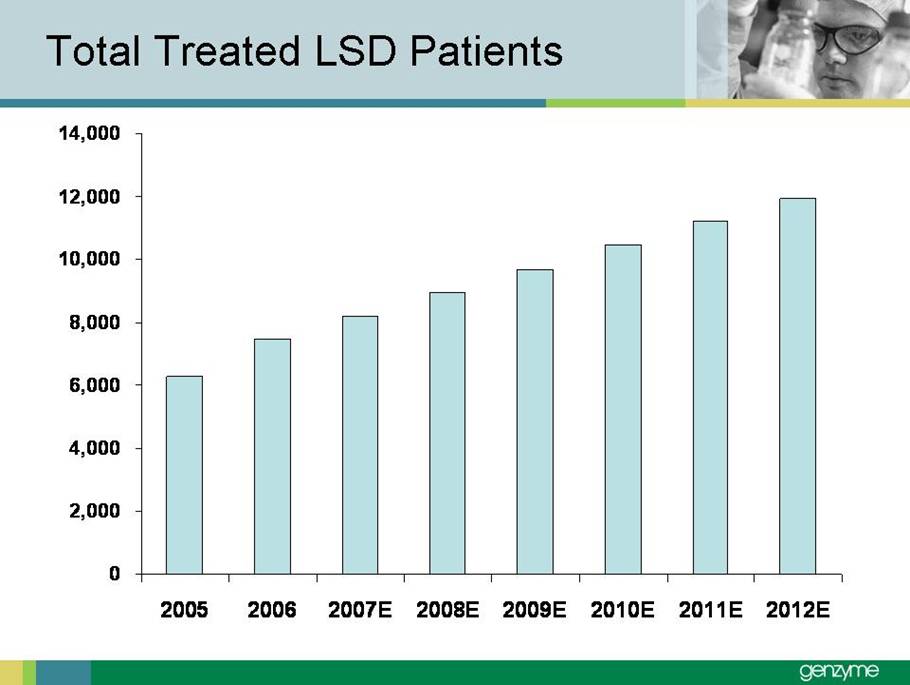
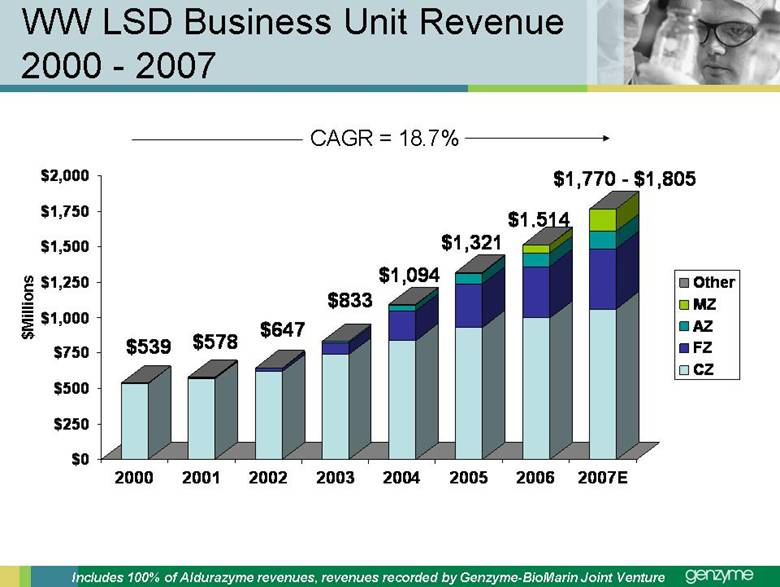
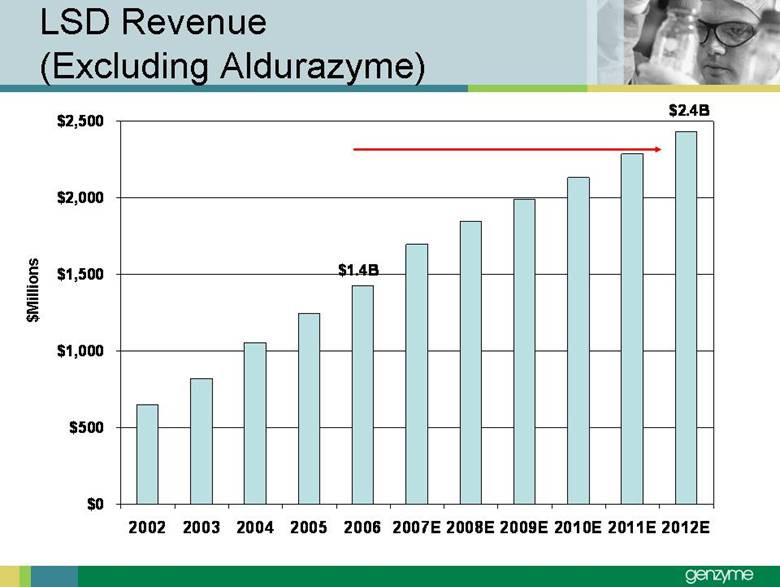
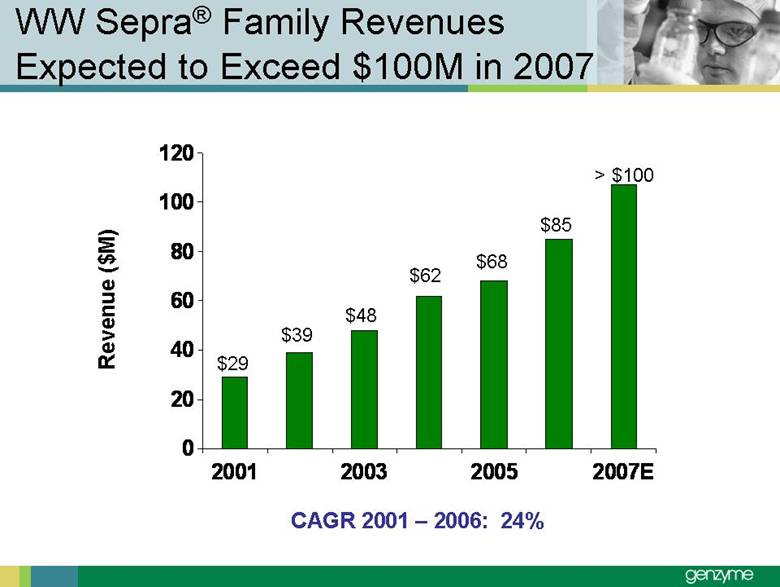
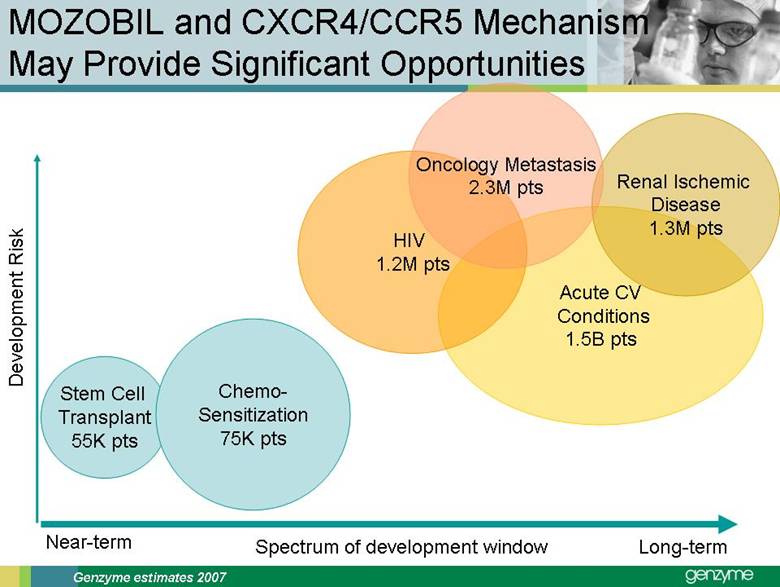
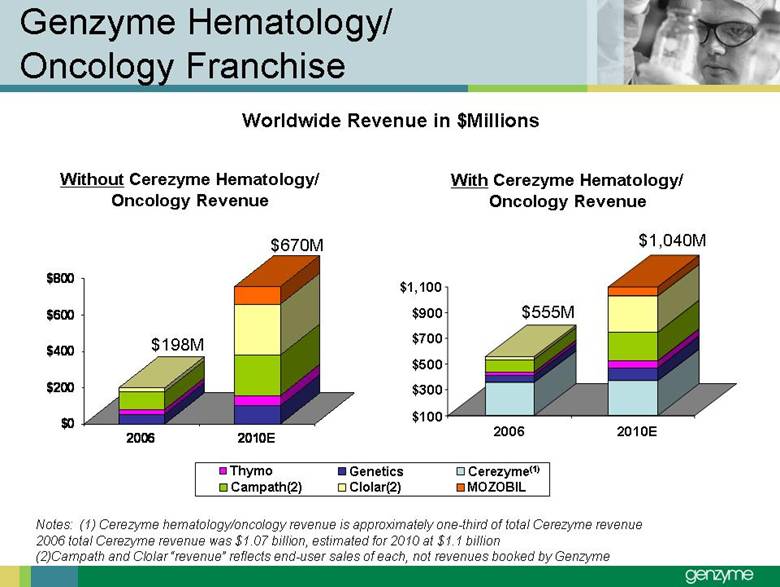
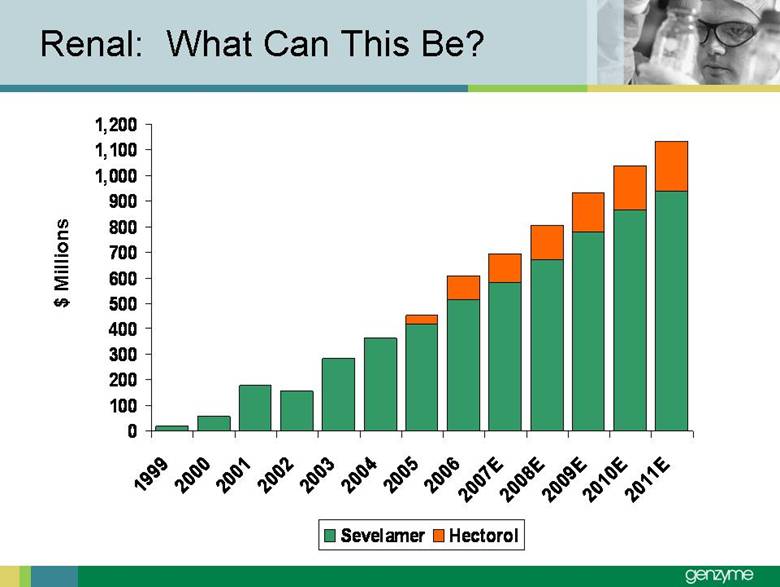
| Total Treated LSD Patients 0 2,000 4,000 6Forward-Looking Statements Our presentations today contain forward-looking statements regarding our financial outlook and business strategies, including without limitation, statements regarding: our projected product development, regulatory filing and action estimates, manufacturing and commercialization plans and timetables, including for MyozymeÒ, tolevamer™, Renvela™ tablets and powder, alemtuzumab (MS), CampathÒ, Mozobil™, Synvisc-One™, ClolarÒ, Renagel PD, and hylastanTM; estimated revenues and growth rates for certain products, including Cerezyme and Sepra, and certain businesses, including oncology, renal and LSDs; the expected drivers of future growth for our revenues, including for Myozyme, Renagel/Renvela, Synvisc, Campath, Clolar, and Sepra; the timing and availability of data from clinical trials, including alemtuzumab (MS), Mozobil, Synvisc-One and hylastan; and the expected patient populations to be treated by