Exhibit 99.1

| Shaping Medicine, Changing Lives Welcome to OSI's Analyst Day December 4, 2008 |

| OSI Pharmaceuticals Forward-looking Statements OSI's presentation today contain forward-looking statements. These statements are subject to known and unknown risks and uncertainties that may cause actual future experience and results to differ materially from the statements made. Factors that might cause such a difference include, among others, the ability to effectively market products, competition from other pharmaceutical companies, the FDA review process and other governmental regulation, OSI's and its collaborators' abilities to successfully develop and commercialize drug candidates, and other factors described in OSI Pharmaceuticals' filings with the Securities and Exchange Commission. OSI is a Profitable Mid-cap Biotechnology Company that Discovers, Develops and Commercializes Innovative Molecular Targeted Therapies Addressing Major Unmet Medical Needs in Oncology, Diabetes & Obesity |

| Shaping Medicine, Changing Lives Colin Goddard, Ph.D. Chief Executive Officer Analyst Day December 4, 2008 CEO Gold Standard Founding Member Strategic Overview |

| OSIP: 2008 Investor R&D Day "Strategy and Future Direction": AGENDA Time Activity Speaker 12:30 Welcome Kathy Galante 12:35 Overview and Strategy Colin Goddard, Ph.D. 1:20 Diabetes & Obesity Anker Lundemose, M.D., Ph.D. Jonathan Rachman, M.D., Ph.D. 1:55 Oncology David Epstein, Ph.D. Mark Miglarese, Ph.D. Karsten Witt, M.D. 2:45 Executing the Tarceva Life Cycle Plan Gabriel Leung 3:25 Wrap-up / Q&A Colin Goddard, Ph.D. 3:35 Poster Session and Cocktail Reception |

| A Look Back: Key Goals For OSIP in 2007/8: Financial Growth & Disciplined Investment Over the Last Two Years There Has Been Significant Investor Focus on: Financial Performance in 2007 and 2008 Two Major Tarceva Phase III Trial Read-Outs (SATURN and BeTa- Lung) in 2H2008 Divestiture of Eye Business Finalized in 3Q2008 "Establishing An Effective Balance Between Financial Performance and Disciplined Reinvestment in the Business for Sustained Growth" |
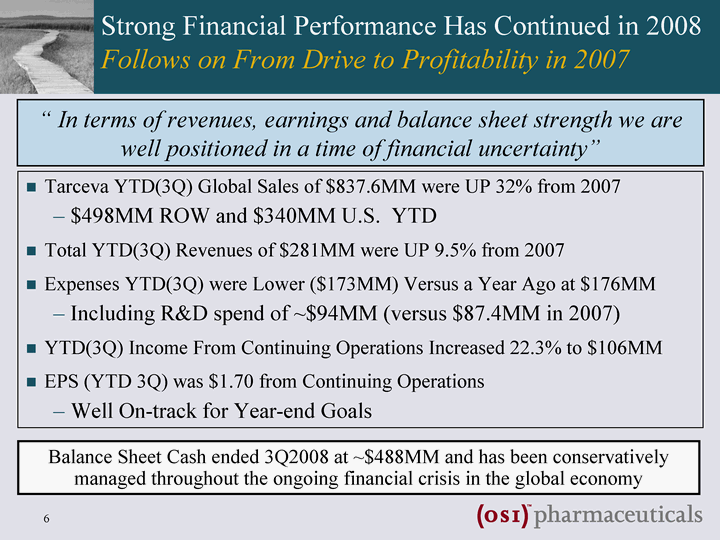
| Strong Financial Performance Has Continued in 2008 Follows on From Drive to Profitability in 2007 Tarceva YTD(3Q) Global Sales of $837.6MM were UP 32% from 2007 $498MM ROW and $340MM U.S. YTD Total YTD(3Q) Revenues of $281MM were UP 9.5% from 2007 Expenses YTD(3Q) were Lower ($173MM) Versus a Year Ago at $176MM Including R&D spend of ~$94MM (versus $87.4MM in 2007) YTD(3Q) Income From Continuing Operations Increased 22.3% to $106MM EPS (YTD 3Q) was $1.70 from Continuing Operations Well On-track for Year-end Goals " In terms of revenues, earnings and balance sheet strength we are well positioned in a time of financial uncertainty" Balance Sheet Cash ended 3Q2008 at ~$488MM and has been conservatively managed throughout the ongoing financial crisis in the global economy |
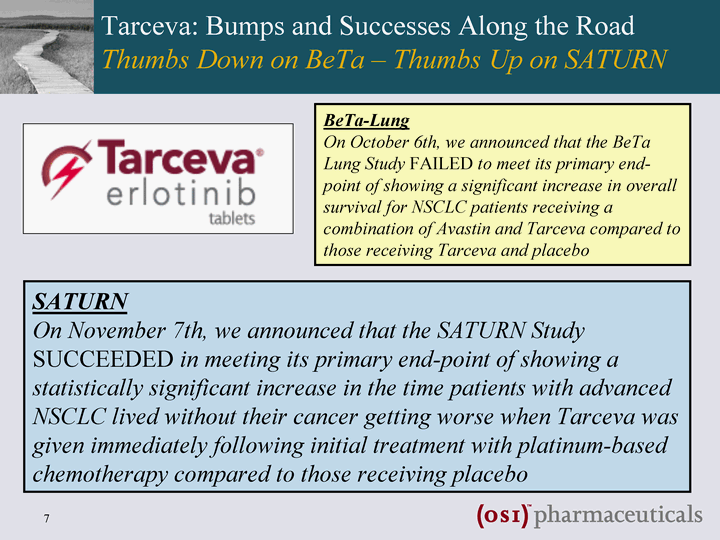
| Tarceva: Bumps and Successes Along the Road Thumbs Down on BeTa - Thumbs Up on SATURN BeTa-Lung On October 6th, we announced that the BeTa Lung Study FAILED to meet its primary end- point of showing a significant increase in overall survival for NSCLC patients receiving a combination of Avastin and Tarceva compared to those receiving Tarceva and placebo SATURN On November 7th, we announced that the SATURN Study SUCCEEDED in meeting its primary end-point of showing a statistically significant increase in the time patients with advanced NSCLC lived without their cancer getting worse when Tarceva was given immediately following initial treatment with platinum-based chemotherapy compared to those receiving placebo |

| The Key Question: Where Do We Go From Here? Discuss Overlying Strategic Themes Outline Plan for Strategic Execution Provide an Assessment of Risk & Reward Around Tarceva Franchise and Reassure You of Our Commitment to Delivering Tarceva Related Shareholder Value Walk Through Some Key Accounting Changes for 2009 Introduce Key Pipeline and Platform Elements That Will be the Focus of the Day's Presentations Agenda for This Presentation |

| OSI Exiting 2008: Strategic Focus Building Meaningfully on Tarceva "Turning the promise of innovative, personalized medicine into practice in our industry" The Premise: Delivering innovative, differentiated new medicines to the right patients, in the right combinations at the right doses should lead to: Speedier and more cost-effective drug development; Delivery of meaningful clinical benefit; and Rapid availability of innovative medicines to the patients who can benefit the most. Building Rapidly on Our Insights From Tarceva in Oncology and Pioneering Personalized Medicine in Diabetes/Obesity Represent Tangible Opportunities for OSIP |

| OSI Exiting 2008: Strategic Focus Building Meaningfully on Tarceva "Turning the promise of innovative, personalized medicine into practice in our industry" The Premise: Delivering innovative, differentiated new medicines to the right patients, in the right combinations at the right doses should lead to: Speedier and more cost-effective drug development; Delivery of meaningful clinical benefit; and Rapid availability of innovative medicines to the patients who can benefit the most. Building Rapidly on Our Insights From Tarceva in Oncology and Pioneering Personalized Medicine in Diabetes/Obesity Represent Tangible Opportunities for OSIP We Are Certainly Not Alone BUT We Believe That We Have The Right Combination of Size, Focus and Financial Strength to Execute a Successful Business Outcome More Effectively Than Most |

| OSIP Exiting 2008: Strategic Execution Delivering Future Shareholder Value From a Strong Base We Believe We Can Build OSIP into the Premier Mid-Cap Biotechnology Company Based Upon: Anchoring Growth Around Tarceva Strong P&L and Balance Sheet Maintaining an effective balance between financial performance & reinvestment in R&D Delivering on the Significant Future Potential From a Differentiated and Innovative Pipeline and Establishing a Reputation for Scientific Excellence Four internally discovered, differentiated, high quality assets advanced to clinical development in last 18 months Leadership position in EMT (oncology) & Neuroendocrine control (diabetes/obesity) We are a profitable, $2-$3 billion market cap company with ~$380MM in annual revenues & generating approximately $150MM in cash flow, primarily from Tarceva & DP-IV royalties |

| OSIP Exiting 2008: Strategic Execution Delivering Future Shareholder Value From a Strong Base We Believe We Can Build OSIP into the Premier Mid-Cap Biotechnology Company Based Upon: Anchoring Growth Around Tarceva Strong P&L and Balance Sheet Maintaining an effective balance between financial performance & reinvestment in R&D Delivering on the Significant Future Potential From a Differentiated and Innovative Pipeline and Establishing a Reputation for Scientific Excellence Four internally discovered, differentiated, high quality assets advanced to clinical development in last 18 months Leadership position in EMT (oncology) & Neuroendocrine control (diabetes/obesity) We are a profitable, $2-$3 billion market cap company with ~$380MM in annual revenues & generating approximately $150MM in cash flow, primarily from Tarceva & DP-IV royalties |
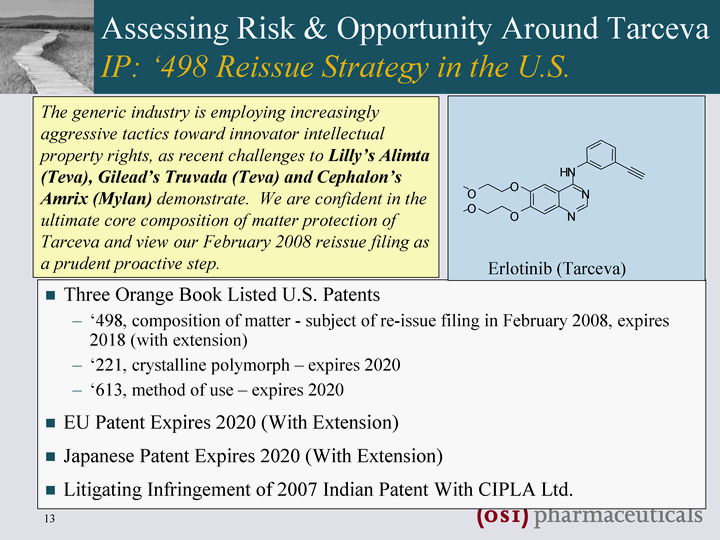
| Assessing Risk & Opportunity Around Tarceva IP: '498 Reissue Strategy in the U.S. Three Orange Book Listed U.S. Patents '498, composition of matter - subject of re-issue filing in February 2008, expires 2018 (with extension) '221, crystalline polymorph - expires 2020 '613, method of use - expires 2020 EU Patent Expires 2020 (With Extension) Japanese Patent Expires 2020 (With Extension) Litigating Infringement of 2007 Indian Patent With CIPLA Ltd. Litigating Infringement of 2007 Indian Patent With CIPLA Ltd. Litigating Infringement of 2007 Indian Patent With CIPLA Ltd. Litigating Infringement of 2007 Indian Patent With CIPLA Ltd. Litigating Infringement of 2007 Indian Patent With CIPLA Ltd. Litigating Infringement of 2007 Indian Patent With CIPLA Ltd. Litigating Infringement of 2007 Indian Patent With CIPLA Ltd. Litigating Infringement of 2007 Indian Patent With CIPLA Ltd. Litigating Infringement of 2007 Indian Patent With CIPLA Ltd. N N N H O O O O Erlotinib (Tarceva) The generic industry is employing increasingly aggressive tactics toward innovator intellectual property rights, as recent challenges to Lilly's Alimta (Teva), Gilead's Truvada (Teva) and Cephalon's Amrix (Mylan) demonstrate. We are confident in the ultimate core composition of matter protection of Tarceva and view our February 2008 reissue filing as a prudent proactive step. |

| Assessing Risk & Opportunity Around Tarceva IP: '498 Reissue Strategy in the U.S. In February 2008 we filed two requests to the PTO to address certain errors in the original '498 patent A certificate of correction related to claim one This was granted in 3Q 2008 A reissue application was filed in order to correct certain errors relating to the claiming of compounds, other than Tarceva, which fall outside of the scope of the main claim in the patent. Tarceva itself is correctly described in the '498 patent Given the prevailing climate we believe that it is likely that a generic company will file an ANDA with a Paragraph IV Certification after the fourth anniversary of Tarceva approval (November, 18th 2008) - should this occur you can expect: They will notify us and we will issue a press release We will file suit within 45 days If we bring suit by Nov. 18, 2009, FDA cannot approve an ANDA until 7.5 years have elapsed from initial Tarceva approval, or May 18, 2012. This period may end early if we were to lose the patent infringement case We, and our collaborators, strongly believe in the inventiveness of Tarceva |

| Assessing Risk & Opportunity Around Tarceva 2009 Market Dynamics C.S.I. Selling Model (US) Patient Share Up Female Adeno Never-smokers Squam share up slightly % Non-prescriber dropped (US) Increased 1st line NSCLC use PanCan stable NICE approval in UK Growth potential in Japan Cost-containment pressure mounts (US) Patient rejection rate up due to co-pay/donut hole Payer control increasing Increasing use of more chemo in earlier lines (US) Slowing growth in some major EU markets Potential for currency impact to go against us in 2009 But Real Growth Drivers Exist Some Challenges...... |
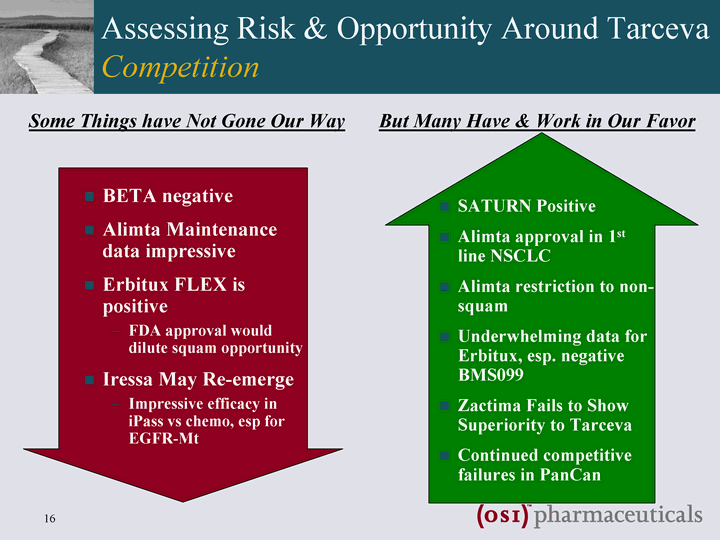
| Assessing Risk & Opportunity Around Tarceva Competition SATURN Positive Alimta approval in 1st line NSCLC Alimta restriction to non- squam Underwhelming data for Erbitux, esp. negative BMS099 Zactima Fails to Show Superiority to Tarceva Continued competitive failures in PanCan BETA negative Alimta Maintenance data impressive Erbitux FLEX is positive FDA approval would dilute squam opportunity Iressa May Re-emerge Impressive efficacy in iPass vs chemo, esp for EGFR-Mt But Many Have & Work in Our Favor Some Things have Not Gone Our Way |

