UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a)
of the Securities Exchange Act of 1934
Filed by the Registrantx
Filed by a Party other than the Registrant¨
Check the appropriate box:
| | |
¨ Preliminary Proxy Statement | | ¨ Confidential, for Use of the Commission Only (as permitted by
Rule 14a-6(e)(2)) |
x Definitive Proxy Statement | |
¨ Definitive Additional Materials | | |
¨ Soliciting Material Pursuant to §240.14a-12 | | |
MDRNA, Inc.
(Name of Registrant as Specified in its Charter)
(Name of Person(s) Filing Proxy Statement if other than the Registrant)
Payment of Filing Fee (Check in the appropriate box):
| x | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | (1) | Title of each class of securities to which transaction applies: |
Common stock, par value $0.006 per share.
| | (2) | Aggregate number of securities to which transaction applies: |
38,350,620 shares of common stock.
| | (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
The maximum aggregate value was determined based upon 38,350,620 shares of MDRNA, Inc. common stock being issued in the transaction multiplied by $1.16, which is the average of the high and low trading prices as reported on The NASDAQ Global Market on May 10, 2010. The filing fee was determined by multiplying $0.00007130 by the maximum aggregate value of the transaction as determined in accordance with the preceding sentence.
| | (4) | Proposed maximum aggregate value of transaction: |
$44,486,719
$3,171.90
| x | Fee paid previously with preliminary materials. |
| ¨ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | (1) | Amount Previously Paid: |
| | (2) | Form, Schedule or Registration No.: |
MDRNA, Inc.
3830 Monte Villa Parkway
Bothell, WA 98021
(425) 908-3600
YOUR VOTE IS VERY IMPORTANT
MDRNA, Inc., which we refer to as “we,” “us,” “our company” and “MDRNA,” and Cequent Pharmaceuticals, Inc., which we refer to as “Cequent,” have entered into a merger agreement pursuant to which Calais Acquisition Corp., a wholly-owned subsidiary of our company, will merge with and into Cequent. Cequent will then continue as a wholly-owned subsidiary of our company. We and Cequent believe that the merger will result in the creation of a leading biopharmaceutical company focused on the discovery, development and commercialization of pharmaceuticals based on RNA interference, or RNAi.
Subject to the terms and conditions of the merger agreement, at the effective time of the merger, each share of Cequent Series A-1 convertible preferred stock and common stock outstanding immediately prior to the merger will be exchanged for shares of the common stock, par value $0.006 per share, of our company, which we refer to as “common stock”, at an exchange ratio that implies a purchase price for Cequent common and preferred stockholders of approximately $44 million, plus an additional value of approximately $2 million to Cequent’s warrant and option holders, based on the 10-day volume-weighted average price of our common stock on The NASDAQ Global Market ending on March 31, 2010. This represents an approximate 56% equity ownership of the combined company for the stockholders of our company and an approximate 44% equity ownership of the combined company for the securityholders of Cequent immediately following the merger.
In addition, each stock option or warrant exercisable for shares of Cequent common stock that is outstanding at the effective time of the merger will be assumed by us and converted into a stock option or warrant to purchase the number of shares of common stock that the holder would have received if such holder had exercised such stock option or warrant for shares of Cequent common stock prior to the merger and exchanged such shares for shares of our common stock in accordance with the terms and provisions of the merger agreement.
Our common stock is currently listed on The Nasdaq Global Market under the symbol “MRNA”. We anticipate that the closing of the merger will occur not later than five business days following the affirmative vote of our stockholders.
We are asking our stockholders to approve the issuance of shares of common stock at the annual meeting of stockholders to take place on Wednesday, July 21, 2010, at 10:00 am Eastern Time, at the offices of Pryor Cashman LLP, 7 Times Square, New York, New York 10036. At this meeting, which will be the annual meeting of the stockholders of MDRNA, you will be asked to vote on the following items:
| 1. | To approve the issuance of shares of common stock pursuant to the Agreement and Plan of Merger, dated as of March 31, 2010, by and among our company, Cequent Pharmaceuticals, Inc., Calais Acquisition Corp. and a representative of the stockholders of Cequent Pharmaceuticals, Inc., as described in the attached proxy statement; |
| 2. | To consider and vote upon an adjournment of the meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes in favor of Proposal No. 1, Proposal No. 3 or Proposal No. 8; |
| 3. | To consider and vote upon a proposed amendment to our certificate of incorporation to change the name of our company from MDRNA, Inc. to Marina Biotech, Inc.; |
| 4. | To elect six (6) persons to our Board of Directors, each to hold office until the 2011 annual meeting of stockholders and until their respective successors shall have been duly elected or appointed and qualify; |
| 5. | To consider and vote upon a proposal to ratify the appointment of KPMG LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2010; |
| 6. | To consider and vote upon a proposal to amend our 2008 Stock Incentive Plan to increase the number of shares available for issuance thereunder from 4,500,000 to 8,500,000; |
| 7. | To consider and vote upon a proposal to amend our 2007 Employee Stock Purchase Plan to increase the number of shares available for issuance thereunder from 300,000 to 600,000; |
| 8. | To consider and vote upon a proposal to change our capital structure by increasing the number of authorized shares of common stock from 90,000,000 to 180,000,000, and by increasing the number of authorized shares of preferred stock from 100,000 to 200,000; |
| 9. | To consider and vote upon a proposal to amend the Rights Agreement, dated February 22, 2000, between our company and American Stock Transfer & Trust Company as Rights Agent, as amended, to extend the term of the Rights Agreement from March 17, 2010 to March 17, 2013; |
| 10. | To consider and vote upon a proposal to amend the Amended and Restated Common Stock Purchase Warrants issued to investors and the placement agent in April 2008 to reduce and, in certain instances, eliminate the floor exercise price of such warrants, and to issue the additional shares of common stock that would be issuable upon full exercise of such warrants as a result thereof; |
| 11. | To consider and vote upon a proposal to amend the Common Stock Purchase Warrants issued to investors in January 2010 to eliminate the floor exercise price of such warrants; and |
| 12. | To transact such other business as may properly come before the meeting or any adjournment or postponement thereof. |
After careful consideration, the Board of Directors has unanimously approved the merger agreement and the respective proposals referred to above, and the Board of Directors has determined that it is advisable and in the best interest of our stockholders to enter into the merger. The Board of Directors recommends that you vote “FOR” the proposals described in the accompanying proxy statement.
Please give all of the detailed information on MDRNA, Cequent and the merger contained in this Proxy Statement your careful attention, especially the discussion in the section entitled “Risk Factors” beginning on page I-23 of this Proxy Statement.
This Proxy Statement is dated June 8, 2010 and is first being mailed to the stockholders of MDRNA on or about June 11, 2010.
Please also see “Where You Can Find More Information” on page VI-1.
MDRNA, Inc.
3830 Monte Villa Parkway
Bothell, WA 98021
(425) 908-3600
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS OF
MDRNA, INC.
TO BE HELD ON Wednesday, July 21, 2010
To the Stockholders of MDRNA, Inc.:
The annual meeting of MDRNA, Inc., a Delaware corporation, will be held on Wednesday, July 21, 2010, at 10:00 a.m., Eastern Time, at the offices of Pryor Cashman LLP, 7 Times Square, New York, New York 10036 for the following purposes:
| 1. | To approve the issuance of shares of common stock pursuant to the Agreement and Plan of Merger, dated as of March 31, 2010, by and among MDRNA, Cequent Pharmaceuticals, Inc., Calais Acquisition Corp. and a representative of the stockholders of Cequent Pharmaceuticals, Inc., as described in the attached proxy statement; |
| 2. | To consider and vote upon an adjournment of the meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes in favor of Proposal No. 1, Proposal No. 3 or Proposal No. 8; |
| 3. | To consider and vote upon a proposed amendment to our certificate of incorporation to change the name of our company from MDRNA, Inc. to Marina Biotech, Inc.; |
| 4. | To elect six (6) persons to our Board of Directors, each to hold office until the 2011 annual meeting of stockholders and until their respective successors shall have been duly elected or appointed and qualify; |
| 5. | To consider and vote upon a proposal to ratify the appointment of KPMG LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2010; |
| 6. | To consider and vote upon a proposal to amend our 2008 Stock Incentive Plan to increase the number of shares available for issuance thereunder from 4,500,000 to 8,500,000; |
| 7. | To consider and vote upon a proposal to amend our 2007 Employee Stock Purchase Plan to increase the number of shares available for issuance thereunder from 300,000 to 600,000; |
| 8. | To consider and vote upon a proposal to change our capital structure by increasing the number of authorized shares of common stock from 90,000,000 to 180,000,000, and by increasing the number of authorized shares of preferred stock from 100,000 to 200,000; |
| 9. | To consider and vote upon a proposal to amend the Rights Agreement, dated February 22, 2000, between our company and American Stock Transfer & Trust Company as Rights Agent, as amended, to extend the term of the Rights Agreement from March 17, 2010 to March 17, 2013; |
| 10. | To consider and vote upon a proposal to amend the Amended and Restated Common Stock Purchase Warrants issued to investors and the placement agent in April 2008 to reduce and, in certain instances, eliminate the floor exercise price of such warrants, and to issue the additional shares of common stock that would be issuable upon full exercise of such warrants as a result thereof; |
| 11. | To consider and vote upon a proposal to amend the Common Stock Purchase Warrants issued to investors in January 2010 to eliminate the floor exercise price of such warrants; and |
| 12. | To transact such other business as may properly come before the meeting or any adjournment or postponement thereof. |
The Board of Directors has fixed June 2, 2010 as the record date for the determination of stockholders entitled to notice of, and to vote at, the annual meeting and any adjournment or postponement thereof. Only stockholders of
record at the close of business on the record date are entitled to notice of, and to vote at, the annual meeting. Only stockholders or their proxy holders and guests may attend the meeting. A list of stockholders entitled to vote will be kept at the offices of MDRNA, Inc., 3830 Monte Villa Parkway, Bothell, WA for ten days before the annual meeting. At the close of business on the record date, our company had 48,896,483 shares of common stock outstanding and entitled to vote.
|
/s/ Peter S. Garcia |
Peter S. Garcia Chief Financial Officer and Secretary |
June 8, 2010
Your vote is important.
The affirmative vote of the holders of a majority of the shares present at the annual meeting, either in person or represented by proxy, and entitled to vote, is required to approve Proposal Nos. 1, 2, 4, 5, 6, 7, 9, 10 and 11. The affirmative vote of the holders of a majority of our issued and outstanding shares of common stock entitled to vote is required to approve Proposal Nos. 3 and 8. For the election of directors (Proposal No. 4), the six nominees receiving the most “For” votes from the shares having voting power present in person or represented by proxy at the annual meting will be elected.
You are urged to attend the annual meeting in person, but if you are unable to do so, the Board of Directors would appreciate the prompt return of the enclosed proxy card, dated and signed, or, if your proxy card or voting instruction form so indicates, your prompt vote electronically via the Internet or telephone.We strongly encourage you to vote electronically if you have that option.
TABLE OF CONTENTS
i
| | |
How to Vote by Proxy | | II-5 |
Other Business; Adjournments | | II-7 |
Appraisal Rights | | II-7 |
Chapter Three-Other Information Regarding Cequent | | |
Overview and Business Strategy | | III-1 |
Background | | III-1 |
Product Development | | III-2 |
RNAi-Based Therapeutics | | III-2 |
RNAi Partnering and Licensing Agreements | | III-6 |
Property Rights and Intellectual Property | | III-7 |
Research | | III-8 |
Employees | | III-8 |
Competition | | III-8 |
Government Regulation | | III-9 |
Development Stage Company | | III-10 |
Management’s Discussion and Analysis of Financial Condition and Results of Operations | | III-10 |
Chapter Four-Beneficial Ownership Information | | |
Ownership of our Common Stock Prior to the Merger | | IV-1 |
Chapter Five-Annual Meeting Proposals | | |
Proposal No. 1-Merger Proposal | | V-1 |
Proposal No. 2-Possible Adjournment of the Annual Meeting | | V-2 |
Proposal No. 3-Approval of the Amendment to our Certificate of Incorporation to Change the Name of our Company to Marina Biotech, Inc. | | V-3 |
Proposal No. 4-Election of Directors | | V-4 |
Proposal No. 5-Ratification of Appointment of Independent Registered Public Accounting Firm | | V-12 |
Proposal No. 6-Approval of an Amendment to our 2008 Stock Incentive Plan to Increase the Number of Shares Available Thereunder from 4,500,000 to 8,500,000 | | V-14 |
Proposal No. 7-Approval of an Amendment to our 2007 Employee Stock Purchase Plan to Increase the Number of Shares Available for Issuance Thereunder from 300,000 to 600,000 | | V-19 |
Proposal No. 8-Approval of a Change to our Capital Structure that Would Increase the Number of Authorized Shares of Common Stock from 90,000,000 to 180,000,000 and that Would Increase the Number of Authorized Shares of Preferred Stock from 100,000 to 200,000 | | V-23 |
Proposal No. 9-Approval of the Amendment to the Stockholder Rights Agreement | | V-26 |
Proposal No. 10-Approval of the Reduction and, in Certain Instances, the Elimination of the Floor Exercise Price of Common Stock Purchase Warrants Issued to Investors and the Placement Agent in April 2008, and the Issuance of Shares of Common Stock upon the Full Exercise of Such Warrants | | V-30 |
Proposal No. 11-Approval of the Elimination of the Floor Exercise Price of Common Stock Purchase Warrants Issued to Investors in January 2010 | | V-35 |
Section 16(A) Beneficial Ownership Reporting Compliance | | V-38 |
Summary Compensation Table | | V-38 |
2009 Outstanding Equity Awards at Fiscal Year-End Table | | V-40 |
Employment Agreements | | V-41 |
Option Repricings | | V-45 |
Payments Upon Termination or Change-in-Control | | V-45 |
2009 Potential Payments Upon Termination or Change In Control Table | | V-47 |
Compensation of Directors | | V-49 |
Transactions with Related Persons, Promoters and Certain Control Persons | | V-51 |
Report of the Audit Committee of the Board of Directors | | V-52 |
Equity Compensation Plan Information | | V-53 |
Householding of Proxy Materials | | V-54 |
Other Matters | | V-54 |
ii
| | | | |
| Annex A | | — | | Agreement and Plan of Merger |
| Annex B | | — | | Fairness Opinion of Canaccord Adams Inc. |
| Annex C | | — | | Audited Financial Statements of Cequent Pharmaceuticals, Inc. for the year ended December 31, 2009 |
| Annex D | | — | | Consent of Independent Auditor |
| Annex E | | — | | Unaudited Financial Statements of Cequent Pharmaceuticals, Inc. for the quarter ended March 31, 2010 |
iii
CHAPTER ONE — THE MERGER
QUESTIONS AND ANSWERS ABOUT THE MERGER
| A: | We and Cequent have entered into an Agreement and Plan of Merger, dated as of March 31, 2010, which is referred to as the merger agreement. The merger agreement contains the terms and conditions of the proposed business combination of our company and Cequent. Under the merger agreement, Calais Acquisition Corp., a wholly-owned subsidiary of our company, which is referred to as the merger sub, will merge with and into Cequent. This transaction is referred to as the merger. Cequent will then continue as a wholly-owned subsidiary of our company. |
At the effective time of the merger, each share of Cequent Series A-1 convertible preferred stock and common stock outstanding immediately prior to the effective time of the merger will be exchanged for shares of our common stock at an exchange ratio that implies a purchase price for Cequent common and preferred stockholders of approximately $44 million, plus an additional value of approximately $2 million to Cequent warrant and option holders, based on the 10-day volume-weighted average price of our common stock on The NASDAQ Global Market ending on March 31, 2010. This represents an approximate 56% equity ownership of the combined company for the stockholders of our company and an approximate 44% equity ownership of the combined company for the common and preferred stockholders of Cequent immediately following the effective time of the merger.
| Q: | Why are we proposing to merge with Cequent? |
| A: | We are proposing the merger because, among other things, we believe that the merger provides us with three key capabilities: (1) an additional RNAi drug discovery platform, i.e. TransKingdom RNAi for oral delivery of RNAi-based therapeutics for gastrointestinal diseases such as colon cancer and Crohn’s disease; (2) a clinical stage program in Familial Adenomatus Polyposis (FAP) which we believe we may be able to commercialize in or near 2014; and (3) an experienced and successful clinical and regulatory team who have advanced Cequent’s FAP program through U.S. Food and Drug Administration (FDA) and National Institutes of Health review pending Phase 1 human clinical testing. We also anticipate that the financial resources available to Cequent at the effective time of the merger will be sufficient to fund the operations of the combined company through December 2010. For a discussion of our reasons for the merger, please see the section entitled “Chapter One — The Merger — Reasons for the Merger” in this proxy statement. |
| Q: | Why am I receiving this Proxy Statement? |
| A: | You are receiving this proxy statement because you have been identified as a stockholder of our company as of the record date, and you are entitled to vote at the annual meeting. This document serves as a proxy statement of our company used to solicit proxies for the annual meeting. This proxy statement contains important information about the merger and the annual meeting, and you should read it carefully. |
| Q: | On what matters are our stockholders being asked to vote? |
| A: | In order to complete the merger, our stockholders must vote to approve the issuance of shares of common stock to Cequent stockholders in the merger. At the annual meeting, our stockholders are being asked to vote on the following items: |
| | • | | the issuance of shares of common stock pursuant to the merger agreement, described under “Chapter One — The Merger Agreement” on page I-52; |
| | • | | the adjournment of the annual meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes in favor of Proposal No. 1, Proposal No. 3 or Proposal No. 8; |
| | • | | the amendment to our certificate of incorporation to change the name of the company from MDRNA, Inc. to Marina Biotech, Inc.; |
I-1
| | • | | the election of six (6) persons to our Board of Directors, each to hold office until the 2011 annual meeting of stockholders and until their respective successors shall have been duly elected or appointed and qualify; |
| | • | | a proposal to ratify the appointment of KPMG LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2010; |
| | • | | a proposal to amend our 2008 Stock Incentive Plan to increase the number of shares available for issuance thereunder from 4,500,000 to 8,500,000; |
| | • | | a proposal to amend our 2007 Employee Stock Purchase Plan to increase the number of shares available for issuance thereunder from 300,000 to 600,000; |
| | • | | a proposal to change our capital structure by increasing the number of authorized shares of common stock from 90,000,000 to 180,000,000, and by increasing the number of authorized shares of preferred stock from 100,000 to 200,000; |
| | • | | a proposal to amend the Rights Agreement, dated February 22, 2000, between our company and American Stock Transfer & Trust Company as Rights Agent, as amended, to extend the term of the Rights Agreement from March 17, 2010 to March 17, 2013; |
| | • | | a proposal to amend the Amended and Restated Common Stock Purchase Warrants issued to investors and the placement agent in April 2008 to reduce and, in certain instances, eliminate the floor exercise price of such warrants, and to issue the additional shares of common stock that would be issuable upon full exercise of such warrants as a result thereof; |
| | • | | a proposal to amend the Common Stock Purchase Warrants issued to investors in January 2010 to eliminate the floor exercise price of such warrants; and |
| | • | | such other matters as may properly come before the annual meeting. |
| Q: | What will happen in the merger? |
| A: | In the merger, Cequent will become a wholly-owned subsidiary of our company. Based upon the outstanding shares of our common stock on March 31, 2010, and assuming the conversion of all of Cequent’s preferred stock on March 31, 2010, immediately following the completion of the merger, Cequent common and preferred stockholders will own approximately 44% of the combined company’s common stock and our stockholders will own approximately 56% of the combined company’s common stock. |
Based upon the outstanding shares of our common stock and assuming the exercise or conversion of all of our outstanding exercisable and non-exercisable warrants and stock options on March 31, 2010, and assuming the exercise or conversion of all of Cequent’s exercisable and non-exercisable warrants, stock options and preferred stock on March 31, 2010, immediately following the completion of the merger, Cequent securityholders would own approximately 37% of the combined company’s common stock and our securityholders would own approximately 63% of the combined company’s common stock. This number assumes the exercise for cash of all warrants and stock options. See “The Merger Agreement — Merger Consideration.”
| Q: | Is stockholder approval of the merger required by the rules of The NASDAQ Stock Market? |
| A: | Under NASDAQ Marketplace Rule 5635(a)(1), a company listed on NASDAQ is required to obtain stockholder approval prior to the issuance of common stock in connection with the acquisition of another company’s stock, if the number of shares of common stock to be issued is in excess of 20% of the number of shares of common stock then outstanding. The newly issued shares of our common stock to be issued in the merger exceed the 20% threshold under the NASDAQ Marketplace Rules. Thus, in order to ensure compliance with NASDAQ Marketplace Rule 5635(a)(1), we must obtain the approval of our stockholders for the issuance of the shares of our common stock in the merger. |
I-2
| Q: | When and where is the annual meeting? |
| A: | The annual meeting will take place on Wednesday, July 21, 2010 at 10:00 a.m., Eastern Time, at the offices of Pryor Cashman LLP, 7 Times Square, New York, New York 10036. |
| Q: | How does the Board of Directors recommend I vote? |
| A: | The Board recommends that stockholders vote “Yes” for the issuance of shares of our common stock pursuant to the merger agreement and for all of the other proposals to be voted on by stockholders at the annual meeting. After careful consideration, the Board of Directors has determined by unanimous vote the merger to be fair to our stockholders and in their best interests, and declared the merger advisable. The Board of Directors approved the merger agreement and recommends that stockholders approve the issuance of the shares pursuant to the merger agreement. |
| A: | You may vote by mail by completing, signing and dating your proxy card and returning it in the enclosed, postage-paid and addressed envelope. If you mark your voting instructions on the proxy card, your shares will be voted: |
| | • | | according to the best judgment of the proxy holders if a proposal comes up for a vote at the annual meeting that is not on the proxy card. |
If you return a signed card, but do not provide voting instructions, your shares will be voted:
| | • | | FOR the issuance of shares of MDRNA common stock in the merger; |
| | • | | FOR an adjournment of the meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes in favor of Proposal No. 1, Proposal No. 3 or Proposal No. 8; |
| | • | | FOR the amendment of our certificate of incorporation to change the name of our company to Marina Biotech, Inc.; |
| | • | | FOR the election of six (6) persons to our Board of Directors, each to hold office until the 2011 annual meeting of stockholders and until their respective successors shall have been duly elected or appointed and qualify; |
| | • | | FOR the ratification of the appointment of KPMG LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2010; |
| | • | | FOR the amendment of our 2008 Stock Incentive Plan to increase the number of shares available for issuance thereunder from 4,500,000 to 8,500,000; |
| | • | | FOR the amendment of our 2007 Employee Stock Purchase Plan to increase the number of shares available for issuance thereunder from 300,000 to 600,000; |
| | • | | FOR the change of our capital structure by increasing the number of authorized shares of common stock from 90,000,000 to 180,000,000, and by increasing the number of authorized shares of preferred stock from 100,000 to 200,000; |
| | • | | FOR the amendment to the Rights Agreement, dated February 22, 2000, between our company and American Stock Transfer & Trust Company as Rights Agent, as amended, to extend the term of the Rights Agreement from March 17, 2010 to March 17, 2013; |
| | • | | FOR the amendment to the Amended and Restated Common Stock Purchase Warrants issued to investors and the placement agent in April 2008 to reduce and, in certain instances, eliminate the floor exercise price of such warrants, and to issue the additional shares of common stock that would be issuable upon full exercise of such warrants as a result thereof; |
I-3
| | • | | FOR the amendment to the Common Stock Purchase Warrants issued to investors in January 2010 to eliminate the floor exercise price of such warrants; and |
| | • | | according to the best judgment of the proxy holders if a proposal properly comes up for a vote at the annual meeting that is not on the proxy card. |
If you are a stockholder of record, you may also vote by telephone at the toll-free number1-800-PROXIESor on the Internet atwww.voteproxy.com. See the instructions on your proxy card or voting instruction form.You are strongly encouraged to vote electronically if you are given that option.
| Q: | What do I do if I want to change my vote? |
| A: | You may send in a later-dated, signed proxy or proxy card to the Secretary of our company before the meeting or you can attend the meeting in person and vote. You may also revoke your proxy by sending a notice of revocation to the Secretary of our company at 3830 Monte Villa Parkway, Bothell, WA 98021, Attention: Secretary. If you voted by the Internet or telephone, you can submit a later vote using those same methods. |
| Q: | If my shares are held in “street name” by my broker, bank or other nominee, will my broker, bank or other nominee vote my shares for me? |
| A: | If you do not provide your broker, bank or nominee with instructions on how to vote your “street name” shares, your broker, bank or nominee will not be permitted to vote them on the matters that are to be considered at the annual meeting relating to the merger, the election of directors, the amendments to our 2008 Stock Incentive Plan and our 2007 Employee Stock Purchase Plan, the amendments to the common stock purchase warrants issued by our company in April 2008 and January 2010, and certain other non-routine matters. You should therefore be sure to provide your broker with instructions on how to vote your shares. |
If you wish to vote your shares in person, you must bring to the meeting a letter from the broker, bank or nominee confirming your beneficial ownership of the shares to be voted.
| Q: | What appraisal rights do stockholders have in connection with the merger? |
| A: | Under Delaware law, holders of our common stock do not have any right to an appraisal of the fair value of their shares in connection with the merger or any other proposal discussed in this proxy statement. |
| Q: | What happens if I do not return a proxy card or otherwise provide proxy instructions? |
| A: | The failure to return your proxy card or otherwise provide proxy instructions could be a factor in establishing a quorum for the annual meeting for purposes of approving the issuance of shares pursuant to the merger agreement or other actions sought to be taken, which is required to transact business at the meeting. |
| Q: | When do you expect the merger to be completed? |
| A: | We are working towards completing the merger as quickly as possible. We hope to complete the merger by the end of June 2010. However, the exact timing of completion of the merger cannot be determined yet because completion of the merger is subject to a number of conditions, as discussed in this proxy statement. |
| Q: | How many authorized but unissued shares of common stock will exist after the closing of the merger? |
| A: | Following the closing of the merger, and assuming the approval of Proposal No. 6 to increase the number of shares available for issuance under our 2008 Stock Incentive Plan to 8,500,000, the approval of Proposal |
I-4
| | No. 7 to increase the number of shares available for issuance under our 2007 Employee Stock Purchase Plan to 600,000, and the approval of Proposal No. 8 to increase the number of authorized shares of our common stock to 180,000,000, we anticipate that there will be approximately 69,000,000 shares of authorized but unissued common stock. |
In addition to approximately 87,247,103 issued and outstanding shares of common stock after the closing of the merger, we will be required to reserve approximately 28,389,646 shares for future issuance following the merger as follows: (i) approximately 8,319,731 shares for issuance upon the exercise of outstanding stock options; (ii) approximately 4,682,344 shares for the issuance of future stock options, restricted stock or other equity awards under our existing stock incentive plans, including our Employee Stock Purchase Plan; (iii) approximately 12,533,472 shares for issuance upon the exercise of outstanding warrants; (iv) approximately 50,944 shares for issuance upon the exercise of outstanding Cequent warrants to be assumed in connection with the merger and (v) approximately 2,803,155 shares for issuance upon the exercise of outstanding Cequent stock options assumed in connection with the merger.
| Q: | What are the federal income tax consequences of the merger? |
| A: | We expect the merger to be treated as a tax-free reorganization pursuant to Section 368(a) of the Internal Revenue Code of 1986, as amended, or the Code. If the merger is treated as a tax-free reorganization, generally the stockholders of Cequent, for federal income tax purposes, will recognize no gain or loss upon their receipt of common stock in the merger, except with respect to cash received by Cequent stockholders instead of fractional shares of common stock, or upon exercise of their appraisal rights. As a stockholder of our company, you will not incur any tax liability as a result of the merger. |
| Q: | Will there be any changes to my shares of MDRNA common stock as a result of the merger? |
| A: | No. All shares of MDRNA common stock will remain outstanding after the merger, and no changes will be made to the shares of MDRNA common stock currently outstanding as a result of the merger. |
| Q: | Should I send in my MDRNA stock certificates with my proxy card? |
| A: | No. Please DO NOT send your MDRNA common stock certificates with your proxy card. No changes will be made to shares of MDRNA common stock in the merger and you will not be asked to send your MDRNA stock certificates to anyone in connection with the merger. |
| Q: | Whom do I call if I have questions about the annual meeting or the merger? |
| A: | You may call MDRNA Investor Relations at (425) 908-3600. |
I-5
SUMMARY
This summary highlights selected information from this proxy statement and may not contain all of the information that is important to you. This summary discusses all of the material aspects of the merger. However, to understand the merger fully and for a more complete description of the legal terms of the merger, you should read this proxy statement and the documents we have referred you to carefully. See “Chapter Six — Additional Information for Stockholders — Where You Can Find More Information.” We have included page references to direct you to a more complete description of the topics presented in this summary.
The Companies
MDRNA, Inc.
3830 Monte Villa Parkway
Bothell, WA 98021
(425) 908-3600
We are a biotechnology company focused on the discovery, development and commercialization of pharmaceuticals based on RNA interference (“RNAi”). Our goal is to be the leader in RNAi therapeutics and improve human health through the development of RNAi-based compounds that provide superior therapeutic options for patients. Our team, of approximately 30 scientists, brings expertise in the discovery, evaluation and optimization of small interfering RNAs (“siRNAs”) as well as siRNA delivery. We have the requisite experience in the areas of RNAi, molecular and cellular biology, lipid, oligonucleotide and peptide chemistry, pharmacology and bioinformatics necessary to discover and develop tailored RNAi-based compounds designed to elicit specific therapeutic effects on a target-by-target basis. Our infrastructure provides for pre-clinical scale manufacturing of both siRNAs and delivery materials, the comprehensive analysis and optimization of these compounds both individually and as drug candidates, and the filing of Investigational New Drug Applications. In addition to our own, internally developed technologies, we strategically in-license and further develop RNAi- and delivery-related technologies, forming a single integrated drug discovery platform. In order to protect our innovations, which encompass a broad platform of both siRNA and delivery technologies, and the eventual drug products that emerge from that platform, we will aggressively continue to build upon our extensive and enabling intellectual property estate.
Calais Acquisition Corp.
3830 Monte Villa Parkway
Bothell, WA 98021
(425) 908-3600
Calais Acquisition Corp., which we refer to as merger sub, is a wholly-owned subsidiary of our company that was incorporated in Delaware on March 29, 2010. Merger sub does not engage in any operations and exists solely to facilitate the merger.
Cequent Pharmaceuticals, Inc.
One Kendall Square, Bldg. 700
Cambridge, MA 02139
(617) 995-7940
Cequent is pioneering the development of novel therapeutics to prevent and treat a wide range of human disorders – from inflammatory disease to cancer – based on Cequent’s proprietary technology, TransKingdom RNA interference (tkRNAi). Cequent has one clinical-stage program, one late preclinical program, and other assets currently under development. Cequent designed its tkRNAi technology as a therapeutic to deactivate specific disease-causing genes safely and effectively, using non-pathogenic bacteria as an engine to produce and
I-6
deliver RNAi directly into cells. tkRNAi is based on scientific research originating at the Institut Pasteur (Paris, France) and at the Beth Israel Deaconess Medical Center/Harvard Medical School. A privately held company based in Cambridge, Massachusetts, Cequent was incorporated on November 10, 2003 and commenced operations in November 2006.
The Merger Agreement (see page I-52)
The merger agreement is attached as Annex A to this proxy statement. You are encouraged to read the merger agreement as it is the legal document that governs the merger.
Merger Consideration (see page I-52)
Under the terms of the merger agreement, in exchange for Cequent’s outstanding shares of common stock and Series A-1 convertible preferred stock, we will issue such number of shares of our common stock equal to approximately 44% of the issued and outstanding shares of the common stock of the combined company, together with cash in lieu of fractional shares of common stock. In addition, each outstanding warrant to purchase Cequent common stock and each option exercisable for Cequent common stock will be assumed by us in the merger and will become exercisable for the same number of shares of common stock as would have been issuable with respect to Cequent shares issued upon the exercise of such warrant or option immediately prior to the closing of the merger and the exercise price will be adjusted in accordance with the terms and conditions of the merger agreement. Generally, the original term of each outstanding stock option and warrant to purchase Cequent common stock that is assumed by us in the merger will remain unchanged.
Risks Relating to the Merger (see page I-23)
In evaluating the issuance of shares of common stock in the merger, you should carefully read this proxy statement and especially consider the factors discussed in the section titled “Risk Factors,” starting on page I-23, for a description of risks relating to the merger.
Factors Considered by, and Recommendation of, the Board of Directors (see page I-33)
The Board of Directors has identified potential benefits of the merger that it believes will contribute to the success of the combined company, including the following:
| | • | | expanded research, development, commercialization and intellectual property expertise and capabilities; |
| | • | | the complementary nature of the businesses of our company and Cequent in the RNAi field, giving the combined company two validated RNAi Drug Discovery Platforms utilizing two distinct and proprietary delivery technologies which can be used for systemic, local and oral delivery; |
| | • | | the potential to leverage the combined technology expertise of our company and Cequent to provide enhanced research and development activities; |
| | • | | synergies associated with combining the skills and capabilities of the two companies; |
| | • | | the forward integration of our company with a biotherapeutic product-focused company such as Cequent to expand our oncology disease pipeline with a clinical stage Phase 1 program as well as expand our pipeline to include inflammatory disease programs with one pre-clinical program; |
| | • | | the potential to commercialize an RNAi-based therapeutic as early as 2014; |
| | • | | world class scientific advisors at the Board of Director and Scientific Advisory Board levels; |
| | • | | an expanded intellectual property estate with RNAi-based therapeutics using UsiRNA and TransKingdom construct technologies; |
I-7
| | • | | the opportunity for our stockholders to participate in a significantly larger, more diversified company, with greater share liquidity; |
| | • | | the potential for realizing annual cost savings through consolidation of administrative functions; |
| | • | | the combined company is anticipated to have sufficient financial resources to fund operations through approximately December 2010; and |
| | • | | the combined company being appropriately capitalized to further develop its clinical programs. |
Opinion of our Financial Advisor (see page I-35)
In connection with the merger, our Board of Directors received an opinion, dated March 31, 2010, from Canaccord Genuity Inc., formerly known as Canaccord Adams Inc., our financial advisor, as to the fairness, from a financial point of view and as of the date of such opinion, to our company of the consideration to be paid in the merger. The full text of Canaccord’s opinion is attached to this proxy statement as Annex B and is incorporated into this proxy statement by reference. Holders of common stock are encouraged to read Canaccord’s opinion carefully in its entirety for a description of the procedures followed, assumptions made, matters considered and qualifications and limitations on the review undertaken by Canaccord in connection with its opinion.
Canaccord’s opinion was addressed to the Board of Directors, and was only one of many factors considered by the Board of Directors in its evaluation of the merger and only addresses the fairness from a financial point of view to our company. Canaccord’s opinion does not address the merits of the underlying decision by us to engage in the merger or related transactions or the relative merits of the merger or related transactions as compared to any other transaction or business strategy in which we might engage and is not intended to, and does not, constitute a recommendation to any stockholder as to how such stockholder should vote or act with respect to the merger or related transactions or any other transaction or business strategy in which we might engage.
Recommendation of the Board of Directors
The Board of Directors has considered all of the proposals that are set forth in this proxy statement, and recommends that our stockholders vote FOR the issuance of the shares pursuant to the merger agreement;FOR the adjournment of the annual meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes in favor of Proposal No. 1, Proposal No. 3 or Proposal No. 8;FOR the amendment of our certificate of incorporation to change the name of our company to Marina Biotech, Inc.;FOR the election of six (6) persons to our Board of Directors, each to hold office until the 2011 annual meeting of stockholders and until their respective successors shall have been duly elected or appointed and qualify;FOR the ratification of KPMG as our independent registered public accounting firm for the fiscal year ending December 31, 2010;FOR the amendment of our 2008 Stock Incentive Plan to increase the number of shares available for issuance thereunder from 4,500,000 to 8,500,000;FOR the amendment of our 2007 Employee Stock Purchase Plan to increase the number of shares available for issuance thereunder from 300,000 to 600,000;FOR the change of our capital structure by increasing the number of authorized shares of common stock from 90,000,000 to 180,000,000, and by increasing the number of authorized shares of preferred stock from 100,000 to 200,000;FOR the amendment to the Rights Agreement, dated February 22, 2000, between our company and American Stock Transfer & Trust Company as Rights Agent, as amended, to extend the term of the Rights Agreement from March 17, 2010 to March 17, 2013;FOR the amendment to the Amended and Restated Common Stock Purchase Warrants issued to investors and the placement agent in April 2008 to reduce and, in certain instances, eliminate the floor exercise price of such warrants, and to issue the additional shares of common stock that would be issuable upon full exercise of such warrants as a result thereof; andFOR the amendment to the Common Stock Purchase Warrants issued to investors in January 2010 to eliminate the floor exercise price of such warrants.
I-8
Directors and Management of MDRNA Following the Merger (page I-53)
At the effective time of the merger, we will enter into a stockholders’ agreement with certain of the principal stockholders of Cequent, which we refer to collectively as the designators. As required by the stockholders’ agreement, upon completion of the merger we will expand the size of our Board of Directors from six to seven, and three of our existing directors will resign from their positions on the Board. Then, if the Nominating and Corporate Governance Committee of our Board of Directors deems, in the exercise of its reasonable good faith discretion, the persons designated by the designators to be qualified, we will appoint the three directors designated by the designators to fill the vacancies and to serve on the Board until our 2011 Annual Meeting of Stockholders.
It is expected that Peter D. Parker, the President, Chief Executive Officer, and a member of the Board of Directors of Cequent, Dr. Chiang Li, a member of the Board of Directors of Cequent and a Founder of Cequent, and Dr. Michael Taylor, a member of the Board of Directors of Cequent, will be designated to serve on the Board of Directors of the combined company. The three directors to be designated by the designators will select a seventh member of the Board of Directors, which person shall be independent under the rules of The NASDAQ Stock Market and shall serve as the Chairman of the Board.
The Board of Directors of the combined company following the merger will also include two members from the existing Board of our company, who are expected to be James M. Karis and Gregory Sessler. J. Michael French will serve as the President, Chief Executive Officer and as a member of the Board of Directors of the combined company.
Consideration to Cequent Stockholders
Subject to the terms and conditions of the merger agreement, at the effective time of the merger, each share of Cequent Series A-1 convertible preferred stock and common stock outstanding immediately prior to the merger will be exchanged for shares of our common stock at an exchange ratio that implies a purchase price for Cequent common and preferred stockholders of approximately $44 million, plus an additional value of approximately $2 million to Cequent warrant and option holders, based on the 10-day volume-weighted average price of our common stock on The NASDAQ Global Market ending on March 31, 2010. This represents an approximate 56% equity ownership of the combined company for our stockholders and an approximate 44% equity ownership of the combined company for the common and preferred stockholders of Cequent immediately following the merger.
In addition, each stock option or warrant exercisable for shares of Cequent common stock that is outstanding at the effective time of the merger will be assumed by us and converted into a stock option or warrant to purchase the number of shares of common stock that the holder would have received if such holder had exercised such stock option or warrant for shares of Cequent common stock prior to the merger and exchanged such shares for shares of our common stock in accordance with the terms and provisions of the merger agreement.
Per-Share Market Price and Dividend Information (see page I-41)
Our common stock is currently listed on The Nasdaq Global Market. Cequent is a private company, and as such, there is no trading market for its common stock. On March 31, 2010, the last full trading day before we and Cequent issued a press release announcing the merger agreement, the closing price of our common stock as reported on The Nasdaq Global Market was $1.10 per share. See “Per Share Market Price and Dividend Information” on page I-41.
Listing of MDRNA Common Stock
The shares of our common stock to be issued in the merger will be listed on The Nasdaq Global Market under the symbol “MRNA”.
I-9
Registration of Merger Consideration (see page I-72)
Upon the closing of the merger, we will enter into a Registration Rights Agreement with certain of the principal stockholders of Cequent pursuant to which we will agree to file, not later than 45 calendar days following the closing date of the merger, a shelf registration statement with the Securities and Exchange Commission relating to the offer and sale of all of the shares of our common stock issued to such stockholders in the merger, and to cause such shelf registration statement to be declared effective within 90 calendar days of the closing date of the merger. We will also grant demand and piggy-back registration rights to such stockholders.
Ownership of MDRNA After the Merger
In the merger, Cequent will become a wholly-owned subsidiary of our company. Based upon the outstanding shares of our common stock on March 31, 2010, and assuming the conversion of all of Cequent’s preferred stock on March 31, 2010, immediately following the completion of the merger, Cequent common and preferred stockholders will own approximately 44% of the combined company’s common stock and our stockholders will own approximately 56% of the combined company’s common stock.
Based upon the outstanding shares of our common stock and assuming the exercise or conversion of all of our outstanding exercisable and non-exercisable warrants and stock options on March 31, 2010, and assuming the exercise or conversion of all of Cequent’s exercisable and non-exercisable warrants, stock options and preferred stock on March 31, 2010, immediately following the completion of the merger, Cequent securityholders would own approximately 37% of the combined company’s common stock and our securityholders would own approximately 63% of the combined company’s common stock. This number assumes the exercise for cash of all warrants and stock options.
For a more detailed discussion of the securities to be issued by us to the securityholders of Cequent in the merger, see “— The Merger — The Merger Agreement — Merger Consideration” on page I-52. Our stockholders will continue to own their existing shares after the merger.
Vote Necessary to Approve the Proposals
The six nominees for director receiving the highest number of affirmative votes will be elected as directors. The vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote, is required for approval of Proposal Nos. 1, 2, 4, 5, 6, 7, 9, 10 and 11. The vote of a majority of the issued and outstanding shares of our common stock entitled to vote is required for approval of Proposal Nos. 3 and 8.
Lock Up Agreements (see page I-67)
Upon the consummation of the merger certain principal stockholders of Cequent, who held approximately 64% of the issued and outstanding shares of Cequent common stock on a fully-diluted basis as of the date of the merger agreement, will enter into lock-up agreements pursuant to which they will agree that they shall not, without our prior approval, offer, pledge, sell, contract to sell, grant, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock received in the merger during the period commencing on the effective date of the merger and ending on the earlier of (A) one hundred and eighty (180) days after the effective date of the merger or (B) thirty (30) days following the closing of an equity financing by our company for the issuance of shares of equity or securities convertible into or exchangeable or exercisable for shares of equity of our company which, when added together with all other equity financings from and after the date of execution of the merger agreement, results in aggregate gross proceeds to us of at least $10 million.
Prior to the execution of the merger agreement, our directors and executive officers entered into lock-up agreements providing for similar restrictions on sales and other dispositions of common stock commencing upon the signing of the merger agreement.
I-10
Interest of Certain of our Executive Officers and Directors in the Merger (see page I-51)
Certain of our directors and executive officers may have interests in the merger that differ from, are in addition to, or conflict with interests of our stockholders. For example, it is anticipated that all of our executive officers will continue in their current positions with the combined company, and that three of the current members of our Board of Directors will continue to serve on the board of directors of the combined company. The Board has also determined that all outstanding common stock purchase options held by the three current members of the Board who will not continue to serve on the Board of the combined company will vest immediately upon the consummation of the merger, and that all of such options will remain exercisable until the earlier of the four-year anniversary of the effective time and the expiry of such options. You should be aware of these interests when considering the recommendation of our Board of Directors that you vote in favor of the issuance of shares of our common stock pursuant to the merger agreement.
Waivers of Change of Control (see page I-51)
On March 31, 2010, each of J. Michael French, our President and Chief Executive Officer, Peter S. Garcia, our Chief Financial Officer and Secretary, and Barry Polisky, Ph.D., our Chief Scientific Officer, entered into waiver agreements with us pursuant to which they agreed that the merger will not constitute a “change of control” for purposes of their employment agreements or for purposes of any plan or non-plan equity awards that they have received from us, and have thereby waived the enhanced protections provided under their employment agreements with respect to the merger. On April 22, 2010, Alison D. Silva, the Vice President, Drug Development of Cequent, entered into a similar waiver agreement with Cequent.
Treatment of Cequent Stock Options and Warrants (see page I-53)
We will assume all stock options exercisable for Cequent common stock and warrants to purchase Cequent common stock that are outstanding at the effective time of the merger. Cequent’s outstanding stock options and warrants will be converted into stock options and warrants, respectively, of our company, which will be exercisable for, upon their timely exercise, the number of shares of our common stock that the holder of such options or warrants would have received if such holder had exercised such warrants or options for shares of Cequent common stock prior to the merger. The exercise price of the assumed Cequent stock options and warrants will be adjusted to give effect to the merger.
Accounting Treatment
We will account for the merger as a purchase of the business, which requires the assets and liabilities of Cequent to be measured and recorded at their fair values as of the date of the merger. The results of operations of Cequent will be included in our Consolidated Statement of Operations from and after the effective time that control of Cequent transfers to our company, which will occur on the date of the merger.
Conditions to the Completion of the Merger (see page I-63)
The completion of the merger depends upon the satisfaction or waiver of a number of conditions, including the following:
| | • | | The stockholders of our company approve Proposal No. 1 relating to the issuance of common stock pursuant to the merger agreement and Proposal No. 8 relating to the amendment of our certificate of incorporation to increase the number of authorized shares of common stock to 180,000,000; |
| | • | | The stockholders of Cequent approve the merger and the transactions contemplated by the merger agreement, which approval was obtained on April 2, 2010; |
I-11
| | • | | The representations and warranties of our company contained in the merger agreement shall be true, complete and accurate in all material respects as of the date when made and at and as of the closing date of the merger as though such representations and warranties were made at and as of such date (except to the extent such representations are made as of a date specific); |
| | • | | We, Cequent and merger sub shall have performed and complied in all material respects with all agreements, obligations and conditions required by the merger agreement to be performed or complied with prior to the closing of the merger; |
| | • | | No suit, action, investigation, inquiry or other proceeding by any governmental body or other person or legal or administrative proceeding shall have been instituted or threatened, which seeks to restrain, enjoin, prevent the consummation of or otherwise affect the transactions contemplated by the merger agreement or which questions the validity or legality of the transactions contemplated thereby; |
| | • | | From December 31, 2009 to the closing date set forth in the merger agreement, neither we nor Cequent shall have suffered any material adverse event; |
| | • | | Cequent shall have received in writing any and all consents, approvals, authorizations, exemptions or waivers required by the merger agreement; |
| | • | | Cequent shall have delivered to us, or caused to be delivered to us, the other items required to be delivered to us in accordance with the merger agreement; |
| | • | | Cequent shall have available an amount in cash equal to (i) $5,100,000, minus (ii) the amount of Cequent’s ordinary course operating expenses from June 2, 2010 through the closing date of the merger; plus (iii) the difference of $3,000,000 and the aggregate principal amount loaned to us by Cequent under the loan agreement; |
| | • | | The shares of our common stock issuable in the merger shall have been approved for listing on The NASDAQ Global Market; |
| | • | | We shall have delivered to Cequent, or caused to be delivered to Cequent, the other items required to be delivered to Cequent in accordance with the merger agreement; |
| | • | | We and merger sub shall have furnished to Cequent all consents, approvals, authorizations, exemptions or waivers required by the merger agreement; |
| | • | | We shall have executed the Registration Rights Agreement; and |
| | • | | We shall have executed the Stockholders’ Agreement. |
The parties to the merger agreement do not anticipate that any material conditions to the merger will be waived by either party. However, if a material condition is waived, the parties may, depending on the specific condition being waived, delay the stockholder votes and circulate revised proxy materials in order to give stockholders the opportunity to consider the effect of the condition being waived. Notwithstanding the foregoing, the conditions requiring the approval of each company’s stockholders cannot be waived.
Regulatory Approvals (see page I-40)
We are not aware of any government regulatory approval required to be obtained with respect to the consummation of the merger, except for the filing of a certificate of merger with the office of the Secretary of State of the State of Delaware, and compliance with all applicable state securities laws regarding the offering and issuance of the merger shares.
I-12
Termination of the Merger Agreement (see page I-65)
We and Cequent can jointly agree to terminate the merger agreement at any time. Either party may terminate the merger agreement if, subject to certain exceptions set forth in the merger agreement, the merger has not been completed by August 31, 2010. In addition, we and Cequent will have the termination rights set forth below:
We may terminate the merger agreement if:
| | • | | the closing of the merger shall fail to occur by reason of the failure of any condition precedent to our obligation to close set forth in the merger agreement (unless the failure results from a breach by us of any representation, warranty or covenant contained in the merger agreement); |
| | • | | stockholders holding more than 10% of the outstanding shares of Cequent common stock exercise their appraisal rights in accordance with Section 262 of the Delaware General Corporation Law; |
| | • | | the closing price of our common stock on The NASDAQ Global Market is in excess of $1.437 for any ten (10) consecutive trading days following the date of the merger agreement; |
| | • | | Cequent is in material breach of the merger agreement and such breach is not cured within a ten (10) day cure period following written notice; |
| | • | | there shall have occurred any material adverse effect with respect to Cequent since the date of the merger agreement; or |
| | • | | the required approval of the stockholders of our company or Cequent contemplated by the merger agreement shall not have been obtained by reason of the failure to obtain the requisite vote upon a vote taken at a meeting of stockholders convened therefor or at any adjournment thereof. |
Cequent may terminate the merger agreement if:
| | • | | the closing of the merger shall not have occurred by reason of the failure of any condition precedent to Cequent’s obligation to close set forth in the merger agreement (unless the failure results primarily from a breach by Cequent of any representation, warranty or covenant contained in the merger agreement); |
| | • | | the closing price of our common stock on The NASDAQ Global Market is below $0.8622 for any ten (10) consecutive trading days following the date of the merger agreement; |
| | • | | our common stock is no longer listed on The NASDAQ Global Market or The NASDAQ Capital Market; |
| | • | | we are in material breach of the merger agreement and such breach is not cured within a ten (10) day period following written notice; |
| | • | | there shall have occurred any material adverse effect with respect to our company since the date of the merger agreement; or |
| | • | | the required approval of the stockholders of our company or Cequent contemplated by the merger agreement shall not have been obtained by reason of the failure to obtain the requisite vote upon a vote taken at a meeting of stockholders convened therefor or at any adjournment thereof. |
Effect of Termination (see page I-65)
If either we, on the one hand, or Cequent, on the other hand, terminate the merger agreement:
| | • | | each of our company, merger sub and Cequent will redeliver to the party furnishing the same or destroy all documents, work papers and other material of any other party relating to the transactions contemplated by the merger agreement, whether so obtained before or after the execution of the merger agreement; |
I-13
| | • | | neither Cequent nor our company, nor merger sub shall make or issue, or cause to be made or issued, any announcement or written statement concerning termination of the merger agreement or the transactions contemplated thereby for dissemination to the general public without the prior written consent of the other parties except as required by law or legal process; and |
| | • | | the merger agreement shall become wholly void and of no force or effect, without any liability or further obligation on the part of Cequent, our company or merger sub or any director, officer, or principal thereof, except for liabilities arising from a breach of the merger agreement prior to termination. |
In addition, if Cequent terminates the merger agreement due to the fact that the required approval of our stockholders contemplated by the merger agreement shall not have been obtained by reason of the failure to obtain the requisite vote upon a vote taken at a meeting of our stockholders convened therefor or at any adjournment thereof, we shall pay to Cequent, on or prior to December 31, 2010, a termination fee equal to the lesser of (i) $500,000 and (ii) Cequent’s reasonable and actual out-of-pocket expenses (including, without limitation, all fees and expenses of counsel, accountants, investment bankers, experts and consultants to Cequent) incurred by Cequent in connection with or related to the authorization, preparation, negotiation, execution and performance of the merger agreement and the transactions contemplated thereby. In no event shall we be required to pay the termination fee if, immediately prior to the termination of the merger agreement, Cequent was in material breach of its obligations under the merger agreement. If we do not pay the termination fee when due, then the termination fee shall be added to the outstanding principal amount of the term note discussed under “Loan Agreement” immediately below, shall be deemed principal under such term note, and shall bear interest from the date thereof until the date when paid at a rate equal to 4% per annum plus the per annum rate otherwise payable under the term note.
Loan Agreement (see page I-68)
Concurrently and in connection with the execution of the merger agreement, we and Cequent entered into a Loan Agreement, which we refer to as the loan agreement, pursuant to which, among other things, Cequent shall extend one or more term loans to us in the aggregate principal amount of up to $3 million to fund our operations prior to the merger. On April 30, 2010, Cequent loaned $1 million to us under the loan agreement as the initial draw-down under the loan agreement and on May 31, 2010, Cequent loaned an additional $1 million to us. The term loans are evidenced by a secured promissory note, which we refer to as the term note, issued by us to Cequent, which bears interest absent an event of default, at a rate of ten percent per annum. We shall repay the principal and interest of the term loans in three equal consecutive monthly installments, commencing on August 15, 2010 and continuing on the 15th day of each month thereafter through and including October 15, 2010. Notwithstanding the foregoing, if the merger is consummated prior to August 15, 2010, then, on the closing date of the merger, we shall not owe to any third party any obligations with respect to the term loans, including, without limitation, the then outstanding principal balance of the term loans and any interest then accrued but unpaid thereon. We may prepay any term loan in whole or in part without premium or penalty.
If we do not pay the termination fee discussed under the section entitled “Effect of Termination” immediately above when due, then the termination fee shall be added to the outstanding principal amount of the term note, shall be deemed principal under the term note, and shall bear interest from the date thereof until the date when paid at a rate equal to 4% per annum plus the per annum rate otherwise payable under the term note.
Loan Warrants (see page I-70)
We agreed in the loan agreement to issue to Cequent on or about the date of each term loan a warrant to purchase shares of common stock, with each such warrant to be exercisable for a number of shares of common stock equal to sixty-five percent (65%) of the principal amount of the term loan being made on such date divided by $1.1496.
I-14
The loan warrants are only exercisable upon the occurrence of both of the following: (x) the merger is not consummated (other than by reason of Cequent’s material breach of the merger agreement), and (y) the occurrence of an Acquisition Transaction (as defined below) prior to the one year anniversary of the termination of the merger agreement. Our obligations and the rights of Cequent under each loan warrant will be effective upon the issuance of such loan warrants and will survive payment of the term loans and our other obligations to Cequent under the loan agreement and the documents related thereto, and the termination of the loan agreement.
An Acquisition Transaction is any one of the following:
| | (1) | a merger or consolidation in which: |
| | • | | our company is a constituent party, or |
| | • | | a subsidiary of our company is a constituent party, |
and either (A) we issue shares of our capital stock pursuant to such merger or consolidation representing 40% or more of our outstanding capital stock, or (B) as a result of such merger or consolidation of a subsidiary, our company’s ownership interest in the surviving entity is reduced by 40% or more;
| | (2) | the sale, lease, transfer, exclusive out-license or other disposition, in a single transaction or series of related transactions, by our company or any subsidiary of our company of 40% or more of the assets or intellectual property of our company and its subsidiaries taken as a whole, or the sale or disposition (whether by merger or otherwise) of one or more subsidiaries of our company if 40% or more of the assets of our company and its subsidiaries taken as a whole are held by such subsidiary or subsidiaries, except where such sale, lease, transfer, exclusive out-license or other disposition is to a wholly-owned subsidiary; or |
| | (3) | the acquisition by a person of beneficial ownership (as defined under Rule 13(d) of the Exchange Act) of equity interests representing a 40% or greater voting interest in our company or a tender offer or exchange offer that, if consummated, would result in any person or group (as defined in Rule 13(d) of the Exchange Act) beneficially owning equity interests representing a 40% or greater economic or voting interest in our company. |
Approval of a Change to Our Capital Structure to Increase the Number of Authorized Shares of Common Stock to 180,000,000 (see Proposal No. 8 beginning on page V-23)
In connection with the merger, you are being asked to approve an amendment to our amended and restated certificate of incorporation to increase the number of authorized shares of our common stock from 90,000,000 to 180,000,000. The approval of this proposal is necessary so that we will have a sufficient number of shares of common stock available to issue to the holders of Cequent common stock in the merger, and is a condition to the obligations of the parties under the merger agreement.
Approval of an Amendment to our Certificate of Incorporation to change the name of our company to Marina Biotech, Inc. (see Proposal No. 3 beginning on page V-3)
In connection with the merger, and in order to better reflect the business and new identity of the combined company following the merger, you are being asked to approve an amendment to our amended and restated certificate of incorporation to change the name of our company from MDRNA, Inc. to Marina Biotech, Inc.
Other Business to be Conducted at the Annual Meeting
As this will be the annual meeting of our stockholders, you will also be asked to vote upon various other matters in addition to the merger, including (i) the adjournment of the annual meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes in favor of Proposal No. 1, Proposal No. 3 or
I-15
Proposal No. 8; (ii) the amendment of our certificate of incorporation to change the name of our company to Marina Biotech, Inc.; (iii) the election of six (6) persons to our Board of Directors, each to hold office until the 2011 annual meeting of stockholders and until their respective successors shall have been duly elected or appointed and qualify; (iv) the ratification of KPMG as our independent registered public accounting firm for the fiscal year ending December 31, 2010; (v) the amendment of our 2008 Stock Incentive Plan to increase the number of shares available for issuance thereunder from 4,500,000 to 8,500,000; (vi) the amendment of our 2007 Employee Stock Purchase Plan to increase the number of shares available for issuance thereunder from 300,000 to 600,000; (vii) the change of our capital structure by increasing the number of authorized shares of common stock from 90,000,000 to 180,000,000, and by increasing the number of authorized shares of preferred stock from 100,000 to 200,000; (viii) the amendment to the Rights Agreement, dated February 22, 2000, between our company and American Stock Transfer & Trust Company as Rights Agent, as amended, to extend the term of the Rights Agreement from March 17, 2010 to March 17, 2013; (ix) the amendment to the Amended and Restated Common Stock Purchase Warrants issued to investors and the placement agent in April 2008 to reduce and, in certain instances, eliminate the floor exercise price of such warrants, and to issue the additional shares of common stock that would be issuable upon full exercise of such warrants as a result thereof; (x) the amendment to the Common Stock Purchase Warrants issued to investors in January 2010 to eliminate the floor exercise price of such warrants; and (xi) other business if properly presented.
I-16
SELECTED HISTORICAL AND PRO FORMA FINANCIAL DATA
How the Financial Statements Were Prepared
The unaudited pro forma condensed combined consolidated statements of operations and unaudited pro forma condensed combined consolidated balance sheets were prepared by combining the historical amounts of each company and the addition of pro forma adjustments related to accounting for the merger. The companies may have performed differently had they been combined in the past. You should not rely on the unaudited pro forma condensed combined consolidated financial information as being indicative of the historical results that we would have had or the future results that the combined company will experience after the merger. See “Unaudited Pro Forma Condensed Combined Consolidated Financial Statements” on page I-42.
We are providing the following information to aid you in your analysis of the financial aspects of the merger. This information is only a summary and should be read in conjunction with:
| | • | | Our consolidated financial statements and related notes, and our “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” contained in our Annual Report on Form 10-K for the year ended December 31, 2009 and in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2010, which are incorporated herein by reference; and |
| | • | | Cequent’s financial statements and related notes, which are attached as Annex C and Annex E to this proxy statement, and its “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, which are included under the heading “Chapter Three — Other Information Regarding Cequent — Business of Cequent” beginning on page III-1. |
Merger-Related Expenses
We estimate that merger-related fees and expenses, consisting primarily of fees and expenses of investment bankers, attorneys, accountants, financial printing, SEC filing fees and other related charges, will be approximately $1.5 million for the merger. These costs will be expensed as incurred. See “Notes to Unaudited Pro Forma Condensed Combined Consolidated Financial Statements” on page I-46.
Periods Covered
The unaudited pro forma condensed combined consolidated balance sheet as of March 31, 2010 gives effect to our merger with Cequent as if it had occurred on that date. The unaudited pro forma condensed combined consolidated statements of operations for the quarter ended March 31, 2010 and the year ended December 31, 2009 give effect to our merger with Cequent as if it had occurred on January 1, 2009.
I-17
SELECTED HISTORICAL CONSOLIDATED FINANCIAL DATA OF MDRNA
You should read the following tables in conjunction with our financial statements and related notes and our “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” contained in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2010 and our Annual Report on Form 10-K for the year ended December 31, 2009. Historical results are not necessarily indicative of the results to be expected in the future.
The statement of operations data for the years ended December 31, 2008 and 2009 and the balance sheet data as of December 31, 2008 and 2009 have been derived from our audited financial statements contained in our Annual Report on Form 10-K for the year ended December 31, 2009. Our consolidated financial statements have been audited by KPMG LLP, independent registered public accounting firm. KPMG LLP’s report on the consolidated financial statements for the year ended December 31, 2009 includes an explanatory paragraph which describes an uncertainty about our ability to continue as a going concern. The balance sheet data as of December 31, 2005, 2006 and 2007 and the statement of operations data for the years ended December 31, 2005, 2006 and 2007 are derived from audited financial statements not included or incorporated by reference in this proxy statement. The statement of operations data for the three months ended March 31, 2010 and 2009, and the balance sheet data as of March 31, 2010 have been derived from unaudited financial statements contained in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2010.
MDRNA, INC. AND SUBSIDIARIES
Consolidated Statements of Operations Data:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended
March 31, 2010
(Unaudited) | | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | |
| | | | | | | | | (In thousands, except per share data) | |
Revenue | | $ | 184 | | | $ | 14,151 | | | $ | 14,732 | | | $ | 2,609 | | | $ | 18,137 | | | $ | 28,490 | | | $ | 7,449 | |
Operating expenses | | | 6,184 | | | | 6,356 | | | | 25,425 | | | | 61,551 | | | | 72,668 | | | | 57,807 | | | | 41,250 | |
Income (loss) from operations | | | (6,000 | ) | | | 7,795 | | | | (10,693 | ) | | | (58,942 | ) | | | (54,531 | ) | | | (29,317 | ) | | | (33,801 | ) |
Other income (expense), net | | | (3,490 | ) | | | (514 | ) | | | 2,647 | | | | (278 | ) | | | 2,159 | | | | 2,149 | | | | 1,638 | |
Loss before cumulative effect of change in accounting principle | | | (9,490 | ) | | | 7,281 | | | | (8,046 | ) | | | (59,220 | ) | | | (52,372 | ) | | | (27,168 | ) | | | (32,163 | ) |
Cumulative effect of change in accounting principle | | | — | | | | — | | | | — | | | | — | | | | — | | | | 291 | | | | — | |
Net income (loss) | | $ | (9,490 | ) | | $ | 7,281 | | | $ | (8,046 | ) | | $ | (59,220 | ) | | $ | (52,372 | ) | | $ | (26,877 | ) | | $ | (32,163 | ) |
Net income (loss) per common share — basic and diluted: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) before cumulative effect of change in accounting principle | | $ | (0.20 | ) | | $ | 0.23 | | | $ | (0.21 | ) | | $ | (2.01 | ) | | $ | (2.10 | ) | | $ | (1.28 | ) | | $ | (1.72 | ) |
Cumulative effect of change in accounting principle | | | — | | | | — | | | | — | | | | — | | | | — | | | | 0.01 | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) per common share — basic and diluted | | $ | (0.20 | ) | | $ | 0.23 | | | $ | (0.21 | ) | | $ | (2.01 | ) | | $ | (2.10 | ) | | $ | (1.27 | ) | | $ | (1.72 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Shares used in computing net income (loss) per share — basic and diluted | | | 47,328 | | | | 32,243 | | | | 37,457 | | | | 29,529 | | | | 24,995 | | | | 21,218 | | | | 18,719 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
I-18
Consolidated Balance Sheet Data:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | March 31,
2010 | | | As of December 31, | |
| | | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | |
| | | (Unaudited) | | | (In thousands) | |
Cash, cash equivalents, restricted cash and investments | | $ | 3,786 | | | $ | 1,746 | | | $ | 3,352 | | | $ | 41,573 | | | $ | 50,993 | | | $ | 59,909 | |
Property and equipment, net | | | 4,258 | | | | 4,569 | | | | 7,844 | | | | 15,004 | | | | 15,444 | | | | 8,173 | |
Total assets | | | 8,597 | | | | 7,229 | | | | 13,137 | | | | 61,616 | | | | 73,832 | | | | 72,953 | |
Total current liabilities | | | 5,309 | | | | 5,130 | | | | 12,524 | | | | 13,568 | | | | 14,725 | | | | 9,178 | |
Fair value liability for price adjustable warrants | | | 11,214 | | | | 7,243 | | | | — | | | | — | | | | — | | | | — | |
Total liabilities | | | 18,120 | | | | 14,115 | | | | 16,396 | | | | 22,396 | | | | 30,496 | | | | 17,386 | |
Accumulated deficit | | | (272,507 | ) | | | (263,017 | ) | | | (254,085 | ) | | | (194,865 | ) | | | (142,493 | ) | | | (115,616 | ) |
Total stockholders’ equity (deficit) | | $ | (9,523 | ) | | $ | (6,886 | ) | | $ | (3,259 | ) | | $ | 39,220 | | | $ | 43,336 | | | $ | 55,567 | |
I-19
SELECTED HISTORICAL FINANCIAL DATA OF CEQUENT
You should read the following tables in conjunction with Cequent’s financial statements and related notes attached as Annex C and Annex E to this proxy statement, and Cequent’s “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” which are included under the heading “Business of Cequent — Management’s Discussion and Analysis of Financial Condition and Results of Operations” beginning on page III-10. Historical results are not necessarily indicative of the results to be expected in the future.
The statement of operations data for the period from November 10, 2003 (inception) through December 31, 2009 and the years ended December 31, 2008 and 2009 and the balance sheet data as of December 31, 2008 and 2009 have been derived from Cequent’s audited financial statements contained in Annex C to this proxy statement. Cequent’s financial statements have been audited by Wolf & Company, P.C. independent registered public accounting firm. Wolf & Company’s report on the financial statements for the year ended December 31, 2009 is included elsewhere herein. The data should be read in conjunction with the financial statements, related notes, and other financial information included in Annex C to this proxy statement. The statement of operations data for the three months ended March 31, 2010 and 2009, and the period from November 10, 2003 (inception) to March 31, 2010, and the balance sheet data as of March 31, 2010, have been derived from unaudited financial statements included as Annex E to this proxy statement.
Cequent Pharmaceuticals, Inc.
(A Development Stage Company)
Statement of Operations Data:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended
March 31,
(Unaudited) | | | Period from November 10,
2003 (Inception) through
March 31, 2010
(Unaudited) | | | Year Ended
December 31, | | | Period from November 10,
2003 (Inception) through
December 31, 2009 | |
| | | 2010 | | | 2009 | | | | 2009 | | | 2008 | | |
| | | (In thousands) | |
Revenue | | $ | 144 | | | $ | 141 | | | $ | 1,241 | | | $ | 581 | | | $ | 391 | | | $ | 1,097 | |
Operating expenses | | | 1,055 | | | | 1,532 | | | | 18,797 | | | | 5,812 | | | | 6,412 | | | | 17,743 | |
Loss from operations | | | (911 | ) | | | (1,391 | ) | | | (17,556 | ) | | | (5,231 | ) | | | (6,021 | ) | | | (16,646 | ) |
Other income (expense), net | | | — | | | | 7 | | | | 384 | | | | (24 | ) | | | 146 | | | | 384 | |
Net loss | | $ | (911 | ) | | $ | (1,384 | ) | | | (17,172 | ) | | $ | (5,256 | ) | | $ | (5,875 | ) | | $ | (16,261 | ) |
Balance Sheet Data:
| | | | | | | | | | | | |
| | | March 31, 2010
(Unaudited) | | | As of December 31, | |
| | | | 2009 | | | 2008 | |
| | | (In thousands) | |
Cash and cash equivalents | | $ | 2,11 | 6 | | $ | 3,214 | | | $ | 5,581 | |
Property and equipment, net | | | 36 | 7 | | | 417 | | | | 574 | |
Total assets | | | 2,56 | 0 | | | 3,713 | | | | 6,328 | |
Total current liabilities | | | 85 | 4 | | | 1,053 | | | | 1,081 | |
Total liabilities | | | 1,55 | 9 | | | 1,907 | | | | 2,525 | |
Deficit accumulated during the development stage | | | (17,79 | 7) | | | (16,886 | ) | | | (11,630 | ) |
Total stockholders’ equity | | $ | 1,00 | 1 | | $ | 1,806 | | | $ | 3,803 | |
I-20
SELECTED UNAUDITED PRO FORMA
CONDENSED COMBINED CONSOLIDATED FINANCIAL DATA
The following selected unaudited pro forma condensed combined consolidated financial information was prepared using the purchase method of accounting. For accounting purposes, our company is considered to be acquiring Cequent in this merger. The unaudited pro forma condensed combined consolidated balance sheet data of our company and Cequent assume that the merger of our company and Cequent took place on March 31, 2010, and combines the historical balance sheet of our company at March 31, 2010 with the historical balance sheet of Cequent at March 31, 2010. The unaudited pro forma condensed combined consolidated statement of operations data for our company and Cequent assume that the merger of our company and Cequent took place as of January 1, 2009. The unaudited pro forma condensed combined consolidated statement of operations data for the year ended December 31, 2009 combines our company’s historical statement of operations for 2009 with Cequent’s historical statement of operations for 2009. The unaudited pro forma condensed combined consolidated statement of operations data for the quarter ended March 31, 2010 combines our company’s historical statement of operations data for the quarter ended March 31, 2010 with Cequent’s historical statement of operations data for the quarter ended March 31, 2010.
The selected unaudited pro forma condensed combined consolidated financial data are presented for illustrative purposes only and are not necessarily indicative of the combined financial position or results of operations of future periods or the results that actually would have been realized had the entities been a single entity during these periods. The pro forma net loss per share is computed by dividing the pro forma net loss by the pro forma weighted average number of shares outstanding. The pro forma per share data are not necessarily indicative of the results that would have occurred, your financial interest in such results, or the future results that will occur after the merger. Our last fiscal year ended on December 31, 2009 and Cequent’s last fiscal year also ended on December 31, 2009. The selected unaudited pro forma condensed combined consolidated financial data as of and for the quarter ended March 31, 2010 and for the year ended December 31, 2009 are derived from the unaudited pro forma condensed combined consolidated financial statements at page I-42 of this proxy statement and should be read in conjunction with those statements and the related notes. See “Unaudited Pro Forma Condensed Combined Consolidated Financial Statements.”
Statement of Operations Data (Unaudited):
| | | | | | | | |
| | | Quarter Ended
March 31, 2010 | | | Year Ended
December 31, 2009 | |
| | | (In thousands, except
per share data) | | | (In thousands, except
per share data) | |
Revenue | | $ | 328 | | | $ | 15,313 | |
Operating expenses | | | 7,239 | | | | 31,237 | |
Loss from operations | | | (6,911 | ) | | | (15,924 | ) |
Other income (expense), net | | | (3,490 | ) | | | 2,622 | |
Net loss | | | (10,401 | ) | | | (13,302 | ) |
Net loss per common share — basic and diluted | | $ | (0.12 | ) | | $ | (0.18 | ) |
Shares used in computing net loss per share — basic and diluted | | | 85,679 | | | | 75,808 | |
I-21
Balance Sheet Data (Unaudited):
| | | | |
| | | As of March 31,
2010 | |
| | |
| | | (In thousands) | |
Cash and cash equivalents | | $ | 10,730 | |
Intangible assets and goodwill | | | 49,978 | |
Property and equipment, net | | | 4,625 | |
Total assets | | | 67,119 | |
Total current liabilities | | | 7,136 | |
Fair value liability for price adjustable warrants | | | 11,214 | |
Deferred taxes | | | 14,595 | |
Total liabilities | | | 34,554 | |
Accumulated deficit | | | (274,056 | ) |
Total stockholders’ equity | | $ | 32,565 | |
Recent Share Prices
Our common stock is listed on The Nasdaq Global Market under the symbol “MRNA”. Cequent is a privately-held company, and there is no trading market for its common stock.
The following table sets forth the high and low price per share of our common stock as reported on The Nasdaq Global Market on March 31, 2010, the last completed trading day prior to the announcement of the merger, on April 1, 2010, the trading day on which we announced the merger, and on June 2, 2010.
| | | | | | |
| | | MDRNA
Common Stock |
| | | High | | Low |
March 31, 2010 | | $ | 1.12 | | $ | 1.05 |
April 1, 2010 | | $ | 1.33 | | $ | 1.11 |
June 2, 2010 | | $ | 1.08 | | $ | 1.01 |
Because the market price of our common stock is subject to fluctuation prior to the closing date of the merger, the market value of the shares of our common stock that holders of Cequent common stock will receive in the merger may increase or decrease.
I-22
RISK FACTORS
You should consider the following risk factors in determining how to vote at the annual meeting.
The following factors should be considered carefully by you in evaluating whether to approve the issuance of shares of common stock in the merger. These factors should be considered in conjunction with any other information included or incorporated by reference herein, including in conjunction with forward-looking statements made herein. See “Chapter Six — Additional Information for Stockholders — Where You Can Find More Information” on page VI-1.
Risks Relating to the Merger
We may not realize all of the anticipated benefits of the Cequent acquisition.
The success of the merger will depend, in part, on our ability to realize the anticipated growth opportunities and strategic synergies from combining the businesses of our company and Cequent. Our ability to realize these benefits, and the timing of this realization, depend upon a number of factors and future events, many of which we and Cequent, individually or collectively, cannot control. These factors and events include:
| | • | | the results of future preclinical studies and clinical trials of Cequent’s programs; |
| | • | | the U.S. Food and Drug Administration, or FDA, agreeing to allow us to continue the development of the acquired programs; |
| | • | | effectively consolidating research and development operations; |
| | • | | retaining and attracting key employees; |
| | • | | consolidating corporate and administrative functions; |
| | • | | effective business development efforts; |
| | • | | preserving our and Cequent’s research and development and other important relationships; and |
| | • | | minimizing the diversion of management’s attention from ongoing business concerns. |
The number of shares of our common stock to be issued to the securityholders of Cequent is not adjustable based on the market price of our common stock, so the merger consideration at the closing may have a greater or lesser value than at the time the merger agreement was signed.
The parties to the merger agreement have determined the number of shares of our common stock to be issued to Cequent’s securityholders based on the VWAP of our common stock on The NASDAQ Global Market as of the date the merger agreement was signed, and this number is not adjustable based on changes in the market price of our common stock. Any changes in the market price of our common stock will not affect the number of shares that holders of Cequent securities will be entitled to receive pursuant to the merger. Therefore, if the market price of our common stock declines from the market price on the date of the merger agreement prior to the consummation of the merger, Cequent securityholders could receive merger consideration with considerably less value. Similarly, if the market price of our common stock increases from the market price on the date of the merger agreement prior to the consummation of the merger, Cequent securityholders could receive merger consideration with considerably more value than their Cequent securities and our stockholders immediately prior to the merger will not be compensated for the increased market value of our common stock. Because the number of shares of our common stock to be issued to the securityholders of Cequent does not adjust as a result of changes in the value of our common stock, for each one percentage point that the market value of our common stock rises or declines, there is a corresponding one percentage point rise or decline, respectively, in the value of the total merger consideration issued to the Cequent securityholders. For example, the volume weighted average price of our common stock on The NASDAQ Global Market for the 10-day period ending on March 31, 2010, the last trading date before the announcement of the merger agreement, was $1.1496 per share. Assuming that a
I-23
total of 40 million shares of our common stock are issued to Cequent securityholders upon the closing of the merger at a per share value of $1.1496 per share (excluding the value of assumed stock options and warrants), the aggregate merger consideration to be issued to Cequent securityholders in the merger would be approximately $45.98 million. If, however, the closing price of our common stock on the date of closing of the merger had declined to, for example, $0.91968 per share, a decline of 20% from the 10-day VWAP as of March 31, 2010, the aggregate merger consideration to be issued to Cequent securityholders in the merger would decrease to approximately $36.79 million in total.
Notwithstanding the foregoing, if the closing price of our common stock on The NASDAQ Global Market is in excess of $1.437 for ten (10) consecutive trading days after the date of the merger agreement, we shall have the right to terminate the merger agreement, and if the closing price of our common stock on The NASDAQ Global Market is below $0.8622 for ten (10) consecutive trading days after the date of the merger agreement, then Cequent shall have the right to terminate the merger agreement.
You may not realize a benefit from the merger commensurate with the ownership dilution you will experience in connection with the merger.
If the combined company is unable to realize the strategic, operational and financial benefits currently anticipated from the merger, you may experience substantial dilution of your ownership interest in our company without receiving any commensurate benefit.
The merger is subject to conditions to closing that could result in the merger being delayed or not consummated, which could negatively impact our stock price and future business and operations.
The merger is subject to conditions to closing as set forth in the merger agreement, including obtaining the requisite stockholder approval of our company. The requisite approval of the stockholders of Cequent to the merger was obtained on April 2, 2010. If any of the conditions to the merger are not satisfied or, where permissible, not waived, the merger will not be consummated. Failure to consummate the merger could negatively impact our stock price, future business and operations, and financial condition. Any delay in the consummation of the merger or any uncertainty about the consummation of the merger may adversely affect the future business, growth, revenue and results of operations of the combined company.
Changes to our financial statements resulting from accounting for the acquisition might adversely affect the market value of our common stock following the merger.
In accordance with U.S. GAAP, the merger will be accounted for as a purchase of a business, which will result in changes to our financial statements that could have an adverse impact on the market value of our common stock following completion of the merger. The total estimated purchase price will be allocated to Cequent’s net tangible assets, identifiable intangible assets and in-process research and development assets based on their fair values as of the date of completion of the merger. Any excess of the purchase price over those fair values will be allocated to goodwill.
The pro forma financial statements are presented for illustrative purposes only and may not be an indication of the combined company’s financial condition or results of operations following the merger.
The pro forma financial statements contained in this proxy statement are presented for illustrative purposes only and may not be an indication of the combined company’s financial condition or results of operations following the merger for several reasons. For example, the pro forma financial statements have been derived from the historical financial statements of both our company and Cequent and certain adjustments and assumptions have been made regarding the combined company after giving effect to the merger. The information upon which these adjustments and assumptions have been made is preliminary, and such adjustments and assumptions are difficult to make with complete accuracy. Moreover, the pro forma financial statements do not reflect all costs that are expected to be incurred by the combined company in connection with the merger. For example, the impact of any
I-24
incremental costs incurred in integrating the two companies is not reflected in the pro forma financial statements. As a result, the actual financial condition and results of operations of the combined company following the merger may differ significantly from these pro forma financial statements.
In addition, the assumptions used in preparing the pro forma financial information may not prove to be accurate, and other factors may affect the combined company’s financial condition or results of operations following the merger. Any potential decline in the combined company’s financial condition or results of operations may cause significant variations in the stock price of the combined company. See the section entitled “Unaudited Pro Forma Condensed Combined Consolidated Financial Statements” beginning on page I-42.
If Cequent’s former stockholders immediately sell our common stock received in the merger, they could cause our common stock price to decline.
We have agreed to file a shelf registration statement on Form S-3 to register the shares of our common stock to be issued to certain of the principal stockholders of Cequent in the merger under the federal securities laws. It is anticipated that all of such principal stockholders will enter into lock-up agreements with our company pursuant to which they will agree that they shall not, without our prior approval, transfer or dispose any shares of our common stock received in the merger during the period commencing on the effective date of the merger and ending on the earlier of (A) one hundred and eighty (180) days after the effective date of the merger or (B) thirty (30) days following the closing of an equity financing by our company for the issuance of shares of equity or securities convertible into or exchangeable or exercisable for shares of equity of our company which, when added together with all other equity financings from and after the date of execution of the merger agreement, results in aggregate gross proceeds to us of at least $10 million.
Once the shelf registration statement is declared effective, and the lock-up period has expired, all of the shares issued to the Cequent principal stockholders will be available for resale in the public market, except for shares of our common stock that will be subject to additional transfer restrictions because those shares were issued to Cequent’s former stockholders who become affiliates of our company after the merger.
If the principal stockholders, or other holders of our stock, sell significant amounts of our common stock following the effectiveness of the shelf registration statement, the market price of our common stock could decline. These sales may also make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate to raise funds through future offerings of common stock.
Our business and stock price may be adversely affected if the acquisition of Cequent is not completed.
Our acquisition of Cequent is subject to several customary conditions, including the approvals of the transaction by our stockholders. If our acquisition of Cequent is not completed, we could be subject to a number of risks that may adversely affect our business and stock price, including:
| | • | | the current market price of shares of our common stock reflects a market assumption that the acquisition will be completed; |
| | • | | under certain circumstances, we could be required to pay the costs and expenses of Cequent; and |
| | • | | we would not realize the benefits we expect from acquiring Cequent. |
If we are required but are unable to repay any amounts owed to Cequent under the loan agreement entered into in connection with the merger agreement, we may be required to consummate an equity or debt financing on terms that are not favorable to our current stockholders or to reduce or terminate our operations and declare bankruptcy.
Concurrently and in connection with the execution of the merger agreement, we entered into a loan agreement with Cequent for a $3 million working capital facility. On April 30, 2010, Cequent loaned $1 million to us under the loan agreement, and on May 31, 2010, Cequent loaned an additional $1 million to us under the loan agreement. Our obligations under the loan agreement are secured by all assets of our company and our wholly-owned subsidiaries. If any amounts become due and payable by us under the loan agreement prior to the
I-25
consummation of the merger or in connection with the termination of the merger agreement and we are unable to pay such amounts, we may be required to consummate an equity or debt financing on terms that are not favorable to our current stockholders or to reduce or terminate our operations and declare bankruptcy.
Certain of our directors and executive officers have interests in the merger that may be different from, or in addition to, or conflict with interests of our stockholders generally.
Certain of our directors and executive officers may have interests in the merger that differ from, are in addition to, or conflict with interests of our stockholders. For example, it is anticipated that all of our executive officers will continue in their current positions with the combined company, and that three of the current members of our board of directors will continue to serve on the board of directors of the combined company. The Board has also determined that all outstanding common stock purchase options held by the three current members of the Board who will not continue to serve on the Board of the combined company will vest immediately upon the consummation of the merger, and that all of such options will remain exercisable until the earlier of the four-year anniversary of the effective time and the expiry of such options. You should be aware of these interests when considering the recommendation of the board of directors that you vote in favor of the issuance of shares of our common stock pursuant to the merger agreement.
After the completion of the merger, the surviving corporation, which will be a wholly-owned subsidiary of our company, will possess not only all of the assets but also all of the liabilities of Cequent. Discovery of previously undisclosed or unknown liabilities could have an adverse effect on the combined company’s business, operating results and financial condition.
Acquisitions involve risks, including inaccurate assessment of undisclosed, contingent or other liabilities or problems. After the completion of the merger, the surviving corporation, which will be a wholly-owned subsidiary of our company, will possess not only all of the assets, but also all of the liabilities of Cequent. Although we conducted a due diligence investigation of Cequent and its known and potential liabilities and obligations, it is possible that undisclosed, contingent or other liabilities or problems may arise after the completion of the merger, which could have an adverse effect on the combined company’s business, operating results and financial condition. The amount of such liabilities may be in excess of the value of the shares of our common stock that will be placed into escrow until January 1, 2011 to cover Cequent’s indemnification obligations under the merger agreement, or such liabilities may not be uncovered until after such shares have been released from escrow.
I-26
SPECIAL NOTE ON FORWARD-LOOKING STATEMENTS
This document contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. These forward-looking statements reflect our current views with respect to future events or our financial performance, and involve certain known and unknown risks, uncertainties and other factors, including those identified below, which may cause our or our industry’s actual or future results, levels of activity, performance or achievements to differ materially from those expressed or implied by any forward-looking statements or from historical results. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Exchange Act. Forward-looking statements include statements preceded by, followed by, or that include the words “may,” “will,” “could,” “would,” “should,” “believe,” “expect,” “plan,” “anticipate,” “intend,” “estimate,” “predict,” “potential” or similar expressions.
Forward-looking statements in this proxy statement include, but are not limited to, statements about: (i) the expected benefits of, and potential value created by, the proposed merger for the our stockholders; (ii) the likelihood of the satisfaction of certain conditions to the completion of the merger and whether and when the merger will be consummated; (iii) the number of shares we expect to issue in the merger and the capitalization of the combined company after the merger; (iv) each of our and Cequent’s results of operations, financial condition and businesses and our and Cequent’s objectives, plans and expectations; and (v) information about the combined company and the expected impact of the proposed merger on the combined company and its future business, operating results and financial condition.
Forward-looking statements are inherently subject to risks and uncertainties, many of which we cannot predict with accuracy and some of which we might not even anticipate. Although we believe that the expectations reflected in such forward-looking statements are based upon reasonable assumptions at the time made, we can give no assurance that such expectations will be achieved. Future events and actual results, financial and otherwise, may differ materially from the results discussed in the forward-looking statements. Readers are cautioned not to place undue reliance on these forward-looking statements. We have no duty to update or revise any forward-looking statements after the date of this proxy statement or to conform them to actual results, new information, future events or otherwise.
The following factors, among others, could cause our or our industry’s future results to differ materially from historical results or those anticipated:
| | • | | our ability to obtain additional funding for our company; |
| | • | | the potential inability of our company and Cequent to close the merger and successfully execute our and their integration strategies; |
| | • | | uncertainties regarding the combined company’s future operating results; |
| | • | | the success or failure of our research and development programs or the programs of our partners; |
| | • | | our efforts to collaborate with pharmaceutical and biotechnology companies to develop products; |
| | • | | our ability to obtain governmental approvals, including product and patent approvals; |
| | • | | our ability to attract and retain our key officers and employees; |
| | • | | costs associated with any product liability claims, patent prosecution, patent infringement lawsuits and other lawsuits; |
| | • | | our ability to maintain our listing on The Nasdaq Global Market; and |
| | • | | our ability to develop and commercialize our products before our competitors. |
I-27
These factors are the important factors of which we are currently aware that could cause actual results, performance or achievements to differ materially from those expressed in any of our forward-looking statements. We operate in a continually changing business environment, and new risk factors emerge from time to time. Other unknown or unpredictable factors also could have material adverse effects on our future results, performance or achievements. We cannot assure you that projected results or events will be achieved or will occur.
I-28
THE MERGER TRANSACTION
General
At the effective time, Calais Acquisition Corp., a wholly-owned subsidiary of our company, will merge with and into Cequent. Cequent will be the surviving entity and will continue as a wholly-owned subsidiary of our company. Subject to the terms and conditions of the merger agreement, at the effective time of the merger, each share of Cequent Series A-1 convertible preferred stock and common stock outstanding immediately prior to the merger will be exchanged for shares of our common stock at an exchange ratio that implies a purchase price for Cequent common and preferred stockholders of approximately $44 million, plus an additional value of approximately $2 million to Cequent’s warrant and option holders, based on the 10-day volume-weighted average price of our common stock on The NASDAQ Global Market ending on March 31, 2010.
Based upon the outstanding shares of our common stock on March 31, 2010, and assuming the conversion of all of Cequent’s preferred stock on March 31, 2010, immediately following the completion of the merger, Cequent common and preferred stockholders will own approximately 44% of the combined company’s common stock and our stockholders will own approximately 56% of the combined company’s common stock.
Based upon the outstanding shares of our common stock and assuming the exercise or conversion of all of our outstanding exercisable and non-exercisable warrants and stock options on March 31, 2010, and assuming the exercise or conversion of all of Cequent’s exercisable and non-exercisable warrants, stock options and preferred stock on March 31, 2010, immediately following the completion of the merger, Cequent securityholders would own approximately 37% of the combined company’s common stock and our securityholders would own approximately 63% of the combined company’s common stock. This number assumes the exercise for cash of all warrants and stock options.