our products, including Synvisc and LSDs products. These statements are subject to risks and uncertainties, and our actual results may differ materially from those projected in the forward looking statements. Those risks and uncertainties include: our ability to successfully complete preclinical and clinical development and post-marketing commitments for our products and services; our ability to expand the use of current products in existing and new indications and in new geographies, including Renagel, Synvisc, Campath and Clolar; the content and timing of actual submissions to and decisions made by the FDA, EMEA and other regulatory agencies; our ability to maintain and obtain regulatory approvals for products, services and manufacturing facilities; our ability to manufacture sufficient amounts of products for development and commercialization activities, and to manage our product inventories; our ability to successfully identify and market to new patients; the accuracy of our estimates of the size and characteristics of the markets to be addressed by our products and services, including growth projections; the accuracy of our information regarding the products and resources of our competitors; our ability to secure reimbursement for our products and services from third-party payors, the extent of coverage and the accuracy of our estimates of the payor mix for our products and services; our ability to obtain, maintain and successfully enforce adequate patent and other proprietary rights protection of our products and services; the scope, validity and enforceability of patents and other proprietary rights held by third parties and their impact on our ability to commercialize our products and services; our ability to successfully expand our sales and marketing teams in existing and new markets; our ability to establish and maintain strategic license, collaboration and distribution arrangements; and the risks and uncertainties described in our SEC reports filed under the Securities Exchange Act of 1934, including the factors discussed under the caption “Risk Factors" in Genzyme's Quarterly Report on Form 10-Q for the period ended March 31, 2007. We caution investors not to place substantial reliance on forward-looking statements. These statements speak only as of today (other than 2007 guidance, which was given February 14th and updated April 25, 2007). We undertake no obligation to update or revise these statements. ,000 8,000 10,000 12,000 14,000 2005 2006 2007E 2008E 2009E 2010E 2011E 2012E |