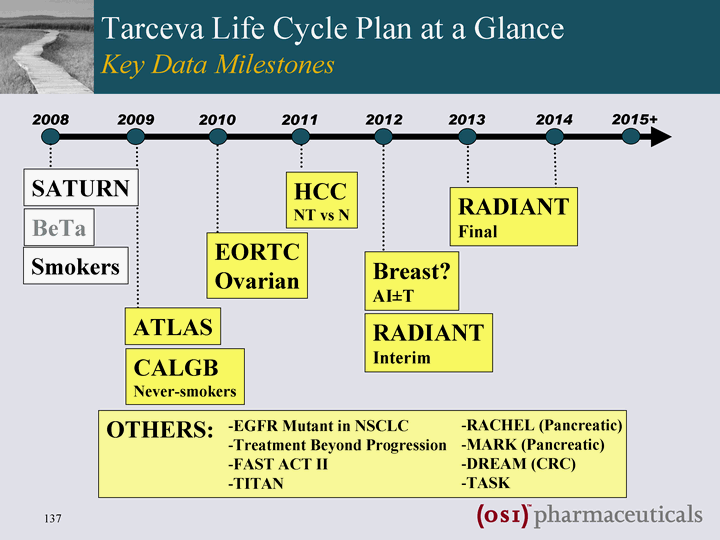
| Smokers Tarceva Life Cycle Plan Has Robust Potential Multiple Future Opportunities for Growth 2008 2014 2013 2012 2011 2010 2009 SATURN BeTa ATLAS CALGB Never-smokers EORTC Ovarian HCC NT vs N Breast? AI+-T RADIANT Interim RADIANT Final OTHERS: EGFR Mutant in NSCLC Treatment Beyond Progression FAST ACT II TITAN RACHEL (Pancreatic) MARK (Pancreatic) DREAM (CRC) TASK 2015+ |

| Assessing Risk & Opportunity Around Tarceva Competition, Market Dynamics & Life Cycle We Believe Tarceva is an Established, Entrenched Part of the Treatment Paradigm for NSCLC and Pancreatic Cancer and That It Should Continue to Grow in 2009 The Extent of Growth in 2009 Will be Impacted by Regulatory and Data Events Surrounding the Competition & Economic Environment A Robust Life Cycle Plan Continues to Hold Out Multiple Opportunities For Expansion of The Tarceva Franchise in the Medium and Longer Term SUMMARY |

| BUT: We Are Committed to Realizing Tarceva Value Building The Overall Business With Discipline 2008 2020 NPV (Note: Plots are conceptual to exemplify theme) Successful Execution of Strategic Business Plan Can Materially Grow the Value of the Overall Business Beyond the Anchoring Tarceva Franchise Continued Life Cycle Investments Mean That Annualized Tarceva NPV Continues to Build for Several Years Building a Successful & Sustainable Business Around Tarceva Requires a Disciplined Approach to Risk Management BUT, Should We Fall Short of This Goal As Tarceva Approaches Full Value We Are Committed to Monetizing Tarceva Value For Our Shareholders Critical Assessment Period |
| OSIP Exiting 2008: Strategic Execuition Delivering Future Shareholder Value From a Strong Base We Believe We Can Build OSIP into the Premier Mid-Cap Biotechnology Company Based Upon Anchoring Growth Around Tarceva Strong P&L and Balance Sheet Maintaining an effective balance between financial performance & reinvestment in R&D Delivering on the Significant Future Potential From a Differentiated and Innovative Pipeline and Establishing a Reputation for Scientific Excellence Four internally discovered, differentiated, high quality assets advanced to clinical development in last 18 months Leadership position in EMT (oncology) & Neuroendocrine control (diabetes/obesity) We are a profitable, $2-$3 billion market cap company with ~$380MM in annual revenues & generating approximately $150MM in cash flow, primarily from Tarceva & DP-IV royalties |
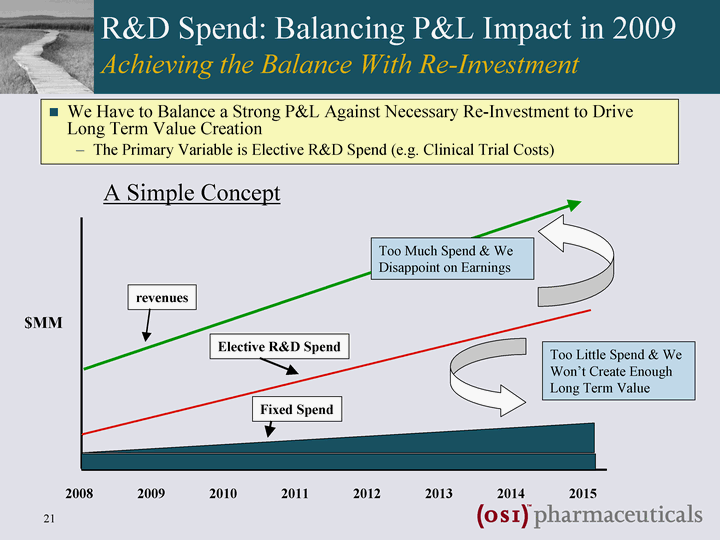
| R&D Spend: Balancing P&L Impact in 2009 Achieving the Balance With Re-Investment We Have to Balance a Strong P&L Against Necessary Re-Investment to Drive Long Term Value Creation The Primary Variable is Elective R&D Spend (e.g. Clinical Trial Costs) revenues Fixed Spend Elective R&D Spend 2008 2009 2010 2011 2012 2013 2014 2015 $MM Too Much Spend & We Disappoint on Earnings Too Little Spend & We Won't Create Enough Long Term Value A Simple Concept |
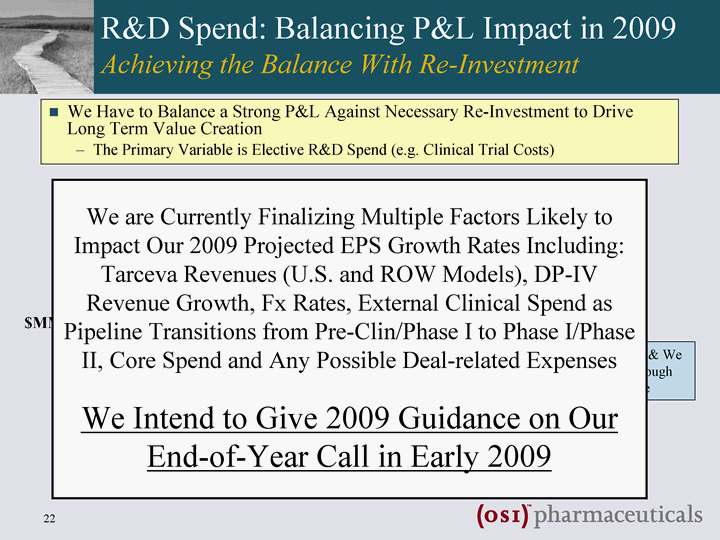
| R&D Spend: Balancing P&L Impact in 2009 Achieving the Balance With Re-Investment We Have to Balance a Strong P&L Against Necessary Re-Investment to Drive Long Term Value Creation The Primary Variable is Elective R&D Spend (e.g. Clinical Trial Costs) revenues Fixed Spend Elective R&D Spend 2008 2009 2010 2011 2012 2013 2014 2015 $MM Too Much Spend & We Disappoint on Earnings Too Little Spend & We Won't Create Enough Long Term Value It's Really a Simple Concept We are Currently Finalizing Multiple Factors Likely to Impact Our 2009 Projected EPS Growth Rates Including: Tarceva Revenues (U.S. and ROW Models), DP-IV Revenue Growth, Fx Rates, External Clinical Spend as Pipeline Transitions from Pre-Clin/Phase I to Phase I/Phase II, Core Spend and Any Possible Deal-related Expenses We Intend to Give 2009 Guidance on Our End-of-Year Call in Early 2009 |
| Assessing Financial Performance in 2009 Key Accounting Adjustments to the GAAP Baseline Two significant accounting changes for 2008/9 will affect how we should view the business High likelihood that 2008 Financial Statements will include a reversal of the tax valuation allowance on Deferred Tax Assets (DTA's) Accumulated NOL's ~ $1.1bn & DTA's ~$560MM 2008 potential one-time adjustment Future years' Tax Impact will show up on P&L Interest expense accounting changes for convertible bonds 2009 Financial Statements will include the impact of these two accounting changes, both of which are non-cash items Full Tax Provision at Statutory Tax Rates Higher Convertible Debt Interest Expense Reflecting the Pro-Forma impact of these accounting changes on 2008 and 2009 P&L's in your models may result in a better comparison of the performance of the underlying business |
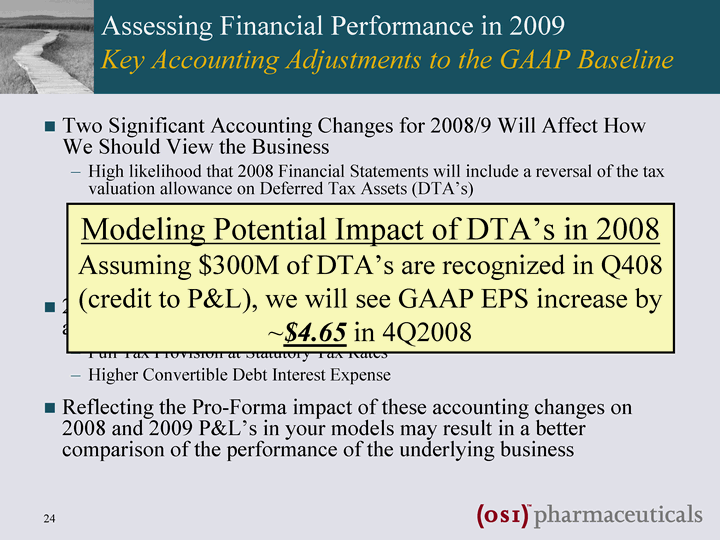
| Assessing Financial Performance in 2009 Key Accounting Adjustments to the GAAP Baseline Two Significant Accounting Changes for 2008/9 Will Affect How We Should View the Business High likelihood that 2008 Financial Statements will include a reversal of the tax valuation allowance on Deferred Tax Assets (DTA's) Accumulated NOL's ~ $1.1bn & DTA's ~$560MM 2008 Potential One-Time Adjustment Future Years' Tax Impact will show up on P&L Interest Expense Accounting Changes for Convertible Bonds 2009 Financial Statements will include the impact of these two accounting changes, both of which are non-cash items Full Tax Provision at Statutory Tax Rates Higher Convertible Debt Interest Expense Reflecting the Pro-Forma impact of these accounting changes on 2008 and 2009 P&L's in your models may result in a better comparison of the performance of the underlying business Modeling Potential Impact of DTA's in 2008 Assuming $300M of DTA's are recognized in Q408 (credit to P&L), we will see GAAP EPS increase by ~$4.65 in 4Q2008 |

| Pro-Forma Impact of DTA's in 2009 Assuming DTA's Are Recognized in 2008 2009 Income Statement will reflect full tax provision at statutory rates (~ 40%) Represents a significant change from 2007/2008, where tax expense was based on AMT rates of ~3% Sensitivity: For every $100M of pre-tax income, EPS will be ~$0.58 lower compared with 2008 calculations The Tax Provision in 2009 could create an apparent distortion in the comparative performance of the underlying business when compared to 2008 THIS IS A NON-CASH IMPACT: the actual Cash tax liability will remain at the low AMT rates until NOL's are consumed (we estimate this will not occur until after 2011) |
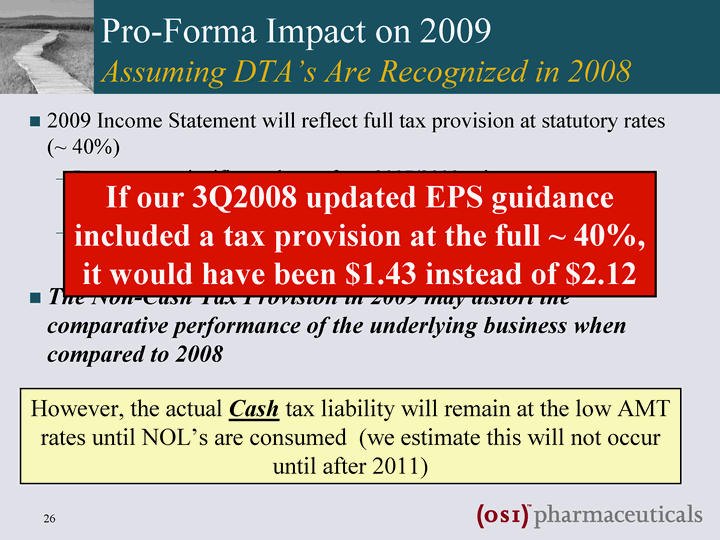
| Pro-Forma Impact on 2009 Assuming DTA's Are Recognized in 2008 2009 Income Statement will reflect full tax provision at statutory rates (~ 40%) Represents a significant change from 2007/2008, where tax expense was based on the significantly lower AMT rates of ~3% Sensitivity: For every $100M of pre-tax income, EPS impact is ~$0.57 compared with 2008 calculations The Non-Cash Tax Provision in 2009 may distort the comparative performance of the underlying business when compared to 2008 However, the actual Cash tax liability will remain at the low AMT rates until NOL's are consumed (we estimate this will not occur until after 2011) If our 3Q2008 updated EPS guidance included a tax provision at the full ~ 40%, it would have been $1.43 instead of $2.12 |
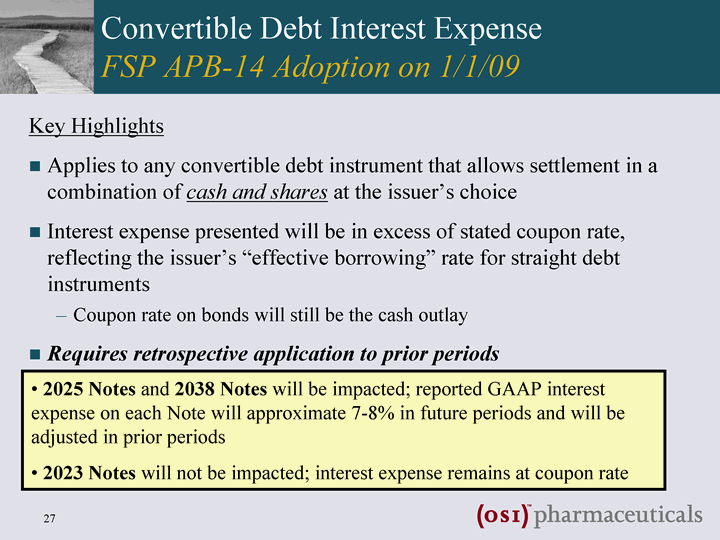
| Convertible Debt Interest Expense FSP APB-14 Adoption on 1/1/09 Key Highlights Applies to any convertible debt instrument that allows settlement in a combination of cash and shares at the issuer's choice Interest expense presented will be in excess of stated coupon rate, reflecting the issuer's "effective borrowing" rate for straight debt instruments Coupon rate on bonds will still be the cash outlay Requires retrospective application to prior periods 2025 Notes and 2038 Notes will be impacted; reported GAAP interest expense on each Note will approximate 7-8% in future periods and will be adjusted in prior periods 2023 Notes will not be impacted; interest expense remains at coupon rate |
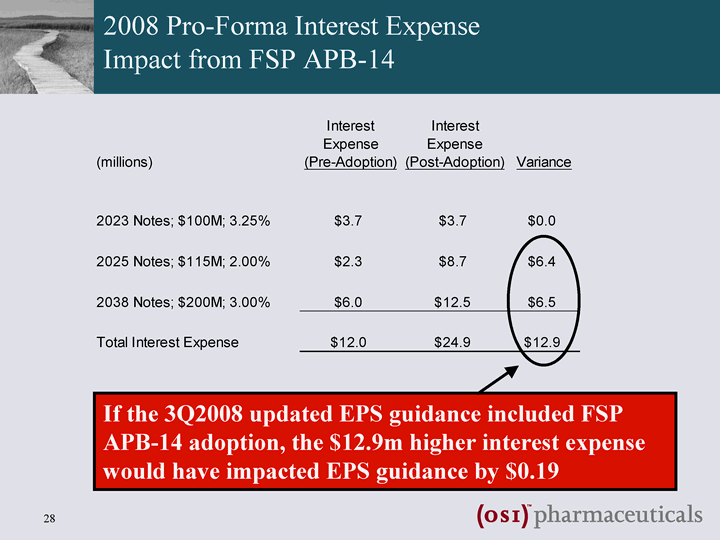
| 2008 Pro-Forma Interest Expense Impact from FSP APB-14 If the 3Q2008 updated EPS guidance included FSP APB-14 adoption, the $12.9m higher interest expense would have impacted EPS guidance by $0.19 |

| Assessing Financial Performance in 2009 Key Accounting Adjustments to the GAAP Baseline Modeling Potential Impact This Pro-Forma EPS estimate attempts to reflect the performance of underlying business in 2008 for comparative purposes with 2009 Note: Excludes Potential One-Time Reversal of DTA's |
| OSIP Exiting 2008: Strategic Execution Delivering Future Shareholder Value From a Strong Base We Believe We Can Build OSIP into the Premier Mid-Cap Biotechnology Company Based Upon: Anchoring Growth Around Tarceva Strong P&L and Balance Sheet Maintaining an effective balance between financial performance & reinvestment in R&D Delivering on the Significant Future Potential From a Differentiated and Innovative Pipeline and Establishing a Reputation for Scientific Excellence Four internally discovered, differentiated, high quality assets advanced to clinical development in last 18 months Leadership position in EMT (oncology) & Neuroendocrine control (diabetes/obesity) We are a profitable, $2-$3 billion market cap company with ~$380MM in annual revenues & generating approximately $150MM in cash flow, primarily from Tarceva & DP-IV royalties |
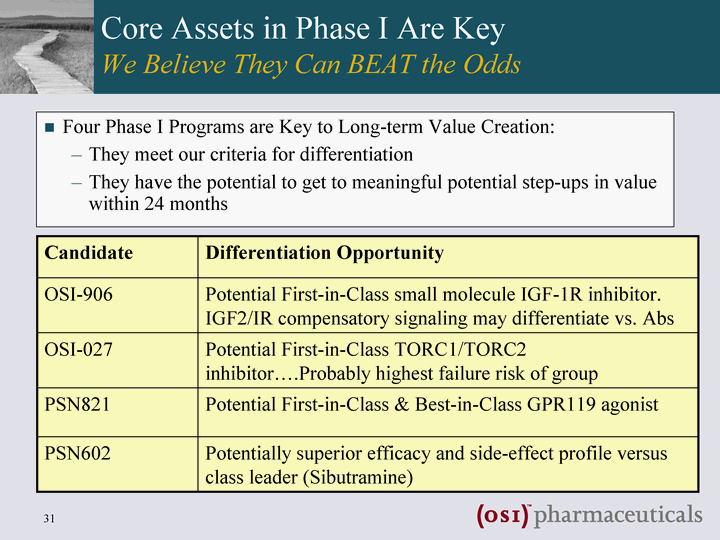
| Core Assets in Phase I Are Key We Believe They Can BEAT the Odds Four Phase I Programs are Key to Long-term Value Creation: They meet our criteria for differentiation They have the potential to get to meaningful potential step-ups in value within 24 months Candidate Differentiation Opportunity OSI-906 Potential First-in-Class small molecule IGF-1R inhibitor. IGF2/IR compensatory signaling may differentiate vs. Abs OSI-027 Potential First-in-Class TORC1/TORC2 inhibitor....Probably highest failure risk of group PSN821 Potential First-in-Class & Best-in-Class GPR119 agonist PSN602 Potentially superior efficacy and side-effect profile versus class leader (Sibutramine) |

