Exhibit 99.2

JPM HEALTHCARE CONFERENCE 2022 Jack Phillips President & CEO Accelerate Diagnostics, Inc January 2022

2 CAUTIONARY NOTE ON FORWARD - LOOKING STATEMENTS © Copyright 2022 Accelerate Diagnostics, Inc. All Rights Reserved. The “ACCELERATE DIAGNOSTICS” and “ACCELERATE PHENO” and “A CCE LERATE PHENOTEST” and “ACCELERATE ARC” and diamond shaped logos and marks are registered trademarks of Accelerate Diagnostics, Inc. All other t rad emarks are the property of their respective owners. Certain statements in this presentation may constitute “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 , including statements such as our intent to produce further innovations, our goal of disrupting the infectious disease diagnostic market, increasing demand for antibiotic resistance solutions, accelerating our market penetration with new product extensions (AST Focused Test and ARC) and Pheno 2 . 0 , continued demand for a combined rapid ID/AST solution from a significant segment of the hospital market, successful development, approval, launch, availability and market demand of new products ARC and Pheno 2 . 0 , and 2022 guidance regarding instrument placement, revenue cash use, burn rate and debt . Forward - looking statements may contain words such as “will,” “may,” “expect,” “believe,” “likely,” “anticipate,” similar expressions, and variations or negatives of these words . Forward - looking statements are made based on management’s views and assumptions regarding future events and business performance as of the time the statements are made . Management is not under any obligation, and we expressly disclaim any obligation, to update, alter, or otherwise revise any forward - looking statements, whether as a result of new information, future events, or otherwise . You are cautioned not to place undue reliance on these forward - looking statements which speak only as of the date hereof . Forward - looking statements include projections, statements about our future and those that are not historical facts . All forward - looking statements that are made in this presentation are subject to risks, uncertainties and other factors that could cause our actual results to differ materially . These are discussed in greater detail in our Annual Report on Form 10 - K for the year ended December 31 , 2020 , and other reports we file with the SEC .

