Competition is intense in all of the Company’s markets and includes numerous large companies and many smaller regional competitors. In many cases, the Company’s primary competition comes from alternative, often older, technologies, such as chemical, sand filtration, and pasteurization as opposed to the finer level of membrane filtration that the Company provides. In many markets, there are significant barriers to entry limiting the number of qualified suppliers. These barriers result from stringent product performance standards, product qualification protocols and requirements for consistent levels of global service and support. The Company’s broad array of patented materials and product designs coupled with its engineering and manufacturing expertise and global reach enable it to provide customers with differentiated product performance and value and global customer support.
The Company’s Life Sciences technologies facilitate the process of drug discovery, development, regulatory validation and production. They are used extensively in the research laboratory, pharmaceutical and biotechnology industries, in blood centers and in hospitals at the point of patient care. The Company’s broad capability in the life sciences industry is a competitive strength and an important element of its strategy going forward. Sales in the Medical and BioPharmaceuticals markets are made through direct sales and distributors. The BioPharmaceuticals market includes the Laboratory market previously reported in the Medical market. Prior year amounts conform to current classification.
Safety, quality, efficacy, ease of use, technical support, product delivery and price are all important considerations among the Company’s Life Sciences customers. Pricing for blood filtration products has become more of a consideration as the Company’s customers have increasingly become large centralized procurers, such as blood centers in the Western Hemisphere and nationalized blood services in Europe and Asia. The backlog for the Life Sciences segment at July 31, 2009 was approximately $140,082,000 (all of which is expected to be shipped in fiscal year 2010) compared with $140,285,000 at July 31, 2008.
The Company’s medical products improve the safety of the use of blood products in patient care and help control the spread of infections in hospitals. The Company’s cell therapy product portfolio provides efficient enabling technologies for the emerging regenerative medicine market.
The backlog for the Medical market at July 31, 2009 was approximately $39,809,000 (all of which is expected to be shipped in fiscal year 2010) compared with $41,311,000 at July 31, 2008. The Company’s principal competitors in the Medical market include Fenwal, Inc., MacoPharma Group, Fresenius Medical Care AG & Co., Millipore Corporation, GE Healthcare (a unit of General Electric Company (“GE”)), Tyco International Ltd., Teleflex Incorporated, Terumo Medical Corporation, and Capital Health Inc.
The Company sells separation systems and disposable filtration and purification technologies primarily to pharmaceutical and biotechnology companies for use by them in the development and commercialization of chemically synthesized and biologically derived drugs and vaccines. The Company provides a broad range of advanced filtration solutions for each critical stage of drug development through drug production. Its filtration systems and validation services assist drug manufacturers through the regulatory process and on to the market. The Company’s broad laboratory product line is used in drug discovery, gene manipulation and proteomics applications.
Company management believes that the Company’s established record of product performance and innovation, as well as its ability to sell and globally support a complete range of products, including its engineered systems, give it a strong competitive advantage among BioPharmaceuticals customers because of the high costs and safety risks associated with drug development and production. The backlog for the BioPharmaceuticals market at July 31, 2009 was approximately $100,273,000 (all of which is expected to be shipped in fiscal year 2010) compared with $98,974,000 at July 31, 2008. Principal competitors in the BioPharmaceuticals market include Millipore Corporation, The Sartorius Group, CUNO (a 3M company) and GE Healthcare.
INDUSTRIAL SEGMENT:
The Company provides enabling and process enhancing technologies throughout the industrial marketplace. This includes the aerospace and transportation, microelectronics and consumer electronics, municipal and industrial water, fuels, chemicals, energy, and food and beverage markets. The Company has the capability to provide customers with integrated solutions for their process fluids. The backlog for the Industrial segment at July 31, 2009 was approximately $388,115,000 (of which approximately $286,800,000 is expected to be shipped in fiscal year 2010) compared with $476,153,000 at July 31, 2008.
ENERGY, WATER & PROCESS TECHNOLOGIES MARKET:
Included in this diverse market are sales of filters, coalescers and integrated separation systems for hydraulic, fuel and lubrication systems on mechanical equipment to many industries, as well as to producers of energy (i.e., oil, gas, renewable and alternative fuels, electricity and chemicals), food and beverages and municipal and industrial water. Virtually all of the raw materials, process fluids and waste streams that course through industry are candidates for multiple stages of filtration, separation and purification. The growing demand for “clean” and “green” technology and increasing demand for water and energy also create growth opportunities for the Company.
Technologies that purify water for use and reuse represent an important opportunity. Governments around the world are implementing stringent new regulations governing drinking water standards and Company management believes that the Company’s filters and systems provide a solution for these requirements. These standards apply to municipal water supplies throughout the U.S. and in a growing number of countries. Industry, which consumes enormous quantities of water, also increasingly needs to filter water before, during and after use both to conserve it and to ensure it meets discharge requirements.
Within the energy market, demand is driven by oil and gas producers, refineries and power generating stations work to increase production, produce cleaner burning fuels, conserve water, meet environmental regulations and develop alternative fuel sources. Each of these applications provides opportunities for the Company.
Within the Food & Beverage market, filtration solutions are provided to the wine, beer, soft drink, bottled water and food ingredient markets. A growing filtration opportunity in this market is the need of wine and beer manufacturers to eliminate the use of diatomaceous earth and pasteurization in their processes.
The backlog at July 31, 2009 was approximately $249,342,000 (of which approximately $215,000,000 is expected to be shipped in fiscal year 2010) compared with $290,931,000 at July 31, 2008. Sales to Energy, Water & Process Technologies customers are made through Company personnel, distributors and manufacturers’ representatives. The Company believes that its TFM strategy and ability to engineer fully integrated systems solutions, underscored by product performance and quality, customer service, and price, are the principal competitive factors in this market. The Company’s primary competitors in the Energy, Water & Process Technologies market include CUNO (a 3M company), GE Infrastructure (a unit of GE), U.S. Filter (a Siemens business), The Sartorius Group, Parker Hannifin Corporation and Rohm and Haas Company.
AEROSPACE & TRANSPORTATION MARKET:
The Company sells filtration and fluid monitoring equipment to the aerospace industry for use on commercial and military aircraft, ships and land-based military vehicles to help protect critical systems and components. Commercial, Military and Transportation sales represented 34%, 49% and 17%, respectively, of total Aerospace & Transportation sales in fiscal year 2009. Key drivers in this market include passenger air miles flown, military budgets, new military and commercial aircraft, and demand for new aircraft and mobile construction equipment in emerging geographic markets, particularly in Asia. Increasing environmental regulation faced by the Company’s customers, as well as customer requirements for improved equipment reliability and fuel efficiency impact demand.
6
The Company’s products are sold to customers through a combination of direct sales to airframe manufacturers and other customers, including the U.S. military, and through the Company’s distribution partner, Satair A/S, for the commercial aerospace “aftermarket,” such as sales to commercial airlines. The backlog at July 31, 2009 was approximately $119,695,000 (of which approximately $58,000,000 is expected to be shipped in fiscal year 2010) compared with $160,792,000 at July 31, 2008. Competition varies by product. The Company’s principal competitors in the Aerospace & Transportation markets include Donaldson Company, Inc., ESCO Technologies Inc. and CLARCOR Inc.
Company management believes that efficacy, performance and quality of product and service, as well as price, are determinative in most sales.
MICROELECTRONICS MARKET:
The Company sells highly sophisticated filtration and purification technologies for the semiconductor, data storage, fiber optic, advanced display and materials markets. The Company provides a comprehensive suite of contamination control solutions for chemical, gas, water, chemical mechanical polishing and photolithography processes to meet the needs of this demanding industry. Integrated circuits, which control almost every device or machine in use today, require exceedingly high levels of filtration technologies, which the Company provides. Diversification into the macroelectronics side of the market is enabling the Company to capitalize on demand for computer gaming consoles, MP3 players, flat screen TVs and monitors, multimedia cell phones and ink jet printers and cartridges. A newer application served by Microelectronics is the growing production of solar cells.
The Company’s products are sold to customers in this market through its own personnel, distributors and manufacturers’ representatives. The backlog at July 31, 2009 was approximately $19,078,000 (of which approximately $13,800,000 is expected to be shipped in fiscal year 2010) compared with $24,430,000 at July 31, 2008. Company management believes that performance, product quality, innovation and service are the most important factors in the majority of sales in this market. The Company’s principal competitors of the Company’s Microelectronics technologies market include Entegris, Inc. and Mott Corporation.
The following comments relate to the two operating segments discussed above:
RAW MATERIALS:
Most raw materials used by the Company are available from multiple sources. A limited number of materials are proprietary products of major chemical companies. Management believes that the Company could obtain satisfactory substitutes for these materials should they become unavailable.
INTELLECTUAL PROPERTY:
The Company owns numerous U.S. and foreign patents and has patent applications pending in the U.S. and abroad. The Company also licenses intellectual property rights from third parties, some of which bear royalties and are terminable in specified circumstances. In addition to the Company’s patent portfolio, the Company possesses a wide array of proprietary technology and know-how. The Company also owns numerous U.S. and foreign trademarks covering its diverse array of products, and has applications pending for the registration of trademarks. The Company believes that patents and other proprietary rights are important to the strength of the Company. The Company also relies upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain its competitive position. The Company does not believe that the expiration of any individual patent or any patents due to expire in the foreseeable future will have a material adverse impact on its business, financial condition or results of operations in any one year.
7
ITEM 1A. RISK FACTORS.
The risk factors described below are not inclusive of all risk factors but highlight those that the Company believes are the most significant and that could impact its performance and financial results. These risk factors should be considered together with all other information presented in this Form 10-K report.
Litigation and regulatory inquiries associated with the restatement of the Company’s prior period financial statements could result in substantial costs, penalties and other adverse effects.
Substantial costs may be incurred to defend and resolve regulatory proceedings and litigation arising out of or relating to matters underlying the Company’s restatement of prior period financial statements as described in its 2007 Form 10-K. These proceedings include the ongoing audits of the Company’s tax returns, as well as audits expected to commence of the Company’s tax returns for some of the periods affected by the restatement. In September 2007, the Company deposited $135 million with the U.S. Treasury, which reflected management’s preliminary assessment of additional taxes and interest that the Company might owe the Internal Revenue Service (“IRS”) for prior years as a result of tax compliance matters identified at the time and did not include any amount with respect to potential penalties. In completing the restatement, the Company examined the appropriateness of the Company’s accounting treatment of the tax consequences of each type of intercompany transaction in the various taxing jurisdictions in which the Company operates. As a result of this analysis, the Company determined that additional financial statement reserves were required with respect to certain other lesser tax compliance matters. The Company cannot predict when the ongoing IRS audit will be completed or the amount or timing of the final resolution with the IRS or other relevant taxing authorities of the matters that gave rise to the restatement, including the amount of any penalties that may be imposed, which could be substantial.
The Company is also subject to other regulatory and litigation proceedings relating to, or arising out of, the restatement, including pending investigations by the SEC and the Department of Justice, purported securities class action lawsuits and derivative lawsuits seeking relief against certain of the Company’s officers and directors. These proceedings could also result in civil or criminal fines and other non-monetary penalties. The Company has not reserved any amount in respect of these matters in its consolidated financial statements.
The Company cannot predict whether any monetary losses it experiences in the proceedings will be covered by insurance or whether insurance proceeds recovered will be sufficient to offset such losses. Pending civil, regulatory and criminal proceedings may also divert the efforts and attention of the Company’s management from business operations, particularly if adverse developments are experienced in any of them, such as an expansion of the investigations being conducted by the SEC and the Department of Justice. See Part I – Item 3 – Legal Proceedings, for further discussion of these pending matters.
If the Company experiences a disruption of its information technology systems, or if the Company fails to successfully implement, continue to manage and integrate its information technology systems, it could harm the Company’s business.
The Company’s information technology (“IT”) systems are an integral part of its business. A serious disruption of its IT systems, whether caused by fire, storm, flood, telecommunications failures, physical or software break-ins or viruses, or any other events, could have a material adverse effect on the Company’s business and results of operations. The Company depends on its IT systems to process transactions, prepare its financial reporting and effectively manage and monitor its business. The Company cannot provide assurance that its contingency plans will allow it to operate at its current level of efficiency in the event of a serious IT disruption.
Additionally, the Company’s ability to most effectively implement its business plans in a rapidly evolving market requires effective planning, reporting and analytical processes and systems. The Company is improving and expects that it will need to continue to improve and further integrate its IT systems, reporting systems and operating procedures on an ongoing basis. If the Company fails to incrementally improve, manage and integrate its IT systems, reporting systems and operating procedures, it could adversely affect the Company’s ability to achieve its objectives.
8
The Company may be adversely affected by global and regional economic conditions and legislative, regulatory and political developments.
The Company conducts operations around the globe. The Company expects to continue to derive a substantial portion of sales and earnings from outside the U.S. The recession in the U.S. and other countries around the globe in which the Company derives significant sales adversely affected the Company’s results for fiscal year 2009 and could continue to have a negative impact on demand for the Company’s products as the prospects, strength and timing of any recovery remain uncertain. Customers or suppliers may experience serious cash flow problems and as a result, may modify, delay or cancel plans to purchase the Company’s products and suppliers may significantly and quickly increase their prices or reduce their output. Additionally, if customers are not successful in generating sufficient revenue or are precluded from securing financing, they may not be able to pay, or may delay payment of, accounts receivable that are owed to the Company. Any inability of current and/or potential customers to purchase the Company’s products and/or to pay the Company for its products may adversely affect the Company’s earnings and cash flow. Sales and earnings could also be affected by the Company’s ability to manage the risks and uncertainties associated with the application of local legal requirements or the enforceability of laws and contractual obligations, trade protection measures, changes in tax laws, regional political instability, war, terrorist activities, severe or prolonged adverse weather conditions and natural disasters as well as health epidemics or pandemics.
Changes in demand for the Company’s products and business relationships with key customers and suppliers, including delays or cancellations in shipments, may affect operating results.
To achieve its objectives, the Company must develop and sell products that are subject to the demands of customers. This is dependent on many factors including, but not limited to, managing and maintaining relationships with key customers, responding to the rapid pace of technological change and obsolescence, which may require increased investment by or greater pressure to commercialize developments rapidly or at prices that may not fully recover the associated investment, and the effect on demand resulting from customers’ research and development, capital expenditure plans and capacity utilization.
The manufacturing of the Company’s products is dependent on an adequate supply of raw materials. The Company’s ability to maintain an adequate supply of raw materials could be impacted by the availability and price of those raw materials and maintaining relationships with key suppliers.
Fluctuations in foreign currency exchange rates and interest rates may materially affect operating results.
In fiscal year 2009, the Company derived 69% of sales from outside the U.S. Although sales and expenditures outside the U.S. are typically made in the local currencies of those countries providing a natural hedge against fluctuations in foreign currency rates, the company retains significant exposure to the value of foreign currencies relative to the U.S. dollar and operating results may be materially affected by changes in foreign currency rates. The primary foreign currency exposures relate to adverse changes in the relationships of the U.S. dollar to the Euro, the British Pound, the Japanese Yen, the Australian Dollar, the Canadian Dollar, the Swiss Franc and the Singapore Dollar, as well as adverse changes in the relationship of the Pound to the Euro.
Giving effect to the Company’s interest rate swap, the Company’s debt portfolio was approximately 45% variable rate at July 31, 2009. Fluctuations in interest rates may also materially affect operating results.
Changes in product mix and product pricing may affect the Company’s operating results particularly with the expansion of the systems business, in which the Company experiences significantly longer sales cycles with less predictable revenue and no certainty of future revenue streams from related consumable product offerings and services.
The Company’s TFM strategy is partially reliant on sales of integrated systems. Because systems are generally sold at lower gross margins than many other products, gross margins could decline if systems sales continue to grow as a percentage of total sales and the anticipated future revenue streams from related consumable product offerings and services are not realized.
9
The Company’s systems platform generally also experiences significantly longer sales cycles and involves less predictable revenue and uncertainty of future revenue streams from related consumable product offerings and services. In addition, the profitability of the Company’s systems sales depends substantially on the ability of management to estimate accurately the costs involved in manufacturing and implementing the relevant system according to the customer’s specifications. Company estimates can be adversely affected by disruptions in a customer’s plans or operations and unforeseen events, such as manufacturing defects. Failure to accurately estimate the Company’s cost of system sales can adversely affect the profitability of those sales, and the Company may not be able to recover lost profits through pricing or other actions.
Increases in costs of manufacturing and operating costs may affect operating results.
The Company’s costs are subject to fluctuations, particularly due to changes in commodity prices, raw materials, energy and related utilities and cost of labor. The achievement of the Company’s financial objectives is reliant on its ability to manage these fluctuations through cost savings or recovery actions and efficiency initiatives.
The Company may not be able to achieve the savings anticipated from its cost reduction and gross margin improvement initiatives.
Since fiscal year 2006, the Company has launched a number of cost reduction and gross margin improvement initiatives, including its facilities rationalization, AmeriPall cost reduction, and EuroPall cost reduction initiatives. Unexpected delays or other factors in these cost reduction and lean manufacturing initiatives could impact the Company’s ability to realize the anticipated savings and to improve or maintain gross margins.
The Company may not be able to obtain regulatory approval or market acceptance of new technologies.
Part of the Company’s planned growth is dependent on new products and technologies. Some of those new products may require regulatory approval. Growth from those new technologies may not be realized if regulatory approval is not granted or customer demand for those products or technologies does not materialize.
The Company may not successfully enforce patents and protect proprietary products and manufacturing techniques.
Some of the Company’s products, as well as some competitor’s products, are based on patented technology and other intellectual property rights. Some of these patented technologies and intellectual property require substantial resources to develop. Operating results may be affected by the costs associated with the Company’s defense of its intellectual property against unauthorized use by others, as well as third-party challenges to its intellectual property. The Company could also experience disruptions in its business, including loss of revenues and adverse effects on its prospects, if its patented or other proprietary technologies are successfully challenged.
Changes in the Company’s effective tax rate may affect operating results.
Fluctuations in the Company’s effective tax rate may affect operating results. The Company’s effective tax rate is subject to fluctuation based on a variety of factors, such as:
the geographical mix of income derived from the countries in which it operates;
currently applicable tax rates, particularly in the U.S.;
the nature, timing and impact of permanent or temporary changes in tax laws or regulations;
the timing and amount of the Company’s repatriation of foreign earnings;
the timing and nature of the Company’s resolution of uncertain income tax positions; and
the Company’s success in managing its effective tax rate through the implementation of global tax and cash management strategies.
The Company operates in numerous countries and is subject to taxation in all of the countries in which it operates. The tax rules and regulations in such countries can be complex and, in many cases, uncertain in their application. In addition to challenges to the Company’s tax positions arising during routine audits, disputes can arise with the taxing authorities over the interpretation or application of certain rules to the Company’s business conducted within the country involved and with respect to intercompany transactions when the parties are taxed in different jurisdictions. Pending proceedings to which the Company is subject include ongoing audits of the Company’s tax returns for some of the periods affected by the restatement, and the Company cannot predict the timing or outcome of the completion of those audits, which could result in the imposition of additional taxes and substantial penalties. See “Litigation and regulatory inquiries associated with the restatement of the Company’s prior period financial statements could result in substantial costs, penalties and other adverse effects” risk factor above.
10
The Company may not be able to successfully complete or integrate acquisitions.
In so far as acquisition opportunities are identified, there is no assurance of the Company’s ability to complete any such transactions and successfully integrate the acquired business as planned.
The Company is subject to domestic and international competition in all of its global markets.
The Company is subject to competition in all of the global markets in which it operates. The Company’s achievement of its objectives is reliant on its ability to successfully respond to many competitive factors including, but not limited to, pricing, technological innovations, product quality, customer service, manufacturing capabilities and hiring and retention of qualified personnel.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
11
ITEM 2. PROPERTIES.
The following are the Company’s principal facilities (i.e., facilities with square footage in excess of 25,000 square feet), which in the opinion of management are suitable and adequate to meet the Company’s requirements:
| | | | Principally Supports | | |
| | | | the Following | | Fiscal Year 2009 |
| Location | | Principal Activities (1) | | Business Groups (2) | | Square Footage |
| OWNED: | | | | | | |
| Western Hemisphere | | | | | | |
| Cortland, NY | | A | | PI | | 338,000 |
| DeLand, FL | | M | | PI | | 279,000 |
| Fajardo & Luquillo, Puerto Rico | | M,W | | PLS | | 261,000 |
| Pt. Washington, NY | | L,S | | A | | 280,000 |
| Ann Arbor, MI | | L,W,S | | PLS | | 186,000 |
| New Port Richey, FL | | A | | PI | | 179,000 |
| Timonium, MD | | M,W,S | | PI | | 160,000 |
| Ft. Myers, FL | | A | | PI | | 111,000 |
| Pensacola, FL | | A | | PLS | | 98,000 |
| Hauppauge, NY | | M | | PLS | | 75,000 |
| Covina, CA | | M,L | | PLS | | 71,000 |
| Putnam, CT | | M | | PI | | 63,000 |
| Europe | | | | | | |
| Bad Kreuznach, Germany | | A | | PI | | 390,000 |
| Portsmouth, U.K. | | A | | A | | 270,000 |
| Crailsheim, Germany | | A | | PI | | 215,000 |
| Ascoli, Buccinasco & Verona, Italy | | A | | A | | 189,000 |
| Tipperary, Ireland | | M | | PI | | 178,000 |
| Redruth, U.K. | | M | | PI | | 163,000 |
| Ilfracombe, U.K. | | M | | PLS | | 125,000 |
| Newquay, U.K. | | M | | PLS | | 110,000 |
| Bazet, France | | A | | PI | | 96,000 |
| Frankfurt, Germany | | W,S | | A | | 75,000 |
| Saint Germain, France | | L,W,S | | A | | 60,000 |
| Ternay, France | | A | | PI | | 33,000 |
| Asia | | | | | | |
| Tsukuba, Japan | | M,L,W | | PI | | 122,000 |
| |
| LEASED: | | | | | | |
| Western Hemisphere | | | | | | |
| Fajardo, Puerto Rico | | W | | PLS | | 114,000 |
| Cortland, NY | | M,W | | PI | | 111,000 |
| Timonium, MD | | M,W | | PI | | 71,000 |
| Tijuana, Mexico | | W | | PLS | | 63,000 |
| Baltimore, MD | | W | | PI | | 41,000 |
| Covina, CA | | W | | PLS | | 40,000 |
| Northborough, MA | | M,W | | A | | 38,000 |
| San Diego, CA | | A | | PI | | 26,000 |
| Europe | | | | | | |
| Johannesburg, South Africa | | W,S | | PI | | 99,000 |
| Madrid, Spain | | L,W,S | | A | | 44,000 |
| Cergy, France | | A | | PLS | | 43,000 |
| Ascoli, Italy | | W | | PLS | | 35,000 |
| Asia | | | | | | |
| Beijing, China | | A | | PI | | 318,000 |
| Melbourne & Somersby, Australia | | A | | A | | 102,000 |
| Mumbai, Banglore, Pune & Bhiwandi, India | | L,W,S | | A | | 73,000 |
| Tokyo, Osaka & Nagoya, Japan | | L,S | | A | | 47,000 |
12
| (1)Definition of Principal Activities | | (2)Definition of Business Groups |
| M: Manufacturing activities | | PLS: Pall Life Sciences |
| L: Laboratories for research & development and validation activities | | PI: Pall Industrial |
| W: Warehousing activities | | CS: Corporate and Shared Services |
| S: Sales, marketing and administrative activities | | A: All of the above |
| A: All of the above | | |
ITEM 3. LEGAL PROCEEDINGS.
Federal Securities Class Actions:
Four putative class action lawsuits were filed against the Company and certain members of its management team alleging violations of the federal securities laws relating to the Company’s understatement of certain of its U.S. income tax payments and of its provision for income taxes in certain prior periods as described in Note 2, Audit Committee Inquiry and Restatement to the consolidated financial statements included in the 2007 Form 10-K. These lawsuits were filed between August 14, 2007 and October 11, 2007 in the U.S. District Court for the Eastern District of New York. By Order dated May 28, 2008, the Court consolidated the cases under the caption “In re Pall Corp,” No. 07-CV-3359 (E.D.N.Y.) (JS) (ARL), appointed a lead plaintiff and ordered that the lead plaintiff file a consolidated amended complaint. The lead plaintiff filed its consolidated amended complaint on August 4, 2008. The lead plaintiff seeks to act as representative for a class consisting of purchasers of the Company’s stock between April 20, 2007, and August 2, 2007, inclusive. The consolidated amended complaint names the Company and its current chief executive officer and chief financial officer as defendants and alleges violations of Section 10(b) and 20(a) of the Exchange Act, as amended, and Rule 10b-5 promulgated by the Securities and Exchange Commission. It alleges that the defendants violated these provisions of the federal securities laws by issuing materially false and misleading public statements about the Company’s financial results and financial statements, including the Company’s income tax liability, effective tax rate, internal controls and accounting practices. The plaintiffs seek unspecified compensatory damages, costs and expenses. The Company moved to dismiss the consolidated amended complaint on September 19, 2008, and filed its reply brief to the lead plaintiff’s opposition to the Company’s motion to dismiss on December 2, 2008. By Memorandum and Order dated September 21, 2009, the Court denied the Company’s motion to dismiss the consolidated amended complaint and granted the lead plaintiff leave to amend the consolidated amended complaint by filing a second amended complaint.
Shareholder Derivative Lawsuits:
On October 5, 2007, two plaintiffs filed identical derivative lawsuits in New York Supreme Court, Nassau County relating to the tax matter described above. These actions purported to bring claims on behalf of the Company based on allegations that certain current and former directors and officers of the Company breached their fiduciary duties by failing to evaluate and otherwise inform themselves about the Company’s internal controls and financial reporting systems and procedures. In addition, plaintiffs allege that certain officers of the Company were unjustly enriched as a result of the Company’s inaccurate financial results over fiscal years 1999-2006 and the first three quarters of fiscal year 2007. The complaints sought unspecified compensatory damages on behalf of the Company, disgorgement of defendants’ salaries, bonuses, stock grants and stock options, equitable relief and costs and expenses. The Company, acting in its capacity as nominal defendant, moved to dismiss the complaints for failure to make a demand upon the Company’s board of directors, which motions were granted on April 30 and May 2, 2008. On September 19, 2008, the same two plaintiffs filed a derivative lawsuit in New York Supreme Court, Nassau County, which was served on the Company on September 26, 2008 (“September Derivative”). This action purports to bring claims on behalf of the Company based on allegations that certain current and former directors and officers of the Company breached their fiduciary duties and were unjustly enriched in connection with the tax matter. In addition, the plaintiffs allege that the Board’s refusal of their demand to commence an action against the defendants was not made in good faith. The plaintiffs and the Company agreed to stay these proceedings pending resolution of the Company’s motion to dismiss in the federal securities class action lawsuit discussed above, after which resolution the plaintiffs and the Company agreed to confer about a schedule for the defendants’ time to answer or otherwise respond to the complaint.
On November 13, 2008, another shareholder filed a derivative lawsuit in New York Supreme Court, Nassau County, against certain current and former directors and officers of the Company, and against the Company, as nominal defendant, which was served on the Company on December 4, 2008. This action purports to bring similar claims as the September Derivative. The plaintiffs and the Company have agreed to an identical stay as in the September Derivative.
Other Proceedings:
The SEC and U.S. Attorney’s Office for the Eastern District of New York are conducting investigations in connection with the tax matter described above. The Company is cooperating with these investigations.
13
Environmental Matters:
The Company has environmental matters, discussed below, at the following four U.S. sites: Ann Arbor, Michigan; Pinellas Park, Florida; Glen Cove, New York and Hauppauge, New York.
The Company’s balance sheet at July 31, 2009 contains environmental liabilities of $12,454,000, which relate to the items discussed below. In the opinion of Company management, the Company is in substantial compliance with applicable environmental laws and regulatory orders and its accruals for environmental remediation are adequate at this time.
Reference is also made to Note 15, Contingencies and Commitments, to the accompanying consolidated financial statements.
Ann Arbor, Michigan:
In February 1988, an action was filed in the Circuit Court for Washtenaw County, Michigan (the “Court”) by the State of Michigan (the “State”) against Gelman Sciences Inc. (“Gelman”), a subsidiary acquired by the Company in February 1997. The action sought to compel Gelman to investigate and remediate contamination near Gelman’s Ann Arbor facility and requested reimbursement of costs the State had expended in investigating the contamination, which the State alleged was caused by Gelman’s disposal of waste water from its manufacturing process. Pursuant to a consent judgment entered into by Gelman and the State in October 1992 (amended September 1996 and October 1999) (the “Consent Judgment”), which resolved that litigation, Gelman is remediating the contamination without admitting wrongdoing. In February 2000, the State Assistant Attorney General filed a Motion to Enforce Consent Judgment in the Court seeking approximately $4,900,000 in stipulated penalties for the alleged violations of the Consent Judgment and additional injunctive relief. Gelman disputed these assertions. Following an evidentiary hearing in July 2000, the Court took the matter of penalties “under advisement.” The Court issued a Remediation Enforcement Order requiring Gelman to submit and implement a detailed plan to reduce the contamination to acceptable levels within five years. Gelman’s plan has been approved by both the Court and the State. Although groundwater concentrations remain above acceptable levels in much of the affected area, the Court has expressed its satisfaction with Gelman’s progress during hearings both before and after the five-year period expired. Neither the State nor the Court has sought or suggested that Gelman should be penalized based on the continued presence of groundwater contamination at the site.
In February 2004, the Court instructed Gelman to submit its Final Feasibility Study describing how it intends to address an area of groundwater contamination not addressed by the previously approved plan. Gelman submitted its Feasibility Study as instructed. The State also submitted its plan for remediating this area of contamination to the Court. On December 17, 2004, the Court issued its Order and Opinion Regarding Remediation and Contamination of the Unit E Aquifer (the “Order”) to address an area of groundwater contamination not addressed in the previously approved plan. Gelman is now in the process of implementing the requirements of the Order.
In correspondence dated June 5, 2001, the State asserted that stipulated penalties in the amount of $142,000 were owed for a separate alleged violation of the Consent Judgment. The Court found that a “substantial basis” for Gelman’s position existed and again took the State’s request “under advisement,” pending the results of certain groundwater monitoring data. That data has been submitted to the Court, but no ruling has been issued.
On August 9, 2001, the State made a written demand for reimbursement of $227,000 it has allegedly incurred for groundwater monitoring. On October 23, 2006, the State made another written demand for reimbursement of these costs, which now total $494,000, with interest. In February 2007, the Company met with the State to discuss whether the State would be interested in a proposal for a “global settlement” to include, among other matters, the claim for past monitoring costs ($494,000). Gelman is engaged in discussion with the State with regard to this demand, however, Gelman considers this claim barred by the Consent Judgment.
14
By letter dated June 15, 2007, the Michigan Department of Environmental Quality (“DEQ”) claimed Gelman was in violation of the Consent Judgment and related work plans due to its failure to operate a groundwater extraction well in the Evergreen Subdivision at the approved minimum purge rate. The DEQ sought to assess stipulated penalties. Gelman filed a Petition for Dispute Resolution with the Court on July 6, 2007 contesting these penalties. Prior to the hearing on Gelman’s petition, the parties met and the DEQ agreed to waive these penalties in exchange for Gelman’s agreement to perform additional investigations in the area. The Court entered a Stipulated Order to this effect on August 7, 2007. Since then, Gelman has installed several monitoring wells requested by the State. Representatives of Gelman and the State met on December 10, 2007 to discuss the data obtained from these wells and to plan further investigative activities. These discussions are ongoing. On April 15, 2008, Gelman submitted two reports summarizing the results of the investigation to date. Gelman also submitted a “capture zone analysis” that confirmed that Gelman was achieving the cleanup objective for the Evergreen Subdivision system. On June 23, 2008, the State provided its response to these reports. The response also addressed outstanding issues regarding several other areas of the site. In its response, the State asked the Company to undertake additional investigation in the Evergreen Subdivision area and in other areas of the site to more fully delineate the extent of contamination. The State also asked the Company to capture additional contaminated groundwater in the Wagner Road area, near the Gelman property, unless the Company can show that it is not feasible to do so. Gelman proposed to the DEQ several modifications to the Consent Judgment on August 1, 2008 and met with the DEQ to discuss these modifications (and other outstanding issues) on September 15, 2008. The parties agreed that Gelman would prepare and submit to the DEQ an outline for modifications to the existing Consent Judgment (and Administrative Orders) by October 15, 2008 and that the parties would meet thereafter to discuss. On April 29, 2009, the Court issued an order that sets forth a schedule for the various steps that must be taken to implement agreed upon modifications to the cleanup program. Pursuant to that schedule, the Company submitted its Comprehensive Proposal to Modify Cleanup Program (the “Proposal”) to the State on May 4, 2009. On June 15, 2009, the State refused to approve the Company’s Proposal. Pursuant to the Court-imposed schedule, the Company filed pleadings identifying areas of dispute and motions seeking approval of its Proposal on August 18, 2009. The DEQ did not file any pleadings regarding the Company’s Proposal, but did file a motion to enforce the existing Consent Judgment that asks the Court to order the Company to undertake additional response activities with regard to certain portions of the site. The DEQ’s motion does not seek monetary damages. The Court has not indicated the exact process by which it will resolve these disputes.
Pinellas Park, Florida:
In 1995, as part of a facility closure, an environmental site assessment was conducted to evaluate potential soil and groundwater impacts from chemicals that may have been used at the Company’s Pinellas Park facility during the previous 24-year period of manufacturing and testing operations. Methyl Isobutyl Ketone (“MIBK”) concentrations in groundwater were found to be higher than regulatory levels. Soil excavation was conducted in 1998 and subsequent groundwater sampling showed MIBK concentrations below the regulatory limits.
In October 2000, environmental consultants for a prospective buyer of the property found groundwater contamination at the Company’s property. In October 2001, a Site Assessment Report conducted by the Company’s consultants, which detailed contamination concentrations and distributions, was submitted to the Florida Department of Environmental Protection (“FDEP”).
In July 2002, a Supplemental Contamination Assessment Plan and an Interim Remedial Action Plan (“IRAP”) were prepared by the Company’s consultants and submitted to the FDEP. A revised IRAP was submitted by the Company in December 2003, and it was accepted by the FDEP in January 2004. A Remedial Action Plan (“RAP”) was submitted by the Company to the FDEP in June 2004. Final approval by the FDEP of the Company’s RAP was received by the Company on August 26, 2006. Pursuant to the approved RAP, the Company began active remediation on the property.
On March 31, 2006, the FDEP requested that the Company investigate potential off-site migration of contaminants. Off-site contamination was identified and the FDEP was notified. On April 13, 2007, the FDEP reclassified the previously approved RAP as an Interim Source Removal Plan (“ISRP”) because a RAP can only be submitted after all contamination is defined.
Pursuant to FDEP requirements, the Company installed additional on-site and off-site monitoring wells during 2006, 2007, 2008 and 2009. Additional monitoring wells will be installed in fiscal year 2010. After they have been installed and sampled, the results will be provided to FDEP. Once the delineation has been declared complete by FDEP, the Company will complete and submit a Site Assessment Report Addendum, summarizing the soil and groundwater contamination, delineation and remediation.
15
Active remediation through the fourth quarter of fiscal year 2009 was performed in accordance with work defined in the ISRP and addenda approved by FDEP. Additional remediation is scheduled to satisfy site closure requirements, which include (1) no free product contaminants, (2) shrinking or stable plumes, and (3) prevention of future exposure of the public or environment through recordation of restrictive covenants prohibiting groundwater use. The first two requirements will be demonstrated through groundwater monitoring; a local law firm is assisting Company management during negotiations with the owners of adjacent properties regarding the third requirement.
Once the contamination has been delineated and active remediation has stopped, groundwater sampling and analysis must continue for at least the legislative minimum of one year. After groundwater sampling is complete, a closure application will be submitted to FDEP.
