rights, public concern as to the safety of products developed by CEL-SCI or other biotechnology and pharmaceutical companies, and general market conditions may have a significant effect on the market price of CEL-SCI’s common stock.
| 17 |
SELLING SHAREHOLDERS
CEL-SCI has issued (or may in the future issue) shares of its common stock to various persons pursuant to certain employee compensation plans adopted by CEL-SCI. The employee compensation plans provide for the grant or issuance to selected employees of CEL-SCI and other persons of shares of CEL-SCI’s common stock or options to purchase shares of CEL-SCI’s common stock. Persons who received shares pursuant to the Plans and who are offering such shares to the public by means of this Prospectus are referred to as the “Selling Shareholders”.
CEL-SCI has Incentive Stock Option Plans, Non-Qualified Stock Option Plans, Stock Bonus Plans, Stock Compensation Plans and an Incentive Stock Bonus Plan. All Stock Option, Bonus and Compensation Plans have been approved by CEL-SCI’s stockholders. A summary description of these Plans follows. In some cases, these Plans are collectively referred to as the “Plans”.
Incentive Stock Option Plans. The has Incentive Stock Option Plans authorize the issuance of shares of CEL-SCI’s Common Stock to persons who exercise options granted pursuant to the Plans. Only CEL-SCI’s employees may be granted options pursuant to the Incentive Stock Option Plans.
Options may not be exercised until one year following the date of grant. Options granted to an employee then owning more than 10% of the common stock of CEL-SCI may not be exercisable by its terms after five years from the date of grant. Any other option granted pursuant to the Plans may not be exercisable by its terms after ten years from the date of grant.
The option exercise price is determined by CEL-SCI’s Compensation Committee but cannot be less than the fair market value of the common stock on the date of the grant of the option (or 110% of the fair market value in the case of a person owning more than 10% of CEL-SCI's outstanding shares).
Non-Qualified Stock Option Plans. The has Non-Qualified Stock Option Plans authorize the issuance of shares of CEL-SCI’s Common Stock to persons who exercise options granted pursuant to the Plans. CEL-SCI’s employees, directors, officers, consultants and advisors are eligible to be granted options pursuant to the Plans, provided however that bona fide services must be rendered by such consultants or advisors and such services must not be in connection with a capital-raising transaction or promoting CEL-SCI’s common stock. The option exercise price is determined by CEL-SCI’s Compensation Committee.
Stock Bonus Plans. Under the Stock Bonus Plans shares of CEL-SCI’s common stock may be issued to CEL-SCI’s employees, directors, officers, consultants and advisors provided however that bona fide services must be rendered by consultants or advisors and such services must not be in connection with a capital-raising transaction or promoting CEL-SCI’s common stock.
Stock Compensation Plans. Under the Stock Compensation Plans shares of CEL-SCI’s common stock may be issued to CEL-SCI’s employees, directors, officers, consultants and advisors in payment of salaries, fees or other compensation owed to these persons. However, bona fide services must be rendered by consultants or advisors and such services must not be in connection with a capital-raising transaction or promoting CEL-SCI’s common stock.
Incentive Stock Bonus Plan. Under the 2014 Incentive Stock Bonus Plan shares of CEL-SCI’s common stock may be issued to executive officers and other employees who contribute significantly to the success of CEL-SCI. The purpose of the Plan is to provide long term incentive for outstanding service to CEL-SCI and its shareholders, to assist in recruiting and retaining people of outstanding ability and initiative in executive and management positions to allow officers and employees to participate in CEL-SCI's future prosperity and growth, and to align the interests of CEL-SCI’s officers and employees with those of its shareholders.
| 18 |
Summary. The following lists, as of August 20, 2024, the options and shares available pursuant to the Plans. Each option represents the right to purchase one share of CEL-SCI's common stock.
Name of Plan |
| Total Options Reserved Under Plans |
| |
Incentive Stock Option Plans |
|
| 138,400 |
|
Non-Qualified Stock Option Plans |
|
| 17,787,200 |
|
Stock Bonus Plans |
|
| 1,283,760 |
|
Stock Compensation Plans |
|
| 634,000 |
|
Incentive Stock Bonus Plan |
|
| 640,000 |
|
Shares issuable upon the exercise of options granted to CEL-SCI’s officers and directors pursuant to its stock option plans are being offered by means of this Prospectus. The following table lists the shareholdings of CEL-SCI’s officers and directors and the shares offered by means of this prospectus as of August 20, 2024.