3 Solid finish to 2021 in a challenging hospital environment Key market learnings translated into a bold product strategy Significant R&D milestones achieved Optimistic about 2022 commercial success and new product milestones Executive Summary
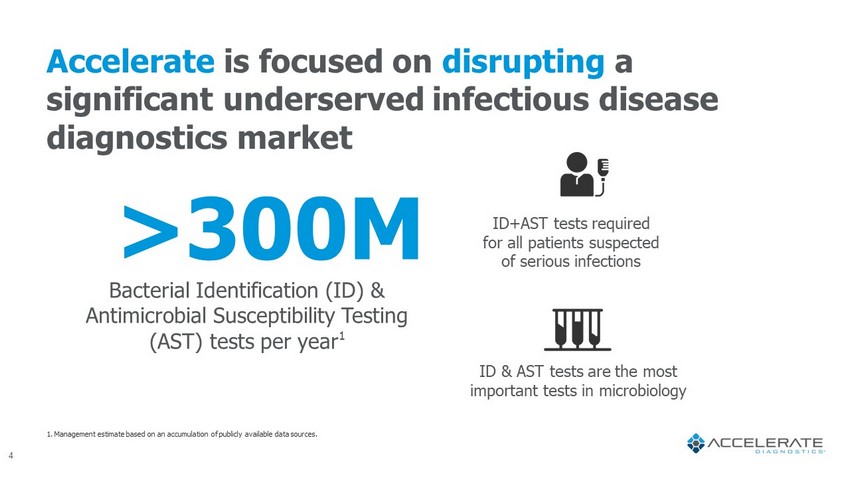
Accelerate is focused on disrupting a significant underserved infectious disease diagnostics market 1. Management estimate based on an accumulation of publicly available data sources. >300M Bacterial Identification (ID) & Antimicrobial Susceptibility Testing (AST) tests per year 1 4 ID+AST tests required for all patients suspected of serious infections ID & AST tests are the most important tests in microbiology
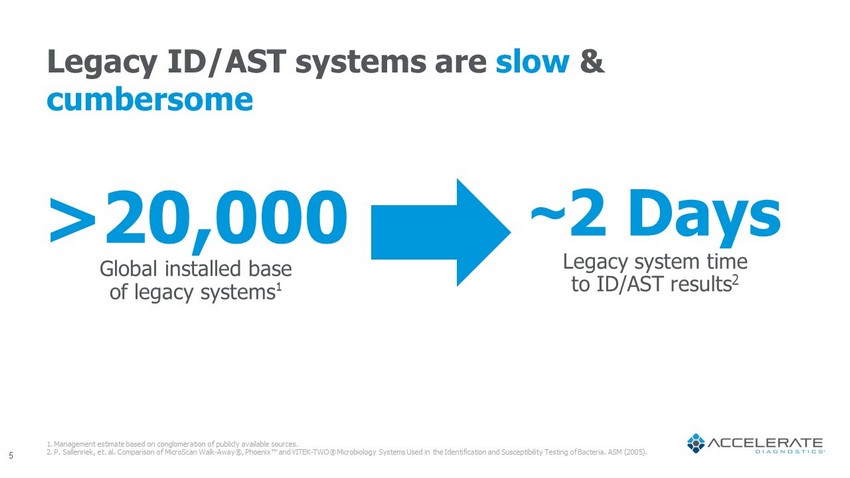
~ 2 Days Legacy system time to ID/AST results 2 5 >20,000 Global installed base of legacy systems 1 1. Management estimate based on conglomeration of publicly available sources. 2. P. Sallenriek , et. al. Comparison of MicroScan Walk - Away®, Phoenix Œ and VITEK - TWO® Microbiology Systems Used in the Identification and Susceptibility Testing of Bacteria. ASM (2005). Legacy ID/AST systems are slow & cumbersome
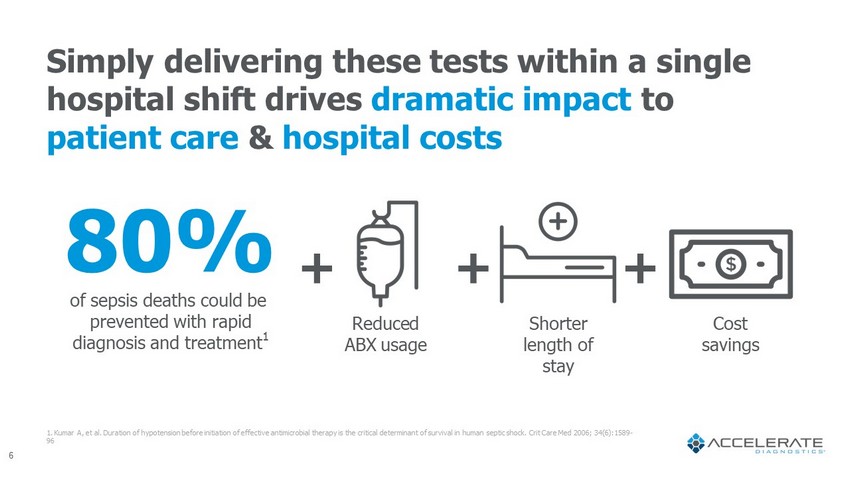
Cost savings 6 80% of sepsis deaths could be prevented with rapid diagnosis and treatment 1 1. Kumar A, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant o f s urvival in human septic shock. Crit Care Med 2006; 34(6):1589 - 96 Shorter length of stay Reduced ABX usage + + + Simply delivering these tests within a single hospital shift drives dramatic impact to patient care & hospital costs
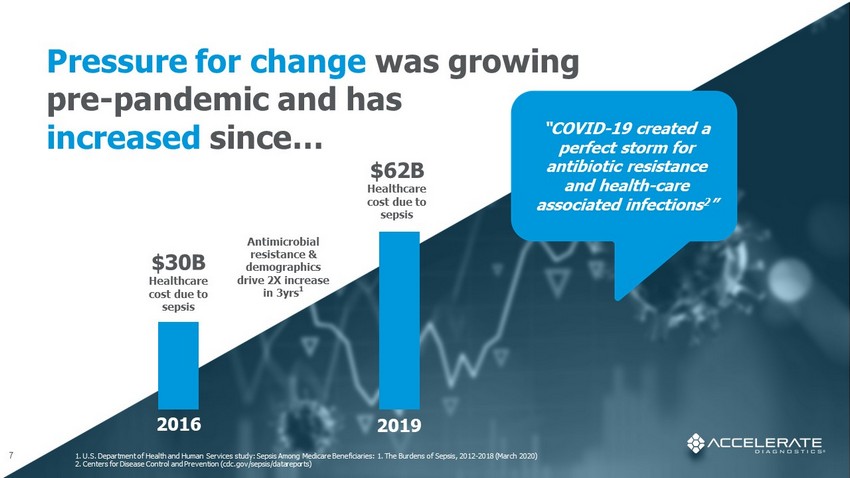
1. U.S. Department of Health and Human Services study: Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012 - 2018 (March 2020) 2. Centers for Disease Control and Prevention (cdc.gov/sepsis/ datareports ) 7 Antimicrobial resistance & demographics drive 2X increase in 3yrs 1 $30B Healthcare cost due to sepsis “COVID - 19 created a perfect storm for antibiotic resistance and health - care associated infections 2 ” Pressure for change was growing pre - pandemic and has increased since… $62B Healthcare cost due to sepsis 2016 2019