Glen Cove, New York:
A March 1994 report indicated groundwater contamination consisting of chlorinated solvents at a neighboring site to the Company’s Glen Cove facility, and later reports found groundwater contamination in both the shallow and intermediate zones at the facility. In 1999, the Company entered into an Order on Consent with the New York State Department of Environmental Conservation (“NYSDEC”), and completed a Phase II Remedial Investigation at the Glen Cove facility.
The NYSDEC has designated two operable units (“OUs”) associated with the Glen Cove facility. In March 2004, the NYSDEC finalized the Record of Decision (“ROD”) for the shallow and intermediate groundwater zones, termed OU-1. The Company signed an Order on Consent for OU-1 effective July 5, 2004, which requires the Company to prepare a Remedial Design/Remedial Action (“RD/RA”) Work Plan to address groundwater conditions at the Glen Cove facility.
The Company completed a pilot test involving the injection of a chemical oxidant into on-site groundwaterand, on May 31, 2006, submitted a report to NYSDEC entitled “In-Situ Chemical Oxidation Phase II Pilot Test and Source Evaluation Report” (the “Report”). The Report contained data which demonstrated that (1) in general, the pilot test successfully reduced contaminant levels and (2) the hydraulic controls installed on the upgradient Photocircuits Corporation (“Photocircuits”) site are not effective and contaminated groundwater continues to migrate from that site. On July 31, 2006, the Company received comments from NYSDEC on the Report. On September 27, 2006, the Company submitted responses to the NYSDEC comments. On November 16, 2006, the Company met with the NYSDEC representatives to discuss the Report and the impact of the continued migration of contaminated groundwater from the upgradient Photocircuits site onto the Glen Cove facility. On January 26, 2007, the Company submitted a draft conceptual remedial design document for the Glen Cove facility to NYSDEC for its technical review.
The Company met with NYSDEC representatives on April 12, 2007 to discuss a possible settlement of liability for OU-1 and for the contamination in the deep groundwater zone, termed OU-2. NYSDEC would not agree to settle OU-2 because a remedial investigation has not been completed. On October 23, 2007, NYSDEC requested submittal of a RD/RA Work Plan, which the Company submitted on December 20, 2007. The Company has pursued possible settlement of liability for OU-1 and met with NYSDEC again on November 30, 2007 to present a settlement framework. On December 20, 2007, the Company submitted a description of the settlement framework for NYSDEC’s further review. On April 15, 2008, the Company met with NYSDEC staff to discuss settlement terms and reached conceptual agreement on settlement for liability for OU-1. In an April 18, 2008 letter, NYSDEC confirmed its acceptance of the conceptual settlement. On August 13, 2008, the Company received for review and comment a proposed Consent Decree to settle this matter. Since August 13, 2008, the Company and NYSDEC have been discussing the terms of the Consent Decree for the settlement of liability for OU-1 and are in the process of finalizing a Consent Decree that would provide for a $2 million payment by the Company (which amount does not exceed the Company’s reserve for this matter) in exchange for a broad release of OU-1 claims and liability (claims and losses arising out of or in connection with OU-2 or any damages to the State’s natural resources would be excluded from the settlement). The Company expects to execute a Consent Decree by October 31, 2009, the end of its first quarter.
The ROD for OU-2 has been deferred by NYSDEC until additional data is available to delineate contamination and select an appropriate remedy. NYSDEC requested that the Company and Photocircuits enter into a joint Order on Consent for the remedial investigation. Photocircuits was not willing to enter into an Order and the Company was informed by NYSDEC that it would undertake the OU-2 investigation at the Photocircuits property. Photocircuits is now in Chapter 11 bankruptcy and, in or about March 2006, the assets of Photocircuits’ Glen Cove facility were sold to American Pacific Financial Corporation (“AMPAC”). AMPAC operated the facility under the Photocircuits name, but closed it on or about April 15, 2007.
16
In July 2007, NYSDEC commenced the OU-2 investigation at both the Photocircuits and Pall sites. The Company has retained an engineering consultant to oversee NYSDEC’s OU-2 work. NYSDEC's OU-2 investigation continues to be ongoing.
Hauppauge, New York:
On December 3, 2004, a third-party action was commenced against the Company in the U.S. District Court for the Eastern District of New York in connection with groundwater contamination. In the primary action, plaintiff Anwar Chitayat (“Chitayat” or the “plaintiff”) seeks recovery against defendants Vanderbilt Associates and Walter Gross for environmental costs allegedly incurred, and to be incurred, in connection with the disposal of hazardous substances from a property located in Hauppauge, New York (the “Site”). The Site is a property located in the same industrial park as a Company facility. Vanderbilt Associates is the prior owner of the site and Walter Gross was a partner in Vanderbilt Associates. Following Mr. Gross’ death in 2005, Barbara Gross was substituted as a third-party plaintiff. Ms. Gross claims that the Company is responsible for releasing hazardous substances into the soil and groundwater at its property, which then migrated to the Site, and seeks indemnification and contribution under Section 113 of CERCLA from third-party defendants, including the Company, in the event she is liable to Chitayat.
Chitayat alleges that prior to 1985, Vanderbilt Associates leased the Site to Sands Textiles Finishers, Inc. for textile manufacturing and dry cleaning. Chitayat alleges that hazardous substances were disposed at the Site during the time period that Mr. Gross and Vanderbilt Associates owned and/or operated the Site, which migrated from the Site to surrounding areas. Chitayat alleges that in August 1998, he entered into a Consent Order with the NYSDEC which resulted in NYSDEC investigating the Site and developing a remediation plan, and required Chitayat to reimburse the State via a periodic payment plan. Chitayat alleges that the total response costs will exceed $3,000,000, and that he has incurred more than $500,000 in costs to date.
In 2005, the plaintiff moved to amend his complaint to add a claim for contribution under Section 113 of CERCLA against the Company, and the Company opposed the proposed amendment. In March 2006, the Court terminated the plaintiff’s motion to amend, and plaintiff has not renewed his motion. As a result, the only claim asserted against the Company is by Barbara Gross.
The NYSDEC has designated two operable units (“OU”) associated with the Site. OU-1 relates to the “on-site” contamination at 90, 100 and 110 Oser Avenue, and represents the geographic area which Chitayat alleges will result in response costs in excess of $3,000,000. OU-2 relates to off-site groundwater contamination migrating away from the Site. In January 2006, the NYSDEC issued a ROD selecting a remedial program for OU-2 which is projected to cost approximately $4,500,000 to implement.
Fact discovery in the case was completed in January 2006. Experts for plaintiff, Barbara Gross, Vanderbilt Associates and the Company served expert reports in March and April 2006, and expert discovery was concluded in May 2006. There is a dispute among the experts as to whether contaminants from the Company’s facility have contributed to cleanup costs at the Site and, if so, to what extent. In September 2006, the Court established a briefing schedule for all parties to submit summary judgment motions, and for Barbara Gross and the Company to make motions to strike certain expert testimony. Third-party defendants, including the Company, filed motions for summary judgment on October 6, 2006. The Company also filed motions to strike certain expert testimony. Plaintiff filed opposition papers with the Court on November 6, 2006, and the moving third-party defendants, including the Company, filed reply papers on November 20, 2006.
While the motions were pending, the parties enlisted the aid of a mediator to negotiate a settlement of the case. The parties met with the mediator on July 30 through August 1, 2007, which resulted in a tentative settlement agreement, subject to drafting of definitive settlement documents. During the process of negotiating the settlement documents, a disagreement developed between the plaintiff and the primary defendants as to the terms of establishment of the settlement fund that had been agreed upon at the mediation. Although the plaintiff and the primary defendants continued in discussions for several months, this dispute was not resolved. The discussions have ceased and the proposed settlement has not yet been achieved.
The summary judgment motions remains pending without a decision. On September 27, 2007, the Court issued a decision on the Company’s motionsin limine to preclude testimony by the experts for plaintiff and third-party plaintiff Barbara Gross, granting the motions in part and denying them in part.
If the settlement were completed as contemplated, the Company’s responsibility would be fixed and it would be released from further liability to the plaintiff or third-party plaintiffs. Because it is not completed, if the Company’s motion for summary judgment is denied, the case will continue. If that happens, the Company will remain subject to potential liability and an allocation of some portion of the response costs paid by plaintiff to the State of New York.
17
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS.
There were no matters submitted to a vote of the Company’s shareholders during the fourth quarter of fiscal year 2009.
EXECUTIVE OFFICERS OF THE REGISTRANT
| | | | | | | First Appointed an |
| Name | | Age(1) | | Current Positions Held | | Executive Officer |
| Eric Krasnoff | | 57 | | Chairman and Chief Executive Officer | | 1986 |
| Lisa McDermott | | 44 | | Chief Financial Officer and Treasurer | | 2006 |
| Donald B. Stevens | | 64 | | President, Chief Operating Officer and President, Industrial | | 1994 |
| Roberto Perez | | 60 | | Group Vice President and President, Life Sciences | | 2003 |
| Sandra Marino | | 39 | | Senior Vice President, General Counsel and Corporate Secretary | | 2008 |
| | (1) | | Age as of September 18, 2009. |
None of the persons listed above is related.
Eric Krasnoff has served as Chairman and Chief Executive Officer since July 1994. Since joining the Company in 1975, Mr. Krasnoff served in several corporate management positions, including Group Vice President and Executive Vice President. He was elected President and Chief Operating Officer of the Company in 1993. Mr. Krasnoff is a director of the Company and member of the board’s executive committee.
Lisa McDermott has served as Chief Financial Officer and Treasurer since January 2006. Ms. McDermott began her employment with the Company in 1999 as Corporate Controller and was promoted to Vice President - Finance in July 2004.
Donald B. Stevens has served as President of the Company since April 2008 and also serves as Chief Operating Officer and President, Industrial. Mr. Stevens has held many key management positions since joining the Company in 1968, including that of Executive Vice President responsible for the Company’s Industrial business.
Roberto Perez has served as Group Vice President and President, Life Sciences, since November 2004. Mr. Perez joined the Company in January 2000 as President of the Medical Products Manufacturing Group. In March 2001, Mr. Perez was promoted to Vice President of the Company’s blood filtration business.
Sandra Marino has served as Senior Vice President and General Counsel since September 2008 and as Corporate Secretary since March 2008. Ms. Marino joined the Company in January 2005 as Corporate Counsel and Assistant Corporate Secretary. Prior to that, Ms. Marino was employed as a corporate attorney at Carter Ledyard & Milburn LLP.
None of the above persons has been involved in those legal proceedings required to be disclosed by Item 401(f) of Regulation S-K during the past five years.
18
PART II
ITEM 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDERMATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
The Company’s common stock is listed on the New York Stock Exchange under the symbol PLL. The table below sets forth quarterly data relating to the Company’s common stock prices and cash dividends declared per share for the past two fiscal years.
| | | | | | | | | | | | | Cash Dividends |
| 2009 | | 2008 | | Declared Per Share |
| Price per share | High | | Low | | High | | Low | | 2009 | | 2008 |
| Quarter: | First | $ | 42.72 | | $ | 21.79 | | $ | 44.55 | | $ | 33.46 | | $ | 0.130 | | $ | 0.24 |
| | Second | | 29.59 | | | 21.61 | | | 42.26 | | | 33.37 | | | 0.145 | | | 0.12 |
| Third | | 28.68 | | | 18.20 | | | 41.48 | | | 34.01 | | | 0.145 | | | 0.13 |
| Fourth | | 30.63 | | | 24.00 | | | 43.19 | | | 34.33 | | | 0.145 | | | 0.13 |
As of September 18, 2009 there were approximately 3,326 holders of record of the Company’s common stock. Dividends are paid when, as and if declared by the board of directors of the Company.
PERFORMANCE GRAPH
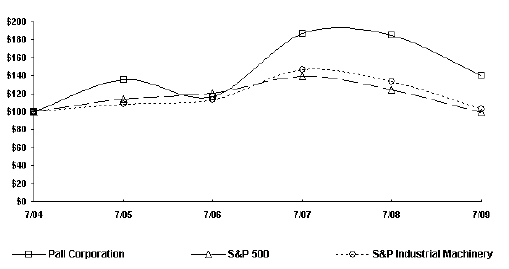
The following graph compares the annual change in the cumulative total return on the Company’s common stock during the Company’s last five fiscal years with the annual change in the cumulative total return of the Standard & Poor’s Composite-500 Index and the Standard & Poor’s Industrial Machinery Index (which includes the Company). The graph assumes an investment of $100 on July 30, 2004 (the last trading day of the Company’s fiscal year 2004) and the reinvestment of all dividends paid during the last five fiscal years.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN
Among Pall Corporation, The S&P 500 Index
And The S&P Industrial Machinery Index
Copyright© 2009 S&P, a division of The McGraw-Hill Companies Inc. All rights reserved.
| | 30-Jul-04 | 29-Jul-05 | 31-Jul-06 | 31-Jul-07 | 31-Jul-08 | 31-Jul-09 |
| Pall Corporation | $ | 100 | $ | 136 | $ | 116 | $ | 187 | $ | 185 | $ | 140 |
| S&P 500 | $ | 100 | $ | 114 | $ | 120 | $ | 140 | $ | 124 | $ | 99 |
| S&P Industrial Machinery | $ | 100 | $ | 108 | $ | 113 | $ | 147 | $ | 134 | $ | 103 |
19
The following table provides information with respect to purchases made by or on behalf of the Company or any “affiliated purchaser” of the Company’s common stock during the quarter ended July 31, 2009.
| | | (In thousands, except per share data) |
| | | | | | | Total Number of | | Approximate |
| | | | | | | Shares Purchased as | | Dollar Value of Shares |
| | | Total Number | | | | Part of Publicly | | that May Yet Be |
| | | of Shares | | Average Price | | Announced Plans or | | Purchased Under the |
| Period | | Purchased | | Paid Per Share | | Programs (1) | | Plans or Programs (1) |
| May 1, 2009 to May 31, 2009 | | — | | $ | — | | — | | $ | 484,498 |
| June 1, 2009 toJune 30, 2009 | | — | | $ | — | | — | | | 484,498 |
| July 1, 2009 toJuly 31, 2009 | | 1,208 | | $ | 26.12 | | 1,208 | | | 452,943 |
| |
| Total | | 1,208 | | $ | 26.12 | | 1,208 | | | |
| | (1) | | On November 15, 2006, the board authorized an expenditure of $250,000 to repurchase shares. On October 16, 2008, the board authorized an additional expenditure of $350,000 to repurchase shares. The Company’s shares may be purchased over time, as market and business conditions warrant. There is no time restriction on this authorization. During the fourth quarter of fiscal year 2009, the Company purchased 1,208 shares in open-market transactions at an aggregate cost of $31,555, with an average price per share of $26.12. Total repurchases in fiscal year 2009 were 3,347 shares at an aggregate cost of $96,439, with an average price per share of $28.81. The aggregate cost of repurchases in fiscal years 2008 and 2007 was $148,850 (4,056 shares at an average price per share of $36.70) and $61,795 (1,586 shares at an average price per share of $38.98), respectively. As of July 31, 2009, $452,943 remains to be expended under the current board repurchase authorizations. Repurchased shares are held in treasury for use in connection with the Company’s stock plans and for general corporate purposes. |
| |
| | | During the fiscal year 2009, 1 share was traded in by an employee in payment of a stock option exercise at a price of $39.84 per share and an aggregate cost of $13. |
20
ITEM 6. SELECTED FINANCIAL DATA.
The following table sets forth selected financial data for the last five fiscal years. This selected financial data is not necessarily indicative of results of future operations and should be read in conjunction with “Item 7–Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the accompanying consolidated financial statements and related notes included elsewhere in this Form 10-K.
| (In millions, except per share data) | | 2009 | | 2008 | | 2007 | | 2006 | | 2005 |
| RESULTS FOR THE YEAR: | | | | | | | | | | | | | | | | | | | | |
| |
| Net sales | | $ | 2,329.2 | | | $ | 2,571.6 | | | $ | 2,249.9 | | | $ | 2,016.8 | | | $ | 1,902.3 | |
| Cost of sales | | | 1,228.5 | | | | 1,360.8 | | | | 1,190.5 | (a) | | | 1,072.8 | (a) | | | 978.9 | (a) |
| Gross profit | | | 1,100.7 | | | | 1,210.8 | | | | 1,059.4 | | | | 944.0 | | | | 923.4 | |
| Selling, general and administrative expenses | | | 699.9 | | | | 749.5 | | | | 675.0 | | | | 641.0 | | | | 621.4 | |
| Research and development | | | 71.2 | | | | 71.6 | | | | 62.4 | | | | 57.3 | | | | 56.2 | |
| Restructuring and other charges, net | | | 30.7 | | | | 31.5 | | | | 22.4 | | | | 12.3 | | | | 38.8 | |
| Interest expense, net | | | 28.1 | | | | 32.6 | | | | 39.1 | | | | 30.2 | | | | 30.0 | |
| Earnings before income taxes | | | 270.8 | | | | 325.6 | | | | 260.5 | | | | 203.2 | | | | 177.0 | |
| Provision for income taxes | | | 75.2 | | | | 108.3 | | | | 133.0 | | | | 151.1 | | | | 63.3 | |
| Net earnings | | $ | 195.6 | (b) | | $ | 217.3 | (b) | | $ | 127.5 | (b) | | $ | 52.1 | (b) | | $ | 113.7 | |
| Earnings per share: | | | | | | | | | | | | | | | | | | | | |
| Basic | | $ | 1.65 | | | $ | 1.77 | | | $ | 1.04 | | | $ | 0.42 | | | $ | 0.91 | |
| Diluted | | $ | 1.64 | | | $ | 1.76 | | | $ | 1.02 | | | $ | 0.41 | | | $ | 0.91 | |
| Dividends declared per share | | $ | 0.565 | | | $ | 0.62 | | | $ | 0.35 | | | $ | 0.43 | | | $ | 0.39 | |
| |
| Capital expenditures | | $ | 133.0 | | | $ | 123.9 | | | $ | 97.8 | | | $ | 96.0 | | | $ | 86.2 | |
| Depreciation and amortization of long-lived assets | | $ | 89.4 | | | $ | 93.2 | | | $ | 94.0 | | | $ | 95.7 | | | $ | 90.9 | |
| |
| YEAR-END POSITION: | | | | | | | | | | | | | | | | | | | | |
| Working capital | | $ | 853.1 | | | $ | 1,085.7 | (c) | | $ | 774.2 | | | $ | 653.3 | | | $ | 598.1 | |
| Property, plant and equipment | | | 681.7 | | | | 663.0 | | | | 607.9 | | | | 621.0 | | | | 608.8 | |
| Total assets | | | 2,840.8 | | | | 2,956.7 | | | | 2,708.8 | | | | 2,461.3 | | | | 2,185.3 | |
| Long-term debt, net of current portion | | | 577.7 | | | | 747.1 | | | | 591.6 | | | | 640.0 | | | | 510.2 | |
| Total liabilities | | | 1,726.2 | | | | 1,817.5 | | | | 1,648.2 | | | | 1,524.2 | | | | 1,193.2 | |
| Stockholders’ equity | | | 1,114.6 | | | | 1,139.2 | | | | 1,060.6 | | | | 937.1 | | | | 992.1 | |
| | a) | | Includes $2.8, $1.7 and $0.8 of adjustments recorded in cost of sales in fiscal years 2007, 2006 and 2005, respectively. The adjustments include a one-time purchase accounting adjustment to record, at market value, inventory acquired from the BioSepra® Process Division (“BioSepra”) of Ciphergen Biosystems, Inc. This resulted in a $2.4 increase in acquired inventories in fiscal year 2005, in accordance with SFAS No. 141, Business Combinations (“SFAS No. 141”), in the opening balance sheet and an increase in cost of sales of $0.6, $0.9 and $0.8 in fiscal years 2007, 2006 and 2005, respectively, concurrent with the sale of a portion of the underlying inventory. The adjustment is considered non-recurring in nature because, although the Company acquired the manufacturing operations of BioSepra, this adjustment was required by SFAS No. 141 as an elimination of the manufacturing profit in inventory acquired from BioSepra and subsequently sold in the period. The adjustments recorded in cost of sales also reflect $2.2 and $0.8 in fiscal years 2007 and 2006, respectively, primarily comprised of incremental depreciation from the planned early retirement of certain fixed assets recorded in conjunction with the Company’s facilities rationalization initiatives in accordance with SFAS No. 144, Accounting for the Impairment or Disposal of Long-Lived Assets. |
| |
| b) | | Effective August 1, 2005, the Company adopted SFAS 123(R), Share Based Payment. The years ended July 31, 2009, July 31, 2008, July 31, 2007 and July 31, 2006 include stock-based compensation expense related to stock options and the employee stock purchase plan of $7.6, $5.2, $4.7 and $7.2, respectively, after pro forma tax effect (6, 4, 4 and 6 cents per share, respectively). |
| |
| c) | | Non-cash working capital at July 31, 2008 has been impacted by the adoption of a new accounting standard, FIN No. 48, Accounting for Uncertainty in Income Taxes (“FIN No. 48”). Consistent with the provisions of FIN No. 48, the Company has reclassified certain tax related assets and liabilities from current to non-current. Such reclassifications had the effect of increasing non-cash working capital at July 31, 2008 by approximately $137.0. |
21
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION ANDRESULTS OF OPERATIONS.
Forward-Looking Statements and Risk Factors
You should read the following discussion together with the accompanying consolidated financial statements and notes thereto and other financial information in this Form 10-K. The discussions under the subheadings “Review of Operating Segments” below are in local currency unless indicated otherwise. Company management considers local currency growth an important measure because by excluding the volatility of exchange rates, underlying volume change is clearer. Dollar amounts discussed below are in thousands, unless otherwise indicated, except per share dollar amounts. In addition, per share dollar amounts are discussed on a diluted basis.
The matters discussed in this Annual Report on Form 10-K contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995. Forward-looking statements contained in this and other written and oral reports are based on current Company expectations and are subject to risks and uncertainties, which could cause actual results to differ materially. All statements regarding future performance, earnings projections, earnings guidance, management’s expectations about its future cash needs and effective tax rate, and other future events or developments are forward-looking statements. Such risks and uncertainties include, but are not limited to, those discussed in “Part I–Item 1A–Risk Factors” in this Form 10-K. The Company makes these statements as of the date of this disclosure and undertakes no obligation to update them.
Critical Accounting Policies and Estimates
The Company’s accompanying consolidated financial statements are prepared in accordance with U.S. generally accepted accounting principles (“GAAP”). These accounting principles require the Company to make certain estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the accompanying consolidated financial statements, as well as the reported amounts of revenues and expenses during the periods presented. Although these estimates are based on Company management’s knowledge of current events and actions it may undertake in the future, actual results may differ from estimates. The following discussion addresses the Company’s critical accounting policies, which are those that are most important to the portrayal of the Company’s financial condition and results, and that require judgment. See also the notes to the accompanying consolidated financial statements, which contain additional information regarding the Company’s accounting policies.
Income Taxes
Significant judgment is required in determining the worldwide provision for income taxes. In the ordinary course of a global business, there are many transactions and calculations where the ultimate tax outcome is uncertain. Some of these uncertainties arise as a consequence of revenue sharing and cost reimbursement arrangements among related entities, the process of identifying items of revenue and expense that qualify for preferential tax treatment and appropriate segregation of foreign and domestic income and expense to avoid double taxation. No assurance can be given that the final tax outcome of these matters will not be different than that which is reflected in the Company’s historical income tax provisions and accruals. Such differences could have a material effect on the Company’s income tax provision and net earnings in the period in which a final determination is made.
The Company records a valuation allowance to reduce deferred tax assets to the amount of the future tax benefit that is more likely than not to be realized. While Company management has considered future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for the valuation allowance, there is no assurance that the valuation allowance would not need to be increased to cover additional deferred tax assets that may not be realizable. Any increase in the valuation allowance could have a material adverse impact on the Company’s income tax provision and net earnings in the period in which such determination is made.
Purchase Accounting and Goodwill
Determining the fair value of certain assets and liabilities acquired in a business combination in accordance with SFAS No. 141 is judgmental in nature and often involves the use of significant estimates and assumptions. There are various methods used to estimate the value of tangible and intangible assets acquired, such as discounted cash flow and market multiple approaches. Some of the more significant estimates and assumptions inherent in the two approaches include: projected future cash flows (including timing); discount rates reflecting the risk inherent in the future cash flows; perpetual growth rate; determination of appropriate market comparables; and the determination of whether a premium or a discount should be applied to comparables. There are also judgments made to determine the expected useful lives assigned to each class of assets and liabilities acquired.
22
The Company accounts for its goodwill and intangible assets in accordance with SFAS No. 142, Goodwill and Other Intangible Assets (“SFAS No. 142”). As such, goodwill is assessed for impairment at least annually during the Company’s fiscal third quarter, or more frequently if certain events or circumstances indicate impairment might have occurred. The Company evaluates the recoverability of goodwill using a two-step impairment test approach at the reporting unit level. In the first step, the overall fair value for the reporting unit is compared to its book value including goodwill. In the event that the overall fair value of the reporting unit was determined to be less than the book value, a second step is performed which compares the implied fair value of the reporting unit’s goodwill to the book value of the goodwill. The implied fair value for the goodwill is determined based on the difference between the overall fair value of the reporting unit and the fair value of the net identifiable assets. If the implied fair value of the goodwill is less than its book value, the difference is recognized as an impairment. In fiscal years 2009 and 2008, the fair value of the Company’s reporting units exceeded the book value of the reporting units and as such, step two was not performed.
When testing for impairment, the Company uses significant estimates and assumptions to estimate the fair values of its reporting units. Fair value of the Company’s reporting units is determined using market multiples (derived from trailing-twelve-month revenue, earnings before interest and taxes (“EBIT”) and earnings before interest, taxes depreciation and amortization (“EBITDA”)), of publicly traded companies with similar operating and investment characteristics as the Company’s reporting units. These various market multiples are applied to the operating performance of the reporting unit being tested to determine a range of fair values for the reporting unit. The fair value of the reporting units is then determined using the average of the fair values derived from the minimum and median market multiples. The minimum and median market multiples used in the fiscal year 2009 impairment testing ranged from 0.2 to 2.4 times revenue, 5.4 to 14.5 times EBIT and 2.1 to 10.7 times EBITDA. The median and minimum market multiples used in the fiscal year 2008 impairment testing ranged from 1.1 to 3.6 times revenue, 8.3 to 22.4 times EBIT and 6.5 to 15.0 times EBITDA. To further substantiate the reasonableness of the fair value of its reporting units, the Company compares enterprise value (outstanding shares multiplied by the closing market price per share, plus debt, less cash and cash equivalents) to the aggregate fair value of its reporting units.
Revenue Recognition
Revenue is recognized when title and risk of loss have transferred to the customer and when contractual terms have been fulfilled, except for certain long-term contracts, whereby revenue is recognized under the percentage of completion method (see below). Transfer of title and risk of loss occurs when the product is delivered in accordance with the contractual shipping terms. In instances where contractual terms include a provision for customer acceptance, revenue is recognized when either (i) the Company has previously demonstrated that the product meets the specified criteria based on either seller or customer-specified objective criteria or (ii) upon formal acceptance received from the customer where the product has not been previously demonstrated to meet customer-specified objective criteria.
For contracts accounted for under the percentage of completion method, revenue is based upon the ratio of costs incurred to date compared with estimated total costs to complete. The cumulative impact of revisions to total estimated costs is reflected in the period of the change, including anticipated losses.
Allowance for Doubtful Accounts
Company management evaluates its ability to collect outstanding receivables and provide allowances when collection becomes doubtful. In performing this evaluation, significant estimates are involved, including an analysis of specific risks on a customer-by-customer basis. Based upon this information, Company management records in earnings an amount believed to be uncollectible. If the factors used to estimate the allowance provided for doubtful accounts do not reflect the future ability to collect outstanding receivables, additional provisions for doubtful accounts may be needed and the future results of operations could be materially affected.
Inventories
Inventories are valued at the lower of cost (principally on the first-in, first-out method) or market. The Company records adjustments to the carrying value of inventory based upon assumptions about historic usage, future demand and market conditions. These adjustments are estimates which could vary significantly, either favorably or unfavorably, from actual requirements if future conditions, customer inventory levels or competitive conditions differ from the Company’s expectations.
23
Recoverability of Available-for-Sale Investments
Other than temporary losses relating to available-for-sale investments are recognized in earnings when Company management determines that the recoverability of the cost of the investment is unlikely. Such losses could result in a material adjustment in the period of the change. Company management considers numerous factors, on a case-by-case basis, in evaluating whether the decline in market value of an available-for-sale security below cost is other than temporary. Such factors include, but are not limited to, (i) the length of time and the extent to which the market value has been less than cost; (ii) the financial condition and the near-term prospects of the issuer of the investment; and (iii) whether Company management intends to retain the investment for a period of time that is sufficient to allow for any anticipated recovery in market value.
Defined Benefit Retirement Plans
The Company sponsors defined benefit retirement plans in various forms covering substantially all employees who meet eligibility requirements. Several statistical and other factors that attempt to anticipate future events are used in calculating the expense and liabilities related to those plans for which the benefit is actuarially determined. These factors include assumptions about the discount rate, expected return on plan assets and rate of future compensation increases as determined by the Company, within certain guidelines. In addition, the Company’s actuarial consultants also use subjective factors, such as withdrawal and mortality rates, to calculate the liabilities and expense. The actuarial assumptions used by the Company are long-term assumptions and may differ materially from actual experience in the short-term due to changing market and economic conditions and changing participant demographics. These differences may have a significant effect on the amount of pension expense and pension assets/(liabilities) recorded by the Company.
Pension expense associated with the Company’s defined benefit plans was $23,240 in fiscal year2009, which was based on a weighted average discount rate of 6.22% (calculated using the projected benefit obligation) and a weighted average expected long-term rate of return on plan assets of 6.55% (calculated using the fair value of plan assets).
The expected rates of return on the various defined benefit pension plans’ assets are based on the asset allocation of each plan and the long-term projected return of those assets. If the expected long-term rate of return on plan assets was reduced by 50 basis points, projected pension expense in fiscal year 2009 would have increased approximately $1,700.
The objective of the discount rate assumption is to reflect the rate at which the pension benefits could be effectively settled. The Company’s methodology for selecting the discount rate for the U.S. plans as of July 31, 2009 was to match the plan’s cash flows to that of a yield curve that provides the equivalent yields on zero-coupon corporate bonds for each maturity. Benefit cash flows due in a particular year can be “settled” theoretically by “investing” them in the zero-coupon bond that matures in the same year. The discount rate is the single rate that produces the same present value of cash flows. The discount rate assumption for non-U.S. plans reflects the market rate for high-quality, fixed-income debt instruments. Both discount rate assumptions are based on the expected duration of benefit payments for each of the Company’s pension plans as of the annual measurement date and is subject to change each year. If the weighted average discount rate was reduced by 50 basis points, pension expense in fiscal year 2009 would have increased by approximately $1,600.
Accrued Expenses and Contingencies
Company management estimates certain material expenses in an effort to record those expenses in the period incurred. When no estimate in a given range is deemed to be better than any other, the low end of the range is accrued. Differences between estimates and assumptions and actual results could result in an accrual requirement materially different from the calculated accrual.
Environmental accruals are recorded based upon historical costs incurred and estimates for future costs of remediation and on-going legal expenses which have a high degree of uncertainty.
Self-insured workers’ compensation insurance accruals are recorded based on insurance claims processed, including applied loss development factors as well as historical claims experience for claims incurred but not yet reported. Self-insured employee medical insurance accruals are recorded based on medical claims processed as well as historical medical claims experience for claims incurred but not yet reported.
24
Results of Operations 2009 Compared with 2008
Review of Consolidated Results
Sales for the fiscal year 2009 decreased 9.4% to $2.3 billion from $2.6 billion in fiscal year 2008. Exchange rates used to translate foreign subsidiary results into U.S. dollars, reduced reported sales by $155,096, primarily due to the strengthening of the U.S. dollar against the Euro, the British Pound and several Asian currencies, partly offset by the weakening of the U.S. dollar against the Japanese Yen and Chinese Renminbi. In local currency, sales decreased 3.4%. Increased pricing achieved in both the Life Sciences and Industrial segments contributed $30,905 to overall sales in the year. In the first quarter of fiscal year 2009, the Company launched its Pricing Excellence initiative that is focused on optimizing prices and product margins by, among other things, better defining the value equation to the benefit of the Company and its customers. Life Sciences segment sales increased 3% (in local currency), primarily attributable to growth in the BioPharmaceuticals market. Sales in the Medical market were up slightly (in local currency). Industrial segment sales decreased 7.3% (in local currency) in the year reflecting declines in the Energy, Water & Process Technologies (“EWPT”) and Microelectronics markets. The Aerospace & Transportation market increased 1.3% (in local currency) in the year. Overall systems sales increased 6.9% (in local currency) reflecting growth in the Pharmaceuticals market as well as in EWPT’s Municipal Water, Power Generation and Food & Beverage markets. Systems sales represented 13.3% of total sales compared to 12.4% in fiscal year 2008. For a detailed discussion of sales, refer to the section “Review of Operating Segments” below.
Gross margin was 47.3% in fiscal year 2009 compared to 47.1% in fiscal year 2008. The improvement in gross margin reflects improved pricing, which contributed about 70 basis points in margin, and the effects of the ongoing cost reduction and lean manufacturing initiatives, partly offset by a shift in product mix to a higher percentage of systems sales as discussed above, a change in market mix within the Industrial segment resulting from decreased sales in higher margin markets such as Microelectronics and Industrial Manufacturing and reduced absorption of manufacturing overhead in Industrial, related to lower volumes. For a detailed discussion of gross margin by segment, refer to the section “Review of Operating Segments” below.
Selling, general and administrative (“SG&A”) expenses in fiscal year 2009 decreased by $49,687, or 6.6% (1% in local currency). As a percentage of sales, SG&A expenses were 30% compared to 29.1% in fiscal year 2008. The increase in SG&A as a percentage of sales primarily reflects the impact of decreased sales period over period, increased selling and marketing personnel-related costs, including those related to the expansion into Latin American and other geographies, increased stock compensation expense, as well as consulting costs, mainly related to the Company’s Pricing Excellence and Enterprise Risk Management initiatives, partly offset by the impact of the Company’s cost reduction initiatives described below. In fiscal year 2009, the Company began implementing the second phase of its European cost reduction initiative (“EuroPall II”). Furthermore, in fiscal year 2009, the Company implemented plans to reduce its workforce globally in response to current economic conditions.
Research and development (“R&D”) expenses were $71,213 in fiscal year 2009 compared to $71,647 in fiscal year 2008, a decrease of less than 1% (an increase of 3.5% in local currency). As a percentage of sales, R&D expenses were 3.1% compared to 2.8% in fiscal year 2008.
In fiscal year 2009, the Company recorded restructuring and other charges (“ROTC”) of $30,723. ROTC in the year was primarily comprised of severance and other costs related to the Company’s on-going cost reduction initiatives, a charge to write-off in-process R&D acquired in the acquisition of GeneSystems, SA (“GeneSystems”) (refer to Note 2, Acquisitions, to the accompanying consolidated financial statements for further discussion of purchase accounting), a charge primarily for the other-than-temporary diminution in value of certain equity investment securities held by the Company’s benefits protection trust, a charge for the impairment of capitalized software, increases to previously established environmental reserves, net of an insurance settlement and legal fees related to matters that were under inquiry by the audit committee, net of an insurance settlement (see Note 2, Audit Committee Inquiry and Restatement, to the consolidated financial statements included in the 2007 Form 10-K). Such charges were partly offset by the reversal of excess restructuring reserves that were previously recorded in the Company’s consolidated statements of earnings in fiscal years 2008 and 2007.