|
|
|
| Number of Shares Being Offered |
|
| Number of shares which will be owned on |
|
|
| ||||||||||||||
Name of Selling Shareholder |
| Number of Shares Owned |
|
| Option Shares (2) |
|
| Bonus Shares (1) |
|
| Stock Bonus Shares |
|
| completion of the Offering |
|
| Percent of Class |
| ||||||
Geert R. Kersten |
|
| 1,199,770 | (3) |
|
| 250,000 |
|
|
| 58,000 |
|
|
| -- |
|
|
| 1,141,770 |
|
|
| 1.82 | % |
Patricia B. Prichep |
|
| 236,024 |
|
|
| 167,000 |
|
|
| 31,000 |
|
|
| -- |
|
|
| 205,024 |
|
| * |
| |
Eyal Talor, Ph.D. |
|
| 116,332 |
|
|
| 182,000 |
|
|
| 31,000 |
|
|
| -- |
|
|
| 85,332 |
|
| * |
| |
Daniel H. Zimmerman, Ph.D. |
|
| 120,226 |
|
|
| -- |
|
|
| -- |
|
|
| -- |
|
|
| 120,226 |
|
| * |
| |
John Cipriano |
|
| 63,108 |
|
|
| -- |
|
|
| 16,000 |
|
|
| -- |
|
|
| 47,108 |
|
| * |
| |
Bruno Baillavoine |
|
| 5,973 |
|
|
| 64,000 |
|
|
| -- |
|
|
| -- |
|
|
| 5,973 |
|
| * |
| |
Robert Watson |
|
| 24,431 |
|
|
| 64,000 |
|
|
| -- |
|
|
| -- |
|
|
| 24,431 |
|
| * |
| |
Mario Gobbo |
|
| -- |
|
|
| 64,000 |
|
|
| -- |
|
|
| -- |
|
|
| -- |
|
| * |
| |
* Less than 1%.
(1) | Includes shares awarded pursuant to CEL-SCI’s 2014 Incentive Stock Bonus Plan that have not vested. |
(2) | Represents shares issued or issuable upon exercise of stock options. The options held by CEL-SCI’s officers and directors are exercisable at prices of between $1.36 and $20.61 per share. |
(3) | Includes shares held in trusts for the benefit of Mr. Kersten’s children, and shares held in the de Clara Trust, for which Mr. Kersten is a beneficiary. |
Mr. Kersten is an officer and director of CEL-SCI. Mr. Baillavoine, Mr. Watson and Mr. Gobbo are directors of CEL-SCI. The other persons in the foregoing table are officers of CEL-SCI.
CEL-SCI has filed with the Commission under the Securities Act of 1933 a Form S-8 registration statement of which this prospectus forms a part with respect to the resale of the shares from time to time in the public market or in privately negotiated transactions.
| 19 |
PLAN OF DISTRIBUTION
The Selling Shareholders may sell the Shares offered by this Prospectus from time to time in negotiated transactions in the public market at fixed prices which may be changed from time to time, at market prices prevailing at the time of sale, at prices related to such prevailing market prices or at negotiated prices. The Selling Shareholders may effect such transactions by selling the Shares to or through broker/dealers, and such broker/dealers may receive compensation in the form of discounts, concessions, or commissions from the Selling Shareholders and/or the purchasers of the Shares for which such broker/dealers may act as agent or to whom they may sell, as principal, or both (which compensation as to a particular broker/dealer may be in excess of customary compensation).
The Selling Shareholders and any broker/dealers who act in connection with the sale of the Shares hereunder may be deemed to be “underwriters” within the meaning of Section 2(11) of the Securities Act of 1933, and any commissions received by them and profit on any resale of the Shares as principal might be deemed to be underwriting discounts and commissions under the Securities Act.
CEL-SCI has advised the Selling Shareholders that they and any securities broker/dealers or others who may be deemed to be statutory underwriters will be subject to the prospectus delivery requirements under the Securities Act of 1933. CEL-SCI has also advised each Selling Shareholder that in the event of a “distribution” of the shares owned by the Selling Shareholder, such Selling Shareholder, any “affiliated purchasers”, and any broker/ dealer or other person who participates in such distribution may be subject to Rule 102 under the Securities Exchange Act of 1934 (“1934 Act”) until their participation in that distribution is completed. A “distribution” is defined in Rule 102 as an offering of securities “that is distinguished from ordinary trading transactions by the magnitude of the offering and the presence of special selling efforts and selling methods". CEL-SCI has also advised the Selling Shareholders that Rule 101 under the 1934 Act prohibits any “stabilizing bid” or “stabilizing purchase” for the purpose of pegging, fixing or stabilizing the price of the common stock in connection with the sale of the Shares by any Selling Shareholder.
DESCRIPTION OF SECURITIES
Common Stock
CEL-SCI is authorized to issue 600,000,000 shares of common stock, (the "common stock"). Holders of common stock are each entitled to cast one vote for each share held of record on all matters presented to shareholders. Cumulative voting is not allowed; hence, the holders of a majority of the outstanding shares of common stock can elect all directors.
Holders of common stock are entitled to receive such dividends as may be declared by the Board of Directors out of funds legally available therefor and, in the event of liquidation, to share pro rata in any distribution of CEL-SCI's assets after payment of liabilities. The board is not obligated to declare a dividend. It is not anticipated that dividends will be paid in the foreseeable future.
Holders of common stock do not have preemptive rights to subscribe to additional shares if issued by CEL-SCI. There are no conversion, redemption, sinking fund or similar provisions regarding the common stock. All of the outstanding shares of common stock are fully paid and non-assessable.