Background of the Merger
It is the practice of management to periodically review with the Board of Directors strategic alternatives to: (1) enhance stockholder value; (2) expand scientific, technical and clinical capability; and (3) remain competitive in the RNAi sector. As part of its ongoing assessment of its strategic alternatives, management developed an acquisition strategy in early 2009 to help our company achieve its strategic alternatives, and first presented the strategy to the Board of Directors in May 2009.
At a meeting of the Board of Directors on May 20, 2009, and at subsequent board meetings, J. Michael French, our President and Chief Executive Officer, discussed the acquisition strategy with members of the Board. Mr. French presented to the Board of Directors a comprehensive list of companies with whom he recommended our company discuss the possibility of a collaboration or business combination. Based on input of both management and the Board of Directors, we determined to use the following criteria to identify potential merger candidates: (a) focus of each candidate’s therapeutic pipeline and stage of clinical development; (b) the ability of each candidate to bring additional cash resources to a combined company to help fund ongoing operations; (c) the quality and depth of each candidate’s management, board of directors, and Scientific Advisory Board members; (d) the quality of each candidate’s research and development team; and (e) the ability for the technology of each candidate to be applied as a drug discovery platform for the development of novel RNAi-based therapeutics. Based on these criteria, Mr. French and the Board of Directors agreed to develop a specific list of potential merger candidates.
During October 2009, we identified approximately 12 companies that broadly matched our criteria. Seven of these companies, including Cequent, were prioritized as the companies that most closely matched our criteria.
Mr. French contacted potential candidates to discuss the possibility of a combination with our company. Each company contacted during this process was invited to meet with senior management. As part of the process of contacting these companies, on October 12, 2009, Mr. French requested and had a meeting with Ted Hibben,
I-29
Chief Business Officer of Cequent, in London, England. During this conversation, Mr. French and Mr. Hibben discussed the potential interest of our company and Cequent in learning more about the science, technology and business of one another. In preparation for an exchange of research data and business strategies, we and Cequent signed a mutual confidential disclosure agreement (CDA) on October 30, 2009.
From October 2009 until we received the signed copy of the letter of intent from Cequent on March 15, 2010, management was engaged in confidential discussions with four other RNAi-based companies to identify complementary or synergistic technologies as well as to establish appropriate evaluations for either a merger or acquisition transaction. We did not enter into a letter of intent with any of these four companies.
Based upon this initial meeting, a subsequent meeting was arranged in Boston, Massachusetts on November 4, 2009 among Peter Parker, Chief Executive Officer of Cequent, Johannes Fruehauf, Vice President of Cequent, Jens Harbourth, Research Director of Cequent, Mr. Hibben, Dr. Barry Polisky, our Chief Scientific Officer, and June Ameen, our Vice President of Corporate Development. At this meeting, it was mutually agreed that both our company and Cequent were based on a solid scientific foundation and that the drug discovery platforms of both companies were complementary. We and Cequent agreed that this was a strong basis for a strategic relationship between the two companies, and agreed to explore the possibility further.
In mid to late December, Mr. Parker and Mr. French met once, exchanged correspondence and had several phone conversations regarding the potential structure of a strategic collaboration between our company and Cequent. On December 17, 2009, Mr. French presented a draft, non-binding letter of intent to Mr. Parker proposing a business combination between the two companies. Mr. Parker and Mr. French agreed to meet again to further discuss the letter of intent and the opportunity in January in San Francisco.
On January 12, 2010, Mr. Parker and Mr. Hibben met with Mr. French and Peter Garcia, our Chief Financial Officer, and had general discussions regarding a possible strategic transaction between our company and Cequent. It was agreed to continue to exchange information between the two companies for expressly this purpose. Mr. Parker and Mr. French continued to have telephone conversations in the days following the January 12, 2010 meeting, and met again on January 17, 2010 to discuss details surrounding the draft letter of intent.
Between January 14, 2010 and April 1, 2010, our management team held regular internal meetings and meetings with our Board of Directors, and met regularly with our outside legal and financial advisors to discuss the status of on-going negotiations and due diligence with the merger candidates, including Cequent.
On February 1, 2010, Mr. French and Mr. Garcia had a conference call with representatives from Canaccord Genuity to discuss potential deal terms for an acquisition by our company of Cequent.
On February 4, 2010, we formally engaged Canaccord Genuity as a financial advisor, to assist us in evaluating strategic acquisition opportunities, including the potential acquisition of Cequent.
On February 8, 2010, Mr. French presented Mr. Parker with a revised, draft non-binding letter of intent to acquire Cequent, reflecting the key terms that had been discussed over that prior month.
On February 9, 2010, Mr. French and Mr. Garcia met in New York City with Scott Weiner, a Senior Principal of Pappas Ventures, and gave an overview of the business, technology and operations of our company, and discussed a potential business combination with Cequent. Pappas Ventures is a venture investor in, and a major holder of the preferred stock of, Cequent.
On February 22, 2010, Mr. French met with Mr. Parker and Mr. Hibben in Cambridge, MA to discuss business development and financing issues related to both companies.
I-30
Beginning the week of March 1, 2010, representatives of our company and Cequent began to exchange additional information about our respective companies, and to discuss in greater detail the proposed structure and terms of a potential business combination, including the financial valuation of Cequent, the consideration to be paid to the stockholders of Cequent, and the treatment of outstanding options and warrants.
On March 4, 2010, Mr. French met in Raleigh, North Carolina with Rosina Maar Pavia, a Partner at Pappas Ventures, and Scott Weiner, to discuss potential terms of the transaction. On March 5, 2010, Mr. Garcia and Mr. Weiner had a phone conversation to finalize the form and amount of consideration to be paid by us and the potential transaction structure, including the financial valuation of Cequent, the consideration to be paid to the stockholders of Cequent, the amount of cash that Cequent would bring to the combined company, and the treatment of outstanding options and warrants.
On March 5, 2010, at a special telephonic meeting of the Deal Committee of the Board of Directors, Mr. French and Mr. Garcia reviewed for such committee the ongoing discussions with Cequent as well as revised terms of the deal based on interactions with Cequent. The Deal Committee asked questions with respect to the structure of the potential transaction, the business and technology of Cequent, the anticipated closing date of the transaction, the amount and form of consideration to be paid, and the pros and cons of the transaction. Messrs. French and Garcia specifically noted that it was expected that Cequent would provide a bridge loan to our company to fund continuing operations until the closing date, and that they anticipated that Cequent would have approximately $8.1 million in cash available on the closing date, net the aggregate amount of any bridge loans made to us. It was estimated that the bridge loan and the additional funds available upon closing would be sufficient to finance the combined company into December 2010. Following discussion of the proposed transaction and other strategic alternatives for business combinations and equity financings available to our company at that time, the Deal Committee authorized Mr. French to continue discussions and negotiations with Cequent, subject to later review by the Board of Directors.
Following the Board meeting on March 5, 2010, Mr. French delivered to Cequent a revised, non-binding letter of intent with proposed key transaction terms, including transaction structure, integration issues, various governance issues and valuation for the purpose of determining an exchange ratio.
On March 8 and 9, 2010, a regulatory consultant hired by us met with representatives from Cequent at their offices in Cambridge to review Cequent’s regulatory filings and to perform additional due diligence.
On March 10 and 11, 2010, representatives of our company met with representatives of Cequent and exchanged additional information covering Cequent’s research and development, business, financial, and intellectual property affairs and to conduct further due diligence. Additionally, on March 10, 2010, Mr. French met with Lauren Silverman, a member of Cequent’s Board of Directors and a Managing Director of Novartis BioVentures Ltd., to present our company’s science, research and oncology programs. Novartis BioVentures Ltd. is a venture investor in, and a major holder of the preferred stock of, Cequent.
On March 15, 2010, we received a signed copy of the letter of intent from Cequent.
On March 17, 2010, Mr. French distributed to Cequent an initial draft of the merger agreement. From March 17, 2010 through April 1, 2010, we and Cequent, together with our and their respective advisors and counsel, engaged in negotiations regarding the merger agreement and related documentation, including termination rights and fees, non-solicitation provisions, indemnification and escrow provisions, representations and warranties, and covenants. In addition, the parties negotiated a loan agreement pursuant to which, among other things, Cequent shall extend one or more loans to our company in the aggregate principal amount of up to $3 million to fund our operations prior to the merger, which loans would be secured by all of the assets of our company and our subsidiaries.
On March 18, 2010, representatives of our company, including Mr. French, Mr. Garcia and Dr. Polisky, met internally to discuss the ongoing due diligence of Cequent, including its development programs and the financial, strategic and operational implications of combining the two companies.
I-31
On March 19, 2010, at a telephonic meeting of our Board of Directors, Mr. French made a presentation to the Board on the status of the proposed merger between our company and Cequent, and reviewed the methodology and manner pursuant to which the consummation of the merger would be pursued.
On March 19, 2010, Mr. Garcia and Mr. Hibben conducted a meeting with Pryor Cashman LLP, legal counsel to our company, and Edwards Angell Palmer & Dodge LLP, legal counsel to Cequent, to discuss a plan for finalizing the merger agreement, the loan agreement and the ancillary agreements.
Through the remaining month of March 2010, Mr. French held a number of telephone conversations with Mr. Parker regarding the proposed transaction. Mr. French and Mr. Garcia also had numerous conference calls with representatives of Canaccord Genuity and Pryor Cashman regarding terms of the merger agreement and the related documents.
On March 25, 2010, representatives from Cequent, including Mr. Parker, Mr. Hibben and Ms. Silva, visited our offices in Bothell, WA to meet our senior management, to continue their on-going due diligence of our company and to discuss additional business issues related to the transaction.
On March 27, 2010, Mr. French had a conversation with Dr. Herbert Hooper, Chairman of the Board of Directors of Cequent and Managing General Partner at Ampersand Ventures, to discuss the proposed transaction. Ampersand Ventures is a venture investor in, and a major holder of the preferred stock of, Cequent.
On March 29, 2010, the Board of Directors held a special telephonic meeting to consider proposed modifications of the merger agreement as well as receive a report of the due diligence performed on the Cequent technology and development programs by Dr. Carl Novina, a member of our Scientific Advisory Board. Management reported to the Board on the status of negotiations, the key terms of the deal and the advisability of entering into the transaction. Following discussion, the Board provided guidance with respect to how to resolve certain open issues in the negotiations between our company and Cequent.
On March 30, 2010, Mr. French and Mr. Parker met to discuss proposed modifications to the merger agreement and related documents. Similar conversations occurred between the legal and financial advisors of both companies over the next two days to resolve outstanding issues and complete negotiation of the merger agreement and ancillary agreements.
On March 31, 2010, the Board of Directors held a special telephonic meeting, at which five out of six of our directors, Mr. Garcia, and our financial and legal advisors were present. At the meeting, representatives of Pryor Cashman reviewed with the directors their fiduciary duties under Delaware law. Mr. French and representatives of Pryor Cashman then updated the Board on the developments since the March 29 telephonic meeting of the Board. Also at this meeting, representatives of Canaccord Genuity, our financial advisor, made a financial presentation to the Board and discussed the results of their analyses of stock trading history, selected recent mergers and acquisitions of pre-clinical companies in the biotechnology industry, and valuations of selected publicly traded pre-clinical biotechnology companies comparable to Cequent. Based on these analyses, a representative of Canaccord Genuity delivered to the Board an oral opinion, which was confirmed by delivery of a written opinion dated March 31, 2010, to the effect that as of such date and subject to the assumptions, qualifications and limitations set forth in its opinion, the consideration to be paid by us in the proposed acquisition of Cequent is fair, from a financial point of view, to our company.
Mr. French, Mr. Garcia and our representatives updated the Board on the results of our due diligence review, and representatives of Pryor Cashman reviewed legal matters, including terms of the proposed merger agreement, loan agreement and security agreements. Following questions and a lengthy discussion, the Board of Directors unanimously: (1) determined that the proposed merger agreement and the transactions contemplated thereby, including the merger and the issuance of shares of our common stock in connection with the merger, were advisable and in the best interests of our company and its stockholders; (2) adopted resolutions approving the
I-32
proposed merger agreement and the transactions contemplated thereby, including approving the transaction under our Rights Agreement; and (3) recommended, subject to the terms and conditions in the proposed merger agreement, that the stockholders of our company approve the issuance of shares in connection with the merger. The Board also authorized the appropriate officers of our company to finalize, execute and deliver the merger agreement and related documentation.
On the evening of March 31, 2010, the parties finalized the terms of the merger agreement and related documentation and the definitive merger agreement and ancillary agreements were executed and delivered by both parties.
On the morning of April 1, 2010, we and Cequent issued a joint press release announcing the acquisition of Cequent by us and held a joint analyst conference call later that morning.
Factors Considered by, and Recommendation of, the MDRNA Board of Directors
The Board of Directors has determined that the terms of the merger and the merger agreement are fair to, and in the best interests of, our company and its stockholders. Accordingly, the Board of Directors has approved the merger agreement and the consummation of the merger and recommends that you voteFORapproval of the issuance of the shares to be issued in connection with the merger agreement and the merger. In evaluating the transaction, the Board of Directors consulted with and received information from management and our legal and financial advisors, and considered the material factors described below.
Reasons for the Merger Identified by the MDRNA Board of Directors
The Board of Directors has identified potential benefits of the merger that it believes will contribute to the success of the combined company, including the following:
| | • | | expanded research, development, commercialization and intellectual property expertise and capabilities; |
| | • | | the complementary nature of the businesses of our company and Cequent in the RNAi field, giving the combined company two validated RNAi Drug Discovery Platforms utilizing two distinct and proprietary delivery technologies which can be used for systemic, local and oral delivery; |
| | • | | the potential to leverage the combined technology expertise of our company and Cequent to provide enhanced research and development activities; |
| | • | | synergies associated with combining the skills and capabilities of the two companies; |
| | • | | the forward integration of our company with a biotherapeutic product-focused company such as Cequent to expand our oncology disease pipeline with a clinical stage Phase 1 program as well as expand our pipeline to include inflammatory disease programs with one pre-clinical program under option; |
| | • | | the potential to commercialize an RNAi-based therapeutic as early as 2014; |
| | • | | world class scientific advisors at the Board of Director and Scientific Advisory Board levels; |
| | • | | an expanded intellectual property estate with RNAi-based therapeutics using UsiRNA and TransKingdom construct technologies; |
| | • | | the opportunity for our stockholders to participate in a significantly larger, more diversified company, with greater share liquidity; |
| | • | | the potential for realizing annual cost savings through consolidation of administrative functions; |
| | • | | the combined company is anticipated to have sufficient financial resources to fund operations through approximately December 2010; and |
| | • | | the combined company being appropriately capitalized to further develop its clinical programs. |
I-33
Other Factors Considered by the MDRNA Board of Directors
In the course of its deliberations, the Board reviewed with management and our legal and financial advisors a number of additional factors relevant to the merger, including the following:
| | • | | historical information concerning our and Cequent’s respective businesses, financial performance and condition, operations, management and competitive position, including results of operations during the most recent fiscal year for each company; |
| | • | | management’s view of the financial condition, results of operations and businesses of Cequent and our company before and after giving effect to the merger, based on management’s due diligence; |
| | • | | the prospects of our company as an independent company with no current clinical stage product candidates; |
| | • | | current financial market conditions and historical market prices, sales prices, volatility and trading information with respect to our common stock; |
| | • | | management’s view as to the potential for other third parties to enter into strategic relationships with or to acquire Cequent; |
| | • | | certain terms of the merger agreement, including the provisions that prohibit Cequent from soliciting other acquisition offers and the composition of the Board of Directors after closing; |
| | • | | the likelihood of completing the merger on the anticipated schedule; |
| | • | | an assessment of alternatives to the merger, including acquiring or in-licensing other drug development opportunities and other possible acquisition and merger candidates, liquidation of our company and the determination that the merger represented a good strategic fit and presented a unique opportunity to enhance and expand our operations and position our company for future growth in the RNAi sector of the biopharmaceutical market; |
| | • | | the opinion of our financial advisor, Canaccord Genuity, and its financial presentation, dated March 31, 2010, to the Board of Directors as to the fairness, from a financial point of view and as of the date of the opinion, to our company of the consideration to be paid in the merger, as more fully described below under the caption “Opinion of MDRNA’s Financial Advisor” beginning on page I-35; and |
| | • | | the impact of the merger on our stockholders and employees. |
Potentially Negative Factors Considered by the MDRNA Board of Directors
The Board of Directors also identified and considered a variety of potentially negative factors in its deliberations concerning the merger, including, but not limited to:
| | • | | the risks that the potential benefits sought in the merger might not be fully realized; |
| | • | | the inherent difficulties in successfully integrating the operation of the companies; |
| | • | | the potential need for additional funding if development does not proceed as anticipated; |
| | • | | the possibility that the merger might not be completed, and the potential adverse effect of the public announcement of the merger on our reputation and ability to obtain financing in the future; |
| | • | | the significant number of shares to be issued to Cequent stockholders; and |
| | • | | other risks described under “Risk Factors” beginning on page I-23 of this proxy statement. |
The Board of Directors believes that these risks were outweighed by the potential benefits of the merger. The foregoing discussion is not exhaustive of all factors considered by the Board of Directors, but it does describe all material factors considered by the Board of Directors. Individual members of the Board of Directors may have considered different factors, and the Board of Directors evaluated these factors as a whole and did not quantify or otherwise assign relative weights to factors considered.
I-34
There can be no assurance that the potential synergies or opportunities considered by the Board of Directors will be achieved through consummation of the merger. See “Risk Factors” beginning on page I-23.
Recommendation of the Board of Directors
After careful consideration, the Board of Directors has unanimously determined the merger to be fair, advisable and in the best interest of our company and its stockholders. The Board of Directors has approved the merger agreement and recommends stockholder approval of the issuance of the shares pursuant to the merger agreement.
In considering the recommendation of the Board of Directors with respect to the merger agreement, you should be aware that certain directors and officers have certain interests in the merger that are in addition to the interests of stockholders generally. For example, it is anticipated that all of the current executive officers of our company will remain in the same positions with the combined company, and that three of the members of our Board of Directors will continue to serve on the Board of Directors of the combined company. The Board has also determined that all outstanding common stock purchase options held by the three current members of the Board who will not continue to serve on the Board of the combined company will vest immediately upon the consummation of the merger, and that all of such options will remain exercisable until the earlier of the four-year anniversary of the effective time and the expiry of such options.
In addition, prior to the execution of the merger agreement, each of J. Michael French, our President and Chief Executive Officer, Peter S. Garcia, our Chief Financial Officer and Secretary, and Barry Polisky, Ph.D., our Chief Scientific Officer, entered into a Waiver Agreement with us pursuant to which each such executive officer waived any and all right, title, claim and interest that he may have to receive any payments or accelerated vesting of equity awards under such executive officer’s employment agreement with us or under any equity compensation plan of our company, in each case as a result of the merger being deemed a change of control (as defined in the respective employment agreement or plan). Moreover, we and each of the aforementioned executive officers agreed that if the executive’s employment is terminated on or before December 31, 2010 for any reason other than termination for cause, then notwithstanding anything to the contrary contained in any employment agreement or in any of our equity compensation plans, any and all unvested common stock options held by the executive shall immediately vest in full upon the effective date of such termination, and shall remain exercisable for a period of two (2) years thereafter (but in no event after the original expiration date of the award). Furthermore, each executive acknowledged that any cash received by us as a result of the consummation of the merger shall not be counted toward the $5 million in unrestricted cash threshold necessary to trigger payment by us to the executive of the retention bonuses previously approved by the Board of Directors.
Opinion of MDRNA’s Financial Advisor
Scope of the Assignment
Our Board of Directors engaged Canaccord Genuity to provide investment banking services in connection with the potential merger with Cequent, including to render an opinion as to whether the merger consideration to be paid by us in the merger was fair to our company from a financial point of view. Canaccord Genuity rendered its oral and written opinion to the Board of Directors that, as of March 31, 2010, and based upon the assumptions made, matters considered, and qualifications and limitations of the review set forth in its written opinion, the merger consideration to be paid by us to Cequent stockholders pursuant to the merger agreement, was fair to our company from a financial point of view.
The full text of Canaccord Genuity’s written opinion, which sets forth the procedures followed, assumptions made, matters considered, and qualifications and limitations of the review undertaken in connection with the opinion, is attached as Annex B to this proxy statement and is incorporated herein by reference. Canaccord Genuity’s opinion was intended for the use and benefit of the Board of Directors in
I-35
connection with its evaluation of the merger. Canaccord Genuity’s opinion does not address other business strategies that may be available to our company, the decision of our company to proceed with the merger, or the financial performance or financial condition of our company following the announcement and the consummation of the merger, and does not constitute a recommendation to the Board of Directors or any stockholder as to how that person should vote on the issuance of shares of common stock in the merger or any related matter. The following summary of Canaccord Genuity’s opinion is qualified in its entirety by reference to the full text of the opinion, and you are urged to read the opinion in its entirety.
For purposes of its opinion and in connection with its review of the merger, Canaccord Genuity, among other things:
| | • | | reviewed certain statements and other business and financial information of our company and Cequent and assumed that there had been no material change in the assets, financial condition, business or prospects of our company, since the respective dates of the most recent financial statement made available to Canaccord Genuity; |
| | • | | considered certain financial forecasts, projections and analyses and other forward-looking financial information for our company and Cequent and assumed that the financial results reflected in such forecasts, projections and analyses and other forward-looking financial information will be realized in the amounts and at the time s projected; |
| | • | | reviewed transaction multiples, to the extent publicly available, of certain precedent merger and acquisitions transactions that Canaccord Genuity deemed relevant; |
| | • | | reviewed trading statistics, to the extent publicly available, of selected public companies that Canaccord Genuity deemed relevant; |
| | • | | reviewed the draft merger agreement, dated March 29, 2010, and the financial terms and conditions set forth therein, as well as the exhibits thereto, and assumed that the final forms of the merger agreement and related documentation and the exhibits thereto will not vary in any regard that is material to Canaccord Genuity’s analysis from the versions included in the draft merger agreement that Canaccord Genuity reviewed; and |
| | • | | made such other studies and inquiries, and reviewed such other data, and considered such other factors as Canaccord Genuity deemed, in its sole judgment, to be necessary, appropriate or relevant. |
In addition to the foregoing, Canaccord Genuity discussed with our management their views as to the financial and other information described in the bullet points above and conducted such other analyses and examinations and considered such other financial, economic and market criteria as Canaccord Genuity deemed appropriate to arrive at its opinion.
In arriving at its opinion, Canaccord Genuity assumed and relied upon the accuracy and completeness of all financial and other information provided to or reviewed by it or publicly available, and did not assume any responsibility for independent verification of any such information. With respect to financial projections and other information provided to or reviewed by it, Canaccord Genuity was advised by our management and Cequent’s management that such projections and other information were reasonably prepared on bases reflecting the best currently available estimates and judgments of our management and Cequent’s management as to expected future financial performance. Canaccord Genuity further relied on the assurances of our management and Cequent’s management that we are unaware of any facts that would make the information or projections provided to Canaccord Genuity incomplete or misleading. Canaccord Genuity did not make any independent evaluations or appraisals of any of our or Cequent’s assets, properties, liabilities or securities, nor did Canaccord Genuity make any physical inspection of our or Cequent’s properties or assets. Canaccord Genuity does not have any opinion on any financial forecast or the assumptions upon which they were based, by our management or Cequent’s management, nor does it have any opinion as to the price of our common stock in the future. Canaccord Genuity assumed that the final form of the merger agreement and related documents would be similar in all material respects to the draft agreements reviewed by it.
I-36
The opinion is based on financial, economic, securities market and other conditions prevailing as of the date of the opinion, and on the conditions and prospects, financial and otherwise, of our company as known to Canaccord Genuity as of the date of the opinion. Canaccord Genuity also relied on the accuracy and completeness of both our and Cequent’s representations and warranties in the merger agreement and assumed that each party will perform all of the covenants and agreements required to be performed by it under the merger agreement without material alteration or waiver thereof and that all conditions to the consummation of the merger will be satisfied without waiver thereof or material alteration of the terms of the merger. Events occurring after the date of the opinion could materially affect the assumptions used in preparing the opinion and Canaccord Genuity undertook no obligation to advise any person of any change in any matter affecting its opinion that may come to its attention after the date of the opinion. Canaccord Genuity did not undertake to reaffirm or revise the opinion or otherwise comment upon any events occurring after the date of the opinion and has no obligation to do so.
Canaccord Genuity and its affiliates, as part of their investment banking business, are continually engaged in performing financial analyses with respect to businesses and their securities in connection with mergers and acquisitions, negotiated underwritings, competitive biddings, secondary distributions of listed and unlisted securities, private placements and other transactions, as well as for private, corporate and other purposes. Canaccord Genuity has acted as financial advisor to our company in connection with the transactions contemplated by the merger agreement.
Canaccord Genuity is a full service securities firm engaged, either directly or through its affiliates, in securities trading, investment management, financial planning and benefits counseling, risk management, hedging, financing and brokerage activities for both companies and individuals. In the ordinary course of these activities, Canaccord Genuity and its affiliates may provide such services to us, may actively trade the debt and/or equity securities (or related derivative securities) of our company for their own account and for the accounts of their customers and may at any time hold long and short positions in such securities. Canaccord Genuity was selected by the Board of Directors based on Canaccord Genuity’s experience, expertise, reputation and familiarity with our company.
Canaccord Genuity received a fee for rendering its opinion for the merger, which was not contingent upon completion of the merger. In addition, we agreed to reimburse Canaccord Genuity’s reasonable expenses and to indemnify Canaccord Genuity and related parties for certain liabilities arising out of its engagement. Canaccord Genuity will receive additional compensation upon the closing of the merger. Canaccord Genuity received compensation or payment as a result of its relationship with us in the form of placement agency fees in connection with securities offerings by our company on January 14, 2010, December 24, 2009 and June 9, 2009. In the ordinary course of its trading and brokerage activities, Canaccord Genuity or their affiliates have in the past and may in the future hold positions, for their own account or the accounts of their customers, in equity, debt or other securities of our company. Canaccord Genuity also may provide investment banking services to our company in the future.
In rendering its opinion, (i) Canaccord Genuity expressed no opinion regarding the fairness, from a financial point of view, of (A) any fees or other amounts paid by our company or (B) the issuance by our company of any other securities or the exercisability of any such securities, in each case in connection with the merger, including, without limitation, the transactions contemplated by the loan agreement between our company and Cequent and the issuance of the warrant pursuant thereto, and (ii) Canaccord Genuity expressed no opinion as to the fairness of the amount or nature of any compensation to any officers, directors or employees of our company, or any class of such persons. Canaccord Genuity did not opine as to the merits of the merger compared to any alternative transactions that may be available to our company should we desire to pursue such alternatives.
Summary of Analyses
The following is a summary of the financial analyses performed by Canaccord Genuity in connection with reaching its opinion:
| | • | | Selected Public Pre-Clinical Company Analysis |
| | • | | Selected Precedent Pre-Clinical Acquisition Analysis |
I-37
| | • | | Equity Ownership Analysis |
While the following summaries describe all of the analyses and examinations that Canaccord Genuity deems material to the opinion, they are not a comprehensive description of all analyses and examinations actually conducted by Canaccord Genuity. The preparation of an opinion necessarily is not susceptible to partial analysis or summary description. Canaccord Genuity believes that such analyses and the following summaries must be considered as a whole and that selecting portions of such analyses and of the factors considered, without considering all such analyses and factors, would create an incomplete view of the process underlying the analyses.
In performing its analyses, Canaccord Genuity made numerous assumptions with respect to industry performance and general business and economic conditions such as industry growth, inflation, interest rates and many other matters, many of which are beyond our control and the control of Canaccord Genuity. Any estimates contained in Canaccord Genuity’s analyses are not necessarily indicative of actual values or future results, which may be significantly more or less favorable than suggested by such analyses.
Selected Public Pre-Clinical Company Analysis
Using publicly available information, Canaccord Genuity compared selected financial and operational information of Cequent with similar information of five publicly-traded companies that are in the biopharmaceutical industry considered by Canaccord Genuity to have therapeutics in similar stages of development as Cequent. In this regard, Canaccord Genuity noted that although such companies were considered similar, none of the companies has the same management, make-up, size or mix of business as Cequent. Canaccord Genuity reviewed and analyzed the following publicly-traded companies: NanoViricides, Inc., International Stem Cell Corporation, RXi Pharmaceuticals Corporation, Cellceutix Corporation and Champions Biotechnology, Inc. (collectively, the “Selected Public Pre-Clinical Company Analysis”).
The Selected Public Pre-Clinical Company Analysis produced a range of equity values of $34.9 million to $225.6 million and a range of enterprise values of $34.5 million to $221.5 million, as of March 30, 2010 (where enterprise value is defined as equity value plus face value of outstanding debt and preferred stock, less cash). As a result of this Selected Public Pre-Clinical Company Analysis, Canaccord Genuity derived a mean equity value of approximately $110.7 million and a median equity value of $90.3 million. Canaccord Genuity also derived a mean enterprise value of $108.1 million and a median enterprise value of $81.5 million. Canaccord Genuity observed that the mean and median equity values and enterprise values were greater than Cequent’s implied equity value of $46 million and enterprise value of $37.9 million, based on the merger agreement.
Selected Precedent Pre-Clinical Acquisition Analysis
Canaccord Genuity reviewed certain publicly available information relating to seven selected recent merger and acquisition transactions (the “Selected Precedent Pre-Clinical Acquisition Analysis”) involving companies that had therapeutics in similar stages of development as Cequent or focused on similar indications at the time the transaction was announced. The Selected Precedent Pre-Clinical Acquisition Analysis considered the following transactions:
| | |
Acquiror | | Target |
Silence Therapeutics | | Intradigm |
Pharmaceutical Product Development | | Magen BioSciences |
Roche | | Arius Research |
Eli Lilly | | SGX Pharmaceuticals |
Teva Pharmaceuticals | | CoGenesys |
Inhibitex | | FermaVir Pharmaceuticals |
Roche | | Therapeutic Human Polyclonals |
I-38
Information reviewed in the Selected Precedent Pre-Clinical Acquisition Analysis consisted of, if available, common equity value, defined as the publicly disclosed value of the consideration issued in the relevant acquisition. The Selected Precedent Pre-Clinical Acquisition Analysis produced a range of equity values of $14.5 million to $400.0 million and a range of enterprise values of $14.5 million to $400.0 million. As a result of this, Canaccord Genuity derived a mean and median equity value of $110.5 million and $56.5 million, respectively, and mean and median enterprise values of $105.5 million and $34.4 million, respectively.
Canaccord Genuity observed that the mean and median equity values of the Selected Precedent Pre-Clinical Acquisition Analysis were higher than Cequent’s implied equity value of $46.0 million, based on the merger agreement. Canaccord Genuity also observed that mean enterprise value was higher than the implied enterprise value of $37.9 million, based on the merger agreement and the median enterprise value was in the range of the enterprise values derived by the Selected Precedent Pre-Clinical Acquisition Analysis.
Equity Ownership Analysis
Using the Selected Public Pre-Clinical Company Analysis and Selected Precedent Pre-Clinical Acquisition Analysis, Canaccord Genuity derived a pro forma equity ownership analysis. Canaccord Genuity derived a stripped mean and median enterprise value of $94.9 million and $81.5 million and $64.7 million and $34.4 million for the Selected Public Pre-Clinical Company Analysis and Selected Precedent Pre-Clinical Acquisition Analysis, respectfully (when stripped mean is defined as the average of the enterprise values determined in each analysis, excluding the highest and lowest values). Canaccord Genuity observed the trading history of our common stock over the past 30 days and noted our equity value of $50.2 million on February 26, 2010, $53.2 million on March 30, 2010, and $56.1 million based on the 10-day volume weighted average price as of March 30, 2010.
Canaccord Genuity added Cequent’s cash, estimated at closing to be $8.1 million, to the stripped mean and median enterprise values derived from the Selected Public Pre-Clinical Company Analysis and Selected Precedent Pre-Clinical Acquisition Analysis to produce implied equity values for Cequent of $43.5 million to $72.8 million based on the Selected Public Pre-Clinical Company Analysis and $89.6 million to $113.0 million based on the Selected Precedent Pre-Clinical Acquisition Analysis.
Canaccord Genuity derived the ranges of pro forma ownership for our company based on the implied values for Cequent and the recent trading values of our company. Based on the Selected Public Pre-Clinical Company Analysis, the derived equity ownership range for our company was 32.8% to 38.5% and Canaccord Genuity observed that our implied ownership of 54.9%, based on the merger agreement, was higher than the range. Based on the Selected Precedent Pre-Clinical Acquisition Analysis, the derived ownership range for our company was 40.8% to 56.9% and Canaccord Genuity observed that our implied ownership of 54.9%, based on the merger agreement, was within this range.
Canaccord Genuity noted that these analyses necessarily involve complex considerations and judgments concerning differences in financial and operating characteristics of Cequent and the companies included in the Selected Public Pre-Clinical Company Analysis and Selected Precedent Pre-Clinical Acquisition Analysis and other factors that could affect the acquisition value of the companies to which Cequent is being compared. A mathematical analysis such as determining the median or mean is not necessarily in itself a meaningful method of using comparable transaction data.
Conclusion
Based upon its analyses, and subject to the assumptions made, matters considered, and qualifications and limitations of the review undertaken in connection with the opinion, Canaccord Genuity is of the opinion that, as of the date of the opinion, the merger consideration to be paid by us in connection with the merger as provided in the merger agreement is fair to our company from a financial point of view.
I-39
Certain U.S. Federal Income Tax Consequences of the Merger
Material United States Federal Income Tax Consequences to Cequent Stockholders
We expect the merger to be treated as a tax-free reorganization pursuant to Section 368(a) of the Internal Revenue Code of 1986, as amended. If the merger is treated as a tax-free reorganization, generally the stockholders of Cequent, for federal income tax purposes, will recognize no gain or loss upon their receipt of common stock in the merger, except with respect to cash received by Cequent stockholders instead of fractional shares of common stock, or upon exercise of their appraisal rights. As a stockholder of our company, you will not incur any tax liability as a result of the merger.
Regulatory Approvals
We are not aware of any government regulatory approvals required to be obtained with respect to the consummation of the merger, other than the filing and acceptance of a certificate of merger with the office of the Secretary of State of the State of Delaware, and compliance with all applicable state securities laws regarding the offering and issuance of the merger shares.
Federal Securities Laws Consequences
The shares of our common stock that are being issued to the Cequent stockholders are being issued to such stockholders pursuant to an exemption from registration provided by Section 4(2) of the Securities Act and Rule 506 of Regulation D promulgated thereunder. At the effective time of the merger, we will enter into a registration rights agreement pursuant to which we will agree to file a shelf registration statement with the SEC under the Securities Act relating to shares of our common stock that we are issuing to certain of the principal stockholders of Cequent no later than 45 calendar days from the closing date of the merger, and to provide such stockholders with other demand and piggy-back registration rights. We have also agreed in the merger agreement to file a registration statement on Form S-8 with the SEC no later than 90 calendar days from the closing date registering the shares of our common stock issuable upon the exercise of the Cequent options or warrants assumed by us in the merger.
I-40
PER SHARE MARKET PRICE AND DIVIDEND INFORMATION
Recent Share Prices
Our common stock is listed on The Nasdaq Global Market under the symbol “MRNA”. Cequent is a private company, and thus its common stock is not publicly traded.
The following table sets forth the high and low price per share of our common stock as reported on The Nasdaq Global Market on March 31, 2010, the last completed trading day prior to the announcement of the merger, on April 1, 2010, the trading day on which the merger was announced, and on June 2, 2010.
| | | | | | |
| | | MDRNA
Common Stock |
| | | High | | Low |
March 31, 2010 | | $ | 1.12 | | $ | 1.05 |
April 1, 2010 | | $ | 1.33 | | | 1.16 |
June 2, 2010 | | $ | 1.08 | | | 1.01 |
Because the market price of our common stock is subject to fluctuation prior to the closing date of the merger, the market value of the shares of our common stock that holders of Cequent common stock will receive in the merger may increase or decrease.
Historical Share Prices
The following table shows the range of high and low prices for our common stock for each of the two most-recently completed fiscal years.
| | | | | | |
| | | 2008 |
| | | High | | Low |
First Quarter | | $ | 3.94 | | $ | 1.91 |
Second Quarter | | | 2.85 | | | 1.11 |
Third Quarter | | | 1.25 | | | 0.38 |
Fourth Quarter | | | 0.85 | | | 0.14 |
| | | | | | |
| | | 2009 |
| | | High | | Low |
First Quarter | | $ | 0.64 | | $ | 0.21 |
Second Quarter | | | 3.55 | | | 0.64 |
Third Quarter | | | 1.93 | | | 1.21 |
Fourth Quarter | | | 1.83 | | | 0.77 |
| | | | | | |
| | | 2010 |
| | | High | | Low |
First Quarter | | $ | 1.87 | | $ | 0.82 |
Second Quarter (through June 2, 2010) | | | 1.42 | | | 0.96 |
Dividend Information
We have never paid cash dividends. If the merger is not consummated, the Board of Directors presently intends to continue a policy of retaining all earnings to finance the expansion of our business. Following the merger, it is expected that the Board of Directors will continue the policy of not paying cash dividends in order to retain earnings for reinvestment in the business of the combined company.
Holders
As of June 1, 2010, there were approximately 17,000 beneficial holders of record of our common stock.
I-41
UNAUDITED PRO FORMA CONDENSED COMBINED CONSOLIDATED FINANCIAL STATEMENTS
The following unaudited pro forma condensed combined consolidated financial statements give effect to the transaction between our company and Cequent using the purchase method of accounting for the business combination.
We cannot assure you that we and Cequent will not incur charges in excess of those included in the pro forma total consideration related to the transaction or that management will be successful in its efforts to integrate the operations of the companies.
In addition to the impact of ongoing integration activities, the timing of completion of the transaction and other changes in net tangible and intangible assets that occur prior to completion of the transaction could cause material differences in the information presented.
The merger has not been consummated as of the date of the preparation of this unaudited pro forma condensed combined consolidated financial information and there can be no assurances that the merger transaction will be consummated.
The unaudited pro forma condensed combined consolidated financial information does not include any adjustments related to restructuring or one-time charges, potential profit improvements, potential cost savings or other costs which may result from the merger, or the result of final valuations of intangible assets and liabilities, which will not be determined until after the consummation of the merger, or the result of amounts which may be borrowed by our company from Cequent pursuant to terms of the loan agreement, which was entered into concurrent and in connection with the execution of the merger agreement.
I-42
UNAUDITED PRO FORMA CONDENSED COMBINED CONSOLIDATED
BALANCE SHEET
As of March 31, 2010
(in thousands)
| | | | | | | | | | | | | | | | | | |
| | | Historical | | | Pro Forma
Adjustments | | | | | Pro Forma
Combined | |
| | | MDRNA | | | Cequent | | | | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | | | (Unaudited) | |
Current assets: | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 2,630 | | | $ | 2,116 | | | $ | 5,984 | | | B | | $ | 10,730 | |
Restricted cash | | | 1,156 | | | | — | | | | — | | | | | | 1,156 | |
Prepaid expenses and other current assets | | | 553 | | | | 32 | | | | — | | | | | | 585 | |
| | | | | | | | | | | | | | | | | | |
Total current assets | | | 4,339 | | | | 2,148 | | | | 5,984 | | | | | | 12,471 | |
Property and equipment, net | | | 4,258 | | | | 367 | | | | — | | | C | | | 4,625 | |
Intangible assets | | | — | | | | — | | | | 41,700 | | | D | | | 41,700 | |
Goodwill | | | — | | | | — | | | | 8,278 | | | E | | | 8,278 | |
Other assets | | | — | | | | 45 | | | | — | | | | | | 45 | |
| | | | | | | | | | | | | | | | | | |
Total assets | | $ | 8,597 | | | $ | 2,560 | | | $ | 55,962 | | | | | $ | 67,119 | |
| | | | | | | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | | | | | |
Accounts payable | | $ | 2,139 | | | $ | 46 | | | | 1,549 | | | F | | $ | 3,734 | |
Other accrued liabilities | | | 3,044 | | | | 232 | | | | — | | | | | | 3,276 | |
Deferred revenue — current portion | | | 126 | | | | 576 | | | | (576 | ) | | G | | | 126 | |
| | | | | | | | | | | | | | | | | | |
Total current liabilities | | | 5,309 | | | | 854 | | | | 973 | | | | | | 7,136 | |
Deferred revenue, net of current portion | | | — | | | | 693 | | | | (693 | ) | | G | | | — | |
Deferred rent and other liabilities | | | 1,597 | | | | 12 | | | | — | | | | | | 1,609 | |
Fair value liability for price adjustable warrants | | | 11,214 | | | | — | | | | — | | | | | | 11,214 | |
Deferred taxes | | | — | | | | — | | | | 14,595 | | | D | | | 14,595 | |
| | | | | | | | | | | | | | | | | | |
Total liabilities | | | 18,120 | | | | 1,559 | | | | 14,875 | | | | | | 34,554 | |
| | | | | | | | | | | | | | | | | | |
Commitments and contingencies | | | | | | | | | | | | | | | | | | |
Stockholders’ equity (deficit): | | | | | | | | | | | | | | | | | | |
Preferred stock | | | — | | | | 16,897 | | | | (16,897 | ) | | H | | | — | |
Common stock and additional paid in capital | | | 262,984 | | | | 1,901 | | | | 43,637 | | | A | | | 306,621 | |
| | | | | | | | | | | (1,901 | ) | | H | | | | |
Accumulated deficit | | | (272,507 | ) | | | (17,797 | ) | | | (1,549 | ) | | F | | | (274,056 | ) |
| | | | | | | | | | | 17,797 | | | H | | | | |
| | | | | | | | | | | | | | | | | | |
Total stockholders’ equity (deficit) | | | (9,523 | ) | | | 1,001 | | | | 41,087 | | | | | | 32,565 | |
| | | | | | | | | | | | | | | | | | |
Total liabilities and stockholders’ equity (deficit) | | $ | 8,597 | | | $ | 2,560 | | | $ | 55,962 | | | | | $ | 67,119 | |
| | | | | | | | | | | | | | | | | | |
See accompanying notes to unaudited pro forma condensed combined consolidated financial statements.
I-43
UNAUDITED PRO FORMA CONDENSED COMBINED CONSOLIDATED
STATEMENT OF OPERATIONS
For the quarter ended March 31, 2010
(in thousands, except per share data)
| | | | | | | | | | | | | | | | |
| | | Historical | | | Pro Forma
Adjustments | | | | Pro Forma
Combined | |
| | | MDRNA | | | Cequent | | | | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | | (Unaudited) | |
License and other revenue | | $ | 184 | | | $ | 144 | | | — | | | | $ | 328 | |
| | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | |
Research and development | | | 3,599 | | | | 291 | | | — | | | | | 3,890 | |
Selling, general and administrative | | | 2,559 | | | | 764 | | | — | | | | | 3,323 | |
Restructuring | | | 26 | | | | — | | | — | | | | | 26 | |
| | | | | | | | | | | | | | | | |
Total operating expenses | | | 6,184 | | | | 1,055 | | | — | | | | | 7,239 | |
| | | | | | | | | | | | | | | | |
Loss from operations | | | (6,000 | ) | | | (911 | ) | | — | | | | | (6,911 | ) |
| | | | | | | | | | | | | | | | |
Other income (expense): | | | | | | | | | | | | | | | | |
Interest and other expense | | | (780 | ) | | | — | | | — | | | | | (780 | ) |
Change in fair value liability for price adjustable warrants | | | (2,710 | ) | | | — | | | — | | | | | (2,710 | ) |
| | | | | | | | | | | | | | | | |
Total other expense, net | | | (3,490 | ) | | | — | | | — | | | | | (3,490 | ) |
| | | | | | | | | | | | | | | | |
Net loss | | $ | (9,490 | ) | | $ | (911 | ) | | — | | | | | (10,401 | ) |
| | | | | | | | | | | | | | | | |
Net loss per common share — basic and diluted | | $ | (0.20 | ) | | | | | | — | | | | $ | (0.12 | ) |
| | | | | | | | | | | | | | | | |
Shares used in computing net loss per share — basic and diluted | | | 47,328 | | | | | | | 38,351 | | I | | | 85,679 | |
| | | | | | | | | | | | | | | | |
See accompanying notes to unaudited pro forma condensed combined consolidated financial statements.
I-44
UNAUDITED PRO FORMA CONDENSED COMBINED CONSOLIDATED
STATEMENT OF OPERATIONS
For the year ended December 31, 2009
(in thousands, except per share data)
| | | | | | | | | | | | | | | | |
| | | Historical | | | Pro Forma
Adjustments | | | | Pro Forma
Combined | |
| | | MDRNA | | | Cequent | | | | |
| | | | | | | | | (Unaudited) | | | | (Unaudited) | |
License and other revenue | | $ | 14,732 | | | $ | 581 | | | — | | | | $ | 15,313 | |
| | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | |
Research and development | | | 14,882 | | | | 3,498 | | | — | | | | | 18,380 | |
Selling, general and administrative | | | 10,088 | | | | 2,314 | | | — | | | | | 12,402 | |
Restructuring | | | 455 | | | | — | | | — | | | | | 455 | |
| | | | | | | | | | | | | | | | |
Total operating expenses | | | 25,425 | | | | 5,812 | | | — | | | | | 31,237 | |
| | | | | | | | | | | | | | | | |
Loss from operations | | | (10,693 | ) | | | (5,231 | ) | | — | | | | | (15,924 | ) |
| | | | | | | | | | | | | | | | |
Other income (expense): | | | | | | | | | | | | | | | | |
Interest income | | | 5 | | | | 11 | | | — | | | | | 16 | |
Interest and other expense | | | (538 | ) | | | (36 | ) | | — | | | | | (574 | ) |
Change in fair value liability for price adjustable warrants | | | 2,526 | | | | — | | | — | | | | | 2,526 | |
Gain on settlement of liabilities, net | | | 654 | | | | — | | | — | | | | | 654 | |
| | | | | | | | | | | | | | | | |
Total other income (expense), net | | | 2,647 | | | | (25 | ) | | — | | | | | 2,622 | |
| | | | | | | | | | | | | | | | |
Net loss | | $ | (8,046 | ) | | $ | (5,256 | ) | | — | | | | | (13,302 | ) |
| | | | | | | | | | | | | | | | |
Net loss per common share — basic and diluted | | $ | (0.21 | ) | | | | | | — | | | | $ | (0.18 | ) |
| | | | | | | | | | | | | | | | |
Shares used in computing net loss per share — basic and diluted | | | 37,457 | | | | | | | 38,351 | | I | | | 75,808 | |
| | | | | | | | | | | | | | | | |
See accompanying notes to unaudited pro forma condensed combined consolidated financial statements.
I-45
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED CONSOLIDATED
FINANCIAL STATEMENTS
1. Description of Transaction
We and Cequent have entered into an Agreement and Plan of Merger, dated as of March 31, 2010, which is referred to as the merger agreement. The merger agreement contains the terms and conditions of the proposed business combination of our company and Cequent. Under the merger agreement, Calais Acquisition Corp., a wholly-owned subsidiary of our company, which is referred to as the merger sub, will merge with and into Cequent. This transaction is referred to as the merger. Cequent will then continue as a wholly-owned subsidiary of our company.
At the effective time of the merger, each share of Cequent Series A-1 convertible preferred stock and common stock outstanding immediately prior to the effective time of the merger will be exchanged for shares of our common stock at an exchange ratio that implies a purchase price for Cequent common and preferred stockholders of approximately $44 million, plus an additional value of approximately $2 million to Cequent warrant and option holders, based on the 10-day volume-weighted average price of our common stock on The NASDAQ Global Market ending on March 31, 2010.
Based upon the outstanding shares of our common stock and assuming the exercise or conversion of all of our outstanding exercisable and non-exercisable warrants and stock options, and assuming the exercise or conversion of all of Cequent’s exercisable and non-exercisable warrants, stock options and preferred stock, immediately following the completion of the merger, Cequent securityholders would own approximately 37% of the combined company’s common stock and our securityholders would own approximately 63% of the combined company’s common stock. In addition to considering these relative securityholdings, MDRNA and Cequent management also considered the proposed composition of the Board of Directors, the proposed structure and members of the executive management team, the size of the combining entities and the terms of the exchange of equity interests in determining the accounting acquirer. Based on the weight of these factors, it was concluded that MDRNA is the accounting acquirer.
2. Basis of Presentation
The unaudited pro forma condensed combined consolidated financial statements, which have been prepared by MDRNA have been derived from historical consolidated financial statements of MDRNA and historical financial statements of Cequent.
The unaudited pro forma condensed combined consolidated financial statements were prepared in accordance with Securities and Exchange Commission Regulation S-X Article 11, using the purchase method of accounting based on Accounting Standards Codification (ASC) 805, Business Combinations, and are based on the historical consolidated financial statements of MDRNA and the acquired business lines after giving effect to the stock to be issued by MDRNA to consummate the acquisition, as well as pro forma adjustments.
ASC 805 requires, among other things, that most assets acquired and liabilities assumed be recognized at their fair values, as determined in accordance with ASC 820, Fair Value Measurements, as of the acquisition date and that the fair value of acquired in-process research and development be recorded on the balance sheet regardless of the likelihood of success as of the acquisition date. In addition, ASC 805 establishes that the consideration transferred be measured at the closing date of the asset acquisition at the then-current market price, which may be different than the amount of consideration assumed in these unaudited pro forma condensed combined consolidated financial statements.
ASC 820, as amended, defines the term “fair value” and sets forth the valuation requirements for any asset or liability measured at fair value, expands related disclosure requirements and specifies a hierarchy of valuation techniques based on the nature of the inputs used to develop the fair value measures. Fair value is defined in
I-46
ASC 820, as amended, as “the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.” This is an exit price concept for the valuation of the asset or liability. In addition, market participants are assumed to be buyers and sellers in the principal (or the most advantageous) market for the asset or liability. Fair value measurements for an asset assume the highest and best use by these market participants. As a result of these standards, MDRNA may be required to record assets which are not intended to be used or sold and/or to value assets at fair value measures that do not reflect MDRNA’s intended use of those assets. Many of these fair value measurements can be highly subjective and it is also possible that other professionals, applying reasonable judgment to the same facts and circumstances, could develop and support a range of alternative estimated amounts.
Under the purchase method of accounting, the assets acquired and liabilities assumed will be recorded as of the completion of the asset acquisition, primarily at their respective fair values and added to those of MDRNA. Financial statements and reported results of operations of MDRNA issued after completion of the asset acquisition will reflect these values, but will not be retroactively restated to reflect the historical financial position or results of operations of Cequent.
Under ASC 805, acquisition-related transaction costs (i.e., advisory, legal, valuation, other professional fees) and certain acquisition-related restructuring charges impacting the target company are expensed in the period in which the costs are incurred. Total advisory, legal, regulatory and valuation costs expected to be incurred by MDRNA are estimated to be approximately $1.5 million. As of March 31, 2010, MDRNA has incurred approximately $0.6 million in acquisition related transaction costs.
3. Accounting Policies
Upon consummation of the merger, MDRNA will perform a detailed review of Cequent’s accounting policies. As a result of that review, it may become necessary to conform the combined company’s financial statements to be consistent with those accounting policies that are determined to be more appropriate for the combined company. The unaudited pro forma condensed combined consolidated financial statements do not assume any differences in accounting policies.
4. Preliminary Purchase Price Determination
The following is a preliminary estimate of consideration expected to be transferred to effect the acquisition of Cequent:
The total preliminary purchase price is estimated at $43.7 million based on the closing price of our common stock on March 31, 2010 of $1.10 per share and is comprised of (in millions):
| | | | |
Equity Component | | $ | 44.7 | |
Exercise price of vested Cequent options and warrants | | | (1.0 | ) |
| | | | |
Total purchase consideration | | $ | 43.7 | |
| | | | |
5. Preliminary Allocation of Consideration Transferred
The estimated total purchase price of $43.7 million was allocated to the net tangible and intangible assets acquired and liabilities assumed based on their fair values as follows (in millions):
| | | | |
Cash (a) | | $ | 8.1 | |
Identifiable Intangible Assets (b) | | | 41.7 | |
Fixed Assets | | | 0.4 | |
Other Assets | | | 0.1 | |
Deferred Tax Liability (c) | | | (14.6 | ) |
Other Liabilities | | | (0.3 | ) |
Goodwill (d) | | | 8.3 | |
| | | | |
Total purchase consideration | | $ | 43.7 | |
| | | | |
I-47
| (a) | The merger agreement contains a condition that as of the closing date, Cequent must have a cash balance of at least (i) $5,100,000, minus the amount of Cequent’s ordinary course operating expenses from June 2, 2010 through the Closing Date; plus (iii) the difference of $3,000,000 and the aggregate principal amount loaned to MDRNA by Cequent under the Loan Agreement. The merger agreement permits Cequent to sell additional shares of Cequent capital stock, or debt or warrants convertible into capital stock of Cequent, to new and existing investors of Cequent, including but not limited to the sale of up to $7,520,000 of Series A-1 Convertible Preferred Stock. In April 2010, Cequent raised proceeds of approximately $2.5 million through the sale of 2,517,173 shares of Series A-1 Convertible Preferred Stock. Cequent is seeking to raise additional proceeds through additional sales of Series A-1 Convertible Preferred Stock. |
| (b) | As of the completion of the merger, identifiable intangible assets are required to be measured at fair value consistent with ASC Subtopic 820-10. The fair value measurements were performed after considering the highest and best use of the acquired intangible assets by market participants. |
The fair value of the identifiable intangible assets was determined using either the income or cost approach.
For purposes of these unaudited pro forma condensed combined consolidated financial statements and using certain high-level assumptions, the fair value of the identifiable intangible assets and their weighted-average useful lives have been estimated as follows:
| | |
In-Process Technology — Program #1 | | $33.5 million (Indefinite Life) |
In-Process Technology — Program #2 | | 6.2 million (Indefinite Life) |
In-Process Technology — Program #3 | | 2.0 million (Indefinite Life) |
| |
| | $41.7 million |
The income approach, which relies on future estimates of cash flows, was used to estimate the fair value of the Program #1.
MDRNA believes that the information gathered during the due diligence process and from discussions with Cequent were adequate to perform preliminary fair value measurements of the primary intangible assets.
MDRNA valued the in-process technology of Program #1 and #2 utilizing a discounted cash flow model, which uses forecasts of future revenues and expenses related to the intangible assets. Program #3 was valued using the cost approach due to the early stage of development.
In-process technology will be accounted for as indefinite-lived intangible assets until the projects are completed or abandoned. Once the projects are completed or abandoned, MDRNA will determine the useful lives of these intangible assets, if any, and the intangible assets will be amortized over the useful lives.
Intangible assets with indefinite useful lives and goodwill will not be amortized but will be tested for impairment at least annually. All intangible assets and goodwill are also tested for impairment when certain indicators are present. In the future, if it were determined that intangible assets or goodwill are impaired, an impairment charge would be recorded at that time.
| (c) | As of the completion of the merger, MDRNA will provide deferred taxes and other tax adjustments as part of the accounting for the acquisition, primarily related to the estimated fair value adjustments for net acquired intangibles (see Note 6. Pro Forma Adjustments). |
| (d) | Goodwill is calculated as the difference between the acquisition date fair value of the consideration expected to be transferred and the values assigned to the assets acquired and liabilities assumed. Goodwill is not amortized, but is evaluated annually for impairment. |
6. Pro Forma Adjustments
Pro forma adjustments reflect those matters that are a direct result of the merger, which are factually supportable and, for pro forma adjustments to the pro forma condensed combined consolidated statement of operations, are expected to have continuing impact. The pro forma adjustments are based on preliminary estimates which may change as additional information is obtained.
I-48
Adjustments included in the column under the heading “Pro Forma Adjustments” represent the following:
| (A) | The value of our shares to be issued is based on a per share value of $1.10, which is equal to the closing price per share of our common stock as reported on The Nasdaq Global Market on March 31, 2010. The equity component of the purchase price has been estimated at approximately $43.7 million consisting of the following shares to be issued, offset by the exercise price of vested stock options and warrants: |
| | • | | Approximately 38,350,620 shares of our common stock to be issued to holders of shares of Cequent preferred and common stock. |
| | • | | Approximately 2,274,832 shares of our common stock to be reserved for Cequent warrant holders, and Cequent option holders, for options vested or expected to vest as of the date of the merger which will be replaced by warrants and vested options to purchase shares of our common stock. The total exercise price for these options and warrants is estimated at $1.0 million. |
The pro forma adjustment for shares and replacement warrants and vested options expected to be issued upon the merger includes the estimated value for the holders of the common stock, preferred stock, warrants and vested options, which represents an estimated 40,625,452 shares valued at approximately $44.7 million based on the March 31, 2010 closing price per share of our common stock, less proceeds from the exercise of vested stock options and warrants estimated at $1.0 million.
Approximately 579,267 shares of our common stock will be reserved for holders of unvested Cequent options as of the date of the merger which will be replaced by unvested options to purchase shares of our common stock. The stock-based compensation expense for the replacement options will be reflected in our statement of operations after the merger is consummated.
| (B) | The merger agreement contains a condition that as of the closing date, Cequent must have a cash balance of at least (i) $5,100,000, minus the amount of Cequent’s ordinary course operating expenses from June 2, 2010 through the Closing Date; plus (iii) the difference of $3,000,000 and the aggregate principal amount loaned to MDRNA by Cequent under the Loan Agreement. The merger agreement permits Cequent to sell additional shares of Cequent capital stock, or debt or warrants convertible into capital stock of Cequent, to new and existing investors of Cequent, including but not limited to the sale of up to $7,520,000 of Series A-1 Convertible Preferred Stock. In April 2010, Cequent raised proceeds of approximately $2.5 million through the sale of 2,517,173 shares of Series A-1 Convertible Preferred Stock. Cequent is seeking to raise additional proceeds through additional sales of Series A-1 Convertible Preferred Stock. The pro forma adjustment for cash includes proceeds of approximately $2.5 million raised in April 2010 and an estimated $3.5 million for additional proceeds to be raised prior to the closing. |
| (C) | Management believes based upon its preliminary assessment that the estimated fair value of Cequent property and equipment, which is comprised primarily of lab and office equipment, reasonably approximates the Cequent net book value of the assets. |
| (D) | Portions of the purchase price are expected to be allocated to indefinite-lived intangible assets which were identified by management and have been valued on a number of factors in a preliminary appraisal. The pro forma adjustment amounts reflect management’s preliminary estimates of the fair values of assets to be acquired and liabilities to be assumed at the date of the merger. These estimates are preliminary because the merger has not yet occurred and management has not yet obtained all of the information that it has arranged to obtain and that is known to be available. |
We have estimated a deferred tax liability of approximately $14.6 million relating to certain indefinite-lived intangible assets using a 35% tax rate.
| (E) | Goodwill represents the excess of purchase price over the fair value of Cequent’s net assets, including identifiable intangible assets, acquired in the merger. Goodwill to be recorded in connection with the merger will differ from the amount presented here as management obtains all information that it has arranged to obtain and that is known to be available, and adjusts the allocation of purchase price accordingly and because the purchase price accounting will be based on MDRNA’s stock price at closing. |
I-49
| (F) | Expenses related to the merger including legal fees, investment banking fees, accounting fees, printing, filing and other costs and fees will be expensed as incurred. A pro forma adjustment estimated in the amount of $1,549,000 has been included on the unaudited pro forma condensed combined consolidated balance sheet as an increase in accounts payable, and as an increase to accumulated deficit. |
| (G) | Deferred revenue previously recorded by Cequent of approximately $1.3 million at March 31, 2010 has been eliminated as there are no future performance obligations in connection with the Definitive Research and License Agreement for selected Cequent Modulators, the right of which to enter into until June 13, 2012 has been granted to Novartis BioVentures Ltd. (or its affiliate). |
| (H) | The pro forma adjustments represent the elimination of Cequent’s stockholders equity accounts. |
| (I) | The weighted average number of shares is based on our historical weighted average shares outstanding and increased to give effect to the issuance of approximately 38,350,620 shares in connection with the merger. This excludes the effect of 2,854,099 common stock equivalents (stock options and warrants) to be issued in connection with the merger since such inclusion in the computation would be anti-dilutive. |
I-50
INTERESTS OF CERTAIN PERSONS IN THE MERGER
Interests of MDRNA’s Executive Officers and Directors in the Merger
Certain of our directors and executive officers may have interests in the merger that differ from, are in addition to, or conflict with interests of our stockholders. For example, it is anticipated that all of the current executive officers of our company will remain in the same positions with the combined company, and that three of the members of our Board of Directors will continue to serve on the Board of Directors of the combined company. The Board has also determined that all outstanding common stock purchase options held by the three current members of the Board who will not continue to serve on the Board of the combined company will vest immediately upon the consummation of the merger, and that all of such options will remain exercisable until the earlier of the four-year anniversary of the effective time and the expiry of such options. You should be aware of these interests when considering the recommendation of our Board of Directors that you vote in favor of the issuance of shares of our common stock pursuant to the merger agreement. The Board of Directors was aware of these potential conflicts of interest during its deliberations on the merits of the merger and in making its decision to approve the merger, the merger agreement and the related transactions.
Waiver of Potential Changes of Control
Prior to the execution of the merger agreement, each of J. Michael French, our President and Chief Executive Officer, Peter S. Garcia, our Chief Financial Officer and Secretary, and Barry Polisky, Ph.D., our Chief Scientific Officer, entered into a waiver agreement with us pursuant to which each such executive officer waived any and all right, title, claim and interest that he may have to receive any payments or accelerated vesting of equity awards under such executive officer’s employment agreement with us or under any equity compensation plan of our company, in each case as a result of the merger being deemed a change of control (as defined in the respective employment agreement or plan). In addition, we and each of Mr. French, Mr. Garcia and Dr. Polisky agreed that if the executive’s employment is terminated on or before December 31, 2010 for any reason other than termination for cause, then notwithstanding anything to the contrary contained in any employment agreement, grant agreement or in any of our equity compensation plans, any and all unvested common stock options held by the executive shall immediately vest in full upon the effective date of such termination, and shall remain exercisable for a period of two (2) years thereafter (but in no event after the original expiration date of the award). Moreover, each executive acknowledged that any cash received by us as a result of the consummation of the merger shall not be counted toward the $5 million in unrestricted cash threshold necessary to trigger payment by us to the executive of the retention bonuses previously approved by the Board of Directors.
On April 22, 2010, Alison D. Silva, the Vice President, Drug Development of Cequent, entered into a similar waiver agreement with Cequent.
I-51
THE MERGER AGREEMENT
The following summary of the merger agreement is qualified by reference to the complete text of the merger agreement, which is incorporated by reference and attached hereto as Annex A. The merger agreement has been included to provide you with information regarding its terms. You are encouraged to read the entire merger agreement. The merger agreement is not intended to provide any other factual information about our company or Cequent. Such information can be found elsewhere in this proxy statement and in the other public filings that we make with the SEC, which are available without charge atwww.sec.gov.
Structure of the Merger
Under the merger agreement, merger sub will merge with and into Cequent, with Cequent becoming the surviving corporation and a wholly-owned subsidiary of our company.
Timing of Closing; Effective Time of the Merger
The closing will occur at a time and date to be specified by the parties, which shall be no later than the fifth business day after the day on which the last of the conditions set forth in the merger agreement has been satisfied or waived, unless we and Cequent mutually agree to a different date or the merger agreement has been terminated prior to such date. We expect that, as soon as practicable following the approval by our stockholders of Proposal Nos.1 and 8 at the annual meeting, the parties will file a certificate of merger with the Secretary of State of Delaware, at which time the merger will be effective.
Merger Consideration
At the effective time of the merger, each share of Cequent Series A-1 convertible preferred stock and common stock outstanding immediately prior to the merger will be exchanged for shares of our common stock at an exchange ratio that implies a purchase price for Cequent common and preferred stockholders of approximately $44 million, plus an additional value of approximately $2 million to Cequent’s warrant and option holders, based on the 10-day volume-weighted average price of our common stock on The NASDAQ Global Market ending on March 31, 2010.
Based upon the outstanding shares of our common stock on March 31, 2010, and assuming the conversion of all of Cequent’s preferred stock on March 31, 2010, immediately following the completion of the merger, Cequent common and preferred stockholders will own approximately 44% of the combined company’s common stock and our stockholders will own approximately 56% of the combined company’s common stock.
Based upon the outstanding shares of our common stock and assuming the exercise or conversion of all of our outstanding exercisable and non-exercisable warrants and stock options on March 31, 2010, and assuming the exercise or conversion of all of Cequent’s exercisable and non-exercisable warrants, stock options and preferred stock on March 31, 2010, immediately following the completion of the merger, Cequent securityholders would own approximately 37% of the combined company’s common stock and our securityholders would own approximately 63% of the combined company’s common stock. This number assumes the exercise for cash of all warrants and stock options.
Cash in Lieu of Fractional Shares or Warrants
We will not issue any fractional shares. In lieu of fractional shares, Cequent stockholders will receive cash in an amount equal to the sum of the fractional share amount multiplied by $1.1496.
I-52
Treatment of Cequent Stock Options and Warrants
At the effective time of the merger, all unexercised outstanding options granted by Cequent and warrants to purchase shares of Cequent common stock will be assumed by us and will be exercisable for the number of shares that the holder would have received if the option or warrant had been exercised prior to the effective time. The exercise price of such assumed options and warrants shall be appropriate adjusted to give effect to the terms and conditions of the merger agreement.
Exchange of Shares
Promptly after the effective time of the merger, we will provide to each holder of Cequent stock instructions explaining how to surrender Cequent stock certificates to us. Holders of Cequent stock that surrender their certificates to us, together with a properly completed letter of transmittal, will receive the appropriate merger consideration. Holders of unexchanged shares of Cequent stock will not be entitled to receive any dividends or other distributions payable by us after the closing until their certificates are surrendered.
Board of Directors and Related Matters
At the effective time of the merger, we will enter into a stockholders’ agreement with certain of the principal stockholders of Cequent, which we refer to collectively as the designators. As required by the stockholders’ agreement, upon completion of the merger we will expand the size of our Board of Directors from six to seven, and three of our existing directors will resign from their positions on the Board. Then, if the Nominating and Corporate Governance Committee of our Board of Directors deems, in the exercise of its reasonable good faith discretion, the persons designated by the designators to be qualified, we will appoint the three directors designated by the designators to fill the vacancies and to serve on the Board until our 2011 Annual Meeting of Stockholders.
It is expected that Peter D. Parker, the President, Chief Executive Officer, and a member of the Board of Directors of Cequent, Dr. Chiang Li, a member of the Board of Directors of Cequent and a Founder of Cequent, and Dr. Michael Taylor, a member of the Board of Directors of Cequent, will be designated to serve on the Board of Directors of the combined company. The three directors to be designated by the designators will select a seventh member of the Board of Directors, which person shall be independent under the rules of The NASDAQ Stock Market and shall serve as the Chairman of the Board.
The Board of Directors of the combined company following the merger will also include two members from the existing Board of our company, who are expected to be James M. Karis and Gregory Sessler. J. Michael French will serve as the President, Chief Executive Officer and as a member of the Board of Directors of the combined company.
Biographical information as to each of Mr. Parker, Dr. Chiang and Dr. Taylor is set forth below:
Peter D. Parker — Mr. Parker has served as the President and CEO, and as a member of the Board of Directors, of Cequent since September 2006. Prior to joining Cequent, Mr. Parker was a General Partner at Ampersand Ventures where he focused on the firm’s Life Sciences activities. He has served as a director of numerous companies including ACLARA BioSciences, Tomah Products, VITEX, Magellan Biosciences, Dynex and Pentose Pharmaceuticals and as Chairman of Alexis, NOVEX, CoPharma, Huntington Laboratories, Protein Ingredient Technologies, Cyclis Pharmaceuticals, Nanodyne, Panacos Pharmaceuticals, AC Tech, Boston Heart Lab and TekCel. Prior to Ampersand, Mr. Parker spent fourteen years at AMAX, Inc. where he was President of Climax Performance Materials Corporation and Corporate Director of Research and Development. He holds B.S. and M.S. degrees from Columbia University.
Chiang J. Li, M.D — Dr. Li is the founder of Cequent and has served as a member of the Board of Directors of Cequent since November 2006. Dr. Li is Chairman and Chief Executive Officer, Boston Biomedical, Inc. From
I-53
September 2003 to January 2007, Dr. Li was Chief Scientific Officer, Executive Vice President and Head of ArQule Biomedical Institute, ArQule Inc. (Nasdaq:ARQL). Dr. Li joined ArQule in September 2003. He previously served as the scientific founder and Vice President of Research at Cyclis Pharmaceuticals, Inc.
His research team at Harvard invented transkingdom RNAi technology, which promises to accelerate biomedical research and medical therapies based on RNA interference. Dr. Li graduated from the Harvard-MIT Division of Health Science and Technology and received his M.D. degree Magna Cum Laude from Harvard Medical School, completed medical residency and fellowship at Harvard’s Brigham Women’s Hospital/Dana-Farber Cancer Institute and Beth Israel Deaconess Medical center.
Michael D. Taylor, Ph.D. — Dr. Taylor has served as a member of the Board of Directors of Cequent since May 2008. Dr. Taylor has been in the pharmaceutical industry for more than twenty years with extensive experience in drug discovery and development, licensing and business development, and managing R&D alliances with pharmaceutical and biotech partners. Dr. Taylor is currently President and CEO of Ensemble Discovery Corp. in Cambridge, MA. Prior to joining Ensemble Discovery in 2007, Dr. Taylor served as Senior Vice President for Pfizer’s Global R&D division where he was responsible for global project and portfolio management. In other positions with Pfizer (and previously Warner-Lambert/Parke-Davis), Dr. Taylor led discovery, and early- and late-stage development projects across multiple therapeutic areas, including Lipitor® and Neurontin.® He has authored or coauthored 65 articles, reviews, and published abstracts and holds six patents. Dr. Taylor earned a Ph.D. in Medicinal Chemistry from the State University of New York at Buffalo and was awarded a National Institute of Health postdoctoral fellowship in natural products synthesis and structure elucidation at the University of Pennsylvania.
Covenants
Limitation on Soliciting, Discussing or Negotiating Other Acquisition Proposals
The merger agreement contains detailed provisions prohibiting Cequent from seeking or entering into an alternative transaction to the merger. Under these “no solicitation” and related provisions, subject to specific exceptions described below, Cequent has agreed that it will not, directly or indirectly (and that it will ensure that its representatives do not directly or indirectly):
| | • | | initiate, solicit, knowingly encourage or knowingly facilitate (including by way of furnishing nonpublic information or access to properties or assets) any acquisition proposal; |
| | • | | furnish any nonpublic information regarding Cequent to any person in connection with or in response to an acquisition proposal or an inquiry or indication of interest that would reasonably be expected to lead to an acquisition proposal (which shall include any acquisition proposal received prior to the date of the merger agreement); |
| | • | | enter into discussions or negotiate with any person in furtherance of an acquisition proposal; |
| | • | | approve, endorse or recommend any acquisition proposal; or |
| | • | | enter into any letter of intent, memorandum of understanding, agreement in principle, merger agreement, acquisition agreement or other similar agreement or any other contract relating to any acquisition proposal, with respect to an acquisition proposal. |
If Cequent should receive an acquisition proposal at any time prior to the effective time, Cequent shall promptly (and in all cases within one (1) business day) advise us in writing of such acquisition proposal, the terms and conditions of any such acquisition proposal and the identity of the person making any such acquisition proposal.
Cequent shall, and shall cause its representatives to, cease immediately and cause to be terminated any and all existing soliciting activities, discussions or negotiations and non-public information access, if any, with or to any person conducted prior to the date of the merger agreement with respect to any acquisition proposal. Cequent shall promptly request that each person, if any, in possession of the confidential information about Cequent that
I-54
was furnished by or on behalf of Cequent in connection with its consideration of any potential acquisition proposal to return or destroy all confidential information furnished to such person prior to the date of the merger agreement.
For the purposes of the merger agreement, an acquisition proposal means any proposal or offer from any person or group of persons (other than Cequent, MDRNA, merger sub or their affiliates) relating to:
| | • | | any merger, consolidation, amalgamation, share exchange, business combination, issuance of securities, acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or other similar transaction: (i) in which Cequent (or any subsidiary thereof) is a constituent corporation; (ii) in which a person or “group” (as defined in the Securities Exchange Act of 1934, as amended and the rules promulgated thereunder) of persons directly or indirectly acquires beneficial or record ownership of securities representing more than 20% of the outstanding securities of any class of voting securities of Cequent; or (iii) in which Cequent issues securities representing more than 20% of the outstanding securities of any class of voting securities of Cequent; |
| | • | | any sale, lease, exchange, transfer, license, acquisition or disposition of any business or businesses or assets that constitute or account for: (i) 20% or more of the consolidated net revenues of Cequent, or consolidated book value of the assets of Cequent; or (ii) 20% or more of the fair market value of the assets (including intangible assets) of Cequent; or |
| | • | | any liquidation or dissolution of Cequent. |
“Acquisition Proposal” does not include the merger or any of the other transactions contemplated by the merger agreement.
Obligations of the Board of Directors with Respect to Holding Meetings of Stockholders
We have agreed, as promptly as practicable after the execution of the merger agreement (but in any event within thirty (30) days after the date of the merger agreement), that we shall prepare and shall file with the SEC a proxy statement with respect to a meeting of our stockholders to be held to consider approval of the issuance of common stock to the stockholders of Cequent pursuant to the merger agreement and the merger (Proposal No. 1 in this proxy statement) and an amendment to our amended and restated certificate of incorporation to increase the number of authorized shares of our common stock to 180,000,000 (Proposal No. 8 in this proxy statement).
Cequent Covenants; Conduct of Business Prior to the Merger
Cequent will, so far as it is within its power to do so, carry on its business diligently and substantially in the same manner as theretofore conducted, and Cequent shall not institute any new methods of acquisition, production, marketing, distribution, sale, lease, license, management, operation, or engage in any transaction or activity, enter into any agreement or make any commitment, except in the ordinary course of business and consistent with past practice.
Cequent shall not institute any new methods of accounting except in accordance with generally accepted accounting principles.
Cequent shall use its commercially reasonable efforts to preserve its business intact, to protect and preserve its assets, and to preserve for us its relationships with licensors, suppliers, distributors, customers, contractors and employees.
Except as required pursuant to applicable law, any contract in existence prior to the date of the merger agreement binding upon Cequent or by the merger agreement, Cequent shall not:
| | • | | borrow or agree to borrow any funds, or incur, or assume or become subject to, whether directly or by way of guarantee or otherwise, any obligation or liability (absolute or contingent), other than obligations and liabilities incurred in the ordinary course of business and consistent with past practice; |
I-55
| | • | | permit or allow any of its assets to be subjected to any encumbrance of any kind or description, other than encumbrances in existence on the date of the merger agreement or mechanics liens or similar encumbrances for service or work in process; |
| | • | | sell, assign, transfer, license, dispose of or permit to lapse any rights to the use of any intellectual property (and Cequent shall take all actions necessary in respect of any infringement of any intellectual property of which it has knowledge), or dispose of or disclose to any person any trade secret, formula, process or know how not theretofore a matter of public knowledge; |
| | • | | make any single capital expenditure or future commitment in excess of $20,000 for additions to property, plant or equipment or make aggregate capital expenditures or future commitments in excess of $50,000 for additions to property, plant or equipment; |
| | • | | pay, loan or advance any amount to, or sell, transfer or lease any properties or assets to, or enter into any agreement or arrangement with, any affiliate; |
| | • | | change any of Cequent’s banking or safe deposit arrangements; |
| | • | | grant or extend any power of attorney or act as guarantor, surety, co-signer, endorser, co maker, indemnitor or otherwise in respect of the obligation of any person; |
| | • | | pay any amounts (whether in cash or property) to any employee of Cequent other than in the ordinary course of business and consistent with past practice or pursuant to any contract; |
| | • | | grant to any officer or employee any increase in compensation or benefits, other than increases in compensation or benefits for employees in the ordinary course of business and consistent with past practice; |
| | • | | pay any pension, retirement allowance or other employee benefit not required by any Cequent plan, policy or program; |
| | • | | adopt, agree to adopt, or make any announcement regarding the adoption of (i) any new pension, retirement or other employee benefit plan, policy or program or (ii) any amendments to any existing plan, policy or program unless required by applicable law; |
| | • | | (a) declare, set aside or pay any dividend or distribution that is payable in cash, stock, or other property with respect to Cequent; (b) redeem, purchase or otherwise acquire, directly or indirectly, any shares of Cequent, or any other securities or any rights, warrants, or options to acquire any such shares or other securities; (c) authorize for issuance, issue, sell, pledge, deliver or agree to commit to issue, sell or pledge (whether through the issuance or granting of any options, warrants, calls, subscriptions, equity appreciation rights or other rights or other agreements) any equity or debt securities of Cequent, or any other securities that are convertible into or exchangeable for shares of any class of Cequent; or (d) split, combine or reclassify the outstanding shares of Cequent, or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of any equity securities of Cequent; |
| | • | | adjust, settle or compromise any claim, obligation, debt, demand, suit or judgment against or on behalf of Cequent, except for claims, obligations, debts, demands, suits or judgments that do not involve an affiliate of Cequent and do not exceed more than $20,000 in any single instance or more than $50,000 in the aggregate; or |
| | • | | agree, whether in writing or otherwise, to do any of the foregoing. |
No contract or commitment will be entered into, and no purchase of supplies and no sale, lease, license, assignment, transfer or disposition of any of Cequent’s assets will be made, by or on behalf of Cequent, except (a) normal contracts or commitments for the purchase of, and normal purchases of, supplies or inventory or for the sale of inventory, in each case made in the ordinary course of business and consistent with past practice, and (b) other contracts, commitments, purchases or sales in the ordinary course of business, consistent with past practice and involving assets having a value not in excess of $20,000.
I-56
Cequent shall maintain the same insurance coverage (to the extent commercially reasonable) as in existence on the date of the merger agreement and all property insured thereby shall be used, operated, maintained and repaired in the ordinary course of business and consistent with past practice.
Cequent shall not do any act or omit to do any act, or permit any act or omission to act, which will cause a breach of any material contract or commitment of Cequent or which would cause the breach by Cequent of any representation, warranty, covenant or agreement made under the merger agreement.
Cequent shall duly comply in all material respects with all laws applicable to it and its properties, operations, business and employees.
Cequent shall file all federal, state, local and foreign tax returns and amendments thereto required to be filed by it and shall pay all taxes shown as due and payable thereon. All returns and reports in respect of employee withholdings, FICA, unemployment and other similar items and other applicable taxes shall be timely made as shall all deposits and payments due in respect of such taxes and obligations.
Cequent shall operate its business according to the operating budget for the expected period from the date of the merger agreement through the closing date, previously provided to us, in all material respects.
The foregoing restrictions, all of which are set forth in the merger agreement, shall not prohibit Cequent from doing any of the following between the date of the merger agreement through the closing date:
| | • | | authorize, issue, and sell additional shares of Cequent’s capital stock, or debt or warrants convertible into capital stock, to new and existing investors in Cequent, including but not limited to the sale of up to $7,520,000 of Cequent’s Series A-1 Convertible preferred Stock; |
| | • | | accelerating the vesting of certain outstanding stock options; and |
| | • | | loan money to us as contemplated under the loan agreement. |
MDRNA Covenants; Conduct of Business Prior to the Merger
We will, so far as it is within our power to do so, carry on our business diligently and substantially in the same manner as theretofore conducted, and we shall not institute any new methods of acquisition, production, marketing, distribution, sale, lease, license, management, operation, or engage in any transaction or activity, enter into any agreement or make any commitment, except in the ordinary course of business and consistent with past practice.
We shall not institute any new methods of accounting except in accordance with generally accepted accounting principles.
We shall use (and shall cause each of our subsidiaries to use) its commercially reasonable efforts to preserve our business and each subsidiary’s business intact, to protect and preserve our and each subsidiary’s assets, and to preserve for Cequent our and each subsidiary’s relationships with licensors, suppliers, distributors, customers, contractors and employees.
Except as required pursuant to applicable law, any contract in existence prior to the date of the merger agreement binding upon us or by the merger agreement, we shall not:
| | • | | borrow or agree to borrow any funds, or incur, or assume or become subject to, whether directly or by way of guarantee or otherwise, any obligation or liability (absolute or contingent), other than obligations and liabilities incurred in the ordinary course of business and consistent with past practice; |
| | • | | permit or allow any of our assets to be subjected to any encumbrance of any kind or description, other than encumbrances in existence on the date of the merger agreement or mechanics liens or similar encumbrances for service or work in process; |
I-57
| | • | | sell, assign, transfer, license, dispose of or permit to lapse any rights to the use of any intellectual property (and we shall take (and shall cause each of our subsidiaries to take) all actions necessary in respect of any infringement of any intellectual property of which we have knowledge), or dispose of or disclose to any person any trade secret, formula, process or know how not theretofore a matter of public knowledge, other than (x) any sale, assignment, transfer, license or other disposition of any of our legacy intranasal assets, and (y) licenses by us of any of our intellectual property in the ordinary course of business consistent with past practice (other than licenses solely to our Di-Alkylated Amino Acid delivery technology);provided,however, that nothing in item (y) above shall prohibit us from licensing any of our intellectual property, including our Di-Alkylated Amino Acid delivery technology, in connection with any multi-year target-based or therapeutic-based collaboration between us and a pharmaceutical company substantially similar to those collaborations previously discussed with Cequent; |
| | • | | make any single capital expenditure or future commitment in excess of $100,000 for additions to property, plant or equipment or make aggregate capital expenditures or future commitments in excess of $200,000 with respect to us and our subsidiaries as a whole for additions to property, plant or equipment, or make any expenditure with respect to the acquisition of the intellectual property of a third party in excess of $250,000 with respect to us and our subsidiaries as a whole (but not including future commitments with respect thereto); |
| | • | | pay, loan or advance any amount to, or sell, transfer or lease any properties or assets to, or enter into any agreement or arrangement with, any affiliate or with any subsidiary other than a subsidiary of our company; |
| | • | | change any of our or any of our subsidiary’s banking or safe deposit arrangements; |
| | • | | grant or extend any power of attorney or act as guarantor, surety, co-signer, endorser, co maker, indemnitor or otherwise in respect of the obligation of any person; |
| | • | | pay any amounts (whether in cash or property) to any employee of our company or our subsidiaries other than in the ordinary course of business and consistent with past practice or pursuant to any contract; |
| | • | | grant to any officer or employee any increase in compensation or benefits, other than increases in compensation or benefits for employees in the ordinary course of business and consistent with past practice; |
| | • | | pay any pension, retirement allowance or other employee benefit not required by any plan, policy or program of our company; |
| | • | | adopt, agree to adopt, or make any announcement regarding the adoption of (i) any new pension, retirement or other employee benefit plan, policy or program or (ii) any amendments to any existing plan, policy or program unless required by applicable law; |
| | • | | (a) declare, set aside or pay any dividend or distribution that is payable in cash, stock, or other property with respect to our company; (b) redeem, purchase or otherwise acquire, directly or indirectly, any shares of our company or any of our subsidiaries, or any other securities or any rights, warrants, or options to acquire any such shares or other securities; (c) except for any pledge of equity securities of each of our subsidiaries to Cequent, authorize for issuance, issue, sell, pledge, deliver or agree to commit to issue, sell or pledge (whether through the issuance or granting of any options, warrants, calls, subscriptions, equity appreciation rights or other rights or other agreements) any equity or debt securities of our company or any of our subsidiaries, or any other securities that are convertible into or exchangeable for shares of any class of our company; or (d) split, combine or reclassify the outstanding shares of our company or any of our subsidiaries, or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of any equity securities of our company or any of our subsidiaries; |
I-58
However, the restrictions set forth in item (c) of the immediately preceding paragraph shall not apply to the issuance by our company of (1) shares of common stock and options to purchase shares of common stock to our employees, officers or directors in the ordinary course of business and consistent with past practice pursuant to any stock or option plan duly adopted for such purpose by a majority of the non-employee members of the Board or a majority of the members of a committee of non-employee directors established for such purpose; (2) shares of common stock upon the exercise or exchange of or conversion of any securities exercisable or exchangeable for or convertible into shares of common stock issued and outstanding on the date of the merger agreement; (3) securities of our company, including any warrants and other convertible securities, having a fair market value on the date on which definitive agreements are executed of not greater than $5 million in connection with the acquisition by our company of any other entity or the assets of any such entity, or the grant by any other person to our company of a license to all or a portion of the intellectual property of such person; or (4) securities of our company, including any warrants and other convertible securities, in a public or private offering of securities to investors having a fair market value on the date on which definitive agreements are executed of not greater than $5 million and at a price of not less than 85% of the fair market value of such securities on the date on which definitive agreements are executed. For purposes of items (3) and (4) of this paragraph, the fair market value of the common stock shall be deemed to be the last reported closing price of the common stock on The Nasdaq Global Market prior to the execution of definitive agreements.
| | • | | adjust, settle or compromise any claim, obligation, debt, demand, suit or judgment against or on behalf of our company or any of our subsidiaries, except for claims, obligations, debts, demands, suits or judgments that do not involve an affiliate of our company and do not exceed more than $50,000 in any single instance or more than $100,000 in the aggregate with respect to our company and our subsidiaries as a whole; or |
| | • | | agree, whether in writing or otherwise, to do any of the foregoing. |
We shall maintain (and shall cause each of our subsidiaries to maintain) the same insurance coverage (to the extent commercially reasonable) as on the date of the merger agreement and all property insured thereby shall be used, operated, maintained and repaired in the ordinary course of business and consistent with past practice.
We shall not do any act or omit to do any act, or permit any act or omission to act (or permit any of our subsidiaries to so do, or omit to do, or to so permit any act or omission), which will cause a breach of any material contract or commitment of our company or any of our subsidiaries or which would cause the breach by us or any of our subsidiaries of any representation, warranty, covenant or agreement made under the merger agreement.
We shall duly comply (and shall cause each of our subsidiaries to duly comply) in all material respects with all laws applicable to us or them, as applicable, and our or their properties, operations, business and employees.
We shall file (and shall cause each of our subsidiaries to file) all federal, state, local and foreign tax returns and amendments thereto required to be filed by us or them, as applicable, and shall pay (and shall cause each of our subsidiaries to pay) all taxes shown as due and payable thereon. All returns and reports in respect of employee withholdings, FICA, unemployment and other similar items and other applicable taxes shall be timely made as shall all deposits and payments due in respect of such taxes and obligations.
No contract or commitment will be entered into, and no purchase of supplies and no sale, lease, license, assignment, transfer or disposition of any of our assets will be made, by or on behalf of our company, except (a) normal contracts or commitments for the purchase of, and normal purchases of, supplies or inventory or for the sale of inventory, in each case made in the ordinary course of business and consistent with past practice, and (b) other contracts, commitments, purchases or sales in the ordinary course of business, consistent with past practice and involving assets having a value not in excess of $20,000.