In fiscal year 2008, the Company recorded ROTC of $31,538. ROTC in the year was primarily comprised of legal and other professional fees related to matters that were under inquiry by the audit committee (see Note 2, Audit Committee Inquiry and Restatement, to the consolidated financial statements included in the 2007 Form 10-K). Additionally, ROTC includes severance and other exit costs related to the Company’s on-going cost reduction initiatives (including its facilities rationalization, EuroPall and AmeriPall cost reduction initiatives), as well as an increase to previously established environmental reserves. Such charges were partly offset by the reversal of excess restructuring reserves previously recorded in the Company’s consolidated statements of earnings in fiscal years 2007, 2006 and 2005.
25
The details of ROTC for the years ended July 31, 2009 and July 31, 2008 can be found in Note 3, Restructuring and Other Charges, Net, to the accompanying consolidated financial statements.
The following table summarizes the activity related to restructuring liabilities that were recorded in fiscal years 2009, 2008, 2007 and 2006.
| | | | | | | | Lease | | | | |
| | | | | | Termination | | | | |
| | | | | | Liabilities & | | | | |
| | Severance | | | Other | | | Total |
| 2009 | | | | | | | | | | | | |
| | Original charge | $ | 18,938 | | | $ | 4,734 | | | $ | 23,672 | |
| Utilized | | (12,757 | ) | | | (4,133 | ) | | | (16,890 | ) |
| Other changes (a) | | 412 | | | | 20 | | | | 432 | |
| Balance at Jul. 31, 2009 | $ | 6,593 | | | $ | 621 | | | $ | 7,214 | |
| |
| 2008 | | | | | | | | | | | | |
| Original charge | $ | 8,814 | | | $ | 3,110 | | | $ | 11,924 | |
| Utilized | | (8,059 | ) | | | (2,849 | ) | | | (10,908 | ) |
| Other changes (a) | | 220 | | | | 6 | | | | 226 | |
| Balance at Jul. 31, 2008 | | 975 | | | | 267 | | | | 1,242 | |
| Utilized | | (741 | ) | | | (201 | ) | | | (942 | ) |
| Reversal of excess reserves (b) | | (24 | ) | | | (4 | ) | | | (28 | ) |
| Other changes (a) | | (96 | ) | | | (19 | ) | | | (115 | ) |
| Balance at Jul. 31, 2009 | $ | 114 | | | $ | 43 | | | $ | 157 | |
| |
| 2007 | | | | | | | | | | | | |
| Original charge | $ | 22,083 | | | $ | 4,321 | | | $ | 26,404 | |
| Utilized | | (6,146 | ) | | | (3,573 | ) | | | (9,719 | ) |
| Other changes (a) | | 611 | | | | 9 | | | | 620 | |
| Balance at Jul. 31, 2007 | | 16,548 | | | | 757 | | | | 17,305 | |
| Utilized | | (13,994 | ) | | | (727 | ) | | | (14,721 | ) |
| Reversal of excess reserves (b) | | (297 | ) | | | (65 | ) | | | (362 | ) |
| Other changes (a) | | 1,281 | | | | 57 | | | | 1,338 | |
| Balance at Jul. 31, 2008 | | 3,538 | | | | 22 | | | | 3,560 | |
| Utilized | | (1,984 | ) | | | (13 | ) | | | (1,997 | ) |
| Reversal of excess reserves (b) | | (136 | ) | | | — | | | | (136 | ) |
| Other changes (a) | | (158 | ) | | | (4 | ) | | | (162 | ) |
| Balance at Jul. 31, 2009 | $ | 1,260 | | | $ | 5 | | | $ | 1,265 | |
| |
| 2006 | | | | | | | | | | | | |
| Original charge | $ | 13,335 | | | $ | 3,043 | | | $ | 16,378 | |
| Utilized | | (7,221 | ) | | | (2,900 | ) | | | (10,121 | ) |
| Other changes (a) | | 182 | | | | 9 | | | | 191 | |
| Balance at Jul. 31, 2006 | | 6,296 | | | | 152 | | | | 6,448 | |
| Utilized | | (2,712 | ) | | | (108 | ) | | | (2,820 | ) |
| Reversal of excess reserves (b) | | (1,385 | ) | | | (40 | ) | | | (1,425 | ) |
| Other changes (a) | | 126 | | | | 2 | | | | 128 | |
| Balance at Jul. 31, 2007 | | 2,325 | | | | 6 | | | | 2,331 | |
| Utilized | | (1,414 | ) | | | (6 | ) | | | (1,420 | ) |
| Reversal of excess reserves (b) | | (56 | ) | | | — | | | | (56 | ) |
| Other changes (a) | | (4 | ) | | | — | | | | (4 | ) |
| Balance at Jul. 31, 2008 | | 851 | | | | — | | | | 851 | |
| Utilized | | (706 | ) | | | — | | | | (706 | ) |
| Balance at Jul. 31, 2009 | $ | 145 | | | $ | — | | | $ | 145 | |
| | (a) | | Other changes primarily reflect translation impact. |
| (b) | | Reflects the reversal of excess restructuring reserves originally recorded in fiscal years 2008, 2007 and 2006. |
26
Earnings before interest and income taxes (“EBIT”) were $298,922 in fiscal year 2009 compared to $358,131 in fiscal year 2008, reflecting the factors discussed above. The impact of foreign currency translation reduced EBIT by $25,314 in fiscal year 2009. As a percentage of sales, EBIT were 12.8% compared to 13.9% in fiscal year 2008.
Net interest expense in fiscal year 2009 decreased to $28,136 from $32,576 in fiscal year 2008. The reduction in net interest expense was primarily attributable to a decrease in interest expense, which was related to lower interest rates and a reduced level of debt due to the repayment of higher interest bearing European debt. A decrease in interest income related to reduced cash balances and lower returns compared to the same period last year partly offset the above.
In fiscal year 2009, the Company’s effective tax rate was 27.8% as compared to 33.2% in fiscal year 2008. For the year ended July 31, 2009, the effective tax rate varied from the U.S. federal statutory rate primarily due to the benefits related to foreign operations, the repatriation of foreign earnings, the restructuring of certain foreign operations, the retroactive extension of the federal research credit provided for in the Emergency Economic Stabilization Act of 2008 and a favorable tax settlement. For the year ended July 31, 2008, the effective tax rate varied from the U.S. federal statutory rate primarily due to the net impact of foreign operations and a tax charge resulting from new tax legislation in Germany.
Net earnings in fiscal year 2009 were $195,619, or $1.64 per share, compared with net earnings of $217,279, or $1.76 per share in fiscal year 2008. In summary, the decline in net earnings dollars reflects the decrease in EBIT partly offset by a decline in net interest expense and a decrease in the effective tax rate. The decline in earnings per share reflects the decrease in net earnings partly offset by the impact of reduced shares outstanding due to stock buybacks. Company management estimates that foreign currency translation reduced net earnings per share by 16 cents in the year. The acquisition of GeneSystems was dilutive to earnings by 5 cents per share in the year.
Review of Operating Segments
The following table presents sales and operating profit by segment, reconciled to earnings before income taxes, for the fiscal years ended July 31, 2009 and July 31, 2008.
| | | | | | % | | | | | % | | % |
| | | 2009 | | Margin | | | 2008 | | Margin | | Change |
| SALES: | | | | | | | | | | | | |
| Life Sciences | $ | 940,461 | | | | $ | 975,231 | | | | (3.6 | ) |
| Industrial | | 1,388,697 | | | | | 1,596,414 | | | | (13.0 | ) |
| Total | $ | 2,329,158 | | | | $ | 2,571,645 | | | | (9.4 | ) |
| | OPERATING PROFIT: | | | | | | | | | | | | |
| Life Sciences | | 200,070 | | 21.3 | | $ | 197,774 | | 20.3 | | 1.2 | |
| Industrial | | 186,053 | | 13.4 | | | 245,855 | | 15.4 | | (24.3 | ) |
| Total operating profit | | 386,123 | | 16.6 | | | 443,629 | | 17.3 | | (13.0 | ) |
| General corporate expenses | | 56,478 | | | | | 53,960 | | | | 4.7 | |
| Earnings before ROTC, interest expense, net | | | | | | | | | | | | |
| and income taxes | | 329,645 | | 14.2 | | | 389,669 | | 15.2 | | (15.4 | ) |
| ROTC | | 30,723 | | | | | 31,538 | | | | | |
| Interest expense, net | | 28,136 | | | | | 32,576 | | | | | |
| Earnings before income taxes | $ | 270,786 | | | | $ | 325,555 | | | | | |
Life Sciences:
Presented below are Summary Statements of Operating Profit for the Life Sciences segment for the fiscal years ended July 31, 2009 and July 31, 2008:
| | | | | | % of | | | | | % of |
| | | 2009 | | Sales | | | 2008 | | Sales |
| | Sales | $ | 940,461 | | | | $ | 975,231 | | |
| Cost of sales | | 450,925 | | 47.9 | | | 473,298 | | 48.5 |
| Gross margin | | 489,536 | | 52.1 | | | 501,933 | | 51.5 |
| SG&A | | 248,559 | | 26.4 | | | 263,233 | | 27.0 |
| R&D | | 40,907 | | 4.4 | | | 40,926 | | 4.2 |
| Operating profit | $ | 200,070 | | 21.3 | | $ | 197,774 | | 20.3 |
27
The tables below present sales by market and geography within the Life Sciences segment for the fiscal years ended July 31, 2009 and July 31, 2008, including the effect of exchange rates for comparative purposes.
| | | | | | | | | | | | | | | % |
| | | | | | | | | | | Exchange | | Change in |
| | | | | | | | % | | Rate | | Local |
| | 2009 | | 2008 | | Change | | Impact | | Currency |
| By Market | | | | | | | | | | | | | | | | |
| Medical (a) | | $ | 389,841 | | $ | 410,416 | | (5.0 | ) | | $ | (23,190 | ) | | 0.6 | |
| BioPharmaceuticals (a) | | | 550,620 | | | 564,815 | | (2.5 | ) | | | (40,858 | ) | | 4.7 | |
| Total Life Sciences | | $ | 940,461 | | $ | 975,231 | | (3.6 | ) | | $ | (64,048 | ) | | 3.0 | |
| |
| By Geography | | | | | | | | | | | | | | | | |
| Western Hemisphere | | $ | 360,975 | | $ | 379,591 | | (4.9 | ) | | $ | (2,169 | ) | | (4.3 | ) |
| Europe | | | 441,514 | | | 469,450 | | (6.0 | ) | | | (58,651 | ) | | 6.5 | |
| Asia | | | 137,972 | | | 126,190 | | 9.3 | | | | (3,228 | ) | | 11.9 | |
| Total Life Sciences | | $ | 940,461 | | $ | 975,231 | | (3.6 | ) | | $ | (64,048 | ) | | 3.0 | |
| (a) | | Amounts reflect inclusion of the Laboratory market within the BioPharmaceuticals market effective August 1, 2008. |
Life Sciences segment sales increased 3% in fiscal year 2009 compared to fiscal year 2008. Increased pricing (driven by the Biopharmaceuticals market) contributed $14,464, or 1.5% to overall sales growth in the year. Life Sciences sales represented approximately 40% of total sales in fiscal year 2009 compared to 38% in fiscal year 2008.
Within Life Sciences, Medical market sales, which now excludes the Laboratory market and represented approximately 41% of Life Sciences sales, was flat compared to fiscal year 2008 as growth in the Hospital, Original Equipment Manufacturer (“OEM”) and Cell Therapy markets was offset by a decline in the Blood Filtration market, the largest market served by Medical. The decline in the Blood Filtration market (-2.4%) primarily relates to decreased volume to several large blood center customers in the Western Hemisphere and Europe (Spain, Germany and Switzerland) partly offset by increased sales to independent blood centers in the U.S., growth in Asia, driven by the adoption of universal leukoreduction in Australia and increased sales in Singapore as well as increased Hospital Transfusion and Cardiovascular sales in Europe. The growth in Hospital sales (+5%) was driven by all geographies, with increased point of use water filter sales a key contributor to growth, particularly in the U.S. Growth in OEM sales (+1.5%) was driven by Europe and Asia.
Sales in the BioPharmaceuticals market, which now includes the Laboratory market, previously reported in Medical, increased 4.7% compared to fiscal year 2008. By geography, growth in Europe (+7.5%), the Company’s largest geographic BioPharmaceuticals market, and in Asia (+14.4%), was partly offset by a decline in the Western Hemisphere (-4.7%). Within BioPharmaceuticals, sales in the Pharmaceuticals market (formerly named BioPharmaceuticals market) increased 5%, while sales in the Laboratory market grew 2.8%. The increase in the Pharmaceuticals market reflects an increase in systems sales of 7.3% (Europe and Asia) accompanied by growth in consumables sales of 4.9% contributed by all geographies. Sales growth in the Pharmaceuticals market was driven by increased demand in the vaccine and plasma derivatives marketplace (including investment by customers in new production facilities). The Company continues to benefit from expanding adoption of single-use technologies for biotechnology and vaccine production. The increase in Laboratory sales reflects strong growth in Europe (Spain, Germany, France and Switzerland) and Asia (China, Korea and Singapore) partly offset by softness in the end-user Western Hemisphere market as customers compressed their inventories.
Life Sciences gross margins increased 60 basis points to 52.1% from 51.5% in fiscal year 2008. The improvement in gross margins was principally driven by improved pricing that contributed approximately 75 basis points in margin, a change in market mix (higher percentage of BioPharmaceuticals versus Medical sales) and savings from the Company’s cost reduction and lean manufacturing initiatives partly offset by a shift in product mix to a higher percentage of systems sales (about 5.6% of total Life Sciences sales compared to 5.4% in fiscal year 2008) and inflation of manufacturing costs.
SG&A expenses decreased by $14,674, or 5.6% (an increase of 1.2% in local currency), compared to fiscal year 2008. The increase in SG&A in local currency was primarily due to increased selling expenses. SG&A as a percentage of sales decreased to 26.4% from 27% in fiscal year 2008. The improvement in SG&A as a percentage of sales reflects the impact of the Company’s cost reduction initiatives.
28
R&D expenses were essentially flat at $40,907 compared to $40,926 in fiscal year 2008. In local currency, R&D expenses increased 6.3%. As a percentage of sales, R&D expenses were 4.4% compared to 4.2% in fiscal year 2008. Increased spending primarily reflects investments in the BioPharmaceuticals market, including spending at GeneSystems, which was acquired on September 2, 2008.
Operating profit dollars increased about 1.2% to $200,070. In local currency, operating profit increased 8.5% in the year. Operating margin improved to 21.3% from 20.3% in fiscal year 2008.
Industrial:
Presented below are summary Statements of Operating Profit for the Industrial segment for the fiscal years ended July 31, 2009 and July 31, 2008:
| | | | | % of | | | | | % of |
| | 2009 | | Sales | | 2008 | | Sales |
| Sales | | $ | 1,388,697 | | | | $ | 1,596,414 | | |
| Cost of sales | | | 777,543 | | 56.0 | | | 887,512 | | 55.6 |
| Gross margin | | | 611,154 | | 44.0 | | | 708,902 | | 44.4 |
| SG&A | | | 394,795 | | 28.4 | | | 432,326 | | 27.1 |
| R&D | | | 30,306 | | 2.2 | | | 30,721 | | 1.9 |
| Operating profit | | $ | 186,053 | | 13.4 | | $ | 245,855 | | 15.4 |
The tables below present sales by market and geography within the Industrial segment for the fiscal years ended July 31, 2009 and July 31, 2008, including the effect of exchange rates for comparative purposes.
| | | | | | | | | | | | | | | % |
| | | | | | | | | | | Exchange | | Change in |
| | | | | | | | % | | Rate | | Local |
| | 2009 | | 2008 | | Change | | Impact | | Currency |
| By Market | | | | | | | | | | | | | | | | |
| Energy, Water & | | | | | | | | | | | | | | | | |
| Process Technologies | | $ | 887,662 | | $ | 981,291 | | (9.5 | ) | | $ | (66,111 | ) | | (2.8 | ) |
| Aerospace & | | | | | | | | | | | | | | | | |
| Transportation | | | 290,006 | | | 306,571 | | (5.4 | ) | | | (20,493 | ) | | 1.3 | |
| Microelectronics | | | 211,029 | | | 308,552 | | (31.6 | ) | | | (4,444 | ) | | (30.2 | ) |
| Total Industrial | | $ | 1,388,697 | | $ | 1,596,414 | | (13.0 | ) | | $ | (91,048 | ) | | (7.3 | ) |
| |
| By Geography | | | | | | | | | | | | | | | | |
| Western Hemisphere | | $ | 408,727 | | $ | 431,068 | | (5.2 | ) | | $ | (6,626 | ) | | (3.6 | ) |
| Europe | | | 518,793 | | | 637,533 | | (18.6 | ) | | | (69,445 | ) | | (7.7 | ) |
| Asia | | | 461,177 | | | 527,813 | | (12.6 | ) | | | (14,977 | ) | | (9.8 | ) |
| Total Industrial | | $ | 1,388,697 | | $ | 1,596,414 | | (13.0 | ) | | $ | (91,048 | ) | | (7.3 | ) |
Industrial segment sales decreased 7.3% in fiscal year 2009, as declines in the EWPT and Microelectronics markets were partly offset by growth in the Aerospace & Transportation market. Increased pricing, largely driven by the EWPT and Aerospace & Transportation markets, contributed $16,441 to overall sales in the year. Industrial systems sales increased 6.8% compared to fiscal year 2008. The increase in systems sales reflects growth in the Municipal Water, Food & Beverage and Power Generation markets reported within EWPT, partly offset by declines in all other Industrial markets. Industrial consumables sales decreased 10.2% in the year, reflecting declines in all markets with the exception of Power Generation, within the EWPT market, and Military Aerospace, within the Aerospace & Transportation market. Industrial represented approximately 60% of the Company’s total sales in fiscal year 2009, compared to 62% in fiscal year 2008.
EWPTmarket sales, which account for about two-thirds of the Industrial segment, decreased 2.8% as declines in the Industrial Manufacturing and Food & Beverage markets were partly offset by growth in the Municipal Water market. Sales in the energy-related markets were flat.
29
Municipal Water sales increased 11.8% in fiscal year 2009 compared to fiscal year 2008. By geography, the sales growth was driven by the Western Hemisphere and Asia. The growth in the Western Hemisphere was primarily attributable to surface water treatment projects driven by federal and local government regulations and scarcity of potable water. The growth in Asia was attributable to systems projects for drinking water driven by emerging regulations. Municipal Water sales were down in Europe primarily related to a slowdown of projects in Eastern Europe, due to economic conditions in the region, as well as in the Middle East, which is a result of decreased funding related to lower oil prices.
Sales in the energy-related markets were flat in fiscal year 2009 compared to fiscal year 2008 as growth in the Power Generation market of 11.6% was offset by a decline in the larger Fuels & Chemicals market of 2.8%. The growth in Power Generation sales was driven by an increase in systems sales of 36.7% (contributed by Europe and Asia) and an increase in consumables sales of 7.2% (all geographies contributing). Growth in systems sales was driven by customer investment in new plant capacity, especially nuclear and wind, as well as in water treatment and condensate systems. The growth in consumables sales reflects good aftermarket demand and growth in capital goods, such as housings. Fuels & Chemicals sales were down as growth in Asia was offset by declines in the Western Hemisphere and Europe related to reduced element demand by chemical producers, particularly related to the automotive, electronics and housing and construction sectors. Asia benefited from increased systems sales due to robust investment activity in the region.
Food and Beverage sales in fiscal year 2009 decreased 2.5% reflecting a decline in consumables of 5.5% (all geographies), partly offset by growth in systems sales of 10.9% (Western Hemisphere and Asia contributing). By geography, sales in Europe (the largest market) were down 8.7% partly mitigated by growth in the Western Hemisphere of 17.2% and in Asia of 4.9%. The decline in sales in Europe reflects decreased sales in Eastern Europe due to economic conditions in the region, a slowdown in the beer and bottled water sector and a general slowing in capital projects. The growth in the Western Hemisphere and Asia were driven by systems sales in the wine and beer sectors. These two regions also have benefited from expanded market share.
Sales in the Industrial Manufacturing market decreased 18.2% in fiscal year 2009. All geographies reported decreased sales compared to the prior period. Sales growth was negatively impacted by the global macroeconomic recessionary environment, particularly in the steel, automotive, metals and paper sectors.
Aerospace & Transportation sales increased 1.3% reflecting growth in the Military and Commercial markets of 18.1% and 1%, respectively, partly offset by a decline in the Transportation market of 26.7%. The growth in Military sales was primarily driven by CH-47 helicopter product shipments, increased OEM platform builds in the Western Hemisphere and growth in Europe, primarily in Germany and France. The Commercial market result primarily reflects strong sales in Europe largely offset by a reduction in the Western Hemisphere. The decline in the Transportation market primarily reflects decreased sales to the construction and truck industries in Europe.
Microelectronics sales declined 30.2% reflecting decreases in all geographies. Overall, the sales decrease reflects the weakness in the semiconductor and consumer electronics markets related to the global economic environment.
Industrial gross margins in fiscal year 2009 decreased 40 basis points to 44% from 44.4% in fiscal year 2008. The decrease in gross margins reflects underabsorption of manufacturing overhead due to volume reduction, a change in market mix resulting from decreased sales in higher margin markets such as Microelectronics and Industrial Manufacturing, and a shift in product mix to a higher percentage of systems sales (about 18.6% of total Industrial sales compared to about 16.8% in fiscal year 2008). These negative factors were partly offset by improved pricing, which contributed about 67 basis points in margin as well as the effects of cost reduction and lean manufacturing initiatives which offset inflationary increases in manufacturing costs.
SG&A expenses decreased by $37,531 or 8.7% (3% in local currency), compared to fiscal year 2008. The decrease in SG&A reflects the impact of cost reduction initiatives as discussed above. SG&A expenses as a percentage of sales were 28.4% compared to 27.1% in fiscal year 2008 reflecting the decline in sales.
R&D expenses decreased 1.4% (flat in local currency) to $30,306 from $30,721 in fiscal year 2008 primarily related to control of short-term spending due to the economic downturn. As a percentage of sales, R&D expenses were 2.2% compared to 1.9% in fiscal year 2008.
As a result of the above factors, operating profit dollars decreased 24.3% to $186,053. In local currency, operating profit decreased 18.8%. Operating margin decreased to 13.4% from 15.4% in fiscal year 2008.
30
Corporate:
Corporate expenses in fiscal year 2009 increased by $2,518 or 4.7% to $56,478 from $53,960 in fiscal year 2008. The increase in Corporate expenses primarily reflects increased consulting costs related to the Company’s pricing and enterprise risk management initiatives, foreign currency transaction losses, increased stock compensation and increased payroll related to additions to Corporate staff.
Results of Operations 2008 Compared with 2007
Review of Consolidated Results
Sales for the fiscal year 2008 increased 14.3% to $2.6 billion from $2.2 billion in fiscal year 2007. Exchange rates used to translate foreign currency results into U.S. dollars, increased reported sales by $159,134, primarily due to the weakening of the U.S. dollar against the Euro, the British Pound, the Yen and various other Asian currencies. In local currency, sales increased 7.2%. Increased pricing achieved in both the Life Sciences and Industrial segments contributed 1% to overall sales growth in the year.
Life Sciences segment sales increased 4.5% (in local currency), attributable to growth in the BioPharmaceuticals market, partly offset by a decrease in the Medical market. Industrial segment sales increased 9% (in local currency) driven by growth in the EWPT and Aerospace & Transportation markets, partially offset by a small decrease in sales to the Microelectronics market. Overall systems sales increased 26.4% (in local currency), representing 12.4% of total sales in fiscal year 2008 compared to 10.4% in fiscal year 2007, primarily attributable to strong sales in the BioPharmaceuticals, EWPT and Aerospace & Transportation markets. For a detailed discussion of sales, refer to the section “Review of Operating Segments” below.
Gross margin, as a percentage of sales, was 47.1% in fiscal year 2008 on par with fiscal year 2007. Improved pricing in both segments contributed approximately 50 basis points in margin. Gross margin has been favorably impacted by a change in market mix within consumables in the Life Sciences segment, improved profitability on certain systems sales in the Industrial segment and savings generated from the Company’s facilities rationalization initiative and other manufacturing cost reduction and efficiency programs, including lean initiatives to improve labor productivity, that dampened estimated inflationary pressures on manufacturing costs. These factors were offset by the impact of a shift in product mix, to a higher percentage of systems sales (as discussed above), which are typically at lower margins than consumables, mix change within consumables to lower margin products in Industrial, and incremental costs related to the facilities rationalization initiative. For a detailed discussion of gross margin by segment, refer to the section “Review of Operating Segments” below.
SG&A expenses in fiscal year 2008 increased by $74,514, or about 11% (approximately 5% in local currency). As a percentage of sales, SG&A expenses decreased to 29.1% from 30% in fiscal year 2007. The decrease in SG&A as a percentage of sales reflects the leveraging of growth in sales, and the impact of cost reduction initiatives partly offset by the impact of increased selling related expenses in the Industrial segment and an increase in Corporate expenses primarily attributable to increased professional fees related to tax and audit services as well as the addition of tax and treasury function personnel.
R&D expenses were $71,647 in fiscal year 2008 compared to $62,414 in fiscal year 2007, up about 15% year over year (approximately 13% in local currency). As a percentage of sales, R&D expenses were 2.8% on par with fiscal year 2007.
In fiscal year 2008, the Company recorded ROTC of $31,538. ROTC in the year was primarily comprised of legal and other professional fees related to matters that were under inquiry by the audit committee (see Note 2, Audit Committee Inquiry and Restatement, to the consolidated financial statements included in the 2007 Form 10-K). Additionally, ROTC includes severance and other exit costs related to the Company’s on-going cost reduction initiatives (including its facilities rationalization, EuroPall and AmeriPall initiatives), as well as an increase to previously established environmental reserves. Such charges were partly offset by the reversal of excess restructuring reserves previously recorded in the Company’s consolidated statements of earnings in fiscal years 2007, 2006 and 2005.
In fiscal year 2007, the Company recorded ROTC of $22,352, primarily related to the Company’s on-going cost reduction initiatives (including its facilities rationalization, EuroPall and AmeriPall initiatives). The ROTC recorded in fiscal year 2007 was primarily comprised of severance, impairment charges related to the planned disposal of buildings and early retirement of certain long-lived assets, and other costs in connection with such initiatives. Additionally, the charges in fiscal year 2007 include an increase to previously established environmental reserves. Such charges were partly offset by the gain on the sale of the Company’s corporate headquarters, an insurance settlement related to an environmental matter, and the reversal of excess restructuring reserves previously recorded in the Company’s consolidated statements of earnings in fiscal years 2006 and 2005.
31
The details of ROTC for the years ended July 31, 2008 and July 31, 2007 can be found in Note 3, Restructuring and Other Charges, Net, to the accompanying consolidated financial statements.
EBIT were $358,131 in fiscal year 2008 compared to $299,585 in fiscal year 2007, reflecting the factors discussed above. As a percentage of sales, EBIT were 13.9% compared to 13.3% in fiscal year 2007.
Net interest expense in fiscal year 2008 decreased to $32,576 from $39,056 in fiscal year 2007. The decline in net interest expense was principally attributable to a decrease in the amount of interest expense recorded due to a payment of $135,000 to the IRS related to the tax matter (for discussion of the tax matter see Note 2, Audit Committee Inquiry and Restatement, to the consolidated financial statements included in the 2007 Form 10-K) partly offset by increased interest expense primarily related to higher debt levels in the U.S. compared to the same period last year.
In fiscal year 2008, the Company’s effective tax rate was 33.2% as compared to 51.1% in fiscal year 2007. The decrease in the effective tax rate was primarily due to charges taken in fiscal year 2007 for the net tax cost of the anticipated repatriation of approximately $160,000 of foreign earnings and additional taxes provided on intercompany transactions net of an increase related to the mix of foreign earnings. The effective tax rate in fiscal year 2008 includes charges resulting from new tax legislation in certain foreign jurisdictions and an incremental tax expense of $2,436 related to cash repatriated in fiscal year 2008 under the plan discussed above. See Note 12, Income Taxes, to the accompanying consolidated financial statements for further details on the components of the Company’s effective tax rate.
Net earnings in fiscal year 2008 were $217,279, or $1.76 per share, compared with net earnings of $127,497, or $1.02 per share in fiscal year 2007. In summary, net earnings reflect the growth in EBIT, a decrease in net interest expense and a decrease in the effective tax rate. Company management estimates that foreign currency translation increased net earnings by approximately 14 cents per share in the year.
Review of Operating Segments
The following table presents sales and operating profit by segment, reconciled to earnings before income taxes, for the fiscal years ended July 31, 2008 and July 31, 2007.
| | | | | % | | | | | | % | | % |
| | 2008 | | Margin | | 2007 | | Margin | | Change |
| SALES: | | | | | | | | | | | | | |
| Life Sciences | | $ | 975,231 | | | | $ | 880,187 | | | | | 10.8 |
| Industrial | | | 1,596,414 | | | | | 1,369,718 | | | | | 16.6 |
| Total | | $ | 2,571,645 | | | | $ | 2,249,905 | | | | | 14.3 |
| OPERATING PROFIT: | | | | | | | | | | | | | |
| Life Sciences | | $ | 197,774 | | 20.3 | | $ | 165,286 | | | 18.8 | | 19.7 |
| Industrial | | | 245,855 | | 15.4 | | | 204,114 | | | 14.9 | | 20.4 |
| Total operating profit | | | 443,629 | | 17.3 | | | 369,400 | | | 16.4 | | 20.1 |
| General corporate expenses | | | 53,960 | | | | | 44,718 | | | | | 20.7 |
| Earnings before ROTC, interest expense, net | | | | | | | | | | | | | |
| and income taxes | | | 389,669 | | 15.2 | | | 324,682 | (a) | | 14.4 | | 20.0 |
| ROTC | | | 31,538 | | | | | 25,097 | (a) | | | | |
| Interest expense, net | | | 32,576 | | | | | 39,056 | | | | | |
| Earnings before income taxes | | $ | 325,555 | | | | $ | 260,529 | | | | | |
| (a) | | Included in ROTC, for the purposes of evaluation of segment profitability, are other adjustments recorded in cost of sales of $2,745 for the year ended July 31, 2007. Such adjustments include incremental depreciation and other adjustments recorded primarily in conjunction with the Company’s facilities rationalization initiative. |
32
Life Sciences:
Presented below are Summary Statements of Operating Profit for the Life Sciences segment for the fiscal years ended July 31, 2008 and July 31, 2007:
| | | | | % of | | | | | % of |
| | 2008 | | Sales | | 2007 | | Sales |
| Sales | | $ | 975,231 | | | | $ | 880,187 | | |
| Cost of sales | | | 473,298 | | 48.5 | | | 432,190 | | 49.1 |
| Gross margin | | | 501,933 | | 51.5 | | | 447,997 | | 50.9 |
| SG&A | | | 263,233 | | 27.0 | | | 248,851 | | 28.3 |
| R&D | | | 40,926 | | 4.2 | | | 33,860 | | 3.8 |
| Operating profit | | $ | 197,774 | | 20.3 | | $ | 165,286 | | 18.8 |
The tables below present sales by market and geography within the Life Sciences segment for the fiscal years ended July 31, 2008 and July 31, 2007, including the effect of exchange rates for comparative purposes.
| | | | | | | | | | | | | % |
| | | | | | | | | | Exchange | | Change in |
| | | | | | | | % | | Rate | | Local |
| | 2008 | | 2007 | | Change | | Impact | | Currency |
| By Market | | | | | | | | | | | | | | |
| Medical (a) | | $ | 410,416 | | $ | 400,060 | | 2.6 | | $ | 18,252 | | (2.0 | ) |
| BioPharmaceuticals (a) | | | 564,815 | | | 480,127 | | 17.6 | | | 37,302 | | 9.9 | |
| Total Life Sciences | | $ | 975,231 | | $ | 880,187 | | 10.8 | | $ | 55,554 | | 4.5 | |
| |
| By Geography | | | | | | | | | | | | | | |
| Western Hemisphere | | $ | 379,591 | | $ | 377,301 | | 0.6 | | $ | 1,432 | | 0.2 | |
| Europe | | | 469,450 | | | 391,500 | | 19.9 | | | 44,905 | | 8.4 | |
| Asia | | | 126,190 | | | 111,386 | | 13.3 | | | 9,217 | | 5.0 | |
| Total Life Sciences | | $ | 975,231 | | $ | 880,187 | | 10.8 | | $ | 55,554 | | 4.5 | |
| (a) | | Amounts reflect inclusion of the Laboratory market within the BioPharmaceuticals market effective August 1, 2008. |
Life Sciences segment sales increased 4.5% in fiscal year 2008 compared to fiscal year 2007. Overall, increased pricing in the Medical and BioPharmaceuticals markets contributed 1.8% to sales growth in the year, and as such, the overall volume increase was 2.7%. Life Sciences represented approximately 38% of total sales in fiscal year 2008 compared with 39% in fiscal year 2007.
Within Life Sciences, Medical market sales, which now excludes the Laboratory market and represented approximately 40% of Life Sciences sales, were down 2% reflecting a 5.5% decrease in Blood Filtration, the largest market served by Medical. Increases in the OEM and Hospital markets of 2.8% and 4.5%, respectively, partly mitigated this impact.
The decrease in the Blood Filtration market primarily relates to decreased volume to several major customers as they have transitioned to alternate suppliers at the natural end of their contractual commitments or as a result of a new tender process. This has been partially offset with a price increase. The growth in OEM sales was largely attributable to drug delivery products in France, as well as new business in Scandinavia. The growth in Hospital sales was primarily driven by increased Aquasafe and breathing filter sales in the Western Hemisphere and Asia, specifically in Japan.
33
Sales in the BioPharmaceuticals market, which now includes the Laboratory market, previously reported in Medical, increased 9.9% compared to fiscal year 2007. By geography, growth was led by Europe (+15%), the Company’s largest geographic BioPharmaceuticals market, accompanied by increases in the Western Hemisphere (+3.3%) and Asia (+7.7%). Within BioPharmaceuticals, sales in the Pharmaceuticals market (formerly named BioPharmaceuticals market) grew 10.8% and sales in the Laboratory market increased 5.1%. The increase in the Pharmaceuticals market reflects an increase in systems sales of 41.1% (contributed by all geographies accompanied by growth in consumables sales of 8.0%). The growth in systems sales primarily reflects investment by the biotechnology sector. The growth in consumables sales was attributable to growth in Europe and Asia of 15.3% and 5.4%, respectively, partly offset by a decrease of 4.3% in the Western Hemisphere caused by sales downturns and inventory reduction programs in certain large U.S. biotechnology customers. Overall, key products driving growth are the Company’s virus removal filters for biotechnology and plasma derived therapeutics, its increasing portfolio of single-use processing technologies including the Kleenpak connector, and its Chromatography portfolio for both systems and consumables. Sales growth in the Laboratory market was contributed by all geographies, with Europe the strongest. Key growth drivers in the Laboratory market were new product initiatives in life sciences research and laboratory water.
Life Sciences gross margins increased 60 basis points to 51.5% from 50.9% in fiscal year 2007. The improvement in gross margins was principally driven by improved pricing that contributed approximately 80 basis points in margin, a change in market mix within consumables (46% of total Life Sciences consumable sales were in the higher margin BioPharmaceuticals market compared to 43.5% in fiscal year 2007), an improvement in systems margins and savings generated from cost reduction initiatives which dampened estimated inflationary pressures on manufacturing and related overhead costs. These factors were partly offset by a shift in product mix, to a higher percentage of systems sales (about 5.4% of total Life Sciences sales compared to 3.9% in fiscal year 2007) which are generally at lower margins than consumables.
SG&A expenses in fiscal year 2008 increased by $14,382, or about 6% (almost 1% in local currency), compared to fiscal year 2007. SG&A as a percentage of sales decreased to 27% from 28.3% in fiscal year 2007. The decrease in SG&A as a percentage of sales reflects the impact of the Company’s cost reduction and efficiency initiatives and the leveraging of growth in sales.
R&D expenses were up about 21% (approximately 19% in local currency) at $40,926 compared to $33,860 in fiscal year 2007. As a percentage of sales, R&D expenses were 4.2% compared to 3.8% in fiscal year 2007. Increased spending reflects investments in both Medical and BioPharmaceuticals projects, including prion and cell harvesting.