Our solution digitizes microbiology for rapid , automated , and accurate ID/AST Yesterday's microbiology Manual workflows Trained staff intensive Slow time to result 8 Digital microbiology Fastest possible AST results Automated workflows Direct from sample capable
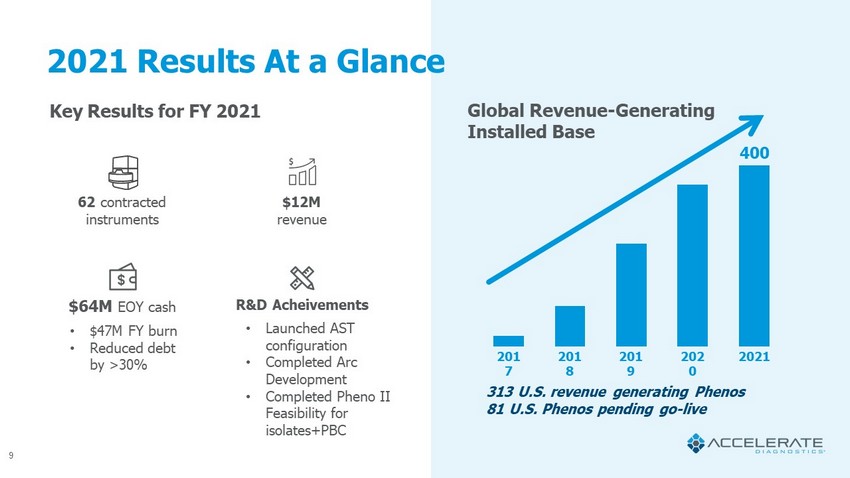
9 313 U.S. revenue generating Phenos 81 U.S. Phenos pending go - live Key Results for FY 2021 Global Revenue - Generating Installed Base 2021 Results At a Glance 62 contracted instruments $12M revenue $64M EOY cash R&D Acheivements • Launched AST configuration • Completed Arc Development • Completed Pheno II Feasibility for isolates+PBC 201 7 201 8 201 9 202 0 2021 • $47M FY burn • Reduced debt by >30% 400

Pheno System & Integrated ID/AST Test + New AST Test Continue to deliver an INTEGRATED Rapid ID/AST solution for those who need it & Offer FLEXIBILITY for those labs who want to LEVERAGE existing ID workflow Accelerate Arc Œ Enter an UNTAPPED MARKET with AUTOMATED SAMPLE PREPARATION to ENABLE RAPID MALDI ID from PBC and in the future from other samples Accelerate Pheno ® II Address ALL acute bacterial Infections and sample types beyond PBC with PHENO II PLATFORM & Menu A bold product strategy capitalizes on this 300M test market opportunity 10