I-59
Affirmative Covenants of MDRNA. Subject to certain exceptions, we have agreed in the merger agreement:
| | • | | To use commercially reasonable efforts to cause the shares of our common stock to be issued as consideration in the merger to be approved for listing on The NASDAQ Global Market; and |
| | • | | Within 30 days after the execution of the merger agreement, to prepare and file with the SEC a proxy statement with respect to the merger relating to the meeting of our stockholders to be held to consider approval of the issuance of common stock to the Cequent stockholders pursuant to the merger agreement and an amendment to our amended and restated certificate of incorporation to increase the number of authorized shares of common stock. We have agreed to use commercially reasonable efforts to cause the proxy statement to be cleared by the SEC as promptly as practicable after the date of the merger agreement, and, prior to such date, we have agreed to take all action required under any applicable laws in connection with the stock issuance contemplated by the merger agreement. We have agreed to mail, or cause to be mailed, to our stockholders, and to duly call the stockholders’ meeting, as promptly as reasonably practicable in accordance with applicable laws following the date on which the SEC clears the proxy statement. |
Affirmative Covenants of MDRNA and Cequent. During the period commencing on the date of the merger agreement and ending upon the earlier to occur of the termination of the merger agreement and the closing date:
| | • | | each party to the merger agreement agrees to cooperate fully with the other parties to the merger agreement and to give to such other parties, their officers, employees, auditors, legal counsel, representatives and agents reasonable access during normal business hours to all such information, documents, premises and employees as the requesting party reasonably considers necessary or advisable for purposes of its investigation of our company and Cequent, as applicable; |
| | • | | each party to the merger agreement will use commercially reasonable efforts to (A) take, or cause to be taken, all appropriate action, and do, or cause to be done, all things necessary, proper or advisable under applicable law or otherwise to consummate and make effective the transactions contemplated by the merger agreement as promptly as practicable, (B) obtain from any governmental entity any consents, licenses, permits, waivers, approvals, authorizations or orders required to be obtained or made by the parties to the merger agreement or any of their respective subsidiaries, or to avoid any action or proceeding by any governmental entity, in connection with the authorization, execution and delivery of the merger agreement and the consummation of the transactions contemplated thereby, including, without limitation, the merger, and (C) make all necessary filings, and thereafter make any other required submissions, with respect to the merger agreement and the transactions contemplated thereby, including the merger, required under (x) the Securities Act and the Exchange Act, and any other applicable federal or state securities laws, and (y) any other applicable law; and |
| | • | | each party to the merger agreement shall use reasonable best efforts to cause the merger to qualify, and will not take any actions, or fail to take any action, which could reasonably be expected to prevent the merger from qualifying as a reorganization under the provisions of Section 368(a) of the Internal Revenue Code of 1986, as amended. |
Representations and Warranties
The merger agreement contains customary representations and warranties of each of our company and merger sub, on the one hand, and Cequent, on the other hand, made solely for the benefit of the other. Many of the representations and warranties may not be accurate or complete as of any particular date because they are subject to a contractual standard of materiality or material adverse effect different from that generally applicable to public disclosures to stockholders. The representations and warranties were used for the purpose of allocating risk between the parties to the merger agreement rather than establishing matters of fact. For the foregoing reasons, you should not rely on the representations and warranties contained in the merger agreement as statements of factual information. The representations and warranties in the merger agreement and the
I-60
description of them in this proxy statement should be read in conjunction with the other information contained in the reports, statements and filings that we publicly file with the SEC. This description of the representations and warranties is included to provide stockholders with information regarding the terms of the merger agreement.
The merger agreement contains representations and warranties made by Cequent to us with respect to, among other things, Cequent’s:
| | • | | Capital Stock and Related Matters; No Investments |
| | • | | Undisclosed Liabilities |
| | • | | Contracts and Commitments |
| | • | | Employees and Labor Relations |
| | • | | Court Orders, Decrees, and Laws |
| | • | | Employee Benefit Plans; ERISA |
| | • | | Related-Party Transactions |
The merger agreement contains representations and warranties made by us and merger sub to Cequent with respect to, among other things, our:
| | • | | Capital Stock and Related Matters; No Investments |
I-61
| | • | | Consents and Approvals, |
| | • | | Undisclosed Liabilities |
| | • | | Contracts and Commitments |
| | • | | Employees and Labor Relations |
| | • | | Court Orders, Decrees, and Laws |
| | • | | Employee Benefit Plans; ERISA |
| | • | | Authorization of MDRNA Common Stock |
| | • | | No Creditor Assignment; No Bankruptcy |
| | • | | Reports and Financial Statements |
| | • | | Section 203 of the DGCL Not Applicable |
| | • | | Interim Operations of Merger Sub |
Survival of Representations and Warranties
The representations and warranties of our company and merger sub in the merger agreement do not survive the closing of the merger. The representations and warranties of Cequent in the merger agreement survive the closing of the merger through December 31, 2010.
Indemnification by the Stockholders of Cequent
The stockholders of Cequent (acting through Ampersand 2006 Limited Partnership, the Stockholders’ Representative) have agreed, severally and not jointly, to indemnify our company and our affiliates (including any officer, director, stockholder, partner, member, employee, agent or representative of our company or any affiliate) from and against all assessments, losses, damages, liabilities, costs and expenses, including, but not limited to, interest, penalties and reasonable fees and expenses of legal counsel imposed upon or incurred by us, the surviving corporation, or any of our or its subsidiaries arising out of, in connection with or resulting from: (i) any breach of any representation or warranty of Cequent contained in or made pursuant to the merger agreement or any document related to the merger agreement, or any certificate or other instrument furnished or to be furnished to us or merger sub under the merger agreement; or (ii) any nonfulfillment of any covenant or agreement of Cequent contained in or made pursuant to the merger agreement or any document related to the merge agreement, or any certificate or other instrument furnished or to be furnished to us or merger sub under the merger agreement.
I-62
The aggregate amount of damages for which the stockholders of Cequent may be obligated to indemnify any person with respect to all claims pursuant to the merger agreement shall not exceed ten percent (10%) of the total value of the aggregate number of shares of common stock issued pursuant to the merger agreement. However, this limitation shall not apply in the event of fraud or willful misrepresentation by Cequent.
Escrow Arrangement
At the effective time of the merger, we shall deduct from the number of shares of common stock to be issued to the stockholders of Cequent a number of shares of our common stock as is equal to ten percent (10%) of the aggregate number of shares of common stock issued in the merger. We shall deposit such shares of our common stock, which we refer to as the escrow shares, with an internationally recognized escrow agent or bank mutually agreed by the parties, for the purpose of securing Cequent’s indemnification obligations under the merger agreement. The escrow shares shall be held by the escrow agent in accordance with the terms of the merger agreement and of an escrow agreement in a form mutually agreed by the parties, which we refer to as the escrow agreement. On January 1, 2011, the escrow agent shall release the escrow shares and deliver to the stockholders of Cequent all of the escrow shares, minus such number of escrow shares having a value (based on a value of $1.1496 per share) equal to the amount of any claim or claims that have been set forth in a notice delivered pursuant to the merger agreement (whether or not such claim or claims have been determined to be valid as of such date) (the remaining escrow shares after January 1, 2011 that either have not been released to the stockholders of Cequent or have not been released to us in respect of any claims being referred to herein as the escrow balance), and the escrow balance shall be retained in escrow pending a final and non-appealable judgment or order of a court of competent jurisdiction of such claim.
Upon the final adjudication of a claim, if the escrow balance exceeds the aggregate amount of all remaining unresolved claims, we shall instruct the escrow agent to release, to the stockholders of Cequent, such number of escrow shares representing such excess from the escrow shares retained by the escrow agent, it being understood that if at such time the aggregate number of escrow shares being retained by the escrow agent have a value that is less than or equal to the aggregate amount of all claims pending at such time, we shall not be obligated to release any escrow shares to the stockholders of Cequent,subject,however to the final adjudication of such claims.
The release of escrow shares by the escrow agent to our company in accordance with the terms and conditions of the merger agreement and the escrow agreement shall be the sole and exclusive remedy available to us to satisfy any of our rights to indemnification set forth in the merger agreement.
Conditions to the Completion of the Merger
Conditions Precedent to the Obligations of Cequent. All obligations of Cequent with respect to the merger are subject to the fulfillment to the satisfaction of Cequent and its legal counsel, prior to or at the closing, of each of the following conditions, except to the extent that Cequent may waive any one or more thereof:
| | • | | Our stockholders approve Proposal No. 1 (relating to the issuance of common stock pursuant to the merger agreement) and Proposal No. 8 (relating to an amendment to our certificate of incorporation to increase the number of authorized shares of common stock to 180,000,000); |
| | • | | The stockholders of Cequent approve the merger and the transactions contemplated by the merger agreement, which approval was obtained on April 2, 2010; |
| | • | | The representations and warranties of our company and merger sub contained in the merger agreement shall be true, complete and accurate in all material respects as of the date when made and at and as of the closing date as though such representations and warranties were made at and as of such date (except to the extent such representations are made as of a date specific); |
| | • | | We and merger sub shall have performed and complied in all material respects with all agreements, obligations and conditions required by the merger agreement to be performed or complied with by us and it on or prior to the closing; |
I-63
| | • | | No suit, action, investigation, inquiry or other proceeding by any governmental body or other person or legal or administrative proceeding shall have been instituted or threatened which seeks to restrain, enjoin, prevent the consummation or otherwise affect the transactions contemplated by the merger agreement or which questions the validity or legality of the transactions contemplated by the merger agreement; |
| | • | | From December 31, 2009 to the closing date, our business shall not have suffered any material adverse effect; |
| | • | | The shares of our common stock issuable to the stockholders of Cequent in the merger shall have been approved for listing on The NASDAQ Global Market, subject to official notice of issuance; |
| | • | | We shall have delivered to Cequent, or cause to be delivered to Cequent, the other items required to be delivered to Cequent in accordance with the merger agreement; |
| | • | | We and merger sub shall have furnished Cequent with all consents, approvals, authorizations, exemptions or waivers set forth on the applicable disclosure schedule to the merger agreement; |
| | • | | We shall have executed the registration rights agreement in the form attached to the merger agreement; and |
| | • | | We shall have executed the stockholders’ agreement in the form attached to the merger agreement. |
Conditions Precedent to the Obligations of MDRNA and Merger Sub. All obligations of our company and merger sub with respect to the merger are subject to the fulfillment to the satisfaction of us and our legal counsel, prior to or at the closing, of each of the following conditions, except to the extent that we may waive any one or more thereof:
| | • | | Our stockholders approve Proposal No. 1 (relating to the issuance of common stock pursuant to the merger agreement) and Proposal No. 8 (relating to an amendment to our certificate of incorporation to increase the number of authorized shares of common stock to 180,000,000); |
| | • | | The stockholders of Cequent approve the merger and the transactions contemplated by the merger agreement, which approval was obtained on April 2, 2010; |
| | • | | The representations and warranties of Cequent contained in the merger agreement shall be true, complete and accurate in all material respects as of the date when made and at and as of the closing date as though such representations and warranties were made at and as of such date; |
| | • | | Cequent shall have performed and complied in all material respects with all agreements, obligations and conditions required by the merger agreement to be performed or complied with by it on or prior to the closing; |
| | • | | No suit, action, investigation, inquiry or other proceeding by any governmental body or other person or legal or administrative proceeding shall have been instituted or, to knowledge, threatened which seeks to restrain, enjoin, prevent the consummation of or otherwise affect the transactions contemplated by the merger agreement or which questions the validity or legality of the transactions contemplated by the merger agreement; |
| | • | | From December 31, 2009 to the closing date, Cequent’s business shall not have suffered any material adverse effect; |
| | • | | We shall have received in writing any and all consents, approvals, authorizations, exemptions or waivers set forth on the applicable disclosure schedule to the merger agreement; |
| | • | | Cequent shall have delivered to us, or caused to be delivered to us, the other items required to be delivered to us in accordance with the merger agreement; and |
| | • | | Cequent shall have available an amount in cash equal to (i) $5,100,000, minus (ii) the amount of Cequent’s ordinary course operating expenses from June 2, 2010 through the closing of the merger; plus (iii) the difference of $3,000,000 and the aggregate principal amount loaned to our company by Cequent under the loan agreement. |
I-64
Material Adverse Effect
Several of the representations, warranties, covenants and closing conditions of our company, merger sub and Cequent in the merger agreement are qualified by reference to whether the item in question has had or could reasonably be expected to have a “material adverse effect” on the applicable company. The merger agreement provides that “material adverse effect” means, when used in connection with our company or Cequent, a material adverse effect, either individually or when aggregated with other such effects, on the assets, business, operations, financial condition or results of operations of Cequent or our company, but excluding changes in the general economy in the United States.
Termination of the Merger Agreement
The merger agreement provides that the parties may terminate the merger agreement prior to the closing as follows:
| | • | | We and Cequent may terminate the merger agreement by mutual written consent; |
| | • | | We may terminate the merger agreement by giving written notice to Cequent if the closing shall fail to occur by reason of the failure of any condition precedent to our obligations under the merger agreement (unless the failure results from a breach by us of any representation, warranty or covenant contained in the merger agreement); |
| | • | | We may terminate the merger agreement by giving written notice to Cequent if stockholders holding more than 10% of the shares of Cequent exercise their appraisal rights in accordance with Section 262 of the Delaware General Corporation Law; |
| | • | | We may terminate the merger agreement by giving written notice to Cequent if the closing price of our common stock on The NASDAQ Global Market is in excess of $1.437 for any ten (10) consecutive trading days following the date of the merger agreement; |
| | • | | Cequent may terminate the merger agreement by giving written notice to us if the closing shall not have occurred by reason of the failure of any condition precedent to Cequent’s obligations under the merger agreement (unless the failure results primarily from a breach by Cequent of any representation, warranty or covenant contained in the merger agreement); |
| | • | | Cequent may terminate the merger agreement by giving written notice to us if (i) the closing price of our common stock on The NASDAQ Global Market is below $0.8622 for any ten (10) consecutive trading days following the date of the merger agreement or (ii) our common stock is no longer listed on The NASDAQ Global Market or The NASDAQ Capital Market; |
| | • | | Either we, on the one hand, or Cequent, on the other hand, may terminate the merger agreement on ten (10) days’ prior written notice if the other party is in material breach of the merger agreement and such breach is not cured within such ten (10) day period; |
| | • | | Either we, on the one hand, or Cequent, on the other hand, may terminate the merger agreement upon written notice if the closing shall not have occurred on or prior to August 31, 2010; |
| | • | | We may terminate the merger agreement if there shall have occurred any material adverse effect with respect to Cequent since the date of the merger agreement; |
| | • | | Cequent may terminate the merger agreement if there shall have occurred any material adverse effect with respect to our company since the date of the merger agreement; or |
| | • | | Either we, on the one hand, or Cequent, on the other hand, may terminate the merger agreement if the required approval of the stockholders of our company or Cequent contemplated by the merger agreement shall not have been obtained by reason of the failure to obtain the requisite vote upon a vote taken at a meeting of stockholders convened therefor or at any adjournment thereof. |
I-65
Termination Fees; Expenses
The merger agreement provides that all expenses incurred by the parties to the merger agreement shall be paid by the party incurring such expenses. However, if Cequent terminates the merger agreement due to the fact that the required approval of our stockholders contemplated by the merger agreement shall not have been obtained by reason of the failure to obtain the requisite vote upon a vote taken at a meeting of our stockholders convened for such purpose or at any adjournment of such meeting, we shall pay to Cequent, on or prior to December 31, 2010, a termination fee equal to the lesser of (i) $500,000 and (ii) Cequent’s reasonable and actual out-of-pocket expenses (including, without limitation, all fees and expenses of counsel, accountants, investment bankers, experts and consultants to Cequent) incurred by Cequent in connection with or related to the authorization, preparation, negotiation, execution and performance of the merger agreement and the transactions contemplated by the merger agreement. In no event shall we be required to pay the termination fee if, immediately prior to the termination of the merger agreement, Cequent was in material breach of its obligations under the merger agreement.
If we do not pay the termination fee when due, then the termination fee shall be added to the outstanding principal amount of the term note issued by us to Cequent on the date of the merger agreement, shall be deemed principal under such term note, and shall bear interest from the date thereof until the date when paid at a rate equal to 4% per annum plus the per annum rate otherwise payable under the term note. In such event, the termination fee shall be an obligation of our company and shall be secured by that certain Security Agreement (All Assets) dated as of March 31, 2010 from our company and each of our subsidiaries to Cequent until all outstanding principal and all interest accrued and unpaid under the term note (other than that representing, or arising on such portion of principal representing, the termination fee), and the fees, costs and expenses our company due Cequent under the loan agreement in connection with the term loans, have been indefeasibly paid in full in cash. Upon the indefeasible payment in full in cash of such principal, interest and fees, costs and expenses, the termination fee shall become unsecured, and all liens and security interests on the collateral securing the termination fee shall be released upon the written request of, and at the cost and expense of, our company.
Amendment, Extension and Waiver of the Merger Agreement
The merger agreement may be amended by the parties, by action taken or authorized by their respective Boards of Directors, at any time before or after approval of the matters presented in connection with the merger by stockholders of our company and Cequent, provided, that after any such approval, no amendment shall be made that by law requires further approval by the stockholders of our company or Cequent, as the case may be, without such further approval. The merger agreement may not be amended except by an instrument in writing signed on behalf of the party to be charged with such amendment.
At any time prior to the effective time of the merger, any party to the merger agreement may, by written consent, extend the other party’s time for the performance of any of the obligations or other acts under the merger agreement, waive any inaccuracies in the other party’s representations and warranties contained in the merger agreement or in any document delivered pursuant to the merger agreement and waive compliance by the other party with any of the agreements or conditions contained in the merger agreement. The conditions requiring the approval of the stockholders of our company and of the stockholders of Cequent, however, cannot be waived.
I-66
LOCK UP AGREEMENTS
Upon the consummation of the merger, certain principal stockholders of Cequent, who collectively held approximately 64% of the issued and outstanding shares of Cequent common stock on the date of the merger agreement on a fully-diluted basis, will enter into lock-up agreements pursuant to which they will agree that they shall not, without our prior approval, offer, pledge, sell, contract to sell, grant, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock received in the merger during the period commencing on the effective date of the merger and ending on the earlier of (A) one hundred and eighty (180) days after the effective date of the merger or (B) thirty (30) days following the closing of an equity financing by our company for the issuance of shares of equity or securities convertible into or exchangeable or exercisable for shares of equity of our company which, when added together with all other equity financings from and after the date of execution of the merger agreement, results in aggregate gross proceeds to our company of at least $10 million.
Prior to the execution of the merger agreement, our directors and executive officers entered into lock-up agreements providing for similar restrictions on sales and other dispositions of common stock by them commencing upon the signing of the merger agreement.
I-67
LOAN AGREEMENT
Concurrently and in connection with the execution of the merger agreement, we and Cequent entered into a loan agreement, which we refer to as the loan agreement, pursuant to which, among other things, Cequent shall extend one or more term loans to us in the aggregate principal amount of up to $3 million to fund our operations prior to the effective date of the merger. On April 30, 2010, Cequent loaned $1 million to us under the loan agreement and on May 31, 2010, Cequent loaned an additional $1 million to us under the loan agreement. The term loans are evidenced by a secured promissory note, which we refer to as the term note, issued by us to Cequent, which bears interest absent an event of default, at a rate of ten percent per annum. We shall repay the principal and interest of the term loans in three equal consecutive monthly installments, commencing on August 15, 2010 and continuing on the 15th day of each month thereafter through and including October 15, 2010. Notwithstanding the foregoing, if the merger is consummated prior to August 15, 2010, then, on the closing date of the merger, we shall not owe to any third party any obligations with respect to the term loans, including, without limitation, the then outstanding principal balance of the term loans and any interest then accrued but unpaid thereon. We may prepay any term loan in whole or in part without premium or penalty.
The term loans are secured by a first priority security interest in substantially all the assets of our company and our wholly-owned subsidiaries pursuant to (i) a Security Agreement (All Assets) by our company and our wholly-owned subsidiaries in favor of Cequent dated as of March 31, 2010, which we refer to as the All Assets Security Agreement, (ii) a Security Agreement (Patents) by our company in favor of Cequent dated as of March 31, 2010, which we refer to as the MDRNA Patent Security Agreement, and (iii) a Security Agreement (Patents) by MDRNA Research, Inc., our wholly-owned subsidiary, in favor of Cequent dated as of March 31, 2010, which we refer to as the MDRNA Research Patent Security Agreement. Our wholly-owned subsidiaries also entered into a Guaranty Agreement dated as of March 31, 2010 in favor of Cequent to guaranty all obligations of our company and our subsidiaries to Cequent in connection with the term loans. The All Assets Security Agreement, the MDRNA Patent Security Agreement and the MDRNA Research Patent Security Agreement collectively provide Cequent with a security interest in substantially all of the assets of our company and our subsidiaries, subject to certain permitted encumbrances as set forth in the loan agreement. The security interest created in the collateral will be first priority, subject to the permitted encumbrances provided in the All Assets Security Agreement, the MDRNA Patent Security Agreement and the MDRNA Research Patent Security Agreement, and will be perfected to the extent such security interest can be perfected by the filing of a financing statement and filings with the United States Patent and Trademark Office. The security interest created in the collateral will be released at such time as the term notes and certain related fees are paid in full.
Events of default under the loan agreement include:
| | • | | Our failure to make any payment of principal or interest under the loan agreement or term note on or before the date when due; or |
| | • | | Any representation or warranty made by us under either the loan agreement or term note is incorrect in any material respect; or |
| | • | | Our default in the performance of certain obligations under the loan agreement; or |
| | • | | Our, or any of our subsidiary’s, default under any other contract, agreement or undertaking existing as of the time we entered into the loan agreement or thereafter entered into; or |
| | • | | Any default shall exist and remain unwaived or uncured with respect to any indebtedness in principal amount in excess of $50,000 of our company or any of our subsidiaries or with respect to any instrument evidencing, guaranteeing, securing or otherwise relating to any such indebtedness, or any such indebtedness shall not have been paid when due; or |
| | • | | We shall be dissolved, or our company or any of our subsidiaries shall become insolvent or bankrupt or shall cease paying its debts as they mature or shall make an assignment for the benefit of creditors, or a trustee, receiver or liquidator shall be appointed for our company or any of our subsidiaries or for a |
I-68
| | substantial part of the property of our company or any of our subsidiaries, or bankruptcy, reorganization, arrangement, insolvency or similar proceedings shall be instituted by or against our company or any of our subsidiaries under the laws of any jurisdiction (except for an involuntary proceeding filed against our company or any of our subsidiaries which is dismissed within 60 days following the institution thereof); or |
| | • | | Any attachment, execution or similar process shall be issued or levied against any property of our company or any of our subsidiaries and such attachment, execution or similar process shall not be paid, stayed, released, vacated or fully bonded within 10 days after its issue or levy; or |
| | • | | Any final uninsured judgment in excess of $25,000 shall be entered against our company or any of our subsidiaries by any court of competent jurisdiction; or |
| | • | | Our company or any of our subsidiaries shall fail to meet its minimum funding requirements under the Employee Retirement Income Security Act with respect to any employee benefit plan (or other class of benefit which the Pension Benefit Guaranty Corporation, or the PBGC, has elected to insure) or any such plan shall be the subject of termination proceedings (whether voluntary or involuntary) and there shall result from such termination proceedings a liability of our company or any of our subsidiaries to the PBGC which, in each case, in the reasonable opinion of Cequent may have a material adverse effect upon the financial condition of our company or any of its subsidiaries; or |
| | • | | The security agreement or any other loan document shall for any reason (other than due to payment in full of all amounts secured or evidenced thereby or due to discharge in writing by Cequent) not remain in full force and effect; or |
| | • | | The security interest and liens of Cequent in and on any of the collateral covered or intended to be covered by the all assets security agreement and/or any patent security agreement shall for any reason (other than written release by Cequent) not be fully perfected liens and security interests; or |
| | • | | Except as contemplated by the merger agreement, a change of control of our company shall occur; or |
| | • | | If the loan agreement or any of the other loan documents shall prove to be illegal or unenforceable in any material respect; or |
| | • | | An environmental event shall have occurred that in the reasonable opinion of Cequent materially adversely affects the financial condition, prospects, business or operations of our company or any of our subsidiaries or any of our or their respective premises; or |
| | • | | Our company or any of our subsidiaries shall suffer substantial loss, theft, taking, damage or destruction to or of any of our or its property which, after taking into account any insurance, would have a material adverse effect upon the business, operations or financial condition of our company or any such subsidiary; or our company or any of our subsidiaries shall suffer the loss (or proceedings shall be commenced which could result in the loss) of any permit, license or contract material to the operation of our company or any of our subsidiaries or any of our or their respective facilities; or |
| | • | | There shall occur any other material adverse change in the condition (financial or otherwise), operations, properties, assets, or liabilities of our company. |
I-69
LOAN WARRANTS
We agreed in the loan agreement to issue to Cequent on or about the date of each term loan a warrant to purchase shares of common stock, with each such warrant to be exercisable for a number of shares of common stock equal to sixty-five percent (65%) of the principal amount of the term loan being made on such date divided by $1.1496. The loan warrants are only exercisable upon the occurrence of both of the following: (x) the merger is not consummated (other than by reason of Cequent’s material breach of the merger agreement), and (y) the occurrence of an Acquisition Transaction (as defined below) prior to the one year anniversary of the termination of the merger agreement. If the merger is consummated, we will have no obligation to issue shares of our common stock to Cequent in connection with the loan warrants.
An Acquisition Transaction, for purposes of the loan warrants, is any one of the following:
| | (1) | a merger or consolidation in which: |
| | • | | our company is a constituent party, or |
| | • | | a subsidiary of our company is a constituent party, |
and either (A) we issue shares of our capital stock pursuant to such merger or consolidation representing 40% or more of our outstanding capital stock, or (B) as a result of such merger or consolidation of a subsidiary, our company’s ownership interest in the surviving entity is reduced by 40% or more;
| | (2) | the sale, lease, transfer, exclusive out-license or other disposition, in a single transaction or series of related transactions, by our company or any subsidiary of our company of 40% or more of the assets or intellectual property of our company and our subsidiaries taken as a whole, or the sale or disposition (whether by merger or otherwise) of one or more subsidiaries of our company if 40% or more of the assets of our company and its subsidiaries taken as a whole are held by such subsidiary or subsidiaries, except where such sale, lease, transfer, exclusive out-license or other disposition is to a wholly-owned subsidiary; or |
| | (3) | the acquisition by a person of beneficial ownership (as defined under Rule 13(d) of the Exchange Act) of equity interests representing a 40% or greater voting interest in our company or a tender offer or exchange offer that, if consummated, would result in any person or group (as defined in Rule 13(d) of the Exchange Act) beneficially owning equity interests representing a 40% or greater economic or voting interest in our company. |
On April 30, 2010, in connection with the $1 million loan by Cequent to us pursuant to the loan agreement, we issued to Cequent a warrant to purchase up to 565,414 shares of our common stock at an exercise price of $1.1496 per share. On May 31, 2010, in connection with an additional $1 million loan by Cequent to us pursuant to the loan agreement, we issued to Cequent an additional warrant to purchase up to 565,414 shares of our common stock at an exercise price of $1.1496 per share.
I-70
STOCKHOLDERS’ AGREEMENT
At the effective time of the merger, we will enter into a stockholders’ agreement, which we refer to as the stockholders’ agreement, with Ampersand 2006 Limited Partnership, A.M. Pappas Life Science Ventures III, LP, PVIII CEO Fund, LP and Novartis BioVentures Ltd., each of whom is referred to as a designator, pursuant to which such designators shall have the right to designate a total of three (3) members of our Board of Directors during the period beginning at the effective time of the merger and ending immediately prior to our 2011 Annual Meeting of Stockholders. During that period of time we will maintain a Board of Directors consisting of no more than seven (7) individuals, and each committee of the Board shall be comprised of four (4) directors. The directors serving on each committee shall be selected in good faith by the Nominating and Corporate Governance Committee, or the Nominating Committee, and in the selection of the members of each committee the Nominating Committee shall ensure that the composition of each committee shall be in accordance with the rules, regulations and requirements of The NASDAQ Stock Market.
Under the stockholders’ agreement, the designators shall have the right to submit to the Nominating Committee for consideration as a director the name of three director nominees, and if the Nominating Committee deems, in the exercise of its reasonable good faith discretion, the director nominees qualified, the Nominating Committee shall appoint the director nominees to serve as directors of our company until our 2011 Annual Meeting of Stockholders. We shall fill any vacancies that may arise upon the resignation, removal, death or disability of any such director with a new director designated in accordance with the foregoing sentence, provided that the designator who designated such director continues to own at least twenty-five percent (25%) of the shares of common stock issued to it in connection with the merger. The initial director nominees of the designators shall be Peter D. Parker, Michael D. Taylor and Chiang Li. During such time as the stockholders’ agreement remains in effect, all of our subsidiaries shall maintain a Board of Directors with a composition identical to that of the Board of Directors of our company.
I-71
REGISTRATION RIGHTS AGREEMENT
Shelf Registration.
At the effective time of the merger, we will enter into a Registration Rights Agreement, which we refer to as the registration rights agreement, with certain of the principal stockholders of Cequent, who we refer to as the Investors, pursuant to which we will agree to use reasonable best efforts (i) to file with the SEC a shelf registration statement under the Securities Act relating to the offer and sale of all of the registrable shares (as defined below) no later than forty-five (45) calendar days from the closing date of the merger and (ii) to cause such shelf registration statement to be declared effective within ninety (90) calendar days of the closing date of the merger. These Investors collectively held approximately 80% of the issued and outstanding shares of Cequent common stock on a fully diluted basis on the date of the merger agreement.
The Investors each shall be entitled, at any time and from time to time when a shelf registration statement is effective, to sell such registrable shares as are then registered pursuant to a shelf takedown from the shelf registration statement, upon not less than five (5) business days’ prior written notice to us. The Investors shall be entitled to request, in the aggregate, six (6) underwritten shelf takedowns under the registration rights agreement, but only if (i) the number of registrable shares to be sold in each such offering would reasonably be expected to yield gross proceeds to such Investor of at least $5 million and (ii) without our consent, such consent not to be unreasonably withheld, the request is not made within ninety (90) days after such Investor has sold shares in another underwritten registered offering under the shelf registration or demand registration provisions of the registration rights agreement.
For purposes of the registration rights agreement, “registrable shares” means, at any time, with respect to a particular Investor, (i) the shares of common stock issued to such Investor in the merger that are held of record by such Investor at such time and (ii) any securities issued by us after the date of the registration rights agreement in respect of the common stock issued in the merger by way of a share dividend or share split or other distribution or in connection with a combination of shares, recapitalization, merger, consolidation or other reorganization. However, registrable shares shall not include any shares of common stock or other securities held by such Investor that are eligible for resale by such Investor pursuant to Rule 144 under the Securities Act without limitation on volume or manner of sale.
Demand Registrations.
If we have not filed, and caused to be effective and maintained the effectiveness of a shelf registration statement relating to the registrable shares , the Investors may request in writing registration for resale under the Securities Act of all or part of the registrable shares;provided,however, that (based on the then-current market prices) the number of registrable shares included in such demand registration would, if fully sold, reasonably be expected to yield gross proceeds of at least $5 million. If we receive a request for a demand registration, we shall use reasonable best efforts (i) to file a registration statement registering for resale such number of registrable shares as requested to be so registered within thirty (30) days after receipt of the request for such registration and (ii) to cause such registration statement to be declared effective by the SEC as soon as practical following such request. The right of any Investor to request a demand registration will end on the first date that such Investor no longer owns any registrable shares. We will not be required to comply with more than one demand request in any rolling period of 180 days, and shall only be obligated to comply with three (3) demand requests in total.
The number of registrable shares to be included in any demand registration that is an underwritten offering shall be subject to limitation if the managing underwriter(s) of the requested demand registration advise us and the Investor(s) requesting such demand registration that in their opinion the number of registrable shares proposed to be included in the demand registration exceeds the number of shares of common stock that can be sold in such underwritten offering without materially delaying or jeopardizing the success of the offering.
I-72
Piggyback Registrations.
Subject to certain exceptions, whenever we propose (i) to register any shares of common stock under the Securities Act for sale to the public solely for cash, and the form of registration statement to be used may be used for any registration of registrable shares or (ii) to sell any shares of common stock that have already been registered “off the shelf” by means of a prospectus supplement, we shall give prompt written notice to the Investors of our intention to effect such a registration and/or shelf takedown and shall include in such registration statement and in any offering of common stock to be made pursuant to that registration statement and/or shelf takedown all registrable shares with respect to which we have received a written request for inclusion therein from any Investor within twenty (20) days after such Investor’s receipt of our notice.
The number of registrable shares to be included in any piggyback registration that is initiated as a primary or secondary underwritten offering shall be subject to limitation if the managing underwriter(s) advise us and, in the case of a primary offering, any Investors, that in their opinion the number of registrable shares proposed to be included in such offering exceeds the number of shares of common stock that can be sold in such offering without materially delaying or jeopardizing the success of the offering.
Suspension Periods.
We may delay the filing or effectiveness of, or by written notice to the applicable Investor(s) suspend the use of, a registration statement in conjunction with a demand registration or a shelf registration (and, if reasonably required, withdraw any registration statement that has been filed), if we determine in good faith that (x) such delay would enable us to avoid disclosure of material information, the disclosure of which at that time would be materially adverse to our best interests or (y) obtaining any financial statements (including required consents) required to be included in any such registration statement would be impracticable. In no event shall there be more than two suspension periods during any rolling period of 365 days, the number of days covered by any one suspension period shall not exceed ninety (90) days, and the number of days covered by all suspension periods shall not exceed 180 days in the aggregate during any rolling period of 365 days.
Expenses.
Subject to certain exceptions, we will pay all registration expenses in connection with each registration of securities pursuant to the registration rights agreement. Each Investor shall bear its pro rata cost of all underwriting discounts and commissions associated with any sale of registrable shares.
APPRAISAL RIGHTS
Under Delaware law, holders of our common stock do not have any right to an appraisal of the fair value of their shares in connection with the merger.
I-73
CHAPTER TWO — INFORMATION ABOUT THE MEETING AND VOTING
The Board of Directors is using this proxy statement to solicit proxies from the holders of our common stock for use at the annual meeting. This proxy statement and accompanying form of proxy is being first mailed to stockholders on or about June 11, 2010.
Matters Relating to the Meeting
Date, Time and Place: | Wednesday, July 21, 2010 |
10:00 a.m., Eastern Time
Offices of Pryor Cashman LLP
7 Times Square
New York, New York 10036
Purpose of Meeting is to Vote on the Following Items: | 1. the issuance of shares of common stock pursuant to the merger agreement, described under “Chapter One — The Merger — The Merger Transaction” on page I-29; |
2. the adjournment of the annual meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes in favor of Proposal No. 1, Proposal No. 3 or Proposal No. 8;
3. the amendment to our certificate of incorporation to change the name of our company from MDRNA, Inc. to Marina Biotech, Inc.;
4. the election of six (6) persons to our Board of Directors, each to hold office until the 2011 annual meeting of stockholders and until their respective successors shall have been duly elected or appointed and qualify;
5. the ratification of the appointment of KPMG LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2010;
6. amendment to our 2008 Stock Incentive Plan to increase the number of shares available for issuance thereunder from 4,500,000 to 8,500,000;
7. amendment to our 2007 Employee Stock Purchase Plan to increase the number of shares available for issuance thereunder from 300,000 to 600,000;
8. change our capital structure by increasing the number of authorized shares of common stock from 90,000,000 to 180,000,000, and by increasing the number of authorized shares of preferred stock from 100,000 to 200,000;
9. amendment to the Rights Agreement, dated February 22, 2000, between our company and American Stock Transfer & Trust Company as Rights Agent, as amended, to extend the term of the Rights Agreement from March 17, 2010 to March 17, 2013;
10. amendment to the Amended and Restated Common Stock Purchase Warrants issued to investors and the placement agent in April 2008 to reduce and, in certain instances, eliminate the floor exercise price of such warrants, and to issue the additional shares of common stock that would be issuable upon full exercise of such warrants as a result thereof;
II-1
11. amendment to the Common Stock Purchase Warrants issued to investors in January 2010 to eliminate the floor exercise price of such warrants; and
12. such other matters as may properly come before the meeting, including the approval of any adjournment of the meeting.
Record Date: | The record date for shares entitled to vote June 2, 2010. |
Outstanding Shares Held on Record Date: | As of June 2, 2010, there were 48,896,483 shares of our common stock outstanding. |
Shares Entitled to Vote: | Shares entitled to vote are shares of common stock held at the close of business on the record date, June 2, 2010. Each share of common stock that you own entitles you to one vote. Shares held by us in treasury, if any, are not voted. |
Quorum Requirement: | A quorum of stockholders is necessary to hold a valid meeting. The presence in person or by proxy at the annual meeting of shares representing a majority in interest of the common stock issued and outstanding and entitled to vote at the annual meeting is a quorum. Abstentions and broker non-votes count as present for establishing a quorum. A broker non-vote occurs on an item when a broker is not permitted to vote on that item without instruction from the beneficial owner of the shares and no instruction is given. |
Outstanding Shares Entitled to Vote and Owned by Directors, Executive Officers and their Affiliates as of June 2, 2010: | 142,773 shares of common stock outstanding and entitled to vote at the annual meeting. These shares represent in total less than 1% of the voting power of our common stock outstanding and entitled to vote at the annual meeting. |
Vote Necessary to Approve the Proposals
| | |
Item | | Vote Necessary |
| |
| Merger Proposal | | Approval of the issuance of the shares of common stock pursuant to the merger agreement described in “Chapter One — The Merger,” requires an affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote. Abstentions will be counted towards the vote total for this proposal, and will have the same effect as “Against” votes. Broker non-votes have no effect and will not be counted towards the vote total for this proposal. |
II-2
| | |
Item | | Vote Necessary |
| |
Adjournment of the meeting, if necessary | | Approval of the adjournment of the annual meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes to approve Proposal No. 1, Proposal No. 3 or Proposal No. 8 requires the affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote, regardless of whether a quorum is present. Abstentions will be counted towards the vote total for this proposal, and will have the same effect as “Against” votes. Broker non-votes have no effect and will not be counted towards the vote total for this proposal. |
| |
| Name Change | | The amendment to our certificate of incorporation to change the name of our company from MDRNA, Inc. to Marina Biotech, Inc., as described in “Chapter Five — Annual Meeting Proposals —Proposal No. 3 — Name Change,” requires the affirmative vote of a majority of the issued and outstanding shares of common stock entitled to vote as of the record date. Both abstentions and broker non-votes will have the same effect as “Against” votes. |
| |
| Election of Directors | | The re-election of six current directors to our Board of Directors, as described in “Chapter Five — Annual Meeting Proposals — Proposal No. 4 — Election of Directors,” requires the affirmative vote of a plurality of the votes cast. Both abstentions and broker non-votes have no effect and will not be counted towards the vote total for this proposal. |
| |
Ratification of the appointment of KPMG LLP as our independent registered public accounting firm | | The ratification of the appointment of KPMG LLP as our independent registered public accounting firm, as described in “Chapter Five — Annual Meeting Proposals — Proposal No. 5 — Ratification of Appointment of Independent Registered Public Accounting Firm,” requires the affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote. Abstentions will be counted towards the vote total for this proposal, and will have the same effect as “Against” votes. Broker non-votes have no effect and will not be counted towards the vote total for this proposal. |
| |
Amendment to our 2008 Stock Incentive Plan | | The approval of the amendment to our 2008 Stock Incentive Plan to increase the number of shares available for issuance thereunder from 4,500,000 to 8,500,000 as described in “Chapter Five — Annual Meeting Proposals — Proposal No. 6 — Approval of an Amendment to our 2008 Stock Incentive Plan to Increase the Number of Shares Available Thereunder from 4,500,000 to 8,500,000,” requires the affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote. Abstentions will be counted towards the vote total for this proposal, and will have the same effect as “Against” votes. Broker non-votes have no effect and will not be counted towards the vote total for this proposal. |
II-3
| | |
Item | | Vote Necessary |
| |
Amendment to our 2007 Employee Stock Purchase Plan | | The approval of the amendment to our 2007 Employee Stock Purchase Plan to increase the number of shares available for issuance thereunder from 300,000 to 600,000 as described in “Chapter Five — Annual Meeting Proposals — Proposal No. 7 — Approval of an Amendment to our 2007 Employee Stock Purchase Plan to Increase the Number of Shares Available Thereunder from 300,000 to 600,000,” requires the affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote. Abstentions will be counted towards the vote total for this proposal, and will have the same effect as “Against” votes. Broker non-votes have no effect and will not be counted towards the vote total for this proposal. |
| |
| Change of our capital structure | | The approval of a change to our capital structure that would increase the number of authorized shares of common stock from 90,000,000 to 180,000,000 and the number of authorized shares of preferred stock from 100,000 to 200,000 as described in “Chapter Five —Annual Meeting Proposals — Proposal No. 8 — Approval of a Change to our Capital Structure that would Increase the Number of Authorized Shares of Common Stock from 90,000,000 to 180,000,000 and the Number of Authorized Shares of Preferred Stock from 100,000 to 200,000,” requires the affirmative vote of a majority of the issued and outstanding shares of common stock entitled to vote as of the record date. Both abstentions and broker non-votes will have the same effect as “Against” votes. |
| |
Amendment to the Rights Agreement | | The approval of the amendment to our Stockholder Rights Agreement as described in “Chapter Five — Annual Meeting Proposals — Proposal No. 9 — Approval of an Amendment to our Stockholder Rights Agreement,” requires the affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote. Abstentions will be counted towards the vote total for this proposal, and will have the same effect as “Against” votes. Broker non-votes have no effect and will not be counted towards the vote total for this proposal. |
| |
Amendment to the April 2008 Common Stock Purchase Warrants | | The approval of the amendment to our Amended and Restated Common Stock Purchase Warrants issued in April 2008 as described in “Chapter Five — Annual Meeting Proposals —Proposal No. 10 — Approval of the Reduction and, in Certain Instances, the Elimination of the Floor Exercise Price of Common Stock Purchase Warrants Issued to Investors and the Placement Agent in April 2008, and the Issuance of Shares of Common Stock Upon the Full Exercise of Such Warrants,” requires the affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote. Abstentions will be counted towards the vote total for this proposal, and will have the same effect as “Against” votes. Broker non-votes have no effect and will not be counted towards the vote total for this proposal. |
II-4
| | |
Item | | Vote Necessary |
| |
Amendment to the January 2010 Common Stock Purchase Warrants | | The approval of the amendment to our Common Stock Purchase Warrants issued in January 2010 as described in “Chapter Five —Annual Meeting Proposals — Proposal No. 11 — Approval of the Elimination of the Floor Exercise Price of Common Stock Purchase Warrants Issued to Investors in January 2010,” requires the affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote. Abstentions will be counted towards the vote total for this proposal, and will have the same effect as “Against” votes. Broker non-votes have no effect and will not be counted towards the vote total for this proposal. |
Voting
You may vote in person at the annual meeting or by proxy. We recommend you vote by proxy even if you plan to attend the meeting. You can always change your vote at the meeting.
Voting instructions are included on your proxy or proxy card. If you properly give your proxy and submit it in time to vote (or vote electronically via the Internet or telephone), one of the individuals named as your proxy will vote your shares as you have directed. You may vote for or against the proposals or abstain from voting. Other than in respect of election of directors, if you mark your proxy “abstain” with respect to any proposal, you will be in effect voting against the proposal. In addition, if you fail to send in your proxy, this, too, will have the same negative effect. If your shares are held in “street name” by a broker, bank or other nominee, the broker cannot vote your shares on any proposal without your instructions. This is a “broker non-vote.” A “broker non-vote” with respect to a proposal may have the effect of a vote against that proposal.
How to Vote by Proxy
Complete, sign, date and return your proxy card in the enclosed envelope. You may also vote electronically by Internet or telephone if your proxy card so indicates.You are encouraged to vote electronically if you have that option.
If you submit your proxy but do not make specific choices, your proxy will follow the Board of Directors’ recommendations and vote your shares:
“FOR” the issuance of shares of common stock in the merger;
“FOR” the adjournment of the annual meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes to approve Proposal No. 1, Proposal No. 3 or Proposal No. 8;
“FOR” the amendment to our certificate of incorporation to change the name of our company from MDRNA, Inc. to Marina Biotech, Inc.;
“FOR” the election of six (6) persons to our Board of Directors, each to hold office until the 2011 annual meeting of stockholders and until their respective successors shall have been duly elected or appointed and qualify;
“FOR” the ratification of KPMG LLP as the independent registered public accounting firm of our company;
“FOR” the amendment of our 2008 Stock Incentive Plan to increase the number of shares available for issuance thereunder from 4,500,000 to 8,500,000;
“FOR” the amendment of our 2007 Employee Stock Purchase Plan to increase the number of shares available for issuance thereunder from 300,000 to 600,000;
II-5
“FOR” the change of our capital structure by increasing the number of authorized shares of common stock from 90,000,000 to 180,000,000, and by increasing the number of authorized shares of preferred stock from 100,000 to 200,000;
“FOR” the amendment to the Rights Agreement, dated February 22, 2000, between our company and American Stock Transfer & Trust Company as Rights Agent, as amended, to extend the term of the Rights Agreement from March 17, 2010 to March 17, 2013;
“FOR” the amendment to the Amended and Restated Common Stock Purchase Warrants issued to investors and the placement agent in April 2008 to reduce and, in certain instances, eliminate the floor exercise price of such warrants, and to issue the additional shares of common stock that would be issuable upon full exercise of such warrants as a result thereof;
“FOR” the amendment to the Common Stock Purchase Warrants issued to investors in January 2010 to eliminate the floor exercise price of such warrants; and
In its discretion as to any other business that may properly come before the annual meeting.
Revoking Your Proxy. You may revoke your proxy before it is voted by:
| | • | | submitting a new proxy with a later date, |
| | • | | submitting a vote electronically via the Internet or by telephone with a later date, if that was how the original vote was submitted, |
| | • | | notifying the Secretary of MDRNA in writing before the meeting that you have revoked your proxy, or |
| | • | | voting in person at the meeting. |
Voting in person. If you plan to attend the meeting and wish to vote in person, we will give you a ballot at the meeting. However, if your shares are held in the name of your broker, bank or other nominee, and you are a stockholder of our company, you must bring an account statement or letter from the nominee indicating that you are the beneficial owner of the shares on June 2, 2010, the record date for shares entitled to vote at the annual meeting.
People with disabilities. We can provide reasonable assistance to help you participate in the meeting if you tell us about your needs and your plan to attend. Please call or write to the Secretary of MDRNA at least two weeks before the meeting at the number or address under “The Companies” on page I-6.
Proxy solicitation. We will pay our own costs, if any, of soliciting proxies.
Solicitation.The solicitation of proxies will be conducted by mail, and we will bear all attendant costs. These costs will include the expense of preparing and mailing proxy materials for the annual meeting and reimbursements paid to brokerage firms and others for their expenses incurred in forwarding solicitation materials regarding the annual meeting to beneficial owners of our common stock. We intend to use the services of Morrow & Co., Inc., 445 Park Avenue, 5th Floor, New York, New York, 10022, in soliciting proxies and, as a result, we expect to pay an amount not to exceed $10,000, plus out-of-pocket expenses, for such services. We may conduct further solicitation personally, telephonically, electronically or by facsimile through our officers, directors and regular employees, none of whom would receive additional compensation for assisting with the solicitation.
The extent to which these proxy soliciting efforts will be necessary depends entirely upon how promptly proxies are submitted. You should send in your proxy without delay. We also reimburse brokers and other nominees for their expenses in sending these materials to you and getting your voting instructions.
II-6
Other Business; Adjournments
We are not currently aware of any other business to be acted upon at the meeting other than as discussed in this proxy statement. If other matters are properly brought before the annual meeting, or any adjourned meeting, your proxies will have discretion to vote or act on those matters according to their best judgment, including adjourning the meeting.
Adjournments may be made for the purpose of, among other things, soliciting additional proxies. Any adjournment may be made from time to time by approval of the holders of shares representing a majority of the votes present in person or by proxy at the meeting, whether or not a quorum exists, without further notice other than by an announcement made at the meeting. We do not currently intend to seek an adjournment of the meeting.
Appraisal Rights
Holders of our common stock do not have any right to an appraisal under Delaware law in connection with any matters to be voted on at the annual meeting.
II-7
CHAPTER THREE — OTHER INFORMATION REGARDING CEQUENT
BUSINESS OF CEQUENT
Overview and Business Strategy
Cequent develops therapeutics based on RNA interference (RNAi) for the treatment of human diseases. Cequent has one clinical-stage program, one late preclinical program, and other assets currently under development.
Familial Adenomatous Polyposis and Colon Cancer Prevention — CEQ508 is Cequent’s lead clinical drug candidate. CEQ508 targets the ß-catenin gene to retard polyp formation in patients with an inherited form of colon cancer called familial adenomatous polyposis, or FAP. A CEQ508 Investigational New Drug Application was accepted by Center for Biologics Evaluation and Research (CBER) of the U.S. Food and Drug Administration (FDA) in mid-December, 2009, and a Phase I safety, tolerability and biomarker trial in FAP patients is slated to begin in the second quarter of 2010. FAP is an orphan disease, which affects about 80,000 persons worldwide. CEQ508 may also prove useful for the prevention of polyp formation and recurrence of disease in colon cancer patients who have had surgery.
Inflammatory Bowel Disease (IBD) — Cequent has received funding from Novartis BioVentures Ltd. to develop a treatment against one target involved in Crohn’s disease and Ulcerative Colitis (collectively, IBD). In May 2009 Cequent demonstratedin vivo proof of concept for this target, which is now the subject of an option held by Novartis BioVentures Ltd. to an exclusive license to Cequent’s technology against this target. Cequent is working on drug candidates targeting several other IBD-implicated genes including TNF-a, IL-6Ra and IL-18, all of whose rights are exclusively retained by Cequent. Given Cequent’s intellectual property estate, IBD is a promising program for Cequent as there are no truly effective treatments and little competition in local delivery.
These programs utilize Cequent’s proprietary transkingdom RNAi (tkRNAiTM) delivery technology. In this approach, the types of bacteria normally present at the site of treatment are engineered to deliver mediators of RNAi called short hairpin RNA, or shRNA. Cequent was the first company to demonstrate oral delivery of RNAi in non-human primates.tkRNA-based therapeutics will be administered orally to patients with both FAP and IBD. Cequent expects to initiate additional clinical programs as the capabilities of its development programs evolve.
Background
Proteins perform many of the vital functions of the cell and of the human body. Although the roles they play are generally beneficial, in certain circumstances, proteins can be harmful. Many human diseases are caused by the inappropriate behavior of proteins. A particular protein may, for example, be present in too great a quantity, be too active, be a mutated version of a normal protein, or appear in the wrong place or at the wrong time. In these circumstances, the ability to stop or reduce production of the protein by selectively silencing the gene that directs its synthesis could be very beneficial in the treatment of a disease. In 1998, scientists discovered that double-stranded RNA can be used to trigger very potent and specific gene silencing inC. elegans, nematode worm species used as a biology model. Subsequent work by a group at Max Planck Institute (Tuschl et al, Nature 2002), showed that this endogenous gene silencing pathway was also present in mammalian cells, including human, and could be harnessed by the introduction of small double-stranded RNA of approximately 20 nucleotides in length (so-called small, interfering RNA, or siRNA), into cells. Subsequent research showed that in order to carry out siRNA’s gene knockdown function, the siRNA is incorporated into the RNA-induced silencing complex (RiSC) that RiSC then guides to a complementary section of the messenger RNA (mRNA) responsible for the production of the protein target. Once RiSC matches the siRNA guide strand to the complementary section of the mRNA, the mRNA is then cleaved and destroyed by RiSC nuclease activity. As a result, gene expression is inhibited at the level of the mRNA before its information can be translated into proteins. Moreover, the RiSC complex is stable and can catalyze multiple rounds of target mRNA cleavages, essentially re-using the original siRNA molecule, enabling highly effective gene silencing. These findings immediately suggested new pharmaceutical approaches.
III-1
Scientific Basis and Products:transkingdomRNA interference (tkRNAi)
Cequent uses a new technology developed at the GI Cancer Laboratory of Beth Israel Deaconess Medical Center (BIDMC), Harvard Medical School, to simultaneously manufacture and effect RNA interference. In this proprietary process, known astranskingdomRNA interference (tkRNAi), non-pathogenic bacteria are engineered to produce siRNA in the form of shRNA. Following oral or topical administration of the engineered bacteria, the bacteria are taken up by the specified cells of the target tissue, release the payload of shRNA, and trigger RNAi to silence specific disease-related genes. The vector bacteria are subsequently degraded by the host cells. The areas of the body which already contain commensal bacteria will be simplest to treat withtkRNAi. Based on work to date, applications oftkRNAi include treatment of inflammatory, infectious, and proliferative diseases. Additional applications are potentially very broad, encompassing many diseases that could be treated through silencing of causative genes. While targeted therapeutics such as small molecules (e.g. Gleevec® and Tarceva®) or monoclonal antibodies (e.g. Herceptin®, Erbitux® and Avastin®) have led to therapeutic successes, these approaches are not suitable for all cellular targets. For example, small molecules have limited capacity to block non-enzymatic cellular events and monoclonal antibodies cannot effectively act on intracellular targets. In contrast, RNAi can potently and selectively silence essentially any cellular gene or combination of genes, making it an ideal approach to influence the behavior of cells where the role of an individual protein or proteins in a disease process is known. In their earliest work, BIDMC researchers validated this approach by demonstrating potent silencing of specific genes associated with diseases of the gastrointestinal tract usingtkRNAi. BIDMC researchers later showed therapeutic activity in animal models of colon polyps and colon cancer based on silencing of theß-cateningene and theK-rasoncogene — two targets previously considered “undruggable.”
Subsequently, Cequent has extended this approach to address two cervical cancer targets (E6 and E7 oncoproteins) as well as many targets implicated in IBD as part of its preclinical pipeline. All of these applications utilize Cequent’s proprietary method for engineering and delivering non-pathogenic bacteria (oral delivery ofE. coliis planned for treating colon polyps and IBD; and topical delivery of a commensal bacteria from the female genitourinary tract (GU) such as lactobacillus is planned for cervical cancer targets) to selectively silence genes.
Product Development
Cequent seeks to be the leader in the delivery of RNAi using bacterial vectors. This form of delivery is ideally suited to the creation of safe natural therapies for diseases that affect any surface or cavity of the body which already contain comensal bacteria — notably the GI tract, the GU track and the skin. Based on Cequent’s broad intellectual property position, it is possible that Cequent’s platform can enable the development of numerous novel drug products over the next several years.
RNAi-Based Therapeutics
Overview
RNAi is widely considered to have the potential to become the next major drug development platform after small molecules, recombinant proteins, and most recently, monoclonal antibodies. In contrast to the current drug paradigm of modulating the function of certain druggable proteins, RNAi can in theory suppress the function of any gene, specifically at the mRNA level, upstream of the protein itself. Through the completion of the human genome project and ensuing work, the number of genes identified and implicated in disease has grown dramatically. These genes have become potential targets for which RNAi approaches can be applied to design therapies in a rapid and predictable manner. Nevertheless, attempts to develop drugs based on RNAi have encountered significant challenges, most notably related to the difficulty of delivering siRNA to the interior of the cell where RNAi operates, particularly into specific target cells in the intact, living organism.
III-2
RNAi Delivery
Unlike previous technologies which are restricted to being able to target only a small subset of well validated drug targets, RNAi can in theory address almost all diseases since it should be possible to modify, if not correct, a disease by suppressing certain genes. This potential, however, is essentially limited by one major factor: delivery.
The opportunity to address so many and varied diseases by RNAi means that there is considerable demand and room for a wide range of RNAi delivery methods for the different cell types, tissues, and organs implicated in disease. In the absence of cell specific targeting techniques, the most advanced synthetic RNAi delivery technologies, liposomes and polymers, are currently limited to applications for diseases of the liver and lung, as well as certain cancers. This creates an opportunity for Cequent since many of the diseases that it can address and the specific approach taken (dosing, route of administration, etc.) will be in the absence of direct competition by other RNAi therapeutic approaches while addressing significant unmet medical needs. This applies particularly to areas of the body that are naturally at the interface with commensal bacteria such as the skin and mucosa of the mouth, nose, cervix and gut. As such, Cequent’s lead program, CEQ-508 for FAP/Colon polyps, targets RNAi to the epithelial lining of the gut where, to date,tkRNAi is the only orally administered RNAi mediator of gene knockdown.
III-3
tkRNAi Drug Discovery Platform
In 2004, work by Drs. Shuanlin Xiang, Johannes Fruehauf, Chiang J. Li, and colleagues at BIDMC showed for the first time that RNAi can be mediated by a live vector system in which innocuous bacteria function both as factories for the production of RNAi triggers as encoded on a plasmid, and to effectively deliver them to animal cells and to live animals. Because the effect is transmitted across animal kingdoms (from bacteria to mammals), it is calledtranskingdomRNA interference. A graphic depiction of the system is shown in FIGURE 1 below for one embodiment in which a protein known as invasin is used for cellular uptake into epithelial cells. Other cell-targeting ligands have been tried by Cequent for delivery to different cell types.
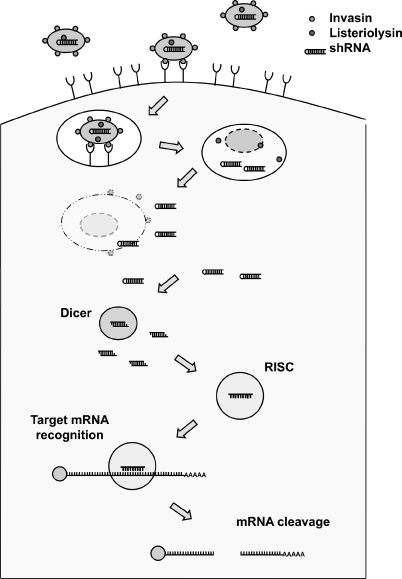
Figure 1.TranskingdomRNAi: bacteria are engineered to act as carriers of shRNA. In this example, invasin expressed on the bacterial surface interacts with beta-integrin receptors on the surface of epithelial cells to trigger uptake into the host cell. Inside of the endosome, the bacteria are lysed rapidly. Listeriolysin released from the bacteria ruptures the membrane of the endosome and allows the shRNA to get access into the host cell cytosol. After reaching the cytosol, shRNA is cleaved by DICER into active siRNA, and one of the two strands (the guide
III-4
strand) is integrated into RiSC (RNA-induced silencing complex). RiSC recognizes homologous regions within the mRNA present in the cytosol and leads to its cleavage and subsequent degradation. The production of a disease-associated protein can thus be significantly reduced.
Work at Cequent has confirmed that suitably engineered bacteria can transmittkRNAi to intact animals and that significant therapeutic effects can be achieved. At BIDMC,tkRNAi was shown to be active in a variety of tumor modelsin vivowith various oncogenes as treatment targets (e.g., k-RAS, beta-catenin). Most notably, Cequent scientists have conducted the first-ever experiments showing RNAi activity after oral application in non-human primates. This study demonstrated that epithelial cells in the GI tract of cynomolgous monkeys showed reduced expression levels of the target gene, beta-catenin, during and after oral administration oftkRNAi bacteria for 28 days. In a mouse model for the prevention of human colon cancer (APCmin), scientists at BIDMC have shown that bacterial mediated RNA interference treatments given orally over long periods (8-16 weeks) can reduce beta-catenin levels in the epithelial tissues of the GI tract and prevent the formation of new intestinal polyps. As an additional observation, this treatment resulted in fewer polyps progressing from low to high grade dysplasia (abnormal cell development). Treatment with tkRNAi against beta-cateninin vitroresulted in decreased levels of beta-catenin as well as its downstream effectors, such as c-myc, c-jun and Cyclin D1. This result was confirmed in samples of tissue from the ileum and colon of monkeys following four weeks of oral administration. Cequent is developing carrier bacteria which include various strains ofE. coli, including probiotic variants, as well asLactobacillus andS. typhimurium. Ongoing research at Cequent is directed at targeting specific subsets of organs and tissues aftertkRNAi treatment through modification of the surface properties of the carrier bacteria. This will enabletkRNAi to be targeted preferentially towards specific diseased tissues or cell types (e.g. cancer cells) and will expand the range of clinical indications available fortkRNAi treatment.tkRNAi bacteria are highly attenuated (weakened) and do not colonize in the GI tract, making this a very controllable system.In vivostudies show that the therapeutic bacteria are cleared from the GI tract within 24-36 hours. Long term studies in live animals have been conducted to establish the safety and immunology profile of chronic treatment withtkRNAi. Notably, daily treatment with oraltkRNAi over 13 weeks did not induce a proinflammatory cytokine response in either healthy or sick (APCmin) mice. In IND-enabling toxicology studies for Cequent’s lead program, daily dosing of cynomolgous monkeys for 28 days also did not induce a proinflammatory cytokine response.
Market for Colon Cancer Prevention and Familial Adenomatous Polyposis (FAP)
According to the U.S. Centers for Disease Control, approximately 140,000 Americans were diagnosed with colorectal cancer (CRC) in 2006, and about 53,000 died from CRC that year. Globally, it is estimated that a half-million people worldwide are diagnosed with CRC each year. Polyp surveillance by colonoscopy every 10 years for Americans over the age of 50 has been shown to be an effective preventative method for CRC. However, preventative approaches in addition to removal of pre-cancerous polyps by colonoscopy/polypectomy are needed to control or eradicate overlooked or rapidly-forming cancerous polyps.
About 1% of CRC cases are related to a specific hereditary disorder called FAP, a rare autosomal disorder affecting 1 in 10,000 individuals. Various reports estimate that there are about 30,000 FAP patients in the U.S. and a total of 80,000 FAP patients throughout the developed world. Because of a mutation in the Adenomatous Polyposis Coli (APC) gene that defines an FAP patient, hundreds to thousands of polyps form in the large and small intestine of FAP patients that become malignant over time. Without surgical removal of the colon, typically in the late teenage years, CRC is almost inevitable for FAP patients with a mean age of CRC onset of 39 years. Currently, the only available treatment for FAP is complete colectomy (removal of the large intestine), though patients remain at risk of polyp and cancer formation in the remaining sections of the rectum, large intestine, ilial pouch and small intestine. To limit polyp formation as well as reduce and eradicate polyps with the aim of preventing adenoma formation, pharmacological therapies, pre-coloectomy and post-colectomy are called for. Cyclooxygenase-2 (COX-2) inhibitors were almost universally chronically administered to FAP patients post-puberty until the discovery that cardiovascular side effects were possible at the high-dose regimen necessary for utility in FAP patients. The safety profile of other pharmacological prophylaxis and treatments for FAP patients (i.e., Nonsteroidal anti-inflammatory drugs, or NSAIDs) generally prohibit their use.
III-5
Current Treatments for FAP
Routine polyp surveillance for and removal of premalignant adenomas by colonoscopy with polypectomy is an effective prevention method for CRC. However, in FAP patients, polyps can escape detection or rapidly appear between colonoscopies.
NSAIDs and in particular COX-2 inhibitors have been used to prevent, slow or reduce polyp formation in FAP and other hyper-polyp patient populations. However, the gastrointestinal and hemorrhagic side effects of NSAIDs generally preclude their use. Although celecoxib (Celebrex®) was approved for use in FAP patients, most COX-2 inhibitors were pulled from the market when cardiovascular issues were identified at the high dose levels needed to impact polyps. While celecoxib is still indicated for FAP, according to specialists who care for FAP patients, it is no longer generally prescribed.
Market for IBD Therapeutics
The prevalence of IBD patients is about 1 million in the U.S. and 4 million worldwide, about equally divided between Crohn´s disease and ulcerative colitis. Prevalence in the developed and the developing worlds is increasing. Typically diagnosed before the age of 30, the primary symptoms of both conditions are abdominal pain and diarrhea which typically become more severe during periodic flares for the duration of the patient’s life. In severe cases, ulcerative colitis can be fatal. There is no pharmacological cure for IBD. Colectomy is curative for ulcerative colitis and can be life-saving in Crohn’s disease, but the incidental surgical damage and altered intestine is life-changing for the patient. Although chronic and acute oral therapies, as well as injectible biologics, can often control symptoms in many patients, there remains a significant unmet need for better pharmacotherapies to effectively treat moderate to severe disease and maintain disease remission. The worldwide market for IBD therapeutic products was estimated at $2 billion in 2005 and, with the introduction of systemic tumor necrosis factor-alpha (TNFa) antibody products, grew to over $6 billion in 2009.
Current Treatments
Treatments for IBD include long-term and flare-control administration of largely generic oral small molecules such as aminosalicylates, corticosteroids, immunomodulators and antibiotics. Anti-TNFa agents are also used chronically, typically when small molecule approaches fail to control both the severity and incidence of IBD flares. About a third of patients do not respond to anti-TNFa therapies and over time many patients fail to respond to treatment. In addition, anti-TNFa therapies include risk of infection and increased risk of certain cancers.
RNAi Partnering and Licensing Agreements
Cequent’s business strategy includes collaborations and strategic partnerships with academic laboratories, research foundations, pharmaceutical companies and biotechnology companies, as well as the pursuit of licenses to key intellectual property. Three important relationships are described below:
Novartis BioVentures Ltd. — In 2007, Cequent entered into a set of agreements with Novartis BioVentures Ltd, in which Novartis BioVentures Ltd. invested in Cequent’s initial equity financing, and also provided non-dilutive financing for Cequent to develop treatments for IBD using Cequent’s tkRNAi technology. One of these agreements, the Option Agreement, grants Novartis BioVentures Ltd. an exclusive option to Cequent’s proprietary tkRNAi technology in conjunction with a single gene target implicated in IBD. Other than Novartis BioVentures Ltd.’s equity interest in Cequent and the Option Agreement, Novartis BioVentures Ltd. has no further rights to any Cequent technology or other gene targets. The specific gene target that is the subject of the option was mutually selected during the course of a funded research project. Various milestones in this research project have been announced. In September 2008, Cequent announced it had achieved the second milestone in the option agreement’s project whereby Cequent completed the simultaneous evaluation of eight targets as potentialtkRNAi therapeutics for IBD, and winnowed the available Novartis BioVentures Ltd. targets down to
III-6
three. By early 2010, the single target subject to the option had been designated, and in April 2010 Cequent announced that it had begun dosing in an IND-enabling non-human primate GLP toxicology study with a therapeutic candidate for the designated target. The IND-enabling non-human GLP toxicology study is on-going with a Novartis target-specific delivery vehicle but Cequent is re-optimizing the therapeutic candidate.
BIDMC — In December 2004, Cequent concluded an exclusive license agreement with BIDMC which included all intellectual property related to the tkRNAi approach, as well as the rights to any improvements to the technology which might be developed at BIDMC for a period of time. The license agreement included several milestones which Cequent needed to achieve to maintain exclusivity, and all of these have now been met. The license includes maintenance payments, as well as milestone and royalty payments to BIDMC for the commercialization of drugs based on the licensed technology.
ViThera Laboratories — In 2010, Cequent assisted its former Vice President of Research in the creation of ViThera, an independent research company. ViThera has a sublicense to tkRNAi technology from Cequent for use in certain non-human applications such as animal health and crop pest control. Cequent is a small equity owner in ViThera and ViThera also performs certain contract research projects for Cequent.
Proprietary Rights and Intellectual Property
Cequent relies primarily on patents and contractual obligations with employees and third parties to protect its proprietary rights. Cequent has sought, and intends to continue to seek, appropriate patent protection for important and strategic components of its proprietary technologies by filing patent applications in the U.S. and certain foreign countries. There can be no assurance that any of Cequent’s patents will guarantee protection or market exclusivity for its products and product candidates. Cequent also uses license agreements both to access external technologies and to convey certain intellectual property rights to others. The financial success of Cequent and, following the consummation of the merger, the combined company, will be dependent in part on Cequent’s and the combined company’s ability to obtain commercially valuable patent claims and to protect intellectual property rights and to operate without infringing upon the proprietary rights of others. As of May 10, 2010, Cequent owned or controlled two issued or allowed patents, five pending U.S. patent applications, including provisional patent applications, one pending Patent Cooperation Treaty (PCT) application and five pending foreign patent applications, to protect its RNAi proprietary technologies.
Cequent’s patents and patent applications are directed to compositions of matter, formulations, methods of use and/or methods of manufacturing, as appropriate. The patent positions of pharmaceutical and biotechnology companies, including Cequent, are generally uncertain and involve complex legal and factual questions. The business of Cequent could be negatively impacted by any of the following:
| | • | | the claims of any patents that are issued may not provide meaningful protection, may not provide a basis for commercially viable products or may not provide Cequent with any competitive advantages; |
| | • | | Cequent’s patents may be challenged by third parties; |
| | • | | others may have patents that relate to Cequent’s technology or business that may prevent Cequent from marketing its product candidates unless it is able to obtain a license to those patents; |
| | • | | the pending patent applications to which Cequent has rights may not result in issued patents; and |
| | • | | Cequent may not be successful in developing additional proprietary technologies that are patentable. |
In addition, others may independently develop similar or alternative technologies, duplicate any of Cequent’s technologies and, if patents are licensed or issued to Cequent, design around the patented technologies licensed to or developed by Cequent. Moreover, Cequent could incur substantial costs in litigation if it has to defend itself in patent suits brought by third parties or if it initiates such suits.
III-7
Research
Much of the original research necessary to establish the mechanism and validity of Cequent’s platform tkRNAi technology had been developed at BIDMC in a program which began at the GI Cancer Laboratory there in 2002. At Cequent, a group of approximately 20 professionals refined the technology during the period from inception of laboratory activities in the early summer of 2007 until the fall of 2009. At that point, Cequent had finalized the delivery system for its first three products and was able to redeploy resources from bench research to drug development, pre-clinical research and clinical research, much of which has been outsourced to industry leading contract research organizations.
Employees
As of June 1, 2010, Cequent had 10 full-time employees. As a drug development company, Cequent has shifted away from a research-centric model with attendant large fixed costs in the form of scientific employees, and moved within the past year to a more development-oriented and “virtual” model in which many pre-clinical development tasks are outsourced to contract research organizations (CRO’s) on a project-by-project basis. In addition, under the leadership of Cequent’s Vice President of Drug Development, Alison Silva, Cequent has retained the services of experienced regulatory and clinical development consultants to assist with the preparation and filing of documents with its regulator — the Center for Biologics Evaluation and Research at the FDA, as well as other regulatory groups at the National Institutes of Health.
Competition
tkRNAi: a fundamentally distinct method of triggering RNAi
BeforetkRNAi, RNAi induction methods could essentially be categorized into either synthetic siRNA or DNA-directed RNAi (ddRNAi). IntkRNAi, Cequent has introduced a fundamentally distinct third method of inducing RNAi and for which Cequent enjoys a strong IP position and know-how.
ddRNAi is a “gene therapy” approach to RNAi therapeutics. Here, a DNA vector is delivered to the host cell nucleus by either viral or non-viral means. The DNA encodes for a hairpin RNA that is transcribed by nuclear RNA polymerases which are then processed by the RNAi-related microRNA processing machinery of the host cell into small interfering RNAs (siRNAs). The siRNAs are incorporated into the RiSC complex to seek out and target complementary mRNAs for degradation. The advantage of this approach is that once the RNAi trigger has been successfully introduced into the cell, long-term gene knockdown could be achieved without the need for frequent repeat-administration. The wide adoption of ddRNAi as a therapeutic modality, however, has been hampered by safety concerns related to oncogenic potential, immunological challenges, and the difficulty of stopping the gene silencing should unacceptable side-effects emerge. This limits the applicability of ddRNAi to relatively few severe diseases with often small patient populations. Non-virally delivered ddRNAi may lessen some of the concerns, but greatly suffers from the difficulty of delivering DNA into sufficient numbers of host cell nuclei.
Most companies developing RNAi therapeutics therefore focus on the use of synthetic double-stranded RNAs (dsRNA) for gene knockdown by RNAi. These dsRNAs feed into the endogenous RNAi process primarily at the RiSC processing stage. Unlike DNA-directed RNAi, synthetic RNAi shares many of the features of traditional drug treatment such as the administration of a synthetic active pharmaceutical ingredient at regular intervals. Although the functional delivery of synthetic dsRNAs is not as difficult as non-viral DNA delivery in ddRNAi, there remain certain delivery challenges. Chemical modifications typically enhance the stability and therefore pharmacology of dsRNAs in the body in addition to mitigating some of their unwanted immuno-stimulatory properties. Sophisticated formulations can facilitate dsRNA’s passing through membranes to reach the cytoplasm. In addition, a number of cell types, tissues, and organs (e.g. gastrointestinal tract, the skin, muscle) are not adequately addressed by current synthetic RNAi delivery methods but can be addressed bytkRNAi.
III-8
tkRNAi is sufficiently different from both ddRNAi and synthetic RNAi to qualify as a third distinct method of inducing RNAi.tkRNAi is distinct from other RNAi induction methods both with respect to the origin of the RNAi trigger and its mode of delivery. With ddRNAi and synthetic RNAi the RNAi trigger is generated by either the host cell (ddRNAi) or chemically synthesized (siRNA). The RNAi trigger intkRNAi originates from the bacteria. Moreover, withtkRNAi there is the flexibility to deliver the bacterially made RNAi trigger to the target cell either in the form of a shRNA or mature siRNA. The ease of bacterial genetic engineering critically facilitates such flexibility and is based on decades of bacterial molecular genetics. This is a major advantage oftkRNAi, as it allows it to be fine-tuned to each indication. It is also by genetic engineering that these bacteria can be used to not only make the RNAi trigger, but to simultaneously deliver the payload into mammalian cells. This is achieved by expressing certain molecules on the surface of the bacteria that interact with specific cell surface receptors, and allows for the efficient and specific delivery of thetkRNAi trigger to target cells. Payload delivery is completed by the escape of shRNA from the endosomes to the cytoplasm within the target cells by the bacteria’s production of an additional genetically engineered pore-forming protein. Thus, shRNA are released into the cytoplasm for processing by DICER and incorporation into RiSC.
Government Regulation
Government authorities in the U.S. and other countries extensively regulate the research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing, among other things, of drugs and biologic products. All of Cequent’s foreseeable product candidates are expected to be regulated as drug products.
In the U.S., the FDA regulates drug products under the Federal Food, Drug and Cosmetic Act (the “FDCA”), and other laws within the Public Health Service Act. Failure to comply with applicable U.S. requirements, both before and after approval, may subject Cequent to administrative and judicial sanctions, such as a delay in approving or refusal by the FDA to approve pending applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, and/or criminal prosecutions. Before Cequent’s drug products are marketed they must be approved by the FDA. The steps required before a novel drug product is approved by the FDA include: (1) pre-clinical laboratory, animal, and formulation tests; (2) submission to the FDA of an Investigational New Drug Application (“IND”) for human clinical testing, which must become effective before human clinical trials may begin; (3) adequate and well-controlled clinical trials to establish the safety and effectiveness of the product for each indication for which approval is sought; (4) submission to the FDA of an New Drug Application (“NDA”); (5) satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug product is produced to assess compliance with cGMP; and FDA review and finally (6) approval of an NDA.
Pre-clinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. The results of the pre-clinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND, which must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions, such as the conduct of the trials as outlined in the IND. In such a case, the IND sponsor and the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed. There can be no assurance that submission of an IND will result in FDA authorization to commence clinical trials. Once an IND is in effect, each clinical trial to be conducted under the IND must be submitted to the FDA, which may or may not allow the trial to proceed.
Clinical trials involve the administration of the investigational drug to human subjects under the supervision of qualified physician-investigators and healthcare personnel. Clinical trials are typically conducted in three defined phases, but the phases may overlap or be combined. Phase 1 usually involves the initial administration of the investigational drug or biologic product to healthy individuals to evaluate its safety, dosage tolerance and pharmacodynamics. Phase 2 usually involves trials in a limited patient population, with the disease or condition for which the test material is being developed, to evaluate dosage tolerance and appropriate dosage; identify possible adverse side effects and safety risks; and preliminarily evaluate the effectiveness of the drug or biologic
III-9
for specific indications. Phase 3 trials usually further evaluate effectiveness and test further for safety by administering the drug or biologic candidate in its final form in an expanded patient population. Cequent’s product development partners, the FDA, or Cequent may suspend clinical trials at any time on various grounds, including any situation where Cequent believes that patients are being exposed to an unacceptable health risk or are obtaining no medical benefit from the test material.
Assuming successful completion of the required clinical testing, the results of the pre-clinical trials and the clinical trials, together with other detailed information, including information on the manufacture and composition of the product, are submitted to the FDA in the form of an NDA requesting approval to market the product for one or more indications. Before approving an application, the FDA will usually inspect the facilities where the product is manufactured, and will not approve the product unless cGMP compliance is satisfactory. If the FDA determines the NDA is not acceptable, the FDA may outline the deficiencies in the NDA and often will request additional information. If the FDA approves the NDA, certain changes to the approved product, such as adding new indications, manufacturing changes or additional labeling claims are subject to further FDA review and approval. The testing and approval process requires substantial time, effort and financial resources, and there can be no assurance that any approval will be granted on a timely basis, if at all.
In addition, regardless of the type of approval, Cequent and its partners are required to comply with a number of FDA requirements both before and after approval. For example, Cequent is required to report certain adverse reactions and production problems, if any, to the FDA, and to comply with certain requirements concerning advertising and promotion for its products. In addition, quality control and manufacturing procedures must continue to conform to cGMP after approval, and the FDA periodically inspects manufacturing facilities to assess compliance with cGMP. Accordingly, manufacturers must continue to expend time, money and effort in all areas of regulatory compliance, including production and quality control to comply with cGMP. In addition, discovery of problems, such as safety problems, may result in changes in labeling or restrictions on a product manufacturer or NDA holder, including removal of the product from the market.
Development Stage Company
Cequent is a development stage company; however, the approach it is taking to discover and develop drugs is novel and unproven. Cequent’s product candidates are in clinical and preclinical development where failure is common. Neither Cequent nor any other company has received regulatory approval to market therapeutics utilizing RNA interference. Cequent does not expect any product candidates, if successfully developed, to receive regulatory approval for commercial sale until at least 2014.
Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of financial condition and results of operations should be read together with Cequent’s financial statements and accompanying notes included in this proxy statement. This discussion contains forward-looking statements, based on current expectations and related to future events and Cequent’s future financial performance, that involve risks and uncertainties. Cequent’s actual results may differ materially from those anticipated in these forward-looking statements as a result of many important factors, including those set forth under “Risk Factors” and elsewhere herein. See “Special Note on Forward-Looking Statements.”
Overview
Cequent develops therapeutics based on RNA interference (RNAi) for the treatment of human diseases. Cequent has one clinical-stage program, one late preclinical program, and other assets currently under development. These programs utilize Cequent’s proprietary transkingdom RNAi (tkRNAiTM) delivery technology. In this approach, the types of bacteria normally present at the site of treatment are engineered to deliver mediators of RNAi called short hairpin RNA, or shRNA. Cequent was the first company to demonstrate oral delivery of RNAi in non-human primates. tkRNA-based therapeutics will be administered orally to patients with both Familial Adenomatous
III-10
Polyposis (FAP) and Inflammatory Bowel Disease (IBD). Cequent believes that RNAi therapeutics have the potential to become a major class of drugs with applications in a wide range of therapeutic areas.
Cequent was incorporated on November 10, 2003 and commenced operations in November 2006. Since inception, Cequent has generated significant losses. As of March 31, 2010, Cequent had an accumulated deficit of approximately $17.8 million. Cequent has funded its operations through March 31, 2010 primarily through proceeds of approximately $17.9 million from the sale of equity securities to investors. Cequent is a development stage company and has focused its efforts since inception primarily on business planning, research and development, acquiring intellectual property rights, recruiting management and technical staff, and raising capital. The FDA recently approved Cequent’s first IND (investigational new drug) application for the first ever trial of an orally administered RNA interference drug in humans. The clinical trial is expected to begin in the second quarter of 2010; however, Cequent is unable to predict when, if ever, it will be able to commence sales of any product. Cequent has not achieved profitability on a quarterly or annual basis and it expects to incur significant additional losses over the next several years. Cequent expects its net losses to increase primarily due to research, development and preclinical activities relating to its collaborations, drug development programs and other general corporate activities. Cequent anticipates that its operating results will fluctuate for the foreseeable future. Therefore, period-to-period comparisons should not be relied upon as predictive of the results in future periods. Cequent’s sources of potential funding for the next several years are expected to include proceeds from the sale of equity, license and other fees, funded research and development payments, and milestone payments under future collaborative arrangements.
Cequent’s drug candidates may encounter problems during clinical trials that could result in the lack of regulatory approval to market its products. Even if Cequent receives regulatory approval, it may not achieve market acceptance for its products, which will prevent it from becoming profitable. In addition, Cequent does not have any sales, marketing or distribution capabilities. Accordingly, Cequent intends to enter into collaborations with other companies that can provide such capabilities.
Critical Accounting Policies and Estimates
While Cequent’s significant accounting policies are more fully described in the notes to its financial statements, Cequent believes the following accounting policies to be the most critical in understanding the judgments and estimates it uses in preparing its financial statements.
Use of Estimates
The process of preparing financial statements in conformity with accounting principles generally accepted in the United States of America requires Cequent’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of assets and liabilities at the date of financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates and changes in estimates may occur.
With respect to Cequent’s estimates involved in the determination of the fair value of its common stock, the fair value of each option grant was estimated as of the date of grant using the Black-Scholes option-pricing model. The fair value is amortized as compensation cost on a straight-line basis over the requisite service period of the awards, which is generally the vesting period. Due to its limited operating history and limited number of sales of its common stock, Cequent estimated its volatility in consideration of a number of factors including the volatility of comparable public companies.
Research and Development Expenses
Costs incurred for research and development are expensed as incurred. Cequent’s R&D expenses consist of costs incurred for internal and external research and development. These costs include direct and research-related overhead expenses. The ability to estimate total development costs and effort can vary significantly for each product candidate due to the inherent complexities and uncertainties in drug development.
III-11
General and Administrative Expenses
General and administrative expenses consist primarily of compensation, including stock-based compensation, for employees in executive and operational functions, including business development. Other significant costs include facilities costs, employee-related travel expenses, and professional fees for administrative financial and legal services.
Revenue Recognition
Through March 31, 2010, Cequent’s revenue primarily relates to an exclusive option agreement with Novartis BioVentures Ltd. Revenue is recognized when persuasive evidence of an arrangement exists, the price is fixed or determinable, delivery has occurred and collectability is reasonably assured. Non-refundable, upfront payments received in connection with license option agreements are recognized over the option term on a straight-line basis.
Impairment of Long-lived Assets
Cequent continually monitors events and changes in circumstances that could indicate carrying amounts of long-lived assets may not be recoverable. An impairment loss is recognized when expected cash flows are less than an asset’s carrying value. Accordingly, when indicators of impairment are present, Cequent evaluates the carrying value of such assets in relation to the operating performance and future undiscounted cash flows of the underlying assets. Cequent’s policy is to record an impairment loss when it is determined that the carrying amount of the asset may not be recoverable. No impairment charges were recorded in the years ended December 31, 2009 and 2008 or in the quarters ended March 31, 2010 or March 31, 2009.
Property and equipment are carried at cost. Depreciation and amortization expense is provided over the estimated useful lives of the assets using the straight-line method. Cequent generally depreciates property and equipment using the straight-line method over the assets estimated useful life, which ranges from three to five years. Determining the useful lives of property and equipment requires Cequent to make significant judgments that can impact its operating results.
Licensing Agreement
On December 30, 2004, Cequent entered into an exclusive licensing agreement with BIDMC for patented technology in the field of Bacterial-Mediated Gene Silencing and related methods of use. This agreement was amended on October 20, 2006. The amended agreement obligates Cequent to issue shares of Cequent’s common stock to BIDMC upon a Qualified Financing until BIDMC holds five percent of the issued and outstanding equity securities of Cequent as of the date of the Qualified Financing. Cequent’s obligation to issue additional shares of common stock ceased when the total equity financing reached $7,500,000. As of March 31, 2010, Cequent had Qualified Financing equal to $18,176,000, as a result of the Series A and Series A-1 Convertible preferred Stock issuances. In the years ended December 31, 2007 and 2006, Cequent issued 78,947 and 437,059 shares of common stock to BIDMC with a fair value of $9,474 and $52,447, respectively, and recorded research and development expense in the same amounts upon issuance of the common stock. There was no additional stock issued to BIDMC in 2009 or 2008 or in the quarter ended March 31, 2010.
The licensing agreement contains a requirement that Cequent must file an IND application with the FDA by December 31, 2009 or Cequent will forfeit the exclusive use of the licensed technology. In compliance with the licensing agreement, Cequent filed its first IND application in November 2009.
Accretion of Convertible Preferred Stock
The accretion of convertible preferred stock relates primarily to dividends which accrue at the compounding rate of eight percent (8%) of the original issue price per annum on each outstanding share of preferred stock. Such
III-12
dividends shall begin accruing on the first anniversary of the original preferred stock issuance date, and shall be on a cumulative basis, compounding annually. Dividends are payable when declared by the Board of Directors, upon a liquidation event and upon conversion.
To date, the Board of Directors has not declared any dividends. At March 31, 2010, Cequent had cumulative accreted dividends on the Series A-1 Convertible Preferred Stock of $1,793,805 that represent dividends accrued on Series A Convertible Preferred Stock of $1,183,805, and $610,000 for the years ended December 31, 2009 and 2008, respectively. There was no accretion of dividends for the quarter ended March 31, 2010.
Results of Operations
Comparison of Results of Operations (in thousands)
| | | | | | | | | | | | |
| | | Quarter Ended
March 31, | | | Change | |
| | | |
| | | 2010 | | | 2009 | | |
Revenue | | $ | 144 | | | $ | 141 | | | $ | 3 | |
Operating Expenses: | | | | | | | | | | | | |
Research and development | | | 291 | | | | 974 | | | | (683 | ) |
General and administrative | | | 764 | | | | 558 | | | | 206 | |
| | | | | | | | | | | | |
Total operating expenses | | | 1,055 | | | | 1,532 | | | | (477 | ) |
| | | | | | | | | | | | |
Operating loss | | | (911 | ) | | | (1,391 | ) | | | 480 | |
Other income (expense): | | | | | | | | | | | | |
Interest income | | | — | | | | 8 | | | | (8 | ) |
Interest expense | | | — | | | | (1 | ) | | | 1 | |
| | | | | | | | | | | | |
Other income (expense), net | | | — | | | | 7 | | | | (7 | ) |
| | | | | | | | | | | | |
Net loss | | $ | (911 | ) | | $ | (1,384 | ) | | $ | 473 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | Years Ended
December 31, | | | Change | |
| | | |
| | | 2009 | | | 2008 | | |
Revenue | | $ | 581 | | | $ | 391 | | | $ | 190 | |
Operating Expenses: | | | | | | | | | | | | |
Research and development | | | 3,498 | | | | 4,294 | | | | (796 | ) |
General and administrative | | | 2,314 | | | | 2,118 | | | | 196 | |
| | | | | | | | | | | | |
Total operating expenses | | | 5,812 | | | | 6,412 | | | | (600 | ) |
| | | | | | | | | | | | |
Operating loss | | | (5,231 | ) | | | (6,021 | ) | | | 790 | |
| | | |
Other income (expense): | | | | | | | | | | | | |
Other income (expense) | | | (2 | ) | | | — | | | | (2 | ) |
Interest income | | | 12 | | | | 149 | | | | (137 | ) |
Interest expense | | | (34 | ) | | | (4 | ) | | | (30 | ) |
| | | | | | | | | | | | |
Other income (expense), net | | | (24 | ) | | | 145 | | | | 169 | |
| | | | | | | | | | | | |
Net loss | | $ | (5,256 | ) | | $ | (5,875 | ) | | $ | 619 | |
| | | | | | | | | | | | |
Comparison of Quarter Ended March 31, 2010 to Quarter Ended March 31, 2009 and Year Ended December 31, 2009 to the Year Ended December 31, 2008
Revenue
Cequent does not currently sell any products. To date, its revenue has been derived primarily from the option agreement with Novartis BioVentures Ltd. The option gives Novartis BioVentures Ltd. (or its affiliate) the right to enter into a Definitive Research and License Agreement for selected Cequent Modulators related to the
III-13
Selected Target, as defined. Novartis BioVentures Ltd. paid a non-refundable fee and was obligated to make an additional non-refundable payment within thirty days of Cequent’s completion of a designated milestone. The option expires on the fifth anniversary of the agreement, June 13, 2012. On June 15, 2007, Cequent received the first payment and recorded the receipt as deferred revenue. On August 28, 2008, Cequent received the second payment and recorded the receipt as deferred revenue. Cequent is amortizing the payments to revenue over the life of the option. Over time, Cequent expects to generate revenue from sales of therapeutic products and from milestones and royalties on the development and sales of therapeutic products by third parties.