As a result of the above factors, operating profit dollars increased about 20% to $197,774. In local currency operating profit increased 13% compared to fiscal year 2007. Operating margin improved to 20.3% from 18.8% in fiscal year 2007.
Industrial:
Presented below are summary Statements of Operating Profit for the Industrial segment for the fiscal years ended July 31, 2008 and July 31, 2007:
| | | | | | | % of | | | | | % of |
| | | | 2008 | | Sales | | | 2007 | | Sales |
| Sales | | $ | 1,596,414 | | | | $ | 1,369,718 | | |
| | Cost of sales | | | 887,512 | | 55.6 | | | 755,614 | | 55.2 |
| Gross margin | | | 708,902 | | 44.4 | | | 614,104 | | 44.8 |
| SG&A | | | 432,326 | | 27.1 | | | 381,436 | | 27.8 |
| R&D | | | 30,721 | | 1.9 | | | 28,554 | | 2.1 |
| Operating profit | | $ | 245,855 | | 15.4 | | $ | 204,114 | | 14.9 |
34
The tables below present sales by market and geography within the Industrial segment for the fiscal years ended July 31, 2008 and July 31, 2007, including the effect of exchange rates for comparative purposes.
| | | | | | | | | | | | | | % |
| | | | | | | | | | Exchange | | Change in |
| | | | | | | | % | | Rate | | Local |
| | | 2008 | | | 2007 | | Change | | Impact | | Currency |
| By Market | | | | | | | | | | | | | |
| Energy, Water & | | | | | | | | | | | | | |
| Process Technologies | $ | 981,291 | | $ | 821,957 | | 19.4 | | $ | 72,644 | | 10.6 | |
| Aerospace & | | | | | | | | | | | | | |
| Transportation | | 306,571 | | | 254,675 | | 20.4 | | | 14,498 | | 14.7 | |
| Microelectronics | | 308,552 | | | 293,086 | | 5.3 | | | 16,438 | | (0.3 | ) |
| Total Industrial | $ | 1,596,414 | | $ | 1,369,718 | | 16.6 | | $ | 103,580 | | 9.0 | |
| |
| By Geography | | | | | | | | | | | | | |
| Western Hemisphere | $ | 431,068 | | $ | 398,428 | | 8.2 | | $ | 3,297 | | 7.4 | |
| Europe | | 637,533 | | | 536,094 | | 18.9 | | | 64,388 | | 6.9 | |
| | Asia | | 527,813 | | | 435,196 | | 21.3 | | | 35,895 | | 13.0 | |
| Total Industrial | $ | 1,596,414 | | $ | 1,369,718 | | 16.6 | | $ | 103,580 | | 9.0 | |
Industrial segment sales grew 9% in fiscal year 2008, driven by growth in the EWPT and Aerospace & Transportation markets, while sales in the Microelectronics market were down slightly. Overall, increased pricing contributed about 0.5% to the overall sales growth in the year and as such, the overall volume growth was 8.5%.
Industrial systems sales increased 23.9% compared to fiscal year 2007. All markets in Industrial with the exception of the energy-related market contributed to the growth in systems sales (Municipal Water, Aerospace & Transportation and Food & Beverage markets were the most significant contributors). Industrial consumables sales grew 6.5%, with all markets contributing with the exception of Microelectronics. Industrial represented about 62% of total sales in fiscal year 2008 compared with 61% in fiscal year 2007.
Within the Industrial segment, EWPT market sales, which account for about 61% of the Industrial segment, were up 10.6%, with all markets contributing to this gain.
Municipal Water sales, which are primarily comprised of systems, increased 36.6% compared to fiscal year 2007, generated by strong growth in all geographies. Sales growth in the Western Hemisphere of 24.9% was primarily attributable to increasing numbers of surface water treatment projects driven by government regulations. In Europe, sales growth of 21.8% was driven by surface water treatment in the United Kingdom, sales of leachate treatment systems to developing countries in Eastern Europe and North Africa, and OEM cartridge sales in France and Germany. In Asia, sales increased just over 160% driven by drought-related projects in Australia and sales of leachate treatment systems in various countries in the region.
Sales in the energy-related market increased 7.8% reflecting growth in consumables in all geographies partly offset by a decline in systems sales. The decrease in systems sales reflects a decline in Europe due to particularly strong sales growth last year, partly offset by growth in the Western Hemisphere and Asia. Growth drivers in the energy-related market include alternative energy, including wind and coal gasification.
Food and Beverage sales were up 5.5% reflecting double-digit growth in systems sales (contributed by Europe and Asia) and low-single digit growth in consumables (all geographies contributing). Sales in Europe, the Company’s largest geographic Food & Beverage market, were up about 4%. Sales growth in Europe reflects the sale of a water system in Hungary and a large sale to a brewery in Romania to provide for all their filtration needs. In general, key growth drivers in Europe include sales of OenoFlow product to the wine market, Aria systems for process water and expanding sales to the beer market. In Asia, sales increased in the low double-digit range attributable to the overall growth in this region in various countries. In the Western Hemisphere, sales increased in the low-single digit range.
Sales in the Industrial Manufacturing market increased 10% generated by growth in all geographies, with the most significant growth achieved in Asia. Demand in the metal and mining sectors were key growth drivers.
35
Aerospace & Transportation sales increased 14.7% with all markets contributing to this gain. The growth in Military sales (+15.4%) was primarily driven by strong growth in Asia, where sales increased 72.4% primarily related to a large water systems project with the Australian military. The growth in Military sales in the year also reflects CH-47 helicopter upgrade projects in the Western Hemisphere. The growth in the Commercial portion of this market (+14.5%) primarily reflects strong after-market sales and increased OEM sales generated by an increase in airframe build rates. The increase in the Transportation market (+13.8%) was driven by demand in the construction and mining equipment sectors.
Microelectronics sales decreased slightly as a decline in the Western Hemisphere was partly offset by low single-digit growth in Europe as well as in Asia, the Company’s largest geographic Microelectronics’ market. Overall, the sales decrease in the year reflects tough comparables against the strong growth achieved in fiscal year 2007 and the cyclical downturn in the semiconductor market, which impacted OEM sales. These factors were partly mitigated by sales growth in the thin film rigid disc, inkjet, solar cell and home server markets.
Industrial gross margins decreased 40 basis points to 44.4% from 44.8% in fiscal year 2007. The decrease in gross margins reflects the impact of a shift in product mix (systems sales were 16.8% of total Industrial sales compared to 14.6% in fiscal year 2007) and market mix within consumables, and incremental costs in Europe related to the facilities rationalization initiative. These negative impacts were partially offset by improved profitability of certain systems sales (some of which is related to standardization of systems and product portfolio rationalization), improved pricing that contributed about 20 basis points in margin and the impact of the Company’s manufacturing cost reduction programs, which dampened the estimated inflation of manufacturing costs.
SG&A expenses increased by $50,890, or about 13% (approximately 6% in local currency) compared to fiscal year 2007. The increase in SG&A reflects an increase in selling related costs. SG&A expenses improved to 27.1% as a percentage of sales from 27.8% in fiscal year 2007. The improvement in SG&A as a percentage of sales reflects the impact of cost reduction and efficiency initiatives and the leveraging of growth in sales offset by the impact of increased selling expenses.
R&D expenses were up about 8% (approximately 5% in local currency) at $30,721 compared to $28,554 in fiscal year 2007. As a percentage of sales, R&D expenses were 1.9% compared to 2.1% in fiscal year 2007. Increased spending in dollars reflects investments in new technologies across various markets within Industrial.
As a result of the above factors, operating profit dollars increased about 20% to $245,855. In local currency operating profit increased about 17% compared to fiscal year 2007. Operating margin was 15.4% compared with 14.9% in fiscal year 2007.
Corporate:
Corporate expenses increased by $9,242, or about 21% to $53,960 from $44,718 in fiscal year 2007. The increase in Corporate expenses primarily reflects the addition of tax and treasury function personnel and increased professional fees related to tax and audit services.
Liquidity and Capital Resources
Non-cash working capital, which is defined as working capital excluding cash and cash equivalents, notes receivable, notes payable and the current portion of long-term debt, was approximately $577,900 at July 31, 2009 as compared with $660,000 at July 31, 2008. Based on discussions with various tax authorities, the Company believes it is reasonably possible that the gross amount of unrecognized tax benefits will decrease by approximately $103,650 within the next 12 months. As a result, the Company has reclassified $97,283 from non-current income taxes payable to current income taxes payable and $2,860 of non-current income taxes receivable to current income taxes receivable. In addition, the Company reclassified $65,985 of non-current prepaid income taxes included in other non-current assets as of July 31, 2008 to other current assets as of July 31, 2009 as this amount could be utilized in the resolution of the unrecognized tax benefits. These reclassifications reduced non-cash working capital by $28,438 compared to July 31, 2008. Excluding these reclassifications and the effect of foreign exchange (discussed below), non-cash working capital decreased approximately $19,000 compared to July 31, 2008.
The Company’s balance sheet is affected by spot exchange rates used to translate local currency amounts into U.S. dollars. In comparing spot exchange rates at July 31, 2009 to those at July 31, 2008, the Euro and the British Pound have weakened against the U.S. dollar, while the Japanese Yen has strengthened against the U.S. dollar. The effect of foreign currency translation decreased non-cash working capital by $34,819, including net inventory, net accounts receivable and other current assets by $22,590, $31,908 and $6,614, respectively, as compared to July 31, 2008. Additionally, foreign currency translation decreased accounts payable and other current liabilities by $21,641 and current income taxes payable by $4,652.
36
Net cash provided by operating activities in fiscal year 2009 was $327,495 as compared to $190,806 in fiscal year 2008, an increase of $136,689. Net cash provided by operating activities in fiscal year 2008 reflected a tax payment of $135,000 to the U.S. Internal Revenue Service. Excluding this item, net cash provided by operating activities increased $1,689 reflecting improvements in working capital partly offset by the reduction in net earnings.
Accounts receivable days sales outstanding (“DSO”) at July 31, 2009 improved slightly from July 31, 2008. Inventory turns were 2.6 for the four quarters ended July 31, 2009, on par with the four quarters ended July 31, 2008.
Free cash flow, which is defined as net cash provided by operating activities less capital expenditures, was $194,446 in fiscal year 2009, as compared with $66,952 in fiscal year 2008. The increase in free cash flow reflects the increase in net cash provided by operating activities as discussed above, partly offset by an increase in capital expenditures. The increase in capital expenditures is primarily related to the Company’s facilities rationalization initiative. The Company closed its East Hills, NY headquarters and combined its operations into an existing facility in nearby Port Washington, NY. The Company utilizes free cash flow as one way to measure its current and future financial performance. Company management believes this measure is important because it is a key element of its planning. The following table reconciles net cash provided by operating activities to free cash flow.
| | | | 2009 | | | 2008 | | | 2007 |
| | Net cash provided by operating activities | $ | 327,495 | | $ | 190,806 | | $ | 332,928 |
| Less capital expenditures | | 133,049 | | | 123,854 | | | 97,763 |
| Free cash flow | $ | 194,446 | | $ | 66,952 | | $ | 235,165 |
Overall, net debt (debt net of cash and cash equivalents) as a percentage of total capitalization (net debt plus equity) was 21.4% at July 31, 2009 as compared to 22.1% at July 31, 2008. Net debt decreased by approximately $18,800 compared with July 31, 2008, comprised of a decrease in gross debt of $57,000 partly offset by a decrease in cash and cash equivalents of $21,000. The impact of foreign exchange rates increased net debt by about $17,200. Significant uses of cash in fiscal year 2009 included the acquisition of GeneSystems ($37,249), repurchases of stock ($96,439) and the repayment of approximately $185,000 of foreign debt, which bear higher rates than U.S. borrowing rates, partly offset by borrowings in the U.S. The Company has a loan of Yen 9 billion (approximately $95,121 as of July 31, 2009) which is due on June 20, 2010. The Company was in compliance with all financial covenants of its various debt agreements as of July 31, 2009.
The Company manages certain financial exposures through a risk management program that includes the use of foreign exchange and interest rate derivative financial instruments. Derivatives are executed with counterparties with a minimum credit rating of “A” by Standard and Poors and Moody’s Investor Services, in accordance with the Company’s policies. The Company does not utilize derivative instruments for trading or speculative purposes. The risk management objective of holding a floating-to-fixed interest rate swap is to lock in fixed interest cash outflows on a floating rate debt obligation. The associated risk is created by changes in market interest rates in Japan. The Company has an outstanding Yen loan with variable interest rates based on JPY-LIBOR-BBA. The Company meets the stated risk management objective by holding a floating-to-fixed interest rate swap resulting in a fixed interest cash flow for the Yen loan. The cash flow hedge consists of an interest rate swap with a notional value of Yen 9 billion. Including the impact of this floating-to-fixed interest rate swap, the Company’s ratio of fixed to variable rate debt is 55% to 45%.
The Company conducts transactions in currencies other than their functional currency. These transactions include non-functional intercompany and external sales as well as intercompany and external purchases. The Company uses foreign exchange forward contracts, matching the notional amounts and durations of the receivables and liabilities resulting from the aforementioned underlying foreign currency transactions, to mitigate the exposure to earnings and cash flows caused by changing foreign exchange rates. The risk management objective of holding foreign exchange derivatives is to mitigate volatility to earnings and cash flows due to changes in foreign exchange rates. The notional amount of foreign currency forward contracts entered into during fiscal year 2009 was $707,910. The notional amount of foreign currency forward contracts outstanding as of July 31, 2009 was $152,856. The Company’s foreign currency balance sheet exposures resulted in the recognition within SG&A of a gain of $4,347 in fiscal year 2009, before the impact of the measures described above. Including the impact of the Company’s foreign exchange derivative instruments, the net recognition within SG&A was a gain of approximately $191 in fiscal year 2009.
The Company utilizes cash flow generated from operations and its revolving credit facility to meet its short-term liquidity needs. Company management considers its existing lines of credit, along with the cash typically generated from operations, to be sufficient to meet its short-term liquidity needs.
37
Capital expenditures were $133,049 in fiscal year 2009. Depreciation expense was $79,420 and amortization expense was $10,019 in fiscal year 2009.
On November 15, 2006, the board of directors authorized an expenditure of $250,000 to repurchase shares of the Company’s common stock. At July 31, 2008 there was $199,382 available to be expended under this authorization. On October 16, 2008, the board authorized an additional expenditure of $350,000 to repurchase shares. The Company repurchased stock of $96,439 in fiscal year 2009, and there was $452,943 remaining at July 31, 2009 under the current stock repurchase programs. Net proceeds from stock plans were $15,757 in fiscal year 2009.
In fiscal year 2009, the Company paid dividends of $64,914, an increase of about 8% compared to fiscal year 2008. The Company increased its quarterly dividend by 11.5% from 13 cents to 14.5 cents per share, effective with the dividend declared on January 22, 2009.
The following is a summary of the Company’s contractual payment commitments as of July 31, 2009 (Interest on long-term debt includes the amount of interest due to be paid during the respective fiscal year based upon the amount of debt outstanding as of July 31, 2009):
| Year Ended | | | | |
| | 2010 | | | 2011 | | | 2012 | | | 2013 | | | 2014 | | Thereafter | | Total |
| Long-term debt | $ | 97,432 | | $ | 282,205 | | $ | 1,723 | | $ | 281,707 | | $ | 1,792 | | $ | 10,239 | | $ | 675,098 |
| Interest on long-term debt | | 20,798 | | | 19,171 | | | 17,526 | | | 9,043 | | | 558 | | | 1,354 | | | 68,450 |
| Operating leases | | 21,982 | | | 14,335 | | | 9,922 | | | 6,056 | | | 4,617 | | | 5,587 | | | 62,499 |
| Purchase commitments | | 60,882 | | | 5,585 | | | 4,705 | | | 4,676 | | | 3,495 | | | 2,662 | | | 82,005 |
| Other commitments | | 4,269 | | | 508 | | | 390 | | | 300 | | | 300 | | | 2,724 | | | 8,491 |
| Total commitments | $ | 205,363 | | $ | 321,804 | | $ | 34,266 | | $ | 301,782 | | $ | 10,762 | | $ | 22,566 | | $ | 896,543 |
The Company had gross liabilities for unrecognized tax benefits of approximately $240,683 and related accrued interest of $52,531 as of July 31, 2009, which were excluded from the table above. See Note 12, Income Taxes, to the accompanying consolidated financial statements for further discussion of these amounts.
Adoption of New Accounting Pronouncements
Effective August 1, 2008, the Company adopted, on a prospective basis, certain required provisions of Financial Accounting Standards Board (“FASB”) Statement of Financial Accounting Standards (“SFAS”) No. 157, Fair Value Measurements (“SFAS No. 157”). The provisions not yet adopted by the Company relate to non-financial assets and liabilities that are recognized or disclosed at fair value on a non-recurring basis, as permitted under FASB Staff Position (“FSP”) No. 157-2, Effective Date of FASB Statement No. 157 (“FSP FAS No. 157-2”). Those remaining aspects of SFAS No. 157 for which the effective date was deferred by FSP FAS No. 157-2 are being evaluated by the Company and will be effective for the first quarter of fiscal year 2010. See Note 10, Fair Value Measurements, for the disclosures required under SFAS No. 157.
Effective August 1, 2008, the Company also adopted the provisions of SFAS No. 159, The Fair Value Option for Financial Assets and Financial Liabilities, including an amendment of FASB Statement No. 115 (“SFAS No. 159”). SFAS No. 159 permits entities to elect to measure specified financial instruments and certain other items at fair value with changes in fair value recognized in earnings each reporting period. The Company has not opted to apply the fair value option to any of the applicable financial assets or liabilities.
Effective with the Company’s third quarter of fiscal year 2009, the Company adopted the provisions of SFAS No. 161, Disclosures about Derivative Instruments and Hedging Activities—an amendment of FASB Statement No. 133 (“SFAS No. 161”). SFAS No. 161 requires entities to provide enhanced disclosures about how and why an entity uses derivative instruments, how derivative instruments and related hedged items are accounted for under SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities (“SFAS No. 133”), and its related interpretations, and how derivative instruments and related hedged items affect an entity’s financial position, financial performance, and cash flows. The adoption did not have an impact on the Company’s consolidated financial position and results of operations as it is a disclosure-only standard. See Note 11, Financial Instruments and Risks & Uncertainties, for the disclosures required under SFAS No. 161.
38
Effective with the Company’s fourth quarter of fiscal year 2009, the Company adopted the provisions of SFAS No. 165, Subsequent Events (“SFAS No. 165”). SFAS No. 165 established general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. In particular, SFAS No. 165 sets forth the period after the balance sheet date during which management of a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements, the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements and the disclosures that an entity should make about events or transactions that occurred after the balance sheet date. In addition, an entity is required to disclose the date through which subsequent events have been evaluated. As of September 28, 2009, there were no subsequent events reportable under SFAS No. 165.
Effective with the Company’s fourth quarter of fiscal year 2009, the Company adopted the provisions of FSP No. FAS 115-2 and FAS 124-2, Recognition and Presentation of Other-Than-Temporary Impairments, which modified existing requirements regarding the recognition of other-than-temporary impairments on debt securities. Under the modified guidance, an entity must assess if it (a) has the intent to sell the debt security, or (b) is more likely than not that the entity will be required to sell the debt security before its anticipated recovery (for example, if its cash or working capital requirements or contractual or regulatory obligations indicate that the debt security will be required to be sold before the forecasted recovery occurs). The adoption of FSP No. FAS 115-2 and FAS 124-2 did not have a material impact on the Company’s consolidated financial statements.
Recently Issued Accounting Pronouncements
In December 2007, the FASB issued SFAS No. 141 (revised 2007), Business Combinations (“SFAS No. 141(R)”). SFAS No. 141(R) establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, and any non-controlling interest in the acquiree, recognizes and measures the goodwill acquired in the business combination or a gain from a bargain purchase, and determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. SFAS No. 141(R) is effective for the Company beginning with fiscal year 2010.
In December 2007, the FASB issued SFAS No. 160, Noncontrolling Interests in Consolidated Financial Statements—an amendment of Accounting Research Bulletin No. 51 (“SFAS No. 160”). SFAS No. 160 establishes accounting and reporting standards for ownership interests in subsidiaries held by parties other than the parent, the amount of consolidated net income attributable to the parent and to the noncontrolling interest, changes in a parent’s ownership interest, and the valuation of retained noncontrolling equity investments when a subsidiary is deconsolidated. SFAS No. 160 also establishes disclosure requirements that clearly identify and distinguish between the interests of the parent and the interests of the noncontrolling owners. SFAS No. 160 is effective for the Company beginning with fiscal year 2010.
In April 2008, the FASB issued FSP No. FAS 142-3, Determination of the Useful Life of Intangible Assets (“FSP No. 142-3”). FSP No. 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under SFAS No. 142, Goodwill and Other Intangible Assets. FSP No. 142-3 is effective for the Company beginning with fiscal year 2010. The Company is in the process of assessing the effect FSP No. 142-3 may have on its consolidated financial statements.
In December 2008, the FASB issued FSP No. FAS 132(R)-1, Employers’ Disclosures about Postretirement Benefit Plan Assets (“FSP No. FAS 132(R)-1”) to require employers to provide additional disclosures about plan assets of a defined benefit pension or other post-retirement plan. These disclosures should principally include information detailing investment policies and strategies, the major categories of plan assets, the inputs and valuation techniques used to measure the fair value of plan assets and an understanding of significant concentrations of risk within plan assets. The Company will provide the disclosure required under FSP No. FAS 132(R)-1 beginning with its annual report on Form 10-K for fiscal year 2010. Upon initial application, the provisions of this FSP are not required for earlier periods that are presented for comparative purposes.
In April 2009, the FASB issued FSP No. FAS 107-1 and APB 28-1, Interim Disclosures about Fair Value of Financial Instruments, principally to require publicly traded companies to provide disclosures about fair value of financial instruments in interim financial information. The adoption of this disclosure-only guidance will not have an impact on the Company’s consolidated financial results and is effective beginning with its first quarter of fiscal year 2010.
39
In June 2009, the FASB issued SFAS No. 168, The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles, a replacement of FASB Statement No. 162 (“SFAS No. 168”). SFAS No. 168 establishes the FASB Accounting Standards Codification as the source of authoritative accounting principles recognized by the FASB to be applied in the preparation of financial statements in conformity with GAAP. SFAS No. 168 explicitly recognizes rules and interpretive releases of the SEC under federal securities laws as authoritative GAAP for SEC registrants. SFAS No. 168 is effective beginning with the Company’s first quarter of fiscal year 2010. The Company does not expect that the adoption of Statement No. 168 will have a material effect on the Company’s consolidated financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK.
The Company’s primary market risks relate to adverse changes in foreign currency exchange rates and interest rates. The sensitivity analyses presented below assume simultaneous shifts in each respective rate, and quantify the impact on the Company’s earnings and cash flows. The changes used for these analyses reflect the Company’s view of changes that are reasonably possible over a one-year period. Actual changes that differ from the changes used for these analyses could yield materially different results.
Foreign Currency
The Company’s reporting currency is the U.S. dollar. Because the Company operates through subsidiaries or branches in over thirty countries around the world, its earnings are exposed to translation risk when the financial statements of the subsidiaries or branches, as stated in their functional currencies, are translated into the U.S. dollar. Company management estimates that foreign exchange translation decreased earnings per share by 16 cents in fiscal year 2009.
Most of the Company’s products are manufactured in the U.S., Puerto Rico, Germany and the United Kingdom, and then sold into many countries. The primary foreign currency exposures relate to adverse changes in the relationships of the U.S. dollar to the British Pound (the “Pound”), the Euro, the Japanese Yen (the “Yen”), Swiss Franc, the Australian Dollar, the Canadian Dollar and the Singapore Dollar, as well as adverse changes in the relationship of the Pound to the Euro. Exposure exists when the functional currency of the buying subsidiaries weakens against the U.S. dollar, the Pound or the Euro, thus causing an increase of the product cost to the buying subsidiary or a reduction in the sales price from the selling subsidiary, which adversely affects the Company’s consolidated gross margin and net earnings. The effect of foreign exchange is partially mitigated because of the significant level of manufacturing done in Europe. In fiscal year 2009, the Euro, Pound, Australian Dollar, Canadian Dollar, Swiss Franc and Singapore Dollar weakened by approximately 9.2%, 20.7%, 19.4%, 14.3%, 3.1%, and 3.3%, respectively, against the U.S. dollar compared with the average exchange rates in effect in fiscal year 2008. Additionally, the Yen strengthened by approximately 11.0% against the U.S. dollar and the Euro strengthened against the Pound by approximately 14.5%. Due to the difficulty in estimating the economic effect of foreign currency rates, particularly in periods of high volatility of such rates, Company management does not provide such estimated effects and reports only the translation effect to earnings per share disclosed above.
The Company is also exposed to transaction risk from adverse changes in exchange rates. These short-term transaction exposures are primarily Yen, Euro, Pound and Swiss Franc denominated receivables and payables. These short-term exposures to changing foreign currency exchange rates are managed by opening forward foreign exchange contracts (“forwards”) to offset the earnings and cash flow impact of non-functional currency denominated receivables and payables as well as the expeditious payment of balances. The Company does not enter into forwards for trading purposes. At July 31, 2009, these exposures amounted to approximately $168,741 and were offset by forwards with a notional principal amount of $152,856. If a hypothetical 10% simultaneous adverse change had occurred in exchange rates as of July 31, 2009, net earnings would have decreased by approximately $3,101, or approximately 3 cents per share.
Interest Rates
The Company is exposed to changes in interest rates, primarily due to its financing and cash management activities, which include long and short-term debt as well as cash and certain short-term, highly liquid investments considered to be cash equivalents.
40
The Company’s debt portfolio is comprised of both fixed and variable rate borrowings. The Company manages interest rate exposure by portfolio balancing including employing interest rate swaps. Including the effect of interest rate swaps, the Company’s debt portfolio was approximately 45% variable rate at July 31, 2009, compared to 50% variable rate at July 31, 2008. As of July 31, 2009, the Company had a cash flow interest rate swap (i.e., variable to fixed rate swap) with a notional amount of $95,121. The fair value of the interest rate swap at July 31, 2009 was a liability of $688. The cash flows on the above mentioned interest rate swap mirror the cash flows of the hedged underlying debt instrument. The Company does not enter into interest rate swaps for trading purposes.
For the year ended July 31, 2009, interest expense, net of interest income, was $28,136. A hypothetical 10% shift in market interest rates for fiscal year 2009 (e.g., if an assumed market interest rate of 5.0% increased to 5.5%) could have an adverse affect on interest of approximately $718.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
The financial statements required by this item are located immediately following the signature pages of this Form 10-K. See Item 15(a)(1) for a listing of financial statements provided.
QUARTERLY FINANCIAL INFORMATION (UNAUDITED)
| | (In thousands, | | First | | Second | | Third | | Fourth | | Full | |
| except per share data) | | Quarter | | Quarter | | Quarter | | Quarter | | Year | |
| 2009: | | | | | | | | | | | | | | | | |
| Net sales | | $ | 578,022 | | $ | 543,296 | | $ | 555,883 | | $ | 651,957 | | $ | 2,329,158 | |
| Gross profit | | | 279,391 | | | 256,349 | | | 264,230 | | | 300,720 | | | 1,100,690 | |
| Restructuring and other | | | | | | | | | | | | | | | | |
| charges, net (a) | | | 8,175 | | | 8,747 | | | 8,369 | | | 5,432 | | | 30,723 | |
| Earnings before income taxes | | | 62,351 | | | 56,546 | | | 64,320 | | | 87,569 | | | 270,786 | |
| Net earnings | | | 43,087 | | | 38,871 | | | 44,162 | | | 69,499 | | | 195,619 | |
| Earnings per share: | | | | | | | | | | | | | | | | |
| Basic | | $ | 0.36 | | $ | 0.33 | | $ | 0.37 | | $ | 0.59 | | $ | 1.65 | |
| Diluted | | $ | 0.36 | | $ | 0.33 | | $ | 0.37 | | $ | 0.58 | | $ | 1.64 | |
| | |
| 2008: | | | | | | | | | | | | | | | | |
| Net sales | | $ | 561,007 | | $ | 625,747 | | $ | 661,680 | | $ | 723,211 | | $ | 2,571,645 | |
| Gross profit | | | 261,316 | | | 288,276 | | | 322,966 | | | 338,277 | | | 1,210,835 | |
| | Restructuring and other | | | | | | | | | | | | | | | | |
| charges, net (a) | | | 8,769 | | | 13,859 | | | 5,495 | | | 3,415 | | | 31,538 | |
| Earnings before income taxes | | | 56,944 | | | 69,417 | | | 93,505 | | | 105,689 | | | 325,555 | |
| Net earnings | | | 36,102 | | | 47,988 | | | 63,274 | | | 69,915 | | | 217,279 | |
| Earnings per share: | | | | | | | | | | | | | | | | |
| Basic | | $ | 0.29 | | $ | 0.39 | | $ | 0.51 | | $ | 0.58 | | $ | 1.77 | |
| Diluted | | $ | 0.29 | | $ | 0.39 | | $ | 0.51 | | $ | 0.57 | | $ | 1.76 | |
| (a) | Refer to Note 3, Restructuring and Other Charges, Net, to the accompanying consolidated financial statements. |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTINGAND FINANCIAL DISCLOSURE.
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES.
DISCLOSURE CONTROLS AND PROCEDURES
The Company carried out an evaluation, under the supervision and with the participation of the Company’s management, including the Company’s chief executive officer and chief financial officer, of the effectiveness of the Company’s disclosure controls and procedures as of July 31, 2009. Based on this evaluation, the chief executive officer and chief financial officer concluded that the Company’s disclosure controls and procedures were effective as of such date to ensure that information required to be disclosed in the reports that it files or submits under the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms.
41
INTERNAL CONTROL OVER FINANCIAL REPORTING
(a)Management’s annual report on internal control over financial reporting.
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities and Exchange Act of 1934. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. The Company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework inInternal Control–Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, management concluded that the Company’s internal control over financial reporting was effective as of July 31, 2009.
42
(b)Attestation report of the registered public accounting firm.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Pall Corporation:
We have audited Pall Corporation and subsidiaries’ internal control over financial reporting as of July 31, 2009, based on criteria established inInternal Control–Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Pall Corporation’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s report on internal control over financial reporting (Item 9A(a)). Our responsibility is to express an opinion on the effectiveness of the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Pall Corporation and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of July 31, 2009, based on criteria established inInternal Control–Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Pall Corporation and subsidiaries as of July 31, 2009 and 2008, and the related consolidated statements of earnings, stockholders’ equity, and cash flows for each of the years in the three-year period ended July 31, 2009 and our report dated September 28, 2009 expressed an unqualified opinion on those consolidated financial statements.
Melville, New York
September 28, 2009
43
(c)Changes in internal control over financial reporting.
There are some changes to the company’s controls and procedures which, taken together, are expected to have a favorable impact on the Company’s controls over a multi-year period. There are a number of significant business improvement initiatives designed to improve processes and enhance customer and supplier relationships and opportunities. These include information systems upgrades and integrations that are in various phases of planning or implementation and contemplate enhancements of ongoing activities to support the growth of the Company’s financial shared service capabilities and standardization of its financial systems. The Company is employing a project management and phased implementation approach that will provide continued monitoring and assessment in order to maintain the effectiveness of internal control over financial reporting during and subsequent to implementation of these initiatives.
In connection with the business improvement initiatives discussed above, during the second, third and fourth quarters of fiscal year 2009, certain subsidiaries in the Western Hemisphere implemented a modified procurement-to-payment process, which includes a new procurement-to-payment system.
Except as noted above, there have been no changes in the Company’s internal control over financial reporting during the fourth quarter of fiscal year 2009 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
ITEM 9B. OTHER INFORMATION.
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
(a) Identification of directors and corporate governance:
Information required by this item is included in the Proxy Statement under the captions “Proposal 1 - Election of Directors,” “Structure and Practices of the Board” and “Section 16(a) Beneficial Ownership Reporting Compliance” and is incorporated by reference in this report.
(b) Identification of executive officers:
Information regarding executive officers is contained in Part I, Item 4 of this report, pursuant to General Instruction G of this form.
* * *
The Company has adopted a code of ethics applicable to its chief executive officer, chief financial officer, controller and other employees with important roles in the financial reporting process. The code of ethics is available on the Company’s website located at www.pall.com/policies. In addition, the Company will provide to any person, without charge, upon request, a copy of the code of ethics, by addressing your request in writing to the Corporate Compliance and Ethics Officer, Pall Corporation, 25 Harbor Park Drive, Port Washington, NY, 11050.
The Company intends to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding an amendment to, or waiver from, a provision of this code of ethics by posting such information on the website specified above.
ITEM 11. EXECUTIVE COMPENSATION.
The information required by this item is included in the Proxy Statement under the caption “Executive Compensation,” “Director Compensation for Fiscal Year 2009” and “Structure and Practices of the Board–Board Committees” and is incorporated by reference in this report.
44
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT ANDRELATED STOCKHOLDER MATTERS.
The information required by this item is included in the Proxy Statement under the captions “Beneficial Ownership of Common Stock and Restricted Stock Units” and “Executive Compensation–Equity Compensation Plans,” and is incorporated by reference in this report.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTORINDEPENDENCE.
The information required by this item is included in the Proxy Statement under the captions “Proposal 1 – Election of Directors,” “Structure and Practices of the Board,” “Policies and Procedures for Related Person Transactions” and “Related Person Transactions,” and is incorporated by reference in this report.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
The information required by this item is included in the Proxy Statement under the captions “Audit and Non-Audit Fees” and “Policy on Audit Committee Pre-Approval of Audit and Permitted Non-Audit Services,” and is incorporated by reference in this report.