| Core Assets in Phase I Are Key We Believe They Can BEAT the Odds? Four Phase I Programs are Key to Long-term Value Creation: They are Differentiated They have the Potential to Get to Meaningful Potential Step-Ups in Value Within 24 Months Candidate Differentiation Opportunity OSI-906 Potential First-in-Class small molecule IGF-1R inhibitor. IGF2/IR compensatory signaling may differentiate vs. Abs OSI-027 Potential First-in-Class TORC1/TORC2 inhibitor....Probably highest failure risk of group PSN821 Potential First-in-Class & Best-in-Class GPR119 agonist PSN602 Potentially superior efficacy and side-effect profile versus class leader (Sibutramine) OSI discovered and advanced all four of these potential "First/Best in Class" product candidates into clinical development within the last 18 months |
| Focusing Resources to Enable a Leadership Role The EMT Platform in Oncology Oncology has become extremely competitive Pfizer, Lilly & Novartis alone are collectively spending billions of dollars annually on Oncology R&D None-the-less, changing treatment paradigms and reducing personalized medicine to practice has proven to be a significant challenge throughout the oncology community OSI's EMT platform has allowed us to focus resources into an important emerging scientific area to establish a leadership position in terms of IP and know-how Our EMT platform facilitates patient enrichment approaches and logically links to related science allowing complementary patient selection opportunities Compensatory signaling Ligand/receptor driven selection around key targets Knowing "Who to Treat and With What Combination" AND "Who Not to Treat" |

| Focusing Resources to Enable a Leadership Role The EMT Platform in Oncology Oncology has become extremely competitive Pfizer, Lilly & Novartis alone are collectively spending billions of dollars annually on Oncology R&D None-the-less, changing treatment paradigms and reducing personalized medicine to practice has proven to be a significant challenge throughout the oncology community OSI's EMT platform has allowed us to focus resources into an important emerging scientific area to establish a leadership position in terms of IP and know-how Our EMT platform facilitates patient enrichment approaches and logically links to related science allowing complementary patient selection opportunities Compensatory signaling Ligand/receptor driven selection around key targets Knowing "Who to Treat and With What Combination" AND "Who Not to Treat" Our Goal is to Repeat the Same Approach in Diabetes/Obesity Where We Believe Our Emerging Neuroendocrine Control Platform Can be Used to Pioneer Personalized Medicine Approaches in Diabetes and Obesity |

| OSI-906: Driving Differentiation With Science Clinical Correlates of Disease to EMT-Status Tarceva & '906 in NSCLC HR=0.37 p=0.0028 Weeks Pre-Clinical Data Suggests Synergistic inhibition of E-Like Tumors With a Combination of Tarceva & OSI-906 Combination Study Planned in NSCLC Collaboration Agreement Signed This Week w/Dako to Develop E- Cadherin/Vimentin Biomarker kit to Support Clinical/Commercial Program E-Cadherin in TRIBUTE A Clue to an Early Marker Tool for EMT |
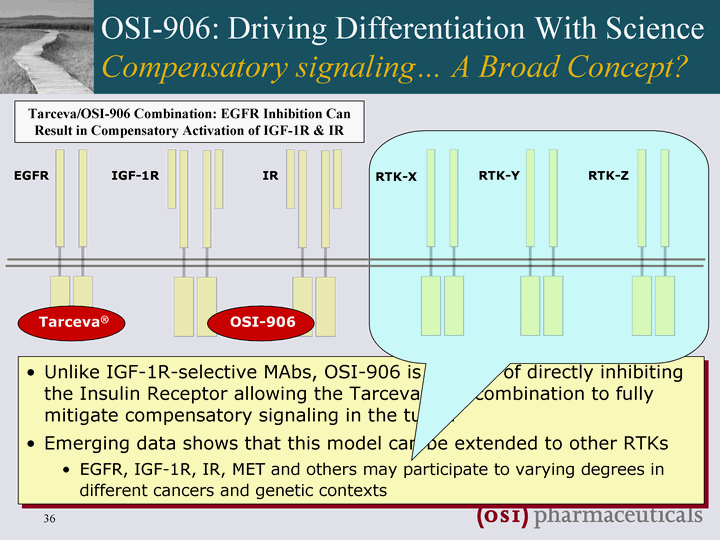
| Unlike IGF-1R-selective MAbs, OSI-906 is capable of directly inhibiting the Insulin Receptor allowing the Tarceva/'906 combination to fully mitigate compensatory signaling in the tumor Emerging data shows that this model can be extended to other RTKs EGFR, IGF-1R, IR, MET and others may participate to varying degrees in different cancers and genetic contexts EGFR IGF-1R IR RTK-X RTK-Y RTK-Z Tarceva(r) OSI-906 OSI-906: Driving Differentiation With Science Compensatory signaling... A Broad Concept? Tarceva/OSI-906 Combination: EGFR Inhibition Can Result in Compensatory Activation of IGF-1R & IR |

| GPR119 receptor expressed in gut & pancreas GPR119 agonism leads to increased b- cell cAMP and release of GLP-1, leading (pre-clinically) to: Improved glucose homeostasis Delayed gastric emptying Appetite suppression Competition: Arena (J&J) Metabolex PSN821: Driving Differentiation With Science Leading GPR119 Agonist Developed From Cadus Acquisition PSN821: GPR119 agonist for treatment of type II diabetes PSN821 Entered Clinical Development on 31st August 2008 |
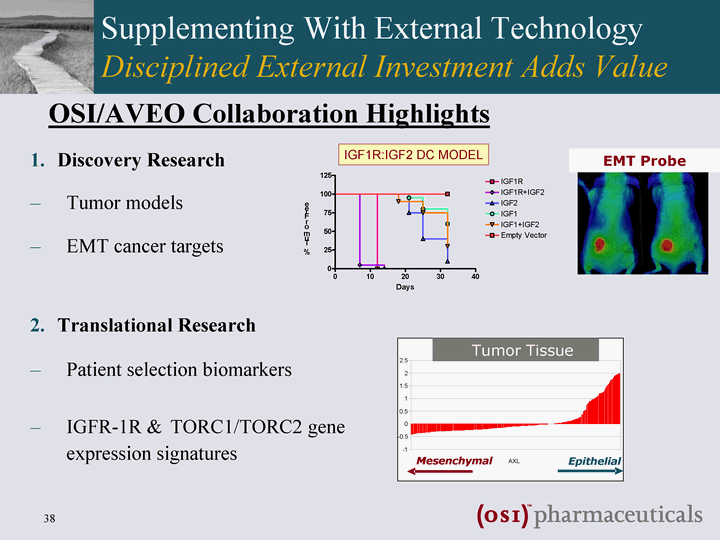
| Supplementing With External Technology Disciplined External Investment Adds Value Discovery Research Tumor models EMT cancer targets Translational Research Patient selection biomarkers IGFR-1R & TORC1/TORC2 gene expression signatures Mesenchymal Epithelial AVEO BH ARCHIVE AXL EMT INDEX - -1 - -0.5 0 0.5 1 1.5 2 2.5 Mesenchymal Epithelial Tumor Tissue AXL EMT Probe OSI/AVEO Collaboration Highlights |
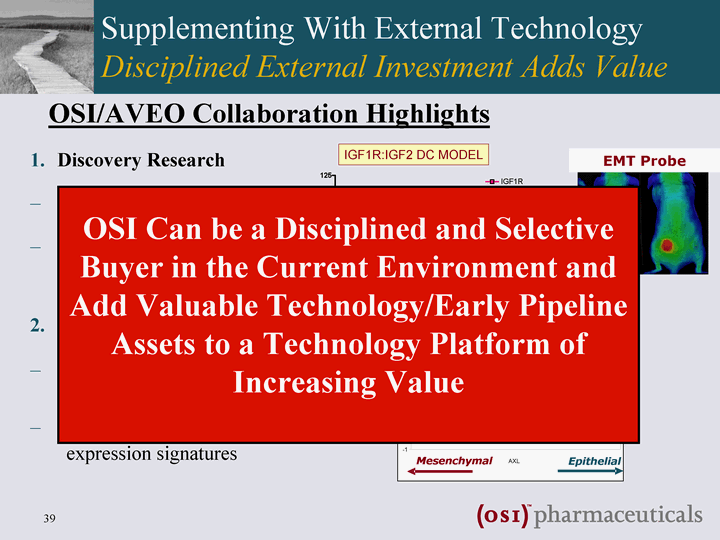
| Supplementing With External Technology Disciplined External Investment Adds Value Discovery Research Tumor models EMT cancer targets Translational Research Patient selection biomarkers IGFR-1R & TORC1/TORC2 gene expression signatures Mesenchymal Epithelial AVEO BH ARCHIVE AXL EMT INDEX - -1 - -0.5 0 0.5 1 1.5 2 2.5 Mesenchymal Epithelial Tumor Tissue AXL EMT Probe OSI/AVEO Collaboration Highlights OSI Can be a Disciplined and Selective Buyer in the Current Environment and Add Valuable Technology/Early Pipeline Assets to a Technology Platform of Increasing Value |
| OSIP Exiting 2008: Summary Strategically Positioned in Challenging Times A profitable, $2-$3 billion market cap, company with ~$380MM in annual revenues & generating approximately $150MM in cash flow, primarily from Tarceva revenues & DP-IV royalties Tarceva has annualized global sales that have exceeded the $1bn/yr run rate and is firmly established in the treatment of lung and pancreatic cancer patients SATURN should drive further growth if successfully approved Additional near (ATLAS), medium (ovarian, never smokers) and long-term (RADIANT, HCC) major clinical studies can further expand utility Emerging pipeline of OSI-discovered, wholly owned & differentiated core development assets with clear paths to create "step-ups" in value PSN821 & PSN602 in Diabetes/Obesity OSI-906 & OSI-027 in Oncology Corporate strategy that seeks to build shareholder value by appropriately balancing financial performance with re-investment in the core Oncology & Diabetes/Obesity asset base via: Continued execution on Tarceva program Highly disciplined R&D investments focused on an internal pipeline of innovative and differentiated core assets and technology platforms and alignment around a commitment to turn the promise of innovative personalized medicine into practice |

| Q&A |

| Overview of Diabetes/Obesity Strategy & Programs Anker Lundemose, M.D., Ph.D. President, OSI Prosidion |

| Challenges Facing Diabetes & Obesity What Are the Opportunities The Challenges Cheap, modestly effective drugs available for diabetes FDA has raised the regulatory hurdle with focus on cardiovascular safety studies General IP/generic and pricing pressure on the industry The Opportunities Diabetes & obesity pandemic- The market opportunity is as large as ever & continues to grow Huge unmet need for therapeutics with effect on both diabetes & weight loss and better & safer obesity drugs Drugs with dramatically improved efficacy/safety are likely to command higher prices |

| Addressing the Challenges Facing Diabetes & Obesity Capturing the Opportunities PSN821 Diabetes/Obesity Potential "Best-in-Class" GPR119 agonist. Increases GLP-1 & Improves glucose homeostasis, delays gastric emptying & induces weight loss - In Phase I PSN602 Obesity Potential "Best-in-class" Serotonin/noradrenaline reuptake inhibitor w/5HT1a agonism - In Phase I PSN010 Diabetes Potential "Best-in-Class" Glucokinase Activator. Increases insulin secretion and glucose uptake in the liver. Licensed to Eli Lilly in 2007 Clinical Assets Address true unmet need and the growing market opportunity Potential to add significant value to OSI's pipeline in coming years It is OSI's strategy to retain control of development of our own assets For a costly traditional development path we have an option to partner programs prior to entering Phase III Increased focus on the ability to change the paradigm Pioneering personalized medicine for Diabetes & Obesity Selecting subgroups of obesity patients who are high responders to therapy |
| Targeted & Personalized Medicines for Diabetes & Obesity The Right Medicine to the Right Patient Biomarker Strategy is the driver of Selection of patients with high response potential Generation of selection IP and diagnostic platforms Personalized Medicine is the driver of Better & more predictable efficacy and potentially better safety Better compliance and better outcome Better pricing and reimbursement potential Deployment of specialized & smaller sales forces OSI Pharmaceuticals will seek to develop a Biomarker/Personalized Medicine strategy Identification of patients who are high responders to treatment Identification of phenotypes and/or biomarkers of such responders Use of biomarkers in future clinical trials and as part of the regulatory strategy Creation of IP around patient selection, biomarkers and corresponding diagnostics |

| R&D Focus Area - "Neuroendocrine Control of Body Weight and Glycaemia" Target Profiles Focus On Both Diabetes & Weight Loss And Better & Safer Obesity Drugs Phase II Identification of High Responders Why? Phase II Identification of High Responders Why? Translational Research Biomarkers BODY WEIGHT OBESITY Better efficacy and/or fewer side effects GLYCAEMIA TYPE 2 DIABETES Glucose lowering plus meaningful weight loss PSN821 PSN602 DEVELOPMENT Expertise in pharmacodynamic measures of appetite / food intake / gastric emptying Phase I Phase I NEURO ENDOCRINE Biomarker Exploration Biomarker Exploration |

| R&D Focus Area - "Neuroendocrine Control of Body Weight and Glycaemia" Target Profiles Focus On Both Diabetes & Weight Loss And Better & Safer Obesity Drugs Phase II Identification of High Responders Why? Phase II Identification of High Responders Why? Translational Research Biomarkers BODY WEIGHT OBESITY Better efficacy and/or fewer side effects GLYCAEMIA TYPE 2 DIABETES Glucose lowering plus meaningful weight loss PSN821 PSN602 DEVELOPMENT Expertise in pharmacodynamic measures of appetite / food intake / gastric emptying Phase I Phase I NEURO ENDOCRINE In vivo pharmacology expertise Rodent models of food intake / obesity / glucose control DISCOVERY Discovery Neuroendocrine Programs |
| R&D Focus Area - "Neuroendocrine Control of Body Weight and Glycaemia" Target Profiles Focus On Both Diabetes & Weight Loss And Better & Safer Obesity Drugs Phase II Identification of High Responders Why? Phase II Identification of High Responders Why? Translational Research Biomarkers BODY WEIGHT OBESITY Better efficacy and/or fewer side effects GLYCAEMIA TYPE 2 DIABETES Glucose lowering plus meaningful weight loss PSN821 PSN602 DEVELOPMENT Expertise in pharmacodynamic measures of appetite / food intake / gastric emptying Phase I Phase I NEURO ENDOCRINE In vivo pharmacology expertise Rodent models of food intake / obesity / glucose control DISCOVERY Discovery Neuroendocrine Programs Neuroendocrine Control of Body Weight and Glycaemia offers: Focus in Key Area of Interest & Current Expertise An Area from Which We Believe We can Deliver 1st and/or Best in Class/Differentiated Therapeutics |
| R&D Focus Area - "Neuroendocrine Control of Body Weight and Glycaemia" Target Profiles Focus On Both Diabetes & Weight Loss And Better & Safer Obesity Drugs Phase II Identification of High Responders Why? Phase II Identification of High Responders Why? Translational Research Biomarkers BODY WEIGHT OBESITY Better efficacy and/or fewer side effects GLYCAEMIA TYPE 2 DIABETES Glucose lowering plus meaningful weight loss PSN821 PSN602 DEVELOPMENT Expertise in pharmacodynamic measures of appetite / food intake / gastric emptying Phase I Phase I NEURO ENDOCRINE In vivo pharmacology expertise Rodent models of food intake / obesity / glucose control DISCOVERY Discovery Neuroendocrine Programs Neuroendocrine Control of Body Weight and Glycaemia potentially offers the: Ability to Change the Paradigm The Possibility of building Translational Medicine/Biomarkers leading to potential Personalized Medicine for our programs |

| Update on PSN821 and PSN602 Jonathan Rachman, M.D., Ph.D. Senior Vice President, OSI Prosidion |