Research and Development Expenses
Cequent expenses research and development costs as incurred. Research and development expensesdecreased to approximately $0.3 million in the quarter ended March 31, 2010 compared to $1.0 million in the quarter ended March 31, 2010 due to a reduction in Cequent’s research and development work force at the end of 2009. Research and development expenses were approximately $3.5 million in 2009 compared to $4.3 million in 2008. Cequent expects to continue to devote substantial resources to research and development and Cequent expects that research and development expenses will increase in the future.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related expenses, professional fees, and general corporate activities. General and administrative expenses increased to approximately $0.8 million in the quarter ended March 31, 2010 compared to $0.6 million in the quarter ended March 31, 2009 due to approximately $0.1 million in transaction costs recorded in the 2010 period relating to the proposed merger with MDRNA and due to higher personnel costs. General and administrative expenses were approximately $2.3 million in 2009 compared to $2.1 million in 2008. This increase was primarily due to the hiring of an additional key member of the Cequent management team.
Liquidity and Capital Resources
Cash Flows
Cequent was incorporated on November 10, 2003 and commenced operations in November 2006. Since its inception, Cequent has generated significant losses. As of March 31, 2010, Cequent had an accumulated deficit in the development stage of $17.8 million. Cequent has funded its operations through March 31, 2010 primarily through the proceeds of approximately $17.9 million from the sale of equity securities. As of March 31, 2010, Cequent had cash and cash equivalents of approximately $2.1 million, consisting primarily of money market instruments, compared to cash and cash equivalents of $3.2 million as of December 31, 2009.
Net cash used in operating activities decreased to approximately $1.1 million in the quarter ended March 31, 2010 compared to $1.6 million in the quarter ended March 31, 2009. The decrease was due primarily to a lower net loss in the 2010 period. Net cash used in operating activities was approximately $5.5 million in 2009 and $4.7 million in 2008. The use of cash in both periods resulted primarily from funding Cequent’s efforts in business planning, research and development, acquiring intellectual property rights, recruiting management and technical staff and raising capital.
In the quarter ended March 31, 2010, investing activities provided cash of approximately $1,000 compared to using cash of $9,000 in the quarter ended March 31, 2009. Net cash used in investing activities was approximately $50,000 in 2009 and $240,000 in 2008. Cash provided by (used in) investing activities in each period resulted primarily from the purchase or sale of research and development equipment.
Net cash used in financing activities was approximately $3,500 in the quarter ended March 31, 2010 and $3,400 in the quarter ended March 31, 2009 consisting primarily of capital lease payments. Net cash provided by financing activities was approximately $3.2 million in 2009 and $3.4 million in 2008. The net cash provided by financing activities resulted primarily from the sale of convertible preferred stock.
III-14
On July 7, 2008, Cequent entered into a Loan and Security Agreement (“Agreement”) for a $3,500,000 Growth Capital Facility (“Growth Capital Line”) with Silicon Valley Bank and Gold Hill Venture Lending 03, L.P (“Lenders”). The Agreement gave Cequent the right to draw advances through September 30, 2009 in an aggregate amount not to exceed the Growth Capital Line. Each advance must be repaid within thirty-six months commencing on the applicable amortization date through consecutive equal monthly payments of principal and interest. The principal amount of each advance accrues interest at a fixed per annum rate equal to 10%, payable monthly. The Agreement was secured by substantially all of Cequent’s assets with the exception of its intellectual property. For the years ended December 31, 2009 and 2008 there were no advances made under this Agreement and the Agreement expired on September 30, 2009.
Based on its current operating plan, Cequent believes that its existing resources will be sufficient to fund its planned operations through at least the end of 2011. However, Cequent may require significant additional funds earlier than it currently expects in order to develop and commence clinical trials for any additional product candidates it identifies.
Recent Financing Activities
In October and November 2009, Cequent received net proceeds of approximately $3.3 million for the sale of 3,326,263 of Series A-1 Convertible Preferred Stock. In 2008, Cequent received net proceeds of $3.35 million for the sale of 3,350,000 shares of Series A Convertible Preferred Stock.
On April 8, 2010, Cequent received proceeds of approximately $2.5 million for the sale of Series A-1 Convertible Preferred Stock at $1.00 per share to existing investors.
In order to fund Cequent’s obligations to our company under the Loan Agreement (as described on page I-68) and to satisfy the closing condition that Cequent must have the Required Cash Amount (as defined in the merger agreement) available at closing, Cequent is seeking to raise additional proceeds of approximately $4 million through additional sales of Series A-1 Convertible Preferred Stock. On April 30, 2010 Cequent loaned our company $1.0 million under the loan agreement and on May 31, 2010 Cequent loaned us an additional $1.0 million.
The merger agreement permits Cequent to sell additional shares of Cequent capital stock, or debt or warrants convertible into capital stock of Cequent, to new and existing investors of Cequent, including but not limited to the sale of up to $7,520,000 of Series A-1 Convertible Preferred Stock.
Off-Balance Sheet Arrangements
Cequent does not have any off-balance sheet arrangements or relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities.
Recent Accounting Pronouncements
In October 2009, the Financial Accounting Standards Board (“FASB”) issued two related accounting pronouncements, ASU 2009-13 and ASU 2009-14, relating to revenue recognition. One pronouncement provides guidance on allocating the consideration in a multiple-deliverable revenue arrangement and requires additional disclosure, while the other pronouncement provides guidance specific to revenue arrangements that include software elements. Both of these pronouncements are effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010 and both must be adopted together. Early adoption is permitted. If a company elects early adoption and the period of adoption is not the beginning of the company’s fiscal year, the company is required to apply the guidance in these pronouncements retrospectively from the beginning of the company’s fiscal year. A company may also elect to adopt the guidance in these pronouncements retrospectively to prior periods. Cequent does not expect the adoption of these pronouncements to have a material impact on its financial statements.
III-15
In January 2010, the FASB issued ASU 2010-06, Fair Value Measurements and Disclosures (Topic 820), Improving Disclosures about Fair Value Measurements. This Update requires new disclosures and clarifies existing disclosures regarding recurring and nonrecurring fair value measurements to provide increased transparency to users of the financial statements. The new disclosures and clarification of existing disclosures are effective for interim and annual periods beginning after December 15, 2009, except for the disclosures pertaining to the roll forward of activity for Level 3 fair value measurements, which are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. Cequent does not expect the adoption of this Update to have a material impact on its financial statements.
Quantitative and Qualitative Disclosures about Market Risk
Cequent is exposed to market risk related to changes in interest rates. Cequent’s current investment policy is to maintain an investment portfolio consisting mainly of U.S. money market and government-grade securities, directly or through managed funds, with maturities of one year or less. Cequent’s cash is
deposited in and primarily invested through highly rated financial institutions in North America, primarily in money market funds as of March 31, 2010.
III-16
CHAPTER FOUR — BENEFICIAL OWNERSHIP INFORMATION
Ownership of our Common Stock Prior to the Merger
The following table sets forth certain information regarding the ownership of our common stock as of May 31, 2010, by: (i) each director and nominee for director of our company; (ii) each of our executive officers named in the Summary Compensation Table; (iii) all current executive officers and directors of our company as a group; and (iv) all those known by us to be beneficial owners of more than five percent of our common stock.
As of May 31, 2010, we were not aware of any beneficial owners with greater than 5% of our outstanding shares of common stock.
Except as otherwise noted, the persons or entities in this table have sole voting and investing power with respect to all of the shares of common stock beneficially owned by them. Unless otherwise indicated, the business address of each person in the table below is c/o MDRNA, Inc., 3830 Monte Villa Parkway, Bothell, Washington 98021. No shares identified below are subject to a pledge.
| | | | | | | | |
Name | | Age | | Number of
Shares (1) | | | Percent of Shares
Outstanding (%) (1) | |
J. Michael French, Director, President and CEO | | 50 | | 933,501 | (2) | | 1.9 | % |
James M. Karis, Director | | 62 | | — | (3) | | * | |
Daniel Peters, Director | | 58 | | 91,000 | (4) | | * | |
James E. Rothman, Director | | 59 | | 195,371 | (5) | | * | |
Gregory Sessler, Director | | 57 | | 111,750 | (6) | | * | |
Bruce R. Thaw, Chairman of the Board | | 57 | | 354,791 | (7) | | * | |
Peter S. Garcia, CFO and Secretary | | 49 | | 120,000 | (8) | | * | |
Barry Polisky, CSO | | 64 | | 220,001 | (9) | | * | |
Bruce R. York, Former CFO | | 55 | | 544,232 | (10) | | 1.1 | % |
All directors and executive officers as a group (9 persons) | | | | 2,570,646 | (11) | | 5.0 | % |
| * | Beneficial ownership of less than 1.0% is omitted. |
| (1) | Except as otherwise noted below, includes all outstanding shares of common stock, shares of common stock underlying vested options, and all outstanding restricted shares of common stock (both vested and unvested), that are owned beneficially by the individual listed with sole voting and/or investment power. All references to “vested” options shall include all such options that are exercisable as of May 31, 2010, as well as those options that will become exercisable within 60 days of May 31, 2010. |
| (2) | Includes vested options to purchase 920,001 shares of common stock. |
| (3) | None of Mr. Karis’ 34,500 options to purchase common stock are vested as of, or will vest within 60 days of, May 31, 2010. |
| (4) | Includes vested options to purchase 91,000 shares of common stock. |
| (5) | Includes vested options to purchase 195,371 shares of common stock, of which 104,371 were granted to Dr. Rothman in connection with his service on our Scientific Advisory Board. |
| (6) | Includes vested options to purchase 111,750 shares of common stock. |
| (7) | Includes vested options to purchase 260,750 shares of common stock. |
| (8) | Includes vested options to purchase 120,000 shares of common stock. |
| (9) | Includes vested options to purchase 220,001 shares of common stock. |
| (10) | Includes vested options to purchase 509,000 shares of common stock. |
| (11) | Includes vested options to purchase 2,427,873 shares of common stock. |
IV-1
CHAPTER FIVE — ANNUAL MEETING PROPOSALS
PROPOSAL NO. 1 — MERGER PROPOSAL
For summary and detailed information regarding the merger proposal, see “Chapter One — The Merger.”
Vote Required and Board of Directors’ Recommendation
Assuming a quorum is present, the affirmative vote of the holders of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote, is required for approval of proposal No. 1. For purposes of this Proposal No. 1, abstentions will have the same effect as a vote against this proposal, and broker non-votes will have no effect on the result of the vote.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” PROPOSAL NO. 1
V-1
PROPOSAL NO. 2 — POSSIBLE ADJOURNMENT OF THE ANNUAL MEETING
If we fail to receive a sufficient number of votes to approve Proposal No. 1, Proposal No. 3 or Proposal No. 8, we may propose to adjourn the annual meeting, if a quorum is present, for the purpose of soliciting additional proxies to approve Proposal No. 1, Proposal No. 3 or Proposal No. 8. We currently do not intend to propose adjournment of the annual meeting if there are sufficient votes to approve Proposal No. 1, Proposal No. 3 and Proposal No. 8. If approval of the proposal to adjourn the annual meeting for the purpose of soliciting additional proxies is submitted to stockholders for approval, such approval requires the affirmative vote of the holders of a majority of the votes cast in person or by proxy at the annual meeting.
Vote Required and Board of Directors’ Recommendation
Assuming a quorum is present, the affirmative vote of the holders of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote, is required for approval of Proposal No. 2. For purposes of this Proposal No. 2, abstentions will have the same effect as a vote against this proposal, and broker non-votes will have no effect on the result of the vote.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” PROPOSAL NO. 2
V-2
PROPOSAL NO. 3
APPROVAL OF THE AMENDMENT TO OUR CERTIFICATE OF INCORPORATION TO
CHANGE THE NAME OF OUR COMPANY TO MARINA BIOTECH, INC.
In connection with the merger, the Board of Directors has adopted resolutions approving, declaring advisable and recommending that our stockholders approve an amendment to our current Certificate of Incorporation, as amended and restated to date (the “Current Certificate”), to change our corporate name from “MDRNA, Inc.” to “Marina Biotech, Inc.” If approved by our stockholders, Proposal No. 3 will become effective upon the filing of a certificate of amendment of the Current Certificate with the Secretary of State of the State of Delaware. We plan to file the certificate of amendment as soon as reasonably practicable after receiving approval of the amendment from our stockholders. However, if Proposal No. 1 is not approved by our stockholders, we will not change the name of our company.
If this Proposal No. 3 is approved, Article First of the Current Certificate will be amended to read in its entirety as follows:
“The name of the Corporation is Marina Biotech, Inc.”
Purpose and Rationale for the Proposed Amendment
The Board is recommending the approval of the company name change to better reflect the independent identity of the combined company following the completion of the merger with Cequent. The Board believes that changing our name to reflect the merger will promote the awareness of the combined company in the minds of strategic partners, stockholders and the investment community.
Effect of the Proposed Amendment
If approved by stockholders, the change in corporate name will not affect the validity or transferability of any existing stock certificates that bear the name “MDRNA, Inc.” If the proposed name change is approved, stockholders with certificated shares should continue to hold their existing stock certificates, and will not be required to submit their stock certificates for exchange. The rights of stockholders holding certificated shares under existing stock certificates and the number of shares represented by those certificates will remain unchanged. Direct registration accounts and any new stock certificates that are issued after the name change becomes effective will bear the name “Marina Biotech, Inc.”
Currently our common stock is quoted on The NASDAQ Global Market under the symbol “MRNA.” If the proposed name change is approved, the stock will continue to trade under the symbol “MRNA.” A new CUSIP number will be assigned to the common stock following the name change.
If the proposal to change our corporate name is not approved, or if Proposal No. 1 is not approved, the amendment to the Current Certificate described in this Proposal No. 3 will not be made and our corporate name and ticker symbol will remain unchanged. However, if Proposal No. 8 is approved, we will file an amendment to the Current Certificate to reflect the increased number of shares of authorized capital stock, as further discussed in Proposal No. 8. If Proposal Nos. 1, 3 and 8 are all approved, then we shall file an amendment to the Current Certificate to reflect both the change in our company name as described in this Proposal No. 3 and the increase in authorized capital stock described in Proposal No. 8.
Vote Required and Board of Directors’ Recommendation
Assuming a quorum is present, the affirmative vote of the holders of a majority of the total issued and outstanding shares of common stock entitled to vote as of the record date, either in person or by proxy, is required for approval of this Proposal No. 3. Abstentions and broker non-votes will be counted as present for purposes of determining if a quorum is present, but will have the same effect as a negative vote on the outcome of this proposal.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” PROPOSAL NO. 3.
V-3
PROPOSAL NO. 4
ELECTION OF DIRECTORS
General
Our Amended and Restated Bylaws (the “Bylaws”) provide that the Board of Directors shall consist of not less than five (5) members and not more than eleven (11) members, as fixed by the Board of Directors. The number of our Board of Directors is currently fixed at six (6).
At the annual meeting, six (6) directors are to be elected by the holders of the common stock to serve until the 2011 annual meeting of our stockholders and until such directors’ respective successors are elected or appointed and qualify or until any such director’s earlier resignation or removal. The Board of Directors, acting upon the recommendation of its Nominating and Corporate Governance Committee, has nominated J. Michael French, James M. Karis, Daniel Peters, James Rothman, Ph.D., Gregory Sessler and Bruce R. Thaw for election to the Board of Directors at the annual meeting. In the event any nominee is unable or unwilling to serve as a director at the time of the annual meeting, the proxies may be voted for the balance of those nominees named and for any substitute nominee designated by the current Board of Directors or the proxy holders to fill such vacancy or for the balance of those nominees named without the nomination of a substitute, or the size of the Board of Directors may be reduced in accordance with our Bylaws.
Nominees
The following information is submitted concerning the nominees for election as directors based upon information received by us from such persons:
J. Michael French. Mr. French has served as our Chief Executive Officer (“CEO”) since June 23, 2008, as our President since October 1, 2008, and as a member of our Board of Directors since September 11, 2008. Prior to joining us, Mr. French served as President of Rosetta Genomics, Inc. from May 2007 to August 2007. Mr. French also served as Senior Vice President of Corporate Development for Sirna Therapeutics, Inc. (“Sirna”) from July 2005 to January 2007, when Sirna was acquired by Merck and Co., Inc. (“Merck”), and he served in various executive positions, including Chief Business Officer, Senior Vice President of Business Development and Vice President of Strategic Alliances, of Entelos, Inc., a pre-IPO biotechnology company, from 2000 to 2005. Mr. French, age 50, holds a B.S. in aerospace engineering from the U.S. Military Academy at West Point and a M.S. in physiology and biophysics from Georgetown University.
James M. Karis. Mr. Karis has served on our Board of Directors since August 2009, and he currently serves on the Audit Committee, the Compensation Committee, and the Nominating and Corporate Governance Committee. Mr. Karis has spent 30 years in the pharmaceutical, healthcare services and medical device industries and brings extensive corporate strategy, operations, M&A and financing experience to our company. Previously, Mr. Karis served as President, Chief Executive Officer and a Director of Entelos from January 2000 until May 2009. Prior to Entelos, he held senior positions in the contract research industry, serving as Chief Operating Officer and President of PAREXEL International Corporation, and earlier, as Chief Operating Officer of Pharmaco International. He was the Vice President of International Operations for Baxter International and a founder of KMR Group, a leading pharmaceutical R&D benchmarking consulting firm. Mr. Karis serves on the Board of BayBio, an advocacy group for Northern California’s life science community. Mr. Karis, age 62, has a B.S. in management and economics from Purdue University and a Masters in applied economics from The American University.
Daniel Peters. Mr. Peters has served on our Board of Directors since June 2008 and currently serves on the Audit and Compensation Committees and serves as Chair of the Nominating and Corporate Governance Committee. Mr. Peters has served as the President, Chief Executive Officer and a member of the board of directors of Molecular Insight Pharmaceuticals, Inc. since June 2009. Prior to his current role, Mr. Peters was President and CEO of Medical Diagnostics at GE Healthcare and a corporate officer at GE. Prior to his role at GE, Mr. Peters served as Chief Operating Officer at Amersham Health. Previously, Mr. Peters served as the President of Nycomed Amersham Imaging Inc., where he was responsible for managing the company’s diagnostic pharmaceutical
V-4
operations in North, South and Central America. Mr. Peters had been President of Nycomed Imaging Inc. in the Americas from 1994 to 1997 and President of the U.S. pharmaceuticals business of Sterling Winthrop from 1993 to 1994. Mr. Peters is currently on the board of Phadia AB in Uppsala Sweden, serving as Chairman. Previously, Mr. Peters served as a Trustee and founding member of the Health Care Institute of New Jersey from 1996 to 2006, a board member of the Pharmaceutical Research and Manufacturers of America from 1995 to 2005, and a board member of the National Pharmaceutical Council from 1990 to 1993. Mr. Peters also served on the board of Diatide Inc. from 1994 to 1997. Mr. Peters, age 58, holds a bachelors degree from Western Illinois University.
James E. Rothman, Ph.D. Dr. Rothman has served on our Board of Directors since June 2008, and as the Scientific Advisor to the Board since May 2009. He also served as a member of our Scientific Advisory Board from April 2008 until December 2008. Dr. Rothman is one of the world’s most distinguished biochemists and cell biologists and is currently the Wallace Professor and Chairman of the Department of Cell Biology at The Yale University School of Medicine. From 2004 until 2007, Dr. Rothman served as Chief Science Advisor of GE Healthcare. He is renowned for discovering the molecular machinery responsible for the transfer of materials among compartments within cells. Prior to joining Yale University in 2008, Dr. Rothman held Professorships at Stanford University from 1978 to 1988; Princeton University from 1988 to 1991; Memorial Sloan-Kettering Cancer Center from 1991 to 2004; and Columbia University from 2004 to 2008. Dr. Rothman’s pioneering research in cell biology has been recognized by election to the U.S. National Academy of Sciences in 1993. He has also received numerous international awards, including the Lasker Award in 2002. Dr. Rothman, age 59, is currently a senior advisor to GE and Eli Lilly.
Gregory Sessler. Mr. Sessler has served on our Board of Directors since June 2008 and currently serves as Chair of the Audit Committee and as a member of the Compensation Committee and of the Nominating and Corporate Governance Committee of the Board of Directors. Mr. Sessler has served as the Chief Operating Officer since December 2008, and as the Executive Vice President and Chief Financial Officer (“CFO”) since 2002, of Spiration, Inc., and is also currently a director and chairman of the audit committee of VLST, Corp. Prior to joining Spiration, Mr. Sessler served as Senior Vice President and CFO of Rosetta Inpharmatics, a leader in informational genomics, from March 2000 until its acquisition by Merck & Co., Inc. in July 2001 for $540 million. Mr. Sessler is a member of the AICPA and FEI, and he previously served on the board of directors of Corixa Corporation. He also serves on the Executive Committee and is a past chairman of the board of directors of the Washington Biotechnology and Biomedical Association. Mr. Sessler, age 57, holds a bachelors degree, magna cum laude, from Syracuse University and an M.B.A. from the Stanford Graduate School of Business.
Bruce R. Thaw. Mr. Thaw has been a member of our Board of Directors since June 1991 and currently serves as Chairman of the Board, Chairman of the Compensation Committee, and as a member of the Audit Committee and the Nominating and Corporate Governance Committee. Since January 2000, Mr. Thaw has served as the President and CEO of Bulbtronics, Inc., a national distributor of technical and specialty light sources and related products to the medical, scientific, entertainment and industrial markets. Mr. Thaw is a practicing attorney and was admitted to the bar of the State of New York in 1978 and the California State Bar in 1983. From 1984 to 2001, Mr. Thaw served as our general counsel. From 1990 until April 2007, Mr. Thaw served as a member of the board of directors of SafeNet, Inc., a company that designs, manufactures and markets information security systems, products and services that protect and secure digital identities, communications, intellectual property and applications over wide area networks and virtual private networks. Mr. Thaw, age 57, holds a B.B.A. degree in Banking and Finance from Hofstra University and a J.D. degree from the Hofstra University School of Law.
Vote Required and Board of Directors’ Recommendation
Assuming a quorum is present, the affirmative vote of a plurality of the votes cast at the annual meeting, either in person or by proxy, is required for the election of a director. For purposes of the election of directors, abstentions and broker non-votes will have no effect on the result of the vote.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” ALL OF THE NOMINEES NAMED IN PROPOSAL NO. 4.
V-5
Biographical Information Concerning our Executive Officers
Biographical information concerning our CEO is set forth above under the caption “Proposal No. 4 — Election of Directors.” Biographical information concerning our CFO and CSO is set forth below.
Peter S. Garcia. Mr. Garcia has served as our Chief Financial Officer since July 2009, and as our Secretary since August 2009. Mr. Garcia served as Chief Financial Officer of both public and private life science and high technology companies for the 13 years prior to joining our company. From 2004 to 2008, Mr. Garcia served as Chief Financial Officer of Nanosys Inc., a privately held nanotechnology company based in Palo Alto, California, where he was responsible for finance, facilities, information technology, and investor and government relations. From 2001 to 2004, Mr. Garcia served as Senior Vice President and Chief Financial Officer of Nuvelo Inc., a publicly held biopharmaceutical company. During his tenure at Nuvelo, Mr. Garcia helped Nuvelo raise over $150 million, helped Nuvelo acquire development stage products, and led Nuvelo’s merger and acquisition strategy. Mr. Garcia has also served as Chief Financial Officer at Novacept, IntraBiotics, and Dendreon; and held senior financial roles at Amgen. Mr. Garcia, age 49, has an M.B.A. from the Anderson School at the University of California Los Angeles and a B.A. in Economics and Sociology from Stanford University.
Barry Polisky, Ph.D. Dr. Polisky has served as our Chief Scientific Officer since January 2, 2009. Previously, he served as a consultant to Merck from February 2008 to August 2008, and served as Research Vice President of Merck from January 2007 to January 2008. Dr. Polisky also served as Chief Scientific Officer and Senior Vice President of Sirna from March 2005 to January 2007, when Sirna was acquired by Merck, and served Sirna as Senior Vice President of Research from December 2003 to February 2005 and as Vice President of Research from June 2002 to December 2003. Prior to joining Sirna, Dr. Polisky served as Vice-President of Research at ThermoBiostar, Inc. from 1999 to 2002, where he developed a non-instrumented SNP diagnostic platform. Dr. Polisky, age 64, received his Ph.D. in molecular biology from the University of Colorado and conducted post-doctoral work in the Department of Biochemistry and Biophysics, University of California, San Francisco.
Director’s Qualifications
In selecting a particular candidate to serve on our Board of Directors we consider the needs of our company based on particular attributes that we believe would be advantageous for our Board members to have and would qualify such candidate to serve on our Board given our business profile and the environment in which we operate. The table below sets forth such attributes and identifies which attributes each director possesses.
| | | | | | | | | | | | |
Attributes | | Mr. French | | Mr. Karis | | Mr. Peters | | Dr. Rothman | | Mr. Sessler | | Mr. Thaw |
Financial Experience | | | | X | | | | | | X | | X |
Public Board Experience | | | | X | | X | | | | X | | X |
Industry Experience | | X | | X | | X | | X | | X | | |
Scientific Experience | | | | | | | | X | | | | |
Commercial Experience | | X | | X | | X | | | | X | | X |
Corporate Governance Experience | | | | X | | | | | | X | | X |
Capital Markets Experience | | | | X | | X | | | | X | | |
Legal Experience | | | | | | | | | | | | X |
Management Experience | | X | | X | | X | | X | | X | | X |
Certain Relationships and Related Transactions
Contractual Arrangements. Pursuant to the terms and conditions of Mr. French’s employment agreement, we agreed, for the term of Mr. French’s employment with us, to nominate Mr. French for successive terms as a member of the Board of Directors. We are obligated to use all best efforts to cause Mr. French to be elected to the Board of Directors at the annual meeting.
V-6
Family Relationships
There are no familial relationships between any of our officers and directors.
Director or Officer Involvement in Certain Legal Proceedings
Our directors and executive officers were not involved in any legal proceedings as described in Item 401(f) of Regulation S-K in the past ten years.
Independence of the Board of Directors
The Board of Directors has adopted NASDAQ’s standards for determining the independence of its members and believes that it interprets these requirements conservatively. In applying these standards, the Board of Directors considers commercial, industrial, banking, consulting, legal, accounting, charitable and familial relationships, among others, in assessing the independence of directors, and must disclose any basis for determining that a relationship is not material. The Board of Directors has determined that a majority of the current members of the Board of Directors, namely James M. Karis, Daniel Peters, Gregory Sessler and Bruce R. Thaw, are independent directors within the meaning of such NASDAQ independence standards in terms of independence from management, such members constituting four (4) of the six (6) current members of, and nominees for, the Board of Directors. In making these independence determinations, the Board of Directors did not exclude from consideration as immaterial any relationship potentially compromising the independence of any of the above directors.
Meetings of the Board of Directors
The Board of Directors held 22 meetings during 2009. During 2009, all incumbent directors attended more than 75% of the aggregate number of meetings of the Board of Directors that were held during the period when such persons served on our Board of Directors. We do not have a formal policy regarding attendance by members of the Board of Directors at the annual meeting of stockholders, but we strongly encourage all members of the Board of Directors to attend our annual meeting and expect such attendance except in the event of extraordinary circumstances. All incumbent members of the Board of Directors attended our annual meeting of stockholders on May 20, 2009.
Executive Sessions of the Board of Directors consisting only of independent directors will be held at least twice per year, and periodically as determined by the independent directors. Such Executive Sessions will typically occur immediately following regularly scheduled meetings of the Board of Directors or at any other time and place as the independent directors may determine. The Board of Directors had previously designated Bruce R. Thaw, the Chairman of our Board of Directors, as generally responsible for organizing, managing and presiding over the Executive Sessions of the Board of Directors and performing such other oversight functions from time to time as the independent directors deem necessary or appropriate, and reporting on outcomes of the Executive Sessions and such other activities to the Board of Directors and CEO as appropriate. Interested parties may submit matters for consideration to the independent directors by utilizing the procedures identified under “Stockholder Communications” in this proxy statement. During 2009, the independent directors met in Executive Session two times.
Committees of the Board of Directors
The Board of Directors has three standing committees: the Audit Committee, the Compensation Committee and the Nominating and Corporate Governance Committee. The Board of Directors has adopted written charters for each of these committees, which we make available free of charge on or through our Internet website, as well as items related to corporate governance matters, including our Code of Business Conduct and Ethics applicable to all employees, officers and directors. We maintain our Internet website at www.mdrnainc.com. You can access our committee charters and code of conduct on our website by first clicking “About MDRNA” and then “Corporate Governance.” We intend to disclose on our Internet website any amendments to or waivers from our Code of Business Conduct and Ethics, as well as any amendments to the charters of any of the Audit,
V-7
Compensation or Nominating and Corporate Governance Committees of the Board of Directors. Any stockholder also may obtain copies of these documents, free of charge, by sending a request in writing to: MDRNA, Inc., Investor Relations Department, 3830 Monte Villa Parkway, Bothell, Washington 98021. The current members of these three committees are identified in the following table:
| | | | | | | | |
Director | | Chairman | | Audit
Committee | | Compensation
Committee | | Nominating
and Corporate
Governance
Committee |
J. Michael French | | | | | | | | |
James M. Karis | | | | X | | X | | X |
Daniel Peters | | | | X | | X | | Chair |
James E. Rothman | | | | | | | | |
Gregory Sessler | | | | Chair | | X | | X |
Bruce R. Thaw | | X | | X | | Chair | | X |
Audit Committee. The Audit Committee, which currently consists of Gregory Sessler, Chairman, James M. Karis, Daniel Peters and Bruce R. Thaw, held seven meetings during 2009. All incumbent directors who served as members of the Audit Committee attended at least 75% of the meetings during the periods served as committee members in 2009. Among other functions, the Audit Committee authorizes and approves the engagement of the independent registered public accounting firm, reviews the results and scope of the audit and other services provided by the independent registered public accounting firm, reviews our financial statements, reviews and evaluates our internal control functions, approves or establishes pre-approval policies and procedures for all professional audit and permissible non-audit services provided by the independent registered public accounting firm and reviews and approves any proposed related party transactions.
The Board of Directors has determined that each of Gregory Sessler, James M. Karis, Daniel Peters and Bruce R. Thaw is an independent director within the meaning of the NASDAQ independence standards and Rule 10A-3 promulgated by the SEC under the Securities Exchange Act of 1934, as amended, or the Exchange Act. In addition, the Board of Directors has determined that each member of the Audit Committee qualifies as an Audit Committee Financial Expert under applicable SEC Rules and satisfies the NASDAQ standards of financial literacy and financial or accounting expertise or experience.
Compensation Committee. The Compensation Committee, which currently consists of Bruce R. Thaw, Chairman, James M. Karis, Daniel Peters and Gregory Sessler, held six meetings during 2009. All incumbent directors who served as members of the Compensation Committee attended at least 75% of the meetings during the periods served as committee members in 2009. The Board of Directors has determined that each of the members of the Compensation Committee is an independent director within the meaning of the NASDAQ independence standards.
The Compensation Committee’s functions include reviewing and approving the compensation and benefits for our executive officers, administering our equity compensation plans and making recommendations to the Board of Directors regarding these matters. The CEO does not participate in the determination of his own compensation or the compensation of directors. However, he makes recommendations to the committee regarding the amount and form of the compensation of the other executive officers and key employees, and he often participates in the committee’s deliberations about their compensation. No other executive officers participate in the determination of the amount or form of the compensation of executive officers or directors. In prior years, the Compensation Committee retained Mercer Human Resource Consulting, a human resource and compensation consulting firm, as its independent compensation consultant, to assist it in reviewing and approving the compensation and benefits for certain of our executive officers. The consultant served at the request of the Compensation Committee, and its fees were approved by the Compensation Committee. The consultant provided the Compensation Committee with information regarding the compensation paid by our competitors and other employers who compete with us for executives.
V-8
Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee, which currently consists of Daniel Peters, Chairman, James M. Karis, Gregory Sessler and Bruce R. Thaw, held five meetings during 2009. All incumbent directors who served as members of the Nominating and Corporate Governance Committee attended at least 75% of the meetings during the periods served as committee members in 2009. The Nominating and Corporate Governance Committee searches for and recommends to the Board of Directors potential nominees for director positions and makes recommendations to the Board of Directors regarding the size, composition and compensation of the Board of Directors and its committees. The Board of Directors has determined that each of the members of the Nominating and Corporate Governance Committee are independent directors within the meaning of the NASDAQ independence standards.
In selecting candidates for the Board of Directors, the Nominating and Corporate Governance Committee begins by determining whether the incumbent directors whose terms expire at the annual meeting of stockholders desire and are qualified to continue their service on the Board of Directors. We are of the view that the continuing service of qualified incumbents promotes stability and continuity in the board room, giving us the benefit of the familiarity and insight into our affairs that our directors have accumulated during their tenure, while contributing to the Board of Directors’ ability to work as a collective body. Accordingly, it is the policy of the Nominating and Corporate Governance Committee, absent special circumstances, to nominate qualified incumbent directors who continue to satisfy the Nominating and Corporate Governance Committee’s criteria for membership on the Board of Directors, whom the Nominating and Corporate Governance Committee believes will continue to make important contributions to the Board of Directors and who consent to stand for re-election and, if re-elected, will continue their service on the Board of Directors. If there are positions on the Board of Directors for which the Nominating and Corporate Governance Committee will not be re-nominating an incumbent director, or if there is a vacancy on the Board of Directors, the Nominating and Corporate Governance Committee will solicit recommendations for nominees from persons whom the Nominating and Corporate Governance Committee believes are likely to be familiar with qualified candidates, including members of our Board of Directors and our senior management. The Nominating and Corporate Governance Committee may also engage a search firm to assist in the identification of qualified candidates. The Nominating and Corporate Governance Committee will review and evaluate each candidate whom it believes merits serious consideration, taking into account all available information concerning the candidate, the existing composition and mix of talent and expertise on the Board of Directors and other factors that it deems relevant. In conducting its review and evaluation, the committee may solicit the views of management and other members of the Board of Directors and may, if deemed helpful, conduct interviews of proposed candidates.
The Nominating and Corporate Governance Committee generally requires that all candidates for the Board of Directors be of the highest personal and professional integrity and have demonstrated exceptional ability and judgment. The Nominating and Corporate Governance Committee will consider whether such candidate will be effective, in conjunction with the other members of the Board of Directors, in collectively serving the long-term interests of our stockholders. In addition, the Nominating and Corporate Governance Committee requires that all candidates have no interests that materially conflict with our interests and those of our stockholders, have meaningful management, advisory or policy making experience, have a general appreciation of the major business issues facing us and have adequate time to devote to service on the Board of Directors. We also require that a majority of our directors be independent, at least three directors have the financial literacy necessary for service on the Audit Committee under applicable NASDAQ rules and at least one director qualifies as an Audit Committee Financial Expert in accordance with applicable SEC rules.
The Nominating and Corporate Governance Committee will consider stockholder recommendations for nominees to fill director positions, provided that the Nominating and Corporate Governance Committee will not entertain stockholder nominations from stockholders who do not meet the eligibility criteria for submission of stockholder proposals under SEC Rule 14a-8 of Regulation 14A under the Exchange Act. Stockholders may submit written recommendations for committee appointments or recommendations for nominees to the Board of Directors, together with appropriate biographical information and qualifications of such nominees as required by our Bylaws, to our Corporate Secretary following the same procedures as described in “Stockholder Communications”
V-9
beginning on page V-10 of this proxy statement. In order for the Nominating and Corporate Governance Committee to consider a nominee for directorship submitted by a stockholder, such recommendation must be received by the Corporate Secretary by the time period set forth in our most recent proxy statement for the submission of stockholder proposals under SEC Rule 14a-8 of Regulation 14A under the Exchange Act. The Corporate Secretary shall then deliver any such communications to the Chairman of the Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee will evaluate stockholder recommendations for candidates for the Board of Directors using the same criteria as for other candidates, except that the Nominating and Corporate Governance Committee may consider, as one of the factors in its evaluation of stockholder recommended candidates, the size and duration of the interest of the recommending stockholder or stockholder group in our equity.
Board Leadership Structure and Role in Risk Oversight
Although we have not adopted a formal policy on whether the Chairman of the Board (“Chairman”) and Chief Executive Officer (“CEO”) positions should be separate or combined, we have determined that it is in the best interests of our company and its stockholders to separate those roles. Mr. Thaw, our Chairman, is an independent director who has served in that role since October 2008, and as a member of our Board of Directors since June 1991. Prior to becoming Chairman, Mr. Thaw served as our Lead Independent Director from December 2007 to October 2008. During the time that Mr. Thaw has served as our Chairman, Mr. French has served as CEO. We believe it is the CEO’s responsibility to run the day-to-day operations of our company, and the Chairman’s responsibility to manage the Board of Directors. As directors continue to have more oversight responsibilities than ever before, we believe it is beneficial to have an experienced, independent Chairman whose sole job is leading the Board. Also, given the numerous responsibilities of the CEO of a biotechnology company, such as ours, we believe it is beneficial to have a CEO whose sole job is to manage the company. By having Mr. Thaw serve as Chairman, and Mr. French serve as CEO, we believe that each will be able to focus his entire energy on managing the Board or running our company, as appropriate. Additionally, we believe the separation of offices is beneficial because a separate Chairman can provide the CEO with guidance and feedback on his performance, and he provides a more effective channel for the Board to express its views on management. The Board of Directors continually evaluates our leadership structure and could in the future decide to combine the Chairman and CEO positions if it believes that doing so would serve the best interests of our company.
Our Audit Committee is primarily responsible for overseeing our risk management processes on behalf of the full Board. The Audit Committee receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate regarding our company’s assessment of risks. In addition, the Audit Committee reports regularly to the full Board of Directors, which also considers our risk profile. The Audit Committee and the full Board of Directors focus on the most significant risks facing our company and our company’s general risk management strategy, and also ensure that risks undertaken by our company are consistent with the Board’s appetite for risk. While the Board oversees our company’s risk management, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing our company and that our Board leadership structure supports this approach.
Stockholder Communications
All stockholder communications must (i) be addressed to our Corporate Secretary at MDRNA, Inc., 3830 Monte Villa Parkway, Bothell, WA 98021, (ii) be in writing either in print or electronic format, (iii) be signed by the stockholder sending the communication, (iv) indicate whether the communication is intended for the entire Board of Directors, the Nominating and Corporate Governance Committee, or the independent directors, (v) if the communication relates to a stockholder proposal or director nominee, identify the number of shares held by the stockholder, the length of time such shares have been held, and the stockholder’s intention to hold or dispose of such shares, provided that the Board of Directors and the Nominating and Corporate Governance Committee will not entertain stockholder proposals or stockholder nominations from stockholders who do not meet the eligibility
V-10
and procedural criteria for submission of stockholder proposals under Commission Rule 14a-8 of Regulation 14A under the Exchange Act and (vi) if the communication relates to a director nominee being recommended by the stockholder, must include appropriate biographical information of the candidate as is required by our Bylaws.
Upon receipt of a stockholder communication that is compliant with the requirements identified above, the Corporate Secretary shall promptly deliver such communication to the appropriate member(s) of the Board of Directors or committee member(s) identified by the stockholder as the intended recipient of such communication by forwarding the communication to either the chairman of the Board of Directors with a copy to the CEO, the chairman of the Nominating and Corporate Governance Committee, or to each of the independent directors, as the case may be.
The Corporate Secretary may, in his or her sole discretion and acting in good faith, provide copies of any such stockholder communication to any one or more of our directors and executive officers, except that in processing any stockholder communication addressed to the independent directors, the Corporate Secretary may not copy any member of management in forwarding such communications. In addition, the Secretary may, in his or her sole discretion and acting in good faith, not forward certain items if they are deemed of a commercial or frivolous nature or otherwise inappropriate for consideration by the intended recipient and any such correspondence may be forwarded elsewhere in our company for review and possible response.
V-11
PROPOSAL NO. 5
RATIFICATION OF APPOINTMENT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
KPMG LLP served as our independent registered public accounting firm for the year ended December 31, 2009, has been our independent registered public accounting firm for each completed fiscal year beginning with the year ended December 31, 1996, and has been appointed by the Audit Committee to continue as our independent registered public accounting firm for the fiscal year ending December 31, 2010. In the event that ratification of this appointment of independent registered public accounting firm is not approved by the affirmative vote of a majority of votes cast on the matter, then the appointment of our independent registered public accounting firm will be reconsidered by the Audit Committee. Representatives of KPMG LLP are expected to be present at the annual meeting to respond to appropriate questions and will be given the opportunity to make a statement if they desire to do so.
Your ratification of the appointment of KPMG LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2010 does not preclude the Audit Committee from terminating its engagement of KPMG LLP and retaining a new independent registered public accounting firm, if it determines that such an action would be in our best interest. Total fees billed to us by KPMG LLP for the years ended December 31, 2009 and 2008 were $310,338 and $329,850, respectively, and were comprised of the following:
Audit Fees. The aggregate fees billed for professional services rendered in connection with (i) the audit of our annual financial statements, (ii) the review of the financial statements included in our Quarterly Reports on Form 10-Q for the quarters ended March 31, June 30 and September 30, (iii) consents and comfort letters issued in connection with equity offerings and (iv) services provided in connection with statutory and regulatory filings or engagements were $285,208 for the year ended December 31, 2009 and $309,655 for the year ended December 31, 2008.
Audit-Related Fees. We incurred $25,130 in audit-related fees in 2009 for the audit of our 401(k) plan for the year ended December 31, 2008. We incurred $20,195 in audit-related fees in 2008 for the audit of our 401(k) plan for the year ended December 31, 2007.
Tax Fees. We did not incur any fees for professional services rendered in connection with tax compliance, tax planning and federal and state tax advice for the years ended December 31, 2009 and December 31, 2008.
All Other Fees. We did not incur any such other fees for the years ended December 31, 2009 and December 31, 2008.
Pre-Approval Policies and Procedures
Pursuant to its charter, the Audit Committee has the sole authority to appoint or replace our independent registered public accounting firm (subject, if applicable, to stockholder ratification). The Audit Committee is directly responsible for the compensation and oversight of the work of the independent registered public accounting firm (including resolution of disagreements between management and the independent registered public accounting firm regarding financial reporting) for the purpose of preparing or issuing an audit report or related work. The independent registered public accounting firm is engaged by, and reports directly to, the Audit Committee.
The Audit Committee pre-approves all auditing services and permitted non-audit services (including the fees and terms thereof) to be performed for us by our independent registered public accounting firm, subject to the de minimis exceptions for non-audit services described in Section 10A(i)(1)(B) of the Exchange Act and SEC Rule 2-01(c)(7)(i)(C) of Regulation S-X, provided that all such excepted services are subsequently approved by the Audit Committee prior to the completion of the audit. In the event pre-approval for such auditing services
V-12
and permitted non-audit services cannot be obtained as a result of inherent time constraints in the matter for which such services are required, the Chairman of the Audit Committee has been granted the authority to pre-approve such services, provided that the estimated cost of such services on each such occasion does not exceed $15,000, and the Chairman of the Audit Committee reports for ratification such pre-approval to the Audit Committee at its next scheduled meeting. The Audit Committee has complied with the procedures set forth above, and has otherwise complied with the provisions of its charter.
Vote Required and Board of Directors’ Recommendation
Assuming a quorum is present, the affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote, is required for approval of this Proposal No. 5. For purposes of Proposal No. 5, abstentions will have the same effect as a vote against this proposal, and broker non-votes will have no effect on the result of the vote.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” PROPOSAL NO. 5.
V-13
PROPOSAL NO. 6
APPROVAL OF AN AMENDMENT TO OUR 2008 STOCK INCENTIVE PLAN TO INCREASE THE NUMBER OF SHARES AVAILABLE THEREUNDER FROM 4,500,000 TO 8,500,000
On April 28, 2010, the Compensation Committee of the Board of Directors unanimously recommended, and the Board of Directors unanimously adopted, subject to stockholder approval at the annual meeting, an amendment to the MDRNA, Inc. 2008 Stock Incentive Plan (the “2008 Plan”) to increase the number of shares of Common Stock authorized for issuance thereunder from 4,500,000 shares to 8,500,000 shares. As part of this process, the Compensation Committee and the Board of Directors reviewed the number of shares available under our 2000 Non-Qualified Stock Option Plan, our 2002 Stock Option Plan, our 2004 Stock Incentive Plan and the 2008 Plan (collectively, the “Plans”), and determined that the 245,278 shares available for grant under the Plans represented an insufficient number of shares to enable us to provide sufficient future grants of stock options or other stock awards. As a result, we believe that the amendment is necessary and in the best interests of our company and its long-term strategic growth to permit us to continue to attract, retain and motivate officers, employees, non-employee directors and consultants.
The purpose of the 2008 Plan is to attract and retain the best available employees and directors for our company and to encourage the highest level of performance by such persons, thereby enhancing the value of our company for the benefit of our stockholders. The 2008 Plan is also intended to motivate such persons to contribute to our future growth and profitability, to reward the performance of these individuals and increase the proprietary and vested interest of all such persons in our growth and performance in a manner that provides them with a means to increase their holdings of common stock and aligns their interests with the interests of our stockholders.
Potentially all of our employees, officers and directors are eligible to participate in the 2008 Plan. Because participation in, and the types of awards that may be made under, the 2008 Plan are subject to the discretion of the Compensation Committee, we cannot determine the dollar value or number of shares of common stock that will in the future be received by or allocated to any participant or groups of participants, including our directors, executive officers and other employees. As of May 31, 2010, there were three executive officers, approximately 44 employees and five non-employee directors of our company who were eligible to participate in the 2008 Plan. During the 2009 fiscal year, we granted options to purchase up to an aggregate of 1,200,000 shares of common stock to our named executive officers as a group, of which 240,000 were granted to Mr. French, 360,000 were granted to Mr. Garcia, 480,000 were granted to Dr. Polisky and 120,000 were granted to Mr. York. During the 2009 fiscal year we also granted options to purchase up to an aggregate of 253,000 shares to our non-employee directors as a group, and options to purchase up to 1,963,000 shares to all of our employees, including all current officers who are not executive officers, as a group. As of June 2, 2010, the closing price of our common stock on the NASDAQ Global Market (“NASDAQ”) was $1.08 per share.
The 2008 Plan provides for the granting of stock options, restricted stock awards, stock appreciation rights, and performance-share awards to our employees and our non-employee directors. The 2008 Plan does not permit the repricing of options or the granting of discounted options, and does not contain an evergreen provision (which would automatically increase the number of shares available under the 2008 Plan). Provisions have been included to meet the requirements for deductibility of executive compensation under Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code, with respect to options and other awards by qualifying payments under the 2008 Plan as performance-based compensation.
The following is a brief description of the 2008 Plan. The full text of the 2008 Plan is attached as Annex A to the Proxy Statement for our 2008 Annual Meeting of Stockholders held on June 10, 2008, and the following description is qualified in its entirety by reference thereto. We urge you to refer to the 2008 Plan document and to read it carefully for a complete statement of the provisions summarized herein.
It is the judgment of the Board of Directors that approval of the amendment to the 2008 Plan to increase the number of shares available for issuance thereunder from 4,500,000 shares to 8,500,000 shares is in the best interests of our company and our stockholders.
V-14
Administration and Duration
The administration of the 2008 Plan is the responsibility of the Compensation Committee. It is anticipated that each member of the Compensation Committee will be a “non-employee Director” within the meaning of Rule 16b-3 under the Securities Exchange Act of 1934, as amended, and an “outside director” within the meaning of Section 162(m) of the Code. Currently, the Compensation Committee is comprised of four independent Directors. Nevertheless, if the Compensation Committee is not so composed it will not invalidate any award. The Board of Directors also may act in place of the Compensation Committee. The Compensation Committee will have the authority to interpret the 2008 Plan, to establish and revise rules and regulations relating to the 2008 Plan, and to make any other determinations that it believes necessary or advisable for the administration of the 2008 Plan.
Limit on Awards under the 2008 Plan
Following approval of this Proposal No. 6, the maximum number of shares of common stock as to which stock options and other stock awards may be granted under the 2008 Plan is 8,500,000 shares. No individual may be granted stock options, stock appreciation rights or other stock-based awards with respect to more than 2,250,000 shares in any calendar year. The shares to be delivered under the 2008 Plan will be made available from authorized but unissued shares of common stock, from treasury shares, or from shares purchased in the open market or otherwise. Shares that are subject to awards under the 2008 Plan but are not actually issued (for example because the award lapsed or was cancelled) and shares of unvested restricted stock that are forfeited, will be available for further awards and options.
Eligibility for Awards
All employees of our company and its non-employee directors will be eligible to participate in the 2008 Plan. From time to time, the Compensation Committee will determine who will be granted awards and the number of shares subject to such awards. The Compensation Committee may delegate to one or more officers the authority to designate the employees eligible to receive awards (other than the key officers) and the size of each such award. Each individual who receives an award under the 2008 Plan is referred to as a “Recipient.”
Stock Options
Options granted under the 2008 Plan may be either non-qualified stock options or incentive stock options qualifying under Section 422 of the Code. The exercise price of any stock option may not be less than the fair market value of the stock on the date the option is granted. The option price is payable in cash or, with the consent of the Compensation Committee, in common stock.
The Compensation Committee determines the terms of each stock option grant at the time of grant. Unless the option agreement granting an option specifies otherwise, options to employees: (i) will be exercisable as to one-third of the shares on the first anniversary of the option grant; (ii) the remaining shares will vest quarterly over the two-year period following the first anniversary of the option grant; and (iii) will remain exercisable until the tenth anniversary of the date of the grant. Options granted to non-employee directors will be fully exercisable on the first anniversary of grant, except that an option granted in conjunction with the annual stockholders meeting will be exercisable at the earlier of the first anniversary of grant and the next annual stockholders meeting (which may be slightly earlier than the first anniversary). No option may be exercised before the first anniversary of date of grant (or the next stockholders meeting in the case of non-employee directors) or after the tenth anniversary of the date of grant.
Stock Appreciation Rights
A stock appreciation right (“SAR”) entitles the Recipient to receive — in cash or shares of stock, at the Compensation Committee’s discretion — the excess of the fair market value of a share of stock on the date of
V-15
exercise over the fair market value on the date of grant. A SAR may, but need not, relate to an option. The Compensation Committee determines the terms of each SAR at the time of the grant. A SAR cannot have a term longer than ten years.
Restricted Stock
The Compensation Committee, in its discretion, may grant awards of restricted stock. A share of restricted stock is a share of our company’s stock that may not be transferred before it is vested and may be subject to such other conditions as the Compensation Committee sets forth in the agreement evidencing the award. In addition, if the Recipient terminates employment, he or she will forfeit any unvested shares. Unless the agreement granting restricted stock specifies otherwise, one third of a restricted stock award will vest on each of the first three anniversaries of the grant date. The grant or vesting of a restricted stock award may be made contingent on achievement of performance goals established by the Compensation Committee. If the Compensation Committee determines that a restricted stock award is intended to constitute “performance-based compensation” for purposes of Code Section 162(m) (see “Code Section 162(m)” below), the grant or vesting of the restricted stock award will be contingent on achievement of objective performance targets based on corporate or divisional earnings-based measures (which may be based on net income, operating income, cash flow, residual income or any combination thereof) and/or one or more corporate, divisional or individual scientific or inventive measures.
Performance Shares
The Compensation Committee, in its discretion, may grant awards of performance shares. A performance share entitles the Recipient to receive shares of our company’s stock or to be paid the value of such shares in cash, in the Compensation Committee’s discretion, if specified performance goals are met. If the Compensation Committee determines that a performance share award is intended to constitute “performance-based compensation” for purposes of Code Section 162(m) (see “Code Section 162(m)” below), the specified performance goals will be based on the criteria listed above under “Restricted Stock.”
Amendment or Termination
Subject to applicable Nasdaq rules, the Board of Directors may amend, alter or terminate the 2008 Plan without stockholder approval. Under the Nasdaq rules, the Board of Directors may not, without stockholder approval, increase the total number of shares reserved for issuance under the 2008 Plan or make any other material changes to the 2008 Plan. In addition, no amendment, alteration or termination by the Board of Directors may adversely affect the rights of a holder of a stock incentive award without the holder’s consent. Unless terminated earlier, the 2008 Plan will terminate on April 4, 2018. Upon termination of the 2008 Plan, outstanding grants and awards made before termination will continue in accordance with their terms. However, no new grants or awards may be made following termination.
Federal Income Tax Consequences
The following discussion outlines generally the current federal income tax consequences of the 2008 Plan. Applicable tax laws and their interpretations are subject to change at any time and application of such laws may vary in individual circumstances.
Incentive Stock Options
A Recipient who is granted an incentive stock option does not recognize taxable income upon the grant or exercise of the option. However, the difference between the fair market value of our common stock on the date of exercise and the option exercise price is a tax preference item that may subject the Recipient to alternative minimum tax. A Recipient generally will receive long-term capital gain or loss treatment on the disposition of shares acquired upon exercise of the option, provided that the disposition occurs more than two years from the
V-16
date the option is granted, and the Recipient holds the stock acquired for more than one year. A Recipient who disposes of shares acquired by exercise prior to the expiration of the forgoing holding periods realizes ordinary income upon the disposition equal to the difference between the option price and the lesser of the fair market value of the shares on the date of exercise and the disposition price. Any appreciation between the fair market value of the shares on the date of exercise and the disposition price is taxed to the Recipient as long or short-term capital gain, depending on the length of the holding period. To the extent the Recipient recognizes ordinary income, we receive a corresponding tax compensation deduction.
Nonqualified Stock Options
A Recipient will not recognize income upon the grant of a nonqualified option. Upon exercise, the Recipient will recognize ordinary income equal to the excess of the fair market value of the stock on the date of exercise over the price paid for the stock. We are entitled to a tax compensation deduction equal to the ordinary income recognized by the Recipient. Any taxable income recognized by a Recipient in connection with an option exercise is subject to income and employment tax withholding. When the Recipient disposes of shares acquired by the exercise of a nonqualified option, any amount received in excess of the fair market value of the shares on the date of exercise will be treated as capital gain. Dispositions made after one year from the exercise date will be treated as long-term capital gain. Dispositions made less than one year from the exercise date will be treated as short-term capital gain.
Stock Appreciation Rights
A Recipient will not recognize income upon the grant of an SAR. Upon exercise, the Recipient will recognize ordinary income equal to the cash or fair market value of the shares of common stock received from the exercise, which will be subject to income and employment tax withholding. We will receive a tax compensation deduction equal to the ordinary income recognized by the Recipient.
Restricted Stock
Generally, a Recipient will not recognize income upon the grant of restricted stock. When the shares of restricted stock vest, the Recipient will recognize ordinary income equal to the fair market value of the stock and also will be subject to income and employment tax withholding. We will receive a tax compensation deduction equal to the amount of ordinary income recognized by the Recipient. A Recipient who receives a restricted stock award may elect to accelerate his or her tax obligation by submitting a Code Section 83(b) election within 30 days after the grant date, pursuant to which the Recipient will be taxed on the fair market value of the restricted stock as of the grant date, and we will receive a tax compensation deduction as of the grant date equal to the ordinary income recognized by the Recipient. Any gain or loss upon a subsequent disposition of the shares will be long-term capital gain or loss if the shares are held for more than one year and otherwise will be short-term capital gain or loss. If, after making the Section 83(b) election, the shares are forfeited, the Recipient will not be entitled to a loss deduction.
Performance Shares
A Recipient will not recognize income upon the grant of performance shares. At the time that the performance goals are achieved and the individual receives the shares or cash, he or she will recognize ordinary income equal to the cash or fair market value of common stock, or combination thereof, received, at which time the Recipient also will be subject to income and employment tax withholding. We will receive a tax compensation deduction equal to the amount of ordinary income recognized by the Recipient.
Code Section 162(m)
Code Section 162(m) denies a federal income tax deduction for certain compensation in excess of $1 million per year paid to the CEO and the four other most highly paid executive officers of a publicly traded corporation. Certain types of compensation, including compensation based on performance criteria that are approved in
V-17
advance by stockholders, are excluded from the computation of the deduction limit. Options and SARs granted under the 2008 Plan are excluded from the computation of the deduction limit and the Compensation Committee can cause other awards under the 2008 Plan to be similarly excluded from the computation of the deduction limit by conditioning the grant or vesting upon specified performance goals.
Vote Required and Board of Directors’ Recommendation
Assuming a quorum is present, the affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote, is required for approval of this Proposal No. 6. For purposes of the approval of Proposal No. 6, abstentions will have the same effect as a vote against this proposal, and broker non-votes will have no effect on the result of the vote.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” PROPOSAL NO. 6.
V-18
PROPOSAL NO. 7
APPROVAL OF AN AMENDMENT TO OUR 2007 EMPLOYEE STOCK PURCHASE PLAN TO INCREASE THE NUMBER OF SHARES AVAILABLE FOR ISSUANCE THEREUNDER FROM 300,000 TO 600,000
We seek to retain the services of current employees, to promote employee morale and to encourage employee ownership of our common stock, and to secure and retain the services of new employees. In order to maximize our success, we look to provide incentives to certain employees through the means of various stock ownership plans, including the MDRNA, Inc. 2007 Employee Stock Purchase Plan (the “Stock Purchase Plan”). The Stock Purchase Plan permits employees to purchase shares at a discount through payroll deductions.
As of April 28, 2010, there were an aggregate of 116,566 shares available for sale under the Stock Purchase Plan. As a result, on April 28, 2010, the Compensation Committee of the Board of Directors unanimously recommended, and the Board of Directors unanimously adopted, subject to stockholder approval at the annual meeting, an amendment to the Stock Purchase Plan to increase the number of shares of common stock available thereunder from 300,000 shares to 600,000 shares.
The following is a brief description of the Stock Purchase Plan. The full text of the Stock Purchase Plan is attached as Annex A to the Proxy Statement for our 2007 Annual Meeting of Stockholders held on June 13, 2007, and the following description is qualified in its entirety by reference thereto. We urge you to refer to the Stock Purchase Plan document and to read it carefully for a complete statement of the provisions summarized herein.
Stock Subject to the Plan
The stock subject to purchase under the Stock Purchase Plan may be unissued shares of common stock or shares of common stock that have been bought on the open market at prevailing market prices or otherwise. Following the approval of this amendment, the number of shares of common stock that may be sold under the Stock Purchase Plan shall not exceed, in the aggregate, 600,000 shares. If any option to purchase shares of common stock granted pursuant to the Stock Purchase Plan shall for any reason terminate without having been exercised, the shares not purchased under such option shall again become available for issuance under the Stock Purchase Plan.
Eligibility
Employees of our company who meet the eligibility requirements under the Stock Purchase Plan may participate in the plan. Because participation in the Stock Purchase Plan is subject to the discretion of both the Compensation Committee and the participants in the Stock Purchase Plan, we cannot determine the dollar value or number of shares of common stock that will in the future be acquired by any participant or groups of participants, including our directors, executive officers and other employees. As of May 31, 2010 there were approximately 47 employees eligible to participate in the Stock Purchase Plan, including three executive officers. During the 2009 fiscal year, our named executive officers as a group acquired 3,500 shares of common stock under the Stock Purchase Plan, all of which were acquired by Mr. French. Also during the 2009 fiscal year, our employees, including all current officers who are not executive officers, as a group, acquired 56,358 shares of common stock under the Stock Purchase Plan.
An employee is eligible to participate in the Stock Purchase plan if the employee is employed by us for more than five months in any calendar year for more than 20 hours per week and has continuously worked for us for a length of time to be set by the Board of Directors, but not to exceed a period of greater than two years, except that no employee may participate (i) if immediately upon participation in the offering the employee owns stock possessing 5% or more of the total combined voting power or value of all classes of our stock, or (ii) if the rights granted to the employee under the Stock Purchase Plan together with rights granted to the employee under all of
V-19
our other employee stock purchase plans create rights to purchase our stock at a rate which exceeds $25,000 per year of the fair market value of the stock as determined at the time such rights are granted, for each calendar year in which such rights are outstanding at any time. In addition, no participant may purchase more than 7,500 shares in any Offering Period.
Offerings Under the Plan
Our Board of Directors may grant or provide for the grant of purchase rights to purchase shares of our common stock under the Stock Purchase Plan to eligible employees on such offering date or dates as selected by the Board. The terms of each offering shall be set by our Board of Directors, but all employees granted purchase rights under the Stock Purchase Plan shall have the same rights and privileges. The provisions of separate offerings need not be identical, but all offerings shall include the period during which the offering shall be effective, which period shall not exceed 27 months beginning with the offering date, and the provisions set forth in the Stock Purchase Plan with respect to eligibility, purchase rights, purchase price, participation, withdrawal, termination and exercise. An eligible employee may become a participant in the Stock Purchase Plan pursuant to an offering by delivering to us a participation agreement within the time specified in the offering. Each participant shall authorize payroll deductions to be held in an account to purchase shares of our common stock on the purchase date.
On each offering date, each eligible employee shall be granted a purchase right to purchase up to the number of shares of common stock as designated by our Board of Directors, not to exceed 15% of such employee’s earnings during the period as stated in the offering. Our Board of Directors shall specify a maximum number of shares of common stock that may be (i) purchased by any participant during the offering and (ii) purchased in aggregate by all participants during the offering. The purchase price of the shares of common stock acquired pursuant to purchase rights granted under the Stock Purchase Plan shall be not less than the lesser of (i) an amount equal to 85% of the fair market value of the shares of common stock on the offering date or (ii) an amount equal to 85% of the fair market value of the shares of common stock on the applicable purchase date.
Termination of Enrollment
A participant in the offering may terminate his or her payroll deductions under the Stock Purchase Plan and withdraw from the offering by delivering to us a notice of withdrawal at any time except as provided in the offering. Upon withdrawal from the offering, we shall distribute to such participant all of his or her accumulated payroll deductions. Purchase rights granted pursuant to any offering under the Stock Purchase Plan shall terminate immediately upon a participant ceasing to be an employee or other lack of eligibility. We will distribute to such terminated or otherwise ineligible employee all of his or her accumulated payroll deductions. A participant may file a written designation of a beneficiary who is to receive shares of Common Stock and/or cash from the participant’s account under the Stock Purchase Plan in the event of such participant’s death.
Plan Administration
The Stock Purchase Plan is administered by our Board of Directors or such committee or employee(s) as the Board of Directors may delegate. Our Board of Directors, or such committee or employee(s) as the Board of Directors may delegate, is vested with full power to determine all questions that may arise under, construe the terms of, adopt rules of procedure and enforce the provisions of the Stock Purchase Plan; provided, however, if our Board of Directors delegates administration to a committee or employee(s) the Board of Directors may remove such administration duties from the committee or employee(s) at any time.
Amendment and Termination
Our Board of Directors may amend the Stock Purchase Plan from time to time. Except as provided in the Stock Purchase Plan, no amendment shall be effective until we have obtained the approval of our stockholders if Section 423 of the Internal Revenue Code of 1986, as amended (the “Code”), or other applicable laws or
V-20
regulations require such approval. The rights and obligations under any purchase rights granted before amendment of the Stock Purchase Plan shall not be impaired by any amendment of the Plan except (i) with the consent of the person to whom such purchase rights were granted or (ii) as necessary to comply with any laws, governmental regulations or requirements of the Section 423 of the Code.
Our Board of Directors in its discretion may suspend or terminate the Stock Purchase Plan at any time. Unless sooner terminated, the Stock Purchase Plan shall terminate at the time that all of the shares of common stock reserved for issuance under the Stock Purchase Plan have been issued. No purchase rights may be granted under the Stock Purchase Plan while the Plan is suspended or after it is terminated. Any benefits, privileges, entitlements and obligations under any purchase rights granted under the Stock Purchase Plan while the Stock Purchase Plan is in effect shall not be impaired by suspension or termination of the Stock Purchase Plan except (i) as expressly provided in the Stock Purchase Plan or with the consent of the person to whom such purchase rights were granted, (ii) as necessary to comply with any laws, regulations, or listing requirements, or (iii) as necessary to ensure that the Stock Purchase Plan and/or purchase rights granted under the Stock Purchase Plan comply with the requirements of Section 423 of the Code.
Federal Income Tax Consequences
Generally, no federal income tax consequences will arise at the time an employee purchases common stock under the Stock Purchase Plan. If an employee disposes of common stock purchased under the ESPP less than one year after the common stock is purchased or within two years of the Grant Date, the employee will be deemed to have received compensation taxable as ordinary income for the taxable year in which the disposition occurs in the amount of the difference between the fair market value of the common stock at the time of purchase and the amount paid by the employee for the common stock. The amount of such ordinary income recognized by the employee will be added to the employee’s basis in the common stock for purposes of determining capital gain or loss upon the disposition of the common stock by the employee. If an employee holds the shares of common stock purchased under the Stock Purchase Plan for at least one year after the common stock is purchased and at least two years after the Grant Date before disposing of such shares, then the employee will be deemed to have received compensation taxable as ordinary income for the taxable year in which the disposition occurs in an amount equal to the lesser of (a) the excess of the fair market value of the common stock on the date of disposition over the purchase price paid by the employee, or (b) the excess of the fair market value of the common stock on the offering date over the purchase price paid by the employee. The amount of such ordinary income recognized by the employee will be added to the employee’s basis in the common stock for purposes of determining capital gain or loss upon the disposition of the common stock by the employee. If an employee dies before disposing of the common stock purchased under the Stock Purchase Plan, he or she will be deemed to have received compensation taxable as ordinary income in the taxable year closing with the employee’s death in an amount equal to the lesser of clauses (a) or (b) as set forth in the first sentence of this paragraph. The employee will not realize any capital gain or loss at death.
We generally will not be entitled to a deduction with respect to the common stock purchased by an employee under the Stock Purchase Plan, unless the employee disposes of the common stock less than one year after the common stock is transferred to the employee or less than two years after the Grant Date.
Accounting Treatment Under Generally Accepted Accounting Principles
The Stock Purchase Plan is classified under FASB ASC Topic 718 (formerly SFAS 123(R)) as a “compensatory” plan because participants have the right to purchase common stock at less than 95% of the fair market value on the Grant Date and because the plan allows for a “look-back” to allow participants to purchase stock based upon the fair market value on the Grant Date as opposed to the Purchase Date. Under FASB ASC Topic 718, we must record a charge to earnings equal to the fair value of the right to purchase common stock under the Stock Purchase Plan determined as of the Grant Date.
V-21
Vote Required and Board of Directors’ Recommendation
Assuming a quorum is present, the affirmative vote of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote, is required for approval of this Proposal No. 7. For purposes of Proposal No. 7, abstentions will have the same effect as a vote against this proposal, and broker non-votes will have no effect on the result of the vote.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” PROPOSAL NO. 7.
V-22
PROPOSAL NO. 8
APPROVAL OF A CHANGE TO OUR CAPITAL STRUCTURE THAT WOULD INCREASE THE NUMBER OF AUTHORIZED SHARES OF COMMON STOCK FROM 90,000,000 TO 180,000,000
AND THAT WOULD INCREASE THE NUMBER OF AUTHORIZED SHARES OF PREFERRED STOCK FROM 100,000 TO 200,000
General
The Board of Directors is proposing to amend our current Certificate of Incorporation, as amended and restated to date (the “Current Certificate”), to increase the number of our authorized shares of common stock from 90,000,000 to 180,000,000, and to increase the number of our authorized shares of preferred stock from 100,000 to 200,000, in each case as more fully described below. Other than the proposed increase in the number of shares of our authorized common stock and the proposed increased in the number of shares of our authorized preferred stock, the proposed amendment is not intended to modify the rights of existing stockholders in any material respect. The Board of Directors approved the proposed increase in the number of authorized shares of common stock and the proposed increase in the number of authorized shares of preferred stock, and recommends the approval and adoption of Proposal No. 8 by the stockholders.
Approval of this Proposal No. 8 is necessary in order to consummate, and is a condition to the consummation of, the merger with Cequent. If this Proposal No. 8 is not approved, we will not have a sufficient number of authorized shares of common stock available to issue to stockholders of Cequent pursuant to the merger agreement and the merger.
If approved, the proposed amendment to the Current Certificate (the “Authorized Capital Amendment”) under this Proposal No. 8 will become effective upon the filing of the Authorized Capital Amendment with the Secretary of State of the State of Delaware, which we would process promptly after the annual meeting. If Proposal No. 8 is not approved, the Authorized Capital Amendment would not be filed, and the Current Certificate would remain in effect, subject to approval of Proposal No. 3 regarding a change of the name of our company from “MDRNA, Inc.” to “Marina Biotech, Inc.”
Background of Proposed Increase in the Number of Authorized Shares
Under Delaware law, we may only issue shares of our capital stock to the extent such shares have been authorized for issuance under our Current Certificate. The Current Certificate authorizes the issuance of up to 90,000,000 shares of common stock and up to 100,000 shares of preferred stock, having a par value of $0.01 per share. As of May 31, 2010, 48,896,483 shares of common stock were issued and outstanding, 346,844 unissued shares of common stock were reserved for future issuance under our equity compensation plans, including 116,566 unissued shares of common stock which were reserved for future issuance under our Employee Stock Purchase Plan, and 12,533,472 unissued shares of common stock which were reserved for issuance upon the exercise of outstanding warrants, leaving approximately 28,223,201 shares of common stock unissued and unreserved.
In addition, as of March 31, 2010, 90,000 shares of the authorized preferred stock have been designated as Series A Junior Participating Preferred Stock, which shares will become issuable upon the occurrence of certain events specified in that certain Rights Agreement, dated as of February 22, 2000, between our company and American Stock Transfer & Trust Company, as rights agent. If this Proposal No. 8 is approved by our stockholders, we intend to designate 180,000 shares of our authorized preferred stock as Series A Junior Participating Preferred Stock.
In order to ensure that a sufficient number of shares of common stock will be available for issuance by us, including for issuance to the stockholders of Cequent in connection with the merger, and that a sufficient number of shares of Series A Junior participating Preferred Stock will be designated to cover any potential issuances of Series A Junior Participating Preferred Stock as a result of certain events discussed in the Rights Plan, the Board
V-23
of Directors has approved, subject to stockholder approval, the Authorized Capital Amendment to increase the number of shares of common stock authorized for issuance from 90,000,000 to 180,000,000 and to increase the number of shares of preferred stock authorized for issuance from 100,000 to 200,000.
Purpose and Effect of the Authorized Capital Amendment
The Board of Directors believes it desirable to increase the authorized number of shares of common stock in order to issue shares of common stock to the stockholders of Cequent upon the closing of the merger, and to provide us with adequate flexibility in corporate planning and strategies. As noted elsewhere in this proxy statement, the approval of the Authorized Capital Amendment is a condition to the closing of the merger with Cequent.
The availability of additional shares of common stock for issuance could be used for a number of purposes, including corporate financing, public or private offerings of common stock, future acquisitions, stock dividends, stock splits, strategic relationships with corporate partners, stock options, and other stock-based compensation. The availability of additional shares of common stock and preferred stock is particularly important in the event that the Board of Directors needs to undertake any of the foregoing actions on an expedited basis and thus to avoid the time and expense of seeking stockholder approval in connection with the contemplated issuance of common stock and preferred stock.
Other than the transactions contemplated by the merger agreement with Cequent, there are currently no plans, agreements or understandings regarding the issuance of any of the additional shares of common stock or preferred stock that would be available if this Proposal No. 8 is approved. Such additional authorized shares may be issued for such purposes and for such consideration as the Board of Directors may determine without further stockholder approval, unless such action is required by applicable law or the rules of Nasdaq or any stock exchange on which our securities may be listed.
The increase in authorized common stock or preferred stock will not have any immediate effect on the rights of existing stockholders. The additional shares of common stock for which authorization is sought would be part of the existing class of common stock. Of the additional 100,000 shares of preferred stock for which authorization is sought, 80,000 would be designated as Series A Junior Participating Preferred Stock. There will be no change in voting rights, dividend rights, liquidation rights, preemptive rights or any other stockholder rights as a result of the Authorized Capital Amendment. However, the Board of Directors will have the authority to issue authorized common stock or preferred stock without requiring future stockholder approval of such issuances, except as may be required by applicable law or the rules of Nasdaq or any stock exchange on which our securities may be listed. To the extent that additional authorized shares are issued in the future, they may decrease the existing stockholders’ percentage equity ownership and, depending on the price at which they are issued, could be dilutive to the existing stockholders. The holders of common stock have no preemptive rights and the Board of Directors has no plans to grant such rights with respect to any such shares.
The increase in our authorized but unissued shares of common stock and preferred stock that would result from adoption of the Authorized Capital Amendment could have a potential anti-takeover effect with respect to our company, although management is not presenting the proposal for this reason and does not presently anticipate using the increased authorized shares for such a purpose. The potential anti-takeover effect of the Authorized Capital Amendment arises because it would enable us to issue additional shares of common stock and additional shares of preferred stock convertible into common stock up to the total authorized number with the effect that stockholdings and related voting rights of then existing stockholders would be diluted to an extent proportionate to the number of additional shares of common stock or common stock equivalents that may be issued. In addition, if we were the subject of a hostile takeover attempt, we could try to impede the takeover by issuing shares of common stock or preferred stock, thereby diluting the voting power of the other outstanding shares and increasing the potential cost of the takeover. The availability of this defensive strategy to us could discourage
V-24
unsolicited takeover attempts, thereby limiting the opportunity for our stockholders to realize a higher price for their shares than is generally available in the public markets. This proposal is not being presented with the intent that it be utilized as a type of anti-takeover device with respect to any attempt or contemplated attempt to acquire control of our company.
Vote Required and Board of Directors Recommendation
Assuming a quorum is present, the affirmative vote of the holders of a majority of the issued and outstanding shares of common stock entitled to vote as of the record date, either in person or by proxy, is required for approval of this Proposal No. 8. Abstentions and broker non-votes will be counted as present for purposes of determining if a quorum is present, but will have the same effect as a negative vote on the outcome of this proposal.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” PROPOSAL NO. 8.
V-25
PROPOSAL NO. 9
APPROVAL OF THE AMENDMENT TO THE STOCKHOLDER RIGHTS AGREEMENT
Background
We are a party to that certain Rights Agreement with American Stock Transfer & Trust Company, as rights agent, dated as of February 22, 2000 (as amended from time to time, the “Rights Agreement”). On March 3, 2010, we entered into an amendment (the “Rights Amendment”) to the Rights Agreement. The Rights Amendment was effective as of March 17, 2010 and extended the Final Expiration Date (as defined in the Rights Agreement) of the Rights Agreement from March 17, 2010 to March 17, 2013, subject to stockholder approval. Specifically, the Rights Amendment contains a provision that requires that the Rights Amendment be submitted for stockholder approval on or before the first anniversary of the date of the Rights Amendment. If the Rights Amendment is not submitted for stockholder approval within that time frame, or if stockholder approval is not obtained, then the Rights Amendment will terminate pursuant to its terms, in which case the Rights Agreement will expire.
The Rights Amendment was entered into in the normal course and not in response to any acquisition proposal. In addition, our Board of Directors has waived the applicability of the Rights Agreement to the merger, the merger agreement and the transactions contemplated thereby.
Over the last 25 years, a substantial number of corporations have adopted similar stockholder rights plans as an anti-takeover device in an attempt to defend against abusive or otherwise undesirable attempts to acquire control, such as:
| | • | | taking control through open-market purchases without giving the stockholders a control premium for their shares or the protections of the federal tender offer rules; |
| | • | | attempting to acquire the company at a time when the company’s common stock is undervalued and at a price that is less than the stock’s intrinsic value; and |
| | • | | attempting, through a partial tender offer, to acquire a majority interest in the company and then forcing the remaining public stockholders to accept cash and/or securities of lesser value. |
The Rights Agreement seeks to discourage such attempts by making an acquisition of our company prohibitively expensive unless it is approved by our Board of Directors. This is accomplished by significantly diluting the prospective acquiror’s stock interest in our company and increasing the number of shares of common stock that would have to be acquired, unless the Board of Directors approves the acquisition.
The Rights Agreement works as follows. Generally, when an acquiror accumulates 15% or more of our common stock, each holder of a Right (as defined in the Rights Agreement) will thereafter have the right to receive, upon exercise of a Right, a number of shares of common stock having a value equal to two times the exercise price of $50. For example, if a stockholder owned 100 shares of common stock at the time an acquiror acquired 15% of the outstanding common stock, and the market value of the common stock at that time was $5.00 per share, the stockholder could exercise Rights to acquire a total of 20 shares of common stock for $2.50 per share. In effect, our stockholders (other than the acquiror, who is not permitted to exercise Rights) would be able to purchase a substantial amount of our stock at a 50% discount. Therefore, if most stockholders exercised these valuable Rights, the stock interest of the acquiror would be dramatically diluted. However, if an acquiror were to open negotiations with the Board of Directors before acquiring 15% or more of the outstanding common stock, the Board could redeem the Rights or modify the terms of the Rights to prevent the acquiror’s stock interest from being diluted. Therefore, the existence of the Rights Agreement encourages potential acquirors to negotiate with the Board of Directors before crossing the 15% ownership threshold.
V-26
Board Consideration
The Board of Directors believes that the Rights Amendment is in the best interests of our stockholders because the Rights Agreement, as so amended:
| | • | | Provides a way for the Board of Directors to defend stockholders against abusive tactics used to gain control of our company without paying all stockholders a fair premium, and to ensure that all of our stockholders are treated fairly and equally in an acquisition of our company; |
| | • | | Encourages anyone seeking to acquire control of our company to negotiate in good faith with the Board of Directors and gives the Board of Directors significant negotiating power on behalf of our stockholders, thereby enabling the Board of Directors to negotiate a fair premium for stockholders that is consistent with the intrinsic value of our company and to block any transaction by a potential acquiror who is unwilling to pay a fair price; |
| | • | | Reduces the likelihood that a potential acquiror who is unwilling to pay a sufficient premium will attempt to acquire our company by means of an open market accumulation, a partial bid for our company, a front-end loaded tender offer or other coercive or unfair takeover tactics, since it limits the size of the position the acquiror may take without the concurrence of the Board; |
| | • | | Slows the process by which a potential acquiror may gain control of our company, thereby affording the Board of Directors additional time to evaluate a proposed transaction and, if necessary, seek alternative transactions or implement other courses of action to maximize stockholder value; and |
| | • | | Does not prevent the making of unsolicited offers or the acquisition of our company at a full and fair price since the existence of the Rights Agreement does not eliminate the Board’s responsibility to consider acquisition proposals in a manner consistent with the directors’ fiduciary duties to stockholders. |
Summary of Rights Agreement
The following description of the material terms of the Rights Agreement, as amended by the Rights Amendment, does not purport to be complete and is qualified in its entirety by reference to the Rights Agreement, Amendment No. 1 to the Rights Agreement, the Rights Amendment, and Amendment No. 3 to the Rights Agreement, which were attached as Exhibit No. 1 to our Current Report on Form 8-K filed on March 16, 2000, Exhibit 4.1 to our Current Report on Form 8-K filed on January 19, 2007, Exhibit 4.1 to our Current Report on Form 8-K filed on March 5, 2010, and Exhibit 4.3 to our Current Report on Form 8-K filed on April 6, 2010, respectively.
Distribution Date; Exercisability
The Rights are currently attached to all Common Share certificates and no separate Rights certificates have been issued. Separate certificates evidencing the Rights (“Right Certificates”) will be mailed to holders of record of the Common Shares as of the close of business on the earlier to occur of (i) a public announcement that a person or group of affiliated or associated persons (an “Acquiring Person”) has acquired beneficial ownership of 15% or more of the outstanding Common Shares or (ii) such date as may be determined by action of the Board of Directors, upon approval of a majority of the Continuing Directors (as defined below), following the commencement of, or announcement of an intention to make, a tender offer or exchange offer the consummation of which would result in the beneficial ownership by a person or group of 15% or more of the outstanding Common Shares (the earlier of such dates being the “Distribution Date”).
The Rights Agreement provides that, until the Distribution Date (or earlier redemption or expiration of the Rights), (i) the Rights will be transferred with and only with the Common Shares, (ii) new Common Share certificates issued after the Record Date upon transfer or new issuance of Common Shares will contain a notation incorporating the Rights Agreement by reference and (iii) the surrender for transfer of any certificates for Common Shares outstanding as of the Record Date will also constitute the transfer of the Rights associated with the Common Shares represented by such certificate.
V-27
The Rights are not exercisable until the Distribution Date. Assuming approval of this Proposal No. 9, the Rights will expire on March 17, 2013 (the “Expiration Date”), unless the Expiration Date is extended or unless the Rights are earlier redeemed or exchanged by our company, in each case, as described below.
Flip-In
If a person or group becomes an Acquiring Person, each holder of a Right will thereafter have the right to receive, upon exercise, Common Shares (or, in certain circumstances, Preferred Shares or other similar securities of our company) having a value equal to two times the exercise price of the Right. Notwithstanding any of the foregoing, following the existence of an Acquiring Person, all Rights that are, or (under certain circumstances specified in the Rights Agreement) were, beneficially owned by any Acquiring Person will be null and void.
For example, at an exercise price of $50 per Right, each Right not owned by an Acquiring Person following an event set forth in the preceding paragraph would entitle its holder to purchase $100 worth of Common Shares (or other consideration, as noted above) for $50. Assuming a value of $5 per Common Share at such time, the holder of each valid Right would be entitled to purchase 20 Common Shares for $50.
Flip-Over
In the event that we are acquired in a merger or other business combination transaction or 50% or more of our consolidated assets or earning power are sold after a person or group has become an Acquiring Person, proper provision will be made so that each holder of a Right will thereafter have the right to receive, upon the exercise thereof at the then current exercise price of the Right, that number of shares of common stock of the acquiring company which at the time of such transaction will have a market value of two times the exercise price of the Right. In the event that any person or group becomes an Acquiring Person, proper provision shall be made so that each holder of a Right, other than Rights beneficially owned by the Acquiring Person (which will thereafter be void), will thereafter have the right to receive upon exercise that number of Common Shares having a market value of two times the exercise price of the Right.
Exchange
At any time after any person or group becomes an Acquiring Person and prior to the acquisition by such person or group of 50% or more of the outstanding Common Shares, the Board of Directors may exchange the Rights (other than Rights owned by such person or group which will have become void), in whole or in part, at an exchange ratio of one Common Share, or one one-thousandth of a Preferred Share (or of a share of a class or series of our preferred stock having equivalent rights, preferences and privileges), per Right (subject to adjustment).
Redemption
At any time prior to the existence of an Acquiring Person, the Board of Directors may redeem the Rights, in whole but not in part, at a price of $.01 per Right (the “Redemption Price”). The redemption of the Rights may be made effective at such time on such basis with such conditions as the Board of Directors, in its sole discretion, may establish. Immediately upon any redemption of the Rights, the right to exercise the Rights will terminate and the only right of the holders of Rights will be to receive the Redemption Price.
Continuing Directors
A determination, approval, consent or other action of the “Board of Directors” shall require approval or consent by a majority of the Continuing Directors (as defined below), provided that no such approval or consent would be required after six months from the date the Continuing Directors cease to constitute a majority of the members of the Board of Directors.
“Continuing Director” means a member of the Board of Directors, who is not an Acquiring Person or a representative or nominee of an Acquiring Person, and who either (i) was a member of the Board of Directors on the date of the Rights Agreement or (ii) subsequently became a member of the Board of Directors, and whose
V-28
nomination for election or election to the Board of Directors was recommended or approved by a majority of the Continuing Directors then on the Board of Directors. For purposes of the Rights Agreement, a determination, calculation, interpretation or other action shall not be deemed approved by a majority of the Continuing Directors unless there is at least one Continuing Director in office who approved such action.
Adjustment
The number of outstanding Rights and the number of one one-thousandths of a Preferred Share issuable upon exercise of each Right are subject to adjustment under certain circumstances.
Preferred Stock
Because of the nature of the Preferred Shares’ dividend, liquidation and voting rights, the value of the one one-thousandth interest in a Preferred Share purchasable upon exercise of each Right should approximate the value of one Common Share.
Rights of Holders
Until a Right is exercised, the holder thereof, as such, will have no rights as a stockholder of our company, including, without limitation, the right to vote or to receive dividends.
Amendment
The Rights Agreement may be amended by the Board of Directors, with the consent of the Rights Agent but without the consent of the holders of the Rights, except that from and after the existence of an Acquiring Person no such amendment may adversely affect the interests of the holders of the Rights (other than the Acquiring Person).
Certain Effects
The Board of Directors believes that the continuation of the Rights Agreement pursuant to the Amendment is in the stockholders’ best interests for the reasons described above. In making your voting decision, however, stockholders should consider that, while the Rights Agreement is not intended to prevent a takeover of our company, it may discourage the accumulation by any person or group of more than 15% of the outstanding common shares and may have the effect of rendering more difficult or discouraging any acquisition of our company deemed undesirable by the Board of Directors, even if stockholders disagree with the Board of Directors’ conclusion.
The Rights Agreement will cause substantial dilution to a person or group that attempts to acquire our company on terms or in a manner not approved, except pursuant to an offer conditioned upon the elimination, purchase or redemption of the Rights. Also, because the Rights Agreement may increase the price required to be paid by a potential acquiror in order to obtain control of our company and thus discourage certain transactions, the continuing effectiveness of the Rights Agreement may reduce the likelihood of a takeover proposal being made for our common stock and discourage some offers from being made at all. This effect and the perception that it may be less likely that our company will be acquired could have an adverse impact on the market price of our common stock.
Vote Required and Board of Directors’ Recommendation
Assuming a quorum is present, the affirmative vote of the holders of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote, is required for approval of this Proposal No. 9. For purposes of Proposal No. 9, abstentions will have the same effect as a vote against this proposal, and broker non-votes will have no effect on the result of the vote.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” PROPOSAL NO. 9.
V-29
PROPOSAL NO. 10
APPROVAL OF THE REDUCTION AND, IN CERTAIN INSTANCES, THE ELIMINATION OF THE FLOOR EXERCISE PRICE OF COMMON STOCK PURCHASE WARRANTS ISSUED TO INVESTORS AND THE PLACEMENT AGENT IN APRIL 2008, AND THE ISSUANCE OF SHARES OF COMMON STOCK UPON THE FULL EXERCISE OF SUCH WARRANTS
Introduction
The purpose of this Proposal No. 10 is to obtain the stockholder approval necessary under NASDAQ Marketplace Rule 5635(d) to allow for: (i) the reduction (and potential elimination, in certain instances discussed below) of the minimum exercise price per share of the Amended and Restated Common Stock Purchase Warrants issued pursuant to that certain Securities Purchase Agreement dated as of April 25, 2008 (the “2008 Purchase Agreement”) between our company and the investors identified on the signature pages thereto (the “2008 Investors”) from $2.17 to $1.00; (ii) the reduction (and potential elimination, in certain instances discussed below) of the minimum exercise price per share of the Amended and Restated Common Stock Purchase Warrants issued to the placement agent in connection with the financing effected pursuant to the 2008 Purchase Agreement (such warrants, the “Agent Warrants”) from $2.17 to $1.00; and (iii) the issuance of additional shares of common stock to the 2008 Investors and the placement agent upon the exercise in full of such warrants following approval of this Proposal No. 10.