Accelerate Pheno ® System Integrated ID/AST Test + New AST Test 11
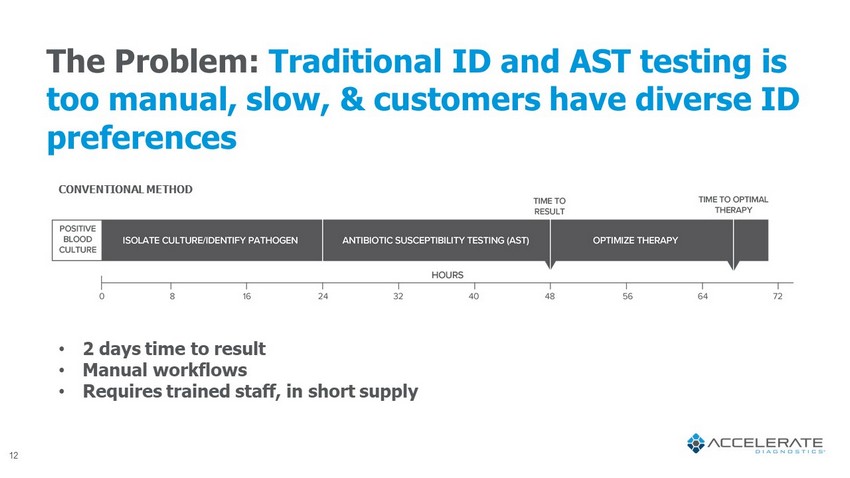
The Problem: Traditional ID and AST testing is too manual, slow, & customers have diverse ID preferences • 2 days time to result • Manual workflows • Requires trained staff, in short supply CONVENTIONAL METHOD 12
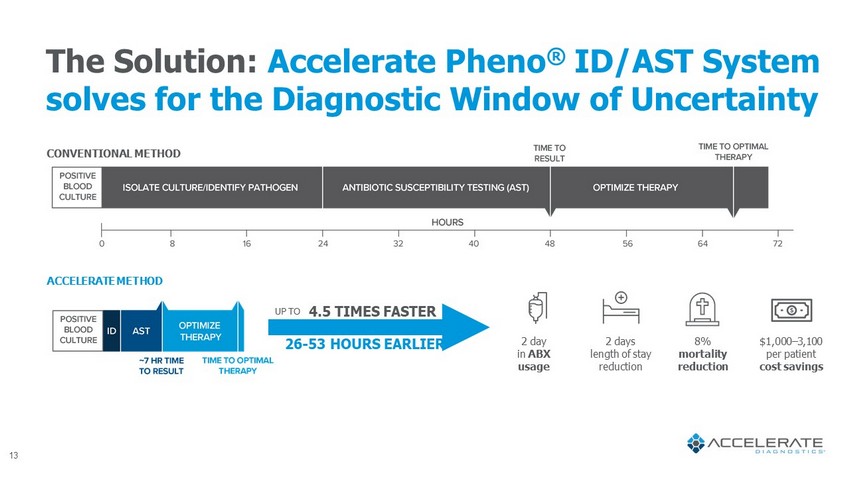
The Solution: Accelerate Pheno ® ID/AST System solves for the Diagnostic Window of Uncertainty CONVENTIONAL METHOD ACCELERATE METHOD 26 - 53 HOURS EARLIER UP TO 8% mortality reduction 2 day in ABX usage 2 days length of stay reduction $1,000 – 3,100 per patient cost savings 4.5 TIMES FASTER 13
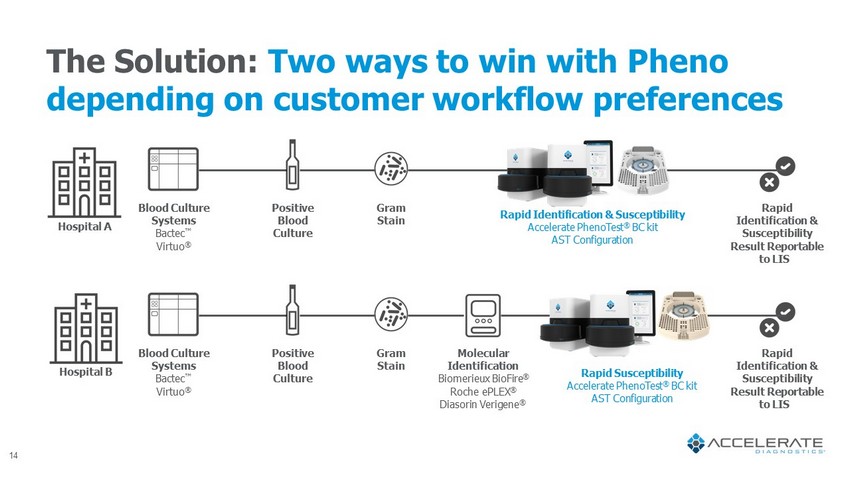
The Solution: Two ways to win with Pheno depending on customer workflow preferences Blood Culture Systems Bactec Œ Virtuo ® Positive Blood Culture Gram Stain Molecular Identification Biomerieux BioFire ® Roche ePLEX ® Diasorin Verigene ® Rapid Susceptibility Accelerate PhenoTest ® BC kit AST Configuration Rapid Identification & Susceptibility Result Reportable to LIS Blood Culture Systems Bactec Œ Virtuo ® Positive Blood Culture Gram Stain Rapid Identification & Susceptibility Accelerate PhenoTest ® BC kit AST Configuration Rapid Identification & Susceptibility Result Reportable to LIS Hospital A Hospital B 14
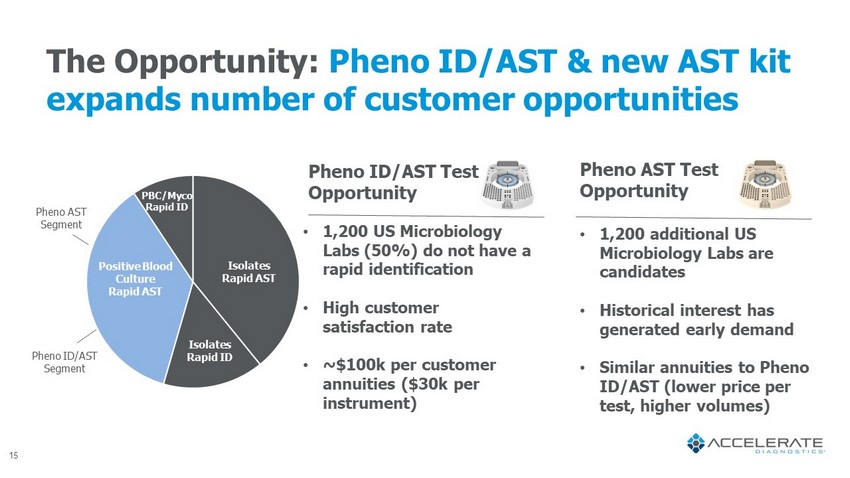
The Opportunity: Pheno ID/AST & new AST kit expands number of customer opportunities • 1,200 US Microbiology Labs (50%) do not have a rapid identification • High customer satisfaction rate • ~$100k per customer annuities ($30k per instrument) • 1,200 additional US Microbiology Labs are candidates • Historical interest has generated early demand • Similar annuities to Pheno ID/AST (lower price per test, higher volumes) Pheno ID/AST Test Opportunity Pheno AST Test Opportunity Pheno ID/AST Segment Pheno AST Segment Isolates Rapid ID Isolates Rapid AST Positive Blood Culture Rapid AST PBC/ Myco Rapid ID 15