| PSN821 |

| GPR119 receptor expressed in gut & pancreas GPR119 agonism leads to increased b- cell cAMP and release of GLP-1, leading (pre-clinically) to: Improved glucose homeostasis Delayed gastric emptying Appetite suppression Competition: Arena (J&J) Metabolex Diabetes and Obesity Clinical Pipeline - PSN821 PSN821: GPR119 agonist for treatment of type II diabetes PSN821 entered Clinical Development on 31st August 2008 |
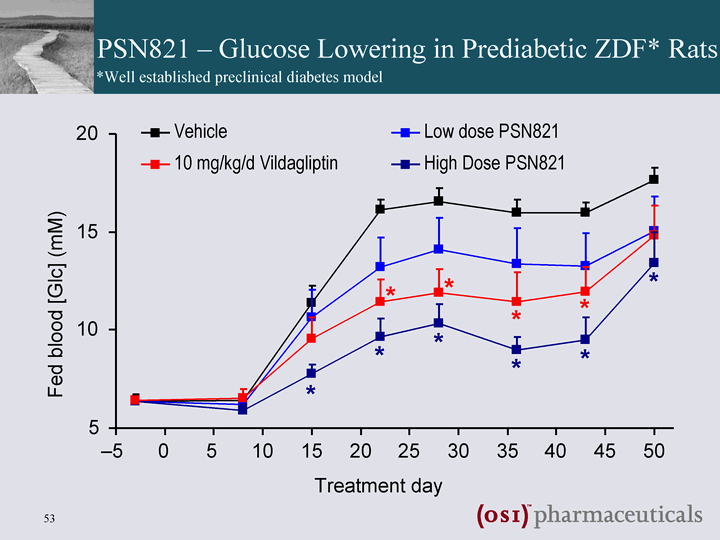
| PSN821 - Glucose Lowering in Prediabetic ZDF* Rats 5 10 15 20 - -5 0 5 10 15 20 25 30 35 40 45 50 Treatment day Fed blood [Glc] (mM) Vehicle Low dose PSN821 High Dose PSN821 10 mg/kg/d Vildagliptin * * * * * * * * * * *Well established preclinical diabetes model |
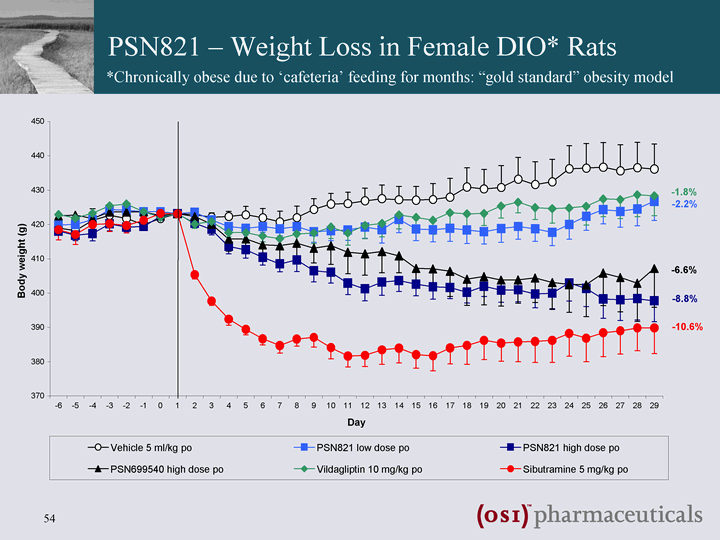
| 370 380 390 400 410 420 430 440 450 - -6 - -5 - -4 - -3 - -2 - -1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Day Body weight (g) Vehicle 5 ml/kg po PSN821 low dose po PSN821 high dose po PSN699540 high dose po Vildagliptin 10 mg/kg po Sibutramine 5 mg/kg po - -2.2% - -1.8% - -10.6% - -6.6% - -8.8% PSN821 - Weight Loss in Female DIO* Rats *Chronically obese due to 'cafeteria' feeding for months: "gold standard" obesity model |

| Indication Monotherapy in type 2 diabetics whose hyperglycemia can't be controlled by diet, weight reduction and exercise In combination with metformin or a TZD or a DPP-IV inhibitor in type 2 diabetics whose hyperglycemia can't be controlled satisfactorily by the single agent alone Dosing Once or twice daily oral dosing Efficacy Glucose lowering at least as good as DPP-IV inhibitors Efficacy Weight loss at least as good as GLP-1 analogues Tolerability At least as good as GLP-1 analogues Differentiation Oral therapy with (at least) equivalent glucose lowering to DPP-IV inhibitors, and (at least) equivalent weight loss to GLP-1 analogues PSN821 - Target Product Profile |

| May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan 2008 2010 Approval PSN821-101 (Phase Ia) PSN821-102 (Phase Ib) 2009 Part 1 SAD (6 doses) (lean males) PD measures: nutrient challenge ? glucose & insulin P3 (overweight/obese males) weight effect (PK) Part 1 ext SAD (3 doses) (lean males) Part 2 MAD (14 days; 3 doses) (overweight/obese males) Part 3 MAD (14 days; 2 doses) (type 2 diabetics) PD measures: fasting glucose meal GTT gastric emptying test meal intake appetite VAS Part 1 Single Dose 3-Way Crossover (overweight/obese males) food effect (PK) gastric emptying PSN821-103 Bioequivalence suspension vs. capsule (PK) PSN821 - GPR119 Agonist for Treatment of Type 2 Diabetes Initial indication of glucose lowering efficacy in patients Effects in patients on: fasting and pp glucose appetite and food intake gastric emptying Note: estimated timeline |

| PSN602 |

| Diabetes and Obesity Clinical Pipeline - PSN602 Sibutramine - Norepinephrine & serotonin reuptake inhibitor ^ appetite reduction and weight loss Sibutramine uptake and efficacy limited by association with increases in HR and BP (increased norepinephrine activity) 5-HT1A agonism included to potentially counter-balance undesirable CV effects ? Improved CV safety profile ? Superior efficacy PSN602: Norepinephrine & serotonin reuptake inhibitor & 5-HT1A agonist for the treatment of obesity PSN602 entered Clinical Development on 17th June 2008 |
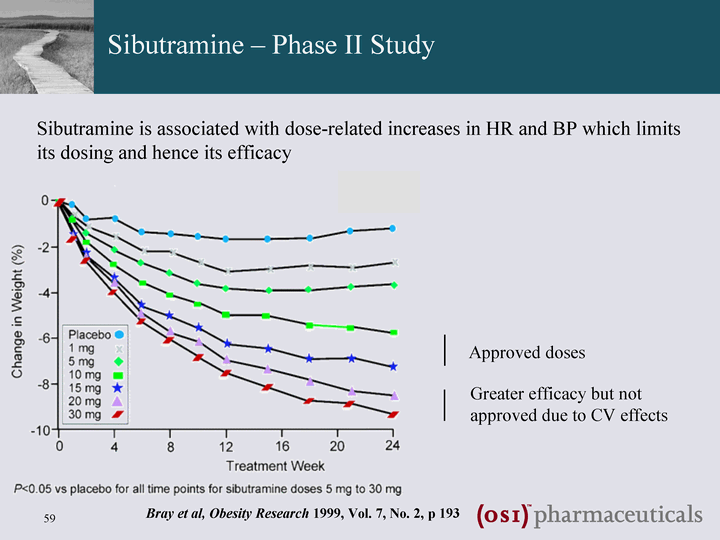
| Sibutramine - Phase II Study Approved doses Greater efficacy but not approved due to CV effects Bray et al, Obesity Research 1999, Vol. 7, No. 2, p 193 Sibutramine is associated with dose-related increases in HR and BP which limits its dosing and hence its efficacy |
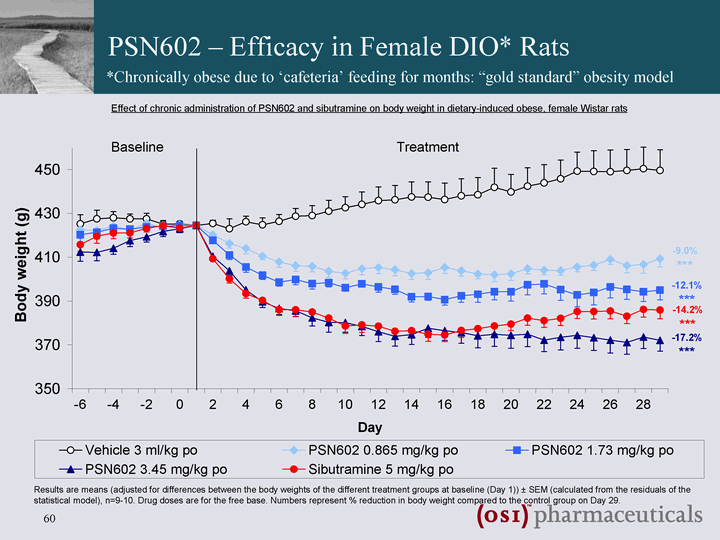
| PSN602 - Efficacy in Female DIO* Rats *Chronically obese due to 'cafeteria' feeding for months: "gold standard" obesity model |

| At up to 3xED50 24h in acute feeding - -2 0 2 4 6 8 10 12 6.9 mg/kg 13.8 mg/kg PSN602 po 20.7 mg/kg Sibutramine 15 mg/kg po Mean arterial blood pressure (difference from vehicle) *** BP - -20 - -10 0 10 20 30 40 50 60 70 6.9 mg/kg 13.8 mg/kg 20.7 mg/kg Sibutramine 15 mg/kg po Heart rate (difference from vehicle) * *** PSN602 po HR Mean arterial BP (mmHg) & HR (bpm) (mean 2 to 8 hours after dosing, D from vehicle) PSN602 - Sibutramine CV Effects Not Seen in Rat Telemetry |

| PSN602 - Target Product Profile Indication Adjunct to dietary intervention and exercise for the treatment of obese patients (BMI ^ 30 kg/m2) with associated risk factors such as type 2 diabetes or dyslipidemia Dosing Once daily oral dosing Efficacy Weight loss superior to sibutramine with attendant improvements in metabolic parameters (lipids, BP, glucose control in type 2 diabetes) Safety Similar to sibutramine but without increases in BP or HR or requirement for regular monitoring of BP and HR Differentiation Superior efficacy to sibutramine without the adverse CV profile |
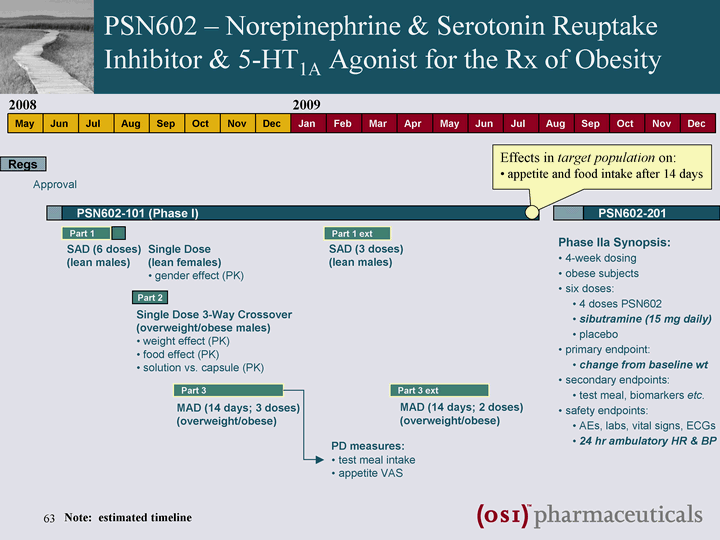
| May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2008 2009 Regs Approval PSN602-101 (Phase I) Phase IIa Synopsis: 4-week dosing obese subjects six doses: 4 doses PSN602 sibutramine (15 mg daily) placebo primary endpoint: change from baseline wt secondary endpoints: test meal, biomarkers etc. safety endpoints: AEs, labs, vital signs, ECGs 24 hr ambulatory HR & BP Part 1 SAD (6 doses) (lean males) Single Dose (lean females) gender effect (PK) Part 2 Single Dose 3-Way Crossover (overweight/obese males) weight effect (PK) food effect (PK) solution vs. capsule (PK) Part 3 MAD (14 days; 3 doses) (overweight/obese) PD measures: test meal intake appetite VAS Part 1 ext SAD (3 doses) (lean males) Part 3 ext MAD (14 days; 2 doses) (overweight/obese) PSN602-201 Effects in target population on: appetite and food intake after 14 days PSN602 - Norepinephrine & Serotonin Reuptake Inhibitor & 5-HT1A Agonist for the Rx of Obesity Note: estimated timeline |
| PSN821 and PSN602 - Summary Clear differentiation thesis based on compelling preclinical data Successful progress through preclinical safety testing and initial CMC development Successful progress through single ascending doses (both programs) and multiple ascending doses (PSN602) in man Both compounds well tolerated ? escalating dosing to establish MTD 4-week dosing studies will include active comparators (PSN602 - sibutramine; PSN821 - sitagliptin) - potentially provide clear demonstration of step-up in value Expected data in 2009: PSN821 - glucose responses to nutrient challenge after SD in diabetic patients - changes in glucose, test meal intake & GE after 14d in diabetic patients PSN602 - changes in appetite & test meal intake after 14d in obese patients |

| Diabetes/Obesity Q&A |

| Epithelial-Mesenchymal Transition Platform David Epstein, Ph.D. Senior Vice President, Oncology |

| Outline Discovery & Translational Research Summary EMT Platform Update Proprietary technology for Discovery and Translational Research New development compound OSI-296 a Proprietary EMT-Targeted Agent OSI-AVEO Collaboration Update Translational Research Highlights |
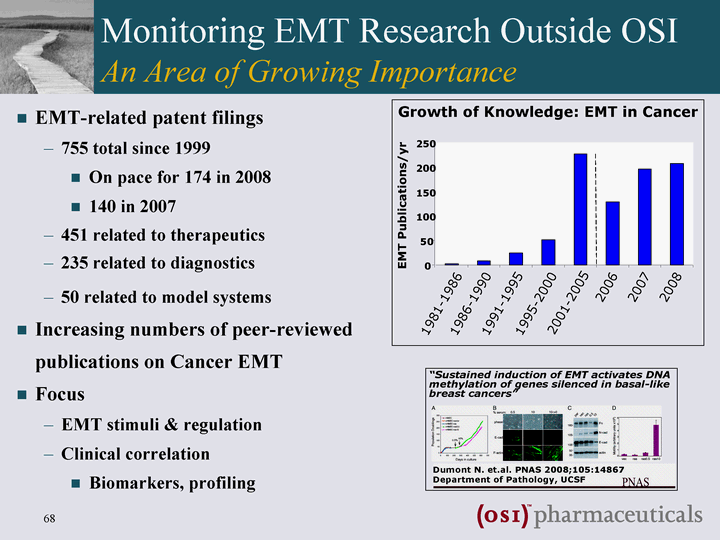
| Monitoring EMT Research Outside OSI An Area of Growing Importance EMT-related patent filings 755 total since 1999 On pace for 174 in 2008 140 in 2007 451 related to therapeutics 235 related to diagnostics 50 related to model systems Increasing numbers of peer-reviewed publications on Cancer EMT Focus EMT stimuli & regulation Clinical correlation Biomarkers, profiling |
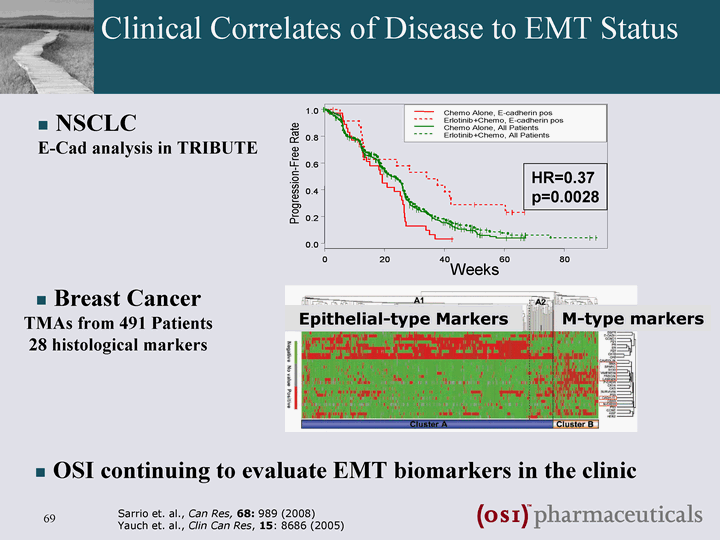
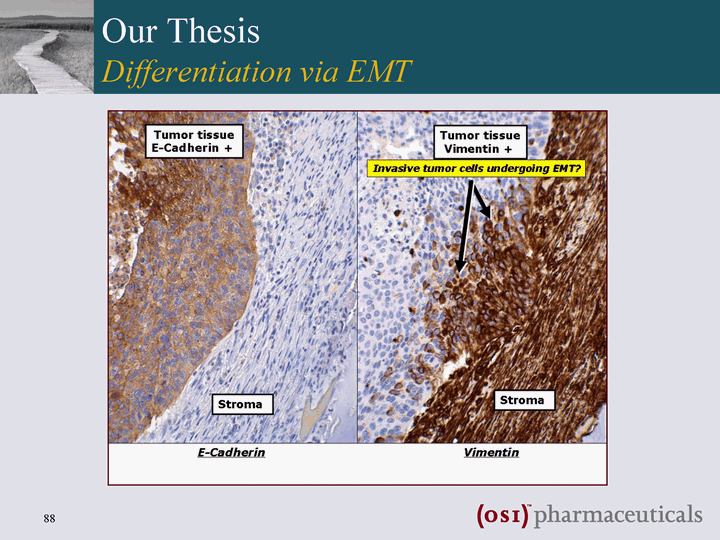
| Clinical Correlates of Disease to EMT Status Sarrio et. al., Can Res, 68: 989 (2008) Yauch et. al., Clin Can Res, 15: 8686 (2005) Epithelial-type Markers M-type markers NSCLC E-Cad analysis in TRIBUTE Breast Cancer TMAs from 491 Patients 28 histological markers HR=0.37 p=0.0028 Weeks OSI continuing to evaluate EMT biomarkers in the clinic |
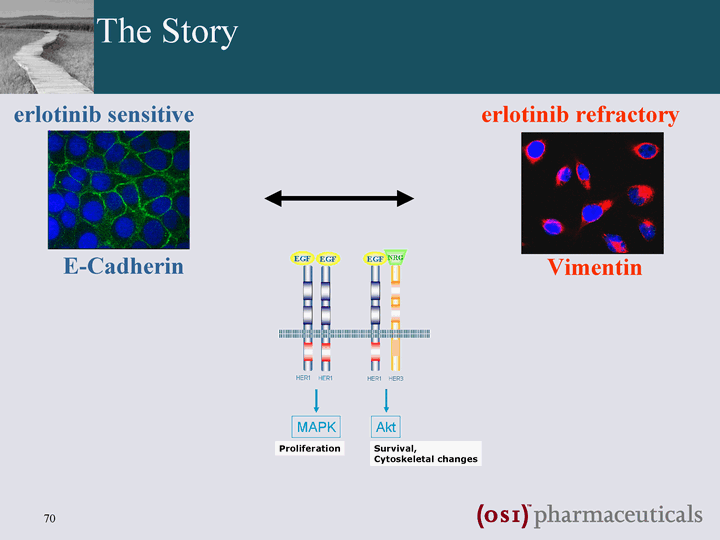
| The Story E-Cadherin erlotinib sensitive Vimentin erlotinib refractory |