If stockholder approval of this Proposal No. 10 is not acquired, the minimum exercise price of the warrants will remain at $2.17 per share, and the number of shares of common stock that the 2008 Investors and the placement agent will be able to acquire upon exercise of such warrants will remain at 5,222,591 shares. If stockholder approval of this Proposal No. 10 is acquired and the floor exercise price is eliminated for the reasons discussed below, there will be no limit to the number of shares of common stock that could potentially be issuable to the 2008 Investors and the placement agent upon the exercise of the warrants.
The discussion in this proxy statement of the terms of the financing consummated pursuant to the 2008 Purchase Agreement and the terms of the Amended and Restated Common Stock Purchase Warrants is qualified in its entirety by the full text of the 2008 Purchase Agreement, Amendment No. 1 to the 2008 Purchase Agreement and the Amended and Restated Common Stock Purchase Warrants, each of which is incorporated into this proxy statement by reference. A copy of the 2008 Purchase Agreement is attached as Exhibit 10.2 to our Current Report on Form 8-K filed with the SEC on April 30, 2008, a copy of Amendment No. 1 to the 2008 Purchase Agreement is attached as Exhibit 10.1 to our Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2009, and a copy of the Amended and Restated Common Stock Purchase Warrant is attached as Exhibit 10.2 to our Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2009.
Description of the Financing
On April 25, 2008, we entered into the 2008 Purchase Agreement with the 2008 Investors pursuant to which we issued to the 2008 Investors 4,590,277 shares of common stock, warrants to purchase up to 4,590,277 shares of common stock that are exercisable during the seven-year period beginning on October 25, 2008 (the “7-Year Warrants”), and warrants to purchase up to 1,377,084 shares of common stock that are exercisable during the 90-day period beginning on October 25, 2008 (the “90-Day Warrants”) for an aggregate purchase price of approximately $7,932,000 (the “Financing”). The initial exercise price of the 7-Year Warrants was $2.376 and the initial exercise price of the 90-Day Warrants was $2.17. The 90-Day Warrants expired by their terms on January 25, 2009. A total of 4,590,277 shares of common stock, plus warrants to purchase an additional 5,967,361 shares of common stock, were issued in the Financing. The proceeds of the Financing were used for expenses related to the financing and working capital.
The 7-Year Warrants, which were amended and restated in June 2009, contain a full ratchet anti-dilution provision that provides that the exercise price of the 7-Year Warrants will be adjusted at any time, during the term of the 7-Year Warrants, if we issue securities below the then current exercise price of the 7-Year Warrants,
V-30
subject to a minimum exercise price (such price, the “Floor Price”) of $2.17 per share. Further, the full ratchet anti-dilution provision requires a corresponding adjustment of the number of shares of common stock that may be acquired upon the exercise of such 7-Year Warrants (such that the total consideration payable by the Investor remains unchanged upon full exercise regardless of a downward adjustment in the exercise price).
In connection with the Financing we issued the Agent Warrants to the placement agent. The Agent Warrants, which were originally exercisable for 229,514 shares of common stock and are exercisable during the five-year period beginning on October 25, 2008, contain terms that are substantially similar to the terms of the 7-Year Warrants. On June 9, 2009, 50,000 of the Agent Warrants were exercised. Following such exercise, 179,514 Agent Warrants remained outstanding.
The per share exercise price of the 7-Year Warrants and the Agent Warrants was adjusted to $2.17 in connection with our issuance of 12% secured promissory notes in the aggregate principal amount of $1,000,000 and warrants to purchase up to 1,075,269 shares of common stock at an initial exercise price of $1.02 per share in December 2009 (the “December 2009 Financing”). Correspondingly, the number of shares that could be acquired upon full exercise of the 7-Year Warrants increased from 4,590,277 to 5,026,036, and the number of shares of common stock that could be acquired upon full exercise of the Agent Warrants increased from 179,514 to 196,555.
Amendment of the 7-Year Warrants and Agent Warrants
In June 2009, we amended and restated the 7-Year Warrants and the Agent Warrants to provide that we would solicit the affirmative vote of our stockholders at our next meeting of stockholders for approval of resolutions providing for the reduction of the Floor Price from $2.17 to $1.00, and the corresponding issuance of additional shares of common stock in connection therewith;provided,however, that the Floor Price shall be eliminated on the first date (if any) (such date, the “Null and Void Date”) on or after June 9, 2009 on which we issue or are deemed to have issued in the aggregate during any immediately preceding 365 days shares of common stock or Common Stock Equivalents (as defined in the 2008 Purchase Agreement), or any combination thereof, which exceed 25% of the issued and outstanding shares of common stock as of the first day of such 365 days. On the Null and Void Date the exercise price of the 7-Year Warrants and the Agent Warrants would be automatically adjusted to the lowest price to which it would have been adjusted had the $1.00 Floor Price not applied during such period. Issuances solely pursuant to clauses (a), (c) and (d) of the defined term Exempt Issuance (defined below) will not be counted in determining whether such 25% was exceeded.
If the exercise price of the 7-Year Warrants and the Agent Warrants is reduced to $1.00 per share, the total number of shares of common stock that would be issuable upon exercise of the 7-Year Warrants would be increased from 5,026,036 to 10,906,498, and the number of shares of common stock that would be issuable upon exercise of the Agent Warrants would be increased from 196,555 to 426,524.
The reduction of the Floor Price from $2.17 to $1.00, and the corresponding increase in the number of shares potentially issuable upon exercise of the 7-Year Warrants and the Agent Warrants, will be effective if and only if we seek and acquire stockholder approval of this Proposal No. 10. Should our stockholders fail to approve this Proposal No. 10, the Floor Price will remain at $2.17 per share, and the maximum number of shares of common stock issuable upon exercise of the 7-Year Warrants and the Agent Warrants would remain at 5,222,591.
Description of Securities
The terms of the 7-Year Warrants and the Agent Warrants each contain full-ratchet anti-dilution protections. Full-ratchet anti-dilution protection is afforded by providing for both an adjustment of the exercise price and a corresponding adjustment in the number of shares of common stock for which the 7-Year Warrants and the Agent Warrants may be exercised in certain circumstances, subject to a Floor Price of $2.17 per share. The exercise price shall be adjusted for issuances of shares, or securities convertible into or exercisable for shares, of common stock at an effective price per share that is less than the then-current exercise price of the 7-Year
V-31
Warrants and the Agent Warrants. In the event of any such reduction, the number of shares of common stock that are issuable upon exercise of the 7-Year Warrants and the Agent Warrants shall be inversely proportionately increased such that the aggregate exercise price remains the same.
The foregoing provision does not apply to “Exempt Issuances,” which are defined as any issuances or sales of: (a) shares of common stock or options to employees, officers or directors of our company pursuant to any stock or option plan duly adopted for such purpose, by a majority of the non-employee members of our Board of Directors or a majority of the members of a committee of non-employee directors established for such purpose; (b) securities upon the exercise or exchange of or conversion of any securities issued under the 2008 Purchase Agreement and/or other securities exercisable or exchangeable for or convertible into shares of common stock issued and outstanding on the date of the 2008 Purchase Agreement, provided that such securities have not been amended since the date of the 2008 Purchase Agreement to increase the number of such securities or to decrease the exercise, exchange or conversion price of such securities; (c) securities issued pursuant to acquisitions or strategic transactions approved by a majority of the disinterested directors of our company, provided that any such issuance shall only be to a person which is, itself or through its subsidiaries, an operating company in a business synergistic with the business of our company and in which we receive benefits in addition to the investment of funds, but shall not include a transaction in which we are issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities; and (d) shares of common stock and warrants to purchase common stock (including common stock issuable upon exercise of such warrants) issued pursuant to that certain Securities Purchase Agreement, dated as of June 9, 2009, by and among our company and the other parties thereto. Exercise of the 7-Year Warrants and the Agent Warrants is subject to a beneficial ownership cap of 4.99% of the total number of shares of common stock then issued and outstanding.
The 7-Year Warrants, upon full exercise at the current exercise price of $2.17 per share, would result in the issuance of 5,026,036 shares of common stock, and the Agent Warrants, upon full exercise at the current exercise price of $2.17 per share, would result in the issuance of 196,555 shares of common stock, in each case except as limited by the beneficial ownership cap and the prohibition of any issuance in violation of NASDAQ Listing Rules.
The adjustment of the Floor Price from $2.17 to $1.00 per share contemplated by this Proposal No. 10, assuming stockholder approval, would result, (A) upon full exercise of the 7-Year Warrants, in the potential issuance of up to 10,906,498 shares of common stock and (B) upon full exercise of the Agent Warrants, in the potential issuance of up to 426,524 shares of common stock (in each case, except as limited by the beneficial ownership cap). This is an aggregate increase of 6,110,431 shares. If stockholder approval of this Proposal No. 10 is acquired and the Floor Price is eliminated for the reasons discussed herein, there will be no limit to the number of shares of common stock that could potentially be issuable upon the exercise of the warrants.
The 7-Year Warrants may be exercised until October 25, 2015 and the Agent Warrants may be exercised until October 25, 2013.
NASDAQ Listing Requirements and the Necessity of Stockholder Approval
As a company listed on the NASDAQ Global Market, we are required to follow the NASDAQ listing rules. One such rule is Marketplace Rule 5635(d)(2), which requires stockholder approval prior to the issuance of securities in connection with a transaction other than a public offering that involves the issuance or potential issuance by an issuer of common stock (or securities convertible into or exercisable for common stock) equal to 20% or more of the common stock or the voting power outstanding before the issuance for less than the greater of book or market value of such common stock. Stockholder approval was not required in connection with the Financing because the terms of the Financing did not result an issuance of 20% or more of our then-outstanding shares of common stock for a price less than the greater of book or market value.
As noted above, we have agreed to seek stockholder approval of an adjustment to the exercise price of the 7-Year Warrants and the Agent Warrants. Following such adjustment, and the potential reduction of the exercise price to
V-32
$1.00 per share, the number of shares of common stock that could be acquired upon full exercise of the 7-Year Warrants and the Agent Warrants, together with the shares that were issued in the Financing, for less than the greater of book or market value, would exceed 20%, thereby requiring stockholder approval.
On the trading date immediately prior to the execution of the 2008 Purchase Agreement, there were 26,713,124 shares of common stock issued and outstanding, the last reported sale price of our common stock on the NASDAQ Global Market was $2.18, and the closing bid price of our common stock on the NASDAQ Global Market was $2.16. In the Financing, we issued to the 2008 Investors a total of 4,590,277 shares of common stock and warrants to purchase up to 5,967,361 shares of common stock, and we issued to the placement agent warrants to purchase up to 229,514 shares of common stock. The exercise price of all of the warrants was greater than the market value of our common stock and none of the warrants were exercisable until at least six months after their issuance. As a result, none of the shares issuable upon exercise of the warrants were included in the calculation under Marketplace Rule 5635(d)(2). With the warrant shares excluded from the 20% calculation as per NASDAQ rules and interpretations, the total number of shares that were issued in the Financing for less than the greater of book or market value represented less than 20% of the then-outstanding shares of common stock. Accordingly, we were not required to seek stockholder approval of the Financing at that time.
However, the 7-Year Warrants and the Agent Warrants, as amended and restated, contain two provisions that are important to this situation. The first provision provides full ratchet, anti-dilution protection to the holders. Such protection acts to adjust the exercise price of the warrants and the number of shares issuable upon exercise thereof when we issue any securities at or below the then current exercise price. As a result of the December 2009 Financing, the exercise price of the 7-Year Warrants and the Agent Warrants was adjusted down to $2.17 and the number of shares that can be acquired upon exercise of the 7-Year Warrants and the Agent Warrants has been adjusted up to 5,222,591.
The second provision provides that we will solicit the affirmative vote of our stockholders at the Meeting for approval of resolutions providing for the reduction of the Floor Price from $2.17 to $1.00, and the corresponding issuance of additional shares of common stock in connection therewith;provided,however, that the Floor Price shall be eliminated on the first date (if any) (the “Null and Void Date”) on or after June 9, 2009 on which we issue or are deemed to have issued in the aggregate during any immediately preceding 365 days shares of common stock or Common Stock Equivalents (as defined in the 2008 Purchase Agreement), or any combination thereof, which exceed 25% of the issued and outstanding shares of common stock as of the first day of such 365 days, and on the Null and Void Date the exercise price of the 7-Year Warrants and the Agent Warrants would be automatically adjusted to the lowest price that it would have been adjusted had the $1.00 Floor Price not applied during such period (it being understood that issuances solely pursuant to clauses (a), (c) and (d) of the defined term Exempt Issuance shall not be counted in determining whether such 25% was exceeded).
If the exercise price of the 7-Year Warrants and the Agent Warrants is reduced to $1.00 per share, the total number of shares of common stock that would be issuable upon exercise of the 7-Year Warrants would be increased by 5,880,462 shares to 10,906,498 shares, and the total number of shares of common stock that would be issuable upon exercise of the Agent Warrants would be increased by 229,969 shares to 426,524 shares. If the Floor Price is eliminated for the reasons discussed herein, there will be no limit to the number of shares of common stock that could potentially be issuable upon the exercise of the warrants.
Stockholder approval of this Proposal No. 10 is required because, since the exercise price of the 7-Year Warrants and the Agent Warrants will be reduced below the market price of our common stock at the time of the Financing, the total number of shares of common stock potentially issuable for less than the greater of book or market value in the Financing (including the 4,590,277 shares previously issued to the 2008 Investors) would exceed 19.99% of the shares of common stock outstanding prior to the Financing.
If the stockholders do not approve this Proposal No. 10, the 2008 Investors and the placement agent will retain their current rights, namely the 2008 Investors will still be entitled to acquire 5,026,036 shares of common stock
V-33
at the current exercise price of $2.17 per share upon full exercise of the 7-Year Warrants, and the placement agent (or its designees) will still be entitled to acquire 196,555 shares of common stock at the current exercise price of $2.17 per share upon full exercise of the Agent Warrants.
Vote Required and Board of Directors’ Recommendation
Assuming a quorum is present, the affirmative vote of the holders of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote, is required for approval of this Proposal No. 10. For purposes of Proposal No. 10, abstentions will have the same effect as votes against this proposal, and broker non-votes will have no effect on the result of the vote. Shares of common stock acquired by the 2008 Investors in the Financing may not be voted in connection with this proposal.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” PROPOSAL NO. 10.
V-34
PROPOSAL NO. 11
APPROVAL OF THE ELIMINATION OF THE FLOOR EXERCISE PRICE OF COMMON STOCK PURCHASE WARRANTS ISSUED TO INVESTORS IN JANUARY 2010
Introduction
The purpose of this Proposal No. 11 is to obtain the stockholder approval necessary under NASDAQ Marketplace Rule 5635(d) to allow for the elimination of the floor exercise price of $0.94 per share (the “Floor Price”) of the Common Stock Purchase Warrants (the “Warrants”) issued pursuant to that certain Securities Purchase Agreement dated as of January 13, 2010 (the “2010 Purchase Agreement”) between our company and the investors identified on the signature pages thereto (the “2010 Investors”). If stockholder approval of this proposal is not acquired, the Floor Price will remain at $0.94 per share.
The discussion in this proxy statement of the terms of the financing consummated pursuant to the 2010 Purchase Agreement and the terms of the Warrants is qualified in its entirety by the full text of the 2010 Purchase Agreement and the Warrants, each of which is incorporated into this proxy statement by reference. A copy of the Warrant and the 2010 Purchase Agreement are attached as Exhibits 4.1 and 10.2, respectively, to our Current Report on Form 8-K filed with the SEC on January 14, 2010.
Description of the Financing
On January 13, 2010, we entered the 2010 Purchase Agreement with the Investors pursuant to which we issued to the 2010 Investors 5,385,557 shares of common stock and warrants to purchase up to 3,500,612 shares of common stock that are exercisable during the five-year period beginning on January 14, 2010 (the “Warrants”) for an aggregate purchase price of approximately $5,500,000 (the “Financing”). The exercise price of the Warrants is $1.00 per share. The proceeds of the Financing were used for expenses related to the financing and working capital.
The Warrants contain a full ratchet anti-dilution provision that provides that the exercise price of the Warrants will be adjusted at any time, during the term of the Warrants, if we issue securities below the then current exercise price of the Warrants, subject to a minimum exercise price (such price, the “Floor Price”) of $0.94 per share. However, the full ratchet anti-dilution provision does not require a corresponding adjustment of the number of shares of common stock that may be acquired upon the exercise of the Warrants. Thus, even if the Floor Price is eliminated, the number of shares issuable upon exercise of the Warrants will remain the same.
The Warrants provide that we will solicit the affirmative vote of our stockholders at the next meeting of our stockholders for approval of resolutions providing for the elimination of the Floor Price.
The elimination of the Floor Price will be effective if and only if we acquire stockholder approval of this Proposal No. 11. Should our stockholders fail to approve this Proposal No. 11, the Floor Price will remain at $0.94. Regardless of the outcome of this Proposal No. 11, the number of shares of common stock issuable upon exercise of the Warrants will remain the same.
Description of Securities
The terms of the Warrants permit exercise for a period of five years and contain full-ratchet anti-dilution protections. Full-ratchet anti-dilution protection is afforded to the 2010 Investors by providing for an adjustment of the exercise price, subject to a Floor Price of $0.94 per share. However, there is no corresponding adjustment in the number of shares of common stock for which the Warrants may be exercised. The exercise price of the Warrants shall be adjusted for issuances of shares, or securities convertible into or exercisable for shares, of common stock at an effective price per share that is less than the then-current exercise price of the Warrants.
V-35
The foregoing provision does not apply to “Exempt Issuances,” which are defined as any issuances or sales of: (a) shares of common stock or options to employees, officers or directors of our company pursuant to any stock or option plan duly adopted for such purpose, by a majority of the non-employee members of our Board of Directors or a majority of the members of a committee of non-employee directors established for such purpose; (b) securities upon the exercise or exchange of or conversion of any securities issued under the 2010 Purchase Agreement and/or other securities exercisable or exchangeable for or convertible into shares of common stock issued and outstanding on the date of the 2010 Purchase Agreement, provided that such securities have not been amended since the date of the 2010 Purchase Agreement to increase the number of such securities or to decrease the exercise, exchange or conversion price of such securities; and (c) securities issued pursuant to acquisitions or strategic transactions approved by a majority of the disinterested directors of our company, provided that any such issuance shall only be to a person which is, itself or through its subsidiaries, an operating company in a business synergistic with the business of our company and in which we receive benefits in addition to the investment of funds, but shall not include a transaction in which we are is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities. Exercise of the Warrants is subject to a beneficial ownership cap of 9.9% of the total number of shares of common stock then issued and outstanding.
NASDAQ Listing Requirements and the Necessity of Stockholder Approval
As a company listed on the NASDAQ Global Market, we are required to follow the NASDAQ listing rules. One such rule is Marketplace Rule 5635(d)(2), which requires stockholder approval prior to the issuance of securities in connection with a transaction other than a public offering that involves the issuance or potential issuance by an issuer of common stock (or securities convertible into or exercisable for common stock) equal to 20% or more of the common stock or the voting power outstanding before the issuance for less than the greater of book or market value of such common stock. Stockholder approval was not required in connection with the Financing because the terms of the Financing did not result an issuance of 20% or more of our then-outstanding shares of common stock for a price less than the greater of book or market value.
As noted above, we have agreed to seek stockholder approval of the Floor Price of the Warrants. Following such adjustment, and the potential reduction of the exercise price of the Warrants below the Floor Price, the number of shares of common stock that could be acquired upon full exercise of the Warrants, together with the shares that were issued in the Financing, for less than the greater of book or market value, would exceed 20%, thereby requiring stockholder approval.
On the trading date immediately prior to the execution of the 2010 Purchase Agreement, there were 40,806,941 shares of common stock issued and outstanding and the last reported sale price of our common stock on the NASDAQ Global Market was $0.95. In the Financing, we issued a total of 5,385,557 shares of common stock and warrants to purchase up to 3,500,612 shares of common stock. The initial exercise price of all of the warrants was greater than the market value of our common stock, and the price per unit was $1.02125. As a result, the Financing was an above market financing under Marketplace Rule 5635(d)(2), and we were not required to seek stockholder approval of the Financing at that time.
However, the Warrants contain two provisions that are important to this situation. The first provision provides full ratchet, anti-dilution protection to the 2010 Investors. Such protection acts to adjust the exercise price of the Warrants (but not the number of shares issuable upon exercise thereof) when we issue any securities at or below the then current exercise price of the Warrants. The second provision provides that we will solicit the affirmative vote of our stockholders at the Meeting for approval of resolutions providing for the elimination of the Floor Price.
Stockholder approval of this Proposal No. 11 is required because, since the exercise price of the Warrants may be reduced below the market price of our common stock at the time of the Financing, the total number of shares of
V-36
common stock potentially issuable for less than the greater of book or market value in the Financing (including the 5,385,557 shares previously issued to the 2010 Investors) would exceed 19.99% of the shares of common stock outstanding prior to the Financing.
If our stockholders do not approve this Proposal No. 11, the Floor Price of the Warrants will remain at $0.94 per share. Regardless of the outcome of this Proposal No. 11, the number of shares issuable upon exercise of the Warrants will remain the same.
Vote Required and Board of Directors Recommendation
Assuming a quorum is present, the affirmative vote of the holders of a majority of the shares present at the annual meeting, either in person or by proxy, and entitled to vote, is required for approval of this Proposal No. 11. For purposes of Proposal No. 11, abstentions will have the same effect as votes against this proposal, and broker non-votes will have no effect on the result of the vote. Shares of common stock acquired by the Investors in the Financing may not be voted in connection with this proposal.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS
VOTE “FOR” PROPOSAL NO. 11.
V-37
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our executive officers and directors, and persons who own more than 10% of a registered class of our equity securities (“Reporting Persons”), to file reports of ownership and changes in ownership with the SEC and with NASDAQ. Such persons are required by the SEC to furnish us with copies of all Section 16(a) forms they file.
Based solely on our review of the reports filed by Reporting Persons, and written representations from certain Reporting Persons that no other reports were required for those persons, we believe that, during the year ended December 31, 2009, the Reporting Persons met all applicable Section 16(a) filing requirements.
Summary Compensation Table
The following table sets forth information regarding compensation earned during 2009 and 2008 by our CEO, our CFO and our other most highly compensated executive officers (“Named Executive Officers”).
SUMMARY COMPENSATION TABLE
| | | | | | | | | | | | | | | | | | |
Name and Principal Position | | Year | | Salary
($) | | Bonus
($) | | Stock
Awards
($) (1) | | Option
Grants
($) (1) | | Non-Equity
Incentive Plan
Compensation
($) | | Change in
Pension
Value and
Non-qualified
Deferred
Compensation
Earnings
($) | | All Other
Compensation
($) (2) | | Total
($) |
J. Michael French, | | 2009 | | 340,000 | | — | | — | | 298,848 | | — | | — | | 102,000 | | 740,848 |
President, CEO and Director (3) | | 2008 | | 176,286 | | — | | — | | 855,162 | | — | | — | | — | | 1,031,448 |
| | | | | | | | | |
Peter S. Garcia, | | 2009 | | 140,961 | | — | | — | | 416,280 | | — | | — | | 45,000 | | 602,241 |
CFO and Secretary (4) | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
Barry Polisky, Ph.D., | | 2009 | | 375,000 | | — | | — | | 228,024 | | — | | — | | 25,000 | | 628,024 |
Chief Scientific Officer (5) | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
Bruce R. York | | 2009 | | 188,461 | | — | | — | | 588,704 | | — | | — | | 272,239 | | 1,049,404 |
Former CFO and Secretary (6) | | 2008 | | 224,156 | | — | | 31,882 | | 276,731 | | — | | — | | 3,735 | | 536,504 |
| (1) | The amounts listed in the Stock Awards and Option Grants columns reflect the dollar amount of the aggregate grant date fair value, in accordance with FASB ASC Topic 718 (formerly SFAS 123(R)). The assumptions used to calculate the stock option awards value may be found in Note 6 to the Consolidated Financial Statements, which is in Part II, Item 8 of our Annual Report on form 10-K for the fiscal year ended December 31, 2009. The dollar amounts do not necessarily reflect the dollar amounts of compensation actually realized or that may be realized by our named executive officers. |
| (2) | The amounts listed in the All Other Compensation column are relocation allowances in the amount of $102,000 to Mr. French and $45,000 to Mr. Garcia, and a $25,000 signing bonus for Dr. Polisky. Such amounts also include a $250,000 severance payment made to Mr. York, plus payments in the amount of $22,083 to Mr. York for accrued unused vacation, each of which was paid out on October 30, 2009, along with 401(k) matching contributions made by us to Mr. York’s 401(k) plan account in the amounts of $156 and $3,735 in 2009 and 2008, respectively. |
| (3) | Mr. French joined our company as CEO on June 23, 2008, became a Director on September 11, 2008 and became President on October 1, 2008. The amount listed under All Other Compensation represents a relocation allowance in the amount of $102,000 approved by the Compensation Committee. |
V-38
| (4) | Mr. Garcia joined our company as CFO effective July 13, 2009. The amount listed under All Other Compensation represents a relocation allowance in the amount of $45,000 approved by the Compensation Committee. |
| (5) | Dr. Polisky joined our company as Chief Scientific Officer on January 2, 2009. The amount listed under All Other Compensation represents a signing bonus in the amount of $25,000. |
| (6) | Mr. York left our company effective October 1, 2009. The 2009 amount in the All Other Compensation column includes $250,000 in cash severance and $22,083 in accrued unused vacation that was paid out on October 30, 2009. The All Other Compensation column also includes 401(k) matching contributions in the amounts of $156 and $3,735 in 2009 and 2008, respectively. The 2009 amount in the Option Grants column includes $439,280, which is the incremental fair value of a modification made to Mr. York’s outstanding equity awards to extend the amount of time to exercise options from 90 days to two years. |
V-39
2009 OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END TABLE
The following table sets forth information regarding the outstanding equity awards held by our Named Executive Officers as of December 31, 2009:
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Option Awards | | Stock Awards |
Name | | | | | Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable | | Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable | | Equity
Incentive
Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options (#) | | Option
Exercise
Price
($) | | Option
Expiration
Date | | Number
of
Shares
or Units
of Stock
That
Have
Not
Vested
(#) | | Market
Value of
Shares
or Units
of Stock
That
Have
Not
Vested
($) | | Equity
Incentive
Plan
Awards:
Number
of
Unearned
Shares,
Units or
Other
Rights
That
Have Not
Vested
(#) | | Equity
Incentive
Plan
Awards:
Market
or Payout
Value of
Unearned
Shares,
Units or
Other
Rights
That
Have Not
Vested
($) |
J. Michael French | | (1 | ) | | 420,000 | | — | | — | | $ | 1.27 | | 6/23/18 | | — | | — | | — | | — |
| | (2 | ) | | 210,000 | | 210,000 | | — | | $ | 2.27 | | 6/23/18 | | — | | — | | — | | — |
| | (3 | ) | | — | | 420,000 | | — | | $ | 3.27 | | 6/23/18 | | — | | — | | — | | — |
| | (4 | ) | | — | | 80,000 | | — | | $ | 1.52 | | 5/20/19 | | — | | — | | — | | — |
| | (5 | ) | | — | | 160,000 | | — | | $ | 1.52 | | 5/20/19 | | — | | — | | — | | — |
| | | | | | | | | | |
Peter S. Garcia | | (6 | ) | | — | | 120,000 | | — | | $ | 1.39 | | 7/13/19 | | — | | — | | — | | — |
| | (7 | ) | | — | | 120,000 | | — | | $ | 2.39 | | 7/13/19 | | — | | — | | — | | — |
| | (8 | ) | | — | | 120,000 | | — | | $ | 3.39 | | 7/13/19 | | — | | — | | — | | — |
| | | | | | | | | | |
Barry Polisky, Ph.D. | | (9 | ) | | — | | 120,000 | | — | | $ | 0.35 | | 1/2/19 | | — | | — | | — | | — |
| | (10 | ) | | — | | 120,000 | | — | | $ | 1.35 | | 1/2/19 | | — | | — | | — | | — |
| | (11 | ) | | — | | 120,000 | | — | | $ | 2.35 | | 1/2/19 | | — | | — | | — | | — |
| | (12 | ) | | — | | 40,000 | | — | | $ | 1.52 | | 5/20/19 | | — | | — | | — | | — |
| | (13 | ) | | — | | 80,000 | | — | | $ | 1.52 | | 5/20/19 | | — | | — | | — | | — |
| | | | | | | | | | |
Bruce R. York | | (14 | ) | | 29,000 | | — | | — | | $ | 2.22 | | 10/1/11 | | — | | — | | — | | — |
| | (14 | ) | | 120,000 | | — | | — | | $ | 1.19 | | 10/1/11 | | — | | — | | — | | — |
| | (14 | ) | | 120,000 | | — | | — | | $ | 2.19 | | 10/1/11 | | — | | — | | — | | — |
| | (14 | ) | | 120,000 | | — | | — | | $ | 3.19 | | 10/1/11 | | — | | — | | — | | — |
| | (14 | ) | | 120,000 | | — | | — | | $ | 1.52 | | 10/1/11 | | — | | — | | — | | — |
| (1) | The options became exercisable on June 23, 2009. |
| (2) | The options vest in four even quarterly increments on September 10, 2009, December 10, 2009, March 10, 2010 and June 10, 2010. |
| (3) | The options vest in four even quarterly increments on September 10, 2010, December 10, 2010, March 10, 2011 and June 10, 2011. |
| (4) | The options vest on May 20, 2010. |
| (5) | The options vest in eight even quarterly increments on August 20, 2010, November 20, 2010, February 20, 2010, May 20, 2011, August 20, 2011, November 20, 2011, February 20, 2012 and May 20, 2012. |
| (6) | The options vest on July 13, 2010. |
| (7) | The options vest in four even quarterly increments on October 13, 2010, January 13, 2011, April 13, 2011 and July 13, 2011. |
| (8) | The options vest in four even quarterly increments on October 13, 2011, January 13, 2012, April 13, 2012 and July 12, 2012. |
| (9) | The options vested on January 4, 2010. |
| (10) | The options vest in four even quarterly increments on April 2, 2010, July 2, 2010, October 2, 2010 and January 3, 2011. |
| (11) | The options vest in four even quarterly increments on April 2, 2011, July 2, 2011, October 2, 2011 and January 3, 2012. |
| (12) | The options vest on May 20, 2010. |
| (13) | The options vest in eight even quarterly increments on August 20, 2010, November 20, 2010, February 20, 2011, August 20, 2011, November 20, 2011, February 20, 2012 and May 20, 2012. |
| (14) | All of the options vested effective October 1, 2009. |
V-40
Employment Agreements
We have entered into employment agreements with each of our Named Executive Officers. These agreements are summarized below and provide that such executive officers shall receive certain payments from us in the event of certain change of control or termination events. For a description of the potential payments upon termination or change of control to be paid to Mr. French, Mr. Garcia and Dr. Polisky, and of the payments paid to Mr. York in connection with his separation from our company, please see “Potential payments upon termination or change in control arrangements” and “2009 Potential Payments upon Termination or Change in Control Tables” below.
J. Michael French
On June 10, 2008, we entered into an employment agreement (the “French Agreement”) with J. Michael French pursuant to which he will serve as our Chief Executive Officer for a term beginning on June 23, 2008 and ending on June 9, 2011. Mr. French was subsequently elected President effective October 1, 2008, and became a Director after election by the Board on September 11, 2008. A copy of the French Agreement was filed as Exhibit 10.2 to our Current Report on Form 8-K dated June 10, 2008.
Pursuant to the French Agreement, Mr. French is entitled to annual base compensation of $340,000, with any increase in base compensation to be set by the Board from time to time as determined by the Board or the Compensation Committee thereof, with the target for each year being the 50th percentile of the Radford survey. He is also eligible to receive annual performance-based incentive cash compensation, with the targeted amount of such incentive cash compensation being 40% of his annual base compensation for the year, but with the actual amount to be determined by the Board or the Compensation Committee. Mr. French also received a relocation allowance in the amount of $102,000.
Under the French Agreement, we granted options to Mr. French to purchase up to 1,260,000 shares of common stock. The options have a term of 10 years beginning on June 23, 2008, and will vest according to the following schedule:
| | • | | 420,000 options became exercisable on June 23, 2009 at an exercise price equal to $1.27 per share, which was the closing price of the common stock on the NASDAQ Global Market on June 23, 2008; |
| | • | | 105,000 options vested or will vest, as applicable, on each of September 10, 2009, December 10, 2009, March 10, 2010 and June 10, 2010 (for an aggregate of 420,000 options during such period) at an exercise price equal to $2.27 per share; and |
| | • | | 105,000 options will vest on each of September 10, 2010, December 10, 2010, March 10, 2011 and June 9, 2011 (for an aggregate of 420,000 options during such period) at an exercise price equal to $3.27 per share. |
If Mr. French’s employment is terminated without cause or he chooses to terminate his employment for good reason, all of Mr. French’s options that are outstanding on the date of termination shall be fully vested and exercisable upon such termination and shall remain exercisable for the remainder of their terms. In addition, he will receive (i) base salary, (ii) incentive cash compensation determined on a pro-rated basis as to the year in which the termination occurs, (iii) pay for accrued but unused paid time off, and (iv) reimbursement for expenses through the date of termination, plus an amount equal to 12 months of his specified base salary at the rate in effect on the date of termination.
If Mr. French’s employment is terminated for cause or he chooses to terminate his employment other than for good reason, vesting of the options shall cease on the date of termination and any then unvested options shall terminate, however the then-vested options shall remain vested and exercisable for the remainder of their respective terms. He will also receive salary, a pro-rated amount of incentive cash compensation for the fiscal year in which the termination occurs, pay for accrued but unused paid time off, and reimbursement of expenses through the date of termination.
V-41
If Mr. French’s employment is terminated due to death or disability, Mr. French or his estate, as applicable, is entitled to receive (i) salary, reimbursement of expenses, and pay for accrued but unused paid time off; (ii) incentive cash compensation determined on a pro-rated basis as to the year in which the termination occurs; and (iii) a lump sum equal to base salary at the rate in effect on the date of termination for the lesser of (a) twelve (12) months and (b) the remaining term of the French Agreement at the time of such termination. In addition, vesting of all of Mr. French’s options that are outstanding on the date of termination shall cease, and any then vested options shall remain exercisable as specified in the applicable grant agreements.
If Mr. French’s employment is terminated by us (other than for cause) or by Mr. French (for good reason), and in either case other than because of death or disability, during the one-year period following a change in control of our company, then Mr. French will be entitled to receive as severance: (i) salary, expense reimbursement and pay for unused paid time off through the date of termination; (ii) a lump-sum amount equal to the greater of (x) twelve (12) months of base salary, and (y) the balance of his base salary to the end of the term of the French Agreement, in each case at the rate in effect on the date of termination; (iii) the amount of his incentive cash compensation for the fiscal year in which the date of termination occurs (determined on a pro-rated basis); and (iv) an additional lump-sum payment equal to fifty percent (50%) of his base salary for such year. In addition, all of Mr. French’s outstanding stock options shall be fully vested and exercisable upon a change of control and shall remain exercisable as specified in the option grant agreements.
Pursuant to the French Agreement, a change in control generally means (i) the acquisition by any person or group of 40% or more of our voting securities, (ii) our reorganization, merger or consolidation, or sale of all or substantially all of our assets, following which our stockholders prior to the consummation of such transaction hold 60% or less of the voting securities of the surviving or acquiring entity, as applicable, (iii) a turnover of the majority of the Board as currently constituted, provided that under most circumstances any individual approved by a majority of the incumbent Board shall be considered as a member of the incumbent Board of Directors for this purpose, or (iv) a complete liquidation or dissolution of us.
The French Agreement also provides that we will, in connection with each election of our directors during the term of the French Agreement, nominate, recommend and use our best efforts to cause the election to the Board of Directors of Mr. French.
In general, Mr. French has agreed not to compete with us for six months following the end of the employment term or to solicit our partners, clients or employees for one year following the end of the employment term. These non-compete and non-solicitation agreements may not be enforceable in some jurisdictions.
Peter S. Garcia
In connection with his appointment as Chief Financial Officer, we entered into an employment agreement (the “Garcia Agreement”) with Mr. Garcia pursuant to which he will serve as our Chief Financial Officer for a three year term beginning on July 13, 2009 (the “Effective Date”). Mr. Garcia was subsequently elected Secretary effective August 11, 2009. A copy of the Garcia Agreement was filed as Exhibit 10.1 to our Current Report on Form 8-K dated July 13, 2009.
Pursuant to the Garcia Agreement, Mr. Garcia is entitled to annual base compensation of $300,000, with any increase in base compensation to be set by the Board of Directors and/or the Chief Executive Officer from time to time as determined by the Board of Directors and/or the Chief Executive Officer. Mr. Garcia is also eligible to receive annual incentive cash compensation, with a target of 30% of his annual base compensation for the year, with the actual amount to be determined by the Board of Directors and/or the Chief Executive Officer.
Moreover, we agreed to pay Mr. Garcia a total of $90,000 in connection with his relocation to the Seattle, Washington metropolitan area, which amount was paid in two payments of $45,000 each through our regular payroll practices on September 15, 2009 and January 29, 2010. We also reimbursed Mr. Garcia for his reasonable
V-42
travel expenses from his home residence in Palo Alto, California to our headquarters in Bothell, Washington until September 2, 2009. Under the Garcia Agreement, we granted to Mr. Garcia options to purchase up to 360,000 shares of the common stock. The options have a term of 10 years beginning on July 13, 2009, and vest according to the following schedule:
| | • | | 120,000 options will vest and become exercisable on July 13, 2010 at an exercise price of $1.39 per share; |
| | • | | 30,000 options will vest and become exercisable on each of October 13, 2010, January 13, 2011, April 13, 2011 and July 13, 2011 (for an aggregate 120,000 options during such period) at an exercise price of $2.39 per share; and |
| | • | | 30,000 options will vest and become exercisable on each of October 13, 2011, January 13, 2012, April 13, 2012 and July 12, 2012 (for an aggregate 120,000 options during such period) at an exercise price of $3.39 per share. |
If we terminate Mr. Garcia’s employment without cause, or Mr. Garcia terminates his employment for good reason, then (i) Mr. Garcia shall be entitled to receive base salary, incentive cash compensation if performance targets established have been met (determined on a pro-rated basis as to the year in which the termination occurs), pay for accrued but unused paid time off, and reimbursement for expenses through the termination date, (ii) a lump sum equal to twelve (12) months of Mr. Garcia’s specified base salary at the rate in effect on the termination date, and (iii) all common stock purchase options granted to Mr. Garcia shall be fully vested and exercisable upon such termination and shall remain exercisable in accordance with the grant agreements, and all shares of restricted stock granted to Mr. Garcia shall become immediately and fully vested.
If we terminate Mr. Garcia’s employment for cause or Mr. Garcia terminates his employment other than for good reason, then (i) Mr. Garcia shall be entitled to receive salary, pay for accrued but unused paid time off, and reimbursement of expenses through the termination date, (ii) the vesting of any outstanding options or shares of restricted stock shall cease on the termination date and (iii) any then un-vested outstanding options shall terminate (with the then-vested outstanding options vested and exercisable as specified in the option grant agreements).
If Mr. Garcia’s employment is terminated due to death or disability, Mr. Garcia (or his estate or legal representative as the case may be) shall be entitled to receive (i) salary, reimbursement of expenses and pay for any unused paid time off accrued through the termination date, (ii) a pro-rated amount of incentive cash compensation for the fiscal year in which the termination date occurs and (iii) a lump sum equal to base salary at the rate in effect on the termination date for the lesser of (x) twelve (12) months and (y) the remaining portion of the initial employment term on the termination date. In addition, vesting of any outstanding options and shares of restricted stock shall cease on the termination date, and any then un-vested outstanding options shall terminate (with the then-vested outstanding options vested and exercisable as specified in the option grant agreements).
In general, Mr. Garcia has agreed: (i) not to compete with our company during the employment term and for twelve (12) months thereafter, (ii) not to solicit any of our partners, consultants, certified research organizations, principal vendors, licensees or employees for twelve (12) months following the end of the employment term, and (iii) not to solicit or accept business from, or perform or supervise the performance of any services related to such business from, certain of our clients, former clients and prospective clients during the employment term and for twelve (12) months thereafter. These non-compete and non-solicitation agreements may not be enforceable in some jurisdictions.
If Mr. Garcia’s employment is terminated either by us or by Mr. Garcia (other than because of Mr. Garcia’s death or disability) following the occurrence of a change of control of our company (as defined in the Garcia Agreement) and the date of such termination is prior to July 13, 2012 and within one (1) year following the occurrence of such change of control, then Mr. Garcia shall be entitled to receive from our company, in lieu of
V-43
the severance payment otherwise payable pursuant to the Garcia Agreement, salary, expense reimbursement and pay for unused paid time off through the termination date. In addition, Mr. Garcia shall be entitled to receive a lump sum amount equal to twelve (12) months of his specified base salary under the Garcia Agreement, and the amount of his incentive cash compensation for the fiscal year in which the termination occurs (determined on a pro-rata basis), plus an additional lump-sum amount equal to 30% of his base salary for such year. Furthermore, notwithstanding the vesting and/or exercisability provisions otherwise applicable to outstanding options and the vesting and restriction provisions applicable to outstanding restricted shares, all such stock options shall be fully vested and exercisable, and all such restricted shares shall be fully vested, upon a change of control and, in the case of options, shall remain exercisable as specified in the option grant agreements, and subject to our right to direct the sale of shares in connection with a change of control.
Barry Polisky, Ph.D.
On October 27, 2008, we entered into an employment agreement (the “Polisky Agreement”) with Dr. Polisky, pursuant to which Dr. Polisky serves as our Chief Scientific Officer for a term beginning on January 2, 2009 (the “Effective Date”) and ending on January 3, 2012. A copy of the Polisky Agreement was filed as Exhibit 10.1 to our Current Report on Form 8-K dated October 27, 2008.
Pursuant to the Polisky Agreement, Dr. Polisky is entitled to annual base compensation of $375,000, with any increase in base compensation to be set by the Board of Directors from time to time as determined by the Board. Dr. Polisky received a one-time signing bonus of $25,000 payable within 30 days of the Effective Date. In addition, Dr. Polisky is eligible to receive annual incentive cash compensation of up to 40 percent of his annual base compensation for the year, with the actual amount to be determined by the Board of Directors or the Compensation Committee.
Under the Polisky Agreement, Dr. Polisky was granted options to purchase up to 360,000 shares of common stock. Dr. Polisky’s options have a term of 10 years beginning on the Effective Date, and vest according to the following schedule:
| | • | | 120,000 options vested and became exercisable on January 4, 2010 at an exercise price of $0.35 per share; |
| | • | | 30,000 options vested and became exercisable, or will vest and become exercisable, as applicable, on each of April 2, 2010, July 2, 2010, October 2, 2010 and January 3, 2011 (for an aggregate 120,000 options during such period) at an exercise price equal to $1.35 per share; and |
| | • | | 30,000 options will vest and become exercisable on each of April 2, 2011, July 2, 2011, October 2, 2011 and January 3, 2012 (for an aggregate 120,000 options during such period) at an exercise price equal to $2.35 per share. |
If Dr. Polisky’s employment is terminated without cause, or upon the expiration of any employment period we fail to offer to renew or extend the employment period (other than if Dr. Polisky shall then have reached our mandatory retirement age), or Dr. Polisky chooses to terminate his employment for good reason, (i) all options granted to Dr. Polisky pursuant to the Polisky Agreement shall be fully vested and exercisable upon such termination and shall remain exercisable in accordance with the grant agreements and (ii) Dr. Polisky will receive an amount equal to 12 months of his specified base salary at the rate in effect on the date of termination.
If Dr. Polisky’s employment is terminated for cause or Dr. Polisky chooses to terminate his employment other than for good reason, vesting of the options shall cease on the date of termination and any then unvested options shall terminate, however the then-vested options shall remain vested and exercisable in accordance with the grant agreements. Regardless of the nature of his separation from our company, Dr. Polisky will also receive salary, a pro-rated amount of incentive cash compensation for the fiscal year in which the termination occurs, pay for accrued but unused paid time off, and reimbursement of expenses through the date of termination.
V-44
In general, Dr. Polisky has agreed not to compete with our company for six months following the end of the employment term or to solicit our partners, vendors or employees for one year following the end of the employment term, unless such employment is terminated by us without cause or by Dr. Polisky for good reason. These non-compete and non-solicitation agreements may not be enforceable in some jurisdictions.
Bruce R. York
On March 7, 2008, we entered into an employment agreement (the “York Agreement”) with Bruce R. York in connection with Mr. York being named our Secretary and Chief Financial Officer, for a term of three years ending on March 7, 2011. A copy of the York Agreement was filed as Exhibit 10.1 to our Current Report on Form 8-K dated March 7, 2008. Mr. York’s employment with our company was terminated effective October 1, 2009.
Pursuant to the York Agreement, Mr. York was entitled to annual base compensation of $250,000, and he was eligible for increases in his base salary as determined by our Board of Directors. Effective for our fiscal year that began on January 1, 2008, and for each calendar year thereafter during the term of the York Agreement, Mr. York’s targeted incentive cash compensation was 40% of his annual base compensation for the year, with the actual amount determined by the Board and our Chief Executive Officer.
Under the York Agreement, in the event that, prior to March 7, 2011, we terminate Mr. York’s employment without cause or if Mr. York terminates his employment as the result of a substantial diminution in his authority or role as Chief Financial Officer, our failure to pay any amounts of base salary and/or incentive cash compensation, our failure to honor promptly any of our other material obligations under the York Agreement, or a material demotion in Mr. York’s title or status, then Mr. York will be entitled to receive as severance a lump sum payment equal to twelve (12) months of his specified base salary at the rate in effect on the date of termination. Upon such event, Mr. York’s options and shares of restricted stock shall become fully vested and such options shall become fully exercisable and shall remain exercisable as specified in the applicable grant agreements.
We amended the York Agreement on July 13, 2009 in connection with Mr. York’s resignation as our Chief Financial Officer and his appointment as our Vice President — Finance and Chief Accounting Officer to provide that, in the event that Mr. York’s employment is terminated on or prior to December 31, 2009 for any reason other than for cause, any and all unvested common stock purchase options held by Mr. York shall immediately vest in full upon the effective date of such termination, and shall remain exercisable for a period of two years thereafter, and any and all unvested shares of restricted common stock held by Mr. York shall immediately vest in full upon the effective date of such termination.
Option repricings
We have not engaged in any option repricings or other modifications, other than as noted related to vesting upon terminations as described in this document, to any of our outstanding equity awards to our Named Executive Officers during fiscal year 2009.
Payments upon Termination or Change-in-Control
The discussion below sets forth the potential termination or change-in-control payments that would be due to certain of our executive officers upon the occurrence of certain specified events. All information described below is presented as if a triggering event occurred on December 31, 2009.
Potential payments upon termination or change in control arrangements
See “Employment Agreements” above for a description of the severance and change in control arrangements for our Named Executive Officers. Each of our Named Executive Officers will be eligible to receive severance
V-45
payments only if each officer signs a general release of claims. The Compensation Committee, as plan administrator of our Stock Option Plans, has the authority to provide for accelerated vesting of options or restricted stock held by our Named Executive Officers and any other person in connection with certain changes in control of our company.
In those employment agreements with our Named Executive Officers containing a change in control provision, subject to certain exceptions, a change in control is generally defined as (i) the acquisition by any person or group of 40% or more of our voting securities, (ii) our reorganization, merger or consolidation, or sale of all or substantially all of our assets, following which our stockholders prior to the consummation of such transaction hold 60% or less of the voting securities of the surviving or acquiring entity, as applicable, (iii) a turnover of the majority of the Board as currently constituted, provided that under most circumstances any individual approved by a majority of the incumbent Board shall be considered as a member of the incumbent Board of Directors for this purpose, or (iv) a complete liquidation or dissolution of our company.
Estimated payments and benefits upon termination
The amount of compensation and benefits payable to each Named Executive Officer who remained an employee of our company on December 31, 2009 under various termination events and circumstances has been estimated in the table below. The amounts shown assume that such termination was effective as of December 31, 2009, our last business day of 2009, and thus includes amounts earned through such time and are estimates of the amounts that would be paid out to the executive officers upon their termination. Amounts under equity awards are determined based on the closing price of our common stock on December 31, 2009, which was $0.81 per share. The actual amounts to be paid out can only be determined at the time of such executive officer’s separation from our company.
Unless otherwise provided by our plan administrator in grant agreements or in employment contracts with our Named Executive Officers, upon termination of a participant’s employment or service, participants generally will forfeit any outstanding awards, except that a participant will have (i) 90 days (but in no event after the original expiration date of the award) following termination of employment or service to exercise any then-vested options and (ii) the earlier of one year or the original expiration of the grant if termination of employment or service is as a result of the participant’s disability or death. In the event of the death or disability of a Named Executive Officer, the Named Executive Officer will receive benefits under our disability plan or payments under our life insurance plan, as appropriate. The terms “cause”, “good reason”, “change of control” and “disability” have the meanings given to such terms in the employment agreements with our Named Executive Officers.
V-46
2009 POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL TABLE
| | | | | | | | | | | | |
| | | Involuntary Not For
Cause Termination
or For Good Reason | | Voluntary or For
Cause | | Death or
Disability | | Termination following
Change-in-Control |
Mr. French | | | | | | | | | | | | |
Lump-sum payment | | $ | 340,000 | | $ | — | | $ | 340,000 | | $ | 489,041 |
Accrued Vacation | | | 32,692 | | | 32,692 | | | 32,692 | | | 32,692 |
Bonus | | | 136,000 | | | 136,000 | | | 136,000 | | | 306,000 |
Stock Options | | | — | | | — | | | — | | | — |
Cobra reimbursement | | | 21,590 | | | — | | | 21,590 | | | — |
| | | | | | | | | | | | |
Total | | $ | 530,282 | | $ | 168,692 | | $ | 530,282 | | $ | 827,733 |
| | | | | | | | | | | | |
Mr. Garcia | | | | | | | | | | | | |
Lump-sum payment | | $ | 300,000 | | $ | — | | $ | 300,000 | | $ | 760,274 |
Accrued Vacation | | | 12,226 | | | 12,226 | | | 12,226 | | | 12,226 |
Bonus | | | 42,288 | | | — | | | 42,288 | | | 180,000 |
Stock Options | | | — | | | — | | | — | | | — |
Cobra reimbursement | | | 21,590 | | | — | | | — | | | — |
| | | | | | | | | | | | |
Total | | $ | 376,104 | | $ | 12,226 | | $ | 354,514 | | $ | 952,500 |
| | | | | | | | | | | | |
Dr. Polisky | | | | | | | | | | | | |
Lump-sum payment | | $ | 375,000 | | $ | — | | $ | 375,000 | | $ | 750,000 |
Accrued Vacation | | | 21,635 | | | 21,635 | | | 21,635 | | | 21,635 |
Bonus | | | 150,000 | | | 150,000 | | | 150,000 | | | 337,500 |
Stock Options | | | 55,200 | | | — | | | — | | | 55,200 |
Cobra reimbursement | | | 21,590 | | | — | | | — | | | — |
| | | | | | | | | | | | |
Total | | $ | 623,425 | | $ | 171,635 | | $ | 546,635 | | $ | 1,164,335 |
| | | | | | | | | | | | |
Lump Sum Payment: The lump sum payments represent contractual payments due to the named executives in accordance with their employment contracts based upon their base salaries in effect as of December 31, 2009:
| | • | | The amounts of $340,000 and $489,041 for Mr. French represent either one year’s pay at the rate in effect on December 31, 2009 or, in the case of a termination following a change-in-control, the amount due through June 9, 2011, the end of his employment contract. |
| | • | | The amount of $300,000 for Mr. Garcia represents one year’s pay at the rate in effect on December 31, 2009, or, in the case of a termination following a change-in-control, the amount due through July 13, 2012, the end of the term specified in his employment contract. |
| | • | | The amounts of $375,000 and $750,000 for Dr. Polisky represent either one year’s pay at the rate in effect on December 31, 2009 or, in the case of a termination following a change-in-control, the amount due through January 3, 2012, the end of his employment contract. |
Accrued Vacation: Accrued vacation amounts represent the unpaid days of personal time off accrued for each named executive officer as of December 31, 2009.
Bonus: All bonus amounts are based upon employment contracts, and are calculated using base salaries in effect as of December 31, 2009. For terminations not involving a change-in-control, bonus amounts are 40% of base salary for each of Mr. French and Dr. Polisky, and 30% of base salary for Mr. Garcia. For terminations following a change-in-control, bonus amounts are (i) one year at 40% and one year at 50% for each of Mr. French and Dr. Polisky, and two years at 30% for Mr. Garcia.
Stock Options: Stock option amounts are valued at $0.81, the closing price on December 31, 2009, less the applicable option exercise price, multiplied by the number of outstanding unvested options assumed to vest on
V-47
such date. As of December 31, 2009, outstanding options to purchase up to 120,000 shares of common stock were in-the-money, all of which were held by Dr. Polisky.
Cobra Reimbursement: Cobra reimbursements represent twelve months of continued company contributions for employer-paid medical insurance.
Payments to Terminated Named Executive Officers
In connection with Mr. York’s separation from our company effective October 1, 2009, and pursuant to the terms of the York Agreement, as amended, we paid to Mr. York severance in the amount of $250,000, all of Mr. York’s unvested options and restricted stock vested effective October 1, 2009, and Mr. York was eligible for COBRA (for which we contributed the employer cost portion for six months).
V-48
COMPENSATION OF DIRECTORS
2009 DIRECTOR COMPENSATION TABLE
| | | | | | | | | | | | | | | | | |
Name | | Fees Earned or
Paid in Cash
($) | | Stock
Awards
($) | | Option
Awards
($) (1) | | Non-Equity
Incentive Plan
Compensation
($) | | Change in
Pension
Value and
Nonqualified
Deferred
Compensation
Earnings
($) | | All Other
Compensation
($) | | Total
($) |
Current Directors | | | | | | | | | | | | | | | | | |
James M. Karis | | $ | 25,500 | | — | | $ | 43,967 | | — | | — | | — | | $ | 69,467 |
Daniel Peters | | | 34,000 | | — | | | 55,918 | | — | | — | | — | | | 89,918 |
James E. Rothman | | | 34,000 | | — | | | 55,918 | | — | | — | | — | | | 89,918 |
Gregory Sessler | | | 38,250 | | — | | | 62,907 | | — | | — | | — | | | 101,157 |
Bruce R. Thaw | | | 55,250 | | — | | | 90,867 | | — | | — | | — | | | 146,117 |
| | | | | | | | | | | | | | | | | |
Subtotal | | $ | 187,000 | | — | | $ | 309,577 | | — | | — | | — | | $ | 496,577 |
| | | | | | | | | | | | | | | | | |
Former Directors (2) | | | | | | | | | | | | | | | | | |
Alexander D. Cross | | | — | | — | | | — | | — | | — | | — | | | — |
John V. Pollock | | | — | | — | | | — | | — | | — | | — | | | — |
Steven C. Quay, M.D., Ph.D. | | | — | | — | | | — | | — | | — | | — | | | — |
| | | | | | | | | | | | | | | | | |
Subtotal | | | — | | — | | | — | | — | | — | | — | | | — |
| | | | | | | | | | | | | | | | | |
Total | | $ | 187,000 | | — | | $ | 309,577 | | — | | — | | — | | $ | 496,577 |
| | | | | | | | | | | | | | | | | |
| (1) | Reflects the dollar amount of the aggregate grant date fair value of the option awards granted to the directors in 2009, in accordance with FASB ASC Topic 718 (formerly SFAS 123 (R)). The assumptions used to calculate the stock option awards value may be found in Note 6 to the Consolidated Financial Statements, Part II, Item 8 of our Annual Report on Form 10-K for the fiscal year ended December 31, 2009. |
| (2) | Dr. Cross, Mr. Pollock and Dr. Quay served as directors of our company until our 2009 Annual Meeting, which was held on May 20, 2009. |
J. Michael French, current director, President and CEO, has not been included in the Director Compensation Tables because he is a Named Executive Officer and does not receive any additional compensation for services provided as a director.
Supplemental Director Award and Option Data Including 2009 Grants and
Outstanding Awards at Year-End
| | | | | | | | | | | | | | |
Name | | 2009 Restricted
Stock Awards
(# shares) | | Fair Value of
2009 Restricted
Stock Awards
($) | | 2009 Stock
Option Grants
(# shares) | | Fair Value of
Options
Granted in
2009
($) (1) | | Aggregate
Number of
Unvested
Restricted
Stock Awards
Outstanding at
December 31,
2009
(# shares) | | Aggregate
Number of
Stock Options
Outstanding at
December 31,
2009
(# shares) | |
Current Directors | | | | | | | | | | | | | | |
James M. Karis | | — | | — | | 34,500 | | $ | 43,967 | | — | | 34,500 | |
Daniel Peters | | — | | — | | 46,000 | | | 55,918 | | — | | 91,000 | |
James E. Rothman | | — | | — | | 46,000 | | | 55,918 | | — | | 195,371 | (2) |
Gregory Sessler | | — | | — | | 51,750 | | | 62,907 | | — | | 111,750 | |
Bruce R. Thaw | | — | | — | | 74,750 | | | 90,867 | | 1,666 | | 190,750 | |
Former Directors | | | | | | | | | | | | | | |
Alexander D. Cross | | — | | — | | — | | | — | | — | | 83,000 | |
John V. Pollock | | — | | — | | — | | | — | | — | | 107,500 | |
Steven C. Quay, M.D., Ph.D. | | — | | — | | — | | | — | | — | | 1,704,247 | |
V-49
| (1) | All of the options granted to our non-executive directors during 2009 were granted on May 20, 2009, the date of our annual meeting of stockholders, when the fair market value was $1.52 per share with the exception of James Karis whose options were granted on August 11, 2009 when the fair market value was $1.53 per share. The weighted average grant date fair value for 2009 option awards was $1.22 per share, calculated using Black Scholes-Merton methodology under FASB ASC Topic 718 (formerly SFAS 123(R)). No grants of restricted stock awards were made to directors during 2009. |
| (2) | Includes vested options to purchase 104,371 shares of common stock granted to Dr. Rothman in connection with his service on our Scientific Advisory Board. |
On May 20, 2009, the Board of Directors approved the recommendations and ratified the determinations of the Nominating and Corporate Governance Committee and authorized us to reduce all aspects of the cash compensation currently paid to non-employee members of the Board by 15%, and to replace the amount of such cash reductions with corresponding increases to the equity grants to such directors. As a result of these changes, the annual cash retainer for non-employee members of the Board was reduced from $30,000 to $25,500, the annual cash retainer for the Chairman of the Audit Committee was reduced from $15,000 to $12,750, the annual cash retainer for each of the Chairman of the Compensation Committee, the Chairman of the Nominating and Corporate Governance Committee and the Scientific Advisor to the Board was reduced from $10,000 to $8,500, and the annual cash retainer for the Chairman of the Board was reduced from $25,000 to $21,250.
Correspondingly, the annual equity grant to non-employee members of the Board was increased from 30,000 options to 34,500 options, the equity grant to the Chairman of the Audit Committee was increased from 15,000 options to 17,250 options, the equity grant to the chairman of the Compensation Committee, the Chairman of the Nominating and Corporate Governance Committee and the Scientific Advisor to the Board was increased from 10,000 options to 11,500 options, and the equity grant to the Chairman of the Board was increased from 25,000 options to 28,750 options.
The Nominating and Corporate Governance Committee recommended, and the Board approved, these changes in director compensation after reviewing the compensation practices of other companies of comparable size in our peer group, and after considering general economic conditions.
Directors’ Stock Compensation Plans. We maintain four compensation plans under which equity compensation awards may be made to directors: the Amended and Restated MDRNA, Inc. 2000 Nonqualified Stock Option Plan (the “2000 Plan”), the MDRNA, Inc. 2002 Stock Option Plan (the “2002 Plan”), the MDRNA, Inc. 2004 Stock Incentive Plan (the “2004 Plan”) and the MDRNA, Inc. 2008 Stock Incentive Plan (the “2008 Plan”). References to the “Director Option Plans” herein refer to the 2000 Plan, the 2002 Plan, the 2004 Plan and the 2008 Plan, collectively. It is our current practice that, upon becoming a member of the Board of Directors, each non-employee director may receive a discretionary award of options to purchase common stock and/or restricted shares of common stock as is determined at such time by the Compensation Committee of the Board of Directors. The discretionary stock option grants under the Director Option Plans are made at an exercise price per share of no less than the “fair market value” (as defined under the Director Option Plans) of a share of common stock on the date the option is granted, and both discretionary stock option and restricted stock grants are generally subject to a vesting period determined by the Compensation Committee in accordance with the applicable Director Option Plan. The Compensation Committee may make additional discretionary grants to eligible directors, consistent with the terms of the Director Option Plans. The Board of Directors may amend, suspend or terminate the Director Option Plans at any time, except that prior approval of our stockholders must be obtained pursuant to applicable NASDAQ rules for any amendments that would constitute a material revision to any of the Director Option Plans, and certain changes require the consent of the affected grantees. In 2009, 253,000 options were granted to the non-employee members of the Board of Directors pursuant to the Director Option Plans. The stock options were granted on May 20, 2009 when the fair market value of the common stock was $1.52 per share, with the exception of the grant to James M. Karis, which was granted on August 11, 2009 when the fair market value of the stock was $1.53 per share.
V-50
Transactions with Related Persons, Promoters and Certain Control Persons
Our Code of Business Conduct and Ethics requires that all employees, including officers and directors, disclose to the CFO the nature of any company business that is conducted with any related party of such employee, officer or director. If the transaction involves an officer or director, the CFO must bring the transaction to the attention of the Audit Committee, which must review and approve the transaction in writing in advance.
V-51
REPORT OF THE AUDIT COMMITTEE OF THE BOARD OF DIRECTORS
The Audit Committee of the Board of Directors, on behalf of the Board of Directors, serves as an independent and objective party to monitor and provide general oversight of the integrity of our financial statements, the independent registered public accounting firm’s qualifications and independence, the performance of the independent registered public accounting firm, the compliance by us with legal and regulatory requirements and our standards of business conduct. The Audit Committee performs these oversight responsibilities in accordance with its Amended and Restated Audit Committee Charter.
Our management is responsible for preparing our financial statements and our financial reporting process. Our independent registered public accounting firm is responsible for performing an independent audit of our consolidated financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). The Audit Committee’s responsibility is to administer and oversee these processes.
The Audit Committee met with the independent registered public accounting firm, with and without management present, to discuss the audit plan, the results of their examinations, and the overall quality of our financial reporting.
In this context, the Audit Committee has reviewed and discussed the audited financial statements for the year ended December 31, 2009 with management and with the independent registered public accounting firm. The Audit Committee has discussed with the independent registered public accounting firm the matters required to be discussed by Statement on Auditing Standards No. 61 (Communications with Audit Committees), which includes, among other items, matters related to the conduct of the audit of our annual financial statements.
The Audit Committee has also received the written disclosures and the letter from the independent registered public accounting firm required by applicable requirements of the Public Company Accounting Oversight Board regarding the independent accountant’s communications with the Audit Committee concerning independence, and has discussed with the independent registered public accounting firm the issue of its independence from us and management. In addition, the Audit Committee has considered whether the provision of non-audit services by the independent registered public accounting firm in 2009 is compatible with maintaining the registered public accounting firm’s independence and has concluded that it is.
Based on its review of the audited financial statements and the various discussions noted above, the Audit Committee recommended to the Board of Directors that the audited financial statements be included in our Annual Report on Form 10-K for the year ended December 31, 2009.
Each of the members of the Audit Committee is independent as defined under the standards of the SEC and NASDAQ, and meets all other requirements of NASDAQ and of such rules of the SEC.
Respectfully submitted by the Audit Committee,
/s/ Gregory Sessler, Chairman
/s/ James M. Karis
/s/ Daniel Peters
/s/ Bruce R. Thaw
The foregoing Audit Committee Report does not constitute soliciting material and shall not be deemed filed or incorporated by reference into any other filing of our company under the Securities Act or the Exchange Act, except to the extent we specifically incorporate this Audit Committee Report by reference therein.
V-52
EQUITY COMPENSATION PLAN INFORMATION
The following table provides aggregate information as of December 31, 2009 about common stock that may be issued upon the exercise of options under all of our equity compensation plans, including the 2000 Plan, the 2002 Plan, the 2004 Plan, the 2008 Plan and the 2007 Employee Stock Purchase Plan (the “ESPP”), along with options granted outside of our equity compensation plans.
| | | | | | | | |
| | | (a) | | | (b) | | (c) |
| | | Number of Securities to be
Issued Upon Exercise of
Outstanding Options | | | Weighted-Average
Exercise Price of
Outstanding Options | | Number of Securities
Remaining Available for
Future Issuance Under
Equity Compensation Plans
(Excluding Securities
Reflected in Column(a)) |
Equity compensation plans approved by security holders | | 6,958,431 | (1) | | $ | 2.12 | | 239,845 |
Equity compensation plans not approved by security holders | | 1,471,800 | (2) | | $ | 3.20 | | 5,433 |
| | | | | | | | |
Total | | 8,430,231 | | | $ | 2.31 | | 245,278 |
| | | | | | | | |
| (1) | Consists of 1,217,193 shares of common stock underlying awards made pursuant to the 2002 Plan, 1,463,217 shares of common stock underlying awards made pursuant to the 2004 Plan and 4,278,021 shares of common stock underlying awards made pursuant to the 2008 Plan. The Board of Directors has delegated authority to the Compensation Committee to serve as administrator of the 2002 Plan, the 2004 Plan, the 2008 Plan and the ESPP. |
| (2) | Consists of 371,837 shares of common stock underlying awards made pursuant to the 2000 Plan and 1,099,963 shares of common stock underlying options awarded to J. Michael French, CEO and President, as an inducement to enter into his employment contract with us in June 2008. Under the 2000 Plan, we are authorized to grant non-qualified stock options to purchase a maximum of 1,000,000 shares of common stock (subject to adjustment in the event of stock splits, stock dividends, recapitalization and other capital adjustments) to our employees, officers, directors and consultants. The Board of Directors has delegated authority to the Compensation Committee to serve as administrator of the 2000 Plan. The Compensation Committee has discretion as to the persons to be granted options, the number of shares subject to the options and the vesting schedules of the options. The 2000 Plan also provides that options shall be exercisable during a period of no more than ten years from the date of grant, and that the option exercise price shall be at least equal to 100% of the fair market value of the common stock on the date of grant. |
V-53
HOUSEHOLDING OF PROXY MATERIALS
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for proxy statements and annual reports with respect to two or more stockholders sharing the same address by delivering a single proxy statement addressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
This year, a number of brokers with account holders who are stockholders of our company will be “householding” our proxy materials. A single proxy statement will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once you have received notice from your broker that they will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in “householding” and would prefer to receive a separate proxy statement and annual report, you should contact your bank, broker or other nominee record holder, or you may contact our Secretary at the address of our principal executive office given under “The Companies” on page I-6.
OTHER MATTERS
We will furnish without charge to each person whose proxy is being solicited, upon the written request of any such person, a copy of our Annual Report on Form 10-K for the fiscal year ended December 31, 2009, as filed with the SEC, including the financial statements. Requests for copies of such Annual Report on Form 10-K should be directed to our Secretary at the address of our principal executive office given under “The Companies” on page I-6.
Our Board of Directors does not know of any other matters that are to be presented for action at the annual meeting. If any other matters are properly brought before the annual meeting or any adjournments thereof, the persons named in the enclosed proxy will have the discretionary authority to vote all proxies received with respect to such matters in accordance with their best judgment.
It is important that the proxies be returned promptly and that your shares are represented at the annual meeting. You are urged to mark, date, execute and promptly return the accompanying proxy card in the enclosed envelope.
V-54
CHAPTER SIX — ADDITIONAL INFORMATION FOR STOCKHOLDERS
FUTURE STOCKHOLDER PROPOSALS
To be considered for inclusion in our notice of annual meeting and proxy statement for, and for presentation at, the 2011 annual meeting of our stockholders, a stockholder proposal must be received by our Secretary at the address of our principal executive office given under “the Companies” on page I-6 no later than February 8, 2011, and must otherwise comply with applicable rules and regulations of the SEC, including Rule 14a-8 of Regulation 14A under the Exchange Act.
Our Bylaws require advance notice of any proposal by a stockholder intended to be presented at an annual meeting that is not included in our notice of annual meeting and proxy statement because it was not timely submitted under the preceding paragraph, or made by or at the direction of any member of the Board of Directors, including any proposal for the nomination for election as a director. To be considered for such presentation at the 2011 annual meeting of our stockholders, any such stockholder proposal must be received by our Corporate Secretary no earlier than March 23, 2011 and no later than May 7, 2011, and discretionary authority may be used if untimely submitted.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and special reports, proxy statements and other information with the SEC. You may read and copy any document we file at the SEC’s Public Reference Room located at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room. We are also required to file electronic versions of these documents with the SEC, which may be accessed from the SEC’s Internet site at http://www.sec.gov or at our website http://www.mdrnainc.com. The information contained on our website is not a part of this proxy statement.
VI-1
Annex A
AGREEMENT AND PLAN OF MERGER
DATED AS OF MARCH 31, 2010
BY AND AMONG
MDRNA, INC.,
CALAIS ACQUISITION CORP.,
CEQUENT PHARMACEUTICALS, INC.,
TABLE OF CONTENTS
iii
iv
SCHEDULES
Company Disclosure Schedule
Purchaser Disclosure Schedule
| | |
| Schedule 1 | | List of Purchaser Management for purposes of knowledge qualifiers |
| Schedule 2 | | List of Company Management for purposes of knowledge qualifiers |
| Schedule 3 | | List of Company Stockholders who are signatories to the Lock-up Agreement |
| Schedule 4 | | List of Purchaser Management and Directors who are signatories to the Lock-up Agreement |
| |
| Exhibit A | | Form of Certificate of Incorporation |
| Exhibit B | | Form of Delaware Certificate of Merger |
| Exhibit C-1 | | Form of Lock-up Agreement for Company Stockholders |
| Exhibit C-2 | | Form of Lock-up Agreement for Purchaser Stockholders |
| Exhibit D | | Form of Accredited Investor Questionnaire |
| Exhibit E | | Form of Voting Agreements for certain Company Stockholders |
| Exhibit F | | Form of Stockholders Agreement Relating to Company Director Nominees |
| Exhibit G | | Form of Registration Rights Agreement |
v
AGREEMENT AND PLAN OF MERGER (this “Agreement”) dated as of March 31, 2010, by and among MDRNA, Inc., a Delaware corporation (“Purchaser”), Calais Acquisition Corp., a Delaware corporation and a wholly-owned subsidiary of Purchaser (“Merger Sub”), and Cequent Pharmaceuticals, Inc., a Delaware corporation (the “Company”).
WHEREAS, the Company is in the business of the development and commercialization of novel therapeutics to prevent and treat a wide range of human diseases, including, without limitation, inflammatory diseases and cancer, based on the Company’s proprietary technology, TransKingdom RNA interference (the “Company Business”); and
WHEREAS, the respective Boards of Directors of Purchaser, Merger Sub and the Company have, subject to the conditions of this Agreement, determined that the Merger (as defined in Section 2.01 below) is advisable and in the best interests of their respective companies and stockholders and approved this Agreement and the transactions contemplated hereby; and
WHEREAS, pursuant to the Merger, Purchaser will acquire all of the outstanding equity securities of Company by way of merger of Company with and into Merger Sub and Purchaser will issue not more than 41,200,000 shares (subject to adjustment in accordance with Section 2.07(j) of the Agreement) of Purchaser Common Stock to the Company (and cash in lieu of fractional shares) in consideration for the Merger, with such shares to include approximately 2,897,629 shares of Purchaser Common Stock subject to stock options and warrants of the Company outstanding on the date of this Agreement;
WHEREAS, within seventy-two (72) hours of the execution and delivery of this Agreement and as a condition and inducement to the parties’ willingness to enter into this Agreement, the stockholders of the Company specified in Section 4.12(b) of this Agreement shall have entered into the Voting Agreement in the form attached hereto asExhibit E, pursuant to which such stockholders will, among other things, agree to vote in favor of the Merger, and the executive officers and directors of Purchaser specified in Section 4.12(a) shall have entered into the Lock-up Agreement within such 72-hour period in the form attached hereto asExhibit C-2;
WHEREAS, Purchaser, Merger Sub and the Company intend, by approving resolutions authorizing this Agreement, to adopt this Agreement as a plan of reorganization within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (the “Code”), and the regulations thereunder, and to cause the Merger to qualify as a reorganization under the provisions of Section 368(a) of the Code;
WHEREAS, Purchaser, Merger Sub and the Company desire to make certain representations, warranties, covenants and agreements in connection with the Merger;
NOW, THEREFORE, in consideration of the mutual premises recited above and the representations, warranties, covenants and agreements contained in this Agreement, the parties hereto, intending to be legally bound, hereby agree as follows:
ARTICLE I
CERTAIN DEFINITIONS
As used in this Agreement each of the following terms shall have the following meaning:
“Acquisition Proposal” means any proposal or offer from any Person or group of Persons (other than the Company, Purchaser, Merger Sub or their Affiliates) relating to:
(a) any merger, consolidation, amalgamation, share exchange, business combination, issuance of securities, acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or other similar transaction: (i) in which the Company (or any subsidiary thereof) is a constituent corporation; (ii) in which a Person or “group” (as defined in the Exchange Act and the rules promulgated thereunder) of Persons directly or indirectly acquires beneficial or record ownership of securities representing more than 20% of the outstanding securities of any class of voting securities of the Company; or (iii) in which the Company issues securities representing more than 20% of the outstanding securities of any class of voting securities of the Company;
(b) any sale, lease, exchange, transfer, license, acquisition or disposition of any business or businesses or assets that constitute or account for: (i) 20% or more of the consolidated net revenues of the Company, or consolidated book value of the assets of the Company; or (ii) 20% or more of the fair market value of the assets (including intangible assets) of the Company; or
(c) any liquidation or dissolution of the Company;provided,however, that the term “Acquisition Proposal” shall not include the Merger or any of the other transactions contemplated by this Agreement.
“Adjudication” shall have the meaning ascribed to such term in Section 2.07(b).
“Affiliate” shall mean an affiliate of an individual or entity as the term “affiliate” is defined in the rules and regulations promulgated under the Securities Act of 1933, as amended.
“Aggregate Merger Consideration Amount” shall mean $46,000,000 minus the Aggregate Option and Warrant Consideration Amount.
“Aggregate Option and Warrant Consideration Amount” shall mean (A) aggregate number of shares of Company Common Stock for which all Company Stock Options and Warrants that are outstanding immediately prior to Effective Time are exercisable (assuming for such purposes that such Company Stock Options and Warrants are fully vested) (the “Aggregate Option and Warrant Common Equivalents”)multiplied by (B) the quotient of (i) $46,000,000, minus the sum of the aggregate Series A-1 Per Share Preference and Series A-1 Accrued Dividends for all shares of Series A-1 Preferred Stock, and plus the Aggregate Option and Warrant Exercise Price Amount and (ii) the sum of the number of outstanding shares of Company Common Stock and Series A-1 Preferred Stock immediately prior to the Effective Time (assuming for purposes of such calculation conversion of the Series A-1 Preferred Stock to Company Common Stock in accordance with the Company Certificate of Incorporation and assuming no unpaid dividends thereon) plus the Aggregate Option and Warrant Common Equivalentsminus (C) the Aggregate Option and Warrant Exercise Amount.
“Aggregate Option and Warrant Exercise Amount” shall mean the aggregate exercise price for the Aggregate Option and Warrant Common Equivalents immediately prior to the Effective Time (assuming for such purposes that such Company Stock Options and Warrants are fully vested).
“Agreement” shall mean this Agreement and Plan of Merger and all schedules and exhibits hereto.
“Ancillary Copyrights” shall mean Intellectual Property constituting unregistered copyrights that either (i) do not embody Proprietary Information or Trade Secrets, or (ii) are not used in the ordinary course of the conduct of the Company Business or Purchaser Business, as applicable.
A-7
“Audit” shall mean any audit, any other examination by any Tax Authority, or any judicial, administrative or other proceeding or litigation (including any appeal of any such judicial, administrative or other proceeding or litigation) relating to Taxes and/or Tax Returns.
“Charter Amendment” shall have the meaning ascribed to such term in Section 4.04(a).
“Closing” shall have the meaning ascribed to such term in Section 2.05.
“Closing Date” shall have the meaning ascribed to such term in Section 3.01.
“Closing Date Tax Period” means any Taxable Periods ending on the Closing Date.
“Code” shall have the meaning ascribed to such term in the preamble of this Agreement.
“Commonly Controlled Entity” shall mean any entity which is under common control with the Company within the meaning of Sections 414(b), (c), (m), (o) or (t) of the Code.
“Company” shall have the meaning ascribed to such term in the preamble of this Agreement.
“Company Business” shall have the meaning ascribed to such term in the first WHEREAS clause of this Agreement.
“Company Certificates” shall have the meaning ascribed to such term in Section 2.07(c).
“Company Certificate of Incorporation” shall mean the Amended and Restated Certificate of Incorporation immediately prior to the Effective Time (and immediately prior to being amended and restated in accordance with Section 2.02).
“Company Common Stock” shall mean each outstanding share of common stock, par value $0.001 per share, of the Company.
“Company Financial Statements” shall have the meaning ascribed to such term in Section 5.07.
“Company Knowledge” means the actual knowledge, after reasonable inquiry, of the persons set forth inSchedule 2.
“Company Leases” shall have the meaning ascribed to such term in Section 5.12(b).
“Company Option” shall have the meaning ascribed to such term in Section 4.09(a).
“Company Preferred Stock” shall mean, collectively, each outstanding share of Series A Preferred Sock and Series A-1 Preferred Stock.
“Company Shares” shall have the meaning ascribed to such term in Section 5.02(a).
“Company Stockholder Approval” shall mean the approval, by the holders of the requisite number of Company Shares, of the Merger and the transactions contemplated by this Agreement.
“Company Warrant” shall have the meaning ascribed to such term in Section 2.07(i).
“Contract” shall mean any contract, agreement, license, commitment, lease, or restriction of any kind to which the Company or Purchaser, as applicable, is a party or by which the Company or Purchaser, as applicable, is bound, including, but not limited to, Third-Party Licenses.
A-8
“Copyrights” shall mean the unregistered copyrights, copyright registrations and applications therefor of the Company or Purchaser, as applicable, all of which are set forth onSchedule 5.13(a) orSchedule 6.10(a).
“CSE Exchange Ratio” shall have the meaning ascribed to such term in Section 2.07(a).
“Delaware Certificate of Merger” shall have the meaning ascribed to such term in Section 2.05.
“DGCL” shall have the meaning ascribed to such term in Section 2.01.
“Dissenting Shares” shall have the meaning ascribed to such term in Section 2.13.
“Effective Time” shall have the meaning ascribed to such term in Section 2.05.
“Employee” and “Employees” shall have the meaning ascribed to such term in Section 5.18.
“Encumbrance” shall mean any claim, mortgage, pledge, lien, security or other third party right or interest of any kind whatsoever, conditional sales agreement, option, encumbrance or charge of any kind affecting real or personal property.
“Environmental Claims” shall mean any and all claims, actions, causes of action, or other written notices by any Person or entity alleging potential liability (including, but not limited to, potential liability for investigatory costs, cleanup costs, governmental response costs, natural resources damages, property damages, personal injuries, or civil or criminal penalties) arising out of or resulting from (i) circumstances forming the basis of any violation of any Environmental Laws or (ii) any releases of Hazardous Materials at any real or personal property presently or formerly owned, leased or managed by the Company or at any disposal facility which may have received Hazardous Materials generated by the Company.
“Environmental Laws” shall mean any applicable federal, state, local or foreign law, treaty, judicial decision, regulation, rule, judgment, order, decree, injunction, permit or governmental restriction, each as in effect on or prior to the Closing Date, relating to the environment.
“Environmental Permits” shall mean Permits required by Environmental Laws.
“ERISA” shall mean the Employee Retirement Income Security Act of 1974, as amended.
“Escrow Agent” shall have the meaning ascribed to such term in Section 2.07(b).
“Escrow Agreement” shall have the meaning ascribed to such term in Section 2.07(b).
“Escrow Balance” shall have the meaning ascribed to such term in Section 2.07(b).
“Escrow Shares” shall have the meaning ascribed to such term in Section 2.07(b).
“Exchange Act” shall mean the Securities Exchange Act of 1934, as amended.
“Exempt Issuance” shall mean the issuance by Purchaser of (1) shares of Purchaser Common Stock and options to purchase shares of Purchaser Common Stock to employees, officers or directors of Purchaser in the ordinary course of business and consistent with past practice pursuant to any stock or option plan duly adopted for such purpose by a majority of the non-employee members of the Purchaser Board or a majority of the members of a committee of non-employee directors established for such purpose; (2) shares of Purchaser Common Stock upon the exercise or exchange of or conversion of any securities exercisable or exchangeable for or convertible into shares of Purchaser Common Stock issued and outstanding on the date of this Agreement; (3) securities of Purchaser, including any warrants and other convertible securities, having a fair market value on
A-9
the date on which definitive agreements are executed of not greater than $5,000,000 in connection with the acquisition by Purchaser of any other entity or the assets of any such entity, or the grant by any other Person to Purchaser of a license to all or a portion of the intellectual property of such Person; or (4) securities of Purchaser, including any warrants and other convertible securities, in a public or private offering of securities to investors having a fair market value on the date on which definitive agreements are executed of not greater than $5,000,000 and at a price of not less than 85% of the fair market value of such securities on the date on which definitive agreements are executed. For purposes of items (3) and (4) of the definition of “Exempt Issuance”, the fair market value of the Purchaser Common Stock shall be deemed to be the last reported closing price of the Purchaser Common Stock on the Nasdaq Global Market prior to the execution of definitive agreements.
“GAAP” shall mean United States generally accepted accounting principles, consistently applied.
“Governmental Authorizations” shall mean all governmental approvals, authorizations, certifications, consents, variances, permissions, licenses, directives, and permits to or from, or filings, notices, or recordings to or with United States federal, state, and local governmental authorities.
“Hazardous Materials” shall include (a) any element, compound, or chemical that is defined, listed or otherwise classified as a contaminant, pollutant, toxic pollutant, toxic or hazardous substance, extremely hazardous substance or chemical, hazardous waste, biohazardous or infectious waste, special waste, or solid waste under Environmental Laws; (b) petroleum, petroleum-based or petroleum-derived products; (c) polychlorinated biphenyls; (d) any substance exhibiting a hazardous waste characteristic including but not limited to corrosivity, ignitability, toxicity or reactivity as well as any radioactive or explosive materials; and (e) any asbestos-containing materials.
“INA” shall have the meaning ascribed to such term in Section 5.18(h).
“ISO’s” shall have the meaning ascribed to such term in Section 4.09(c).
“Intellectual Property” shall mean any and all of the following: (i) Patents; (ii) Trade Secrets; (iii) Copyrights; and (iv) Trademarks.
“Intellectual Property Registrations” shall mean any and all of the following related to the Intellectual Property: (i) issued Patents and applications for Patents; (ii) Copyright registrations and applications to register Copyrights to the extent eligible for registration; (iii) registered Trademarks, applications to register Trademarks, including intent-to-use applications, or other registrations or applications related to Trademarks; and (iv) any other application, certificate (including supplemental protection certificates), filing, registration or other document issued by, filed with, or recorded by, any governmental entity at any time, which document (when so filed or recorded) creates or conveys legally enforceable rights with respect to any Intellectual Property or proprietary right anywhere in the world.
“Investment” shall mean, as applied to any Person, (i) any direct or indirect purchase or other acquisition by such Person of any notes, obligations, instruments, stock, securities or ownership interest (including without limitation partnership interests and joint venture interests) of any Person and (ii) any capital contribution by such Person to any other Person.
“Law” means any federal, state, foreign, material local or municipal or other law, statute, constitution, principle of common law, resolution, ordinance, code, edict, decree, rule, regulation, ruling or requirement issued enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any governmental entity (or under the authority of the NASDAQ Stock Market, Inc. or the Financial Industry Regulatory Authority).
“Leased Tangible Property” shall mean all machinery, furniture, equipment and other tangible personal property, in each case which is subject to a leasehold interest held by the Company or Purchaser, as applicable.
A-10
“Licensed Intellectual Property” shall mean Intellectual Property which the Company or Purchaser, as applicable, has the right to use by agreement (such as a Third-Party License) with a Person (or another Person acting as an authorized representative of such Person) claiming to own (or control the Company’s or Purchaser’s, as applicable, use of) such Intellectual Property.
“Licensed Rights” shall mean the Company’s or Purchaser’s, as applicable, rights to practice or otherwise exploit any intellectual property owned by a third party pursuant to any Contract.
“Material Adverse Effect” shall mean a material adverse effect, either individually or when aggregated with other such effects, on the assets, business, operations, financial condition or results of operations of the Company or Purchaser, as applicable, but excluding changes in the general economy in the United States.
“Material Contract” shall mean the following Contracts:
(a) all Contracts, including, without limitation, broker, distributor, dealer, manufacturer’s representative, franchise, agency, sales promotion, market research, marketing consulting and advertising Contracts to which the Company is a party involving aggregate annual payments by or to the Company of more than $25,000;
(b) all management Contracts (other than employment agreements) and Contracts with other consultants, including any Contracts involving the payment of royalties or other amounts calculated based upon (x) the revenues or income of the Company or (y) the revenues or income of any product of the Company to which the Company is a party, excepting those Contracts that involve aggregate annual consideration payable by the Company of less than $5,000;
(c) all Contracts that limit or purport to limit the ability of the Company or any key executives of the Company (except those between such key executive and the Company) to compete in any line of business or with any Person or in any geographic area or location or during any period of time;
(d) all Contracts obligating the Company to (A) make a future purchase of materials, supplies or equipment that (I) has annual payments by the Company in excess of $25,000, or (II) has a term extending for more than one year from the date of this Agreement, or (B) deliver materials, products or supplies (including sales orders) that (x) has annual payments to the Company in excess of $25,000, or (y) has a term extending for more than one year from the date of this Agreement;
(e) all joint venture Contracts or partnership arrangements involving a sharing of profits, losses, costs or liabilities by the Company;
(f) all contractual obligations to sell or otherwise dispose of any assets;
(g) all Contracts providing for the acquisition by the Company of any Intellectual Property rights from any Person, the grant by any Person to the Company of a license to use any Intellectual Property rights, and the grant by the Company of any Intellectual Property rights to any other Person;
(h) all Contracts between the Company and any other party providing for the acquisition or disposition by the Company (including, without limitation, by merger, consolidation, acquisition or disposition of stock or assets or any other business combination) of any corporation, partnership, other business organization or division thereof or an amount of assets material to the business or to such other party; and
(i) all Contracts under which (i) the Company has any obligation for indebtedness for borrowed money or (ii) the Company has any obligation constituting a guarantee of any indebtedness for borrowed money owing by any other Person.
“Merger” shall have the meaning ascribed to such term in Section 2.01.
“Merger Consideration” shall have the meaning ascribed to such term in Section 2.07(a).
A-11
“Merger Sub” shall have the meaning ascribed to such term in the preamble of this Agreement.
“Option Holder” and “Option Holders” shall mean those individuals who hold Company Options, all of whom are listed onSchedule 5.02 hereof.