45
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
| (a) Documents filed as part of the Form 10-K: |
| | |
| (1) The following items are filed as part of this report: |
| | Report of Independent Registered Public Accounting Firm |
| Consolidated Balance Sheets – July 31, 2009 and July 31, 2008 |
| Consolidated Statements of Earnings – years ended July 31, 2009, July 31, 2008 and July 31, 2007 |
| Consolidated Statements of Stockholders’ Equity – years ended July 31, 2009, July 31, 2008 and July 31, 2007 |
| Consolidated Statements of Cash Flows – years ended July 31, 2009, July 31, 2008 and July 31, 2007 |
| Notes to consolidated financial statements |
| |
| (2) The following financial statement schedule is filed as part of this report: |
| Schedule II – Valuation and Qualifying Accounts |
| |
| All other schedules are omitted because they are not applicable or the required information is shown in the financial statements or in the notes thereto. |
| |
| (3) Exhibits: |
| The exhibits listed in the accompanying Exhibit Index are filed or incorporated by reference as part of this report. |
Exhibit Index
| Exhibit | | |
| Number | | Description of Exhibit | |
| 3.1(i)* | | Restated Certificate of Incorporation of the Registrant as amended through November 23, 1993, filed as Exhibit 3(i) to the Registrant’s 1994 Form 10-K. |
| |
| 3.1(ii)* | | By-Laws of the Registrant as amended through July 16, 2009, filed as Exhibit 3(ii) to the Registrant’s Form 8-K filed on July 22, 2009. |
| |
| 4.1(i)* | | Indenture dated as of August 1, 2002, by and among the Registrant, as Issuer, the Guarantors named therein, as Guarantors, and The Bank of New York, as Trustee, relating to the Registrant’s 6% Senior Notes due August 1, 2012 filed as Exhibit 4(iii) to the Registrant’s 2002 Form 10-K. |
| |
| 4.1(ii)* | | First Supplemental Indenture, dated as of October 9, 2007, to the Indenture, dated as of August 1, 2002, by and among the Registrant, the guarantors named therein and The Bank of New York, as trustee, filed as Exhibit 4.1 to the Registrant’s Form 8-K filed on October 11, 2007. |
| |
| 4.2(i)* | | Rights Agreement dated as of November 17, 1989, between the Registrant and United States Trust Company of New York, as Rights Agent, filed as an Exhibit to the Registrant’s Form 8-A filed on September 10, 1992. |
| |
| 4.2(ii)* | | Amendment No. 1, dated as of April 20, 1999, to the Rights Agreement dated as of November 17, 1989, between the Registrant and United States Trust Company of New York, as Rights Agent, filed as Exhibit II to the Registrant’s Form 8-A/A filed on April 22, 1999. |
| |
| | The exhibits filed herewith do not include other instruments with respect to long-term debt of the Registrant and its subsidiaries, inasmuch as the total amount of debt authorized under any such instrument does not exceed 10% of the total assets of the Registrant and its subsidiaries on a consolidated basis. The Registrant agrees, pursuant to Item 601(b) (4) (iii) of Regulation S-K, that it will furnish a copy of any such instrument to the Securities and Exchange Commission upon request. |
| |
| 10.1(i)* | | Credit Agreement dated June 21, 2006, between the Registrant and JPMorgan Chase Bank and the Other Lenders Party Thereto, filed as Exhibit 4(ii) to the Registrant’s Form 8-K filed on June 27, 2006. |
46
| 10.1(ii)* | | First Amendment and Waiver, dated as of August 16, 2007 to the Five-Year Credit Agreement, dated as of June 21, 2006, among Pall Corporation, the subsidiaries of the Company named on the signature pages thereto, the lenders from time to time party thereto, JPMorgan Chase Bank, N.A., as facility agent for the Lenders, and J.P. Morgan Europe Limited, as London agent for the Lenders, filed as Exhibit 10 to the Registrant’s Form 8-K filed on August 20, 2007. |
| |
| 10.1(iii)* | | Second Amendment and Waiver, dated as of December 7, 2007 to the Five-Year Credit Agreement, dated as of June 21, 2006, among the Registrant, the subsidiaries of the Registrant named on the signature pages thereto, the lenders from time to time party thereto, JPMorgan Chase Bank, N.A., as facility agent for the Lenders, and J.P. Morgan Europe Limited, as London agent for the Lenders, filed as Exhibit 10 to the Registrant’s Form 8-K filed on December 11, 2007. |
| |
| 10.1(iv)* | | Third Amendment and Waiver, dated as of March 25, 2008 to the Five-Year Credit Agreement, dated as of June 21, 2006, among the Registrant, the subsidiaries of the Registrant named on the signature pages thereto, the lenders from time to time party thereto, JPMorgan Chase Bank, N.A., as facility agent for the Lenders, and J.P. Morgan Europe Limited, as London agent for the Lenders, filed as Exhibit 10.1 (iv) to the Registrant’s 2007 Form 10-K. |
| |
| 10.2*‡ | | Employment Agreement dated January 21, 2004, as amended and restated effective July 20, 2005, between the Registrant and Eric Krasnoff, filed as Exhibit 10.5 to the Registrant’s Form 8-K filed on July 25, 2005. |
| |
| 10.3*‡ | | Amendment dated May 3, 2006 to Employment Agreement dated January 21, 2004, as amended and restated effective July 20, 2005, between the Registrant and Eric Krasnoff, filed as Exhibit 10.28 to the Registrant’s 2006 Form 10-K. |
| |
| 10.4*‡ | | Amendment dated July 18, 2006 to Employment Agreement dated January 21, 2004, as amended and restated effective July 20, 2005, between the Registrant and Eric Krasnoff, filed as Exhibit 10.29 to the Registrant’s 2006 Form 10-K. |
| |
| 10.5*‡ | | Amendment to Employment Agreement effective December 31, 2008 between the Company and Eric Krasnoff, filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2009. |
| |
| 10.6*‡ | | Employment Agreement dated April 24, 2008, between the Registrant and Donald B. Stevens, filed as Exhibit 10 to the Registrant’s Form 8-K filed on April 28, 2008. |
| | |
| 10.7*‡(a) | | Loan Agreement between the Company and Donald Stevens, effective on or about May 27, 2005, filed as Exhibit 10.5 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2009. |
| | |
| 10.8*‡ | | Employment Agreement dated September 12, 2005 between the Registrant and Roberto Perez, filed as Exhibit 10.7 to the 2006 Form 10-K. |
| | |
| 10.9*‡ | | Amendment dated May 3, 2006 to Employment Agreement dated September 12, 2005, between the Registrant and Roberto Perez, filed as Exhibit 10.36 to the Registrant’s 2006 Form 10-K. |
| | |
| 10.10*‡ | | Amendment dated July 18, 2006 to Employment Agreement dated September 12, 2005, between the Registrant and Roberto Perez, filed as Exhibit 10.37 to the Registrant’s 2006 Form 10-K. |
| | |
| 10.11*‡ | | Amendment to Employment Agreement effective December 31, 2008 between the Company and Roberto Perez, filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2009. |
| | |
| 10.12*‡(a) | | Mortgage Note by Roberto Perez and Astrid Perez in favor of the Company, dated March 2000, filed as Exhibit 10.6 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2009. |
| | |
| 10.13*‡ | | Employment Agreement dated June 1, 2004 between the Registrant and Lisa McDermott, filed as Exhibit 10.11 to the Registrant’s 2005 Form 10-K. |
| | |
| 10.14*‡ | | Amendment dated May 3, 2006 to Employment Agreement dated June 1, 2004 between the Registrant and Lisa McDermott, filed as Exhibit 10.38 to the Registrant’s 2006 Form 10-K. |
| | |
| 10.15*‡ | | Amendment dated July 18, 2006 to Employment Agreement dated June 1, 2004 between the Registrant and Lisa McDermott, filed as Exhibit 10.39 to the Registrant’s 2006 Form 10-K. |
47
| 10.16*‡ | | Amendment to Employment Agreement effective December 31, 2008 between the Company and Lisa McDermott, filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2009. |
| | |
| 10.17*‡ | | Pall Corporation Supplementary Pension Plan, effective December 31, 2008, filed as Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2009. |
| | |
| 10.18*‡ | | Pall Corporation Supplementary Profit-Sharing Plan as amended effective July 19, 2005, filed as Exhibit 10.3 to the Registrant’s Form 8-K filed on July 25, 2005. |
| | |
| 10.19*‡ | | Pall Corporation Profit-Sharing Plan as amended and restated as of July 1, 1998, filed as Exhibit 10.15 to the Registrant’s 2002 Form 10-K. |
| | |
| 10.20*‡ | | Pall Corporation Profit-Sharing Plan amended pursuant to provisions of the Economic Growth and Tax Relief Reconciliation Act of 2001, filed as Exhibit 10.17 to the Registrant’s 2003 Form 10-K. |
| | |
| 10.21*‡ | | Pall Corporation 2004 Executive Incentive Bonus Plan, as amended effective January 18, 2006, filed as Exhibit 10.15 to the Registrant’s 2006 Form 10-K. |
| | |
| 10.22*‡ | | Pall Corporation 1991 Stock Option Plan, as amended effective April 17, 2002, filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended April 27, 2002. |
| | |
| 10.23*‡ | | Pall Corporation 1993 Stock Option Plan, as amended effective April 17, 2002, filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended April 27, 2002. |
| | |
| 10.24*‡ | | Pall Corporation 1995 Stock Option Plan, as amended effective April 17, 2002, filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended April 27, 2002. |
| | |
| 10.25*‡ | | Pall Corporation 1998 Stock Option Plan, as amended effective April 17, 2002, filed as Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended April 27, 2002. |
| | |
| 10.26*‡ | | Form of Notice of Grant of Restricted Stock Units Under Pall Corporation 2005 Stock Compensation Plan, filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2005. |
| | |
| 10.27*‡ | | Form of Notice of Grant of Annual Award Units Under Pall Corporation 2005 Stock Compensation Plan, filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2005. |
| | |
| 10.28*‡ | | Form of Notice of Grant of Stock Option Grant Agreement Under Pall Corporation 2005 Stock Compensation Plan, filed as Exhibit 10.20 to the Registrant’s 2007 Form 10-K. |
| | |
| 10.29*‡ | | Pall Corporation 2005 Stock Compensation Plan, as amended effective January 19, 2006, filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2006. |
| | |
| 10.30*‡ | | Pall Corporation Stock Option Plan for Non-Employee Directors, as amended effective November 19, 1998, filed as Exhibit 10.5 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended October 31, 1998. |
| | |
| 10.31*‡ | | Pall Corporation 2001 Stock Option Plan for Non-Employee Directors, as amended September 17, 2004, filed as Exhibit 10.25 to the Registrant’s 2004 Form 10-K. |
| | |
| 10.32*‡ | | Pall Corporation Management Stock Purchase Plan as amended effective July 19, 2005, filed as Exhibit 10.4 to the Registrant’s Form 8-K filed on July 25, 2005. |
| | |
| 10.33*‡ | | Pall Corporation Employee Stock Purchase Plan as amended effective October 17, 2003, filed as Exhibit 10.27 to the Registrant’s 2003 Form 10-K. |
| | |
| 10.34*‡ | | Principal Rules of the Pall Supplementary Pension Scheme, filed as Exhibit 10.25 to the Registrant’s 1995 Form 10-K. |
48
| 14* | | Pall Corporation Code of Ethics applicable to its Chief Executive Officer, Chief Financial Officer, Controller and other employees with important roles in the financial reporting process, filed as Exhibit 99.1 to the Registrant’s 2004 Form 10-K. |
| |
| 21† | | Subsidiaries of the Registrant. |
| |
| 23† | | Consent of Independent Registered Public Accounting Firm. |
| |
| 31.1† | | Certification of Chief Executive Officer pursuant to section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 31.2† | | Certification of Chief Financial Officer pursuant to section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.1† | | Certification of Chief Executive Officer furnished pursuant to 18 U.S.C. Section 1350 as adopted pursuant to section 906 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.2† | | Certification of Chief Financial Officer furnished pursuant to 18 U.S.C. Section 1350 as adopted pursuant to section 906 of the Sarbanes-Oxley Act of 2002. |
| * | | Incorporated herein by reference. The Registrant’s SEC file number is 001- 04311. |
| | | |
| † | | Filed herewith. |
| | | |
| ‡ | | Denotes management contract or compensatory plan or arrangement. |
| | | |
| (a) | | Confidential treatment has been granted for certain information contained in the document. Such information has been omitted and filed separately with the Securities and Exchange Commission. |
49
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | Pall Corporation |
| |
| September 28, 2009 | By: | /s/ | LISA MCDERMOTT |
| | | Lisa McDermott, |
| | | Chief Financial Officer and Treasurer |
| |
| | /s/ | FRANCIS MOSCHELLA |
| | | Francis Moschella, |
| | | Vice President – Corporate Controller |
| | | Chief Accounting Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| /s/ | ERIC KRASNOFF | | Chairman of the Board and | | September 28, 2009 |
| Eric Krasnoff | | Chief Executive Officer | | |
| |
| /s/ | LISA MCDERMOTT | | Chief Financial Officer and Treasurer | | September 28, 2009 |
| Lisa McDermott | | | | |
| |
| /s/ | FRANCIS MOSCHELLA | | Vice President – Corporate Controller | | September 28, 2009 |
| Francis Moschella | | Chief Accounting Officer | | |
| |
| /s/ | DANIEL J. CARROLL, JR. | | Director | | September 28, 2009 |
| Daniel J. Carroll, Jr. | | | | |
| | |
| /s/ | ROBERT B. COUTTS | | Director | | September 28, 2009 |
| Robert B. Coutts | | | | |
| |
| /s/ | CHERYL W. GRISÉ | | Director | | September 28, 2009 |
| Cheryl W. Grisé | | | | |
| |
| /s/ | ULRIC S. HAYNES, JR. | | Director | | September 28, 2009 |
| Ulric S. Haynes, Jr. | | | | |
| |
| /s/ | RONALD HOFFMAN | | Director | | September 28, 2009 |
| Ronald Hoffman | | | | |
| |
| /s/ | DENNIS N. LONGSTREET | | Director | | September 28, 2009 |
| Dennis N. Longstreet | | | | |
| |
| /s/ | EDWIN W. MARTIN, JR. | | Director | | September 28, 2009 |
| Edwin W. Martin, Jr. | | | | |
| |
| /s/ | KATHARINE L. PLOURDE | | Director | | September 28, 2009 |
| Katharine L. Plourde | | | | |
| |
| /s/ | HEYWOOD SHELLEY | | Director | | September 28, 2009 |
| Heywood Shelley | | | | |
| |
| /s/ | EDWARD L. SNYDER | | Director | | September 28, 2009 |
| Edward L. Snyder | | | | |
| |
| /s/ | EDWARD TRAVAGLIANTI | | Director | | September 28, 2009 |
| Edward Travaglianti | | | | |
50
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Pall Corporation:
We have audited the accompanying consolidated balance sheets of Pall Corporation and subsidiaries as of July 31, 2009 and 2008, and the related consolidated statements of earnings, stockholders’ equity, and cash flows for each of the years in the three-year period ended July 31, 2009. In connection with our audits of the consolidated financial statements, we also have audited the accompanying financial statement schedule. These consolidated financial statements and the financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and the financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Pall Corporation and subsidiaries as of July 31, 2009 and 2008, and the results of their operations and their cash flows for each of the years in the three-year period ended July 31, 2009, in conformity with U.S. generally accepted accounting principles. Also in our opinion, the related financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Pall Corporation and subsidiaries’ internal control over financial reporting as of July 31, 2009, based on criteria established inInternal Control–Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated September 28, 2009 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
As discussed in the notes to the consolidated financial statements, effective August 1, 2008, the Company changed its method of accounting for fair value measurements due to the adoption of Statement of Financial Accounting Standards No. 157, “Fair Value Measurements”, effective August 1, 2007, the Company changed its method of accounting for uncertainty in income taxes due to the adoption of Financial Accounting Standards Board Interpretation No. 48, “Accounting for Uncertainty in Income Taxes”, effective July 31, 2007, the Company changed its method of accounting for pension and other postretirement benefits due to the adoption of Statement of Financial Accounting Standards No. 158, “Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans”, and effective August 1, 2006, the Company changed its method for quantifying errors based on Securities and Exchange Commission Staff Accounting Bulletin No. 108, “Considering the Effects of Prior Year Misstatements When Quantifying Misstatements in Current Year Financial Statements”.
Melville, New York
September 28, 2009
51
PALL CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(In thousands, except per share data)
| July 31, 2009 | | July 31, 2008 |
| ASSETS | | | | | | | |
| Current assets: | | | | | | | |
| Cash and cash equivalents | $ | 414,011 | | | $ | 454,065 | |
| Accounts receivable | | 561,063 | | | | 617,079 | |
| Inventories | | 413,278 | | | | 492,977 | |
| Other current assets | | 182,098 | | | | 95,518 | |
| Total current assets | | 1,570,450 | | | | 1,659,639 | |
| Property, plant and equipment | | 681,658 | | | | 662,985 | |
| Goodwill | | 282,777 | | | | 265,893 | |
| Intangible assets | | 63,751 | | | | 46,204 | |
| Other non-current assets | | 242,176 | | | | 322,025 | |
| Total assets | $ | 2,840,812 | | | $ | 2,956,746 | |
| |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | |
| Current liabilities: | | | | | | | |
| Notes payable | $ | 42,371 | | | $ | 26,062 | |
| Accounts payable | | 171,956 | | | | 200,744 | |
| Accrued liabilities | | 250,838 | | | | 270,522 | |
| Income taxes payable | | 137,846 | | | | 57,882 | |
| Current portion of long-term debt | | 97,432 | | | | 3,252 | |
| Dividends payable | | 16,947 | | | | 15,501 | |
| Total current liabilities | | 717,390 | | | | 573,963 | |
| Long-term debt, net of current portion | | 577,666 | | | | 747,051 | |
| Income taxes payable – non-current | | 133,919 | | | | 233,420 | |
| Deferred income taxes | | 9,293 | | | | 10,979 | |
| Other non-current liabilities | | 287,946 | | | | 252,098 | |
| Total liabilities | | 1,726,214 | | | | 1,817,511 | |
| |
| Stockholders’ equity: | | | | | | | |
| Common stock, par value $.10 per share; 500,000 shares | | | | | | | |
| authorized; 127,958 shares issued | | 12,796 | | | | 12,796 | |
| Capital in excess of par value | | 197,759 | | | | 178,608 | |
| Retained earnings | | 1,237,735 | | | | 1,118,616 | |
| Treasury stock, at cost (2009 - 11,083 shares, 2008 - 8,717 shares) | | (354,274 | ) | | | (290,508 | ) |
| Stock option loans | | (435 | ) | | | (450 | ) |
| Accumulated other comprehensive income/(loss): | | | | | | | |
| Foreign currency translation | | 127,015 | | | | 179,429 | |
| Pension liability adjustment | | (108,977 | ) | | | (61,322 | ) |
| Unrealized investment gains | | 3,423 | | | | 2,343 | |
| Unrealized losses on derivatives | | (444 | ) | | | (277 | ) |
| | | 21,017 | | | | 120,173 | |
| Total stockholders’ equity | | 1,114,598 | | | | 1,139,235 | |
| Total liabilities and stockholders’ equity | $ | 2,840,812 | | | $ | 2,956,746 | |
See accompanying notes to consolidated financial statements.
52
PALL CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EARNINGS
(In thousands, except per share data)
| Years Ended |
| July 31, 2009 | | July 31, 2008 | | July 31, 2007 |
| Net sales | $ | 2,329,158 | | $ | 2,571,645 | | $ | 2,249,905 |
| Cost of sales | | 1,228,468 | | | 1,360,810 | | | 1,190,549 |
| Gross profit | | 1,100,690 | | | 1,210,835 | | | 1,059,356 |
| |
| Selling, general and administrative expenses | | 699,832 | | | 749,519 | | | 675,005 |
| Research and development | | 71,213 | | | 71,647 | | | 62,414 |
| Restructuring and other charges, net | | 30,723 | | | 31,538 | | | 22,352 |
| Interest expense, net | | 28,136 | | | 32,576 | | | 39,056 |
| Earnings before income taxes | | 270,786 | | | 325,555 | | | 260,529 |
| Provision for income taxes | | 75,167 | | | 108,276 | | | 133,032 |
| |
| Net earnings | $ | 195,619 | | $ | 217,279 | | $ | 127,497 |
| |
| Earnings per share: | | | | | | | | |
| Basic | $ | 1.65 | | $ | 1.77 | | $ | 1.04 |
| Diluted | $ | 1.64 | | $ | 1.76 | | $ | 1.02 |
| |
| Average shares outstanding: | | | | | | | | |
| Basic | | 118,631 | | | 122,445 | | | 123,115 |
| Diluted | | 119,571 | | | 123,686 | | | 124,393 |
See accompanying notes to consolidated financial statements.
53
PALL CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands)
| | | | Capital | | | | | | | | Accumulated | | | | |
| | | | in Excess | | | | | | Stock | | Other | | | | |
| Years Ended July 31, 2007, July 31, 2008 | | Common | | of Par | | Retained | | Treasury | | Option | | Comprehensive | | | | Comprehensive |
| and July 31, 2009 | | Stock | | Value | | Earnings | | Stock | | Loans | | Income/(Loss) | | Total | | Income |
| Balance at August 1, 2006 | | $ | 12,796 | | $ | 137,165 | | | $ | 909,810 | | | $ | (156,775 | ) | | $ | (1,311 | ) | | $ | 35,412 | | | $ | 937,097 | | | | | |
| Impact of adoption of SAB No. 108 (Note 14) | | | | | | | | | | (5,957 | ) | | | | | | | | | | | | | | | (5,957 | ) | | | | |
| Balance at August 1, 2006, as adjusted | | | 12,796 | | | 137,165 | | | | 903,853 | | | | (156,775 | ) | | | (1,311 | ) | | | 35,412 | | | | 931,140 | | | | | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net earnings | | | | | | | | | | 127,497 | | | | | | | | | | | | | | | | 127,497 | | | $ | 127,497 | |
| Other comprehensive income/(loss): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation | | | | | | | | | | | | | | | | | | | | | | 35,923 | | | | 35,923 | | | | 35,923 | |
| Minimum pension liability | | | | | | | | | | | | | | | | | | | | | | 26,325 | | | | 26,325 | | | | 26,325 | |
| Unrealized investment gains | | | | | | | | | | | | | | | | | | | | | | 852 | | | | 852 | | | | 852 | |
| Unrealized gain on derivatives | | | | | | | | | | | | | | | | | | | | | | 138 | | | | 138 | | | | 138 | |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | $ | 190,735 | |
| Adjustment to initially adopt the provisions of | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| SFAS No. 158 (net of tax) | | | | | | | | | | | | | | | | | | | | | | (20,277 | ) | | | (20,277 | ) | | | | |
| Dividends declared | | | | | | | | | | (43,345 | ) | | | | | | | | | | | | | | | (43,345 | ) | | | | |
| Issuance of 1,973 shares for stock plans | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| and tax benefit related to stock plans | | | | | | 5,852 | | | | (13,060 | ) | | | 54,116 | | | | | | | | | | | | 46,908 | | | | | |
| Restricted stock units related to | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| stock plans | | | | | | 2,481 | | | | | | | | | | | | | | | | | | | | 2,481 | | | | | |
| Stock based compensation expense | | | | | | 14,122 | | | | | | | | | | | | | | | | | | | | 14,122 | | | | | |
| Purchase of 1,585 shares | | | | | | | | | | | | | | (61,795 | ) | | | | | | | | | | | (61,795 | ) | | | | |
| Stock option loans | | | | | | | | | | | | | | | | | | 632 | | | | | | | | 632 | | | | | |
| Balance at July 31, 2007 | | | 12,796 | | | 159,620 | | | | 974,945 | | | | (164,454 | ) | | | (679 | ) | | | 78,373 | | | | 1,060,601 | | | | | |
| Impact of adoption of FIN No. 48 | | | | | | | | | | 5,570 | | | | | | | | | | | | | | | | 5,570 | | | | | |
| Balance at August 1, 2007 | | | 12,796 | | | 159,620 | | | | 980,515 | | | | (164,454 | ) | | | (679 | ) | | | 78,373 | | | | 1,066,171 | | | | | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net earnings | | | | | | | | | | 217,279 | | | | | | | | | | | | | | | | 217,279 | | | $ | 217,279 | |
| Other comprehensive income/(loss): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation | | | | | | | | | | | | | | | | | | | | | | 36,738 | | | | 36,738 | | | | 36,738 | |
| Pension liability adjustment | | | | | | | | | | | | | | | | | | | | | | 5,714 | | | | 5,714 | | | | 5,714 | |
| Unrealized investment losses | | | | | | | | | | | | | | | | | | | | | | (458 | ) | | | (458 | ) | | | (458 | ) |
| Unrealized loss on derivatives | | | | | | | | | | | | | | | | | | | | | | (194 | ) | | | (194 | ) | | | (194 | ) |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | $ | 259,079 | |
| Dividends declared | | | | | | | | | | (76,407 | ) | | | | | | | | | | | | | | | (76,407 | ) | | | | |
| Issuance of 751 shares for stock plans and tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| benefit related to stock plans | | | | | | (2,058 | ) | | | (2,771 | ) | | | 22,796 | | | | | | | | | | | | 17,967 | | | | | |
| Restricted stock units related to stock plans | | | | | | 4,987 | | | | | | | | | | | | | | | | | | | | 4,987 | | | | | |
| Stock based compensation expense | | | | | | 16,059 | | | | | | | | | | | | | | | | | | | | 16,059 | | | | | |
| Purchase of 4,056 shares | | | | | | | | | | | | | | (148,850 | ) | | | | | | | | | | | (148,850 | ) | | | | |
| Stock option loans | | | | | | | | | | | | | | | | | | 229 | | | | | | | | 229 | | | | | |
| Balance at July 31, 2008 | | | 12,796 | | | 178,608 | | | | 1,118,616 | | | | (290,508 | ) | | | (450 | ) | | | 120,173 | | | | 1,139,235 | | | | | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net earnings | | | | | | | | | | 195,619 | | | | | | | | | | | | | | | | 195,619 | | | $ | 195,619 | |
| Other comprehensive income/(loss): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation | | | | | | | | | | | | | | | | | | | | | | (52,414 | ) | | | (52,414 | ) | | | (52,414 | ) |
| Pension liability adjustment | | | | | | | | | | | | | | | | | | | | | | (47,655 | ) | | | (47,655 | ) | | | (47,655 | ) |
| Unrealized investment gains | | | | | | | | | | | | | | | | | | | | | | 1,080 | | | | 1,080 | | | | 1,080 | |
| Unrealized loss on derivatives | | | | | | | | | | | | | | | | | | | | | | (167 | ) | | | (167 | ) | | | (167 | ) |
| Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | $ | 96,463 | |
| Dividends declared | | | | | | | | | | (67,523 | ) | | | | | | | | | | | | | | | (67,523 | ) | | | | |
| Issuance of 981 shares for stock plans and tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| benefit related to stock plans | | | | | | (10,872 | ) | | | (8,977 | ) | | | 32,673 | | | | | | | | | | | | 12,824 | | | | | |
| Restricted stock units related to stock plans | | | | | | 6,539 | | | | | | | | | | | | | | | | | | | | 6,539 | | | | | |
| Stock based compensation expense | | | | | | 23,484 | | | | | | | | | | | | | | | | | | | | 23,484 | | | | | |
| Purchase of 3,347 shares | | | | | | | | | | | | | | (96,439 | ) | | | | | | | | | | | (96,439 | ) | | | | |
| Stock option loans | | | | | | | | | | | | | | | | | | 15 | | | | | | | | 15 | | | | | |
| Balance at July 31, 2009 | | $ | 12,796 | | $ | 197,759 | | | $ | 1,237,735 | | | $ | (354,274 | ) | | $ | (435 | ) | | $ | 21,017 | | | $ | 1,114,598 | | | | | |
See accompanying notes to consolidated financial statements.
54
PALL CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | Years Ended | | |
| July 31, 2009 | | July 31, 2008 | | July 31, 2007 |
| Operating activities: | | | | | | | | | | | |
| Net earnings | $ | 195,619 | | | $ | 217,279 | | | $ | 127,497 | |
| Adjustments to reconcile net earnings to net cash provided | | | | | | | | | | | |
| by operating activities: | | | | | | | | | | | |
| Restructuring and other charges, net | | 4,317 | | | | 1,721 | | | | 21,235 | |
| Depreciation and amortization of long-lived assets | | 89,439 | | | | 93,205 | | | | 93,977 | |
| Non-cash stock compensation | | 23,484 | | | | 16,059 | | | | 14,122 | |
| Excess tax benefits from stock based compensation | | | | | | | | | | | |
| arrangements | | (457 | ) | | | (1,802 | ) | | | (7,929 | ) |
| Amortization of deferred revenue | | (2,154 | ) | | | (2,154 | ) | | | (2,154 | ) |
| Deferred income taxes | | (10,642 | ) | | | (19,374 | ) | | | 1,891 | |
| Provisions for doubtful accounts | | 2,864 | | | | 2,544 | | | | 2,924 | |
| Other | | (355 | ) | | | (27 | ) | | | (27 | ) |
| Changes in operating assets and liabilities, net of | | | | | | | | | | | |
| effects of acquisitions and dispositions: | | | | | | | | | | | |
| Inventories | | 57,147 | | | | (4,996 | ) | | | (47,471 | ) |
| Accounts receivable | | 19,954 | | | | (31,996 | ) | | | (16,084 | ) |
| Income taxes receivable/payable | | 656 | | | | (113,756 | ) | | | 100,920 | |
| Accounts payable and accrued expenses | | (42,071 | ) | | | (11,003 | ) | | | 34,086 | |
| Other assets | | 20,412 | | | | 27,756 | | | | 3,936 | |
| Other liabilities | | (30,718 | ) | | | 17,350 | | | | 6,005 | |
| Net cash provided by operating activities | | 327,495 | | | | 190,806 | | | | 332,928 | |
| Investing activities: | | | | | | | | | | | |
| Capital expenditures | | (133,049 | ) | | | (123,854 | ) | | | (97,763 | ) |
| Purchases of retirement benefit assets | | (20,555 | ) | | | (26,177 | ) | | | (22,841 | ) |
| Proceeds from sale of retirement benefit assets | | 18,737 | | | | 23,055 | | | | 22,824 | |
| Disposals of fixed assets | | 4,241 | | | | 10,137 | | | | 47,734 | |
| Acquisitions of businesses, net of disposals | | | | | | | | | | | |
| and cash acquired | | (37,249 | ) | | | — | | | | (406 | ) |
| Other | | (14,155 | ) | | | (4,848 | ) | | | (4,975 | ) |
| Net cash used by investing activities | | (182,030 | ) | | | (121,687 | ) | | | (55,427 | ) |
| Financing activities: | | | | | | | | | | | |
| Long-term borrowings | | 171,010 | | | | 211,549 | | | | 80,328 | |
| Repayments of long-term debt | | (213,974 | ) | | | (82,884 | ) | | | (173,596 | ) |
| Notes payable | | 19,493 | | | | (16,420 | ) | | | 1,303 | |
| Purchase of treasury stock | | (96,439 | ) | | | (148,850 | ) | | | (61,795 | ) |
| Dividends paid | | (64,914 | ) | | | (59,945 | ) | | | (56,228 | ) |
| Net proceeds from stock plans | | 15,757 | | | | 18,407 | | | | 39,611 | |
| Excess tax benefits from stock based compensation | | | | | | | | | | | |
| arrangements | | 457 | | | | 1,802 | | | | 7,929 | |
| Net cash used by financing activities | | (168,610 | ) | | | (76,341 | ) | | | (162,448 | ) |
| Cash flow for year | | (23,145 | ) | | | (7,222 | ) | | | 115,053 | |
| Cash and cash equivalents at beginning of year | | 454,065 | | | | 443,036 | | | | 317,657 | |
| Effect of exchange rate changes on cash | | (16,909 | ) | | | 18,251 | | | | 10,326 | |
| Cash and cash equivalents at end of year | $ | 414,011 | | | $ | 454,065 | | | $ | 443,036 | |
| Supplemental disclosures: | | | | | | | | | | | |
| Interest paid | $ | 40,740 | | | $ | 43,287 | | | $ | 34,176 | |
| Income taxes paid (net of refunds) | $ | 84,680 | | | $ | 231,030 | | | $ | 48,825 | |
See accompanying notes to consolidated financial statements.
55
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share data)
NOTE 1– ACCOUNTING POLICIES AND RELATED MATTERS
The Company
Pall Corporation and its subsidiaries (hereinafter collectively called the “Company” unless the context requires otherwise) manufacture and market filtration, purification and separation products and integrated systems solutions throughout the world to a diverse group of customers. As discussed in Note 19, Segment Information and Geographies, management has determined that the Company’s reportable segments, that are also its operating segments, consist of its two vertically integrated businesses: Life Sciences and Industrial.
The Company’s fiscal year ends on July 31, and the Company’s fiscal quarters end on October 31, January 31 and April 30.
Presentation and Use of Estimates
The financial statements of the Company are presented on a consolidated basis with its subsidiaries, substantially all ofwhich are wholly-owned. All significant intercompany balances and transactions have been eliminated in consolidation.
Financial statements of foreign subsidiaries have been translated into U.S. dollars at exchange rates as follows: (i) balance sheet accounts at year-end rates, except equity accounts which are translated at historic rates, and (ii) income statement accounts at weighted average rates. Translation gains and losses are reflected in stockholders’ equity, while transaction gains and losses, which result from the settlement of foreign denominated receivables and payables at rates that differ from rates in effect at the transaction date, are reflected in earnings. Net transaction gains/(losses) inclusive of offsetting gains/losses on foreign currency forward contracts in fiscal years 2009, 2008 and 2007 amounted to $191, ($2,949) and ($2,266), respectively, and were recorded in selling, general and administrative expenses.
To prepare the Company’s consolidated financial statements in accordance with U.S. generally accepted accounting principles, management is required to make assumptions that may affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Estimates are used for, but not limited to, inventory valuation; provisions for doubtful accounts; asset recoverability; depreciable lives of fixed assets and useful lives of patents and amortizable intangibles; fair value of financial instruments; income tax assets and liabilities; pension valuations; restructuring and other charges; valuation of assets acquired and liabilities assumed in business combinations; allocation of costs to operating segments; revenue recognition and liabilities for items such as environmental remediation. The Company is subject to uncertainties such as the impact of future events, economic, environmental and political factors, and changes in the business climate; therefore, actual results may differ from those estimates. When no estimate in a given range is deemed to be better than any other when estimating contingent liabilities, the low end of the range is accrued. Accordingly, the accounting estimates used in the preparation of the Company’s consolidated financial statements will change as new events occur, as more experience is acquired, as additional information is obtained and as the Company’s operating environment changes. Changes in estimates are made when circumstances warrant. Such changes and refinements in estimation methodologies are reflected in reported results of operations; if material, the effects of changes in estimates are disclosed in the notes to the consolidated financial statements.
Cash and Cash Equivalents
All financial instruments purchased with a maturity of three months or less, other than amounts held in the benefits protection trust, are considered cash equivalents.
Inventories
Inventories are valued at the lower of cost (on the first-in, first-out method) or market.
56
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
Investments
Investments (which represent an equity interest of less than 20% and have readily determinable market values) are considered available-for-sale securities as such, these investments are carried at fair value in accordance with Financial Accounting Standards Board (“FASB”) Statement of Financial Accounting Standards (“SFAS”) No. 115, Accounting for Certain Investments in Debt and Equity Securities (“SFAS No. 115”). Unrealized gains and losses on these securities are reported as a separate component of stockholders’ equity until realized from sale or when unrealized losses are deemed to be other than temporary. Other than temporary losses are recognized in earnings when management determines that the recoverability of the cost of the investment is unlikely. The Company considers numerous factors, on a case-by-case basis, in evaluating whether the decline in market value of an available-for-sale security below cost is other than temporary. Such factors include, but are not limited to, (i) the length of time and the extent to which the market value has been less than cost; (ii) the financial condition and the near-term prospects of the issuer of the investment; and (iii) whether the Company’s intent to retain the investment for the period of time is sufficient to allow for any anticipated recovery in market value. Investments are included in “Other non-current assets” in the Consolidated Balance Sheets.
Acquisition Accounting
Acquisitions are accounted for using the purchase method of accounting in accordance with SFAS No. 141, Business Combinations (“SFAS No. 141”). SFAS No. 141 requires that the total cost of an acquisition be allocated to the tangible and intangible assets acquired and liabilities assumed based upon their respective fair values at the date of acquisition. The allocation of the purchase price is dependent upon valuations and other studies.
Long-Lived Assets
The Company accounts for its goodwill and intangible assets in accordance with SFAS No. 142, Goodwill and Other Intangible Assets (“SFAS No. 142”). As such, goodwill is not amortized and is assessed for impairment at least annually, including whenever events or circumstances indicate impairment might have occurred. The Company evaluates the recoverability of goodwill using a two-step impairment test approach at the reporting unit level. In the first step, the overall fair value for the reporting unit is compared to its book value including goodwill. In the event that the overall fair value of the reporting unit was determined to be less than the book value, a second step is performed which compares the implied fair value of the reporting unit’s goodwill to the book value of the goodwill. The implied fair value for the goodwill is determined based on the difference between the overall fair value of the reporting unit and the fair value of the net identifiable assets. If the implied fair value of the goodwill is less than its book value, the difference is recognized as an impairment. In fiscal years 2009 and 2008, the fair value of the Company’s reporting units exceeded the book value of the reporting units and as such, step two was not performed.
The Company’s amortizable intangible assets, which are comprised almost entirely of patented and unpatented technology and trademarks, are subject to amortization for periods ranging up to 20 years, principally on a straight-line basis. Property, plant and equipment are stated at cost. Depreciation is provided over the estimated useful lives of the respective assets, principally on the straight-line basis. The estimated useful lives range from 30 to 50 years for buildings, three to ten years for machinery and equipment and eight to ten years for furniture and fixtures. Leasehold improvements are depreciated over the shorter of the remaining life or the remaining lease term.