| OSI's MTT portfolio targets tumor cells with an "Epithelial" profile Gene Transcription AKT MAPK EGFR mut/wt Her1:Her1 Her1:Her3 IGF-1R PI3K Ras a-Cat b-Cat g-Cat a-Cat g-Cat b-Cat E-cadherin a-Cat b-Cat g-Cat a-Cat g-Cat b-Cat PDK-1 m-TOR OSI-906 EMT-Status Influences Sensitivity of TKIs OSI-296 Erlotinib OSI-027 EMT-Kinase |
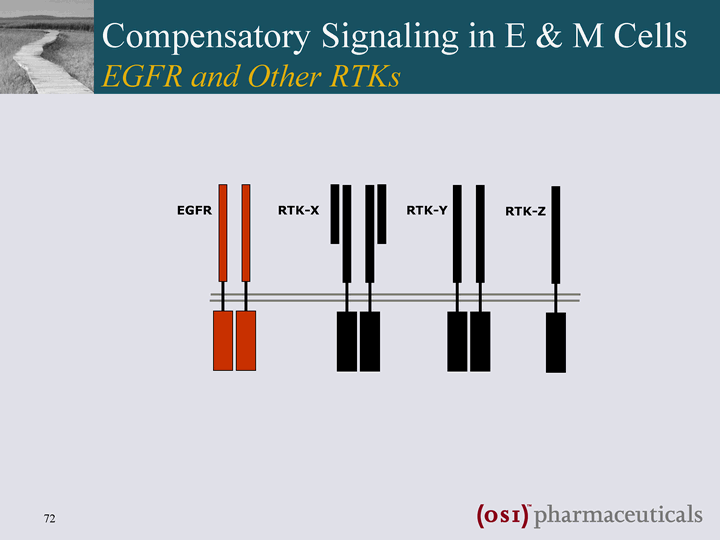
| EGFR RTK-X RTK-Y RTK-Z Compensatory Signaling in E & M Cells EGFR and Other RTKs |
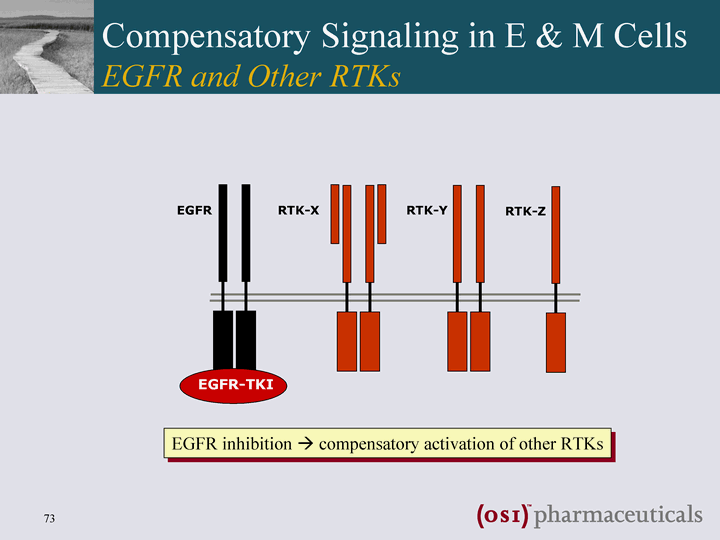
| EGFR RTK-X RTK-Y RTK-Z EGFR-TKI EGFR inhibition ? compensatory activation of other RTKs Compensatory Signaling in E & M Cells EGFR and Other RTKs |

| (EGFR) Her1:Her1 AKT MAPK IGF1R PI3K Ras ZEB1, Snail, Twist Integrins Cox2 PDGFR TGFbR Gene transcription ILK b-Cat MMPs PDK-1 m-TOR Src OSI-027 EMT-Status Influences Sensitivity of TKIs We believe there is a strategic need to be able to study and target the "Mesenchymal" tumor cell Mesenchymal-like tumor cells Share stem cell properties Resistant to SOC therapy Associated with advanced disease Sensitive to TORC1/TORC2 inhibition (OSI-027) |
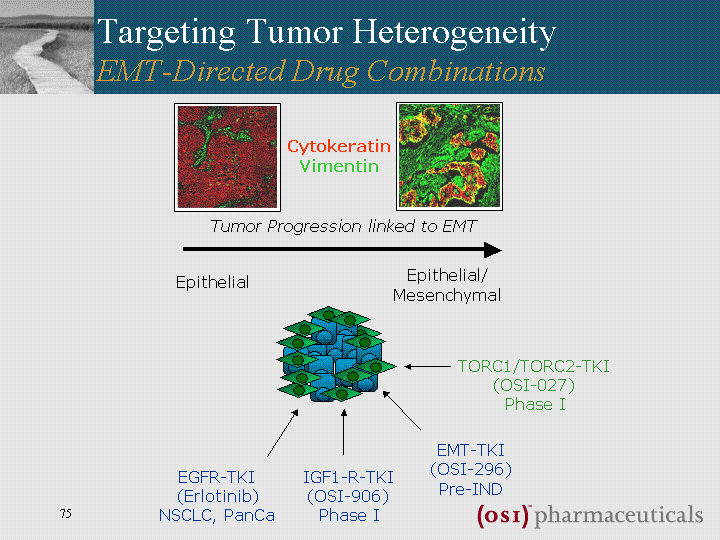
| EGFR-TKI (Erlotinib) NSCLC, PanCa IGF1-R-TKI (OSI-906) Phase I TORC1/TORC2-TKI (OSI-027) Phase I Epithelial Epithelial/ Mesenchymal Tumor Progression linked to EMT Cytokeratin Vimentin Targeting Tumor Heterogeneity EMT-Directed Drug Combinations cMET-TKI (OSI-296) Pre-IND |

| EMT Discovery & Translational Research Vision of a Successful Initiative Proprietary EMT Programs Emanating from OSI & AVEO- Collaboration OSI EMT Hypotheses Vetted (e.g., OSI-906 & OSI-296) The EMT Platform Know-How Deployed in all Programs (e.g., OSI-296) EMT & Tumor Growth Blocked in Tumor Models EMT & Pathway Signatures Deployed in Clinical Programs Biomarkers from OSI & AVEO Vetted by OSI-906 and OSI-027 Translational Research |
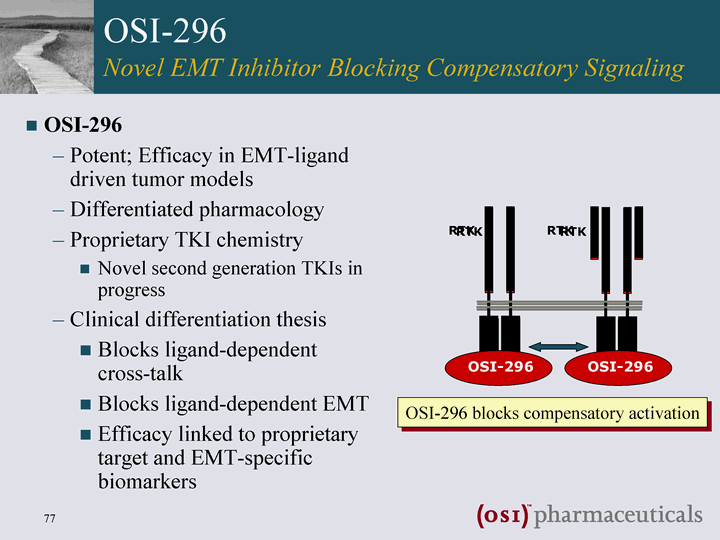
| OSI-296 Novel EMT Inhibitor Blocking Compensatory Signaling OSI-296 Potent; Efficacy in EMT-ligand driven tumor models Differentiated pharmacology Proprietary TKI chemistry Novel second generation TKIs in progress Clinical differentiation thesis Blocks ligand-dependent cross-talk Blocks ligand-dependent EMT Efficacy linked to proprietary target and EMT-specific biomarkers RTK RTK OSI-296 blocks compensatory activation OSI-296 |
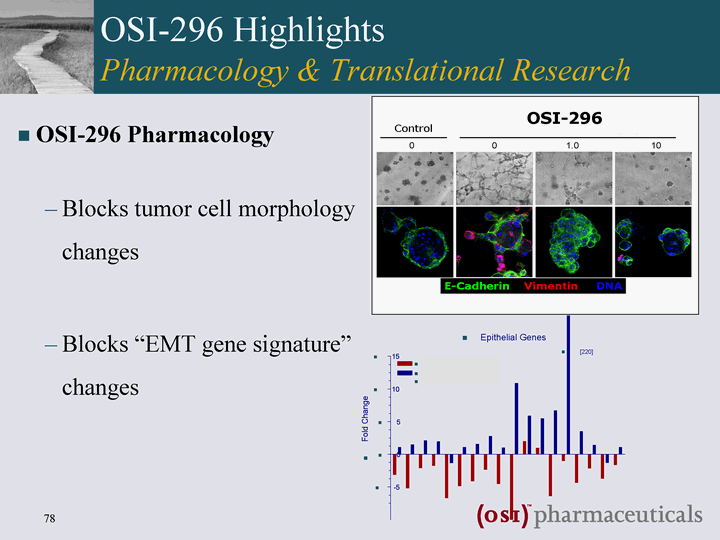
| OSI-296 Pharmacology Blocks tumor cell morphology changes Blocks "EMT gene signature" changes OSI-296 Highlights Pharmacology & Translational Research OSI-296 |

| OSI-AVEO Collaboration Highlights Discovery Research Tumor models EMT cancer targets Translational Research Patient selection biomarkers IGFR-1R & TORC1/TORC2 gene expression signatures Mesenchymal Epithelial AVEO BH ARCHIVE AXL EMT INDEX - -1 - -0.5 0 0.5 1 1.5 2 2.5 Mesenchymal Epithelial Tumor Tissue AXL EMT Probe |

| OSI-AVEO Collaboration Highlights Discovery Research Tumor models EMT cancer targets Translational Research Patient selection biomarkers IGFR-1R & TORC1/TORC2 gene expression signatures Mesenchymal Epithelial AVEO BH ARCHIVE AXL EMT INDEX - -1 - -0.5 0 0.5 1 1.5 2 2.5 Mesenchymal Epithelial Tumor Tissue AXL EMT Probe Evaluating option to in-license 6 targets in 2008 Provides exclusive IP rights on targets for non-MAbs OSI owns exclusively all small molecule IP OSI owns exclusively all IP emanating from 3 optioned tumor models in 2008 |
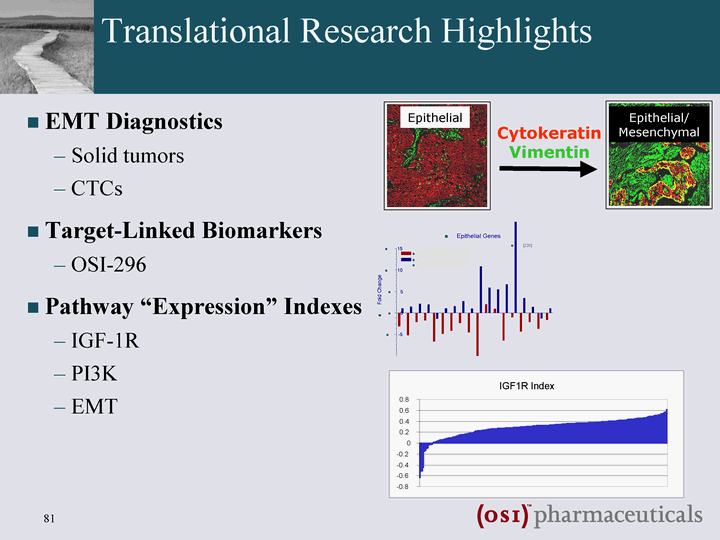
| Translational Research Highlights EMT Diagnostics Solid tumors CTCs Target-Linked Biomarkers OSI-296 Pathway "Expression" Indexes IGF-1R PI3K EMT Cytokeratin Vimentin Epithelial Epithelial/ Mesenchymal |
| Translational Research Highlights EMT Diagnostics Solid tumors CTCs Target-Linked Biomarkers OSI-296 Pathway "Expression" Indexes IGF-1R PI3K EMT Cytokeratin Vimentin Epithelial Epithelial/ Mesenchymal OSI Exclusive Technology & Proprietary IP Compounds Pharmacodynamic biomarkers Patient-Selection biomarkers linked to target and compounds EMT & Target-linked Biomarkers Gene and Pathway Signatures Developed solely at OSI Developed through OSI-AVEO agreement (for non-MAbs) |
| Summary The EMT R & D Initiative at OSI EMT-status of tumors is a determinant of therapeutic response Erlotinib preclinical and clinical data OSI-906, OSI-027 & OSI-296 preclinical data E and M-tumor cells utilize alternate signaling pathways Blockade of signaling in E and M-tumor cells ? compensatory activation of RTKs as an outcome of receptor cross-talk Rationale for blocking next generation resistance mechanisms EGFR ? activation of IGF-1R and cMET IGF-1R ? p-EGFR and p-IR cMET ? p-XR |

| Translational Research Mark Miglarese, Ph.D. Senior Director, Translational Research, Oncology Bench Bedside |

| Our Vision and Key Themes Compensatory signaling and TKI pharmacology in the context of EMT Pathway- and ligand-based predictive biomarkers to provide maximum benefit to our patients Translational Research as a driver of our near-term clinical plans for OSI-906 Combination with Tarceva(r) for NSCLC Combination with paclitaxel for OvCa Single-agent OSI-906 for ACC Translational Research Outline |
| Translational Research at OSI Our mission is to design and execute innovative non-clinical and clinical research strategies to rapidly determine the Right Regimen Right Dose Right Patients Right Time |

| OSI Translational Research Confronting TKI Resistance Epithelial-Mesenchymal Transition Transcriptional reprogramming Alternate RTK Activation Decreased ErbB3 activity Compensatory activation of RTKs IGF-1R, IR, EGFR, MET, others? Acute signaling feedback mechanisms Genetic/epigenetic adaptation Genomic amplification (MET) Genetic mutations (EGFR, PIK3CA, KRAS, PTEN) Transcriptional modulation (PTEN, IGF2) |
| Our Thesis Differentiation via EMT |
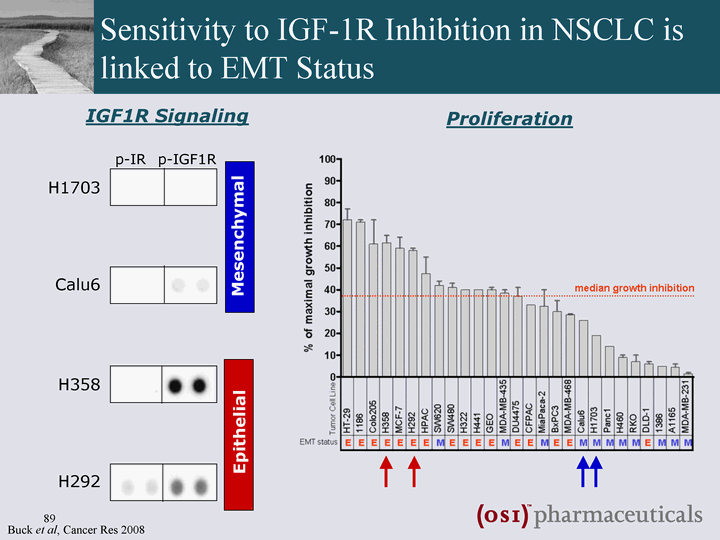
| Sensitivity to IGF-1R Inhibition in NSCLC is linked to EMT Status IGF1R Signaling p-IR p-IGF1R H1703 Calu6 H358 H292 Epithelial Mesenchymal Proliferation Buck et al, Cancer Res 2008 |

| EMT and Sensitivity to Combined Inhibition of EGFR and IGF-1R Synergistic induction of apoptosis in an epithelial background EMT biomarkers may be predictive for response to Tarceva(r) + OSI-906 epithelial mesenchymal |

| Compensatory Signaling in E Tumor Cells EGFR, IGF-1R and IR EGFR IGF-1R IR |

| EGFR IGF-1R IR Tarceva(r) EGFR inhibition can result in compensatory activation of IGF-1R and IR Compensatory Signaling in E Tumor Cells EGFR, IGF-1R and IR |
| Compensatory Signaling in E Tumor Cells EGFR, IGF-1R and IR EGFR IGF-1R IR |