Accelerate Arc ΠRapid MALDI Prep 16
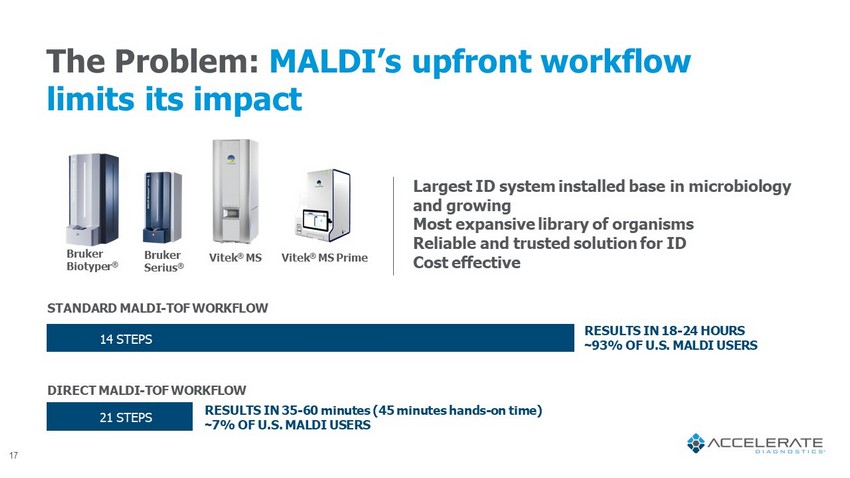
The Problem: MALDI’s upfront workflow limits its impact Bruker Biotyper ® Largest ID system installed base in microbiology and growing Most expansive library of organisms Reliable and trusted solution for ID Cost effective 14 STEPS 21 STEPS Vitek ® MS Prime Bruker Serius ® Vitek ® MS STANDARD MALDI - TOF WORKFLOW DIRECT MALDI - TOF WORKFLOW RESULTS IN 35 - 60 minutes (45 minutes hands - on time) ~ 7% OF U.S. MALDI USERS RESULTS IN 18 - 24 HOURS ~ 93% OF U.S. MALDI USERS 17
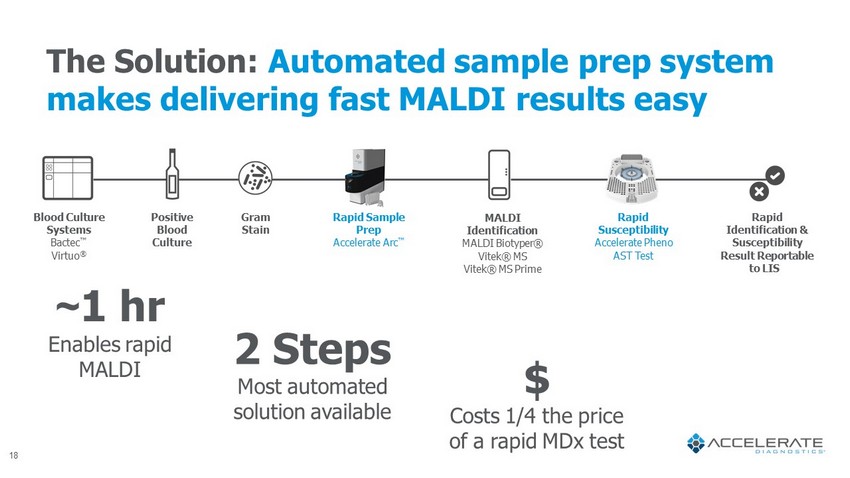
The Solution: Automated sample prep system makes delivering fast MALDI results easy ~ 1 hr Enables rapid MALDI Blood Culture Systems Bactec Œ Virtuo ® Positive Blood Culture Gram Stain Rapid Susceptibility Accelerate Pheno AST Test Rapid Identification & Susceptibility Result Reportable to LIS 2 Steps Most automated solution available $ Costs 1/4 the price of a rapid MDx test Rapid Sample Prep Accelerate Arc Œ MALDI Identification MALDI Biotyper ® Vitek ® MS Vitek ® MS Prime 18
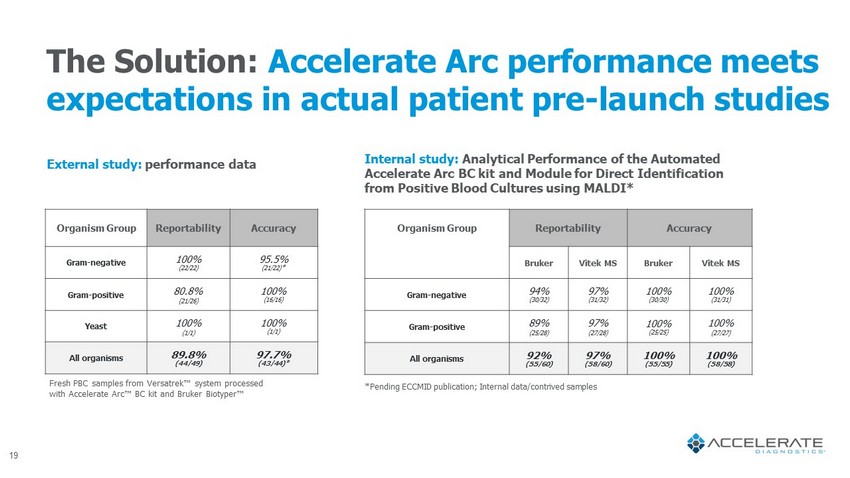
The Solution: Accelerate Arc performance meets expectations in actual patient pre - launch studies Organism Group Reportability Accuracy Gram - negative 100% (22/22) 95.5% (21/22)* Gram - positive 80.8% (21/26) 100% (16/16) Yeast 100% (1/1) 100% (1/1) All organisms 89.8% (44/49) 97.7% (43/44)* External study: performance data Fresh PBC samples from Versatrek Πsystem processed with Accelerate Arc ΠBC kit and Bruker Biotyper ΠOrganism Group Reportability Accuracy Bruker Vitek MS Bruker Vitek MS Gram - negative 94% (30/32) 97% (31/32) 100% (30/30) 100% (31/31) Gram - positive 89% (25/28) 97% (27/28) 100% (25/25) 100% (27/27) All organisms 92% (55/60) 97% (58/60) 100% (55/55) 100% (58/58) Internal study: Analytical Performance of the Automated Accelerate Arc BC kit and Module for Direct Identification from Positive Blood Cultures using MALDI* *Pending ECCMID publication; Internal data/contrived samples 19
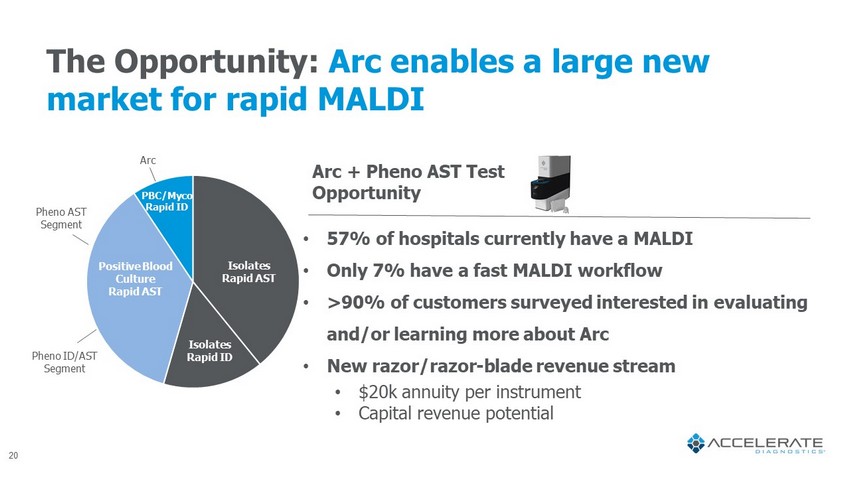
The Opportunity: Arc enables a large new market for rapid MALDI • 57% of hospitals currently have a MALDI • Only 7% have a fast MALDI workflow • >90% of customers surveyed interested in evaluating and/or learning more about Arc • New razor/razor - blade revenue stream • $20k annuity per instrument • Capital revenue potential Arc + Pheno AST Test Opportunity Pheno ID/AST Segment Pheno AST Segment Arc Isolates Rapid ID Isolates Rapid AST Positive Blood Culture Rapid AST PBC/ Myco Rapid ID 20