The Company periodically reviews its depreciable and amortizable long-lived assets for impairment whenever events or circumstances indicate that the carrying amount of an asset (or asset group) may not be recoverable. If the sum of the expected cash flows, undiscounted, is less than the carrying amount of the asset (or asset group), an impairment loss is recognized as the amount by which the carrying amount of the asset (or asset group) exceeds its fair value.
57
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
Revenue Recognition
Revenue is recognized when title and risk of loss have transferred to the customer and when contractual terms have been fulfilled, except for certain long-term contracts, whereby revenue is recognized under the percentage of completion method (see below). Transfer of title and risk of loss occurs when the product is delivered in accordance with the contractual shipping terms. In instances where contractual terms include a provision for customer acceptance, revenue is recognized when either (i) the Company has previously demonstrated that the product meets the specified criteria based on either seller or customer-specified objective criteria or (ii) upon formal acceptance received from the customer where the product has not been previously demonstrated to meet customer-specified objective criteria.
For contracts accounted for under the percentage of completion method, revenue is based upon the ratio of costs incurred to date compared with estimated total costs to complete. The cumulative impact of revisions to total estimated costs is reflected in the period of the change, including anticipated losses.
Stock Plans
The Company currently has four stock-based employee compensation plans (collectively, the “Stock Plans”), which are described more fully in Note 16, Common Stock. Effective August 1, 2005, the Company adopted the fair value recognition provisions of SFAS No. 123(R), Share-Based Payment (“SFAS No. 123(R)”), using the modified-prospective-transition method. Under that transition method, compensation cost recognized for the years ended July 31, 2009, July 31, 2008 and July 31, 2007 include: (a) compensation cost for all share-based payments granted prior to, but not yet vested as of, August 1, 2005, based on the grant-date fair value estimated in accordance with the original provisions of SFAS No. 123, and (b) compensation cost for the vested portion of share-based payments granted subsequent to August 1, 2005, based on the grant-date fair value estimated in accordance with the provisions of SFAS No. 123(R).
Environmental Matters
Accruals for environmental matters are recorded when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated, based on current law and existing technologies. These accruals are adjusted periodically as facts and circumstances change, assessment and remediation efforts progress or as additional technical or legal information becomes available. Costs of future expenditures for environmental remediation obligations are not discounted to their present value and are expected to be disbursed over an extended period of time. Accruals for environmental liabilities are included in “Accrued liabilities” and “Other non-current liabilities” in the Consolidated Balance Sheets.
Income Taxes
Taxes on income are provided using the asset and liability method. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the years in which the differences are expected to reverse.
For further discussion, refer to Note 12, Income Taxes.
Earnings Per Share
The Consolidated Statements of Earnings present basic and diluted earnings per share. Basic earnings per share is determined by dividing income available to common stockholders by the weighted average number of common shares outstanding for the period. Diluted earnings per share considers the potential effect of dilution on basic earnings per share assuming potentially dilutive securities that meet certain criteria, such as stock options, were outstanding since issuance. The treasury stock method is used to determine the dilutive effect of potentially dilutive securities. Employee stock options and restricted stock units of 2,933, 1,235 and 546 for fiscal years 2009, 2008 and 2007, respectively, were not included in the computation of diluted shares because their effect would have been antidilutive.
The following is a reconciliation between basic shares outstanding and diluted shares outstanding:
| | 2009 | | 2008 | | 2007 |
| Basic shares outstanding | | 118,631 | | 122,445 | | 123,115 |
| Effect of dilutive securities (a) | | 940 | | 1,241 | | 1,278 |
Diluted shares outstanding | | 119,571 | | 123,686 | | 124,393 |
| (a) | | Refer to Note 16, Common Stock, for a description of the Company’s stock plans. |
58
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
Derivative Instruments
The Company accounts for its derivative instruments in accordance with SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities, as amended (“SFAS No. 133”). SFAS No. 133 establishes accounting and reporting standards for derivative instruments as either assets or liabilities in the statement of financial position based on their fair values. Changes in the fair values are reported in earnings or other comprehensive income depending on the use of the derivative and whether it qualifies for hedge accounting. Derivative instruments are designated and accounted for as either a hedge of a recognized asset or liability (fair value hedge) or a hedge of a forecasted transaction (cash flow hedge). For derivatives designated as effective cash flow hedges, changes in fair values are recognized in other comprehensive income/(loss). Changes in fair values related to fair value hedges as well as the ineffective portion of cash flow hedges are recognized in earnings. Changes in the fair value of the underlying hedged item of a fair value hedge are also recognized in earnings. For further discussion, refer to Note 11, Financial Instruments and Risks & Uncertainties.
Adoption of New Accounting Pronouncement
Effective August 1, 2008, the Company adopted, on a prospective basis, certain required provisions of SFAS No. 157. The provisions not yet adopted by the Company relate to non-financial assets and liabilities that are recognized or disclosed at fair value on a non-recurring basis, as permitted under FASB Staff Position No. 157-2, Effective Date of FASB Statement No. 157 (“FSP FAS No. 157-2”). Those remaining aspects of SFAS No. 157 for which the effective date was deferred by FSP FAS No. 157-2 are being evaluated by the Company and will be effective for the first quarter of fiscal year 2010. See Note 10, Fair Value Measurements, for the disclosures required under SFAS No. 157.
Effective August 1, 2008, the Company also adopted the provisions of SFAS No. 159, The Fair Value Option for Financial Assets and Financial Liabilities, including an amendment of FASB Statement No. 115 (“SFAS No. 159”). SFAS No. 159 permits entities to elect to measure specified financial instruments and certain other items at fair value with changes in fair value recognized in earnings each reporting period. The Company has not opted to apply the fair value option to any of the applicable financial assets or liabilities.
Effective with the Company’s third quarter of fiscal year 2009, the Company adopted the provisions of SFAS No. 161, Disclosures about Derivative Instruments and Hedging Activities—an amendment of FASB Statement No. 133 (“SFAS No. 161”). SFAS No. 161 requires entities to provide enhanced disclosures about how and why an entity uses derivative instruments, how derivative instruments and related hedged items are accounted for under SFAS No. 133, and its related interpretations, and how derivative instruments and related hedged items affect an entity’s financial position, financial performance, and cash flows. The adoption did not have an impact on the Company’s consolidated financial position and results of operations as it is a disclosure-only standard. See Note 11, Financial Instruments and Risks & Uncertainties, for the disclosures required under SFAS No. 161.
Effective with the Company’s fourth quarter of fiscal year 2009, the Company adopted the provisions of SFAS No. 165, Subsequent Events (“SFAS No. 165”). SFAS No. 165 established general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. In particular, SFAS No. 165 sets forth the period after the balance sheet date during which management of a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements, the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements and the disclosures that an entity should make about events or transactions that occurred after the balance sheet date. In addition, an entity is required to disclose the date through which subsequent events have been evaluated. For the fiscal year ended July 31, 2009, the Company has evaluated subsequent events for potential recognition and disclosure through September 28, 2009, the date of financial statement issuance. As of September 28, 2009, there were no subsequent events reportable under SFAS No. 165.
Effective with the Company’s fourth quarter of fiscal year 2009, the Company adopted the provisions of FSP No. FAS 115-2 and FAS 124-2, Recognition and Presentation of Other-Than-Temporary Impairments, which modified existing requirements regarding the recognition of other-than-temporary impairments on debt securities. Under the modified guidance, an entity must assess if it (a) has the intent to sell the debt security, or (b) is more likely than not that the entity will be required to sell the debt security before its anticipated recovery (for example, if its cash or working capital requirements or contractual or regulatory obligations indicate that the debt security will be required to be sold before the forecasted recovery occurs). The adoption of FSP No. FAS 115-2 and FAS 124-2 did not have a material impact on the Company’s consolidated financial statements.
59
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
NOTE 2 - ACQUISITIONS
On September 2, 2008 (the “Closing Date”), the Company acquired 100% of the share capital and voting rights, on a fully diluted basis, of GeneSystems, SA (“GeneSystems”), a privately held French biotechnology company that has developed a patented approach to rapid microbiological detection equipment and disposables. On the Closing Date, the Company paid a cash purchase price of 25,000 Euros ($36,265 U.S. dollar equivalent at the foreign exchange rate on the Closing Date), subject to a post closing working capital adjustment. In the second quarter of fiscal year 2009, the Company paid the working capital adjustment of 289 Euros ($382 equivalent).
In the event that French regulations relating to the monitoring of possible contamination of hot water systems and/or water cooling towers by Legionella are amended by the second anniversary of the Closing Date, with effect within 12 months of such amendment, to either (i) make the use of Polymerase Chain Reaction technology mandatory for such monitoring in France or (ii) validate its use as the only or preferred method for such monitoring in France (the “Legionella Regulation”), a post closing payment equal to 11,500 Euros (less any indemnity related payments of up to 2,000 Euros) will also be paid. If the Legionella Regulation is published after the second anniversary of the Closing Date, but prior to the third anniversary of the Closing Date, and becomes effective within 12 months of publication, the sellers will be paid 5,000 Euros (less any indemnity related payments of up to 2,000 Euros). None of the aforementioned events that would require any post closing payments occurred through July 31, 2009. Accordingly, no liabilities for such payments have been recorded as of July 31, 2009.
The acquisition was accounted for using the purchase method of accounting in accordance with SFAS No. 141. SFAS No. 141 requires that the total cost of an acquisition be allocated to the tangible and intangible assets acquired and liabilities assumed based upon their respective fair values at the date of acquisition.
The following table summarizes the final allocation of the purchase price to the assets acquired and liabilities assumed at the date of the acquisition:
| Purchase price | | $ | 36,647 |
| Transaction costs | | | 698 |
| Total purchase price | | | 37,345 |
| Cash acquired | | | 96 |
| Total purchase price, net of cash acquired | | | 37,249 |
| |
| Accounts receivable | | | 909 |
| Inventories | | | 1,883 |
| Other current assets | | | 683 |
| Property plant and equipment | | | 491 |
| In-process research and development | | | 1,743 |
| Intangible assets | | | 16,618 |
| Total assets and in-process research and development acquired | | | 22,327 |
| |
| Accounts payable and other current liabilities | | | 2,260 |
| Other non-current liabilities | | | 4,785 |
| Total liabilities assumed | | | 7,045 |
| |
| Goodwill | | $ | 21,967 |
Based upon the valuation of in-process research and development, the Company recorded a charge to earnings of approximately $1,743, which has been included in Restructuring and other charges, net (see Note 3, Restructuring and Other Charges, Net) for the year ended July 31, 2009.
The amount of in-process research and development was determined by identifying research projects for which technological feasibility had not been established and for which no alternative future uses existed. As of the acquisition date, there was one project that met the above criteria. The project identified is targeted for the BioPharmaceuticals market. The value of the research project identified to be in-process was determined by estimating the future cash flows from the project once commercially feasible and discounting the net cash flows back to their present value. The key assumptions specifically underlying the valuation for purchased in-process research and development consist of an expected completion date for the in-process project, estimated costs to complete the project, revenue and expense projections, and discount rates based on the risks associated with the development life cycle of the in-process technology acquired. The weighted average discount rate used was approximately 40%. The project is expected to be completed by the end of calendar year 2010.
60
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
Based upon the markets GeneSystems serves, the goodwill was assigned to the Company’s Life Sciences segment. The goodwill is not tax deductible. Pro forma financial information has not been provided as it would not be materially different from the financial information that was previously reported. The results of GeneSystems have been included in the results of operations of the Company since the date of acquisition.
NOTE 3 – RESTRUCTURING AND OTHER CHARGES, NET
The following tables summarize the restructuring related items and other charges/(gains) recorded in fiscal years 2009, 2008 and 2007:
| | | | | | Other | | | | |
| | | | | | Charges/(Gains) | | | | |
| 2009 | | Restructuring (1) | | (2) | | Total |
| Severance | | $ | 18,938 | | | $ | — | | | $ | 18,938 | |
| Impairment and loss on disposal of assets (2a) | | | 174 | | | | 3,477 | | | | 3,651 | |
| Other | | | 4,734 | | | | (942 | ) | | | 3,792 | |
| In-process research and development (2b) | | | — | | | | 1,743 | | | | 1,743 | |
| Costs related to inquiry (2c) | | | — | | | | 955 | | | | 955 | |
| Environmental matters (2d) | | | — | | | | 1,808 | | | | 1,808 | |
| | | 23,846 | | | | 7,041 | | | | 30,887 | |
| Reversal of excess restructuring reserves | | | (164 | ) | | | — | | | | (164 | ) |
| | $ | 23,682 | | | $ | 7,041 | | | $ | 30,723 | |
| |
| Cash | | $ | 24,585 | | | $ | 1,821 | | | $ | 26,406 | |
| Non-cash | | | (903 | ) | | | 5,220 | | | | 4,317 | |
| | $ | 23,682 | | | $ | 7,041 | | | $ | 30,723 | |
| |
| 2008 | | | | | | | | | | | | |
| Costs related to inquiry (2c) | | $ | — | | | $ | 19,081 | | | $ | 19,081 | |
| Severance | | | 8,814 | | | | — | | | | 8,814 | |
| Other | | | 3,110 | | | | 482 | | | | 3,592 | |
| Gain on disposal of assets, net | | | (158 | ) | | | (484 | ) | | | (642 | ) |
| Environmental matters (2d) | | | — | | | | 1,275 | | | | 1,275 | |
| | | 11,766 | | | | 20,354 | | | | 32,120 | |
| Reversal of excess restructuring reserves | | | (582 | ) | | | — | | | | (582 | ) |
| | $ | 11,184 | | | $ | 20,354 | | | $ | 31,538 | |
| |
| Cash | | $ | 11,154 | | | $ | 19,895 | | | $ | 31,049 | |
| Non-cash | | | 30 | | | | 459 | | | | 489 | |
| | $ | 11,184 | | | $ | 20,354 | | | $ | 31,538 | |
61
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
| | | | | | Other | | | | |
| | | | | | Charges/(Gains) | | | | |
| 2007 | | Restructuring (1) | | (2) | | Total |
| Severance | | $ | 22,840 | | | $ | — | | | $ | 22,840 | |
| Gain on sale and impairment of assets, net | | | (3,219 | ) | | | — | | | | (3,219 | ) |
| Other | | | 5,438 | | | | (1,076 | ) | | | 4,362 | |
| Environmental matters (2d) | | | — | | | | 644 | | | | 644 | |
| | | 25,059 | | | | (432 | ) | | | 24,627 | |
| Reversal of excess restructuring reserves | | | (2,275 | ) | | | — | | | | (2,275 | ) |
| | $ | 22,784 | | | $ | (432 | ) | | $ | 22,352 | |
| |
| Cash | | $ | 17,672 | | | $ | (628 | ) | | $ | 17,044 | |
| Non-cash | | | 5,112 | | | | 196 | | | | 5,308 | |
| | $ | 22,784 | | | $ | (432 | ) | | $ | 22,352 | |
Company management examined the overall structure of the Company and the manner in which it conducts business activities with the objective of increasing revenue growth, improving efficiency and achieving cost reduction. This resulted in various restructuring activities, including the Company’s facilities rationalization initiative and European cost reduction initiative (“EuroPall”), which commenced in fiscal year 2006, and the Western Hemisphere cost reduction initiative (“AmeriPall”), which commenced in fiscal year 2007. In fiscal year 2009, the Company commenced the second phase of its European cost reduction initiative (“EuroPall II”) and implemented plans to reduce its workforce globally in response to current economic conditions.
2007:
- The Company recorded severance liabilities for the termination of certain employees worldwide as well as other costs primarily related to its facilities rationalization, EuroPall and AmeriPall initiatives.
- The Company recorded impairment charges of $7,667 related to the planned disposal of buildings and the early retirement of certain long-lived assets, as part of the Company’s facilities rationalization initiative. Furthermore, the Company recorded a gain on the sale of its corporate headquarters of $10,886.
2008:
- The Company recorded severance liabilities for the termination of certain employees worldwide as well as other costs primarily related to its facilities rationalization, EuroPall and AmeriPall initiatives.
2009:
- The Company recorded severance liabilities for the termination of certain employees worldwide as well as other costs primarily related to its EuroPall II, AmeriPall and economic workforce reduction initiatives.
The following table summarizes the activity related to restructuring liabilities that were recorded in fiscal years 2009, 2008, 2007 and 2006:
| | | | | | Lease | | | | |
| | | | | | Termination | | | | |
| | | | | | Liabilities & | | | | |
| 2009 | | | Severance | | Other | | Total |
| Original charge | | $ | 18,938 | | | $ | 4,734 | | | $ | 23,672 | |
| Utilized | | | (12,757 | ) | | | (4,133 | ) | | | (16,890 | ) |
| Other changes (a) | | | 412 | | | | 20 | | | | 432 | |
| Balance at Jul. 31, 2009 | | $ | 6,593 | | | $ | 621 | | | $ | 7,214 | |
62
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
| | | | | | Lease | | | | |
| | | | | | Termination | | | | |
| | | | | | Liabilities & | | | | |
| 2008 | | | Severance | | Other | | Total |
| Original charge | | $ | 8,814 | | | $ | 3,110 | | | $ | 11,924 | |
| Utilized | | | (8,059 | ) | | | (2,849 | ) | | | (10,908 | ) |
| Other changes (a) | | | 220 | | | | 6 | | | | 226 | |
| Balance at Jul. 31, 2008 | | | 975 | | | | 267 | | | | 1,242 | |
| Utilized | | | (741 | ) | | | (201 | ) | | | (942 | ) |
| Reversal of excess reserves (b) | | | (24 | ) | | | (4 | ) | | | (28 | ) |
| Other changes (a) | | | (96 | ) | | | (19 | ) | | | (115 | ) |
| Balance at Jul. 31, 2009 | | $ | 114 | | | $ | 43 | | | $ | 157 | |
| |
| 2007 | | | | | | | | | | | | | |
| Original charge | | $ | 22,083 | | | $ | 4,321 | | | $ | 26,404 | |
| Utilized | | | (6,146 | ) | | | (3,573 | ) | | | (9,719 | ) |
| Other changes (a) | | | 611 | | | | 9 | | | | 620 | |
| Balance at Jul. 31, 2007 | | | 16,548 | | | | 757 | | | | 17,305 | |
| Utilized | | | (13,994 | ) | | | (727 | ) | | | (14,721 | ) |
| Reversal of excess reserves (b) | | | (297 | ) | | | (65 | ) | | | (362 | ) |
| Other changes (a) | | | 1,281 | | | | 57 | | | | 1,338 | |
| Balance at Jul. 31, 2008 | | | 3,538 | | | | 22 | | | | 3,560 | |
| Utilized | | | (1,984 | ) | | | (13 | ) | | | (1,997 | ) |
| Reversal of excess reserves (b) | | | (136 | ) | | | — | | | | (136 | ) |
| Other changes (a) | | | (158 | ) | | | (4 | ) | | | (162 | ) |
| Balance at Jul. 31, 2009 | | $ | 1,260 | | | $ | 5 | | | $ | 1,265 | |
| |
| 2006 | | | | | | | | | | | | | |
| Original charge | | $ | 13,335 | | | $ | 3,043 | | | $ | 16,378 | |
| Utilized | | | (7,221 | ) | | | (2,900 | ) | | | (10,121 | ) |
| Other changes (a) | | | 182 | | | | 9 | | | | 191 | |
| Balance at Jul. 31, 2006 | | | 6,296 | | | | 152 | | | | 6,448 | |
| Utilized | | | (2,712 | ) | | | (108 | ) | | | (2,820 | ) |
| Reversal of excess reserves (b) | | | (1,385 | ) | | | (40 | ) | | | (1,425 | ) |
| Other changes (a) | | | 126 | | | | 2 | | | | 128 | |
| Balance at Jul. 31, 2007 | | | 2,325 | | | | 6 | | | | 2,331 | |
| Utilized | | | (1,414 | ) | | | (6 | ) | | | (1,420 | ) |
| Reversal of excess reserves (b) | | | (56 | ) | | | — | | | | (56 | ) |
| Other changes (a) | | | (4 | ) | | | — | | | | (4 | ) |
| Balance at Jul. 31, 2008 | | | 851 | | | | — | | | | 851 | |
| Utilized | | | (706 | ) | | | — | | | | (706 | ) |
| Balance at Jul. 31, 2009 | | $ | 145 | | | $ | — | | | $ | 145 | |
| (a) | | Other changes primarily reflect translation impact. |
| | |
| (b) | | Reflects the reversal of excess restructuring reserves originally recorded in fiscal years 2008, 2007 and 2006. |
(2)Other Charges/(Gains):
(a)Impairment of assets:
In fiscal year 2009, the Company recorded a charge of $1,500 for the impairment of capitalized software development costs related to discontinued projects and a charge of $1,977 primarily for the other-than-temporary diminution in value of certain equity securities held by its benefits protection trust.
63
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
(b)In-process research and development:
In fiscal year 2009, the Company wrote off of in-process research and development acquired in the acquisition of GeneSystems (refer to Note 2, Acquisitions, for further discussion of purchase accounting).
(c)Costs related to inquiry:
In fiscal years 2008 and 2009, the Company recorded legal and other professional fees related to matters that were under audit committee inquiry. See Note 2, Audit Committee Inquiry and Restatement, to the consolidated financial statements included in the Company’s Annual Report on Form 10-K for the fiscal year ended July 31, 2007 (“2007 Form 10-K”) for a description of this inquiry.
(d)Environmental Matters:
In fiscal years 2007, 2008 and 2009, the Company increased its previously established environmental reserves, primarily related to environmental matters in Pinellas Park, Florida and Ann Arbor, Michigan. The increase to the environmental reserves recorded in fiscal year 2009 is net of an insurance recovery received. For more detail regarding environmental matters, please refer to Note 15, Contingencies and Commitments.
NOTE 4 – ACCOUNTS RECEIVABLE
Accounts receivable are summarized as follows:
| | 2009 | | 2008 |
| Billed | | $ | 464,023 | | | $ | 572,262 | |
| Unbilled | | | 107,642 | | | | 55,746 | |
| Total | | | 571,665 | | | | 628,008 | |
| Less: allowance for doubtful accounts | | | (10,602 | ) | | | (10,929 | ) |
| Accounts receivable | | $ | 561,063 | | | $ | 617,079 | |
Unbilled receivables principally relate to revenues accrued for long-term contracts recorded under the percentage-of-completion method of accounting.
NOTE 5 – INVENTORIES
The major classes of inventory, net, are as follows:
| | 2009 | | 2008 |
| Raw materials and components | | $ | 115,274 | | $ | 138,146 |
| Work-in-process | | | 55,409 | | | 77,245 |
| Finished goods | | | 242,595 | | | 277,586 |
| | $ | 413,278 | | $ | 492,977 |
NOTE 6 – PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consist of the following:
| | 2009 | | 2008 |
| Land | | $ | 49,047 | | | $ | 47,900 | |
| Buildings and improvements | | | 498,893 | | | | 471,910 | |
| Machinery and equipment | | | 879,493 | | | | 887,862 | |
| Furniture and fixtures | | | 85,191 | | | | 88,449 | |
| | | 1,512,624 | | | | 1,496,121 | |
| Less: Accumulated depreciation and amortization | | | (830,966 | ) | | | (833,136 | ) |
| Property, plant and equipment, net | | $ | 681,658 | | | $ | 662,985 | |
64
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
NOTE 7 – GOODWILL AND INTANGIBLE ASSETS
The following table presents goodwill, net of accumulated amortization recorded prior to adopting SFAS No. 142, allocated by reportable segment, in accordance with SFAS No. 142.
| | 2009 | | 2008 |
| Life Sciences | | $ | 91,361 | | $ | 72,629 |
| Industrial | | | 191,416 | | | 193,264 |
| | $ | 282,777 | | $ | 265,893 |
The change in the carrying amount of goodwill is primarily attributable to the acquisition of GeneSystems, as discussed in Note 2, Acquisitions, and to changes in foreign exchange rates used to translate the goodwill contained in the financial statements of foreign subsidiaries using the rates at each respective balance sheet date.
Intangible assets consist of the following:
| | 2009 |
| | | | | Accumulated | | | |
| | Gross | | Amortization | | Net |
| Patents and unpatented technology | | $ | 96,421 | | $ | 49,680 | | $ | 46,741 |
| Trademarks | | | 6,379 | | | 3,712 | | | 2,667 |
| Other | | | 17,690 | | | 3,347 | | | 14,343 |
| | $ | 120,490 | | $ | 56,739 | | $ | 63,751 |
| |
| | 2008 |
| | | | | Accumulated | | | |
| | Gross | | Amortization | | Net |
| Patents and unpatented technology | | $ | 85,336 | | $ | 43,853 | | $ | 41,483 |
| Trademarks | | | 4,902 | | | 3,123 | | | 1,779 |
| Other | | | 5,058 | | | 2,116 | | | 2,942 |
| | $ | 95,296 | | $ | 49,092 | | $ | 46,204 |
The change in the carrying amount of patents and unpatented technology is primarily attributable to the acquisition of GeneSystems, as discussed in Note 2, Acquisitions. The change in the carrying amount of other intangibles is primarily related to the purchase of certain distribution rights to a customer base related to the BioPharmaceuticals market. Patents and trademarks include costs to register new patents and trademarks. Patents also include expenditures to defend certain patents.
Amortization expense for intangible assets for fiscal years 2009, 2008 and 2007 was $9,751, $7,887 and $8,183, respectively. Amortization expense is estimated to be approximately $10,207 in fiscal year 2010, $9,987 in fiscal year 2011, $9,720 in fiscal year 2012, $6,813 in fiscal year 2013 and $5,778 in fiscal year 2014.
65
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
NOTE 8 – OTHER CURRENT AND NON-CURRENT ASSETS
Other current assets consist of the following:
| | 2009 | | 2008 |
| Deferred income taxes (a) | | $ | 58,026 | | $ | 35,914 |
| Income taxes receivable | | | 5,893 | | | 2,899 |
| Prepaid income taxes (a) | | | 65,985 | | | — |
| Prepaid expenses | | | 32,204 | | | 34,026 |
| Other receivables | | | 19,990 | | | 22,679 |
| | $ | 182,098 | | $ | 95,518 |
Other non-current assets consist of the following:
| | 2009 | | 2008 |
| Deferred income taxes (a) | | $ | 100,492 | | $ | 92,656 |
| Retirement benefit assets (b) | | | 77,751 | | | 82,940 |
| Investments (b) | | | 2,620 | | | 4,518 |
| Prepaid pension expenses (c) | | | 250 | | | 4,184 |
| Prepaid income taxes (a) | | | — | | | 65,985 |
| Income taxes receivable | | | 40,317 | | | 51,982 |
| Other | | | 20,746 | | | 19,760 |
| | $ | 242,176 | | $ | 322,025 |
| (a) | | Refer to Note 12, Income Taxes, for further discussion of these amounts. |
| | |
| (b) | | Retirement benefit assets are held to satisfy obligations related to certain retirement benefit plans, which provide benefits to eligible employees in Germany and the U.S. Included therein are guaranteed investment contracts of $20,414 and $25,618 as of July 31, 2009 and July 31, 2008, respectively. The guaranteed investment contracts were established to pay for supplementary retirement benefits related to plans in Germany. The July 31, 2009 and July 31, 2008 consolidated balance sheets reflect related liabilities in the amounts of $53,560 and $52,484, respectively. |
| |
| | | Also included within retirement benefit assets is a benefits protection trust, with assets aggregating $57,337 and $57,322 as of July 31, 2009 and July 31, 2008, respectively. The trust was established for the purpose of satisfying certain supplemental post-employment benefit obligations in the U.S. for eligible executives in the event of a change of control of the Company. In addition to holding cash equivalents primarily to satisfy short-term cash requirements relating to benefit payments, the trust primarily invests in U.S. government obligations, debt obligations of corporations and financial institutions with high credit ratings and equity mutual fund shares. Contractual maturity dates of debt securities held by the trust range from 2009 to 2043. Such debt and equity securities are classified as available-for-sale and aggregated $56,170 and $55,979 as of July 31, 2009 and July 31, 2008, respectively. The July 31, 2009 and July 31, 2008 balance sheets reflect retirement benefit assets held in the trust of $50,351 and $50,740, related to retirement benefit liabilities of $70,999 and $59,666, respectively. Included in investments is the Company’s investment in Satair A/S (“Satair”) of $2,588 and $4,477, at July 31, 2009 and July 31, 2008, respectively, which is classified as available-for-sale. |
66
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
The following is a summary of the Company’s available-for-sale investments by category at July 31, 2009 and July 31, 2008:
| | | | | | | | Gross | | Gross | | Net |
| | Cost/ | | | | | Unrealized | | Unrealized | | Unrealized |
| | Amortized | | | | | Holding | | Holding | | Holding |
| 2009 | | | Cost Basis | | Fair Value | | Gains | | Losses | | Gains |
| Equity securities | | $ | 5,550 | | $ | 7,114 | | $ | 1,566 | | $ | (2 | ) | | $ | 1,564 |
| Debt securities: | | | | | | | | | | | | | | | | |
| U.S. Treasury | | | 14,417 | | | 15,210 | | | 841 | | | (48 | ) | | | 793 |
| Other U.S.government | | | 11,609 | | | 12,467 | | | 868 | | | (10 | ) | | | 858 |
| CMO/mortgage-backed | | | 298 | | | 319 | | | 21 | | | — | | | | 21 |
| Corporate | | | 22,367 | | | 23,680 | | | 1,314 | | | (1 | ) | | | 1,313 |
| | $ | 54,241 | | $ | 58,790 | | $ | 4,610 | | $ | (61 | ) | | $ | 4,549 |
| |
| 2008 | | | | | | | | | | | | | | | | | |
| Equity securities | | $ | 7,353 | | $ | 9,560 | | $ | 3,125 | | $ | (918 | ) | | $ | 2,207 |
| Debt securities: | | | | | | | | | | | | | | | | |
| U.S. Treasury | | | 15,587 | | | 16,511 | | | 943 | | | (19 | ) | | | 924 |
| Other U.S.government | | | 10,111 | | | 10,370 | | | 331 | | | (72 | ) | | | 259 |
| CMO/mortgage-backed | | | 371 | | | 378 | | | 7 | | | — | | | | 7 |
| Corporate | | | 23,484 | | | 23,678 | | | 386 | | | (192 | ) | | | 194 |
| | $ | 56,906 | | $ | 60,497 | | $ | 4,792 | | $ | (1,201 | ) | | $ | 3,591 |
The following table shows the gross unrealized losses and fair value of the Company’s available-for-sale investments with unrealized losses aggregated by investment category and length of time that individual securities have been in a continuous unrealized loss position:
| | Less than 12 months | | 12 months or greater | | Total |
| | | | | Gross | | | | | Gross | | | | | Gross |
| | | | | Unrealized | | | | | Unrealized | | | | | Unrealized |
| | Fair | | Holding | | Fair | | Holding | | Fair | | Holding |
| 2009 | | | Value | | Losses | | Value | | Losses | | Value | | Losses |
| Equity securities | | $ | 32 | | $ | 2 | | $ | — | | $ | — | | $ | 32 | | $ | 2 |
| Debt securities: | | | | | | | | | | | | | | | | | | |
| U.S. Treasury | | | 1,085 | | | 48 | | | — | | | — | | | 1,085 | | | 48 |
| Other U.S.government | | | 1,152 | | | 10 | | | — | | | — | | | 1,152 | | | 10 |
| CMO/mortgage-backed | | | — | | | — | | | — | | | — | | | — | | | — |
| Corporate | | | 297 | | | 1 | | | — | | | — | | | 297 | | | 1 |
| | $ | 2,566 | | $ | 61 | | $ | — | | $ | — | | $ | 2,566 | | $ | 61 |
67
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
| | Less than 12 months | | 12 months or greater | | Total |
| | | | | | Gross | | | | | | Gross | | | | | | Gross |
| | | | | | Unrealized | | | | | | Unrealized | | | | | | Unrealized |
| | | Fair | | | Holding | | | Fair | | | Holding | | | Fair | | | Holding |
| 2008 | | | Value | | | Losses | | | Value | | | Losses | | | Value | | | Losses |
| Equity securities | $ | 4,051 | | $ | 918 | | $ | — | | $ | — | | $ | 4,051 | | $ | 918 |
| Debt securities: | | | | | | | | | | | | | | | | | |
| U.S. Treasury | | 696 | | | 19 | | | — | | | — | | | 696 | | | 19 |
| Other U.S.government | | 3,029 | | | 72 | | | — | | | — | | | 3,029 | | | 72 |
| CMO/mortgage-backed | | — | | | — | | | — | | | — | | | — | | | — |
| Corporate | | 6,745 | | | 192 | | | — | | | — | | | 6,745 | | | 192 |
| | | $ | 14,521 | | $ | 1,201 | | $ | — | | $ | — | | $ | 14,521 | | $ | 1,201 |
The following table shows the proceeds and gross gains and losses from the sale of available-for-sale investments for the years ended July 31, 2009, July 31, 2008 and July 31, 2007:
| | | 2009 | | | 2008 | | | 2007 |
| | Proceeds from sales | $ | 11,229 | | $ | 15,132 | | $ | 13,344 |
| | Realized gross gains on sales | | 497 | | | 278 | | | 4 |
| Realized gross losses on sales | | 376 | | | 31 | | | 61 |
| (c) | | Prepaid pension expenses represent the non-current amounts arising from the excess of cumulative employer contributions over accrued net pension expenses. Refer to Note 14, Pension and Profit Sharing Plans and Arrangements for further discussion. |
NOTE 9 – NOTES PAYABLE AND LONG-TERM DEBT
Notes payable is comprised of overdraft borrowings from a financial institution of $41,030, and borrowings under various short-term unsecured credit facilities of $1,341 as of July 31, 2009. The Company had available unsecured credit facilities which require no compensating balances, totaling approximately $210,363. In addition to providing short-term liquidity and overdraft protection, these facilities also support various programs (such as guarantee, performance bond and warranty) mandated by customers. At July 31, 2009, borrowings under these facilities were included in notes payable and an additional $42,291 was committed to various other programs. The weighted average interest rates on notes payable at the end of fiscal years 2009 and 2008 were 0.3% and 5.9%, respectively.