| EGFR IGF-1R IR Y a-IGF1R Anti-IGF-IR MAbs can induce compensatory activation of EGFR and IR Compensatory Signaling in E Tumor Cells EGFR, IGF-1R and IR |
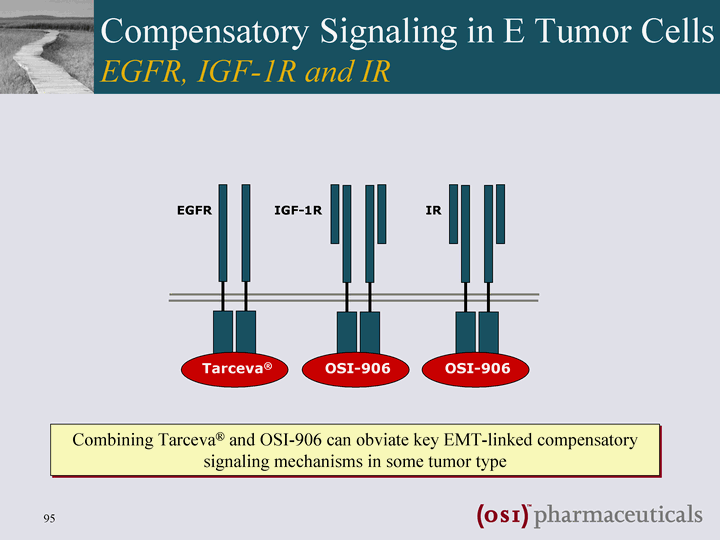
| EGFR IGF-1R IR Tarceva(r) OSI-906 OSI-906 Combining Tarceva(r) and OSI-906 can obviate key EMT-linked compensatory signaling mechanisms in some tumor type Compensatory Signaling in E Tumor Cells EGFR, IGF-1R and IR |

| Scientific Rationale for OSI-906 + Tarceva(r) Reciprocal signaling between EGFR and IGF-1R is well-established HER3 HER1/EGFR ERK IGF1R IRS1 PI3K OSI-906 AKT Tarceva(r) IR/IGF1R control Tarceva(r) IGF-1R inh p-IGF-1R control Tarceva(r) IGF-1R inh T + I p-EGFR EGFR p-HER3 |
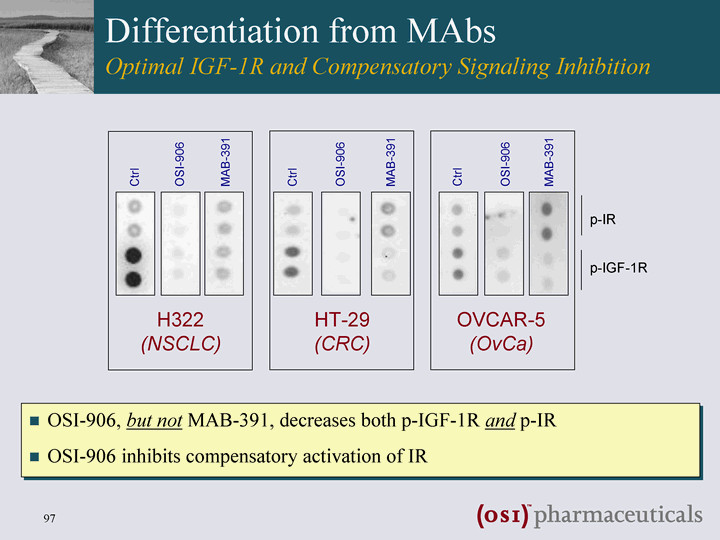
| Differentiation from MAbs Optimal IGF-1R and Compensatory Signaling Inhibition OSI-906 MAB-391 Ctrl H322 (NSCLC) HT-29 (CRC) OVCAR-5 (OvCa) OSI-906 MAB-391 Ctrl OSI-906 MAB-391 Ctrl p-IR p-IGF-1R OSI-906, but not MAB-391, decreases both p-IGF-1R and p-IR OSI-906 inhibits compensatory activation of IR |

| IGF-1R expression occurs in up to 80-100% of OvCa Over-expression of IGF-2 is a poor prognostic indicator in OvCa IGF-1R activates the AKT pathway and is a driver of growth and survival in many cancer types Paclitaxel induces AKT signaling in many human tumor cell lines OSI-906 can suppress paclitaxel-induced p-AKT OSI-906 cooperates with paclitaxel in cisplatin-resistant OvCa cell lines Published data indicate a role for Insulin Receptors (IR) in OvCa IGF-2 activates both IGF-1R and IR Differentiation from MAbs that target only IGF-1R OSI-906 + paclitaxel for OvCa |
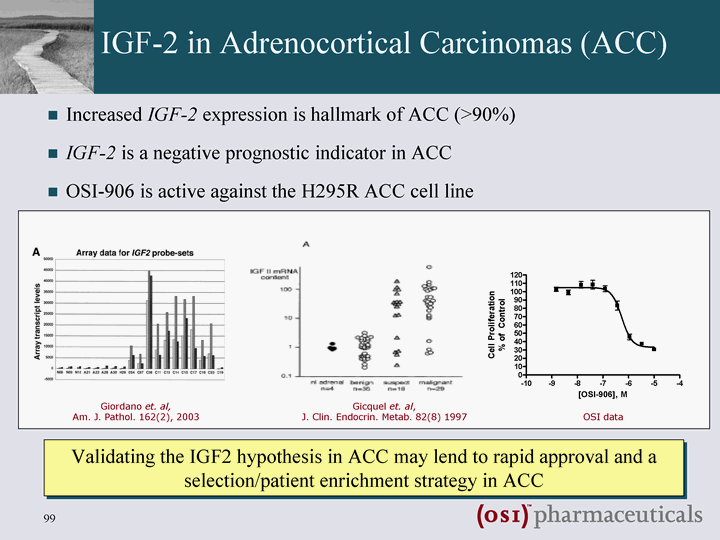
| IGF-2 in Adrenocortical Carcinomas (ACC) Increased IGF-2 expression is hallmark of ACC (>90%) IGF-2 is a negative prognostic indicator in ACC OSI-906 is active against the H295R ACC cell line Validating the IGF2 hypothesis in ACC may lend to rapid approval and a selection/patient enrichment strategy in ACC Giordano et. al, Am. J. Pathol. 162(2), 2003 Gicquel et. al, J. Clin. Endocrin. Metab. 82(8) 1997 OSI data |
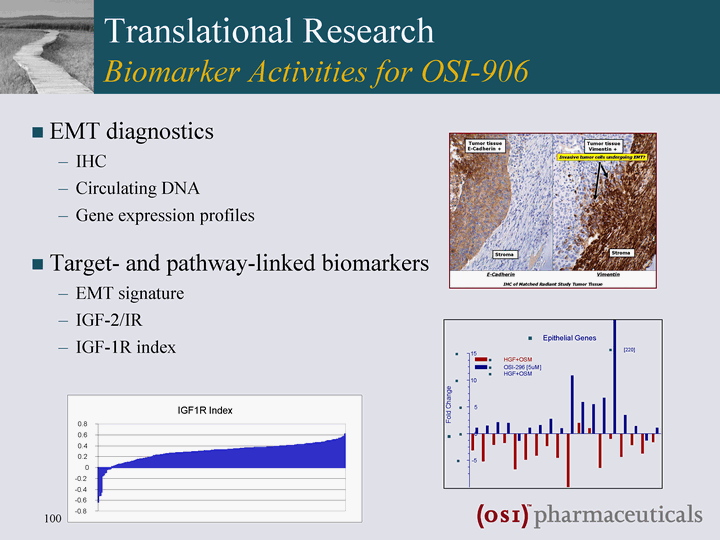
| Translational Research Biomarker Activities for OSI-906 EMT diagnostics IHC Circulating DNA Gene expression profiles Target- and pathway-linked biomarkers EMT signature IGF-2/IR IGF-1R index |

| Oncology Portfolio: R&D Strategy and Update on Pipeline Karsten Witt, M.D. Vice President, Clinical Development, Oncology |
| Agenda OSI-906 (IGF-1R Inhibitor) Program Status Clinical Development Initiatives OSI-027 (TORC1/TORC2 Inhibitor) Program Status Clinical Development Initiatives |
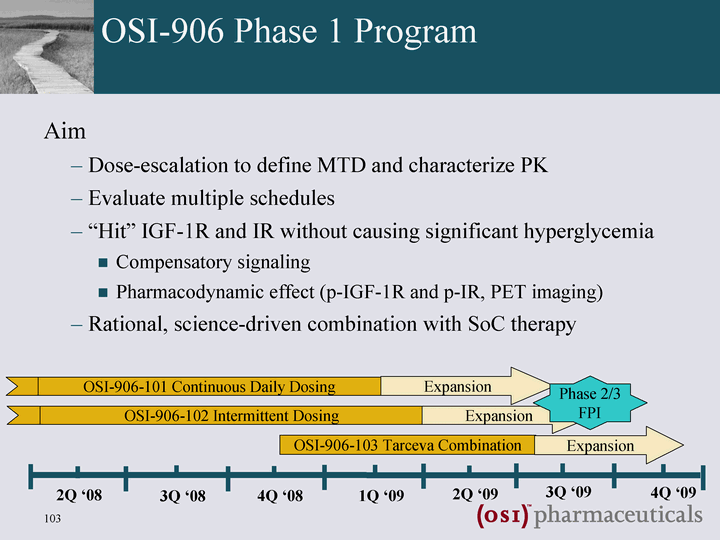
| OSI-906 Phase 1 Program Aim Dose-escalation to define MTD and characterize PK Evaluate multiple schedules "Hit" IGF-1R and IR without causing significant hyperglycemia Compensatory signaling Pharmacodynamic effect (p-IGF-1R and p-IR, PET imaging) Rational, science-driven combination with SoC therapy 3Q '08 4Q '08 1Q '09 2Q '09 3Q '09 4Q '09 2Q '08 OSI-906-101 Continuous Daily Dosing OSI-906-102 Intermittent Dosing OSI-906-103 Tarceva Combination Expansion Expansion Expansion Phase 2/3 FPI |
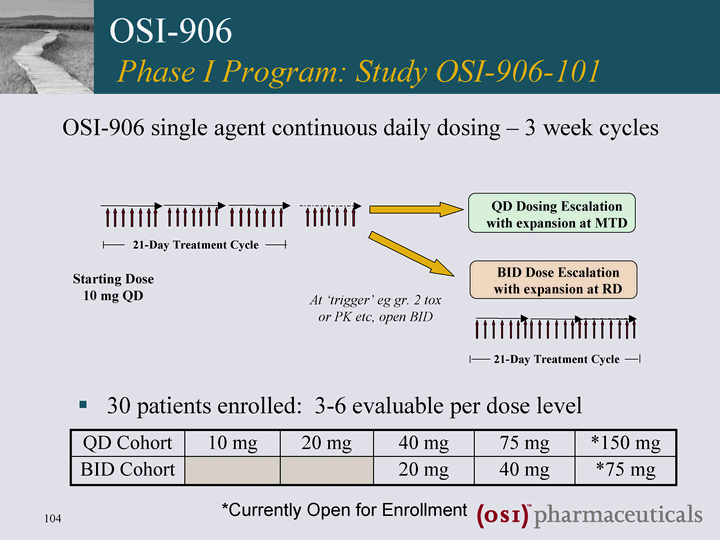
| 30 patients enrolled: 3-6 evaluable per dose level OSI-906 single agent continuous daily dosing - 3 week cycles OSI-906 Phase I Program: Study OSI-906-101 QD Cohort 10 mg 20 mg 40 mg 75 mg *150 mg BID Cohort 20 mg 40 mg *75 mg *Currently Open for Enrollment BID Dose Escalation with expansion at RD At 'trigger' eg gr. 2 tox or PK etc, open BID QD Dosing Escalation with expansion at MTD Starting Dose 10 mg QD 21-Day Treatment Cycle 21-Day Treatment Cycle |
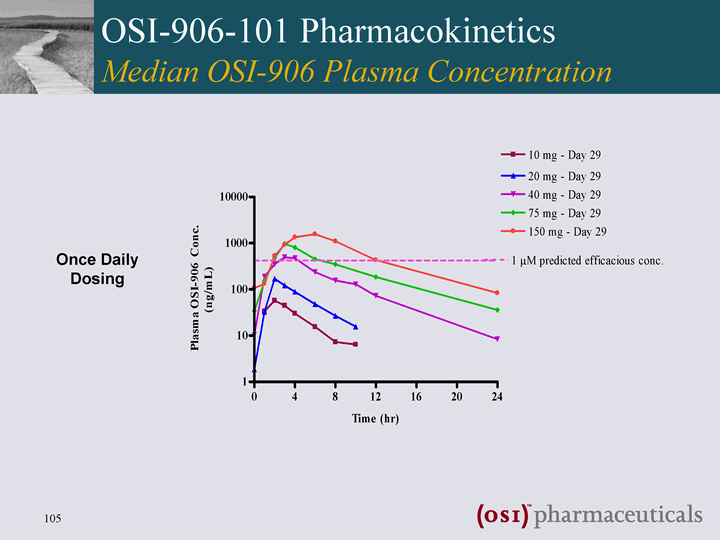
| OSI-906-101 Pharmacokinetics Median OSI-906 Plasma Concentration Once Daily Dosing |
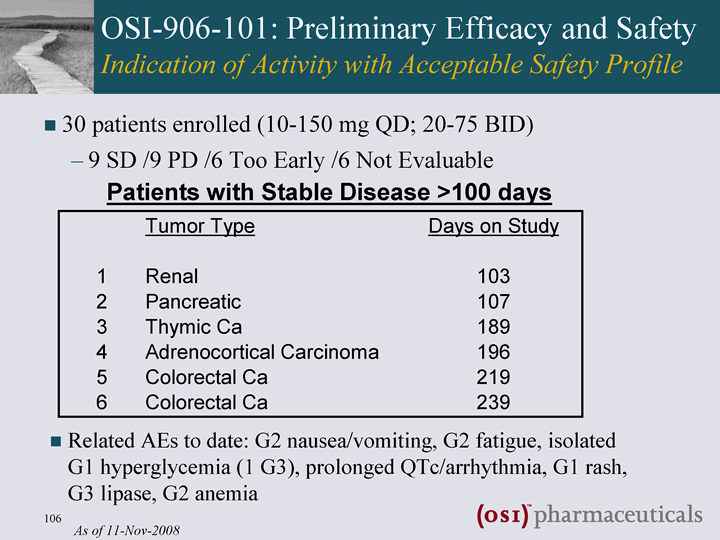
| OSI-906-101: Preliminary Efficacy and Safety Indication of Activity with Acceptable Safety Profile 30 patients enrolled (10-150 mg QD; 20-75 BID) 9 SD /9 PD /6 Too Early /6 Not Evaluable Patients with Stable Disease >100 days As of 11-Nov-2008 Related AEs to date: G2 nausea/vomiting, G2 fatigue, isolated G1 hyperglycemia (1 G3), prolonged QTc/arrhythmia, G1 rash, G3 lipase, G2 anemia |
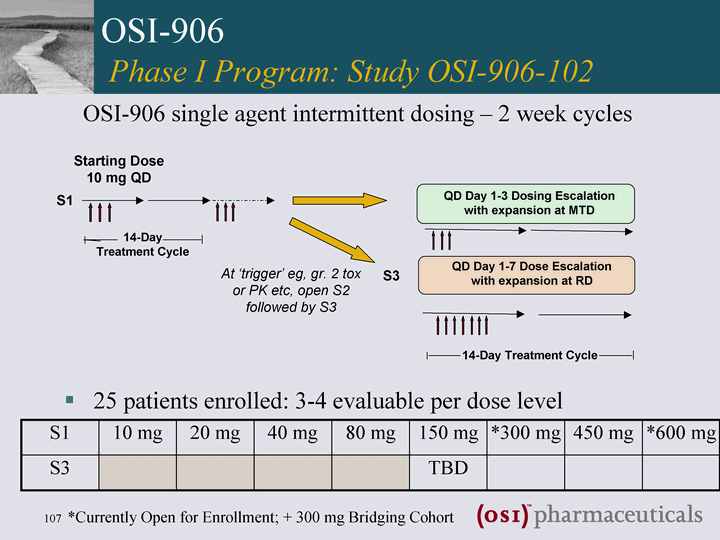
| 25 patients enrolled: 3-4 evaluable per dose level OSI-906 single agent intermittent dosing - 2 week cycles OSI-906 Phase I Program: Study OSI-906-102 At 'trigger' eg, gr. 2 tox or PK etc, open S2 followed by S3 QD Day 1-3 Dosing Escalation with expansion at MTD Starting Dose 10 mg QD 14-Day Treatment Cycle 14-Day Treatment Cycle S1 S3 S1 10 mg 20 mg 40 mg 80 mg 150 mg *300 mg 450 mg *600 mg S3 TBD *Currently Open for Enrollment; + 300 mg Bridging Cohort |
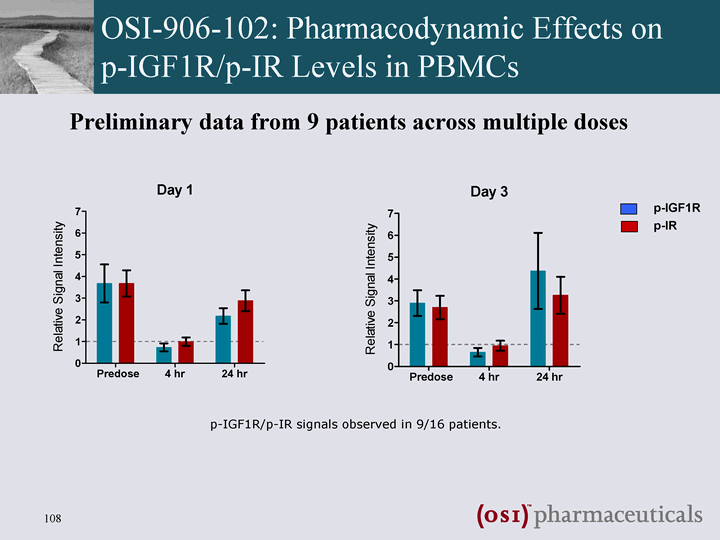
| OSI-906-102: Pharmacodynamic Effects on p-IGF1R/p-IR Levels in PBMCs p-IGF1R/p-IR signals observed in 9/16 patients. Preliminary data from 9 patients across multiple doses p-IGF1R p-IR |