Preferred Stock
CEL-SCI is authorized to issue up to 200,000 shares of preferred stock. CEL-SCI's Articles of Incorporation provide that the Board of Directors has the authority to divide the preferred stock into series and, within the limitations provided by Colorado statute, to fix by resolution the voting power, designations, preferences, and relative participation, special rights, and the qualifications, limitations or restrictions of the shares of any series so established. As the Board of Directors has authority to establish the terms of, and to issue, the preferred stock without shareholder approval, the preferred stock could be issued to defend against any attempted takeover of CEL-SCI. As of the date of this prospectus no shares of preferred stock were outstanding.
| 20 |
Rights Agreement
In October 2020, CEL-SCI declared a dividend of one Series A Right and one Series B Right, or collectively the Rights, for each share of CEL-SCI’s common stock which was outstanding on October 30, 2025. When the Rights become exercisable, each Series A Right will entitle the registered holder, subject to the terms of a Rights Agreement, to purchase from CEL-SCI one share of CEL-SCI’s common stock at a price equal to 20% of the market price of CEL-SCI’s common stock on the distribution date, although the price may be adjusted pursuant to the terms of the Rights Agreement. If after a person or group of affiliated persons has acquired 15% or more of CEL-SCI’s common stock or following the commencement of a tender offer for 15% or more of CEL-SCI’s outstanding common stock (i) CEL-SCI is acquired in a merger or other business combination and CEL-SCI is not the surviving corporation, (ii) any person consolidates or merges with CEL-SCI and all or part of CEL-SCI’s common shares are converted or exchanged for securities, cash or property of any other person, or (iii) 50% or more of CEL-SCI’s consolidated assets or earning power are sold, proper provision will be made so that each holder of a Series B Right will thereafter have the right to receive, upon payment of the exercise price of $100 (subject to adjustment), that number of shares of common stock of the acquiring company which at the time of such transaction has a market value that is twice the exercise price of the Series B Right.
The description and terms of the Rights are set forth in a Rights Agreement between the Company and Computershare Trust Company, N.A., as Rights Agent.
Distribution of Rights
Initially, stockholders will not receive separate certificates for the Rights as the Rights will be represented by outstanding common stock certificates. Until the exercise date, the Rights cannot be bought, sold or otherwise traded separately from the common stock. Certificates for common stock will carry a notation that indicates that Rights are attached to the common stock and incorporate the terms of the Rights Agreement.
Separate certificates representing the Rights will be distributed as soon as practicable after the earliest to occur of:
| · | 15 business days following a public announcement that a person or group of affiliated or associated persons has acquired beneficial ownership of 15% or more of CEL-SCI’s outstanding common stock, or |
|
|
|
| · | 15 business days (or such later date as may be determined by action of CEL-SCI’s board of directors prior to such time as any person or group of affiliated persons has acquired 15% or more of CEL-SCI’s common stock) following the commencement of, or announcement of an intention to make, a tender offer or exchange offer the consummation of which would result in the beneficial ownership by a person or group of 15% or more of such outstanding common stock. |
The earlier of such dates described above is called the “distribution date.”
Until the distribution date (or earlier redemption or expiration of the Rights), the surrender for transfer of any certificates for common stock outstanding as of the record date, even without such notation, will also constitute the transfer of the Rights associated with the common stock represented by such certificate. As soon as practicable following the distribution date, separate certificates evidencing the Rights will be mailed to holders of record of the common stock as of the close of business on the distribution date, and such separate right certificates alone will evidence the Rights.
Exercise and Expiration
The holders of the Rights are not required to take any action until the Rights become exercisable. The Rights are not exercisable until the distribution date. Holders of the Rights will be notified by CEL-SCI that the Rights have become exercisable. The Rights will expire on October 30, 2025, unless the expiration date is extended or unless the Rights are earlier redeemed by CEL-SCI as described below.
| 21 |
Redemption
At any time prior to the distribution date, CEL-SCI’s board of directors may redeem the Rights in whole, but not in part, at a price of $0.0001 per Right. Subject to the foregoing, the redemption of the Rights may be made effective at such time, on such basis and with such conditions as CEL-SCI’s board of directors in its sole discretion may establish. Immediately upon any redemption of the Rights, the right to exercise the Rights will terminate and the only entitlement of the holders of Rights will be to receive the redemption price.
Exchange Option
At any time after a person or group of affiliated persons has acquired 15% or more of CEL-SCI’s common stock or following the commencement of a tender offer for 15% or more of CEL-SCI’s outstanding common stock, and prior to the acquisition by such person of 50% or more of the outstanding common stock, CEL-SCI’s board of directors may exchange the Rights (other than Rights owned by such person or group which have become void), in whole or in part, at an exchange ratio of one share of common stock per Right (subject to adjustment).
Transfer Agent
Computershare Trust Company, Inc., of Denver, Colorado, is the transfer agent for CEL-SCI's common stock.
| 22 |