Long-term debt consists of:
| | | | 2009 | | 2008 |
| Senior revolving credit facility, due in fiscal year 2011 (a) | $ | 280,000 | | | $ | 356,737 | |
| Private placement senior notes, due in fiscal year 2013 (b) | | 280,000 | | | | 280,000 | |
| Yen denominated loan, due in fiscal year 2010 (c) | | 95,121 | | | | 83,421 | |
| | Other | | 19,897 | | | | 30,038 | |
| | | 675,018 | | | | 750,196 | |
| SFAS No. 133 fair value adjustment, net (d) | | 80 | | | | 107 | |
| Total long-term debt | | 675,098 | | | | 750,303 | |
| Current portion | | (97,432 | ) | | | (3,252 | ) |
| Long-term debt, net of current portion | $ | 577,666 | | | $ | 747,051 | |
68
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
| (a) | | On June 21, 2006, the Company and certain wholly-owned subsidiary borrowers, entered into a multi-currency (U.S.$, Pound, Euro), five-year $500,000 unsecured senior revolving credit facility with a syndicate of banks, which expires on June 21, 2011. Letters of credit outstanding against the $500,000 revolving credit facility as of July 31, 2009 were approximately $12,725. |
| |
| | | Borrowings under the current facility bear interest at either a variable rate based upon LIBOR (U.S.$ and £ borrowings) or EURIBOR (Euro borrowings) or at the prime rate of the Administrative Agent (U.S.$ borrowing only). The current facility contains customary affirmative and negative covenants, financial covenants, representations and warranties and events of defaults. The financial covenants are as follows: |
| |
| | | i. | | Minimum interest coverage ratio: The Ratio of Earnings Before Net Interest, Taxes, Depreciation, Amortization and the Non-Cash Portion of Non-Recurring Charges and Income (“EBITDA”) to Net Interest Expense shall not be less than 3.50 to 1.00, computed on the basis of cumulative results for the most recently ended four consecutive quarters. |
| |
| | | ii. | | Maximum funded debt ratio: The Ratio of Consolidated Funded Debt to EBITDA shall not exceed 3.50 to 1.00, EBITDA computed on the basis of cumulative results for the most recently ended four consecutive quarters. |
| |
| | | The Company is in compliance with these financial covenants. |
| |
| (b) | | On August 6, 2002, the Company completed a private placement offering of $280,000 6% senior notes due on August 1, 2012. The notes are unsecured and unsubordinated obligations of the Company and rank pari passu to its other outstanding unsecured and unsubordinated indebtedness. |
| |
| (c) | | The Company has a loan of Yen 9 billion (approximately $95,121 as of July 31, 2009) which is due on June 20, 2010. At July 31, 2009, the balance of this loan is reported as part of the current portion of long-term debt. Interest payments are at a variable rate based upon Yen LIBOR. The Company designated this borrowing as a non-derivative hedge of a portion of its net Yen investment in a Japanese subsidiary. |
| |
| (d) | | Refer to Note 11, Financial Instruments and Risks & Uncertainties, for further discussion of the Company’s derivative financial instruments and hedging activities. |
| |
| | | The aggregate annual maturities of long-term debt during fiscal years 2010 through 2014 are approximately as follows: |
| |
| | 2010 | | $ | 97,432 |
| | 2011 | | | 282,205 |
| 2012 | | | 1,723 |
| 2013 | | | 281,707 |
| 2014 | | | 1,792 |
Interest expense, net, for fiscal years 2009, 2008 and 2007 is comprised of:
| | | | | 2009 | | | 2008 | | | 2007 |
| | Interest expense(i) | | $ | 36,864 | | $ | 50,937 | | $ | 56,837 |
| Interest income | | | 8,728 | | | 18,361 | | | 17,781 |
| Interest expense, net | | $ | 28,136 | | $ | 32,576 | | $ | 39,056 |
| (i) | | For fiscal years 2009, 2008 and 2007, interest expense included $7,684, $11,470 and $22,273, respectively, related to Income taxes payable. |
69
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
NOTE 10 – FAIR VALUE MEASUREMENTS
SFAS No. 157 defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements. SFAS No. 157 does not require any new fair value measurements; rather, it applies to all other accounting pronouncements that require or permit fair value, except for those pronouncements specifically excluded from its scope. SFAS No. 157 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
SFAS No. 157 discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost). The standard utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
- Level 1: Use of observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
- Level 2: Use of inputs other than quoted prices included in Level 1, which are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data.
- Level 3: Use of inputs that are unobservable.
The following table presents, for each of these hierarchy levels, the Company’s financial assets and liabilities that are measured at fair value on a recurring basis as of July 31, 2009:
| | | | Fair Value Measurements |
| As of | | | | | | | | | |
| Jul. 31, 2009 | | Level 1 | | Level 2 | | Level 3 |
| Financial assets carried at fair value | | | | | | | | | | | |
| Available-for-sale debt securities | $ | 51,676 | | $ | 51,676 | | $ | — | | $ | — |
| Available-for-sale equity securities | | 7,114 | | | 7,114 | | | — | | | — |
| Derivative financial instruments | | 1,307 | | | — | | | 1,307 | | | — |
| |
| Financial liabilities carried at fair value | | | | | | | | | | | |
| Derivative financial instruments | | 1,059 | | | — | | | 1,059 | | | — |
The Company’s available-for-sale debt and equity securities are valued using quoted market prices and, as such, are classified within Level 1 of the fair value hierarchy.
The derivative financial instruments classified within Level 2 of the fair value hierarchy are comprised of an interest rate swap and foreign currency forward contracts. The fair value of the Company’s outstanding interest rate swap contract was determined based upon a non-binding valuation from the counterparty that is corroborated by observable market data such as Japanese Yen interest rates and yield curves. The fair values of the Company’s foreign currency forward contracts were valued using pricing models, with all significant inputs derived from or corroborated by observable market data such as yield curves, currency spot and forward rates and currency volatilities.
NOTE 11 – FINANCIAL INSTRUMENTS AND RISKS & UNCERTAINTIES
As of July 31, 2009, the Company had an interest rate swap and foreign currency forward contracts outstanding with notional amounts aggregating $95,121 and $152,856 respectively, whose fair values were a liability of $688 and an asset of $936, respectively. Accumulated other comprehensive income includes $444, net of tax, of cumulative unrealized losses on its variable to fixed rate interest rate swap (i.e., cash flow hedge).
The Company manages certain financial exposures through a risk management program that includes the use of foreign exchange and interest rate derivative financial instruments. Derivatives are executed with counterparties with a minimum credit rating of “A” by Standard & Poors and Moody’s Investor Services, in accordance with the Company’s policies. The Company does not utilize derivative instruments for trading or speculative purposes.
70
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
Foreign Exchange
a. Derivatives Not Designated as Hedging Instruments under SFAS No. 133
The risk management objective of holding foreign exchange derivatives is to mitigate volatility to earnings and cash flows due to changes in foreign exchange rates. The Company and its subsidiaries conduct transactions in currencies other than their functional currencies. These transactions include non-functional intercompany and external sales as well as intercompany and external purchases. The Company uses foreign exchange forward contracts, matching the notional amounts and durations of the receivables and liabilities resulting from the aforementioned underlying foreign currency transactions, to mitigate the exposure to earnings and cash flows caused by changing foreign exchange rates. The notional amount of foreign currency forward contracts entered into during the twelve months ended July 31, 2009 was $707,910. The notional amount of foreign currency forward contracts outstanding as of July 31, 2009 was $152,856.
b. Net Investment Hedges under SFAS No. 133
The risk management objective of designating the Company’s foreign currency loan as a hedge of its net investment in a wholly owned Japanese subsidiary is to mitigate the change in the fair value of the Company’s net investment due to changes in foreign exchange rates. The Company uses a Japanese Yen (“JPY”) loan outstanding to hedge its equity of the same amount in the Japanese wholly owned subsidiary. The hedge of net investment consists of a JPY 9 billion loan.
Interest Rates
a. Cash Flow Hedges under SFAS No. 133
The risk management objective of holding a floating-to-fixed interest rate swap is to lock in fixed interest cash outflows on a floating rate debt obligation. The associated risk is created by changes in market interest rates in Japan. The Company has an outstanding JPY loan with variable interest rates based on JPY-LIBOR-BBA. The Company meets the stated risk management objective through a “receive variable, pay fixed” interest rate swap entered into on June 20, 2007 related to the Yen 9 billion loan that matures in June 2010, whereby the Company receives payments at a variable rate based upon Yen LIBOR and makes payments at a fixed rate of 1.58% on a notional amount of Yen 9 billion.
The fair values of the Company’s derivative financial instruments included in the consolidated balance sheet as of July 31, 2009 are presented as follows:
| Asset Derivatives | | Liability Derivatives |
| Balance Sheet Location | | Fair Value | | Balance Sheet Location | | Fair Value |
| Derivatives designated as hedging instruments |
| Interest rate swap contract | Other current assets | | $ | — | | Other current liabilities | | $ | 688 |
| |
| Derivatives not designated as hedging instruments |
| Foreign exchange forward contracts | Other current assets | | $ | 1,307 | | Other current liabilities | | $ | 371 |
| Total derivatives | | | $ | 1,307 | | | | $ | 1,059 |
| |
| Nonderivative instruments designated as hedging instruments |
| Net investment hedge | | | | | | Current portion of long-term debt | | $ | 95,121 |
The amounts of the gains and losses related to the Company’s derivative financial instruments designated as hedging instruments for the year ended July 31, 2009 are presented as follows:
| | | | | | | Location of Gain or (Loss) | | | | | | |
| Amount of Gain or (Loss) | | Reclassified from | | Amount of Gain or (Loss) |
| Recognized in OCI on | | Accumulated OCI into | | Reclassified from Accumulated |
| Derivatives | | Earnings (Effective | | OCI into Earnings |
| (Effective Portion) | �� | Portion) | | (Effective Portion)(a) |
| Derivatives in cash flow hedging | | | | | | | | | | | | | |
| relationships | | | | | | | | | | | | | |
| Interest rate swap contract | | $ | (167 | ) | | | Interest expense | | | $ | (483 | ) | |
| (a) | | There were no gains or losses recognized in earnings related to the ineffective portion of the hedging relationship or related to the amount excluded from the assessment of hedge effectiveness for the twelve months ended July 31, 2009. |
71
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
The amounts of the gains and losses related to the Company’s derivative financial instruments not designated as hedging instruments for the year ended July 31, 2009 are presented as follows:
| | Location of Gain or (Loss) Recognized in | | Amount of Gain or (Loss) Recognized |
| | Earnings on Derivatives | | in Earnings on Derivatives |
Derivatives not designated as
hedging relationships | | | | | | | | |
| Foreign exchange forward contracts | | Selling, general and administrative | | | | | | |
| | expenses | | | $ | (4,156 | ) | |
The amounts of the gains and losses related to the Company’s nonderivative financial instruments designated as hedging instruments for the year ended July 31, 2009 are presented as follows:
| | | | | | | Location of Gain or (Loss) | | | | | |
| Amount of Gain or (Loss) | | Reclassified from | | Amount of Gain or (Loss) |
| Recognized in OCI on | | Accumulated OCI into | | Reclassified from Accumulated |
| Derivatives | | Earnings (Effective | | OCI into Earnings |
| (Effective Portion) | | Portion) | | (Effective Portion)(a) |
| Nonderivatives designated as | | | | | | | | | | | | |
| hedging relationships | | | | | | | | | | | | |
| Net investment hedge | | $ | (7,488 | ) | | | N/A | | | $ | — | |
| (a) | | There were no gains or losses recognized in earnings related to the ineffective portion of the hedging relationship or related to the amount excluded from the assessment of hedge effectiveness for the year ended July 31, 2009. |
The credit risk related to the interest rate swaps and the forwards is considered low because such instruments are entered into only with financial institutions having high credit ratings and are generally settled on a net basis.
The Company’s cash and cash equivalents are in high-quality securities placed with a wide array of financial institutions with high credit ratings limiting the Company’s exposure to concentration of credit risks.
The Company’s products are sold to a diverse group of customers throughout the world. The Company is subject to certain risks and uncertainties as a result of changes in general economic conditions, sources of supply, competition, foreign exchange rates, tax reform, litigation and regulatory developments. The diversity and breadth of the Company’s products and geographic operations mitigate the risk that adverse changes in any likely event would materially affect the Company’s financial position. Additionally, as a result of the diversity of its customer base, the Company does not consider itself exposed to concentration of credit risks. These risks are further minimized by placing credit limits, ongoing monitoring of customers’ account balances, and assessment of customers’ financial strength.
NOTE 12 – INCOME TAXES
The components of earnings before income taxes are as follows:
| 2009 | | 2008 | | 2007 |
| Domestic operations | $ | (9,193 | ) | | $ | 27,437 | | | $ | 44,675 | |
| Foreign operations | | 279,979 | | | | 298,118 | | | | 215,854 | |
| $ | 270,786 | | | $ | 325,555 | | | $ | 260,529 | |
| |
| The provisions for income taxes consist of the following | | | | | | | | | | | |
| items: | | | | | | | | | | | |
| Current: | | | | | | | | | | | |
| Federal, state and local | $ | 2,843 | | | $ | 49,166 | | | $ | 51,620 | |
| Foreign | | 82,966 | | | | 78,484 | | | | 79,521 | |
| | | 85,809 | | | | 127,650 | | | | 131,141 | |
| Deferred: | | | | | | | | | | | |
| Federal, state and local | | (11,623 | ) | | | (24,326 | ) | | | 4,756 | |
| Foreign | | 981 | | | | 4,952 | | | | (2,865 | ) |
| | | (10,642 | ) | | | (19,374 | ) | | | 1,891 | |
| | $ | 75,167 | | | $ | 108,276 | | | $ | 133,032 | |
72
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
A reconciliation of the provisions for income taxes follows:
| % of Pretax Earnings |
| 2009 | | 2008 | | 2007 |
| Computed “expected” tax expense | 35.0 | % | | 35.0 | % | | 35.0 | % |
| |
| Foreign income and withholding taxes, | | | | | | | | |
| net of U.S. foreign tax credits | (7.4 | ) | | (2.6 | ) | | 6.0 | |
| Taxes on undistributed earnings | — | | | — | | | 8.5 | |
| U.S. tax credits | (0.9 | ) | | (0.2 | ) | | (0.6 | ) |
| Change in valuation allowance | — | | | — | | | (0.3 | ) |
| Other, net | 1.1 | | | 1.0 | | | 2.5 | |
| Effective tax rate | 27.8 | % | | 33.2 | % | | 51.1 | % |
In fiscal year 2007, the Company provided $22,000 of U.S. tax on approximately $160,000 of undistributed foreign earnings that previously have not been subject to U.S. tax. This provision resulted in an effective tax rate reconciling item of 8.5%. U.S. tax was not previously provided on these earnings as they were considered by the Company to be indefinitely reinvested in its foreign operations. The decision was made to provide taxes for this portion of undistributed foreign earnings in anticipation of those foreign funds being remitted to the U.S. in the future to fund tax payments that resulted from the previously announced understatement of U.S. federal income tax payments. During fiscal year 2009, approximately $21,000 was repatriated under this plan leaving approximately $39,000 remaining of the $160,000 to be repatriated.
As of July 31, 2009, the Company has not provided deferred taxes on approximately $772,600 of undistributed foreign subsidiaries’ earnings because it intends to invest substantially all such earnings in its foreign operations indefinitely. The additional U.S. and non-U.S. income and withholding taxes that would arise on the reversal of the temporary differences could be offset, in part, by tax credits. Because the determination of the amount of available tax credits and the limitations imposed on the annual utilization of such credits are subject to a highly complex series of calculations and expense allocations, it is impractical to estimate the amount of net income taxes and withholding taxes that might be payable on the remaining pool of undistributed earnings if a reversal of temporary differences occurred.
The components of the net deferred tax asset at July 31, are as follows:
| | | 2009 | | 2008 |
| Deferred tax asset: | | | | | | | |
| Tax loss and tax credit carry-forwards | $ | 33,286 | | | $ | 27,607 | |
| Inventories | | 28,162 | | | | 24,682 | |
| Compensation and benefits | | 30,045 | | | | 25,943 | |
| Environmental | | 4,601 | | | | 5,390 | |
| Accrued expenses | | 28,466 | | | | 27,573 | |
| Amortization | | 9,264 | | | | 11,185 | |
| Net pensions | | 67,847 | | | | 43,917 | |
| Other | | 30,609 | | | | 28,798 | |
| Gross deferred tax asset | | 232,280 | | | | 195,095 | |
| Valuation allowance | | (28,090 | ) | | | (22,327 | ) |
| Total deferred tax asset | | 204,190 | | | | 172,768 | |
| Deferred tax liability: | | | | | | | |
| Plant and equipment | | (32,189 | ) | | | (36,610 | ) |
| Undistributed foreign earnings | | (4,466 | ) | | | (8,858 | ) |
| Amortization | | (8,065 | ) | | | (3,817 | ) |
| | Other | | (15,894 | ) | | | (9,540 | ) |
| Total deferred tax liability | | (60,614 | ) | | | (58,825 | ) |
| Net deferred tax asset | $ | 143,576 | | | $ | 113,943 | |
73
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
As of July 31, 2009, the Company had available tax net operating loss, tax capital loss and tax credit carry forwards subject to expiration as follows:
| | | Losses | | | |
| Year of Expiration | | Operating | | Capital | | Tax Credits |
| 2010 | $ | — | | $ | — | | $ | 4,786 |
| | 2011-2019 | | 3,766 | | | 3,392 | | | 1,819 |
| 2020-2029 | | 5,750 | | | — | | | 2,672 |
| | Subtotal | | 9,516 | | | 3,392 | | | 9,277 |
| Indefinite | | 94,728 | | | — | | | — |
| Total | $ | 104,244 | | $ | 3,392 | | $ | 9,277 |
In addition, the Company has various state net operating loss carryforwards that expire in varying amounts through fiscal year 2029.
The valuation allowance has been increased by $5,763 during the fiscal year ended July 31, 2009. This increase primarily resulted from the acquisition of GeneSystems (as discussed in Note 2, Acquisition) and its historical tax losses.
In evaluating the reasonableness of the valuation allowance, management assesses whether it is more likely than not that some portion, or all, of its deferred tax assets will not be realized. Ultimately, the realization of deferred tax assets is dependent upon generation of sufficient future taxable income during those periods in which temporary differences become deductible and/or net operating loss and tax credit carryforwards can be utilized. To this end, management considers the level of historical taxable income, the scheduled reversal of taxable temporary differences, tax-planning strategies and projected future taxable income. Based on these considerations, management believes it is more likely than not that the Company will realize the benefit of its deferred tax asset, net of the July 31, 2009 valuation allowance.
Effective August 1, 2007, the Company adopted a new accounting standard, FIN No. 48, Accounting for Uncertainty in Income Taxes, resulting in a cumulative effect adjustment of $5,570, reducing its liability for unrecognized income tax benefits and interest and increasing the August 1, 2007 balance of Retained Earnings.
A reconciliation of the beginning and ending gross amounts of unrecognized tax benefits is as follows:
| | | 2009 | | 2008 |
| Beginning balance | $ | 242,287 | | | $ | 210,000 | |
| Increases for tax positions taken during the current year | | 17,753 | | | | 26,232 | |
| | Increases for tax positions taken in prior years | | 8,203 | | | | 376 | |
| Decreases for tax positions taken in prior years | | (16,951 | ) | | | (1,039 | ) |
| Settlements with tax authorities | | (643 | ) | | | (463 | ) |
| Translation and other | | (9,966 | ) | | | 7,181 | |
| Ending balance | $ | 240,683 | | | $ | 242,287 | |
The total amount of net unrecognized tax benefits that would reduce the effective tax rate, if recognized, was $158,890 at July 31, 2009.
The Company files income tax returns in the United States and multiple foreign jurisdictions with varying statutes of limitations. In the normal course of business, the Company and its subsidiaries are subject to examination by various taxing authorities. As of July 31, 2009, the Company is subject to U.S. federal and state local income tax examinations for the fiscal tax years ended in 1996 through 2008, and to non-U.S. income tax examinations for the fiscal tax years ended in 2000 through 2008.
74
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
The Company recognizes accrued interest expense related to unrecognized income tax benefits in interest expense and the balance at the end of a reporting period is recorded in interest payable on the Company’s consolidated balance sheet. Penalties are accrued as part of the provision for income taxes and the unpaid balance at the end of a reporting period is recorded as part of current or non-current income taxes payable. During the fiscal years ended July 31, 2009 and July 31, 2008, $7,011 and $13,149 of interest and penalties were recognized in the statement of earnings, respectively. As of July 31, 2009, the Company has accrued $75,157 for the potential payment of interest and penalties.
In September 2007, the Company deposited $135,000 with the Internal Revenue Service. A portion of this deposit has been reflected as a reduction of current income taxes payable and interest payable, with the remainder reflected as prepaid income taxes in other current assets on the Company’s consolidated balance sheet.
Due to the potential resolution of tax examinations and the expiration of various statutes of limitation, the Company believes that it is reasonably possible that the gross amount of unrecognized tax benefits may decrease within the next twelve months by a range of zero to $103,650. As a result, the Company has reclassified $97,283 from non-current income taxes payable to current income taxes payable and $2,860 of non-current income taxes receivable to current income taxes receivable. In addition, the Company reclassified $65,985 of non-current prepaid income taxes included in other non-current assets as of July 31, 2008 to prepaid income taxes in other current assets as of July 31, 2009 as this amount could be utilized in the resolution of the unrecognized tax benefits. Consistent with the reclassification of liabilities, interest was reclassified from non-current interest payable to current interest payable.
The amounts recorded in the consolidated financial statements reflect the Company’s current estimate of income tax liabilities as of July 31, 2009 including interest and penalties. As previously disclosed in Note 2, Audit Committee Inquiry and Restatement, to the consolidated financial statements included in the 2007 Form 10-K, the actual amounts due and payable upon final settlement of the matters that are under review by taxing authorities in the U.S. and other taxing jurisdictions may differ materially from the Company’s estimate. In particular, the Company may be subject to potential additional penalties that may be asserted by the U.S. and foreign taxing authorities of up to $126,000, which has not been reflected in the consolidated financial statements as of July 31, 2009.
75
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
NOTE 13 – ACCRUED AND OTHER NON-CURRENT LIABILITIES
Accrued liabilities consist of the following:
| | | | 2009 | | | 2008 |
| Payroll and related taxes | $ | 113,401 | | $ | 136,675 |
| Benefits | | 16,490 | | | 18,075 |
| Interest payable (a) | | 36,568 | | | 18,854 |
| Environmental remediation (b) | | 5,530 | | | 5,180 |
| Deferred income taxes | | 5,649 | | | 3,648 |
| Other | | 73,200 | | | 88,090 |
| | $ | 250,838 | | $ | 270,522 |
| |
| Other non-current liabilities consist of the following: | | | |
| |
| | | 2009 | | | 2008 |
| Retirement benefits (c) | $ | 231,405 | | $ | 167,737 |
| Interest payable – non-current (a) | | 24,733 | | | 46,323 |
| Deferred revenue (d) | | 11,667 | | | 13,821 |
| Environmental remediation (b) | | 6,924 | | | 9,569 |
| Other | | 13,217 | | | 14,648 |
| | $ | 287,946 | | $ | 252,098 |
| | (a) | | Accrued liabilities include interest payable related to income taxes as of July 31, 2009 which was previously included in interest payable–non-current as of July 31, 2008; refer to Note 12, Income Taxes, for further discussion. |
| |
| (b) | | For further discussion regarding environmental remediation liabilities refer to Note 15, Contingencies and Commitments. |
| |
| (c) | | For discussion regarding retirement benefits refer to Note 14, Pension and Profit Sharing Plans and Arrangements. |
| |
| (d) | | On December 16, 2005, the Company sold the rights to its Western Hemisphere commercial aerospace aftermarket distribution channel for the Company’s products for a ten-year period to Satair. The proceeds received for the distribution rights were recorded as deferred revenue and are being amortized as an increase to sales over the life of the distribution agreement. |
NOTE 14 – PENSION AND PROFIT SHARING PLANS AND ARRANGEMENTS
Pension Plans
The Company provides substantially all domestic and foreign employees with retirement benefits. Funding policy for domestic plans, which is primarily comprised of a cash balance pension plan, is in accordance with the Employee Retirement Income Security Act of 1974 (“ERISA”); for foreign plans, funding is determined by local tax laws and other regulations. Pension costs charged to operations totaled $23,240, $25,363 and $30,314 in fiscal years 2009, 2008 and 2007, respectively.
In fiscal year 2007, the Company adopted the provisions of Staff Accounting Bulletin No. 108, Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements (“SAB No. 108”) and recorded a cumulative effect adjustment to the opening balance of retained earnings of $5,957, an increase to noncurrent deferred tax assets of $2,982 and an increase to other non-current liabilities of $8,939, substantially all of which relates to periods prior to fiscal 2005. The uncorrected misstatement for consideration under SAB No. 108, which was considered immaterial to the Company’s financial position and results of operations in prior reporting periods under the income statement approach, related to an understatement of pension liabilities in a foreign subsidiary and the related understatement of pension expense which had not been recognized in accordance with SFAS No. 87, Employers’ Accounting for Pensions.
76
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
As of July 31, 2007, the Company adopted the provisions of SFAS No. 158. In accordance with SFAS No. 158, the Company is required to record the difference between its benefit obligations and any plan assets of its defined benefit plans. Upon adoption, SFAS No. 158 requires the recognition of previously unrecognized actuarial gains and losses, prior service costs or credits and net transition amounts within accumulated other comprehensive income (expense), net of tax. Additionally, SFAS No. 158 requires companies to measure plan assets and benefit obligations as of the date of the Company’s fiscal year-end statement of financial position, which is July 31. The Company’s defined benefit pension plans were already measured as of July 31; therefore, the measurement date provisions of SFAS No. 158 did not affect the Company’s existing valuation practices.
The following table reflects the change in benefit obligations, change in plan assets and funded status for these plans:
| U.S. Plans | | Foreign Plans |
| | 2009 | | | 2008 | | | 2009 | | | 2008 |
| Change in benefit obligation: | | | | | | | | | | | | | | | |
| Benefit obligation - beginning of year | $ | 190,686 | | | $ | 192,017 | | | $ | 337,206 | | | $ | 356,701 | |
| Curtailments and settlements | | — | | | | — | | | | (1,098 | ) | | | (462 | ) |
| Service cost | | 8,132 | | | | 7,998 | | | | 4,723 | | | | 4,074 | |
| Interest cost | | 12,426 | | | | 11,586 | | | | 16,025 | | | | 19,044 | |
| Plan participant contributions | | — | | | | — | | | | 25 | | | | 27 | |
| Plan amendments | | 3,147 | | | | 962 | | | | — | | | | (1,871 | ) |
| Actuarial loss (gain) | | 7,657 | | | | (9,311 | ) | | | 25,402 | | | | (34,819 | ) |
| Total benefits paid | | (11,473 | ) | | | (12,566 | ) | | | (13,068 | ) | | | (13,657 | ) |
| Plan transfer | | — | | | | — | | | | — | | | | 186 | |
| Effect of exchange rates | | — | | | | — | | | | (36,726 | ) | | | 7,983 | |
| Benefit obligation – end of year | | 210,575 | | | | 190,686 | | | | 332,489 | | | | 337,206 | |
| Change in plan assets (a): | | | | | | | | | | | | | | | |
| Fair value of plan assets – beginning of year | | 117,370 | | | | 134,085 | | | | 242,384 | | | | 258,815 | |
| Curtailments and settlements | | — | | | | — | | | | (1,069 | ) | | | — | |
| Actual return on plan assets | | (14,547 | ) | | | (7,614 | ) | | | (4,785 | ) | | | (14,098 | ) |
| Company contributions | | 4,423 | | | | 3,465 | | | | 17,865 | | | | 11,929 | |
| Plan participant contributions | | — | | | | — | | | | 25 | | | | 27 | |
| Benefits paid from plan assets | | (11,473 | ) | | | (12,566 | ) | | | (13,068 | ) | | | (13,657 | ) |
| Plan transfer | | — | | | | — | | | | 75 | | | | 186 | |
| Effect of exchange rates | | — | | | | — | | | | (29,991 | ) | | | (818 | ) |
| Fair value of plan assets - end of year | | 95,773 | | | | 117,370 | | | | 211,436 | | | | 242,384 | |
| |
| Funded status (a) | $ | (114,802 | ) | | $ | (73,316 | ) | | $ | (121,053 | ) | | $ | (94,822 | ) |
| |
| Accumulated benefit obligation | $ | 188,570 | | | $ | 171,095 | | | $ | 322,358 | | | $ | 327,541 | |
| |
| Plans with accumulated benefit obligations in excess | | | | | | | | | | | | | | | |
| of plan assets consist of the following: | | | | | | | | | | | | | | | |
| Accumulated benefit obligation | $ | 188,570 | | | $ | 149,985 | | | $ | 318,689 | | | $ | 322,402 | |
| Projected benefit obligation | | 210,575 | | | | 169,576 | | | | 327,740 | | | | 330,478 | |
| Plan assets at fair value | | 95,773 | | | | 92,405 | | | | 207,499 | | | | 236,336 | |
| | (a) | | The Company has certain supplemental defined benefit plans, which provide benefits to eligible executives in the U.S. and employees abroad. As such, the above tables do not include the Company’s assets relating to these plans of $49,481 and $49,719 for the U.S. plans and $20,414 and $25,618 for the foreign plans as of July 31, 2009 and July 31, 2008, respectively. Liabilities, included in the tables above, related to these plans were $70,129 and $58,362 for the U.S. plans and $53,560 and $52,484 for the foreign plans as of July 31, 2009 and July 31, 2008, respectively. |
77
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
| | | U.S. Plans | | Foreign Plans |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 |
| | Amounts recognized in the balance sheet consists of: | | | | | | | | | | | | | | | |
| Non-current assets | $ | — | | | $ | 3,855 | | | $ | 250 | | | $ | 329 | |
| Current liabilities | | (3,298 | ) | | | (2,985 | ) | | | (1,402 | ) | | | (1,884 | ) |
| Non-current liabilities | | (111,504 | ) | | | (74,186 | ) | | | (119,901 | ) | | | (93,267 | ) |
| Net amount recognized | $ | (114,802 | ) | | $ | (73,316 | ) | | $ | (121,053 | ) | | $ | (94,822 | ) |
Net periodic benefit cost for the Company’s defined benefit pension plans includes the following components:
| | | U.S. Plans | | Foreign Plans |
| | 2009 | | 2008 | | 2007 | | 2009 | | 2008 | | 2007 |
| Service cost | $ | 8,132 | | | $ | 7,998 | | | $ | 7,818 | | | $ | 4,723 | | | $ | 4,074 | | | $ | 3,941 | |
| Interest cost | | 12,426 | | | | 11,586 | | | | 11,051 | | | | 16,025 | | | | 19,044 | | | | 16,493 | |
| Expected return on plan assets | | (8,455 | ) | | | (8,760 | ) | | | (8,497 | ) | | | (13,132 | ) | | | (15,862 | ) | | | (13,074 | ) |
| Amortization of prior service cost | | 1,538 | | | | 1,221 | | | | 1,115 | | | | 235 | | | | 256 | | | | 612 | |
| | Amortization of net transition asset | | — | | | | — | | | | (42 | ) | | | — | | | | — | | | | — | |
| Amortization of actuarial loss | | 1,056 | | | | 1,869 | | | | 2,315 | | | | 701 | | | | 4,399 | | | | 8,582 | |
| Gain due to curtailments and | | | | | | | | | | | | | | | | | | | | | | | |
| settlements | | — | | | | — | | | | — | | | | (9 | ) | | | (462 | ) | | | — | |
| Net periodic benefit cost | $ | 14,697 | | | $ | 13,914 | | | $ | 13,760 | | | $ | 8,543 | | | $ | 11,449 | | | $ | 16,554 | |
Other changes in plan assets and benefit obligations recognized in other comprehensive income (loss) for the year ending July 31, 2009 are as follows:
| | | | U.S. Plans | | | Foreign Plans |
| Net actuarial loss | $ | 30,659 | | | $ | 43,291 | |
| Recognized actuarial loss | | (1,056 | ) | | | (692 | ) |
| | Prior service cost | | 3,147 | | | | — | |
| Recognized prior service credit | | (1,538 | ) | | | (235 | ) |
| Effect of exchange rates on amounts included in accumulated other | | | | | | | |
| comprehensive income | | — | | | | (4,283 | ) |
| Total recognized in other comprehensive loss (before tax effects) | $ | 31,212 | | | $ | 38,081 | |
| Total recognized in other comprehensive loss, net of tax effects | $ | 20,367 | | | $ | 27,288 | |
| Total recognized in net periodic benefit cost and other comprehensive | | | | | | | |
| loss (before tax effects) | $ | 45,909 | | | $ | 46,624 | |
| Total recognized in net periodic benefit cost and other comprehensive | | | | | | | |
| loss, net of tax effects | $ | 29,773 | | | $ | 32,930 | |
Amounts recognized in accumulated other comprehensive loss as of July 31, 2009 are as follows:
| | | | | | | Foreign | | | |
| | | U.S. Plans | | | Plans | | | Total |
| Prior service cost | $ | 9,411 | | $ | 1,227 | | $ | 10,638 |
| Net actuarial loss | | 62,325 | | | 90,663 | | | 152,988 |
| Total amounts recognized in accumulated other | | | | | | | | |
| comprehensive loss | $ | 71,736 | | $ | 91,890 | | $ | 163,626 |
| |
| Amounts recognized in accumulated other comprehensive income as of July 31, 2008 are as follows: |
| | |
| | | | | | Foreign | | | |
| | | U.S. Plans | | | Plans | | | Total |
| Prior service cost | $ | 7,802 | | $ | 1,044 | | $ | 8,846 |
| Net actuarial loss | | 32,722 | | | 52,765 | | | 85,487 |
| Total amounts recognized in accumulated other | | | | | | | | |
| comprehensive income | $ | 40,524 | | $ | 53,809 | | $ | 94,333 |
78
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
Amounts in accumulated other comprehensive income expected to be amortized as components of net periodic benefit cost during fiscal year 2010 are as follows:
| | | | | | | Foreign | | | |
| | | U.S. Plans | | | Plans | | | Total |
| Prior service cost | $ | 1,783 | | $ | 240 | | $ | 2,023 |
| | Net actuarial loss | $ | 2,434 | | $ | 2,926 | | $ | 5,360 |
The following table provides the weighted-average assumptions used to determine benefit obligations and net periodic benefit cost:
| | | U.S. Plans | | Foreign Plans |
| | 2009 | | 2008 | | 2007 | | 2009 | | 2008 | | 2007 |
| Assumptions used to determine | | | | | | | | | | | | | | | | | |
| benefit obligations | | | | | | | | | | | | | | | | | |
| Discount rate | 6.00 | % | | 6.75 | % | | 6.25 | % | | 5.66 | % | | 5.92 | % | | 5.40 | % |
| Rate of compensation increase | 4.66 | % | | 4.69 | % | | 4.68 | % | | 3.01 | % | | 3.15 | % | | 2.94 | % |
| |
| | Assumptions used to determine | | | | | | | | | | | | | | | | | |
| net periodic benefit cost | | | | | | | | | | | | | | | | | |
| Discount rate | 6.75 | % | | 6.25 | % | | 6.25 | % | | 5.92 | % | | 5.40 | % | | 4.83 | % |
| Expected long-term rate of return on | | | | | | | | | | | | | | | | | |
| plan assets | 7.00 | % | | 7.00 | % | | 7.00 | % | | 6.33 | % | | 6.44 | % | | 6.47 | % |
| Rate of compensation increase | 4.69 | % | | 4.68 | % | | 4.68 | % | | 3.15 | % | | 2.94 | % | | 2.93 | % |
The Company determines its actuarial assumptions on an annual basis. To develop the expected long-term rate of return on plan assets assumption, the Company considers the current level of expected returns on risk free investments (primarily government bonds), the historical level of the risk premium associated with the other asset classes in which the portfolio is invested and the expectations for future returns of each asset class. The expected return for each asset class was then weighted based upon the target asset allocation to develop the expected long-term rate of return on plan assets assumption for the portfolio.
The following table provides the Company’s weighted average target plan asset allocation and actual asset allocation by asset category:
| | | 2009 | | 2008 |
| | Target | | Actual | | Actual |
| | | Allocation | | Allocation | | Allocation |
| Equity securities | 53 | % | | 51 | % | | 54 | % |
| Debt securities | 30 | % | | 32 | % | | 31 | % |
| Other | 17 | % | | 17 | % | | 15 | % |
The Company’s investment objective for defined benefit plan assets is to meet the plans’ benefit obligations, while preserving plan assets. The investment strategies focus on asset class diversification, liquidity to meet benefit payments and an appropriate balance of long-term return and risk. Plan assets are diversified across several investment managers and are generally invested in liquid funds that track broad market equity and bond indices. Plan fiduciaries oversee the investment allocation process, which includes selecting investment managers, commissioning periodic asset-liability studies, setting long-term strategic targets and monitoring asset allocations.
Management’s estimate of the Company’s cash requirements for the defined benefit plans for the year ending July 31, 2010 is $33,508. This is comprised of expected benefit payments of $6,566, which will be paid directly to plan participants from Company assets, as well as expected Company contributions of $26,942. Expected contributions are dependent on many variables, including the variability of the market value of the assets as compared to the obligation and other market or regulatory conditions. Accordingly, actual funding may differ from current estimates.