| OSI-906-102: Preliminary Efficacy and Safety Indication of Activity with Acceptable Safety Profile 25 patients enrolled (10-450 mg Day 1-3 q 14) 12 SD /10 PD /3 Too Early Patients with Stable Disease >100 days As of 11-Nov-2008 Related AEs to date: G1 fatigue, G1 rash, G1 hyperglycemia, G1 proteinuria, G1 tachycardia, G1 neuropathy |

| A 4th-line NSCLC Patient Receiving OSI-906 post-Tarceva 73yr M patient diagnosed with Adeno Ca in January 2007 Prior treatment: carboplatin/paclitaxel/bevacizumab (2 mo); single agent erlotinib (3 mo) then single agent pemetrexed (1 mo) Initiated OSI-906 40 mg Day 1-3: 12/2007 Documented minor response by RECIST Patient has SD for >44 weeks |

| OSI-906-101 and -102 Studies Plasma Concentrations vs. Glucose & Insulin Glucose values (uncontrolled for fasting) do not increase with OSI- 906 plasma concentration Trend of insulin increasing with OSI-906 plasma concentration appears similar between monkeys and humans 1 µM predicted efficacious concentration |
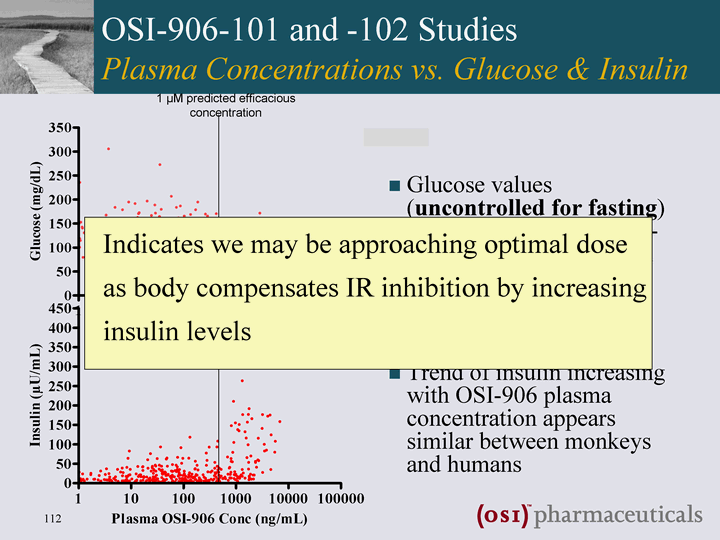
| OSI-906-101 and -102 Studies Plasma Concentrations vs. Glucose & Insulin Glucose values (uncontrolled for fasting) do not correlate with OSI- 906 plasma concentration Trend of insulin increasing with OSI-906 plasma concentration appears similar between monkeys and humans 1 µM predicted efficacious concentration Indicates we may be approaching optimal dose as body compensates IR inhibition by increasing insulin levels |

| OSI-906-103: Phase I OSI-906 + Tarceva Combination Study All Comers/ NSCLC pts predominantly Tarceva + OSI-906 Starting Dose: 100 mg Tarceva(r) + 50 mg 906 n=12-24 Tumor type TBD n=20 Dose Escalation Dose Expansion Dosing of 1st patient Nov 10, 2008 |

| OSI-906 Development Plan Prioritization Scheme Tarceva combos NSCLC PaCa SoC combos Front-line NSCLC OvCa CRC SCCHN Biomarker studies BrCa, PrCa Speed ACC, Ewing's sarcoma (EwS) Other 3rd/4th line NSCLC, HCC, MM Compensatory Signaling Differentiation Ligand Selection EMT Link Clinical Feasibility & Speed Leveraging Tarceva Opportunities for Patient Selection Strategic Focus |
| Summary OSI-906 Clinical Development Plan Strategic/tactical positioning First in class oral IGF-1R inhibitor, validated target, strong IP position Two opportunities for patient selection/enhanced efficacy based on EMT and/or IGF-II/compensatory signaling Translational research guiding personalized medicine Differentiation First small molecule to clinic, flexibility to dose with other agents Rapid cessation of drug exposure in case of adverse effects (unlike MAb's) Ability to target both IGF-1R and IR Compensatory and hybrid signaling Broader spectrum of activity and greater efficacy Speed to POC and registration POC in ACC with possible accelerated approval opportunity Larger commercial biomarker/patient selection initiatives Leverage existing EMT platform |

| Agenda OSI-906 (IGF-1R Inhibitor) Program Status Clinical Development Initiatives OSI-027 (TORC1/TORC2 Inhibitor) Program Status Clinical Development Initiatives |

| Differentiation of OSI-027 from Rapalogs Potent Signal Transduction Inhibitor with EMT Fit First molecular targeted therapy we have seen that equally targets E and M cells Attenuates AKT activation through inhibition of TORC2 Induces apoptosis in many models, including those resistant to rapamycin Greater maximal efficacy in vitro Potentiates chemotherapy and anti- angiogenic therapy More pronounced effect on vessel normalization More pronounced reduction in VEGF production OSI-027 PDK1 TSC1/2 TORC1 TORC2 AKT 4E-BP1 S6K S6 AKT PI3K OSI-027 |

| OSI-027 OSI-027-101 Study Outline S1 Dose will be adjusted downwards for S3 daily S2 S3 At 'non-DLT event' eg G2 tox or PK etc, open S2 and S3 Dose can be maintained in S2 weekly Weekly Phase 2 RD established Cont. Phase 2 RD established D1-3 q7d Phase 2 RD established Initiate study with D1-3 q7d Starting Dose 10 mg 3 patients dosed with 10 mg Day 1-3 weekly Initial safety findings Decreased cardiac function (LVEF) = DLT Reversible Grade 2 QTc prolongation Exposures were higher than anticipated from pre-clinical studies |
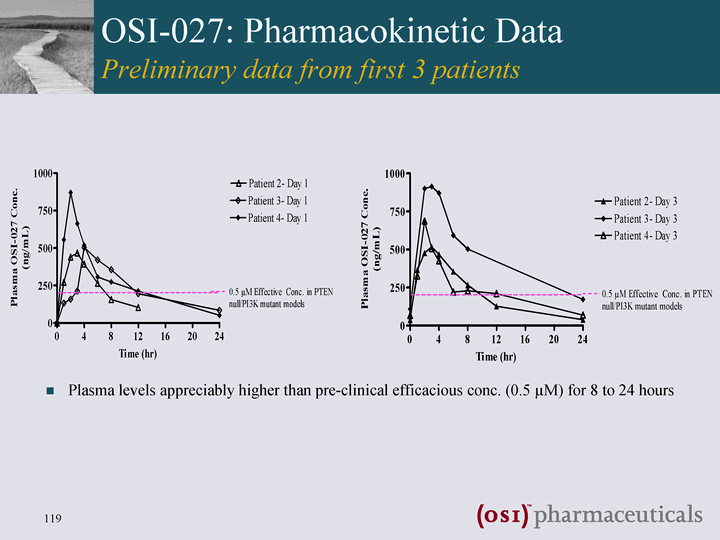
| OSI-027: Pharmacokinetic Data Preliminary data from first 3 patients Plasma levels appreciably higher than pre-clinical efficacious conc. (0.5 µM) for 8 to 24 hours |
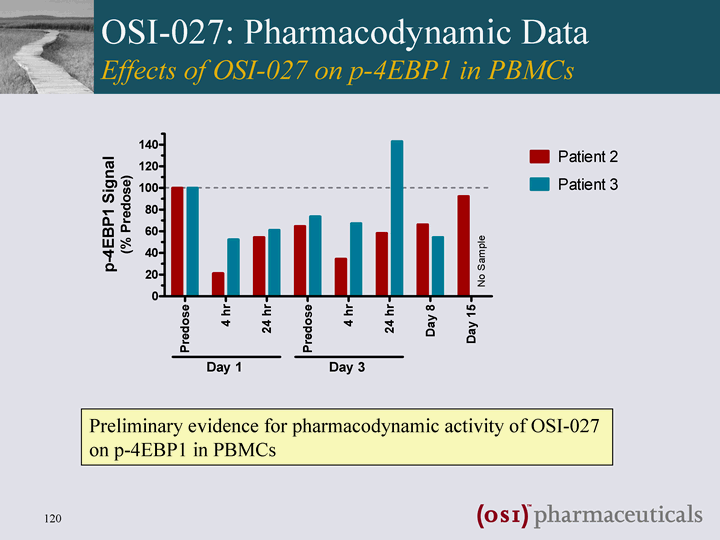
| OSI-027: Pharmacodynamic Data Effects of OSI-027 on p-4EBP1 in PBMCs Preliminary evidence for pharmacodynamic activity of OSI-027 on p-4EBP1 in PBMCs |
| OSI-027 Program Summary OSI-027 is a potent and selective inhibitor of TORC1/TORC2 Differentiation Rapalogs lack activity against TORC2 Complete, effective TORC1/TORC2 blockade may have advantages over PI3K inhibitors Potential value in validating EMT clinically OSI-027 proprietary gene signature from AVEO to be deployed in clinical trials Phase I program underway Cautious dose-escalating due to preclinical toxicity Evaluating Phase II strategy |

| Oncology Q&A |

| Gabriel Leung President, OSI Oncology Executing the Tarceva Life Cycle Plan |

| Smokers Tarceva Life Cycle Plan at a Glance Key Data Milestones 2008 2014 2013 2012 2011 2010 2009 SATURN BeTa ATLAS CALGB Never-smokers EORTC Ovarian HCC NT vs N Breast? AI+-T RADIANT Interim RADIANT Final OTHERS: EGFR Mutant in NSCLC Treatment Beyond Progression FAST ACT II TITAN RACHEL (Pancreatic) MARK (Pancreatic) DREAM (CRC) TASK 2015+ |
| Tarceva: Bumps and Challenges Along the Road The BeTa-Lung Disappointment On October 6th, we announced that the BeTa Lung Study - the first of two major Tarceva Phase III trials set to read-out in 2H2008 - FAILED to meet its primary end-point of showing a significant increase in overall survival for NSCLC patients receiving a combination of Avastin and Tarceva compared to those receiving Tarceva and placebo |
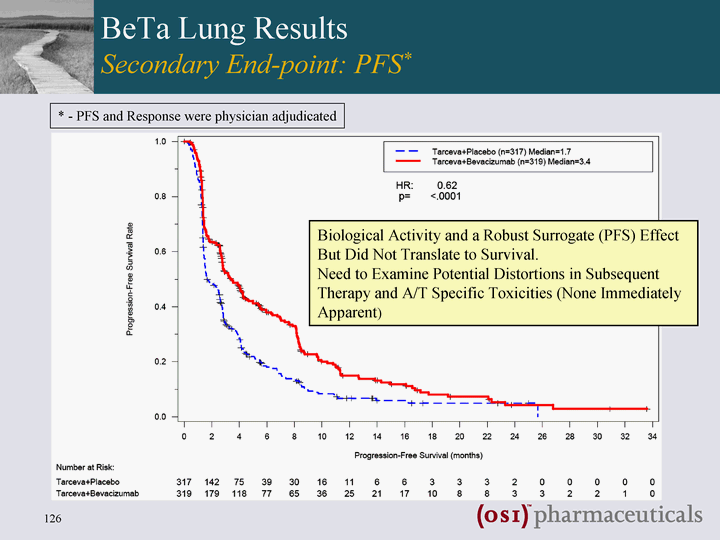
| BeTa Lung Results Secondary End-point: PFS* Secondary End-point: PFS* Secondary End-point: PFS* Biological Activity and a Robust Surrogate (PFS) Effect But Did Not Translate to Survival. Need to Examine Potential Distortions in Subsequent Therapy and A/T Specific Toxicities (None Immediately Apparent) * - PFS and Response were physician adjudicated |
| BeTa Lung: Conclusions & Commentary Clinical Activity But a Failed Study Conclusions from an OSI perspective This WAS a disappointing surprise (based on Phase II Data) The Avastin/Tarceva combination failed to improve overall survival compared to Tarceva alone (T performed well) Study design different from the pending ATLAS study where Tarceva plus Avastin is compared to Avastin plus Placebo in a front-line maintenance setting There is a silver lining in Tarceva data Confirms BR.21 PS0/1 second-line sub-set in prospective Phase III setting Assessing business impact Negatively impacts, but does not negate, our Tarceva growth projections We believed it could have been a valuable combination in the U.S. We believed it would have been of less importance outside the U.S. |

| BeTa Lung: What is The Silver Lining? Phase III Data for Tarceva Supports Chemo Comparison Comparative Trials vs. BeTa Phase III trials BeTa (636 pts) PS 0-2 One prior chemo Primary endpoint: OS BeTa (636 pts) PS 0-2 One prior chemo Primary endpoint: OS Alimta 2nd line (571 pts) PS 0-2 One prior chemo Primary endpoint: OS Alimta 2nd line (571 pts) PS 0-2 One prior chemo Primary endpoint: OS BR.21 (731 pts) PS 0-3 > one prior chemo Primary endpoint: OS A / T Tarceva Alimta Docetaxel Tarceva RR (%) 12.6 6.2 9.1 8.8 8.9 PFS (months) 3.4 1.7 2.9 2.9 2.2 Medium Survival (months) 9.3 9.2 8.3 7.9 6.7 Hazard Ratio (survival) .97 .97 .99 .99 .73 |

| Tarceva: Successes Along the Road Onwards to SATURN and a Better Outcome! On November 7th, we announced that the SATURN Study - the second of two major Tarceva Phase III trials set to read-out in 2H2008 - SUCCEEDED in meeting its primary end-point of showing a statistically significant increase in the time patients with advanced NSCLC lived without their cancer getting worse when Tarceva was given immediately following initial treatment with platinum-based chemotherapy compared to those receiving placebo |
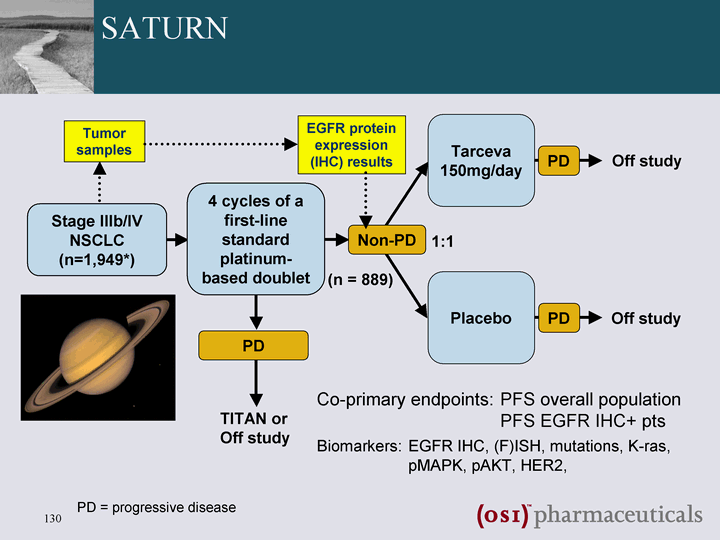
| SATURN Placebo Stage IIIb/IV NSCLC (n=1,949*) Non-PD TITAN or Off study Tarceva 150mg/day PD Off study (n = 889) PD 1:1 4 cycles of a first-line standard platinum- based doublet PD PD = progressive disease Off study Tumor samples EGFR protein expression (IHC) results Biomarkers: EGFR IHC, (F)ISH, mutations, K-ras, pMAPK, pAKT, HER2, Co-primary endpoints: PFS overall population PFS EGFR IHC+ pts |
| Disclosure on SATURN Data to be Presented at a Major Scientific Meeting Initial first-tier data analysis are complete and both the study's co-primary end-points have been met PFS in all-comers PFS in patients who are EGFR protein expression positive (IHC) Independent review results are consistent with investigator assessments Data for overall survival (the key secondary end-point) are still immature We expect the data to be mature in a timeframe consistent with regulatory review Filing discussions will be held with key regulatory agencies We expect FDA will want to see mature survival data as part of the review process but do not necessarily believe that it will be needed prior to submission There are no new or unexpected safety signals |