“Owned Tangible Property” shall mean all machinery, furniture, fixtures, equipment and other tangible personal property owned by the Company or Purchaser, as applicable.
“Patent Agency” means any federal, national, multinational, state, provincial or local regulatory agency, department, bureau or other governmental entity with authority to grant legally enforceable protection to inventions or discoveries.
“Patents” shall mean all of the Company’s or Parent’s, as applicable, patents, patent applications, utility models, certificates of invention, patents of addition or substitution, and other governmental grants for the protection of inventions anywhere in the world, including any reissue, renewal, re-examination, or extension thereof, and applications for any of the foregoing, including any international, regional, national, provisional, divisional, continuation, continuation in part, continued prosecution, supplemental protection certificates and petty patent applications, all of which are set forth onSchedule 5.13(a) orSchedule 6.10(a).
“Permit” shall mean any license, franchise, permit, consent, order, approval, authorization or registration from, of or with a governmental entity.
“Person” shall mean an individual, partnership, corporation, limited liability company, association, joint stock company, trust, joint venture, unincorporated organization or governmental entity or any department, agency or political subdivision thereof.
“Plan” and “Plans” shall have the meaning ascribed to such term in Section 5.21.
“Post-2006 Period” shall have the meaning ascribed to such term in Section 5.10(j).
“Pre-Closing Straddle Tax Liability” shall mean any Straddle Period Taxes.
“Pre-Closing Tax Periods” shall mean Taxable Periods ending before the Closing Date.
“Principal Stockholder” and “Principal Stockholders” shall mean the stockholders of the Company identified on the signature pages to the Voting Agreement.
“Proceeding” shall mean any notice of investigation or claim or any action, suit, proceeding, hearing, investigation, charge, complaint, claim or demand, pending or threatened, of any nature.
“Proprietary Information” shall mean the technical, commercial, marketing or other information, data and material of the Company or Purchaser, as applicable, as of the Closing Date to the extent that such information, data or material was generally considered by the Company or Purchaser, as applicable, prior to the Effective Date to be confidential or proprietary in nature including any algorithm; procedure; idea; concept; strategic, business and other plan; research; invention or invention disclosure (whether patentable or unpatentable); test, engineering and technical materials, customer lists, know-how, show-how or methodology; trade secret, process, design, formula, software source code and other non-public programming such as applets, assemblers and compilers, and other information or data which has not entered the public domain, and all records or fixations of any of the foregoing, including but not limited to, laboratory notes and software documentation, if any.
“Proxy Statement” shall have the meaning ascribed to such term in Section 4.04(a).
“Purchaser” shall have the meaning ascribed to such term in the preamble of this Agreement.
A-12
“Purchaser Board” shall mean the Board of Directors of Purchaser.
“Purchaser Business” shall mean the business of discovery, development and commercialization of pharmaceuticals based on RNA interference.
“Purchaser Certificates” shall have the meaning ascribed to such term in Section 2.07(c).
“Purchaser Common Stock” shall mean the common stock, par value $0.006 per share, of Purchaser.
“Purchaser Financial Statements” shall have the meaning ascribed to such term in Section 6.21(b).
“Purchaser Knowledge” shall mean the actual knowledge, after reasonable inquiry, of the persons set forth inSchedule 1.
“Purchaser Leases” shall have the meaning ascribed to such term in Section 6.10(b).
“Purchaser Material Contract” shall have the meaning ascribed to such term in Section 6.10(a).
“Purchaser Preferred Stock” shall have the meaning ascribed to such term in Section 6.02(a).
“Purchaser Shares” shall have the meaning ascribed to such term in Section 6.02(a).
“Purchaser Stockholder Approval” shall mean the approval by the stockholders of Purchaser of the Charter Amendment and the Purchaser Stock Issuance at the Purchaser Stockholders’ Meeting.
“Purchaser Stockholders’ Meeting” shall have the meaning ascribed to such term in Section 4.04(a).
“Purchaser Stock Issuance” shall have the meaning ascribed to such term in Section 4.04(a).
“Purchaser Subsidiaries” shall mean, individually or collectively as the context may require, Atossa Healthcare, Inc., a Delaware corporation, MDRNA Research, Inc., a Delaware corporation, and Merger Sub, each of which are wholly-owned Subsidiaries of Purchaser.
“Real Property” means all fee or leasehold interests, easements, real estate licenses, rights to access and other rights with respect to real property.
“Related Documents” shall mean all agreements, instruments, documents and certificates to be executed and delivered pursuant to this Agreement.
“Release” shall mean any spilling, leaking, pumping, emitting, emptying, discharging, injecting, escaping, leaching, migrating, dumping, or disposing of Hazardous Materials (including the abandonment or discarding of barrels, containers or other closed receptacles containing Hazardous Materials) into the environment in violation of any applicable Environmental Law.
“Release Date” shall have the meaning ascribed to such term in Section 2.07(b).
“Required Cash Amount” shall mean, as of the Closing Date, the amount equal to (i) $5,100,000, minus (ii) the amount of the Company’s ordinary course operating expenses from June 2, 2010 through the Closing Date; plus (iii) the difference of $3,000,000 and the aggregate principal amount loaned to the Purchaser by the Company under the Loan Agreement.
“SEC” shall mean the United States Securities and Exchange Commission.
“SEC Documents” shall have the meaning ascribed to such term in Section 6.21.
A-13
“Securities Act” shall mean the Securities Act of 1933, as amended.
“Series A-1 Per Share Accrued Dividends” shall have the meaning as ascribed in Section 2.07(a)(ii).
“Series A-1 Per Share Preference” shall mean one dollar ($1.00) per share.
“Series A Preferred Stock” shall mean each shares of Series A convertible preferred stock, par value $0.001 per share, of the Company.
“Series A-1 Preferred Stock” shall mean each share of Series A-1 convertible preferred stock, par value $0.001 per share, of the Company.
“Signing Date Stock Price” shall mean $1.1496.
“Stockholders” shall mean those Persons who hold shares of (i) Company Common Stock and (ii) Company Preferred Stock.
“Stockholders’ Representative” means Ampersand 2006 Limited Partnership.
“Straddle Period” shall mean any tax period which includes, but does not end, on the Closing Date.
“Subsidiary” shall mean with respect to any Person, each entity of which a majority of the voting power or equity interest is owned, directly or indirectly, by such Person.
“Subsidiary Business” shall have the meaning ascribed to such term in Section 4.03.
“Surviving Corporation” shall have the meaning ascribed to such term in Section 2.01.
“Tangible Property” shall mean the Owned Tangible Property and the Leased Tangible Property.
“Tax” shall mean any federal, territorial, state, local, or foreign income, gross receipts, license, payroll, wage, employment, excise, utility, communications, production, occupancy, severance, stamp, occupation, premium, windfall profits, environmental, customs duties, capital stock, capital levy, franchise, profits, withholding, social security (or similar), unemployment, disability, real property, real property gains, recordation, business license, workers’ compensation, Pension Benefit Guaranty Corporation, personal property, sales, use, transfer, registration, value added, ad valorem, alternative or add-on minimum, estimated, or other tax, fee, charge, premium, imposition of any kind whatsoever in the nature of taxes, however denominated, imposed by any Tax Authority, including any obligation to pay any such amount owed by another Person, together with any interest, penalties or other additions to tax and any interest on any such interest, penalties and additions to tax that may become payable in respect thereof.
“Tax Authority” shall mean the Internal Revenue Service (“IRS”) and any other federal, territorial, state, local or foreign government and any agency, authority or political subdivision of any of the foregoing.
“Tax Law” shall mean the Code, any federal, territorial, state, county, local or foreign laws related to Taxes and any regulations or official administrative pronouncements released under any such laws.
“Tax Returns” shall mean all reports, estimates, declarations of estimated tax, information statements and returns relating to, or required to be filed in connection with, any Taxes, including information returns or reports with respect to backup withholding and other payments to third parties.
A-14
“Taxable Period” means any taxable year or any other period that is treated as a taxable year with respect to which any Tax may be imposed under any Tax Law.
“Third-Party License” shall mean all licenses, agreements, obligations or other commitments under which a Person has granted the Company or Purchaser, as applicable, a right to use any Licensed Intellectual Property in connection with the Company Business or Purchaser Business, as applicable.
“Trademarks” shall mean the trade names, trade dress, logos, common law trademarks and service marks, trademark and service mark registrations and applications therefor, World Wide Web addresses and domain names and applications and registrations therefor, and all goodwill associated with all of the foregoing throughout the world, in each case of the Company or Purchaser, as applicable, all of which are set forth onSchedule 5.13(a) orSchedule 6.10(a).
“Trade Secrets” shall mean non-public information, know-how and other Proprietary Information which, as a result of its not being generally known to the public or to a particular industry, confer upon those to whom it has been disclosed a competitive advantage or other valuable benefit in connection with the conduct of the Company Business or Purchaser Business, as applicable, all of which are set forth on, or generally described in,Schedule 5.13(a) orSchedule 6.10(a).
“Treasury Regulations” shall mean the regulations of the United States Treasury promulgated under the Code.
“USPTO” means the United States Patent and Trademark Office.
ARTICLE II
THE MERGER
2.01The Merger. Subject to the terms and conditions of this Agreement and in accordance with the Delaware General Corporation Law (the “DGCL”), at the Effective Time (as defined in Section 2.05), Merger Sub shall be merged with and into the Company (the “Merger”), and the Company shall be the surviving corporation in the Merger (the “Surviving Corporation”) and shall continue its corporate existence under the Laws of the State of Delaware with all its rights, privileges, immunities, powers and franchises continuing unaffected by the Merger. At the Effective Time, the separate existence of Merger Sub shall cease.
2.02Certificate of Incorporation. At the Effective Time, the certificate of incorporation of the Surviving Corporation shall be amended and restated to read as set forth onExhibit A hereto, and as so amended shall be the certificate of incorporation of the Surviving Corporation until thereafter amended in accordance with applicable Law.
2.03By-Laws. At the Effective Time, the by-laws of Merger Sub, as in effect immediately prior to the Effective Time, shall be the by-laws of the Surviving Corporation until thereafter amended or restated as provided therein or by applicable Law.
2.04Directors and Officers.
(a) At the Effective Time, the directors and officers of Merger Sub immediately prior to the Effective Time shall become the directors and officers of the Surviving Corporation. Each director and officer of the Surviving Corporation shall hold office in accordance with the certificate of incorporation and by-laws of the Surviving Corporation.
(b) Immediately prior to the Effective Time, the Board of Directors of Purchaser shall cause Purchaser’s Board of Directors to consist of no more than seven persons, and with respect to such Board of Directors: (i) to appoint three Company nominees in accordance with the Stockholders Agreement attached
A-15
as Exhibit F (the “Company Nominees”), to the Board of Directors, (ii) to appoint three Purchaser nominees, which may include Purchaser’s directors immediately prior to the Effective Time and (iii) to appoint a nominee selected by both Company and Purchaser, subject in each case to the independence requirements and any other qualification requirements of directors set forth in the applicable Marketplace Rules of the NASDAQ Global Market. In addition, immediately prior to the Effective Time, Purchaser shall take all necessary action so that, following the Effective Time, Peter D. Parker, or another person selected by the directors designated by the Company, shall serve as Chairman of the Purchaser Board, and J. Michael French shall continue to serve as Chief Executive Officer of Purchaser.
2.05Effective Time. The Merger shall be effected by the filing, at the time of the Closing as provided in Section 3.01 (the “Closing”), of a certificate of merger, substantially in the form ofExhibit B hereto (the “Delaware Certificate of Merger”), with the Secretary of State of the State of Delaware in accordance with the provisions of Section 251 of the DGCL. The Merger shall become effective at the time of such filing or at such later time as is set forth in the Delaware Certificate of Merger (the “Effective Time”).
2.06Effect of Merger. At and after the Effective Time, the effect of the Merger shall, in all respects, be as provided in this Agreement, the Delaware Certificate of Merger, and the DGCL. Without limiting the generality of the foregoing, and subject thereto, at the Effective Time, all of the property, rights, privileges, powers and franchises of the Company and Merger Sub shall vest in the Surviving Corporation, and all debts, liabilities and duties of the Company and Merger Sub shall become the debts, liabilities and duties of the Surviving Corporation.
2.07Conversion and Exchange of Capital Stock.
(a)Merger Consideration. At the Effective Time, by virtue of the Merger and automatically without any action on the part of Parent, Company, Merger Sub, or any Stockholder, each share of Series A-1 Preferred Stock and Company Common Stock issued and outstanding immediately prior to the Effective Time, excluding (i) Dissenting Shares and (ii) shares held in the treasury of the Company and all rights in respect thereof, shall, forthwith cease to exist and be converted into and become exchangeable for the right to receive the number of shares of Purchaser Common Stock (the “Merger Consideration”) equal to:
(i) With respect to each outstanding share of Series A-1 Preferred Stock the sum of the following:
(A) the quotient of (1) the sum of the Series A-1 Per Share Preference and the Series A-1 Per Share Accrued Dividends that the holder of such share of Series A-1 Preferred Stock is entitled to receive in accordance with the Company Certificate of Incorporation divided by (2) the Signing Date Stock Price. “Series A-1 Per Share Accrued Dividends” is determined on a share-by-share basis based on date of issuance of the share in accordance with the Company Certificate of Incorporation; plus
(B) the product of the number of shares of Common Stock issuable upon the conversion of such share of Series A-1 Preferred Stock immediately prior to the Effective Time pursuant to the Company Certificate of Incorporation in accordance with the Company Certificate of Incorporation (assuming no unpaid dividends thereon) multiplied by the CSE Exchange Ratio;
(ii) With respect to each outstanding share of Company Common Stock, a number equal to the CSE Exchange Ratio. “CSE Per Share Merger Consideration” shall equal the quotient of (a) the difference of (i) the Aggregate Merger Consideration Amount minus (ii) the sum of the aggregate Series A-1 Per Share Preference and Series A-1 Accrued Dividends for all shares of Series A-1 Preferred Stock and (b) the number of outstanding shares of Company Common Stock and Series A-1 Preferred Stock immediately prior to the Effective Time (assuming for purposes of such calculation conversion of the Series A-1 Preferred Stock to Company Common Stock in accordance with the Company Certificate of Incorporation and assuming no unpaid dividends thereon). The “CSE Exchange Ratio” shall equal the CSE Per Share Merger Consideration divided by the Signing Date Stock Price.
A-16
(b)Escrow Arrangement. At the Effective Time, Purchaser shall deduct from the Merger Consideration such number of shares of Purchaser Common Stock as is equal to ten percent (10%) of the aggregate Merger Consideration (such shares, the “Escrow Shares”), which Purchaser shall deposit with an internationally recognized escrow agent or bank mutually agreed by the parties (the “Escrow Agent”), for the purpose of securing the Company’s indemnification obligations hereunder. The Escrow Shares shall be held by the Escrow Agent in accordance with the terms hereof and of an escrow agreement in a form mutually agreed by the parties (the “Escrow Agreement”). On January 1, 2011 (the “Release Date”), in accordance with the terms of the Escrow Agreement, the Escrow Agent shall release the Escrow Shares and deliver to the Stockholders all of the Escrow Shares, minus such number of Escrow Shares having a value (based on the Signing Date Stock Price) equal to the amount of any Claim or Claims that have been set forth in a Notice pursuant to Article VIII of this Agreement (whether or not such Claim or Claims have been determined to be valid as of such date) (the remaining Escrow Shares after the Release Date that either have not been released to the Stockholders or have not been released to Purchaser in respect of any Claims being referred to herein as the “Escrow Balance”), and the Escrow Balance shall be retained in escrow pending Adjudication of such Claim. “Adjudication” shall mean, unless otherwise agreed among the parties hereto, a final judgment or order of a court of competent jurisdiction not subject to any further appeals. Upon the Adjudication of a Claim, if the Escrow Balance exceeds the aggregate amount of all remaining unresolved Claims, Purchaser shall instruct the Escrow Agent to release, in accordance with the terms of the Escrow Agreement, to the Stockholders such number of Escrow Shares representing such excess from the Escrow Shares retained by the Escrow Agent, it being understood that if at such time the aggregate number of Escrow Shares being retained by the Escrow Agent have a value that is less than or equal to the aggregate amount of all Claims pending at such time, Purchaser shall not be obligated to release any Escrow Shares to the Stockholders,subject,however to the Adjudication of such Claims. The parties agree that the mechanism set forth in this Section 2.07(b) shall be the sole and exclusive remedy available to Purchaser to satisfy any of its rights to indemnification set forth in Article VIII of this Agreement.
(c)Exchange Procedures. Promptly (but in any event no more than five (5) business days) after the Effective Time, Purchaser shall mail to each holder of record of certificates of Company Common Stock and Company Preferred Stock (collectively, “Company Certificates”), whose shares were converted into the right to receive shares of Purchaser Common Stock (and cash in lieu of fractional shares pursuant to Section 2.09): (i) a letter of transmittal in form and substance satisfactory to the Company, such approval not to be unreasonably withheld (which shall specify that delivery shall be effected, and risk of loss and title to the Company Certificates shall pass, only upon receipt of the Company Certificates by Purchaser, and shall be in such form and have such other provisions as Purchaser may reasonably specify); and (ii) instructions for use in effecting the surrender of the Company Certificates in exchange for the applicable Merger Consideration. Upon surrender of a Company Certificate for cancellation to Purchaser or to such agent or agents as may be appointed by Purchaser, together with such letter of transmittal, duly completed and validly executed, and such other documents as may be reasonably required by Purchaser, the holder of such Company Certificate shall be entitled to receive in exchange therefor a certificate of Purchaser Common Stock (“Purchaser Certificates”) representing the number of whole shares of Purchaser Common Stock that such holder has the right to receive pursuant to this Article II (together with payment of cash in lieu of fractional shares which such holder has the right to receive pursuant to Section 2.09) and the Company Certificate so surrendered shall forthwith be canceled. Until so surrendered, each outstanding Company Certificate that, prior to the Effective Time, represented shares of Company Common Stock or Company Preferred Stock, as the case may be, will be deemed from and after the Effective Time, for all purposes other than the payment of dividends and distributions, to evidence the ownership of the number of full shares of Purchaser Common Stock into which such shares of Company Common Stock or Company Preferred Stock, as the case may be, shall have been so converted (together with payment of cash in lieu of fractional shares which such holder has the right to receive pursuant to Section 2.09). Notwithstanding any other provision of this Agreement, no interest will be paid or will accrue on any cash payable to holders of Company Certificates pursuant to the provisions of this Article II.
A-17
(d)Distributions With Respect to Unexchanged Shares. No dividends or other distributions with respect to Purchaser Common Stock with a record date after the Effective Time will be paid to the holder of any unsurrendered Company Certificate with respect to the shares of Purchaser Common Stock represented thereby until such holder surrenders such Company Certificate. Subject to the effect of applicable escheat or similar Laws, following the surrender of any such Company Certificate, there shall be paid to the record holder of the Purchaser Certificates issued in exchange therefor, without interest, (i) at the time of such surrender, the amount of any such dividends or other distributions with a record date after the Effective Time theretofore payable (but for the provisions of this Section 2.07(d)) with respect to such shares of Purchaser Common Stock and (ii) at the appropriate payment date the amount of dividends or other distributions with a record date after the Effective Time but prior to such surrender and with a payment date subsequent to such surrender payable with respect to such whole Purchaser Common Stock.
(e)Transfer of Ownership. If any Purchaser Certificate is to be issued in a name, or cash in lieu of fractional shares paid to a person, other than that in which the Company Certificate surrendered in exchange therefor is registered, it will be a condition of the issuance and/or payment thereof that the Company Certificate so surrendered will be properly endorsed and otherwise in proper form for transfer and that the person requesting such exchange will have paid to Purchaser or any agent designated by it any transfer or other Taxes required by reason of the issuance of a Purchaser Certificate for shares of Purchaser Common Stock in any name other than that of the registered holder of the Company Certificate surrendered, or established to the satisfaction of Purchaser or any agent designated by it that such Tax has been paid or is not payable.
(f)Stockholders’ Rights. At and after the Effective Time, the Stockholders shall cease to have any rights as stockholders of the Company, and the Company’s capital stock shall cease to be outstanding and shall be cancelled and retired.
(g)Treasury Stock. Each share of capital stock of the Company held in the Company’s treasury, and each share of capital stock of the Company owned by Purchaser or any wholly-owned subsidiary of Purchaser, in each case immediately prior to the Effective Time shall, by virtue of the Merger and without any action on the part of the Company, be cancelled and retired and cease to exist, without any conversion thereof or any payment of any consideration therefor.
(h)Treatment of Stock Options. All options to purchase Company Common Stock then outstanding under the Company’s 2006 Stock Incentive Plan shall be assumed by Purchaser in accordance with Section 4.09.
(i)Treatment of Warrants. All warrants outstanding at the Effective Time to purchase Company Common Stock (collectively, the “Company Warrants”), shall be treated in accordance with Section 4.09.
(j)Adjustment for Additional Cash. If, as of the Closing Date, the Company has available more than the Required Cash Amount, then Purchaser may determine in its sole discretion to accept such additional cash, in exchange for adjusting the CSE Exchange Ratio to reflect such additional value, as may be agreed upon by the parties. If Purchaser does not decide to make such adjustment or the parties cannot agree on the amount of such adjustment, then, notwithstanding anything in Section 4.02, Company shall have the right to distribute (by means of a dividend or otherwise), immediately prior to the Effective Time, such excess Required Cash Amount to the Company stockholders, in accordance with its certificate of incorporation in effect at such time.
(k)Treatment of Merger Sub Capital Stock. Each share of capital stock of Merger Sub issued and outstanding immediately prior to the Effective Time shall, by virtue of the Merger and without any action on the part of the holder thereof, be converted into one validly issued, fully paid and non-assessable share of common stock of the Surviving Corporation.
(l)Limitations. Notwithstanding anything contained in this Section 2.07, the aggregate number of shares of Purchaser Common Stock issuable under this Section 2.07 (as a result of the conversion of the Company Shares and the exercise of the options and warrants, if any, to purchase Company Common Stock
A-18
or Company Preferred Stock assumed by Purchaser) shall in no event exceed forty-nine and ninety-nine hundredths percent (49.99%) of the outstanding shares of Purchaser Common Stock immediately after the Effective Time.
2.08Stock Transfer Books. As of the Effective Time, the stock transfer books of the Company shall each be closed, and there shall be no further registration of transfers of shares of Company Common Stock thereafter on the records of any such stock transfer books. In the event of a transfer of ownership of shares of Company Common Stock that is not registered in the stock transfer records of the Company at the Effective Time, a certificate or certificates representing the number of full shares of Purchaser Common Stock into which such shares of Company Common Stock, as the case may be, shall have been converted, if any, shall be issued to the transferee together with a cash payment in lieu of fractional shares, if any, in accordance with Section 2.09 hereof, and a cash payment in the amount of dividends, if any, in accordance with Section 2.07(d) hereof, if the certificate or certificates representing such shares of Company Common Stock, as the case may be, is or are surrendered as provided in Section 2.07(c) hereof, accompanied by all documents required to evidence and effect such transfer and by evidence of payment of any applicable stock transfer tax.
2.09No Fractional Share Certificates. No scrip or fractional share Purchaser Certificate shall be issued upon the surrender for exchange of Company Certificates, and an outstanding fractional share interest shall not entitle the owner thereof to vote, to receive dividends or to any rights of a stockholder of Purchaser or of the Surviving Corporation with respect to such fractional share interest. As promptly as practicable following the Effective Time, Purchaser shall pay each holder of Company Common Stock and Company Preferred Stock, as applicable, net of withholding taxes, an amount in cash, rounded to the nearest whole cent, equal to the product obtained by multiplying (i) the fractional share interest to which such holder would otherwise be entitled (after taking into account all shares of Company Common Stock and Company Preferred Stock held at the Effective Time by such holder) by (ii) the Signing Date Stock Price.
2.10Certain Adjustments. If between the date of this Agreement and the Effective Time, the outstanding shares of Purchaser Common Stock or Company Common Stock shall be changed into a different number of shares by reason of any reclassification, recapitalization, split-up, combination or exchange of shares, or any dividend payable in stock or other securities shall be declared thereon with a record date within such period, then the CSE Exchange Ratio shall be adjusted accordingly to provide to Purchaser and the Company the same economic effect as contemplated by this Agreement prior to such reclassification, recapitalization, split-up, combination, exchange or dividend.
2.11Lost, Stolen or Destroyed Certificates. In the event any Company Certificates shall have been lost, stolen or destroyed, Purchaser shall issue in exchange for such lost, stolen or destroyed Company Certificates, upon the making of an affidavit of that fact by the holder thereof, such shares of Purchaser Common Stock (and cash in lieu of fractional shares) as may be required pursuant to Section 2.07(a),provided,however, that Purchaser may, in its discretion and as a condition precedent to the issuance thereof, require the owner of such lost, stolen or destroyed Company Certificates to indemnify Purchaser against any claim that may be made against Purchaser or the Surviving Corporation with respect to the Company Certificates alleged to have been lost, stolen or destroyed.
2.12Required Deduction or Withholding. Each of Purchaser and the Surviving Corporation shall be entitled to deduct and withhold from the consideration otherwise payable to any holder of Company Common Stock pursuant to this Agreement such amounts as may be required to be deducted or withheld with respect to the making of such payment or any other payment in connection with the transactions contemplated by this Agreement under the Code or any applicable provision of state, local or foreign Tax Law. To the extent that amounts are so deducted or withheld and paid over to the appropriate taxing authority by Purchaser or the Surviving Corporation, such amounts shall be treated for all purposes of this Agreement as having been paid to the person to whom such amounts would otherwise have been paid.
A-19
2.13Dissenting Shares. Notwithstanding anything in this Agreement to the contrary, shares of the Company’s capital stock outstanding immediately prior to the Effective Time and held by a Stockholder who has not voted in favor of the Merger or consented thereto in writing and who has demanded appraisal for such shares in accordance with Section 262 of the DGCL, if such Section 262 provides for appraisal rights for such capital stock in the Merger (“Dissenting Shares”), shall not be converted into the right to receive a pro rata portion of the Merger Consideration as provided in Section 2.07, unless and until such holder fails to perfect or withdraws or otherwise loses his or her right to appraisal and payment under the DGCL. If, after the Effective Time, any such holder fails to perfect or withdraws or loses his or her right to appraisal, such Dissenting Shares shall thereupon be treated as if they had been converted as of the Effective Time into the right to receive a pro rata portion of the Merger Consideration, if any, to which such holder is entitled, without interest or dividends thereon. The Company shall give Purchaser prompt notice of any demands received by the Company for appraisal of capital stock and, prior to the Effective Time, Purchaser shall have the right to participate in all negotiations and proceedings with respect to such demands. Prior to the Effective Time, the Company shall not, except with the prior written consent of Purchaser, make any payment with respect to, or settle or offer to settle, any such demands.
2.14Additional Actions. If, at any time after the Effective Time, the Surviving Corporation shall consider or be advised that any further assignments or assurances in law or any other acts are reasonably necessary or desirable (a) to vest, perfect or confirm, of record or otherwise, in the Surviving Corporation, title to and possession of any property or right of the Company acquired or to be acquired by reason of, or as a result of, the Merger, or (b) otherwise to carry out the purposes of this Agreement, the Company and its proper officers and directors shall be deemed to have granted to the Surviving Corporation an irrevocable power of attorney to execute and deliver all such proper deeds, assignments and assurances in law and to do all acts necessary or proper to vest, perfect or confirm title to and possession of such property or rights in the Surviving Corporation and otherwise reasonable to carry out the purposes of this Agreement; and the proper officers and directors of the Surviving Corporation are fully authorized in the name of the Company or otherwise to take any and all such action.
ARTICLE III
CLOSING AND PAYMENT OBLIGATION
3.01Closing. The consummation of the Merger contemplated by this Agreement (the “Closing”) shall be held at the offices of Pryor Cashman LLP, 7 Times Square, New York, New York, at 11:00 A.M., local time, no later than the fifth (5th) business day following the satisfaction or waiver (by Purchaser and Merger Sub, in the case of the conditions in Section 7.01 and by the Company in the case of the conditions in Section 7.02) of all of the conditions to Closing set forth in Article VII hereof, or such other time, place and date as may be mutually agreed upon by the parties hereto. The date of the Closing is sometimes herein referred to as the “Closing Date”. By mutual agreement of the parties, the Closing may be alternatively accomplished by facsimile transmission to the respective offices of legal counsel for the parties of the requisite documents, duly executed where required, with originals to be delivered by overnight courier service on the next business day following the Closing.
3.02Deliveries by Company. The Company agrees to deliver (or cause to be delivered) to Purchaser and Merger Sub at the Closing on the Closing Date the following agreements and documents, all reasonably satisfactory in form and substance to Purchaser, Merger Sub and their legal counsel:
(a) a certificate of good standing and/or subsistence, dated as of a recent date prior to the Closing, issued by the Secretary of State of the State of Delaware and of each other jurisdiction in which the Company is required to be qualified to do business;
(b) all corporate minute and stock books, stock ledgers and corporate seals of the Company;
(c) written resignations of all officers and members of the Board of Directors of the Company;
A-20
(d) evidence of receipt of all consents set forth onSchedule 5.06;
(e) a certificate of an officer of the Company in a form approved in advance by Purchaser, dated the Closing Date, certifying that attached thereto is (A) a true, correct and complete certified copy of the Certificate of Incorporation of the Company, (B) a true, correct and complete copy of the by-laws of the Company, and (C) a true, correct and complete copy of any resolutions adopted by the Board of Directors of the Company or the Stockholders relating to this Agreement or the transactions contemplated hereby, in each case as are then in full force and effect;
(f) a duly executed Lock-Up Agreement signed by each of the Principal Stockholders, in the form ofExhibit C-1 hereto;
(g) Accredited Investor Questionnaires duly signed and completed by all Company Stockholders in the form ofExhibit D hereto;
(h) a duly executed Voting Agreement signed by each of the Principal Stockholders, in the form ofExhibit E hereto;
(i) a duly executed Escrow Agreement signed by the Company, in the form ofExhibit H hereto;
(j) such other documents and instruments as may be reasonably required to effectuate the terms of this Agreement and to comply with the terms hereof.
3.03Deliveries by Purchaser and Merger Sub. Subject to the terms and conditions of this Agreement, Purchaser and Merger Sub agree to deliver at the Closing to the Company and the Stockholders’ Representative the following:
(a) the Merger Consideration;
(b) a duly executed Escrow Agreement signed by Purchaser, in the form ofExhibit H hereto; and
(c) such other documents and instruments as may be reasonably required to effectuate the terms of this Agreement and to comply with the terms hereof.
ARTICLE IV
ADDITIONAL AGREEMENTS
4.01Investigation. During the period commencing on the date of this Agreement and ending upon the earlier to occur of the termination of this Agreement and the Effective Time, each party to this Agreement agrees to cooperate fully with the other parties to this Agreement and to give to such other parties, their officers, employees, auditors, legal counsel, representatives and agents reasonable access during normal business hours to all such information, documents, premises and employees as the requesting party reasonably considers necessary or advisable for purposes of its investigation of Purchaser, the Company and the Company Business, as applicable. The parties hereto agree to consult with each other in an effort to establish procedures designed to implement the provisions of this Section 4.01 in order to minimize disruption to Purchaser, the Company and the Company Business, as applicable. Pending the Closing, each party hereto shall preserve the confidentiality of any information provided to such party relating to Purchaser, the Company and the Company Business which is confidential in nature. In the event of termination of this Agreement, the parties hereto agree to maintain the confidentiality of such information except to the extent that such item:
(a) is or becomes publicly known or generally known in the industry through no act of the receiving party;
(b) is required to be disclosed to or by order of a governmental agency or a court of law or otherwise as required by Law; provided that prior to any such disclosure notice of such requirement of disclosure is provided to the disclosing party and the disclosing party is afforded the reasonable opportunity to object to such disclosure; or
A-21
(c) is required to be disclosed to the parties’ attorneys, accountants or other agents or employees working on this transaction.
4.02Conduct of the Company’s Business Pending the Closing. The Company agrees that from the date hereof through the Closing Date, the Company Business will be conducted only in the ordinary course consistent with past practice, and, except as may be permitted by this Agreement, or as otherwise shown onSchedule 4.02 or approved in writing in advance by Purchaser, the Company agrees as follows:
(a) The Company will, so far as it is within its power to do so, carry on the Company Business diligently and substantially in the same manner as heretofore conducted, and the Company shall not institute any new methods of acquisition, production, marketing, distribution, sale, lease, license, management, operation, or engage in any transaction or activity, enter into any agreement or make any commitment, except in the ordinary course of business and consistent with past practice.
(b) The Company shall not institute any new methods of accounting except in accordance with generally accepted accounting principles.
(c) The Company shall use its commercially reasonable efforts to preserve the Company Business intact, to protect and preserve the Company’s assets, and to preserve for Purchaser the Company’s relationships with licensors, suppliers, distributors, customers, contractors and employees.
(d) Except as required pursuant to (i) applicable Law, (ii) any Contract in existence prior to the date hereof binding upon the Company, or (iii) by this Agreement, the Company shall not:
(i)(A) borrow or agree to borrow any funds or (B) incur, or assume or become subject to, whether directly or by way of guarantee or otherwise, any obligation or liability (absolute or contingent), except in the case of clause (B) obligations and liabilities incurred in the ordinary course of business and consistent with past practice;
(ii) permit or allow any of its assets to be subjected to any Encumbrance of any kind or description, other than Encumbrances in existence on the date hereof or mechanics liens or similar Encumbrances for service or work in process;
(iii) sell, assign, transfer, license, dispose of or permit to lapse any rights to the use of any Intellectual Property (and the Company shall take all actions necessary in respect of any infringement of any Intellectual Property of which it has Company Knowledge), or dispose of or disclose to any Person any trade secret, formula, process or know how not theretofore a matter of public knowledge;
(iv) make any single capital expenditure or future commitment in excess of $20,000 for additions to property, plant or equipment or make aggregate capital expenditures or future commitments in excess of $50,000 for additions to property, plant or equipment;
(v) pay, loan or advance any amount to, or sell, transfer or lease any properties or assets to, or enter into any agreement or arrangement with, any Affiliate;
(vi) change any of the Company’s banking or safe deposit arrangements;
(vii) grant or extend any power of attorney or act as guarantor, surety, co-signer, endorser, co maker, indemnitor or otherwise in respect of the obligation of any Person;
(viii) pay any amounts (whether in cash or property) to any employee of the Company other than in the ordinary course of business and consistent with past practice or pursuant to any Contract;
(ix) grant to any officer or employee any increase in compensation or benefits, other than increases in compensation or benefits for employees in the ordinary course of business and consistent with past practice;
(x) pay any pension, retirement allowance or other employee benefit not required by any plan, policy or program identified onSchedule 5.21(a) hereto;
A-22
(xi) adopt, agree to adopt, or make any announcement regarding the adoption of (i) any new pension, retirement or other employee benefit plan, policy or program or (ii) any amendments to any existing plan, policy or program identified onSchedule 5.21(a) unless required by applicable Law;
(xii)(A) declare, set aside or pay any dividend or distribution that is payable in cash, stock, or other property with respect to the Company; (B) redeem, purchase or otherwise acquire, directly or indirectly, any shares of the Company, or any other securities thereof or any rights, warrants, or options to acquire any such shares or other securities; (C) other than as set forth at the end of this Section 4.02, authorize for issuance, issue, sell, pledge, deliver or agree to commit to issue, sell or pledge (whether through the issuance or granting of any options, warrants, calls, subscriptions, equity appreciation rights or other rights or other agreements) any equity or debt securities of the Company, or any other securities that are convertible into or exchangeable for shares of any class of the company; or (D) split, combine or reclassify the outstanding shares of the Company, or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of any equity securities of the Company;
(xiii) adjust, settle or compromise any claim, obligation, debt, demand, suit or judgment against or on behalf of the Company, except for claims, obligations, debts, demands, suits or judgments that do not involve an Affiliate of the Company and do not exceed more than $20,000 in any single instance or more than $50,000 in the aggregate; or
(xiv) agree, whether in writing or otherwise, to do any of the foregoing.
(e) No contract or commitment will be entered into, and no purchase of supplies and no sale, lease, license, assignment, transfer or disposition of any of the Company’s assets will be made, by or on behalf of the Company, except (i) normal contracts or commitments for the purchase of, and normal purchases of, supplies or inventory or for the sale of inventory, in each case made in the ordinary course of business and consistent with past practice, and (ii) other contracts, commitments, purchases or sales in the ordinary course of business, consistent with past practice and involving assets having a value not in excess of $20,000.
(f) The Company shall maintain the same insurance coverage (to the extent commercially reasonable) set forth onSchedule 5.17 hereto and all property insured thereby shall be used, operated, maintained and repaired in the ordinary course of business and consistent with past practice.
(g) The Company shall not do any act or omit to do any act, or permit any act or omission to act, which will cause a breach of any Material Contract or commitment of the Company or which would cause the breach by the Company of any representation, warranty, covenant or agreement made hereunder.
(h) The Company shall duly comply in all material respects with all Laws applicable to it and its properties, operations, business and employees.
(i) The Company shall file all Federal, state, local and foreign Tax Returns and amendments thereto required to be filed by them and shall pay all Taxes shown as due and payable thereon. All returns and reports in respect of employee withholdings, FICA, unemployment and other similar items and other applicable Taxes shall be timely made as shall all deposits and payments due in respect of such Taxes and obligations.
(j) The Company has provided the Purchaser with an operating budget for the expected period from the date hereof through the Closing Date, as set forth inSchedule 4.02(j), and agrees it will operate its business according to such operating budget in all material respects.
Notwithstanding anything to the contrary in this Section 4.02, this Section 4.02 shall not prohibit the Company from doing any of the following between the date hereof through the Closing Date:
(1) authorize, issue, and sell additional shares of Company capital stock, or debt or warrants convertible into capital stock of the Company, to new and existing investors in the Company, including but not limited through the sale of up to $7,520,000 of Series A-1 Preferred Convertible Stock of the Company;
A-23
(2) accelerating the vesting of outstanding Company Stock Options as described inSchedule 5.21(e); and
(3) loan money to the Purchaser as contemplated under that certain Loan Agreement being entered into concurrently with this Agreement between Purchaser and the Company (the “Loan Agreement”) pursuant to the terms of the Loan Documents (as defined in such Loan Agreement).
4.03Conduct of Purchaser’s Business Pending the Closing. Purchaser agrees that from the date hereof through the Closing Date, the Purchaser Business and the respective businesses of each Purchaser Subsidiary (each, a “Subsidiary Business”) will be conducted only in the ordinary course consistent with past practice, and, except as may be permitted by this Agreement, or as otherwise shown onSchedule 4.03 or approved in writing in advance by the Company, Purchaser agrees as follows:
(a) Purchaser will, so far as it is within its power to do so, carry on the Purchaser Business (and will cause each Purchaser Subsidiary to carry on its respective Subsidiary Business) diligently and substantially in the same manner as heretofore conducted, and Purchaser shall not (and shall not permit any Purchaser Subsidiary to) institute any new methods of acquisition, production, marketing, distribution, sale, lease, license, management, operation, or engage in any transaction or activity, enter into any agreement or make any commitment, except in the ordinary course of business and consistent with past practice.
(b) Purchaser shall not (and shall not permit any Purchaser Subsidiary to) institute any new methods of accounting except in accordance with generally accepted accounting principles.
(c) Purchaser shall use (and shall cause each Purchaser Subsidiary to use) its commercially reasonable efforts to preserve the Purchaser Business and each Subsidiary business intact, to protect and preserve Purchaser’s and each Purchaser Subsidiary’s assets, and to preserve for the Company Purchaser’s and each Purchaser Subsidiary’s relationships with licensors, suppliers, distributors, customers, contractors and employees.
(d) Except as required pursuant to (i) applicable Law (ii) any Contract in existence prior to the date hereof binding upon Purchaser, or (iii) this Agreement, Purchaser shall not (and shall not permit any Purchaser Subsidiary to):
(i) (A) borrow or agree to borrow any funds or (B) incur, or assume or become subject to, whether directly or by way of guarantee or otherwise, any obligation or liability (absolute or contingent), except in the case of clause (B) obligations and liabilities incurred in the ordinary course of business and consistent with past practice;
(ii) permit or allow any of its assets to be subjected to any Encumbrance of any kind or description, other than Encumbrances in existence on the date hereof or contemplated hereby, or mechanics liens or similar Encumbrances for service or work in process, and other than in connection with the transactions contemplated by this Agreement;
(iii) sell, assign, transfer, license, dispose of or permit to lapse any rights to the use of any Intellectual Property (and Purchaser shall take (and shall cause each Purchaser Subsidiary to take) all actions necessary in respect of any infringement of any Intellectual Property of which it has Purchaser Knowledge), or dispose of or disclose to any Person any trade secret, formula, process or know how not theretofore a matter of public knowledge, other than (x) any sale, assignment, transfer, license or other disposition of any of Purchaser’s legacy intranasal assets, and (y) licenses by Purchaser of any of its Intellectual Property in the ordinary course of Purchaser’s Business consistent with past practice (other than licenses solely to Purchaser’s Di-Alkylated Amino Acid delivery technology);provided,however, that nothing in item (y) above shall prohibit Purchaser from licensing any of its Intellectual Property, including its Di-Alkylated Amino Acid delivery technology, in connection with any multi-year target-based or therapeutic-based collaboration between Purchaser and a pharmaceutical company substantially similar to those collaborations previously discussed with the Company;
A-24
(iv) make any single capital expenditure or future commitment in excess of $100,000 for additions to property, plant or equipment, make aggregate capital expenditures or future commitments in excess of $200,000 with respect to Purchaser and the Purchaser Subsidiaries as a whole for additions to property, plant or equipment, or make any expenditure with respect to the acquisition of the intellectual property of a third party in excess of $250,000 with respect to Purchaser and the Purchaser Subsidiaries as a whole (but not including future commitments with respect thereto);
(v) pay, loan or advance any amount to, or sell, transfer or lease any properties or assets to, or enter into any agreement or arrangement with, any Affiliate or with any Subsidiary other than a Purchaser Subsidiary;
(vi) change any of Purchaser’s or such Purchaser Subsidiary’s banking or safe deposit arrangements;
(vii) grant or extend any power of attorney or act as guarantor, surety, co-signer, endorser, co maker, indemnitor or otherwise in respect of the obligation of any Person;
(viii) pay any amounts (whether in cash or property) to any employee of Purchaser or any Purchaser Subsidiary other than in the ordinary course of business and consistent with past practice or pursuant to any Contract;
(ix) grant to any officer or employee any increase in compensation or benefits, other than increases in compensation or benefits for employees in the ordinary course of business and consistent with past practice;
(x) pay any pension, retirement allowance or other employee benefit not required by any plan, policy or program identified onSchedule 6.17(a) hereto;
(xi) adopt, agree to adopt, or make any announcement regarding the adoption of (i) any new pension, retirement or other employee benefit plan, policy or program or (ii) any amendments to any existing plan, policy or program identified onSchedule 6.17(a) unless required by applicable Law;
(xii)(A) declare, set aside or pay any dividend or distribution that is payable in cash, stock, or other property with respect to Purchaser (provided that nothing herein shall prohibit the payments of dividends or distributions by any Purchaser Subsidiary to Purchaser); (B) redeem, purchase or otherwise acquire, directly or indirectly, any shares of Purchaser or any Purchaser Subsidiary, or any other securities thereof or any rights, warrants, or options to acquire any such shares or other securities; (C) except for any pledge of equity securities of each Purchaser Subsidiary to the Company, authorize for issuance, issue, sell, pledge, deliver or agree to commit to issue, sell or pledge (whether through the issuance or granting of any options, warrants, calls, subscriptions, equity appreciation rights or other rights or other agreements) any equity or debt securities of Purchaser or any Purchaser Subsidiary, or any other securities that are convertible into or exchangeable for shares of any class of Purchaser or any Purchaser Subsidiary, other than an Exempt Issuance; or (D) split, combine or reclassify the outstanding shares of Purchaser or any Purchaser Subsidiary, or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of any equity securities of Purchaser or any Purchaser Subsidiary;
(xiii) adjust, settle or compromise any claim, obligation, debt, demand, suit or judgment against or on behalf of Purchaser or any Purchaser Subsidiary, except for claims, obligations, debts, demands, suits or judgments that do not involve an Affiliate of Purchaser and do not exceed more than $50,000 in any single instance or more than $100,000 in the aggregate with respect to Purchaser and the Purchaser Subsidiaries as a whole; or
(xiv) agree, whether in writing or otherwise, to do any of the foregoing.
(e) Purchaser shall maintain (and shall cause each Purchaser Subsidiary to maintain) the same insurance coverage (to the extent commercially reasonable) as on the date of this Agreement and all property insured thereby shall be used, operated, maintained and repaired in the ordinary course of business and consistent with past practice.
A-25
(f) Purchaser shall not do any act or omit to do any act, or permit any act or omission to act (or permit any Purchaser Subsidiary to so do, or omit to do, or to so permit any act or omission), which will cause a breach of any Purchaser Material Contract or commitment of Purchaser or any Purchaser Subsidiary or which would cause the breach by Purchaser or any Purchaser Subsidiary of any representation, warranty, covenant or agreement made hereunder.
(g) Purchaser shall duly comply (and shall cause each Purchaser Subsidiary to duly comply) in all material respects with all Laws applicable to it and its properties, operations, business and employees.
(h) Purchaser shall file (and shall cause each Purchaser Subsidiary to file) all Federal, state, local and foreign Tax Returns and amendments thereto required to be filed by them and shall pay (and shall cause each Purchaser Subsidiary to pay) all Taxes shown as due and payable thereon. All returns and reports in respect of employee withholdings, FICA, unemployment and other similar items and other applicable Taxes shall be timely made as shall all deposits and payments due in respect of such Taxes and obligations.
4.04Proxy Statement; Company Approval.
(a) As promptly as practicable after the execution of this Agreement, (but in any event within 30 days after the date hereof) Purchaser shall prepare and shall file with the SEC a document or documents that will constitute the proxy statement with respect to the Merger (together with any amendments thereto, the “Proxy Statement”) relating to the special meeting of the stockholders of Purchaser (such meeting, the “Purchaser Stockholders’ Meeting”) to be held to consider approval of an amendment to the Certificate of Incorporation of Purchaser to increase the number of authorized shares of Purchaser Common Stock to 180,000,000 (the “Charter Amendment”) and the issuance of Purchaser Common Stock to the Stockholders pursuant to this Agreement and the Merger (the “Purchaser Stock Issuance”). Purchaser shall use commercially reasonable efforts to cause the Proxy Statement to be cleared by the SEC as promptly as practicable after the date hereof, and, prior to such date, Purchaser shall take all action required under any applicable Laws in connection with the Purchaser Stock Issuance. The Company shall furnish all information concerning the Company as Purchaser may reasonably request in connection with such actions and the preparation of the Proxy Statement. Purchaser shall notify the Company of the receipt of any comments from the SEC on the Proxy Statement and of any requests by the SEC for any amendments or supplements thereto or for additional information and shall provide to the Company promptly copies of all correspondence between Purchaser or any of its representatives and advisors and the SEC. Purchaser shall mail, or cause to be mailed, to the stockholders of Purchaser, and shall duly call the Purchaser Stockholders’ Meeting, as promptly as reasonably practicable in accordance with applicable Law following the date on which the SEC clears the Proxy Statement.
(b) None of the information supplied by the Company for inclusion or incorporation by reference in the Proxy Statement shall, (a) at the time filed with the SEC or other regulatory agency, (b) at the date it or any amendments or supplements thereto are first mailed to stockholders of Purchaser, (c) at the time of the Purchaser Stockholders’ Meeting and (d) at the Effective Time, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they are made, not misleading. If at any time prior to the Effective Time any event or circumstance relating to the Company, or any of its officers or directors, should be discovered by the Company that should be set forth in an amendment or a supplement to the Proxy Statement, the Company shall promptly inform Purchaser. All documents that the Company is responsible for filing with the SEC in connection with the Merger, if any, will comply as to form in all material respects with the applicable requirements of the rules and regulations of the Securities Act and the Exchange Act.
(c) Purchaser shall promptly notify the Company of the receipt of all comments of the SEC staff with respect to the Proxy Statement and of any request by the SEC staff for any amendment or supplement thereto or for additional information and shall promptly provide the Company copies of all correspondence between Purchaser and/or any of its representatives, on the one hand, and the SEC staff, on the other hand, with respect to the Proxy Statement.
A-26
(d) Subject to applicable Laws, notwithstanding anything to the contrary stated above, prior to filing or mailing the Proxy Statement or filing any other required filings (or, in each case, any amendment or supplement thereto) or responding to any comments of the SEC staff with respect thereto, Purchaser shall promptly provide the Company with an opportunity to review and comment on such document or response and shall in good faith consider for inclusion in such document or response comments reasonably and timely proposed by the Company.
(e) As promptly as practicable following the execution of this Agreement, and in no event more than seventy-two (72) hours following the execution of this Agreement, the Company shall take, or cause to be taken, all appropriate action, and do, or cause to be done, all things necessary, proper or advisable to obtain the Company Stockholder Approval.
4.05NASDAQ Global Market Listing. Purchaser shall promptly prepare and submit to The NASDAQ Stock Market, Inc. a listing application covering the shares of Purchaser Common Stock to be issued in the transactions contemplated by this Agreement, such listing to be effective at or prior to the Effective Time and shall use commercially reasonable efforts to cause such shares to be approved for listing on The NASDAQ Global Market. The Company shall furnish such information concerning it, any of its Affiliates and the holders of the Company’s capital stock as Purchaser may reasonably request in connection with such actions and the preparation of the listing application.
4.06Appropriate Action; Consents; Filings.
(a) Purchaser and the Company shall use their commercially reasonable efforts to (A) take, or cause to be taken, all appropriate action, and do, or cause to be done, all things necessary, proper or advisable under applicable Law or otherwise to consummate and make effective the transactions contemplated by this Agreement as promptly as practicable, (B) obtain from any governmental entity any consents, licenses, permits, waivers, approvals, authorizations or orders required to be obtained or made by Purchaser or the Company or any of their respective Subsidiaries, or to avoid any action or proceeding by any governmental entity, in connection with the authorization, execution and delivery of this Agreement and the consummation of the transactions contemplated hereby, including, without limitation, the Merger, and (C) make all necessary filings, and thereafter make any other required submissions, with respect to this Agreement and the transactions contemplated hereby, including the Merger, required under (x) the Securities Act and the Exchange Act, and any other applicable federal or state securities Laws, and (y) any other applicable Law.
(b) Notwithstanding anything to the contrary contained in this Agreement, but subject to any agreement between Purchaser and the Company contained in any document evidencing or relating to the term loan to be made by the Company to Purchaser on or about the date of this Agreement, no party shall have any obligation under this Agreement: (i) to dispose of or transfer or cause any of its Subsidiaries to dispose of or transfer any assets; (ii) to discontinue, or cause any its Subsidiaries to discontinue, offering any product or service; (iii) to license or otherwise make available, or cause any its Subsidiaries to license or otherwise make available, to any person any Intellectual Property; (iv) to hold, or cause any of its Subsidiaries to hold, separate any assets or operations (either before or after the Effective Time); (v) to make, or cause any of its Subsidiaries to make, any commitment (to any governmental entity or otherwise) regarding its future operations or to contest any Legal Proceeding or any order, writ, injunction or decree relating to the transactions contemplated hereby if such party determines in good faith that contesting such Legal Proceeding or order, writ, injunction or decree could materially adversely affect such party.
(c) Purchaser and the Company shall give (or shall cause any of their respective Subsidiaries to give) any notices to third parties, and use all commercially reasonable best efforts to obtain any third party consents necessary, proper or advisable to consummate the transactions contemplated in this Agreement.
A-27
4.07Certain Notices.
(a) Purchaser shall give prompt notice to the Company, and the Company shall give prompt notice to Purchaser, of (i) the occurrence, or non-occurrence, of any event the occurrence or non-occurrence of which would be reasonably likely to cause any representation or warranty contained in this Agreement to be materially untrue or inaccurate, and (ii) any failure of Purchaser, any Purchaser Subsidiary or the Company, as the case may be, materially to comply with or satisfy any covenant, condition or agreement to be complied with or satisfied by it hereunder; provided, however, that the delivery of any notice pursuant to this Section 4.07 shall not limit or otherwise affect the remedies available hereunder to the party receiving such notice; and provided, further, that failure to give such notice shall not be treated as a breach of covenant for the purposes of Sections 7.01(c) and 7.02(c) unless the failure to give such notice results in a Material Adverse Effect on the other party.
(b) Each of Purchaser and the Company shall give prompt notice to the other of: (i) any material notice or other communication from any person alleging that the consent of such person is or may be required in connection with the Merger or other transactions contemplated by this Agreement; (ii) any material notice or other communication from any governmental entity in connection with the Merger or other transactions contemplated by this Agreement; (iii) any Legal Proceeding relating to or involving or otherwise affecting Purchaser, any Purchaser Subsidiary, Merger Sub or the Company that relates to the Merger or other transactions contemplated by this Agreement; (iv) the occurrence of a default or event that, with notice or lapse of time or both, is reasonably likely to become a default under a Material Contract; and (v) any change that would be considered reasonably likely to result in a Material Adverse Effect, or is likely to impair in any material respect the ability of either Purchaser or the Company to consummate the transactions contemplated by this Agreement; provided, that failure to give such notice shall not be treated as a breach of covenant for the purposes of Sections 7.01(c) and 7.02(c) unless the failure to give such notice results in a Material Adverse Effect on the other party.
4.08No Solicitation of Transactions.
(a) At all times during the period commencing with the execution and delivery of this Agreement and continuing until the earlier to occur of the termination of this Agreement pursuant to Article VIII hereof and the Effective Time, the Company shall not, nor shall it authorize any officer, trustee, director, employee, investment banker, financial advisor, attorney, broker, finder or other agent, representative or affiliate of the Company to: (i) initiate, solicit, knowingly encourage or knowingly facilitate (including by way of furnishing nonpublic information or access to properties or assets) any Acquisition Proposal; (ii) furnish any nonpublic information regarding the Company to any Person in connection with or in response to an Acquisition Proposal or an inquiry or indication of interest that would reasonably be expected to lead to an Acquisition Proposal (which shall include any Acquisition Proposal received prior to the date hereof); (iii) enter into discussions or negotiate with any Person in furtherance of an Acquisition Proposal; (iv) approve, endorse or recommend any Acquisition Proposal; or (v) enter into any letter of intent, memorandum of understanding, agreement in principle, merger agreement, acquisition agreement or other similar agreement or any other contract relating to any Acquisition Proposal, with respect to an Acquisition Proposal. Notwithstanding the foregoing or any other provision of this Agreement to the contrary, if the Company should receive an Acquisition Proposal at any time prior to the Effective Time, the Company shall promptly (and in all cases within one (1) business day) advise Purchaser in writing of such Acquisition Proposal, the terms and conditions of any such Acquisition Proposal and the identity of the Person making any such Acquisition Proposal.
(b) The Company shall, and shall cause its representatives to, cease immediately and cause to be terminated any and all existing soliciting activities, discussions or negotiations and non-public information access, if any, with or to any Person conducted prior to the date hereof with respect to any Acquisition Proposal. The Company shall promptly request that each Person, if any, in possession of the confidential information about the Company that was furnished by or on behalf of the Company in connection with its consideration of any potential Acquisition Proposal to return or destroy all confidential information heretofore furnished to such Person.
A-28
4.09Stock Options and Warrants.
(a) At the Effective Time, the Company’s obligations with respect to each outstanding option to purchase shares of Company Common Stock (each, a “Company Option” and collectively, the “Company Options”) under the Company Plans, whether vested or unvested, and the Company’s obligations with respect to each warrant to purchase shares of Company Common Stock (each, a “Warrant” and collectively, the “Warrants”) will be assumed by Purchaser. Each Company Option so assumed by Purchaser under this Agreement shall be subject to substantially the same terms and conditions set forth in the Company Plans (which plans shall be adopted upon substantially the same terms and conditions by Purchaser) or agreement pursuant to which such Company Option was issued as in effect immediately prior to the Effective Time, and each Warrant so assumed by Purchaser under this Agreement shall be subject to substantially the same terms and conditions set forth in such applicable Warrant agreement, except as follows (i) such Company Option or Warrant will be exercisable for that number of shares of Purchaser Common Stock equal to the product of the number of shares of Company Common Stock that were purchasable under such Company Option or Warrant immediately prior to the Effective Time multiplied by the CSE Exchange Ratio, rounded down to the nearest whole number of shares of Purchaser Common Stock, and (ii) the per share exercise price for the shares of Purchaser Common Stock issuable upon exercise of such assumed Company Option or Warrant will be equal to the quotient determined by dividing the exercise price per share of Company Common Stock at which such Company Option or Warrant was exercisable immediately prior to the Effective Time by the CSE Exchange Ratio, and rounding the resulting exercise price up to the nearest whole cent. Following the Effective Time, Purchaser will send to the holders of the assumed Company Options and Warrants a written notice setting forth (i) the number of shares of Purchaser Common Stock that are subject to such assumed Company Option, and (ii) the exercise price per share of Purchaser Common Stock issuable upon exercise of such assumed Company Option. In addition, Purchaser shall file with the SEC, no later than ninety (90) days after the Effective Time, a registration statement on Form S-8 registering the exercise of any Company Options issued under the Company Stock Plans assumed by Purchaser pursuant to this Section 4.09 (to the extent the exercise of such options is eligible to be registered using a Form S-8 registration statement).
(b) Purchaser and Company shall take all action that may be reasonably necessary to effectuate the provisions of this Section 4.09. The Company Options and Warrants assumed by Purchaser shall retain their existing vesting schedules following the Effective Time.
(c) It is the intention of the parties that Company Options assumed by Purchaser qualify following the Effective Time as incentive stock options as defined in the Code (“ISO’s”) to the extent such Company Options qualified as ISO’s prior to the Effective Time.
(d) Purchaser will reserve sufficient shares of Purchaser Common Stock for issuance under this Section 4.09.
4.10Tax-Free Reorganization. Notwithstanding anything herein to the contrary, each of Merger Sub, Purchaser and Company shall use reasonable best efforts to cause the Merger to qualify, and will not take any actions, or fail to take any action, which could reasonably be expected to prevent the Merger from qualifying as a reorganization under the provisions of Section 368(a) of the Code. Merger Sub shall, and shall cause the Surviving Corporation and Purchaser to, report, to the extent required by the Code or the regulations thereunder, the Merger for United States federal income tax purposes as a reorganization within the meaning of Section of 368(a) of the Code. Purchaser and Company will each make available to the other party and their respective legal counsel copies of all returns requested by the other party
4.11Tax Matters.
(a)Tax Returns. The Company and Stockholders’ Representative shall duly prepare, or cause to be prepared, and file, or cause to be filed, on a timely basis, all Tax Returns with respect to the Company for Taxable Periods ending before the Closing Date (“Pre-Closing Tax Periods”) and for any Taxable Periods ending on the Closing Date (“Closing Date Tax Period”). Such Tax Returns shall be filed on a timely basis
A-29
consistent with the Company’s past practice in filing its Tax Returns and shall not be filed without the approval of the Stockholders’ Representative. Purchaser shall duly prepare, or cause to be prepared, and file, or cause to be filed, on a timely basis all Tax Returns with respect to the Company for any taxable period which includes but does not end on the Closing Date (“Straddle Period”) and for any taxable periods beginning after the Closing Date (the “Post-Closing Tax Periods”). Purchaser shall permit the Stockholders’ Representative to review and comment on each Tax Return with respect to the Company for any Straddle Period and shall make such revisions as the Stockholders’ Representative shall reasonably request. Tax Returns for a Straddle Period shall be prepared consistent with the Company’s past practice. Unless the prior written consent of the Stockholders’ Representative is first obtained, Purchaser shall not take any action (including without limitation, file any amended Tax Returns or claim any Tax refunds) which would in any way alter the balance of Taxes owing or Tax refunds or credits with respect any Pre-Closing Tax Period or any Closing Date Tax Period. For purposes of this Agreement, in the case of any Straddle Period, Taxes of the Company (“Pre-Closing Straddle Tax Liability”) for the portion of any Straddle Period ending on and including the Closing Date (a “Pre-Closing Straddle Period”) shall, where possible, be computed as if such taxable period ended as of the close of business on the Closing Date. For purposes of the foregoing, any items attributable to a Straddle Period which cannot be taken into account in the manner so provided (i.e. Taxes not based upon income or receipts) shall be allocated to the Pre-Closing Straddle Period for purposes of determining the Pre-Closing Straddle Tax Liability, pro rata, based upon the number of days in the Pre-Closing Straddle Period, as compared to the total number of days in the Straddle Period, provided that if any Straddle Period Tax is based on income or revenue, then such allocation shall be based upon the actual activities of the Company as determined from the books and records of the Company for such Pre-Closing Straddle Period. Unless otherwise indicated, a Pre-Closing Straddle Period shall be treated as a “Pre-Closing Tax Period” for purposes of this Agreement.
(b)Cooperation on Tax Matters. Purchaser and the Stockholders’ Representative shall cooperate fully, as and to the extent reasonably requested by the other party, in connection with the filing of Tax Returns pursuant to this Section 4.11 (including amended Tax Returns for Pre-Closing Tax Periods and the Closing Date Tax Period (or portions thereof) that the Stockholders’ Representative may reasonably request Purchaser to file) and any Audit. Such cooperation shall include the retention and (upon the other party’s request) the provision of records and information which are reasonably relevant to such Tax Returns or Audit and making employees available on a mutually convenient basis to provide additional information and explanations of materials provided hereunder. Purchaser shall not dispose of any records relating to Taxes paid or payable by the Company and which are attributable to Pre-Closing Tax Periods and the Closing Date Tax Period prior to the later of six months after the expiration of the applicable limitations period on assessment with respect to any such Taxes, or the final resolution of all Audits or litigation initiated prior to the expiration of the applicable limitations period.
(c)Pre-Closing Tax Period Audits. Purchaser will promptly notify Stockholders’ Representative if it receives written notice of any Audits relating to Pre-Closing Tax Periods (a “Pre-Closing Audit”) and/or the Closing Date Tax Period (a “Closing Date Audit”). With respect to such Audits the Stockholders (at their expense) through the Stockholders’ Representative may elect (in its sole discretion) to control all proceedings and may make all decisions taken in connection therewith at the Stockholders’ Representative sole discretion, provided that any such proceeding and any such decision taken in connection therewith does not increase the Tax liability for any portion of a Straddle Period beginning after the Closing Date (the “Post Closing Straddle Period”) or any Post-Closing Tax Period. In the event a decision with respect to a Pre-Closing Audit or Closing Date Audit would increase a tax liability for a Post-Closing Straddle Period or a Post-Closing Tax Period, no such decision shall be implemented or effectuated without the prior written consent of Purchaser (which consent shall not be unreasonably withheld or delayed). The Stockholders’ Representative shall exercise reasonable efforts to keep Purchaser fully apprised of any such Pre-Closing Audit or Closing Date Audit. With respect to any such Pre-Closing Audit or Closing Date Audit, Purchaser shall furnish the Stockholders’ Representative with the usual form of power of attorney (Form 2848) and provide to the Stockholders’ Representative such records and information as may be necessary for the Stockholders’ Representative to control such Pre-Closing Audit or Closing Date Audit proceeding. If the
A-30
Stockholders’ Representative has failed to take reasonable steps necessary to control a Pre-Closing Audit or Closing Date Audit, as the case may be, or to timely respond to any requests, demands or proceedings in connection therewith, then Purchaser shall have the right to assume control of such Pre-Closing Audit or Closing Date Audit (at the Stockholders’ expense), as the case may be, and make all decisions taken in connection therewith at Purchaser’s sole discretion, provided that any such proceeding and any such decision taken in connection therewith does not increase the Tax liability for any Pre-Closing Tax Period or the Closing Date Tax Period. Otherwise, no such decision shall be implemented or effectuated without the prior written consent of the Stockholders’ Representative (which consent shall not be unreasonably withheld or delayed). Purchaser shall exercise reasonable efforts to keep the Stockholders’ Representative apprised of all aspects of any such Pre-Closing Audit or Closing Date Audit.
(d) Purchaser and the Surviving Corporation shall not amend any Tax Return of the Company for Pre-Closing Tax Periods, or file a claim for refund of Taxes attributable to a Pre-Closing Tax Period, without the Stockholders’ Representative’s consent.
4.12Purchaser Management and Directors Lock-up Agreements; Voting Agreement for Certain Company Stockholders.
(a) As promptly as practicable following the execution of this Agreement, and in no event more than seventy-two (72) hours following the execution of this Agreement, the executive officers and directors of Purchaser set forth inSchedule 4 shall have each executed and delivered the Lock-up Agreement set forth asExhibit C-2.
(b) As promptly as practicable following the execution of this Agreement, and in no event more than seventy-two (72) hours following the execution of this Agreement, the Company Stockholders set forth inSchedule 3 shall have each executed and delivered the Voting Agreement set forth asExhibit E.
ARTICLE V
REPRESENTATIONS AND WARRANTIES
OF THE COMPANY
The Company hereby represents and warrants to Purchaser and Merger Sub that the statements contained in this Article 5 are complete and accurate as of the date hereof, except as set forth in the written disclosure schedule delivered by the Company to the Purchaser. Such Company disclosure schedule shall be arranged in sections corresponding to the numbered and lettered sections and subsections contained in this Article 5, and the disclosures in any section or subsection of the Company’s disclosure schedule shall qualify only the corresponding section or subsection of this Article 5, unless the disclosures in one section or subsection reasonably appear to apply to another section or subsection of the Company’s disclosure schedule.
5.01Organization. The Company is a corporation duly organized, validly existing and in good standing under the Laws of the State of Delaware. The Company has all requisite corporate power and authority to enable it to own, lease or otherwise hold its properties and assets and to carry on its business as presently conducted. The Company is duly qualified to do business and in good standing in each jurisdiction in which the nature of its business or the ownership, leasing or holding of its properties makes such qualification necessary. A list of the jurisdictions in which the Company is so qualified is set forth onSchedule 5.01. The Company does not have any Subsidiaries.
5.02Capital Stock and Related Matters; No Investments.
(a) The authorized capital stock of the Company consists of 172,434,420 authorized shares consisting of: (i) 130,000,000 shares of Company Common Stock, (ii) 14,850,000 shares of Series A Preferred Stock, (iii) 27,584,420 shares of Series A-1 Preferred Stock and (iv) 0 shares of undesignated stock, all par value $0.001, of which as of the date hereof 5,824,710 shares of Company Common Stock are issued and
A-31
outstanding, 0 shares of the Series A Preferred Stock are issued and outstanding and 15,176,263 shares of the Series A-1 Preferred Stock are issued and outstanding (collectively, the “Company Shares”). The Company Shares constitute all of the issued and outstanding shares of capital stock of the Company and are held by the Stockholders in the amounts set forth onSchedule 5.02 hereto.
(b) Except as set forth onSchedule 5.02 hereto, the Company has no outstanding stock or securities convertible into or exchangeable for any shares for its capital stock or containing any profit participation features, nor does it have outstanding any rights or options to subscribe for or purchase its capital stock or any stock or securities convertible into or exchangeable for any shares for its capital stock or any stock appreciation rights or phantom stock plans. The Company is not subject to any obligation (contingent or otherwise) to repurchase or otherwise acquire or retire any shares of its capital stock or any warrants, options or other rights to acquire its capital stock. All of the outstanding shares of the Company’s capital stock have been validly issued and are fully paid and nonassessable.
(c) Except as set forth onSchedule 5.02 there are no statutory or contractual shareholders’ preemptive rights or rights of refusal with respect to the Merger. The Company has not violated any applicable federal or state securities Laws in connection with the offer or sale of the Shares.
(d) The Company does not own or hold any Investment in any Person.
5.03Authorization. Subject to approvals as are required by Law, the Company has all requisite power and authority (or legal capacity, as the case may be) to enter into this Agreement and the Related Documents to be executed and delivered by the Company pursuant hereto or in connection with the transactions contemplated hereby or thereby, and to consummate the transactions contemplated hereby and thereby. Subject only to Company Stockholder Approval, all acts and other proceedings required to be taken by the Company to authorize the execution, delivery and performance of this Agreement and the Related Documents to which it is a party, and the consummation of the transactions contemplated hereby and thereby have been (or by the Closing will have been) duly and properly taken.
5.04Valid and Binding. This Agreement constitutes (and, when executed and delivered at Closing, each Related Document, to the extent that the Company is a party thereto, will constitute) a valid and binding obligation of the Company, enforceable against the signatory in accordance with its terms, except that (i) such enforcement may be limited by or subject to any bankruptcy, insolvency, reorganization, moratorium or similar Laws now or hereafter in effect relating to or limiting creditors’ rights generally and (ii) the remedy of specific performance and injunctive and other forms of equitable relief are subject to certain equitable defenses and to the discretion of the court before which any proceeding therefor may be brought.
5.05No Violation. The execution and delivery of this Agreement and each Related Document by the Company, and the consummation of the transactions contemplated hereby and thereby and compliance with the terms hereof and thereof does not and will not (subject only to obtaining any required consents, approvals, authorizations, exemptions or waivers set forth onSchedule 5.06 hereto) conflict with, or result in any violation of or default (with or without notice or lapse of time, or both) under, or give rise to a right of termination, cancellation or acceleration of any obligation or to loss of a material benefit under or result in the creation of any Encumbrance of any kind upon any of the Company’s assets under, any provision of (i) the certificate of incorporation or by-laws of the Company, (ii) any note, bond, mortgage, indenture, deed of trust, license, lease, contract, commitment or loan or other agreement to which the Company is a party or by which any of its properties or assets are bound, or (iii) any statute, regulation, rule, injunction, judgment, order, Law, ordinance, decree, ruling, charge or other restriction of any government, governmental agency, or court applicable to the Company or its property or assets, except in the case of clauses (ii) and (iii) for any such violations, breaches, defaults, rights of termination, cancellation or acceleration or requirements which, individually or in the aggregate would not have a Material Adverse Effect or would not adversely affect the ability of the Company to consummate the transactions contemplated by this Agreement.
A-32
5.06Consents and Approvals. Other than the filing of the Delaware Certificate of Merger with the Secretary of State of the State of Delaware, or as otherwise set forth inSchedule 5.06 hereto, no consent, approval or authorization of, or declaration, filing or registration with, any governmental or regulatory authority or any court or other tribunal, and no consent or waiver of any party to any Contract is required to be obtained by the Company in connection with the execution, delivery and performance of this Agreement and the Related Documents or the consummation of the transactions contemplated hereby or thereby.
5.07Financial Statements. The Company has furnished to Purchaser true, correct and complete copies of (i) audited balance sheets of the Company as of December 31, 2007 and December 31, 2008, a draft audited balance sheet of the Company as of December 31, 2009 and an unaudited consolidated balance sheet of the Company as of February 28, 2010, and (ii) audited consolidated income statements of the Company for the years ended December 31, 2007 and December 31, 2008, a draft audited consolidated income statement of the Company for the year ended December 31, 2009 and an unaudited consolidated income statement of the Company for the two-month period ended February 28, 2010 (collectively, the “Company Financial Statements”), copies of which are attached hereto asSchedule 5.07, with the exception of the draft audited balance sheet of the Company as of December 31, 2009 and the draft audited consolidated income statement of the Company for the year ended December 31, 2009, which have been separately furnished to the Purchaser and shall be provided by the Company to the Purchaser as soon as practicable after they are issued to the Company by the Company’s independent auditors. The Company Financial Statements have been prepared by the Company on the basis of the books and records maintained by the Company in the ordinary course of business in a manner consistently used and applied throughout the periods involved. The Company Financial Statements have been prepared in accordance with GAAP and present fairly the assets, liabilities and the financial condition of the Company as at the respective dates thereof, except that the Company’s unaudited consolidated balance sheet as of February 28, 2010 and income statement for the two-month period then ended do not contain footnotes and information related thereto and are subject to normal year end adjustments in the ordinary course of business. The books and records of the Company to which such statements relate fairly reflect in all material respects the assets, liabilities and operations of the Company.
5.08Interim Operations. Except as set forth onSchedule 5.08, since December 31, 2009:
(a) the Company Business has been conducted by the Company only in the ordinary course consistent with past practices;
(i) with respect to the Company Business, the Company has not:
(ii) suffered any Material Adverse Effect;
(iii) incurred any liabilities or obligations (absolute, accrued, contingent or otherwise), except in the ordinary and usual course of business and consistent with past practice, or increased, or experienced any change in any assumptions underlying or methods of calculating, any bad debt, contingency or other reserves;
(iv) paid, discharged or satisfied any claims, liabilities or obligations (absolute, accrued, contingent or otherwise), other than the payment, discharge or satisfaction in the ordinary and usual course of business and consistent with past practice liabilities and obligations reflected or reserved against in the Company Financial Statements or incurred in the ordinary course of business and consistent with past practice;
(v) permitted or allowed the Company’s assets to be subjected to any Encumbrance (except Encumbrances created by Law);
(vi) canceled any debts owing to the Company or waived any claims or rights, except for amounts each individually under $5,000;
(vii) sold, transferred, or otherwise disposed of, or transferred or granted any rights under any lease, license or agreement with respect to, any of its assets, except in the ordinary course of business and consistent with past practices;
A-33
(viii) disposed of, failed to take reasonable steps to protect, or permitted to lapse, any rights for the use of, the Company’s Intellectual Property, or disposed of, failed to take reasonable steps to protect any Proprietary Information;
(ix) made any change in any method of accounting or accounting practice;
(x) made any single capital expenditure or future commitment in excess of $20,000, or made aggregate capital expenditures or future commitments in excess of $50,000;
(xi) made any material change in the manner in which the Company Business is conducted;
(xii) made any material change in policies relating to the Company Business (whether or not in the ordinary and usual course of business);
(xiii) had any labor dispute or received notice of any grievance;
(xiv) borrowed or agreed to borrow any funds;
(xv) paid and/or declared any dividends with respect to its shares of capital stock, whether in shares of capital stock or other property;
(xvi) granted to any officer or employee any increase in compensation or benefits, other than increases in compensation or benefits to employees in the ordinary and usual course of business;
(xvii) paid any pension, retirement allowance or other employee benefit not required by any plan, policy or program identified onSchedule 5.21 hereto or any employment agreement set forth onSchedule 5.21 hereto;
(xviii) adopted, agreed to adopt, or made any announcement regarding the adoption of (i) any new pension, retirement or other employee benefit plan, program or policy, or (ii) any amendments to any existing pension, retirement or other employee benefit plan, policy or program identified onSchedule 5.21 unless otherwise required by applicable Law; or
(xix) suffered or agreed to take any of the actions set forth in this subparagraph (ii).
(b) None of the assets of the Company have been affected in any way as a result of fire, explosion or other casualty (whether or not covered by insurance).
5.09Undisclosed Liabilities. The Company does not have any liabilities or obligations of any nature (whether accrued, absolute, contingent or otherwise), except for liabilities or obligations (i) disclosed onSchedule 5.09 hereto, (ii) disclosed in the Company Financial Statements, (iii) arising in the ordinary course of business consistent with past practice under any Material Contract, or (iv) incurred in the ordinary course of business consistent with past practices since December 31, 2009.
5.10Taxes.
(a) Except as set forth onSchedule 5.10(a), the Company has timely filed all Tax Returns required by applicable Law to be filed by or on behalf of the Company on or prior to the date hereof, and such Tax Returns are true, complete, correct and in conformity with applicable Tax Laws in all material respects. The Company has paid all Taxes (whether or not required to be shown on any Tax Return) required to be paid by the Company on or before the date hereof, or where payment is not yet due, has established or will establish, on or before the Closing Date, in accordance with GAAP, an adequate reserve on its books and financial records for the payment of all Taxes due from the Company, with respect to any Pre-Closing Tax Period (including any Pre-Closing Straddle Tax Liability) and the Closing Date Tax Period. All Taxes that the Company is or was required by Law to withhold, deposit or collect have been duly withheld, deposited or collected and, to the extent required, have been paid to the relevant Tax Authority. The Company has timely complied with all information and reporting and backup withholding requirements, including maintenance of any required records with respect thereto, in connection with amounts paid or owing to any employee, creditor, independent contractor or other third party.
A-34
(b) Except as described inSchedule 5.10(b), the Tax Returns with respect to the Company have not been subject to an Audit since its incorporation, and there are no ongoing Audits of the Company. The Company has not received written notice by any Tax Authority of an Audit nor to Knowledge is any such Audit contemplated, threatened or pending.
(c) to the Company’s Knowledge, there are no claims, investigations, actions or proceedings pending or threatened, against the Company by any Tax Authority for any past due Taxes with respect to which the Company would be liable. There has been no waiver by the Company of any applicable statute of limitations nor any consent for the extension of the time for the assessment of any Tax against the Company.
(d) The Company is not delinquent in the payment of any amount of Taxes and there are no Tax liens upon any property or assets of the Company, except liens for Taxes not yet due and payable.
(e) The Company has not agreed, nor is it required, to make any adjustment under Section 481(a) of the Code by reason of a change in accounting method or otherwise.
(f) The Company is not liable for the Taxes of any Person other than the Company, including, without limitation, (i) under U.S. Treasury Regulations Section 1.1502-6 (or comparable provision of state, local or foreign Law), (ii) as a transferee or successor, or (iii) by contract, indemnity or otherwise. Except as set forth onSchedule 5.10(f), the Company has not ever been a member of a consolidated, combined or unitary group for federal, state, local or foreign Tax purposes or have ever been included as part of a consolidated, combined or unitary Tax Return.
(g) The Company is not, nor has it ever been, a party to any Tax sharing agreement, Tax indemnity agreement or other similar Tax sharing arrangement.
(h) Except as set forth inSchedule 5.10(h), since January 1, 2004 no claim has been asserted in writing by a Tax Authority in a jurisdiction where the Company has not filed Tax Returns that the Company is or may be subject to taxation by that jurisdiction. To Knowledge no such claim was asserted prior to 2004.
(i)Schedule 5.10(i) sets forth each of the states for which the Company is currently filing income or franchise Tax Returns (or similar type of Tax Returns) or is required to file for the current Taxable Period.
(j) The Company has provided Purchaser with copies of: (i) all Tax Returns filed by, or on behalf of, the Company for periods beginning on or after January 1, 2006 (the “Post-2006 Period”); (ii) all notices, protests or other correspondence relating to any Post-2006 Period Taxes or Tax Returns; (iii) any letter rulings, determination letters or similar documents issued by any Tax Authority with respect to the Company; (iv) any closing agreement entered into by the Company with any Tax Authority; and (vi any Tax Return workpapers relating to the Tax Returns referred to in clause (i). The Company has not entered into an exchange under Code Section 1031 with a “related person” (within the meaning of Code Section 1031(f)(3)) which could result in the Company being required to recognize gain under Code Section 1031(f)(1). The Company is not taking into account any gain under the installment method of Code Section 453.
(k) The Company is not, and has never been, a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code and Purchaser is not required to withhold tax on the purchase of the stock of the Company by reason of Section 1445 of the Code. Except as set forth inSchedule 5.10(k), the Company has not entered into any compensatory agreements with respect to the performance of services which payment thereunder would result in a nondeductible expense pursuant to Section 162(m) or 280G of the Code or an excise tax to the recipient of such payment pursuant to Section 4999 of the Code. The Company has not participated in an international boycott as defined in Section 999 of the Code.
(l) The Company has not participated in any transaction that is a reportable transaction within the meaning of Treasury Regulation Section 1.6011-4 or Section 6707A of the Code.
(m) Neither the Company nor, to the Company’s Knowledge, any of the Company’s affiliates has taken or agreed to take any action that would prevent the Merger from qualifying as a reorganization within the
A-35
meaning of Section 368(a) of the Code. The Company is not aware of any agreement, plan or other circumstance that would prevent the Merger from qualifying as a reorganization within the meaning of Section 368(a) of the Code.
5.11Condition of Property. Except as set forth onSchedule 5.11, all Tangible Property of the Company is in good operating condition and repair, reasonable wear and tear excepted, and all such Tangible Property is adequate for the uses to which it is being put. None of such Tangible Property is in need of maintenance or repairs except for ordinary, routine maintenance and repairs which are not material in nature or cost.
5.12Contracts and Commitments.
(a)Schedule 5.12(a) lists all Material Contracts (copies of which have heretofore been made available to Purchaser) and describes all currently effective oral agreements and commitments, if any, to which the Company is a party. Except as set forth onSchedule 5.12(a) hereto, (i) all Material Contracts constitute valid and binding agreements of the Company, and, to the Company’s Knowledge, each other party thereto, enforceable in accordance with their terms except that (A) such enforcement may be limited by or subject to any bankruptcy, insolvency, reorganization, moratorium or similar Laws now or hereafter in effect relating to or limiting creditors’ rights generally and (B) the remedy of specific performance and injunctive and other forms of equitable relief are subject to certain equitable defenses and to the discretion of the court before which any proceeding therefor may be brought, (ii) with respect to the Material Contracts there are no existing material defaults by the Company, or, to the Company’s Knowledge, by any other party thereto and there is no event which (whether with or without notice, lapse of time or the happening or occurrence of any other event) would constitute a material default under the Material Contracts by the Company, or, to the Company’s Knowledge, by any other party thereto, (iii) the Company is not restricted by agreement from carrying on the Company Business in any geographical location, and (iv) there are no negotiations pending or in progress to revise any Material Contract.
(b) The Company does not own any Real Property. The only leases for Real Property to which the Company is a party is set forth onSchedule 5.12(b) (collectively, and together with all addenda, the “Company Leases”). With respect to the Company Leases, (i) each such Company Lease is in full force and effect and is binding and enforceable against the Company, and to the Company’s Knowledge, the lessor, in accordance with its terms except that (A) such enforcement may be limited by or subject to any bankruptcy, insolvency, reorganization, moratorium or similar Laws now or hereafter in effect relating to or limiting creditors’ rights generally and (B) the remedy of specific performance and injunctive and other forms of equitable relief are subject to certain equitable defenses and to the discretion of the court before which any proceeding therefor may be brought; (ii) all rental and other charges payable pursuant to the terms and conditions of each such Company Lease have been paid and no rent has been paid in advance more than 30 days; (iii) there are no charges, offsets or defenses against the enforcement by the respective lessors thereunder of any agreement, covenant or condition on the part of the Company to be performed or observed pursuant to the terms of each Company Lease; (iv) there are no defaults by the Company of any agreement, covenant or condition on the part of the Company to be performed or observed pursuant to the terms of each Company Lease; (v) there are no actions or proceedings pending or, to the Company’s Knowledge, threatened, by the lessor under each such Company Lease; (vi) except for security deposits required by the Company Leases and identified onSchedule 5.12(b), the respective lessor does not hold any deposits for the Company’s accounts under each such Company Lease; (vii) the Merger and the transactions contemplated hereby will not constitute a prohibited transfer under any Company Lease; and (viii) to the Company’s Knowledge, there are no defaults by respective lessors of any agreement, covenant or condition on the part of such lessor to be performed or observed pursuant to the terms of any such Company Lease. The current expiration date and remaining options to extend each Company Lease are as set forth onSchedule 5.12(b) hereto.
5.13Intellectual Property.
(a)General.Schedule 5.13(a) contains a complete and accurate list of all Intellectual Property Registrations, including all Patents. Except as disclosed onSchedule 5.13(a), the Company is not aware of
A-36
any actions which, if not taken by Purchaser within ninety (90) days of the Closing Date, would limit or preclude Purchaser from obtaining, perfecting, preserving, renewing or maintaining any Intellectual Property Registrations, including the payment of any registration, maintenance or renewal fees or the filing of any responses to Patent Agency office actions, documents, applications or certificates.
(b)Valid Assignment. In each case in which the Company has acquired ownership of (rather than licenses or other rights to) any Intellectual Property from any Person, the Company has obtained a valid and enforceable assignment sufficient to irrevocably transfer to Purchaser all rights in such Intellectual Property (including the Company’s right to seek past and future damages for infringement with respect thereto to the extent permitted by applicable Law). To the extent required by, and in accordance with, applicable Laws and regulations, the Company has recorded each assignment to it of any Intellectual Property Registrations with the USPTO or equivalent Patent Agencies outside the United States.
(c)No Infringement. To the Company’s Knowledge, no Person is infringing or misappropriating any Intellectual Property or Licensed Rights as of the Effective Date. The Company has not received written notice of a claim from any Person claiming that any Intellectual Property or Licensed Rights infringes or misappropriates any rights of such Person or constitutes unfair competition or trade practices under the Laws of any jurisdiction. With respect to the Patents, although the validity of claims regarding novelty, non-obviousness and enablement have not been established, the Company’s claims and representations made to the USPTO and other Patent Agencies in its applications for Patents regarding novelty, non-obviousness and enablement were made in good faith, and the Company has no Company Knowledge of any facts which the Company reasonably believes would cause such claims or representations to be untrue or misleading. To the Company’s Knowledge, there is no unexpired Patent of any third party that includes claims that would be infringed by the Company Business as currently conducted.
(d)Confidentiality. The Company has taken all actions reasonably necessary to maintain and protect its rights in the Intellectual Property, including in Trade Secrets and other Proprietary Information. Without limiting the foregoing, the Company has and enforces a policy requiring each employee, consultant, contractor and other third parties with access to the Trade Secrets to execute proprietary information and confidentiality agreements, and all such current and former employees, consultants, contractors and third parties have executed such an agreement. To the Company’s Knowledge, there has been no violation or unauthorized disclosure or use of any Intellectual Property.
(e)No Transfer of Rights. Except as disclosed onSchedule 5.13(e), the Company is the exclusive owner of all the Intellectual Property owned by the Company and no other person owns or has any rights to any of the Intellectual Property and no Person has ownership rights or license rights to improvements made by the Company in any Patents. The Company has not transferred to any other Person any interest in, granted to any other Person any license, sublicense or other right to use, authorized the retention by any Person of any rights to use or joint ownership of, or entered into a covenant, in favor of any other Person, not to sue for infringement of, any Intellectual Property, except as disclosed onSchedule 5.13(e).
(f)Exclusive Rights. All of the Intellectual Property, excluding any Licensed Rights, was created solely by either (A) Employees of the Company acting within the scope of their employment or (B) employees of third parties or consultants or contractors of the Company who have validly and irrevocably assigned all of their rights, including rights to all Intellectual Property therein, to the Company.
(g)No Adverse Proceedings. To the Company’s Knowledge, there are no interference action or other Proceedings pending, or any written communication that threatens an interference action or other Proceeding, before any Patent Agency or other governmental entity in any jurisdiction in regard to any Intellectual Property.
(h)No Undisclosed Inventions. To the Company’s Knowledge, the Company has not received any notice under any Contract that constitutes a research or other collaborative agreement regarding the Intellectual Property that the other party to such Contract has developed an invention under such Contract for which the Company would have ownership or license rights under such Contract.
A-37
(i)Payment of Intellectual Property Costs. Except as described onSchedule 5.13(i), the Company has paid in full any and all costs whatsoever, including attorney’s fees, associated with the preparation, filing, prosecution and maintenance of the Patents and covering the period ending on the Closing Date, including any maintenance fees which become due for the Patents on or prior to the Closing Date.
5.14Title to the Assets.