79
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
The following table provides the pension benefits expected to be paid to participants, which include payments funded from the Company’s assets, as discussed above, as well as payments paid from plan assets:
| Expected pension benefit payments | | | |
| 2010 | $ | 23,119 |
| 2011 | | 23,943 |
| 2012 | | 26,122 |
| 2013 | | 33,856 |
| 2014 | | 28,363 |
| 2015-2019 | | 169,854 |
Defined Contribution Plans
The Company’s 401(k) and profit sharing plan covers substantially all domestic employees of the Company and its participating subsidiaries, other than those employees covered by a union retirement plan. The Plan provides that participants may voluntarily contribute a percentage of their compensation and the Company will make a matching contribution equal to 100% of the first 3% of each participant’s contributions. The expense associated with the plan for fiscal years 2009, 2008, and 2007 was $6,134, $6,038 and $4,636, respectively.
The Company and its subsidiaries also participate in defined contribution pension plans primarily for the benefit of certain foreign employees. The expense associated with these plans was $9,544, $10,084 and $7,809 for fiscal years 2009, 2008 and 2007, respectively.
NOTE 15 – CONTINGENCIES AND COMMITMENTS
With respect to the matters described below under the headings Federal Securities Class Actions, Shareholder Derivative Lawsuits and Other Proceedings, no liabilities or insurance recoveries have been reflected in the consolidated financial statements as of July 31, 2009 as these amounts are not probable or estimable.
Federal Securities Class Actions:
Four putative class action lawsuits were filed against the Company and certain members of its management team alleging violations of the federal securities laws relating to the Company’s understatement of certain of its U.S. income tax payments and of its provision for income taxes in certain prior periods as described in Note 2, Audit Committee Inquiry and Restatement to the consolidated financial statements included in the 2007 Form 10-K. These lawsuits were filed between August 14, 2007 and October 11, 2007 in the United States District Court for the Eastern District of New York. By Order dated May 28, 2008, the Court consolidated the cases under the caption “In re Pall Corp,” No. 07-CV-3359 (E.D.N.Y.) (JS) (ARL), appointed a lead plaintiff and ordered that the lead plaintiff file a consolidated amended complaint. The lead plaintiff filed its consolidated amended complaint on August 4, 2008. The lead plaintiff seeks to act as representative for a class consisting of purchasers of the Company’s stock between April 20, 2007, and August 2, 2007, inclusive. The consolidated amended complaint names the Company, its current chief executive officer and chief financial officer as defendants and alleges violations of Section 10(b) and 20(a) of the Exchange Act, as amended, and Rule 10b-5 promulgated by the Securities and Exchange Commission. It alleges that the defendants violated these provisions of the federal securities laws by issuing materially false and misleading public statements about the Company’s financial results and financial statements, including the Company’s income tax liability, effective tax rate, internal controls and accounting practices. The plaintiffs seek unspecified compensatory damages, costs and expenses. The Company moved to dismiss the consolidated amended complaint on September 19, 2008, and filed its reply brief to the lead plaintiff’s opposition to the Company’s motion to dismiss on December 2, 2008. By Memorandum and Order dated September 21, 2009, the Court denied the Company’s motion to dismiss the consolidated amended complaint and granted the lead plaintiff leave to amend the consolidated amended complaint by filing a second amended complaint.
80
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
Shareholder Derivative Lawsuits:
On October 5, 2007, two plaintiffs filed identical derivative lawsuits in New York Supreme Court, Nassau County relating to the tax matter described above. These actions purported to bring claims on behalf of the Company based on allegations that certain current and former directors and officers of the Company breached their fiduciary duties by failing to evaluate and otherwise inform themselves about the Company’s internal controls and financial reporting systems and procedures. In addition, plaintiffs allege that certain officers of the Company were unjustly enriched as a result of the Company’s inaccurate financial results over fiscal years 1999-2006 and the first three quarters of fiscal year 2007. The complaints sought unspecified compensatory damages on behalf of the Company, disgorgement of defendants’ salaries, bonuses, stock grants and stock options, equitable relief and costs and expenses. The Company, acting in its capacity as nominal defendant, moved to dismiss the complaints for failure to make a demand upon the Company’s board of directors, which motions were granted on April 30 and May 2, 2008. On September 19, 2008, the same two plaintiffs filed a derivative lawsuit in New York Supreme Court, Nassau County, which was served on the Company on September 26, 2008 (“September Derivative”). This action purports to bring claims on behalf of the Company based on allegations that certain current and former directors and officers of the Company breached their fiduciary duties and were unjustly enriched in connection with the tax matter. In addition, the plaintiffs allege that the Board’s refusal of their demand to commence an action against the defendants was not made in good faith. The plaintiffs and the Company agreed to stay these proceedings pending resolution of the Company’s motion to dismiss in the federal securities class action lawsuit discussed above, after which resolution the plaintiffs and the Company agreed to confer about a schedule for the defendants’ time to answer or otherwise respond to the complaint.
On November 13, 2008, another shareholder filed a derivative lawsuit in New York Supreme Court, Nassau County, against certain current and former directors and officers of the Company, and against the Company, as nominal defendant, which was served on the Company on December 4, 2008. This action purports to bring similar claims as the September Derivative. The plaintiffs and the Company have agreed to an identical stay as in the September Derivative.
Other Proceedings:
The SEC and U.S. Attorney’s Office for the Eastern District of New York are conducting investigations in connection with the tax matter described above. The Company is cooperating with these investigations.
Environmental Matters:
Certain facilities of the Company are involved in environmental proceedings. The most significant matter pertains to the Company’s subsidiary, Gelman Sciences Inc. (“Gelman”), which constitutes the majority of the $12,454 and $14,749 of accruals in the Company’s Consolidated Balance Sheets at July 31, 2009, and July 31, 2008, respectively. The Company recorded charges of $2,179 and $1,275 in fiscal years 2009 and 2008, respectively, related to environmental matters. The increases recorded to the environmental liabilities represent management’s best estimate of the cost to be incurred to perform remediation. The estimates are based upon the feasibility of the use of certain remediation technologies and processes as well as the facts known to management at the time the estimates are made. (Refer to Note 1, Accounting Policies and Related Matters).
In February 1988, an action was filed in the Circuit Court for Washtenaw County, Michigan (the “Court”) by the State of Michigan (the “State”) against Gelman Sciences Inc. (“Gelman”), a subsidiary acquired by the Company in February 1997. The action sought to compel Gelman to investigate and remediate contamination near Gelman’s Ann Arbor facility and requested reimbursement of costs the State had expended in investigating the contamination, which the State alleged was caused by Gelman’s disposal of waste water from its manufacturing process. Pursuant to a consent judgment entered into by Gelman and the State in October 1992 (amended September 1996 and October 1999) (the “Consent Judgment”), which resolved that litigation, Gelman is remediating the contamination without admitting wrongdoing. In February 2000, the State Assistant Attorney General filed a Motion to Enforce Consent Judgment in the Court seeking approximately $4,900 in stipulated penalties for the alleged violations of the Consent Judgment and additional injunctive relief. Gelman disputed these assertions. Following an evidentiary hearing in July 2000, the Court took the matter of penalties “under advisement.” The Court issued a Remediation Enforcement Order (the “REO”) requiring Gelman to submit and implement a detailed plan to reduce the contamination to acceptable levels within five years. Gelman’s plan has been approved by both the Court and the State. Although groundwater concentrations remain above acceptable levels in much of the affected area, the Court has expressed its satisfaction with Gelman’s progress during hearings both before and after the five-year period expired. Neither the State nor the Court has sought or suggested that Gelman should be penalized based on the continued presence of groundwater contamination at the site.
81
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
In February 2004, the Court instructed Gelman to submit its Final Feasibility Study describing how it intends to address an area of groundwater contamination not addressed by the previously approved plan. Gelman submitted its Feasibility Study as instructed. The State also submitted its plan for remediating this area of contamination to the Court. On December 17, 2004, the Court issued its Order and Opinion Regarding Remediation and Contamination of the Unit E Aquifer (the “Order”) to address an area of groundwater contamination not addressed in the previously approved plan. Gelman is now in the process of implementing the requirements of the Order.
In correspondence dated June 5, 2001, the State asserted that stipulated penalties in the amount of $142 were owed for a separate alleged violation of the Consent Judgment. The Court found that a “substantial basis” for Gelman’s position existed and again took the State’s request “under advisement,” pending the results of certain groundwater monitoring data. That data has been submitted to the Court, but no ruling has been issued.
On August 9, 2001, the State made a written demand for reimbursement of $227 it has allegedly incurred for groundwater monitoring. On October 23, 2006, the State made another written demand for reimbursement of these costs, which now total $494, with interest. In February 2007, the Company met with the State to discuss whether the State would be interested in a proposal for a “global settlement” to include, among other matters, the claim for past monitoring costs ($494). Gelman is engaged in discussion with the State with regard to this demand, however, Gelman considers this claim barred by the Consent Judgment.
By letter dated June 15, 2007, the Michigan Department of Environmental Quality (“DEQ”) claimed Gelman was in violation of the Consent Judgment and related work plans due to its failure to operate a groundwater extraction well in the Evergreen Subdivision at the approved minimum purge rate. The DEQ sought to assess stipulated penalties. Gelman filed a Petition for Dispute Resolution with the Court on July 6, 2007 contesting these penalties. Prior to the hearing on Gelman’s petition, the parties met and the DEQ agreed to waive these penalties in exchange for Gelman’s agreement to perform additional investigations in the area. The Court entered a Stipulated Order to this effect on August 7, 2007. Since then, Gelman has installed several monitoring wells requested by the State. Representatives of Gelman and the State met on December 10, 2007 to discuss the data obtained from these wells and to plan further investigative activities. These discussions are ongoing. On April 15, 2008, Gelman submitted two reports summarizing the results of the investigation to date. Gelman also submitted a “capture zone analysis” that confirmed that Gelman was achieving the cleanup objective for the Evergreen Subdivision system. On June 23, 2008, the State provided its response to these reports. The response also addressed outstanding issues regarding several other areas of the site. In its response, the State asked the Company to undertake additional investigation in the Evergreen Subdivision area and in other areas of the site to more fully delineate the extent of contamination. The State also asked the Company to capture additional contaminated groundwater in the Wagner Road area, near the Gelman property, unless the Company can show that it is not feasible to do so. Gelman proposed to the DEQ several modifications to the Consent Judgment on August 1, 2008 and met with the DEQ to discuss these modifications (and other outstanding issues) on September 15, 2008. The parties agreed that Gelman would prepare and submit to the DEQ an outline for modifications to the existing Consent Judgment (and Administrative Orders) by October 15, 2008 and that the parties would meet thereafter to discuss. On April 29, 2009, the Court issued an order that sets forth a schedule for the various steps that must be taken to implement agreed upon modifications to the cleanup program. Pursuant to that schedule, the Company submitted its Comprehensive Proposal to Modify Cleanup Program to the State on May 4, 2009. On June 15, 2009, the State refused to approve the Company's Proposal. Pursuant to the Court-imposed schedule, the Company filed pleadings identifying areas of dispute and motions seeking approval of its Proposal on August 18, 2009. The DEQ did not file any pleadings regarding the Company’s Proposal, but did file a motion to enforce the existing Consent Judgment that asks the Court to order the Company to undertake additional response activities with regard to certain portions of the site. The DEQ’s motion does not seek monetary damages. The Court has not indicated the exact process by which it will resolve these disputes.
In the opinion of management, the Company is in substantial compliance with applicable environmental laws and its accruals for environmental remediation are adequate at this time. Because regulatory standards under environmental laws are becoming increasingly stringent, there can be no assurance that future developments, additional information and experience gained will not cause the Company to incur material environmental liabilities or costs beyond those accrued in its consolidated financial statements.
82
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
Other Commitments and Contingencies:
The Company and its subsidiaries are subject to certain other legal actions that arise in the normal course of business. It is management’s opinion that these other actions will not have a material effect on the Company’s financial position.
The Company warrants its products against defect in design, materials and workmanship over various time periods. Warranty costs are recorded based upon experience. The warranty accrual as of July 31, 2009 and July 31, 2008 is immaterial to the financial position of the Company as is the change in the accrual for fiscal year 2009 to the Company’s consolidated results of operations, cash flows and financial position.
As of July 31, 2009, the Company had surety bonds outstanding relating primarily to its long-term contracts with governmental agencies of approximately $128,319.
The Company and its subsidiaries lease office and warehouse space, automobiles, computers and office equipment. Rent expense for all operating leases amounted to approximately $31,601 in 2009, $30,818 in 2008 and $25,664 in 2007. Future minimum rental commitments at July 31, 2009, for all non-cancelable operating leases with initial terms exceeding one year are $21,982 in 2010; $14,335 in 2011; $9,922 in 2012; $6,056 in 2013, $4,617 in 2014 and $5,587 thereafter.
The Company and its subsidiaries have various non-cancelable purchase commitments for goods or services with various vendors that have terms in excess of one year. Future purchase commitments at July 31, 2009, for the aforementioned purchase commitments are $60,882 in 2010; $5,585 in 2011; $4,705 in 2012, $4,676 in 2013 and $3,495 in 2014 and $2,662 thereafter.
NOTE 16 – COMMON STOCK
Shareholder Rights Plan
In 1989, the board of directors adopted, and the Company’s shareholders approved, a Shareholder Rights Plan. Under the Plan, as amended on April 20, 1999, one right is attached to each outstanding share of the Company’s common stock. Each right, when it becomes exercisable, will entitle the registered holder to purchase one share of the Company’s common stock at an initial exercise price of $80 per share, subject to adjustment in certain events. The rights will become exercisable and will trade separately from the common stock (1) ten days after any person or group acquires 20% or more of the Company’s outstanding common stock (an Acquiring Person), or (2) ten business days after any person or group commences or announces a tender offer for 20% or more of the outstanding common stock. If any person or group becomes an Acquiring Person, each holder of a right, other than rights owned by the Acquiring Person, would thereafter be entitled, upon exercise of the right at the exercise price, to receive a number of shares of common stock of the Company having a market value at that time of twice the exercise price of the right. Alternatively, the board of directors could exchange the rights not owned by the Acquiring Person for common stock at an exchange ratio of one share of common stock per right. In addition, if the Company is acquired in a merger or other business combination, or 50% or more of its consolidated assets or earning power are sold, each holder of a right would thereafter be entitled, upon exercise of the right at the exercise price, to receive a number of shares of the most powerful voting capital stock of the acquiring company, which at the time of the business combination or sale had a market value of twice the exercise price of the right.
The rights will expire on December 1, 2009, unless earlier redeemed. The rights are redeemable by the board of directors for one-third of a cent per right at any time until a person or group becomes an Acquiring Person.
Stock Repurchase Programs
On November 15, 2006, the board authorized an expenditure of $250,000 to repurchase shares. On October 16, 2008, the board authorized an additional expenditure of $350,000 to repurchase shares. The Company’s shares may be purchased over time, as market and business conditions warrant. There is no time restriction on this authorization. Total repurchases in fiscal year 2009 were 3,347 shares at an aggregate cost of $96,439, with an average price per share of $28.81. The aggregate cost of repurchases in fiscal years 2008 and 2007 was $148,850 (4,056 shares at an average price per share of $36.70) and $61,795 (1,586 shares at an average price per share of $38.98), respectively. As of July 31, 2009, $452,943 remains to be expended under the current board repurchase authorizations. Repurchased shares are held in treasury for use in connection with the Company’s stock plans and for general corporate purposes.
83
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
Stock Plans
The Company currently has four stock-based employee compensation plans which are described more fully below under the captions Stock Purchase Plans and Stock Option and Restricted Stock Unit Plans. The detailed components of stock-based compensation expense recorded in the Consolidated Statements of Earnings for the years ended July 31, 2009, July 31, 2008 and July 31, 2007 are illustrated in the table below.
| | July 31, 2009 | | July 31, 2008 | | July 31, 2007 |
| Stock options | | $ | 4,627 | | $ | 3,092 | | $ | 4,034 |
| Restricted stock units | | | 10,136 | | | 6,609 | | | 4,881 |
| Employee stock purchase plan (“ESPP”) | | | 4,939 | | | 3,957 | | | 2,955 |
| Management stock purchase plan (“MSPP”) | | | 3,782 | | | 2,401 | | | 2,252 |
| Total | | $ | 23,484 | | $ | 16,059 | | $ | 14,122 |
The following table illustrates the income tax effects related to stock-based compensation for the fiscal years:
| | 2009 | | 2008 | | 2007 |
| Excess tax benefit in cash flows from | | | | | | | | | |
| financing activities | | $ | 457 | | $ | 1,802 | | $ | 7,929 |
| Tax benefit recognized related to | | | | | | | | | |
| total stock-based compensation expense | | | 6,288 | | | 4,710 | | | 4,728 |
| Actual tax benefit realized for tax deductions | | | | | | | | | |
| from stock-basedpayment arrangements | | | 3,949 | | | 3,738 | | | 9,965 |
The following weighted average assumptions were used in estimating the fair value of stock options and ESPP shares granted during the fiscal years using a Black-Scholes-Merton option pricing formula:
| | 2009 | | 2008 | | 2007 |
| Stock Options | | | | | | | | | | | | |
| Weighted average fair value at | | | | | | | | | | | | |
| grant date | | $ | 6.79 | | | $ | 8.29 | | | $ | 10.03 | |
| Valuation assumptions: | | | | | | | | | | | | |
| Expected dividend yield | | | 1.8 | % | | | 1.7 | % | | | 1.8 | % |
| Expected volatility | | | 31.9 | % | | | 24.9 | % | | | 25.6 | % |
| Expected life (years) | | | 5 | | | | 5 | | | | 5 | |
| Risk-free interest rate | | | 1.8 | % | | | 2.9 | % | | | 4.8 | % |
| | | | | | | | | | | | | |
| | 2009 | | 2008 | | 2007 |
| ESPP Shares | | | | | | | | | | | | |
| Weighted average fair value at | | | | | | | | | | | | |
| grant date | | $ | 7.99 | | | $ | 9.14 | | | $ | 7.77 | |
| Valuation assumptions: | | | | | | | | | | | | |
| Expected dividend yield | | | 1.9 | % | | | 1.3 | % | | | 1.5 | % |
| Expected volatility | | | 56.1 | % | | | 33.7 | % | | | 21.0 | % |
| Expected life (years) | | | ½ year | | | | ½ year | | | | ½ year | |
| Risk-free interest rate | | | 0.7 | % | | | 2.7 | % | | | 5.1 | % |
The Company has placed exclusive reliance on historical volatility in its estimate of expected volatility. The Company used a sequential period of historical data equal to the expected term (or expected life) of the options using a simple average calculation based upon the daily closing prices of the aforementioned period.
84
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
The expected life (years) represents the period of time for which the options granted are expected to be outstanding. This estimate was derived from historical share option exercise experience, which management believes provides the best estimate of the expected term.
The following paragraphs describe each of the aforementioned stock-based compensation plans in detail:
Stock Purchase Plans
During fiscal year 2000, the Company’s shareholders approved two stock purchase plans, the MSPP and the ESPP. Participation in the MSPP is limited to certain executives as approved by the compensation committee of the board of directors, which also established common stock ownership targets for participants. Participation in the ESPP is available to all employees except those that are included in the MSPP.
The purpose of the MSPP is to encourage key employees of the Company to increase their ownership of shares of the Company’s common stock by providing such employees with an opportunity to elect to have portions of their total annual compensation paid in the form of restricted units, to make cash purchases of restricted units and to earn additional matching restricted units which vest over a three year period for matches prior to August 1, 2003 and vest over four years for matches made thereafter. Such restricted units aggregated 1,020 and 838 as of July 31, 2009 and July 31, 2008, respectively. As of July 31, 2009, there was $7,500 of total unrecognized compensation cost related to nonvested restricted stock units granted under the MSPP, which is expected to be recognized over a weighted-average period of 2.7 years.
The following is a summary of MSPP activity during the fiscal years:
| | 2009 | | 2008 | | 2007 |
| Deferred compensation and cash | | | | | | | | | |
| contributions | | $ | 5,382 | | $ | 4,034 | | $ | 3,259 |
| Fair value of restricted stock units vested | | $ | 1,757 | | $ | 1,172 | | $ | 306 |
| Vested units distributed | | | 158 | | | 159 | | | 141 |
The ESPP enables participants to purchase shares of the Company’s common stock through payroll deductions at a price equal to 85% of the lower of the market price at the beginning or end of each semi-annual stock purchase period. The semi-annual offering periods end in April and October. For the years ended July 31, 2009, July 31, 2008 and July 31, 2007, the Company issued 567, 462 and 498 shares at an average price of $22.28, $31.75 and $26.37, respectively.
Both plans provide for accelerated vesting if there is a change in control (as defined in the plans). All of the above shares were issued from treasury stock.
Stock Option and Restricted Stock Unit Plans
The Company has adopted several plans that provide for the granting of stock options to employees and non-employee directors at option prices equal to the market price of the common stock at the date of grant. On November 17, 2004, the Company’s shareholders approved the 2005 Stock Plan, which had been developed in contemplation of adopting the provisions of SFAS No. 123(R). As a result of such approval, the compensation committee of the board of directors (a) amended the 2001 Stock Option Plan for non-employee directors to reduce the total number of shares remaining available for grants from 261 to 150, and (b) terminated all other stock plans, except that options then outstanding thereunder remained in effect in accordance with their terms. Up to 5,000 shares are issuable under the 2005 Stock Plan. Both plans provide for accelerated vesting if there is a change in control (as defined in the plans). The 2005 Stock Plan permits the Company to grant to its employees and non-employee directors other forms of equity compensation in addition to stock options (that is, restricted shares, restricted units, performance shares and performance units).
The fair value of the restricted unit awards are determined by reference to the closing price of the stock on the date of the award, and are charged to earnings over the service periods during which the awards are deemed to be earned; four years, in the case of units awarded to employees and upon grant, in the case of the annual award units to non-employee directors. The annual award units granted to non-employee directors of the Company (and any related dividends paid in the form of additional units) are converted to shares once the director ceases to be a member of the board of directors. A total of 44 and 19 annual award units were granted during the years ended July 31, 2009 and July 31, 2008, respectively, with weighted-average fair market values of $27.98 and $39.03 per share, respectively. Restricted stock units granted to employees cliff-vest after the fourth anniversary of the date of grant. Dividends paid on unvested restricted stock units vest at the same time as the restricted units for which the dividends were recorded.
85
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
A summary of restricted stock unit activity, excluding annual award units, for the 2005 Stock Plan during the year ended July 31, 2009, is presented below:
| | | | | Weighted- |
| | | | | Average |
| | | | | Grant-Date |
| | Shares | | Fair Value |
| Nonvested at August 1, 2008 | | 1,025 | | | $ | 34.80 |
| Granted | | 432 | | | | 27.54 |
| Vested | | (224 | ) | | | 30.11 |
| Forfeited | | (17 | ) | | | 34.57 |
| Nonvested at July 31, 2009 | | 1,216 | | | $ | 33.10 |
As of July 31, 2009 there was $25,987 of total unrecognized compensation cost related to nonvested restricted stock units granted under the 2005 Stock Plan, which is expected to be recognized over a weighted-average period of 3.1 years.
The forms of options adopted provide that the options may not be exercised within one year from the date of grant, and expire if not completely exercised within seven years from the date of grant. Generally, in any year after the first year, the options can be exercised with respect to only up to 25% of the shares subject to the option, computed cumulatively. The Company’s shareholders have approved all of the Company’s stock plans.
A summary of option activity for all stock option plans during the year ended July 31, 2009 is presented below:
| | | | | Weighted | | Weighted | | | |
| | | | | -Average | | Average | | Aggregate |
| | | | | Exercise | | Remaining | | Intrinsic |
| Options | | Shares | | Price | | Contractual Term | | Value |
| Outstanding at August 1, 2008 | | 3,357 | | | $ | 28.15 | | | | | |
| Granted | | 748 | | | | 26.68 | | | | | |
| Exercised | | (127 | ) | | | 22.43 | | | | | |
| Forfeited or Expired | | (43 | ) | | | 29.10 | | | | | |
| Outstanding at July 31, 2009 | | 3,935 | | | $ | 28.04 | | 4.3 | | $ | 17,100 |
| Expected to vest at July 31, 2009 | | 1,582 | | | $ | 31.84 | | 5.9 | | $ | 2,780 |
| Exercisable at July 31, 2009 | | 2,315 | | | $ | 25.34 | | 3.2 | | $ | 14,278 |
As of July 31, 2009, there was $10,288 of total unrecognized compensation cost related to nonvested stock options, which is expected to be recognized over a weighted-average period of 2.8 years. The total intrinsic value of options exercised during the years ended July 31, 2009, July 31, 2008 and July 31, 2007 was $1,387, $3,760, and $23,883, respectively. The intrinsic value is the result of multiplying shares by the amount by which the current market value of the underlying stock exceeds the exercise price of the option.
As of July 31, 2009, approximately 6,100 shares of common stock of the Company were reserved for stock-based compensation plans. Of the 6,100 shares, approximately 3,000 shares are reserved for vested awards and approximately 3,100 shares are reserved for unvested awards.The Company currently uses treasury shares that have been repurchased through the Company’s stock repurchase program to satisfy share award exercises.
NOTE 17 – INCENTIVE COMPENSATION PLANS
The plans provide additional compensation to officers and key employees of the Company and its subsidiaries based upon the achievement of specified goals. The compensation committee of the board of directors establishes the goals on which the Company’s executive officers are compensated, and management establishes the goals for other covered employees. The aggregate amounts charged to expense in connection with the plans were $19,857, $27,394 and $28,437 for fiscal years 2009, 2008 and 2007, respectively.
86
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
NOTE 18 – OTHER COMPREHENSIVE INCOME (LOSS)
The Company has elected to report comprehensive income in the Consolidated Statement of Stockholders’ Equity. The changes in the components of other comprehensive income (loss) are as follows:
| | Pretax | | | | | | Net |
| | Amount | | Tax Effect | | Amount |
| 2009 | | | | | | | | | | | | | |
| Foreign currency translation | | $ | (50,115 | ) | | $ | (2,299 | ) | | $ | (52,414 | ) |
| Pension liability adjustment | | | (69,293 | ) | | | 21,638 | | | | (47,655 | ) |
| Unrealized investment gains(a) | | | 958 | | | | 122 | | | | 1,080 | |
| Unrealized losses on derivatives | | | (256 | ) | | | 89 | | | | (167 | ) |
| Other comprehensive loss | | $ | (118,706 | ) | | $ | 19,550 | | | $ | (99,156 | ) |
| |
| 2008 | | | | | | | | | | | | | |
| Foreign currency translation | | $ | 32,440 | | | $ | 4,298 | | | $ | 36,738 | |
| Pension liability adjustment | | | 6,853 | | | | (1,139 | ) | | | 5,714 | |
| Unrealized investment losses(a) | | | (808 | ) | | | 350 | | | | (458 | ) |
| Unrealized losses on derivatives | | | (277 | ) | | | 83 | | | | (194 | ) |
| Other comprehensive income | | $ | 38,208 | | | $ | 3,592 | | | $ | 41,800 | |
| |
| 2007 | | | | | | | | | | | | | |
| Foreign currency translation | | $ | 32,204 | | | $ | 3,719 | | | $ | 35,923 | |
| Minimum pension liability adjustment(b) | | | 36,993 | | | | (10,668 | ) | | | 26,325 | |
| Unrealized investment gains(a) | | | 2,450 | | | | (1,598 | ) | | | 852 | |
| Unrealized gains on derivatives | | | 34 | | | | 104 | | | | 138 | |
| Other comprehensive income | | $ | 71,681 | | | $ | (8,443 | ) | | $ | 63,238 | |
| (a) | | The unrealized (losses) gains on available-for-sale securities, net of related taxes, consisted of the following: |
| | 2009 | | 2008 | | 2007 |
| Net unrealized gains/(losses) arising during the period, net of tax | | | | | | | | | | | |
| (expense) benefit of $122, $350 and $(1,598) in 2009, 2008 and | | | | | | | | | | | |
| 2007, respectively | | $ | (958 | ) | | $ | (509 | ) | | $ | 533 |
| Adjustment for unrealized loss included in net earnings due to | | | | | | | | | | | |
| impairment | | | 2,038 | | | | 51 | | | | 319 |
| Other comprehensive income/(loss) | | $ | 1,080 | | | $ | (458 | ) | | $ | 852 |
| (b) | | During the fourth quarter of fiscal year 2007, the Company adopted SFAS No. 158. The initial impact of adopting the provisions of SFAS No. 158 was a charge to accumulated other comprehensive income of $20,277, an increase to noncurrent deferred tax assets of $11,580 and an increase to other non-current liabilities of $31,857. These amounts are not included in the changes in components of other comprehensive income above. |
87
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
NOTE 19 – SEGMENT INFORMATION AND GEOGRAPHIES
The Company serves customers through two global vertically integrated businesses: Life Sciences and Industrial. The Life Sciences business group is focused on developing, manufacturing and selling products to customers in the Medical and BioPharmaceuticals markets. The Industrial business group is focused on developing, manufacturing and selling products to customers in the Aerospace & Transportation, Microelectronics and Energy, Water & Process Technologies markets. The chief executive officer manages the Company and makes key decisions about the allocation of Company resources based on the two businesses. The Company’s reportable segments, that are also its operating segments, consist of its two vertically integrated businesses, Life Sciences and Industrial, in accordance with the provisions of SFAS No. 131, Disclosures about Segments of an Enterprise and Related Information.
The Company’s subsidiaries sell both Life Sciences and Industrial products. As such, certain overhead costs of these subsidiaries are shared by the businesses. Additionally these business groups are supported by shared and corporate services groups that facilitate the Company’s corporate governance and business activities globally.
Cash and cash equivalents, short-term investments, investments and retirement benefit assets and income taxes all of which are managed at the Corporate level, are included in Corporate/Shared Services assets. Furthermore assets not specifically identified to a business group are also included in Corporate/Shared Services assets. Accounts receivable and inventory are in all cases specifically identified to a business group.
Expenses associated with the headquarters operations, interest expense, net, the provision for income taxes, as well as restructuring and other charges are currently excluded from the measurement and evaluation of the profitability of the Company’s reportable segments.
88
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
| SEGMENT INFORMATION: | | 2009 | | 2008 | | 2007 |
| SALES: | | | | | | | | | | | |
| Life Sciences | | $ | 940,461 | | | $ | 975,231 | | | $ | 880,187 |
| Industrial | | | 1,388,697 | | | | 1,596,414 | | | | 1,369,718 |
| Total | | $ | 2,329,158 | | | $ | 2,571,645 | | | $ | 2,249,905 |
| |
| OPERATING PROFIT: | | | | | | | | | | | |
| Life Sciences | | $ | 200,070 | | | $ | 197,774 | | | $ | 165,286 |
| Industrial | | | 186,053 | | | | 245,855 | | | | 204,114 |
| Total operating profit | | | 386,123 | | | | 443,629 | | | | 369,400 |
| General corporate expenses | | | 56,478 | | | | 53,960 | | | | 44,718 |
| Earnings before ROTC, interest expense, net and income taxes (a) | | | 329,645 | | | | 389,669 | | | | 324,682 |
| ROTC (a) | | | 30,723 | | | | 31,538 | | | | 25,097 |
| Interest expense, net | | | 28,136 | | | | 32,576 | | | | 39,056 |
| Earnings before income taxes | | $ | 270,786 | | | $ | 325,555 | | | $ | 260,529 |
| |
| DEPRECIATION AND AMORTIZATION: | | | | | | | | | | | |
| Life Sciences | | $ | 45,593 | | | $ | 47,090 | | | $ | 48,033 |
| Industrial | | | 42,415 | | | | 44,717 | | | | 44,023 |
| Subtotal | | | 88,008 | | | | 91,807 | | | | 92,056 |
| Corporate | | | 1,431 | | | | 1,398 | | | | 1,921 |
| Total | | $ | 89,439 | | | $ | 93,205 | | | $ | 93,977 |
| |
| CAPITAL EXPENDITURES: | | | | | | | | | | | |
| Life Sciences | | $ | 70,865 | | | $ | 67,765 | | | $ | 51,462 |
| Industrial | | | 45,083 | | | | 37,570 | | | | 31,147 |
| Subtotal | | | 115,948 | | | | 105,335 | | | | 82,609 |
| Corporate/Shared Services | | | 17,101 | | | | 18,519 | | | | 15,154 |
| Total | | $ | 133,049 | | | $ | 123,854 | | | $ | 97,763 |
| |
| IDENTIFIABLE ASSETS: | | | | | | | | | | | |
| Life Sciences | | $ | 847,515 | | | $ | 791,272 | | | | |
| Industrial | | | 1,097,627 | | | | 1,187,156 | | | | |
| Subtotal | | | 1,945,142 | | | | 1,978,428 | | | | |
| Corporate/Shared Services | | | 895,670 | | | | 978,318 | | | | |
| Total | | $ | 2,840,812 | | | $ | 2,956,746 | | | | |
| |
| GEOGRAPHIC INFORMATION: | | | | | | | | | | | |
| SALES: | | | | | | | | | | | |
| Western Hemisphere | | $ | 769,702 | | | $ | 810,659 | | | $ | 775,729 |
| Europe | | | 960,307 | | | | 1,106,983 | | | | 927,594 |
| Asia | | | 599,149 | | | | 654,003 | | | | 546,582 |
| Total | | $ | 2,329,158 | | | $ | 2,571,645 | | | $ | 2,249,905 |
| |
| IDENTIFIABLE ASSETS: | | | | | | | | | | | |
| Western Hemisphere | | $ | 1,074,641 | | | $ | 1,000,302 | | | | |
| Europe | | | 643,184 | | | | 737,279 | | | | |
| Asia | | | 271,212 | | | | 293,584 | | | | |
| Eliminations | | | (43,895 | ) | | | (52,737 | ) | | | |
| Subtotal | | | 1,945,142 | | | | 1,978,428 | | | | |
| Corporate/Shared Services | | | 895,670 | | | | 978,318 | | | | |
| Total | | $ | 2,840,812 | | | $ | 2,956,746 | | | | |
89
PALL CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands, except per share data)
| | (a) | | Included in ROTC for the purposes of evaluation of segment profitability are other adjustments recorded in cost of sales of $2,745 for the year ended July 31, 2007. Such adjustments include incremental depreciation and other adjustments recorded primarily in conjunction with the Company’s facilities rationalization initiative. |
Sales by the Company’s U.S. operations to unaffiliated customers totaled approximately $715,000, $754,000 and $726,000 in fiscal years 2009, 2008 and 2007, respectively. Included therein are export sales of approximately $86,000, $66,000 and $71,000 in fiscal years 2009, 2008 and 2007, respectively. Sales by the Company’s subsidiaries in Germany amounted to approximately $234,000, $278,000 and $244,000 in fiscal years 2009, 2008 and 2007, respectively. Sales by the Company’s subsidiary in Japan amounted to approximately $214,000, $235,000 and $215,000 in fiscal years 2009, 2008 and 2007, respectively. The Company considers its foreign operations to be of major importance to its future growth prospects. The risks related to the Company’s foreign operations include the local political and regulatory developments as well as the regional economic climate.
90
PALL CORPORATION AND SUBSIDIARIES
FINANCIAL STATEMENT SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS
(In thousands)
| | Balance at | | | | | | | | | | | | | Balance at |
| | Beginning | | Additions to | | | | | | Translation | | End |
| Description | | of Year | | Reserve | | Write-offs | | Adjustments | | of Year |
| Allowance for doubtful | | | | | | | | | | | | | | | | | |
| accounts: | | | | | | | | | | | | | | | | | |
| Year Ended: | | | | | | | | | | | | | | | | | |
| July 31, 2009 | | $ | 10,929 | | $ | 2,864 | | $ | (2,584 | ) | | $ | (607 | ) | | $ | 10,602 |
| July 31, 2008 | | $ | 11,810 | | $ | 2,544 | | $ | (4,151 | ) | | $ | 726 | | | $ | 10,929 |
| July 31, 2007 | | $ | 11,902 | | $ | 2,924 | | $ | (3,472 | ) | | $ | 456 | | | $ | 11,810 |
91