Accelerate Pheno ® II Total Lab AST Solution 21
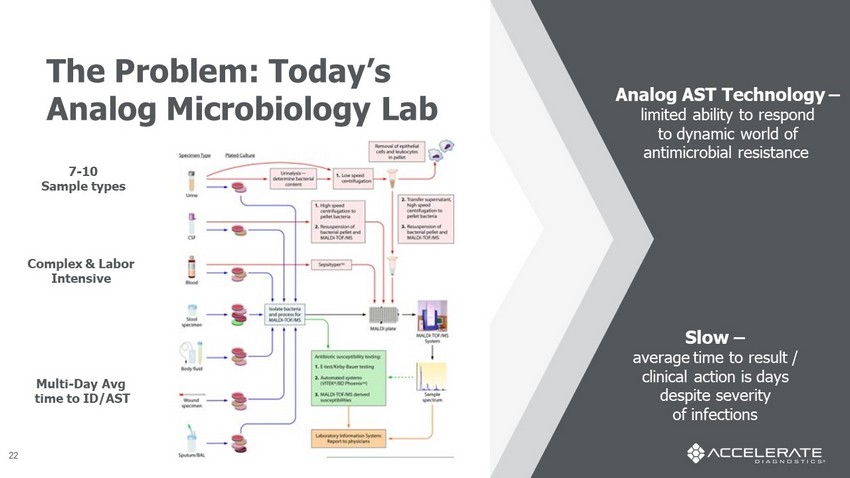
7 - 10 Sample types Complex & Labor Intensive Multi - Day Avg time to ID/AST Analog AST Technology – limited ability to respond to dynamic world of antimicrobial resistance Slow – average time to result / clinical action is days despite severity of infections The Problem: Today’s Analog Microbiology Lab 22