(a) Except as described onSchedule 5.14 or in the Company Financial Statements, the Company has good and marketable title to, or have a valid leasehold interest or valid license or other contractual rights in, the properties and assets used by it in the Business, located on their premises or shown on the most recent Company Financial Statement or acquired thereafter, free and clear of all Encumbrances, except for (i) properties and assets disposed of in the ordinary course of business since December 31, 2009, (ii) Encumbrances for Taxes not yet due and payable or Encumbrances for Taxes which are being contested in good faith, and (iii) Encumbrances which are not material to the value of the properties or assets encumbered and which do not impair in any material respect the current use or operation of such properties and assets. The Company owns, or has a valid leasehold or other interest in, all assets (including all Intellectual Property) necessary for the conduct of the Company Business as currently conducted by the Company, and immediately after the Closing the Company will continue to own, or have a valid leasehold or other interest in all such assets so as to be able to conduct the Company Business in all respects after the Closing in substantially the same manner as the Company Business has been conducted prior to the Closing.
(b) The assets and properties owned by the Company together with the rights enjoyed by the Company, on the date hereof, constitute all of the assets, properties and rights which were used to achieve the financial results set forth on the Company Financial Statements, excepting only those assets and properties disposed of or otherwise transferred by the Company in the ordinary course of business.
5.15Land Use Matters. There are no pending or, to the Company’s Knowledge, threatened, legal actions or proceedings in the nature of condemnation proceedings that might prohibit, restrict or impair the use and occupancy of the property covered by any Company Leases, or result in the suspension, revocation, impairment, forfeiture or non-renewal of any required licenses, permits, certificates and approvals for the use and occupancy and operation of the property covered by any Company Leases, other than such prohibitions, restrictions, suspensions, revocations, impairments, forfeitures and non-renewals that individually or in the aggregate would not result in a Material Adverse Effect.
5.16Environmental Matters.
(a)Compliance. (i) The Company is in compliance in all material respects with all applicable Environmental Laws; (ii) the Company has not received any written communication from any Person or governmental entity that alleges that the Company is not in compliance with applicable Environmental Laws; and (iii) there have not been any Releases of Hazardous Materials by the Company, or, by any other party, at any property currently or formerly owned or operated by the Company that occurred during the period of the Company’s ownership or operation of such property.
(b)Environmental Permits. The Company has all Environmental Permits necessary for the conduct and operation of the Company Business. All such permits are in good standing or, where applicable, a renewal application has been timely filed and is pending agency approval. The Company is in compliance with all terms and conditions of all such Environmental Permits and are not required to make any expenditure in order to obtain or renew any Environmental Permits, except where the failure to obtain or be in such compliance and the requirement to make such expenditure do not or will not have a Material Adverse Effect on the Company Business.
(c)Environmental Claims. There are no Environmental Claims pending or, to the Company’s Knowledge, threatened, against the Company or against any real or personal property or operation that the Company owns, leases or manages.
A-38
5.17Insurance.Schedule 5.17 (i) lists the insurance policies currently maintained with respect to the Company and its assets and properties (summaries of which have been provided or made available to Purchaser), and (ii) specifically describes all claims made by the Company during the past three years under any such insurance policies maintained by the Company. All such policies listed onSchedule 5.17 are in full force and effect, all premiums due and payable thereon have been paid and no written or oral notice of cancellation or termination has been received with respect to any such policy which was not replaced on substantially similar terms prior to the date of such cancellation. All such policies will remain in full force and effect at least until the Closing Date.
5.18Employees and Labor Relations.
(a)Schedule 5.18(a) contains the names of all persons employed by the Company as of the date hereof (each, an “Employee” and collectively, the “Employees”), lists which Employees are leased, part-time or temporary employees, the salary, commission, bonus opportunity and current vacation accrual for each Employee, and indicates which Employees are currently on short-term or long-term disability, the date of commencement of employment and title. Except for the persons listed onSchedule 5.18(a), the Company does not have any employment, compensation, noncompetition, nonsolicitation or other similar arrangements with any individuals who perform services for the Company.
(b) Except as set forth onSchedule 5.18(b), all employees of the Company are “at will” employees, each of whom can be terminated at any time (subject to all applicable Laws) without penalty or premium and whose employment terms are solely governed by the current policy manual of the Company, a true and complete copy of which has been provided to Purchaser.
(c) Except as set forth onSchedule 5.18(c), (i) there is no current labor strike or work stoppage or lockout against or materially affecting the Company; during the past five (5) years there has not been any such action against the Company; and to the Company’s Knowledge, there has not been, and is not now, any such action threatened against or materially affecting the Company; (ii) none of the Employees of the Company are represented by a union or subject to a collective bargaining agreement and to the Company’s Knowledge, no union organizational campaign is in progress with respect to the Employees and no question concerning representation exists respecting such Employees; (iii) the Company is in compliance in all material respects with all applicable Laws respecting employment and employment practices, terms and conditions of employment and wages and hours and is not engaged in any unfair labor practice; and (iv) other than with Stockholders, Option Holders and Warrant holders (solely in their capacity as such) there are no agreements or arrangements between (x) the Company and (y) an individual consultant, former consultant, employee or former employee obligating the Company to make any payment to any such individual as a result of the transactions contemplated by this Agreement.
(d) Except as set forth onSchedule 5.18(d), there are no loans outstanding from the Company to any of the Employees.
(e) The Company is not in breach of any material terms of employment of any of the Employees nor to the Company’s Knowledge is any Employee in breach of any material term of his or her employment relationship.
(f) As of the date hereof, none of the Employees has given or received notice of termination of his or her employment.
(g) None of the Employees is the subject of any material disciplinary action nor is any Employee engaged in any grievance procedure and to the Company’s Knowledge, there is no matter or fact in existence which can be reasonably foreseen as likely to give rise to the same.
(h) The Company has complied in all material respects with the employment eligibility verification form requirements under the Immigration and Naturalization Act, as amended (“INA”), in recruiting, hiring, reviewing and documenting prospective employees for employment eligibility verification purposes and the Company has complied in all material respects with the paperwork provisions and anti-discrimination
A-39
provisions of the INA. The Company has obtained and maintained the employee records and I-9 forms in proper order as required by United States Law. To the Company’s Knowledge the Company does not employ any workers unauthorized to work in the United States.
5.19Litigation. Except as set forth onSchedule 5.19, as of the date hereof, there is no pending or, to the Company’s Knowledge, threatened action, proceeding or investigation by or before any court, governmental agency or arbitrator or other tribunal, which is or may be brought against or which involves the Company or the Company Business:
(a) which questions or challenges the validity of, or seeks damages or equitable relief on the basis of, this Agreement or any action taken or to be taken by the Company or the Principal Stockholders pursuant to this Agreement or in connection with the transactions contemplated hereby; or
(b) which might affect the right of Purchaser after the Closing Date to own the Company or to conduct the Company Business as presently conducted;
(c) nor to the Company’s Knowledge is there any valid basis for any such action, proceeding or investigation.
5.20Court Orders, Decrees, and Laws. Except as described inSchedule 5.20, there is no outstanding, or to the Company’s Knowledge threatened, order, writ, injunction, or decree of any court, governmental agency, or arbitration tribunal against the Company. The Company is in compliance in all material respects with all applicable federal, state or local Laws, rules, regulations, ordinances, zoning requirements, governmental restrictions, orders, judgments and decrees affecting, involving or relating to the Company Business, and the Company has not received any notices alleging any such violation. The foregoing shall be deemed to include Laws, rules and regulations relating to the federal patent, copyright, and trademark Laws, state trade secret and unfair competition Laws, and to all other applicable Laws, rules and regulations including, but not limited to, equal opportunity, wage and hour, and other employment matters, and antitrust and trade regulations, safety (including OSHA), building, zoning or health Laws, ordinances and regulations.
5.21Employee Benefit Plans; ERISA.
(a)Schedule 5.21(a) contains a list of each of the Company’s employee benefit plan, arrangement, policy or commitment within the meaning of Section 3(3) of ERISA and any employment, consulting or deferred compensation agreement, incentive compensation, bonus, executive compensation, severance, termination or post-employment pay, disability, hospitalization or other medical, dental, vision, life or other insurance, stock purchase, stock option, stock appreciation, stock award, pension, profit sharing, savings or retirement plan, program or arrangement, or any holiday or vacation practice, whether written or oral, tax-qualified under the Code or non-qualified, whether covered by ERISA or not, maintained or contributed to by the Company covering its employees, former employees, retirees or sales personnel or with respect to which the Company or any Commonly Controlled Entity, respectively, has or in the future could have any direct or indirect, actual or contingent liability (each, a “Plan” and collectively, the “Plans”). Except as set forth onSchedule 5.21(a), the Company will not incur any liability in connection with any Plan solely as a result of the consummation of the transactions contemplated by this Agreement. Except as set forth onSchedule 5.21(a), neither the Company nor any Commonly Controlled Entity has any legally binding oral or written plan or other commitment, whether covered by ERISA or not, to create or participate in any additional plan, agreement or arrangement or to modify or change any existing Plan in any manner. The Company has made available to Purchaser true and complete copies of the Plans, the trust agreements and other contracts (including any amendments to any of the foregoing) relating to the Plans and all other relevant documents governing or relating to the Plans (including, but not limited to, the latest summary plan descriptions, all other material employee communications, the latest annual report (and all schedules and attachments) filed with the IRS with respect to each of the Plans, as applicable, all material communication with any governmental entity or agency (including, without limitation, the Department of Labor and the IRS) for the last three fiscal years, and, if the Plan is intended to qualify under Section 401(a) of the Code, the most recent determination letter received from the IRS).
A-40
(b) Any Plan, including, but not limited to, any Plan that was an “employee pension benefit plan” as defined in Section 3(2) of ERISA, that the Company or any Commonly Controlled Entity has ever sponsored or maintained, or in or to which the Company or any Commonly Controlled Entity has ever participated or contributed, on behalf of its respective employees, former employees, retirees or sales personnel which was subsequently terminated, was terminated in compliance with the requirements of the Code and ERISA and neither the Company nor any Commonly Controlled Entity has incurred any liability with respect to such Plan or the termination of such Plan that is due and owing and has not yet been satisfied under the terms of the Plan, the Code, ERISA or any other Law or regulation pursuant to which Purchaser may incur liability or have liability attributed to it under any federal, state or local Law as a result of the consummation of the transactions contemplated by this Agreement. Neither the Company nor any Commonly Controlled Entity maintains or contributes to, nor, within the past five years has the Company or any Commonly Controlled Entity maintained or contributed to, (i) a “multiemployer plan,” as that term is defined in Section 414(f) of the Code or Sections 3(37) or 4001(a)(31) of ERISA or (ii) an “employee benefit pension plan,” as defined in Section 3(2) of ERISA, that is subject to Section 412 of the Code, Section 302 of ERISA or Title IV of ERISA. No Plan is a multiple employer plan within the meaning of Section 413(c) of the Code or Sections 4063, 4064 or 4066 of ERISA. The Company has not terminated any employee benefit plan as described in Section 3(3) of ERISA.
(c) Full payment has been made as of the Closing Date of all contributions or amounts (other than current outstanding routine claims for benefits) which the Company is required to contribute or pay under the terms of any Plan, and all contributions to any Plan which are required or recommended with respect to any period of time prior to the Closing have been made or such amounts have been accrued in accordance with GAAP. There are no funded benefit obligations for which contributions have not been made or properly accrued and there are no unfunded benefit obligations that have not been accounted for by reserves, or otherwise properly footnoted in accordance with GAAP on the Company Financial Statements.
Each of the Plans is and has been operated and administered in all material respects in accordance with applicable Laws, including but not limited to, ERISA and the Code, and all required material governmental filings and material participant disclosures have been made on a timely basis. With respect to each Plan, no event has occurred, and there exists no condition or set of circumstances in connection with which the Company could, directly or indirectly (through a Commonly Controlled Entity or otherwise), be subject to any liability under ERISA, the Code or any other applicable Law, except liability for benefit claims and funding obligations payable in the ordinary course. No prohibited transaction within the meaning of Section 406 of ERISA or 4975 of the Code, or breach of fiduciary duty under Title I of ERISA, has occurred with respect to any Plan or with respect to the Company or any Commonly Controlled Entity that could reasonably be expected to result in liability to the Company. Each Plan that is intended to qualify under Section 401(a) of the Code has received a currently effective favorable determination letter from the IRS, and such Plan and its related trust are exempt from taxation under Section 501(a) of the Code.
There are no pending, or, to the Company’s Knowledge, threatened or anticipated claims, litigation, administrative actions or proceedings against or otherwise involving any of the Plans or related trusts, or any fiduciary thereof, by any governmental agency, or by any employee, former employee, leased employee, former leased employee, retiree or sales personnel or by any participant or beneficiary covered under any of the Plans, or otherwise involving the Plans (other than routine claims for benefits). There is no judgment, decree, injunction, rule or order of any court, governmental body, commission, agency or arbitrator outstanding against or in favor of any Plan or, to the Company’s Knowledge, any fiduciary thereof in that capacity. No assets of the Company are allocated to or held in a “rabbi trust” or similar funding vehicle or subject to any lien under ERISA.
(d) Each Plan that is a “group health plan” (as defined in Section 607(1) of ERISA) has been operated in compliance in all material respects with the provisions of COBRA (Section 4980B of the Code), the Health Insurance Portability and Accountability Act of 1996 and, to the extent required, any applicable similar state Law. Each Plan that is an “employee welfare benefit plan” within the meaning of Section 3(1) of ERISA (i) is a fully insured plan, (ii) may be terminated after the Closing Date in accordance with the
A-41
terms of any underlying contract without liability to the Company, other than liabilities relating to claims incurred prior to the effective date of the termination of such Plan, (iii) is not a multiple employer welfare arrangement within the meaning of Section 3(40) of ERISA and (iv) has no reserves, assets, surpluses or prepaid premiums. Except as set forth onSchedule 5.21(d), the Company does not currently provide or have a current obligation to provide for any material post-retirement or post-employment health and welfare benefits, including but not limited to, severance, salary continuation, termination, disability, death, or retiree health or medical benefits except as required by applicable Law.
(e) Except as set forth onSchedule 5.21(e), the consummation of the transactions contemplated by this Agreement will not, of itself, entitle any current or former employee or leased employee of the Company to severance pay, unemployment compensation or any similar payment or accelerate the time of payment or vesting, or increase the amount of compensation due to, or in respect of, any current or former employee or leased employee, nor will it result in the breach of any agreement with any such employee.
(f) None of the assets of the Company is subject to any lien under Section 302(f) of ERISA or Section 412(n) of the Code.
5.22Broker’s Fees. Neither the Company nor any of its representatives or Persons acting or its behalf have made any commitment or done any other act which would create any liability for any brokerage, finder’s or similar fee or commission in connection with the transactions contemplated by this Agreement.
5.23Related-Party Transactions. Except as set forth onSchedule 5.23, the Company is not to the Company’s Knowledge, a party to any contract, agreement, license, lease, or arrangement with, or any other commitment to, directly or indirectly, (i) any Stockholder or any Affiliate of any Stockholder of the Company; (ii) any officer or employee of the Company; (iii) any corporation, trust, or other entity in which any Stockholder or any such officer or employee has a material equity or participating interest; (iv) any partnership in which any Stockholder or any such officer or employee has a partnership or participating interest, or (v) any such officer or employee, in each case, relating to or involving the Company Business, except, in each instance, for existing compensation arrangements listed inSchedule 5.21(a). Each contract, agreement, license, lease, arrangement, and commitment listed inSchedule 5.23 was entered into by the Company in the ordinary course of business.
5.24Licenses; Permits.Schedule 5.24 sets forth, with respect to the Company, all Governmental Authorizations and Permits. Such Governmental Authorizations and Permits constitute all approvals, authorizations, certifications, consents, variances, permissions, licenses, or permits to or from, or filings, notices, or recordings to or with, federal, state, or local governmental authorities that are required for the ownership and use of the assets of the Company and the conduct of the Company Business under federal, state, and local Law, regulation, ordinance, zoning requirement, governmental restriction, order, judgment, or decree, except where the failure to obtain such Governmental Authorizations and/or Permits does not or will not, individually or in the aggregate, have a Material Adverse Effect. The Company is in compliance in all material respects with all terms and conditions of such Governmental Authorizations and Permits. All of such Governmental Authorizations and Permits are in full force and effect, and to Knowledge, no suspension or cancellation of any of them is being threatened, nor will any of such Governmental Authorizations and/or Permits be affected by the consummation of the transactions described in this Agreement. The Company is in compliance in all material respects with all other applicable limitations, restrictions, conditions, standards, prohibitions, requirements, obligations, schedules, and timetables contained in any Law governing the Company Business.
5.25Accounts Receivable. All Accounts Receivable of the Company have arisen in the ordinary course of business in arms length transactions for goods actually sold and services actually performed or to be performed. The Company has available in its records copies of invoices or electronic records of services performed and all existing Contracts with respect to all such Accounts Receivable.
5.26Disclosure. The representations and warranties of the Company in this Agreement and each document (including, but not limited to, financial statements, exhibits and schedules), certificate, or other writing furnished
A-42
or to be furnished by the Company pursuant to the provisions hereof or in connection with the transactions contemplated hereby, when considered as a whole, do not contain any untrue statement of material fact and do not omit to state any material fact necessary in order to make the statements contained herein or therein not misleading.
ARTICLE VI
REPRESENTATIONS AND WARRANTIES OF PURCHASER AND MERGER SUB
Purchaser and Merger Sub hereby represent and warrant to the Company and the Stockholders’ Representative on behalf of the Stockholders that the statements contained in this Article 6 are complete and accurate as of the date hereof, except as set forth in the SEC Documents (as defined in Section 6.21 below), or in the written disclosure schedule delivered by Purchaser to the Company. Such Purchaser disclosure schedule shall be arranged in sections corresponding to the numbered and lettered sections and subsections contained in this Article 6, and the disclosures in any section or subsection of the Purchaser’s disclosure schedule shall qualify only the corresponding section or subsection of this Article 6, unless the disclosures in one section or subsection reasonably appear to apply to another section or subsection of the Purchaser’s disclosure schedule.
6.01Organization. Each of Purchaser and Merger Sub is a corporation duly organized, validly existing and in good standing under the Laws of the State of Delaware. Each of Purchaser and Merger Sub has all requisite corporate power and authority to enable it to own, lease or otherwise hold its properties and assets and to carry on its business as presently conducted. Each of Purchaser and Merger Sub is duly qualified to do business and in good standing in each jurisdiction in which the nature of its business or the ownership, leasing or holding of its properties makes such qualification necessary. A list of the jurisdictions in which each of Purchaser and Merger Sub is so qualified is set forth onSchedule 6.01. A list of all of the Subsidiaries of Purchaser is set forth onSchedule 6.01. Merger Sub does not have any Subsidiaries.
6.02Capital Stock and Related Matters; No Investments.
(a) The authorized capital stock of Purchaser consists of 90,100,000 authorized shares consisting of: (i) 90,000,00 shares of Purchaser Common Stock, and (ii) 100,000 shares of preferred stock, par value $0.01 (“Purchaser Preferred Stock”), of which 90,000 shares have been designated as Series A Junior Participating Preferred Stock. As of the date hereof 48,881,483 shares of Purchaser Common Stock are issued and outstanding, and no shares of Purchaser Preferred Stock are issued and outstanding (collectively, the “Purchaser Shares”). The Purchaser Shares constitute all of the issued and outstanding shares of capital stock of Purchaser and are held by Purchaser’s stockholders. The authorized capital stock of Merger Sub consists of 1,000 shares of common stock, $0.001 par value per share, 100 of which are issued and outstanding and held by Purchaser.
(b) Except as set forth onSchedule 6.02 hereto, neither Purchaser nor Merger Sub has any outstanding stock or securities convertible into or exchangeable for any shares of its capital stock or containing any profit participation features, nor does it have outstanding any rights or options to subscribe for or purchase its capital stock or any stock or securities convertible into or exchangeable for any shares of its capital stock or any stock appreciation rights or phantom stock plans. Neither Purchaser nor Merger Sub is subject to any obligation (contingent or otherwise) to repurchase or otherwise acquire or retire any shares of its capital stock or any warrants, options or other rights to acquire its capital stock. All of the outstanding shares of Purchaser’s and Merger Sub’s capital stock have been validly issued and are fully paid and nonassessable.
(c) Except as set forth onSchedule 6.02, there are no statutory or contractual shareholders’ preemptive rights or rights of refusal with respect to the Merger. Neither Purchaser nor Merger Sub has violated any applicable federal or state securities Laws in connection with the offer or sale of the Shares.
(d) Except for the Subsidiaries of Purchaser set forth onSchedule 6.01, neither Purchaser nor Merger Sub owns or holds any Investment in any Person.
A-43
6.03Authorization. Subject to approvals as are required by Law, each of Purchaser and Merger Sub has all requisite power and authority (or legal capacity, as the case may be) to enter into this Agreement and the Related Documents to be executed and delivered by Purchaser and Merger Sub pursuant hereto or in connection with the transactions contemplated hereby or thereby, and to consummate the transactions contemplated hereby and thereby. Subject only to Purchaser Stockholder Approval, all acts and other proceedings required to be taken by Purchaser to authorize the execution, delivery and performance of this Agreement and the Related Documents to which it is a party, and the consummation of the transactions contemplated hereby and thereby have been (or by the Closing will have been) duly and properly taken.
6.04Valid and Binding. This Agreement constitutes (and, when executed and delivered at Closing, each Related Document, to the extent that Purchaser or Merger Sub is a party thereto, will constitute) a valid and binding obligation of Purchaser or Merger Sub, enforceable against the signatory in accordance with its terms, except that (i) such enforcement may be limited by or subject to any bankruptcy, insolvency, reorganization, moratorium or similar Laws now or hereafter in effect relating to or limiting creditors’ rights generally and (ii) the remedy of specific performance and injunctive and other forms of equitable relief are subject to certain equitable defenses and to the discretion of the court before which any proceeding therefor may be brought.
6.05No Violation. The execution and delivery of this Agreement and each Related Document by Purchaser and Merger Sub, and the consummation of the transactions contemplated hereby and thereby and compliance with the terms hereof and thereof does not and will not (subject only to obtaining any required consents, approvals, authorizations, exemptions or waivers set forth onSchedule 6.05 hereto) conflict with, or result in any violation of or default (with or without notice or lapse of time, or both) under, or give rise to a right of termination, cancellation or acceleration of any obligation or to loss of a material benefit under or result in the creation of any Encumbrance of any kind upon any of Purchaser’s assets under, any provision of (i) the certificate of incorporation or by-laws of Purchaser, (ii) any note, bond, mortgage, indenture, deed of trust, license, lease, contract, commitment or loan or other agreement to which Purchaser is a party or by which any of its properties or assets are bound, or (iii) any statute, regulation, rule, injunction, judgment, order, Law, ordinance, decree, ruling, charge or other restriction of any government, governmental agency, or court applicable to Purchaser or its property or assets, except in the case of clauses (ii) and (iii) for any such violations, breaches, defaults, rights of termination, cancellation or acceleration or requirements which, individually or in the aggregate would not have a Material Adverse Effect or would not adversely affect the ability of Purchaser to consummate the transactions contemplated by this Agreement.
6.06Consents and Approvals. Other than the filing of the Delaware Certificate of Merger with the Secretary of State of the State of Delaware, or as otherwise set forth inSchedule 6.06 hereto, no consent, approval or authorization of, or declaration, filing or registration with, any governmental or regulatory authority or any court or other tribunal, and no consent or waiver of any party to any Contract is required to be obtained by Purchaser or Merger Sub in connection with the execution, delivery and performance of this Agreement and the Related Documents or the consummation of the transactions contemplated hereby or thereby.
6.07Interim Operations. Except as set forth onSchedule 6.07, during the period from December 31, 2009 until the date of this Agreement:
(a) the Purchaser Business has been conducted by Purchaser only in the ordinary course consistent with past practices;
(i) with respect to the Purchaser Business, Purchaser has not (except as otherwise permitted by this Agreement):
(ii) suffered any Material Adverse Effect;
(iii) incurred any liabilities or obligations (absolute, accrued, contingent or otherwise), except in the ordinary and usual course of business and consistent with past practice, or increased, or experienced any change in any assumptions underlying or methods of calculating, any bad debt, contingency or other reserves;
A-44
(iv) paid, discharged or satisfied any claims, liabilities or obligations (absolute, accrued, contingent or otherwise), other than the payment, discharge or satisfaction in the ordinary and usual course of business and consistent with past practice liabilities and obligations reflected or reserved against in the Purchaser Financial Statements or incurred in the ordinary course of business and consistent with past practice;
(v) permitted or allowed Purchaser’s assets to be subjected to any Encumbrance (except Encumbrances created by Law);
(vi) canceled any debts owing to Purchaser or waived any claims or rights, except for amounts each individually under $5,000;
(vii) sold, transferred, or otherwise disposed of, or transferred or granted any rights under any lease, license or agreement with respect to, any of its assets, except in the ordinary course of business and consistent with past practices;
(viii) disposed of, failed to take reasonable steps to protect, or permitted to lapse, any rights for the use of, the Purchaser’s Intellectual Property, or disposed of, failed to take reasonable steps to protect any Proprietary Information;
(ix) made any change in any method of accounting or accounting practice;
(x) made any single capital expenditure or future commitment in excess of $20,000, or made aggregate capital expenditures or future commitments in excess of $50,000;
(xi) made any material change in the manner in which the Purchaser Business is conducted;
(xii) made any material change in policies relating to the Purchaser Business (whether or not in the ordinary and usual course of business);
(xiii) had any labor dispute or received notice of any grievance;
(xiv) borrowed or agreed to borrow any funds;
(xv) paid and/or declared any dividends with respect to its shares of capital stock, whether in shares of capital stock or other property;
(xvi) granted to any officer or employee any increase in compensation or benefits, other than increases in compensation or benefits to employees in the ordinary and usual course of business;
(xvii) paid any pension, retirement allowance or other employee benefit not required by any plan, policy or program identified onSchedule 6.07 hereto or any employment agreement set forth onSchedule 6.07 hereto;
(xviii) adopted, agreed to adopt, or made any announcement regarding the adoption of (i) any new pension, retirement or other employee benefit plan, program or policy, or (ii) any amendments to any existing pension, retirement or other employee benefit plan, policy or program identified onSchedule 6.07 unless otherwise required by applicable Law; or
(xix) suffered or agreed to take any of the actions set forth in this subparagraph (ii).
(b) None of the assets of Purchaser have been affected in any way as a result of fire, explosion or other casualty (whether or not covered by insurance).
6.08Undisclosed Liabilities. The Purchaser does not have any liabilities or obligations of any nature (whether accrued, absolute, contingent or otherwise), except for liabilities or obligations (i) disclosed onSchedule 6.08 hereto, (ii) disclosed in the Purchaser Financial Statements, (iii) arising in the ordinary course of business consistent with past practice under any Material Contract, or (iv) incurred in the ordinary course of business consistent with past practices since December 31, 2009.
A-45
6.09Taxes.
(a) Except as set forth onSchedule 6.09(a), Purchaser has timely filed all Tax Returns required by applicable Law to be filed by or on behalf of Purchaser on or prior to the date hereof, and such Tax Returns are true, complete, correct and in conformity with applicable Tax Laws in all material respects. Purchaser has paid all Taxes (whether or not required to be shown on any Tax Return) required to be paid by Purchaser on or before the date hereof, or where payment is not yet due, has established or will establish, on or before the Closing Date, in accordance with GAAP, an adequate reserve on its books and financial records for the payment of all Taxes due from the Company, with respect to any Pre-Closing Tax Period (including any Pre-Closing Straddle Tax Liability) and the Closing Date Tax Period. All Taxes that Purchaser is or was required by Law to withhold, deposit or collect have been duly withheld, deposited or collected and, to the extent required, have been paid to the relevant Tax Authority. Purchaser has timely complied with all information and reporting and backup withholding requirements, including maintenance of any required records with respect thereto, in connection with amounts paid or owing to any employee, creditor, independent contractor or other third party.
(b) Except as described inSchedule 6.09(b), the Tax Returns with respect to Purchaser have not been subject to an Audit since its incorporation, and there are no ongoing Audits of Purchaser. Purchaser has not received written notice by any Tax Authority of an Audit nor to Purchaser’s Knowledge is any such Audit contemplated, threatened or pending.
(c) To Purchaser’s Knowledge, there are no claims, investigations, actions or proceedings pending or, to Purchaser’s Knowledge, threatened, against Purchaser by any Tax Authority for any past due Taxes with respect to which Purchaser would be liable. There has been no waiver by Purchaser of any applicable statute of limitations nor any consent for the extension of the time for the assessment of any Tax against Purchaser.
(d) Purchaser is not delinquent in the payment of any amount of Taxes and there are no Tax liens upon any property or assets of Purchaser, except liens for Taxes not yet due and payable.
(e) Purchaser has not agreed, nor is it required, to make any adjustment under Section 481(a) of the Code by reason of a change in accounting method or otherwise.
(f) Purchaser is not liable for the Taxes of any Person other than Purchaser including, without limitation, (i) under U.S. Treasury Regulations Section 1.1502-6 (or comparable provision of state, local or foreign Law), (ii) as a transferee or successor, or (iii) by contract, indemnity or otherwise. Except as set forth onSchedule 6.09(f), Purchaser has not ever been a member of a consolidated, combined or unitary group for federal, state, local or foreign Tax purposes or have ever been included as part of a consolidated, combined or unitary Tax Return.
(g) Purchaser is not, nor has it ever been, a party to any Tax sharing agreement, Tax indemnity agreement or other similar Tax sharing arrangement.
(h) Except as set forth inSchedule 6.09(h), since January 1, 2004 no claim has been asserted in writing by a Tax Authority in a jurisdiction where Purchaser has not filed Tax Returns that Purchaser is or may be subject to taxation by that jurisdiction. To Purchaser’s Knowledge no such claim was asserted prior to 2004.
(i)Schedule 6.09(i) sets forth each of the states for which Purchaser is currently filing income or franchise Tax Returns (or similar type of Tax Returns) or is required to file for the current Taxable Period.
(j) Purchaser has provided the Company with copies of: (i) all Tax Returns filed by, or on behalf of, Purchaser for the Post-2006 Period; (ii) all notices, protests or other correspondence relating to any Post-2006 Period Taxes or Tax Returns; (iii) any letter rulings, determination letters or similar documents issued by any Tax Authority with respect to Purchaser; (iv) any closing agreement entered into by Purchaser with any Tax Authority; and (vi) any Tax Return workpapers relating to the Tax Returns referred to in clause (i). Purchaser has not entered into an exchange under Code Section 1031 with a “related person” (within the meaning of Code Section 1031(f)(3)) which could result in Purchaser being required to recognize gain under Code Section 1031(f)(1). Purchaser is not taking into account any gain under the installment method of Code Section 453.
A-46
(k) Purchaser is not, and has never been, a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code and the Company is not required to withhold tax on the purchase of the stock of Purchaser by reason of Section 1445 of the Code. Except as set forth inSchedule 6.10(l), Purchaser has not entered into any compensatory agreements with respect to the performance of services which payment thereunder would result in a nondeductible expense pursuant to Section 162(m) or 280G of the Code or an excise tax to the recipient of such payment pursuant to Section 4999 of the Code. Purchaser has not participated in an international boycott as defined in Section 999 of the Code.
(l) Purchaser has not participated in any transaction that is a reportable transaction within the meaning of Treasury Regulation Section 1.6011-4 or Section 6707A of the Code.
(m) Neither Purchaser nor, to the knowledge of Purchaser, any of Purchaser’s affiliates has taken or agreed to take any action that would prevent the Merger from qualifying as a reorganization within the meaning of Section 368(a) of the Code. Purchaser is not aware of any agreement, plan or other circumstance that would prevent the Merger from qualifying as a reorganization within the meaning of Section 368(a) of the Code.
6.10Contracts and Commitments.
(a)Schedule 6.10(a) lists any Contract to which Purchaser or any Subsidiary of Purchaser is a party and (i) that is a “material contract” as defined in Section 601(b)(10) of Regulation S-K promulgated by the SEC, (ii) that involves payment or receipt by Purchaser or any Subsidiary of Purchaser under any such Contract of $100,000 or more in the aggregate or obligations after the date of this Agreement in excess of $100,000 in the aggregate or (iii) that is material to the Purchaser Business. Each contract of the type described in this Section 6.10(a), whether or not set forth on Schedule 6.10(a), is referred to herein as a “Purchaser Material Contract.” Except as set forth onSchedule 6.10(a) hereto, (i) all Purchaser Material Contracts constitute valid and binding agreements of Purchaser or any Subsidiary of Purchaser, and, to Purchaser’s Knowledge, each other party thereto, enforceable in accordance with their terms except that (A) such enforcement may be limited by or subject to any bankruptcy, insolvency, reorganization, moratorium or similar Laws now or hereafter in effect relating to or limiting creditors’ rights generally and (B) the remedy of specific performance and injunctive and other forms of equitable relief are subject to certain equitable defenses and to the discretion of the court before which any proceeding therefor may be brought, (ii) with respect to the Purchaser Material Contracts there are no existing material defaults by Purchaser or any Subsidiary of Purchaser, or, to Purchaser’s Knowledge, by any other party thereto and there is no event which (whether with or without notice, lapse of time or the happening or occurrence of any other event) would constitute a material default under the Purchaser Material Contracts by Purchaser or any Subsidiary of Purchaser or, to Purchaser’s Knowledge, by any other party thereto, (iii) neither Purchaser nor any Subsidiary of Purchaser is restricted by agreement from carrying on the Purchaser Business in any geographical location, and (iv) there are no negotiations pending or in progress to revise any Purchaser Material Contract.
(b) Purchaser does not own any Real Property. The only leases for Real Property to which Purchaser is a party are set forth onSchedule 6.10(b) (collectively, and together with all addenda, the “Purchaser Leases”). With respect to the Purchaser Leases, (i) each such Purchaser Lease is in full force and effect and is binding and enforceable against Purchaser, and to Purchaser’s Knowledge, the lessor, in accordance with its terms except that (A) such enforcement may be limited by or subject to any bankruptcy, insolvency, reorganization, moratorium or similar Laws now or hereafter in effect relating to or limiting creditors’ rights generally and (B) the remedy of specific performance and injunctive and other forms of equitable relief are subject to certain equitable defenses and to the discretion of the court before which any proceeding therefor may be brought; (ii) all rental and other charges payable pursuant to the terms and conditions of each such Purchaser Lease have been paid and no rent has been paid in advance more than 30 days; (iii) there are no charges, offsets or defenses against the enforcement by the respective lessors thereunder of any agreement, covenant or condition on the part of Purchaser to be performed or observed pursuant to the terms of each Purchaser Lease; (iv) there are no defaults by Purchaser of any agreement, covenant or condition on the part
A-47
of Purchaser to be performed or observed pursuant to the terms of each Purchaser Lease; (v) there are no actions or proceedings pending or, to Purchaser’s Knowledge, threatened, by the lessor under each such Purchaser Lease; (vi) except for security deposits required by the Purchaser Leases and identified onSchedule 6.10(b), the respective lessor does not hold any deposits for Purchaser’s accounts under each such Purchaser Lease; (vii) the Merger and the transactions contemplated hereby will not constitute a prohibited transfer under any Purchaser Lease; and (viii) to Purchaser’s Knowledge, there are no defaults by respective lessors of any agreement, covenant or condition on the part of such lessor to be performed or observed pursuant to the terms of any such Purchaser Lease. The current expiration date and remaining options to extend each Purchaser Lease are as set forth onSchedule 6.10(b) hereto.
6.11Intellectual Property.
(a)General.Schedule 6.11(a) contains a complete and accurate list of all Intellectual Property Registrations, including all Patents.
(b)Valid Assignment. In each case in which Purchaser has acquired ownership of (rather than licenses or other rights to) any Intellectual Property from any Person, Purchaser has obtained rights in the interest and title of the Intellectual Property. To the extent required by, and in accordance with, applicable Laws and regulations, Purchaser has recorded each assignment to it of any Intellectual Property Registrations with the USPTO or equivalent Patent Agencies outside the United States.
(c)No Infringement. To Purchaser’s Knowledge, no Person is infringing or misappropriating any Intellectual Property or Licensed Rights as of the Effective Date. Purchaser has not received written notice of a claim from any Person claiming that any Intellectual Property or Licensed Rights infringes or misappropriates any rights of such Person or constitutes unfair competition or trade practices under the Laws of any jurisdiction. With respect to the Patents, although the validity of claims regarding novelty, non-obviousness and enablement have not been established, Purchaser’s claims and representations made to the USPTO and other Patent Agencies in its applications for Patents regarding novelty, non-obviousness and enablement were made in good faith, and Purchaser has no Purchaser Knowledge of any facts which Purchaser reasonably believes would cause such claims or representations to be untrue or misleading. To Purchaser’s Knowledge, there is no unexpired Patent of any third party that includes claims that would be infringed by or that would or do limit the scope of the Purchaser Business as currently conducted.
(d)Confidentiality. Purchaser has taken all actions reasonably necessary to maintain and protect its rights in the Intellectual Property, including in Trade Secrets and other Proprietary Information. Without limiting the foregoing, Purchaser has and enforces a policy requiring each employee, consultant, contractor and other third parties with access to the Trade Secrets to execute proprietary information and confidentiality agreements, and all such current and former employees, consultants, contractors and third parties have executed such an agreement. To Purchaser’s Knowledge, there has been no violation or unauthorized disclosure or use of any Intellectual Property.
(e)No Transfer of Rights. Except as disclosed onSchedule 6.11(e), Purchaser is the exclusive owner of all the Intellectual Property owned by Purchaser and no other person owns or has any rights to any of the Intellectual Property and no Person has ownership rights or license rights to improvements made by Purchaser in any Patents. Purchaser has not transferred to any other Person any interest in, granted to any other Person any license, sublicense or other right to use, authorized the retention by any Person of any rights to use or joint ownership of, or entered into a covenant, in favor of any other Person, not to sue for infringement of, any Intellectual Property, except as disclosed onSchedule 6.11(e).
(f)Exclusive Rights. All of the Intellectual Property, excluding any Licensed Rights, was created solely by either (A) Employees of Purchaser acting within the scope of their employment or (B) employees of third parties or consultants or contractors of Purchaser who have validly and irrevocably assigned all of their rights, including rights to all Intellectual Property therein, to Purchaser.
A-48
(g)No Adverse Proceedings. To Purchaser’s Knowledge, there are no interference actions or other Proceedings pending, or any written communication that threatens an interference action or other Proceeding, before any Patent Agency or other governmental entity in any jurisdiction in regard to any Intellectual Property.
(h)No Undisclosed Inventions. To Purchaser’s Knowledge, Purchaser has not received any notice under any Contract that constitutes a research or other collaborative agreement regarding the Intellectual Property that the other party to such Contract has developed an invention under such Contract for which Purchaser would have ownership or license rights under such Contract.
(i)Payment of Intellectual Property Costs. Except as described onSchedule 6.11(i), Purchaser has paid in full any and all costs whatsoever, including attorney’s fees, associated with the preparation, filing, prosecution and maintenance of the Patents and covering the period ending on the Closing Date, including any maintenance fees which become due for the Patents on or prior to the Closing Date.
6.12Title to the Assets.
(a) Except as described onSchedule 6.12 or in the SEC Documents or in the Purchaser Financial Statements, Purchaser has good and marketable title to, or has a valid leasehold interest or valid license or other contractual rights in, the properties and assets used by it in the Purchaser Business, located on their premises or shown on the most recent Purchaser Financial Statement or acquired thereafter, free and clear of all Encumbrances, except for (i) properties and assets disposed of in the ordinary course of business since December 31, 2009, (ii) Encumbrances for Taxes not yet due and payable or Encumbrances for Taxes which are being contested in good faith, and (iii) Encumbrances which are not material to the value of the properties or assets encumbered and which do not impair in any material respect the current use or operation of such properties and assets. Purchaser owns, or has a valid leasehold or other interest in, all assets (including all Intellectual Property) necessary for the conduct of the Purchaser Business as currently conducted by Purchaser, and immediately after the Closing Purchaser will continue to own, or have a valid leasehold or other interest in all such assets so as to be able to conduct the Purchaser Business in all respects after the Closing in substantially the same manner as the Purchaser Business has been conducted prior to the Closing.
(b) The assets and properties owned by Purchaser, together with the rights enjoyed by Purchaser, on the date hereof, constitute all of the assets, properties and rights which were used to achieve the financial results set forth on the Purchaser Financial Statements, excepting only those assets and properties disposed of or otherwise transferred by Purchaser in the ordinary course of business.
6.13Employees and Labor Relations.
(a) The SEC Documents contain the names of all the executive officers employed by Purchaser as of the date hereof, and list the salary and compensation arrangements for each named executive officer (as such term is defined in Item 402 of Regulation S-K).
(b) Except as set forth onSchedule 6.13(b), all employees of Purchaser are “at will” employees, each of whom can be terminated at any time (subject to all applicable Laws) without penalty or premium and whose employment terms are solely governed by the current policy manual of the Company, a true and complete copy of which has been provided to Purchaser.
(c) Except as set forth onSchedule 6.13(c), (i) there is no current labor strike or work stoppage or lockout against or materially affecting Purchaser; during the past five (5) years there has not been any such action against Purchaser; and to Purchaser’s Knowledge, there has not been, and is not now, any such action threatened against or materially affecting Purchaser; (ii) none of the employees of Purchaser are represented by a union or subject to a collective bargaining agreement and to Purchaser’s Knowledge, no union organizational campaign is in progress with respect to such employees and no question concerning representation exists respecting such employees; (iii) Purchaser is in compliance in all material respects with
A-49
all applicable Laws respecting employment and employment practices, terms and conditions of employment and wages and hours and is not engaged in any unfair labor practice; and (iv) other than with Purchaser’s stockholders, option holders and warrant holders (solely in their capacity as such) there are no agreements or arrangements between (x) Purchaser and (y) an individual consultant, former consultant, employee or former employee obligating Purchaser to make any payment to any such individual as a result of the transactions contemplated by this Agreement.
(d) Except as set forth onSchedule 6.13(d), there are no loans outstanding from Purchaser to any of its employees.
(e) Purchaser is not in breach of any material terms of employment of any of its employees nor to Purchaser’s Knowledge is any employee in breach of any material term of his or her employment relationship.
(f) As of the date hereof, none of Purchaser’s employees has given or received notice of termination of his or her employment.
(g) None of Purchaser’s employees is the subject of any material disciplinary action nor is any employee engaged in any grievance procedure and to Purchaser’s Knowledge, there is no matter or fact in existence which can be reasonably foreseen as likely to give rise to the same.
(h) Purchaser has complied in all material respects with the employment eligibility verification form requirements under the INA, in recruiting, hiring, reviewing and documenting prospective employees for employment eligibility verification purposes and Purchaser has complied in all material respects with the paperwork provisions and anti-discrimination provisions of the INA. Purchaser has obtained and maintained the employee records and I-9 forms in proper order as required by United States Law. To Purchaser’s Knowledge Purchaser does not employ any workers unauthorized to work in the United States.
6.14Litigation. Except as set forth in the SEC Documents and onSchedule 6.14, as of the date hereof, there is no pending or, to Purchaser’s Knowledge, threatened action, proceeding or investigation by or before any court, governmental agency or arbitrator or other tribunal, which is or may be brought against or which involves Purchaser or the Purchaser’s Business:
(a) which questions or challenges the validity of, or seeks damages or equitable relief on the basis of, this Agreement or any action taken or to be taken by Purchaser or Merger Sub pursuant to this Agreement or in connection with the transactions contemplated hereby; or
(b) which might affect the right of Purchaser to conduct the Purchaser’s Business as presently conducted;
(c) nor to Purchaser’s Knowledge is there any valid basis for any such action, proceeding or investigation.
6.15Court Orders, Decrees, and Laws. Except as described inSchedule 6.15, there is no outstanding, or to the Purchaser’s Knowledge threatened, order, writ, injunction, or decree of any court, governmental agency, or arbitration tribunal against the Purchaser. The Purchaser is in compliance in all material respects with all applicable federal, state or local Laws, rules, regulations, ordinances, zoning requirements, governmental restrictions, orders, judgments and decrees affecting, involving or relating to the Purchaser’s Business, and the Purchaser has not received any notices alleging any such violation. The foregoing shall be deemed to include Laws, rules and regulations relating to the federal patent, copyright, and trademark Laws, state trade secret and unfair competition Laws, and to all other applicable Laws, rules and regulations including, but not limited to, equal opportunity, wage and hour, and other employment matters, and antitrust and trade regulations, safety (including OSHA), building, zoning or health Laws, ordinances and regulations.
6.16Licenses Permits.Schedule 6.16 sets forth, with respect to the Purchaser, all Governmental Authorizations and Permits. Such Governmental Authorizations and Permits constitute all approvals, authorizations, certifications, consents, variances, permissions, licenses, or permits to or from, or filings, notices,
A-50
or recordings to or with, federal, state, or local governmental authorities that are required for the ownership and use of the assets of the Purchaser and the conduct of the Purchaser’s Business under federal, state, and local Law, regulation, ordinance, zoning requirement, governmental restriction, order, judgment, or decree, except where the failure to obtain such Governmental Authorizations and/or Permits does not or will not, individually or in the aggregate, have a Material Adverse Effect. The Purchaser is in compliance in all material respects with all terms and conditions of such Governmental Authorizations and Permits. All of such Governmental Authorizations and Permits are in full force and effect, and to the Purchaser’s Knowledge, no suspension or cancellation of any of them is being threatened, nor will any of such Governmental Authorizations and/or Permits be affected by the consummation of the transactions described in this Agreement. The Purchaser is in compliance in all material respects with all other applicable limitations, restrictions, conditions, standards, prohibitions, requirements, obligations, schedules, and timetables contained in any Law governing the Purchaser’s Business.
6.17Employee Benefit Plans; ERISA.
(a)Schedule 6.17(a) contains a list of each Plan maintained or contributed to by Purchaser covering its employees, former employees, retirees or sales personnel or with respect to which Purchaser or any Commonly Controlled Entity, respectively, has or in the future could have any direct or indirect, actual or contingent liability. Except as set forth onSchedule 6.17(a), Purchaser will not incur any liability in connection with any Plan solely as a result of the consummation of the transactions contemplated by this Agreement. Except as set forth onSchedule 6.17, neither Purchaser nor any Commonly Controlled Entity has any legally binding oral or written plan or other commitment, whether covered by ERISA or not, to create or participate in any additional plan, agreement or arrangement or to modify or change any existing Plan in any manner. Purchaser has made available to Company true and complete copies of the Plans, the trust agreements and other contracts (including any amendments to any of the foregoing) relating to the Plans and all other relevant documents governing or relating to the Plans (including, but not limited to, the latest summary plan descriptions, all other material employee communications, the latest annual report (and all schedules and attachments) filed with the IRS with respect to each of the Plans, as applicable, all material communication with any governmental entity or agency (including, without limitation, the Department of Labor and the IRS) for the last three fiscal years, and, if the Plan is intended to qualify under Section 401(a) of the Code, the most recent determination letter received from the IRS).
(b) Any Plan, including, but not limited to, any Plan that was an “employee pension benefit plan” as defined in Section 3(2) of ERISA, that Purchaser or any Commonly Controlled Entity has ever sponsored or maintained, or in or to which Purchaser or any Commonly Controlled Entity has ever participated or contributed, on behalf of its respective employees, former employees, retirees or sales personnel which was subsequently terminated, was terminated in compliance with the requirements of the Code and ERISA and neither Purchaser nor any Commonly Controlled Entity has incurred any liability with respect to such Plan or the termination of such Plan that is due and owing and has not yet been satisfied under the terms of the Plan, the Code, ERISA or any other Law or regulation pursuant to which Purchaser may incur liability or have liability attributed to it under any federal, state or local Law as a result of the consummation of the transactions contemplated by this Agreement. Neither Purchaser nor any Commonly Controlled Entity maintains or contributes to, nor, within the past five years has Purchaser or any Commonly Controlled Entity maintained or contributed to, (i) a “multiemployer plan,” as that term is defined in Section 414(f) of the Code or Sections 3(37) or 4001(a)(31) of ERISA or (ii) an “employee benefit pension plan,” as defined in Section 3(2) of ERISA, that is subject to Section 412 of the Code, Section 302 of ERISA or Title IV of ERISA. No Plan is a multiple employer plan within the meaning of Section 413(c) of the Code or Sections 4063, 4064 or 4066 of ERISA. Purchaser has not terminated any employee benefit plan as described in Section 3(3) of ERISA.
(c) Full payment has been made as of the Closing Date of all contributions or amounts (other than current outstanding routine claims for benefits) which Purchaser is required to contribute or pay under the terms of any Plan, and all contributions to any Plan which are required or recommended with respect to any period of time prior to the Closing have been made or such amounts have been accrued in accordance with
A-51
GAAP. There are no funded benefit obligations for which contributions have not been made or properly accrued and there are no unfunded benefit obligations that have not been accounted for by reserves, or otherwise properly footnoted in accordance with GAAP on the Purchaser Financial Statements.
Each of the Purchaser’s Plans is and has been operated and administered in all material respects in accordance with applicable Laws, including but not limited to, ERISA and the Code, and all required material governmental filings and material participant disclosures have been made on a timely basis. With respect to each Plan, no event has occurred, and there exists no condition or set of circumstances in connection with which Purchaser could, directly or indirectly (through a Commonly Controlled Entity or otherwise), be subject to any liability under ERISA, the Code or any other applicable Law, except liability for benefit claims and funding obligations payable in the ordinary course. No prohibited transaction within the meaning of Section 406 of ERISA or 4975 of the Code, or breach of fiduciary duty under Title I of ERISA, has occurred with respect to any Plan or with respect to Purchaser or any Commonly Controlled Entity that could reasonably be expected to result in liability to Purchaser. Each Plan that is intended to qualify under Section 401(a) of the Code has received a currently effective favorable determination letter from the IRS, and such Plan and its related trust are exempt from taxation under Section 501(a) of the Code.
There are no pending, or, to Purchaser’s Knowledge, threatened or anticipated claims, litigation, administrative actions or proceedings against or otherwise involving any of the Plans or related trusts, or any fiduciary thereof, by any governmental agency, or by any employee, former employee, leased employee, former leased employee, retiree or sales personnel or by any participant or beneficiary covered under any of the Plans, or otherwise involving the Plans (other than routine claims for benefits). There is no judgment, decree, injunction, rule or order of any court, governmental body, commission, agency or arbitrator outstanding against or in favor of any Plan or, to Purchaser’s Knowledge, any fiduciary thereof in that capacity. No assets of Purchaser are allocated to or held in a “rabbi trust” or similar funding vehicle or subject to any lien under ERISA.
(d) Each Purchaser Plan that is a “group health plan” (as defined in Section 607(1) of ERISA) has been operated in compliance in all material respects with the provisions of COBRA (Section 4980B of the Code), the Health Insurance Portability and Accountability Act of 1996 and, to the extent required, any applicable similar state Law. Each Purchaser Plan that is an “employee welfare benefit plan” within the meaning of Section 3(1) of ERISA (i) is a fully insured plan, (ii) is not a multiple employer welfare arrangement within the meaning of Section 3(40) of ERISA and (iii) has no reserves, assets, surpluses or prepaid premiums. Except as set forth onSchedule 6.17(d), Purchaser does not currently provide or have a current obligation to provide for any material post-retirement or post-employment health and welfare benefits, including but not limited to, severance, salary continuation, termination, disability, death, or retiree health or medical benefits except as required by applicable Law.
(e) Except as set forth onSchedule 6.17(e), the consummation of the transactions contemplated by this Agreement will not, of itself, entitle any current or former employee or leased employee of Purchaser to severance pay, unemployment compensation or any similar payment or accelerate the time of payment or vesting, or increase the amount of compensation due to, or in respect of, any current or former employee or leased employee, nor will it result in the breach of any agreement with any such employee.
(f) None of the assets of Purchaser is subject to any lien under Section 302(f) of ERISA or Section 412(n) of the Code.
6.18Broker’s Fees. Except as set forth onSchedule 6.18, neither Purchaser, Merger Sub nor anyone acting on its behalf has made any commitment or done any other act which would create any liability for any brokerage, finder’s or similar fees or commissions in connection with the transactions contemplated by this Agreement.
6.19Authorization of Purchaser Common Stock. The shares of Purchaser Common Stock to be issued at the Closing have been duly authorized and, when issued by Purchaser, will be fully paid and non-assessable, free and clear of any Encumbrance of any third party of any nature whatsoever.
A-52
6.20No Creditor Assignment; No Bankruptcy. Neither Purchaser nor Merger Sub has made a general assignment for the benefit of creditors, filed any voluntary petition in bankruptcy or suffered the filing of any involuntary petition by its creditors, suffered the appointment of a receiver to take possession of all, or substantially all, of its assets, suffered the attachment or seizure of all, or substantially all, of its assets, admitted in writing its inability to pay its debts as they come due or made an offer of settlement, extension or composition to its creditors generally.
6.21Reports and Financial Statements.
(a) Purchaser has filed all forms, reports and documents required to be filed with the SEC since January 1, 2007 and all such required forms, reports and documents are referred to herein as the “SEC Documents.” As of their respective dates, the SEC Documents (i) were prepared in accordance with the requirements of the Securities Act or the Exchange Act, as the case may be, and the rules and regulations of the SEC thereunder applicable to such SEC Documents, and (ii) did not at the time they were filed (or if amended or superseded by a filing prior to the date of this Agreement, then on the date of such filing) contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. The certifications and statements required by (A) Rule 13a-14 under the Exchange Act and (B) 18 U.S.C. §1350 (Section 906 of the Sarbanes-Oxley Act) relating to the SEC Documents are accurate and complete and comply as to form and content with all applicable legal requirements.
(b) The audited financial statements (including any related notes thereto) contained in the SEC Documents or delivered to the Company representing the balance sheet of Purchaser at December 31, 2009 and the statements of operations, stockholders’ equity and cash flows for the three-year period then ended (the “Purchaser Financial Statements”), (x) complied with the published rules and regulations of the SEC with respect thereto, (y) were prepared in accordance with GAAP applied on a consistent basis throughout the periods involved (except as may be indicated in the notes thereto) and (z) fairly presented the results of its operations and cash flows for the periods indicated. The balance sheet of Purchaser as of December 31, 2009 is hereinafter referred to as the “Purchaser Balance Sheet.”
(c) Purchaser maintains adequate disclosure controls and procedures designed to ensure that material information relating to Purchaser, is made known to the Chief Executive Officer and the Principal Accounting Officer of Purchaser by others within that entity. To Purchaser’s knowledge, there are no (i) significant deficiencies and material weaknesses in the design or operation of internal controls over financial reporting which are reasonably likely to adversely affect in any material respect Purchaser’s ability to record, process, summarize and report financial information and (ii) fraud, or allegation of fraud, whether or not material, that involves management or other employees who have a significant role in Purchaser’s internal controls over financial reporting.
(d) Purchaser maintains a system of internal accounting controls designed to provide reasonable assurance that: (i) transactions are executed in accordance with management’s general or specific authorizations; (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with GAAP principles and to maintain asset accountability; (iii) access to assets is permitted only in accordance with management’s general or specific authorization; and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences.
6.22Section 203 of the DGCL Not Applicable. Purchaser’s Board has taken all actions so that the restrictions contained in Section 203 of the DGCL applicable to a “business combination” (as defined in Section 203 of the DGCL) shall not apply to the execution, delivery or performance of this Agreement, the Voting Agreements or the consummation of the Merger or the other transactions contemplated by this Agreement or the Voting Agreements.
A-53
6.23Rights Agreement. Purchaser has duly entered into an amendment to the Purchaser Rights Plan, a signed copy of which has been delivered to the Company (the “Purchaser’s Rights Agreement Amendment”) and taken all other action necessary or appropriate so that the entering into of this Agreement or the Voting Agreements do not and will not result in the ability of any person to exercise any of Purchaser’s rights under the Purchaser Rights Plan (the “Purchaser Rights”) or enable or require Purchaser Rights issued thereunder to separate from the shares of Purchaser Common Stock to which they are attached or to be triggered or become exercisable or cease to be redeemable.
6.24Interim Operations of Merger Sub. Merger Sub was formed solely for the purpose of engaging in the transactions contemplated by this Agreement, has engaged in no other business activities and has conducted no operations except in connection with the transactions contemplated hereby.
ARTICLE VII
CONDITIONS OF CLOSING; CERTAIN COVENANTS
7.01Conditions Precedent to the Obligations of Purchaser and Merger Sub. All obligations of Purchaser and Merger Sub hereunder with respect to the Merger are subject to the fulfillment to the satisfaction of Purchaser and its legal counsel, prior to or at the Closing, of each of the following conditions, except to the extent that Purchaser may waive any one or more thereof:
(a) The Purchaser Stockholder Approval and the Company Stockholder Approval shall have been obtained.
(b) The representations and warranties contained in Article V hereof shall be true, complete and accurate in all material respects as of the date when made and at and as of the Closing Date as though such representations and warranties were made at and as of such date (except to the extent such representations are made as of a date specific).
(c) The Company shall have performed and complied in all material respects with all agreements, obligations and conditions required by this Agreement to be performed or complied with by it or him on or prior to the Closing.
(d) No suit, action, investigation, inquiry or other proceeding by any governmental body or other Person or legal or administrative proceeding shall have been instituted or, to Knowledge, threatened, which seeks to restrain, enjoin, prevent the consummation or otherwise affect the transactions contemplated by this Agreement or which questions the validity or legality of the transactions contemplated hereby.
(e) From December 31, 2009 to the Closing Date, the Company Business shall not have suffered any Material Adverse Effect.
(f) The Company shall have received in writing any and all consents, approvals, authorizations, exemptions or waivers set forth onSchedule 5.06 hereto.
(g) The Company shall have delivered to Purchaser, or cause to be delivered to Purchaser, the other items required to be delivered to Purchaser in accordance with Section 3.02 hereof.
(h) The Company shall have available the Required Cash Amount.
(i) The Company shall have furnished Purchaser with such certificates to evidence compliance with the conditions set forth in this Section 7.01 as may reasonably be requested by Purchaser or its legal counsel.
7.02Conditions Precedent to the Obligations of the Company. All obligations of the Company hereunder with respect to the Merger and the other agreements hereunder are subject to the fulfillment to the satisfaction of the Company and its legal counsel, prior to or at the Closing, of each of the following conditions, except to the extent that the Company may waive any one or more thereof:
(a) The Purchaser Stockholder Approval and the Company Stockholder Approval shall have been obtained.
A-54
(b) The representations and warranties contained in Article VI hereof shall be true, complete and accurate in all material respects as of the date when made and at and as of the Closing Date as though such representations and warranties were made at and as of such date (except to the extent such representations are made as of a date specific).
(c) Purchaser and Merger Sub shall have performed and complied in all material respects with all agreements, obligations and conditions required by this Agreement to be performed or complied with by Purchaser or Merger Sub on or prior to the Closing.
(d) No suit, action, investigation, inquiry or other proceeding by any governmental body or other Person or legal or administrative proceeding shall have been instituted or threatened which seeks to restrain, enjoin, prevent the consummation of or otherwise affect the transactions contemplated by this Agreement or which questions the validity or legality of the transactions contemplated hereby.
(e) From December 31, 2009 to the Closing Date, the Purchaser Business shall not have suffered any Material Adverse Effect.
(f) The shares of Purchaser Common Stock issuable to the Stockholders in the Merger shall have been approved for listing on the NASDAQ Global Market, subject to official notice of issuance.
(g) Purchaser shall have delivered to the Company, or cause to be delivered to the Company, the other items required to be delivered to the Company in accordance with Section 3.03 hereof.
(h) Purchaser and Merger Sub shall have furnished the Company with such certificates of Purchaser and Merger Sub to evidence compliance with the conditions set forth in this Section 7.02 as may be requested by the Company or its legal counsel and all consents, approvals, authorizations, exemptions or waivers set forth onSchedule 6.06.
(i) Purchaser shall have executed the Registration Rights Agreement in the form ofExhibit G hereto, providing certain registration rights to the Company’s stockholders.
(j) Purchaser shall have executed the Stockholders Agreement in the form ofExhibit F hereto.
ARTICLE VIII
SURVIVAL OF REPRESENTATIONS
AND WARRANTIES; INDEMNIFICATION
8.01Survival of Representations and Warranties. All representations and warranties made by the Company in this Agreement or in each of the Related Documents delivered pursuant hereto, shall survive the Closing hereunder and any investigation heretofore made by or on behalf of Purchaser through December 31, 2010. All representations and warranties made by Purchaser in this Agreement shall terminate at the earlier of the Effective Time or upon the termination of this Agreement pursuant to Section 9.01. No investigation by Purchaser or the Company, as the case may be, shall relieve the Purchaser or the Company, as the case may be from any liability for any misrepresentation made in this Agreement.
8.02Notice of Damages. A party seeking indemnity hereunder (the “Indemnified Party”) will give the party from whom indemnity is sought hereunder (such party, the “Indemnitor”), prompt notice (hereinafter, the “Indemnification Notice”) of any demands, claims, actions or causes of action (collectively, “Claims”) asserted against the Indemnified Party. Failure to give such notice shall not relieve the Indemnitor of any obligations which the Indemnitor may have to the Indemnified Party under this Article VII, except to the extent that such failure has prejudiced the Indemnitor under the provisions for indemnification contained in this Agreement. For purposes of this Article VIII, notice to the Stockholders’ Representative shall constitute notice to the Stockholders.
8.03Agreements to Indemnify. (a) Subject to the terms and conditions of this Article VIII, the Stockholders (acting through the Stockholders’ Representative) shall be responsible, severally and not jointly, to the limited
A-55
extent described below, to indemnify, defend and hold harmless Purchaser and its Affiliates (including any officer, director, shareholder, partner, member, employee, agent or representative of any thereof) (a “Purchaser Affiliate”) from and against all assessments, losses, damages, liabilities, costs and expenses, including, but not limited to, interest, penalties and reasonable fees and expenses of legal counsel (collectively, “Damages”) imposed upon or incurred by Purchaser, the Surviving Corporation, or any of its Subsidiaries arising out of, in connection with or resulting from: (i) any breach of any representation or warranty of the Company contained in or made pursuant to this Agreement or any Related Document, or any certificate or other instrument furnished or to be furnished to Purchaser or Merger Sub hereunder; or (ii) any nonfulfillment of any covenant or agreement of the Company contained in or made pursuant to this Agreement or any Related Document, or any certificate or other instrument furnished or to be furnished to Purchaser or Merger Sub hereunder.
(b) The Indemnitor shall reimburse an Indemnified Party promptly after delivery of an Indemnification Notice certifying that the Indemnified Party has incurred Damages after compliance with the terms of this Article VIII;provided,however, that the Indemnitor shall have the right to contest any such Damages or its obligations to indemnify therefor in accordance with the terms of this Agreement.
(c) The right of Purchaser to indemnification pursuant to this Section 8.03 shall be the sole and exclusive right and remedy exercisable against the Company and the Stockholders in connection with the transactions contemplated by this Agreement, regardless of the legal theory advanced in support of such claims, excepting only the case of fraud;provided, that this provision shall not waive or limit any party’s right to injunctive relief or other equitable relief for the limited purpose of avoiding immediate and irreparable harm
8.04Conditions of Indemnification of Third-Party Claims. The obligations and liabilities of the Stockholders and the Company under Section 8.03 hereof with respect to Damages resulting from Claims by persons not party to this Agreement shall be subject to the following terms and conditions:
(a) Promptly after delivery of an Indemnification Notice to the Stockholders’ Representative in respect of a Claim and subject to paragraph (c) of this Section 8.04, the Stockholders’ Representative may elect, by written notice to the Indemnified Party, to undertake the defense and/or settlement thereof with counsel reasonably satisfactory to the Indemnified Party, at the sole cost and expense of the Indemnitor. If the Stockholders’ Representative chooses to defend any claim, the Indemnified Party shall cooperate with all reasonable requests of the Stockholders’ Representative and shall make available to the Stockholders’ Representative any books, records or other documents within its control that are necessary or appropriate for such defense.
(b) In the event that the Stockholders’ Representative, acting on behalf of the Indemnitor, within a reasonable time after receipt of an Indemnification Notice, does not so elect to defend such Claim, the Indemnified Party will have the right (upon further notice to the Stockholders’ Representative) to undertake the defense, compromise or settlement of such Claim for the account of the Indemnitor, subject to the right of the Stockholders’ Representative, acting on behalf of the Indemnitor, to assume the defense of such Claim pursuant to the terms of paragraph (a) of this Section 8.04 at any time prior to settlement, compromise or final determination thereof, provided, that the Indemnitor reimburses in full all costs of the Indemnified Party (including reasonable attorney’s fees and expenses) incurred by it in connection with such defense prior to such assumption.
(c) Anything in this Section 8.04 to the contrary notwithstanding, (i) if the Stockholders’ Representative, acting on behalf of the Indemnitor, assumes the defense of any Claim, any Indemnified Party shall be entitled to participate in the defense, compromise or settlement of such Claim with counsel of its own choice at its own expense,provided,however, if representation by counsel selected by the Stockholders’ Representative would present a conflict of interest, then such Indemnified Party shall be entitled to participate in the defense, compromise or settlement of such Claim with counsel of its own choice at the expense of the Indemnitor, (ii) no person who has undertaken to defend a Claim under Section 8.04(a) hereof shall, without written consent of all Indemnified Parties, settle or compromise any Claim or consent
A-56
to entry of any judgment which (A) does not include as an unconditional term thereof the release by the claimant or the plaintiff of all Indemnified Parties from all liability arising from events which allegedly give rise to such Claim or (B) imposes any restrictions of any kind on the continuing operations of the Business.
8.05Limitations on Indemnification.
(a) Notwithstanding anything to the contrary provided elsewhere in this Agreement, the obligations of the Stockholders under this Agreement to indemnify any Indemnified Party with respect to any Claim pursuant to Section 8.03 and Section 8.04 shall be of no force and forever barred unless the Indemnified Party has given the Stockholders’ Representative notice of such claim on or prior to December 31, 2010. In any event, the parties shall fully cooperate with each other and their respective counsel in accordance with Section 8.03 and Section 8.04 in connection with any such litigation, defense, settlement or other attempted resolution.
(b) The aggregate amount of Damages for which the Stockholders may be obligated to indemnify any Indemnified Party with respect to all Claims pursuant to Section 8.03 and 8.04 shall not exceed ten percent (10%) of the total value of the aggregate Merger Consideration (the “Cap”);provided,however, that the Cap shall not apply in the event of fraud or willful misrepresentation by the Company. The parties agree that any liability that the Stockholders may incur for Damages pursuant to this Article VIII shall be satisfied solely and exclusively by the release by the Escrow Agent to Purchaser for cancellation of that number of Escrow Shares having a value equal to the amount of Damages, as set forth in Section 2.07(b).
ARTICLE IX
TERMINATION
9.01Termination of Agreement. The parties may terminate this Agreement prior to the Closing as follows:
(a) Purchaser and the Company may terminate this Agreement by mutual written consent;
(b) Purchaser may terminate this Agreement by giving written notice to the Company if the Closing shall fail to occur by reason of the failure of any condition precedent under Section 7.01 hereof (unless the failure results from a breach by Purchaser of any representation, warranty or covenant contained in this Agreement);
(c) Purchaser may terminate this Agreement by giving written notice to the Company if Stockholders holding more than 10% of the Shares exercise their appraisal rights in accordance with Section 262 of the DGCL;
(d) Purchaser may terminate this Agreement by giving written notice to the Company if the closing price of the Purchaser Common Stock on The NASDAQ Global Market is in excess of 125% of the Signing Date Stock Price for any ten (10) consecutive trading days following the date hereof;
(e) the Company may terminate this Agreement by giving written notice to Purchaser if the Closing shall not have occurred by reason of the failure of any condition precedent under Section 7.02 hereof (unless the failure results primarily from a breach by the Company of any representation, warranty or covenant contained in this Agreement);
(f) the Company may terminate this Agreement by giving written notice to Purchaser if (i) the closing price of the Purchaser Common Stock on The NASDAQ Global Market is below 75% of the Signing Date Stock Price for any ten (10) consecutive trading days following the date hereof or (ii) the Purchase Common Stock is no longer listed on The NASDAQ Global Market or The NASDAQ Capital Market;
(g) either Purchaser, on the one hand, or the Company, on the other hand, may terminate this Agreement on ten (10) days’ prior written notice if the other party is in material breach of this Agreement and such breach is not cured within such ten (10) day period;
A-57
(h) either Purchaser, on the one hand, or the Company, on the other hand, may terminate this Agreement upon written notice if the Closing shall not have occurred on or prior to August 31, 2010;
(i) by Purchaser, if there shall have occurred any Material Adverse Effect with respect to Company since the date of this Agreement;
(j) by the Company, if there shall have occurred any Material Adverse Effect with respect to Purchaser since the date of this Agreement; or
(k) by either Purchaser, on the one hand, or Company, on the other hand, if the required approval of the stockholders of Purchaser or Company contemplated by this Agreement shall not have been obtained by reason of the failure to obtain the requisite vote upon a vote taken at a meeting of stockholders convened therefor or at any adjournment thereof;provided, that the right to terminate this Agreement under this Section 8.01(k) shall not be available to any party where the failure to obtain stockholder approval of such party shall have been caused by the action or failure to act of such party in breach of this Agreement.
9.02Effect of Termination. If either Purchaser and Merger Sub, on the one hand, or the Company on the other hand, terminate this Agreement pursuant to Section 9.01:
(a) each of Purchaser, Merger Sub and the Company will redeliver to the party furnishing the same or destroy all documents, work papers and other material of any other party relating to the transactions contemplated hereby, whether so obtained before or after the execution hereof;
(b) neither the Company, nor Purchaser, nor Merger Sub shall make or issue, or cause to be made or issued, any announcement or written statement concerning termination of this Agreement or the transactions contemplated hereby for dissemination to the general public without the prior written consent of the other parties except as required by Law or legal process;
(c) this Agreement shall become wholly void and of no force or effect, without any liability or further obligation on the part of the Company, Purchaser or Merger Sub or any director, officer, or principal thereof, except for liabilities of one party hereto to another arising from a breach of this Agreement prior to termination in accordance with Section 9.01 (including without limitation the breach of a representation or a covenant that results in the failure of the Closing to occur) and except that the provisions set forth in this Section 9.02 shall survive such termination; and
(d) if the Company shall terminate this Agreement pursuant to Section 9.01(k) due to the fact that the required approval of the stockholders of Purchaser contemplated by this Agreement shall not have been obtained by reason of the failure to obtain the requisite vote upon a vote taken at a meeting of stockholders of Purchaser convened therefor or at any adjournment thereof, Purchaser shall pay to the Company, on or prior to December 31, 2010, an amount equal to the lesser of (i) $500,000 and (ii) the Company’s reasonable and actual out-of-pocket expenses (including, without limitation, all fees and expenses of counsel, accountants, investment bankers, experts and consultants to the Company) incurred by the Company in connection with or related to the authorization, preparation, negotiation, execution and performance of this Agreement and the transactions contemplated hereby. For the avoidance of doubt, in no event shall Purchaser be required to pay the amounts set forth in this Section 9.02(d) if, immediately prior to the termination of this Agreement, the Company was in material breach of its obligations under this Agreement.
ARTICLE X
MISCELLANEOUS PROVISIONS
10.01Expenses. Except as otherwise provided herein, each of the parties hereto will pay its own expenses incurred by or on its behalf in connection with this Agreement or any transaction contemplated by this Agreement, whether or not such transaction shall be consummated, including without limitation all fees of its respective legal counsel and accountants
A-58
10.02Notices. (a) All notices, requests, demands, consents or waivers and other communications required or permitted hereunder shall be in writing and shall be deemed to have been duly given if delivered by hand or by telecopy (with immediate confirmation), one business day after being sent if by nationally recognized overnight courier or if mailed, then four days after being sent by certified or registered mail, return receipt requested with postage prepaid:
If to the Company:
(Prior to Closing)
Cequent Pharmaceuticals, Inc.
One Kendall Square, Bldg. 700
Cambridge, MA 02139
Attention: Peter D. Parker, President and CEO
Telecopy: 617-995-7941
(After Closing)
Ampersand 2006 Limited Partnership
c/o Ampersand Ventures
55 William Street, Suite 240
Wellesley, MA 02481
Attention: Herbert H. Hooper, Stockholders’ Representative
Telecopy: 781-239-0824
With a copy to:
Edwards Angel Palmer & Dodge LLP
111 Huntington Avenue
Boston, MA 02199-7613
Attention: James T. Barrett, Esq. Matthew J. Gardella, Esq.
Telecopy: 617-227-4420
If to Purchaser or Merger Sub, to:
MDRNA, Inc.
3830 Monte Villa Parkway
Bothell, WA 98021
Attention: J. Michael French, President and CEO
Telecopy: 425-908-3650
With a copy to:
Pryor Cashman LLP
7 Times Square
New York, NY 10036
Attention: Lawrence Remmel, Esq.
Telecopy: (212) 798-6365
or, in each case, to such other person or address as any party shall furnish to the other parties in writing.
10.03Binding; No Assignment. This Agreement and all of the provisions hereof shall be binding upon and inure to the benefit of the parties hereto and their respective successors and permitted assigns, but neither this Agreement nor any of the rights, interests or obligations hereunder shall be assigned by any of the parties hereto without the prior written consent of the other parties, except by operation of Law and except that Purchaser may assign all or part of this Agreement and its rights hereunder (a) to an Affiliate of Purchaser or (b) from and after
A-59
the Closing to a person, not a party to this Agreement, who acquires substantially all of the assets of such Purchaser and who assumes all of the obligations of such Purchaser hereunder, provided in each such case that no such assignment shall release Purchaser from its duties and obligations hereunder.
10.04Severability. If in any jurisdiction, any provision of this Agreement or its application to any party or circumstance is restricted, prohibited or unenforceable, such provision shall, as to such jurisdiction, be ineffective only to the extent of such restriction, prohibition or unenforceability without invalidating the remaining provisions hereof and without affecting the validity or enforceability of such provision in any other jurisdiction or its application to other parties or circumstances. In addition, if any one or more of the provisions contained in this Agreement shall for any reason in any jurisdiction be held to be excessively broad as to time, duration, geographical scope, activity or subject, it shall be construed by limiting and reducing it, so as to be enforceable to the extent compatible with the applicable Law of such jurisdiction as it shall then appear.
10.05Governing Law; Consent to Jurisdiction and Venue.
(a) The parties acknowledge and agree that this Agreement shall be governed by the Laws of the State of Delaware as to all matters including, but not limited to, matters of validity, construction, effect, performance and liability, without consideration of conflicts of laws provisions contained therein and that the courts of the State of Delaware shall have exclusive jurisdiction of all disputes with respect to this Agreement, including without limitation, any dispute relating to the construction or interpretation of the rights and obligations of any party, which is not resolved through discussion between the parties.
(b) Each of the parties hereto hereby irrevocably and unconditionally submit to the non-exclusive jurisdiction of any Delaware State or Federal court sitting in New Castle County in any action or proceeding arising out of or relating to this Agreement, any Related Document or any transaction contemplated hereby or thereby. The Company, Surviving Corporation, Purchaser and the Stockholders’ Representative hereby irrevocably waive, to the fullest extent they may effectively do so, the defense of an inconvenient forum to the maintenance of such action or proceeding, and also irrevocably and unconditionally consent to the service of any and all process in any such action or proceeding by the mailing of copies of such process by certified mail to their respective addresses specified in Section 10.02. The Company, Surviving Corporation, Purchaser and the Stockholders’ Representative further irrevocably and unconditionally agree that a final judgment in any such action or proceeding (after exhaustion of all appeals or expiration of the time for appeal) shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by Law.
10.06Counterparts. This Agreement may be executed simultaneously in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
10.07Headings. The title of this Agreement and the headings of the Sections and Articles of this Agreement are for reference purposes only and shall not be used in construing or interpreting this Agreement.
10.08Entire Agreement; Amendment; Waiver. This Agreement and the Related Documents delivered pursuant to the terms hereof, sets forth the entire agreement and understanding of the parties hereto in respect of the subject matter hereof, and supersedes all prior agreements, promises, covenants, arrangements, representations or warranties, whether oral or written, by any party hereto or any officer, director, employee or representative of any party hereto. No modification or waiver of any provision of this Agreement shall be valid unless it is in writing and signed by the party to be charged therewith. The waiver of breach of any term or condition of this Agreement shall not be deemed to constitute a waiver of any other breach of the same or any other term or condition.
10.09Third Parties. Except as specifically set forth or referred to herein, nothing herein expressed or implied is intended or shall be construed to confer upon or give to any person or corporation other than the parties hereto and their respective successors or assigns any rights or remedies under or by reason of this Agreement.
A-60
10.10Publicity. The press release announcing the execution of this Agreement shall be issued in the form as has been mutually agreed upon by Purchaser and the Company and each of Purchaser and the Company shall consult with the other party before issuing any other press release or otherwise making any public statement with respect to the Merger or this Agreement and shall not issue any such press release or make any such public statement prior to consulting with and obtaining the prior consent of the other party (which shall not be unreasonably withheld or delayed); provided, that Purchaser may, without consulting with or obtaining the prior consent of the other party, issue such press release or make such public statement as may be required by applicable Law or any listing agreement with a national securities exchange or automated quotation system to which it is a party.
10.11No Presumption. The Company, Purchaser and Merger Sub have each participated in the negotiation and drafting of this Agreement and have each been represented throughout to its satisfaction by legal counsel of its choosing. In the event any ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the parties hereto and no presumption or burden of proof shall arise favoring or disfavoring any party by virtue of the authorship of any of the provisions of this Agreement.
10.12Gender; Tense, Etc. Where the context or construction requires, all words applied in the plural shall be deemed to have been used in the singular, and vice versa; the masculine shall include the feminine and neuter, and vice versa; and the present tense shall include the past and future tense, and vice versa.
10.13Reference to Days. All references to days in this Agreement shall be deemed to refer to calendar days, unless otherwise specified.
[remainder of page intentionally left blank; signature page follows]
A-61
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be duly executed and delivered in New York, New York, all on the day and year first above written.
| | |
| PURCHASER: |
|
| MDRNA, INC. |
| |
| By: | | /s/ J. MICHAEL FRENCH |
| Name: | | J. Michael French |
| Title: | | President and CEO |
|
| MERGER SUB: |
|
| CALAIS ACQUISITION CORP. |
| |
| By: | | /s/ J. MICHAEL FRENCH |
| Name: | | J. Michael French |
| Title: | | President |
|
| THE COMPANY: |
|
| CEQUENT PHARMACEUTICALS, INC. |
| |
| By: | | /s/ PETER D. PARKER |
| Name: | | Peter D. Parker |
| Title: | | President and CEO |
|
| STOCKHOLDERS’ REPRESENTATIVE: |
|
| AMPERSAND 2006 LIMITED PARTNERSHIP |
| |
| By: | | AMP-06 Management Company Limited Partnership, its General Partner |
| |
| By: | | AMP-06 MC LLC, its General Partner |
| |
| By: | | /s/ HERBERT H. HOOPER |
| Name: | | Herbert H. Hooper |
| Title: | | Member |
Annex B

99 High Street
11tth Floor
Boston, MA 02110
Phone: (617) 371-3900
Fax: (617) 227-2119
www.canacordadams.com
March 31, 2010
The Board of Directors
MDRNA, Inc.
3830 Monte Villa Parkway
Bothell, WA 98021
Members of the Board of Directors:
We understand that MDRNA, Inc. (“MDRNA” or the “Company”) proposes to enter into an Agreement and Plan of Merger (the “Agreement”) by and among the Company, Merger Sub and Cequent Pharmaceuticals, Inc. (“Cequent”) pursuant to which Merger Sub shall be merged with and into Cequent (the “Merger”), and Cequent shall be the surviving corporation. The terms and conditions of the Merger and other related transactions are set out more fully in the Agreement (the “Transaction”). All capitalized terms shall have the meanings ascribed to such terms in the Agreement unless the context clearly provides otherwise.
The Agreement provides, among other things, that upon consummation of the Merger, all of the capital stock of Cequent shall be converted into the right to receive shares of common stock, $0.006 par value per share, of MDRNA in an aggregate amount equal to $46 million, representing approximately 45.1% of the outstanding securities of the combined company on a fully diluted basis (the “Merger Consideration”).
You have requested our opinion as to the fairness to the Company, from a financial point of view, of the Merger Consideration to be paid by the Company in connection with the Merger (the “Fairness Opinion”). Our Fairness Opinion only addresses the Merger Consideration to be paid by the Company in connection with the Transaction and does not address the fairness, from a financial point of view, of (i) any fees or other amounts paid or payable by the Company or (ii) the issuance by the Company of any other securities or the exercisability of any such securities, in each case in connection with the Transaction, including without limitation the transactions contemplated by that certain Loan Agreement (the “Loan Agreement”) by and between the Company and Cequent and the agreements contemplated thereby and the issuance of the Warrant for the Purchase of Common Stock contemplated by the Loan Agreement that is exercisable upon termination of the Agreement. This letter and our Fairness Opinion have been authorized by our Fairness Opinion Review Committee.
Canaccord Adams Inc. (“Canaccord Adams”), as part of its investment banking activities, is regularly engaged in the valuation of businesses and their securities in connection with mergers and acquisitions, underwritings, distributions of listed and unlisted securities, private placements and valuations for corporate and other purposes. Canaccord Adams has acted as a financial advisor to the Company in the Transaction and will receive a fee, which is not contingent upon the closing of the Transaction, upon delivery for rendering this Fairness Opinion. Canaccord Adams will also receive a financial advisory fee contingent upon the successful completion of the Transaction. Canaccord Adams will not receive any other compensation or payment upon successful completion of the Transaction. Canaccord Adams has received compensation or payment as a result of its relationship with the Company in the form of placement agency fees in connection with the January 14, 2010 offering of 3,500,612
B-1
MDRNA, Inc.
March 31, 2010
Page 2 of 4
common shares resulting in gross proceeds of $5,500,000, the December 24, 2009 offering of promissory notes and warrants resulting in gross proceeds of $1,000,000 and the June 9, 2009 offering of 5,520,000 common shares resulting in gross proceeds of $10,500,000. In the ordinary course of our trading and brokerage activities, we or our affiliates have in the past and may in the future hold positions, for our own account or the accounts of our customers, in equity, debt or other securities of the Company.
For purposes of developing our Fairness Opinion, we have among other things:
| | i) | reviewed certain financial statements and other business and financial information of MDRNA and Cequent and have assumed that there has been no material change in the assets, financial condition, business or prospects of the Company, since the respective dates of the most recent financial statements made available to us; |
| | ii) | considered certain financial forecasts, projections and analyses and other forward-looking financial information for MDRNA and Cequent and have assumed that the financial results reflected in such forecasts, projections and analyses and other forward-looking financial information will be realized in the amounts and at the times projected; |
| | iii) | reviewed transaction multiples, to the extent publicly available, of certain precedent merger and acquisitions transactions that we deemed relevant; |
| | iv) | reviewed trading statistics, to the extent publicly available, of selected public companies that we deemed relevant; |
| | v) | reviewed the draft Agreement, dated March 29, 2010, and the financial terms and conditions set forth therein, as well as the exhibits thereto, and have assumed that the final forms of the Agreement and related documentation and the exhibits thereto will not vary in any regard that is material to our analysis from the versions included in the draft Agreement that we reviewed; and |
| | vi) | made such other studies and inquiries, and reviewed such other data, and considered such other factors as we have deemed, in our sole judgment, to be necessary, appropriate or relevant. |
In connection with our review and arriving at our Fairness Opinion, we have not independently verified any information received from MDRNA, Cequent or either of their representatives or otherwise made available to us, have relied on such information, and have assumed that all such information is complete and accurate in all respects. We have also relied on the assurances of the management team of MDRNA that they are not aware of any facts that would make such information misleading. With respect to any forecasts, projections and analyses and other forward-looking financial information reviewed by us relating to the prospects of MDRNA and Cequent, including information relating to the strategic, financial and operational benefits anticipated from the Transaction, we have assumed, with your permission, that such forecasts, projections and analyses and other forward-looking information have been reasonably prepared or developed on bases reflecting the best currently available estimates and judgments of the management team of MDRNA and Cequent. We have not conducted any valuation or appraisal of any of the assets of MDRNA or Cequent. We have not been requested to conduct and have not conducted a physical inspection of the properties or facilities of MDRNA or Cequent. We have assumed, with your permission, that any material liabilities (contingent or otherwise, known or unknown) of MDRNA are as set forth in the financial statements of the Company provided to us. We have made no independent investigation of any legal matters involving MDRNA or Cequent.
Our Fairness Opinion is necessarily based on financial, economic and securities market conditions prevailing as of the date hereof, and on the conditions and prospects, financial and otherwise, of MDRNA as known to us on the date hereof. In developing our Fairness Opinion, we have taken into account our assessment of general
B-2
MDRNA, Inc.
March 31, 2010
Page 3 of 4
economic, market and financial conditions and our experience in similar transactions, as well as our experience in securities valuation in general. It should be understood that (i) subsequent developments may affect the conclusions expressed in this Fairness Opinion if it were to be rendered as of a later date, and (ii) we disclaim any obligation to advise any person of any change in any matter affecting this Fairness Opinion that may come to our attention after the date of this Fairness Opinion. We have not undertaken to reaffirm or revise this Fairness Opinion or otherwise comment upon any events occurring after the delivery hereof and do not have any obligation to update, revise or reaffirm this Fairness Opinion. We express no opinion as to the fairness of the amount or nature of any compensation to be any of the Company’s officers, directors or employees, or classes of such persons.
For purposes of rendering our Fairness Opinion, we have assumed in all respects material to our analysis, that the Merger Consideration payable pursuant to the Agreement was determined through arm’s-length negotiations between the appropriate parties, that the representations and warranties of each party contained in the Agreement are true and correct, that each party will perform all of the covenants and agreements required to be performed by it under the Agreement without material alteration or waiver thereof and that all conditions to the consummation of the proposed Transaction will be satisfied without waiver thereof or material alteration to the terms of the proposed Transaction. In addition, we have assumed, with your consent, that the historical financial statements reviewed by us have been prepared and fairly presented in accordance with U.S. generally accepted accounting principles consistently applied.
In preparing the Fairness Opinion, we performed a variety of financial and comparative analyses. The preparation of a fairness opinion is a complex process involving various determinations as to the most appropriate and relevant methods of financial analysis and the application of those methods to the particular circumstances and, therefore, a fairness opinion is not readily susceptible to partial analysis or summary description. We arrived at our ultimate Fairness Opinion based on the results of all analyses we undertook and assessed as a whole and did not draw, in isolation, conclusions from or with regard to any one factor or method of analysis. Accordingly, we believe that our analyses must be considered as a whole and that selecting portions of our analyses and factors or focusing on information presented in tabular format, without considering all analyses and factors or the narrative description of the analyses, could create a misleading or incomplete view of the processes underlying our analyses and our Fairness Opinion.
In our analyses, we considered industry performance, general business, economic, market and financial conditions and other matters, many of which are beyond the Company’s control. No company, transaction or business used in our analyses as a comparison is identical to the Company or the proposed Transaction, and an evaluation of the results of those analyses is not entirely mathematical. Rather, the analyses involve complex considerations and judgments concerning financial and operating characteristics and other factors that could affect the acquisition or other values of the companies, business segments or transactions analyzed. The estimates contained in our analyses and the ranges of valuations resulting from any particular analysis are not necessarily indicative of actual values or predictive of future results or values, which may be significantly more or less favorable than those suggested by the analyses. In addition, analyses relating to the value of businesses or securities do not purport to be appraisals or to reflect the prices at which businesses or securities actually may be sold. Accordingly, the estimates used in, and the results derived from, our analyses are inherently subject to substantial uncertainty.
This Fairness Opinion does not address other business strategies that might be available to MDRNA, the decision of the Company to proceed with the Transaction, or the financial performance or financial condition of MDRNA following the announcement and the consummation of the Transaction.
B-3
MDRNA, Inc.