| OSI Smoking Initiative Resulted in Label Update FDA Updated Label in September 2008 MTD determined to be 300mg/day in current smokers versus 150 mg/day FDA Updated Label: The Tarceva label now states that "Cigarette smoking has been shown to reduce erlotinib exposure" and that a "cautious increase in the dose of Tarceva, not exceeding 300mg" is an available option to physicians treating lung cancer patients who continue to smoke. The label points out efficacy and long- term safety of a higher dose has not been established in patients who continue to smoke. Correlation of Smoking, Exposure and Rash Warrants Continued Investigation in Further Studies Single 150 mg Dose in Healthy Male Subjects |
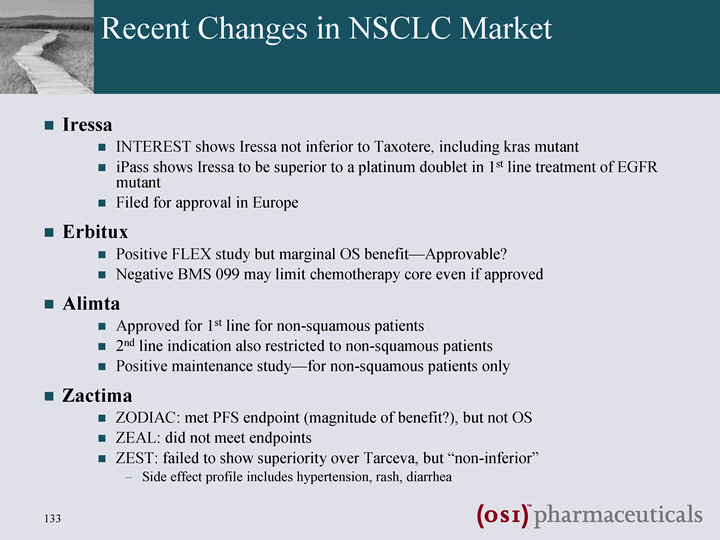
| Recent Changes in NSCLC Market Iressa INTEREST shows Iressa not inferior to Taxotere, including kras mutant iPass shows Iressa to be superior to a platinum doublet in 1st line treatment of EGFR mutant Filed for approval in Europe Erbitux Positive FLEX study but marginal OS benefit-Approvable? Negative BMS 099 may limit chemotherapy core even if approved Alimta Approved for 1st line for non-squamous patients 2nd line indication also restricted to non-squamous patients Positive maintenance study-for non-squamous patients only Zactima ZODIAC: met PFS endpoint (magnitude of benefit?), but not OS ZEAL: did not meet endpoints ZEST: failed to show superiority over Tarceva, but "non-inferior" Side effect profile includes hypertension, rash, diarrhea |

| Ch eligible Ch ineligible Non-sq/Adeno Sq Avastin-eligible Avastin-ineligible EFGR Mutant NSCLC 1st-line Maint 2nd-line Al = Alimta Ch = Chemo Pt = Platinum TBP = Treatment Beyond Progression Av = Avastin E = Erbitux T = Tarceva Tarceva in NSCLC Potential & Existing Opportunities Note: slide represents OSI's view of the developing NSCLC market and not necessarily that of our collaborators |

| Ch eligible Ch ineligible Non-sq/Adeno Sq Avastin-eligible Avastin-ineligible Pt+Al+Av Pt+Al Ch+-E T EFGR Mutant NSCLC 1st-line Maint 2nd-line Al = Alimta Ch = Chemo Pt = Platinum TBP = Treatment Beyond Progression Av = Avastin E = Erbitux T = Tarceva T Tarceva in NSCLC Potential & Existing Opportunities Note: slide represents OSI's view of the developing NSCLC market and not necessarily that of our collaborators |
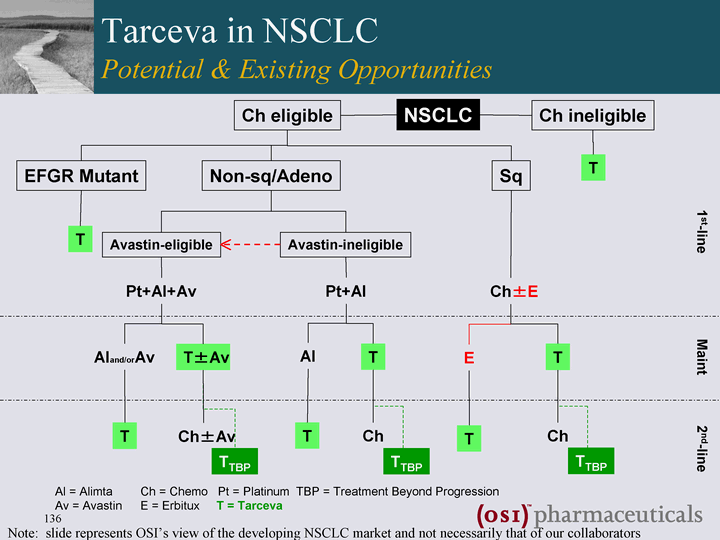
| Ch eligible Ch ineligible Non-sq/Adeno Sq Avastin-eligible Avastin-ineligible Pt+Al+Av Pt+Al Aland/orAv T+-Av T Ch+-Av Al T T Ch Ch+-E T Ch TTBP TTBP TTBP T EFGR Mutant NSCLC 1st-line Maint 2nd-line Al = Alimta Ch = Chemo Pt = Platinum TBP = Treatment Beyond Progression Av = Avastin E = Erbitux T = Tarceva T E T Tarceva in NSCLC Potential & Existing Opportunities Note: slide represents OSI's view of the developing NSCLC market and not necessarily that of our collaborators |
| Smokers Tarceva Life Cycle Plan at a Glance Key Data Milestones 2008 2014 2013 2012 2011 2010 2009 SATURN BeTa ATLAS CALGB Never-smokers EORTC Ovarian HCC NT vs N Breast? AI+-T RADIANT Interim RADIANT Final OTHERS: EGFR Mutant in NSCLC Treatment Beyond Progression FAST ACT II TITAN RACHEL (Pancreatic) MARK (Pancreatic) DREAM (CRC) TASK 2015+ |

| 1o Endpoint: Progression-free survival (from the time of randomization) 2o Safety * Specified regimens: Carboplatin / paclitaxel Carboplatin / gemcitabine Carboplatin / docetaxel Cisplatin / gemcitabine Cisplatin / docetaxel Cisplatin / vinorelbine Arm 1 Bev. / placebo to PD Arm 2 Bev. / Erlotinib to PD 1:1 Randomization (patients w/o PD or significant toxicity) Chemotherapy* + Avastin(r) x 4 cycles (N=1150) Erlotinib Off-Study Therapy ATLAS Study Schema |
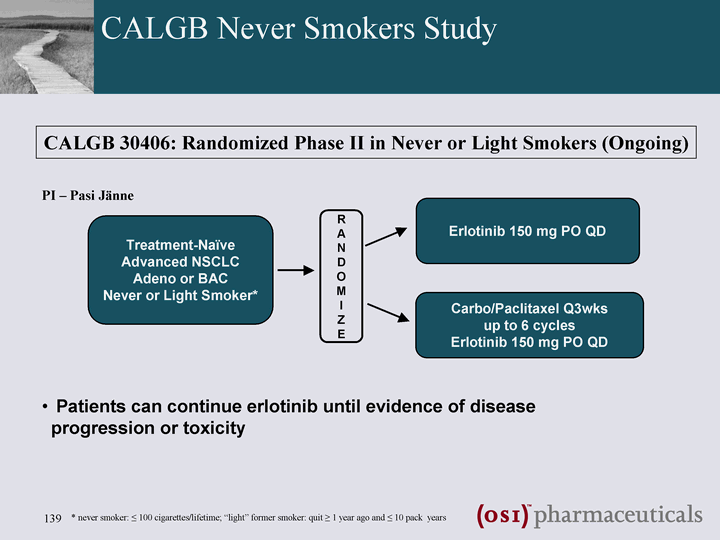
| CALGB Never Smokers Study Treatment-Naive Advanced NSCLC Adeno or BAC Never or Light Smoker* Erlotinib 150 mg PO QD R A N D O M I Z E Carbo/Paclitaxel Q3wks up to 6 cycles Erlotinib 150 mg PO QD CALGB 30406: Randomized Phase II in Never or Light Smokers (Ongoing) Patients can continue erlotinib until evidence of disease progression or toxicity * never smoker: ^ 100 cigarettes/lifetime; "light" former smoker: quit ^ 1 year ago and ^ 10 pack years PI - Pasi Janne |

| EORTC Phase III Ovarian Study Tarceva Maintenance: SATURN Concept (n = 830) High-risk ovarian / FT cancer* CR / PR / SD within 6w Platinum based 1st line therapy PD Placebo 150 mg/d (up to 24m) PD Off study Tarceva 150 mg/day (up to 24m) PD Off study Primary endpoint = PFS Secondary endpoints: OS, safety, QoL, cutaneous toxicity Translational biomarker component Fully accrued as of Feb 2008 Off study * high-risk stage I and stages II-IV ovarian epithelial, primary peritoneal, or fallopian tube cancer |
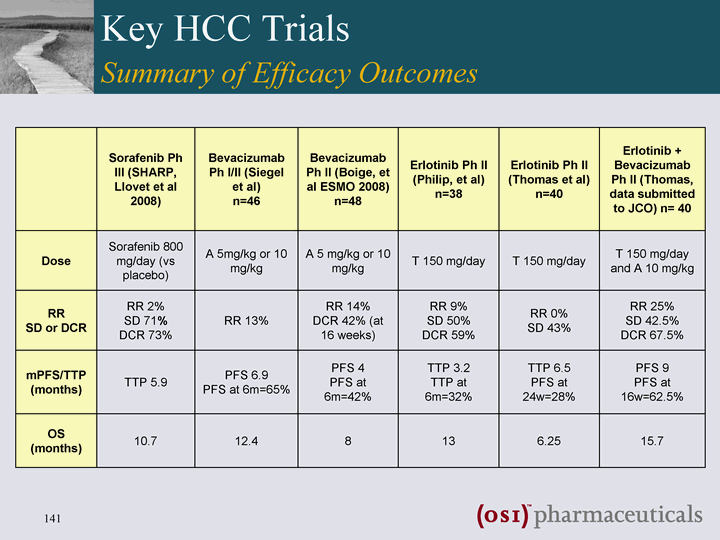
| Key HCC Trials Summary of Efficacy Outcomes Sorafenib Ph III (SHARP, Llovet et al 2008) Bevacizumab Ph I/II (Siegel et al) n=46 Bevacizumab Ph II (Boige, et al ESMO 2008) n=48 Erlotinib Ph II (Philip, et al) n=38 Erlotinib Ph II (Thomas et al) n=40 Erlotinib + Bevacizumab Ph II (Thomas, data submitted to JCO) n= 40 Dose Sorafenib 800 mg/day (vs placebo) A 5mg/kg or 10 mg/kg A 5 mg/kg or 10 mg/kg T 150 mg/day T 150 mg/day T 150 mg/day and A 10 mg/kg RR SD or DCR RR 2% SD 71% DCR 73% RR 13% RR 14% DCR 42% (at 16 weeks) RR 9% SD 50% DCR 59% RR 0% SD 43% RR 25% SD 42.5% DCR 67.5% mPFS/TTP (months) TTP 5.9 PFS 6.9 PFS at 6m=65% PFS 4 PFS at 6m=42% TTP 3.2 TTP at 6m=32% TTP 6.5 PFS at 24w=28% PFS 9 PFS at 16w=62.5% OS (months) 10.7 12.4 8 13 6.25 15.7 |

| HCC Phase III Trials Proposed Study Advanced HCC CP-A PS 0/1 No prior therapy Nexavar 400 mg BID Tarceva 150 mg QD R A N D O M I Z E Nexavar 400 mg BID Placebo QD Primary: OS Secondary: Safety, TTP, DCR and QoL Other: RR, BM, PK FPI Expected in 1H09 |
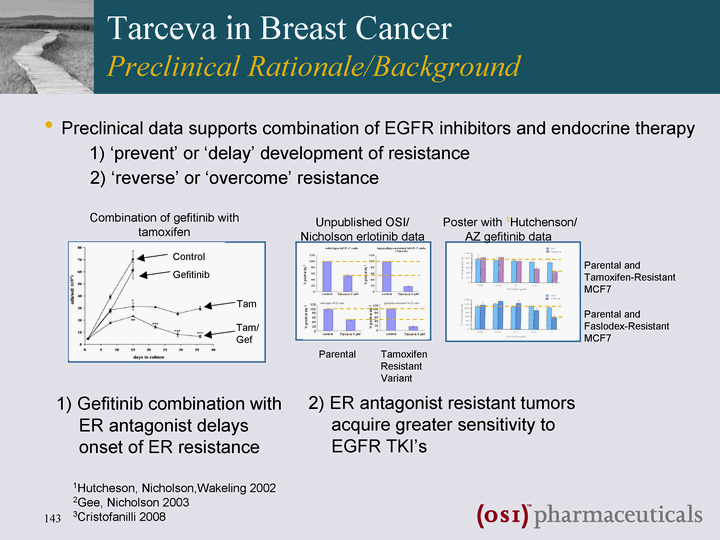
| Tarceva in Breast Cancer Preclinical Rationale/Background 2) ER antagonist resistant tumors acquire greater sensitivity to EGFR TKI's Combination of gefitinib with tamoxifen significantly delays onset of ER inhibitor resistance Unpublished OSI/ Nicholson erlotinib data Poster with 1Hutchenson/ AZ gefitinib data Parental Tamoxifen Resistant Variant Parental and Tamoxifen-Resistant MCF7 Parental and Faslodex-Resistant MCF7 Control Gefitinib Tam Tam/ Gef 1) Gefitinib combination with ER antagonist delays onset of ER resistance 1Hutcheson, Nicholson,Wakeling 2002 2Gee, Nicholson 2003 3Cristofanilli 2008 Preclinical data supports combination of EGFR inhibitors and endocrine therapy 1) 'prevent' or 'delay' development of resistance 2) 'reverse' or 'overcome' resistance |

| Tarceva in Breast Cancer Rationale/Background Recent Cristofinalli Iressa/Anastrazole (ASCO 2008) results have generated interest to investigate role of Tarceva + HT in MBC Improvement in PFS and DCR for combination vs anastrazole alone Two Tarceva ISTs investigate endocrine therapy combinations: Mayer/Arteaga 1L Phase II erlotinib/letrozole 48 pts accrued over 5 years and closed early in July 2008 (planned to accrue 150 pts) PFS = Primary endpoint 27 events to date, anticipating PFS > 12 months, some receiving therapy for as long as 4 yrs, 21 pts have yet to progress Toxicity Primarily skin rash and fingertip skin "cracking", minimal diarrhea Reductions to 100mg for same reason (approx 30%) DC rate due to toxicity unknown Makhoul 2L randomized Phase II fulvestrant +/- Tarceva - 12/130; 4/8 sites open |

| Tarceva in Breast Cancer Study Concept: 1L Inoperable LA/MBC Postmenopausal Inoperable LA or MBC ER+ (and PR +ve?) Her2 -ve No prior Chemo for MBC No Prior hormonal therapy for MBC or >12 mos after adjuvant treatment Letrozole or Dealer's Choice + Tarceva Primary Objective TTP Secondary Objectives ORR CBR Survival Safety / Tolerability Biological / Marker Correlates RANDOMIZED 1:1 Letrozole or Dealer's Choice + Placebo Final Commitment Gated On Feasibility Analysis |
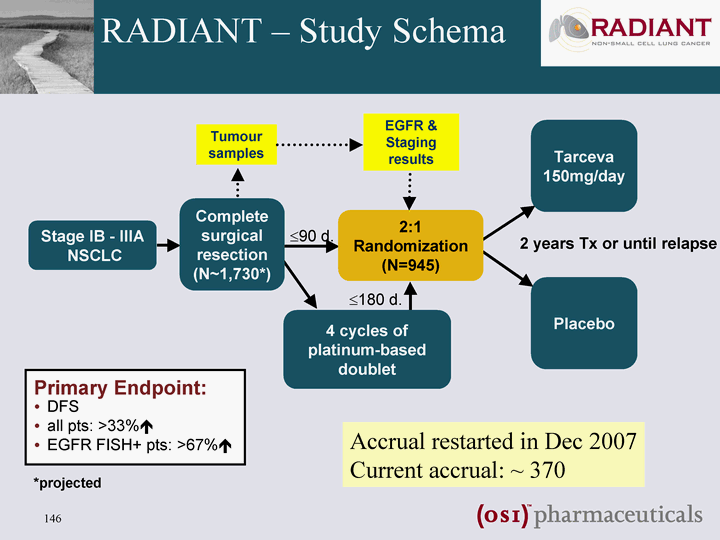
| RADIANT - Study Schema Placebo Stage IB - IIIA NSCLC 2:1 Randomization (N=945) Tarceva 150mg/day 4 cycles of platinum-based doublet *projected 2 years Tx or until relapse Tumour samples EGFR & Staging results Complete surgical resection (N~1,730*) ?90 d. ?180 d. Primary Endpoint: DFS all pts: >33%? EGFR FISH+ pts: >67%? Accrual restarted in Dec 2007 Current accrual: ~ 370 |

| Smokers Tarceva Life Cyle Plan at a Glance Key Data Milestones 2008 2014 2013 2012 2011 2010 2009 SATURN BeTa ATLAS CALGB Never-smokers EORTC Ovarian HCC NT vs N Breast? AI+-T RADIANT Interim RADIANT Final OTHERS: EGFR Mutant in NSCLC Treatment Beyond Progression FAST ACT II TITAN RACHEL (Pancreatic) MARK (Pancreatic) DREAM (CRC) TASK 2015+ |

| Tarceva Q&A |

| Wrap-up and Final Q&A |