The Solution: high throughput, broad menu, and best in class speed enable a total lab solution 23
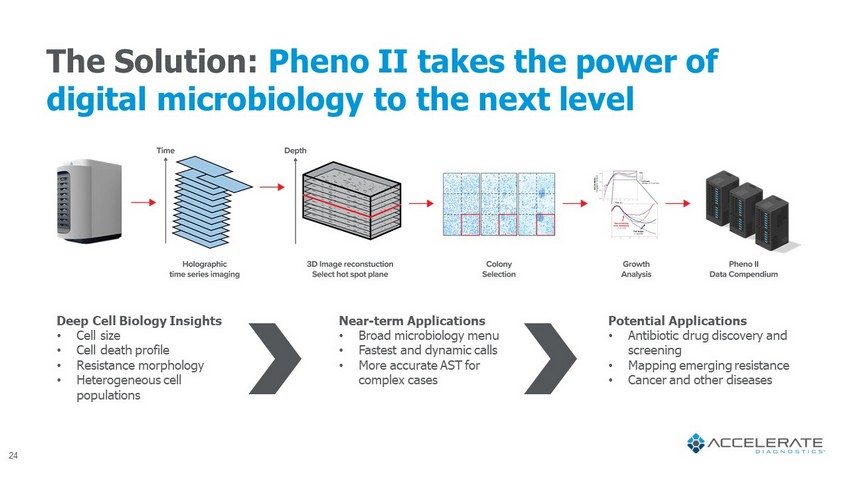
Deep Cell Biology Insights • Cell size • Cell death profile • Resistance morphology • Heterogeneous cell populations Near - term Applications • Broad microbiology menu • Fastest and dynamic calls • More accurate AST for complex cases Potential Applications • Antibiotic drug discovery and screening • Mapping emerging resistance • Cancer and other diseases The Solution: Pheno II takes the power of digital microbiology to the next level 24

INSTRUMENTATION The Solution: Pheno II will deliver a step - function improvement in throughput and cost CONSUMABLE Pheno I Pheno II 10 times the throughput per footprint Less mechanical complexity Solid state imaging vs lens based Pheno I Pheno II 1/30 th the size 1/15 th the average unit cost 3 times the AST analyses per consumable Ambient vs. cold storage 25

The Solution: Pheno II now in assay development on back of strong feasibility data 2018 Solid - State Imaging Feasibility 2019 Prototype Design 2020 Prototype Build & Test 2021 Rapid Isolates Feasibility Rapid PBC Feasibility 2022 Final Instrument Design Assay Development 2023 Clinical Trials Regulatory LAUNCH 26
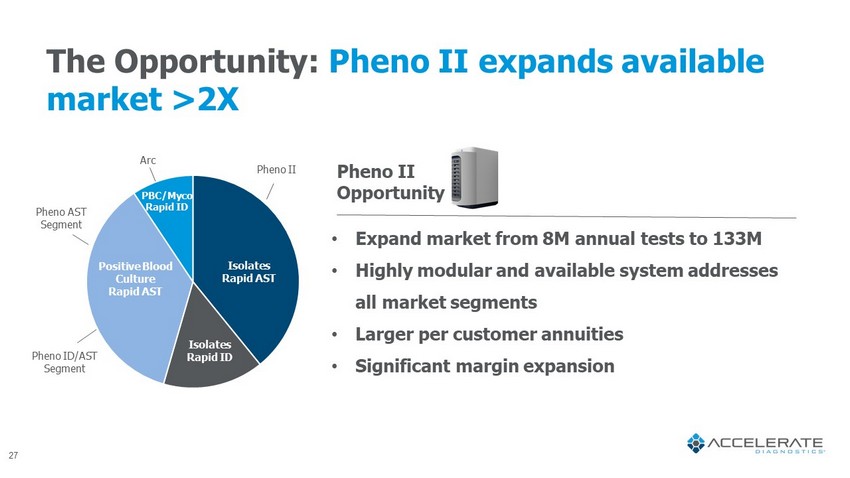
The Opportunity: Pheno II expands available market >2X Pheno ID/AST Segment Pheno AST Segment • Expand market from 8M annual tests to 133M • Highly modular and available system addresses all market segments • Larger per customer annuities • Significant margin expansion Pheno II Opportunity Pheno II Arc Isolates Rapid ID Isolates Rapid AST Positive Blood Culture Rapid AST PBC/ Myco Rapid ID 27