March 31, 2010
Page 4 of 4
This letter and our Fairness Opinion set forth herein are directed to and for the information of the Board of Directors only and shall not be reproduced, summarized, described or referred to or given to any other person or otherwise made public or used for any other purpose, or published or referred to at any time, in whole or in part, without our prior written consent, except that this opinion may be referenced and included in its entirety in any proxy statement to be distributed to holders of the Company’s common stock in connection with the Transaction provided that it is reproduced in full, and that any description of or reference to Canaccord Adams, and any summary of our Fairness Opinion in the proxy statement is in a form reasonably acceptable to Canacccord Adams. Our Fairness Opinion does not constitute a recommendation to any holder of securities of the Company on how to vote such holder’s securities with respect to any matter in connection with the Transaction.
Based upon and subject to the foregoing, including the various assumptions and limitations set forth herein, it is our opinion that, as of the date hereof, the Merger Consideration to be paid by the Company is fair, from a financial point of view, to the Company.
Sincerely,
CANACCORD ADAMS INC.
B-4
Annex C
INDEX TO FINANCIAL STATEMENTS
OF
CEQUENT PHARMACEUTICALS, INC.
(A Development State Company)
Years ended December 31, 2009 and 2008 and the Period from
November 10, 2003 (Inception) through December 31, 2009
CONTENTS
| | |
| | | Page |
Independent Auditors’ Report | | C-2 |
| |
Balance Sheets | | C-3 |
| |
Statements of Operations | | C-4 |
| |
Statements of Changes in Stockholders’ Equity | | C-5 |
| |
Statements of Cash Flows | | C-7 |
| |
Notes to Financial Statements | | C-8 |
C-1
INDEPENDENT AUDITORS’ REPORT
To the Board of Directors
Cequent Pharmaceuticals, Inc.
Cambridge, Massachusetts
We have audited the accompanying balance sheets of Cequent Pharmaceuticals, Inc. (a development stage company) as of December 31, 2009 and 2008, and the related statements of operations, changes in stockholders’ equity and cash flows for the years then ended and for the period from November 10, 2003 (inception) to December 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Cequent Pharmaceuticals, Inc. as of December 31, 2009 and 2008, and the results of its operations and its cash flows for the years then ended and for the period from November 10, 2003 (inception) to December 31, 2009, in conformity with accounting principles generally accepted in the United States of America.
/s/ Wolf & Company, P.C.
Boston, Massachusetts
April 12, 2010
C-2
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
BALANCE SHEETS
ASSETS
| | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 3,213,748 | | | $ | 5,580,661 | |
Prepaid expenses | | | 37,211 | | | | 92,889 | |
| | | | | | | | |
Total current assets | | | 3,250,959 | | | | 5,673,550 | |
| | |
Property and equipment, net | | | 417,224 | | | | 573,967 | |
Other assets | | | 45,133 | | | | 80,859 | |
| | | | | | | | |
Total assets | | $ | 3,713,316 | | | $ | 6,328,376 | |
| | | | | | | | |
|
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |
| | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 131,009 | | | $ | 119,285 | |
Accrued expenses | | | 331,938 | | | | 371,871 | |
Capital lease—current portion | | | 13,656 | | | | 14,057 | |
Deferred revenue—current portion | | | 576,087 | | | | 576,087 | |
| | | | | | | | |
Total current liabilities | | | 1,052,690 | | | | 1,081,300 | |
| | |
Capital lease, net of current portion | | | 17,769 | | | | 30,984 | |
Deferred revenue, net of current portion | | | 836,956 | | | | 1,413,043 | |
| | | | | | | | |
Total liabilities | | | 1,907,415 | | | | 2,525,327 | |
| | | | | | | | |
Commitments | | | | | | | | |
| | |
Stockholders’ equity: | | | | | | | | |
Series A redeemable, convertible preferred stock, $0.001 par value; authorized 14,850,000 shares, issued and outstanding none and 14,850,000 shares, at December 31, 2009 and 2008, respectively | | | — | | | | 15,430,682 | |
Series A-1 redeemable, convertible preferred stock, $0.001 par value; authorized 27,584,420 shares and none, issued and outstanding 15,176,263 shares and none, at December 31, 2009 and 2008, respectively (preference in liquidation of $16,970,068 at December 31, 2009) | | | 16,966,615 | | | | — | |
Common stock , $0.001 par value; authorized 130,000,000 shares and 22,550,000 shares, issued and outstanding 5,820,127 and 2,820,127 at December 31, 2009 and 2008, respectively | | | 5,820 | | | | 2,820 | |
Additional paid-in capital | | | 1,719,445 | | | | — | |
Deficit accumulated during the development stage | | | (16,885,979 | ) | | | (11,630,453 | ) |
| | | | | | | | |
Total stockholders’ equity | | | 1,805,901 | | | | 3,803,049 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 3,713,316 | | | $ | 6,328,376 | |
| | | | | | | | |
See accompanying notes to the financial statements.
C-3
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
STATEMENTS OF OPERATIONS
| | | | | | | | | | | | |
| | | Years Ended December 31, | | | Period from
November 10,
2003 (Inception)
through December 31,
2009 | |
| | | 2009 | | | 2008 | | |
Revenue | | $ | 581,087 | | | $ | 390,872 | | | $ | 1,096,957 | |
| | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 3,498,223 | | | | 4,293,766 | | | | 11,272,709 | |
General and administrative | | | 2,314,262 | | | | 2,118,403 | | | | 6,469,902 | |
| | | | | | | | | | | | |
Total operating expenses | | | 5,812,485 | | | | 6,412,169 | | | | 17,742,611 | |
| | | | | | | | | | | | |
Operating loss | | | (5,231,398 | ) | | | (6,021,297 | ) | | | (16,645,654 | ) |
| | | | | | | | | | | | |
Other income (expense): | | | | | | | | | | | | |
Other income (expense) | | | (2,313 | ) | | | — | | | | 10,287 | |
Interest income | | | 11,753 | | | | 149,840 | | | | 445,884 | |
Interest expense | | | (33,568 | ) | | | (3,910 | ) | | | (71,731 | ) |
| | | | | | | | | | | | |
Other income (expense), net | | | (24,128 | ) | | | 145,930 | | | | 384,440 | |
| | | | | | | | | | | | |
Net loss | | $ | (5,255,526 | ) | | $ | (5,875,367 | ) | | $ | (16,261,214 | ) |
| | | | | | | | | | | | |
See accompanying notes to the financial statements.
C-4
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
Years Ended December 31, 2009 and 2008 and the Period from November 10, 2003 (Inception) through December 31, 2009
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Series A and A-1 Preferred Stock | | Common Stock | | | Treasury Stock | | | Additional
Paid-in
Capital | | | Deficit
Accumulated
During the
Development
Stage | | | Total
Stockholders’
Equity | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | Shares | | Amount | | Shares | | | Amount | | | Shares | | | Amount | | | | |
Issuance of common stock for cash | | — | | $ | — | | 918,253 | | | $ | 918 | | | — | | | $ | — | | | $ | 1,332 | | | $ | — | | | $ | 2,250 | |
Net loss | | — | | | — | | — | | | | — | | | — | | | | — | | | | — | | | | (1,579 | ) | | | (1,579 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2004 | | — | | | — | | 918,253 | | | | 918 | | | — | | | | — | | | | 1,332 | | | | (1,579 | ) | | | 671 | |
Issuance of common stock for cash | | — | | | — | | 103,048 | | | | 103 | | | — | | | | — | | | | 4,386 | | | | — | | | | 4,489 | |
Net loss | | — | | | — | | — | | | | — | | | — | | | | — | | | | — | | | | (247,665 | ) | | | (247,665 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2005 | | — | | | — | | 1,021,301 | | | | 1,021 | | | — | | | | — | | | | 5,718 | | | | (249,244 | ) | | | (242,505 | ) |
Issuance of Series A preferred stock, net of issuance costs of $83,038 | | 6,000,000 | | | 5,916,962 | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | 5,916,962 | |
Issuance of common stock for cash | | — | | | — | | 1,368,910 | | | | 1,369 | | | — | | | | — | | | | 3,318 | | | | — | | | | 4,687 | |
Issuance of common stock in conjunction with license agreement | | — | | | — | | 437,059 | | | | 437 | | | — | | | | — | | | | 52,010 | | | | — | | | | 52,447 | |
Repurchase of common stock | | — | | | — | | — | | | | — | | | (86,090 | ) | | | (86 | ) | | | — | | | | — | | | | (86 | ) |
Net loss | | — | | | — | | — | | | | — | | | — | | | | — | | | | — | | | | (684,205 | ) | | | (684,205 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2006 | | 6,000,000 | | | 5,916,962 | | 2,827,270 | | | | 2,827 | | | (86,090 | ) | | | (86 | ) | | | 61,046 | | | | (933,449 | ) | | | 5,047,300 | |
Issuance of Series A preferred stock, net of issuance costs of $91,399 | | 5,500,000 | | | 5,408,601 | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | 5,408,601 | |
Accretion of Series A issuance costs | | — | | | 23,061 | | — | | | | — | | | — | | | | — | | | | (23,061 | ) | | | — | | | | — | |
Accretion of Series A dividends | | — | | | 80,000 | | — | | | | — | | | — | | | | — | | | | (61,107 | ) | | | (18,893 | ) | | | — | |
Issuance of common stock in conjunction with license agreement | | — | | | — | | 78,947 | | | | 79 | | | — | | | | — | | | | 9,395 | | | | — | | | | 9,474 | |
Share-based compensation expense | | — | | | — | | — | | | | — | | | — | | | | — | | | | 13,727 | | | | — | | | | 13,727 | |
Retirement of treasury stock | | — | | | — | | (86,090 | ) | | | (86 | ) | | 86,090 | | | | 86 | | | | — | | | | — | | | | — | |
Net loss | | — | | | — | | — | | | | — | | | — | | | | — | | | | — | | | | (4,196,872 | ) | | | (4,196,872 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2007 | | 11,500,000 | | | 11,428,624 | | 2,820,127 | | | | 2,820 | | | — | | | | — | | | | — | | | | (5,149,214 | ) | | | 6,282,230 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
(continued)
See accompanying notes to financial statements.
C-5
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (Concluded)
Years Ended December 31, 2009 and 2008 and the Period from November 10, 2003 (Inception) through December 31, 2009
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Series A and A-1 Preferred Stock | | | Common Stock | | Treasury Stock | | Additional
Paid-in
Capital | | | Deficit
Accumulated
During the
Development
Stage | | | Total
Stockholders’
Equity | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | Shares | | | Amount | | | Shares | | Amount | | Shares | | Amount | | | |
Issuance of Series A preferred stock | | 3,350,000 | | | | 3,350,000 | | | — | | | — | | — | | | — | | | — | | | | — | | | | 3,350,000 | |
Issuance of warrants | | — | | | | — | | | — | | | — | | — | | | — | | | 5,533 | | | | — | | | | 5,533 | |
Accretion of Series A issuance costs | | — | | | | 42,058 | | | — | | | — | | — | | | — | | | (37,667 | ) | | | — | | | | 4,391 | |
Accretion of Series A dividends | | — | | | | 610,000 | | | — | | | — | | — | | | — | | | (4,128 | ) | | | (605,872 | ) | | | — | |
Share-based compensation expense | | — | | | | — | | | — | | | — | | — | | | — | | | 36,262 | | | | — | | | | 36,262 | |
Net loss | | — | | | | — | | | — | | | — | | — | | | — | | | — | | | | (5,875,367 | ) | | | (5,875,367 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2008 | | 14,850,000 | | | | 15,430,682 | | | 2,820,127 | | | 2,820 | | — | | | — | | | — | | | | (11,630,453 | ) | | | 3,803,049 | |
Accretion of Series A dividends | | — | | | | 1,183,805 | | | — | | | — | | — | | | — | | | (1,183,805 | ) | | | — | | | | — | |
Series A preferred stock converted to common stock | | (3,000,000 | ) | | | (3,000,000 | ) | | 3,000,000 | | | 3,000 | | — | | | — | | | 2,997,000 | | | | — | | | | — | |
Issuance of Series A-1 preferred stock, net of issuance costs of $89,132 | | 3,326,263 | | | | 3,237,131 | | | — | | | — | | — | | | — | | | — | | | | — | | | | 3,237,131 | |
Accretion of Series A and Series A-1 issuance costs | | — | | | | 114,997 | | | — | | | — | | — | | | — | | | (114,997 | ) | | | — | | | | — | |
Share-based compensation expense | | — | | | | — | | | — | | | — | | — | | | — | | | 21,247 | | | | — | | | | 21,247 | |
Net loss | | — | | | | — | | | — | | | — | | — | | | — | | | — | | | | (5,255,526 | ) | | | (5,255,526 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2009 | | 15,176,263 | | | $ | 16,966,615 | | | 5,820,127 | | $ | 5,820 | | — | | $ | — | | $ | 1,719,445 | | | $ | (16,885,979 | ) | | $ | 1,805,901 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes to financial statements.
C-6
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | |
| | | Years Ended December 31, | | | Period from
November 10,
2003 (Inception)
through December 31,
2009 | |
| | | 2009 | | | 2008 | | |
Cash flows from operating activities: | | | | | | | | | | | | |
Net loss | | $ | (5,255,526 | ) | | $ | (5,875,367 | ) | | $ | (16,261,214 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Depreciation and amortization expense | | | 202,863 | | | | 166,284 | | | | 448,097 | |
Non-cash interest expense | | | 27,469 | | | | 3,910 | | | | 31,379 | |
Share-based compensation expense | | | 21,247 | | | | 36,262 | | | | 71,236 | |
Fair value of common stock issued in conjunction with license agreement | | | — | | | | — | | | | 61,921 | |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Restricted cash | | | — | | | | 41,229 | | | | — | |
Prepaid expenses and other assets | | | 63,935 | | | | 37,832 | | | | (108,190 | ) |
Accounts payable | | | 11,724 | | | | (122,685 | ) | | | 131,009 | |
Accrued expenses | | | (39,933 | ) | | | 178,629 | | | | 336,329 | |
Deferred revenue | | | (576,087 | ) | | | 864,128 | | | | 1,413,043 | |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (5,544,308 | ) | | | (4,669,778 | ) | | | (13,876,390 | ) |
| | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | |
Purchases of property and equipment | | | (46,120 | ) | | | (239,773 | ) | | | (820,280 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (46,120 | ) | | | (239,773 | ) | | | (820,280 | ) |
| | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | |
Proceeds from issuance of preferred stock, net of issuance costs | | | 3,237,131 | | | | 3,350,000 | | | | 17,912,694 | |
Repayments of capital lease | | | (13,616 | ) | | | — | | | | (13,616 | ) |
Proceeds from issuance of common stock | | | — | | | | — | | | | 11,426 | |
Repurchase of common stock | | | — | | | | — | | | | (86 | ) |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 3,223,515 | | | | 3,350,000 | | | | 17,910,418 | |
| | | | | | | | | | | | |
(Decrease) increase in cash and cash equivalents | | | (2,366,913 | ) | | | (1,559,551 | ) | | | 3,213,748 | |
Cash and cash equivalents at beginning of period | | | 5,580,661 | | | | 7,140,212 | | | | — | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of period | | $ | 3,213,748 | | | $ | 5,580,661 | | | $ | 3,213,748 | |
| | | | | | | | | | | | |
Supplemental disclosure of cash flow information and non-cash transactions: | | | | | | | | | | | | |
Cash paid for interest | | $ | 1,696 | | | $ | — | | | $ | 35,949 | |
| | | | | | | | | | | | |
Exchange of Series A Preferred stock and dividends for Series A-1 Preferred stock and dividends | | $ | 13,643,805 | | | $ | — | | | $ | 13,643,805 | |
| | | | | | | | | | | | |
Exchange of Series A Preferred stock for Common Stock | | $ | 3,000,000 | | | $ | — | | | $ | 3,000,000 | |
| | | | | | | | | | | | |
Accretion of dividend and issuance costs on Series A Preferred stock | | $ | 1,298,802 | | | $ | 652,058 | | | $ | 2,053,921 | |
| | | | | | | | | | | | |
Relative fair value of warrants recorded as deferred financing costs | | $ | — | | | $ | 5,533 | | | $ | 5,533 | |
| | | | | | | | | | | | |
Property and equipment acquired under capital leases | | $ | — | | | $ | 45,041 | | | $ | 45,041 | |
| | | | | | | | | | | | |
See accompanying notes to financial statements.
C-7
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
Years Ended December 31, 2009 and 2008, and the Period from
November 10, 2003 (Inception) to December 31, 2009
Business
Cequent Pharmaceuticals, Inc. (“Cequent”) was incorporated on November 10, 2003 under the laws of the State of Delaware.
Cequent is an early-stage biopharmaceutical company developing novel therapeutics to prevent and treat a wide range of human diseases based on Cequent’s proprietary technology, TransKingdom RNA interference (tkRNAiTM). The FDA recently approved Cequent’s IND (investigational new drug) application for the first trial of an orally administered RNA interference drug in humans.
Since its inception, Cequent has devoted substantially all of its efforts to business planning, research and development, recruiting management and technical staff, acquiring operating assets and raising capital. Accordingly, Cequent is considered to be in the development stage.
Cequent is subject to a number of risks similar to other companies in its industry including rapid technological change, uncertainty of market acceptance of the product, competition from larger companies with substitute products and dependence on key personnel.
| 2. | SIGNIFICANT ACCOUNTING POLICIES |
A summary of the significant accounting policies followed by Cequent in the preparation of the accompanying consolidated financial statements follows:
Use of estimates
The process of preparing financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of assets and liabilities at the date of financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates and changes in estimates may occur.
Cash and cash equivalents
Cequent considers all highly liquid investments with maturities of three months or less at the date of purchase to be cash equivalents.
Financial instruments
Cequent’s financial instruments, consisting of cash and cash equivalents, accounts payable and accrued liabilities are carried at cost, which approximates fair value due to the short-term nature of these instruments. The carrying value of capital lease obligations approximates its fair value due to the market terms of the arrangements.
C-8
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
Derivative instruments
Cequent generally does not use derivative instruments to hedge exposures to cash-flow or market risks; however, certain warrants to purchase common stock that do not meet the requirements for classification as equity are classified as liabilities. In such instances, net-cash settlement is assumed for financial reporting purposes, even when the terms of the underlying contracts do not provide for a net-cash settlement. Such financial instruments are initially recorded at fair value, or relative fair value when issued with other instruments, with subsequent changes in fair value charged (credited) to operations in each reporting period. If these instruments subsequently meet the requirements for classification as equity, Cequent reclassifies the fair value to equity.
At December 31, 2009, Cequent had outstanding warrants to purchase 198,249 shares of its common stock with an exercise price of $0.07 per share. These warrants are considered to be derivative instruments since the agreements contain “down round” provisions whereby the number of shares covered by the warrants is subject to change in the event of certain dilutive stock issuances. The fair value of these derivative instruments at January 1, 2009 and December 31, 2009 was immaterial, as was the change in the fair value during that period. The fair value of these warrants was estimated using the Black-Scholes option pricing model and assumptions consistent with those disclosed in “Share-based payments” below.
The primary underlying risk exposure pertaining to the warrants is the change in fair value of the underlying common stock for each reporting period.
Property and equipment
Property and equipment are carried at cost. Depreciation and amortization expense is provided over the estimated useful lives of the assets using the straight-line method. A summary of the estimated useful lives is as follows:
| | |
Classification | | Estimated Useful Life |
Computer hardware and software | | 3 years |
Office furniture and equipment | | 5 years |
Lab equipment | | 5 years |
Leasehold improvements | | Shorter of lease term
or useful life |
Depreciation and amortization expense was $202,863 and $166,284 for the years ended December 31, 2009 and 2008, respectively.
Maintenance and repairs are charged to expense as incurred, while any additions or improvements are capitalized.
Research and development expenses
Costs incurred for research and development are expensed as incurred.
Income taxes
For federal and state income taxes, deferred tax assets and liabilities are recognized based upon temporary differences between the financial statement and the tax basis of assets and liabilities. Deferred income taxes are based upon prescribed rates and enacted laws applicable to periods in which differences are expected to
C-9
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
reverse. A valuation allowance is recorded when it is more likely than not that some portion or all of the deferred tax assets will not be realized. Accordingly, Cequent provides a valuation allowance, if necessary, to reduce deferred tax assets to amounts that are realizable.
Tax positions taken or expected to be taken in the course of preparing Cequent’s tax returns are required to be evaluated to determine whether the tax positions are “more-likely-than-not” of being sustained by the applicable tax authority. Tax positions not deemed to meet a more-likely-than-not threshold would be recorded as a tax expense in the current year. There were no uncertain tax positions that require accrual or disclosure to the financial statements as of December 31, 2009.
Concentrations of credit risk
Cequent has no significant off-balance-sheet concentration of credit risk such as foreign exchange contracts, option contracts or other foreign hedging arrangements. Cequent may from time to time have cash in banks in excess of FDIC insurance limits.
Impairment of long-lived assets
Cequent continually monitors events and changes in circumstances that could indicate carrying amounts of long-lived assets may not be recoverable. An impairment loss is recognized when expected cash flows are less than an asset’s carrying value. Accordingly, when indicators of impairment are present, Cequent evaluates the carrying value of such assets in relation to the operating performance and future undiscounted cash flows of the underlying assets. Cequent’s policy is to record an impairment loss when it is determined that the carrying amount of the asset may not be recoverable. No impairment charges were recorded in the years ended December 31, 2009 and 2008.
Revenue recognition
Through December 31, 2009, Cequent’s revenue primarily relates to a single option agreement (see Note 10). Revenue is recognized when persuasive evidence of an arrangement exists, the price is fixed or determinable, delivery has occurred and collectibility is reasonably assured. Non-refundable, upfront payments received in connection with license option agreements are recognized over the option term on a straight-line basis.
Share-based payments
Cequent recognizes compensation costs resulting from the issuance of stock-based awards to employees, non-employees and directors as an expense in the statement of operations over the service period based on a measurement of fair value for each stock-based award.
The fair value of each option grant was estimated as of the date of grant using the Black-Scholes option-pricing model. The fair value is amortized as compensation cost on a straight-line basis over the requisite service period of the awards, which is generally the vesting period. Due to its limited operating history and limited number of sales of its common stock, Cequent estimated its volatility in consideration of a number of factors including the volatility of comparable public companies.
C-10
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
The following assumptions were used to estimate the fair value of stock options granted using the Black-Scholes option pricing model:
| | | | |
| | | 2009 | | 2008 |
Risk-free interest rate | | 1.87 – 2.57% | | 1.52 – 3.3% |
Expected life | | 5 years | | 5 years |
Volatility | | 69% | | 46% |
Dividend rate | | 0% | | 0% |
Recent accounting pronouncements
In June 2008, the Financial Accounting Standards Board (“FASB”) ratified an accounting pronouncement that provides that an entity should use a two step approach to evaluate whether an equity-linked financial instrument (or embedded feature) is indexed to its own stock, including evaluating the instrument’s contingent exercise and settlement provisions. This accounting pronouncement is effective for fiscal years beginning after December 15, 2008. The consensus must be applied to outstanding instruments as of the beginning of the fiscal year in which the consensus is adopted and should be treated as a cumulative-effect adjustment to the opening balance of retained earnings. Early adoption is not permitted. On January 1, 2009, Cequent adopted this pronouncement and it did not have a material impact on Cequent’s financial statements or related disclosures.
In October 2009, the FASB issued two related accounting pronouncements, ASU 2009-13 and ASU 2009-14, relating to revenue recognition. One pronouncement provides guidance on allocating the consideration in a multiple-deliverable revenue arrangement and requires additional disclosure, while the other pronouncement provides guidance specific to revenue arrangements that include software elements.
For multiple-deliverable arrangements, the pronouncement eliminates the requirement that there be objective and reliable evidence of fair value of the undelivered item in order for the delivered item to be considered a separate unit of accounting. Now, the delivered item shall be considered a separate unit of accounting if the delivered item has value to the customer on a standalone basis and, if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item or items is considered probable and substantially in the control of the vendor. Arrangement consideration shall be allocated at the inception of the arrangement to all deliverables on the basis of their relative selling price. When applying the relative selling price method, the selling price of each deliverable shall be determined using vendor-specific objective evidence, if it exists; third party evidence of selling price; or if neither of those exist, the vendor shall use its best estimate of the selling price for that deliverable.
The pronouncement specific to certain revenue arrangements that include software elements amends the scope of arrangements that are accounted for under software revenue recognition rules and provides guidance for allocating the arrangement consideration. The pronouncement clarifies that the following arrangements are outside the scope of accounting under software revenue recognition rules and must follow the general revenue recognition rules for multiple-deliverable arrangements: 1) non-software components of tangible products, 2) software components of tangible products that are sold, licensed or leased with tangible products when the software components and non-software components of the tangible product function together to deliver the tangible product’s essential functionality, and 3) undelivered elements that relate to software that is essential to the tangible product’s functionality in 2 above. If an arrangement contains software and non-software deliverables, consideration must be allocated to the non-software deliverables individually and to the software deliverables as a group based on the relative selling price method.
C-11
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
Both of these pronouncements are effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010 and both must be adopted together. Early adoption is permitted. If a company elects early adoption and the period of adoption is not the beginning of the company’s fiscal year, the company is required to apply the guidance in these pronouncements retrospectively from the beginning of the company’s fiscal year. A company may also elect to adopt the guidance in these pronouncements retrospectively to prior periods. Cequent does not expect the adoption of these pronouncements to have a material impact on its financial statements.
In January 2010, the FASB issued ASU 2010-06, Fair Value Measurements and Disclosures (Topic 820), Improving Disclosures about Fair Value Measurements. This Update requires new disclosures and clarifies existing disclosures regarding recurring and nonrecurring fair value measurements to provide increased transparency to users of the financial statements. The new disclosures and clarification of existing disclosures are effective for interim and annual periods beginning after December 15, 2009, except for the disclosures pertaining to the roll forward of activity for Level 3 fair value measurements, which are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. Cequent does not expect the adoption of this Update to have a material impact on its financial statements.
Included in other assets are the following:
| | | | | | | |
| | | 2009 | | 2008 | |
Deposit on leased office space | | $ | 45,133 | | $ | 45,133 | |
Deferred financing costs | | | — | | | 31,379 | |
Other assets | | | — | | | 8,257 | |
| | | | | | | |
| | | 45,133 | | | 84,769 | |
Less accumulated amortization on deferred financing costs | | | — | | | (3,910 | ) |
| | | | | | | |
| | $ | 45,133 | | $ | 80,859 | |
| | | | | | | |
Amortization of deferred financing costs of $27,469 and $3,910 is included in interest expense in the years ended December 31, 2009 and 2008, respectively.
Property and equipment consisted of the following:
| | | | | | | | |
| | | 2009 | | | 2008 | |
Computer software and hardware | | $ | 143,158 | | | $ | 160,830 | |
Office furniture and equipment | | | 101,642 | | | | 101,642 | |
Lab equipment | | | 523,127 | | | | 480,375 | |
Leasehold improvements | | | 76,354 | | | | 76,354 | |
| | | | | | | | |
| | | 844,281 | | | | 819,201 | |
Less accumulated depreciation and amortization | | | (427,057 | ) | | | (245,234 | ) |
| | | | | | | | |
| | $ | 417,224 | | | $ | 573,967 | |
| | | | | | | | |
C-12
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
Accrued expenses consisted of the following:
| | | | | | |
| | | 2009 | | 2008 |
Accrued bonus | | $ | 195,800 | | $ | 277,640 |
Accrued expenses | | | 40,042 | | | 55,748 |
Accrued severance | | | 46,375 | | | — |
Accrued legal | | | 35,000 | | | 16,560 |
Accrued vacation | | | 9,788 | | | 21,923 |
Accrued other | | | 4,933 | | | — |
| | | | | | |
| | $ | 331,938 | | $ | 371,871 |
| | | | | | |
| 6. | LOAN AND SECURITY AGREEMENT |
On July 7, 2008, Cequent entered into a Loan and Security Agreement (“Agreement”) for a $3,500,000 Growth Capital Facility (“Growth Capital Line”) with Silicon Valley Bank and Gold Hill Venture Lending 03, L.P (“Lenders”). The Agreement gave Cequent the right to draw advances through September 30, 2009 in an aggregate amount not to exceed the Growth Capital Line. Each advance must be repaid within thirty-six months commencing on the applicable amortization date through consecutive equal monthly payments of principal and interest. The principal amount of each advance accrues interest at a fixed per annum rate equal to 10%, payable monthly. The Agreement was secured by substantially all of Cequent’s assets with the exception of its intellectual property. For the years ended December 31, 2009 and 2008 there were no advances made under this Agreement and the Agreement expired on September 30, 2009.
In connection with the Agreement, Cequent issued to the Lenders, after the effect of certain anti-dilution rights, warrants to purchase 198,249 shares of common stock at an exercise price of $0.07 per share. The warrants have a ten year term and expire in 2018. The fair value was determined at issuance to be $5,533 which was recorded as a deferred financing cost and additional paid-in-capital. The fair value of the warrant was determined using the Black-Scholes option pricing model and assumptions consistent with those described in Note 2.
In December 2009, Cequent leased certain equipment under a capital lease agreement that is included in property and equipment and accumulated depreciation on the balance sheet at December 31, 2009:
| | | | |
Leased equipment | | $ | 45,041 | |
Less accumulated depreciation | | | (9,759 | ) |
| | | | |
| | $ | 35,282 | |
| | | | |
C-13
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
The term of the lease calls for monthly payments in the amount of $1,380 until December 2011. The minimum lease payments under the capital lease at December 31, 2009 are as follows:
| | | | |
Year Ending December 31, | | | |
2010 | | $ | 16,555 | |
2011 | | | 16,555 | |
| | | | |
Total | | | 33,110 | |
Less: amount representing interest | | | (1,685 | ) |
| | | | |
| | $ | 31,425 | |
| | | | |
For the years ended December 31, 2009 and 2008, Cequent expensed $456 and $542, respectively, related to the Massachusetts excise tax, which is included in general and administrative expenses. At December 31, 2009 and 2008, Cequent had accrued amounts related to the Massachusetts excise tax of $4,933 and none, respectively.
Deferred taxes
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The only significant component of Cequent’s deferred tax asset relates to its net operating loss carryforward.
Cequent has provided a valuation allowance against the deferred tax assets, since it has a history of losses. Cequent currently believes that it is more likely than not that the deferred tax assets relating to the loss carryforwards and other temporary differences will not be realized in the future. In the year ended December 31, 2009, the valuation allowance increase approximately $1,907,000.
Operating loss carryforward
Through December 31, 2009, Cequent had U.S. federal and state net operating loss carryforwards of approximately $14,871,000 that can be carried forward and offset against taxable income. Federal net operating losses can be carried forward for 20 years and begin to expire in 2024 while Massachusetts net operating losses can be carried forward for five years and began to expire in 2009. Utilization of net operating losses may be subject to substantial annual limitations due to the “change in ownership” provisions of the Internal Revenue Code, and similar state provisions. The annual limitations may result in the expiration of net operating losses before utilization.
Series A and A-1 Redeemable, Convertible Preferred Stock
In October 2006, Cequent entered into a Securities Purchase Agreement (the “Agreement”) authorizing the issuance of up to 8,000,000 shares of Series A Redeemable, Convertible Preferred Stock (“Series A Preferred Stock”). In June and December 2007 and August 2008, Cequent authorized additional closings of Series A Preferred Stock increasing the number of authorized shares of Series A Preferred to 14,850,000 shares as of December 31, 2008.
C-14
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
In October 2009, Cequent entered into a Series A-1 Convertible Preferred Stock Purchase Agreement (the “Series A-1 Agreement) authorizing the conversion of up to 14,850,000 of original Series A Preferred Stock to Series A-1 Preferred Stock. In addition, the Series A-1 Agreement also authorized the issuance of up to an additional 12,734,420 of Series A-1 shares for a total of 27,584,420 authorized shares of Series A-1 Preferred Stock.
Under the Series A-1 Agreement, 11,850,000 shares of Series A Preferred Stock converted to an equal number of shares of Series A-1 Preferred Stock and 3,000,000 shares of Series A Preferred Stock converted into an equal number of shares of common stock. In addition, Cequent sold 3,326,263 shares of Series A-1 Preferred Stock for cash proceeds of $3,237,131, net of issuance costs of $89,132.
Significant terms of the Series A-1 Preferred Stock are as follows:
Conversion
Each share of Series A-1 Preferred Stock is initially convertible into four shares of common stock, subject to adjustments as applicable from time to time, at the option of the holder, at any time after the day of issuance of such share or automatically upon the earlier of (i) a qualified initial public offering (“IPO”) in which shares are sold at not less than $4.00 per share and gross proceeds to Cequent are not less than $35 million; or (ii) when the holders of at least a majority of the then outstanding Series A-1 Preferred Stock so agree.
Liquidation preference
Upon any liquidation, dissolution, or winding up of Cequent, either voluntary or involuntary, the holders of Series A-1 Preferred Stock shall be entitled to receive, prior and in preference to holders of Series A Preferred Stock or common stock, an amount per share equal to (i) the Series A-1 Original Issues Price ($1.00, subject to adjustment) per share of Series A-1 Preferred Stock; plus (ii) an amount equal to all accrued but unpaid dividends and all declared but unpaid dividends.
After the payment of all preferential amounts required to be paid to the holders of Series A-1 Preferred Stock, the holders of Series A Preferred Stock shall be entitled to receive, prior and in preference to holders of common stock, an amount per share equal to (i) the Series A Original Issues Price ($1.00, subject to adjustment) per share of Series A-1 Preferred Stock; plus (ii) an amount equal to all accrued but unpaid dividends and all declared but unpaid dividends.
After the payment of all preferential amounts required to be paid to the holders of Series A and A-1 Preferred Stock, the remaining assets of Cequent available for distribution to its stockholders shall be distributed among the holders of shares of Series A and A-1 Preferred Stock, common stock and any other class or series of stock entitled to participate in liquidation distributions with the holders of common stock, pro rata based on the number of shares of common stock held by each (assuming conversion into common stock of all such shares); provided, however, that the holders of Series A and A-1 Preferred Stock are not entitled to receive an amount in excess of four times the Original Issue Price.
Dividends
The holders of Series A-1 Preferred Stock shall be entitled to receive dividends, out of any assets legally available if and when declared by the Board of Directors, at the rate of eight percent (8%) of the Series A-1 Original Issue Price per annum on each outstanding share of Series A-1 Preferred Stock. Such dividends shall begin accruing on the first anniversary of the original Series A-1 Preferred Stock
C-15
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
issuance date, and shall be on a cumulative basis, compounding annually. Dividends are payable when declared by the Board of Directors, upon a liquidation event and upon conversion. Dividends with respect to the Series A Preferred Stock exchanged for Series A-1 Preferred Stock that were accrued but unpaid at the time of such exchange shall be deemed to have accrued to the Series A-1 Preferred Stock for which such Series A Preferred Stock was exchanged and such share of Series A-1 Preferred Stock thereafter accrue dividends as though such accrued but unpaid dividends had accrued originally on such Series A-1 Preferred Stock shares.
To date, the Board of Directors has not declared any dividends. At December 31, 2009, Cequent had cumulative accreted dividends on the Series A-1 Preferred Stock of $1,793,805 that represent dividends accrued on Series A Preferred Stock of $1,183,805 and $610,000 for the years ended December 31, 2009 and 2008, respectively.
Voting
The holders of each share of Series A-1 Preferred Stock have the right to one vote for each share of common stock into which such Series A-1 Preferred Stock could then convert. As long as any of the Series A-1 Preferred Stock is outstanding, the holders of a majority of Series A-1 Preferred Stock have the right, exclusively and as a separate class, to elect four members of Cequent’s Board of Directors.
Redemption
On each of the fourth, fifth and sixth anniversaries of the Series A-1 Preferred Stock issuance date, any holder of Series A-1 Preferred Stock may elect to redeem up to a maximum of 33%, 67% and 100%, respectively, of the holder’s shares of Series A-1 Preferred Stock at an amount per share equal to the Series A-1 Original Issue Price per share, plus all accrued but unpaid dividends on such share of Series A-1 Preferred Stock. The amount of the redemption is subject to appropriate adjustments in the event of any stock dividend, stock split, combination or other similar recapitalization affecting Series A-1 Preferred Stock.
Cequent considers the Series A-1 Preferred Stock contingently redeemable as a result of the conversion feature and has therefore classified the Series A-1 Preferred Stock as equity in the balance sheet.
Cequent will accrete the Series A-1 Preferred Stock to its redemption value over the period from issuance to redemption, such that the carrying amounts of the Series A-1 Preferred Stock will equal the redemption amount at the earliest redemption date.
Demand registration and participation rights
At any time two years after the date of issuance, the holders of Series A-1 Preferred Stock, in the aggregate and forming at least a majority of total equity securities outstanding, had the right to request that Cequent file a qualified initial public offering (“IPO”). No such filing has occurred. The holders of Series A-1 Preferred Stock also have certain participation rights in future financings.
Participation rights
The holders of Series A-1 Preferred Stock have the right, for a period of thirty days following the delivery of an equity offering by Cequent, to purchase or acquire at a price and upon the other terms specified in the offer, up to said holder’s pro rata portion of the equity securities plus such additional
C-16
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
portion of the securities up to the total of all of the holders of Series A-1 Preferred Stock pro rata portion of the equity securities offered for sale.
Common stock
The common stock confers upon its holders the right to receive dividends out of any assets legally available, when and as declared by the Board of Directors. The holders of the common stock have the right, exclusively and as a separate class, to elect one director. In addition, the holders of the common stock have the right to participate and vote in the general meeting of stockholders and in extraordinary meetings of Cequent in such manner that each share confers upon its holder one vote therein.
Restricted common stock
In March 2004, Cequent sold 918,253 shares of restricted common stock to its two founders for total proceeds of $2,250. Cequent sold 9,182 shares of restricted common stock to a consultant in January 2006 for total proceeds of $400. Following the termination of a founder’s employment, Cequent has the right to repurchase all unvested restricted shares at a price equal to the original purchase price. Cequent’s repurchase rights vary by each restricted stockholders’ respective agreement and also include vesting schedules of between three and four years. At December 31, 2009, there were no shares of restricted common stock subject to repurchase.
If a holder of restricted stock proposes to transfer shares that are no longer subject to repurchase by Cequent, then the holder shall give written notice to Cequent of the proposed transfer. For a specified period of time following the delivery of the request, Cequent shall have the option to purchase all, but not less than all, of the shares proposed for transfer at the price and upon the terms set forth in the transfer notice, as defined.
Other common stock issuances
In 2005, Cequent sold 103,048 shares of its common stock for proceeds of $4,489. In 2006, Cequent sold 1,368,910 shares of its common stock for proceeds of $4,687.
Licensing agreement
On December 30, 2004, Cequent entered into an exclusive licensing agreement with Beth Israel Deaconess Medical Center (“BIDMC”) for patented technology in the field of Bacterial-Mediated Gene Silencing and related methods of use. This agreement was amended on October 20, 2006. The amended agreement obligates Cequent to issue shares of common stock to BIDMC upon a Qualified Financing until BIDMC holds five percent of the issued and outstanding equity securities of Cequent as of the date of the Qualified Financing. Cequent’s obligation to issue additional shares of common stock ceased when the total equity financing reached $7,500,000. As of December 31, 2009, Cequent had Qualified Financing equal to $18,176,000, as a result of the Series A and Series A-1 Preferred Stock issuances. In the years ended December 31, 2007 and 2006, Cequent issued 78,947 and 437,059 shares of common stock to BIDMC with a fair value of $9,474 and $52,447, respectively, and recorded research and development expense in the same amounts upon issuance of the common stock. There was no additional stock issued to BIDMC in 2009 or 2008.
The licensing agreement contains a requirement that Cequent must file an IND application with the FDA by December 31, 2009 or Cequent will forfeit the exclusive use of the licensed technology. In compliance with the licensing agreement, Cequent filed its first IND application in November 2009.
C-17
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
Treasury stock
In 2006, as a result of the death of one of Cequent’s founders, Cequent repurchased unvested shares of restricted stock. Cequent repurchased 86,090 shares at their original purchase price of $0.001 or $86. These shares were held in treasury in accordance with Delaware law until May 9, 2007 when Cequent returned the 86,090 shares to the status of authorized but unissued.
Stock option plan
In 2006, Cequent adopted the 2006 Stock Incentive Plan (the “Plan”). Under the Plan, the Board of Directors may grant options and establish the terms of each grant in accordance with provisions of the Plan. Plan options are exercisable for up to 10 years from the date of issuance.
As of December 31, 2009, the aggregate number of common shares which may be issued under the Plan was 15,673,052 shares. A summary of the activity under Cequent’s stock option plan is as follows:
| | | | | | | | | | | |
| | | Shares | | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual
Term
(Months) | | Aggregate
Intrinsic
Value |
Outstanding at December 31, 2008 | | 3,207,106 | | | $ | 0.118 | | | | | |
Granted | | 435,000 | | | $ | 0.130 | | | | | |
Exercised | | — | | | | — | | | | | |
Forfeited | | (1,058,334 | ) | | | 0.126 | | | | | |
| | | | | | | | | | | |
Outstanding at December 31, 2009 | | 2,583,772 | | | $ | 0.121 | | 98 | | $ | 3,568 |
| | | | | | | | | | | |
| | | | |
Options exercisable at December 31, 2009 | | 1,366,997 | | | $ | 0.116 | | 114 | | $ | 3,568 |
| | | | | | | | | | | |
Reserved for future grants | | 11,990,834 | | | | | | | | | |
| | | | | | | | | | | |
Cequent recorded $21,247 and $36,262 as compensation expense in 2009 and 2008, respectively, for stock option awards granted to employees and consultants under the Plan. As of December 31, 2009, there was $47,680 of total unrecognized compensation cost related to nonvested share-based compensation arrangements granted under the Plan which will be recognized over a weighted average period of approximately three years.
The weighted-average grant-date fair value of options granted during 2009 and 2008 was $0.07 and $0.04, respectively. As of December 31, 2009 no stock options have been exercised under the Plan.
The exercise prices for options granted in 2009 and 2008 were $0.13 and $0.12 per share, respectively.
Fair value was determined using the Black-Scholes option pricing model and the assumptions described in Note 2.
Included in the 1,368,910 shares of common stock sold in 2006 (see “Other Common Stock Issuances” above) were 1,098,446 shares of common stock issued under the Plan.
Cequent entered into an exclusive option agreement with Novartis BioVentures Ltd., a related party, for an exclusive license. The option gives Novartis BioVentures Ltd. the right to enter into a Definitive Research
C-18
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
and License Agreement for selected Cequent Modulators related to the Selected Target, as defined. Novartis BioVentures Ltd. paid a non-refundable fee of $1,250,000 and is obligated to make an additional non-refundable payment of $1,250,000 within thirty days of Cequent’s completion of a designated milestone. The option expires on the fifth anniversary of the agreement, June 13, 2012. On June 15, 2007, Cequent received the first payment and recorded the receipt as deferred revenue. On August 28, 2008, Cequent received the second payment and recorded the receipt as deferred revenue. Cequent is amortizing the payments to revenue over the life of the option. In the years ended December 31, 2009 and 2008, Cequent recognized revenue of $581,087 and $390,872, respectively, in connection with the agreement.
Cequent leases office space in Cambridge, Massachusetts under a lease agreement with a 39 month term commencing on March 1, 2007. This lease was amended in September 2007 to increase the square footage. The lease has escalating monthly payments over its life of between $20,201 and $21,267.
The aggregate future minimum lease payment under this lease is as follows:
| | | |
Year Ending December 31, | | |
2010 | | $ | 106,335 |
| | | |
Rent expense for operating leases with escalation provisions is recognized on a straight-line basis over the term of the lease. Total rent expense was $247,324 and $248,382 for the years ended December 31, 2009 and 2008, respectively.
In January 2007, Cequent adopted a 401(k) plan (the “Plan”) covering all employees. Employees must be 21 years of age in order to participate in the Plan. Under the Plan, Cequent may make matching contributions. In 2009 and 2008 Cequent incurred expenses of $37,892 and $37,157, respectively, in connection with the Plan.
Management has evaluated subsequent events through April 12, 2010, which is the date the financial statements were available to be issued. Other than as discussed below there were no subsequent events that require adjustment to or disclosure in the financial statements.
Stock option and incentive stock
On February 5, 2010, pursuant to Cequent’s 2006 Incentive Stock Plan, Cequent granted to employees and consultants stock options for the purchase of 5,807,354 shares of Cequent’s common stock at an exercise price of $0.05 per share. Subject to the terms of the Plan and the stock option agreement, such options will become exercisable for 25% of the shares on the first anniversary of the vesting start date and thereafter for 1/48 (rounded up to the next whole number) of the shares on the same day of each month following the vesting start date set forth below until such options are fully exercisable and such options shall expire ten years from the date of grant.
In addition, Cequent reissued as Non-qualified Stock Options (NSO) certain Incentive Stock Options (ISO) for the purchase of 655,000 shares of Cequent’s common stock. The ISO’s were issued by Cequent prior to
C-19
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2009 to certain individuals who have since converted in status from employees of Cequent to consultants. The previous vesting schedules of the ISO’s were preserved until their consulting agreements are terminated.
On March 18, 2010, pursuant to Cequent’s 2006 Incentive Stock Plan, Cequent granted to employees and consultants stock options for the purchase of 5,888,000 shares of Cequent’s common stock at an exercise price of $0.13 per share. Subject to the terms of the Plan and the stock option agreement, such options will become exercisable for 25% of the shares on the earlier of a Change of Control or the first anniversary of the vesting start date and thereafter for 1/48 (rounded up to the next whole number) of the shares on the same day of each month following the vesting start date set forth below until such options are fully exercisable and such options shall expire ten years from the date of grant.
Agreement and Plan of Merger
On March 31, 2010, Cequent entered into an Agreement and Plan of Merger (“Merger Agreement”) with MDRNA, Inc. (“MDRNA”), a leading RNAi-based drug discovery and development company, and with Calais Acquisition Corp. (“merger sub”), a wholly-owned subsidiary of MDRNA, pursuant to which merger sub would merge with and into Cequent, with Cequent surviving as a wholly-owned subsidiary of MDRNA. The transaction includes certain loan provisions that will fund MDRNA’s operations through the anticipated closing of the merger in early July 2010. (See below).
Under the terms of the Merger Agreement, each outstanding share of Cequent’s common stock will be exchanged for MDRNA common stock at an exchange ratio that implies a purchase price for Cequent’s common and preferred stockholders of approximately $44 million, plus an additional value of approximately $2 million to warrant and option holders, based on the 10 day Volume-Weighted Average Price (VWAP) of MDRNA shares on March 31, 2010. This represents an approximate 56% equity ownership in the consolidated entity for MDRNA stockholders and a 44% equity ownership for Cequent’s securityholders.
The Merger Agreement anticipates that the combined company will be headquartered in Bothell, Washington with offices in Cambridge, Massachusetts. RNAi drug discovery, research and biology will continue to be conducted at MDRNA’s R&D facility in Bothell while clinical operations will reside in Cambridge.
In connection with the execution of the Merger Agreement described above, MDRNA and Cequent entered into a Loan Agreement (the “Loan Agreement”), pursuant to which, among other things, Cequent shall extend one or more loans to MDRNA in the aggregate principal amount of up to $3,000,000 (the “Term Loans”) to fund MDRNA’s operations prior to the completion or closing of the merger. The Term Loans are evidenced by a secured promissory note issued by MDRNA to Cequent (the “Term Note”), which bears interest absent an event of default, at a rate of ten percent per annum. MDRNA will repay the principal and interest of the Term Loans in three equal consecutive monthly installments, commencing on August 15, 2010 and continuing on the 15th day of each month thereafter through and including October 15, 2010. Notwithstanding the foregoing, if the Merger is consummated prior to August 15, 2010, then, on the closing date of the Merger, MDRNA shall not owe to any third party any obligations with respect to the Term Loans, including, without limitation the then outstanding principal balance of the Term Loans and any interest then accrued but unpaid thereon. MDRNA may prepay any Term Loan in whole or in part without premium or penalty.
As consideration for the Loan Agreement, MDRNA agrees to issue to Cequent on the date of each Term Loan a five-year warrant to purchase shares of MDRNA’s capital stock with an exercise price subject to
C-20
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Concluded)
adjustments as defined in the Merger Agreement. Each warrant is exercisable for a number of shares of MDRNA’s common stock equal to sixty-five percent (65%) of the principal amount of the Term Loan being made on such date divided by $1.1496 (subject to an equitable adjustment in the event of any stock split, stock dividend, stock combination or the like). The warrant cannot be exercised unless the Merger Agreement has been terminated, without the consummation of the transaction contemplated by the Merger Agreement. Upon the completion of the Merger Agreement the warrants will terminate and be invalid.
Preferred stock issuance
On April 8, 2010, pursuant to the Series A-1 Convertible Preferred Stock Purchase Agreement, Cequent issued 2,517,173 Series A-1 Convertible Preferred Stock for gross proceeds of $2,517,173.
C-21
Annex D
CONSENT OF INDEPENDENT AUDITOR
We have issued our report dated April 12, 2010, with respect to the financial statements of Cequent Pharmaceuticals, Inc. for the years ended December 31, 2009 and 2008 and for the period from November 10, 2003 (inception) to December 31, 2009 which is included in this Proxy Statement. We consent to the inclusion in this Proxy Statement of the aforementioned report.
/s/ Wolf & Company, P.C.
Boston, Massachusetts
June 4, 2010
D-1
Annex E
INDEX TO FINANCIAL STATEMENTS
OF
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
Three-month Periods ended March 31, 2010 and 2009 and the Period from
November 10, 2003 (Inception) through March 31, 2010
(Unaudited)
CONTENTS
E-1
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
BALANCE SHEETS
(Unaudited)
| | | | | | | | |
| | | As of, | |
| | | March 31,
2010 | | | December 31,
2009 | |
| ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 2,116,378 | | | $ | 3,213,748 | |
Prepaid expenses | | | 31,399 | | | | 37,211 | |
| | | | | | | | |
Total current assets | | | 2,147,777 | | | | 3,250,959 | |
Property and equipment, net | | | 367,067 | | | | 417,224 | |
Other assets | | | 45,133 | | | | 45,133 | |
| | | | | | | | |
Total assets | | $ | 2,559,977 | | | $ | 3,713,316 | |
| | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 45,599 | | | $ | 131,009 | |
Accrued expenses | | | 216,972 | | | | 331,938 | |
Capital lease—current portion | | | 15,236 | | | | 13,656 | |
Deferred revenue—current portion | | | 576,087 | | | | 576,087 | |
| | | | | | | | |
Total current liabilities | | | 853,894 | | | | 1,052,690 | |
Capital lease, net of current portion | | | 12,089 | | | | 17,769 | |
Deferred revenue, net of current portion | | | 692,935 | | | | 836,956 | |
| | | | | | | | |
Total liabilities | | | 1,558,918 | | | | 1,907,415 | |
| | | | | | | | |
Commitments | | | | | | | | |
Stockholders’ equity: | | | | | | | | |
Series A redeemable, convertible preferred stock, $0.001 par value; authorized 14,850,000 shares, none issued and outstanding at March 31, 2010 and December 31, 2009 | | | — | | | | — | |
Series A-1 redeemable, convertible preferred stock, $0.001 par value; authorized 27,584,420 shares, issued and outstanding 15,176,263 at March 31, 2010 and December 31, 2009 (preference in liquidation of $16,970,068 at March 31, 2010) | | | 16,896,705 | | | | 16,966,615 | |
Common stock , $0.001 par value; authorized 130,000,000 shares, issued and outstanding 5,824,710 and 5,820,127 at March 31, 2010 and December 31, 2009, respectively | | | 5,825 | | | | 5,820 | |
Additional paid-in capital | | | 1,895,119 | | | | 1,719,445 | |
Deficit accumulated during the development stage | | | (17,796,590 | ) | | | (16,885,979 | ) |
| | | | | | | | |
Total stockholders’ equity | | | 1,001,059 | | | | 1,805,901 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 2,559,977 | | | $ | 3,713,316 | |
| | | | | | | | |
The accompanying notes are an integral part of these financial statements.
E-2
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
STATEMENTS OF OPERATIONS
(Unaudited)
| | | | | | | | | | | | |
| | | Three Months Ended March 31, | | | Period from
November 10,
2003 (Inception)
through March 31,
2010 | |
| | | 2010 | | | 2009 | | |
Revenue | | $ | 144,021 | | | $ | 140,625 | | | $ | 1,240,978 | |
| | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 291,153 | | | | 973,815 | | | | 11,563,862 | |
General and administrative | | | 763,365 | | | | 557,658 | | | | 7,233,267 | |
| | | | | | | | | | | | |
Total operating expenses | | | 1,054,518 | | | | 1,531,473 | | | | 18,797,129 | |
| | | | | | | | | | | | |
Operating loss | | | (910,497 | ) | | | (1,390,848 | ) | | | (17,556,151 | ) |
| | | | | | | | | | | | |
Other income (expense): | | | | | | | | | | | | |
Other income (expense) | | | — | | | | — | | | | 10,287 | |
Interest income | | | 63 | | | | 7,986 | | | | 445,947 | |
Interest expense | �� | | (177 | ) | | | (727 | ) | | | (71,908 | ) |
| | | | | | | | | | | | |
Other income (expense), net | | | (114 | ) | | | 7,259 | | | | 384,326 | |
| | | | | | | | | | | | |
Net loss | | $ | (910,611 | ) | | $ | (1,383,589 | ) | | $ | (17,171,825 | ) |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements
E-3
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
STATEMENTS OF CASH FLOWS
Unaudited
| | | | | | | | | | | | |
| | | Three Months Ended March 31, | | | Period from
November 10,
2003 (Inception)
through March 31,
2010 | |
| | | 2010 | | | 2009 | | |
Cash flows from operating activities: | | | | | | | | | | | | |
Net loss | | $ | (910,611 | ) | | $ | (1,383,589 | ) | | $ | (17,171,825 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Depreciation and amortization expense | | | 48,948 | | | | 36,000 | | | | 497,045 | |
Non-cash interest expense | | | — | | | | — | | | | 31,379 | |
Share-based compensation expense | | | 105,173 | | | | 14,957 | | | | 176,409 | |
Fair value of common stock issued in conjunction with license agreement | | | — | | | | — | | | | 61,921 | |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Prepaid expenses and other assets | | | 5,812 | | | | 47,978 | | | | (102,378 | ) |
Accounts payable | | | (85,410 | ) | | | 52,235 | | | | 45,599 | |
Accrued expenses | | | (114,966 | ) | | | (263,185 | ) | | | 221,363 | |
Deferred revenue | | | (144,021 | ) | | | (140,625 | ) | | | 1,269,022 | |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (1,095,075 | ) | | | (1,636,229 | ) | | | (14,971,465 | ) |
| | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | |
Purchases of property and equipment | | | — | | | | (8,900 | ) | | | (820,280 | ) |
Disposal of property and equipment | | | 1,209 | | | | — | | | | 1,209 | |
| | | | | | | | | | | | |
Net cash provided (used) in investing activities | | | 1,209 | | | | (8,900 | ) | | | (819,071 | ) |
| | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | |
Proceeds from issuance of preferred stock, net of issuance costs | | | — | | | | — | | | | 17,912,694 | |
Repayments of capital lease | | | (4,100 | ) | | | (3,411 | ) | | | (17,716 | ) |
Proceeds from issuance of common stock | | | 596 | | | | — | | | | 12,022 | |
Repurchase of common stock | | | — | | | | — | | | | (86 | ) |
| | | | | | | | | | | | |
Net cash (used) provided by financing activities | | | (3,504 | ) | | | (3,411 | ) | | | 17,906,914 | |
| | | | | | | | | | | | |
(Decrease) increase in cash and cash equivalents | | | (1,097,370 | ) | | | (1,648,540 | ) | | | 2,116,378 | |
Cash and cash equivalents at beginning of period | | | 3,213,748 | | | | 5,580,661 | | | | — | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of period | | $ | 2,116,378 | | | $ | 3,932,121 | | | $ | 2,116,378 | |
| | | | | | | | | | | | |
Supplemental disclosure of cash flow information and non-cash transactions: | | | | | | | | | | | | |
Cash paid for interest | | $ | 177 | | | $ | 565 | | | $ | 36,126 | |
| | | | | | | | | | | | |
Exchange of Series A Preferred stock and dividends for Series A-1 Preferred stock and dividends | | $ | — | | | $ | — | | | $ | 13,643,805 | |
| | | | | | | | | | | | |
Exchange of Series A Preferred stock for Common Stock | | $ | — | | | $ | — | | | $ | 3,000,000 | |
| | | | | | | | | | | | |
| | | — | | | | | | | | | |
Accretion of dividend and issuance costs on Series A Preferred stock | | $ | — | | | $ | 180,000 | | | $ | 2,053,921 | |
| | | | | | | | | | | | |
Relative fair value of warrants recorded as deferred financing costs | | $ | — | | | $ | 5,533 | | | $ | 5,533 | |
| | | | | | | | | | | | |
Property and equipment acquired under capital leases | | $ | — | | | $ | 45,041 | | | $ | 45,041 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
E-4
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
Period Ended March 31, 2010 and 2009, and the Period from
November 10, 2003 (Inception) to March 31, 2010 (Unaudited)
1. NATURE OF OPERATIONS
Business
Cequent Pharmaceuticals, Inc. (“Cequent”) was incorporated on November 10, 2003 under the laws of the State of Delaware.
Cequent is an early-stage biopharmaceutical company developing novel therapeutics to prevent and treat a wide range of human diseases based on Cequent’s proprietary technology, TransKingdom RNA interference (tkRNAiTM). The FDA recently approved Cequent’s IND (investigational new drug) application for the first trial of an orally administered RNA interference drug in humans.
Since its inception, Cequent has devoted substantially all of its efforts to business planning, research and development, recruiting management and technical staff, acquiring operating assets and raising capital. Accordingly, Cequent is considered to be in the development stage.
Cequent is subject to a number of risks similar to other companies in its industry including rapid technological change, uncertainty of market acceptance of the product, competition from larger companies with substitute products and dependence on key personnel.
2. SIGNIFICANT ACCOUNTING POLICIES
A summary of the significant accounting policies followed by Cequent in the preparation of the accompanying consolidated financial statements follows:
Basis of presentation
The accompanying unaudited financial statements of Cequent have been prepared in accordance with U.S. generally accepted accounting principles for interim financial information and do not include all the information and notes required by accounting principles generally accepted in the United States for complete financial statements. In the opinion of management, the accompanying unaudited financial statements include all adjustments, consisting of normal recurring adjustments, which are necessary to present fairly our financial position, results of operations and cash flows. Operating results for the three month period ended March 31, 2010, are not necessarily indicative of the results that may be expected for a full fiscal year.
Certain amounts in the 2009 financial statements have been reclassified to conform with the 2010 presentation.
Use of estimates
The process of preparing financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of assets and liabilities at the date of financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates and changes in estimates may occur.
E-5
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
Cash and cash equivalents
Cequent considers all highly liquid investments with maturities of three months or less at the date of purchase to be cash equivalents.
Financial instruments
Cequent’s financial instruments, consisting of cash and cash equivalents, accounts payable and accrued liabilities are carried at cost, which approximates fair value due to the short-term nature of these instruments. The carrying value of capital lease obligations approximates its fair value due to the market terms of the arrangements.
Derivative instruments
Cequent generally does not use derivative instruments to hedge exposures to cash-flow or market risks; however, certain warrants to purchase common stock that do not meet the requirements for classification as equity are classified as liabilities. In such instances, net-cash settlement is assumed for financial reporting purposes, even when the terms of the underlying contracts do not provide for a net-cash settlement. Such financial instruments are initially recorded at fair value, or relative fair value when issued with other instruments, with subsequent changes in fair value charged (credited) to operations in each reporting period. If these instruments subsequently meet the requirements for classification as equity, Cequent reclassifies the fair value to equity.
At March 31, 2010, Cequent had outstanding warrants to purchase 198,249 shares of its common stock with an exercise price of $0.07 per share. These warrants are considered to be derivative instruments since the agreements contain “down round” provisions whereby the number of shares covered by the warrants is subject to change in the event of certain dilutive stock issuances. The fair value of these derivative instruments at March 31, 2010 and December 31, 2009 was immaterial, as was the change in the fair value during that period. The fair value of these warrants was estimated using the Black-Scholes option pricing model and assumptions consistent with those disclosed in “Share-based payments” below.
The primary underlying risk exposure pertaining to the warrants is the change in fair value of the underlying common stock for each reporting period.
Property and equipment
Property and equipment are carried at cost. Depreciation and amortization expense is provided over the estimated useful lives of the assets using the straight-line method. A summary of the estimated useful lives is as follows:
| | |
Classification | | Estimated Useful Life |
Computer hardware and software | | 3 years |
Office furniture and equipment | | 5 years |
Lab equipment | | 5 years |
Leasehold improvements | | Shorter of lease term
or useful life |
E-6
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
Depreciation and amortization expense was $48,948 and $36,000 for the three-month periods ended March 31, 2010 and 2009, respectively.
Maintenance and repairs are charged to expense as incurred, while any additions or improvements are capitalized.
Research and development expenses
Costs incurred for research and development are expensed as incurred.
Income taxes
For federal and state income taxes, deferred tax assets and liabilities are recognized based upon temporary differences between the financial statement and the tax basis of assets and liabilities. Deferred income taxes are based upon prescribed rates and enacted laws applicable to periods in which differences are expected to reverse. A valuation allowance is recorded when it is more likely than not that some portion or all of the deferred tax assets will not be realized. Accordingly, Cequent provides a valuation allowance, if necessary, to reduce deferred tax assets to amounts that are realizable.
Tax positions taken or expected to be taken in the course of preparing Cequent’s tax returns are required to be evaluated to determine whether the tax positions are “more-likely-than-not” of being sustained by the applicable tax authority. Tax positions not deemed to meet a more-likely-than-not threshold would be recorded as a tax expense in the current year. There were no uncertain tax positions that require accrual or disclosure to the financial statements as of March 31, 2010.
Concentrations of credit risk
Cequent has no significant off-balance-sheet concentration of credit risk such as foreign exchange contracts, option contracts or other foreign hedging arrangements. Cequent may from time to time have cash in banks in excess of FDIC insurance limits.
Impairment of long-lived assets
Cequent continually monitors events and changes in circumstances that could indicate carrying amounts of long-lived assets may not be recoverable. An impairment loss is recognized when expected cash flows are less than an asset’s carrying value. Accordingly, when indicators of impairment are present, Cequent evaluates the carrying value of such assets in relation to the operating performance and future undiscounted cash flows of the underlying assets. Cequent’s policy is to record an impairment loss when it is determined that the carrying amount of the asset may not be recoverable. No impairment charges were recorded in the three month periods ended March 31, 2010 and 2009.
Revenue recognition
Through March 31, 2010, Cequent’s revenue primarily relates to a single option agreement (see Note 7). Revenue is recognized when persuasive evidence of an arrangement exists, the price is fixed or determinable, delivery has occurred and collectibility is reasonably assured. Non-refundable, upfront payments received in connection with license option agreements are recognized over the option term on a straight-line basis.
E-7
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
Share-based payments
Cequent recognizes compensation costs resulting from the issuance of stock-based awards to employees, non-employees and directors as an expense in the statement of operations over the service period based on a measurement of fair value for each stock-based award.
The fair value of each option grant was estimated as of the date of grant using the Black-Scholes option-pricing model. The fair value is amortized as compensation cost on a straight-line basis over the requisite service period of the awards, which is generally the vesting period. Due to its limited operating history and limited number of sales of its common stock, Cequent estimated its volatility in consideration of a number of factors including the volatility of comparable public companies.
The following assumptions were used to estimate the fair value of stock options granted using the Black-Scholes option pricing model:
| | | | |
| | | March 31, 2010 | | March 31, 2009 |
Risk-free interest rate | | 3.00 – 3.68% | | 1.87% |
Expected life | | 5 – 10 years | | 5 years |
Volatility | | 69% | | 69% |
Dividend rate | | 0% | | 0% |
Recent accounting pronouncements
In January 2010, the FASB issued ASU 2010-06, Fair Value Measurements and Disclosures (Topic 820), Improving Disclosures about Fair Value Measurements. This Update requires new disclosures and clarifies existing disclosures regarding recurring and nonrecurring fair value measurements to provide increased transparency to users of the financial statements. The new disclosures and clarification of existing disclosures are effective for interim and annual periods beginning after December 15, 2009, except for the disclosures pertaining to the roll forward of activity for Level 3 fair value measurements, which are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. Cequent does not expect the adoption of this Update to have a material impact on its financial statements.
3. PROPERTY AND EQUIPMENT
Property and equipment consisted of the following:
| | | | | | | | |
| | | March 31, 2010 | | | December 31, 2009 | |
Computer software and hardware | | $ | 141,949 | | | $ | 143,158 | |
Office furniture and equipment | | | 101,642 | | | | 101,642 | |
Lab equipment | | | 523,127 | | | | 523,127 | |
Leasehold improvements | | | 76,354 | | | | 76,354 | |
| | | | | | | | |
| | | 843,072 | | | | 844,281 | |
Less accumulated depreciation and amortization | | | (476,005 | ) | | | (427,057 | ) |
| | | | | | | | |
| | $ | 367,067 | | | $ | 417,224 | |
| | | | | | | | |
E-8
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
4. ACCRUED EXPENSES
Accrued expenses consisted of the following:
| | | | | | |
| | | March 31, 2010 | | December 31, 2009 |
Accrued bonus | | $ | — | | $ | 195,800 |
Accrued expenses | | | 55,353 | | | 40,042 |
Accrued severance | | | — | | | 46,375 |
Accrued legal | | | 94,037 | | | 35,000 |
Accrued vacation | | | 67,582 | | | 9,788 |
Accrued other | | | — | | | 4,933 |
| | | | | | |
| | $ | 216,972 | | $ | 331,938 |
| | | | | | |
5. CAPITAL LEASE
In March 31, 20010, Cequent leased certain equipment under a capital lease agreement that is included in property and equipment and accumulated depreciation on the balance sheet at March 31, 2010:
| | | | |
Leased equipment | | $ | 45,041 | |
Less accumulated depreciation | | | (12,011 | ) |
| | | | |
| | $ | 33,030 | |
| | | | |
The term of the lease calls for monthly payments in the amount of $1,380 until December 2011. The minimum lease payments under the capital lease are as follows:
| | | | |
Year Ending December 31, | | | |
2010 | | $ | 12,416 | |
2011 | | | 16,555 | |
| | | | |
Total | | | 28,971 | |
Less: amount representing interest | | | (1,646 | ) |
| | | | |
| | $ | 27,325 | |
| | | | |
6. STOCKHOLDERS’ EQUITY
Series A and A-1 Redeemable, Convertible Preferred Stock
In October 2006, Cequent entered into a Securities Purchase Agreement (the “Agreement”) authorizing the issuance of up to 8,000,000 shares of Series A Redeemable, Convertible Preferred Stock (“Series A Preferred Stock”). In June and December 2007 and August 2008, Cequent authorized additional closings of Series A Preferred Stock increasing the number of authorized shares of Series A Preferred to 14,850,000 shares as of December 31, 2008.
In October 2009, Cequent entered into a Series A-1 Convertible Preferred Stock Purchase Agreement (the “Series A-1 Agreement) authorizing the conversion of up to 14,850,000 of original Series A Preferred Stock to
E-9
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
Series A-1 Preferred Stock. In addition, the Series A-1 Agreement also authorized the issuance of up to an additional 12,734,420 of Series A-1 shares for a total of 27,584,420 authorized shares of Series A-1 Preferred Stock.
Under the Series A-1 Agreement, 11,850,000 shares of Series A Preferred Stock converted to an equal number of shares of Series A-1 Preferred Stock and 3,000,000 shares of Series A Preferred Stock converted into an equal number of shares of common stock. In addition, Cequent sold 3,326,263 shares of Series A-1 Preferred Stock for cash proceeds of $3,237,131, net of issuance costs of $89,132.
Significant terms of the Series A-1 Preferred Stock are as follows:
Conversion
Each share of Series A-1 Preferred Stock is initially convertible into four shares of common stock, subject to adjustments as applicable from time to time, at the option of the holder, at any time after the day of issuance of such share or automatically upon the earlier of (i) a qualified initial public offering (“IPO”) in which shares are sold at not less than $4.00 per share and gross proceeds to Cequent are not less than $35 million; or (ii) when the holders of at least a majority of the then outstanding Series A-1 Preferred Stock so agree.
Liquidation preference
Upon any liquidation, dissolution, or winding up of Cequent, either voluntary or involuntary, the holders of Series A-1 Preferred Stock shall be entitled to receive, prior and in preference to holders of Series A Preferred Stock or common stock, an amount per share equal to (i) the Series A-1 Original Issues Price ($1.00, subject to adjustment) per share of Series A-1 Preferred Stock; plus (ii) an amount equal to all accrued but unpaid dividends and all declared but unpaid dividends.
After the payment of all preferential amounts required to be paid to the holders of Series A-1 Preferred Stock, the holders of Series A Preferred Stock shall be entitled to receive, prior and in preference to holders of common stock, an amount per share equal to (i) the Series A Original Issue Price ($1.00, subject to adjustment) per share of Series A-1 Preferred Stock; plus (ii) an amount equal to all accrued but unpaid dividends and all declared but unpaid dividends.
After the payment of all preferential amounts required to be paid to the holders of Series A and A-1 Preferred Stock, the remaining assets of Cequent available for distribution to its stockholders shall be distributed among the holders of shares of Series A and A-1 Preferred Stock, common stock and any other class or series of stock entitled to participate in liquidation distributions with the holders of common stock, pro rata based on the number of shares of common stock held by each (assuming conversion into common stock of all such shares); provided, however, that the holders of Series A and A-1 Preferred Stock are not entitled to receive an amount in excess of four times the Original Issue Price.
Dividends
The holders of Series A-1 Preferred Stock shall be entitled to receive dividends, out of any assets legally available if and when declared by the Board of Directors, at the rate of eight percent (8%) of the Series A-1 Original Issue Price per annum on each outstanding share of Series A-1 Preferred Stock. Such dividends shall begin accruing on the first anniversary of the original Series A-1 Preferred Stock issuance date, and shall be on a cumulative basis, compounding annually. Dividends are payable when declared by the Board
E-10
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
of Directors, upon a liquidation event and upon conversion. Dividends with respect to the Series A Preferred Stock exchanged for Series A-1 Preferred Stock that were accrued but unpaid at the time of such exchange shall be deemed to have accrued to the Series A-1 Preferred Stock for which such Series A Preferred Stock was exchanged and such share of Series A-1 Preferred Stock thereafter accrue dividends as though such accrued but unpaid dividends had accrued originally on such Series A-1 Preferred Stock shares.
To date, the Board of Directors has not declared any dividends. At March 31, 2010, Cequent had cumulative accreted dividends on the Series A-1 Preferred Stock of $1,793,805. There was no accretion of dividends for the three-month periods ended March 31, 2010 and 2009.
Voting
The holders of each share of Series A-1 Preferred Stock have the right to one vote for each share of common stock into which such Series A-1 Preferred Stock could then convert. As long as any of the Series A-1 Preferred Stock is outstanding, the holders of a majority of Series A-1 Preferred Stock have the right, exclusively and as a separate class, to elect four members of Cequent’s Board of Directors.
Redemption
On each of the fourth, fifth and sixth anniversaries of the Series A-1 Preferred Stock issuance date, any holder of Series A-1 Preferred Stock may elect to redeem up to a maximum of 33%, 67% and 100%, respectively, of the holder’s shares of Series A-1 Preferred Stock at an amount per share equal to the Series A-1 Original Issue Price per share, plus all accrued but unpaid dividends on such share of Series A-1 Preferred Stock. The amount of the redemption is subject to appropriate adjustments in the event of any stock dividend, stock split, combination or other similar recapitalization affecting Series A-1 Preferred Stock.
Cequent considers the Series A-1 Preferred Stock contingently redeemable as a result of the conversion feature and has therefore classified the Series A-1 Preferred Stock as equity in the balance sheet.
Cequent will accrete the Series A-1 Preferred Stock to its redemption value over the period from issuance to redemption, such that the carrying amounts of the Series A-1 Preferred Stock will equal the redemption amount at the earliest redemption date.
Demand registration and participation rights
At any time two years after the date of issuance, the holders of Series A-1 Preferred Stock, in the aggregate and forming at least a majority of total equity securities outstanding, had the right to request that Cequent file a qualified initial public offering (“IPO”). No such filing has occurred. The holders of Series A-1 Preferred Stock also have certain participation rights in future financings.
Participation rights
The holders of Series A-1 Preferred Stock have the right, for a period of thirty days following the delivery of an equity offering by Cequent, to purchase or acquire at a price and upon the other terms specified in the offer, up to said holder’s pro rata portion of the equity securities plus such additional portion of the securities up to the total of all of the holders of Series A-1 Preferred Stock pro rata portion of the equity securities offered for sale.
E-11
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
Common stock
The common stock confers upon its holders the right to receive dividends out of any assets legally available, when and as declared by the Board of Directors. The holders of the common stock have the right, exclusively and as a separate class, to elect one director. In addition, the holders of the common stock have the right to participate and vote in the general meeting of stockholders and in extraordinary meetings of Cequent in such manner that each share confers upon its holder one vote therein.
Stock option plan
In 2006, Cequent adopted the 2006 Stock Incentive Plan (the “Plan”). Under the Plan, the Board of Directors may grant options and establish the terms of each grant in accordance with provisions of the Plan. Plan options are exercisable for up to 10 years from the date of issuance.
As of March 31, 2010, the aggregate number of common shares which may be issued under the Plan was 15,673,052 shares. A summary of the activity under Cequent’s stock option plan for the quarter ended March 31, 2010 is as follows:
| | | | | | | | | | | |
| | | Shares | | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual
Term
(Months) | | Aggregate
Intrinsic
Value |
Outstanding at December 31, 2009 | | 2,583,772 | | | $ | 0.121 | | | | | |
Granted | | 12,350,355 | | | $ | 0.088 | | | | | |
Exercised | | (4,583 | ) | | $ | 0.130 | | | | | |
Forfeited | | (444,583 | ) | | | 0.123 | | | | | |
| | | | | | | | | | | |
Outstanding at March 31, 2010 | | 14,484,961 | | | $ | 0.093 | | 115 | | $ | 547,125 |
| | | | | | | | | | | |
Options exercisable at March 31, 2010 | | 4,130,720 | | | $ | 0.069 | | 110 | | $ | 259,760 |
| | | | | | | | | | | |
Reserved for future grants | | 85,062 | | | | | | | | | |
| | | | | | | | | | | |
Cequent recorded $105,173 and $14,957 as compensation expense in three-month period ended March 31, 2010 and 2009, respectively, for stock option awards granted to employees and consultants under the Plan. As of March 31, 2010, there was $606,305 of total unrecognized compensation cost related to nonvested share-based compensation arrangements granted under the Plan which will be recognized over a weighted average period of approximately three years.
The weighted-average grant-date fair value of options granted during the three month period ended March 31, 2010 was $0.07. The exercise prices for options granted in the three-month period ended March 31, 2010 were $0.05 and $0.13 per share. Fair value was determined using the Black-Scholes option pricing model and the assumptions described in Note 2.
On March 18, 2010, Cequent granted options to purchase a total of 5,888,000 shares of common stock at $0.13 per share. These options vest as to 25% of the original number of shares on the earlier of (1) the first anniversary of the grant date or (2) immediately upon a change of control, as defined. Cequent has made an initial best estimate of the requisite service period for these options which may be changed in subsequent periods.
E-12
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
Option Modification
In the three months ended March 31, 2010, in conjunction with the change in status from employees to consultants, Cequent effectively modified and re-priced options with three individuals for the purchase of a total of 655,000 shares of common stock. The re-issued options retained the vesting terms of the original options, have an exercise price of $0.05 per share and a ten-year term. The total expense related to these options will equal the portion of the grant-date fair value of the original award for which the requisite service is expected to be or has already been rendered ($4,631) plus the incremental cost resulting from the modification ($20,671). Incremental cost was measured as the excess of the fair value of the modified award over the fair value of the original award immediately before its terms were modified. The portion of the newly measured cost attributable to the remaining portion of the requisite service period will be recognized as expense over the service period. As the modified options were granted to non-employees, the fair value will be recomputed throughout the remaining vesting period as measurement dates occur.
7. DEFERRED REVENUE
Cequent entered into an exclusive option agreement with Novartis BioVentures Ltd., a related party, for an exclusive license. The option gives Novartis BioVentures Ltd. the right to enter into a Definitive Research and License Agreement for selected Cequent Modulators related to the Selected Target, as defined. Novartis BioVentures Ltd. paid a non-refundable fee of $1,250,000 and is obligated to make an additional non-refundable payment of $1,250,000 within thirty days of Cequent’s completion of a designated milestone. The option expires on the fifth anniversary of the agreement, June 13, 2012. On June 15, 2007, Cequent received the first payment and recorded the receipt as deferred revenue. On August 28, 2008, Cequent received the second payment and recorded the receipt as deferred revenue. Cequent is amortizing the payments to revenue over the life of the option. In the three-month period ended March 31, 2010 and 2009, Cequent recognized revenue of $144,021 and $140,625, respectively, in connection with the agreement.
8. SUBSEQUENT EVENTS
Management has evaluated subsequent events through June 4, 2010, which is the date the financial statements were available to be issued. Other than as discussed below there were no subsequent events that require adjustment to or disclosure in the financial statements.
Agreement and Plan of Merger
On March 31, 2010, Cequent entered into an Agreement and Plan of Merger (“Merger Agreement”) with MDRNA, Inc. (“MDRNA”), a leading RNAi-based drug discovery and development company, and with Calais Acquisition Corp. (“merger sub”), a wholly-owned subsidiary of MDRNA, pursuant to which merger sub would merge with and into Cequent, with Cequent surviving as a wholly-owned subsidiary of MDRNA. The transaction includes certain loan provisions that will fund MDRNA’s operations through the anticipated closing of the merger in early July 2010. (See below).
Under the terms of the Merger Agreement, each outstanding share of Cequent’s common stock will be exchanged for MDRNA common stock at an exchange ratio that implies a purchase price for Cequent’s common and preferred stockholders of approximately $44 million, plus an additional value of approximately $2 million to warrant and option holders, based on the 10 day Volume-Weighted Average Price (VWAP) of MDRNA shares on March 31, 2010. This represents an approximate 56% equity ownership in the consolidated entity for MDRNA stockholders and a 44% equity ownership for Cequent’s securityholders.
E-13
CEQUENT PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS (Continued)
The Merger Agreement anticipates that the combined company will be headquartered in Bothell, Washington with offices in Cambridge, Massachusetts. RNAi drug discovery, research and biology will continue to be conducted at MDRNA’s R&D facility in Bothell while clinical operations will reside in Cambridge.
In connection with the execution of the Merger Agreement described above, MDRNA and Cequent entered into a Loan Agreement (the “Loan Agreement”), pursuant to which, among other things, Cequent shall extend one or more loans to MDRNA in the aggregate principal amount of up to $3,000,000 (the “Term Loans”) to fund MDRNA’s operations prior to the completion or closing of the merger. The Term Loans are evidenced by a secured promissory note issued by MDRNA to Cequent (the “Term Note”), which bears interest absent an event of default, at a rate of ten percent per annum. MDRNA will repay the principal and interest of the Term Loans in three equal consecutive monthly installments, commencing on August 15, 2010 and continuing on the 15th day of each month thereafter through and including October 15, 2010. Notwithstanding the foregoing, if the Merger is consummated prior to August 15, 2010, then, on the closing date of the Merger, MDRNA shall not owe to any third party any obligations with respect to the Term Loans, including, without limitation the then outstanding principal balance of the Term Loans and any interest then accrued but unpaid thereon. MDRNA may prepay any Term Loan in whole or in part without premium or penalty.
As consideration for the Loan Agreement, MDRNA agrees to issue to Cequent on the date of each Term Loan a five-year warrant to purchase shares of MDRNA’s capital stock with an exercise price subject to adjustments as defined in the Merger Agreement. Each warrant is exercisable for a number of shares of MDRNA’s common stock equal to sixty-five percent (65%) of the principal amount of the Term Loan being made on such date divided by $1.1496 (subject to an equitable adjustment in the event of any stock split, stock dividend, stock combination or the like). The loan warrants are only exercisable, for a five year term, if the merger is not consummated, other than by reason of Cequent’s material breach of the Merger Agreement, and MDRNA engages in a transaction with another party that is similar to the proposed merger, prior to the one year anniversary of the termination of the Merger Agreement. Upon the completion of the Merger Agreement the warrants will terminate and be invalid.
Preferred stock issuance
On April 8, 2010, pursuant to the Series A-1 Convertible Preferred Stock Purchase Agreement, Cequent issued 2,517,173 Series A-1 Convertible Preferred Stock for gross proceeds of $2,517,173.
Loan Agreement
On April 30, 2010, Cequent extended MDRNA a Term Loan in the amount of $1,000,000 and on May 31, 2010, Cequent extended MDRNA an additional Term Loan in the amount of $1,000,000 under the Loan Agreement.
E-14
| | |
MDRNA, INC. 3830 MONTE VILLA PARKWAY BOTHELL, WA 98021 | | VOTE BY INTERNET -www.proxyvote.com |
| | Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 p.m. Eastern Time the day before the cut-off date or meeting date. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form. |
| | ELECTRONIC DELIVERY OF FUTURE PROXY MATERIALS |
| | If you would like to reduce the costs incurred by our company in mailing proxy materials, you can consent to receiving all future proxy statements, proxy cards and annual reports electronically via e-mail or the Internet. To sign up for electronic delivery, please follow the instructions above to vote using the Internet and, when prompted, indicate that you agree to receive or access proxy materials electronically in future years. |
| | VOTE BY PHONE - 1-800-690-6903 |
| | Use any touch-tone telephone to transmit your voting instructions up until 11:59 p.m. Eastern Time the day before the cut-off date or meeting date. Have your proxy card in hand when you call and then follow the instructions. |
| | VOTE BY MAIL |
| | Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717. |
| | | | |
| TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: |
| | M25537-P99256 | | KEEP THIS PORTION FOR YOUR RECORDS |
| DETACH AND RETURN THIS PORTION ONLY |
| THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED. |
| | | | | | | | | | | | | | | | | | | | |
| MDRNA, INC. | | For All | | Withhold All | | For All Except | | | | To withhold authority to vote for any individual nominee(s), mark “For All Except” and write the number(s) of the nominee(s) on the line below. | | | | | | |
| | | | | | | | | | | | | | | | | |
The Board of Directors recommends that you vote FOR the following: | | ¨ | | ¨ | | ¨ | | | | | | | | | | | | |
Vote on Directors | | | | | | | | | | | | | | | | |
4. | | To elect six (6) persons to our Board of Directors, each to hold office until the 2011 annual meeting of stockholders and until their respective successors shall have been duly elected or appointed and qualify; | | | | | | | | | | | | | | | | |
| | | | | | | | | | | |
| | | 01) J. Michael French 04) James E. Rothman, Ph.D. | | | | | | | | | | | | | | For | | Against | | Abstain |
| | | 02) James M. Karis 05) Gregory Sessler | | | | | | | | | | | | | | | | |
| | | 03) Daniel Peters 06) Bruce R. Thaw | | For | | Against | | Abstain | | | | 8. | | To consider and vote upon a proposal to change our capital structure by increasing the number of authorized shares of common stock from 90,000,000 to 180,000,000, and by increasing the number of authorized shares of preferred stock from 100,000 to 200,000; | | ¨ | | ¨ | | ¨ |
Vote on Proposals | | | | | | | | | | | | | | | | |
| | | | | | | | | | | |
| 1. | | To approve the issuance of shares of common stock pursuant to the Agreement and Plan of Merger, dated as of March 31, 2010, by and among MDRNA, Cequent Pharmaceuticals, Inc., Calais Acquisition Corp. and a representative of the stockholders of Cequent Pharmaceuticals, Inc., as described in the attached proxy statement; | | ¨ | | ¨ | | ¨ | | | | 9. | | To consider and vote upon a proposal to amend the Rights Agreement, dated February 22, 2000, between our company and American Stock Transfer & Trust Company as Rights Agent, as amended, to extend the term of the Rights Agreement from March 17, 2010 to March 17, 2013; | | ¨ | | ¨ | | ¨ |
| | | | | | | | | | | |
| 2. | | To consider and vote upon an adjournment of the meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes in favor of Proposal No. 1, Proposal No. 3 or Proposal No. 8; | | ¨ | | ¨ | | ¨ | | | | 10. | | To consider and vote upon a proposal to amend the Amended and Restated Common Stock Purchase Warrants issued to investors and the placement agent in April 2008 to reduce and, in certain instances, eliminate the floor exercise price of such warrants, and to issue the additional shares of common stock that would be issuable upon full exercise of such warrants as a result thereof; | | ¨ | | ¨ | | ¨ |
| | | | | | | | | | | |
| 3. | | To consider and vote upon a proposed amendment to our certificate of incorporation to change the name of our company from MDRNA, Inc. to Marina Biotech, Inc.; | | ¨ | | ¨ | | ¨ | | | | 11. | | To consider and vote upon a proposal to amend the Common Stock Purchase Warrants issued to investors in January 2010 to eliminate the floor exercise price of such warrants; and | | ¨ | | ¨ | | ¨ |
| | | | | | | | | | | |
| 5. | | To consider and vote upon a proposal to ratify the appointment of KPMG LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2010; | | ¨ | | ¨ | | ¨ | | | | 12. | | To transact such other business as may properly come before the meeting or any adjournment or postponement thereof. | | ¨ | | ¨ | | ¨ |
| | | | | | | | |
| 6. | | To consider and vote upon a proposal to amend our 2008 Stock Incentive Plan to increase the number of shares available for issuance thereunder from 4,500,000 to 8,500,000; | | ¨ | | ¨ | | ¨ | | | | For address changes and/or comments, please check this box and write them on the back where indicated. | | ¨ |
| | | | | | | | | | | |
| 7. | | To consider and vote upon a proposal to amend our 2007 Employee Stock Purchase Plan to increase the number of shares available for issuance thereunder from 300,000 to 600,000; | | ¨ | | ¨ | | ¨ | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | Signature [PLEASE SIGN WITHIN BOX] | | Date | | | | | | | | | | Signature (Joint Owners) | | Date | | | | |
Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting:
The Notice and Proxy Statement and Annual Report are available at www.proxyvote.com.
| | | | | | | | | | |
MDRNA, INC. |
The undersigned hereby appoint(s) J. Michael French and Peter S. Garcia, or either of them, lawful attorneys and proxies of the undersigned, with full power of substitution, for and in the name, place and stead of the undersigned to attend the Annual Meeting of Stockholders (the “Annual Meeting”) of MDRNA, Inc. (the “Company”) to be held at the offices of Pryor Cashman LLP, 7 Times Square, New York, N.Y. 10036, on Wednesday, July 21, 2010, at 10:00 a.m., Eastern Daylight Time, and any adjournment(s) or postponement(s) thereof, with all powers the undersigned would possess if personally present, and to vote the number of shares the undersigned would be entitled to vote if personally present. This proxy when properly executed will be voted in the manner directed herein by the undersigned stockholder. If no direction is made, this proxy will be voted “FOR” proposal numbers 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 and 11. Any prior proxies are hereby revoked. |
| | | | | |
| | | Address Changes/Comments: | | | | | | |
| | | | |
| | | | | | | |
| | | | |
| | | | | | | |
| | | (If you noted any Address Changes/Comments above, please mark corresponding box on the reverse side.) | | | | |