In Summary 28
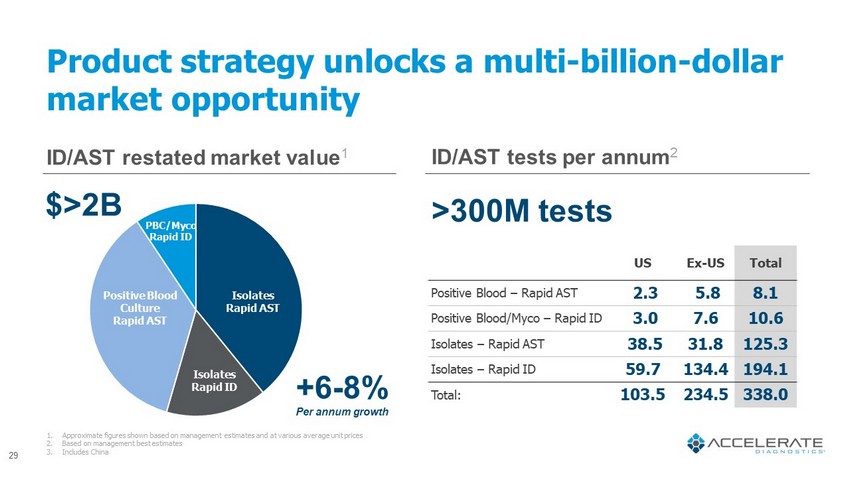
Product strategy unlocks a multi - billion - dollar market opportunity US Ex - US Total Positive Blood – Rapid AST 2.3 5.8 8.1 Positive Blood/ Myco – Rapid ID 3.0 7.6 10.6 Isolates – Rapid AST 38.5 31.8 125.3 Isolates – Rapid ID 59.7 134.4 194.1 Total: 103.5 234.5 338.0 1. Approximate figures shown based on management estimates and at various average unit prices 2. Based on management best estimates 3. Includes China ID/AST restated market value 1 $>2B ID/AST tests per annum 2 >300M tests +6 - 8% Per annum growth Isolates Rapid ID Isolates Rapid AST Positive Blood Culture Rapid AST PBC/ Myco Rapid ID 29

Broadening product offering & a decade of technical/ market experience sets AXDX apart 30 Product Breadth Rapid ID (FDA approved) Rapid AST (FDA approved) Arc (Rapid MALDI ID) NexGen Clinical data >75 publications & proof sources Commercial Experience >100 customers Notable KOLs Optimized implementation process w/ LIS interfaces R&D Expertise 5 petabytes of bacteria data Cold bank of 10,000 bacteria Data from 100,000 field runs 30

2022 guidance reflects diminished backlog but strong funnel and accelerating new product interest Revenue $13 - 14M Cash use $45 - $55M Key R&D Milestones • Q1 Launch Arc • Pheno II instrument final • Pheno II workflow final Continued Balance Improvements • Continue to manage debt • Focused investment strategy 31

Summary WHO WE ARE A diagnostics company based in Arizona, founded in 2012, with the purpose of delivering lifesaving answers for patients with serious infections. THE PROBLEM Due to the overuse of antibiotics, the human and hospital costs of treating conditions like sepsis are increasing. Each year , s epsis causes hundreds of thousands of potentially avoidable deaths and adds an estimated $62 billion in costs to the U.S. healthcare syste m. OUR SOLUTION The Accelerate Pheno system uses proprietary technology to identify pathogens and report antibiotic susceptibility testing results in about 7 hours, allowing antibiotic optimization days earlier than conventional methods. MARKET OPPORTUNITY / FUNDAMENTALS First - mover advantage with high barriers to entry in a multi - billion - dollar market. Razor/blade business model should support no rmalized high GM with significant FCF at scale, all while advancing our mission to save lives and reduce healthcare costs RUNWAY FOR GROWTH Entered 2022 with single - digit penetration after two years of limited commercial activity during the pandemic. Large runway for growth as >300M slow test market is restated with rapid testing. 32































